User login
Puffy fingers improves predictiveness of very early systemic sclerosis diagnosis
The presence of puffy fingers in patients with Raynaud’s phenomenon who are antinuclear antibody positive was further validated as an important sign of possible early systemic sclerosis in a recent study.
The findings support the value of using the European League Against Rheumatism Scleroderma Trial and Research Group’s (EUSTAR’s) new criteria of ANA positivity, Raynaud’s phenomenon, and puffy fingers as three red flags that raise suspicion for very early systemic sclerosis, reported Dr. Tünde Minier of the University of Pécs in Hungary and her colleagues (Ann. Rheum. Dis. 2013 Aug. 12 [doi: 10.1136/annrheumdis-2013-203716]). They calculated that the positive predictive value of ANA positivity for developing systemic sclerosis in patients with Raynaud’s phenomenon increased from 33.9% to 88.5% when combined with the presence of puffy fingers.
The researchers examined 469 patients with Raynaud’s phenomenon who were enrolled in the multicenter, prospective, observational Very Early Diagnosis of Systemic Sclerosis (VEDOSS) study at 33 EUSTAR centers throughout and outside Europe. About a third of the patients were ANA negative (32.2%), and 67.8% were ANA positive. Among the ANA-positive participants, 53.6% had a systemic sclerosis pattern on nailfold capillaroscopy, compared with only 13.4% of the ANA-negative patients (P less than .001).
Just over half of the ANA-negative patients lacked a systemic sclerosis pattern on nailfold capillaroscopy, systemic sclerosis–related specific clinical symptoms, or an erythrocyte sedimentation rate of 25 mm/hr or greater, and so were diagnosed with primary Raynaud’s.
The examinations of the patients also included assessments for digital ulcers, digital pitting scars, telangiectases, calcinosis, tendon friction rubs, esophageal symptoms, and symptoms consistent with median nerve compression syndrome.
The most common clinical features in the ANA-positive patients were previous or current puffy fingers, found in 38.5% of the ANA-positive patients versus 23.3% of the ANA-negative patients, and esophageal symptoms, identified in 35.2% of the ANA-positive patients and 18.4% of the ANA-negative patients.
"Almost 90% of ANA-positive Raynaud’s phenomenon patients with previous or current finger edematous skin changes (puffy fingers) already had a nailfold capillaroscopy systemic sclerosis pattern and/or systemic sclerosis–specific autoantibodies," Dr. Minier’s team wrote. Specifically, 73.3% of ANA-positive patients with Raynaud’s and puffy fingers had a nailfold capillaroscopy systemic sclerosis pattern, compared with only 41.2% of ANA-positive patients without puffy fingers. Overall, 88.5% of ANA-positive patients with Raynaud’s and puffy fingers met the criteria for very early systemic sclerosis.
The ANA-positive patients with puffy fingers were also more likely to have other symptoms than were the ANA-positive patients without puffy fingers. Sclerodactyly was identified in 17.8% of the ANA-positive patients with puffy fingers, compared with 6.2% of the ANA-positive patients without current or previous puffy fingers (P = .002). Telangiectases also appeared on 17.3% of the ANA-positive patients with puffy fingers, compared with 9.2% of those without puffy fingers (P = .033). Similarly, 42.1% of ANA-positive patients with puffy fingers had esophageal symptoms, compared with 30.9% of those without puffy fingers (P = .043).
The researchers also noted that even puffy fingers in ANA-negative patients may require more careful follow-up, because 17% of the ANA-negative patients with current or previous puffy fingers had a nailfold capillaroscopy systemic sclerosis pattern and 20% of the ANA-negative patients with puffy fingers had sclerodactyly with other systemic sclerosis symptoms.
The authors noted that one limitation of their study is the inability to generalize their findings to the broader population of Raynaud’s phenomenon patients seen by general physicians, since a higher percentage of the patients enrolled in this cohort meet very early systemic sclerosis classification.
"Patients identified with the very early diagnostic criteria may also have scleroderma with limited cutaneous involvement or even undifferentiated connective tissue disease," a limitation that longer-term follow-up data may clarify with a comparison of the ACR 1980 classification criteria and the new ACR-EULAR criteria.
The study did not use external funding. The authors had no disclosures.
* This story was updated 8/20/2013.
It is appreciated among rheumatologists that although
Raynaud’s phenomenon is common in the general population, it occurs more
commonly in patients with connective tissue disorders and in particular in those
with scleroderma. Recently, preliminary criteria have been proposed by the European
League Against Rheumatism (EULAR) Scleroderma Trials and Research Group to
identify very early systemic sclerosis. Three red-flag features were identified
as those which would raise suspicion for early scleroderma: Raynaud’s phenomenon,
puffy fingers, and a positive ANA. In Dr. Minier’s study, puffy fingers emerged
as an important feature suggesting a higher likelihood of systemic sclerosis.

|
| Dr. Robert Spiera |
It has already been appreciated that patients with Raynaud’s phenomenon who have a positive ANA have a higher likelihood of developing a
connective tissue disorder. In this cohort study, the presence of puffy fingers
in the past or at the time of evaluation markedly increased a positive
predictive value that scleroderma would be present or develop. Perhaps even
more important was their observation that among patients with Raynaud’s phenomenon
without detectable ANA (in whom the likelihood of a connective tissue disorder
evolving was traditionally felt to be low), if puffy fingers were present,
as many as 20% ultimately developed features suggestive of systemic sclerosis.
Rheumatologists have typically recognized the presence
of puffy fingers as potentially an important clinical finding, indicating a
likelihood of the patient having an inflammatory arthritis, connective tissue
disorder, or scleroderma. Of course there is a differential diagnosis of
puffy hands, including thyroid disease, edema, or other metabolic
abnormalities. Nevertheless, in the context of Raynaud’s phenomenon and
detectable ANA, vigilance for evolution toward a scleroderma spectrum disorder
is warranted.
Ultimately, the balance of disease criteria can be
difficult. More liberal diagnostic criteria allow the recognition and diagnosis
of the disorder in patients earlier in the disease course. That must be
balanced against the downside of establishing a diagnosis of a potentially dangerous
and debilitating rheumatic disease in a patient in whom it might not more fully
evolve. Ultimately, the very diagnosis itself could be a major cause of anxiety
and, indeed, morbidity to those patients.
The finding, however, of the importance of puffy
fingers as a predictive factor in establishing a diagnosis of systemic
sclerosis is important, and would be particularly of value if recognized in the
general medical community in terms of identifying patients worthy of further
investigation or rheumatologic referral.
Dr. Robert Spiera is a professor of clinical medicine at
Weill Cornell Medical College and director of the Scleroderma, Vasculitis and Myositis Center at the Hospital for Special Surgery in New York.
It is appreciated among rheumatologists that although
Raynaud’s phenomenon is common in the general population, it occurs more
commonly in patients with connective tissue disorders and in particular in those
with scleroderma. Recently, preliminary criteria have been proposed by the European
League Against Rheumatism (EULAR) Scleroderma Trials and Research Group to
identify very early systemic sclerosis. Three red-flag features were identified
as those which would raise suspicion for early scleroderma: Raynaud’s phenomenon,
puffy fingers, and a positive ANA. In Dr. Minier’s study, puffy fingers emerged
as an important feature suggesting a higher likelihood of systemic sclerosis.

|
| Dr. Robert Spiera |
It has already been appreciated that patients with Raynaud’s phenomenon who have a positive ANA have a higher likelihood of developing a
connective tissue disorder. In this cohort study, the presence of puffy fingers
in the past or at the time of evaluation markedly increased a positive
predictive value that scleroderma would be present or develop. Perhaps even
more important was their observation that among patients with Raynaud’s phenomenon
without detectable ANA (in whom the likelihood of a connective tissue disorder
evolving was traditionally felt to be low), if puffy fingers were present,
as many as 20% ultimately developed features suggestive of systemic sclerosis.
Rheumatologists have typically recognized the presence
of puffy fingers as potentially an important clinical finding, indicating a
likelihood of the patient having an inflammatory arthritis, connective tissue
disorder, or scleroderma. Of course there is a differential diagnosis of
puffy hands, including thyroid disease, edema, or other metabolic
abnormalities. Nevertheless, in the context of Raynaud’s phenomenon and
detectable ANA, vigilance for evolution toward a scleroderma spectrum disorder
is warranted.
Ultimately, the balance of disease criteria can be
difficult. More liberal diagnostic criteria allow the recognition and diagnosis
of the disorder in patients earlier in the disease course. That must be
balanced against the downside of establishing a diagnosis of a potentially dangerous
and debilitating rheumatic disease in a patient in whom it might not more fully
evolve. Ultimately, the very diagnosis itself could be a major cause of anxiety
and, indeed, morbidity to those patients.
The finding, however, of the importance of puffy
fingers as a predictive factor in establishing a diagnosis of systemic
sclerosis is important, and would be particularly of value if recognized in the
general medical community in terms of identifying patients worthy of further
investigation or rheumatologic referral.
Dr. Robert Spiera is a professor of clinical medicine at
Weill Cornell Medical College and director of the Scleroderma, Vasculitis and Myositis Center at the Hospital for Special Surgery in New York.
It is appreciated among rheumatologists that although
Raynaud’s phenomenon is common in the general population, it occurs more
commonly in patients with connective tissue disorders and in particular in those
with scleroderma. Recently, preliminary criteria have been proposed by the European
League Against Rheumatism (EULAR) Scleroderma Trials and Research Group to
identify very early systemic sclerosis. Three red-flag features were identified
as those which would raise suspicion for early scleroderma: Raynaud’s phenomenon,
puffy fingers, and a positive ANA. In Dr. Minier’s study, puffy fingers emerged
as an important feature suggesting a higher likelihood of systemic sclerosis.

|
| Dr. Robert Spiera |
It has already been appreciated that patients with Raynaud’s phenomenon who have a positive ANA have a higher likelihood of developing a
connective tissue disorder. In this cohort study, the presence of puffy fingers
in the past or at the time of evaluation markedly increased a positive
predictive value that scleroderma would be present or develop. Perhaps even
more important was their observation that among patients with Raynaud’s phenomenon
without detectable ANA (in whom the likelihood of a connective tissue disorder
evolving was traditionally felt to be low), if puffy fingers were present,
as many as 20% ultimately developed features suggestive of systemic sclerosis.
Rheumatologists have typically recognized the presence
of puffy fingers as potentially an important clinical finding, indicating a
likelihood of the patient having an inflammatory arthritis, connective tissue
disorder, or scleroderma. Of course there is a differential diagnosis of
puffy hands, including thyroid disease, edema, or other metabolic
abnormalities. Nevertheless, in the context of Raynaud’s phenomenon and
detectable ANA, vigilance for evolution toward a scleroderma spectrum disorder
is warranted.
Ultimately, the balance of disease criteria can be
difficult. More liberal diagnostic criteria allow the recognition and diagnosis
of the disorder in patients earlier in the disease course. That must be
balanced against the downside of establishing a diagnosis of a potentially dangerous
and debilitating rheumatic disease in a patient in whom it might not more fully
evolve. Ultimately, the very diagnosis itself could be a major cause of anxiety
and, indeed, morbidity to those patients.
The finding, however, of the importance of puffy
fingers as a predictive factor in establishing a diagnosis of systemic
sclerosis is important, and would be particularly of value if recognized in the
general medical community in terms of identifying patients worthy of further
investigation or rheumatologic referral.
Dr. Robert Spiera is a professor of clinical medicine at
Weill Cornell Medical College and director of the Scleroderma, Vasculitis and Myositis Center at the Hospital for Special Surgery in New York.
The presence of puffy fingers in patients with Raynaud’s phenomenon who are antinuclear antibody positive was further validated as an important sign of possible early systemic sclerosis in a recent study.
The findings support the value of using the European League Against Rheumatism Scleroderma Trial and Research Group’s (EUSTAR’s) new criteria of ANA positivity, Raynaud’s phenomenon, and puffy fingers as three red flags that raise suspicion for very early systemic sclerosis, reported Dr. Tünde Minier of the University of Pécs in Hungary and her colleagues (Ann. Rheum. Dis. 2013 Aug. 12 [doi: 10.1136/annrheumdis-2013-203716]). They calculated that the positive predictive value of ANA positivity for developing systemic sclerosis in patients with Raynaud’s phenomenon increased from 33.9% to 88.5% when combined with the presence of puffy fingers.
The researchers examined 469 patients with Raynaud’s phenomenon who were enrolled in the multicenter, prospective, observational Very Early Diagnosis of Systemic Sclerosis (VEDOSS) study at 33 EUSTAR centers throughout and outside Europe. About a third of the patients were ANA negative (32.2%), and 67.8% were ANA positive. Among the ANA-positive participants, 53.6% had a systemic sclerosis pattern on nailfold capillaroscopy, compared with only 13.4% of the ANA-negative patients (P less than .001).
Just over half of the ANA-negative patients lacked a systemic sclerosis pattern on nailfold capillaroscopy, systemic sclerosis–related specific clinical symptoms, or an erythrocyte sedimentation rate of 25 mm/hr or greater, and so were diagnosed with primary Raynaud’s.
The examinations of the patients also included assessments for digital ulcers, digital pitting scars, telangiectases, calcinosis, tendon friction rubs, esophageal symptoms, and symptoms consistent with median nerve compression syndrome.
The most common clinical features in the ANA-positive patients were previous or current puffy fingers, found in 38.5% of the ANA-positive patients versus 23.3% of the ANA-negative patients, and esophageal symptoms, identified in 35.2% of the ANA-positive patients and 18.4% of the ANA-negative patients.
"Almost 90% of ANA-positive Raynaud’s phenomenon patients with previous or current finger edematous skin changes (puffy fingers) already had a nailfold capillaroscopy systemic sclerosis pattern and/or systemic sclerosis–specific autoantibodies," Dr. Minier’s team wrote. Specifically, 73.3% of ANA-positive patients with Raynaud’s and puffy fingers had a nailfold capillaroscopy systemic sclerosis pattern, compared with only 41.2% of ANA-positive patients without puffy fingers. Overall, 88.5% of ANA-positive patients with Raynaud’s and puffy fingers met the criteria for very early systemic sclerosis.
The ANA-positive patients with puffy fingers were also more likely to have other symptoms than were the ANA-positive patients without puffy fingers. Sclerodactyly was identified in 17.8% of the ANA-positive patients with puffy fingers, compared with 6.2% of the ANA-positive patients without current or previous puffy fingers (P = .002). Telangiectases also appeared on 17.3% of the ANA-positive patients with puffy fingers, compared with 9.2% of those without puffy fingers (P = .033). Similarly, 42.1% of ANA-positive patients with puffy fingers had esophageal symptoms, compared with 30.9% of those without puffy fingers (P = .043).
The researchers also noted that even puffy fingers in ANA-negative patients may require more careful follow-up, because 17% of the ANA-negative patients with current or previous puffy fingers had a nailfold capillaroscopy systemic sclerosis pattern and 20% of the ANA-negative patients with puffy fingers had sclerodactyly with other systemic sclerosis symptoms.
The authors noted that one limitation of their study is the inability to generalize their findings to the broader population of Raynaud’s phenomenon patients seen by general physicians, since a higher percentage of the patients enrolled in this cohort meet very early systemic sclerosis classification.
"Patients identified with the very early diagnostic criteria may also have scleroderma with limited cutaneous involvement or even undifferentiated connective tissue disease," a limitation that longer-term follow-up data may clarify with a comparison of the ACR 1980 classification criteria and the new ACR-EULAR criteria.
The study did not use external funding. The authors had no disclosures.
* This story was updated 8/20/2013.
The presence of puffy fingers in patients with Raynaud’s phenomenon who are antinuclear antibody positive was further validated as an important sign of possible early systemic sclerosis in a recent study.
The findings support the value of using the European League Against Rheumatism Scleroderma Trial and Research Group’s (EUSTAR’s) new criteria of ANA positivity, Raynaud’s phenomenon, and puffy fingers as three red flags that raise suspicion for very early systemic sclerosis, reported Dr. Tünde Minier of the University of Pécs in Hungary and her colleagues (Ann. Rheum. Dis. 2013 Aug. 12 [doi: 10.1136/annrheumdis-2013-203716]). They calculated that the positive predictive value of ANA positivity for developing systemic sclerosis in patients with Raynaud’s phenomenon increased from 33.9% to 88.5% when combined with the presence of puffy fingers.
The researchers examined 469 patients with Raynaud’s phenomenon who were enrolled in the multicenter, prospective, observational Very Early Diagnosis of Systemic Sclerosis (VEDOSS) study at 33 EUSTAR centers throughout and outside Europe. About a third of the patients were ANA negative (32.2%), and 67.8% were ANA positive. Among the ANA-positive participants, 53.6% had a systemic sclerosis pattern on nailfold capillaroscopy, compared with only 13.4% of the ANA-negative patients (P less than .001).
Just over half of the ANA-negative patients lacked a systemic sclerosis pattern on nailfold capillaroscopy, systemic sclerosis–related specific clinical symptoms, or an erythrocyte sedimentation rate of 25 mm/hr or greater, and so were diagnosed with primary Raynaud’s.
The examinations of the patients also included assessments for digital ulcers, digital pitting scars, telangiectases, calcinosis, tendon friction rubs, esophageal symptoms, and symptoms consistent with median nerve compression syndrome.
The most common clinical features in the ANA-positive patients were previous or current puffy fingers, found in 38.5% of the ANA-positive patients versus 23.3% of the ANA-negative patients, and esophageal symptoms, identified in 35.2% of the ANA-positive patients and 18.4% of the ANA-negative patients.
"Almost 90% of ANA-positive Raynaud’s phenomenon patients with previous or current finger edematous skin changes (puffy fingers) already had a nailfold capillaroscopy systemic sclerosis pattern and/or systemic sclerosis–specific autoantibodies," Dr. Minier’s team wrote. Specifically, 73.3% of ANA-positive patients with Raynaud’s and puffy fingers had a nailfold capillaroscopy systemic sclerosis pattern, compared with only 41.2% of ANA-positive patients without puffy fingers. Overall, 88.5% of ANA-positive patients with Raynaud’s and puffy fingers met the criteria for very early systemic sclerosis.
The ANA-positive patients with puffy fingers were also more likely to have other symptoms than were the ANA-positive patients without puffy fingers. Sclerodactyly was identified in 17.8% of the ANA-positive patients with puffy fingers, compared with 6.2% of the ANA-positive patients without current or previous puffy fingers (P = .002). Telangiectases also appeared on 17.3% of the ANA-positive patients with puffy fingers, compared with 9.2% of those without puffy fingers (P = .033). Similarly, 42.1% of ANA-positive patients with puffy fingers had esophageal symptoms, compared with 30.9% of those without puffy fingers (P = .043).
The researchers also noted that even puffy fingers in ANA-negative patients may require more careful follow-up, because 17% of the ANA-negative patients with current or previous puffy fingers had a nailfold capillaroscopy systemic sclerosis pattern and 20% of the ANA-negative patients with puffy fingers had sclerodactyly with other systemic sclerosis symptoms.
The authors noted that one limitation of their study is the inability to generalize their findings to the broader population of Raynaud’s phenomenon patients seen by general physicians, since a higher percentage of the patients enrolled in this cohort meet very early systemic sclerosis classification.
"Patients identified with the very early diagnostic criteria may also have scleroderma with limited cutaneous involvement or even undifferentiated connective tissue disease," a limitation that longer-term follow-up data may clarify with a comparison of the ACR 1980 classification criteria and the new ACR-EULAR criteria.
The study did not use external funding. The authors had no disclosures.
* This story was updated 8/20/2013.
FROM ANNALS OF THE RHEUMATIC DISEASES
Major finding: Puffy fingers were identified in more Raynaud's phenomenon patients who were antinuclear antibody positive (38.5%) than ANA negative (23.3%, P less than .01).
Data source: The findings are based on an analysis of signs and symptoms in 469 Reynaud’s phenomenon patients enrolled in the Very Early Diagnosis of Systemic Sclerosis cohort from 33 EUSTAR centers throughout and outside Europe.
Disclosures: Information on the study’s funding was unavailable. The authors had no disclosures.
Venous eczema and lipodermatosclerosis common in venous insufficiency
Venous eczema and lipodermatosclerosis should be considered in patients with painful dermatitis or pruritic erythematous eruptions on their lower extremities, especially if there are other signs of venous insufficiency, according to Dr. Laurel M. Morton and Dr. Tania J. Phillips.
"Venous eczema and lipodermatosclerosis are relatively common conditions caused by chronic venous insufficiency, yet at times they can be a challenge to diagnose and treat," said Dr. Morton in an interview.
Writing in the September issue of Seminars in Cutaneous Medicine and Surgery, Dr. Morton and Dr. Phillips of the department of dermatology, Boston University, reviewed the challenges associated with the diagnosis and treatment of these conditions (Semin. Cutan. Med. Surg. 2013;32:169-76).
Venous eczema presents as erythematous, scaly, pruritic skin on the lower legs and ankles, often in association with other signs of venous disease such as varicose veins, edema, hemosiderin pigmentation, atrophie blanche, and lipodermatosclerosis.
Lipodermatosclerosis is a progressive fibrotic process affecting the dermis and subcutaneous fat of the lower leg, resulting in hyperpigmentation and induration.
There are few data on the prevalence of venous eczema and lipodermatosclerosis, or even for chronic venous disease in general, the researchers noted. However, they suggested that as many as 17% of men and 40% of women suffer from chronic venous insufficiency, and of the 23% of Americans with varicose veins, 2 million will develop skin changes.
"Perhaps the most challenging condition to rule out is allergic contact dermatitis, since this may be seen in conjunction with venous eczema, which is characterized by a decreased skin barrier that may increase the rate of sensitization."
Venous eczema can be confused with other papulosquamous conditions such as nummular eczema and psoriasis, as well as xerosis, eczema craquele, and cellulitis.
"Perhaps the most challenging condition to rule out is allergic contact dermatitis, since this may be seen in conjunction with venous eczema, which is characterized by a decreased skin barrier that may increase the rate of sensitization," the researchers reported.
Consider patch testing in cases where allergy is suspected and, if irritant contact dermatitis is a possibility, patients should be asked about topical applications, they added.
Acute lipodermatosclerosis presents as painful, erythematous, purple indurated plaques – well demarcated from normal skin – confined to the lower extremity, possibly with white scale, while chronic lipodermatosclerosis is generally associated with a classic "inverted champagne bottle" appearance of the distal third of the lower leg.
Acute lipodermatosclerosis is also often misdiagnosed as cellulitis, although a key distinguishing factor is lack of improvement with antibiotics. Acute lipodermatosclerosis also can be confused with conditions such as erythema nodosum, thrombophlebitis, and fibrosing conditions such as inflammatory morphea.
As venous eczema and lipodermatosclerosis are caused by venous insufficiency, the authors argue that compression should be the first line of treatment.
"The most important treatment for both conditions is graduated compression, but oral, topical, and surgical interventions should be considered as adjunctive approaches," Dr. Morton said.
Venous eczema also can be managed topically with emollients and immunomodulators – including corticosteroids and calcineurin inhibitors – although scant data support this approach.
However, the researchers highlighted one study in which patients treated with a combination of oral doxycycline and topical tacrolimus showed statistically significant improvement in pain, edema, erythema, pigmentation, pruritus, and exudate (Indian J. Pharmacol. 2012;44:111-13).
Patients with lipodermatosclerosis may not tolerate compression due to extreme skin tenderness; however, there is reasonable evidence in favor of stanozolol, an anabolic steroid with fibrinolytic properties, the researchers noted. Other agents that may help these patients include danazol, oxandrolone, pentoxifylline, and intralesional triamcinolone.
There is little evidence to support the use of ultrasound, the researchers said. However, it is safe and may offer an alternative to other therapeutic options in recalcitrant disease, they added.
The researchers had no financial conflicts to disclose.
Venous eczema and lipodermatosclerosis should be considered in patients with painful dermatitis or pruritic erythematous eruptions on their lower extremities, especially if there are other signs of venous insufficiency, according to Dr. Laurel M. Morton and Dr. Tania J. Phillips.
"Venous eczema and lipodermatosclerosis are relatively common conditions caused by chronic venous insufficiency, yet at times they can be a challenge to diagnose and treat," said Dr. Morton in an interview.
Writing in the September issue of Seminars in Cutaneous Medicine and Surgery, Dr. Morton and Dr. Phillips of the department of dermatology, Boston University, reviewed the challenges associated with the diagnosis and treatment of these conditions (Semin. Cutan. Med. Surg. 2013;32:169-76).
Venous eczema presents as erythematous, scaly, pruritic skin on the lower legs and ankles, often in association with other signs of venous disease such as varicose veins, edema, hemosiderin pigmentation, atrophie blanche, and lipodermatosclerosis.
Lipodermatosclerosis is a progressive fibrotic process affecting the dermis and subcutaneous fat of the lower leg, resulting in hyperpigmentation and induration.
There are few data on the prevalence of venous eczema and lipodermatosclerosis, or even for chronic venous disease in general, the researchers noted. However, they suggested that as many as 17% of men and 40% of women suffer from chronic venous insufficiency, and of the 23% of Americans with varicose veins, 2 million will develop skin changes.
"Perhaps the most challenging condition to rule out is allergic contact dermatitis, since this may be seen in conjunction with venous eczema, which is characterized by a decreased skin barrier that may increase the rate of sensitization."
Venous eczema can be confused with other papulosquamous conditions such as nummular eczema and psoriasis, as well as xerosis, eczema craquele, and cellulitis.
"Perhaps the most challenging condition to rule out is allergic contact dermatitis, since this may be seen in conjunction with venous eczema, which is characterized by a decreased skin barrier that may increase the rate of sensitization," the researchers reported.
Consider patch testing in cases where allergy is suspected and, if irritant contact dermatitis is a possibility, patients should be asked about topical applications, they added.
Acute lipodermatosclerosis presents as painful, erythematous, purple indurated plaques – well demarcated from normal skin – confined to the lower extremity, possibly with white scale, while chronic lipodermatosclerosis is generally associated with a classic "inverted champagne bottle" appearance of the distal third of the lower leg.
Acute lipodermatosclerosis is also often misdiagnosed as cellulitis, although a key distinguishing factor is lack of improvement with antibiotics. Acute lipodermatosclerosis also can be confused with conditions such as erythema nodosum, thrombophlebitis, and fibrosing conditions such as inflammatory morphea.
As venous eczema and lipodermatosclerosis are caused by venous insufficiency, the authors argue that compression should be the first line of treatment.
"The most important treatment for both conditions is graduated compression, but oral, topical, and surgical interventions should be considered as adjunctive approaches," Dr. Morton said.
Venous eczema also can be managed topically with emollients and immunomodulators – including corticosteroids and calcineurin inhibitors – although scant data support this approach.
However, the researchers highlighted one study in which patients treated with a combination of oral doxycycline and topical tacrolimus showed statistically significant improvement in pain, edema, erythema, pigmentation, pruritus, and exudate (Indian J. Pharmacol. 2012;44:111-13).
Patients with lipodermatosclerosis may not tolerate compression due to extreme skin tenderness; however, there is reasonable evidence in favor of stanozolol, an anabolic steroid with fibrinolytic properties, the researchers noted. Other agents that may help these patients include danazol, oxandrolone, pentoxifylline, and intralesional triamcinolone.
There is little evidence to support the use of ultrasound, the researchers said. However, it is safe and may offer an alternative to other therapeutic options in recalcitrant disease, they added.
The researchers had no financial conflicts to disclose.
Venous eczema and lipodermatosclerosis should be considered in patients with painful dermatitis or pruritic erythematous eruptions on their lower extremities, especially if there are other signs of venous insufficiency, according to Dr. Laurel M. Morton and Dr. Tania J. Phillips.
"Venous eczema and lipodermatosclerosis are relatively common conditions caused by chronic venous insufficiency, yet at times they can be a challenge to diagnose and treat," said Dr. Morton in an interview.
Writing in the September issue of Seminars in Cutaneous Medicine and Surgery, Dr. Morton and Dr. Phillips of the department of dermatology, Boston University, reviewed the challenges associated with the diagnosis and treatment of these conditions (Semin. Cutan. Med. Surg. 2013;32:169-76).
Venous eczema presents as erythematous, scaly, pruritic skin on the lower legs and ankles, often in association with other signs of venous disease such as varicose veins, edema, hemosiderin pigmentation, atrophie blanche, and lipodermatosclerosis.
Lipodermatosclerosis is a progressive fibrotic process affecting the dermis and subcutaneous fat of the lower leg, resulting in hyperpigmentation and induration.
There are few data on the prevalence of venous eczema and lipodermatosclerosis, or even for chronic venous disease in general, the researchers noted. However, they suggested that as many as 17% of men and 40% of women suffer from chronic venous insufficiency, and of the 23% of Americans with varicose veins, 2 million will develop skin changes.
"Perhaps the most challenging condition to rule out is allergic contact dermatitis, since this may be seen in conjunction with venous eczema, which is characterized by a decreased skin barrier that may increase the rate of sensitization."
Venous eczema can be confused with other papulosquamous conditions such as nummular eczema and psoriasis, as well as xerosis, eczema craquele, and cellulitis.
"Perhaps the most challenging condition to rule out is allergic contact dermatitis, since this may be seen in conjunction with venous eczema, which is characterized by a decreased skin barrier that may increase the rate of sensitization," the researchers reported.
Consider patch testing in cases where allergy is suspected and, if irritant contact dermatitis is a possibility, patients should be asked about topical applications, they added.
Acute lipodermatosclerosis presents as painful, erythematous, purple indurated plaques – well demarcated from normal skin – confined to the lower extremity, possibly with white scale, while chronic lipodermatosclerosis is generally associated with a classic "inverted champagne bottle" appearance of the distal third of the lower leg.
Acute lipodermatosclerosis is also often misdiagnosed as cellulitis, although a key distinguishing factor is lack of improvement with antibiotics. Acute lipodermatosclerosis also can be confused with conditions such as erythema nodosum, thrombophlebitis, and fibrosing conditions such as inflammatory morphea.
As venous eczema and lipodermatosclerosis are caused by venous insufficiency, the authors argue that compression should be the first line of treatment.
"The most important treatment for both conditions is graduated compression, but oral, topical, and surgical interventions should be considered as adjunctive approaches," Dr. Morton said.
Venous eczema also can be managed topically with emollients and immunomodulators – including corticosteroids and calcineurin inhibitors – although scant data support this approach.
However, the researchers highlighted one study in which patients treated with a combination of oral doxycycline and topical tacrolimus showed statistically significant improvement in pain, edema, erythema, pigmentation, pruritus, and exudate (Indian J. Pharmacol. 2012;44:111-13).
Patients with lipodermatosclerosis may not tolerate compression due to extreme skin tenderness; however, there is reasonable evidence in favor of stanozolol, an anabolic steroid with fibrinolytic properties, the researchers noted. Other agents that may help these patients include danazol, oxandrolone, pentoxifylline, and intralesional triamcinolone.
There is little evidence to support the use of ultrasound, the researchers said. However, it is safe and may offer an alternative to other therapeutic options in recalcitrant disease, they added.
The researchers had no financial conflicts to disclose.
FROM SEMINARS IN CUTANEOUS MEDICINE AND SURGERY
The chicken or the egg: Obesity or psoriasis?
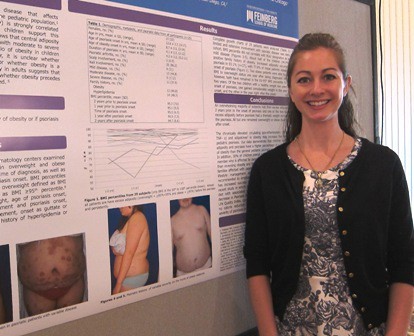
MILWAUKEE – Excess adiposity occurred prior to psoriasis in 93% of 29 overweight and obese psoriatic children, in a chart review examining the relationship between psoriasis and obesity.
Further, 78% of patients were obese before developing psoriasis, Dr. Lauren Becker reported at the annual meeting of the Society for Pediatric Dermatology.
Although the authors anticipated that most of the children would show increased adiposity before psoriasis based on clinical observations, "none of us predicted that it would be 27 of 29," she said in an interview. "It really was incredible, and even the ones who were normal weight became overweight or obese within a year of their psoriasis."
The review was sparked by a recent international study, led by senior author Dr. Amy Paller of Northwestern University in Chicago, in which 38% of psoriatic children had excess central adiposity (high waist-to-height ratio) compared with 21% of controls. The odds of obesity were also several times higher than those reported for adults with psoriasis, jumping 4.29-fold overall in psoriatic children versus controls, 4.92-fold in those with severe versus mild psoriasis, and 7.6-fold in the United States. (JAMA Dermatol. 2013;149:166-76).
Although other studies in children support an association between obesity and psoriasis, the international study was the first to measure central adiposity, a more sensitive indicator of cardiovascular risk in children than BMI, Dr. Kelly Cordoro remarked in a separate lecture on the comorbidities of pediatric psoriasis at the meeting.
"Adiposity, hypertension, hyperlipidemia, and diabetes are increased in prevalence among pediatric psoriasis patients, but the obesity association is the strongest," she said. "It’s global, and the effect is most pronounced in the United States, where in particular, central obesity is highest and has the accompanying higher cardiovascular risk."
Previous research has shown that obesity is strongly correlated with psoriasis in adults. A recent meta-analysis showed that psoriatic adults were more likely to be obese than those without psoriasis (pooled odds ratio, 1.66), and that the odds were even higher in patients with severe versus mild psoriasis (OR, 2.23, vs. OR, 1.46) (Nutr. Diabetes 2012 ;2:e54).
Both clinicians observed that psoriasis and obesity are chronic inflammatory states, marked by overexpression of circulating proinflammatory cytokines derived from Th1 and Th17 lymphocyte subsets, as well as tumor necrosis factor–alpha and adipokines. In addition, a fat cell is a microenvironment of inflammation, with adipose tissue releasing proinflammatory cytokines such as interleukin-6 and TNF-alpha.
The two diseases drive one another, but the exact relationship remains unclear, said Dr. Becker, a pediatrician and dermatology fellow at Northwestern at the time of the study. In addition, the impact on quality of life and social interactions of being both obese or overweight and having psoriasis may predispose patients to mental and physical health problems.
"Children with excess adiposity or with the highly visible lesions of psoriasis are more often teased or bullied, which may contribute to the tendency to become socially isolated, decrease physical activity, and increase eating," she said. "It really is a vicious circle."
Although the data are clear that psoriasis and obesity are associated, the current chart review suggesting that obesity precedes psoriasis is too small to definitively answer the question, Dr. Cordoro, a pediatric dermatologist at the University of California, San Francisco, said in an interview.
Dr. Becker said obesity clearly came before psoriasis in their study cohort, but agreed that further study is needed to confirm the results. She also stressed the need for biomarker analyses to identify overweight/obese children who are at risk for psoriasis, and studies to address whether weight loss can reverse the severity of pediatric psoriasis.
For example, data from a study of overweight psoriatic adults showed that eating a low-calorie diet improved psoriasis severity and quality of life after 4 months (JAMA Dermatol. 2013;149:795-801).
However, in the absence of adequate data, clinicians should consider metabolic testing to determine whether their overweight pediatric patients are on the path to the metabolic syndrome, Dr. Becker and Dr. Cordoro suggested. Metabolic testing was not performed on any of the 29 children in the chart review, although 48% had a family history of hyperlipidemia and 45% had a family history of obesity.
The average age of the children was 12.6 years; the average age of onset of obesity was 4 years (range, 2-12 years), and the average age of onset of psoriasis was 9 years (range, 2-14 years).
Two obese patients were able to reduce their BMIs from obese to overweight status 1 year after being diagnosed with psoriasis; however, both have remained in the 85th-95th BMI percentile for more than 2 years, Dr. Becker said. Both of the children who had a normal BMI prior to their psoriasis had a BMI in the overweight or obese range within 1 year after diagnosis, Dr. Becker noted.
Dr. Cordoro also made an impassioned plea for clinicians to address the significant psychosocial comorbidity present in psoriatic children.
"You can think about obesity, psoriasis, and depression as being reciprocal exacerbating factors, such that each triggers the other and represents an insult to self-esteem and the overall well-being of these patients," she said. "They end up having high stress levels and really dismal quality of life. These issues are as important in the management of the child as the medical considerations."
Dr. Becker, her coauthors, and Dr. Cordoro reported having no relevant financial disclosures.
MILWAUKEE – Excess adiposity occurred prior to psoriasis in 93% of 29 overweight and obese psoriatic children, in a chart review examining the relationship between psoriasis and obesity.
Further, 78% of patients were obese before developing psoriasis, Dr. Lauren Becker reported at the annual meeting of the Society for Pediatric Dermatology.
Although the authors anticipated that most of the children would show increased adiposity before psoriasis based on clinical observations, "none of us predicted that it would be 27 of 29," she said in an interview. "It really was incredible, and even the ones who were normal weight became overweight or obese within a year of their psoriasis."
The review was sparked by a recent international study, led by senior author Dr. Amy Paller of Northwestern University in Chicago, in which 38% of psoriatic children had excess central adiposity (high waist-to-height ratio) compared with 21% of controls. The odds of obesity were also several times higher than those reported for adults with psoriasis, jumping 4.29-fold overall in psoriatic children versus controls, 4.92-fold in those with severe versus mild psoriasis, and 7.6-fold in the United States. (JAMA Dermatol. 2013;149:166-76).
Although other studies in children support an association between obesity and psoriasis, the international study was the first to measure central adiposity, a more sensitive indicator of cardiovascular risk in children than BMI, Dr. Kelly Cordoro remarked in a separate lecture on the comorbidities of pediatric psoriasis at the meeting.
"Adiposity, hypertension, hyperlipidemia, and diabetes are increased in prevalence among pediatric psoriasis patients, but the obesity association is the strongest," she said. "It’s global, and the effect is most pronounced in the United States, where in particular, central obesity is highest and has the accompanying higher cardiovascular risk."
Previous research has shown that obesity is strongly correlated with psoriasis in adults. A recent meta-analysis showed that psoriatic adults were more likely to be obese than those without psoriasis (pooled odds ratio, 1.66), and that the odds were even higher in patients with severe versus mild psoriasis (OR, 2.23, vs. OR, 1.46) (Nutr. Diabetes 2012 ;2:e54).
Both clinicians observed that psoriasis and obesity are chronic inflammatory states, marked by overexpression of circulating proinflammatory cytokines derived from Th1 and Th17 lymphocyte subsets, as well as tumor necrosis factor–alpha and adipokines. In addition, a fat cell is a microenvironment of inflammation, with adipose tissue releasing proinflammatory cytokines such as interleukin-6 and TNF-alpha.
The two diseases drive one another, but the exact relationship remains unclear, said Dr. Becker, a pediatrician and dermatology fellow at Northwestern at the time of the study. In addition, the impact on quality of life and social interactions of being both obese or overweight and having psoriasis may predispose patients to mental and physical health problems.
"Children with excess adiposity or with the highly visible lesions of psoriasis are more often teased or bullied, which may contribute to the tendency to become socially isolated, decrease physical activity, and increase eating," she said. "It really is a vicious circle."
Although the data are clear that psoriasis and obesity are associated, the current chart review suggesting that obesity precedes psoriasis is too small to definitively answer the question, Dr. Cordoro, a pediatric dermatologist at the University of California, San Francisco, said in an interview.
Dr. Becker said obesity clearly came before psoriasis in their study cohort, but agreed that further study is needed to confirm the results. She also stressed the need for biomarker analyses to identify overweight/obese children who are at risk for psoriasis, and studies to address whether weight loss can reverse the severity of pediatric psoriasis.
For example, data from a study of overweight psoriatic adults showed that eating a low-calorie diet improved psoriasis severity and quality of life after 4 months (JAMA Dermatol. 2013;149:795-801).
However, in the absence of adequate data, clinicians should consider metabolic testing to determine whether their overweight pediatric patients are on the path to the metabolic syndrome, Dr. Becker and Dr. Cordoro suggested. Metabolic testing was not performed on any of the 29 children in the chart review, although 48% had a family history of hyperlipidemia and 45% had a family history of obesity.
The average age of the children was 12.6 years; the average age of onset of obesity was 4 years (range, 2-12 years), and the average age of onset of psoriasis was 9 years (range, 2-14 years).
Two obese patients were able to reduce their BMIs from obese to overweight status 1 year after being diagnosed with psoriasis; however, both have remained in the 85th-95th BMI percentile for more than 2 years, Dr. Becker said. Both of the children who had a normal BMI prior to their psoriasis had a BMI in the overweight or obese range within 1 year after diagnosis, Dr. Becker noted.
Dr. Cordoro also made an impassioned plea for clinicians to address the significant psychosocial comorbidity present in psoriatic children.
"You can think about obesity, psoriasis, and depression as being reciprocal exacerbating factors, such that each triggers the other and represents an insult to self-esteem and the overall well-being of these patients," she said. "They end up having high stress levels and really dismal quality of life. These issues are as important in the management of the child as the medical considerations."
Dr. Becker, her coauthors, and Dr. Cordoro reported having no relevant financial disclosures.
MILWAUKEE – Excess adiposity occurred prior to psoriasis in 93% of 29 overweight and obese psoriatic children, in a chart review examining the relationship between psoriasis and obesity.
Further, 78% of patients were obese before developing psoriasis, Dr. Lauren Becker reported at the annual meeting of the Society for Pediatric Dermatology.
Although the authors anticipated that most of the children would show increased adiposity before psoriasis based on clinical observations, "none of us predicted that it would be 27 of 29," she said in an interview. "It really was incredible, and even the ones who were normal weight became overweight or obese within a year of their psoriasis."
The review was sparked by a recent international study, led by senior author Dr. Amy Paller of Northwestern University in Chicago, in which 38% of psoriatic children had excess central adiposity (high waist-to-height ratio) compared with 21% of controls. The odds of obesity were also several times higher than those reported for adults with psoriasis, jumping 4.29-fold overall in psoriatic children versus controls, 4.92-fold in those with severe versus mild psoriasis, and 7.6-fold in the United States. (JAMA Dermatol. 2013;149:166-76).
Although other studies in children support an association between obesity and psoriasis, the international study was the first to measure central adiposity, a more sensitive indicator of cardiovascular risk in children than BMI, Dr. Kelly Cordoro remarked in a separate lecture on the comorbidities of pediatric psoriasis at the meeting.
"Adiposity, hypertension, hyperlipidemia, and diabetes are increased in prevalence among pediatric psoriasis patients, but the obesity association is the strongest," she said. "It’s global, and the effect is most pronounced in the United States, where in particular, central obesity is highest and has the accompanying higher cardiovascular risk."
Previous research has shown that obesity is strongly correlated with psoriasis in adults. A recent meta-analysis showed that psoriatic adults were more likely to be obese than those without psoriasis (pooled odds ratio, 1.66), and that the odds were even higher in patients with severe versus mild psoriasis (OR, 2.23, vs. OR, 1.46) (Nutr. Diabetes 2012 ;2:e54).
Both clinicians observed that psoriasis and obesity are chronic inflammatory states, marked by overexpression of circulating proinflammatory cytokines derived from Th1 and Th17 lymphocyte subsets, as well as tumor necrosis factor–alpha and adipokines. In addition, a fat cell is a microenvironment of inflammation, with adipose tissue releasing proinflammatory cytokines such as interleukin-6 and TNF-alpha.
The two diseases drive one another, but the exact relationship remains unclear, said Dr. Becker, a pediatrician and dermatology fellow at Northwestern at the time of the study. In addition, the impact on quality of life and social interactions of being both obese or overweight and having psoriasis may predispose patients to mental and physical health problems.
"Children with excess adiposity or with the highly visible lesions of psoriasis are more often teased or bullied, which may contribute to the tendency to become socially isolated, decrease physical activity, and increase eating," she said. "It really is a vicious circle."
Although the data are clear that psoriasis and obesity are associated, the current chart review suggesting that obesity precedes psoriasis is too small to definitively answer the question, Dr. Cordoro, a pediatric dermatologist at the University of California, San Francisco, said in an interview.
Dr. Becker said obesity clearly came before psoriasis in their study cohort, but agreed that further study is needed to confirm the results. She also stressed the need for biomarker analyses to identify overweight/obese children who are at risk for psoriasis, and studies to address whether weight loss can reverse the severity of pediatric psoriasis.
For example, data from a study of overweight psoriatic adults showed that eating a low-calorie diet improved psoriasis severity and quality of life after 4 months (JAMA Dermatol. 2013;149:795-801).
However, in the absence of adequate data, clinicians should consider metabolic testing to determine whether their overweight pediatric patients are on the path to the metabolic syndrome, Dr. Becker and Dr. Cordoro suggested. Metabolic testing was not performed on any of the 29 children in the chart review, although 48% had a family history of hyperlipidemia and 45% had a family history of obesity.
The average age of the children was 12.6 years; the average age of onset of obesity was 4 years (range, 2-12 years), and the average age of onset of psoriasis was 9 years (range, 2-14 years).
Two obese patients were able to reduce their BMIs from obese to overweight status 1 year after being diagnosed with psoriasis; however, both have remained in the 85th-95th BMI percentile for more than 2 years, Dr. Becker said. Both of the children who had a normal BMI prior to their psoriasis had a BMI in the overweight or obese range within 1 year after diagnosis, Dr. Becker noted.
Dr. Cordoro also made an impassioned plea for clinicians to address the significant psychosocial comorbidity present in psoriatic children.
"You can think about obesity, psoriasis, and depression as being reciprocal exacerbating factors, such that each triggers the other and represents an insult to self-esteem and the overall well-being of these patients," she said. "They end up having high stress levels and really dismal quality of life. These issues are as important in the management of the child as the medical considerations."
Dr. Becker, her coauthors, and Dr. Cordoro reported having no relevant financial disclosures.
AT THE SPD ANNUAL MEETING
Major finding: Among 29 children, 93% met criteria for excess adiposity prior to developing psoriasis, and 78% of these were obese before their psoriasis.
Data source: A growth chart review of 29 obese/overweight children with psoriasis.
Disclosures: Dr. Becker, her coauthors, and Dr. Cordoro reported having no relevant financial disclosures.
Methotrexate assay useful in severe pediatric dermatitis, psoriasis
MILWAUKEE – In children with severe atopic dermatitis or psoriasis, the methotrexate polyglutamate assay appears useful in guiding dose modification in those failing to respond to methotrexate at 12 weeks, a retrospective study has shown.
The methotrexate polyglutamate 3 assay (MTX PG3) measures concentrations of methotrexate polyglutamates, the active metabolites of methotrexate, Syed Rahman said at the annual meeting of the Society for Pediatric Dermatology.
Methotrexate, a prodrug, requires enzymatic conversion via sequential addition of glutamic acid residues. Those residues form a glutamate "tail" that enhances intracellular methotrexate retention. The 3-tail metabolite, MTX PG3, is the predominant species in most patients, explained Mr. Rahman of St. Louis University.
"This study supports the usefulness of a commercially available tool for customizing optimal methotrexate dosing, coauthor Dr. Elaine Siegfried, professor of pediatrics and dermatology at the university, said in an interview.
The MTX PG3 assay has been validated in adults with rheumatoid arthritis where patients with a therapeutic methotrexate polyglutamate level of more than 60 nmol/L were fivefold more likely to have a good response to methotrexate than those with levels below this threshold, according to poster results presented at the 2008 American College of Rheumatology annual meeting (cited on the company website). However, the test was not predictive of response in 36% of patients whose arthritis improved with treatment despite subtherapeutic levels.
For the current analysis, the investigators reviewed the charts of 46 children with severe atopic dermatitis, psoriasis, or atopic dermatitis–psoriasis overlap, who were treated with a starting dose of oral methotrexate 0.5 mg/kg per week (maximum, 15 mg) and assessed by MTX PG3 assay at week 12. In a subset of children who failed to respond, the dose was modified and the level rechecked at 8 weeks. Disease severity was rated using a 5-point physician global assessment scale, with a good to excellent response defined by a 2-point improvement.
In all, 83% of children achieved a good to excellent response, with 27 patients doing so within 12 weeks and 11 responding after dose adjustment involving either a higher oral dose or switching to subcutaneous administration, Mr. Rahman reported.
Response rates to methotrexate were slightly higher in children with psoriasis or overlap (the two groups were combined) than those with atopic dermatitis (94% vs. 77%; no P value given). Overall, 8 children failed to respond.
The mean MTX PG3 level significantly correlated with efficacy in patients with atopic dermatitis (responders, 34.8 nmol/L, vs. 17.8 nmol/L, nonresponders; P = .018), but not in those with psoriasis–atopic dermatitis overlap (responders, 26.5 nmol/L, vs. 20.7, nonresponders; P = .724), Mr. Rahman reported.
The data suggest that compared with the therapeutic level of 60 nmol/L identified in adults, "pediatric patients have a lower therapeutic value," he said.
A subset analysis of MTX PG3 levels based on response identified a significantly higher mean maximum MTX PG3 level for responders than nonresponders (31.5 vs. 18.1 nmol/L; P = .035).
More importantly, late responders had the highest methotrexate levels. All late responders achieved levels greater than 30 nmol/L, and mean levels were significantly higher than for early responders (41.9 vs. 27.3 nmol/L; P = .024), suggesting that the test is most valuable for the subset of children requiring dose adjustment, Mr. Rahman said.
"Patients who respond won’t even need the test, but for those at the 12-week juncture who do not respond and their levels are less than 30 nmol/L, this [study] suggests that if we increase the PO [dose] or change to subcutaneous [administration], we will eventually have a response," he said.
During a discussion of the results, an audience member questioned whether the test, with its added expense, really enhances clinical judgment because most clinicians would already adjust the dose in their patients failing to respond at 12 weeks.
Mr. Rahman replied that not all patients will respond to continued treatment with methotrexate, and that the test could differentiate between responders and nonresponders, thereby directing the patient to alternative therapy earlier.
Session moderator Dr. Amy Paller, chair of dermatology and professor of pediatrics and dermatology at Northwestern University in Chicago, agreed that the test is most valuable for the subset of children who are nonresponders at 12 weeks.
"In these children, this test can guide dose modification," she said in an interview. "Pharmacogenetic variation can require relatively higher oral doses or subcutaneous administration."
In contrast to a therapeutic level of more than 60 nmol/L established for adults, a level of 30 nmol/L may be sufficient in children, Dr. Paller said.
"A MTX PG3 less than 30 nmol/L in a child who has failed to respond within 12 weeks suggests that an increase in oral dose, or even a change to subcutaneous [administration], will boost efficacy," she commented.
Mr. Rahman, Dr. Siegfried, and Dr. Paller reported having no relevant financial disclosures.
MILWAUKEE – In children with severe atopic dermatitis or psoriasis, the methotrexate polyglutamate assay appears useful in guiding dose modification in those failing to respond to methotrexate at 12 weeks, a retrospective study has shown.
The methotrexate polyglutamate 3 assay (MTX PG3) measures concentrations of methotrexate polyglutamates, the active metabolites of methotrexate, Syed Rahman said at the annual meeting of the Society for Pediatric Dermatology.
Methotrexate, a prodrug, requires enzymatic conversion via sequential addition of glutamic acid residues. Those residues form a glutamate "tail" that enhances intracellular methotrexate retention. The 3-tail metabolite, MTX PG3, is the predominant species in most patients, explained Mr. Rahman of St. Louis University.
"This study supports the usefulness of a commercially available tool for customizing optimal methotrexate dosing, coauthor Dr. Elaine Siegfried, professor of pediatrics and dermatology at the university, said in an interview.
The MTX PG3 assay has been validated in adults with rheumatoid arthritis where patients with a therapeutic methotrexate polyglutamate level of more than 60 nmol/L were fivefold more likely to have a good response to methotrexate than those with levels below this threshold, according to poster results presented at the 2008 American College of Rheumatology annual meeting (cited on the company website). However, the test was not predictive of response in 36% of patients whose arthritis improved with treatment despite subtherapeutic levels.
For the current analysis, the investigators reviewed the charts of 46 children with severe atopic dermatitis, psoriasis, or atopic dermatitis–psoriasis overlap, who were treated with a starting dose of oral methotrexate 0.5 mg/kg per week (maximum, 15 mg) and assessed by MTX PG3 assay at week 12. In a subset of children who failed to respond, the dose was modified and the level rechecked at 8 weeks. Disease severity was rated using a 5-point physician global assessment scale, with a good to excellent response defined by a 2-point improvement.
In all, 83% of children achieved a good to excellent response, with 27 patients doing so within 12 weeks and 11 responding after dose adjustment involving either a higher oral dose or switching to subcutaneous administration, Mr. Rahman reported.
Response rates to methotrexate were slightly higher in children with psoriasis or overlap (the two groups were combined) than those with atopic dermatitis (94% vs. 77%; no P value given). Overall, 8 children failed to respond.
The mean MTX PG3 level significantly correlated with efficacy in patients with atopic dermatitis (responders, 34.8 nmol/L, vs. 17.8 nmol/L, nonresponders; P = .018), but not in those with psoriasis–atopic dermatitis overlap (responders, 26.5 nmol/L, vs. 20.7, nonresponders; P = .724), Mr. Rahman reported.
The data suggest that compared with the therapeutic level of 60 nmol/L identified in adults, "pediatric patients have a lower therapeutic value," he said.
A subset analysis of MTX PG3 levels based on response identified a significantly higher mean maximum MTX PG3 level for responders than nonresponders (31.5 vs. 18.1 nmol/L; P = .035).
More importantly, late responders had the highest methotrexate levels. All late responders achieved levels greater than 30 nmol/L, and mean levels were significantly higher than for early responders (41.9 vs. 27.3 nmol/L; P = .024), suggesting that the test is most valuable for the subset of children requiring dose adjustment, Mr. Rahman said.
"Patients who respond won’t even need the test, but for those at the 12-week juncture who do not respond and their levels are less than 30 nmol/L, this [study] suggests that if we increase the PO [dose] or change to subcutaneous [administration], we will eventually have a response," he said.
During a discussion of the results, an audience member questioned whether the test, with its added expense, really enhances clinical judgment because most clinicians would already adjust the dose in their patients failing to respond at 12 weeks.
Mr. Rahman replied that not all patients will respond to continued treatment with methotrexate, and that the test could differentiate between responders and nonresponders, thereby directing the patient to alternative therapy earlier.
Session moderator Dr. Amy Paller, chair of dermatology and professor of pediatrics and dermatology at Northwestern University in Chicago, agreed that the test is most valuable for the subset of children who are nonresponders at 12 weeks.
"In these children, this test can guide dose modification," she said in an interview. "Pharmacogenetic variation can require relatively higher oral doses or subcutaneous administration."
In contrast to a therapeutic level of more than 60 nmol/L established for adults, a level of 30 nmol/L may be sufficient in children, Dr. Paller said.
"A MTX PG3 less than 30 nmol/L in a child who has failed to respond within 12 weeks suggests that an increase in oral dose, or even a change to subcutaneous [administration], will boost efficacy," she commented.
Mr. Rahman, Dr. Siegfried, and Dr. Paller reported having no relevant financial disclosures.
MILWAUKEE – In children with severe atopic dermatitis or psoriasis, the methotrexate polyglutamate assay appears useful in guiding dose modification in those failing to respond to methotrexate at 12 weeks, a retrospective study has shown.
The methotrexate polyglutamate 3 assay (MTX PG3) measures concentrations of methotrexate polyglutamates, the active metabolites of methotrexate, Syed Rahman said at the annual meeting of the Society for Pediatric Dermatology.
Methotrexate, a prodrug, requires enzymatic conversion via sequential addition of glutamic acid residues. Those residues form a glutamate "tail" that enhances intracellular methotrexate retention. The 3-tail metabolite, MTX PG3, is the predominant species in most patients, explained Mr. Rahman of St. Louis University.
"This study supports the usefulness of a commercially available tool for customizing optimal methotrexate dosing, coauthor Dr. Elaine Siegfried, professor of pediatrics and dermatology at the university, said in an interview.
The MTX PG3 assay has been validated in adults with rheumatoid arthritis where patients with a therapeutic methotrexate polyglutamate level of more than 60 nmol/L were fivefold more likely to have a good response to methotrexate than those with levels below this threshold, according to poster results presented at the 2008 American College of Rheumatology annual meeting (cited on the company website). However, the test was not predictive of response in 36% of patients whose arthritis improved with treatment despite subtherapeutic levels.
For the current analysis, the investigators reviewed the charts of 46 children with severe atopic dermatitis, psoriasis, or atopic dermatitis–psoriasis overlap, who were treated with a starting dose of oral methotrexate 0.5 mg/kg per week (maximum, 15 mg) and assessed by MTX PG3 assay at week 12. In a subset of children who failed to respond, the dose was modified and the level rechecked at 8 weeks. Disease severity was rated using a 5-point physician global assessment scale, with a good to excellent response defined by a 2-point improvement.
In all, 83% of children achieved a good to excellent response, with 27 patients doing so within 12 weeks and 11 responding after dose adjustment involving either a higher oral dose or switching to subcutaneous administration, Mr. Rahman reported.
Response rates to methotrexate were slightly higher in children with psoriasis or overlap (the two groups were combined) than those with atopic dermatitis (94% vs. 77%; no P value given). Overall, 8 children failed to respond.
The mean MTX PG3 level significantly correlated with efficacy in patients with atopic dermatitis (responders, 34.8 nmol/L, vs. 17.8 nmol/L, nonresponders; P = .018), but not in those with psoriasis–atopic dermatitis overlap (responders, 26.5 nmol/L, vs. 20.7, nonresponders; P = .724), Mr. Rahman reported.
The data suggest that compared with the therapeutic level of 60 nmol/L identified in adults, "pediatric patients have a lower therapeutic value," he said.
A subset analysis of MTX PG3 levels based on response identified a significantly higher mean maximum MTX PG3 level for responders than nonresponders (31.5 vs. 18.1 nmol/L; P = .035).
More importantly, late responders had the highest methotrexate levels. All late responders achieved levels greater than 30 nmol/L, and mean levels were significantly higher than for early responders (41.9 vs. 27.3 nmol/L; P = .024), suggesting that the test is most valuable for the subset of children requiring dose adjustment, Mr. Rahman said.
"Patients who respond won’t even need the test, but for those at the 12-week juncture who do not respond and their levels are less than 30 nmol/L, this [study] suggests that if we increase the PO [dose] or change to subcutaneous [administration], we will eventually have a response," he said.
During a discussion of the results, an audience member questioned whether the test, with its added expense, really enhances clinical judgment because most clinicians would already adjust the dose in their patients failing to respond at 12 weeks.
Mr. Rahman replied that not all patients will respond to continued treatment with methotrexate, and that the test could differentiate between responders and nonresponders, thereby directing the patient to alternative therapy earlier.
Session moderator Dr. Amy Paller, chair of dermatology and professor of pediatrics and dermatology at Northwestern University in Chicago, agreed that the test is most valuable for the subset of children who are nonresponders at 12 weeks.
"In these children, this test can guide dose modification," she said in an interview. "Pharmacogenetic variation can require relatively higher oral doses or subcutaneous administration."
In contrast to a therapeutic level of more than 60 nmol/L established for adults, a level of 30 nmol/L may be sufficient in children, Dr. Paller said.
"A MTX PG3 less than 30 nmol/L in a child who has failed to respond within 12 weeks suggests that an increase in oral dose, or even a change to subcutaneous [administration], will boost efficacy," she commented.
Mr. Rahman, Dr. Siegfried, and Dr. Paller reported having no relevant financial disclosures.
AT THE ANNUAL MEETING OF THE SOCIETY FOR PEDIATRIC DERMATOLOGY
Major finding: Late responders had the highest methotrexate levels (41.9 nmol/L vs. 27.3 nmol/L in early responders; P = .024) on the MTX PG3 assay.
Data source: A retrospective study of 46 children on methotrexate for atopic dermatitis, psoriasis, or atopic dermatitis–psoriasis overlap who were assessed by MTX PG3 assay.
Disclosures: Mr. Rahman, Dr. Siegfried, and Dr. Paller reported having no relevant financial disclosures.
Expert panel sets broad SpA treat-to-target goals
MADRID – A panel of clinicians with expertise in the management of patients with spondyloarthritis took another step toward better defining this disease category and the goals of its treatment by producing the first "treat-to-target" recommendations for spondyloarthritis.
The new recommendations will "guide physicians, patients, and other stakeholders on how to optimize reduction of signs and symptoms and, ideally, outcomes, and will drive" the current research agenda, Dr. Josef S. Smolen said while presenting the panel’s recommendations at the annual European Congress of Rheumatology.
"Treat to target proposes a stepwise approach to achieving optimal outcomes based on available evidence and expert opinion. Treat to target has been successfully applied to management of diabetes, hypertension, hyperlipidemia, and rheumatoid arthritis," and the new task force set out to address whether it could be used when treating patients with spondyloarthritis (SpA), a question that required the expert task force to review the evidence for setting specific treatment goals for patients with SpA, said Dr. Smolen, professor of medicine at the Medical University of Vienna.
The task force eventually decided that the treat-to-target concept was applicable to SpA, but that the data available so far prevented the task force from setting specific treatment goals. While the recommendations underscore the importance of treating SpA patients with a goal of remission or inactive disease, and failing that, at least low disease activity, they fall short of giving clinicians guidance on how to best measure and define remission or low disease activity or how to achieve these goals.
For example, for axial SpA – the subtype that received the most specific recommendations – the panel advised clinicians to guide treatment decisions by using a "validated composite measure of disease activity" such as the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) plus acute phase reactants, or the Ankylosing Spondylitis Disease Activity Score (ASDAS) with or without functional measures such as the Bath Ankylosing Spondylitis Functional Index (BASFI). But the recommendations say nothing about what scores by these measures would define remission or low disease activity. The recommendations also talk about using other factors to further gauge axial SpA disease activity such as MRI analysis of inflammation and radiographic progression, but again Dr. Smolen provided no specific targets when using these measures.
For other, less well studied types of SpA, the new recommendations were even more nebulous. For psoriatic arthritis, the panel indicated that "validated measures of musculoskeletal disease activity" should be "performed and documented regularly" as should other factors such as spinal and extraarticular manifestations, imaging findings, and comorbidities, but the recommendations gave no specifics on what any of these measures might be or what level might equal remission or low disease. The same held true for peripheral SpA.
Other factors touched on by the new recommendations include the need for individualized and shared decision making between physicians and patients, the need for coordination of care among rheumatologists and other medical specialists who treat SpA patients (dermatologists, gastroenterologists, and ophthalmologists), and the need to take into account extraarticular manifestations of SpA diseases, comorbidities, and treatment risks along with the goal of low disease activity.
"I think it was important to document that there is a paucity of trials that have looked at strategies to treat" SpA. "For rheumatoid arthritis we have strategies, but not in spondyloarthritis, and I think it is important to say that," said Dr. Jürgen Braun, medical director of Rheumazentrum Ruhrgebiet in Herne, Germany.
SpA also lags behind rheumatoid arthritis by not having any treatments proven to slow radiographic progression. "Although we say that it is presumably important to treat inflammation [in patients with SpA], we are not sure," said Dr. Braun, who served as a member of the treat-to-target task force.
Even though the task force’s report highlights the absence of evidence for most facets of SpA management, "in the absence of evidence you need eminence, and that’s what was produced." Dr. Braun predicted that rheumatologists and other clinicians who care for patients with SpA would welcome these new recommendations despite their shortcomings. "What we say is what clinicians feel: If a patient’s CRP is elevated we must do something about it, but the evidence supporting this is limited. Presumably, it is important to treat inflammation, but we are not yet sure," he said in an interview.
"We don’t have trial results yet where you set up a quantifiable endpoint as your target, but that is coming," said Dr. Philip J. Mease, director of the rheumatology clinical research division at Swedish Medical Center in Seattle and a member of the task force. For example, the results from the Tight Control of Psoriatic Arthritis (TICOPA) trial "will tell us whether more aggressive treatment to a quantifiable target is appropriate and makes a difference. We anticipate that it will, but evidence is currently lacking." The primary endpoint of the TICOPA trial is change in the ACR20 response, but the trial includes several other clinical measures as secondary endpoints, including the Assessment in Ankylosing Spondylitis (ASAS), the BASDAI, and the psoriasis area severity index (PASI).
"The rheumatoid model is prompting us to develop quantifiable measures like the ASDAS and the new Psoriatic Arthritis Disease Activity Score (PASDAS) that we’ll start to see used. I just spoke with a colleague about the need to also develop a similar measure for peripheral SpA," Dr. Mease said in an interview.
The task force that developed the treat-to-target recommendations included 16 physicians and patients on its steering committee and 16 on an advisory committee. The majority came from various European locations, but about a quarter of the task force members were from the United States. To arrive at its recommendations, the task force used a comprehensive literature review that identified 22 published reports that addressed treatment targets for SpA (Ann. Rheum. Dis. 2013 June 10 [doi:10.1136/annrheumdis-2013-203860]). The treat-to-target recommendations were published online a few days before Dr. Smolen’s presentation at the meeting (Ann. Rheum. Dis. 2013 June 8 [doi:10.1136/annrheumdis-2013-203419]).
Dr. Smolen, Dr. Braun, and Dr. Mease said that they had no disclosures relevant to the topic.
On Twitter @mitchelzoler
MADRID – A panel of clinicians with expertise in the management of patients with spondyloarthritis took another step toward better defining this disease category and the goals of its treatment by producing the first "treat-to-target" recommendations for spondyloarthritis.
The new recommendations will "guide physicians, patients, and other stakeholders on how to optimize reduction of signs and symptoms and, ideally, outcomes, and will drive" the current research agenda, Dr. Josef S. Smolen said while presenting the panel’s recommendations at the annual European Congress of Rheumatology.
"Treat to target proposes a stepwise approach to achieving optimal outcomes based on available evidence and expert opinion. Treat to target has been successfully applied to management of diabetes, hypertension, hyperlipidemia, and rheumatoid arthritis," and the new task force set out to address whether it could be used when treating patients with spondyloarthritis (SpA), a question that required the expert task force to review the evidence for setting specific treatment goals for patients with SpA, said Dr. Smolen, professor of medicine at the Medical University of Vienna.
The task force eventually decided that the treat-to-target concept was applicable to SpA, but that the data available so far prevented the task force from setting specific treatment goals. While the recommendations underscore the importance of treating SpA patients with a goal of remission or inactive disease, and failing that, at least low disease activity, they fall short of giving clinicians guidance on how to best measure and define remission or low disease activity or how to achieve these goals.
For example, for axial SpA – the subtype that received the most specific recommendations – the panel advised clinicians to guide treatment decisions by using a "validated composite measure of disease activity" such as the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) plus acute phase reactants, or the Ankylosing Spondylitis Disease Activity Score (ASDAS) with or without functional measures such as the Bath Ankylosing Spondylitis Functional Index (BASFI). But the recommendations say nothing about what scores by these measures would define remission or low disease activity. The recommendations also talk about using other factors to further gauge axial SpA disease activity such as MRI analysis of inflammation and radiographic progression, but again Dr. Smolen provided no specific targets when using these measures.
For other, less well studied types of SpA, the new recommendations were even more nebulous. For psoriatic arthritis, the panel indicated that "validated measures of musculoskeletal disease activity" should be "performed and documented regularly" as should other factors such as spinal and extraarticular manifestations, imaging findings, and comorbidities, but the recommendations gave no specifics on what any of these measures might be or what level might equal remission or low disease. The same held true for peripheral SpA.
Other factors touched on by the new recommendations include the need for individualized and shared decision making between physicians and patients, the need for coordination of care among rheumatologists and other medical specialists who treat SpA patients (dermatologists, gastroenterologists, and ophthalmologists), and the need to take into account extraarticular manifestations of SpA diseases, comorbidities, and treatment risks along with the goal of low disease activity.
"I think it was important to document that there is a paucity of trials that have looked at strategies to treat" SpA. "For rheumatoid arthritis we have strategies, but not in spondyloarthritis, and I think it is important to say that," said Dr. Jürgen Braun, medical director of Rheumazentrum Ruhrgebiet in Herne, Germany.
SpA also lags behind rheumatoid arthritis by not having any treatments proven to slow radiographic progression. "Although we say that it is presumably important to treat inflammation [in patients with SpA], we are not sure," said Dr. Braun, who served as a member of the treat-to-target task force.
Even though the task force’s report highlights the absence of evidence for most facets of SpA management, "in the absence of evidence you need eminence, and that’s what was produced." Dr. Braun predicted that rheumatologists and other clinicians who care for patients with SpA would welcome these new recommendations despite their shortcomings. "What we say is what clinicians feel: If a patient’s CRP is elevated we must do something about it, but the evidence supporting this is limited. Presumably, it is important to treat inflammation, but we are not yet sure," he said in an interview.
"We don’t have trial results yet where you set up a quantifiable endpoint as your target, but that is coming," said Dr. Philip J. Mease, director of the rheumatology clinical research division at Swedish Medical Center in Seattle and a member of the task force. For example, the results from the Tight Control of Psoriatic Arthritis (TICOPA) trial "will tell us whether more aggressive treatment to a quantifiable target is appropriate and makes a difference. We anticipate that it will, but evidence is currently lacking." The primary endpoint of the TICOPA trial is change in the ACR20 response, but the trial includes several other clinical measures as secondary endpoints, including the Assessment in Ankylosing Spondylitis (ASAS), the BASDAI, and the psoriasis area severity index (PASI).
"The rheumatoid model is prompting us to develop quantifiable measures like the ASDAS and the new Psoriatic Arthritis Disease Activity Score (PASDAS) that we’ll start to see used. I just spoke with a colleague about the need to also develop a similar measure for peripheral SpA," Dr. Mease said in an interview.
The task force that developed the treat-to-target recommendations included 16 physicians and patients on its steering committee and 16 on an advisory committee. The majority came from various European locations, but about a quarter of the task force members were from the United States. To arrive at its recommendations, the task force used a comprehensive literature review that identified 22 published reports that addressed treatment targets for SpA (Ann. Rheum. Dis. 2013 June 10 [doi:10.1136/annrheumdis-2013-203860]). The treat-to-target recommendations were published online a few days before Dr. Smolen’s presentation at the meeting (Ann. Rheum. Dis. 2013 June 8 [doi:10.1136/annrheumdis-2013-203419]).
Dr. Smolen, Dr. Braun, and Dr. Mease said that they had no disclosures relevant to the topic.
On Twitter @mitchelzoler
MADRID – A panel of clinicians with expertise in the management of patients with spondyloarthritis took another step toward better defining this disease category and the goals of its treatment by producing the first "treat-to-target" recommendations for spondyloarthritis.
The new recommendations will "guide physicians, patients, and other stakeholders on how to optimize reduction of signs and symptoms and, ideally, outcomes, and will drive" the current research agenda, Dr. Josef S. Smolen said while presenting the panel’s recommendations at the annual European Congress of Rheumatology.
"Treat to target proposes a stepwise approach to achieving optimal outcomes based on available evidence and expert opinion. Treat to target has been successfully applied to management of diabetes, hypertension, hyperlipidemia, and rheumatoid arthritis," and the new task force set out to address whether it could be used when treating patients with spondyloarthritis (SpA), a question that required the expert task force to review the evidence for setting specific treatment goals for patients with SpA, said Dr. Smolen, professor of medicine at the Medical University of Vienna.
The task force eventually decided that the treat-to-target concept was applicable to SpA, but that the data available so far prevented the task force from setting specific treatment goals. While the recommendations underscore the importance of treating SpA patients with a goal of remission or inactive disease, and failing that, at least low disease activity, they fall short of giving clinicians guidance on how to best measure and define remission or low disease activity or how to achieve these goals.
For example, for axial SpA – the subtype that received the most specific recommendations – the panel advised clinicians to guide treatment decisions by using a "validated composite measure of disease activity" such as the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) plus acute phase reactants, or the Ankylosing Spondylitis Disease Activity Score (ASDAS) with or without functional measures such as the Bath Ankylosing Spondylitis Functional Index (BASFI). But the recommendations say nothing about what scores by these measures would define remission or low disease activity. The recommendations also talk about using other factors to further gauge axial SpA disease activity such as MRI analysis of inflammation and radiographic progression, but again Dr. Smolen provided no specific targets when using these measures.
For other, less well studied types of SpA, the new recommendations were even more nebulous. For psoriatic arthritis, the panel indicated that "validated measures of musculoskeletal disease activity" should be "performed and documented regularly" as should other factors such as spinal and extraarticular manifestations, imaging findings, and comorbidities, but the recommendations gave no specifics on what any of these measures might be or what level might equal remission or low disease. The same held true for peripheral SpA.
Other factors touched on by the new recommendations include the need for individualized and shared decision making between physicians and patients, the need for coordination of care among rheumatologists and other medical specialists who treat SpA patients (dermatologists, gastroenterologists, and ophthalmologists), and the need to take into account extraarticular manifestations of SpA diseases, comorbidities, and treatment risks along with the goal of low disease activity.
"I think it was important to document that there is a paucity of trials that have looked at strategies to treat" SpA. "For rheumatoid arthritis we have strategies, but not in spondyloarthritis, and I think it is important to say that," said Dr. Jürgen Braun, medical director of Rheumazentrum Ruhrgebiet in Herne, Germany.
SpA also lags behind rheumatoid arthritis by not having any treatments proven to slow radiographic progression. "Although we say that it is presumably important to treat inflammation [in patients with SpA], we are not sure," said Dr. Braun, who served as a member of the treat-to-target task force.
Even though the task force’s report highlights the absence of evidence for most facets of SpA management, "in the absence of evidence you need eminence, and that’s what was produced." Dr. Braun predicted that rheumatologists and other clinicians who care for patients with SpA would welcome these new recommendations despite their shortcomings. "What we say is what clinicians feel: If a patient’s CRP is elevated we must do something about it, but the evidence supporting this is limited. Presumably, it is important to treat inflammation, but we are not yet sure," he said in an interview.
"We don’t have trial results yet where you set up a quantifiable endpoint as your target, but that is coming," said Dr. Philip J. Mease, director of the rheumatology clinical research division at Swedish Medical Center in Seattle and a member of the task force. For example, the results from the Tight Control of Psoriatic Arthritis (TICOPA) trial "will tell us whether more aggressive treatment to a quantifiable target is appropriate and makes a difference. We anticipate that it will, but evidence is currently lacking." The primary endpoint of the TICOPA trial is change in the ACR20 response, but the trial includes several other clinical measures as secondary endpoints, including the Assessment in Ankylosing Spondylitis (ASAS), the BASDAI, and the psoriasis area severity index (PASI).
"The rheumatoid model is prompting us to develop quantifiable measures like the ASDAS and the new Psoriatic Arthritis Disease Activity Score (PASDAS) that we’ll start to see used. I just spoke with a colleague about the need to also develop a similar measure for peripheral SpA," Dr. Mease said in an interview.
The task force that developed the treat-to-target recommendations included 16 physicians and patients on its steering committee and 16 on an advisory committee. The majority came from various European locations, but about a quarter of the task force members were from the United States. To arrive at its recommendations, the task force used a comprehensive literature review that identified 22 published reports that addressed treatment targets for SpA (Ann. Rheum. Dis. 2013 June 10 [doi:10.1136/annrheumdis-2013-203860]). The treat-to-target recommendations were published online a few days before Dr. Smolen’s presentation at the meeting (Ann. Rheum. Dis. 2013 June 8 [doi:10.1136/annrheumdis-2013-203419]).
Dr. Smolen, Dr. Braun, and Dr. Mease said that they had no disclosures relevant to the topic.
On Twitter @mitchelzoler
AT THE EULAR CONGRESS 2013
Squamous Cell Carcinoma of the Lip Associated With Adalimumab Therapy for Ankylosing Spondylitis: A Case Report and Review of TNF-α Inhibitors and Cutaneous Carcinoma Risk
Scleroderma patients suffer from small bowel bacterial overgrowth
MADRID – More than a third of patients with systemic sclerosis and intestinal symptoms have an increase in gastrointestinal tract bacteria, an alteration in the type of gut microbes present, or both, based on data from a French study presented at the annual European Congress of Rheumatology.
Small intestinal bacterial overgrowth (SIBO) was found to affect 14 (38%) of 37 patients included in the study. These patients had been recruited from a larger group of 120 scleroderma patients who complained of gastrointestinal symptoms over a 2-year period.
"SIBO can compromise patients’ quality of life and be responsible for mortality," study investigator Dr. Marie Tauber said. SIBO can cause GI symptoms such as bloating, diarrhea, malabsorption, weight loss, and malnutrition, which may have a significant impact on patients’ overall prognosis, she noted.
Dr. Tauber, a dermatologist who took part in the research as part of an internship within the rheumatology department of the Hôpital Cochin in Paris, explained the three goals of the study. The first was to examine the prevalence of SIBO in patients with systemic sclerosis exhibiting GI symptoms, and the second was to identify subsets of patients who might be at increased risk. A third goal was to observe the impact of optimal SIBO treatment on the patients’ conditions.
The median age of participants was 59 years, and 79% were women. The median disease duration was approximately 10 years, and 49% of patients had diffuse cutaneous disease.
A diagnosis of SIBO was based on positive hydrogen and methane breath tests, and blood assays were used to assess the presence of malabsorption.
The researchers also administered two questionnaires – the generic SF-36 (Short Form 36) Health Survey and the disease-specific UCLA SCTC GIT (University of California, Los Angeles, Scleroderma Clinical Trial Consortium Gastrointestinal Tract Instrument) – to patients at the time of their breath testing visits.
All patients with SIBO were treated with a rotating regimen of amoxicillin, ciprofloxacin, and metronidazole, each given for 1 month at a time. Breath tests, as well as the UCLA SCTC GIT, were repeated. Fewer than 50% of patients had a negative breath test after antibody treatment, which highlighted a need to repeat the test after antibiotic treatment to determine whether a second course is required, Dr. Tauber noted.
Three clinical parameters separated patients with and without SIBO: longer disease duration (11 vs. 7 years; P = .02), lower prevalence of anti-topoisomerase I antibodies (Anti-Scl-70 Ab, 7% vs. 39%; P = .04), and higher prevalence of definite pulmonary arterial hypertension (PAH, 21% vs. 0%; P = .04).
Total UCLA SCTC GIT scores were higher in patients with SIBO than in those without it (0.79 vs. 0.31; P = .03). SIBO-affected patients also were more likely to have weight loss of 5% or more (43% vs. 8%; P = .03).
Given the small number of patients, it is difficult to draw firm conclusions from these data, and larger studies are needed, Dr. Tauber observed. However, the findings suggest that there may be factors associated with SIBO that could be targeted in an intervention program.
Two patients with SIBO died despite antibiotic treatment, which "underscores, unfortunately, the association between SIBO and mortality," Dr. Tauber said.
"To our knowledge, this is the first study using the UCLA SCTC GIT to identify patients at risk of SIBO in systemic sclerosis," and the data support the systematic use of this score, together with regular weight evaluation in these patients, she concluded.
Dr. Tauber had no financial conflicts to disclose.
MADRID – More than a third of patients with systemic sclerosis and intestinal symptoms have an increase in gastrointestinal tract bacteria, an alteration in the type of gut microbes present, or both, based on data from a French study presented at the annual European Congress of Rheumatology.
Small intestinal bacterial overgrowth (SIBO) was found to affect 14 (38%) of 37 patients included in the study. These patients had been recruited from a larger group of 120 scleroderma patients who complained of gastrointestinal symptoms over a 2-year period.
"SIBO can compromise patients’ quality of life and be responsible for mortality," study investigator Dr. Marie Tauber said. SIBO can cause GI symptoms such as bloating, diarrhea, malabsorption, weight loss, and malnutrition, which may have a significant impact on patients’ overall prognosis, she noted.
Dr. Tauber, a dermatologist who took part in the research as part of an internship within the rheumatology department of the Hôpital Cochin in Paris, explained the three goals of the study. The first was to examine the prevalence of SIBO in patients with systemic sclerosis exhibiting GI symptoms, and the second was to identify subsets of patients who might be at increased risk. A third goal was to observe the impact of optimal SIBO treatment on the patients’ conditions.
The median age of participants was 59 years, and 79% were women. The median disease duration was approximately 10 years, and 49% of patients had diffuse cutaneous disease.
A diagnosis of SIBO was based on positive hydrogen and methane breath tests, and blood assays were used to assess the presence of malabsorption.
The researchers also administered two questionnaires – the generic SF-36 (Short Form 36) Health Survey and the disease-specific UCLA SCTC GIT (University of California, Los Angeles, Scleroderma Clinical Trial Consortium Gastrointestinal Tract Instrument) – to patients at the time of their breath testing visits.
All patients with SIBO were treated with a rotating regimen of amoxicillin, ciprofloxacin, and metronidazole, each given for 1 month at a time. Breath tests, as well as the UCLA SCTC GIT, were repeated. Fewer than 50% of patients had a negative breath test after antibody treatment, which highlighted a need to repeat the test after antibiotic treatment to determine whether a second course is required, Dr. Tauber noted.
Three clinical parameters separated patients with and without SIBO: longer disease duration (11 vs. 7 years; P = .02), lower prevalence of anti-topoisomerase I antibodies (Anti-Scl-70 Ab, 7% vs. 39%; P = .04), and higher prevalence of definite pulmonary arterial hypertension (PAH, 21% vs. 0%; P = .04).
Total UCLA SCTC GIT scores were higher in patients with SIBO than in those without it (0.79 vs. 0.31; P = .03). SIBO-affected patients also were more likely to have weight loss of 5% or more (43% vs. 8%; P = .03).
Given the small number of patients, it is difficult to draw firm conclusions from these data, and larger studies are needed, Dr. Tauber observed. However, the findings suggest that there may be factors associated with SIBO that could be targeted in an intervention program.
Two patients with SIBO died despite antibiotic treatment, which "underscores, unfortunately, the association between SIBO and mortality," Dr. Tauber said.
"To our knowledge, this is the first study using the UCLA SCTC GIT to identify patients at risk of SIBO in systemic sclerosis," and the data support the systematic use of this score, together with regular weight evaluation in these patients, she concluded.
Dr. Tauber had no financial conflicts to disclose.
MADRID – More than a third of patients with systemic sclerosis and intestinal symptoms have an increase in gastrointestinal tract bacteria, an alteration in the type of gut microbes present, or both, based on data from a French study presented at the annual European Congress of Rheumatology.
Small intestinal bacterial overgrowth (SIBO) was found to affect 14 (38%) of 37 patients included in the study. These patients had been recruited from a larger group of 120 scleroderma patients who complained of gastrointestinal symptoms over a 2-year period.
"SIBO can compromise patients’ quality of life and be responsible for mortality," study investigator Dr. Marie Tauber said. SIBO can cause GI symptoms such as bloating, diarrhea, malabsorption, weight loss, and malnutrition, which may have a significant impact on patients’ overall prognosis, she noted.
Dr. Tauber, a dermatologist who took part in the research as part of an internship within the rheumatology department of the Hôpital Cochin in Paris, explained the three goals of the study. The first was to examine the prevalence of SIBO in patients with systemic sclerosis exhibiting GI symptoms, and the second was to identify subsets of patients who might be at increased risk. A third goal was to observe the impact of optimal SIBO treatment on the patients’ conditions.
The median age of participants was 59 years, and 79% were women. The median disease duration was approximately 10 years, and 49% of patients had diffuse cutaneous disease.
A diagnosis of SIBO was based on positive hydrogen and methane breath tests, and blood assays were used to assess the presence of malabsorption.
The researchers also administered two questionnaires – the generic SF-36 (Short Form 36) Health Survey and the disease-specific UCLA SCTC GIT (University of California, Los Angeles, Scleroderma Clinical Trial Consortium Gastrointestinal Tract Instrument) – to patients at the time of their breath testing visits.
All patients with SIBO were treated with a rotating regimen of amoxicillin, ciprofloxacin, and metronidazole, each given for 1 month at a time. Breath tests, as well as the UCLA SCTC GIT, were repeated. Fewer than 50% of patients had a negative breath test after antibody treatment, which highlighted a need to repeat the test after antibiotic treatment to determine whether a second course is required, Dr. Tauber noted.
Three clinical parameters separated patients with and without SIBO: longer disease duration (11 vs. 7 years; P = .02), lower prevalence of anti-topoisomerase I antibodies (Anti-Scl-70 Ab, 7% vs. 39%; P = .04), and higher prevalence of definite pulmonary arterial hypertension (PAH, 21% vs. 0%; P = .04).
Total UCLA SCTC GIT scores were higher in patients with SIBO than in those without it (0.79 vs. 0.31; P = .03). SIBO-affected patients also were more likely to have weight loss of 5% or more (43% vs. 8%; P = .03).
Given the small number of patients, it is difficult to draw firm conclusions from these data, and larger studies are needed, Dr. Tauber observed. However, the findings suggest that there may be factors associated with SIBO that could be targeted in an intervention program.
Two patients with SIBO died despite antibiotic treatment, which "underscores, unfortunately, the association between SIBO and mortality," Dr. Tauber said.
"To our knowledge, this is the first study using the UCLA SCTC GIT to identify patients at risk of SIBO in systemic sclerosis," and the data support the systematic use of this score, together with regular weight evaluation in these patients, she concluded.
Dr. Tauber had no financial conflicts to disclose.
AT THE EULAR CONGRESS 2013
Major finding: SIBO affected 14 (38%) of 37 scleroderma patients with gastrointestinal symptoms.
Data source: Observational study of 120 adults with systemic sclerosis and intestinal symptoms.
Disclosures: Dr. Tauber had no financial conflicts to disclose.
Apremilast effects sustained at 1 year in psoriatic arthritis
MADRID – Apremilast improves the signs and symptoms of psoriatic arthritis in about 60% of patients at 1 year, according to long-term data from the PALACE 1 trial.
At week 52, a 20% improvement in disease symptoms according to American College of Rheumatology (ACR 20) response criteria was achieved by 57%-63% of patients treated with apremilast, providing evidence of sustained treatment effects.
The PALACE 1 trial’s primary endpoint of an ACR 20 at 16 weeks, already reported last year, was achieved by 31% of patients treated with apremilast 20 mg and 40% of those given apremilast 30 mg, compared with 19% of those given placebo.
"Oral apremilast demonstrated long-term efficacy, including improvement in signs and symptoms and physical function, and skin manifestations," Dr. Arthur Kavanaugh, professor of medicine at the University of California, San Diego, said at the annual European Congress of Rheumatology.
Apremilast is an oral phosphodiesterase 4 inhibitor under investigation as a treatment for active psoriatic arthritis (PsA). It is being evaluated as a possible treatment for skin psoriasis, ankylosing spondylitis, rheumatoid arthritis, and Behçet’s disease.
PALACE 1 was a phase III, multicenter, double-blind, placebo-controlled study of apremilast for the treatment of active PsA. A total of 504 patients with a documented diagnosis of PsA for at least 6 months were recruited into the study (Ann. Rheum. Dis. 2013;72[Suppls3]:163).
Functional outcomes improved
Physical function improved according to measurements on the Health Assessment Questionnaire–Disability Index (HAQ-DI). At baseline, HAQ-DI scores were 1.21, and "we saw that patients improved by –0.35, which is certainly the level that patients can say, ‘I feel better and I can do my daily activities better,’ " Dr. Kavanaugh said.
Additionally, 25% and 37% of patients treated with apremilast 20 mg and 30 mg, respectively, achieved a 75% improvement in the Psoriasis Area and Severity Index at 52 weeks, which is a "very high bar" to achieve, he noted.
The main side effect seen was diarrhea, affecting 11%-19% of patients given apremilast and 2.4% given placebo. However, diarrhea occurred mainly in the first 6 months of therapy and could be managed by taking appropriate measures on an individual patient basis, Dr. Kavanaugh said. This might include prescription of an antidiarrheal agent.
"Prolonged exposure to apremilast did not result in any unexpected increased incidence of adverse events or laboratory abnormalities," he noted. The latter could mean that, if approved, apremilast might not need routine laboratory monitoring.
The PALACE development program
PALACE 1 is one of several clinical trials that have investigated the efficacy and safety of apremilast in active PsA. In these studies, 24 weeks’ treatment with one of two oral doses (20 mg or 30 mg twice daily) of apremilast was compared to placebo. The primary endpoint of the studies was the percentage of patients achieving ACR 20 at 16 weeks.
For inclusion in the trials, patients had to have active disease despite prior therapy with disease-modifying antirheumatic drugs (DMARDs), biologic agents, or both. Dr. Kavanaugh noted that the majority of patients had failed DMARD therapy in PALACE 1, with almost one-quarter receiving prior biologic therapy.
Patients who had a less than 20% reduction from baseline in swollen/tender joint counts at 16 weeks were re-randomized to receive apremilast 20 mg or 30 mg if they had originally been treated with placebo, while patients originally randomized to active treatment stayed on their initial dose if they failed to respond significantly. At the end of the planned 24-week treatment period, all remaining patients on placebo were re-randomized to apremilast 20 mg or 30 mg until 1 year of follow up.
PALACE 3 data also reported
The results of PALACE 3 and combined 6-month safety data from the PALACE 1, PALACE 2, and PALACE 3 trials were also reported at the congress.
In PALACE 3 (Ann. Rheum. Dis. 2013;72[Suppl. 3]:685), significantly more patients achieved the primary endpoint of an ACR 20 at 16 weeks if they were treated with either the 20-mg dose (29.4%, P = .02) or 30-mg dose (42.8%, P less than .0001) of apremilast, compared with those given placebo (18.9%). There was also statistically significant and "clinically meaningful" improvement in physical function and pain. "The results of PALACE 3 support the efficacy and safety findings of the PALACE 1 study and help establish the profile of apremilast in PsA," the PALACE 3 investigators concluded.
The pooled safety findings revealed no new safety concerns and showed apremilast was generally well tolerated (Ann. Rheum. Dis. 2013;72[Suppl. 3]:85). Rates of diarrhea at 24 weeks were 12.6% and 16.5% for the 20-mg and 30-mg doses of apremilast, and 2.8% for placebo, respectively. Other side effects of note included nausea (10% and 16.1% vs. 4.6%), headache (8.4% and 11.5% vs. 4.6%), and upper respiratory tract infection (7% and 6% vs. 3%).
Time for regulatory approval
Based on the positive findings of the PALACE 1, 2, and 3 studies, apremilast’s developer, Celgene, is expected to file for regulatory approval in the treatment of active PsA. In doing so, apremilast will join another novel agent, ustekinumab, in the queue for approval for this indication.
Ustekinumab is a human interleukin-12 and -23 antagonist produced by Janssen that is already approved in Europe and in the United States for skin psoriasis. One-year data also show that it is effective and well tolerated for PsA. It is given subcutaneously, whereas apremilast is an oral agent.
Dr. Kavanaugh has provided expert advice to and/or received research grants from the following companies: AstraZeneca, Bristol-Myers Squibb, Celgene, Centocor-Janssen, Pfizer, Roche, and UCB.
MADRID – Apremilast improves the signs and symptoms of psoriatic arthritis in about 60% of patients at 1 year, according to long-term data from the PALACE 1 trial.
At week 52, a 20% improvement in disease symptoms according to American College of Rheumatology (ACR 20) response criteria was achieved by 57%-63% of patients treated with apremilast, providing evidence of sustained treatment effects.
The PALACE 1 trial’s primary endpoint of an ACR 20 at 16 weeks, already reported last year, was achieved by 31% of patients treated with apremilast 20 mg and 40% of those given apremilast 30 mg, compared with 19% of those given placebo.
"Oral apremilast demonstrated long-term efficacy, including improvement in signs and symptoms and physical function, and skin manifestations," Dr. Arthur Kavanaugh, professor of medicine at the University of California, San Diego, said at the annual European Congress of Rheumatology.
Apremilast is an oral phosphodiesterase 4 inhibitor under investigation as a treatment for active psoriatic arthritis (PsA). It is being evaluated as a possible treatment for skin psoriasis, ankylosing spondylitis, rheumatoid arthritis, and Behçet’s disease.
PALACE 1 was a phase III, multicenter, double-blind, placebo-controlled study of apremilast for the treatment of active PsA. A total of 504 patients with a documented diagnosis of PsA for at least 6 months were recruited into the study (Ann. Rheum. Dis. 2013;72[Suppls3]:163).
Functional outcomes improved
Physical function improved according to measurements on the Health Assessment Questionnaire–Disability Index (HAQ-DI). At baseline, HAQ-DI scores were 1.21, and "we saw that patients improved by –0.35, which is certainly the level that patients can say, ‘I feel better and I can do my daily activities better,’ " Dr. Kavanaugh said.
Additionally, 25% and 37% of patients treated with apremilast 20 mg and 30 mg, respectively, achieved a 75% improvement in the Psoriasis Area and Severity Index at 52 weeks, which is a "very high bar" to achieve, he noted.
The main side effect seen was diarrhea, affecting 11%-19% of patients given apremilast and 2.4% given placebo. However, diarrhea occurred mainly in the first 6 months of therapy and could be managed by taking appropriate measures on an individual patient basis, Dr. Kavanaugh said. This might include prescription of an antidiarrheal agent.
"Prolonged exposure to apremilast did not result in any unexpected increased incidence of adverse events or laboratory abnormalities," he noted. The latter could mean that, if approved, apremilast might not need routine laboratory monitoring.
The PALACE development program
PALACE 1 is one of several clinical trials that have investigated the efficacy and safety of apremilast in active PsA. In these studies, 24 weeks’ treatment with one of two oral doses (20 mg or 30 mg twice daily) of apremilast was compared to placebo. The primary endpoint of the studies was the percentage of patients achieving ACR 20 at 16 weeks.
For inclusion in the trials, patients had to have active disease despite prior therapy with disease-modifying antirheumatic drugs (DMARDs), biologic agents, or both. Dr. Kavanaugh noted that the majority of patients had failed DMARD therapy in PALACE 1, with almost one-quarter receiving prior biologic therapy.
Patients who had a less than 20% reduction from baseline in swollen/tender joint counts at 16 weeks were re-randomized to receive apremilast 20 mg or 30 mg if they had originally been treated with placebo, while patients originally randomized to active treatment stayed on their initial dose if they failed to respond significantly. At the end of the planned 24-week treatment period, all remaining patients on placebo were re-randomized to apremilast 20 mg or 30 mg until 1 year of follow up.
PALACE 3 data also reported
The results of PALACE 3 and combined 6-month safety data from the PALACE 1, PALACE 2, and PALACE 3 trials were also reported at the congress.
In PALACE 3 (Ann. Rheum. Dis. 2013;72[Suppl. 3]:685), significantly more patients achieved the primary endpoint of an ACR 20 at 16 weeks if they were treated with either the 20-mg dose (29.4%, P = .02) or 30-mg dose (42.8%, P less than .0001) of apremilast, compared with those given placebo (18.9%). There was also statistically significant and "clinically meaningful" improvement in physical function and pain. "The results of PALACE 3 support the efficacy and safety findings of the PALACE 1 study and help establish the profile of apremilast in PsA," the PALACE 3 investigators concluded.
The pooled safety findings revealed no new safety concerns and showed apremilast was generally well tolerated (Ann. Rheum. Dis. 2013;72[Suppl. 3]:85). Rates of diarrhea at 24 weeks were 12.6% and 16.5% for the 20-mg and 30-mg doses of apremilast, and 2.8% for placebo, respectively. Other side effects of note included nausea (10% and 16.1% vs. 4.6%), headache (8.4% and 11.5% vs. 4.6%), and upper respiratory tract infection (7% and 6% vs. 3%).
Time for regulatory approval
Based on the positive findings of the PALACE 1, 2, and 3 studies, apremilast’s developer, Celgene, is expected to file for regulatory approval in the treatment of active PsA. In doing so, apremilast will join another novel agent, ustekinumab, in the queue for approval for this indication.
Ustekinumab is a human interleukin-12 and -23 antagonist produced by Janssen that is already approved in Europe and in the United States for skin psoriasis. One-year data also show that it is effective and well tolerated for PsA. It is given subcutaneously, whereas apremilast is an oral agent.
Dr. Kavanaugh has provided expert advice to and/or received research grants from the following companies: AstraZeneca, Bristol-Myers Squibb, Celgene, Centocor-Janssen, Pfizer, Roche, and UCB.
MADRID – Apremilast improves the signs and symptoms of psoriatic arthritis in about 60% of patients at 1 year, according to long-term data from the PALACE 1 trial.
At week 52, a 20% improvement in disease symptoms according to American College of Rheumatology (ACR 20) response criteria was achieved by 57%-63% of patients treated with apremilast, providing evidence of sustained treatment effects.
The PALACE 1 trial’s primary endpoint of an ACR 20 at 16 weeks, already reported last year, was achieved by 31% of patients treated with apremilast 20 mg and 40% of those given apremilast 30 mg, compared with 19% of those given placebo.
"Oral apremilast demonstrated long-term efficacy, including improvement in signs and symptoms and physical function, and skin manifestations," Dr. Arthur Kavanaugh, professor of medicine at the University of California, San Diego, said at the annual European Congress of Rheumatology.
Apremilast is an oral phosphodiesterase 4 inhibitor under investigation as a treatment for active psoriatic arthritis (PsA). It is being evaluated as a possible treatment for skin psoriasis, ankylosing spondylitis, rheumatoid arthritis, and Behçet’s disease.
PALACE 1 was a phase III, multicenter, double-blind, placebo-controlled study of apremilast for the treatment of active PsA. A total of 504 patients with a documented diagnosis of PsA for at least 6 months were recruited into the study (Ann. Rheum. Dis. 2013;72[Suppls3]:163).
Functional outcomes improved
Physical function improved according to measurements on the Health Assessment Questionnaire–Disability Index (HAQ-DI). At baseline, HAQ-DI scores were 1.21, and "we saw that patients improved by –0.35, which is certainly the level that patients can say, ‘I feel better and I can do my daily activities better,’ " Dr. Kavanaugh said.
Additionally, 25% and 37% of patients treated with apremilast 20 mg and 30 mg, respectively, achieved a 75% improvement in the Psoriasis Area and Severity Index at 52 weeks, which is a "very high bar" to achieve, he noted.
The main side effect seen was diarrhea, affecting 11%-19% of patients given apremilast and 2.4% given placebo. However, diarrhea occurred mainly in the first 6 months of therapy and could be managed by taking appropriate measures on an individual patient basis, Dr. Kavanaugh said. This might include prescription of an antidiarrheal agent.
"Prolonged exposure to apremilast did not result in any unexpected increased incidence of adverse events or laboratory abnormalities," he noted. The latter could mean that, if approved, apremilast might not need routine laboratory monitoring.
The PALACE development program
PALACE 1 is one of several clinical trials that have investigated the efficacy and safety of apremilast in active PsA. In these studies, 24 weeks’ treatment with one of two oral doses (20 mg or 30 mg twice daily) of apremilast was compared to placebo. The primary endpoint of the studies was the percentage of patients achieving ACR 20 at 16 weeks.
For inclusion in the trials, patients had to have active disease despite prior therapy with disease-modifying antirheumatic drugs (DMARDs), biologic agents, or both. Dr. Kavanaugh noted that the majority of patients had failed DMARD therapy in PALACE 1, with almost one-quarter receiving prior biologic therapy.
Patients who had a less than 20% reduction from baseline in swollen/tender joint counts at 16 weeks were re-randomized to receive apremilast 20 mg or 30 mg if they had originally been treated with placebo, while patients originally randomized to active treatment stayed on their initial dose if they failed to respond significantly. At the end of the planned 24-week treatment period, all remaining patients on placebo were re-randomized to apremilast 20 mg or 30 mg until 1 year of follow up.
PALACE 3 data also reported
The results of PALACE 3 and combined 6-month safety data from the PALACE 1, PALACE 2, and PALACE 3 trials were also reported at the congress.
In PALACE 3 (Ann. Rheum. Dis. 2013;72[Suppl. 3]:685), significantly more patients achieved the primary endpoint of an ACR 20 at 16 weeks if they were treated with either the 20-mg dose (29.4%, P = .02) or 30-mg dose (42.8%, P less than .0001) of apremilast, compared with those given placebo (18.9%). There was also statistically significant and "clinically meaningful" improvement in physical function and pain. "The results of PALACE 3 support the efficacy and safety findings of the PALACE 1 study and help establish the profile of apremilast in PsA," the PALACE 3 investigators concluded.
The pooled safety findings revealed no new safety concerns and showed apremilast was generally well tolerated (Ann. Rheum. Dis. 2013;72[Suppl. 3]:85). Rates of diarrhea at 24 weeks were 12.6% and 16.5% for the 20-mg and 30-mg doses of apremilast, and 2.8% for placebo, respectively. Other side effects of note included nausea (10% and 16.1% vs. 4.6%), headache (8.4% and 11.5% vs. 4.6%), and upper respiratory tract infection (7% and 6% vs. 3%).
Time for regulatory approval
Based on the positive findings of the PALACE 1, 2, and 3 studies, apremilast’s developer, Celgene, is expected to file for regulatory approval in the treatment of active PsA. In doing so, apremilast will join another novel agent, ustekinumab, in the queue for approval for this indication.
Ustekinumab is a human interleukin-12 and -23 antagonist produced by Janssen that is already approved in Europe and in the United States for skin psoriasis. One-year data also show that it is effective and well tolerated for PsA. It is given subcutaneously, whereas apremilast is an oral agent.
Dr. Kavanaugh has provided expert advice to and/or received research grants from the following companies: AstraZeneca, Bristol-Myers Squibb, Celgene, Centocor-Janssen, Pfizer, Roche, and UCB.
AT THE EULAR CONGRESS 2013
Major finding: ACR 20 response at 1 year was 57%-63% for patients given apremilast.
Data source: PALACE 1, a 504-patient, phase III, multicenter, double blind, placebo-controlled study of apremilast for the treatment of active psoriatic arthritis.
Disclosures: Celgene funded the study. Dr. Kavanaugh has provided expert advice to and/or received research grants from the following companies: AstraZeneca, Bristol-Myers Squibb, Celgene, Centocor-Janssen, Pfizer, Roche, and UCB.
Annual pulmonary hypertension screening recommended for systemic sclerosis
MADRID – Patients with systemic sclerosis should undergo annual screening for pulmonary arterial hypertension using a combination of transthoracic echocardiography and pulmonary function tests, an international expert panel said.
These are the first evidence- and consensus-based recommendations for pulmonary arterial hypertension (PAH) screening in patients with systemic sclerosis, and the panel also called for screening patients with mixed or other connective tissue diseases with scleroderma features. "Our hope is that these recommendations will lead to earlier detection of PAH in connective tissue diseases and improve patient outcomes," Dr. Dinesh Khanna said while presenting the screening recommendations at the annual European Congress of Rheumatology.
About 5%-15% of patients with systemic sclerosis develop PAH, and once PAH occurs, up to 30% of patients will die within 3 years, said Dr. Khanna, director of the scleroderma program at the University of Michigan, Ann Arbor.
"Despite having approved drugs available" to treat systemic sclerosis and other scleroderma-spectrum disorder connective tissue diseases, these treatments "have not had a huge impact on survival. The only thing we can offer patients is screening, followed by early diagnosis and treatment," Dr. Khanna said in an interview.
The new recommendations say that patients with a tricuspid regurgitant velocity measured by transthoracic echocardiography greater than 2.8 m/s require assessment for PAH by right heart catheterization. Right heart catheterization is also needed for patients with a tricuspid regurgitant velocity of 2.5-2.8 m/s if they also have signs or symptoms of PAH such as dyspnea, fatigue, chest pain, dizziness, loud pulmonary sound, or peripheral edema. Another echo finding that should trigger right heart catheterization regardless of signs or symptoms or tricuspid regurgitation is right atrial or ventricular enlargement.
The key measures on pulmonary function tests that trigger right heart catheterization is a forced vital capacity (FVC) to diffusion capacity of lungs for carbon monoxide (DLCO) ratio of more than 1.6, or a DLCO of less than 60% if either appears in the setting of PAH signs or symptoms. Alternatively, meeting either of these pulmonary criteria should lead to right heart catheterization regardless of signs and symptoms if the patient’s most recent blood level of N-terminal pro-brain natriuretic peptide (NT-ProBNP) was greater than twice the upper limit of normal.
The panel also said that patients should undergo right heart catheterization regardless of PAH signs and symptoms if they fulfill the screening algorithm developed for the DETECT study (Ann. Rheum. Dis. 2013 May 18 [doi:10.1036/annrheumdis-2013-203301]).
The panel recommended annual transthoracic echo and pulmonary function test screening, or more frequently if a patient shows new signs or symptoms. Measurement of NT-ProBNP should happen at baseline, and then be repeated if new signs or symptoms of PAH appear. They also recommended applying the full DETECT screening algorithm in patients diagnosed with systemic sclerosis or other scleroderma spectrum connective-tissue disease for more than 3 years and a DLCO that is less than 60%. Right heart catheterization is mandatory to definitively diagnose PAH, Dr. Khanna stressed. The panel also said screening is not needed in patients with mixed- or other connective tissue disorders who did not have scleroderma-like features.
In a separate report at the meeting Dr. Khanna and his associates assessed the ability of transthoracic echocardiography and pulmonary function tests to screen patients with PAH. They used data from 69 patients with PAH in two separate reported series that together had 347 patients with systemic sclerosis who underwent assessment for suspected PAH (J. Rheumatol. 2011;38:2172-9 and J. Rheumatol. 2010;37:2290-8).
The new, retrospective analysis showed that combining transthoracic echo and pulmonary-function test screens can have a negative predictive accuracy of 98% for correctly ruling out PAH in patients with systemic sclerosis, reported Dr. Heather Gladue, a rheumatology fellow at the University of Michigan.
The recommendations panel cautioned that its proposals should not substitute for individualized, direct assessment of each patient. The panel also noted that the cost effectiveness of its recommendations had not yet been assessed. In addition to representatives from the University of Michigan, the task force included members from the University of California, Los Angeles; Massachusetts General Hospital, Boston; Stanford (Calif.) University; the University of Zurich; University Hospital in Lille, France; the University of Paris-South; McGill University, Montreal; Johns Hopkins University, Baltimore; and St. Joseph Hospital, Phoenix.
The task force was supported by the Scleroderma Foundation and the Pulmonary Hypertension Association. Dr. Khanna said that he has been a consultant to several drug companies including Actelion, Bayer, Genentech/Roche, Gilead, Merck, and DIGNA. Dr. Gladue said that she had no disclosures.
Twitter @mitchelzoler
MADRID – Patients with systemic sclerosis should undergo annual screening for pulmonary arterial hypertension using a combination of transthoracic echocardiography and pulmonary function tests, an international expert panel said.
These are the first evidence- and consensus-based recommendations for pulmonary arterial hypertension (PAH) screening in patients with systemic sclerosis, and the panel also called for screening patients with mixed or other connective tissue diseases with scleroderma features. "Our hope is that these recommendations will lead to earlier detection of PAH in connective tissue diseases and improve patient outcomes," Dr. Dinesh Khanna said while presenting the screening recommendations at the annual European Congress of Rheumatology.
About 5%-15% of patients with systemic sclerosis develop PAH, and once PAH occurs, up to 30% of patients will die within 3 years, said Dr. Khanna, director of the scleroderma program at the University of Michigan, Ann Arbor.
"Despite having approved drugs available" to treat systemic sclerosis and other scleroderma-spectrum disorder connective tissue diseases, these treatments "have not had a huge impact on survival. The only thing we can offer patients is screening, followed by early diagnosis and treatment," Dr. Khanna said in an interview.
The new recommendations say that patients with a tricuspid regurgitant velocity measured by transthoracic echocardiography greater than 2.8 m/s require assessment for PAH by right heart catheterization. Right heart catheterization is also needed for patients with a tricuspid regurgitant velocity of 2.5-2.8 m/s if they also have signs or symptoms of PAH such as dyspnea, fatigue, chest pain, dizziness, loud pulmonary sound, or peripheral edema. Another echo finding that should trigger right heart catheterization regardless of signs or symptoms or tricuspid regurgitation is right atrial or ventricular enlargement.
The key measures on pulmonary function tests that trigger right heart catheterization is a forced vital capacity (FVC) to diffusion capacity of lungs for carbon monoxide (DLCO) ratio of more than 1.6, or a DLCO of less than 60% if either appears in the setting of PAH signs or symptoms. Alternatively, meeting either of these pulmonary criteria should lead to right heart catheterization regardless of signs and symptoms if the patient’s most recent blood level of N-terminal pro-brain natriuretic peptide (NT-ProBNP) was greater than twice the upper limit of normal.
The panel also said that patients should undergo right heart catheterization regardless of PAH signs and symptoms if they fulfill the screening algorithm developed for the DETECT study (Ann. Rheum. Dis. 2013 May 18 [doi:10.1036/annrheumdis-2013-203301]).
The panel recommended annual transthoracic echo and pulmonary function test screening, or more frequently if a patient shows new signs or symptoms. Measurement of NT-ProBNP should happen at baseline, and then be repeated if new signs or symptoms of PAH appear. They also recommended applying the full DETECT screening algorithm in patients diagnosed with systemic sclerosis or other scleroderma spectrum connective-tissue disease for more than 3 years and a DLCO that is less than 60%. Right heart catheterization is mandatory to definitively diagnose PAH, Dr. Khanna stressed. The panel also said screening is not needed in patients with mixed- or other connective tissue disorders who did not have scleroderma-like features.
In a separate report at the meeting Dr. Khanna and his associates assessed the ability of transthoracic echocardiography and pulmonary function tests to screen patients with PAH. They used data from 69 patients with PAH in two separate reported series that together had 347 patients with systemic sclerosis who underwent assessment for suspected PAH (J. Rheumatol. 2011;38:2172-9 and J. Rheumatol. 2010;37:2290-8).
The new, retrospective analysis showed that combining transthoracic echo and pulmonary-function test screens can have a negative predictive accuracy of 98% for correctly ruling out PAH in patients with systemic sclerosis, reported Dr. Heather Gladue, a rheumatology fellow at the University of Michigan.
The recommendations panel cautioned that its proposals should not substitute for individualized, direct assessment of each patient. The panel also noted that the cost effectiveness of its recommendations had not yet been assessed. In addition to representatives from the University of Michigan, the task force included members from the University of California, Los Angeles; Massachusetts General Hospital, Boston; Stanford (Calif.) University; the University of Zurich; University Hospital in Lille, France; the University of Paris-South; McGill University, Montreal; Johns Hopkins University, Baltimore; and St. Joseph Hospital, Phoenix.
The task force was supported by the Scleroderma Foundation and the Pulmonary Hypertension Association. Dr. Khanna said that he has been a consultant to several drug companies including Actelion, Bayer, Genentech/Roche, Gilead, Merck, and DIGNA. Dr. Gladue said that she had no disclosures.
Twitter @mitchelzoler
MADRID – Patients with systemic sclerosis should undergo annual screening for pulmonary arterial hypertension using a combination of transthoracic echocardiography and pulmonary function tests, an international expert panel said.
These are the first evidence- and consensus-based recommendations for pulmonary arterial hypertension (PAH) screening in patients with systemic sclerosis, and the panel also called for screening patients with mixed or other connective tissue diseases with scleroderma features. "Our hope is that these recommendations will lead to earlier detection of PAH in connective tissue diseases and improve patient outcomes," Dr. Dinesh Khanna said while presenting the screening recommendations at the annual European Congress of Rheumatology.
About 5%-15% of patients with systemic sclerosis develop PAH, and once PAH occurs, up to 30% of patients will die within 3 years, said Dr. Khanna, director of the scleroderma program at the University of Michigan, Ann Arbor.
"Despite having approved drugs available" to treat systemic sclerosis and other scleroderma-spectrum disorder connective tissue diseases, these treatments "have not had a huge impact on survival. The only thing we can offer patients is screening, followed by early diagnosis and treatment," Dr. Khanna said in an interview.
The new recommendations say that patients with a tricuspid regurgitant velocity measured by transthoracic echocardiography greater than 2.8 m/s require assessment for PAH by right heart catheterization. Right heart catheterization is also needed for patients with a tricuspid regurgitant velocity of 2.5-2.8 m/s if they also have signs or symptoms of PAH such as dyspnea, fatigue, chest pain, dizziness, loud pulmonary sound, or peripheral edema. Another echo finding that should trigger right heart catheterization regardless of signs or symptoms or tricuspid regurgitation is right atrial or ventricular enlargement.
The key measures on pulmonary function tests that trigger right heart catheterization is a forced vital capacity (FVC) to diffusion capacity of lungs for carbon monoxide (DLCO) ratio of more than 1.6, or a DLCO of less than 60% if either appears in the setting of PAH signs or symptoms. Alternatively, meeting either of these pulmonary criteria should lead to right heart catheterization regardless of signs and symptoms if the patient’s most recent blood level of N-terminal pro-brain natriuretic peptide (NT-ProBNP) was greater than twice the upper limit of normal.
The panel also said that patients should undergo right heart catheterization regardless of PAH signs and symptoms if they fulfill the screening algorithm developed for the DETECT study (Ann. Rheum. Dis. 2013 May 18 [doi:10.1036/annrheumdis-2013-203301]).
The panel recommended annual transthoracic echo and pulmonary function test screening, or more frequently if a patient shows new signs or symptoms. Measurement of NT-ProBNP should happen at baseline, and then be repeated if new signs or symptoms of PAH appear. They also recommended applying the full DETECT screening algorithm in patients diagnosed with systemic sclerosis or other scleroderma spectrum connective-tissue disease for more than 3 years and a DLCO that is less than 60%. Right heart catheterization is mandatory to definitively diagnose PAH, Dr. Khanna stressed. The panel also said screening is not needed in patients with mixed- or other connective tissue disorders who did not have scleroderma-like features.
In a separate report at the meeting Dr. Khanna and his associates assessed the ability of transthoracic echocardiography and pulmonary function tests to screen patients with PAH. They used data from 69 patients with PAH in two separate reported series that together had 347 patients with systemic sclerosis who underwent assessment for suspected PAH (J. Rheumatol. 2011;38:2172-9 and J. Rheumatol. 2010;37:2290-8).
The new, retrospective analysis showed that combining transthoracic echo and pulmonary-function test screens can have a negative predictive accuracy of 98% for correctly ruling out PAH in patients with systemic sclerosis, reported Dr. Heather Gladue, a rheumatology fellow at the University of Michigan.
The recommendations panel cautioned that its proposals should not substitute for individualized, direct assessment of each patient. The panel also noted that the cost effectiveness of its recommendations had not yet been assessed. In addition to representatives from the University of Michigan, the task force included members from the University of California, Los Angeles; Massachusetts General Hospital, Boston; Stanford (Calif.) University; the University of Zurich; University Hospital in Lille, France; the University of Paris-South; McGill University, Montreal; Johns Hopkins University, Baltimore; and St. Joseph Hospital, Phoenix.
The task force was supported by the Scleroderma Foundation and the Pulmonary Hypertension Association. Dr. Khanna said that he has been a consultant to several drug companies including Actelion, Bayer, Genentech/Roche, Gilead, Merck, and DIGNA. Dr. Gladue said that she had no disclosures.
Twitter @mitchelzoler
AT THE EULAR CONGRESS 2013
Algorithm helps to DETECT pulmonary hypertension in systemic sclerosis
MADRID – The use of a two-step algorithm significantly increased the rate at which pulmonary arterial hypertension was diagnosed in patients with systemic sclerosis in a prospective, observational, cross-sectional study.
The results of the DETECT study, presented at the annual European Congress of Rheumatology, showed that the two-step algorithm had a sensitivity of 96% for correctly identifying the condition, which was higher than the 71% sensitivity obtained using methods recommended currently by the European Society of Cardiology/European Respiratory Society (ESC/ERS) guidelines. The ESC/ERS recommendations are mainly based on consensus rather than robust evidence, and focus on the use of transthoracic echocardiography.
"DETECT is unique because it shows that if you just do an echocardiogram that you miss 29% of people who subsequently have pulmonary arterial hypertension [PAH], whereas if you apply the DETECT algorithm you miss only 4% of the people," Dr. Dinesh Khanna, director of the scleroderma program at the University of Michigan, Ann Arbor, said in an interview.
Dr. Khanna, who was a coinvestigator in the study, added: "PAH is a leading cause of mortality; it has high prevalence [and] it has a median survival of 2 to 3 years. ... You don’t want to miss these patients." Dr. Khanna presented recommendations for annual screening of PAH in systemic sclerosis patients at another session at the meeting.
Although 4% of patients are still being missed, this is a dramatic improvement over current clinical practice, said DETECT investigator Dr. Christopher Denton, who presented the findings of the international, multicenter trial. The study was also recently published online (Ann. Rheum. Dis. 2013 May 18 [doi: 10.1136/annrheumdis-2013-203301]).
"There is a general feeling that patients need to be screened so that diagnoses can be made and licensed therapies can be initiated," said Dr. Denton of the Royal Free Hospital in London. "The goal of the study was to rationalize a large number of potential variables into a small number that could be developed into a risk score," he added, and "ultimately to ensure that the most appropriate patients are referred for diagnostic right heart catheter studies." Right heart catheterization (RHC) remains the only method for confirming a diagnosis of PAH.
A total of 646 adult patients with established scleroderma (greater than 3 years) and reduced diffusing capacity of the lung for carbon monoxide (DLCO less than 60% of predicted) were screened and 466 enrolled in the study. All of them underwent RHC, and 145 (31%) were found to have PAH. This was defined as a mean pulmonary arterial pressure of 25 mm Hg or higher.
Of the 145 patients with PAH, 87 met World Health Organization (WHO) group 1 criteria for mild PAH, and this was the group of interest, as a diagnosis of PAH "had been robustly excluded" by normal methods, Dr. Denton said. This group of patients was compared with the group that did not have PAH (n = 321).
Patients with WHO group 1 PAH were slightly older than patients who did not have PAH (mean ages, 61 and 56 years). The PAH patients also tended to have a longer disease duration (163 vs. 130 months) and had slightly lower DLCO (43% vs. 48% of predicted).
The DETECT investigators examined 112 variables, including demographic and clinical parameters, serum tests, and electro- and echocardiogram results, that they thought might be able to help differentiate patients with PAH from those without it. After expert analysis and various types of statistical modeling, they ended up with eight items that were used to develop the two-step algorithm.
Step 1 of the algorithm involves testing for lung function, expressed as a ratio of the percentage predicted forced vital capacity and DLCO; the presence of current or past telangiectasia; serum anticentromere antibody positivity; serum levels of N-terminal prohormone brain natriuretic peptide (NT-proBNP); serum urate levels; and right axis deviation on an electrocardiogram. Step 2 involves measurement of two echocardiographic parameters: right atrium area and tricuspid regurgitation velocity.
"The aim is to make this a computer-based system," Dr. Denton explained. A trial electronic version of the tool is being tested, which involves the aforementioned clinical variables being entered first to determine if an echocardiogram is warranted, and then determining if the results of the echocardiogram warrant further referral for RHC.
The rates of referral for RHC were higher if the two-step algorithm was used, compared with the use of ESC/ERS guideline-recommended methods (62% and 40%). The specificity of the algorithm was 48%, with positive and negative predictive values of 35% and 98%, respectively. The values for guideline-recommended methods were 69%, 40%, and 89%.
The DETECT algorithm has the potential to revise standards of care in patients with systemic sclerosis. Dr. Denton noted that not only was it a sensitive, noninvasive screening tool, but that it also had the potential to reduce the number of missed diagnoses and to potentially identify PAH earlier in mildly symptomatic patients.
"The reason to use a two-step approach is that this potentially will improve the use of echocardiography as well as the more invasive test of right heart catheterization," he commented. "So we hope that this sort of approach will ultimately improve the approach and the standard of care for systemic sclerosis."
DETECT was an academic-led study funded by Actelion. Dr. Denton has received consulting and speaker fees and/or research funding from, or has been a clinical trial investigator for, several companies including Actelion, Boehringer Ingelheim, and CSL Behring. Dr. Khanna disclosed acting as a consultant for several companies including Actelion, Bayer, and Celgene.
MADRID – The use of a two-step algorithm significantly increased the rate at which pulmonary arterial hypertension was diagnosed in patients with systemic sclerosis in a prospective, observational, cross-sectional study.
The results of the DETECT study, presented at the annual European Congress of Rheumatology, showed that the two-step algorithm had a sensitivity of 96% for correctly identifying the condition, which was higher than the 71% sensitivity obtained using methods recommended currently by the European Society of Cardiology/European Respiratory Society (ESC/ERS) guidelines. The ESC/ERS recommendations are mainly based on consensus rather than robust evidence, and focus on the use of transthoracic echocardiography.
"DETECT is unique because it shows that if you just do an echocardiogram that you miss 29% of people who subsequently have pulmonary arterial hypertension [PAH], whereas if you apply the DETECT algorithm you miss only 4% of the people," Dr. Dinesh Khanna, director of the scleroderma program at the University of Michigan, Ann Arbor, said in an interview.
Dr. Khanna, who was a coinvestigator in the study, added: "PAH is a leading cause of mortality; it has high prevalence [and] it has a median survival of 2 to 3 years. ... You don’t want to miss these patients." Dr. Khanna presented recommendations for annual screening of PAH in systemic sclerosis patients at another session at the meeting.
Although 4% of patients are still being missed, this is a dramatic improvement over current clinical practice, said DETECT investigator Dr. Christopher Denton, who presented the findings of the international, multicenter trial. The study was also recently published online (Ann. Rheum. Dis. 2013 May 18 [doi: 10.1136/annrheumdis-2013-203301]).
"There is a general feeling that patients need to be screened so that diagnoses can be made and licensed therapies can be initiated," said Dr. Denton of the Royal Free Hospital in London. "The goal of the study was to rationalize a large number of potential variables into a small number that could be developed into a risk score," he added, and "ultimately to ensure that the most appropriate patients are referred for diagnostic right heart catheter studies." Right heart catheterization (RHC) remains the only method for confirming a diagnosis of PAH.
A total of 646 adult patients with established scleroderma (greater than 3 years) and reduced diffusing capacity of the lung for carbon monoxide (DLCO less than 60% of predicted) were screened and 466 enrolled in the study. All of them underwent RHC, and 145 (31%) were found to have PAH. This was defined as a mean pulmonary arterial pressure of 25 mm Hg or higher.
Of the 145 patients with PAH, 87 met World Health Organization (WHO) group 1 criteria for mild PAH, and this was the group of interest, as a diagnosis of PAH "had been robustly excluded" by normal methods, Dr. Denton said. This group of patients was compared with the group that did not have PAH (n = 321).
Patients with WHO group 1 PAH were slightly older than patients who did not have PAH (mean ages, 61 and 56 years). The PAH patients also tended to have a longer disease duration (163 vs. 130 months) and had slightly lower DLCO (43% vs. 48% of predicted).
The DETECT investigators examined 112 variables, including demographic and clinical parameters, serum tests, and electro- and echocardiogram results, that they thought might be able to help differentiate patients with PAH from those without it. After expert analysis and various types of statistical modeling, they ended up with eight items that were used to develop the two-step algorithm.
Step 1 of the algorithm involves testing for lung function, expressed as a ratio of the percentage predicted forced vital capacity and DLCO; the presence of current or past telangiectasia; serum anticentromere antibody positivity; serum levels of N-terminal prohormone brain natriuretic peptide (NT-proBNP); serum urate levels; and right axis deviation on an electrocardiogram. Step 2 involves measurement of two echocardiographic parameters: right atrium area and tricuspid regurgitation velocity.
"The aim is to make this a computer-based system," Dr. Denton explained. A trial electronic version of the tool is being tested, which involves the aforementioned clinical variables being entered first to determine if an echocardiogram is warranted, and then determining if the results of the echocardiogram warrant further referral for RHC.
The rates of referral for RHC were higher if the two-step algorithm was used, compared with the use of ESC/ERS guideline-recommended methods (62% and 40%). The specificity of the algorithm was 48%, with positive and negative predictive values of 35% and 98%, respectively. The values for guideline-recommended methods were 69%, 40%, and 89%.
The DETECT algorithm has the potential to revise standards of care in patients with systemic sclerosis. Dr. Denton noted that not only was it a sensitive, noninvasive screening tool, but that it also had the potential to reduce the number of missed diagnoses and to potentially identify PAH earlier in mildly symptomatic patients.
"The reason to use a two-step approach is that this potentially will improve the use of echocardiography as well as the more invasive test of right heart catheterization," he commented. "So we hope that this sort of approach will ultimately improve the approach and the standard of care for systemic sclerosis."
DETECT was an academic-led study funded by Actelion. Dr. Denton has received consulting and speaker fees and/or research funding from, or has been a clinical trial investigator for, several companies including Actelion, Boehringer Ingelheim, and CSL Behring. Dr. Khanna disclosed acting as a consultant for several companies including Actelion, Bayer, and Celgene.
MADRID – The use of a two-step algorithm significantly increased the rate at which pulmonary arterial hypertension was diagnosed in patients with systemic sclerosis in a prospective, observational, cross-sectional study.
The results of the DETECT study, presented at the annual European Congress of Rheumatology, showed that the two-step algorithm had a sensitivity of 96% for correctly identifying the condition, which was higher than the 71% sensitivity obtained using methods recommended currently by the European Society of Cardiology/European Respiratory Society (ESC/ERS) guidelines. The ESC/ERS recommendations are mainly based on consensus rather than robust evidence, and focus on the use of transthoracic echocardiography.
"DETECT is unique because it shows that if you just do an echocardiogram that you miss 29% of people who subsequently have pulmonary arterial hypertension [PAH], whereas if you apply the DETECT algorithm you miss only 4% of the people," Dr. Dinesh Khanna, director of the scleroderma program at the University of Michigan, Ann Arbor, said in an interview.
Dr. Khanna, who was a coinvestigator in the study, added: "PAH is a leading cause of mortality; it has high prevalence [and] it has a median survival of 2 to 3 years. ... You don’t want to miss these patients." Dr. Khanna presented recommendations for annual screening of PAH in systemic sclerosis patients at another session at the meeting.
Although 4% of patients are still being missed, this is a dramatic improvement over current clinical practice, said DETECT investigator Dr. Christopher Denton, who presented the findings of the international, multicenter trial. The study was also recently published online (Ann. Rheum. Dis. 2013 May 18 [doi: 10.1136/annrheumdis-2013-203301]).
"There is a general feeling that patients need to be screened so that diagnoses can be made and licensed therapies can be initiated," said Dr. Denton of the Royal Free Hospital in London. "The goal of the study was to rationalize a large number of potential variables into a small number that could be developed into a risk score," he added, and "ultimately to ensure that the most appropriate patients are referred for diagnostic right heart catheter studies." Right heart catheterization (RHC) remains the only method for confirming a diagnosis of PAH.
A total of 646 adult patients with established scleroderma (greater than 3 years) and reduced diffusing capacity of the lung for carbon monoxide (DLCO less than 60% of predicted) were screened and 466 enrolled in the study. All of them underwent RHC, and 145 (31%) were found to have PAH. This was defined as a mean pulmonary arterial pressure of 25 mm Hg or higher.
Of the 145 patients with PAH, 87 met World Health Organization (WHO) group 1 criteria for mild PAH, and this was the group of interest, as a diagnosis of PAH "had been robustly excluded" by normal methods, Dr. Denton said. This group of patients was compared with the group that did not have PAH (n = 321).
Patients with WHO group 1 PAH were slightly older than patients who did not have PAH (mean ages, 61 and 56 years). The PAH patients also tended to have a longer disease duration (163 vs. 130 months) and had slightly lower DLCO (43% vs. 48% of predicted).
The DETECT investigators examined 112 variables, including demographic and clinical parameters, serum tests, and electro- and echocardiogram results, that they thought might be able to help differentiate patients with PAH from those without it. After expert analysis and various types of statistical modeling, they ended up with eight items that were used to develop the two-step algorithm.
Step 1 of the algorithm involves testing for lung function, expressed as a ratio of the percentage predicted forced vital capacity and DLCO; the presence of current or past telangiectasia; serum anticentromere antibody positivity; serum levels of N-terminal prohormone brain natriuretic peptide (NT-proBNP); serum urate levels; and right axis deviation on an electrocardiogram. Step 2 involves measurement of two echocardiographic parameters: right atrium area and tricuspid regurgitation velocity.
"The aim is to make this a computer-based system," Dr. Denton explained. A trial electronic version of the tool is being tested, which involves the aforementioned clinical variables being entered first to determine if an echocardiogram is warranted, and then determining if the results of the echocardiogram warrant further referral for RHC.
The rates of referral for RHC were higher if the two-step algorithm was used, compared with the use of ESC/ERS guideline-recommended methods (62% and 40%). The specificity of the algorithm was 48%, with positive and negative predictive values of 35% and 98%, respectively. The values for guideline-recommended methods were 69%, 40%, and 89%.
The DETECT algorithm has the potential to revise standards of care in patients with systemic sclerosis. Dr. Denton noted that not only was it a sensitive, noninvasive screening tool, but that it also had the potential to reduce the number of missed diagnoses and to potentially identify PAH earlier in mildly symptomatic patients.
"The reason to use a two-step approach is that this potentially will improve the use of echocardiography as well as the more invasive test of right heart catheterization," he commented. "So we hope that this sort of approach will ultimately improve the approach and the standard of care for systemic sclerosis."
DETECT was an academic-led study funded by Actelion. Dr. Denton has received consulting and speaker fees and/or research funding from, or has been a clinical trial investigator for, several companies including Actelion, Boehringer Ingelheim, and CSL Behring. Dr. Khanna disclosed acting as a consultant for several companies including Actelion, Bayer, and Celgene.
AT THE EULAR CONGRESS 2013
Major finding: Only 4% of cases were missed using the two-step algorithm, compared with 29% for guideline-recommended detection.
Data source: DETECT is an international, multicenter, prospective, observational, cross-sectional study of 87 systemic sclerosis patients with and 321 without pulmonary arterial hypertension.
Disclosures: DETECT was an academic-led study funded by Actelion. Dr. Denton has received consulting and speaker fees and/or research funding from, or has been a clinical trial investigator for, several companies including Actelion, Boehringer Ingelheim, and CSL Behring. Dr. Khanna disclosed acting as a consultant for several companies including Actelion, Bayer, and Celgene.