User login
Secukinumab tames psoriatic arthritis in FUTURE 2 trial
BOSTON – Secukinumab improved quality of life and symptoms in patients with psoriatic arthritis, regardless of whether they had received prior anti-tumor-necrosis factor therapy or were concurrently receiving methotrexate, Dr. Ian B. McInnes reported at the annual meeting of the American College of Rheumatology.
A 300-mg dose of the investigational drug, which is given subcutaneously, proved to be the most effective dose, Dr. McInnes, of the University of Glasgow, Scotland, said in his presentation of the 24-week results of the FUTURE 2 study. The primary endpoint, a response of at least ACR20, was achieved by 54% of 100 patients given the 300-mg dose and by 51% of 100 patients given the 150-mg dose. The 75-mg dose was far less effective, with a 29% response in 99 patients; the 98 patients given placebo had a 15% response.
At the 150-mg and 300-mg doses, the rates of ACR20 responses were comparable whether or not patients were also taking concomitant methotrexate. Further, the drug’s safety profile was comparable to placebo, Dr. McInnes said.
An ACR50 was achieved by 35% of patients given secukinumab at either 300 mg or 150 mg, and by 18% of those given 75 mg and 7% of those given placebo. About 20% of patients given the higher doses had an ACR70 response, as did 6% of those given 75 mg and 1% of those given placebo.
At the 300-mg dose, secukinumab also resolved dactylitis and enthesitis in approximately half of the affected patients.
Mean improvements in quality of life based on patients’ SF 36 PCS score (Short Form-36 Physical Component Summary) at 24 weeks from baseline were 7.25 in those on the 300-mg dose and 6.39 in those on the 150-mg dose. Those on the 75-mg dose had a 4.38-point mean improvement, whereas patients on placebo had a 1.34-point mean improvement in SF 36 PCS.
No safety signals were noted; adverse events were few and comparable to placebo. Five subjects on the active drug had mild to moderate candidal infections that responded to oral therapy. Neutropenia occurred in one patient in the 300-mg dose group and in one patient in the placebo group, but was transient and patients continued on therapy.
Secukinumab is a fully-human IgG1k monoclonal antibody that selectively targets IL-17A. The drug, manufactured by Novartis, was unanimously recommended for approval by an advisory committee to the Food and Drug Administration. It was administered weekly as a subcutaneous injection for the first 4 weeks of the study, then given again at week 8 and once every 4 weeks thereafter in patients assigned to one of the secukinumab arms of the double-blind, randomized study. Patient assigned to the placebo group were either responders and assigned to receive secukinumab at week 24 and every 4 weeks thereafter or were nonresponders assigned to receive secukinumab at week 16 and every 4 weeks thereafter. Only patients with at least 20% reductions in the number of tender joints or swollen joints continue to receive the drug beyond 1 year.
To be eligible for the study, patients needed to have a diagnosis of active psoriatic arthritis classified by CASPAR criteria and tenderness in at least 3 of 78 joints and swelling of at least 3 of 76 joints. They additionally needed to have an inadequate response to nonsteroidal anti-inflammatory drugs, methotrexate, or anti-TNF therapy
The primary endpoint of the study was ACR20 response at 24 weeks. Secondary endpoints included PASI 75 and PASI 90 responses, change in DAS28-CRP (28-joint Disease Activity Score using C-reactive protein) from baseline, change in SF-36 PCS and HAQ-DI (Health Assessment Questionnaire-Disability Index) from baseline, ACR50 response, the proportion of subjects with dactylitis and enthesitis, and overall safety and tolerability.
As of press time, the FDA was expected to take additional action in January 2015, according to Novartis.
The study was sponsored by Novartis, the maker of secukinumab. Dr. McInnes receives consulting fees from Novartis as well as multiple other pharmaceutical companies.
BOSTON – Secukinumab improved quality of life and symptoms in patients with psoriatic arthritis, regardless of whether they had received prior anti-tumor-necrosis factor therapy or were concurrently receiving methotrexate, Dr. Ian B. McInnes reported at the annual meeting of the American College of Rheumatology.
A 300-mg dose of the investigational drug, which is given subcutaneously, proved to be the most effective dose, Dr. McInnes, of the University of Glasgow, Scotland, said in his presentation of the 24-week results of the FUTURE 2 study. The primary endpoint, a response of at least ACR20, was achieved by 54% of 100 patients given the 300-mg dose and by 51% of 100 patients given the 150-mg dose. The 75-mg dose was far less effective, with a 29% response in 99 patients; the 98 patients given placebo had a 15% response.
At the 150-mg and 300-mg doses, the rates of ACR20 responses were comparable whether or not patients were also taking concomitant methotrexate. Further, the drug’s safety profile was comparable to placebo, Dr. McInnes said.
An ACR50 was achieved by 35% of patients given secukinumab at either 300 mg or 150 mg, and by 18% of those given 75 mg and 7% of those given placebo. About 20% of patients given the higher doses had an ACR70 response, as did 6% of those given 75 mg and 1% of those given placebo.
At the 300-mg dose, secukinumab also resolved dactylitis and enthesitis in approximately half of the affected patients.
Mean improvements in quality of life based on patients’ SF 36 PCS score (Short Form-36 Physical Component Summary) at 24 weeks from baseline were 7.25 in those on the 300-mg dose and 6.39 in those on the 150-mg dose. Those on the 75-mg dose had a 4.38-point mean improvement, whereas patients on placebo had a 1.34-point mean improvement in SF 36 PCS.
No safety signals were noted; adverse events were few and comparable to placebo. Five subjects on the active drug had mild to moderate candidal infections that responded to oral therapy. Neutropenia occurred in one patient in the 300-mg dose group and in one patient in the placebo group, but was transient and patients continued on therapy.
Secukinumab is a fully-human IgG1k monoclonal antibody that selectively targets IL-17A. The drug, manufactured by Novartis, was unanimously recommended for approval by an advisory committee to the Food and Drug Administration. It was administered weekly as a subcutaneous injection for the first 4 weeks of the study, then given again at week 8 and once every 4 weeks thereafter in patients assigned to one of the secukinumab arms of the double-blind, randomized study. Patient assigned to the placebo group were either responders and assigned to receive secukinumab at week 24 and every 4 weeks thereafter or were nonresponders assigned to receive secukinumab at week 16 and every 4 weeks thereafter. Only patients with at least 20% reductions in the number of tender joints or swollen joints continue to receive the drug beyond 1 year.
To be eligible for the study, patients needed to have a diagnosis of active psoriatic arthritis classified by CASPAR criteria and tenderness in at least 3 of 78 joints and swelling of at least 3 of 76 joints. They additionally needed to have an inadequate response to nonsteroidal anti-inflammatory drugs, methotrexate, or anti-TNF therapy
The primary endpoint of the study was ACR20 response at 24 weeks. Secondary endpoints included PASI 75 and PASI 90 responses, change in DAS28-CRP (28-joint Disease Activity Score using C-reactive protein) from baseline, change in SF-36 PCS and HAQ-DI (Health Assessment Questionnaire-Disability Index) from baseline, ACR50 response, the proportion of subjects with dactylitis and enthesitis, and overall safety and tolerability.
As of press time, the FDA was expected to take additional action in January 2015, according to Novartis.
The study was sponsored by Novartis, the maker of secukinumab. Dr. McInnes receives consulting fees from Novartis as well as multiple other pharmaceutical companies.
BOSTON – Secukinumab improved quality of life and symptoms in patients with psoriatic arthritis, regardless of whether they had received prior anti-tumor-necrosis factor therapy or were concurrently receiving methotrexate, Dr. Ian B. McInnes reported at the annual meeting of the American College of Rheumatology.
A 300-mg dose of the investigational drug, which is given subcutaneously, proved to be the most effective dose, Dr. McInnes, of the University of Glasgow, Scotland, said in his presentation of the 24-week results of the FUTURE 2 study. The primary endpoint, a response of at least ACR20, was achieved by 54% of 100 patients given the 300-mg dose and by 51% of 100 patients given the 150-mg dose. The 75-mg dose was far less effective, with a 29% response in 99 patients; the 98 patients given placebo had a 15% response.
At the 150-mg and 300-mg doses, the rates of ACR20 responses were comparable whether or not patients were also taking concomitant methotrexate. Further, the drug’s safety profile was comparable to placebo, Dr. McInnes said.
An ACR50 was achieved by 35% of patients given secukinumab at either 300 mg or 150 mg, and by 18% of those given 75 mg and 7% of those given placebo. About 20% of patients given the higher doses had an ACR70 response, as did 6% of those given 75 mg and 1% of those given placebo.
At the 300-mg dose, secukinumab also resolved dactylitis and enthesitis in approximately half of the affected patients.
Mean improvements in quality of life based on patients’ SF 36 PCS score (Short Form-36 Physical Component Summary) at 24 weeks from baseline were 7.25 in those on the 300-mg dose and 6.39 in those on the 150-mg dose. Those on the 75-mg dose had a 4.38-point mean improvement, whereas patients on placebo had a 1.34-point mean improvement in SF 36 PCS.
No safety signals were noted; adverse events were few and comparable to placebo. Five subjects on the active drug had mild to moderate candidal infections that responded to oral therapy. Neutropenia occurred in one patient in the 300-mg dose group and in one patient in the placebo group, but was transient and patients continued on therapy.
Secukinumab is a fully-human IgG1k monoclonal antibody that selectively targets IL-17A. The drug, manufactured by Novartis, was unanimously recommended for approval by an advisory committee to the Food and Drug Administration. It was administered weekly as a subcutaneous injection for the first 4 weeks of the study, then given again at week 8 and once every 4 weeks thereafter in patients assigned to one of the secukinumab arms of the double-blind, randomized study. Patient assigned to the placebo group were either responders and assigned to receive secukinumab at week 24 and every 4 weeks thereafter or were nonresponders assigned to receive secukinumab at week 16 and every 4 weeks thereafter. Only patients with at least 20% reductions in the number of tender joints or swollen joints continue to receive the drug beyond 1 year.
To be eligible for the study, patients needed to have a diagnosis of active psoriatic arthritis classified by CASPAR criteria and tenderness in at least 3 of 78 joints and swelling of at least 3 of 76 joints. They additionally needed to have an inadequate response to nonsteroidal anti-inflammatory drugs, methotrexate, or anti-TNF therapy
The primary endpoint of the study was ACR20 response at 24 weeks. Secondary endpoints included PASI 75 and PASI 90 responses, change in DAS28-CRP (28-joint Disease Activity Score using C-reactive protein) from baseline, change in SF-36 PCS and HAQ-DI (Health Assessment Questionnaire-Disability Index) from baseline, ACR50 response, the proportion of subjects with dactylitis and enthesitis, and overall safety and tolerability.
As of press time, the FDA was expected to take additional action in January 2015, according to Novartis.
The study was sponsored by Novartis, the maker of secukinumab. Dr. McInnes receives consulting fees from Novartis as well as multiple other pharmaceutical companies.
AT THE ACR ANNUAL MEETING
Key clinical point: Secukinumab could prove to be a major new therapy for psoriatic arthritis.
Major finding: After 24 weeks, a response of at least ACR20 was achieved by 54% of 100 patients given the 300-mg dose of secukinumab.
Data source: The FUTURE 2 study group of nearly 400 patients with psoriatic arthritis who were randomized either to one of three doses of secukinumab or placebo.
Disclosures: The study was sponsored by Novartis, the maker of secukinumab. Dr. McInnes receives consulting fees from Novartis as well as multiple other pharmaceutical companies.
Few psoriatic arthritis patients achieve minimal disease activity on methotrexate
BOSTON – It’s time to test whether methotrexate is really up to snuff as a first-line therapy for psoriatic arthritis, Canadian investigators say.
They base that recommendation on findings from a retrospective study showing that fewer than 18% of patients with psoriatic arthritis treated with methotrexate achieved minimal disease activity (MDA) after 6 months.
They also found evidence to suggest that physicians may overestimate the benefits of methotrexate for psoriatic arthritis.
“Physician-dependent measures reveal good response to methotrexate at 6 months, but patient-reported measures are less responsive, possibly due to side effects, back pain, disease, [and/or] disability,” said Dr. Barry J. Sheane of the division of rheumatology in the department of medicine at the University of Toronto.
The data on the effects of methotrexate in psoriatic arthritis are considerably less impressive than are those seen with tumor necrosis factor–alpha inhibitors (TNFi), Dr. Sheane said. For example, at 24 weeks, 39% of patients treated with adalimumab (Humira) and 52% treated with infliximab (Remicade) had achieved MDA, he noted.
“We suggest that a randomized controlled trial of methotrexate compared to a TNF inhibitor is warranted,” Dr. Sheane said at the annual meeting of the American College of Rheumatology.
He and his colleagues reviewed records on 204 consecutive patients treated for psoriatic arthritis with methotrexate from January 2004 through April 2014. The patients, who had a mean duration of psoriatic arthritis of 6.2 years, were all naive to biological antirheumatic drugs and were initiating methotrexate therapy.
Of the 204 patients, 167 had data sufficient for a 6-month analysis.
The investigators defined MDA after 6 months on methotrexate, the primary endpoint, as the presence of at least five out of the following seven domains: 0-1 tender joints, 0-1 swollen joints, Psoriasis Area Severity Index (PASI) 1 or less or body surface area involved 3% or less, 0-1 tender entheseal points, Health Assessment Questionnaire (HAQ) score of 0.5 or less, patient global disease activity Visual Analog Scale score of 20 or lower, and patient pain Visual Analog Scale score of 15 or lower.
They found that 17.4% of patients (29 of 167) met the physician-rated criteria for MDA at 6 months, even though 82.6% of the patients (138) had a PASI score of 1 or lower, and 58.1% (97) had a swollen joint count of 1 or fewer.
When the patients were asked for their assessment of global disease activity, 13.2% (22) rated disease activity with a prespecified score of 20 or lower. Only 12% of patients (20) reported an HAQ score of 0.5 or less.
The mean methotrexate dose was similar between patients who achieved MDA (17.8 mg/week) and those who did not (17.3 mg/week). The median dose was 17.5 mg/week in each group.
After controlling for sex, baseline sacroiliitis, and duration of psoriasis and psoriatic arthritis at the start of methotrexate in a multivariate model, the authors found that only dactylitis, inflammatory back pain, and mechanical back pain were significantly associated with a lower probability of patients reaching MDA. The variables that did not prove to be associated with achieving MDA after 6 months on methotrexate included erythrocyte sedimentation rate, presence of nail disease, number of clinically damaged joints, and body mass index.
The study was internally funded. Dr. Sheane reported having no relevant disclosures.
BOSTON – It’s time to test whether methotrexate is really up to snuff as a first-line therapy for psoriatic arthritis, Canadian investigators say.
They base that recommendation on findings from a retrospective study showing that fewer than 18% of patients with psoriatic arthritis treated with methotrexate achieved minimal disease activity (MDA) after 6 months.
They also found evidence to suggest that physicians may overestimate the benefits of methotrexate for psoriatic arthritis.
“Physician-dependent measures reveal good response to methotrexate at 6 months, but patient-reported measures are less responsive, possibly due to side effects, back pain, disease, [and/or] disability,” said Dr. Barry J. Sheane of the division of rheumatology in the department of medicine at the University of Toronto.
The data on the effects of methotrexate in psoriatic arthritis are considerably less impressive than are those seen with tumor necrosis factor–alpha inhibitors (TNFi), Dr. Sheane said. For example, at 24 weeks, 39% of patients treated with adalimumab (Humira) and 52% treated with infliximab (Remicade) had achieved MDA, he noted.
“We suggest that a randomized controlled trial of methotrexate compared to a TNF inhibitor is warranted,” Dr. Sheane said at the annual meeting of the American College of Rheumatology.
He and his colleagues reviewed records on 204 consecutive patients treated for psoriatic arthritis with methotrexate from January 2004 through April 2014. The patients, who had a mean duration of psoriatic arthritis of 6.2 years, were all naive to biological antirheumatic drugs and were initiating methotrexate therapy.
Of the 204 patients, 167 had data sufficient for a 6-month analysis.
The investigators defined MDA after 6 months on methotrexate, the primary endpoint, as the presence of at least five out of the following seven domains: 0-1 tender joints, 0-1 swollen joints, Psoriasis Area Severity Index (PASI) 1 or less or body surface area involved 3% or less, 0-1 tender entheseal points, Health Assessment Questionnaire (HAQ) score of 0.5 or less, patient global disease activity Visual Analog Scale score of 20 or lower, and patient pain Visual Analog Scale score of 15 or lower.
They found that 17.4% of patients (29 of 167) met the physician-rated criteria for MDA at 6 months, even though 82.6% of the patients (138) had a PASI score of 1 or lower, and 58.1% (97) had a swollen joint count of 1 or fewer.
When the patients were asked for their assessment of global disease activity, 13.2% (22) rated disease activity with a prespecified score of 20 or lower. Only 12% of patients (20) reported an HAQ score of 0.5 or less.
The mean methotrexate dose was similar between patients who achieved MDA (17.8 mg/week) and those who did not (17.3 mg/week). The median dose was 17.5 mg/week in each group.
After controlling for sex, baseline sacroiliitis, and duration of psoriasis and psoriatic arthritis at the start of methotrexate in a multivariate model, the authors found that only dactylitis, inflammatory back pain, and mechanical back pain were significantly associated with a lower probability of patients reaching MDA. The variables that did not prove to be associated with achieving MDA after 6 months on methotrexate included erythrocyte sedimentation rate, presence of nail disease, number of clinically damaged joints, and body mass index.
The study was internally funded. Dr. Sheane reported having no relevant disclosures.
BOSTON – It’s time to test whether methotrexate is really up to snuff as a first-line therapy for psoriatic arthritis, Canadian investigators say.
They base that recommendation on findings from a retrospective study showing that fewer than 18% of patients with psoriatic arthritis treated with methotrexate achieved minimal disease activity (MDA) after 6 months.
They also found evidence to suggest that physicians may overestimate the benefits of methotrexate for psoriatic arthritis.
“Physician-dependent measures reveal good response to methotrexate at 6 months, but patient-reported measures are less responsive, possibly due to side effects, back pain, disease, [and/or] disability,” said Dr. Barry J. Sheane of the division of rheumatology in the department of medicine at the University of Toronto.
The data on the effects of methotrexate in psoriatic arthritis are considerably less impressive than are those seen with tumor necrosis factor–alpha inhibitors (TNFi), Dr. Sheane said. For example, at 24 weeks, 39% of patients treated with adalimumab (Humira) and 52% treated with infliximab (Remicade) had achieved MDA, he noted.
“We suggest that a randomized controlled trial of methotrexate compared to a TNF inhibitor is warranted,” Dr. Sheane said at the annual meeting of the American College of Rheumatology.
He and his colleagues reviewed records on 204 consecutive patients treated for psoriatic arthritis with methotrexate from January 2004 through April 2014. The patients, who had a mean duration of psoriatic arthritis of 6.2 years, were all naive to biological antirheumatic drugs and were initiating methotrexate therapy.
Of the 204 patients, 167 had data sufficient for a 6-month analysis.
The investigators defined MDA after 6 months on methotrexate, the primary endpoint, as the presence of at least five out of the following seven domains: 0-1 tender joints, 0-1 swollen joints, Psoriasis Area Severity Index (PASI) 1 or less or body surface area involved 3% or less, 0-1 tender entheseal points, Health Assessment Questionnaire (HAQ) score of 0.5 or less, patient global disease activity Visual Analog Scale score of 20 or lower, and patient pain Visual Analog Scale score of 15 or lower.
They found that 17.4% of patients (29 of 167) met the physician-rated criteria for MDA at 6 months, even though 82.6% of the patients (138) had a PASI score of 1 or lower, and 58.1% (97) had a swollen joint count of 1 or fewer.
When the patients were asked for their assessment of global disease activity, 13.2% (22) rated disease activity with a prespecified score of 20 or lower. Only 12% of patients (20) reported an HAQ score of 0.5 or less.
The mean methotrexate dose was similar between patients who achieved MDA (17.8 mg/week) and those who did not (17.3 mg/week). The median dose was 17.5 mg/week in each group.
After controlling for sex, baseline sacroiliitis, and duration of psoriasis and psoriatic arthritis at the start of methotrexate in a multivariate model, the authors found that only dactylitis, inflammatory back pain, and mechanical back pain were significantly associated with a lower probability of patients reaching MDA. The variables that did not prove to be associated with achieving MDA after 6 months on methotrexate included erythrocyte sedimentation rate, presence of nail disease, number of clinically damaged joints, and body mass index.
The study was internally funded. Dr. Sheane reported having no relevant disclosures.
AT THE ACR ANNUAL MEETING
Key clinical point: It may be time to rethink methotrexate’s role as first-line therapy for psoriatic arthritis.
Major finding: Only 17.4% of patients with psoriatic arthritis treated with methotrexate achieved minimal disease activity at 6 months.
Data source: Retrospective study of 204 patients, 167 of whom had data sufficient for an efficacy and dosing analysis at 6 months.
Disclosures: The study was internally funded. Dr. Sheane reported having no relevant disclosures.
TNF-alpha blockers effective in sustaining reduced PsA activity
A state of minimal disease activity in patients with psoriatic arthritis is sustainable over several years by using treatments with tumor necrosis factor–alpha blockers, according to findings from a single-center, retrospective cohort study.
The study is the first to report on the predictors of minimal disease activity (MDA) in patients with psoriatic arthritis (PsA) taking TNF-alpha inhibitors in a clinical setting and “is also the first “to report about PsA patients who achieved and continued to be in MDA state after treatment change,” wrote the study investigators, who were led by Dr. Amir Haddad of the University of Toronto.
The investigators reported on 226 patients with PsA who were not in an MDA state when they presented to the University of Toronto and were treated with TNF-alpha inhibitors during 2000-2012. They had excluded 23 patients who had MDA when treatment began and 57 who were on TNF-alpha blockers prior to enrollment (Arthritis Care Res. 2014 Dec. 2 [doi:10.1002/acr.22529]).
The patients were 65% male with an average diagnosis age of 36.0 years and were followed at 6-12 month intervals. The authors defined MDA according to the criteria provided by Coates et al. (Ann. Rheum. Dis. 2010;69:48-53), which required patients to meet at least five of seven criteria: 0-1 tender joints ; 0-1 swollen joints; Psoriasis Activity and Severity Index of 1 or less or body surface area of 3 or less; patient pain visual analogue score (VAS) of 15 or less; patient global disease activity VAS of 20 or less; health assessment questionnaire of 0.5 or less; tender entheseal points of 1 or less.
A total of 145 patients (64%) achieved MDA status after an average of 1.30 months (standard deviation, 1.51), and 88 (61%) achieved sustained MDA (defined as at least 1 year) for a mean of 3.46 years (standard deviation, 2.25). Another 17 patients remained in an MDA state after reducing their anti–TNF-alpha drug dose, including 9 who withdrew anti–TNF-alpha treatment completely, and these patients remained in an MDA state for a mean of 2.11 years. The investigators noted that “no protocol was used for tapering the dose and it was left to patient’s preference,” and that MDA was sustained for longer periods of time in patients who reduced the TNF-alpha inhibitor dose and for shorter periods in patients who withdrew the treatment.
Male sex and a normal erythrocyte sedimentation rate (ESR) were found to be reliable predictors of achieving MDA. The authors cited previous studies on ankylosing spondylitis or axial spondyloarthritis, and noted that low ESR/C-reactive protein is considered a predictor for nonresponse to TNF-alpha blockers, but caution that the results of their own study may “simply [be] from using different outcome measures for response.”
The authors disclosed that all but one of them are members of the University of Toronto’s Psoriatic Arthritis Program, which is partly funded by the Arthritis Society, the Canadian Institutes of Health Research, and the Krembil Foundation. Additionally, Dr. Haddad disclosed that he was supported by unrestricted educational grants from Janssen and UCB.
A state of minimal disease activity in patients with psoriatic arthritis is sustainable over several years by using treatments with tumor necrosis factor–alpha blockers, according to findings from a single-center, retrospective cohort study.
The study is the first to report on the predictors of minimal disease activity (MDA) in patients with psoriatic arthritis (PsA) taking TNF-alpha inhibitors in a clinical setting and “is also the first “to report about PsA patients who achieved and continued to be in MDA state after treatment change,” wrote the study investigators, who were led by Dr. Amir Haddad of the University of Toronto.
The investigators reported on 226 patients with PsA who were not in an MDA state when they presented to the University of Toronto and were treated with TNF-alpha inhibitors during 2000-2012. They had excluded 23 patients who had MDA when treatment began and 57 who were on TNF-alpha blockers prior to enrollment (Arthritis Care Res. 2014 Dec. 2 [doi:10.1002/acr.22529]).
The patients were 65% male with an average diagnosis age of 36.0 years and were followed at 6-12 month intervals. The authors defined MDA according to the criteria provided by Coates et al. (Ann. Rheum. Dis. 2010;69:48-53), which required patients to meet at least five of seven criteria: 0-1 tender joints ; 0-1 swollen joints; Psoriasis Activity and Severity Index of 1 or less or body surface area of 3 or less; patient pain visual analogue score (VAS) of 15 or less; patient global disease activity VAS of 20 or less; health assessment questionnaire of 0.5 or less; tender entheseal points of 1 or less.
A total of 145 patients (64%) achieved MDA status after an average of 1.30 months (standard deviation, 1.51), and 88 (61%) achieved sustained MDA (defined as at least 1 year) for a mean of 3.46 years (standard deviation, 2.25). Another 17 patients remained in an MDA state after reducing their anti–TNF-alpha drug dose, including 9 who withdrew anti–TNF-alpha treatment completely, and these patients remained in an MDA state for a mean of 2.11 years. The investigators noted that “no protocol was used for tapering the dose and it was left to patient’s preference,” and that MDA was sustained for longer periods of time in patients who reduced the TNF-alpha inhibitor dose and for shorter periods in patients who withdrew the treatment.
Male sex and a normal erythrocyte sedimentation rate (ESR) were found to be reliable predictors of achieving MDA. The authors cited previous studies on ankylosing spondylitis or axial spondyloarthritis, and noted that low ESR/C-reactive protein is considered a predictor for nonresponse to TNF-alpha blockers, but caution that the results of their own study may “simply [be] from using different outcome measures for response.”
The authors disclosed that all but one of them are members of the University of Toronto’s Psoriatic Arthritis Program, which is partly funded by the Arthritis Society, the Canadian Institutes of Health Research, and the Krembil Foundation. Additionally, Dr. Haddad disclosed that he was supported by unrestricted educational grants from Janssen and UCB.
A state of minimal disease activity in patients with psoriatic arthritis is sustainable over several years by using treatments with tumor necrosis factor–alpha blockers, according to findings from a single-center, retrospective cohort study.
The study is the first to report on the predictors of minimal disease activity (MDA) in patients with psoriatic arthritis (PsA) taking TNF-alpha inhibitors in a clinical setting and “is also the first “to report about PsA patients who achieved and continued to be in MDA state after treatment change,” wrote the study investigators, who were led by Dr. Amir Haddad of the University of Toronto.
The investigators reported on 226 patients with PsA who were not in an MDA state when they presented to the University of Toronto and were treated with TNF-alpha inhibitors during 2000-2012. They had excluded 23 patients who had MDA when treatment began and 57 who were on TNF-alpha blockers prior to enrollment (Arthritis Care Res. 2014 Dec. 2 [doi:10.1002/acr.22529]).
The patients were 65% male with an average diagnosis age of 36.0 years and were followed at 6-12 month intervals. The authors defined MDA according to the criteria provided by Coates et al. (Ann. Rheum. Dis. 2010;69:48-53), which required patients to meet at least five of seven criteria: 0-1 tender joints ; 0-1 swollen joints; Psoriasis Activity and Severity Index of 1 or less or body surface area of 3 or less; patient pain visual analogue score (VAS) of 15 or less; patient global disease activity VAS of 20 or less; health assessment questionnaire of 0.5 or less; tender entheseal points of 1 or less.
A total of 145 patients (64%) achieved MDA status after an average of 1.30 months (standard deviation, 1.51), and 88 (61%) achieved sustained MDA (defined as at least 1 year) for a mean of 3.46 years (standard deviation, 2.25). Another 17 patients remained in an MDA state after reducing their anti–TNF-alpha drug dose, including 9 who withdrew anti–TNF-alpha treatment completely, and these patients remained in an MDA state for a mean of 2.11 years. The investigators noted that “no protocol was used for tapering the dose and it was left to patient’s preference,” and that MDA was sustained for longer periods of time in patients who reduced the TNF-alpha inhibitor dose and for shorter periods in patients who withdrew the treatment.
Male sex and a normal erythrocyte sedimentation rate (ESR) were found to be reliable predictors of achieving MDA. The authors cited previous studies on ankylosing spondylitis or axial spondyloarthritis, and noted that low ESR/C-reactive protein is considered a predictor for nonresponse to TNF-alpha blockers, but caution that the results of their own study may “simply [be] from using different outcome measures for response.”
The authors disclosed that all but one of them are members of the University of Toronto’s Psoriatic Arthritis Program, which is partly funded by the Arthritis Society, the Canadian Institutes of Health Research, and the Krembil Foundation. Additionally, Dr. Haddad disclosed that he was supported by unrestricted educational grants from Janssen and UCB.
FROM ARTHRITIS CARE & RESEARCH
Key clinical point: A majority of PsA patients seen in a clinical setting can achieve minimal disease activity on TNF-alpha inhibitors.
Major finding: A total of 64% of PsA patients taking TNF-alpha inhibitors achieved MDA within a mean duration of 1.30 years.
Data source: A retrospective, observational cohort study of 226 patients with PsA.
Disclosures: The authors reported several potential conflicts.
Psoriasis is independently associated with advanced liver fibrosis
AMSTERDAM – Older psoriasis patients have an increased risk of advanced hepatic fibrosis even if they have mild skin disease that has never required systemic therapy.
This finding from the Rotterdam Study raises a red flag: “It could be suggested to screen for liver fibrosis ... before and during potentially hepatotoxic therapies in psoriasis patients, especially in those with metabolic syndrome,” Dr. Ella van der Voort said in presenting the study findings at the annual congress of the European Academy of Dermatology and Venereology.
The Rotterdam Study is an ongoing large, prospective, population-based cohort study which began in 1998. Routine liver screening by FibroScan was incorporated into the study protocol in 2011. The proprietary FibroScan transient elastography device is noninvasive and yields results in 5 minutes, explained Dr. van der Voort, a dermatologist at Erasmus University, Rotterdam, the Netherlands.
In her analysis, Dr. van der Voort reported on 1,535 Rotterdam Study participants with FibroScan results, including 75 with psoriasis. The mean age was 71 years, both in the psoriasis patients and the 1,461 nonpsoriatic controls. The psoriasis patients had mild skin disease, with a mean Psoriasis Area and Severity Index score of 2.0. None of the psoriasis patients had ever received methotrexate or any other systemic therapy for their psoriasis.
The prevalence of advanced liver fibrosis was 3.6% in controls and 8.1% in the psoriasis patients. In a multivariate analysis adjusted for alcohol consumption, alanine aminotransferase levels, steatosis, metabolic syndrome, age, and gender, psoriasis was independently associated with a 2.6-fold increased risk of advanced liver fibrosis.
Among the subgroup of subjects with nonalcoholic fatty liver disease (NAFLD), psoriasis was associated with an even greater likelihood of advanced hepatic fibrosis. This liver finding was present in 15% of 20 patients with mild psoriasis and NAFLD, compared with 4% of 375 nonpsoriatic controls with NAFLD, for an adjusted 4.1-fold increased risk among the group with psoriasis.
Psoriasis patients with advanced liver fibrosis had a relatively high prevalence of the metabolic syndrome, so hepatic screening efforts could be focused on this subgroup, in Dr. van der Voort’s view.
In contrast, serum ALTs were generally within normal range in affected patients. “Using elevated ALTs to trigger screening for liver fibrosis in psoriasis patients is not good advice,” she cautioned.
Citing the advanced age of the Rotterdam Study cohort, Dr. van der Voort noted that her study findings may not be applicable to younger patients with mild psoriasis.
Dr. van der Voort presented another analysis from the Rotterdam Study at the 2013 EADV Congress in Istanbul. In that analysis, she reported that psoriasis was independently associated with a 70% increased risk of NAFLD. Since patients with NAFLD are at increased risk for progression to fibrosis and cirrhosis, the new finding of a 2.6-fold increased risk for advanced liver fibrosis in psoriasis patients doesn’t come as a total surprise. The biggest concern, of course, is that advanced liver fibrosis is, in turn, associated with an increased risk of hepatocellular carcinoma.
The Rotterdam Study is funded by Dutch governmental grants and foundations. Dr. van der Voort reported having no financial conflicts.
AMSTERDAM – Older psoriasis patients have an increased risk of advanced hepatic fibrosis even if they have mild skin disease that has never required systemic therapy.
This finding from the Rotterdam Study raises a red flag: “It could be suggested to screen for liver fibrosis ... before and during potentially hepatotoxic therapies in psoriasis patients, especially in those with metabolic syndrome,” Dr. Ella van der Voort said in presenting the study findings at the annual congress of the European Academy of Dermatology and Venereology.
The Rotterdam Study is an ongoing large, prospective, population-based cohort study which began in 1998. Routine liver screening by FibroScan was incorporated into the study protocol in 2011. The proprietary FibroScan transient elastography device is noninvasive and yields results in 5 minutes, explained Dr. van der Voort, a dermatologist at Erasmus University, Rotterdam, the Netherlands.
In her analysis, Dr. van der Voort reported on 1,535 Rotterdam Study participants with FibroScan results, including 75 with psoriasis. The mean age was 71 years, both in the psoriasis patients and the 1,461 nonpsoriatic controls. The psoriasis patients had mild skin disease, with a mean Psoriasis Area and Severity Index score of 2.0. None of the psoriasis patients had ever received methotrexate or any other systemic therapy for their psoriasis.
The prevalence of advanced liver fibrosis was 3.6% in controls and 8.1% in the psoriasis patients. In a multivariate analysis adjusted for alcohol consumption, alanine aminotransferase levels, steatosis, metabolic syndrome, age, and gender, psoriasis was independently associated with a 2.6-fold increased risk of advanced liver fibrosis.
Among the subgroup of subjects with nonalcoholic fatty liver disease (NAFLD), psoriasis was associated with an even greater likelihood of advanced hepatic fibrosis. This liver finding was present in 15% of 20 patients with mild psoriasis and NAFLD, compared with 4% of 375 nonpsoriatic controls with NAFLD, for an adjusted 4.1-fold increased risk among the group with psoriasis.
Psoriasis patients with advanced liver fibrosis had a relatively high prevalence of the metabolic syndrome, so hepatic screening efforts could be focused on this subgroup, in Dr. van der Voort’s view.
In contrast, serum ALTs were generally within normal range in affected patients. “Using elevated ALTs to trigger screening for liver fibrosis in psoriasis patients is not good advice,” she cautioned.
Citing the advanced age of the Rotterdam Study cohort, Dr. van der Voort noted that her study findings may not be applicable to younger patients with mild psoriasis.
Dr. van der Voort presented another analysis from the Rotterdam Study at the 2013 EADV Congress in Istanbul. In that analysis, she reported that psoriasis was independently associated with a 70% increased risk of NAFLD. Since patients with NAFLD are at increased risk for progression to fibrosis and cirrhosis, the new finding of a 2.6-fold increased risk for advanced liver fibrosis in psoriasis patients doesn’t come as a total surprise. The biggest concern, of course, is that advanced liver fibrosis is, in turn, associated with an increased risk of hepatocellular carcinoma.
The Rotterdam Study is funded by Dutch governmental grants and foundations. Dr. van der Voort reported having no financial conflicts.
AMSTERDAM – Older psoriasis patients have an increased risk of advanced hepatic fibrosis even if they have mild skin disease that has never required systemic therapy.
This finding from the Rotterdam Study raises a red flag: “It could be suggested to screen for liver fibrosis ... before and during potentially hepatotoxic therapies in psoriasis patients, especially in those with metabolic syndrome,” Dr. Ella van der Voort said in presenting the study findings at the annual congress of the European Academy of Dermatology and Venereology.
The Rotterdam Study is an ongoing large, prospective, population-based cohort study which began in 1998. Routine liver screening by FibroScan was incorporated into the study protocol in 2011. The proprietary FibroScan transient elastography device is noninvasive and yields results in 5 minutes, explained Dr. van der Voort, a dermatologist at Erasmus University, Rotterdam, the Netherlands.
In her analysis, Dr. van der Voort reported on 1,535 Rotterdam Study participants with FibroScan results, including 75 with psoriasis. The mean age was 71 years, both in the psoriasis patients and the 1,461 nonpsoriatic controls. The psoriasis patients had mild skin disease, with a mean Psoriasis Area and Severity Index score of 2.0. None of the psoriasis patients had ever received methotrexate or any other systemic therapy for their psoriasis.
The prevalence of advanced liver fibrosis was 3.6% in controls and 8.1% in the psoriasis patients. In a multivariate analysis adjusted for alcohol consumption, alanine aminotransferase levels, steatosis, metabolic syndrome, age, and gender, psoriasis was independently associated with a 2.6-fold increased risk of advanced liver fibrosis.
Among the subgroup of subjects with nonalcoholic fatty liver disease (NAFLD), psoriasis was associated with an even greater likelihood of advanced hepatic fibrosis. This liver finding was present in 15% of 20 patients with mild psoriasis and NAFLD, compared with 4% of 375 nonpsoriatic controls with NAFLD, for an adjusted 4.1-fold increased risk among the group with psoriasis.
Psoriasis patients with advanced liver fibrosis had a relatively high prevalence of the metabolic syndrome, so hepatic screening efforts could be focused on this subgroup, in Dr. van der Voort’s view.
In contrast, serum ALTs were generally within normal range in affected patients. “Using elevated ALTs to trigger screening for liver fibrosis in psoriasis patients is not good advice,” she cautioned.
Citing the advanced age of the Rotterdam Study cohort, Dr. van der Voort noted that her study findings may not be applicable to younger patients with mild psoriasis.
Dr. van der Voort presented another analysis from the Rotterdam Study at the 2013 EADV Congress in Istanbul. In that analysis, she reported that psoriasis was independently associated with a 70% increased risk of NAFLD. Since patients with NAFLD are at increased risk for progression to fibrosis and cirrhosis, the new finding of a 2.6-fold increased risk for advanced liver fibrosis in psoriasis patients doesn’t come as a total surprise. The biggest concern, of course, is that advanced liver fibrosis is, in turn, associated with an increased risk of hepatocellular carcinoma.
The Rotterdam Study is funded by Dutch governmental grants and foundations. Dr. van der Voort reported having no financial conflicts.
AT THE EADV CONGRESS
Key clinical point: Noninvasive screening for advanced hepatic fibrosis may be in order for older patients with mild psoriasis never treated systemically.
Major finding: In a multivariate analysis adjusted for alcohol consumption, alanine aminotransferase levels, steatosis, metabolic syndrome, age, and gender, psoriasis was independently associated with a 2.6-fold increased risk of advanced liver fibrosis.
Data source: This analysis involved noninvasive screening for hepatic fibrosis in 1,535 elderly participants in the Rotterdam Study, an ongoing prospective population-based cohort study.
Disclosures: The Rotterdam Study is funded by Dutch governmental research grants and foundations. The presenter reported having no financial conflicts.
Psoriasis: Brodalumab maintains efficacy through 144 weeks
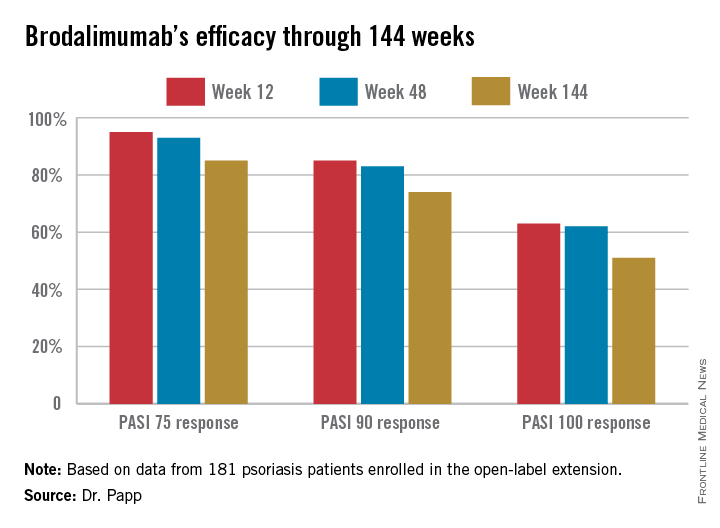
AMSTERDAM – The majority of psoriasis patients placed on the investigational biologic agent brodalumab maintained a PASI 100 response throughout 144 weeks in a long-term, open-label extension of a phase II study.
“The benefit/risk ratio of brodalumab remains very favorable, and warrants continued development of this anti–interleukin-17 receptor A human monoclonal antibody as a potential treatment for psoriasis,” Dr. Kim Papp declared in presenting the study results at the annual congress of the European Academy of Dermatology and Venereology.
Large phase III clinical trials of brodalumab for the treatment of moderate to severe plaque psoriasis are ongoing.
The parent phase II study was 16 weeks long, double blinded, and placebo controlled. At the study’s end, 181 participants enrolled in the open-label extension, in which they received subcutaneous brodalumab at 210 mg every 2 weeks. The study protocol was amended after about a year, with the dose reduced to 140 mg every 2 weeks in the 119 patients weighing 100 kg or less. Six of those patients subsequently had an inadequate response to the lower dose, and were returned to the higher-dose regimen, explained Dr. Papp of Probity Medical Research in Waterloo, Ont.
Roughly 95% of patients became PASI 75 responders within the first few weeks. There was very little drop-off over time. The PASI 75 response rate was 95% at week 12, 93% at week 48, and 85% at week 144.
At week 144, roughly two-thirds of subjects were deemed clear or almost clear according to Physician Global Assessment.
No new safety signals emerged during this long-term study. The most common adverse events were minor upper respiratory infections. Two percent of patients developed a grade 2 absolute neutrophil count of less than 1,500 per 109/L; however, these were transitory events that resolved without a change in treatment.
In response to an audience question, Dr. Papp said antidrug antibodies did develop in some patients during the course of 144 weeks of treatment, but he had no information as to the clinical effect, if any.
“This is a fairly small study population. I think it makes sense to wait for the phase III results to see if the antibodies affect safety and/or efficacy,” he added.
Dr. Papp reported receiving research support from Amgen, which is codeveloping brodalumab with AstraZeneca/MedImmune.
AMSTERDAM – The majority of psoriasis patients placed on the investigational biologic agent brodalumab maintained a PASI 100 response throughout 144 weeks in a long-term, open-label extension of a phase II study.
“The benefit/risk ratio of brodalumab remains very favorable, and warrants continued development of this anti–interleukin-17 receptor A human monoclonal antibody as a potential treatment for psoriasis,” Dr. Kim Papp declared in presenting the study results at the annual congress of the European Academy of Dermatology and Venereology.
Large phase III clinical trials of brodalumab for the treatment of moderate to severe plaque psoriasis are ongoing.
The parent phase II study was 16 weeks long, double blinded, and placebo controlled. At the study’s end, 181 participants enrolled in the open-label extension, in which they received subcutaneous brodalumab at 210 mg every 2 weeks. The study protocol was amended after about a year, with the dose reduced to 140 mg every 2 weeks in the 119 patients weighing 100 kg or less. Six of those patients subsequently had an inadequate response to the lower dose, and were returned to the higher-dose regimen, explained Dr. Papp of Probity Medical Research in Waterloo, Ont.
Roughly 95% of patients became PASI 75 responders within the first few weeks. There was very little drop-off over time. The PASI 75 response rate was 95% at week 12, 93% at week 48, and 85% at week 144.
At week 144, roughly two-thirds of subjects were deemed clear or almost clear according to Physician Global Assessment.
No new safety signals emerged during this long-term study. The most common adverse events were minor upper respiratory infections. Two percent of patients developed a grade 2 absolute neutrophil count of less than 1,500 per 109/L; however, these were transitory events that resolved without a change in treatment.
In response to an audience question, Dr. Papp said antidrug antibodies did develop in some patients during the course of 144 weeks of treatment, but he had no information as to the clinical effect, if any.
“This is a fairly small study population. I think it makes sense to wait for the phase III results to see if the antibodies affect safety and/or efficacy,” he added.
Dr. Papp reported receiving research support from Amgen, which is codeveloping brodalumab with AstraZeneca/MedImmune.
AMSTERDAM – The majority of psoriasis patients placed on the investigational biologic agent brodalumab maintained a PASI 100 response throughout 144 weeks in a long-term, open-label extension of a phase II study.
“The benefit/risk ratio of brodalumab remains very favorable, and warrants continued development of this anti–interleukin-17 receptor A human monoclonal antibody as a potential treatment for psoriasis,” Dr. Kim Papp declared in presenting the study results at the annual congress of the European Academy of Dermatology and Venereology.
Large phase III clinical trials of brodalumab for the treatment of moderate to severe plaque psoriasis are ongoing.
The parent phase II study was 16 weeks long, double blinded, and placebo controlled. At the study’s end, 181 participants enrolled in the open-label extension, in which they received subcutaneous brodalumab at 210 mg every 2 weeks. The study protocol was amended after about a year, with the dose reduced to 140 mg every 2 weeks in the 119 patients weighing 100 kg or less. Six of those patients subsequently had an inadequate response to the lower dose, and were returned to the higher-dose regimen, explained Dr. Papp of Probity Medical Research in Waterloo, Ont.
Roughly 95% of patients became PASI 75 responders within the first few weeks. There was very little drop-off over time. The PASI 75 response rate was 95% at week 12, 93% at week 48, and 85% at week 144.
At week 144, roughly two-thirds of subjects were deemed clear or almost clear according to Physician Global Assessment.
No new safety signals emerged during this long-term study. The most common adverse events were minor upper respiratory infections. Two percent of patients developed a grade 2 absolute neutrophil count of less than 1,500 per 109/L; however, these were transitory events that resolved without a change in treatment.
In response to an audience question, Dr. Papp said antidrug antibodies did develop in some patients during the course of 144 weeks of treatment, but he had no information as to the clinical effect, if any.
“This is a fairly small study population. I think it makes sense to wait for the phase III results to see if the antibodies affect safety and/or efficacy,” he added.
Dr. Papp reported receiving research support from Amgen, which is codeveloping brodalumab with AstraZeneca/MedImmune.
AT THE EADV CONGRESS
Key clinical point: The investigational interleukin-17 receptor A inhibitor brodalumab maintained strong clinical efficacy throughout 144 weeks of psoriasis treatment.
Major finding: The PASI 90 response rate to brodalumab was 85% at week 12, 83% at week 48, and 74% at week 144.
Data source: A 181-patient, prospective, open-label extension of a phase II study.
Disclosures: Dr. Papp reported receiving financial support from Amgen, which is codeveloping brodalumab with AstraZeneca/MedImmune.
The Top 100
In the October 2014 issue of the Journal of Clinical and Aesthetic Dermatology (2014;7:10-19), Wu et al published the top 100 most-cited psoriasis articles in clinical dermatologic journals from 1970 to 2012. Given the explosion of literature in this area, I was very excited to rush to find the list online.
The authors conducted a citation analysis of major clinical dermatologic journals from 1970 to 2012 limited to the subject of psoriasis. They used the search term psoriasis in the Science Citation Index from 1970 to 2012 and included articles that have received 100 or more citations. The top 100 articles were further stratified by country, institution, and study type.
The authors found that half of the top 100 cited articles were from the United States; 81 of them were original articles. The majority of the top 100 articles were from dermatology programs in the United States, but institutions in the United Kingdom and Germany also made notable contributions.
There were 2 periods of particular note in their analysis. The high numbers of citations from 1985 to 1989 correlated with the elucidation of the immune-mediated pathogenesis of psoriasis at that time. The high number of top citations from 2000 to 2004 correlated with the development of biologic agents in psoriasis therapy.
What’s the issue?
It is interesting to see which studies have been most influential in this highly active area of dermatology. What articles have most influenced your approach to psoriasis?
In the October 2014 issue of the Journal of Clinical and Aesthetic Dermatology (2014;7:10-19), Wu et al published the top 100 most-cited psoriasis articles in clinical dermatologic journals from 1970 to 2012. Given the explosion of literature in this area, I was very excited to rush to find the list online.
The authors conducted a citation analysis of major clinical dermatologic journals from 1970 to 2012 limited to the subject of psoriasis. They used the search term psoriasis in the Science Citation Index from 1970 to 2012 and included articles that have received 100 or more citations. The top 100 articles were further stratified by country, institution, and study type.
The authors found that half of the top 100 cited articles were from the United States; 81 of them were original articles. The majority of the top 100 articles were from dermatology programs in the United States, but institutions in the United Kingdom and Germany also made notable contributions.
There were 2 periods of particular note in their analysis. The high numbers of citations from 1985 to 1989 correlated with the elucidation of the immune-mediated pathogenesis of psoriasis at that time. The high number of top citations from 2000 to 2004 correlated with the development of biologic agents in psoriasis therapy.
What’s the issue?
It is interesting to see which studies have been most influential in this highly active area of dermatology. What articles have most influenced your approach to psoriasis?
In the October 2014 issue of the Journal of Clinical and Aesthetic Dermatology (2014;7:10-19), Wu et al published the top 100 most-cited psoriasis articles in clinical dermatologic journals from 1970 to 2012. Given the explosion of literature in this area, I was very excited to rush to find the list online.
The authors conducted a citation analysis of major clinical dermatologic journals from 1970 to 2012 limited to the subject of psoriasis. They used the search term psoriasis in the Science Citation Index from 1970 to 2012 and included articles that have received 100 or more citations. The top 100 articles were further stratified by country, institution, and study type.
The authors found that half of the top 100 cited articles were from the United States; 81 of them were original articles. The majority of the top 100 articles were from dermatology programs in the United States, but institutions in the United Kingdom and Germany also made notable contributions.
There were 2 periods of particular note in their analysis. The high numbers of citations from 1985 to 1989 correlated with the elucidation of the immune-mediated pathogenesis of psoriasis at that time. The high number of top citations from 2000 to 2004 correlated with the development of biologic agents in psoriasis therapy.
What’s the issue?
It is interesting to see which studies have been most influential in this highly active area of dermatology. What articles have most influenced your approach to psoriasis?
Certolizumab achieves sustained skin improvement in psoriatic arthritis
AMSTERDAM – Certolizumab pegol maintained significant improvement in dermatologic outcomes in psoriatic arthritis patients through 96 weeks of treatment in the phase III RAPID-PsA trial.
Moreover, the safety profile of this tumor necrosis factor (TNF) inhibitor was in line with findings from shorter-term studies, including the week 24 report from RAPID-PsA. Treatment-emergent adverse events were similar in type and frequency to those in placebo-treated controls, with the exception of an increased rate of minor upper respiratory tract infections. No cases of tuberculosis occurred.
“There were no new safety issues despite the increased exposure time out to 96 weeks,” Dr. Owen Davies reported at the annual congress of the European Academy of Dermatology and Venereology.
RAPID-PsA is an ongoing 216-week phase III study. It was double-blind and placebo-controlled through the first 24 weeks. The study started out with 409 psoriatic arthritis patients, half of whom had previously failed to response to one nonbiologic disease-modifying antirheumatic drug (DMARD), while the other half had been nonresponders to two or more. Of the 273 patients placed on certolizumab, 80% completed both 48 and 96 weeks of the study, explained Dr. Davies of UCB Pharma in Slough, England.
He focused on the dermatologic outcomes because the arthritis outcomes have previously been reported and served to support certolizumab’s regulatory approval for the treatment of psoriatic arthritis. The biologic is also approved for treatment of rheumatoid arthritis, ankylosing spondylitis, and Crohn’s disease. However, certolizumab’s durability of effect on the psoriatic skin manifestations of psoriatic arthritis hasn’t previously been addressed.
Briefly, at week 12 – the primary endpoint for the joint-related outcomes – 55% of patients achieved an ACR 20 response, compared with 24% on placebo. Moreover, 35% of certolizumab-treated patients had an ACR 50 response at that point, and 20% had an ACR 70 response. The ACR response rate in certolizumab-treated patients was similar regardless of whether or not they had previously been on another anti-TNF biologic.
Dr. Davies addressed in detail the dermatologic outcomes in the 166 psoriatic arthritis patients with at least 3% psoriasis body surface area involvement at baseline. They had an average 10-year disease duration, 24% body surface area involvement, and a baseline Psoriasis Area Severity Index (PASI) score of 12.0.
Dermatologic responses to certolizumab were comparable regardless of whether patients had been randomized to the biologic at 200 mg subcutaneously every 2 weeks or 400 mg once every 4 weeks. As was true for the joint-related responses to certolizumab, the skin responses were similar both in anti-TNF–naive and anti-TNF–experienced patients, he noted.
The PASI 75 response rate in this group of psoriatic arthritis patients with significant skin involvement was 61% at week 24, 65% at week 48, and 53% at week 96. The improvement was even greater in the 71 patients with more severe skin involvement as defined by a baseline PASI score of 10 or more. Certolizumab-treated patients also showed important improvements on the Physician Global Assessment and Dermatology Life Quality Index.
Certolizumab is a pegylated Fab’ fragment of a humanized TNF inhibitor monoclonal antibody.
“Certolizumab is structurally different from other currently available anti-TNF agents, which are either IgG1 monoclonal antibodies or, in the case of etanercept, a receptor fusion protein. Whether or not these structural differences will translate into clinical differences is a question being addressed in ongoing clinical trials,” Dr. Davies said.
The RAPID-PsA study is sponsored by UCB Pharma, where Dr. Davies is employed.
AMSTERDAM – Certolizumab pegol maintained significant improvement in dermatologic outcomes in psoriatic arthritis patients through 96 weeks of treatment in the phase III RAPID-PsA trial.
Moreover, the safety profile of this tumor necrosis factor (TNF) inhibitor was in line with findings from shorter-term studies, including the week 24 report from RAPID-PsA. Treatment-emergent adverse events were similar in type and frequency to those in placebo-treated controls, with the exception of an increased rate of minor upper respiratory tract infections. No cases of tuberculosis occurred.
“There were no new safety issues despite the increased exposure time out to 96 weeks,” Dr. Owen Davies reported at the annual congress of the European Academy of Dermatology and Venereology.
RAPID-PsA is an ongoing 216-week phase III study. It was double-blind and placebo-controlled through the first 24 weeks. The study started out with 409 psoriatic arthritis patients, half of whom had previously failed to response to one nonbiologic disease-modifying antirheumatic drug (DMARD), while the other half had been nonresponders to two or more. Of the 273 patients placed on certolizumab, 80% completed both 48 and 96 weeks of the study, explained Dr. Davies of UCB Pharma in Slough, England.
He focused on the dermatologic outcomes because the arthritis outcomes have previously been reported and served to support certolizumab’s regulatory approval for the treatment of psoriatic arthritis. The biologic is also approved for treatment of rheumatoid arthritis, ankylosing spondylitis, and Crohn’s disease. However, certolizumab’s durability of effect on the psoriatic skin manifestations of psoriatic arthritis hasn’t previously been addressed.
Briefly, at week 12 – the primary endpoint for the joint-related outcomes – 55% of patients achieved an ACR 20 response, compared with 24% on placebo. Moreover, 35% of certolizumab-treated patients had an ACR 50 response at that point, and 20% had an ACR 70 response. The ACR response rate in certolizumab-treated patients was similar regardless of whether or not they had previously been on another anti-TNF biologic.
Dr. Davies addressed in detail the dermatologic outcomes in the 166 psoriatic arthritis patients with at least 3% psoriasis body surface area involvement at baseline. They had an average 10-year disease duration, 24% body surface area involvement, and a baseline Psoriasis Area Severity Index (PASI) score of 12.0.
Dermatologic responses to certolizumab were comparable regardless of whether patients had been randomized to the biologic at 200 mg subcutaneously every 2 weeks or 400 mg once every 4 weeks. As was true for the joint-related responses to certolizumab, the skin responses were similar both in anti-TNF–naive and anti-TNF–experienced patients, he noted.
The PASI 75 response rate in this group of psoriatic arthritis patients with significant skin involvement was 61% at week 24, 65% at week 48, and 53% at week 96. The improvement was even greater in the 71 patients with more severe skin involvement as defined by a baseline PASI score of 10 or more. Certolizumab-treated patients also showed important improvements on the Physician Global Assessment and Dermatology Life Quality Index.
Certolizumab is a pegylated Fab’ fragment of a humanized TNF inhibitor monoclonal antibody.
“Certolizumab is structurally different from other currently available anti-TNF agents, which are either IgG1 monoclonal antibodies or, in the case of etanercept, a receptor fusion protein. Whether or not these structural differences will translate into clinical differences is a question being addressed in ongoing clinical trials,” Dr. Davies said.
The RAPID-PsA study is sponsored by UCB Pharma, where Dr. Davies is employed.
AMSTERDAM – Certolizumab pegol maintained significant improvement in dermatologic outcomes in psoriatic arthritis patients through 96 weeks of treatment in the phase III RAPID-PsA trial.
Moreover, the safety profile of this tumor necrosis factor (TNF) inhibitor was in line with findings from shorter-term studies, including the week 24 report from RAPID-PsA. Treatment-emergent adverse events were similar in type and frequency to those in placebo-treated controls, with the exception of an increased rate of minor upper respiratory tract infections. No cases of tuberculosis occurred.
“There were no new safety issues despite the increased exposure time out to 96 weeks,” Dr. Owen Davies reported at the annual congress of the European Academy of Dermatology and Venereology.
RAPID-PsA is an ongoing 216-week phase III study. It was double-blind and placebo-controlled through the first 24 weeks. The study started out with 409 psoriatic arthritis patients, half of whom had previously failed to response to one nonbiologic disease-modifying antirheumatic drug (DMARD), while the other half had been nonresponders to two or more. Of the 273 patients placed on certolizumab, 80% completed both 48 and 96 weeks of the study, explained Dr. Davies of UCB Pharma in Slough, England.
He focused on the dermatologic outcomes because the arthritis outcomes have previously been reported and served to support certolizumab’s regulatory approval for the treatment of psoriatic arthritis. The biologic is also approved for treatment of rheumatoid arthritis, ankylosing spondylitis, and Crohn’s disease. However, certolizumab’s durability of effect on the psoriatic skin manifestations of psoriatic arthritis hasn’t previously been addressed.
Briefly, at week 12 – the primary endpoint for the joint-related outcomes – 55% of patients achieved an ACR 20 response, compared with 24% on placebo. Moreover, 35% of certolizumab-treated patients had an ACR 50 response at that point, and 20% had an ACR 70 response. The ACR response rate in certolizumab-treated patients was similar regardless of whether or not they had previously been on another anti-TNF biologic.
Dr. Davies addressed in detail the dermatologic outcomes in the 166 psoriatic arthritis patients with at least 3% psoriasis body surface area involvement at baseline. They had an average 10-year disease duration, 24% body surface area involvement, and a baseline Psoriasis Area Severity Index (PASI) score of 12.0.
Dermatologic responses to certolizumab were comparable regardless of whether patients had been randomized to the biologic at 200 mg subcutaneously every 2 weeks or 400 mg once every 4 weeks. As was true for the joint-related responses to certolizumab, the skin responses were similar both in anti-TNF–naive and anti-TNF–experienced patients, he noted.
The PASI 75 response rate in this group of psoriatic arthritis patients with significant skin involvement was 61% at week 24, 65% at week 48, and 53% at week 96. The improvement was even greater in the 71 patients with more severe skin involvement as defined by a baseline PASI score of 10 or more. Certolizumab-treated patients also showed important improvements on the Physician Global Assessment and Dermatology Life Quality Index.
Certolizumab is a pegylated Fab’ fragment of a humanized TNF inhibitor monoclonal antibody.
“Certolizumab is structurally different from other currently available anti-TNF agents, which are either IgG1 monoclonal antibodies or, in the case of etanercept, a receptor fusion protein. Whether or not these structural differences will translate into clinical differences is a question being addressed in ongoing clinical trials,” Dr. Davies said.
The RAPID-PsA study is sponsored by UCB Pharma, where Dr. Davies is employed.
AT THE EADV CONGRESS
Key clinical point: Certolizumab pegol maintains sustained improvement in the dermatologic manifestations of psoriatic arthritis through 96 weeks.
Major finding: Sixty-two percent of psoriatic arthritis patients with at least 3% body surface area involvement at a baseline PASI score of 10 or more still had a PASI 75 dermatologic response after 96 weeks on certolizumab.
Data source: The RAPID-PsA study is an ongoing 216-week, prospective, randomized, multicenter trial involving 409 psoriatic arthritis patients, including 166 with significant skin involvement at baseline.
Disclosures: The study is sponsored by UCB Pharma. The presenter is a full-time company employee.
Oral curcumin shown effective in psoriasis
AMSTERDAM – Oral curcumin proved safe and effective as adjunctive therapy in patients on topical corticosteroids for mild to moderate psoriasis vulgaris in a 12-week, randomized, placebo-controlled clinical trial.
This agent helps fill an unmet need in psoriasis, Dr. Emiliano Antiga observed in presenting the study findings at the annual congress of the European Academy of Dermatology and Venereology.
That’s because by far most of the action in the development of new treatments for psoriasis focuses on biologics and other extremely costly agents targeting patients at the moderate to severe end of the disease spectrum. But psoriasis is a chronic condition, and the many patients with milder disease require long-term therapies that are nontoxic and won’t break the bank. Enter curcumin.
“Oral curcumin is effective, safe, and it is cheap,” declared Dr. Antiga, a dermatologist at the University of Florence (Italy).
Moreover, it has a biologically plausible mechanism of benefit in psoriasis, as was demonstrated in his 60-patient randomized trial. Serum levels of the proinflammatory cytokine interleukin-22 were cut in half in the group assigned to 12 weeks of daily oral curcumin while remaining unchanged in the control group.
Curcumin is derived from turmeric, the dried rhizome of a plant, Curcuma longa. Turmeric is a yellowish Indian spice used in curries. But curcumin has long been used therapeutically in traditional Indian and Chinese medicine. Studies have shown that curcumin has antiproliferative, antiangiogenic, and anti-inflammatory effects. In a small study by other investigators, topical turmeric not only successfully cleared psoriasis lesions, it also suppressed phosphorylase kinase activity, which is important to keratinocyte proliferation (Br. J. Dermatol. 2000;143:937-49).
Dr. Antiga presented a study of 60 patients with mild to moderate psoriasis vulgaris as defined by a baseline median Psoriasis Area and Severity Index (PASI) score of 5.5 who were randomly assigned to 12 weeks of treatment with topical corticosteroids plus 3 g per day of oral curcumin or topical steroids plus placebo. The active-treatment capsules contained curcumin embedded in nanoparticle liposomes to enhance bioavailability.
Forty-nine patients completed the study. The primary endpoint was reduction in PASI values over 12 weeks. Both groups showed improvement – after all, the controls were on active treatment with topical steroids – but the change in PASI scores was significantly greater in the curcumin-treated patients.
Adverse events in the curcumin group were limited to one case of diarrhea. There was one case of nausea and one papular eruption in the control group.
Although IL-22 levels at 12 weeks were halved in the curcumin group and unchanged in controls, levels of the inflammatory cytokines IL-10 and -17 and transforming growth factor–beta remained unchanged in both groups over time.
Dr. Antiga reported having no financial conflicts regarding this study.
AMSTERDAM – Oral curcumin proved safe and effective as adjunctive therapy in patients on topical corticosteroids for mild to moderate psoriasis vulgaris in a 12-week, randomized, placebo-controlled clinical trial.
This agent helps fill an unmet need in psoriasis, Dr. Emiliano Antiga observed in presenting the study findings at the annual congress of the European Academy of Dermatology and Venereology.
That’s because by far most of the action in the development of new treatments for psoriasis focuses on biologics and other extremely costly agents targeting patients at the moderate to severe end of the disease spectrum. But psoriasis is a chronic condition, and the many patients with milder disease require long-term therapies that are nontoxic and won’t break the bank. Enter curcumin.
“Oral curcumin is effective, safe, and it is cheap,” declared Dr. Antiga, a dermatologist at the University of Florence (Italy).
Moreover, it has a biologically plausible mechanism of benefit in psoriasis, as was demonstrated in his 60-patient randomized trial. Serum levels of the proinflammatory cytokine interleukin-22 were cut in half in the group assigned to 12 weeks of daily oral curcumin while remaining unchanged in the control group.
Curcumin is derived from turmeric, the dried rhizome of a plant, Curcuma longa. Turmeric is a yellowish Indian spice used in curries. But curcumin has long been used therapeutically in traditional Indian and Chinese medicine. Studies have shown that curcumin has antiproliferative, antiangiogenic, and anti-inflammatory effects. In a small study by other investigators, topical turmeric not only successfully cleared psoriasis lesions, it also suppressed phosphorylase kinase activity, which is important to keratinocyte proliferation (Br. J. Dermatol. 2000;143:937-49).
Dr. Antiga presented a study of 60 patients with mild to moderate psoriasis vulgaris as defined by a baseline median Psoriasis Area and Severity Index (PASI) score of 5.5 who were randomly assigned to 12 weeks of treatment with topical corticosteroids plus 3 g per day of oral curcumin or topical steroids plus placebo. The active-treatment capsules contained curcumin embedded in nanoparticle liposomes to enhance bioavailability.
Forty-nine patients completed the study. The primary endpoint was reduction in PASI values over 12 weeks. Both groups showed improvement – after all, the controls were on active treatment with topical steroids – but the change in PASI scores was significantly greater in the curcumin-treated patients.
Adverse events in the curcumin group were limited to one case of diarrhea. There was one case of nausea and one papular eruption in the control group.
Although IL-22 levels at 12 weeks were halved in the curcumin group and unchanged in controls, levels of the inflammatory cytokines IL-10 and -17 and transforming growth factor–beta remained unchanged in both groups over time.
Dr. Antiga reported having no financial conflicts regarding this study.
AMSTERDAM – Oral curcumin proved safe and effective as adjunctive therapy in patients on topical corticosteroids for mild to moderate psoriasis vulgaris in a 12-week, randomized, placebo-controlled clinical trial.
This agent helps fill an unmet need in psoriasis, Dr. Emiliano Antiga observed in presenting the study findings at the annual congress of the European Academy of Dermatology and Venereology.
That’s because by far most of the action in the development of new treatments for psoriasis focuses on biologics and other extremely costly agents targeting patients at the moderate to severe end of the disease spectrum. But psoriasis is a chronic condition, and the many patients with milder disease require long-term therapies that are nontoxic and won’t break the bank. Enter curcumin.
“Oral curcumin is effective, safe, and it is cheap,” declared Dr. Antiga, a dermatologist at the University of Florence (Italy).
Moreover, it has a biologically plausible mechanism of benefit in psoriasis, as was demonstrated in his 60-patient randomized trial. Serum levels of the proinflammatory cytokine interleukin-22 were cut in half in the group assigned to 12 weeks of daily oral curcumin while remaining unchanged in the control group.
Curcumin is derived from turmeric, the dried rhizome of a plant, Curcuma longa. Turmeric is a yellowish Indian spice used in curries. But curcumin has long been used therapeutically in traditional Indian and Chinese medicine. Studies have shown that curcumin has antiproliferative, antiangiogenic, and anti-inflammatory effects. In a small study by other investigators, topical turmeric not only successfully cleared psoriasis lesions, it also suppressed phosphorylase kinase activity, which is important to keratinocyte proliferation (Br. J. Dermatol. 2000;143:937-49).
Dr. Antiga presented a study of 60 patients with mild to moderate psoriasis vulgaris as defined by a baseline median Psoriasis Area and Severity Index (PASI) score of 5.5 who were randomly assigned to 12 weeks of treatment with topical corticosteroids plus 3 g per day of oral curcumin or topical steroids plus placebo. The active-treatment capsules contained curcumin embedded in nanoparticle liposomes to enhance bioavailability.
Forty-nine patients completed the study. The primary endpoint was reduction in PASI values over 12 weeks. Both groups showed improvement – after all, the controls were on active treatment with topical steroids – but the change in PASI scores was significantly greater in the curcumin-treated patients.
Adverse events in the curcumin group were limited to one case of diarrhea. There was one case of nausea and one papular eruption in the control group.
Although IL-22 levels at 12 weeks were halved in the curcumin group and unchanged in controls, levels of the inflammatory cytokines IL-10 and -17 and transforming growth factor–beta remained unchanged in both groups over time.
Dr. Antiga reported having no financial conflicts regarding this study.
AT THE EADV CONGRESS
Key clinical point: A common ingredient in Indian curry spice mixes is safe and effective as adjuvant therapy for mild-to-moderate psoriasis.
Major finding: Daily oral curcumin capsules plus topical corticosteroids resulted in a PASI 75 improvement rate of 48% compared with a 12% rate in patients who got topical steroids plus placebo.
Data source: A prospective randomized 12-week clinical trial involving 60 patients with mild to moderate psoriasis vulgaris.
Disclosures: The presenter reported no financial conflicts regarding this study, conducted free of commercial support.
Chronic inflammatory disease patients at greater risk of major CV events
Patients with rheumatoid arthritis, psoriatic arthritis, or psoriasis are at an increased risk of major adverse cardiovascular events when compared with the general population, according to findings from a large cohort study.
All three diseases had statistically similar risks for major adverse cardiovascular events (MACE) after adjustment for age, gender, and traditional CV risk factors, Dr. Alexis Ogdie-Beatty of the University of Pennsylvania, Philadelphia, and her colleagues reported (Ann. Rheum. Dis. 2014 Oct. 30 [doi: 10.1136/annrheumdis-2014-205675]).
The investigators noted that most studies of CV risk in psoriatic arthritis (PsA) patients have been cross-sectional and that three previous population-based cohort studies have evaluated CV risk in psoriasis patients, with PsA patients as a subgroup; however, these three psoriasis studies did not include incident MACE and matched internal control patients with adjustments for traditional CV risk factors.
The investigators used data from the Health Improvement Network, a U.K. primary care medical record database, and compared the number of MACE (myocardial infarction, cerebrovascular accident, and CV death) that occurred during a mean 5 years of follow-up in 41,752 patients with rheumatoid arthritis (RA), 8,706 with PsA, 138,424 with psoriasis, and 81,573 matched controls. There was significant interaction between disease-modifying antirheumatic drug (DMARD) use and disease group (P < .001 for MACE and two components, CV death and cerebrovascular accident; and P = .01 for MI).
The risk of MACE was higher in patients with PsA not prescribed a DMARD (hazard ratio, 1.24; 95% confidence interval, 1.03-1.49). This risk was elevated in RA patients both with DMARD prescriptions (HR, 1.58; 95% CI, 1.46-1.70) and without (HR, 1.39; 95% CI, 1.28-1.50). Patients with severe psoriasis who were prescribed a DMARD had an HR of 1.42 (95% CI, 1.17-1.73), whereas psoriasis patients not prescribed a DMARD had an HR of 1.08 (95% CI, 1.02-1.15).
The results highlight a need for improved screening and management of traditional CV risk factors in patients with inflammatory diseases, the researchers said.
Study limitations included not being able to measure disease severity or the use of over-the-counter NSAIDs, as well as having few records on biologic medications and possibly missing DMARD prescriptions.
The researchers were supported by various grants from the Rheumatology Research Foundation, the National Institutes of Health, the Doris Duke Charitable Foundation, and the Icelandic Research Fund. Several authors reported financial relationships with companies that market drugs for chronic inflammatory diseases.
Patients with rheumatoid arthritis, psoriatic arthritis, or psoriasis are at an increased risk of major adverse cardiovascular events when compared with the general population, according to findings from a large cohort study.
All three diseases had statistically similar risks for major adverse cardiovascular events (MACE) after adjustment for age, gender, and traditional CV risk factors, Dr. Alexis Ogdie-Beatty of the University of Pennsylvania, Philadelphia, and her colleagues reported (Ann. Rheum. Dis. 2014 Oct. 30 [doi: 10.1136/annrheumdis-2014-205675]).
The investigators noted that most studies of CV risk in psoriatic arthritis (PsA) patients have been cross-sectional and that three previous population-based cohort studies have evaluated CV risk in psoriasis patients, with PsA patients as a subgroup; however, these three psoriasis studies did not include incident MACE and matched internal control patients with adjustments for traditional CV risk factors.
The investigators used data from the Health Improvement Network, a U.K. primary care medical record database, and compared the number of MACE (myocardial infarction, cerebrovascular accident, and CV death) that occurred during a mean 5 years of follow-up in 41,752 patients with rheumatoid arthritis (RA), 8,706 with PsA, 138,424 with psoriasis, and 81,573 matched controls. There was significant interaction between disease-modifying antirheumatic drug (DMARD) use and disease group (P < .001 for MACE and two components, CV death and cerebrovascular accident; and P = .01 for MI).
The risk of MACE was higher in patients with PsA not prescribed a DMARD (hazard ratio, 1.24; 95% confidence interval, 1.03-1.49). This risk was elevated in RA patients both with DMARD prescriptions (HR, 1.58; 95% CI, 1.46-1.70) and without (HR, 1.39; 95% CI, 1.28-1.50). Patients with severe psoriasis who were prescribed a DMARD had an HR of 1.42 (95% CI, 1.17-1.73), whereas psoriasis patients not prescribed a DMARD had an HR of 1.08 (95% CI, 1.02-1.15).
The results highlight a need for improved screening and management of traditional CV risk factors in patients with inflammatory diseases, the researchers said.
Study limitations included not being able to measure disease severity or the use of over-the-counter NSAIDs, as well as having few records on biologic medications and possibly missing DMARD prescriptions.
The researchers were supported by various grants from the Rheumatology Research Foundation, the National Institutes of Health, the Doris Duke Charitable Foundation, and the Icelandic Research Fund. Several authors reported financial relationships with companies that market drugs for chronic inflammatory diseases.
Patients with rheumatoid arthritis, psoriatic arthritis, or psoriasis are at an increased risk of major adverse cardiovascular events when compared with the general population, according to findings from a large cohort study.
All three diseases had statistically similar risks for major adverse cardiovascular events (MACE) after adjustment for age, gender, and traditional CV risk factors, Dr. Alexis Ogdie-Beatty of the University of Pennsylvania, Philadelphia, and her colleagues reported (Ann. Rheum. Dis. 2014 Oct. 30 [doi: 10.1136/annrheumdis-2014-205675]).
The investigators noted that most studies of CV risk in psoriatic arthritis (PsA) patients have been cross-sectional and that three previous population-based cohort studies have evaluated CV risk in psoriasis patients, with PsA patients as a subgroup; however, these three psoriasis studies did not include incident MACE and matched internal control patients with adjustments for traditional CV risk factors.
The investigators used data from the Health Improvement Network, a U.K. primary care medical record database, and compared the number of MACE (myocardial infarction, cerebrovascular accident, and CV death) that occurred during a mean 5 years of follow-up in 41,752 patients with rheumatoid arthritis (RA), 8,706 with PsA, 138,424 with psoriasis, and 81,573 matched controls. There was significant interaction between disease-modifying antirheumatic drug (DMARD) use and disease group (P < .001 for MACE and two components, CV death and cerebrovascular accident; and P = .01 for MI).
The risk of MACE was higher in patients with PsA not prescribed a DMARD (hazard ratio, 1.24; 95% confidence interval, 1.03-1.49). This risk was elevated in RA patients both with DMARD prescriptions (HR, 1.58; 95% CI, 1.46-1.70) and without (HR, 1.39; 95% CI, 1.28-1.50). Patients with severe psoriasis who were prescribed a DMARD had an HR of 1.42 (95% CI, 1.17-1.73), whereas psoriasis patients not prescribed a DMARD had an HR of 1.08 (95% CI, 1.02-1.15).
The results highlight a need for improved screening and management of traditional CV risk factors in patients with inflammatory diseases, the researchers said.
Study limitations included not being able to measure disease severity or the use of over-the-counter NSAIDs, as well as having few records on biologic medications and possibly missing DMARD prescriptions.
The researchers were supported by various grants from the Rheumatology Research Foundation, the National Institutes of Health, the Doris Duke Charitable Foundation, and the Icelandic Research Fund. Several authors reported financial relationships with companies that market drugs for chronic inflammatory diseases.
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point: Patients with RA, PsA, and psoriasis have similarly elevated risk for major adverse cardiovascular events when compared with the general population.
Major finding: The risk of MACE was higher in patients with PsA not prescribed a DMARD (hazard ratio, 1.24; 95% confidence interval, 1.03-1.49), compared with matched controls.
Data source: A population-based, longitudinal cohort study of 41,752 RA patients, 8,706 PsA patients, 138,424 psoriasis patients, and 81,573 matched controls.
Disclosures: The researchers were supported by various grants from the Rheumatology Research Foundation, the National Institutes of Health, the Doris Duke Charitable Foundation, and the Icelandic Research Fund. Several authors reported financial relationships with companies that market drugs for chronic inflammatory diseases.
Recent Findings About Plaque Psoriasis
More Evidence Needed on Combined Use of Systemic Agents for Plaque Psoriasis
To evaluate the combined use of systemic agents to achieve disease control in therapy-resistant patients with plaque-type psoriasis, Busard et al (JAMA Dermatol. doi:10.1001/jamadermatol.2014.1111) conducted an electronic search of randomized clinical trials. They found that most studies favored combination therapy, but the significance level and quality of evidence was low. Etanercept plus methotrexate was investigated with an adequate sample size and demonstrated superior efficacy compared to etanercept monotherapy. Although it was associated with an increase in adverse events, the overall safety profile was acceptable.
Practice Point: Patients with plaque psoriasis may benefit from combination therapy with systemic agents, but there is insufficient evidence. Etanercept plus methotrexate may be beneficial, with dose reductions to minimize adverse effects.
>>Read more at JAMA Dermatology
Plaque Psoriasis Familial Risk Highest in First-Degree Relatives
Psoriasis is known to be a highly heritable disease. In a cross-sectional study of 640 consecutive, unrelated adult patients with chronic plaque psoriasis, Di Lernia et al (Clin Exp Dermatol. 2014;39:801-805) found a positive familial history of psoriasis in more than half (59.37%) of patients. Of these patients, 27.18% had at least 1 parent with psoriasis, 16.56% had at least 1 second-degree relative with psoriasis, and 5.31% had 1 third-degree relative with psoriasis.
Practice Point: There is a high familial recurrence risk for plaque psoriasis.
>>Read more at Clinical and Experimental Dermatology
Secukinumab Efficacy for Plaque Psoriasis
Two phase 3, double-blind, 52-week trials (ERASURE and FIXTURE) evaluated subcutaneous secukinumab at a dose of 300 or 150 mg (administed once weekly for 5 weeks, then every 4 weeks), placebo, or (in the FIXTURE study only) etanercept at a dose of 50 mg (administered twice weekly for 12 weeks, then once weekly) in patients with moderate to severe plaque psoriasis. Langley et al, in conjunction with the ERASURE Study Group and FIXTURE Study Group, reported (N Engl J Med. 2014;371:326-338) that the proportion of patients who met the criterion for a reduction of 75% or more from baseline in the psoriasis area and severity index score (PASI 75) at week 12 was higher with each secukinumab dose than with placebo or etanercept. The proportion of patients with a response of 0 (clear) or 1 (almost clear) on the modified investigator global assessment at week 12 was higher with each secukinumab dose than with placebo or etanercept.
This study was funded by Novartis Pharmaceuticals.
Practice Point: Efficacy of secukinumab has been documented in randomized trials of patients with moderate to severe plaque psoriasis, validating IL-17A as a therapeutic target.
>>Read more at the New England Journal of Medicine
More Evidence Needed on Combined Use of Systemic Agents for Plaque Psoriasis
To evaluate the combined use of systemic agents to achieve disease control in therapy-resistant patients with plaque-type psoriasis, Busard et al (JAMA Dermatol. doi:10.1001/jamadermatol.2014.1111) conducted an electronic search of randomized clinical trials. They found that most studies favored combination therapy, but the significance level and quality of evidence was low. Etanercept plus methotrexate was investigated with an adequate sample size and demonstrated superior efficacy compared to etanercept monotherapy. Although it was associated with an increase in adverse events, the overall safety profile was acceptable.
Practice Point: Patients with plaque psoriasis may benefit from combination therapy with systemic agents, but there is insufficient evidence. Etanercept plus methotrexate may be beneficial, with dose reductions to minimize adverse effects.
>>Read more at JAMA Dermatology
Plaque Psoriasis Familial Risk Highest in First-Degree Relatives
Psoriasis is known to be a highly heritable disease. In a cross-sectional study of 640 consecutive, unrelated adult patients with chronic plaque psoriasis, Di Lernia et al (Clin Exp Dermatol. 2014;39:801-805) found a positive familial history of psoriasis in more than half (59.37%) of patients. Of these patients, 27.18% had at least 1 parent with psoriasis, 16.56% had at least 1 second-degree relative with psoriasis, and 5.31% had 1 third-degree relative with psoriasis.
Practice Point: There is a high familial recurrence risk for plaque psoriasis.
>>Read more at Clinical and Experimental Dermatology
Secukinumab Efficacy for Plaque Psoriasis
Two phase 3, double-blind, 52-week trials (ERASURE and FIXTURE) evaluated subcutaneous secukinumab at a dose of 300 or 150 mg (administed once weekly for 5 weeks, then every 4 weeks), placebo, or (in the FIXTURE study only) etanercept at a dose of 50 mg (administered twice weekly for 12 weeks, then once weekly) in patients with moderate to severe plaque psoriasis. Langley et al, in conjunction with the ERASURE Study Group and FIXTURE Study Group, reported (N Engl J Med. 2014;371:326-338) that the proportion of patients who met the criterion for a reduction of 75% or more from baseline in the psoriasis area and severity index score (PASI 75) at week 12 was higher with each secukinumab dose than with placebo or etanercept. The proportion of patients with a response of 0 (clear) or 1 (almost clear) on the modified investigator global assessment at week 12 was higher with each secukinumab dose than with placebo or etanercept.
This study was funded by Novartis Pharmaceuticals.
Practice Point: Efficacy of secukinumab has been documented in randomized trials of patients with moderate to severe plaque psoriasis, validating IL-17A as a therapeutic target.
>>Read more at the New England Journal of Medicine
More Evidence Needed on Combined Use of Systemic Agents for Plaque Psoriasis
To evaluate the combined use of systemic agents to achieve disease control in therapy-resistant patients with plaque-type psoriasis, Busard et al (JAMA Dermatol. doi:10.1001/jamadermatol.2014.1111) conducted an electronic search of randomized clinical trials. They found that most studies favored combination therapy, but the significance level and quality of evidence was low. Etanercept plus methotrexate was investigated with an adequate sample size and demonstrated superior efficacy compared to etanercept monotherapy. Although it was associated with an increase in adverse events, the overall safety profile was acceptable.
Practice Point: Patients with plaque psoriasis may benefit from combination therapy with systemic agents, but there is insufficient evidence. Etanercept plus methotrexate may be beneficial, with dose reductions to minimize adverse effects.
>>Read more at JAMA Dermatology
Plaque Psoriasis Familial Risk Highest in First-Degree Relatives
Psoriasis is known to be a highly heritable disease. In a cross-sectional study of 640 consecutive, unrelated adult patients with chronic plaque psoriasis, Di Lernia et al (Clin Exp Dermatol. 2014;39:801-805) found a positive familial history of psoriasis in more than half (59.37%) of patients. Of these patients, 27.18% had at least 1 parent with psoriasis, 16.56% had at least 1 second-degree relative with psoriasis, and 5.31% had 1 third-degree relative with psoriasis.
Practice Point: There is a high familial recurrence risk for plaque psoriasis.
>>Read more at Clinical and Experimental Dermatology
Secukinumab Efficacy for Plaque Psoriasis
Two phase 3, double-blind, 52-week trials (ERASURE and FIXTURE) evaluated subcutaneous secukinumab at a dose of 300 or 150 mg (administed once weekly for 5 weeks, then every 4 weeks), placebo, or (in the FIXTURE study only) etanercept at a dose of 50 mg (administered twice weekly for 12 weeks, then once weekly) in patients with moderate to severe plaque psoriasis. Langley et al, in conjunction with the ERASURE Study Group and FIXTURE Study Group, reported (N Engl J Med. 2014;371:326-338) that the proportion of patients who met the criterion for a reduction of 75% or more from baseline in the psoriasis area and severity index score (PASI 75) at week 12 was higher with each secukinumab dose than with placebo or etanercept. The proportion of patients with a response of 0 (clear) or 1 (almost clear) on the modified investigator global assessment at week 12 was higher with each secukinumab dose than with placebo or etanercept.
This study was funded by Novartis Pharmaceuticals.
Practice Point: Efficacy of secukinumab has been documented in randomized trials of patients with moderate to severe plaque psoriasis, validating IL-17A as a therapeutic target.
>>Read more at the New England Journal of Medicine