User login
Improvement in patient-reported outcomes comparable with ustekinumab and TNFi in PsA
Key clinical point: Improvements in patient-reported outcomes were generally comparable with ustekinumab and tumor necrosis factor inhibitor (TNFi) treatments in patients with psoriatic arthritis (PsA).
Major finding: At 3 years, ustekinumab and TNFi were associated with comparable improvements in EuroQol-5 dimensions health state visual analogue scale scores (ustekinumab: mean change from baseline [Δ], 11.0 [95% CI, 6.5-15.4]; TNFi: Δ, 18.9 [95% CI, 14.0-23.9]) and work productivity (ustekinumab: Δ, 24.9% [95% CI, 15.8%-34.0%]; TNFi: Δ, 44.5% [95% CI, 38.4%-50.6%]).
Study details: This study evaluated 437 patients with PsA from the PsABio study who initiated first- to third-line ustekinumab (n=219) or TNFi (n=218) and continued the initial treatment for 3 years.
Disclosures: This study was sponsored by Janssen. Several authors reported ties with various sources, including Janssen. E Theander reported being a former employee of Janssen. M Sharaf and W Noel declared being employees of or owning stocks in Johnson & Johnson.
Source: Gossec L et al. Improvement in patient-reported outcomes and work productivity following 3-year ustekinumab or tumour necrosis factor inhibitor treatment in patients with psoriatic arthritis: Results from the PsABio real-world study. Arthritis Res Ther. 2023;25(1):109 (Jun 23). Doi: 10.1186/s13075-023-03058-y.
Key clinical point: Improvements in patient-reported outcomes were generally comparable with ustekinumab and tumor necrosis factor inhibitor (TNFi) treatments in patients with psoriatic arthritis (PsA).
Major finding: At 3 years, ustekinumab and TNFi were associated with comparable improvements in EuroQol-5 dimensions health state visual analogue scale scores (ustekinumab: mean change from baseline [Δ], 11.0 [95% CI, 6.5-15.4]; TNFi: Δ, 18.9 [95% CI, 14.0-23.9]) and work productivity (ustekinumab: Δ, 24.9% [95% CI, 15.8%-34.0%]; TNFi: Δ, 44.5% [95% CI, 38.4%-50.6%]).
Study details: This study evaluated 437 patients with PsA from the PsABio study who initiated first- to third-line ustekinumab (n=219) or TNFi (n=218) and continued the initial treatment for 3 years.
Disclosures: This study was sponsored by Janssen. Several authors reported ties with various sources, including Janssen. E Theander reported being a former employee of Janssen. M Sharaf and W Noel declared being employees of or owning stocks in Johnson & Johnson.
Source: Gossec L et al. Improvement in patient-reported outcomes and work productivity following 3-year ustekinumab or tumour necrosis factor inhibitor treatment in patients with psoriatic arthritis: Results from the PsABio real-world study. Arthritis Res Ther. 2023;25(1):109 (Jun 23). Doi: 10.1186/s13075-023-03058-y.
Key clinical point: Improvements in patient-reported outcomes were generally comparable with ustekinumab and tumor necrosis factor inhibitor (TNFi) treatments in patients with psoriatic arthritis (PsA).
Major finding: At 3 years, ustekinumab and TNFi were associated with comparable improvements in EuroQol-5 dimensions health state visual analogue scale scores (ustekinumab: mean change from baseline [Δ], 11.0 [95% CI, 6.5-15.4]; TNFi: Δ, 18.9 [95% CI, 14.0-23.9]) and work productivity (ustekinumab: Δ, 24.9% [95% CI, 15.8%-34.0%]; TNFi: Δ, 44.5% [95% CI, 38.4%-50.6%]).
Study details: This study evaluated 437 patients with PsA from the PsABio study who initiated first- to third-line ustekinumab (n=219) or TNFi (n=218) and continued the initial treatment for 3 years.
Disclosures: This study was sponsored by Janssen. Several authors reported ties with various sources, including Janssen. E Theander reported being a former employee of Janssen. M Sharaf and W Noel declared being employees of or owning stocks in Johnson & Johnson.
Source: Gossec L et al. Improvement in patient-reported outcomes and work productivity following 3-year ustekinumab or tumour necrosis factor inhibitor treatment in patients with psoriatic arthritis: Results from the PsABio real-world study. Arthritis Res Ther. 2023;25(1):109 (Jun 23). Doi: 10.1186/s13075-023-03058-y.
Apremilast offers a safe long-term oral treatment option for psoriatic arthritis
Key clinical point: Apremilast appeared safe for long-term use with a consistent safety profile in patients with psoriatic arthritis (PsA), indicating a favorable benefit-risk profile.
Major finding: The overall incidence of serious treatment-emergent adverse events (TEAEs; exposure-adjusted incidence rate/100 patient-years, 6.9 and 8.9, respectively) and special interest TEAEs, such as major adverse cardiac events (0.1% and 0.1%, respectively), serious opportunistic infections (0.1% and 0.1%, respectively), and malignancies (0.3% and 0.4%, respectively), were similar in the apremilast and placebo groups and remained low throughout the apremilast exposure period.
Study details: This pooled analysis of 15 randomized trials included patients with plaque psoriasis (n=2,881), PsA (n=1,564), and Behçet’s syndrome (n=318) who received either apremilast or placebo.
Disclosures: This study was sponsored by Amgen Inc. Seven authors declared being employees and stockholders of Amgen. The other authors reported receiving honoraria, grants, or research funding as speakers, investigators, or advisory board members from various sources, including Amgen.
Source: Mease PJ et al. Apremilast long-term safety up to 5 years from 15 pooled randomized, placebo-controlled studies of psoriasis, psoriatic arthritis, and Behçet's syndrome. Am J Clin Dermatol. 2023;1-12 (Jun 14). Doi: 10.1007/s40257-023-00783-7.
Key clinical point: Apremilast appeared safe for long-term use with a consistent safety profile in patients with psoriatic arthritis (PsA), indicating a favorable benefit-risk profile.
Major finding: The overall incidence of serious treatment-emergent adverse events (TEAEs; exposure-adjusted incidence rate/100 patient-years, 6.9 and 8.9, respectively) and special interest TEAEs, such as major adverse cardiac events (0.1% and 0.1%, respectively), serious opportunistic infections (0.1% and 0.1%, respectively), and malignancies (0.3% and 0.4%, respectively), were similar in the apremilast and placebo groups and remained low throughout the apremilast exposure period.
Study details: This pooled analysis of 15 randomized trials included patients with plaque psoriasis (n=2,881), PsA (n=1,564), and Behçet’s syndrome (n=318) who received either apremilast or placebo.
Disclosures: This study was sponsored by Amgen Inc. Seven authors declared being employees and stockholders of Amgen. The other authors reported receiving honoraria, grants, or research funding as speakers, investigators, or advisory board members from various sources, including Amgen.
Source: Mease PJ et al. Apremilast long-term safety up to 5 years from 15 pooled randomized, placebo-controlled studies of psoriasis, psoriatic arthritis, and Behçet's syndrome. Am J Clin Dermatol. 2023;1-12 (Jun 14). Doi: 10.1007/s40257-023-00783-7.
Key clinical point: Apremilast appeared safe for long-term use with a consistent safety profile in patients with psoriatic arthritis (PsA), indicating a favorable benefit-risk profile.
Major finding: The overall incidence of serious treatment-emergent adverse events (TEAEs; exposure-adjusted incidence rate/100 patient-years, 6.9 and 8.9, respectively) and special interest TEAEs, such as major adverse cardiac events (0.1% and 0.1%, respectively), serious opportunistic infections (0.1% and 0.1%, respectively), and malignancies (0.3% and 0.4%, respectively), were similar in the apremilast and placebo groups and remained low throughout the apremilast exposure period.
Study details: This pooled analysis of 15 randomized trials included patients with plaque psoriasis (n=2,881), PsA (n=1,564), and Behçet’s syndrome (n=318) who received either apremilast or placebo.
Disclosures: This study was sponsored by Amgen Inc. Seven authors declared being employees and stockholders of Amgen. The other authors reported receiving honoraria, grants, or research funding as speakers, investigators, or advisory board members from various sources, including Amgen.
Source: Mease PJ et al. Apremilast long-term safety up to 5 years from 15 pooled randomized, placebo-controlled studies of psoriasis, psoriatic arthritis, and Behçet's syndrome. Am J Clin Dermatol. 2023;1-12 (Jun 14). Doi: 10.1007/s40257-023-00783-7.
Real-world study confirms clinical efficacy of ixekizumab in PsA
Key clinical point: Ixekizumab improved musculoskeletal disease activity and patient-reported outcomes in a real-world cohort of patients with psoriatic arthritis.
Major finding: The Clinical Disease Activity Index improved significantly at 6 months (mean change [Δ], −3.5) and 12 months (Δ, −4.3; both P < .0001) after ixekizumab initiation, along with significant improvements in tender joint count, swollen joint count, and Physician’s Global Assessment scores (all P < .05). All patient-reported outcomes, including Patient’s Global Assessment, pain Visual Analog Scale, and Multidimensional Health Assessment Questionnaire Functional Index scores, improved at both time points.
Study details: This retrospective study included 1,812 patients with PsA from the OM1 PremiOMTM PsA dataset who initiated ixekizumab.
Disclosures: This study was sponsored by Eli Lilly and Company Pharmaceuticals. Three authors declared being employees and shareholders of Eli Lilly and Company, and 3 other authors declared being employees of OM1, Inc. W Tillett declared being a paid consultant for various sources, including Eli Lilly. The other authors declared no conflict of interests.
Source: Tillett W et al. Changes in musculoskeletal disease activity and patient-reported outcomes in patients with psoriatic arthritis treated with ixekizumab: Results from a real-world US cohort. Front Med (Lausanne). 2023;10:1184028 (Jun 21). Doi: 10.3389/fmed.2023.1184028.
Key clinical point: Ixekizumab improved musculoskeletal disease activity and patient-reported outcomes in a real-world cohort of patients with psoriatic arthritis.
Major finding: The Clinical Disease Activity Index improved significantly at 6 months (mean change [Δ], −3.5) and 12 months (Δ, −4.3; both P < .0001) after ixekizumab initiation, along with significant improvements in tender joint count, swollen joint count, and Physician’s Global Assessment scores (all P < .05). All patient-reported outcomes, including Patient’s Global Assessment, pain Visual Analog Scale, and Multidimensional Health Assessment Questionnaire Functional Index scores, improved at both time points.
Study details: This retrospective study included 1,812 patients with PsA from the OM1 PremiOMTM PsA dataset who initiated ixekizumab.
Disclosures: This study was sponsored by Eli Lilly and Company Pharmaceuticals. Three authors declared being employees and shareholders of Eli Lilly and Company, and 3 other authors declared being employees of OM1, Inc. W Tillett declared being a paid consultant for various sources, including Eli Lilly. The other authors declared no conflict of interests.
Source: Tillett W et al. Changes in musculoskeletal disease activity and patient-reported outcomes in patients with psoriatic arthritis treated with ixekizumab: Results from a real-world US cohort. Front Med (Lausanne). 2023;10:1184028 (Jun 21). Doi: 10.3389/fmed.2023.1184028.
Key clinical point: Ixekizumab improved musculoskeletal disease activity and patient-reported outcomes in a real-world cohort of patients with psoriatic arthritis.
Major finding: The Clinical Disease Activity Index improved significantly at 6 months (mean change [Δ], −3.5) and 12 months (Δ, −4.3; both P < .0001) after ixekizumab initiation, along with significant improvements in tender joint count, swollen joint count, and Physician’s Global Assessment scores (all P < .05). All patient-reported outcomes, including Patient’s Global Assessment, pain Visual Analog Scale, and Multidimensional Health Assessment Questionnaire Functional Index scores, improved at both time points.
Study details: This retrospective study included 1,812 patients with PsA from the OM1 PremiOMTM PsA dataset who initiated ixekizumab.
Disclosures: This study was sponsored by Eli Lilly and Company Pharmaceuticals. Three authors declared being employees and shareholders of Eli Lilly and Company, and 3 other authors declared being employees of OM1, Inc. W Tillett declared being a paid consultant for various sources, including Eli Lilly. The other authors declared no conflict of interests.
Source: Tillett W et al. Changes in musculoskeletal disease activity and patient-reported outcomes in patients with psoriatic arthritis treated with ixekizumab: Results from a real-world US cohort. Front Med (Lausanne). 2023;10:1184028 (Jun 21). Doi: 10.3389/fmed.2023.1184028.
Predictors of treatment response in PsA patients initiating a first TNFi
Key clinical point: In biologic-naïve patients with psoriatic arthritis (PsA) who initiated a first tumor necrosis factor inhibitor (TNFi), sex, disease duration, C-reactive protein level, age at treatment initiation, and fatigue predicted the achievement of the Disease Activity index for PsA in 28 joints (DAPSA28) remission at 6 months.
Major finding: Male sex (odds ratio [OR], 1.85; 95% CI, 1.54-2.23), longer disease duration (OR, 1.66; 95% CI, 1.26-2.20), and higher C-reactive protein (OR, 1.52; 95% CI, 1.22-1.89) positively predicted the achievement of DAPSA28 remission at 6 months, whereas older age at treatment initiation (OR, 0.97; 95% CI, 0.96-0.98) and higher fatigue score (OR, 0.99; 95% CI, 0.98-0.99) were negative predictors.
Study details: This study evaluated the data of 13,369 biologic-naïve patients registered with a PsA diagnosis from 13 European registries who initiated a first TNFi treatment.
Disclosures: This study was sponsored by Novartis Pharma AG. Several authors declared receiving speaker or consulting fees and research grants from various sources, including Novartis.
Source: Linde L et al. Predictors of DAPSA28 remission in patients with psoriatic arthritis initiating a first TNF inhibitor: Results from 13 European registries. Rheumatology (Oxford). 2023 (Jun 14). Doi: 10.1093/rheumatology/kead284.
Key clinical point: In biologic-naïve patients with psoriatic arthritis (PsA) who initiated a first tumor necrosis factor inhibitor (TNFi), sex, disease duration, C-reactive protein level, age at treatment initiation, and fatigue predicted the achievement of the Disease Activity index for PsA in 28 joints (DAPSA28) remission at 6 months.
Major finding: Male sex (odds ratio [OR], 1.85; 95% CI, 1.54-2.23), longer disease duration (OR, 1.66; 95% CI, 1.26-2.20), and higher C-reactive protein (OR, 1.52; 95% CI, 1.22-1.89) positively predicted the achievement of DAPSA28 remission at 6 months, whereas older age at treatment initiation (OR, 0.97; 95% CI, 0.96-0.98) and higher fatigue score (OR, 0.99; 95% CI, 0.98-0.99) were negative predictors.
Study details: This study evaluated the data of 13,369 biologic-naïve patients registered with a PsA diagnosis from 13 European registries who initiated a first TNFi treatment.
Disclosures: This study was sponsored by Novartis Pharma AG. Several authors declared receiving speaker or consulting fees and research grants from various sources, including Novartis.
Source: Linde L et al. Predictors of DAPSA28 remission in patients with psoriatic arthritis initiating a first TNF inhibitor: Results from 13 European registries. Rheumatology (Oxford). 2023 (Jun 14). Doi: 10.1093/rheumatology/kead284.
Key clinical point: In biologic-naïve patients with psoriatic arthritis (PsA) who initiated a first tumor necrosis factor inhibitor (TNFi), sex, disease duration, C-reactive protein level, age at treatment initiation, and fatigue predicted the achievement of the Disease Activity index for PsA in 28 joints (DAPSA28) remission at 6 months.
Major finding: Male sex (odds ratio [OR], 1.85; 95% CI, 1.54-2.23), longer disease duration (OR, 1.66; 95% CI, 1.26-2.20), and higher C-reactive protein (OR, 1.52; 95% CI, 1.22-1.89) positively predicted the achievement of DAPSA28 remission at 6 months, whereas older age at treatment initiation (OR, 0.97; 95% CI, 0.96-0.98) and higher fatigue score (OR, 0.99; 95% CI, 0.98-0.99) were negative predictors.
Study details: This study evaluated the data of 13,369 biologic-naïve patients registered with a PsA diagnosis from 13 European registries who initiated a first TNFi treatment.
Disclosures: This study was sponsored by Novartis Pharma AG. Several authors declared receiving speaker or consulting fees and research grants from various sources, including Novartis.
Source: Linde L et al. Predictors of DAPSA28 remission in patients with psoriatic arthritis initiating a first TNF inhibitor: Results from 13 European registries. Rheumatology (Oxford). 2023 (Jun 14). Doi: 10.1093/rheumatology/kead284.
Beta-defensin-2 may serve as a predictive biomarker for clinical response to secukinumab in PsA
Key clinical point: A significant quantitative association exists between baseline serum beta-defensin-2 (BD-2) levels and the clinical response to secukinumab in patients with psoriatic arthritis (PsA).
Major finding: Baseline serum BD-2 levels were significantly associated with the American College of Rheumatology (ACR) response to secukinumab (Spearman’s rho, 27%; P = 3.8e-5) but not to placebo at week 16, with the trend being consistent for ≥1 year. The addition of BD-2 to the clinical model improved the prediction of the 16-week ACR 20% improvement response to secukinumab by increasing the area under the receiver operating characteristic curve by 11 percentage points.
Study details: This retrospective analysis of the phase 3 FUTURE 1-5 trials included 1,989 patients with PsA who received secukinumab or placebo.
Disclosures: This study did not receive any funding. Seven authors declared being employees of or holding shares or stock options in Novartis AG, and M Cardner declared being an employee of AstraZeneca AB. Two authors reported ties unrelated to this study.
Source: Cardner M et al. Analysis of serum proteomics data identifies a quantitative association between beta-defensin 2 at baseline and clinical response to IL-17 blockade in psoriatic arthritis. RMD Open. 2023;9(2):e003042 (Jun 15). Doi: 10.1136/rmdopen-2023-003042.
Key clinical point: A significant quantitative association exists between baseline serum beta-defensin-2 (BD-2) levels and the clinical response to secukinumab in patients with psoriatic arthritis (PsA).
Major finding: Baseline serum BD-2 levels were significantly associated with the American College of Rheumatology (ACR) response to secukinumab (Spearman’s rho, 27%; P = 3.8e-5) but not to placebo at week 16, with the trend being consistent for ≥1 year. The addition of BD-2 to the clinical model improved the prediction of the 16-week ACR 20% improvement response to secukinumab by increasing the area under the receiver operating characteristic curve by 11 percentage points.
Study details: This retrospective analysis of the phase 3 FUTURE 1-5 trials included 1,989 patients with PsA who received secukinumab or placebo.
Disclosures: This study did not receive any funding. Seven authors declared being employees of or holding shares or stock options in Novartis AG, and M Cardner declared being an employee of AstraZeneca AB. Two authors reported ties unrelated to this study.
Source: Cardner M et al. Analysis of serum proteomics data identifies a quantitative association between beta-defensin 2 at baseline and clinical response to IL-17 blockade in psoriatic arthritis. RMD Open. 2023;9(2):e003042 (Jun 15). Doi: 10.1136/rmdopen-2023-003042.
Key clinical point: A significant quantitative association exists between baseline serum beta-defensin-2 (BD-2) levels and the clinical response to secukinumab in patients with psoriatic arthritis (PsA).
Major finding: Baseline serum BD-2 levels were significantly associated with the American College of Rheumatology (ACR) response to secukinumab (Spearman’s rho, 27%; P = 3.8e-5) but not to placebo at week 16, with the trend being consistent for ≥1 year. The addition of BD-2 to the clinical model improved the prediction of the 16-week ACR 20% improvement response to secukinumab by increasing the area under the receiver operating characteristic curve by 11 percentage points.
Study details: This retrospective analysis of the phase 3 FUTURE 1-5 trials included 1,989 patients with PsA who received secukinumab or placebo.
Disclosures: This study did not receive any funding. Seven authors declared being employees of or holding shares or stock options in Novartis AG, and M Cardner declared being an employee of AstraZeneca AB. Two authors reported ties unrelated to this study.
Source: Cardner M et al. Analysis of serum proteomics data identifies a quantitative association between beta-defensin 2 at baseline and clinical response to IL-17 blockade in psoriatic arthritis. RMD Open. 2023;9(2):e003042 (Jun 15). Doi: 10.1136/rmdopen-2023-003042.
Humira biosimilars: Five things to know
The best-selling drug Humira (adalimumab) now faces competition in the United States after a 20-year monopoly. The first adalimumab biosimilar, Amjevita, launched in the United States on January 31, and in July, seven additional biosimilars became available. These drugs have the potential to lower prescription drug prices, but when and by how much remains to be seen.
Here’s what you need to know about adalimumab biosimilars.
What Humira biosimilars are now available?
Eight different biosimilars have launched in 2023 with discounts as large at 85% from Humira’s list price of $6,922. A few companies also offer two price points.
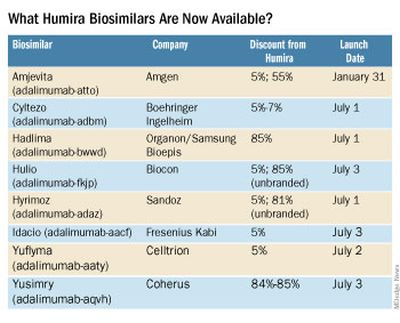
Three of these biosimilars – Hadlima, Hyrimoz, and Yuflyma – are available in high concentration formulations. This high concentration formulation makes up 85% of Humira prescriptions, according to a report from Goodroot, a collection of companies focused on lowering health care costs.
Cyltezo is currently the only adalimumab biosimilar with an interchangeability designation, meaning that a pharmacist can substitute the biosimilar for an equivalent Humira prescription without the intervention of a clinician. A total of 47 states allow for these substitutions without prior approval from a clinician, according to Goodroot, and the clinician must be notified of the switch within a certain time frame. A total of 40 states require that patients be notified of the switch before substitution.
However, it’s not clear if this interchangeability designation will prove an advantage for Cyltezo, as it is interchangeable with the lower concentration version of Humira that makes up just 15% of prescriptions.
Most of the companies behind these biosimilars are pursuing interchangeability designations for their drugs, except for Fresenius Kabi (Idacio) and Coherus (Yusimry).
A ninth biosimilar, Pfizer’s adalimumab-afzb (Abrilada), is not yet on the market and is currently awaiting an approval decision from the Food and Drug Administration to add an interchangeability designation to its prior approval for a low-concentration formulation.
Why are they priced differently?
The two price points offer different deals to payers. Pharmacy benefit managers make confidential agreements with drug manufacturers to get a discount – called a rebate – to get the drug on the PBM’s formulary. The PBM keeps a portion of that rebate, and the rest is passed on to the insurance company and patients. Biosimilars at a higher price point will likely offer larger rebates. Biosimilars offered at lower price points incorporate this discount up front in their list pricing and likely will not offer large rebates.
Will biosimilars be covered by payers?
Currently, biosimilars are being offered on formularies at parity with Humira, meaning they are on the same tier. The PBM companies OptumRx and Cigna Group’s Express Scripts will offer Amjevita (at both price points), Cyltezo, and Hyrimoz (at both price points).
“This decision allows our clients flexibility to provide access to the lower list price, so members in high-deductible plans and benefit designs with coinsurance can experience lower out-of-pocket costs,” said OptumRx spokesperson Isaac Sorensen in an email.
Mark Cuban Cost Plus Drug Company, which uses a direct-to-consumer model, will offer Yusimry for $567.27 on its website. SmithRx, a PBM based in San Francisco, announced it would partner with Cost Plus Drugs to offer Yusimry, adding that SmithRx members can use their insurance benefits to further reduce out-of-pocket costs. RxPreferred, another PBM, will also offer Yusimry through its partnership with Cuban’s company.
The news website Formulary Watch previously reported that CVS Caremark, another of the biggest PBMs, will be offering Amjevita, but as a nonpreferred brand, while Humira remains the preferred brand. CVS Caremark did not respond to a request for comment.
Will patients pay less?
Biosimilars have been touted as a potential solution to lower spending on biologic drugs, but it’s unknown if patients will ultimately benefit with lower out-of-pocket costs. It’s “impossible to predict” if the discount that third-party payers pay will be passed on to consumers, said Mark Fendrick, MD, who directs the University of Michigan Center for Value-based Insurance Design in Ann Arbor.
Generally, a consumer’s copay is a percentage of a drug’s list price, so it stands to reason that a low drug price would result in lower out-of-pocket payments. While this is mostly true, Humira has a successful copay assistance program to lower prescription costs for consumers. According to a 2022 IQVIA report, 82% of commercial prescriptions cost patients less than $10 for Humira because of this program.
To appeal to patients, biosimilar companies will need to offer similar savings, Dr. Fendrick added. “There will be some discontent if patients are actually asked to pay more out-of-pocket for a less expensive drug,” he said.
All eight companies behind these biosimilars are offering or will be launching copay saving programs, many which advertise copays as low as $0 per month for eligible patients.
How will Humira respond?
Marta Wosińska, PhD, a health care economist at the Brookings Institute, Washington, predicts payers will use these lower biosimilar prices to negotiate better deals with AbbVie, Humira’s manufacturer. “We have a lot of players coming into [the market] right now, so the competition is really fierce,” she said. In response, AbbVie will need to increase rebates on Humira and/or lower its price to compete with these biosimilars.
“The ball is in AbbVie’s court,” she said. “If [the company] is not willing to drop price sufficiently, then payers will start switching to biosimilars.”
Dr. Fendrick reported past financial relationships and consulting arrangements with AbbVie, Amgen, Arnold Ventures, Bayer, CareFirst, BlueCross BlueShield, and many other companies. Dr. Wosińska has received funding from Arnold Ventures and serves as an expert witness on antitrust cases involving generic medication.
A version of this article first appeared on Medscape.com.
The best-selling drug Humira (adalimumab) now faces competition in the United States after a 20-year monopoly. The first adalimumab biosimilar, Amjevita, launched in the United States on January 31, and in July, seven additional biosimilars became available. These drugs have the potential to lower prescription drug prices, but when and by how much remains to be seen.
Here’s what you need to know about adalimumab biosimilars.
What Humira biosimilars are now available?
Eight different biosimilars have launched in 2023 with discounts as large at 85% from Humira’s list price of $6,922. A few companies also offer two price points.

Three of these biosimilars – Hadlima, Hyrimoz, and Yuflyma – are available in high concentration formulations. This high concentration formulation makes up 85% of Humira prescriptions, according to a report from Goodroot, a collection of companies focused on lowering health care costs.
Cyltezo is currently the only adalimumab biosimilar with an interchangeability designation, meaning that a pharmacist can substitute the biosimilar for an equivalent Humira prescription without the intervention of a clinician. A total of 47 states allow for these substitutions without prior approval from a clinician, according to Goodroot, and the clinician must be notified of the switch within a certain time frame. A total of 40 states require that patients be notified of the switch before substitution.
However, it’s not clear if this interchangeability designation will prove an advantage for Cyltezo, as it is interchangeable with the lower concentration version of Humira that makes up just 15% of prescriptions.
Most of the companies behind these biosimilars are pursuing interchangeability designations for their drugs, except for Fresenius Kabi (Idacio) and Coherus (Yusimry).
A ninth biosimilar, Pfizer’s adalimumab-afzb (Abrilada), is not yet on the market and is currently awaiting an approval decision from the Food and Drug Administration to add an interchangeability designation to its prior approval for a low-concentration formulation.
Why are they priced differently?
The two price points offer different deals to payers. Pharmacy benefit managers make confidential agreements with drug manufacturers to get a discount – called a rebate – to get the drug on the PBM’s formulary. The PBM keeps a portion of that rebate, and the rest is passed on to the insurance company and patients. Biosimilars at a higher price point will likely offer larger rebates. Biosimilars offered at lower price points incorporate this discount up front in their list pricing and likely will not offer large rebates.
Will biosimilars be covered by payers?
Currently, biosimilars are being offered on formularies at parity with Humira, meaning they are on the same tier. The PBM companies OptumRx and Cigna Group’s Express Scripts will offer Amjevita (at both price points), Cyltezo, and Hyrimoz (at both price points).
“This decision allows our clients flexibility to provide access to the lower list price, so members in high-deductible plans and benefit designs with coinsurance can experience lower out-of-pocket costs,” said OptumRx spokesperson Isaac Sorensen in an email.
Mark Cuban Cost Plus Drug Company, which uses a direct-to-consumer model, will offer Yusimry for $567.27 on its website. SmithRx, a PBM based in San Francisco, announced it would partner with Cost Plus Drugs to offer Yusimry, adding that SmithRx members can use their insurance benefits to further reduce out-of-pocket costs. RxPreferred, another PBM, will also offer Yusimry through its partnership with Cuban’s company.
The news website Formulary Watch previously reported that CVS Caremark, another of the biggest PBMs, will be offering Amjevita, but as a nonpreferred brand, while Humira remains the preferred brand. CVS Caremark did not respond to a request for comment.
Will patients pay less?
Biosimilars have been touted as a potential solution to lower spending on biologic drugs, but it’s unknown if patients will ultimately benefit with lower out-of-pocket costs. It’s “impossible to predict” if the discount that third-party payers pay will be passed on to consumers, said Mark Fendrick, MD, who directs the University of Michigan Center for Value-based Insurance Design in Ann Arbor.
Generally, a consumer’s copay is a percentage of a drug’s list price, so it stands to reason that a low drug price would result in lower out-of-pocket payments. While this is mostly true, Humira has a successful copay assistance program to lower prescription costs for consumers. According to a 2022 IQVIA report, 82% of commercial prescriptions cost patients less than $10 for Humira because of this program.
To appeal to patients, biosimilar companies will need to offer similar savings, Dr. Fendrick added. “There will be some discontent if patients are actually asked to pay more out-of-pocket for a less expensive drug,” he said.
All eight companies behind these biosimilars are offering or will be launching copay saving programs, many which advertise copays as low as $0 per month for eligible patients.
How will Humira respond?
Marta Wosińska, PhD, a health care economist at the Brookings Institute, Washington, predicts payers will use these lower biosimilar prices to negotiate better deals with AbbVie, Humira’s manufacturer. “We have a lot of players coming into [the market] right now, so the competition is really fierce,” she said. In response, AbbVie will need to increase rebates on Humira and/or lower its price to compete with these biosimilars.
“The ball is in AbbVie’s court,” she said. “If [the company] is not willing to drop price sufficiently, then payers will start switching to biosimilars.”
Dr. Fendrick reported past financial relationships and consulting arrangements with AbbVie, Amgen, Arnold Ventures, Bayer, CareFirst, BlueCross BlueShield, and many other companies. Dr. Wosińska has received funding from Arnold Ventures and serves as an expert witness on antitrust cases involving generic medication.
A version of this article first appeared on Medscape.com.
The best-selling drug Humira (adalimumab) now faces competition in the United States after a 20-year monopoly. The first adalimumab biosimilar, Amjevita, launched in the United States on January 31, and in July, seven additional biosimilars became available. These drugs have the potential to lower prescription drug prices, but when and by how much remains to be seen.
Here’s what you need to know about adalimumab biosimilars.
What Humira biosimilars are now available?
Eight different biosimilars have launched in 2023 with discounts as large at 85% from Humira’s list price of $6,922. A few companies also offer two price points.

Three of these biosimilars – Hadlima, Hyrimoz, and Yuflyma – are available in high concentration formulations. This high concentration formulation makes up 85% of Humira prescriptions, according to a report from Goodroot, a collection of companies focused on lowering health care costs.
Cyltezo is currently the only adalimumab biosimilar with an interchangeability designation, meaning that a pharmacist can substitute the biosimilar for an equivalent Humira prescription without the intervention of a clinician. A total of 47 states allow for these substitutions without prior approval from a clinician, according to Goodroot, and the clinician must be notified of the switch within a certain time frame. A total of 40 states require that patients be notified of the switch before substitution.
However, it’s not clear if this interchangeability designation will prove an advantage for Cyltezo, as it is interchangeable with the lower concentration version of Humira that makes up just 15% of prescriptions.
Most of the companies behind these biosimilars are pursuing interchangeability designations for their drugs, except for Fresenius Kabi (Idacio) and Coherus (Yusimry).
A ninth biosimilar, Pfizer’s adalimumab-afzb (Abrilada), is not yet on the market and is currently awaiting an approval decision from the Food and Drug Administration to add an interchangeability designation to its prior approval for a low-concentration formulation.
Why are they priced differently?
The two price points offer different deals to payers. Pharmacy benefit managers make confidential agreements with drug manufacturers to get a discount – called a rebate – to get the drug on the PBM’s formulary. The PBM keeps a portion of that rebate, and the rest is passed on to the insurance company and patients. Biosimilars at a higher price point will likely offer larger rebates. Biosimilars offered at lower price points incorporate this discount up front in their list pricing and likely will not offer large rebates.
Will biosimilars be covered by payers?
Currently, biosimilars are being offered on formularies at parity with Humira, meaning they are on the same tier. The PBM companies OptumRx and Cigna Group’s Express Scripts will offer Amjevita (at both price points), Cyltezo, and Hyrimoz (at both price points).
“This decision allows our clients flexibility to provide access to the lower list price, so members in high-deductible plans and benefit designs with coinsurance can experience lower out-of-pocket costs,” said OptumRx spokesperson Isaac Sorensen in an email.
Mark Cuban Cost Plus Drug Company, which uses a direct-to-consumer model, will offer Yusimry for $567.27 on its website. SmithRx, a PBM based in San Francisco, announced it would partner with Cost Plus Drugs to offer Yusimry, adding that SmithRx members can use their insurance benefits to further reduce out-of-pocket costs. RxPreferred, another PBM, will also offer Yusimry through its partnership with Cuban’s company.
The news website Formulary Watch previously reported that CVS Caremark, another of the biggest PBMs, will be offering Amjevita, but as a nonpreferred brand, while Humira remains the preferred brand. CVS Caremark did not respond to a request for comment.
Will patients pay less?
Biosimilars have been touted as a potential solution to lower spending on biologic drugs, but it’s unknown if patients will ultimately benefit with lower out-of-pocket costs. It’s “impossible to predict” if the discount that third-party payers pay will be passed on to consumers, said Mark Fendrick, MD, who directs the University of Michigan Center for Value-based Insurance Design in Ann Arbor.
Generally, a consumer’s copay is a percentage of a drug’s list price, so it stands to reason that a low drug price would result in lower out-of-pocket payments. While this is mostly true, Humira has a successful copay assistance program to lower prescription costs for consumers. According to a 2022 IQVIA report, 82% of commercial prescriptions cost patients less than $10 for Humira because of this program.
To appeal to patients, biosimilar companies will need to offer similar savings, Dr. Fendrick added. “There will be some discontent if patients are actually asked to pay more out-of-pocket for a less expensive drug,” he said.
All eight companies behind these biosimilars are offering or will be launching copay saving programs, many which advertise copays as low as $0 per month for eligible patients.
How will Humira respond?
Marta Wosińska, PhD, a health care economist at the Brookings Institute, Washington, predicts payers will use these lower biosimilar prices to negotiate better deals with AbbVie, Humira’s manufacturer. “We have a lot of players coming into [the market] right now, so the competition is really fierce,” she said. In response, AbbVie will need to increase rebates on Humira and/or lower its price to compete with these biosimilars.
“The ball is in AbbVie’s court,” she said. “If [the company] is not willing to drop price sufficiently, then payers will start switching to biosimilars.”
Dr. Fendrick reported past financial relationships and consulting arrangements with AbbVie, Amgen, Arnold Ventures, Bayer, CareFirst, BlueCross BlueShield, and many other companies. Dr. Wosińska has received funding from Arnold Ventures and serves as an expert witness on antitrust cases involving generic medication.
A version of this article first appeared on Medscape.com.
Remote teams offer chance to improve difficult-to-treat PsA
DUBLIN – according to presenters at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
In the same session at the meeting, GRAPPA also announced a new initiative to define difficult-to-treat PsA.
Deepak Jadon, MBBCh, PhD, a rheumatologist with Cambridge (England) University Hospitals NHS Foundation Trust, described his experience of running a clinic for patients with difficult-to-treat PsA in eastern England, covering a catchment area of approximately 6 million people between six and seven hospitals. He discussed how the MDT in his region operates to discuss the management of such patients, whose treatment options may also have indications for comorbidities such as inflammatory bowel disease or uveitis, or have complicating factors such as metabolic syndrome.
“You have to have an interested and engaged colleague to form that collaboration,” Dr. Jadon said. “If you are working in isolation, without your colleagues in the same building, that becomes harder. We have been running remote multispecialty meetings without the patient being present, and I have had the good fortune of having medical students brought into our practice. We discussed approximately 220 patients, initially in our psoriasis-spondyloarthritis MDT and subsequently in our inflammatory bowel disease–spondyloarthritis MDT.”
There are also MDTs with hepatologist colleagues carried out on an ad hoc basis to discuss patients with nonalcoholic fatty liver disease, as well as patients with hepatitis or a transplanted liver, who have psoriatic disease.
This difficult-to-treat cohort is discussed in MDT meetings conducted on Zoom. At MDT meetings, carried out with frequencies ranging from monthly to bimonthly, Dr. Jadon said there would be two dermatologists, two rheumatologists, one to four dermatology and rheumatology trainees and fellows, one to four specialist nurses, one to three research nurses, and one biologics pharmacist. They record the meetings and discuss anywhere from 4 to 18 patients, reviewing items in their electronic medical record, calling or writing patients and/or their primary care clinician as needed. They take about an hour to meet, with a half hour of prep time and another 1.5 hours to undertake necessary actions.
“Generally, the question is, how can we change treatment to best cover the domains of disease?” Dr. Jadon said. “Progressively, more patients are being put onto biologics as a result of these conversations, and I do feel that it has helped our patients and us to consolidate their management plan. Naturally, as all clinicians do, we doubt ourselves and wonder if we are missing something. Is there an aspect of the disease [being missed]? Is there a treatment that I haven’t been using? [The meetings have] been reassuring in that regard. I also learn from my colleagues who have earlier access to treatments, especially in dermatology.”
In a small number of patients, some combinations of advanced therapies, such as combining a Janus kinase inhibitor with a biologic, have been used as a result of these collaborations, “and to discuss this in an MDT has been reassuring, including from a medico-legal perspective,” Dr. Jadon said. “One of the main things we found to be useful is having a brief referral pro forma. Usually, by the time patients reach this forum, they have used a lot of treatments, and it can be difficult to remember that on the spot. It is also important to focus on what the actual question is. Naturally, in these discussions, where you talk about the complexities and various facets of disease, you can get a bit lost and sometimes you actually don’t address the original question.”
He also said it has been very beneficial to use screen sharing in the remote MDTs so that different disciplines can review images together, such as with radiology colleagues. “There are varying skill sets among our colleagues, especially in radiology, and it has been quite nice to review their peripheral imaging, their axial imaging, laboratory markers, and skin lesions together.”
New GRAPPA project to provide clarity
A new GRAPPA project has been devised to help physicians identify and define difficult-to-treat and difficult-to-manage PsA in order to help physicians to categorize and treat these patients.
“We have a growing treatment armamentarium ... but we still do not reach all the patients that we would like to,” said Fabian Proft, MD, of Charité University Medicine, Berlin. “We set our targets, but we see in the real world that we are only reaching them in 40% or 50% of our patients. So, we need to do better, and in order to do better, we need to understand better.”
“We should not only make a definition of difficult-to-treat PsA, which is nonresponse to treatment with objective signs of inflammation, but also we need to address and acknowledge difficult-to-manage [patients],” Dr. Proft said. “We should not stop as soon as we come up with a definition. This will be a working definition and will need to be validated.”
The speakers reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
DUBLIN – according to presenters at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
In the same session at the meeting, GRAPPA also announced a new initiative to define difficult-to-treat PsA.
Deepak Jadon, MBBCh, PhD, a rheumatologist with Cambridge (England) University Hospitals NHS Foundation Trust, described his experience of running a clinic for patients with difficult-to-treat PsA in eastern England, covering a catchment area of approximately 6 million people between six and seven hospitals. He discussed how the MDT in his region operates to discuss the management of such patients, whose treatment options may also have indications for comorbidities such as inflammatory bowel disease or uveitis, or have complicating factors such as metabolic syndrome.
“You have to have an interested and engaged colleague to form that collaboration,” Dr. Jadon said. “If you are working in isolation, without your colleagues in the same building, that becomes harder. We have been running remote multispecialty meetings without the patient being present, and I have had the good fortune of having medical students brought into our practice. We discussed approximately 220 patients, initially in our psoriasis-spondyloarthritis MDT and subsequently in our inflammatory bowel disease–spondyloarthritis MDT.”
There are also MDTs with hepatologist colleagues carried out on an ad hoc basis to discuss patients with nonalcoholic fatty liver disease, as well as patients with hepatitis or a transplanted liver, who have psoriatic disease.
This difficult-to-treat cohort is discussed in MDT meetings conducted on Zoom. At MDT meetings, carried out with frequencies ranging from monthly to bimonthly, Dr. Jadon said there would be two dermatologists, two rheumatologists, one to four dermatology and rheumatology trainees and fellows, one to four specialist nurses, one to three research nurses, and one biologics pharmacist. They record the meetings and discuss anywhere from 4 to 18 patients, reviewing items in their electronic medical record, calling or writing patients and/or their primary care clinician as needed. They take about an hour to meet, with a half hour of prep time and another 1.5 hours to undertake necessary actions.
“Generally, the question is, how can we change treatment to best cover the domains of disease?” Dr. Jadon said. “Progressively, more patients are being put onto biologics as a result of these conversations, and I do feel that it has helped our patients and us to consolidate their management plan. Naturally, as all clinicians do, we doubt ourselves and wonder if we are missing something. Is there an aspect of the disease [being missed]? Is there a treatment that I haven’t been using? [The meetings have] been reassuring in that regard. I also learn from my colleagues who have earlier access to treatments, especially in dermatology.”
In a small number of patients, some combinations of advanced therapies, such as combining a Janus kinase inhibitor with a biologic, have been used as a result of these collaborations, “and to discuss this in an MDT has been reassuring, including from a medico-legal perspective,” Dr. Jadon said. “One of the main things we found to be useful is having a brief referral pro forma. Usually, by the time patients reach this forum, they have used a lot of treatments, and it can be difficult to remember that on the spot. It is also important to focus on what the actual question is. Naturally, in these discussions, where you talk about the complexities and various facets of disease, you can get a bit lost and sometimes you actually don’t address the original question.”
He also said it has been very beneficial to use screen sharing in the remote MDTs so that different disciplines can review images together, such as with radiology colleagues. “There are varying skill sets among our colleagues, especially in radiology, and it has been quite nice to review their peripheral imaging, their axial imaging, laboratory markers, and skin lesions together.”
New GRAPPA project to provide clarity
A new GRAPPA project has been devised to help physicians identify and define difficult-to-treat and difficult-to-manage PsA in order to help physicians to categorize and treat these patients.
“We have a growing treatment armamentarium ... but we still do not reach all the patients that we would like to,” said Fabian Proft, MD, of Charité University Medicine, Berlin. “We set our targets, but we see in the real world that we are only reaching them in 40% or 50% of our patients. So, we need to do better, and in order to do better, we need to understand better.”
“We should not only make a definition of difficult-to-treat PsA, which is nonresponse to treatment with objective signs of inflammation, but also we need to address and acknowledge difficult-to-manage [patients],” Dr. Proft said. “We should not stop as soon as we come up with a definition. This will be a working definition and will need to be validated.”
The speakers reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
DUBLIN – according to presenters at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
In the same session at the meeting, GRAPPA also announced a new initiative to define difficult-to-treat PsA.
Deepak Jadon, MBBCh, PhD, a rheumatologist with Cambridge (England) University Hospitals NHS Foundation Trust, described his experience of running a clinic for patients with difficult-to-treat PsA in eastern England, covering a catchment area of approximately 6 million people between six and seven hospitals. He discussed how the MDT in his region operates to discuss the management of such patients, whose treatment options may also have indications for comorbidities such as inflammatory bowel disease or uveitis, or have complicating factors such as metabolic syndrome.
“You have to have an interested and engaged colleague to form that collaboration,” Dr. Jadon said. “If you are working in isolation, without your colleagues in the same building, that becomes harder. We have been running remote multispecialty meetings without the patient being present, and I have had the good fortune of having medical students brought into our practice. We discussed approximately 220 patients, initially in our psoriasis-spondyloarthritis MDT and subsequently in our inflammatory bowel disease–spondyloarthritis MDT.”
There are also MDTs with hepatologist colleagues carried out on an ad hoc basis to discuss patients with nonalcoholic fatty liver disease, as well as patients with hepatitis or a transplanted liver, who have psoriatic disease.
This difficult-to-treat cohort is discussed in MDT meetings conducted on Zoom. At MDT meetings, carried out with frequencies ranging from monthly to bimonthly, Dr. Jadon said there would be two dermatologists, two rheumatologists, one to four dermatology and rheumatology trainees and fellows, one to four specialist nurses, one to three research nurses, and one biologics pharmacist. They record the meetings and discuss anywhere from 4 to 18 patients, reviewing items in their electronic medical record, calling or writing patients and/or their primary care clinician as needed. They take about an hour to meet, with a half hour of prep time and another 1.5 hours to undertake necessary actions.
“Generally, the question is, how can we change treatment to best cover the domains of disease?” Dr. Jadon said. “Progressively, more patients are being put onto biologics as a result of these conversations, and I do feel that it has helped our patients and us to consolidate their management plan. Naturally, as all clinicians do, we doubt ourselves and wonder if we are missing something. Is there an aspect of the disease [being missed]? Is there a treatment that I haven’t been using? [The meetings have] been reassuring in that regard. I also learn from my colleagues who have earlier access to treatments, especially in dermatology.”
In a small number of patients, some combinations of advanced therapies, such as combining a Janus kinase inhibitor with a biologic, have been used as a result of these collaborations, “and to discuss this in an MDT has been reassuring, including from a medico-legal perspective,” Dr. Jadon said. “One of the main things we found to be useful is having a brief referral pro forma. Usually, by the time patients reach this forum, they have used a lot of treatments, and it can be difficult to remember that on the spot. It is also important to focus on what the actual question is. Naturally, in these discussions, where you talk about the complexities and various facets of disease, you can get a bit lost and sometimes you actually don’t address the original question.”
He also said it has been very beneficial to use screen sharing in the remote MDTs so that different disciplines can review images together, such as with radiology colleagues. “There are varying skill sets among our colleagues, especially in radiology, and it has been quite nice to review their peripheral imaging, their axial imaging, laboratory markers, and skin lesions together.”
New GRAPPA project to provide clarity
A new GRAPPA project has been devised to help physicians identify and define difficult-to-treat and difficult-to-manage PsA in order to help physicians to categorize and treat these patients.
“We have a growing treatment armamentarium ... but we still do not reach all the patients that we would like to,” said Fabian Proft, MD, of Charité University Medicine, Berlin. “We set our targets, but we see in the real world that we are only reaching them in 40% or 50% of our patients. So, we need to do better, and in order to do better, we need to understand better.”
“We should not only make a definition of difficult-to-treat PsA, which is nonresponse to treatment with objective signs of inflammation, but also we need to address and acknowledge difficult-to-manage [patients],” Dr. Proft said. “We should not stop as soon as we come up with a definition. This will be a working definition and will need to be validated.”
The speakers reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
AT GRAPPA 2023
Keep depression, anxiety screening top of mind in patients with psoriatic disease
DUBLIN – , warranting routine screening and having community contacts for mental health professional referrals, Elizabeth Wallace, MD, said at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
Dr. Wallace, of Cherry Hills Dermatology, Englewood, Colo., discussed the complex interactions between mental illness and psoriatic disease and the potential pitfalls of this comorbidity for these patients.
The topic of mental health is “consistently at the top of our patients’ minds, and certainly our minds too,” said session comoderator and GRAPPA president-elect Joseph F. Merola, MD, MMSc.
“In the U.S., around 17% of people with psoriasis have depression vs. 9% in those without psoriasis,” Dr. Wallace explained. “Psoriasis patients are twice as likely to have depression, compared to those without psoriasis, and psoriasis patients are 33% more likely to attempt suicide and 20% more likely to complete suicide, compared to those without psoriasis.” More severe psoriasis and younger age of onset are also associated with a greater likelihood of suicidality, she added.
Mediators of depression
“The inflammatory mechanisms driving PsD can drive depression and anxiety, and vice-versa,” she said. “There are often also genetic links, for example genetic variations in serotonin receptors, and psychological issues in psoriatic disease are predictably worsened by feelings of stigmatization, embarrassment, and social isolation.”
There are also efforts underway in clinics to “normalize” screening for anxiety and depression among this patient cohort, Dr. Wallace said. “We know that our psoriasis patients face social stigma from the visibility of their disease, and that stress can lead to flares of their condition,” she told the attendees. “We also know that patients who experience stigma also have an increased risk of depressive symptoms. We all know now that psoriasis has well-established pathways with upregulated proinflammatory cytokines.
“Increased cytokines stimulate indoleamine 2,3-dioxygenase, which converts tryptophan to kynurenine. Kynurenine is metabolized to quinolinic acid, which is neurotoxic.” She explained that because serotonin derives from tryptophan, decreases in tryptophan lead to reduced serotonin, and therefore increased risk of depression.
Interleukin-6 is known to be upregulated in depression and downregulated with the use of antidepressant medications, Dr. Wallace said. Mouse models in research have shown that deletion of the IL-6 gene produces antidepressant effects, and studies in humans have shown that IL-6, more than any other serum cytokine, is found at higher levels in humans with depression and psoriatic disease.
IL-17 is also implicated in psoriatic disease and mental health problems, Dr. Wallace said. “With stress, you get upregulation of the Tc17 cells, which produce IL-17,” she explained. “IL-17, along with other inflammatory markers, can actually make the blood-brain barrier more permeable, and when you get more permeability to the blood-brain barrier, you get these cytokines that can cross from the periphery and into the brain.
“With this crossing into the brain, you get further activation of more Th17 [cells] and that, on neurons, leads to increased potassium production, which is directly neurotoxic, so you get neuron destruction.”
Talking about depression
“So, what can we share with our patients?” Dr. Wallace asked. “We can discuss with them that psoriatic patients in general are more likely to be depressed or to have higher rates of suicide. The literature consistently shows that patients whose psoriasis is successfully treated experience reduced depression, and we can provide an understandable review of systemic medications, with warnings on depression and/or suicidality.”
Dr. Wallace advised to screen for depression with the Patient Health Questionnaire-2 (PHQ-2), a validated, two-item tool that asks, “Over the past 2 weeks, how often have you been bothered by having little interest or pleasure in doing things?” and “Over the past 2 weeks, how often have you been bothered by feeling down, depressed, or hopeless?”
She presented a case study illustrative of the type of presentation she sees in her clinic. It involved a 32-year-old man with plaque psoriasis and a high degree of body surface affected. “It’s now July in Colorado, it’s getting warm, people want to wear their shorts and T-shirts, but he said he could no longer hide his psoriasis,” said Dr. Wallace. “Further, it’s in areas that he cannot hide, such as his scalp, his beard, and he also has nail disease. Often, these patients don’t want to shake hands with their bosses or their colleagues and that’s very embarrassing for them.”
Dr. Wallace explained that this patient had seen advertisements for biologic drugs and requested to commence a treatment course. “During the exam, and now that you are developing some rapport with him, you discover that he is feeling down, is embarrassed at work, and has started to avoid social situations.” This is illustrative of a patient who should be screened for mental health conditions, specifically using PHQ-2, she said.
“You can be the person at the front line to screen these patients for mental health conditions, and, specifically for depression, with PHQ-2,” she said. PHQ-2 scores range from 0 to 6, and a score of 3 or higher is considered a positive screen.
“This is where your relationship with another health provider who is most qualified to care for these patients and validate them for their mental health condition can be absolutely critical,” Dr. Wallace said.
Successful PsD treatment lessens the risk for mental health comorbidities, and this is also seen in psoriatic arthritis, Dr. Wallace pointed out. Patient education is critical regarding their increased risk for depression and potential suicidal ideation, she added.
“It’s our job as clinicians to provide patients with an understandable, easy-to-digest review of systemic medications and warnings on depression and suicidality so that they can be aware of these factors.”
Perspective from Dr. Merola
In an interview, Dr. Merola, a double board-certified dermatologist and rheumatologist at Brigham and Women’s Hospital, Boston, discussed the interactions between mental and physical illness.
“One of the things we are learning is that it’s very much a multifactorial issue, in that skin and joints contribute, in some obvious ways, to anxiety and depression, like the fact that somebody doesn’t feel good about their appearance, or they can’t complete daily activities,” he said. “Those are the more obvious ones. But there is data and evidence that there is a biology behind that as well – inflammatory cytokines that drive skin disease probably also have a direct impact on the CNS and probably also drive anxiety and depression.
“We know that disordered sleep contributes to anxiety – think about how we feel if we get a horrible night’s sleep ... it’s hard to pick apart: ‘Am I depressed, am I anxious because I am having too much coffee? Because I am fatigued?’ So, we get into these circles, but the point is, we have to break these cycles, and we have to do it in multiple places. Yes, we have to fix the skin and the joints, but we also have to have interventions and think about how to screen for anxiety and depression. We also have to think about identifying disordered sleep, and how we intervene there as well.”
These challenges require a collaborative approach among physicians. “We can help patients to build their team that gets them help for their skin, for their joints, for their anxiety or depression, their disordered sleep, for their nutritional disorders, their obesity, and so on. So, we are trying to pick apart and unpack those complexities,” he said.
In regard to the potential impacts of this holistic strategy on physician workloads, Dr. Merola acknowledged it is important to consider physician wellness. “There’s no question that we want to be doing the best we can for our colleagues, but we don’t want to overload our colleagues by saying, ‘By the way, not only should we be treating their skin and joints,’ which of course we should be doing, but ‘could you also manage their diabetes, their obesity, their disordered sleep, their anxiety, their depression, difficulties with insurance, getting access to treatments, etc.’
“This is where effective collaboration between physicians becomes important,” he stressed. “We can’t manage every single piece, but we can make sure our patients are informed, are aware, and assist them to get the help that they need.”
In the United States, there “is a real issue” with access to mental health care and greater awareness needs to be created around this issue, he added.
Dr. Wallace and Dr. Merola report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
DUBLIN – , warranting routine screening and having community contacts for mental health professional referrals, Elizabeth Wallace, MD, said at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
Dr. Wallace, of Cherry Hills Dermatology, Englewood, Colo., discussed the complex interactions between mental illness and psoriatic disease and the potential pitfalls of this comorbidity for these patients.
The topic of mental health is “consistently at the top of our patients’ minds, and certainly our minds too,” said session comoderator and GRAPPA president-elect Joseph F. Merola, MD, MMSc.
“In the U.S., around 17% of people with psoriasis have depression vs. 9% in those without psoriasis,” Dr. Wallace explained. “Psoriasis patients are twice as likely to have depression, compared to those without psoriasis, and psoriasis patients are 33% more likely to attempt suicide and 20% more likely to complete suicide, compared to those without psoriasis.” More severe psoriasis and younger age of onset are also associated with a greater likelihood of suicidality, she added.
Mediators of depression
“The inflammatory mechanisms driving PsD can drive depression and anxiety, and vice-versa,” she said. “There are often also genetic links, for example genetic variations in serotonin receptors, and psychological issues in psoriatic disease are predictably worsened by feelings of stigmatization, embarrassment, and social isolation.”
There are also efforts underway in clinics to “normalize” screening for anxiety and depression among this patient cohort, Dr. Wallace said. “We know that our psoriasis patients face social stigma from the visibility of their disease, and that stress can lead to flares of their condition,” she told the attendees. “We also know that patients who experience stigma also have an increased risk of depressive symptoms. We all know now that psoriasis has well-established pathways with upregulated proinflammatory cytokines.
“Increased cytokines stimulate indoleamine 2,3-dioxygenase, which converts tryptophan to kynurenine. Kynurenine is metabolized to quinolinic acid, which is neurotoxic.” She explained that because serotonin derives from tryptophan, decreases in tryptophan lead to reduced serotonin, and therefore increased risk of depression.
Interleukin-6 is known to be upregulated in depression and downregulated with the use of antidepressant medications, Dr. Wallace said. Mouse models in research have shown that deletion of the IL-6 gene produces antidepressant effects, and studies in humans have shown that IL-6, more than any other serum cytokine, is found at higher levels in humans with depression and psoriatic disease.
IL-17 is also implicated in psoriatic disease and mental health problems, Dr. Wallace said. “With stress, you get upregulation of the Tc17 cells, which produce IL-17,” she explained. “IL-17, along with other inflammatory markers, can actually make the blood-brain barrier more permeable, and when you get more permeability to the blood-brain barrier, you get these cytokines that can cross from the periphery and into the brain.
“With this crossing into the brain, you get further activation of more Th17 [cells] and that, on neurons, leads to increased potassium production, which is directly neurotoxic, so you get neuron destruction.”
Talking about depression
“So, what can we share with our patients?” Dr. Wallace asked. “We can discuss with them that psoriatic patients in general are more likely to be depressed or to have higher rates of suicide. The literature consistently shows that patients whose psoriasis is successfully treated experience reduced depression, and we can provide an understandable review of systemic medications, with warnings on depression and/or suicidality.”
Dr. Wallace advised to screen for depression with the Patient Health Questionnaire-2 (PHQ-2), a validated, two-item tool that asks, “Over the past 2 weeks, how often have you been bothered by having little interest or pleasure in doing things?” and “Over the past 2 weeks, how often have you been bothered by feeling down, depressed, or hopeless?”
She presented a case study illustrative of the type of presentation she sees in her clinic. It involved a 32-year-old man with plaque psoriasis and a high degree of body surface affected. “It’s now July in Colorado, it’s getting warm, people want to wear their shorts and T-shirts, but he said he could no longer hide his psoriasis,” said Dr. Wallace. “Further, it’s in areas that he cannot hide, such as his scalp, his beard, and he also has nail disease. Often, these patients don’t want to shake hands with their bosses or their colleagues and that’s very embarrassing for them.”
Dr. Wallace explained that this patient had seen advertisements for biologic drugs and requested to commence a treatment course. “During the exam, and now that you are developing some rapport with him, you discover that he is feeling down, is embarrassed at work, and has started to avoid social situations.” This is illustrative of a patient who should be screened for mental health conditions, specifically using PHQ-2, she said.
“You can be the person at the front line to screen these patients for mental health conditions, and, specifically for depression, with PHQ-2,” she said. PHQ-2 scores range from 0 to 6, and a score of 3 or higher is considered a positive screen.
“This is where your relationship with another health provider who is most qualified to care for these patients and validate them for their mental health condition can be absolutely critical,” Dr. Wallace said.
Successful PsD treatment lessens the risk for mental health comorbidities, and this is also seen in psoriatic arthritis, Dr. Wallace pointed out. Patient education is critical regarding their increased risk for depression and potential suicidal ideation, she added.
“It’s our job as clinicians to provide patients with an understandable, easy-to-digest review of systemic medications and warnings on depression and suicidality so that they can be aware of these factors.”
Perspective from Dr. Merola
In an interview, Dr. Merola, a double board-certified dermatologist and rheumatologist at Brigham and Women’s Hospital, Boston, discussed the interactions between mental and physical illness.
“One of the things we are learning is that it’s very much a multifactorial issue, in that skin and joints contribute, in some obvious ways, to anxiety and depression, like the fact that somebody doesn’t feel good about their appearance, or they can’t complete daily activities,” he said. “Those are the more obvious ones. But there is data and evidence that there is a biology behind that as well – inflammatory cytokines that drive skin disease probably also have a direct impact on the CNS and probably also drive anxiety and depression.
“We know that disordered sleep contributes to anxiety – think about how we feel if we get a horrible night’s sleep ... it’s hard to pick apart: ‘Am I depressed, am I anxious because I am having too much coffee? Because I am fatigued?’ So, we get into these circles, but the point is, we have to break these cycles, and we have to do it in multiple places. Yes, we have to fix the skin and the joints, but we also have to have interventions and think about how to screen for anxiety and depression. We also have to think about identifying disordered sleep, and how we intervene there as well.”
These challenges require a collaborative approach among physicians. “We can help patients to build their team that gets them help for their skin, for their joints, for their anxiety or depression, their disordered sleep, for their nutritional disorders, their obesity, and so on. So, we are trying to pick apart and unpack those complexities,” he said.
In regard to the potential impacts of this holistic strategy on physician workloads, Dr. Merola acknowledged it is important to consider physician wellness. “There’s no question that we want to be doing the best we can for our colleagues, but we don’t want to overload our colleagues by saying, ‘By the way, not only should we be treating their skin and joints,’ which of course we should be doing, but ‘could you also manage their diabetes, their obesity, their disordered sleep, their anxiety, their depression, difficulties with insurance, getting access to treatments, etc.’
“This is where effective collaboration between physicians becomes important,” he stressed. “We can’t manage every single piece, but we can make sure our patients are informed, are aware, and assist them to get the help that they need.”
In the United States, there “is a real issue” with access to mental health care and greater awareness needs to be created around this issue, he added.
Dr. Wallace and Dr. Merola report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
DUBLIN – , warranting routine screening and having community contacts for mental health professional referrals, Elizabeth Wallace, MD, said at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
Dr. Wallace, of Cherry Hills Dermatology, Englewood, Colo., discussed the complex interactions between mental illness and psoriatic disease and the potential pitfalls of this comorbidity for these patients.
The topic of mental health is “consistently at the top of our patients’ minds, and certainly our minds too,” said session comoderator and GRAPPA president-elect Joseph F. Merola, MD, MMSc.
“In the U.S., around 17% of people with psoriasis have depression vs. 9% in those without psoriasis,” Dr. Wallace explained. “Psoriasis patients are twice as likely to have depression, compared to those without psoriasis, and psoriasis patients are 33% more likely to attempt suicide and 20% more likely to complete suicide, compared to those without psoriasis.” More severe psoriasis and younger age of onset are also associated with a greater likelihood of suicidality, she added.
Mediators of depression
“The inflammatory mechanisms driving PsD can drive depression and anxiety, and vice-versa,” she said. “There are often also genetic links, for example genetic variations in serotonin receptors, and psychological issues in psoriatic disease are predictably worsened by feelings of stigmatization, embarrassment, and social isolation.”
There are also efforts underway in clinics to “normalize” screening for anxiety and depression among this patient cohort, Dr. Wallace said. “We know that our psoriasis patients face social stigma from the visibility of their disease, and that stress can lead to flares of their condition,” she told the attendees. “We also know that patients who experience stigma also have an increased risk of depressive symptoms. We all know now that psoriasis has well-established pathways with upregulated proinflammatory cytokines.
“Increased cytokines stimulate indoleamine 2,3-dioxygenase, which converts tryptophan to kynurenine. Kynurenine is metabolized to quinolinic acid, which is neurotoxic.” She explained that because serotonin derives from tryptophan, decreases in tryptophan lead to reduced serotonin, and therefore increased risk of depression.
Interleukin-6 is known to be upregulated in depression and downregulated with the use of antidepressant medications, Dr. Wallace said. Mouse models in research have shown that deletion of the IL-6 gene produces antidepressant effects, and studies in humans have shown that IL-6, more than any other serum cytokine, is found at higher levels in humans with depression and psoriatic disease.
IL-17 is also implicated in psoriatic disease and mental health problems, Dr. Wallace said. “With stress, you get upregulation of the Tc17 cells, which produce IL-17,” she explained. “IL-17, along with other inflammatory markers, can actually make the blood-brain barrier more permeable, and when you get more permeability to the blood-brain barrier, you get these cytokines that can cross from the periphery and into the brain.
“With this crossing into the brain, you get further activation of more Th17 [cells] and that, on neurons, leads to increased potassium production, which is directly neurotoxic, so you get neuron destruction.”
Talking about depression
“So, what can we share with our patients?” Dr. Wallace asked. “We can discuss with them that psoriatic patients in general are more likely to be depressed or to have higher rates of suicide. The literature consistently shows that patients whose psoriasis is successfully treated experience reduced depression, and we can provide an understandable review of systemic medications, with warnings on depression and/or suicidality.”
Dr. Wallace advised to screen for depression with the Patient Health Questionnaire-2 (PHQ-2), a validated, two-item tool that asks, “Over the past 2 weeks, how often have you been bothered by having little interest or pleasure in doing things?” and “Over the past 2 weeks, how often have you been bothered by feeling down, depressed, or hopeless?”
She presented a case study illustrative of the type of presentation she sees in her clinic. It involved a 32-year-old man with plaque psoriasis and a high degree of body surface affected. “It’s now July in Colorado, it’s getting warm, people want to wear their shorts and T-shirts, but he said he could no longer hide his psoriasis,” said Dr. Wallace. “Further, it’s in areas that he cannot hide, such as his scalp, his beard, and he also has nail disease. Often, these patients don’t want to shake hands with their bosses or their colleagues and that’s very embarrassing for them.”
Dr. Wallace explained that this patient had seen advertisements for biologic drugs and requested to commence a treatment course. “During the exam, and now that you are developing some rapport with him, you discover that he is feeling down, is embarrassed at work, and has started to avoid social situations.” This is illustrative of a patient who should be screened for mental health conditions, specifically using PHQ-2, she said.
“You can be the person at the front line to screen these patients for mental health conditions, and, specifically for depression, with PHQ-2,” she said. PHQ-2 scores range from 0 to 6, and a score of 3 or higher is considered a positive screen.
“This is where your relationship with another health provider who is most qualified to care for these patients and validate them for their mental health condition can be absolutely critical,” Dr. Wallace said.
Successful PsD treatment lessens the risk for mental health comorbidities, and this is also seen in psoriatic arthritis, Dr. Wallace pointed out. Patient education is critical regarding their increased risk for depression and potential suicidal ideation, she added.
“It’s our job as clinicians to provide patients with an understandable, easy-to-digest review of systemic medications and warnings on depression and suicidality so that they can be aware of these factors.”
Perspective from Dr. Merola
In an interview, Dr. Merola, a double board-certified dermatologist and rheumatologist at Brigham and Women’s Hospital, Boston, discussed the interactions between mental and physical illness.
“One of the things we are learning is that it’s very much a multifactorial issue, in that skin and joints contribute, in some obvious ways, to anxiety and depression, like the fact that somebody doesn’t feel good about their appearance, or they can’t complete daily activities,” he said. “Those are the more obvious ones. But there is data and evidence that there is a biology behind that as well – inflammatory cytokines that drive skin disease probably also have a direct impact on the CNS and probably also drive anxiety and depression.
“We know that disordered sleep contributes to anxiety – think about how we feel if we get a horrible night’s sleep ... it’s hard to pick apart: ‘Am I depressed, am I anxious because I am having too much coffee? Because I am fatigued?’ So, we get into these circles, but the point is, we have to break these cycles, and we have to do it in multiple places. Yes, we have to fix the skin and the joints, but we also have to have interventions and think about how to screen for anxiety and depression. We also have to think about identifying disordered sleep, and how we intervene there as well.”
These challenges require a collaborative approach among physicians. “We can help patients to build their team that gets them help for their skin, for their joints, for their anxiety or depression, their disordered sleep, for their nutritional disorders, their obesity, and so on. So, we are trying to pick apart and unpack those complexities,” he said.
In regard to the potential impacts of this holistic strategy on physician workloads, Dr. Merola acknowledged it is important to consider physician wellness. “There’s no question that we want to be doing the best we can for our colleagues, but we don’t want to overload our colleagues by saying, ‘By the way, not only should we be treating their skin and joints,’ which of course we should be doing, but ‘could you also manage their diabetes, their obesity, their disordered sleep, their anxiety, their depression, difficulties with insurance, getting access to treatments, etc.’
“This is where effective collaboration between physicians becomes important,” he stressed. “We can’t manage every single piece, but we can make sure our patients are informed, are aware, and assist them to get the help that they need.”
In the United States, there “is a real issue” with access to mental health care and greater awareness needs to be created around this issue, he added.
Dr. Wallace and Dr. Merola report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT GRAPPA 2023
Oral IL-23 receptor antagonist for psoriasis promising: Phase 2b study
SINGAPORE – across all doses, compared with placebo, according to results of the FRONTIER 1 trial.
In the 16-week phase 2b study, 255 adults with moderate to severe plaque psoriasis were randomly assigned into six treatment groups: placebo (n = 43), JNJ-2113 25 mg daily (n = 43), 25 mg twice daily (n = 41), 50 mg daily (n = 43), 100 mg daily (n = 43), or 100 mg twice daily (n = 42).
Of those who took the placebo, only 9.3% achieved the study’s primary endpoint of a 75% or greater improvement in the Psoriasis Area and Severity Index (PASI-75) by week 16. This was compared with 78.6% in the group that took the highest dose.
“Additionally, the onset of action was fairly fast: at week 4, more than 20% of patients had achieved PASI 75,” said Robert Bissonnette, MD, CEO of Innovaderm Research in Montreal, who presented the findings during a late-breaker session at the World Congress of Dermatology.
Patients in the remaining groups demonstrated a response that corresponded to dosing level: with 37.2%, 51.2%, 58.1%, and 65.1% achieving PASI-75 in the 25 mg daily, 25 mg twice-daily, 50 mg daily, and 100 mg daily groups, respectively.
“These results are very interesting because in terms of psoriasis treatment, if this is confirmed in phase 3, it would give us an oral alternative that would be selective for IL-23,” said Dr. Bissonnette, referring to the signaling pathway that plays a critical role in the pathogenesis of several immune-mediated inflammatory diseases, including plaque psoriasis.
Although rarely life-threatening, the skin disorder is often intractable to treatment. In recent years, therapies that block IL-23 signaling and downstream inflammatory cytokine production have proven useful. “We have on the market a number of biological agents targeting IL-23 that we use on a regular basis,” said Dr. Bissonnette. “However, there are currently no orally delivered therapies.”
If successful, JNJ-2113 – a first-in-class oral IL-23 antagonist peptide developed by Janssen – could change the treatment paradigm for patients with moderate to severe plaque psoriasis. “When I was first introduced to the concept, I thought it wouldn’t work as it’s a peptide, that it would be digested by the stomach,” he told the audience. “But because of its GI stability and its potency, when you administer it orally, you can detect pharmacological activity.”
A well-tolerated alternative
Participants in the FRONTIER 1 trial were on average about 44 years old and weighed 88.9 kg (195 lb). Most had been living with psoriasis for about 18 years, with a total PASI score of 19.05. In addition, 43.1% had been treated with phototherapy in the past, 22% with biologics, and 78.4% with systemics.
PASI 90 and 100 were among some of the secondary outcomes measured. Similar to the primary outcome of PASI 75, all treatment groups demonstrated a statistically significant dose-response in PASI 90, compared with placebo. For those on the highest dose of JNJ-2113, 59.5% and 40.5% achieved PASI 90 and PASI 100, respectively, by week 16. The corresponding figures for those receiving placebo were 2.3% and 0%.
The safety profile for JNJ-2113 across all doses was similar to that of placebo, with no evidence of a dose-dependent increase in the occurrence of adverse events (AEs). The most frequently reported AEs were COVID-19 and nasopharyngitis. There were three serious AEs (COVID-19, infected cyst, suicide attempt) among those on the active drug, but the investigators assessed that they were not related to the study intervention. No deaths, major adverse cardiac events, or malignancies were reported during the study.
Approached for an independent comment, Marius-Anton Ionescu, MD, PhD, from the University Hospital Saint Louis, Paris, who specializes in psoriasis, told this news organization that the new development with JNJ-2113 “is really promising.”
Treatment for plaque psoriasis has improved to the point where some biologics, such as risankizumab (Skyrizi), only require patients to have “four shots a year,” he says. “This is the future of psoriasis treatment; it might go down to two shots a year” – a regimen that will be easier than taking an oral medication once or twice a day.
“But it’s good to have an oral option because you will always have some patients who say: ‘Shots are not for me, I’m afraid,’ ” he says.
However, Dr. Ionescu noted that if JNJ-2113 were to pass phase 3 trials, it might face stiff competition from the selective tyrosine kinase 2 (TYK2) inhibitor deucravacitinib (Sotyktu), which the U.S. Food and Drug Administration approved for use in adults with moderate to severe plaque psoriasis last September. “It has very good results and is the first oral therapy that is comparable with biologics for plaque psoriasis,” he says.
But Dr. Bissonnette remains hopeful for the future. “I think JNJ-2113 goes way beyond psoriasis because this type of strategy using oral peptide–blocking receptors could be used in other immune-mediated diseases, including atopic dermatitis and other diseases outside of dermatology.” In addition to running a phase 3 study for moderate to severe plaque psoriasis, Janssen is planning to initiate a phase 2b clinical trial of JNJ-2113 in adults with ulcerative colitis.
The study was funded by Janssen. Dr. Bissonnette reports consulting and investigating for Janssen, and being on advisory panels and receiving research funding from multiple other pharmaceutical companies. Dr. Ionescu is an investigator for Psoriasis National Register France Psobioteq (no honoraria), and an investigator and speaker for Uriage cosmetics (honoraria).
A version of this article first appeared on Medscape.com.
SINGAPORE – across all doses, compared with placebo, according to results of the FRONTIER 1 trial.
In the 16-week phase 2b study, 255 adults with moderate to severe plaque psoriasis were randomly assigned into six treatment groups: placebo (n = 43), JNJ-2113 25 mg daily (n = 43), 25 mg twice daily (n = 41), 50 mg daily (n = 43), 100 mg daily (n = 43), or 100 mg twice daily (n = 42).
Of those who took the placebo, only 9.3% achieved the study’s primary endpoint of a 75% or greater improvement in the Psoriasis Area and Severity Index (PASI-75) by week 16. This was compared with 78.6% in the group that took the highest dose.
“Additionally, the onset of action was fairly fast: at week 4, more than 20% of patients had achieved PASI 75,” said Robert Bissonnette, MD, CEO of Innovaderm Research in Montreal, who presented the findings during a late-breaker session at the World Congress of Dermatology.
Patients in the remaining groups demonstrated a response that corresponded to dosing level: with 37.2%, 51.2%, 58.1%, and 65.1% achieving PASI-75 in the 25 mg daily, 25 mg twice-daily, 50 mg daily, and 100 mg daily groups, respectively.
“These results are very interesting because in terms of psoriasis treatment, if this is confirmed in phase 3, it would give us an oral alternative that would be selective for IL-23,” said Dr. Bissonnette, referring to the signaling pathway that plays a critical role in the pathogenesis of several immune-mediated inflammatory diseases, including plaque psoriasis.
Although rarely life-threatening, the skin disorder is often intractable to treatment. In recent years, therapies that block IL-23 signaling and downstream inflammatory cytokine production have proven useful. “We have on the market a number of biological agents targeting IL-23 that we use on a regular basis,” said Dr. Bissonnette. “However, there are currently no orally delivered therapies.”
If successful, JNJ-2113 – a first-in-class oral IL-23 antagonist peptide developed by Janssen – could change the treatment paradigm for patients with moderate to severe plaque psoriasis. “When I was first introduced to the concept, I thought it wouldn’t work as it’s a peptide, that it would be digested by the stomach,” he told the audience. “But because of its GI stability and its potency, when you administer it orally, you can detect pharmacological activity.”
A well-tolerated alternative
Participants in the FRONTIER 1 trial were on average about 44 years old and weighed 88.9 kg (195 lb). Most had been living with psoriasis for about 18 years, with a total PASI score of 19.05. In addition, 43.1% had been treated with phototherapy in the past, 22% with biologics, and 78.4% with systemics.
PASI 90 and 100 were among some of the secondary outcomes measured. Similar to the primary outcome of PASI 75, all treatment groups demonstrated a statistically significant dose-response in PASI 90, compared with placebo. For those on the highest dose of JNJ-2113, 59.5% and 40.5% achieved PASI 90 and PASI 100, respectively, by week 16. The corresponding figures for those receiving placebo were 2.3% and 0%.
The safety profile for JNJ-2113 across all doses was similar to that of placebo, with no evidence of a dose-dependent increase in the occurrence of adverse events (AEs). The most frequently reported AEs were COVID-19 and nasopharyngitis. There were three serious AEs (COVID-19, infected cyst, suicide attempt) among those on the active drug, but the investigators assessed that they were not related to the study intervention. No deaths, major adverse cardiac events, or malignancies were reported during the study.
Approached for an independent comment, Marius-Anton Ionescu, MD, PhD, from the University Hospital Saint Louis, Paris, who specializes in psoriasis, told this news organization that the new development with JNJ-2113 “is really promising.”
Treatment for plaque psoriasis has improved to the point where some biologics, such as risankizumab (Skyrizi), only require patients to have “four shots a year,” he says. “This is the future of psoriasis treatment; it might go down to two shots a year” – a regimen that will be easier than taking an oral medication once or twice a day.
“But it’s good to have an oral option because you will always have some patients who say: ‘Shots are not for me, I’m afraid,’ ” he says.
However, Dr. Ionescu noted that if JNJ-2113 were to pass phase 3 trials, it might face stiff competition from the selective tyrosine kinase 2 (TYK2) inhibitor deucravacitinib (Sotyktu), which the U.S. Food and Drug Administration approved for use in adults with moderate to severe plaque psoriasis last September. “It has very good results and is the first oral therapy that is comparable with biologics for plaque psoriasis,” he says.
But Dr. Bissonnette remains hopeful for the future. “I think JNJ-2113 goes way beyond psoriasis because this type of strategy using oral peptide–blocking receptors could be used in other immune-mediated diseases, including atopic dermatitis and other diseases outside of dermatology.” In addition to running a phase 3 study for moderate to severe plaque psoriasis, Janssen is planning to initiate a phase 2b clinical trial of JNJ-2113 in adults with ulcerative colitis.
The study was funded by Janssen. Dr. Bissonnette reports consulting and investigating for Janssen, and being on advisory panels and receiving research funding from multiple other pharmaceutical companies. Dr. Ionescu is an investigator for Psoriasis National Register France Psobioteq (no honoraria), and an investigator and speaker for Uriage cosmetics (honoraria).
A version of this article first appeared on Medscape.com.
SINGAPORE – across all doses, compared with placebo, according to results of the FRONTIER 1 trial.
In the 16-week phase 2b study, 255 adults with moderate to severe plaque psoriasis were randomly assigned into six treatment groups: placebo (n = 43), JNJ-2113 25 mg daily (n = 43), 25 mg twice daily (n = 41), 50 mg daily (n = 43), 100 mg daily (n = 43), or 100 mg twice daily (n = 42).
Of those who took the placebo, only 9.3% achieved the study’s primary endpoint of a 75% or greater improvement in the Psoriasis Area and Severity Index (PASI-75) by week 16. This was compared with 78.6% in the group that took the highest dose.
“Additionally, the onset of action was fairly fast: at week 4, more than 20% of patients had achieved PASI 75,” said Robert Bissonnette, MD, CEO of Innovaderm Research in Montreal, who presented the findings during a late-breaker session at the World Congress of Dermatology.
Patients in the remaining groups demonstrated a response that corresponded to dosing level: with 37.2%, 51.2%, 58.1%, and 65.1% achieving PASI-75 in the 25 mg daily, 25 mg twice-daily, 50 mg daily, and 100 mg daily groups, respectively.
“These results are very interesting because in terms of psoriasis treatment, if this is confirmed in phase 3, it would give us an oral alternative that would be selective for IL-23,” said Dr. Bissonnette, referring to the signaling pathway that plays a critical role in the pathogenesis of several immune-mediated inflammatory diseases, including plaque psoriasis.
Although rarely life-threatening, the skin disorder is often intractable to treatment. In recent years, therapies that block IL-23 signaling and downstream inflammatory cytokine production have proven useful. “We have on the market a number of biological agents targeting IL-23 that we use on a regular basis,” said Dr. Bissonnette. “However, there are currently no orally delivered therapies.”
If successful, JNJ-2113 – a first-in-class oral IL-23 antagonist peptide developed by Janssen – could change the treatment paradigm for patients with moderate to severe plaque psoriasis. “When I was first introduced to the concept, I thought it wouldn’t work as it’s a peptide, that it would be digested by the stomach,” he told the audience. “But because of its GI stability and its potency, when you administer it orally, you can detect pharmacological activity.”
A well-tolerated alternative
Participants in the FRONTIER 1 trial were on average about 44 years old and weighed 88.9 kg (195 lb). Most had been living with psoriasis for about 18 years, with a total PASI score of 19.05. In addition, 43.1% had been treated with phototherapy in the past, 22% with biologics, and 78.4% with systemics.
PASI 90 and 100 were among some of the secondary outcomes measured. Similar to the primary outcome of PASI 75, all treatment groups demonstrated a statistically significant dose-response in PASI 90, compared with placebo. For those on the highest dose of JNJ-2113, 59.5% and 40.5% achieved PASI 90 and PASI 100, respectively, by week 16. The corresponding figures for those receiving placebo were 2.3% and 0%.
The safety profile for JNJ-2113 across all doses was similar to that of placebo, with no evidence of a dose-dependent increase in the occurrence of adverse events (AEs). The most frequently reported AEs were COVID-19 and nasopharyngitis. There were three serious AEs (COVID-19, infected cyst, suicide attempt) among those on the active drug, but the investigators assessed that they were not related to the study intervention. No deaths, major adverse cardiac events, or malignancies were reported during the study.
Approached for an independent comment, Marius-Anton Ionescu, MD, PhD, from the University Hospital Saint Louis, Paris, who specializes in psoriasis, told this news organization that the new development with JNJ-2113 “is really promising.”
Treatment for plaque psoriasis has improved to the point where some biologics, such as risankizumab (Skyrizi), only require patients to have “four shots a year,” he says. “This is the future of psoriasis treatment; it might go down to two shots a year” – a regimen that will be easier than taking an oral medication once or twice a day.
“But it’s good to have an oral option because you will always have some patients who say: ‘Shots are not for me, I’m afraid,’ ” he says.
However, Dr. Ionescu noted that if JNJ-2113 were to pass phase 3 trials, it might face stiff competition from the selective tyrosine kinase 2 (TYK2) inhibitor deucravacitinib (Sotyktu), which the U.S. Food and Drug Administration approved for use in adults with moderate to severe plaque psoriasis last September. “It has very good results and is the first oral therapy that is comparable with biologics for plaque psoriasis,” he says.
But Dr. Bissonnette remains hopeful for the future. “I think JNJ-2113 goes way beyond psoriasis because this type of strategy using oral peptide–blocking receptors could be used in other immune-mediated diseases, including atopic dermatitis and other diseases outside of dermatology.” In addition to running a phase 3 study for moderate to severe plaque psoriasis, Janssen is planning to initiate a phase 2b clinical trial of JNJ-2113 in adults with ulcerative colitis.
The study was funded by Janssen. Dr. Bissonnette reports consulting and investigating for Janssen, and being on advisory panels and receiving research funding from multiple other pharmaceutical companies. Dr. Ionescu is an investigator for Psoriasis National Register France Psobioteq (no honoraria), and an investigator and speaker for Uriage cosmetics (honoraria).
A version of this article first appeared on Medscape.com.
AT WCD 2023
Study finds subcutaneous spesolimab reduces flares in patients with GPP
SINGAPORE – presented in a late-breaker session at the World Congress of Dermatology,
In the phase 2b study, patients who received the high-dose regimen (a 600-mg subcutaneous loading dose, then 300-mg SC every 4 weeks) of spesolimab experienced 84% fewer GPP fares over 48 weeks, compared with those on placebo, reported Bruce Strober, MD, PhD, Central Connecticut Dermatology, Cromwell, and clinical professor of dermatology, Yale University, New Haven, Conn. “Additionally, no flares occurred after week 4, and this, in turn, translated into improved patient outcomes.”
GPP is a rare, chronic, systemic neutrophilic skin disease. The resulting flares, characterized by painful pustules all over the body, can lead to sepsis, shock, and other life-threatening complications. “People who have it are considerably burdened by it, so targeted therapy of this disease is incredibly important because it leads to lessened morbidity and, importantly, mortality for these patients,” Dr. Strober said.
“It’s important not only to treat the flares but also to prevent them,” he noted.
The intravenous formulation of spesolimab (Spevigo) was approved for the treatment of GPP flares in adults by the Food and Drug Administration in September 2022. It is now authorized in nearly 40 countries, including Japan, China, and the European Union.
The phase 2 Effisayil 2 study presented at the meeting evaluated the subcutaneous formulation of spesolimab. Data on subcutaneous spesolimab has been submitted to the FDA, and has received breakthrough therapy designation, according to the manufacturer, Boehringer Ingelheim.
Flare prevention
In the study, 123 patients with GPP were randomly assigned 1:1:1:1 to one of four groups: high-dose spesolimab, medium-dose (600-mg SC loading dose, then 300-mg SC every 12 weeks), low-dose (300-mg SC loading dose, then 150-mg SC every 12 weeks), or placebo. In the event of a flare during the randomized treatment period, a patient was administered a single, 900-mg intravenous dose of spesolimab.
Nearly two-thirds of the participants were female and nearly two-thirds were Asian, with a mean age of about 39-43 years.
The mean numbers of GPP flares experienced annually by those in the low-, medium-, and high-dose spesolimab groups were 2.7, 1.9, and 2.4, respectively (2.4% in the placebo group). Fewer than a third had concurrent plaque psoriasis at baseline. Most (48.4%-63.3%) did not have an IL-36RN mutation.
Additionally, the Generalized Pustular Psoriasis Physician Global Assessment total score was 1 in 74.2%-93.5% of participants, and 0 in the remainder.
The primary study endpoint was the time to GPP flare by week 48. The risk of developing a flare among those on high-dose spesolimab was 84% lower, compared with that of those on placebo (hazard ratio, 0.16; 95% confidence interval, 0.05-0.54; P = .0005). No patients on the high dose had a flare after the 4th week of the study.
Similarly, for the secondary endpoint (occurrence of at least one GPP flare by week 48). Dr. Strober and his colleagues reported that high-dose spesolimab was superior to placebo with a risk difference of -39% (95% CI, –0.62 to –0.16; P = .0013). By contrast, the risk differences for the medium- and low-dose spesolimab arms were –0.23 (95% CI, –0.46 to 0.01) and -0.31 (95% CI, –0.54 to –0.08), respectively.
The safety profile of subcutaneous spesolimab across all three doses was similar to that of placebo, and there was no dose-dependent trend. Reported adverse events (AEs) were mild. There were five (5.4%) AEs leading to discontinuation of the drug in the medium- and high-dose groups, but none in the low-dose group. Overall, there were nine (9.7%) serious AEs reported in the spesolimab groups, and three (10%) in the high-dose group; no deaths occurred on any dose.
Participants most often reported injection-site erythema, reported in 13 (14%) of the patients on spesolimab versus 1 (3.3%) of those on placebo.
“Overall, the study demonstrates that subcutaneous spesolimab is effective at controlling GPP flares, especially at a high dose relative to placebo, and supports subcutaneous spesolimab for the therapy for GPP flare prevention,” Dr. Strober said at the meeting.
Targeting the IL-36 pathway
In a comment, Todd Schlesinger, MD, Clinical Research Center of the Carolinas, Charleston, S.C., who moderated the session, said: “It’s very exciting to be able to have a subcutaneous version of the medication.”
“I think the IL-36 is a great pathway,” he said, referring to the signaling pathway within the immune system that is central to the pathogenesis of GPP and several other autoinflammatory diseases.
However, Dr. Schlesinger said that he would have liked to have seen data on how many patients ended up treated with intravenous spesolimab.
He added that he would like future studies of subcutaneous spesolimab to examine the effect in different populations that vary by parameters such as weight, race, and disease severity. “Just seeing how somebody who’s flaring five times a year and you give them this medication and they’re now flaring once a year – that’s interesting data that we might like to know in the future.”
Other than for preventing GPP flares, spesolimab is being studied for treating other IL-36–mediated skin diseases, such as palmoplantar pustulosis.
The study was funded by Boehringer Ingelheim; both Dr. Strober and Dr. Schlesinger do research and consulting for BI, and receive funding from multiple other pharmaceutical companies.
A version of this article first appeared on Medscape.com.
SINGAPORE – presented in a late-breaker session at the World Congress of Dermatology,
In the phase 2b study, patients who received the high-dose regimen (a 600-mg subcutaneous loading dose, then 300-mg SC every 4 weeks) of spesolimab experienced 84% fewer GPP fares over 48 weeks, compared with those on placebo, reported Bruce Strober, MD, PhD, Central Connecticut Dermatology, Cromwell, and clinical professor of dermatology, Yale University, New Haven, Conn. “Additionally, no flares occurred after week 4, and this, in turn, translated into improved patient outcomes.”
GPP is a rare, chronic, systemic neutrophilic skin disease. The resulting flares, characterized by painful pustules all over the body, can lead to sepsis, shock, and other life-threatening complications. “People who have it are considerably burdened by it, so targeted therapy of this disease is incredibly important because it leads to lessened morbidity and, importantly, mortality for these patients,” Dr. Strober said.
“It’s important not only to treat the flares but also to prevent them,” he noted.
The intravenous formulation of spesolimab (Spevigo) was approved for the treatment of GPP flares in adults by the Food and Drug Administration in September 2022. It is now authorized in nearly 40 countries, including Japan, China, and the European Union.
The phase 2 Effisayil 2 study presented at the meeting evaluated the subcutaneous formulation of spesolimab. Data on subcutaneous spesolimab has been submitted to the FDA, and has received breakthrough therapy designation, according to the manufacturer, Boehringer Ingelheim.
Flare prevention
In the study, 123 patients with GPP were randomly assigned 1:1:1:1 to one of four groups: high-dose spesolimab, medium-dose (600-mg SC loading dose, then 300-mg SC every 12 weeks), low-dose (300-mg SC loading dose, then 150-mg SC every 12 weeks), or placebo. In the event of a flare during the randomized treatment period, a patient was administered a single, 900-mg intravenous dose of spesolimab.
Nearly two-thirds of the participants were female and nearly two-thirds were Asian, with a mean age of about 39-43 years.
The mean numbers of GPP flares experienced annually by those in the low-, medium-, and high-dose spesolimab groups were 2.7, 1.9, and 2.4, respectively (2.4% in the placebo group). Fewer than a third had concurrent plaque psoriasis at baseline. Most (48.4%-63.3%) did not have an IL-36RN mutation.
Additionally, the Generalized Pustular Psoriasis Physician Global Assessment total score was 1 in 74.2%-93.5% of participants, and 0 in the remainder.
The primary study endpoint was the time to GPP flare by week 48. The risk of developing a flare among those on high-dose spesolimab was 84% lower, compared with that of those on placebo (hazard ratio, 0.16; 95% confidence interval, 0.05-0.54; P = .0005). No patients on the high dose had a flare after the 4th week of the study.
Similarly, for the secondary endpoint (occurrence of at least one GPP flare by week 48). Dr. Strober and his colleagues reported that high-dose spesolimab was superior to placebo with a risk difference of -39% (95% CI, –0.62 to –0.16; P = .0013). By contrast, the risk differences for the medium- and low-dose spesolimab arms were –0.23 (95% CI, –0.46 to 0.01) and -0.31 (95% CI, –0.54 to –0.08), respectively.
The safety profile of subcutaneous spesolimab across all three doses was similar to that of placebo, and there was no dose-dependent trend. Reported adverse events (AEs) were mild. There were five (5.4%) AEs leading to discontinuation of the drug in the medium- and high-dose groups, but none in the low-dose group. Overall, there were nine (9.7%) serious AEs reported in the spesolimab groups, and three (10%) in the high-dose group; no deaths occurred on any dose.
Participants most often reported injection-site erythema, reported in 13 (14%) of the patients on spesolimab versus 1 (3.3%) of those on placebo.
“Overall, the study demonstrates that subcutaneous spesolimab is effective at controlling GPP flares, especially at a high dose relative to placebo, and supports subcutaneous spesolimab for the therapy for GPP flare prevention,” Dr. Strober said at the meeting.
Targeting the IL-36 pathway
In a comment, Todd Schlesinger, MD, Clinical Research Center of the Carolinas, Charleston, S.C., who moderated the session, said: “It’s very exciting to be able to have a subcutaneous version of the medication.”
“I think the IL-36 is a great pathway,” he said, referring to the signaling pathway within the immune system that is central to the pathogenesis of GPP and several other autoinflammatory diseases.
However, Dr. Schlesinger said that he would have liked to have seen data on how many patients ended up treated with intravenous spesolimab.
He added that he would like future studies of subcutaneous spesolimab to examine the effect in different populations that vary by parameters such as weight, race, and disease severity. “Just seeing how somebody who’s flaring five times a year and you give them this medication and they’re now flaring once a year – that’s interesting data that we might like to know in the future.”
Other than for preventing GPP flares, spesolimab is being studied for treating other IL-36–mediated skin diseases, such as palmoplantar pustulosis.
The study was funded by Boehringer Ingelheim; both Dr. Strober and Dr. Schlesinger do research and consulting for BI, and receive funding from multiple other pharmaceutical companies.
A version of this article first appeared on Medscape.com.
SINGAPORE – presented in a late-breaker session at the World Congress of Dermatology,
In the phase 2b study, patients who received the high-dose regimen (a 600-mg subcutaneous loading dose, then 300-mg SC every 4 weeks) of spesolimab experienced 84% fewer GPP fares over 48 weeks, compared with those on placebo, reported Bruce Strober, MD, PhD, Central Connecticut Dermatology, Cromwell, and clinical professor of dermatology, Yale University, New Haven, Conn. “Additionally, no flares occurred after week 4, and this, in turn, translated into improved patient outcomes.”
GPP is a rare, chronic, systemic neutrophilic skin disease. The resulting flares, characterized by painful pustules all over the body, can lead to sepsis, shock, and other life-threatening complications. “People who have it are considerably burdened by it, so targeted therapy of this disease is incredibly important because it leads to lessened morbidity and, importantly, mortality for these patients,” Dr. Strober said.
“It’s important not only to treat the flares but also to prevent them,” he noted.
The intravenous formulation of spesolimab (Spevigo) was approved for the treatment of GPP flares in adults by the Food and Drug Administration in September 2022. It is now authorized in nearly 40 countries, including Japan, China, and the European Union.
The phase 2 Effisayil 2 study presented at the meeting evaluated the subcutaneous formulation of spesolimab. Data on subcutaneous spesolimab has been submitted to the FDA, and has received breakthrough therapy designation, according to the manufacturer, Boehringer Ingelheim.
Flare prevention
In the study, 123 patients with GPP were randomly assigned 1:1:1:1 to one of four groups: high-dose spesolimab, medium-dose (600-mg SC loading dose, then 300-mg SC every 12 weeks), low-dose (300-mg SC loading dose, then 150-mg SC every 12 weeks), or placebo. In the event of a flare during the randomized treatment period, a patient was administered a single, 900-mg intravenous dose of spesolimab.
Nearly two-thirds of the participants were female and nearly two-thirds were Asian, with a mean age of about 39-43 years.
The mean numbers of GPP flares experienced annually by those in the low-, medium-, and high-dose spesolimab groups were 2.7, 1.9, and 2.4, respectively (2.4% in the placebo group). Fewer than a third had concurrent plaque psoriasis at baseline. Most (48.4%-63.3%) did not have an IL-36RN mutation.
Additionally, the Generalized Pustular Psoriasis Physician Global Assessment total score was 1 in 74.2%-93.5% of participants, and 0 in the remainder.
The primary study endpoint was the time to GPP flare by week 48. The risk of developing a flare among those on high-dose spesolimab was 84% lower, compared with that of those on placebo (hazard ratio, 0.16; 95% confidence interval, 0.05-0.54; P = .0005). No patients on the high dose had a flare after the 4th week of the study.
Similarly, for the secondary endpoint (occurrence of at least one GPP flare by week 48). Dr. Strober and his colleagues reported that high-dose spesolimab was superior to placebo with a risk difference of -39% (95% CI, –0.62 to –0.16; P = .0013). By contrast, the risk differences for the medium- and low-dose spesolimab arms were –0.23 (95% CI, –0.46 to 0.01) and -0.31 (95% CI, –0.54 to –0.08), respectively.
The safety profile of subcutaneous spesolimab across all three doses was similar to that of placebo, and there was no dose-dependent trend. Reported adverse events (AEs) were mild. There were five (5.4%) AEs leading to discontinuation of the drug in the medium- and high-dose groups, but none in the low-dose group. Overall, there were nine (9.7%) serious AEs reported in the spesolimab groups, and three (10%) in the high-dose group; no deaths occurred on any dose.
Participants most often reported injection-site erythema, reported in 13 (14%) of the patients on spesolimab versus 1 (3.3%) of those on placebo.
“Overall, the study demonstrates that subcutaneous spesolimab is effective at controlling GPP flares, especially at a high dose relative to placebo, and supports subcutaneous spesolimab for the therapy for GPP flare prevention,” Dr. Strober said at the meeting.
Targeting the IL-36 pathway
In a comment, Todd Schlesinger, MD, Clinical Research Center of the Carolinas, Charleston, S.C., who moderated the session, said: “It’s very exciting to be able to have a subcutaneous version of the medication.”
“I think the IL-36 is a great pathway,” he said, referring to the signaling pathway within the immune system that is central to the pathogenesis of GPP and several other autoinflammatory diseases.
However, Dr. Schlesinger said that he would have liked to have seen data on how many patients ended up treated with intravenous spesolimab.
He added that he would like future studies of subcutaneous spesolimab to examine the effect in different populations that vary by parameters such as weight, race, and disease severity. “Just seeing how somebody who’s flaring five times a year and you give them this medication and they’re now flaring once a year – that’s interesting data that we might like to know in the future.”
Other than for preventing GPP flares, spesolimab is being studied for treating other IL-36–mediated skin diseases, such as palmoplantar pustulosis.
The study was funded by Boehringer Ingelheim; both Dr. Strober and Dr. Schlesinger do research and consulting for BI, and receive funding from multiple other pharmaceutical companies.
A version of this article first appeared on Medscape.com.
AT WCD 2023




