User login
STEMI: Prereperfusion IV metoprolol shows long-term benefits
WASHINGTON – Early administration of intravenous metoprolol prior to primary percutaneous coronary intervention in patients with Killip class I or II anterior ST-elevation myocardial infarction reaped impressive long-term benefits in updated results from the Spanish METOCARD-CNIC trial.
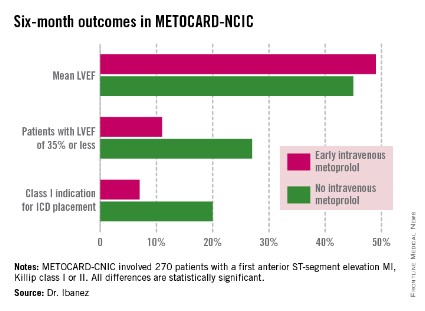
At 6 months post infarct, the mean left ventricular ejection fraction (LVEF) was significantly higher in the early IV beta-blocker group than in controls. Also, the prevalence of a severely depressed LVEF of 35% or less was significantly lower, as was the proportion of patients having a class I indication for an implantable cardioverter-defibrillator, Dr. Borja Ibanez reported at the annual meeting of the American College of Cardiology.
METOCARD-CNIC
The primary study endpoint was infarct size as measured by magnetic resonance imaging 7 days post STEMI. As previously reported, infarct size was 20% smaller in the IV metoprolol recipients (Circulation 2013;128:1495-1503). This finding was encouraging, Dr. Ibanez observed, because infarct size is a major determinant of long-term morbidity and mortality. And while primary PCI for STEMI results in very low acute mortality, there is a high residual risk of subsequent heart failure and death. So the search is on for treatments that reduce infarct size. And prior to METOCARD-CNIC there had been no randomized controlled trials of early IV beta-blocker therapy during the primary PCI era.
At ACC 14, Dr. Ibanez presented prespecified secondary endpoints based upon outcomes at 6 months (see chart) and 2 years post STEMI.
At a median follow-up of 2 years, the composite endpoint comprised of death, reinfarction, hospital admission for heart failure, or malignant arrhythmia had occurred in 10.8% of the IV metoprolol group, compared with 18.3% of controls. This translated to an adjusted 45% relative risk reduction which didn’t quite reach statistical significance, but then again the trial wasn’t of sufficient size to be powered to evaluate clinical endpoints.
Of note, the heart failure hospital admission component of the composite endpoint occurred in 2.2% of the IV beta-blocker group, compared with 6.9% of controls, for a 68% relative risk reduction that was statistically significant. The curves began to split at 12 months and continued to diverge through 24 months, according to Dr. Ibanez.
Based upon the encouraging findings of METOCARD-CNIC, planning is underway for a large randomized trial powered to evaluate hard clinical endpoints. It will be called the MOVE ON! trial, the cardiologist added.
METOCARD-CNIC was funded by the Carlos III National Center for Cardiovascular Investigations and the Spanish Ministry of Health and Social Policy. Dr. Ibanez reported having no financial conflicts of interest.
WASHINGTON – Early administration of intravenous metoprolol prior to primary percutaneous coronary intervention in patients with Killip class I or II anterior ST-elevation myocardial infarction reaped impressive long-term benefits in updated results from the Spanish METOCARD-CNIC trial.
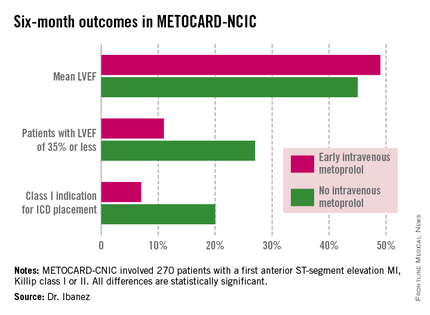
At 6 months post infarct, the mean left ventricular ejection fraction (LVEF) was significantly higher in the early IV beta-blocker group than in controls. Also, the prevalence of a severely depressed LVEF of 35% or less was significantly lower, as was the proportion of patients having a class I indication for an implantable cardioverter-defibrillator, Dr. Borja Ibanez reported at the annual meeting of the American College of Cardiology.

METOCARD-CNIC
The primary study endpoint was infarct size as measured by magnetic resonance imaging 7 days post STEMI. As previously reported, infarct size was 20% smaller in the IV metoprolol recipients (Circulation 2013;128:1495-1503). This finding was encouraging, Dr. Ibanez observed, because infarct size is a major determinant of long-term morbidity and mortality. And while primary PCI for STEMI results in very low acute mortality, there is a high residual risk of subsequent heart failure and death. So the search is on for treatments that reduce infarct size. And prior to METOCARD-CNIC there had been no randomized controlled trials of early IV beta-blocker therapy during the primary PCI era.
At ACC 14, Dr. Ibanez presented prespecified secondary endpoints based upon outcomes at 6 months (see chart) and 2 years post STEMI.
At a median follow-up of 2 years, the composite endpoint comprised of death, reinfarction, hospital admission for heart failure, or malignant arrhythmia had occurred in 10.8% of the IV metoprolol group, compared with 18.3% of controls. This translated to an adjusted 45% relative risk reduction which didn’t quite reach statistical significance, but then again the trial wasn’t of sufficient size to be powered to evaluate clinical endpoints.
Of note, the heart failure hospital admission component of the composite endpoint occurred in 2.2% of the IV beta-blocker group, compared with 6.9% of controls, for a 68% relative risk reduction that was statistically significant. The curves began to split at 12 months and continued to diverge through 24 months, according to Dr. Ibanez.
Based upon the encouraging findings of METOCARD-CNIC, planning is underway for a large randomized trial powered to evaluate hard clinical endpoints. It will be called the MOVE ON! trial, the cardiologist added.
METOCARD-CNIC was funded by the Carlos III National Center for Cardiovascular Investigations and the Spanish Ministry of Health and Social Policy. Dr. Ibanez reported having no financial conflicts of interest.
WASHINGTON – Early administration of intravenous metoprolol prior to primary percutaneous coronary intervention in patients with Killip class I or II anterior ST-elevation myocardial infarction reaped impressive long-term benefits in updated results from the Spanish METOCARD-CNIC trial.
At 6 months post infarct, the mean left ventricular ejection fraction (LVEF) was significantly higher in the early IV beta-blocker group than in controls. Also, the prevalence of a severely depressed LVEF of 35% or less was significantly lower, as was the proportion of patients having a class I indication for an implantable cardioverter-defibrillator, Dr. Borja Ibanez reported at the annual meeting of the American College of Cardiology.

METOCARD-CNIC
The primary study endpoint was infarct size as measured by magnetic resonance imaging 7 days post STEMI. As previously reported, infarct size was 20% smaller in the IV metoprolol recipients (Circulation 2013;128:1495-1503). This finding was encouraging, Dr. Ibanez observed, because infarct size is a major determinant of long-term morbidity and mortality. And while primary PCI for STEMI results in very low acute mortality, there is a high residual risk of subsequent heart failure and death. So the search is on for treatments that reduce infarct size. And prior to METOCARD-CNIC there had been no randomized controlled trials of early IV beta-blocker therapy during the primary PCI era.
At ACC 14, Dr. Ibanez presented prespecified secondary endpoints based upon outcomes at 6 months (see chart) and 2 years post STEMI.
At a median follow-up of 2 years, the composite endpoint comprised of death, reinfarction, hospital admission for heart failure, or malignant arrhythmia had occurred in 10.8% of the IV metoprolol group, compared with 18.3% of controls. This translated to an adjusted 45% relative risk reduction which didn’t quite reach statistical significance, but then again the trial wasn’t of sufficient size to be powered to evaluate clinical endpoints.
Of note, the heart failure hospital admission component of the composite endpoint occurred in 2.2% of the IV beta-blocker group, compared with 6.9% of controls, for a 68% relative risk reduction that was statistically significant. The curves began to split at 12 months and continued to diverge through 24 months, according to Dr. Ibanez.
Based upon the encouraging findings of METOCARD-CNIC, planning is underway for a large randomized trial powered to evaluate hard clinical endpoints. It will be called the MOVE ON! trial, the cardiologist added.
METOCARD-CNIC was funded by the Carlos III National Center for Cardiovascular Investigations and the Spanish Ministry of Health and Social Policy. Dr. Ibanez reported having no financial conflicts of interest.
AT ACC 14
Key clinical point: Prereperfusion IV metoprolol may reduce heart failure readmissions.
Major finding: Patients with anterior ST-elevation MI who received intravenous metoprolol prior to primary PCI had a significantly greater left ventricular ejection fraction at 6 months follow-up than those who didn’t. They also were 68% less likely to be hospitalized for heart failure during 2 years of follow-up.
Data source: A six-center prospective trial in which 270 Spanish patients with a first anterior STEMI were randomized to have administration of IV metoprolol or not while being transported for primary PCI.
Disclosures: The METOCARD-CNIC trial was sponsored by the Spanish Ministry of Health and Social Policy and the Carlos III National Center for Cardiovascular Investigations. The presenter reported having no financial conflicts.
Tests may help before liver transplant, not after
CHICAGO – Two laboratory measurements that are commonly used to assess the cause of symptomatic ascites before liver transplant may be deceptive when used to assess posttransplant ascites, a retrospective study of 15 patients suggested.
Before liver transplant, a serum-ascites albumin gradient (SAAG) greater than 1.1 g/dL differentiates ascites due to portal hypertension rather than other causes 97% of the time. An ascites total protein (aTP) measurement has a lower accuracy for portal hypertension of 57%, but when used in conjunction with SAAG, an aTP of 2.5 g/dL or greater suggests that the cause is cardiac ascites, tuberculous ascites, or peritoneal carcinomatosis, Dr. Jeffrey LaFond explained at the annual Digestive Disease Week.
He and his associates studied the records of 15 patients who developed symptomatic post-transplant ascites that had enough volume to require therapeutic paracentesis. The ascites occurred a mean of 515 days after transplantation (ranging from 14 to 2,744 days). In the work-up for ascites, the sensitivity of SAAG for portal hypertension was 82% and the sensitivity of aTP for portal hypertension was 50%.
Three of 12 patients who had a posttransplant SAAG had a gradient below 1.1 g/dL even though other tests found no evidence of another cause for ascites besides portal hypertension, and two of those three patients had confirmed portal hypertension, he reported. Five of 10 patients with an aTP had a value greater than 2.5 g/dL, even though they had confirmed portal hypertension and normal cardiac function, he reported.
"Assessment of ascites due to portal hypertension and/or vascular stricture in the posttransplant period using SAAG and aTP can be deceiving and cannot be relied upon to make diagnostic interventional decisions," said Dr. LaFond of the University of Virginia, Charlottesville. "A hepatic venogram should be performed early on in patients with posttransplant ascites to evaluate for strictures and portal hypertension.
Records showed that all patients in the study had ascites confirmed by imaging and/or paracenteses and had diagnostic studies to rule out heart failure, opportunistic infection, or malignancy as the source of ascites. Suspected portal hypertension was confirmed by pressure measurements or the presence of vascular strictures on venogram, with portal hypertension defined as a sinusoidal or portosystemic gradient greater than 5 or the presence of a stricture with a gradient of at least 3.
An estimated 3%-7% of patients develop ascites after liver transplant, which has been associated with an increased risk of renal impairment, prolonged hospitalization, and abdominal infections, he said.
Dr. LaFond reported having no financial disclosures.
On Twitter @sherryboschert
*This story was updated 6/3/2014.
CHICAGO – Two laboratory measurements that are commonly used to assess the cause of symptomatic ascites before liver transplant may be deceptive when used to assess posttransplant ascites, a retrospective study of 15 patients suggested.
Before liver transplant, a serum-ascites albumin gradient (SAAG) greater than 1.1 g/dL differentiates ascites due to portal hypertension rather than other causes 97% of the time. An ascites total protein (aTP) measurement has a lower accuracy for portal hypertension of 57%, but when used in conjunction with SAAG, an aTP of 2.5 g/dL or greater suggests that the cause is cardiac ascites, tuberculous ascites, or peritoneal carcinomatosis, Dr. Jeffrey LaFond explained at the annual Digestive Disease Week.
He and his associates studied the records of 15 patients who developed symptomatic post-transplant ascites that had enough volume to require therapeutic paracentesis. The ascites occurred a mean of 515 days after transplantation (ranging from 14 to 2,744 days). In the work-up for ascites, the sensitivity of SAAG for portal hypertension was 82% and the sensitivity of aTP for portal hypertension was 50%.
Three of 12 patients who had a posttransplant SAAG had a gradient below 1.1 g/dL even though other tests found no evidence of another cause for ascites besides portal hypertension, and two of those three patients had confirmed portal hypertension, he reported. Five of 10 patients with an aTP had a value greater than 2.5 g/dL, even though they had confirmed portal hypertension and normal cardiac function, he reported.
"Assessment of ascites due to portal hypertension and/or vascular stricture in the posttransplant period using SAAG and aTP can be deceiving and cannot be relied upon to make diagnostic interventional decisions," said Dr. LaFond of the University of Virginia, Charlottesville. "A hepatic venogram should be performed early on in patients with posttransplant ascites to evaluate for strictures and portal hypertension.
Records showed that all patients in the study had ascites confirmed by imaging and/or paracenteses and had diagnostic studies to rule out heart failure, opportunistic infection, or malignancy as the source of ascites. Suspected portal hypertension was confirmed by pressure measurements or the presence of vascular strictures on venogram, with portal hypertension defined as a sinusoidal or portosystemic gradient greater than 5 or the presence of a stricture with a gradient of at least 3.
An estimated 3%-7% of patients develop ascites after liver transplant, which has been associated with an increased risk of renal impairment, prolonged hospitalization, and abdominal infections, he said.
Dr. LaFond reported having no financial disclosures.
On Twitter @sherryboschert
*This story was updated 6/3/2014.
CHICAGO – Two laboratory measurements that are commonly used to assess the cause of symptomatic ascites before liver transplant may be deceptive when used to assess posttransplant ascites, a retrospective study of 15 patients suggested.
Before liver transplant, a serum-ascites albumin gradient (SAAG) greater than 1.1 g/dL differentiates ascites due to portal hypertension rather than other causes 97% of the time. An ascites total protein (aTP) measurement has a lower accuracy for portal hypertension of 57%, but when used in conjunction with SAAG, an aTP of 2.5 g/dL or greater suggests that the cause is cardiac ascites, tuberculous ascites, or peritoneal carcinomatosis, Dr. Jeffrey LaFond explained at the annual Digestive Disease Week.
He and his associates studied the records of 15 patients who developed symptomatic post-transplant ascites that had enough volume to require therapeutic paracentesis. The ascites occurred a mean of 515 days after transplantation (ranging from 14 to 2,744 days). In the work-up for ascites, the sensitivity of SAAG for portal hypertension was 82% and the sensitivity of aTP for portal hypertension was 50%.
Three of 12 patients who had a posttransplant SAAG had a gradient below 1.1 g/dL even though other tests found no evidence of another cause for ascites besides portal hypertension, and two of those three patients had confirmed portal hypertension, he reported. Five of 10 patients with an aTP had a value greater than 2.5 g/dL, even though they had confirmed portal hypertension and normal cardiac function, he reported.
"Assessment of ascites due to portal hypertension and/or vascular stricture in the posttransplant period using SAAG and aTP can be deceiving and cannot be relied upon to make diagnostic interventional decisions," said Dr. LaFond of the University of Virginia, Charlottesville. "A hepatic venogram should be performed early on in patients with posttransplant ascites to evaluate for strictures and portal hypertension.
Records showed that all patients in the study had ascites confirmed by imaging and/or paracenteses and had diagnostic studies to rule out heart failure, opportunistic infection, or malignancy as the source of ascites. Suspected portal hypertension was confirmed by pressure measurements or the presence of vascular strictures on venogram, with portal hypertension defined as a sinusoidal or portosystemic gradient greater than 5 or the presence of a stricture with a gradient of at least 3.
An estimated 3%-7% of patients develop ascites after liver transplant, which has been associated with an increased risk of renal impairment, prolonged hospitalization, and abdominal infections, he said.
Dr. LaFond reported having no financial disclosures.
On Twitter @sherryboschert
*This story was updated 6/3/2014.
AT DDW 2014
Key clinical point: Serum-ascites albumin gradient and ascites total protein cannot be relied upon to differentiate the cause of ascites after liver transplant.
Major finding: The sensitivity for portal hypertension as the cause of ascites after liver transplant was 82% for SAAG and 50% for aTP.
Data source: A retrospective study of 15 patients who developed symptomatic ascites after liver transplantation that required intervention.
Disclosures: Dr. LaFond reported having no financial disclosures.
Off-pump CABG reduces acute kidney injury, but not long-term function loss
Compared with on-pump coronary artery bypass graft surgery, off-pump CABG reduced the risk of postoperative acute kidney injury, but was not associated with better-preserved kidney function at 1 year in a substudy of the randomized CORONARY trial.
Acute kidney injury within 30 days of surgery occurred in 17.5% of 1,472 patients in the off-pump group, compared with 20.8% of 1,460 in the on-pump group (relative risk, 0.83). Loss of kidney function at 1 year occurred in 17.1% and 15.3% of patients in the groups, respectively (relative risk, 1.10), Dr. Amit X. Garg of the London (Ontario)Health Sciences Center, and his colleagues reported on behalf of the CORONARY (Coronary Artery Bypass Grafting Surgery Off- or On-pump Revascularization Study) investigators.
The observed relative risks for both acute kidney injury and long-term loss of kidney function were consistent when the data were reanalyzed using multiple alternate definitions of the two outcomes, as well as when using the composite outcomes of acute kidney injury or death, or kidney function loss or death, respectively, the authors said.
The findings were presented at the annual European Renal Association-European Dialysis and Transplant Association Congress, and were published simultaneously in JAMA.
CORONARY participants were 4,732 adults undergoing first isolated CABG surgery at 79 sites in 19 countries. Previously published results from the CORONARY trial showed no significant difference between on- and off-pump CABG with respect to a composite outcome of death, nonfatal myocardial infarction, stroke or new dialysis for kidney failure (or in any of these individual components) within 30 days or at 1 year postrandomization. Those included in the current analysis were 2,932 patients from 63 sites in 16 countries who were enrolled into the kidney function substudy between January, 2010 and November, 2011.
Acute kidney injury was defined as an increase of at least 50% in serum creatinine concentration from prerandomization. Loss of kidney function at 1 year was defined as at least a 20% loss in estimated glomerular filtration rate from prerandomization level, the investigators said (JAMA 2014 June 2[doi:10.1001/jama.2014.4952]).
The current findings, along with those from a recent meta-analysis of 22 prior randomized, controlled trials provide convincing evidence that off-pump vs. on-pump CABG surgery reduces the risk of mild to moderate acute kidney injury, particularly in those with preoperative chronic kidney disease, they said.
Mild or moderate acute kidney injury affects about 30% of patients after cardiac surgery, with about 1% requiring acute dialysis for severe kidney injury, but the effects of mild or moderate acute kidney injury on long-term kidney function are not clear.
Animal studies have suggested a causal link, and several human observational studies have shown a link as early as 3 months after injury, but "it remains unproven in a randomized clinical trial that an intervention that prevents such acute kidney injury better preserves long-term kidney function," they said.
The lack of an effect on long-term kidney function in the CORONARY trial may reflect inadequate follow-up, errors with serum creatinine concentration as a measure of kidney function, nonacute kidney injury effects of off-pump CABG surgery or differential care in follow-up between the groups, small magnitude of injury reduction with off-pump CABG, or lack of association between mild to moderate acute kidney injury and substantial chronic kidney disease.
"Regardless of the reason attributed to the observed 1-year kidney results from the CORONARY trial, the findings emphasize proof is needed to claim an intervention that reduces the risk of mild acute kidney injury better preserves long-term kidney function for the group that received it," the investigators wrote, adding that this has implications for the development, testing, and use of interventions to prevent acute kidney injury.
The findings support the current position of regulatory agencies – including the Food and Drug Administration – that no intervention will be approved based solely on its ability to prevent modest acute kidney injury, but rather that proof is required that the intervention has an effect on long-term permanent kidney function or other clinically meaningful events, they explained.
"This provides pause for interventions such as N-acetylcysteine and intravenous sodium bicarbonate, which have been broadly adopted because smaller trials demonstrated a reduced risk of modest acute kidney injury without proof of an effect on permanent kidney function. Our results also have implications for trials currently examining intervention effects on mild acute kidney injury without assessing long-term kidney function," they concluded. Furthermore, future trials should consider multiple measures of kidney function over time, examine trajectories of kidney function loss, and use new markers of kidney function or injury, and enroll a greater number of patients with baseline chronic kidney disease to better ascertain whether a causal relationship exists between acute kidney injury and long-term kidney function, they said.
CORONARY and the kidney substudy were supported by grants from the Canadian Institutes of Health Research.
Dr. Garg reported receiving grant funding from Astellas, Roche, and Pfizer. Another author (Dr. P.J. Devereaux) reported receiving grants from Abbott Diagnostics, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Covidien, Roche Diagnostics, and Stryker, and one (Dr. Chirag R. Parikh) reported participating on a data monitoring committee for a phase II trial sponsored by AbbVie. The remaining authors reported having no disclosures.
Compared with on-pump coronary artery bypass graft surgery, off-pump CABG reduced the risk of postoperative acute kidney injury, but was not associated with better-preserved kidney function at 1 year in a substudy of the randomized CORONARY trial.
Acute kidney injury within 30 days of surgery occurred in 17.5% of 1,472 patients in the off-pump group, compared with 20.8% of 1,460 in the on-pump group (relative risk, 0.83). Loss of kidney function at 1 year occurred in 17.1% and 15.3% of patients in the groups, respectively (relative risk, 1.10), Dr. Amit X. Garg of the London (Ontario)Health Sciences Center, and his colleagues reported on behalf of the CORONARY (Coronary Artery Bypass Grafting Surgery Off- or On-pump Revascularization Study) investigators.
The observed relative risks for both acute kidney injury and long-term loss of kidney function were consistent when the data were reanalyzed using multiple alternate definitions of the two outcomes, as well as when using the composite outcomes of acute kidney injury or death, or kidney function loss or death, respectively, the authors said.
The findings were presented at the annual European Renal Association-European Dialysis and Transplant Association Congress, and were published simultaneously in JAMA.
CORONARY participants were 4,732 adults undergoing first isolated CABG surgery at 79 sites in 19 countries. Previously published results from the CORONARY trial showed no significant difference between on- and off-pump CABG with respect to a composite outcome of death, nonfatal myocardial infarction, stroke or new dialysis for kidney failure (or in any of these individual components) within 30 days or at 1 year postrandomization. Those included in the current analysis were 2,932 patients from 63 sites in 16 countries who were enrolled into the kidney function substudy between January, 2010 and November, 2011.
Acute kidney injury was defined as an increase of at least 50% in serum creatinine concentration from prerandomization. Loss of kidney function at 1 year was defined as at least a 20% loss in estimated glomerular filtration rate from prerandomization level, the investigators said (JAMA 2014 June 2[doi:10.1001/jama.2014.4952]).
The current findings, along with those from a recent meta-analysis of 22 prior randomized, controlled trials provide convincing evidence that off-pump vs. on-pump CABG surgery reduces the risk of mild to moderate acute kidney injury, particularly in those with preoperative chronic kidney disease, they said.
Mild or moderate acute kidney injury affects about 30% of patients after cardiac surgery, with about 1% requiring acute dialysis for severe kidney injury, but the effects of mild or moderate acute kidney injury on long-term kidney function are not clear.
Animal studies have suggested a causal link, and several human observational studies have shown a link as early as 3 months after injury, but "it remains unproven in a randomized clinical trial that an intervention that prevents such acute kidney injury better preserves long-term kidney function," they said.
The lack of an effect on long-term kidney function in the CORONARY trial may reflect inadequate follow-up, errors with serum creatinine concentration as a measure of kidney function, nonacute kidney injury effects of off-pump CABG surgery or differential care in follow-up between the groups, small magnitude of injury reduction with off-pump CABG, or lack of association between mild to moderate acute kidney injury and substantial chronic kidney disease.
"Regardless of the reason attributed to the observed 1-year kidney results from the CORONARY trial, the findings emphasize proof is needed to claim an intervention that reduces the risk of mild acute kidney injury better preserves long-term kidney function for the group that received it," the investigators wrote, adding that this has implications for the development, testing, and use of interventions to prevent acute kidney injury.
The findings support the current position of regulatory agencies – including the Food and Drug Administration – that no intervention will be approved based solely on its ability to prevent modest acute kidney injury, but rather that proof is required that the intervention has an effect on long-term permanent kidney function or other clinically meaningful events, they explained.
"This provides pause for interventions such as N-acetylcysteine and intravenous sodium bicarbonate, which have been broadly adopted because smaller trials demonstrated a reduced risk of modest acute kidney injury without proof of an effect on permanent kidney function. Our results also have implications for trials currently examining intervention effects on mild acute kidney injury without assessing long-term kidney function," they concluded. Furthermore, future trials should consider multiple measures of kidney function over time, examine trajectories of kidney function loss, and use new markers of kidney function or injury, and enroll a greater number of patients with baseline chronic kidney disease to better ascertain whether a causal relationship exists between acute kidney injury and long-term kidney function, they said.
CORONARY and the kidney substudy were supported by grants from the Canadian Institutes of Health Research.
Dr. Garg reported receiving grant funding from Astellas, Roche, and Pfizer. Another author (Dr. P.J. Devereaux) reported receiving grants from Abbott Diagnostics, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Covidien, Roche Diagnostics, and Stryker, and one (Dr. Chirag R. Parikh) reported participating on a data monitoring committee for a phase II trial sponsored by AbbVie. The remaining authors reported having no disclosures.
Compared with on-pump coronary artery bypass graft surgery, off-pump CABG reduced the risk of postoperative acute kidney injury, but was not associated with better-preserved kidney function at 1 year in a substudy of the randomized CORONARY trial.
Acute kidney injury within 30 days of surgery occurred in 17.5% of 1,472 patients in the off-pump group, compared with 20.8% of 1,460 in the on-pump group (relative risk, 0.83). Loss of kidney function at 1 year occurred in 17.1% and 15.3% of patients in the groups, respectively (relative risk, 1.10), Dr. Amit X. Garg of the London (Ontario)Health Sciences Center, and his colleagues reported on behalf of the CORONARY (Coronary Artery Bypass Grafting Surgery Off- or On-pump Revascularization Study) investigators.
The observed relative risks for both acute kidney injury and long-term loss of kidney function were consistent when the data were reanalyzed using multiple alternate definitions of the two outcomes, as well as when using the composite outcomes of acute kidney injury or death, or kidney function loss or death, respectively, the authors said.
The findings were presented at the annual European Renal Association-European Dialysis and Transplant Association Congress, and were published simultaneously in JAMA.
CORONARY participants were 4,732 adults undergoing first isolated CABG surgery at 79 sites in 19 countries. Previously published results from the CORONARY trial showed no significant difference between on- and off-pump CABG with respect to a composite outcome of death, nonfatal myocardial infarction, stroke or new dialysis for kidney failure (or in any of these individual components) within 30 days or at 1 year postrandomization. Those included in the current analysis were 2,932 patients from 63 sites in 16 countries who were enrolled into the kidney function substudy between January, 2010 and November, 2011.
Acute kidney injury was defined as an increase of at least 50% in serum creatinine concentration from prerandomization. Loss of kidney function at 1 year was defined as at least a 20% loss in estimated glomerular filtration rate from prerandomization level, the investigators said (JAMA 2014 June 2[doi:10.1001/jama.2014.4952]).
The current findings, along with those from a recent meta-analysis of 22 prior randomized, controlled trials provide convincing evidence that off-pump vs. on-pump CABG surgery reduces the risk of mild to moderate acute kidney injury, particularly in those with preoperative chronic kidney disease, they said.
Mild or moderate acute kidney injury affects about 30% of patients after cardiac surgery, with about 1% requiring acute dialysis for severe kidney injury, but the effects of mild or moderate acute kidney injury on long-term kidney function are not clear.
Animal studies have suggested a causal link, and several human observational studies have shown a link as early as 3 months after injury, but "it remains unproven in a randomized clinical trial that an intervention that prevents such acute kidney injury better preserves long-term kidney function," they said.
The lack of an effect on long-term kidney function in the CORONARY trial may reflect inadequate follow-up, errors with serum creatinine concentration as a measure of kidney function, nonacute kidney injury effects of off-pump CABG surgery or differential care in follow-up between the groups, small magnitude of injury reduction with off-pump CABG, or lack of association between mild to moderate acute kidney injury and substantial chronic kidney disease.
"Regardless of the reason attributed to the observed 1-year kidney results from the CORONARY trial, the findings emphasize proof is needed to claim an intervention that reduces the risk of mild acute kidney injury better preserves long-term kidney function for the group that received it," the investigators wrote, adding that this has implications for the development, testing, and use of interventions to prevent acute kidney injury.
The findings support the current position of regulatory agencies – including the Food and Drug Administration – that no intervention will be approved based solely on its ability to prevent modest acute kidney injury, but rather that proof is required that the intervention has an effect on long-term permanent kidney function or other clinically meaningful events, they explained.
"This provides pause for interventions such as N-acetylcysteine and intravenous sodium bicarbonate, which have been broadly adopted because smaller trials demonstrated a reduced risk of modest acute kidney injury without proof of an effect on permanent kidney function. Our results also have implications for trials currently examining intervention effects on mild acute kidney injury without assessing long-term kidney function," they concluded. Furthermore, future trials should consider multiple measures of kidney function over time, examine trajectories of kidney function loss, and use new markers of kidney function or injury, and enroll a greater number of patients with baseline chronic kidney disease to better ascertain whether a causal relationship exists between acute kidney injury and long-term kidney function, they said.
CORONARY and the kidney substudy were supported by grants from the Canadian Institutes of Health Research.
Dr. Garg reported receiving grant funding from Astellas, Roche, and Pfizer. Another author (Dr. P.J. Devereaux) reported receiving grants from Abbott Diagnostics, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Covidien, Roche Diagnostics, and Stryker, and one (Dr. Chirag R. Parikh) reported participating on a data monitoring committee for a phase II trial sponsored by AbbVie. The remaining authors reported having no disclosures.
FROM THE ERA-EDTA ANNUAL CONGRESS
Key clinical point: Preservation of kidney function is not a reason to choose off-pump CABG over on-pump.
Major finding: Relative risk of acute kidney injury, 0.83; relative risk of kidney function loss at 1 year, 1.10 with off- vs. on-pump CABG.
Data source: A substudy of 2,932 patients from the randomized CORONARY trial.
Disclosures: CORONARY and the kidney substudy were supported by grants from the Canadian Institutes of Health Research. Dr. Garg reported receiving grant funding from Astellas, Roche, and Pfizer. Another author (Dr. P.J. Devereaux) reported receiving grants from Abbott Diagnostics, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Covidien, Roche Diagnostics, and Stryker, and one (Dr. Chirag R. Parikh) reported participating on a data monitoring committee for a phase II trial sponsored by AbbVie. The remaining authors reported having no disclosures.
FDA approves generic celecoxib
The first generic versions of celecoxib received approval May 30 by the Food and Drug Administration.
Teva Pharmaceutical Industries received approval to market celecoxib capsules in 50 mg, 100 mg, 200 mg, and 400 mg strengths, and has 180-day exclusivity on the 100 mg, 200 mg, and 400 mg strength products. Mylan Pharmaceuticals received approval to market 50 mg celecoxib capsules.
Citing the importance of "affordable treatment options for chronic conditions," Dr. Janet Woodcock, director of the FDA’s Center for Drug Evaluation and Research, added in a written statement, the assurance "that these FDA-approved generic drugs have met our rigorous approval standards." Generic prescription drugs approved by the FDA are of the same strength as brand-name drugs, and generic drug manufacturing and packaging sites must pass the same quality standards as those of brand-name drugs.
As with its trade version Celebrex and all nonsteroidal anti-inflammatory drugs, the two new generics will carry a boxed warning in their prescribing information regarding the risks of heart attack or stroke and serious gastrointestinal bleeding. These labels also will note that risk is increased for patients who have heart disease or heart disease risk factors, as it is for patients who take NSAIDs for prolonged periods of time.
Information about the availability of generic celecoxib is available from the companies.
On Twitter @maryjodales
The first generic versions of celecoxib received approval May 30 by the Food and Drug Administration.
Teva Pharmaceutical Industries received approval to market celecoxib capsules in 50 mg, 100 mg, 200 mg, and 400 mg strengths, and has 180-day exclusivity on the 100 mg, 200 mg, and 400 mg strength products. Mylan Pharmaceuticals received approval to market 50 mg celecoxib capsules.
Citing the importance of "affordable treatment options for chronic conditions," Dr. Janet Woodcock, director of the FDA’s Center for Drug Evaluation and Research, added in a written statement, the assurance "that these FDA-approved generic drugs have met our rigorous approval standards." Generic prescription drugs approved by the FDA are of the same strength as brand-name drugs, and generic drug manufacturing and packaging sites must pass the same quality standards as those of brand-name drugs.
As with its trade version Celebrex and all nonsteroidal anti-inflammatory drugs, the two new generics will carry a boxed warning in their prescribing information regarding the risks of heart attack or stroke and serious gastrointestinal bleeding. These labels also will note that risk is increased for patients who have heart disease or heart disease risk factors, as it is for patients who take NSAIDs for prolonged periods of time.
Information about the availability of generic celecoxib is available from the companies.
On Twitter @maryjodales
The first generic versions of celecoxib received approval May 30 by the Food and Drug Administration.
Teva Pharmaceutical Industries received approval to market celecoxib capsules in 50 mg, 100 mg, 200 mg, and 400 mg strengths, and has 180-day exclusivity on the 100 mg, 200 mg, and 400 mg strength products. Mylan Pharmaceuticals received approval to market 50 mg celecoxib capsules.
Citing the importance of "affordable treatment options for chronic conditions," Dr. Janet Woodcock, director of the FDA’s Center for Drug Evaluation and Research, added in a written statement, the assurance "that these FDA-approved generic drugs have met our rigorous approval standards." Generic prescription drugs approved by the FDA are of the same strength as brand-name drugs, and generic drug manufacturing and packaging sites must pass the same quality standards as those of brand-name drugs.
As with its trade version Celebrex and all nonsteroidal anti-inflammatory drugs, the two new generics will carry a boxed warning in their prescribing information regarding the risks of heart attack or stroke and serious gastrointestinal bleeding. These labels also will note that risk is increased for patients who have heart disease or heart disease risk factors, as it is for patients who take NSAIDs for prolonged periods of time.
Information about the availability of generic celecoxib is available from the companies.
On Twitter @maryjodales
Prevention bundle led to drop in postoperative pressure ulcers
BALTIMORE – Implementing a bundle of preventive interventions led to a significant drop in pressure ulcers acquired during hospitalization among high-risk surgical patients, according to a prospective study.
Moreover, the beneficial effect of the program was sustained for more than a year after the program was implemented, reported Dr. Sylvia Martinez of Baylor College of Medicine and the Michael E. DeBakey VA Medical Center in Houston.
Dr. Martinez pointed out that strategies used to prevent hospital-acquired pressure ulcers are not standardized, and that in an effort to improve quality of care, save money, and reduce mortality, the Centers for Medicare & Medicaid Services no longer reimburses medical facilities for stage III and IV hospital-acquired pressure ulcers.
More than 7,000 high-risk patients were included in the study. During the 6 months before the plan was implemented at the medical center, the mean pressure ulcer rate overall was 3.37, dropping to 1.45 during the 6 months after implementation, a significant difference (P = .004). At 18 months, the rate was 0.89. During this time, the mean rates of hospital-acquired pressure ulcers in the entire VA system did not significantly change, ranging from 1.68 to 1.85, Dr. Martinez reported at the annual meeting of the Surgical Infection Society.
The study was done to address the relatively high rate of pressure ulcers in the medical center’s surgical service. Dr. Martinez and her associates evaluated the impact of an evidence-based hospital-acquired pressure ulcer prevention bundle, developed by an interdisciplinary team.
Patients were classified as high risk if they met at least two of four criteria (age greater than 62 years, albumin less than 3.5 g/dL, ASA score greater than 3, and surgery lasting for more than 3 hours).
The components of the program included:
• Comprehensive skin assessment and documentation and identification of any ulcers on admission, transfer, and discharge.
• Use of operating room beds with pressure redistributing mattresses, with careful positioning and padding of pressure points.
• Turning of patients every 2 hours with pressure point relief via wedges, heel pads, and pillows, as well as massages of bony prominences.
• Assessment of patients for pressure ulcers on daily rounds (led by the unit nurse manager) and use of daily checklists in high-risk patients.
The pressure ulcer rate was calculated by the number of discharged acute-care patients with a stage II or greater hospital-acquired pressure ulcer, divided by number patient days in the hospital (the discharge date minus the admission date for all discharged acute-care patients who were hospitalized for at least 48 hours).
During the period studied, 21,377 operations were performed. Almost 5,000 (4,692) were during the 6-month period prior to implementation of the program, from January to June 2012, and 16,685 were done in the 18 months after implementation (including 4,461 during the first 6 months). Overall, 34% of the patients were determined to be at high risk.
The rates dropped significantly in different parts of the surgical service: In the surgical intensive care unit, the rate dropped from 2.93 before implementation to 1.25 at 6 months and 0.86 at 18 months post implementation. In the surgical wards, the rate dropped from 2.93 to 0.83 at 6 months, and was 0.86 at 18 months.
Implementation of this bundle "may decrease hospital-acquired pressure ulcer rates in high risk surgical patients," with sustained lower rates, which "can lead to decreased costs, complications, and length of stay, and improved quality of life for our patients," Dr. Martinez concluded.
Among the study’s limitations were that it was conducted at one hospital and it did not evaluate which elements of the prevention bundle were most important, she said.
Dr. Martinez had no disclosures. The study was funded by the U.S. Department of Veterans Affairs.
BALTIMORE – Implementing a bundle of preventive interventions led to a significant drop in pressure ulcers acquired during hospitalization among high-risk surgical patients, according to a prospective study.
Moreover, the beneficial effect of the program was sustained for more than a year after the program was implemented, reported Dr. Sylvia Martinez of Baylor College of Medicine and the Michael E. DeBakey VA Medical Center in Houston.
Dr. Martinez pointed out that strategies used to prevent hospital-acquired pressure ulcers are not standardized, and that in an effort to improve quality of care, save money, and reduce mortality, the Centers for Medicare & Medicaid Services no longer reimburses medical facilities for stage III and IV hospital-acquired pressure ulcers.
More than 7,000 high-risk patients were included in the study. During the 6 months before the plan was implemented at the medical center, the mean pressure ulcer rate overall was 3.37, dropping to 1.45 during the 6 months after implementation, a significant difference (P = .004). At 18 months, the rate was 0.89. During this time, the mean rates of hospital-acquired pressure ulcers in the entire VA system did not significantly change, ranging from 1.68 to 1.85, Dr. Martinez reported at the annual meeting of the Surgical Infection Society.
The study was done to address the relatively high rate of pressure ulcers in the medical center’s surgical service. Dr. Martinez and her associates evaluated the impact of an evidence-based hospital-acquired pressure ulcer prevention bundle, developed by an interdisciplinary team.
Patients were classified as high risk if they met at least two of four criteria (age greater than 62 years, albumin less than 3.5 g/dL, ASA score greater than 3, and surgery lasting for more than 3 hours).
The components of the program included:
• Comprehensive skin assessment and documentation and identification of any ulcers on admission, transfer, and discharge.
• Use of operating room beds with pressure redistributing mattresses, with careful positioning and padding of pressure points.
• Turning of patients every 2 hours with pressure point relief via wedges, heel pads, and pillows, as well as massages of bony prominences.
• Assessment of patients for pressure ulcers on daily rounds (led by the unit nurse manager) and use of daily checklists in high-risk patients.
The pressure ulcer rate was calculated by the number of discharged acute-care patients with a stage II or greater hospital-acquired pressure ulcer, divided by number patient days in the hospital (the discharge date minus the admission date for all discharged acute-care patients who were hospitalized for at least 48 hours).
During the period studied, 21,377 operations were performed. Almost 5,000 (4,692) were during the 6-month period prior to implementation of the program, from January to June 2012, and 16,685 were done in the 18 months after implementation (including 4,461 during the first 6 months). Overall, 34% of the patients were determined to be at high risk.
The rates dropped significantly in different parts of the surgical service: In the surgical intensive care unit, the rate dropped from 2.93 before implementation to 1.25 at 6 months and 0.86 at 18 months post implementation. In the surgical wards, the rate dropped from 2.93 to 0.83 at 6 months, and was 0.86 at 18 months.
Implementation of this bundle "may decrease hospital-acquired pressure ulcer rates in high risk surgical patients," with sustained lower rates, which "can lead to decreased costs, complications, and length of stay, and improved quality of life for our patients," Dr. Martinez concluded.
Among the study’s limitations were that it was conducted at one hospital and it did not evaluate which elements of the prevention bundle were most important, she said.
Dr. Martinez had no disclosures. The study was funded by the U.S. Department of Veterans Affairs.
BALTIMORE – Implementing a bundle of preventive interventions led to a significant drop in pressure ulcers acquired during hospitalization among high-risk surgical patients, according to a prospective study.
Moreover, the beneficial effect of the program was sustained for more than a year after the program was implemented, reported Dr. Sylvia Martinez of Baylor College of Medicine and the Michael E. DeBakey VA Medical Center in Houston.
Dr. Martinez pointed out that strategies used to prevent hospital-acquired pressure ulcers are not standardized, and that in an effort to improve quality of care, save money, and reduce mortality, the Centers for Medicare & Medicaid Services no longer reimburses medical facilities for stage III and IV hospital-acquired pressure ulcers.
More than 7,000 high-risk patients were included in the study. During the 6 months before the plan was implemented at the medical center, the mean pressure ulcer rate overall was 3.37, dropping to 1.45 during the 6 months after implementation, a significant difference (P = .004). At 18 months, the rate was 0.89. During this time, the mean rates of hospital-acquired pressure ulcers in the entire VA system did not significantly change, ranging from 1.68 to 1.85, Dr. Martinez reported at the annual meeting of the Surgical Infection Society.
The study was done to address the relatively high rate of pressure ulcers in the medical center’s surgical service. Dr. Martinez and her associates evaluated the impact of an evidence-based hospital-acquired pressure ulcer prevention bundle, developed by an interdisciplinary team.
Patients were classified as high risk if they met at least two of four criteria (age greater than 62 years, albumin less than 3.5 g/dL, ASA score greater than 3, and surgery lasting for more than 3 hours).
The components of the program included:
• Comprehensive skin assessment and documentation and identification of any ulcers on admission, transfer, and discharge.
• Use of operating room beds with pressure redistributing mattresses, with careful positioning and padding of pressure points.
• Turning of patients every 2 hours with pressure point relief via wedges, heel pads, and pillows, as well as massages of bony prominences.
• Assessment of patients for pressure ulcers on daily rounds (led by the unit nurse manager) and use of daily checklists in high-risk patients.
The pressure ulcer rate was calculated by the number of discharged acute-care patients with a stage II or greater hospital-acquired pressure ulcer, divided by number patient days in the hospital (the discharge date minus the admission date for all discharged acute-care patients who were hospitalized for at least 48 hours).
During the period studied, 21,377 operations were performed. Almost 5,000 (4,692) were during the 6-month period prior to implementation of the program, from January to June 2012, and 16,685 were done in the 18 months after implementation (including 4,461 during the first 6 months). Overall, 34% of the patients were determined to be at high risk.
The rates dropped significantly in different parts of the surgical service: In the surgical intensive care unit, the rate dropped from 2.93 before implementation to 1.25 at 6 months and 0.86 at 18 months post implementation. In the surgical wards, the rate dropped from 2.93 to 0.83 at 6 months, and was 0.86 at 18 months.
Implementation of this bundle "may decrease hospital-acquired pressure ulcer rates in high risk surgical patients," with sustained lower rates, which "can lead to decreased costs, complications, and length of stay, and improved quality of life for our patients," Dr. Martinez concluded.
Among the study’s limitations were that it was conducted at one hospital and it did not evaluate which elements of the prevention bundle were most important, she said.
Dr. Martinez had no disclosures. The study was funded by the U.S. Department of Veterans Affairs.
AT THE SIS ANNUAL MEETING
Key clinical point: Use preventive measures to reduce pressure ulcers in high-risk surgical patients.
Major finding: The rate of pressure ulcers acquired during hospitalization dropped from 3.37 during the 6 months before a prevention bundle was implemented to 0.89 18 months afterward.
Data source: A prospective study of surgical patients at high risk for developing bedsores, comparing rates of hospital-acquired pressure ulcers 6 and 18 months after preventive interventions with the 6-month period beforehand.
Disclosures: Dr. Martinez had no disclosures. The study was funded by the U.S. Department of Veterans Affairs.
Pasireotide decreases incidence of postoperative fistula
The somatostatin analogue pasireotide reduced postoperative pancreatic fistula leak or abscess by 56%, compared with placebo, a randomized study has determined.
Pasireotide (Signifor) was effective after both pancreaticoduodenectomy and distal pancreatectomy, whether or not the pancreatic duct was dilated, Dr. Peter J. Allen and his colleagues wrote in the May 21 issue of the New England Journal of Medicine (N. Engl. J. Med. 2014;370:2014-22).
In those patients who did develop fistulas or leaks, pasireotide was associated with fewer grade 3 occurrences.
"These results suggest that ... not only were many leaks and fistulas prevented, but when they did occur they were less clinically relevant," wrote Dr. Allen of the Memorial Sloan Kettering Cancer Center, New York, and his coauthors.
The study randomized 300 patients to subcutaneous injections of either placebo or pasireotide twice daily for 7 days after pancreatic surgery. The primary endpoint was the development of a pancreatic leak, fistula, or abscess of at least grade 3. Secondary endpoints included the overall rate of pancreatic complications (all grades) and the rate of grade B or grade C pancreatic fistula.
Patients were a mean of 64 years old. Most (73%) underwent a pancreaticoduodenectomy. The average length of stay for these patients was about 10 days. The active group received 900 mcg of pasireotide subcutaneously twice daily for 7 days, beginning on the morning of surgery.
Mean postoperative serum glucose levels were significantly higher in patients taking pasireotide (258 mg/dL vs. 215 mg/dL). Readmission occurred in significantly fewer pasireotide patients (17% vs. 29%).
Significantly fewer of those taking the active drug were able to finish the entire course of 14 doses (76% vs. 86% given placebo). The lower completion rate was mostly due to nausea and vomiting, which caused 26 patients in the active group and 3 in the placebo group to withdraw from the study.
A leak or fistula of grade 3 or higher developed in 45 patients. The outcome was significantly less common among those taking pasireotide than among those on placebo (9% vs. 21%; relative risk, 0.44). "This corresponded to an absolute risk reduction of 11.7 percentage points," with a number needed to treat of 8, the investigators said.
Pasireotide was significantly more effective than placebo in surgical subgroups, including pancreaticoduodenectomy (RR, 0.49) and distal pancreatectomy (RR, 0.32). The effect was also positive whether the pancreatic duct was dilated (RR, 0.11) or nondilated (RR, 0.55).
The secondary outcome (grade B or C postoperative fistula) occurred in 37 patients (12%). In the pasireotide group, there were 12 grade B fistulas and no grade C fistulas. In the placebo group, there were 20 grade B and 5 grade C fistulas.
Overall 60-day mortality was 0.7% (one death in each treatment group). Grade 3 and 4 complications were common, occurring in 92% of the pasireotide group and 90% of the placebo group. Most of these were expected postoperative serum abnormalities.
The investigators said that the other approved somatostatin analogue, octreotide, has not been clearly associated with pancreatic leak reduction. They suggested that pasireotide may be more effective because it has a longer half-life and binds to four of the five somatostatin-receptor subtypes, rather than just two, as octreotide does.
They added that the octreotide studies were conducted before 2005, when there was no consistent definition of postoperative pancreatic fistula. Therefore, they concluded, the extant data cannot be used to identify octreotide efficacy in this application.
Pasireotide, which is made by Novartis Pharmaceuticals, is currently approved as an injection for the treatment of Cushing’s disease patients who cannot be helped through surgery.
Novartis Pharmaceuticals sponsored the trial. Dr. Allen received Novartis grant funding but had no other financial ties with the company.
The somatostatin analogue pasireotide reduced postoperative pancreatic fistula leak or abscess by 56%, compared with placebo, a randomized study has determined.
Pasireotide (Signifor) was effective after both pancreaticoduodenectomy and distal pancreatectomy, whether or not the pancreatic duct was dilated, Dr. Peter J. Allen and his colleagues wrote in the May 21 issue of the New England Journal of Medicine (N. Engl. J. Med. 2014;370:2014-22).
In those patients who did develop fistulas or leaks, pasireotide was associated with fewer grade 3 occurrences.
"These results suggest that ... not only were many leaks and fistulas prevented, but when they did occur they were less clinically relevant," wrote Dr. Allen of the Memorial Sloan Kettering Cancer Center, New York, and his coauthors.
The study randomized 300 patients to subcutaneous injections of either placebo or pasireotide twice daily for 7 days after pancreatic surgery. The primary endpoint was the development of a pancreatic leak, fistula, or abscess of at least grade 3. Secondary endpoints included the overall rate of pancreatic complications (all grades) and the rate of grade B or grade C pancreatic fistula.
Patients were a mean of 64 years old. Most (73%) underwent a pancreaticoduodenectomy. The average length of stay for these patients was about 10 days. The active group received 900 mcg of pasireotide subcutaneously twice daily for 7 days, beginning on the morning of surgery.
Mean postoperative serum glucose levels were significantly higher in patients taking pasireotide (258 mg/dL vs. 215 mg/dL). Readmission occurred in significantly fewer pasireotide patients (17% vs. 29%).
Significantly fewer of those taking the active drug were able to finish the entire course of 14 doses (76% vs. 86% given placebo). The lower completion rate was mostly due to nausea and vomiting, which caused 26 patients in the active group and 3 in the placebo group to withdraw from the study.
A leak or fistula of grade 3 or higher developed in 45 patients. The outcome was significantly less common among those taking pasireotide than among those on placebo (9% vs. 21%; relative risk, 0.44). "This corresponded to an absolute risk reduction of 11.7 percentage points," with a number needed to treat of 8, the investigators said.
Pasireotide was significantly more effective than placebo in surgical subgroups, including pancreaticoduodenectomy (RR, 0.49) and distal pancreatectomy (RR, 0.32). The effect was also positive whether the pancreatic duct was dilated (RR, 0.11) or nondilated (RR, 0.55).
The secondary outcome (grade B or C postoperative fistula) occurred in 37 patients (12%). In the pasireotide group, there were 12 grade B fistulas and no grade C fistulas. In the placebo group, there were 20 grade B and 5 grade C fistulas.
Overall 60-day mortality was 0.7% (one death in each treatment group). Grade 3 and 4 complications were common, occurring in 92% of the pasireotide group and 90% of the placebo group. Most of these were expected postoperative serum abnormalities.
The investigators said that the other approved somatostatin analogue, octreotide, has not been clearly associated with pancreatic leak reduction. They suggested that pasireotide may be more effective because it has a longer half-life and binds to four of the five somatostatin-receptor subtypes, rather than just two, as octreotide does.
They added that the octreotide studies were conducted before 2005, when there was no consistent definition of postoperative pancreatic fistula. Therefore, they concluded, the extant data cannot be used to identify octreotide efficacy in this application.
Pasireotide, which is made by Novartis Pharmaceuticals, is currently approved as an injection for the treatment of Cushing’s disease patients who cannot be helped through surgery.
Novartis Pharmaceuticals sponsored the trial. Dr. Allen received Novartis grant funding but had no other financial ties with the company.
The somatostatin analogue pasireotide reduced postoperative pancreatic fistula leak or abscess by 56%, compared with placebo, a randomized study has determined.
Pasireotide (Signifor) was effective after both pancreaticoduodenectomy and distal pancreatectomy, whether or not the pancreatic duct was dilated, Dr. Peter J. Allen and his colleagues wrote in the May 21 issue of the New England Journal of Medicine (N. Engl. J. Med. 2014;370:2014-22).
In those patients who did develop fistulas or leaks, pasireotide was associated with fewer grade 3 occurrences.
"These results suggest that ... not only were many leaks and fistulas prevented, but when they did occur they were less clinically relevant," wrote Dr. Allen of the Memorial Sloan Kettering Cancer Center, New York, and his coauthors.
The study randomized 300 patients to subcutaneous injections of either placebo or pasireotide twice daily for 7 days after pancreatic surgery. The primary endpoint was the development of a pancreatic leak, fistula, or abscess of at least grade 3. Secondary endpoints included the overall rate of pancreatic complications (all grades) and the rate of grade B or grade C pancreatic fistula.
Patients were a mean of 64 years old. Most (73%) underwent a pancreaticoduodenectomy. The average length of stay for these patients was about 10 days. The active group received 900 mcg of pasireotide subcutaneously twice daily for 7 days, beginning on the morning of surgery.
Mean postoperative serum glucose levels were significantly higher in patients taking pasireotide (258 mg/dL vs. 215 mg/dL). Readmission occurred in significantly fewer pasireotide patients (17% vs. 29%).
Significantly fewer of those taking the active drug were able to finish the entire course of 14 doses (76% vs. 86% given placebo). The lower completion rate was mostly due to nausea and vomiting, which caused 26 patients in the active group and 3 in the placebo group to withdraw from the study.
A leak or fistula of grade 3 or higher developed in 45 patients. The outcome was significantly less common among those taking pasireotide than among those on placebo (9% vs. 21%; relative risk, 0.44). "This corresponded to an absolute risk reduction of 11.7 percentage points," with a number needed to treat of 8, the investigators said.
Pasireotide was significantly more effective than placebo in surgical subgroups, including pancreaticoduodenectomy (RR, 0.49) and distal pancreatectomy (RR, 0.32). The effect was also positive whether the pancreatic duct was dilated (RR, 0.11) or nondilated (RR, 0.55).
The secondary outcome (grade B or C postoperative fistula) occurred in 37 patients (12%). In the pasireotide group, there were 12 grade B fistulas and no grade C fistulas. In the placebo group, there were 20 grade B and 5 grade C fistulas.
Overall 60-day mortality was 0.7% (one death in each treatment group). Grade 3 and 4 complications were common, occurring in 92% of the pasireotide group and 90% of the placebo group. Most of these were expected postoperative serum abnormalities.
The investigators said that the other approved somatostatin analogue, octreotide, has not been clearly associated with pancreatic leak reduction. They suggested that pasireotide may be more effective because it has a longer half-life and binds to four of the five somatostatin-receptor subtypes, rather than just two, as octreotide does.
They added that the octreotide studies were conducted before 2005, when there was no consistent definition of postoperative pancreatic fistula. Therefore, they concluded, the extant data cannot be used to identify octreotide efficacy in this application.
Pasireotide, which is made by Novartis Pharmaceuticals, is currently approved as an injection for the treatment of Cushing’s disease patients who cannot be helped through surgery.
Novartis Pharmaceuticals sponsored the trial. Dr. Allen received Novartis grant funding but had no other financial ties with the company.
FROM NEJM
Key clinical point: Pasireotide reduced the incidence of postoperative pancreatic fistula, leak, or abscess.
Major finding: Compared with placebo, pasireotide reduced the rate of fistula, leak, or abscess by 56%.
Data source: The randomized, placebo-controlled study included 300 patients.
Disclosures: Novartis Pharmaceuticals sponsored the trial. Dr. Allen received Novartis grant funding but had no other financial ties with the company.
Shorter antibiotic course treats periotoneal infections
BALTIMORE – A study of patients with intraabdominal infection indicates that a shorter course of antibiotics is as effective as the standard, longer course, leading researchers to recommend a new standard of care.
The rates of recurrent infections and other outcomes were similar in patients with intraabdominal infections treated with antibiotics for 4 days following source control and among those who received a longer course of treatment based on when their clinical symptoms resolved, in a study reported at the annual meeting of the Surgical Infection Society.
The results support the use of the shorter treatment strategy in this setting, said Dr. Robert Sawyer, who presented the results of the study, SIS Multicenter Study of Duration of Antibiotics for Intraabdominal Infection.
While antibiotics are used as an adjunct to treat intraabdominal infections, the appropriate duration of treatment is not clear, and reducing the time exposed to antibiotics "could be worthwhile," said Dr. Sawyer, professor of surgery and public health sciences and chief of acute care surgery at the University of Virginia, Charlottesville.
The randomized, multicenter, unblinded study compared a shorter vs. a longer course of antibiotic therapy in 518 patients with intraabdominal infections: 4 days in 258 patients or treatment that continued for 2 days after resolution of the patient’s fever, leukocytosis, and ileus, with a maximum of 10 days of treatment in total, in 260 patients. The patients had similar APACHE II scores; their mean age was in the early 50s; and the colon or rectum was the most common source of infection, followed by the appendix and the small bowel. Antibiotics were administered intravenously or orally. Patients with inadequate source control and a high risk of death within 72 hours were excluded.
Within 30 days, the primary outcome – a composite of surgical site infection, recurrent intraabdominal infection, and death – was similar between the two groups, at about 21.7% in the 4-day treatment group vs. 22.7% in the group treated for 2 days after symptoms resolved.
There were no significant differences in the individual endpoints between the two groups. There were also no differences in the composite endpoints in different subgroups, and when those with percutaneous drainage or patients whose source of infection was the appendix were excluded. Among sicker patients with APACHE scores of 10 or higher, there was a numerical benefit favoring those treated for 4 days (22.1% vs. 29.8% among those treated for the longer duration), but the difference was not statistically significant, although it was reassuring, Dr. Sawyer said.
There were no differences between the two groups in the rates of secondary infections or subsequent infection with resistant pathogens or Clostridium difficile, which were secondary endpoints.
Based on these results, "after source control is obtained, we recommend 4 days of antimicrobial therapy for all patients with intraabdominal infections as the new standard of care," Dr. Sawyer concluded.
Although they had positive comments about the study, several members of the audience commented that a larger study would be a better base for a recommendation to change the standard of care.
This study was one of several studies presented at the meeting that addressed the issue of reducing the time on antibiotic therapy in patients with surgical infections.
The study was sponsored by the National Institutes of Health. Dr. Sawyer had no disclosures.
BALTIMORE – A study of patients with intraabdominal infection indicates that a shorter course of antibiotics is as effective as the standard, longer course, leading researchers to recommend a new standard of care.
The rates of recurrent infections and other outcomes were similar in patients with intraabdominal infections treated with antibiotics for 4 days following source control and among those who received a longer course of treatment based on when their clinical symptoms resolved, in a study reported at the annual meeting of the Surgical Infection Society.
The results support the use of the shorter treatment strategy in this setting, said Dr. Robert Sawyer, who presented the results of the study, SIS Multicenter Study of Duration of Antibiotics for Intraabdominal Infection.
While antibiotics are used as an adjunct to treat intraabdominal infections, the appropriate duration of treatment is not clear, and reducing the time exposed to antibiotics "could be worthwhile," said Dr. Sawyer, professor of surgery and public health sciences and chief of acute care surgery at the University of Virginia, Charlottesville.
The randomized, multicenter, unblinded study compared a shorter vs. a longer course of antibiotic therapy in 518 patients with intraabdominal infections: 4 days in 258 patients or treatment that continued for 2 days after resolution of the patient’s fever, leukocytosis, and ileus, with a maximum of 10 days of treatment in total, in 260 patients. The patients had similar APACHE II scores; their mean age was in the early 50s; and the colon or rectum was the most common source of infection, followed by the appendix and the small bowel. Antibiotics were administered intravenously or orally. Patients with inadequate source control and a high risk of death within 72 hours were excluded.
Within 30 days, the primary outcome – a composite of surgical site infection, recurrent intraabdominal infection, and death – was similar between the two groups, at about 21.7% in the 4-day treatment group vs. 22.7% in the group treated for 2 days after symptoms resolved.
There were no significant differences in the individual endpoints between the two groups. There were also no differences in the composite endpoints in different subgroups, and when those with percutaneous drainage or patients whose source of infection was the appendix were excluded. Among sicker patients with APACHE scores of 10 or higher, there was a numerical benefit favoring those treated for 4 days (22.1% vs. 29.8% among those treated for the longer duration), but the difference was not statistically significant, although it was reassuring, Dr. Sawyer said.
There were no differences between the two groups in the rates of secondary infections or subsequent infection with resistant pathogens or Clostridium difficile, which were secondary endpoints.
Based on these results, "after source control is obtained, we recommend 4 days of antimicrobial therapy for all patients with intraabdominal infections as the new standard of care," Dr. Sawyer concluded.
Although they had positive comments about the study, several members of the audience commented that a larger study would be a better base for a recommendation to change the standard of care.
This study was one of several studies presented at the meeting that addressed the issue of reducing the time on antibiotic therapy in patients with surgical infections.
The study was sponsored by the National Institutes of Health. Dr. Sawyer had no disclosures.
BALTIMORE – A study of patients with intraabdominal infection indicates that a shorter course of antibiotics is as effective as the standard, longer course, leading researchers to recommend a new standard of care.
The rates of recurrent infections and other outcomes were similar in patients with intraabdominal infections treated with antibiotics for 4 days following source control and among those who received a longer course of treatment based on when their clinical symptoms resolved, in a study reported at the annual meeting of the Surgical Infection Society.
The results support the use of the shorter treatment strategy in this setting, said Dr. Robert Sawyer, who presented the results of the study, SIS Multicenter Study of Duration of Antibiotics for Intraabdominal Infection.
While antibiotics are used as an adjunct to treat intraabdominal infections, the appropriate duration of treatment is not clear, and reducing the time exposed to antibiotics "could be worthwhile," said Dr. Sawyer, professor of surgery and public health sciences and chief of acute care surgery at the University of Virginia, Charlottesville.
The randomized, multicenter, unblinded study compared a shorter vs. a longer course of antibiotic therapy in 518 patients with intraabdominal infections: 4 days in 258 patients or treatment that continued for 2 days after resolution of the patient’s fever, leukocytosis, and ileus, with a maximum of 10 days of treatment in total, in 260 patients. The patients had similar APACHE II scores; their mean age was in the early 50s; and the colon or rectum was the most common source of infection, followed by the appendix and the small bowel. Antibiotics were administered intravenously or orally. Patients with inadequate source control and a high risk of death within 72 hours were excluded.
Within 30 days, the primary outcome – a composite of surgical site infection, recurrent intraabdominal infection, and death – was similar between the two groups, at about 21.7% in the 4-day treatment group vs. 22.7% in the group treated for 2 days after symptoms resolved.
There were no significant differences in the individual endpoints between the two groups. There were also no differences in the composite endpoints in different subgroups, and when those with percutaneous drainage or patients whose source of infection was the appendix were excluded. Among sicker patients with APACHE scores of 10 or higher, there was a numerical benefit favoring those treated for 4 days (22.1% vs. 29.8% among those treated for the longer duration), but the difference was not statistically significant, although it was reassuring, Dr. Sawyer said.
There were no differences between the two groups in the rates of secondary infections or subsequent infection with resistant pathogens or Clostridium difficile, which were secondary endpoints.
Based on these results, "after source control is obtained, we recommend 4 days of antimicrobial therapy for all patients with intraabdominal infections as the new standard of care," Dr. Sawyer concluded.
Although they had positive comments about the study, several members of the audience commented that a larger study would be a better base for a recommendation to change the standard of care.
This study was one of several studies presented at the meeting that addressed the issue of reducing the time on antibiotic therapy in patients with surgical infections.
The study was sponsored by the National Institutes of Health. Dr. Sawyer had no disclosures.
AT THE SIS ANNUAL MEETING
Major finding: In patients with intraabdominal infections, the rates of a composite of adverse outcomes were similar – about 22% – after a 4-day course of antibiotic therapy and after continuing antibiotics for 2 days after clinical symptoms resolved.
Data source: A multicenter, randomized controlled, unblinded study of 518 adults with intraabdominal infections compared the effects of two different approaches to antibiotic therapy after source control on the composite endpoint of surgical site infection, recurrent intraabdominal infection, and death within 30 days.
Disclosures: Dr. Robert Sawyer had no disclosures. The study was sponsored by the National Institutes of Health.
System overhaul eliminated CABG surgical site infections
ORLANDO – A system-wide quality improvement process that utilized define, measure, analyze, improve, and control and rapid adoption methodology eliminated surgical site infections among patients undergoing isolated coronary artery bypass with donor site surgery at a regional medical center.
Between March 2012 and March 2013 the overall decline in the rate of surgical site infections among 250 patients surpassed the center’s goal of a 40% decrease in the infection rate to a rate of 1.61; instead, the center achieved a rate of 0.7 during that time period. Since May, 2012, no surgical site infections have occurred among coronary artery bypass graft patients, Candis Kles, a critical care nurse at Athens (Ga.) Regional Medical Center, reported at the annual meeting of the Society of Thoracic Surgeons.
"We are now into our 23rd month without a deep/organ space infection with over 454 patients," Ms. Kles said in a May interview.
The quality improvement process was initiated after the infection rates at the center were found to be high (ranging up to 6.1), compared with National Healthcare Safety Network data. Using the define, measure, analyze, improve, and control methodology, a multidisciplinary team was convened, including cardiothoracic surgeons, nurses, and support personnel, and a 6-month process was undertaken to identify areas for improvement, said Ms. Kles who chaired the team.
"We also at this time partnered with the VHA National Hospital Engagement Network for some extra training and to help us along the process," she noted.
The team flow-charted processes – from scheduled surgery to discharge – and posted the flow charts in the operating room and cardiac intensive care unit to identify inconsistencies and potential problem areas. Frontline staff members were engaged and encouraged to participate in the improvement process by posting notes, suggestions, and questions on the flow charts.
A literature review was conducted to ensure that current practices mirrored best practices.
Despite an extensive evaluation of possible patient-, procedure-, and hospital-related factors that might be associated with the infection rate, no single cause of the high rate was identified, although a number of areas for improvement were recognized. Among the changes that were implemented were use of chlorhexidine oral rinse, which was administered until discharge; use of disposable EKG leads (after high bacterial counts were found on the leads in use prior to program initiation); use of silver-impregnated midsternal dressings; a change in surgical and suture techniques; development of patient educational materials, and standardization of all processes, Ms. Kles said, noting that the changes were implemented rapidly.
The success of the program is largely attributable to input from all levels and disciplines within the organization – including physicians who championed the process, and to staff engagement and buy in, she said.
The improvement in the infection rate resulted in cost avoidance of $212,355 (compared with a total excess cost of surgical site infections of $250,965 in 2011), and in a reduction from 156 to 24 excess hospital days.
Ms. Kles reported that as of February 2014 she is a member of the speakers bureau for Mölnlycke Health Care, a maker of surgical and wound care products.
ORLANDO – A system-wide quality improvement process that utilized define, measure, analyze, improve, and control and rapid adoption methodology eliminated surgical site infections among patients undergoing isolated coronary artery bypass with donor site surgery at a regional medical center.
Between March 2012 and March 2013 the overall decline in the rate of surgical site infections among 250 patients surpassed the center’s goal of a 40% decrease in the infection rate to a rate of 1.61; instead, the center achieved a rate of 0.7 during that time period. Since May, 2012, no surgical site infections have occurred among coronary artery bypass graft patients, Candis Kles, a critical care nurse at Athens (Ga.) Regional Medical Center, reported at the annual meeting of the Society of Thoracic Surgeons.
"We are now into our 23rd month without a deep/organ space infection with over 454 patients," Ms. Kles said in a May interview.
The quality improvement process was initiated after the infection rates at the center were found to be high (ranging up to 6.1), compared with National Healthcare Safety Network data. Using the define, measure, analyze, improve, and control methodology, a multidisciplinary team was convened, including cardiothoracic surgeons, nurses, and support personnel, and a 6-month process was undertaken to identify areas for improvement, said Ms. Kles who chaired the team.
"We also at this time partnered with the VHA National Hospital Engagement Network for some extra training and to help us along the process," she noted.
The team flow-charted processes – from scheduled surgery to discharge – and posted the flow charts in the operating room and cardiac intensive care unit to identify inconsistencies and potential problem areas. Frontline staff members were engaged and encouraged to participate in the improvement process by posting notes, suggestions, and questions on the flow charts.
A literature review was conducted to ensure that current practices mirrored best practices.
Despite an extensive evaluation of possible patient-, procedure-, and hospital-related factors that might be associated with the infection rate, no single cause of the high rate was identified, although a number of areas for improvement were recognized. Among the changes that were implemented were use of chlorhexidine oral rinse, which was administered until discharge; use of disposable EKG leads (after high bacterial counts were found on the leads in use prior to program initiation); use of silver-impregnated midsternal dressings; a change in surgical and suture techniques; development of patient educational materials, and standardization of all processes, Ms. Kles said, noting that the changes were implemented rapidly.
The success of the program is largely attributable to input from all levels and disciplines within the organization – including physicians who championed the process, and to staff engagement and buy in, she said.
The improvement in the infection rate resulted in cost avoidance of $212,355 (compared with a total excess cost of surgical site infections of $250,965 in 2011), and in a reduction from 156 to 24 excess hospital days.
Ms. Kles reported that as of February 2014 she is a member of the speakers bureau for Mölnlycke Health Care, a maker of surgical and wound care products.
ORLANDO – A system-wide quality improvement process that utilized define, measure, analyze, improve, and control and rapid adoption methodology eliminated surgical site infections among patients undergoing isolated coronary artery bypass with donor site surgery at a regional medical center.
Between March 2012 and March 2013 the overall decline in the rate of surgical site infections among 250 patients surpassed the center’s goal of a 40% decrease in the infection rate to a rate of 1.61; instead, the center achieved a rate of 0.7 during that time period. Since May, 2012, no surgical site infections have occurred among coronary artery bypass graft patients, Candis Kles, a critical care nurse at Athens (Ga.) Regional Medical Center, reported at the annual meeting of the Society of Thoracic Surgeons.
"We are now into our 23rd month without a deep/organ space infection with over 454 patients," Ms. Kles said in a May interview.
The quality improvement process was initiated after the infection rates at the center were found to be high (ranging up to 6.1), compared with National Healthcare Safety Network data. Using the define, measure, analyze, improve, and control methodology, a multidisciplinary team was convened, including cardiothoracic surgeons, nurses, and support personnel, and a 6-month process was undertaken to identify areas for improvement, said Ms. Kles who chaired the team.
"We also at this time partnered with the VHA National Hospital Engagement Network for some extra training and to help us along the process," she noted.
The team flow-charted processes – from scheduled surgery to discharge – and posted the flow charts in the operating room and cardiac intensive care unit to identify inconsistencies and potential problem areas. Frontline staff members were engaged and encouraged to participate in the improvement process by posting notes, suggestions, and questions on the flow charts.
A literature review was conducted to ensure that current practices mirrored best practices.
Despite an extensive evaluation of possible patient-, procedure-, and hospital-related factors that might be associated with the infection rate, no single cause of the high rate was identified, although a number of areas for improvement were recognized. Among the changes that were implemented were use of chlorhexidine oral rinse, which was administered until discharge; use of disposable EKG leads (after high bacterial counts were found on the leads in use prior to program initiation); use of silver-impregnated midsternal dressings; a change in surgical and suture techniques; development of patient educational materials, and standardization of all processes, Ms. Kles said, noting that the changes were implemented rapidly.
The success of the program is largely attributable to input from all levels and disciplines within the organization – including physicians who championed the process, and to staff engagement and buy in, she said.
The improvement in the infection rate resulted in cost avoidance of $212,355 (compared with a total excess cost of surgical site infections of $250,965 in 2011), and in a reduction from 156 to 24 excess hospital days.
Ms. Kles reported that as of February 2014 she is a member of the speakers bureau for Mölnlycke Health Care, a maker of surgical and wound care products.
AT THE STS ANNUAL MEETING
Key clinical point: Cross-discipline buy in obliterated CABG surgical site infections.
Major finding: Since May, 2012, no surgical site infections have occurred among CABG patients.
Data source: An overview of a quality improvement process at a regional medical center.
Disclosures: Ms. Kles reported that as of February, 2014 she is a member of the speakers bureau for Mölnlycke Health Care.
Big Data and the art of medicine
Life is short, and the Art long to learn.
–Chaucer
If you are anything like us, you still hold firmly to the notion that medicine, at its core, is an art. Sure, medicine is informed by cutting-edge sciences, such as biochemistry and molecular biology. But one can’t capture the nuances of the patient interaction by studying the Krebs cycle, nor define the motivating forces driving physicians by looking through a microscope. On the contrary, physicians are artists, much like musicians, sculptors, or dancers. And, like any of these artists, it would seem that a physician’s craft should improve through "practice," not by studying data or using a computer. So how do we reconcile this in the era of "Big Data"? How can the "ones and zeros" living deep in the "guts" of our electronic health records (EHRs) promise to revolutionize an art that has relied solely on the judgment – or gut sense – of physicians for centuries? Here we will attempt to answer these questions and ponder whether or not we really can improve the art of medicine with the help of data.
It’s more than the ‘ones and zeros’
With the right information and the proper tools to analyze it, it is possible to conceive of an improvement in our ability to care for patients. Take, for example, the prevention of malignancies. Currently, early detection of cancer relies heavily on a physician’s knowledge of – and compliance with – current cancer screening guidelines. Success also depends on a patient’s willingness to come in for annual visits to receive the instruction. If the patient doesn’t show up for a physical, or if the provider neglects to mention the need for a colonoscopy when the patient does appear, the test may go unordered (much to the patient’s relief, perhaps!). But the right tools and analytics won’t let that happen. Instead, the technology will identify the highest-risk populations with ever-improving accuracy and notify both physician and patient of the need for action. With enough data, we may even be able to make observations in trends of cancer inheritance never before possible and predict cancer long before it might be detected by conventional screening protocols.
It pays to care about the data
Physicians may not realize it, but data can have a significant financial advantage, by improving reimbursement and decreasing the overall cost of care. This can be achieved in two ways. The first way is by using the data to paint a more accurate picture of patient complexity. Medicare assigns a risk-adjusted score to patient cohorts based on the severity of their diagnoses and reimburses Medicare Advantage plans based on that score: the higher the score, the better the reimbursement. Occasionally, those additional dollars are passed along to the treating physicians. But all too often physicians do not use ICD-9 codes properly on their claims, making their patients appear less complicated and thereby receiving lower reimbursement. Through emerging data collection tools, improper coding can be identified and corrected, and missed opportunities can be discovered early enough to capture additional funds.
The second way these tools can be used leads to direct benefit to the health care system in general, through improved medical cost management. By interfacing with insurers and analyzing claims, the software can identify patients who are high utilizers and can show trends in medical costs across a community or health system. This allows providers to target certain patients or disease states around which to build cost-containment strategies and create win-win scenarios that decrease hospital readmissions, limit cost, and improve patient quality of life.
We recently learned of a great example of this. Using a population management data tool, a community health system was able to identify a geographic area in their region with a large uninsured population. This group had a disproportionately high utilization of emergency medical services and very low care quality markers (such as diabetes control, vaccination rates, etc.). Through targeted outreach based on these data, the system was able to direct individuals into low-cost, high-quality primary care sites and reduce emergency service utilization to levels below the surrounding neighborhoods. Simultaneously, the health of the community improved through better disease management and care coordination. Finally, data analytics tools uncovered additional opportunities for savings by identifying expensive brand-name drug prescriptions that could be replaced with generic drug alternatives.
A reluctant revolution
As we have lamented on previous occasions, the adoption of health care information technology is often driven by artificial external forces, such as stimulus programs or regulatory requirements. The government has routinely used incentive payments and reimbursement adjustments in order to spur widespread acceptance of EHRs. Most infamously, the Meaningful Use Regulations program has become the poster child for government involvement in direct patient care. Through the use of annual payments over a 5-year period (combined with the threat of penalties for lack of compliance), Meaningful Use has almost single-handedly enabled the Big Data revolution in health care by requiring physicians to purchase electronic health records systems and use them for population management. While seemingly a good thing, most physicians would hardly regard these systems as "meaningful." In fact, many question if there is any value in having an electronic record at all.
Whether or not their detractors admit it, EHRs do form the backbone of a new and very powerful information network – one which many believe has the power to revolutionize health care. While we certainly do not view the "data revolution" as the panacea others have claimed it to be, we do recognize that the right tools are emerging to enable physicians to learn from data and implement new, novel, and "disruptive" strategies to improve patient care.
Art is not static; the canvas, the paints, and the viewpoints change over time as experience evolves. Leonardo da Vinci furthered the world’s understanding of perspective. Pablo Picasso led a revolution in modern art. Each was different from his predecessors, and each expressed a human need to understand and portray the world in a manner consistent with his age. The same is true of our age and the art of medicine. The science has changed, as has the viewpoint and perspective from which we provide care. Our ability to record, retrieve, and understand health and disease will never be the same. But the attention to the patient is ever present. The necessity of interpreting the shifting world of health and disease to provide an empathic understanding of each patient’s individual and unique place in the world will never go away. Therein lies the Art.
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is associate director of the family medicine residency program at Abington Memorial Hospital and professor of family and community medicine at Temple University, Philadelphia. He is editor in chief of Redi-Reference Inc., a software company that creates mobile apps.
Life is short, and the Art long to learn.
–Chaucer
If you are anything like us, you still hold firmly to the notion that medicine, at its core, is an art. Sure, medicine is informed by cutting-edge sciences, such as biochemistry and molecular biology. But one can’t capture the nuances of the patient interaction by studying the Krebs cycle, nor define the motivating forces driving physicians by looking through a microscope. On the contrary, physicians are artists, much like musicians, sculptors, or dancers. And, like any of these artists, it would seem that a physician’s craft should improve through "practice," not by studying data or using a computer. So how do we reconcile this in the era of "Big Data"? How can the "ones and zeros" living deep in the "guts" of our electronic health records (EHRs) promise to revolutionize an art that has relied solely on the judgment – or gut sense – of physicians for centuries? Here we will attempt to answer these questions and ponder whether or not we really can improve the art of medicine with the help of data.
It’s more than the ‘ones and zeros’
With the right information and the proper tools to analyze it, it is possible to conceive of an improvement in our ability to care for patients. Take, for example, the prevention of malignancies. Currently, early detection of cancer relies heavily on a physician’s knowledge of – and compliance with – current cancer screening guidelines. Success also depends on a patient’s willingness to come in for annual visits to receive the instruction. If the patient doesn’t show up for a physical, or if the provider neglects to mention the need for a colonoscopy when the patient does appear, the test may go unordered (much to the patient’s relief, perhaps!). But the right tools and analytics won’t let that happen. Instead, the technology will identify the highest-risk populations with ever-improving accuracy and notify both physician and patient of the need for action. With enough data, we may even be able to make observations in trends of cancer inheritance never before possible and predict cancer long before it might be detected by conventional screening protocols.
It pays to care about the data
Physicians may not realize it, but data can have a significant financial advantage, by improving reimbursement and decreasing the overall cost of care. This can be achieved in two ways. The first way is by using the data to paint a more accurate picture of patient complexity. Medicare assigns a risk-adjusted score to patient cohorts based on the severity of their diagnoses and reimburses Medicare Advantage plans based on that score: the higher the score, the better the reimbursement. Occasionally, those additional dollars are passed along to the treating physicians. But all too often physicians do not use ICD-9 codes properly on their claims, making their patients appear less complicated and thereby receiving lower reimbursement. Through emerging data collection tools, improper coding can be identified and corrected, and missed opportunities can be discovered early enough to capture additional funds.
The second way these tools can be used leads to direct benefit to the health care system in general, through improved medical cost management. By interfacing with insurers and analyzing claims, the software can identify patients who are high utilizers and can show trends in medical costs across a community or health system. This allows providers to target certain patients or disease states around which to build cost-containment strategies and create win-win scenarios that decrease hospital readmissions, limit cost, and improve patient quality of life.
We recently learned of a great example of this. Using a population management data tool, a community health system was able to identify a geographic area in their region with a large uninsured population. This group had a disproportionately high utilization of emergency medical services and very low care quality markers (such as diabetes control, vaccination rates, etc.). Through targeted outreach based on these data, the system was able to direct individuals into low-cost, high-quality primary care sites and reduce emergency service utilization to levels below the surrounding neighborhoods. Simultaneously, the health of the community improved through better disease management and care coordination. Finally, data analytics tools uncovered additional opportunities for savings by identifying expensive brand-name drug prescriptions that could be replaced with generic drug alternatives.
A reluctant revolution
As we have lamented on previous occasions, the adoption of health care information technology is often driven by artificial external forces, such as stimulus programs or regulatory requirements. The government has routinely used incentive payments and reimbursement adjustments in order to spur widespread acceptance of EHRs. Most infamously, the Meaningful Use Regulations program has become the poster child for government involvement in direct patient care. Through the use of annual payments over a 5-year period (combined with the threat of penalties for lack of compliance), Meaningful Use has almost single-handedly enabled the Big Data revolution in health care by requiring physicians to purchase electronic health records systems and use them for population management. While seemingly a good thing, most physicians would hardly regard these systems as "meaningful." In fact, many question if there is any value in having an electronic record at all.
Whether or not their detractors admit it, EHRs do form the backbone of a new and very powerful information network – one which many believe has the power to revolutionize health care. While we certainly do not view the "data revolution" as the panacea others have claimed it to be, we do recognize that the right tools are emerging to enable physicians to learn from data and implement new, novel, and "disruptive" strategies to improve patient care.
Art is not static; the canvas, the paints, and the viewpoints change over time as experience evolves. Leonardo da Vinci furthered the world’s understanding of perspective. Pablo Picasso led a revolution in modern art. Each was different from his predecessors, and each expressed a human need to understand and portray the world in a manner consistent with his age. The same is true of our age and the art of medicine. The science has changed, as has the viewpoint and perspective from which we provide care. Our ability to record, retrieve, and understand health and disease will never be the same. But the attention to the patient is ever present. The necessity of interpreting the shifting world of health and disease to provide an empathic understanding of each patient’s individual and unique place in the world will never go away. Therein lies the Art.
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is associate director of the family medicine residency program at Abington Memorial Hospital and professor of family and community medicine at Temple University, Philadelphia. He is editor in chief of Redi-Reference Inc., a software company that creates mobile apps.
Life is short, and the Art long to learn.
–Chaucer
If you are anything like us, you still hold firmly to the notion that medicine, at its core, is an art. Sure, medicine is informed by cutting-edge sciences, such as biochemistry and molecular biology. But one can’t capture the nuances of the patient interaction by studying the Krebs cycle, nor define the motivating forces driving physicians by looking through a microscope. On the contrary, physicians are artists, much like musicians, sculptors, or dancers. And, like any of these artists, it would seem that a physician’s craft should improve through "practice," not by studying data or using a computer. So how do we reconcile this in the era of "Big Data"? How can the "ones and zeros" living deep in the "guts" of our electronic health records (EHRs) promise to revolutionize an art that has relied solely on the judgment – or gut sense – of physicians for centuries? Here we will attempt to answer these questions and ponder whether or not we really can improve the art of medicine with the help of data.
It’s more than the ‘ones and zeros’
With the right information and the proper tools to analyze it, it is possible to conceive of an improvement in our ability to care for patients. Take, for example, the prevention of malignancies. Currently, early detection of cancer relies heavily on a physician’s knowledge of – and compliance with – current cancer screening guidelines. Success also depends on a patient’s willingness to come in for annual visits to receive the instruction. If the patient doesn’t show up for a physical, or if the provider neglects to mention the need for a colonoscopy when the patient does appear, the test may go unordered (much to the patient’s relief, perhaps!). But the right tools and analytics won’t let that happen. Instead, the technology will identify the highest-risk populations with ever-improving accuracy and notify both physician and patient of the need for action. With enough data, we may even be able to make observations in trends of cancer inheritance never before possible and predict cancer long before it might be detected by conventional screening protocols.
It pays to care about the data
Physicians may not realize it, but data can have a significant financial advantage, by improving reimbursement and decreasing the overall cost of care. This can be achieved in two ways. The first way is by using the data to paint a more accurate picture of patient complexity. Medicare assigns a risk-adjusted score to patient cohorts based on the severity of their diagnoses and reimburses Medicare Advantage plans based on that score: the higher the score, the better the reimbursement. Occasionally, those additional dollars are passed along to the treating physicians. But all too often physicians do not use ICD-9 codes properly on their claims, making their patients appear less complicated and thereby receiving lower reimbursement. Through emerging data collection tools, improper coding can be identified and corrected, and missed opportunities can be discovered early enough to capture additional funds.
The second way these tools can be used leads to direct benefit to the health care system in general, through improved medical cost management. By interfacing with insurers and analyzing claims, the software can identify patients who are high utilizers and can show trends in medical costs across a community or health system. This allows providers to target certain patients or disease states around which to build cost-containment strategies and create win-win scenarios that decrease hospital readmissions, limit cost, and improve patient quality of life.
We recently learned of a great example of this. Using a population management data tool, a community health system was able to identify a geographic area in their region with a large uninsured population. This group had a disproportionately high utilization of emergency medical services and very low care quality markers (such as diabetes control, vaccination rates, etc.). Through targeted outreach based on these data, the system was able to direct individuals into low-cost, high-quality primary care sites and reduce emergency service utilization to levels below the surrounding neighborhoods. Simultaneously, the health of the community improved through better disease management and care coordination. Finally, data analytics tools uncovered additional opportunities for savings by identifying expensive brand-name drug prescriptions that could be replaced with generic drug alternatives.
A reluctant revolution
As we have lamented on previous occasions, the adoption of health care information technology is often driven by artificial external forces, such as stimulus programs or regulatory requirements. The government has routinely used incentive payments and reimbursement adjustments in order to spur widespread acceptance of EHRs. Most infamously, the Meaningful Use Regulations program has become the poster child for government involvement in direct patient care. Through the use of annual payments over a 5-year period (combined with the threat of penalties for lack of compliance), Meaningful Use has almost single-handedly enabled the Big Data revolution in health care by requiring physicians to purchase electronic health records systems and use them for population management. While seemingly a good thing, most physicians would hardly regard these systems as "meaningful." In fact, many question if there is any value in having an electronic record at all.
Whether or not their detractors admit it, EHRs do form the backbone of a new and very powerful information network – one which many believe has the power to revolutionize health care. While we certainly do not view the "data revolution" as the panacea others have claimed it to be, we do recognize that the right tools are emerging to enable physicians to learn from data and implement new, novel, and "disruptive" strategies to improve patient care.
Art is not static; the canvas, the paints, and the viewpoints change over time as experience evolves. Leonardo da Vinci furthered the world’s understanding of perspective. Pablo Picasso led a revolution in modern art. Each was different from his predecessors, and each expressed a human need to understand and portray the world in a manner consistent with his age. The same is true of our age and the art of medicine. The science has changed, as has the viewpoint and perspective from which we provide care. Our ability to record, retrieve, and understand health and disease will never be the same. But the attention to the patient is ever present. The necessity of interpreting the shifting world of health and disease to provide an empathic understanding of each patient’s individual and unique place in the world will never go away. Therein lies the Art.
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is associate director of the family medicine residency program at Abington Memorial Hospital and professor of family and community medicine at Temple University, Philadelphia. He is editor in chief of Redi-Reference Inc., a software company that creates mobile apps.
Federal legislation would provide doctors litigation safe harbor
Physician leaders are voicing support for a proposed federal law that aims to reduce litigation against doctors, lower health care costs, and establish more fairness in the analyzing of malpractice claims. The Saving Lives, Saving Costs Act, introduced in March by Rep. Dr. Ami Bera, (D-CA), would provide safe harbor protection to doctors who are sued if they followed evidence-based clinical guidelines.
"The Bera/Barr bill would accomplish two very important things," said Dr. John C. Jennings, president of the American Congress of Obstetricians and Gynecologists and professor of obstetrics and gynecology at Texas Tech University Health Sciences Center at the Permian Basin. "First, it would provide an incentive for every physician to follow the best clinical guidelines developed by his or her specialty, reducing care variations and potentially increasing patient safety and quality of care. Second, it would provide an affirmative defense for physicians who have followed their specialties’ guidelines and find themselves in the middle of a malpractice suit."
Under the bill, clinical guidelines developed by professional medical organizations would be used to determine whether a plaintiff’s lawsuit could continue against a physician defendant. If a doctor adhered to the approved guidelines during the time of the alleged malpractice event, the case would be removed from court proceedings, while a medical review panel investigated the claim. The bill would also allow for relevant cases to be moved from state to federal court if they involved federal dollars such as Medicare.The bill was referred to the Subcommittee on the Constitution and Civil Justice on March 20 for review.
Dr. Bera declined to comment for this article. In a statement, he said the measure is a practical way to decrease the skyrocketing cost of health care and to ensure the malpractice system works better for patients and physicians.
"As a doctor, I know that physicians want to do what’s best for their patients, and promoting evidence-based medicine will help us do that," he said.
The proposed safe harbor measure is a beneficial initiative that would potentially have a positive impact on multiple aspects of the health care system, adds Dr. David A. Fleming, president-elect of the American College of Physicians and chair of the department of medicine for the University of Missouri–Columbia.
"I think anything that standardized a patient-centered and evidence-based approach to care will serve only to improve clinical outcomes and decrease health care costs, as well as decrease liability costs at every level," Dr. Fleming said in an interview. "Encouraging the use of generally accepted and evidence-based clinical guidelines, as promoted by the Saving Lives, Saving Costs federal bill, is a good way to reach that."
Dr. Fleming noted an ACP position paper published in April that discusses the medical liability crisis physicians continue to face and outlines innovative solutions for a better malpractice environment. "Medical Liability Reform – Innovative Solutions for New Health Care System," also provides an update on state-based medical liability activities and summarizes traditional and newer tort reform proposals.
The paper highlights the positive effect of such state reforms as caps on noneconomic damages, injury funds, stronger expert witness rules, and alternative dispute resolution initiatives such as apology, disclosure, and compensation programs. The report provides nine approaches that should be incorporated into a multifaceted medical malpractice reform initiative, including passage of a comprehensive tort reform package, oversight of medical liability insurers, and development of effective safe harbor protections that improve quality of care, increase efficiency, and reduce costs.
As for federal reform, Dr. Fleming said legislation at the congressional level often faces successful challenges by trial attorneys and advocacy groups that argue plaintiffs’ rights would be violated. The politicization of the issue is also a problem, he said. However, federal tort reform is still possible and physicians should keep advocating significant changes by Congress, he said.
"The litigious environment in which we live continues to contribute to a sense of fear and consternation by practicing physicians that affects how they relate to patients and undoubtedly adds to health care costs," Dr. Fleming said.
Along with the Saving Lives, Saving Costs bills, doctors are closely watching several other federal malpractice reform measures under review. For instance, the Health Care Safety Net Enhancement Act would help ensure that physicians furnishing medical services, pursuant to the Emergency Medical Treatment & Active Labor Act (EMTALA), receive the same liability coverage currently extended to health professionals who provide Medicaid services at free clinics. The bill has been referred to the Subcommittee on Health.
Meanwhile, the Standard of Care Protection Act of 2013 would ensure that provisions of the Affordable Care Act and other federal laws cannot be used to create new standards of care for medical liability lawsuits. The proposed law was included in the recent Medicare Sustainable Growth Rate Formula bill, which passed out of the Energy and Commerce Committee.
"With so many changes occurring in the health care system, physicians are rightly concerned that federal rules and regulations could result in new, unwarranted, liability exposures," Dr. Jennings said. "This legislation helps safeguard physicians."
Physician leaders are voicing support for a proposed federal law that aims to reduce litigation against doctors, lower health care costs, and establish more fairness in the analyzing of malpractice claims. The Saving Lives, Saving Costs Act, introduced in March by Rep. Dr. Ami Bera, (D-CA), would provide safe harbor protection to doctors who are sued if they followed evidence-based clinical guidelines.
"The Bera/Barr bill would accomplish two very important things," said Dr. John C. Jennings, president of the American Congress of Obstetricians and Gynecologists and professor of obstetrics and gynecology at Texas Tech University Health Sciences Center at the Permian Basin. "First, it would provide an incentive for every physician to follow the best clinical guidelines developed by his or her specialty, reducing care variations and potentially increasing patient safety and quality of care. Second, it would provide an affirmative defense for physicians who have followed their specialties’ guidelines and find themselves in the middle of a malpractice suit."
Under the bill, clinical guidelines developed by professional medical organizations would be used to determine whether a plaintiff’s lawsuit could continue against a physician defendant. If a doctor adhered to the approved guidelines during the time of the alleged malpractice event, the case would be removed from court proceedings, while a medical review panel investigated the claim. The bill would also allow for relevant cases to be moved from state to federal court if they involved federal dollars such as Medicare.The bill was referred to the Subcommittee on the Constitution and Civil Justice on March 20 for review.
Dr. Bera declined to comment for this article. In a statement, he said the measure is a practical way to decrease the skyrocketing cost of health care and to ensure the malpractice system works better for patients and physicians.
"As a doctor, I know that physicians want to do what’s best for their patients, and promoting evidence-based medicine will help us do that," he said.
The proposed safe harbor measure is a beneficial initiative that would potentially have a positive impact on multiple aspects of the health care system, adds Dr. David A. Fleming, president-elect of the American College of Physicians and chair of the department of medicine for the University of Missouri–Columbia.
"I think anything that standardized a patient-centered and evidence-based approach to care will serve only to improve clinical outcomes and decrease health care costs, as well as decrease liability costs at every level," Dr. Fleming said in an interview. "Encouraging the use of generally accepted and evidence-based clinical guidelines, as promoted by the Saving Lives, Saving Costs federal bill, is a good way to reach that."
Dr. Fleming noted an ACP position paper published in April that discusses the medical liability crisis physicians continue to face and outlines innovative solutions for a better malpractice environment. "Medical Liability Reform – Innovative Solutions for New Health Care System," also provides an update on state-based medical liability activities and summarizes traditional and newer tort reform proposals.
The paper highlights the positive effect of such state reforms as caps on noneconomic damages, injury funds, stronger expert witness rules, and alternative dispute resolution initiatives such as apology, disclosure, and compensation programs. The report provides nine approaches that should be incorporated into a multifaceted medical malpractice reform initiative, including passage of a comprehensive tort reform package, oversight of medical liability insurers, and development of effective safe harbor protections that improve quality of care, increase efficiency, and reduce costs.
As for federal reform, Dr. Fleming said legislation at the congressional level often faces successful challenges by trial attorneys and advocacy groups that argue plaintiffs’ rights would be violated. The politicization of the issue is also a problem, he said. However, federal tort reform is still possible and physicians should keep advocating significant changes by Congress, he said.
"The litigious environment in which we live continues to contribute to a sense of fear and consternation by practicing physicians that affects how they relate to patients and undoubtedly adds to health care costs," Dr. Fleming said.
Along with the Saving Lives, Saving Costs bills, doctors are closely watching several other federal malpractice reform measures under review. For instance, the Health Care Safety Net Enhancement Act would help ensure that physicians furnishing medical services, pursuant to the Emergency Medical Treatment & Active Labor Act (EMTALA), receive the same liability coverage currently extended to health professionals who provide Medicaid services at free clinics. The bill has been referred to the Subcommittee on Health.
Meanwhile, the Standard of Care Protection Act of 2013 would ensure that provisions of the Affordable Care Act and other federal laws cannot be used to create new standards of care for medical liability lawsuits. The proposed law was included in the recent Medicare Sustainable Growth Rate Formula bill, which passed out of the Energy and Commerce Committee.
"With so many changes occurring in the health care system, physicians are rightly concerned that federal rules and regulations could result in new, unwarranted, liability exposures," Dr. Jennings said. "This legislation helps safeguard physicians."
Physician leaders are voicing support for a proposed federal law that aims to reduce litigation against doctors, lower health care costs, and establish more fairness in the analyzing of malpractice claims. The Saving Lives, Saving Costs Act, introduced in March by Rep. Dr. Ami Bera, (D-CA), would provide safe harbor protection to doctors who are sued if they followed evidence-based clinical guidelines.
"The Bera/Barr bill would accomplish two very important things," said Dr. John C. Jennings, president of the American Congress of Obstetricians and Gynecologists and professor of obstetrics and gynecology at Texas Tech University Health Sciences Center at the Permian Basin. "First, it would provide an incentive for every physician to follow the best clinical guidelines developed by his or her specialty, reducing care variations and potentially increasing patient safety and quality of care. Second, it would provide an affirmative defense for physicians who have followed their specialties’ guidelines and find themselves in the middle of a malpractice suit."
Under the bill, clinical guidelines developed by professional medical organizations would be used to determine whether a plaintiff’s lawsuit could continue against a physician defendant. If a doctor adhered to the approved guidelines during the time of the alleged malpractice event, the case would be removed from court proceedings, while a medical review panel investigated the claim. The bill would also allow for relevant cases to be moved from state to federal court if they involved federal dollars such as Medicare.The bill was referred to the Subcommittee on the Constitution and Civil Justice on March 20 for review.
Dr. Bera declined to comment for this article. In a statement, he said the measure is a practical way to decrease the skyrocketing cost of health care and to ensure the malpractice system works better for patients and physicians.
"As a doctor, I know that physicians want to do what’s best for their patients, and promoting evidence-based medicine will help us do that," he said.
The proposed safe harbor measure is a beneficial initiative that would potentially have a positive impact on multiple aspects of the health care system, adds Dr. David A. Fleming, president-elect of the American College of Physicians and chair of the department of medicine for the University of Missouri–Columbia.
"I think anything that standardized a patient-centered and evidence-based approach to care will serve only to improve clinical outcomes and decrease health care costs, as well as decrease liability costs at every level," Dr. Fleming said in an interview. "Encouraging the use of generally accepted and evidence-based clinical guidelines, as promoted by the Saving Lives, Saving Costs federal bill, is a good way to reach that."
Dr. Fleming noted an ACP position paper published in April that discusses the medical liability crisis physicians continue to face and outlines innovative solutions for a better malpractice environment. "Medical Liability Reform – Innovative Solutions for New Health Care System," also provides an update on state-based medical liability activities and summarizes traditional and newer tort reform proposals.
The paper highlights the positive effect of such state reforms as caps on noneconomic damages, injury funds, stronger expert witness rules, and alternative dispute resolution initiatives such as apology, disclosure, and compensation programs. The report provides nine approaches that should be incorporated into a multifaceted medical malpractice reform initiative, including passage of a comprehensive tort reform package, oversight of medical liability insurers, and development of effective safe harbor protections that improve quality of care, increase efficiency, and reduce costs.
As for federal reform, Dr. Fleming said legislation at the congressional level often faces successful challenges by trial attorneys and advocacy groups that argue plaintiffs’ rights would be violated. The politicization of the issue is also a problem, he said. However, federal tort reform is still possible and physicians should keep advocating significant changes by Congress, he said.
"The litigious environment in which we live continues to contribute to a sense of fear and consternation by practicing physicians that affects how they relate to patients and undoubtedly adds to health care costs," Dr. Fleming said.
Along with the Saving Lives, Saving Costs bills, doctors are closely watching several other federal malpractice reform measures under review. For instance, the Health Care Safety Net Enhancement Act would help ensure that physicians furnishing medical services, pursuant to the Emergency Medical Treatment & Active Labor Act (EMTALA), receive the same liability coverage currently extended to health professionals who provide Medicaid services at free clinics. The bill has been referred to the Subcommittee on Health.
Meanwhile, the Standard of Care Protection Act of 2013 would ensure that provisions of the Affordable Care Act and other federal laws cannot be used to create new standards of care for medical liability lawsuits. The proposed law was included in the recent Medicare Sustainable Growth Rate Formula bill, which passed out of the Energy and Commerce Committee.
"With so many changes occurring in the health care system, physicians are rightly concerned that federal rules and regulations could result in new, unwarranted, liability exposures," Dr. Jennings said. "This legislation helps safeguard physicians."