User login
FDA Rejects MDMA-AT for PTSD, but Lykos, Others, Vow to Push on
The Food and Drug Administration’s (FDA) decision not to approve midomafetamine-assisted therapy (MDMA-AT) for posttraumatic stress disorder (PTSD) puts the therapy’s near-term future in doubt, but officials say the rejection may not knock it out of contention as an eventual therapeutic tool for a variety of conditions.
In August the agency declined to approve the drug with currently available study data and requested that the company conduct an additional phase 3 trial. The agency’s action had potentially devastating consequences for MDMA-AT’s sponsor, Lykos Therapeutics, and was a huge disappointment for researchers, clinicians, and patients who were optimistic that it would be a new option for a condition that affects 13-17 million Americans.
For now, no other company is poised to imminently seek FDA approval for MDMA.
Despite the setback, research into MDMA that combines different psychotherapeutic approaches continues. Currently, there are seven US studies actively recruiting participants, and another 13 are registered with an eye toward starting recruitment, as reported on ClinicalTrials.gov.
The lack of FDA approval “actually increases the opportunity now for us to do trials,” said Michael Ostacher, MD, professor of psychiatry and behavioral sciences at Stanford Medicine in California. Researchers won’t have to be sponsored by Lykos to get access to MDMA.
“There’s a lot of energy and interest in doing these studies,” he said in an interview, adding that philanthropic organizations and Veterans Affairs (VA) are contributing funds to support such studies.
The VA provided a statement saying that it “intends to gather rigorous scientific evidence on the potential efficacy and safety of psychedelic compounds when used in conjunction with psychotherapy.” It also noted that “these studies will be conducted under stringent safety protocols and will mark the first time since the 1960’s that VA is funding research on such compounds.”
Rachel Yehuda, PhD, director of the Center for Psychedelic Therapy Research at Icahn School of Medicine at Mount Sinai in New York City, said in an interview that the FDA rejection “raises questions about how to keep the work going.”
Without the FDA’s imprimatur, MDMA remains a schedule 1 drug, which means it has no valid medical use.
“It’s a lot more complicated and expensive to work with a scheduled compound than to work with a compound that has been approved,” Dr. Yehuda said.
Also, without Lykos or another drug company sponsor, investigators have to find an acceptable MDMA source on their own, said Dr. Yehuda, who was an investigator on a study in which Lykos provided MDMA but was not involved in study design, data collection, analysis, or manuscript preparation.
Lykos in Disarray
Within a week of the FDA’s decision, Lykos announced it was cutting its staff by 75% and that Rick Doblin, PhD, the founder and president of the Multidisciplinary Association for Psychedelic Studies (MAPS) that gave rise to Lykos, had resigned from the Lykos board.
A frequently controversial figure, Doblin has been attempting to legitimize MDMA as a therapy since the mid-1980s. He formed a public benefit corporation (PBC) in 2014 with an eye toward FDA approval. The PBC fully separated from MAPS in 2024 and became Lykos.
Although the FDA has left the door open to approval, Lykos has not released the agency’s complete response letter, so it’s not clear exactly what the FDA is seeking. In a statement, the company said it believes the issues “can be addressed with existing data, postapproval requirements, or through reference to the scientific literature.”
Lykos said in an email that it is working on “securing the meeting with the FDA” and that it “will work with the agency to determine what needs to be done to fulfill their requests.”
Soon after the FDA decision, Lykos was hit with another blow. The journal Psychopharmacology retracted an article that pooled six Lykos phase 2 studies, claiming the paper’s authors knew about unethical conduct before submission but did not inform the publisher.
Lykos said the issues could have been addressed through a correction and that it has filed a complaint with the Committee on Publication Ethics. It also noted that the misconduct at issue was reported to the FDA and Health Canada.
“However, we did not disclose the violations to the journal itself, an additional step we should have taken and regret not doing,” the company said. It added that the efficacy data in the paper were not part of the FDA submission.
Author Allison A. Feduccia, PhD, cofounder of Psychedelic Support, agreed with the retraction but disagreed with the wording. In a post on LinkedIn, she said she and other authors were not informed about the misconduct until years after the study’s submission.
Four authors — including Dr. Doblin — disagreed with the retraction.
Dr. Doblin said in a statement that he’d resigned from Lykos to escape the restrictions that came with being a fiduciary. “Now I can advocate and speak freely,” he said, adding that he could also return to his activist roots.
He predicted that Lykos would eventually gain FDA approval. But if Lykos can’t convince the agency, it have the necessary data already in hand; “potential FDA approval is now at least 2 years away, possibly more,” Dr. Doblin said in his statement.
Research Continues
Lykos is not the only company hoping to commercialize MDMA. Toronto-based Awakn Life Sciences has an MDMA preclinical development program for addiction. In addition, some companies are offering MDMA therapy through clinics, such as Numinus in Utah and Sunstone Therapies in Rockville, Maryland.
But Lykos was the closest to bringing a product to market. The company is still a sponsor of four MDMA-related clinical trials, three of which appear to be on hold. One study at the VA San Diego Healthcare System, San Diego, that is actively recruiting is an open-label trial to assess MDMA-AT in combination with brief Cognitive-Behavioral Conjoint Therapy for PTSD.
Those studies are among 13 US trials listed in ClinicalTrials.gov that have not yet begun recruiting and 7 that are actively recruiting.
Among them is a study of MDMA plus exposure therapy, funded by and conducted at Emory University in Atlanta. One of the Emory principal investigators, Barbara Rothbaum, MD, has also been named to a Lykos’ panel that would help ensure oversight of MDMA-AT post FDA approval.
Dr. Ostacher is an investigator in a study planned at VA Palo Alto Health Care System in California, that will compare MDMA-AT with cognitive processing therapy in veterans with severe PTSD. He said it will be open label in an effort to minimize expectation bias and issues with blinding — both problems that tripped up the Lykos application. Although placebo-controlled trials are the gold standard, it’s not ideal when “the purpose of the drug is for it to change how you see the world and yourself,” Dr. Ostacher said.
The study aims to see whether MDMA-AT is better than “a much shorter, less onerous, but quite evidence-based psychotherapy for PTSD,” he said.
The FDA’s decision is not the end of the road, said Dr. Ostacher. “Even though I think this makes for an obvious delay, I don’t think that it’s a permanent one,” he said.
Dr. Yehuda also said she is not ready to give up.
“We don’t plan on stopping — we plan on finding a way,” she said.
“In our experience, this is a very powerful approach that helps a lot of people that haven’t found help using other approaches, and when it’s in the hands of really trusted, experienced, ethical clinicians in a trusted environment, this could be a real game changer for people who have not been able to find belief by traditional methods,” she said.
Dr. Ostacher reported no relevant financial relationships. Dr. Yahuda is the principal investigator on clinical trials for the Center for Psychedelic Psychotherapy and Trauma Research that are sponsored by the Multidisciplinary Association for Psychedelic Studies and COMPASS Pathways.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration’s (FDA) decision not to approve midomafetamine-assisted therapy (MDMA-AT) for posttraumatic stress disorder (PTSD) puts the therapy’s near-term future in doubt, but officials say the rejection may not knock it out of contention as an eventual therapeutic tool for a variety of conditions.
In August the agency declined to approve the drug with currently available study data and requested that the company conduct an additional phase 3 trial. The agency’s action had potentially devastating consequences for MDMA-AT’s sponsor, Lykos Therapeutics, and was a huge disappointment for researchers, clinicians, and patients who were optimistic that it would be a new option for a condition that affects 13-17 million Americans.
For now, no other company is poised to imminently seek FDA approval for MDMA.
Despite the setback, research into MDMA that combines different psychotherapeutic approaches continues. Currently, there are seven US studies actively recruiting participants, and another 13 are registered with an eye toward starting recruitment, as reported on ClinicalTrials.gov.
The lack of FDA approval “actually increases the opportunity now for us to do trials,” said Michael Ostacher, MD, professor of psychiatry and behavioral sciences at Stanford Medicine in California. Researchers won’t have to be sponsored by Lykos to get access to MDMA.
“There’s a lot of energy and interest in doing these studies,” he said in an interview, adding that philanthropic organizations and Veterans Affairs (VA) are contributing funds to support such studies.
The VA provided a statement saying that it “intends to gather rigorous scientific evidence on the potential efficacy and safety of psychedelic compounds when used in conjunction with psychotherapy.” It also noted that “these studies will be conducted under stringent safety protocols and will mark the first time since the 1960’s that VA is funding research on such compounds.”
Rachel Yehuda, PhD, director of the Center for Psychedelic Therapy Research at Icahn School of Medicine at Mount Sinai in New York City, said in an interview that the FDA rejection “raises questions about how to keep the work going.”
Without the FDA’s imprimatur, MDMA remains a schedule 1 drug, which means it has no valid medical use.
“It’s a lot more complicated and expensive to work with a scheduled compound than to work with a compound that has been approved,” Dr. Yehuda said.
Also, without Lykos or another drug company sponsor, investigators have to find an acceptable MDMA source on their own, said Dr. Yehuda, who was an investigator on a study in which Lykos provided MDMA but was not involved in study design, data collection, analysis, or manuscript preparation.
Lykos in Disarray
Within a week of the FDA’s decision, Lykos announced it was cutting its staff by 75% and that Rick Doblin, PhD, the founder and president of the Multidisciplinary Association for Psychedelic Studies (MAPS) that gave rise to Lykos, had resigned from the Lykos board.
A frequently controversial figure, Doblin has been attempting to legitimize MDMA as a therapy since the mid-1980s. He formed a public benefit corporation (PBC) in 2014 with an eye toward FDA approval. The PBC fully separated from MAPS in 2024 and became Lykos.
Although the FDA has left the door open to approval, Lykos has not released the agency’s complete response letter, so it’s not clear exactly what the FDA is seeking. In a statement, the company said it believes the issues “can be addressed with existing data, postapproval requirements, or through reference to the scientific literature.”
Lykos said in an email that it is working on “securing the meeting with the FDA” and that it “will work with the agency to determine what needs to be done to fulfill their requests.”
Soon after the FDA decision, Lykos was hit with another blow. The journal Psychopharmacology retracted an article that pooled six Lykos phase 2 studies, claiming the paper’s authors knew about unethical conduct before submission but did not inform the publisher.
Lykos said the issues could have been addressed through a correction and that it has filed a complaint with the Committee on Publication Ethics. It also noted that the misconduct at issue was reported to the FDA and Health Canada.
“However, we did not disclose the violations to the journal itself, an additional step we should have taken and regret not doing,” the company said. It added that the efficacy data in the paper were not part of the FDA submission.
Author Allison A. Feduccia, PhD, cofounder of Psychedelic Support, agreed with the retraction but disagreed with the wording. In a post on LinkedIn, she said she and other authors were not informed about the misconduct until years after the study’s submission.
Four authors — including Dr. Doblin — disagreed with the retraction.
Dr. Doblin said in a statement that he’d resigned from Lykos to escape the restrictions that came with being a fiduciary. “Now I can advocate and speak freely,” he said, adding that he could also return to his activist roots.
He predicted that Lykos would eventually gain FDA approval. But if Lykos can’t convince the agency, it have the necessary data already in hand; “potential FDA approval is now at least 2 years away, possibly more,” Dr. Doblin said in his statement.
Research Continues
Lykos is not the only company hoping to commercialize MDMA. Toronto-based Awakn Life Sciences has an MDMA preclinical development program for addiction. In addition, some companies are offering MDMA therapy through clinics, such as Numinus in Utah and Sunstone Therapies in Rockville, Maryland.
But Lykos was the closest to bringing a product to market. The company is still a sponsor of four MDMA-related clinical trials, three of which appear to be on hold. One study at the VA San Diego Healthcare System, San Diego, that is actively recruiting is an open-label trial to assess MDMA-AT in combination with brief Cognitive-Behavioral Conjoint Therapy for PTSD.
Those studies are among 13 US trials listed in ClinicalTrials.gov that have not yet begun recruiting and 7 that are actively recruiting.
Among them is a study of MDMA plus exposure therapy, funded by and conducted at Emory University in Atlanta. One of the Emory principal investigators, Barbara Rothbaum, MD, has also been named to a Lykos’ panel that would help ensure oversight of MDMA-AT post FDA approval.
Dr. Ostacher is an investigator in a study planned at VA Palo Alto Health Care System in California, that will compare MDMA-AT with cognitive processing therapy in veterans with severe PTSD. He said it will be open label in an effort to minimize expectation bias and issues with blinding — both problems that tripped up the Lykos application. Although placebo-controlled trials are the gold standard, it’s not ideal when “the purpose of the drug is for it to change how you see the world and yourself,” Dr. Ostacher said.
The study aims to see whether MDMA-AT is better than “a much shorter, less onerous, but quite evidence-based psychotherapy for PTSD,” he said.
The FDA’s decision is not the end of the road, said Dr. Ostacher. “Even though I think this makes for an obvious delay, I don’t think that it’s a permanent one,” he said.
Dr. Yehuda also said she is not ready to give up.
“We don’t plan on stopping — we plan on finding a way,” she said.
“In our experience, this is a very powerful approach that helps a lot of people that haven’t found help using other approaches, and when it’s in the hands of really trusted, experienced, ethical clinicians in a trusted environment, this could be a real game changer for people who have not been able to find belief by traditional methods,” she said.
Dr. Ostacher reported no relevant financial relationships. Dr. Yahuda is the principal investigator on clinical trials for the Center for Psychedelic Psychotherapy and Trauma Research that are sponsored by the Multidisciplinary Association for Psychedelic Studies and COMPASS Pathways.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration’s (FDA) decision not to approve midomafetamine-assisted therapy (MDMA-AT) for posttraumatic stress disorder (PTSD) puts the therapy’s near-term future in doubt, but officials say the rejection may not knock it out of contention as an eventual therapeutic tool for a variety of conditions.
In August the agency declined to approve the drug with currently available study data and requested that the company conduct an additional phase 3 trial. The agency’s action had potentially devastating consequences for MDMA-AT’s sponsor, Lykos Therapeutics, and was a huge disappointment for researchers, clinicians, and patients who were optimistic that it would be a new option for a condition that affects 13-17 million Americans.
For now, no other company is poised to imminently seek FDA approval for MDMA.
Despite the setback, research into MDMA that combines different psychotherapeutic approaches continues. Currently, there are seven US studies actively recruiting participants, and another 13 are registered with an eye toward starting recruitment, as reported on ClinicalTrials.gov.
The lack of FDA approval “actually increases the opportunity now for us to do trials,” said Michael Ostacher, MD, professor of psychiatry and behavioral sciences at Stanford Medicine in California. Researchers won’t have to be sponsored by Lykos to get access to MDMA.
“There’s a lot of energy and interest in doing these studies,” he said in an interview, adding that philanthropic organizations and Veterans Affairs (VA) are contributing funds to support such studies.
The VA provided a statement saying that it “intends to gather rigorous scientific evidence on the potential efficacy and safety of psychedelic compounds when used in conjunction with psychotherapy.” It also noted that “these studies will be conducted under stringent safety protocols and will mark the first time since the 1960’s that VA is funding research on such compounds.”
Rachel Yehuda, PhD, director of the Center for Psychedelic Therapy Research at Icahn School of Medicine at Mount Sinai in New York City, said in an interview that the FDA rejection “raises questions about how to keep the work going.”
Without the FDA’s imprimatur, MDMA remains a schedule 1 drug, which means it has no valid medical use.
“It’s a lot more complicated and expensive to work with a scheduled compound than to work with a compound that has been approved,” Dr. Yehuda said.
Also, without Lykos or another drug company sponsor, investigators have to find an acceptable MDMA source on their own, said Dr. Yehuda, who was an investigator on a study in which Lykos provided MDMA but was not involved in study design, data collection, analysis, or manuscript preparation.
Lykos in Disarray
Within a week of the FDA’s decision, Lykos announced it was cutting its staff by 75% and that Rick Doblin, PhD, the founder and president of the Multidisciplinary Association for Psychedelic Studies (MAPS) that gave rise to Lykos, had resigned from the Lykos board.
A frequently controversial figure, Doblin has been attempting to legitimize MDMA as a therapy since the mid-1980s. He formed a public benefit corporation (PBC) in 2014 with an eye toward FDA approval. The PBC fully separated from MAPS in 2024 and became Lykos.
Although the FDA has left the door open to approval, Lykos has not released the agency’s complete response letter, so it’s not clear exactly what the FDA is seeking. In a statement, the company said it believes the issues “can be addressed with existing data, postapproval requirements, or through reference to the scientific literature.”
Lykos said in an email that it is working on “securing the meeting with the FDA” and that it “will work with the agency to determine what needs to be done to fulfill their requests.”
Soon after the FDA decision, Lykos was hit with another blow. The journal Psychopharmacology retracted an article that pooled six Lykos phase 2 studies, claiming the paper’s authors knew about unethical conduct before submission but did not inform the publisher.
Lykos said the issues could have been addressed through a correction and that it has filed a complaint with the Committee on Publication Ethics. It also noted that the misconduct at issue was reported to the FDA and Health Canada.
“However, we did not disclose the violations to the journal itself, an additional step we should have taken and regret not doing,” the company said. It added that the efficacy data in the paper were not part of the FDA submission.
Author Allison A. Feduccia, PhD, cofounder of Psychedelic Support, agreed with the retraction but disagreed with the wording. In a post on LinkedIn, she said she and other authors were not informed about the misconduct until years after the study’s submission.
Four authors — including Dr. Doblin — disagreed with the retraction.
Dr. Doblin said in a statement that he’d resigned from Lykos to escape the restrictions that came with being a fiduciary. “Now I can advocate and speak freely,” he said, adding that he could also return to his activist roots.
He predicted that Lykos would eventually gain FDA approval. But if Lykos can’t convince the agency, it have the necessary data already in hand; “potential FDA approval is now at least 2 years away, possibly more,” Dr. Doblin said in his statement.
Research Continues
Lykos is not the only company hoping to commercialize MDMA. Toronto-based Awakn Life Sciences has an MDMA preclinical development program for addiction. In addition, some companies are offering MDMA therapy through clinics, such as Numinus in Utah and Sunstone Therapies in Rockville, Maryland.
But Lykos was the closest to bringing a product to market. The company is still a sponsor of four MDMA-related clinical trials, three of which appear to be on hold. One study at the VA San Diego Healthcare System, San Diego, that is actively recruiting is an open-label trial to assess MDMA-AT in combination with brief Cognitive-Behavioral Conjoint Therapy for PTSD.
Those studies are among 13 US trials listed in ClinicalTrials.gov that have not yet begun recruiting and 7 that are actively recruiting.
Among them is a study of MDMA plus exposure therapy, funded by and conducted at Emory University in Atlanta. One of the Emory principal investigators, Barbara Rothbaum, MD, has also been named to a Lykos’ panel that would help ensure oversight of MDMA-AT post FDA approval.
Dr. Ostacher is an investigator in a study planned at VA Palo Alto Health Care System in California, that will compare MDMA-AT with cognitive processing therapy in veterans with severe PTSD. He said it will be open label in an effort to minimize expectation bias and issues with blinding — both problems that tripped up the Lykos application. Although placebo-controlled trials are the gold standard, it’s not ideal when “the purpose of the drug is for it to change how you see the world and yourself,” Dr. Ostacher said.
The study aims to see whether MDMA-AT is better than “a much shorter, less onerous, but quite evidence-based psychotherapy for PTSD,” he said.
The FDA’s decision is not the end of the road, said Dr. Ostacher. “Even though I think this makes for an obvious delay, I don’t think that it’s a permanent one,” he said.
Dr. Yehuda also said she is not ready to give up.
“We don’t plan on stopping — we plan on finding a way,” she said.
“In our experience, this is a very powerful approach that helps a lot of people that haven’t found help using other approaches, and when it’s in the hands of really trusted, experienced, ethical clinicians in a trusted environment, this could be a real game changer for people who have not been able to find belief by traditional methods,” she said.
Dr. Ostacher reported no relevant financial relationships. Dr. Yahuda is the principal investigator on clinical trials for the Center for Psychedelic Psychotherapy and Trauma Research that are sponsored by the Multidisciplinary Association for Psychedelic Studies and COMPASS Pathways.
A version of this article first appeared on Medscape.com.
Involuntary flashbacks
The correct diagnosis is posttraumatic stress disorder (PTSD). The patient's anxiety, irritability, sleep difficulties, and other symptoms are directly related to the recent traumatic event (car crash), and he has no significant physical injuries or neurologic abnormalities.
Generalized anxiety disorder is incorrect because it involves chronic worry about various life aspects for at least 6 months, unrelated to a specific trauma.
Postconcussion syndrome is not applicable because of the lack of concussion evidence and other symptoms, such as headaches or dizziness.
Acute stress disorder is similar to PTSD but is diagnosed when symptoms occur within 3 days to 1 month after a trauma. Because this patient's symptoms have persisted beyond 1 month, PTSD is the most likely diagnosis.
Patients with PTSD exhibit pronounced cognitive, affective, or behavioral responses to trauma reminders; these responses may include severe anxiety, dissociative episodes, flashbacks, and hyperreactive behaviors. The intensity of these symptoms and the resulting psychosocial impairment are more severe in individuals with PTSD compared with people who experience trauma without developing the disorder. To manage such heightened arousal, individuals with PTSD often engage in avoidance behaviors, leading to emotional numbing; reduced interest in daily activities; and, in severe cases, detachment from others.
The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision (DSM-5-TR) outlines specific criteria for diagnosing PTSD in individuals older than 6 years. These criteria include: (A) exposure to actual or threatened death, serious injury, or sexual violence; (B) the presence of one or more intrusion symptoms related to the traumatic event; (C) persistent avoidance of stimuli associated with the trauma; (D) negative alterations in cognitions and mood related to the trauma; and (E) marked alterations in arousal and reactivity, evidenced by two or more specific symptoms.
Early intervention is key in the treatment of PTSD to prevent the condition from becoming chronic. Although more empirical data are needed, especially regarding pharmacotherapy, early supportive interventions such as psychoeducation and case management have shown promise in acutely traumatized individuals.
Trauma-focused psychotherapy is recommended as the first-line treatment for most adults with PTSD. This approach, which includes exposure-based therapies, is generally preferred over other therapies or pharmacologic treatments, such as selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors. However, in patients with comorbid conditions (eg, depression, psychosis) that impair their ability to engage in trauma-focused therapy, initial pharmacologic management is advised until symptoms stabilize, after which psychotherapy can be introduced.
Clinical trials and meta-analyses have demonstrated the efficacy of various trauma-focused therapies, including trauma-focused cognitive-behavioral therapy, prolonged exposure therapy, and eye movement desensitization and reprocessing. The treatment choice should be collaborative, based on patient presentation, preference, and therapist expertise.
For individuals with PTSD experiencing significant sleep disturbances, particularly nightmares, prazosin is suggested. Clinical studies demonstrate that prazosin effectively reduces overall PTSD symptoms, nightmares, and sleep disturbances in approximately half of the patients treated.
Medication regimens effective for PTSD should be continued for at least 6 months to 1 year to prevent relapse or recurrence. Multiple clinical trials in patients with PTSD who completed acute treatment with SSRIs have demonstrated that those who continued with SSRIs were less likely to have relapse compared with those receiving placebo.
Jasvinder Chawla, MD, Professor of Neurology, Loyola University Medical Center, Maywood; Director, Clinical Neurophysiology Lab, Department of Neurology, Hines VA Hospital, Hines, IL.
Jasvinder Chawla, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The correct diagnosis is posttraumatic stress disorder (PTSD). The patient's anxiety, irritability, sleep difficulties, and other symptoms are directly related to the recent traumatic event (car crash), and he has no significant physical injuries or neurologic abnormalities.
Generalized anxiety disorder is incorrect because it involves chronic worry about various life aspects for at least 6 months, unrelated to a specific trauma.
Postconcussion syndrome is not applicable because of the lack of concussion evidence and other symptoms, such as headaches or dizziness.
Acute stress disorder is similar to PTSD but is diagnosed when symptoms occur within 3 days to 1 month after a trauma. Because this patient's symptoms have persisted beyond 1 month, PTSD is the most likely diagnosis.
Patients with PTSD exhibit pronounced cognitive, affective, or behavioral responses to trauma reminders; these responses may include severe anxiety, dissociative episodes, flashbacks, and hyperreactive behaviors. The intensity of these symptoms and the resulting psychosocial impairment are more severe in individuals with PTSD compared with people who experience trauma without developing the disorder. To manage such heightened arousal, individuals with PTSD often engage in avoidance behaviors, leading to emotional numbing; reduced interest in daily activities; and, in severe cases, detachment from others.
The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision (DSM-5-TR) outlines specific criteria for diagnosing PTSD in individuals older than 6 years. These criteria include: (A) exposure to actual or threatened death, serious injury, or sexual violence; (B) the presence of one or more intrusion symptoms related to the traumatic event; (C) persistent avoidance of stimuli associated with the trauma; (D) negative alterations in cognitions and mood related to the trauma; and (E) marked alterations in arousal and reactivity, evidenced by two or more specific symptoms.
Early intervention is key in the treatment of PTSD to prevent the condition from becoming chronic. Although more empirical data are needed, especially regarding pharmacotherapy, early supportive interventions such as psychoeducation and case management have shown promise in acutely traumatized individuals.
Trauma-focused psychotherapy is recommended as the first-line treatment for most adults with PTSD. This approach, which includes exposure-based therapies, is generally preferred over other therapies or pharmacologic treatments, such as selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors. However, in patients with comorbid conditions (eg, depression, psychosis) that impair their ability to engage in trauma-focused therapy, initial pharmacologic management is advised until symptoms stabilize, after which psychotherapy can be introduced.
Clinical trials and meta-analyses have demonstrated the efficacy of various trauma-focused therapies, including trauma-focused cognitive-behavioral therapy, prolonged exposure therapy, and eye movement desensitization and reprocessing. The treatment choice should be collaborative, based on patient presentation, preference, and therapist expertise.
For individuals with PTSD experiencing significant sleep disturbances, particularly nightmares, prazosin is suggested. Clinical studies demonstrate that prazosin effectively reduces overall PTSD symptoms, nightmares, and sleep disturbances in approximately half of the patients treated.
Medication regimens effective for PTSD should be continued for at least 6 months to 1 year to prevent relapse or recurrence. Multiple clinical trials in patients with PTSD who completed acute treatment with SSRIs have demonstrated that those who continued with SSRIs were less likely to have relapse compared with those receiving placebo.
Jasvinder Chawla, MD, Professor of Neurology, Loyola University Medical Center, Maywood; Director, Clinical Neurophysiology Lab, Department of Neurology, Hines VA Hospital, Hines, IL.
Jasvinder Chawla, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The correct diagnosis is posttraumatic stress disorder (PTSD). The patient's anxiety, irritability, sleep difficulties, and other symptoms are directly related to the recent traumatic event (car crash), and he has no significant physical injuries or neurologic abnormalities.
Generalized anxiety disorder is incorrect because it involves chronic worry about various life aspects for at least 6 months, unrelated to a specific trauma.
Postconcussion syndrome is not applicable because of the lack of concussion evidence and other symptoms, such as headaches or dizziness.
Acute stress disorder is similar to PTSD but is diagnosed when symptoms occur within 3 days to 1 month after a trauma. Because this patient's symptoms have persisted beyond 1 month, PTSD is the most likely diagnosis.
Patients with PTSD exhibit pronounced cognitive, affective, or behavioral responses to trauma reminders; these responses may include severe anxiety, dissociative episodes, flashbacks, and hyperreactive behaviors. The intensity of these symptoms and the resulting psychosocial impairment are more severe in individuals with PTSD compared with people who experience trauma without developing the disorder. To manage such heightened arousal, individuals with PTSD often engage in avoidance behaviors, leading to emotional numbing; reduced interest in daily activities; and, in severe cases, detachment from others.
The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision (DSM-5-TR) outlines specific criteria for diagnosing PTSD in individuals older than 6 years. These criteria include: (A) exposure to actual or threatened death, serious injury, or sexual violence; (B) the presence of one or more intrusion symptoms related to the traumatic event; (C) persistent avoidance of stimuli associated with the trauma; (D) negative alterations in cognitions and mood related to the trauma; and (E) marked alterations in arousal and reactivity, evidenced by two or more specific symptoms.
Early intervention is key in the treatment of PTSD to prevent the condition from becoming chronic. Although more empirical data are needed, especially regarding pharmacotherapy, early supportive interventions such as psychoeducation and case management have shown promise in acutely traumatized individuals.
Trauma-focused psychotherapy is recommended as the first-line treatment for most adults with PTSD. This approach, which includes exposure-based therapies, is generally preferred over other therapies or pharmacologic treatments, such as selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors. However, in patients with comorbid conditions (eg, depression, psychosis) that impair their ability to engage in trauma-focused therapy, initial pharmacologic management is advised until symptoms stabilize, after which psychotherapy can be introduced.
Clinical trials and meta-analyses have demonstrated the efficacy of various trauma-focused therapies, including trauma-focused cognitive-behavioral therapy, prolonged exposure therapy, and eye movement desensitization and reprocessing. The treatment choice should be collaborative, based on patient presentation, preference, and therapist expertise.
For individuals with PTSD experiencing significant sleep disturbances, particularly nightmares, prazosin is suggested. Clinical studies demonstrate that prazosin effectively reduces overall PTSD symptoms, nightmares, and sleep disturbances in approximately half of the patients treated.
Medication regimens effective for PTSD should be continued for at least 6 months to 1 year to prevent relapse or recurrence. Multiple clinical trials in patients with PTSD who completed acute treatment with SSRIs have demonstrated that those who continued with SSRIs were less likely to have relapse compared with those receiving placebo.
Jasvinder Chawla, MD, Professor of Neurology, Loyola University Medical Center, Maywood; Director, Clinical Neurophysiology Lab, Department of Neurology, Hines VA Hospital, Hines, IL.
Jasvinder Chawla, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
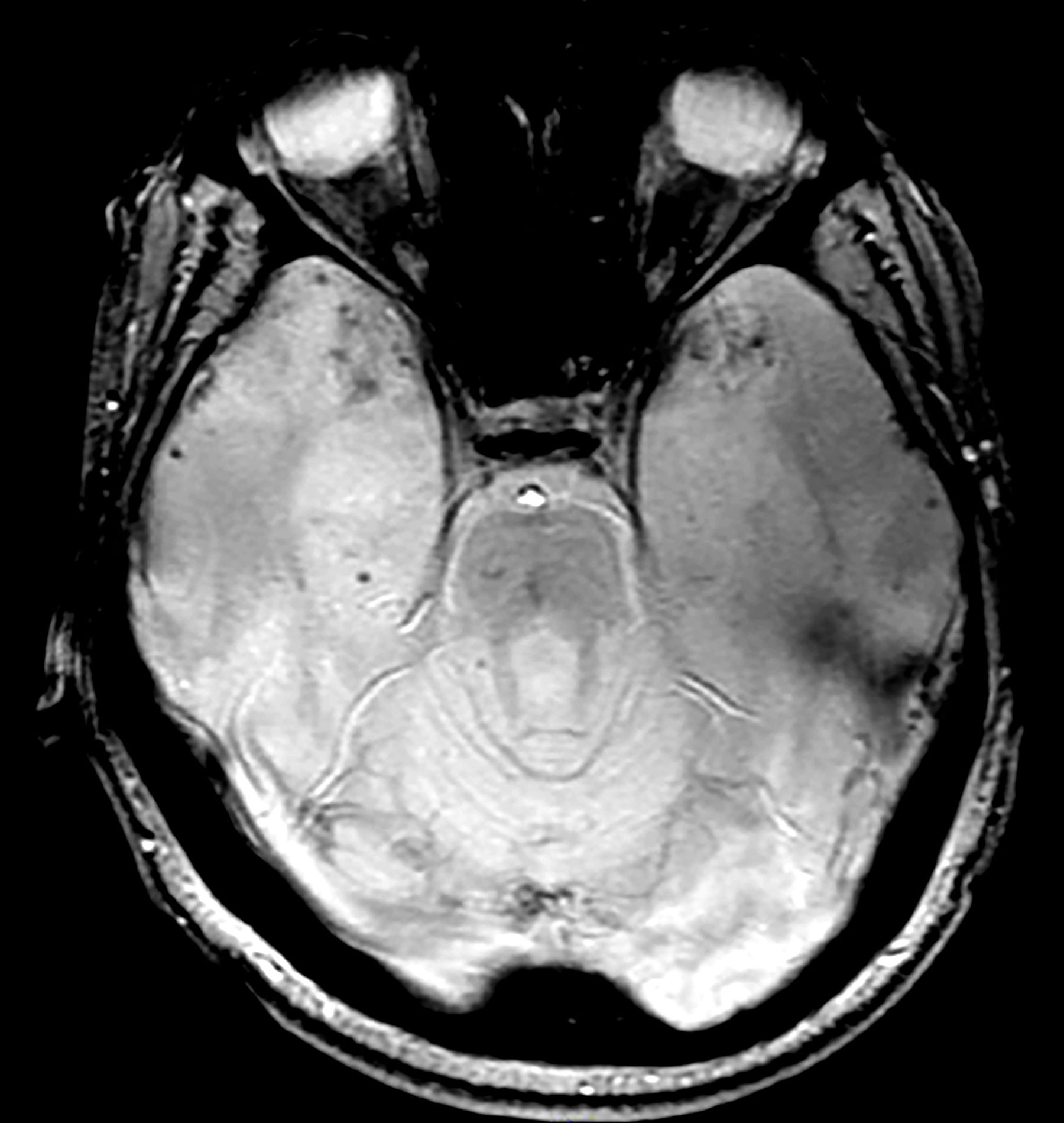
A 28-year-old man presented to the emergency department following a high-speed motor vehicle accident 2 months ago. He sustained no major physical injuries but had minor lacerations and bruising. The patient reported feeling unusually irritable and having difficulty sleeping since the accident, citing frequent flashbacks to the accident and occasional nightmares. He has started to feel more anxious and withdrawn, losing interest in hobbies such as swimming and biking that he previously enjoyed.
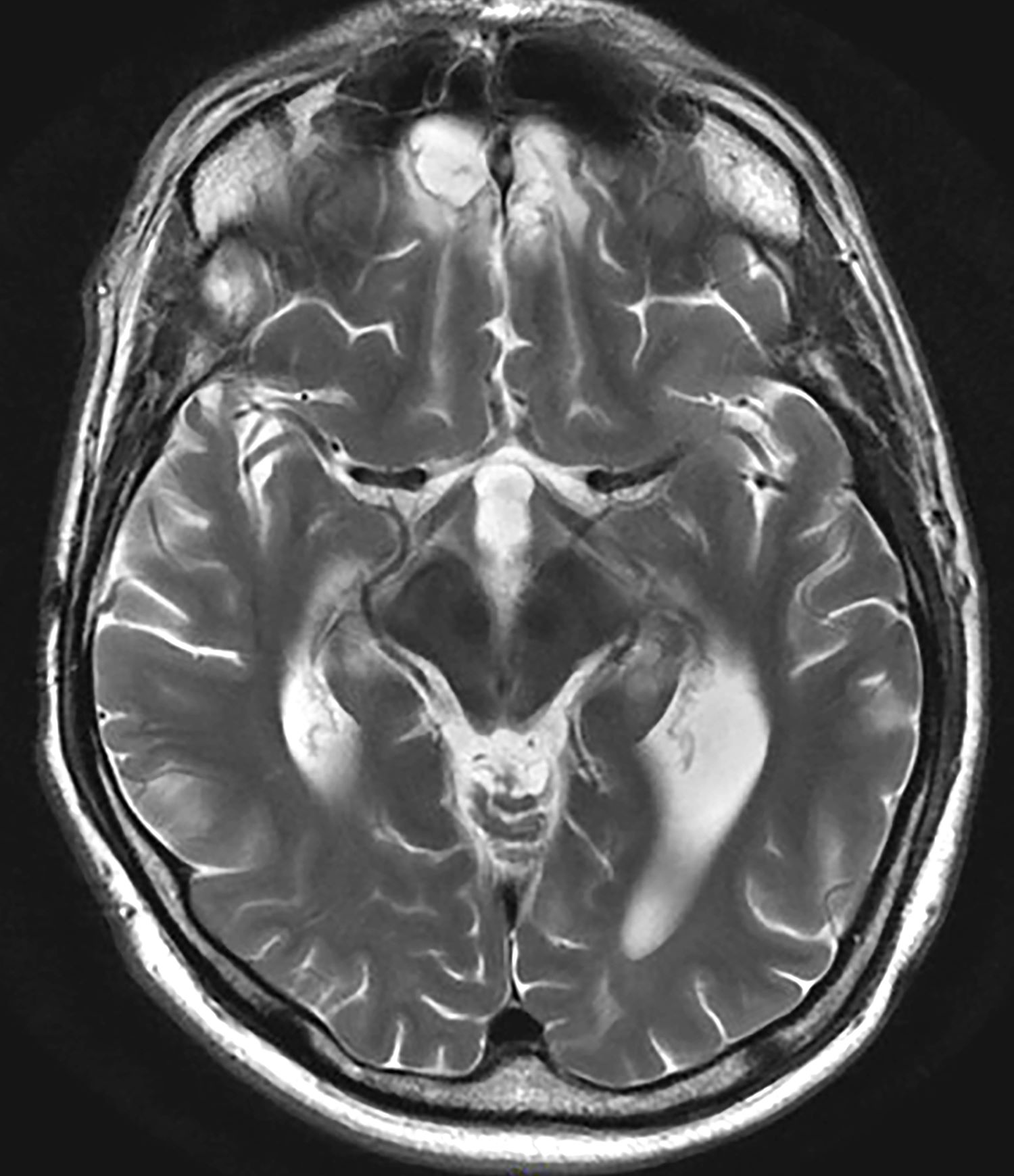
The patient's medical history is unremarkable, with no previous psychiatric or neurologic conditions. His neurologic examination was normal. An initial axial T2-weighted brain MRI demonstrated multiple small areas of hemorrhage, indicative of a diffuse axonal injury or shear-type injury. Despite the lack of significant physical injuries, the patient expressed ongoing distress related to the traumatic event.
Persistent mood swings
The most likely diagnosis for this patient is veteran posttraumatic stress disorder (PTSD), given his history of combat exposure and symptoms, such as severe headaches, difficulty concentrating, mood swings, nightmares, flashbacks, increased startle response, and hypervigilance. MRI findings showing significant changes in the limbic system and hippocampal regions support this diagnosis. Other potential diagnoses, like traumatic brain injury, chronic migraine, and major depressive disorder, are less likely because of their inability to account for the full range of his symptoms and specific MRI abnormalities.
PTSD, experienced by a subset of individuals after exposure to life-threatening events, has a lifetime prevalence of 4%-7% and a current prevalence of 1%-3%, with higher rates in older women, those with more trauma, and combat veterans. Nearly half of US veterans are aged 65 or older, many being Vietnam-era veterans at elevated risk for PTSD. Prevalence rates in older veterans range between 1% and 22%.
PTSD is characterized by intrusive thoughts, flashbacks, nightmares, avoidance of reminders, hypervigilance, and sleep difficulties, significantly disrupting interpersonal and occupational functioning. Screening tools like the primary care (PC) PTSD-5 and PCL-5, used in primary care settings, are effective for early detection, provisional diagnosis, and monitoring of symptom changes. The clinician-administered PTSD scale for Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition DSM-5 (CAPS-5) is the gold standard for diagnosis, particularly among veterans, with multimethod assessments combining self-report measures and semi-structured interviews recommended for accuracy. The DSM-5 criteria for PTSD diagnosis describe exposure to traumatic events, intrusion symptoms, avoidance behaviors, negative mood, and altered arousal, with symptoms persisting for over a month and causing significant distress or functional impairment.
Research has identified consistent anatomical and functional changes in PTSD patients, such as smaller hippocampi, decreased corpus callosum and prefrontal cortex, increased amygdala reactivity, and decreased prefrontal cortex activity. PTSD, linked to alterations in brain regions involved in fear learning and memory, shows diminished structural integrity in executive function areas, reduced cortical volumes in the cingulate brain cortex and frontal regions, and reduced white matter integrity in key brain pathways. Neuroimaging findings, however, are primarily used for research currently and have yet to be widely implemented in clinical guidelines.
International PTSD treatment guidelines consistently recognize trauma-focused cognitive-behavioral therapies (CBTs), such as cognitive processing therapy (CPT), prolonged exposure (PE), and eye movement desensitization and reprocessing (EMDR) as the gold standard. Recent guidelines have expanded the list of recommended treatments: The 2023 Department of Veterans Affairs and Department of Defense guidelines in the United States also endorse therapies like written narrative exposure and brief eclectic therapy. Internationally, guidelines do not perfectly coincide, as the 2018 update from the United Kingdom's National Institute for Health and Care Excellence (NICE) gives the highest recommendations to PE and CPT but rates EMDR slightly lower for military veterans because of limited evidence. Overall, guidelines consistently advocate for trauma-focused psychological interventions as the primary treatment for PTSD.
Guidelines from NICE and the World Health Organization do not recommend medications as the primary treatment; the American Psychiatric Association and the US Department of Veterans Affairs support selective serotonin reuptake inhibitors and prazosin but advise against benzodiazepines. Inpatient care may be necessary for individuals who pose a danger to themselves or others, or for those with severe PTSD from childhood abuse, to aid in emotional regulation and treatment.
Jasvinder Chawla, MD, Professor of Neurology, Loyola University Medical Center, Maywood; Director, Clinical Neurophysiology Lab, Department of Neurology, Hines VA Hospital, Hines, IL.
Jasvinder Chawla, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The most likely diagnosis for this patient is veteran posttraumatic stress disorder (PTSD), given his history of combat exposure and symptoms, such as severe headaches, difficulty concentrating, mood swings, nightmares, flashbacks, increased startle response, and hypervigilance. MRI findings showing significant changes in the limbic system and hippocampal regions support this diagnosis. Other potential diagnoses, like traumatic brain injury, chronic migraine, and major depressive disorder, are less likely because of their inability to account for the full range of his symptoms and specific MRI abnormalities.
PTSD, experienced by a subset of individuals after exposure to life-threatening events, has a lifetime prevalence of 4%-7% and a current prevalence of 1%-3%, with higher rates in older women, those with more trauma, and combat veterans. Nearly half of US veterans are aged 65 or older, many being Vietnam-era veterans at elevated risk for PTSD. Prevalence rates in older veterans range between 1% and 22%.
PTSD is characterized by intrusive thoughts, flashbacks, nightmares, avoidance of reminders, hypervigilance, and sleep difficulties, significantly disrupting interpersonal and occupational functioning. Screening tools like the primary care (PC) PTSD-5 and PCL-5, used in primary care settings, are effective for early detection, provisional diagnosis, and monitoring of symptom changes. The clinician-administered PTSD scale for Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition DSM-5 (CAPS-5) is the gold standard for diagnosis, particularly among veterans, with multimethod assessments combining self-report measures and semi-structured interviews recommended for accuracy. The DSM-5 criteria for PTSD diagnosis describe exposure to traumatic events, intrusion symptoms, avoidance behaviors, negative mood, and altered arousal, with symptoms persisting for over a month and causing significant distress or functional impairment.
Research has identified consistent anatomical and functional changes in PTSD patients, such as smaller hippocampi, decreased corpus callosum and prefrontal cortex, increased amygdala reactivity, and decreased prefrontal cortex activity. PTSD, linked to alterations in brain regions involved in fear learning and memory, shows diminished structural integrity in executive function areas, reduced cortical volumes in the cingulate brain cortex and frontal regions, and reduced white matter integrity in key brain pathways. Neuroimaging findings, however, are primarily used for research currently and have yet to be widely implemented in clinical guidelines.
International PTSD treatment guidelines consistently recognize trauma-focused cognitive-behavioral therapies (CBTs), such as cognitive processing therapy (CPT), prolonged exposure (PE), and eye movement desensitization and reprocessing (EMDR) as the gold standard. Recent guidelines have expanded the list of recommended treatments: The 2023 Department of Veterans Affairs and Department of Defense guidelines in the United States also endorse therapies like written narrative exposure and brief eclectic therapy. Internationally, guidelines do not perfectly coincide, as the 2018 update from the United Kingdom's National Institute for Health and Care Excellence (NICE) gives the highest recommendations to PE and CPT but rates EMDR slightly lower for military veterans because of limited evidence. Overall, guidelines consistently advocate for trauma-focused psychological interventions as the primary treatment for PTSD.
Guidelines from NICE and the World Health Organization do not recommend medications as the primary treatment; the American Psychiatric Association and the US Department of Veterans Affairs support selective serotonin reuptake inhibitors and prazosin but advise against benzodiazepines. Inpatient care may be necessary for individuals who pose a danger to themselves or others, or for those with severe PTSD from childhood abuse, to aid in emotional regulation and treatment.
Jasvinder Chawla, MD, Professor of Neurology, Loyola University Medical Center, Maywood; Director, Clinical Neurophysiology Lab, Department of Neurology, Hines VA Hospital, Hines, IL.
Jasvinder Chawla, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The most likely diagnosis for this patient is veteran posttraumatic stress disorder (PTSD), given his history of combat exposure and symptoms, such as severe headaches, difficulty concentrating, mood swings, nightmares, flashbacks, increased startle response, and hypervigilance. MRI findings showing significant changes in the limbic system and hippocampal regions support this diagnosis. Other potential diagnoses, like traumatic brain injury, chronic migraine, and major depressive disorder, are less likely because of their inability to account for the full range of his symptoms and specific MRI abnormalities.
PTSD, experienced by a subset of individuals after exposure to life-threatening events, has a lifetime prevalence of 4%-7% and a current prevalence of 1%-3%, with higher rates in older women, those with more trauma, and combat veterans. Nearly half of US veterans are aged 65 or older, many being Vietnam-era veterans at elevated risk for PTSD. Prevalence rates in older veterans range between 1% and 22%.
PTSD is characterized by intrusive thoughts, flashbacks, nightmares, avoidance of reminders, hypervigilance, and sleep difficulties, significantly disrupting interpersonal and occupational functioning. Screening tools like the primary care (PC) PTSD-5 and PCL-5, used in primary care settings, are effective for early detection, provisional diagnosis, and monitoring of symptom changes. The clinician-administered PTSD scale for Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition DSM-5 (CAPS-5) is the gold standard for diagnosis, particularly among veterans, with multimethod assessments combining self-report measures and semi-structured interviews recommended for accuracy. The DSM-5 criteria for PTSD diagnosis describe exposure to traumatic events, intrusion symptoms, avoidance behaviors, negative mood, and altered arousal, with symptoms persisting for over a month and causing significant distress or functional impairment.
Research has identified consistent anatomical and functional changes in PTSD patients, such as smaller hippocampi, decreased corpus callosum and prefrontal cortex, increased amygdala reactivity, and decreased prefrontal cortex activity. PTSD, linked to alterations in brain regions involved in fear learning and memory, shows diminished structural integrity in executive function areas, reduced cortical volumes in the cingulate brain cortex and frontal regions, and reduced white matter integrity in key brain pathways. Neuroimaging findings, however, are primarily used for research currently and have yet to be widely implemented in clinical guidelines.
International PTSD treatment guidelines consistently recognize trauma-focused cognitive-behavioral therapies (CBTs), such as cognitive processing therapy (CPT), prolonged exposure (PE), and eye movement desensitization and reprocessing (EMDR) as the gold standard. Recent guidelines have expanded the list of recommended treatments: The 2023 Department of Veterans Affairs and Department of Defense guidelines in the United States also endorse therapies like written narrative exposure and brief eclectic therapy. Internationally, guidelines do not perfectly coincide, as the 2018 update from the United Kingdom's National Institute for Health and Care Excellence (NICE) gives the highest recommendations to PE and CPT but rates EMDR slightly lower for military veterans because of limited evidence. Overall, guidelines consistently advocate for trauma-focused psychological interventions as the primary treatment for PTSD.
Guidelines from NICE and the World Health Organization do not recommend medications as the primary treatment; the American Psychiatric Association and the US Department of Veterans Affairs support selective serotonin reuptake inhibitors and prazosin but advise against benzodiazepines. Inpatient care may be necessary for individuals who pose a danger to themselves or others, or for those with severe PTSD from childhood abuse, to aid in emotional regulation and treatment.
Jasvinder Chawla, MD, Professor of Neurology, Loyola University Medical Center, Maywood; Director, Clinical Neurophysiology Lab, Department of Neurology, Hines VA Hospital, Hines, IL.
Jasvinder Chawla, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
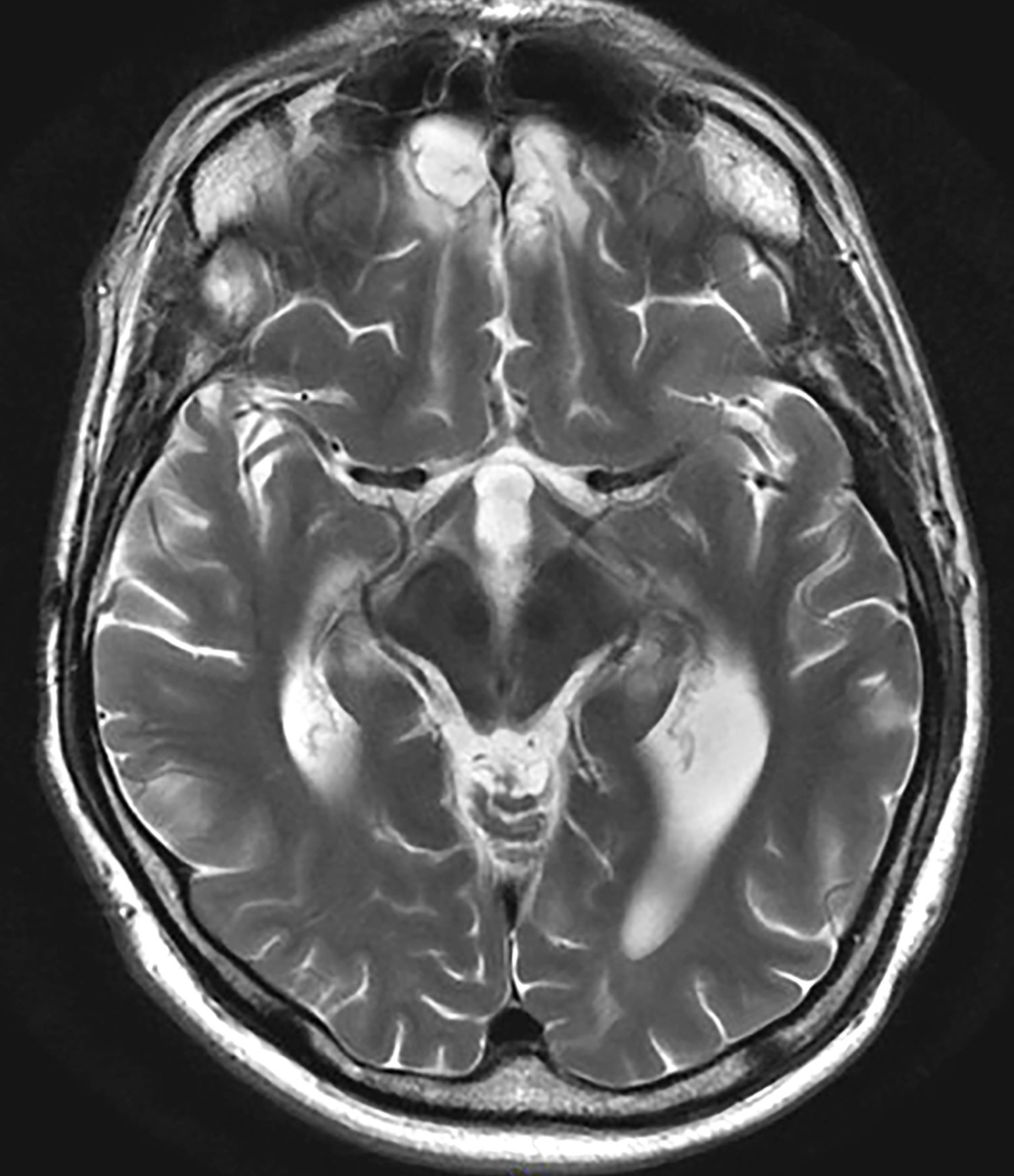
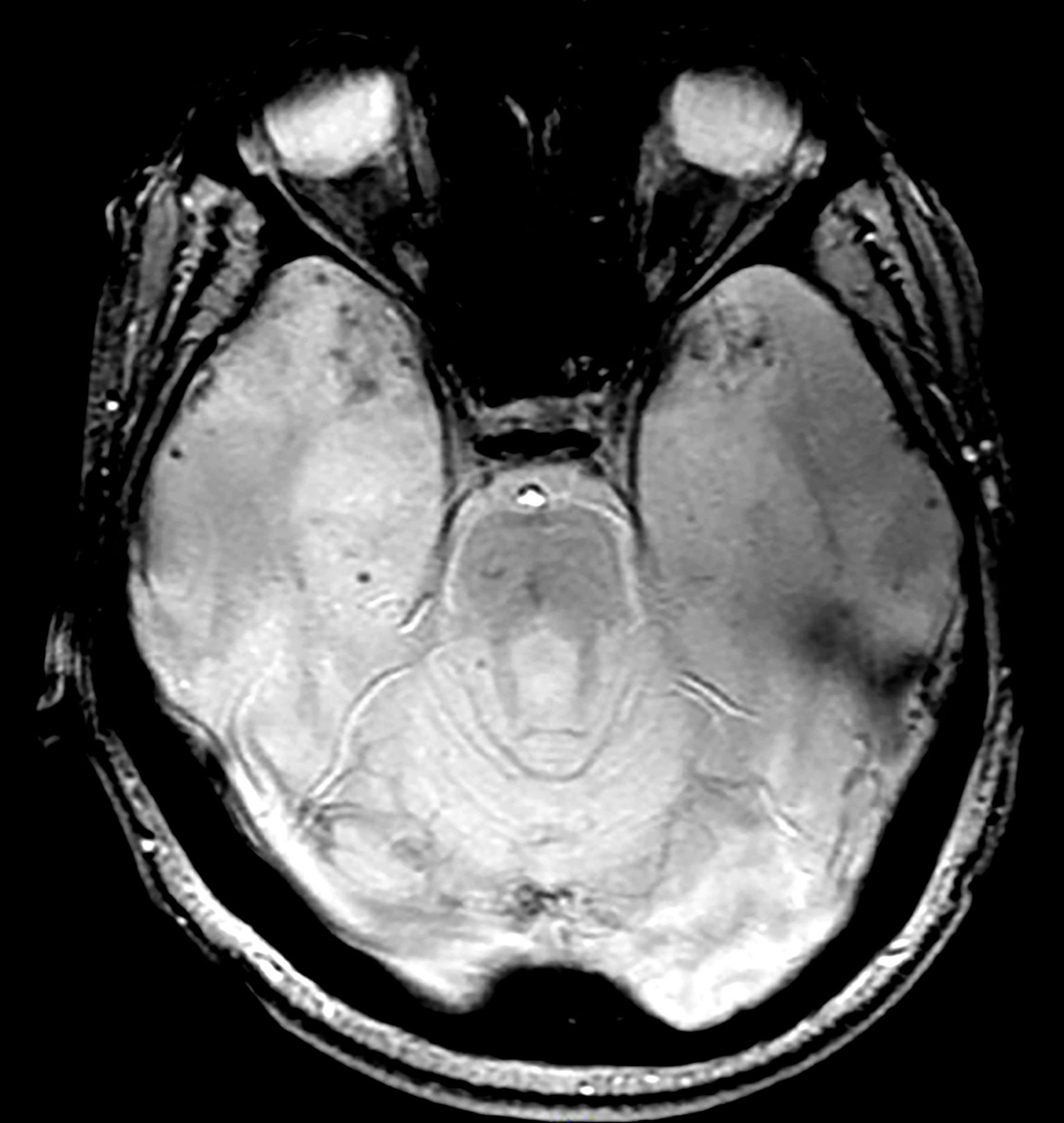
A 35-year-old male veteran presents with a history of severe headaches, difficulty concentrating, and persistent mood swings. He served multiple tours in a combat zone, where he was exposed to several traumatic events, including the loss of close friends. His medical history reveals previous diagnoses of insomnia and anxiety, for which he has been prescribed various medications over the years with limited success. During his clinical evaluation, he describes frequent nightmares and flashbacks related to his time in service. He reports an increased startle response and hypervigilance, often feeling on edge and irritable. A recent MRI of the brain, as shown in the image here, reveals significant changes in the limbic system, with abnormalities in the hippocampal regions. Laboratory tests and physical exams are otherwise unremarkable, but his mental health assessment indicates severe distress, which is affecting his daily functioning and interpersonal relationships.
PTSD Workup
Editor's Note: This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
Editor's Note: This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
Editor's Note: This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
PTSD: The Basics
Editor's Note: This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
Editor's Note: This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
Editor's Note: This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
PTSD Needs a New Name, Experts Say — Here’s Why
In a bid to reduce stigma and improve treatment rates, for inclusion in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision (DSM-5-TR). The APA’s policy is that a rolling name change is available if the current term is determined to be harmful.
Currently led by anesthesiologist Eugene Lipov, MD, clinical assistant professor, University of Illinois Chicago, and chief medical officer of Stella Center, also in Chicago, the formal request for the proposed name change to the APA’s DSM-5-TR Steering Committee in August 2023.
The APA Steering Committee rejected the proposed name change in November 2023, citing a “lack of convincing evidence.” However, Dr. Lipov and colleagues remain undeterred and continue to advocate for the change.
“The word ‘disorder’ is both imprecise and stigmatizing,” Dr. Lipov said. “Because of stigma, many people with PTSD — especially those in the military — don’t get help, which my research has demonstrated.”
Patients are more likely to seek help if their symptoms are framed as manifestations of an injury that is diagnosable and treatable, like a broken leg, Dr. Lipov said. “Stigma can kill in very real ways, since delayed care or lack of care can directly lead to suicides, thus satisfying the reduce harm requirement for the name change.”
Neurobiology of Trauma
Dr. Lipov grew up with a veteran father affected by PTSD and a mother with debilitating depression who eventually took her life. “I understand the impact of trauma very well,” he said.
Although not a psychiatrist, Dr. Lipov pioneered a highly successful treatment for PTSD by adapting an anesthetic technique — the stellate ganglion block (SGB) — to reverse many trauma symptoms through the process of “rebooting.”
This involves reversing the activity of the sympathetic nervous system — the fight-or-flight response — to the pretrauma state by anesthetizing the sympathetic ganglion in the neck. Investigating how SGB can help ameliorate the symptoms of PTSD led him to investigate and describe the neurobiology of PTSD and the mechanism of action of SGB.
The impact of SGD on PTSD was supported by a small neuroimaging study demonstrating that the right amygdala — the area of the brain associated with the fear response — was overactivated in patients with PTSD but that this region was deactivated after the administration of SGB, Dr. Lipov said.
“I believe that psychiatric conditions are actually physiologic brain changes that can be measured by advanced neuroimaging technologies and then physiologically treated,” he stated.
He noted that a growing body of literature suggests that use of the SGB for PTSD can be effective “because PTSD has a neurobiological basis and is essentially caused by an actual injury to the brain.”
A Natural Response, Not a Disorder
Dr. Lipov’s clinical work treating PTSD as a brain injury led him to connect with Frank Ochberg, MD, a founding board member of the International Society for Traumatic Stress Studies, former associate director of the National Institute of Mental Health, and former director of the Michigan Department of Mental Health.
In 2012, Dr. Ochberg teamed up with retired Army General Peter Chiarelli and Jonathan Shay, MD, PhD, author of Achilles in Vietnam: Combat Trauma and the Undoing of Character, to petition the DSM-5 Steering Committee to change the name of PTSD to PTSI in the upcoming DSM-5.
Dr. Ochberg explained that Gen. Chiarelli believed the term “disorder” suggests a preexisting issue prior to enlistment, potentially making an individual appear “weak.” He noted that this stigma is particularly troubling for military personnel, who often avoid seeking so they are not perceived as vulnerable, which can lead to potentially dire consequences, including suicide.
“We received endorsements from many quarters, not only advocates for service members or veterans,” Dr. Ochberg said.
This included feminists like Gloria Steinem, who championed the rights of women who had survived rape, incest, and domestic violence. As one advocate put it: “The natural human reaction to a life-threatening event should not be labeled a disorder.”
The DSM-5 Steering Committee declined to change the name. “Their feeling was that if we change the word ‘disorder’ to something else, we’d have to change every condition in the DSM that’s called a ‘disorder’. And they felt there really was nothing wrong with the word,” said Dr. Ochberg.
However, Dr. Lipov noted that other diagnoses have undergone name changes in the DSM for the sake of accuracy or stigma reduction. For example, the term mental retardation (DSM-IV) was changed to intellectual disability in DSM-5, and gender identity disorder was changed to gender dysphoria.
A decade later, Dr. Lipov decided to try again. To bolster his contention, he conducted a telephone survey of 1025 individuals. Of these, about 50% had a PTSD diagnosis.
Approximately two thirds of respondents agreed that a name change to PTSI would reduce the stigma associated with the term “PTSD.” Over half said it would increase the likelihood they would seek medical help. Those diagnosed with PTSD were most likely to endorse the name change.
Dr. Lipov conducts an ongoing survey of psychiatrists to ascertain their views on the potential name change and hopes to include findings in future research and communication with the DSM-5 Steering Committee. In addition, he has developed a new survey that expands upon his original survey, which specifically looked at individuals with PTSD.
“The new survey includes a wide range of people, many of whom have never been diagnosed. One of the questions we ask is whether they’ve ever heard of PTSD, and then we ask them about their reaction to the term,” he said.
A Barrier to Care
Psychiatrist Marcel Green, MD, director of Hudson Mind in New York City, refers to himself as an “interventional psychiatrist,” as he employs a comprehensive approach that includes not only medication and psychotherapy but also specialized techniques like SBG for severe anxiety-related physical symptoms and certain pain conditions.
Dr. Green, who is not involved in the name change initiative, agrees that the term “disorder” carries more stigma than “injury” for many groups, including those who have experienced childhood trauma, those struggling with substance abuse, or who are from backgrounds or peer groups where seeking mental health care is stigmatized.
Patients like these “are looking to me to give them a language to frame what they’re going through, and I tell them their symptoms are consistent with PTSD,” he said. “But they tell me don’t see themselves as having a disorder, which hinders their pursuit of care.”
Framing the condition as an “injury” also aligns with the approach of using biologic interventions to address the injury. Dr. Green has found SGB helpful in treating substance abuse disorder too, “which is a form of escape from the hyperactivation that accompanies PTSD.” And after the procedure, “they’re more receptive to therapy.”
Unfortunately, said Dr. Lipov, the DSM Steering Committee rejected his proposed name change, stating that the “concept of disorder as a dividing line from, eg, normal reactions to stress, is a core concept in the DSM, and the term has only rarely been removed.”
Moreover, the committee “did not see sufficient evidence ... that the name PTSD is stigmatizing and actually deters people with the disorder from seeking treatment who would not be deterred from doing so by PTSI.”
‘An Avenue for Dignity’
Ken Duckworth, MD, chief medical officer of the National Alliance on Mental Illness (NAMI), noted that the organization does not have an official position on this issue. However, he shared his own personal perspective.
There may be merit in the proposed name change, said Dr. Duckworth, but more evidence is needed. “If it’s clear, after rigorous studies have been performed and there’s compelling data, that calling it a ‘disorder’ rather than an ‘injury’ is actually preventing people from getting the care they need, then it merits serious attention.”
If so, Dr. Duckworth would be “interested in having a conversation with the policy team at NAMI to start to see if we could activate the DSM Committee.”
Roger McIntyre, MD, professor of psychiatry and pharmacology at the University of Toronto in Ontario, Canada, and head of the Mood Disorders Psychopharmacology Unit, said the name change initiative is a “really interesting proposal.”
Dr. McIntyre, chairman and executive director of the Brain and Cognition Discovery Foundation, also in Toronto, who is not involved in the initiative, has also heard “many people say that the term ‘disorder’ is stigmatizing and might even come across as pejorative in some ways.”
By contrast, “the word ‘injury’ parallels physical injury, and what we currently call ‘PTSD’ is a psychological or emotional injury no less devastating than torn tissue or broken bones,” added Dr. McIntyre, who is also the chairman of the board of the Depression and Bipolar Support Alliance.
Dr. Ochberg agreed. “In the military, ‘injury’ opens up an avenue for dignity, for a medal. Being injured and learning how to deal with an injury is part of having yet another honorable task that comes from being an honorable person who did an honorable thing.”
While disappointed, Dr. Lipov does not plan to give up on his vision. “I will continue to amass evidence that the word ‘PTSD’ is stigmatizing and indeed does prevent people from seeking care and will resubmit the proposal to the DSM Steering Committee when I have gathered a larger body of compelling evidence.”
Currently, Dr. Lipov is in active discussions with the special operations force of the US Army to obtain more evidence. “This will be the follow-up to bolster the opinion of Peter Chiarelli,” he said. “It is known that suicide and PTSD are highly related. This is especially urgent and relevant because recent data suggest suicide rate of military personnel in the VA may be as high as 44 per day,” Dr. Lipov said.
Dr. Lipov is the chief medical officer and an investor in the Stella Center. Dr. Green performs SGBs as part of his psychiatric practice. Drs. Ochberg, McIntyre, and Duckworth reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
In a bid to reduce stigma and improve treatment rates, for inclusion in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision (DSM-5-TR). The APA’s policy is that a rolling name change is available if the current term is determined to be harmful.
Currently led by anesthesiologist Eugene Lipov, MD, clinical assistant professor, University of Illinois Chicago, and chief medical officer of Stella Center, also in Chicago, the formal request for the proposed name change to the APA’s DSM-5-TR Steering Committee in August 2023.
The APA Steering Committee rejected the proposed name change in November 2023, citing a “lack of convincing evidence.” However, Dr. Lipov and colleagues remain undeterred and continue to advocate for the change.
“The word ‘disorder’ is both imprecise and stigmatizing,” Dr. Lipov said. “Because of stigma, many people with PTSD — especially those in the military — don’t get help, which my research has demonstrated.”
Patients are more likely to seek help if their symptoms are framed as manifestations of an injury that is diagnosable and treatable, like a broken leg, Dr. Lipov said. “Stigma can kill in very real ways, since delayed care or lack of care can directly lead to suicides, thus satisfying the reduce harm requirement for the name change.”
Neurobiology of Trauma
Dr. Lipov grew up with a veteran father affected by PTSD and a mother with debilitating depression who eventually took her life. “I understand the impact of trauma very well,” he said.
Although not a psychiatrist, Dr. Lipov pioneered a highly successful treatment for PTSD by adapting an anesthetic technique — the stellate ganglion block (SGB) — to reverse many trauma symptoms through the process of “rebooting.”
This involves reversing the activity of the sympathetic nervous system — the fight-or-flight response — to the pretrauma state by anesthetizing the sympathetic ganglion in the neck. Investigating how SGB can help ameliorate the symptoms of PTSD led him to investigate and describe the neurobiology of PTSD and the mechanism of action of SGB.
The impact of SGD on PTSD was supported by a small neuroimaging study demonstrating that the right amygdala — the area of the brain associated with the fear response — was overactivated in patients with PTSD but that this region was deactivated after the administration of SGB, Dr. Lipov said.
“I believe that psychiatric conditions are actually physiologic brain changes that can be measured by advanced neuroimaging technologies and then physiologically treated,” he stated.
He noted that a growing body of literature suggests that use of the SGB for PTSD can be effective “because PTSD has a neurobiological basis and is essentially caused by an actual injury to the brain.”
A Natural Response, Not a Disorder
Dr. Lipov’s clinical work treating PTSD as a brain injury led him to connect with Frank Ochberg, MD, a founding board member of the International Society for Traumatic Stress Studies, former associate director of the National Institute of Mental Health, and former director of the Michigan Department of Mental Health.
In 2012, Dr. Ochberg teamed up with retired Army General Peter Chiarelli and Jonathan Shay, MD, PhD, author of Achilles in Vietnam: Combat Trauma and the Undoing of Character, to petition the DSM-5 Steering Committee to change the name of PTSD to PTSI in the upcoming DSM-5.
Dr. Ochberg explained that Gen. Chiarelli believed the term “disorder” suggests a preexisting issue prior to enlistment, potentially making an individual appear “weak.” He noted that this stigma is particularly troubling for military personnel, who often avoid seeking so they are not perceived as vulnerable, which can lead to potentially dire consequences, including suicide.
“We received endorsements from many quarters, not only advocates for service members or veterans,” Dr. Ochberg said.
This included feminists like Gloria Steinem, who championed the rights of women who had survived rape, incest, and domestic violence. As one advocate put it: “The natural human reaction to a life-threatening event should not be labeled a disorder.”
The DSM-5 Steering Committee declined to change the name. “Their feeling was that if we change the word ‘disorder’ to something else, we’d have to change every condition in the DSM that’s called a ‘disorder’. And they felt there really was nothing wrong with the word,” said Dr. Ochberg.
However, Dr. Lipov noted that other diagnoses have undergone name changes in the DSM for the sake of accuracy or stigma reduction. For example, the term mental retardation (DSM-IV) was changed to intellectual disability in DSM-5, and gender identity disorder was changed to gender dysphoria.
A decade later, Dr. Lipov decided to try again. To bolster his contention, he conducted a telephone survey of 1025 individuals. Of these, about 50% had a PTSD diagnosis.
Approximately two thirds of respondents agreed that a name change to PTSI would reduce the stigma associated with the term “PTSD.” Over half said it would increase the likelihood they would seek medical help. Those diagnosed with PTSD were most likely to endorse the name change.
Dr. Lipov conducts an ongoing survey of psychiatrists to ascertain their views on the potential name change and hopes to include findings in future research and communication with the DSM-5 Steering Committee. In addition, he has developed a new survey that expands upon his original survey, which specifically looked at individuals with PTSD.
“The new survey includes a wide range of people, many of whom have never been diagnosed. One of the questions we ask is whether they’ve ever heard of PTSD, and then we ask them about their reaction to the term,” he said.
A Barrier to Care
Psychiatrist Marcel Green, MD, director of Hudson Mind in New York City, refers to himself as an “interventional psychiatrist,” as he employs a comprehensive approach that includes not only medication and psychotherapy but also specialized techniques like SBG for severe anxiety-related physical symptoms and certain pain conditions.
Dr. Green, who is not involved in the name change initiative, agrees that the term “disorder” carries more stigma than “injury” for many groups, including those who have experienced childhood trauma, those struggling with substance abuse, or who are from backgrounds or peer groups where seeking mental health care is stigmatized.
Patients like these “are looking to me to give them a language to frame what they’re going through, and I tell them their symptoms are consistent with PTSD,” he said. “But they tell me don’t see themselves as having a disorder, which hinders their pursuit of care.”
Framing the condition as an “injury” also aligns with the approach of using biologic interventions to address the injury. Dr. Green has found SGB helpful in treating substance abuse disorder too, “which is a form of escape from the hyperactivation that accompanies PTSD.” And after the procedure, “they’re more receptive to therapy.”
Unfortunately, said Dr. Lipov, the DSM Steering Committee rejected his proposed name change, stating that the “concept of disorder as a dividing line from, eg, normal reactions to stress, is a core concept in the DSM, and the term has only rarely been removed.”
Moreover, the committee “did not see sufficient evidence ... that the name PTSD is stigmatizing and actually deters people with the disorder from seeking treatment who would not be deterred from doing so by PTSI.”
‘An Avenue for Dignity’
Ken Duckworth, MD, chief medical officer of the National Alliance on Mental Illness (NAMI), noted that the organization does not have an official position on this issue. However, he shared his own personal perspective.
There may be merit in the proposed name change, said Dr. Duckworth, but more evidence is needed. “If it’s clear, after rigorous studies have been performed and there’s compelling data, that calling it a ‘disorder’ rather than an ‘injury’ is actually preventing people from getting the care they need, then it merits serious attention.”
If so, Dr. Duckworth would be “interested in having a conversation with the policy team at NAMI to start to see if we could activate the DSM Committee.”
Roger McIntyre, MD, professor of psychiatry and pharmacology at the University of Toronto in Ontario, Canada, and head of the Mood Disorders Psychopharmacology Unit, said the name change initiative is a “really interesting proposal.”
Dr. McIntyre, chairman and executive director of the Brain and Cognition Discovery Foundation, also in Toronto, who is not involved in the initiative, has also heard “many people say that the term ‘disorder’ is stigmatizing and might even come across as pejorative in some ways.”
By contrast, “the word ‘injury’ parallels physical injury, and what we currently call ‘PTSD’ is a psychological or emotional injury no less devastating than torn tissue or broken bones,” added Dr. McIntyre, who is also the chairman of the board of the Depression and Bipolar Support Alliance.
Dr. Ochberg agreed. “In the military, ‘injury’ opens up an avenue for dignity, for a medal. Being injured and learning how to deal with an injury is part of having yet another honorable task that comes from being an honorable person who did an honorable thing.”
While disappointed, Dr. Lipov does not plan to give up on his vision. “I will continue to amass evidence that the word ‘PTSD’ is stigmatizing and indeed does prevent people from seeking care and will resubmit the proposal to the DSM Steering Committee when I have gathered a larger body of compelling evidence.”
Currently, Dr. Lipov is in active discussions with the special operations force of the US Army to obtain more evidence. “This will be the follow-up to bolster the opinion of Peter Chiarelli,” he said. “It is known that suicide and PTSD are highly related. This is especially urgent and relevant because recent data suggest suicide rate of military personnel in the VA may be as high as 44 per day,” Dr. Lipov said.
Dr. Lipov is the chief medical officer and an investor in the Stella Center. Dr. Green performs SGBs as part of his psychiatric practice. Drs. Ochberg, McIntyre, and Duckworth reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
In a bid to reduce stigma and improve treatment rates, for inclusion in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision (DSM-5-TR). The APA’s policy is that a rolling name change is available if the current term is determined to be harmful.
Currently led by anesthesiologist Eugene Lipov, MD, clinical assistant professor, University of Illinois Chicago, and chief medical officer of Stella Center, also in Chicago, the formal request for the proposed name change to the APA’s DSM-5-TR Steering Committee in August 2023.
The APA Steering Committee rejected the proposed name change in November 2023, citing a “lack of convincing evidence.” However, Dr. Lipov and colleagues remain undeterred and continue to advocate for the change.
“The word ‘disorder’ is both imprecise and stigmatizing,” Dr. Lipov said. “Because of stigma, many people with PTSD — especially those in the military — don’t get help, which my research has demonstrated.”
Patients are more likely to seek help if their symptoms are framed as manifestations of an injury that is diagnosable and treatable, like a broken leg, Dr. Lipov said. “Stigma can kill in very real ways, since delayed care or lack of care can directly lead to suicides, thus satisfying the reduce harm requirement for the name change.”
Neurobiology of Trauma
Dr. Lipov grew up with a veteran father affected by PTSD and a mother with debilitating depression who eventually took her life. “I understand the impact of trauma very well,” he said.
Although not a psychiatrist, Dr. Lipov pioneered a highly successful treatment for PTSD by adapting an anesthetic technique — the stellate ganglion block (SGB) — to reverse many trauma symptoms through the process of “rebooting.”
This involves reversing the activity of the sympathetic nervous system — the fight-or-flight response — to the pretrauma state by anesthetizing the sympathetic ganglion in the neck. Investigating how SGB can help ameliorate the symptoms of PTSD led him to investigate and describe the neurobiology of PTSD and the mechanism of action of SGB.
The impact of SGD on PTSD was supported by a small neuroimaging study demonstrating that the right amygdala — the area of the brain associated with the fear response — was overactivated in patients with PTSD but that this region was deactivated after the administration of SGB, Dr. Lipov said.
“I believe that psychiatric conditions are actually physiologic brain changes that can be measured by advanced neuroimaging technologies and then physiologically treated,” he stated.
He noted that a growing body of literature suggests that use of the SGB for PTSD can be effective “because PTSD has a neurobiological basis and is essentially caused by an actual injury to the brain.”
A Natural Response, Not a Disorder
Dr. Lipov’s clinical work treating PTSD as a brain injury led him to connect with Frank Ochberg, MD, a founding board member of the International Society for Traumatic Stress Studies, former associate director of the National Institute of Mental Health, and former director of the Michigan Department of Mental Health.
In 2012, Dr. Ochberg teamed up with retired Army General Peter Chiarelli and Jonathan Shay, MD, PhD, author of Achilles in Vietnam: Combat Trauma and the Undoing of Character, to petition the DSM-5 Steering Committee to change the name of PTSD to PTSI in the upcoming DSM-5.
Dr. Ochberg explained that Gen. Chiarelli believed the term “disorder” suggests a preexisting issue prior to enlistment, potentially making an individual appear “weak.” He noted that this stigma is particularly troubling for military personnel, who often avoid seeking so they are not perceived as vulnerable, which can lead to potentially dire consequences, including suicide.
“We received endorsements from many quarters, not only advocates for service members or veterans,” Dr. Ochberg said.
This included feminists like Gloria Steinem, who championed the rights of women who had survived rape, incest, and domestic violence. As one advocate put it: “The natural human reaction to a life-threatening event should not be labeled a disorder.”
The DSM-5 Steering Committee declined to change the name. “Their feeling was that if we change the word ‘disorder’ to something else, we’d have to change every condition in the DSM that’s called a ‘disorder’. And they felt there really was nothing wrong with the word,” said Dr. Ochberg.
However, Dr. Lipov noted that other diagnoses have undergone name changes in the DSM for the sake of accuracy or stigma reduction. For example, the term mental retardation (DSM-IV) was changed to intellectual disability in DSM-5, and gender identity disorder was changed to gender dysphoria.
A decade later, Dr. Lipov decided to try again. To bolster his contention, he conducted a telephone survey of 1025 individuals. Of these, about 50% had a PTSD diagnosis.
Approximately two thirds of respondents agreed that a name change to PTSI would reduce the stigma associated with the term “PTSD.” Over half said it would increase the likelihood they would seek medical help. Those diagnosed with PTSD were most likely to endorse the name change.
Dr. Lipov conducts an ongoing survey of psychiatrists to ascertain their views on the potential name change and hopes to include findings in future research and communication with the DSM-5 Steering Committee. In addition, he has developed a new survey that expands upon his original survey, which specifically looked at individuals with PTSD.
“The new survey includes a wide range of people, many of whom have never been diagnosed. One of the questions we ask is whether they’ve ever heard of PTSD, and then we ask them about their reaction to the term,” he said.
A Barrier to Care
Psychiatrist Marcel Green, MD, director of Hudson Mind in New York City, refers to himself as an “interventional psychiatrist,” as he employs a comprehensive approach that includes not only medication and psychotherapy but also specialized techniques like SBG for severe anxiety-related physical symptoms and certain pain conditions.
Dr. Green, who is not involved in the name change initiative, agrees that the term “disorder” carries more stigma than “injury” for many groups, including those who have experienced childhood trauma, those struggling with substance abuse, or who are from backgrounds or peer groups where seeking mental health care is stigmatized.
Patients like these “are looking to me to give them a language to frame what they’re going through, and I tell them their symptoms are consistent with PTSD,” he said. “But they tell me don’t see themselves as having a disorder, which hinders their pursuit of care.”
Framing the condition as an “injury” also aligns with the approach of using biologic interventions to address the injury. Dr. Green has found SGB helpful in treating substance abuse disorder too, “which is a form of escape from the hyperactivation that accompanies PTSD.” And after the procedure, “they’re more receptive to therapy.”
Unfortunately, said Dr. Lipov, the DSM Steering Committee rejected his proposed name change, stating that the “concept of disorder as a dividing line from, eg, normal reactions to stress, is a core concept in the DSM, and the term has only rarely been removed.”
Moreover, the committee “did not see sufficient evidence ... that the name PTSD is stigmatizing and actually deters people with the disorder from seeking treatment who would not be deterred from doing so by PTSI.”
‘An Avenue for Dignity’
Ken Duckworth, MD, chief medical officer of the National Alliance on Mental Illness (NAMI), noted that the organization does not have an official position on this issue. However, he shared his own personal perspective.
There may be merit in the proposed name change, said Dr. Duckworth, but more evidence is needed. “If it’s clear, after rigorous studies have been performed and there’s compelling data, that calling it a ‘disorder’ rather than an ‘injury’ is actually preventing people from getting the care they need, then it merits serious attention.”
If so, Dr. Duckworth would be “interested in having a conversation with the policy team at NAMI to start to see if we could activate the DSM Committee.”
Roger McIntyre, MD, professor of psychiatry and pharmacology at the University of Toronto in Ontario, Canada, and head of the Mood Disorders Psychopharmacology Unit, said the name change initiative is a “really interesting proposal.”
Dr. McIntyre, chairman and executive director of the Brain and Cognition Discovery Foundation, also in Toronto, who is not involved in the initiative, has also heard “many people say that the term ‘disorder’ is stigmatizing and might even come across as pejorative in some ways.”
By contrast, “the word ‘injury’ parallels physical injury, and what we currently call ‘PTSD’ is a psychological or emotional injury no less devastating than torn tissue or broken bones,” added Dr. McIntyre, who is also the chairman of the board of the Depression and Bipolar Support Alliance.
Dr. Ochberg agreed. “In the military, ‘injury’ opens up an avenue for dignity, for a medal. Being injured and learning how to deal with an injury is part of having yet another honorable task that comes from being an honorable person who did an honorable thing.”
While disappointed, Dr. Lipov does not plan to give up on his vision. “I will continue to amass evidence that the word ‘PTSD’ is stigmatizing and indeed does prevent people from seeking care and will resubmit the proposal to the DSM Steering Committee when I have gathered a larger body of compelling evidence.”
Currently, Dr. Lipov is in active discussions with the special operations force of the US Army to obtain more evidence. “This will be the follow-up to bolster the opinion of Peter Chiarelli,” he said. “It is known that suicide and PTSD are highly related. This is especially urgent and relevant because recent data suggest suicide rate of military personnel in the VA may be as high as 44 per day,” Dr. Lipov said.
Dr. Lipov is the chief medical officer and an investor in the Stella Center. Dr. Green performs SGBs as part of his psychiatric practice. Drs. Ochberg, McIntyre, and Duckworth reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Data Trends 2024: Depression and PTSD
- Inoue C, Shawler E, Jordan CH, Moore MJ, Jackson CA. Veteran and military mental health issues. StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing. Updated August 17, 2023. Accessed April 1, 2024. https://www.ncbi.nlm.nih.gov/books/NBK572092/
- Panaite V, Cohen NJ, Luter SL, et al. Mental health treatment utilization patterns among 108,457 Afghanistan and Iraq veterans with depression. Psychol Serv. 2024 Feb 1. doi:10.1037/ser0000819
- Holder N, Holliday R, Ranney RM, et al. Relationship of social determinants of health with symptom severity among veterans and non-veterans with probable posttraumatic stress disorder or depression. Soc Psychiatry Psychiatr Epidemiol. 2023;58(10):1523-1534. doi:10.1007/s00127-023-02478-0
- Merians AN, Gross G, Spoont MR, Bellamy CD, Harpaz-Rotem I, Pietrzak RH. Racial and ethnic mental health disparities in U.S. military veterans: results from the National Health and Resilience in Veterans Study. J Psychiatr Res. 2023;161:71-76. doi:10.1016/j.jpsychires.2023.03.005
- Fischer IC, Schnurr PP, Pietrzak RH. Employment status among US military veterans with a history of posttraumatic stress disorder: results from the National Health and Resilience in Veterans Study. J Trauma Stress. 2023;36(6):1167-1175. doi:10.1002/jts.22977
- Portnoy GA, Relyea MR, Presseau C, et al. Screening for intimate partner violence experience and use in the Veterans Health Administration. JAMA Netw Open. 2023;6(10):e2337685. doi:10.1001/jamanetworkopen.2023.37685
- Cowlishaw S, Freijah I, Kartal D, et al. Intimate Partner Violence (IPV) in Military and Veteran Populations: A Systematic Review of Population-Based Surveys and Population Screening Studies. Int J Environ Res Public Health. 2022;19(14):8853. Published 2022 Jul 21. doi:10.3390/ijerph19148853
- Ranney RM, Maguen S, Bernhard PA, et al. Treatment utilization for posttraumatic stress disorder in a national sample of veterans and nonveterans. Med Care. 2023;61(2):87-94. doi:10.1097/MLR.0000000000001793
- Inoue C, Shawler E, Jordan CH, Moore MJ, Jackson CA. Veteran and military mental health issues. StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing. Updated August 17, 2023. Accessed April 1, 2024. https://www.ncbi.nlm.nih.gov/books/NBK572092/
- Panaite V, Cohen NJ, Luter SL, et al. Mental health treatment utilization patterns among 108,457 Afghanistan and Iraq veterans with depression. Psychol Serv. 2024 Feb 1. doi:10.1037/ser0000819
- Holder N, Holliday R, Ranney RM, et al. Relationship of social determinants of health with symptom severity among veterans and non-veterans with probable posttraumatic stress disorder or depression. Soc Psychiatry Psychiatr Epidemiol. 2023;58(10):1523-1534. doi:10.1007/s00127-023-02478-0
- Merians AN, Gross G, Spoont MR, Bellamy CD, Harpaz-Rotem I, Pietrzak RH. Racial and ethnic mental health disparities in U.S. military veterans: results from the National Health and Resilience in Veterans Study. J Psychiatr Res. 2023;161:71-76. doi:10.1016/j.jpsychires.2023.03.005
- Fischer IC, Schnurr PP, Pietrzak RH. Employment status among US military veterans with a history of posttraumatic stress disorder: results from the National Health and Resilience in Veterans Study. J Trauma Stress. 2023;36(6):1167-1175. doi:10.1002/jts.22977
- Portnoy GA, Relyea MR, Presseau C, et al. Screening for intimate partner violence experience and use in the Veterans Health Administration. JAMA Netw Open. 2023;6(10):e2337685. doi:10.1001/jamanetworkopen.2023.37685
- Cowlishaw S, Freijah I, Kartal D, et al. Intimate Partner Violence (IPV) in Military and Veteran Populations: A Systematic Review of Population-Based Surveys and Population Screening Studies. Int J Environ Res Public Health. 2022;19(14):8853. Published 2022 Jul 21. doi:10.3390/ijerph19148853
- Ranney RM, Maguen S, Bernhard PA, et al. Treatment utilization for posttraumatic stress disorder in a national sample of veterans and nonveterans. Med Care. 2023;61(2):87-94. doi:10.1097/MLR.0000000000001793
- Inoue C, Shawler E, Jordan CH, Moore MJ, Jackson CA. Veteran and military mental health issues. StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing. Updated August 17, 2023. Accessed April 1, 2024. https://www.ncbi.nlm.nih.gov/books/NBK572092/
- Panaite V, Cohen NJ, Luter SL, et al. Mental health treatment utilization patterns among 108,457 Afghanistan and Iraq veterans with depression. Psychol Serv. 2024 Feb 1. doi:10.1037/ser0000819
- Holder N, Holliday R, Ranney RM, et al. Relationship of social determinants of health with symptom severity among veterans and non-veterans with probable posttraumatic stress disorder or depression. Soc Psychiatry Psychiatr Epidemiol. 2023;58(10):1523-1534. doi:10.1007/s00127-023-02478-0
- Merians AN, Gross G, Spoont MR, Bellamy CD, Harpaz-Rotem I, Pietrzak RH. Racial and ethnic mental health disparities in U.S. military veterans: results from the National Health and Resilience in Veterans Study. J Psychiatr Res. 2023;161:71-76. doi:10.1016/j.jpsychires.2023.03.005
- Fischer IC, Schnurr PP, Pietrzak RH. Employment status among US military veterans with a history of posttraumatic stress disorder: results from the National Health and Resilience in Veterans Study. J Trauma Stress. 2023;36(6):1167-1175. doi:10.1002/jts.22977
- Portnoy GA, Relyea MR, Presseau C, et al. Screening for intimate partner violence experience and use in the Veterans Health Administration. JAMA Netw Open. 2023;6(10):e2337685. doi:10.1001/jamanetworkopen.2023.37685
- Cowlishaw S, Freijah I, Kartal D, et al. Intimate Partner Violence (IPV) in Military and Veteran Populations: A Systematic Review of Population-Based Surveys and Population Screening Studies. Int J Environ Res Public Health. 2022;19(14):8853. Published 2022 Jul 21. doi:10.3390/ijerph19148853
- Ranney RM, Maguen S, Bernhard PA, et al. Treatment utilization for posttraumatic stress disorder in a national sample of veterans and nonveterans. Med Care. 2023;61(2):87-94. doi:10.1097/MLR.0000000000001793
Federal Health Care Data Trends 2024
Federal Health Care Data Trends is a special supplement to Federal Practitioner, showcasing the latest research in health care for veterans and active-duty military members via compelling infographics. Click below to view highlights from the issue:
Federal Health Care Data Trends is a special supplement to Federal Practitioner, showcasing the latest research in health care for veterans and active-duty military members via compelling infographics. Click below to view highlights from the issue:
Federal Health Care Data Trends is a special supplement to Federal Practitioner, showcasing the latest research in health care for veterans and active-duty military members via compelling infographics. Click below to view highlights from the issue:
How Clinicians Can Help Patients Navigate Psychedelics/Microdosing
Peter Grinspoon, MD, has some advice for clinicians when patients ask questions about microdosing of psychedelics: Keep the lines of communication open — and don’t be judgmental.
“If you’re dismissive or critical or sound like you’re judging them, then the patients just clam up,” said Dr. Grinspoon, a professor of medicine at Harvard Medical School and a primary care physician at Massachusetts General Hospital, both in Boston.
Psychedelic drugs are still illegal in the majority of states despite the growth of public interest in and use of these substances. That growth is evidenced by a flurry of workshops, reports, law enforcement seizures, and pressure by Congressional members for the Food and Drug Administration to approve new psychedelic drugs, just in the past year.
A recent study in JAMA Health Forum showed a nearly 14-fold increase in Google searches — from 7.9 to 105.6 per 10 million nationwide — for the term “microdosing” and related wording, between 2015 and 2023.
Two states — Oregon and Colorado — have decriminalized certain psychedelic drugs and are in various stages of establishing regulations and centers for prospective clients. Almost two dozen localities, like Ann Arbor, Michigan, have decriminalized psychedelic drugs. A handful of states have active legislation to decriminalize use, while others have bills that never made it out of committee.
But no definitive studies have reported that microdosing produces positive mental effects at a higher rate than placebo, according to Dr. Grinspoon. So
“We’re in this renaissance where everybody is idealizing these medications, as opposed to 20 years ago when we were in the war on drugs and everybody was dismissing them,” Dr. Grinspoon said. “The truth is somewhere in between.”
The Science
Microdosing is defined as taking doses of 1/5 to 1/20 of the conventional recreational amount, which might include a dried psilocybin mushroom, lysergic acid diethylamide, or 3,4-methylenedioxymethamphetamine. But even that much may be neither effective nor safe.
Dr. Grinspoon said clinicians should tell patients that psychedelics may cause harm, although the drugs are relatively nontoxic and are not addictive. An illegally obtained psilocybin could cause negative reactions, especially if the drug has been adulterated with other substances and if the actual dose is higher than what was indicated by the seller.
He noted that people have different reactions to psychedelics, just as they have to prescription medications. He cited one example of a woman who microdosed and could not sleep for 2 weeks afterward. Only recently have randomized, double-blinded studies begun on benefits and harms.
Researchers have also begun investigating whether long-term microdosing of psilocybin could lead to valvular heart disease (VHD), said Kevin Yang, MD, a psychiatry resident at the University of California San Diego School of Medicine. A recent review of evidence concluded that microdosing various psychedelics over a period of months can lead to drug-induced VHD.
“It’s extremely important to emphasize with patients that not only do we not know if it works or not, we also don’t really know how safe it is,” Dr. Yang said.
Dr. Yang also said clinicians should consider referring patients to a mental health professional, and especially those that may have expertise in psychedelic therapies.
One of those experts is Rachel Yehuda, PhD, director of the Center for Psychedelic Psychotherapy and Trauma Research at Icahn School of Medicine at Mount Sinai in New York City. She said therapists should be able to assess the patient’s perceived need for microdosing and “invite reflections about why current approaches are falling short.”
“I would also not actively discourage it either but remain curious until both of you have a better understanding of the reasons for seeking this out and potential alternative strategies for obtaining more therapeutic benefits,” she said. “I think it is really important to study the effects of both micro- and macrodosing of psychedelics but not move in advance of the data.”
Navigating Legality
Recent ballot measures in Oregon and Colorado directed the states to develop regulated and licensed psilocybin-assisted therapy centers for legal “trips.” Oregon’s first center was opened in 2023, and Colorado is now developing its own licensing model.
According to the Oregon Health Authority, the centers are not medical facilities, and prescription or referral from a medical professional is not required.
The Oregon Academy of Family Physicians (OAFP) has yet to release guidance to clinicians on how to talk to their patients about these drugs or potential interest in visiting a licensed therapy center.
However, Betsy Boyd-Flynn, executive director of OAFP, said the organization is working on continuing medical education for what the average family physician needs to know if a patient asks about use.
“We suspect that many of our members have interest and want to learn more,” she said.
Dr. Grinspoon said clinicians should talk with patients about legality during these conversations.
“The big question I get is: ‘I really want to try microdosing, but how do I obtain the mushrooms?’ ” he said. “You can’t really as a physician tell them to do anything illegal. So you tell them to be safe, be careful, and to use their judgment.”
Patients who want to pursue microdosing who do not live in Oregon have two legal and safe options, Dr. Grinspoon said: Enroll in a clinical study or find a facility in a state or country — such as Oregon or Jamaica — that offers microdosing with psilocybin.
Clinicians also should warn their patients that the consequences of obtaining illicit psilocybin could exacerbate the mental health stresses they are seeking to alleviate.
“It’s going to get worse if they get tangled up with law enforcement or take something that’s contaminated and they get real sick,” he said.
Lisa Gillespie contributed reporting to this story. A version of this article appeared on Medscape.com.
Peter Grinspoon, MD, has some advice for clinicians when patients ask questions about microdosing of psychedelics: Keep the lines of communication open — and don’t be judgmental.
“If you’re dismissive or critical or sound like you’re judging them, then the patients just clam up,” said Dr. Grinspoon, a professor of medicine at Harvard Medical School and a primary care physician at Massachusetts General Hospital, both in Boston.
Psychedelic drugs are still illegal in the majority of states despite the growth of public interest in and use of these substances. That growth is evidenced by a flurry of workshops, reports, law enforcement seizures, and pressure by Congressional members for the Food and Drug Administration to approve new psychedelic drugs, just in the past year.
A recent study in JAMA Health Forum showed a nearly 14-fold increase in Google searches — from 7.9 to 105.6 per 10 million nationwide — for the term “microdosing” and related wording, between 2015 and 2023.
Two states — Oregon and Colorado — have decriminalized certain psychedelic drugs and are in various stages of establishing regulations and centers for prospective clients. Almost two dozen localities, like Ann Arbor, Michigan, have decriminalized psychedelic drugs. A handful of states have active legislation to decriminalize use, while others have bills that never made it out of committee.
But no definitive studies have reported that microdosing produces positive mental effects at a higher rate than placebo, according to Dr. Grinspoon. So
“We’re in this renaissance where everybody is idealizing these medications, as opposed to 20 years ago when we were in the war on drugs and everybody was dismissing them,” Dr. Grinspoon said. “The truth is somewhere in between.”
The Science
Microdosing is defined as taking doses of 1/5 to 1/20 of the conventional recreational amount, which might include a dried psilocybin mushroom, lysergic acid diethylamide, or 3,4-methylenedioxymethamphetamine. But even that much may be neither effective nor safe.
Dr. Grinspoon said clinicians should tell patients that psychedelics may cause harm, although the drugs are relatively nontoxic and are not addictive. An illegally obtained psilocybin could cause negative reactions, especially if the drug has been adulterated with other substances and if the actual dose is higher than what was indicated by the seller.
He noted that people have different reactions to psychedelics, just as they have to prescription medications. He cited one example of a woman who microdosed and could not sleep for 2 weeks afterward. Only recently have randomized, double-blinded studies begun on benefits and harms.
Researchers have also begun investigating whether long-term microdosing of psilocybin could lead to valvular heart disease (VHD), said Kevin Yang, MD, a psychiatry resident at the University of California San Diego School of Medicine. A recent review of evidence concluded that microdosing various psychedelics over a period of months can lead to drug-induced VHD.
“It’s extremely important to emphasize with patients that not only do we not know if it works or not, we also don’t really know how safe it is,” Dr. Yang said.
Dr. Yang also said clinicians should consider referring patients to a mental health professional, and especially those that may have expertise in psychedelic therapies.
One of those experts is Rachel Yehuda, PhD, director of the Center for Psychedelic Psychotherapy and Trauma Research at Icahn School of Medicine at Mount Sinai in New York City. She said therapists should be able to assess the patient’s perceived need for microdosing and “invite reflections about why current approaches are falling short.”
“I would also not actively discourage it either but remain curious until both of you have a better understanding of the reasons for seeking this out and potential alternative strategies for obtaining more therapeutic benefits,” she said. “I think it is really important to study the effects of both micro- and macrodosing of psychedelics but not move in advance of the data.”
Navigating Legality
Recent ballot measures in Oregon and Colorado directed the states to develop regulated and licensed psilocybin-assisted therapy centers for legal “trips.” Oregon’s first center was opened in 2023, and Colorado is now developing its own licensing model.
According to the Oregon Health Authority, the centers are not medical facilities, and prescription or referral from a medical professional is not required.
The Oregon Academy of Family Physicians (OAFP) has yet to release guidance to clinicians on how to talk to their patients about these drugs or potential interest in visiting a licensed therapy center.
However, Betsy Boyd-Flynn, executive director of OAFP, said the organization is working on continuing medical education for what the average family physician needs to know if a patient asks about use.
“We suspect that many of our members have interest and want to learn more,” she said.
Dr. Grinspoon said clinicians should talk with patients about legality during these conversations.
“The big question I get is: ‘I really want to try microdosing, but how do I obtain the mushrooms?’ ” he said. “You can’t really as a physician tell them to do anything illegal. So you tell them to be safe, be careful, and to use their judgment.”
Patients who want to pursue microdosing who do not live in Oregon have two legal and safe options, Dr. Grinspoon said: Enroll in a clinical study or find a facility in a state or country — such as Oregon or Jamaica — that offers microdosing with psilocybin.
Clinicians also should warn their patients that the consequences of obtaining illicit psilocybin could exacerbate the mental health stresses they are seeking to alleviate.
“It’s going to get worse if they get tangled up with law enforcement or take something that’s contaminated and they get real sick,” he said.
Lisa Gillespie contributed reporting to this story. A version of this article appeared on Medscape.com.
Peter Grinspoon, MD, has some advice for clinicians when patients ask questions about microdosing of psychedelics: Keep the lines of communication open — and don’t be judgmental.
“If you’re dismissive or critical or sound like you’re judging them, then the patients just clam up,” said Dr. Grinspoon, a professor of medicine at Harvard Medical School and a primary care physician at Massachusetts General Hospital, both in Boston.
Psychedelic drugs are still illegal in the majority of states despite the growth of public interest in and use of these substances. That growth is evidenced by a flurry of workshops, reports, law enforcement seizures, and pressure by Congressional members for the Food and Drug Administration to approve new psychedelic drugs, just in the past year.
A recent study in JAMA Health Forum showed a nearly 14-fold increase in Google searches — from 7.9 to 105.6 per 10 million nationwide — for the term “microdosing” and related wording, between 2015 and 2023.
Two states — Oregon and Colorado — have decriminalized certain psychedelic drugs and are in various stages of establishing regulations and centers for prospective clients. Almost two dozen localities, like Ann Arbor, Michigan, have decriminalized psychedelic drugs. A handful of states have active legislation to decriminalize use, while others have bills that never made it out of committee.
But no definitive studies have reported that microdosing produces positive mental effects at a higher rate than placebo, according to Dr. Grinspoon. So
“We’re in this renaissance where everybody is idealizing these medications, as opposed to 20 years ago when we were in the war on drugs and everybody was dismissing them,” Dr. Grinspoon said. “The truth is somewhere in between.”
The Science
Microdosing is defined as taking doses of 1/5 to 1/20 of the conventional recreational amount, which might include a dried psilocybin mushroom, lysergic acid diethylamide, or 3,4-methylenedioxymethamphetamine. But even that much may be neither effective nor safe.
Dr. Grinspoon said clinicians should tell patients that psychedelics may cause harm, although the drugs are relatively nontoxic and are not addictive. An illegally obtained psilocybin could cause negative reactions, especially if the drug has been adulterated with other substances and if the actual dose is higher than what was indicated by the seller.
He noted that people have different reactions to psychedelics, just as they have to prescription medications. He cited one example of a woman who microdosed and could not sleep for 2 weeks afterward. Only recently have randomized, double-blinded studies begun on benefits and harms.
Researchers have also begun investigating whether long-term microdosing of psilocybin could lead to valvular heart disease (VHD), said Kevin Yang, MD, a psychiatry resident at the University of California San Diego School of Medicine. A recent review of evidence concluded that microdosing various psychedelics over a period of months can lead to drug-induced VHD.
“It’s extremely important to emphasize with patients that not only do we not know if it works or not, we also don’t really know how safe it is,” Dr. Yang said.
Dr. Yang also said clinicians should consider referring patients to a mental health professional, and especially those that may have expertise in psychedelic therapies.
One of those experts is Rachel Yehuda, PhD, director of the Center for Psychedelic Psychotherapy and Trauma Research at Icahn School of Medicine at Mount Sinai in New York City. She said therapists should be able to assess the patient’s perceived need for microdosing and “invite reflections about why current approaches are falling short.”
“I would also not actively discourage it either but remain curious until both of you have a better understanding of the reasons for seeking this out and potential alternative strategies for obtaining more therapeutic benefits,” she said. “I think it is really important to study the effects of both micro- and macrodosing of psychedelics but not move in advance of the data.”
Navigating Legality
Recent ballot measures in Oregon and Colorado directed the states to develop regulated and licensed psilocybin-assisted therapy centers for legal “trips.” Oregon’s first center was opened in 2023, and Colorado is now developing its own licensing model.
According to the Oregon Health Authority, the centers are not medical facilities, and prescription or referral from a medical professional is not required.
The Oregon Academy of Family Physicians (OAFP) has yet to release guidance to clinicians on how to talk to their patients about these drugs or potential interest in visiting a licensed therapy center.
However, Betsy Boyd-Flynn, executive director of OAFP, said the organization is working on continuing medical education for what the average family physician needs to know if a patient asks about use.
“We suspect that many of our members have interest and want to learn more,” she said.
Dr. Grinspoon said clinicians should talk with patients about legality during these conversations.
“The big question I get is: ‘I really want to try microdosing, but how do I obtain the mushrooms?’ ” he said. “You can’t really as a physician tell them to do anything illegal. So you tell them to be safe, be careful, and to use their judgment.”
Patients who want to pursue microdosing who do not live in Oregon have two legal and safe options, Dr. Grinspoon said: Enroll in a clinical study or find a facility in a state or country — such as Oregon or Jamaica — that offers microdosing with psilocybin.
Clinicians also should warn their patients that the consequences of obtaining illicit psilocybin could exacerbate the mental health stresses they are seeking to alleviate.
“It’s going to get worse if they get tangled up with law enforcement or take something that’s contaminated and they get real sick,” he said.
Lisa Gillespie contributed reporting to this story. A version of this article appeared on Medscape.com.
More Evidence PTSD Tied to Obstructive Sleep Apnea Risk
Posttraumatic stress disorder (PTSD) may enhance the risk for obstructive sleep apnea (OSA) in older male veterans, the results of a cross-sectional twin study suggested. However, additional high-quality research is needed and may yield important mechanistic insights into both conditions and improve treatment, experts said.
“The strength of the association was a bit surprising,” said study investigator Amit J. Shah, MD, MSCR, Emory University, Atlanta, Georgia. “Many physicians and scientists may otherwise assume that the relationship between PTSD and sleep apnea would be primarily mediated by obesity, but we did not find that obesity explained our findings.”
The study was published online in JAMA Network Open.
A More Rigorous Evaluation
“Prior studies have shown an association between PTSD and sleep apnea, but the size of the association was not as strong,” Dr. Shah said, possibly because many were based on symptomatic patients referred for clinical evaluation of OSA and some relied on self-report of a sleep apnea diagnosis.
The current study involved 181 male twins, aged 61-71 years, including 66 pairs discordant for PTSD symptoms and 15 pairs discordant for PTSD diagnosis, who were recruited from the Vietnam Era Twin Registry and underwent a formal psychiatric and polysomnography evaluation as follow-up of the Emory Twin Study.
PTSD symptom severity was assessed using the self-administered Posttraumatic Stress Disorder Checklist (PCL). OSA was mild in 74% of participants, moderate to severe in 40%, and severe in 18%.
The mean apnea-hypopnea index (AHI) was 17.7 events per hour, and the mean proportion of the night with SaO2 less than 90% was 8.9%.
In fully adjusted models, each 15-point within-pair difference in PCL score was associated with a 4.6 events-per-hour higher AHI, a 6.4 events-per-hour higher oxygen desaturation index, and a 4.8% greater sleep duration with SaO2 less than 90%.
A current PTSD diagnosis is associated with an approximate 10-unit higher adjusted AHI in separate models involving potential cardiovascular mediators (10.5-unit; 95% CI, 5.7-15.3) and sociodemographic and psychiatric confounders (10.7-unit; 95% CI, 4.0-17.4).
The investigators called for more research into the underlying mechanisms but speculated that pharyngeal collapsibility and exaggerated loop gain, among others, may play a role.
“Our findings broaden the concept of OSA as one that may involve stress pathways in addition to the traditional mechanisms involving airway collapse and obesity,” Dr. Shah said. “We should be more suspicious of OSA as an important comorbidity in PTSD, given the high OSA prevalence that we found in PTSD veterans.”
Questions Remain
In an accompanying editorial, Steven H. Woodward, PhD, and Ruth M. Benca, MD, PhD, VA Palo Alto Health Care Systems, Palo Alto, California, noted the study affirmatively answers the decades-old question of whether rates of OSA are elevated in PTSD and “eliminates many potential confounders that might cast doubt on the PTSD-OSA association.”
However, they noted, it’s difficult to ascertain the directionality of this association and point out that, in terms of potential mechanisms, the oft-cited 1994 study linking sleep fragmentation with upper airway collapsibility has never been replicated and that a recent study found no difference in airway collapsibility or evidence of differential loop gain in combat veterans with and without PTSD.
Dr. Woodward and Dr. Benca also highlighted the large body of evidence that psychiatric disorders such as bipolar disorder, schizophrenia, and, in particular, major depressive disorder, are strongly associated with higher rates of OSA.
“In sum, we do not believe that a fair reading of the current literature supports a conclusion that PTSD bears an association with OSA that does not overlap with those manifested by other psychiatric disorders,” they wrote.
“This commentary is not intended to discourage any specific line of inquiry. Rather, we seek to keep the door open as wide as possible to hypotheses and research designs aimed at elucidating the relationships between OSA and psychiatric disorders,” Dr. Woodward and Dr. Benca concluded.
In response, Dr. Shah said the editorialists’ “point about psychiatric conditions other than PTSD also being important in OSA is well taken. In our own cohort, we did not see such an association, but that does not mean that this does not exist.
“Autonomic physiology, which we plan to study next, may underlie not only the PTSD-OSA relationship but also the relationship between other psychiatric factors and OSA,” he added.
The study was funded by grants from the National Institutes of Health (NIH). One study author reported receiving personal fees from Idorsia, and another reported receiving personal fees from Clinilabs, Eisai, Ferring Pharmaceuticals, Huxley, Idorsia, and Merck Sharp & Dohme. Dr. Benca reported receiving grants from the NIH and Eisai and personal fees from Eisai, Idorsia, Haleon, and Sage Therapeutics. Dr. Woodward reported having no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Posttraumatic stress disorder (PTSD) may enhance the risk for obstructive sleep apnea (OSA) in older male veterans, the results of a cross-sectional twin study suggested. However, additional high-quality research is needed and may yield important mechanistic insights into both conditions and improve treatment, experts said.
“The strength of the association was a bit surprising,” said study investigator Amit J. Shah, MD, MSCR, Emory University, Atlanta, Georgia. “Many physicians and scientists may otherwise assume that the relationship between PTSD and sleep apnea would be primarily mediated by obesity, but we did not find that obesity explained our findings.”
The study was published online in JAMA Network Open.
A More Rigorous Evaluation
“Prior studies have shown an association between PTSD and sleep apnea, but the size of the association was not as strong,” Dr. Shah said, possibly because many were based on symptomatic patients referred for clinical evaluation of OSA and some relied on self-report of a sleep apnea diagnosis.
The current study involved 181 male twins, aged 61-71 years, including 66 pairs discordant for PTSD symptoms and 15 pairs discordant for PTSD diagnosis, who were recruited from the Vietnam Era Twin Registry and underwent a formal psychiatric and polysomnography evaluation as follow-up of the Emory Twin Study.
PTSD symptom severity was assessed using the self-administered Posttraumatic Stress Disorder Checklist (PCL). OSA was mild in 74% of participants, moderate to severe in 40%, and severe in 18%.
The mean apnea-hypopnea index (AHI) was 17.7 events per hour, and the mean proportion of the night with SaO2 less than 90% was 8.9%.
In fully adjusted models, each 15-point within-pair difference in PCL score was associated with a 4.6 events-per-hour higher AHI, a 6.4 events-per-hour higher oxygen desaturation index, and a 4.8% greater sleep duration with SaO2 less than 90%.
A current PTSD diagnosis is associated with an approximate 10-unit higher adjusted AHI in separate models involving potential cardiovascular mediators (10.5-unit; 95% CI, 5.7-15.3) and sociodemographic and psychiatric confounders (10.7-unit; 95% CI, 4.0-17.4).
The investigators called for more research into the underlying mechanisms but speculated that pharyngeal collapsibility and exaggerated loop gain, among others, may play a role.
“Our findings broaden the concept of OSA as one that may involve stress pathways in addition to the traditional mechanisms involving airway collapse and obesity,” Dr. Shah said. “We should be more suspicious of OSA as an important comorbidity in PTSD, given the high OSA prevalence that we found in PTSD veterans.”
Questions Remain
In an accompanying editorial, Steven H. Woodward, PhD, and Ruth M. Benca, MD, PhD, VA Palo Alto Health Care Systems, Palo Alto, California, noted the study affirmatively answers the decades-old question of whether rates of OSA are elevated in PTSD and “eliminates many potential confounders that might cast doubt on the PTSD-OSA association.”
However, they noted, it’s difficult to ascertain the directionality of this association and point out that, in terms of potential mechanisms, the oft-cited 1994 study linking sleep fragmentation with upper airway collapsibility has never been replicated and that a recent study found no difference in airway collapsibility or evidence of differential loop gain in combat veterans with and without PTSD.
Dr. Woodward and Dr. Benca also highlighted the large body of evidence that psychiatric disorders such as bipolar disorder, schizophrenia, and, in particular, major depressive disorder, are strongly associated with higher rates of OSA.
“In sum, we do not believe that a fair reading of the current literature supports a conclusion that PTSD bears an association with OSA that does not overlap with those manifested by other psychiatric disorders,” they wrote.
“This commentary is not intended to discourage any specific line of inquiry. Rather, we seek to keep the door open as wide as possible to hypotheses and research designs aimed at elucidating the relationships between OSA and psychiatric disorders,” Dr. Woodward and Dr. Benca concluded.
In response, Dr. Shah said the editorialists’ “point about psychiatric conditions other than PTSD also being important in OSA is well taken. In our own cohort, we did not see such an association, but that does not mean that this does not exist.
“Autonomic physiology, which we plan to study next, may underlie not only the PTSD-OSA relationship but also the relationship between other psychiatric factors and OSA,” he added.
The study was funded by grants from the National Institutes of Health (NIH). One study author reported receiving personal fees from Idorsia, and another reported receiving personal fees from Clinilabs, Eisai, Ferring Pharmaceuticals, Huxley, Idorsia, and Merck Sharp & Dohme. Dr. Benca reported receiving grants from the NIH and Eisai and personal fees from Eisai, Idorsia, Haleon, and Sage Therapeutics. Dr. Woodward reported having no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Posttraumatic stress disorder (PTSD) may enhance the risk for obstructive sleep apnea (OSA) in older male veterans, the results of a cross-sectional twin study suggested. However, additional high-quality research is needed and may yield important mechanistic insights into both conditions and improve treatment, experts said.
“The strength of the association was a bit surprising,” said study investigator Amit J. Shah, MD, MSCR, Emory University, Atlanta, Georgia. “Many physicians and scientists may otherwise assume that the relationship between PTSD and sleep apnea would be primarily mediated by obesity, but we did not find that obesity explained our findings.”
The study was published online in JAMA Network Open.
A More Rigorous Evaluation
“Prior studies have shown an association between PTSD and sleep apnea, but the size of the association was not as strong,” Dr. Shah said, possibly because many were based on symptomatic patients referred for clinical evaluation of OSA and some relied on self-report of a sleep apnea diagnosis.
The current study involved 181 male twins, aged 61-71 years, including 66 pairs discordant for PTSD symptoms and 15 pairs discordant for PTSD diagnosis, who were recruited from the Vietnam Era Twin Registry and underwent a formal psychiatric and polysomnography evaluation as follow-up of the Emory Twin Study.
PTSD symptom severity was assessed using the self-administered Posttraumatic Stress Disorder Checklist (PCL). OSA was mild in 74% of participants, moderate to severe in 40%, and severe in 18%.
The mean apnea-hypopnea index (AHI) was 17.7 events per hour, and the mean proportion of the night with SaO2 less than 90% was 8.9%.
In fully adjusted models, each 15-point within-pair difference in PCL score was associated with a 4.6 events-per-hour higher AHI, a 6.4 events-per-hour higher oxygen desaturation index, and a 4.8% greater sleep duration with SaO2 less than 90%.
A current PTSD diagnosis is associated with an approximate 10-unit higher adjusted AHI in separate models involving potential cardiovascular mediators (10.5-unit; 95% CI, 5.7-15.3) and sociodemographic and psychiatric confounders (10.7-unit; 95% CI, 4.0-17.4).
The investigators called for more research into the underlying mechanisms but speculated that pharyngeal collapsibility and exaggerated loop gain, among others, may play a role.
“Our findings broaden the concept of OSA as one that may involve stress pathways in addition to the traditional mechanisms involving airway collapse and obesity,” Dr. Shah said. “We should be more suspicious of OSA as an important comorbidity in PTSD, given the high OSA prevalence that we found in PTSD veterans.”
Questions Remain
In an accompanying editorial, Steven H. Woodward, PhD, and Ruth M. Benca, MD, PhD, VA Palo Alto Health Care Systems, Palo Alto, California, noted the study affirmatively answers the decades-old question of whether rates of OSA are elevated in PTSD and “eliminates many potential confounders that might cast doubt on the PTSD-OSA association.”
However, they noted, it’s difficult to ascertain the directionality of this association and point out that, in terms of potential mechanisms, the oft-cited 1994 study linking sleep fragmentation with upper airway collapsibility has never been replicated and that a recent study found no difference in airway collapsibility or evidence of differential loop gain in combat veterans with and without PTSD.
Dr. Woodward and Dr. Benca also highlighted the large body of evidence that psychiatric disorders such as bipolar disorder, schizophrenia, and, in particular, major depressive disorder, are strongly associated with higher rates of OSA.
“In sum, we do not believe that a fair reading of the current literature supports a conclusion that PTSD bears an association with OSA that does not overlap with those manifested by other psychiatric disorders,” they wrote.
“This commentary is not intended to discourage any specific line of inquiry. Rather, we seek to keep the door open as wide as possible to hypotheses and research designs aimed at elucidating the relationships between OSA and psychiatric disorders,” Dr. Woodward and Dr. Benca concluded.
In response, Dr. Shah said the editorialists’ “point about psychiatric conditions other than PTSD also being important in OSA is well taken. In our own cohort, we did not see such an association, but that does not mean that this does not exist.
“Autonomic physiology, which we plan to study next, may underlie not only the PTSD-OSA relationship but also the relationship between other psychiatric factors and OSA,” he added.
The study was funded by grants from the National Institutes of Health (NIH). One study author reported receiving personal fees from Idorsia, and another reported receiving personal fees from Clinilabs, Eisai, Ferring Pharmaceuticals, Huxley, Idorsia, and Merck Sharp & Dohme. Dr. Benca reported receiving grants from the NIH and Eisai and personal fees from Eisai, Idorsia, Haleon, and Sage Therapeutics. Dr. Woodward reported having no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN