User login
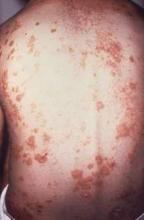
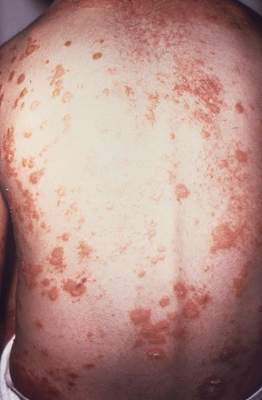
Annual costs of psoriasis costs top $112 billion
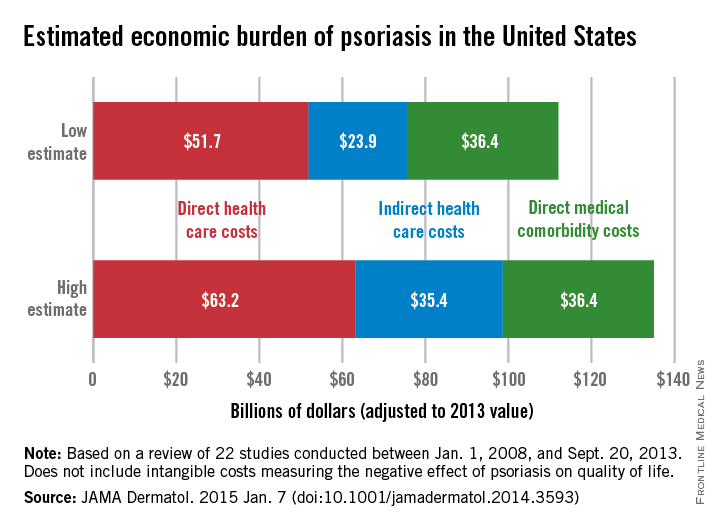
The total economic burden of psoriasis in the United States is at least $112 billion per year, and possibly as high as $135 billion, investigators estimated in a study published Jan. 7 in JAMA Dermatology.
Dr. Elizabeth A. Brezinski of the University of California, Davis, in Sacramento, and her associates, reviewed 22 studies conducted between Jan. 1, 2008, and Sept. 20, 2013, adjusting the results to 2013 dollars.
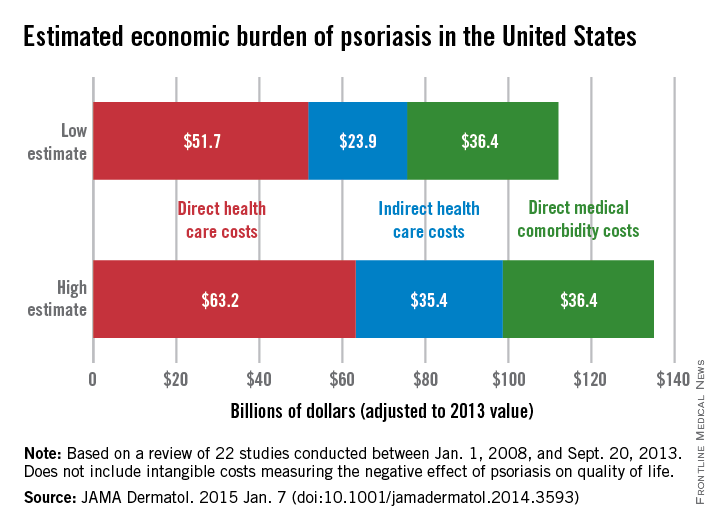
Estimates for the direct medical cost of psoriasis care ranged from $51.7 billion to $63.2 billion per year. Indirect costs from absenteeism or working while sick contributed another $23.9-$35.4 billion, with comorbidity costs estimated at $36.4 billion annually (JAMA Dermatol. 2014 Jan. 7 [doi:10.1001/jamadermatol.2014.3593]).
Intangible costs on quality of life, which were not included in the annual figures, were estimated to be $85.1 billion over the lifetimes of the psoriasis patient population (7.4 million as of 2013), they said.
“Defining the economic burden of psoriasis from a societal perspective is the foundation for innovating and providing access to cost-effective therapies that will result in improved patient outcomes,” Dr. Brezinski and her coauthors wrote.
One of the researchers reported serving as an investigator for, or consultant to, AbbVie, Amgen, Celgene, Janssen, Lilly, Merck, Pfizer, and UCB. No other disclosures were reported.
The total economic burden of psoriasis in the United States is at least $112 billion per year, and possibly as high as $135 billion, investigators estimated in a study published Jan. 7 in JAMA Dermatology.
Dr. Elizabeth A. Brezinski of the University of California, Davis, in Sacramento, and her associates, reviewed 22 studies conducted between Jan. 1, 2008, and Sept. 20, 2013, adjusting the results to 2013 dollars.

Estimates for the direct medical cost of psoriasis care ranged from $51.7 billion to $63.2 billion per year. Indirect costs from absenteeism or working while sick contributed another $23.9-$35.4 billion, with comorbidity costs estimated at $36.4 billion annually (JAMA Dermatol. 2014 Jan. 7 [doi:10.1001/jamadermatol.2014.3593]).
Intangible costs on quality of life, which were not included in the annual figures, were estimated to be $85.1 billion over the lifetimes of the psoriasis patient population (7.4 million as of 2013), they said.
“Defining the economic burden of psoriasis from a societal perspective is the foundation for innovating and providing access to cost-effective therapies that will result in improved patient outcomes,” Dr. Brezinski and her coauthors wrote.
One of the researchers reported serving as an investigator for, or consultant to, AbbVie, Amgen, Celgene, Janssen, Lilly, Merck, Pfizer, and UCB. No other disclosures were reported.
The total economic burden of psoriasis in the United States is at least $112 billion per year, and possibly as high as $135 billion, investigators estimated in a study published Jan. 7 in JAMA Dermatology.
Dr. Elizabeth A. Brezinski of the University of California, Davis, in Sacramento, and her associates, reviewed 22 studies conducted between Jan. 1, 2008, and Sept. 20, 2013, adjusting the results to 2013 dollars.

Estimates for the direct medical cost of psoriasis care ranged from $51.7 billion to $63.2 billion per year. Indirect costs from absenteeism or working while sick contributed another $23.9-$35.4 billion, with comorbidity costs estimated at $36.4 billion annually (JAMA Dermatol. 2014 Jan. 7 [doi:10.1001/jamadermatol.2014.3593]).
Intangible costs on quality of life, which were not included in the annual figures, were estimated to be $85.1 billion over the lifetimes of the psoriasis patient population (7.4 million as of 2013), they said.
“Defining the economic burden of psoriasis from a societal perspective is the foundation for innovating and providing access to cost-effective therapies that will result in improved patient outcomes,” Dr. Brezinski and her coauthors wrote.
One of the researchers reported serving as an investigator for, or consultant to, AbbVie, Amgen, Celgene, Janssen, Lilly, Merck, Pfizer, and UCB. No other disclosures were reported.
FROM JAMA DERMATOLOGY
Immunogenicity to TNF-alpha blockers varies in psoriatic arthritis
Immunogenicity to three TNF-alpha blocking agents seems to vary substantially among patients with psoriatic arthritis, and the use of methotrexate appears to attenuate the presence of antidrug antibodies, according to findings from a cross-sectional study.
Although researchers have looked at the immunogenicity of TNF-alpha blockers in patients with diseases such as rheumatoid arthritis (RA), ankylosing spondylitis, psoriasis, and inflammatory bowel disease, their immunogenicity in psoriatic arthritis (PsA) patients has not been fully investigated, noted the current study’s investigators, led by Dr. Michael Zisapel of the department of rheumatology at Tel Aviv University (J. Rheumatol. 2014 Nov. 15 [doi:10.3899/jrheum.140685]).
The prevalence of antidrug antibodies (ADAb) to TNF-alpha blockers has been reported to be 20%-40% in RA, 25%-64% in ankylosing spondylitis, and about 33% in psoriasis, and evidence has shown that this is significantly reduced by the use of methotrexate, they said.
The study involved 93 patients with PsA who were taking adalimumab (n = 48), infliximab (n = 24), and etanercept (n = 21). A quarter of the patients were taking methotrexate at an average dose of 13.3 mg/week.
Overall, 77% of the patients had therapeutic drug levels. The prevalence of immunogenicity in the entire group was 33.3%, and one-fifth of patients had high concentrations of antibodies.
High levels of ADAb were found in 29% of patients taking adalimumab and 21% of the patients taking infliximab. No ADAb levels were found in patients taking etanercept.
Interestingly, the patients taking adalimumab demonstrated an immunogenicity of 54%, but only half of them (29%) showed high concentrations of antibodies.
“This finding suggests that a variety of levels might be found that might affect the significance of immunogenicity differently among patients producing ADAb,” the investigators wrote.
Fewer methotrexate-treated patients had high ADAb concentrations, compared with patients not taking methotrexate (16.7% vs. 21.7%).
A clear correlation was found between the presence of immunogenicity, lower drug levels, and decreased clinical response in PsA patients, just as other studies have found in RA and ankylosing spondylitis patients, the authors noted.
“Our results suggest that the use of methotrexate should be strongly considered in addition to monoclonal antibodies,” they said.
Limitations of their study included the small number of patients and the use of a bridging ELISA test which is reliable but considered to be less accurate than radioimmunoassay.
No disclosure information was available.
Immunogenicity to three TNF-alpha blocking agents seems to vary substantially among patients with psoriatic arthritis, and the use of methotrexate appears to attenuate the presence of antidrug antibodies, according to findings from a cross-sectional study.
Although researchers have looked at the immunogenicity of TNF-alpha blockers in patients with diseases such as rheumatoid arthritis (RA), ankylosing spondylitis, psoriasis, and inflammatory bowel disease, their immunogenicity in psoriatic arthritis (PsA) patients has not been fully investigated, noted the current study’s investigators, led by Dr. Michael Zisapel of the department of rheumatology at Tel Aviv University (J. Rheumatol. 2014 Nov. 15 [doi:10.3899/jrheum.140685]).
The prevalence of antidrug antibodies (ADAb) to TNF-alpha blockers has been reported to be 20%-40% in RA, 25%-64% in ankylosing spondylitis, and about 33% in psoriasis, and evidence has shown that this is significantly reduced by the use of methotrexate, they said.
The study involved 93 patients with PsA who were taking adalimumab (n = 48), infliximab (n = 24), and etanercept (n = 21). A quarter of the patients were taking methotrexate at an average dose of 13.3 mg/week.
Overall, 77% of the patients had therapeutic drug levels. The prevalence of immunogenicity in the entire group was 33.3%, and one-fifth of patients had high concentrations of antibodies.
High levels of ADAb were found in 29% of patients taking adalimumab and 21% of the patients taking infliximab. No ADAb levels were found in patients taking etanercept.
Interestingly, the patients taking adalimumab demonstrated an immunogenicity of 54%, but only half of them (29%) showed high concentrations of antibodies.
“This finding suggests that a variety of levels might be found that might affect the significance of immunogenicity differently among patients producing ADAb,” the investigators wrote.
Fewer methotrexate-treated patients had high ADAb concentrations, compared with patients not taking methotrexate (16.7% vs. 21.7%).
A clear correlation was found between the presence of immunogenicity, lower drug levels, and decreased clinical response in PsA patients, just as other studies have found in RA and ankylosing spondylitis patients, the authors noted.
“Our results suggest that the use of methotrexate should be strongly considered in addition to monoclonal antibodies,” they said.
Limitations of their study included the small number of patients and the use of a bridging ELISA test which is reliable but considered to be less accurate than radioimmunoassay.
No disclosure information was available.
Immunogenicity to three TNF-alpha blocking agents seems to vary substantially among patients with psoriatic arthritis, and the use of methotrexate appears to attenuate the presence of antidrug antibodies, according to findings from a cross-sectional study.
Although researchers have looked at the immunogenicity of TNF-alpha blockers in patients with diseases such as rheumatoid arthritis (RA), ankylosing spondylitis, psoriasis, and inflammatory bowel disease, their immunogenicity in psoriatic arthritis (PsA) patients has not been fully investigated, noted the current study’s investigators, led by Dr. Michael Zisapel of the department of rheumatology at Tel Aviv University (J. Rheumatol. 2014 Nov. 15 [doi:10.3899/jrheum.140685]).
The prevalence of antidrug antibodies (ADAb) to TNF-alpha blockers has been reported to be 20%-40% in RA, 25%-64% in ankylosing spondylitis, and about 33% in psoriasis, and evidence has shown that this is significantly reduced by the use of methotrexate, they said.
The study involved 93 patients with PsA who were taking adalimumab (n = 48), infliximab (n = 24), and etanercept (n = 21). A quarter of the patients were taking methotrexate at an average dose of 13.3 mg/week.
Overall, 77% of the patients had therapeutic drug levels. The prevalence of immunogenicity in the entire group was 33.3%, and one-fifth of patients had high concentrations of antibodies.
High levels of ADAb were found in 29% of patients taking adalimumab and 21% of the patients taking infliximab. No ADAb levels were found in patients taking etanercept.
Interestingly, the patients taking adalimumab demonstrated an immunogenicity of 54%, but only half of them (29%) showed high concentrations of antibodies.
“This finding suggests that a variety of levels might be found that might affect the significance of immunogenicity differently among patients producing ADAb,” the investigators wrote.
Fewer methotrexate-treated patients had high ADAb concentrations, compared with patients not taking methotrexate (16.7% vs. 21.7%).
A clear correlation was found between the presence of immunogenicity, lower drug levels, and decreased clinical response in PsA patients, just as other studies have found in RA and ankylosing spondylitis patients, the authors noted.
“Our results suggest that the use of methotrexate should be strongly considered in addition to monoclonal antibodies,” they said.
Limitations of their study included the small number of patients and the use of a bridging ELISA test which is reliable but considered to be less accurate than radioimmunoassay.
No disclosure information was available.
FROM JOURNAL OF RHEUMATOLOGY
Key clinical point: The use of methotrexate should be strongly considered in addition to TNF-alpha blockers in patients with psoriatic arthritis to reduce the presence and influence of antidrug antibodies.
Major finding: High levels of antidrug antibodies were found in 29% of patients taking adalimumab, 21% of the patients taking infliximab, and no patients taking etanercept.
Data source: A cross-sectional study of 93 consecutive psoriatic arthritis patients.
Disclosures: No disclosure information was available.
Psoriasis patients have higher rate of low back pain
The prevalence of low back pain appears to be higher in people with psoriasis than in the general population, according to an analysis of national survey data.
The findings may change the way psoriasis patients are managed when they present to primary care or specialty clinics with sudden-onset back and buttock pain, said first author of the study, Dr. Nicole Thom of the division of rheumatology at Cedars-Sinai Medical Center, Los Angeles, and her colleagues (Arthritis Care Res. 2014 Dec. 2 [doi:10.1002/acr.22528]).
Using data from the 2009-2010 U.S. National Health and Nutrition Examination Survey of 6,684 adults, the researchers identified 5,103 people who had answered questions on back pain. A total of 148 had psoriasis and 5 had psoriatic arthritis (PsA).
People with psoriasis/PsA had a significantly higher prevalence of axial pain as measured using the 3-month duration criteria, compared with people without the disease (31.1% vs. 18.9%; P = .04). They were also more likely to have alternating buttock pain (7.2% vs. 2.4%; P = .03) and meet Berlin 7b and 8a criteria for inflammatory back pain (P = .04 and P = .02, respectively). The prevalence of spondyloarthritis was significantly higher in the psoriasis/PsA group when using Amor or European Spondyloarthritis Study Group criteria (14.3% vs. 1.5%; P = .001). Sudden onset of axial pain was also higher in the psoriasis/PsA group (23.3% vs. 13.0%; P = .01), the researchers reported.
“The internist or family medicine physician should include inflammatory back pain in their differential diagnosis,” the study authors suggest.
With more and more research continuing to support multiple comorbidities in psoriasis, it also raises the question as to whether rheumatologists, dermatologists, and other health care professionals should be screening for them, they said.
The work was supported in part by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and a National Center for Research Resources grant to the Clinical Translational Science Institute at the University of California, Los Angeles.
The prevalence of low back pain appears to be higher in people with psoriasis than in the general population, according to an analysis of national survey data.
The findings may change the way psoriasis patients are managed when they present to primary care or specialty clinics with sudden-onset back and buttock pain, said first author of the study, Dr. Nicole Thom of the division of rheumatology at Cedars-Sinai Medical Center, Los Angeles, and her colleagues (Arthritis Care Res. 2014 Dec. 2 [doi:10.1002/acr.22528]).
Using data from the 2009-2010 U.S. National Health and Nutrition Examination Survey of 6,684 adults, the researchers identified 5,103 people who had answered questions on back pain. A total of 148 had psoriasis and 5 had psoriatic arthritis (PsA).
People with psoriasis/PsA had a significantly higher prevalence of axial pain as measured using the 3-month duration criteria, compared with people without the disease (31.1% vs. 18.9%; P = .04). They were also more likely to have alternating buttock pain (7.2% vs. 2.4%; P = .03) and meet Berlin 7b and 8a criteria for inflammatory back pain (P = .04 and P = .02, respectively). The prevalence of spondyloarthritis was significantly higher in the psoriasis/PsA group when using Amor or European Spondyloarthritis Study Group criteria (14.3% vs. 1.5%; P = .001). Sudden onset of axial pain was also higher in the psoriasis/PsA group (23.3% vs. 13.0%; P = .01), the researchers reported.
“The internist or family medicine physician should include inflammatory back pain in their differential diagnosis,” the study authors suggest.
With more and more research continuing to support multiple comorbidities in psoriasis, it also raises the question as to whether rheumatologists, dermatologists, and other health care professionals should be screening for them, they said.
The work was supported in part by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and a National Center for Research Resources grant to the Clinical Translational Science Institute at the University of California, Los Angeles.
The prevalence of low back pain appears to be higher in people with psoriasis than in the general population, according to an analysis of national survey data.
The findings may change the way psoriasis patients are managed when they present to primary care or specialty clinics with sudden-onset back and buttock pain, said first author of the study, Dr. Nicole Thom of the division of rheumatology at Cedars-Sinai Medical Center, Los Angeles, and her colleagues (Arthritis Care Res. 2014 Dec. 2 [doi:10.1002/acr.22528]).
Using data from the 2009-2010 U.S. National Health and Nutrition Examination Survey of 6,684 adults, the researchers identified 5,103 people who had answered questions on back pain. A total of 148 had psoriasis and 5 had psoriatic arthritis (PsA).
People with psoriasis/PsA had a significantly higher prevalence of axial pain as measured using the 3-month duration criteria, compared with people without the disease (31.1% vs. 18.9%; P = .04). They were also more likely to have alternating buttock pain (7.2% vs. 2.4%; P = .03) and meet Berlin 7b and 8a criteria for inflammatory back pain (P = .04 and P = .02, respectively). The prevalence of spondyloarthritis was significantly higher in the psoriasis/PsA group when using Amor or European Spondyloarthritis Study Group criteria (14.3% vs. 1.5%; P = .001). Sudden onset of axial pain was also higher in the psoriasis/PsA group (23.3% vs. 13.0%; P = .01), the researchers reported.
“The internist or family medicine physician should include inflammatory back pain in their differential diagnosis,” the study authors suggest.
With more and more research continuing to support multiple comorbidities in psoriasis, it also raises the question as to whether rheumatologists, dermatologists, and other health care professionals should be screening for them, they said.
The work was supported in part by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and a National Center for Research Resources grant to the Clinical Translational Science Institute at the University of California, Los Angeles.
FROM ARTHRITIS CARE & RESEARCH
Calcipotriene 0.005%–Betamethasone Dipropionate 0.064% Ointment Versus Topical Suspension in the Treatment of Plaque Psoriasis: A Randomized Pilot Study of Patient Preference
Psoriasis is a chronic relapsing inflammatory skin and joint disease that affects 1% to 3% of the US population.1 In cases of mild to moderate disease, topical agents including corticosteroids, vitamin D analogues, and retinoids are the mainstay of therapy. The need for long-term treatment can be frustrating for patients and treatment adherence often is problematic, resulting in poor outcomes. Reported adherence rates to topical psoriasis treatments range from 27% to 73%.2-6
Topical agents for treatment of psoriasis are available in various formulations, including creams, lotions, gels, ointments, solutions, and shampoos. For topical psoriasis therapies, vehicle formulation plays a major role in both delivery of the active drug and treatment adherence. Patients often cite poor cosmetic characteristics (eg, product feels too sticky or greasy, product feels unpleasant/has a bad texture, product is too messy, product application is too time consuming/takes too long to rub in) as reasons for poor treatment adherence.2,6-9 Psoriasis patients tend to prefer formulations that are not as messy such as solutions and foams versus creams, gels, and ointments.10
Ointments have been favored by physicians for the treatment of psoriasis because of the belief that their occlusive properties result in greater potency; however, a systematic review of clinical trials of different formulations of clobetasol propionate did not find that ointments were more effective than other vehicles.11 Furthermore, if a patient finds an ointment to be cosmetically unacceptable, he/she will be less inclined to use the medication as prescribed, regardless of its potency.
The objective of this study was to conduct a preliminary assessment of patient preference for ointment versus topical suspension formulations of calcipotriene 0.005%–betamethasone dipropionate 0.064% for treatment of plaque psoriasis. The specific attributes that were found to be appealing or unappealing by participants for each formulation also were evaluated.
Methods
Study Design and Participants
This open-label, investigator-blinded, crossover, prospective, single-center study evaluated patient preference for ointment versus topical suspension formulations of calcipo-triene 0.005%–betamethasone dipropionate 0.064% in the treatment of plaque psoriasis. The study protocol was approved by the Wake Forest School of Medicine (Winston-Salem, North Carolina) institutional review board.
Participants were recruited from the Wake Forest School of Medicine dermatology clinic. Inclusion criteria included men and women with mild to moderate plaque-type psoriasis who were 18 years of age or older; participants also were required to have an investigator global assessment (IGA) score of 2 (mild) or 3 (moderate) on a 5-point scale and 1% to 10% body surface involvement on the trunk or extremities.
Exclusion criteria included use of a topical or systemic psoriasis treatment within 2 or 4 weeks of baseline, respectively. Women who were pregnant, breastfeeding, planning to become pregnant, or could potentially become pregnant and were not using a medically accepted form of contraception also were excluded from the study. Patients with other serious skin conditions or any other chronic medical conditions that were not well controlled also were considered ineligible.
As a pilot study, a sample size of 20 participants was needed based on available funding.
Assessments
At baseline, the diagnosis of plaque-type psoriasis in each participant was confirmed by the investigator. Each participant’s medical history was obtained and all prior and current medications were reviewed to ensure eligibility criteria were met. Female participants of childbearing potential also underwent a urine pregnancy test. Consent was obtained from all enrolled participants. Investigators assessed the severity of psoriasis at baseline using the IGA.
All participants then were randomized (1:1 randomization) for treatment with either calcipotriene 0.005%–betamethasone dipropionate 0.064% ointment or calcipotriene 0.005%–betamethasone dipropionate 0.064% topical suspension. A simple random sampling chart was prepared and used by the study staff to determine treatment group allotment. Participants were instructed to apply the assigned study drug to affected areas of the body, excluding the scalp, face, and intertriginous areas, once daily for 3 days. Participants and study staff were aware of the study product being used, while investigators remained blinded. Participants also were asked to maintain a daily medication diary noting when the medication was applied.
Participants returned on day 3 for crossover treatment. Participants were asked to complete a subjective participant preference survey and study staff reviewed concomitant medications and adverse events. The packaging for the initial study drug was collected and weighed, and the crossover drug was dispensed to each participant to be applied once daily for 3 days.
Participants returned on day 6 or 7 for follow-up and were again asked to complete the subjective participant preference survey; study staff reviewed concomitant medications and adverse events. The packaging for the crossover study drug was collected and weighed, and the participant’s medication diary also was collected.
Subjective Participant Preference Survey
The subjective participant preference survey consisted of 15 questions relating to the participant’s experience with the study drug (eg, how the product felt to touch, amount of greasiness, time it took to apply). The final survey question asked participants to rate the overall appeal of the vehicle. Participants responded to the questions using a 7-point grading scale (1=extremely unappealing; 4=neutral; 7=extremely appealing). Total preference scores could range from 15 to 105.
End Points
The primary end point was the mean total preference score for each study drug obtained from the subjective participant preference surveys. Secondary end points included median values for individual survey questions and treatment adherence, which was measured from self-reported medication diary entries.
Statistical Analysis
Participant characteristics were reported with percentages for dichotomous data and median and ranges for other data. Subjective participant preference survey scores were calculated by taking the mean (standard deviation [SD]) sum of the scores for each individual survey item for both products. A generalized linear model that accounted for possible carryover and period effects was used to compare the difference of individual participant scores for the 2 products using SAS software. The mean (SD) amount of product used was reported and correlated to the preference score using a Spearman rank correlation. Total and individual survey scores were compared between sexes using Wilcoxon rank sum tests.
Results
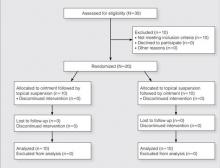
Participants were enrolled in and completed the study from January 2013 to March 2013. The Figure presents a diagram of the Consolidated Standards of Reporting Trials. Thirty patients were screened; 10 patients did not meet eligibility criteria. Twenty patients were enrolled in the study with 10 patients randomized to each study arm. All 20 participants completed the study.
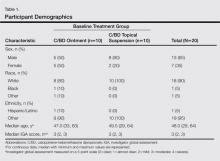
Participant demographics are described in Table 1. The median age was 48 years (range 29–64 years). The majority of participants were male (13/20) and white (18/20). The median IGA score was 3 (range, 2–3).
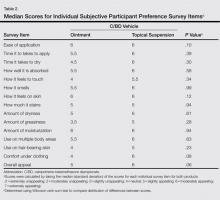
The mean (SD) total preference score for the calcipotriene 0.005%–betamethasone dipropionate 0.064% ointment formulation was 73.5 (19.4) and 80 (20.2) for the topical suspension formulation. The difference between means was -6.5 (95% confidence interval, -19.7-6.8; P=.32 after adjusting for possible carryover and period effects). Participants used a mean (SD) of 11 (11.4) g of product per study arm for the ointment formulation and 8.8 (6.6) g for the topical suspension formulation. There was no correlation between the amount of product used and preference for product (Spearman r=-0.01; P=.94). No statistically significant difference in product preference among men versus women was noted when considering total preference score or median scores of individual survey questions. Median overall appeal rating for the ointment formulation was 5 (slightly appealing) versus 6 (moderately appealing) for the topical suspension formulation, approaching statistical significance with P=.06 (Table 2). No significant carryover effects from one product to the other were noted (P=.64). The mean (SD) total preference scores were 81.1 (18.4) and 77.6 (21.2) in participants who used the topical suspension first followed by the ointment. In participants who used the ointment first followed by the topical suspension, the mean (SD) total preference scores were 69.4 (17.6) and 78.9 (22.8). Self-reported treatment adherence according to the participant’s daily medication diary was 100%.
Adverse effects during the study included 1 report of neck and back muscle pain and 1 report of sinusitis; neither was considered to be related to the study drugs.
Comment
Psoriasis is a chronic disease that can be difficult to treat, and treatment compliance often is poor. Multiple topical agents often are needed for adequate disease control, and adherence can be an even greater hurdle than with monotherapy. Combination products such as calcipotriene–betamethasone dipropionate offer the potential advantage of once-daily application. Adherence to once-daily application regimens for treatment of psoriasis has been shown to be greater than twice-daily application (82% vs 44%).4 However, the vehicle may be an adherence barrier for some patients.
Calcipotriene 0.005%–betamethasone dipropionate 0.064% topical suspension originally was indicated for the treatment of psoriasis of the scalp; however, it is now also indicated for treatment of psoriasis of the body.12 This topical suspension formulation is less messy, which could potentially be more cosmetically appealing and useful for improving treatment adherence.
Overall, the participants in our small pilot study showed a preference for the topical suspension versus the ointment formulation. The difference was substantial but was not statistically significant. This result is consistent with a previous study in which patients were found to prefer solutions that were less greasy compared to messy sticky ointments.10 Although the topical suspension received a higher average rating from participants for how it felt to touch and how it felt under clothing than the ointment and the ointment was rated on average as more greasy (none of these individual items achieved statistical significance), the calcipotriene–betamethasone dipropionate ointment was still rated as slightly appealing overall (Table 2). This result supports the need for physicians to discuss individual patient preferences when choosing the most appropriate vehicle for topical psoriasis treatment.
In our study, participants were found to use less product during treatment with the topical suspension versus the ointment, likely because the topical suspension formulation is thinner and spreads easier; however, participants rated the ease of application of the 2 products equally (Table 2). Ease of application was rated moderately appealing and time to apply was rated slightly to moderately appealing, which is important because patients often cite these factors as barriers to treatment adherence.2,6,13
The occlusive properties of ointment formulations provide moisturization by preventing water loss, a property that can be desirable when treating psoriatic plaques. Unlike the ointment, which was formulated with petrolatum and mineral oil, the calcipotriene–betamethasone dipropionate topical suspension was formulated with hydrogenated castor oil and mineral oil to provide moisturization. Nevertheless, participants found both the ointment and topical suspension to be moderately appealing (median score, 6; P=.94) for moisturization.
Limitations of this pilot study include the small sample size, which restricted the extent of subgroup analyses and the generalizability of our results. The small sample size also may or may not have contributed to the lack of statistical significance in the majority of the outcomes. This study provides pilot data that can be used to define a larger study; however, we do not think that a larger study is needed, as patients can be offered both vehicles in a practical clinical setting and can choose the product that is right for them. The short treatment duration of 3 days for each formulation also is a limitation, as a patient’s preference may change over time with longer use of the product. Treatment efficacy, which was not measured in this study, also could have an effect on patient preference, which could be assessed over a longer treatment period. Additionally, the study drugs may not be representative of all ointments or topical suspensions in their cosmetic appeal.
Conclusion
In this small cohort of plaque psoriasis patients, a calcipotriene–betamethasone dipropionate topical suspension was preferred over an ointment formulation, but in clinical practice it may be best to allow patients to choose the vehicle formulation that is most desirable on an individual basis. The topical suspension provides clinicians with an alternative that not only has the benefits of a combination product but also has been found to be appealing to patients.
1. Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385.
2. Brown KK, Rehmus WE, Kimball AB. Determining the relative importance of patient motivations for nonadherence to topical corticosteroid therapy in psoriasis. J Am Acad Dermatol. 2006;55:607-613.
3. Carroll CL, Feldman SR, Camacho FT, et al. Adherence to topical therapy decreases during the course of an 8-week psoriasis clinical trial: commonly used methods of measuring adherence to topical therapy overestimate actual use. J Am Acad Dermatol. 2004;51:212-216.
4. Zaghloul SS, Goodfield MJ. Objective assessment of compliance with psoriasis treatment. Arch Dermatol. 2004;140:408-414.
5. Richards HL, Fortune DG, O’Sullivan TM, et al. Patients with psoriasis and their compliance with medication. J Am Acad Dermatol. 1999;41:581-583.
6. Fouere S, Adjadj L, Pawin H. How patients experience psoriasis: results from a European survey. J Eur Acad Dermatol Venereol. 2005;19:S2-S6.
7. van de Kerkhof PC, Steegers-Theunissen RP, Kuipers MV. Evaluation of topical drug treatment in psoriasis. Dermatology. 1998;197:31-36.
8. Devaux S, Castela A, Archier E, et al. Adherence to topical treatment in psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):S61-S67.
9. Chan SA, Hussain F, Lawson LG, et al. Factors affecting adherence to treatment of psoriasis: comparing biologic therapy to other modalities. J Dermatolog Treat. 2013;24:64-69.
10. Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
11. Warino L, Balkrishnan R, Feldman SR. Clobetasol propionate for psoriasis: are ointments really more potent? J Drugs Dermatol. 2006;5:527-532.
12. Menter A, Gold LS, Bukhalo M, et al. Calcipotriene plus betamethasone dipropionate topical suspension for the treatment of mild to moderate psoriasis vulgaris on the body: a randomized, double-blind, vehicle-controlled trial. J Drugs Dermatol. 2013;12:92-98.
13. Krueger G, Koo J, Lebwohl M, et al. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol. 2001;137:280-284.
Psoriasis is a chronic relapsing inflammatory skin and joint disease that affects 1% to 3% of the US population.1 In cases of mild to moderate disease, topical agents including corticosteroids, vitamin D analogues, and retinoids are the mainstay of therapy. The need for long-term treatment can be frustrating for patients and treatment adherence often is problematic, resulting in poor outcomes. Reported adherence rates to topical psoriasis treatments range from 27% to 73%.2-6
Topical agents for treatment of psoriasis are available in various formulations, including creams, lotions, gels, ointments, solutions, and shampoos. For topical psoriasis therapies, vehicle formulation plays a major role in both delivery of the active drug and treatment adherence. Patients often cite poor cosmetic characteristics (eg, product feels too sticky or greasy, product feels unpleasant/has a bad texture, product is too messy, product application is too time consuming/takes too long to rub in) as reasons for poor treatment adherence.2,6-9 Psoriasis patients tend to prefer formulations that are not as messy such as solutions and foams versus creams, gels, and ointments.10
Ointments have been favored by physicians for the treatment of psoriasis because of the belief that their occlusive properties result in greater potency; however, a systematic review of clinical trials of different formulations of clobetasol propionate did not find that ointments were more effective than other vehicles.11 Furthermore, if a patient finds an ointment to be cosmetically unacceptable, he/she will be less inclined to use the medication as prescribed, regardless of its potency.
The objective of this study was to conduct a preliminary assessment of patient preference for ointment versus topical suspension formulations of calcipotriene 0.005%–betamethasone dipropionate 0.064% for treatment of plaque psoriasis. The specific attributes that were found to be appealing or unappealing by participants for each formulation also were evaluated.
Methods
Study Design and Participants
This open-label, investigator-blinded, crossover, prospective, single-center study evaluated patient preference for ointment versus topical suspension formulations of calcipo-triene 0.005%–betamethasone dipropionate 0.064% in the treatment of plaque psoriasis. The study protocol was approved by the Wake Forest School of Medicine (Winston-Salem, North Carolina) institutional review board.
Participants were recruited from the Wake Forest School of Medicine dermatology clinic. Inclusion criteria included men and women with mild to moderate plaque-type psoriasis who were 18 years of age or older; participants also were required to have an investigator global assessment (IGA) score of 2 (mild) or 3 (moderate) on a 5-point scale and 1% to 10% body surface involvement on the trunk or extremities.
Exclusion criteria included use of a topical or systemic psoriasis treatment within 2 or 4 weeks of baseline, respectively. Women who were pregnant, breastfeeding, planning to become pregnant, or could potentially become pregnant and were not using a medically accepted form of contraception also were excluded from the study. Patients with other serious skin conditions or any other chronic medical conditions that were not well controlled also were considered ineligible.
As a pilot study, a sample size of 20 participants was needed based on available funding.
Assessments
At baseline, the diagnosis of plaque-type psoriasis in each participant was confirmed by the investigator. Each participant’s medical history was obtained and all prior and current medications were reviewed to ensure eligibility criteria were met. Female participants of childbearing potential also underwent a urine pregnancy test. Consent was obtained from all enrolled participants. Investigators assessed the severity of psoriasis at baseline using the IGA.
All participants then were randomized (1:1 randomization) for treatment with either calcipotriene 0.005%–betamethasone dipropionate 0.064% ointment or calcipotriene 0.005%–betamethasone dipropionate 0.064% topical suspension. A simple random sampling chart was prepared and used by the study staff to determine treatment group allotment. Participants were instructed to apply the assigned study drug to affected areas of the body, excluding the scalp, face, and intertriginous areas, once daily for 3 days. Participants and study staff were aware of the study product being used, while investigators remained blinded. Participants also were asked to maintain a daily medication diary noting when the medication was applied.
Participants returned on day 3 for crossover treatment. Participants were asked to complete a subjective participant preference survey and study staff reviewed concomitant medications and adverse events. The packaging for the initial study drug was collected and weighed, and the crossover drug was dispensed to each participant to be applied once daily for 3 days.
Participants returned on day 6 or 7 for follow-up and were again asked to complete the subjective participant preference survey; study staff reviewed concomitant medications and adverse events. The packaging for the crossover study drug was collected and weighed, and the participant’s medication diary also was collected.
Subjective Participant Preference Survey
The subjective participant preference survey consisted of 15 questions relating to the participant’s experience with the study drug (eg, how the product felt to touch, amount of greasiness, time it took to apply). The final survey question asked participants to rate the overall appeal of the vehicle. Participants responded to the questions using a 7-point grading scale (1=extremely unappealing; 4=neutral; 7=extremely appealing). Total preference scores could range from 15 to 105.
End Points
The primary end point was the mean total preference score for each study drug obtained from the subjective participant preference surveys. Secondary end points included median values for individual survey questions and treatment adherence, which was measured from self-reported medication diary entries.
Statistical Analysis
Participant characteristics were reported with percentages for dichotomous data and median and ranges for other data. Subjective participant preference survey scores were calculated by taking the mean (standard deviation [SD]) sum of the scores for each individual survey item for both products. A generalized linear model that accounted for possible carryover and period effects was used to compare the difference of individual participant scores for the 2 products using SAS software. The mean (SD) amount of product used was reported and correlated to the preference score using a Spearman rank correlation. Total and individual survey scores were compared between sexes using Wilcoxon rank sum tests.
Results
Participants were enrolled in and completed the study from January 2013 to March 2013. The Figure presents a diagram of the Consolidated Standards of Reporting Trials. Thirty patients were screened; 10 patients did not meet eligibility criteria. Twenty patients were enrolled in the study with 10 patients randomized to each study arm. All 20 participants completed the study.
Participant demographics are described in Table 1. The median age was 48 years (range 29–64 years). The majority of participants were male (13/20) and white (18/20). The median IGA score was 3 (range, 2–3).
The mean (SD) total preference score for the calcipotriene 0.005%–betamethasone dipropionate 0.064% ointment formulation was 73.5 (19.4) and 80 (20.2) for the topical suspension formulation. The difference between means was -6.5 (95% confidence interval, -19.7-6.8; P=.32 after adjusting for possible carryover and period effects). Participants used a mean (SD) of 11 (11.4) g of product per study arm for the ointment formulation and 8.8 (6.6) g for the topical suspension formulation. There was no correlation between the amount of product used and preference for product (Spearman r=-0.01; P=.94). No statistically significant difference in product preference among men versus women was noted when considering total preference score or median scores of individual survey questions. Median overall appeal rating for the ointment formulation was 5 (slightly appealing) versus 6 (moderately appealing) for the topical suspension formulation, approaching statistical significance with P=.06 (Table 2). No significant carryover effects from one product to the other were noted (P=.64). The mean (SD) total preference scores were 81.1 (18.4) and 77.6 (21.2) in participants who used the topical suspension first followed by the ointment. In participants who used the ointment first followed by the topical suspension, the mean (SD) total preference scores were 69.4 (17.6) and 78.9 (22.8). Self-reported treatment adherence according to the participant’s daily medication diary was 100%.
Adverse effects during the study included 1 report of neck and back muscle pain and 1 report of sinusitis; neither was considered to be related to the study drugs.
Comment
Psoriasis is a chronic disease that can be difficult to treat, and treatment compliance often is poor. Multiple topical agents often are needed for adequate disease control, and adherence can be an even greater hurdle than with monotherapy. Combination products such as calcipotriene–betamethasone dipropionate offer the potential advantage of once-daily application. Adherence to once-daily application regimens for treatment of psoriasis has been shown to be greater than twice-daily application (82% vs 44%).4 However, the vehicle may be an adherence barrier for some patients.
Calcipotriene 0.005%–betamethasone dipropionate 0.064% topical suspension originally was indicated for the treatment of psoriasis of the scalp; however, it is now also indicated for treatment of psoriasis of the body.12 This topical suspension formulation is less messy, which could potentially be more cosmetically appealing and useful for improving treatment adherence.
Overall, the participants in our small pilot study showed a preference for the topical suspension versus the ointment formulation. The difference was substantial but was not statistically significant. This result is consistent with a previous study in which patients were found to prefer solutions that were less greasy compared to messy sticky ointments.10 Although the topical suspension received a higher average rating from participants for how it felt to touch and how it felt under clothing than the ointment and the ointment was rated on average as more greasy (none of these individual items achieved statistical significance), the calcipotriene–betamethasone dipropionate ointment was still rated as slightly appealing overall (Table 2). This result supports the need for physicians to discuss individual patient preferences when choosing the most appropriate vehicle for topical psoriasis treatment.
In our study, participants were found to use less product during treatment with the topical suspension versus the ointment, likely because the topical suspension formulation is thinner and spreads easier; however, participants rated the ease of application of the 2 products equally (Table 2). Ease of application was rated moderately appealing and time to apply was rated slightly to moderately appealing, which is important because patients often cite these factors as barriers to treatment adherence.2,6,13
The occlusive properties of ointment formulations provide moisturization by preventing water loss, a property that can be desirable when treating psoriatic plaques. Unlike the ointment, which was formulated with petrolatum and mineral oil, the calcipotriene–betamethasone dipropionate topical suspension was formulated with hydrogenated castor oil and mineral oil to provide moisturization. Nevertheless, participants found both the ointment and topical suspension to be moderately appealing (median score, 6; P=.94) for moisturization.
Limitations of this pilot study include the small sample size, which restricted the extent of subgroup analyses and the generalizability of our results. The small sample size also may or may not have contributed to the lack of statistical significance in the majority of the outcomes. This study provides pilot data that can be used to define a larger study; however, we do not think that a larger study is needed, as patients can be offered both vehicles in a practical clinical setting and can choose the product that is right for them. The short treatment duration of 3 days for each formulation also is a limitation, as a patient’s preference may change over time with longer use of the product. Treatment efficacy, which was not measured in this study, also could have an effect on patient preference, which could be assessed over a longer treatment period. Additionally, the study drugs may not be representative of all ointments or topical suspensions in their cosmetic appeal.
Conclusion
In this small cohort of plaque psoriasis patients, a calcipotriene–betamethasone dipropionate topical suspension was preferred over an ointment formulation, but in clinical practice it may be best to allow patients to choose the vehicle formulation that is most desirable on an individual basis. The topical suspension provides clinicians with an alternative that not only has the benefits of a combination product but also has been found to be appealing to patients.
Psoriasis is a chronic relapsing inflammatory skin and joint disease that affects 1% to 3% of the US population.1 In cases of mild to moderate disease, topical agents including corticosteroids, vitamin D analogues, and retinoids are the mainstay of therapy. The need for long-term treatment can be frustrating for patients and treatment adherence often is problematic, resulting in poor outcomes. Reported adherence rates to topical psoriasis treatments range from 27% to 73%.2-6
Topical agents for treatment of psoriasis are available in various formulations, including creams, lotions, gels, ointments, solutions, and shampoos. For topical psoriasis therapies, vehicle formulation plays a major role in both delivery of the active drug and treatment adherence. Patients often cite poor cosmetic characteristics (eg, product feels too sticky or greasy, product feels unpleasant/has a bad texture, product is too messy, product application is too time consuming/takes too long to rub in) as reasons for poor treatment adherence.2,6-9 Psoriasis patients tend to prefer formulations that are not as messy such as solutions and foams versus creams, gels, and ointments.10
Ointments have been favored by physicians for the treatment of psoriasis because of the belief that their occlusive properties result in greater potency; however, a systematic review of clinical trials of different formulations of clobetasol propionate did not find that ointments were more effective than other vehicles.11 Furthermore, if a patient finds an ointment to be cosmetically unacceptable, he/she will be less inclined to use the medication as prescribed, regardless of its potency.
The objective of this study was to conduct a preliminary assessment of patient preference for ointment versus topical suspension formulations of calcipotriene 0.005%–betamethasone dipropionate 0.064% for treatment of plaque psoriasis. The specific attributes that were found to be appealing or unappealing by participants for each formulation also were evaluated.
Methods
Study Design and Participants
This open-label, investigator-blinded, crossover, prospective, single-center study evaluated patient preference for ointment versus topical suspension formulations of calcipo-triene 0.005%–betamethasone dipropionate 0.064% in the treatment of plaque psoriasis. The study protocol was approved by the Wake Forest School of Medicine (Winston-Salem, North Carolina) institutional review board.
Participants were recruited from the Wake Forest School of Medicine dermatology clinic. Inclusion criteria included men and women with mild to moderate plaque-type psoriasis who were 18 years of age or older; participants also were required to have an investigator global assessment (IGA) score of 2 (mild) or 3 (moderate) on a 5-point scale and 1% to 10% body surface involvement on the trunk or extremities.
Exclusion criteria included use of a topical or systemic psoriasis treatment within 2 or 4 weeks of baseline, respectively. Women who were pregnant, breastfeeding, planning to become pregnant, or could potentially become pregnant and were not using a medically accepted form of contraception also were excluded from the study. Patients with other serious skin conditions or any other chronic medical conditions that were not well controlled also were considered ineligible.
As a pilot study, a sample size of 20 participants was needed based on available funding.
Assessments
At baseline, the diagnosis of plaque-type psoriasis in each participant was confirmed by the investigator. Each participant’s medical history was obtained and all prior and current medications were reviewed to ensure eligibility criteria were met. Female participants of childbearing potential also underwent a urine pregnancy test. Consent was obtained from all enrolled participants. Investigators assessed the severity of psoriasis at baseline using the IGA.
All participants then were randomized (1:1 randomization) for treatment with either calcipotriene 0.005%–betamethasone dipropionate 0.064% ointment or calcipotriene 0.005%–betamethasone dipropionate 0.064% topical suspension. A simple random sampling chart was prepared and used by the study staff to determine treatment group allotment. Participants were instructed to apply the assigned study drug to affected areas of the body, excluding the scalp, face, and intertriginous areas, once daily for 3 days. Participants and study staff were aware of the study product being used, while investigators remained blinded. Participants also were asked to maintain a daily medication diary noting when the medication was applied.
Participants returned on day 3 for crossover treatment. Participants were asked to complete a subjective participant preference survey and study staff reviewed concomitant medications and adverse events. The packaging for the initial study drug was collected and weighed, and the crossover drug was dispensed to each participant to be applied once daily for 3 days.
Participants returned on day 6 or 7 for follow-up and were again asked to complete the subjective participant preference survey; study staff reviewed concomitant medications and adverse events. The packaging for the crossover study drug was collected and weighed, and the participant’s medication diary also was collected.
Subjective Participant Preference Survey
The subjective participant preference survey consisted of 15 questions relating to the participant’s experience with the study drug (eg, how the product felt to touch, amount of greasiness, time it took to apply). The final survey question asked participants to rate the overall appeal of the vehicle. Participants responded to the questions using a 7-point grading scale (1=extremely unappealing; 4=neutral; 7=extremely appealing). Total preference scores could range from 15 to 105.
End Points
The primary end point was the mean total preference score for each study drug obtained from the subjective participant preference surveys. Secondary end points included median values for individual survey questions and treatment adherence, which was measured from self-reported medication diary entries.
Statistical Analysis
Participant characteristics were reported with percentages for dichotomous data and median and ranges for other data. Subjective participant preference survey scores were calculated by taking the mean (standard deviation [SD]) sum of the scores for each individual survey item for both products. A generalized linear model that accounted for possible carryover and period effects was used to compare the difference of individual participant scores for the 2 products using SAS software. The mean (SD) amount of product used was reported and correlated to the preference score using a Spearman rank correlation. Total and individual survey scores were compared between sexes using Wilcoxon rank sum tests.
Results
Participants were enrolled in and completed the study from January 2013 to March 2013. The Figure presents a diagram of the Consolidated Standards of Reporting Trials. Thirty patients were screened; 10 patients did not meet eligibility criteria. Twenty patients were enrolled in the study with 10 patients randomized to each study arm. All 20 participants completed the study.
Participant demographics are described in Table 1. The median age was 48 years (range 29–64 years). The majority of participants were male (13/20) and white (18/20). The median IGA score was 3 (range, 2–3).
The mean (SD) total preference score for the calcipotriene 0.005%–betamethasone dipropionate 0.064% ointment formulation was 73.5 (19.4) and 80 (20.2) for the topical suspension formulation. The difference between means was -6.5 (95% confidence interval, -19.7-6.8; P=.32 after adjusting for possible carryover and period effects). Participants used a mean (SD) of 11 (11.4) g of product per study arm for the ointment formulation and 8.8 (6.6) g for the topical suspension formulation. There was no correlation between the amount of product used and preference for product (Spearman r=-0.01; P=.94). No statistically significant difference in product preference among men versus women was noted when considering total preference score or median scores of individual survey questions. Median overall appeal rating for the ointment formulation was 5 (slightly appealing) versus 6 (moderately appealing) for the topical suspension formulation, approaching statistical significance with P=.06 (Table 2). No significant carryover effects from one product to the other were noted (P=.64). The mean (SD) total preference scores were 81.1 (18.4) and 77.6 (21.2) in participants who used the topical suspension first followed by the ointment. In participants who used the ointment first followed by the topical suspension, the mean (SD) total preference scores were 69.4 (17.6) and 78.9 (22.8). Self-reported treatment adherence according to the participant’s daily medication diary was 100%.
Adverse effects during the study included 1 report of neck and back muscle pain and 1 report of sinusitis; neither was considered to be related to the study drugs.
Comment
Psoriasis is a chronic disease that can be difficult to treat, and treatment compliance often is poor. Multiple topical agents often are needed for adequate disease control, and adherence can be an even greater hurdle than with monotherapy. Combination products such as calcipotriene–betamethasone dipropionate offer the potential advantage of once-daily application. Adherence to once-daily application regimens for treatment of psoriasis has been shown to be greater than twice-daily application (82% vs 44%).4 However, the vehicle may be an adherence barrier for some patients.
Calcipotriene 0.005%–betamethasone dipropionate 0.064% topical suspension originally was indicated for the treatment of psoriasis of the scalp; however, it is now also indicated for treatment of psoriasis of the body.12 This topical suspension formulation is less messy, which could potentially be more cosmetically appealing and useful for improving treatment adherence.
Overall, the participants in our small pilot study showed a preference for the topical suspension versus the ointment formulation. The difference was substantial but was not statistically significant. This result is consistent with a previous study in which patients were found to prefer solutions that were less greasy compared to messy sticky ointments.10 Although the topical suspension received a higher average rating from participants for how it felt to touch and how it felt under clothing than the ointment and the ointment was rated on average as more greasy (none of these individual items achieved statistical significance), the calcipotriene–betamethasone dipropionate ointment was still rated as slightly appealing overall (Table 2). This result supports the need for physicians to discuss individual patient preferences when choosing the most appropriate vehicle for topical psoriasis treatment.
In our study, participants were found to use less product during treatment with the topical suspension versus the ointment, likely because the topical suspension formulation is thinner and spreads easier; however, participants rated the ease of application of the 2 products equally (Table 2). Ease of application was rated moderately appealing and time to apply was rated slightly to moderately appealing, which is important because patients often cite these factors as barriers to treatment adherence.2,6,13
The occlusive properties of ointment formulations provide moisturization by preventing water loss, a property that can be desirable when treating psoriatic plaques. Unlike the ointment, which was formulated with petrolatum and mineral oil, the calcipotriene–betamethasone dipropionate topical suspension was formulated with hydrogenated castor oil and mineral oil to provide moisturization. Nevertheless, participants found both the ointment and topical suspension to be moderately appealing (median score, 6; P=.94) for moisturization.
Limitations of this pilot study include the small sample size, which restricted the extent of subgroup analyses and the generalizability of our results. The small sample size also may or may not have contributed to the lack of statistical significance in the majority of the outcomes. This study provides pilot data that can be used to define a larger study; however, we do not think that a larger study is needed, as patients can be offered both vehicles in a practical clinical setting and can choose the product that is right for them. The short treatment duration of 3 days for each formulation also is a limitation, as a patient’s preference may change over time with longer use of the product. Treatment efficacy, which was not measured in this study, also could have an effect on patient preference, which could be assessed over a longer treatment period. Additionally, the study drugs may not be representative of all ointments or topical suspensions in their cosmetic appeal.
Conclusion
In this small cohort of plaque psoriasis patients, a calcipotriene–betamethasone dipropionate topical suspension was preferred over an ointment formulation, but in clinical practice it may be best to allow patients to choose the vehicle formulation that is most desirable on an individual basis. The topical suspension provides clinicians with an alternative that not only has the benefits of a combination product but also has been found to be appealing to patients.
1. Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385.
2. Brown KK, Rehmus WE, Kimball AB. Determining the relative importance of patient motivations for nonadherence to topical corticosteroid therapy in psoriasis. J Am Acad Dermatol. 2006;55:607-613.
3. Carroll CL, Feldman SR, Camacho FT, et al. Adherence to topical therapy decreases during the course of an 8-week psoriasis clinical trial: commonly used methods of measuring adherence to topical therapy overestimate actual use. J Am Acad Dermatol. 2004;51:212-216.
4. Zaghloul SS, Goodfield MJ. Objective assessment of compliance with psoriasis treatment. Arch Dermatol. 2004;140:408-414.
5. Richards HL, Fortune DG, O’Sullivan TM, et al. Patients with psoriasis and their compliance with medication. J Am Acad Dermatol. 1999;41:581-583.
6. Fouere S, Adjadj L, Pawin H. How patients experience psoriasis: results from a European survey. J Eur Acad Dermatol Venereol. 2005;19:S2-S6.
7. van de Kerkhof PC, Steegers-Theunissen RP, Kuipers MV. Evaluation of topical drug treatment in psoriasis. Dermatology. 1998;197:31-36.
8. Devaux S, Castela A, Archier E, et al. Adherence to topical treatment in psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):S61-S67.
9. Chan SA, Hussain F, Lawson LG, et al. Factors affecting adherence to treatment of psoriasis: comparing biologic therapy to other modalities. J Dermatolog Treat. 2013;24:64-69.
10. Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
11. Warino L, Balkrishnan R, Feldman SR. Clobetasol propionate for psoriasis: are ointments really more potent? J Drugs Dermatol. 2006;5:527-532.
12. Menter A, Gold LS, Bukhalo M, et al. Calcipotriene plus betamethasone dipropionate topical suspension for the treatment of mild to moderate psoriasis vulgaris on the body: a randomized, double-blind, vehicle-controlled trial. J Drugs Dermatol. 2013;12:92-98.
13. Krueger G, Koo J, Lebwohl M, et al. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol. 2001;137:280-284.
1. Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385.
2. Brown KK, Rehmus WE, Kimball AB. Determining the relative importance of patient motivations for nonadherence to topical corticosteroid therapy in psoriasis. J Am Acad Dermatol. 2006;55:607-613.
3. Carroll CL, Feldman SR, Camacho FT, et al. Adherence to topical therapy decreases during the course of an 8-week psoriasis clinical trial: commonly used methods of measuring adherence to topical therapy overestimate actual use. J Am Acad Dermatol. 2004;51:212-216.
4. Zaghloul SS, Goodfield MJ. Objective assessment of compliance with psoriasis treatment. Arch Dermatol. 2004;140:408-414.
5. Richards HL, Fortune DG, O’Sullivan TM, et al. Patients with psoriasis and their compliance with medication. J Am Acad Dermatol. 1999;41:581-583.
6. Fouere S, Adjadj L, Pawin H. How patients experience psoriasis: results from a European survey. J Eur Acad Dermatol Venereol. 2005;19:S2-S6.
7. van de Kerkhof PC, Steegers-Theunissen RP, Kuipers MV. Evaluation of topical drug treatment in psoriasis. Dermatology. 1998;197:31-36.
8. Devaux S, Castela A, Archier E, et al. Adherence to topical treatment in psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):S61-S67.
9. Chan SA, Hussain F, Lawson LG, et al. Factors affecting adherence to treatment of psoriasis: comparing biologic therapy to other modalities. J Dermatolog Treat. 2013;24:64-69.
10. Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
11. Warino L, Balkrishnan R, Feldman SR. Clobetasol propionate for psoriasis: are ointments really more potent? J Drugs Dermatol. 2006;5:527-532.
12. Menter A, Gold LS, Bukhalo M, et al. Calcipotriene plus betamethasone dipropionate topical suspension for the treatment of mild to moderate psoriasis vulgaris on the body: a randomized, double-blind, vehicle-controlled trial. J Drugs Dermatol. 2013;12:92-98.
13. Krueger G, Koo J, Lebwohl M, et al. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol. 2001;137:280-284.
Practice Points
- Patient preference plays an important role in adherence to treatment regimens in chronic skin diseases such as psoriasis.
- The topical suspension formulation of calcipotriene 0.005%–betamethasone dipropionate 0.064% was preferred by psoriasis patients over the ointment; however, the availability of the 2 formulations provides patients with options and allows them to choose which product they prefer.
Ixekizumab linked to decrease in psoriasis-related sexual difficulties
AMSTERDAM – Patient complaints of psoriasis-related sexual difficulties decreased significantly in response to treatment with the investigational biologic agent ixekizumab in a phase 2 dose-ranging study.
Sexual problems attributed by psoriasis patients to their skin condition are a common, underappreciated, and understudied problem. Most physicians simply don’t ask. But when they do, as was done formally in this study, it turned out that at baseline roughly one-third of the 142 participating subjects with moderate to severe chronic plaque psoriasis reported some degree of sexual problems they believed due specifically to their skin disease, Dr. Lyn Guenther reported at the annual congress of the European Academy of Dermatology and Venereology.
That rate dropped precipitously and in dose-dependent fashion in response to subcutaneous therapy with ixekizumab, a super-potent psoriasis medication directed against interleukin 17A, according to Dr. Guenther, professor of dermatology at the University of Western Ontario, London.
The study entailed double-blind randomization of the 142 participants to subcutaneous injections of ixekizumab at 10, 25, 75, or 150 mg or placebo at 0, 2, 4, 8, 12, and 16 weeks. The standard primary outcome -- the proportion of patients with a PASI 75 improvement at 12 weeks -- has previously been reported as highly positive (N. Engl. J. Med. 2012;366:1190-9).
For her secondary analysis of psoriasis-related sexual difficulties and their response to treatment, Dr. Guenther used as her metric the patients’ response to item 9 on the Dermatology Life Quality Index, which all subjects completed at weeks 0, 8, and 16. Item 9 asks the extent to which the responder’s skin “caused any sexual difficulties” during the past week. The options range from 0 (none at all) to 3 (very much). She categorized a response of 1 or more as evidence of sexual difficulties. And because of the relatively small sample size in this study, she lumped together as the low-dose therapy group those patients assigned to ixekizumab at 10 or 25 mg, with the high-dose group being comprised of patients on 75 or
After 8 weeks on low-dose ixekizumab, the proportion of patients reporting any skin disease-related sexual difficulties within the past week fell from a baseline of 30% to 16%. After 8 weeks of high-dose ixekizumab, the figure was just 7%. Those rates remained unchanged at 16 weeks. In contrast, the placebo group remained unchanged over time, with 32% of patients still reporting sexual difficulties caused by their skin disease at week 8.
The degree to which a patient’s psoriasis improved in response to therapy with the humanized monoclonal antibody was closely related to the reduction in skin-related sexual problems. Among the 76 ixekizumab-treated patients who achieved a PASI 75 response at week 16, the rate of self-reported sexual difficulties within the previous week was 7%. For those with less than a PASI 75 response, the rate was 24%.
An impressively high 38% percent of patients on high-dose ixekizumab achieved a PASI 100 response. Only 5% of them reported any skin-related sexual difficulties at week 8, as did 9% at week 16.
Dr. Guenther also looked at the data restricting the analysis to patients with more severe baseline sexual impairment as defined by a response of 2 or 3 on item 9 of the DLQI. Among patients on high-dose izekizumab, the rate dropped from 10.5% at baseline to 1.8% at week 8 to zero at week 16. For patients on low-dose izekizumab, the progression was 13.8% to 8.8% to 3.5% at week 16. Rates remained unchanged over time in the control group.
Although this phase 2 study was limited in size, Dr. Guenther found much the same thing earlier in the much larger phase 3 PHOENIX 1 and 2 trials of ustekinumab (Stelara), which together featured 1,334 psoriasis patients randomized to the human anti-interkelukin-12/23 monoclonal antibody. In that analysis, the proportion of ustekinumab-treated patients with impaired sexual function as assessed by DLQI item 9 plunged from 22.4% at baseline to 2.7% at week 12, compared with no change in placebo-treated controls. The bigger the PASI improvement, the greater the reduction in psoriasis-related sexual dysfunction (J. Eur. Acad. Dermatol. Venereol. 2011;25:851-7).
The phase 2 ixekizumab study was funded by Eli Lilly. Dr. Guenther is a consultant to the company. Positive primary outcomes in three pivotal phase 3 clinical trials of ixekizumab totalling nearly 3,900 randomized psoriasis patients have since been reported. The company plans to apply for marketing approval of the biologic in the first half of 2015.
AMSTERDAM – Patient complaints of psoriasis-related sexual difficulties decreased significantly in response to treatment with the investigational biologic agent ixekizumab in a phase 2 dose-ranging study.
Sexual problems attributed by psoriasis patients to their skin condition are a common, underappreciated, and understudied problem. Most physicians simply don’t ask. But when they do, as was done formally in this study, it turned out that at baseline roughly one-third of the 142 participating subjects with moderate to severe chronic plaque psoriasis reported some degree of sexual problems they believed due specifically to their skin disease, Dr. Lyn Guenther reported at the annual congress of the European Academy of Dermatology and Venereology.
That rate dropped precipitously and in dose-dependent fashion in response to subcutaneous therapy with ixekizumab, a super-potent psoriasis medication directed against interleukin 17A, according to Dr. Guenther, professor of dermatology at the University of Western Ontario, London.
The study entailed double-blind randomization of the 142 participants to subcutaneous injections of ixekizumab at 10, 25, 75, or 150 mg or placebo at 0, 2, 4, 8, 12, and 16 weeks. The standard primary outcome -- the proportion of patients with a PASI 75 improvement at 12 weeks -- has previously been reported as highly positive (N. Engl. J. Med. 2012;366:1190-9).
For her secondary analysis of psoriasis-related sexual difficulties and their response to treatment, Dr. Guenther used as her metric the patients’ response to item 9 on the Dermatology Life Quality Index, which all subjects completed at weeks 0, 8, and 16. Item 9 asks the extent to which the responder’s skin “caused any sexual difficulties” during the past week. The options range from 0 (none at all) to 3 (very much). She categorized a response of 1 or more as evidence of sexual difficulties. And because of the relatively small sample size in this study, she lumped together as the low-dose therapy group those patients assigned to ixekizumab at 10 or 25 mg, with the high-dose group being comprised of patients on 75 or
After 8 weeks on low-dose ixekizumab, the proportion of patients reporting any skin disease-related sexual difficulties within the past week fell from a baseline of 30% to 16%. After 8 weeks of high-dose ixekizumab, the figure was just 7%. Those rates remained unchanged at 16 weeks. In contrast, the placebo group remained unchanged over time, with 32% of patients still reporting sexual difficulties caused by their skin disease at week 8.
The degree to which a patient’s psoriasis improved in response to therapy with the humanized monoclonal antibody was closely related to the reduction in skin-related sexual problems. Among the 76 ixekizumab-treated patients who achieved a PASI 75 response at week 16, the rate of self-reported sexual difficulties within the previous week was 7%. For those with less than a PASI 75 response, the rate was 24%.
An impressively high 38% percent of patients on high-dose ixekizumab achieved a PASI 100 response. Only 5% of them reported any skin-related sexual difficulties at week 8, as did 9% at week 16.
Dr. Guenther also looked at the data restricting the analysis to patients with more severe baseline sexual impairment as defined by a response of 2 or 3 on item 9 of the DLQI. Among patients on high-dose izekizumab, the rate dropped from 10.5% at baseline to 1.8% at week 8 to zero at week 16. For patients on low-dose izekizumab, the progression was 13.8% to 8.8% to 3.5% at week 16. Rates remained unchanged over time in the control group.
Although this phase 2 study was limited in size, Dr. Guenther found much the same thing earlier in the much larger phase 3 PHOENIX 1 and 2 trials of ustekinumab (Stelara), which together featured 1,334 psoriasis patients randomized to the human anti-interkelukin-12/23 monoclonal antibody. In that analysis, the proportion of ustekinumab-treated patients with impaired sexual function as assessed by DLQI item 9 plunged from 22.4% at baseline to 2.7% at week 12, compared with no change in placebo-treated controls. The bigger the PASI improvement, the greater the reduction in psoriasis-related sexual dysfunction (J. Eur. Acad. Dermatol. Venereol. 2011;25:851-7).
The phase 2 ixekizumab study was funded by Eli Lilly. Dr. Guenther is a consultant to the company. Positive primary outcomes in three pivotal phase 3 clinical trials of ixekizumab totalling nearly 3,900 randomized psoriasis patients have since been reported. The company plans to apply for marketing approval of the biologic in the first half of 2015.
AMSTERDAM – Patient complaints of psoriasis-related sexual difficulties decreased significantly in response to treatment with the investigational biologic agent ixekizumab in a phase 2 dose-ranging study.
Sexual problems attributed by psoriasis patients to their skin condition are a common, underappreciated, and understudied problem. Most physicians simply don’t ask. But when they do, as was done formally in this study, it turned out that at baseline roughly one-third of the 142 participating subjects with moderate to severe chronic plaque psoriasis reported some degree of sexual problems they believed due specifically to their skin disease, Dr. Lyn Guenther reported at the annual congress of the European Academy of Dermatology and Venereology.
That rate dropped precipitously and in dose-dependent fashion in response to subcutaneous therapy with ixekizumab, a super-potent psoriasis medication directed against interleukin 17A, according to Dr. Guenther, professor of dermatology at the University of Western Ontario, London.
The study entailed double-blind randomization of the 142 participants to subcutaneous injections of ixekizumab at 10, 25, 75, or 150 mg or placebo at 0, 2, 4, 8, 12, and 16 weeks. The standard primary outcome -- the proportion of patients with a PASI 75 improvement at 12 weeks -- has previously been reported as highly positive (N. Engl. J. Med. 2012;366:1190-9).
For her secondary analysis of psoriasis-related sexual difficulties and their response to treatment, Dr. Guenther used as her metric the patients’ response to item 9 on the Dermatology Life Quality Index, which all subjects completed at weeks 0, 8, and 16. Item 9 asks the extent to which the responder’s skin “caused any sexual difficulties” during the past week. The options range from 0 (none at all) to 3 (very much). She categorized a response of 1 or more as evidence of sexual difficulties. And because of the relatively small sample size in this study, she lumped together as the low-dose therapy group those patients assigned to ixekizumab at 10 or 25 mg, with the high-dose group being comprised of patients on 75 or
After 8 weeks on low-dose ixekizumab, the proportion of patients reporting any skin disease-related sexual difficulties within the past week fell from a baseline of 30% to 16%. After 8 weeks of high-dose ixekizumab, the figure was just 7%. Those rates remained unchanged at 16 weeks. In contrast, the placebo group remained unchanged over time, with 32% of patients still reporting sexual difficulties caused by their skin disease at week 8.
The degree to which a patient’s psoriasis improved in response to therapy with the humanized monoclonal antibody was closely related to the reduction in skin-related sexual problems. Among the 76 ixekizumab-treated patients who achieved a PASI 75 response at week 16, the rate of self-reported sexual difficulties within the previous week was 7%. For those with less than a PASI 75 response, the rate was 24%.
An impressively high 38% percent of patients on high-dose ixekizumab achieved a PASI 100 response. Only 5% of them reported any skin-related sexual difficulties at week 8, as did 9% at week 16.
Dr. Guenther also looked at the data restricting the analysis to patients with more severe baseline sexual impairment as defined by a response of 2 or 3 on item 9 of the DLQI. Among patients on high-dose izekizumab, the rate dropped from 10.5% at baseline to 1.8% at week 8 to zero at week 16. For patients on low-dose izekizumab, the progression was 13.8% to 8.8% to 3.5% at week 16. Rates remained unchanged over time in the control group.
Although this phase 2 study was limited in size, Dr. Guenther found much the same thing earlier in the much larger phase 3 PHOENIX 1 and 2 trials of ustekinumab (Stelara), which together featured 1,334 psoriasis patients randomized to the human anti-interkelukin-12/23 monoclonal antibody. In that analysis, the proportion of ustekinumab-treated patients with impaired sexual function as assessed by DLQI item 9 plunged from 22.4% at baseline to 2.7% at week 12, compared with no change in placebo-treated controls. The bigger the PASI improvement, the greater the reduction in psoriasis-related sexual dysfunction (J. Eur. Acad. Dermatol. Venereol. 2011;25:851-7).
The phase 2 ixekizumab study was funded by Eli Lilly. Dr. Guenther is a consultant to the company. Positive primary outcomes in three pivotal phase 3 clinical trials of ixekizumab totalling nearly 3,900 randomized psoriasis patients have since been reported. The company plans to apply for marketing approval of the biologic in the first half of 2015.
AT THE EADV CONGRESS
Key clinical point: Psoriasis patients have a high rate of sexual problems they attribute to their skin disease, and which decrease with effective psoriasis therapy.
Major finding: The prevalence of self-reported psoriasis-related sexual difficulties within the previous week fell from 32% at baseline to 7% among patients with a PASI 75 response to ixekizumab at week 16.
Data source: This was a double-blind, phase 2, dose-ranging study involving 142 patients with moderate to severe chronic plaque psoriasis randomized to 16 weeks of ixekizumab or placebo.
Disclosures: The study was funded by Eli Lilly. The presenter is a consultant to the company.
Consider phototherapy for certain psoriasis patients
LAS VEGAS – Psoriasis patients who are unresponsive to topical therapy, do not have psoriatic arthritis, and can make regular office visits may be candidates for successful treatment with phototherapy.
A patient with type II skin and extensive plaque psoriasis could be treated with a targeted NB-UVB laser, Dr. Kenneth B. Gordon said at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
However, it’s important to evaluate each patient individually on phototherapy decisions, rather than relying on published protocols, noted Dr. Gordon of Northwestern University Feinberg School of Medicine in Chicago.
The advantages of the NB-UVB laser for psoriasis include a high rate of response (data show more than 75% of patients achieve PASI 75), the ability to avoid treating uninvolved skin, and potential long-term clearing of lesions, he said.
For someone with extensive plaque psoriasis, Dr. Gordon said he would start with 3 treatments per week, at 200 mJ, and increase the fluence by 25 mJ if necessary. “A good response can be expected in 4-6 weeks,” he said.
As for long-term treatment, “I leave it up to the patient but offer long-term maintenance,” said Dr. Gordon. Long-term efficacy of NB-UVB for plaque psoriasis has not been well studied, and safety data are unknown. “It is unclear whether there is a skin cancer risk,” he said. Reducing the number of exposures for a patient on maintenance therapy may be an option, he added.
Short-term treatment with NB-UVB can be effective in some psoriasis patients, such as in cases of an acute flare of guttate psoriasis, Dr. Gordon noted. Psoralen and UV light therapy (PUVA) is another option, he said.
Phototherapy also has a role in managing psoriasis in conjunction with retinoids, Dr. Gordon said. He cited an example of a 59-year-old woman with palmar psoriasis. For this patient, he said he would recommend starting with acitretin for approximately 1 month, if the patient tolerates it. “If the acitretin works by itself, no need to add phototherapy,” he said. However, if UVB is added, account for the photosensitizing agent, he emphasized. “Decrease the starting dose by 25-50 mJ or decrease the skin type by one,” he said.
Some psoriasis patients will not benefit from phototherapy, particularly those with erythroderma, said Dr. Gordon. “If skin is highly inflamed, phototherapy could induce easy burning, Koebnerization,” or other complications, he noted.
Dr. Gordon disclosed that his department at Northwestern derives income from phototherapy, but he personally does not. He also disclosed receiving research grants from and/or serving as a consultant to multiple companies including AbbVie, Amgen, Celgene, Eli Lilly, and Janssen.
LAS VEGAS – Psoriasis patients who are unresponsive to topical therapy, do not have psoriatic arthritis, and can make regular office visits may be candidates for successful treatment with phototherapy.
A patient with type II skin and extensive plaque psoriasis could be treated with a targeted NB-UVB laser, Dr. Kenneth B. Gordon said at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
However, it’s important to evaluate each patient individually on phototherapy decisions, rather than relying on published protocols, noted Dr. Gordon of Northwestern University Feinberg School of Medicine in Chicago.
The advantages of the NB-UVB laser for psoriasis include a high rate of response (data show more than 75% of patients achieve PASI 75), the ability to avoid treating uninvolved skin, and potential long-term clearing of lesions, he said.
For someone with extensive plaque psoriasis, Dr. Gordon said he would start with 3 treatments per week, at 200 mJ, and increase the fluence by 25 mJ if necessary. “A good response can be expected in 4-6 weeks,” he said.
As for long-term treatment, “I leave it up to the patient but offer long-term maintenance,” said Dr. Gordon. Long-term efficacy of NB-UVB for plaque psoriasis has not been well studied, and safety data are unknown. “It is unclear whether there is a skin cancer risk,” he said. Reducing the number of exposures for a patient on maintenance therapy may be an option, he added.
Short-term treatment with NB-UVB can be effective in some psoriasis patients, such as in cases of an acute flare of guttate psoriasis, Dr. Gordon noted. Psoralen and UV light therapy (PUVA) is another option, he said.
Phototherapy also has a role in managing psoriasis in conjunction with retinoids, Dr. Gordon said. He cited an example of a 59-year-old woman with palmar psoriasis. For this patient, he said he would recommend starting with acitretin for approximately 1 month, if the patient tolerates it. “If the acitretin works by itself, no need to add phototherapy,” he said. However, if UVB is added, account for the photosensitizing agent, he emphasized. “Decrease the starting dose by 25-50 mJ or decrease the skin type by one,” he said.
Some psoriasis patients will not benefit from phototherapy, particularly those with erythroderma, said Dr. Gordon. “If skin is highly inflamed, phototherapy could induce easy burning, Koebnerization,” or other complications, he noted.
Dr. Gordon disclosed that his department at Northwestern derives income from phototherapy, but he personally does not. He also disclosed receiving research grants from and/or serving as a consultant to multiple companies including AbbVie, Amgen, Celgene, Eli Lilly, and Janssen.
LAS VEGAS – Psoriasis patients who are unresponsive to topical therapy, do not have psoriatic arthritis, and can make regular office visits may be candidates for successful treatment with phototherapy.
A patient with type II skin and extensive plaque psoriasis could be treated with a targeted NB-UVB laser, Dr. Kenneth B. Gordon said at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
However, it’s important to evaluate each patient individually on phototherapy decisions, rather than relying on published protocols, noted Dr. Gordon of Northwestern University Feinberg School of Medicine in Chicago.
The advantages of the NB-UVB laser for psoriasis include a high rate of response (data show more than 75% of patients achieve PASI 75), the ability to avoid treating uninvolved skin, and potential long-term clearing of lesions, he said.
For someone with extensive plaque psoriasis, Dr. Gordon said he would start with 3 treatments per week, at 200 mJ, and increase the fluence by 25 mJ if necessary. “A good response can be expected in 4-6 weeks,” he said.
As for long-term treatment, “I leave it up to the patient but offer long-term maintenance,” said Dr. Gordon. Long-term efficacy of NB-UVB for plaque psoriasis has not been well studied, and safety data are unknown. “It is unclear whether there is a skin cancer risk,” he said. Reducing the number of exposures for a patient on maintenance therapy may be an option, he added.
Short-term treatment with NB-UVB can be effective in some psoriasis patients, such as in cases of an acute flare of guttate psoriasis, Dr. Gordon noted. Psoralen and UV light therapy (PUVA) is another option, he said.
Phototherapy also has a role in managing psoriasis in conjunction with retinoids, Dr. Gordon said. He cited an example of a 59-year-old woman with palmar psoriasis. For this patient, he said he would recommend starting with acitretin for approximately 1 month, if the patient tolerates it. “If the acitretin works by itself, no need to add phototherapy,” he said. However, if UVB is added, account for the photosensitizing agent, he emphasized. “Decrease the starting dose by 25-50 mJ or decrease the skin type by one,” he said.
Some psoriasis patients will not benefit from phototherapy, particularly those with erythroderma, said Dr. Gordon. “If skin is highly inflamed, phototherapy could induce easy burning, Koebnerization,” or other complications, he noted.
Dr. Gordon disclosed that his department at Northwestern derives income from phototherapy, but he personally does not. He also disclosed receiving research grants from and/or serving as a consultant to multiple companies including AbbVie, Amgen, Celgene, Eli Lilly, and Janssen.
EXPERT ANALYSIS FROM SDEF LAS VEGAS DERMATOLOGY SEMINAR
MACE Update
Over the last several years, we have come to understand that patients with inflammatory diseases may be at higher risk for major adverse cardiovascular events (MACEs) and cardiovascular death. In an article published online on October 28 in the Annals of the Rheumatic Diseases, Ogdie et al reported on a study of the risk for MACEs among patients with psoriatic arthritis (PsA), rheumatoid arthritis (RA), and psoriasis without known PsA compared with the general population after adjusting for traditional cardiovascular risk factors.
The authors performed a population-based longitudinal cohort study (1994-2010) in The Health Improvement Network (THIN), a primary care medical record database in the United Kingdom. Patients aged 18 to 89 years with the applicable diagnoses were included. Up to 10 unexposed controls were selected for each patient with PsA, matched on practice and index date.
The study outcomes included the following: cardiovascular death, myocardial infarction, cerebrovascular accidents, and the composite outcome (MACE). Cox proportional hazards models were utilized to calculate the hazard ratios (HRs) for each of the outcomes after adjusting for traditional risk factors. The authors’ preliminary hypothesis was that there was an interaction between disease status and disease-modifying antirheumatic drug (DMARD) use.
In the analysis, the authors identified individuals with PsA (N=8706), RA (N=41,752), psoriasis (N=138,424), and unexposed controls (N=81,573). After adjusting for traditional risk factors, the risk for MACE was higher in the following populations: patients with PsA not prescribed a DMARD (HR, 1.24; 95% confidence interval [CI], 1.03-1.49), patients with RA (no DMARD: HR, 1.39; 95% CI, 1.28-1.50; DMARD: HR, 1.58; 95% CI, 1.46-1.70), patients with psoriasis not prescribed a DMARD (HR, 1.08; 95% CI, 1.02-1.15), and patients with severe psoriasis (DMARD: HR, 1.42; 95% CI, 1.17-1.73).
The authors concluded that cardiovascular risk should be addressed with all patients affected by psoriasis, PsA, or RA. These results suggest the need for improved screening and management of traditional cardiovascular risk factors in patients with inflammatory diseases.
What’s the issue?
Although there has been literature on MACE in these diseases, existing studies have not examined the risk for incident MACE including myocardial infarction, cerebrovascular accidents, and cardiovascular death in PsA compared with matched internal controls from a population-based perspective after accounting for the presence of traditional cardiovascular risk factors. This study is an additional piece of evidence that supports the need for comprehensive management of these patients. How do you currently address the concern for MACE?
Over the last several years, we have come to understand that patients with inflammatory diseases may be at higher risk for major adverse cardiovascular events (MACEs) and cardiovascular death. In an article published online on October 28 in the Annals of the Rheumatic Diseases, Ogdie et al reported on a study of the risk for MACEs among patients with psoriatic arthritis (PsA), rheumatoid arthritis (RA), and psoriasis without known PsA compared with the general population after adjusting for traditional cardiovascular risk factors.
The authors performed a population-based longitudinal cohort study (1994-2010) in The Health Improvement Network (THIN), a primary care medical record database in the United Kingdom. Patients aged 18 to 89 years with the applicable diagnoses were included. Up to 10 unexposed controls were selected for each patient with PsA, matched on practice and index date.
The study outcomes included the following: cardiovascular death, myocardial infarction, cerebrovascular accidents, and the composite outcome (MACE). Cox proportional hazards models were utilized to calculate the hazard ratios (HRs) for each of the outcomes after adjusting for traditional risk factors. The authors’ preliminary hypothesis was that there was an interaction between disease status and disease-modifying antirheumatic drug (DMARD) use.
In the analysis, the authors identified individuals with PsA (N=8706), RA (N=41,752), psoriasis (N=138,424), and unexposed controls (N=81,573). After adjusting for traditional risk factors, the risk for MACE was higher in the following populations: patients with PsA not prescribed a DMARD (HR, 1.24; 95% confidence interval [CI], 1.03-1.49), patients with RA (no DMARD: HR, 1.39; 95% CI, 1.28-1.50; DMARD: HR, 1.58; 95% CI, 1.46-1.70), patients with psoriasis not prescribed a DMARD (HR, 1.08; 95% CI, 1.02-1.15), and patients with severe psoriasis (DMARD: HR, 1.42; 95% CI, 1.17-1.73).
The authors concluded that cardiovascular risk should be addressed with all patients affected by psoriasis, PsA, or RA. These results suggest the need for improved screening and management of traditional cardiovascular risk factors in patients with inflammatory diseases.
What’s the issue?
Although there has been literature on MACE in these diseases, existing studies have not examined the risk for incident MACE including myocardial infarction, cerebrovascular accidents, and cardiovascular death in PsA compared with matched internal controls from a population-based perspective after accounting for the presence of traditional cardiovascular risk factors. This study is an additional piece of evidence that supports the need for comprehensive management of these patients. How do you currently address the concern for MACE?
Over the last several years, we have come to understand that patients with inflammatory diseases may be at higher risk for major adverse cardiovascular events (MACEs) and cardiovascular death. In an article published online on October 28 in the Annals of the Rheumatic Diseases, Ogdie et al reported on a study of the risk for MACEs among patients with psoriatic arthritis (PsA), rheumatoid arthritis (RA), and psoriasis without known PsA compared with the general population after adjusting for traditional cardiovascular risk factors.
The authors performed a population-based longitudinal cohort study (1994-2010) in The Health Improvement Network (THIN), a primary care medical record database in the United Kingdom. Patients aged 18 to 89 years with the applicable diagnoses were included. Up to 10 unexposed controls were selected for each patient with PsA, matched on practice and index date.
The study outcomes included the following: cardiovascular death, myocardial infarction, cerebrovascular accidents, and the composite outcome (MACE). Cox proportional hazards models were utilized to calculate the hazard ratios (HRs) for each of the outcomes after adjusting for traditional risk factors. The authors’ preliminary hypothesis was that there was an interaction between disease status and disease-modifying antirheumatic drug (DMARD) use.
In the analysis, the authors identified individuals with PsA (N=8706), RA (N=41,752), psoriasis (N=138,424), and unexposed controls (N=81,573). After adjusting for traditional risk factors, the risk for MACE was higher in the following populations: patients with PsA not prescribed a DMARD (HR, 1.24; 95% confidence interval [CI], 1.03-1.49), patients with RA (no DMARD: HR, 1.39; 95% CI, 1.28-1.50; DMARD: HR, 1.58; 95% CI, 1.46-1.70), patients with psoriasis not prescribed a DMARD (HR, 1.08; 95% CI, 1.02-1.15), and patients with severe psoriasis (DMARD: HR, 1.42; 95% CI, 1.17-1.73).
The authors concluded that cardiovascular risk should be addressed with all patients affected by psoriasis, PsA, or RA. These results suggest the need for improved screening and management of traditional cardiovascular risk factors in patients with inflammatory diseases.
What’s the issue?
Although there has been literature on MACE in these diseases, existing studies have not examined the risk for incident MACE including myocardial infarction, cerebrovascular accidents, and cardiovascular death in PsA compared with matched internal controls from a population-based perspective after accounting for the presence of traditional cardiovascular risk factors. This study is an additional piece of evidence that supports the need for comprehensive management of these patients. How do you currently address the concern for MACE?
Most Common Dermatologic Conditions Encountered by Dermatologists and Nondermatologists
Skin diseases are highly prevalent in the United States, affecting an estimated 1 in 3 Americans at any given time.1,2 In 2009 the direct medical costs associated with skin-related diseases, including health services and prescriptions, was approximately $22 billion; the annual total economic burden was estimated to be closer to $96 billion when factoring in the cost of lost productivity and pay for symptom relief.3,4 Effective and efficient management of skin disease is essential to minimizing cost and morbidity. Nondermatologists traditionally have diagnosed the majority of skin diseases.5,6 In particular, primary care physicians commonly manage dermatologic conditions and often are the first health care providers to encounter patients presenting with skin problems. A predicted shortage of dermatologists will likely contribute to an increase in this trend.7,8 Therefore, it is important to adequately prepare nondermatologists to evaluate and treat the skin conditions that they are most likely to encounter in their scope of practice.
Residents, particularly in primary care specialties, often have opportunities to spend 2 to 4 weeks with a dermatologist to learn about skin diseases; however, the skin conditions most often encountered by dermatologists may differ from those most often encountered by physicians in other specialties. For instance, one study demonstrated a disparity between the most common skin problems seen by dermatologists and internists.9 These dissimilarities should be recognized and addressed in curriculum content. The purpose of this study was to identify and compare the 20 most common dermatologic conditions reported by dermatologists versus those reported by nondermatologists (ie, internists, pediatricians, family physicians, emergency medicine physicians, general surgeons, otolaryngologists) from 2001 to 2010. Data also were analyzed to determine the top 20 conditions referred to dermatologists by nondermatologists as a potential indicator for areas of further improvement within medical education. With this knowledge, we hope educational curricula and self-study can be modified to reflect the current epidemiology of cutaneous diseases, thereby improving patient care.
Methods
Data from 2001 to 2010 were extracted from the National Ambulatory Medical Care Survey (NAMCS), which is an ongoing survey conducted by the National Center for Health Statistics. The NAMCS collects descriptive data regarding ambulatory visits to nonfederal office-based physicians in the United States. Participating physicians are instructed to record information about patient visits for a 1-week period, including patient demographics, insurance status, reason for visit, diagnoses, procedures, therapeutics, and referrals made at that time. Data collected for the NAMCS are entered into a multistage probability sample to produce national estimates. Within dermatology, an average of 118 dermatologists are sampled each year, and over the last 10 years, participation rates have ranged from 47% to 77%.
International Classification of Diseases, Ninth Revision, Clinical Modification codes were identified to determine the diagnoses that could be classified as dermatologic conditions. Select infectious and neoplastic disorders of the skin and mucous membrane conditions were included as well as the codes for skin diseases. Nondermatologic diagnoses and V codes were not included in the study. Data for all providers were studied to identify outpatient visits associated with the primary diagnosis of a dermatologic condition. Minor diagnoses that were considered to be subsets of major diagnoses were combined to allow better analysis of the data. For example, all tinea infections (ie, dermatophytosis of various sites, dermatomycosis unspecified) were combined into 1 diagnosis referred to as tinea because the recognition and treatment of this disease does not vary tremendously by anatomic location. Visits to dermatologists that listed nonspecific diagnoses and codes (eg, other postsurgical status [V45.89], neoplasm of uncertain behavior site unspecified [238.9]) were assumed to be for dermatologic problems.
Sampling weights were applied to obtain estimates for the number of each diagnosis made nationally. All data analyses were performed using SAS software and linear regression models were generated using SAS PROC SURVEYREG.
Data were analyzed to determine the dermatologic conditions most commonly encountered by dermatologists and nondermatologists in emergency medicine, family medicine, general surgery, internal medicine, otolaryngology, and pediatrics; these specialties include physicians who are known to commonly diagnose and treat skin diseases.10 Data also were analyzed to determine the most common conditions referred to dermatologists for treatment by nondermatologists from the selected specialties. Permission to conduct this study was obtained from the Wake Forest University institutional review board (Winston-Salem, North Carolina).
Results
From 2001 to 2010, more than 700 million outpatient visits for skin-related problems were identified, with 676.3 million visits to dermatologists, emergency medicine physicians, family practitioners, general surgeons, internists, otolaryngologists, and pediatricians. More than half (52.9%) of all skin-related visits were addressed by nondermatologists during this time. Among nondermatologists, family practitioners encountered the greatest number of skin diseases (20.5%), followed by pediatricians (11.3%), internists (9.2%), general surgeons (3.4%), otolaryngologists (1.0%), and emergency medicine physicians (0.2%)(Table 1).
Benign tumors and acne were the most common cutaneous conditions referred to dermatologists by nondermatologists (10.6% and 10.1% of all dermatology referrals, respectively), followed by nonmelanoma skin cancers (9.7%), contact dermatitis (8.8%), and actinic keratosis (7.8%)(Table 2). The top 20 conditions referred to dermatologists accounted for 83.7% of all outpatient referrals to dermatologists.
Among the diseases most frequently reported by nondermatologists, contact dermatitis was the most common (12.0%), with twice the number of visits to nondermatologists for contact dermatitis than to dermatologists (51.6 million vs 25.3 million). In terms of disease categories, infectious skin diseases (ie, bacterial [cellulitis/abscess], viral [warts, herpesvirus], fungal [tinea] and yeast [candida] etiologies) were the most common dermatologic conditions reported by nondermatologists (Table 2).
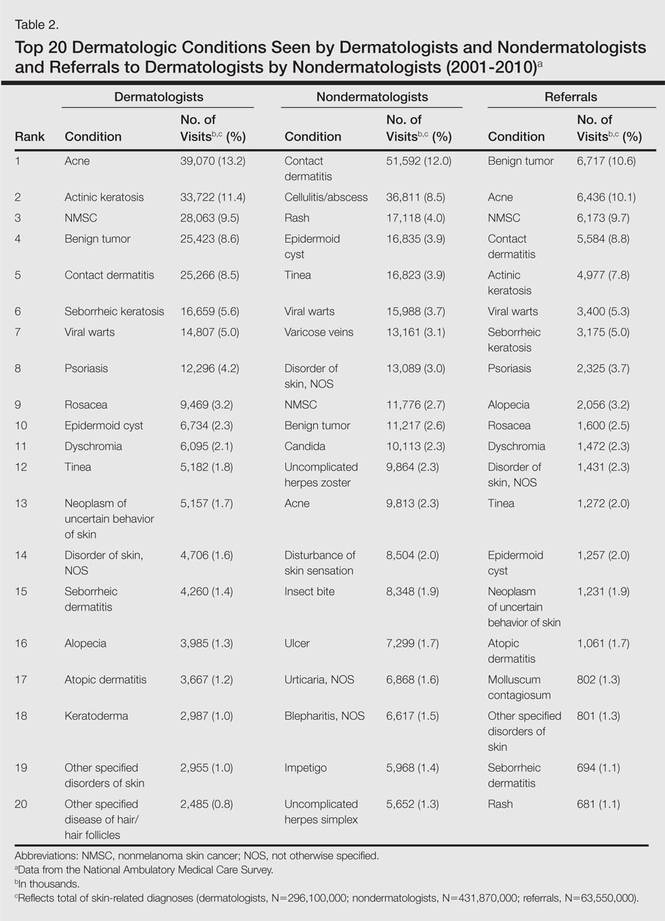
The top 20 dermatologic conditions reported by dermatologists accounted for 85.4% of all diagnoses made by dermatologists. Diseases that were among the top 20 conditions encountered by dermatologists but were not among the top 20 for nondermatologists included actinic keratosis, seborrheic keratosis, atopic dermatitis, psoriasis, alopecia, rosacea, dyschromia, seborrheic dermatitis, follicular disease, and neoplasm of uncertain behavior of skin. Additionally, 5 of the top 20 conditions encountered by dermatologists also were among the top 20 for only 1 individual nondermatologic specialty; these included atopic dermatitis (pediatrics), seborrheic dermatitis (pediatrics), psoriasis (internal medicine), rosacea (otolaryngology), and keratoderma (general surgery). Seborrheic dermatitis, psoriasis, and rosacea also were among the top 20 conditions most commonly referred to dermatologists for treatment by nondermatologists. Table 3 shows the top 20 dermatologic conditions encountered by nondermatologists by comparison.
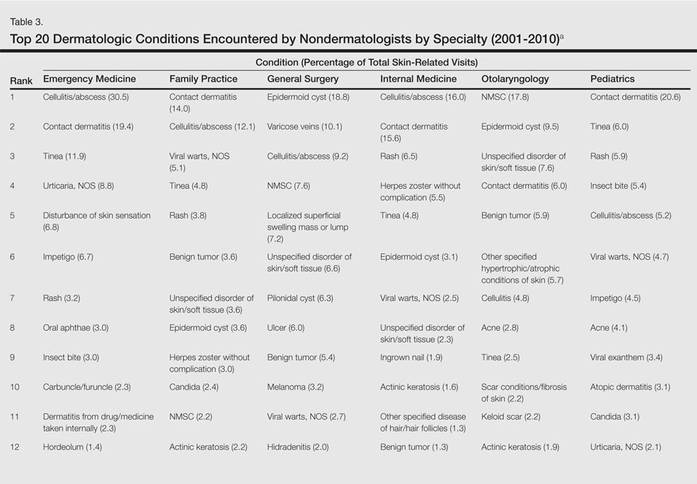
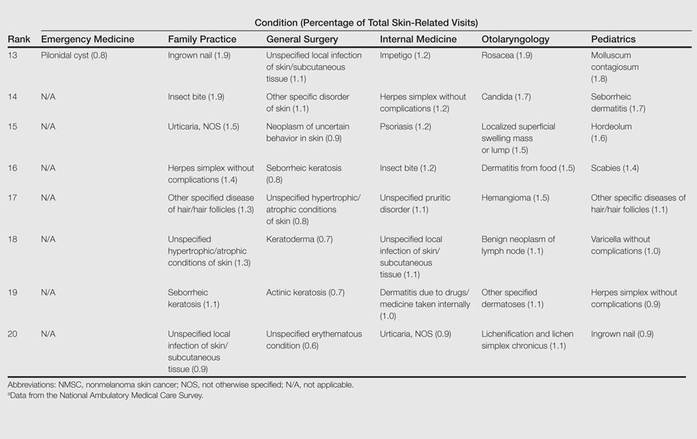
Comment
According to NAMCS data from 2001 to 2010, visits to nondermatologists accounted for more than half of total outpatient visits for cutaneous diseases in the United States, whereas visits to dermatologists accounted for 47.1%. These findings are consistent with historical data indicating that 30% to 40% of skin-related visits are to dermatologists, and the majority of patients with skin disease are diagnosed by nondermatologists.5,6
Past data indicate that most visits to dermatologists were for evaluation of acne, infections, psoriasis, and neoplasms, whereas most visits to nondermatologists were for evaluation of epidermoid cysts, impetigo, plant dermatitis, cellulitis, and diaper rash.9 Over the last 10 years, acne has been more commonly encountered by nondermatologists, especially pediatricians. Additionally, infectious etiologies have been seen in larger volume by nondermatologists.9 Together, infectious cutaneous conditions make up nearly one-fourth of dermatologic encounters by emergency medicine physicians, internists, and family practitioners but are not within the top 20 diagnoses referred to dermatologists, which suggests that uncomplicated cases of cellulitis, herpes zoster, and other skin-related infections are largely managed by nondermatologists.5,6 Contact dermatitis, often caused by specific allergens such as detergents, solvents, and topical products, was one of the most common reported dermatologic encounters among dermatologists and nondermatologists and also was the fourth most common condition referred to dermatologists by nondermatologists for treatment; however, there may be an element of overuse of the International Classification of Diseases, Ninth Revision code, as any presumed contact dermatitis of unspecified cause can be reported under 692.9 defined as contact dermatitis and other eczema, unspecified cause. The high rate of referrals to dermatologists by nondermatologists may be for patch testing and further management. Additionally, there are no specific codes for allergic or irritant dermatitis, thus these diseases may be lumped together.
Although nearly half of all dermatologic encounters were seen by nondermatologists, dermatologists see a much larger proportion of patients with skin disease than nondermatologists and nondermatologists often have limited exposure to the field of dermatology during residency training. Studies have demonstrated differences in the abilities of dermatologists and nondermatologists to correctly diagnose common cutaneous diseases, which unsurprisingly revealed greater diagnostic accuracy demonstrated by dermatologists.11-16 The increase in acne and skin-related infections reported by nondermatologists is consistent with possible efforts to increase formal training in frequently encountered skin diseases. In one study evaluating the impact of a formal 3-week dermatology curriculum on an internal medicine department, internists demonstrated 100% accuracy in the diagnosis of acne and herpes zoster in contrast to 29% for tinea and 12% for lichen planus.5,6
The current Accreditation Council for Graduate Medical Education guidelines place little emphasis on exposure to dermatology training during residency for internists and pediatricians, as this training is not a required component of these programs.17 Two core problems with current training regarding the evaluation and management of cutaneous disease are minimal exposure to dermatologic conditions in medical school and residency and lack of consensus on the core topics that should be taught to nondermatologists.18 Exposure to dermatologic conditions through rotations in medical school has been shown to increase residents’ self-reported confidence in diagnosing and treating alopecia, cutaneous drug eruptions, warts, acne, rosacea, nonmelanoma skin cancers, sun damage, psoriasis, seborrhea, atopic dermatitis, and contact dermatitis; however, the majority of primary care residents surveyed still felt that this exposure in medical school was inadequate.19
In creating a core curriculum for dermatology training for nondermatologists, it is important to consider the dermatologic conditions that are most frequently encountered by these specialties. Our study revealed that the most commonly encountered dermatologic conditions differ among dermatologists and nondermatologists, with a fair degree of variation even among individual specialties. Failure to recognize these discrepancies has likely contributed to the challenges faced by nondermatologists in the diagnosis and management of dermatologic disease. In this study, contact dermatitis, epidermoid cysts, and skin infections were the most common dermatologic conditions encountered by nondermatologists and also were among the top skin diseases referred to dermatologists by nondermatologists. This finding suggests that nondermatologists are able to identify these conditions but have a tendency to refer approximately 10% of these patients to dermatology for further management. Clinical evaluation and medical management of these cutaneous diseases may be an important area of focus for medical school curricula, as the treatment of these diseases is within the capabilities of the nondermatologist. For example, initial management of dermatitis requires determination of the type of dermatitis (ie, essential, contact, atopic, seborrheic, stasis) and selection of an appropriate topical steroid, with referral to a dermatologist needed for questionable or refractory cases. Although a curriculum cannot be built solely on a list of the top 20 diagnoses provided here, these data may serve as a preliminary platform for medical school dermatology curriculum design. The curriculum also should include serious skin diseases, such as melanoma and severe drug eruptions. Although these conditions are less commonly encountered by nondermatologists, missed diagnosis and/or improper management can be life threatening.
The use of NAMCS data presents a few limitations. For instance, these data only represent outpatient management of skin disease. There is the potential for misdiagnosis and coding errors by the reporting physicians. The volume of data (ie, billions of office visits) prevents verification of diagnostic accuracy. The coding system requires physicians to give a diagnosis but does not provide any means by which to determine the physician’s confidence in that diagnosis. There is no code for “uncertain” or “diagnosis not determined.” Additionally, an “unspecified” diagnosis may reflect uncertainty or may simply imply that no other code accurately described the condition. Despite these limitations, the NAMCS database is a large, nationally representative survey of actual patient visits and represents some of the best data available for a study such as ours.
Conclusion
This study provides an important analysis of the most common outpatient dermatologic conditions encountered by dermatologists and nondermatologists of various specialties and offers a foundation from which to construct curricula for dermatology training tailored to individual specialties based on their needs. In the future, identification of the most common inpatient dermatologic conditions managed by each specialty also may benefit curriculum design.
- Thorpe KE, Florence CS, Joski P. Which medical conditions account for the rise in health care spending? Health Aff (Millwood). 2004;(suppl web exclusives):W4-437-445.
- Johnson ML. Defining the burden of skin disease in the United States—a historical perspective. J Investig Dermatol Symp Proc. 2004;9:108-110.
- Agency for Healthcare Research and Quality. Medical expenditure panel survey. US Department of Health & Human Services Web site. http://meps.ahrq.gov. Accessed November 17, 2014.
- Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490-500.
- Johnson ML. On teaching dermatology to nondermatologists. Arch Dermatol. 1994;130:850-852.
- Ramsay DL, Weary PE. Primary care in dermatology: whose role should it be? J Am Acad Dermatol. 1996;35:1005-1008.
- Kimball AB, Resneck JS Jr. The US dermatology workforce: a specialty remains in shortage. J Am Acad Dermatol. 2008;59:741-745.
- Resneck JS Jr, Kimball AB. Who else is providing care in dermatology practices? trends in the use of nonphysician clinicians. J Am Acad Dermatol. 2008;58:211-216.
- Feldman SR, Fleischer AB Jr, McConnell RC. Most common dermatologic problems identified by internists, 1990-1994. Arch Intern Med. 1998;158:726-730.
- Ahn CS, Davis SA, Debade TS, et al. Noncosmetic skin-related procedures performed in the United States: an analysis of national ambulatory medical care survey data from 1995 to 2010. Dermatol Surg. 2013;39:1912-1921.
- Antic M, Conen D, Itin PH. Teaching effects of dermatological consultations on nondermatologists in the field of internal medicine. a study of 1290 inpatients. Dermatology. 2004;208:32-37.
- Federman DG, Concato J, Kirsner RS. Comparison of dermatologic diagnoses by primary care practitioners and dermatologists. a review of the literature. Arch Fam Med. 1999;8:170-172.
- Fleischer AB Jr, Herbert CR, Feldman SR, et al. Diagnosis of skin disease by nondermatologists. Am J Manag Care. 2000;6:1149-1156.
- Kirsner RS, Federman DG. Lack of correlation between internists’ ability in dermatology and their patterns of treating patients with skin disease. Arch Dermatol. 1996;132:1043-1046.
- McCarthy GM, Lamb GC, Russell TJ, et al. Primary care-based dermatology practice: internists need more training. J Gen Intern Med. 1991;6:52-56.
- Sellheyer K, Bergfeld WF. A retrospective biopsy study of the clinical diagnostic accuracy of common skin diseases by different specialties compared with dermatology. J Am Acad Dermatol. 2005;52:823-830.
- Medical specialties. Accreditation Council for Graduate Medical Education Web site. http://www.acgme.org/acgmeweb/tabid/368ProgramandInstitutionalGuidelines/MedicalAccreditation.aspx. Accessed November 17, 2014.
- McCleskey PE, Gilson RT, DeVillez RL. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.
- Hansra NK, O’Sullivan P, Chen CL, et al. Medical school dermatology curriculum: are we adequately preparing primary care physicians? J Am Acad Dermatol. 2009;61:23-29.
Skin diseases are highly prevalent in the United States, affecting an estimated 1 in 3 Americans at any given time.1,2 In 2009 the direct medical costs associated with skin-related diseases, including health services and prescriptions, was approximately $22 billion; the annual total economic burden was estimated to be closer to $96 billion when factoring in the cost of lost productivity and pay for symptom relief.3,4 Effective and efficient management of skin disease is essential to minimizing cost and morbidity. Nondermatologists traditionally have diagnosed the majority of skin diseases.5,6 In particular, primary care physicians commonly manage dermatologic conditions and often are the first health care providers to encounter patients presenting with skin problems. A predicted shortage of dermatologists will likely contribute to an increase in this trend.7,8 Therefore, it is important to adequately prepare nondermatologists to evaluate and treat the skin conditions that they are most likely to encounter in their scope of practice.
Residents, particularly in primary care specialties, often have opportunities to spend 2 to 4 weeks with a dermatologist to learn about skin diseases; however, the skin conditions most often encountered by dermatologists may differ from those most often encountered by physicians in other specialties. For instance, one study demonstrated a disparity between the most common skin problems seen by dermatologists and internists.9 These dissimilarities should be recognized and addressed in curriculum content. The purpose of this study was to identify and compare the 20 most common dermatologic conditions reported by dermatologists versus those reported by nondermatologists (ie, internists, pediatricians, family physicians, emergency medicine physicians, general surgeons, otolaryngologists) from 2001 to 2010. Data also were analyzed to determine the top 20 conditions referred to dermatologists by nondermatologists as a potential indicator for areas of further improvement within medical education. With this knowledge, we hope educational curricula and self-study can be modified to reflect the current epidemiology of cutaneous diseases, thereby improving patient care.
Methods
Data from 2001 to 2010 were extracted from the National Ambulatory Medical Care Survey (NAMCS), which is an ongoing survey conducted by the National Center for Health Statistics. The NAMCS collects descriptive data regarding ambulatory visits to nonfederal office-based physicians in the United States. Participating physicians are instructed to record information about patient visits for a 1-week period, including patient demographics, insurance status, reason for visit, diagnoses, procedures, therapeutics, and referrals made at that time. Data collected for the NAMCS are entered into a multistage probability sample to produce national estimates. Within dermatology, an average of 118 dermatologists are sampled each year, and over the last 10 years, participation rates have ranged from 47% to 77%.
International Classification of Diseases, Ninth Revision, Clinical Modification codes were identified to determine the diagnoses that could be classified as dermatologic conditions. Select infectious and neoplastic disorders of the skin and mucous membrane conditions were included as well as the codes for skin diseases. Nondermatologic diagnoses and V codes were not included in the study. Data for all providers were studied to identify outpatient visits associated with the primary diagnosis of a dermatologic condition. Minor diagnoses that were considered to be subsets of major diagnoses were combined to allow better analysis of the data. For example, all tinea infections (ie, dermatophytosis of various sites, dermatomycosis unspecified) were combined into 1 diagnosis referred to as tinea because the recognition and treatment of this disease does not vary tremendously by anatomic location. Visits to dermatologists that listed nonspecific diagnoses and codes (eg, other postsurgical status [V45.89], neoplasm of uncertain behavior site unspecified [238.9]) were assumed to be for dermatologic problems.
Sampling weights were applied to obtain estimates for the number of each diagnosis made nationally. All data analyses were performed using SAS software and linear regression models were generated using SAS PROC SURVEYREG.
Data were analyzed to determine the dermatologic conditions most commonly encountered by dermatologists and nondermatologists in emergency medicine, family medicine, general surgery, internal medicine, otolaryngology, and pediatrics; these specialties include physicians who are known to commonly diagnose and treat skin diseases.10 Data also were analyzed to determine the most common conditions referred to dermatologists for treatment by nondermatologists from the selected specialties. Permission to conduct this study was obtained from the Wake Forest University institutional review board (Winston-Salem, North Carolina).
Results
From 2001 to 2010, more than 700 million outpatient visits for skin-related problems were identified, with 676.3 million visits to dermatologists, emergency medicine physicians, family practitioners, general surgeons, internists, otolaryngologists, and pediatricians. More than half (52.9%) of all skin-related visits were addressed by nondermatologists during this time. Among nondermatologists, family practitioners encountered the greatest number of skin diseases (20.5%), followed by pediatricians (11.3%), internists (9.2%), general surgeons (3.4%), otolaryngologists (1.0%), and emergency medicine physicians (0.2%)(Table 1).

Benign tumors and acne were the most common cutaneous conditions referred to dermatologists by nondermatologists (10.6% and 10.1% of all dermatology referrals, respectively), followed by nonmelanoma skin cancers (9.7%), contact dermatitis (8.8%), and actinic keratosis (7.8%)(Table 2). The top 20 conditions referred to dermatologists accounted for 83.7% of all outpatient referrals to dermatologists.
Among the diseases most frequently reported by nondermatologists, contact dermatitis was the most common (12.0%), with twice the number of visits to nondermatologists for contact dermatitis than to dermatologists (51.6 million vs 25.3 million). In terms of disease categories, infectious skin diseases (ie, bacterial [cellulitis/abscess], viral [warts, herpesvirus], fungal [tinea] and yeast [candida] etiologies) were the most common dermatologic conditions reported by nondermatologists (Table 2).

The top 20 dermatologic conditions reported by dermatologists accounted for 85.4% of all diagnoses made by dermatologists. Diseases that were among the top 20 conditions encountered by dermatologists but were not among the top 20 for nondermatologists included actinic keratosis, seborrheic keratosis, atopic dermatitis, psoriasis, alopecia, rosacea, dyschromia, seborrheic dermatitis, follicular disease, and neoplasm of uncertain behavior of skin. Additionally, 5 of the top 20 conditions encountered by dermatologists also were among the top 20 for only 1 individual nondermatologic specialty; these included atopic dermatitis (pediatrics), seborrheic dermatitis (pediatrics), psoriasis (internal medicine), rosacea (otolaryngology), and keratoderma (general surgery). Seborrheic dermatitis, psoriasis, and rosacea also were among the top 20 conditions most commonly referred to dermatologists for treatment by nondermatologists. Table 3 shows the top 20 dermatologic conditions encountered by nondermatologists by comparison.


Comment
According to NAMCS data from 2001 to 2010, visits to nondermatologists accounted for more than half of total outpatient visits for cutaneous diseases in the United States, whereas visits to dermatologists accounted for 47.1%. These findings are consistent with historical data indicating that 30% to 40% of skin-related visits are to dermatologists, and the majority of patients with skin disease are diagnosed by nondermatologists.5,6
Past data indicate that most visits to dermatologists were for evaluation of acne, infections, psoriasis, and neoplasms, whereas most visits to nondermatologists were for evaluation of epidermoid cysts, impetigo, plant dermatitis, cellulitis, and diaper rash.9 Over the last 10 years, acne has been more commonly encountered by nondermatologists, especially pediatricians. Additionally, infectious etiologies have been seen in larger volume by nondermatologists.9 Together, infectious cutaneous conditions make up nearly one-fourth of dermatologic encounters by emergency medicine physicians, internists, and family practitioners but are not within the top 20 diagnoses referred to dermatologists, which suggests that uncomplicated cases of cellulitis, herpes zoster, and other skin-related infections are largely managed by nondermatologists.5,6 Contact dermatitis, often caused by specific allergens such as detergents, solvents, and topical products, was one of the most common reported dermatologic encounters among dermatologists and nondermatologists and also was the fourth most common condition referred to dermatologists by nondermatologists for treatment; however, there may be an element of overuse of the International Classification of Diseases, Ninth Revision code, as any presumed contact dermatitis of unspecified cause can be reported under 692.9 defined as contact dermatitis and other eczema, unspecified cause. The high rate of referrals to dermatologists by nondermatologists may be for patch testing and further management. Additionally, there are no specific codes for allergic or irritant dermatitis, thus these diseases may be lumped together.
Although nearly half of all dermatologic encounters were seen by nondermatologists, dermatologists see a much larger proportion of patients with skin disease than nondermatologists and nondermatologists often have limited exposure to the field of dermatology during residency training. Studies have demonstrated differences in the abilities of dermatologists and nondermatologists to correctly diagnose common cutaneous diseases, which unsurprisingly revealed greater diagnostic accuracy demonstrated by dermatologists.11-16 The increase in acne and skin-related infections reported by nondermatologists is consistent with possible efforts to increase formal training in frequently encountered skin diseases. In one study evaluating the impact of a formal 3-week dermatology curriculum on an internal medicine department, internists demonstrated 100% accuracy in the diagnosis of acne and herpes zoster in contrast to 29% for tinea and 12% for lichen planus.5,6
The current Accreditation Council for Graduate Medical Education guidelines place little emphasis on exposure to dermatology training during residency for internists and pediatricians, as this training is not a required component of these programs.17 Two core problems with current training regarding the evaluation and management of cutaneous disease are minimal exposure to dermatologic conditions in medical school and residency and lack of consensus on the core topics that should be taught to nondermatologists.18 Exposure to dermatologic conditions through rotations in medical school has been shown to increase residents’ self-reported confidence in diagnosing and treating alopecia, cutaneous drug eruptions, warts, acne, rosacea, nonmelanoma skin cancers, sun damage, psoriasis, seborrhea, atopic dermatitis, and contact dermatitis; however, the majority of primary care residents surveyed still felt that this exposure in medical school was inadequate.19
In creating a core curriculum for dermatology training for nondermatologists, it is important to consider the dermatologic conditions that are most frequently encountered by these specialties. Our study revealed that the most commonly encountered dermatologic conditions differ among dermatologists and nondermatologists, with a fair degree of variation even among individual specialties. Failure to recognize these discrepancies has likely contributed to the challenges faced by nondermatologists in the diagnosis and management of dermatologic disease. In this study, contact dermatitis, epidermoid cysts, and skin infections were the most common dermatologic conditions encountered by nondermatologists and also were among the top skin diseases referred to dermatologists by nondermatologists. This finding suggests that nondermatologists are able to identify these conditions but have a tendency to refer approximately 10% of these patients to dermatology for further management. Clinical evaluation and medical management of these cutaneous diseases may be an important area of focus for medical school curricula, as the treatment of these diseases is within the capabilities of the nondermatologist. For example, initial management of dermatitis requires determination of the type of dermatitis (ie, essential, contact, atopic, seborrheic, stasis) and selection of an appropriate topical steroid, with referral to a dermatologist needed for questionable or refractory cases. Although a curriculum cannot be built solely on a list of the top 20 diagnoses provided here, these data may serve as a preliminary platform for medical school dermatology curriculum design. The curriculum also should include serious skin diseases, such as melanoma and severe drug eruptions. Although these conditions are less commonly encountered by nondermatologists, missed diagnosis and/or improper management can be life threatening.
The use of NAMCS data presents a few limitations. For instance, these data only represent outpatient management of skin disease. There is the potential for misdiagnosis and coding errors by the reporting physicians. The volume of data (ie, billions of office visits) prevents verification of diagnostic accuracy. The coding system requires physicians to give a diagnosis but does not provide any means by which to determine the physician’s confidence in that diagnosis. There is no code for “uncertain” or “diagnosis not determined.” Additionally, an “unspecified” diagnosis may reflect uncertainty or may simply imply that no other code accurately described the condition. Despite these limitations, the NAMCS database is a large, nationally representative survey of actual patient visits and represents some of the best data available for a study such as ours.
Conclusion
This study provides an important analysis of the most common outpatient dermatologic conditions encountered by dermatologists and nondermatologists of various specialties and offers a foundation from which to construct curricula for dermatology training tailored to individual specialties based on their needs. In the future, identification of the most common inpatient dermatologic conditions managed by each specialty also may benefit curriculum design.
Skin diseases are highly prevalent in the United States, affecting an estimated 1 in 3 Americans at any given time.1,2 In 2009 the direct medical costs associated with skin-related diseases, including health services and prescriptions, was approximately $22 billion; the annual total economic burden was estimated to be closer to $96 billion when factoring in the cost of lost productivity and pay for symptom relief.3,4 Effective and efficient management of skin disease is essential to minimizing cost and morbidity. Nondermatologists traditionally have diagnosed the majority of skin diseases.5,6 In particular, primary care physicians commonly manage dermatologic conditions and often are the first health care providers to encounter patients presenting with skin problems. A predicted shortage of dermatologists will likely contribute to an increase in this trend.7,8 Therefore, it is important to adequately prepare nondermatologists to evaluate and treat the skin conditions that they are most likely to encounter in their scope of practice.
Residents, particularly in primary care specialties, often have opportunities to spend 2 to 4 weeks with a dermatologist to learn about skin diseases; however, the skin conditions most often encountered by dermatologists may differ from those most often encountered by physicians in other specialties. For instance, one study demonstrated a disparity between the most common skin problems seen by dermatologists and internists.9 These dissimilarities should be recognized and addressed in curriculum content. The purpose of this study was to identify and compare the 20 most common dermatologic conditions reported by dermatologists versus those reported by nondermatologists (ie, internists, pediatricians, family physicians, emergency medicine physicians, general surgeons, otolaryngologists) from 2001 to 2010. Data also were analyzed to determine the top 20 conditions referred to dermatologists by nondermatologists as a potential indicator for areas of further improvement within medical education. With this knowledge, we hope educational curricula and self-study can be modified to reflect the current epidemiology of cutaneous diseases, thereby improving patient care.
Methods
Data from 2001 to 2010 were extracted from the National Ambulatory Medical Care Survey (NAMCS), which is an ongoing survey conducted by the National Center for Health Statistics. The NAMCS collects descriptive data regarding ambulatory visits to nonfederal office-based physicians in the United States. Participating physicians are instructed to record information about patient visits for a 1-week period, including patient demographics, insurance status, reason for visit, diagnoses, procedures, therapeutics, and referrals made at that time. Data collected for the NAMCS are entered into a multistage probability sample to produce national estimates. Within dermatology, an average of 118 dermatologists are sampled each year, and over the last 10 years, participation rates have ranged from 47% to 77%.
International Classification of Diseases, Ninth Revision, Clinical Modification codes were identified to determine the diagnoses that could be classified as dermatologic conditions. Select infectious and neoplastic disorders of the skin and mucous membrane conditions were included as well as the codes for skin diseases. Nondermatologic diagnoses and V codes were not included in the study. Data for all providers were studied to identify outpatient visits associated with the primary diagnosis of a dermatologic condition. Minor diagnoses that were considered to be subsets of major diagnoses were combined to allow better analysis of the data. For example, all tinea infections (ie, dermatophytosis of various sites, dermatomycosis unspecified) were combined into 1 diagnosis referred to as tinea because the recognition and treatment of this disease does not vary tremendously by anatomic location. Visits to dermatologists that listed nonspecific diagnoses and codes (eg, other postsurgical status [V45.89], neoplasm of uncertain behavior site unspecified [238.9]) were assumed to be for dermatologic problems.
Sampling weights were applied to obtain estimates for the number of each diagnosis made nationally. All data analyses were performed using SAS software and linear regression models were generated using SAS PROC SURVEYREG.
Data were analyzed to determine the dermatologic conditions most commonly encountered by dermatologists and nondermatologists in emergency medicine, family medicine, general surgery, internal medicine, otolaryngology, and pediatrics; these specialties include physicians who are known to commonly diagnose and treat skin diseases.10 Data also were analyzed to determine the most common conditions referred to dermatologists for treatment by nondermatologists from the selected specialties. Permission to conduct this study was obtained from the Wake Forest University institutional review board (Winston-Salem, North Carolina).
Results
From 2001 to 2010, more than 700 million outpatient visits for skin-related problems were identified, with 676.3 million visits to dermatologists, emergency medicine physicians, family practitioners, general surgeons, internists, otolaryngologists, and pediatricians. More than half (52.9%) of all skin-related visits were addressed by nondermatologists during this time. Among nondermatologists, family practitioners encountered the greatest number of skin diseases (20.5%), followed by pediatricians (11.3%), internists (9.2%), general surgeons (3.4%), otolaryngologists (1.0%), and emergency medicine physicians (0.2%)(Table 1).

Benign tumors and acne were the most common cutaneous conditions referred to dermatologists by nondermatologists (10.6% and 10.1% of all dermatology referrals, respectively), followed by nonmelanoma skin cancers (9.7%), contact dermatitis (8.8%), and actinic keratosis (7.8%)(Table 2). The top 20 conditions referred to dermatologists accounted for 83.7% of all outpatient referrals to dermatologists.
Among the diseases most frequently reported by nondermatologists, contact dermatitis was the most common (12.0%), with twice the number of visits to nondermatologists for contact dermatitis than to dermatologists (51.6 million vs 25.3 million). In terms of disease categories, infectious skin diseases (ie, bacterial [cellulitis/abscess], viral [warts, herpesvirus], fungal [tinea] and yeast [candida] etiologies) were the most common dermatologic conditions reported by nondermatologists (Table 2).

The top 20 dermatologic conditions reported by dermatologists accounted for 85.4% of all diagnoses made by dermatologists. Diseases that were among the top 20 conditions encountered by dermatologists but were not among the top 20 for nondermatologists included actinic keratosis, seborrheic keratosis, atopic dermatitis, psoriasis, alopecia, rosacea, dyschromia, seborrheic dermatitis, follicular disease, and neoplasm of uncertain behavior of skin. Additionally, 5 of the top 20 conditions encountered by dermatologists also were among the top 20 for only 1 individual nondermatologic specialty; these included atopic dermatitis (pediatrics), seborrheic dermatitis (pediatrics), psoriasis (internal medicine), rosacea (otolaryngology), and keratoderma (general surgery). Seborrheic dermatitis, psoriasis, and rosacea also were among the top 20 conditions most commonly referred to dermatologists for treatment by nondermatologists. Table 3 shows the top 20 dermatologic conditions encountered by nondermatologists by comparison.


Comment
According to NAMCS data from 2001 to 2010, visits to nondermatologists accounted for more than half of total outpatient visits for cutaneous diseases in the United States, whereas visits to dermatologists accounted for 47.1%. These findings are consistent with historical data indicating that 30% to 40% of skin-related visits are to dermatologists, and the majority of patients with skin disease are diagnosed by nondermatologists.5,6
Past data indicate that most visits to dermatologists were for evaluation of acne, infections, psoriasis, and neoplasms, whereas most visits to nondermatologists were for evaluation of epidermoid cysts, impetigo, plant dermatitis, cellulitis, and diaper rash.9 Over the last 10 years, acne has been more commonly encountered by nondermatologists, especially pediatricians. Additionally, infectious etiologies have been seen in larger volume by nondermatologists.9 Together, infectious cutaneous conditions make up nearly one-fourth of dermatologic encounters by emergency medicine physicians, internists, and family practitioners but are not within the top 20 diagnoses referred to dermatologists, which suggests that uncomplicated cases of cellulitis, herpes zoster, and other skin-related infections are largely managed by nondermatologists.5,6 Contact dermatitis, often caused by specific allergens such as detergents, solvents, and topical products, was one of the most common reported dermatologic encounters among dermatologists and nondermatologists and also was the fourth most common condition referred to dermatologists by nondermatologists for treatment; however, there may be an element of overuse of the International Classification of Diseases, Ninth Revision code, as any presumed contact dermatitis of unspecified cause can be reported under 692.9 defined as contact dermatitis and other eczema, unspecified cause. The high rate of referrals to dermatologists by nondermatologists may be for patch testing and further management. Additionally, there are no specific codes for allergic or irritant dermatitis, thus these diseases may be lumped together.
Although nearly half of all dermatologic encounters were seen by nondermatologists, dermatologists see a much larger proportion of patients with skin disease than nondermatologists and nondermatologists often have limited exposure to the field of dermatology during residency training. Studies have demonstrated differences in the abilities of dermatologists and nondermatologists to correctly diagnose common cutaneous diseases, which unsurprisingly revealed greater diagnostic accuracy demonstrated by dermatologists.11-16 The increase in acne and skin-related infections reported by nondermatologists is consistent with possible efforts to increase formal training in frequently encountered skin diseases. In one study evaluating the impact of a formal 3-week dermatology curriculum on an internal medicine department, internists demonstrated 100% accuracy in the diagnosis of acne and herpes zoster in contrast to 29% for tinea and 12% for lichen planus.5,6
The current Accreditation Council for Graduate Medical Education guidelines place little emphasis on exposure to dermatology training during residency for internists and pediatricians, as this training is not a required component of these programs.17 Two core problems with current training regarding the evaluation and management of cutaneous disease are minimal exposure to dermatologic conditions in medical school and residency and lack of consensus on the core topics that should be taught to nondermatologists.18 Exposure to dermatologic conditions through rotations in medical school has been shown to increase residents’ self-reported confidence in diagnosing and treating alopecia, cutaneous drug eruptions, warts, acne, rosacea, nonmelanoma skin cancers, sun damage, psoriasis, seborrhea, atopic dermatitis, and contact dermatitis; however, the majority of primary care residents surveyed still felt that this exposure in medical school was inadequate.19
In creating a core curriculum for dermatology training for nondermatologists, it is important to consider the dermatologic conditions that are most frequently encountered by these specialties. Our study revealed that the most commonly encountered dermatologic conditions differ among dermatologists and nondermatologists, with a fair degree of variation even among individual specialties. Failure to recognize these discrepancies has likely contributed to the challenges faced by nondermatologists in the diagnosis and management of dermatologic disease. In this study, contact dermatitis, epidermoid cysts, and skin infections were the most common dermatologic conditions encountered by nondermatologists and also were among the top skin diseases referred to dermatologists by nondermatologists. This finding suggests that nondermatologists are able to identify these conditions but have a tendency to refer approximately 10% of these patients to dermatology for further management. Clinical evaluation and medical management of these cutaneous diseases may be an important area of focus for medical school curricula, as the treatment of these diseases is within the capabilities of the nondermatologist. For example, initial management of dermatitis requires determination of the type of dermatitis (ie, essential, contact, atopic, seborrheic, stasis) and selection of an appropriate topical steroid, with referral to a dermatologist needed for questionable or refractory cases. Although a curriculum cannot be built solely on a list of the top 20 diagnoses provided here, these data may serve as a preliminary platform for medical school dermatology curriculum design. The curriculum also should include serious skin diseases, such as melanoma and severe drug eruptions. Although these conditions are less commonly encountered by nondermatologists, missed diagnosis and/or improper management can be life threatening.
The use of NAMCS data presents a few limitations. For instance, these data only represent outpatient management of skin disease. There is the potential for misdiagnosis and coding errors by the reporting physicians. The volume of data (ie, billions of office visits) prevents verification of diagnostic accuracy. The coding system requires physicians to give a diagnosis but does not provide any means by which to determine the physician’s confidence in that diagnosis. There is no code for “uncertain” or “diagnosis not determined.” Additionally, an “unspecified” diagnosis may reflect uncertainty or may simply imply that no other code accurately described the condition. Despite these limitations, the NAMCS database is a large, nationally representative survey of actual patient visits and represents some of the best data available for a study such as ours.
Conclusion
This study provides an important analysis of the most common outpatient dermatologic conditions encountered by dermatologists and nondermatologists of various specialties and offers a foundation from which to construct curricula for dermatology training tailored to individual specialties based on their needs. In the future, identification of the most common inpatient dermatologic conditions managed by each specialty also may benefit curriculum design.
- Thorpe KE, Florence CS, Joski P. Which medical conditions account for the rise in health care spending? Health Aff (Millwood). 2004;(suppl web exclusives):W4-437-445.
- Johnson ML. Defining the burden of skin disease in the United States—a historical perspective. J Investig Dermatol Symp Proc. 2004;9:108-110.
- Agency for Healthcare Research and Quality. Medical expenditure panel survey. US Department of Health & Human Services Web site. http://meps.ahrq.gov. Accessed November 17, 2014.
- Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490-500.
- Johnson ML. On teaching dermatology to nondermatologists. Arch Dermatol. 1994;130:850-852.
- Ramsay DL, Weary PE. Primary care in dermatology: whose role should it be? J Am Acad Dermatol. 1996;35:1005-1008.
- Kimball AB, Resneck JS Jr. The US dermatology workforce: a specialty remains in shortage. J Am Acad Dermatol. 2008;59:741-745.
- Resneck JS Jr, Kimball AB. Who else is providing care in dermatology practices? trends in the use of nonphysician clinicians. J Am Acad Dermatol. 2008;58:211-216.
- Feldman SR, Fleischer AB Jr, McConnell RC. Most common dermatologic problems identified by internists, 1990-1994. Arch Intern Med. 1998;158:726-730.
- Ahn CS, Davis SA, Debade TS, et al. Noncosmetic skin-related procedures performed in the United States: an analysis of national ambulatory medical care survey data from 1995 to 2010. Dermatol Surg. 2013;39:1912-1921.
- Antic M, Conen D, Itin PH. Teaching effects of dermatological consultations on nondermatologists in the field of internal medicine. a study of 1290 inpatients. Dermatology. 2004;208:32-37.
- Federman DG, Concato J, Kirsner RS. Comparison of dermatologic diagnoses by primary care practitioners and dermatologists. a review of the literature. Arch Fam Med. 1999;8:170-172.
- Fleischer AB Jr, Herbert CR, Feldman SR, et al. Diagnosis of skin disease by nondermatologists. Am J Manag Care. 2000;6:1149-1156.
- Kirsner RS, Federman DG. Lack of correlation between internists’ ability in dermatology and their patterns of treating patients with skin disease. Arch Dermatol. 1996;132:1043-1046.
- McCarthy GM, Lamb GC, Russell TJ, et al. Primary care-based dermatology practice: internists need more training. J Gen Intern Med. 1991;6:52-56.
- Sellheyer K, Bergfeld WF. A retrospective biopsy study of the clinical diagnostic accuracy of common skin diseases by different specialties compared with dermatology. J Am Acad Dermatol. 2005;52:823-830.
- Medical specialties. Accreditation Council for Graduate Medical Education Web site. http://www.acgme.org/acgmeweb/tabid/368ProgramandInstitutionalGuidelines/MedicalAccreditation.aspx. Accessed November 17, 2014.
- McCleskey PE, Gilson RT, DeVillez RL. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.
- Hansra NK, O’Sullivan P, Chen CL, et al. Medical school dermatology curriculum: are we adequately preparing primary care physicians? J Am Acad Dermatol. 2009;61:23-29.
- Thorpe KE, Florence CS, Joski P. Which medical conditions account for the rise in health care spending? Health Aff (Millwood). 2004;(suppl web exclusives):W4-437-445.
- Johnson ML. Defining the burden of skin disease in the United States—a historical perspective. J Investig Dermatol Symp Proc. 2004;9:108-110.
- Agency for Healthcare Research and Quality. Medical expenditure panel survey. US Department of Health & Human Services Web site. http://meps.ahrq.gov. Accessed November 17, 2014.
- Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490-500.
- Johnson ML. On teaching dermatology to nondermatologists. Arch Dermatol. 1994;130:850-852.
- Ramsay DL, Weary PE. Primary care in dermatology: whose role should it be? J Am Acad Dermatol. 1996;35:1005-1008.
- Kimball AB, Resneck JS Jr. The US dermatology workforce: a specialty remains in shortage. J Am Acad Dermatol. 2008;59:741-745.
- Resneck JS Jr, Kimball AB. Who else is providing care in dermatology practices? trends in the use of nonphysician clinicians. J Am Acad Dermatol. 2008;58:211-216.
- Feldman SR, Fleischer AB Jr, McConnell RC. Most common dermatologic problems identified by internists, 1990-1994. Arch Intern Med. 1998;158:726-730.
- Ahn CS, Davis SA, Debade TS, et al. Noncosmetic skin-related procedures performed in the United States: an analysis of national ambulatory medical care survey data from 1995 to 2010. Dermatol Surg. 2013;39:1912-1921.
- Antic M, Conen D, Itin PH. Teaching effects of dermatological consultations on nondermatologists in the field of internal medicine. a study of 1290 inpatients. Dermatology. 2004;208:32-37.
- Federman DG, Concato J, Kirsner RS. Comparison of dermatologic diagnoses by primary care practitioners and dermatologists. a review of the literature. Arch Fam Med. 1999;8:170-172.
- Fleischer AB Jr, Herbert CR, Feldman SR, et al. Diagnosis of skin disease by nondermatologists. Am J Manag Care. 2000;6:1149-1156.
- Kirsner RS, Federman DG. Lack of correlation between internists’ ability in dermatology and their patterns of treating patients with skin disease. Arch Dermatol. 1996;132:1043-1046.
- McCarthy GM, Lamb GC, Russell TJ, et al. Primary care-based dermatology practice: internists need more training. J Gen Intern Med. 1991;6:52-56.
- Sellheyer K, Bergfeld WF. A retrospective biopsy study of the clinical diagnostic accuracy of common skin diseases by different specialties compared with dermatology. J Am Acad Dermatol. 2005;52:823-830.
- Medical specialties. Accreditation Council for Graduate Medical Education Web site. http://www.acgme.org/acgmeweb/tabid/368ProgramandInstitutionalGuidelines/MedicalAccreditation.aspx. Accessed November 17, 2014.
- McCleskey PE, Gilson RT, DeVillez RL. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.
- Hansra NK, O’Sullivan P, Chen CL, et al. Medical school dermatology curriculum: are we adequately preparing primary care physicians? J Am Acad Dermatol. 2009;61:23-29.
Practice Points
- Approximately half of skin-related visits are to nondermatologists, such as family medicine physicians, pediatricians, and internists.
- Skin conditions that most frequently present to nondermatologists are different from those seen by dermatologists.
- Education efforts in nondermatology specialties should be targeted toward the common skin diseases that present to these specialties to maximize the yield of medical education and improve diagnostic accuracy and patient outcomes.
Oral tofacitinib scores against psoriasis in phase III trial
AMSTERDAM – Oral tofacitinib for psoriasis proved as effective as – and in certain domains better than – subcutaneous etanercept in improving patient-reported quality of life endpoints in a phase III clinical trial.
The double-blind study included 1,101 patients with moderate to severe chronic plaque psoriasis who were randomized to 12 weeks of the oral Janus kinase inhibitor tofacitinib at 5 mg twice daily, 10 mg twice daily, subcutaneous etanercept (Enbrel) at 50 mg twice weekly, or placebo. The findings were presented by Dr. Fernando Valenzuela at the annual congress of the European Academy of Dermatology and Venereology.
Earlier in 2014, at the annual meeting of the American Academy of Dermatology in Denver, Dr. Valenzuela presented the phase III study’s primary results, in which the oral small molecule proved noninferior to the tumor necrosis factor inhibitor in improving PASI scores. At the EADV Congress in Amsterdam, he focused on the secondary outcomes, which arguably matter more to patients than do changes in PASI scores, namely, measures of quality of life and itchiness.
From a baseline Itch Severity Item score of 5, indicative of moderate to severe itching, week 12 scores fell by a mean of 3.2, 4.0, 3.5, and 0.4 points, respectively, in patients on low- or high-dose tofacitinib, etanercept, and placebo. The improvement in itch in patients randomized to tofacitinib at 10 mg twice daily was not only greater than with etanercept, it occurred faster as well, with significant reduction in itch scores documented on day 2 of therapy, reported Dr. Valenzuela, a dermatologist at the University of Chile in Santiago.
The median baseline Dermatology Life Quality Index (DLQI) score was 12. Significant improvement was seen from week 4 in all three active treatment study arms. By week 12, DLQI scores had dropped by an average of 7.3 points in patients on tofacitinib at 5 mg twice daily, 9.7 points in those on 10 mg twice daily, 9.0 points in etanercept-treated patients, and 1.9 points with placebo. Seventy-eight percent of patients on tofacitinib at 10 mg twice daily experienced a 5-point or larger drop in the DLQI by week 12, as did 75% of those on etanercept, 66% of those on low-dose tofacitinib, and 32% of those on placebo.
At baseline, 30% of patients rated their psoriasis as moderate and 70% rated their psoriasis as severe based on Patient Global Assessment. By week 12, more than 50% of patients on tofacitinib at 10 mg twice daily or etanercept rated their skin as clear or almost clear.
More than 70% of patients in all three active treatment arms indicated at week 12 that they were satisfied with their medication. Satisfaction scores were highest, and equally so, in those on high-dose tofacitinib and etanercept.
“Patients using tofacitinib at the low dose had not that good an improvement. It was better improvement than with placebo, but it doesn’t look like etanercept,” Dr. Valenzuela concluded.
Tofacitinib is approved as Xeljanz for the treatment of rheumatoid arthritis, but remains investigational for psoriasis. According to a company statement, Pfizer intends to submit a supplemental New Drug Application (sNDA) to the Food and Drug Administration by early 2015.
The study was funded by Pfizer. Dr. Valenzuela is an adviser to Pfizer, AbbVie, Eli Lilly, Janssen, Merck, and Novartis.
AMSTERDAM – Oral tofacitinib for psoriasis proved as effective as – and in certain domains better than – subcutaneous etanercept in improving patient-reported quality of life endpoints in a phase III clinical trial.
The double-blind study included 1,101 patients with moderate to severe chronic plaque psoriasis who were randomized to 12 weeks of the oral Janus kinase inhibitor tofacitinib at 5 mg twice daily, 10 mg twice daily, subcutaneous etanercept (Enbrel) at 50 mg twice weekly, or placebo. The findings were presented by Dr. Fernando Valenzuela at the annual congress of the European Academy of Dermatology and Venereology.
Earlier in 2014, at the annual meeting of the American Academy of Dermatology in Denver, Dr. Valenzuela presented the phase III study’s primary results, in which the oral small molecule proved noninferior to the tumor necrosis factor inhibitor in improving PASI scores. At the EADV Congress in Amsterdam, he focused on the secondary outcomes, which arguably matter more to patients than do changes in PASI scores, namely, measures of quality of life and itchiness.
From a baseline Itch Severity Item score of 5, indicative of moderate to severe itching, week 12 scores fell by a mean of 3.2, 4.0, 3.5, and 0.4 points, respectively, in patients on low- or high-dose tofacitinib, etanercept, and placebo. The improvement in itch in patients randomized to tofacitinib at 10 mg twice daily was not only greater than with etanercept, it occurred faster as well, with significant reduction in itch scores documented on day 2 of therapy, reported Dr. Valenzuela, a dermatologist at the University of Chile in Santiago.
The median baseline Dermatology Life Quality Index (DLQI) score was 12. Significant improvement was seen from week 4 in all three active treatment study arms. By week 12, DLQI scores had dropped by an average of 7.3 points in patients on tofacitinib at 5 mg twice daily, 9.7 points in those on 10 mg twice daily, 9.0 points in etanercept-treated patients, and 1.9 points with placebo. Seventy-eight percent of patients on tofacitinib at 10 mg twice daily experienced a 5-point or larger drop in the DLQI by week 12, as did 75% of those on etanercept, 66% of those on low-dose tofacitinib, and 32% of those on placebo.
At baseline, 30% of patients rated their psoriasis as moderate and 70% rated their psoriasis as severe based on Patient Global Assessment. By week 12, more than 50% of patients on tofacitinib at 10 mg twice daily or etanercept rated their skin as clear or almost clear.
More than 70% of patients in all three active treatment arms indicated at week 12 that they were satisfied with their medication. Satisfaction scores were highest, and equally so, in those on high-dose tofacitinib and etanercept.
“Patients using tofacitinib at the low dose had not that good an improvement. It was better improvement than with placebo, but it doesn’t look like etanercept,” Dr. Valenzuela concluded.
Tofacitinib is approved as Xeljanz for the treatment of rheumatoid arthritis, but remains investigational for psoriasis. According to a company statement, Pfizer intends to submit a supplemental New Drug Application (sNDA) to the Food and Drug Administration by early 2015.
The study was funded by Pfizer. Dr. Valenzuela is an adviser to Pfizer, AbbVie, Eli Lilly, Janssen, Merck, and Novartis.
AMSTERDAM – Oral tofacitinib for psoriasis proved as effective as – and in certain domains better than – subcutaneous etanercept in improving patient-reported quality of life endpoints in a phase III clinical trial.
The double-blind study included 1,101 patients with moderate to severe chronic plaque psoriasis who were randomized to 12 weeks of the oral Janus kinase inhibitor tofacitinib at 5 mg twice daily, 10 mg twice daily, subcutaneous etanercept (Enbrel) at 50 mg twice weekly, or placebo. The findings were presented by Dr. Fernando Valenzuela at the annual congress of the European Academy of Dermatology and Venereology.
Earlier in 2014, at the annual meeting of the American Academy of Dermatology in Denver, Dr. Valenzuela presented the phase III study’s primary results, in which the oral small molecule proved noninferior to the tumor necrosis factor inhibitor in improving PASI scores. At the EADV Congress in Amsterdam, he focused on the secondary outcomes, which arguably matter more to patients than do changes in PASI scores, namely, measures of quality of life and itchiness.
From a baseline Itch Severity Item score of 5, indicative of moderate to severe itching, week 12 scores fell by a mean of 3.2, 4.0, 3.5, and 0.4 points, respectively, in patients on low- or high-dose tofacitinib, etanercept, and placebo. The improvement in itch in patients randomized to tofacitinib at 10 mg twice daily was not only greater than with etanercept, it occurred faster as well, with significant reduction in itch scores documented on day 2 of therapy, reported Dr. Valenzuela, a dermatologist at the University of Chile in Santiago.
The median baseline Dermatology Life Quality Index (DLQI) score was 12. Significant improvement was seen from week 4 in all three active treatment study arms. By week 12, DLQI scores had dropped by an average of 7.3 points in patients on tofacitinib at 5 mg twice daily, 9.7 points in those on 10 mg twice daily, 9.0 points in etanercept-treated patients, and 1.9 points with placebo. Seventy-eight percent of patients on tofacitinib at 10 mg twice daily experienced a 5-point or larger drop in the DLQI by week 12, as did 75% of those on etanercept, 66% of those on low-dose tofacitinib, and 32% of those on placebo.
At baseline, 30% of patients rated their psoriasis as moderate and 70% rated their psoriasis as severe based on Patient Global Assessment. By week 12, more than 50% of patients on tofacitinib at 10 mg twice daily or etanercept rated their skin as clear or almost clear.
More than 70% of patients in all three active treatment arms indicated at week 12 that they were satisfied with their medication. Satisfaction scores were highest, and equally so, in those on high-dose tofacitinib and etanercept.
“Patients using tofacitinib at the low dose had not that good an improvement. It was better improvement than with placebo, but it doesn’t look like etanercept,” Dr. Valenzuela concluded.
Tofacitinib is approved as Xeljanz for the treatment of rheumatoid arthritis, but remains investigational for psoriasis. According to a company statement, Pfizer intends to submit a supplemental New Drug Application (sNDA) to the Food and Drug Administration by early 2015.
The study was funded by Pfizer. Dr. Valenzuela is an adviser to Pfizer, AbbVie, Eli Lilly, Janssen, Merck, and Novartis.
AT THE EADV CONGRESS
Key clinical point: Multiple measures of quality of life and disease burden improved similarly in psoriasis patients regardless of whether they were on subcutaneous etanercept or the oral Janus kinase inhibitor tofacitinib.
Major finding: From a median baseline Dermatology Life Quality Index score of 12, scores improved by an average of 7.3 points after 12 weeks of tofacitinib at 5 mg twice daily, 9.7 points with tofacitinib at 10 mg twice daily, 9.0 points with etanercept at 50 mg twice weekly, and 1.9 points with placebo.
Data source: A phase III randomized, double-blind prospective study involving 1,101 patients with moderate to severe chronic plaque psoriasis.
Disclosures: The presenter is an adviser to Pfizer, which sponsored the study, and other pharmaceutical companies.
Dermatologists counsel psoriasis patients on obesity, less on alcohol and tobacco
Less than half of dermatologists screen or counsel psoriasis patients about alcohol or tobacco use, compared with all patients, but approximately 60% screen or counsel psoriasis patients about obesity, based on data from an online survey of 171 dermatologists and dermatology residents.
The findings were published in a letter to the editor in the November issue of the Journal of the American Academy of Dermatology (2014;71:1028-9).
However, among patients with psoriasis, more than two-thirds of the respondents said they were more likely to both screen and counsel patients with moderate to severe psoriasis about alcohol, tobacco, and obesity, compared with psoriasis patients overall.
Previous research suggests that if psoriasis patients’ issues with alcohol, tobacco, and obesity are addressed, “patients may experience disease improvement, emphasizing the importance of screening and counseling,” wrote Brandon L. Adler and Aimee E. Krausz, medical students at Albert Einstein College of Medicine, Bronx, N.Y., and their colleagues.
Overall, 87% of respondents believed themselves responsible for screening for the three risk factors, but 56% believed themselves responsible for counseling.
Although the results were limited by the focus on academic dermatologists, the findings suggest a need to improve dermatologists’ confidence in counseling patients, the researchers noted. “Systematic training and effective counseling instruments would empower practitioners to translate this knowledge into clinical practice.”
The study was supported by the Dermatology Foundation Career Development Awards Program.
The researchers had no financial conflicts to disclose.
|
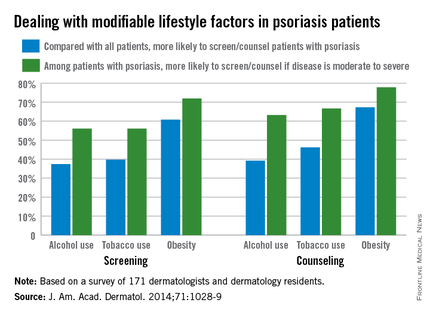
Less than half of dermatologists screen or counsel psoriasis patients about alcohol or tobacco use, compared with all patients, but approximately 60% screen or counsel psoriasis patients about obesity, based on data from an online survey of 171 dermatologists and dermatology residents.
The findings were published in a letter to the editor in the November issue of the Journal of the American Academy of Dermatology (2014;71:1028-9).
However, among patients with psoriasis, more than two-thirds of the respondents said they were more likely to both screen and counsel patients with moderate to severe psoriasis about alcohol, tobacco, and obesity, compared with psoriasis patients overall.
Previous research suggests that if psoriasis patients’ issues with alcohol, tobacco, and obesity are addressed, “patients may experience disease improvement, emphasizing the importance of screening and counseling,” wrote Brandon L. Adler and Aimee E. Krausz, medical students at Albert Einstein College of Medicine, Bronx, N.Y., and their colleagues.
Overall, 87% of respondents believed themselves responsible for screening for the three risk factors, but 56% believed themselves responsible for counseling.
Although the results were limited by the focus on academic dermatologists, the findings suggest a need to improve dermatologists’ confidence in counseling patients, the researchers noted. “Systematic training and effective counseling instruments would empower practitioners to translate this knowledge into clinical practice.”
The study was supported by the Dermatology Foundation Career Development Awards Program.
The researchers had no financial conflicts to disclose.
|

Less than half of dermatologists screen or counsel psoriasis patients about alcohol or tobacco use, compared with all patients, but approximately 60% screen or counsel psoriasis patients about obesity, based on data from an online survey of 171 dermatologists and dermatology residents.
The findings were published in a letter to the editor in the November issue of the Journal of the American Academy of Dermatology (2014;71:1028-9).
However, among patients with psoriasis, more than two-thirds of the respondents said they were more likely to both screen and counsel patients with moderate to severe psoriasis about alcohol, tobacco, and obesity, compared with psoriasis patients overall.
Previous research suggests that if psoriasis patients’ issues with alcohol, tobacco, and obesity are addressed, “patients may experience disease improvement, emphasizing the importance of screening and counseling,” wrote Brandon L. Adler and Aimee E. Krausz, medical students at Albert Einstein College of Medicine, Bronx, N.Y., and their colleagues.
Overall, 87% of respondents believed themselves responsible for screening for the three risk factors, but 56% believed themselves responsible for counseling.
Although the results were limited by the focus on academic dermatologists, the findings suggest a need to improve dermatologists’ confidence in counseling patients, the researchers noted. “Systematic training and effective counseling instruments would empower practitioners to translate this knowledge into clinical practice.”
The study was supported by the Dermatology Foundation Career Development Awards Program.
The researchers had no financial conflicts to disclose.
|

FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY