User login
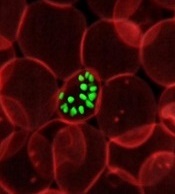
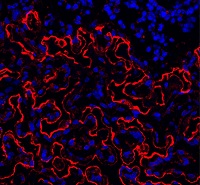
Team identifies proteins that give malaria parasites ‘superpower’
Researchers say they have identified proteins that enable the malaria parasite Plasmodium falciparum to “walk through cell walls.”
The team believes the proteins—SPECT and PLP1—could be targeted to develop antimalarial drugs or vaccines.
Justin Boddey, PhD, of the Walter and Eliza Hall Institute of Medical Research in Parkville, Victoria, Australia, and his colleagues described this research in Cell Reports.
“The malaria infection cycle begins with a mosquito bite, when parasites are injected into the skin, and then rapidly move to the liver,” Dr Boddey explained. “We have shown that P falciparum employs a technique called cell traversal to quickly move through host cells in their path as they seek out liver cells to infect.”
“Our study identified that P falciparum parasites traverse human cells—effectively walking through cell walls—using 2 proteins called SPECT and PLP1 to achieve this superpower. This allows parasites to get from the skin to the liver very quickly following a mosquito bite.”
Dr Boddey said pinpointing these proteins was a good avenue for new therapies.
“Our long-term goal is to eradicate malaria, so we have to look at ways of breaking the cycle of infection,” he said. “A vaccine or treatment that halts the liver-stage infection offers the best chance of eradication because it stops parasites before they take hold.” ![]()
Researchers say they have identified proteins that enable the malaria parasite Plasmodium falciparum to “walk through cell walls.”
The team believes the proteins—SPECT and PLP1—could be targeted to develop antimalarial drugs or vaccines.
Justin Boddey, PhD, of the Walter and Eliza Hall Institute of Medical Research in Parkville, Victoria, Australia, and his colleagues described this research in Cell Reports.
“The malaria infection cycle begins with a mosquito bite, when parasites are injected into the skin, and then rapidly move to the liver,” Dr Boddey explained. “We have shown that P falciparum employs a technique called cell traversal to quickly move through host cells in their path as they seek out liver cells to infect.”
“Our study identified that P falciparum parasites traverse human cells—effectively walking through cell walls—using 2 proteins called SPECT and PLP1 to achieve this superpower. This allows parasites to get from the skin to the liver very quickly following a mosquito bite.”
Dr Boddey said pinpointing these proteins was a good avenue for new therapies.
“Our long-term goal is to eradicate malaria, so we have to look at ways of breaking the cycle of infection,” he said. “A vaccine or treatment that halts the liver-stage infection offers the best chance of eradication because it stops parasites before they take hold.” ![]()
Researchers say they have identified proteins that enable the malaria parasite Plasmodium falciparum to “walk through cell walls.”
The team believes the proteins—SPECT and PLP1—could be targeted to develop antimalarial drugs or vaccines.
Justin Boddey, PhD, of the Walter and Eliza Hall Institute of Medical Research in Parkville, Victoria, Australia, and his colleagues described this research in Cell Reports.
“The malaria infection cycle begins with a mosquito bite, when parasites are injected into the skin, and then rapidly move to the liver,” Dr Boddey explained. “We have shown that P falciparum employs a technique called cell traversal to quickly move through host cells in their path as they seek out liver cells to infect.”
“Our study identified that P falciparum parasites traverse human cells—effectively walking through cell walls—using 2 proteins called SPECT and PLP1 to achieve this superpower. This allows parasites to get from the skin to the liver very quickly following a mosquito bite.”
Dr Boddey said pinpointing these proteins was a good avenue for new therapies.
“Our long-term goal is to eradicate malaria, so we have to look at ways of breaking the cycle of infection,” he said. “A vaccine or treatment that halts the liver-stage infection offers the best chance of eradication because it stops parasites before they take hold.” ![]()
NCCN launches radiation therapy resource
The National Comprehensive Cancer Network® (NCCN®) recently launched the NCCN Radiation Therapy Compendium™, which provides a single access point for NCCN recommendations pertaining to radiation therapy.
The compendium provides guidance on all radiation therapy modalities recommended within NCCN guidelines, including intensity modulated radiation therapy, intra-operative radiation therapy, stereotactic radiosurgery/stereotactic body radiotherapy/stereotactic ablative radiotherapy, image-guided radiotherapy, low dose-rate brachytherapy/high dose-rate brachytherapy, radioisotope, and particle therapy.
“As a single source for all radiation therapy recommendations within the NCCN guidelines, the compendium benefits patients with cancer by assisting providers and payers in making evidence-based treatment and coverage decisions,” said Robert W. Carlson, MD, chief executive officer of NCCN.
The NCCN Radiation Therapy Compendium™ includes recommendations for the following 24 cancer types:
Acute myeloid leukemia
Anal cancer
B-cell lymphomas
Bladder cancer
Breast cancer
Chronic lymphocytic leukemia/small lymphoblastic lymphoma
Colon cancer
Hepatobiliary cancers
Kidney cancer
Malignant pleural mesothelioma
Melanoma
Multiple myeloma
Neuroendocrine tumors
Non-small cell lung cancer
Occult primary cancer
Pancreatic adenocarcinoma
Penile cancer
Primary cutaneous B-cell lymphomas
Prostate cancer
Rectal cancer
Small cell lung cancer
Soft tissue sarcoma
T-cell lymphomas
Testicular cancer
NCCN said additional cancer types will be published on a rolling basis over the coming months. ![]()
The National Comprehensive Cancer Network® (NCCN®) recently launched the NCCN Radiation Therapy Compendium™, which provides a single access point for NCCN recommendations pertaining to radiation therapy.
The compendium provides guidance on all radiation therapy modalities recommended within NCCN guidelines, including intensity modulated radiation therapy, intra-operative radiation therapy, stereotactic radiosurgery/stereotactic body radiotherapy/stereotactic ablative radiotherapy, image-guided radiotherapy, low dose-rate brachytherapy/high dose-rate brachytherapy, radioisotope, and particle therapy.
“As a single source for all radiation therapy recommendations within the NCCN guidelines, the compendium benefits patients with cancer by assisting providers and payers in making evidence-based treatment and coverage decisions,” said Robert W. Carlson, MD, chief executive officer of NCCN.
The NCCN Radiation Therapy Compendium™ includes recommendations for the following 24 cancer types:
Acute myeloid leukemia
Anal cancer
B-cell lymphomas
Bladder cancer
Breast cancer
Chronic lymphocytic leukemia/small lymphoblastic lymphoma
Colon cancer
Hepatobiliary cancers
Kidney cancer
Malignant pleural mesothelioma
Melanoma
Multiple myeloma
Neuroendocrine tumors
Non-small cell lung cancer
Occult primary cancer
Pancreatic adenocarcinoma
Penile cancer
Primary cutaneous B-cell lymphomas
Prostate cancer
Rectal cancer
Small cell lung cancer
Soft tissue sarcoma
T-cell lymphomas
Testicular cancer
NCCN said additional cancer types will be published on a rolling basis over the coming months. ![]()
The National Comprehensive Cancer Network® (NCCN®) recently launched the NCCN Radiation Therapy Compendium™, which provides a single access point for NCCN recommendations pertaining to radiation therapy.
The compendium provides guidance on all radiation therapy modalities recommended within NCCN guidelines, including intensity modulated radiation therapy, intra-operative radiation therapy, stereotactic radiosurgery/stereotactic body radiotherapy/stereotactic ablative radiotherapy, image-guided radiotherapy, low dose-rate brachytherapy/high dose-rate brachytherapy, radioisotope, and particle therapy.
“As a single source for all radiation therapy recommendations within the NCCN guidelines, the compendium benefits patients with cancer by assisting providers and payers in making evidence-based treatment and coverage decisions,” said Robert W. Carlson, MD, chief executive officer of NCCN.
The NCCN Radiation Therapy Compendium™ includes recommendations for the following 24 cancer types:
Acute myeloid leukemia
Anal cancer
B-cell lymphomas
Bladder cancer
Breast cancer
Chronic lymphocytic leukemia/small lymphoblastic lymphoma
Colon cancer
Hepatobiliary cancers
Kidney cancer
Malignant pleural mesothelioma
Melanoma
Multiple myeloma
Neuroendocrine tumors
Non-small cell lung cancer
Occult primary cancer
Pancreatic adenocarcinoma
Penile cancer
Primary cutaneous B-cell lymphomas
Prostate cancer
Rectal cancer
Small cell lung cancer
Soft tissue sarcoma
T-cell lymphomas
Testicular cancer
NCCN said additional cancer types will be published on a rolling basis over the coming months. ![]()
Model could advance fight against malaria
A new mathematical model could aid the development of drugs that target malaria parasites’ metabolic processes, according to researchers.
The team said they developed the first model of a malaria parasite that accurately integrates its genetics and metabolism.
They described this model in PLOS Computational Biology.
“The model integrates all available knowledge on the genetics and metabolism of the parasites and allows the formulation of testable hypotheses behind the parasites’ essential functions,” said study author Vassily Hatzimanikatis, PhD, of École Polytechnique Fédérale de Lausanne in Switzerland.
“Ultimately, it can accelerate the discovery toward novel antimalarial drug targets.”
To create the model, Dr Hatzimanikatis and his colleagues studied Plasmodium falciparum parasites, focusing on the way they produce and use energy for their metabolic reactions.
This revealed which metabolic functions were essential at each stage of infection and which were energetically coupled through key metabolites.
The team was therefore able to model the bioenergetics of the metabolism of P falciparum, predicting which genes are indispensable for every biological function in the parasite.
By integrating metabolomics and genetics data, the model revealed the complex interactions between gene products, reactions, and metabolites in the parasite, and it revealed potential mechanisms to target with drugs.
“The design of efficient antimalarial drugs that target the parasite’s and not the patient’s metabolism requires an in-depth understanding of the mechanisms that make a particular enzyme essential,” said Anush Chiappino-Pepe, a PhD student at École Polytechnique Fédérale de Lausanne.
“So mathematical modeling of the parasite’s metabolism becomes a very powerful tool.”
The researchers said they will continue to calibrate and improve the predictive capabilities of their model with additional genetics and metabolomics data.
They hope to reveal the mechanisms behind host-pathogen interactions and gain insight into the physiology of P falciparum while it is dormant. ![]()
A new mathematical model could aid the development of drugs that target malaria parasites’ metabolic processes, according to researchers.
The team said they developed the first model of a malaria parasite that accurately integrates its genetics and metabolism.
They described this model in PLOS Computational Biology.
“The model integrates all available knowledge on the genetics and metabolism of the parasites and allows the formulation of testable hypotheses behind the parasites’ essential functions,” said study author Vassily Hatzimanikatis, PhD, of École Polytechnique Fédérale de Lausanne in Switzerland.
“Ultimately, it can accelerate the discovery toward novel antimalarial drug targets.”
To create the model, Dr Hatzimanikatis and his colleagues studied Plasmodium falciparum parasites, focusing on the way they produce and use energy for their metabolic reactions.
This revealed which metabolic functions were essential at each stage of infection and which were energetically coupled through key metabolites.
The team was therefore able to model the bioenergetics of the metabolism of P falciparum, predicting which genes are indispensable for every biological function in the parasite.
By integrating metabolomics and genetics data, the model revealed the complex interactions between gene products, reactions, and metabolites in the parasite, and it revealed potential mechanisms to target with drugs.
“The design of efficient antimalarial drugs that target the parasite’s and not the patient’s metabolism requires an in-depth understanding of the mechanisms that make a particular enzyme essential,” said Anush Chiappino-Pepe, a PhD student at École Polytechnique Fédérale de Lausanne.
“So mathematical modeling of the parasite’s metabolism becomes a very powerful tool.”
The researchers said they will continue to calibrate and improve the predictive capabilities of their model with additional genetics and metabolomics data.
They hope to reveal the mechanisms behind host-pathogen interactions and gain insight into the physiology of P falciparum while it is dormant. ![]()
A new mathematical model could aid the development of drugs that target malaria parasites’ metabolic processes, according to researchers.
The team said they developed the first model of a malaria parasite that accurately integrates its genetics and metabolism.
They described this model in PLOS Computational Biology.
“The model integrates all available knowledge on the genetics and metabolism of the parasites and allows the formulation of testable hypotheses behind the parasites’ essential functions,” said study author Vassily Hatzimanikatis, PhD, of École Polytechnique Fédérale de Lausanne in Switzerland.
“Ultimately, it can accelerate the discovery toward novel antimalarial drug targets.”
To create the model, Dr Hatzimanikatis and his colleagues studied Plasmodium falciparum parasites, focusing on the way they produce and use energy for their metabolic reactions.
This revealed which metabolic functions were essential at each stage of infection and which were energetically coupled through key metabolites.
The team was therefore able to model the bioenergetics of the metabolism of P falciparum, predicting which genes are indispensable for every biological function in the parasite.
By integrating metabolomics and genetics data, the model revealed the complex interactions between gene products, reactions, and metabolites in the parasite, and it revealed potential mechanisms to target with drugs.
“The design of efficient antimalarial drugs that target the parasite’s and not the patient’s metabolism requires an in-depth understanding of the mechanisms that make a particular enzyme essential,” said Anush Chiappino-Pepe, a PhD student at École Polytechnique Fédérale de Lausanne.
“So mathematical modeling of the parasite’s metabolism becomes a very powerful tool.”
The researchers said they will continue to calibrate and improve the predictive capabilities of their model with additional genetics and metabolomics data.
They hope to reveal the mechanisms behind host-pathogen interactions and gain insight into the physiology of P falciparum while it is dormant. ![]()
ASCO reports progress, challenges in cancer care
The US cancer care delivery system is undergoing changes to better meet the needs of cancer patients, but persistent hurdles threaten to slow progress, according to the American Society of Clinical Oncology (ASCO).
ASCO’s “The State of Cancer Care in America, 2017” report describes areas of progress, including new approaches for cancer diagnosis and treatment, improved data sharing to drive innovation, and an increased focus on value-based healthcare.
However, the report also suggests that access and affordability challenges, along with increased practice burdens, continue to pose barriers to high-value, high-quality cancer care.
The report was published in the Journal of Oncology Practice.
Challenges
The report notes that the US population is growing rapidly, changing demographically, and living longer. And all of these factors contribute to a record number of cancer cases/survivors.
It has been estimated that the number of cancer survivors in the US will grow from 15.5 million to 20.3 million by 2026.
Unfortunately, the report says, cancer care is unaffordable for many patients, even those with health insurance.
And significant health disparities persist that are independent of insurance status. Socioeconomic status, geography, and race/ethnicity all impact patient health outcomes.
The report also suggests that oncology practices are facing increased administrative burdens that divert time and resources from their patients.
Progress
Despite the aforementioned challenges, the report paints an optimistic vision about the future of cancer care and highlights activity in the past year aimed at improving care.
For instance, the Food and Drug Administration approved 5 new anticancer therapies, expanded the use of 13, and approved several diagnostic tests in 2016.
In addition, overall cancer incidence and mortality rates were lower in 2016 than in previous decades.
“Since 1991, we’ve been able to save 2.1 million lives because of significant advances in prevention, diagnosis, and treatment—something unimaginable even a decade ago,” said ASCO President Daniel F. Hayes, MD.
“But there’s still more work to be done to ensure that every patient with cancer, no matter who they are or where they live, has access to high-quality, high-value cancer care.”
The report includes a list of recommendations that, ASCO believes, could help bring the US closer to achieving that goal. ![]()
The US cancer care delivery system is undergoing changes to better meet the needs of cancer patients, but persistent hurdles threaten to slow progress, according to the American Society of Clinical Oncology (ASCO).
ASCO’s “The State of Cancer Care in America, 2017” report describes areas of progress, including new approaches for cancer diagnosis and treatment, improved data sharing to drive innovation, and an increased focus on value-based healthcare.
However, the report also suggests that access and affordability challenges, along with increased practice burdens, continue to pose barriers to high-value, high-quality cancer care.
The report was published in the Journal of Oncology Practice.
Challenges
The report notes that the US population is growing rapidly, changing demographically, and living longer. And all of these factors contribute to a record number of cancer cases/survivors.
It has been estimated that the number of cancer survivors in the US will grow from 15.5 million to 20.3 million by 2026.
Unfortunately, the report says, cancer care is unaffordable for many patients, even those with health insurance.
And significant health disparities persist that are independent of insurance status. Socioeconomic status, geography, and race/ethnicity all impact patient health outcomes.
The report also suggests that oncology practices are facing increased administrative burdens that divert time and resources from their patients.
Progress
Despite the aforementioned challenges, the report paints an optimistic vision about the future of cancer care and highlights activity in the past year aimed at improving care.
For instance, the Food and Drug Administration approved 5 new anticancer therapies, expanded the use of 13, and approved several diagnostic tests in 2016.
In addition, overall cancer incidence and mortality rates were lower in 2016 than in previous decades.
“Since 1991, we’ve been able to save 2.1 million lives because of significant advances in prevention, diagnosis, and treatment—something unimaginable even a decade ago,” said ASCO President Daniel F. Hayes, MD.
“But there’s still more work to be done to ensure that every patient with cancer, no matter who they are or where they live, has access to high-quality, high-value cancer care.”
The report includes a list of recommendations that, ASCO believes, could help bring the US closer to achieving that goal. ![]()
The US cancer care delivery system is undergoing changes to better meet the needs of cancer patients, but persistent hurdles threaten to slow progress, according to the American Society of Clinical Oncology (ASCO).
ASCO’s “The State of Cancer Care in America, 2017” report describes areas of progress, including new approaches for cancer diagnosis and treatment, improved data sharing to drive innovation, and an increased focus on value-based healthcare.
However, the report also suggests that access and affordability challenges, along with increased practice burdens, continue to pose barriers to high-value, high-quality cancer care.
The report was published in the Journal of Oncology Practice.
Challenges
The report notes that the US population is growing rapidly, changing demographically, and living longer. And all of these factors contribute to a record number of cancer cases/survivors.
It has been estimated that the number of cancer survivors in the US will grow from 15.5 million to 20.3 million by 2026.
Unfortunately, the report says, cancer care is unaffordable for many patients, even those with health insurance.
And significant health disparities persist that are independent of insurance status. Socioeconomic status, geography, and race/ethnicity all impact patient health outcomes.
The report also suggests that oncology practices are facing increased administrative burdens that divert time and resources from their patients.
Progress
Despite the aforementioned challenges, the report paints an optimistic vision about the future of cancer care and highlights activity in the past year aimed at improving care.
For instance, the Food and Drug Administration approved 5 new anticancer therapies, expanded the use of 13, and approved several diagnostic tests in 2016.
In addition, overall cancer incidence and mortality rates were lower in 2016 than in previous decades.
“Since 1991, we’ve been able to save 2.1 million lives because of significant advances in prevention, diagnosis, and treatment—something unimaginable even a decade ago,” said ASCO President Daniel F. Hayes, MD.
“But there’s still more work to be done to ensure that every patient with cancer, no matter who they are or where they live, has access to high-quality, high-value cancer care.”
The report includes a list of recommendations that, ASCO believes, could help bring the US closer to achieving that goal. ![]()
New resource designed to help prevent CINV
The Hematology/Oncology Pharmacy Association (HOPA) has announced the release of a toolkit intended to help prevent chemotherapy-induced nausea and vomiting (CINV) in cancer patients.
The Time to Talk CINV™ toolkit was designed to facilitate dialogue between patients and their healthcare teams to ensure no patient is needlessly suffering from CINV.
The tools in the kit, which are targeted to both patients and healthcare providers, were created with guidance from patients, caregivers, pharmacists, and nurses.
The entire toolkit is available for free download at TimeToTalkCINV.com.
The toolkit is part of the Time to Talk CINV™ campaign, which is a collaboration between HOPA, Eisai Inc., and Helsinn Therapeutics (US), Inc. (funded by Eisai and Helsinn Therapeutics).
The campaign began in response to results from a survey of cancer patients.
“Our research revealed a vast majority of patients on chemotherapy who experience nausea and vomiting expect to have this side effect, and 95% of these patients said, at some point, chemo-induced nausea and vomiting had an impact on their daily lives,” said Sarah Peters, PharmD, president of HOPA.
“In response to these findings, as well as the finding that healthcare team members are looking for better communication tools, the Time to Talk CINV toolkit was created to facilitate efficient and effective conversations about nausea and vomiting from chemotherapy to ensure that each patient is receiving the right information and effective management approaches.”
The Time to Talk CINV toolkit includes the following resources:
- A list of myths and truths about CINV intended to eliminate common misperceptions
- A checklist of questions patients can ask healthcare providers to better understand CINV
- A chemotherapy side effect tracker, which enables patients to track their experience and report back to their healthcare team
- A communication checklist for the healthcare team outlining best practices for communicating with patients to prevent CINV.
Each tool can be printed or filled out digitally.
Additional information about CINV, including videos and infographics, can be found on the Time to Talk CINV website. ![]()
The Hematology/Oncology Pharmacy Association (HOPA) has announced the release of a toolkit intended to help prevent chemotherapy-induced nausea and vomiting (CINV) in cancer patients.
The Time to Talk CINV™ toolkit was designed to facilitate dialogue between patients and their healthcare teams to ensure no patient is needlessly suffering from CINV.
The tools in the kit, which are targeted to both patients and healthcare providers, were created with guidance from patients, caregivers, pharmacists, and nurses.
The entire toolkit is available for free download at TimeToTalkCINV.com.
The toolkit is part of the Time to Talk CINV™ campaign, which is a collaboration between HOPA, Eisai Inc., and Helsinn Therapeutics (US), Inc. (funded by Eisai and Helsinn Therapeutics).
The campaign began in response to results from a survey of cancer patients.
“Our research revealed a vast majority of patients on chemotherapy who experience nausea and vomiting expect to have this side effect, and 95% of these patients said, at some point, chemo-induced nausea and vomiting had an impact on their daily lives,” said Sarah Peters, PharmD, president of HOPA.
“In response to these findings, as well as the finding that healthcare team members are looking for better communication tools, the Time to Talk CINV toolkit was created to facilitate efficient and effective conversations about nausea and vomiting from chemotherapy to ensure that each patient is receiving the right information and effective management approaches.”
The Time to Talk CINV toolkit includes the following resources:
- A list of myths and truths about CINV intended to eliminate common misperceptions
- A checklist of questions patients can ask healthcare providers to better understand CINV
- A chemotherapy side effect tracker, which enables patients to track their experience and report back to their healthcare team
- A communication checklist for the healthcare team outlining best practices for communicating with patients to prevent CINV.
Each tool can be printed or filled out digitally.
Additional information about CINV, including videos and infographics, can be found on the Time to Talk CINV website. ![]()
The Hematology/Oncology Pharmacy Association (HOPA) has announced the release of a toolkit intended to help prevent chemotherapy-induced nausea and vomiting (CINV) in cancer patients.
The Time to Talk CINV™ toolkit was designed to facilitate dialogue between patients and their healthcare teams to ensure no patient is needlessly suffering from CINV.
The tools in the kit, which are targeted to both patients and healthcare providers, were created with guidance from patients, caregivers, pharmacists, and nurses.
The entire toolkit is available for free download at TimeToTalkCINV.com.
The toolkit is part of the Time to Talk CINV™ campaign, which is a collaboration between HOPA, Eisai Inc., and Helsinn Therapeutics (US), Inc. (funded by Eisai and Helsinn Therapeutics).
The campaign began in response to results from a survey of cancer patients.
“Our research revealed a vast majority of patients on chemotherapy who experience nausea and vomiting expect to have this side effect, and 95% of these patients said, at some point, chemo-induced nausea and vomiting had an impact on their daily lives,” said Sarah Peters, PharmD, president of HOPA.
“In response to these findings, as well as the finding that healthcare team members are looking for better communication tools, the Time to Talk CINV toolkit was created to facilitate efficient and effective conversations about nausea and vomiting from chemotherapy to ensure that each patient is receiving the right information and effective management approaches.”
The Time to Talk CINV toolkit includes the following resources:
- A list of myths and truths about CINV intended to eliminate common misperceptions
- A checklist of questions patients can ask healthcare providers to better understand CINV
- A chemotherapy side effect tracker, which enables patients to track their experience and report back to their healthcare team
- A communication checklist for the healthcare team outlining best practices for communicating with patients to prevent CINV.
Each tool can be printed or filled out digitally.
Additional information about CINV, including videos and infographics, can be found on the Time to Talk CINV website. ![]()
AYAs struggle socially in early years after cancer diagnosis
A new study indicates that adolescent and young adult (AYA) cancer survivors continue to face social difficulties for more than 2 years after their diagnosis.
The research, published in Cancer, suggests these patients may see some improvement in their social lives during the first year after diagnosis.
However, their social functioning tends to remain constant after that, leaving them socially impaired relative to their cancer-free peers.
Previous studies have shown that AYAs with cancer experience greater challenges in social functioning than their cancer-free peers or even compared to older cancer patients.
But few studies have examined this phenomenon by following the same patients over time.
Olga Husson, PhD, of the Radboud University Medical Center in The Netherlands, and her colleagues set out to examine changes in social functioning among AYAs in the early years after a cancer diagnosis.
The researchers asked AYA cancer patients at 5 US medical institutions to complete a survey about social functioning within 4 months of their diagnosis, 12 months later, and 24 months later.
There were 141 patients (ages 14 to 39 at diagnosis) who completed the surveys.
The researchers found that, when compared to population norms, the cancer patients had inferior social functioning at all the time points studied.
Among the cancer patients, the mean social functioning score from the Medical Outcomes Study Short Form 36 Health Survey (version 2) was 52.0 around the time of cancer diagnosis, 73.1 at the 12-month follow-up, and 69.2 at the 24-month follow-up. In comparison, the population norm (for people ages 18 to 44) is 85.1 (P<0.001 for all time points).
The researchers did note that cancer patients experienced significant improvements in social functioning from baseline to the 12-month follow-up, but there was no further improvement after that.
The researchers also examined the different trajectories of social functioning over time. They found that social functioning improved over time for 47% of the cancer patients but worsened for 13%. In addition, 32% of patients had consistently low social functioning, and 9% had consistently high social functioning.
The cancer patients with consistently low social functioning were more likely to be off treatment at the time of follow-up, report more physical symptoms and higher levels of psychological distress (at both baseline and follow-up), and perceive themselves to receive less social support.
“Reducing physical symptoms and psychological distress and enhancing social support by interventions in the period after treatment may potentially help these young survivors to better reintegrate into society,” Dr Husson said. ![]()
A new study indicates that adolescent and young adult (AYA) cancer survivors continue to face social difficulties for more than 2 years after their diagnosis.
The research, published in Cancer, suggests these patients may see some improvement in their social lives during the first year after diagnosis.
However, their social functioning tends to remain constant after that, leaving them socially impaired relative to their cancer-free peers.
Previous studies have shown that AYAs with cancer experience greater challenges in social functioning than their cancer-free peers or even compared to older cancer patients.
But few studies have examined this phenomenon by following the same patients over time.
Olga Husson, PhD, of the Radboud University Medical Center in The Netherlands, and her colleagues set out to examine changes in social functioning among AYAs in the early years after a cancer diagnosis.
The researchers asked AYA cancer patients at 5 US medical institutions to complete a survey about social functioning within 4 months of their diagnosis, 12 months later, and 24 months later.
There were 141 patients (ages 14 to 39 at diagnosis) who completed the surveys.
The researchers found that, when compared to population norms, the cancer patients had inferior social functioning at all the time points studied.
Among the cancer patients, the mean social functioning score from the Medical Outcomes Study Short Form 36 Health Survey (version 2) was 52.0 around the time of cancer diagnosis, 73.1 at the 12-month follow-up, and 69.2 at the 24-month follow-up. In comparison, the population norm (for people ages 18 to 44) is 85.1 (P<0.001 for all time points).
The researchers did note that cancer patients experienced significant improvements in social functioning from baseline to the 12-month follow-up, but there was no further improvement after that.
The researchers also examined the different trajectories of social functioning over time. They found that social functioning improved over time for 47% of the cancer patients but worsened for 13%. In addition, 32% of patients had consistently low social functioning, and 9% had consistently high social functioning.
The cancer patients with consistently low social functioning were more likely to be off treatment at the time of follow-up, report more physical symptoms and higher levels of psychological distress (at both baseline and follow-up), and perceive themselves to receive less social support.
“Reducing physical symptoms and psychological distress and enhancing social support by interventions in the period after treatment may potentially help these young survivors to better reintegrate into society,” Dr Husson said. ![]()
A new study indicates that adolescent and young adult (AYA) cancer survivors continue to face social difficulties for more than 2 years after their diagnosis.
The research, published in Cancer, suggests these patients may see some improvement in their social lives during the first year after diagnosis.
However, their social functioning tends to remain constant after that, leaving them socially impaired relative to their cancer-free peers.
Previous studies have shown that AYAs with cancer experience greater challenges in social functioning than their cancer-free peers or even compared to older cancer patients.
But few studies have examined this phenomenon by following the same patients over time.
Olga Husson, PhD, of the Radboud University Medical Center in The Netherlands, and her colleagues set out to examine changes in social functioning among AYAs in the early years after a cancer diagnosis.
The researchers asked AYA cancer patients at 5 US medical institutions to complete a survey about social functioning within 4 months of their diagnosis, 12 months later, and 24 months later.
There were 141 patients (ages 14 to 39 at diagnosis) who completed the surveys.
The researchers found that, when compared to population norms, the cancer patients had inferior social functioning at all the time points studied.
Among the cancer patients, the mean social functioning score from the Medical Outcomes Study Short Form 36 Health Survey (version 2) was 52.0 around the time of cancer diagnosis, 73.1 at the 12-month follow-up, and 69.2 at the 24-month follow-up. In comparison, the population norm (for people ages 18 to 44) is 85.1 (P<0.001 for all time points).
The researchers did note that cancer patients experienced significant improvements in social functioning from baseline to the 12-month follow-up, but there was no further improvement after that.
The researchers also examined the different trajectories of social functioning over time. They found that social functioning improved over time for 47% of the cancer patients but worsened for 13%. In addition, 32% of patients had consistently low social functioning, and 9% had consistently high social functioning.
The cancer patients with consistently low social functioning were more likely to be off treatment at the time of follow-up, report more physical symptoms and higher levels of psychological distress (at both baseline and follow-up), and perceive themselves to receive less social support.
“Reducing physical symptoms and psychological distress and enhancing social support by interventions in the period after treatment may potentially help these young survivors to better reintegrate into society,” Dr Husson said. ![]()
Funders could do more to reduce research waste, team says
A study published in The Lancet suggests agencies that distribute public funds for research could do more to reduce waste.
Investigators evaluated 11 agencies from various countries and found the agencies aren’t always transparent about what they are doing to prevent waste in research.
The investigators also found evidence to suggest that some agencies are not taking certain steps that could reduce waste, and the governments responsible for the public money these agencies distribute are not holding them to account.
“Our investigation has shown that, on the whole, information about the policies and processes used by national funding agencies across the funding landscape are not transparent or readily available,” said Mona Nasser, DDS, of Plymouth University in the UK.
“It would appear that governments around the world often do not hold these agencies accountable for adding value to research and reducing research waste. This is not a call for governments to reduce spending on medical research, but, rather, as public funds become increasingly squeezed, there is no better time for funding agencies and governments to work together to ensure that we will all get the best ‘bang for the buck.’”
For this study, Dr Nasser and her colleagues investigated how research funders monitor and take steps to reduce waste in the research they support. The team also examined how funders support methodology research and the development of research infrastructures to reduce waste.
The investigators looked through the websites of 11 national research funders that distribute public funds in the US, UK, Australia, Canada, Germany, France, The Netherlands, Denmark, and Norway.
The team looked for information on how the agencies decide what to fund and how they ensure what they fund is not wasteful. The investigators also contacted these agencies to verify their findings.
The team found that approaches vary among the funders, but there are weaknesses that are applicable across all funding bodies.
One weakness is that grant committees tend to be dominated by academics and clinicians, which is a problem because patients’ interests may be overlooked.
The funders with the “most extensive” involvement of the general public are the National Institute of Health Research (NIHR) in the UK and ZonMW in The Netherlands.
Another weakness is the fact that practice and policy decisions are often made without the systematic assessment of existing research evidence.
The only funder to require reference to relevant systematic reviews in all funding applications is NIHR.
Yet another weakness is that only 6 of the 11 funding agencies require the publication of full reports of the research they have funded. And none of the funders have a comprehensive strategy to make data from all research projects freely available.
Based on these results, Dr Nasser and her colleagues concluded that more should be done to ensure transparency and accountability.
“In simple terms, there is a 2-pronged requirement for medical research funding bodies which distribute public funds,” Dr Nasser said. “The first is that they need to be fully responsible for how and why those funds are distributed because they are ultimately answerable to every tax payer in their home countries.”
“The second is that they need to ensure that public funds are not only invested wisely in research projects which represent both good value and waste-limited practice, but also to ensure that the results of these studies are made available in a usable format to the people who need them.” ![]()
A study published in The Lancet suggests agencies that distribute public funds for research could do more to reduce waste.
Investigators evaluated 11 agencies from various countries and found the agencies aren’t always transparent about what they are doing to prevent waste in research.
The investigators also found evidence to suggest that some agencies are not taking certain steps that could reduce waste, and the governments responsible for the public money these agencies distribute are not holding them to account.
“Our investigation has shown that, on the whole, information about the policies and processes used by national funding agencies across the funding landscape are not transparent or readily available,” said Mona Nasser, DDS, of Plymouth University in the UK.
“It would appear that governments around the world often do not hold these agencies accountable for adding value to research and reducing research waste. This is not a call for governments to reduce spending on medical research, but, rather, as public funds become increasingly squeezed, there is no better time for funding agencies and governments to work together to ensure that we will all get the best ‘bang for the buck.’”
For this study, Dr Nasser and her colleagues investigated how research funders monitor and take steps to reduce waste in the research they support. The team also examined how funders support methodology research and the development of research infrastructures to reduce waste.
The investigators looked through the websites of 11 national research funders that distribute public funds in the US, UK, Australia, Canada, Germany, France, The Netherlands, Denmark, and Norway.
The team looked for information on how the agencies decide what to fund and how they ensure what they fund is not wasteful. The investigators also contacted these agencies to verify their findings.
The team found that approaches vary among the funders, but there are weaknesses that are applicable across all funding bodies.
One weakness is that grant committees tend to be dominated by academics and clinicians, which is a problem because patients’ interests may be overlooked.
The funders with the “most extensive” involvement of the general public are the National Institute of Health Research (NIHR) in the UK and ZonMW in The Netherlands.
Another weakness is the fact that practice and policy decisions are often made without the systematic assessment of existing research evidence.
The only funder to require reference to relevant systematic reviews in all funding applications is NIHR.
Yet another weakness is that only 6 of the 11 funding agencies require the publication of full reports of the research they have funded. And none of the funders have a comprehensive strategy to make data from all research projects freely available.
Based on these results, Dr Nasser and her colleagues concluded that more should be done to ensure transparency and accountability.
“In simple terms, there is a 2-pronged requirement for medical research funding bodies which distribute public funds,” Dr Nasser said. “The first is that they need to be fully responsible for how and why those funds are distributed because they are ultimately answerable to every tax payer in their home countries.”
“The second is that they need to ensure that public funds are not only invested wisely in research projects which represent both good value and waste-limited practice, but also to ensure that the results of these studies are made available in a usable format to the people who need them.” ![]()
A study published in The Lancet suggests agencies that distribute public funds for research could do more to reduce waste.
Investigators evaluated 11 agencies from various countries and found the agencies aren’t always transparent about what they are doing to prevent waste in research.
The investigators also found evidence to suggest that some agencies are not taking certain steps that could reduce waste, and the governments responsible for the public money these agencies distribute are not holding them to account.
“Our investigation has shown that, on the whole, information about the policies and processes used by national funding agencies across the funding landscape are not transparent or readily available,” said Mona Nasser, DDS, of Plymouth University in the UK.
“It would appear that governments around the world often do not hold these agencies accountable for adding value to research and reducing research waste. This is not a call for governments to reduce spending on medical research, but, rather, as public funds become increasingly squeezed, there is no better time for funding agencies and governments to work together to ensure that we will all get the best ‘bang for the buck.’”
For this study, Dr Nasser and her colleagues investigated how research funders monitor and take steps to reduce waste in the research they support. The team also examined how funders support methodology research and the development of research infrastructures to reduce waste.
The investigators looked through the websites of 11 national research funders that distribute public funds in the US, UK, Australia, Canada, Germany, France, The Netherlands, Denmark, and Norway.
The team looked for information on how the agencies decide what to fund and how they ensure what they fund is not wasteful. The investigators also contacted these agencies to verify their findings.
The team found that approaches vary among the funders, but there are weaknesses that are applicable across all funding bodies.
One weakness is that grant committees tend to be dominated by academics and clinicians, which is a problem because patients’ interests may be overlooked.
The funders with the “most extensive” involvement of the general public are the National Institute of Health Research (NIHR) in the UK and ZonMW in The Netherlands.
Another weakness is the fact that practice and policy decisions are often made without the systematic assessment of existing research evidence.
The only funder to require reference to relevant systematic reviews in all funding applications is NIHR.
Yet another weakness is that only 6 of the 11 funding agencies require the publication of full reports of the research they have funded. And none of the funders have a comprehensive strategy to make data from all research projects freely available.
Based on these results, Dr Nasser and her colleagues concluded that more should be done to ensure transparency and accountability.
“In simple terms, there is a 2-pronged requirement for medical research funding bodies which distribute public funds,” Dr Nasser said. “The first is that they need to be fully responsible for how and why those funds are distributed because they are ultimately answerable to every tax payer in their home countries.”
“The second is that they need to ensure that public funds are not only invested wisely in research projects which represent both good value and waste-limited practice, but also to ensure that the results of these studies are made available in a usable format to the people who need them.”
HDAC6 inhibitors reverse CIPN in rodents
Histone deacetylase (HDAC) inhibitors can treat chemotherapy-induced peripheral neuropathy (CIPN) in mice and rats, according to research published in PAIN.
The animals developed CIPN after treatment with cisplatin or paclitaxel.
But treatment with an HDAC6 inhibitor—ACY-1083 or ricolinostat (ACY-1215)—was able to reverse and sometimes prevent the symptoms of CIPN in the animals.
The researchers said these findings are “especially promising” because one of the HDAC6 inhibitors, ricolinostat, is already being tested in clinical trials.
This research was funded, in part, by Acetylon Pharmaceuticals Inc., and employees of the company were involved in the research.
The HDAC6 inhibitors are being developed by Regenacy Pharmaceuticals, LLC, a spinout of Acetylon Pharmaceuticals.
The researchers found that ACY-1083 was able to reverse cisplatin-induced mechanical allodynia (pain in response to touch) in mice.
ACY-1083 also prevented mechanical allodynia when given an hour prior to treatment with cisplatin.
The team noted that ricolinostat, which is less selective for HDAC6 than ACY-1083, also reversed cisplatin-induced mechanical allodynia.
The beneficial effect of ricolinostat was still seen a week after treatment, which was the last time point tested.
The researchers also found that ACY-1083 was able to reverse paclitaxel-induced mechanical allodynia in rats.
And ACY-1083 could reverse cisplatin-induced spontaneous pain and numbness in mice.
“These data demonstrate that HDAC6 is a novel therapeutic target for the treatment of chemotherapy-induced neuropathies, for which there are currently no FDA-approved drugs,” said Matthew B. Jarpe, PhD, associate vice-president of biology at Regenacy Pharmaceuticals.
Dr Jarpe said this research suggests an HDAC6 inhibitor could potentially be used in conjunction with platinum- or taxane-based chemotherapy to allow for higher and/or longer chemotherapy dosing regimens.
“Additionally, the reversal of both pain and numbness in this preclinical model suggests that an HDAC6 inhibitor could be used after a course of chemotherapy is completed to treat and potentially reverse resultant debilitating CIPN symptoms that impact function and quality of life for many patients,” he said.
“Preclinical results seen with the clinical candidate ricolinostat highlight the translational potential of these findings to the clinic.”
Histone deacetylase (HDAC) inhibitors can treat chemotherapy-induced peripheral neuropathy (CIPN) in mice and rats, according to research published in PAIN.
The animals developed CIPN after treatment with cisplatin or paclitaxel.
But treatment with an HDAC6 inhibitor—ACY-1083 or ricolinostat (ACY-1215)—was able to reverse and sometimes prevent the symptoms of CIPN in the animals.
The researchers said these findings are “especially promising” because one of the HDAC6 inhibitors, ricolinostat, is already being tested in clinical trials.
This research was funded, in part, by Acetylon Pharmaceuticals Inc., and employees of the company were involved in the research.
The HDAC6 inhibitors are being developed by Regenacy Pharmaceuticals, LLC, a spinout of Acetylon Pharmaceuticals.
The researchers found that ACY-1083 was able to reverse cisplatin-induced mechanical allodynia (pain in response to touch) in mice.
ACY-1083 also prevented mechanical allodynia when given an hour prior to treatment with cisplatin.
The team noted that ricolinostat, which is less selective for HDAC6 than ACY-1083, also reversed cisplatin-induced mechanical allodynia.
The beneficial effect of ricolinostat was still seen a week after treatment, which was the last time point tested.
The researchers also found that ACY-1083 was able to reverse paclitaxel-induced mechanical allodynia in rats.
And ACY-1083 could reverse cisplatin-induced spontaneous pain and numbness in mice.
“These data demonstrate that HDAC6 is a novel therapeutic target for the treatment of chemotherapy-induced neuropathies, for which there are currently no FDA-approved drugs,” said Matthew B. Jarpe, PhD, associate vice-president of biology at Regenacy Pharmaceuticals.
Dr Jarpe said this research suggests an HDAC6 inhibitor could potentially be used in conjunction with platinum- or taxane-based chemotherapy to allow for higher and/or longer chemotherapy dosing regimens.
“Additionally, the reversal of both pain and numbness in this preclinical model suggests that an HDAC6 inhibitor could be used after a course of chemotherapy is completed to treat and potentially reverse resultant debilitating CIPN symptoms that impact function and quality of life for many patients,” he said.
“Preclinical results seen with the clinical candidate ricolinostat highlight the translational potential of these findings to the clinic.”
Histone deacetylase (HDAC) inhibitors can treat chemotherapy-induced peripheral neuropathy (CIPN) in mice and rats, according to research published in PAIN.
The animals developed CIPN after treatment with cisplatin or paclitaxel.
But treatment with an HDAC6 inhibitor—ACY-1083 or ricolinostat (ACY-1215)—was able to reverse and sometimes prevent the symptoms of CIPN in the animals.
The researchers said these findings are “especially promising” because one of the HDAC6 inhibitors, ricolinostat, is already being tested in clinical trials.
This research was funded, in part, by Acetylon Pharmaceuticals Inc., and employees of the company were involved in the research.
The HDAC6 inhibitors are being developed by Regenacy Pharmaceuticals, LLC, a spinout of Acetylon Pharmaceuticals.
The researchers found that ACY-1083 was able to reverse cisplatin-induced mechanical allodynia (pain in response to touch) in mice.
ACY-1083 also prevented mechanical allodynia when given an hour prior to treatment with cisplatin.
The team noted that ricolinostat, which is less selective for HDAC6 than ACY-1083, also reversed cisplatin-induced mechanical allodynia.
The beneficial effect of ricolinostat was still seen a week after treatment, which was the last time point tested.
The researchers also found that ACY-1083 was able to reverse paclitaxel-induced mechanical allodynia in rats.
And ACY-1083 could reverse cisplatin-induced spontaneous pain and numbness in mice.
“These data demonstrate that HDAC6 is a novel therapeutic target for the treatment of chemotherapy-induced neuropathies, for which there are currently no FDA-approved drugs,” said Matthew B. Jarpe, PhD, associate vice-president of biology at Regenacy Pharmaceuticals.
Dr Jarpe said this research suggests an HDAC6 inhibitor could potentially be used in conjunction with platinum- or taxane-based chemotherapy to allow for higher and/or longer chemotherapy dosing regimens.
“Additionally, the reversal of both pain and numbness in this preclinical model suggests that an HDAC6 inhibitor could be used after a course of chemotherapy is completed to treat and potentially reverse resultant debilitating CIPN symptoms that impact function and quality of life for many patients,” he said.
“Preclinical results seen with the clinical candidate ricolinostat highlight the translational potential of these findings to the clinic.”
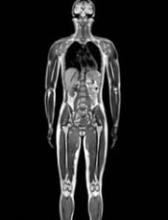
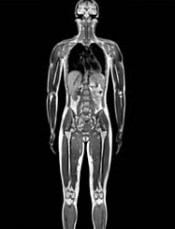
PRAC recommends suspending gadolinium agents
The European Medicines Agency’s (EMA) Pharmacovigilance and Risk Assessment Committee (PRAC) has recommended suspending marketing authorizations for 4 linear gadolinium contrast agents because of evidence that small amounts of the gadolinium they contain are deposited in the brain.
The agents concerned are intravenous injections of gadobenic acid, gadodiamide, gadopentetic acid, and gadoversetamide, which are given to patients to enhance images from magnetic resonance imaging (MRI) body scans.
The PRAC’s review of gadolinium agents found “convincing evidence” of accumulation of gadolinium in the brain from studies directly measuring gadolinium in brain tissues and areas of increased signal intensity seen on MRI scan images many months after the last injection of a gadolinium contrast agent.
Although no symptoms or diseases linked to gadolinium in the brain have been reported, the PRAC took a precautionary approach, noting that data on the long-term effects in the brain are limited.
Companies concerned by this review have the right to request that the PRAC re-examine its recommendations.
The PRAC’s recommendations will be sent to the Committee for Medicinal Products for Human Use (CHMP) for its opinion. Further details will be published at the time of the CHMP opinion.
The final stage of the review procedure is the adoption by the European Commission of a legally binding decision applicable in all European Union member states.
Details of the review, recommendations
The PRAC’s review was initiated on March 17, 2016, at the request of the European Commission, under Article 31 of Directive 2001/83/EC.
The review covers agents containing the following active substances: gadobenic acid, gadobutrol, gadodiamide, gadopentetic acid, gadoteric acid, gadoteridol, gadoversetamide, and gadoxetic acid.
The 4 agents recommended for suspension (gadobenic acid, gadodiamide, gadopentetic acid, and gadoversetamide) are linear agents. They have a structure more likely to release gadolinium, which can build up in body tissues.
Other agents, known as macrocyclic agents, are more stable and have a much lower propensity to release gadolinium.
The PRAC recommends that macrocyclic agents (gadobutrol, gadoteric acid, and gadoteridol) be used at the lowest dose that enhances images sufficiently to make diagnoses and only when unenhanced body scans are not suitable.
The PRAC has recommended that some linear agents remain available. The committee said that gadoxetic acid, a linear agent used at a low dose for liver scans, can remain on the market as it meets an important diagnostic need in patients with few alternatives.
In addition, a formulation of gadopentetic acid injected directly into joints should remain available because its gadolinium concentration is very low—around 200 times lower than those of intravenous products.
Both agents should be used at the lowest dose that enhances images sufficiently to make diagnoses and only if unenhanced scans are not suitable.
For those marketing authorizations recommended for suspension, the suspensions can be lifted if the respective companies provide evidence of new benefits in an identified patient group that outweigh its risks or show that their product (modified or not) does not release gadolinium significantly or lead to its retention in tissues. ![]()
The European Medicines Agency’s (EMA) Pharmacovigilance and Risk Assessment Committee (PRAC) has recommended suspending marketing authorizations for 4 linear gadolinium contrast agents because of evidence that small amounts of the gadolinium they contain are deposited in the brain.
The agents concerned are intravenous injections of gadobenic acid, gadodiamide, gadopentetic acid, and gadoversetamide, which are given to patients to enhance images from magnetic resonance imaging (MRI) body scans.
The PRAC’s review of gadolinium agents found “convincing evidence” of accumulation of gadolinium in the brain from studies directly measuring gadolinium in brain tissues and areas of increased signal intensity seen on MRI scan images many months after the last injection of a gadolinium contrast agent.
Although no symptoms or diseases linked to gadolinium in the brain have been reported, the PRAC took a precautionary approach, noting that data on the long-term effects in the brain are limited.
Companies concerned by this review have the right to request that the PRAC re-examine its recommendations.
The PRAC’s recommendations will be sent to the Committee for Medicinal Products for Human Use (CHMP) for its opinion. Further details will be published at the time of the CHMP opinion.
The final stage of the review procedure is the adoption by the European Commission of a legally binding decision applicable in all European Union member states.
Details of the review, recommendations
The PRAC’s review was initiated on March 17, 2016, at the request of the European Commission, under Article 31 of Directive 2001/83/EC.
The review covers agents containing the following active substances: gadobenic acid, gadobutrol, gadodiamide, gadopentetic acid, gadoteric acid, gadoteridol, gadoversetamide, and gadoxetic acid.
The 4 agents recommended for suspension (gadobenic acid, gadodiamide, gadopentetic acid, and gadoversetamide) are linear agents. They have a structure more likely to release gadolinium, which can build up in body tissues.
Other agents, known as macrocyclic agents, are more stable and have a much lower propensity to release gadolinium.
The PRAC recommends that macrocyclic agents (gadobutrol, gadoteric acid, and gadoteridol) be used at the lowest dose that enhances images sufficiently to make diagnoses and only when unenhanced body scans are not suitable.
The PRAC has recommended that some linear agents remain available. The committee said that gadoxetic acid, a linear agent used at a low dose for liver scans, can remain on the market as it meets an important diagnostic need in patients with few alternatives.
In addition, a formulation of gadopentetic acid injected directly into joints should remain available because its gadolinium concentration is very low—around 200 times lower than those of intravenous products.
Both agents should be used at the lowest dose that enhances images sufficiently to make diagnoses and only if unenhanced scans are not suitable.
For those marketing authorizations recommended for suspension, the suspensions can be lifted if the respective companies provide evidence of new benefits in an identified patient group that outweigh its risks or show that their product (modified or not) does not release gadolinium significantly or lead to its retention in tissues. ![]()
The European Medicines Agency’s (EMA) Pharmacovigilance and Risk Assessment Committee (PRAC) has recommended suspending marketing authorizations for 4 linear gadolinium contrast agents because of evidence that small amounts of the gadolinium they contain are deposited in the brain.
The agents concerned are intravenous injections of gadobenic acid, gadodiamide, gadopentetic acid, and gadoversetamide, which are given to patients to enhance images from magnetic resonance imaging (MRI) body scans.
The PRAC’s review of gadolinium agents found “convincing evidence” of accumulation of gadolinium in the brain from studies directly measuring gadolinium in brain tissues and areas of increased signal intensity seen on MRI scan images many months after the last injection of a gadolinium contrast agent.
Although no symptoms or diseases linked to gadolinium in the brain have been reported, the PRAC took a precautionary approach, noting that data on the long-term effects in the brain are limited.
Companies concerned by this review have the right to request that the PRAC re-examine its recommendations.
The PRAC’s recommendations will be sent to the Committee for Medicinal Products for Human Use (CHMP) for its opinion. Further details will be published at the time of the CHMP opinion.
The final stage of the review procedure is the adoption by the European Commission of a legally binding decision applicable in all European Union member states.
Details of the review, recommendations
The PRAC’s review was initiated on March 17, 2016, at the request of the European Commission, under Article 31 of Directive 2001/83/EC.
The review covers agents containing the following active substances: gadobenic acid, gadobutrol, gadodiamide, gadopentetic acid, gadoteric acid, gadoteridol, gadoversetamide, and gadoxetic acid.
The 4 agents recommended for suspension (gadobenic acid, gadodiamide, gadopentetic acid, and gadoversetamide) are linear agents. They have a structure more likely to release gadolinium, which can build up in body tissues.
Other agents, known as macrocyclic agents, are more stable and have a much lower propensity to release gadolinium.
The PRAC recommends that macrocyclic agents (gadobutrol, gadoteric acid, and gadoteridol) be used at the lowest dose that enhances images sufficiently to make diagnoses and only when unenhanced body scans are not suitable.
The PRAC has recommended that some linear agents remain available. The committee said that gadoxetic acid, a linear agent used at a low dose for liver scans, can remain on the market as it meets an important diagnostic need in patients with few alternatives.
In addition, a formulation of gadopentetic acid injected directly into joints should remain available because its gadolinium concentration is very low—around 200 times lower than those of intravenous products.
Both agents should be used at the lowest dose that enhances images sufficiently to make diagnoses and only if unenhanced scans are not suitable.
For those marketing authorizations recommended for suspension, the suspensions can be lifted if the respective companies provide evidence of new benefits in an identified patient group that outweigh its risks or show that their product (modified or not) does not release gadolinium significantly or lead to its retention in tissues. ![]()
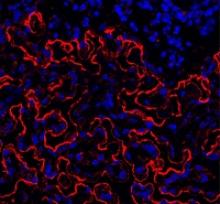
Group reports rejuvenation of aging HSCs
Preclinical research suggests osteopontin, a protein made by osteoblasts, plays a role in hematopoietic stem cell (HSC) aging.
Experiments in mice revealed that, with age, there is a reduction in the expression of osteopontin in the bone marrow stroma.
A lack of osteopontin induced young HSCs to “act” older, while treating older HSCs with recombinant osteopontin restored youthful properties.
Hartmut Geiger, PhD, of the University of Ulm in Germany, and his colleagues reported these findings in EMBO.
“We show that the place where HSCs form in the bone marrow loses osteopontin upon aging, but if you give back the missing protein to the blood-forming cells, they suddenly rejuvenate and act younger,” Dr Geiger said.
“Our study points to exciting, novel ways to have a better immune system and possibly less blood cancer upon aging by therapeutically targeting the place where blood stem cells form.”
The researchers conducted a number of experiments to test the formation and vitality of cells in and near the bone marrow microenvironment.
The team looked at the formation of endosteum stroma cells in aging mice and monitored levels of osteopontin and other proteins linked to distinct cells in the bone marrow during the aging process.
The researchers said they observed reduced production of osteoblasts and other stroma cells in the endosteum of older mice. They also saw decreased osteopontin levels in the bone marrow of older animals, which was associated with reduced vigor and function of HSCs.
The team followed up these experiments by transplanting bone marrow cells from older mice (19 to 21 months) into young mice (8 to 10 weeks).
The researchers also transplanted aged HSCs from older mice into younger mice, and the team treated aged HSCs with a recombinant form of the osteopontin protein.
Transplantation into the younger animals caused HSCs to behave in a younger, more vital manner, the researchers said. This meant smaller numbers of HSCs with greater potential for forming different blood cells, which included larger populations of B and T cells and decreased production of myeloid cells.
The researchers also saw aged HSCs treated with recombinant osteopontin regain their youthful characteristics and capacity to form different blood cell types. Also observed was diminished signaling of Cdc42, a protein previously shown to cause aging in HSCs.
Osteopontin levels are not only low in the bone marrow niche but also in the blood upon aging. As a follow-up to the current study, the researchers are investigating the possibility of using osteopontin replacement therapy in mice to counter the influence of an aging niche directly in the animals. ![]()
Preclinical research suggests osteopontin, a protein made by osteoblasts, plays a role in hematopoietic stem cell (HSC) aging.
Experiments in mice revealed that, with age, there is a reduction in the expression of osteopontin in the bone marrow stroma.
A lack of osteopontin induced young HSCs to “act” older, while treating older HSCs with recombinant osteopontin restored youthful properties.
Hartmut Geiger, PhD, of the University of Ulm in Germany, and his colleagues reported these findings in EMBO.
“We show that the place where HSCs form in the bone marrow loses osteopontin upon aging, but if you give back the missing protein to the blood-forming cells, they suddenly rejuvenate and act younger,” Dr Geiger said.
“Our study points to exciting, novel ways to have a better immune system and possibly less blood cancer upon aging by therapeutically targeting the place where blood stem cells form.”
The researchers conducted a number of experiments to test the formation and vitality of cells in and near the bone marrow microenvironment.
The team looked at the formation of endosteum stroma cells in aging mice and monitored levels of osteopontin and other proteins linked to distinct cells in the bone marrow during the aging process.
The researchers said they observed reduced production of osteoblasts and other stroma cells in the endosteum of older mice. They also saw decreased osteopontin levels in the bone marrow of older animals, which was associated with reduced vigor and function of HSCs.
The team followed up these experiments by transplanting bone marrow cells from older mice (19 to 21 months) into young mice (8 to 10 weeks).
The researchers also transplanted aged HSCs from older mice into younger mice, and the team treated aged HSCs with a recombinant form of the osteopontin protein.
Transplantation into the younger animals caused HSCs to behave in a younger, more vital manner, the researchers said. This meant smaller numbers of HSCs with greater potential for forming different blood cells, which included larger populations of B and T cells and decreased production of myeloid cells.
The researchers also saw aged HSCs treated with recombinant osteopontin regain their youthful characteristics and capacity to form different blood cell types. Also observed was diminished signaling of Cdc42, a protein previously shown to cause aging in HSCs.
Osteopontin levels are not only low in the bone marrow niche but also in the blood upon aging. As a follow-up to the current study, the researchers are investigating the possibility of using osteopontin replacement therapy in mice to counter the influence of an aging niche directly in the animals. ![]()
Preclinical research suggests osteopontin, a protein made by osteoblasts, plays a role in hematopoietic stem cell (HSC) aging.
Experiments in mice revealed that, with age, there is a reduction in the expression of osteopontin in the bone marrow stroma.
A lack of osteopontin induced young HSCs to “act” older, while treating older HSCs with recombinant osteopontin restored youthful properties.
Hartmut Geiger, PhD, of the University of Ulm in Germany, and his colleagues reported these findings in EMBO.
“We show that the place where HSCs form in the bone marrow loses osteopontin upon aging, but if you give back the missing protein to the blood-forming cells, they suddenly rejuvenate and act younger,” Dr Geiger said.
“Our study points to exciting, novel ways to have a better immune system and possibly less blood cancer upon aging by therapeutically targeting the place where blood stem cells form.”
The researchers conducted a number of experiments to test the formation and vitality of cells in and near the bone marrow microenvironment.
The team looked at the formation of endosteum stroma cells in aging mice and monitored levels of osteopontin and other proteins linked to distinct cells in the bone marrow during the aging process.
The researchers said they observed reduced production of osteoblasts and other stroma cells in the endosteum of older mice. They also saw decreased osteopontin levels in the bone marrow of older animals, which was associated with reduced vigor and function of HSCs.
The team followed up these experiments by transplanting bone marrow cells from older mice (19 to 21 months) into young mice (8 to 10 weeks).
The researchers also transplanted aged HSCs from older mice into younger mice, and the team treated aged HSCs with a recombinant form of the osteopontin protein.
Transplantation into the younger animals caused HSCs to behave in a younger, more vital manner, the researchers said. This meant smaller numbers of HSCs with greater potential for forming different blood cells, which included larger populations of B and T cells and decreased production of myeloid cells.
The researchers also saw aged HSCs treated with recombinant osteopontin regain their youthful characteristics and capacity to form different blood cell types. Also observed was diminished signaling of Cdc42, a protein previously shown to cause aging in HSCs.
Osteopontin levels are not only low in the bone marrow niche but also in the blood upon aging. As a follow-up to the current study, the researchers are investigating the possibility of using osteopontin replacement therapy in mice to counter the influence of an aging niche directly in the animals. ![]()