User login
ICD-10 codes for EVALI released
The Centers for Disease Control and Prevention has issued coding guidance to help track e-cigarette, or vaping, product use–associated lung injury (EVALI).
The purpose of the coding guidelines “is to provide official diagnosis coding guidance for healthcare encounters related to the 2019 health care encounters and deaths related to” EVALI, CDC stated in a document detailing the coding update. The document was posted on the CDC website. The guidance is consistent with current clinical knowledge about e-cigarette, or vaping, related disorders.
CDC noted in the document that the guidance “is intended to be used in conjunction with current ICD-10-CM classification,” and the codes provided “are intended to provide e-cigarette, or vaping, product use coding guidance only.”
The codes are intended to track a number of areas related to EVALI, including lung-related complications, poisoning and toxicity, and substance use, abuse, and dependence.
The following conditions associated with EVALI are covered in the new coding guidance:
- Bronchitis and pneumonitis caused by chemicals, gases, and fumes.
- Bronchitis and pneumonitis caused by chemicals, gases, fumes, and vapors; includes chemical pneumonitis.
- Pneumonitis caused by inhalation of oils and essences; includes lipoid pneumonia.
- Acute respiratory distress syndrome.
- Pulmonary eosinophilia, not elsewhere classified.
- Acute interstitial pneumonitis.
The document notes that the coding guidance has been approved by the National Center for Health Statistics, the American Health Information Management Association, the American Hospital Association, and the Centers for Medicare & Medicaid Services.
The Centers for Disease Control and Prevention has issued coding guidance to help track e-cigarette, or vaping, product use–associated lung injury (EVALI).
The purpose of the coding guidelines “is to provide official diagnosis coding guidance for healthcare encounters related to the 2019 health care encounters and deaths related to” EVALI, CDC stated in a document detailing the coding update. The document was posted on the CDC website. The guidance is consistent with current clinical knowledge about e-cigarette, or vaping, related disorders.
CDC noted in the document that the guidance “is intended to be used in conjunction with current ICD-10-CM classification,” and the codes provided “are intended to provide e-cigarette, or vaping, product use coding guidance only.”
The codes are intended to track a number of areas related to EVALI, including lung-related complications, poisoning and toxicity, and substance use, abuse, and dependence.
The following conditions associated with EVALI are covered in the new coding guidance:
- Bronchitis and pneumonitis caused by chemicals, gases, and fumes.
- Bronchitis and pneumonitis caused by chemicals, gases, fumes, and vapors; includes chemical pneumonitis.
- Pneumonitis caused by inhalation of oils and essences; includes lipoid pneumonia.
- Acute respiratory distress syndrome.
- Pulmonary eosinophilia, not elsewhere classified.
- Acute interstitial pneumonitis.
The document notes that the coding guidance has been approved by the National Center for Health Statistics, the American Health Information Management Association, the American Hospital Association, and the Centers for Medicare & Medicaid Services.
The Centers for Disease Control and Prevention has issued coding guidance to help track e-cigarette, or vaping, product use–associated lung injury (EVALI).
The purpose of the coding guidelines “is to provide official diagnosis coding guidance for healthcare encounters related to the 2019 health care encounters and deaths related to” EVALI, CDC stated in a document detailing the coding update. The document was posted on the CDC website. The guidance is consistent with current clinical knowledge about e-cigarette, or vaping, related disorders.
CDC noted in the document that the guidance “is intended to be used in conjunction with current ICD-10-CM classification,” and the codes provided “are intended to provide e-cigarette, or vaping, product use coding guidance only.”
The codes are intended to track a number of areas related to EVALI, including lung-related complications, poisoning and toxicity, and substance use, abuse, and dependence.
The following conditions associated with EVALI are covered in the new coding guidance:
- Bronchitis and pneumonitis caused by chemicals, gases, and fumes.
- Bronchitis and pneumonitis caused by chemicals, gases, fumes, and vapors; includes chemical pneumonitis.
- Pneumonitis caused by inhalation of oils and essences; includes lipoid pneumonia.
- Acute respiratory distress syndrome.
- Pulmonary eosinophilia, not elsewhere classified.
- Acute interstitial pneumonitis.
The document notes that the coding guidance has been approved by the National Center for Health Statistics, the American Health Information Management Association, the American Hospital Association, and the Centers for Medicare & Medicaid Services.
Readmission for COPD exacerbation upped in-hospital mortality risk
NEW ORLEANS – Reduction of readmission rates among individuals hospitalized for an acute exacerbation of COPD could reduce mortality and health care expenditures, results of a large, retrospective study suggest.
said researcher Anand Muthu Krishnan, MBBS, an from the University of Connecticut, Farmington.
“This is not a small problem,” Dr. Krishnan said in a podium presentation at the annual meeting of the American College of Chest Physicians. “The amount of money that can be saved can be put into primary care for curbing COPD and better patient outcomes, basically, if you’re able to put in checkpoints to stop this problem.”
Bundled care interventions by interdisciplinary teams have thus far proven effective at improving quality of care and improving process measures in this setting, said Dr. Krishnan.
The retrospective cohort study by Dr. Krishnan and colleagues included 530,229 adult patients in the 2016 National Readmission Database who had a principal diagnosis of acute COPD exacerbation. The mean age of the patients was 68 years, and 58% were female.
The rates of readmission at 30 days after discharge were 16.3% for any cause and 5.4% specifically for COPD, the researchers found. Of note, the in-hospital mortality rate increased from 1.1% to 3.8% during readmission (P less than .01), Dr. Krishnan said.
Readmissions were linked to a cumulative length of stay of 458,677 days, with corresponding hospital costs of $0.97 billion and charges of $4.0 billion; the COPD-specific readmissions were associated with cumulative length of stay of 132,026 days, costs of $253 million, and charges of $1 billion, Dr. Krishnan reported.
Dr. Krishnan and coauthors disclosed no relationships relevant to their study.
SOURCE: Krishnan AM et al. CHEST 2019. Abstract, doi: 10.1016/j.chest.2019.08.229.
NEW ORLEANS – Reduction of readmission rates among individuals hospitalized for an acute exacerbation of COPD could reduce mortality and health care expenditures, results of a large, retrospective study suggest.
said researcher Anand Muthu Krishnan, MBBS, an from the University of Connecticut, Farmington.
“This is not a small problem,” Dr. Krishnan said in a podium presentation at the annual meeting of the American College of Chest Physicians. “The amount of money that can be saved can be put into primary care for curbing COPD and better patient outcomes, basically, if you’re able to put in checkpoints to stop this problem.”
Bundled care interventions by interdisciplinary teams have thus far proven effective at improving quality of care and improving process measures in this setting, said Dr. Krishnan.
The retrospective cohort study by Dr. Krishnan and colleagues included 530,229 adult patients in the 2016 National Readmission Database who had a principal diagnosis of acute COPD exacerbation. The mean age of the patients was 68 years, and 58% were female.
The rates of readmission at 30 days after discharge were 16.3% for any cause and 5.4% specifically for COPD, the researchers found. Of note, the in-hospital mortality rate increased from 1.1% to 3.8% during readmission (P less than .01), Dr. Krishnan said.
Readmissions were linked to a cumulative length of stay of 458,677 days, with corresponding hospital costs of $0.97 billion and charges of $4.0 billion; the COPD-specific readmissions were associated with cumulative length of stay of 132,026 days, costs of $253 million, and charges of $1 billion, Dr. Krishnan reported.
Dr. Krishnan and coauthors disclosed no relationships relevant to their study.
SOURCE: Krishnan AM et al. CHEST 2019. Abstract, doi: 10.1016/j.chest.2019.08.229.
NEW ORLEANS – Reduction of readmission rates among individuals hospitalized for an acute exacerbation of COPD could reduce mortality and health care expenditures, results of a large, retrospective study suggest.
said researcher Anand Muthu Krishnan, MBBS, an from the University of Connecticut, Farmington.
“This is not a small problem,” Dr. Krishnan said in a podium presentation at the annual meeting of the American College of Chest Physicians. “The amount of money that can be saved can be put into primary care for curbing COPD and better patient outcomes, basically, if you’re able to put in checkpoints to stop this problem.”
Bundled care interventions by interdisciplinary teams have thus far proven effective at improving quality of care and improving process measures in this setting, said Dr. Krishnan.
The retrospective cohort study by Dr. Krishnan and colleagues included 530,229 adult patients in the 2016 National Readmission Database who had a principal diagnosis of acute COPD exacerbation. The mean age of the patients was 68 years, and 58% were female.
The rates of readmission at 30 days after discharge were 16.3% for any cause and 5.4% specifically for COPD, the researchers found. Of note, the in-hospital mortality rate increased from 1.1% to 3.8% during readmission (P less than .01), Dr. Krishnan said.
Readmissions were linked to a cumulative length of stay of 458,677 days, with corresponding hospital costs of $0.97 billion and charges of $4.0 billion; the COPD-specific readmissions were associated with cumulative length of stay of 132,026 days, costs of $253 million, and charges of $1 billion, Dr. Krishnan reported.
Dr. Krishnan and coauthors disclosed no relationships relevant to their study.
SOURCE: Krishnan AM et al. CHEST 2019. Abstract, doi: 10.1016/j.chest.2019.08.229.
REPORTING FROM CHEST 2019
Opioids, benzodiazepines carry greater risk of COPD-related hospitalization
according to recent research from Annals of the American Thoracic Society.
In addition, the risk of hospitalization because of respiratory events for patients with chronic obstructive pulmonary disease (COPD) was greater when opioid and benzodiazepine medications were combined, compared with patients who did not take either medication, Jacques G. Baillargeon, PhD, of the department of preventive medicine and community health at the University of Texas, Galveston, and colleagues wrote.
“Patients with COPD and their physicians should judiciously assess the risks and benefits of opioids and benzodiazepines, alone and in combination, and preferentially recommend nonopioid and nonbenzodiazepine approaches for pain, sleep, and anxiety management in patients with COPD,” the investigators wrote.
The researchers performed a case-control study of 3,232 Medicare beneficiary cases of COPD patients who were aged at least 66 years. Patients were included if they experienced a hospitalization related to a COPD-related adverse event with a respiratory diagnosis in 2014 and then matched to one or two control patients (total, 6,247 patients) based on age at hospitalization, gender, COPD medication, COPD complexity, obstructive sleep apnea, and socioeconomic status. COPD complexity was assigned to three levels (low, moderate, high) and calculated using the patient’s comorbid respiratory conditions and associated medical procedures in the 12 months prior to their hospitalization.
They found that, in the 30 days before COPD-related hospitalization, use of opioids was associated with greater likelihood of hospitalization (adjusted odds ratio, 1.73; 95% confidence interval, 1.52-1.97), as was use of benzodiazepines (aOR, 1.42; 95% CI, 1.21-1.66). When patients used both opioids and benzodiazepines, they had a significantly higher risk of hospitalization, compared with patients who did not use opioids or benzodiazepines (aOR, 2.32; 95% CI, 1.94-2.77).
In the 60 days prior to hospitalization, there was also a greater likelihood of hospitalization among COPD patients who used opioids (aOR, 1.66; 95% CI, 1.47-1.88), benzodiazepines (aOR, 1.44; 95% CI, 1.24-1.67), and both opioids and benzodiazepines (aOR, 2.27; 95% CI, 1.93-2.67); at 90 days, this higher risk of hospitalization persisted among COPD patients taking opioids (aOR, 1.58; 95% CI, 1.40-1.78), benzodiazepines (aOR, 1.40; 95% CI, 1.20-1.63), and both opioids and benzodiazepines (aOR, 2.21; 95% CI, 1.88-2.59).
The researchers acknowledged that one potential limitation in the study was how COPD diagnoses were obtained through coding performed by clinicians instead of from laboratory testing. Confounding by COPD indication and severity; use of over-the-counter medication or opioids and benzodiazepines received illegally; and lack of analyses of potential confounders such as diet, alcohol use, smoking status and herbal supplement use were other limitations.
This study was supported by an award from the National Center for Advancing Translational Sciences and National Institutes of Health. Dr. Baillargeon had no disclosures.
SOURCE: Baillargeon JG et al. Ann Am Thorac Soc. 2019 Oct 1. doi: 10.1513/AnnalsATS.201901-024OC.
according to recent research from Annals of the American Thoracic Society.
In addition, the risk of hospitalization because of respiratory events for patients with chronic obstructive pulmonary disease (COPD) was greater when opioid and benzodiazepine medications were combined, compared with patients who did not take either medication, Jacques G. Baillargeon, PhD, of the department of preventive medicine and community health at the University of Texas, Galveston, and colleagues wrote.
“Patients with COPD and their physicians should judiciously assess the risks and benefits of opioids and benzodiazepines, alone and in combination, and preferentially recommend nonopioid and nonbenzodiazepine approaches for pain, sleep, and anxiety management in patients with COPD,” the investigators wrote.
The researchers performed a case-control study of 3,232 Medicare beneficiary cases of COPD patients who were aged at least 66 years. Patients were included if they experienced a hospitalization related to a COPD-related adverse event with a respiratory diagnosis in 2014 and then matched to one or two control patients (total, 6,247 patients) based on age at hospitalization, gender, COPD medication, COPD complexity, obstructive sleep apnea, and socioeconomic status. COPD complexity was assigned to three levels (low, moderate, high) and calculated using the patient’s comorbid respiratory conditions and associated medical procedures in the 12 months prior to their hospitalization.
They found that, in the 30 days before COPD-related hospitalization, use of opioids was associated with greater likelihood of hospitalization (adjusted odds ratio, 1.73; 95% confidence interval, 1.52-1.97), as was use of benzodiazepines (aOR, 1.42; 95% CI, 1.21-1.66). When patients used both opioids and benzodiazepines, they had a significantly higher risk of hospitalization, compared with patients who did not use opioids or benzodiazepines (aOR, 2.32; 95% CI, 1.94-2.77).
In the 60 days prior to hospitalization, there was also a greater likelihood of hospitalization among COPD patients who used opioids (aOR, 1.66; 95% CI, 1.47-1.88), benzodiazepines (aOR, 1.44; 95% CI, 1.24-1.67), and both opioids and benzodiazepines (aOR, 2.27; 95% CI, 1.93-2.67); at 90 days, this higher risk of hospitalization persisted among COPD patients taking opioids (aOR, 1.58; 95% CI, 1.40-1.78), benzodiazepines (aOR, 1.40; 95% CI, 1.20-1.63), and both opioids and benzodiazepines (aOR, 2.21; 95% CI, 1.88-2.59).
The researchers acknowledged that one potential limitation in the study was how COPD diagnoses were obtained through coding performed by clinicians instead of from laboratory testing. Confounding by COPD indication and severity; use of over-the-counter medication or opioids and benzodiazepines received illegally; and lack of analyses of potential confounders such as diet, alcohol use, smoking status and herbal supplement use were other limitations.
This study was supported by an award from the National Center for Advancing Translational Sciences and National Institutes of Health. Dr. Baillargeon had no disclosures.
SOURCE: Baillargeon JG et al. Ann Am Thorac Soc. 2019 Oct 1. doi: 10.1513/AnnalsATS.201901-024OC.
according to recent research from Annals of the American Thoracic Society.
In addition, the risk of hospitalization because of respiratory events for patients with chronic obstructive pulmonary disease (COPD) was greater when opioid and benzodiazepine medications were combined, compared with patients who did not take either medication, Jacques G. Baillargeon, PhD, of the department of preventive medicine and community health at the University of Texas, Galveston, and colleagues wrote.
“Patients with COPD and their physicians should judiciously assess the risks and benefits of opioids and benzodiazepines, alone and in combination, and preferentially recommend nonopioid and nonbenzodiazepine approaches for pain, sleep, and anxiety management in patients with COPD,” the investigators wrote.
The researchers performed a case-control study of 3,232 Medicare beneficiary cases of COPD patients who were aged at least 66 years. Patients were included if they experienced a hospitalization related to a COPD-related adverse event with a respiratory diagnosis in 2014 and then matched to one or two control patients (total, 6,247 patients) based on age at hospitalization, gender, COPD medication, COPD complexity, obstructive sleep apnea, and socioeconomic status. COPD complexity was assigned to three levels (low, moderate, high) and calculated using the patient’s comorbid respiratory conditions and associated medical procedures in the 12 months prior to their hospitalization.
They found that, in the 30 days before COPD-related hospitalization, use of opioids was associated with greater likelihood of hospitalization (adjusted odds ratio, 1.73; 95% confidence interval, 1.52-1.97), as was use of benzodiazepines (aOR, 1.42; 95% CI, 1.21-1.66). When patients used both opioids and benzodiazepines, they had a significantly higher risk of hospitalization, compared with patients who did not use opioids or benzodiazepines (aOR, 2.32; 95% CI, 1.94-2.77).
In the 60 days prior to hospitalization, there was also a greater likelihood of hospitalization among COPD patients who used opioids (aOR, 1.66; 95% CI, 1.47-1.88), benzodiazepines (aOR, 1.44; 95% CI, 1.24-1.67), and both opioids and benzodiazepines (aOR, 2.27; 95% CI, 1.93-2.67); at 90 days, this higher risk of hospitalization persisted among COPD patients taking opioids (aOR, 1.58; 95% CI, 1.40-1.78), benzodiazepines (aOR, 1.40; 95% CI, 1.20-1.63), and both opioids and benzodiazepines (aOR, 2.21; 95% CI, 1.88-2.59).
The researchers acknowledged that one potential limitation in the study was how COPD diagnoses were obtained through coding performed by clinicians instead of from laboratory testing. Confounding by COPD indication and severity; use of over-the-counter medication or opioids and benzodiazepines received illegally; and lack of analyses of potential confounders such as diet, alcohol use, smoking status and herbal supplement use were other limitations.
This study was supported by an award from the National Center for Advancing Translational Sciences and National Institutes of Health. Dr. Baillargeon had no disclosures.
SOURCE: Baillargeon JG et al. Ann Am Thorac Soc. 2019 Oct 1. doi: 10.1513/AnnalsATS.201901-024OC.
FROM ANNALS OF THE AMERICAN THORACIC SOCIETY
Adolescent lung inflammation may trigger later MS
STOCKHOLM – Scott Montgomery, PhD, said at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis.
This is speculative, he readily acknowledged, but it is a hypothesis supported by multiple lines of evidence provided by separate Swedish national health care registry studies he has led that showed associations between pneumonia or infectious mononucleosis occurring in early adolescence and increased risk of later MS.
These findings are consistent with the well-established observations that two other causes of lung irritation – cigarette smoking and exposure to organic solvents – are also linked to increased risk of MS (Neurology. 2018 Jul 31;91[5]:e455-62), noted Dr. Montgomery, head of the clinical epidemiology research group at Örebro (Sweden) University.
Moreover, he and his coinvestigators also found in yet another Swedish national registry cohort study that one concussion during adolescence was independently associated with a statistically significant 1.22-fold increased risk of later MS, while two or more were linked to a 2.33-fold increased risk. In contrast, concussions occurring before age 11 years were not associated with any increased risk of MS, which suggests an age-defined period of susceptibility (Ann Neurol. 2017 Oct;82[4]:554-61).
“There seems to be greater brain resilience in childhood as compared to adolescence,” Dr. Montgomery commented.
The new Swedish registry pneumonia study included 6,109 Swedish MS patients and 49,479 controls matched for age, gender, and locale. In an analysis adjusted for education level and history of infectious mononucleosis, history of having pneumonia at age 11-15 years was independently associated with a 2.8-fold increased risk of subsequent MS. Pneumonia occurring at age 16-20 years was associated with a more modest 1.38-fold increased risk, which did not achieve statistical significance, while pneumonia up to age 5 years or at age 6-10 years conferred no increased risk. The investigators restricted their analysis to cases of pneumonia occurring up to age 20 years because that is younger than the typical age of MS onset. The age restriction sidestepped the potential for confounding by reverse causation since it is known that pneumonia occurs with increased frequency in patients with MS.
Because MS patients also have an increased risk of urinary tract infections, Dr. Montgomery and coinvestigators also analyzed the same pediatric data set for UTI rates broken down by 5-year age groups. Rates were similar in individuals who later developed MS and in controls, which suggests that the observed increase in MS risk associated with pneumonia in early adolescence was not an expression of an MS prodromal illness, he explained.
The investigators focused on pneumonia in childhood and adolescence as a potential trigger for MS because pneumonia results in more profound and prolonged inflammation than do other common respiratory illnesses. For example, pneumonia has been shown to be linked to increased risks of cardiovascular disease and chronic kidney disease for up to 5 years after the infection.
Developmentally, age 11-15 years is a period defined by peripubertal reorganization and synaptogenesis, while synaptic pruning and axonal myelination are on the agenda at age 16-20 years, Dr. Montgomery observed.
The study of infectious mononucleosis as a potential risk factor for MS included 4,527 Swedish MS patients and 3.2 million controls, all born during 1970-2000 and followed until 2014. In this analysis, infectious mononucleosis occurring at age 11-15 years was associated with the greatest risk of subsequent MS, with an associated 3.47-fold greater risk of the neurologic disease versus that seen in patients who did not have infectious mononucleosis at age 11-15 years
“It does look like a causal association between Epstein-Barr virus infection and subsequent MS,” according to Dr. Montgomery.
He noted that a plausible mechanism by which lung inflammation could predispose future MS has been put forth by German investigators. Using an animal model, they demonstrated that autoreactive T cells are prepared in bronchus-associated lymphoid tissue and attain a migratory profile allowing them to cross the blood-brain barrier and induce CNS autoimmune disease (Nature. 2012 Aug 30;488[7413]:675-9).
All of this, as Dr. Montgomery emphasized, is speculative at this point in regard to MS pathogenesis. What is not speculative, he continued, is the solid evidence that infection-related mortality after diagnosis of MS has gone down substantially in the current era of newer disease-modifying treatments, as he and his coinvestigators have demonstrated (Neurology. 2017 Aug 8;89[6]:555-62).
“People with MS, compared to the general population, are still at increased risk, but not nearly as much as the infection-related mortality risk present back in the 1960s-80s. So things have improved somewhat,” Dr. Montgomery said.
Which MS patients are at increased risk for mortality caused by infection? His Swedish national registry research demonstrates that the risk is essentially confined to patients with secondary or primary progressive MS or an Expanded Disability Status Scale score of 6 or more.
Another new study he presented at the meeting focused on the types of infections that are more common in a contemporary MS population than in MS-free individuals. This Swedish national cohort study included 6,602 patients diagnosed with MS during 2008-2016 and 61,828 age-, sex-, and location-matched controls. Infections serious enough to have resulted in hospitalization occurred 2.59 times more frequently in the MS population. The risk of meningitis and encephalitis was increased 6.16-fold, opportunistic infections were 2.72-fold more frequent, the risk of urinary tract and kidney infections was increased 2.44-fold, herpes virus infections were increased 2.32-fold, and the combined rate of pneumonia and influenza was roughly double that seen in the matched general population.
Dr. Montgomery reported receiving research funding from F. Hoffmann–La Roche, Novartis, and AstraZeneca and serving on an advisory board for IQVIA.
SOURCE: Montgomery S. ECTRIMS 2019, Abstract 270.
STOCKHOLM – Scott Montgomery, PhD, said at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis.
This is speculative, he readily acknowledged, but it is a hypothesis supported by multiple lines of evidence provided by separate Swedish national health care registry studies he has led that showed associations between pneumonia or infectious mononucleosis occurring in early adolescence and increased risk of later MS.
These findings are consistent with the well-established observations that two other causes of lung irritation – cigarette smoking and exposure to organic solvents – are also linked to increased risk of MS (Neurology. 2018 Jul 31;91[5]:e455-62), noted Dr. Montgomery, head of the clinical epidemiology research group at Örebro (Sweden) University.
Moreover, he and his coinvestigators also found in yet another Swedish national registry cohort study that one concussion during adolescence was independently associated with a statistically significant 1.22-fold increased risk of later MS, while two or more were linked to a 2.33-fold increased risk. In contrast, concussions occurring before age 11 years were not associated with any increased risk of MS, which suggests an age-defined period of susceptibility (Ann Neurol. 2017 Oct;82[4]:554-61).
“There seems to be greater brain resilience in childhood as compared to adolescence,” Dr. Montgomery commented.
The new Swedish registry pneumonia study included 6,109 Swedish MS patients and 49,479 controls matched for age, gender, and locale. In an analysis adjusted for education level and history of infectious mononucleosis, history of having pneumonia at age 11-15 years was independently associated with a 2.8-fold increased risk of subsequent MS. Pneumonia occurring at age 16-20 years was associated with a more modest 1.38-fold increased risk, which did not achieve statistical significance, while pneumonia up to age 5 years or at age 6-10 years conferred no increased risk. The investigators restricted their analysis to cases of pneumonia occurring up to age 20 years because that is younger than the typical age of MS onset. The age restriction sidestepped the potential for confounding by reverse causation since it is known that pneumonia occurs with increased frequency in patients with MS.
Because MS patients also have an increased risk of urinary tract infections, Dr. Montgomery and coinvestigators also analyzed the same pediatric data set for UTI rates broken down by 5-year age groups. Rates were similar in individuals who later developed MS and in controls, which suggests that the observed increase in MS risk associated with pneumonia in early adolescence was not an expression of an MS prodromal illness, he explained.
The investigators focused on pneumonia in childhood and adolescence as a potential trigger for MS because pneumonia results in more profound and prolonged inflammation than do other common respiratory illnesses. For example, pneumonia has been shown to be linked to increased risks of cardiovascular disease and chronic kidney disease for up to 5 years after the infection.
Developmentally, age 11-15 years is a period defined by peripubertal reorganization and synaptogenesis, while synaptic pruning and axonal myelination are on the agenda at age 16-20 years, Dr. Montgomery observed.
The study of infectious mononucleosis as a potential risk factor for MS included 4,527 Swedish MS patients and 3.2 million controls, all born during 1970-2000 and followed until 2014. In this analysis, infectious mononucleosis occurring at age 11-15 years was associated with the greatest risk of subsequent MS, with an associated 3.47-fold greater risk of the neurologic disease versus that seen in patients who did not have infectious mononucleosis at age 11-15 years
“It does look like a causal association between Epstein-Barr virus infection and subsequent MS,” according to Dr. Montgomery.
He noted that a plausible mechanism by which lung inflammation could predispose future MS has been put forth by German investigators. Using an animal model, they demonstrated that autoreactive T cells are prepared in bronchus-associated lymphoid tissue and attain a migratory profile allowing them to cross the blood-brain barrier and induce CNS autoimmune disease (Nature. 2012 Aug 30;488[7413]:675-9).
All of this, as Dr. Montgomery emphasized, is speculative at this point in regard to MS pathogenesis. What is not speculative, he continued, is the solid evidence that infection-related mortality after diagnosis of MS has gone down substantially in the current era of newer disease-modifying treatments, as he and his coinvestigators have demonstrated (Neurology. 2017 Aug 8;89[6]:555-62).
“People with MS, compared to the general population, are still at increased risk, but not nearly as much as the infection-related mortality risk present back in the 1960s-80s. So things have improved somewhat,” Dr. Montgomery said.
Which MS patients are at increased risk for mortality caused by infection? His Swedish national registry research demonstrates that the risk is essentially confined to patients with secondary or primary progressive MS or an Expanded Disability Status Scale score of 6 or more.
Another new study he presented at the meeting focused on the types of infections that are more common in a contemporary MS population than in MS-free individuals. This Swedish national cohort study included 6,602 patients diagnosed with MS during 2008-2016 and 61,828 age-, sex-, and location-matched controls. Infections serious enough to have resulted in hospitalization occurred 2.59 times more frequently in the MS population. The risk of meningitis and encephalitis was increased 6.16-fold, opportunistic infections were 2.72-fold more frequent, the risk of urinary tract and kidney infections was increased 2.44-fold, herpes virus infections were increased 2.32-fold, and the combined rate of pneumonia and influenza was roughly double that seen in the matched general population.
Dr. Montgomery reported receiving research funding from F. Hoffmann–La Roche, Novartis, and AstraZeneca and serving on an advisory board for IQVIA.
SOURCE: Montgomery S. ECTRIMS 2019, Abstract 270.
STOCKHOLM – Scott Montgomery, PhD, said at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis.
This is speculative, he readily acknowledged, but it is a hypothesis supported by multiple lines of evidence provided by separate Swedish national health care registry studies he has led that showed associations between pneumonia or infectious mononucleosis occurring in early adolescence and increased risk of later MS.
These findings are consistent with the well-established observations that two other causes of lung irritation – cigarette smoking and exposure to organic solvents – are also linked to increased risk of MS (Neurology. 2018 Jul 31;91[5]:e455-62), noted Dr. Montgomery, head of the clinical epidemiology research group at Örebro (Sweden) University.
Moreover, he and his coinvestigators also found in yet another Swedish national registry cohort study that one concussion during adolescence was independently associated with a statistically significant 1.22-fold increased risk of later MS, while two or more were linked to a 2.33-fold increased risk. In contrast, concussions occurring before age 11 years were not associated with any increased risk of MS, which suggests an age-defined period of susceptibility (Ann Neurol. 2017 Oct;82[4]:554-61).
“There seems to be greater brain resilience in childhood as compared to adolescence,” Dr. Montgomery commented.
The new Swedish registry pneumonia study included 6,109 Swedish MS patients and 49,479 controls matched for age, gender, and locale. In an analysis adjusted for education level and history of infectious mononucleosis, history of having pneumonia at age 11-15 years was independently associated with a 2.8-fold increased risk of subsequent MS. Pneumonia occurring at age 16-20 years was associated with a more modest 1.38-fold increased risk, which did not achieve statistical significance, while pneumonia up to age 5 years or at age 6-10 years conferred no increased risk. The investigators restricted their analysis to cases of pneumonia occurring up to age 20 years because that is younger than the typical age of MS onset. The age restriction sidestepped the potential for confounding by reverse causation since it is known that pneumonia occurs with increased frequency in patients with MS.
Because MS patients also have an increased risk of urinary tract infections, Dr. Montgomery and coinvestigators also analyzed the same pediatric data set for UTI rates broken down by 5-year age groups. Rates were similar in individuals who later developed MS and in controls, which suggests that the observed increase in MS risk associated with pneumonia in early adolescence was not an expression of an MS prodromal illness, he explained.
The investigators focused on pneumonia in childhood and adolescence as a potential trigger for MS because pneumonia results in more profound and prolonged inflammation than do other common respiratory illnesses. For example, pneumonia has been shown to be linked to increased risks of cardiovascular disease and chronic kidney disease for up to 5 years after the infection.
Developmentally, age 11-15 years is a period defined by peripubertal reorganization and synaptogenesis, while synaptic pruning and axonal myelination are on the agenda at age 16-20 years, Dr. Montgomery observed.
The study of infectious mononucleosis as a potential risk factor for MS included 4,527 Swedish MS patients and 3.2 million controls, all born during 1970-2000 and followed until 2014. In this analysis, infectious mononucleosis occurring at age 11-15 years was associated with the greatest risk of subsequent MS, with an associated 3.47-fold greater risk of the neurologic disease versus that seen in patients who did not have infectious mononucleosis at age 11-15 years
“It does look like a causal association between Epstein-Barr virus infection and subsequent MS,” according to Dr. Montgomery.
He noted that a plausible mechanism by which lung inflammation could predispose future MS has been put forth by German investigators. Using an animal model, they demonstrated that autoreactive T cells are prepared in bronchus-associated lymphoid tissue and attain a migratory profile allowing them to cross the blood-brain barrier and induce CNS autoimmune disease (Nature. 2012 Aug 30;488[7413]:675-9).
All of this, as Dr. Montgomery emphasized, is speculative at this point in regard to MS pathogenesis. What is not speculative, he continued, is the solid evidence that infection-related mortality after diagnosis of MS has gone down substantially in the current era of newer disease-modifying treatments, as he and his coinvestigators have demonstrated (Neurology. 2017 Aug 8;89[6]:555-62).
“People with MS, compared to the general population, are still at increased risk, but not nearly as much as the infection-related mortality risk present back in the 1960s-80s. So things have improved somewhat,” Dr. Montgomery said.
Which MS patients are at increased risk for mortality caused by infection? His Swedish national registry research demonstrates that the risk is essentially confined to patients with secondary or primary progressive MS or an Expanded Disability Status Scale score of 6 or more.
Another new study he presented at the meeting focused on the types of infections that are more common in a contemporary MS population than in MS-free individuals. This Swedish national cohort study included 6,602 patients diagnosed with MS during 2008-2016 and 61,828 age-, sex-, and location-matched controls. Infections serious enough to have resulted in hospitalization occurred 2.59 times more frequently in the MS population. The risk of meningitis and encephalitis was increased 6.16-fold, opportunistic infections were 2.72-fold more frequent, the risk of urinary tract and kidney infections was increased 2.44-fold, herpes virus infections were increased 2.32-fold, and the combined rate of pneumonia and influenza was roughly double that seen in the matched general population.
Dr. Montgomery reported receiving research funding from F. Hoffmann–La Roche, Novartis, and AstraZeneca and serving on an advisory board for IQVIA.
SOURCE: Montgomery S. ECTRIMS 2019, Abstract 270.
REPORTING FROM ECTRIMS 2019
ACIP approves child and adolescent vaccination schedule for 2020
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted unanimously to approve the child and adolescent immunization schedule for 2020.
by busy providers,” Candice Robinson, MD, MPH, of the CDC’s National Center for Immunization and Respiratory Diseases, said at the CDC’s October meeting of ACIP. Updates reflect changes in language in the adult vaccination schedule, notably the change in the definition of “contraindication.” The updated wording in the Notes substitutes “not recommended or contraindicated” instead of the word “contraindicated” only.
Another notable change was the addition of information on adolescent vaccination of children who received the meningococcal ACWY vaccine before 10 years of age. For “children in whom boosters are not recommended due to an ongoing or increased risk of meningococcal disease” (such as a healthy child traveling to an endemic area), they should receive MenACWY according to the recommended adolescent schedule. But those children for whom boosters are recommended because of increased disease risk from conditions including complement deficiency, HIV, or asplenia should “follow the booster schedule for persons at increased risk.”
Other changes include restructuring of the notes for the live attenuated influenza vaccine (LAIV) in special situations. The schedule now uses a bulleted list to show that LAIV should not be used in the following circumstances:
- Having history of severe allergic reaction to a previous vaccine or vaccine component.
- Using aspirin or a salicylate-containing medication.
- Being aged 2-4 years with a history of asthma or wheezing.
- Having immunocompromised conditions.
- Having anatomic or functional asplenia.
- Having cochlear implants.
- Experiencing cerebrospinal fluid–oropharyngeal communication.
- Having immunocompromised close contacts or caregivers.
- Being pregnant.
- Having received flu antivirals within the previous 48 hours.
In addition, language on shared clinical decision-making was added to the notes on the meningococcal B vaccine for adolescents and young adults aged 18-23 years not at increased risk. Based on shared clinical decision making, the recommendation is a “two-dose series of Bexsero at least 1 month apart” or “two-dose series of Trumenba at least 6 months apart; if dose two is administered earlier than 6 months, administer a third dose at least 4 months after dose two.”
Several vaccines’ Notes sections, including hepatitis B and meningococcal disease, added links to detailed recommendations in the corresponding issues of the CDC’s Morbidity and Mortality Weekly Report, to allow clinicians easy access to additional information.
View the current Child & Adolescent Vaccination Schedule here.
The ACIP members had no financial conflicts to disclose.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted unanimously to approve the child and adolescent immunization schedule for 2020.
by busy providers,” Candice Robinson, MD, MPH, of the CDC’s National Center for Immunization and Respiratory Diseases, said at the CDC’s October meeting of ACIP. Updates reflect changes in language in the adult vaccination schedule, notably the change in the definition of “contraindication.” The updated wording in the Notes substitutes “not recommended or contraindicated” instead of the word “contraindicated” only.
Another notable change was the addition of information on adolescent vaccination of children who received the meningococcal ACWY vaccine before 10 years of age. For “children in whom boosters are not recommended due to an ongoing or increased risk of meningococcal disease” (such as a healthy child traveling to an endemic area), they should receive MenACWY according to the recommended adolescent schedule. But those children for whom boosters are recommended because of increased disease risk from conditions including complement deficiency, HIV, or asplenia should “follow the booster schedule for persons at increased risk.”
Other changes include restructuring of the notes for the live attenuated influenza vaccine (LAIV) in special situations. The schedule now uses a bulleted list to show that LAIV should not be used in the following circumstances:
- Having history of severe allergic reaction to a previous vaccine or vaccine component.
- Using aspirin or a salicylate-containing medication.
- Being aged 2-4 years with a history of asthma or wheezing.
- Having immunocompromised conditions.
- Having anatomic or functional asplenia.
- Having cochlear implants.
- Experiencing cerebrospinal fluid–oropharyngeal communication.
- Having immunocompromised close contacts or caregivers.
- Being pregnant.
- Having received flu antivirals within the previous 48 hours.
In addition, language on shared clinical decision-making was added to the notes on the meningococcal B vaccine for adolescents and young adults aged 18-23 years not at increased risk. Based on shared clinical decision making, the recommendation is a “two-dose series of Bexsero at least 1 month apart” or “two-dose series of Trumenba at least 6 months apart; if dose two is administered earlier than 6 months, administer a third dose at least 4 months after dose two.”
Several vaccines’ Notes sections, including hepatitis B and meningococcal disease, added links to detailed recommendations in the corresponding issues of the CDC’s Morbidity and Mortality Weekly Report, to allow clinicians easy access to additional information.
View the current Child & Adolescent Vaccination Schedule here.
The ACIP members had no financial conflicts to disclose.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted unanimously to approve the child and adolescent immunization schedule for 2020.
by busy providers,” Candice Robinson, MD, MPH, of the CDC’s National Center for Immunization and Respiratory Diseases, said at the CDC’s October meeting of ACIP. Updates reflect changes in language in the adult vaccination schedule, notably the change in the definition of “contraindication.” The updated wording in the Notes substitutes “not recommended or contraindicated” instead of the word “contraindicated” only.
Another notable change was the addition of information on adolescent vaccination of children who received the meningococcal ACWY vaccine before 10 years of age. For “children in whom boosters are not recommended due to an ongoing or increased risk of meningococcal disease” (such as a healthy child traveling to an endemic area), they should receive MenACWY according to the recommended adolescent schedule. But those children for whom boosters are recommended because of increased disease risk from conditions including complement deficiency, HIV, or asplenia should “follow the booster schedule for persons at increased risk.”
Other changes include restructuring of the notes for the live attenuated influenza vaccine (LAIV) in special situations. The schedule now uses a bulleted list to show that LAIV should not be used in the following circumstances:
- Having history of severe allergic reaction to a previous vaccine or vaccine component.
- Using aspirin or a salicylate-containing medication.
- Being aged 2-4 years with a history of asthma or wheezing.
- Having immunocompromised conditions.
- Having anatomic or functional asplenia.
- Having cochlear implants.
- Experiencing cerebrospinal fluid–oropharyngeal communication.
- Having immunocompromised close contacts or caregivers.
- Being pregnant.
- Having received flu antivirals within the previous 48 hours.
In addition, language on shared clinical decision-making was added to the notes on the meningococcal B vaccine for adolescents and young adults aged 18-23 years not at increased risk. Based on shared clinical decision making, the recommendation is a “two-dose series of Bexsero at least 1 month apart” or “two-dose series of Trumenba at least 6 months apart; if dose two is administered earlier than 6 months, administer a third dose at least 4 months after dose two.”
Several vaccines’ Notes sections, including hepatitis B and meningococcal disease, added links to detailed recommendations in the corresponding issues of the CDC’s Morbidity and Mortality Weekly Report, to allow clinicians easy access to additional information.
View the current Child & Adolescent Vaccination Schedule here.
The ACIP members had no financial conflicts to disclose.
FROM AN ACIP MEETING
Vaping-linked injuries top 1,600 cases
according to the latest update provided by the Centers for Disease Control and Prevention. Thirty-four deaths have been confirmed.
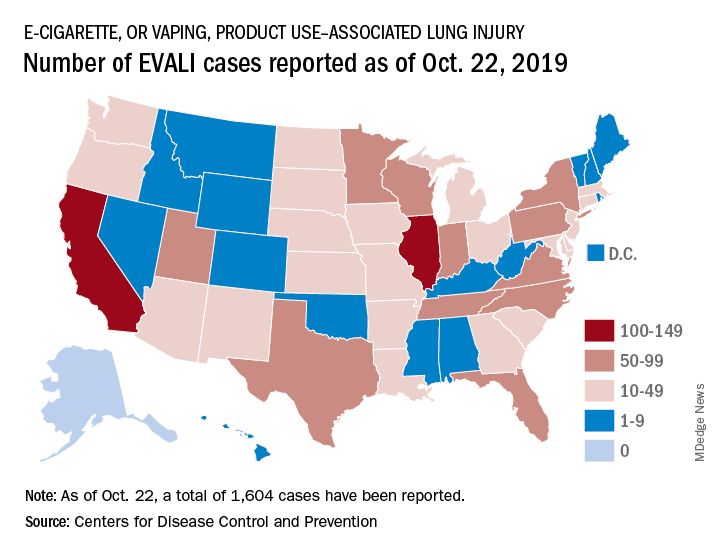
E-cigarette–linked lung injuries, now called EVALI, occurred in all U.S. states (except Alaska), the District of Columbia, and the U.S. Virgin Islands. Deaths have occurred in 24 states: Alabama, California (3), Connecticut, Delaware, Florida, Georgia (2), Illinois (2), Indiana (3), Kansas (2), Massachusetts, Michigan, Minnesota (3), Mississippi, Missouri, Montana, Nebraska, New Jersey, New York, Oregon (2), Pennsylvania, Tennessee, Texas, Utah, and Virginia. More deaths are under investigation.
The median age of deceased patients was 49 years and ranged from 17 to 75 years.
Data on age, sex, and substances used in e-cigarette, or vaping, products will be updated in the Morbidity and Mortality Weekly Report (MMWR) report being released on Friday, Oct. 25, 2019.
The CDC is now doing additional testing on available samples for chemical in the bronchoalveolar lavage fluid, blood, or urine, as well as lung biopsy or autopsy specimens. It also is validating methods for aerosol emission testing of case-associated product samples from vaping products and e-liquids.
For more information and resources visit For the Public, For Healthcare Providers, and For State and Local Health Departments pages, as well as the CDC’s Publications and Resources page.
according to the latest update provided by the Centers for Disease Control and Prevention. Thirty-four deaths have been confirmed.

E-cigarette–linked lung injuries, now called EVALI, occurred in all U.S. states (except Alaska), the District of Columbia, and the U.S. Virgin Islands. Deaths have occurred in 24 states: Alabama, California (3), Connecticut, Delaware, Florida, Georgia (2), Illinois (2), Indiana (3), Kansas (2), Massachusetts, Michigan, Minnesota (3), Mississippi, Missouri, Montana, Nebraska, New Jersey, New York, Oregon (2), Pennsylvania, Tennessee, Texas, Utah, and Virginia. More deaths are under investigation.
The median age of deceased patients was 49 years and ranged from 17 to 75 years.
Data on age, sex, and substances used in e-cigarette, or vaping, products will be updated in the Morbidity and Mortality Weekly Report (MMWR) report being released on Friday, Oct. 25, 2019.
The CDC is now doing additional testing on available samples for chemical in the bronchoalveolar lavage fluid, blood, or urine, as well as lung biopsy or autopsy specimens. It also is validating methods for aerosol emission testing of case-associated product samples from vaping products and e-liquids.
For more information and resources visit For the Public, For Healthcare Providers, and For State and Local Health Departments pages, as well as the CDC’s Publications and Resources page.
according to the latest update provided by the Centers for Disease Control and Prevention. Thirty-four deaths have been confirmed.

E-cigarette–linked lung injuries, now called EVALI, occurred in all U.S. states (except Alaska), the District of Columbia, and the U.S. Virgin Islands. Deaths have occurred in 24 states: Alabama, California (3), Connecticut, Delaware, Florida, Georgia (2), Illinois (2), Indiana (3), Kansas (2), Massachusetts, Michigan, Minnesota (3), Mississippi, Missouri, Montana, Nebraska, New Jersey, New York, Oregon (2), Pennsylvania, Tennessee, Texas, Utah, and Virginia. More deaths are under investigation.
The median age of deceased patients was 49 years and ranged from 17 to 75 years.
Data on age, sex, and substances used in e-cigarette, or vaping, products will be updated in the Morbidity and Mortality Weekly Report (MMWR) report being released on Friday, Oct. 25, 2019.
The CDC is now doing additional testing on available samples for chemical in the bronchoalveolar lavage fluid, blood, or urine, as well as lung biopsy or autopsy specimens. It also is validating methods for aerosol emission testing of case-associated product samples from vaping products and e-liquids.
For more information and resources visit For the Public, For Healthcare Providers, and For State and Local Health Departments pages, as well as the CDC’s Publications and Resources page.
REPORTING FROM THE CDC
ACIP approves 2020 adult vaccination schedule
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted unanimously to approve the adult immunization schedule for 2020, although some fine-tuning may occur before publication.
“Some of the wordsmithing may be done later,” ACIP executive secretary Amanda Cohn, MD, said at the ACIP October meeting.
Key updates to the schedule included a change in wording for the definition of the red bars on the table to include “not recommended or contraindicated” instead of only the word “contraindicated.” Committee members were especially interested in changing this wording to guide clinicians in use of the live attenuated influenza vaccine because of its potential value in vaccinating health care personnel.
Other updates include language that vaccination of adolescents and young adults aged 16-23 years who are not at increased risk for meningococcal disease should be vaccinated as follows: “Based on shared clinical decision making, 2-dose series MenB-4C at least 1 month apart or 2-dose series MenB-FHbp at 0, 6 months.”
Similarly, clinical decision-making language was added to the notes for the pneumococcal polysaccharide vaccine (PPSV23) and the 13-valent pneumococcal conjugate vaccine (PCV13).
The routine vaccination calls for only one dose of PPSV23 given on or after the individual’s 65th birthday. Then, based on shared clinical decision making, a dose of PCV13 is recommended for immunocompetent individuals aged 65 years and older. The notes also state that, based on shared clinical decision making, PCV13 and PPSV23 should not be given in the same visit and, if both will be given, PCV13 should be first and should be given 1 year before PPSV23. In addition, “PPSV23 should be given at least 5 years after any previous PPSV23 dose.”
The schedule also adds shared clinical decision making to the notes on human papillomavirus vaccination for adults aged 27-45 years.
The committee members acknowledged the increasing complexity of the adult vaccination schedule, but several members agreed that it is accessible to many clinicians.
“We can’t let the perfect be the enemy of the good” said Jason Goldman, MD, liaison representing the American College of Physicians. “Those who want to learn the schedule will learn it; the health system will learn it,” even if not every specialist does.
The table “is something to draw you in,” said Sandra Fryhofer, MD, an internist who is liaison for the American Medical Association. The notes provide more details.
More specific information about contraindications for patients with cochlear implants, which also came up in the discussion, may be added to the schedule at a later date.
View the current adult vaccination schedule here.
The ACIP members had no financial conflicts to disclose.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted unanimously to approve the adult immunization schedule for 2020, although some fine-tuning may occur before publication.
“Some of the wordsmithing may be done later,” ACIP executive secretary Amanda Cohn, MD, said at the ACIP October meeting.
Key updates to the schedule included a change in wording for the definition of the red bars on the table to include “not recommended or contraindicated” instead of only the word “contraindicated.” Committee members were especially interested in changing this wording to guide clinicians in use of the live attenuated influenza vaccine because of its potential value in vaccinating health care personnel.
Other updates include language that vaccination of adolescents and young adults aged 16-23 years who are not at increased risk for meningococcal disease should be vaccinated as follows: “Based on shared clinical decision making, 2-dose series MenB-4C at least 1 month apart or 2-dose series MenB-FHbp at 0, 6 months.”
Similarly, clinical decision-making language was added to the notes for the pneumococcal polysaccharide vaccine (PPSV23) and the 13-valent pneumococcal conjugate vaccine (PCV13).
The routine vaccination calls for only one dose of PPSV23 given on or after the individual’s 65th birthday. Then, based on shared clinical decision making, a dose of PCV13 is recommended for immunocompetent individuals aged 65 years and older. The notes also state that, based on shared clinical decision making, PCV13 and PPSV23 should not be given in the same visit and, if both will be given, PCV13 should be first and should be given 1 year before PPSV23. In addition, “PPSV23 should be given at least 5 years after any previous PPSV23 dose.”
The schedule also adds shared clinical decision making to the notes on human papillomavirus vaccination for adults aged 27-45 years.
The committee members acknowledged the increasing complexity of the adult vaccination schedule, but several members agreed that it is accessible to many clinicians.
“We can’t let the perfect be the enemy of the good” said Jason Goldman, MD, liaison representing the American College of Physicians. “Those who want to learn the schedule will learn it; the health system will learn it,” even if not every specialist does.
The table “is something to draw you in,” said Sandra Fryhofer, MD, an internist who is liaison for the American Medical Association. The notes provide more details.
More specific information about contraindications for patients with cochlear implants, which also came up in the discussion, may be added to the schedule at a later date.
View the current adult vaccination schedule here.
The ACIP members had no financial conflicts to disclose.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted unanimously to approve the adult immunization schedule for 2020, although some fine-tuning may occur before publication.
“Some of the wordsmithing may be done later,” ACIP executive secretary Amanda Cohn, MD, said at the ACIP October meeting.
Key updates to the schedule included a change in wording for the definition of the red bars on the table to include “not recommended or contraindicated” instead of only the word “contraindicated.” Committee members were especially interested in changing this wording to guide clinicians in use of the live attenuated influenza vaccine because of its potential value in vaccinating health care personnel.
Other updates include language that vaccination of adolescents and young adults aged 16-23 years who are not at increased risk for meningococcal disease should be vaccinated as follows: “Based on shared clinical decision making, 2-dose series MenB-4C at least 1 month apart or 2-dose series MenB-FHbp at 0, 6 months.”
Similarly, clinical decision-making language was added to the notes for the pneumococcal polysaccharide vaccine (PPSV23) and the 13-valent pneumococcal conjugate vaccine (PCV13).
The routine vaccination calls for only one dose of PPSV23 given on or after the individual’s 65th birthday. Then, based on shared clinical decision making, a dose of PCV13 is recommended for immunocompetent individuals aged 65 years and older. The notes also state that, based on shared clinical decision making, PCV13 and PPSV23 should not be given in the same visit and, if both will be given, PCV13 should be first and should be given 1 year before PPSV23. In addition, “PPSV23 should be given at least 5 years after any previous PPSV23 dose.”
The schedule also adds shared clinical decision making to the notes on human papillomavirus vaccination for adults aged 27-45 years.
The committee members acknowledged the increasing complexity of the adult vaccination schedule, but several members agreed that it is accessible to many clinicians.
“We can’t let the perfect be the enemy of the good” said Jason Goldman, MD, liaison representing the American College of Physicians. “Those who want to learn the schedule will learn it; the health system will learn it,” even if not every specialist does.
The table “is something to draw you in,” said Sandra Fryhofer, MD, an internist who is liaison for the American Medical Association. The notes provide more details.
More specific information about contraindications for patients with cochlear implants, which also came up in the discussion, may be added to the schedule at a later date.
View the current adult vaccination schedule here.
The ACIP members had no financial conflicts to disclose.
ACIP plans flu review for older adults
according to data presented at a meeting of the Centers for Disease Control and Prevention’s ACIP.
Lynette Brammer of the CDC’s National Center for Immunization and Respiratory Diseases (NCIRD) presented a surveillance update of the flu season in the United States so far. Overall, the influenza A(H3N2) viruses are predominant, although dominance varies in different regions of the country, and it is too soon to predict what strain will dominate later in the season.
“While two of the four vaccine components were updated for the Southern Hemisphere, the components selected for the 2019-2020 Northern Hemisphere vaccine, at this time, look appropriate for the season,” she said.
In other flu news, Lisa Groskopf, MD, of the NCIRD discussed the influenza work group’s plans for a meta-analysis to assess the relative benefit of different vaccines for older adults, in light of the growing variety of products available.
Currently, no preferential recommendations have been made for a specific vaccine for a particular age group. “There’s a dearth of data comparing these vaccines to one another,” said Dr. Groskopf. She added that, because vaccine effectiveness varies by season, the generalizability of effectiveness data is another challenge.
The work group’s systematic review and meta-analysis is designed to compare the high-dose inactivated influenza vaccine (HD-IIV), the adjuvanted inactivated influenza vaccine (aIIV), and the recombinant influenza vaccine (RIV). The study will include adults aged 65 years and older who receive trivalent or quadrivalent HD-IIV, aIIV, or RIV, compared with those who receive another influenza vaccine, a noninfluenza control vaccine, placebo, or no vaccine. The outcomes will include data on safety and effectiveness of the vaccines, Dr. Groskopf said.
In addition to safety and effectiveness, manufacturers such as Sanofi Pasteur continue to collect data on the success of available vaccines and develop new ones. Lee-Jah Chang, MD, of Sanofi Pasteur presented results of a noninferiority study of the company’s investigational high-dose quadrivalent influenza vaccine (QIV-HD; including two prevailing B viruses) versus the high-dose trivalent influenza vaccine (TID-HD). The study was conducted at 35 sites in the United States and included 2,670 adults aged 65 years and older.
Overall, the reactogenicity profile for patients given QIV-HD was similar to that of TID-HD, and approximately 5% of patients in the QIV group reported an immediate adverse event, Dr. Chang said. However, no related deaths or related adverse events of special interest occurred in any of the study groups.
Sanofi plans to pursue licensure of the QIV-HD vaccine, with a Center for Biologics Evaluation and Research action date of Nov. 4, 2019, said Dr. Chang. If the vaccine is licensed, it should be available for purchase by health care providers in the first quarter of 2020.
The ACIP members had no financial conflicts to disclose.
according to data presented at a meeting of the Centers for Disease Control and Prevention’s ACIP.
Lynette Brammer of the CDC’s National Center for Immunization and Respiratory Diseases (NCIRD) presented a surveillance update of the flu season in the United States so far. Overall, the influenza A(H3N2) viruses are predominant, although dominance varies in different regions of the country, and it is too soon to predict what strain will dominate later in the season.
“While two of the four vaccine components were updated for the Southern Hemisphere, the components selected for the 2019-2020 Northern Hemisphere vaccine, at this time, look appropriate for the season,” she said.
In other flu news, Lisa Groskopf, MD, of the NCIRD discussed the influenza work group’s plans for a meta-analysis to assess the relative benefit of different vaccines for older adults, in light of the growing variety of products available.
Currently, no preferential recommendations have been made for a specific vaccine for a particular age group. “There’s a dearth of data comparing these vaccines to one another,” said Dr. Groskopf. She added that, because vaccine effectiveness varies by season, the generalizability of effectiveness data is another challenge.
The work group’s systematic review and meta-analysis is designed to compare the high-dose inactivated influenza vaccine (HD-IIV), the adjuvanted inactivated influenza vaccine (aIIV), and the recombinant influenza vaccine (RIV). The study will include adults aged 65 years and older who receive trivalent or quadrivalent HD-IIV, aIIV, or RIV, compared with those who receive another influenza vaccine, a noninfluenza control vaccine, placebo, or no vaccine. The outcomes will include data on safety and effectiveness of the vaccines, Dr. Groskopf said.
In addition to safety and effectiveness, manufacturers such as Sanofi Pasteur continue to collect data on the success of available vaccines and develop new ones. Lee-Jah Chang, MD, of Sanofi Pasteur presented results of a noninferiority study of the company’s investigational high-dose quadrivalent influenza vaccine (QIV-HD; including two prevailing B viruses) versus the high-dose trivalent influenza vaccine (TID-HD). The study was conducted at 35 sites in the United States and included 2,670 adults aged 65 years and older.
Overall, the reactogenicity profile for patients given QIV-HD was similar to that of TID-HD, and approximately 5% of patients in the QIV group reported an immediate adverse event, Dr. Chang said. However, no related deaths or related adverse events of special interest occurred in any of the study groups.
Sanofi plans to pursue licensure of the QIV-HD vaccine, with a Center for Biologics Evaluation and Research action date of Nov. 4, 2019, said Dr. Chang. If the vaccine is licensed, it should be available for purchase by health care providers in the first quarter of 2020.
The ACIP members had no financial conflicts to disclose.
according to data presented at a meeting of the Centers for Disease Control and Prevention’s ACIP.
Lynette Brammer of the CDC’s National Center for Immunization and Respiratory Diseases (NCIRD) presented a surveillance update of the flu season in the United States so far. Overall, the influenza A(H3N2) viruses are predominant, although dominance varies in different regions of the country, and it is too soon to predict what strain will dominate later in the season.
“While two of the four vaccine components were updated for the Southern Hemisphere, the components selected for the 2019-2020 Northern Hemisphere vaccine, at this time, look appropriate for the season,” she said.
In other flu news, Lisa Groskopf, MD, of the NCIRD discussed the influenza work group’s plans for a meta-analysis to assess the relative benefit of different vaccines for older adults, in light of the growing variety of products available.
Currently, no preferential recommendations have been made for a specific vaccine for a particular age group. “There’s a dearth of data comparing these vaccines to one another,” said Dr. Groskopf. She added that, because vaccine effectiveness varies by season, the generalizability of effectiveness data is another challenge.
The work group’s systematic review and meta-analysis is designed to compare the high-dose inactivated influenza vaccine (HD-IIV), the adjuvanted inactivated influenza vaccine (aIIV), and the recombinant influenza vaccine (RIV). The study will include adults aged 65 years and older who receive trivalent or quadrivalent HD-IIV, aIIV, or RIV, compared with those who receive another influenza vaccine, a noninfluenza control vaccine, placebo, or no vaccine. The outcomes will include data on safety and effectiveness of the vaccines, Dr. Groskopf said.
In addition to safety and effectiveness, manufacturers such as Sanofi Pasteur continue to collect data on the success of available vaccines and develop new ones. Lee-Jah Chang, MD, of Sanofi Pasteur presented results of a noninferiority study of the company’s investigational high-dose quadrivalent influenza vaccine (QIV-HD; including two prevailing B viruses) versus the high-dose trivalent influenza vaccine (TID-HD). The study was conducted at 35 sites in the United States and included 2,670 adults aged 65 years and older.
Overall, the reactogenicity profile for patients given QIV-HD was similar to that of TID-HD, and approximately 5% of patients in the QIV group reported an immediate adverse event, Dr. Chang said. However, no related deaths or related adverse events of special interest occurred in any of the study groups.
Sanofi plans to pursue licensure of the QIV-HD vaccine, with a Center for Biologics Evaluation and Research action date of Nov. 4, 2019, said Dr. Chang. If the vaccine is licensed, it should be available for purchase by health care providers in the first quarter of 2020.
The ACIP members had no financial conflicts to disclose.
REPORTING FROM AN ACIP MEETING
ACIP recommends two options for pertussis vaccination
Either the Tdap or Td vaccine is an acceptable option for pertussis vaccination in most situations, recommended the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
In a unanimous 14-0 vote at the October meeting, based on the immunization schedule for persons aged 7 years and older.
Safety data showed no differences in safety concerns between Tdap and Td, including data from pregnant women, said Fiona Havers, MD, of the CDC’s National Center for Immunization and Respiratory Diseases (NCIRD), Atlanta.
Several of the ACIP members noted that the revised language to include both Tdap and Td reflects the increased use of Tdap and allows for maximum flexibility in clinical settings.
The revised language advises that booster doses of “either Td or Tdap” every 10 years throughout life are recommended for continued protection against tetanus and diphtheria. In addition, either Td or Tdap should be used if a tetanus toxoid–containing vaccine is indicated for prophylaxis in nonpregnant individuals.
For catch-up recommendations, which also apply to pregnant women, the committee approved the following wording for a series of three doses for individuals aged 7-18 years and 19 years and older who have never been vaccinated, that “the preferred schedule is a dose of Tdap (preferably the first dose), followed by either Tdap or Td at least 4 weeks afterward and another dose of either Td or Tdap 6-12 months later.” Individuals in these same age groups who are not fully vaccinated should receive one dose of Tdap, and a dose of either Td or Tdap if additional doses are needed.
The committee also voted unanimously 14-0 to accept the updated wording for pertussis vaccination in the Vaccines for Children program.
The ACIP members had no financial conflicts to disclose.
Either the Tdap or Td vaccine is an acceptable option for pertussis vaccination in most situations, recommended the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
In a unanimous 14-0 vote at the October meeting, based on the immunization schedule for persons aged 7 years and older.
Safety data showed no differences in safety concerns between Tdap and Td, including data from pregnant women, said Fiona Havers, MD, of the CDC’s National Center for Immunization and Respiratory Diseases (NCIRD), Atlanta.
Several of the ACIP members noted that the revised language to include both Tdap and Td reflects the increased use of Tdap and allows for maximum flexibility in clinical settings.
The revised language advises that booster doses of “either Td or Tdap” every 10 years throughout life are recommended for continued protection against tetanus and diphtheria. In addition, either Td or Tdap should be used if a tetanus toxoid–containing vaccine is indicated for prophylaxis in nonpregnant individuals.
For catch-up recommendations, which also apply to pregnant women, the committee approved the following wording for a series of three doses for individuals aged 7-18 years and 19 years and older who have never been vaccinated, that “the preferred schedule is a dose of Tdap (preferably the first dose), followed by either Tdap or Td at least 4 weeks afterward and another dose of either Td or Tdap 6-12 months later.” Individuals in these same age groups who are not fully vaccinated should receive one dose of Tdap, and a dose of either Td or Tdap if additional doses are needed.
The committee also voted unanimously 14-0 to accept the updated wording for pertussis vaccination in the Vaccines for Children program.
The ACIP members had no financial conflicts to disclose.
Either the Tdap or Td vaccine is an acceptable option for pertussis vaccination in most situations, recommended the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
In a unanimous 14-0 vote at the October meeting, based on the immunization schedule for persons aged 7 years and older.
Safety data showed no differences in safety concerns between Tdap and Td, including data from pregnant women, said Fiona Havers, MD, of the CDC’s National Center for Immunization and Respiratory Diseases (NCIRD), Atlanta.
Several of the ACIP members noted that the revised language to include both Tdap and Td reflects the increased use of Tdap and allows for maximum flexibility in clinical settings.
The revised language advises that booster doses of “either Td or Tdap” every 10 years throughout life are recommended for continued protection against tetanus and diphtheria. In addition, either Td or Tdap should be used if a tetanus toxoid–containing vaccine is indicated for prophylaxis in nonpregnant individuals.
For catch-up recommendations, which also apply to pregnant women, the committee approved the following wording for a series of three doses for individuals aged 7-18 years and 19 years and older who have never been vaccinated, that “the preferred schedule is a dose of Tdap (preferably the first dose), followed by either Tdap or Td at least 4 weeks afterward and another dose of either Td or Tdap 6-12 months later.” Individuals in these same age groups who are not fully vaccinated should receive one dose of Tdap, and a dose of either Td or Tdap if additional doses are needed.
The committee also voted unanimously 14-0 to accept the updated wording for pertussis vaccination in the Vaccines for Children program.
The ACIP members had no financial conflicts to disclose.
FROM AN ACIP MEETING
New test edges closer to rapid, accurate ID of active TB
A new point-of-care assay designed with machine learning offers improved accuracy for rapid identification of active tuberculosis (TB) infection, according to investigators.
, reported lead author Rushdy Ahmad, PhD, of the Broad Institute of MIT and Harvard in Cambridge, Mass., and colleagues. When fully developed, such a test could improve interventions for the most vulnerable patients, such as those with HIV, among whom TB often goes undiagnosed.
“Rapid and accurate diagnosis of active TB with current sputum-based diagnostic tools remains challenging in high-burden, resource-limited settings,” the investigators wrote. Their report is in Science Translational Medicine.
They went on to explain the gap that currently exists between microscopy, which is operator dependent and insensitive, and newer technologies, such as nucleic acid amplification, which are more sensitive but heavily resource dependent. “Furthermore, two of the most vulnerable and highly affected groups – young children and adults with HIV infection – are unlikely to be diagnosed using sputum because of difficulty obtaining sputum and low bacillary loads in the sample.”
To look for a more practical option, the investigators drew blood from 406 patients with chronic cough. Then, using a bead-based immunoassay with machine learning, the investigators identified four blood proteins associated with active TB infection: interleukin-6 (IL-6), IL-8, IL-18, and vascular endothelial growth factor (VEGF). Blind validation of 317 samples from patients with chronic cough in Asia, Africa, and South America showed that the four biomarkers offered a sensitivity of 80% and a specificity of 65%. By adding a fifth biomarker, an antibody against TB antigen Ag85B, the investigators were able to raise accuracy figures to 86% sensitivity and 69% specificity.
Adding even more biomarkers could theoretically raise accuracy even further, according to the investigators. The WHO minimal performance thresholds are 90% sensitivity and 70% specificity, with optimal targets slightly higher, at 95% sensitivity and 80% specificity. Although these standards have not yet been met, the investigators plan on testing the existing assay in real-world scenarios while simultaneously aiming to make it better.
“A near-term goal is ... to incrementally improve the marker panel up to an anticipated 6- to 10-plex assay,” the investigators wrote. “However, given the urgency of the problem, the possibility of incremental improvements will not delay platform refinement and field testing.”
The Bill and Melinda Gates Foundation funded the study. The investigators reported additional relationships with Quanterix Corporation and FIND.
SOURCE: Ahmad et al. Sci Transl Med. 2019 Oct 23. doi: 10.1126/scitranslmed.aaw8287.
A new point-of-care assay designed with machine learning offers improved accuracy for rapid identification of active tuberculosis (TB) infection, according to investigators.
, reported lead author Rushdy Ahmad, PhD, of the Broad Institute of MIT and Harvard in Cambridge, Mass., and colleagues. When fully developed, such a test could improve interventions for the most vulnerable patients, such as those with HIV, among whom TB often goes undiagnosed.
“Rapid and accurate diagnosis of active TB with current sputum-based diagnostic tools remains challenging in high-burden, resource-limited settings,” the investigators wrote. Their report is in Science Translational Medicine.
They went on to explain the gap that currently exists between microscopy, which is operator dependent and insensitive, and newer technologies, such as nucleic acid amplification, which are more sensitive but heavily resource dependent. “Furthermore, two of the most vulnerable and highly affected groups – young children and adults with HIV infection – are unlikely to be diagnosed using sputum because of difficulty obtaining sputum and low bacillary loads in the sample.”
To look for a more practical option, the investigators drew blood from 406 patients with chronic cough. Then, using a bead-based immunoassay with machine learning, the investigators identified four blood proteins associated with active TB infection: interleukin-6 (IL-6), IL-8, IL-18, and vascular endothelial growth factor (VEGF). Blind validation of 317 samples from patients with chronic cough in Asia, Africa, and South America showed that the four biomarkers offered a sensitivity of 80% and a specificity of 65%. By adding a fifth biomarker, an antibody against TB antigen Ag85B, the investigators were able to raise accuracy figures to 86% sensitivity and 69% specificity.
Adding even more biomarkers could theoretically raise accuracy even further, according to the investigators. The WHO minimal performance thresholds are 90% sensitivity and 70% specificity, with optimal targets slightly higher, at 95% sensitivity and 80% specificity. Although these standards have not yet been met, the investigators plan on testing the existing assay in real-world scenarios while simultaneously aiming to make it better.
“A near-term goal is ... to incrementally improve the marker panel up to an anticipated 6- to 10-plex assay,” the investigators wrote. “However, given the urgency of the problem, the possibility of incremental improvements will not delay platform refinement and field testing.”
The Bill and Melinda Gates Foundation funded the study. The investigators reported additional relationships with Quanterix Corporation and FIND.
SOURCE: Ahmad et al. Sci Transl Med. 2019 Oct 23. doi: 10.1126/scitranslmed.aaw8287.
A new point-of-care assay designed with machine learning offers improved accuracy for rapid identification of active tuberculosis (TB) infection, according to investigators.
, reported lead author Rushdy Ahmad, PhD, of the Broad Institute of MIT and Harvard in Cambridge, Mass., and colleagues. When fully developed, such a test could improve interventions for the most vulnerable patients, such as those with HIV, among whom TB often goes undiagnosed.
“Rapid and accurate diagnosis of active TB with current sputum-based diagnostic tools remains challenging in high-burden, resource-limited settings,” the investigators wrote. Their report is in Science Translational Medicine.
They went on to explain the gap that currently exists between microscopy, which is operator dependent and insensitive, and newer technologies, such as nucleic acid amplification, which are more sensitive but heavily resource dependent. “Furthermore, two of the most vulnerable and highly affected groups – young children and adults with HIV infection – are unlikely to be diagnosed using sputum because of difficulty obtaining sputum and low bacillary loads in the sample.”
To look for a more practical option, the investigators drew blood from 406 patients with chronic cough. Then, using a bead-based immunoassay with machine learning, the investigators identified four blood proteins associated with active TB infection: interleukin-6 (IL-6), IL-8, IL-18, and vascular endothelial growth factor (VEGF). Blind validation of 317 samples from patients with chronic cough in Asia, Africa, and South America showed that the four biomarkers offered a sensitivity of 80% and a specificity of 65%. By adding a fifth biomarker, an antibody against TB antigen Ag85B, the investigators were able to raise accuracy figures to 86% sensitivity and 69% specificity.
Adding even more biomarkers could theoretically raise accuracy even further, according to the investigators. The WHO minimal performance thresholds are 90% sensitivity and 70% specificity, with optimal targets slightly higher, at 95% sensitivity and 80% specificity. Although these standards have not yet been met, the investigators plan on testing the existing assay in real-world scenarios while simultaneously aiming to make it better.
“A near-term goal is ... to incrementally improve the marker panel up to an anticipated 6- to 10-plex assay,” the investigators wrote. “However, given the urgency of the problem, the possibility of incremental improvements will not delay platform refinement and field testing.”
The Bill and Melinda Gates Foundation funded the study. The investigators reported additional relationships with Quanterix Corporation and FIND.
SOURCE: Ahmad et al. Sci Transl Med. 2019 Oct 23. doi: 10.1126/scitranslmed.aaw8287.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: A new point-of-care assay designed with machine learning offers improved accuracy for rapid identification of active tuberculosis (TB) infection.
Major finding: The assay had a sensitivity of 86%.
Study details: A machine learning and validation study involving patients with chronic cough from multiple countries.
Disclosures: The Bill and Melinda Gates Foundation funded the study. The investigators reported relationships with Quanterix Corporation and FIND.
Source: Ahmad et al. Sci Transl Med. 2019 Oct 23. doi: 10.1126/scitranslmed.aaw8287.