User login
Water safety: Drowning isn’t the only concern
References
1. CDC Childhood Injury Report: Patterns of Unintentional Injuries among 0-19 Year Olds in the United States, 2000-2006. Centers for Disease Control and Prevention Web site. https://www.cdc.gov/safechild/pdf/cdc-childhoodinjury.pdf. Accessed June 26, 2019.
2. World Health Organization. Global Report on Drowning: Preventing a Leading Killer. https://apps.who.int/iris/bitstream/handle/10665/143893/9789241564786_eng.pdf;jsessionid=0B51AAEB51E29A603A0CABB41FCD96B5?sequence=1. Accessed June 26, 2019.
3. Vanden Esschert K, Haileyesus T, Tarrier AL, et al. Pool chemical injuries in public and residential settings—United States, 2008–2017, and New York, 2018. MMWR Morb Mortal Wkly Rep. 2019;68:433–438.
4. Healthy Swimming. Centers for Disease Control and Prevention Web site. https://www.cdc.gov/healthywater/swimming/index.html. Accessed June 26, 2019.
References
1. CDC Childhood Injury Report: Patterns of Unintentional Injuries among 0-19 Year Olds in the United States, 2000-2006. Centers for Disease Control and Prevention Web site. https://www.cdc.gov/safechild/pdf/cdc-childhoodinjury.pdf. Accessed June 26, 2019.
2. World Health Organization. Global Report on Drowning: Preventing a Leading Killer. https://apps.who.int/iris/bitstream/handle/10665/143893/9789241564786_eng.pdf;jsessionid=0B51AAEB51E29A603A0CABB41FCD96B5?sequence=1. Accessed June 26, 2019.
3. Vanden Esschert K, Haileyesus T, Tarrier AL, et al. Pool chemical injuries in public and residential settings—United States, 2008–2017, and New York, 2018. MMWR Morb Mortal Wkly Rep. 2019;68:433–438.
4. Healthy Swimming. Centers for Disease Control and Prevention Web site. https://www.cdc.gov/healthywater/swimming/index.html. Accessed June 26, 2019.
References
1. CDC Childhood Injury Report: Patterns of Unintentional Injuries among 0-19 Year Olds in the United States, 2000-2006. Centers for Disease Control and Prevention Web site. https://www.cdc.gov/safechild/pdf/cdc-childhoodinjury.pdf. Accessed June 26, 2019.
2. World Health Organization. Global Report on Drowning: Preventing a Leading Killer. https://apps.who.int/iris/bitstream/handle/10665/143893/9789241564786_eng.pdf;jsessionid=0B51AAEB51E29A603A0CABB41FCD96B5?sequence=1. Accessed June 26, 2019.
3. Vanden Esschert K, Haileyesus T, Tarrier AL, et al. Pool chemical injuries in public and residential settings—United States, 2008–2017, and New York, 2018. MMWR Morb Mortal Wkly Rep. 2019;68:433–438.
4. Healthy Swimming. Centers for Disease Control and Prevention Web site. https://www.cdc.gov/healthywater/swimming/index.html. Accessed June 26, 2019.
Treatment for pediatric low-grade glioma is associated with poor cognitive and socioeconomic outcomes
Children who underwent surgery alone had better neuropsychologic and socioeconomic outcomes than those who also underwent radiotherapy, but their outcomes were worse than those of unaffected siblings. These findings were published online June 24 in Cancer.
“Late effects in adulthood are evident even for children with the least malignant types of brain tumors who were treated with the least toxic therapies available at the time,” said M. Douglas Ris, PhD, professor of pediatrics and psychology at Baylor College of Medicine in Houston, in a press release. “As pediatric brain tumors become more survivable with continued advances in treatments, we need to improve surveillance of these populations so that survivors continue to receive the best interventions during their transition to adulthood and well beyond.”
Clinicians generally have assumed that children with low-grade CNS tumors who receive less toxic treatment will have fewer long-term effects than survivors of more malignant tumors who undergo neurotoxic therapies. Yet research has indicated that the former patients can have lasting neurobehavioral or functional morbidity.
Dr. Ris and colleagues invited survivors of pediatric low-grade gliomas participating in the Childhood Cancer Survivor Study (CCSS) and a sibling comparison group to undergo a direct, comprehensive neurocognitive assessment. Of 495 eligible survivors, 257 participated. Seventy-six patients did not travel to a study site, but completed a questionnaire, and the researchers did not include data for this group in their analysis. Dr. Ris and colleagues obtained information about surgery and radiotherapy from participants’ medical records. Patients underwent standardized, age-normed neuropsychologic tests. The primary neuropsychologic outcomes were the Composite Neuropsychological Index (CNI) and estimated IQ. To evaluate socioeconomic outcomes, Dr. Ris and colleagues measured participants’ educational attainment, income, and occupational prestige.
After the researchers adjusted the data for age and sex, they found that siblings had higher mean scores than survivors treated with surgery plus radiotherapy or surgery alone on all neuropsychologic outcomes, including the CNI (siblings, 106.8; surgery only, 95.6; surgery plus radiotherapy, 88.3) and estimated IQ. Survivors who had been diagnosed at younger ages had low scores for all outcomes except for attention/processing speed.
Furthermore, surgery plus radiotherapy was associated with a 7.7-fold higher risk of having an occupation in the lowest sibling quartile, compared with siblings. Survivors who underwent surgery alone had a 2.8-fold higher risk than siblings of having an occupation in the lowest quartile. Surgery plus radiotherapy was associated with a 2.6-fold increased risk of a low occupation score, compared with survivors who underwent surgery alone.
Compared with siblings, surgery plus radiotherapy was associated with a 4.5-fold risk of an annual income of less than $20,000, while the risk for survivors who underwent surgery alone did not differ significantly from that for siblings. Surgery plus radiotherapy was associated with a 2.6-fold higher risk than surgery alone. Surgery plus radiotherapy was also associated with a significantly increased risk for an education level lower than a bachelor’s degree, compared with siblings, but surgery alone was not.
The National Cancer Institute supported the study. The authors had no disclosures.
SOURCE: Ris MD et al. Cancer. 2019 Jun 24. doi: 10.1002/cncr.32186.
Children who underwent surgery alone had better neuropsychologic and socioeconomic outcomes than those who also underwent radiotherapy, but their outcomes were worse than those of unaffected siblings. These findings were published online June 24 in Cancer.
“Late effects in adulthood are evident even for children with the least malignant types of brain tumors who were treated with the least toxic therapies available at the time,” said M. Douglas Ris, PhD, professor of pediatrics and psychology at Baylor College of Medicine in Houston, in a press release. “As pediatric brain tumors become more survivable with continued advances in treatments, we need to improve surveillance of these populations so that survivors continue to receive the best interventions during their transition to adulthood and well beyond.”
Clinicians generally have assumed that children with low-grade CNS tumors who receive less toxic treatment will have fewer long-term effects than survivors of more malignant tumors who undergo neurotoxic therapies. Yet research has indicated that the former patients can have lasting neurobehavioral or functional morbidity.
Dr. Ris and colleagues invited survivors of pediatric low-grade gliomas participating in the Childhood Cancer Survivor Study (CCSS) and a sibling comparison group to undergo a direct, comprehensive neurocognitive assessment. Of 495 eligible survivors, 257 participated. Seventy-six patients did not travel to a study site, but completed a questionnaire, and the researchers did not include data for this group in their analysis. Dr. Ris and colleagues obtained information about surgery and radiotherapy from participants’ medical records. Patients underwent standardized, age-normed neuropsychologic tests. The primary neuropsychologic outcomes were the Composite Neuropsychological Index (CNI) and estimated IQ. To evaluate socioeconomic outcomes, Dr. Ris and colleagues measured participants’ educational attainment, income, and occupational prestige.
After the researchers adjusted the data for age and sex, they found that siblings had higher mean scores than survivors treated with surgery plus radiotherapy or surgery alone on all neuropsychologic outcomes, including the CNI (siblings, 106.8; surgery only, 95.6; surgery plus radiotherapy, 88.3) and estimated IQ. Survivors who had been diagnosed at younger ages had low scores for all outcomes except for attention/processing speed.
Furthermore, surgery plus radiotherapy was associated with a 7.7-fold higher risk of having an occupation in the lowest sibling quartile, compared with siblings. Survivors who underwent surgery alone had a 2.8-fold higher risk than siblings of having an occupation in the lowest quartile. Surgery plus radiotherapy was associated with a 2.6-fold increased risk of a low occupation score, compared with survivors who underwent surgery alone.
Compared with siblings, surgery plus radiotherapy was associated with a 4.5-fold risk of an annual income of less than $20,000, while the risk for survivors who underwent surgery alone did not differ significantly from that for siblings. Surgery plus radiotherapy was associated with a 2.6-fold higher risk than surgery alone. Surgery plus radiotherapy was also associated with a significantly increased risk for an education level lower than a bachelor’s degree, compared with siblings, but surgery alone was not.
The National Cancer Institute supported the study. The authors had no disclosures.
SOURCE: Ris MD et al. Cancer. 2019 Jun 24. doi: 10.1002/cncr.32186.
Children who underwent surgery alone had better neuropsychologic and socioeconomic outcomes than those who also underwent radiotherapy, but their outcomes were worse than those of unaffected siblings. These findings were published online June 24 in Cancer.
“Late effects in adulthood are evident even for children with the least malignant types of brain tumors who were treated with the least toxic therapies available at the time,” said M. Douglas Ris, PhD, professor of pediatrics and psychology at Baylor College of Medicine in Houston, in a press release. “As pediatric brain tumors become more survivable with continued advances in treatments, we need to improve surveillance of these populations so that survivors continue to receive the best interventions during their transition to adulthood and well beyond.”
Clinicians generally have assumed that children with low-grade CNS tumors who receive less toxic treatment will have fewer long-term effects than survivors of more malignant tumors who undergo neurotoxic therapies. Yet research has indicated that the former patients can have lasting neurobehavioral or functional morbidity.
Dr. Ris and colleagues invited survivors of pediatric low-grade gliomas participating in the Childhood Cancer Survivor Study (CCSS) and a sibling comparison group to undergo a direct, comprehensive neurocognitive assessment. Of 495 eligible survivors, 257 participated. Seventy-six patients did not travel to a study site, but completed a questionnaire, and the researchers did not include data for this group in their analysis. Dr. Ris and colleagues obtained information about surgery and radiotherapy from participants’ medical records. Patients underwent standardized, age-normed neuropsychologic tests. The primary neuropsychologic outcomes were the Composite Neuropsychological Index (CNI) and estimated IQ. To evaluate socioeconomic outcomes, Dr. Ris and colleagues measured participants’ educational attainment, income, and occupational prestige.
After the researchers adjusted the data for age and sex, they found that siblings had higher mean scores than survivors treated with surgery plus radiotherapy or surgery alone on all neuropsychologic outcomes, including the CNI (siblings, 106.8; surgery only, 95.6; surgery plus radiotherapy, 88.3) and estimated IQ. Survivors who had been diagnosed at younger ages had low scores for all outcomes except for attention/processing speed.
Furthermore, surgery plus radiotherapy was associated with a 7.7-fold higher risk of having an occupation in the lowest sibling quartile, compared with siblings. Survivors who underwent surgery alone had a 2.8-fold higher risk than siblings of having an occupation in the lowest quartile. Surgery plus radiotherapy was associated with a 2.6-fold increased risk of a low occupation score, compared with survivors who underwent surgery alone.
Compared with siblings, surgery plus radiotherapy was associated with a 4.5-fold risk of an annual income of less than $20,000, while the risk for survivors who underwent surgery alone did not differ significantly from that for siblings. Surgery plus radiotherapy was associated with a 2.6-fold higher risk than surgery alone. Surgery plus radiotherapy was also associated with a significantly increased risk for an education level lower than a bachelor’s degree, compared with siblings, but surgery alone was not.
The National Cancer Institute supported the study. The authors had no disclosures.
SOURCE: Ris MD et al. Cancer. 2019 Jun 24. doi: 10.1002/cncr.32186.
FROM CANCER
ACIP approves meningococcal booster for persons at increased risk
according to the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
The committee voted unanimously in favor of a booster dose of MenB vaccine 1 year after completion of the primary series, with additional boosters every 2-3 years “for as long as risk remains” for high-risk persons, including microbiologists and persons with complement deficiency, complement inhibitor use, or asplenia.
The committee also voted unanimously in favor of a one-time MenB booster for individuals aged 10 years and older who are at least a year beyond completion of a MenB primary series and deemed at increased risk by public health officials in an outbreak situation.
In addition, “a booster dose interval of 6 months or more may be considered by public health officials depending on the specific outbreak, vaccine strategy, and projected duration of elevated risk” according to the language, which was included in the unanimously approved statement “Meningococcal Vaccination: Recommendations of The Advisory Committee on Immunization Practices.”
The updated statement on meningococcal vaccination was developed in 2019 “to consolidate all existing ACIP recommendations for MenACWY and MenB vaccines in a single document,” said Sarah Mbaeyi, MD, of the CDC’s National Center for Immunization and Respiratory Diseases, who presented immunogenicity data and the proposed recommendations.
The statement includes the recommendation of a MenB primary series for individuals aged 16-23 years based on shared clinical decision making. Kelly Moore, MD, of Vanderbilt University, Nashville, Tenn., noted the importance of ongoing data collection, and said clinicians must make clear to patients that, “if they want protection, they need the booster.”
Approximately 7% of serogroup B cases in the United States are related to disease outbreaks, mainly among college students, Dr. Mbaeyi said. All 13 universities that experienced outbreaks between 2013 and 2019 have implemented a MenB primary series, and one university has implemented an off-label booster program.
The work group concluded that a MenB booster dose is necessary to sustain protection against serogroup B disease in persons at increased risk during an outbreak, and that the potential benefits outweighed the harms given the seriousness of meningococcal disease.
Paul Hunter, MD, of the City of Milwaukee Health Department, noted that “the booster recommendation gives more flexibility” in an outbreak response.
The committee also voted unanimously to approve the Vaccines for Children resolution for the meningococcal vaccine that updates language to align with the new recommendations.
The ACIP members had no financial conflicts to disclose.
according to the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
The committee voted unanimously in favor of a booster dose of MenB vaccine 1 year after completion of the primary series, with additional boosters every 2-3 years “for as long as risk remains” for high-risk persons, including microbiologists and persons with complement deficiency, complement inhibitor use, or asplenia.
The committee also voted unanimously in favor of a one-time MenB booster for individuals aged 10 years and older who are at least a year beyond completion of a MenB primary series and deemed at increased risk by public health officials in an outbreak situation.
In addition, “a booster dose interval of 6 months or more may be considered by public health officials depending on the specific outbreak, vaccine strategy, and projected duration of elevated risk” according to the language, which was included in the unanimously approved statement “Meningococcal Vaccination: Recommendations of The Advisory Committee on Immunization Practices.”
The updated statement on meningococcal vaccination was developed in 2019 “to consolidate all existing ACIP recommendations for MenACWY and MenB vaccines in a single document,” said Sarah Mbaeyi, MD, of the CDC’s National Center for Immunization and Respiratory Diseases, who presented immunogenicity data and the proposed recommendations.
The statement includes the recommendation of a MenB primary series for individuals aged 16-23 years based on shared clinical decision making. Kelly Moore, MD, of Vanderbilt University, Nashville, Tenn., noted the importance of ongoing data collection, and said clinicians must make clear to patients that, “if they want protection, they need the booster.”
Approximately 7% of serogroup B cases in the United States are related to disease outbreaks, mainly among college students, Dr. Mbaeyi said. All 13 universities that experienced outbreaks between 2013 and 2019 have implemented a MenB primary series, and one university has implemented an off-label booster program.
The work group concluded that a MenB booster dose is necessary to sustain protection against serogroup B disease in persons at increased risk during an outbreak, and that the potential benefits outweighed the harms given the seriousness of meningococcal disease.
Paul Hunter, MD, of the City of Milwaukee Health Department, noted that “the booster recommendation gives more flexibility” in an outbreak response.
The committee also voted unanimously to approve the Vaccines for Children resolution for the meningococcal vaccine that updates language to align with the new recommendations.
The ACIP members had no financial conflicts to disclose.
according to the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
The committee voted unanimously in favor of a booster dose of MenB vaccine 1 year after completion of the primary series, with additional boosters every 2-3 years “for as long as risk remains” for high-risk persons, including microbiologists and persons with complement deficiency, complement inhibitor use, or asplenia.
The committee also voted unanimously in favor of a one-time MenB booster for individuals aged 10 years and older who are at least a year beyond completion of a MenB primary series and deemed at increased risk by public health officials in an outbreak situation.
In addition, “a booster dose interval of 6 months or more may be considered by public health officials depending on the specific outbreak, vaccine strategy, and projected duration of elevated risk” according to the language, which was included in the unanimously approved statement “Meningococcal Vaccination: Recommendations of The Advisory Committee on Immunization Practices.”
The updated statement on meningococcal vaccination was developed in 2019 “to consolidate all existing ACIP recommendations for MenACWY and MenB vaccines in a single document,” said Sarah Mbaeyi, MD, of the CDC’s National Center for Immunization and Respiratory Diseases, who presented immunogenicity data and the proposed recommendations.
The statement includes the recommendation of a MenB primary series for individuals aged 16-23 years based on shared clinical decision making. Kelly Moore, MD, of Vanderbilt University, Nashville, Tenn., noted the importance of ongoing data collection, and said clinicians must make clear to patients that, “if they want protection, they need the booster.”
Approximately 7% of serogroup B cases in the United States are related to disease outbreaks, mainly among college students, Dr. Mbaeyi said. All 13 universities that experienced outbreaks between 2013 and 2019 have implemented a MenB primary series, and one university has implemented an off-label booster program.
The work group concluded that a MenB booster dose is necessary to sustain protection against serogroup B disease in persons at increased risk during an outbreak, and that the potential benefits outweighed the harms given the seriousness of meningococcal disease.
Paul Hunter, MD, of the City of Milwaukee Health Department, noted that “the booster recommendation gives more flexibility” in an outbreak response.
The committee also voted unanimously to approve the Vaccines for Children resolution for the meningococcal vaccine that updates language to align with the new recommendations.
The ACIP members had no financial conflicts to disclose.
REPORTING FROM AN ACIP MEETING
Cryptosporidiosis infections spike during summer swim season
Outbreaks of cryptosporidiosis increased in the United States by an average of 13% each year between 2009 and 2017, based on data from the Centers for Disease Control and Prevention.
In a study published in the CDC’s Morbidity and Mortality Weekly Report, researchers reviewed data from 444 reported outbreaks submitted to the CDC’s National Outbreak Reporting System totaling 7,465 cases, including 287 hospitalizations and one death.
The outbreaks during this period were most commonly associated with pools and water parks (35%), exposure to cattle (15%), and child care settings (13%). Another 3% of outbreaks were associated with drinking unpasteurized milk or apple cider. An outbreak was defined as two or more cases linked to a common source.
The profuse, watery diarrhea associated with infection from the cryptosporidium parasite can last for 3 weeks in healthy individuals and can cause life-threatening malnutrition in the immunocompromised, wrote Radhika Gharpure, DVM, of the CDC’s National Center for Emerging and Zoonotic Infectious Diseases, and colleagues.
The overall number of outbreaks peaked during July and August each year; the number associated with pools and water parks peaked between June and August, the number associated with cattle peaked between March and May, and the number associated with child care settings peaked between July and September.
The results were limited by several factors including likely underestimation of the number of outbreaks, the use of multipathogen testing panels that could have inflated the number of outbreaks, and the variation in the ability of jurisdictions to detect, investigate, and report outbreaks, the researchers noted. CryptoNet, a molecularly-based surveillance system, has shown potential to track disease transmission, they said.
However, primary prevention is important to prevent the spread of disease, and strategies include refraining from swimming when one has diarrhea and for 2 weeks after resolution of diarrhea, not sending children to child care when they have diarrhea, and washing hands thoroughly after contact with animals, the researchers said.
“If a cryptosporidiosis outbreak occurs, substantial decontamination measures are needed, including hyperchlorinating public treated recreational water venues (e.g., swimming pools at a hotel, apartment complex, or water park) and using hydrogen peroxide to disinfect surfaces in child care settings to inactivate Cryptosporidium oocysts,” they emphasized.
The researchers had no financial conflicts to disclose.
SOURCE: Gharpure R et al. MMWR. 2019 June 28. 68:568-72.
Outbreaks of cryptosporidiosis increased in the United States by an average of 13% each year between 2009 and 2017, based on data from the Centers for Disease Control and Prevention.
In a study published in the CDC’s Morbidity and Mortality Weekly Report, researchers reviewed data from 444 reported outbreaks submitted to the CDC’s National Outbreak Reporting System totaling 7,465 cases, including 287 hospitalizations and one death.
The outbreaks during this period were most commonly associated with pools and water parks (35%), exposure to cattle (15%), and child care settings (13%). Another 3% of outbreaks were associated with drinking unpasteurized milk or apple cider. An outbreak was defined as two or more cases linked to a common source.
The profuse, watery diarrhea associated with infection from the cryptosporidium parasite can last for 3 weeks in healthy individuals and can cause life-threatening malnutrition in the immunocompromised, wrote Radhika Gharpure, DVM, of the CDC’s National Center for Emerging and Zoonotic Infectious Diseases, and colleagues.
The overall number of outbreaks peaked during July and August each year; the number associated with pools and water parks peaked between June and August, the number associated with cattle peaked between March and May, and the number associated with child care settings peaked between July and September.
The results were limited by several factors including likely underestimation of the number of outbreaks, the use of multipathogen testing panels that could have inflated the number of outbreaks, and the variation in the ability of jurisdictions to detect, investigate, and report outbreaks, the researchers noted. CryptoNet, a molecularly-based surveillance system, has shown potential to track disease transmission, they said.
However, primary prevention is important to prevent the spread of disease, and strategies include refraining from swimming when one has diarrhea and for 2 weeks after resolution of diarrhea, not sending children to child care when they have diarrhea, and washing hands thoroughly after contact with animals, the researchers said.
“If a cryptosporidiosis outbreak occurs, substantial decontamination measures are needed, including hyperchlorinating public treated recreational water venues (e.g., swimming pools at a hotel, apartment complex, or water park) and using hydrogen peroxide to disinfect surfaces in child care settings to inactivate Cryptosporidium oocysts,” they emphasized.
The researchers had no financial conflicts to disclose.
SOURCE: Gharpure R et al. MMWR. 2019 June 28. 68:568-72.
Outbreaks of cryptosporidiosis increased in the United States by an average of 13% each year between 2009 and 2017, based on data from the Centers for Disease Control and Prevention.
In a study published in the CDC’s Morbidity and Mortality Weekly Report, researchers reviewed data from 444 reported outbreaks submitted to the CDC’s National Outbreak Reporting System totaling 7,465 cases, including 287 hospitalizations and one death.
The outbreaks during this period were most commonly associated with pools and water parks (35%), exposure to cattle (15%), and child care settings (13%). Another 3% of outbreaks were associated with drinking unpasteurized milk or apple cider. An outbreak was defined as two or more cases linked to a common source.
The profuse, watery diarrhea associated with infection from the cryptosporidium parasite can last for 3 weeks in healthy individuals and can cause life-threatening malnutrition in the immunocompromised, wrote Radhika Gharpure, DVM, of the CDC’s National Center for Emerging and Zoonotic Infectious Diseases, and colleagues.
The overall number of outbreaks peaked during July and August each year; the number associated with pools and water parks peaked between June and August, the number associated with cattle peaked between March and May, and the number associated with child care settings peaked between July and September.
The results were limited by several factors including likely underestimation of the number of outbreaks, the use of multipathogen testing panels that could have inflated the number of outbreaks, and the variation in the ability of jurisdictions to detect, investigate, and report outbreaks, the researchers noted. CryptoNet, a molecularly-based surveillance system, has shown potential to track disease transmission, they said.
However, primary prevention is important to prevent the spread of disease, and strategies include refraining from swimming when one has diarrhea and for 2 weeks after resolution of diarrhea, not sending children to child care when they have diarrhea, and washing hands thoroughly after contact with animals, the researchers said.
“If a cryptosporidiosis outbreak occurs, substantial decontamination measures are needed, including hyperchlorinating public treated recreational water venues (e.g., swimming pools at a hotel, apartment complex, or water park) and using hydrogen peroxide to disinfect surfaces in child care settings to inactivate Cryptosporidium oocysts,” they emphasized.
The researchers had no financial conflicts to disclose.
SOURCE: Gharpure R et al. MMWR. 2019 June 28. 68:568-72.
FROM MMWR
Key risk factors of pediatric cervical spinal injury identified
Risk factors that have good test accuracy in recognizing pediatric cervical spinal injury (CSI) exist, and they can be incorporated into a clinical prediction rule that has the potential to greatly lower the need for cervical spine imaging during trauma evaluation, Julie C Leonard, MD, MPH, and associates reported in Pediatrics.
Though rare, cervical spine injuries in children lead to significant morbidity and mortality, and the vast majority of these children screened radiographically have no injury at all, which makes the inherent lifetime risk of malignancy from unnecessary radiation exposure troubling to many.
In their 2014-2016 prospective observational study in which 4,091 children aged 0-17 years were evaluated for blunt trauma in one of four U.S. tertiary care children’s hospitals, 2% had CSIs.
The mean age in the cohort was 9 years; the mean age of CSI patients was 11 years. Fully 39% of patients were under 8 years of age and 23 (1%) had CSIs. Among those with CSIs, more were boys, white, and non-Hispanic. Motor vehicle crash and sports-related injuries were reported to be the most common route of injury in all children.
The main goal of the study was to “establish the infrastructure for conducting a larger cohort study,” said Dr. Leonard of Ohio State University Nationwide Children’s Hospital in Columbus, and associates. They were successful in confirming the existence of an association between CSI and head injury. Specifically, the greatest independent associations with pediatric CSI were substantial head injury, namely basilar skull fracture; signs of traumatic brain injury, such as altered mental status; respiratory failure and intubation; and head-first impacts.
The authors were careful to point out that risk factors identified in their study differed from other studies focused on adult injury, specifically with regard to neck findings. They speculated that the increased neck and spine tenderness that commonly increase following restrictive supine positioning in a cervical collar on a rigid long board could play a key role. They also speculated that ED clinicians may be more likely to “defer aspects of the neck examination” in cases where children present wearing cervical collars, which limits assessment to self reporting.
As with adult evaluation, in which adult CSI prediction rules for cervical imaging depend upon determining the extent of normal mental status after blunt trauma, successful identification of pediatric candidates will require a similar set of CSI prediction rules. “Future development of a robust pediatric CSI prediction rule should be focused on stratification based on mental status because it may be meaningful in determining which children to triage to CT scan,” the investigators advised.
Future research exploring how these risk factors can be used to build a clear, pediatric CSI prediction rule that is prospective and observational in nature is crucial to improving the timeliness and accuracy of CSI diagnosis, Dr. Leonard and associates said.
In an accompanying editorial, Mark I. Neuman, MD, MPH and Rebekah C. Mannix, MD, MPH, noted that evidence uncovered by Leonard et al. will, no doubt, provide the conceptual foundation for a future multicenter trial that can establish effective criteria needed to consider the use of imaging when evaluating CSI in children.
Previously, the National Emergency X-Ray Utilization Study (NEXUS) was the largest prospective study of CSI that also included children. The Leonard et al. study includes a much higher proportion of children, 39% of whom were younger than 8 years of age. Although the sensitivities of the models used in this latest study are lower than for those used in the NEXUS study, the specificity is much higher at 46%-50%, which has noteworthy implications for classifying children at risk of CSI. “If validated, these findings have the potential to spare imaging in over one-third of children,” said Dr. Neuman and Dr. Mannix, both of the division of emergency medicine at Boston Children’s Hospital, and the department of pediatrics at Harvard Medical School, Boston.
“The complex and varying nature of CSI in children, the result of differences in the intrinsic biomechanics of the pediatric cervical spine, mechanism of injury, and variable presentations between younger and older children pose challenges for the development of a universal, simple, and highly sensitive clinical prediction rule,” they concluded.
The National Institutes of Health funded the study, and Dr. Leonard received a grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The authors had no relevant disclosures. There was no external funding for the accompanying editorial and Dr. Neuman and Dr. Mannix said they had no relevant disclosures.
SOURCE: Leonard J et al. Pediatrics. 2019;144(1):e20183221; Neuman MI et al. Pediatrics. 2019;144(1):e20184052.
Risk factors that have good test accuracy in recognizing pediatric cervical spinal injury (CSI) exist, and they can be incorporated into a clinical prediction rule that has the potential to greatly lower the need for cervical spine imaging during trauma evaluation, Julie C Leonard, MD, MPH, and associates reported in Pediatrics.
Though rare, cervical spine injuries in children lead to significant morbidity and mortality, and the vast majority of these children screened radiographically have no injury at all, which makes the inherent lifetime risk of malignancy from unnecessary radiation exposure troubling to many.
In their 2014-2016 prospective observational study in which 4,091 children aged 0-17 years were evaluated for blunt trauma in one of four U.S. tertiary care children’s hospitals, 2% had CSIs.
The mean age in the cohort was 9 years; the mean age of CSI patients was 11 years. Fully 39% of patients were under 8 years of age and 23 (1%) had CSIs. Among those with CSIs, more were boys, white, and non-Hispanic. Motor vehicle crash and sports-related injuries were reported to be the most common route of injury in all children.
The main goal of the study was to “establish the infrastructure for conducting a larger cohort study,” said Dr. Leonard of Ohio State University Nationwide Children’s Hospital in Columbus, and associates. They were successful in confirming the existence of an association between CSI and head injury. Specifically, the greatest independent associations with pediatric CSI were substantial head injury, namely basilar skull fracture; signs of traumatic brain injury, such as altered mental status; respiratory failure and intubation; and head-first impacts.
The authors were careful to point out that risk factors identified in their study differed from other studies focused on adult injury, specifically with regard to neck findings. They speculated that the increased neck and spine tenderness that commonly increase following restrictive supine positioning in a cervical collar on a rigid long board could play a key role. They also speculated that ED clinicians may be more likely to “defer aspects of the neck examination” in cases where children present wearing cervical collars, which limits assessment to self reporting.
As with adult evaluation, in which adult CSI prediction rules for cervical imaging depend upon determining the extent of normal mental status after blunt trauma, successful identification of pediatric candidates will require a similar set of CSI prediction rules. “Future development of a robust pediatric CSI prediction rule should be focused on stratification based on mental status because it may be meaningful in determining which children to triage to CT scan,” the investigators advised.
Future research exploring how these risk factors can be used to build a clear, pediatric CSI prediction rule that is prospective and observational in nature is crucial to improving the timeliness and accuracy of CSI diagnosis, Dr. Leonard and associates said.
In an accompanying editorial, Mark I. Neuman, MD, MPH and Rebekah C. Mannix, MD, MPH, noted that evidence uncovered by Leonard et al. will, no doubt, provide the conceptual foundation for a future multicenter trial that can establish effective criteria needed to consider the use of imaging when evaluating CSI in children.
Previously, the National Emergency X-Ray Utilization Study (NEXUS) was the largest prospective study of CSI that also included children. The Leonard et al. study includes a much higher proportion of children, 39% of whom were younger than 8 years of age. Although the sensitivities of the models used in this latest study are lower than for those used in the NEXUS study, the specificity is much higher at 46%-50%, which has noteworthy implications for classifying children at risk of CSI. “If validated, these findings have the potential to spare imaging in over one-third of children,” said Dr. Neuman and Dr. Mannix, both of the division of emergency medicine at Boston Children’s Hospital, and the department of pediatrics at Harvard Medical School, Boston.
“The complex and varying nature of CSI in children, the result of differences in the intrinsic biomechanics of the pediatric cervical spine, mechanism of injury, and variable presentations between younger and older children pose challenges for the development of a universal, simple, and highly sensitive clinical prediction rule,” they concluded.
The National Institutes of Health funded the study, and Dr. Leonard received a grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The authors had no relevant disclosures. There was no external funding for the accompanying editorial and Dr. Neuman and Dr. Mannix said they had no relevant disclosures.
SOURCE: Leonard J et al. Pediatrics. 2019;144(1):e20183221; Neuman MI et al. Pediatrics. 2019;144(1):e20184052.
Risk factors that have good test accuracy in recognizing pediatric cervical spinal injury (CSI) exist, and they can be incorporated into a clinical prediction rule that has the potential to greatly lower the need for cervical spine imaging during trauma evaluation, Julie C Leonard, MD, MPH, and associates reported in Pediatrics.
Though rare, cervical spine injuries in children lead to significant morbidity and mortality, and the vast majority of these children screened radiographically have no injury at all, which makes the inherent lifetime risk of malignancy from unnecessary radiation exposure troubling to many.
In their 2014-2016 prospective observational study in which 4,091 children aged 0-17 years were evaluated for blunt trauma in one of four U.S. tertiary care children’s hospitals, 2% had CSIs.
The mean age in the cohort was 9 years; the mean age of CSI patients was 11 years. Fully 39% of patients were under 8 years of age and 23 (1%) had CSIs. Among those with CSIs, more were boys, white, and non-Hispanic. Motor vehicle crash and sports-related injuries were reported to be the most common route of injury in all children.
The main goal of the study was to “establish the infrastructure for conducting a larger cohort study,” said Dr. Leonard of Ohio State University Nationwide Children’s Hospital in Columbus, and associates. They were successful in confirming the existence of an association between CSI and head injury. Specifically, the greatest independent associations with pediatric CSI were substantial head injury, namely basilar skull fracture; signs of traumatic brain injury, such as altered mental status; respiratory failure and intubation; and head-first impacts.
The authors were careful to point out that risk factors identified in their study differed from other studies focused on adult injury, specifically with regard to neck findings. They speculated that the increased neck and spine tenderness that commonly increase following restrictive supine positioning in a cervical collar on a rigid long board could play a key role. They also speculated that ED clinicians may be more likely to “defer aspects of the neck examination” in cases where children present wearing cervical collars, which limits assessment to self reporting.
As with adult evaluation, in which adult CSI prediction rules for cervical imaging depend upon determining the extent of normal mental status after blunt trauma, successful identification of pediatric candidates will require a similar set of CSI prediction rules. “Future development of a robust pediatric CSI prediction rule should be focused on stratification based on mental status because it may be meaningful in determining which children to triage to CT scan,” the investigators advised.
Future research exploring how these risk factors can be used to build a clear, pediatric CSI prediction rule that is prospective and observational in nature is crucial to improving the timeliness and accuracy of CSI diagnosis, Dr. Leonard and associates said.
In an accompanying editorial, Mark I. Neuman, MD, MPH and Rebekah C. Mannix, MD, MPH, noted that evidence uncovered by Leonard et al. will, no doubt, provide the conceptual foundation for a future multicenter trial that can establish effective criteria needed to consider the use of imaging when evaluating CSI in children.
Previously, the National Emergency X-Ray Utilization Study (NEXUS) was the largest prospective study of CSI that also included children. The Leonard et al. study includes a much higher proportion of children, 39% of whom were younger than 8 years of age. Although the sensitivities of the models used in this latest study are lower than for those used in the NEXUS study, the specificity is much higher at 46%-50%, which has noteworthy implications for classifying children at risk of CSI. “If validated, these findings have the potential to spare imaging in over one-third of children,” said Dr. Neuman and Dr. Mannix, both of the division of emergency medicine at Boston Children’s Hospital, and the department of pediatrics at Harvard Medical School, Boston.
“The complex and varying nature of CSI in children, the result of differences in the intrinsic biomechanics of the pediatric cervical spine, mechanism of injury, and variable presentations between younger and older children pose challenges for the development of a universal, simple, and highly sensitive clinical prediction rule,” they concluded.
The National Institutes of Health funded the study, and Dr. Leonard received a grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The authors had no relevant disclosures. There was no external funding for the accompanying editorial and Dr. Neuman and Dr. Mannix said they had no relevant disclosures.
SOURCE: Leonard J et al. Pediatrics. 2019;144(1):e20183221; Neuman MI et al. Pediatrics. 2019;144(1):e20184052.
FROM PEDIATRICS
ACIP adds hexavalent vaccine to VFC program
The pediatric hexavalent vaccine (DTaP-[inactivated poliovirus] IPV-[hepatitis B] HepB-[Haemophilis influenzae type b] Hib) should be included as an option in the Vaccines for Children (VFC) program for the infant series at ages 2, 4, and 6 months, according to unanimous votes at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
The addition of the vaccine to the VFC program required no motions on the part of the committee, but involved separate votes on each component of the vaccine.
Combination vaccination has been associated with increased coverage and more likely completion of the full infant vaccine series, said Sara Oliver, MD, of the CDC’s National Center for Immunization and Respiratory Diseases.
The new vaccine is being developed jointly by Sanofi and Merck, and has been approved by the Food and Drug Administration for use in children through age 4 years.
Dr. Oliver presented evidence that the safety profile of the combination vaccine is consistent with that of the component vaccines. In addition, “use of combination vaccines can reduce the number of injections patient receive and alleviate concern associated with the number of injections,” she said. However, “considerations should include provider assessment, patient preference, and the potential for adverse events.”
although it will not be available until 2021 in order to ensure sufficient supply, Dr. Oliver noted.
The combination vaccination work group considered whether the new vaccine should be preferentially recommended for American Indian and Alaskan Native populations, but they concluded that post–dose one immunogenicity data are needed before such a preferential recommendation can be made.
The ACIP members had no financial conflicts to disclose.
The pediatric hexavalent vaccine (DTaP-[inactivated poliovirus] IPV-[hepatitis B] HepB-[Haemophilis influenzae type b] Hib) should be included as an option in the Vaccines for Children (VFC) program for the infant series at ages 2, 4, and 6 months, according to unanimous votes at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
The addition of the vaccine to the VFC program required no motions on the part of the committee, but involved separate votes on each component of the vaccine.
Combination vaccination has been associated with increased coverage and more likely completion of the full infant vaccine series, said Sara Oliver, MD, of the CDC’s National Center for Immunization and Respiratory Diseases.
The new vaccine is being developed jointly by Sanofi and Merck, and has been approved by the Food and Drug Administration for use in children through age 4 years.
Dr. Oliver presented evidence that the safety profile of the combination vaccine is consistent with that of the component vaccines. In addition, “use of combination vaccines can reduce the number of injections patient receive and alleviate concern associated with the number of injections,” she said. However, “considerations should include provider assessment, patient preference, and the potential for adverse events.”
although it will not be available until 2021 in order to ensure sufficient supply, Dr. Oliver noted.
The combination vaccination work group considered whether the new vaccine should be preferentially recommended for American Indian and Alaskan Native populations, but they concluded that post–dose one immunogenicity data are needed before such a preferential recommendation can be made.
The ACIP members had no financial conflicts to disclose.
The pediatric hexavalent vaccine (DTaP-[inactivated poliovirus] IPV-[hepatitis B] HepB-[Haemophilis influenzae type b] Hib) should be included as an option in the Vaccines for Children (VFC) program for the infant series at ages 2, 4, and 6 months, according to unanimous votes at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
The addition of the vaccine to the VFC program required no motions on the part of the committee, but involved separate votes on each component of the vaccine.
Combination vaccination has been associated with increased coverage and more likely completion of the full infant vaccine series, said Sara Oliver, MD, of the CDC’s National Center for Immunization and Respiratory Diseases.
The new vaccine is being developed jointly by Sanofi and Merck, and has been approved by the Food and Drug Administration for use in children through age 4 years.
Dr. Oliver presented evidence that the safety profile of the combination vaccine is consistent with that of the component vaccines. In addition, “use of combination vaccines can reduce the number of injections patient receive and alleviate concern associated with the number of injections,” she said. However, “considerations should include provider assessment, patient preference, and the potential for adverse events.”
although it will not be available until 2021 in order to ensure sufficient supply, Dr. Oliver noted.
The combination vaccination work group considered whether the new vaccine should be preferentially recommended for American Indian and Alaskan Native populations, but they concluded that post–dose one immunogenicity data are needed before such a preferential recommendation can be made.
The ACIP members had no financial conflicts to disclose.
REPORTING FROM AN ACIP MEETING
Substantial reductions in HPV infections, CIN2+ after vaccination
The introduction of the human papillomavirus according to a meta-analysis of data from more than 60 million individuals worldwide.
Mélanie Drolet, PhD, from the Centre de recherche du CHU de Québec–Université Laval, and coauthors of the HPV Vaccination Impact Study Group reported the results of a systematic review and meta-analysis of 65 studies showing pre- and postvaccination frequency of at least one HPV-related endpoint published in the Lancet. The studies were conducted in 14 high-income countries, 12 of which were vaccinating only women and girls, with the results at 5-8 years published in the Lancet.
At 5-8 years after a vaccination program was implemented, there was a significant 83% reduction in the prevalence of HPV 16 and 18, both of which are targeted by the vaccine, among girls aged 13-19 years; a 66% reduction among women aged 20-24 years; and a 37% reduction in women aged 25-29 years, even though most of these women were unvaccinated.
There also were significant decreases at 5-8 years in the prevalence of HPV subtypes 31, 33, and 45, which are not included in the vaccine but against which the vaccine appears to offer cross-protection. Among girls aged 13-19 years, there was a significant 54% reduction in the prevalence of these subtypes, among women aged 20-24 years there was a nonsignificant 28% decrease, but among women aged 25-29 years, there was no significant decrease.
The analysis also found significant declines in the prevalence of cervical intraepithelial neoplasias (CINs) of grade 2 or above. At 5-9 years after vaccination was introduced, CIN2+ decreased by 51% among girls aged 15-19 years who also were screened for cervical cancer, and by 31% among women aged 20-24 years.
However, over the same time period, the rates of CIN2+ increased by a significant 19% among mostly unvaccinated women aged 25-29 years and 23% among mostly unvaccinated women aged 30-39 years, despite both groups being screened for cervical abnormalities.
While most of the countries in the study vaccinated only girls and women, two studies did find nonsignificant decreases in the prevalence of HPV 16, 18, 31, 33, and 45 among boys aged 16-19 years, but not among men aged 20-24 years.
HPV vaccination also was associated with significant declines in the incidence of anogenital warts among both males and females. In the first 4 years alone, vaccination was associated with significant reductions in anogenital wart diagnoses among females aged 15-29 years, as well as nonsignificant but “substantial” reductions in unvaccinated boys aged 15-19 years.
After 5-8 years, anogenital wart diagnoses decreased by 67% among girls aged 15-19 years, significantly by 54% among women aged 20-24 years, and 31% among women aged 25-29 years – all significant changes. Among boys aged 15-19 years, anogenital wart diagnoses decreased by a significant 48%, and among men aged 20-24 years they decreased by a significant 32%.
The decreases in anogenital wart diagnoses were even greater in countries that implemented vaccination among multiple cohorts simultaneously and achieved high vaccination coverage, compared with countries that vaccinated only one cohort at a time or had low routine vaccination coverage.
“Our study is the first to show the real-world additional benefit of multicohort HPV vaccination and high routine vaccination coverage, and the fast and substantial herd effects of vaccination in countries which implement these measures,” wrote Dr. Drolet and coauthors. “The greater impact of multicohort vaccination was similar when restricting the analyses to countries with high routine vaccination coverage.”
They pointed to the World Health Organization’s recently revised position on HPV vaccination, which now recommends vaccination of multiple cohorts of girls aged 9-14 years, although they raised the question of what might be the optimal number of age cohorts. “Number needed to vaccinate and cost-effectiveness analyses in high-income countries suggest that vaccinating multiple cohorts of individuals up to 18 years of age is highly efficient and cost effective.”
This analysis by Drolet et al. “provides compelling evidence for HPV vaccine efficacy on all outcomes explored and for almost all age strata,” Dr. Silvia de Sanjose, of PATH in Seattle, and Dr. Sinead Delany-Moretlwe of the Wits Reproductive Health and HIV Institute at the University of Witwatersrand in Johannesburg, said in an accompanying editorial (Lancet. 2019 Jun 26. doi: 10.1016/ S0140-6736[19]30549-5). This study shows just how effective HPV vaccination can be across a range of outcomes and ages, and also demonstrates the herd immunity benefits, particularly when multiple cohorts are vaccinated and there is high vaccination coverage.
One key limitation of this analysis is the lack of data from low- and middle-income countries. The data by Drolet et al. “emphasise the importance of redoubling our efforts to tackle the fiscal, supply, and programmatic barriers that currently limit HPV vaccine programmes; with these efforts, HPV vaccination could become a hallmark investment of cancer prevention in the 21st century,” Dr. de Sanjose and Dr. Delany-Moretlwe concluded.
The study was funded by WHO, Canadian Institutes of Health Research, and Fonds de recherche du Québec–Santé. No conflicts of interest were declared.
Dr. de Sanjose declared previous institutional support from Merck.
SOURCE: Drolet M et al. Lancet 2019 Jun 26. doi: 10.1016/ S0140-6736(19)30298-3.
The introduction of the human papillomavirus according to a meta-analysis of data from more than 60 million individuals worldwide.
Mélanie Drolet, PhD, from the Centre de recherche du CHU de Québec–Université Laval, and coauthors of the HPV Vaccination Impact Study Group reported the results of a systematic review and meta-analysis of 65 studies showing pre- and postvaccination frequency of at least one HPV-related endpoint published in the Lancet. The studies were conducted in 14 high-income countries, 12 of which were vaccinating only women and girls, with the results at 5-8 years published in the Lancet.
At 5-8 years after a vaccination program was implemented, there was a significant 83% reduction in the prevalence of HPV 16 and 18, both of which are targeted by the vaccine, among girls aged 13-19 years; a 66% reduction among women aged 20-24 years; and a 37% reduction in women aged 25-29 years, even though most of these women were unvaccinated.
There also were significant decreases at 5-8 years in the prevalence of HPV subtypes 31, 33, and 45, which are not included in the vaccine but against which the vaccine appears to offer cross-protection. Among girls aged 13-19 years, there was a significant 54% reduction in the prevalence of these subtypes, among women aged 20-24 years there was a nonsignificant 28% decrease, but among women aged 25-29 years, there was no significant decrease.
The analysis also found significant declines in the prevalence of cervical intraepithelial neoplasias (CINs) of grade 2 or above. At 5-9 years after vaccination was introduced, CIN2+ decreased by 51% among girls aged 15-19 years who also were screened for cervical cancer, and by 31% among women aged 20-24 years.
However, over the same time period, the rates of CIN2+ increased by a significant 19% among mostly unvaccinated women aged 25-29 years and 23% among mostly unvaccinated women aged 30-39 years, despite both groups being screened for cervical abnormalities.
While most of the countries in the study vaccinated only girls and women, two studies did find nonsignificant decreases in the prevalence of HPV 16, 18, 31, 33, and 45 among boys aged 16-19 years, but not among men aged 20-24 years.
HPV vaccination also was associated with significant declines in the incidence of anogenital warts among both males and females. In the first 4 years alone, vaccination was associated with significant reductions in anogenital wart diagnoses among females aged 15-29 years, as well as nonsignificant but “substantial” reductions in unvaccinated boys aged 15-19 years.
After 5-8 years, anogenital wart diagnoses decreased by 67% among girls aged 15-19 years, significantly by 54% among women aged 20-24 years, and 31% among women aged 25-29 years – all significant changes. Among boys aged 15-19 years, anogenital wart diagnoses decreased by a significant 48%, and among men aged 20-24 years they decreased by a significant 32%.
The decreases in anogenital wart diagnoses were even greater in countries that implemented vaccination among multiple cohorts simultaneously and achieved high vaccination coverage, compared with countries that vaccinated only one cohort at a time or had low routine vaccination coverage.
“Our study is the first to show the real-world additional benefit of multicohort HPV vaccination and high routine vaccination coverage, and the fast and substantial herd effects of vaccination in countries which implement these measures,” wrote Dr. Drolet and coauthors. “The greater impact of multicohort vaccination was similar when restricting the analyses to countries with high routine vaccination coverage.”
They pointed to the World Health Organization’s recently revised position on HPV vaccination, which now recommends vaccination of multiple cohorts of girls aged 9-14 years, although they raised the question of what might be the optimal number of age cohorts. “Number needed to vaccinate and cost-effectiveness analyses in high-income countries suggest that vaccinating multiple cohorts of individuals up to 18 years of age is highly efficient and cost effective.”
This analysis by Drolet et al. “provides compelling evidence for HPV vaccine efficacy on all outcomes explored and for almost all age strata,” Dr. Silvia de Sanjose, of PATH in Seattle, and Dr. Sinead Delany-Moretlwe of the Wits Reproductive Health and HIV Institute at the University of Witwatersrand in Johannesburg, said in an accompanying editorial (Lancet. 2019 Jun 26. doi: 10.1016/ S0140-6736[19]30549-5). This study shows just how effective HPV vaccination can be across a range of outcomes and ages, and also demonstrates the herd immunity benefits, particularly when multiple cohorts are vaccinated and there is high vaccination coverage.
One key limitation of this analysis is the lack of data from low- and middle-income countries. The data by Drolet et al. “emphasise the importance of redoubling our efforts to tackle the fiscal, supply, and programmatic barriers that currently limit HPV vaccine programmes; with these efforts, HPV vaccination could become a hallmark investment of cancer prevention in the 21st century,” Dr. de Sanjose and Dr. Delany-Moretlwe concluded.
The study was funded by WHO, Canadian Institutes of Health Research, and Fonds de recherche du Québec–Santé. No conflicts of interest were declared.
Dr. de Sanjose declared previous institutional support from Merck.
SOURCE: Drolet M et al. Lancet 2019 Jun 26. doi: 10.1016/ S0140-6736(19)30298-3.
The introduction of the human papillomavirus according to a meta-analysis of data from more than 60 million individuals worldwide.
Mélanie Drolet, PhD, from the Centre de recherche du CHU de Québec–Université Laval, and coauthors of the HPV Vaccination Impact Study Group reported the results of a systematic review and meta-analysis of 65 studies showing pre- and postvaccination frequency of at least one HPV-related endpoint published in the Lancet. The studies were conducted in 14 high-income countries, 12 of which were vaccinating only women and girls, with the results at 5-8 years published in the Lancet.
At 5-8 years after a vaccination program was implemented, there was a significant 83% reduction in the prevalence of HPV 16 and 18, both of which are targeted by the vaccine, among girls aged 13-19 years; a 66% reduction among women aged 20-24 years; and a 37% reduction in women aged 25-29 years, even though most of these women were unvaccinated.
There also were significant decreases at 5-8 years in the prevalence of HPV subtypes 31, 33, and 45, which are not included in the vaccine but against which the vaccine appears to offer cross-protection. Among girls aged 13-19 years, there was a significant 54% reduction in the prevalence of these subtypes, among women aged 20-24 years there was a nonsignificant 28% decrease, but among women aged 25-29 years, there was no significant decrease.
The analysis also found significant declines in the prevalence of cervical intraepithelial neoplasias (CINs) of grade 2 or above. At 5-9 years after vaccination was introduced, CIN2+ decreased by 51% among girls aged 15-19 years who also were screened for cervical cancer, and by 31% among women aged 20-24 years.
However, over the same time period, the rates of CIN2+ increased by a significant 19% among mostly unvaccinated women aged 25-29 years and 23% among mostly unvaccinated women aged 30-39 years, despite both groups being screened for cervical abnormalities.
While most of the countries in the study vaccinated only girls and women, two studies did find nonsignificant decreases in the prevalence of HPV 16, 18, 31, 33, and 45 among boys aged 16-19 years, but not among men aged 20-24 years.
HPV vaccination also was associated with significant declines in the incidence of anogenital warts among both males and females. In the first 4 years alone, vaccination was associated with significant reductions in anogenital wart diagnoses among females aged 15-29 years, as well as nonsignificant but “substantial” reductions in unvaccinated boys aged 15-19 years.
After 5-8 years, anogenital wart diagnoses decreased by 67% among girls aged 15-19 years, significantly by 54% among women aged 20-24 years, and 31% among women aged 25-29 years – all significant changes. Among boys aged 15-19 years, anogenital wart diagnoses decreased by a significant 48%, and among men aged 20-24 years they decreased by a significant 32%.
The decreases in anogenital wart diagnoses were even greater in countries that implemented vaccination among multiple cohorts simultaneously and achieved high vaccination coverage, compared with countries that vaccinated only one cohort at a time or had low routine vaccination coverage.
“Our study is the first to show the real-world additional benefit of multicohort HPV vaccination and high routine vaccination coverage, and the fast and substantial herd effects of vaccination in countries which implement these measures,” wrote Dr. Drolet and coauthors. “The greater impact of multicohort vaccination was similar when restricting the analyses to countries with high routine vaccination coverage.”
They pointed to the World Health Organization’s recently revised position on HPV vaccination, which now recommends vaccination of multiple cohorts of girls aged 9-14 years, although they raised the question of what might be the optimal number of age cohorts. “Number needed to vaccinate and cost-effectiveness analyses in high-income countries suggest that vaccinating multiple cohorts of individuals up to 18 years of age is highly efficient and cost effective.”
This analysis by Drolet et al. “provides compelling evidence for HPV vaccine efficacy on all outcomes explored and for almost all age strata,” Dr. Silvia de Sanjose, of PATH in Seattle, and Dr. Sinead Delany-Moretlwe of the Wits Reproductive Health and HIV Institute at the University of Witwatersrand in Johannesburg, said in an accompanying editorial (Lancet. 2019 Jun 26. doi: 10.1016/ S0140-6736[19]30549-5). This study shows just how effective HPV vaccination can be across a range of outcomes and ages, and also demonstrates the herd immunity benefits, particularly when multiple cohorts are vaccinated and there is high vaccination coverage.
One key limitation of this analysis is the lack of data from low- and middle-income countries. The data by Drolet et al. “emphasise the importance of redoubling our efforts to tackle the fiscal, supply, and programmatic barriers that currently limit HPV vaccine programmes; with these efforts, HPV vaccination could become a hallmark investment of cancer prevention in the 21st century,” Dr. de Sanjose and Dr. Delany-Moretlwe concluded.
The study was funded by WHO, Canadian Institutes of Health Research, and Fonds de recherche du Québec–Santé. No conflicts of interest were declared.
Dr. de Sanjose declared previous institutional support from Merck.
SOURCE: Drolet M et al. Lancet 2019 Jun 26. doi: 10.1016/ S0140-6736(19)30298-3.
FROM THE LANCET
Key clinical point: Significant declines in HPV infections, CIN2+, and anogenital warts have occurred after the introduction of HPV vaccine programs, some because of herd effects.
Major finding: The HPV vaccination program is associated with a significant 83% reduction in the prevalence of HPV 16 and 18 among girls aged 13-19 years in 14 high-income countries.
Study details: Systematic review and meta-analysis of 65 studies involving more than 60 million individuals in 14 countries.
Disclosures: The study was funded by World Health Organization, Canadian Institutes of Health Research, and Fonds de recherche du Québec – Santé. No conflicts of interest were declared.
Source: Drolet M et al. Lancet 2019 Jun 26. doi: 10.1016/ S0140-6736(19)30298-3.
‘Robust antitumor immune responses’ observed in pediatric ALL
Pediatric acute lymphoblastic leukemia (ALL) may be more vulnerable to immunotherapies than previously thought, according to researchers.
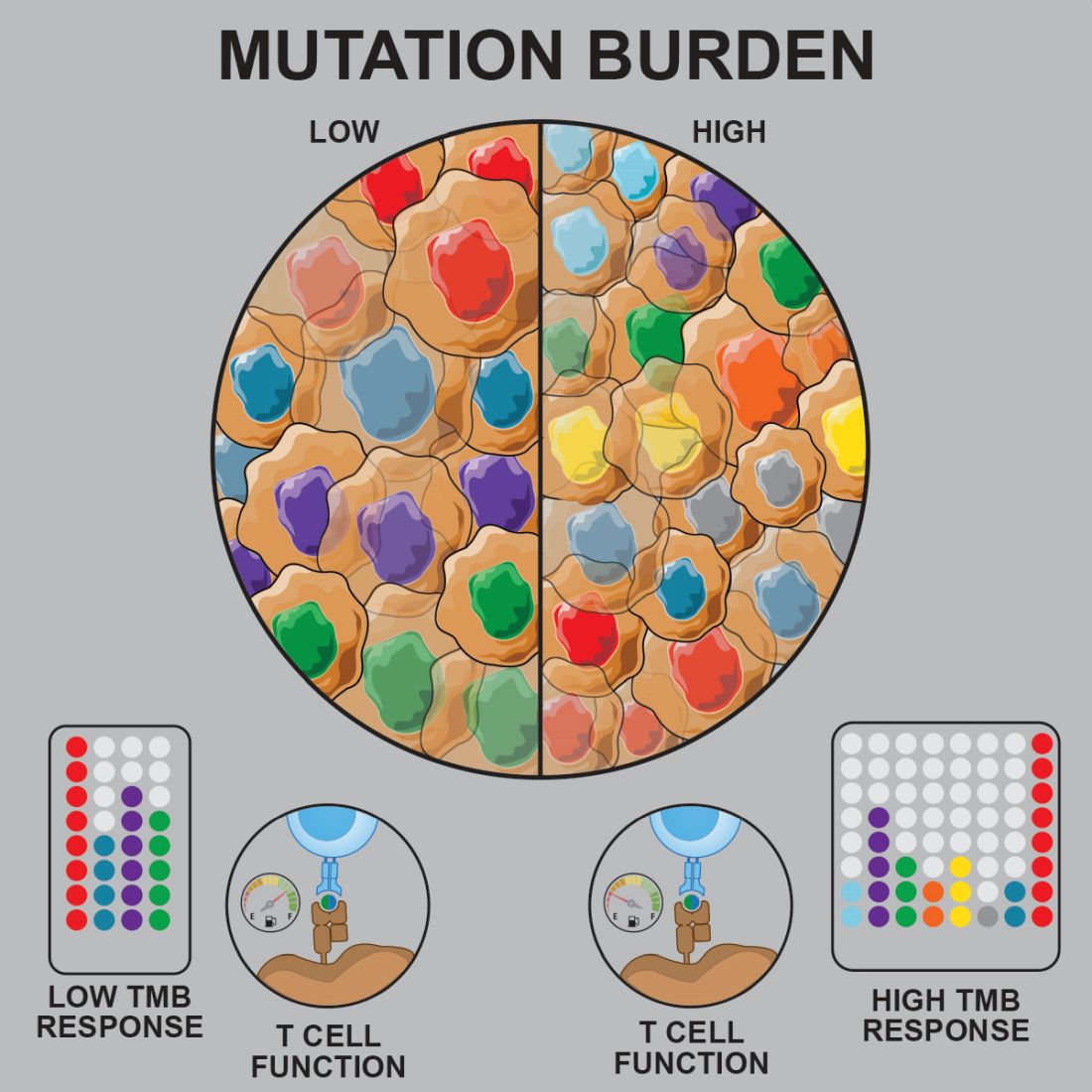
Prior studies suggested that tumors with a low mutational burden don’t elicit strong antitumor responses and therefore aren’t very susceptible to immunotherapy.
Now, researchers have found evidence to suggest that pediatric ALL induces “robust antitumor immune responses” despite a low mutational burden. The investigators identified tumor-associated CD8+ T cells that responded to 86% of neoantigens tested and recognized 68% of neoepitopes tested.
Anthony E. Zamora, PhD, of St. Jude Children’s Research Hospital in Memphis, Tenn., and colleagues recounted these findings in Science Translational Medicine.
The researchers analyzed samples from pediatric patients with ETV-associated ALL (n = 9) or ERG-associated ALL (n = 2) to determine how endogenous CD8+ T cells respond to patient-specific cancer neoantigens.
The investigators first assessed the ability of tumor-specific mutations and gene fusions to generate neoepitopes, or neoantigens predicted to bind patient-specific human leukocyte antigen (HLA) proteins. The team identified 5-28 neoepitopes per patient, including epitopes that spanned the fusion junction in patients with ETV6-RUNX1 fusions.
The researchers then tested whether CD8+ tumor infiltrating lymphocytes (TILs) were directly responsive to mutated neoepitopes. They observed cytokine responses across patient samples, noting that 31 of the 36 putative neoantigens tested (86%) were “immunogenic and capable of inducing robust cytokine responses.”
Next, the investigators mapped TIL responses to specific epitopes using patient-specific tetramers that corresponded to the previously identified neoepitopes. Seventeen of the 25 patient-specific tetramers (68%) bound to TILs above the background set by irrelevant HLA-matched tetramers.
“Within those responses, we observed immunodominance hierarchies among the distinct TIL populations, with a majority of tetramer-bound CD8+ T cells restricted to one or two putative neoepitopes,” the researchers noted.
The team also pointed out that seven of nine patients tested had CD8+ T cells that responded to ETV6-RUNX1.
Finally, the investigators performed transcriptional profiling of ALL-specific CD8+ TILs to assess inter- and intrapatient heterogeneity. The team identified three hierarchical clusters, which were characterized by transcriptional factors and regulators associated with:
- Functional effector CD8+ T cells (TBX21 and EOMES).
- Dysfunctional CD8+ T cells (STAT1/3/4, NR4A2/3, and BCL6).
- Exhausted CD8+ T cells (EOMES, MAF, PRDM1, and BATF).
Considering these findings together, the researchers concluded that “pediatric ALL elicits a potent neoepitope-specific CD8+ T-cell response.” Therefore, adoptive T-cell, monoclonal antibody, and targeted T-cell receptor therapies “should be explored” in pediatric ALL.
This research was supported by the National Institutes of Health, National Cancer Institute, National Institute of General Medical Sciences, Key for a Cure Foundation, and American Lebanese Syrian Associated Charities. The researchers disclosed patent applications and relationships with Pfizer, Amgen, and other companies.
SOURCE: Zamora AE et al. Sci. Transl. Med. 2019 Jun 26. doi: 10.1126/scitranslmed.aat8549.
Pediatric acute lymphoblastic leukemia (ALL) may be more vulnerable to immunotherapies than previously thought, according to researchers.

Prior studies suggested that tumors with a low mutational burden don’t elicit strong antitumor responses and therefore aren’t very susceptible to immunotherapy.
Now, researchers have found evidence to suggest that pediatric ALL induces “robust antitumor immune responses” despite a low mutational burden. The investigators identified tumor-associated CD8+ T cells that responded to 86% of neoantigens tested and recognized 68% of neoepitopes tested.
Anthony E. Zamora, PhD, of St. Jude Children’s Research Hospital in Memphis, Tenn., and colleagues recounted these findings in Science Translational Medicine.
The researchers analyzed samples from pediatric patients with ETV-associated ALL (n = 9) or ERG-associated ALL (n = 2) to determine how endogenous CD8+ T cells respond to patient-specific cancer neoantigens.
The investigators first assessed the ability of tumor-specific mutations and gene fusions to generate neoepitopes, or neoantigens predicted to bind patient-specific human leukocyte antigen (HLA) proteins. The team identified 5-28 neoepitopes per patient, including epitopes that spanned the fusion junction in patients with ETV6-RUNX1 fusions.
The researchers then tested whether CD8+ tumor infiltrating lymphocytes (TILs) were directly responsive to mutated neoepitopes. They observed cytokine responses across patient samples, noting that 31 of the 36 putative neoantigens tested (86%) were “immunogenic and capable of inducing robust cytokine responses.”
Next, the investigators mapped TIL responses to specific epitopes using patient-specific tetramers that corresponded to the previously identified neoepitopes. Seventeen of the 25 patient-specific tetramers (68%) bound to TILs above the background set by irrelevant HLA-matched tetramers.
“Within those responses, we observed immunodominance hierarchies among the distinct TIL populations, with a majority of tetramer-bound CD8+ T cells restricted to one or two putative neoepitopes,” the researchers noted.
The team also pointed out that seven of nine patients tested had CD8+ T cells that responded to ETV6-RUNX1.
Finally, the investigators performed transcriptional profiling of ALL-specific CD8+ TILs to assess inter- and intrapatient heterogeneity. The team identified three hierarchical clusters, which were characterized by transcriptional factors and regulators associated with:
- Functional effector CD8+ T cells (TBX21 and EOMES).
- Dysfunctional CD8+ T cells (STAT1/3/4, NR4A2/3, and BCL6).
- Exhausted CD8+ T cells (EOMES, MAF, PRDM1, and BATF).
Considering these findings together, the researchers concluded that “pediatric ALL elicits a potent neoepitope-specific CD8+ T-cell response.” Therefore, adoptive T-cell, monoclonal antibody, and targeted T-cell receptor therapies “should be explored” in pediatric ALL.
This research was supported by the National Institutes of Health, National Cancer Institute, National Institute of General Medical Sciences, Key for a Cure Foundation, and American Lebanese Syrian Associated Charities. The researchers disclosed patent applications and relationships with Pfizer, Amgen, and other companies.
SOURCE: Zamora AE et al. Sci. Transl. Med. 2019 Jun 26. doi: 10.1126/scitranslmed.aat8549.
Pediatric acute lymphoblastic leukemia (ALL) may be more vulnerable to immunotherapies than previously thought, according to researchers.

Prior studies suggested that tumors with a low mutational burden don’t elicit strong antitumor responses and therefore aren’t very susceptible to immunotherapy.
Now, researchers have found evidence to suggest that pediatric ALL induces “robust antitumor immune responses” despite a low mutational burden. The investigators identified tumor-associated CD8+ T cells that responded to 86% of neoantigens tested and recognized 68% of neoepitopes tested.
Anthony E. Zamora, PhD, of St. Jude Children’s Research Hospital in Memphis, Tenn., and colleagues recounted these findings in Science Translational Medicine.
The researchers analyzed samples from pediatric patients with ETV-associated ALL (n = 9) or ERG-associated ALL (n = 2) to determine how endogenous CD8+ T cells respond to patient-specific cancer neoantigens.
The investigators first assessed the ability of tumor-specific mutations and gene fusions to generate neoepitopes, or neoantigens predicted to bind patient-specific human leukocyte antigen (HLA) proteins. The team identified 5-28 neoepitopes per patient, including epitopes that spanned the fusion junction in patients with ETV6-RUNX1 fusions.
The researchers then tested whether CD8+ tumor infiltrating lymphocytes (TILs) were directly responsive to mutated neoepitopes. They observed cytokine responses across patient samples, noting that 31 of the 36 putative neoantigens tested (86%) were “immunogenic and capable of inducing robust cytokine responses.”
Next, the investigators mapped TIL responses to specific epitopes using patient-specific tetramers that corresponded to the previously identified neoepitopes. Seventeen of the 25 patient-specific tetramers (68%) bound to TILs above the background set by irrelevant HLA-matched tetramers.
“Within those responses, we observed immunodominance hierarchies among the distinct TIL populations, with a majority of tetramer-bound CD8+ T cells restricted to one or two putative neoepitopes,” the researchers noted.
The team also pointed out that seven of nine patients tested had CD8+ T cells that responded to ETV6-RUNX1.
Finally, the investigators performed transcriptional profiling of ALL-specific CD8+ TILs to assess inter- and intrapatient heterogeneity. The team identified three hierarchical clusters, which were characterized by transcriptional factors and regulators associated with:
- Functional effector CD8+ T cells (TBX21 and EOMES).
- Dysfunctional CD8+ T cells (STAT1/3/4, NR4A2/3, and BCL6).
- Exhausted CD8+ T cells (EOMES, MAF, PRDM1, and BATF).
Considering these findings together, the researchers concluded that “pediatric ALL elicits a potent neoepitope-specific CD8+ T-cell response.” Therefore, adoptive T-cell, monoclonal antibody, and targeted T-cell receptor therapies “should be explored” in pediatric ALL.
This research was supported by the National Institutes of Health, National Cancer Institute, National Institute of General Medical Sciences, Key for a Cure Foundation, and American Lebanese Syrian Associated Charities. The researchers disclosed patent applications and relationships with Pfizer, Amgen, and other companies.
SOURCE: Zamora AE et al. Sci. Transl. Med. 2019 Jun 26. doi: 10.1126/scitranslmed.aat8549.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: Preclinical research suggests pediatric acute lymphoblastic leukemia (ALL) induces “robust antitumor immune responses” despite a low mutational burden.
Major finding: Investigators identified tumor-associated CD8+ T cells that responded to 86% of neoantigens tested and recognized 68% of neoepitopes tested.
Study details: Analysis of samples from pediatric patients with ETV-associated ALL (n = 9) or ERG-associated ALL (n = 2).
Disclosures: The research was supported by the National Institutes of Health, National Cancer Institute, National Institute of General Medical Sciences, Key for a Cure Foundation, and American Lebanese Syrian Associated Charities. The researchers disclosed patent applications and relationships with Pfizer, Amgen, and other companies.
Source: Zamora AE et al. Sci. Transl. Med. 2019 Jun 26. doi: 10.1126/scitranslmed.aat8549.
Parent education improves quick disposal of children’s unused prescription opioids
SAN ANTONIO – Interventions aimed at educating parents about proper disposal methods for leftover prescription opioids and on explaining the risks of retaining opioids can increase the likelihood that parents will dispose of opioids when their children no longer need them, according to new research.
“Cost-effective disposal methods can nudge parents to dispose of their child’s leftover opioids promptly after use, but risk messaging is needed to best affect both early disposal and planned retention,” concluded Terri Voepel-Lewis, PhD, RN, of the University of Michigan, Ann Arbor, and colleagues.
“Such strategies can effectively reduce the presence of risky leftover medications in the home and decrease the risks posed to children and adolescents,” they wrote in a research poster at the annual meeting of College on Problems of Drug Dependence.
The researchers recruited 517 parents of children prescribed a short course of opioids, excluding children with chronic pain or the inability to report their pain.
The 255 parents randomly assigned to the nudge group received visual instructions on how to properly dispose of drugs while the 262 parents in the control group did not receive information on a disposal method. The groups were otherwise similar in terms of parent education, race/ethnicity, the child’s age and past opioid use, the parents’ past opioid use or misuse, whether opioids were kept in the home and whether the child’s procedure had been orthopedic/sports medicine–related.
Parents also were randomly assigned to routine care or to a Scenario-Tailored Opioid Messaging Program (STOMP). The STOMP group received tailored opioid risk information.
After a baseline survey on the child’s past pain, opioid use, misuse of opioids and risk perceptions, parents completed follow-up surveys at 7 and 14 days on opioid use, child pain, and behaviors related to retaining or disposing of opioids.
Just over a third of parents in the nudge group (34.7%) disposed of leftover opioids immediately after use, compared with 24% in the control group (odds ratio, 1.68; P = .01). Parents with the highest rate of disposal were those in the nudge group who participated in STOMP; they were more than twice as likely to dispose of opioids immediately after they were no longer needed (OR, 2.55; compared with control/non-STOMP).
A higher likelihood of disposal for parents in the nudge group alone, however, barely missed significance (OR, 1.77; P = .06) before adjustment. Parents’ intention to dispose of opioids was significantly different only among those who received STOMP education.
After the researchers controlled for child and parent factors, actual early disposal was significantly more likely in both the nudge and STOMP groups.
“Parental past opioid behaviors (kept an opioid in the home and past misuse) as well as orthopedic/sports medicine procedure were strongly associated with parents’ intention to retain [opioids],” the authors reported.
The study results revealed a divergence in parents’ intentions versus their behavior for one of the intervention groups.
“The nudge intervention improved parents’ prompt disposal of leftover prescription opioids but had no effect on planned retention rates,” the researchers reported. “In contrast, STOMP education had significant effects on early disposal behavior and planned retention. ”
Additional findings regarding parents’ past behaviors suggested that those who have kept leftover opioids or previously misused them may see the risks of doing so as low, the authors noted.
“Importantly, parents’ past prescription opioid retention behavior doubled the risk for planned retention, and their past opioid misuse more than tripled the risk,” the researchers wrote. “Assessing parents’ past behaviors and enhancing their perceptions of the real risks posed to children are important targets for risk reduction.”
The National Institute on Drug Addiction funded the research. The authors reported having no conflicts of interest.
SAN ANTONIO – Interventions aimed at educating parents about proper disposal methods for leftover prescription opioids and on explaining the risks of retaining opioids can increase the likelihood that parents will dispose of opioids when their children no longer need them, according to new research.
“Cost-effective disposal methods can nudge parents to dispose of their child’s leftover opioids promptly after use, but risk messaging is needed to best affect both early disposal and planned retention,” concluded Terri Voepel-Lewis, PhD, RN, of the University of Michigan, Ann Arbor, and colleagues.
“Such strategies can effectively reduce the presence of risky leftover medications in the home and decrease the risks posed to children and adolescents,” they wrote in a research poster at the annual meeting of College on Problems of Drug Dependence.
The researchers recruited 517 parents of children prescribed a short course of opioids, excluding children with chronic pain or the inability to report their pain.
The 255 parents randomly assigned to the nudge group received visual instructions on how to properly dispose of drugs while the 262 parents in the control group did not receive information on a disposal method. The groups were otherwise similar in terms of parent education, race/ethnicity, the child’s age and past opioid use, the parents’ past opioid use or misuse, whether opioids were kept in the home and whether the child’s procedure had been orthopedic/sports medicine–related.
Parents also were randomly assigned to routine care or to a Scenario-Tailored Opioid Messaging Program (STOMP). The STOMP group received tailored opioid risk information.
After a baseline survey on the child’s past pain, opioid use, misuse of opioids and risk perceptions, parents completed follow-up surveys at 7 and 14 days on opioid use, child pain, and behaviors related to retaining or disposing of opioids.
Just over a third of parents in the nudge group (34.7%) disposed of leftover opioids immediately after use, compared with 24% in the control group (odds ratio, 1.68; P = .01). Parents with the highest rate of disposal were those in the nudge group who participated in STOMP; they were more than twice as likely to dispose of opioids immediately after they were no longer needed (OR, 2.55; compared with control/non-STOMP).
A higher likelihood of disposal for parents in the nudge group alone, however, barely missed significance (OR, 1.77; P = .06) before adjustment. Parents’ intention to dispose of opioids was significantly different only among those who received STOMP education.
After the researchers controlled for child and parent factors, actual early disposal was significantly more likely in both the nudge and STOMP groups.
“Parental past opioid behaviors (kept an opioid in the home and past misuse) as well as orthopedic/sports medicine procedure were strongly associated with parents’ intention to retain [opioids],” the authors reported.
The study results revealed a divergence in parents’ intentions versus their behavior for one of the intervention groups.
“The nudge intervention improved parents’ prompt disposal of leftover prescription opioids but had no effect on planned retention rates,” the researchers reported. “In contrast, STOMP education had significant effects on early disposal behavior and planned retention. ”
Additional findings regarding parents’ past behaviors suggested that those who have kept leftover opioids or previously misused them may see the risks of doing so as low, the authors noted.
“Importantly, parents’ past prescription opioid retention behavior doubled the risk for planned retention, and their past opioid misuse more than tripled the risk,” the researchers wrote. “Assessing parents’ past behaviors and enhancing their perceptions of the real risks posed to children are important targets for risk reduction.”
The National Institute on Drug Addiction funded the research. The authors reported having no conflicts of interest.
SAN ANTONIO – Interventions aimed at educating parents about proper disposal methods for leftover prescription opioids and on explaining the risks of retaining opioids can increase the likelihood that parents will dispose of opioids when their children no longer need them, according to new research.
“Cost-effective disposal methods can nudge parents to dispose of their child’s leftover opioids promptly after use, but risk messaging is needed to best affect both early disposal and planned retention,” concluded Terri Voepel-Lewis, PhD, RN, of the University of Michigan, Ann Arbor, and colleagues.
“Such strategies can effectively reduce the presence of risky leftover medications in the home and decrease the risks posed to children and adolescents,” they wrote in a research poster at the annual meeting of College on Problems of Drug Dependence.
The researchers recruited 517 parents of children prescribed a short course of opioids, excluding children with chronic pain or the inability to report their pain.
The 255 parents randomly assigned to the nudge group received visual instructions on how to properly dispose of drugs while the 262 parents in the control group did not receive information on a disposal method. The groups were otherwise similar in terms of parent education, race/ethnicity, the child’s age and past opioid use, the parents’ past opioid use or misuse, whether opioids were kept in the home and whether the child’s procedure had been orthopedic/sports medicine–related.
Parents also were randomly assigned to routine care or to a Scenario-Tailored Opioid Messaging Program (STOMP). The STOMP group received tailored opioid risk information.
After a baseline survey on the child’s past pain, opioid use, misuse of opioids and risk perceptions, parents completed follow-up surveys at 7 and 14 days on opioid use, child pain, and behaviors related to retaining or disposing of opioids.
Just over a third of parents in the nudge group (34.7%) disposed of leftover opioids immediately after use, compared with 24% in the control group (odds ratio, 1.68; P = .01). Parents with the highest rate of disposal were those in the nudge group who participated in STOMP; they were more than twice as likely to dispose of opioids immediately after they were no longer needed (OR, 2.55; compared with control/non-STOMP).
A higher likelihood of disposal for parents in the nudge group alone, however, barely missed significance (OR, 1.77; P = .06) before adjustment. Parents’ intention to dispose of opioids was significantly different only among those who received STOMP education.
After the researchers controlled for child and parent factors, actual early disposal was significantly more likely in both the nudge and STOMP groups.
“Parental past opioid behaviors (kept an opioid in the home and past misuse) as well as orthopedic/sports medicine procedure were strongly associated with parents’ intention to retain [opioids],” the authors reported.
The study results revealed a divergence in parents’ intentions versus their behavior for one of the intervention groups.
“The nudge intervention improved parents’ prompt disposal of leftover prescription opioids but had no effect on planned retention rates,” the researchers reported. “In contrast, STOMP education had significant effects on early disposal behavior and planned retention. ”
Additional findings regarding parents’ past behaviors suggested that those who have kept leftover opioids or previously misused them may see the risks of doing so as low, the authors noted.
“Importantly, parents’ past prescription opioid retention behavior doubled the risk for planned retention, and their past opioid misuse more than tripled the risk,” the researchers wrote. “Assessing parents’ past behaviors and enhancing their perceptions of the real risks posed to children are important targets for risk reduction.”
The National Institute on Drug Addiction funded the research. The authors reported having no conflicts of interest.
REPORTING FROM CPDD 2019
What’s new in pediatric sepsis
LJUBLJANA, SLOVENIA – The dogma of the “Golden Hour” for the immediate management of pediatric sepsis has been oversold and actually is based upon weak evidence, Luregn J. Schlapbach, MD, asserted at the annual meeting of the European Society for Paediatric Infectious Diseases.
The true Golden Hour – that is, the time frame within which it’s imperative to administer the sepsis bundle comprised of appropriate antibiotics, fluids, and inotropes – is probably more like 3 hours.
“The evidence suggests that up to 3 hours you don’t really have a big difference in outcomes for sepsis. If you recognize shock there’s no question: You should not even wait 1 hour. But if you’re not certain, it may be better to give up to 3 hours to work up the child and get the senior clinician involved before you make decisions about treatment. So I’m not advocating to delay anything, said Dr. Schlapbach, a pediatric intensivist at the Child Health Research Center at the University of Queensland in South Brisbane, Australia.
The problem with a 1-hour mandate for delivery of the sepsis bundle, as recommended in guidelines by the Surviving Sepsis Campaign and the American College of Critical Care Medicine, and endorsed in quality improvement initiatives, is that the time pressure pushes physicians to overprescribe antibiotics to children who don’t actually have a serious bacterial infection. And that, he noted, contributes to the growing problem of antimicrobial resistance.
“You may have a child where you’re not too sure. Usually you would have done a urine culture because UTI [urinary tract infection] is quite a common cause of these infections, and many of these kids aren’t necessarily septic. But if people tell you that within 1 hour you need to treat, are you going to take the time to do the urine culture, or are you just going to decide to treat?” he asked rhetorically.
Dr. Schlapbach is a world-renowned pediatric sepsis researcher. He is far from alone in his reservations about the Golden Hour mandate.
“This is one of the reasons why IDSA [the Infectious Diseases Society of America] has not endorsed the Surviving Sepsis Campaign,” according to the physician, who noted that, in a position statement, IDSA officials have declared that discrimination of sepsis from noninfectious conditions remains a challenge, and that a 60-minute time to antibiotics may jeopardize patient reassessment (Clin Infect Dis. 2018 May 15;66[10]:1631-5).
Dr. Schlapbach highlighted other recent developments in pediatric sepsis.
The definition of adult sepsis has changed, and the pediatric version needs to as well
The revised definition of sepsis, known as Sepsis-3, issued by the International Sepsis Definition Task Force in 2016 notably dropped systemic inflammatory response syndrome (SIRS), as a requirement for sepsis (JAMA. 2016;315[8]:801-10). The revised definition characterizes sepsis as a dysregulated host response to infection resulting in life-threatening organ dysfunction. But Sepsis-3 is based entirely on adult data and is not considered applicable to children.
The current Pediatric Sepsis Consensus Conference definition dates back to 2005. A comprehensive revision is getting underway. It, too, is likely to drop SIRS into the wastebasket, Dr. Schlapbach said.
“It is probably time to abandon the old view of sepsis disease progression, which proposes a progression from infection to SIRS to severe sepsis with organ dysfunction to septic shock, because most children with infection do manifest signs of SIRS, such as tachycardia, tachypnea, and fever, and these probably should be considered as more of an adaptive rather than a maladaptive response,” he explained.
The goal of the pediatric sepsis redefinition project is to come up with something more useful for clinicians than the Sepsis-3 definition. While the Sepsis-3 concept of a dysregulated host response to infection sounds nice, he explained, “we don’t actually know what it is.
“One of the challenges that you all know as pediatricians is that children who develop sepsis get sick very, very quickly. We all have memories of children who we saw and may have discharged, and they were dead 12 hours later,” he noted.
Indeed, he and others have shown in multiple studies that up to 50% of pediatric deaths caused by sepsis happen within 24 hours of presentation.
“So whatever happens, it happens very quickly. The true question for us is actually how and why do children progress from no organ dysfunction, where the mortality is close to zero, to organ dysfunction, where all of a sudden mortality jumps up dramatically. It’s this progression that we don’t understand at all,” according to Dr. Schlapbach.
The genetic contribution to fulminant sepsis in children may be substantial
One-third of pediatric sepsis deaths in high-income countries happen in previously healthy children. In a proof-of-concept study, Dr. Schlapbach and coinvestigators in the Swiss Pediatric Sepsis Study Group conducted exome-sequencing genetic studies in eight previously healthy children with no family history of immunodeficiency who died of severe sepsis because of community-acquired Pseudomonas aeruginosa infection. Two of the eight had rare loss-of-function mutations in genes known to cause primary immunodeficiencies. The investigators proposed that unusually severe sepsis in previously healthy children warrants exome sequencing to look for underlying previously undetected primary immunodeficiencies. That’s important information for survivors and/or affected families to have, they argued (Front Immunol. 2016 Sep 20;7:357. eCollection 2016).
“There are some indications that the genetic contribution in children with sepsis may be larger than previously assumed,” he said.
The longstanding practice of fluid bolus therapy for resuscitation in pediatric sepsis is being reexamined
The FEAST (Fluid Expansion As Supportive Therapy) study, a randomized trial of more than 3,000 children with severe febrile illness and impaired perfusion in sub-Saharan Africa, turned heads with its finding that fluid boluses significantly increased 48-hour mortality (BMC Med. 2013 Mar 14;11:67).
Indeed, the FEAST findings, supported by mechanistic animal studies, were sufficiently compelling that the use of fluid boluses in both pediatric and adult septic shock is now under scrutiny in two major randomized trials: RIFTS (the Restrictive IV Fluid Trial in Severe Sepsis and Septic Shock), and CLOVERS (Crystalloid Liberal or Vasopressors Early Resuscitation in Sepsis). Stay tuned.
Dr. Schlapbach reported having no financial conflicts regarding his presentation.
LJUBLJANA, SLOVENIA – The dogma of the “Golden Hour” for the immediate management of pediatric sepsis has been oversold and actually is based upon weak evidence, Luregn J. Schlapbach, MD, asserted at the annual meeting of the European Society for Paediatric Infectious Diseases.
The true Golden Hour – that is, the time frame within which it’s imperative to administer the sepsis bundle comprised of appropriate antibiotics, fluids, and inotropes – is probably more like 3 hours.
“The evidence suggests that up to 3 hours you don’t really have a big difference in outcomes for sepsis. If you recognize shock there’s no question: You should not even wait 1 hour. But if you’re not certain, it may be better to give up to 3 hours to work up the child and get the senior clinician involved before you make decisions about treatment. So I’m not advocating to delay anything, said Dr. Schlapbach, a pediatric intensivist at the Child Health Research Center at the University of Queensland in South Brisbane, Australia.
The problem with a 1-hour mandate for delivery of the sepsis bundle, as recommended in guidelines by the Surviving Sepsis Campaign and the American College of Critical Care Medicine, and endorsed in quality improvement initiatives, is that the time pressure pushes physicians to overprescribe antibiotics to children who don’t actually have a serious bacterial infection. And that, he noted, contributes to the growing problem of antimicrobial resistance.
“You may have a child where you’re not too sure. Usually you would have done a urine culture because UTI [urinary tract infection] is quite a common cause of these infections, and many of these kids aren’t necessarily septic. But if people tell you that within 1 hour you need to treat, are you going to take the time to do the urine culture, or are you just going to decide to treat?” he asked rhetorically.
Dr. Schlapbach is a world-renowned pediatric sepsis researcher. He is far from alone in his reservations about the Golden Hour mandate.
“This is one of the reasons why IDSA [the Infectious Diseases Society of America] has not endorsed the Surviving Sepsis Campaign,” according to the physician, who noted that, in a position statement, IDSA officials have declared that discrimination of sepsis from noninfectious conditions remains a challenge, and that a 60-minute time to antibiotics may jeopardize patient reassessment (Clin Infect Dis. 2018 May 15;66[10]:1631-5).
Dr. Schlapbach highlighted other recent developments in pediatric sepsis.
The definition of adult sepsis has changed, and the pediatric version needs to as well
The revised definition of sepsis, known as Sepsis-3, issued by the International Sepsis Definition Task Force in 2016 notably dropped systemic inflammatory response syndrome (SIRS), as a requirement for sepsis (JAMA. 2016;315[8]:801-10). The revised definition characterizes sepsis as a dysregulated host response to infection resulting in life-threatening organ dysfunction. But Sepsis-3 is based entirely on adult data and is not considered applicable to children.
The current Pediatric Sepsis Consensus Conference definition dates back to 2005. A comprehensive revision is getting underway. It, too, is likely to drop SIRS into the wastebasket, Dr. Schlapbach said.
“It is probably time to abandon the old view of sepsis disease progression, which proposes a progression from infection to SIRS to severe sepsis with organ dysfunction to septic shock, because most children with infection do manifest signs of SIRS, such as tachycardia, tachypnea, and fever, and these probably should be considered as more of an adaptive rather than a maladaptive response,” he explained.
The goal of the pediatric sepsis redefinition project is to come up with something more useful for clinicians than the Sepsis-3 definition. While the Sepsis-3 concept of a dysregulated host response to infection sounds nice, he explained, “we don’t actually know what it is.
“One of the challenges that you all know as pediatricians is that children who develop sepsis get sick very, very quickly. We all have memories of children who we saw and may have discharged, and they were dead 12 hours later,” he noted.
Indeed, he and others have shown in multiple studies that up to 50% of pediatric deaths caused by sepsis happen within 24 hours of presentation.
“So whatever happens, it happens very quickly. The true question for us is actually how and why do children progress from no organ dysfunction, where the mortality is close to zero, to organ dysfunction, where all of a sudden mortality jumps up dramatically. It’s this progression that we don’t understand at all,” according to Dr. Schlapbach.
The genetic contribution to fulminant sepsis in children may be substantial
One-third of pediatric sepsis deaths in high-income countries happen in previously healthy children. In a proof-of-concept study, Dr. Schlapbach and coinvestigators in the Swiss Pediatric Sepsis Study Group conducted exome-sequencing genetic studies in eight previously healthy children with no family history of immunodeficiency who died of severe sepsis because of community-acquired Pseudomonas aeruginosa infection. Two of the eight had rare loss-of-function mutations in genes known to cause primary immunodeficiencies. The investigators proposed that unusually severe sepsis in previously healthy children warrants exome sequencing to look for underlying previously undetected primary immunodeficiencies. That’s important information for survivors and/or affected families to have, they argued (Front Immunol. 2016 Sep 20;7:357. eCollection 2016).
“There are some indications that the genetic contribution in children with sepsis may be larger than previously assumed,” he said.
The longstanding practice of fluid bolus therapy for resuscitation in pediatric sepsis is being reexamined
The FEAST (Fluid Expansion As Supportive Therapy) study, a randomized trial of more than 3,000 children with severe febrile illness and impaired perfusion in sub-Saharan Africa, turned heads with its finding that fluid boluses significantly increased 48-hour mortality (BMC Med. 2013 Mar 14;11:67).
Indeed, the FEAST findings, supported by mechanistic animal studies, were sufficiently compelling that the use of fluid boluses in both pediatric and adult septic shock is now under scrutiny in two major randomized trials: RIFTS (the Restrictive IV Fluid Trial in Severe Sepsis and Septic Shock), and CLOVERS (Crystalloid Liberal or Vasopressors Early Resuscitation in Sepsis). Stay tuned.
Dr. Schlapbach reported having no financial conflicts regarding his presentation.
LJUBLJANA, SLOVENIA – The dogma of the “Golden Hour” for the immediate management of pediatric sepsis has been oversold and actually is based upon weak evidence, Luregn J. Schlapbach, MD, asserted at the annual meeting of the European Society for Paediatric Infectious Diseases.
The true Golden Hour – that is, the time frame within which it’s imperative to administer the sepsis bundle comprised of appropriate antibiotics, fluids, and inotropes – is probably more like 3 hours.
“The evidence suggests that up to 3 hours you don’t really have a big difference in outcomes for sepsis. If you recognize shock there’s no question: You should not even wait 1 hour. But if you’re not certain, it may be better to give up to 3 hours to work up the child and get the senior clinician involved before you make decisions about treatment. So I’m not advocating to delay anything, said Dr. Schlapbach, a pediatric intensivist at the Child Health Research Center at the University of Queensland in South Brisbane, Australia.
The problem with a 1-hour mandate for delivery of the sepsis bundle, as recommended in guidelines by the Surviving Sepsis Campaign and the American College of Critical Care Medicine, and endorsed in quality improvement initiatives, is that the time pressure pushes physicians to overprescribe antibiotics to children who don’t actually have a serious bacterial infection. And that, he noted, contributes to the growing problem of antimicrobial resistance.
“You may have a child where you’re not too sure. Usually you would have done a urine culture because UTI [urinary tract infection] is quite a common cause of these infections, and many of these kids aren’t necessarily septic. But if people tell you that within 1 hour you need to treat, are you going to take the time to do the urine culture, or are you just going to decide to treat?” he asked rhetorically.
Dr. Schlapbach is a world-renowned pediatric sepsis researcher. He is far from alone in his reservations about the Golden Hour mandate.
“This is one of the reasons why IDSA [the Infectious Diseases Society of America] has not endorsed the Surviving Sepsis Campaign,” according to the physician, who noted that, in a position statement, IDSA officials have declared that discrimination of sepsis from noninfectious conditions remains a challenge, and that a 60-minute time to antibiotics may jeopardize patient reassessment (Clin Infect Dis. 2018 May 15;66[10]:1631-5).
Dr. Schlapbach highlighted other recent developments in pediatric sepsis.
The definition of adult sepsis has changed, and the pediatric version needs to as well
The revised definition of sepsis, known as Sepsis-3, issued by the International Sepsis Definition Task Force in 2016 notably dropped systemic inflammatory response syndrome (SIRS), as a requirement for sepsis (JAMA. 2016;315[8]:801-10). The revised definition characterizes sepsis as a dysregulated host response to infection resulting in life-threatening organ dysfunction. But Sepsis-3 is based entirely on adult data and is not considered applicable to children.
The current Pediatric Sepsis Consensus Conference definition dates back to 2005. A comprehensive revision is getting underway. It, too, is likely to drop SIRS into the wastebasket, Dr. Schlapbach said.
“It is probably time to abandon the old view of sepsis disease progression, which proposes a progression from infection to SIRS to severe sepsis with organ dysfunction to septic shock, because most children with infection do manifest signs of SIRS, such as tachycardia, tachypnea, and fever, and these probably should be considered as more of an adaptive rather than a maladaptive response,” he explained.
The goal of the pediatric sepsis redefinition project is to come up with something more useful for clinicians than the Sepsis-3 definition. While the Sepsis-3 concept of a dysregulated host response to infection sounds nice, he explained, “we don’t actually know what it is.
“One of the challenges that you all know as pediatricians is that children who develop sepsis get sick very, very quickly. We all have memories of children who we saw and may have discharged, and they were dead 12 hours later,” he noted.
Indeed, he and others have shown in multiple studies that up to 50% of pediatric deaths caused by sepsis happen within 24 hours of presentation.
“So whatever happens, it happens very quickly. The true question for us is actually how and why do children progress from no organ dysfunction, where the mortality is close to zero, to organ dysfunction, where all of a sudden mortality jumps up dramatically. It’s this progression that we don’t understand at all,” according to Dr. Schlapbach.
The genetic contribution to fulminant sepsis in children may be substantial
One-third of pediatric sepsis deaths in high-income countries happen in previously healthy children. In a proof-of-concept study, Dr. Schlapbach and coinvestigators in the Swiss Pediatric Sepsis Study Group conducted exome-sequencing genetic studies in eight previously healthy children with no family history of immunodeficiency who died of severe sepsis because of community-acquired Pseudomonas aeruginosa infection. Two of the eight had rare loss-of-function mutations in genes known to cause primary immunodeficiencies. The investigators proposed that unusually severe sepsis in previously healthy children warrants exome sequencing to look for underlying previously undetected primary immunodeficiencies. That’s important information for survivors and/or affected families to have, they argued (Front Immunol. 2016 Sep 20;7:357. eCollection 2016).
“There are some indications that the genetic contribution in children with sepsis may be larger than previously assumed,” he said.
The longstanding practice of fluid bolus therapy for resuscitation in pediatric sepsis is being reexamined
The FEAST (Fluid Expansion As Supportive Therapy) study, a randomized trial of more than 3,000 children with severe febrile illness and impaired perfusion in sub-Saharan Africa, turned heads with its finding that fluid boluses significantly increased 48-hour mortality (BMC Med. 2013 Mar 14;11:67).
Indeed, the FEAST findings, supported by mechanistic animal studies, were sufficiently compelling that the use of fluid boluses in both pediatric and adult septic shock is now under scrutiny in two major randomized trials: RIFTS (the Restrictive IV Fluid Trial in Severe Sepsis and Septic Shock), and CLOVERS (Crystalloid Liberal or Vasopressors Early Resuscitation in Sepsis). Stay tuned.
Dr. Schlapbach reported having no financial conflicts regarding his presentation.
EXPERT ANALYSIS FROM ESPID 2019












