User login
Emapalumab produces major responses in macrophage activation syndrome
MADRID – Initial results of an ongoing small pilot study of the anti-interferon gamma (IFN-gamma) monoclonal antibody emapalumab has demonstrated efficacy in the treatment of glucocorticoid-refractory macrophage activation syndrome (MAS) in patients with systemic juvenile idiopathic arthritis (SJIA).
“By week 4 there was a complete response in all six patients treated. All of them had failed conventional therapy,” Fabrizio De Benedetti, MD, PhD, head of the division of rheumatology at IRCCS Ospedale Pediatrico Bambino Gesù, Rome, reported at the European Congress of Rheumatology.
The results are the first of a multicenter, pilot study with twin protocols in Europe and North America. Emapalumab (Gamifant) is already approved by the Food and Drug Administration for the treatment of primary hemophagocytic lymphohistiocytosis (HLH) unresponsive to conventional therapy.
The study is enrolling children with MAS complicating SJIA that is unresponsive to high-dose intravenous glucocorticoids. Emapalumab, which has been shown to neutralize IFN-gamma in animal models, is being administered in an initial dose of 6 mg/kg followed by doses of 3 mg/kg every 3 days for 4 weeks.
MAS is a common complication of rheumatic diseases, particularly SJIA, according to Dr. De Benedetti. It has been characterized as a secondary form of HLH involving an excessive activation and expansion of macrophages as well as T cells. It can produce a wide variety of complications, including hepatosplenomegaly, liver dysfunction, and coagulation abnormalities. If uncontrolled, it can lead to organ failure and death.
Among the first six patients, four had confirmed SJIA and two had presumptive SJIA. The average age was 11 years with a range of 2 to 25 years. Four of the patients were female.
Many of the patients had failed therapies in addition to glucocorticoids, such as cyclosporine and anakinra. A diagnosis of HLH and prior treatment with a biologic therapy were exclusion criteria.
By 8 weeks, six had a complete response, which included the resolution of symptoms by normalization of ferritin, liver enzymes, and D-dimers. In three of the six patients, a complete response was achieved by week 4.
“Steroid tapering by investigator discretion was permitted, and four of the six patients had a meaningful tapering of steroids within 8 weeks,” Dr. De Benedetti reported.
Of the three serious adverse events recorded so far, only reactivation of cytomegalovirus (CMV) infection was attributed to emapalumab. This infection resolved with treatment. Several other infections observed over the course of the study were not thought to be related to treatment.
The initial results have encouraged an expansion of the study protocol in Europe where several treatment centers are expected to begin enrolling patients shortly. A second parallel study protocol will begin soon in North America, but no patient had been treated at the time that Dr. De Benedetti presented these initial findings.
Based on evidence that IFN-gamma drives hyperinflammation and hypercytokinemia in MAS, the initial results with emapalumab are encouraging, according to Dr. De Benedetti. He said the results not only provide evidence that emapalumab is active in MAS but support the pathogenic role of IFN-gamma in this disease.
Dr. De Benedetti reported financial relationships with multiple pharmaceutical companies, including SOBI, the sponsor of this study.
SOURCE: De Benedetti F et al. Ann Rheum Dis. Jun 2019;78(Suppl2):178. Abstract OPO204, doi: 10.1136/annrheumdis-2019-eular.3341.
MADRID – Initial results of an ongoing small pilot study of the anti-interferon gamma (IFN-gamma) monoclonal antibody emapalumab has demonstrated efficacy in the treatment of glucocorticoid-refractory macrophage activation syndrome (MAS) in patients with systemic juvenile idiopathic arthritis (SJIA).
“By week 4 there was a complete response in all six patients treated. All of them had failed conventional therapy,” Fabrizio De Benedetti, MD, PhD, head of the division of rheumatology at IRCCS Ospedale Pediatrico Bambino Gesù, Rome, reported at the European Congress of Rheumatology.
The results are the first of a multicenter, pilot study with twin protocols in Europe and North America. Emapalumab (Gamifant) is already approved by the Food and Drug Administration for the treatment of primary hemophagocytic lymphohistiocytosis (HLH) unresponsive to conventional therapy.
The study is enrolling children with MAS complicating SJIA that is unresponsive to high-dose intravenous glucocorticoids. Emapalumab, which has been shown to neutralize IFN-gamma in animal models, is being administered in an initial dose of 6 mg/kg followed by doses of 3 mg/kg every 3 days for 4 weeks.
MAS is a common complication of rheumatic diseases, particularly SJIA, according to Dr. De Benedetti. It has been characterized as a secondary form of HLH involving an excessive activation and expansion of macrophages as well as T cells. It can produce a wide variety of complications, including hepatosplenomegaly, liver dysfunction, and coagulation abnormalities. If uncontrolled, it can lead to organ failure and death.
Among the first six patients, four had confirmed SJIA and two had presumptive SJIA. The average age was 11 years with a range of 2 to 25 years. Four of the patients were female.
Many of the patients had failed therapies in addition to glucocorticoids, such as cyclosporine and anakinra. A diagnosis of HLH and prior treatment with a biologic therapy were exclusion criteria.
By 8 weeks, six had a complete response, which included the resolution of symptoms by normalization of ferritin, liver enzymes, and D-dimers. In three of the six patients, a complete response was achieved by week 4.
“Steroid tapering by investigator discretion was permitted, and four of the six patients had a meaningful tapering of steroids within 8 weeks,” Dr. De Benedetti reported.
Of the three serious adverse events recorded so far, only reactivation of cytomegalovirus (CMV) infection was attributed to emapalumab. This infection resolved with treatment. Several other infections observed over the course of the study were not thought to be related to treatment.
The initial results have encouraged an expansion of the study protocol in Europe where several treatment centers are expected to begin enrolling patients shortly. A second parallel study protocol will begin soon in North America, but no patient had been treated at the time that Dr. De Benedetti presented these initial findings.
Based on evidence that IFN-gamma drives hyperinflammation and hypercytokinemia in MAS, the initial results with emapalumab are encouraging, according to Dr. De Benedetti. He said the results not only provide evidence that emapalumab is active in MAS but support the pathogenic role of IFN-gamma in this disease.
Dr. De Benedetti reported financial relationships with multiple pharmaceutical companies, including SOBI, the sponsor of this study.
SOURCE: De Benedetti F et al. Ann Rheum Dis. Jun 2019;78(Suppl2):178. Abstract OPO204, doi: 10.1136/annrheumdis-2019-eular.3341.
MADRID – Initial results of an ongoing small pilot study of the anti-interferon gamma (IFN-gamma) monoclonal antibody emapalumab has demonstrated efficacy in the treatment of glucocorticoid-refractory macrophage activation syndrome (MAS) in patients with systemic juvenile idiopathic arthritis (SJIA).
“By week 4 there was a complete response in all six patients treated. All of them had failed conventional therapy,” Fabrizio De Benedetti, MD, PhD, head of the division of rheumatology at IRCCS Ospedale Pediatrico Bambino Gesù, Rome, reported at the European Congress of Rheumatology.
The results are the first of a multicenter, pilot study with twin protocols in Europe and North America. Emapalumab (Gamifant) is already approved by the Food and Drug Administration for the treatment of primary hemophagocytic lymphohistiocytosis (HLH) unresponsive to conventional therapy.
The study is enrolling children with MAS complicating SJIA that is unresponsive to high-dose intravenous glucocorticoids. Emapalumab, which has been shown to neutralize IFN-gamma in animal models, is being administered in an initial dose of 6 mg/kg followed by doses of 3 mg/kg every 3 days for 4 weeks.
MAS is a common complication of rheumatic diseases, particularly SJIA, according to Dr. De Benedetti. It has been characterized as a secondary form of HLH involving an excessive activation and expansion of macrophages as well as T cells. It can produce a wide variety of complications, including hepatosplenomegaly, liver dysfunction, and coagulation abnormalities. If uncontrolled, it can lead to organ failure and death.
Among the first six patients, four had confirmed SJIA and two had presumptive SJIA. The average age was 11 years with a range of 2 to 25 years. Four of the patients were female.
Many of the patients had failed therapies in addition to glucocorticoids, such as cyclosporine and anakinra. A diagnosis of HLH and prior treatment with a biologic therapy were exclusion criteria.
By 8 weeks, six had a complete response, which included the resolution of symptoms by normalization of ferritin, liver enzymes, and D-dimers. In three of the six patients, a complete response was achieved by week 4.
“Steroid tapering by investigator discretion was permitted, and four of the six patients had a meaningful tapering of steroids within 8 weeks,” Dr. De Benedetti reported.
Of the three serious adverse events recorded so far, only reactivation of cytomegalovirus (CMV) infection was attributed to emapalumab. This infection resolved with treatment. Several other infections observed over the course of the study were not thought to be related to treatment.
The initial results have encouraged an expansion of the study protocol in Europe where several treatment centers are expected to begin enrolling patients shortly. A second parallel study protocol will begin soon in North America, but no patient had been treated at the time that Dr. De Benedetti presented these initial findings.
Based on evidence that IFN-gamma drives hyperinflammation and hypercytokinemia in MAS, the initial results with emapalumab are encouraging, according to Dr. De Benedetti. He said the results not only provide evidence that emapalumab is active in MAS but support the pathogenic role of IFN-gamma in this disease.
Dr. De Benedetti reported financial relationships with multiple pharmaceutical companies, including SOBI, the sponsor of this study.
SOURCE: De Benedetti F et al. Ann Rheum Dis. Jun 2019;78(Suppl2):178. Abstract OPO204, doi: 10.1136/annrheumdis-2019-eular.3341.
REPORTING FROM EULAR 2019 CONGRESS
July: An important month for pediatric hospital medicine
National conferences and grassroots initiatives
Each July, the largest gathering of pediatric hospitalists occurs, and 2019 is no different! This year, hospitalists who care for children will gather at Pediatric Hospital Medicine (PHM) in Seattle from July 25 to 28, with the goal of enhancing participants’ knowledge and competence in the areas of innovation, clinical medicine, education, health services, practice management, quality improvement, and research.
But what makes this year particularly special is the launch of the subspecialty exam for certification in pediatric hospital medicine coming later this fall, solidifying its growth and importance within hospital medicine and the entire health care landscape. The American Board of Pediatrics (ABP) has approved PHM as the newest board subspecialty with a 2-year fellowship accredited by the Accreditation Council for Graduate Medical Education (ACGME). This conference will be a great opportunity to join with others to review competencies for board review, as well as to network with those who are also navigating the road ahead.
During 2019, the Pediatric Hospitalist Special Interest Group (SIG) of SHM has been working tirelessly on several initiatives, including a revision of the Pediatric Hospital Medicine Core Competencies as well as additional work to develop Choosing Wisely 2.0 recommendations. These will help us ensure we are developing the best curricula for the next generation of pediatric hospitalists, while cutting back on unnecessary tests and procedures for those practicing today. Each of these initiatives, as well as the July conference, highlights the opportunities that we have within SHM to work with other like-minded providers who care for children. While we partner with all professionals across many organizations, like the American Academy of Pediatrics and the Academic Pediatric Association to name a few, I wanted to share my reflections on SHM and my appreciation for the “big tent” philosophy that has served us so well thus far.
Having an opportunity to sit on the board of SHM has allowed me a chance to really appreciate the efforts that this organization invests in all who care for patients in the hospital; we have an active group of advanced-practice providers, practice administrators, residents, students, academic hospitalists, and the list goes on and on. We collaborate with a number of spectacular societies dedicated to medical specialties, and we are always open to new ways of improving the methods of delivering care to patients, in hospitals, post-acute care facilities, homes – you name it! As health care delivery models continue to evolve, I believe we are well positioned to be leaders in the delivery of acute care medicine in the hospital and beyond.
I have also learned of happenings at the grassroots level by attending SHM chapter meetings across the United States. For example, the Hampton Roads Chapter led a great Point-of-Care Ultrasound (POCUS) workshop, and influenced by that, I shared an idea at home in Nashville – borrowing my son as a model to demonstrate ultrasound techniques that hospitalists can use to assist in clinical care. I hope you, as pediatric hospitalists, will see if you have a local chapter and attend a meeting; whether you are a member of SHM or not, you can mingle with those who provide acute care treatments to all your communities and share best practices. If you don’t see an SHM chapter close by, let’s get one going! SHM is here to help launch a chapter that can help bring your community together and provide education and networking closer to home.
If you can’t attend PHM in Seattle this year, I hope you will make every effort to be at PHM 2020, where our own SIG leader, Dr. Jeffrey Grill from Louisville, Ky., will be chairing the next rendition of this amazing conference. The SHM Meetings team led by Michelle Kann will be working tirelessly to make it a great event with continued growth in content and attendance.
Dr. Rehm is associate professor, pediatrics, and director, division of pediatric outreach medicine at Vanderbilt University and Monroe Carell Jr. Children’s Hospital at Vanderbilt, both in Nashville, Tenn. She is also a member of the SHM board of directors.
National conferences and grassroots initiatives
National conferences and grassroots initiatives
Each July, the largest gathering of pediatric hospitalists occurs, and 2019 is no different! This year, hospitalists who care for children will gather at Pediatric Hospital Medicine (PHM) in Seattle from July 25 to 28, with the goal of enhancing participants’ knowledge and competence in the areas of innovation, clinical medicine, education, health services, practice management, quality improvement, and research.
But what makes this year particularly special is the launch of the subspecialty exam for certification in pediatric hospital medicine coming later this fall, solidifying its growth and importance within hospital medicine and the entire health care landscape. The American Board of Pediatrics (ABP) has approved PHM as the newest board subspecialty with a 2-year fellowship accredited by the Accreditation Council for Graduate Medical Education (ACGME). This conference will be a great opportunity to join with others to review competencies for board review, as well as to network with those who are also navigating the road ahead.
During 2019, the Pediatric Hospitalist Special Interest Group (SIG) of SHM has been working tirelessly on several initiatives, including a revision of the Pediatric Hospital Medicine Core Competencies as well as additional work to develop Choosing Wisely 2.0 recommendations. These will help us ensure we are developing the best curricula for the next generation of pediatric hospitalists, while cutting back on unnecessary tests and procedures for those practicing today. Each of these initiatives, as well as the July conference, highlights the opportunities that we have within SHM to work with other like-minded providers who care for children. While we partner with all professionals across many organizations, like the American Academy of Pediatrics and the Academic Pediatric Association to name a few, I wanted to share my reflections on SHM and my appreciation for the “big tent” philosophy that has served us so well thus far.
Having an opportunity to sit on the board of SHM has allowed me a chance to really appreciate the efforts that this organization invests in all who care for patients in the hospital; we have an active group of advanced-practice providers, practice administrators, residents, students, academic hospitalists, and the list goes on and on. We collaborate with a number of spectacular societies dedicated to medical specialties, and we are always open to new ways of improving the methods of delivering care to patients, in hospitals, post-acute care facilities, homes – you name it! As health care delivery models continue to evolve, I believe we are well positioned to be leaders in the delivery of acute care medicine in the hospital and beyond.
I have also learned of happenings at the grassroots level by attending SHM chapter meetings across the United States. For example, the Hampton Roads Chapter led a great Point-of-Care Ultrasound (POCUS) workshop, and influenced by that, I shared an idea at home in Nashville – borrowing my son as a model to demonstrate ultrasound techniques that hospitalists can use to assist in clinical care. I hope you, as pediatric hospitalists, will see if you have a local chapter and attend a meeting; whether you are a member of SHM or not, you can mingle with those who provide acute care treatments to all your communities and share best practices. If you don’t see an SHM chapter close by, let’s get one going! SHM is here to help launch a chapter that can help bring your community together and provide education and networking closer to home.
If you can’t attend PHM in Seattle this year, I hope you will make every effort to be at PHM 2020, where our own SIG leader, Dr. Jeffrey Grill from Louisville, Ky., will be chairing the next rendition of this amazing conference. The SHM Meetings team led by Michelle Kann will be working tirelessly to make it a great event with continued growth in content and attendance.
Dr. Rehm is associate professor, pediatrics, and director, division of pediatric outreach medicine at Vanderbilt University and Monroe Carell Jr. Children’s Hospital at Vanderbilt, both in Nashville, Tenn. She is also a member of the SHM board of directors.
Each July, the largest gathering of pediatric hospitalists occurs, and 2019 is no different! This year, hospitalists who care for children will gather at Pediatric Hospital Medicine (PHM) in Seattle from July 25 to 28, with the goal of enhancing participants’ knowledge and competence in the areas of innovation, clinical medicine, education, health services, practice management, quality improvement, and research.
But what makes this year particularly special is the launch of the subspecialty exam for certification in pediatric hospital medicine coming later this fall, solidifying its growth and importance within hospital medicine and the entire health care landscape. The American Board of Pediatrics (ABP) has approved PHM as the newest board subspecialty with a 2-year fellowship accredited by the Accreditation Council for Graduate Medical Education (ACGME). This conference will be a great opportunity to join with others to review competencies for board review, as well as to network with those who are also navigating the road ahead.
During 2019, the Pediatric Hospitalist Special Interest Group (SIG) of SHM has been working tirelessly on several initiatives, including a revision of the Pediatric Hospital Medicine Core Competencies as well as additional work to develop Choosing Wisely 2.0 recommendations. These will help us ensure we are developing the best curricula for the next generation of pediatric hospitalists, while cutting back on unnecessary tests and procedures for those practicing today. Each of these initiatives, as well as the July conference, highlights the opportunities that we have within SHM to work with other like-minded providers who care for children. While we partner with all professionals across many organizations, like the American Academy of Pediatrics and the Academic Pediatric Association to name a few, I wanted to share my reflections on SHM and my appreciation for the “big tent” philosophy that has served us so well thus far.
Having an opportunity to sit on the board of SHM has allowed me a chance to really appreciate the efforts that this organization invests in all who care for patients in the hospital; we have an active group of advanced-practice providers, practice administrators, residents, students, academic hospitalists, and the list goes on and on. We collaborate with a number of spectacular societies dedicated to medical specialties, and we are always open to new ways of improving the methods of delivering care to patients, in hospitals, post-acute care facilities, homes – you name it! As health care delivery models continue to evolve, I believe we are well positioned to be leaders in the delivery of acute care medicine in the hospital and beyond.
I have also learned of happenings at the grassroots level by attending SHM chapter meetings across the United States. For example, the Hampton Roads Chapter led a great Point-of-Care Ultrasound (POCUS) workshop, and influenced by that, I shared an idea at home in Nashville – borrowing my son as a model to demonstrate ultrasound techniques that hospitalists can use to assist in clinical care. I hope you, as pediatric hospitalists, will see if you have a local chapter and attend a meeting; whether you are a member of SHM or not, you can mingle with those who provide acute care treatments to all your communities and share best practices. If you don’t see an SHM chapter close by, let’s get one going! SHM is here to help launch a chapter that can help bring your community together and provide education and networking closer to home.
If you can’t attend PHM in Seattle this year, I hope you will make every effort to be at PHM 2020, where our own SIG leader, Dr. Jeffrey Grill from Louisville, Ky., will be chairing the next rendition of this amazing conference. The SHM Meetings team led by Michelle Kann will be working tirelessly to make it a great event with continued growth in content and attendance.
Dr. Rehm is associate professor, pediatrics, and director, division of pediatric outreach medicine at Vanderbilt University and Monroe Carell Jr. Children’s Hospital at Vanderbilt, both in Nashville, Tenn. She is also a member of the SHM board of directors.
Y 2 the ED? (Why patients go to the emergency department)
Along with terminal care and inflated drug prices, the excessive number of “inappropriate” ED visits often is cited as a major driver of health care costs in the United States. Why do so many patients choose to go to the ED for complaints that might be better or more economically treated in another setting?
A report by two researchers in the division of emergency medicine at the Boston Children’s Hospital that appeared in the June 2019 Pediatrics suggests that, at least for pediatric patients, “increased insurance coverage neither drove nor counteracted” the recent trends in ED visits. (“Trends in Pediatric Emergency Department Use After the Affordable Care Act,” Pediatrics. 2019 Jun 1. doi: 10.1542/peds.2018-3542).
I guess it’s not surprising – and somewhat comforting – to learn that, when parents believe their child has an emergent condition they give little thought to the cost of care. Is the trend of increasing ED use a result of an evolving definition of an “emergency”? Your grandparents, or certainly your great grandparents, might claim that, when they were young most minor injuries were handled at home, or at least in the neighborhood by someone with first aid experience who wasn’t put off by the sight of blood. However, a trend away from self-reliance in everything from food preparation to auto repair, combined with media overexposure to the serious complications of apparently minor illness and injury, has left most parents feeling fearful and helpless in the face of adversity.
We have to accept as a given that many parents are going to interpret their child’s situation as emergent, even though you and I might not. But what are the factors that prompt a concerned parent to take his child to the ED instead of a physician’s office? It may simply be the path of least resistance. The parent’s past experience may include frustrating and time-consuming attempts to navigate a clunky phone system only to be met by a receptionist or triage nurse who seems more committed to deflecting calls and protecting the physician’s schedule than getting the patient seen.
The call may miraculously get through to someone with a caring voice and the patience to listen, but the parent then learns that the office doesn’t do minor wound care or he is told that the physician almost certainly will want to do an x-ray of any injured extremity and that the ED is a better choice. It doesn’t take very many scenarios like this to prompt a parent to make his first and only call to the ED. To some extent, physician behavior has helped mold parents’ definition of an emergency.
We are encouraged to make our offices a “medical home.” However, it appears the medical home model is one that is built around chronic conditions and behavioral problems and gives little attention to the acute complaints. When you came running into the house with a skinned knee, did your mother tell to you go across the street to the neighbor’s house because blood made her squeamish and she didn’t have any bandages?
There are ways to structure an office and a schedule which are more welcoming to patients with minor emergencies, and I know it is a difficult sell to physicians who are handcuffed by their EHRs and already overwhelmed by patients with time-consuming behavioral complaints. However, if your practice is facing competition from pop-up urgent care centers or if you are increasingly troubled that your patients are receiving fragmented care, it may not be too late to make your practice into a true medical home that welcomes minor emergencies.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Along with terminal care and inflated drug prices, the excessive number of “inappropriate” ED visits often is cited as a major driver of health care costs in the United States. Why do so many patients choose to go to the ED for complaints that might be better or more economically treated in another setting?
A report by two researchers in the division of emergency medicine at the Boston Children’s Hospital that appeared in the June 2019 Pediatrics suggests that, at least for pediatric patients, “increased insurance coverage neither drove nor counteracted” the recent trends in ED visits. (“Trends in Pediatric Emergency Department Use After the Affordable Care Act,” Pediatrics. 2019 Jun 1. doi: 10.1542/peds.2018-3542).
I guess it’s not surprising – and somewhat comforting – to learn that, when parents believe their child has an emergent condition they give little thought to the cost of care. Is the trend of increasing ED use a result of an evolving definition of an “emergency”? Your grandparents, or certainly your great grandparents, might claim that, when they were young most minor injuries were handled at home, or at least in the neighborhood by someone with first aid experience who wasn’t put off by the sight of blood. However, a trend away from self-reliance in everything from food preparation to auto repair, combined with media overexposure to the serious complications of apparently minor illness and injury, has left most parents feeling fearful and helpless in the face of adversity.
We have to accept as a given that many parents are going to interpret their child’s situation as emergent, even though you and I might not. But what are the factors that prompt a concerned parent to take his child to the ED instead of a physician’s office? It may simply be the path of least resistance. The parent’s past experience may include frustrating and time-consuming attempts to navigate a clunky phone system only to be met by a receptionist or triage nurse who seems more committed to deflecting calls and protecting the physician’s schedule than getting the patient seen.
The call may miraculously get through to someone with a caring voice and the patience to listen, but the parent then learns that the office doesn’t do minor wound care or he is told that the physician almost certainly will want to do an x-ray of any injured extremity and that the ED is a better choice. It doesn’t take very many scenarios like this to prompt a parent to make his first and only call to the ED. To some extent, physician behavior has helped mold parents’ definition of an emergency.
We are encouraged to make our offices a “medical home.” However, it appears the medical home model is one that is built around chronic conditions and behavioral problems and gives little attention to the acute complaints. When you came running into the house with a skinned knee, did your mother tell to you go across the street to the neighbor’s house because blood made her squeamish and she didn’t have any bandages?
There are ways to structure an office and a schedule which are more welcoming to patients with minor emergencies, and I know it is a difficult sell to physicians who are handcuffed by their EHRs and already overwhelmed by patients with time-consuming behavioral complaints. However, if your practice is facing competition from pop-up urgent care centers or if you are increasingly troubled that your patients are receiving fragmented care, it may not be too late to make your practice into a true medical home that welcomes minor emergencies.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Along with terminal care and inflated drug prices, the excessive number of “inappropriate” ED visits often is cited as a major driver of health care costs in the United States. Why do so many patients choose to go to the ED for complaints that might be better or more economically treated in another setting?
A report by two researchers in the division of emergency medicine at the Boston Children’s Hospital that appeared in the June 2019 Pediatrics suggests that, at least for pediatric patients, “increased insurance coverage neither drove nor counteracted” the recent trends in ED visits. (“Trends in Pediatric Emergency Department Use After the Affordable Care Act,” Pediatrics. 2019 Jun 1. doi: 10.1542/peds.2018-3542).
I guess it’s not surprising – and somewhat comforting – to learn that, when parents believe their child has an emergent condition they give little thought to the cost of care. Is the trend of increasing ED use a result of an evolving definition of an “emergency”? Your grandparents, or certainly your great grandparents, might claim that, when they were young most minor injuries were handled at home, or at least in the neighborhood by someone with first aid experience who wasn’t put off by the sight of blood. However, a trend away from self-reliance in everything from food preparation to auto repair, combined with media overexposure to the serious complications of apparently minor illness and injury, has left most parents feeling fearful and helpless in the face of adversity.
We have to accept as a given that many parents are going to interpret their child’s situation as emergent, even though you and I might not. But what are the factors that prompt a concerned parent to take his child to the ED instead of a physician’s office? It may simply be the path of least resistance. The parent’s past experience may include frustrating and time-consuming attempts to navigate a clunky phone system only to be met by a receptionist or triage nurse who seems more committed to deflecting calls and protecting the physician’s schedule than getting the patient seen.
The call may miraculously get through to someone with a caring voice and the patience to listen, but the parent then learns that the office doesn’t do minor wound care or he is told that the physician almost certainly will want to do an x-ray of any injured extremity and that the ED is a better choice. It doesn’t take very many scenarios like this to prompt a parent to make his first and only call to the ED. To some extent, physician behavior has helped mold parents’ definition of an emergency.
We are encouraged to make our offices a “medical home.” However, it appears the medical home model is one that is built around chronic conditions and behavioral problems and gives little attention to the acute complaints. When you came running into the house with a skinned knee, did your mother tell to you go across the street to the neighbor’s house because blood made her squeamish and she didn’t have any bandages?
There are ways to structure an office and a schedule which are more welcoming to patients with minor emergencies, and I know it is a difficult sell to physicians who are handcuffed by their EHRs and already overwhelmed by patients with time-consuming behavioral complaints. However, if your practice is facing competition from pop-up urgent care centers or if you are increasingly troubled that your patients are receiving fragmented care, it may not be too late to make your practice into a true medical home that welcomes minor emergencies.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Acquired MMR immunity doesn’t last to age 1 year
according to results of a study in Vaccine.
María José Cilleruelo, PhD, of Hospital Universitario Puerta de Hierro in Majadahonda, Spain, and colleagues showed that, although most infants acquire some protective antibodies against MMR from their mothers during gestation, most have lost this protection as early as 3 months of age. This single-center, observational, prospective study was conducted between October 2013 and December 2014, and it began with 146 mother-child pairs, with 99 remaining in follow-up at 3 months, 77 at 6 months, 63 at 9 months, and 30 at 12 months. For measles, 88% of newborns were seropositive, but only 19% were at 3 months; for mumps, 70% of newborns were seropositive, but only 11% were at 3 months; and for rubella, 91% of newborns were seropositive, but only 13% were at 3 months. No infants were seropositive for mumps or rubella at 9 months, and only 2% were for measles. No infants were seropositive for any of these viruses by 12 months of age.
The investigators noted that, given Spain (where the study was conducted) is a country that gives the first MMR vaccine at 12 months of life, these declining titers can leave most infants vulnerable to those viruses before then.
“We suggest that it may be worth considering administering the first dose of MMR vaccine before 12 months of age,” the investigators concluded, although they advised studies be undertaken into the efficacy and safety of administration of that vaccine in infants younger than 12 months. They noted that the biggest limitation of this study was the high percentage of loss to follow-up, which limited the statistical power to make comparisons.
The study was funded by the Fondo de Investigación Sanitaria, and one of the authors was funded by CIBER de Epidemiología y Salud Pública. The authors declared that there are no conflicts of interest.
SOURCE: Cilleruelo MJ et al. Vaccine. 2019;37:4164-71.
according to results of a study in Vaccine.
María José Cilleruelo, PhD, of Hospital Universitario Puerta de Hierro in Majadahonda, Spain, and colleagues showed that, although most infants acquire some protective antibodies against MMR from their mothers during gestation, most have lost this protection as early as 3 months of age. This single-center, observational, prospective study was conducted between October 2013 and December 2014, and it began with 146 mother-child pairs, with 99 remaining in follow-up at 3 months, 77 at 6 months, 63 at 9 months, and 30 at 12 months. For measles, 88% of newborns were seropositive, but only 19% were at 3 months; for mumps, 70% of newborns were seropositive, but only 11% were at 3 months; and for rubella, 91% of newborns were seropositive, but only 13% were at 3 months. No infants were seropositive for mumps or rubella at 9 months, and only 2% were for measles. No infants were seropositive for any of these viruses by 12 months of age.
The investigators noted that, given Spain (where the study was conducted) is a country that gives the first MMR vaccine at 12 months of life, these declining titers can leave most infants vulnerable to those viruses before then.
“We suggest that it may be worth considering administering the first dose of MMR vaccine before 12 months of age,” the investigators concluded, although they advised studies be undertaken into the efficacy and safety of administration of that vaccine in infants younger than 12 months. They noted that the biggest limitation of this study was the high percentage of loss to follow-up, which limited the statistical power to make comparisons.
The study was funded by the Fondo de Investigación Sanitaria, and one of the authors was funded by CIBER de Epidemiología y Salud Pública. The authors declared that there are no conflicts of interest.
SOURCE: Cilleruelo MJ et al. Vaccine. 2019;37:4164-71.
according to results of a study in Vaccine.
María José Cilleruelo, PhD, of Hospital Universitario Puerta de Hierro in Majadahonda, Spain, and colleagues showed that, although most infants acquire some protective antibodies against MMR from their mothers during gestation, most have lost this protection as early as 3 months of age. This single-center, observational, prospective study was conducted between October 2013 and December 2014, and it began with 146 mother-child pairs, with 99 remaining in follow-up at 3 months, 77 at 6 months, 63 at 9 months, and 30 at 12 months. For measles, 88% of newborns were seropositive, but only 19% were at 3 months; for mumps, 70% of newborns were seropositive, but only 11% were at 3 months; and for rubella, 91% of newborns were seropositive, but only 13% were at 3 months. No infants were seropositive for mumps or rubella at 9 months, and only 2% were for measles. No infants were seropositive for any of these viruses by 12 months of age.
The investigators noted that, given Spain (where the study was conducted) is a country that gives the first MMR vaccine at 12 months of life, these declining titers can leave most infants vulnerable to those viruses before then.
“We suggest that it may be worth considering administering the first dose of MMR vaccine before 12 months of age,” the investigators concluded, although they advised studies be undertaken into the efficacy and safety of administration of that vaccine in infants younger than 12 months. They noted that the biggest limitation of this study was the high percentage of loss to follow-up, which limited the statistical power to make comparisons.
The study was funded by the Fondo de Investigación Sanitaria, and one of the authors was funded by CIBER de Epidemiología y Salud Pública. The authors declared that there are no conflicts of interest.
SOURCE: Cilleruelo MJ et al. Vaccine. 2019;37:4164-71.
FROM VACCINE
LAIV doesn’t up asthmatic children’s risk of lower respiratory events
, according to an analysis published in Vaccine.
The data corroborate other research indicating that live attenuated influenza vaccine (LAIV) is safe for children with asthma older than 2 years and suggest that the choice of vaccination in this population should be based on effectiveness, according to James D. Nordin, MD, MPH, a clinical researcher at HealthPartners Institute in Minneapolis, and colleagues.
Children and adolescents with asthma have an increased risk of morbidity if they contract influenza. They represent a disproportionate number of pediatric influenza hospitalizations and have been a focus of efforts to vaccinate children against influenza. Since 2003, the inactivated influenza vaccine (IIV) and the LAIV have been available. Research indicates that LAIV is more effective than IIV at preventing culture-confirmed influenza in children. Two studies found an increased risk of wheezing in children who received LAIV, but other studies failed to replicate these findings.
A retrospective cohort study
Dr. Nordin and associates conducted a retrospective observational cohort study to investigate whether use of a guideline recommending LAIV for children aged 2 years and older with asthma increased the risk of lower respiratory events within 21 or 42 days of vaccination, compared with standard guidelines to administer IIV in children with asthma. The investigators drew data from two large medical groups with independent clinical leadership that serve demographically similar populations in Minnesota. One group (the LAIV group) switched its preference for all children from IIV to LAIV in 2010. The control group continued using IIV for children with asthma throughout the study period. Each group operates more than 20 clinics.
The investigators included children and adolescents aged 2-17 years who presented during one or more influenza season from 2007-2008 through 2014-2015. Eligible participants had a diagnosis of asthma or wheezing, received one or more influenza vaccines, had continuous insurance enrollment, and had at least one primary care or asthma related subspecialty encounter. They excluded patients with contraindications for LAIV (e.g., pregnancy, malignancy, and cystic fibrosis) and those with any hospitalization, ED visit, or outpatient encounter for a lower respiratory event in the 42 days before influenza vaccination.
Dr. Nordin and colleagues used a generalized estimating equation regression to estimate the ratio of rate ratios (RORs) comparing events before and after vaccination between the LAIV guideline and control groups. The researchers examined covariates such as age, gender, race or ethnicity, Medicaid insurance for at least 1 month in the previous year, neighborhood poverty, and neighborhood rates of asthma.
No increased risk
The investigators included 4,771 children and 7,851 child-influenza records in their analysis. During the period from 2007 to 2010, there were 2,215 child-influenza records from children and adolescents included from the LAIV group and 735 from the IIV guideline group. From 2010 to 2015, there were 3,767 child-influenza records in children and adolescents from the LAIV group and 1,134 from the IIV guideline group. After the LAIV group adopted the new guideline, the proportion of patients receiving LAIV increased from 23% to 68% in the LAIV group and from 7% to 11% in the control group.
About 88% of lower respiratory events included diagnoses for asthma exacerbations. When the investigators adjusted the data for age, asthma severity, asthma control, race or ethnicity, and Medicaid coverage, they found no increase in lower respiratory events associated with the LAIV guideline. The adjusted ROR was 0.74 for lower respiratory events within 21 days of vaccination and 0.77 for lower respiratory events within 42 days of vaccination. The results were similar when Dr. Nordin and colleagues stratified the data by age group, and including additional covariates did not alter the ROR estimates. In all, 21 hospitalizations occurred within 42 days of influenza vaccination, and the LAIV guideline did not increase the risk for hospitalization.
“Findings from this study are consistent with several recent observational studies of LAIV in children and adolescents with asthma,” said Dr. Nordin and colleagues.
One limitation of the current study was that the data were restricted to the information available in electronic health care or claims records. The researchers therefore were able to observe only medically attended lower respiratory events. Furthermore, the exclusion of asthma management encounters and the classification of asthma severity were based on diagnoses, visits, and medication orders and fills. The estimates thus are prone to misclassification, which may have biased the results. Finally, information on important variables such as daycare attendance, presence of school-age siblings, and exposure to secondhand smoke was not available.
The research was funded by a grant from the National Institute of Allergy and Infectious Diseases. The authors had no relevant financial disclosures.
SOURCE: Nordin JD et al. Vaccine. 2019 Jun 10. doi: 10.1016/j.vaccine.2019.05.081.
, according to an analysis published in Vaccine.
The data corroborate other research indicating that live attenuated influenza vaccine (LAIV) is safe for children with asthma older than 2 years and suggest that the choice of vaccination in this population should be based on effectiveness, according to James D. Nordin, MD, MPH, a clinical researcher at HealthPartners Institute in Minneapolis, and colleagues.
Children and adolescents with asthma have an increased risk of morbidity if they contract influenza. They represent a disproportionate number of pediatric influenza hospitalizations and have been a focus of efforts to vaccinate children against influenza. Since 2003, the inactivated influenza vaccine (IIV) and the LAIV have been available. Research indicates that LAIV is more effective than IIV at preventing culture-confirmed influenza in children. Two studies found an increased risk of wheezing in children who received LAIV, but other studies failed to replicate these findings.
A retrospective cohort study
Dr. Nordin and associates conducted a retrospective observational cohort study to investigate whether use of a guideline recommending LAIV for children aged 2 years and older with asthma increased the risk of lower respiratory events within 21 or 42 days of vaccination, compared with standard guidelines to administer IIV in children with asthma. The investigators drew data from two large medical groups with independent clinical leadership that serve demographically similar populations in Minnesota. One group (the LAIV group) switched its preference for all children from IIV to LAIV in 2010. The control group continued using IIV for children with asthma throughout the study period. Each group operates more than 20 clinics.
The investigators included children and adolescents aged 2-17 years who presented during one or more influenza season from 2007-2008 through 2014-2015. Eligible participants had a diagnosis of asthma or wheezing, received one or more influenza vaccines, had continuous insurance enrollment, and had at least one primary care or asthma related subspecialty encounter. They excluded patients with contraindications for LAIV (e.g., pregnancy, malignancy, and cystic fibrosis) and those with any hospitalization, ED visit, or outpatient encounter for a lower respiratory event in the 42 days before influenza vaccination.
Dr. Nordin and colleagues used a generalized estimating equation regression to estimate the ratio of rate ratios (RORs) comparing events before and after vaccination between the LAIV guideline and control groups. The researchers examined covariates such as age, gender, race or ethnicity, Medicaid insurance for at least 1 month in the previous year, neighborhood poverty, and neighborhood rates of asthma.
No increased risk
The investigators included 4,771 children and 7,851 child-influenza records in their analysis. During the period from 2007 to 2010, there were 2,215 child-influenza records from children and adolescents included from the LAIV group and 735 from the IIV guideline group. From 2010 to 2015, there were 3,767 child-influenza records in children and adolescents from the LAIV group and 1,134 from the IIV guideline group. After the LAIV group adopted the new guideline, the proportion of patients receiving LAIV increased from 23% to 68% in the LAIV group and from 7% to 11% in the control group.
About 88% of lower respiratory events included diagnoses for asthma exacerbations. When the investigators adjusted the data for age, asthma severity, asthma control, race or ethnicity, and Medicaid coverage, they found no increase in lower respiratory events associated with the LAIV guideline. The adjusted ROR was 0.74 for lower respiratory events within 21 days of vaccination and 0.77 for lower respiratory events within 42 days of vaccination. The results were similar when Dr. Nordin and colleagues stratified the data by age group, and including additional covariates did not alter the ROR estimates. In all, 21 hospitalizations occurred within 42 days of influenza vaccination, and the LAIV guideline did not increase the risk for hospitalization.
“Findings from this study are consistent with several recent observational studies of LAIV in children and adolescents with asthma,” said Dr. Nordin and colleagues.
One limitation of the current study was that the data were restricted to the information available in electronic health care or claims records. The researchers therefore were able to observe only medically attended lower respiratory events. Furthermore, the exclusion of asthma management encounters and the classification of asthma severity were based on diagnoses, visits, and medication orders and fills. The estimates thus are prone to misclassification, which may have biased the results. Finally, information on important variables such as daycare attendance, presence of school-age siblings, and exposure to secondhand smoke was not available.
The research was funded by a grant from the National Institute of Allergy and Infectious Diseases. The authors had no relevant financial disclosures.
SOURCE: Nordin JD et al. Vaccine. 2019 Jun 10. doi: 10.1016/j.vaccine.2019.05.081.
, according to an analysis published in Vaccine.
The data corroborate other research indicating that live attenuated influenza vaccine (LAIV) is safe for children with asthma older than 2 years and suggest that the choice of vaccination in this population should be based on effectiveness, according to James D. Nordin, MD, MPH, a clinical researcher at HealthPartners Institute in Minneapolis, and colleagues.
Children and adolescents with asthma have an increased risk of morbidity if they contract influenza. They represent a disproportionate number of pediatric influenza hospitalizations and have been a focus of efforts to vaccinate children against influenza. Since 2003, the inactivated influenza vaccine (IIV) and the LAIV have been available. Research indicates that LAIV is more effective than IIV at preventing culture-confirmed influenza in children. Two studies found an increased risk of wheezing in children who received LAIV, but other studies failed to replicate these findings.
A retrospective cohort study
Dr. Nordin and associates conducted a retrospective observational cohort study to investigate whether use of a guideline recommending LAIV for children aged 2 years and older with asthma increased the risk of lower respiratory events within 21 or 42 days of vaccination, compared with standard guidelines to administer IIV in children with asthma. The investigators drew data from two large medical groups with independent clinical leadership that serve demographically similar populations in Minnesota. One group (the LAIV group) switched its preference for all children from IIV to LAIV in 2010. The control group continued using IIV for children with asthma throughout the study period. Each group operates more than 20 clinics.
The investigators included children and adolescents aged 2-17 years who presented during one or more influenza season from 2007-2008 through 2014-2015. Eligible participants had a diagnosis of asthma or wheezing, received one or more influenza vaccines, had continuous insurance enrollment, and had at least one primary care or asthma related subspecialty encounter. They excluded patients with contraindications for LAIV (e.g., pregnancy, malignancy, and cystic fibrosis) and those with any hospitalization, ED visit, or outpatient encounter for a lower respiratory event in the 42 days before influenza vaccination.
Dr. Nordin and colleagues used a generalized estimating equation regression to estimate the ratio of rate ratios (RORs) comparing events before and after vaccination between the LAIV guideline and control groups. The researchers examined covariates such as age, gender, race or ethnicity, Medicaid insurance for at least 1 month in the previous year, neighborhood poverty, and neighborhood rates of asthma.
No increased risk
The investigators included 4,771 children and 7,851 child-influenza records in their analysis. During the period from 2007 to 2010, there were 2,215 child-influenza records from children and adolescents included from the LAIV group and 735 from the IIV guideline group. From 2010 to 2015, there were 3,767 child-influenza records in children and adolescents from the LAIV group and 1,134 from the IIV guideline group. After the LAIV group adopted the new guideline, the proportion of patients receiving LAIV increased from 23% to 68% in the LAIV group and from 7% to 11% in the control group.
About 88% of lower respiratory events included diagnoses for asthma exacerbations. When the investigators adjusted the data for age, asthma severity, asthma control, race or ethnicity, and Medicaid coverage, they found no increase in lower respiratory events associated with the LAIV guideline. The adjusted ROR was 0.74 for lower respiratory events within 21 days of vaccination and 0.77 for lower respiratory events within 42 days of vaccination. The results were similar when Dr. Nordin and colleagues stratified the data by age group, and including additional covariates did not alter the ROR estimates. In all, 21 hospitalizations occurred within 42 days of influenza vaccination, and the LAIV guideline did not increase the risk for hospitalization.
“Findings from this study are consistent with several recent observational studies of LAIV in children and adolescents with asthma,” said Dr. Nordin and colleagues.
One limitation of the current study was that the data were restricted to the information available in electronic health care or claims records. The researchers therefore were able to observe only medically attended lower respiratory events. Furthermore, the exclusion of asthma management encounters and the classification of asthma severity were based on diagnoses, visits, and medication orders and fills. The estimates thus are prone to misclassification, which may have biased the results. Finally, information on important variables such as daycare attendance, presence of school-age siblings, and exposure to secondhand smoke was not available.
The research was funded by a grant from the National Institute of Allergy and Infectious Diseases. The authors had no relevant financial disclosures.
SOURCE: Nordin JD et al. Vaccine. 2019 Jun 10. doi: 10.1016/j.vaccine.2019.05.081.
FROM VACCINE
The FDA has revised its guidance on fish consumption
The revision touts the health benefits of fish and shellfish and promotes safer fish choices for those who should limit mercury exposure – including women who are or might become pregnant, women who are breastfeeding, and young children.
Those individuals should avoid commercial fish with the highest levels of mercury and should instead choose from “the many types of fish that are lower in mercury – including ones commonly found in grocery stores, such as salmon, shrimp, pollock, canned light tuna, tilapia, catfish, and cod,” according to an FDA press statement.
The potential health benefits of eating fish were highlighted in the U.S. Department of Health and Human Services/Department of Agriculture 2015-2020 Dietary Guidelines for Americans, and in 2017 the FDA and the Environmental Protection Agency released advice on fish consumption, including a user-friendly reference chart regarding mercury levels in various types of fish.
Although the information in the chart has not changed, the FDA revised its advice to expand on the “information about the benefits of fish as part of healthy eating patterns by promoting the science-based recommendations of the 2015-2020 Dietary Guidelines for Americans.”
The advice calls for consumption of at least 8 ounces of seafood per week for adults (less for children) based on a 2,000 calorie diet, and for women who are pregnant or breastfeeding, 8-12 ounces of a variety of seafood per week selected from choices lower in mercury.
“The FDA’s revised advice highlights the many nutrients found in fish, several of which have important roles in growth and development during pregnancy and early childhood. It also highlights the potential health benefits of eating fish as part of a healthy eating pattern, particularly for improving heart health and lowering the risk of obesity,” the press release states.
Despite these benefits – and the recommendations for intake – concerns about mercury in fish have led many pregnant women in the United States to consume far less than the recommended amount of seafood, according to Susan Mayne, PhD, director of the FDA’s Center for Food Safety and Applied Nutrition.
“Our goal is to make sure Americans are equipped with this knowledge so that they can reap the benefits of eating fish, while choosing types of fish that are safe for them and their families to eat,” Dr. Mayne said in the FDA statement.
In response to the revised guidance, John S. Cullen, MD, president of the American Academy of Family Physicians, said that all women should be counseled to eat a well-balanced and varied diet including meats, dairy products, fruits, vegetables, and grains, and pregnant women should limit their intake of fish and seafood products to 8-12 ounces, or about 2-3 fish meals, per week.
Pregnant women may eat salmon in moderation, but should avoid raw seafood of any type because of possible contamination with parasites and Norwalk-like viruses, he said, adding that seafood like shark, swordfish, king mackerel, tilefish, Bigeye (Ahi) tuna steaks, and other long-lived fish high on the food chain should be avoided completely because of high mercury levels.
“While the AAFP did not review the revised advice to the dietary guidelines, family physicians are on the front lines encouraging healthy nutrition for pregnant and breastfeeding women and young children. It’s an ongoing, important part of the patient-physician conversation that begins with the initial prenatal visit,” Dr. Cullen said in a statement.
Similarly, Christopher M. Zahn, MD, vice president of practice activities for the American College of Obstetricians and Gynecologists, said the FDA/EPA updated guidance is in line with ACOG recommendations.
“The guidance continues to underscore the value of eating seafood 2-3 times per week during pregnancy and the importance of avoiding fish products that are high in mercury. The additional emphasis on healthy eating patterns mirrors ACOG’s long-standing guidance on the importance of a well-balanced, varied, nutritional diet that is consistent with a woman’s access to food and food preferences,” he said in a statement, noting that “seafood is a nutrient-rich food that has proven beneficial to women and in aiding the development of a fetus throughout pregnancy.”
The revision touts the health benefits of fish and shellfish and promotes safer fish choices for those who should limit mercury exposure – including women who are or might become pregnant, women who are breastfeeding, and young children.
Those individuals should avoid commercial fish with the highest levels of mercury and should instead choose from “the many types of fish that are lower in mercury – including ones commonly found in grocery stores, such as salmon, shrimp, pollock, canned light tuna, tilapia, catfish, and cod,” according to an FDA press statement.
The potential health benefits of eating fish were highlighted in the U.S. Department of Health and Human Services/Department of Agriculture 2015-2020 Dietary Guidelines for Americans, and in 2017 the FDA and the Environmental Protection Agency released advice on fish consumption, including a user-friendly reference chart regarding mercury levels in various types of fish.
Although the information in the chart has not changed, the FDA revised its advice to expand on the “information about the benefits of fish as part of healthy eating patterns by promoting the science-based recommendations of the 2015-2020 Dietary Guidelines for Americans.”
The advice calls for consumption of at least 8 ounces of seafood per week for adults (less for children) based on a 2,000 calorie diet, and for women who are pregnant or breastfeeding, 8-12 ounces of a variety of seafood per week selected from choices lower in mercury.
“The FDA’s revised advice highlights the many nutrients found in fish, several of which have important roles in growth and development during pregnancy and early childhood. It also highlights the potential health benefits of eating fish as part of a healthy eating pattern, particularly for improving heart health and lowering the risk of obesity,” the press release states.
Despite these benefits – and the recommendations for intake – concerns about mercury in fish have led many pregnant women in the United States to consume far less than the recommended amount of seafood, according to Susan Mayne, PhD, director of the FDA’s Center for Food Safety and Applied Nutrition.
“Our goal is to make sure Americans are equipped with this knowledge so that they can reap the benefits of eating fish, while choosing types of fish that are safe for them and their families to eat,” Dr. Mayne said in the FDA statement.
In response to the revised guidance, John S. Cullen, MD, president of the American Academy of Family Physicians, said that all women should be counseled to eat a well-balanced and varied diet including meats, dairy products, fruits, vegetables, and grains, and pregnant women should limit their intake of fish and seafood products to 8-12 ounces, or about 2-3 fish meals, per week.
Pregnant women may eat salmon in moderation, but should avoid raw seafood of any type because of possible contamination with parasites and Norwalk-like viruses, he said, adding that seafood like shark, swordfish, king mackerel, tilefish, Bigeye (Ahi) tuna steaks, and other long-lived fish high on the food chain should be avoided completely because of high mercury levels.
“While the AAFP did not review the revised advice to the dietary guidelines, family physicians are on the front lines encouraging healthy nutrition for pregnant and breastfeeding women and young children. It’s an ongoing, important part of the patient-physician conversation that begins with the initial prenatal visit,” Dr. Cullen said in a statement.
Similarly, Christopher M. Zahn, MD, vice president of practice activities for the American College of Obstetricians and Gynecologists, said the FDA/EPA updated guidance is in line with ACOG recommendations.
“The guidance continues to underscore the value of eating seafood 2-3 times per week during pregnancy and the importance of avoiding fish products that are high in mercury. The additional emphasis on healthy eating patterns mirrors ACOG’s long-standing guidance on the importance of a well-balanced, varied, nutritional diet that is consistent with a woman’s access to food and food preferences,” he said in a statement, noting that “seafood is a nutrient-rich food that has proven beneficial to women and in aiding the development of a fetus throughout pregnancy.”
The revision touts the health benefits of fish and shellfish and promotes safer fish choices for those who should limit mercury exposure – including women who are or might become pregnant, women who are breastfeeding, and young children.
Those individuals should avoid commercial fish with the highest levels of mercury and should instead choose from “the many types of fish that are lower in mercury – including ones commonly found in grocery stores, such as salmon, shrimp, pollock, canned light tuna, tilapia, catfish, and cod,” according to an FDA press statement.
The potential health benefits of eating fish were highlighted in the U.S. Department of Health and Human Services/Department of Agriculture 2015-2020 Dietary Guidelines for Americans, and in 2017 the FDA and the Environmental Protection Agency released advice on fish consumption, including a user-friendly reference chart regarding mercury levels in various types of fish.
Although the information in the chart has not changed, the FDA revised its advice to expand on the “information about the benefits of fish as part of healthy eating patterns by promoting the science-based recommendations of the 2015-2020 Dietary Guidelines for Americans.”
The advice calls for consumption of at least 8 ounces of seafood per week for adults (less for children) based on a 2,000 calorie diet, and for women who are pregnant or breastfeeding, 8-12 ounces of a variety of seafood per week selected from choices lower in mercury.
“The FDA’s revised advice highlights the many nutrients found in fish, several of which have important roles in growth and development during pregnancy and early childhood. It also highlights the potential health benefits of eating fish as part of a healthy eating pattern, particularly for improving heart health and lowering the risk of obesity,” the press release states.
Despite these benefits – and the recommendations for intake – concerns about mercury in fish have led many pregnant women in the United States to consume far less than the recommended amount of seafood, according to Susan Mayne, PhD, director of the FDA’s Center for Food Safety and Applied Nutrition.
“Our goal is to make sure Americans are equipped with this knowledge so that they can reap the benefits of eating fish, while choosing types of fish that are safe for them and their families to eat,” Dr. Mayne said in the FDA statement.
In response to the revised guidance, John S. Cullen, MD, president of the American Academy of Family Physicians, said that all women should be counseled to eat a well-balanced and varied diet including meats, dairy products, fruits, vegetables, and grains, and pregnant women should limit their intake of fish and seafood products to 8-12 ounces, or about 2-3 fish meals, per week.
Pregnant women may eat salmon in moderation, but should avoid raw seafood of any type because of possible contamination with parasites and Norwalk-like viruses, he said, adding that seafood like shark, swordfish, king mackerel, tilefish, Bigeye (Ahi) tuna steaks, and other long-lived fish high on the food chain should be avoided completely because of high mercury levels.
“While the AAFP did not review the revised advice to the dietary guidelines, family physicians are on the front lines encouraging healthy nutrition for pregnant and breastfeeding women and young children. It’s an ongoing, important part of the patient-physician conversation that begins with the initial prenatal visit,” Dr. Cullen said in a statement.
Similarly, Christopher M. Zahn, MD, vice president of practice activities for the American College of Obstetricians and Gynecologists, said the FDA/EPA updated guidance is in line with ACOG recommendations.
“The guidance continues to underscore the value of eating seafood 2-3 times per week during pregnancy and the importance of avoiding fish products that are high in mercury. The additional emphasis on healthy eating patterns mirrors ACOG’s long-standing guidance on the importance of a well-balanced, varied, nutritional diet that is consistent with a woman’s access to food and food preferences,” he said in a statement, noting that “seafood is a nutrient-rich food that has proven beneficial to women and in aiding the development of a fetus throughout pregnancy.”
Self-harm with bupropion linked to greater risk compared to SSRIs
Adolescents who attempt self-harm using bupropion are at a significantly higher risk of serious morbidity and poor outcomes, compared with those who attempt self-harm with selective serotonin reuptake inhibitors (SSRIs), according to Adam Overberg, PharmD, of the Indiana Poison Center in Indianapolis and associates.
In a study published in Pediatrics, the researchers analyzed 30,026 cases that were coded as “suspected suicide” and were reported to the National Poison Data System between June 2013 and December 2017. All cases were in adolescents aged 10-19 years. A total of 3,504 cases were exposures to bupropion; the rest were exposures to SSRIs.
Cases involving SSRIs were significantly more likely to result in either minor or no outcomes, compared with bupropion (68.0% vs 33.2%); cases resulting in moderate or major outcomes were much more likely to involve bupropion, compared with SSRIs (58.1% vs 19.0%). Among the 10 most common effects in cases with a moderate or major outcome, bupropion was more likely to cause tachycardia (83.7% vs. 59.9%), vomiting (24.8% vs. 20.6%), cardiac conduction disturbances (20.0% vs. 17.1%), agitation (20.2% vs. 11.7%), seizures (27.0% vs. 8.5%), and hallucinations (28.6% vs. 4.3%). Cases involving SSRIs were more likely to cause hypertension (25.3% vs. 17.6%). Eight deaths were reported in the study population; all were caused by bupropion ingestion.
Medical therapies that were more common with bupropion overdose included intubation (4.9% vs. 0.3%), vasopressor use (1.1% vs. 0.2%), benzodiazepine administration (34.2% vs. 5.4%), supplemental oxygen requirement (8.2% vs. 0.8%), and CPR (0.5% vs. 0.01%); three patients in the bupropion group required extracorporeal membrane oxygenation, compared with none in the SSRI group.
“Suicidal ingestions are increasing steadily, as are the numbers of adolescents treated with medication for depression. In light of bupropion’s disproportionately significant morbidity and mortality risk, it would be prudent for practitioners to avoid the use of this medication in adolescents that are at risk for self-harm,” the investigators concluded.
The study investigators reported that there were no conflicts of interest.
SOURCE: Overberg A et al. Pediatrics. 2019 Jul 5. doi: 10.1542/peds.2018-3295.
Adolescents who attempt self-harm using bupropion are at a significantly higher risk of serious morbidity and poor outcomes, compared with those who attempt self-harm with selective serotonin reuptake inhibitors (SSRIs), according to Adam Overberg, PharmD, of the Indiana Poison Center in Indianapolis and associates.
In a study published in Pediatrics, the researchers analyzed 30,026 cases that were coded as “suspected suicide” and were reported to the National Poison Data System between June 2013 and December 2017. All cases were in adolescents aged 10-19 years. A total of 3,504 cases were exposures to bupropion; the rest were exposures to SSRIs.
Cases involving SSRIs were significantly more likely to result in either minor or no outcomes, compared with bupropion (68.0% vs 33.2%); cases resulting in moderate or major outcomes were much more likely to involve bupropion, compared with SSRIs (58.1% vs 19.0%). Among the 10 most common effects in cases with a moderate or major outcome, bupropion was more likely to cause tachycardia (83.7% vs. 59.9%), vomiting (24.8% vs. 20.6%), cardiac conduction disturbances (20.0% vs. 17.1%), agitation (20.2% vs. 11.7%), seizures (27.0% vs. 8.5%), and hallucinations (28.6% vs. 4.3%). Cases involving SSRIs were more likely to cause hypertension (25.3% vs. 17.6%). Eight deaths were reported in the study population; all were caused by bupropion ingestion.
Medical therapies that were more common with bupropion overdose included intubation (4.9% vs. 0.3%), vasopressor use (1.1% vs. 0.2%), benzodiazepine administration (34.2% vs. 5.4%), supplemental oxygen requirement (8.2% vs. 0.8%), and CPR (0.5% vs. 0.01%); three patients in the bupropion group required extracorporeal membrane oxygenation, compared with none in the SSRI group.
“Suicidal ingestions are increasing steadily, as are the numbers of adolescents treated with medication for depression. In light of bupropion’s disproportionately significant morbidity and mortality risk, it would be prudent for practitioners to avoid the use of this medication in adolescents that are at risk for self-harm,” the investigators concluded.
The study investigators reported that there were no conflicts of interest.
SOURCE: Overberg A et al. Pediatrics. 2019 Jul 5. doi: 10.1542/peds.2018-3295.
Adolescents who attempt self-harm using bupropion are at a significantly higher risk of serious morbidity and poor outcomes, compared with those who attempt self-harm with selective serotonin reuptake inhibitors (SSRIs), according to Adam Overberg, PharmD, of the Indiana Poison Center in Indianapolis and associates.
In a study published in Pediatrics, the researchers analyzed 30,026 cases that were coded as “suspected suicide” and were reported to the National Poison Data System between June 2013 and December 2017. All cases were in adolescents aged 10-19 years. A total of 3,504 cases were exposures to bupropion; the rest were exposures to SSRIs.
Cases involving SSRIs were significantly more likely to result in either minor or no outcomes, compared with bupropion (68.0% vs 33.2%); cases resulting in moderate or major outcomes were much more likely to involve bupropion, compared with SSRIs (58.1% vs 19.0%). Among the 10 most common effects in cases with a moderate or major outcome, bupropion was more likely to cause tachycardia (83.7% vs. 59.9%), vomiting (24.8% vs. 20.6%), cardiac conduction disturbances (20.0% vs. 17.1%), agitation (20.2% vs. 11.7%), seizures (27.0% vs. 8.5%), and hallucinations (28.6% vs. 4.3%). Cases involving SSRIs were more likely to cause hypertension (25.3% vs. 17.6%). Eight deaths were reported in the study population; all were caused by bupropion ingestion.
Medical therapies that were more common with bupropion overdose included intubation (4.9% vs. 0.3%), vasopressor use (1.1% vs. 0.2%), benzodiazepine administration (34.2% vs. 5.4%), supplemental oxygen requirement (8.2% vs. 0.8%), and CPR (0.5% vs. 0.01%); three patients in the bupropion group required extracorporeal membrane oxygenation, compared with none in the SSRI group.
“Suicidal ingestions are increasing steadily, as are the numbers of adolescents treated with medication for depression. In light of bupropion’s disproportionately significant morbidity and mortality risk, it would be prudent for practitioners to avoid the use of this medication in adolescents that are at risk for self-harm,” the investigators concluded.
The study investigators reported that there were no conflicts of interest.
SOURCE: Overberg A et al. Pediatrics. 2019 Jul 5. doi: 10.1542/peds.2018-3295.
FROM PEDIATRICS
Legislative, educational interventions influenced vaccine status of California kindergartners
After California lawmakers implemented policies to limit and eventually eliminate nonmedical exemptions for childhood vaccinations, the proportion of kindergartners who were not up to date for recommended vaccinations fell from 10% in 2013 to 5% in 2017.
At the same time, the
The findings come from an observational study that used cross-sectional school-entry data from 2000 to 2017 to calculate the rates of kindergartners attending California schools who were not up to date on required vaccinations.
“Large-scale vaccination programs that included school-entry mandates have been essential to maintaining high levels of immunization coverage and low rates of vaccine-preventable diseases,” researchers led by S. Cassandra Pingali, MPH, MS, wrote in JAMA. “However, an increasing number of parents are not vaccinating their children over concerns about potential adverse effects. These parental actions threaten the herd immunity established by decades of high vaccine uptake and increase the potential for disease outbreaks.”
Ms. Pingali, of the department of epidemiology at Emory University, Atlanta, and colleagues conducted an observational analysis of California kindergartners who were not up to date on one or more of the required vaccinations during the course of three interventions implemented in the state. The first was Assembly Bill 2109 (AB 2109), which was passed in 2014. It required parents to show proof they had discussed the risks of not vaccinating their children with a health care practitioner before they obtained a personal belief exemption. The second intervention was a campaign carried out in 2015 by the California Department of Public Health and local health departments, designed to educate school staff on the proper application of the conditional admission criteria, which allowed students additional time to catch up on vaccination. The third intervention was the implementation of Senate bill 277 (SB 277), which banned all personal belief exemptions.
Between 2000 and 2017, the researchers reported that the yearly mean kindergarten enrollment in California was 517,962 and the mean number of schools was 7,278. Over this time, the yearly rate of students without up-to-date vaccination status rose from 8% during 2000 to 10% during 2013, before decreasing to 5% during 2017. Ms. Pingali and associates also found that average percentage chance of any within-school contact for a student without up-to-date vaccination status with another student with the same status was 19% during 2000, and increased steadily to 26% during 2014, the first year of AB 2109. The values decreased to 3% (the first year of SB 277), before increasing slightly to 5% during 2017.
“Across the interventions, the percentage of kindergartners attending schools with an up-to-date vaccination status percentage that was greater than the herd immunity threshold also increased for various vaccine-preventable diseases,” the researchers wrote. “Overall, the results suggest that the risk of disease outbreak via potential contact among susceptible children decreased over the course of the interventions.”
The way Matthew M. Davis, MD and Seema K. Shah, JD, see it, the current outbreak of measles in the United States is rooted in the failure of parents to vaccinate their children against the disease based on their beliefs rather than medical contraindications.
“The public health implications of such decisions are amplified because parents who share belief systems about childhood vaccinations tend to congregate socially and residentially, thereby forming clusters of unvaccinated children who are at elevated health risks when exposed to vaccine-preventable diseases,” the authors wrote in an accompanying editorial.
While the study reported by Pingali et al. did not measure actual outbreaks of disease, “reductions in children’s risk of contracting measles are a promising outcome in California resulting from policy changes,” wrote Dr. Davis and Ms. Shah, both of Northwestern University, Chicago (JAMA. 2019;322[1]:33-4). “Yet, because of the ease of domestic and international travel, the mobile nature of young families, and the inability of all states to implement this approach, changes made in each state for nonmedical exemptions may not ensure sufficiently high protection against measles for children across all jurisdictions in the United States. Although states have historically made their own decisions about vaccination exemptions linked to day care or school entry because states exercise primary authority over educational matters, childhood vaccination is a national matter in many respects.”
The best way to remedy the current system failure regarding measles vaccination, they continued, may be to adopt a unified national approach to prohibit nonmedical exemptions. They pointed to the fact that the United States previously achieved virtual eradication of measles as recently as 2000. “Following that achievement, state-level policy changes relaxed immunization requirements and set the stage for progressively larger outbreaks in the United States in recent years. Such system failures result when the products, processes, and people (including the public) that comprise systems do not function or behave in ways that protect health optimally.”
The study was supported by a grant from the National Institutes of Health. One coauthor reported having received consulting fees from Merck and grants from Pfizer and Walgreens. Another reported receiving grants from Pfizer, Merck, GlaxoSmithKline, Sanofi Pasteur, Protein Science, Dynavax, and MedImmune. The remaining coauthors reported no relevant financial disclosures.
The editorialists reported having no financial disclosures.
SOURCE: Pingali SC et al. JAMA. 2019 Jul 2. doi: 10.1001/jama.2019.7924.
After California lawmakers implemented policies to limit and eventually eliminate nonmedical exemptions for childhood vaccinations, the proportion of kindergartners who were not up to date for recommended vaccinations fell from 10% in 2013 to 5% in 2017.
At the same time, the
The findings come from an observational study that used cross-sectional school-entry data from 2000 to 2017 to calculate the rates of kindergartners attending California schools who were not up to date on required vaccinations.
“Large-scale vaccination programs that included school-entry mandates have been essential to maintaining high levels of immunization coverage and low rates of vaccine-preventable diseases,” researchers led by S. Cassandra Pingali, MPH, MS, wrote in JAMA. “However, an increasing number of parents are not vaccinating their children over concerns about potential adverse effects. These parental actions threaten the herd immunity established by decades of high vaccine uptake and increase the potential for disease outbreaks.”
Ms. Pingali, of the department of epidemiology at Emory University, Atlanta, and colleagues conducted an observational analysis of California kindergartners who were not up to date on one or more of the required vaccinations during the course of three interventions implemented in the state. The first was Assembly Bill 2109 (AB 2109), which was passed in 2014. It required parents to show proof they had discussed the risks of not vaccinating their children with a health care practitioner before they obtained a personal belief exemption. The second intervention was a campaign carried out in 2015 by the California Department of Public Health and local health departments, designed to educate school staff on the proper application of the conditional admission criteria, which allowed students additional time to catch up on vaccination. The third intervention was the implementation of Senate bill 277 (SB 277), which banned all personal belief exemptions.
Between 2000 and 2017, the researchers reported that the yearly mean kindergarten enrollment in California was 517,962 and the mean number of schools was 7,278. Over this time, the yearly rate of students without up-to-date vaccination status rose from 8% during 2000 to 10% during 2013, before decreasing to 5% during 2017. Ms. Pingali and associates also found that average percentage chance of any within-school contact for a student without up-to-date vaccination status with another student with the same status was 19% during 2000, and increased steadily to 26% during 2014, the first year of AB 2109. The values decreased to 3% (the first year of SB 277), before increasing slightly to 5% during 2017.
“Across the interventions, the percentage of kindergartners attending schools with an up-to-date vaccination status percentage that was greater than the herd immunity threshold also increased for various vaccine-preventable diseases,” the researchers wrote. “Overall, the results suggest that the risk of disease outbreak via potential contact among susceptible children decreased over the course of the interventions.”
The way Matthew M. Davis, MD and Seema K. Shah, JD, see it, the current outbreak of measles in the United States is rooted in the failure of parents to vaccinate their children against the disease based on their beliefs rather than medical contraindications.
“The public health implications of such decisions are amplified because parents who share belief systems about childhood vaccinations tend to congregate socially and residentially, thereby forming clusters of unvaccinated children who are at elevated health risks when exposed to vaccine-preventable diseases,” the authors wrote in an accompanying editorial.
While the study reported by Pingali et al. did not measure actual outbreaks of disease, “reductions in children’s risk of contracting measles are a promising outcome in California resulting from policy changes,” wrote Dr. Davis and Ms. Shah, both of Northwestern University, Chicago (JAMA. 2019;322[1]:33-4). “Yet, because of the ease of domestic and international travel, the mobile nature of young families, and the inability of all states to implement this approach, changes made in each state for nonmedical exemptions may not ensure sufficiently high protection against measles for children across all jurisdictions in the United States. Although states have historically made their own decisions about vaccination exemptions linked to day care or school entry because states exercise primary authority over educational matters, childhood vaccination is a national matter in many respects.”
The best way to remedy the current system failure regarding measles vaccination, they continued, may be to adopt a unified national approach to prohibit nonmedical exemptions. They pointed to the fact that the United States previously achieved virtual eradication of measles as recently as 2000. “Following that achievement, state-level policy changes relaxed immunization requirements and set the stage for progressively larger outbreaks in the United States in recent years. Such system failures result when the products, processes, and people (including the public) that comprise systems do not function or behave in ways that protect health optimally.”
The study was supported by a grant from the National Institutes of Health. One coauthor reported having received consulting fees from Merck and grants from Pfizer and Walgreens. Another reported receiving grants from Pfizer, Merck, GlaxoSmithKline, Sanofi Pasteur, Protein Science, Dynavax, and MedImmune. The remaining coauthors reported no relevant financial disclosures.
The editorialists reported having no financial disclosures.
SOURCE: Pingali SC et al. JAMA. 2019 Jul 2. doi: 10.1001/jama.2019.7924.
After California lawmakers implemented policies to limit and eventually eliminate nonmedical exemptions for childhood vaccinations, the proportion of kindergartners who were not up to date for recommended vaccinations fell from 10% in 2013 to 5% in 2017.
At the same time, the
The findings come from an observational study that used cross-sectional school-entry data from 2000 to 2017 to calculate the rates of kindergartners attending California schools who were not up to date on required vaccinations.
“Large-scale vaccination programs that included school-entry mandates have been essential to maintaining high levels of immunization coverage and low rates of vaccine-preventable diseases,” researchers led by S. Cassandra Pingali, MPH, MS, wrote in JAMA. “However, an increasing number of parents are not vaccinating their children over concerns about potential adverse effects. These parental actions threaten the herd immunity established by decades of high vaccine uptake and increase the potential for disease outbreaks.”
Ms. Pingali, of the department of epidemiology at Emory University, Atlanta, and colleagues conducted an observational analysis of California kindergartners who were not up to date on one or more of the required vaccinations during the course of three interventions implemented in the state. The first was Assembly Bill 2109 (AB 2109), which was passed in 2014. It required parents to show proof they had discussed the risks of not vaccinating their children with a health care practitioner before they obtained a personal belief exemption. The second intervention was a campaign carried out in 2015 by the California Department of Public Health and local health departments, designed to educate school staff on the proper application of the conditional admission criteria, which allowed students additional time to catch up on vaccination. The third intervention was the implementation of Senate bill 277 (SB 277), which banned all personal belief exemptions.
Between 2000 and 2017, the researchers reported that the yearly mean kindergarten enrollment in California was 517,962 and the mean number of schools was 7,278. Over this time, the yearly rate of students without up-to-date vaccination status rose from 8% during 2000 to 10% during 2013, before decreasing to 5% during 2017. Ms. Pingali and associates also found that average percentage chance of any within-school contact for a student without up-to-date vaccination status with another student with the same status was 19% during 2000, and increased steadily to 26% during 2014, the first year of AB 2109. The values decreased to 3% (the first year of SB 277), before increasing slightly to 5% during 2017.
“Across the interventions, the percentage of kindergartners attending schools with an up-to-date vaccination status percentage that was greater than the herd immunity threshold also increased for various vaccine-preventable diseases,” the researchers wrote. “Overall, the results suggest that the risk of disease outbreak via potential contact among susceptible children decreased over the course of the interventions.”
The way Matthew M. Davis, MD and Seema K. Shah, JD, see it, the current outbreak of measles in the United States is rooted in the failure of parents to vaccinate their children against the disease based on their beliefs rather than medical contraindications.
“The public health implications of such decisions are amplified because parents who share belief systems about childhood vaccinations tend to congregate socially and residentially, thereby forming clusters of unvaccinated children who are at elevated health risks when exposed to vaccine-preventable diseases,” the authors wrote in an accompanying editorial.
While the study reported by Pingali et al. did not measure actual outbreaks of disease, “reductions in children’s risk of contracting measles are a promising outcome in California resulting from policy changes,” wrote Dr. Davis and Ms. Shah, both of Northwestern University, Chicago (JAMA. 2019;322[1]:33-4). “Yet, because of the ease of domestic and international travel, the mobile nature of young families, and the inability of all states to implement this approach, changes made in each state for nonmedical exemptions may not ensure sufficiently high protection against measles for children across all jurisdictions in the United States. Although states have historically made their own decisions about vaccination exemptions linked to day care or school entry because states exercise primary authority over educational matters, childhood vaccination is a national matter in many respects.”
The best way to remedy the current system failure regarding measles vaccination, they continued, may be to adopt a unified national approach to prohibit nonmedical exemptions. They pointed to the fact that the United States previously achieved virtual eradication of measles as recently as 2000. “Following that achievement, state-level policy changes relaxed immunization requirements and set the stage for progressively larger outbreaks in the United States in recent years. Such system failures result when the products, processes, and people (including the public) that comprise systems do not function or behave in ways that protect health optimally.”
The study was supported by a grant from the National Institutes of Health. One coauthor reported having received consulting fees from Merck and grants from Pfizer and Walgreens. Another reported receiving grants from Pfizer, Merck, GlaxoSmithKline, Sanofi Pasteur, Protein Science, Dynavax, and MedImmune. The remaining coauthors reported no relevant financial disclosures.
The editorialists reported having no financial disclosures.
SOURCE: Pingali SC et al. JAMA. 2019 Jul 2. doi: 10.1001/jama.2019.7924.
FROM JAMA
‘Tis the season … for fireworks injuries
according to the Consumer Product Safety Commission.
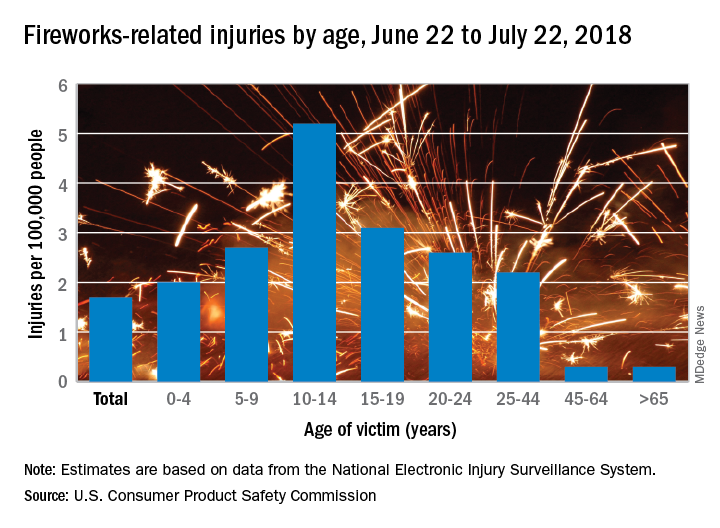
Of the estimated 9,100 fireworks-related injuries treated in emergency departments last year, 5,600 (62%) occurred between June 22 and July 22, 2018. That works out to a rate of 1.7 ED-treated injuries per 100,000 people for that 1 month and a rate of 2.8 per 100,000 for the entire year, the CPSC said in its 2018 Fireworks Annual Report.
Children had higher injury rates than adults in the Fourth of July window, and those aged 10-14 years had the highest rate of all, 5.2 injuries per 100,000 population. They were followed by teens aged 15-19 years (3.1 per 100,000) and children aged 5-9 (2.7 per 100,000), the CPSC investigators said based on data from the National Electronic Injury Surveillance System.
A deeper dive into the data pool shows that firecrackers caused more injuries – 19% of the total for the month – than any other type of firework device (reloadable shells were second at 12%). Burns were the most common type of injury, making up 44% of the total, and hands and fingers were the body parts most often injured (28% of the total), they reported.
There were five fireworks-related deaths last year – below the average of 7.6 per year since 2003 – but the total for 2018 may go up because reporting for the year is not yet complete. In one of the 2018 cases, an 18-year-old taped a tube to a football helmet and tried to launch a mortar shell while wearing the helmet. The first one worked, but the second shell got stuck and exploded in the tube, the CPSC said.
“CPSC works year-round to help prevent deaths and injuries from fireworks, by verifying fireworks meet safety regulations in our ports, marketplace, and on the road,” acting CPSC Chairman Ann Marie Buerkle said in a written statement. “Beyond CPSC’s efforts, we want to make sure everyone takes simple safety steps to celebrate safely with their family and friends.”
according to the Consumer Product Safety Commission.

Of the estimated 9,100 fireworks-related injuries treated in emergency departments last year, 5,600 (62%) occurred between June 22 and July 22, 2018. That works out to a rate of 1.7 ED-treated injuries per 100,000 people for that 1 month and a rate of 2.8 per 100,000 for the entire year, the CPSC said in its 2018 Fireworks Annual Report.
Children had higher injury rates than adults in the Fourth of July window, and those aged 10-14 years had the highest rate of all, 5.2 injuries per 100,000 population. They were followed by teens aged 15-19 years (3.1 per 100,000) and children aged 5-9 (2.7 per 100,000), the CPSC investigators said based on data from the National Electronic Injury Surveillance System.
A deeper dive into the data pool shows that firecrackers caused more injuries – 19% of the total for the month – than any other type of firework device (reloadable shells were second at 12%). Burns were the most common type of injury, making up 44% of the total, and hands and fingers were the body parts most often injured (28% of the total), they reported.
There were five fireworks-related deaths last year – below the average of 7.6 per year since 2003 – but the total for 2018 may go up because reporting for the year is not yet complete. In one of the 2018 cases, an 18-year-old taped a tube to a football helmet and tried to launch a mortar shell while wearing the helmet. The first one worked, but the second shell got stuck and exploded in the tube, the CPSC said.
“CPSC works year-round to help prevent deaths and injuries from fireworks, by verifying fireworks meet safety regulations in our ports, marketplace, and on the road,” acting CPSC Chairman Ann Marie Buerkle said in a written statement. “Beyond CPSC’s efforts, we want to make sure everyone takes simple safety steps to celebrate safely with their family and friends.”
according to the Consumer Product Safety Commission.

Of the estimated 9,100 fireworks-related injuries treated in emergency departments last year, 5,600 (62%) occurred between June 22 and July 22, 2018. That works out to a rate of 1.7 ED-treated injuries per 100,000 people for that 1 month and a rate of 2.8 per 100,000 for the entire year, the CPSC said in its 2018 Fireworks Annual Report.
Children had higher injury rates than adults in the Fourth of July window, and those aged 10-14 years had the highest rate of all, 5.2 injuries per 100,000 population. They were followed by teens aged 15-19 years (3.1 per 100,000) and children aged 5-9 (2.7 per 100,000), the CPSC investigators said based on data from the National Electronic Injury Surveillance System.
A deeper dive into the data pool shows that firecrackers caused more injuries – 19% of the total for the month – than any other type of firework device (reloadable shells were second at 12%). Burns were the most common type of injury, making up 44% of the total, and hands and fingers were the body parts most often injured (28% of the total), they reported.
There were five fireworks-related deaths last year – below the average of 7.6 per year since 2003 – but the total for 2018 may go up because reporting for the year is not yet complete. In one of the 2018 cases, an 18-year-old taped a tube to a football helmet and tried to launch a mortar shell while wearing the helmet. The first one worked, but the second shell got stuck and exploded in the tube, the CPSC said.
“CPSC works year-round to help prevent deaths and injuries from fireworks, by verifying fireworks meet safety regulations in our ports, marketplace, and on the road,” acting CPSC Chairman Ann Marie Buerkle said in a written statement. “Beyond CPSC’s efforts, we want to make sure everyone takes simple safety steps to celebrate safely with their family and friends.”
New research in otitis media
New research was presented at the International Society for Otitis Media meeting in June 2019, which I attended. I would like to share a selection of new findings from the many presentations.
Transtympanic antibiotic delivery
Topical therapy has been used to treat only otitis externa and acute otitis media (AOM) with ear discharge. Giving antibiotics through the tympanic membrane could mitigate many of the concerns about antibiotic use driving antibiotic resistance of bacteria among children. Up to now, using antibiotics in the ear canal to treat AOM has not been considered because the tympanic membrane is highly impermeable to the transtympanic diffusion of any drugs. However, in recent years, a number of different drug delivery systems have been developed, and in some cases, animal studies have shown that noninvasive transtympanic delivery is possible so that drugs can reach high concentrations in the middle ear without damage. Nanovesicles and nanoliposomes that contain antibiotics and are small enough to pass through the eardrum have been developed and tested in animal models; these show promise. Ototopical administration of a drug called vinpocetine that was repurposed has been tested in mice and shown to reduce inflammation and mucus production in the middle ear during otitis media.
Biofilms
Antibiotic treatment failure can occur in AOM for several reasons. The treatment of choice, amoxicillin, for example may fail to achieve an adequate concentration because of poor absorption in the gastrointestinal tract or poor penetration into the middle ear. Or, the antibiotic chosen may not be effective because of resistance of the strain causing the infection. Another explanation, especially in recurrent AOM and chronic AOM, could be the presence of biofilms. Biofilms are multicellular bacterial communities incorporated in a polymeric, plasticlike matrix in which pathogens are protected from antibiotic activity. The biofilm provides a physical barrier to antibiotic penetration, and bacteria can persist in the middle ear and periodically cause a new AOM. If AOM persists or becomes a more chronic otitis media with effusion, the “glue ear” causes an environment in the middle ear that is low in oxygen. A low-oxygen environment is favorable to biofilms. Also one might expect that middle ear pus would have a low pH, but actual measurements show the pH is highly alkaline. Species of Haemophilus influenzae have been identified as more virulent when in an alkaline pH or the alkaline pH makes the H. influenzae persist better in the middle ear, perhaps in a biofilm. To eliminate biofilms and improve antibiotic efficacy, a vaccine against a protein expressed by H. influenzae has been developed. Antibodies against this protein have been shown to disrupt and prevent the formation of biofilms in an animal model.
Probiotics
The normal bacteria that live in the nasopharynx of children with recurrent AOM is now known to differ from that of children who experience infrequent AOM or remain AOM-free throughout childhood. The use of oral pre- and probiotics for AOM prophylaxis remains debated because the results of studies are conflicting and frequently show no effect. So the idea of using prebiotics or probiotics to create a favorable “microbiome” of the nose is under investigation. Two species of bacteria that are gathering the most attention are Corynebacterium species (a few types in particular) and a bacteria called Dolosigranulum pigrum. Delivery of the commensal species would be as a nose spray.
Vaccines
The use of pneumococcal conjugate vaccines (PCVs) has reduced the frequency of AOM caused by Streptococcus pneumoniae. PCVs are not as effective against AOM as they are against invasive pneumococcal disease, but they still help a lot. However, because there are now at least 96 different serotypes of the pneumococcus based on different capsular types, we see a pattern of replacement of disease-causing strains by new strains within a few years of introduction of a new formulation. We started with 7 serotypes (Prevnar 7) in year 2000, and it was replaced by the current formulation with 13 serotypes (Prevnar 13) in 2010. Replacements have occurred again so vaccine companies are making new formulations for the future that include more serotypes, up to 20 serotypes. But, technically and feasibility-wise there is a limit to making such vaccines. A vaccine based on killed unencapsulated bacteria has been tested for safety and immunogenicity in young children. There is no test so far for prevention of AOM. Another type of vaccine based on proteins expressed by the pneumococcus that could be vaccine targets was tested in American Navajo children, and it failed to be as efficacious as hoped.
Biomarkers.
Due to recurrent AOM or persistent otitis media with effusion, about 15% of children in the United States receive tympanostomy tubes. Among those who receive tubes, about 20% go on to receive a second set of tubes, often with adenotonsillectomy. To find a biomarker that could identify children likely to require a second set of tubes, the fluid in the middle ear was tested when a first set of tubes were inserted. If bacteria were detected by polymerase chain reaction (PCR) testing or if a profile of specific inflammatory cytokines was measured, those results could be used to predict a high likelihood for a second set of tubes.
Overdiagnosis
Diagnosis of AOM is challenging in young children, in whom it most frequently occurs. The ear canal is typically about 3 mm wide, the child struggles during the examination, and diagnostic skills are not taught in training, resulting in a high overdiagnosis rate. I presented data that suggest too many children who are not truly otitis prone have been classified as otitis prone based on incorrect clinical diagnosis. My colleagues and I found that 30% of children reach the threshold of three episodes of AOM in 6 months or four within a year when diagnosed by community pediatricians, similar to many other studies. Validated otoscopists (trained by experts with diagnosis definitively proven as at least 85% accurate using tympanocentesis) classify 15% of children as otitis prone – half as many. If tympanocentesis is used to prove middle ear fluid has bacterial pathogens (about 95% yield a bacterial otopathogen using culture and PCR), then about 10% of children are classified as otitis prone – one-third as many. This suggests that children clinically diagnosed by community-based pediatricians are overdiagnosed with AOM, perhaps three times more often than true. And that leads to overuse of antibiotics and referrals for tympanostomy tube surgery more often than should occur. So we need to improve diagnostic methods beyond otoscopy. New types of imaging for the eardrum and middle ear using novel technologies are in early clinical trials.
Immunity
The notion that young children get AOM because of Eustachian tube dysfunction in their early years of life (horizontal anatomy) may be true, but there is more to the story. After 10 years of work, the scientists in my research group have shown that children in the first 3 years of life can have an immune system that is suppressed – it is poorly responsive to pathogens and routine pediatric vaccines. Many features resemble a neonatal immune system, beginning life with a suppressed immune system or being in cytokine storm from birth. We introduced the term “prolonged neonatal-like immune profile (PNIP)” to give a general description of the immune responses we have found in otitis-prone children. They outgrow this. So the immune maturation is delayed but not permanent. It is mostly resolved by age 3 years. We found problems in both innate and adaptive immunity. It may be that the main explanation for recurrent AOM in the first years of life is PNIP. Scientists from Australia also reported immunity problems in Aboriginal children and they are very otitis prone, often progressing to chronic suppurative otitis media. Animal model studies of AOM show inadequate innate and adaptive immunity importantly contribute to the infection as well.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts to declare. Email him at [email protected].
New research was presented at the International Society for Otitis Media meeting in June 2019, which I attended. I would like to share a selection of new findings from the many presentations.
Transtympanic antibiotic delivery
Topical therapy has been used to treat only otitis externa and acute otitis media (AOM) with ear discharge. Giving antibiotics through the tympanic membrane could mitigate many of the concerns about antibiotic use driving antibiotic resistance of bacteria among children. Up to now, using antibiotics in the ear canal to treat AOM has not been considered because the tympanic membrane is highly impermeable to the transtympanic diffusion of any drugs. However, in recent years, a number of different drug delivery systems have been developed, and in some cases, animal studies have shown that noninvasive transtympanic delivery is possible so that drugs can reach high concentrations in the middle ear without damage. Nanovesicles and nanoliposomes that contain antibiotics and are small enough to pass through the eardrum have been developed and tested in animal models; these show promise. Ototopical administration of a drug called vinpocetine that was repurposed has been tested in mice and shown to reduce inflammation and mucus production in the middle ear during otitis media.
Biofilms
Antibiotic treatment failure can occur in AOM for several reasons. The treatment of choice, amoxicillin, for example may fail to achieve an adequate concentration because of poor absorption in the gastrointestinal tract or poor penetration into the middle ear. Or, the antibiotic chosen may not be effective because of resistance of the strain causing the infection. Another explanation, especially in recurrent AOM and chronic AOM, could be the presence of biofilms. Biofilms are multicellular bacterial communities incorporated in a polymeric, plasticlike matrix in which pathogens are protected from antibiotic activity. The biofilm provides a physical barrier to antibiotic penetration, and bacteria can persist in the middle ear and periodically cause a new AOM. If AOM persists or becomes a more chronic otitis media with effusion, the “glue ear” causes an environment in the middle ear that is low in oxygen. A low-oxygen environment is favorable to biofilms. Also one might expect that middle ear pus would have a low pH, but actual measurements show the pH is highly alkaline. Species of Haemophilus influenzae have been identified as more virulent when in an alkaline pH or the alkaline pH makes the H. influenzae persist better in the middle ear, perhaps in a biofilm. To eliminate biofilms and improve antibiotic efficacy, a vaccine against a protein expressed by H. influenzae has been developed. Antibodies against this protein have been shown to disrupt and prevent the formation of biofilms in an animal model.
Probiotics
The normal bacteria that live in the nasopharynx of children with recurrent AOM is now known to differ from that of children who experience infrequent AOM or remain AOM-free throughout childhood. The use of oral pre- and probiotics for AOM prophylaxis remains debated because the results of studies are conflicting and frequently show no effect. So the idea of using prebiotics or probiotics to create a favorable “microbiome” of the nose is under investigation. Two species of bacteria that are gathering the most attention are Corynebacterium species (a few types in particular) and a bacteria called Dolosigranulum pigrum. Delivery of the commensal species would be as a nose spray.
Vaccines
The use of pneumococcal conjugate vaccines (PCVs) has reduced the frequency of AOM caused by Streptococcus pneumoniae. PCVs are not as effective against AOM as they are against invasive pneumococcal disease, but they still help a lot. However, because there are now at least 96 different serotypes of the pneumococcus based on different capsular types, we see a pattern of replacement of disease-causing strains by new strains within a few years of introduction of a new formulation. We started with 7 serotypes (Prevnar 7) in year 2000, and it was replaced by the current formulation with 13 serotypes (Prevnar 13) in 2010. Replacements have occurred again so vaccine companies are making new formulations for the future that include more serotypes, up to 20 serotypes. But, technically and feasibility-wise there is a limit to making such vaccines. A vaccine based on killed unencapsulated bacteria has been tested for safety and immunogenicity in young children. There is no test so far for prevention of AOM. Another type of vaccine based on proteins expressed by the pneumococcus that could be vaccine targets was tested in American Navajo children, and it failed to be as efficacious as hoped.
Biomarkers.
Due to recurrent AOM or persistent otitis media with effusion, about 15% of children in the United States receive tympanostomy tubes. Among those who receive tubes, about 20% go on to receive a second set of tubes, often with adenotonsillectomy. To find a biomarker that could identify children likely to require a second set of tubes, the fluid in the middle ear was tested when a first set of tubes were inserted. If bacteria were detected by polymerase chain reaction (PCR) testing or if a profile of specific inflammatory cytokines was measured, those results could be used to predict a high likelihood for a second set of tubes.
Overdiagnosis
Diagnosis of AOM is challenging in young children, in whom it most frequently occurs. The ear canal is typically about 3 mm wide, the child struggles during the examination, and diagnostic skills are not taught in training, resulting in a high overdiagnosis rate. I presented data that suggest too many children who are not truly otitis prone have been classified as otitis prone based on incorrect clinical diagnosis. My colleagues and I found that 30% of children reach the threshold of three episodes of AOM in 6 months or four within a year when diagnosed by community pediatricians, similar to many other studies. Validated otoscopists (trained by experts with diagnosis definitively proven as at least 85% accurate using tympanocentesis) classify 15% of children as otitis prone – half as many. If tympanocentesis is used to prove middle ear fluid has bacterial pathogens (about 95% yield a bacterial otopathogen using culture and PCR), then about 10% of children are classified as otitis prone – one-third as many. This suggests that children clinically diagnosed by community-based pediatricians are overdiagnosed with AOM, perhaps three times more often than true. And that leads to overuse of antibiotics and referrals for tympanostomy tube surgery more often than should occur. So we need to improve diagnostic methods beyond otoscopy. New types of imaging for the eardrum and middle ear using novel technologies are in early clinical trials.
Immunity
The notion that young children get AOM because of Eustachian tube dysfunction in their early years of life (horizontal anatomy) may be true, but there is more to the story. After 10 years of work, the scientists in my research group have shown that children in the first 3 years of life can have an immune system that is suppressed – it is poorly responsive to pathogens and routine pediatric vaccines. Many features resemble a neonatal immune system, beginning life with a suppressed immune system or being in cytokine storm from birth. We introduced the term “prolonged neonatal-like immune profile (PNIP)” to give a general description of the immune responses we have found in otitis-prone children. They outgrow this. So the immune maturation is delayed but not permanent. It is mostly resolved by age 3 years. We found problems in both innate and adaptive immunity. It may be that the main explanation for recurrent AOM in the first years of life is PNIP. Scientists from Australia also reported immunity problems in Aboriginal children and they are very otitis prone, often progressing to chronic suppurative otitis media. Animal model studies of AOM show inadequate innate and adaptive immunity importantly contribute to the infection as well.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts to declare. Email him at [email protected].
New research was presented at the International Society for Otitis Media meeting in June 2019, which I attended. I would like to share a selection of new findings from the many presentations.
Transtympanic antibiotic delivery
Topical therapy has been used to treat only otitis externa and acute otitis media (AOM) with ear discharge. Giving antibiotics through the tympanic membrane could mitigate many of the concerns about antibiotic use driving antibiotic resistance of bacteria among children. Up to now, using antibiotics in the ear canal to treat AOM has not been considered because the tympanic membrane is highly impermeable to the transtympanic diffusion of any drugs. However, in recent years, a number of different drug delivery systems have been developed, and in some cases, animal studies have shown that noninvasive transtympanic delivery is possible so that drugs can reach high concentrations in the middle ear without damage. Nanovesicles and nanoliposomes that contain antibiotics and are small enough to pass through the eardrum have been developed and tested in animal models; these show promise. Ototopical administration of a drug called vinpocetine that was repurposed has been tested in mice and shown to reduce inflammation and mucus production in the middle ear during otitis media.
Biofilms
Antibiotic treatment failure can occur in AOM for several reasons. The treatment of choice, amoxicillin, for example may fail to achieve an adequate concentration because of poor absorption in the gastrointestinal tract or poor penetration into the middle ear. Or, the antibiotic chosen may not be effective because of resistance of the strain causing the infection. Another explanation, especially in recurrent AOM and chronic AOM, could be the presence of biofilms. Biofilms are multicellular bacterial communities incorporated in a polymeric, plasticlike matrix in which pathogens are protected from antibiotic activity. The biofilm provides a physical barrier to antibiotic penetration, and bacteria can persist in the middle ear and periodically cause a new AOM. If AOM persists or becomes a more chronic otitis media with effusion, the “glue ear” causes an environment in the middle ear that is low in oxygen. A low-oxygen environment is favorable to biofilms. Also one might expect that middle ear pus would have a low pH, but actual measurements show the pH is highly alkaline. Species of Haemophilus influenzae have been identified as more virulent when in an alkaline pH or the alkaline pH makes the H. influenzae persist better in the middle ear, perhaps in a biofilm. To eliminate biofilms and improve antibiotic efficacy, a vaccine against a protein expressed by H. influenzae has been developed. Antibodies against this protein have been shown to disrupt and prevent the formation of biofilms in an animal model.
Probiotics
The normal bacteria that live in the nasopharynx of children with recurrent AOM is now known to differ from that of children who experience infrequent AOM or remain AOM-free throughout childhood. The use of oral pre- and probiotics for AOM prophylaxis remains debated because the results of studies are conflicting and frequently show no effect. So the idea of using prebiotics or probiotics to create a favorable “microbiome” of the nose is under investigation. Two species of bacteria that are gathering the most attention are Corynebacterium species (a few types in particular) and a bacteria called Dolosigranulum pigrum. Delivery of the commensal species would be as a nose spray.
Vaccines
The use of pneumococcal conjugate vaccines (PCVs) has reduced the frequency of AOM caused by Streptococcus pneumoniae. PCVs are not as effective against AOM as they are against invasive pneumococcal disease, but they still help a lot. However, because there are now at least 96 different serotypes of the pneumococcus based on different capsular types, we see a pattern of replacement of disease-causing strains by new strains within a few years of introduction of a new formulation. We started with 7 serotypes (Prevnar 7) in year 2000, and it was replaced by the current formulation with 13 serotypes (Prevnar 13) in 2010. Replacements have occurred again so vaccine companies are making new formulations for the future that include more serotypes, up to 20 serotypes. But, technically and feasibility-wise there is a limit to making such vaccines. A vaccine based on killed unencapsulated bacteria has been tested for safety and immunogenicity in young children. There is no test so far for prevention of AOM. Another type of vaccine based on proteins expressed by the pneumococcus that could be vaccine targets was tested in American Navajo children, and it failed to be as efficacious as hoped.
Biomarkers.
Due to recurrent AOM or persistent otitis media with effusion, about 15% of children in the United States receive tympanostomy tubes. Among those who receive tubes, about 20% go on to receive a second set of tubes, often with adenotonsillectomy. To find a biomarker that could identify children likely to require a second set of tubes, the fluid in the middle ear was tested when a first set of tubes were inserted. If bacteria were detected by polymerase chain reaction (PCR) testing or if a profile of specific inflammatory cytokines was measured, those results could be used to predict a high likelihood for a second set of tubes.
Overdiagnosis
Diagnosis of AOM is challenging in young children, in whom it most frequently occurs. The ear canal is typically about 3 mm wide, the child struggles during the examination, and diagnostic skills are not taught in training, resulting in a high overdiagnosis rate. I presented data that suggest too many children who are not truly otitis prone have been classified as otitis prone based on incorrect clinical diagnosis. My colleagues and I found that 30% of children reach the threshold of three episodes of AOM in 6 months or four within a year when diagnosed by community pediatricians, similar to many other studies. Validated otoscopists (trained by experts with diagnosis definitively proven as at least 85% accurate using tympanocentesis) classify 15% of children as otitis prone – half as many. If tympanocentesis is used to prove middle ear fluid has bacterial pathogens (about 95% yield a bacterial otopathogen using culture and PCR), then about 10% of children are classified as otitis prone – one-third as many. This suggests that children clinically diagnosed by community-based pediatricians are overdiagnosed with AOM, perhaps three times more often than true. And that leads to overuse of antibiotics and referrals for tympanostomy tube surgery more often than should occur. So we need to improve diagnostic methods beyond otoscopy. New types of imaging for the eardrum and middle ear using novel technologies are in early clinical trials.
Immunity
The notion that young children get AOM because of Eustachian tube dysfunction in their early years of life (horizontal anatomy) may be true, but there is more to the story. After 10 years of work, the scientists in my research group have shown that children in the first 3 years of life can have an immune system that is suppressed – it is poorly responsive to pathogens and routine pediatric vaccines. Many features resemble a neonatal immune system, beginning life with a suppressed immune system or being in cytokine storm from birth. We introduced the term “prolonged neonatal-like immune profile (PNIP)” to give a general description of the immune responses we have found in otitis-prone children. They outgrow this. So the immune maturation is delayed but not permanent. It is mostly resolved by age 3 years. We found problems in both innate and adaptive immunity. It may be that the main explanation for recurrent AOM in the first years of life is PNIP. Scientists from Australia also reported immunity problems in Aboriginal children and they are very otitis prone, often progressing to chronic suppurative otitis media. Animal model studies of AOM show inadequate innate and adaptive immunity importantly contribute to the infection as well.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts to declare. Email him at [email protected].