User login
Rate of sling removal 9 years after MUS for SUI over 3%
a British study found.
Ipek Gurol-Urganci, PhD, of the London School of Hygiene and Tropical Medicine, and her coauthors said their study comes as a result of safety concerns around the procedure, which resulted in a suspension of the operation in the United Kingdom.
“There is concern about problems that some women experience following MUS insertion, including pain, dyspareunia, persistent urinary incontinence, and exposure or erosion. However, there is little randomized, clinical trial evidence on these longer-term outcomes,” they wrote in JAMA, noting that an estimated 250,000 MUS operations were performed in 2010 in the United States.
The current study involved 95,057 women in England who underwent an MUS insertion procedure for SUI for the first time in a National Health Service hospital between 2006 and 2015. Overall, 60,194 of the women had a retropubic insertion and 34,863 had a transobturator insertion.
At 9 years after the initial insertion, the mesh was removed in 3.3% of women. The risk of removal was higher for women who had a retropubic insertion (3.6%), compared with those who had a transobturator insertion (2.7%).
“The risk of a removal was about 30% lower if the mesh sling had been inserted via the transobturator route, which may be explained by the removal of transobturator sling being a more complicated procedure,” Dr. Gurol-Urganci and her associates noted.
Mesh sling removal risk decreased with age, with the risk at 4.4% for women aged 18-39 years, compared with 2.1% in women aged 70 years and older at 9 years after insertion.
The authors wrote that the risks of removal and any reoperation (mesh removal and/or reoperation for SUI) were higher among women from a white racial/ethnic background. However, it was not possible to “disentangle explanations” for these possible differences in risk seen with patient characteristics, which ranged from higher morbidity to differences in the reasons for surgery.
Results also showed that the risk of reoperation was 4.5% at 9 years after the initial insertion, and was slightly higher for a transobturator insertion at 5.3%, compared with 4.1% for a retropubic insertion.
The risk of any reoperation, including mesh removal and/or reoperation for SUI, following the initial MUS insertion was 6.9% at 9 years (95% confidence interval, 6.7%-7.1%), but no statistically significant difference was observed between retropubic and transobturator insertion.
“The present results demonstrate that removal and reoperation risks were associated with the insertion route and patient factors,” Dr. Gurol-Urganci and her associates wrote.
“These findings may guide women and their surgeons when making decisions about surgical treatment of stress urinary incontinence,” they concluded.
The study was supported by a grant from the National Institute for Health Research Health Services and Delivery Research Programme and several of the authors reported receiving National Institute for Health Research research grants. One author reported providing consultancy services to Cambridge Medical Robotics, Femeda, and Astellas.
SOURCE: Gurol-Urganci I et al. JAMA. 2018 Oct 23. doi:10.1001/jama.2018.14997.
a British study found.
Ipek Gurol-Urganci, PhD, of the London School of Hygiene and Tropical Medicine, and her coauthors said their study comes as a result of safety concerns around the procedure, which resulted in a suspension of the operation in the United Kingdom.
“There is concern about problems that some women experience following MUS insertion, including pain, dyspareunia, persistent urinary incontinence, and exposure or erosion. However, there is little randomized, clinical trial evidence on these longer-term outcomes,” they wrote in JAMA, noting that an estimated 250,000 MUS operations were performed in 2010 in the United States.
The current study involved 95,057 women in England who underwent an MUS insertion procedure for SUI for the first time in a National Health Service hospital between 2006 and 2015. Overall, 60,194 of the women had a retropubic insertion and 34,863 had a transobturator insertion.
At 9 years after the initial insertion, the mesh was removed in 3.3% of women. The risk of removal was higher for women who had a retropubic insertion (3.6%), compared with those who had a transobturator insertion (2.7%).
“The risk of a removal was about 30% lower if the mesh sling had been inserted via the transobturator route, which may be explained by the removal of transobturator sling being a more complicated procedure,” Dr. Gurol-Urganci and her associates noted.
Mesh sling removal risk decreased with age, with the risk at 4.4% for women aged 18-39 years, compared with 2.1% in women aged 70 years and older at 9 years after insertion.
The authors wrote that the risks of removal and any reoperation (mesh removal and/or reoperation for SUI) were higher among women from a white racial/ethnic background. However, it was not possible to “disentangle explanations” for these possible differences in risk seen with patient characteristics, which ranged from higher morbidity to differences in the reasons for surgery.
Results also showed that the risk of reoperation was 4.5% at 9 years after the initial insertion, and was slightly higher for a transobturator insertion at 5.3%, compared with 4.1% for a retropubic insertion.
The risk of any reoperation, including mesh removal and/or reoperation for SUI, following the initial MUS insertion was 6.9% at 9 years (95% confidence interval, 6.7%-7.1%), but no statistically significant difference was observed between retropubic and transobturator insertion.
“The present results demonstrate that removal and reoperation risks were associated with the insertion route and patient factors,” Dr. Gurol-Urganci and her associates wrote.
“These findings may guide women and their surgeons when making decisions about surgical treatment of stress urinary incontinence,” they concluded.
The study was supported by a grant from the National Institute for Health Research Health Services and Delivery Research Programme and several of the authors reported receiving National Institute for Health Research research grants. One author reported providing consultancy services to Cambridge Medical Robotics, Femeda, and Astellas.
SOURCE: Gurol-Urganci I et al. JAMA. 2018 Oct 23. doi:10.1001/jama.2018.14997.
a British study found.
Ipek Gurol-Urganci, PhD, of the London School of Hygiene and Tropical Medicine, and her coauthors said their study comes as a result of safety concerns around the procedure, which resulted in a suspension of the operation in the United Kingdom.
“There is concern about problems that some women experience following MUS insertion, including pain, dyspareunia, persistent urinary incontinence, and exposure or erosion. However, there is little randomized, clinical trial evidence on these longer-term outcomes,” they wrote in JAMA, noting that an estimated 250,000 MUS operations were performed in 2010 in the United States.
The current study involved 95,057 women in England who underwent an MUS insertion procedure for SUI for the first time in a National Health Service hospital between 2006 and 2015. Overall, 60,194 of the women had a retropubic insertion and 34,863 had a transobturator insertion.
At 9 years after the initial insertion, the mesh was removed in 3.3% of women. The risk of removal was higher for women who had a retropubic insertion (3.6%), compared with those who had a transobturator insertion (2.7%).
“The risk of a removal was about 30% lower if the mesh sling had been inserted via the transobturator route, which may be explained by the removal of transobturator sling being a more complicated procedure,” Dr. Gurol-Urganci and her associates noted.
Mesh sling removal risk decreased with age, with the risk at 4.4% for women aged 18-39 years, compared with 2.1% in women aged 70 years and older at 9 years after insertion.
The authors wrote that the risks of removal and any reoperation (mesh removal and/or reoperation for SUI) were higher among women from a white racial/ethnic background. However, it was not possible to “disentangle explanations” for these possible differences in risk seen with patient characteristics, which ranged from higher morbidity to differences in the reasons for surgery.
Results also showed that the risk of reoperation was 4.5% at 9 years after the initial insertion, and was slightly higher for a transobturator insertion at 5.3%, compared with 4.1% for a retropubic insertion.
The risk of any reoperation, including mesh removal and/or reoperation for SUI, following the initial MUS insertion was 6.9% at 9 years (95% confidence interval, 6.7%-7.1%), but no statistically significant difference was observed between retropubic and transobturator insertion.
“The present results demonstrate that removal and reoperation risks were associated with the insertion route and patient factors,” Dr. Gurol-Urganci and her associates wrote.
“These findings may guide women and their surgeons when making decisions about surgical treatment of stress urinary incontinence,” they concluded.
The study was supported by a grant from the National Institute for Health Research Health Services and Delivery Research Programme and several of the authors reported receiving National Institute for Health Research research grants. One author reported providing consultancy services to Cambridge Medical Robotics, Femeda, and Astellas.
SOURCE: Gurol-Urganci I et al. JAMA. 2018 Oct 23. doi:10.1001/jama.2018.14997.
FROM JAMA
Key clinical point: The findings of this study may inform decision making when choosing treatment for stress urinary incontinence.
Major finding: Within 9 years of a mesh insertion for stress urinary incontinence, the rate of sling removal was 3.3% and the rate of reoperation was 4.5%.
Study details: A prospective, observational study examining long-term mesh removal and reoperations in over 95,000 women who underwent midurethral mesh operations for stress urinary incontinence between 2006 and 2015.
Disclosures: The study was supported by a grant from the National Institute for Health Research Health Services and Delivery Research Programme and several of the authors reported receiving National Institute for Health Research research grants. One author reported providing consultancy services to Cambridge Medical Robotics, Femeda, and Astellas Pharma.
Source: Gurol-Urganci I et al. JAMA. 2018 Oct 23. doi:10.1001/jama.2018.14997.
New and promising GSM treatments, more clinical takeaways from NAMS 2018
Learn more about NAMS: http://www.menopause.org/home
Learn more about NAMS: http://www.menopause.org/home
Learn more about NAMS: http://www.menopause.org/home
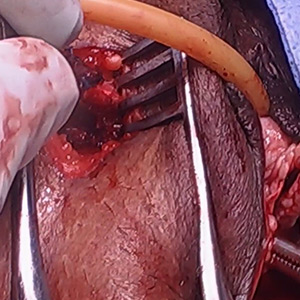
Vaginal and bilateral thigh removal of a transobturator sling

Additional videos from SGS are available here, including these recent offerings:
- Morcellation at the time of vaginal hysterectomy
- Surgical management of non-tubal ectopic pregnancies
- Size can matter: Laparoscopic hysterectomy for the very large uterus
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.

Additional videos from SGS are available here, including these recent offerings:
- Morcellation at the time of vaginal hysterectomy
- Surgical management of non-tubal ectopic pregnancies
- Size can matter: Laparoscopic hysterectomy for the very large uterus
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.

Additional videos from SGS are available here, including these recent offerings:
- Morcellation at the time of vaginal hysterectomy
- Surgical management of non-tubal ectopic pregnancies
- Size can matter: Laparoscopic hysterectomy for the very large uterus
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
This video is brought to you by
2018 Update on pelvic floor dysfunction
Using cystoscopy to evaluate ureteral efflux and bladder integrity following benign gynecologic surgery increases the detection rate of urinary tract injuries.1 Currently, it is standard of care to perform a cystoscopy following anti-incontinence procedures, but there is no consensus among ObGyns regarding the use of universal cystoscopy following benign gynecologic surgery.2 A number of studies, however, have suggested potential best practices for evaluating urinary tract injury during pelvic surgery for benign gynecologic conditions.
Pelvic surgeries for benign gynecologic conditions, including fibroids, menorrhagia, and pelvic organ prolapse (POP), are common. More than 500,000 hysterectomies are performed annually in the United States, and up to 11% of women will undergo at least one surgery for POP or urinary incontinence in their lifetime.3,4 During gynecologic surgery, the urinary tract is at risk, and the injury rate ranges from 0.02% to 2% for ureteral injury and from 1% to 5% for bladder injury.5,6
In a recent large randomized controlled trial, the rate of intraoperative ureteral obstruction following uterosacral ligament suspension (USLS) was 3.2%.7 Vaginal vault suspensions, as well as other vaginal cuff closure techniques, are common procedures associated with urinary tract injury.8 Additionally, ureteral injury during surgery for POP occurs in as many as 2% of anterior vaginal wall repairs.9
It is well documented that a delay in diagnosis of ureteral and/or bladder injuries is associated with increased morbidity, including the need for additional surgery to repair the injury; in addition, significant delay in identifying an injury may lead to subsequent sequela, such as renal injury and fistula formation.8
A large study in California found that 36.5% of hysterectomies performed for POP were performed by general gynecologists.10 General ObGyns performing these surgeries therefore must understand the risk of urinary tract injury during hysterectomy and reconstructive pelvic procedures so that they can appropriately identify, evaluate, and repair injuries in a timely fashion.
The best way to identify urinary tract injury at the time of gynecologic surgery is by cystoscopy, including a bladder survey and ureteral efflux evaluation. When should a cystoscopy be performed, and what is the best method for visualizing ureteral efflux? Can instituting universal cystoscopy for all gynecologic procedures make a difference in the rate of injury detection? In this Update, we summarize the data from 4 studies that help to answer these questions.
Continue to: About 30% of urinary tract injuries...
About 30% of urinary tract injuries identified prior to cystoscopy at hysterectomy (which detected 5 of 6 injuries)
Vakili B, Chesson RR, Kyle BL, et al. The incidence of urinary tract injury during hysterectomy: a prospective analysis based on universal cystoscopy. Am J Obstet Gynecol. 2005;192(5):1599–1604.
Vakili and colleagues conducted a multicenter prospective cohort study of women undergoing hysterectomy for benign indications; cystoscopy was performed in all cases. The 3 hospitals involved were all part of the Louisiana State University Health system. The investigators’ goal was to determine the rate of urinary tract injury in this patient population at the time of intraoperative cystoscopy.
Intraoperative cystoscopy beats visual evaluation
Four hundred and seventy-one women underwent hysterectomy and had intraoperative cystoscopy, including evaluation of ureteral patency with administration of intravenous (IV) indigo carmine. Patients underwent abdominal, vaginal, or laparoscopic hysterectomy, and 54 (11.4%) had concurrent POP or anti-incontinence procedures. The majority underwent an abdominal hysterectomy (59%), 31% had a vaginal hysterectomy, and 10% had a laparoscopic-assisted vaginal hysterectomy or total laparoscopic hysterectomy.
Rate of urinary tract injuries. The total urinary tract injury rate detected by cystoscopy was 4.8%. The ureteral injury rate was 1.7%, and the bladder injury rate was 3.6%. A combined ureteral and bladder injury occurred in 2 women.
Surgery for POP significantly increased the risk of ureteral injury (7.3% vs 1.2%; P = .025). All cases of ureteral injury during POP surgery occurred during USLS. There was a trend toward a higher rate of bladder injury in the group with concurrent anti-incontinence surgery (12.5% vs 3.1%; P = .049). Regarding the route of hysterectomy, the vaginal approach had the highest rate of ureteral injury; however, when prolapse cases were removed from the analysis, there were no differences between the abdominal, vaginal, and laparoscopic approaches for ureteral or bladder injuries.
Injury detection with cystoscopy. Importantly, the authors found that only 30% of injuries were identified prior to performing intraoperative cystoscopy. The majority of these were bladder injuries. In addition, despite visual confirmation of ureteral peristalsis during abdominal hysterectomy, when intraoperative cystoscopy was performed with evaluation for ureteral efflux, 5 of 6 ureteral injury cases were identified. The authors reported 1 postoperative vesicovaginal fistula and concluded that it was likely due to an unrecognized bladder injury. No other undetected injuries were identified.
Notably, no complications occurred as a result of cystoscopy.
Multiple surgical indications reflect real-world scenario
The study included physicians from 3 different hospitals and all routes of hysterectomy for multiple benign gynecologic indications as well as concomitant pelvic reconstructive procedures. While this enhances the generalizability of the study results, all study sites were located in Louisiana at hospitals with resident trainee involvement. Additionally, this study confirms previous retrospective studies that reported higher rates of injury with pelvic reconstructive procedures.
The study is limited by the inability to blind surgeons, which may have resulted in the surgeons altering their techniques and/or having a heightened awareness of the urinary tract. However, their rates of ureteral and bladder injuries were slightly higher than previously reported rates, suggesting that the procedures inherently carry risk. The study is further limited by the lack of a retrospective comparison group of hysterectomy without routine cystoscopy and a longer follow-up period that may have revealed additional missed delayed urologic injuries.
The rate of urinary tract injury, including both bladder and ureteral injuries, was more than 4% at the time of hysterectomy for benign conditions. Using intraoperative peristalsis or normal ureteral caliber could result in a false sense of security since these are not reliable signs of ureteral integrity. The majority of urinary tract injuries will not be identified without cystoscopic evaluation.
Continue to: Universal cystoscopy policy...
Universal cystoscopy policy proves protective, surgeon adherence is high
Chi AM, Curran DS, Morgan DM, Fenner DE, Swenson CW. Universal cystoscopy after benign hysterectomy: examining the effects of an institutional policy. Obstet Gynecol. 2016;127(2):369–375.
In a retrospective cohort study, Chi and colleagues evaluated urinary tract injuries at the time of hysterectomy before and after the institution of a universal cystoscopy policy. At the time of policy implementation at the University of Michigan, all faculty who performed hysterectomies attended a cystoscopy workshop. Attending physicians without prior cystoscopy training also were proctored in the operating room for 3 sessions and were required to demonstrate competency with bladder survey, visualizing ureteral efflux, and urethral assessment. Indigo carmine was used to visualize ureteral efflux.
Detection of urologic injury almost doubled with cystoscopy
A total of 2,822 hysterectomies were included in the study, with 973 in the pre–universal cystoscopy group and 1,849 in the post–universal cystoscopy group. The study period was 7 years, and data on complications were abstracted for 1 year after the completion of the study period.
The primary outcome had 3 components:
- the rate of urologic injury before and after the policy
- the cystoscopy detection rate of urologic injury
- the adherence rate to the policy.
The overall rate of bladder and ureteral injury was 2.1%; the rate of injury during pre–universal screening was 2.6%, and during post–universal screening was 1.8%. The intraoperative detection rate of injury nearly doubled, from 24% to 47%, when intraoperative cystoscopy was utilized. In addition, the percentage of delayed urologic complications decreased from 28% to 5.9% (P = .03) following implementation of the universal cystoscopy policy. With regard to surgeon adherence, cystoscopy was documented in 86.1% of the hysterectomy cases after the policy was implemented compared with 35.7% of cases before the policy.
The investigators performed a cost analysis and found that hospital costs were nearly twice as much if a delayed urologic injury was diagnosed.
Study had many strengths
This study evaluated aspects of implementing quality initiatives after proper training and proctoring of a procedure. The authors compared very large cohorts from a busy academic medical center in which surgeon adherence with routine cystoscopy was high. The majority of patient outcomes were tracked for an extended period following surgery, thereby minimizing the risk of missing delayed urologic injuries. Notably, however, there was shorter follow-up time for the post–universal cystoscopy group, which could result in underestimating the rate of delayed urologic injuries in this cohort.
Instituting a universal cystoscopy policy for hysterectomy was associated with a significant decrease in delayed postoperative urinary tract complications and an increase in the intraoperative detection rate of urologic injuries. Intraoperative detection and repair of a urinary tract injury is cost-effective compared with a delayed diagnosis.
Continue to: Cystoscopy reveals ureteral obstruction...
Cystoscopy reveals ureteral obstruction during various vaginal POP repair procedures
Gustilo-Ashby AM, Jelovsek JE, Barber MD, Yoo EH, Paraiso MF, Walters MD. The incidence of ureteral obstruction and the value of intraoperative cystoscopy during vaginal surgery for pelvic organ prolapse. Am J Obstet Gynecol. 2006;194(5):1478–1485.
To determine the rate of ureteral obstruction and ureteral injury during vaginal surgery for POP and the accuracy of using intraoperative cystoscopy to prevent upper urinary tract morbidity, Gustilo-Ashby and colleagues performed a retrospective review study of a large patient cohort.
Cystoscopy with indigo carmine is highly sensitive
The study included 700 patients who underwent vaginal surgery for anterior and/or apical POP. Patients had 1 or more of the following anterior and apical prolapse repair procedures: USLS (51%), distal McCall culdeplasty (26%), proximal McCall culdeplasty (29%), anterior colporrhaphy (82%), and colpocleisis (1.4%). Of note, distal McCall culdeplasty was defined as incorporation of the “vaginal epithelium into the uterosacral plication,” while proximal McCall culdeplasty involved plication of “the uterosacral ligaments in the midline proximal to the vaginal cuff.” All patients were given IV indigo carmine to aid in visualizing ureteral efflux.
The majority of patients had a hysterectomy (56%). When accounting for rare false-positive and negative cystoscopy results, the overall ureteral obstruction rate was 5.1% and the ureteral injury rate was 0.9%. The majority of obstructions occurred with USLS (5.9%), proximal McCall culdeplasty (4.4%), and colpocleisis (4.2%). Ureteral injuries occurred only in 6 cases: 3 USLS and 3 proximal McCall culdeplasty procedures.
Based on these findings, the authors calculated that cystoscopy at the time of vaginal surgery for anterior and/or apical prolapse has a sensitivity of 94.4% and a specificity of 99.5% for detecting ureteral obstruction. The positive predictive value of cystoscopy with the use of indigo carmine for detection of ureteral obstruction is 91.9% and the negative predictive value is 99.7%.
Impact of indigo carmine’s unavailability
This study’s strengths include its large sample size and the variety of surgical approaches used for repair of anterior vaginal wall and apical prolapse. Its retrospective design, however, is a limitation; this could result in underreporting of ureteral injuries if patients received care at another institution after surgery. Furthermore, it is unclear if cystoscopy would be as predictive of ureteral injury without the use of indigo carmine, which is no longer available at most institutions.
The utility of cystoscopy with IV indigo carmine as a screening test for ureteral obstruction is highlighted by the fact that most obstructions were relieved by intraoperative suture removal following positive cystoscopy. McCall culdeplasty procedures are commonly performed by general ObGyns at the time of vaginal hysterectomy. It is therefore important to note that rates of ureteral obstruction after proximal McCall culdeplasty were only slightly lower than those after USLS.
Continue to: Sodium fluorescein and 10% dextrose...
Sodium fluorescein and 10% dextrose provide clear visibility of ureteral jets in cystoscopy
Espaillat-Rijo L, Siff L, Alas AN, et al. Intraoperative cystoscopic evaluation of ureteral patency: a randomized controlled trial. Obstet Gynecol. 2016;128(6):1378–1383.
In a multicenter randomized controlled trial, Espaillat-Rijo and colleagues compared various methods for visualizing ureteral efflux in participants who underwent gynecologic or urogynecologic procedures in which cystoscopy was performed.
Study compared 4 media
The investigators enrolled 176 participants (174 completed the trial) and randomly assigned them to receive 1 of 4 modalities: 1) normal saline as a bladder distention medium (control), 2) 10% dextrose as a bladder distention medium, 3) 200 mg oral phenazopyridine given 30 minutes prior to cystoscopy, or 4) 50 mg IV sodium fluorescein at the start of cystoscopy. Indigo carmine was not included in this study because it has not been routinely available since 2014.
Surgeons were asked to categorize the ureteral jets as “clearly visible,” “somewhat visible,” or “not visible.”
The primary outcome was subjective visibility of the ureteral jet with each modality during cystoscopy. Secondary outcomes included surgeon satisfaction, adverse reactions to the modality used, postoperative urinary tract infection, postoperative urinary retention, and delayed diagnosis of ureteral injury.
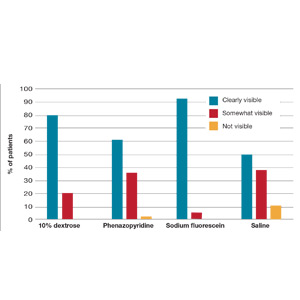
Visibility assessment results. Overall, ureteral jets were “clearly visible” in 125 cases (71%) compared with “somewhat visible” in 45 (25.6%) and “not visible” in 4 (2.3%) cases. There was a statistically significant difference between the 4 groups. Use of sodium fluorescein and 10% dextrose resulted in significantly better visualization of ureteral jets (P < .001 and P = .004, respectively) compared with the control group. Visibility with phenazopyridine was not significantly different from that in the control group or in the 10% dextrose group (FIGURE).
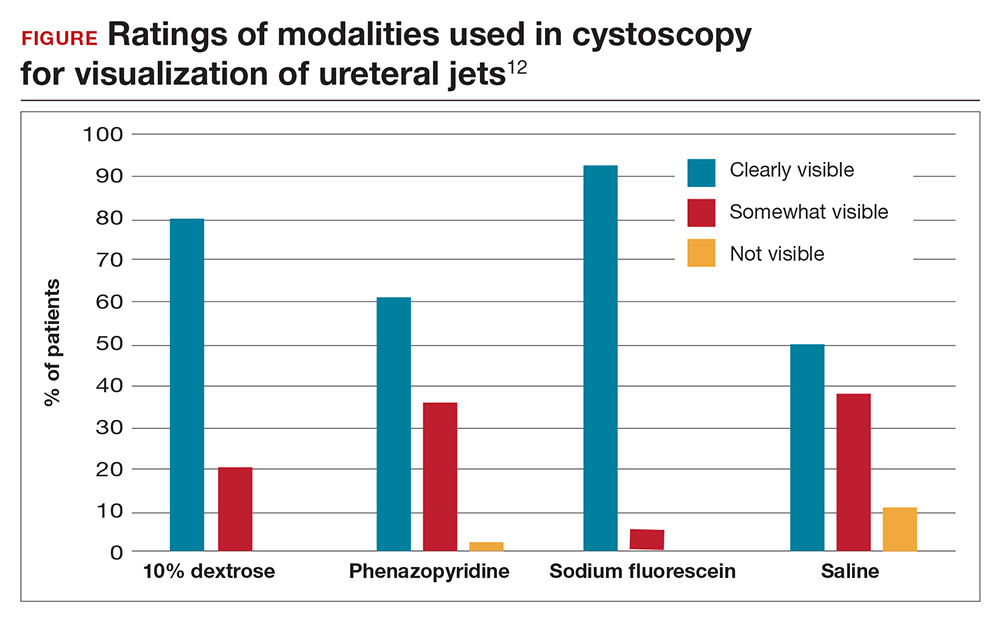
Surgeon satisfaction was highest with 10% dextrose and sodium fluorescein. In 6 cases, the surgeon was not satisfied with visualization of the ureteral jets and relied on fluorescein (5 times) or 10% dextrose (1 time) to ultimately see efflux. No significant adverse events occurred; the rate of urinary tract infection was 24.1% and did not differ between groups.
Results are widely generalizable
This was a well-designed randomized multicenter trial that included both benign gynecologic and urogynecologic procedures, thus strengthening the generalizability of the study. The study was timely since proven methods for visualization of ureteral patency became limited with the withdrawal of commercially available indigo carmine, the previous gold standard.
Intravenous sodium fluorescein and 10% dextrose as bladder distention media can both safely be used to visualize ureteral efflux and result in high surgeon satisfaction. Although 10% dextrose has been associated with higher rates of postoperative urinary tract infection,11 this was not found to be the case in this study. Preoperative administration of oral phenazopyridine was no different from the control modality with regard to visibility and surgeon satisfaction.
Continue to: The cost-effectiveness consideration
The cost-effectiveness consideration
The debate around universal cystoscopy following benign gynecologic surgery is ongoing.
The studies discussed in this Update demonstrate that cystoscopy following hysterectomy for benign indications:
- is superior to visualizing ureteral peristalsis
- increases detection of urinary tract injuries, and
- decreases delayed urologic injuries.
Although these articles emphasize the importance of detecting urologic injury, the picture would not be complete without mention of cost-effectiveness. Only one study, from 2001, has evaluated the cost-effectiveness of universal cystoscopy.1 Those authors concluded that universal cystoscopy is cost-effective only when the rate of urologic injury is 1.5% to 2%, but this conclusion, admittedly, was limited by the lack of data on medicolegal settlements, outpatient expenses, and nonmedical-related economic loss from decreased productivity. Given the extensive changes that have occurred in medical practice over the last 17 years and the emphasis on quality metrics and safety, an updated analysis would be needed to make definitive conclusions about cost-effectiveness.
While this Update cannot settle the ongoing debate of universal cystoscopy in gynecology, it is important to remember that the American College of Obstetricians and Gynecologists and the American Urogynecologic Society recommend cystoscopy following all surgeries for pelvic organ prolapse and stress urinary incontinence.2
References
- Visco AG, Taber KH, Weidner AD, Barber MD, Myers ER. Cost-effectiveness of universal cystoscopy to identify ureteral injury at hysterectomy. Obstet Gynecol. 2001;97(5 pt 1):685–692.
- ACOG Committee on Practice Bulletins–Gynecology and the American Urogynecologic Society. Urinary incontinence in women. Female Pelvic Med Reconstr Surg. 2015;21(6):304–314.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Ibeanu OA, Chesson RR, Echols KT, Nieves M, Busangu F, Nolan TE. Urinary tract injury during hysterectomy based on universal cystoscopy. Obstet Gynecol. 2009;113(1):6–10.
- ACOG Committee on Practice Bulletins–Gynecology and the American Urogynecologic Society. Urinary incontinence in women. Female Pelvic Med Reconstr Surg. 2015;21(6):304–314.
- Wilcox LS, Koonin LM, Pokras R, Strauss LT, Xia Z, Peterson HB. Hysterectomy in the United States, 1988–1990. Obstet Gynecol. 1994;83(4):549–555.
- Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89(4):501–506.
- Mäkinen J, Johansson J, Tomás C, et al. Morbidity of 10,110 hysterectomies by type of approach. Hum Reprod. 2001;16(7):1473–1478.
- Gilmour DT, Dwyer PL, Carey MP. Lower urinary tract injury during gynecologic surgery and its detection by intraoperative cystoscopy. Obstet Gynecol. 1999;94(5 pt 2):883–889.
- Barber MD, Brubaker L, Burgio KL, et al; Eunice Kennedy Schriver National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Comparison of 2 transvaginal surgical approaches and perioperative behavioral therapy for apical vaginal prolapse: the OPTIMAL randomized trial. JAMA. 2014;311(10):1023–1034.
- Brandes S, Coburn M, Armenakas N, McAninch J. Diagnosis and management of ureteric injury: an evidence-based analysis. BJU Int. 2004;94(3):277–289.
- Kwon CH, Goldberg RP, Koduri S, Sand PK. The use of intraoperative cystoscopy in major vaginal and urogynecologic surgeries. Am J Obstet Gynecol. 2002;187(6):1466–1471.
- Adams-Piper ER, Guaderrama NM, Chen Q, Whitcomb EL. Impact of surgical training on the performance of proposed quality measures for hysterectomy for pelvic organ prolapse. Am J Obstet Gynecol. 2017;216(6):588.e1–588.e5.
- Siff LN, Unger CA, Jelovsek JE, Paraiso MF, Ridgeway BM Barber MD. Assessing ureteral patency using 10% dextrose cystoscopy fluid: evaluation of urinary tract infection rates. Am J Obstet Gynecol. 2016;215(1):74.e1–74.e6.
- Espaillat-Rijo L, Siff L, Alas AN, et al. Intraoperative cystoscopic evaluation of ureteral patency: a randomized controlled trial. Obstet Gynecol. 2016;128(6):1378–1383.
Using cystoscopy to evaluate ureteral efflux and bladder integrity following benign gynecologic surgery increases the detection rate of urinary tract injuries.1 Currently, it is standard of care to perform a cystoscopy following anti-incontinence procedures, but there is no consensus among ObGyns regarding the use of universal cystoscopy following benign gynecologic surgery.2 A number of studies, however, have suggested potential best practices for evaluating urinary tract injury during pelvic surgery for benign gynecologic conditions.
Pelvic surgeries for benign gynecologic conditions, including fibroids, menorrhagia, and pelvic organ prolapse (POP), are common. More than 500,000 hysterectomies are performed annually in the United States, and up to 11% of women will undergo at least one surgery for POP or urinary incontinence in their lifetime.3,4 During gynecologic surgery, the urinary tract is at risk, and the injury rate ranges from 0.02% to 2% for ureteral injury and from 1% to 5% for bladder injury.5,6
In a recent large randomized controlled trial, the rate of intraoperative ureteral obstruction following uterosacral ligament suspension (USLS) was 3.2%.7 Vaginal vault suspensions, as well as other vaginal cuff closure techniques, are common procedures associated with urinary tract injury.8 Additionally, ureteral injury during surgery for POP occurs in as many as 2% of anterior vaginal wall repairs.9
It is well documented that a delay in diagnosis of ureteral and/or bladder injuries is associated with increased morbidity, including the need for additional surgery to repair the injury; in addition, significant delay in identifying an injury may lead to subsequent sequela, such as renal injury and fistula formation.8
A large study in California found that 36.5% of hysterectomies performed for POP were performed by general gynecologists.10 General ObGyns performing these surgeries therefore must understand the risk of urinary tract injury during hysterectomy and reconstructive pelvic procedures so that they can appropriately identify, evaluate, and repair injuries in a timely fashion.
The best way to identify urinary tract injury at the time of gynecologic surgery is by cystoscopy, including a bladder survey and ureteral efflux evaluation. When should a cystoscopy be performed, and what is the best method for visualizing ureteral efflux? Can instituting universal cystoscopy for all gynecologic procedures make a difference in the rate of injury detection? In this Update, we summarize the data from 4 studies that help to answer these questions.
Continue to: About 30% of urinary tract injuries...
About 30% of urinary tract injuries identified prior to cystoscopy at hysterectomy (which detected 5 of 6 injuries)
Vakili B, Chesson RR, Kyle BL, et al. The incidence of urinary tract injury during hysterectomy: a prospective analysis based on universal cystoscopy. Am J Obstet Gynecol. 2005;192(5):1599–1604.
Vakili and colleagues conducted a multicenter prospective cohort study of women undergoing hysterectomy for benign indications; cystoscopy was performed in all cases. The 3 hospitals involved were all part of the Louisiana State University Health system. The investigators’ goal was to determine the rate of urinary tract injury in this patient population at the time of intraoperative cystoscopy.
Intraoperative cystoscopy beats visual evaluation
Four hundred and seventy-one women underwent hysterectomy and had intraoperative cystoscopy, including evaluation of ureteral patency with administration of intravenous (IV) indigo carmine. Patients underwent abdominal, vaginal, or laparoscopic hysterectomy, and 54 (11.4%) had concurrent POP or anti-incontinence procedures. The majority underwent an abdominal hysterectomy (59%), 31% had a vaginal hysterectomy, and 10% had a laparoscopic-assisted vaginal hysterectomy or total laparoscopic hysterectomy.
Rate of urinary tract injuries. The total urinary tract injury rate detected by cystoscopy was 4.8%. The ureteral injury rate was 1.7%, and the bladder injury rate was 3.6%. A combined ureteral and bladder injury occurred in 2 women.
Surgery for POP significantly increased the risk of ureteral injury (7.3% vs 1.2%; P = .025). All cases of ureteral injury during POP surgery occurred during USLS. There was a trend toward a higher rate of bladder injury in the group with concurrent anti-incontinence surgery (12.5% vs 3.1%; P = .049). Regarding the route of hysterectomy, the vaginal approach had the highest rate of ureteral injury; however, when prolapse cases were removed from the analysis, there were no differences between the abdominal, vaginal, and laparoscopic approaches for ureteral or bladder injuries.
Injury detection with cystoscopy. Importantly, the authors found that only 30% of injuries were identified prior to performing intraoperative cystoscopy. The majority of these were bladder injuries. In addition, despite visual confirmation of ureteral peristalsis during abdominal hysterectomy, when intraoperative cystoscopy was performed with evaluation for ureteral efflux, 5 of 6 ureteral injury cases were identified. The authors reported 1 postoperative vesicovaginal fistula and concluded that it was likely due to an unrecognized bladder injury. No other undetected injuries were identified.
Notably, no complications occurred as a result of cystoscopy.
Multiple surgical indications reflect real-world scenario
The study included physicians from 3 different hospitals and all routes of hysterectomy for multiple benign gynecologic indications as well as concomitant pelvic reconstructive procedures. While this enhances the generalizability of the study results, all study sites were located in Louisiana at hospitals with resident trainee involvement. Additionally, this study confirms previous retrospective studies that reported higher rates of injury with pelvic reconstructive procedures.
The study is limited by the inability to blind surgeons, which may have resulted in the surgeons altering their techniques and/or having a heightened awareness of the urinary tract. However, their rates of ureteral and bladder injuries were slightly higher than previously reported rates, suggesting that the procedures inherently carry risk. The study is further limited by the lack of a retrospective comparison group of hysterectomy without routine cystoscopy and a longer follow-up period that may have revealed additional missed delayed urologic injuries.
The rate of urinary tract injury, including both bladder and ureteral injuries, was more than 4% at the time of hysterectomy for benign conditions. Using intraoperative peristalsis or normal ureteral caliber could result in a false sense of security since these are not reliable signs of ureteral integrity. The majority of urinary tract injuries will not be identified without cystoscopic evaluation.
Continue to: Universal cystoscopy policy...
Universal cystoscopy policy proves protective, surgeon adherence is high
Chi AM, Curran DS, Morgan DM, Fenner DE, Swenson CW. Universal cystoscopy after benign hysterectomy: examining the effects of an institutional policy. Obstet Gynecol. 2016;127(2):369–375.
In a retrospective cohort study, Chi and colleagues evaluated urinary tract injuries at the time of hysterectomy before and after the institution of a universal cystoscopy policy. At the time of policy implementation at the University of Michigan, all faculty who performed hysterectomies attended a cystoscopy workshop. Attending physicians without prior cystoscopy training also were proctored in the operating room for 3 sessions and were required to demonstrate competency with bladder survey, visualizing ureteral efflux, and urethral assessment. Indigo carmine was used to visualize ureteral efflux.
Detection of urologic injury almost doubled with cystoscopy
A total of 2,822 hysterectomies were included in the study, with 973 in the pre–universal cystoscopy group and 1,849 in the post–universal cystoscopy group. The study period was 7 years, and data on complications were abstracted for 1 year after the completion of the study period.
The primary outcome had 3 components:
- the rate of urologic injury before and after the policy
- the cystoscopy detection rate of urologic injury
- the adherence rate to the policy.
The overall rate of bladder and ureteral injury was 2.1%; the rate of injury during pre–universal screening was 2.6%, and during post–universal screening was 1.8%. The intraoperative detection rate of injury nearly doubled, from 24% to 47%, when intraoperative cystoscopy was utilized. In addition, the percentage of delayed urologic complications decreased from 28% to 5.9% (P = .03) following implementation of the universal cystoscopy policy. With regard to surgeon adherence, cystoscopy was documented in 86.1% of the hysterectomy cases after the policy was implemented compared with 35.7% of cases before the policy.
The investigators performed a cost analysis and found that hospital costs were nearly twice as much if a delayed urologic injury was diagnosed.
Study had many strengths
This study evaluated aspects of implementing quality initiatives after proper training and proctoring of a procedure. The authors compared very large cohorts from a busy academic medical center in which surgeon adherence with routine cystoscopy was high. The majority of patient outcomes were tracked for an extended period following surgery, thereby minimizing the risk of missing delayed urologic injuries. Notably, however, there was shorter follow-up time for the post–universal cystoscopy group, which could result in underestimating the rate of delayed urologic injuries in this cohort.
Instituting a universal cystoscopy policy for hysterectomy was associated with a significant decrease in delayed postoperative urinary tract complications and an increase in the intraoperative detection rate of urologic injuries. Intraoperative detection and repair of a urinary tract injury is cost-effective compared with a delayed diagnosis.
Continue to: Cystoscopy reveals ureteral obstruction...
Cystoscopy reveals ureteral obstruction during various vaginal POP repair procedures
Gustilo-Ashby AM, Jelovsek JE, Barber MD, Yoo EH, Paraiso MF, Walters MD. The incidence of ureteral obstruction and the value of intraoperative cystoscopy during vaginal surgery for pelvic organ prolapse. Am J Obstet Gynecol. 2006;194(5):1478–1485.
To determine the rate of ureteral obstruction and ureteral injury during vaginal surgery for POP and the accuracy of using intraoperative cystoscopy to prevent upper urinary tract morbidity, Gustilo-Ashby and colleagues performed a retrospective review study of a large patient cohort.
Cystoscopy with indigo carmine is highly sensitive
The study included 700 patients who underwent vaginal surgery for anterior and/or apical POP. Patients had 1 or more of the following anterior and apical prolapse repair procedures: USLS (51%), distal McCall culdeplasty (26%), proximal McCall culdeplasty (29%), anterior colporrhaphy (82%), and colpocleisis (1.4%). Of note, distal McCall culdeplasty was defined as incorporation of the “vaginal epithelium into the uterosacral plication,” while proximal McCall culdeplasty involved plication of “the uterosacral ligaments in the midline proximal to the vaginal cuff.” All patients were given IV indigo carmine to aid in visualizing ureteral efflux.
The majority of patients had a hysterectomy (56%). When accounting for rare false-positive and negative cystoscopy results, the overall ureteral obstruction rate was 5.1% and the ureteral injury rate was 0.9%. The majority of obstructions occurred with USLS (5.9%), proximal McCall culdeplasty (4.4%), and colpocleisis (4.2%). Ureteral injuries occurred only in 6 cases: 3 USLS and 3 proximal McCall culdeplasty procedures.
Based on these findings, the authors calculated that cystoscopy at the time of vaginal surgery for anterior and/or apical prolapse has a sensitivity of 94.4% and a specificity of 99.5% for detecting ureteral obstruction. The positive predictive value of cystoscopy with the use of indigo carmine for detection of ureteral obstruction is 91.9% and the negative predictive value is 99.7%.
Impact of indigo carmine’s unavailability
This study’s strengths include its large sample size and the variety of surgical approaches used for repair of anterior vaginal wall and apical prolapse. Its retrospective design, however, is a limitation; this could result in underreporting of ureteral injuries if patients received care at another institution after surgery. Furthermore, it is unclear if cystoscopy would be as predictive of ureteral injury without the use of indigo carmine, which is no longer available at most institutions.
The utility of cystoscopy with IV indigo carmine as a screening test for ureteral obstruction is highlighted by the fact that most obstructions were relieved by intraoperative suture removal following positive cystoscopy. McCall culdeplasty procedures are commonly performed by general ObGyns at the time of vaginal hysterectomy. It is therefore important to note that rates of ureteral obstruction after proximal McCall culdeplasty were only slightly lower than those after USLS.
Continue to: Sodium fluorescein and 10% dextrose...
Sodium fluorescein and 10% dextrose provide clear visibility of ureteral jets in cystoscopy
Espaillat-Rijo L, Siff L, Alas AN, et al. Intraoperative cystoscopic evaluation of ureteral patency: a randomized controlled trial. Obstet Gynecol. 2016;128(6):1378–1383.
In a multicenter randomized controlled trial, Espaillat-Rijo and colleagues compared various methods for visualizing ureteral efflux in participants who underwent gynecologic or urogynecologic procedures in which cystoscopy was performed.
Study compared 4 media
The investigators enrolled 176 participants (174 completed the trial) and randomly assigned them to receive 1 of 4 modalities: 1) normal saline as a bladder distention medium (control), 2) 10% dextrose as a bladder distention medium, 3) 200 mg oral phenazopyridine given 30 minutes prior to cystoscopy, or 4) 50 mg IV sodium fluorescein at the start of cystoscopy. Indigo carmine was not included in this study because it has not been routinely available since 2014.
Surgeons were asked to categorize the ureteral jets as “clearly visible,” “somewhat visible,” or “not visible.”
The primary outcome was subjective visibility of the ureteral jet with each modality during cystoscopy. Secondary outcomes included surgeon satisfaction, adverse reactions to the modality used, postoperative urinary tract infection, postoperative urinary retention, and delayed diagnosis of ureteral injury.
Visibility assessment results. Overall, ureteral jets were “clearly visible” in 125 cases (71%) compared with “somewhat visible” in 45 (25.6%) and “not visible” in 4 (2.3%) cases. There was a statistically significant difference between the 4 groups. Use of sodium fluorescein and 10% dextrose resulted in significantly better visualization of ureteral jets (P < .001 and P = .004, respectively) compared with the control group. Visibility with phenazopyridine was not significantly different from that in the control group or in the 10% dextrose group (FIGURE).

Surgeon satisfaction was highest with 10% dextrose and sodium fluorescein. In 6 cases, the surgeon was not satisfied with visualization of the ureteral jets and relied on fluorescein (5 times) or 10% dextrose (1 time) to ultimately see efflux. No significant adverse events occurred; the rate of urinary tract infection was 24.1% and did not differ between groups.
Results are widely generalizable
This was a well-designed randomized multicenter trial that included both benign gynecologic and urogynecologic procedures, thus strengthening the generalizability of the study. The study was timely since proven methods for visualization of ureteral patency became limited with the withdrawal of commercially available indigo carmine, the previous gold standard.
Intravenous sodium fluorescein and 10% dextrose as bladder distention media can both safely be used to visualize ureteral efflux and result in high surgeon satisfaction. Although 10% dextrose has been associated with higher rates of postoperative urinary tract infection,11 this was not found to be the case in this study. Preoperative administration of oral phenazopyridine was no different from the control modality with regard to visibility and surgeon satisfaction.
Continue to: The cost-effectiveness consideration
The cost-effectiveness consideration
The debate around universal cystoscopy following benign gynecologic surgery is ongoing.
The studies discussed in this Update demonstrate that cystoscopy following hysterectomy for benign indications:
- is superior to visualizing ureteral peristalsis
- increases detection of urinary tract injuries, and
- decreases delayed urologic injuries.
Although these articles emphasize the importance of detecting urologic injury, the picture would not be complete without mention of cost-effectiveness. Only one study, from 2001, has evaluated the cost-effectiveness of universal cystoscopy.1 Those authors concluded that universal cystoscopy is cost-effective only when the rate of urologic injury is 1.5% to 2%, but this conclusion, admittedly, was limited by the lack of data on medicolegal settlements, outpatient expenses, and nonmedical-related economic loss from decreased productivity. Given the extensive changes that have occurred in medical practice over the last 17 years and the emphasis on quality metrics and safety, an updated analysis would be needed to make definitive conclusions about cost-effectiveness.
While this Update cannot settle the ongoing debate of universal cystoscopy in gynecology, it is important to remember that the American College of Obstetricians and Gynecologists and the American Urogynecologic Society recommend cystoscopy following all surgeries for pelvic organ prolapse and stress urinary incontinence.2
References
- Visco AG, Taber KH, Weidner AD, Barber MD, Myers ER. Cost-effectiveness of universal cystoscopy to identify ureteral injury at hysterectomy. Obstet Gynecol. 2001;97(5 pt 1):685–692.
- ACOG Committee on Practice Bulletins–Gynecology and the American Urogynecologic Society. Urinary incontinence in women. Female Pelvic Med Reconstr Surg. 2015;21(6):304–314.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Using cystoscopy to evaluate ureteral efflux and bladder integrity following benign gynecologic surgery increases the detection rate of urinary tract injuries.1 Currently, it is standard of care to perform a cystoscopy following anti-incontinence procedures, but there is no consensus among ObGyns regarding the use of universal cystoscopy following benign gynecologic surgery.2 A number of studies, however, have suggested potential best practices for evaluating urinary tract injury during pelvic surgery for benign gynecologic conditions.
Pelvic surgeries for benign gynecologic conditions, including fibroids, menorrhagia, and pelvic organ prolapse (POP), are common. More than 500,000 hysterectomies are performed annually in the United States, and up to 11% of women will undergo at least one surgery for POP or urinary incontinence in their lifetime.3,4 During gynecologic surgery, the urinary tract is at risk, and the injury rate ranges from 0.02% to 2% for ureteral injury and from 1% to 5% for bladder injury.5,6
In a recent large randomized controlled trial, the rate of intraoperative ureteral obstruction following uterosacral ligament suspension (USLS) was 3.2%.7 Vaginal vault suspensions, as well as other vaginal cuff closure techniques, are common procedures associated with urinary tract injury.8 Additionally, ureteral injury during surgery for POP occurs in as many as 2% of anterior vaginal wall repairs.9
It is well documented that a delay in diagnosis of ureteral and/or bladder injuries is associated with increased morbidity, including the need for additional surgery to repair the injury; in addition, significant delay in identifying an injury may lead to subsequent sequela, such as renal injury and fistula formation.8
A large study in California found that 36.5% of hysterectomies performed for POP were performed by general gynecologists.10 General ObGyns performing these surgeries therefore must understand the risk of urinary tract injury during hysterectomy and reconstructive pelvic procedures so that they can appropriately identify, evaluate, and repair injuries in a timely fashion.
The best way to identify urinary tract injury at the time of gynecologic surgery is by cystoscopy, including a bladder survey and ureteral efflux evaluation. When should a cystoscopy be performed, and what is the best method for visualizing ureteral efflux? Can instituting universal cystoscopy for all gynecologic procedures make a difference in the rate of injury detection? In this Update, we summarize the data from 4 studies that help to answer these questions.
Continue to: About 30% of urinary tract injuries...
About 30% of urinary tract injuries identified prior to cystoscopy at hysterectomy (which detected 5 of 6 injuries)
Vakili B, Chesson RR, Kyle BL, et al. The incidence of urinary tract injury during hysterectomy: a prospective analysis based on universal cystoscopy. Am J Obstet Gynecol. 2005;192(5):1599–1604.
Vakili and colleagues conducted a multicenter prospective cohort study of women undergoing hysterectomy for benign indications; cystoscopy was performed in all cases. The 3 hospitals involved were all part of the Louisiana State University Health system. The investigators’ goal was to determine the rate of urinary tract injury in this patient population at the time of intraoperative cystoscopy.
Intraoperative cystoscopy beats visual evaluation
Four hundred and seventy-one women underwent hysterectomy and had intraoperative cystoscopy, including evaluation of ureteral patency with administration of intravenous (IV) indigo carmine. Patients underwent abdominal, vaginal, or laparoscopic hysterectomy, and 54 (11.4%) had concurrent POP or anti-incontinence procedures. The majority underwent an abdominal hysterectomy (59%), 31% had a vaginal hysterectomy, and 10% had a laparoscopic-assisted vaginal hysterectomy or total laparoscopic hysterectomy.
Rate of urinary tract injuries. The total urinary tract injury rate detected by cystoscopy was 4.8%. The ureteral injury rate was 1.7%, and the bladder injury rate was 3.6%. A combined ureteral and bladder injury occurred in 2 women.
Surgery for POP significantly increased the risk of ureteral injury (7.3% vs 1.2%; P = .025). All cases of ureteral injury during POP surgery occurred during USLS. There was a trend toward a higher rate of bladder injury in the group with concurrent anti-incontinence surgery (12.5% vs 3.1%; P = .049). Regarding the route of hysterectomy, the vaginal approach had the highest rate of ureteral injury; however, when prolapse cases were removed from the analysis, there were no differences between the abdominal, vaginal, and laparoscopic approaches for ureteral or bladder injuries.
Injury detection with cystoscopy. Importantly, the authors found that only 30% of injuries were identified prior to performing intraoperative cystoscopy. The majority of these were bladder injuries. In addition, despite visual confirmation of ureteral peristalsis during abdominal hysterectomy, when intraoperative cystoscopy was performed with evaluation for ureteral efflux, 5 of 6 ureteral injury cases were identified. The authors reported 1 postoperative vesicovaginal fistula and concluded that it was likely due to an unrecognized bladder injury. No other undetected injuries were identified.
Notably, no complications occurred as a result of cystoscopy.
Multiple surgical indications reflect real-world scenario
The study included physicians from 3 different hospitals and all routes of hysterectomy for multiple benign gynecologic indications as well as concomitant pelvic reconstructive procedures. While this enhances the generalizability of the study results, all study sites were located in Louisiana at hospitals with resident trainee involvement. Additionally, this study confirms previous retrospective studies that reported higher rates of injury with pelvic reconstructive procedures.
The study is limited by the inability to blind surgeons, which may have resulted in the surgeons altering their techniques and/or having a heightened awareness of the urinary tract. However, their rates of ureteral and bladder injuries were slightly higher than previously reported rates, suggesting that the procedures inherently carry risk. The study is further limited by the lack of a retrospective comparison group of hysterectomy without routine cystoscopy and a longer follow-up period that may have revealed additional missed delayed urologic injuries.
The rate of urinary tract injury, including both bladder and ureteral injuries, was more than 4% at the time of hysterectomy for benign conditions. Using intraoperative peristalsis or normal ureteral caliber could result in a false sense of security since these are not reliable signs of ureteral integrity. The majority of urinary tract injuries will not be identified without cystoscopic evaluation.
Continue to: Universal cystoscopy policy...
Universal cystoscopy policy proves protective, surgeon adherence is high
Chi AM, Curran DS, Morgan DM, Fenner DE, Swenson CW. Universal cystoscopy after benign hysterectomy: examining the effects of an institutional policy. Obstet Gynecol. 2016;127(2):369–375.
In a retrospective cohort study, Chi and colleagues evaluated urinary tract injuries at the time of hysterectomy before and after the institution of a universal cystoscopy policy. At the time of policy implementation at the University of Michigan, all faculty who performed hysterectomies attended a cystoscopy workshop. Attending physicians without prior cystoscopy training also were proctored in the operating room for 3 sessions and were required to demonstrate competency with bladder survey, visualizing ureteral efflux, and urethral assessment. Indigo carmine was used to visualize ureteral efflux.
Detection of urologic injury almost doubled with cystoscopy
A total of 2,822 hysterectomies were included in the study, with 973 in the pre–universal cystoscopy group and 1,849 in the post–universal cystoscopy group. The study period was 7 years, and data on complications were abstracted for 1 year after the completion of the study period.
The primary outcome had 3 components:
- the rate of urologic injury before and after the policy
- the cystoscopy detection rate of urologic injury
- the adherence rate to the policy.
The overall rate of bladder and ureteral injury was 2.1%; the rate of injury during pre–universal screening was 2.6%, and during post–universal screening was 1.8%. The intraoperative detection rate of injury nearly doubled, from 24% to 47%, when intraoperative cystoscopy was utilized. In addition, the percentage of delayed urologic complications decreased from 28% to 5.9% (P = .03) following implementation of the universal cystoscopy policy. With regard to surgeon adherence, cystoscopy was documented in 86.1% of the hysterectomy cases after the policy was implemented compared with 35.7% of cases before the policy.
The investigators performed a cost analysis and found that hospital costs were nearly twice as much if a delayed urologic injury was diagnosed.
Study had many strengths
This study evaluated aspects of implementing quality initiatives after proper training and proctoring of a procedure. The authors compared very large cohorts from a busy academic medical center in which surgeon adherence with routine cystoscopy was high. The majority of patient outcomes were tracked for an extended period following surgery, thereby minimizing the risk of missing delayed urologic injuries. Notably, however, there was shorter follow-up time for the post–universal cystoscopy group, which could result in underestimating the rate of delayed urologic injuries in this cohort.
Instituting a universal cystoscopy policy for hysterectomy was associated with a significant decrease in delayed postoperative urinary tract complications and an increase in the intraoperative detection rate of urologic injuries. Intraoperative detection and repair of a urinary tract injury is cost-effective compared with a delayed diagnosis.
Continue to: Cystoscopy reveals ureteral obstruction...
Cystoscopy reveals ureteral obstruction during various vaginal POP repair procedures
Gustilo-Ashby AM, Jelovsek JE, Barber MD, Yoo EH, Paraiso MF, Walters MD. The incidence of ureteral obstruction and the value of intraoperative cystoscopy during vaginal surgery for pelvic organ prolapse. Am J Obstet Gynecol. 2006;194(5):1478–1485.
To determine the rate of ureteral obstruction and ureteral injury during vaginal surgery for POP and the accuracy of using intraoperative cystoscopy to prevent upper urinary tract morbidity, Gustilo-Ashby and colleagues performed a retrospective review study of a large patient cohort.
Cystoscopy with indigo carmine is highly sensitive
The study included 700 patients who underwent vaginal surgery for anterior and/or apical POP. Patients had 1 or more of the following anterior and apical prolapse repair procedures: USLS (51%), distal McCall culdeplasty (26%), proximal McCall culdeplasty (29%), anterior colporrhaphy (82%), and colpocleisis (1.4%). Of note, distal McCall culdeplasty was defined as incorporation of the “vaginal epithelium into the uterosacral plication,” while proximal McCall culdeplasty involved plication of “the uterosacral ligaments in the midline proximal to the vaginal cuff.” All patients were given IV indigo carmine to aid in visualizing ureteral efflux.
The majority of patients had a hysterectomy (56%). When accounting for rare false-positive and negative cystoscopy results, the overall ureteral obstruction rate was 5.1% and the ureteral injury rate was 0.9%. The majority of obstructions occurred with USLS (5.9%), proximal McCall culdeplasty (4.4%), and colpocleisis (4.2%). Ureteral injuries occurred only in 6 cases: 3 USLS and 3 proximal McCall culdeplasty procedures.
Based on these findings, the authors calculated that cystoscopy at the time of vaginal surgery for anterior and/or apical prolapse has a sensitivity of 94.4% and a specificity of 99.5% for detecting ureteral obstruction. The positive predictive value of cystoscopy with the use of indigo carmine for detection of ureteral obstruction is 91.9% and the negative predictive value is 99.7%.
Impact of indigo carmine’s unavailability
This study’s strengths include its large sample size and the variety of surgical approaches used for repair of anterior vaginal wall and apical prolapse. Its retrospective design, however, is a limitation; this could result in underreporting of ureteral injuries if patients received care at another institution after surgery. Furthermore, it is unclear if cystoscopy would be as predictive of ureteral injury without the use of indigo carmine, which is no longer available at most institutions.
The utility of cystoscopy with IV indigo carmine as a screening test for ureteral obstruction is highlighted by the fact that most obstructions were relieved by intraoperative suture removal following positive cystoscopy. McCall culdeplasty procedures are commonly performed by general ObGyns at the time of vaginal hysterectomy. It is therefore important to note that rates of ureteral obstruction after proximal McCall culdeplasty were only slightly lower than those after USLS.
Continue to: Sodium fluorescein and 10% dextrose...
Sodium fluorescein and 10% dextrose provide clear visibility of ureteral jets in cystoscopy
Espaillat-Rijo L, Siff L, Alas AN, et al. Intraoperative cystoscopic evaluation of ureteral patency: a randomized controlled trial. Obstet Gynecol. 2016;128(6):1378–1383.
In a multicenter randomized controlled trial, Espaillat-Rijo and colleagues compared various methods for visualizing ureteral efflux in participants who underwent gynecologic or urogynecologic procedures in which cystoscopy was performed.
Study compared 4 media
The investigators enrolled 176 participants (174 completed the trial) and randomly assigned them to receive 1 of 4 modalities: 1) normal saline as a bladder distention medium (control), 2) 10% dextrose as a bladder distention medium, 3) 200 mg oral phenazopyridine given 30 minutes prior to cystoscopy, or 4) 50 mg IV sodium fluorescein at the start of cystoscopy. Indigo carmine was not included in this study because it has not been routinely available since 2014.
Surgeons were asked to categorize the ureteral jets as “clearly visible,” “somewhat visible,” or “not visible.”
The primary outcome was subjective visibility of the ureteral jet with each modality during cystoscopy. Secondary outcomes included surgeon satisfaction, adverse reactions to the modality used, postoperative urinary tract infection, postoperative urinary retention, and delayed diagnosis of ureteral injury.
Visibility assessment results. Overall, ureteral jets were “clearly visible” in 125 cases (71%) compared with “somewhat visible” in 45 (25.6%) and “not visible” in 4 (2.3%) cases. There was a statistically significant difference between the 4 groups. Use of sodium fluorescein and 10% dextrose resulted in significantly better visualization of ureteral jets (P < .001 and P = .004, respectively) compared with the control group. Visibility with phenazopyridine was not significantly different from that in the control group or in the 10% dextrose group (FIGURE).

Surgeon satisfaction was highest with 10% dextrose and sodium fluorescein. In 6 cases, the surgeon was not satisfied with visualization of the ureteral jets and relied on fluorescein (5 times) or 10% dextrose (1 time) to ultimately see efflux. No significant adverse events occurred; the rate of urinary tract infection was 24.1% and did not differ between groups.
Results are widely generalizable
This was a well-designed randomized multicenter trial that included both benign gynecologic and urogynecologic procedures, thus strengthening the generalizability of the study. The study was timely since proven methods for visualization of ureteral patency became limited with the withdrawal of commercially available indigo carmine, the previous gold standard.
Intravenous sodium fluorescein and 10% dextrose as bladder distention media can both safely be used to visualize ureteral efflux and result in high surgeon satisfaction. Although 10% dextrose has been associated with higher rates of postoperative urinary tract infection,11 this was not found to be the case in this study. Preoperative administration of oral phenazopyridine was no different from the control modality with regard to visibility and surgeon satisfaction.
Continue to: The cost-effectiveness consideration
The cost-effectiveness consideration
The debate around universal cystoscopy following benign gynecologic surgery is ongoing.
The studies discussed in this Update demonstrate that cystoscopy following hysterectomy for benign indications:
- is superior to visualizing ureteral peristalsis
- increases detection of urinary tract injuries, and
- decreases delayed urologic injuries.
Although these articles emphasize the importance of detecting urologic injury, the picture would not be complete without mention of cost-effectiveness. Only one study, from 2001, has evaluated the cost-effectiveness of universal cystoscopy.1 Those authors concluded that universal cystoscopy is cost-effective only when the rate of urologic injury is 1.5% to 2%, but this conclusion, admittedly, was limited by the lack of data on medicolegal settlements, outpatient expenses, and nonmedical-related economic loss from decreased productivity. Given the extensive changes that have occurred in medical practice over the last 17 years and the emphasis on quality metrics and safety, an updated analysis would be needed to make definitive conclusions about cost-effectiveness.
While this Update cannot settle the ongoing debate of universal cystoscopy in gynecology, it is important to remember that the American College of Obstetricians and Gynecologists and the American Urogynecologic Society recommend cystoscopy following all surgeries for pelvic organ prolapse and stress urinary incontinence.2
References
- Visco AG, Taber KH, Weidner AD, Barber MD, Myers ER. Cost-effectiveness of universal cystoscopy to identify ureteral injury at hysterectomy. Obstet Gynecol. 2001;97(5 pt 1):685–692.
- ACOG Committee on Practice Bulletins–Gynecology and the American Urogynecologic Society. Urinary incontinence in women. Female Pelvic Med Reconstr Surg. 2015;21(6):304–314.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Ibeanu OA, Chesson RR, Echols KT, Nieves M, Busangu F, Nolan TE. Urinary tract injury during hysterectomy based on universal cystoscopy. Obstet Gynecol. 2009;113(1):6–10.
- ACOG Committee on Practice Bulletins–Gynecology and the American Urogynecologic Society. Urinary incontinence in women. Female Pelvic Med Reconstr Surg. 2015;21(6):304–314.
- Wilcox LS, Koonin LM, Pokras R, Strauss LT, Xia Z, Peterson HB. Hysterectomy in the United States, 1988–1990. Obstet Gynecol. 1994;83(4):549–555.
- Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89(4):501–506.
- Mäkinen J, Johansson J, Tomás C, et al. Morbidity of 10,110 hysterectomies by type of approach. Hum Reprod. 2001;16(7):1473–1478.
- Gilmour DT, Dwyer PL, Carey MP. Lower urinary tract injury during gynecologic surgery and its detection by intraoperative cystoscopy. Obstet Gynecol. 1999;94(5 pt 2):883–889.
- Barber MD, Brubaker L, Burgio KL, et al; Eunice Kennedy Schriver National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Comparison of 2 transvaginal surgical approaches and perioperative behavioral therapy for apical vaginal prolapse: the OPTIMAL randomized trial. JAMA. 2014;311(10):1023–1034.
- Brandes S, Coburn M, Armenakas N, McAninch J. Diagnosis and management of ureteric injury: an evidence-based analysis. BJU Int. 2004;94(3):277–289.
- Kwon CH, Goldberg RP, Koduri S, Sand PK. The use of intraoperative cystoscopy in major vaginal and urogynecologic surgeries. Am J Obstet Gynecol. 2002;187(6):1466–1471.
- Adams-Piper ER, Guaderrama NM, Chen Q, Whitcomb EL. Impact of surgical training on the performance of proposed quality measures for hysterectomy for pelvic organ prolapse. Am J Obstet Gynecol. 2017;216(6):588.e1–588.e5.
- Siff LN, Unger CA, Jelovsek JE, Paraiso MF, Ridgeway BM Barber MD. Assessing ureteral patency using 10% dextrose cystoscopy fluid: evaluation of urinary tract infection rates. Am J Obstet Gynecol. 2016;215(1):74.e1–74.e6.
- Espaillat-Rijo L, Siff L, Alas AN, et al. Intraoperative cystoscopic evaluation of ureteral patency: a randomized controlled trial. Obstet Gynecol. 2016;128(6):1378–1383.
- Ibeanu OA, Chesson RR, Echols KT, Nieves M, Busangu F, Nolan TE. Urinary tract injury during hysterectomy based on universal cystoscopy. Obstet Gynecol. 2009;113(1):6–10.
- ACOG Committee on Practice Bulletins–Gynecology and the American Urogynecologic Society. Urinary incontinence in women. Female Pelvic Med Reconstr Surg. 2015;21(6):304–314.
- Wilcox LS, Koonin LM, Pokras R, Strauss LT, Xia Z, Peterson HB. Hysterectomy in the United States, 1988–1990. Obstet Gynecol. 1994;83(4):549–555.
- Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89(4):501–506.
- Mäkinen J, Johansson J, Tomás C, et al. Morbidity of 10,110 hysterectomies by type of approach. Hum Reprod. 2001;16(7):1473–1478.
- Gilmour DT, Dwyer PL, Carey MP. Lower urinary tract injury during gynecologic surgery and its detection by intraoperative cystoscopy. Obstet Gynecol. 1999;94(5 pt 2):883–889.
- Barber MD, Brubaker L, Burgio KL, et al; Eunice Kennedy Schriver National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Comparison of 2 transvaginal surgical approaches and perioperative behavioral therapy for apical vaginal prolapse: the OPTIMAL randomized trial. JAMA. 2014;311(10):1023–1034.
- Brandes S, Coburn M, Armenakas N, McAninch J. Diagnosis and management of ureteric injury: an evidence-based analysis. BJU Int. 2004;94(3):277–289.
- Kwon CH, Goldberg RP, Koduri S, Sand PK. The use of intraoperative cystoscopy in major vaginal and urogynecologic surgeries. Am J Obstet Gynecol. 2002;187(6):1466–1471.
- Adams-Piper ER, Guaderrama NM, Chen Q, Whitcomb EL. Impact of surgical training on the performance of proposed quality measures for hysterectomy for pelvic organ prolapse. Am J Obstet Gynecol. 2017;216(6):588.e1–588.e5.
- Siff LN, Unger CA, Jelovsek JE, Paraiso MF, Ridgeway BM Barber MD. Assessing ureteral patency using 10% dextrose cystoscopy fluid: evaluation of urinary tract infection rates. Am J Obstet Gynecol. 2016;215(1):74.e1–74.e6.
- Espaillat-Rijo L, Siff L, Alas AN, et al. Intraoperative cystoscopic evaluation of ureteral patency: a randomized controlled trial. Obstet Gynecol. 2016;128(6):1378–1383.
What works best for genitourinary syndrome of menopause: vaginal estrogen, vaginal laser, or combined laser and estrogen therapy?
EXPERT COMMENTARY
GSM encompasses a constellation of symptoms involving the vulva, vagina, urethra, and bladder, and it can affect quality of life in more than half of women by 3 years past menopause.1,2 Local estrogen creams, tablets, and rings are considered the gold standard treatment for GSM.3 The rising cost of many of these pharmacologic treatments has created headlines and concerns over price gouging for drugs used to treat female sexual dysfunction.4 Recent alternatives to local estrogens include vaginal moisturizers and lubricants, vaginal dehydroepiandrosterone (DHEA) suppositories, oral ospemifene, and vaginal laser therapy.
Laser treatment (with fractionated CO2, erbium, and hybrid lasers) activates heat shock proteins and tissue growth factors to stimulateneocollagenesis and neovascularization within the vaginal epithelium,but it is expensive and not covered by insurance because it is considered a cosmetic procedure.5Most evidence on laser therapy for GSM comes from prospective case series with small numbers and short-term follow-up with no comparison arms.6,7 A recent trial by Cruz and colleagues, however, is notable because it is one of the first published studies that compared vaginal laser with vaginal estrogen alone and with a combination laser plus estrogen arm. We need level 1 comparative data from studies such as this to help us counsel the millions of US women with GSM.
Details of the study
In this single-site randomized, double-blind, placebo-controlled trial conducted in Brazil, postmenopausal women were assigned to 1 of 3 treatment groups (15 per group):
- CO2 laser (MonaLisa Touch, SmartXide 2 system; DEKA Laser; Florence, Italy): 2 treatments total, 1 month apart, plus placebo cream (laser arm)
- estriol cream (1 mg estriol 3 times per week for 20 weeks) plus sham laser (estriol arm)
- CO2 laser plus estriol cream 3 times per week (laser plus estriol combination arm).
The primary outcome included a change in visual analog scale (VAS) score for symptoms related to vulvovaginal atrophy (VVA), including dyspareunia, dryness, and burning (0–10 scale with 0 = no symptoms and 10 = most severe symptoms), and change in the objective Vaginal Health Index (VHI). Assessments were made at baseline and at 8 and 20 weeks. Participants were included if they were menopausal for at least 2 years and had at least 1 moderately bothersome VVA symptom (based on a VAS score of 4 or greater).
Secondary outcomes included the objective FSFI questionnaire evaluating desire, arousal, lubrication, orgasm, satisfaction, and pain. FSFI scores can range from 2 (severe dysfunction) to 36 (no dysfunction). A total FSFI score less than 26 was deemed equivalent to dysfunction. Cytologic smear evaluation using a vaginal maturation index was included in all 3 treatment arms. Sample size calculation of 45 patients (15 per arm) for this trial was based on a 3-point difference in the VHI.
The baseline characteristics for participants in each treatment arm were similar, except that participants in the vaginal estriol group were less symptomatic at baseline. This group had less burning at baseline based on the FSFI and less dyspareunia based on the VAS.
On July 30, 2018, the US Food and Drug Administration (FDA) issued a safety warning against the use of energy-based devices for vaginal "rejuvenation"1 and sent warning letters to 7 companies--Alma Lasers; BTL Aesthetics; BTL Industries, Inc; Cynosure, Inc; InMode MD; Sciton, Inc; and Thermigen, Inc.2 The concern relates to marketing claims made on many of these companies' websites on the use of radiofrequency and laser technology for such specific conditions as vaginal laxity, vaginal dryness, urinary incontinence, and sexual function and response. These devices are neither cleared nor approved by the FDA for these specific indications; they are rather approved for general gynecologic conditions, such as the treatment of genital warts and precancerous conditions.
The FDA sent the safety warning related to energy-based vaginal therapies to patients and providers and have encouraged them to submit any adverse events to MedWatch, the FDA Safety Information and Adverse Event Reporting system.1 The "It has come to our attention letters" issued by the FDA to the above manufacturers request additional information and FDA clearance or approval numbers for claims made on their websites--specifically, referenced benefits of energy-based devices for vaginal, vulvar, and sexual health.2 This information is requested from manufacturers in writing by August 30, 2018 (30 days).
References
- FDA warns against use of energy-based devices to perform vaginal 'rejuvenation' or vaginal cosmetic procedures: FDA safety communication. US Food and Drug Administration website. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm615013.htm. Updated July 30, 2018. Accessed July 30, 2018.
- Letters to industry. US Food and Drug Administration website. https://www.fda.gov/MedicalDevices/ResourcesforYou/Industry/ucm111104.htm. Updated July 30, 2018. Accessed July 30, 2018.
Laser treatment improved dryness, burning, and dyspareunia but caused more pain
All 3 treatment groups showed statistically significant improvement in vaginal dryness at 20 weeks, but only the laser-alone arm and the laser plus estriol arms showed improvement in dyspareunia and burning. The total FSFI scores improved significantly only in the laser plus estriol arm (TABLE). No difference in the vaginal maturation index was noted between groups; however, improved numbers of parabasal cells were found in participants in the laser treatment arms.
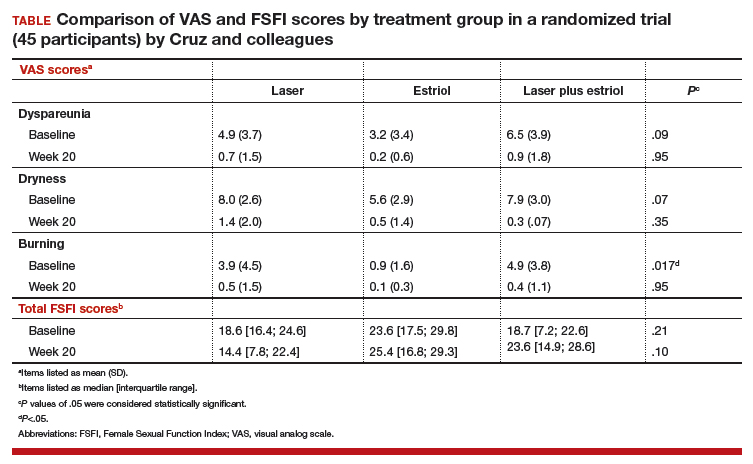
While participants in the laser treatment arms (alone and in combination with estriol) showed significant improvement in the VAS domains of dyspareunia and burning compared with those treated with estriol alone, there was a contradictory finding of more pain in both laser arms at 20 weeks compared with the estriol-alone group, based on the FSFI. The FSFI is a validated, objective quality-of-life questionnaire, and the finding of more pain with laser treatment is a concern.
Exercise caution when interpreting these study findings. While this preliminary study showed that fractionated CO2 laser treatment had favorable outcomes for dyspareunia, dryness, and burning, the propensity for increased vaginal pain with this treatment is a concern. This study was not adequately powered to analyze multiple comparisons in postmenopausal women with GSM symptoms. There were significant baseline differences, with less bothersome burning and sexual complaints based on the FSFI and VAS, in the vaginal estriol arm. The finding of more pain in the laser treatment arms at 20 weeks compared with that in the vaginal estriol arm is of concern and warrants further investigation.
-- Cheryl B. Iglesia, MD
Study strengths and weaknesses
This study is one of the first of its kind to compare laser therapy alone and in combination with local estriol to vaginal estriol alone for the treatment of GSM. The trial’s strength is in its design as a double-blind, placebo-controlled block randomized trial, which adds to the prospective cohort trials that generally show favorable outcomes for fractionated laser for the treatment of GSM.
The study’s weaknesses include its small sample size, single trial site, and short-term follow-up. Findings from this trial should be considered preliminary and not generalizable. Other weaknesses are the 3 of 45 participants lost to follow-up and the significant baseline differences among the women, with lower bothersome baseline VAS scores in the estriol arm.
Furthermore, this study was not powered for multiple comparisons, and conclusions favoring laser therapy cannot be overinflated. Lasers such as CO2 target the chromophore water, and indiscriminate use in severely dry vaginal epithelium may cause more pain or scarring. Longer-term follow-up is needed.
More research also is needed to develop guidelines related to pre-laser treatment to achieve optimal vaginal pH and ideal vaginal maturation, including, for example, vaginal priming with estrogen, DHEA, or other moisturizers.
This study also suggests the use of vaginal laser therapy as a drug delivery mechanism for combination therapy. Many vaginal estrogen treatments are expensive (despite prescription drug coverage), and laser treatments are very expensive (and not covered by insurance), so research to optimize outcomes and minimize patient expense is needed.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med. 2013;10(7):1790–1799.
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21(10):1063–1068.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24(7):728–753.
- Thomas K. Prices keep rising for drugs treating painful sex in women. New York Times. June 3, 2018. https://www.nytimes.com/2018/06/03/health/vagina-womens-health-drug-prices.html. Accessed July 15, 2018.
- Tadir Y, Gaspar A, Lev-Sagie A, et al. Light and energy based therapeutics for genitourinary syndrome of meno-pause: consensus and controversies. Lasers Surg Med. 2017;49(2):137–159.
- Athanasiou S, Pitsouni E, Antonopoulou S, et al. The effect of microablative fractional CO2 laser on vaginal flora of postmenopausal women. Climacteric. 2016;19(5):512–518.
- Sokol ER, Karram MM. Use of a novel fractional CO2 laser for the treatment of genitourinary syndrome of menopause: 1-year outcomes. Menopause. 2017;24(7):810–814.
EXPERT COMMENTARY
GSM encompasses a constellation of symptoms involving the vulva, vagina, urethra, and bladder, and it can affect quality of life in more than half of women by 3 years past menopause.1,2 Local estrogen creams, tablets, and rings are considered the gold standard treatment for GSM.3 The rising cost of many of these pharmacologic treatments has created headlines and concerns over price gouging for drugs used to treat female sexual dysfunction.4 Recent alternatives to local estrogens include vaginal moisturizers and lubricants, vaginal dehydroepiandrosterone (DHEA) suppositories, oral ospemifene, and vaginal laser therapy.
Laser treatment (with fractionated CO2, erbium, and hybrid lasers) activates heat shock proteins and tissue growth factors to stimulateneocollagenesis and neovascularization within the vaginal epithelium,but it is expensive and not covered by insurance because it is considered a cosmetic procedure.5Most evidence on laser therapy for GSM comes from prospective case series with small numbers and short-term follow-up with no comparison arms.6,7 A recent trial by Cruz and colleagues, however, is notable because it is one of the first published studies that compared vaginal laser with vaginal estrogen alone and with a combination laser plus estrogen arm. We need level 1 comparative data from studies such as this to help us counsel the millions of US women with GSM.
Details of the study
In this single-site randomized, double-blind, placebo-controlled trial conducted in Brazil, postmenopausal women were assigned to 1 of 3 treatment groups (15 per group):
- CO2 laser (MonaLisa Touch, SmartXide 2 system; DEKA Laser; Florence, Italy): 2 treatments total, 1 month apart, plus placebo cream (laser arm)
- estriol cream (1 mg estriol 3 times per week for 20 weeks) plus sham laser (estriol arm)
- CO2 laser plus estriol cream 3 times per week (laser plus estriol combination arm).
The primary outcome included a change in visual analog scale (VAS) score for symptoms related to vulvovaginal atrophy (VVA), including dyspareunia, dryness, and burning (0–10 scale with 0 = no symptoms and 10 = most severe symptoms), and change in the objective Vaginal Health Index (VHI). Assessments were made at baseline and at 8 and 20 weeks. Participants were included if they were menopausal for at least 2 years and had at least 1 moderately bothersome VVA symptom (based on a VAS score of 4 or greater).
Secondary outcomes included the objective FSFI questionnaire evaluating desire, arousal, lubrication, orgasm, satisfaction, and pain. FSFI scores can range from 2 (severe dysfunction) to 36 (no dysfunction). A total FSFI score less than 26 was deemed equivalent to dysfunction. Cytologic smear evaluation using a vaginal maturation index was included in all 3 treatment arms. Sample size calculation of 45 patients (15 per arm) for this trial was based on a 3-point difference in the VHI.
The baseline characteristics for participants in each treatment arm were similar, except that participants in the vaginal estriol group were less symptomatic at baseline. This group had less burning at baseline based on the FSFI and less dyspareunia based on the VAS.
On July 30, 2018, the US Food and Drug Administration (FDA) issued a safety warning against the use of energy-based devices for vaginal "rejuvenation"1 and sent warning letters to 7 companies--Alma Lasers; BTL Aesthetics; BTL Industries, Inc; Cynosure, Inc; InMode MD; Sciton, Inc; and Thermigen, Inc.2 The concern relates to marketing claims made on many of these companies' websites on the use of radiofrequency and laser technology for such specific conditions as vaginal laxity, vaginal dryness, urinary incontinence, and sexual function and response. These devices are neither cleared nor approved by the FDA for these specific indications; they are rather approved for general gynecologic conditions, such as the treatment of genital warts and precancerous conditions.
The FDA sent the safety warning related to energy-based vaginal therapies to patients and providers and have encouraged them to submit any adverse events to MedWatch, the FDA Safety Information and Adverse Event Reporting system.1 The "It has come to our attention letters" issued by the FDA to the above manufacturers request additional information and FDA clearance or approval numbers for claims made on their websites--specifically, referenced benefits of energy-based devices for vaginal, vulvar, and sexual health.2 This information is requested from manufacturers in writing by August 30, 2018 (30 days).
References
- FDA warns against use of energy-based devices to perform vaginal 'rejuvenation' or vaginal cosmetic procedures: FDA safety communication. US Food and Drug Administration website. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm615013.htm. Updated July 30, 2018. Accessed July 30, 2018.
- Letters to industry. US Food and Drug Administration website. https://www.fda.gov/MedicalDevices/ResourcesforYou/Industry/ucm111104.htm. Updated July 30, 2018. Accessed July 30, 2018.
Laser treatment improved dryness, burning, and dyspareunia but caused more pain
All 3 treatment groups showed statistically significant improvement in vaginal dryness at 20 weeks, but only the laser-alone arm and the laser plus estriol arms showed improvement in dyspareunia and burning. The total FSFI scores improved significantly only in the laser plus estriol arm (TABLE). No difference in the vaginal maturation index was noted between groups; however, improved numbers of parabasal cells were found in participants in the laser treatment arms.

While participants in the laser treatment arms (alone and in combination with estriol) showed significant improvement in the VAS domains of dyspareunia and burning compared with those treated with estriol alone, there was a contradictory finding of more pain in both laser arms at 20 weeks compared with the estriol-alone group, based on the FSFI. The FSFI is a validated, objective quality-of-life questionnaire, and the finding of more pain with laser treatment is a concern.
Exercise caution when interpreting these study findings. While this preliminary study showed that fractionated CO2 laser treatment had favorable outcomes for dyspareunia, dryness, and burning, the propensity for increased vaginal pain with this treatment is a concern. This study was not adequately powered to analyze multiple comparisons in postmenopausal women with GSM symptoms. There were significant baseline differences, with less bothersome burning and sexual complaints based on the FSFI and VAS, in the vaginal estriol arm. The finding of more pain in the laser treatment arms at 20 weeks compared with that in the vaginal estriol arm is of concern and warrants further investigation.
-- Cheryl B. Iglesia, MD
Study strengths and weaknesses
This study is one of the first of its kind to compare laser therapy alone and in combination with local estriol to vaginal estriol alone for the treatment of GSM. The trial’s strength is in its design as a double-blind, placebo-controlled block randomized trial, which adds to the prospective cohort trials that generally show favorable outcomes for fractionated laser for the treatment of GSM.
The study’s weaknesses include its small sample size, single trial site, and short-term follow-up. Findings from this trial should be considered preliminary and not generalizable. Other weaknesses are the 3 of 45 participants lost to follow-up and the significant baseline differences among the women, with lower bothersome baseline VAS scores in the estriol arm.
Furthermore, this study was not powered for multiple comparisons, and conclusions favoring laser therapy cannot be overinflated. Lasers such as CO2 target the chromophore water, and indiscriminate use in severely dry vaginal epithelium may cause more pain or scarring. Longer-term follow-up is needed.
More research also is needed to develop guidelines related to pre-laser treatment to achieve optimal vaginal pH and ideal vaginal maturation, including, for example, vaginal priming with estrogen, DHEA, or other moisturizers.
This study also suggests the use of vaginal laser therapy as a drug delivery mechanism for combination therapy. Many vaginal estrogen treatments are expensive (despite prescription drug coverage), and laser treatments are very expensive (and not covered by insurance), so research to optimize outcomes and minimize patient expense is needed.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
EXPERT COMMENTARY
GSM encompasses a constellation of symptoms involving the vulva, vagina, urethra, and bladder, and it can affect quality of life in more than half of women by 3 years past menopause.1,2 Local estrogen creams, tablets, and rings are considered the gold standard treatment for GSM.3 The rising cost of many of these pharmacologic treatments has created headlines and concerns over price gouging for drugs used to treat female sexual dysfunction.4 Recent alternatives to local estrogens include vaginal moisturizers and lubricants, vaginal dehydroepiandrosterone (DHEA) suppositories, oral ospemifene, and vaginal laser therapy.
Laser treatment (with fractionated CO2, erbium, and hybrid lasers) activates heat shock proteins and tissue growth factors to stimulateneocollagenesis and neovascularization within the vaginal epithelium,but it is expensive and not covered by insurance because it is considered a cosmetic procedure.5Most evidence on laser therapy for GSM comes from prospective case series with small numbers and short-term follow-up with no comparison arms.6,7 A recent trial by Cruz and colleagues, however, is notable because it is one of the first published studies that compared vaginal laser with vaginal estrogen alone and with a combination laser plus estrogen arm. We need level 1 comparative data from studies such as this to help us counsel the millions of US women with GSM.
Details of the study
In this single-site randomized, double-blind, placebo-controlled trial conducted in Brazil, postmenopausal women were assigned to 1 of 3 treatment groups (15 per group):
- CO2 laser (MonaLisa Touch, SmartXide 2 system; DEKA Laser; Florence, Italy): 2 treatments total, 1 month apart, plus placebo cream (laser arm)
- estriol cream (1 mg estriol 3 times per week for 20 weeks) plus sham laser (estriol arm)
- CO2 laser plus estriol cream 3 times per week (laser plus estriol combination arm).
The primary outcome included a change in visual analog scale (VAS) score for symptoms related to vulvovaginal atrophy (VVA), including dyspareunia, dryness, and burning (0–10 scale with 0 = no symptoms and 10 = most severe symptoms), and change in the objective Vaginal Health Index (VHI). Assessments were made at baseline and at 8 and 20 weeks. Participants were included if they were menopausal for at least 2 years and had at least 1 moderately bothersome VVA symptom (based on a VAS score of 4 or greater).
Secondary outcomes included the objective FSFI questionnaire evaluating desire, arousal, lubrication, orgasm, satisfaction, and pain. FSFI scores can range from 2 (severe dysfunction) to 36 (no dysfunction). A total FSFI score less than 26 was deemed equivalent to dysfunction. Cytologic smear evaluation using a vaginal maturation index was included in all 3 treatment arms. Sample size calculation of 45 patients (15 per arm) for this trial was based on a 3-point difference in the VHI.
The baseline characteristics for participants in each treatment arm were similar, except that participants in the vaginal estriol group were less symptomatic at baseline. This group had less burning at baseline based on the FSFI and less dyspareunia based on the VAS.
On July 30, 2018, the US Food and Drug Administration (FDA) issued a safety warning against the use of energy-based devices for vaginal "rejuvenation"1 and sent warning letters to 7 companies--Alma Lasers; BTL Aesthetics; BTL Industries, Inc; Cynosure, Inc; InMode MD; Sciton, Inc; and Thermigen, Inc.2 The concern relates to marketing claims made on many of these companies' websites on the use of radiofrequency and laser technology for such specific conditions as vaginal laxity, vaginal dryness, urinary incontinence, and sexual function and response. These devices are neither cleared nor approved by the FDA for these specific indications; they are rather approved for general gynecologic conditions, such as the treatment of genital warts and precancerous conditions.
The FDA sent the safety warning related to energy-based vaginal therapies to patients and providers and have encouraged them to submit any adverse events to MedWatch, the FDA Safety Information and Adverse Event Reporting system.1 The "It has come to our attention letters" issued by the FDA to the above manufacturers request additional information and FDA clearance or approval numbers for claims made on their websites--specifically, referenced benefits of energy-based devices for vaginal, vulvar, and sexual health.2 This information is requested from manufacturers in writing by August 30, 2018 (30 days).
References
- FDA warns against use of energy-based devices to perform vaginal 'rejuvenation' or vaginal cosmetic procedures: FDA safety communication. US Food and Drug Administration website. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm615013.htm. Updated July 30, 2018. Accessed July 30, 2018.
- Letters to industry. US Food and Drug Administration website. https://www.fda.gov/MedicalDevices/ResourcesforYou/Industry/ucm111104.htm. Updated July 30, 2018. Accessed July 30, 2018.
Laser treatment improved dryness, burning, and dyspareunia but caused more pain
All 3 treatment groups showed statistically significant improvement in vaginal dryness at 20 weeks, but only the laser-alone arm and the laser plus estriol arms showed improvement in dyspareunia and burning. The total FSFI scores improved significantly only in the laser plus estriol arm (TABLE). No difference in the vaginal maturation index was noted between groups; however, improved numbers of parabasal cells were found in participants in the laser treatment arms.

While participants in the laser treatment arms (alone and in combination with estriol) showed significant improvement in the VAS domains of dyspareunia and burning compared with those treated with estriol alone, there was a contradictory finding of more pain in both laser arms at 20 weeks compared with the estriol-alone group, based on the FSFI. The FSFI is a validated, objective quality-of-life questionnaire, and the finding of more pain with laser treatment is a concern.
Exercise caution when interpreting these study findings. While this preliminary study showed that fractionated CO2 laser treatment had favorable outcomes for dyspareunia, dryness, and burning, the propensity for increased vaginal pain with this treatment is a concern. This study was not adequately powered to analyze multiple comparisons in postmenopausal women with GSM symptoms. There were significant baseline differences, with less bothersome burning and sexual complaints based on the FSFI and VAS, in the vaginal estriol arm. The finding of more pain in the laser treatment arms at 20 weeks compared with that in the vaginal estriol arm is of concern and warrants further investigation.
-- Cheryl B. Iglesia, MD
Study strengths and weaknesses
This study is one of the first of its kind to compare laser therapy alone and in combination with local estriol to vaginal estriol alone for the treatment of GSM. The trial’s strength is in its design as a double-blind, placebo-controlled block randomized trial, which adds to the prospective cohort trials that generally show favorable outcomes for fractionated laser for the treatment of GSM.
The study’s weaknesses include its small sample size, single trial site, and short-term follow-up. Findings from this trial should be considered preliminary and not generalizable. Other weaknesses are the 3 of 45 participants lost to follow-up and the significant baseline differences among the women, with lower bothersome baseline VAS scores in the estriol arm.
Furthermore, this study was not powered for multiple comparisons, and conclusions favoring laser therapy cannot be overinflated. Lasers such as CO2 target the chromophore water, and indiscriminate use in severely dry vaginal epithelium may cause more pain or scarring. Longer-term follow-up is needed.
More research also is needed to develop guidelines related to pre-laser treatment to achieve optimal vaginal pH and ideal vaginal maturation, including, for example, vaginal priming with estrogen, DHEA, or other moisturizers.
This study also suggests the use of vaginal laser therapy as a drug delivery mechanism for combination therapy. Many vaginal estrogen treatments are expensive (despite prescription drug coverage), and laser treatments are very expensive (and not covered by insurance), so research to optimize outcomes and minimize patient expense is needed.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med. 2013;10(7):1790–1799.
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21(10):1063–1068.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24(7):728–753.
- Thomas K. Prices keep rising for drugs treating painful sex in women. New York Times. June 3, 2018. https://www.nytimes.com/2018/06/03/health/vagina-womens-health-drug-prices.html. Accessed July 15, 2018.
- Tadir Y, Gaspar A, Lev-Sagie A, et al. Light and energy based therapeutics for genitourinary syndrome of meno-pause: consensus and controversies. Lasers Surg Med. 2017;49(2):137–159.
- Athanasiou S, Pitsouni E, Antonopoulou S, et al. The effect of microablative fractional CO2 laser on vaginal flora of postmenopausal women. Climacteric. 2016;19(5):512–518.
- Sokol ER, Karram MM. Use of a novel fractional CO2 laser for the treatment of genitourinary syndrome of menopause: 1-year outcomes. Menopause. 2017;24(7):810–814.
- Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med. 2013;10(7):1790–1799.
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21(10):1063–1068.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24(7):728–753.
- Thomas K. Prices keep rising for drugs treating painful sex in women. New York Times. June 3, 2018. https://www.nytimes.com/2018/06/03/health/vagina-womens-health-drug-prices.html. Accessed July 15, 2018.
- Tadir Y, Gaspar A, Lev-Sagie A, et al. Light and energy based therapeutics for genitourinary syndrome of meno-pause: consensus and controversies. Lasers Surg Med. 2017;49(2):137–159.
- Athanasiou S, Pitsouni E, Antonopoulou S, et al. The effect of microablative fractional CO2 laser on vaginal flora of postmenopausal women. Climacteric. 2016;19(5):512–518.
- Sokol ER, Karram MM. Use of a novel fractional CO2 laser for the treatment of genitourinary syndrome of menopause: 1-year outcomes. Menopause. 2017;24(7):810–814.
Ovarian masses: Surgery or surveillance?
A meaningful evolution has occurred over the past 30 years in the evaluation of ovarian tumors. In the 1980s, any palpable ovarian tumor was recommended for surgical removal.1 In the early 2000s, studies showed that unilocular cysts were at very low risk for malignancy, and surveillance was recommended.2 In the following decade, septate cysts were added to the list of ovarian tumors unlikely to be malignant, and nonsurgical therapy was suggested.3 It is estimated that 10% of women will undergo surgery for an adnexal mass in their lifetime, despite the fact that only 1 in 6 (13%–21%) of these masses is found to be malignant.4,5
A comprehensive, morphology-based pelvic ultrasonography is the first and most important step in evaluating an ovarian tumor’s risk of malignancy to determine whether surgery or surveillance is required.
Ovarian cancer continues to be the leading cause of gynecologic cancer death. Despite achieving superior surgical and cancer outcomes, a gynecologic oncologist performs only 40% of the initial ovarian cancer surgeries.6 Premenopausal and menopausal ovarian tumors are different in cause and consequence. Only 15% of premenopausal tumors are malignant, most commonly germ cell tumors, borderline ovarian tumors, and epithelial ovarian cancers. Tumors in menopausal women are less common but are more likely to be malignant. In actuality, up to 50% of tumors in this population are malignant. The most common of these malignancies are epithelial ovarian cancers, cancers metastatic to the ovary, and malignant stromal tumors.
Effective and evidence-based preoperative evaluations are available to help the clinician estimate a tumor’s risk of malignancy and determine which tumors are appropriate for referral to a specialist for surgery.
The actual incidence and prevalence of ovarian tumors are not known. From a review of almost 40,000 ultrasonography scans performed in the University of Kentucky Ovarian Cancer Screening Program, the estimated incidence and prevalence of ovarian abnormalities are 8.2 per 100 women annuallyand 17%, respectively.7 Seventy percent of these abnormalities have a unilocular or simple septate morphology and are at low risk for malignancy.7 The remaining 30% of abnormalities are high risk, although this represents only 9% of the total population evaluated. Since the vast majority of these abnormalities are expected to be asymptomatic, most will go unrecognized in the general population. For women who have an ovarian abnormality on ultrasonography, the majority will be at low risk for malignancy and will not require surgery.
Ovarian ultrasonography plus morphologic scoring comprise a comprehensive approach
The recently published recommendations of the First International Consensus Conference report on adnexal masses are summarized in TABLE 1.8 The expert panel reviewed the evidence and concluded that effective ultrasonography strategies exist and are well validated, and that low-risk asymptomatic ovarian cysts do not require surgical removal.

While no single ultrasonographic findingcan differentiate a benign from a malignant mass, morphologic scoring systems improve our ability to estimate a tumor’s malignant potential. In the United States, most practitioners in women’s health have ready access to gynecologic ultrasonography, but individual training and proficiency vary. Since not everyone is an expert sonographer, it is useful to employ an objective strategy when evaluating an ovarian tumor. The focus of a comprehensive ovarian ultrasonography is to recognize morphologic patterns that reflect a tumor’s malignant potential. While tumor volume is useful, tumor morphology is the most prognostic feature.
International Ovarian Tumor Analysis group
The International Ovarian Tumor Analysis (IOTA) group has published extensively on sonographic definitions and patterns that categorize tumors based on appearance.9 Simple rules and the ADNEX risk model are 2 of the group’s approaches (FIGURE 1).10,11 Both methods have been validated as effective for differentiating benign from malignant ovarian tumors, but neither has been used to study serial changes in ovarian morphology.

Regardless of the strategy employed, 25% of ovarian ultrasonography evaluations will be interpreted as “indeterminate” or “risk unknown.”10 The IOTA strategies have been successfully used in Europe for years, but they have not yet been studied or adopted in the United States.
Kentucky morphology index
The morphology index (MI) from the University of Kentucky is an ultrasonography-based scoring system that combines tumor volume and tumor structure into a simple and effective index with a score ranging from 0 to 10 (FIGURE 2).12 A rising Kentucky MI score has a linear and predictable increase in the risk of ovarian malignancy. In a review of almost 40,000 sonograms, 85% of the malignancies had an MI score of 5 or greater (TABLE 2).12 Using this as a cutoff, the sensitivity and specificity for predicting malignancy was 86% and 98%, respectively.12
When comparing the ADNEX risk model with the Kentucky MI, investigators reviewed 45,000 ultrasound results and found that the majority of cancers were categorized by the ADNEX model in the lowest 4 of the 10 risk-of-malignancy groups, compared with only 15% for the MI.13 This clustering or skew is potentially problematic, since we expect higher scores to be more predictive of cancer than lower scores. It also infers that the ADNEX model may not be useful in serial surveillance strategies. Moreover, the ADNEX model identified only 30% of early stage cancers compared with identification of 80% with use of the MI.13
Serial ultrasonography
Serial ultrasonography is a concept similar to any longitudinal biomarker evaluation. In the United Kingdom Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) program, the Risk of Ovarian Cancer Algorithm (ROCA) employs serial measurements of cancer antigen 125 (CA 125) to improve cancer detection. Serial ultrasonography similarly can be applied to better characterize a tumor’s physiology as well as its morphology. Over time, malignant ovarian tumors grow naturally in volume and complexity, and they do so at a rate faster than nonmalignant tumors. If this physical change can be measured objectively with ultrasonography, then serial sonography becomes a valuable diagnostic aid.
In comparing serial MI scores with clinical outcomes, studies have shown that malignant tumors exhibit a rapid increase, nonmalignant tumors have a stable or gradual rise, and resolving cysts show a decrease in MI score over time (FIGURE 3).12 An increase in the MI score of 1 or more per month (≥1 per month) is concerning for malignancy, and surgical removal should be considered. If the MI score of an asymptomatic ovarian tumor does not increase by 1 per month, it can be surveilled with intermittent ultrasonography.
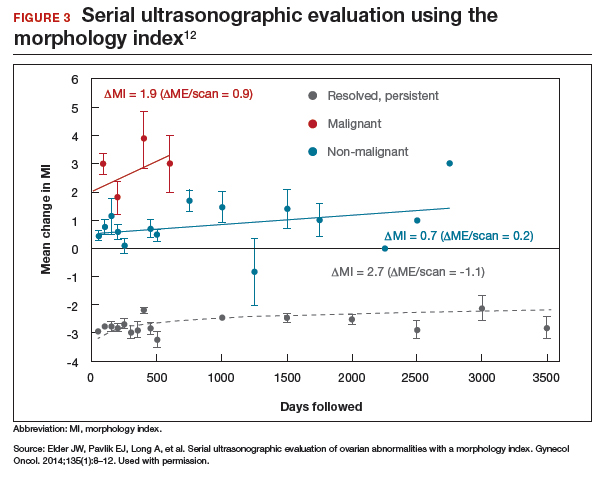
Read about evaluating with serum biomarkers and sonography.
Serum biomarkers useful for determining risk, need for referral
Serum biomarkers can be used to complement an ultrasonographic evaluation. They are particularly useful when surgery is recommended but the sonographic evaluation is indeterminate for malignancy risk. Many serum biomarkers are commonly used for the preoperative evaluation of an ovarian tumor or for surveillance of a malignancy following diagnosis (TABLE 3).
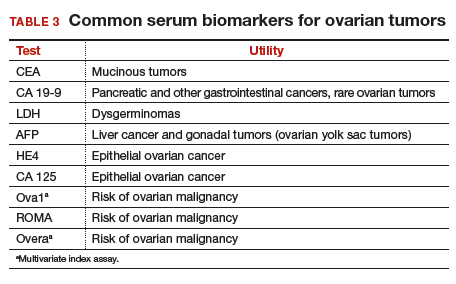
CA 125 is the most commonly ordered serum biomarker test for ovarian cancer. It is estimated that three‐quarters of CA 125 tests are ordered for preoperative use, which is not the US Food and Drug Administration (FDA) approved indication. Despite our clinical reliance on CA 125 as a diagnostic test prior to surgery, its utility is limited because of a low sensitivity for predicting cancer in premenopausal women and early stage disease.14,15 CA 125 specificity also varies widely, depending on patient age and other clinical factors, ranging from as low as 26% in premenopausal women to as high as 100% in postmenopausal women.16 Because CA 125 often is negative when early stage cancer is present, or positive when cancer is not, it is not recommended for preoperative use for determining whether an ovarian tumor is malignant or whether surgery is indicated. CA 125 should be used to monitor patients with a known ovarian malignancy.
The new triage serum biomarkers, Overa, Ova1, and ROMA (Risk of Ovarian Malignancy Algorithm), are FDA cleared for preoperative use to help determine whether a woman needing surgery for an ovarian mass should be referred to a gynecologic oncologist.17–20 These tests should not be used to decide if surgery is indicated, but rather should be considered when the decision for surgery has already been made but the malignancy risk is unknown. A woman with a “high risk” result should be referred to a gynecologic oncologist, while one with a “low risk” score is very unlikely to have a malignancy and referral to a specialist is not necessary. TABLE 4 lists a comparison of the relative performance of these serum biomarkers.14,15,17–20 There are no published data on the use of serial triage biomarkers.
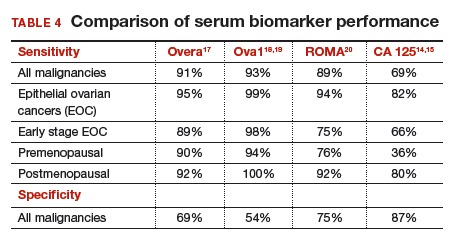
How to evaluate an ovarian tumor
Approximately 65% of the time, ovarian cystic tumors can be identified accurately as low risk based on the initial sonographic evaluation (TABLE 5). In this scenario, the risk of malignancy is very low (<1%), no secondary testing is needed, and no surgery is recommended.1,3,21
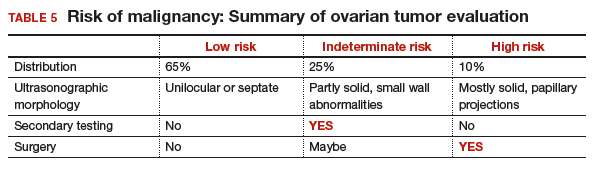
About 10% of tumors are expected to have a high-risk morphology on ultrasonography, where the risk of malignancy exceeds 25% and referral to a gynecologic oncologist is required.
The remaining 25% of tumors cannot be accurately classified with a single ultrasonographic evaluation and are considered indeterminate.22 Indeterminate tumors require secondary testing to ascertain whether surgery is indicated. Secondary testing may consist of serial ultrasonography, magnetic resonance imaging (MRI), or serum triage biomarker testing if the decision for surgery has been made.
A 2-step process is recommended for evaluating an ovarian tumor.
Step 1. Perform a detailed ultrasonography study using a morphology-based system. Classify the tumor as:
- low risk (65%): unilocular, simple septate, no flow on color Doppler
- simple rules: benign
- MI score 0–3
- no secondary testing; no referral is recommended
- high risk (10%): irregular, mostly solid, papillary projections, very strong flow on color Doppler
- simple rules: malignant
- MI score ≥5
- no secondary testing; refer to a gynecologic oncologist
- indeterminate (25%): partly solid, small wall abnormalities, minimal or moderate flow on color Doppler
- simple rules: both M and B rules apply or no rule applies
- MI score usually 4–6
- perform secondary testing (step 2).
Step 2. Perform secondary testing as follows:
- serum triage biomarkers if surgery is planned (Ova1, ROMA, Overa), or
- MRI, or
- serial sonography.
The 3 case scenarios that follow illustrate how the ovarian tumor evaluation process may be applied in clinical practice, with referral to a gynecologic oncologist as appropriate.
CASE 1 Postmenopausal woman with urinary symptoms and pelvic pressure
A 61-year-old woman is referred with a newly identified ovarian tumor. She has had 1 month of urinary urgency, frequency, and pelvic pressure, but she denies vaginal bleeding or fever. She has no family history of cancer. The referring physician included results of a serum CA 125 (48 U/mL; normal, ≤35 U/mL). A pelvic examination reveals a palpable, irregular mass in the anterior pelvis with limited mobility.
What would be your next step in the evaluation of this patient?
Start with ultrasonography
Step 1. Perform pelvic ultrasonography. In this patient, transvaginal sonography revealed a 6-cm (volume, 89 mL) mostly solid tumor (FIGURE 4). The maximum solid diameter of the tumor was 4.0 cm. There was a 20-mL pocket of pelvic ascites.
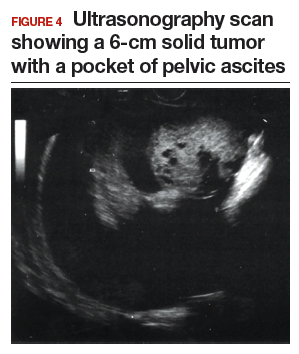
Results of morphology-based classification were as follows:
- simple rules: M1 and M5 positive; B rules: negative (malignant; high risk)
- ADNEX: 51.6% risk of malignancy (high risk)
- MI: 7 (high risk).
Step 2. Consider secondary testing. In this case, no secondary testing was recommended. Treatment plan. The patient was referred to a gynecologic oncologist for surgery and was found to have a stage IIA serous ovarian carcinoma.
CASE 2 Woman with history of pelvic symptoms and worsening pain
A 46-year-old woman presents with worsening pelvic pain over the last month. She has a long-standing history of pelvic pain, dysmenorrhea, and dyspareunia from suspected endometriosis. She has no family history of cancer. The referring physician included the following serum biomarker results: CA 125, 48 U/mL (normal, ≤35 U/mL), and HE4, 60 pM (normal, ≤150 pM). On pelvic examination, there is a palpable mass with limited mobility in the posterior cul-de-sac.
Based on the patient’s available history, physical examination, and biomarker information, how would you proceed?
Follow the 2-step process
Step 1. Perform pelvic ultrasonography. Transvaginal sonography revealed a 6-cm (volume, 89 mL) partly solid tumor with regular internal borders (FIGURE 5). The maximum solid diameter of the tumor was 4.5 cm. There was no pelvic ascites.
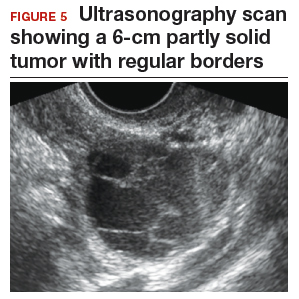
Morphology classification was as follows:
- simple rules: M5 equivocal; B4 positive (indeterminate risk)
- ADNEX: 42.7% risk of malignancy (high risk)
- MI: 6 (indeterminate risk).
Step 2. Secondary testing was recommended for this patient. Test results were:
- repeat ultrasonography in 4 weeks with MI of 7 (volume score increase from 2 to 3, structure score unchanged at 4). Change in MI score +1 per month (high risk)
- Overa: 5.2 (high risk)
- ROMA: 11.8% (low risk).
Treatment plan. The patient was referred to a gynecologic oncologist because of an increasing MI score on serial sonography. Surgery revealed a stage IA grade 2 endometrioid adenocarcinoma of the ovary with surrounding endometriosis.
Read about treating a woman with postmenstrual bleeding.
CASE 3 Woman with postmenopausal bleeding seeks medical care
A 62-year-old woman is referred with new-onset postmenopausal spotting for 1 month. She was recently prescribed antibiotics for diverticulitis. She has no family history of cancer. The referring physician included the results of a serum CA 125, which was 48 U/mL (normal, ≤35 U/mL). On pelvic examination, a mobile cystic mass is noted in the posterior cul-de-sac.
Use the stepwise protocol to sort out findings
Step 1. Pelvic ultrasonography. Transvaginal sonography suggested the presence of an endometrial polyp and revealed a 6-cm (volume, 89 mL) septate ovarian cyst (FIGURE 6).
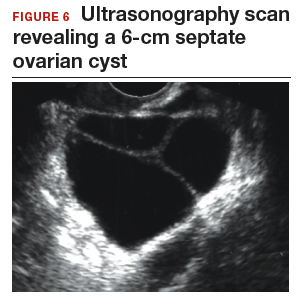
Based on morphology classification, risk was categorized as:
- simple rules: M rules negative; B2, B4, B5 positive (benign; low risk)
- ADNEX: 2.9% risk of malignancy (low risk)
- MI: 2 (low risk).
Step 2. No secondary testing was recommended in this case.
Treatment plan. The patient’s gynecologist performed a hysteroscopic polypectomy that revealed no cancer. Serial monitoring was recommended for the low-risk ovarian cyst. The next ultrasonography scan, at 6 months, was unchanged; a subsequent scan was ordered for 12 months later, and at that time the cyst had resolved.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Barber HR, Graber EA. The PMPO syndrome (postmenopausal palpable ovary syndrome). Obstet Gynecol. 1971;38(6):921–923.
- Modesitt SC, Pavlik EJ, Ueland FR, DePriest PD, Kryscio RJ, van Nagell JR Jr. Risk of malignancy in unilocular ovarian cystic tumors less than 10 centimeters in diameter. Obstet Gynecol. 2003;102(3):594–599.
- Saunders BA, Podzielinski I, Ware RA, et al. Risk of malignancy in sonographically confirmed septated cystic ovarian tumors. Gynecol Oncol. 2010;118(3):278–282.
- Moore RG, McMeekin DS, Brown AK, et al. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol Oncol. 2009;112(1):40–46.
- Jordan SM, Bristow RE. Ovarian cancer biomarkers as diagnostic triage tests. Current Biomarker Findings. 2013;3:35–42.
- Giede KC, Kieser K, Dodge J, Rosen B. Who should operate on patients with ovarian cancer? An evidence-based review. Gynecol Oncol. 2005;99(2):447–461.
- Pavlik EJ, Ueland FR, Miller RW, et al. Frequency and disposition of ovarian abnormalities followed with serial transvaginal ultrasonography. Obstet Gynecol. 2013;122(2 pt 1):210–217.
- Glanc P, Benacerraf B, Bourne T, et al. First International Consensus Report on adnexal masses: management recommendations. J Ultrasound Med. 2017;36(5):849–863.
- Timmerman D, Valentin L, Bourne TGH, Collins WP, Verrelst H, Vergote I; International Ovarian Tumor Analysis (IOTA) Group. Terms, definitions and measurements to describe the sonographic features of adnexal tumors: a consensus opinion from the International Ovarian Tumor Analysis (IOTA) group. Ultrasound Obstet Gynecol. 2000;6(5):500–505.
- Timmerman D, Testa AC, Bourne T, et al. Simple ultrasound-based rules for the diagnosis of ovarian cancer. Ultrasound Obstet Gynecol. 2008;31(6):681–690.
- Van Calser B, Van Hoorde K, Valentin L, et al. Evaluating the risk of ovarian cancer before surgery using the ADNEX model to differentiate between benign, borderline, early and advanced stage invasive, and secondary metastatic tumours: prospective multicentre diagnostic study. BMJ. 2014;349:g5920.
- Elder JW, Pavlik EJ, Long A, et al. Serial ultrasonographic evaluation of ovarian abnormalities with a morphology index. Gynecol Oncol. 2014;135(1):8–12.
- Lefringhouse J, Ueland F, Ore R, et al. Comparing 2 sonographic scoring systems for distinguishing benign from malignant ovarian tumors [abstract]. Gynecol Oncol. 2016;141(suppl 1):57.
- Bast RC Jr, Klug TL, St John E, et al. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N Engl J Med. 1983;309(15):883–887.
- Jacobs I, Bast RC Jr. The CA 125 tumour-associated antigen: a review of the literature. Hum Reprod. 1989;4(1):1–12.
- Myers ER, Bastian LA, Havrilesky LJ, et al. Management of adnexal mass. Agency for Healthcare Research and Quality. https://archive.ahrq.gov/downloads/pub/evidence/pdf/adnexal/adnexal.pdf. Published February 2006. Accessed May 15, 2018.
- Coleman RL, Herzog TJ, Chan DW, et al. Validation of a second-generation multivariate index assay for malignancy risk of adnexal masses. Am J Obstet Gynecol. 2016;215(1):82.e1–e11.
- Ueland FR, Desimone CP, Seamon LG, et al. Effectiveness of a multivariate index assay in the preoperative assessment of ovarian tumors. Obstet Gynecol. 2011;117(6):1289–1297.
- Bristow RE, Smith A, Zhang Z, et al. Ovarian malignancy risk stratification of the adnexal mass using a multivariate index assay. Gynecol Oncol. 2013;128(2):252–259.
- Moore RG, McMeekin DS, Brown AK, et al. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol Oncol. 2009;112(1):40–46.
- Valentin L, Ameye L, Franchi D, et al. Risk of malignancy in unilocular cysts: a study of 1148 adnexal masses classified as unilocular cysts on transvaginal ultrasound and review of the literature. Ultrasound Obstet Gynecol. 2013;41(1):80–89.
- Timmerman D, Ameye L, Fischerova D, et al. Simple ultrasound rules to distinguish between benign and malignant adnexal masses before surgery: prospective validation by IOTA group. BMJ. 2010;341:c6839.
A meaningful evolution has occurred over the past 30 years in the evaluation of ovarian tumors. In the 1980s, any palpable ovarian tumor was recommended for surgical removal.1 In the early 2000s, studies showed that unilocular cysts were at very low risk for malignancy, and surveillance was recommended.2 In the following decade, septate cysts were added to the list of ovarian tumors unlikely to be malignant, and nonsurgical therapy was suggested.3 It is estimated that 10% of women will undergo surgery for an adnexal mass in their lifetime, despite the fact that only 1 in 6 (13%–21%) of these masses is found to be malignant.4,5
A comprehensive, morphology-based pelvic ultrasonography is the first and most important step in evaluating an ovarian tumor’s risk of malignancy to determine whether surgery or surveillance is required.
Ovarian cancer continues to be the leading cause of gynecologic cancer death. Despite achieving superior surgical and cancer outcomes, a gynecologic oncologist performs only 40% of the initial ovarian cancer surgeries.6 Premenopausal and menopausal ovarian tumors are different in cause and consequence. Only 15% of premenopausal tumors are malignant, most commonly germ cell tumors, borderline ovarian tumors, and epithelial ovarian cancers. Tumors in menopausal women are less common but are more likely to be malignant. In actuality, up to 50% of tumors in this population are malignant. The most common of these malignancies are epithelial ovarian cancers, cancers metastatic to the ovary, and malignant stromal tumors.
Effective and evidence-based preoperative evaluations are available to help the clinician estimate a tumor’s risk of malignancy and determine which tumors are appropriate for referral to a specialist for surgery.
The actual incidence and prevalence of ovarian tumors are not known. From a review of almost 40,000 ultrasonography scans performed in the University of Kentucky Ovarian Cancer Screening Program, the estimated incidence and prevalence of ovarian abnormalities are 8.2 per 100 women annuallyand 17%, respectively.7 Seventy percent of these abnormalities have a unilocular or simple septate morphology and are at low risk for malignancy.7 The remaining 30% of abnormalities are high risk, although this represents only 9% of the total population evaluated. Since the vast majority of these abnormalities are expected to be asymptomatic, most will go unrecognized in the general population. For women who have an ovarian abnormality on ultrasonography, the majority will be at low risk for malignancy and will not require surgery.
Ovarian ultrasonography plus morphologic scoring comprise a comprehensive approach
The recently published recommendations of the First International Consensus Conference report on adnexal masses are summarized in TABLE 1.8 The expert panel reviewed the evidence and concluded that effective ultrasonography strategies exist and are well validated, and that low-risk asymptomatic ovarian cysts do not require surgical removal.

While no single ultrasonographic findingcan differentiate a benign from a malignant mass, morphologic scoring systems improve our ability to estimate a tumor’s malignant potential. In the United States, most practitioners in women’s health have ready access to gynecologic ultrasonography, but individual training and proficiency vary. Since not everyone is an expert sonographer, it is useful to employ an objective strategy when evaluating an ovarian tumor. The focus of a comprehensive ovarian ultrasonography is to recognize morphologic patterns that reflect a tumor’s malignant potential. While tumor volume is useful, tumor morphology is the most prognostic feature.
International Ovarian Tumor Analysis group
The International Ovarian Tumor Analysis (IOTA) group has published extensively on sonographic definitions and patterns that categorize tumors based on appearance.9 Simple rules and the ADNEX risk model are 2 of the group’s approaches (FIGURE 1).10,11 Both methods have been validated as effective for differentiating benign from malignant ovarian tumors, but neither has been used to study serial changes in ovarian morphology.

Regardless of the strategy employed, 25% of ovarian ultrasonography evaluations will be interpreted as “indeterminate” or “risk unknown.”10 The IOTA strategies have been successfully used in Europe for years, but they have not yet been studied or adopted in the United States.
Kentucky morphology index
The morphology index (MI) from the University of Kentucky is an ultrasonography-based scoring system that combines tumor volume and tumor structure into a simple and effective index with a score ranging from 0 to 10 (FIGURE 2).12 A rising Kentucky MI score has a linear and predictable increase in the risk of ovarian malignancy. In a review of almost 40,000 sonograms, 85% of the malignancies had an MI score of 5 or greater (TABLE 2).12 Using this as a cutoff, the sensitivity and specificity for predicting malignancy was 86% and 98%, respectively.12


When comparing the ADNEX risk model with the Kentucky MI, investigators reviewed 45,000 ultrasound results and found that the majority of cancers were categorized by the ADNEX model in the lowest 4 of the 10 risk-of-malignancy groups, compared with only 15% for the MI.13 This clustering or skew is potentially problematic, since we expect higher scores to be more predictive of cancer than lower scores. It also infers that the ADNEX model may not be useful in serial surveillance strategies. Moreover, the ADNEX model identified only 30% of early stage cancers compared with identification of 80% with use of the MI.13
Serial ultrasonography
Serial ultrasonography is a concept similar to any longitudinal biomarker evaluation. In the United Kingdom Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) program, the Risk of Ovarian Cancer Algorithm (ROCA) employs serial measurements of cancer antigen 125 (CA 125) to improve cancer detection. Serial ultrasonography similarly can be applied to better characterize a tumor’s physiology as well as its morphology. Over time, malignant ovarian tumors grow naturally in volume and complexity, and they do so at a rate faster than nonmalignant tumors. If this physical change can be measured objectively with ultrasonography, then serial sonography becomes a valuable diagnostic aid.
In comparing serial MI scores with clinical outcomes, studies have shown that malignant tumors exhibit a rapid increase, nonmalignant tumors have a stable or gradual rise, and resolving cysts show a decrease in MI score over time (FIGURE 3).12 An increase in the MI score of 1 or more per month (≥1 per month) is concerning for malignancy, and surgical removal should be considered. If the MI score of an asymptomatic ovarian tumor does not increase by 1 per month, it can be surveilled with intermittent ultrasonography.

Read about evaluating with serum biomarkers and sonography.
Serum biomarkers useful for determining risk, need for referral
Serum biomarkers can be used to complement an ultrasonographic evaluation. They are particularly useful when surgery is recommended but the sonographic evaluation is indeterminate for malignancy risk. Many serum biomarkers are commonly used for the preoperative evaluation of an ovarian tumor or for surveillance of a malignancy following diagnosis (TABLE 3).

CA 125 is the most commonly ordered serum biomarker test for ovarian cancer. It is estimated that three‐quarters of CA 125 tests are ordered for preoperative use, which is not the US Food and Drug Administration (FDA) approved indication. Despite our clinical reliance on CA 125 as a diagnostic test prior to surgery, its utility is limited because of a low sensitivity for predicting cancer in premenopausal women and early stage disease.14,15 CA 125 specificity also varies widely, depending on patient age and other clinical factors, ranging from as low as 26% in premenopausal women to as high as 100% in postmenopausal women.16 Because CA 125 often is negative when early stage cancer is present, or positive when cancer is not, it is not recommended for preoperative use for determining whether an ovarian tumor is malignant or whether surgery is indicated. CA 125 should be used to monitor patients with a known ovarian malignancy.
The new triage serum biomarkers, Overa, Ova1, and ROMA (Risk of Ovarian Malignancy Algorithm), are FDA cleared for preoperative use to help determine whether a woman needing surgery for an ovarian mass should be referred to a gynecologic oncologist.17–20 These tests should not be used to decide if surgery is indicated, but rather should be considered when the decision for surgery has already been made but the malignancy risk is unknown. A woman with a “high risk” result should be referred to a gynecologic oncologist, while one with a “low risk” score is very unlikely to have a malignancy and referral to a specialist is not necessary. TABLE 4 lists a comparison of the relative performance of these serum biomarkers.14,15,17–20 There are no published data on the use of serial triage biomarkers.

How to evaluate an ovarian tumor
Approximately 65% of the time, ovarian cystic tumors can be identified accurately as low risk based on the initial sonographic evaluation (TABLE 5). In this scenario, the risk of malignancy is very low (<1%), no secondary testing is needed, and no surgery is recommended.1,3,21

About 10% of tumors are expected to have a high-risk morphology on ultrasonography, where the risk of malignancy exceeds 25% and referral to a gynecologic oncologist is required.
The remaining 25% of tumors cannot be accurately classified with a single ultrasonographic evaluation and are considered indeterminate.22 Indeterminate tumors require secondary testing to ascertain whether surgery is indicated. Secondary testing may consist of serial ultrasonography, magnetic resonance imaging (MRI), or serum triage biomarker testing if the decision for surgery has been made.
A 2-step process is recommended for evaluating an ovarian tumor.
Step 1. Perform a detailed ultrasonography study using a morphology-based system. Classify the tumor as:
- low risk (65%): unilocular, simple septate, no flow on color Doppler
- simple rules: benign
- MI score 0–3
- no secondary testing; no referral is recommended
- high risk (10%): irregular, mostly solid, papillary projections, very strong flow on color Doppler
- simple rules: malignant
- MI score ≥5
- no secondary testing; refer to a gynecologic oncologist
- indeterminate (25%): partly solid, small wall abnormalities, minimal or moderate flow on color Doppler
- simple rules: both M and B rules apply or no rule applies
- MI score usually 4–6
- perform secondary testing (step 2).
Step 2. Perform secondary testing as follows:
- serum triage biomarkers if surgery is planned (Ova1, ROMA, Overa), or
- MRI, or
- serial sonography.
The 3 case scenarios that follow illustrate how the ovarian tumor evaluation process may be applied in clinical practice, with referral to a gynecologic oncologist as appropriate.
CASE 1 Postmenopausal woman with urinary symptoms and pelvic pressure
A 61-year-old woman is referred with a newly identified ovarian tumor. She has had 1 month of urinary urgency, frequency, and pelvic pressure, but she denies vaginal bleeding or fever. She has no family history of cancer. The referring physician included results of a serum CA 125 (48 U/mL; normal, ≤35 U/mL). A pelvic examination reveals a palpable, irregular mass in the anterior pelvis with limited mobility.
What would be your next step in the evaluation of this patient?
Start with ultrasonography
Step 1. Perform pelvic ultrasonography. In this patient, transvaginal sonography revealed a 6-cm (volume, 89 mL) mostly solid tumor (FIGURE 4). The maximum solid diameter of the tumor was 4.0 cm. There was a 20-mL pocket of pelvic ascites.

Results of morphology-based classification were as follows:
- simple rules: M1 and M5 positive; B rules: negative (malignant; high risk)
- ADNEX: 51.6% risk of malignancy (high risk)
- MI: 7 (high risk).
Step 2. Consider secondary testing. In this case, no secondary testing was recommended. Treatment plan. The patient was referred to a gynecologic oncologist for surgery and was found to have a stage IIA serous ovarian carcinoma.
CASE 2 Woman with history of pelvic symptoms and worsening pain
A 46-year-old woman presents with worsening pelvic pain over the last month. She has a long-standing history of pelvic pain, dysmenorrhea, and dyspareunia from suspected endometriosis. She has no family history of cancer. The referring physician included the following serum biomarker results: CA 125, 48 U/mL (normal, ≤35 U/mL), and HE4, 60 pM (normal, ≤150 pM). On pelvic examination, there is a palpable mass with limited mobility in the posterior cul-de-sac.
Based on the patient’s available history, physical examination, and biomarker information, how would you proceed?
Follow the 2-step process
Step 1. Perform pelvic ultrasonography. Transvaginal sonography revealed a 6-cm (volume, 89 mL) partly solid tumor with regular internal borders (FIGURE 5). The maximum solid diameter of the tumor was 4.5 cm. There was no pelvic ascites.

Morphology classification was as follows:
- simple rules: M5 equivocal; B4 positive (indeterminate risk)
- ADNEX: 42.7% risk of malignancy (high risk)
- MI: 6 (indeterminate risk).
Step 2. Secondary testing was recommended for this patient. Test results were:
- repeat ultrasonography in 4 weeks with MI of 7 (volume score increase from 2 to 3, structure score unchanged at 4). Change in MI score +1 per month (high risk)
- Overa: 5.2 (high risk)
- ROMA: 11.8% (low risk).
Treatment plan. The patient was referred to a gynecologic oncologist because of an increasing MI score on serial sonography. Surgery revealed a stage IA grade 2 endometrioid adenocarcinoma of the ovary with surrounding endometriosis.
Read about treating a woman with postmenstrual bleeding.
CASE 3 Woman with postmenopausal bleeding seeks medical care
A 62-year-old woman is referred with new-onset postmenopausal spotting for 1 month. She was recently prescribed antibiotics for diverticulitis. She has no family history of cancer. The referring physician included the results of a serum CA 125, which was 48 U/mL (normal, ≤35 U/mL). On pelvic examination, a mobile cystic mass is noted in the posterior cul-de-sac.
Use the stepwise protocol to sort out findings
Step 1. Pelvic ultrasonography. Transvaginal sonography suggested the presence of an endometrial polyp and revealed a 6-cm (volume, 89 mL) septate ovarian cyst (FIGURE 6).

Based on morphology classification, risk was categorized as:
- simple rules: M rules negative; B2, B4, B5 positive (benign; low risk)
- ADNEX: 2.9% risk of malignancy (low risk)
- MI: 2 (low risk).
Step 2. No secondary testing was recommended in this case.
Treatment plan. The patient’s gynecologist performed a hysteroscopic polypectomy that revealed no cancer. Serial monitoring was recommended for the low-risk ovarian cyst. The next ultrasonography scan, at 6 months, was unchanged; a subsequent scan was ordered for 12 months later, and at that time the cyst had resolved.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
A meaningful evolution has occurred over the past 30 years in the evaluation of ovarian tumors. In the 1980s, any palpable ovarian tumor was recommended for surgical removal.1 In the early 2000s, studies showed that unilocular cysts were at very low risk for malignancy, and surveillance was recommended.2 In the following decade, septate cysts were added to the list of ovarian tumors unlikely to be malignant, and nonsurgical therapy was suggested.3 It is estimated that 10% of women will undergo surgery for an adnexal mass in their lifetime, despite the fact that only 1 in 6 (13%–21%) of these masses is found to be malignant.4,5
A comprehensive, morphology-based pelvic ultrasonography is the first and most important step in evaluating an ovarian tumor’s risk of malignancy to determine whether surgery or surveillance is required.
Ovarian cancer continues to be the leading cause of gynecologic cancer death. Despite achieving superior surgical and cancer outcomes, a gynecologic oncologist performs only 40% of the initial ovarian cancer surgeries.6 Premenopausal and menopausal ovarian tumors are different in cause and consequence. Only 15% of premenopausal tumors are malignant, most commonly germ cell tumors, borderline ovarian tumors, and epithelial ovarian cancers. Tumors in menopausal women are less common but are more likely to be malignant. In actuality, up to 50% of tumors in this population are malignant. The most common of these malignancies are epithelial ovarian cancers, cancers metastatic to the ovary, and malignant stromal tumors.
Effective and evidence-based preoperative evaluations are available to help the clinician estimate a tumor’s risk of malignancy and determine which tumors are appropriate for referral to a specialist for surgery.
The actual incidence and prevalence of ovarian tumors are not known. From a review of almost 40,000 ultrasonography scans performed in the University of Kentucky Ovarian Cancer Screening Program, the estimated incidence and prevalence of ovarian abnormalities are 8.2 per 100 women annuallyand 17%, respectively.7 Seventy percent of these abnormalities have a unilocular or simple septate morphology and are at low risk for malignancy.7 The remaining 30% of abnormalities are high risk, although this represents only 9% of the total population evaluated. Since the vast majority of these abnormalities are expected to be asymptomatic, most will go unrecognized in the general population. For women who have an ovarian abnormality on ultrasonography, the majority will be at low risk for malignancy and will not require surgery.
Ovarian ultrasonography plus morphologic scoring comprise a comprehensive approach
The recently published recommendations of the First International Consensus Conference report on adnexal masses are summarized in TABLE 1.8 The expert panel reviewed the evidence and concluded that effective ultrasonography strategies exist and are well validated, and that low-risk asymptomatic ovarian cysts do not require surgical removal.

While no single ultrasonographic findingcan differentiate a benign from a malignant mass, morphologic scoring systems improve our ability to estimate a tumor’s malignant potential. In the United States, most practitioners in women’s health have ready access to gynecologic ultrasonography, but individual training and proficiency vary. Since not everyone is an expert sonographer, it is useful to employ an objective strategy when evaluating an ovarian tumor. The focus of a comprehensive ovarian ultrasonography is to recognize morphologic patterns that reflect a tumor’s malignant potential. While tumor volume is useful, tumor morphology is the most prognostic feature.
International Ovarian Tumor Analysis group
The International Ovarian Tumor Analysis (IOTA) group has published extensively on sonographic definitions and patterns that categorize tumors based on appearance.9 Simple rules and the ADNEX risk model are 2 of the group’s approaches (FIGURE 1).10,11 Both methods have been validated as effective for differentiating benign from malignant ovarian tumors, but neither has been used to study serial changes in ovarian morphology.

Regardless of the strategy employed, 25% of ovarian ultrasonography evaluations will be interpreted as “indeterminate” or “risk unknown.”10 The IOTA strategies have been successfully used in Europe for years, but they have not yet been studied or adopted in the United States.
Kentucky morphology index
The morphology index (MI) from the University of Kentucky is an ultrasonography-based scoring system that combines tumor volume and tumor structure into a simple and effective index with a score ranging from 0 to 10 (FIGURE 2).12 A rising Kentucky MI score has a linear and predictable increase in the risk of ovarian malignancy. In a review of almost 40,000 sonograms, 85% of the malignancies had an MI score of 5 or greater (TABLE 2).12 Using this as a cutoff, the sensitivity and specificity for predicting malignancy was 86% and 98%, respectively.12


When comparing the ADNEX risk model with the Kentucky MI, investigators reviewed 45,000 ultrasound results and found that the majority of cancers were categorized by the ADNEX model in the lowest 4 of the 10 risk-of-malignancy groups, compared with only 15% for the MI.13 This clustering or skew is potentially problematic, since we expect higher scores to be more predictive of cancer than lower scores. It also infers that the ADNEX model may not be useful in serial surveillance strategies. Moreover, the ADNEX model identified only 30% of early stage cancers compared with identification of 80% with use of the MI.13
Serial ultrasonography
Serial ultrasonography is a concept similar to any longitudinal biomarker evaluation. In the United Kingdom Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) program, the Risk of Ovarian Cancer Algorithm (ROCA) employs serial measurements of cancer antigen 125 (CA 125) to improve cancer detection. Serial ultrasonography similarly can be applied to better characterize a tumor’s physiology as well as its morphology. Over time, malignant ovarian tumors grow naturally in volume and complexity, and they do so at a rate faster than nonmalignant tumors. If this physical change can be measured objectively with ultrasonography, then serial sonography becomes a valuable diagnostic aid.
In comparing serial MI scores with clinical outcomes, studies have shown that malignant tumors exhibit a rapid increase, nonmalignant tumors have a stable or gradual rise, and resolving cysts show a decrease in MI score over time (FIGURE 3).12 An increase in the MI score of 1 or more per month (≥1 per month) is concerning for malignancy, and surgical removal should be considered. If the MI score of an asymptomatic ovarian tumor does not increase by 1 per month, it can be surveilled with intermittent ultrasonography.

Read about evaluating with serum biomarkers and sonography.
Serum biomarkers useful for determining risk, need for referral
Serum biomarkers can be used to complement an ultrasonographic evaluation. They are particularly useful when surgery is recommended but the sonographic evaluation is indeterminate for malignancy risk. Many serum biomarkers are commonly used for the preoperative evaluation of an ovarian tumor or for surveillance of a malignancy following diagnosis (TABLE 3).

CA 125 is the most commonly ordered serum biomarker test for ovarian cancer. It is estimated that three‐quarters of CA 125 tests are ordered for preoperative use, which is not the US Food and Drug Administration (FDA) approved indication. Despite our clinical reliance on CA 125 as a diagnostic test prior to surgery, its utility is limited because of a low sensitivity for predicting cancer in premenopausal women and early stage disease.14,15 CA 125 specificity also varies widely, depending on patient age and other clinical factors, ranging from as low as 26% in premenopausal women to as high as 100% in postmenopausal women.16 Because CA 125 often is negative when early stage cancer is present, or positive when cancer is not, it is not recommended for preoperative use for determining whether an ovarian tumor is malignant or whether surgery is indicated. CA 125 should be used to monitor patients with a known ovarian malignancy.
The new triage serum biomarkers, Overa, Ova1, and ROMA (Risk of Ovarian Malignancy Algorithm), are FDA cleared for preoperative use to help determine whether a woman needing surgery for an ovarian mass should be referred to a gynecologic oncologist.17–20 These tests should not be used to decide if surgery is indicated, but rather should be considered when the decision for surgery has already been made but the malignancy risk is unknown. A woman with a “high risk” result should be referred to a gynecologic oncologist, while one with a “low risk” score is very unlikely to have a malignancy and referral to a specialist is not necessary. TABLE 4 lists a comparison of the relative performance of these serum biomarkers.14,15,17–20 There are no published data on the use of serial triage biomarkers.

How to evaluate an ovarian tumor
Approximately 65% of the time, ovarian cystic tumors can be identified accurately as low risk based on the initial sonographic evaluation (TABLE 5). In this scenario, the risk of malignancy is very low (<1%), no secondary testing is needed, and no surgery is recommended.1,3,21

About 10% of tumors are expected to have a high-risk morphology on ultrasonography, where the risk of malignancy exceeds 25% and referral to a gynecologic oncologist is required.
The remaining 25% of tumors cannot be accurately classified with a single ultrasonographic evaluation and are considered indeterminate.22 Indeterminate tumors require secondary testing to ascertain whether surgery is indicated. Secondary testing may consist of serial ultrasonography, magnetic resonance imaging (MRI), or serum triage biomarker testing if the decision for surgery has been made.
A 2-step process is recommended for evaluating an ovarian tumor.
Step 1. Perform a detailed ultrasonography study using a morphology-based system. Classify the tumor as:
- low risk (65%): unilocular, simple septate, no flow on color Doppler
- simple rules: benign
- MI score 0–3
- no secondary testing; no referral is recommended
- high risk (10%): irregular, mostly solid, papillary projections, very strong flow on color Doppler
- simple rules: malignant
- MI score ≥5
- no secondary testing; refer to a gynecologic oncologist
- indeterminate (25%): partly solid, small wall abnormalities, minimal or moderate flow on color Doppler
- simple rules: both M and B rules apply or no rule applies
- MI score usually 4–6
- perform secondary testing (step 2).
Step 2. Perform secondary testing as follows:
- serum triage biomarkers if surgery is planned (Ova1, ROMA, Overa), or
- MRI, or
- serial sonography.
The 3 case scenarios that follow illustrate how the ovarian tumor evaluation process may be applied in clinical practice, with referral to a gynecologic oncologist as appropriate.
CASE 1 Postmenopausal woman with urinary symptoms and pelvic pressure
A 61-year-old woman is referred with a newly identified ovarian tumor. She has had 1 month of urinary urgency, frequency, and pelvic pressure, but she denies vaginal bleeding or fever. She has no family history of cancer. The referring physician included results of a serum CA 125 (48 U/mL; normal, ≤35 U/mL). A pelvic examination reveals a palpable, irregular mass in the anterior pelvis with limited mobility.
What would be your next step in the evaluation of this patient?
Start with ultrasonography
Step 1. Perform pelvic ultrasonography. In this patient, transvaginal sonography revealed a 6-cm (volume, 89 mL) mostly solid tumor (FIGURE 4). The maximum solid diameter of the tumor was 4.0 cm. There was a 20-mL pocket of pelvic ascites.

Results of morphology-based classification were as follows:
- simple rules: M1 and M5 positive; B rules: negative (malignant; high risk)
- ADNEX: 51.6% risk of malignancy (high risk)
- MI: 7 (high risk).
Step 2. Consider secondary testing. In this case, no secondary testing was recommended. Treatment plan. The patient was referred to a gynecologic oncologist for surgery and was found to have a stage IIA serous ovarian carcinoma.
CASE 2 Woman with history of pelvic symptoms and worsening pain
A 46-year-old woman presents with worsening pelvic pain over the last month. She has a long-standing history of pelvic pain, dysmenorrhea, and dyspareunia from suspected endometriosis. She has no family history of cancer. The referring physician included the following serum biomarker results: CA 125, 48 U/mL (normal, ≤35 U/mL), and HE4, 60 pM (normal, ≤150 pM). On pelvic examination, there is a palpable mass with limited mobility in the posterior cul-de-sac.
Based on the patient’s available history, physical examination, and biomarker information, how would you proceed?
Follow the 2-step process
Step 1. Perform pelvic ultrasonography. Transvaginal sonography revealed a 6-cm (volume, 89 mL) partly solid tumor with regular internal borders (FIGURE 5). The maximum solid diameter of the tumor was 4.5 cm. There was no pelvic ascites.

Morphology classification was as follows:
- simple rules: M5 equivocal; B4 positive (indeterminate risk)
- ADNEX: 42.7% risk of malignancy (high risk)
- MI: 6 (indeterminate risk).
Step 2. Secondary testing was recommended for this patient. Test results were:
- repeat ultrasonography in 4 weeks with MI of 7 (volume score increase from 2 to 3, structure score unchanged at 4). Change in MI score +1 per month (high risk)
- Overa: 5.2 (high risk)
- ROMA: 11.8% (low risk).
Treatment plan. The patient was referred to a gynecologic oncologist because of an increasing MI score on serial sonography. Surgery revealed a stage IA grade 2 endometrioid adenocarcinoma of the ovary with surrounding endometriosis.
Read about treating a woman with postmenstrual bleeding.
CASE 3 Woman with postmenopausal bleeding seeks medical care
A 62-year-old woman is referred with new-onset postmenopausal spotting for 1 month. She was recently prescribed antibiotics for diverticulitis. She has no family history of cancer. The referring physician included the results of a serum CA 125, which was 48 U/mL (normal, ≤35 U/mL). On pelvic examination, a mobile cystic mass is noted in the posterior cul-de-sac.
Use the stepwise protocol to sort out findings
Step 1. Pelvic ultrasonography. Transvaginal sonography suggested the presence of an endometrial polyp and revealed a 6-cm (volume, 89 mL) septate ovarian cyst (FIGURE 6).

Based on morphology classification, risk was categorized as:
- simple rules: M rules negative; B2, B4, B5 positive (benign; low risk)
- ADNEX: 2.9% risk of malignancy (low risk)
- MI: 2 (low risk).
Step 2. No secondary testing was recommended in this case.
Treatment plan. The patient’s gynecologist performed a hysteroscopic polypectomy that revealed no cancer. Serial monitoring was recommended for the low-risk ovarian cyst. The next ultrasonography scan, at 6 months, was unchanged; a subsequent scan was ordered for 12 months later, and at that time the cyst had resolved.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Barber HR, Graber EA. The PMPO syndrome (postmenopausal palpable ovary syndrome). Obstet Gynecol. 1971;38(6):921–923.
- Modesitt SC, Pavlik EJ, Ueland FR, DePriest PD, Kryscio RJ, van Nagell JR Jr. Risk of malignancy in unilocular ovarian cystic tumors less than 10 centimeters in diameter. Obstet Gynecol. 2003;102(3):594–599.
- Saunders BA, Podzielinski I, Ware RA, et al. Risk of malignancy in sonographically confirmed septated cystic ovarian tumors. Gynecol Oncol. 2010;118(3):278–282.
- Moore RG, McMeekin DS, Brown AK, et al. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol Oncol. 2009;112(1):40–46.
- Jordan SM, Bristow RE. Ovarian cancer biomarkers as diagnostic triage tests. Current Biomarker Findings. 2013;3:35–42.
- Giede KC, Kieser K, Dodge J, Rosen B. Who should operate on patients with ovarian cancer? An evidence-based review. Gynecol Oncol. 2005;99(2):447–461.
- Pavlik EJ, Ueland FR, Miller RW, et al. Frequency and disposition of ovarian abnormalities followed with serial transvaginal ultrasonography. Obstet Gynecol. 2013;122(2 pt 1):210–217.
- Glanc P, Benacerraf B, Bourne T, et al. First International Consensus Report on adnexal masses: management recommendations. J Ultrasound Med. 2017;36(5):849–863.
- Timmerman D, Valentin L, Bourne TGH, Collins WP, Verrelst H, Vergote I; International Ovarian Tumor Analysis (IOTA) Group. Terms, definitions and measurements to describe the sonographic features of adnexal tumors: a consensus opinion from the International Ovarian Tumor Analysis (IOTA) group. Ultrasound Obstet Gynecol. 2000;6(5):500–505.
- Timmerman D, Testa AC, Bourne T, et al. Simple ultrasound-based rules for the diagnosis of ovarian cancer. Ultrasound Obstet Gynecol. 2008;31(6):681–690.
- Van Calser B, Van Hoorde K, Valentin L, et al. Evaluating the risk of ovarian cancer before surgery using the ADNEX model to differentiate between benign, borderline, early and advanced stage invasive, and secondary metastatic tumours: prospective multicentre diagnostic study. BMJ. 2014;349:g5920.
- Elder JW, Pavlik EJ, Long A, et al. Serial ultrasonographic evaluation of ovarian abnormalities with a morphology index. Gynecol Oncol. 2014;135(1):8–12.
- Lefringhouse J, Ueland F, Ore R, et al. Comparing 2 sonographic scoring systems for distinguishing benign from malignant ovarian tumors [abstract]. Gynecol Oncol. 2016;141(suppl 1):57.
- Bast RC Jr, Klug TL, St John E, et al. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N Engl J Med. 1983;309(15):883–887.
- Jacobs I, Bast RC Jr. The CA 125 tumour-associated antigen: a review of the literature. Hum Reprod. 1989;4(1):1–12.
- Myers ER, Bastian LA, Havrilesky LJ, et al. Management of adnexal mass. Agency for Healthcare Research and Quality. https://archive.ahrq.gov/downloads/pub/evidence/pdf/adnexal/adnexal.pdf. Published February 2006. Accessed May 15, 2018.
- Coleman RL, Herzog TJ, Chan DW, et al. Validation of a second-generation multivariate index assay for malignancy risk of adnexal masses. Am J Obstet Gynecol. 2016;215(1):82.e1–e11.
- Ueland FR, Desimone CP, Seamon LG, et al. Effectiveness of a multivariate index assay in the preoperative assessment of ovarian tumors. Obstet Gynecol. 2011;117(6):1289–1297.
- Bristow RE, Smith A, Zhang Z, et al. Ovarian malignancy risk stratification of the adnexal mass using a multivariate index assay. Gynecol Oncol. 2013;128(2):252–259.
- Moore RG, McMeekin DS, Brown AK, et al. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol Oncol. 2009;112(1):40–46.
- Valentin L, Ameye L, Franchi D, et al. Risk of malignancy in unilocular cysts: a study of 1148 adnexal masses classified as unilocular cysts on transvaginal ultrasound and review of the literature. Ultrasound Obstet Gynecol. 2013;41(1):80–89.
- Timmerman D, Ameye L, Fischerova D, et al. Simple ultrasound rules to distinguish between benign and malignant adnexal masses before surgery: prospective validation by IOTA group. BMJ. 2010;341:c6839.
- Barber HR, Graber EA. The PMPO syndrome (postmenopausal palpable ovary syndrome). Obstet Gynecol. 1971;38(6):921–923.
- Modesitt SC, Pavlik EJ, Ueland FR, DePriest PD, Kryscio RJ, van Nagell JR Jr. Risk of malignancy in unilocular ovarian cystic tumors less than 10 centimeters in diameter. Obstet Gynecol. 2003;102(3):594–599.
- Saunders BA, Podzielinski I, Ware RA, et al. Risk of malignancy in sonographically confirmed septated cystic ovarian tumors. Gynecol Oncol. 2010;118(3):278–282.
- Moore RG, McMeekin DS, Brown AK, et al. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol Oncol. 2009;112(1):40–46.
- Jordan SM, Bristow RE. Ovarian cancer biomarkers as diagnostic triage tests. Current Biomarker Findings. 2013;3:35–42.
- Giede KC, Kieser K, Dodge J, Rosen B. Who should operate on patients with ovarian cancer? An evidence-based review. Gynecol Oncol. 2005;99(2):447–461.
- Pavlik EJ, Ueland FR, Miller RW, et al. Frequency and disposition of ovarian abnormalities followed with serial transvaginal ultrasonography. Obstet Gynecol. 2013;122(2 pt 1):210–217.
- Glanc P, Benacerraf B, Bourne T, et al. First International Consensus Report on adnexal masses: management recommendations. J Ultrasound Med. 2017;36(5):849–863.
- Timmerman D, Valentin L, Bourne TGH, Collins WP, Verrelst H, Vergote I; International Ovarian Tumor Analysis (IOTA) Group. Terms, definitions and measurements to describe the sonographic features of adnexal tumors: a consensus opinion from the International Ovarian Tumor Analysis (IOTA) group. Ultrasound Obstet Gynecol. 2000;6(5):500–505.
- Timmerman D, Testa AC, Bourne T, et al. Simple ultrasound-based rules for the diagnosis of ovarian cancer. Ultrasound Obstet Gynecol. 2008;31(6):681–690.
- Van Calser B, Van Hoorde K, Valentin L, et al. Evaluating the risk of ovarian cancer before surgery using the ADNEX model to differentiate between benign, borderline, early and advanced stage invasive, and secondary metastatic tumours: prospective multicentre diagnostic study. BMJ. 2014;349:g5920.
- Elder JW, Pavlik EJ, Long A, et al. Serial ultrasonographic evaluation of ovarian abnormalities with a morphology index. Gynecol Oncol. 2014;135(1):8–12.
- Lefringhouse J, Ueland F, Ore R, et al. Comparing 2 sonographic scoring systems for distinguishing benign from malignant ovarian tumors [abstract]. Gynecol Oncol. 2016;141(suppl 1):57.
- Bast RC Jr, Klug TL, St John E, et al. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N Engl J Med. 1983;309(15):883–887.
- Jacobs I, Bast RC Jr. The CA 125 tumour-associated antigen: a review of the literature. Hum Reprod. 1989;4(1):1–12.
- Myers ER, Bastian LA, Havrilesky LJ, et al. Management of adnexal mass. Agency for Healthcare Research and Quality. https://archive.ahrq.gov/downloads/pub/evidence/pdf/adnexal/adnexal.pdf. Published February 2006. Accessed May 15, 2018.
- Coleman RL, Herzog TJ, Chan DW, et al. Validation of a second-generation multivariate index assay for malignancy risk of adnexal masses. Am J Obstet Gynecol. 2016;215(1):82.e1–e11.
- Ueland FR, Desimone CP, Seamon LG, et al. Effectiveness of a multivariate index assay in the preoperative assessment of ovarian tumors. Obstet Gynecol. 2011;117(6):1289–1297.
- Bristow RE, Smith A, Zhang Z, et al. Ovarian malignancy risk stratification of the adnexal mass using a multivariate index assay. Gynecol Oncol. 2013;128(2):252–259.
- Moore RG, McMeekin DS, Brown AK, et al. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol Oncol. 2009;112(1):40–46.
- Valentin L, Ameye L, Franchi D, et al. Risk of malignancy in unilocular cysts: a study of 1148 adnexal masses classified as unilocular cysts on transvaginal ultrasound and review of the literature. Ultrasound Obstet Gynecol. 2013;41(1):80–89.
- Timmerman D, Ameye L, Fischerova D, et al. Simple ultrasound rules to distinguish between benign and malignant adnexal masses before surgery: prospective validation by IOTA group. BMJ. 2010;341:c6839.
Complete MUS mesh removal not linked to incontinence
SAN FRANCISCO – Stress urinary incontinence (SUI) following removal of a mid-urethral sling (MUS) mesh is not necessarily associated with increased risk of postsurgical urinary incontinence, according to a retrospective study at a high-volume, tertiary medical center.
Follow-up procedures occurred more often in women with preoperative urodynamic SUI and less often in women who were stress continent.
Among women who were stress continent, obesity and postmenopausal status were linked to postsurgical SUI. There was no association between postsurgical SUI and the extent of mesh excision or prior revisions.
The study grew out of observations that SUI occurred less often than expected.
“There’s an increasing recognition of complications related to synthetic MUS,” said Janine Oliver, MD, who presented the study at the annual meeting of the American Urological Association. “As mesh removal procedures were being performed, we assumed that the majority of patients, if not all, would be incontinent afterward, since we were removing the sling that was put in to fix stress incontinence in most cases.”
In a patient who would benefit from a complete mesh removal, “the fear that it may lead to a higher risk of urinary incontinence is not a good justification to not do it,” Dr. Oliver said. She did note, however, that the procedures were done by specialists, so findings may not be applicable to general practitioners.
The study was performed while Dr. Oliver was a fellow at the University of California, Los Angeles. She is now with the division of urology at the University of Colorado, Anschutz, in Aurora.
Dr. Oliver and her colleagues analyzed data from 233 patients who underwent MUS excision at UCLA for MUS-related complications during 2013-2015, and who had at least 3 months of follow-up. The average patient age was 55.4 years; an average of 5.4 years passed between the placement and excision of MUS. The mean body mass index was 28.9, and mean follow-up was 23.5 months.
A total of 84% of patients underwent a total excision; 45% of MUS were retropubic, 35% were transobturator, 10% were single incision, and 10% were multiple incision.
Nearly half (49%) of patients required a second procedure for SUI, such as bulking agent injection, bladder neck suspension, or repeat sling procedure.
In the entire cohort, multivariate analyses found significant associations between heightened risk of postoperative SUI and increasing time to MUS excision (odds ratio, 1.16; 95% confidence interval, 1.03-1.30), total MUS excision (OR, 4.14; 95% CI, 1.38-12.37), and preoperative urodynamic SUI (OR, 4.66; 95% CI, 2.13-10.19).
Of 51 patients who had preoperative urodynamic SUI, 39 (76%) ultimately underwent another surgery. Although increased time to MUS excision and total mesh removal were associated with urinary incontinence in this group in univariate analyses, they were no longer significant following a multivariate analysis.
Of 140 patients with a negative preoperative urodynamic testing for SUI, 59 (42%) went on to have another SUI procedure. After multivariate analysis, the only risk factors for urinary incontinence were obesity (OR, 4.74; 95% CI, 1.73-13.02) and postmenopausal status (OR, 3.78; 95% CI, 1.16-12.33).
“I think there’s a lot of fear, even among urologists and specialists who see these problems, that complete mesh removal is associated with a higher risk of complications and a higher risk of incontinence,” said Dr. Oliver. “These data would suggest that, in certain subgroups, that’s not true. The risks factors that we identified in a multivariate analysis were being obese and being postmenopausal, but not complete mesh removal.”
The study received no external funding. Dr. Oliver reported having no financial conflicts of interest.
SOURCE: Oliver J et al. AUA Annual Meeting. Abstract PD05-10.
SAN FRANCISCO – Stress urinary incontinence (SUI) following removal of a mid-urethral sling (MUS) mesh is not necessarily associated with increased risk of postsurgical urinary incontinence, according to a retrospective study at a high-volume, tertiary medical center.
Follow-up procedures occurred more often in women with preoperative urodynamic SUI and less often in women who were stress continent.
Among women who were stress continent, obesity and postmenopausal status were linked to postsurgical SUI. There was no association between postsurgical SUI and the extent of mesh excision or prior revisions.
The study grew out of observations that SUI occurred less often than expected.
“There’s an increasing recognition of complications related to synthetic MUS,” said Janine Oliver, MD, who presented the study at the annual meeting of the American Urological Association. “As mesh removal procedures were being performed, we assumed that the majority of patients, if not all, would be incontinent afterward, since we were removing the sling that was put in to fix stress incontinence in most cases.”
In a patient who would benefit from a complete mesh removal, “the fear that it may lead to a higher risk of urinary incontinence is not a good justification to not do it,” Dr. Oliver said. She did note, however, that the procedures were done by specialists, so findings may not be applicable to general practitioners.
The study was performed while Dr. Oliver was a fellow at the University of California, Los Angeles. She is now with the division of urology at the University of Colorado, Anschutz, in Aurora.
Dr. Oliver and her colleagues analyzed data from 233 patients who underwent MUS excision at UCLA for MUS-related complications during 2013-2015, and who had at least 3 months of follow-up. The average patient age was 55.4 years; an average of 5.4 years passed between the placement and excision of MUS. The mean body mass index was 28.9, and mean follow-up was 23.5 months.
A total of 84% of patients underwent a total excision; 45% of MUS were retropubic, 35% were transobturator, 10% were single incision, and 10% were multiple incision.
Nearly half (49%) of patients required a second procedure for SUI, such as bulking agent injection, bladder neck suspension, or repeat sling procedure.
In the entire cohort, multivariate analyses found significant associations between heightened risk of postoperative SUI and increasing time to MUS excision (odds ratio, 1.16; 95% confidence interval, 1.03-1.30), total MUS excision (OR, 4.14; 95% CI, 1.38-12.37), and preoperative urodynamic SUI (OR, 4.66; 95% CI, 2.13-10.19).
Of 51 patients who had preoperative urodynamic SUI, 39 (76%) ultimately underwent another surgery. Although increased time to MUS excision and total mesh removal were associated with urinary incontinence in this group in univariate analyses, they were no longer significant following a multivariate analysis.
Of 140 patients with a negative preoperative urodynamic testing for SUI, 59 (42%) went on to have another SUI procedure. After multivariate analysis, the only risk factors for urinary incontinence were obesity (OR, 4.74; 95% CI, 1.73-13.02) and postmenopausal status (OR, 3.78; 95% CI, 1.16-12.33).
“I think there’s a lot of fear, even among urologists and specialists who see these problems, that complete mesh removal is associated with a higher risk of complications and a higher risk of incontinence,” said Dr. Oliver. “These data would suggest that, in certain subgroups, that’s not true. The risks factors that we identified in a multivariate analysis were being obese and being postmenopausal, but not complete mesh removal.”
The study received no external funding. Dr. Oliver reported having no financial conflicts of interest.
SOURCE: Oliver J et al. AUA Annual Meeting. Abstract PD05-10.
SAN FRANCISCO – Stress urinary incontinence (SUI) following removal of a mid-urethral sling (MUS) mesh is not necessarily associated with increased risk of postsurgical urinary incontinence, according to a retrospective study at a high-volume, tertiary medical center.
Follow-up procedures occurred more often in women with preoperative urodynamic SUI and less often in women who were stress continent.
Among women who were stress continent, obesity and postmenopausal status were linked to postsurgical SUI. There was no association between postsurgical SUI and the extent of mesh excision or prior revisions.
The study grew out of observations that SUI occurred less often than expected.
“There’s an increasing recognition of complications related to synthetic MUS,” said Janine Oliver, MD, who presented the study at the annual meeting of the American Urological Association. “As mesh removal procedures were being performed, we assumed that the majority of patients, if not all, would be incontinent afterward, since we were removing the sling that was put in to fix stress incontinence in most cases.”
In a patient who would benefit from a complete mesh removal, “the fear that it may lead to a higher risk of urinary incontinence is not a good justification to not do it,” Dr. Oliver said. She did note, however, that the procedures were done by specialists, so findings may not be applicable to general practitioners.
The study was performed while Dr. Oliver was a fellow at the University of California, Los Angeles. She is now with the division of urology at the University of Colorado, Anschutz, in Aurora.
Dr. Oliver and her colleagues analyzed data from 233 patients who underwent MUS excision at UCLA for MUS-related complications during 2013-2015, and who had at least 3 months of follow-up. The average patient age was 55.4 years; an average of 5.4 years passed between the placement and excision of MUS. The mean body mass index was 28.9, and mean follow-up was 23.5 months.
A total of 84% of patients underwent a total excision; 45% of MUS were retropubic, 35% were transobturator, 10% were single incision, and 10% were multiple incision.
Nearly half (49%) of patients required a second procedure for SUI, such as bulking agent injection, bladder neck suspension, or repeat sling procedure.
In the entire cohort, multivariate analyses found significant associations between heightened risk of postoperative SUI and increasing time to MUS excision (odds ratio, 1.16; 95% confidence interval, 1.03-1.30), total MUS excision (OR, 4.14; 95% CI, 1.38-12.37), and preoperative urodynamic SUI (OR, 4.66; 95% CI, 2.13-10.19).
Of 51 patients who had preoperative urodynamic SUI, 39 (76%) ultimately underwent another surgery. Although increased time to MUS excision and total mesh removal were associated with urinary incontinence in this group in univariate analyses, they were no longer significant following a multivariate analysis.
Of 140 patients with a negative preoperative urodynamic testing for SUI, 59 (42%) went on to have another SUI procedure. After multivariate analysis, the only risk factors for urinary incontinence were obesity (OR, 4.74; 95% CI, 1.73-13.02) and postmenopausal status (OR, 3.78; 95% CI, 1.16-12.33).
“I think there’s a lot of fear, even among urologists and specialists who see these problems, that complete mesh removal is associated with a higher risk of complications and a higher risk of incontinence,” said Dr. Oliver. “These data would suggest that, in certain subgroups, that’s not true. The risks factors that we identified in a multivariate analysis were being obese and being postmenopausal, but not complete mesh removal.”
The study received no external funding. Dr. Oliver reported having no financial conflicts of interest.
SOURCE: Oliver J et al. AUA Annual Meeting. Abstract PD05-10.
REPORTING FROM THE AUA ANNUAL MEETING
Key clinical point: There was no association between postsurgical incontinence risk and complete mesh removal.
Major finding: In patients without presurgical urodynamic incontinence, only obesity (OR, 4.74) and postmenopausal status (OR, 3.78) were linked to incontinence risk.
Study details: A retrospective analysis of 233 patients.
Disclosures: The study received no external funding. Dr. Oliver reported having no financial conflicts of interest.
Source: Oliver J et al. AUA Annual Meeting. Abstract PD05-10.
ACOG updates guidance on pelvic organ prolapse
Using polypropylene mesh to augment surgical repair of anterior vaginal wall prolapse improves anatomic and some subjective outcomes, compared with native tissue repair, but it also comes with increased morbidity, according to new guidance from the American College of Obstetricians and Gynecologists.
(POP) to incorporate recent systematic review evidence.
When using polypropylene mesh for anterior POP repair, 11% of patients develop mesh erosion, of which 7% require surgical correction, according to the updated practice bulletin (Obstet Gynecol. 2017;130:e234-50).
“Referral to an obstetrician-gynecologist with appropriate training and experience, such as a female pelvic medicine and reconstructive surgery specialist, is recommended for surgical treatment of prolapse mesh complications,” ACOG and AUGS wrote.
The practice bulletin updates the recommendations on mesh based on a recent systematic review and meta-analysis that concluded that biological graft repair and absorbable mesh offered minimal benefits compared with native tissue repair, and did not significantly reduce rates of prolapse awareness or repeat surgery (Cochrane Database Syst Rev. 2016 Nov 30;11:CD004014).
Porcine dermis graft, which was used in most of the studies, did not significantly reduce rates of anterior prolapse recurrence compared with native tissue repair. Use of polypropylene mesh also tends to prolong operating times and causes more blood loss than native tissue anterior repair, and is associated with an elevated combined risk of stress urinary incontinence, mesh erosion, and repeat surgery for prolapse, the review concluded.
“Uterosacral and sacrospinous ligament suspension for apical POP with native tissue are equally effective surgical treatments of POP, with comparable anatomic, functional, and adverse outcomes,” the authors wrote in the practice bulletin.
Neither synthetic mesh nor biologic grafts improve outcomes of transvaginal repair of posterior vaginal wall prolapse, they added. As an alternative to surgery, most women can be successfully fitted with a pessary and clinicians should offer them this option, the practice bulletin stated. In up to 9% of cases, pessaries cause local devascularization or erosion, in which case they should be removed for 2-4 weeks while the patient undergoes local estrogen therapy.
Although POP is common and benign, symptomatic cases undermine quality of life by causing vaginal bulge and pressure and problems voiding, defecating, and during sexual activity. Consequently, about 300,000 women in the United States undergo surgery for POP every year. By 2050, population aging in the United States will lead to about a 50% rise in the number of women with POP, according to the practice bulletin.
Using polypropylene mesh to augment surgical repair of anterior vaginal wall prolapse improves anatomic and some subjective outcomes, compared with native tissue repair, but it also comes with increased morbidity, according to new guidance from the American College of Obstetricians and Gynecologists.
(POP) to incorporate recent systematic review evidence.
When using polypropylene mesh for anterior POP repair, 11% of patients develop mesh erosion, of which 7% require surgical correction, according to the updated practice bulletin (Obstet Gynecol. 2017;130:e234-50).
“Referral to an obstetrician-gynecologist with appropriate training and experience, such as a female pelvic medicine and reconstructive surgery specialist, is recommended for surgical treatment of prolapse mesh complications,” ACOG and AUGS wrote.
The practice bulletin updates the recommendations on mesh based on a recent systematic review and meta-analysis that concluded that biological graft repair and absorbable mesh offered minimal benefits compared with native tissue repair, and did not significantly reduce rates of prolapse awareness or repeat surgery (Cochrane Database Syst Rev. 2016 Nov 30;11:CD004014).
Porcine dermis graft, which was used in most of the studies, did not significantly reduce rates of anterior prolapse recurrence compared with native tissue repair. Use of polypropylene mesh also tends to prolong operating times and causes more blood loss than native tissue anterior repair, and is associated with an elevated combined risk of stress urinary incontinence, mesh erosion, and repeat surgery for prolapse, the review concluded.
“Uterosacral and sacrospinous ligament suspension for apical POP with native tissue are equally effective surgical treatments of POP, with comparable anatomic, functional, and adverse outcomes,” the authors wrote in the practice bulletin.
Neither synthetic mesh nor biologic grafts improve outcomes of transvaginal repair of posterior vaginal wall prolapse, they added. As an alternative to surgery, most women can be successfully fitted with a pessary and clinicians should offer them this option, the practice bulletin stated. In up to 9% of cases, pessaries cause local devascularization or erosion, in which case they should be removed for 2-4 weeks while the patient undergoes local estrogen therapy.
Although POP is common and benign, symptomatic cases undermine quality of life by causing vaginal bulge and pressure and problems voiding, defecating, and during sexual activity. Consequently, about 300,000 women in the United States undergo surgery for POP every year. By 2050, population aging in the United States will lead to about a 50% rise in the number of women with POP, according to the practice bulletin.
Using polypropylene mesh to augment surgical repair of anterior vaginal wall prolapse improves anatomic and some subjective outcomes, compared with native tissue repair, but it also comes with increased morbidity, according to new guidance from the American College of Obstetricians and Gynecologists.
(POP) to incorporate recent systematic review evidence.
When using polypropylene mesh for anterior POP repair, 11% of patients develop mesh erosion, of which 7% require surgical correction, according to the updated practice bulletin (Obstet Gynecol. 2017;130:e234-50).
“Referral to an obstetrician-gynecologist with appropriate training and experience, such as a female pelvic medicine and reconstructive surgery specialist, is recommended for surgical treatment of prolapse mesh complications,” ACOG and AUGS wrote.
The practice bulletin updates the recommendations on mesh based on a recent systematic review and meta-analysis that concluded that biological graft repair and absorbable mesh offered minimal benefits compared with native tissue repair, and did not significantly reduce rates of prolapse awareness or repeat surgery (Cochrane Database Syst Rev. 2016 Nov 30;11:CD004014).
Porcine dermis graft, which was used in most of the studies, did not significantly reduce rates of anterior prolapse recurrence compared with native tissue repair. Use of polypropylene mesh also tends to prolong operating times and causes more blood loss than native tissue anterior repair, and is associated with an elevated combined risk of stress urinary incontinence, mesh erosion, and repeat surgery for prolapse, the review concluded.
“Uterosacral and sacrospinous ligament suspension for apical POP with native tissue are equally effective surgical treatments of POP, with comparable anatomic, functional, and adverse outcomes,” the authors wrote in the practice bulletin.
Neither synthetic mesh nor biologic grafts improve outcomes of transvaginal repair of posterior vaginal wall prolapse, they added. As an alternative to surgery, most women can be successfully fitted with a pessary and clinicians should offer them this option, the practice bulletin stated. In up to 9% of cases, pessaries cause local devascularization or erosion, in which case they should be removed for 2-4 weeks while the patient undergoes local estrogen therapy.
Although POP is common and benign, symptomatic cases undermine quality of life by causing vaginal bulge and pressure and problems voiding, defecating, and during sexual activity. Consequently, about 300,000 women in the United States undergo surgery for POP every year. By 2050, population aging in the United States will lead to about a 50% rise in the number of women with POP, according to the practice bulletin.
FROM OBSTETRICS & GYNECOLOGY
Beyond the Kegel: the who, why, and how of pelvic floor PT
PHILADELPHIA – When a woman is referred for pelvic floor physical therapy, what’s involved? Is there evidence behind the treatments, and what exactly does pelvic floor therapy look like?
Denise Hartzell Leggin, a physical therapist who specializes in pelvic floor dysfunction, reviewed how the female pelvic floor can change with age, and provided the rationale for pelvic floor physical therapy (PT) at the annual meeting of the North American Menopause Society.
“Physical therapists treat musculoskeletal and neuromuscular dysfunctions,” said Ms. Hartzell Leggin. So, when a physician suspects a musculoskeletal cause for pelvic floor dysfunction, a PT referral may be appropriate, she said.
Why refer for PT?
As part of the aging process, pelvic floor dysfunction can coexist with the genitourinary syndrome of menopause (GSM), said Ms. Hartzell. Though the pathophysiology is not always clear, aging does have some effect on the pelvic floor musculature and, together with GSM, can contribute to women’s urogenital symptoms in later life.
These symptoms, she said, can be the harbingers of “a host of clinical conditions,” including urinary incontinence and fecal incontinence, constipation, and bladder-emptying problems. Also, changes in the pelvic musculature from childbirth, surgeries, and hypotonicity or hypertonicity can contribute to sexual dysfunction in later life, said Ms. Hartzell Leggin, who is affiliated with Good Shepherd Penn Partners and in private practice in the Philadelphia area.
The musculature of the pelvic floor functions as more than a bowl for carrying the pelvic organs, Ms. Hartzell Leggin said. The collective muscles and fascia form a sling that fills in the pelvic ring and functions as an integrated system with constant resting tone. But the musculature is also active and interactive.
“The diaphragm and the pelvic floor move in symmetry during respiration,” and pelvic floor tone tightens in anticipation of increased intra-abdominal pressure from a cough, a sneeze, or even a laugh. “These are active structures – the brain can talk to the pelvic floor and make it do something,” she said.
Who’s a good candidate?
Looking at risk factors for pelvic organ prolapse alone, Ms. Hartzell Leggin said these can include age, body mass index, a history of occupational or recreational heavy lifting, chronic cough, and even genetics.
However, one of the most significant risk factors for prolapse of pelvic organs is simply having had a vaginal delivery. Up to 50% of women who have delivered a child vaginally may eventually have some degree of pelvic organ prolapse, though not all women will be symptomatic, Ms. Hartzell Leggin said.
Since postsurgical pelvic organ prolapse rates may top 30% within 2 years, an initial referral for pelvic floor PT is a rational conservative approach, she said. And even if a patient progresses to surgery, PT may be a useful adjunct.
Pelvic floor dysfunction may also be considered if a diastasis recti is discovered on physical exam, or if the patient reports a linear abdominal bulge. Patients with diastasis recti are more likely to have pelvic floor dysfunction than the general population, she said, so it’s worth asking about any related symptoms.
For voiding issues, “conservative treatment is first-line,” said Ms. Hartzell Leggin, so a PT referral for pelvic floor therapy and, in some cases, some behavioral retraining can help with issues of urinary frequency and urgency. These are options that may be considered before prescribing anticholinergic medication, she said.
How does pelvic floor PT work?
When a physician refers a patient for pelvic floor PT, what’s the process? The physical therapy evaluation will begin with history taking, including the chief complaint, past medical and surgical history, and an obstetric/gynecologic/sexual history, said Ms. Hartzell Leggin. Medications are also reviewed.
The physical therapist’s examination should encompass a thorough orthopedic examination, with attention to the lumbar spine and hips, and posture and gait. An external and internal examination of the pelvic floor will look for muscle tone at rest and with strain, and for any defects or prolapse.
Pelvic floor strength is assessed according to ability to contract, with some assessment of strength available through palpation. More quantitative means may include manometry, dynamometry, or the use of progressive weighted vaginal cones.
There’s no single standardized measurement tool to assess pelvic floor strength. Palpation is a valuable tool for an experienced clinician, and it also can provide real-time feedback to the patient as she becomes more aware of her pelvic floor. The discipline is moving toward more standardized terminology, with several reporting scales now available to report pelvic floor strength, said Ms. Hartzell Leggin.
The Pelvic Floor Distress Inventory is a validated tool that captures information about the impact of pelvic floor dysfunction on a patient’s daily functioning. “I think I capture a lot when my patient comes in and completes that form,” said Ms. Hartzell Leggin. The Genitourinary Pain Index is another validated tool that measures urinary symptoms, pain, and associated quality of life impacts. Patients may be asked to keep a home therapy and symptom or voiding diary for additional information.
The pelvic floor PT treatment algorithm will vary, depending on whether there’s underlying hypertonicity or hypotonicity, but will involve pelvic floor exercises, soft tissue mobilization, and consideration of a variety of modalities including electrical stimulation and ultrasound. For hypertonicity, vaginal dilators may be used, while weighted vaginal cones may be used for hypotonicity.
Physical therapists should know when to refer a patient back to a physician and should always work as part of an interdisciplinary team, she said.
Ms. Hartzell Leggin reported that she is the president of Elite Rehabilitation Services in Audubon, Pa.
[email protected]
On Twitter @karioakes
PHILADELPHIA – When a woman is referred for pelvic floor physical therapy, what’s involved? Is there evidence behind the treatments, and what exactly does pelvic floor therapy look like?
Denise Hartzell Leggin, a physical therapist who specializes in pelvic floor dysfunction, reviewed how the female pelvic floor can change with age, and provided the rationale for pelvic floor physical therapy (PT) at the annual meeting of the North American Menopause Society.
“Physical therapists treat musculoskeletal and neuromuscular dysfunctions,” said Ms. Hartzell Leggin. So, when a physician suspects a musculoskeletal cause for pelvic floor dysfunction, a PT referral may be appropriate, she said.
Why refer for PT?
As part of the aging process, pelvic floor dysfunction can coexist with the genitourinary syndrome of menopause (GSM), said Ms. Hartzell. Though the pathophysiology is not always clear, aging does have some effect on the pelvic floor musculature and, together with GSM, can contribute to women’s urogenital symptoms in later life.
These symptoms, she said, can be the harbingers of “a host of clinical conditions,” including urinary incontinence and fecal incontinence, constipation, and bladder-emptying problems. Also, changes in the pelvic musculature from childbirth, surgeries, and hypotonicity or hypertonicity can contribute to sexual dysfunction in later life, said Ms. Hartzell Leggin, who is affiliated with Good Shepherd Penn Partners and in private practice in the Philadelphia area.
The musculature of the pelvic floor functions as more than a bowl for carrying the pelvic organs, Ms. Hartzell Leggin said. The collective muscles and fascia form a sling that fills in the pelvic ring and functions as an integrated system with constant resting tone. But the musculature is also active and interactive.
“The diaphragm and the pelvic floor move in symmetry during respiration,” and pelvic floor tone tightens in anticipation of increased intra-abdominal pressure from a cough, a sneeze, or even a laugh. “These are active structures – the brain can talk to the pelvic floor and make it do something,” she said.
Who’s a good candidate?
Looking at risk factors for pelvic organ prolapse alone, Ms. Hartzell Leggin said these can include age, body mass index, a history of occupational or recreational heavy lifting, chronic cough, and even genetics.
However, one of the most significant risk factors for prolapse of pelvic organs is simply having had a vaginal delivery. Up to 50% of women who have delivered a child vaginally may eventually have some degree of pelvic organ prolapse, though not all women will be symptomatic, Ms. Hartzell Leggin said.
Since postsurgical pelvic organ prolapse rates may top 30% within 2 years, an initial referral for pelvic floor PT is a rational conservative approach, she said. And even if a patient progresses to surgery, PT may be a useful adjunct.
Pelvic floor dysfunction may also be considered if a diastasis recti is discovered on physical exam, or if the patient reports a linear abdominal bulge. Patients with diastasis recti are more likely to have pelvic floor dysfunction than the general population, she said, so it’s worth asking about any related symptoms.
For voiding issues, “conservative treatment is first-line,” said Ms. Hartzell Leggin, so a PT referral for pelvic floor therapy and, in some cases, some behavioral retraining can help with issues of urinary frequency and urgency. These are options that may be considered before prescribing anticholinergic medication, she said.
How does pelvic floor PT work?
When a physician refers a patient for pelvic floor PT, what’s the process? The physical therapy evaluation will begin with history taking, including the chief complaint, past medical and surgical history, and an obstetric/gynecologic/sexual history, said Ms. Hartzell Leggin. Medications are also reviewed.
The physical therapist’s examination should encompass a thorough orthopedic examination, with attention to the lumbar spine and hips, and posture and gait. An external and internal examination of the pelvic floor will look for muscle tone at rest and with strain, and for any defects or prolapse.
Pelvic floor strength is assessed according to ability to contract, with some assessment of strength available through palpation. More quantitative means may include manometry, dynamometry, or the use of progressive weighted vaginal cones.
There’s no single standardized measurement tool to assess pelvic floor strength. Palpation is a valuable tool for an experienced clinician, and it also can provide real-time feedback to the patient as she becomes more aware of her pelvic floor. The discipline is moving toward more standardized terminology, with several reporting scales now available to report pelvic floor strength, said Ms. Hartzell Leggin.
The Pelvic Floor Distress Inventory is a validated tool that captures information about the impact of pelvic floor dysfunction on a patient’s daily functioning. “I think I capture a lot when my patient comes in and completes that form,” said Ms. Hartzell Leggin. The Genitourinary Pain Index is another validated tool that measures urinary symptoms, pain, and associated quality of life impacts. Patients may be asked to keep a home therapy and symptom or voiding diary for additional information.
The pelvic floor PT treatment algorithm will vary, depending on whether there’s underlying hypertonicity or hypotonicity, but will involve pelvic floor exercises, soft tissue mobilization, and consideration of a variety of modalities including electrical stimulation and ultrasound. For hypertonicity, vaginal dilators may be used, while weighted vaginal cones may be used for hypotonicity.
Physical therapists should know when to refer a patient back to a physician and should always work as part of an interdisciplinary team, she said.
Ms. Hartzell Leggin reported that she is the president of Elite Rehabilitation Services in Audubon, Pa.
[email protected]
On Twitter @karioakes
PHILADELPHIA – When a woman is referred for pelvic floor physical therapy, what’s involved? Is there evidence behind the treatments, and what exactly does pelvic floor therapy look like?
Denise Hartzell Leggin, a physical therapist who specializes in pelvic floor dysfunction, reviewed how the female pelvic floor can change with age, and provided the rationale for pelvic floor physical therapy (PT) at the annual meeting of the North American Menopause Society.
“Physical therapists treat musculoskeletal and neuromuscular dysfunctions,” said Ms. Hartzell Leggin. So, when a physician suspects a musculoskeletal cause for pelvic floor dysfunction, a PT referral may be appropriate, she said.
Why refer for PT?
As part of the aging process, pelvic floor dysfunction can coexist with the genitourinary syndrome of menopause (GSM), said Ms. Hartzell. Though the pathophysiology is not always clear, aging does have some effect on the pelvic floor musculature and, together with GSM, can contribute to women’s urogenital symptoms in later life.
These symptoms, she said, can be the harbingers of “a host of clinical conditions,” including urinary incontinence and fecal incontinence, constipation, and bladder-emptying problems. Also, changes in the pelvic musculature from childbirth, surgeries, and hypotonicity or hypertonicity can contribute to sexual dysfunction in later life, said Ms. Hartzell Leggin, who is affiliated with Good Shepherd Penn Partners and in private practice in the Philadelphia area.
The musculature of the pelvic floor functions as more than a bowl for carrying the pelvic organs, Ms. Hartzell Leggin said. The collective muscles and fascia form a sling that fills in the pelvic ring and functions as an integrated system with constant resting tone. But the musculature is also active and interactive.
“The diaphragm and the pelvic floor move in symmetry during respiration,” and pelvic floor tone tightens in anticipation of increased intra-abdominal pressure from a cough, a sneeze, or even a laugh. “These are active structures – the brain can talk to the pelvic floor and make it do something,” she said.
Who’s a good candidate?
Looking at risk factors for pelvic organ prolapse alone, Ms. Hartzell Leggin said these can include age, body mass index, a history of occupational or recreational heavy lifting, chronic cough, and even genetics.
However, one of the most significant risk factors for prolapse of pelvic organs is simply having had a vaginal delivery. Up to 50% of women who have delivered a child vaginally may eventually have some degree of pelvic organ prolapse, though not all women will be symptomatic, Ms. Hartzell Leggin said.
Since postsurgical pelvic organ prolapse rates may top 30% within 2 years, an initial referral for pelvic floor PT is a rational conservative approach, she said. And even if a patient progresses to surgery, PT may be a useful adjunct.
Pelvic floor dysfunction may also be considered if a diastasis recti is discovered on physical exam, or if the patient reports a linear abdominal bulge. Patients with diastasis recti are more likely to have pelvic floor dysfunction than the general population, she said, so it’s worth asking about any related symptoms.
For voiding issues, “conservative treatment is first-line,” said Ms. Hartzell Leggin, so a PT referral for pelvic floor therapy and, in some cases, some behavioral retraining can help with issues of urinary frequency and urgency. These are options that may be considered before prescribing anticholinergic medication, she said.
How does pelvic floor PT work?
When a physician refers a patient for pelvic floor PT, what’s the process? The physical therapy evaluation will begin with history taking, including the chief complaint, past medical and surgical history, and an obstetric/gynecologic/sexual history, said Ms. Hartzell Leggin. Medications are also reviewed.
The physical therapist’s examination should encompass a thorough orthopedic examination, with attention to the lumbar spine and hips, and posture and gait. An external and internal examination of the pelvic floor will look for muscle tone at rest and with strain, and for any defects or prolapse.
Pelvic floor strength is assessed according to ability to contract, with some assessment of strength available through palpation. More quantitative means may include manometry, dynamometry, or the use of progressive weighted vaginal cones.
There’s no single standardized measurement tool to assess pelvic floor strength. Palpation is a valuable tool for an experienced clinician, and it also can provide real-time feedback to the patient as she becomes more aware of her pelvic floor. The discipline is moving toward more standardized terminology, with several reporting scales now available to report pelvic floor strength, said Ms. Hartzell Leggin.
The Pelvic Floor Distress Inventory is a validated tool that captures information about the impact of pelvic floor dysfunction on a patient’s daily functioning. “I think I capture a lot when my patient comes in and completes that form,” said Ms. Hartzell Leggin. The Genitourinary Pain Index is another validated tool that measures urinary symptoms, pain, and associated quality of life impacts. Patients may be asked to keep a home therapy and symptom or voiding diary for additional information.
The pelvic floor PT treatment algorithm will vary, depending on whether there’s underlying hypertonicity or hypotonicity, but will involve pelvic floor exercises, soft tissue mobilization, and consideration of a variety of modalities including electrical stimulation and ultrasound. For hypertonicity, vaginal dilators may be used, while weighted vaginal cones may be used for hypotonicity.
Physical therapists should know when to refer a patient back to a physician and should always work as part of an interdisciplinary team, she said.
Ms. Hartzell Leggin reported that she is the president of Elite Rehabilitation Services in Audubon, Pa.
[email protected]
On Twitter @karioakes
EXPERT ANALYSIS FROM NAMS 2017
Pelvic organ prolapse: Effective treatments
Editor’s Note: This is the fifth installment of a six-part series that will review key concepts and articles that ob.gyns. can use to prepare for the American Board of Obstetrics and Gynecology Maintenance of Certification examination. The series is adapted from Ob/Gyn Board Master (obgynboardmaster.com), an online board review course created by Erudyte. This month’s edition of the Board Corner focuses on pelvic organ prolapse.
The American College of Obstetricians and Gynecologists’ “Practice Bulletins” are important practice management guidelines for ob.gyn. clinicians. The Practice Bulletins are rich sources of material that is often tested on board exams. Earlier this year, ACOG released a revised Practice Bulletin (#176) updating its advice on the diagnosis and management of pelvic organ prolapse (POP).1 It is a well-written document summarizing most of the landmark articles published in the field of female pelvic medicine and reconstructive surgery. We recommend you read this bulletin and review this topic carefully.
Let’s begin with a possible medical board question: Which of the following procedures is the most effective for a sexually-active patient with advanced prolapse?
A. Sacrospinous ligament suspension (SSLS)
B. Uterosacral ligament suspension (USLS)
C. Sacrocolpopexy (SCP)
D. Colpocleisis
E. Hysteropexy
A randomized trial comparing SSLS and USLS found the two apical procedures with native tissue repair are equally effective with comparable functional and adverse outcomes (answers A and B are incorrect). However, randomized trials comparing SCP to SSLS show that SCP with synthetic mesh has the lowest recurrence rate for prolapse. Colpocleisis is done for patients who are not sexually active (answer D is incorrect). Hysteropexy is performed for patients who desire preservation of the uterus. There is less available evidence on safety and efficacy, compared with hysterectomy at the time of prolapse repair (answer E is incorrect)
Key points
The key points to remember are:
1. SCP is the most effective prolapse repair technique.
2. USLS and SSLS fixation are equally effective when compared with one another.
3. Colpocleisis is a highly successful procedure for POP in patients who are not sexually active.
Literature summary
The lifetime risk for undergoing surgery for POP or stress incontinence is 20%. POP is the descent of one or more aspects of the vagina or uterus, which allows nearby organs to herniate into the vagina. POP should only be treated if it is symptomatic and bothersome for the patient. The pessary is an alternative to surgical treatment of prolapse.
Proven risk factors for POP are increased parity, vaginal delivery, age, obesity, chronic constipation, and certain congenital anomalies. A history should be taken to elucidate symptoms of prolapse, such as bulge, pressure, sexual dysfunction, lower urinary tract dysfunction, or defecatory dysfunction. It is also important to find out how much the POP is affecting her quality of life. A physical exam is best performed with a split speculum, with bladder empty, while the patient performs a Valsalva maneuver. We recommend using the POP-Q system to grade the severity of prolapse. The tone of the pelvic floor muscle should also be evaluated (absent, weak, normal, or strong) during pelvic exam.
The minimum testing necessary for a patient with POP is urinalysis and a postvoid residual. A stress test with a full bladder should also be done with and without reduction of the prolapse. If you’re considering surgery and the patient has advanced prolapse and/or other complicating factors – such as obstructive symptoms or significant neurologic disorder – you should consider performing urodynamic testing as well.
Native tissue, suture-based reconstructive repairs of the vagina include apical procedures, such as SSLS and USLS, in addition to anterior colporrhaphy and posterior repair. At 2-year follow-up, SSLS and USLS along with anterior colporrhaphy and posterior repair are equally effective for treatment of prolapse with comparable functional and adverse outcomes. SCP is more effective than SSLS but the abdominal procedure (not laparoscopic) may be associated with more complications. Currently, there are no published randomized trials comparing minimally-invasive SCP to USLS, but one is underway (clinicaltrials.gov).
Other procedures for POP include obliterative procedures such as colpocleisis, which is highly effective for patients who do not desire future vaginal intercourse and also has low morbidity. Preservation of the uterus by hysteropexy procedures (either transvaginal or transabdominal) are also options for women desiring to preserve their uterus, but these procedures have little safety and efficacy data. Regardless of the procedure performed, routine intraoperative cystoscopy should be done to assure ureteral patency and to rule out injury to the lower urinary tract.
Some type of prophylactic anti-incontinence procedure – retropubic or Burch – may be done at the time of vaginal prolapse repair or abdominal prolapse repair, respectively, in order to reduce the chance of postoperative stress urinary incontinence in a patient without symptoms of stress incontinence. The exception to this is in a patient who has an elevated postvoid residual or someone with a prior anti-incontinence procedure without symptoms of stress urinary incontinence.
Practice tips
Finally, here are some precautions and words of advice about the following POP procedures:
- Neither synthetic nor biologic grafts should be used to augment posterior repairs as these do not improve outcomes.
- Transvaginal repair of rectocele is superior to the transanal repair techniques.
- Synthetic mesh augmentation of the anterior vaginal wall may improve anatomic outcomes, but this comes at a cost (more reoperations and higher rate of complications). Thus, surgeons performing these procedures should have specialized training and the patient should have a unique indication and must undergo proper consent as recommended by ACOG.
Dr. Siddighi is editor-in-chief of the Ob/Gyn Board Master and director of female pelvic medicine and reconstructive surgery and director of grand rounds at Loma Linda University Health in California. Ob.Gyn. News and Ob/Gyn Board Master are owned by the same parent company, Frontline Medical Communications.
Reference
Editor’s Note: This is the fifth installment of a six-part series that will review key concepts and articles that ob.gyns. can use to prepare for the American Board of Obstetrics and Gynecology Maintenance of Certification examination. The series is adapted from Ob/Gyn Board Master (obgynboardmaster.com), an online board review course created by Erudyte. This month’s edition of the Board Corner focuses on pelvic organ prolapse.
The American College of Obstetricians and Gynecologists’ “Practice Bulletins” are important practice management guidelines for ob.gyn. clinicians. The Practice Bulletins are rich sources of material that is often tested on board exams. Earlier this year, ACOG released a revised Practice Bulletin (#176) updating its advice on the diagnosis and management of pelvic organ prolapse (POP).1 It is a well-written document summarizing most of the landmark articles published in the field of female pelvic medicine and reconstructive surgery. We recommend you read this bulletin and review this topic carefully.
Let’s begin with a possible medical board question: Which of the following procedures is the most effective for a sexually-active patient with advanced prolapse?
A. Sacrospinous ligament suspension (SSLS)
B. Uterosacral ligament suspension (USLS)
C. Sacrocolpopexy (SCP)
D. Colpocleisis
E. Hysteropexy
A randomized trial comparing SSLS and USLS found the two apical procedures with native tissue repair are equally effective with comparable functional and adverse outcomes (answers A and B are incorrect). However, randomized trials comparing SCP to SSLS show that SCP with synthetic mesh has the lowest recurrence rate for prolapse. Colpocleisis is done for patients who are not sexually active (answer D is incorrect). Hysteropexy is performed for patients who desire preservation of the uterus. There is less available evidence on safety and efficacy, compared with hysterectomy at the time of prolapse repair (answer E is incorrect)
Key points
The key points to remember are:
1. SCP is the most effective prolapse repair technique.
2. USLS and SSLS fixation are equally effective when compared with one another.
3. Colpocleisis is a highly successful procedure for POP in patients who are not sexually active.
Literature summary
The lifetime risk for undergoing surgery for POP or stress incontinence is 20%. POP is the descent of one or more aspects of the vagina or uterus, which allows nearby organs to herniate into the vagina. POP should only be treated if it is symptomatic and bothersome for the patient. The pessary is an alternative to surgical treatment of prolapse.
Proven risk factors for POP are increased parity, vaginal delivery, age, obesity, chronic constipation, and certain congenital anomalies. A history should be taken to elucidate symptoms of prolapse, such as bulge, pressure, sexual dysfunction, lower urinary tract dysfunction, or defecatory dysfunction. It is also important to find out how much the POP is affecting her quality of life. A physical exam is best performed with a split speculum, with bladder empty, while the patient performs a Valsalva maneuver. We recommend using the POP-Q system to grade the severity of prolapse. The tone of the pelvic floor muscle should also be evaluated (absent, weak, normal, or strong) during pelvic exam.
The minimum testing necessary for a patient with POP is urinalysis and a postvoid residual. A stress test with a full bladder should also be done with and without reduction of the prolapse. If you’re considering surgery and the patient has advanced prolapse and/or other complicating factors – such as obstructive symptoms or significant neurologic disorder – you should consider performing urodynamic testing as well.
Native tissue, suture-based reconstructive repairs of the vagina include apical procedures, such as SSLS and USLS, in addition to anterior colporrhaphy and posterior repair. At 2-year follow-up, SSLS and USLS along with anterior colporrhaphy and posterior repair are equally effective for treatment of prolapse with comparable functional and adverse outcomes. SCP is more effective than SSLS but the abdominal procedure (not laparoscopic) may be associated with more complications. Currently, there are no published randomized trials comparing minimally-invasive SCP to USLS, but one is underway (clinicaltrials.gov).
Other procedures for POP include obliterative procedures such as colpocleisis, which is highly effective for patients who do not desire future vaginal intercourse and also has low morbidity. Preservation of the uterus by hysteropexy procedures (either transvaginal or transabdominal) are also options for women desiring to preserve their uterus, but these procedures have little safety and efficacy data. Regardless of the procedure performed, routine intraoperative cystoscopy should be done to assure ureteral patency and to rule out injury to the lower urinary tract.
Some type of prophylactic anti-incontinence procedure – retropubic or Burch – may be done at the time of vaginal prolapse repair or abdominal prolapse repair, respectively, in order to reduce the chance of postoperative stress urinary incontinence in a patient without symptoms of stress incontinence. The exception to this is in a patient who has an elevated postvoid residual or someone with a prior anti-incontinence procedure without symptoms of stress urinary incontinence.
Practice tips
Finally, here are some precautions and words of advice about the following POP procedures:
- Neither synthetic nor biologic grafts should be used to augment posterior repairs as these do not improve outcomes.
- Transvaginal repair of rectocele is superior to the transanal repair techniques.
- Synthetic mesh augmentation of the anterior vaginal wall may improve anatomic outcomes, but this comes at a cost (more reoperations and higher rate of complications). Thus, surgeons performing these procedures should have specialized training and the patient should have a unique indication and must undergo proper consent as recommended by ACOG.
Dr. Siddighi is editor-in-chief of the Ob/Gyn Board Master and director of female pelvic medicine and reconstructive surgery and director of grand rounds at Loma Linda University Health in California. Ob.Gyn. News and Ob/Gyn Board Master are owned by the same parent company, Frontline Medical Communications.
Reference
Editor’s Note: This is the fifth installment of a six-part series that will review key concepts and articles that ob.gyns. can use to prepare for the American Board of Obstetrics and Gynecology Maintenance of Certification examination. The series is adapted from Ob/Gyn Board Master (obgynboardmaster.com), an online board review course created by Erudyte. This month’s edition of the Board Corner focuses on pelvic organ prolapse.
The American College of Obstetricians and Gynecologists’ “Practice Bulletins” are important practice management guidelines for ob.gyn. clinicians. The Practice Bulletins are rich sources of material that is often tested on board exams. Earlier this year, ACOG released a revised Practice Bulletin (#176) updating its advice on the diagnosis and management of pelvic organ prolapse (POP).1 It is a well-written document summarizing most of the landmark articles published in the field of female pelvic medicine and reconstructive surgery. We recommend you read this bulletin and review this topic carefully.
Let’s begin with a possible medical board question: Which of the following procedures is the most effective for a sexually-active patient with advanced prolapse?
A. Sacrospinous ligament suspension (SSLS)
B. Uterosacral ligament suspension (USLS)
C. Sacrocolpopexy (SCP)
D. Colpocleisis
E. Hysteropexy
A randomized trial comparing SSLS and USLS found the two apical procedures with native tissue repair are equally effective with comparable functional and adverse outcomes (answers A and B are incorrect). However, randomized trials comparing SCP to SSLS show that SCP with synthetic mesh has the lowest recurrence rate for prolapse. Colpocleisis is done for patients who are not sexually active (answer D is incorrect). Hysteropexy is performed for patients who desire preservation of the uterus. There is less available evidence on safety and efficacy, compared with hysterectomy at the time of prolapse repair (answer E is incorrect)
Key points
The key points to remember are:
1. SCP is the most effective prolapse repair technique.
2. USLS and SSLS fixation are equally effective when compared with one another.
3. Colpocleisis is a highly successful procedure for POP in patients who are not sexually active.
Literature summary
The lifetime risk for undergoing surgery for POP or stress incontinence is 20%. POP is the descent of one or more aspects of the vagina or uterus, which allows nearby organs to herniate into the vagina. POP should only be treated if it is symptomatic and bothersome for the patient. The pessary is an alternative to surgical treatment of prolapse.
Proven risk factors for POP are increased parity, vaginal delivery, age, obesity, chronic constipation, and certain congenital anomalies. A history should be taken to elucidate symptoms of prolapse, such as bulge, pressure, sexual dysfunction, lower urinary tract dysfunction, or defecatory dysfunction. It is also important to find out how much the POP is affecting her quality of life. A physical exam is best performed with a split speculum, with bladder empty, while the patient performs a Valsalva maneuver. We recommend using the POP-Q system to grade the severity of prolapse. The tone of the pelvic floor muscle should also be evaluated (absent, weak, normal, or strong) during pelvic exam.
The minimum testing necessary for a patient with POP is urinalysis and a postvoid residual. A stress test with a full bladder should also be done with and without reduction of the prolapse. If you’re considering surgery and the patient has advanced prolapse and/or other complicating factors – such as obstructive symptoms or significant neurologic disorder – you should consider performing urodynamic testing as well.
Native tissue, suture-based reconstructive repairs of the vagina include apical procedures, such as SSLS and USLS, in addition to anterior colporrhaphy and posterior repair. At 2-year follow-up, SSLS and USLS along with anterior colporrhaphy and posterior repair are equally effective for treatment of prolapse with comparable functional and adverse outcomes. SCP is more effective than SSLS but the abdominal procedure (not laparoscopic) may be associated with more complications. Currently, there are no published randomized trials comparing minimally-invasive SCP to USLS, but one is underway (clinicaltrials.gov).
Other procedures for POP include obliterative procedures such as colpocleisis, which is highly effective for patients who do not desire future vaginal intercourse and also has low morbidity. Preservation of the uterus by hysteropexy procedures (either transvaginal or transabdominal) are also options for women desiring to preserve their uterus, but these procedures have little safety and efficacy data. Regardless of the procedure performed, routine intraoperative cystoscopy should be done to assure ureteral patency and to rule out injury to the lower urinary tract.
Some type of prophylactic anti-incontinence procedure – retropubic or Burch – may be done at the time of vaginal prolapse repair or abdominal prolapse repair, respectively, in order to reduce the chance of postoperative stress urinary incontinence in a patient without symptoms of stress incontinence. The exception to this is in a patient who has an elevated postvoid residual or someone with a prior anti-incontinence procedure without symptoms of stress urinary incontinence.
Practice tips
Finally, here are some precautions and words of advice about the following POP procedures:
- Neither synthetic nor biologic grafts should be used to augment posterior repairs as these do not improve outcomes.
- Transvaginal repair of rectocele is superior to the transanal repair techniques.
- Synthetic mesh augmentation of the anterior vaginal wall may improve anatomic outcomes, but this comes at a cost (more reoperations and higher rate of complications). Thus, surgeons performing these procedures should have specialized training and the patient should have a unique indication and must undergo proper consent as recommended by ACOG.
Dr. Siddighi is editor-in-chief of the Ob/Gyn Board Master and director of female pelvic medicine and reconstructive surgery and director of grand rounds at Loma Linda University Health in California. Ob.Gyn. News and Ob/Gyn Board Master are owned by the same parent company, Frontline Medical Communications.