User login
Contradictions abound in ‘The End of Mental Illness’
Daniel G. Amen, MD, is an American psychiatrist well-known for his eponymous clinics, television appearances, and series of books on mental health. One of his latest books, “The End of Mental Illness,” summarizes many of his views on the causes of and treatments for mental illnesses.
Dr. Amen’s approaches – such as his advocacy for the widespread use of single photon emission computed tomography (SPECT) imaging – are somewhat controversial and at times fall outside the mainstream of current psychiatric thought. So does “The End of Mental Illness” contain anything of value to the average practicing psychiatrist? (It should be noted that I listened to this as an audiobook and took notes as I listened. This does limit my ability to directly quote portions of the text, but I believe my notes are reliable.)
He begins the book by pointing out that the term “mental illness” might be better replaced with the term “brain illness.” With this shift in terminology, Dr. Amen introduces a theme that recurs throughout the book: That mental illnesses ultimately stem from various ways in which the brain can be harmed. While the suggested change in terminology might help reduce the stigma associated with psychiatric illnesses, Dr. Amen is surprisingly timid about implementing this term in his own book. He repeatedly refers to “brain health/mental health” issues instead of discarding the “mental” term altogether. Even his BRIGHT MINDS acronym for risk factors for mental illnesses includes the term “mind” instead of “brain.”
Continuing the theme of challenging terminology, Dr. Amen goes on to decry the weaknesses of the DSM system of nosology. This is a valid point, because under the current system, the same patient may receive differing diagnoses depending on which provider is seen and how certain symptoms are interpreted. Yet, here again, Dr. Amen does not seem to adhere to his own advice: He uses DSM terminology throughout the book, speaking of depression, anxiety, bipolar disorder, and ADHD. An oddity (which, admittedly, could have been the audiobook reader’s mistake rather than an error in the original text) is that the DSM is referred to as the “Diagnostic and Structural Manual” rather than the Diagnostic and Statistical Manual. He criticizes the DSM for its imprecision, pointing out the variety of symptom combinations that can produce the same diagnoses and how similar symptoms may overlap between differing diagnoses. Yet, his descriptions of common SPECT patterns (his preferred tool to assist in diagnosis) make it clear that here, too, there is a lot of overlap. As an example, ADHD was associated with at least three of the imaging patterns he described. It is also somewhat ironic how Dr. Amen obliquely criticizes the American Psychiatric Association for profiting from the use of the DSM, when SPECT imaging is expensive and profits his own organization.
Dr. Amen repeatedly asserts that psychiatry is unique among medical specialties for making diagnoses based on symptom clusters rather than direct visualization of the affected organ. Yet, psychiatry is not, in fact, unique in making diagnoses in this way. Some examples of diagnoses based on symptom clusters from other medical specialties are systemic lupus erythematosus, fibromyalgia, and chronic fatigue syndrome. Although he asserts that SPECT imaging better demonstrates the root cause of mental illnesses, it is unclear from his book whether this is actually the case.
The descriptions for the ways in which Dr. Amen uses SPECT (which, admittedly, are vague and presumably simplified for a general audience) suggest that he has made observations correlating specific imaging patterns with certain emotional/behavioral outcomes. However, the imaging patterns he describes in the book can be interpreted to represent multiple different mental conditions, making it clear that SPECT is not a laserlike diagnostic tool that produces a single, indisputable diagnosis. Accuracy with SPECT seems especially questionable in light of two case examples he shares where brain imaging was interpreted as representing illness, but the patients were not demonstrating any signs of mental dysfunction. In one case, Dr. Amen opined that the patient’s vibrant spiritual life “overrode” the sick brain, but if this is true,
Patient testimonials are provided, asserting that SPECT imaging helped them know “exactly” what treatment would help them. One cannot help but wonder whether part of the benefit of SPECT imaging is a placebo effect, boosting the confidence of patients that the treatment they are receiving is personalized and scientifically sound. A similar trend is currently seen more broadly in psychiatry with the widespread promotion of pharmacogenetic testing. Such testing may bolster patient confidence in their medication, but its value in improving patient outcomes has not been established.1
Dr. Amen outlines a brief history of mental health care, including differing approaches and therapies from the time of Sigmund Freud up to the present. His outline is somewhat critical of the perceived shortcomings of his psychiatric forebears, yet this seems entirely unnecessary. All scientific disciplines must start somewhere and build from limited knowledge to greater. Is it necessary to belittle Freud for not being able to do SPECT imaging in the 1800s?
Interestingly, Dr. Amen leaves cognitive-behavioral therapy (CBT), a landmark, evidence-based form of psychotherapy, out of his overview of the history of psychiatry. He does go on to mention CBT as part of the treatment offerings of the Amen Clinics, which could leave the lay reader with the incorrect impression that CBT is a treatment unique to Amen Clinics. Similarly, at one point Dr. Amen writes about “what I call automatic negative thoughts.” This phrasing could confuse readers who might not know that automatic thoughts are a concept endemic to CBT.
Dr. Amen writes repeatedly about the Amen Clinics 4 Circles, four key areas of life that can contribute to mental health. These areas are biological, psychological, social, and spiritual. While Amen Clinics may have come up with the term “4 Circles,” the biopsychosocial model of understanding illness was developed by George Engel, MD, in 1977, and current discussions of this model frequently incorporate a spiritual dimension as well.2
Dr. Amen’s writing at times mischaracterizes psychotropic medications in unhelpful ways. He speaks of psychotropic medications generally as being addictive. While this is certainly true for stimulants and benzodiazepines, most would agree that this does not apply to many other commonly used medications in psychiatry, including selective serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants, antipsychotics, and mood stabilizers. He also paints with a broad brush when he states that anxiety medications can cause dementia. A concerning link has been demonstrated between benzodiazepine use and dementia,3 but SSRIs (which are considered first-line medications for anxiety) are not known to cause dementia and may actually delay progression from mild cognitive impairment to Alzheimer’s dementia.4 His mention of medication use affecting a patient’s insurability could have the unfortunate effect of scaring away suffering individuals from seeking help. The one category of psychiatric medication he does not seem concerned about is psychostimulants, which is odd – given the addictive, cardiovascular, and other risks associated with that medication class.
In contrast to his skepticism regarding many psychotropic medications, Dr. Amen expresses significant enthusiasm regarding nutraceutical use. While there has been research in this area supporting a role for some nutraceutical interventions, there is still a need for more rigorous studies.5 To support his endorsement of natural remedies, Dr. Amen mentions that Hippocrates recommended herbs and spices for many health conditions. But Hippocrates lived more than 2,000 years ago, and the state of medicine has advanced significantly since then.
Dr. Amen also mentions that 80% of the developing world relies upon natural or herbal remedies as the primary source of medicine. While he frames this statement as supporting his endorsement of such remedies, it could conversely be said that this is evidence of the need to make pharmacological interventions more widely available in the developing world.
Much of “The End of Mental Illness” is dedicated to reviewing specific risk factors that could cause harm to a person’s mental well-being. One example is head trauma. Dr. Amen documents at least one instance in which he was convinced that his patient had experienced head trauma, and questioned the patient again and again about possible brain injuries. One must wonder whether the positive results of such focused, repetitive questioning might be evidence of confirmation bias, as a search to confirm the preexisting belief of head trauma could lead to overlooking alternative explanations for a patient’s symptoms.
Another risk factor dwelt upon is exposure to toxins. One toxin Dr. Amen rightly recommends avoiding is tobacco smoke. Yet, his approach to advocate for a tobacco-free lifestyle is somewhat problematic. He lists chemicals contained in tobacco smoke, and then names unpleasant items that share those ingredients, such as paint. This smacks of the same sloppy logic manifested in social media memes decrying the use of vaccines by listing their ingredients alongside scary-sounding products that contain identical ingredients (for example, vaccines contain formaldehyde, which is used to embalm dead bodies!). This is analogous to saying that water is bad for you because it contains hydrogen, which is also an ingredient in atomic bombs.
Dr. Amen makes the blanket recommendation to avoid products containing “chemicals.” This is a difficult recommendation to interpret, since literally all matter is made of chemicals. It seems that Dr. Amen is leaning into the vague idea of a “chemical” as something artificially created in a lab, which must, therefore, be dangerous.
Along these lines, Dr. Amen suggests that if a person doesn’t know what is in a specific food item, it should not be eaten. Although this sounds reasonable on the surface, if people were told the names of the proteins and chemical compounds that make up many naturally occurring plants or meats, they would likely not recognize many of them. Dr. Amen dedicates space to list seemingly benign exposures – such as eating nonorganic produce, using two or more beauty products each day, or touching grocery store receipts – as possible “toxins.” By contrast, there is a certain irony in the absence of any mention of the risks associated with radiation from the SPECT imaging he staunchly advocates for. One potential risk of the book listing so many “toxins” to avoid is that patients could waste valuable time and energy eliminating exposures that pose little or no risk, rather than focusing efforts on well-established treatments.
In light of the observations and critiques offered above, one might come away with the impression that I would not recommend “The End of Mental Illness.” However, although one can nitpick details in the book, some of its bigger ideas make it worth commending to readers. Dr. Amen rightfully emphasizes the need for psychiatrists and patients to think more broadly about mental health issues beyond the use of pills. He justifiably criticizes the “15-minute med check” model of practice and the idea that medications are the end-all, be-all of treatment. He demonstrates an appropriate appreciation for the serious risks of reliance on benzodiazepines.6 Dr. Amen points out important contributions from Viktor Frankl, MD, to the field of psychiatry, which may go overlooked today. He also helpfully points out that bipolar disorder may often be misdiagnosed (although he attributes the misdiagnosis to traumatic brain injury, whereas other psychiatrists might say the misdiagnosis is due to borderline personality disorder).
Much of what Dr. Amen writes is sensible, and psychiatrists would do well to adopt the following steps he advocates for: Taking a comprehensive biopsychosocial-spiritual approach to the assessment and treatment of patients; thinking broadly in their differential diagnoses and not forgetting their medical training; understanding that medication alone is often not sufficient to make lasting, positive change in a person’s life; paying attention to healthy habits such as diet, exercise, sleep, and social activity; and knowing that CBT is a valuable tool that can change lives.
There is much to appreciate in “The End of Mental Illness,” especially the overarching idea that psychiatry isn’t just a symptom checklist and a prescription pad. Rather, achieving mental well-being often requires broader thinking and sustained lifestyle changes.
Although I did not agree with everything in the book, it did cause me to think and reflect on my own practice. I read “The End of Mental Illness” with colleagues in my department, and it stimulated a lively discussion. Isn’t that ultimately what a psychiatrist would want from a book like this – the opportunity to reflect, discuss, and potentially improve one’s own practice?
Dr. Weber is physician lead in the department of psychiatry at Intermountain Healthcare Budge Clinic, Logan (Utah) Psychiatry. He disclosed no relevant financial relationships.
References
1. JAMA Netw Open. 2020;3(12). doi: 10.1001/jamanetworkopen.2020.27909.
2. Curr Opin Psychiatry. 2014;27:358-63.
3. BMJ 2014. doi: 10.1136/bmj.g5205.
4. Am J Psychiatry. 2018 Mar 1;175:232-41.
5. Am J Psychiatry. 2016 Jun 1;173:575-87.
6. Current Psychiatry. 2018 Feb;17(2):22-7.
Daniel G. Amen, MD, is an American psychiatrist well-known for his eponymous clinics, television appearances, and series of books on mental health. One of his latest books, “The End of Mental Illness,” summarizes many of his views on the causes of and treatments for mental illnesses.
Dr. Amen’s approaches – such as his advocacy for the widespread use of single photon emission computed tomography (SPECT) imaging – are somewhat controversial and at times fall outside the mainstream of current psychiatric thought. So does “The End of Mental Illness” contain anything of value to the average practicing psychiatrist? (It should be noted that I listened to this as an audiobook and took notes as I listened. This does limit my ability to directly quote portions of the text, but I believe my notes are reliable.)
He begins the book by pointing out that the term “mental illness” might be better replaced with the term “brain illness.” With this shift in terminology, Dr. Amen introduces a theme that recurs throughout the book: That mental illnesses ultimately stem from various ways in which the brain can be harmed. While the suggested change in terminology might help reduce the stigma associated with psychiatric illnesses, Dr. Amen is surprisingly timid about implementing this term in his own book. He repeatedly refers to “brain health/mental health” issues instead of discarding the “mental” term altogether. Even his BRIGHT MINDS acronym for risk factors for mental illnesses includes the term “mind” instead of “brain.”
Continuing the theme of challenging terminology, Dr. Amen goes on to decry the weaknesses of the DSM system of nosology. This is a valid point, because under the current system, the same patient may receive differing diagnoses depending on which provider is seen and how certain symptoms are interpreted. Yet, here again, Dr. Amen does not seem to adhere to his own advice: He uses DSM terminology throughout the book, speaking of depression, anxiety, bipolar disorder, and ADHD. An oddity (which, admittedly, could have been the audiobook reader’s mistake rather than an error in the original text) is that the DSM is referred to as the “Diagnostic and Structural Manual” rather than the Diagnostic and Statistical Manual. He criticizes the DSM for its imprecision, pointing out the variety of symptom combinations that can produce the same diagnoses and how similar symptoms may overlap between differing diagnoses. Yet, his descriptions of common SPECT patterns (his preferred tool to assist in diagnosis) make it clear that here, too, there is a lot of overlap. As an example, ADHD was associated with at least three of the imaging patterns he described. It is also somewhat ironic how Dr. Amen obliquely criticizes the American Psychiatric Association for profiting from the use of the DSM, when SPECT imaging is expensive and profits his own organization.
Dr. Amen repeatedly asserts that psychiatry is unique among medical specialties for making diagnoses based on symptom clusters rather than direct visualization of the affected organ. Yet, psychiatry is not, in fact, unique in making diagnoses in this way. Some examples of diagnoses based on symptom clusters from other medical specialties are systemic lupus erythematosus, fibromyalgia, and chronic fatigue syndrome. Although he asserts that SPECT imaging better demonstrates the root cause of mental illnesses, it is unclear from his book whether this is actually the case.
The descriptions for the ways in which Dr. Amen uses SPECT (which, admittedly, are vague and presumably simplified for a general audience) suggest that he has made observations correlating specific imaging patterns with certain emotional/behavioral outcomes. However, the imaging patterns he describes in the book can be interpreted to represent multiple different mental conditions, making it clear that SPECT is not a laserlike diagnostic tool that produces a single, indisputable diagnosis. Accuracy with SPECT seems especially questionable in light of two case examples he shares where brain imaging was interpreted as representing illness, but the patients were not demonstrating any signs of mental dysfunction. In one case, Dr. Amen opined that the patient’s vibrant spiritual life “overrode” the sick brain, but if this is true,
Patient testimonials are provided, asserting that SPECT imaging helped them know “exactly” what treatment would help them. One cannot help but wonder whether part of the benefit of SPECT imaging is a placebo effect, boosting the confidence of patients that the treatment they are receiving is personalized and scientifically sound. A similar trend is currently seen more broadly in psychiatry with the widespread promotion of pharmacogenetic testing. Such testing may bolster patient confidence in their medication, but its value in improving patient outcomes has not been established.1
Dr. Amen outlines a brief history of mental health care, including differing approaches and therapies from the time of Sigmund Freud up to the present. His outline is somewhat critical of the perceived shortcomings of his psychiatric forebears, yet this seems entirely unnecessary. All scientific disciplines must start somewhere and build from limited knowledge to greater. Is it necessary to belittle Freud for not being able to do SPECT imaging in the 1800s?
Interestingly, Dr. Amen leaves cognitive-behavioral therapy (CBT), a landmark, evidence-based form of psychotherapy, out of his overview of the history of psychiatry. He does go on to mention CBT as part of the treatment offerings of the Amen Clinics, which could leave the lay reader with the incorrect impression that CBT is a treatment unique to Amen Clinics. Similarly, at one point Dr. Amen writes about “what I call automatic negative thoughts.” This phrasing could confuse readers who might not know that automatic thoughts are a concept endemic to CBT.
Dr. Amen writes repeatedly about the Amen Clinics 4 Circles, four key areas of life that can contribute to mental health. These areas are biological, psychological, social, and spiritual. While Amen Clinics may have come up with the term “4 Circles,” the biopsychosocial model of understanding illness was developed by George Engel, MD, in 1977, and current discussions of this model frequently incorporate a spiritual dimension as well.2
Dr. Amen’s writing at times mischaracterizes psychotropic medications in unhelpful ways. He speaks of psychotropic medications generally as being addictive. While this is certainly true for stimulants and benzodiazepines, most would agree that this does not apply to many other commonly used medications in psychiatry, including selective serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants, antipsychotics, and mood stabilizers. He also paints with a broad brush when he states that anxiety medications can cause dementia. A concerning link has been demonstrated between benzodiazepine use and dementia,3 but SSRIs (which are considered first-line medications for anxiety) are not known to cause dementia and may actually delay progression from mild cognitive impairment to Alzheimer’s dementia.4 His mention of medication use affecting a patient’s insurability could have the unfortunate effect of scaring away suffering individuals from seeking help. The one category of psychiatric medication he does not seem concerned about is psychostimulants, which is odd – given the addictive, cardiovascular, and other risks associated with that medication class.
In contrast to his skepticism regarding many psychotropic medications, Dr. Amen expresses significant enthusiasm regarding nutraceutical use. While there has been research in this area supporting a role for some nutraceutical interventions, there is still a need for more rigorous studies.5 To support his endorsement of natural remedies, Dr. Amen mentions that Hippocrates recommended herbs and spices for many health conditions. But Hippocrates lived more than 2,000 years ago, and the state of medicine has advanced significantly since then.
Dr. Amen also mentions that 80% of the developing world relies upon natural or herbal remedies as the primary source of medicine. While he frames this statement as supporting his endorsement of such remedies, it could conversely be said that this is evidence of the need to make pharmacological interventions more widely available in the developing world.
Much of “The End of Mental Illness” is dedicated to reviewing specific risk factors that could cause harm to a person’s mental well-being. One example is head trauma. Dr. Amen documents at least one instance in which he was convinced that his patient had experienced head trauma, and questioned the patient again and again about possible brain injuries. One must wonder whether the positive results of such focused, repetitive questioning might be evidence of confirmation bias, as a search to confirm the preexisting belief of head trauma could lead to overlooking alternative explanations for a patient’s symptoms.
Another risk factor dwelt upon is exposure to toxins. One toxin Dr. Amen rightly recommends avoiding is tobacco smoke. Yet, his approach to advocate for a tobacco-free lifestyle is somewhat problematic. He lists chemicals contained in tobacco smoke, and then names unpleasant items that share those ingredients, such as paint. This smacks of the same sloppy logic manifested in social media memes decrying the use of vaccines by listing their ingredients alongside scary-sounding products that contain identical ingredients (for example, vaccines contain formaldehyde, which is used to embalm dead bodies!). This is analogous to saying that water is bad for you because it contains hydrogen, which is also an ingredient in atomic bombs.
Dr. Amen makes the blanket recommendation to avoid products containing “chemicals.” This is a difficult recommendation to interpret, since literally all matter is made of chemicals. It seems that Dr. Amen is leaning into the vague idea of a “chemical” as something artificially created in a lab, which must, therefore, be dangerous.
Along these lines, Dr. Amen suggests that if a person doesn’t know what is in a specific food item, it should not be eaten. Although this sounds reasonable on the surface, if people were told the names of the proteins and chemical compounds that make up many naturally occurring plants or meats, they would likely not recognize many of them. Dr. Amen dedicates space to list seemingly benign exposures – such as eating nonorganic produce, using two or more beauty products each day, or touching grocery store receipts – as possible “toxins.” By contrast, there is a certain irony in the absence of any mention of the risks associated with radiation from the SPECT imaging he staunchly advocates for. One potential risk of the book listing so many “toxins” to avoid is that patients could waste valuable time and energy eliminating exposures that pose little or no risk, rather than focusing efforts on well-established treatments.
In light of the observations and critiques offered above, one might come away with the impression that I would not recommend “The End of Mental Illness.” However, although one can nitpick details in the book, some of its bigger ideas make it worth commending to readers. Dr. Amen rightfully emphasizes the need for psychiatrists and patients to think more broadly about mental health issues beyond the use of pills. He justifiably criticizes the “15-minute med check” model of practice and the idea that medications are the end-all, be-all of treatment. He demonstrates an appropriate appreciation for the serious risks of reliance on benzodiazepines.6 Dr. Amen points out important contributions from Viktor Frankl, MD, to the field of psychiatry, which may go overlooked today. He also helpfully points out that bipolar disorder may often be misdiagnosed (although he attributes the misdiagnosis to traumatic brain injury, whereas other psychiatrists might say the misdiagnosis is due to borderline personality disorder).
Much of what Dr. Amen writes is sensible, and psychiatrists would do well to adopt the following steps he advocates for: Taking a comprehensive biopsychosocial-spiritual approach to the assessment and treatment of patients; thinking broadly in their differential diagnoses and not forgetting their medical training; understanding that medication alone is often not sufficient to make lasting, positive change in a person’s life; paying attention to healthy habits such as diet, exercise, sleep, and social activity; and knowing that CBT is a valuable tool that can change lives.
There is much to appreciate in “The End of Mental Illness,” especially the overarching idea that psychiatry isn’t just a symptom checklist and a prescription pad. Rather, achieving mental well-being often requires broader thinking and sustained lifestyle changes.
Although I did not agree with everything in the book, it did cause me to think and reflect on my own practice. I read “The End of Mental Illness” with colleagues in my department, and it stimulated a lively discussion. Isn’t that ultimately what a psychiatrist would want from a book like this – the opportunity to reflect, discuss, and potentially improve one’s own practice?
Dr. Weber is physician lead in the department of psychiatry at Intermountain Healthcare Budge Clinic, Logan (Utah) Psychiatry. He disclosed no relevant financial relationships.
References
1. JAMA Netw Open. 2020;3(12). doi: 10.1001/jamanetworkopen.2020.27909.
2. Curr Opin Psychiatry. 2014;27:358-63.
3. BMJ 2014. doi: 10.1136/bmj.g5205.
4. Am J Psychiatry. 2018 Mar 1;175:232-41.
5. Am J Psychiatry. 2016 Jun 1;173:575-87.
6. Current Psychiatry. 2018 Feb;17(2):22-7.
Daniel G. Amen, MD, is an American psychiatrist well-known for his eponymous clinics, television appearances, and series of books on mental health. One of his latest books, “The End of Mental Illness,” summarizes many of his views on the causes of and treatments for mental illnesses.
Dr. Amen’s approaches – such as his advocacy for the widespread use of single photon emission computed tomography (SPECT) imaging – are somewhat controversial and at times fall outside the mainstream of current psychiatric thought. So does “The End of Mental Illness” contain anything of value to the average practicing psychiatrist? (It should be noted that I listened to this as an audiobook and took notes as I listened. This does limit my ability to directly quote portions of the text, but I believe my notes are reliable.)
He begins the book by pointing out that the term “mental illness” might be better replaced with the term “brain illness.” With this shift in terminology, Dr. Amen introduces a theme that recurs throughout the book: That mental illnesses ultimately stem from various ways in which the brain can be harmed. While the suggested change in terminology might help reduce the stigma associated with psychiatric illnesses, Dr. Amen is surprisingly timid about implementing this term in his own book. He repeatedly refers to “brain health/mental health” issues instead of discarding the “mental” term altogether. Even his BRIGHT MINDS acronym for risk factors for mental illnesses includes the term “mind” instead of “brain.”
Continuing the theme of challenging terminology, Dr. Amen goes on to decry the weaknesses of the DSM system of nosology. This is a valid point, because under the current system, the same patient may receive differing diagnoses depending on which provider is seen and how certain symptoms are interpreted. Yet, here again, Dr. Amen does not seem to adhere to his own advice: He uses DSM terminology throughout the book, speaking of depression, anxiety, bipolar disorder, and ADHD. An oddity (which, admittedly, could have been the audiobook reader’s mistake rather than an error in the original text) is that the DSM is referred to as the “Diagnostic and Structural Manual” rather than the Diagnostic and Statistical Manual. He criticizes the DSM for its imprecision, pointing out the variety of symptom combinations that can produce the same diagnoses and how similar symptoms may overlap between differing diagnoses. Yet, his descriptions of common SPECT patterns (his preferred tool to assist in diagnosis) make it clear that here, too, there is a lot of overlap. As an example, ADHD was associated with at least three of the imaging patterns he described. It is also somewhat ironic how Dr. Amen obliquely criticizes the American Psychiatric Association for profiting from the use of the DSM, when SPECT imaging is expensive and profits his own organization.
Dr. Amen repeatedly asserts that psychiatry is unique among medical specialties for making diagnoses based on symptom clusters rather than direct visualization of the affected organ. Yet, psychiatry is not, in fact, unique in making diagnoses in this way. Some examples of diagnoses based on symptom clusters from other medical specialties are systemic lupus erythematosus, fibromyalgia, and chronic fatigue syndrome. Although he asserts that SPECT imaging better demonstrates the root cause of mental illnesses, it is unclear from his book whether this is actually the case.
The descriptions for the ways in which Dr. Amen uses SPECT (which, admittedly, are vague and presumably simplified for a general audience) suggest that he has made observations correlating specific imaging patterns with certain emotional/behavioral outcomes. However, the imaging patterns he describes in the book can be interpreted to represent multiple different mental conditions, making it clear that SPECT is not a laserlike diagnostic tool that produces a single, indisputable diagnosis. Accuracy with SPECT seems especially questionable in light of two case examples he shares where brain imaging was interpreted as representing illness, but the patients were not demonstrating any signs of mental dysfunction. In one case, Dr. Amen opined that the patient’s vibrant spiritual life “overrode” the sick brain, but if this is true,
Patient testimonials are provided, asserting that SPECT imaging helped them know “exactly” what treatment would help them. One cannot help but wonder whether part of the benefit of SPECT imaging is a placebo effect, boosting the confidence of patients that the treatment they are receiving is personalized and scientifically sound. A similar trend is currently seen more broadly in psychiatry with the widespread promotion of pharmacogenetic testing. Such testing may bolster patient confidence in their medication, but its value in improving patient outcomes has not been established.1
Dr. Amen outlines a brief history of mental health care, including differing approaches and therapies from the time of Sigmund Freud up to the present. His outline is somewhat critical of the perceived shortcomings of his psychiatric forebears, yet this seems entirely unnecessary. All scientific disciplines must start somewhere and build from limited knowledge to greater. Is it necessary to belittle Freud for not being able to do SPECT imaging in the 1800s?
Interestingly, Dr. Amen leaves cognitive-behavioral therapy (CBT), a landmark, evidence-based form of psychotherapy, out of his overview of the history of psychiatry. He does go on to mention CBT as part of the treatment offerings of the Amen Clinics, which could leave the lay reader with the incorrect impression that CBT is a treatment unique to Amen Clinics. Similarly, at one point Dr. Amen writes about “what I call automatic negative thoughts.” This phrasing could confuse readers who might not know that automatic thoughts are a concept endemic to CBT.
Dr. Amen writes repeatedly about the Amen Clinics 4 Circles, four key areas of life that can contribute to mental health. These areas are biological, psychological, social, and spiritual. While Amen Clinics may have come up with the term “4 Circles,” the biopsychosocial model of understanding illness was developed by George Engel, MD, in 1977, and current discussions of this model frequently incorporate a spiritual dimension as well.2
Dr. Amen’s writing at times mischaracterizes psychotropic medications in unhelpful ways. He speaks of psychotropic medications generally as being addictive. While this is certainly true for stimulants and benzodiazepines, most would agree that this does not apply to many other commonly used medications in psychiatry, including selective serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants, antipsychotics, and mood stabilizers. He also paints with a broad brush when he states that anxiety medications can cause dementia. A concerning link has been demonstrated between benzodiazepine use and dementia,3 but SSRIs (which are considered first-line medications for anxiety) are not known to cause dementia and may actually delay progression from mild cognitive impairment to Alzheimer’s dementia.4 His mention of medication use affecting a patient’s insurability could have the unfortunate effect of scaring away suffering individuals from seeking help. The one category of psychiatric medication he does not seem concerned about is psychostimulants, which is odd – given the addictive, cardiovascular, and other risks associated with that medication class.
In contrast to his skepticism regarding many psychotropic medications, Dr. Amen expresses significant enthusiasm regarding nutraceutical use. While there has been research in this area supporting a role for some nutraceutical interventions, there is still a need for more rigorous studies.5 To support his endorsement of natural remedies, Dr. Amen mentions that Hippocrates recommended herbs and spices for many health conditions. But Hippocrates lived more than 2,000 years ago, and the state of medicine has advanced significantly since then.
Dr. Amen also mentions that 80% of the developing world relies upon natural or herbal remedies as the primary source of medicine. While he frames this statement as supporting his endorsement of such remedies, it could conversely be said that this is evidence of the need to make pharmacological interventions more widely available in the developing world.
Much of “The End of Mental Illness” is dedicated to reviewing specific risk factors that could cause harm to a person’s mental well-being. One example is head trauma. Dr. Amen documents at least one instance in which he was convinced that his patient had experienced head trauma, and questioned the patient again and again about possible brain injuries. One must wonder whether the positive results of such focused, repetitive questioning might be evidence of confirmation bias, as a search to confirm the preexisting belief of head trauma could lead to overlooking alternative explanations for a patient’s symptoms.
Another risk factor dwelt upon is exposure to toxins. One toxin Dr. Amen rightly recommends avoiding is tobacco smoke. Yet, his approach to advocate for a tobacco-free lifestyle is somewhat problematic. He lists chemicals contained in tobacco smoke, and then names unpleasant items that share those ingredients, such as paint. This smacks of the same sloppy logic manifested in social media memes decrying the use of vaccines by listing their ingredients alongside scary-sounding products that contain identical ingredients (for example, vaccines contain formaldehyde, which is used to embalm dead bodies!). This is analogous to saying that water is bad for you because it contains hydrogen, which is also an ingredient in atomic bombs.
Dr. Amen makes the blanket recommendation to avoid products containing “chemicals.” This is a difficult recommendation to interpret, since literally all matter is made of chemicals. It seems that Dr. Amen is leaning into the vague idea of a “chemical” as something artificially created in a lab, which must, therefore, be dangerous.
Along these lines, Dr. Amen suggests that if a person doesn’t know what is in a specific food item, it should not be eaten. Although this sounds reasonable on the surface, if people were told the names of the proteins and chemical compounds that make up many naturally occurring plants or meats, they would likely not recognize many of them. Dr. Amen dedicates space to list seemingly benign exposures – such as eating nonorganic produce, using two or more beauty products each day, or touching grocery store receipts – as possible “toxins.” By contrast, there is a certain irony in the absence of any mention of the risks associated with radiation from the SPECT imaging he staunchly advocates for. One potential risk of the book listing so many “toxins” to avoid is that patients could waste valuable time and energy eliminating exposures that pose little or no risk, rather than focusing efforts on well-established treatments.
In light of the observations and critiques offered above, one might come away with the impression that I would not recommend “The End of Mental Illness.” However, although one can nitpick details in the book, some of its bigger ideas make it worth commending to readers. Dr. Amen rightfully emphasizes the need for psychiatrists and patients to think more broadly about mental health issues beyond the use of pills. He justifiably criticizes the “15-minute med check” model of practice and the idea that medications are the end-all, be-all of treatment. He demonstrates an appropriate appreciation for the serious risks of reliance on benzodiazepines.6 Dr. Amen points out important contributions from Viktor Frankl, MD, to the field of psychiatry, which may go overlooked today. He also helpfully points out that bipolar disorder may often be misdiagnosed (although he attributes the misdiagnosis to traumatic brain injury, whereas other psychiatrists might say the misdiagnosis is due to borderline personality disorder).
Much of what Dr. Amen writes is sensible, and psychiatrists would do well to adopt the following steps he advocates for: Taking a comprehensive biopsychosocial-spiritual approach to the assessment and treatment of patients; thinking broadly in their differential diagnoses and not forgetting their medical training; understanding that medication alone is often not sufficient to make lasting, positive change in a person’s life; paying attention to healthy habits such as diet, exercise, sleep, and social activity; and knowing that CBT is a valuable tool that can change lives.
There is much to appreciate in “The End of Mental Illness,” especially the overarching idea that psychiatry isn’t just a symptom checklist and a prescription pad. Rather, achieving mental well-being often requires broader thinking and sustained lifestyle changes.
Although I did not agree with everything in the book, it did cause me to think and reflect on my own practice. I read “The End of Mental Illness” with colleagues in my department, and it stimulated a lively discussion. Isn’t that ultimately what a psychiatrist would want from a book like this – the opportunity to reflect, discuss, and potentially improve one’s own practice?
Dr. Weber is physician lead in the department of psychiatry at Intermountain Healthcare Budge Clinic, Logan (Utah) Psychiatry. He disclosed no relevant financial relationships.
References
1. JAMA Netw Open. 2020;3(12). doi: 10.1001/jamanetworkopen.2020.27909.
2. Curr Opin Psychiatry. 2014;27:358-63.
3. BMJ 2014. doi: 10.1136/bmj.g5205.
4. Am J Psychiatry. 2018 Mar 1;175:232-41.
5. Am J Psychiatry. 2016 Jun 1;173:575-87.
6. Current Psychiatry. 2018 Feb;17(2):22-7.
Borderline personality disorder diagnosis: To tell or not to tell patients?
News of actor/comedian Pete Davidson expressing relief after finally receiving a diagnosis of borderline personality disorder (BPD) prompted a recent Twitter discussion among physicians regarding the ongoing debate on whether or not to tell a patient he or she has this diagnosis.
“I’ve heard from [many] trainees that they were told never to tell a patient they had BPD, but I can hardly think of anything more paternalistic and stigmatizing,” Amy Barnhorst, MD, vice chair of community psychiatry at University of California, Davis, tweeted.
“Most patients, when I explain it to them, have this kind of reaction – they feel relieved and understood,” she added.
“I was told that as well [not to tell] in one of my practicum placements,” one respondent who identified herself as a clinical/forensic psychologist tweeted back. “I said it anyway and the person was relieved there was a name for what they were living with.”
However, others disagreed with Dr. Barnhorst, noting that BPD is a very serious, stigmatizing, and challenging disorder to treat and, because of this, may cause patients to lose hope.
Still, Dr. Barnhorst stands by her position. Although “there is a negative stigma against a diagnosis of BPD,” that idea more often comes from the clinician instead of the patient, she said.
“I’ve never had a patient say, ‘how dare you call me that!’ like it was an insult,” she said in an interview. Not disclosing a diagnosis “is like you’re not trusting a patient to be a reasonable adult human about this.”
‘Hard diagnosis’
Although BPD is a “hard diagnosis, we would never withhold a diagnosis of cancer or liver disease or something else we knew patients didn’t want but that we were going to try and treat them for,” said Dr. Barnhorst.
BPD is linked to significant morbidity because of its common association with comorbid conditions, such as major depressive disorder, substance use disorders, and dysthymia. A history of self-harm is present in 70%-75% of these patients and some estimates suggest up to 9% of individuals with BPD die by suicide.
In an article published in Innovations in Clinical Neuroscience investigators discussed “ethical and clinical questions psychiatrists should consider” when treating BPD, including whether a diagnosis should be shared with a patient.
After such a diagnosis a patient may “react intensely in negative ways and these responses may be easily triggered,” the researchers wrote.
“A propensity that will likely cause psychiatrists anguish, however, is BPD patients’ increased likelihood of attempting suicide,” they added. Part of the problem has been that, in the past, it was thought that a BPD prognosis was untreatable. However, the researchers note that is no longer the case.
Still, Kaz Nelson, MD, associate professor of psychiatry and behavioral sciences at the University of Minnesota, Minneapolis, has labeled BPD a so-called “asterisk” disorder.
As she wrote in a recent blog, “We tell patients when they meet criteria for a medical diagnosis.* We show compassion and nonjudgmentalism to patients.* We do not discriminate against patients.*” However, the asterisk for each of these statements is: *Except for those with BPD.
Ongoing debate
Starting around the 1980s, the DSM listed personality disorders under the No. 2 Axis, which is for conditions with symptoms that are “not mitigatable,” said Dr. Nelson.
“It really started as well-meaning therapists who care about their patients who wanted to develop some precision in understanding people, and them starting to notice some patterns that can get in the way of optimal function,” she said in an interview.
The thought was not to disclose these diagnoses “because that was for you to understand, and for the patient to discover these patterns over time in the course of your work together,” Dr. Nelson added.
Although treatment for BPD used to be virtually nonexistent, there is now hope – especially with dialectic-behavior therapy (DBT), which uses mindfulness to teach patients how to control emotions and improve relationships.
According to the National Education Alliance for BPD, other useful treatments include mentalization-based therapy, transference-focused therapy, and “good psychiatric management.” Although there are currently no approved medications for BPD, some drugs are used to treat comorbid conditions such as depression or anxiety.
“We now know that people recover, and the whole paradigm has been turned on its head,” Dr. Nelson said. For example, “we no longer categorize these things as treatable or untreatable, which was a very positive move.”
So why is the field still debating the issue of diagnosis disclosure?
“To this day there are different psychiatrists and some medical school curricula that continue to teach that personality disorders are long-term, fixed, and nontreatable – and that it’s kind of disparaging to give this kind of diagnosis to a patient,” Dr. Nelson said.
Dr. Nelson, also the vice chair for education at the University of Minnesota, Minneapolis, medical school, reported that there “we acknowledge BPD’s painful history and that there are these misconceptions. They’re going to be on the front line of combating discrimination and the idea that if you see a patient with possible BPD coming you should run. That’s just unacceptable.”
Dr. Nelson noted that the idea of disclosing a BPD diagnosis is less controversial now than in the past, but “the whole thing is still under debate, and treatment guidelines [on BPD] are old and expired.”
Criteria for BPD were not updated when the DSM-5 was published in 2013, and that needs to be fixed, Dr. Nelson added. “In the meantime, we’re trying to get the word out that it’s okay to interact with people about the diagnosis, discuss treatment plans, and manage it as one would with any other psychiatric or medical illness.”
An evolution, not a debate
Paul Appelbaum, MD, past president of the American Psychiatric Association and current chair of the organization’s DSM steering committee, said in an interview that he hasn’t been involved in any recent debate on this issue.
“I think practice has changed to the point where the general practice is to discuss patient diagnoses with [patients] openly. Patients appreciate that and psychiatrists have come to see the advantages of it,” said Dr. Appelbaum, a professor of psychiatry, medicine, and law at Columbia University, New York.
Dr. Appelbaum noted that patients also increasingly have access to their medical records, “so the reality is that it’s no longer possible in many cases to withhold a diagnosis.”
he said. “Maybe not everyone is entirely on board yet but there has been a sea change in psychiatric practices.”
Asked whether there needs to be some type of guideline update or statement released by the APA regarding BPD, Dr. Appelbaum said he doesn’t think the overall issue is BPD specific but applies to all psychiatric diagnoses.
“To the extent that there are still practitioners today that are telling students or residents [not to disclose], I would guess that they were trained a very long time ago and have not adapted to the new world,” he said.
“I don’t want to speak for the APA, but speaking for myself: I certainly encourage residents that I teach to be open about a diagnosis. It’s not just clinically helpful in some cases, it’s also ethically required from the perspective of allowing patients to make appropriate decisions about their treatment. And arguably it’s legally required as well, as part of the informed consent requirement,” Dr. Appelbaum said.
Regarding DSM updates, he noted that the committee “looks to the field to propose to us additions or changes to the DSM that are warranted by data that have been gathered since the DSM-5 came out.” There is a process set up on the DSM’s website to review such proposals.
In addition, Dr. Appelbaum said that there have been discussions about using a new model “that focuses on dimensions rather than on discreet categories” in order to classify personality disorders.
“There’s a group out there that is formulating a proposal that they will submit to us” on this, he added. “That’s the major discussion that is going on right now and it would clearly have implications for borderline as well as all the other personality disorders.”
In a statement, the APA said practice guidelines for BPD are currently under review and that the organization does not have a “position statement” on BPD for clinicians. The last update to its guideline was in the early 2000s.
A version of this article first appeared on Medscape.com.
News of actor/comedian Pete Davidson expressing relief after finally receiving a diagnosis of borderline personality disorder (BPD) prompted a recent Twitter discussion among physicians regarding the ongoing debate on whether or not to tell a patient he or she has this diagnosis.
“I’ve heard from [many] trainees that they were told never to tell a patient they had BPD, but I can hardly think of anything more paternalistic and stigmatizing,” Amy Barnhorst, MD, vice chair of community psychiatry at University of California, Davis, tweeted.
“Most patients, when I explain it to them, have this kind of reaction – they feel relieved and understood,” she added.
“I was told that as well [not to tell] in one of my practicum placements,” one respondent who identified herself as a clinical/forensic psychologist tweeted back. “I said it anyway and the person was relieved there was a name for what they were living with.”
However, others disagreed with Dr. Barnhorst, noting that BPD is a very serious, stigmatizing, and challenging disorder to treat and, because of this, may cause patients to lose hope.
Still, Dr. Barnhorst stands by her position. Although “there is a negative stigma against a diagnosis of BPD,” that idea more often comes from the clinician instead of the patient, she said.
“I’ve never had a patient say, ‘how dare you call me that!’ like it was an insult,” she said in an interview. Not disclosing a diagnosis “is like you’re not trusting a patient to be a reasonable adult human about this.”
‘Hard diagnosis’
Although BPD is a “hard diagnosis, we would never withhold a diagnosis of cancer or liver disease or something else we knew patients didn’t want but that we were going to try and treat them for,” said Dr. Barnhorst.
BPD is linked to significant morbidity because of its common association with comorbid conditions, such as major depressive disorder, substance use disorders, and dysthymia. A history of self-harm is present in 70%-75% of these patients and some estimates suggest up to 9% of individuals with BPD die by suicide.
In an article published in Innovations in Clinical Neuroscience investigators discussed “ethical and clinical questions psychiatrists should consider” when treating BPD, including whether a diagnosis should be shared with a patient.
After such a diagnosis a patient may “react intensely in negative ways and these responses may be easily triggered,” the researchers wrote.
“A propensity that will likely cause psychiatrists anguish, however, is BPD patients’ increased likelihood of attempting suicide,” they added. Part of the problem has been that, in the past, it was thought that a BPD prognosis was untreatable. However, the researchers note that is no longer the case.
Still, Kaz Nelson, MD, associate professor of psychiatry and behavioral sciences at the University of Minnesota, Minneapolis, has labeled BPD a so-called “asterisk” disorder.
As she wrote in a recent blog, “We tell patients when they meet criteria for a medical diagnosis.* We show compassion and nonjudgmentalism to patients.* We do not discriminate against patients.*” However, the asterisk for each of these statements is: *Except for those with BPD.
Ongoing debate
Starting around the 1980s, the DSM listed personality disorders under the No. 2 Axis, which is for conditions with symptoms that are “not mitigatable,” said Dr. Nelson.
“It really started as well-meaning therapists who care about their patients who wanted to develop some precision in understanding people, and them starting to notice some patterns that can get in the way of optimal function,” she said in an interview.
The thought was not to disclose these diagnoses “because that was for you to understand, and for the patient to discover these patterns over time in the course of your work together,” Dr. Nelson added.
Although treatment for BPD used to be virtually nonexistent, there is now hope – especially with dialectic-behavior therapy (DBT), which uses mindfulness to teach patients how to control emotions and improve relationships.
According to the National Education Alliance for BPD, other useful treatments include mentalization-based therapy, transference-focused therapy, and “good psychiatric management.” Although there are currently no approved medications for BPD, some drugs are used to treat comorbid conditions such as depression or anxiety.
“We now know that people recover, and the whole paradigm has been turned on its head,” Dr. Nelson said. For example, “we no longer categorize these things as treatable or untreatable, which was a very positive move.”
So why is the field still debating the issue of diagnosis disclosure?
“To this day there are different psychiatrists and some medical school curricula that continue to teach that personality disorders are long-term, fixed, and nontreatable – and that it’s kind of disparaging to give this kind of diagnosis to a patient,” Dr. Nelson said.
Dr. Nelson, also the vice chair for education at the University of Minnesota, Minneapolis, medical school, reported that there “we acknowledge BPD’s painful history and that there are these misconceptions. They’re going to be on the front line of combating discrimination and the idea that if you see a patient with possible BPD coming you should run. That’s just unacceptable.”
Dr. Nelson noted that the idea of disclosing a BPD diagnosis is less controversial now than in the past, but “the whole thing is still under debate, and treatment guidelines [on BPD] are old and expired.”
Criteria for BPD were not updated when the DSM-5 was published in 2013, and that needs to be fixed, Dr. Nelson added. “In the meantime, we’re trying to get the word out that it’s okay to interact with people about the diagnosis, discuss treatment plans, and manage it as one would with any other psychiatric or medical illness.”
An evolution, not a debate
Paul Appelbaum, MD, past president of the American Psychiatric Association and current chair of the organization’s DSM steering committee, said in an interview that he hasn’t been involved in any recent debate on this issue.
“I think practice has changed to the point where the general practice is to discuss patient diagnoses with [patients] openly. Patients appreciate that and psychiatrists have come to see the advantages of it,” said Dr. Appelbaum, a professor of psychiatry, medicine, and law at Columbia University, New York.
Dr. Appelbaum noted that patients also increasingly have access to their medical records, “so the reality is that it’s no longer possible in many cases to withhold a diagnosis.”
he said. “Maybe not everyone is entirely on board yet but there has been a sea change in psychiatric practices.”
Asked whether there needs to be some type of guideline update or statement released by the APA regarding BPD, Dr. Appelbaum said he doesn’t think the overall issue is BPD specific but applies to all psychiatric diagnoses.
“To the extent that there are still practitioners today that are telling students or residents [not to disclose], I would guess that they were trained a very long time ago and have not adapted to the new world,” he said.
“I don’t want to speak for the APA, but speaking for myself: I certainly encourage residents that I teach to be open about a diagnosis. It’s not just clinically helpful in some cases, it’s also ethically required from the perspective of allowing patients to make appropriate decisions about their treatment. And arguably it’s legally required as well, as part of the informed consent requirement,” Dr. Appelbaum said.
Regarding DSM updates, he noted that the committee “looks to the field to propose to us additions or changes to the DSM that are warranted by data that have been gathered since the DSM-5 came out.” There is a process set up on the DSM’s website to review such proposals.
In addition, Dr. Appelbaum said that there have been discussions about using a new model “that focuses on dimensions rather than on discreet categories” in order to classify personality disorders.
“There’s a group out there that is formulating a proposal that they will submit to us” on this, he added. “That’s the major discussion that is going on right now and it would clearly have implications for borderline as well as all the other personality disorders.”
In a statement, the APA said practice guidelines for BPD are currently under review and that the organization does not have a “position statement” on BPD for clinicians. The last update to its guideline was in the early 2000s.
A version of this article first appeared on Medscape.com.
News of actor/comedian Pete Davidson expressing relief after finally receiving a diagnosis of borderline personality disorder (BPD) prompted a recent Twitter discussion among physicians regarding the ongoing debate on whether or not to tell a patient he or she has this diagnosis.
“I’ve heard from [many] trainees that they were told never to tell a patient they had BPD, but I can hardly think of anything more paternalistic and stigmatizing,” Amy Barnhorst, MD, vice chair of community psychiatry at University of California, Davis, tweeted.
“Most patients, when I explain it to them, have this kind of reaction – they feel relieved and understood,” she added.
“I was told that as well [not to tell] in one of my practicum placements,” one respondent who identified herself as a clinical/forensic psychologist tweeted back. “I said it anyway and the person was relieved there was a name for what they were living with.”
However, others disagreed with Dr. Barnhorst, noting that BPD is a very serious, stigmatizing, and challenging disorder to treat and, because of this, may cause patients to lose hope.
Still, Dr. Barnhorst stands by her position. Although “there is a negative stigma against a diagnosis of BPD,” that idea more often comes from the clinician instead of the patient, she said.
“I’ve never had a patient say, ‘how dare you call me that!’ like it was an insult,” she said in an interview. Not disclosing a diagnosis “is like you’re not trusting a patient to be a reasonable adult human about this.”
‘Hard diagnosis’
Although BPD is a “hard diagnosis, we would never withhold a diagnosis of cancer or liver disease or something else we knew patients didn’t want but that we were going to try and treat them for,” said Dr. Barnhorst.
BPD is linked to significant morbidity because of its common association with comorbid conditions, such as major depressive disorder, substance use disorders, and dysthymia. A history of self-harm is present in 70%-75% of these patients and some estimates suggest up to 9% of individuals with BPD die by suicide.
In an article published in Innovations in Clinical Neuroscience investigators discussed “ethical and clinical questions psychiatrists should consider” when treating BPD, including whether a diagnosis should be shared with a patient.
After such a diagnosis a patient may “react intensely in negative ways and these responses may be easily triggered,” the researchers wrote.
“A propensity that will likely cause psychiatrists anguish, however, is BPD patients’ increased likelihood of attempting suicide,” they added. Part of the problem has been that, in the past, it was thought that a BPD prognosis was untreatable. However, the researchers note that is no longer the case.
Still, Kaz Nelson, MD, associate professor of psychiatry and behavioral sciences at the University of Minnesota, Minneapolis, has labeled BPD a so-called “asterisk” disorder.
As she wrote in a recent blog, “We tell patients when they meet criteria for a medical diagnosis.* We show compassion and nonjudgmentalism to patients.* We do not discriminate against patients.*” However, the asterisk for each of these statements is: *Except for those with BPD.
Ongoing debate
Starting around the 1980s, the DSM listed personality disorders under the No. 2 Axis, which is for conditions with symptoms that are “not mitigatable,” said Dr. Nelson.
“It really started as well-meaning therapists who care about their patients who wanted to develop some precision in understanding people, and them starting to notice some patterns that can get in the way of optimal function,” she said in an interview.
The thought was not to disclose these diagnoses “because that was for you to understand, and for the patient to discover these patterns over time in the course of your work together,” Dr. Nelson added.
Although treatment for BPD used to be virtually nonexistent, there is now hope – especially with dialectic-behavior therapy (DBT), which uses mindfulness to teach patients how to control emotions and improve relationships.
According to the National Education Alliance for BPD, other useful treatments include mentalization-based therapy, transference-focused therapy, and “good psychiatric management.” Although there are currently no approved medications for BPD, some drugs are used to treat comorbid conditions such as depression or anxiety.
“We now know that people recover, and the whole paradigm has been turned on its head,” Dr. Nelson said. For example, “we no longer categorize these things as treatable or untreatable, which was a very positive move.”
So why is the field still debating the issue of diagnosis disclosure?
“To this day there are different psychiatrists and some medical school curricula that continue to teach that personality disorders are long-term, fixed, and nontreatable – and that it’s kind of disparaging to give this kind of diagnosis to a patient,” Dr. Nelson said.
Dr. Nelson, also the vice chair for education at the University of Minnesota, Minneapolis, medical school, reported that there “we acknowledge BPD’s painful history and that there are these misconceptions. They’re going to be on the front line of combating discrimination and the idea that if you see a patient with possible BPD coming you should run. That’s just unacceptable.”
Dr. Nelson noted that the idea of disclosing a BPD diagnosis is less controversial now than in the past, but “the whole thing is still under debate, and treatment guidelines [on BPD] are old and expired.”
Criteria for BPD were not updated when the DSM-5 was published in 2013, and that needs to be fixed, Dr. Nelson added. “In the meantime, we’re trying to get the word out that it’s okay to interact with people about the diagnosis, discuss treatment plans, and manage it as one would with any other psychiatric or medical illness.”
An evolution, not a debate
Paul Appelbaum, MD, past president of the American Psychiatric Association and current chair of the organization’s DSM steering committee, said in an interview that he hasn’t been involved in any recent debate on this issue.
“I think practice has changed to the point where the general practice is to discuss patient diagnoses with [patients] openly. Patients appreciate that and psychiatrists have come to see the advantages of it,” said Dr. Appelbaum, a professor of psychiatry, medicine, and law at Columbia University, New York.
Dr. Appelbaum noted that patients also increasingly have access to their medical records, “so the reality is that it’s no longer possible in many cases to withhold a diagnosis.”
he said. “Maybe not everyone is entirely on board yet but there has been a sea change in psychiatric practices.”
Asked whether there needs to be some type of guideline update or statement released by the APA regarding BPD, Dr. Appelbaum said he doesn’t think the overall issue is BPD specific but applies to all psychiatric diagnoses.
“To the extent that there are still practitioners today that are telling students or residents [not to disclose], I would guess that they were trained a very long time ago and have not adapted to the new world,” he said.
“I don’t want to speak for the APA, but speaking for myself: I certainly encourage residents that I teach to be open about a diagnosis. It’s not just clinically helpful in some cases, it’s also ethically required from the perspective of allowing patients to make appropriate decisions about their treatment. And arguably it’s legally required as well, as part of the informed consent requirement,” Dr. Appelbaum said.
Regarding DSM updates, he noted that the committee “looks to the field to propose to us additions or changes to the DSM that are warranted by data that have been gathered since the DSM-5 came out.” There is a process set up on the DSM’s website to review such proposals.
In addition, Dr. Appelbaum said that there have been discussions about using a new model “that focuses on dimensions rather than on discreet categories” in order to classify personality disorders.
“There’s a group out there that is formulating a proposal that they will submit to us” on this, he added. “That’s the major discussion that is going on right now and it would clearly have implications for borderline as well as all the other personality disorders.”
In a statement, the APA said practice guidelines for BPD are currently under review and that the organization does not have a “position statement” on BPD for clinicians. The last update to its guideline was in the early 2000s.
A version of this article first appeared on Medscape.com.
A clinical approach to pharmacotherapy for personality disorders
DSM-5 defines personality disorders (PDs) as the presence of an enduring pattern of inner experience and behavior that “deviates markedly from the expectations of the individual’s culture, is pervasive and inflexible, has an onset in adulthood, is stable over time, and leads to distress or impairment.”1 As a general rule, PDs are not limited to episodes of illness, but reflect an individual’s long-term adjustment. These disorders occur in 10% to 15% of the general population; the rates are especially high in health care settings, in criminal offenders, and in those with a substance use disorder (SUD).2 PDs nearly always have an onset in adolescence or early adulthood and tend to diminish in severity with advancing age. They are associated with high rates of unemployment, homelessness, divorce and separation, domestic violence, substance misuse, and suicide.3
Psychotherapy is the first-line treatment for PDs, but there has been growing interest in using pharmacotherapy to treat PDs. While much of the PD treatment literature focuses on borderline PD,4-9 this article describes diagnosis, potential pharmacotherapy strategies, and methods to assess response to treatment for patients with all types of PDs.
Recognizing and diagnosing personality disorders
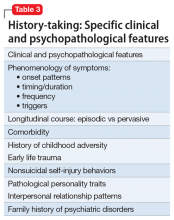
The diagnosis of a PD requires an understanding of DSM-5 criteria combined with a comprehensive psychiatric history and mental status examination. The patient’s history is the most important basis for diagnosing a PD.2 Collateral information from relatives or friends can help confirm the severity and pervasiveness of the individual’s personality problems. In some patients, long-term observation might be necessary to confirm the presence of a PD. Some clinicians are reluctant to diagnose PDs because of stigma, a problem common among patients with borderline PD.10,11
To screen for PDs, a clinician might ask the patient about problems with interpersonal relationships, sense of self, work, affect, impulse control, and reality testing. Table 112 lists general screening questions for the presence of a PD from the Iowa Personality Disorders Screen. Structured diagnostic interviews and self-report assessments could boost recognition of PDs, but these tools are rarely used outside of research settings.13,14
The PD clusters
DSM-5 divides 10 PDs into 3 clusters based on shared phenomenology and diagnostic criteria. Few patients have a “pure” case in which they meet criteria for only a single personality disorder.1
Cluster A. “Eccentric cluster” disorders are united by social aversion, a failure to form close attachments, or paranoia and suspiciousness.15 These include paranoid, schizoid, and schizotypal PD. Low self-awareness is typical. There are no treatment guidelines for these disorders, although there is some clinical trial data for schizotypal PD.
Cluster B. “Dramatic cluster” disorders share dramatic, emotional, and erratic characteristics.14 These include narcissistic, antisocial, borderline, and histrionic PD. Antisocial and narcissistic patients have low self-awareness. There are treatment guidelines for antisocial and borderline PD, and a variety of clinical trial data is available for the latter.15
Continue to: Cluster C
Cluster C. “Anxious cluster” disorders are united by anxiousness, fearfulness, and poor self-esteem. Many of these patients also display interpersonal rigidity.15 These disorders include avoidant, dependent, and obsessive-compulsive PD. There are no treatment guidelines or clinical trial data for these disorders.
Why consider pharmacotherapy for personality disorders?
The consensus among experts is that psychotherapy is the treatment of choice for PDs.15 Despite significant gaps in the evidence base, there has been a growing interest in using psychotropic medication to treat PDs. For example, research shows that >90% of patients with borderline PD are prescribed medication, most typically antidepressants, antipsychotics, mood stabilizers, stimulants, or sedative-hypnotics.16,17
Increased interest in pharmacotherapy for PDs could be related to research showing the importance of underlying neurobiology, particularly for antisocial and borderline PD.18,19 This work is complemented by genetic research showing the heritability of PD traits and disorders.20,21 Another factor could be renewed interest in dimensional approaches to the classification of PDs, as exemplified by DSM-5’s alternative model for PDs.1 This approach aligns with some expert recommendations to focus on treating PD symptom dimensions, rather than the syndrome itself.22
Importantly, no psychotropic medication is FDA-approved for the treatment of any PD. For that reason, prescribing medication for a PD is “off-label,” although prescribing a medication for a comorbid disorder for which the drug has an FDA-approved indication is not (eg, prescribing an antidepressant for major depressive disorder [MDD]).
Principles for prescribing
Despite gaps in research data, general principles for using medication to treat PDs have emerged from treatment guidelines for antisocial and borderline PD, clinical trial data, reviews and meta-analyses, and expert opinion. Clinicians should address the following considerations before prescribing medication to a patient with a PD.
Continue to: PD diagnosis
PD diagnosis. Has the patient been properly assessed and diagnosed? While history is the most important basis for diagnosis, the clinician should be familiar with the PDs and DSM-5 criteria. Has the patient been informed of the diagnosis and its implications for treatment?
Patient interest in medication. Is the patient interested in taking medication? Patients with borderline PD are often prescribed medication, but there are sparse data for the other PDs. The patient might have little interest in the PD diagnosis or its treatment.
Comorbidity. Has the patient been assessed for comorbid psychiatric disorders that could interfere with medication use (ie, an SUD) or might be a focus of treatment (eg, MDD)? Patients with PDs typically have significant comorbidity that a thorough evaluation will uncover.
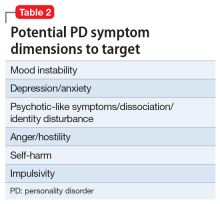
PD symptom dimensions. Has the patient been assessed to determine cognitive or behavioral symptom dimensions of their PD? One or more symptom dimension(s) could be the focus of treatment. Table 2 lists examples of PD symptom dimensions.
Strategies to guide prescribing
Strategies to help guide prescribing include targeting any comorbid disorder(s), targeting important PD symptom dimensions (eg, impulsive aggression), choosing medication based on the similarity of the PD to another disorder known to respond to medication, and targeting the PD itself.
Continue to: Targeting comorbid disorders
Targeting comorbid disorders. National Institute for Health and Care Excellence guidelines for antisocial and borderline PD recommend that clinicians focus on treating comorbid disorders, a position echoed in Cochrane and other reviews.4,9,22-26 For example, a patient with borderline PD experiencing a major depressive episode could be treated with an antidepressant. Targeting the depressive symptoms could boost the patient’s mood, perhaps lessening the individual’s PD symptoms or reducing their severity.
Targeting important symptom dimensions. For patients with borderline PD, several guidelines and reviews have suggested that treatment should focus on emotional dysregulation and impulsive aggression (mood stabilizers, antipsychotics), or cognitive-perceptual symptoms (antipsychotics).4-6,15 There is some evidence that mood stabilizers or second-generation antipsychotics could help reduce impulsive aggression in patients with antisocial PD.27
Choosing medication based on similarity to another disorder known to respond to medication. Avoidant PD overlaps with social anxiety disorder and can be conceptualized as a chronic, pervasive social phobia. Avoidant PD might respond to a medication known to be effective for treating social anxiety disorder, such as a selective serotonin reuptake inhibitor (SSRI) or venlafaxine.28 Treating obsessive-compulsive PD with an SSRI is another example of this strategy, as 1 small study of fluvoxamine suggests.29 Obsessive-compulsive PD is common in persons with obsessive-compulsive disorder, and overlap includes preoccupation with orders, rules, and lists, and an inability to throw things out.
Targeting the PD syndrome. Another strategy is to target the PD itself. Clinical trial data suggest the antipsychotic risperidone can reduce the symptoms of schizotypal PD.30 Considering that this PD has a genetic association with schizophrenia, it is not surprising that the patient’s ideas of reference, odd communication, or transient paranoia might respond to an antipsychotic. Data from randomized controlled trials (RCTs) support the use of the second-generation antipsychotics aripiprazole and quetiapine to treat BPD.31,32 While older guidelines4,5 supported the use of the mood stabilizer lamotrigine, a recent RCT found that it was no more effective than placebo for borderline PD or its symptom dimensions.33
What to do before prescribing
Before writing a prescription, the clinician and patient should discuss the presence of a PD and the desirability of treatment. The patient should understand the limited evidence base and know that medication prescribed for a PD is off-label. The clinician should discuss medication selection and its rationale, and whether the medication is targeting a comorbid disorder, symptom dimension(s), or the PD itself. Additional considerations for prescribing for patients with PDs are listed in Table 3.34

Continue to: Avoid polypharmacy
Avoid polypharmacy. Many patients with borderline PD are prescribed multiple psychotropic medications.16,17 This approach leads to greater expense and more adverse effects, and is not evidence-based.
Avoid benzodiazepines. Many patients with borderline PD are prescribed benzodiazepines, often as part of a polypharmacy regimen. These drugs can cause disinhibition, thereby increasing acting-out behaviors and self-harm.35 Also, patients with PDs often have SUDs, which is a contraindication for benzodiazepine use.
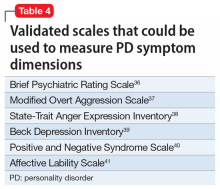
Rate the patient’s improvement. Both the patient and clinician can benefit from monitoring symptomatic improvement. Several validated scales can be used to rate depression, anxiety, impulsivity, mood lability, anger, and aggression (Table 436-41).Some validated scales for borderline PD align with DSM-5 criteria. Two such widely used instruments are the Zanarini Rating Scale for Borderline Personality Disorder (ZAN-BPD)42 and the self-rated Borderline Evaluation of Severity Over Time (BEST).43 Each has questions that could be pulled to rate a symptom dimension of interest, such as affective instability, anger dyscontrol, or abandonment fears (Table 542,43).
A visual analog scale is easy to use and can target symptom dimensions of interest.44 For example, a clinician could use a visual analog scale to rate mood instability by asking a patient to rate their mood severity by making a mark along a 10-cm line (0 = “Most erratic emotions I have experienced,” 10 = “Most stable I have ever experienced my emotions to be”). This score can be recorded at baseline and subsequent visits.
Take-home points
PDs are common in the general population and health care settings. They are underrecognized by the general public and mental health professionals, often because of stigma. Clinicians could boost their recognition of these disorders by embedding simple screening questions in their patient assessments. Many patients with PDs will be interested in pharmacotherapy for their disorder or symptoms. Treatment strategies include targeting the comorbid disorder(s), targeting important PD symptom dimensions, choosing medication based on the similarity of the PD to another disorder known to respond to medication, and targeting the PD itself. Each strategy has its limitations and varying degrees of empirical support. Treatment response can be monitored using validated scales or a visual analog scale.
Continue to: Bottom Line
Bottom Line
Although psychotherapy is the first-line treatment and no medications are FDAapproved for treating personality disorders (PDs), there has been growing interest in using psychotropic medication to treat PDs. Strategies for pharmacotherapy include targeting comorbid disorders, PD symptom dimensions, or the PD itself. Choice of medication can be based on the similarity of the PD with another disorder known to respond to medication.
Related Resources
- Correa Da Costa S, Sanches M, Soares JC. Bipolar disorder or borderline personality disorder? Current Psychiatry. 2019;18(11):26-29,35-39.
- Bateman A, Gunderson J, Mulder R. Treatment of personality disorders. Lancet. 2015;385(9969):735-743.
Drug Brand Names
Aripiprazole • Abilify
Fluvoxamine • Luvox
Lamotrigine • Lamictal
Quetiapine • Seroquel
Risperidone • Risperdal
Venlafaxine • Effexor
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Black DW, Andreasen N. Personality disorders. In: Black DW, Andreasen N. Introductory textbook of psychiatry, 7th edition. American Psychiatric Publishing; 2020:410-423.
3. Black DW, Blum N, Pfohl B, et al. Suicidal behavior in borderline personality disorder: prevalence, risk factors, prediction, and prevention. J Pers Disord 2004;18(3):226-239.
4. Lieb K, Völlm B, Rücker G, et al. Pharmacotherapy for borderline personality disorder: Cochrane systematic review of randomised trials. Br J Psychiatry. 2010;196(1):4-12.
5. Vita A, De Peri L, Sacchetti E. Antipsychotics, antidepressants, anticonvulsants, and placebo on the symptom dimensions of borderline personality disorder – a meta-analysis of randomized controlled and open-label trials. J Clin Psychopharmacol. 2011;31(5):613-624.
6. Stoffers JM, Lieb K. Pharmacotherapy for borderline personality disorder – current evidence and recent trends. Curr Psychiatry Rep. 2015;17(1):534.
7. Hancock-Johnson E, Griffiths C, Picchioni M. A focused systematic review of pharmacological treatment for borderline personality disorder. CNS Drugs. 2017;31(5):345-356.
8. Black DW, Paris J, Schulz SC. Personality disorders: evidence-based integrated biopsychosocial treatment of borderline personality disorder. In: Muse M, ed. Cognitive behavioral psychopharmacology: the clinical practice of evidence-based biopsychosocial integration. John Wiley & Sons; 2018:137-165.
9. Stoffers-Winterling J, Sorebø OJ, Lieb K. Pharmacotherapy for borderline personality disorder: an update of published, unpublished and ongoing studies. Curr Psychiatry Rep. 2020;22(8):37.
10. Lewis G, Appleby L. Personality disorder: the patients psychiatrists dislike. Br J Psychiatry. 1988;153:44-49.
11. Black DW, Pfohl B, Blum N, et al. Attitudes toward borderline personality disorder: a survey of 706 mental health clinicians. CNS Spectr. 2011;16(3):67-74.
12. Langbehn DR, Pfohl BM, Reynolds S, et al. The Iowa Personality Disorder Screen: development and preliminary validation of a brief screening interview. J Pers Disord. 1999;13(1):75-89.
13. Pfohl B, Blum N, Zimmerman M. Structured Interview for DSM-IV Personality (SIDP-IV). American Psychiatric Press; 1997.
14. First MB, Spitzer RL, Gibbon M, et al. The Structured Clinical Interview for DSM-III-R Personality Disorders (SCID-II). Part II: multisite test-retest reliability study. J Pers Disord. 1995;9(2):92-104.
15. Bateman A, Gunderson J, Mulder R. Treatment of personality disorders. Lancet. 2015;385(9969):735-743.
16. Zanarini MC, Frankenburg FR, Reich DB, et al. Treatment rates for patients with borderline personality disorder and other personality disorders: a 16-year study. Psychiatr Serv. 2015;66(1):15-20.
17. Black DW, Allen J, McCormick B, et al. Treatment received by persons with BPD participating in a randomized clinical trial of the Systems Training for Emotional Predictability and Problem Solving programme. Person Ment Health. 2011;5(3):159-168.
18. Yang Y, Glenn AL, Raine A. Brain abnormalities in antisocial individuals: implications for the law. Behav Sci Law. 2008;26(1):65-83.
19. Ruocco AC, Amirthavasagam S, Choi-Kain LW, et al. Neural correlates of negative emotionality in BPD: an activation-likelihood-estimation meta-analysis. Biol Psychiatry. 2013;73(2):153-160.
20. Livesley WJ, Jang KL, Jackson DN, et al. Genetic and environmental contributions to dimensions of personality disorder. Am J Psychiatry. 1993;150(12):1826-1831.
21. Slutske WS. The genetics of antisocial behavior. Curr Psychiatry Rep. 2001;3(2):158-162.
22. Ripoll LH, Triebwasser J, Siever LJ. Evidence-based pharmacotherapy for personality disorders. Int J Neuropsychopharmacol. 2011;14(9):1257-1288.
23. National Institute for Health and Care Excellence (NICE). Borderline personality disorder: recognition and management. Clinical guideline [CG78]. Published January 2009. https://www.nice.org.uk/guidance/cg78
24. National Institute for Health and Care Excellence (NICE). Antisocial personality disorder: prevention and management. Clinical guideline [CG77]. Published January 2009. Updated March 27, 2013. https://www.nice.org.uk/guidance/cg77
25. Khalifa N, Duggan C, Stoffers J, et al. Pharmacologic interventions for antisocial personality disorder. Cochrane Database Syst Rep. 2010;(8):CD007667.
26. Stoffers JM, Völlm BA, Rücker G, et al. Psychological therapies for people with borderline personality disorder. Cochrane Database Syst Rev. 2012;2012(8):CD005652.
27. Black DW. The treatment of antisocial personality disorder. Current Treatment Options in Psychiatry. 2017. https://doi.org/10.1007/s40501-017-0123-z
28. Stein MB, Liebowitz MR, Lydiard RB, et al. Paroxetine treatment of generalized social phobia (social anxiety disorder): a randomized controlled trial. JAMA. 1998;280(8):708-713.
29. Ansseau M. The obsessive-compulsive personality: diagnostic aspects and treatment possibilities. In: Den Boer JA, Westenberg HGM, eds. Focus on obsessive-compulsive spectrum disorders. Syn-Thesis; 1997:61-73.
30. Koenigsberg HW, Reynolds D, Goodman M, et al. Risperidone in the treatment of schizotypal personality disorder. J Clin Psychiatry. 2003;64(6):628-634.
31. Black DW, Zanarini MC, Romine A, et al. Comparison of low and moderate dosages of extended-release quetiapine in borderline personality disorder: a randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 2014;171(11):1174-1182.
32. Nickel MK, Muelbacher M, Nickel C, et al. Aripiprazole in the treatment of patients with borderline personality disorder: a double-blind, placebo-controlled study. Am J Psychiatry. 2006;163(5):833-838.
33. Crawford MJ, Sanatinia R, Barrett B, et al; LABILE study team. The clinical effectiveness and cost-effectiveness of lamotrigine in borderline personality disorder: a randomized placebo-controlled trial. Am J Psychiatry. 2018;175(8):756-764.
34. Frankenburg FR, Zanarini MC. The association between borderline personality disorder and chronic medical illnesses, poor health-related lifestyle choices, and costly forms of health care utilization. J Clin Psychiatry. 2004;65(12)1660-1665.
35. Cowdry RW, Gardner DL. Pharmacotherapy of borderline personality disorder. Alprazolam, carbamazepine, trifluoperazine, and tranylcypromine. Arch Gen Psychiatry. 1988;45(2):111-119.
36. Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:799-812.
37. Ratey JJ, Gutheil CM. The measurement of aggressive behavior: reflections on the use of the Overt Aggression Scale and the Modified Overt Aggression Scale. J Neuropsychiatr Clin Neurosci. 1991;3(2):S57-S60.
38. Spielberger CD, Sydeman SJ, Owen AE, et al. Measuring anxiety and anger with the State-Trait Anxiety Inventory (STAI) and the State-Trait Anger Expression Inventory (STAXI). In: Maruish ME, ed. The use of psychological testing for treatment planning and outcomes assessment. Lawrence Erlbaum Associates Publishers; 1999:993-1021.
39. Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory II. Psychological Corp; 1996.
40. Watson D, Clark LA. The PANAS-X: Manual for the Positive and Negative Affect Schedule – Expanded Form. The University of Iowa; 1999.
41. Harvey D, Greenberg BR, Serper MR, et al. The affective lability scales: development, reliability, and validity. J Clin Psychol. 1989;45(5):786-793.
42. Zanarini MC, Vujanovic AA, Parachini EA, et al. Zanarini Rating Scale for Borderline Personality Disorder (ZAN-BPD): a continuous measure of DSM-IV borderline psychopathology. J Person Disord. 2003:17(3):233-242.
43. Pfohl B, Blum N, St John D, et al. Reliability and validity of the Borderline Evaluation of Severity Over Time (BEST): a new scale to measure severity and change in borderline personality disorder. J Person Disord. 2009;23(3):281-293.
44. Ahearn EP. The use of visual analog scales in mood disorders: a critical review. J Psychiatr Res. 1997;31(5):569-579.
DSM-5 defines personality disorders (PDs) as the presence of an enduring pattern of inner experience and behavior that “deviates markedly from the expectations of the individual’s culture, is pervasive and inflexible, has an onset in adulthood, is stable over time, and leads to distress or impairment.”1 As a general rule, PDs are not limited to episodes of illness, but reflect an individual’s long-term adjustment. These disorders occur in 10% to 15% of the general population; the rates are especially high in health care settings, in criminal offenders, and in those with a substance use disorder (SUD).2 PDs nearly always have an onset in adolescence or early adulthood and tend to diminish in severity with advancing age. They are associated with high rates of unemployment, homelessness, divorce and separation, domestic violence, substance misuse, and suicide.3
Psychotherapy is the first-line treatment for PDs, but there has been growing interest in using pharmacotherapy to treat PDs. While much of the PD treatment literature focuses on borderline PD,4-9 this article describes diagnosis, potential pharmacotherapy strategies, and methods to assess response to treatment for patients with all types of PDs.
Recognizing and diagnosing personality disorders
The diagnosis of a PD requires an understanding of DSM-5 criteria combined with a comprehensive psychiatric history and mental status examination. The patient’s history is the most important basis for diagnosing a PD.2 Collateral information from relatives or friends can help confirm the severity and pervasiveness of the individual’s personality problems. In some patients, long-term observation might be necessary to confirm the presence of a PD. Some clinicians are reluctant to diagnose PDs because of stigma, a problem common among patients with borderline PD.10,11
To screen for PDs, a clinician might ask the patient about problems with interpersonal relationships, sense of self, work, affect, impulse control, and reality testing. Table 112 lists general screening questions for the presence of a PD from the Iowa Personality Disorders Screen. Structured diagnostic interviews and self-report assessments could boost recognition of PDs, but these tools are rarely used outside of research settings.13,14
The PD clusters
DSM-5 divides 10 PDs into 3 clusters based on shared phenomenology and diagnostic criteria. Few patients have a “pure” case in which they meet criteria for only a single personality disorder.1
Cluster A. “Eccentric cluster” disorders are united by social aversion, a failure to form close attachments, or paranoia and suspiciousness.15 These include paranoid, schizoid, and schizotypal PD. Low self-awareness is typical. There are no treatment guidelines for these disorders, although there is some clinical trial data for schizotypal PD.
Cluster B. “Dramatic cluster” disorders share dramatic, emotional, and erratic characteristics.14 These include narcissistic, antisocial, borderline, and histrionic PD. Antisocial and narcissistic patients have low self-awareness. There are treatment guidelines for antisocial and borderline PD, and a variety of clinical trial data is available for the latter.15
Continue to: Cluster C
Cluster C. “Anxious cluster” disorders are united by anxiousness, fearfulness, and poor self-esteem. Many of these patients also display interpersonal rigidity.15 These disorders include avoidant, dependent, and obsessive-compulsive PD. There are no treatment guidelines or clinical trial data for these disorders.
Why consider pharmacotherapy for personality disorders?
The consensus among experts is that psychotherapy is the treatment of choice for PDs.15 Despite significant gaps in the evidence base, there has been a growing interest in using psychotropic medication to treat PDs. For example, research shows that >90% of patients with borderline PD are prescribed medication, most typically antidepressants, antipsychotics, mood stabilizers, stimulants, or sedative-hypnotics.16,17
Increased interest in pharmacotherapy for PDs could be related to research showing the importance of underlying neurobiology, particularly for antisocial and borderline PD.18,19 This work is complemented by genetic research showing the heritability of PD traits and disorders.20,21 Another factor could be renewed interest in dimensional approaches to the classification of PDs, as exemplified by DSM-5’s alternative model for PDs.1 This approach aligns with some expert recommendations to focus on treating PD symptom dimensions, rather than the syndrome itself.22
Importantly, no psychotropic medication is FDA-approved for the treatment of any PD. For that reason, prescribing medication for a PD is “off-label,” although prescribing a medication for a comorbid disorder for which the drug has an FDA-approved indication is not (eg, prescribing an antidepressant for major depressive disorder [MDD]).
Principles for prescribing
Despite gaps in research data, general principles for using medication to treat PDs have emerged from treatment guidelines for antisocial and borderline PD, clinical trial data, reviews and meta-analyses, and expert opinion. Clinicians should address the following considerations before prescribing medication to a patient with a PD.
Continue to: PD diagnosis
PD diagnosis. Has the patient been properly assessed and diagnosed? While history is the most important basis for diagnosis, the clinician should be familiar with the PDs and DSM-5 criteria. Has the patient been informed of the diagnosis and its implications for treatment?
Patient interest in medication. Is the patient interested in taking medication? Patients with borderline PD are often prescribed medication, but there are sparse data for the other PDs. The patient might have little interest in the PD diagnosis or its treatment.
Comorbidity. Has the patient been assessed for comorbid psychiatric disorders that could interfere with medication use (ie, an SUD) or might be a focus of treatment (eg, MDD)? Patients with PDs typically have significant comorbidity that a thorough evaluation will uncover.
PD symptom dimensions. Has the patient been assessed to determine cognitive or behavioral symptom dimensions of their PD? One or more symptom dimension(s) could be the focus of treatment. Table 2 lists examples of PD symptom dimensions.
Strategies to guide prescribing
Strategies to help guide prescribing include targeting any comorbid disorder(s), targeting important PD symptom dimensions (eg, impulsive aggression), choosing medication based on the similarity of the PD to another disorder known to respond to medication, and targeting the PD itself.
Continue to: Targeting comorbid disorders
Targeting comorbid disorders. National Institute for Health and Care Excellence guidelines for antisocial and borderline PD recommend that clinicians focus on treating comorbid disorders, a position echoed in Cochrane and other reviews.4,9,22-26 For example, a patient with borderline PD experiencing a major depressive episode could be treated with an antidepressant. Targeting the depressive symptoms could boost the patient’s mood, perhaps lessening the individual’s PD symptoms or reducing their severity.
Targeting important symptom dimensions. For patients with borderline PD, several guidelines and reviews have suggested that treatment should focus on emotional dysregulation and impulsive aggression (mood stabilizers, antipsychotics), or cognitive-perceptual symptoms (antipsychotics).4-6,15 There is some evidence that mood stabilizers or second-generation antipsychotics could help reduce impulsive aggression in patients with antisocial PD.27
Choosing medication based on similarity to another disorder known to respond to medication. Avoidant PD overlaps with social anxiety disorder and can be conceptualized as a chronic, pervasive social phobia. Avoidant PD might respond to a medication known to be effective for treating social anxiety disorder, such as a selective serotonin reuptake inhibitor (SSRI) or venlafaxine.28 Treating obsessive-compulsive PD with an SSRI is another example of this strategy, as 1 small study of fluvoxamine suggests.29 Obsessive-compulsive PD is common in persons with obsessive-compulsive disorder, and overlap includes preoccupation with orders, rules, and lists, and an inability to throw things out.
Targeting the PD syndrome. Another strategy is to target the PD itself. Clinical trial data suggest the antipsychotic risperidone can reduce the symptoms of schizotypal PD.30 Considering that this PD has a genetic association with schizophrenia, it is not surprising that the patient’s ideas of reference, odd communication, or transient paranoia might respond to an antipsychotic. Data from randomized controlled trials (RCTs) support the use of the second-generation antipsychotics aripiprazole and quetiapine to treat BPD.31,32 While older guidelines4,5 supported the use of the mood stabilizer lamotrigine, a recent RCT found that it was no more effective than placebo for borderline PD or its symptom dimensions.33
What to do before prescribing
Before writing a prescription, the clinician and patient should discuss the presence of a PD and the desirability of treatment. The patient should understand the limited evidence base and know that medication prescribed for a PD is off-label. The clinician should discuss medication selection and its rationale, and whether the medication is targeting a comorbid disorder, symptom dimension(s), or the PD itself. Additional considerations for prescribing for patients with PDs are listed in Table 3.34

Continue to: Avoid polypharmacy
Avoid polypharmacy. Many patients with borderline PD are prescribed multiple psychotropic medications.16,17 This approach leads to greater expense and more adverse effects, and is not evidence-based.
Avoid benzodiazepines. Many patients with borderline PD are prescribed benzodiazepines, often as part of a polypharmacy regimen. These drugs can cause disinhibition, thereby increasing acting-out behaviors and self-harm.35 Also, patients with PDs often have SUDs, which is a contraindication for benzodiazepine use.
Rate the patient’s improvement. Both the patient and clinician can benefit from monitoring symptomatic improvement. Several validated scales can be used to rate depression, anxiety, impulsivity, mood lability, anger, and aggression (Table 436-41).Some validated scales for borderline PD align with DSM-5 criteria. Two such widely used instruments are the Zanarini Rating Scale for Borderline Personality Disorder (ZAN-BPD)42 and the self-rated Borderline Evaluation of Severity Over Time (BEST).43 Each has questions that could be pulled to rate a symptom dimension of interest, such as affective instability, anger dyscontrol, or abandonment fears (Table 542,43).
A visual analog scale is easy to use and can target symptom dimensions of interest.44 For example, a clinician could use a visual analog scale to rate mood instability by asking a patient to rate their mood severity by making a mark along a 10-cm line (0 = “Most erratic emotions I have experienced,” 10 = “Most stable I have ever experienced my emotions to be”). This score can be recorded at baseline and subsequent visits.
Take-home points
PDs are common in the general population and health care settings. They are underrecognized by the general public and mental health professionals, often because of stigma. Clinicians could boost their recognition of these disorders by embedding simple screening questions in their patient assessments. Many patients with PDs will be interested in pharmacotherapy for their disorder or symptoms. Treatment strategies include targeting the comorbid disorder(s), targeting important PD symptom dimensions, choosing medication based on the similarity of the PD to another disorder known to respond to medication, and targeting the PD itself. Each strategy has its limitations and varying degrees of empirical support. Treatment response can be monitored using validated scales or a visual analog scale.
Continue to: Bottom Line
Bottom Line
Although psychotherapy is the first-line treatment and no medications are FDAapproved for treating personality disorders (PDs), there has been growing interest in using psychotropic medication to treat PDs. Strategies for pharmacotherapy include targeting comorbid disorders, PD symptom dimensions, or the PD itself. Choice of medication can be based on the similarity of the PD with another disorder known to respond to medication.
Related Resources
- Correa Da Costa S, Sanches M, Soares JC. Bipolar disorder or borderline personality disorder? Current Psychiatry. 2019;18(11):26-29,35-39.
- Bateman A, Gunderson J, Mulder R. Treatment of personality disorders. Lancet. 2015;385(9969):735-743.
Drug Brand Names
Aripiprazole • Abilify
Fluvoxamine • Luvox
Lamotrigine • Lamictal
Quetiapine • Seroquel
Risperidone • Risperdal
Venlafaxine • Effexor
DSM-5 defines personality disorders (PDs) as the presence of an enduring pattern of inner experience and behavior that “deviates markedly from the expectations of the individual’s culture, is pervasive and inflexible, has an onset in adulthood, is stable over time, and leads to distress or impairment.”1 As a general rule, PDs are not limited to episodes of illness, but reflect an individual’s long-term adjustment. These disorders occur in 10% to 15% of the general population; the rates are especially high in health care settings, in criminal offenders, and in those with a substance use disorder (SUD).2 PDs nearly always have an onset in adolescence or early adulthood and tend to diminish in severity with advancing age. They are associated with high rates of unemployment, homelessness, divorce and separation, domestic violence, substance misuse, and suicide.3
Psychotherapy is the first-line treatment for PDs, but there has been growing interest in using pharmacotherapy to treat PDs. While much of the PD treatment literature focuses on borderline PD,4-9 this article describes diagnosis, potential pharmacotherapy strategies, and methods to assess response to treatment for patients with all types of PDs.
Recognizing and diagnosing personality disorders
The diagnosis of a PD requires an understanding of DSM-5 criteria combined with a comprehensive psychiatric history and mental status examination. The patient’s history is the most important basis for diagnosing a PD.2 Collateral information from relatives or friends can help confirm the severity and pervasiveness of the individual’s personality problems. In some patients, long-term observation might be necessary to confirm the presence of a PD. Some clinicians are reluctant to diagnose PDs because of stigma, a problem common among patients with borderline PD.10,11
To screen for PDs, a clinician might ask the patient about problems with interpersonal relationships, sense of self, work, affect, impulse control, and reality testing. Table 112 lists general screening questions for the presence of a PD from the Iowa Personality Disorders Screen. Structured diagnostic interviews and self-report assessments could boost recognition of PDs, but these tools are rarely used outside of research settings.13,14
The PD clusters
DSM-5 divides 10 PDs into 3 clusters based on shared phenomenology and diagnostic criteria. Few patients have a “pure” case in which they meet criteria for only a single personality disorder.1
Cluster A. “Eccentric cluster” disorders are united by social aversion, a failure to form close attachments, or paranoia and suspiciousness.15 These include paranoid, schizoid, and schizotypal PD. Low self-awareness is typical. There are no treatment guidelines for these disorders, although there is some clinical trial data for schizotypal PD.
Cluster B. “Dramatic cluster” disorders share dramatic, emotional, and erratic characteristics.14 These include narcissistic, antisocial, borderline, and histrionic PD. Antisocial and narcissistic patients have low self-awareness. There are treatment guidelines for antisocial and borderline PD, and a variety of clinical trial data is available for the latter.15
Continue to: Cluster C
Cluster C. “Anxious cluster” disorders are united by anxiousness, fearfulness, and poor self-esteem. Many of these patients also display interpersonal rigidity.15 These disorders include avoidant, dependent, and obsessive-compulsive PD. There are no treatment guidelines or clinical trial data for these disorders.
Why consider pharmacotherapy for personality disorders?
The consensus among experts is that psychotherapy is the treatment of choice for PDs.15 Despite significant gaps in the evidence base, there has been a growing interest in using psychotropic medication to treat PDs. For example, research shows that >90% of patients with borderline PD are prescribed medication, most typically antidepressants, antipsychotics, mood stabilizers, stimulants, or sedative-hypnotics.16,17
Increased interest in pharmacotherapy for PDs could be related to research showing the importance of underlying neurobiology, particularly for antisocial and borderline PD.18,19 This work is complemented by genetic research showing the heritability of PD traits and disorders.20,21 Another factor could be renewed interest in dimensional approaches to the classification of PDs, as exemplified by DSM-5’s alternative model for PDs.1 This approach aligns with some expert recommendations to focus on treating PD symptom dimensions, rather than the syndrome itself.22
Importantly, no psychotropic medication is FDA-approved for the treatment of any PD. For that reason, prescribing medication for a PD is “off-label,” although prescribing a medication for a comorbid disorder for which the drug has an FDA-approved indication is not (eg, prescribing an antidepressant for major depressive disorder [MDD]).
Principles for prescribing
Despite gaps in research data, general principles for using medication to treat PDs have emerged from treatment guidelines for antisocial and borderline PD, clinical trial data, reviews and meta-analyses, and expert opinion. Clinicians should address the following considerations before prescribing medication to a patient with a PD.
Continue to: PD diagnosis
PD diagnosis. Has the patient been properly assessed and diagnosed? While history is the most important basis for diagnosis, the clinician should be familiar with the PDs and DSM-5 criteria. Has the patient been informed of the diagnosis and its implications for treatment?
Patient interest in medication. Is the patient interested in taking medication? Patients with borderline PD are often prescribed medication, but there are sparse data for the other PDs. The patient might have little interest in the PD diagnosis or its treatment.
Comorbidity. Has the patient been assessed for comorbid psychiatric disorders that could interfere with medication use (ie, an SUD) or might be a focus of treatment (eg, MDD)? Patients with PDs typically have significant comorbidity that a thorough evaluation will uncover.
PD symptom dimensions. Has the patient been assessed to determine cognitive or behavioral symptom dimensions of their PD? One or more symptom dimension(s) could be the focus of treatment. Table 2 lists examples of PD symptom dimensions.
Strategies to guide prescribing
Strategies to help guide prescribing include targeting any comorbid disorder(s), targeting important PD symptom dimensions (eg, impulsive aggression), choosing medication based on the similarity of the PD to another disorder known to respond to medication, and targeting the PD itself.
Continue to: Targeting comorbid disorders
Targeting comorbid disorders. National Institute for Health and Care Excellence guidelines for antisocial and borderline PD recommend that clinicians focus on treating comorbid disorders, a position echoed in Cochrane and other reviews.4,9,22-26 For example, a patient with borderline PD experiencing a major depressive episode could be treated with an antidepressant. Targeting the depressive symptoms could boost the patient’s mood, perhaps lessening the individual’s PD symptoms or reducing their severity.
Targeting important symptom dimensions. For patients with borderline PD, several guidelines and reviews have suggested that treatment should focus on emotional dysregulation and impulsive aggression (mood stabilizers, antipsychotics), or cognitive-perceptual symptoms (antipsychotics).4-6,15 There is some evidence that mood stabilizers or second-generation antipsychotics could help reduce impulsive aggression in patients with antisocial PD.27
Choosing medication based on similarity to another disorder known to respond to medication. Avoidant PD overlaps with social anxiety disorder and can be conceptualized as a chronic, pervasive social phobia. Avoidant PD might respond to a medication known to be effective for treating social anxiety disorder, such as a selective serotonin reuptake inhibitor (SSRI) or venlafaxine.28 Treating obsessive-compulsive PD with an SSRI is another example of this strategy, as 1 small study of fluvoxamine suggests.29 Obsessive-compulsive PD is common in persons with obsessive-compulsive disorder, and overlap includes preoccupation with orders, rules, and lists, and an inability to throw things out.
Targeting the PD syndrome. Another strategy is to target the PD itself. Clinical trial data suggest the antipsychotic risperidone can reduce the symptoms of schizotypal PD.30 Considering that this PD has a genetic association with schizophrenia, it is not surprising that the patient’s ideas of reference, odd communication, or transient paranoia might respond to an antipsychotic. Data from randomized controlled trials (RCTs) support the use of the second-generation antipsychotics aripiprazole and quetiapine to treat BPD.31,32 While older guidelines4,5 supported the use of the mood stabilizer lamotrigine, a recent RCT found that it was no more effective than placebo for borderline PD or its symptom dimensions.33
What to do before prescribing
Before writing a prescription, the clinician and patient should discuss the presence of a PD and the desirability of treatment. The patient should understand the limited evidence base and know that medication prescribed for a PD is off-label. The clinician should discuss medication selection and its rationale, and whether the medication is targeting a comorbid disorder, symptom dimension(s), or the PD itself. Additional considerations for prescribing for patients with PDs are listed in Table 3.34

Continue to: Avoid polypharmacy
Avoid polypharmacy. Many patients with borderline PD are prescribed multiple psychotropic medications.16,17 This approach leads to greater expense and more adverse effects, and is not evidence-based.
Avoid benzodiazepines. Many patients with borderline PD are prescribed benzodiazepines, often as part of a polypharmacy regimen. These drugs can cause disinhibition, thereby increasing acting-out behaviors and self-harm.35 Also, patients with PDs often have SUDs, which is a contraindication for benzodiazepine use.
Rate the patient’s improvement. Both the patient and clinician can benefit from monitoring symptomatic improvement. Several validated scales can be used to rate depression, anxiety, impulsivity, mood lability, anger, and aggression (Table 436-41).Some validated scales for borderline PD align with DSM-5 criteria. Two such widely used instruments are the Zanarini Rating Scale for Borderline Personality Disorder (ZAN-BPD)42 and the self-rated Borderline Evaluation of Severity Over Time (BEST).43 Each has questions that could be pulled to rate a symptom dimension of interest, such as affective instability, anger dyscontrol, or abandonment fears (Table 542,43).
A visual analog scale is easy to use and can target symptom dimensions of interest.44 For example, a clinician could use a visual analog scale to rate mood instability by asking a patient to rate their mood severity by making a mark along a 10-cm line (0 = “Most erratic emotions I have experienced,” 10 = “Most stable I have ever experienced my emotions to be”). This score can be recorded at baseline and subsequent visits.
Take-home points
PDs are common in the general population and health care settings. They are underrecognized by the general public and mental health professionals, often because of stigma. Clinicians could boost their recognition of these disorders by embedding simple screening questions in their patient assessments. Many patients with PDs will be interested in pharmacotherapy for their disorder or symptoms. Treatment strategies include targeting the comorbid disorder(s), targeting important PD symptom dimensions, choosing medication based on the similarity of the PD to another disorder known to respond to medication, and targeting the PD itself. Each strategy has its limitations and varying degrees of empirical support. Treatment response can be monitored using validated scales or a visual analog scale.
Continue to: Bottom Line
Bottom Line
Although psychotherapy is the first-line treatment and no medications are FDAapproved for treating personality disorders (PDs), there has been growing interest in using psychotropic medication to treat PDs. Strategies for pharmacotherapy include targeting comorbid disorders, PD symptom dimensions, or the PD itself. Choice of medication can be based on the similarity of the PD with another disorder known to respond to medication.
Related Resources
- Correa Da Costa S, Sanches M, Soares JC. Bipolar disorder or borderline personality disorder? Current Psychiatry. 2019;18(11):26-29,35-39.
- Bateman A, Gunderson J, Mulder R. Treatment of personality disorders. Lancet. 2015;385(9969):735-743.
Drug Brand Names
Aripiprazole • Abilify
Fluvoxamine • Luvox
Lamotrigine • Lamictal
Quetiapine • Seroquel
Risperidone • Risperdal
Venlafaxine • Effexor
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Black DW, Andreasen N. Personality disorders. In: Black DW, Andreasen N. Introductory textbook of psychiatry, 7th edition. American Psychiatric Publishing; 2020:410-423.
3. Black DW, Blum N, Pfohl B, et al. Suicidal behavior in borderline personality disorder: prevalence, risk factors, prediction, and prevention. J Pers Disord 2004;18(3):226-239.
4. Lieb K, Völlm B, Rücker G, et al. Pharmacotherapy for borderline personality disorder: Cochrane systematic review of randomised trials. Br J Psychiatry. 2010;196(1):4-12.
5. Vita A, De Peri L, Sacchetti E. Antipsychotics, antidepressants, anticonvulsants, and placebo on the symptom dimensions of borderline personality disorder – a meta-analysis of randomized controlled and open-label trials. J Clin Psychopharmacol. 2011;31(5):613-624.
6. Stoffers JM, Lieb K. Pharmacotherapy for borderline personality disorder – current evidence and recent trends. Curr Psychiatry Rep. 2015;17(1):534.
7. Hancock-Johnson E, Griffiths C, Picchioni M. A focused systematic review of pharmacological treatment for borderline personality disorder. CNS Drugs. 2017;31(5):345-356.
8. Black DW, Paris J, Schulz SC. Personality disorders: evidence-based integrated biopsychosocial treatment of borderline personality disorder. In: Muse M, ed. Cognitive behavioral psychopharmacology: the clinical practice of evidence-based biopsychosocial integration. John Wiley & Sons; 2018:137-165.
9. Stoffers-Winterling J, Sorebø OJ, Lieb K. Pharmacotherapy for borderline personality disorder: an update of published, unpublished and ongoing studies. Curr Psychiatry Rep. 2020;22(8):37.
10. Lewis G, Appleby L. Personality disorder: the patients psychiatrists dislike. Br J Psychiatry. 1988;153:44-49.
11. Black DW, Pfohl B, Blum N, et al. Attitudes toward borderline personality disorder: a survey of 706 mental health clinicians. CNS Spectr. 2011;16(3):67-74.
12. Langbehn DR, Pfohl BM, Reynolds S, et al. The Iowa Personality Disorder Screen: development and preliminary validation of a brief screening interview. J Pers Disord. 1999;13(1):75-89.
13. Pfohl B, Blum N, Zimmerman M. Structured Interview for DSM-IV Personality (SIDP-IV). American Psychiatric Press; 1997.
14. First MB, Spitzer RL, Gibbon M, et al. The Structured Clinical Interview for DSM-III-R Personality Disorders (SCID-II). Part II: multisite test-retest reliability study. J Pers Disord. 1995;9(2):92-104.
15. Bateman A, Gunderson J, Mulder R. Treatment of personality disorders. Lancet. 2015;385(9969):735-743.
16. Zanarini MC, Frankenburg FR, Reich DB, et al. Treatment rates for patients with borderline personality disorder and other personality disorders: a 16-year study. Psychiatr Serv. 2015;66(1):15-20.
17. Black DW, Allen J, McCormick B, et al. Treatment received by persons with BPD participating in a randomized clinical trial of the Systems Training for Emotional Predictability and Problem Solving programme. Person Ment Health. 2011;5(3):159-168.
18. Yang Y, Glenn AL, Raine A. Brain abnormalities in antisocial individuals: implications for the law. Behav Sci Law. 2008;26(1):65-83.
19. Ruocco AC, Amirthavasagam S, Choi-Kain LW, et al. Neural correlates of negative emotionality in BPD: an activation-likelihood-estimation meta-analysis. Biol Psychiatry. 2013;73(2):153-160.
20. Livesley WJ, Jang KL, Jackson DN, et al. Genetic and environmental contributions to dimensions of personality disorder. Am J Psychiatry. 1993;150(12):1826-1831.
21. Slutske WS. The genetics of antisocial behavior. Curr Psychiatry Rep. 2001;3(2):158-162.
22. Ripoll LH, Triebwasser J, Siever LJ. Evidence-based pharmacotherapy for personality disorders. Int J Neuropsychopharmacol. 2011;14(9):1257-1288.
23. National Institute for Health and Care Excellence (NICE). Borderline personality disorder: recognition and management. Clinical guideline [CG78]. Published January 2009. https://www.nice.org.uk/guidance/cg78
24. National Institute for Health and Care Excellence (NICE). Antisocial personality disorder: prevention and management. Clinical guideline [CG77]. Published January 2009. Updated March 27, 2013. https://www.nice.org.uk/guidance/cg77
25. Khalifa N, Duggan C, Stoffers J, et al. Pharmacologic interventions for antisocial personality disorder. Cochrane Database Syst Rep. 2010;(8):CD007667.
26. Stoffers JM, Völlm BA, Rücker G, et al. Psychological therapies for people with borderline personality disorder. Cochrane Database Syst Rev. 2012;2012(8):CD005652.
27. Black DW. The treatment of antisocial personality disorder. Current Treatment Options in Psychiatry. 2017. https://doi.org/10.1007/s40501-017-0123-z
28. Stein MB, Liebowitz MR, Lydiard RB, et al. Paroxetine treatment of generalized social phobia (social anxiety disorder): a randomized controlled trial. JAMA. 1998;280(8):708-713.
29. Ansseau M. The obsessive-compulsive personality: diagnostic aspects and treatment possibilities. In: Den Boer JA, Westenberg HGM, eds. Focus on obsessive-compulsive spectrum disorders. Syn-Thesis; 1997:61-73.
30. Koenigsberg HW, Reynolds D, Goodman M, et al. Risperidone in the treatment of schizotypal personality disorder. J Clin Psychiatry. 2003;64(6):628-634.
31. Black DW, Zanarini MC, Romine A, et al. Comparison of low and moderate dosages of extended-release quetiapine in borderline personality disorder: a randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 2014;171(11):1174-1182.
32. Nickel MK, Muelbacher M, Nickel C, et al. Aripiprazole in the treatment of patients with borderline personality disorder: a double-blind, placebo-controlled study. Am J Psychiatry. 2006;163(5):833-838.
33. Crawford MJ, Sanatinia R, Barrett B, et al; LABILE study team. The clinical effectiveness and cost-effectiveness of lamotrigine in borderline personality disorder: a randomized placebo-controlled trial. Am J Psychiatry. 2018;175(8):756-764.
34. Frankenburg FR, Zanarini MC. The association between borderline personality disorder and chronic medical illnesses, poor health-related lifestyle choices, and costly forms of health care utilization. J Clin Psychiatry. 2004;65(12)1660-1665.
35. Cowdry RW, Gardner DL. Pharmacotherapy of borderline personality disorder. Alprazolam, carbamazepine, trifluoperazine, and tranylcypromine. Arch Gen Psychiatry. 1988;45(2):111-119.
36. Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:799-812.
37. Ratey JJ, Gutheil CM. The measurement of aggressive behavior: reflections on the use of the Overt Aggression Scale and the Modified Overt Aggression Scale. J Neuropsychiatr Clin Neurosci. 1991;3(2):S57-S60.
38. Spielberger CD, Sydeman SJ, Owen AE, et al. Measuring anxiety and anger with the State-Trait Anxiety Inventory (STAI) and the State-Trait Anger Expression Inventory (STAXI). In: Maruish ME, ed. The use of psychological testing for treatment planning and outcomes assessment. Lawrence Erlbaum Associates Publishers; 1999:993-1021.
39. Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory II. Psychological Corp; 1996.
40. Watson D, Clark LA. The PANAS-X: Manual for the Positive and Negative Affect Schedule – Expanded Form. The University of Iowa; 1999.
41. Harvey D, Greenberg BR, Serper MR, et al. The affective lability scales: development, reliability, and validity. J Clin Psychol. 1989;45(5):786-793.
42. Zanarini MC, Vujanovic AA, Parachini EA, et al. Zanarini Rating Scale for Borderline Personality Disorder (ZAN-BPD): a continuous measure of DSM-IV borderline psychopathology. J Person Disord. 2003:17(3):233-242.
43. Pfohl B, Blum N, St John D, et al. Reliability and validity of the Borderline Evaluation of Severity Over Time (BEST): a new scale to measure severity and change in borderline personality disorder. J Person Disord. 2009;23(3):281-293.
44. Ahearn EP. The use of visual analog scales in mood disorders: a critical review. J Psychiatr Res. 1997;31(5):569-579.
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Black DW, Andreasen N. Personality disorders. In: Black DW, Andreasen N. Introductory textbook of psychiatry, 7th edition. American Psychiatric Publishing; 2020:410-423.
3. Black DW, Blum N, Pfohl B, et al. Suicidal behavior in borderline personality disorder: prevalence, risk factors, prediction, and prevention. J Pers Disord 2004;18(3):226-239.
4. Lieb K, Völlm B, Rücker G, et al. Pharmacotherapy for borderline personality disorder: Cochrane systematic review of randomised trials. Br J Psychiatry. 2010;196(1):4-12.
5. Vita A, De Peri L, Sacchetti E. Antipsychotics, antidepressants, anticonvulsants, and placebo on the symptom dimensions of borderline personality disorder – a meta-analysis of randomized controlled and open-label trials. J Clin Psychopharmacol. 2011;31(5):613-624.
6. Stoffers JM, Lieb K. Pharmacotherapy for borderline personality disorder – current evidence and recent trends. Curr Psychiatry Rep. 2015;17(1):534.
7. Hancock-Johnson E, Griffiths C, Picchioni M. A focused systematic review of pharmacological treatment for borderline personality disorder. CNS Drugs. 2017;31(5):345-356.
8. Black DW, Paris J, Schulz SC. Personality disorders: evidence-based integrated biopsychosocial treatment of borderline personality disorder. In: Muse M, ed. Cognitive behavioral psychopharmacology: the clinical practice of evidence-based biopsychosocial integration. John Wiley & Sons; 2018:137-165.
9. Stoffers-Winterling J, Sorebø OJ, Lieb K. Pharmacotherapy for borderline personality disorder: an update of published, unpublished and ongoing studies. Curr Psychiatry Rep. 2020;22(8):37.
10. Lewis G, Appleby L. Personality disorder: the patients psychiatrists dislike. Br J Psychiatry. 1988;153:44-49.
11. Black DW, Pfohl B, Blum N, et al. Attitudes toward borderline personality disorder: a survey of 706 mental health clinicians. CNS Spectr. 2011;16(3):67-74.
12. Langbehn DR, Pfohl BM, Reynolds S, et al. The Iowa Personality Disorder Screen: development and preliminary validation of a brief screening interview. J Pers Disord. 1999;13(1):75-89.
13. Pfohl B, Blum N, Zimmerman M. Structured Interview for DSM-IV Personality (SIDP-IV). American Psychiatric Press; 1997.
14. First MB, Spitzer RL, Gibbon M, et al. The Structured Clinical Interview for DSM-III-R Personality Disorders (SCID-II). Part II: multisite test-retest reliability study. J Pers Disord. 1995;9(2):92-104.
15. Bateman A, Gunderson J, Mulder R. Treatment of personality disorders. Lancet. 2015;385(9969):735-743.
16. Zanarini MC, Frankenburg FR, Reich DB, et al. Treatment rates for patients with borderline personality disorder and other personality disorders: a 16-year study. Psychiatr Serv. 2015;66(1):15-20.
17. Black DW, Allen J, McCormick B, et al. Treatment received by persons with BPD participating in a randomized clinical trial of the Systems Training for Emotional Predictability and Problem Solving programme. Person Ment Health. 2011;5(3):159-168.
18. Yang Y, Glenn AL, Raine A. Brain abnormalities in antisocial individuals: implications for the law. Behav Sci Law. 2008;26(1):65-83.
19. Ruocco AC, Amirthavasagam S, Choi-Kain LW, et al. Neural correlates of negative emotionality in BPD: an activation-likelihood-estimation meta-analysis. Biol Psychiatry. 2013;73(2):153-160.
20. Livesley WJ, Jang KL, Jackson DN, et al. Genetic and environmental contributions to dimensions of personality disorder. Am J Psychiatry. 1993;150(12):1826-1831.
21. Slutske WS. The genetics of antisocial behavior. Curr Psychiatry Rep. 2001;3(2):158-162.
22. Ripoll LH, Triebwasser J, Siever LJ. Evidence-based pharmacotherapy for personality disorders. Int J Neuropsychopharmacol. 2011;14(9):1257-1288.
23. National Institute for Health and Care Excellence (NICE). Borderline personality disorder: recognition and management. Clinical guideline [CG78]. Published January 2009. https://www.nice.org.uk/guidance/cg78
24. National Institute for Health and Care Excellence (NICE). Antisocial personality disorder: prevention and management. Clinical guideline [CG77]. Published January 2009. Updated March 27, 2013. https://www.nice.org.uk/guidance/cg77
25. Khalifa N, Duggan C, Stoffers J, et al. Pharmacologic interventions for antisocial personality disorder. Cochrane Database Syst Rep. 2010;(8):CD007667.
26. Stoffers JM, Völlm BA, Rücker G, et al. Psychological therapies for people with borderline personality disorder. Cochrane Database Syst Rev. 2012;2012(8):CD005652.
27. Black DW. The treatment of antisocial personality disorder. Current Treatment Options in Psychiatry. 2017. https://doi.org/10.1007/s40501-017-0123-z
28. Stein MB, Liebowitz MR, Lydiard RB, et al. Paroxetine treatment of generalized social phobia (social anxiety disorder): a randomized controlled trial. JAMA. 1998;280(8):708-713.
29. Ansseau M. The obsessive-compulsive personality: diagnostic aspects and treatment possibilities. In: Den Boer JA, Westenberg HGM, eds. Focus on obsessive-compulsive spectrum disorders. Syn-Thesis; 1997:61-73.
30. Koenigsberg HW, Reynolds D, Goodman M, et al. Risperidone in the treatment of schizotypal personality disorder. J Clin Psychiatry. 2003;64(6):628-634.
31. Black DW, Zanarini MC, Romine A, et al. Comparison of low and moderate dosages of extended-release quetiapine in borderline personality disorder: a randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 2014;171(11):1174-1182.
32. Nickel MK, Muelbacher M, Nickel C, et al. Aripiprazole in the treatment of patients with borderline personality disorder: a double-blind, placebo-controlled study. Am J Psychiatry. 2006;163(5):833-838.
33. Crawford MJ, Sanatinia R, Barrett B, et al; LABILE study team. The clinical effectiveness and cost-effectiveness of lamotrigine in borderline personality disorder: a randomized placebo-controlled trial. Am J Psychiatry. 2018;175(8):756-764.
34. Frankenburg FR, Zanarini MC. The association between borderline personality disorder and chronic medical illnesses, poor health-related lifestyle choices, and costly forms of health care utilization. J Clin Psychiatry. 2004;65(12)1660-1665.
35. Cowdry RW, Gardner DL. Pharmacotherapy of borderline personality disorder. Alprazolam, carbamazepine, trifluoperazine, and tranylcypromine. Arch Gen Psychiatry. 1988;45(2):111-119.
36. Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:799-812.
37. Ratey JJ, Gutheil CM. The measurement of aggressive behavior: reflections on the use of the Overt Aggression Scale and the Modified Overt Aggression Scale. J Neuropsychiatr Clin Neurosci. 1991;3(2):S57-S60.
38. Spielberger CD, Sydeman SJ, Owen AE, et al. Measuring anxiety and anger with the State-Trait Anxiety Inventory (STAI) and the State-Trait Anger Expression Inventory (STAXI). In: Maruish ME, ed. The use of psychological testing for treatment planning and outcomes assessment. Lawrence Erlbaum Associates Publishers; 1999:993-1021.
39. Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory II. Psychological Corp; 1996.
40. Watson D, Clark LA. The PANAS-X: Manual for the Positive and Negative Affect Schedule – Expanded Form. The University of Iowa; 1999.
41. Harvey D, Greenberg BR, Serper MR, et al. The affective lability scales: development, reliability, and validity. J Clin Psychol. 1989;45(5):786-793.
42. Zanarini MC, Vujanovic AA, Parachini EA, et al. Zanarini Rating Scale for Borderline Personality Disorder (ZAN-BPD): a continuous measure of DSM-IV borderline psychopathology. J Person Disord. 2003:17(3):233-242.
43. Pfohl B, Blum N, St John D, et al. Reliability and validity of the Borderline Evaluation of Severity Over Time (BEST): a new scale to measure severity and change in borderline personality disorder. J Person Disord. 2009;23(3):281-293.
44. Ahearn EP. The use of visual analog scales in mood disorders: a critical review. J Psychiatr Res. 1997;31(5):569-579.
When should a patient’s violent thoughts trigger your action?
When patients relay their fantasies during psychotherapy sessions, those visions are often rooted in frustration or wish fulfillment, according to Jessica Ferranti, MD.
“[Sigmund] Freud talked about how our fantasy life is invested with large amounts of energy and interest and conveys a true essence of our personality – a truth about what we’re thinking and who we are,” Dr. Ferranti, a forensic psychiatrist in the division of psychiatry and the law at the University of California, Davis, said during an annual psychopharmacology update held by the Nevada Psychiatric Association.
“Fantasy life is one of the most important conveyances of information that we can get from our patients, whether in the general office or in the forensic realm – if we can access it, which is difficult, because fantasies are often intensely personal. They fall into the category of very high resistance topics with many patients.”
Psychiatrists routinely ask about violent thoughts and homicidal ideation, but violent fantasies – especially those that are sexually violent in nature – can be a warning sign of future danger. Dr. Ferranti defined violent fantasies as those depicting the use of physical force with the intent to injure another person or destroy property.
“This would be an individual who fantasizes about sadistically raping a woman, for instance,” said Dr. Ferranti, who directs the UC Davis Workplace Safety and Psychiatric Assessment Clinic. “That is an ominous and psychopathological sign in terms of the preoccupation with that kind of violent crime.”
Aggression, on the other hand, “is a very broad spectrum, with actions like assertion, interpersonal confrontation, or verbal expressions that are angry or hostile, but that do not necessarily lead to violence.”
Dr. Ferranti acknowledged that today’s rushed clinical environment makes it challenging for psychiatrists and psychologists to get patients to share detailed fantasies they may be harboring.
“It’s very difficult to get to deeper material with patients, unless potentially you have more intensive therapy going on, like a psychotherapeutic relationship where you see the patient frequently, an intensive treatment, [or] perhaps an inpatient hospitalization or a partial day program.” The key is that “the patient gets comfortable with relaying more of the truth about what they’re experiencing,” she said. “In some cases, this occurs during the forensic evaluation, because we have the luxury to do very lengthy evaluations. Under the stress of being with another person in the room for many hours, oftentimes the patient will disclose things eventually.
“I’ve been a forensic psychiatrist for the better part of 12 years, and I can tell you after hundreds of evaluations I’ve never had a person not speak. That’s a good thing, because a principle of the work we do, or talk therapy even, is that When we lose symbolism, the ability to represent things in our mind and speak about them, we are at greater risk of collapsing into the real and acting on the things we think about.”
Statutory reporting duties vary from state to state. In California, mandatory reporting duties include child abuse, elder abuse, abuse or neglect of developmentally disabled individuals, domestic violence, and victims of a gunshot wound. “Failing to report any of these crimes is a misdemeanor in California,” she said. “With all these statutory reporting duties, we have no legal obligation to inform the patient of the report. Under California law, patients do not have the right to refuse the report. These are reports we make in our best judgment, whether the patient is happy about that or not.”
What happens if your patient confesses to a past crime? “There’s no legal duty to report this,” Dr. Ferranti said. “The general rule is, unless there’s a current person who’s at risk, it would be violating confidentiality to report. This includes murder, bank robbery, and sexual assault. In addition, you cannot admit a patient to an inpatient setting to help them avoid arrest, even if you think the act in question was due to symptoms of a mental disorder, disease, or defect. You can actually be charged with aiding and abetting a criminal.”
In the 1976 landmark case Tarasoff v. the Regents of the University of California, the California Supreme Court ruled that psychiatrists and other therapists have a duty to do what is reasonably necessary to protect third parties if a patient presents a serious risk of violence to another person.
“Reasonable steps may include warning the third party, notifying police, detaining and hospitalizing the patient, intensifying the treatment to a higher level of care or more frequent outpatient appointments, removing weapons, and changing the medication therapy,” Dr. Ferranti said. “The more you can do of these, the better.”
She also discussed the concept of foreseeability, which she defined as the reasonable anticipation that harm or injury is likely to result from an act or omission to act.
“This is the malpractice standard for negligence,” she said. “In other words, was it foreseeable by a reasonable psychiatrist that this person was going to hurt someone else or themselves?” Another landmark case, Jablonski Pahls v. the United States broadened the reporting obligations of psychiatrists. In this 1983 case, the U.S. Court of Appeals 9th Circuit ruled that mental health professionals have to do more than warn foreseeable victims of an imminent danger of potential harm; they must involuntarily hospitalize the dangerous individual and consult that person’s prior records.
There is no sure-fire way to predict when an individual’s underlying violent fantasies are likely to be acted on, but Dr. Ferranti mentioned several behaviors that should raise alarm. One is a heightened physiological arousal when the person discusses the fantasy, such as rapid heartbeat, sweating; or physical posturing, such as clenching their fists or pounding their hands on an object as they tell you about it. You also want to determine the persistence of the fantasy.
“Can the patient think about it?” she asked. “Can they retain the ability to symbolize and separate themselves from necessarily doing whatever it is they think about?” You also want to determine the individual’s propensity for externalizing behaviors. “Here we’re talking about cluster B personality group patients – antisocial, narcissistic, and borderline patients who by virtue of their aggressivity titer and difficulties with anger, have a higher propensity for acting out and acting violently.”
Then there’s the concept of foreseeability. “Ask yourself, how likely is it that this could actually happen, based on the known risk factors and what you know about the patient?” Dr. Ferranti said. “Past history of violence is also very important. What people have done once before, they’re likely to do again.”
A good violence risk assessment can help you mitigate the potential for one of your patients to carry out harm to self or to others. Key risk factors include psychopathy, past violence, substance abuse, specific person/entity threatened, a history of impulsivity, unemployment, military history, gun possession, and the presence of paranoid and/or persecutory ideation or delusions.
“Know your specific state statutes and case law,” Dr. Ferranti concluded. “Delaying Tarasoff notification may indicate no need to violate confidentiality. If you think it’s warranted, do it without delay. Documentation is important when you’re consulting with therapists back and forth. You also want to attempt to obtain prior records and release only information that is required in a case of violence toward others. The details of the therapy or diagnosis are likely not relevant.”
Dr. Ferranti reported having no disclosures.
When patients relay their fantasies during psychotherapy sessions, those visions are often rooted in frustration or wish fulfillment, according to Jessica Ferranti, MD.
“[Sigmund] Freud talked about how our fantasy life is invested with large amounts of energy and interest and conveys a true essence of our personality – a truth about what we’re thinking and who we are,” Dr. Ferranti, a forensic psychiatrist in the division of psychiatry and the law at the University of California, Davis, said during an annual psychopharmacology update held by the Nevada Psychiatric Association.
“Fantasy life is one of the most important conveyances of information that we can get from our patients, whether in the general office or in the forensic realm – if we can access it, which is difficult, because fantasies are often intensely personal. They fall into the category of very high resistance topics with many patients.”
Psychiatrists routinely ask about violent thoughts and homicidal ideation, but violent fantasies – especially those that are sexually violent in nature – can be a warning sign of future danger. Dr. Ferranti defined violent fantasies as those depicting the use of physical force with the intent to injure another person or destroy property.
“This would be an individual who fantasizes about sadistically raping a woman, for instance,” said Dr. Ferranti, who directs the UC Davis Workplace Safety and Psychiatric Assessment Clinic. “That is an ominous and psychopathological sign in terms of the preoccupation with that kind of violent crime.”
Aggression, on the other hand, “is a very broad spectrum, with actions like assertion, interpersonal confrontation, or verbal expressions that are angry or hostile, but that do not necessarily lead to violence.”
Dr. Ferranti acknowledged that today’s rushed clinical environment makes it challenging for psychiatrists and psychologists to get patients to share detailed fantasies they may be harboring.
“It’s very difficult to get to deeper material with patients, unless potentially you have more intensive therapy going on, like a psychotherapeutic relationship where you see the patient frequently, an intensive treatment, [or] perhaps an inpatient hospitalization or a partial day program.” The key is that “the patient gets comfortable with relaying more of the truth about what they’re experiencing,” she said. “In some cases, this occurs during the forensic evaluation, because we have the luxury to do very lengthy evaluations. Under the stress of being with another person in the room for many hours, oftentimes the patient will disclose things eventually.
“I’ve been a forensic psychiatrist for the better part of 12 years, and I can tell you after hundreds of evaluations I’ve never had a person not speak. That’s a good thing, because a principle of the work we do, or talk therapy even, is that When we lose symbolism, the ability to represent things in our mind and speak about them, we are at greater risk of collapsing into the real and acting on the things we think about.”
Statutory reporting duties vary from state to state. In California, mandatory reporting duties include child abuse, elder abuse, abuse or neglect of developmentally disabled individuals, domestic violence, and victims of a gunshot wound. “Failing to report any of these crimes is a misdemeanor in California,” she said. “With all these statutory reporting duties, we have no legal obligation to inform the patient of the report. Under California law, patients do not have the right to refuse the report. These are reports we make in our best judgment, whether the patient is happy about that or not.”
What happens if your patient confesses to a past crime? “There’s no legal duty to report this,” Dr. Ferranti said. “The general rule is, unless there’s a current person who’s at risk, it would be violating confidentiality to report. This includes murder, bank robbery, and sexual assault. In addition, you cannot admit a patient to an inpatient setting to help them avoid arrest, even if you think the act in question was due to symptoms of a mental disorder, disease, or defect. You can actually be charged with aiding and abetting a criminal.”
In the 1976 landmark case Tarasoff v. the Regents of the University of California, the California Supreme Court ruled that psychiatrists and other therapists have a duty to do what is reasonably necessary to protect third parties if a patient presents a serious risk of violence to another person.
“Reasonable steps may include warning the third party, notifying police, detaining and hospitalizing the patient, intensifying the treatment to a higher level of care or more frequent outpatient appointments, removing weapons, and changing the medication therapy,” Dr. Ferranti said. “The more you can do of these, the better.”
She also discussed the concept of foreseeability, which she defined as the reasonable anticipation that harm or injury is likely to result from an act or omission to act.
“This is the malpractice standard for negligence,” she said. “In other words, was it foreseeable by a reasonable psychiatrist that this person was going to hurt someone else or themselves?” Another landmark case, Jablonski Pahls v. the United States broadened the reporting obligations of psychiatrists. In this 1983 case, the U.S. Court of Appeals 9th Circuit ruled that mental health professionals have to do more than warn foreseeable victims of an imminent danger of potential harm; they must involuntarily hospitalize the dangerous individual and consult that person’s prior records.
There is no sure-fire way to predict when an individual’s underlying violent fantasies are likely to be acted on, but Dr. Ferranti mentioned several behaviors that should raise alarm. One is a heightened physiological arousal when the person discusses the fantasy, such as rapid heartbeat, sweating; or physical posturing, such as clenching their fists or pounding their hands on an object as they tell you about it. You also want to determine the persistence of the fantasy.
“Can the patient think about it?” she asked. “Can they retain the ability to symbolize and separate themselves from necessarily doing whatever it is they think about?” You also want to determine the individual’s propensity for externalizing behaviors. “Here we’re talking about cluster B personality group patients – antisocial, narcissistic, and borderline patients who by virtue of their aggressivity titer and difficulties with anger, have a higher propensity for acting out and acting violently.”
Then there’s the concept of foreseeability. “Ask yourself, how likely is it that this could actually happen, based on the known risk factors and what you know about the patient?” Dr. Ferranti said. “Past history of violence is also very important. What people have done once before, they’re likely to do again.”
A good violence risk assessment can help you mitigate the potential for one of your patients to carry out harm to self or to others. Key risk factors include psychopathy, past violence, substance abuse, specific person/entity threatened, a history of impulsivity, unemployment, military history, gun possession, and the presence of paranoid and/or persecutory ideation or delusions.
“Know your specific state statutes and case law,” Dr. Ferranti concluded. “Delaying Tarasoff notification may indicate no need to violate confidentiality. If you think it’s warranted, do it without delay. Documentation is important when you’re consulting with therapists back and forth. You also want to attempt to obtain prior records and release only information that is required in a case of violence toward others. The details of the therapy or diagnosis are likely not relevant.”
Dr. Ferranti reported having no disclosures.
When patients relay their fantasies during psychotherapy sessions, those visions are often rooted in frustration or wish fulfillment, according to Jessica Ferranti, MD.
“[Sigmund] Freud talked about how our fantasy life is invested with large amounts of energy and interest and conveys a true essence of our personality – a truth about what we’re thinking and who we are,” Dr. Ferranti, a forensic psychiatrist in the division of psychiatry and the law at the University of California, Davis, said during an annual psychopharmacology update held by the Nevada Psychiatric Association.
“Fantasy life is one of the most important conveyances of information that we can get from our patients, whether in the general office or in the forensic realm – if we can access it, which is difficult, because fantasies are often intensely personal. They fall into the category of very high resistance topics with many patients.”
Psychiatrists routinely ask about violent thoughts and homicidal ideation, but violent fantasies – especially those that are sexually violent in nature – can be a warning sign of future danger. Dr. Ferranti defined violent fantasies as those depicting the use of physical force with the intent to injure another person or destroy property.
“This would be an individual who fantasizes about sadistically raping a woman, for instance,” said Dr. Ferranti, who directs the UC Davis Workplace Safety and Psychiatric Assessment Clinic. “That is an ominous and psychopathological sign in terms of the preoccupation with that kind of violent crime.”
Aggression, on the other hand, “is a very broad spectrum, with actions like assertion, interpersonal confrontation, or verbal expressions that are angry or hostile, but that do not necessarily lead to violence.”
Dr. Ferranti acknowledged that today’s rushed clinical environment makes it challenging for psychiatrists and psychologists to get patients to share detailed fantasies they may be harboring.
“It’s very difficult to get to deeper material with patients, unless potentially you have more intensive therapy going on, like a psychotherapeutic relationship where you see the patient frequently, an intensive treatment, [or] perhaps an inpatient hospitalization or a partial day program.” The key is that “the patient gets comfortable with relaying more of the truth about what they’re experiencing,” she said. “In some cases, this occurs during the forensic evaluation, because we have the luxury to do very lengthy evaluations. Under the stress of being with another person in the room for many hours, oftentimes the patient will disclose things eventually.
“I’ve been a forensic psychiatrist for the better part of 12 years, and I can tell you after hundreds of evaluations I’ve never had a person not speak. That’s a good thing, because a principle of the work we do, or talk therapy even, is that When we lose symbolism, the ability to represent things in our mind and speak about them, we are at greater risk of collapsing into the real and acting on the things we think about.”
Statutory reporting duties vary from state to state. In California, mandatory reporting duties include child abuse, elder abuse, abuse or neglect of developmentally disabled individuals, domestic violence, and victims of a gunshot wound. “Failing to report any of these crimes is a misdemeanor in California,” she said. “With all these statutory reporting duties, we have no legal obligation to inform the patient of the report. Under California law, patients do not have the right to refuse the report. These are reports we make in our best judgment, whether the patient is happy about that or not.”
What happens if your patient confesses to a past crime? “There’s no legal duty to report this,” Dr. Ferranti said. “The general rule is, unless there’s a current person who’s at risk, it would be violating confidentiality to report. This includes murder, bank robbery, and sexual assault. In addition, you cannot admit a patient to an inpatient setting to help them avoid arrest, even if you think the act in question was due to symptoms of a mental disorder, disease, or defect. You can actually be charged with aiding and abetting a criminal.”
In the 1976 landmark case Tarasoff v. the Regents of the University of California, the California Supreme Court ruled that psychiatrists and other therapists have a duty to do what is reasonably necessary to protect third parties if a patient presents a serious risk of violence to another person.
“Reasonable steps may include warning the third party, notifying police, detaining and hospitalizing the patient, intensifying the treatment to a higher level of care or more frequent outpatient appointments, removing weapons, and changing the medication therapy,” Dr. Ferranti said. “The more you can do of these, the better.”
She also discussed the concept of foreseeability, which she defined as the reasonable anticipation that harm or injury is likely to result from an act or omission to act.
“This is the malpractice standard for negligence,” she said. “In other words, was it foreseeable by a reasonable psychiatrist that this person was going to hurt someone else or themselves?” Another landmark case, Jablonski Pahls v. the United States broadened the reporting obligations of psychiatrists. In this 1983 case, the U.S. Court of Appeals 9th Circuit ruled that mental health professionals have to do more than warn foreseeable victims of an imminent danger of potential harm; they must involuntarily hospitalize the dangerous individual and consult that person’s prior records.
There is no sure-fire way to predict when an individual’s underlying violent fantasies are likely to be acted on, but Dr. Ferranti mentioned several behaviors that should raise alarm. One is a heightened physiological arousal when the person discusses the fantasy, such as rapid heartbeat, sweating; or physical posturing, such as clenching their fists or pounding their hands on an object as they tell you about it. You also want to determine the persistence of the fantasy.
“Can the patient think about it?” she asked. “Can they retain the ability to symbolize and separate themselves from necessarily doing whatever it is they think about?” You also want to determine the individual’s propensity for externalizing behaviors. “Here we’re talking about cluster B personality group patients – antisocial, narcissistic, and borderline patients who by virtue of their aggressivity titer and difficulties with anger, have a higher propensity for acting out and acting violently.”
Then there’s the concept of foreseeability. “Ask yourself, how likely is it that this could actually happen, based on the known risk factors and what you know about the patient?” Dr. Ferranti said. “Past history of violence is also very important. What people have done once before, they’re likely to do again.”
A good violence risk assessment can help you mitigate the potential for one of your patients to carry out harm to self or to others. Key risk factors include psychopathy, past violence, substance abuse, specific person/entity threatened, a history of impulsivity, unemployment, military history, gun possession, and the presence of paranoid and/or persecutory ideation or delusions.
“Know your specific state statutes and case law,” Dr. Ferranti concluded. “Delaying Tarasoff notification may indicate no need to violate confidentiality. If you think it’s warranted, do it without delay. Documentation is important when you’re consulting with therapists back and forth. You also want to attempt to obtain prior records and release only information that is required in a case of violence toward others. The details of the therapy or diagnosis are likely not relevant.”
Dr. Ferranti reported having no disclosures.
FROM NPA 2021
Aggression is influenced by genetic, environmental factors
Aggression in individuals is influenced by genetic and environmental factors, but can be reduced with treatment, according to Emil F. Coccaro, MD.
“It actually is a complex triad of emotion, cognition, and behavior. The emotion is anger, the cognition is hostility, and the behavior is aggression. And they sort of go in that order,” Dr. Coccaro said at Focus on Neuropsychiatry presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
Although aggression can be thought of in a numerous ways, premeditated and impulsive aggression are most relevant to behavioral studies in psychiatry, Dr. Coccaro explained. Premeditated aggression is goal oriented, while impulsive aggression comes from frustration or a response to a threat. Impulsive aggression is “typically social or frustrative in nature, and studies that we’ve done that show that individuals move toward a threat while nonaggressives move away it,” he said. Both types of aggression can be seen in the same individuals at different times.
Aggression also can be considered using a threshold model. Calm individuals, for example, might have a low baseline of aggression and a high threshold before they act out. An aggressive person, on the other hand, has a lower threshold and a higher baseline level. “Their delta to get to the point where they’re going to explode is much shorter, much lower than it is in someone who is healthy,” Dr. Coccaro said.
“What we think is that the threshold to explode is probably regulated by various neurobiological features. The baseline state of aggression also may be related to baseline neurobiological features, but also what’s going on in the environment, because the neurobiological features that send someone to exploding aggression are there all the time,” he explained.
Individuals with secondary aggression are likely to have an underlying condition, such as a primary disease of the brain, systemic or metabolic disorder, or a psychiatric disorder such as schizophrenia. “If someone’s schizophrenic and they’ve got voices telling them to hurt somebody, or delusions that someone’s going to hurt them, that’s not primary aggression, that’s secondary to the psychosis,” Dr. Coccaro noted.
An individual with primary aggression is likely to have intermittent explosive disorder (IED). IED is not a new diagnosis and has been listed in the DSM since the DSM-I as “passive-aggressive personality.” It was relisted in the DSM-II as “explosive personality,” then changed to IED in the DSM-3 as a diagnosis of exclusion that was poorly operationalized, according to Dr. Coccaro. The criteria for IED under the DSM-III did not define the number of recurrent outbursts needed, what they looked like, the time frame, and excluded people who were generally impulsive.
“That’s not really what these people look like and it’s not what impulsive aggression looks like,” he said. Although the DSM-IV removed the exclusion criteria for general impulsivity and aggression, “it was still purely operational.”
The DSM-5 criteria define IED as “verbal and physical aggression without destruction or assault, twice equally on average for 3 months, or three or more episodes of physical destruction/assault over a 1-year period. These individuals have outbursts “grossly out of proportion to provocation,” the aggression is generally impulsive, and it causes stress and impairment with an age of onset at older than 6 years.
“It’s not better accounted for a whole variety of things, but we actually made some of those exclusion criteria a little less stringent,” compared with criteria in the DSM-IV, Dr. Coccaro said. “That’s because it turns out that it doesn’t really matter much of the time what the comorbidity is. If you have this aggressiveness in the absence of those other conditions, it’s IED.”
According to a reanalysis of the National Comorbidity Survey, 11.7% of adolescents displayed aggressiveness within the last year and 17.3% over a lifetime, compared with 5.1% of adults within the last year and 8.0% within a lifetime. Under DSM-5 criteria, 6.4% of adolescents within the last year and 8.9% over a lifetime currently have IED, compared with 2.6% of adults within the last year and 4.0% over a lifetime, but “could go as high” as the percentage of individuals diagnosed with aggressiveness, Dr. Coccaro noted.
“People who are not called IED many times are not called IED because we didn’t have all the information we needed to actually make the diagnosis,” he said.
Individuals with DSM-5 IED can have as many as 30 episodes in 1 year, compared with those who are nonaggressive and are also more likely to damage property. “These are the big episodes, not simply the episodes where people are getting irritable and snapping at people. These are the big ones, where they’re really destroying objects and pushing or hitting people,” Dr. Coccaro said. About one-fourth of individuals with IED hurt victims badly enough that they require medical attention, one-fifth exhibit aggression toward a partner, and one-fourth receive aggression from their own partner.
In terms of comorbidity with other psychiatric disorders, “IEDs don’t have more comorbidity in general than other disorders,” Dr. Coccaro noted. Personality disorders such as paranoid, antisocial, borderline narcissistic, and obsessive-compulsive disorders are more common in individuals with IED. Aggression in these people present differently depending on the personality disorder. “Someone who’s paranoid might blow up at you if you get in their face. For an antisocial, they’ll blow up at you if you’re preventing them from doing what they want to do. Borderlines, you reject them or you abandon them, they’re going to blow up. Narcissists will blow up when you reject. OCD will also blow up when you mess around with their sense of order,” Dr. Coccaro said.
Genetics also play a role in whether a person may have IED. These percentages were consistent, regardless of whether the individual had a comorbid condition, history of alcohol or drug use, or history of suicide, he said. Other factors that influence likelihood of IED are environment, behaviors such as smoking, and conditions such as traumatic brain injury. Experiencing aggression as a child is another factor.
“IED is the categorical expression of impulsive aggression, and it’s far more common than once thought,” Dr. Coccaro said. “And IED is totally unrecognized in its role in societal violence.”
Treatment can suppress, but not cure aggression
Medications used to treat aggression and impulsive aggression include lithium, SSRIs, mood stabilizers, neuroleptics, and beta-blockers. However, the treatments are not a “magic bullet,” Dr. Coccaro noted. “The meds tend to suppress aggressiveness, but not cure it.”
Timing of treatment is also a factor for medication. In studies of patients taking lithium for aggression, for example, “when they gave the drug to people who liked being aggressive, they didn’t like being on these drugs because it made them feel unprotected. It just was at odds with who they thought they were,” Dr. Coccaro said. “The people who took the drug and did well and really liked being on the drug with people who didn’t like that they were aggressive.”
Neurorehabilitation and cognitive-behavioral therapy specific to aggression, called cognitive relaxation and coping skills therapy, are nonpsychotropic approaches to treating aggression. “These therapeutic approaches are working not only to reduce progression, but also to reduce the social information processing problems that aggressive individuals have,” Dr. Coccaro said.
Another approach, known as interpretation bias training, teaches individuals with aggression to judge slightly angry-looking photos of people as not being angry. After 7-14 days of training, aggressive behavior in adolescents has been shown to be reduced. The changes were also visible on functional MRI.
“What they found was that when you treated them, the change in the amygdala went down when you looked at the angry faces and in the left lateral, post training, they became happier,” Dr. Coccaro said.
Global Academy and this news organization are owned by the same parent company. Dr. Coccaro reported serving as a consultant for Avanir, Azevan, and Bracket. He also reported receiving research grants from the National Institute of Mental Health, the National Institute on Alcohol Abuse and Alcoholism, and the Pritzker Pucker Family Foundation.
Aggression in individuals is influenced by genetic and environmental factors, but can be reduced with treatment, according to Emil F. Coccaro, MD.
“It actually is a complex triad of emotion, cognition, and behavior. The emotion is anger, the cognition is hostility, and the behavior is aggression. And they sort of go in that order,” Dr. Coccaro said at Focus on Neuropsychiatry presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
Although aggression can be thought of in a numerous ways, premeditated and impulsive aggression are most relevant to behavioral studies in psychiatry, Dr. Coccaro explained. Premeditated aggression is goal oriented, while impulsive aggression comes from frustration or a response to a threat. Impulsive aggression is “typically social or frustrative in nature, and studies that we’ve done that show that individuals move toward a threat while nonaggressives move away it,” he said. Both types of aggression can be seen in the same individuals at different times.
Aggression also can be considered using a threshold model. Calm individuals, for example, might have a low baseline of aggression and a high threshold before they act out. An aggressive person, on the other hand, has a lower threshold and a higher baseline level. “Their delta to get to the point where they’re going to explode is much shorter, much lower than it is in someone who is healthy,” Dr. Coccaro said.
“What we think is that the threshold to explode is probably regulated by various neurobiological features. The baseline state of aggression also may be related to baseline neurobiological features, but also what’s going on in the environment, because the neurobiological features that send someone to exploding aggression are there all the time,” he explained.
Individuals with secondary aggression are likely to have an underlying condition, such as a primary disease of the brain, systemic or metabolic disorder, or a psychiatric disorder such as schizophrenia. “If someone’s schizophrenic and they’ve got voices telling them to hurt somebody, or delusions that someone’s going to hurt them, that’s not primary aggression, that’s secondary to the psychosis,” Dr. Coccaro noted.
An individual with primary aggression is likely to have intermittent explosive disorder (IED). IED is not a new diagnosis and has been listed in the DSM since the DSM-I as “passive-aggressive personality.” It was relisted in the DSM-II as “explosive personality,” then changed to IED in the DSM-3 as a diagnosis of exclusion that was poorly operationalized, according to Dr. Coccaro. The criteria for IED under the DSM-III did not define the number of recurrent outbursts needed, what they looked like, the time frame, and excluded people who were generally impulsive.
“That’s not really what these people look like and it’s not what impulsive aggression looks like,” he said. Although the DSM-IV removed the exclusion criteria for general impulsivity and aggression, “it was still purely operational.”
The DSM-5 criteria define IED as “verbal and physical aggression without destruction or assault, twice equally on average for 3 months, or three or more episodes of physical destruction/assault over a 1-year period. These individuals have outbursts “grossly out of proportion to provocation,” the aggression is generally impulsive, and it causes stress and impairment with an age of onset at older than 6 years.
“It’s not better accounted for a whole variety of things, but we actually made some of those exclusion criteria a little less stringent,” compared with criteria in the DSM-IV, Dr. Coccaro said. “That’s because it turns out that it doesn’t really matter much of the time what the comorbidity is. If you have this aggressiveness in the absence of those other conditions, it’s IED.”
According to a reanalysis of the National Comorbidity Survey, 11.7% of adolescents displayed aggressiveness within the last year and 17.3% over a lifetime, compared with 5.1% of adults within the last year and 8.0% within a lifetime. Under DSM-5 criteria, 6.4% of adolescents within the last year and 8.9% over a lifetime currently have IED, compared with 2.6% of adults within the last year and 4.0% over a lifetime, but “could go as high” as the percentage of individuals diagnosed with aggressiveness, Dr. Coccaro noted.
“People who are not called IED many times are not called IED because we didn’t have all the information we needed to actually make the diagnosis,” he said.
Individuals with DSM-5 IED can have as many as 30 episodes in 1 year, compared with those who are nonaggressive and are also more likely to damage property. “These are the big episodes, not simply the episodes where people are getting irritable and snapping at people. These are the big ones, where they’re really destroying objects and pushing or hitting people,” Dr. Coccaro said. About one-fourth of individuals with IED hurt victims badly enough that they require medical attention, one-fifth exhibit aggression toward a partner, and one-fourth receive aggression from their own partner.
In terms of comorbidity with other psychiatric disorders, “IEDs don’t have more comorbidity in general than other disorders,” Dr. Coccaro noted. Personality disorders such as paranoid, antisocial, borderline narcissistic, and obsessive-compulsive disorders are more common in individuals with IED. Aggression in these people present differently depending on the personality disorder. “Someone who’s paranoid might blow up at you if you get in their face. For an antisocial, they’ll blow up at you if you’re preventing them from doing what they want to do. Borderlines, you reject them or you abandon them, they’re going to blow up. Narcissists will blow up when you reject. OCD will also blow up when you mess around with their sense of order,” Dr. Coccaro said.
Genetics also play a role in whether a person may have IED. These percentages were consistent, regardless of whether the individual had a comorbid condition, history of alcohol or drug use, or history of suicide, he said. Other factors that influence likelihood of IED are environment, behaviors such as smoking, and conditions such as traumatic brain injury. Experiencing aggression as a child is another factor.
“IED is the categorical expression of impulsive aggression, and it’s far more common than once thought,” Dr. Coccaro said. “And IED is totally unrecognized in its role in societal violence.”
Treatment can suppress, but not cure aggression
Medications used to treat aggression and impulsive aggression include lithium, SSRIs, mood stabilizers, neuroleptics, and beta-blockers. However, the treatments are not a “magic bullet,” Dr. Coccaro noted. “The meds tend to suppress aggressiveness, but not cure it.”
Timing of treatment is also a factor for medication. In studies of patients taking lithium for aggression, for example, “when they gave the drug to people who liked being aggressive, they didn’t like being on these drugs because it made them feel unprotected. It just was at odds with who they thought they were,” Dr. Coccaro said. “The people who took the drug and did well and really liked being on the drug with people who didn’t like that they were aggressive.”
Neurorehabilitation and cognitive-behavioral therapy specific to aggression, called cognitive relaxation and coping skills therapy, are nonpsychotropic approaches to treating aggression. “These therapeutic approaches are working not only to reduce progression, but also to reduce the social information processing problems that aggressive individuals have,” Dr. Coccaro said.
Another approach, known as interpretation bias training, teaches individuals with aggression to judge slightly angry-looking photos of people as not being angry. After 7-14 days of training, aggressive behavior in adolescents has been shown to be reduced. The changes were also visible on functional MRI.
“What they found was that when you treated them, the change in the amygdala went down when you looked at the angry faces and in the left lateral, post training, they became happier,” Dr. Coccaro said.
Global Academy and this news organization are owned by the same parent company. Dr. Coccaro reported serving as a consultant for Avanir, Azevan, and Bracket. He also reported receiving research grants from the National Institute of Mental Health, the National Institute on Alcohol Abuse and Alcoholism, and the Pritzker Pucker Family Foundation.
Aggression in individuals is influenced by genetic and environmental factors, but can be reduced with treatment, according to Emil F. Coccaro, MD.
“It actually is a complex triad of emotion, cognition, and behavior. The emotion is anger, the cognition is hostility, and the behavior is aggression. And they sort of go in that order,” Dr. Coccaro said at Focus on Neuropsychiatry presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
Although aggression can be thought of in a numerous ways, premeditated and impulsive aggression are most relevant to behavioral studies in psychiatry, Dr. Coccaro explained. Premeditated aggression is goal oriented, while impulsive aggression comes from frustration or a response to a threat. Impulsive aggression is “typically social or frustrative in nature, and studies that we’ve done that show that individuals move toward a threat while nonaggressives move away it,” he said. Both types of aggression can be seen in the same individuals at different times.
Aggression also can be considered using a threshold model. Calm individuals, for example, might have a low baseline of aggression and a high threshold before they act out. An aggressive person, on the other hand, has a lower threshold and a higher baseline level. “Their delta to get to the point where they’re going to explode is much shorter, much lower than it is in someone who is healthy,” Dr. Coccaro said.
“What we think is that the threshold to explode is probably regulated by various neurobiological features. The baseline state of aggression also may be related to baseline neurobiological features, but also what’s going on in the environment, because the neurobiological features that send someone to exploding aggression are there all the time,” he explained.
Individuals with secondary aggression are likely to have an underlying condition, such as a primary disease of the brain, systemic or metabolic disorder, or a psychiatric disorder such as schizophrenia. “If someone’s schizophrenic and they’ve got voices telling them to hurt somebody, or delusions that someone’s going to hurt them, that’s not primary aggression, that’s secondary to the psychosis,” Dr. Coccaro noted.
An individual with primary aggression is likely to have intermittent explosive disorder (IED). IED is not a new diagnosis and has been listed in the DSM since the DSM-I as “passive-aggressive personality.” It was relisted in the DSM-II as “explosive personality,” then changed to IED in the DSM-3 as a diagnosis of exclusion that was poorly operationalized, according to Dr. Coccaro. The criteria for IED under the DSM-III did not define the number of recurrent outbursts needed, what they looked like, the time frame, and excluded people who were generally impulsive.
“That’s not really what these people look like and it’s not what impulsive aggression looks like,” he said. Although the DSM-IV removed the exclusion criteria for general impulsivity and aggression, “it was still purely operational.”
The DSM-5 criteria define IED as “verbal and physical aggression without destruction or assault, twice equally on average for 3 months, or three or more episodes of physical destruction/assault over a 1-year period. These individuals have outbursts “grossly out of proportion to provocation,” the aggression is generally impulsive, and it causes stress and impairment with an age of onset at older than 6 years.
“It’s not better accounted for a whole variety of things, but we actually made some of those exclusion criteria a little less stringent,” compared with criteria in the DSM-IV, Dr. Coccaro said. “That’s because it turns out that it doesn’t really matter much of the time what the comorbidity is. If you have this aggressiveness in the absence of those other conditions, it’s IED.”
According to a reanalysis of the National Comorbidity Survey, 11.7% of adolescents displayed aggressiveness within the last year and 17.3% over a lifetime, compared with 5.1% of adults within the last year and 8.0% within a lifetime. Under DSM-5 criteria, 6.4% of adolescents within the last year and 8.9% over a lifetime currently have IED, compared with 2.6% of adults within the last year and 4.0% over a lifetime, but “could go as high” as the percentage of individuals diagnosed with aggressiveness, Dr. Coccaro noted.
“People who are not called IED many times are not called IED because we didn’t have all the information we needed to actually make the diagnosis,” he said.
Individuals with DSM-5 IED can have as many as 30 episodes in 1 year, compared with those who are nonaggressive and are also more likely to damage property. “These are the big episodes, not simply the episodes where people are getting irritable and snapping at people. These are the big ones, where they’re really destroying objects and pushing or hitting people,” Dr. Coccaro said. About one-fourth of individuals with IED hurt victims badly enough that they require medical attention, one-fifth exhibit aggression toward a partner, and one-fourth receive aggression from their own partner.
In terms of comorbidity with other psychiatric disorders, “IEDs don’t have more comorbidity in general than other disorders,” Dr. Coccaro noted. Personality disorders such as paranoid, antisocial, borderline narcissistic, and obsessive-compulsive disorders are more common in individuals with IED. Aggression in these people present differently depending on the personality disorder. “Someone who’s paranoid might blow up at you if you get in their face. For an antisocial, they’ll blow up at you if you’re preventing them from doing what they want to do. Borderlines, you reject them or you abandon them, they’re going to blow up. Narcissists will blow up when you reject. OCD will also blow up when you mess around with their sense of order,” Dr. Coccaro said.
Genetics also play a role in whether a person may have IED. These percentages were consistent, regardless of whether the individual had a comorbid condition, history of alcohol or drug use, or history of suicide, he said. Other factors that influence likelihood of IED are environment, behaviors such as smoking, and conditions such as traumatic brain injury. Experiencing aggression as a child is another factor.
“IED is the categorical expression of impulsive aggression, and it’s far more common than once thought,” Dr. Coccaro said. “And IED is totally unrecognized in its role in societal violence.”
Treatment can suppress, but not cure aggression
Medications used to treat aggression and impulsive aggression include lithium, SSRIs, mood stabilizers, neuroleptics, and beta-blockers. However, the treatments are not a “magic bullet,” Dr. Coccaro noted. “The meds tend to suppress aggressiveness, but not cure it.”
Timing of treatment is also a factor for medication. In studies of patients taking lithium for aggression, for example, “when they gave the drug to people who liked being aggressive, they didn’t like being on these drugs because it made them feel unprotected. It just was at odds with who they thought they were,” Dr. Coccaro said. “The people who took the drug and did well and really liked being on the drug with people who didn’t like that they were aggressive.”
Neurorehabilitation and cognitive-behavioral therapy specific to aggression, called cognitive relaxation and coping skills therapy, are nonpsychotropic approaches to treating aggression. “These therapeutic approaches are working not only to reduce progression, but also to reduce the social information processing problems that aggressive individuals have,” Dr. Coccaro said.
Another approach, known as interpretation bias training, teaches individuals with aggression to judge slightly angry-looking photos of people as not being angry. After 7-14 days of training, aggressive behavior in adolescents has been shown to be reduced. The changes were also visible on functional MRI.
“What they found was that when you treated them, the change in the amygdala went down when you looked at the angry faces and in the left lateral, post training, they became happier,” Dr. Coccaro said.
Global Academy and this news organization are owned by the same parent company. Dr. Coccaro reported serving as a consultant for Avanir, Azevan, and Bracket. He also reported receiving research grants from the National Institute of Mental Health, the National Institute on Alcohol Abuse and Alcoholism, and the Pritzker Pucker Family Foundation.
FROM FOCUS ON NEUROPSYCHIATRY 2020
Novel drug may lower agitation, aggression in multiple psychiatric disorders
The novel lysine-specific demethylase 1 inhibitor vafidemstat (ORY-2001, Oryzon Genomics) is effective for treating agitation and aggression across a number of psychiatric disorders, new research suggests.
The REIMAGINE trial included 30 patients with autism spectrum disorder (ASD), ADHD, or borderline personality disorder (BPD). Results showed significant improvements after 8 weeks in general functioning and agitation-aggression scores for all three disorders.
The study “supports vafidemstat as an emerging therapeutic option to treat aggression-agitation, as well as the nonaggression features of psychiatric diseases with high unmet medical need,” lead researcher Roger Bullock, MD, Oryzon Genomics, Corneliá De Llobregat, Spain, told Medscape Medical News.
Bullock added.
However, another expert urged prudence when interpreting the findings.
“The study results must be viewed with caution, given the inherent limitations of an open-label trial, small sample size, and weak rationale for the sample selection,” said Nathan Kolla, MD, PhD, a psychiatrist at the University of Toronto, Canada, who was not involved with the research.
The findings were presented at the European Psychiatric Association (EPA) 2020 Congress, which was held online this year because of the COVID-19 pandemic.
Little evidence available
“Epigenetic mechanisms have been proposed in many psychiatric conditions, but so far, little clinical evidence is available,” Bullock said during his presentation.
In preclinical models, vafidemstat has been associated with a reduction in aggressive behavior “and the normal response to stress of immediate early genes in the prefrontal cortex” via the modification of gene transcription, noted Bullock.
“This new approach makes it a good candidate to look at aggression in multiple psychiatric and CNS conditions,” he added.
REIMAGINE was a phase 2a open-label trial that included 30 patients (53% women; mean age, 33.5 years; 87% White) with psychiatric disorders who had significant or persistent agitation or aggression that was disruptive of the patients› daily life.
Among the participants, 12 had BPD, 11 had ADHD, and seven had ASD. All were treated with vafidemstat 1.2 mg for 8 weeks.
In all, 23 patients completed all 8 weeks of treatment, including nine patients with BPD, eight with ADHD, and six with ASD.
Results showed that the study drug was well tolerated, with no serious adverse events reported and no patients withdrawing because of safety-related events.
The most common adverse events were headache (20%) and insomnia (10%), which resolved without intervention or treatment modification.
Significantly improved scores
Across the whole cohort, the drug was associated with significant reductions in scores over baseline on the Clinical Global Impression–Severity (CGI-S) and CGI-Improvement (CGI-I) scales. There were also significant improvements for Neuropsychiatric Inventory (NPI) total scores and agitation-aggression scores (P < .001 for comparisons).
Similar results were observed with respect to individual diagnoses, albeit at varying degrees of significance for each scale.
Patients with BPD experienced significant reductions in scores on the Borderline Personality Disorder Checklist (BPDCL) (P < .01). Patients with ADHD experienced reductions on the ADHD Rating Scale (P < .05).
Patients with BPD also experienced reductions in suicidal ideation, as measured with the Columbia Suicide Severity Rating Scale (P < .01). That is “the only cohort where this trait is relevant,” the researchers note.
In addition, significant correlations were shown between NPI total scores and scores on the BPDCL after treatment with vafidemstat (P = .015), as well as between NPI agitation-aggression scores and both CGI-I (P = .008) and CGI-S scores (P = .0001).
“This convergence of signals in scales of different nature and scope support the pharmacological role of vafidemstat in controlling aggression-agitation in different psychiatric conditions,” the investigators note.
Bullock added that further randomized placebo-controlled clinical trials “to confirm vafidemstat’s potential to treat aggression-agitation in psychiatric disorders are now planned.”
First up will be PORTICO, which is planned to start over the coming months in Spain and will include patients with BPD.
Several limitations
Commenting on the study for Medscape Medical News, Kolla, who is also a researcher at the Center for Addiction and Mental Health, noted that REIMAGINE was originally designed to test vafidemstat for the treatment of agitation and aggression in patients with Alzheimer’s disease (AD).
“It seems peculiar that the study investigators would choose to examine three additional psychiatric disorders that bear little resemblance to AD in terms of phenomenology. Additionally, the etiological underpinnings of the three disorders likely differ markedly from AD,” said Kolla, who was not involved with the research.
In addition, the “very small” sample size in each group makes it difficult to interpret the investigators’ conclusions, he noted.
There are also “many more sophisticated scales” to assess agitation and aggression than what were used in the study, he added.
Kolla also questioned the notion that a drug such as vafidemstat satisfies an unmet clinical need for the treatment of aggression and agitation.
Trials that “purport to reduce aggression in these populations often provide some level of global improvement in functioning that may appear as if they directly treat agitation or aggression,” he said. “However, no drug has ever been developed that directly reduces aggression and agitation.”
That means that, for now, there is insufficient evidence to “conclude that vafidemstat overcomes the unmet medical need of treating aggression/agitation,” he said.
For Kolla, the concept of a psychiatric drug that works by effecting epigenetic changes to the genome is also questionable, although such mechanisms may “play a role in the salubrious effects of certain mood stabilizers or antipsychotics for which better-defined mechanisms of action have been established.”
The study was funded by Oryzon Genomics. Bullock and the other investigators are employees of Oryzon Genomics.
This article first appeared on Medscape.com.
The novel lysine-specific demethylase 1 inhibitor vafidemstat (ORY-2001, Oryzon Genomics) is effective for treating agitation and aggression across a number of psychiatric disorders, new research suggests.
The REIMAGINE trial included 30 patients with autism spectrum disorder (ASD), ADHD, or borderline personality disorder (BPD). Results showed significant improvements after 8 weeks in general functioning and agitation-aggression scores for all three disorders.
The study “supports vafidemstat as an emerging therapeutic option to treat aggression-agitation, as well as the nonaggression features of psychiatric diseases with high unmet medical need,” lead researcher Roger Bullock, MD, Oryzon Genomics, Corneliá De Llobregat, Spain, told Medscape Medical News.
Bullock added.
However, another expert urged prudence when interpreting the findings.
“The study results must be viewed with caution, given the inherent limitations of an open-label trial, small sample size, and weak rationale for the sample selection,” said Nathan Kolla, MD, PhD, a psychiatrist at the University of Toronto, Canada, who was not involved with the research.
The findings were presented at the European Psychiatric Association (EPA) 2020 Congress, which was held online this year because of the COVID-19 pandemic.
Little evidence available
“Epigenetic mechanisms have been proposed in many psychiatric conditions, but so far, little clinical evidence is available,” Bullock said during his presentation.
In preclinical models, vafidemstat has been associated with a reduction in aggressive behavior “and the normal response to stress of immediate early genes in the prefrontal cortex” via the modification of gene transcription, noted Bullock.
“This new approach makes it a good candidate to look at aggression in multiple psychiatric and CNS conditions,” he added.
REIMAGINE was a phase 2a open-label trial that included 30 patients (53% women; mean age, 33.5 years; 87% White) with psychiatric disorders who had significant or persistent agitation or aggression that was disruptive of the patients› daily life.
Among the participants, 12 had BPD, 11 had ADHD, and seven had ASD. All were treated with vafidemstat 1.2 mg for 8 weeks.
In all, 23 patients completed all 8 weeks of treatment, including nine patients with BPD, eight with ADHD, and six with ASD.
Results showed that the study drug was well tolerated, with no serious adverse events reported and no patients withdrawing because of safety-related events.
The most common adverse events were headache (20%) and insomnia (10%), which resolved without intervention or treatment modification.
Significantly improved scores
Across the whole cohort, the drug was associated with significant reductions in scores over baseline on the Clinical Global Impression–Severity (CGI-S) and CGI-Improvement (CGI-I) scales. There were also significant improvements for Neuropsychiatric Inventory (NPI) total scores and agitation-aggression scores (P < .001 for comparisons).
Similar results were observed with respect to individual diagnoses, albeit at varying degrees of significance for each scale.
Patients with BPD experienced significant reductions in scores on the Borderline Personality Disorder Checklist (BPDCL) (P < .01). Patients with ADHD experienced reductions on the ADHD Rating Scale (P < .05).
Patients with BPD also experienced reductions in suicidal ideation, as measured with the Columbia Suicide Severity Rating Scale (P < .01). That is “the only cohort where this trait is relevant,” the researchers note.
In addition, significant correlations were shown between NPI total scores and scores on the BPDCL after treatment with vafidemstat (P = .015), as well as between NPI agitation-aggression scores and both CGI-I (P = .008) and CGI-S scores (P = .0001).
“This convergence of signals in scales of different nature and scope support the pharmacological role of vafidemstat in controlling aggression-agitation in different psychiatric conditions,” the investigators note.
Bullock added that further randomized placebo-controlled clinical trials “to confirm vafidemstat’s potential to treat aggression-agitation in psychiatric disorders are now planned.”
First up will be PORTICO, which is planned to start over the coming months in Spain and will include patients with BPD.
Several limitations
Commenting on the study for Medscape Medical News, Kolla, who is also a researcher at the Center for Addiction and Mental Health, noted that REIMAGINE was originally designed to test vafidemstat for the treatment of agitation and aggression in patients with Alzheimer’s disease (AD).
“It seems peculiar that the study investigators would choose to examine three additional psychiatric disorders that bear little resemblance to AD in terms of phenomenology. Additionally, the etiological underpinnings of the three disorders likely differ markedly from AD,” said Kolla, who was not involved with the research.
In addition, the “very small” sample size in each group makes it difficult to interpret the investigators’ conclusions, he noted.
There are also “many more sophisticated scales” to assess agitation and aggression than what were used in the study, he added.
Kolla also questioned the notion that a drug such as vafidemstat satisfies an unmet clinical need for the treatment of aggression and agitation.
Trials that “purport to reduce aggression in these populations often provide some level of global improvement in functioning that may appear as if they directly treat agitation or aggression,” he said. “However, no drug has ever been developed that directly reduces aggression and agitation.”
That means that, for now, there is insufficient evidence to “conclude that vafidemstat overcomes the unmet medical need of treating aggression/agitation,” he said.
For Kolla, the concept of a psychiatric drug that works by effecting epigenetic changes to the genome is also questionable, although such mechanisms may “play a role in the salubrious effects of certain mood stabilizers or antipsychotics for which better-defined mechanisms of action have been established.”
The study was funded by Oryzon Genomics. Bullock and the other investigators are employees of Oryzon Genomics.
This article first appeared on Medscape.com.
The novel lysine-specific demethylase 1 inhibitor vafidemstat (ORY-2001, Oryzon Genomics) is effective for treating agitation and aggression across a number of psychiatric disorders, new research suggests.
The REIMAGINE trial included 30 patients with autism spectrum disorder (ASD), ADHD, or borderline personality disorder (BPD). Results showed significant improvements after 8 weeks in general functioning and agitation-aggression scores for all three disorders.
The study “supports vafidemstat as an emerging therapeutic option to treat aggression-agitation, as well as the nonaggression features of psychiatric diseases with high unmet medical need,” lead researcher Roger Bullock, MD, Oryzon Genomics, Corneliá De Llobregat, Spain, told Medscape Medical News.
Bullock added.
However, another expert urged prudence when interpreting the findings.
“The study results must be viewed with caution, given the inherent limitations of an open-label trial, small sample size, and weak rationale for the sample selection,” said Nathan Kolla, MD, PhD, a psychiatrist at the University of Toronto, Canada, who was not involved with the research.
The findings were presented at the European Psychiatric Association (EPA) 2020 Congress, which was held online this year because of the COVID-19 pandemic.
Little evidence available
“Epigenetic mechanisms have been proposed in many psychiatric conditions, but so far, little clinical evidence is available,” Bullock said during his presentation.
In preclinical models, vafidemstat has been associated with a reduction in aggressive behavior “and the normal response to stress of immediate early genes in the prefrontal cortex” via the modification of gene transcription, noted Bullock.
“This new approach makes it a good candidate to look at aggression in multiple psychiatric and CNS conditions,” he added.
REIMAGINE was a phase 2a open-label trial that included 30 patients (53% women; mean age, 33.5 years; 87% White) with psychiatric disorders who had significant or persistent agitation or aggression that was disruptive of the patients› daily life.
Among the participants, 12 had BPD, 11 had ADHD, and seven had ASD. All were treated with vafidemstat 1.2 mg for 8 weeks.
In all, 23 patients completed all 8 weeks of treatment, including nine patients with BPD, eight with ADHD, and six with ASD.
Results showed that the study drug was well tolerated, with no serious adverse events reported and no patients withdrawing because of safety-related events.
The most common adverse events were headache (20%) and insomnia (10%), which resolved without intervention or treatment modification.
Significantly improved scores
Across the whole cohort, the drug was associated with significant reductions in scores over baseline on the Clinical Global Impression–Severity (CGI-S) and CGI-Improvement (CGI-I) scales. There were also significant improvements for Neuropsychiatric Inventory (NPI) total scores and agitation-aggression scores (P < .001 for comparisons).
Similar results were observed with respect to individual diagnoses, albeit at varying degrees of significance for each scale.
Patients with BPD experienced significant reductions in scores on the Borderline Personality Disorder Checklist (BPDCL) (P < .01). Patients with ADHD experienced reductions on the ADHD Rating Scale (P < .05).
Patients with BPD also experienced reductions in suicidal ideation, as measured with the Columbia Suicide Severity Rating Scale (P < .01). That is “the only cohort where this trait is relevant,” the researchers note.
In addition, significant correlations were shown between NPI total scores and scores on the BPDCL after treatment with vafidemstat (P = .015), as well as between NPI agitation-aggression scores and both CGI-I (P = .008) and CGI-S scores (P = .0001).
“This convergence of signals in scales of different nature and scope support the pharmacological role of vafidemstat in controlling aggression-agitation in different psychiatric conditions,” the investigators note.
Bullock added that further randomized placebo-controlled clinical trials “to confirm vafidemstat’s potential to treat aggression-agitation in psychiatric disorders are now planned.”
First up will be PORTICO, which is planned to start over the coming months in Spain and will include patients with BPD.
Several limitations
Commenting on the study for Medscape Medical News, Kolla, who is also a researcher at the Center for Addiction and Mental Health, noted that REIMAGINE was originally designed to test vafidemstat for the treatment of agitation and aggression in patients with Alzheimer’s disease (AD).
“It seems peculiar that the study investigators would choose to examine three additional psychiatric disorders that bear little resemblance to AD in terms of phenomenology. Additionally, the etiological underpinnings of the three disorders likely differ markedly from AD,” said Kolla, who was not involved with the research.
In addition, the “very small” sample size in each group makes it difficult to interpret the investigators’ conclusions, he noted.
There are also “many more sophisticated scales” to assess agitation and aggression than what were used in the study, he added.
Kolla also questioned the notion that a drug such as vafidemstat satisfies an unmet clinical need for the treatment of aggression and agitation.
Trials that “purport to reduce aggression in these populations often provide some level of global improvement in functioning that may appear as if they directly treat agitation or aggression,” he said. “However, no drug has ever been developed that directly reduces aggression and agitation.”
That means that, for now, there is insufficient evidence to “conclude that vafidemstat overcomes the unmet medical need of treating aggression/agitation,” he said.
For Kolla, the concept of a psychiatric drug that works by effecting epigenetic changes to the genome is also questionable, although such mechanisms may “play a role in the salubrious effects of certain mood stabilizers or antipsychotics for which better-defined mechanisms of action have been established.”
The study was funded by Oryzon Genomics. Bullock and the other investigators are employees of Oryzon Genomics.
This article first appeared on Medscape.com.
Command hallucinations, but is it really psychosis?
CASE Frequent hospitalizations
Ms. D, age 26, presents to the emergency department (ED) after drinking a bottle of hand sanitizer in a suicide attempt. She is admitted to an inpatient psychiatric unit, where she spends 50 days, followed by a transfer to a step-down unit, where she spends 26 days. Upon discharge, her diagnosis is schizoaffective disorder–bipolar type.
Shortly before this, Ms. D had intentionally ingested 20 vitamin pills to “make her heart stop” after a conflict at home. After ingesting the pills, Ms. D presented to the ED, where she stated that if she were discharged, she would kill herself by taking “better pills.” She was then admitted to an inpatient psychiatric unit, where she spent 60 days before being moved to an extended-care step-down facility, where she resided for 42 days.
HISTORY A challenging past
Ms. D has a history of >25 psychiatric hospitalizations with varying discharge diagnoses, including schizophrenia, schizoaffective disorder, borderline personality disorder (BPD), and borderline intellectual functioning.
Ms. D was raised in a 2-parent home with 3 older half-brothers and 3 sisters. She was sexually assaulted by a cousin when she was 12. Ms. D recalls one event of self-injury/cutting behavior at age 15 after she was bullied by peers. Her family history is significant for schizophrenia (mother), alcohol use disorder (both parents), and bipolar disorder (sister). Her mother, who is now deceased, was admitted to state psychiatric hospitals for extended periods.
Her medication regimen has changed with nearly every hospitalization but generally has included ≥1 antipsychotic, a mood stabilizer, an antidepressant, and a benzodiazepine (often prescribed on an as-needed basis). Ms. D is obese and has difficulty sleeping, hypothyroidism, gastroesophageal reflux disease (GERD), hypertension, and iron deficiency anemia. She receives medications to manage each of these conditions.
Ms. D’s previous psychotic symptoms included auditory command hallucinations. These occurred under stressful circumstances, such as during severe family conflicts that often led to her feeling abandoned. She reported that the “voice” she heard was usually her own instructing her to “take pills.” There was no prior evidence of bizarre delusions, negative symptoms, or disorganized thoughts or speech.
During episodes of decompensation, Ms. D did not report symptoms of mania, sustained depressed mood, or anxiety, nor were these symptoms observed. Although Ms. D endorsed suicidal ideation with a plan, intent, and means, during several of her previous ED presentations, she told clinicians that her intent was not to end her life but rather to evoke concern in her family members.
Continue to: After her mother died...
After her mother died when Ms. D was 19, she began to have nightmares of wanting to hurt herself and others and began experiencing multiple hospitalizations. In 2010, Ms. D was referred to an assertive community treatment (ACT) program for individuals age 16 to 27 because of her inability to participate in traditional community-based services and her historical need for advanced services, in order to provide psychiatric care in the least restrictive means possible.
Despite receiving intensive ACT services, and in addition to the numerous inpatient psychiatric hospitalizations, over 7 years, Ms. D accumulated 8 additional general-medical hospitalizations and >50 visits to hospital EDs and urgent care facilities. These hospitalizations typically followed arguments at home, strained family dynamics, and not feeling wanted. Ms. D would ingest large quantities of prescription or over-the-counter medications as a way of coping, which often occurred while she was residing in a step-down facility after hospital discharge.
[polldaddy:10528342]
The authors’ observations
The treatment team decided to transition Ms. D to an LTSR with full continuum of treatment. While some clinicians might be concerned with potential iatrogenic harm of LTSR placement and might instead recommend less restrictive residential support and an IOP. However, in Ms. D’s case, her numerous admissions to EDs, urgent care facilities, and medical and psychiatric hospitals, her failed step-down facility placements, and her family conflicts and poor dynamics limited the efficacy of her natural support system and drove the recommendation for an LTSR.
Previously, Ms. D’s experience with ACT services had centered on managing acute crises, with brief periods of stabilization that insufficiently engaged her in a consistent and meaningful treatment plan. Ms. D’s insurance company agreed to pay for the LTSR after lengthy discussions with the clinical leadership at the ACT program and the LTSR demonstrated that she was a high utilizer of health care services. They concluded that Ms. D’s stay at the LTSR would be less expensive than the frequent use of expensive hospital services and care.
EVALUATION A consensus on the diagnosis
During the first few weeks of Ms. D’s admission to the LTSR, the treatment team takes a thorough history and reviews her medical records, which they obtained from several past inpatient admissions and therapists who previously treated Ms. D. The team also collects collateral information from Ms. D’s family members. Based on this information, interviews, and composite behavioral observations from the first few weeks of Ms. D’s time at the LTSR, the psychiatrists and treatment team at the LTSR and ACT program determine that Ms. D meets the criteria for a primary diagnosis of BPD. Previous discharge diagnoses of schizoaffective disorder–bipolar type (Table 11), schizophrenia, or bipolar disorder could not be affirmed.

Continue to: The authors' observations
The authors’ observations
During Ms. D’s LTSR placement, it became clear that her self-harm behaviors and numerous visits to the ED and urgent care facilities involved severe and intense emotional dysregulation and maladaptive behaviors. These behaviors had developed over time in response to acute stressors and past trauma, and not as a result of a sustained mood or psychotic disorder. Before her LTSR placement, Ms. D was unable to use more adaptive coping skills, such as skills building, learning, and coaching. Ms. D typically “thrived” with medical attention in the ED or hospital, and once the stressor dissipated, she was discharged back to the same stressful living environment associated with her maladaptive coping.
Table 2 outlines the rationale for long-term residential treatment for Ms. D.

TREATMENT Developing more effective skills
Bolstered by a clearer diagnostic formulation of BPD, Ms. D’s initial treatment goals at the LTSR include developing effective skills (eg, mindfulness, interpersonal effectiveness, emotion regulation, and distress tolerance) to cope with family conflicts and other stressors while she is outside the facility on a therapeutic pass. Ms. D’s treatment focuses on skills learning and coaching, and behavior chain analyses, which are conducted by her therapist from the ACT program.
Ms. D remains clinically stable throughout her LTSR placement, and benefits from ongoing skills building and learning, coaching, and community integration efforts.
[polldaddy:10528348]
The authors’ observations
Several systematic reviews2-5 have found that there is a lack of high-quality evidence for the use of various psychotropic medications for patients with BPD, yet polypharmacy is common. Many patients with BPD receive ≥2 medications and >25% of patients receive ≥4 medications, typically for prolonged periods. Stoffers et al4 suggested that FGAs and antidepressants have marginal effects of for patients with BPD; however, their use cannot be ruled out because they may be helpful for comorbid symptoms that are often observed in patients with BPD. There is better evidence for SGAs, mood stabilizers, and omega-3 fatty acids; however, most effect estimates were based on single studies, and there is minimal data on long-term use of these agents.4
Continue to: A recent review highlighted...
A recent review highlighted 2 trends in medication prescribing for individuals with BPD3:
- a decrease in the use of benzodiazepines and antidepressants
- an increase in or preference for mood stabilizers and SGAs, especially valproate and quetiapine.
In terms of which medications can be used to target specific symptoms, the same researchers also noted from previous studies3:
- The prior use of SSRIs to target affective dysregulation, anxiety, and impulsive- behavior dyscontrol
- mood stabilizers (notably anticonvulsants) and SGAs to target “core symptoms” of BPD, including affective dysregulation, impulsive-behavioral dyscontrol, and cognitive-perceptual distortions
- omega-3 fatty acids for mood stabilization, impulsive-behavior dyscontrol, and possibly to reduce self-harm behaviors.
TREATMENT Medication adjustments
The treatment team reviews the lack of evidence for the long-term use of psychotropic medications in the treatment of BPD with Ms. D and her relatives,2-5 and develops a medication regimen that is clinically appropriate for managing the symptoms of BPD, while also being mindful of adverse effects.
When Ms. D was admitted to the LTSR from the hospital, her psychotropic medication regimen included haloperidol, 150 mg IM every month; olanzapine, 20 mg at bedtime; benztropine, 1 mg twice daily; and melatonin, 9 mg at bedtime.
Following discussions with Ms. D and her older sister, the team initiates a taper of olanzapine because of metabolic concerns. Ms. D has gained >40 lb while receiving this medication and had hypertension. Olanzapine was tapered and discontinued over the course of 3 months with no reemergence of sustained mood or psychotic symptoms (Table 3). During this period, Ms. D also participates in dietary counselling, follows a portion-controlled regimen, and loses >30 lb. Her wellness plan focuses on nutrition and exercise to improve her overall physical health.
Continue to: Six months into her stay...
Six months into her stay at the LTSR, Ms. D remains clinically stable and is able to leave the LTSR placement to go on home passes. At this time, the team begins to taper the haloperidol long-acting injection. One month prior to discharge from the LTSR, haloperidol is discontinued entirely. The treatment team simultaneously tapers and discontinues benztropine. No recurrence of extrapyramidal symptoms is observed by staff or noted by the patient.
A treatment plan is developed to address Ms. D’s medical conditions, including hypothyroidism, GERD, and obesity. Ms. D does not appear to have difficulty sleeping at the LTSR, so melatonin is tapered by 3-mg decrements and stopped after 2 months. However, shortly thereafter, she develops insomnia, so a 3-mg dose is re-initiated, and her complaints abate. Her primary care physician discontinues hydrochlorothiazide, an antihypertensive medication.
Ms. D’s medication regimen consists of melatonin, 3 mg at bedtime; pantoprazole, 40 mg before breakfast, for GERD; senna, 8.6 mg at bedtime, and polyethylene glycol, 17 gm/d, for constipation; levothyroxine, 125 mcg/d, for hypothyroidism; metoprolol extended-release, 50 mg/d, for hypertension; and ferrous sulfate, 325 mg/d, for iron deficiency anemia.
OUTCOME Improved functioning
After 11 months at the LTSR, Ms. D is discharged home. She continues to receive outpatient services in the community through the ACT program, meeting with her therapist for cognitive-behavioral therapy, skills building and learning, and integration.
Approximately 9 months later, Ms. D is re-started on an SSRI (sertraline, 50 mg/d, which is increased to 100 mg/d 9 months later) to target symptoms of anxiety, which primarily manifest as excessive worrying. Hydroxyzine, 50 mg 3 times daily as needed, is added to this regimen, for breakthrough anxiety symptoms. Hydroxyzine is prescribed instead of a benzodiazepine to avoid potential addiction and abuse.
Continue to: Oral ziprasidone...
Oral ziprasidone, 20 mg/d twice daily, is initiated during 2 brief inpatient psychiatric admissions; however, it is successfully tapered within 1 week of discharge, in partnership with the ACT program.
In the 23 months after her discharge, Ms. D has had 1 ED visit and 2 brief inpatient psychiatric hospitalizations, which is markedly fewer encounters than she had in the 2 years before her LTSR placement. She has also lost an additional 30 lb since her LTSR discharge through a healthy diet and exercise.
Ms. D is now considering transitioning to living independently in the community through a residential supported housing program.
Bottom Line
Psychotic symptoms in patients with borderline personality disorder (BPD) are typically fleeting and mostly occur in the context of intense interpersonal conflicts and real or imagined abandonment. Long-term structured residence placement for patients with BPD can allow for careful formulation of a treatment plan, and help patients gain effective skills to cope with difficult family dynamics and other stressors, with the ultimate goal of gradual community integration.
Related Resource
- National Education Alliance for Borderline Personality Disorder. https://www.borderlinepersonalitydisorder.org.
Drug Brand Names
Benztropine • Cogentin
Haloperidol • Haldol
Hydrochlorothiazide • Microzide, HydroDiuril
Hydroxyzine • Vistaril
Levothyroxine • Synthroid,
Metoprolol ER • Toprol XL
Olanzapine • Zyprexa
Pantoprazole • Protonix
Polyethylene glycol • MiraLax, Glycolax
Quetiapine • Seroquel
Senna • Senokot
Sertraline • Zoloft
Valproate • Depakene, Depakote
Ziprasidone • Geodon
1. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Hancock-Johnson E, Griffiths C, Picchioni M. A focused systematic review of pharmacological treatment for borderline personality disorder. CNS Drugs. 2017;31:345-356.
3. Starcevic V, Janca A. Pharmacotherapy of borderline personality disorder: replacing confusion with prudent pragmatism. Curr Opin Psychiatry. 2018;31(1):69-73.
4. Stoffers J, Völlm BA, Rücker G, et al. Pharmacological interventions for borderline personality disorder. Cochrane Database Syst Rev. 2010;6:CD005653. doi: 10.1002/14651858.CD005653.pub2.
5. Stoffers-Winterling JM, Storebo OJ, Völlm BA, et al. Pharmacological interventions for borderline personality disorder. Cochrane Database Syst Rev. 2018;3:CD012956. doi: 10.1002/14651858.CD012956.
CASE Frequent hospitalizations
Ms. D, age 26, presents to the emergency department (ED) after drinking a bottle of hand sanitizer in a suicide attempt. She is admitted to an inpatient psychiatric unit, where she spends 50 days, followed by a transfer to a step-down unit, where she spends 26 days. Upon discharge, her diagnosis is schizoaffective disorder–bipolar type.
Shortly before this, Ms. D had intentionally ingested 20 vitamin pills to “make her heart stop” after a conflict at home. After ingesting the pills, Ms. D presented to the ED, where she stated that if she were discharged, she would kill herself by taking “better pills.” She was then admitted to an inpatient psychiatric unit, where she spent 60 days before being moved to an extended-care step-down facility, where she resided for 42 days.
HISTORY A challenging past
Ms. D has a history of >25 psychiatric hospitalizations with varying discharge diagnoses, including schizophrenia, schizoaffective disorder, borderline personality disorder (BPD), and borderline intellectual functioning.
Ms. D was raised in a 2-parent home with 3 older half-brothers and 3 sisters. She was sexually assaulted by a cousin when she was 12. Ms. D recalls one event of self-injury/cutting behavior at age 15 after she was bullied by peers. Her family history is significant for schizophrenia (mother), alcohol use disorder (both parents), and bipolar disorder (sister). Her mother, who is now deceased, was admitted to state psychiatric hospitals for extended periods.
Her medication regimen has changed with nearly every hospitalization but generally has included ≥1 antipsychotic, a mood stabilizer, an antidepressant, and a benzodiazepine (often prescribed on an as-needed basis). Ms. D is obese and has difficulty sleeping, hypothyroidism, gastroesophageal reflux disease (GERD), hypertension, and iron deficiency anemia. She receives medications to manage each of these conditions.
Ms. D’s previous psychotic symptoms included auditory command hallucinations. These occurred under stressful circumstances, such as during severe family conflicts that often led to her feeling abandoned. She reported that the “voice” she heard was usually her own instructing her to “take pills.” There was no prior evidence of bizarre delusions, negative symptoms, or disorganized thoughts or speech.
During episodes of decompensation, Ms. D did not report symptoms of mania, sustained depressed mood, or anxiety, nor were these symptoms observed. Although Ms. D endorsed suicidal ideation with a plan, intent, and means, during several of her previous ED presentations, she told clinicians that her intent was not to end her life but rather to evoke concern in her family members.
Continue to: After her mother died...
After her mother died when Ms. D was 19, she began to have nightmares of wanting to hurt herself and others and began experiencing multiple hospitalizations. In 2010, Ms. D was referred to an assertive community treatment (ACT) program for individuals age 16 to 27 because of her inability to participate in traditional community-based services and her historical need for advanced services, in order to provide psychiatric care in the least restrictive means possible.
Despite receiving intensive ACT services, and in addition to the numerous inpatient psychiatric hospitalizations, over 7 years, Ms. D accumulated 8 additional general-medical hospitalizations and >50 visits to hospital EDs and urgent care facilities. These hospitalizations typically followed arguments at home, strained family dynamics, and not feeling wanted. Ms. D would ingest large quantities of prescription or over-the-counter medications as a way of coping, which often occurred while she was residing in a step-down facility after hospital discharge.
[polldaddy:10528342]
The authors’ observations
The treatment team decided to transition Ms. D to an LTSR with full continuum of treatment. While some clinicians might be concerned with potential iatrogenic harm of LTSR placement and might instead recommend less restrictive residential support and an IOP. However, in Ms. D’s case, her numerous admissions to EDs, urgent care facilities, and medical and psychiatric hospitals, her failed step-down facility placements, and her family conflicts and poor dynamics limited the efficacy of her natural support system and drove the recommendation for an LTSR.
Previously, Ms. D’s experience with ACT services had centered on managing acute crises, with brief periods of stabilization that insufficiently engaged her in a consistent and meaningful treatment plan. Ms. D’s insurance company agreed to pay for the LTSR after lengthy discussions with the clinical leadership at the ACT program and the LTSR demonstrated that she was a high utilizer of health care services. They concluded that Ms. D’s stay at the LTSR would be less expensive than the frequent use of expensive hospital services and care.
EVALUATION A consensus on the diagnosis
During the first few weeks of Ms. D’s admission to the LTSR, the treatment team takes a thorough history and reviews her medical records, which they obtained from several past inpatient admissions and therapists who previously treated Ms. D. The team also collects collateral information from Ms. D’s family members. Based on this information, interviews, and composite behavioral observations from the first few weeks of Ms. D’s time at the LTSR, the psychiatrists and treatment team at the LTSR and ACT program determine that Ms. D meets the criteria for a primary diagnosis of BPD. Previous discharge diagnoses of schizoaffective disorder–bipolar type (Table 11), schizophrenia, or bipolar disorder could not be affirmed.

Continue to: The authors' observations
The authors’ observations
During Ms. D’s LTSR placement, it became clear that her self-harm behaviors and numerous visits to the ED and urgent care facilities involved severe and intense emotional dysregulation and maladaptive behaviors. These behaviors had developed over time in response to acute stressors and past trauma, and not as a result of a sustained mood or psychotic disorder. Before her LTSR placement, Ms. D was unable to use more adaptive coping skills, such as skills building, learning, and coaching. Ms. D typically “thrived” with medical attention in the ED or hospital, and once the stressor dissipated, she was discharged back to the same stressful living environment associated with her maladaptive coping.
Table 2 outlines the rationale for long-term residential treatment for Ms. D.

TREATMENT Developing more effective skills
Bolstered by a clearer diagnostic formulation of BPD, Ms. D’s initial treatment goals at the LTSR include developing effective skills (eg, mindfulness, interpersonal effectiveness, emotion regulation, and distress tolerance) to cope with family conflicts and other stressors while she is outside the facility on a therapeutic pass. Ms. D’s treatment focuses on skills learning and coaching, and behavior chain analyses, which are conducted by her therapist from the ACT program.
Ms. D remains clinically stable throughout her LTSR placement, and benefits from ongoing skills building and learning, coaching, and community integration efforts.
[polldaddy:10528348]
The authors’ observations
Several systematic reviews2-5 have found that there is a lack of high-quality evidence for the use of various psychotropic medications for patients with BPD, yet polypharmacy is common. Many patients with BPD receive ≥2 medications and >25% of patients receive ≥4 medications, typically for prolonged periods. Stoffers et al4 suggested that FGAs and antidepressants have marginal effects of for patients with BPD; however, their use cannot be ruled out because they may be helpful for comorbid symptoms that are often observed in patients with BPD. There is better evidence for SGAs, mood stabilizers, and omega-3 fatty acids; however, most effect estimates were based on single studies, and there is minimal data on long-term use of these agents.4
Continue to: A recent review highlighted...
A recent review highlighted 2 trends in medication prescribing for individuals with BPD3:
- a decrease in the use of benzodiazepines and antidepressants
- an increase in or preference for mood stabilizers and SGAs, especially valproate and quetiapine.
In terms of which medications can be used to target specific symptoms, the same researchers also noted from previous studies3:
- The prior use of SSRIs to target affective dysregulation, anxiety, and impulsive- behavior dyscontrol
- mood stabilizers (notably anticonvulsants) and SGAs to target “core symptoms” of BPD, including affective dysregulation, impulsive-behavioral dyscontrol, and cognitive-perceptual distortions
- omega-3 fatty acids for mood stabilization, impulsive-behavior dyscontrol, and possibly to reduce self-harm behaviors.
TREATMENT Medication adjustments
The treatment team reviews the lack of evidence for the long-term use of psychotropic medications in the treatment of BPD with Ms. D and her relatives,2-5 and develops a medication regimen that is clinically appropriate for managing the symptoms of BPD, while also being mindful of adverse effects.
When Ms. D was admitted to the LTSR from the hospital, her psychotropic medication regimen included haloperidol, 150 mg IM every month; olanzapine, 20 mg at bedtime; benztropine, 1 mg twice daily; and melatonin, 9 mg at bedtime.
Following discussions with Ms. D and her older sister, the team initiates a taper of olanzapine because of metabolic concerns. Ms. D has gained >40 lb while receiving this medication and had hypertension. Olanzapine was tapered and discontinued over the course of 3 months with no reemergence of sustained mood or psychotic symptoms (Table 3). During this period, Ms. D also participates in dietary counselling, follows a portion-controlled regimen, and loses >30 lb. Her wellness plan focuses on nutrition and exercise to improve her overall physical health.

Continue to: Six months into her stay...
Six months into her stay at the LTSR, Ms. D remains clinically stable and is able to leave the LTSR placement to go on home passes. At this time, the team begins to taper the haloperidol long-acting injection. One month prior to discharge from the LTSR, haloperidol is discontinued entirely. The treatment team simultaneously tapers and discontinues benztropine. No recurrence of extrapyramidal symptoms is observed by staff or noted by the patient.
A treatment plan is developed to address Ms. D’s medical conditions, including hypothyroidism, GERD, and obesity. Ms. D does not appear to have difficulty sleeping at the LTSR, so melatonin is tapered by 3-mg decrements and stopped after 2 months. However, shortly thereafter, she develops insomnia, so a 3-mg dose is re-initiated, and her complaints abate. Her primary care physician discontinues hydrochlorothiazide, an antihypertensive medication.
Ms. D’s medication regimen consists of melatonin, 3 mg at bedtime; pantoprazole, 40 mg before breakfast, for GERD; senna, 8.6 mg at bedtime, and polyethylene glycol, 17 gm/d, for constipation; levothyroxine, 125 mcg/d, for hypothyroidism; metoprolol extended-release, 50 mg/d, for hypertension; and ferrous sulfate, 325 mg/d, for iron deficiency anemia.
OUTCOME Improved functioning
After 11 months at the LTSR, Ms. D is discharged home. She continues to receive outpatient services in the community through the ACT program, meeting with her therapist for cognitive-behavioral therapy, skills building and learning, and integration.
Approximately 9 months later, Ms. D is re-started on an SSRI (sertraline, 50 mg/d, which is increased to 100 mg/d 9 months later) to target symptoms of anxiety, which primarily manifest as excessive worrying. Hydroxyzine, 50 mg 3 times daily as needed, is added to this regimen, for breakthrough anxiety symptoms. Hydroxyzine is prescribed instead of a benzodiazepine to avoid potential addiction and abuse.
Continue to: Oral ziprasidone...
Oral ziprasidone, 20 mg/d twice daily, is initiated during 2 brief inpatient psychiatric admissions; however, it is successfully tapered within 1 week of discharge, in partnership with the ACT program.
In the 23 months after her discharge, Ms. D has had 1 ED visit and 2 brief inpatient psychiatric hospitalizations, which is markedly fewer encounters than she had in the 2 years before her LTSR placement. She has also lost an additional 30 lb since her LTSR discharge through a healthy diet and exercise.
Ms. D is now considering transitioning to living independently in the community through a residential supported housing program.
Bottom Line
Psychotic symptoms in patients with borderline personality disorder (BPD) are typically fleeting and mostly occur in the context of intense interpersonal conflicts and real or imagined abandonment. Long-term structured residence placement for patients with BPD can allow for careful formulation of a treatment plan, and help patients gain effective skills to cope with difficult family dynamics and other stressors, with the ultimate goal of gradual community integration.
Related Resource
- National Education Alliance for Borderline Personality Disorder. https://www.borderlinepersonalitydisorder.org.
Drug Brand Names
Benztropine • Cogentin
Haloperidol • Haldol
Hydrochlorothiazide • Microzide, HydroDiuril
Hydroxyzine • Vistaril
Levothyroxine • Synthroid,
Metoprolol ER • Toprol XL
Olanzapine • Zyprexa
Pantoprazole • Protonix
Polyethylene glycol • MiraLax, Glycolax
Quetiapine • Seroquel
Senna • Senokot
Sertraline • Zoloft
Valproate • Depakene, Depakote
Ziprasidone • Geodon
CASE Frequent hospitalizations
Ms. D, age 26, presents to the emergency department (ED) after drinking a bottle of hand sanitizer in a suicide attempt. She is admitted to an inpatient psychiatric unit, where she spends 50 days, followed by a transfer to a step-down unit, where she spends 26 days. Upon discharge, her diagnosis is schizoaffective disorder–bipolar type.
Shortly before this, Ms. D had intentionally ingested 20 vitamin pills to “make her heart stop” after a conflict at home. After ingesting the pills, Ms. D presented to the ED, where she stated that if she were discharged, she would kill herself by taking “better pills.” She was then admitted to an inpatient psychiatric unit, where she spent 60 days before being moved to an extended-care step-down facility, where she resided for 42 days.
HISTORY A challenging past
Ms. D has a history of >25 psychiatric hospitalizations with varying discharge diagnoses, including schizophrenia, schizoaffective disorder, borderline personality disorder (BPD), and borderline intellectual functioning.
Ms. D was raised in a 2-parent home with 3 older half-brothers and 3 sisters. She was sexually assaulted by a cousin when she was 12. Ms. D recalls one event of self-injury/cutting behavior at age 15 after she was bullied by peers. Her family history is significant for schizophrenia (mother), alcohol use disorder (both parents), and bipolar disorder (sister). Her mother, who is now deceased, was admitted to state psychiatric hospitals for extended periods.
Her medication regimen has changed with nearly every hospitalization but generally has included ≥1 antipsychotic, a mood stabilizer, an antidepressant, and a benzodiazepine (often prescribed on an as-needed basis). Ms. D is obese and has difficulty sleeping, hypothyroidism, gastroesophageal reflux disease (GERD), hypertension, and iron deficiency anemia. She receives medications to manage each of these conditions.
Ms. D’s previous psychotic symptoms included auditory command hallucinations. These occurred under stressful circumstances, such as during severe family conflicts that often led to her feeling abandoned. She reported that the “voice” she heard was usually her own instructing her to “take pills.” There was no prior evidence of bizarre delusions, negative symptoms, or disorganized thoughts or speech.
During episodes of decompensation, Ms. D did not report symptoms of mania, sustained depressed mood, or anxiety, nor were these symptoms observed. Although Ms. D endorsed suicidal ideation with a plan, intent, and means, during several of her previous ED presentations, she told clinicians that her intent was not to end her life but rather to evoke concern in her family members.
Continue to: After her mother died...
After her mother died when Ms. D was 19, she began to have nightmares of wanting to hurt herself and others and began experiencing multiple hospitalizations. In 2010, Ms. D was referred to an assertive community treatment (ACT) program for individuals age 16 to 27 because of her inability to participate in traditional community-based services and her historical need for advanced services, in order to provide psychiatric care in the least restrictive means possible.
Despite receiving intensive ACT services, and in addition to the numerous inpatient psychiatric hospitalizations, over 7 years, Ms. D accumulated 8 additional general-medical hospitalizations and >50 visits to hospital EDs and urgent care facilities. These hospitalizations typically followed arguments at home, strained family dynamics, and not feeling wanted. Ms. D would ingest large quantities of prescription or over-the-counter medications as a way of coping, which often occurred while she was residing in a step-down facility after hospital discharge.
[polldaddy:10528342]
The authors’ observations
The treatment team decided to transition Ms. D to an LTSR with full continuum of treatment. While some clinicians might be concerned with potential iatrogenic harm of LTSR placement and might instead recommend less restrictive residential support and an IOP. However, in Ms. D’s case, her numerous admissions to EDs, urgent care facilities, and medical and psychiatric hospitals, her failed step-down facility placements, and her family conflicts and poor dynamics limited the efficacy of her natural support system and drove the recommendation for an LTSR.
Previously, Ms. D’s experience with ACT services had centered on managing acute crises, with brief periods of stabilization that insufficiently engaged her in a consistent and meaningful treatment plan. Ms. D’s insurance company agreed to pay for the LTSR after lengthy discussions with the clinical leadership at the ACT program and the LTSR demonstrated that she was a high utilizer of health care services. They concluded that Ms. D’s stay at the LTSR would be less expensive than the frequent use of expensive hospital services and care.
EVALUATION A consensus on the diagnosis
During the first few weeks of Ms. D’s admission to the LTSR, the treatment team takes a thorough history and reviews her medical records, which they obtained from several past inpatient admissions and therapists who previously treated Ms. D. The team also collects collateral information from Ms. D’s family members. Based on this information, interviews, and composite behavioral observations from the first few weeks of Ms. D’s time at the LTSR, the psychiatrists and treatment team at the LTSR and ACT program determine that Ms. D meets the criteria for a primary diagnosis of BPD. Previous discharge diagnoses of schizoaffective disorder–bipolar type (Table 11), schizophrenia, or bipolar disorder could not be affirmed.

Continue to: The authors' observations
The authors’ observations
During Ms. D’s LTSR placement, it became clear that her self-harm behaviors and numerous visits to the ED and urgent care facilities involved severe and intense emotional dysregulation and maladaptive behaviors. These behaviors had developed over time in response to acute stressors and past trauma, and not as a result of a sustained mood or psychotic disorder. Before her LTSR placement, Ms. D was unable to use more adaptive coping skills, such as skills building, learning, and coaching. Ms. D typically “thrived” with medical attention in the ED or hospital, and once the stressor dissipated, she was discharged back to the same stressful living environment associated with her maladaptive coping.
Table 2 outlines the rationale for long-term residential treatment for Ms. D.

TREATMENT Developing more effective skills
Bolstered by a clearer diagnostic formulation of BPD, Ms. D’s initial treatment goals at the LTSR include developing effective skills (eg, mindfulness, interpersonal effectiveness, emotion regulation, and distress tolerance) to cope with family conflicts and other stressors while she is outside the facility on a therapeutic pass. Ms. D’s treatment focuses on skills learning and coaching, and behavior chain analyses, which are conducted by her therapist from the ACT program.
Ms. D remains clinically stable throughout her LTSR placement, and benefits from ongoing skills building and learning, coaching, and community integration efforts.
[polldaddy:10528348]
The authors’ observations
Several systematic reviews2-5 have found that there is a lack of high-quality evidence for the use of various psychotropic medications for patients with BPD, yet polypharmacy is common. Many patients with BPD receive ≥2 medications and >25% of patients receive ≥4 medications, typically for prolonged periods. Stoffers et al4 suggested that FGAs and antidepressants have marginal effects of for patients with BPD; however, their use cannot be ruled out because they may be helpful for comorbid symptoms that are often observed in patients with BPD. There is better evidence for SGAs, mood stabilizers, and omega-3 fatty acids; however, most effect estimates were based on single studies, and there is minimal data on long-term use of these agents.4
Continue to: A recent review highlighted...
A recent review highlighted 2 trends in medication prescribing for individuals with BPD3:
- a decrease in the use of benzodiazepines and antidepressants
- an increase in or preference for mood stabilizers and SGAs, especially valproate and quetiapine.
In terms of which medications can be used to target specific symptoms, the same researchers also noted from previous studies3:
- The prior use of SSRIs to target affective dysregulation, anxiety, and impulsive- behavior dyscontrol
- mood stabilizers (notably anticonvulsants) and SGAs to target “core symptoms” of BPD, including affective dysregulation, impulsive-behavioral dyscontrol, and cognitive-perceptual distortions
- omega-3 fatty acids for mood stabilization, impulsive-behavior dyscontrol, and possibly to reduce self-harm behaviors.
TREATMENT Medication adjustments
The treatment team reviews the lack of evidence for the long-term use of psychotropic medications in the treatment of BPD with Ms. D and her relatives,2-5 and develops a medication regimen that is clinically appropriate for managing the symptoms of BPD, while also being mindful of adverse effects.
When Ms. D was admitted to the LTSR from the hospital, her psychotropic medication regimen included haloperidol, 150 mg IM every month; olanzapine, 20 mg at bedtime; benztropine, 1 mg twice daily; and melatonin, 9 mg at bedtime.
Following discussions with Ms. D and her older sister, the team initiates a taper of olanzapine because of metabolic concerns. Ms. D has gained >40 lb while receiving this medication and had hypertension. Olanzapine was tapered and discontinued over the course of 3 months with no reemergence of sustained mood or psychotic symptoms (Table 3). During this period, Ms. D also participates in dietary counselling, follows a portion-controlled regimen, and loses >30 lb. Her wellness plan focuses on nutrition and exercise to improve her overall physical health.

Continue to: Six months into her stay...
Six months into her stay at the LTSR, Ms. D remains clinically stable and is able to leave the LTSR placement to go on home passes. At this time, the team begins to taper the haloperidol long-acting injection. One month prior to discharge from the LTSR, haloperidol is discontinued entirely. The treatment team simultaneously tapers and discontinues benztropine. No recurrence of extrapyramidal symptoms is observed by staff or noted by the patient.
A treatment plan is developed to address Ms. D’s medical conditions, including hypothyroidism, GERD, and obesity. Ms. D does not appear to have difficulty sleeping at the LTSR, so melatonin is tapered by 3-mg decrements and stopped after 2 months. However, shortly thereafter, she develops insomnia, so a 3-mg dose is re-initiated, and her complaints abate. Her primary care physician discontinues hydrochlorothiazide, an antihypertensive medication.
Ms. D’s medication regimen consists of melatonin, 3 mg at bedtime; pantoprazole, 40 mg before breakfast, for GERD; senna, 8.6 mg at bedtime, and polyethylene glycol, 17 gm/d, for constipation; levothyroxine, 125 mcg/d, for hypothyroidism; metoprolol extended-release, 50 mg/d, for hypertension; and ferrous sulfate, 325 mg/d, for iron deficiency anemia.
OUTCOME Improved functioning
After 11 months at the LTSR, Ms. D is discharged home. She continues to receive outpatient services in the community through the ACT program, meeting with her therapist for cognitive-behavioral therapy, skills building and learning, and integration.
Approximately 9 months later, Ms. D is re-started on an SSRI (sertraline, 50 mg/d, which is increased to 100 mg/d 9 months later) to target symptoms of anxiety, which primarily manifest as excessive worrying. Hydroxyzine, 50 mg 3 times daily as needed, is added to this regimen, for breakthrough anxiety symptoms. Hydroxyzine is prescribed instead of a benzodiazepine to avoid potential addiction and abuse.
Continue to: Oral ziprasidone...
Oral ziprasidone, 20 mg/d twice daily, is initiated during 2 brief inpatient psychiatric admissions; however, it is successfully tapered within 1 week of discharge, in partnership with the ACT program.
In the 23 months after her discharge, Ms. D has had 1 ED visit and 2 brief inpatient psychiatric hospitalizations, which is markedly fewer encounters than she had in the 2 years before her LTSR placement. She has also lost an additional 30 lb since her LTSR discharge through a healthy diet and exercise.
Ms. D is now considering transitioning to living independently in the community through a residential supported housing program.
Bottom Line
Psychotic symptoms in patients with borderline personality disorder (BPD) are typically fleeting and mostly occur in the context of intense interpersonal conflicts and real or imagined abandonment. Long-term structured residence placement for patients with BPD can allow for careful formulation of a treatment plan, and help patients gain effective skills to cope with difficult family dynamics and other stressors, with the ultimate goal of gradual community integration.
Related Resource
- National Education Alliance for Borderline Personality Disorder. https://www.borderlinepersonalitydisorder.org.
Drug Brand Names
Benztropine • Cogentin
Haloperidol • Haldol
Hydrochlorothiazide • Microzide, HydroDiuril
Hydroxyzine • Vistaril
Levothyroxine • Synthroid,
Metoprolol ER • Toprol XL
Olanzapine • Zyprexa
Pantoprazole • Protonix
Polyethylene glycol • MiraLax, Glycolax
Quetiapine • Seroquel
Senna • Senokot
Sertraline • Zoloft
Valproate • Depakene, Depakote
Ziprasidone • Geodon
1. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Hancock-Johnson E, Griffiths C, Picchioni M. A focused systematic review of pharmacological treatment for borderline personality disorder. CNS Drugs. 2017;31:345-356.
3. Starcevic V, Janca A. Pharmacotherapy of borderline personality disorder: replacing confusion with prudent pragmatism. Curr Opin Psychiatry. 2018;31(1):69-73.
4. Stoffers J, Völlm BA, Rücker G, et al. Pharmacological interventions for borderline personality disorder. Cochrane Database Syst Rev. 2010;6:CD005653. doi: 10.1002/14651858.CD005653.pub2.
5. Stoffers-Winterling JM, Storebo OJ, Völlm BA, et al. Pharmacological interventions for borderline personality disorder. Cochrane Database Syst Rev. 2018;3:CD012956. doi: 10.1002/14651858.CD012956.
1. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Hancock-Johnson E, Griffiths C, Picchioni M. A focused systematic review of pharmacological treatment for borderline personality disorder. CNS Drugs. 2017;31:345-356.
3. Starcevic V, Janca A. Pharmacotherapy of borderline personality disorder: replacing confusion with prudent pragmatism. Curr Opin Psychiatry. 2018;31(1):69-73.
4. Stoffers J, Völlm BA, Rücker G, et al. Pharmacological interventions for borderline personality disorder. Cochrane Database Syst Rev. 2010;6:CD005653. doi: 10.1002/14651858.CD005653.pub2.
5. Stoffers-Winterling JM, Storebo OJ, Völlm BA, et al. Pharmacological interventions for borderline personality disorder. Cochrane Database Syst Rev. 2018;3:CD012956. doi: 10.1002/14651858.CD012956.
Borderline personality disorder common in chronic pain patients
NATIONAL HARBOR, MD. – A significant proportion of patients who suffer from chronic pain also have features of borderline personality disorder (BPD), new research shows.
Results of a systematic literature review showed 23% of patients with chronic noncancer pain (CNCP) had some features of BPD, including difficulty maintaining relationships, as well as affect and mood instability.
“The fact that one-fourth of individuals with CNCP could have co-occurring BPD underscores the need for improved access to good psychological care,” lead investigator Fei Cao, MD, PhD, University of Missouri at Kansas City, said in an interview.
“If we treat the borderline personality disorder and address the psychiatric needs as well as the pain needs of the patient, then we will be able to treat their pain more successfully,” Cao said.
The findings were presented at the American Academy of Pain Medicine (AAPM) 2020 Annual Meeting.
Treatment resistance
Cao noted that a “significant number” of CNCP patients have at least some resistance to any type of pain treatment and speculated that BPD may increase treatment-resistant chronic pain.
Initially an anesthesiologist and pain medicine specialist, Cao later became a psychiatrist after recognizing the importance of addressing the underlying psychological needs of patients with chronic pain.
He noted that there is a strong psychological component to chronic pain and that many patients with chronic pain have suffered psychological trauma.
“You have to think about what may have happened to these patients. That is most important. I would not say these are difficult patients. I would say we just don’t know what happened to them,” he said.
To gain a better understanding of the prevalence of BPD in patients suffering from chronic pain and potentially provide some unexploited targets for chronic pain management, the investigators analyzed data from 11 studies published between 1994 and 2019. They found the prevalence of BPD among CNCP patients was 23.3%. Pain types included chronic headache (11.3%), arthritis (27.5%), and chronic spinal cord pain (24.3%).
We also have to treat their BPD. This can then make pain easier to control. Chronic pain management is often long-term and requires good compliance. A diagnosis of BPD might suggest poor compliance,” said Cao.
Screen for BPD
The study findings, he added, indicate a need to screen for BPD in patients with chronic pain. Interventions that are effective in the treatment of BPD and CNCP include cognitive-behavioral therapy, dialectical behavior therapy, antidepressants, and anticonvulsants.
“These should be considered as the first-line treatment in persons with comorbid pain and BPD,” Cao said.
Commenting on the findings, Ann E. Hansen, DVM, MD, Chronic Pain Wellness Center, Phoenix VA Health Care System, Arizona, said the study illustrates the multifactorial nature of chronic pain syndromes, and underscores the importance of a multidisciplinary approach to evaluation and treatment.
“The authors present data showing that BPD is a common diagnosis in patients with chronic pain, thus raising provider awareness to consider BPD and to involve behavioral health colleagues in comanaging these complex patients to achieve optimal outcomes,” Hansen said.
Cao and Hansen have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
SOURCE: Cao F et al. American Academy of Pain Medicine (AAPM) 2020 Annual Meeting, Abstract 505.
NATIONAL HARBOR, MD. – A significant proportion of patients who suffer from chronic pain also have features of borderline personality disorder (BPD), new research shows.
Results of a systematic literature review showed 23% of patients with chronic noncancer pain (CNCP) had some features of BPD, including difficulty maintaining relationships, as well as affect and mood instability.
“The fact that one-fourth of individuals with CNCP could have co-occurring BPD underscores the need for improved access to good psychological care,” lead investigator Fei Cao, MD, PhD, University of Missouri at Kansas City, said in an interview.
“If we treat the borderline personality disorder and address the psychiatric needs as well as the pain needs of the patient, then we will be able to treat their pain more successfully,” Cao said.
The findings were presented at the American Academy of Pain Medicine (AAPM) 2020 Annual Meeting.
Treatment resistance
Cao noted that a “significant number” of CNCP patients have at least some resistance to any type of pain treatment and speculated that BPD may increase treatment-resistant chronic pain.
Initially an anesthesiologist and pain medicine specialist, Cao later became a psychiatrist after recognizing the importance of addressing the underlying psychological needs of patients with chronic pain.
He noted that there is a strong psychological component to chronic pain and that many patients with chronic pain have suffered psychological trauma.
“You have to think about what may have happened to these patients. That is most important. I would not say these are difficult patients. I would say we just don’t know what happened to them,” he said.
To gain a better understanding of the prevalence of BPD in patients suffering from chronic pain and potentially provide some unexploited targets for chronic pain management, the investigators analyzed data from 11 studies published between 1994 and 2019. They found the prevalence of BPD among CNCP patients was 23.3%. Pain types included chronic headache (11.3%), arthritis (27.5%), and chronic spinal cord pain (24.3%).
We also have to treat their BPD. This can then make pain easier to control. Chronic pain management is often long-term and requires good compliance. A diagnosis of BPD might suggest poor compliance,” said Cao.
Screen for BPD
The study findings, he added, indicate a need to screen for BPD in patients with chronic pain. Interventions that are effective in the treatment of BPD and CNCP include cognitive-behavioral therapy, dialectical behavior therapy, antidepressants, and anticonvulsants.
“These should be considered as the first-line treatment in persons with comorbid pain and BPD,” Cao said.
Commenting on the findings, Ann E. Hansen, DVM, MD, Chronic Pain Wellness Center, Phoenix VA Health Care System, Arizona, said the study illustrates the multifactorial nature of chronic pain syndromes, and underscores the importance of a multidisciplinary approach to evaluation and treatment.
“The authors present data showing that BPD is a common diagnosis in patients with chronic pain, thus raising provider awareness to consider BPD and to involve behavioral health colleagues in comanaging these complex patients to achieve optimal outcomes,” Hansen said.
Cao and Hansen have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
SOURCE: Cao F et al. American Academy of Pain Medicine (AAPM) 2020 Annual Meeting, Abstract 505.
NATIONAL HARBOR, MD. – A significant proportion of patients who suffer from chronic pain also have features of borderline personality disorder (BPD), new research shows.
Results of a systematic literature review showed 23% of patients with chronic noncancer pain (CNCP) had some features of BPD, including difficulty maintaining relationships, as well as affect and mood instability.
“The fact that one-fourth of individuals with CNCP could have co-occurring BPD underscores the need for improved access to good psychological care,” lead investigator Fei Cao, MD, PhD, University of Missouri at Kansas City, said in an interview.
“If we treat the borderline personality disorder and address the psychiatric needs as well as the pain needs of the patient, then we will be able to treat their pain more successfully,” Cao said.
The findings were presented at the American Academy of Pain Medicine (AAPM) 2020 Annual Meeting.
Treatment resistance
Cao noted that a “significant number” of CNCP patients have at least some resistance to any type of pain treatment and speculated that BPD may increase treatment-resistant chronic pain.
Initially an anesthesiologist and pain medicine specialist, Cao later became a psychiatrist after recognizing the importance of addressing the underlying psychological needs of patients with chronic pain.
He noted that there is a strong psychological component to chronic pain and that many patients with chronic pain have suffered psychological trauma.
“You have to think about what may have happened to these patients. That is most important. I would not say these are difficult patients. I would say we just don’t know what happened to them,” he said.
To gain a better understanding of the prevalence of BPD in patients suffering from chronic pain and potentially provide some unexploited targets for chronic pain management, the investigators analyzed data from 11 studies published between 1994 and 2019. They found the prevalence of BPD among CNCP patients was 23.3%. Pain types included chronic headache (11.3%), arthritis (27.5%), and chronic spinal cord pain (24.3%).
We also have to treat their BPD. This can then make pain easier to control. Chronic pain management is often long-term and requires good compliance. A diagnosis of BPD might suggest poor compliance,” said Cao.
Screen for BPD
The study findings, he added, indicate a need to screen for BPD in patients with chronic pain. Interventions that are effective in the treatment of BPD and CNCP include cognitive-behavioral therapy, dialectical behavior therapy, antidepressants, and anticonvulsants.
“These should be considered as the first-line treatment in persons with comorbid pain and BPD,” Cao said.
Commenting on the findings, Ann E. Hansen, DVM, MD, Chronic Pain Wellness Center, Phoenix VA Health Care System, Arizona, said the study illustrates the multifactorial nature of chronic pain syndromes, and underscores the importance of a multidisciplinary approach to evaluation and treatment.
“The authors present data showing that BPD is a common diagnosis in patients with chronic pain, thus raising provider awareness to consider BPD and to involve behavioral health colleagues in comanaging these complex patients to achieve optimal outcomes,” Hansen said.
Cao and Hansen have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
SOURCE: Cao F et al. American Academy of Pain Medicine (AAPM) 2020 Annual Meeting, Abstract 505.
REPORTING FROM THE AAPM 2020 ANNUAL MEETING
Brain imaging offers new insight into persistent antisocial behavior
Individuals who exhibit antisocial behavior over a lifetime have a thinner cortex and smaller surface area in key brain regions relative to their counterparts who do not engage in antisocial behavior, new research shows.
However, investigators found no widespread structural brain abnormalities in the group of individuals who exhibited antisocial behavior only during adolescence.
These brain differences seem to be “quite specific and unique” to individuals who exhibit persistent antisocial behavior over their life, lead researcher Christina O. Carlisi, PhD, of University College London, said during a press briefing.
“Critically, the findings don’t directly link brain structure abnormalities to antisocial behavior,” she said. Nor do they mean that anyone with a smaller brain or brain area is destined to be antisocial or to commit a crime.
“Our findings support the idea that, for the small proportion of individuals with life-course–persistent antisocial behavior, there may be differences in their brain structure that make it difficult for them to develop social skills that prevent them from engaging in antisocial behavior,” Dr. Carlisi said in a news release. “These people could benefit from more support throughout their lives.”
It was published online Feb. 17 in the Lancet Psychiatry (doi: 10.1016/S2215-0366[20]30002-X).
Support for second chances
Speaking at the press briefing, coauthor Terrie E. Moffitt, PhD, of Duke University, Durham, N.C., said it’s well known that most young criminals are between the ages of 16 and 25.
Breaking the law is not at all rare in this age group, but not all of these young offenders are alike, she noted. Only a few become persistent repeat offenders.
“They start as a young child with aggressive conduct problems and eventually sink into a long-term lifestyle of repetitive serious crime that lasts well into adulthood, but this is a small group,” Dr. Moffitt explained. “In contrast, the larger majority of offenders will have only a short-term brush with lawbreaking and then grow up to become law-abiding members of society.”
The current study suggests that what makes short-term offenders behave differently from long-term offenders might involve some vulnerability at the level of the structure of the brain, Dr. Moffitt said.
The findings stem from 672 individuals in the Dunedin Multidisciplinary Health and Development Study, a population-representative, longitudinal birth cohort that assesses health and behavior.
On the basis of reports from parents, care givers, and teachers, as well as self-reports of conduct problems in persons aged 7-26 years, 80 participants (12%) had “life-course–persistent” antisocial behavior, 151 (23%) had adolescent-only antisocial behavior, and 441 (66%) had “low” antisocial behavior (control group, whose members never had a pervasive or persistent pattern of antisocial behavior).
Brain MRI obtained at age 45 years showed that, among individuals with persistent antisocial behavior, mean surface area was smaller (95% confidence interval, –0.24 to –0.11; P less than .0001) and mean cortical thickness was lower (95% CI, –0.19 to –0.02; P = .020) than was those of their peers in the control group.
For those in the life-course–persistent group, surface area was reduced in 282 of 360 anatomically defined brain parcels, and cortex was thinner in 11 of 360 parcels encompassing frontal and temporal regions (which were associated with executive function, emotion regulation, and motivation), compared with the control group.
Widespread differences in brain surface morphometry were not found in those who exhibited antisocial behavior during adolescence only. Such behavior was likely the result of their having to navigate through socially tough years.
“These findings underscore prior research that really highlights that there are different types of young offenders. They are not all the same; they should not all be treated the same,” coauthor Essi Viding, PhD, who also is affiliated with University College London, told reporters.
The findings support current strategies aimed at giving young offenders “a second chance” as opposed to enforcing harsher policies that prioritize incarceration for all young offenders, Dr. Viding added.
Important contribution
The authors of an accompanying commentary noted that, despite “remarkable progress in the past 3 decades, the etiology of antisocial behavior remains elusive” (Lancet Psychiatry. 2020 Feb 17. doi: 10.1016/S2215-0366[20]30035-3).
This study makes “an important contribution by identifying structural brain correlates of antisocial behavior that could be used to differentiate among individuals with life-course-persistent antisocial behavior, those with adolescence-limited antisocial behavior, and non-antisocial controls,” write Inti A. Brazil, PhD, of the Donders Institute for Brain, Cognition and Behavior, Radboud University, Nijmegen, the Netherlands, and Macià Buades-Rotger, PhD, of the Institute of Psychology II, University of Lübeck, Germany.
They noted that the findings might help to move the field closer to achieving the long-standing goal of incorporating neural data into assessment protocols for antisocial behavior.
The discovery of “meaningful morphologic differences between individuals with life-course–persistent and adolescence-limited antisocial behavior offers an important advance in the use of brain metrics for differentiating among individuals with antisocial dispositions.
“Importantly, however, it remains to be determined whether and how measuring the brain can be used to bridge the different taxometric views and theories on the etiology of antisocial behavior,” Dr. Brazil and Dr. Buades-Rotger concluded.
The study was funded by the U.S. National Institute on Aging; the Health Research Council of New Zealand; the New Zealand Ministry of Business, Innovation and Employment; the U.K. Medical Research Council; the Avielle Foundation; and the Wellcome Trust. The study authors and the authors of the commentary disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Individuals who exhibit antisocial behavior over a lifetime have a thinner cortex and smaller surface area in key brain regions relative to their counterparts who do not engage in antisocial behavior, new research shows.
However, investigators found no widespread structural brain abnormalities in the group of individuals who exhibited antisocial behavior only during adolescence.
These brain differences seem to be “quite specific and unique” to individuals who exhibit persistent antisocial behavior over their life, lead researcher Christina O. Carlisi, PhD, of University College London, said during a press briefing.
“Critically, the findings don’t directly link brain structure abnormalities to antisocial behavior,” she said. Nor do they mean that anyone with a smaller brain or brain area is destined to be antisocial or to commit a crime.
“Our findings support the idea that, for the small proportion of individuals with life-course–persistent antisocial behavior, there may be differences in their brain structure that make it difficult for them to develop social skills that prevent them from engaging in antisocial behavior,” Dr. Carlisi said in a news release. “These people could benefit from more support throughout their lives.”
It was published online Feb. 17 in the Lancet Psychiatry (doi: 10.1016/S2215-0366[20]30002-X).
Support for second chances
Speaking at the press briefing, coauthor Terrie E. Moffitt, PhD, of Duke University, Durham, N.C., said it’s well known that most young criminals are between the ages of 16 and 25.
Breaking the law is not at all rare in this age group, but not all of these young offenders are alike, she noted. Only a few become persistent repeat offenders.
“They start as a young child with aggressive conduct problems and eventually sink into a long-term lifestyle of repetitive serious crime that lasts well into adulthood, but this is a small group,” Dr. Moffitt explained. “In contrast, the larger majority of offenders will have only a short-term brush with lawbreaking and then grow up to become law-abiding members of society.”
The current study suggests that what makes short-term offenders behave differently from long-term offenders might involve some vulnerability at the level of the structure of the brain, Dr. Moffitt said.
The findings stem from 672 individuals in the Dunedin Multidisciplinary Health and Development Study, a population-representative, longitudinal birth cohort that assesses health and behavior.
On the basis of reports from parents, care givers, and teachers, as well as self-reports of conduct problems in persons aged 7-26 years, 80 participants (12%) had “life-course–persistent” antisocial behavior, 151 (23%) had adolescent-only antisocial behavior, and 441 (66%) had “low” antisocial behavior (control group, whose members never had a pervasive or persistent pattern of antisocial behavior).
Brain MRI obtained at age 45 years showed that, among individuals with persistent antisocial behavior, mean surface area was smaller (95% confidence interval, –0.24 to –0.11; P less than .0001) and mean cortical thickness was lower (95% CI, –0.19 to –0.02; P = .020) than was those of their peers in the control group.
For those in the life-course–persistent group, surface area was reduced in 282 of 360 anatomically defined brain parcels, and cortex was thinner in 11 of 360 parcels encompassing frontal and temporal regions (which were associated with executive function, emotion regulation, and motivation), compared with the control group.
Widespread differences in brain surface morphometry were not found in those who exhibited antisocial behavior during adolescence only. Such behavior was likely the result of their having to navigate through socially tough years.
“These findings underscore prior research that really highlights that there are different types of young offenders. They are not all the same; they should not all be treated the same,” coauthor Essi Viding, PhD, who also is affiliated with University College London, told reporters.
The findings support current strategies aimed at giving young offenders “a second chance” as opposed to enforcing harsher policies that prioritize incarceration for all young offenders, Dr. Viding added.
Important contribution
The authors of an accompanying commentary noted that, despite “remarkable progress in the past 3 decades, the etiology of antisocial behavior remains elusive” (Lancet Psychiatry. 2020 Feb 17. doi: 10.1016/S2215-0366[20]30035-3).
This study makes “an important contribution by identifying structural brain correlates of antisocial behavior that could be used to differentiate among individuals with life-course-persistent antisocial behavior, those with adolescence-limited antisocial behavior, and non-antisocial controls,” write Inti A. Brazil, PhD, of the Donders Institute for Brain, Cognition and Behavior, Radboud University, Nijmegen, the Netherlands, and Macià Buades-Rotger, PhD, of the Institute of Psychology II, University of Lübeck, Germany.
They noted that the findings might help to move the field closer to achieving the long-standing goal of incorporating neural data into assessment protocols for antisocial behavior.
The discovery of “meaningful morphologic differences between individuals with life-course–persistent and adolescence-limited antisocial behavior offers an important advance in the use of brain metrics for differentiating among individuals with antisocial dispositions.
“Importantly, however, it remains to be determined whether and how measuring the brain can be used to bridge the different taxometric views and theories on the etiology of antisocial behavior,” Dr. Brazil and Dr. Buades-Rotger concluded.
The study was funded by the U.S. National Institute on Aging; the Health Research Council of New Zealand; the New Zealand Ministry of Business, Innovation and Employment; the U.K. Medical Research Council; the Avielle Foundation; and the Wellcome Trust. The study authors and the authors of the commentary disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Individuals who exhibit antisocial behavior over a lifetime have a thinner cortex and smaller surface area in key brain regions relative to their counterparts who do not engage in antisocial behavior, new research shows.
However, investigators found no widespread structural brain abnormalities in the group of individuals who exhibited antisocial behavior only during adolescence.
These brain differences seem to be “quite specific and unique” to individuals who exhibit persistent antisocial behavior over their life, lead researcher Christina O. Carlisi, PhD, of University College London, said during a press briefing.
“Critically, the findings don’t directly link brain structure abnormalities to antisocial behavior,” she said. Nor do they mean that anyone with a smaller brain or brain area is destined to be antisocial or to commit a crime.
“Our findings support the idea that, for the small proportion of individuals with life-course–persistent antisocial behavior, there may be differences in their brain structure that make it difficult for them to develop social skills that prevent them from engaging in antisocial behavior,” Dr. Carlisi said in a news release. “These people could benefit from more support throughout their lives.”
It was published online Feb. 17 in the Lancet Psychiatry (doi: 10.1016/S2215-0366[20]30002-X).
Support for second chances
Speaking at the press briefing, coauthor Terrie E. Moffitt, PhD, of Duke University, Durham, N.C., said it’s well known that most young criminals are between the ages of 16 and 25.
Breaking the law is not at all rare in this age group, but not all of these young offenders are alike, she noted. Only a few become persistent repeat offenders.
“They start as a young child with aggressive conduct problems and eventually sink into a long-term lifestyle of repetitive serious crime that lasts well into adulthood, but this is a small group,” Dr. Moffitt explained. “In contrast, the larger majority of offenders will have only a short-term brush with lawbreaking and then grow up to become law-abiding members of society.”
The current study suggests that what makes short-term offenders behave differently from long-term offenders might involve some vulnerability at the level of the structure of the brain, Dr. Moffitt said.
The findings stem from 672 individuals in the Dunedin Multidisciplinary Health and Development Study, a population-representative, longitudinal birth cohort that assesses health and behavior.
On the basis of reports from parents, care givers, and teachers, as well as self-reports of conduct problems in persons aged 7-26 years, 80 participants (12%) had “life-course–persistent” antisocial behavior, 151 (23%) had adolescent-only antisocial behavior, and 441 (66%) had “low” antisocial behavior (control group, whose members never had a pervasive or persistent pattern of antisocial behavior).
Brain MRI obtained at age 45 years showed that, among individuals with persistent antisocial behavior, mean surface area was smaller (95% confidence interval, –0.24 to –0.11; P less than .0001) and mean cortical thickness was lower (95% CI, –0.19 to –0.02; P = .020) than was those of their peers in the control group.
For those in the life-course–persistent group, surface area was reduced in 282 of 360 anatomically defined brain parcels, and cortex was thinner in 11 of 360 parcels encompassing frontal and temporal regions (which were associated with executive function, emotion regulation, and motivation), compared with the control group.
Widespread differences in brain surface morphometry were not found in those who exhibited antisocial behavior during adolescence only. Such behavior was likely the result of their having to navigate through socially tough years.
“These findings underscore prior research that really highlights that there are different types of young offenders. They are not all the same; they should not all be treated the same,” coauthor Essi Viding, PhD, who also is affiliated with University College London, told reporters.
The findings support current strategies aimed at giving young offenders “a second chance” as opposed to enforcing harsher policies that prioritize incarceration for all young offenders, Dr. Viding added.
Important contribution
The authors of an accompanying commentary noted that, despite “remarkable progress in the past 3 decades, the etiology of antisocial behavior remains elusive” (Lancet Psychiatry. 2020 Feb 17. doi: 10.1016/S2215-0366[20]30035-3).
This study makes “an important contribution by identifying structural brain correlates of antisocial behavior that could be used to differentiate among individuals with life-course-persistent antisocial behavior, those with adolescence-limited antisocial behavior, and non-antisocial controls,” write Inti A. Brazil, PhD, of the Donders Institute for Brain, Cognition and Behavior, Radboud University, Nijmegen, the Netherlands, and Macià Buades-Rotger, PhD, of the Institute of Psychology II, University of Lübeck, Germany.
They noted that the findings might help to move the field closer to achieving the long-standing goal of incorporating neural data into assessment protocols for antisocial behavior.
The discovery of “meaningful morphologic differences between individuals with life-course–persistent and adolescence-limited antisocial behavior offers an important advance in the use of brain metrics for differentiating among individuals with antisocial dispositions.
“Importantly, however, it remains to be determined whether and how measuring the brain can be used to bridge the different taxometric views and theories on the etiology of antisocial behavior,” Dr. Brazil and Dr. Buades-Rotger concluded.
The study was funded by the U.S. National Institute on Aging; the Health Research Council of New Zealand; the New Zealand Ministry of Business, Innovation and Employment; the U.K. Medical Research Council; the Avielle Foundation; and the Wellcome Trust. The study authors and the authors of the commentary disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Bipolar disorder or borderline personality disorder?
Although evidence suggests that bipolar disorder (BD) and borderline personality disorder (BPD) are distinct entities, their differential diagnosis is often challenging as a result of considerable overlap of phenotypical features. Moreover, BD and BPD frequently co-occur, which makes it even more difficult to differentiate these 2 conditions. Strategies for improving diagnostic accuracy are critical to optimizing patients’ clinical outcomes and long-term prognosis. Misdiagnosing these 2 conditions can be particularly deleterious, and failure to recognize their co-occurrence can result in additional burden to typically complex and severe clinical presentations.
This article describes key aspects of the differential diagnosis between BD and BPD, emphasizing core features and major dissimilarities between these 2 conditions, and discusses the implications of misdiagnosis. The goal is to highlight the clinical and psychopathological aspects of BD and BPD to help clinicians properly distinguish these 2 disorders.
Psychopathological and sociodemographic correlates
Bipolar disorder is a chronic and severe mental illness that is classified based on clusters of symptoms—manic, hypomanic, and depressive.1 It is among the 10 leading causes of disability worldwide, with significant morbidity arising from acute affective episodes and subacute states.2 Data suggest the lifetime prevalence of BPD is 2.1%, and subthreshold forms may affect an additional 2.4% of the US population.3 The onset of symptoms typically occurs during late adolescence or early adulthood, and mood lability and cyclothymic temperament are the most common prodromal features.4
In contrast, personality disorders, such as BPD, are characteristically pervasive and maladaptive patterns of emotional responses that usually deviate from an individual’s stage of development and cultural background.1 These disorders tend to cause significant impairment, particularly in personal, occupational, and social domains. Environmental factors, such as early childhood trauma, seem to play an important role in the genesis of personality disorders, which may be particularly relevant in BPD, a disorder characterized by marked impulsivity and a pattern of instability in personal relationships, self-image, and affect.1,5,6 Similarly to BD, BPD is also chronic and highly disabling.
According to the National Survey on Alcohol and Related Conditions (NESARC), approximately 15% of US adults were found to have at least one type of personality disorder, and 6% met criteria for a cluster B personality disorder (antisocial, borderline, narcissistic, and histrionic).7 The lifetime prevalence of BPD is nearly 2%, with higher estimates observed in psychiatric settings.7,8
As a result of the phenotypical resemblance between BD and BPD (Figure), the differential diagnosis is often difficult. Recent studies suggest that co-occurrence of BD and BPD is common, with rates of comorbid BPD as high as 29% in BD I and 24% in BD II.8,9 On the other hand, nearly 20% of individuals with BPD seem to have comorbid BD.8,9 Several studies suggest that comorbid personality disorders represent a negative prognostic factor in the course of mood disorders, and the presence of BPD in patients with BD seems to be associated with more severe clinical presentations, greater treatment complexity, a higher number of depressive episodes, poor inter-episode functioning, and higher rates of other comorbidities, such as substance use disorders (SUDs).8-11 The effect of BD on the course of BPD is unclear and fairly unexplored, although it has been suggested that better control of mood symptoms may lead to more stable psychosocial functioning in BPD.9
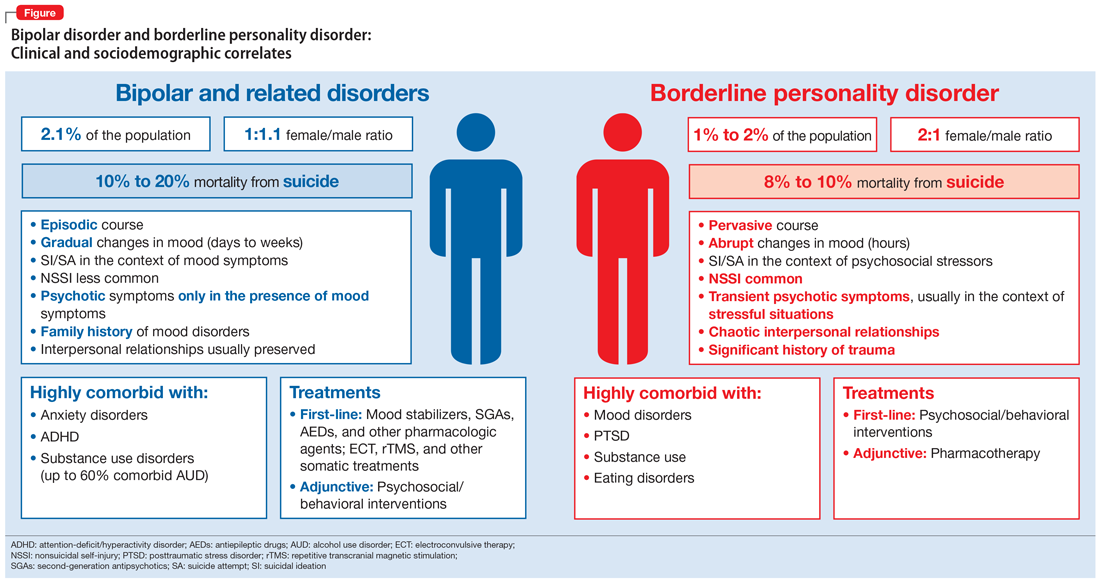
Whether BD and BPD are part of the same spectrum is a matter for debate.12-14 Multidimensional approaches have been proposed to better characterize these disorders in at-risk populations, based on structured interviews, self-administered and clinician-rated clinical scales (Table 1), neuroimaging studies, biological markers, and machine-learning models.15,16 Compelling evidence suggests that BD and BPD have distinct underlying neurobiological and psychopathological mechanisms12,13; however, the differential diagnosis still relies on phenotypical features, since the search for biological markers has not yet identified specific biomarkers that can be used in clinical practice.
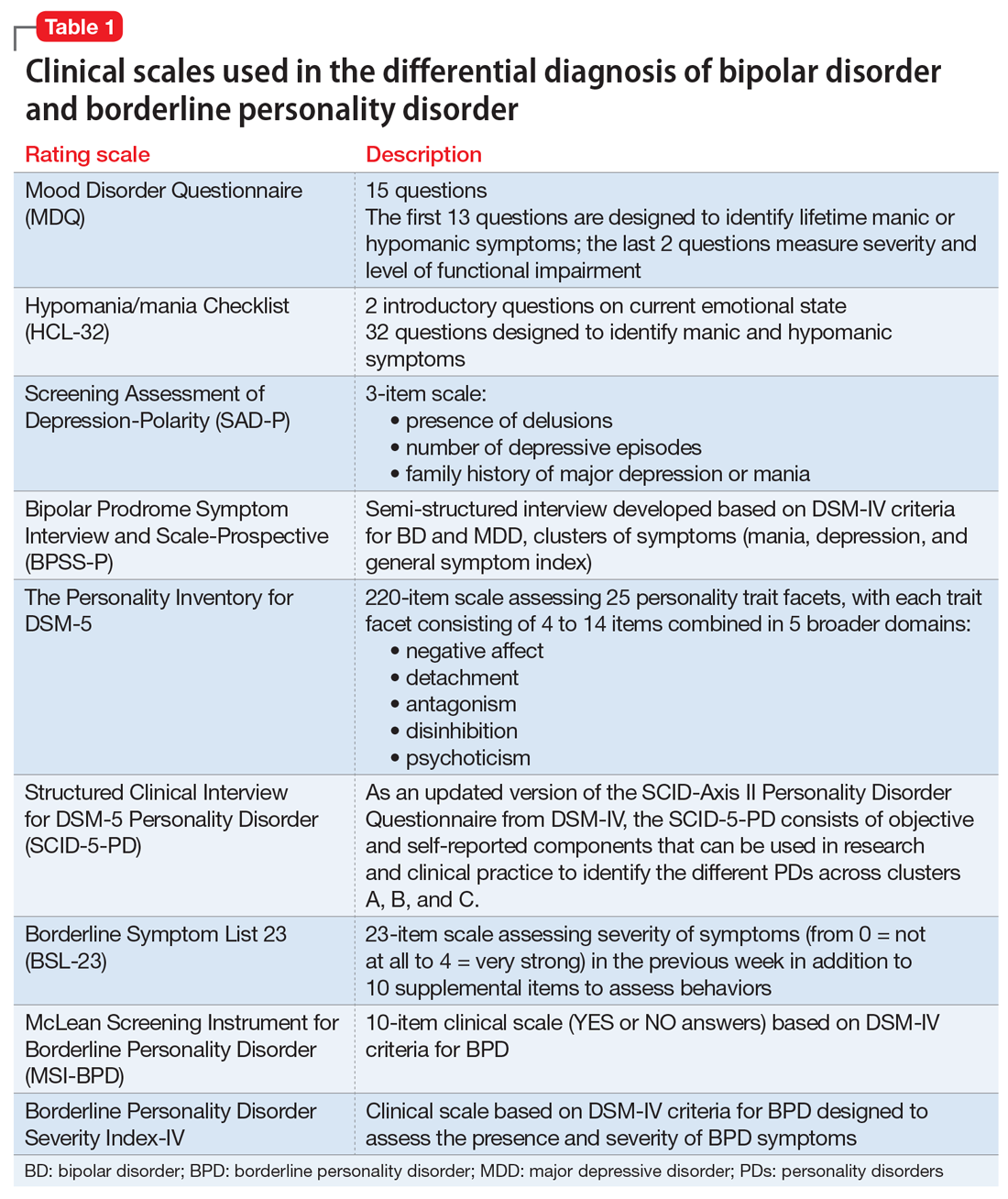
Continue to: Core features of BPD...
Core features of BPD, such as mood lability, impulsivity, and risk-taking behaviors, are also part of the diagnostic criteria for BD (Table 2).1 Similarly, depressive symptoms prevail in the course of BD.17,18 This adds complexity to the differential because “depressivity” is also part of the diagnostic criteria for BPD.1 Therefore, comprehensive psychiatric assessments and longitudinal observations are critical to diagnostic accuracy and treatment planning. Further characterization of symptoms, such as onset patterns, clinical course, phenomenology of symptoms (eg, timing, frequency, duration, triggers), and personality traits, will provide information to properly distinguish these 2 syndromes when, for example, it is unclear if the “mood swings” and impulsivity are part of a mood or a personality disorder (Table 3).
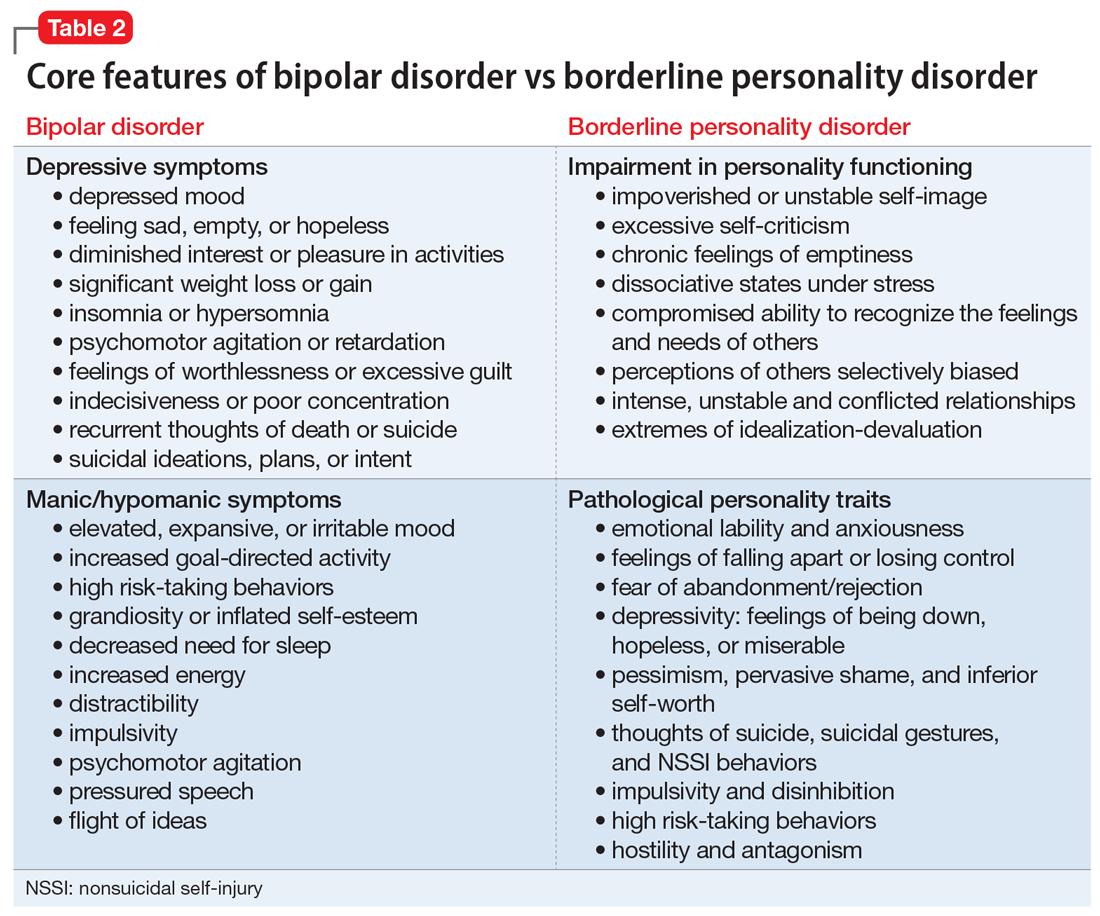
Clinical features: A closer look
Borderline personality disorder. Affect dysregulation, emotional instability, impoverished and unstable self-image, and chronic feelings of emptiness are core features of BPD.1,5,19 These characteristics, when combined with a fear of abandonment or rejection, a compromised ability to recognize the feelings and needs of others, and extremes of idealization-devaluation, tend to culminate in problematic and chaotic relationships.6,19 Individuals with BPD may become suspicious or paranoid under stressful situations. Under these circumstances, individuals with BPD may also experience depersonalization and other dissociative symptoms.6,20 The mood lability and emotional instability observed in patients with BPD usually are in response to environmental factors, and although generally intense and out of proportion, they tend to be ephemeral and short-lived, typically lasting a few hours.1,5 The anxiety and depressive symptoms reported by patients with BPD frequently are associated with feelings of “falling apart” or “losing control,” pessimism, shame, and low self-esteem. Coping strategies tend to be poorly developed and/or maladaptive, and individuals with BPD usually display a hostile and antagonistic demeanor and engage in suicidal or nonsuicidal self-injury (NSSI) behaviors as means to alleviate overwhelming emotional distress. Impulsivity, disinhibition, poor tolerance to frustration, and risk-taking behaviors are also characteristic of BPD.1,5 As a result, BPD is usually associated with significant impairment in functioning, multiple hospitalizations, and high rates of comorbid mood disorders, posttraumatic stress disorder (PTSD), SUDs, and death by suicide.
Bipolar disorder. Conversely, the fluctuations in mood and affect observed in patients with BD are usually episodic rather than pervasive, and tend to last longer (typically days to weeks) compared with the transient mood shifts observed in patients with BPD.4,17,18 The impulsivity, psychomotor agitation, and increased goal-directed activity reported by patients with BD are usually seen in the context of an acute affective episode, and are far less common during periods of stability or euthymic affect.4,17,18 Grandiosity and inflated self-esteem—hallmarks of a manic or hypomanic state—seem to oppose the unstable self-image observed in BPD, although indecisiveness and low self-worth may be observed in individuals with BD during depressive episodes. Antidepressant-induced mania or hypomania, atypical depressive episodes, and disruptions in sleep and circadian rhythms may be predictors of BD.4,21 Furthermore, although psychosocial stressors may be associated with acute affective episodes in early stages of bipolar illness, over time minimal stressors are necessary to ignite new affective episodes.22,23 Although BD is associated with high rates of suicide, suicide attempts are usually seen in the context of an acute depressive episode, and NSSI behaviors are less common among patients with BD.24
Lastly, other biographical data, such as a history of early life trauma, comorbidity, and a family history of psychiatric illnesses, can be particularly helpful in establishing the differential diagnosis between BD and BPD.25 For instance, evidence suggests that the heritability of BD may be as high as 70%, which usually translates into an extensive family history of bipolar and related disorders.26 In addition, studies suggest a high co-occurrence of anxiety disorders, attention-deficit/hyperactivity disorder, and SUDs in patients with BD, whereas PTSD, SUDs, and eating disorders tend to be highly comorbid with BPD.27 Childhood adversity (ie, a history of physical, sexual, or emotional abuse, or neglect) seems to be pivotal in the genesis of BPD and may predispose these individuals to psychotic and dissociative symptoms, particularly those with a history of sexual abuse, while playing a more secondary role in BD.28-31
Implications of misdiagnosis
In the view of the limitations of the existing models, multidimensional approaches are necessary to improve diagnostic accuracy. Presently, the differential diagnosis of BD and BPD continues to rely on clinical findings and syndromic classifications. Misdiagnosing BD and BPD has adverse therapeutic and prognostic implications.32 For instance, while psychotropic medications and neuromodulatory therapies (eg, electroconvulsive therapy, repetitive transcranial magnetic stimulation) are considered first-line treatments for patients with BD, psychosocial interventions tend to be adjunctive treatments in BD.33 Conversely, although pharmacotherapy might be helpful for patients with BPD, psychosocial and behavioral interventions are the mainstay treatment for this disorder, with the strongest evidence supporting cognitive-behavioral therapy, dialectical behavioral therapy, mentalization-based therapy, and transference-focused therapy.34-36 Thus, misdiagnosing BD as BPD with comorbid depression may result in the use of antidepressants, which can be detrimental in BD. Antidepressant treatment of BD, particularly as monotherapy, has been associated with manic or hypomanic switch, mixed states, and frequent cycling.21 Moreover, delays in diagnosis and proper treatment of BD may result in protracted mood symptoms, prolonged affective episodes, higher rates of disability, functional impairment, and overall worse clinical outcomes.24 In addition, because behavioral and psychosocial interventions are usually adjunctive therapies rather than first-line interventions for patients with BD, misdiagnosing BPD as BD may ultimately prevent these individuals from receiving proper treatment, likely resulting in more severe functional impairment, multiple hospitalizations, self-inflicted injuries, and suicide attempts, since psychotropic medications are not particularly effective for improving self-efficacy and coping strategies, nor for correcting cognitive distortions, particularly in self-image, and pathological personality traits, all of which are critical aspects of BPD treatment.
Continue to: Several factors might...
Several factors might make clinicians reluctant to diagnose BPD, or bias them to diagnose BD more frequently. These include a lack of familiarity with the diagnostic criteria for BPD, the phenotypical resemblance between BP and BPD, or even concerns about the stigma and negative implications that are associated with a BPD diagnosis.32,37,38
Whereas BD is currently perceived as a condition with a strong biological basis, there are considerable misconceptions regarding BPD and its nature.4-6,22,26 As a consequence, individuals with BPD tend to be perceived as “difficult-to-treat,” “uncooperative,” or “attention-seeking.” These misconceptions may result in poor clinician-patient relationships, unmet clinical and psychiatric needs, and frustration for both clinicians and patients.37
Through advances in biological psychiatry, precision medicine may someday be a part of psychiatric practice. Biological “signatures” may eventually help clinicians in diagnosing and treating psychiatric disorders. Presently, however, rigorous history-taking and comprehensive clinical assessments are still the most powerful tools a clinician can use to accomplish these goals. Finally, destigmatizing psychiatric disorders and educating patients and clinicians are also critical to improving clinical outcomes and promoting mental health in a compassionate and empathetic fashion.
Bottom Line
Despite the phenotypical resemblance between bipolar disorder (BP) and borderline personality disorder (BPD), the 2 are independent conditions with distinct neurobiological and psychopathological underpinnings. Clinicians can use a rigorous assessment of pathological personality traits and characterization of symptoms, such as onset patterns, clinical course, and phenomenology, to properly distinguish between BP and BPD.
Related Resources
- Fiedorowicz JG, Black DW. Borderline, bipolar, or both? Frame your diagnosis on the patient history. Current Psychiatry. 2010; 9(1):21-24,29-32.
- Zimmerman M. Improving the recognition of borderline personality disorder. Current Psychiatry. 2017;16(10):13-19.
- National Institute of Mental Health. Overview on bipolar disorder. www.nimh.nih.gov/health/publications/bipolar-disorder/index.shtml. Revised October 2018.
- National Institute of Mental Health. Overview on borderline personality disorder. www.nimh.nih.gov/health/publications/borderline-personality-disorder-fact-sheet/index.shtml.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Whiteford HA, Degenhardt L, Rehm J, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1575-1586.
3. Merikangas KR, Akiskal HS, Angst J, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2007;64(5):543-552.
4. Malhi GS, Bargh DM, Coulston CM, et al. Predicting bipolar disorder on the basis of phenomenology: implications for prevention and early intervention. Bipolar Disord. 2014;16(5):455-470.
5. Skodol AE, Gunderson JG, Pfohl B, et al. The borderline diagnosis I: psychopathology. Biol Psychiatry. 2002;51(12):936-950.
6. Skodol AE, Siever LJ, Livesley WJ, et al. The borderline diagnosis II: biology, genetics, and clinical course. Biol Psychiatry. 2002;51(12):951-963.
7. Hasin DS, Grant BF. The National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Waves 1 and 2: review and summary of findings. Soc Psychiatry Psychiatr Epidemiol. 2015;50(11):1609-1640.
8. McDermid J, Sareen J, El-Gabalawy R, et al. Co-morbidity of bipolar disorder and borderline personality disorder: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Compr Psychiatry. 2015;58:18-28.
9. Gunderson JG, Weinberg I, Daversa MT, et al. Descriptive and longitudinal observations on the relationship of borderline personality disorder and bipolar disorder. Am J Psychiatry. 2006;163(7):1173-1178.
10. Swartz HA, Pilkonis PA, Frank E, et al. Acute treatment outcomes in patients with bipolar I disorder and co-morbid borderline personality disorder receiving medication and psychotherapy. Bipolar Disord. 2005;7(2):192-197.
11. Riemann G, Weisscher N, Post RM, et al. The relationship between self-reported borderline personality features and prospective illness course in bipolar disorder. Int J Bipolar Disord. 2017;5(1):31.
12. de la Rosa I, Oquendo MA, García G, et al. Determining if borderline personality disorder and bipolar disorder are alternative expressions of the same disorder. J Clin Psychiatry. 2017;778(8):e994-e999. doi: 10.4088/JCP.16m11190.
13. di Giacomo E, Aspesi F, Fotiadou M, et al. Unblending borderline personality and bipolar disorders. J Psychiatr Res. 2017;91:90-97.
14. Parker G, Bayes A, McClure G, et al. Clinical status of comorbid bipolar disorder and borderline personality disorder. Br J Psychiatry. 2016;209(3):209-215.
15. Perez Arribas I, Goodwin GM, Geddes JR, et al. A signature-based machine learning model for distinguishing bipolar disorder and borderline personality disorder. Transl Psychiatry. 2018;8(1):274.
16. Insel T, Cuthbert B, Garvey M, et al. Research Domain Criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167(7):748-751.
17. Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530-537.
18. Judd LL, Akiskal HS, Schettler PJ, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry. 2003;60(3):261-269.
19. Oldham JM, Skodol AE, Bender DS. A current integrative perspective on personality disorders. American Psychiatric Publishing, Inc. 2005.
20. Herzog JI, Schmahl C. Adverse childhood experiences and the consequences on neurobiological, psychosocial, and somatic conditions across the lifespan. Front Psychiatry. 2018;9:420.
21. Barbuti M, Pacchiarotti I, Vieta E, et al. Antidepressant-induced hypomania/mania in patients with major depression: evidence from the BRIDGE-II-MIX study. J Affect Disord. 2017;219:187-192.
22. Post RM. Mechanisms of illness progression in the recurrent affective disorders. Neurotox Res. 2010;18(3-4):256-271.
23. da Costa SC, Passos IC, Lowri C, et al. Refractory bipolar disorder and neuroprogression. Prog Neuro-Psychopharmacology Biol Psychiatry. 2016;70:103-110.
24. Crump C, Sundquist K, Winkleby MA, et al. Comorbidities and mortality in bipolar disorder: a Swedish national cohort study. JAMA Psychiatry. 2013;70(9):931-939.
25. Zimmerman M, Martinez JH, Morgan TA, et al. Distinguishing bipolar II depression from major depressive disorder with comorbid borderline personality disorder: demographic, clinical, and family history differences. J Clin Psychiatry. 2013;74(9):880-886.
26. Hasler G, Drevets WC, Gould TD, et al. Toward constructing an endophenotype strategy for bipolar disorders. Biol Psychiatry. 2006;60(2):93-105.
27. Brieger P, Ehrt U, Marneros A. Frequency of comorbid personality disorders in bipolar and unipolar affective disorders. Compr Psychiatry. 2003;44(1):28-34.
28. Leverich GS, McElroy SL, Suppes T, et al. Early physical and sexual abuse associated with an adverse course of bipolar illness. Biol Psychiatry. 2002;51(4):288-297.
29. Leverich GS, Post RM. Course of bipolar illness after history of childhood trauma. Lancet. 2006;367(9516):1040-1042.
30. Golier JA, Yehuda R, Bierer LM, et al. The relationship of borderline personality disorder to posttraumatic stress disorder and traumatic events. Am J Psychiatry. 2003;160(11):2018-2024.
31. Nicol K, Pope M, Romaniuk L, et al. Childhood trauma, midbrain activation and psychotic symptoms in borderline personality disorder. Transl Psychiatry. 2015;5:e559. doi:10.1038/tp.2015.53.
32. Ruggero CJ, Zimmerman M, Chelminski I, et al. Borderline personality disorder and the misdiagnosis of bipolar disorder. J Psychiatr Res. 2010;44(6):405-408.
33. Geddes JR, Miklowitz DJ. Treatment of bipolar disorder. Lancet. 2013;381(9878):1672-1682.
34. McMain S, Korman LM, Dimeff L. Dialectical behavior therapy and the treatment of emotion dysregulation. J Clin Psychol. 2001;57(2):183-196.
35. Cristea IA, Gentili C, Cotet CD, et al. Efficacy of psychotherapies for borderline personality disorder: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74(4):319-328.
36. Linehan MM, Korslund KE, Harned MS, et al. Dialectical behavior therapy for high suicide risk in individuals with borderline personality disorder. JAMA Psychiatry. 2015;72(75);475-482.
37. LeQuesne ER, Hersh RG. Disclosure of a diagnosis of borderline personality disorder. J Psychiatr Pract. 2004:10(3):170-176.
38. Young AH. Bipolar disorder: diagnostic conundrums and associated comorbidities. J Clin Psychiatry. 2009;70(8):e26. doi:10.4088/jcp.7067br6c.
Although evidence suggests that bipolar disorder (BD) and borderline personality disorder (BPD) are distinct entities, their differential diagnosis is often challenging as a result of considerable overlap of phenotypical features. Moreover, BD and BPD frequently co-occur, which makes it even more difficult to differentiate these 2 conditions. Strategies for improving diagnostic accuracy are critical to optimizing patients’ clinical outcomes and long-term prognosis. Misdiagnosing these 2 conditions can be particularly deleterious, and failure to recognize their co-occurrence can result in additional burden to typically complex and severe clinical presentations.
This article describes key aspects of the differential diagnosis between BD and BPD, emphasizing core features and major dissimilarities between these 2 conditions, and discusses the implications of misdiagnosis. The goal is to highlight the clinical and psychopathological aspects of BD and BPD to help clinicians properly distinguish these 2 disorders.
Psychopathological and sociodemographic correlates
Bipolar disorder is a chronic and severe mental illness that is classified based on clusters of symptoms—manic, hypomanic, and depressive.1 It is among the 10 leading causes of disability worldwide, with significant morbidity arising from acute affective episodes and subacute states.2 Data suggest the lifetime prevalence of BPD is 2.1%, and subthreshold forms may affect an additional 2.4% of the US population.3 The onset of symptoms typically occurs during late adolescence or early adulthood, and mood lability and cyclothymic temperament are the most common prodromal features.4
In contrast, personality disorders, such as BPD, are characteristically pervasive and maladaptive patterns of emotional responses that usually deviate from an individual’s stage of development and cultural background.1 These disorders tend to cause significant impairment, particularly in personal, occupational, and social domains. Environmental factors, such as early childhood trauma, seem to play an important role in the genesis of personality disorders, which may be particularly relevant in BPD, a disorder characterized by marked impulsivity and a pattern of instability in personal relationships, self-image, and affect.1,5,6 Similarly to BD, BPD is also chronic and highly disabling.
According to the National Survey on Alcohol and Related Conditions (NESARC), approximately 15% of US adults were found to have at least one type of personality disorder, and 6% met criteria for a cluster B personality disorder (antisocial, borderline, narcissistic, and histrionic).7 The lifetime prevalence of BPD is nearly 2%, with higher estimates observed in psychiatric settings.7,8
As a result of the phenotypical resemblance between BD and BPD (Figure), the differential diagnosis is often difficult. Recent studies suggest that co-occurrence of BD and BPD is common, with rates of comorbid BPD as high as 29% in BD I and 24% in BD II.8,9 On the other hand, nearly 20% of individuals with BPD seem to have comorbid BD.8,9 Several studies suggest that comorbid personality disorders represent a negative prognostic factor in the course of mood disorders, and the presence of BPD in patients with BD seems to be associated with more severe clinical presentations, greater treatment complexity, a higher number of depressive episodes, poor inter-episode functioning, and higher rates of other comorbidities, such as substance use disorders (SUDs).8-11 The effect of BD on the course of BPD is unclear and fairly unexplored, although it has been suggested that better control of mood symptoms may lead to more stable psychosocial functioning in BPD.9

Whether BD and BPD are part of the same spectrum is a matter for debate.12-14 Multidimensional approaches have been proposed to better characterize these disorders in at-risk populations, based on structured interviews, self-administered and clinician-rated clinical scales (Table 1), neuroimaging studies, biological markers, and machine-learning models.15,16 Compelling evidence suggests that BD and BPD have distinct underlying neurobiological and psychopathological mechanisms12,13; however, the differential diagnosis still relies on phenotypical features, since the search for biological markers has not yet identified specific biomarkers that can be used in clinical practice.

Continue to: Core features of BPD...
Core features of BPD, such as mood lability, impulsivity, and risk-taking behaviors, are also part of the diagnostic criteria for BD (Table 2).1 Similarly, depressive symptoms prevail in the course of BD.17,18 This adds complexity to the differential because “depressivity” is also part of the diagnostic criteria for BPD.1 Therefore, comprehensive psychiatric assessments and longitudinal observations are critical to diagnostic accuracy and treatment planning. Further characterization of symptoms, such as onset patterns, clinical course, phenomenology of symptoms (eg, timing, frequency, duration, triggers), and personality traits, will provide information to properly distinguish these 2 syndromes when, for example, it is unclear if the “mood swings” and impulsivity are part of a mood or a personality disorder (Table 3).

Clinical features: A closer look
Borderline personality disorder. Affect dysregulation, emotional instability, impoverished and unstable self-image, and chronic feelings of emptiness are core features of BPD.1,5,19 These characteristics, when combined with a fear of abandonment or rejection, a compromised ability to recognize the feelings and needs of others, and extremes of idealization-devaluation, tend to culminate in problematic and chaotic relationships.6,19 Individuals with BPD may become suspicious or paranoid under stressful situations. Under these circumstances, individuals with BPD may also experience depersonalization and other dissociative symptoms.6,20 The mood lability and emotional instability observed in patients with BPD usually are in response to environmental factors, and although generally intense and out of proportion, they tend to be ephemeral and short-lived, typically lasting a few hours.1,5 The anxiety and depressive symptoms reported by patients with BPD frequently are associated with feelings of “falling apart” or “losing control,” pessimism, shame, and low self-esteem. Coping strategies tend to be poorly developed and/or maladaptive, and individuals with BPD usually display a hostile and antagonistic demeanor and engage in suicidal or nonsuicidal self-injury (NSSI) behaviors as means to alleviate overwhelming emotional distress. Impulsivity, disinhibition, poor tolerance to frustration, and risk-taking behaviors are also characteristic of BPD.1,5 As a result, BPD is usually associated with significant impairment in functioning, multiple hospitalizations, and high rates of comorbid mood disorders, posttraumatic stress disorder (PTSD), SUDs, and death by suicide.
Bipolar disorder. Conversely, the fluctuations in mood and affect observed in patients with BD are usually episodic rather than pervasive, and tend to last longer (typically days to weeks) compared with the transient mood shifts observed in patients with BPD.4,17,18 The impulsivity, psychomotor agitation, and increased goal-directed activity reported by patients with BD are usually seen in the context of an acute affective episode, and are far less common during periods of stability or euthymic affect.4,17,18 Grandiosity and inflated self-esteem—hallmarks of a manic or hypomanic state—seem to oppose the unstable self-image observed in BPD, although indecisiveness and low self-worth may be observed in individuals with BD during depressive episodes. Antidepressant-induced mania or hypomania, atypical depressive episodes, and disruptions in sleep and circadian rhythms may be predictors of BD.4,21 Furthermore, although psychosocial stressors may be associated with acute affective episodes in early stages of bipolar illness, over time minimal stressors are necessary to ignite new affective episodes.22,23 Although BD is associated with high rates of suicide, suicide attempts are usually seen in the context of an acute depressive episode, and NSSI behaviors are less common among patients with BD.24
Lastly, other biographical data, such as a history of early life trauma, comorbidity, and a family history of psychiatric illnesses, can be particularly helpful in establishing the differential diagnosis between BD and BPD.25 For instance, evidence suggests that the heritability of BD may be as high as 70%, which usually translates into an extensive family history of bipolar and related disorders.26 In addition, studies suggest a high co-occurrence of anxiety disorders, attention-deficit/hyperactivity disorder, and SUDs in patients with BD, whereas PTSD, SUDs, and eating disorders tend to be highly comorbid with BPD.27 Childhood adversity (ie, a history of physical, sexual, or emotional abuse, or neglect) seems to be pivotal in the genesis of BPD and may predispose these individuals to psychotic and dissociative symptoms, particularly those with a history of sexual abuse, while playing a more secondary role in BD.28-31
Implications of misdiagnosis
In the view of the limitations of the existing models, multidimensional approaches are necessary to improve diagnostic accuracy. Presently, the differential diagnosis of BD and BPD continues to rely on clinical findings and syndromic classifications. Misdiagnosing BD and BPD has adverse therapeutic and prognostic implications.32 For instance, while psychotropic medications and neuromodulatory therapies (eg, electroconvulsive therapy, repetitive transcranial magnetic stimulation) are considered first-line treatments for patients with BD, psychosocial interventions tend to be adjunctive treatments in BD.33 Conversely, although pharmacotherapy might be helpful for patients with BPD, psychosocial and behavioral interventions are the mainstay treatment for this disorder, with the strongest evidence supporting cognitive-behavioral therapy, dialectical behavioral therapy, mentalization-based therapy, and transference-focused therapy.34-36 Thus, misdiagnosing BD as BPD with comorbid depression may result in the use of antidepressants, which can be detrimental in BD. Antidepressant treatment of BD, particularly as monotherapy, has been associated with manic or hypomanic switch, mixed states, and frequent cycling.21 Moreover, delays in diagnosis and proper treatment of BD may result in protracted mood symptoms, prolonged affective episodes, higher rates of disability, functional impairment, and overall worse clinical outcomes.24 In addition, because behavioral and psychosocial interventions are usually adjunctive therapies rather than first-line interventions for patients with BD, misdiagnosing BPD as BD may ultimately prevent these individuals from receiving proper treatment, likely resulting in more severe functional impairment, multiple hospitalizations, self-inflicted injuries, and suicide attempts, since psychotropic medications are not particularly effective for improving self-efficacy and coping strategies, nor for correcting cognitive distortions, particularly in self-image, and pathological personality traits, all of which are critical aspects of BPD treatment.
Continue to: Several factors might...
Several factors might make clinicians reluctant to diagnose BPD, or bias them to diagnose BD more frequently. These include a lack of familiarity with the diagnostic criteria for BPD, the phenotypical resemblance between BP and BPD, or even concerns about the stigma and negative implications that are associated with a BPD diagnosis.32,37,38
Whereas BD is currently perceived as a condition with a strong biological basis, there are considerable misconceptions regarding BPD and its nature.4-6,22,26 As a consequence, individuals with BPD tend to be perceived as “difficult-to-treat,” “uncooperative,” or “attention-seeking.” These misconceptions may result in poor clinician-patient relationships, unmet clinical and psychiatric needs, and frustration for both clinicians and patients.37
Through advances in biological psychiatry, precision medicine may someday be a part of psychiatric practice. Biological “signatures” may eventually help clinicians in diagnosing and treating psychiatric disorders. Presently, however, rigorous history-taking and comprehensive clinical assessments are still the most powerful tools a clinician can use to accomplish these goals. Finally, destigmatizing psychiatric disorders and educating patients and clinicians are also critical to improving clinical outcomes and promoting mental health in a compassionate and empathetic fashion.
Bottom Line
Despite the phenotypical resemblance between bipolar disorder (BP) and borderline personality disorder (BPD), the 2 are independent conditions with distinct neurobiological and psychopathological underpinnings. Clinicians can use a rigorous assessment of pathological personality traits and characterization of symptoms, such as onset patterns, clinical course, and phenomenology, to properly distinguish between BP and BPD.
Related Resources
- Fiedorowicz JG, Black DW. Borderline, bipolar, or both? Frame your diagnosis on the patient history. Current Psychiatry. 2010; 9(1):21-24,29-32.
- Zimmerman M. Improving the recognition of borderline personality disorder. Current Psychiatry. 2017;16(10):13-19.
- National Institute of Mental Health. Overview on bipolar disorder. www.nimh.nih.gov/health/publications/bipolar-disorder/index.shtml. Revised October 2018.
- National Institute of Mental Health. Overview on borderline personality disorder. www.nimh.nih.gov/health/publications/borderline-personality-disorder-fact-sheet/index.shtml.
Although evidence suggests that bipolar disorder (BD) and borderline personality disorder (BPD) are distinct entities, their differential diagnosis is often challenging as a result of considerable overlap of phenotypical features. Moreover, BD and BPD frequently co-occur, which makes it even more difficult to differentiate these 2 conditions. Strategies for improving diagnostic accuracy are critical to optimizing patients’ clinical outcomes and long-term prognosis. Misdiagnosing these 2 conditions can be particularly deleterious, and failure to recognize their co-occurrence can result in additional burden to typically complex and severe clinical presentations.
This article describes key aspects of the differential diagnosis between BD and BPD, emphasizing core features and major dissimilarities between these 2 conditions, and discusses the implications of misdiagnosis. The goal is to highlight the clinical and psychopathological aspects of BD and BPD to help clinicians properly distinguish these 2 disorders.
Psychopathological and sociodemographic correlates
Bipolar disorder is a chronic and severe mental illness that is classified based on clusters of symptoms—manic, hypomanic, and depressive.1 It is among the 10 leading causes of disability worldwide, with significant morbidity arising from acute affective episodes and subacute states.2 Data suggest the lifetime prevalence of BPD is 2.1%, and subthreshold forms may affect an additional 2.4% of the US population.3 The onset of symptoms typically occurs during late adolescence or early adulthood, and mood lability and cyclothymic temperament are the most common prodromal features.4
In contrast, personality disorders, such as BPD, are characteristically pervasive and maladaptive patterns of emotional responses that usually deviate from an individual’s stage of development and cultural background.1 These disorders tend to cause significant impairment, particularly in personal, occupational, and social domains. Environmental factors, such as early childhood trauma, seem to play an important role in the genesis of personality disorders, which may be particularly relevant in BPD, a disorder characterized by marked impulsivity and a pattern of instability in personal relationships, self-image, and affect.1,5,6 Similarly to BD, BPD is also chronic and highly disabling.
According to the National Survey on Alcohol and Related Conditions (NESARC), approximately 15% of US adults were found to have at least one type of personality disorder, and 6% met criteria for a cluster B personality disorder (antisocial, borderline, narcissistic, and histrionic).7 The lifetime prevalence of BPD is nearly 2%, with higher estimates observed in psychiatric settings.7,8
As a result of the phenotypical resemblance between BD and BPD (Figure), the differential diagnosis is often difficult. Recent studies suggest that co-occurrence of BD and BPD is common, with rates of comorbid BPD as high as 29% in BD I and 24% in BD II.8,9 On the other hand, nearly 20% of individuals with BPD seem to have comorbid BD.8,9 Several studies suggest that comorbid personality disorders represent a negative prognostic factor in the course of mood disorders, and the presence of BPD in patients with BD seems to be associated with more severe clinical presentations, greater treatment complexity, a higher number of depressive episodes, poor inter-episode functioning, and higher rates of other comorbidities, such as substance use disorders (SUDs).8-11 The effect of BD on the course of BPD is unclear and fairly unexplored, although it has been suggested that better control of mood symptoms may lead to more stable psychosocial functioning in BPD.9

Whether BD and BPD are part of the same spectrum is a matter for debate.12-14 Multidimensional approaches have been proposed to better characterize these disorders in at-risk populations, based on structured interviews, self-administered and clinician-rated clinical scales (Table 1), neuroimaging studies, biological markers, and machine-learning models.15,16 Compelling evidence suggests that BD and BPD have distinct underlying neurobiological and psychopathological mechanisms12,13; however, the differential diagnosis still relies on phenotypical features, since the search for biological markers has not yet identified specific biomarkers that can be used in clinical practice.

Continue to: Core features of BPD...
Core features of BPD, such as mood lability, impulsivity, and risk-taking behaviors, are also part of the diagnostic criteria for BD (Table 2).1 Similarly, depressive symptoms prevail in the course of BD.17,18 This adds complexity to the differential because “depressivity” is also part of the diagnostic criteria for BPD.1 Therefore, comprehensive psychiatric assessments and longitudinal observations are critical to diagnostic accuracy and treatment planning. Further characterization of symptoms, such as onset patterns, clinical course, phenomenology of symptoms (eg, timing, frequency, duration, triggers), and personality traits, will provide information to properly distinguish these 2 syndromes when, for example, it is unclear if the “mood swings” and impulsivity are part of a mood or a personality disorder (Table 3).

Clinical features: A closer look
Borderline personality disorder. Affect dysregulation, emotional instability, impoverished and unstable self-image, and chronic feelings of emptiness are core features of BPD.1,5,19 These characteristics, when combined with a fear of abandonment or rejection, a compromised ability to recognize the feelings and needs of others, and extremes of idealization-devaluation, tend to culminate in problematic and chaotic relationships.6,19 Individuals with BPD may become suspicious or paranoid under stressful situations. Under these circumstances, individuals with BPD may also experience depersonalization and other dissociative symptoms.6,20 The mood lability and emotional instability observed in patients with BPD usually are in response to environmental factors, and although generally intense and out of proportion, they tend to be ephemeral and short-lived, typically lasting a few hours.1,5 The anxiety and depressive symptoms reported by patients with BPD frequently are associated with feelings of “falling apart” or “losing control,” pessimism, shame, and low self-esteem. Coping strategies tend to be poorly developed and/or maladaptive, and individuals with BPD usually display a hostile and antagonistic demeanor and engage in suicidal or nonsuicidal self-injury (NSSI) behaviors as means to alleviate overwhelming emotional distress. Impulsivity, disinhibition, poor tolerance to frustration, and risk-taking behaviors are also characteristic of BPD.1,5 As a result, BPD is usually associated with significant impairment in functioning, multiple hospitalizations, and high rates of comorbid mood disorders, posttraumatic stress disorder (PTSD), SUDs, and death by suicide.
Bipolar disorder. Conversely, the fluctuations in mood and affect observed in patients with BD are usually episodic rather than pervasive, and tend to last longer (typically days to weeks) compared with the transient mood shifts observed in patients with BPD.4,17,18 The impulsivity, psychomotor agitation, and increased goal-directed activity reported by patients with BD are usually seen in the context of an acute affective episode, and are far less common during periods of stability or euthymic affect.4,17,18 Grandiosity and inflated self-esteem—hallmarks of a manic or hypomanic state—seem to oppose the unstable self-image observed in BPD, although indecisiveness and low self-worth may be observed in individuals with BD during depressive episodes. Antidepressant-induced mania or hypomania, atypical depressive episodes, and disruptions in sleep and circadian rhythms may be predictors of BD.4,21 Furthermore, although psychosocial stressors may be associated with acute affective episodes in early stages of bipolar illness, over time minimal stressors are necessary to ignite new affective episodes.22,23 Although BD is associated with high rates of suicide, suicide attempts are usually seen in the context of an acute depressive episode, and NSSI behaviors are less common among patients with BD.24
Lastly, other biographical data, such as a history of early life trauma, comorbidity, and a family history of psychiatric illnesses, can be particularly helpful in establishing the differential diagnosis between BD and BPD.25 For instance, evidence suggests that the heritability of BD may be as high as 70%, which usually translates into an extensive family history of bipolar and related disorders.26 In addition, studies suggest a high co-occurrence of anxiety disorders, attention-deficit/hyperactivity disorder, and SUDs in patients with BD, whereas PTSD, SUDs, and eating disorders tend to be highly comorbid with BPD.27 Childhood adversity (ie, a history of physical, sexual, or emotional abuse, or neglect) seems to be pivotal in the genesis of BPD and may predispose these individuals to psychotic and dissociative symptoms, particularly those with a history of sexual abuse, while playing a more secondary role in BD.28-31
Implications of misdiagnosis
In the view of the limitations of the existing models, multidimensional approaches are necessary to improve diagnostic accuracy. Presently, the differential diagnosis of BD and BPD continues to rely on clinical findings and syndromic classifications. Misdiagnosing BD and BPD has adverse therapeutic and prognostic implications.32 For instance, while psychotropic medications and neuromodulatory therapies (eg, electroconvulsive therapy, repetitive transcranial magnetic stimulation) are considered first-line treatments for patients with BD, psychosocial interventions tend to be adjunctive treatments in BD.33 Conversely, although pharmacotherapy might be helpful for patients with BPD, psychosocial and behavioral interventions are the mainstay treatment for this disorder, with the strongest evidence supporting cognitive-behavioral therapy, dialectical behavioral therapy, mentalization-based therapy, and transference-focused therapy.34-36 Thus, misdiagnosing BD as BPD with comorbid depression may result in the use of antidepressants, which can be detrimental in BD. Antidepressant treatment of BD, particularly as monotherapy, has been associated with manic or hypomanic switch, mixed states, and frequent cycling.21 Moreover, delays in diagnosis and proper treatment of BD may result in protracted mood symptoms, prolonged affective episodes, higher rates of disability, functional impairment, and overall worse clinical outcomes.24 In addition, because behavioral and psychosocial interventions are usually adjunctive therapies rather than first-line interventions for patients with BD, misdiagnosing BPD as BD may ultimately prevent these individuals from receiving proper treatment, likely resulting in more severe functional impairment, multiple hospitalizations, self-inflicted injuries, and suicide attempts, since psychotropic medications are not particularly effective for improving self-efficacy and coping strategies, nor for correcting cognitive distortions, particularly in self-image, and pathological personality traits, all of which are critical aspects of BPD treatment.
Continue to: Several factors might...
Several factors might make clinicians reluctant to diagnose BPD, or bias them to diagnose BD more frequently. These include a lack of familiarity with the diagnostic criteria for BPD, the phenotypical resemblance between BP and BPD, or even concerns about the stigma and negative implications that are associated with a BPD diagnosis.32,37,38
Whereas BD is currently perceived as a condition with a strong biological basis, there are considerable misconceptions regarding BPD and its nature.4-6,22,26 As a consequence, individuals with BPD tend to be perceived as “difficult-to-treat,” “uncooperative,” or “attention-seeking.” These misconceptions may result in poor clinician-patient relationships, unmet clinical and psychiatric needs, and frustration for both clinicians and patients.37
Through advances in biological psychiatry, precision medicine may someday be a part of psychiatric practice. Biological “signatures” may eventually help clinicians in diagnosing and treating psychiatric disorders. Presently, however, rigorous history-taking and comprehensive clinical assessments are still the most powerful tools a clinician can use to accomplish these goals. Finally, destigmatizing psychiatric disorders and educating patients and clinicians are also critical to improving clinical outcomes and promoting mental health in a compassionate and empathetic fashion.
Bottom Line
Despite the phenotypical resemblance between bipolar disorder (BP) and borderline personality disorder (BPD), the 2 are independent conditions with distinct neurobiological and psychopathological underpinnings. Clinicians can use a rigorous assessment of pathological personality traits and characterization of symptoms, such as onset patterns, clinical course, and phenomenology, to properly distinguish between BP and BPD.
Related Resources
- Fiedorowicz JG, Black DW. Borderline, bipolar, or both? Frame your diagnosis on the patient history. Current Psychiatry. 2010; 9(1):21-24,29-32.
- Zimmerman M. Improving the recognition of borderline personality disorder. Current Psychiatry. 2017;16(10):13-19.
- National Institute of Mental Health. Overview on bipolar disorder. www.nimh.nih.gov/health/publications/bipolar-disorder/index.shtml. Revised October 2018.
- National Institute of Mental Health. Overview on borderline personality disorder. www.nimh.nih.gov/health/publications/borderline-personality-disorder-fact-sheet/index.shtml.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Whiteford HA, Degenhardt L, Rehm J, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1575-1586.
3. Merikangas KR, Akiskal HS, Angst J, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2007;64(5):543-552.
4. Malhi GS, Bargh DM, Coulston CM, et al. Predicting bipolar disorder on the basis of phenomenology: implications for prevention and early intervention. Bipolar Disord. 2014;16(5):455-470.
5. Skodol AE, Gunderson JG, Pfohl B, et al. The borderline diagnosis I: psychopathology. Biol Psychiatry. 2002;51(12):936-950.
6. Skodol AE, Siever LJ, Livesley WJ, et al. The borderline diagnosis II: biology, genetics, and clinical course. Biol Psychiatry. 2002;51(12):951-963.
7. Hasin DS, Grant BF. The National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Waves 1 and 2: review and summary of findings. Soc Psychiatry Psychiatr Epidemiol. 2015;50(11):1609-1640.
8. McDermid J, Sareen J, El-Gabalawy R, et al. Co-morbidity of bipolar disorder and borderline personality disorder: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Compr Psychiatry. 2015;58:18-28.
9. Gunderson JG, Weinberg I, Daversa MT, et al. Descriptive and longitudinal observations on the relationship of borderline personality disorder and bipolar disorder. Am J Psychiatry. 2006;163(7):1173-1178.
10. Swartz HA, Pilkonis PA, Frank E, et al. Acute treatment outcomes in patients with bipolar I disorder and co-morbid borderline personality disorder receiving medication and psychotherapy. Bipolar Disord. 2005;7(2):192-197.
11. Riemann G, Weisscher N, Post RM, et al. The relationship between self-reported borderline personality features and prospective illness course in bipolar disorder. Int J Bipolar Disord. 2017;5(1):31.
12. de la Rosa I, Oquendo MA, García G, et al. Determining if borderline personality disorder and bipolar disorder are alternative expressions of the same disorder. J Clin Psychiatry. 2017;778(8):e994-e999. doi: 10.4088/JCP.16m11190.
13. di Giacomo E, Aspesi F, Fotiadou M, et al. Unblending borderline personality and bipolar disorders. J Psychiatr Res. 2017;91:90-97.
14. Parker G, Bayes A, McClure G, et al. Clinical status of comorbid bipolar disorder and borderline personality disorder. Br J Psychiatry. 2016;209(3):209-215.
15. Perez Arribas I, Goodwin GM, Geddes JR, et al. A signature-based machine learning model for distinguishing bipolar disorder and borderline personality disorder. Transl Psychiatry. 2018;8(1):274.
16. Insel T, Cuthbert B, Garvey M, et al. Research Domain Criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167(7):748-751.
17. Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530-537.
18. Judd LL, Akiskal HS, Schettler PJ, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry. 2003;60(3):261-269.
19. Oldham JM, Skodol AE, Bender DS. A current integrative perspective on personality disorders. American Psychiatric Publishing, Inc. 2005.
20. Herzog JI, Schmahl C. Adverse childhood experiences and the consequences on neurobiological, psychosocial, and somatic conditions across the lifespan. Front Psychiatry. 2018;9:420.
21. Barbuti M, Pacchiarotti I, Vieta E, et al. Antidepressant-induced hypomania/mania in patients with major depression: evidence from the BRIDGE-II-MIX study. J Affect Disord. 2017;219:187-192.
22. Post RM. Mechanisms of illness progression in the recurrent affective disorders. Neurotox Res. 2010;18(3-4):256-271.
23. da Costa SC, Passos IC, Lowri C, et al. Refractory bipolar disorder and neuroprogression. Prog Neuro-Psychopharmacology Biol Psychiatry. 2016;70:103-110.
24. Crump C, Sundquist K, Winkleby MA, et al. Comorbidities and mortality in bipolar disorder: a Swedish national cohort study. JAMA Psychiatry. 2013;70(9):931-939.
25. Zimmerman M, Martinez JH, Morgan TA, et al. Distinguishing bipolar II depression from major depressive disorder with comorbid borderline personality disorder: demographic, clinical, and family history differences. J Clin Psychiatry. 2013;74(9):880-886.
26. Hasler G, Drevets WC, Gould TD, et al. Toward constructing an endophenotype strategy for bipolar disorders. Biol Psychiatry. 2006;60(2):93-105.
27. Brieger P, Ehrt U, Marneros A. Frequency of comorbid personality disorders in bipolar and unipolar affective disorders. Compr Psychiatry. 2003;44(1):28-34.
28. Leverich GS, McElroy SL, Suppes T, et al. Early physical and sexual abuse associated with an adverse course of bipolar illness. Biol Psychiatry. 2002;51(4):288-297.
29. Leverich GS, Post RM. Course of bipolar illness after history of childhood trauma. Lancet. 2006;367(9516):1040-1042.
30. Golier JA, Yehuda R, Bierer LM, et al. The relationship of borderline personality disorder to posttraumatic stress disorder and traumatic events. Am J Psychiatry. 2003;160(11):2018-2024.
31. Nicol K, Pope M, Romaniuk L, et al. Childhood trauma, midbrain activation and psychotic symptoms in borderline personality disorder. Transl Psychiatry. 2015;5:e559. doi:10.1038/tp.2015.53.
32. Ruggero CJ, Zimmerman M, Chelminski I, et al. Borderline personality disorder and the misdiagnosis of bipolar disorder. J Psychiatr Res. 2010;44(6):405-408.
33. Geddes JR, Miklowitz DJ. Treatment of bipolar disorder. Lancet. 2013;381(9878):1672-1682.
34. McMain S, Korman LM, Dimeff L. Dialectical behavior therapy and the treatment of emotion dysregulation. J Clin Psychol. 2001;57(2):183-196.
35. Cristea IA, Gentili C, Cotet CD, et al. Efficacy of psychotherapies for borderline personality disorder: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74(4):319-328.
36. Linehan MM, Korslund KE, Harned MS, et al. Dialectical behavior therapy for high suicide risk in individuals with borderline personality disorder. JAMA Psychiatry. 2015;72(75);475-482.
37. LeQuesne ER, Hersh RG. Disclosure of a diagnosis of borderline personality disorder. J Psychiatr Pract. 2004:10(3):170-176.
38. Young AH. Bipolar disorder: diagnostic conundrums and associated comorbidities. J Clin Psychiatry. 2009;70(8):e26. doi:10.4088/jcp.7067br6c.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Whiteford HA, Degenhardt L, Rehm J, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1575-1586.
3. Merikangas KR, Akiskal HS, Angst J, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2007;64(5):543-552.
4. Malhi GS, Bargh DM, Coulston CM, et al. Predicting bipolar disorder on the basis of phenomenology: implications for prevention and early intervention. Bipolar Disord. 2014;16(5):455-470.
5. Skodol AE, Gunderson JG, Pfohl B, et al. The borderline diagnosis I: psychopathology. Biol Psychiatry. 2002;51(12):936-950.
6. Skodol AE, Siever LJ, Livesley WJ, et al. The borderline diagnosis II: biology, genetics, and clinical course. Biol Psychiatry. 2002;51(12):951-963.
7. Hasin DS, Grant BF. The National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Waves 1 and 2: review and summary of findings. Soc Psychiatry Psychiatr Epidemiol. 2015;50(11):1609-1640.
8. McDermid J, Sareen J, El-Gabalawy R, et al. Co-morbidity of bipolar disorder and borderline personality disorder: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Compr Psychiatry. 2015;58:18-28.
9. Gunderson JG, Weinberg I, Daversa MT, et al. Descriptive and longitudinal observations on the relationship of borderline personality disorder and bipolar disorder. Am J Psychiatry. 2006;163(7):1173-1178.
10. Swartz HA, Pilkonis PA, Frank E, et al. Acute treatment outcomes in patients with bipolar I disorder and co-morbid borderline personality disorder receiving medication and psychotherapy. Bipolar Disord. 2005;7(2):192-197.
11. Riemann G, Weisscher N, Post RM, et al. The relationship between self-reported borderline personality features and prospective illness course in bipolar disorder. Int J Bipolar Disord. 2017;5(1):31.
12. de la Rosa I, Oquendo MA, García G, et al. Determining if borderline personality disorder and bipolar disorder are alternative expressions of the same disorder. J Clin Psychiatry. 2017;778(8):e994-e999. doi: 10.4088/JCP.16m11190.
13. di Giacomo E, Aspesi F, Fotiadou M, et al. Unblending borderline personality and bipolar disorders. J Psychiatr Res. 2017;91:90-97.
14. Parker G, Bayes A, McClure G, et al. Clinical status of comorbid bipolar disorder and borderline personality disorder. Br J Psychiatry. 2016;209(3):209-215.
15. Perez Arribas I, Goodwin GM, Geddes JR, et al. A signature-based machine learning model for distinguishing bipolar disorder and borderline personality disorder. Transl Psychiatry. 2018;8(1):274.
16. Insel T, Cuthbert B, Garvey M, et al. Research Domain Criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167(7):748-751.
17. Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530-537.
18. Judd LL, Akiskal HS, Schettler PJ, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry. 2003;60(3):261-269.
19. Oldham JM, Skodol AE, Bender DS. A current integrative perspective on personality disorders. American Psychiatric Publishing, Inc. 2005.
20. Herzog JI, Schmahl C. Adverse childhood experiences and the consequences on neurobiological, psychosocial, and somatic conditions across the lifespan. Front Psychiatry. 2018;9:420.
21. Barbuti M, Pacchiarotti I, Vieta E, et al. Antidepressant-induced hypomania/mania in patients with major depression: evidence from the BRIDGE-II-MIX study. J Affect Disord. 2017;219:187-192.
22. Post RM. Mechanisms of illness progression in the recurrent affective disorders. Neurotox Res. 2010;18(3-4):256-271.
23. da Costa SC, Passos IC, Lowri C, et al. Refractory bipolar disorder and neuroprogression. Prog Neuro-Psychopharmacology Biol Psychiatry. 2016;70:103-110.
24. Crump C, Sundquist K, Winkleby MA, et al. Comorbidities and mortality in bipolar disorder: a Swedish national cohort study. JAMA Psychiatry. 2013;70(9):931-939.
25. Zimmerman M, Martinez JH, Morgan TA, et al. Distinguishing bipolar II depression from major depressive disorder with comorbid borderline personality disorder: demographic, clinical, and family history differences. J Clin Psychiatry. 2013;74(9):880-886.
26. Hasler G, Drevets WC, Gould TD, et al. Toward constructing an endophenotype strategy for bipolar disorders. Biol Psychiatry. 2006;60(2):93-105.
27. Brieger P, Ehrt U, Marneros A. Frequency of comorbid personality disorders in bipolar and unipolar affective disorders. Compr Psychiatry. 2003;44(1):28-34.
28. Leverich GS, McElroy SL, Suppes T, et al. Early physical and sexual abuse associated with an adverse course of bipolar illness. Biol Psychiatry. 2002;51(4):288-297.
29. Leverich GS, Post RM. Course of bipolar illness after history of childhood trauma. Lancet. 2006;367(9516):1040-1042.
30. Golier JA, Yehuda R, Bierer LM, et al. The relationship of borderline personality disorder to posttraumatic stress disorder and traumatic events. Am J Psychiatry. 2003;160(11):2018-2024.
31. Nicol K, Pope M, Romaniuk L, et al. Childhood trauma, midbrain activation and psychotic symptoms in borderline personality disorder. Transl Psychiatry. 2015;5:e559. doi:10.1038/tp.2015.53.
32. Ruggero CJ, Zimmerman M, Chelminski I, et al. Borderline personality disorder and the misdiagnosis of bipolar disorder. J Psychiatr Res. 2010;44(6):405-408.
33. Geddes JR, Miklowitz DJ. Treatment of bipolar disorder. Lancet. 2013;381(9878):1672-1682.
34. McMain S, Korman LM, Dimeff L. Dialectical behavior therapy and the treatment of emotion dysregulation. J Clin Psychol. 2001;57(2):183-196.
35. Cristea IA, Gentili C, Cotet CD, et al. Efficacy of psychotherapies for borderline personality disorder: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74(4):319-328.
36. Linehan MM, Korslund KE, Harned MS, et al. Dialectical behavior therapy for high suicide risk in individuals with borderline personality disorder. JAMA Psychiatry. 2015;72(75);475-482.
37. LeQuesne ER, Hersh RG. Disclosure of a diagnosis of borderline personality disorder. J Psychiatr Pract. 2004:10(3):170-176.
38. Young AH. Bipolar disorder: diagnostic conundrums and associated comorbidities. J Clin Psychiatry. 2009;70(8):e26. doi:10.4088/jcp.7067br6c.