User login
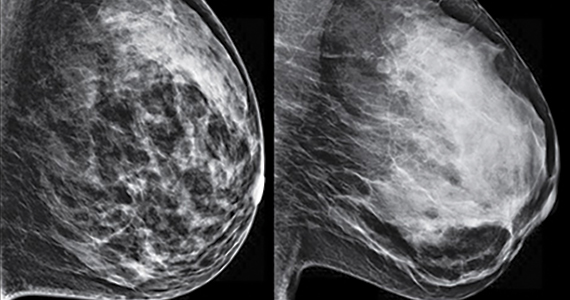
3D vs 2D mammography for detecting cancer in dense breasts
Text copyright DenseBreast-info.org.
Answer
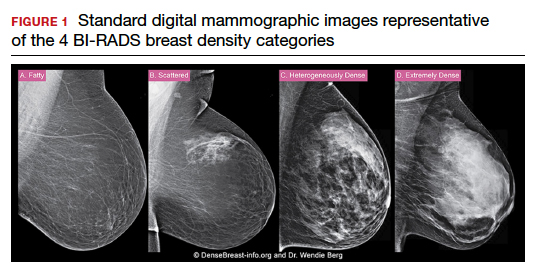
C. Overall, tomosynthesis depicts an additional 1 to 2 cancers per thousand women screened in the first round of screening when added to standard digital mammography;1-3 however, this improvement in cancer detection is only observed in women with fatty breasts (category A), scattered fibroglandular tissue (category B), and heterogeneously dense breasts (category C). Importantly, tomosynthesis does not significantly improve breast cancer detection in women with extremely dense breasts (category D).2,4
Digital breast tomosynthesis, also referred to as “3-dimensional mammography” (3D mammography) or tomosynthesis, uses a dedicated electronic detector system to obtain multiple projection images that are reconstructed by the computer to create thin slices or slabs of multiple slices of the breast. These slices can be individually “scrolled through” by the radiologist to reduce tissue overlap that may obscure breast cancers on a standard mammogram. While tomosynthesis improves breast cancer detection in women with fatty, scattered fibroglandular density, and heterogeneously dense breasts, there is very little soft tissue contrast in extremely dense breasts due to insufficient fat, and some cancers will remain hidden by dense tissue even on sliced images through the breast.
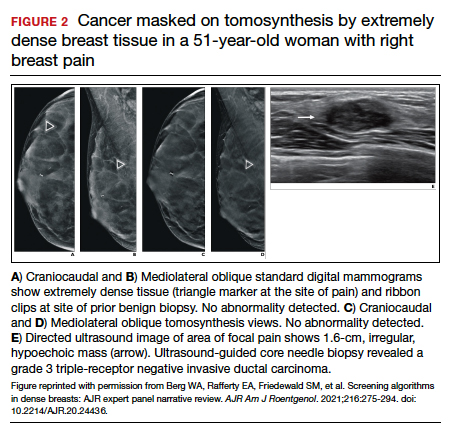
FIGURE 2 shows an example of cancer that was missed on tomosynthesis in a 51-year-old woman with extremely dense breasts and right breast pain. The cancer was masked by extremely dense tissue on standard digital mammography and tomosynthesis; no abnormalities were detected. Ultrasonography showed a 1.6-cm, irregular, hypoechoic mass at the site of pain, and biopsy revealed a grade 3 triple-receptor negative invasive ductal carcinoma.
In women with dense breasts, especially extremely dense breasts, supplemental screening beyond tomosynthesis should be considered. Although tomosynthesis doesn’t improve cancer detection in extremely dense breasts, it does reduce callbacks for additional testing in all breast densities compared with standard digital mammography. Callbacks are reduced from approximately 100‒120 per 1,000 women screened with standard digital mammography alone1,5 to an average of 80 per 1,000 women when tomosynthesis and standard mammography are interpreted together.1-3 ●
For more information, visit medically sourced DenseBreast-info.org. Comprehensive resources include a free CME opportunity, Dense Breasts and Supplemental Screening.
- Conant EF, Zuckerman SP, McDonald ES, et al. Five consecutive years of screening with digital breast tomosynthesis: outcomes by screening year and round. Radiology. 2020;295:285-293.
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA. 2016;315:1784-1786.
- Skaane P, Bandos AI, Niklason LT, et al. Digital mammography versus digital mammography plus tomosynthesis in breast cancer screening: the Oslo Tomosynthesis Screening Trial. Radiology. 2019;291:23-30.
- Lowry KP, Coley RY, Miglioretti DL, et al. Screening performance of digital breast tomosynthesis vs digital mammography in community practice by patient age, screening round, and breast density. JAMA Netw Open. 2020;3:e2011792.
- Lee CS, Sengupta D, Bhargavan-Chatfield M, et al. Association of patient age with outcomes of current-era, large-scale screening mammography: analysis of data from the National Mammography Database. JAMA Oncol. 2017;3:1134-1136.
Text copyright DenseBreast-info.org.
Answer
C. Overall, tomosynthesis depicts an additional 1 to 2 cancers per thousand women screened in the first round of screening when added to standard digital mammography;1-3 however, this improvement in cancer detection is only observed in women with fatty breasts (category A), scattered fibroglandular tissue (category B), and heterogeneously dense breasts (category C). Importantly, tomosynthesis does not significantly improve breast cancer detection in women with extremely dense breasts (category D).2,4
Digital breast tomosynthesis, also referred to as “3-dimensional mammography” (3D mammography) or tomosynthesis, uses a dedicated electronic detector system to obtain multiple projection images that are reconstructed by the computer to create thin slices or slabs of multiple slices of the breast. These slices can be individually “scrolled through” by the radiologist to reduce tissue overlap that may obscure breast cancers on a standard mammogram. While tomosynthesis improves breast cancer detection in women with fatty, scattered fibroglandular density, and heterogeneously dense breasts, there is very little soft tissue contrast in extremely dense breasts due to insufficient fat, and some cancers will remain hidden by dense tissue even on sliced images through the breast.
FIGURE 2 shows an example of cancer that was missed on tomosynthesis in a 51-year-old woman with extremely dense breasts and right breast pain. The cancer was masked by extremely dense tissue on standard digital mammography and tomosynthesis; no abnormalities were detected. Ultrasonography showed a 1.6-cm, irregular, hypoechoic mass at the site of pain, and biopsy revealed a grade 3 triple-receptor negative invasive ductal carcinoma.

In women with dense breasts, especially extremely dense breasts, supplemental screening beyond tomosynthesis should be considered. Although tomosynthesis doesn’t improve cancer detection in extremely dense breasts, it does reduce callbacks for additional testing in all breast densities compared with standard digital mammography. Callbacks are reduced from approximately 100‒120 per 1,000 women screened with standard digital mammography alone1,5 to an average of 80 per 1,000 women when tomosynthesis and standard mammography are interpreted together.1-3 ●
For more information, visit medically sourced DenseBreast-info.org. Comprehensive resources include a free CME opportunity, Dense Breasts and Supplemental Screening.
Text copyright DenseBreast-info.org.
Answer
C. Overall, tomosynthesis depicts an additional 1 to 2 cancers per thousand women screened in the first round of screening when added to standard digital mammography;1-3 however, this improvement in cancer detection is only observed in women with fatty breasts (category A), scattered fibroglandular tissue (category B), and heterogeneously dense breasts (category C). Importantly, tomosynthesis does not significantly improve breast cancer detection in women with extremely dense breasts (category D).2,4
Digital breast tomosynthesis, also referred to as “3-dimensional mammography” (3D mammography) or tomosynthesis, uses a dedicated electronic detector system to obtain multiple projection images that are reconstructed by the computer to create thin slices or slabs of multiple slices of the breast. These slices can be individually “scrolled through” by the radiologist to reduce tissue overlap that may obscure breast cancers on a standard mammogram. While tomosynthesis improves breast cancer detection in women with fatty, scattered fibroglandular density, and heterogeneously dense breasts, there is very little soft tissue contrast in extremely dense breasts due to insufficient fat, and some cancers will remain hidden by dense tissue even on sliced images through the breast.
FIGURE 2 shows an example of cancer that was missed on tomosynthesis in a 51-year-old woman with extremely dense breasts and right breast pain. The cancer was masked by extremely dense tissue on standard digital mammography and tomosynthesis; no abnormalities were detected. Ultrasonography showed a 1.6-cm, irregular, hypoechoic mass at the site of pain, and biopsy revealed a grade 3 triple-receptor negative invasive ductal carcinoma.

In women with dense breasts, especially extremely dense breasts, supplemental screening beyond tomosynthesis should be considered. Although tomosynthesis doesn’t improve cancer detection in extremely dense breasts, it does reduce callbacks for additional testing in all breast densities compared with standard digital mammography. Callbacks are reduced from approximately 100‒120 per 1,000 women screened with standard digital mammography alone1,5 to an average of 80 per 1,000 women when tomosynthesis and standard mammography are interpreted together.1-3 ●
For more information, visit medically sourced DenseBreast-info.org. Comprehensive resources include a free CME opportunity, Dense Breasts and Supplemental Screening.
- Conant EF, Zuckerman SP, McDonald ES, et al. Five consecutive years of screening with digital breast tomosynthesis: outcomes by screening year and round. Radiology. 2020;295:285-293.
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA. 2016;315:1784-1786.
- Skaane P, Bandos AI, Niklason LT, et al. Digital mammography versus digital mammography plus tomosynthesis in breast cancer screening: the Oslo Tomosynthesis Screening Trial. Radiology. 2019;291:23-30.
- Lowry KP, Coley RY, Miglioretti DL, et al. Screening performance of digital breast tomosynthesis vs digital mammography in community practice by patient age, screening round, and breast density. JAMA Netw Open. 2020;3:e2011792.
- Lee CS, Sengupta D, Bhargavan-Chatfield M, et al. Association of patient age with outcomes of current-era, large-scale screening mammography: analysis of data from the National Mammography Database. JAMA Oncol. 2017;3:1134-1136.
- Conant EF, Zuckerman SP, McDonald ES, et al. Five consecutive years of screening with digital breast tomosynthesis: outcomes by screening year and round. Radiology. 2020;295:285-293.
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA. 2016;315:1784-1786.
- Skaane P, Bandos AI, Niklason LT, et al. Digital mammography versus digital mammography plus tomosynthesis in breast cancer screening: the Oslo Tomosynthesis Screening Trial. Radiology. 2019;291:23-30.
- Lowry KP, Coley RY, Miglioretti DL, et al. Screening performance of digital breast tomosynthesis vs digital mammography in community practice by patient age, screening round, and breast density. JAMA Netw Open. 2020;3:e2011792.
- Lee CS, Sengupta D, Bhargavan-Chatfield M, et al. Association of patient age with outcomes of current-era, large-scale screening mammography: analysis of data from the National Mammography Database. JAMA Oncol. 2017;3:1134-1136.
Quiz developed in collaboration with 
Boxed warnings: Legal risks that many physicians never see coming
Almost all physicians write prescriptions, and each prescription requires a physician to assess the risks and benefits of the drug. If an adverse drug reaction occurs, physicians may be called on to defend their risk-benefit assessment in court.
The assessment of risk is complicated when there is a boxed warning that describes potentially serious and life-threatening adverse reactions associated with a drug. Some of our most commonly prescribed drugs have boxed warnings, and drugs that were initially approved by the Food and Drug Administration without boxed warnings may have them added years later.
One serious problem with boxed warnings is that there are no reliable mechanisms for making sure that physicians are aware of them. The warnings are typically not seen by physicians as printed product labels, just as physicians often don’t see the pills and capsules that they prescribe. Pharmacists who receive packaged drugs from manufacturers may be the only ones to see an actual printed boxed warning, but even those pharmacists have little reason to read each label and note changes when handling many bulk packages.
This problem is aggravated by misperceptions that many physicians have about boxed warnings and the increasingly intense scrutiny given to them by mass media and the courts. Lawyers can use boxed warnings to make a drug look dangerous, even when it’s not, and to make physicians look reckless when prescribing it. Therefore, it is important for physicians to understand what boxed warnings are, what they are not, the problems they cause, and how to minimize these problems.
What is a ‘boxed warning’?
The marketing and sale of drugs in the United States requires approval by the FDA. Approval requires manufacturers to prepare a document containing “Full Prescribing Information” for the drug and to include a printed copy in every package of the drug that is sold. This document is commonly called a “package insert,” but the FDA designates this document as the manufacturer’s product “label.”
In 1979, the FDA began requiring some labels to appear within thick, black rectangular borders; these have come to be known as boxed warnings. Boxed warnings are usually placed at the beginning of a label. They may be added to the label of a previously approved drug already on the market or included in the product label when first approved and marketed.
The requirement for a boxed warning most often arises when a signal appears during review of postmarketing surveillance data suggesting a possible and plausible association between a drug and an adverse reaction. Warnings may also be initiated in response to petitions from public interest groups, or upon the discovery of serious toxicity in animals. Regardless of their origin, the intent of a boxed warning is to highlight information that may have important therapeutic consequences and warrants heightened awareness among physicians.
What a boxed warning is not
A boxed warning is not “issued” by the FDA; it is merely required by the FDA. Specific wording or a template may be suggested by the FDA, but product labels and boxed warnings are written and issued by the manufacturer. This distinction may seem minor, but extensive litigation has occurred over whether manufacturers have met their duty to warn consumers about possible risks when using their products, and this duty cannot be shifted to the FDA.
A boxed warning may not be added to a product label at the option of a manufacturer. The FDA allows a boxed warning only if it requires the warning, to preserve its impact. It should be noted that some medical information sources (e.g., PDR.net) may include a “BOXED WARNING” in their drug monographs, but monographs not written by a manufacturer are not regulated by the FDA, and the text of their boxed warnings do not always correspond to the boxed warning that was approved by the FDA.
A boxed warning is not an indication that revocation of FDA approval is being considered or that it is likely to be revoked. FDA approval is subject to ongoing review and may be revoked at any time, without a prior boxed warning.
A boxed warning is not the highest level of warning. The FDA may require a manufacturer to send out a “Dear Health Care Provider” (DHCP) letter when an even higher or more urgent level of warning is deemed necessary. DHCP letters are usually accompanied by revisions of the product label, but most label revisions – and even most boxed warnings – are not accompanied by DHCP letters.
A boxed warning is not a statement about causation. Most warnings describe an “association” between a drug and an adverse effect, or “increased risk,” or instances of a particular adverse effect that “have been reported” in persons taking a drug. The words in a boxed warning are carefully chosen and require careful reading; in most cases they refrain from stating that a drug actually causes an adverse effect. The postmarketing surveillance data on which most warnings are based generally cannot provide the kind of evidence required to establish causation, and an association may be nothing more than an uncommon manifestation of the disorder for which the drug has been prescribed.
A boxed warning is not a statement about the probability of an adverse reaction occurring. The requirement for a boxed warning correlates better to the new recognition of a possible association than to the probability of an association. For example, penicillin has long been known to cause fatal anaphylaxis in 1/100,000 first-time administrations, but it does not have a boxed warning. The adverse consequences described in boxed warnings are often far less frequent – so much so that most physicians will never see them.
A boxed warning does not define the standard of care. The warning is a requirement imposed on the manufacturer, not on the practice of medicine. For legal purposes, the “standard of care” for the practice of medicine is defined state by state and is typically cast in terms such as “what most physicians would do in similar circumstances.” Physicians often prescribe drugs in spite of boxed warnings, just as they often prescribe drugs for “off label” indications, always balancing risk versus benefit.
A boxed warning does not constitute a contraindication to the use of a medication. Some warnings state that a drug is contraindicated in some situations, but product labels have another mandated section for listing contraindications, and most boxed warnings have no corresponding entry in that section.
A boxed warning does not necessarily constitute current information, nor is it always updated when new or contrary information becomes available. Revisions to boxed warnings, and to product labels in general, are made only after detailed review at the FDA, and the process of deciding whether an existing boxed warning continues to be appropriate may divert limited regulatory resources from more urgent priorities. Consequently, revisions to a boxed warning may lag behind the data that justify a revision by months or years. Revisions may never occur if softening or eliminating a boxed warning is deemed to be not worth the cost by a manufacturer.
Boxed warning problems for physicians
There is no reliable mechanism for manufacturers or the FDA to communicate boxed warnings directly to physicians, so it’s not clear how physicians are expected to stay informed about the issuance or revision of boxed warnings. They may first learn about new or revised warnings in the mass media, which is paying ever-increasing attention to press releases from the FDA. However, it can be difficult for the media to accurately convey the subtle and complex nature of a boxed warning in nontechnical terms.
Many physicians subscribe to various medical news alerts and attend continuing medical education (CME) programs, which often do an excellent job of highlighting new warnings, while hospitals, clinics, and pharmacies may broadcast news about boxed warnings in newsletters or other notices. But these notifications are ephemeral and may be missed by physicians who are overwhelmed by email, notices, newsletters, and CME programs.
The warnings that pop up in electronic medical records systems are often so numerous that physicians become trained to ignore them. Printed advertisements in professional journals must include mandated boxed warnings, but their visibility is waning as physicians increasingly read journals online.
Another conundrum is how to inform the public about boxed warnings.
Manufacturers are prohibited from direct-to-consumer advertising of drugs with boxed warnings, although the warnings are easily found on the Internet. Some patients expect and welcome detailed information from their physicians, so it’s a good policy to always and repeatedly review this information with them, especially if they are members of an identified risk group. However, that policy may be counterproductive if it dissuades anxious patients from needed therapy despite risk-benefit considerations that strongly favor it. Boxed warnings are well known to have “spillover effects” in which the aspersions cast by a boxed warning for a relatively small subgroup of patients causes use of a drug to decline among all patients.
Compounding this conundrum is that physicians rarely have sufficient information to gauge the magnitude of a risk, given that boxed warnings are often based on information from surveillance systems that cannot accurately quantify the risk or even establish a causal relationship. The text of a boxed warning generally does not provide the information needed for evidence-based clinical practice such as a quantitative estimate of effect, information about source and trustworthiness of the evidence, and guidance on implementation. For these and other reasons, FDA policies about various boxed warnings have been the target of significant criticism.
Medication guides are one mechanism to address the challenge of informing patients about the risks of drugs they are taking. FDA-approved medication guides are available for most drugs dispensed as outpatient prescriptions, they’re written in plain language for the consumer, and they include paraphrased versions of any boxed warning. Ideally, patients review these guides with their physicians or pharmacists, but the guides may be lengthy and raise questions that may not be answerable (e.g., about incidence rates). Patients may decline to review this information when a drug is prescribed or dispensed, and they may discard printed copies given to them without reading.
What can physicians do to minimize boxed warning problems?
Physicians should periodically review the product labels for drugs they commonly prescribe, including drugs they’ve prescribed for a long time. Prescription renewal requests can be used as a prompt to check for changes in a patient’s condition or other medications that might place a patient in the target population of a boxed warning. Physicians can subscribe to newsletters that announce and discuss significant product label changes, including alerts directly from the FDA. Physicians may also enlist their office staff to find and review boxed warnings for drugs being prescribed, noting which ones should require a conversation with any patient who has been or will be receiving this drug. They may want to make explicit mention in their encounter record that a boxed warning, medication guide, or overall risk-benefit assessment has been discussed.
Summary
The nature of boxed warnings, the means by which they are disseminated, and their role in clinical practice are all in great need of improvement. Until that occurs, boxed warnings offer some, but only very limited, help to patients and physicians who struggle to understand the risks of medications.
Dr. Axelsen is professor in the departments of pharmacology, biochemistry, and biophysics, and of medicine, infectious diseases section, University of Pennsylvania, Philadelphia. He disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
Almost all physicians write prescriptions, and each prescription requires a physician to assess the risks and benefits of the drug. If an adverse drug reaction occurs, physicians may be called on to defend their risk-benefit assessment in court.
The assessment of risk is complicated when there is a boxed warning that describes potentially serious and life-threatening adverse reactions associated with a drug. Some of our most commonly prescribed drugs have boxed warnings, and drugs that were initially approved by the Food and Drug Administration without boxed warnings may have them added years later.
One serious problem with boxed warnings is that there are no reliable mechanisms for making sure that physicians are aware of them. The warnings are typically not seen by physicians as printed product labels, just as physicians often don’t see the pills and capsules that they prescribe. Pharmacists who receive packaged drugs from manufacturers may be the only ones to see an actual printed boxed warning, but even those pharmacists have little reason to read each label and note changes when handling many bulk packages.
This problem is aggravated by misperceptions that many physicians have about boxed warnings and the increasingly intense scrutiny given to them by mass media and the courts. Lawyers can use boxed warnings to make a drug look dangerous, even when it’s not, and to make physicians look reckless when prescribing it. Therefore, it is important for physicians to understand what boxed warnings are, what they are not, the problems they cause, and how to minimize these problems.
What is a ‘boxed warning’?
The marketing and sale of drugs in the United States requires approval by the FDA. Approval requires manufacturers to prepare a document containing “Full Prescribing Information” for the drug and to include a printed copy in every package of the drug that is sold. This document is commonly called a “package insert,” but the FDA designates this document as the manufacturer’s product “label.”
In 1979, the FDA began requiring some labels to appear within thick, black rectangular borders; these have come to be known as boxed warnings. Boxed warnings are usually placed at the beginning of a label. They may be added to the label of a previously approved drug already on the market or included in the product label when first approved and marketed.
The requirement for a boxed warning most often arises when a signal appears during review of postmarketing surveillance data suggesting a possible and plausible association between a drug and an adverse reaction. Warnings may also be initiated in response to petitions from public interest groups, or upon the discovery of serious toxicity in animals. Regardless of their origin, the intent of a boxed warning is to highlight information that may have important therapeutic consequences and warrants heightened awareness among physicians.
What a boxed warning is not
A boxed warning is not “issued” by the FDA; it is merely required by the FDA. Specific wording or a template may be suggested by the FDA, but product labels and boxed warnings are written and issued by the manufacturer. This distinction may seem minor, but extensive litigation has occurred over whether manufacturers have met their duty to warn consumers about possible risks when using their products, and this duty cannot be shifted to the FDA.
A boxed warning may not be added to a product label at the option of a manufacturer. The FDA allows a boxed warning only if it requires the warning, to preserve its impact. It should be noted that some medical information sources (e.g., PDR.net) may include a “BOXED WARNING” in their drug monographs, but monographs not written by a manufacturer are not regulated by the FDA, and the text of their boxed warnings do not always correspond to the boxed warning that was approved by the FDA.
A boxed warning is not an indication that revocation of FDA approval is being considered or that it is likely to be revoked. FDA approval is subject to ongoing review and may be revoked at any time, without a prior boxed warning.
A boxed warning is not the highest level of warning. The FDA may require a manufacturer to send out a “Dear Health Care Provider” (DHCP) letter when an even higher or more urgent level of warning is deemed necessary. DHCP letters are usually accompanied by revisions of the product label, but most label revisions – and even most boxed warnings – are not accompanied by DHCP letters.
A boxed warning is not a statement about causation. Most warnings describe an “association” between a drug and an adverse effect, or “increased risk,” or instances of a particular adverse effect that “have been reported” in persons taking a drug. The words in a boxed warning are carefully chosen and require careful reading; in most cases they refrain from stating that a drug actually causes an adverse effect. The postmarketing surveillance data on which most warnings are based generally cannot provide the kind of evidence required to establish causation, and an association may be nothing more than an uncommon manifestation of the disorder for which the drug has been prescribed.
A boxed warning is not a statement about the probability of an adverse reaction occurring. The requirement for a boxed warning correlates better to the new recognition of a possible association than to the probability of an association. For example, penicillin has long been known to cause fatal anaphylaxis in 1/100,000 first-time administrations, but it does not have a boxed warning. The adverse consequences described in boxed warnings are often far less frequent – so much so that most physicians will never see them.
A boxed warning does not define the standard of care. The warning is a requirement imposed on the manufacturer, not on the practice of medicine. For legal purposes, the “standard of care” for the practice of medicine is defined state by state and is typically cast in terms such as “what most physicians would do in similar circumstances.” Physicians often prescribe drugs in spite of boxed warnings, just as they often prescribe drugs for “off label” indications, always balancing risk versus benefit.
A boxed warning does not constitute a contraindication to the use of a medication. Some warnings state that a drug is contraindicated in some situations, but product labels have another mandated section for listing contraindications, and most boxed warnings have no corresponding entry in that section.
A boxed warning does not necessarily constitute current information, nor is it always updated when new or contrary information becomes available. Revisions to boxed warnings, and to product labels in general, are made only after detailed review at the FDA, and the process of deciding whether an existing boxed warning continues to be appropriate may divert limited regulatory resources from more urgent priorities. Consequently, revisions to a boxed warning may lag behind the data that justify a revision by months or years. Revisions may never occur if softening or eliminating a boxed warning is deemed to be not worth the cost by a manufacturer.
Boxed warning problems for physicians
There is no reliable mechanism for manufacturers or the FDA to communicate boxed warnings directly to physicians, so it’s not clear how physicians are expected to stay informed about the issuance or revision of boxed warnings. They may first learn about new or revised warnings in the mass media, which is paying ever-increasing attention to press releases from the FDA. However, it can be difficult for the media to accurately convey the subtle and complex nature of a boxed warning in nontechnical terms.
Many physicians subscribe to various medical news alerts and attend continuing medical education (CME) programs, which often do an excellent job of highlighting new warnings, while hospitals, clinics, and pharmacies may broadcast news about boxed warnings in newsletters or other notices. But these notifications are ephemeral and may be missed by physicians who are overwhelmed by email, notices, newsletters, and CME programs.
The warnings that pop up in electronic medical records systems are often so numerous that physicians become trained to ignore them. Printed advertisements in professional journals must include mandated boxed warnings, but their visibility is waning as physicians increasingly read journals online.
Another conundrum is how to inform the public about boxed warnings.
Manufacturers are prohibited from direct-to-consumer advertising of drugs with boxed warnings, although the warnings are easily found on the Internet. Some patients expect and welcome detailed information from their physicians, so it’s a good policy to always and repeatedly review this information with them, especially if they are members of an identified risk group. However, that policy may be counterproductive if it dissuades anxious patients from needed therapy despite risk-benefit considerations that strongly favor it. Boxed warnings are well known to have “spillover effects” in which the aspersions cast by a boxed warning for a relatively small subgroup of patients causes use of a drug to decline among all patients.
Compounding this conundrum is that physicians rarely have sufficient information to gauge the magnitude of a risk, given that boxed warnings are often based on information from surveillance systems that cannot accurately quantify the risk or even establish a causal relationship. The text of a boxed warning generally does not provide the information needed for evidence-based clinical practice such as a quantitative estimate of effect, information about source and trustworthiness of the evidence, and guidance on implementation. For these and other reasons, FDA policies about various boxed warnings have been the target of significant criticism.
Medication guides are one mechanism to address the challenge of informing patients about the risks of drugs they are taking. FDA-approved medication guides are available for most drugs dispensed as outpatient prescriptions, they’re written in plain language for the consumer, and they include paraphrased versions of any boxed warning. Ideally, patients review these guides with their physicians or pharmacists, but the guides may be lengthy and raise questions that may not be answerable (e.g., about incidence rates). Patients may decline to review this information when a drug is prescribed or dispensed, and they may discard printed copies given to them without reading.
What can physicians do to minimize boxed warning problems?
Physicians should periodically review the product labels for drugs they commonly prescribe, including drugs they’ve prescribed for a long time. Prescription renewal requests can be used as a prompt to check for changes in a patient’s condition or other medications that might place a patient in the target population of a boxed warning. Physicians can subscribe to newsletters that announce and discuss significant product label changes, including alerts directly from the FDA. Physicians may also enlist their office staff to find and review boxed warnings for drugs being prescribed, noting which ones should require a conversation with any patient who has been or will be receiving this drug. They may want to make explicit mention in their encounter record that a boxed warning, medication guide, or overall risk-benefit assessment has been discussed.
Summary
The nature of boxed warnings, the means by which they are disseminated, and their role in clinical practice are all in great need of improvement. Until that occurs, boxed warnings offer some, but only very limited, help to patients and physicians who struggle to understand the risks of medications.
Dr. Axelsen is professor in the departments of pharmacology, biochemistry, and biophysics, and of medicine, infectious diseases section, University of Pennsylvania, Philadelphia. He disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
Almost all physicians write prescriptions, and each prescription requires a physician to assess the risks and benefits of the drug. If an adverse drug reaction occurs, physicians may be called on to defend their risk-benefit assessment in court.
The assessment of risk is complicated when there is a boxed warning that describes potentially serious and life-threatening adverse reactions associated with a drug. Some of our most commonly prescribed drugs have boxed warnings, and drugs that were initially approved by the Food and Drug Administration without boxed warnings may have them added years later.
One serious problem with boxed warnings is that there are no reliable mechanisms for making sure that physicians are aware of them. The warnings are typically not seen by physicians as printed product labels, just as physicians often don’t see the pills and capsules that they prescribe. Pharmacists who receive packaged drugs from manufacturers may be the only ones to see an actual printed boxed warning, but even those pharmacists have little reason to read each label and note changes when handling many bulk packages.
This problem is aggravated by misperceptions that many physicians have about boxed warnings and the increasingly intense scrutiny given to them by mass media and the courts. Lawyers can use boxed warnings to make a drug look dangerous, even when it’s not, and to make physicians look reckless when prescribing it. Therefore, it is important for physicians to understand what boxed warnings are, what they are not, the problems they cause, and how to minimize these problems.
What is a ‘boxed warning’?
The marketing and sale of drugs in the United States requires approval by the FDA. Approval requires manufacturers to prepare a document containing “Full Prescribing Information” for the drug and to include a printed copy in every package of the drug that is sold. This document is commonly called a “package insert,” but the FDA designates this document as the manufacturer’s product “label.”
In 1979, the FDA began requiring some labels to appear within thick, black rectangular borders; these have come to be known as boxed warnings. Boxed warnings are usually placed at the beginning of a label. They may be added to the label of a previously approved drug already on the market or included in the product label when first approved and marketed.
The requirement for a boxed warning most often arises when a signal appears during review of postmarketing surveillance data suggesting a possible and plausible association between a drug and an adverse reaction. Warnings may also be initiated in response to petitions from public interest groups, or upon the discovery of serious toxicity in animals. Regardless of their origin, the intent of a boxed warning is to highlight information that may have important therapeutic consequences and warrants heightened awareness among physicians.
What a boxed warning is not
A boxed warning is not “issued” by the FDA; it is merely required by the FDA. Specific wording or a template may be suggested by the FDA, but product labels and boxed warnings are written and issued by the manufacturer. This distinction may seem minor, but extensive litigation has occurred over whether manufacturers have met their duty to warn consumers about possible risks when using their products, and this duty cannot be shifted to the FDA.
A boxed warning may not be added to a product label at the option of a manufacturer. The FDA allows a boxed warning only if it requires the warning, to preserve its impact. It should be noted that some medical information sources (e.g., PDR.net) may include a “BOXED WARNING” in their drug monographs, but monographs not written by a manufacturer are not regulated by the FDA, and the text of their boxed warnings do not always correspond to the boxed warning that was approved by the FDA.
A boxed warning is not an indication that revocation of FDA approval is being considered or that it is likely to be revoked. FDA approval is subject to ongoing review and may be revoked at any time, without a prior boxed warning.
A boxed warning is not the highest level of warning. The FDA may require a manufacturer to send out a “Dear Health Care Provider” (DHCP) letter when an even higher or more urgent level of warning is deemed necessary. DHCP letters are usually accompanied by revisions of the product label, but most label revisions – and even most boxed warnings – are not accompanied by DHCP letters.
A boxed warning is not a statement about causation. Most warnings describe an “association” between a drug and an adverse effect, or “increased risk,” or instances of a particular adverse effect that “have been reported” in persons taking a drug. The words in a boxed warning are carefully chosen and require careful reading; in most cases they refrain from stating that a drug actually causes an adverse effect. The postmarketing surveillance data on which most warnings are based generally cannot provide the kind of evidence required to establish causation, and an association may be nothing more than an uncommon manifestation of the disorder for which the drug has been prescribed.
A boxed warning is not a statement about the probability of an adverse reaction occurring. The requirement for a boxed warning correlates better to the new recognition of a possible association than to the probability of an association. For example, penicillin has long been known to cause fatal anaphylaxis in 1/100,000 first-time administrations, but it does not have a boxed warning. The adverse consequences described in boxed warnings are often far less frequent – so much so that most physicians will never see them.
A boxed warning does not define the standard of care. The warning is a requirement imposed on the manufacturer, not on the practice of medicine. For legal purposes, the “standard of care” for the practice of medicine is defined state by state and is typically cast in terms such as “what most physicians would do in similar circumstances.” Physicians often prescribe drugs in spite of boxed warnings, just as they often prescribe drugs for “off label” indications, always balancing risk versus benefit.
A boxed warning does not constitute a contraindication to the use of a medication. Some warnings state that a drug is contraindicated in some situations, but product labels have another mandated section for listing contraindications, and most boxed warnings have no corresponding entry in that section.
A boxed warning does not necessarily constitute current information, nor is it always updated when new or contrary information becomes available. Revisions to boxed warnings, and to product labels in general, are made only after detailed review at the FDA, and the process of deciding whether an existing boxed warning continues to be appropriate may divert limited regulatory resources from more urgent priorities. Consequently, revisions to a boxed warning may lag behind the data that justify a revision by months or years. Revisions may never occur if softening or eliminating a boxed warning is deemed to be not worth the cost by a manufacturer.
Boxed warning problems for physicians
There is no reliable mechanism for manufacturers or the FDA to communicate boxed warnings directly to physicians, so it’s not clear how physicians are expected to stay informed about the issuance or revision of boxed warnings. They may first learn about new or revised warnings in the mass media, which is paying ever-increasing attention to press releases from the FDA. However, it can be difficult for the media to accurately convey the subtle and complex nature of a boxed warning in nontechnical terms.
Many physicians subscribe to various medical news alerts and attend continuing medical education (CME) programs, which often do an excellent job of highlighting new warnings, while hospitals, clinics, and pharmacies may broadcast news about boxed warnings in newsletters or other notices. But these notifications are ephemeral and may be missed by physicians who are overwhelmed by email, notices, newsletters, and CME programs.
The warnings that pop up in electronic medical records systems are often so numerous that physicians become trained to ignore them. Printed advertisements in professional journals must include mandated boxed warnings, but their visibility is waning as physicians increasingly read journals online.
Another conundrum is how to inform the public about boxed warnings.
Manufacturers are prohibited from direct-to-consumer advertising of drugs with boxed warnings, although the warnings are easily found on the Internet. Some patients expect and welcome detailed information from their physicians, so it’s a good policy to always and repeatedly review this information with them, especially if they are members of an identified risk group. However, that policy may be counterproductive if it dissuades anxious patients from needed therapy despite risk-benefit considerations that strongly favor it. Boxed warnings are well known to have “spillover effects” in which the aspersions cast by a boxed warning for a relatively small subgroup of patients causes use of a drug to decline among all patients.
Compounding this conundrum is that physicians rarely have sufficient information to gauge the magnitude of a risk, given that boxed warnings are often based on information from surveillance systems that cannot accurately quantify the risk or even establish a causal relationship. The text of a boxed warning generally does not provide the information needed for evidence-based clinical practice such as a quantitative estimate of effect, information about source and trustworthiness of the evidence, and guidance on implementation. For these and other reasons, FDA policies about various boxed warnings have been the target of significant criticism.
Medication guides are one mechanism to address the challenge of informing patients about the risks of drugs they are taking. FDA-approved medication guides are available for most drugs dispensed as outpatient prescriptions, they’re written in plain language for the consumer, and they include paraphrased versions of any boxed warning. Ideally, patients review these guides with their physicians or pharmacists, but the guides may be lengthy and raise questions that may not be answerable (e.g., about incidence rates). Patients may decline to review this information when a drug is prescribed or dispensed, and they may discard printed copies given to them without reading.
What can physicians do to minimize boxed warning problems?
Physicians should periodically review the product labels for drugs they commonly prescribe, including drugs they’ve prescribed for a long time. Prescription renewal requests can be used as a prompt to check for changes in a patient’s condition or other medications that might place a patient in the target population of a boxed warning. Physicians can subscribe to newsletters that announce and discuss significant product label changes, including alerts directly from the FDA. Physicians may also enlist their office staff to find and review boxed warnings for drugs being prescribed, noting which ones should require a conversation with any patient who has been or will be receiving this drug. They may want to make explicit mention in their encounter record that a boxed warning, medication guide, or overall risk-benefit assessment has been discussed.
Summary
The nature of boxed warnings, the means by which they are disseminated, and their role in clinical practice are all in great need of improvement. Until that occurs, boxed warnings offer some, but only very limited, help to patients and physicians who struggle to understand the risks of medications.
Dr. Axelsen is professor in the departments of pharmacology, biochemistry, and biophysics, and of medicine, infectious diseases section, University of Pennsylvania, Philadelphia. He disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
Is telemedicine here to stay?
Dear colleagues and friends,
I am fortunate to receive the baton from Charles Kahi, MD, in facilitating the fascinating and timely debates that have characterized the AGA Perspective series. Favorable reimbursement changes and the need for social distancing fast-tracked telemedicine, a care delivery model that had been slowly evolving.
In this month’s Perspective column, Dr. Hernaez and Dr. Vaughn discuss the pros and cons of telemedicine in GI. Is it the new office visit? Or simply just good enough for when we really need it? I look forward to hearing your thoughts and experiences on the AGA Community forum as well as by email ([email protected]).
Gyanprakash A. Ketwaroo, MD, is assistant professor of medicine at Baylor College of Medicine, Houston. He is an associate editor for GI & Hepatology News.
It holds promise
BY RUBEN HERNAEZ, MD, MPH, PHD
It was around January 2020, when COVID-19 was something far, far away, and not particularly worrisome. I was performing a routine care visit of one of my out-of-state patients waitlisted for a liver transplant. All was going fine until he stated, “Hey doc, while I appreciate your time and visits, these travels to Houston are quite inconvenient for my family and me: It is a logistical ordeal, and my wife is always afraid of catching something in the airplane. Could you do the same remotely such as a videoconference?” And just like that, it sparked my interest in how to maintain his liver transplant care from a distance. The Federation of State Medical Boards defines telemedicine as “the practice of medicine using electronic communication, information technology, or other means between a physician in one location, and a patient in another location, with or without an intervening health care provider.1 What my patient was asking was to use a mode of telemedicine – a video visit – to receive the same quality of care. He brought up three critical points that I will discuss further: access to specialty care (such as transplant hepatology), reduction of costs (time and money), and improved patient satisfaction.
Arora and colleagues pioneered the Extension for Community Healthcare Outcomes (ECHO) project, providing complex specialty medical care to underserved populations through a model of team-based interdisciplinary development in hepatitis C infection treatment in underserved communities, with cure rates similar to the university settings.1 The University of Michigan–Veterans Affairs Medical Center used a similar approach, called Specialty Care Access Network–Extension of Community Healthcare Outcome (SCAN‐ECHO). They showed that telemedicine improved survival in 513 patients evaluated in this program compared to regular care (hazard ratio [HR]of 0.54, 95% confidence interval 0.36‐0.81, P = .003).2 So the evidence backs my patient’s request in providing advanced medical care using a telemedicine platform.
An extra benefit of telemedicine in the current climate crisis is reducing the carbon footprint: There’s no need to travel. Telemedicine has been shown to be cost effective: A study of claims data from Jefferson Health reported that patients who received care from an on-demand telemedicine program had net cost savings per telemedicine visit between $19 and $121 per visit compared with traditional in-person visits.3 Using telemonitoring, a form of telemedicine, Bloom et al. showed in 100 simulated patients with cirrhosis and ascites over a 6-month horizon that standard of care was $167,500 more expensive than telemonitoring. The net savings of telemonitoring was always superior in different clinical scenarios.4
Further, our patients significantly decrease travel time (almost instant), improve compliance with medical appointments (more flexibility), and no more headaches related to parking or getting lost around the medical campus. Not surprisingly, these perks from telemedicine are associated with patient-reported outcomes. Reed and colleagues reported patients’ experience with video telemedicine visits in Kaiser Permanente Northern California (n = 1,274) and showed that “67% generally needed to make one or more arrangements to attend an in-person office visit (55% time off from work, 29% coverage for another activity or responsibility, 15% child care or caregiving, and 10% another person to accompany them)”; in contrast, 87% reported a video visit as “more convenient for me,” and 93% stated that “my video visit adequately addressed my needs.”5 In liver transplantation, John et al. showed that, in the Veterans Health Administration, telehealth was associated with a significantly shorter time on the liver transplant waitlist (138.8 vs. 249 days), reduction in the time from referral to evaluation (HR, 0.15; 95% CI, 0.09-0.21; P < .01) and listing (HR, 0.26; 95% CI, 0.12-0.40; P < .01) in a study of 232 patients with advanced cirrhosis.6
So, should I change my approach to patients undergoing care for chronic liver/gastrointestinal diseases? I think so. Telemedicine and its tools provide clear benefits to our patients by increasing access to care, time and money savings, and satisfaction. I am fortunate to work within the largest healthcare network in the Nation – the Veterans Health Administration – and therefore, I can cross state lines to provide medical care/advice using the video-visit tool (VA VideoConnect). One could argue that some patients might find it challenging to access telemedicine appointments, but with adequate coaching or support from our teams, telemedicine visits are a click away.
Going back to my patient, I embraced his request and coached him on using VA VideoConnect. We can continue his waitlist medical care in the following months despite the COVID-19 pandemic using telemedicine. I can assess his asterixis and ascites via his cellphone; his primary care team fills in the vitals and labs to complete a virtual visit. There’s no question in my mind that telemedicine is here to stay and that we will continue to adapt e-health tools into video visits (for example, integrating vitals, measurement of frailty, and remote monitoring). The future of our specialty is here, and I envision we will eventually have home-based hospitalizations with daily virtual rounds.
Dr. Hernaez is with the section of gastroenterology and the Center for Innovations in Quality, Effectiveness, and Safety at the Michael E. DeBakey Veterans Affairs Medical Center and in the section of gastroenterology and hepatology in the department of medicine at Baylor College of Medicine, both in Houston. He has no relevant conflicts of interest.
References
1. Arora S et al. N Engl J Med. 2011 Jun 9;364(23):2199-207.
2. Su GL et al. Hepatology. 2018 Dec;68(6):2317-24.
3. Nord G et al. Am J Emerg Med. 2019 May;37(5):890-4.
4. Bloom PP et al. Dig Dis Sci. 2021. doi: 10.1007/s10620-021-07013-2.
5. Reed ME et al. Ann Intern Med. 2019 Aug;171:222-4.
6. John BV et al. Clin Gastroenterol Hepatol. 2020 Jul;18:1822-30.e4.
It has its limits
BY BYRON P. VAUGHN, MD, MS
The post-pandemic world will include telehealth. Technology disrupts business as usual and often brings positive change. But there are consequences. To employ telehealth into routine care equitably and effectively within a gastroenterology practice, we should consider two general questions: “Was the care I provided the same quality as if the patient was seen in person?” and more broadly, “am I satisfied with my practice’s implementation of telehealth?” This perspective will highlight several areas affecting gastroenterology care: lack of physical exam, disproportionate impact on certain populations, development of a patient-provider relationship, impact on physician well-being, and potential financial ramifications. We will all have to adapt to telemedicine to some extent. Understanding the trade-offs of this technology can help us position effectively in a gastroenterology practice.
Perhaps the most obvious limitation of telemedicine is the lack of vital signs and physical exam. Determining if a patient is “sick or not” is often one of the first lessons trainees learn overnight. Vital signs and physical exam are crucial in the complex triaging that occurs when evaluating for diagnoses with potentially urgent interventions. While most outpatient gastroenterology clinics are not evaluating an acute abdomen, in the correct context, the physical exam provides important nuance and often reassurance. My personal estimate is that 90% of a diagnosis is based on the history. But without physical contact, providers may increase costly downstream diagnostic testing or referrals to the emergency department. Increasing use of at-home or wearable health technology could help but requires system investments in infrastructure to implement.
Telemedicine requires a baseline level of equipment and knowledge to participate. A variety of populations will have either a knowledge gap or technology gap. Lack of rural high-speed Internet can lead to poor video quality, inhibiting effective communication and frustrating both provider and patient. In urban areas, there is a drastic wealth divide, and some groups may have difficulty obtaining sufficient equipment to complete a video visit. Even with adequate infrastructure and equipment, certain groups may be disadvantaged because of a lack of the technological savvy or literacy needed to navigate a virtual visit. The addition of interpreter services adds complexity to communication on top of the virtual interaction. These technology and knowledge gaps can produce confusion and potentially lead to worse care.1 Careful selection of appropriate patients for telemedicine is essential. Is the quality of care over a virtual visit the same for a business executive as that of a non-English speaking refugee?
The term “webside manner” precedes the pandemic but will be important in the lexicon of doctor-patient relationships moving forward. We routinely train physicians about the importance of small actions to improve our bedside manner, such as sitting down, reacting to body language, and making eye contact. First impressions matter in relationship building. For many of my established IBD patients, I can easily hop into a comfortable repertoire in person, virtually, or even on the phone. In addition, I know these people well enough to trust that I am providing the same level of care regardless of visit medium. However, a new patient virtual encounter requires nuance. I have met new patients while they are driving (I requested the patient park!), in public places, and at work. Despite instructions given to patients about the appropriate location for a virtual visit, the patient location is not in our control. For some patients this may increase the comfort of the visit. However, for others, it can lead to distractions or potentially limit the amount of information a patient is willing to share. Forming a patient-provider relationship virtually will require a new set of skills and specific training for many practitioners.
Telehealth can contribute to provider burnout. While a busy in-person clinic can be exhausting, I have found I can be more exhausted after a half-day of virtual clinic. There is an element of human connection that is difficult to replace online.2 On top of that loss, video visits are more psychologically demanding than in-person interactions. I also spend more time in a chair, have fewer coffee breaks, and have fewer professional interactions with the clinic staff and professional colleagues. Several other micro-stressors exist in virtual care that may make “Zoom fatigue” a real occupational hazard.3
Lastly, there are implications on reimbursement with telehealth. In Minnesota, a 2015 telemedicine law required private and state employee health plans to provide the same coverage for telemedicine as in-person visits, although patients had to drive to a clinic or facility to use secure telehealth equipment and have vital signs taken. With the pandemic, this stipulation is waived, and it seems likely to become permanent. However, reimbursement questions will arise, as there is a perception that a 30-minute telephone call should not cost as much as a 30-minute in-person visit, regardless of the content of the conversation.
We will have to learn to move forward with telehealth. The strength of telehealth is likely in patients with chronic, well controlled diseases, who have frequent interactions with health care. Examples of this include (although certainly are not limited to) established patients with well-controlled IBD, non-cirrhotic liver disease, and irritable bowel syndrome. Triaging patients who need in-person evaluation, ensuring patient and provider well-being, and creating a financially sustainable model of care are yet unresolved issues. Providers will likely vary in their personal acceptance of telehealth and will need to advocate within their own systems to obtain a hybrid model of telehealth that maximizes quality of care with job satisfaction.
Dr. Vaughn is associate professor of medicine and codirector of the inflammatory bowel disease program in the division of gastroenterology, hepatology, and nutrition at University of Minnesota, Minneapolis. He has received consulting fees from Prometheus and research support from Roche, Takeda, Celgene, Diasorin, and Crestovo.
References
1. George S et al. Stud Health Technol Inform. 2013;192:946.
2. Blank S. “What’s missing from Zoom reminds us what it means to be human,” 2020 Apr 27, Medium.
3. Williams N. Occup Med (Lond). 2021 Apr. doi: 10.1093/occmed/kqab041.
Dear colleagues and friends,
I am fortunate to receive the baton from Charles Kahi, MD, in facilitating the fascinating and timely debates that have characterized the AGA Perspective series. Favorable reimbursement changes and the need for social distancing fast-tracked telemedicine, a care delivery model that had been slowly evolving.
In this month’s Perspective column, Dr. Hernaez and Dr. Vaughn discuss the pros and cons of telemedicine in GI. Is it the new office visit? Or simply just good enough for when we really need it? I look forward to hearing your thoughts and experiences on the AGA Community forum as well as by email ([email protected]).
Gyanprakash A. Ketwaroo, MD, is assistant professor of medicine at Baylor College of Medicine, Houston. He is an associate editor for GI & Hepatology News.
It holds promise
BY RUBEN HERNAEZ, MD, MPH, PHD
It was around January 2020, when COVID-19 was something far, far away, and not particularly worrisome. I was performing a routine care visit of one of my out-of-state patients waitlisted for a liver transplant. All was going fine until he stated, “Hey doc, while I appreciate your time and visits, these travels to Houston are quite inconvenient for my family and me: It is a logistical ordeal, and my wife is always afraid of catching something in the airplane. Could you do the same remotely such as a videoconference?” And just like that, it sparked my interest in how to maintain his liver transplant care from a distance. The Federation of State Medical Boards defines telemedicine as “the practice of medicine using electronic communication, information technology, or other means between a physician in one location, and a patient in another location, with or without an intervening health care provider.1 What my patient was asking was to use a mode of telemedicine – a video visit – to receive the same quality of care. He brought up three critical points that I will discuss further: access to specialty care (such as transplant hepatology), reduction of costs (time and money), and improved patient satisfaction.
Arora and colleagues pioneered the Extension for Community Healthcare Outcomes (ECHO) project, providing complex specialty medical care to underserved populations through a model of team-based interdisciplinary development in hepatitis C infection treatment in underserved communities, with cure rates similar to the university settings.1 The University of Michigan–Veterans Affairs Medical Center used a similar approach, called Specialty Care Access Network–Extension of Community Healthcare Outcome (SCAN‐ECHO). They showed that telemedicine improved survival in 513 patients evaluated in this program compared to regular care (hazard ratio [HR]of 0.54, 95% confidence interval 0.36‐0.81, P = .003).2 So the evidence backs my patient’s request in providing advanced medical care using a telemedicine platform.
An extra benefit of telemedicine in the current climate crisis is reducing the carbon footprint: There’s no need to travel. Telemedicine has been shown to be cost effective: A study of claims data from Jefferson Health reported that patients who received care from an on-demand telemedicine program had net cost savings per telemedicine visit between $19 and $121 per visit compared with traditional in-person visits.3 Using telemonitoring, a form of telemedicine, Bloom et al. showed in 100 simulated patients with cirrhosis and ascites over a 6-month horizon that standard of care was $167,500 more expensive than telemonitoring. The net savings of telemonitoring was always superior in different clinical scenarios.4
Further, our patients significantly decrease travel time (almost instant), improve compliance with medical appointments (more flexibility), and no more headaches related to parking or getting lost around the medical campus. Not surprisingly, these perks from telemedicine are associated with patient-reported outcomes. Reed and colleagues reported patients’ experience with video telemedicine visits in Kaiser Permanente Northern California (n = 1,274) and showed that “67% generally needed to make one or more arrangements to attend an in-person office visit (55% time off from work, 29% coverage for another activity or responsibility, 15% child care or caregiving, and 10% another person to accompany them)”; in contrast, 87% reported a video visit as “more convenient for me,” and 93% stated that “my video visit adequately addressed my needs.”5 In liver transplantation, John et al. showed that, in the Veterans Health Administration, telehealth was associated with a significantly shorter time on the liver transplant waitlist (138.8 vs. 249 days), reduction in the time from referral to evaluation (HR, 0.15; 95% CI, 0.09-0.21; P < .01) and listing (HR, 0.26; 95% CI, 0.12-0.40; P < .01) in a study of 232 patients with advanced cirrhosis.6
So, should I change my approach to patients undergoing care for chronic liver/gastrointestinal diseases? I think so. Telemedicine and its tools provide clear benefits to our patients by increasing access to care, time and money savings, and satisfaction. I am fortunate to work within the largest healthcare network in the Nation – the Veterans Health Administration – and therefore, I can cross state lines to provide medical care/advice using the video-visit tool (VA VideoConnect). One could argue that some patients might find it challenging to access telemedicine appointments, but with adequate coaching or support from our teams, telemedicine visits are a click away.
Going back to my patient, I embraced his request and coached him on using VA VideoConnect. We can continue his waitlist medical care in the following months despite the COVID-19 pandemic using telemedicine. I can assess his asterixis and ascites via his cellphone; his primary care team fills in the vitals and labs to complete a virtual visit. There’s no question in my mind that telemedicine is here to stay and that we will continue to adapt e-health tools into video visits (for example, integrating vitals, measurement of frailty, and remote monitoring). The future of our specialty is here, and I envision we will eventually have home-based hospitalizations with daily virtual rounds.
Dr. Hernaez is with the section of gastroenterology and the Center for Innovations in Quality, Effectiveness, and Safety at the Michael E. DeBakey Veterans Affairs Medical Center and in the section of gastroenterology and hepatology in the department of medicine at Baylor College of Medicine, both in Houston. He has no relevant conflicts of interest.
References
1. Arora S et al. N Engl J Med. 2011 Jun 9;364(23):2199-207.
2. Su GL et al. Hepatology. 2018 Dec;68(6):2317-24.
3. Nord G et al. Am J Emerg Med. 2019 May;37(5):890-4.
4. Bloom PP et al. Dig Dis Sci. 2021. doi: 10.1007/s10620-021-07013-2.
5. Reed ME et al. Ann Intern Med. 2019 Aug;171:222-4.
6. John BV et al. Clin Gastroenterol Hepatol. 2020 Jul;18:1822-30.e4.
It has its limits
BY BYRON P. VAUGHN, MD, MS
The post-pandemic world will include telehealth. Technology disrupts business as usual and often brings positive change. But there are consequences. To employ telehealth into routine care equitably and effectively within a gastroenterology practice, we should consider two general questions: “Was the care I provided the same quality as if the patient was seen in person?” and more broadly, “am I satisfied with my practice’s implementation of telehealth?” This perspective will highlight several areas affecting gastroenterology care: lack of physical exam, disproportionate impact on certain populations, development of a patient-provider relationship, impact on physician well-being, and potential financial ramifications. We will all have to adapt to telemedicine to some extent. Understanding the trade-offs of this technology can help us position effectively in a gastroenterology practice.
Perhaps the most obvious limitation of telemedicine is the lack of vital signs and physical exam. Determining if a patient is “sick or not” is often one of the first lessons trainees learn overnight. Vital signs and physical exam are crucial in the complex triaging that occurs when evaluating for diagnoses with potentially urgent interventions. While most outpatient gastroenterology clinics are not evaluating an acute abdomen, in the correct context, the physical exam provides important nuance and often reassurance. My personal estimate is that 90% of a diagnosis is based on the history. But without physical contact, providers may increase costly downstream diagnostic testing or referrals to the emergency department. Increasing use of at-home or wearable health technology could help but requires system investments in infrastructure to implement.
Telemedicine requires a baseline level of equipment and knowledge to participate. A variety of populations will have either a knowledge gap or technology gap. Lack of rural high-speed Internet can lead to poor video quality, inhibiting effective communication and frustrating both provider and patient. In urban areas, there is a drastic wealth divide, and some groups may have difficulty obtaining sufficient equipment to complete a video visit. Even with adequate infrastructure and equipment, certain groups may be disadvantaged because of a lack of the technological savvy or literacy needed to navigate a virtual visit. The addition of interpreter services adds complexity to communication on top of the virtual interaction. These technology and knowledge gaps can produce confusion and potentially lead to worse care.1 Careful selection of appropriate patients for telemedicine is essential. Is the quality of care over a virtual visit the same for a business executive as that of a non-English speaking refugee?
The term “webside manner” precedes the pandemic but will be important in the lexicon of doctor-patient relationships moving forward. We routinely train physicians about the importance of small actions to improve our bedside manner, such as sitting down, reacting to body language, and making eye contact. First impressions matter in relationship building. For many of my established IBD patients, I can easily hop into a comfortable repertoire in person, virtually, or even on the phone. In addition, I know these people well enough to trust that I am providing the same level of care regardless of visit medium. However, a new patient virtual encounter requires nuance. I have met new patients while they are driving (I requested the patient park!), in public places, and at work. Despite instructions given to patients about the appropriate location for a virtual visit, the patient location is not in our control. For some patients this may increase the comfort of the visit. However, for others, it can lead to distractions or potentially limit the amount of information a patient is willing to share. Forming a patient-provider relationship virtually will require a new set of skills and specific training for many practitioners.
Telehealth can contribute to provider burnout. While a busy in-person clinic can be exhausting, I have found I can be more exhausted after a half-day of virtual clinic. There is an element of human connection that is difficult to replace online.2 On top of that loss, video visits are more psychologically demanding than in-person interactions. I also spend more time in a chair, have fewer coffee breaks, and have fewer professional interactions with the clinic staff and professional colleagues. Several other micro-stressors exist in virtual care that may make “Zoom fatigue” a real occupational hazard.3
Lastly, there are implications on reimbursement with telehealth. In Minnesota, a 2015 telemedicine law required private and state employee health plans to provide the same coverage for telemedicine as in-person visits, although patients had to drive to a clinic or facility to use secure telehealth equipment and have vital signs taken. With the pandemic, this stipulation is waived, and it seems likely to become permanent. However, reimbursement questions will arise, as there is a perception that a 30-minute telephone call should not cost as much as a 30-minute in-person visit, regardless of the content of the conversation.
We will have to learn to move forward with telehealth. The strength of telehealth is likely in patients with chronic, well controlled diseases, who have frequent interactions with health care. Examples of this include (although certainly are not limited to) established patients with well-controlled IBD, non-cirrhotic liver disease, and irritable bowel syndrome. Triaging patients who need in-person evaluation, ensuring patient and provider well-being, and creating a financially sustainable model of care are yet unresolved issues. Providers will likely vary in their personal acceptance of telehealth and will need to advocate within their own systems to obtain a hybrid model of telehealth that maximizes quality of care with job satisfaction.
Dr. Vaughn is associate professor of medicine and codirector of the inflammatory bowel disease program in the division of gastroenterology, hepatology, and nutrition at University of Minnesota, Minneapolis. He has received consulting fees from Prometheus and research support from Roche, Takeda, Celgene, Diasorin, and Crestovo.
References
1. George S et al. Stud Health Technol Inform. 2013;192:946.
2. Blank S. “What’s missing from Zoom reminds us what it means to be human,” 2020 Apr 27, Medium.
3. Williams N. Occup Med (Lond). 2021 Apr. doi: 10.1093/occmed/kqab041.
Dear colleagues and friends,
I am fortunate to receive the baton from Charles Kahi, MD, in facilitating the fascinating and timely debates that have characterized the AGA Perspective series. Favorable reimbursement changes and the need for social distancing fast-tracked telemedicine, a care delivery model that had been slowly evolving.
In this month’s Perspective column, Dr. Hernaez and Dr. Vaughn discuss the pros and cons of telemedicine in GI. Is it the new office visit? Or simply just good enough for when we really need it? I look forward to hearing your thoughts and experiences on the AGA Community forum as well as by email ([email protected]).
Gyanprakash A. Ketwaroo, MD, is assistant professor of medicine at Baylor College of Medicine, Houston. He is an associate editor for GI & Hepatology News.
It holds promise
BY RUBEN HERNAEZ, MD, MPH, PHD
It was around January 2020, when COVID-19 was something far, far away, and not particularly worrisome. I was performing a routine care visit of one of my out-of-state patients waitlisted for a liver transplant. All was going fine until he stated, “Hey doc, while I appreciate your time and visits, these travels to Houston are quite inconvenient for my family and me: It is a logistical ordeal, and my wife is always afraid of catching something in the airplane. Could you do the same remotely such as a videoconference?” And just like that, it sparked my interest in how to maintain his liver transplant care from a distance. The Federation of State Medical Boards defines telemedicine as “the practice of medicine using electronic communication, information technology, or other means between a physician in one location, and a patient in another location, with or without an intervening health care provider.1 What my patient was asking was to use a mode of telemedicine – a video visit – to receive the same quality of care. He brought up three critical points that I will discuss further: access to specialty care (such as transplant hepatology), reduction of costs (time and money), and improved patient satisfaction.
Arora and colleagues pioneered the Extension for Community Healthcare Outcomes (ECHO) project, providing complex specialty medical care to underserved populations through a model of team-based interdisciplinary development in hepatitis C infection treatment in underserved communities, with cure rates similar to the university settings.1 The University of Michigan–Veterans Affairs Medical Center used a similar approach, called Specialty Care Access Network–Extension of Community Healthcare Outcome (SCAN‐ECHO). They showed that telemedicine improved survival in 513 patients evaluated in this program compared to regular care (hazard ratio [HR]of 0.54, 95% confidence interval 0.36‐0.81, P = .003).2 So the evidence backs my patient’s request in providing advanced medical care using a telemedicine platform.
An extra benefit of telemedicine in the current climate crisis is reducing the carbon footprint: There’s no need to travel. Telemedicine has been shown to be cost effective: A study of claims data from Jefferson Health reported that patients who received care from an on-demand telemedicine program had net cost savings per telemedicine visit between $19 and $121 per visit compared with traditional in-person visits.3 Using telemonitoring, a form of telemedicine, Bloom et al. showed in 100 simulated patients with cirrhosis and ascites over a 6-month horizon that standard of care was $167,500 more expensive than telemonitoring. The net savings of telemonitoring was always superior in different clinical scenarios.4
Further, our patients significantly decrease travel time (almost instant), improve compliance with medical appointments (more flexibility), and no more headaches related to parking or getting lost around the medical campus. Not surprisingly, these perks from telemedicine are associated with patient-reported outcomes. Reed and colleagues reported patients’ experience with video telemedicine visits in Kaiser Permanente Northern California (n = 1,274) and showed that “67% generally needed to make one or more arrangements to attend an in-person office visit (55% time off from work, 29% coverage for another activity or responsibility, 15% child care or caregiving, and 10% another person to accompany them)”; in contrast, 87% reported a video visit as “more convenient for me,” and 93% stated that “my video visit adequately addressed my needs.”5 In liver transplantation, John et al. showed that, in the Veterans Health Administration, telehealth was associated with a significantly shorter time on the liver transplant waitlist (138.8 vs. 249 days), reduction in the time from referral to evaluation (HR, 0.15; 95% CI, 0.09-0.21; P < .01) and listing (HR, 0.26; 95% CI, 0.12-0.40; P < .01) in a study of 232 patients with advanced cirrhosis.6
So, should I change my approach to patients undergoing care for chronic liver/gastrointestinal diseases? I think so. Telemedicine and its tools provide clear benefits to our patients by increasing access to care, time and money savings, and satisfaction. I am fortunate to work within the largest healthcare network in the Nation – the Veterans Health Administration – and therefore, I can cross state lines to provide medical care/advice using the video-visit tool (VA VideoConnect). One could argue that some patients might find it challenging to access telemedicine appointments, but with adequate coaching or support from our teams, telemedicine visits are a click away.
Going back to my patient, I embraced his request and coached him on using VA VideoConnect. We can continue his waitlist medical care in the following months despite the COVID-19 pandemic using telemedicine. I can assess his asterixis and ascites via his cellphone; his primary care team fills in the vitals and labs to complete a virtual visit. There’s no question in my mind that telemedicine is here to stay and that we will continue to adapt e-health tools into video visits (for example, integrating vitals, measurement of frailty, and remote monitoring). The future of our specialty is here, and I envision we will eventually have home-based hospitalizations with daily virtual rounds.
Dr. Hernaez is with the section of gastroenterology and the Center for Innovations in Quality, Effectiveness, and Safety at the Michael E. DeBakey Veterans Affairs Medical Center and in the section of gastroenterology and hepatology in the department of medicine at Baylor College of Medicine, both in Houston. He has no relevant conflicts of interest.
References
1. Arora S et al. N Engl J Med. 2011 Jun 9;364(23):2199-207.
2. Su GL et al. Hepatology. 2018 Dec;68(6):2317-24.
3. Nord G et al. Am J Emerg Med. 2019 May;37(5):890-4.
4. Bloom PP et al. Dig Dis Sci. 2021. doi: 10.1007/s10620-021-07013-2.
5. Reed ME et al. Ann Intern Med. 2019 Aug;171:222-4.
6. John BV et al. Clin Gastroenterol Hepatol. 2020 Jul;18:1822-30.e4.
It has its limits
BY BYRON P. VAUGHN, MD, MS
The post-pandemic world will include telehealth. Technology disrupts business as usual and often brings positive change. But there are consequences. To employ telehealth into routine care equitably and effectively within a gastroenterology practice, we should consider two general questions: “Was the care I provided the same quality as if the patient was seen in person?” and more broadly, “am I satisfied with my practice’s implementation of telehealth?” This perspective will highlight several areas affecting gastroenterology care: lack of physical exam, disproportionate impact on certain populations, development of a patient-provider relationship, impact on physician well-being, and potential financial ramifications. We will all have to adapt to telemedicine to some extent. Understanding the trade-offs of this technology can help us position effectively in a gastroenterology practice.
Perhaps the most obvious limitation of telemedicine is the lack of vital signs and physical exam. Determining if a patient is “sick or not” is often one of the first lessons trainees learn overnight. Vital signs and physical exam are crucial in the complex triaging that occurs when evaluating for diagnoses with potentially urgent interventions. While most outpatient gastroenterology clinics are not evaluating an acute abdomen, in the correct context, the physical exam provides important nuance and often reassurance. My personal estimate is that 90% of a diagnosis is based on the history. But without physical contact, providers may increase costly downstream diagnostic testing or referrals to the emergency department. Increasing use of at-home or wearable health technology could help but requires system investments in infrastructure to implement.
Telemedicine requires a baseline level of equipment and knowledge to participate. A variety of populations will have either a knowledge gap or technology gap. Lack of rural high-speed Internet can lead to poor video quality, inhibiting effective communication and frustrating both provider and patient. In urban areas, there is a drastic wealth divide, and some groups may have difficulty obtaining sufficient equipment to complete a video visit. Even with adequate infrastructure and equipment, certain groups may be disadvantaged because of a lack of the technological savvy or literacy needed to navigate a virtual visit. The addition of interpreter services adds complexity to communication on top of the virtual interaction. These technology and knowledge gaps can produce confusion and potentially lead to worse care.1 Careful selection of appropriate patients for telemedicine is essential. Is the quality of care over a virtual visit the same for a business executive as that of a non-English speaking refugee?
The term “webside manner” precedes the pandemic but will be important in the lexicon of doctor-patient relationships moving forward. We routinely train physicians about the importance of small actions to improve our bedside manner, such as sitting down, reacting to body language, and making eye contact. First impressions matter in relationship building. For many of my established IBD patients, I can easily hop into a comfortable repertoire in person, virtually, or even on the phone. In addition, I know these people well enough to trust that I am providing the same level of care regardless of visit medium. However, a new patient virtual encounter requires nuance. I have met new patients while they are driving (I requested the patient park!), in public places, and at work. Despite instructions given to patients about the appropriate location for a virtual visit, the patient location is not in our control. For some patients this may increase the comfort of the visit. However, for others, it can lead to distractions or potentially limit the amount of information a patient is willing to share. Forming a patient-provider relationship virtually will require a new set of skills and specific training for many practitioners.
Telehealth can contribute to provider burnout. While a busy in-person clinic can be exhausting, I have found I can be more exhausted after a half-day of virtual clinic. There is an element of human connection that is difficult to replace online.2 On top of that loss, video visits are more psychologically demanding than in-person interactions. I also spend more time in a chair, have fewer coffee breaks, and have fewer professional interactions with the clinic staff and professional colleagues. Several other micro-stressors exist in virtual care that may make “Zoom fatigue” a real occupational hazard.3
Lastly, there are implications on reimbursement with telehealth. In Minnesota, a 2015 telemedicine law required private and state employee health plans to provide the same coverage for telemedicine as in-person visits, although patients had to drive to a clinic or facility to use secure telehealth equipment and have vital signs taken. With the pandemic, this stipulation is waived, and it seems likely to become permanent. However, reimbursement questions will arise, as there is a perception that a 30-minute telephone call should not cost as much as a 30-minute in-person visit, regardless of the content of the conversation.
We will have to learn to move forward with telehealth. The strength of telehealth is likely in patients with chronic, well controlled diseases, who have frequent interactions with health care. Examples of this include (although certainly are not limited to) established patients with well-controlled IBD, non-cirrhotic liver disease, and irritable bowel syndrome. Triaging patients who need in-person evaluation, ensuring patient and provider well-being, and creating a financially sustainable model of care are yet unresolved issues. Providers will likely vary in their personal acceptance of telehealth and will need to advocate within their own systems to obtain a hybrid model of telehealth that maximizes quality of care with job satisfaction.
Dr. Vaughn is associate professor of medicine and codirector of the inflammatory bowel disease program in the division of gastroenterology, hepatology, and nutrition at University of Minnesota, Minneapolis. He has received consulting fees from Prometheus and research support from Roche, Takeda, Celgene, Diasorin, and Crestovo.
References
1. George S et al. Stud Health Technol Inform. 2013;192:946.
2. Blank S. “What’s missing from Zoom reminds us what it means to be human,” 2020 Apr 27, Medium.
3. Williams N. Occup Med (Lond). 2021 Apr. doi: 10.1093/occmed/kqab041.
80% of Americans research recommendations post-visit
Confusion over health information and doctor advice is even higher among people who care for patients than among those who don’t provide care to their loved ones, the nationally representative survey from the AHIMA Foundation found.
The survey also shows that 80% of Americans – and an even higher portion of caregivers – are likely to research medical recommendations online after a doctor’s visit. But 1 in 4 people don’t know how to access their own medical records or find it difficult to do so.
The findings reflect the same low level of health literacy in the U.S. population that earlier surveys did. The results also indicate that little has changed since the Department of Health and Human Services released a National Action Plan to Improve Health Literacy in 2010.
That plan emphasized the need to develop and share accurate health information that helps people make decisions; to promote changes in the health care system that improve health information, communication, informed decision-making, and access to health services; and to increase the sharing and use of evidence-based health literacy practices.
According to the AHIMA Foundation report, 62% of Americans are not sure they understand their doctor’s advice and the health information discussed during a visit. Twenty-four percent say they don’t comprehend any of it, and 31% can’t remember what was said during the visit. Fifteen percent of those surveyed said they were more confused about their health than they were before the encounter with their doctor.
Caregivers have special issues
Forty-three percent of Americans are caregivers, the report notes, and 91% of those play an active role in managing someone else’s health. Millennials (65%) and Gen Xers (50%) are significantly more likely than Gen Zers (39%) and Boomers (20%) to be a caregiver.
Most caregivers have concerns about their loved ones’ ability to manage their own health. Most of them believe that doctors provide enough information, but 38% don’t believe a doctor can communicate effectively with the patient if the caregiver is not present.
Forty-three percent of caretakers don’t think their loved ones can understand medical information on their own. On the other hand, caregivers are more likely than people who don’t provide care to say the doctor confused them and to research the doctor’s advice after an appointment.
For many patients and caregivers, communications break down when they are with their health care provider. Twenty-two percent of Americans say they do not feel comfortable asking their doctor certain health questions. This inability to have a satisfactory dialogue with their doctor means that many patients leave their appointments without getting clear answers to their questions (24%) or without having an opportunity to ask any questions at all (17%).
This is not surprising, considering that a 2018 study found that doctors spend only 11 seconds, on average, listening to patients before interrupting them.
Depending on the internet
Overall, the AHIMA survey found, 42% of Americans research their doctor’s recommendations after an appointment. A higher percentage of caregivers than noncaregiver peers do so (47% vs. 38%). Eighty percent of respondents say they are “likely” to research their doctor’s advice online after a visit.
When they have a medical problem or a question about their condition, just as many Americans (59%) turn to the internet for an answer as contact their doctor directly, the survey found. Twenty-nine percent of the respondents consult friends, family, or colleagues; 23% look up medical records if they’re easily accessible; 19% ask pharmacists for advice; and 6% call an unspecified 800 number.
Americans feel secure in the health information they find on the internet. Among those who go online to look up information, 86% are confident that it is credible. And 42% report feeling relieved that they can find a lot of information about their health concerns. Respondents also say that the information they gather allows them to feel more confident in their doctor’s recommendations (35%) and that they feel better after having learned more on the internet than their doctor had told them (39%). Men are more likely than women to say that their confidence in their doctor’s recommendations increased after doing online research (40% vs. 30%).
Access to health records
Access to medical records would help people better understand their condition or diagnosis. But nearly half of Americans (48%) admit they don’t usually review their medical records until long after an appointment, and 52% say they rarely access their records at all.
One in four Americans say that they don’t know where to go to access their health information or that they didn’t find the process easy. More than half of those who have never had to find their records think the process would be difficult if they had to try.
Eighty-one percent of Americans use an online platform or portal to access their medical records or health information. Two-thirds of Americans who use an online portal trust that their medical information is kept safe and not shared with other people or organizations.
Four in five respondents agree that if they had access to all of their health information, including medical records, recommendations, conditions, and test results, they’d see an improvement in their health management. Fifty-nine percent of them believe they’d also be more confident about understanding their health, and 47% say they’d have greater trust in their doctor’s recommendations. Higher percentages of caregivers than noncaregivers say the same.
Younger people, those with a high school degree or less, and those who earn less than $50,000 are less likely than older, better educated, and more affluent people to understand their doctor’s health information and to ask questions of their providers.
People of color struggle with their relationships with doctors, are less satisfied than white people with the information they receive during visits, and are more likely than white peers to feel that if they had access to all their health information, they’d manage their health better and be more confident in their doctors’ recommendations, the survey found.
A version of this article first appeared on WebMD.com.
Confusion over health information and doctor advice is even higher among people who care for patients than among those who don’t provide care to their loved ones, the nationally representative survey from the AHIMA Foundation found.
The survey also shows that 80% of Americans – and an even higher portion of caregivers – are likely to research medical recommendations online after a doctor’s visit. But 1 in 4 people don’t know how to access their own medical records or find it difficult to do so.
The findings reflect the same low level of health literacy in the U.S. population that earlier surveys did. The results also indicate that little has changed since the Department of Health and Human Services released a National Action Plan to Improve Health Literacy in 2010.
That plan emphasized the need to develop and share accurate health information that helps people make decisions; to promote changes in the health care system that improve health information, communication, informed decision-making, and access to health services; and to increase the sharing and use of evidence-based health literacy practices.
According to the AHIMA Foundation report, 62% of Americans are not sure they understand their doctor’s advice and the health information discussed during a visit. Twenty-four percent say they don’t comprehend any of it, and 31% can’t remember what was said during the visit. Fifteen percent of those surveyed said they were more confused about their health than they were before the encounter with their doctor.
Caregivers have special issues
Forty-three percent of Americans are caregivers, the report notes, and 91% of those play an active role in managing someone else’s health. Millennials (65%) and Gen Xers (50%) are significantly more likely than Gen Zers (39%) and Boomers (20%) to be a caregiver.
Most caregivers have concerns about their loved ones’ ability to manage their own health. Most of them believe that doctors provide enough information, but 38% don’t believe a doctor can communicate effectively with the patient if the caregiver is not present.
Forty-three percent of caretakers don’t think their loved ones can understand medical information on their own. On the other hand, caregivers are more likely than people who don’t provide care to say the doctor confused them and to research the doctor’s advice after an appointment.
For many patients and caregivers, communications break down when they are with their health care provider. Twenty-two percent of Americans say they do not feel comfortable asking their doctor certain health questions. This inability to have a satisfactory dialogue with their doctor means that many patients leave their appointments without getting clear answers to their questions (24%) or without having an opportunity to ask any questions at all (17%).
This is not surprising, considering that a 2018 study found that doctors spend only 11 seconds, on average, listening to patients before interrupting them.
Depending on the internet
Overall, the AHIMA survey found, 42% of Americans research their doctor’s recommendations after an appointment. A higher percentage of caregivers than noncaregiver peers do so (47% vs. 38%). Eighty percent of respondents say they are “likely” to research their doctor’s advice online after a visit.
When they have a medical problem or a question about their condition, just as many Americans (59%) turn to the internet for an answer as contact their doctor directly, the survey found. Twenty-nine percent of the respondents consult friends, family, or colleagues; 23% look up medical records if they’re easily accessible; 19% ask pharmacists for advice; and 6% call an unspecified 800 number.
Americans feel secure in the health information they find on the internet. Among those who go online to look up information, 86% are confident that it is credible. And 42% report feeling relieved that they can find a lot of information about their health concerns. Respondents also say that the information they gather allows them to feel more confident in their doctor’s recommendations (35%) and that they feel better after having learned more on the internet than their doctor had told them (39%). Men are more likely than women to say that their confidence in their doctor’s recommendations increased after doing online research (40% vs. 30%).
Access to health records
Access to medical records would help people better understand their condition or diagnosis. But nearly half of Americans (48%) admit they don’t usually review their medical records until long after an appointment, and 52% say they rarely access their records at all.
One in four Americans say that they don’t know where to go to access their health information or that they didn’t find the process easy. More than half of those who have never had to find their records think the process would be difficult if they had to try.
Eighty-one percent of Americans use an online platform or portal to access their medical records or health information. Two-thirds of Americans who use an online portal trust that their medical information is kept safe and not shared with other people or organizations.
Four in five respondents agree that if they had access to all of their health information, including medical records, recommendations, conditions, and test results, they’d see an improvement in their health management. Fifty-nine percent of them believe they’d also be more confident about understanding their health, and 47% say they’d have greater trust in their doctor’s recommendations. Higher percentages of caregivers than noncaregivers say the same.
Younger people, those with a high school degree or less, and those who earn less than $50,000 are less likely than older, better educated, and more affluent people to understand their doctor’s health information and to ask questions of their providers.
People of color struggle with their relationships with doctors, are less satisfied than white people with the information they receive during visits, and are more likely than white peers to feel that if they had access to all their health information, they’d manage their health better and be more confident in their doctors’ recommendations, the survey found.
A version of this article first appeared on WebMD.com.
Confusion over health information and doctor advice is even higher among people who care for patients than among those who don’t provide care to their loved ones, the nationally representative survey from the AHIMA Foundation found.
The survey also shows that 80% of Americans – and an even higher portion of caregivers – are likely to research medical recommendations online after a doctor’s visit. But 1 in 4 people don’t know how to access their own medical records or find it difficult to do so.
The findings reflect the same low level of health literacy in the U.S. population that earlier surveys did. The results also indicate that little has changed since the Department of Health and Human Services released a National Action Plan to Improve Health Literacy in 2010.
That plan emphasized the need to develop and share accurate health information that helps people make decisions; to promote changes in the health care system that improve health information, communication, informed decision-making, and access to health services; and to increase the sharing and use of evidence-based health literacy practices.
According to the AHIMA Foundation report, 62% of Americans are not sure they understand their doctor’s advice and the health information discussed during a visit. Twenty-four percent say they don’t comprehend any of it, and 31% can’t remember what was said during the visit. Fifteen percent of those surveyed said they were more confused about their health than they were before the encounter with their doctor.
Caregivers have special issues
Forty-three percent of Americans are caregivers, the report notes, and 91% of those play an active role in managing someone else’s health. Millennials (65%) and Gen Xers (50%) are significantly more likely than Gen Zers (39%) and Boomers (20%) to be a caregiver.
Most caregivers have concerns about their loved ones’ ability to manage their own health. Most of them believe that doctors provide enough information, but 38% don’t believe a doctor can communicate effectively with the patient if the caregiver is not present.
Forty-three percent of caretakers don’t think their loved ones can understand medical information on their own. On the other hand, caregivers are more likely than people who don’t provide care to say the doctor confused them and to research the doctor’s advice after an appointment.
For many patients and caregivers, communications break down when they are with their health care provider. Twenty-two percent of Americans say they do not feel comfortable asking their doctor certain health questions. This inability to have a satisfactory dialogue with their doctor means that many patients leave their appointments without getting clear answers to their questions (24%) or without having an opportunity to ask any questions at all (17%).
This is not surprising, considering that a 2018 study found that doctors spend only 11 seconds, on average, listening to patients before interrupting them.
Depending on the internet
Overall, the AHIMA survey found, 42% of Americans research their doctor’s recommendations after an appointment. A higher percentage of caregivers than noncaregiver peers do so (47% vs. 38%). Eighty percent of respondents say they are “likely” to research their doctor’s advice online after a visit.
When they have a medical problem or a question about their condition, just as many Americans (59%) turn to the internet for an answer as contact their doctor directly, the survey found. Twenty-nine percent of the respondents consult friends, family, or colleagues; 23% look up medical records if they’re easily accessible; 19% ask pharmacists for advice; and 6% call an unspecified 800 number.
Americans feel secure in the health information they find on the internet. Among those who go online to look up information, 86% are confident that it is credible. And 42% report feeling relieved that they can find a lot of information about their health concerns. Respondents also say that the information they gather allows them to feel more confident in their doctor’s recommendations (35%) and that they feel better after having learned more on the internet than their doctor had told them (39%). Men are more likely than women to say that their confidence in their doctor’s recommendations increased after doing online research (40% vs. 30%).
Access to health records
Access to medical records would help people better understand their condition or diagnosis. But nearly half of Americans (48%) admit they don’t usually review their medical records until long after an appointment, and 52% say they rarely access their records at all.
One in four Americans say that they don’t know where to go to access their health information or that they didn’t find the process easy. More than half of those who have never had to find their records think the process would be difficult if they had to try.
Eighty-one percent of Americans use an online platform or portal to access their medical records or health information. Two-thirds of Americans who use an online portal trust that their medical information is kept safe and not shared with other people or organizations.
Four in five respondents agree that if they had access to all of their health information, including medical records, recommendations, conditions, and test results, they’d see an improvement in their health management. Fifty-nine percent of them believe they’d also be more confident about understanding their health, and 47% say they’d have greater trust in their doctor’s recommendations. Higher percentages of caregivers than noncaregivers say the same.
Younger people, those with a high school degree or less, and those who earn less than $50,000 are less likely than older, better educated, and more affluent people to understand their doctor’s health information and to ask questions of their providers.
People of color struggle with their relationships with doctors, are less satisfied than white people with the information they receive during visits, and are more likely than white peers to feel that if they had access to all their health information, they’d manage their health better and be more confident in their doctors’ recommendations, the survey found.
A version of this article first appeared on WebMD.com.
Now Takeda offers rebate if lung cancer drug fails to work
The rebate offer is for brigatinib (Alunbrig) which is approved for the treatment of adults with anaplastic lymphoma kinase positive (ALK+) metastatic non–small cell lung cancer (NSCLC) as detected by an FDA-approved test.
The move follows a rebate offer from Pfizer for crizotinib (Xalkori), which is also approved for ALK+ (as well as ROS1+) NSCLC, and also for ALK+ anaplastic large cell lymphoma
For its offer, Takeda has teamed up with Point32Health, the second-largest health plan in New England with about 2.3 million members. The new agreement will make brigatinib widely available to patients who may benefit from its use, say the companies.
If a patient is unable to remain on brigatinib for 3 months or longer because of effectiveness or tolerability, Takeda will refund a yet unspecified amount of money to Point32Health. Brigatinib’s list price is $17,000 for a month’s treatment.
“Given the importance of facilitating cutting-edge oncology treatment and also the reality that not all patients show a positive response, reimbursement for oncology treatments is an area that is prime for innovative financing approaches,” said Michael Sherman, MD, chief medical officer and executive vice president, Point32Health, in a statement. “Collaborating with Takeda to share risk makes this agreement a crucial milestone in bringing cost-effectiveness to cancer care.”
The Pfizer program for crizotinib is somewhat different. For one thing, Pfizer’s refund is offered to any patient who qualifies and not just those who are covered by a specific plan. Second, Takeda is thus far only refunding money to the insurer, whereas Pfizer will also reimburse patients for out-of-pocket expenses.
There is a similar approach that has been offered by Novartis for tisagenlecleucel (Kymriah), the CAR T-cell therapy that launched with a daunting price tag of $475,000. After receiving backlash over the cost, Novartis announced that if the drug does not work after the first month, patients pay nothing.
In addition, Italy has been using this system for several years. Pharmaceutical companies must refund money if the drug fails to work. In 2015, the state-run health care system collected 200 million euros ($220 million) in refunds.
A version of this article first appeared on Medscape.com.
The rebate offer is for brigatinib (Alunbrig) which is approved for the treatment of adults with anaplastic lymphoma kinase positive (ALK+) metastatic non–small cell lung cancer (NSCLC) as detected by an FDA-approved test.
The move follows a rebate offer from Pfizer for crizotinib (Xalkori), which is also approved for ALK+ (as well as ROS1+) NSCLC, and also for ALK+ anaplastic large cell lymphoma
For its offer, Takeda has teamed up with Point32Health, the second-largest health plan in New England with about 2.3 million members. The new agreement will make brigatinib widely available to patients who may benefit from its use, say the companies.
If a patient is unable to remain on brigatinib for 3 months or longer because of effectiveness or tolerability, Takeda will refund a yet unspecified amount of money to Point32Health. Brigatinib’s list price is $17,000 for a month’s treatment.
“Given the importance of facilitating cutting-edge oncology treatment and also the reality that not all patients show a positive response, reimbursement for oncology treatments is an area that is prime for innovative financing approaches,” said Michael Sherman, MD, chief medical officer and executive vice president, Point32Health, in a statement. “Collaborating with Takeda to share risk makes this agreement a crucial milestone in bringing cost-effectiveness to cancer care.”
The Pfizer program for crizotinib is somewhat different. For one thing, Pfizer’s refund is offered to any patient who qualifies and not just those who are covered by a specific plan. Second, Takeda is thus far only refunding money to the insurer, whereas Pfizer will also reimburse patients for out-of-pocket expenses.
There is a similar approach that has been offered by Novartis for tisagenlecleucel (Kymriah), the CAR T-cell therapy that launched with a daunting price tag of $475,000. After receiving backlash over the cost, Novartis announced that if the drug does not work after the first month, patients pay nothing.
In addition, Italy has been using this system for several years. Pharmaceutical companies must refund money if the drug fails to work. In 2015, the state-run health care system collected 200 million euros ($220 million) in refunds.
A version of this article first appeared on Medscape.com.
The rebate offer is for brigatinib (Alunbrig) which is approved for the treatment of adults with anaplastic lymphoma kinase positive (ALK+) metastatic non–small cell lung cancer (NSCLC) as detected by an FDA-approved test.
The move follows a rebate offer from Pfizer for crizotinib (Xalkori), which is also approved for ALK+ (as well as ROS1+) NSCLC, and also for ALK+ anaplastic large cell lymphoma
For its offer, Takeda has teamed up with Point32Health, the second-largest health plan in New England with about 2.3 million members. The new agreement will make brigatinib widely available to patients who may benefit from its use, say the companies.
If a patient is unable to remain on brigatinib for 3 months or longer because of effectiveness or tolerability, Takeda will refund a yet unspecified amount of money to Point32Health. Brigatinib’s list price is $17,000 for a month’s treatment.
“Given the importance of facilitating cutting-edge oncology treatment and also the reality that not all patients show a positive response, reimbursement for oncology treatments is an area that is prime for innovative financing approaches,” said Michael Sherman, MD, chief medical officer and executive vice president, Point32Health, in a statement. “Collaborating with Takeda to share risk makes this agreement a crucial milestone in bringing cost-effectiveness to cancer care.”
The Pfizer program for crizotinib is somewhat different. For one thing, Pfizer’s refund is offered to any patient who qualifies and not just those who are covered by a specific plan. Second, Takeda is thus far only refunding money to the insurer, whereas Pfizer will also reimburse patients for out-of-pocket expenses.
There is a similar approach that has been offered by Novartis for tisagenlecleucel (Kymriah), the CAR T-cell therapy that launched with a daunting price tag of $475,000. After receiving backlash over the cost, Novartis announced that if the drug does not work after the first month, patients pay nothing.
In addition, Italy has been using this system for several years. Pharmaceutical companies must refund money if the drug fails to work. In 2015, the state-run health care system collected 200 million euros ($220 million) in refunds.
A version of this article first appeared on Medscape.com.
Which specialties get the biggest markups over Medicare rates?
Anesthesiologists charge private insurers more than 300% above Medicare rates, a markup that is higher than that of 16 other specialties, according to a study released by the Urban Institute.
The Washington-based nonprofit institute found that the lowest markups were in psychiatry, ophthalmology, ob.gyn., family medicine, gastroenterology, and internal medicine, at 110%-120% of Medicare rates. .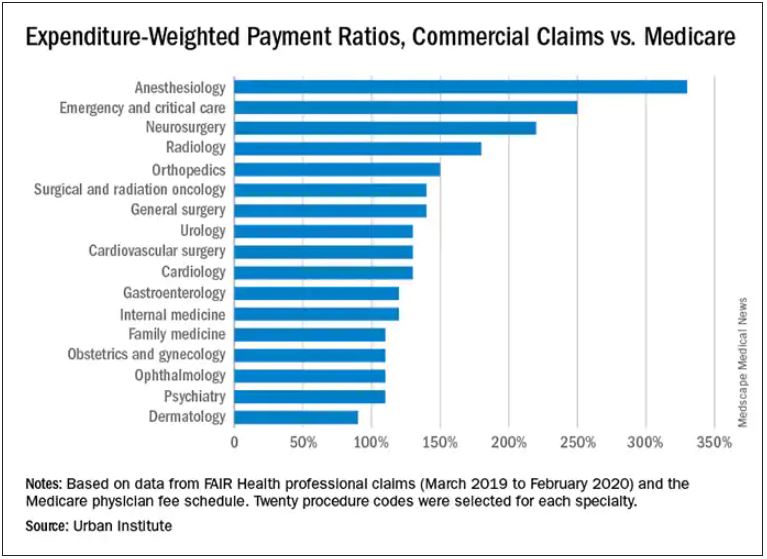
In the middle are cardiology and cardiovascular surgery (130%), urology (130%), general surgery, surgical and radiation oncology (all at 140%), and orthopedics (150%).
At the top end were radiology (180%), neurosurgery (220%), emergency and critical care (250%), and anesthesiology (330%).
The wide variation in payments could be cited in support of the idea of applying Medicare rates across all physician specialties, say the study authors. Although lowering practitioner payments might lead to savings, it “will also create more pushback from providers, especially if these rates are introduced in the employer market,” write researchers Stacey McMorrow, PhD, Robert A. Berenson, MD, and John Holahan, PhD.
It is not known whether lowering commercial payment rates might decrease patient access, they write.
The authors also note that specialties in which the potential for a fee reduction was greatest were also the specialties for which baseline compensation was highest – from $350,000 annually for emergency physicians to $800,000 a year for neurosurgeons. Annual compensation for ob.gyns., dermatologists, and opthalmologists is about $350,000 a year, which suggests that “these specialties are similarly well compensated by both Medicare and commercial insurers,” the authors write.
The investigators assessed the top 20 procedure codes by expenditure in each of 17 physician specialties. They estimated the commercial-to-Medicare payment ratio for each service and constructed weighted averages across services for each specialty at the national level and for 12 states for which data for all the specialties and services were available.
The researchers analyzed claims from the FAIR Health database between March 2019 and March 2020. That database represents 60 insurers covering 150 million people.
Pediatric and geriatric specialties, nonphysician practitioners, out-of-network clinicians, and ambulatory surgery center claims were excluded. Codes with modifiers, J codes, and clinical laboratory services were also not included.
The charges used in the study were not the actual contracted rates. The authors instead used “imputed allowed amounts” for each claim line. That method was used to protect the confidentiality of the negotiated rates.
With regard to all specialties, the lowest compensated services were procedures, evaluation and management, and tests, which received 140%-150% of the Medicare rate. Treatments and imaging were marked up 160%. Anesthesia was reimbursed at a rate 330% higher than the rate Medicare would pay.
The authors also assessed geographic variation for the 12 states for which they had data.
Similar to findings in other studies, the researchers found that the markup was lowest in Pennsylvania (120%) and highest in Wisconsin (260%). The U.S. average was 160%. California and Missouri were at 150%; Michigan was right at the average.
For physicians in Illinois, Louisiana, Colorado, Texas, and New York, markups were 170%-180% over the Medicare rate. Markups for clinicians in New Jersey (190%) and Arizona (200%) were closest to the Wisconsin rate.
The authors note some study limitations, including the fact that they excluded out-of-network practitioners, “and such payments may disproportionately affect certain specialties.”
A version of this article first appeared on Medscape.com.
Anesthesiologists charge private insurers more than 300% above Medicare rates, a markup that is higher than that of 16 other specialties, according to a study released by the Urban Institute.
The Washington-based nonprofit institute found that the lowest markups were in psychiatry, ophthalmology, ob.gyn., family medicine, gastroenterology, and internal medicine, at 110%-120% of Medicare rates. .
In the middle are cardiology and cardiovascular surgery (130%), urology (130%), general surgery, surgical and radiation oncology (all at 140%), and orthopedics (150%).
At the top end were radiology (180%), neurosurgery (220%), emergency and critical care (250%), and anesthesiology (330%).
The wide variation in payments could be cited in support of the idea of applying Medicare rates across all physician specialties, say the study authors. Although lowering practitioner payments might lead to savings, it “will also create more pushback from providers, especially if these rates are introduced in the employer market,” write researchers Stacey McMorrow, PhD, Robert A. Berenson, MD, and John Holahan, PhD.
It is not known whether lowering commercial payment rates might decrease patient access, they write.
The authors also note that specialties in which the potential for a fee reduction was greatest were also the specialties for which baseline compensation was highest – from $350,000 annually for emergency physicians to $800,000 a year for neurosurgeons. Annual compensation for ob.gyns., dermatologists, and opthalmologists is about $350,000 a year, which suggests that “these specialties are similarly well compensated by both Medicare and commercial insurers,” the authors write.
The investigators assessed the top 20 procedure codes by expenditure in each of 17 physician specialties. They estimated the commercial-to-Medicare payment ratio for each service and constructed weighted averages across services for each specialty at the national level and for 12 states for which data for all the specialties and services were available.
The researchers analyzed claims from the FAIR Health database between March 2019 and March 2020. That database represents 60 insurers covering 150 million people.
Pediatric and geriatric specialties, nonphysician practitioners, out-of-network clinicians, and ambulatory surgery center claims were excluded. Codes with modifiers, J codes, and clinical laboratory services were also not included.
The charges used in the study were not the actual contracted rates. The authors instead used “imputed allowed amounts” for each claim line. That method was used to protect the confidentiality of the negotiated rates.
With regard to all specialties, the lowest compensated services were procedures, evaluation and management, and tests, which received 140%-150% of the Medicare rate. Treatments and imaging were marked up 160%. Anesthesia was reimbursed at a rate 330% higher than the rate Medicare would pay.
The authors also assessed geographic variation for the 12 states for which they had data.
Similar to findings in other studies, the researchers found that the markup was lowest in Pennsylvania (120%) and highest in Wisconsin (260%). The U.S. average was 160%. California and Missouri were at 150%; Michigan was right at the average.
For physicians in Illinois, Louisiana, Colorado, Texas, and New York, markups were 170%-180% over the Medicare rate. Markups for clinicians in New Jersey (190%) and Arizona (200%) were closest to the Wisconsin rate.
The authors note some study limitations, including the fact that they excluded out-of-network practitioners, “and such payments may disproportionately affect certain specialties.”
A version of this article first appeared on Medscape.com.
Anesthesiologists charge private insurers more than 300% above Medicare rates, a markup that is higher than that of 16 other specialties, according to a study released by the Urban Institute.
The Washington-based nonprofit institute found that the lowest markups were in psychiatry, ophthalmology, ob.gyn., family medicine, gastroenterology, and internal medicine, at 110%-120% of Medicare rates. .
In the middle are cardiology and cardiovascular surgery (130%), urology (130%), general surgery, surgical and radiation oncology (all at 140%), and orthopedics (150%).
At the top end were radiology (180%), neurosurgery (220%), emergency and critical care (250%), and anesthesiology (330%).
The wide variation in payments could be cited in support of the idea of applying Medicare rates across all physician specialties, say the study authors. Although lowering practitioner payments might lead to savings, it “will also create more pushback from providers, especially if these rates are introduced in the employer market,” write researchers Stacey McMorrow, PhD, Robert A. Berenson, MD, and John Holahan, PhD.
It is not known whether lowering commercial payment rates might decrease patient access, they write.
The authors also note that specialties in which the potential for a fee reduction was greatest were also the specialties for which baseline compensation was highest – from $350,000 annually for emergency physicians to $800,000 a year for neurosurgeons. Annual compensation for ob.gyns., dermatologists, and opthalmologists is about $350,000 a year, which suggests that “these specialties are similarly well compensated by both Medicare and commercial insurers,” the authors write.
The investigators assessed the top 20 procedure codes by expenditure in each of 17 physician specialties. They estimated the commercial-to-Medicare payment ratio for each service and constructed weighted averages across services for each specialty at the national level and for 12 states for which data for all the specialties and services were available.
The researchers analyzed claims from the FAIR Health database between March 2019 and March 2020. That database represents 60 insurers covering 150 million people.
Pediatric and geriatric specialties, nonphysician practitioners, out-of-network clinicians, and ambulatory surgery center claims were excluded. Codes with modifiers, J codes, and clinical laboratory services were also not included.
The charges used in the study were not the actual contracted rates. The authors instead used “imputed allowed amounts” for each claim line. That method was used to protect the confidentiality of the negotiated rates.
With regard to all specialties, the lowest compensated services were procedures, evaluation and management, and tests, which received 140%-150% of the Medicare rate. Treatments and imaging were marked up 160%. Anesthesia was reimbursed at a rate 330% higher than the rate Medicare would pay.
The authors also assessed geographic variation for the 12 states for which they had data.
Similar to findings in other studies, the researchers found that the markup was lowest in Pennsylvania (120%) and highest in Wisconsin (260%). The U.S. average was 160%. California and Missouri were at 150%; Michigan was right at the average.
For physicians in Illinois, Louisiana, Colorado, Texas, and New York, markups were 170%-180% over the Medicare rate. Markups for clinicians in New Jersey (190%) and Arizona (200%) were closest to the Wisconsin rate.
The authors note some study limitations, including the fact that they excluded out-of-network practitioners, “and such payments may disproportionately affect certain specialties.”
A version of this article first appeared on Medscape.com.
Pfizer offers refund if drug ‘doesn’t work’
The high cost of new cancer drugs has been the subject of many debates and discussions, but the issue remains largely unresolved.
Now, one pharmaceutical company is offering a refund if its drug “doesn’t work.”
For what it says is the first time in the industry,
“Through this pilot program, Pfizer will offer a warranty to patients and health plans -- Medicare Part D, commercial and those who pay cash -- who are prescribed Xalkori for an FDA [US Food and Drug Administration]–approved indication,” said a company spokesperson.
Although Pfizer claims that its pilot program is a first in the industry, there have been others that are similar.
In 2017, Novartis offered something similar for tisagenlecleucel (Kymriah), the CAR T-cell therapy that launched with a daunting price tag of $475,000. After receiving backlash over the cost, Novartis announced that if the drug does not work after the first month, patients pay nothing.
Italy has been using this system for several years. Pharmaceutical companies must refund money if the drug fails to work. In 2015, the state-run healthcare system collected €200 million ($220 million) in refunds.
Pfizer pledge
Crizotinib is a selective tyrosine kinase inhibitor used mainly in the treatment of metastatic non–small cell lung cancer for patients whose tumors are positive for ALK or ROS1, as detected by an FDA-approved test. This indication was approved a decade ago. Another indication, ALK-positive anaplastic large cell lymphoma, was added earlier this year.
Details of the Pfizer Pledge are posted on Pfizer’s website. Eligible patients are those for whom crizotinib is discontinued before the fourth 30-day supply is dispensed by the patient’s pharmacy.
“The warranty will reimburse an amount equal to the cost paid for the medicine,” the spokesperson added. “The insurance-backed warranty pilot program will be insured and managed by AIG.”
This program is only available for patients who reside in the United States.
If use of crizotinib is discontinued and documentation of ineffectiveness is provided, Pfizer will refund the out-of-pocket amount that was paid for up to the first three bottles (30-day supply) of crizotinib, up to a maximum of $19,144 for each month’s supply, or a total of $57,432. Pfizer will also refund the cost that was paid by Medicare or a commercial insurer.
“Also, we have made sure to develop a program that also allows for Medicare patients to be eligible, since they are exempt from copay cards and at risk for significant financial burden when starting an oncology treatment,” said the spokesperson.
The pilot program is available to patients who began taking crizotinib from June 1, 2021, through December 31, 2021.
So far, Pfizer is offering this warranty only for crizotinib, but that may change in the future.
“Once the pilot is complete, we will assess learnings and consider whether to build a more robust, scalable program capable of supporting multiple products,” the Pfizer spokesperson commented.
Previous scheme ended in court
Pfizer had previously tried a different approach to reducing drug costs: it had attempted to offer copay support programs to Medicare patients who were prescribed its cardiac drug tafamidis (Vyndaqe, Vyndamax).
Tafamidis, launched in 2019, is used for patients with transthyretin amyloid cardiomyopathy. For those patients, it has been shown to reduce all-cause mortality and cardiovascular hospitalizations. It costs about $225,000 a year and has been described as the most expensive cardiovascular drug in the United States.
Earlier this month, a court dismissed Pfizer’s challenge to an anti-kickback law that prevented the company from offering copay support programs to Medicare patients.
The judge ruled that Pfizer’s plan to offer direct payments to patients violated a federal ban on “knowingly or willfully” providing financial support to induce drug purchases, even in the absence of corrupt intent.
Pharmaceutical manufacturers are forbidden from subsidizing copayments for Medicare beneficiaries but are allowed to donate to independent nonprofit organizations that offer copay assistance. Pfizer sued the U.S. Department of Health and Human Services in June 2020 to get a court ruling that their proposed programs were legal.
The new pledge program for crizotinib operates from a different premise, the Pfizer spokesperson commented.
A version of this article first appeared on Medscape.com.
The high cost of new cancer drugs has been the subject of many debates and discussions, but the issue remains largely unresolved.
Now, one pharmaceutical company is offering a refund if its drug “doesn’t work.”
For what it says is the first time in the industry,
“Through this pilot program, Pfizer will offer a warranty to patients and health plans -- Medicare Part D, commercial and those who pay cash -- who are prescribed Xalkori for an FDA [US Food and Drug Administration]–approved indication,” said a company spokesperson.
Although Pfizer claims that its pilot program is a first in the industry, there have been others that are similar.
In 2017, Novartis offered something similar for tisagenlecleucel (Kymriah), the CAR T-cell therapy that launched with a daunting price tag of $475,000. After receiving backlash over the cost, Novartis announced that if the drug does not work after the first month, patients pay nothing.
Italy has been using this system for several years. Pharmaceutical companies must refund money if the drug fails to work. In 2015, the state-run healthcare system collected €200 million ($220 million) in refunds.
Pfizer pledge
Crizotinib is a selective tyrosine kinase inhibitor used mainly in the treatment of metastatic non–small cell lung cancer for patients whose tumors are positive for ALK or ROS1, as detected by an FDA-approved test. This indication was approved a decade ago. Another indication, ALK-positive anaplastic large cell lymphoma, was added earlier this year.
Details of the Pfizer Pledge are posted on Pfizer’s website. Eligible patients are those for whom crizotinib is discontinued before the fourth 30-day supply is dispensed by the patient’s pharmacy.
“The warranty will reimburse an amount equal to the cost paid for the medicine,” the spokesperson added. “The insurance-backed warranty pilot program will be insured and managed by AIG.”
This program is only available for patients who reside in the United States.
If use of crizotinib is discontinued and documentation of ineffectiveness is provided, Pfizer will refund the out-of-pocket amount that was paid for up to the first three bottles (30-day supply) of crizotinib, up to a maximum of $19,144 for each month’s supply, or a total of $57,432. Pfizer will also refund the cost that was paid by Medicare or a commercial insurer.
“Also, we have made sure to develop a program that also allows for Medicare patients to be eligible, since they are exempt from copay cards and at risk for significant financial burden when starting an oncology treatment,” said the spokesperson.
The pilot program is available to patients who began taking crizotinib from June 1, 2021, through December 31, 2021.
So far, Pfizer is offering this warranty only for crizotinib, but that may change in the future.
“Once the pilot is complete, we will assess learnings and consider whether to build a more robust, scalable program capable of supporting multiple products,” the Pfizer spokesperson commented.
Previous scheme ended in court
Pfizer had previously tried a different approach to reducing drug costs: it had attempted to offer copay support programs to Medicare patients who were prescribed its cardiac drug tafamidis (Vyndaqe, Vyndamax).
Tafamidis, launched in 2019, is used for patients with transthyretin amyloid cardiomyopathy. For those patients, it has been shown to reduce all-cause mortality and cardiovascular hospitalizations. It costs about $225,000 a year and has been described as the most expensive cardiovascular drug in the United States.
Earlier this month, a court dismissed Pfizer’s challenge to an anti-kickback law that prevented the company from offering copay support programs to Medicare patients.
The judge ruled that Pfizer’s plan to offer direct payments to patients violated a federal ban on “knowingly or willfully” providing financial support to induce drug purchases, even in the absence of corrupt intent.
Pharmaceutical manufacturers are forbidden from subsidizing copayments for Medicare beneficiaries but are allowed to donate to independent nonprofit organizations that offer copay assistance. Pfizer sued the U.S. Department of Health and Human Services in June 2020 to get a court ruling that their proposed programs were legal.
The new pledge program for crizotinib operates from a different premise, the Pfizer spokesperson commented.
A version of this article first appeared on Medscape.com.
The high cost of new cancer drugs has been the subject of many debates and discussions, but the issue remains largely unresolved.
Now, one pharmaceutical company is offering a refund if its drug “doesn’t work.”
For what it says is the first time in the industry,
“Through this pilot program, Pfizer will offer a warranty to patients and health plans -- Medicare Part D, commercial and those who pay cash -- who are prescribed Xalkori for an FDA [US Food and Drug Administration]–approved indication,” said a company spokesperson.
Although Pfizer claims that its pilot program is a first in the industry, there have been others that are similar.
In 2017, Novartis offered something similar for tisagenlecleucel (Kymriah), the CAR T-cell therapy that launched with a daunting price tag of $475,000. After receiving backlash over the cost, Novartis announced that if the drug does not work after the first month, patients pay nothing.
Italy has been using this system for several years. Pharmaceutical companies must refund money if the drug fails to work. In 2015, the state-run healthcare system collected €200 million ($220 million) in refunds.
Pfizer pledge
Crizotinib is a selective tyrosine kinase inhibitor used mainly in the treatment of metastatic non–small cell lung cancer for patients whose tumors are positive for ALK or ROS1, as detected by an FDA-approved test. This indication was approved a decade ago. Another indication, ALK-positive anaplastic large cell lymphoma, was added earlier this year.
Details of the Pfizer Pledge are posted on Pfizer’s website. Eligible patients are those for whom crizotinib is discontinued before the fourth 30-day supply is dispensed by the patient’s pharmacy.
“The warranty will reimburse an amount equal to the cost paid for the medicine,” the spokesperson added. “The insurance-backed warranty pilot program will be insured and managed by AIG.”
This program is only available for patients who reside in the United States.
If use of crizotinib is discontinued and documentation of ineffectiveness is provided, Pfizer will refund the out-of-pocket amount that was paid for up to the first three bottles (30-day supply) of crizotinib, up to a maximum of $19,144 for each month’s supply, or a total of $57,432. Pfizer will also refund the cost that was paid by Medicare or a commercial insurer.
“Also, we have made sure to develop a program that also allows for Medicare patients to be eligible, since they are exempt from copay cards and at risk for significant financial burden when starting an oncology treatment,” said the spokesperson.
The pilot program is available to patients who began taking crizotinib from June 1, 2021, through December 31, 2021.
So far, Pfizer is offering this warranty only for crizotinib, but that may change in the future.
“Once the pilot is complete, we will assess learnings and consider whether to build a more robust, scalable program capable of supporting multiple products,” the Pfizer spokesperson commented.
Previous scheme ended in court
Pfizer had previously tried a different approach to reducing drug costs: it had attempted to offer copay support programs to Medicare patients who were prescribed its cardiac drug tafamidis (Vyndaqe, Vyndamax).
Tafamidis, launched in 2019, is used for patients with transthyretin amyloid cardiomyopathy. For those patients, it has been shown to reduce all-cause mortality and cardiovascular hospitalizations. It costs about $225,000 a year and has been described as the most expensive cardiovascular drug in the United States.
Earlier this month, a court dismissed Pfizer’s challenge to an anti-kickback law that prevented the company from offering copay support programs to Medicare patients.
The judge ruled that Pfizer’s plan to offer direct payments to patients violated a federal ban on “knowingly or willfully” providing financial support to induce drug purchases, even in the absence of corrupt intent.
Pharmaceutical manufacturers are forbidden from subsidizing copayments for Medicare beneficiaries but are allowed to donate to independent nonprofit organizations that offer copay assistance. Pfizer sued the U.S. Department of Health and Human Services in June 2020 to get a court ruling that their proposed programs were legal.
The new pledge program for crizotinib operates from a different premise, the Pfizer spokesperson commented.
A version of this article first appeared on Medscape.com.
Patient loses prostate after biopsy slide switched; more
It’s difficult enough when a patient’s prostate is removed because of cancer. But it’s another thing altogether when the prostate is removed because of a medical error, as a report on 3 CBS Philly, among other news outlets, makes clear.
The patient, Eric Spangs, lives in southeastern Pennsylvania. Testing indicated an elevation in prostate-specific antigen (PSA) level. He subsequently underwent biopsy of the prostate, which appeared to indicate cancer. In time, though, Mr. Spangs learned there had been an error: the tissue section used in the microscopic diagnosis had come from the biopsy specimen of a different patient. Mr. Spangs himself didn’t actually have cancer.
Ordinarily, such news would be cause for celebration. But this was far from a normal situ ation: Following his initial cancer diagnosis, Mr. Spangs underwent a radical laparoscopic prostatectomy at a local hospital.
“It’s devastated me emotionally and physically,” Mr. Spangs said. It has also been emotionally devastating for his wife, Melissa. (The couple has five children.)
Their attorney, Aaron Freiwald, has filed a suit against the health system to which the local hospital belongs and the area’s largest urologic practice.
The Spangs wish to caution other patients not to make the same mistake they did: they failed to get a second opinion from an oncology specialist, as recommended by the American Cancer Society. (Eric Spangs did receive a second opinion from someone at the urologic practice, but that practice doesn’t specialize in oncology.)
The Spangs also worry about the patient who received the false-negative biopsy result. They have been assured, however, that that patient will be properly notified of his actual cancer status.
Fertility specialist uses own sperm to impregnate patients
A suit claims that a Rochester, N.Y., gynecologist and fertility specialist used his own sperm to inseminate multiple patients, according to a story reported by the Associated Press and other news outlets.
The suit was filed last month by the daughter — call her “Harriet Jones” — of one of the women who received fertility services from the doctor during the 1980s. Ms. Jones’s suit alleges that at the time, the doctor told her mother that the sperm donor would be a medical student at the University of Rochester. In fact, the donor was the doctor himself. He kept that fact a secret for years, even after Ms. Jones — his own daughter — sought him out for gynecologic services.
The secret gradually began to come to light in 2016, when Ms. Jones’s nonbiological father — the man who had helped to raise her — died. Curious about her biological father, Ms. Jones sought to learn his identity from the Rochester gynecologist who had treated her mother and was now her own gynecologist. The doctor said he couldn’t be of help; he claimed he hadn’t kept the relevant records.
Ms. Jones then submitted a blood sample to a direct-to-consumer genetic testing company. The results surprised her: Not only did she learn of her ethnicity, but she also discovered the existence of two half siblings, who were donor-conceived in 1984 and 1985, respectively, the very period when her own mother was undergoing insemination procedures. Ms. Jones subsequently discovered the existence of additional half siblings, all born in the first half of the 1980s.
Initially elated by the discoveries, Ms. Jones soon grew despondent and anxious. She suffered from migraine headaches, among other symptoms. Her biological father, it seemed, had been “a serial sperm donor.”
Still, she continued to go to her Rochester doctor for treatment, having no reason to suspect anything untoward about him. Her visits, including those for prolonged menstrual bleeding, involved routine breast and pelvic exams, transvaginal ultrasounds, and intrauterine contraceptive placements under sedation.
Over this period, her doctor was friendly, asking her a variety of questions about her personal life. During one especially strange visit, however, he began chuckling and said, “You’re a really good kid, such a good kid.” During this visit, he invited his wife into the exam room, presumably to meet Ms. Jones.
It was at this moment that Ms. Jones had a revelation: Could her gynecologist actually be her biological father?
In May 2021, Ms. Jones and a half brother with whom she had been in touch contacted the gynecologist’s daughter from his first marriage. All three underwent genetic testing. The results showed a 99.99% chance of a genetic link.
Ms. Jones has said in her suit that “no reasonable woman” would have submitted to pelvic examinations and other examinations by a doctor whom she knew to be her father.
Besides fraud, her suit alleges medical malpractice, battery, infliction of mental distress, and lack of informed consent. She is seeking compensation for all harm caused to her, including past and future economic damages, past unreimbursed medical expenses, and future expenses related to her mental health treatment and care.
The story included no further details about the civil litigation. As for criminal charges, it’s unlikely Ms. Jones’s biological father — her gynecologist — will face criminal charges for his alleged crimes because they fall outside of the state’s statute of limitations.
Parents say daughter’s stroke wasn’t identified
The Georgia parents of a young woman who died from a stroke following a series of alleged misdiagnoses are suing multiple practitioners, reports Legal Newswire and other news outlets
In June 2019, Michaela Smith was training for her job as a detention officer when she began experiencing a variety of symptoms, including headache, shortness of breath, throat swelling, and slurred speech. She was taken to the emergency department (ED) at Hamilton Medical Center, in Dalton, Ga.
There, she was examined by an attending ED doctor, who ordered a CT scan. The results were read by radiologist Michael J. Cooney, MD. In his reading, the Smith family’s lawsuit alleges, Dr. Cooney failed to identify the basilar artery sign, which is a key indicator of a vessel occlusion in stroke patients. Dr. Cooney concluded that Ms. Smith’s scan showed no acute intracranial abnormality. He sent her home without further discharge instructions.
At home, Ms. Smith fell asleep but awoke in an altered mental state, one of several classic stroke symptoms that she had been experiencing. She returned to the ED. This time, she was examined by David F. Hawkins, MD, an ED physician. Although his differential diagnosis identified Ms. Smith’s symptoms as most likely stroke related, Dr. Hawkins allegedly failed to immediately corroborate his findings with additional vascular imaging. Later in the day, Ms. Smith did undergo an MRI, which a second radiologist, Kevin F. Johnson, MD, misread as showing no signs of ischemia in her basilar artery, according to the lawsuit.
That same day, Dr. Hawkins conferred with a second neurologist, Jeffrey T. Glass, MD, who recommended that Ms. Smith be admitted to the hospital because of her deteriorating condition. The Smiths’ suit claims that Dr. Glass also failed to diagnosis their daughter’s underlying condition, although he did sign off on her transfer to Baroness Erlanger Hospital, in Chattanooga, Tenn.
There, Ms. Smith’s condition continued to worsen. She soon required mechanical ventilation and tube feeding. On July 3, 2019, she was pronounced dead.
“This is an egregious case of negligence,” said the attorney representing the Smiths, who are suing the physicians involved and their practices, as well as Hamilton Medical Center and several unnamed defendants.
“Although two radiology studies and her clinical presentation indicated that Michaela was having a catastrophic stroke, her doctors repeatedly misread the studies as normal, failed to diagnose the stroke, and failed to treat her deficits as a neurological emergency,” the family’s lawyer stated.
At press time, there had been no response from any of the defendants or their attorneys.
A version of this article first appeared on Medscape.com.
It’s difficult enough when a patient’s prostate is removed because of cancer. But it’s another thing altogether when the prostate is removed because of a medical error, as a report on 3 CBS Philly, among other news outlets, makes clear.
The patient, Eric Spangs, lives in southeastern Pennsylvania. Testing indicated an elevation in prostate-specific antigen (PSA) level. He subsequently underwent biopsy of the prostate, which appeared to indicate cancer. In time, though, Mr. Spangs learned there had been an error: the tissue section used in the microscopic diagnosis had come from the biopsy specimen of a different patient. Mr. Spangs himself didn’t actually have cancer.
Ordinarily, such news would be cause for celebration. But this was far from a normal situ ation: Following his initial cancer diagnosis, Mr. Spangs underwent a radical laparoscopic prostatectomy at a local hospital.
“It’s devastated me emotionally and physically,” Mr. Spangs said. It has also been emotionally devastating for his wife, Melissa. (The couple has five children.)
Their attorney, Aaron Freiwald, has filed a suit against the health system to which the local hospital belongs and the area’s largest urologic practice.
The Spangs wish to caution other patients not to make the same mistake they did: they failed to get a second opinion from an oncology specialist, as recommended by the American Cancer Society. (Eric Spangs did receive a second opinion from someone at the urologic practice, but that practice doesn’t specialize in oncology.)
The Spangs also worry about the patient who received the false-negative biopsy result. They have been assured, however, that that patient will be properly notified of his actual cancer status.
Fertility specialist uses own sperm to impregnate patients
A suit claims that a Rochester, N.Y., gynecologist and fertility specialist used his own sperm to inseminate multiple patients, according to a story reported by the Associated Press and other news outlets.
The suit was filed last month by the daughter — call her “Harriet Jones” — of one of the women who received fertility services from the doctor during the 1980s. Ms. Jones’s suit alleges that at the time, the doctor told her mother that the sperm donor would be a medical student at the University of Rochester. In fact, the donor was the doctor himself. He kept that fact a secret for years, even after Ms. Jones — his own daughter — sought him out for gynecologic services.
The secret gradually began to come to light in 2016, when Ms. Jones’s nonbiological father — the man who had helped to raise her — died. Curious about her biological father, Ms. Jones sought to learn his identity from the Rochester gynecologist who had treated her mother and was now her own gynecologist. The doctor said he couldn’t be of help; he claimed he hadn’t kept the relevant records.
Ms. Jones then submitted a blood sample to a direct-to-consumer genetic testing company. The results surprised her: Not only did she learn of her ethnicity, but she also discovered the existence of two half siblings, who were donor-conceived in 1984 and 1985, respectively, the very period when her own mother was undergoing insemination procedures. Ms. Jones subsequently discovered the existence of additional half siblings, all born in the first half of the 1980s.
Initially elated by the discoveries, Ms. Jones soon grew despondent and anxious. She suffered from migraine headaches, among other symptoms. Her biological father, it seemed, had been “a serial sperm donor.”
Still, she continued to go to her Rochester doctor for treatment, having no reason to suspect anything untoward about him. Her visits, including those for prolonged menstrual bleeding, involved routine breast and pelvic exams, transvaginal ultrasounds, and intrauterine contraceptive placements under sedation.
Over this period, her doctor was friendly, asking her a variety of questions about her personal life. During one especially strange visit, however, he began chuckling and said, “You’re a really good kid, such a good kid.” During this visit, he invited his wife into the exam room, presumably to meet Ms. Jones.
It was at this moment that Ms. Jones had a revelation: Could her gynecologist actually be her biological father?
In May 2021, Ms. Jones and a half brother with whom she had been in touch contacted the gynecologist’s daughter from his first marriage. All three underwent genetic testing. The results showed a 99.99% chance of a genetic link.
Ms. Jones has said in her suit that “no reasonable woman” would have submitted to pelvic examinations and other examinations by a doctor whom she knew to be her father.
Besides fraud, her suit alleges medical malpractice, battery, infliction of mental distress, and lack of informed consent. She is seeking compensation for all harm caused to her, including past and future economic damages, past unreimbursed medical expenses, and future expenses related to her mental health treatment and care.
The story included no further details about the civil litigation. As for criminal charges, it’s unlikely Ms. Jones’s biological father — her gynecologist — will face criminal charges for his alleged crimes because they fall outside of the state’s statute of limitations.
Parents say daughter’s stroke wasn’t identified
The Georgia parents of a young woman who died from a stroke following a series of alleged misdiagnoses are suing multiple practitioners, reports Legal Newswire and other news outlets
In June 2019, Michaela Smith was training for her job as a detention officer when she began experiencing a variety of symptoms, including headache, shortness of breath, throat swelling, and slurred speech. She was taken to the emergency department (ED) at Hamilton Medical Center, in Dalton, Ga.
There, she was examined by an attending ED doctor, who ordered a CT scan. The results were read by radiologist Michael J. Cooney, MD. In his reading, the Smith family’s lawsuit alleges, Dr. Cooney failed to identify the basilar artery sign, which is a key indicator of a vessel occlusion in stroke patients. Dr. Cooney concluded that Ms. Smith’s scan showed no acute intracranial abnormality. He sent her home without further discharge instructions.
At home, Ms. Smith fell asleep but awoke in an altered mental state, one of several classic stroke symptoms that she had been experiencing. She returned to the ED. This time, she was examined by David F. Hawkins, MD, an ED physician. Although his differential diagnosis identified Ms. Smith’s symptoms as most likely stroke related, Dr. Hawkins allegedly failed to immediately corroborate his findings with additional vascular imaging. Later in the day, Ms. Smith did undergo an MRI, which a second radiologist, Kevin F. Johnson, MD, misread as showing no signs of ischemia in her basilar artery, according to the lawsuit.
That same day, Dr. Hawkins conferred with a second neurologist, Jeffrey T. Glass, MD, who recommended that Ms. Smith be admitted to the hospital because of her deteriorating condition. The Smiths’ suit claims that Dr. Glass also failed to diagnosis their daughter’s underlying condition, although he did sign off on her transfer to Baroness Erlanger Hospital, in Chattanooga, Tenn.
There, Ms. Smith’s condition continued to worsen. She soon required mechanical ventilation and tube feeding. On July 3, 2019, she was pronounced dead.
“This is an egregious case of negligence,” said the attorney representing the Smiths, who are suing the physicians involved and their practices, as well as Hamilton Medical Center and several unnamed defendants.
“Although two radiology studies and her clinical presentation indicated that Michaela was having a catastrophic stroke, her doctors repeatedly misread the studies as normal, failed to diagnose the stroke, and failed to treat her deficits as a neurological emergency,” the family’s lawyer stated.
At press time, there had been no response from any of the defendants or their attorneys.
A version of this article first appeared on Medscape.com.
It’s difficult enough when a patient’s prostate is removed because of cancer. But it’s another thing altogether when the prostate is removed because of a medical error, as a report on 3 CBS Philly, among other news outlets, makes clear.
The patient, Eric Spangs, lives in southeastern Pennsylvania. Testing indicated an elevation in prostate-specific antigen (PSA) level. He subsequently underwent biopsy of the prostate, which appeared to indicate cancer. In time, though, Mr. Spangs learned there had been an error: the tissue section used in the microscopic diagnosis had come from the biopsy specimen of a different patient. Mr. Spangs himself didn’t actually have cancer.
Ordinarily, such news would be cause for celebration. But this was far from a normal situ ation: Following his initial cancer diagnosis, Mr. Spangs underwent a radical laparoscopic prostatectomy at a local hospital.
“It’s devastated me emotionally and physically,” Mr. Spangs said. It has also been emotionally devastating for his wife, Melissa. (The couple has five children.)
Their attorney, Aaron Freiwald, has filed a suit against the health system to which the local hospital belongs and the area’s largest urologic practice.
The Spangs wish to caution other patients not to make the same mistake they did: they failed to get a second opinion from an oncology specialist, as recommended by the American Cancer Society. (Eric Spangs did receive a second opinion from someone at the urologic practice, but that practice doesn’t specialize in oncology.)
The Spangs also worry about the patient who received the false-negative biopsy result. They have been assured, however, that that patient will be properly notified of his actual cancer status.
Fertility specialist uses own sperm to impregnate patients
A suit claims that a Rochester, N.Y., gynecologist and fertility specialist used his own sperm to inseminate multiple patients, according to a story reported by the Associated Press and other news outlets.
The suit was filed last month by the daughter — call her “Harriet Jones” — of one of the women who received fertility services from the doctor during the 1980s. Ms. Jones’s suit alleges that at the time, the doctor told her mother that the sperm donor would be a medical student at the University of Rochester. In fact, the donor was the doctor himself. He kept that fact a secret for years, even after Ms. Jones — his own daughter — sought him out for gynecologic services.
The secret gradually began to come to light in 2016, when Ms. Jones’s nonbiological father — the man who had helped to raise her — died. Curious about her biological father, Ms. Jones sought to learn his identity from the Rochester gynecologist who had treated her mother and was now her own gynecologist. The doctor said he couldn’t be of help; he claimed he hadn’t kept the relevant records.
Ms. Jones then submitted a blood sample to a direct-to-consumer genetic testing company. The results surprised her: Not only did she learn of her ethnicity, but she also discovered the existence of two half siblings, who were donor-conceived in 1984 and 1985, respectively, the very period when her own mother was undergoing insemination procedures. Ms. Jones subsequently discovered the existence of additional half siblings, all born in the first half of the 1980s.
Initially elated by the discoveries, Ms. Jones soon grew despondent and anxious. She suffered from migraine headaches, among other symptoms. Her biological father, it seemed, had been “a serial sperm donor.”
Still, she continued to go to her Rochester doctor for treatment, having no reason to suspect anything untoward about him. Her visits, including those for prolonged menstrual bleeding, involved routine breast and pelvic exams, transvaginal ultrasounds, and intrauterine contraceptive placements under sedation.
Over this period, her doctor was friendly, asking her a variety of questions about her personal life. During one especially strange visit, however, he began chuckling and said, “You’re a really good kid, such a good kid.” During this visit, he invited his wife into the exam room, presumably to meet Ms. Jones.
It was at this moment that Ms. Jones had a revelation: Could her gynecologist actually be her biological father?
In May 2021, Ms. Jones and a half brother with whom she had been in touch contacted the gynecologist’s daughter from his first marriage. All three underwent genetic testing. The results showed a 99.99% chance of a genetic link.
Ms. Jones has said in her suit that “no reasonable woman” would have submitted to pelvic examinations and other examinations by a doctor whom she knew to be her father.
Besides fraud, her suit alleges medical malpractice, battery, infliction of mental distress, and lack of informed consent. She is seeking compensation for all harm caused to her, including past and future economic damages, past unreimbursed medical expenses, and future expenses related to her mental health treatment and care.
The story included no further details about the civil litigation. As for criminal charges, it’s unlikely Ms. Jones’s biological father — her gynecologist — will face criminal charges for his alleged crimes because they fall outside of the state’s statute of limitations.
Parents say daughter’s stroke wasn’t identified
The Georgia parents of a young woman who died from a stroke following a series of alleged misdiagnoses are suing multiple practitioners, reports Legal Newswire and other news outlets
In June 2019, Michaela Smith was training for her job as a detention officer when she began experiencing a variety of symptoms, including headache, shortness of breath, throat swelling, and slurred speech. She was taken to the emergency department (ED) at Hamilton Medical Center, in Dalton, Ga.
There, she was examined by an attending ED doctor, who ordered a CT scan. The results were read by radiologist Michael J. Cooney, MD. In his reading, the Smith family’s lawsuit alleges, Dr. Cooney failed to identify the basilar artery sign, which is a key indicator of a vessel occlusion in stroke patients. Dr. Cooney concluded that Ms. Smith’s scan showed no acute intracranial abnormality. He sent her home without further discharge instructions.
At home, Ms. Smith fell asleep but awoke in an altered mental state, one of several classic stroke symptoms that she had been experiencing. She returned to the ED. This time, she was examined by David F. Hawkins, MD, an ED physician. Although his differential diagnosis identified Ms. Smith’s symptoms as most likely stroke related, Dr. Hawkins allegedly failed to immediately corroborate his findings with additional vascular imaging. Later in the day, Ms. Smith did undergo an MRI, which a second radiologist, Kevin F. Johnson, MD, misread as showing no signs of ischemia in her basilar artery, according to the lawsuit.
That same day, Dr. Hawkins conferred with a second neurologist, Jeffrey T. Glass, MD, who recommended that Ms. Smith be admitted to the hospital because of her deteriorating condition. The Smiths’ suit claims that Dr. Glass also failed to diagnosis their daughter’s underlying condition, although he did sign off on her transfer to Baroness Erlanger Hospital, in Chattanooga, Tenn.
There, Ms. Smith’s condition continued to worsen. She soon required mechanical ventilation and tube feeding. On July 3, 2019, she was pronounced dead.
“This is an egregious case of negligence,” said the attorney representing the Smiths, who are suing the physicians involved and their practices, as well as Hamilton Medical Center and several unnamed defendants.
“Although two radiology studies and her clinical presentation indicated that Michaela was having a catastrophic stroke, her doctors repeatedly misread the studies as normal, failed to diagnose the stroke, and failed to treat her deficits as a neurological emergency,” the family’s lawyer stated.
At press time, there had been no response from any of the defendants or their attorneys.
A version of this article first appeared on Medscape.com.
New land mines in your next (and even current) employment contract
Physician employment contracts include some new dangers. This includes physicians taking a new job, but it also includes already-employed doctors who are being asked to resign a new contract that contains new conditions. A number of these new clauses have arisen because of COVID-19. When the pandemic dramatically reduced patient flow, many employers didn’t have enough money to pay doctors and didn’t always have physicians in the right location or practice setting.
Vowing this would never happen again, some employers have rewritten their physician contracts to make it easier to reassign and terminate physicians.
Here are 12 potential land mines in a physician employment contract, some of which were added as a result of the pandemic.
You could be immediately terminated without notice
One outcome of the pandemic is the growing use of “force majeure” clauses, which give the employer the right to reduce your compensation or even terminate you due to a natural disaster, which could include COVID.
“COVID made employers aware of the potential impact of disasters on their operations,” said Dan Shay, a health law attorney at Alice Gosfield & Associates in Philadelphia. “Therefore, even as the threat of COVID abates in many places, employers are continuing to put this provision in the contract.”
What can you do? “One way to get some protection is to rule out a termination without cause in the first year,” said Michael A. Cassidy, a physician contract attorney at Tucker Arensberg in Pittsburgh.
The force majeure clause is less likely to affect salary, but could impact bonus and incentive tied to performance. It’s wise to try to specifically limit how much the force majeure could reduce pay tied to performance, and to be prepared to negotiate that aspect of your contract.
No protections if you’re let go through no fault of your own
You could lose your job if your employer could not generate enough business and has to let some doctors go. This happened quite often in the early days of the COVID pandemic.
In these situations, the doctor has not done anything wrong to prompt the termination, but the restrictive covenant may still apply, meaning that the doctor would have to leave the area to find work.
What can you do? You’re in a good position to get this changed, said Christopher L. Nuland, a solo physician contract attorney in Jacksonville, Fla. “Many employers recognize that it would be draconian to require a restrictive covenant in this case, and they will agree to modify this provision.”
Similarly, the employer may not cover your tail insurance even if you were let go from your work through no fault of your own. Most malpractice policies for employer physicians require buying an extra policy, called a tail, if you leave. In some cases, the employer won’t provide a tail and will make the departing doctor buy it.
In these cases, “try for a compromise, such as stipulating that the party that caused the termination should pay for the tail,” Mr. Nuland said. “The employer may not agree to anything more than that because they want to set up a disincentive against you leaving.”
Employer could unilaterally alter your compensation
Many recent contracts give the employer the option to unilaterally modify compensation, such as changing the base salary or raising the target required for meeting the productivity bonus, said Ericka L. Adler, a physician contract attorney at Roetzel & Andress in Chicago.
Ms. Adler thought this change could have been prompted by employers’ financial problems during the pandemic. In the early months of COVID, many physicians were not making much money for the employer but still had to be paid. So employers added a clause saying they could reduce compensation at any time, she said.
What can you do? Harsh provisions like this often come up in contracts with private equity firms, Mr. Cassidy said. “The contract might say the employer can adjust compensation or even terminate physicians based on productivity or their profitability. And it may say that if they reassign you to a new location and you refuse, they can terminate you.”
“If you can’t get these clauses removed, try to reduce the impact of a termination by providing longer notice periods or by inserting a severance agreement,” Mr. Cassidy said.
Accelerating notice for without-cause terminations
Physicians who are convicted of a felony or other moral issue can usually be terminated immediately. But if you are terminated for other reasons – that is, “without cause” – you are given notice at a certain number of days before you have to leave (typically 60-90 days), so that you have time to find a new job.
Some recent contracts, however, allow for very little notice in without-cause terminations, which allows the employer to fire you in as little as 0 days after providing notice, Ms. Adler said.
“This means that, even if 90 days’ notice is provided in the contract, the employer can decide that your last day will be an earlier date,” she said.
Why is this happening? Ms. Adler said employers want to begin reallocating resources and patients as soon as possible. The problem came to employers’ attention during the COVID pandemic, when they were contractually forced to pay doctors for doing little or nothing during the notice period.
What can you do? Possibly not much, other than attempt to negotiate. “Large employers typically don’t want to drop this provision, but at the least, the doctor needs to understand the risk it creates for them,” she said.
You could be assigned to far-off locations
As patient care needs changed dramatically during the pandemic, employers needed to reassign doctors to new locations.
Some new contracts allow employers to simply inform the doctor that they are changing the work location. However, “you don’t want to be assigned to a new work location that is 50 miles away,” Mr. Nuland said.
What can you do? Mr. Nuland recommended adding new language saying that, if the new assignment is more than 20 miles away, both parties would have to approve it.
You could end up working too many off-hours
“Most employers won’t issue a specific work schedule,” Mr. Nuland said. “They want the flexibility to assign evening or weekend work, and it would be difficult for a young doctor to change this.”
What can you do? Mr. Nuland recommended trying to set some limits. “You can try to limit off-hours work to two times a month or something like that,” she said. And if you need to have a special schedule, such as not working on Fridays, Adler advises that this should be put into the contract.
If you can’t get anything changed in the contract, Mr. Nuland said the next-best thing is to ask employers to tell you specifically what they plan to do with you. “Most employers will give you an informal idea of what’s expected – maybe not an exact schedule, but it’s quite likely they will honor it.”
You wouldn’t be able to work nearby if you left the job
Most contracts have a noncompete clause, also known as a “restrictive covenant,” which prevents employed physicians from working in the area if they left the job.
“Almost every doctor I represent has told me that they’re not concerned about the noncompete clause because, they believe, it is not enforceable anyway,” Ms. Adler said. “This is incorrect.”
Mr. Nuland said the faster pace of job-changing during the pandemic makes it all the more likely that doctors have to deal with a restrictive covenant. At the same time, some employers have been expanding the restriction – either by enlarging the radius where the restriction applies or by making the restriction apply to each of their sites, so that each one has a restricted radius around it.
For example, one contract Mr. Nuland is currently reviewing has a 20-mile radius that in effect becomes a 120-mile radius because the employer is counting four offices.
What can you do? Mr. Nuland advised trying to reduce the impact of the noncompete – for instance, making it apply only to the offices where you worked, or trading more time for less distance. “If you have a 2-year, 20-mile restriction, ask for a 3-year, 10-mile restriction, where the radius could be easier to deal with,” he said.
You might end up with too much call
Contracts rarely detail your call schedule because employers want flexibility to expand call as patient care needs change, but you can try adding some specificity, said Sanja Ord, a physician contract attorney at Greensfelder, Hemker & Gale in St. Louis.
Contracts often use wide-open language to describe call, such as simply making it “subject to the house call policies,” Mr. Cassidy said. Language that is more beneficial to the physician would say that call must be “equal” among “similarly situated” physicians.
But Ms. Ord said even provisions for equal call can turn out to be onerous if there are too few doctors in the call roster, so it’s a good idea to find out just how many doctors will be participating in call.
Still, Adler said even that strategy can’t remove all risk. What happens, she asked, if several physicians participating in call decide to leave? Then you might end up with call every other night.
What can you do? Mr. Cassidy recommends specifying a maximum amount of call – for example, no more frequent than one in four nights.
Physician must pay for reimbursement claw-backs by payers
When auditors for Medicare or other payers find overpayments after the fact, called a ‘claw-back,’ the provider must pay them back. But which provider has to do that – you or your employer?
In many cases, your employer’s billing office may have introduced the error, but there may be a clause in the contract stating that the physician is solely responsible for all claw-backs. That could be costly.
What can you do? Mr. Shay said the clause should state that you have to pay only when it is the result of your own error or omission, and also not when it was made at the direction of the employer.
Some work may be outside of your subspecialty
In some cases, the employer may assign subspecialized doctors to work outside their subspecialty, Mr. Nuland said.
For example, he said he represented an endocrinologist who expected to see only diabetes patients but was assigned to some general internal medicine work as well, and an otolaryngologist client of his who completed a fellowship on facial plastic surgery was expected to do liposuction in a cosmetic surgery group.
What can you do? To prevent this from happening, Mr. Nuland recommends a clause stating that your work will be restricted to your subspecialty.
What the employer promised isn’t in the contract
“Beware of promises that are not in the contract,” Mr. Shay said. “You might feel you can really trust your new boss and what he tells you, but what if that person resigns, or the organization gets a new owner who doesn’t honor unwritten agreements?”
Many contracts have an integration clause, which specifies that the contract constitutes the complete agreement between the two parties, and it nullifies any other oral or written promises made to the physician.
For example, the employer might have promised a relocation bonus and a sign-on bonus, but for some reason it didn’t get into the contract, Ms. Ord said. In those cases, the employer is under no obligation to honor the promise.
What can you do? Mr. Cassidy said it is possible to hold the employer to a commitment made outside the contract. The alternative document, such as an offer letter, has to specifically state that the commitment is protected from the integration clause in the contract, he said, adding: “It is still better to have the commitment put into the contract.”
Contract is simply accepted as is
“Generally, the bigger the employer, the less likely they will alter an agreement just to make you happy,” Mr. Shay said.
But even in these contracts, he said there is still opportunity to fix errors and ambiguities that could harm you later – or even alter a provision if you can’t remove it outright.
The back-and-forth is important, Ms. Adler said. “Negotiation means trying to have some control over your job and your life.”
Mr. Cassidy said a big part of contract review is facing up to the possibility that you may have to resign or be let go.
“Many physicians don’t like to think about leaving when they’re just starting a job, but they need to,” he said. “You need to begin with the end in mind. Think about what would happen if this job didn’t work out.”
A version of this article first appeared on Medscape.com.
Physician employment contracts include some new dangers. This includes physicians taking a new job, but it also includes already-employed doctors who are being asked to resign a new contract that contains new conditions. A number of these new clauses have arisen because of COVID-19. When the pandemic dramatically reduced patient flow, many employers didn’t have enough money to pay doctors and didn’t always have physicians in the right location or practice setting.
Vowing this would never happen again, some employers have rewritten their physician contracts to make it easier to reassign and terminate physicians.
Here are 12 potential land mines in a physician employment contract, some of which were added as a result of the pandemic.
You could be immediately terminated without notice
One outcome of the pandemic is the growing use of “force majeure” clauses, which give the employer the right to reduce your compensation or even terminate you due to a natural disaster, which could include COVID.
“COVID made employers aware of the potential impact of disasters on their operations,” said Dan Shay, a health law attorney at Alice Gosfield & Associates in Philadelphia. “Therefore, even as the threat of COVID abates in many places, employers are continuing to put this provision in the contract.”
What can you do? “One way to get some protection is to rule out a termination without cause in the first year,” said Michael A. Cassidy, a physician contract attorney at Tucker Arensberg in Pittsburgh.
The force majeure clause is less likely to affect salary, but could impact bonus and incentive tied to performance. It’s wise to try to specifically limit how much the force majeure could reduce pay tied to performance, and to be prepared to negotiate that aspect of your contract.
No protections if you’re let go through no fault of your own
You could lose your job if your employer could not generate enough business and has to let some doctors go. This happened quite often in the early days of the COVID pandemic.
In these situations, the doctor has not done anything wrong to prompt the termination, but the restrictive covenant may still apply, meaning that the doctor would have to leave the area to find work.
What can you do? You’re in a good position to get this changed, said Christopher L. Nuland, a solo physician contract attorney in Jacksonville, Fla. “Many employers recognize that it would be draconian to require a restrictive covenant in this case, and they will agree to modify this provision.”
Similarly, the employer may not cover your tail insurance even if you were let go from your work through no fault of your own. Most malpractice policies for employer physicians require buying an extra policy, called a tail, if you leave. In some cases, the employer won’t provide a tail and will make the departing doctor buy it.
In these cases, “try for a compromise, such as stipulating that the party that caused the termination should pay for the tail,” Mr. Nuland said. “The employer may not agree to anything more than that because they want to set up a disincentive against you leaving.”
Employer could unilaterally alter your compensation
Many recent contracts give the employer the option to unilaterally modify compensation, such as changing the base salary or raising the target required for meeting the productivity bonus, said Ericka L. Adler, a physician contract attorney at Roetzel & Andress in Chicago.
Ms. Adler thought this change could have been prompted by employers’ financial problems during the pandemic. In the early months of COVID, many physicians were not making much money for the employer but still had to be paid. So employers added a clause saying they could reduce compensation at any time, she said.
What can you do? Harsh provisions like this often come up in contracts with private equity firms, Mr. Cassidy said. “The contract might say the employer can adjust compensation or even terminate physicians based on productivity or their profitability. And it may say that if they reassign you to a new location and you refuse, they can terminate you.”
“If you can’t get these clauses removed, try to reduce the impact of a termination by providing longer notice periods or by inserting a severance agreement,” Mr. Cassidy said.
Accelerating notice for without-cause terminations
Physicians who are convicted of a felony or other moral issue can usually be terminated immediately. But if you are terminated for other reasons – that is, “without cause” – you are given notice at a certain number of days before you have to leave (typically 60-90 days), so that you have time to find a new job.
Some recent contracts, however, allow for very little notice in without-cause terminations, which allows the employer to fire you in as little as 0 days after providing notice, Ms. Adler said.
“This means that, even if 90 days’ notice is provided in the contract, the employer can decide that your last day will be an earlier date,” she said.
Why is this happening? Ms. Adler said employers want to begin reallocating resources and patients as soon as possible. The problem came to employers’ attention during the COVID pandemic, when they were contractually forced to pay doctors for doing little or nothing during the notice period.
What can you do? Possibly not much, other than attempt to negotiate. “Large employers typically don’t want to drop this provision, but at the least, the doctor needs to understand the risk it creates for them,” she said.
You could be assigned to far-off locations
As patient care needs changed dramatically during the pandemic, employers needed to reassign doctors to new locations.
Some new contracts allow employers to simply inform the doctor that they are changing the work location. However, “you don’t want to be assigned to a new work location that is 50 miles away,” Mr. Nuland said.
What can you do? Mr. Nuland recommended adding new language saying that, if the new assignment is more than 20 miles away, both parties would have to approve it.
You could end up working too many off-hours
“Most employers won’t issue a specific work schedule,” Mr. Nuland said. “They want the flexibility to assign evening or weekend work, and it would be difficult for a young doctor to change this.”
What can you do? Mr. Nuland recommended trying to set some limits. “You can try to limit off-hours work to two times a month or something like that,” she said. And if you need to have a special schedule, such as not working on Fridays, Adler advises that this should be put into the contract.
If you can’t get anything changed in the contract, Mr. Nuland said the next-best thing is to ask employers to tell you specifically what they plan to do with you. “Most employers will give you an informal idea of what’s expected – maybe not an exact schedule, but it’s quite likely they will honor it.”
You wouldn’t be able to work nearby if you left the job
Most contracts have a noncompete clause, also known as a “restrictive covenant,” which prevents employed physicians from working in the area if they left the job.
“Almost every doctor I represent has told me that they’re not concerned about the noncompete clause because, they believe, it is not enforceable anyway,” Ms. Adler said. “This is incorrect.”
Mr. Nuland said the faster pace of job-changing during the pandemic makes it all the more likely that doctors have to deal with a restrictive covenant. At the same time, some employers have been expanding the restriction – either by enlarging the radius where the restriction applies or by making the restriction apply to each of their sites, so that each one has a restricted radius around it.
For example, one contract Mr. Nuland is currently reviewing has a 20-mile radius that in effect becomes a 120-mile radius because the employer is counting four offices.
What can you do? Mr. Nuland advised trying to reduce the impact of the noncompete – for instance, making it apply only to the offices where you worked, or trading more time for less distance. “If you have a 2-year, 20-mile restriction, ask for a 3-year, 10-mile restriction, where the radius could be easier to deal with,” he said.
You might end up with too much call
Contracts rarely detail your call schedule because employers want flexibility to expand call as patient care needs change, but you can try adding some specificity, said Sanja Ord, a physician contract attorney at Greensfelder, Hemker & Gale in St. Louis.
Contracts often use wide-open language to describe call, such as simply making it “subject to the house call policies,” Mr. Cassidy said. Language that is more beneficial to the physician would say that call must be “equal” among “similarly situated” physicians.
But Ms. Ord said even provisions for equal call can turn out to be onerous if there are too few doctors in the call roster, so it’s a good idea to find out just how many doctors will be participating in call.
Still, Adler said even that strategy can’t remove all risk. What happens, she asked, if several physicians participating in call decide to leave? Then you might end up with call every other night.
What can you do? Mr. Cassidy recommends specifying a maximum amount of call – for example, no more frequent than one in four nights.
Physician must pay for reimbursement claw-backs by payers
When auditors for Medicare or other payers find overpayments after the fact, called a ‘claw-back,’ the provider must pay them back. But which provider has to do that – you or your employer?
In many cases, your employer’s billing office may have introduced the error, but there may be a clause in the contract stating that the physician is solely responsible for all claw-backs. That could be costly.
What can you do? Mr. Shay said the clause should state that you have to pay only when it is the result of your own error or omission, and also not when it was made at the direction of the employer.
Some work may be outside of your subspecialty
In some cases, the employer may assign subspecialized doctors to work outside their subspecialty, Mr. Nuland said.
For example, he said he represented an endocrinologist who expected to see only diabetes patients but was assigned to some general internal medicine work as well, and an otolaryngologist client of his who completed a fellowship on facial plastic surgery was expected to do liposuction in a cosmetic surgery group.
What can you do? To prevent this from happening, Mr. Nuland recommends a clause stating that your work will be restricted to your subspecialty.
What the employer promised isn’t in the contract
“Beware of promises that are not in the contract,” Mr. Shay said. “You might feel you can really trust your new boss and what he tells you, but what if that person resigns, or the organization gets a new owner who doesn’t honor unwritten agreements?”
Many contracts have an integration clause, which specifies that the contract constitutes the complete agreement between the two parties, and it nullifies any other oral or written promises made to the physician.
For example, the employer might have promised a relocation bonus and a sign-on bonus, but for some reason it didn’t get into the contract, Ms. Ord said. In those cases, the employer is under no obligation to honor the promise.
What can you do? Mr. Cassidy said it is possible to hold the employer to a commitment made outside the contract. The alternative document, such as an offer letter, has to specifically state that the commitment is protected from the integration clause in the contract, he said, adding: “It is still better to have the commitment put into the contract.”
Contract is simply accepted as is
“Generally, the bigger the employer, the less likely they will alter an agreement just to make you happy,” Mr. Shay said.
But even in these contracts, he said there is still opportunity to fix errors and ambiguities that could harm you later – or even alter a provision if you can’t remove it outright.
The back-and-forth is important, Ms. Adler said. “Negotiation means trying to have some control over your job and your life.”
Mr. Cassidy said a big part of contract review is facing up to the possibility that you may have to resign or be let go.
“Many physicians don’t like to think about leaving when they’re just starting a job, but they need to,” he said. “You need to begin with the end in mind. Think about what would happen if this job didn’t work out.”
A version of this article first appeared on Medscape.com.
Physician employment contracts include some new dangers. This includes physicians taking a new job, but it also includes already-employed doctors who are being asked to resign a new contract that contains new conditions. A number of these new clauses have arisen because of COVID-19. When the pandemic dramatically reduced patient flow, many employers didn’t have enough money to pay doctors and didn’t always have physicians in the right location or practice setting.
Vowing this would never happen again, some employers have rewritten their physician contracts to make it easier to reassign and terminate physicians.
Here are 12 potential land mines in a physician employment contract, some of which were added as a result of the pandemic.
You could be immediately terminated without notice
One outcome of the pandemic is the growing use of “force majeure” clauses, which give the employer the right to reduce your compensation or even terminate you due to a natural disaster, which could include COVID.
“COVID made employers aware of the potential impact of disasters on their operations,” said Dan Shay, a health law attorney at Alice Gosfield & Associates in Philadelphia. “Therefore, even as the threat of COVID abates in many places, employers are continuing to put this provision in the contract.”
What can you do? “One way to get some protection is to rule out a termination without cause in the first year,” said Michael A. Cassidy, a physician contract attorney at Tucker Arensberg in Pittsburgh.
The force majeure clause is less likely to affect salary, but could impact bonus and incentive tied to performance. It’s wise to try to specifically limit how much the force majeure could reduce pay tied to performance, and to be prepared to negotiate that aspect of your contract.
No protections if you’re let go through no fault of your own
You could lose your job if your employer could not generate enough business and has to let some doctors go. This happened quite often in the early days of the COVID pandemic.
In these situations, the doctor has not done anything wrong to prompt the termination, but the restrictive covenant may still apply, meaning that the doctor would have to leave the area to find work.
What can you do? You’re in a good position to get this changed, said Christopher L. Nuland, a solo physician contract attorney in Jacksonville, Fla. “Many employers recognize that it would be draconian to require a restrictive covenant in this case, and they will agree to modify this provision.”
Similarly, the employer may not cover your tail insurance even if you were let go from your work through no fault of your own. Most malpractice policies for employer physicians require buying an extra policy, called a tail, if you leave. In some cases, the employer won’t provide a tail and will make the departing doctor buy it.
In these cases, “try for a compromise, such as stipulating that the party that caused the termination should pay for the tail,” Mr. Nuland said. “The employer may not agree to anything more than that because they want to set up a disincentive against you leaving.”
Employer could unilaterally alter your compensation
Many recent contracts give the employer the option to unilaterally modify compensation, such as changing the base salary or raising the target required for meeting the productivity bonus, said Ericka L. Adler, a physician contract attorney at Roetzel & Andress in Chicago.
Ms. Adler thought this change could have been prompted by employers’ financial problems during the pandemic. In the early months of COVID, many physicians were not making much money for the employer but still had to be paid. So employers added a clause saying they could reduce compensation at any time, she said.
What can you do? Harsh provisions like this often come up in contracts with private equity firms, Mr. Cassidy said. “The contract might say the employer can adjust compensation or even terminate physicians based on productivity or their profitability. And it may say that if they reassign you to a new location and you refuse, they can terminate you.”
“If you can’t get these clauses removed, try to reduce the impact of a termination by providing longer notice periods or by inserting a severance agreement,” Mr. Cassidy said.
Accelerating notice for without-cause terminations
Physicians who are convicted of a felony or other moral issue can usually be terminated immediately. But if you are terminated for other reasons – that is, “without cause” – you are given notice at a certain number of days before you have to leave (typically 60-90 days), so that you have time to find a new job.
Some recent contracts, however, allow for very little notice in without-cause terminations, which allows the employer to fire you in as little as 0 days after providing notice, Ms. Adler said.
“This means that, even if 90 days’ notice is provided in the contract, the employer can decide that your last day will be an earlier date,” she said.
Why is this happening? Ms. Adler said employers want to begin reallocating resources and patients as soon as possible. The problem came to employers’ attention during the COVID pandemic, when they were contractually forced to pay doctors for doing little or nothing during the notice period.
What can you do? Possibly not much, other than attempt to negotiate. “Large employers typically don’t want to drop this provision, but at the least, the doctor needs to understand the risk it creates for them,” she said.
You could be assigned to far-off locations
As patient care needs changed dramatically during the pandemic, employers needed to reassign doctors to new locations.
Some new contracts allow employers to simply inform the doctor that they are changing the work location. However, “you don’t want to be assigned to a new work location that is 50 miles away,” Mr. Nuland said.
What can you do? Mr. Nuland recommended adding new language saying that, if the new assignment is more than 20 miles away, both parties would have to approve it.
You could end up working too many off-hours
“Most employers won’t issue a specific work schedule,” Mr. Nuland said. “They want the flexibility to assign evening or weekend work, and it would be difficult for a young doctor to change this.”
What can you do? Mr. Nuland recommended trying to set some limits. “You can try to limit off-hours work to two times a month or something like that,” she said. And if you need to have a special schedule, such as not working on Fridays, Adler advises that this should be put into the contract.
If you can’t get anything changed in the contract, Mr. Nuland said the next-best thing is to ask employers to tell you specifically what they plan to do with you. “Most employers will give you an informal idea of what’s expected – maybe not an exact schedule, but it’s quite likely they will honor it.”
You wouldn’t be able to work nearby if you left the job
Most contracts have a noncompete clause, also known as a “restrictive covenant,” which prevents employed physicians from working in the area if they left the job.
“Almost every doctor I represent has told me that they’re not concerned about the noncompete clause because, they believe, it is not enforceable anyway,” Ms. Adler said. “This is incorrect.”
Mr. Nuland said the faster pace of job-changing during the pandemic makes it all the more likely that doctors have to deal with a restrictive covenant. At the same time, some employers have been expanding the restriction – either by enlarging the radius where the restriction applies or by making the restriction apply to each of their sites, so that each one has a restricted radius around it.
For example, one contract Mr. Nuland is currently reviewing has a 20-mile radius that in effect becomes a 120-mile radius because the employer is counting four offices.
What can you do? Mr. Nuland advised trying to reduce the impact of the noncompete – for instance, making it apply only to the offices where you worked, or trading more time for less distance. “If you have a 2-year, 20-mile restriction, ask for a 3-year, 10-mile restriction, where the radius could be easier to deal with,” he said.
You might end up with too much call
Contracts rarely detail your call schedule because employers want flexibility to expand call as patient care needs change, but you can try adding some specificity, said Sanja Ord, a physician contract attorney at Greensfelder, Hemker & Gale in St. Louis.
Contracts often use wide-open language to describe call, such as simply making it “subject to the house call policies,” Mr. Cassidy said. Language that is more beneficial to the physician would say that call must be “equal” among “similarly situated” physicians.
But Ms. Ord said even provisions for equal call can turn out to be onerous if there are too few doctors in the call roster, so it’s a good idea to find out just how many doctors will be participating in call.
Still, Adler said even that strategy can’t remove all risk. What happens, she asked, if several physicians participating in call decide to leave? Then you might end up with call every other night.
What can you do? Mr. Cassidy recommends specifying a maximum amount of call – for example, no more frequent than one in four nights.
Physician must pay for reimbursement claw-backs by payers
When auditors for Medicare or other payers find overpayments after the fact, called a ‘claw-back,’ the provider must pay them back. But which provider has to do that – you or your employer?
In many cases, your employer’s billing office may have introduced the error, but there may be a clause in the contract stating that the physician is solely responsible for all claw-backs. That could be costly.
What can you do? Mr. Shay said the clause should state that you have to pay only when it is the result of your own error or omission, and also not when it was made at the direction of the employer.
Some work may be outside of your subspecialty
In some cases, the employer may assign subspecialized doctors to work outside their subspecialty, Mr. Nuland said.
For example, he said he represented an endocrinologist who expected to see only diabetes patients but was assigned to some general internal medicine work as well, and an otolaryngologist client of his who completed a fellowship on facial plastic surgery was expected to do liposuction in a cosmetic surgery group.
What can you do? To prevent this from happening, Mr. Nuland recommends a clause stating that your work will be restricted to your subspecialty.
What the employer promised isn’t in the contract
“Beware of promises that are not in the contract,” Mr. Shay said. “You might feel you can really trust your new boss and what he tells you, but what if that person resigns, or the organization gets a new owner who doesn’t honor unwritten agreements?”
Many contracts have an integration clause, which specifies that the contract constitutes the complete agreement between the two parties, and it nullifies any other oral or written promises made to the physician.
For example, the employer might have promised a relocation bonus and a sign-on bonus, but for some reason it didn’t get into the contract, Ms. Ord said. In those cases, the employer is under no obligation to honor the promise.
What can you do? Mr. Cassidy said it is possible to hold the employer to a commitment made outside the contract. The alternative document, such as an offer letter, has to specifically state that the commitment is protected from the integration clause in the contract, he said, adding: “It is still better to have the commitment put into the contract.”
Contract is simply accepted as is
“Generally, the bigger the employer, the less likely they will alter an agreement just to make you happy,” Mr. Shay said.
But even in these contracts, he said there is still opportunity to fix errors and ambiguities that could harm you later – or even alter a provision if you can’t remove it outright.
The back-and-forth is important, Ms. Adler said. “Negotiation means trying to have some control over your job and your life.”
Mr. Cassidy said a big part of contract review is facing up to the possibility that you may have to resign or be let go.
“Many physicians don’t like to think about leaving when they’re just starting a job, but they need to,” he said. “You need to begin with the end in mind. Think about what would happen if this job didn’t work out.”
A version of this article first appeared on Medscape.com.
Docs: Insurers’ payment delays, downcoding a ‘revenue grab’
Despite reporting record profits during the COVID-19 pandemic, major insurance companies are delaying claims payments and making it more difficult for hospitals and physicians to get paid the full amount of claims, observers and physicians say.
Kaiser Health News recently reported that hospitals, in particular, are affected by the slowdown in claims payments from Anthem Blue Cross, the nation’s second largest health insurer. The investigative piece did not focus on outpatient or independent practices. Research by this news organization shows that the health plans’ new policies are also reducing cash flow and raising costs for ambulatory care groups. In addition, it showed that other payers besides Anthem have engaged in the same practices.
“What we’ve seen is that with complex claims, such as those with -25 modifiers, plans are routinely requiring documentation,” Jim Donohue, senior manager and associate principal at ECG Management Consultants, said in an interview. “It’s not a denial, it’s a request for more information for medical records prior to processing payments. That has the effect of slowing down payments.”
This is exactly what one internal medicine group in the Southeast has noticed. The internist who heads the practice, who asked not to be identified, says that about 4-6 months ago, United, Humana, and other payers started to require documentation for prepayment review on a higher percentage of complex claims such as 99214 (established patient), 99204 (new patient), and claims with -25 modifiers. (The latter are appended to evaluation and management [E/M] claims in which patients had comorbidities that were addressed in the same visit as the main complaint.)
“That’s really frustrating, because you have to print out or take the record for that particular visit and computer fax it to them,” the practice leader notes. “And invariably, they’ll say they didn’t get a certain percentage of them. It’s our fault because they lost the claim.”
In the past, he says, health plans would occasionally ask for the note related to a complex visit where they saw issues, and they’ve always done random postpayment chart audits. But the percentage of prepayment reviews has significantly increased in recent months, he says.
Until a plan does this review, the claim can’t be processed because it’s not regarded as a clean claim. And this has implications for insurers’ compliance with laws that, in most states, require them to pay claims within 30-40 days of submission. (Medicare’s limit is 30 days.) According to Mr. Donohue, the clock doesn’t start ticking on this requirement unless and until a claim is clean. So by requiring documentation on complex claims, the plans can not only justify downcoding a claim, but can also delay payment without triggering state penalties.
Insurer admits ‘challenges’ with claims processing
VCU Health, a health system affiliated with Virginia Commonwealth University, recently filed a complaint against Anthem with Virginia’s insurance commissioner, asking that the Virginia Bureau of Insurance investigate the company’s claims-processing delays. The complaint claimed Anthem owes VCU more than $385 million, of which $171 million is over 90 days old. Much of that consists of commercial claims, which are subject to the state’s 40-day claims payment rule.
VCU cited several problems it said Anthem had created that slowed claims payments:
Any claim over a certain dollar limit requires an itemized bill.
Anthem requests detailed medical records prior to considering payment of even clean claims.
Documents must be uploaded to a web portal that has technical problems, and Anthem has lost some documents as a result.
Claims are being incorrectly processed for some professionals, “resulting in multi-million-dollar underpayments of anesthesia, nurse practitioners, pathology, and behavioral health providers.”
In addition, as the Kaiser Health News article points out, hospitals have blamed the increase in payment delays or denials partly on “preauthorization hurdles for routine procedures and requirements that doctors themselves – not support staffers – speak to insurance gatekeepers.”
In response to an inquiry from this news organization, an Anthem spokesman admitted that some payments to providers have been delayed, partly because of changes in the company’s claims-processing system. “We recognize there have been some challenges as we work with care providers to update claims processing, and readjust and adapt to a new set of dynamics as we continue to manage the pandemic,” said the spokesman.
The Kaiser Health News piece reported that Anthem’s CFO had told stock analysts on a conference call that the company had slowed claims payments to build up its financial reserves during the pandemic – a statement that some physicians called “outrageous.” But the Anthem spokesman told this news organization the quote was taken out of context and that the CFO was talking not about reserves but about “days in claims payable.” The spokesman said, “The payment delays that the article focuses on are not the primary driver or even a material driver of the increase in our overall reserves or DCPs [defined contribution plans] relative to historical levels. In fact, the vast majority of our claims are being processed in a timely manner.”
Some claims routinely downcoded
Even if that were the case, it would not explain why some physicians are seeing their higher-cost claims routinely downcoded. Will Sawyer, MD, a family physician in Cincinnati, told this news organization, “Anthem has been downcoding relentlessly since October 2020.” More often than not, when his office submits a claim with a 99214 code (office visit, 30-39 minutes, moderate medical decision-making), it’s changed to 99213 (office visit, 20-29 minutes, low medical decision-making) before processing, he says.
This has resulted in a significant diminution of his income, he notes. Anthem pays him less than Medicare for E/M visits, and the downcoding reduces his payment from $86 to $68 for a complex visit that may have taken half an hour or more.
In some cases where his office manager has noticed the downcoding, Dr. Sawyer says, she has resubmitted the claim with a copy of the encounter form. But Anthem hasn’t budged. And the refiling effort takes a toll on his solo practice, which doesn’t have sufficient staff, as it is.
Dr. Sawyer acknowledges that he has sent in a higher percentage of complex claims in the past year than he did previously. But much of that is the result of two factors beyond his control: First, many patients avoided coming into the office early in the pandemic, and when they returned, their preventive and chronic care needs were greater. Second, he says, “There are many comorbidities and mental health aspects, which exacerbate many issues and become an issue. We’re not dealing with engines here; they’re human beings. And it takes time.”
In response to Dr. Sawyer’s comments, Anthem said that it uses “analytical tools to review evaluation and management (E/M) codes during the claims adjudication and processing process.” Physicians who believe that certain claims should not have been downcoded can dispute these decisions; they must supply a statement explaining why they disagree with the decision along with documentation to support their statement, the company said. Anthem added that it reviews claims to lower costs for its members.
‘Revenue-grab strategy’
Dr. Sawyer believes that what Anthem is doing to him and other physicians reflects its desire to increase profits by netting extra revenue and keeping physicians’ money while it delays payments to them – a practice known in the trade as “the float.” Moreover, he says, the company depends on many practices not keeping track of their finances during the pandemic.
“When practices are running at warp speed, trying to keep people healthy and getting burned out, they aren’t paying as close attention to the details of payment. It’s an absolute revenue-grab strategy that’s unconscionable,” says Dr. Sawyer.
The Southeast internist also thinks that insurance companies other than Anthem – including United and Humana – are profiting from the float. Besides delaying his payments with gratuitous demands for documentation, he said, they also downcode many claims, forcing the practice to refile the claims and appeal. That forces the practice to pay overtime or bring on more claims staff, which raises administrative costs.
The plans’ strategy, the internist says, is this: “If they downcode millions of claims, a certain number of physicians will give up without appealing, and they’ll raise their profits.”
A United spokesperson said in an interview, “We pay claims appropriately under members’ plans and within the required time frame.” Humana had not responded to this news organization’s request for comment at press time.
Challenge to practice economics
Insurer policies that delay payments or downcode claims, ECG’s Mr. Donohue points out, are especially harmful to primary care and other ambulatory practices that have many small-dollar claims.
“That’s where it’s challenging, because it’s not like a $10,000 case where you add $100 to it [to meet records requests]. You’re talking about something that’s relatively low dollar, where the practice makes a small surplus, and when you add administrative costs, it can change the economics,” he says.
While the economic burden on ambulatory care practices may be greater, Anders Gilberg, senior vice president of government affairs for the Medical Group Management Association (MGMA), said that the payment delays and demands for documentation – along with prior authorization – particularly affect inpatient care. The health plans are questioning big-ticket items more than ever, he said, and most of those services occur in hospitals.
However, the greater level of insurer scrutiny also affects physicians who treat patients in the hospital, including surgeons and emergency department physicians who contract with the facilities, he adds.
Mr. Gilberg views the current situation as an exacerbation of the health plan policies that physicians have long struggled with. “It’s not new to have insurers play the float and not pay claims on time. Unfortunately, this is something that medical practices are used to.”
A version of this article first appeared on Medscape.com.
Despite reporting record profits during the COVID-19 pandemic, major insurance companies are delaying claims payments and making it more difficult for hospitals and physicians to get paid the full amount of claims, observers and physicians say.
Kaiser Health News recently reported that hospitals, in particular, are affected by the slowdown in claims payments from Anthem Blue Cross, the nation’s second largest health insurer. The investigative piece did not focus on outpatient or independent practices. Research by this news organization shows that the health plans’ new policies are also reducing cash flow and raising costs for ambulatory care groups. In addition, it showed that other payers besides Anthem have engaged in the same practices.
“What we’ve seen is that with complex claims, such as those with -25 modifiers, plans are routinely requiring documentation,” Jim Donohue, senior manager and associate principal at ECG Management Consultants, said in an interview. “It’s not a denial, it’s a request for more information for medical records prior to processing payments. That has the effect of slowing down payments.”
This is exactly what one internal medicine group in the Southeast has noticed. The internist who heads the practice, who asked not to be identified, says that about 4-6 months ago, United, Humana, and other payers started to require documentation for prepayment review on a higher percentage of complex claims such as 99214 (established patient), 99204 (new patient), and claims with -25 modifiers. (The latter are appended to evaluation and management [E/M] claims in which patients had comorbidities that were addressed in the same visit as the main complaint.)
“That’s really frustrating, because you have to print out or take the record for that particular visit and computer fax it to them,” the practice leader notes. “And invariably, they’ll say they didn’t get a certain percentage of them. It’s our fault because they lost the claim.”
In the past, he says, health plans would occasionally ask for the note related to a complex visit where they saw issues, and they’ve always done random postpayment chart audits. But the percentage of prepayment reviews has significantly increased in recent months, he says.
Until a plan does this review, the claim can’t be processed because it’s not regarded as a clean claim. And this has implications for insurers’ compliance with laws that, in most states, require them to pay claims within 30-40 days of submission. (Medicare’s limit is 30 days.) According to Mr. Donohue, the clock doesn’t start ticking on this requirement unless and until a claim is clean. So by requiring documentation on complex claims, the plans can not only justify downcoding a claim, but can also delay payment without triggering state penalties.
Insurer admits ‘challenges’ with claims processing
VCU Health, a health system affiliated with Virginia Commonwealth University, recently filed a complaint against Anthem with Virginia’s insurance commissioner, asking that the Virginia Bureau of Insurance investigate the company’s claims-processing delays. The complaint claimed Anthem owes VCU more than $385 million, of which $171 million is over 90 days old. Much of that consists of commercial claims, which are subject to the state’s 40-day claims payment rule.
VCU cited several problems it said Anthem had created that slowed claims payments:
Any claim over a certain dollar limit requires an itemized bill.
Anthem requests detailed medical records prior to considering payment of even clean claims.
Documents must be uploaded to a web portal that has technical problems, and Anthem has lost some documents as a result.
Claims are being incorrectly processed for some professionals, “resulting in multi-million-dollar underpayments of anesthesia, nurse practitioners, pathology, and behavioral health providers.”
In addition, as the Kaiser Health News article points out, hospitals have blamed the increase in payment delays or denials partly on “preauthorization hurdles for routine procedures and requirements that doctors themselves – not support staffers – speak to insurance gatekeepers.”
In response to an inquiry from this news organization, an Anthem spokesman admitted that some payments to providers have been delayed, partly because of changes in the company’s claims-processing system. “We recognize there have been some challenges as we work with care providers to update claims processing, and readjust and adapt to a new set of dynamics as we continue to manage the pandemic,” said the spokesman.
The Kaiser Health News piece reported that Anthem’s CFO had told stock analysts on a conference call that the company had slowed claims payments to build up its financial reserves during the pandemic – a statement that some physicians called “outrageous.” But the Anthem spokesman told this news organization the quote was taken out of context and that the CFO was talking not about reserves but about “days in claims payable.” The spokesman said, “The payment delays that the article focuses on are not the primary driver or even a material driver of the increase in our overall reserves or DCPs [defined contribution plans] relative to historical levels. In fact, the vast majority of our claims are being processed in a timely manner.”
Some claims routinely downcoded
Even if that were the case, it would not explain why some physicians are seeing their higher-cost claims routinely downcoded. Will Sawyer, MD, a family physician in Cincinnati, told this news organization, “Anthem has been downcoding relentlessly since October 2020.” More often than not, when his office submits a claim with a 99214 code (office visit, 30-39 minutes, moderate medical decision-making), it’s changed to 99213 (office visit, 20-29 minutes, low medical decision-making) before processing, he says.
This has resulted in a significant diminution of his income, he notes. Anthem pays him less than Medicare for E/M visits, and the downcoding reduces his payment from $86 to $68 for a complex visit that may have taken half an hour or more.
In some cases where his office manager has noticed the downcoding, Dr. Sawyer says, she has resubmitted the claim with a copy of the encounter form. But Anthem hasn’t budged. And the refiling effort takes a toll on his solo practice, which doesn’t have sufficient staff, as it is.
Dr. Sawyer acknowledges that he has sent in a higher percentage of complex claims in the past year than he did previously. But much of that is the result of two factors beyond his control: First, many patients avoided coming into the office early in the pandemic, and when they returned, their preventive and chronic care needs were greater. Second, he says, “There are many comorbidities and mental health aspects, which exacerbate many issues and become an issue. We’re not dealing with engines here; they’re human beings. And it takes time.”
In response to Dr. Sawyer’s comments, Anthem said that it uses “analytical tools to review evaluation and management (E/M) codes during the claims adjudication and processing process.” Physicians who believe that certain claims should not have been downcoded can dispute these decisions; they must supply a statement explaining why they disagree with the decision along with documentation to support their statement, the company said. Anthem added that it reviews claims to lower costs for its members.
‘Revenue-grab strategy’
Dr. Sawyer believes that what Anthem is doing to him and other physicians reflects its desire to increase profits by netting extra revenue and keeping physicians’ money while it delays payments to them – a practice known in the trade as “the float.” Moreover, he says, the company depends on many practices not keeping track of their finances during the pandemic.
“When practices are running at warp speed, trying to keep people healthy and getting burned out, they aren’t paying as close attention to the details of payment. It’s an absolute revenue-grab strategy that’s unconscionable,” says Dr. Sawyer.
The Southeast internist also thinks that insurance companies other than Anthem – including United and Humana – are profiting from the float. Besides delaying his payments with gratuitous demands for documentation, he said, they also downcode many claims, forcing the practice to refile the claims and appeal. That forces the practice to pay overtime or bring on more claims staff, which raises administrative costs.
The plans’ strategy, the internist says, is this: “If they downcode millions of claims, a certain number of physicians will give up without appealing, and they’ll raise their profits.”
A United spokesperson said in an interview, “We pay claims appropriately under members’ plans and within the required time frame.” Humana had not responded to this news organization’s request for comment at press time.
Challenge to practice economics
Insurer policies that delay payments or downcode claims, ECG’s Mr. Donohue points out, are especially harmful to primary care and other ambulatory practices that have many small-dollar claims.
“That’s where it’s challenging, because it’s not like a $10,000 case where you add $100 to it [to meet records requests]. You’re talking about something that’s relatively low dollar, where the practice makes a small surplus, and when you add administrative costs, it can change the economics,” he says.
While the economic burden on ambulatory care practices may be greater, Anders Gilberg, senior vice president of government affairs for the Medical Group Management Association (MGMA), said that the payment delays and demands for documentation – along with prior authorization – particularly affect inpatient care. The health plans are questioning big-ticket items more than ever, he said, and most of those services occur in hospitals.
However, the greater level of insurer scrutiny also affects physicians who treat patients in the hospital, including surgeons and emergency department physicians who contract with the facilities, he adds.
Mr. Gilberg views the current situation as an exacerbation of the health plan policies that physicians have long struggled with. “It’s not new to have insurers play the float and not pay claims on time. Unfortunately, this is something that medical practices are used to.”
A version of this article first appeared on Medscape.com.
Despite reporting record profits during the COVID-19 pandemic, major insurance companies are delaying claims payments and making it more difficult for hospitals and physicians to get paid the full amount of claims, observers and physicians say.
Kaiser Health News recently reported that hospitals, in particular, are affected by the slowdown in claims payments from Anthem Blue Cross, the nation’s second largest health insurer. The investigative piece did not focus on outpatient or independent practices. Research by this news organization shows that the health plans’ new policies are also reducing cash flow and raising costs for ambulatory care groups. In addition, it showed that other payers besides Anthem have engaged in the same practices.
“What we’ve seen is that with complex claims, such as those with -25 modifiers, plans are routinely requiring documentation,” Jim Donohue, senior manager and associate principal at ECG Management Consultants, said in an interview. “It’s not a denial, it’s a request for more information for medical records prior to processing payments. That has the effect of slowing down payments.”
This is exactly what one internal medicine group in the Southeast has noticed. The internist who heads the practice, who asked not to be identified, says that about 4-6 months ago, United, Humana, and other payers started to require documentation for prepayment review on a higher percentage of complex claims such as 99214 (established patient), 99204 (new patient), and claims with -25 modifiers. (The latter are appended to evaluation and management [E/M] claims in which patients had comorbidities that were addressed in the same visit as the main complaint.)
“That’s really frustrating, because you have to print out or take the record for that particular visit and computer fax it to them,” the practice leader notes. “And invariably, they’ll say they didn’t get a certain percentage of them. It’s our fault because they lost the claim.”
In the past, he says, health plans would occasionally ask for the note related to a complex visit where they saw issues, and they’ve always done random postpayment chart audits. But the percentage of prepayment reviews has significantly increased in recent months, he says.
Until a plan does this review, the claim can’t be processed because it’s not regarded as a clean claim. And this has implications for insurers’ compliance with laws that, in most states, require them to pay claims within 30-40 days of submission. (Medicare’s limit is 30 days.) According to Mr. Donohue, the clock doesn’t start ticking on this requirement unless and until a claim is clean. So by requiring documentation on complex claims, the plans can not only justify downcoding a claim, but can also delay payment without triggering state penalties.
Insurer admits ‘challenges’ with claims processing
VCU Health, a health system affiliated with Virginia Commonwealth University, recently filed a complaint against Anthem with Virginia’s insurance commissioner, asking that the Virginia Bureau of Insurance investigate the company’s claims-processing delays. The complaint claimed Anthem owes VCU more than $385 million, of which $171 million is over 90 days old. Much of that consists of commercial claims, which are subject to the state’s 40-day claims payment rule.
VCU cited several problems it said Anthem had created that slowed claims payments:
Any claim over a certain dollar limit requires an itemized bill.
Anthem requests detailed medical records prior to considering payment of even clean claims.
Documents must be uploaded to a web portal that has technical problems, and Anthem has lost some documents as a result.
Claims are being incorrectly processed for some professionals, “resulting in multi-million-dollar underpayments of anesthesia, nurse practitioners, pathology, and behavioral health providers.”
In addition, as the Kaiser Health News article points out, hospitals have blamed the increase in payment delays or denials partly on “preauthorization hurdles for routine procedures and requirements that doctors themselves – not support staffers – speak to insurance gatekeepers.”
In response to an inquiry from this news organization, an Anthem spokesman admitted that some payments to providers have been delayed, partly because of changes in the company’s claims-processing system. “We recognize there have been some challenges as we work with care providers to update claims processing, and readjust and adapt to a new set of dynamics as we continue to manage the pandemic,” said the spokesman.
The Kaiser Health News piece reported that Anthem’s CFO had told stock analysts on a conference call that the company had slowed claims payments to build up its financial reserves during the pandemic – a statement that some physicians called “outrageous.” But the Anthem spokesman told this news organization the quote was taken out of context and that the CFO was talking not about reserves but about “days in claims payable.” The spokesman said, “The payment delays that the article focuses on are not the primary driver or even a material driver of the increase in our overall reserves or DCPs [defined contribution plans] relative to historical levels. In fact, the vast majority of our claims are being processed in a timely manner.”
Some claims routinely downcoded
Even if that were the case, it would not explain why some physicians are seeing their higher-cost claims routinely downcoded. Will Sawyer, MD, a family physician in Cincinnati, told this news organization, “Anthem has been downcoding relentlessly since October 2020.” More often than not, when his office submits a claim with a 99214 code (office visit, 30-39 minutes, moderate medical decision-making), it’s changed to 99213 (office visit, 20-29 minutes, low medical decision-making) before processing, he says.
This has resulted in a significant diminution of his income, he notes. Anthem pays him less than Medicare for E/M visits, and the downcoding reduces his payment from $86 to $68 for a complex visit that may have taken half an hour or more.
In some cases where his office manager has noticed the downcoding, Dr. Sawyer says, she has resubmitted the claim with a copy of the encounter form. But Anthem hasn’t budged. And the refiling effort takes a toll on his solo practice, which doesn’t have sufficient staff, as it is.
Dr. Sawyer acknowledges that he has sent in a higher percentage of complex claims in the past year than he did previously. But much of that is the result of two factors beyond his control: First, many patients avoided coming into the office early in the pandemic, and when they returned, their preventive and chronic care needs were greater. Second, he says, “There are many comorbidities and mental health aspects, which exacerbate many issues and become an issue. We’re not dealing with engines here; they’re human beings. And it takes time.”
In response to Dr. Sawyer’s comments, Anthem said that it uses “analytical tools to review evaluation and management (E/M) codes during the claims adjudication and processing process.” Physicians who believe that certain claims should not have been downcoded can dispute these decisions; they must supply a statement explaining why they disagree with the decision along with documentation to support their statement, the company said. Anthem added that it reviews claims to lower costs for its members.
‘Revenue-grab strategy’
Dr. Sawyer believes that what Anthem is doing to him and other physicians reflects its desire to increase profits by netting extra revenue and keeping physicians’ money while it delays payments to them – a practice known in the trade as “the float.” Moreover, he says, the company depends on many practices not keeping track of their finances during the pandemic.
“When practices are running at warp speed, trying to keep people healthy and getting burned out, they aren’t paying as close attention to the details of payment. It’s an absolute revenue-grab strategy that’s unconscionable,” says Dr. Sawyer.
The Southeast internist also thinks that insurance companies other than Anthem – including United and Humana – are profiting from the float. Besides delaying his payments with gratuitous demands for documentation, he said, they also downcode many claims, forcing the practice to refile the claims and appeal. That forces the practice to pay overtime or bring on more claims staff, which raises administrative costs.
The plans’ strategy, the internist says, is this: “If they downcode millions of claims, a certain number of physicians will give up without appealing, and they’ll raise their profits.”
A United spokesperson said in an interview, “We pay claims appropriately under members’ plans and within the required time frame.” Humana had not responded to this news organization’s request for comment at press time.
Challenge to practice economics
Insurer policies that delay payments or downcode claims, ECG’s Mr. Donohue points out, are especially harmful to primary care and other ambulatory practices that have many small-dollar claims.
“That’s where it’s challenging, because it’s not like a $10,000 case where you add $100 to it [to meet records requests]. You’re talking about something that’s relatively low dollar, where the practice makes a small surplus, and when you add administrative costs, it can change the economics,” he says.
While the economic burden on ambulatory care practices may be greater, Anders Gilberg, senior vice president of government affairs for the Medical Group Management Association (MGMA), said that the payment delays and demands for documentation – along with prior authorization – particularly affect inpatient care. The health plans are questioning big-ticket items more than ever, he said, and most of those services occur in hospitals.
However, the greater level of insurer scrutiny also affects physicians who treat patients in the hospital, including surgeons and emergency department physicians who contract with the facilities, he adds.
Mr. Gilberg views the current situation as an exacerbation of the health plan policies that physicians have long struggled with. “It’s not new to have insurers play the float and not pay claims on time. Unfortunately, this is something that medical practices are used to.”
A version of this article first appeared on Medscape.com.