User login
FDA puts REMS requirements on hold to ensure continuity of care
In a Nov. 2 notice on its website, the FDA said it is aware that health care professionals and patients continue to experience ongoing difficulties with the clozapine REMS program, including issues with patient access to clozapine following discharge from inpatient care.
A chief concern is that inpatient pharmacies are only allowed to dispense a 7-days’ supply of clozapine to the patient upon discharge.
To address this issue, the FDA said it will now (temporarily) not object if inpatient pharmacies dispense a days’ supply of clozapine that aligns with the patient’s monitoring frequency.
For example, a 7-days’ supply for weekly monitoring, a 14-days’ supply for twice-monthly monitoring, and a 30-days’ supply for monthly monitoring upon discharge from an inpatient facility.
Clozapine is a second-generation (atypical) antipsychotic used to treat schizophrenia that is not well controlled with standard antipsychotics.
While clozapine can be highly effective in some patients, it also carries serious risks, including a decrease in neutrophil count, which can lead to severe neutropenia, serious infections, and death.
As a result, those taking the drug must undergo regular absolute neutrophil count monitoring. Clozapine REMS is intended to maximize the benefits of the drug and minimize risk.
The FDA says it will continue to exercise earlier enforcement discretion regarding the clozapine REMS program announced back in November 2021. This includes allowing pharmacists to dispense clozapine without a REMS dispense authorization and allowing wholesalers to ship clozapine to pharmacies and health care settings without confirming enrollment in the REMS.
“We understand that difficulties with the clozapine REMS program have caused frustration and have led to problems with patient access to clozapine. FDA takes these concerns seriously. Continuity of care, patient access to clozapine, and patient safety are our highest priorities,” the FDA says.
The agency is working closely with the clozapine REMS program administrators to address these challenges and avoid interruptions in patient care.
The FDA encourages pharmacists and prescribers to continue working with the clozapine REMS to complete certification and prescribers to enroll patients in the program.
A version of this article first appeared on Medscape.com.
In a Nov. 2 notice on its website, the FDA said it is aware that health care professionals and patients continue to experience ongoing difficulties with the clozapine REMS program, including issues with patient access to clozapine following discharge from inpatient care.
A chief concern is that inpatient pharmacies are only allowed to dispense a 7-days’ supply of clozapine to the patient upon discharge.
To address this issue, the FDA said it will now (temporarily) not object if inpatient pharmacies dispense a days’ supply of clozapine that aligns with the patient’s monitoring frequency.
For example, a 7-days’ supply for weekly monitoring, a 14-days’ supply for twice-monthly monitoring, and a 30-days’ supply for monthly monitoring upon discharge from an inpatient facility.
Clozapine is a second-generation (atypical) antipsychotic used to treat schizophrenia that is not well controlled with standard antipsychotics.
While clozapine can be highly effective in some patients, it also carries serious risks, including a decrease in neutrophil count, which can lead to severe neutropenia, serious infections, and death.
As a result, those taking the drug must undergo regular absolute neutrophil count monitoring. Clozapine REMS is intended to maximize the benefits of the drug and minimize risk.
The FDA says it will continue to exercise earlier enforcement discretion regarding the clozapine REMS program announced back in November 2021. This includes allowing pharmacists to dispense clozapine without a REMS dispense authorization and allowing wholesalers to ship clozapine to pharmacies and health care settings without confirming enrollment in the REMS.
“We understand that difficulties with the clozapine REMS program have caused frustration and have led to problems with patient access to clozapine. FDA takes these concerns seriously. Continuity of care, patient access to clozapine, and patient safety are our highest priorities,” the FDA says.
The agency is working closely with the clozapine REMS program administrators to address these challenges and avoid interruptions in patient care.
The FDA encourages pharmacists and prescribers to continue working with the clozapine REMS to complete certification and prescribers to enroll patients in the program.
A version of this article first appeared on Medscape.com.
In a Nov. 2 notice on its website, the FDA said it is aware that health care professionals and patients continue to experience ongoing difficulties with the clozapine REMS program, including issues with patient access to clozapine following discharge from inpatient care.
A chief concern is that inpatient pharmacies are only allowed to dispense a 7-days’ supply of clozapine to the patient upon discharge.
To address this issue, the FDA said it will now (temporarily) not object if inpatient pharmacies dispense a days’ supply of clozapine that aligns with the patient’s monitoring frequency.
For example, a 7-days’ supply for weekly monitoring, a 14-days’ supply for twice-monthly monitoring, and a 30-days’ supply for monthly monitoring upon discharge from an inpatient facility.
Clozapine is a second-generation (atypical) antipsychotic used to treat schizophrenia that is not well controlled with standard antipsychotics.
While clozapine can be highly effective in some patients, it also carries serious risks, including a decrease in neutrophil count, which can lead to severe neutropenia, serious infections, and death.
As a result, those taking the drug must undergo regular absolute neutrophil count monitoring. Clozapine REMS is intended to maximize the benefits of the drug and minimize risk.
The FDA says it will continue to exercise earlier enforcement discretion regarding the clozapine REMS program announced back in November 2021. This includes allowing pharmacists to dispense clozapine without a REMS dispense authorization and allowing wholesalers to ship clozapine to pharmacies and health care settings without confirming enrollment in the REMS.
“We understand that difficulties with the clozapine REMS program have caused frustration and have led to problems with patient access to clozapine. FDA takes these concerns seriously. Continuity of care, patient access to clozapine, and patient safety are our highest priorities,” the FDA says.
The agency is working closely with the clozapine REMS program administrators to address these challenges and avoid interruptions in patient care.
The FDA encourages pharmacists and prescribers to continue working with the clozapine REMS to complete certification and prescribers to enroll patients in the program.
A version of this article first appeared on Medscape.com.
Patients with schizophrenia may be twice as likely to develop dementia
Results from a review and meta-analysis of almost 13 million total participants from nine countries showed that, across multiple different psychotic disorders, there was a 2.5-fold higher risk of developing dementia later in life compared with individuals who did not have a disorder. This was regardless of the age at which the patients first developed the mental illness.
Moreover, participants with a psychotic disorder tended to be younger than average when diagnosed with dementia. Two studies showed that those with psychotic disorders were more likely to be diagnosed with dementia as early as in their 60s.
“The findings add to a growing body of evidence linking psychiatric disorders with later cognitive decline and dementia,” senior investigator Jean Stafford, PhD, a research fellow at MRC Unit for Lifelong Health and Ageing, University College London, told this news organization.
Dr. Stafford noted that the results highlight the importance of being aware of and watchful for symptoms of cognitive decline in patients with psychotic disorders in mid- and late life.
“In addition, given that people with psychotic disorders are at higher risk of experiencing multiple health conditions, including dementia, managing overall physical and mental health in this group is crucial,” she said.
The findings were published online in Psychological Medicine.
Bringing the evidence together
There is increasing evidence that multiple psychiatric symptoms and diagnoses are associated with cognitive decline and dementia, with particularly strong evidence for late-life depression, Dr. Stafford said.
“However, the relationship between psychotic disorders and dementia is less well-established,” she added.
Last year, her team published a study showing a strong association between very late onset psychotic disorders, defined as first diagnosed after age 60 years, and increased risk for dementia in Swedish population register data.
“We also became aware of several other large studies on the topic published in the last few years and realized that an up-to-date systematic review and meta-analysis was needed to bring together the evidence, specifically focusing on longitudinal studies,” Dr. Stafford said.
The researchers searched four databases of prospective and retrospective longitudinal studies published through March 2022. Studies were required to focus on adults aged 18 years or older with a clinical diagnosis of a nonaffective psychotic disorder and a comparison group consisting of adults without a nonaffective psychotic disorder.
Of 9,496 papers, the investigators selected 11 published from 2003 to 2022 that met criteria for inclusion in their meta-analysis (12,997,101 participants), with follow-up periods ranging from 1.57 to 33 years.
The studies hailed from Denmark, Finland, Sweden, the United Kingdom, the United States, Australia, Taiwan, New Zealand, and Israel.
Random-effects meta-analyses were used to pool estimates across studies. The researchers assessed the risk of bias for each study. They also included two additional studies in the review, but not the meta-analysis, that focused specifically on late-onset acute and transient psychosis and late-onset delusional disorder.
The other studies focused on late-onset schizophrenia and/or very late onset schizophrenia-like psychoses, schizophrenia, psychotic disorders, and schizophrenia in older people.
Most studies investigated the incidence of all-cause dementia, although one study focused on the incidence of Alzheimer’s disease.
Potential mechanisms
The narrative review showed that most studies (n = 10) were of high methodological quality, although two were rated as fair and one as poor.
Almost all studies accounted for basic sociodemographic confounders. Several also adjusted for comorbidities, alcohol/substance use disorders, medications, smoking status, and income/education level.
Pooled estimates from the meta-analyzed studies showed that only one showed no significant association between psychotic disorders and dementia, whereas 10 reported increased risk (pooled risk ratio, 2.52; 95% confidence interval, 1.67-3.80; I2, 99.7%).
Subgroup analyses showed higher risk in participants with typical and late-onset psychotic disorders (pooled RR, 2.10; 95% CI, 2.33-4.14; I2, 77.5%; P = .004) vs. those with very late onset schizophrenia-like psychoses (pooled RR, 2.77; 95% CI, 1.74-4.40 I2, 98.9%; P < .001).
The effect was larger in studies with a follow-up of less than 10 years vs. those with a follow-up of 10 years or more, and it was also greater in studies conducted in non-European vs. European countries (all P < .001).
Studies with more female participants (≥ 60%) showed higher risk compared with those that had a lower percentage of female participants. Studies published during or after 2020 showed a stronger association than those published before 2020 (all P < .001).
There was also a higher risk for dementia in studies investigating broader nonaffective psychotic disorders compared with studies investigating only schizophrenia, in prospective vs. retrospective studies, and in studies with a minimum age of less than 60 years at baseline vs. a minimum age of 60 or older (all P < .001).
“Several possible mechanisms could underlie these findings, although we were not able to directly test these in our review,” Dr. Stafford said. She noted that psychotic disorders and other psychiatric diagnoses may cause dementia.
“People with psychotic disorders such as schizophrenia are also at higher risk of health conditions including cardiovascular disease and diabetes, which are known risk factors for dementia and could underpin these associations,” said Dr. Stafford.
It is also possible “that psychotic symptoms could be early markers of dementia for some people, rather than causes,” she added.
Neuroimaging evidence lacking
Commenting on the study, Dilip V. Jeste, MD, former senior associate dean for healthy aging and senior care and distinguished professor of psychiatry and neurosciences at the University of California, San Diego, complimented the investigators for “an excellent article on an important but difficult topic.”
Limitations “pertain not to the meta-analysis but to the original studies,” said Dr. Jeste, who was not involved with the review. Diagnosing dementia in individuals with psychotic disorders is “challenging because cognitive deficits and behavioral symptoms in psychotic disorders may be misdiagnosed as dementia in some individuals – and vice versa,” he added.
Moreover, the studies did not specify the type of dementia, such as Alzheimer’s disease, vascular, Lewy body, frontotemporal, or mixed. Together, “they account for 90% of the dementias, and most patients with these dementias have brain abnormalities that can clearly be seen on MRI,” Dr. Jeste said.
However, patients with schizophrenia who are diagnosed with dementia “rarely show severe brain atrophy, even in specific regions commonly observed in nonpsychotic people with these dementias,” Dr. Jeste noted.
Thus, objective neuroimaging-based evidence for dementia and its subtype “is lacking in most of the published studies of persons with psychotic disorders diagnosed as having dementia,” he said.
There is a “clear need for comprehensive studies of dementia in people with psychotic disorders to understand the significance of the results,” Dr. Jeste concluded.
The review did not receive any funding. Dr. Stafford was supported by an NIHR-UCLH BRC Postdoctoral Bridging Fellowship and the National Institute for Health Research Biomedical Research Centre at University College London Hospitals NHS Foundation Trust. Dr. Stafford was also the principal investigator in one of the studies meeting the inclusion criteria of the review. The other investigators and Dr. Jeste reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results from a review and meta-analysis of almost 13 million total participants from nine countries showed that, across multiple different psychotic disorders, there was a 2.5-fold higher risk of developing dementia later in life compared with individuals who did not have a disorder. This was regardless of the age at which the patients first developed the mental illness.
Moreover, participants with a psychotic disorder tended to be younger than average when diagnosed with dementia. Two studies showed that those with psychotic disorders were more likely to be diagnosed with dementia as early as in their 60s.
“The findings add to a growing body of evidence linking psychiatric disorders with later cognitive decline and dementia,” senior investigator Jean Stafford, PhD, a research fellow at MRC Unit for Lifelong Health and Ageing, University College London, told this news organization.
Dr. Stafford noted that the results highlight the importance of being aware of and watchful for symptoms of cognitive decline in patients with psychotic disorders in mid- and late life.
“In addition, given that people with psychotic disorders are at higher risk of experiencing multiple health conditions, including dementia, managing overall physical and mental health in this group is crucial,” she said.
The findings were published online in Psychological Medicine.
Bringing the evidence together
There is increasing evidence that multiple psychiatric symptoms and diagnoses are associated with cognitive decline and dementia, with particularly strong evidence for late-life depression, Dr. Stafford said.
“However, the relationship between psychotic disorders and dementia is less well-established,” she added.
Last year, her team published a study showing a strong association between very late onset psychotic disorders, defined as first diagnosed after age 60 years, and increased risk for dementia in Swedish population register data.
“We also became aware of several other large studies on the topic published in the last few years and realized that an up-to-date systematic review and meta-analysis was needed to bring together the evidence, specifically focusing on longitudinal studies,” Dr. Stafford said.
The researchers searched four databases of prospective and retrospective longitudinal studies published through March 2022. Studies were required to focus on adults aged 18 years or older with a clinical diagnosis of a nonaffective psychotic disorder and a comparison group consisting of adults without a nonaffective psychotic disorder.
Of 9,496 papers, the investigators selected 11 published from 2003 to 2022 that met criteria for inclusion in their meta-analysis (12,997,101 participants), with follow-up periods ranging from 1.57 to 33 years.
The studies hailed from Denmark, Finland, Sweden, the United Kingdom, the United States, Australia, Taiwan, New Zealand, and Israel.
Random-effects meta-analyses were used to pool estimates across studies. The researchers assessed the risk of bias for each study. They also included two additional studies in the review, but not the meta-analysis, that focused specifically on late-onset acute and transient psychosis and late-onset delusional disorder.
The other studies focused on late-onset schizophrenia and/or very late onset schizophrenia-like psychoses, schizophrenia, psychotic disorders, and schizophrenia in older people.
Most studies investigated the incidence of all-cause dementia, although one study focused on the incidence of Alzheimer’s disease.
Potential mechanisms
The narrative review showed that most studies (n = 10) were of high methodological quality, although two were rated as fair and one as poor.
Almost all studies accounted for basic sociodemographic confounders. Several also adjusted for comorbidities, alcohol/substance use disorders, medications, smoking status, and income/education level.
Pooled estimates from the meta-analyzed studies showed that only one showed no significant association between psychotic disorders and dementia, whereas 10 reported increased risk (pooled risk ratio, 2.52; 95% confidence interval, 1.67-3.80; I2, 99.7%).
Subgroup analyses showed higher risk in participants with typical and late-onset psychotic disorders (pooled RR, 2.10; 95% CI, 2.33-4.14; I2, 77.5%; P = .004) vs. those with very late onset schizophrenia-like psychoses (pooled RR, 2.77; 95% CI, 1.74-4.40 I2, 98.9%; P < .001).
The effect was larger in studies with a follow-up of less than 10 years vs. those with a follow-up of 10 years or more, and it was also greater in studies conducted in non-European vs. European countries (all P < .001).
Studies with more female participants (≥ 60%) showed higher risk compared with those that had a lower percentage of female participants. Studies published during or after 2020 showed a stronger association than those published before 2020 (all P < .001).
There was also a higher risk for dementia in studies investigating broader nonaffective psychotic disorders compared with studies investigating only schizophrenia, in prospective vs. retrospective studies, and in studies with a minimum age of less than 60 years at baseline vs. a minimum age of 60 or older (all P < .001).
“Several possible mechanisms could underlie these findings, although we were not able to directly test these in our review,” Dr. Stafford said. She noted that psychotic disorders and other psychiatric diagnoses may cause dementia.
“People with psychotic disorders such as schizophrenia are also at higher risk of health conditions including cardiovascular disease and diabetes, which are known risk factors for dementia and could underpin these associations,” said Dr. Stafford.
It is also possible “that psychotic symptoms could be early markers of dementia for some people, rather than causes,” she added.
Neuroimaging evidence lacking
Commenting on the study, Dilip V. Jeste, MD, former senior associate dean for healthy aging and senior care and distinguished professor of psychiatry and neurosciences at the University of California, San Diego, complimented the investigators for “an excellent article on an important but difficult topic.”
Limitations “pertain not to the meta-analysis but to the original studies,” said Dr. Jeste, who was not involved with the review. Diagnosing dementia in individuals with psychotic disorders is “challenging because cognitive deficits and behavioral symptoms in psychotic disorders may be misdiagnosed as dementia in some individuals – and vice versa,” he added.
Moreover, the studies did not specify the type of dementia, such as Alzheimer’s disease, vascular, Lewy body, frontotemporal, or mixed. Together, “they account for 90% of the dementias, and most patients with these dementias have brain abnormalities that can clearly be seen on MRI,” Dr. Jeste said.
However, patients with schizophrenia who are diagnosed with dementia “rarely show severe brain atrophy, even in specific regions commonly observed in nonpsychotic people with these dementias,” Dr. Jeste noted.
Thus, objective neuroimaging-based evidence for dementia and its subtype “is lacking in most of the published studies of persons with psychotic disorders diagnosed as having dementia,” he said.
There is a “clear need for comprehensive studies of dementia in people with psychotic disorders to understand the significance of the results,” Dr. Jeste concluded.
The review did not receive any funding. Dr. Stafford was supported by an NIHR-UCLH BRC Postdoctoral Bridging Fellowship and the National Institute for Health Research Biomedical Research Centre at University College London Hospitals NHS Foundation Trust. Dr. Stafford was also the principal investigator in one of the studies meeting the inclusion criteria of the review. The other investigators and Dr. Jeste reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results from a review and meta-analysis of almost 13 million total participants from nine countries showed that, across multiple different psychotic disorders, there was a 2.5-fold higher risk of developing dementia later in life compared with individuals who did not have a disorder. This was regardless of the age at which the patients first developed the mental illness.
Moreover, participants with a psychotic disorder tended to be younger than average when diagnosed with dementia. Two studies showed that those with psychotic disorders were more likely to be diagnosed with dementia as early as in their 60s.
“The findings add to a growing body of evidence linking psychiatric disorders with later cognitive decline and dementia,” senior investigator Jean Stafford, PhD, a research fellow at MRC Unit for Lifelong Health and Ageing, University College London, told this news organization.
Dr. Stafford noted that the results highlight the importance of being aware of and watchful for symptoms of cognitive decline in patients with psychotic disorders in mid- and late life.
“In addition, given that people with psychotic disorders are at higher risk of experiencing multiple health conditions, including dementia, managing overall physical and mental health in this group is crucial,” she said.
The findings were published online in Psychological Medicine.
Bringing the evidence together
There is increasing evidence that multiple psychiatric symptoms and diagnoses are associated with cognitive decline and dementia, with particularly strong evidence for late-life depression, Dr. Stafford said.
“However, the relationship between psychotic disorders and dementia is less well-established,” she added.
Last year, her team published a study showing a strong association between very late onset psychotic disorders, defined as first diagnosed after age 60 years, and increased risk for dementia in Swedish population register data.
“We also became aware of several other large studies on the topic published in the last few years and realized that an up-to-date systematic review and meta-analysis was needed to bring together the evidence, specifically focusing on longitudinal studies,” Dr. Stafford said.
The researchers searched four databases of prospective and retrospective longitudinal studies published through March 2022. Studies were required to focus on adults aged 18 years or older with a clinical diagnosis of a nonaffective psychotic disorder and a comparison group consisting of adults without a nonaffective psychotic disorder.
Of 9,496 papers, the investigators selected 11 published from 2003 to 2022 that met criteria for inclusion in their meta-analysis (12,997,101 participants), with follow-up periods ranging from 1.57 to 33 years.
The studies hailed from Denmark, Finland, Sweden, the United Kingdom, the United States, Australia, Taiwan, New Zealand, and Israel.
Random-effects meta-analyses were used to pool estimates across studies. The researchers assessed the risk of bias for each study. They also included two additional studies in the review, but not the meta-analysis, that focused specifically on late-onset acute and transient psychosis and late-onset delusional disorder.
The other studies focused on late-onset schizophrenia and/or very late onset schizophrenia-like psychoses, schizophrenia, psychotic disorders, and schizophrenia in older people.
Most studies investigated the incidence of all-cause dementia, although one study focused on the incidence of Alzheimer’s disease.
Potential mechanisms
The narrative review showed that most studies (n = 10) were of high methodological quality, although two were rated as fair and one as poor.
Almost all studies accounted for basic sociodemographic confounders. Several also adjusted for comorbidities, alcohol/substance use disorders, medications, smoking status, and income/education level.
Pooled estimates from the meta-analyzed studies showed that only one showed no significant association between psychotic disorders and dementia, whereas 10 reported increased risk (pooled risk ratio, 2.52; 95% confidence interval, 1.67-3.80; I2, 99.7%).
Subgroup analyses showed higher risk in participants with typical and late-onset psychotic disorders (pooled RR, 2.10; 95% CI, 2.33-4.14; I2, 77.5%; P = .004) vs. those with very late onset schizophrenia-like psychoses (pooled RR, 2.77; 95% CI, 1.74-4.40 I2, 98.9%; P < .001).
The effect was larger in studies with a follow-up of less than 10 years vs. those with a follow-up of 10 years or more, and it was also greater in studies conducted in non-European vs. European countries (all P < .001).
Studies with more female participants (≥ 60%) showed higher risk compared with those that had a lower percentage of female participants. Studies published during or after 2020 showed a stronger association than those published before 2020 (all P < .001).
There was also a higher risk for dementia in studies investigating broader nonaffective psychotic disorders compared with studies investigating only schizophrenia, in prospective vs. retrospective studies, and in studies with a minimum age of less than 60 years at baseline vs. a minimum age of 60 or older (all P < .001).
“Several possible mechanisms could underlie these findings, although we were not able to directly test these in our review,” Dr. Stafford said. She noted that psychotic disorders and other psychiatric diagnoses may cause dementia.
“People with psychotic disorders such as schizophrenia are also at higher risk of health conditions including cardiovascular disease and diabetes, which are known risk factors for dementia and could underpin these associations,” said Dr. Stafford.
It is also possible “that psychotic symptoms could be early markers of dementia for some people, rather than causes,” she added.
Neuroimaging evidence lacking
Commenting on the study, Dilip V. Jeste, MD, former senior associate dean for healthy aging and senior care and distinguished professor of psychiatry and neurosciences at the University of California, San Diego, complimented the investigators for “an excellent article on an important but difficult topic.”
Limitations “pertain not to the meta-analysis but to the original studies,” said Dr. Jeste, who was not involved with the review. Diagnosing dementia in individuals with psychotic disorders is “challenging because cognitive deficits and behavioral symptoms in psychotic disorders may be misdiagnosed as dementia in some individuals – and vice versa,” he added.
Moreover, the studies did not specify the type of dementia, such as Alzheimer’s disease, vascular, Lewy body, frontotemporal, or mixed. Together, “they account for 90% of the dementias, and most patients with these dementias have brain abnormalities that can clearly be seen on MRI,” Dr. Jeste said.
However, patients with schizophrenia who are diagnosed with dementia “rarely show severe brain atrophy, even in specific regions commonly observed in nonpsychotic people with these dementias,” Dr. Jeste noted.
Thus, objective neuroimaging-based evidence for dementia and its subtype “is lacking in most of the published studies of persons with psychotic disorders diagnosed as having dementia,” he said.
There is a “clear need for comprehensive studies of dementia in people with psychotic disorders to understand the significance of the results,” Dr. Jeste concluded.
The review did not receive any funding. Dr. Stafford was supported by an NIHR-UCLH BRC Postdoctoral Bridging Fellowship and the National Institute for Health Research Biomedical Research Centre at University College London Hospitals NHS Foundation Trust. Dr. Stafford was also the principal investigator in one of the studies meeting the inclusion criteria of the review. The other investigators and Dr. Jeste reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM PSYCHOLOGICAL MEDICINE
Menopause an independent risk factor for schizophrenia relapse
Investigators studied a cohort of close to 62,000 people with SSDs, stratifying individuals by sex and age, and found that starting between the ages of 45 and 50 years – when the menopausal transition is underway – women were more frequently hospitalized for psychosis, compared with men and women younger than 45 years.
In addition, the protective effect of antipsychotic medication was highest in women younger than 45 years and lowest in women aged 45 years or older, even at higher doses.
“Women with schizophrenia who are older than 45 are a vulnerable group for relapse, and higher doses of antipsychotics are not the answer,” lead author Iris Sommer, MD, PhD, professor, department of neuroscience, University Medical Center of Groningen, the Netherlands, told this news organization.
The study was published online in Schizophrenia Bulletin.
Vulnerable period
There is an association between estrogen levels and disease severity throughout the life stages of women with SSDs, with lower estrogen levels associated with psychosis, for example, during low estrogenic phases of the menstrual cycle, the investigators note.
“After menopause, estrogen levels remain low, which is associated with a deterioration in the clinical course; therefore, women with SSD have sex-specific psychiatric needs that differ according to their life stage,” they add.
“Estrogens inhibit an important liver enzyme (cytochrome P-450 [CYP1A2]), which leads to higher blood levels of several antipsychotics like olanzapine and clozapine,” said Dr. Sommer. In addition, estrogens make the stomach less acidic, “leading to easier resorption of medication.”
As a clinician, Dr. Sommer said that she has “often witnessed a worsening of symptoms [of psychosis] after menopause.” As a researcher, she “knew that estrogens can have ameliorating effects on brain health, especially in schizophrenia.”
She and her colleagues were motivated to research the issue because there is a “remarkable paucity” of quantitative data on a “vulnerable period that all women with schizophrenia will experience.”
Detailed, quantitative data
The researchers sought to provide “detailed, quantitative data on life-stage dependent clinical changes occurring in women with SSD, using an intra-individual design to prevent confounding.”
They drew on data from a nationwide, register-based cohort study of all hospitalized patients with SSD between 1972 and 2014 in Finland (n = 61,889), with follow-up from Jan. 1, 1996, to Dec. 31, 2017.
People were stratified according to age (younger than 45 years and 45 years or older), with the same person contributing person-time to both age groups. The cohort was also subdivided into 5-year age groups, starting at age 20 years and ending at age 69 years.
The primary outcome measure was relapse (that is, inpatient hospitalization because of psychosis).
The researchers focused specifically on monotherapies, excluding time periods when two or more antipsychotics were used concomitantly. They also looked at antipsychotic nonuse periods.
Antipsychotic monotherapies were categorized into defined daily doses per day (DDDs/d):
- less than 0.4
- 0.4 to 0.6
- 0.6 to 0.9
- 0.9 to less than 1.1
- 1.1 to less than 1.4
- 1.4 to less than 1.6
- 1.6 or more
The researchers restricted the main analyses to the four most frequently used oral antipsychotic monotherapies: clozapine, olanzapine, quetiapine, and risperidone.
The turning tide
The cohort consisted of more men than women (31,104 vs. 30,785, respectively), with a mean (standard deviation) age of 49.8 (16.6) years in women vs. 43.6 (14.8) in men.
Among both sexes, olanzapine was the most prescribed antipsychotic (roughly one-quarter of patients). In women, the next most common antipsychotic was risperidone, followed by quetiapine and clozapine, whereas in men, the second most common antipsychotic was clozapine, followed by risperidone and quetiapine.
When the researchers compared men and women younger than 45 years, there were “few consistent differences” in proportions hospitalized for psychosis.
Starting at age 45 years and continuing through the oldest age group (65-69 years), higher proportions of women were hospitalized for psychosis, compared with their male peers (all Ps < .00001).
Women 45 or older had significantly higher risk for relapse associated with standard dose use, compared with the other groups.
When the researchers compared men and women older and younger than 45 years, women younger than 45 years showed lower adjusted hazard ratios (aHRs) at doses between of 0.6-0.9 DDDs/d, whereas for doses over 1.1 DDDs/d, women aged 45 years or older showed “remarkably higher” aHRs, compared with women younger than 45 years and men aged 45 years or older, with a difference that increased with increasing dose.
In women, the efficacy of the antipsychotics was decreased at these DDDs/d.
“We ... showed that antipsychotic monotherapy is most effective in preventing relapse in women below 45, as compared to women above that age, and also as compared to men of all ages,” the authors summarize. But after age 45 years, “the tide seems to turn for women,” compared with younger women and with men of the same age group.
One of several study limitations was the use of age as an estimation of menopausal status, they note.
Don’t just raise the dose
Commenting on the research, Mary Seeman, MD, professor emerita, department of psychiatry, University of Toronto, noted the study corroborates her group’s findings regarding the effect of menopause on antipsychotic response.
“When the efficacy of previously effective antipsychotic doses wanes at menopause, raising the dose is not the treatment of choice because it increases the risk of weight gain, cardiovascular, and cerebrovascular events,” said Dr. Seeman, who was not involved with the current research.
“Changing to an antipsychotic that is less affected by estrogen loss may work better,” she continued, noting that amisulpride and aripiprazole “work well post menopause.”
Additional interventions may include changing to a depot or skin-patch antipsychotic that “obviates first-pass metabolism,” adding hormone replacement or a selective estrogen receptor modulator or including phytoestrogens (bioidenticals) in the diet.
The study yields research recommendations, including comparing the effectiveness of different antipsychotics in postmenopausal women with SSDs, recruiting pre- and postmenopausal women in trials of antipsychotic drugs, and stratifying by hormonal status when analyzing results of antipsychotic trials, Dr. Seeman said.
This work was supported by the Finnish Ministry of Social Affairs and Health through the developmental fund for Niuvanniemi Hospital and the Academy of Finland. The Dutch Medical Research Association supported Dr. Sommer. Dr. Sommer declares no relevant financial relationships. The other authors’ disclosures are listed on the original paper. Dr. Seeman declares no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators studied a cohort of close to 62,000 people with SSDs, stratifying individuals by sex and age, and found that starting between the ages of 45 and 50 years – when the menopausal transition is underway – women were more frequently hospitalized for psychosis, compared with men and women younger than 45 years.
In addition, the protective effect of antipsychotic medication was highest in women younger than 45 years and lowest in women aged 45 years or older, even at higher doses.
“Women with schizophrenia who are older than 45 are a vulnerable group for relapse, and higher doses of antipsychotics are not the answer,” lead author Iris Sommer, MD, PhD, professor, department of neuroscience, University Medical Center of Groningen, the Netherlands, told this news organization.
The study was published online in Schizophrenia Bulletin.
Vulnerable period
There is an association between estrogen levels and disease severity throughout the life stages of women with SSDs, with lower estrogen levels associated with psychosis, for example, during low estrogenic phases of the menstrual cycle, the investigators note.
“After menopause, estrogen levels remain low, which is associated with a deterioration in the clinical course; therefore, women with SSD have sex-specific psychiatric needs that differ according to their life stage,” they add.
“Estrogens inhibit an important liver enzyme (cytochrome P-450 [CYP1A2]), which leads to higher blood levels of several antipsychotics like olanzapine and clozapine,” said Dr. Sommer. In addition, estrogens make the stomach less acidic, “leading to easier resorption of medication.”
As a clinician, Dr. Sommer said that she has “often witnessed a worsening of symptoms [of psychosis] after menopause.” As a researcher, she “knew that estrogens can have ameliorating effects on brain health, especially in schizophrenia.”
She and her colleagues were motivated to research the issue because there is a “remarkable paucity” of quantitative data on a “vulnerable period that all women with schizophrenia will experience.”
Detailed, quantitative data
The researchers sought to provide “detailed, quantitative data on life-stage dependent clinical changes occurring in women with SSD, using an intra-individual design to prevent confounding.”
They drew on data from a nationwide, register-based cohort study of all hospitalized patients with SSD between 1972 and 2014 in Finland (n = 61,889), with follow-up from Jan. 1, 1996, to Dec. 31, 2017.
People were stratified according to age (younger than 45 years and 45 years or older), with the same person contributing person-time to both age groups. The cohort was also subdivided into 5-year age groups, starting at age 20 years and ending at age 69 years.
The primary outcome measure was relapse (that is, inpatient hospitalization because of psychosis).
The researchers focused specifically on monotherapies, excluding time periods when two or more antipsychotics were used concomitantly. They also looked at antipsychotic nonuse periods.
Antipsychotic monotherapies were categorized into defined daily doses per day (DDDs/d):
- less than 0.4
- 0.4 to 0.6
- 0.6 to 0.9
- 0.9 to less than 1.1
- 1.1 to less than 1.4
- 1.4 to less than 1.6
- 1.6 or more
The researchers restricted the main analyses to the four most frequently used oral antipsychotic monotherapies: clozapine, olanzapine, quetiapine, and risperidone.
The turning tide
The cohort consisted of more men than women (31,104 vs. 30,785, respectively), with a mean (standard deviation) age of 49.8 (16.6) years in women vs. 43.6 (14.8) in men.
Among both sexes, olanzapine was the most prescribed antipsychotic (roughly one-quarter of patients). In women, the next most common antipsychotic was risperidone, followed by quetiapine and clozapine, whereas in men, the second most common antipsychotic was clozapine, followed by risperidone and quetiapine.
When the researchers compared men and women younger than 45 years, there were “few consistent differences” in proportions hospitalized for psychosis.
Starting at age 45 years and continuing through the oldest age group (65-69 years), higher proportions of women were hospitalized for psychosis, compared with their male peers (all Ps < .00001).
Women 45 or older had significantly higher risk for relapse associated with standard dose use, compared with the other groups.
When the researchers compared men and women older and younger than 45 years, women younger than 45 years showed lower adjusted hazard ratios (aHRs) at doses between of 0.6-0.9 DDDs/d, whereas for doses over 1.1 DDDs/d, women aged 45 years or older showed “remarkably higher” aHRs, compared with women younger than 45 years and men aged 45 years or older, with a difference that increased with increasing dose.
In women, the efficacy of the antipsychotics was decreased at these DDDs/d.
“We ... showed that antipsychotic monotherapy is most effective in preventing relapse in women below 45, as compared to women above that age, and also as compared to men of all ages,” the authors summarize. But after age 45 years, “the tide seems to turn for women,” compared with younger women and with men of the same age group.
One of several study limitations was the use of age as an estimation of menopausal status, they note.
Don’t just raise the dose
Commenting on the research, Mary Seeman, MD, professor emerita, department of psychiatry, University of Toronto, noted the study corroborates her group’s findings regarding the effect of menopause on antipsychotic response.
“When the efficacy of previously effective antipsychotic doses wanes at menopause, raising the dose is not the treatment of choice because it increases the risk of weight gain, cardiovascular, and cerebrovascular events,” said Dr. Seeman, who was not involved with the current research.
“Changing to an antipsychotic that is less affected by estrogen loss may work better,” she continued, noting that amisulpride and aripiprazole “work well post menopause.”
Additional interventions may include changing to a depot or skin-patch antipsychotic that “obviates first-pass metabolism,” adding hormone replacement or a selective estrogen receptor modulator or including phytoestrogens (bioidenticals) in the diet.
The study yields research recommendations, including comparing the effectiveness of different antipsychotics in postmenopausal women with SSDs, recruiting pre- and postmenopausal women in trials of antipsychotic drugs, and stratifying by hormonal status when analyzing results of antipsychotic trials, Dr. Seeman said.
This work was supported by the Finnish Ministry of Social Affairs and Health through the developmental fund for Niuvanniemi Hospital and the Academy of Finland. The Dutch Medical Research Association supported Dr. Sommer. Dr. Sommer declares no relevant financial relationships. The other authors’ disclosures are listed on the original paper. Dr. Seeman declares no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators studied a cohort of close to 62,000 people with SSDs, stratifying individuals by sex and age, and found that starting between the ages of 45 and 50 years – when the menopausal transition is underway – women were more frequently hospitalized for psychosis, compared with men and women younger than 45 years.
In addition, the protective effect of antipsychotic medication was highest in women younger than 45 years and lowest in women aged 45 years or older, even at higher doses.
“Women with schizophrenia who are older than 45 are a vulnerable group for relapse, and higher doses of antipsychotics are not the answer,” lead author Iris Sommer, MD, PhD, professor, department of neuroscience, University Medical Center of Groningen, the Netherlands, told this news organization.
The study was published online in Schizophrenia Bulletin.
Vulnerable period
There is an association between estrogen levels and disease severity throughout the life stages of women with SSDs, with lower estrogen levels associated with psychosis, for example, during low estrogenic phases of the menstrual cycle, the investigators note.
“After menopause, estrogen levels remain low, which is associated with a deterioration in the clinical course; therefore, women with SSD have sex-specific psychiatric needs that differ according to their life stage,” they add.
“Estrogens inhibit an important liver enzyme (cytochrome P-450 [CYP1A2]), which leads to higher blood levels of several antipsychotics like olanzapine and clozapine,” said Dr. Sommer. In addition, estrogens make the stomach less acidic, “leading to easier resorption of medication.”
As a clinician, Dr. Sommer said that she has “often witnessed a worsening of symptoms [of psychosis] after menopause.” As a researcher, she “knew that estrogens can have ameliorating effects on brain health, especially in schizophrenia.”
She and her colleagues were motivated to research the issue because there is a “remarkable paucity” of quantitative data on a “vulnerable period that all women with schizophrenia will experience.”
Detailed, quantitative data
The researchers sought to provide “detailed, quantitative data on life-stage dependent clinical changes occurring in women with SSD, using an intra-individual design to prevent confounding.”
They drew on data from a nationwide, register-based cohort study of all hospitalized patients with SSD between 1972 and 2014 in Finland (n = 61,889), with follow-up from Jan. 1, 1996, to Dec. 31, 2017.
People were stratified according to age (younger than 45 years and 45 years or older), with the same person contributing person-time to both age groups. The cohort was also subdivided into 5-year age groups, starting at age 20 years and ending at age 69 years.
The primary outcome measure was relapse (that is, inpatient hospitalization because of psychosis).
The researchers focused specifically on monotherapies, excluding time periods when two or more antipsychotics were used concomitantly. They also looked at antipsychotic nonuse periods.
Antipsychotic monotherapies were categorized into defined daily doses per day (DDDs/d):
- less than 0.4
- 0.4 to 0.6
- 0.6 to 0.9
- 0.9 to less than 1.1
- 1.1 to less than 1.4
- 1.4 to less than 1.6
- 1.6 or more
The researchers restricted the main analyses to the four most frequently used oral antipsychotic monotherapies: clozapine, olanzapine, quetiapine, and risperidone.
The turning tide
The cohort consisted of more men than women (31,104 vs. 30,785, respectively), with a mean (standard deviation) age of 49.8 (16.6) years in women vs. 43.6 (14.8) in men.
Among both sexes, olanzapine was the most prescribed antipsychotic (roughly one-quarter of patients). In women, the next most common antipsychotic was risperidone, followed by quetiapine and clozapine, whereas in men, the second most common antipsychotic was clozapine, followed by risperidone and quetiapine.
When the researchers compared men and women younger than 45 years, there were “few consistent differences” in proportions hospitalized for psychosis.
Starting at age 45 years and continuing through the oldest age group (65-69 years), higher proportions of women were hospitalized for psychosis, compared with their male peers (all Ps < .00001).
Women 45 or older had significantly higher risk for relapse associated with standard dose use, compared with the other groups.
When the researchers compared men and women older and younger than 45 years, women younger than 45 years showed lower adjusted hazard ratios (aHRs) at doses between of 0.6-0.9 DDDs/d, whereas for doses over 1.1 DDDs/d, women aged 45 years or older showed “remarkably higher” aHRs, compared with women younger than 45 years and men aged 45 years or older, with a difference that increased with increasing dose.
In women, the efficacy of the antipsychotics was decreased at these DDDs/d.
“We ... showed that antipsychotic monotherapy is most effective in preventing relapse in women below 45, as compared to women above that age, and also as compared to men of all ages,” the authors summarize. But after age 45 years, “the tide seems to turn for women,” compared with younger women and with men of the same age group.
One of several study limitations was the use of age as an estimation of menopausal status, they note.
Don’t just raise the dose
Commenting on the research, Mary Seeman, MD, professor emerita, department of psychiatry, University of Toronto, noted the study corroborates her group’s findings regarding the effect of menopause on antipsychotic response.
“When the efficacy of previously effective antipsychotic doses wanes at menopause, raising the dose is not the treatment of choice because it increases the risk of weight gain, cardiovascular, and cerebrovascular events,” said Dr. Seeman, who was not involved with the current research.
“Changing to an antipsychotic that is less affected by estrogen loss may work better,” she continued, noting that amisulpride and aripiprazole “work well post menopause.”
Additional interventions may include changing to a depot or skin-patch antipsychotic that “obviates first-pass metabolism,” adding hormone replacement or a selective estrogen receptor modulator or including phytoestrogens (bioidenticals) in the diet.
The study yields research recommendations, including comparing the effectiveness of different antipsychotics in postmenopausal women with SSDs, recruiting pre- and postmenopausal women in trials of antipsychotic drugs, and stratifying by hormonal status when analyzing results of antipsychotic trials, Dr. Seeman said.
This work was supported by the Finnish Ministry of Social Affairs and Health through the developmental fund for Niuvanniemi Hospital and the Academy of Finland. The Dutch Medical Research Association supported Dr. Sommer. Dr. Sommer declares no relevant financial relationships. The other authors’ disclosures are listed on the original paper. Dr. Seeman declares no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM SCHIZOPHRENIA BULLETIN
Impaired communication predicts coercive inpatient psychiatric care
Despite improvements in reducing coercive measures in psychiatric inpatient care, both involuntary admission and coercive measures remain in use in many countries worldwide, wrote Celline Cole, MSc, a doctoral candidate at Charité Universitätsmedizin, Berlin, and colleagues. Such measures are considered “severe violations of a person’s rights to self-determination and personal freedom,” they wrote.
Previous studies have identified characteristics that increase the risk of involuntary inpatient admission, but the association between patients’ communication ability and coercive measures has not been explored, they noted.
In a study published in the Journal of Psychiatric Research, the investigators reviewed data from 1,556 adults who were admitted to psychiatric inpatient care at a single center in Germany in 2019. Patients’ communication ability was defined and recorded as one of the following: perfect; limited because of language or other reasons; or impossible because of language or other reasons (no communication).
Overall, 23% of patients were admitted involuntarily; the most common reasons for referral to inpatient care in the study population were physical aggression against individuals (8%) or objects (4%), and verbal aggression (7%). A total of 1,085 patients (70%) were able or willing to communicate.
Patients with limited or no communication ability because of language issues were three to four times more likely to be admitted involuntarily (odds ratios, 3.08 and 4.02, respectively), while those with limited or no communication ability because of nonlanguage issues were even more likely to be admitted involuntarily (ORs, 3.10 and 13.71, respectively), compared with patients without communication problems.
Patients with limited communication ability because of language issues also were significantly more likely than those without communication issues to experience coercive measures (OR, 4.53), as were patients with either limited or no communication ability because of no-language issues (ORs, 1.58 and 3.55, respectively).
Involuntary admission was defined as provisional detention, detention initiated by the patient’s legal guardian followed by a court order, or detention by court order “according to the Mental Health Law of the State of Berlin,” the researchers said. The average length of inpatient stay was 19 days. The age of the patients ranged from 18 to 96 years, with a mean age of 41.5 years, and 63% identified as male. Approximately two-thirds (62%) were unemployed or job-seeking during their treatment period, 38% were living alone, and 17% were homeless.
Although most of the study population (84%) was of German nationality, nearly half (48%) had a first- or second-generation migration background, the researchers noted.
“When thinking about effectively targeting this issue it is crucial to consider the different reasons why patients are limited in their ability to communicate,” the researchers wrote in their discussion. “Considering the rising numbers of refugees and persons with a migration background in Germany and many other countries worldwide, it is likely that more and more individuals with a language barrier will present at psychiatric emergency rooms,” they emphasized.
The findings were limited by several factors including the retrospective design, the relatively small number of patients with limitations or complete inability to communicate, and the use of data from a single hospital, and the incomplete data on nonlanguage reasons for limited or no communication ability, the researchers noted. Future studies should include more complete measures for recording these reasons, and data on forced medication, they added.
However, the results were strengthened by the range of sociodemographic, clinical, and admission-related variables in a large and representative sample, and highlight the need for appropriate interventions for patients with communication challenges, they said.
“Adequate financial and human resources need to be allocated to psychiatric hospitals that allow for high quality, available, and accessible interpretation services as well as mobilization of patients’ support networks during and after admission,” they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Despite improvements in reducing coercive measures in psychiatric inpatient care, both involuntary admission and coercive measures remain in use in many countries worldwide, wrote Celline Cole, MSc, a doctoral candidate at Charité Universitätsmedizin, Berlin, and colleagues. Such measures are considered “severe violations of a person’s rights to self-determination and personal freedom,” they wrote.
Previous studies have identified characteristics that increase the risk of involuntary inpatient admission, but the association between patients’ communication ability and coercive measures has not been explored, they noted.
In a study published in the Journal of Psychiatric Research, the investigators reviewed data from 1,556 adults who were admitted to psychiatric inpatient care at a single center in Germany in 2019. Patients’ communication ability was defined and recorded as one of the following: perfect; limited because of language or other reasons; or impossible because of language or other reasons (no communication).
Overall, 23% of patients were admitted involuntarily; the most common reasons for referral to inpatient care in the study population were physical aggression against individuals (8%) or objects (4%), and verbal aggression (7%). A total of 1,085 patients (70%) were able or willing to communicate.
Patients with limited or no communication ability because of language issues were three to four times more likely to be admitted involuntarily (odds ratios, 3.08 and 4.02, respectively), while those with limited or no communication ability because of nonlanguage issues were even more likely to be admitted involuntarily (ORs, 3.10 and 13.71, respectively), compared with patients without communication problems.
Patients with limited communication ability because of language issues also were significantly more likely than those without communication issues to experience coercive measures (OR, 4.53), as were patients with either limited or no communication ability because of no-language issues (ORs, 1.58 and 3.55, respectively).
Involuntary admission was defined as provisional detention, detention initiated by the patient’s legal guardian followed by a court order, or detention by court order “according to the Mental Health Law of the State of Berlin,” the researchers said. The average length of inpatient stay was 19 days. The age of the patients ranged from 18 to 96 years, with a mean age of 41.5 years, and 63% identified as male. Approximately two-thirds (62%) were unemployed or job-seeking during their treatment period, 38% were living alone, and 17% were homeless.
Although most of the study population (84%) was of German nationality, nearly half (48%) had a first- or second-generation migration background, the researchers noted.
“When thinking about effectively targeting this issue it is crucial to consider the different reasons why patients are limited in their ability to communicate,” the researchers wrote in their discussion. “Considering the rising numbers of refugees and persons with a migration background in Germany and many other countries worldwide, it is likely that more and more individuals with a language barrier will present at psychiatric emergency rooms,” they emphasized.
The findings were limited by several factors including the retrospective design, the relatively small number of patients with limitations or complete inability to communicate, and the use of data from a single hospital, and the incomplete data on nonlanguage reasons for limited or no communication ability, the researchers noted. Future studies should include more complete measures for recording these reasons, and data on forced medication, they added.
However, the results were strengthened by the range of sociodemographic, clinical, and admission-related variables in a large and representative sample, and highlight the need for appropriate interventions for patients with communication challenges, they said.
“Adequate financial and human resources need to be allocated to psychiatric hospitals that allow for high quality, available, and accessible interpretation services as well as mobilization of patients’ support networks during and after admission,” they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Despite improvements in reducing coercive measures in psychiatric inpatient care, both involuntary admission and coercive measures remain in use in many countries worldwide, wrote Celline Cole, MSc, a doctoral candidate at Charité Universitätsmedizin, Berlin, and colleagues. Such measures are considered “severe violations of a person’s rights to self-determination and personal freedom,” they wrote.
Previous studies have identified characteristics that increase the risk of involuntary inpatient admission, but the association between patients’ communication ability and coercive measures has not been explored, they noted.
In a study published in the Journal of Psychiatric Research, the investigators reviewed data from 1,556 adults who were admitted to psychiatric inpatient care at a single center in Germany in 2019. Patients’ communication ability was defined and recorded as one of the following: perfect; limited because of language or other reasons; or impossible because of language or other reasons (no communication).
Overall, 23% of patients were admitted involuntarily; the most common reasons for referral to inpatient care in the study population were physical aggression against individuals (8%) or objects (4%), and verbal aggression (7%). A total of 1,085 patients (70%) were able or willing to communicate.
Patients with limited or no communication ability because of language issues were three to four times more likely to be admitted involuntarily (odds ratios, 3.08 and 4.02, respectively), while those with limited or no communication ability because of nonlanguage issues were even more likely to be admitted involuntarily (ORs, 3.10 and 13.71, respectively), compared with patients without communication problems.
Patients with limited communication ability because of language issues also were significantly more likely than those without communication issues to experience coercive measures (OR, 4.53), as were patients with either limited or no communication ability because of no-language issues (ORs, 1.58 and 3.55, respectively).
Involuntary admission was defined as provisional detention, detention initiated by the patient’s legal guardian followed by a court order, or detention by court order “according to the Mental Health Law of the State of Berlin,” the researchers said. The average length of inpatient stay was 19 days. The age of the patients ranged from 18 to 96 years, with a mean age of 41.5 years, and 63% identified as male. Approximately two-thirds (62%) were unemployed or job-seeking during their treatment period, 38% were living alone, and 17% were homeless.
Although most of the study population (84%) was of German nationality, nearly half (48%) had a first- or second-generation migration background, the researchers noted.
“When thinking about effectively targeting this issue it is crucial to consider the different reasons why patients are limited in their ability to communicate,” the researchers wrote in their discussion. “Considering the rising numbers of refugees and persons with a migration background in Germany and many other countries worldwide, it is likely that more and more individuals with a language barrier will present at psychiatric emergency rooms,” they emphasized.
The findings were limited by several factors including the retrospective design, the relatively small number of patients with limitations or complete inability to communicate, and the use of data from a single hospital, and the incomplete data on nonlanguage reasons for limited or no communication ability, the researchers noted. Future studies should include more complete measures for recording these reasons, and data on forced medication, they added.
However, the results were strengthened by the range of sociodemographic, clinical, and admission-related variables in a large and representative sample, and highlight the need for appropriate interventions for patients with communication challenges, they said.
“Adequate financial and human resources need to be allocated to psychiatric hospitals that allow for high quality, available, and accessible interpretation services as well as mobilization of patients’ support networks during and after admission,” they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
FROM JOURNAL OF PSYCHIATRIC RESEARCH
‘Amazing’ phase 3 results for novel schizophrenia combo drug
VIENNA – The investigational agent xanomeline-trospium (KarXT, Karuna Therapeutics) achieves significant and clinically meaningful improvements in schizophrenia symptom scores without causing problematic adverse effects, new research suggests.
Results from the phase 3 EMERGENT-2 trial, which included more than 250 patients with schizophrenia, showed that those who received xanomeline-trospium for 5 weeks achieved a significant reduction in Positive and Negative Syndrome Scale (PANSS) total scores of more than nine points compared with their peers who received placebo. In addition, the improvements started at week 2.
Alongside significant reductions in both positive and negative symptoms, the results suggest the agent was well tolerated, with treatment-emergent adverse events (TEAEs) largely mild to moderate and transient in nature.
Lead investigator Christoph U. Correll, MD, professor of psychiatry at the Zucker School of Medicine at Hofstra/Northwell, Uniondale, New York, told this news organization that the upcoming EMERGENT-3 study will have a “European component” and that the “readout is expected most likely in the first quarter of next year.”
Dr. Correll suggested that if leads to “two positive studies and reasonable safety,” the novel agent may become part of the “next generation of antipsychotics that are not related to postsynaptic dopamine blockade.”
The findings for EMERGENT-2, presented at the 35th European College of Neuropsychopharmacology (ECNP) Congress as a poster and as an oral presentation, were an update of topline results released earlier this year.
Novel compound
Xanomeline-trospium is a novel compound that combines the dual M1/M4-preferring muscarinic receptor agonist effect of xanomeline with the peripherally restricted muscarinic receptor antagonist effect of trospium.
A previous phase 2 trial that compared the drug with placebo in almost 200 patients suggested it significantly reduced psychosis symptoms, leading to the current phase 3 trial.
Dr. Correll noted that xanomeline-trospium reduces psychosis via a “bottom up and top down approach.”
He said that on one hand, M4 agonism decreases acetylcholine in the ventral tegmental area and the associated stratum, “which then decreases dopamine levels from the bottom up,” while the M1 agonism stimulates GABA and decreases dopamine from the “top down.”
M1 agonism, however, also stimulates the cholinergic system peripherally, “which can give you nausea, vomiting, and also some blood pressure and pulse” problems, Dr. Correll said.
That was the limitation when this approach was studied by Lilly as a treatment for patients with Alzheimer’s disease, but the addition of trospium means “you’re buffering somewhat the cholinergic peripheral effects,” he said.
While that can conversely lead to dyspepsia, dry mouth, and constipation, Dr. Correll noted that the adverse effects of the novel agent are “mitigated by titration,” with patients taking up to 8 days to reach the full dose.
The result is that the drug was “overall tolerated, and the effect sizes were quite astounding,” he reported.
Intermittent, time limited TEAEs
The current trial included 252 patients aged 18-65 years (mean age, 45 years) who were confirmed to have schizophrenia and who had recently experienced a worsening of psychotic symptoms that warranted hospitalization. Three-quarters of the participants were men, and a similar proportion were Black. Approximately one quarter were White.
All were randomly assigned in a 1:1 ratio to receive either xanomeline-trospium or placebo following a 2-week screening period.
Xanomeline and trospium were titrated from 50 mg/20 mg twice daily to 125 mg/30 mg twice daily, and patients were treated for a total of 5 weeks. Efficacy and safety analyses were conducted in those who had received at least one dose of the study drug.
At the end of the treatment period, xanomeline-trospium was associated with a significant 9.6-point reduction in PANSS total scores relative to placebo; scores fell by 21.2 points with the active treatment, vs. 11.6 points with placebo (P < .0001).
The significant improvement in PANSS total score began at week 2 (P < .05) and continued to accrue over the course of the study.
Xanomeline-trospium was also associated with significant reductions in PANSS positive subscale scores in comparison with placebo (P < .0001), as well as with reductions in PANSS negative subscale scores (P < .01) and PANSS Marder negative subscale scores (P < .01).
Although 75.4% of patients who received xanomeline-trospium experienced a TEAE, in comparison with 58.4% of the placebo group, very few experienced a serious TEAE (just 1.6% in both groups).
TEAEs leading to discontinuation occurred in 7.1% of the active-treatment group, vs. 5.6% of the placebo group. The overall discontinuation rates from the trial were 25% and 21%, respectively.
The most common TEAEs with xanomeline-trospium were constipation (21.4%), dyspepsia (19.0%), nausea (19.0%), vomiting (14.3%), and headache (13.5%).
The results showed that cholinergic TEAEs typically began within the first 2 weeks of treatment and were “intermittent and time limited in nature,” the investigators noted. Moreover, average blood pressure levels were “similar” between the xanomeline-trospium and placebo groups “at each time point throughout the trial,” they added.
Dr. Correll reported that whereas the EMERGENT studies are testing xanomeline-trospium as a monotherapy, the ARISE program will be examining it as an “augmentation” treatment. “And that’s relevant because, let’s face it, patients do not switch” treatments, he said.
He suggested that if xanomeline-trospium is able to have a synergistic effect with other drugs, “we might be able to treat people who are currently not benefiting enough from postsynaptic dopamine blockade to maybe get a little bit closer” to the benefits seen with clozapine, which “also has problematic side effects.”
‘Really revolutionary’
Following the oral presentation of the study by coauthor Stephen K. Brannan, MD, chief medical officer, Karuna Therapeutics, Boston, the results were warmly received.
Session cochair Mark Weiser, MD, chairman at the department of psychiatry, Sackler School of Medicine, Tel Aviv University, Israel, said the agent is “really revolutionary in the field.
“It’s a non-dopamine compound which helps for schizophrenia, so we’re all very optimistic about it,” Dr. Weiser added.
Nevertheless, he asked Dr. Brannan whether the occurrence of gastrointestinal adverse effects with xanomeline-trospium led to “functional unblinding of the study.”
Dr. Brannan answered that the investigators were “really worried about this prior to EMERGENT-1” but that formal testing suggested it was not a problem.
Dr. Brannan said that although this has not yet been formally tested for the current trial, he believes that it is “highly unlikely” that functional unblinding occurred, inasmuch as the “percentages are about in the same range as we saw in EMERGENT-1.”
Speaking to ECNP Congress Daily in a conference roundup video, session cochair Andreas Reif, MD, PhD, professor of psychiatry, psychosomatic medicine, and psychotherapy at the University Hospital of Frankfurt (Germany), also highlighted the study.
He said that along with a study of dexmedetomidine sublingual film for agitation associated with schizophrenia or bipolar disorder that was also presented in the session, the current trial is “pivotal.”
Dr. Reif noted that the effect size shown with xanomeline-trospium was “really amazing.”
“We are in a really exciting time in treating mental disorders,” he said. “Industry is finally investing again, and really has new compounds that will make it to the market.”
Karuna plans to submit a new drug application with the Food and Drug Administration for KarXT in mid-2023. The drug is also in development for the treatment of psychiatric and neurologic conditions other than schizophrenia, including Alzheimer’s disease.
The study was funded by Karuna Therapeutics. Dr. Correll has reported relationships with Karuna, as well as AbbVie, Acadia, Alkermes, Allergan, Angelini, Aristo, Axsome, Boehringer-Ingelheim, Cardio Diagnostics, Cerevel, CNX Therapeutics, Compass Pathways, Damitsa, Gedeon Richter, Hikma, Holmusk, IntraCellular Therapies, Janssen/J&J, LB Pharma, Lundbeck, MedAvante-ProPhase, MedInCell, Medscape, Merck, Mindpax, Mitsubishi Tanabe Pharma, Mylan, Neurocrine, Newron, Noven, Otsuka, Pfizer, Pharmabrain, PPD Biotech, Recordati, Relmada, Reviva, Rovi, Seqirus, Servier, SK Life Science, Sumitomo Dainippon, Sunovion, Sun Pharma, Supernus, Takeda, Teva, Viatris, Otsuka, and UpToDate.
A version of this article first appeared on Medscape.com.
VIENNA – The investigational agent xanomeline-trospium (KarXT, Karuna Therapeutics) achieves significant and clinically meaningful improvements in schizophrenia symptom scores without causing problematic adverse effects, new research suggests.
Results from the phase 3 EMERGENT-2 trial, which included more than 250 patients with schizophrenia, showed that those who received xanomeline-trospium for 5 weeks achieved a significant reduction in Positive and Negative Syndrome Scale (PANSS) total scores of more than nine points compared with their peers who received placebo. In addition, the improvements started at week 2.
Alongside significant reductions in both positive and negative symptoms, the results suggest the agent was well tolerated, with treatment-emergent adverse events (TEAEs) largely mild to moderate and transient in nature.
Lead investigator Christoph U. Correll, MD, professor of psychiatry at the Zucker School of Medicine at Hofstra/Northwell, Uniondale, New York, told this news organization that the upcoming EMERGENT-3 study will have a “European component” and that the “readout is expected most likely in the first quarter of next year.”
Dr. Correll suggested that if leads to “two positive studies and reasonable safety,” the novel agent may become part of the “next generation of antipsychotics that are not related to postsynaptic dopamine blockade.”
The findings for EMERGENT-2, presented at the 35th European College of Neuropsychopharmacology (ECNP) Congress as a poster and as an oral presentation, were an update of topline results released earlier this year.
Novel compound
Xanomeline-trospium is a novel compound that combines the dual M1/M4-preferring muscarinic receptor agonist effect of xanomeline with the peripherally restricted muscarinic receptor antagonist effect of trospium.
A previous phase 2 trial that compared the drug with placebo in almost 200 patients suggested it significantly reduced psychosis symptoms, leading to the current phase 3 trial.
Dr. Correll noted that xanomeline-trospium reduces psychosis via a “bottom up and top down approach.”
He said that on one hand, M4 agonism decreases acetylcholine in the ventral tegmental area and the associated stratum, “which then decreases dopamine levels from the bottom up,” while the M1 agonism stimulates GABA and decreases dopamine from the “top down.”
M1 agonism, however, also stimulates the cholinergic system peripherally, “which can give you nausea, vomiting, and also some blood pressure and pulse” problems, Dr. Correll said.
That was the limitation when this approach was studied by Lilly as a treatment for patients with Alzheimer’s disease, but the addition of trospium means “you’re buffering somewhat the cholinergic peripheral effects,” he said.
While that can conversely lead to dyspepsia, dry mouth, and constipation, Dr. Correll noted that the adverse effects of the novel agent are “mitigated by titration,” with patients taking up to 8 days to reach the full dose.
The result is that the drug was “overall tolerated, and the effect sizes were quite astounding,” he reported.
Intermittent, time limited TEAEs
The current trial included 252 patients aged 18-65 years (mean age, 45 years) who were confirmed to have schizophrenia and who had recently experienced a worsening of psychotic symptoms that warranted hospitalization. Three-quarters of the participants were men, and a similar proportion were Black. Approximately one quarter were White.
All were randomly assigned in a 1:1 ratio to receive either xanomeline-trospium or placebo following a 2-week screening period.
Xanomeline and trospium were titrated from 50 mg/20 mg twice daily to 125 mg/30 mg twice daily, and patients were treated for a total of 5 weeks. Efficacy and safety analyses were conducted in those who had received at least one dose of the study drug.
At the end of the treatment period, xanomeline-trospium was associated with a significant 9.6-point reduction in PANSS total scores relative to placebo; scores fell by 21.2 points with the active treatment, vs. 11.6 points with placebo (P < .0001).
The significant improvement in PANSS total score began at week 2 (P < .05) and continued to accrue over the course of the study.
Xanomeline-trospium was also associated with significant reductions in PANSS positive subscale scores in comparison with placebo (P < .0001), as well as with reductions in PANSS negative subscale scores (P < .01) and PANSS Marder negative subscale scores (P < .01).
Although 75.4% of patients who received xanomeline-trospium experienced a TEAE, in comparison with 58.4% of the placebo group, very few experienced a serious TEAE (just 1.6% in both groups).
TEAEs leading to discontinuation occurred in 7.1% of the active-treatment group, vs. 5.6% of the placebo group. The overall discontinuation rates from the trial were 25% and 21%, respectively.
The most common TEAEs with xanomeline-trospium were constipation (21.4%), dyspepsia (19.0%), nausea (19.0%), vomiting (14.3%), and headache (13.5%).
The results showed that cholinergic TEAEs typically began within the first 2 weeks of treatment and were “intermittent and time limited in nature,” the investigators noted. Moreover, average blood pressure levels were “similar” between the xanomeline-trospium and placebo groups “at each time point throughout the trial,” they added.
Dr. Correll reported that whereas the EMERGENT studies are testing xanomeline-trospium as a monotherapy, the ARISE program will be examining it as an “augmentation” treatment. “And that’s relevant because, let’s face it, patients do not switch” treatments, he said.
He suggested that if xanomeline-trospium is able to have a synergistic effect with other drugs, “we might be able to treat people who are currently not benefiting enough from postsynaptic dopamine blockade to maybe get a little bit closer” to the benefits seen with clozapine, which “also has problematic side effects.”
‘Really revolutionary’
Following the oral presentation of the study by coauthor Stephen K. Brannan, MD, chief medical officer, Karuna Therapeutics, Boston, the results were warmly received.
Session cochair Mark Weiser, MD, chairman at the department of psychiatry, Sackler School of Medicine, Tel Aviv University, Israel, said the agent is “really revolutionary in the field.
“It’s a non-dopamine compound which helps for schizophrenia, so we’re all very optimistic about it,” Dr. Weiser added.
Nevertheless, he asked Dr. Brannan whether the occurrence of gastrointestinal adverse effects with xanomeline-trospium led to “functional unblinding of the study.”
Dr. Brannan answered that the investigators were “really worried about this prior to EMERGENT-1” but that formal testing suggested it was not a problem.
Dr. Brannan said that although this has not yet been formally tested for the current trial, he believes that it is “highly unlikely” that functional unblinding occurred, inasmuch as the “percentages are about in the same range as we saw in EMERGENT-1.”
Speaking to ECNP Congress Daily in a conference roundup video, session cochair Andreas Reif, MD, PhD, professor of psychiatry, psychosomatic medicine, and psychotherapy at the University Hospital of Frankfurt (Germany), also highlighted the study.
He said that along with a study of dexmedetomidine sublingual film for agitation associated with schizophrenia or bipolar disorder that was also presented in the session, the current trial is “pivotal.”
Dr. Reif noted that the effect size shown with xanomeline-trospium was “really amazing.”
“We are in a really exciting time in treating mental disorders,” he said. “Industry is finally investing again, and really has new compounds that will make it to the market.”
Karuna plans to submit a new drug application with the Food and Drug Administration for KarXT in mid-2023. The drug is also in development for the treatment of psychiatric and neurologic conditions other than schizophrenia, including Alzheimer’s disease.
The study was funded by Karuna Therapeutics. Dr. Correll has reported relationships with Karuna, as well as AbbVie, Acadia, Alkermes, Allergan, Angelini, Aristo, Axsome, Boehringer-Ingelheim, Cardio Diagnostics, Cerevel, CNX Therapeutics, Compass Pathways, Damitsa, Gedeon Richter, Hikma, Holmusk, IntraCellular Therapies, Janssen/J&J, LB Pharma, Lundbeck, MedAvante-ProPhase, MedInCell, Medscape, Merck, Mindpax, Mitsubishi Tanabe Pharma, Mylan, Neurocrine, Newron, Noven, Otsuka, Pfizer, Pharmabrain, PPD Biotech, Recordati, Relmada, Reviva, Rovi, Seqirus, Servier, SK Life Science, Sumitomo Dainippon, Sunovion, Sun Pharma, Supernus, Takeda, Teva, Viatris, Otsuka, and UpToDate.
A version of this article first appeared on Medscape.com.
VIENNA – The investigational agent xanomeline-trospium (KarXT, Karuna Therapeutics) achieves significant and clinically meaningful improvements in schizophrenia symptom scores without causing problematic adverse effects, new research suggests.
Results from the phase 3 EMERGENT-2 trial, which included more than 250 patients with schizophrenia, showed that those who received xanomeline-trospium for 5 weeks achieved a significant reduction in Positive and Negative Syndrome Scale (PANSS) total scores of more than nine points compared with their peers who received placebo. In addition, the improvements started at week 2.
Alongside significant reductions in both positive and negative symptoms, the results suggest the agent was well tolerated, with treatment-emergent adverse events (TEAEs) largely mild to moderate and transient in nature.
Lead investigator Christoph U. Correll, MD, professor of psychiatry at the Zucker School of Medicine at Hofstra/Northwell, Uniondale, New York, told this news organization that the upcoming EMERGENT-3 study will have a “European component” and that the “readout is expected most likely in the first quarter of next year.”
Dr. Correll suggested that if leads to “two positive studies and reasonable safety,” the novel agent may become part of the “next generation of antipsychotics that are not related to postsynaptic dopamine blockade.”
The findings for EMERGENT-2, presented at the 35th European College of Neuropsychopharmacology (ECNP) Congress as a poster and as an oral presentation, were an update of topline results released earlier this year.
Novel compound
Xanomeline-trospium is a novel compound that combines the dual M1/M4-preferring muscarinic receptor agonist effect of xanomeline with the peripherally restricted muscarinic receptor antagonist effect of trospium.
A previous phase 2 trial that compared the drug with placebo in almost 200 patients suggested it significantly reduced psychosis symptoms, leading to the current phase 3 trial.
Dr. Correll noted that xanomeline-trospium reduces psychosis via a “bottom up and top down approach.”
He said that on one hand, M4 agonism decreases acetylcholine in the ventral tegmental area and the associated stratum, “which then decreases dopamine levels from the bottom up,” while the M1 agonism stimulates GABA and decreases dopamine from the “top down.”
M1 agonism, however, also stimulates the cholinergic system peripherally, “which can give you nausea, vomiting, and also some blood pressure and pulse” problems, Dr. Correll said.
That was the limitation when this approach was studied by Lilly as a treatment for patients with Alzheimer’s disease, but the addition of trospium means “you’re buffering somewhat the cholinergic peripheral effects,” he said.
While that can conversely lead to dyspepsia, dry mouth, and constipation, Dr. Correll noted that the adverse effects of the novel agent are “mitigated by titration,” with patients taking up to 8 days to reach the full dose.
The result is that the drug was “overall tolerated, and the effect sizes were quite astounding,” he reported.
Intermittent, time limited TEAEs
The current trial included 252 patients aged 18-65 years (mean age, 45 years) who were confirmed to have schizophrenia and who had recently experienced a worsening of psychotic symptoms that warranted hospitalization. Three-quarters of the participants were men, and a similar proportion were Black. Approximately one quarter were White.
All were randomly assigned in a 1:1 ratio to receive either xanomeline-trospium or placebo following a 2-week screening period.
Xanomeline and trospium were titrated from 50 mg/20 mg twice daily to 125 mg/30 mg twice daily, and patients were treated for a total of 5 weeks. Efficacy and safety analyses were conducted in those who had received at least one dose of the study drug.
At the end of the treatment period, xanomeline-trospium was associated with a significant 9.6-point reduction in PANSS total scores relative to placebo; scores fell by 21.2 points with the active treatment, vs. 11.6 points with placebo (P < .0001).
The significant improvement in PANSS total score began at week 2 (P < .05) and continued to accrue over the course of the study.
Xanomeline-trospium was also associated with significant reductions in PANSS positive subscale scores in comparison with placebo (P < .0001), as well as with reductions in PANSS negative subscale scores (P < .01) and PANSS Marder negative subscale scores (P < .01).
Although 75.4% of patients who received xanomeline-trospium experienced a TEAE, in comparison with 58.4% of the placebo group, very few experienced a serious TEAE (just 1.6% in both groups).
TEAEs leading to discontinuation occurred in 7.1% of the active-treatment group, vs. 5.6% of the placebo group. The overall discontinuation rates from the trial were 25% and 21%, respectively.
The most common TEAEs with xanomeline-trospium were constipation (21.4%), dyspepsia (19.0%), nausea (19.0%), vomiting (14.3%), and headache (13.5%).
The results showed that cholinergic TEAEs typically began within the first 2 weeks of treatment and were “intermittent and time limited in nature,” the investigators noted. Moreover, average blood pressure levels were “similar” between the xanomeline-trospium and placebo groups “at each time point throughout the trial,” they added.
Dr. Correll reported that whereas the EMERGENT studies are testing xanomeline-trospium as a monotherapy, the ARISE program will be examining it as an “augmentation” treatment. “And that’s relevant because, let’s face it, patients do not switch” treatments, he said.
He suggested that if xanomeline-trospium is able to have a synergistic effect with other drugs, “we might be able to treat people who are currently not benefiting enough from postsynaptic dopamine blockade to maybe get a little bit closer” to the benefits seen with clozapine, which “also has problematic side effects.”
‘Really revolutionary’
Following the oral presentation of the study by coauthor Stephen K. Brannan, MD, chief medical officer, Karuna Therapeutics, Boston, the results were warmly received.
Session cochair Mark Weiser, MD, chairman at the department of psychiatry, Sackler School of Medicine, Tel Aviv University, Israel, said the agent is “really revolutionary in the field.
“It’s a non-dopamine compound which helps for schizophrenia, so we’re all very optimistic about it,” Dr. Weiser added.
Nevertheless, he asked Dr. Brannan whether the occurrence of gastrointestinal adverse effects with xanomeline-trospium led to “functional unblinding of the study.”
Dr. Brannan answered that the investigators were “really worried about this prior to EMERGENT-1” but that formal testing suggested it was not a problem.
Dr. Brannan said that although this has not yet been formally tested for the current trial, he believes that it is “highly unlikely” that functional unblinding occurred, inasmuch as the “percentages are about in the same range as we saw in EMERGENT-1.”
Speaking to ECNP Congress Daily in a conference roundup video, session cochair Andreas Reif, MD, PhD, professor of psychiatry, psychosomatic medicine, and psychotherapy at the University Hospital of Frankfurt (Germany), also highlighted the study.
He said that along with a study of dexmedetomidine sublingual film for agitation associated with schizophrenia or bipolar disorder that was also presented in the session, the current trial is “pivotal.”
Dr. Reif noted that the effect size shown with xanomeline-trospium was “really amazing.”
“We are in a really exciting time in treating mental disorders,” he said. “Industry is finally investing again, and really has new compounds that will make it to the market.”
Karuna plans to submit a new drug application with the Food and Drug Administration for KarXT in mid-2023. The drug is also in development for the treatment of psychiatric and neurologic conditions other than schizophrenia, including Alzheimer’s disease.
The study was funded by Karuna Therapeutics. Dr. Correll has reported relationships with Karuna, as well as AbbVie, Acadia, Alkermes, Allergan, Angelini, Aristo, Axsome, Boehringer-Ingelheim, Cardio Diagnostics, Cerevel, CNX Therapeutics, Compass Pathways, Damitsa, Gedeon Richter, Hikma, Holmusk, IntraCellular Therapies, Janssen/J&J, LB Pharma, Lundbeck, MedAvante-ProPhase, MedInCell, Medscape, Merck, Mindpax, Mitsubishi Tanabe Pharma, Mylan, Neurocrine, Newron, Noven, Otsuka, Pfizer, Pharmabrain, PPD Biotech, Recordati, Relmada, Reviva, Rovi, Seqirus, Servier, SK Life Science, Sumitomo Dainippon, Sunovion, Sun Pharma, Supernus, Takeda, Teva, Viatris, Otsuka, and UpToDate.
A version of this article first appeared on Medscape.com.
AT ECNP 2022
‘Disturbing’ lack of follow-up care after psychiatric crises
There is a concerning lack of follow-up care for young people who experience a mental health crisis, new research suggests.
The follow-up rate was less than 30% for those who had visited an ED.
The strongest predictor of follow-up was having received both primary and mental health care during the 6 months prior to using the acute service.
“For people discharging folks after a psychiatric crisis, whether it be in a hospital or emergency room setting, connecting them with their outpatient provider to ensure the transfer of care and continuity of care is vitally important to reduce risks for this population,” coinvestigator Brian Skehan, MD, PhD, assistant professor and psychiatrist, University of Massachusetts, Worcester, said during a press briefing.
If these discharged patients do not have a provider, “make sure they get one,” Lisa Dixon, MD, editor-in-chief of Psychiatric Services, added during the same briefing. “That’s the gift of life potentially for these young people.”
The findings were published online in Psychiatric Services.
Alarming trends
The alarming suicide trends among youths were exacerbated by the COVID-19 pandemic, Dr. Skehan noted.
He cited a 2021 study that showed more than 44% of high school students experienced persistent sadness or hopelessness over the previous year, 1 in 5 seriously considered suicide, and almost 1 in 10 actually attempted suicide.
“When we look at the number of young adults and adolescents struggling with behavioral health issues, the data trend is disturbing nationwide,” Dr. Skehan said.
The current study included participants aged 12-27 years who had private insurance. Many youth in this age category are experiencing significant changes, such as moving from high school to college and from pediatric providers to adult providers – and some “get lost in this transition,” said Dr. Skehan.
He noted many inpatient psychiatric units are not geared to young adults. “They may miss out on some aspects of inpatient care because it’s not geared to their developmental stage,” he said.
Assessing U.S. patient data in the IBM MarketScan commercial database (2013-2018), the researchers created two study samples: 95,153 inpatients and 108,576 patients who used the ED. All had an acute event stemming from a mental health condition.
The investigators explored the role of “established” outpatient care, defined as having had at least one visit with a provider of primary or mental health care in the 6 months prior to the acute psychiatric event.
Covariates included age at time of service (aged 12-17 years or 18-27 years), gender, health care plan type, psychiatric diagnosis, whether the acute event was self-harm or suicide related, and medical complexity.
Low follow-up rates
In the inpatient group, the average age was 18.9 years, the most common length of hospital stay was 4-6 days, and 1.5% left against medical advice. The most common primary diagnosis was major depression (53.7%), followed by bipolar disorder (22.3%). The least common disorders were PTSD, comorbid eating disorders, and disruptive disorders.
About one-third of participants had used both primary and mental health care during the 6 months before hospitalization, whereas 22.8% had no established outpatient care. Established care was most common among those with comorbid eating disorders and least common among those with psychotic disorders.
Results showed 42.7% of the hospitalized patients received follow up within 7 days and 67.4% received follow up within 30 days.
The strongest predictor of mental health follow-up care was established outpatient care. Compared with those who had no such care, those who had received both primary care and mental health care before the acute event had the highest odds of receiving follow-up (within 7 days, adjusted odds ratio, 2.81; 95% confidence interval, 2.68-2.94).
Older age and leaving against medical advice were associated with decreased likelihood of follow-up. Female sex, hospitalizations related to self-harm or suicidality, and longer length of stay were associated with increased likelihood of mental health follow-up care.
Compared with those hospitalized for major depression, those hospitalized for schizophrenia, bipolar disorder, PTSD, disruptive disorders, or comorbid substance use disorder were less likely to receive mental health follow-up. For example, only 23.7% of youth with comorbid substance use discharged from the hospital had follow-up within 7 days.
Similar patterns were observed for 30-day follow-up care.
‘Accessible and appealing’ options needed
In the ED-visit group, the average age was 19.5 years (58% female). Most (70.4%) had no chronic health conditions other than a psychiatric disorder. The primary diagnoses were anxiety disorders or phobias (44.1%) and major depression (23%).
One in four visits included a code for self-harm, suicidal ideation, or suicide attempt. And almost one third lacked established outpatient care before the ED visit.
Results showed 28.6% of the ED group received mental health care follow-up within 7 days and 46.4% received it within 30 days.
Again, the strongest predictor of mental health follow-up was prior outpatient care. For example, compared with participants with no established outpatient care, those with both primary care and mental health care were the most likely to receive follow-up within 7 days (aOR, 4.06; 95% CI, 3.72-4.42).
These numbers “are far from the goal of making sure everybody is getting follow-up care within 7 days of an acute psychiatric event,” Dr. Skehan said.
He stressed the need for “accessible and appealing options for youth.” These could include telehealth services, improved communication among health care providers in the ED, and reducing barriers to access follow-up care.
“This probably highlights the need to have more case management and referral services, and maybe make sure patients have a follow-up appointment before they leave the emergency room,” said Dr. Skehan. “This doesn’t necessarily guarantee they’ll get there but hopefully it makes it more likely they will have that access should they need it.”
The study was funded by grants from the National Institute of General Medical Sciences and the National Center for Advancing Translational Sciences, from the National Institutes of Health. The investigators reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
There is a concerning lack of follow-up care for young people who experience a mental health crisis, new research suggests.
The follow-up rate was less than 30% for those who had visited an ED.
The strongest predictor of follow-up was having received both primary and mental health care during the 6 months prior to using the acute service.
“For people discharging folks after a psychiatric crisis, whether it be in a hospital or emergency room setting, connecting them with their outpatient provider to ensure the transfer of care and continuity of care is vitally important to reduce risks for this population,” coinvestigator Brian Skehan, MD, PhD, assistant professor and psychiatrist, University of Massachusetts, Worcester, said during a press briefing.
If these discharged patients do not have a provider, “make sure they get one,” Lisa Dixon, MD, editor-in-chief of Psychiatric Services, added during the same briefing. “That’s the gift of life potentially for these young people.”
The findings were published online in Psychiatric Services.
Alarming trends
The alarming suicide trends among youths were exacerbated by the COVID-19 pandemic, Dr. Skehan noted.
He cited a 2021 study that showed more than 44% of high school students experienced persistent sadness or hopelessness over the previous year, 1 in 5 seriously considered suicide, and almost 1 in 10 actually attempted suicide.
“When we look at the number of young adults and adolescents struggling with behavioral health issues, the data trend is disturbing nationwide,” Dr. Skehan said.
The current study included participants aged 12-27 years who had private insurance. Many youth in this age category are experiencing significant changes, such as moving from high school to college and from pediatric providers to adult providers – and some “get lost in this transition,” said Dr. Skehan.
He noted many inpatient psychiatric units are not geared to young adults. “They may miss out on some aspects of inpatient care because it’s not geared to their developmental stage,” he said.
Assessing U.S. patient data in the IBM MarketScan commercial database (2013-2018), the researchers created two study samples: 95,153 inpatients and 108,576 patients who used the ED. All had an acute event stemming from a mental health condition.
The investigators explored the role of “established” outpatient care, defined as having had at least one visit with a provider of primary or mental health care in the 6 months prior to the acute psychiatric event.
Covariates included age at time of service (aged 12-17 years or 18-27 years), gender, health care plan type, psychiatric diagnosis, whether the acute event was self-harm or suicide related, and medical complexity.
Low follow-up rates
In the inpatient group, the average age was 18.9 years, the most common length of hospital stay was 4-6 days, and 1.5% left against medical advice. The most common primary diagnosis was major depression (53.7%), followed by bipolar disorder (22.3%). The least common disorders were PTSD, comorbid eating disorders, and disruptive disorders.
About one-third of participants had used both primary and mental health care during the 6 months before hospitalization, whereas 22.8% had no established outpatient care. Established care was most common among those with comorbid eating disorders and least common among those with psychotic disorders.
Results showed 42.7% of the hospitalized patients received follow up within 7 days and 67.4% received follow up within 30 days.
The strongest predictor of mental health follow-up care was established outpatient care. Compared with those who had no such care, those who had received both primary care and mental health care before the acute event had the highest odds of receiving follow-up (within 7 days, adjusted odds ratio, 2.81; 95% confidence interval, 2.68-2.94).
Older age and leaving against medical advice were associated with decreased likelihood of follow-up. Female sex, hospitalizations related to self-harm or suicidality, and longer length of stay were associated with increased likelihood of mental health follow-up care.
Compared with those hospitalized for major depression, those hospitalized for schizophrenia, bipolar disorder, PTSD, disruptive disorders, or comorbid substance use disorder were less likely to receive mental health follow-up. For example, only 23.7% of youth with comorbid substance use discharged from the hospital had follow-up within 7 days.
Similar patterns were observed for 30-day follow-up care.
‘Accessible and appealing’ options needed
In the ED-visit group, the average age was 19.5 years (58% female). Most (70.4%) had no chronic health conditions other than a psychiatric disorder. The primary diagnoses were anxiety disorders or phobias (44.1%) and major depression (23%).
One in four visits included a code for self-harm, suicidal ideation, or suicide attempt. And almost one third lacked established outpatient care before the ED visit.
Results showed 28.6% of the ED group received mental health care follow-up within 7 days and 46.4% received it within 30 days.
Again, the strongest predictor of mental health follow-up was prior outpatient care. For example, compared with participants with no established outpatient care, those with both primary care and mental health care were the most likely to receive follow-up within 7 days (aOR, 4.06; 95% CI, 3.72-4.42).
These numbers “are far from the goal of making sure everybody is getting follow-up care within 7 days of an acute psychiatric event,” Dr. Skehan said.
He stressed the need for “accessible and appealing options for youth.” These could include telehealth services, improved communication among health care providers in the ED, and reducing barriers to access follow-up care.
“This probably highlights the need to have more case management and referral services, and maybe make sure patients have a follow-up appointment before they leave the emergency room,” said Dr. Skehan. “This doesn’t necessarily guarantee they’ll get there but hopefully it makes it more likely they will have that access should they need it.”
The study was funded by grants from the National Institute of General Medical Sciences and the National Center for Advancing Translational Sciences, from the National Institutes of Health. The investigators reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
There is a concerning lack of follow-up care for young people who experience a mental health crisis, new research suggests.
The follow-up rate was less than 30% for those who had visited an ED.
The strongest predictor of follow-up was having received both primary and mental health care during the 6 months prior to using the acute service.
“For people discharging folks after a psychiatric crisis, whether it be in a hospital or emergency room setting, connecting them with their outpatient provider to ensure the transfer of care and continuity of care is vitally important to reduce risks for this population,” coinvestigator Brian Skehan, MD, PhD, assistant professor and psychiatrist, University of Massachusetts, Worcester, said during a press briefing.
If these discharged patients do not have a provider, “make sure they get one,” Lisa Dixon, MD, editor-in-chief of Psychiatric Services, added during the same briefing. “That’s the gift of life potentially for these young people.”
The findings were published online in Psychiatric Services.
Alarming trends
The alarming suicide trends among youths were exacerbated by the COVID-19 pandemic, Dr. Skehan noted.
He cited a 2021 study that showed more than 44% of high school students experienced persistent sadness or hopelessness over the previous year, 1 in 5 seriously considered suicide, and almost 1 in 10 actually attempted suicide.
“When we look at the number of young adults and adolescents struggling with behavioral health issues, the data trend is disturbing nationwide,” Dr. Skehan said.
The current study included participants aged 12-27 years who had private insurance. Many youth in this age category are experiencing significant changes, such as moving from high school to college and from pediatric providers to adult providers – and some “get lost in this transition,” said Dr. Skehan.
He noted many inpatient psychiatric units are not geared to young adults. “They may miss out on some aspects of inpatient care because it’s not geared to their developmental stage,” he said.
Assessing U.S. patient data in the IBM MarketScan commercial database (2013-2018), the researchers created two study samples: 95,153 inpatients and 108,576 patients who used the ED. All had an acute event stemming from a mental health condition.
The investigators explored the role of “established” outpatient care, defined as having had at least one visit with a provider of primary or mental health care in the 6 months prior to the acute psychiatric event.
Covariates included age at time of service (aged 12-17 years or 18-27 years), gender, health care plan type, psychiatric diagnosis, whether the acute event was self-harm or suicide related, and medical complexity.
Low follow-up rates
In the inpatient group, the average age was 18.9 years, the most common length of hospital stay was 4-6 days, and 1.5% left against medical advice. The most common primary diagnosis was major depression (53.7%), followed by bipolar disorder (22.3%). The least common disorders were PTSD, comorbid eating disorders, and disruptive disorders.
About one-third of participants had used both primary and mental health care during the 6 months before hospitalization, whereas 22.8% had no established outpatient care. Established care was most common among those with comorbid eating disorders and least common among those with psychotic disorders.
Results showed 42.7% of the hospitalized patients received follow up within 7 days and 67.4% received follow up within 30 days.
The strongest predictor of mental health follow-up care was established outpatient care. Compared with those who had no such care, those who had received both primary care and mental health care before the acute event had the highest odds of receiving follow-up (within 7 days, adjusted odds ratio, 2.81; 95% confidence interval, 2.68-2.94).
Older age and leaving against medical advice were associated with decreased likelihood of follow-up. Female sex, hospitalizations related to self-harm or suicidality, and longer length of stay were associated with increased likelihood of mental health follow-up care.
Compared with those hospitalized for major depression, those hospitalized for schizophrenia, bipolar disorder, PTSD, disruptive disorders, or comorbid substance use disorder were less likely to receive mental health follow-up. For example, only 23.7% of youth with comorbid substance use discharged from the hospital had follow-up within 7 days.
Similar patterns were observed for 30-day follow-up care.
‘Accessible and appealing’ options needed
In the ED-visit group, the average age was 19.5 years (58% female). Most (70.4%) had no chronic health conditions other than a psychiatric disorder. The primary diagnoses were anxiety disorders or phobias (44.1%) and major depression (23%).
One in four visits included a code for self-harm, suicidal ideation, or suicide attempt. And almost one third lacked established outpatient care before the ED visit.
Results showed 28.6% of the ED group received mental health care follow-up within 7 days and 46.4% received it within 30 days.
Again, the strongest predictor of mental health follow-up was prior outpatient care. For example, compared with participants with no established outpatient care, those with both primary care and mental health care were the most likely to receive follow-up within 7 days (aOR, 4.06; 95% CI, 3.72-4.42).
These numbers “are far from the goal of making sure everybody is getting follow-up care within 7 days of an acute psychiatric event,” Dr. Skehan said.
He stressed the need for “accessible and appealing options for youth.” These could include telehealth services, improved communication among health care providers in the ED, and reducing barriers to access follow-up care.
“This probably highlights the need to have more case management and referral services, and maybe make sure patients have a follow-up appointment before they leave the emergency room,” said Dr. Skehan. “This doesn’t necessarily guarantee they’ll get there but hopefully it makes it more likely they will have that access should they need it.”
The study was funded by grants from the National Institute of General Medical Sciences and the National Center for Advancing Translational Sciences, from the National Institutes of Health. The investigators reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM PSYCHIATRIC SERVICES
Gut microbiota disruption a driver of aggression in schizophrenia?
However, at least one expert expressed concerns over the study’s conclusions.
Results from a study of 50 inpatients with schizophrenia showed significantly higher pro-inflammation, pro-oxidation, and leaky gut biomarkers in those with aggression vs. their peers who did not display aggression.
In addition, those with aggression showed less alpha diversity and evenness of the fecal bacterial community, lower levels of several beneficial gut bacteria, and higher levels of the fecal genera Prevotella.
Six short-chain fatty acids (SCFAs) and six neurotransmitters were also lower in the aggression vs. no-aggression groups.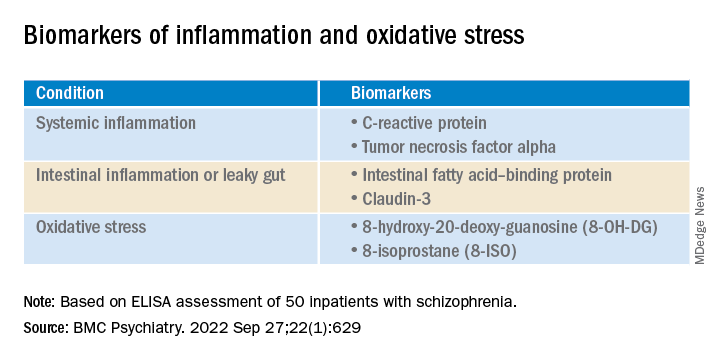
“The present study was the first to compare the state of inflammation, oxidation, intestinal microbiota, and metabolites” in inpatients with schizophrenia and aggression, compared with those who did not show aggression, write the investigators, led by Hongxin Deng, department of psychiatry, Zhumadian (China) Psychiatric Hospital.
“Results indicate pro-inflammation, pro-oxidation and leaky gut phenotypes relating to enteric dysbacteriosis and microbial SCFAs feature the aggression in [individuals with schizophrenia], which provides clues for future microbial-based or anti-inflammatory/oxidative therapies on aggression,” they add.
The findings were published online in BMC Psychiatry.
Unknown pathogenesis
Although emerging evidence suggests that schizophrenia “may augment the propensity for aggression incidence about fourfold to sevenfold,” the pathogenesis of aggression “remains largely unknown,” the investigators note.
The same researchers previously found an association between the systemic pro-inflammation response and the onset or severity of aggression in schizophrenia, “possibly caused by leaky gut-induced bacterial translocation.”
The researchers suggest that peripheral cytokines “could cross the blood-brain barrier, thus precipitating changes in mood and behavior through hypothalamic-pituitary-adrenal axis.”
However, they note that the pro-inflammation phenotype is “often a synergistic effect of multiple causes.” Of these, chronic pro-oxidative stress has been shown to contribute to aggression onset in intermittent explosive disorder, but this association has rarely been confirmed in patients with schizophrenia.
In addition, increasing evidence points to enteric dysbacteriosis and dysbiosis of intestinal flora metabolites, including SCFAs or neurotransmitters, as potentially “integral parts of psychiatric disorders’ pathophysiology” by changing the state of both oxidative stress and inflammation.
The investigators hypothesized that the systemic pro-inflammation phenotype in aggression-affected schizophrenia cases “involves alterations to gut microbiota and its metabolites, leaky gut, and oxidative stress.” However, the profiles of these variables and their interrelationships have been “poorly investigated” in inpatients with schizophrenia and aggression.
To fill this gap, they assessed adult psychiatric inpatients with schizophrenia and aggressive behaviors and inpatients with schizophrenia but no aggressive behavior within 1 week before admission (n = 25 per group; mean age, 33.52 years, and 32.88 years, respectively; 68% and 64% women, respectively).
They collected stool samples from each patient and used enzyme-linked immunoassay (ELISA) to detect fecal calprotectin protein, an indicator of intestinal inflammation. They also collected fasting peripheral blood samples, using ELISA to detect several biomarkers.
The researchers also used the Modified Overt Aggression Scale (MOAS) to characterize aggressive behaviors and the Positive and Negative Syndrome Scale to characterize psychiatric symptoms.
‘Vital role’
Significantly higher biomarkers for systemic pro-inflammation, pro-oxidation and leaky gut were found in the aggression vs the no-aggression group (all P < .05).
After controlling for potential confounders, the researchers also found positive associations between MOAS scores and biomarkers, both serum and fecal.
There were also positive associations between serum 8-hydroxy-20-deoxy-guanosine (8-OH-DG) or 8-isoprostane (8-ISO) and systemic inflammatory biomarkers (all R > 0; P < .05).
In addition, the alpha diversity and evenness of the fecal bacterial community were lower in the aggression vs. no aggression groups.
When the researchers compared the relative abundance of the top 15 genera composition of intestinal microflora in the two groups, Bacteroides, Faecalibacterium, Blautia, Bifidobacterium, Collinsella, and Eubacterium coprostanoligenes were “remarkably reduced” in the group with aggression, whereas the abundance of fecal genera Prevotella was significantly increased (all corrected P < .001).
In the patients who had schizophrenia with aggression, levels of six SCFAs and six neurotransmitters were much lower than in the patients with schizophrenia but no aggression (all P < .05).
Inpatients with schizophrenia and aggression “had dramatically increased serum level of 8-OH-DG (nucleic acid oxidation biomarker) and 8-ISO (lipid oxidation biomarker) than those without, and further correlation analysis also showed positive correlativity between pro-oxidation and systemic pro-inflammation response or aggression severity,” the investigators write.
The findings “collectively suggest the cocontributory role of systemic pro-inflammation and pro-oxidation in the development of aggression” in schizophrenia, they add. “Gut dysbacteriosis with leaky gut seems to play a vital role in the pathophysiology.”
Correlation vs. causality
Commenting for this article, Emeran Mayer, MD, distinguished research professor of medicine at the G. Oppenheimer Center for Neurobiology of Stress and Resilience and UCLA Brain Gut Microbiome Center, Los Angeles, said that “at first glance, it is interesting that the behavioral trait of aggression but not the diagnosis of schizophrenia showed the differences in markers of systemic inflammation, increased gut permeability, and microbiome parameters.”
However, like many such descriptive studies, the research is flawed by comparing two patient groups and concluding causality between the biomarkers and the behavior traits, added Dr. Mayer, who was not involved with the study.
The study’s shortcomings include its small sample size as well as several confounding factors – particularly diet, sleep, exercise, and stress and anxiety levels – that were not considered, he said. The study also lacked a control group with high levels of aggression but without schizophrenia.
“The observed changes in intestinal permeability, unscientifically referred to as ‘leaky gut,’ as well as the gut microbiome differences, could be secondary to chronically increased sympathetic nervous system activation in the high aggression group,” Dr. Mayer said. “This is an interesting hypothesis which should be discussed and should have been addressed in this study.”
The differences in gut microbial composition and SCFA production “could be secondary to differences in plant-based diet components,” Dr. Mayer speculated, wondering how well dietary intake was controlled.
“Overall, it is an interesting descriptive study, which unfortunately does not contribute significantly to a better understanding of the role of the brain-gut microbiome system in schizophrenic patients,” he said.
The study was funded by a grant from China Postdoctoral Science Foundation. The investigators have reported no relevant financial relationships. Dr. Mayer is a scientific advisory board member of Danone, Axial Therapeutics, Viome, Amare, Mahana Therapeutics, Pendulum, Bloom Biosciences, and APC Microbiome Ireland.
A version of this article first appeared on Medscape.com.
However, at least one expert expressed concerns over the study’s conclusions.
Results from a study of 50 inpatients with schizophrenia showed significantly higher pro-inflammation, pro-oxidation, and leaky gut biomarkers in those with aggression vs. their peers who did not display aggression.
In addition, those with aggression showed less alpha diversity and evenness of the fecal bacterial community, lower levels of several beneficial gut bacteria, and higher levels of the fecal genera Prevotella.
Six short-chain fatty acids (SCFAs) and six neurotransmitters were also lower in the aggression vs. no-aggression groups.
“The present study was the first to compare the state of inflammation, oxidation, intestinal microbiota, and metabolites” in inpatients with schizophrenia and aggression, compared with those who did not show aggression, write the investigators, led by Hongxin Deng, department of psychiatry, Zhumadian (China) Psychiatric Hospital.
“Results indicate pro-inflammation, pro-oxidation and leaky gut phenotypes relating to enteric dysbacteriosis and microbial SCFAs feature the aggression in [individuals with schizophrenia], which provides clues for future microbial-based or anti-inflammatory/oxidative therapies on aggression,” they add.
The findings were published online in BMC Psychiatry.
Unknown pathogenesis
Although emerging evidence suggests that schizophrenia “may augment the propensity for aggression incidence about fourfold to sevenfold,” the pathogenesis of aggression “remains largely unknown,” the investigators note.
The same researchers previously found an association between the systemic pro-inflammation response and the onset or severity of aggression in schizophrenia, “possibly caused by leaky gut-induced bacterial translocation.”
The researchers suggest that peripheral cytokines “could cross the blood-brain barrier, thus precipitating changes in mood and behavior through hypothalamic-pituitary-adrenal axis.”
However, they note that the pro-inflammation phenotype is “often a synergistic effect of multiple causes.” Of these, chronic pro-oxidative stress has been shown to contribute to aggression onset in intermittent explosive disorder, but this association has rarely been confirmed in patients with schizophrenia.
In addition, increasing evidence points to enteric dysbacteriosis and dysbiosis of intestinal flora metabolites, including SCFAs or neurotransmitters, as potentially “integral parts of psychiatric disorders’ pathophysiology” by changing the state of both oxidative stress and inflammation.
The investigators hypothesized that the systemic pro-inflammation phenotype in aggression-affected schizophrenia cases “involves alterations to gut microbiota and its metabolites, leaky gut, and oxidative stress.” However, the profiles of these variables and their interrelationships have been “poorly investigated” in inpatients with schizophrenia and aggression.
To fill this gap, they assessed adult psychiatric inpatients with schizophrenia and aggressive behaviors and inpatients with schizophrenia but no aggressive behavior within 1 week before admission (n = 25 per group; mean age, 33.52 years, and 32.88 years, respectively; 68% and 64% women, respectively).
They collected stool samples from each patient and used enzyme-linked immunoassay (ELISA) to detect fecal calprotectin protein, an indicator of intestinal inflammation. They also collected fasting peripheral blood samples, using ELISA to detect several biomarkers.
The researchers also used the Modified Overt Aggression Scale (MOAS) to characterize aggressive behaviors and the Positive and Negative Syndrome Scale to characterize psychiatric symptoms.
‘Vital role’
Significantly higher biomarkers for systemic pro-inflammation, pro-oxidation and leaky gut were found in the aggression vs the no-aggression group (all P < .05).
After controlling for potential confounders, the researchers also found positive associations between MOAS scores and biomarkers, both serum and fecal.
There were also positive associations between serum 8-hydroxy-20-deoxy-guanosine (8-OH-DG) or 8-isoprostane (8-ISO) and systemic inflammatory biomarkers (all R > 0; P < .05).
In addition, the alpha diversity and evenness of the fecal bacterial community were lower in the aggression vs. no aggression groups.
When the researchers compared the relative abundance of the top 15 genera composition of intestinal microflora in the two groups, Bacteroides, Faecalibacterium, Blautia, Bifidobacterium, Collinsella, and Eubacterium coprostanoligenes were “remarkably reduced” in the group with aggression, whereas the abundance of fecal genera Prevotella was significantly increased (all corrected P < .001).
In the patients who had schizophrenia with aggression, levels of six SCFAs and six neurotransmitters were much lower than in the patients with schizophrenia but no aggression (all P < .05).
Inpatients with schizophrenia and aggression “had dramatically increased serum level of 8-OH-DG (nucleic acid oxidation biomarker) and 8-ISO (lipid oxidation biomarker) than those without, and further correlation analysis also showed positive correlativity between pro-oxidation and systemic pro-inflammation response or aggression severity,” the investigators write.
The findings “collectively suggest the cocontributory role of systemic pro-inflammation and pro-oxidation in the development of aggression” in schizophrenia, they add. “Gut dysbacteriosis with leaky gut seems to play a vital role in the pathophysiology.”
Correlation vs. causality
Commenting for this article, Emeran Mayer, MD, distinguished research professor of medicine at the G. Oppenheimer Center for Neurobiology of Stress and Resilience and UCLA Brain Gut Microbiome Center, Los Angeles, said that “at first glance, it is interesting that the behavioral trait of aggression but not the diagnosis of schizophrenia showed the differences in markers of systemic inflammation, increased gut permeability, and microbiome parameters.”
However, like many such descriptive studies, the research is flawed by comparing two patient groups and concluding causality between the biomarkers and the behavior traits, added Dr. Mayer, who was not involved with the study.
The study’s shortcomings include its small sample size as well as several confounding factors – particularly diet, sleep, exercise, and stress and anxiety levels – that were not considered, he said. The study also lacked a control group with high levels of aggression but without schizophrenia.
“The observed changes in intestinal permeability, unscientifically referred to as ‘leaky gut,’ as well as the gut microbiome differences, could be secondary to chronically increased sympathetic nervous system activation in the high aggression group,” Dr. Mayer said. “This is an interesting hypothesis which should be discussed and should have been addressed in this study.”
The differences in gut microbial composition and SCFA production “could be secondary to differences in plant-based diet components,” Dr. Mayer speculated, wondering how well dietary intake was controlled.
“Overall, it is an interesting descriptive study, which unfortunately does not contribute significantly to a better understanding of the role of the brain-gut microbiome system in schizophrenic patients,” he said.
The study was funded by a grant from China Postdoctoral Science Foundation. The investigators have reported no relevant financial relationships. Dr. Mayer is a scientific advisory board member of Danone, Axial Therapeutics, Viome, Amare, Mahana Therapeutics, Pendulum, Bloom Biosciences, and APC Microbiome Ireland.
A version of this article first appeared on Medscape.com.
However, at least one expert expressed concerns over the study’s conclusions.
Results from a study of 50 inpatients with schizophrenia showed significantly higher pro-inflammation, pro-oxidation, and leaky gut biomarkers in those with aggression vs. their peers who did not display aggression.
In addition, those with aggression showed less alpha diversity and evenness of the fecal bacterial community, lower levels of several beneficial gut bacteria, and higher levels of the fecal genera Prevotella.
Six short-chain fatty acids (SCFAs) and six neurotransmitters were also lower in the aggression vs. no-aggression groups.
“The present study was the first to compare the state of inflammation, oxidation, intestinal microbiota, and metabolites” in inpatients with schizophrenia and aggression, compared with those who did not show aggression, write the investigators, led by Hongxin Deng, department of psychiatry, Zhumadian (China) Psychiatric Hospital.
“Results indicate pro-inflammation, pro-oxidation and leaky gut phenotypes relating to enteric dysbacteriosis and microbial SCFAs feature the aggression in [individuals with schizophrenia], which provides clues for future microbial-based or anti-inflammatory/oxidative therapies on aggression,” they add.
The findings were published online in BMC Psychiatry.
Unknown pathogenesis
Although emerging evidence suggests that schizophrenia “may augment the propensity for aggression incidence about fourfold to sevenfold,” the pathogenesis of aggression “remains largely unknown,” the investigators note.
The same researchers previously found an association between the systemic pro-inflammation response and the onset or severity of aggression in schizophrenia, “possibly caused by leaky gut-induced bacterial translocation.”
The researchers suggest that peripheral cytokines “could cross the blood-brain barrier, thus precipitating changes in mood and behavior through hypothalamic-pituitary-adrenal axis.”
However, they note that the pro-inflammation phenotype is “often a synergistic effect of multiple causes.” Of these, chronic pro-oxidative stress has been shown to contribute to aggression onset in intermittent explosive disorder, but this association has rarely been confirmed in patients with schizophrenia.
In addition, increasing evidence points to enteric dysbacteriosis and dysbiosis of intestinal flora metabolites, including SCFAs or neurotransmitters, as potentially “integral parts of psychiatric disorders’ pathophysiology” by changing the state of both oxidative stress and inflammation.
The investigators hypothesized that the systemic pro-inflammation phenotype in aggression-affected schizophrenia cases “involves alterations to gut microbiota and its metabolites, leaky gut, and oxidative stress.” However, the profiles of these variables and their interrelationships have been “poorly investigated” in inpatients with schizophrenia and aggression.
To fill this gap, they assessed adult psychiatric inpatients with schizophrenia and aggressive behaviors and inpatients with schizophrenia but no aggressive behavior within 1 week before admission (n = 25 per group; mean age, 33.52 years, and 32.88 years, respectively; 68% and 64% women, respectively).
They collected stool samples from each patient and used enzyme-linked immunoassay (ELISA) to detect fecal calprotectin protein, an indicator of intestinal inflammation. They also collected fasting peripheral blood samples, using ELISA to detect several biomarkers.
The researchers also used the Modified Overt Aggression Scale (MOAS) to characterize aggressive behaviors and the Positive and Negative Syndrome Scale to characterize psychiatric symptoms.
‘Vital role’
Significantly higher biomarkers for systemic pro-inflammation, pro-oxidation and leaky gut were found in the aggression vs the no-aggression group (all P < .05).
After controlling for potential confounders, the researchers also found positive associations between MOAS scores and biomarkers, both serum and fecal.
There were also positive associations between serum 8-hydroxy-20-deoxy-guanosine (8-OH-DG) or 8-isoprostane (8-ISO) and systemic inflammatory biomarkers (all R > 0; P < .05).
In addition, the alpha diversity and evenness of the fecal bacterial community were lower in the aggression vs. no aggression groups.
When the researchers compared the relative abundance of the top 15 genera composition of intestinal microflora in the two groups, Bacteroides, Faecalibacterium, Blautia, Bifidobacterium, Collinsella, and Eubacterium coprostanoligenes were “remarkably reduced” in the group with aggression, whereas the abundance of fecal genera Prevotella was significantly increased (all corrected P < .001).
In the patients who had schizophrenia with aggression, levels of six SCFAs and six neurotransmitters were much lower than in the patients with schizophrenia but no aggression (all P < .05).
Inpatients with schizophrenia and aggression “had dramatically increased serum level of 8-OH-DG (nucleic acid oxidation biomarker) and 8-ISO (lipid oxidation biomarker) than those without, and further correlation analysis also showed positive correlativity between pro-oxidation and systemic pro-inflammation response or aggression severity,” the investigators write.
The findings “collectively suggest the cocontributory role of systemic pro-inflammation and pro-oxidation in the development of aggression” in schizophrenia, they add. “Gut dysbacteriosis with leaky gut seems to play a vital role in the pathophysiology.”
Correlation vs. causality
Commenting for this article, Emeran Mayer, MD, distinguished research professor of medicine at the G. Oppenheimer Center for Neurobiology of Stress and Resilience and UCLA Brain Gut Microbiome Center, Los Angeles, said that “at first glance, it is interesting that the behavioral trait of aggression but not the diagnosis of schizophrenia showed the differences in markers of systemic inflammation, increased gut permeability, and microbiome parameters.”
However, like many such descriptive studies, the research is flawed by comparing two patient groups and concluding causality between the biomarkers and the behavior traits, added Dr. Mayer, who was not involved with the study.
The study’s shortcomings include its small sample size as well as several confounding factors – particularly diet, sleep, exercise, and stress and anxiety levels – that were not considered, he said. The study also lacked a control group with high levels of aggression but without schizophrenia.
“The observed changes in intestinal permeability, unscientifically referred to as ‘leaky gut,’ as well as the gut microbiome differences, could be secondary to chronically increased sympathetic nervous system activation in the high aggression group,” Dr. Mayer said. “This is an interesting hypothesis which should be discussed and should have been addressed in this study.”
The differences in gut microbial composition and SCFA production “could be secondary to differences in plant-based diet components,” Dr. Mayer speculated, wondering how well dietary intake was controlled.
“Overall, it is an interesting descriptive study, which unfortunately does not contribute significantly to a better understanding of the role of the brain-gut microbiome system in schizophrenic patients,” he said.
The study was funded by a grant from China Postdoctoral Science Foundation. The investigators have reported no relevant financial relationships. Dr. Mayer is a scientific advisory board member of Danone, Axial Therapeutics, Viome, Amare, Mahana Therapeutics, Pendulum, Bloom Biosciences, and APC Microbiome Ireland.
A version of this article first appeared on Medscape.com.
FROM BMC PSYCHIATRY
Faulty fences: Blood-brain barrier dysfunction in schizophrenia
The blood-brain barrier (BBB) is an essential barrier of closely spaced cells that regulates entry into the CNS. What passes should be highly regulated to protect the brain from potentially harmful peripheral cells or molecules from the rest of the body. However, research has revealed that the BBB is pathologically permeable in several disease states, including schizophrenia, epilepsy, traumatic brain injury, autism, and DiGeorge syndrome (22q11.2 deletion syndrome, which often presents with symptoms of schizophrenia).1,2 In this article, we discuss potential markers of BBB dysfunction, the consequences of a porous BBB, the effect of BBB permeability on microglial activation, and possible treatment implications.
Detecting a BBB leak
The BBB is composed of microvascular endothelial cell units. Adherens junctions, astrocyte endfeet, and pericytes are all part of these units, but tight junctions have the most significant role in BBB barrier function. Tight junction protein composition varies depending on the location of the endothelium. In the BBB, they are primarily composed of claudin-5, occludin, zonulin, and junction adhesion molecules (JAMs) (Figure). Claudins and occludins are especially important components of the tight junction because they span plasma membranes.3
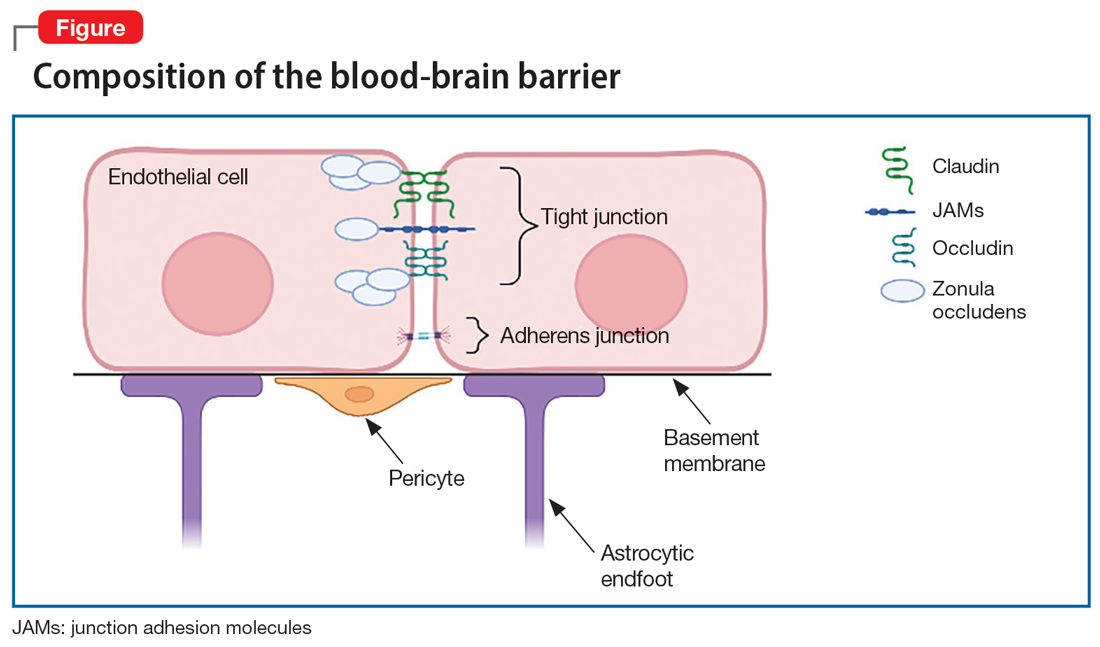
Researchers began to suspect tight junction permeability in schizophrenia while searching for schizophrenia biomarkers. For example, S100B is a marker of astrocytic reactivity to damage. It is increased in schizophrenia, major depressive disorder, and bipolar disorder.4 Studies found elevated S100B specifically in drug-free patients with schizophrenia,5 which prompted research suggesting it could predict the severity of negative symptoms.6 The accuracy of S100B as a biomarker was later complicated by the finding that adipose tissue also secretes S100B. This is problematic due to the high rates of comorbid obesity in psychiatric populations.2
Perhaps a better biomarker is the ratio of albumin in the CSF vs that in peripheral serum. The CSF-to-blood albumin ratio (Q-Alb) is widely considered an acceptable marker of BBB dysfunction because albumin must cross the BBB to alter the ratio. Studies have found a high Q-Alb in neurodegenerative disorders such as multiple sclerosis as well as in schizophrenia, which suggests that some level of BBB dysfunction is occurring. Although the Q-Alb may change slightly when confounded by antipsychotic use or with CSF flow changes,2,4 both S100B and Q-Alb elevation are sufficient for further investigation into tight junction alteration in schizophrenia.
Claudin-5 is a promising factor in detecting BBB permeability. Claudin-5 is deleted in DiGeorge syndrome, which is highly comorbid with schizophrenia and psychosis.1 Mouse knockdown studies show that full suppression of claudin-5 results in psychotic symptoms before fatal seizures,2,7 but a partial absence may enable psychotic symptoms. The same study showed that normally continuous claudin-5 was patchy along blood vessels in the affected sample.7 Follow-up experiments suggest that loss of claudin-5 in schizophrenia is especially prominent in the hippocampus, and there is mixed evidence of a decrease in the prefrontal cortex.8
Outside of claudin-5 alone, JAM-A plays a more regulatory role. It is upstream from an enhancer protein gene that serves as a transcription factor for the claudin-5 promoter, so when JAM-A is deleted, there is less claudin-5.9 However, while this decrease in claudin-5 may be pathological, there could still be various upstream changes that lead to schizophrenia.
What are the consequences of a porous BBB?
Although it is well established that the BBB passes small molecules and solutes, there is significant evidence of inflammatory trafficking in disease states. The BBB moves proinflammatory cytokines, alters transporters, and may even let white blood cells (WBCs) pass through. Immune cell infiltration has different requirements depending on the cell type. T cells rely on integrins, vascular cell adhesion molecule 1 (vCAM1), and intercellular adhesion molecule 1 (iCAM1) for binding, rolling, adhering, crossing, and migration to sites of inflammation.10,11 Both iCAM1 and vCAM1 are elevated in schizophrenia compared to other psychiatric disorders (such as unipolar depression) and correlate with other biomarkers. For example, vCAM1, responsible for recruitment and crossing, is correlated with a high Q-Alb.12 Primarily produced by astrocytes and endothelial cells, iCAM1 plays the largest role in crossing the BBB and migration. Postmortem tissue demonstrates that cytokines upregulate iCAM1 mRNA at the BBB in schizophrenia.13 Increased cytokines are well documented in the inflammatory model of schizophrenia. Interestingly, decreasing claudin-5 also upregulates iCAM1 production.14 Therefore, low baseline claudin-5 may contribute to additional inflammation and symptoms.
Continue to: BBB permeability also results...
BBB permeability also results in a certain pattern of leukocyte and cytokine activity. Interleukin-1 (IL-1), IL-6, and tumor necrosis factor–alpha can all cross the BBB during neuroimmune inflammation,10 but there are abnormal heightened and sustained responses of these molecules in schizophrenia. IL-6 is a proinflammatory cytokine in both acute and chronic inflammation that is expressed by astrocytes, endothelial cells, and microglia.15 IL-6 and its soluble receptor are both elevated in schizophrenia and are associated with white matter degeneration16,17 and an increase in vCAM1.15 This implies that while neuroinflammation in schizophrenia is occurring, additional leukocytes are being recruited and secreting their own cytokines in a chronic destructive positive feedback loop. Meanwhile, atypical IL-10 levels can no longer maintain balanced levels of inflammatory molecules,16 which leads to reduced control of inflammation.
Genetics and immunohistochemistry suggest that the BBB allows the passage of excess B cells and T cells in schizophrenia. Cytokines from WBCs or the BBB during inflammation recruit these additional infiltrating lymphocytes. In gene-wide association studies, there are several genes in schizophrenia important for B cells and T cells in addition to inflammation that interact in a proinflammatory network.16 These cells are also diffusely found in the white matter18 and hippocampal tissue19 of patients with schizophrenia. Taken together, an increase in adhesion molecules, WBCs, and cytokine crosstalk supports a leaky BBB as an important component of the inflammatory model of schizophrenia.
The role of microglia in BBB dysfunction
The effect of BBB permeability on microglial activation is an important caveat in the current research. Although several reports have linked neuroinflammation to confirmed microglial activation in schizophrenia, there is not enough evidence to claim that the BBB alone is the missing link between these theories. Some research suggests that chronic release of cytokines such as IL-6 from macrophages and T cells could increase migration across the BBB for microglial activation.16,20 However, positron emission tomography has shown mixed results at best. Translocator protein (TSPO) is expressed by microglia that are actively secreting cytokines.21 Researchers tracking TSPO changes in relation to BBB alteration have not seen elevated binding in schizophrenia, change due to stage of disease course, or differentiation from low-grade inflammation.21-24 Moreover, TSPO may be confounded by antipsychotic use25 and microglial expression did not correlate with any changes in adhesion molecules.13 TSPO is not an ideal indicator of microglial activation due to BBB breakdown, but that does not bar the possibility of at least a partial contribution to the development of schizophrenia.
Corsi-Zuelli et al26 created a model that attempts to merge BBB permeability and microglial activation through a different medium—T regulatory cells (TRegs). They write that if TRegs mediate interactions between astrocytes and microglia, their hypofunction would impose a prolonged T cell response. The increased access to a high level of IL-6 and its soluble receptors may keep the TRegs hypofunctional in schizophrenia and promote T cell conversion to inflammatory cell types. Experimentally, TReg induction reversed some psychotic symptoms, and greater TReg expression was associated with fewer negative symptoms.26 In an already insufficient BBB, more access to cytokines and leukocytes would sustain inflammation and microglial secretions.
In addition to the issues described regarding the BBB, the blood-CSF barrier at the choroid plexus may also be insufficient in schizophrenia (Box27-31).
Box
The choroid plexus’ primary role is to make CSF, but it also secretes cytokines and to some extent serves as a barrier. Unlike the blood-brain barrier (BBB), the blood-CSF barrier is composed of endothelial cells with fenestrations as well as tight junctions, which make the blood-CSF barrier overall more permeable.27,28 The most unusual finding regarding the choroid plexus in schizophrenia is size. The choroid plexus is physically larger in patients with schizophrenia, and to a lesser extent, in their first-degree relatives.29 A larger choroid plexus is correlated with more severe cognitive symptoms, increased risk for psychosis via biological stress, and significantly higher interleukin-6 (IL-6).27,29 The increased thickness could be an attempt to compensate for hyperactivity and toxic processes in a permeable environment. More circulating cytokines such as IL-6 and tumor necrosis factor–alpha from microglia can trigger an increase in intercellular adhesion molecule 1, resulting in leukocyte attachment and entry.30 Less claudin-5 at the choroid plexus in schizophrenia implicates similar permissive effects as seen at the BBB.31 Although the contribution of blood-CSF barrier dysfunction to schizophrenia requires further study, reduced barrier function outside the BBB is a viable line of inquiry.
Continue to: Caveats about this research
Caveats about this research
There are 3 important points to note about the current research concerning abnormal BBB permeability:
1. BBB dysfunction may exist only in a subset of people diagnosed with schizophrenia. In most human studies, only some patients with schizophrenia demonstrated alterations that suggested pathological BBB permeability. In addition, even when there is BBB dysfunction, it could be a secondary phenomenon, rather than a primary etiologic process.
2. Patient demographics across studies have not always been adequately described. Potential confounds such as obesity, smoking, or antipsychotic use were not consistently recorded or examined as a possible factor.
3. Currently available biomarkers are not perfect. Cytokine elevation, S100B, and Q-Alb are indirect measures of BBB disruption and are found in other disorders. Therefore, they only support the theory of BBB dysfunction in schizophrenia, rather than prove it. They are also not reliable markers for schizophrenia alone. Researchers have pointed out that these markers and proteins work in concert, which necessitates a network analysis approach.16 More research regarding the details of permeability is required to establish more reliable biomarkers and tailored treatment.
Treatment implications
One of the first treatment directions that comes to mind is managing the gaps in the BBB via tight junctions. Presently, there are no FDA-approved medications for altering tight junction proteins, but researchers are exploring potential agents that can induce claudin-5 and reduce inflammation.14 While we wait for such a medication, patients may benefit from existing anti-inflammatory treatments to control immune infiltration and its products. Various anti-inflammatory agents—including cyclooxygenase inhibitors,
Bottom Line
Recent research has revealed that the blood-brain barrier (BBB) is pathologically permeable in several disease states, including schizophrenia. Better characterization of the leaky BBB in schizophrenia has enormous potential in helping us understand how current theories fit together and could serve as a missing puzzle piece in treating schizophrenia.
Related Resources
- Levine A, Strawn JR. The brain’s Twitter system: neuronal extracellular vesicles. Current Psychiatry. 2022;21(6):9-11, 17-19,27. doi:10.12788/cp.0257
Drug Brand Names
Minocycline • Dynacin, Minocin
1. Li Y, Xia Y, Zhu H, et al. Investigation of neurodevelopmental deficits of 22 q11.2 deletion syndrome with a patient-iPSC-derived blood-brain barrier model. Cells. 2021;10(10):2576. doi:10.3390/cells10102576
2. Kealy J, Greene C, Campbell M. Blood-brain barrier regulation in psychiatric disorders. Neurosci Lett. 2020;726:133664. doi:10.1016/j.neulet.2018.06.033
3. Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16(1):1-13. doi:10.1016/j.nbd.2003.12.016
4. Futtrup J, Margolinsky R, Benros ME, et al. Blood-brain barrier pathology in patients with severe mental disorders: a systematic review and meta-analysis of biomarkers in case-control studies. Brain Behav Immun Health. 2020;6:100102. doi:10.1016/j.bbih.2020.100102
5. Chen S, Tian L, Chen N, et al. Cognitive dysfunction correlates with elevated serum S100B concentration in drug-free acutely relapsed patients with schizophrenia. Psychiatry Res. 2017;247:6-11. doi:10.1016/j.psychres.2016.09.029
6. Wu YF, Sytwu HK, Lung FW. Human aquaporin 4 gene polymorphisms and haplotypes are associated with serum S100B level and negative symptoms of schizophrenia in a southern Chinese Han population. Front Psychiatry. 2018;9:657. doi:10.3389/fpsyt.2018.00657
7. Greene C, Kealy J, Humphries MM, et al. Dose-dependent expression of claudin-5 is a modifying factor in schizophrenia. Mol Psychiatry. 2018;23(11):2156-2166. doi:10.1038/MP.2017.156
8. Greene C, Hanley N, Campbell M. Blood-brain barrier associated tight junction disruption is a hallmark feature of major psychiatric disorders. Transl Psychiatry. 2020;10(1):373. doi:10.1038/s41398-020-01054-3
9. Kakogiannos N, Ferrari L, Giampietro C, et al. JAM-A acts via C/EBP-α to promote claudin-5 expression and enhance endothelial barrier function. Circ Res. 2020:1056-1073. doi:10.1161/CIRCRESAHA.120.316742
10. Erickson MA, Dohi K, Banks WA. Neuroinflammation: a common pathway in CNS diseases as mediated at the blood-brain barrier. Neuroimmunomodulation. 2012;19(2):121-130. doi:10.1159/000330247
11. Ao LY, Yan YY, Zhou L, et al. Immune cells after ischemic stroke onset: roles, migration, and target intervention. J Mol Neurosci. 2018;66(3):342-355. doi:10.1007/s12031-018-1173-4
12. Meixensberger S, Kuzior H, Fiebich BL, et al. Upregulation of sICAM-1 and sVCAM-1 levels in the cerebrospinal fluid of patients with schizophrenia spectrum disorders. Diagnostics (Basel). 2021;11(7):1134. doi:10.3390diagnostics11071134
13. Cai HQ, Catts VS, Webster MJ, et al. Increased macrophages and changed brain endothelial cell gene expression in the frontal cortex of people with schizophrenia displaying inflammation. Mol Psychiatry. 2020;25(4):761-775. doi:10.1038/s41380-018-0235-x
14. Greene C, Hanley N, Reschke CR, et al. Microvascular stabilization via blood-brain barrier regulation prevents seizure activity. Nat Commun. 2022;13(1):2003. doi:10.1038/s41467-022-29657-y
15. García-Juárez M, Camacho-Morales A. Defining the role of anti- and pro-inflammatory outcomes of interleukin-6 in mental health. Neuroscience. 2022;492:32-46. doi:10.1016/j.neuroscience.2022.03.020
16. Pong S, Karmacharya R, Sofman M, et al. The role of brain microvascular endothelial cell and blood-brain barrier dysfunction in schizophrenia. Complex Psychiatry. 2020;6(1-2):30-46. doi:10.1159/000511552
17. Patel A, Zhu Y, Kuzhikandathil EV, et al. Soluble interleukin-6 receptor induces motor stereotypies and co-localizes with gp130 in regions linked to cortico-striato-thalamo-cortical circuits. PLoS One. 2012;7(7): e41623. doi:10.1371/journal.pone.0041623
18. Schlaaff K, Dobrowolny H, Frodl T, et al. Increased densities of T and B lymphocytes indicate neuroinflammation in subgroups of schizophrenia and mood disorder patients. Brain Behav Immun. 2020;88:497-506. doi:10.1016/j.bbi.2020.04.021
19. Busse S, Busse M, Schiltz K, et al. Different distribution patterns of lymphocytes and microglia in the hippocampus of patients with residual versus paranoid schizophrenia: further evidence for disease course-related immune alterations? Brain Behav Immun. 2012;26(8):1273-1279. doi:10.1016/j.bbi.2012.08.005
20. Na KS, Jung HY, Kim YK. The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:277-286. doi:10.1016/j.pnpbp.2012.10.022
21. Conen S, Gregory CJ, Hinz R, et al. Neuroinflammation as measured by positron emission tomography in patients with recent onset and established schizophrenia: implications for immune pathogenesis. Mol Psychiatry. 2021;26(9):5398-5406. doi:10.1038/S41380-020-0829-Y
22. Najjar S, Pahlajani S, De Sanctis V, et al. Neurovascular unit dysfunction and blood-brain barrier hyperpermeability contribute to schizophrenia neurobiology: a theoretical integration of clinical and experimental evidence. Front Psychiatry. 2017;8:83. doi:10.3389/fpsyt.2017.00083
23. Pinjari OF, Dasgupta SK, Okusaga OO. Plasma soluble P-selectin, interleukin-6 and S100B protein in patients with schizophrenia: a pilot study. Psychiatr Q. 2022;93(1):335-345. doi:10.1007/s11126-021-09954-3
24. Di Biase MA, Zalesky A, O’keefe G, et al. PET imaging of putative microglial activation in individuals at ultra-high risk for psychosis, recently diagnosed and chronically ill with schizophrenia. Transl Psychiatry. 2017;7(8):e1225. doi:10.1038/tp.2017.193
25. Holmes SE, Hinz R, Drake RJ, et al. In vivo imaging of brain microglial activity in antipsychotic-free and medicated schizophrenia: a [11C](R)-PK11195 positron emission tomography study. Mol Psychiatry. 2016;21(12):1672-1679. doi:10.1038/mp.2016.180
26. Corsi-Zuelli F, Deakin B, de Lima MHF, et al. T regulatory cells as a potential therapeutic target in psychosis? Current challenges and future perspectives. Brain Behav Immun Health. 2021;17:100330. doi:10.1016/j.bbih.2021.100330
27. Bannai D, Lutz O, Lizano P. Neuroimaging considerations when investigating choroid plexus morphology in idiopathic psychosis. Schizophr Res. 2020;224:19-21. doi:10.1016/j.schres.2020.07.013
28. Hladky SB, Barrand MA. Fluid and ion transfer across the blood-brain and blood-cerebrospinal fluid barriers; a comparative account of mechanisms and roles. Fluids Barriers CNS. 2016;13(1):19. doi:10.1186/s12987-016-0040-3
29. Lizano P, Lutz O, Ling G, et al. Association of choroid plexus enlargement with cognitive, inflammatory, and structural phenotypes across the psychosis spectrum. Am J Psychiatry. 2019;176(7):564-572. doi:10.1176/appi.ajp.2019.18070825
30. Castellani G, Contarini G, Mereu M, et al. Dopamine-mediated immunomodulation affects choroid plexus function. Brain Behav Immun. 2019;81:138-150. doi:10.1016/j.bbi.2019.06.006
31. Bitanihirwe BKY, Lizano P, Woo TW. Deconstructing the functional neuroanatomy of the choroid plexus: an ontogenetic perspective for studying neurodevelopmental and neuropsychiatric disorders. Mol Psychiatry. 2022;1-10. doi:10.1038/s41380-022-01623-6
The blood-brain barrier (BBB) is an essential barrier of closely spaced cells that regulates entry into the CNS. What passes should be highly regulated to protect the brain from potentially harmful peripheral cells or molecules from the rest of the body. However, research has revealed that the BBB is pathologically permeable in several disease states, including schizophrenia, epilepsy, traumatic brain injury, autism, and DiGeorge syndrome (22q11.2 deletion syndrome, which often presents with symptoms of schizophrenia).1,2 In this article, we discuss potential markers of BBB dysfunction, the consequences of a porous BBB, the effect of BBB permeability on microglial activation, and possible treatment implications.
Detecting a BBB leak
The BBB is composed of microvascular endothelial cell units. Adherens junctions, astrocyte endfeet, and pericytes are all part of these units, but tight junctions have the most significant role in BBB barrier function. Tight junction protein composition varies depending on the location of the endothelium. In the BBB, they are primarily composed of claudin-5, occludin, zonulin, and junction adhesion molecules (JAMs) (Figure). Claudins and occludins are especially important components of the tight junction because they span plasma membranes.3

Researchers began to suspect tight junction permeability in schizophrenia while searching for schizophrenia biomarkers. For example, S100B is a marker of astrocytic reactivity to damage. It is increased in schizophrenia, major depressive disorder, and bipolar disorder.4 Studies found elevated S100B specifically in drug-free patients with schizophrenia,5 which prompted research suggesting it could predict the severity of negative symptoms.6 The accuracy of S100B as a biomarker was later complicated by the finding that adipose tissue also secretes S100B. This is problematic due to the high rates of comorbid obesity in psychiatric populations.2
Perhaps a better biomarker is the ratio of albumin in the CSF vs that in peripheral serum. The CSF-to-blood albumin ratio (Q-Alb) is widely considered an acceptable marker of BBB dysfunction because albumin must cross the BBB to alter the ratio. Studies have found a high Q-Alb in neurodegenerative disorders such as multiple sclerosis as well as in schizophrenia, which suggests that some level of BBB dysfunction is occurring. Although the Q-Alb may change slightly when confounded by antipsychotic use or with CSF flow changes,2,4 both S100B and Q-Alb elevation are sufficient for further investigation into tight junction alteration in schizophrenia.
Claudin-5 is a promising factor in detecting BBB permeability. Claudin-5 is deleted in DiGeorge syndrome, which is highly comorbid with schizophrenia and psychosis.1 Mouse knockdown studies show that full suppression of claudin-5 results in psychotic symptoms before fatal seizures,2,7 but a partial absence may enable psychotic symptoms. The same study showed that normally continuous claudin-5 was patchy along blood vessels in the affected sample.7 Follow-up experiments suggest that loss of claudin-5 in schizophrenia is especially prominent in the hippocampus, and there is mixed evidence of a decrease in the prefrontal cortex.8
Outside of claudin-5 alone, JAM-A plays a more regulatory role. It is upstream from an enhancer protein gene that serves as a transcription factor for the claudin-5 promoter, so when JAM-A is deleted, there is less claudin-5.9 However, while this decrease in claudin-5 may be pathological, there could still be various upstream changes that lead to schizophrenia.
What are the consequences of a porous BBB?
Although it is well established that the BBB passes small molecules and solutes, there is significant evidence of inflammatory trafficking in disease states. The BBB moves proinflammatory cytokines, alters transporters, and may even let white blood cells (WBCs) pass through. Immune cell infiltration has different requirements depending on the cell type. T cells rely on integrins, vascular cell adhesion molecule 1 (vCAM1), and intercellular adhesion molecule 1 (iCAM1) for binding, rolling, adhering, crossing, and migration to sites of inflammation.10,11 Both iCAM1 and vCAM1 are elevated in schizophrenia compared to other psychiatric disorders (such as unipolar depression) and correlate with other biomarkers. For example, vCAM1, responsible for recruitment and crossing, is correlated with a high Q-Alb.12 Primarily produced by astrocytes and endothelial cells, iCAM1 plays the largest role in crossing the BBB and migration. Postmortem tissue demonstrates that cytokines upregulate iCAM1 mRNA at the BBB in schizophrenia.13 Increased cytokines are well documented in the inflammatory model of schizophrenia. Interestingly, decreasing claudin-5 also upregulates iCAM1 production.14 Therefore, low baseline claudin-5 may contribute to additional inflammation and symptoms.
Continue to: BBB permeability also results...
BBB permeability also results in a certain pattern of leukocyte and cytokine activity. Interleukin-1 (IL-1), IL-6, and tumor necrosis factor–alpha can all cross the BBB during neuroimmune inflammation,10 but there are abnormal heightened and sustained responses of these molecules in schizophrenia. IL-6 is a proinflammatory cytokine in both acute and chronic inflammation that is expressed by astrocytes, endothelial cells, and microglia.15 IL-6 and its soluble receptor are both elevated in schizophrenia and are associated with white matter degeneration16,17 and an increase in vCAM1.15 This implies that while neuroinflammation in schizophrenia is occurring, additional leukocytes are being recruited and secreting their own cytokines in a chronic destructive positive feedback loop. Meanwhile, atypical IL-10 levels can no longer maintain balanced levels of inflammatory molecules,16 which leads to reduced control of inflammation.
Genetics and immunohistochemistry suggest that the BBB allows the passage of excess B cells and T cells in schizophrenia. Cytokines from WBCs or the BBB during inflammation recruit these additional infiltrating lymphocytes. In gene-wide association studies, there are several genes in schizophrenia important for B cells and T cells in addition to inflammation that interact in a proinflammatory network.16 These cells are also diffusely found in the white matter18 and hippocampal tissue19 of patients with schizophrenia. Taken together, an increase in adhesion molecules, WBCs, and cytokine crosstalk supports a leaky BBB as an important component of the inflammatory model of schizophrenia.
The role of microglia in BBB dysfunction
The effect of BBB permeability on microglial activation is an important caveat in the current research. Although several reports have linked neuroinflammation to confirmed microglial activation in schizophrenia, there is not enough evidence to claim that the BBB alone is the missing link between these theories. Some research suggests that chronic release of cytokines such as IL-6 from macrophages and T cells could increase migration across the BBB for microglial activation.16,20 However, positron emission tomography has shown mixed results at best. Translocator protein (TSPO) is expressed by microglia that are actively secreting cytokines.21 Researchers tracking TSPO changes in relation to BBB alteration have not seen elevated binding in schizophrenia, change due to stage of disease course, or differentiation from low-grade inflammation.21-24 Moreover, TSPO may be confounded by antipsychotic use25 and microglial expression did not correlate with any changes in adhesion molecules.13 TSPO is not an ideal indicator of microglial activation due to BBB breakdown, but that does not bar the possibility of at least a partial contribution to the development of schizophrenia.
Corsi-Zuelli et al26 created a model that attempts to merge BBB permeability and microglial activation through a different medium—T regulatory cells (TRegs). They write that if TRegs mediate interactions between astrocytes and microglia, their hypofunction would impose a prolonged T cell response. The increased access to a high level of IL-6 and its soluble receptors may keep the TRegs hypofunctional in schizophrenia and promote T cell conversion to inflammatory cell types. Experimentally, TReg induction reversed some psychotic symptoms, and greater TReg expression was associated with fewer negative symptoms.26 In an already insufficient BBB, more access to cytokines and leukocytes would sustain inflammation and microglial secretions.
In addition to the issues described regarding the BBB, the blood-CSF barrier at the choroid plexus may also be insufficient in schizophrenia (Box27-31).
Box
The choroid plexus’ primary role is to make CSF, but it also secretes cytokines and to some extent serves as a barrier. Unlike the blood-brain barrier (BBB), the blood-CSF barrier is composed of endothelial cells with fenestrations as well as tight junctions, which make the blood-CSF barrier overall more permeable.27,28 The most unusual finding regarding the choroid plexus in schizophrenia is size. The choroid plexus is physically larger in patients with schizophrenia, and to a lesser extent, in their first-degree relatives.29 A larger choroid plexus is correlated with more severe cognitive symptoms, increased risk for psychosis via biological stress, and significantly higher interleukin-6 (IL-6).27,29 The increased thickness could be an attempt to compensate for hyperactivity and toxic processes in a permeable environment. More circulating cytokines such as IL-6 and tumor necrosis factor–alpha from microglia can trigger an increase in intercellular adhesion molecule 1, resulting in leukocyte attachment and entry.30 Less claudin-5 at the choroid plexus in schizophrenia implicates similar permissive effects as seen at the BBB.31 Although the contribution of blood-CSF barrier dysfunction to schizophrenia requires further study, reduced barrier function outside the BBB is a viable line of inquiry.
Continue to: Caveats about this research
Caveats about this research
There are 3 important points to note about the current research concerning abnormal BBB permeability:
1. BBB dysfunction may exist only in a subset of people diagnosed with schizophrenia. In most human studies, only some patients with schizophrenia demonstrated alterations that suggested pathological BBB permeability. In addition, even when there is BBB dysfunction, it could be a secondary phenomenon, rather than a primary etiologic process.
2. Patient demographics across studies have not always been adequately described. Potential confounds such as obesity, smoking, or antipsychotic use were not consistently recorded or examined as a possible factor.
3. Currently available biomarkers are not perfect. Cytokine elevation, S100B, and Q-Alb are indirect measures of BBB disruption and are found in other disorders. Therefore, they only support the theory of BBB dysfunction in schizophrenia, rather than prove it. They are also not reliable markers for schizophrenia alone. Researchers have pointed out that these markers and proteins work in concert, which necessitates a network analysis approach.16 More research regarding the details of permeability is required to establish more reliable biomarkers and tailored treatment.
Treatment implications
One of the first treatment directions that comes to mind is managing the gaps in the BBB via tight junctions. Presently, there are no FDA-approved medications for altering tight junction proteins, but researchers are exploring potential agents that can induce claudin-5 and reduce inflammation.14 While we wait for such a medication, patients may benefit from existing anti-inflammatory treatments to control immune infiltration and its products. Various anti-inflammatory agents—including cyclooxygenase inhibitors,
Bottom Line
Recent research has revealed that the blood-brain barrier (BBB) is pathologically permeable in several disease states, including schizophrenia. Better characterization of the leaky BBB in schizophrenia has enormous potential in helping us understand how current theories fit together and could serve as a missing puzzle piece in treating schizophrenia.
Related Resources
- Levine A, Strawn JR. The brain’s Twitter system: neuronal extracellular vesicles. Current Psychiatry. 2022;21(6):9-11, 17-19,27. doi:10.12788/cp.0257
Drug Brand Names
Minocycline • Dynacin, Minocin
The blood-brain barrier (BBB) is an essential barrier of closely spaced cells that regulates entry into the CNS. What passes should be highly regulated to protect the brain from potentially harmful peripheral cells or molecules from the rest of the body. However, research has revealed that the BBB is pathologically permeable in several disease states, including schizophrenia, epilepsy, traumatic brain injury, autism, and DiGeorge syndrome (22q11.2 deletion syndrome, which often presents with symptoms of schizophrenia).1,2 In this article, we discuss potential markers of BBB dysfunction, the consequences of a porous BBB, the effect of BBB permeability on microglial activation, and possible treatment implications.
Detecting a BBB leak
The BBB is composed of microvascular endothelial cell units. Adherens junctions, astrocyte endfeet, and pericytes are all part of these units, but tight junctions have the most significant role in BBB barrier function. Tight junction protein composition varies depending on the location of the endothelium. In the BBB, they are primarily composed of claudin-5, occludin, zonulin, and junction adhesion molecules (JAMs) (Figure). Claudins and occludins are especially important components of the tight junction because they span plasma membranes.3

Researchers began to suspect tight junction permeability in schizophrenia while searching for schizophrenia biomarkers. For example, S100B is a marker of astrocytic reactivity to damage. It is increased in schizophrenia, major depressive disorder, and bipolar disorder.4 Studies found elevated S100B specifically in drug-free patients with schizophrenia,5 which prompted research suggesting it could predict the severity of negative symptoms.6 The accuracy of S100B as a biomarker was later complicated by the finding that adipose tissue also secretes S100B. This is problematic due to the high rates of comorbid obesity in psychiatric populations.2
Perhaps a better biomarker is the ratio of albumin in the CSF vs that in peripheral serum. The CSF-to-blood albumin ratio (Q-Alb) is widely considered an acceptable marker of BBB dysfunction because albumin must cross the BBB to alter the ratio. Studies have found a high Q-Alb in neurodegenerative disorders such as multiple sclerosis as well as in schizophrenia, which suggests that some level of BBB dysfunction is occurring. Although the Q-Alb may change slightly when confounded by antipsychotic use or with CSF flow changes,2,4 both S100B and Q-Alb elevation are sufficient for further investigation into tight junction alteration in schizophrenia.
Claudin-5 is a promising factor in detecting BBB permeability. Claudin-5 is deleted in DiGeorge syndrome, which is highly comorbid with schizophrenia and psychosis.1 Mouse knockdown studies show that full suppression of claudin-5 results in psychotic symptoms before fatal seizures,2,7 but a partial absence may enable psychotic symptoms. The same study showed that normally continuous claudin-5 was patchy along blood vessels in the affected sample.7 Follow-up experiments suggest that loss of claudin-5 in schizophrenia is especially prominent in the hippocampus, and there is mixed evidence of a decrease in the prefrontal cortex.8
Outside of claudin-5 alone, JAM-A plays a more regulatory role. It is upstream from an enhancer protein gene that serves as a transcription factor for the claudin-5 promoter, so when JAM-A is deleted, there is less claudin-5.9 However, while this decrease in claudin-5 may be pathological, there could still be various upstream changes that lead to schizophrenia.
What are the consequences of a porous BBB?
Although it is well established that the BBB passes small molecules and solutes, there is significant evidence of inflammatory trafficking in disease states. The BBB moves proinflammatory cytokines, alters transporters, and may even let white blood cells (WBCs) pass through. Immune cell infiltration has different requirements depending on the cell type. T cells rely on integrins, vascular cell adhesion molecule 1 (vCAM1), and intercellular adhesion molecule 1 (iCAM1) for binding, rolling, adhering, crossing, and migration to sites of inflammation.10,11 Both iCAM1 and vCAM1 are elevated in schizophrenia compared to other psychiatric disorders (such as unipolar depression) and correlate with other biomarkers. For example, vCAM1, responsible for recruitment and crossing, is correlated with a high Q-Alb.12 Primarily produced by astrocytes and endothelial cells, iCAM1 plays the largest role in crossing the BBB and migration. Postmortem tissue demonstrates that cytokines upregulate iCAM1 mRNA at the BBB in schizophrenia.13 Increased cytokines are well documented in the inflammatory model of schizophrenia. Interestingly, decreasing claudin-5 also upregulates iCAM1 production.14 Therefore, low baseline claudin-5 may contribute to additional inflammation and symptoms.
Continue to: BBB permeability also results...
BBB permeability also results in a certain pattern of leukocyte and cytokine activity. Interleukin-1 (IL-1), IL-6, and tumor necrosis factor–alpha can all cross the BBB during neuroimmune inflammation,10 but there are abnormal heightened and sustained responses of these molecules in schizophrenia. IL-6 is a proinflammatory cytokine in both acute and chronic inflammation that is expressed by astrocytes, endothelial cells, and microglia.15 IL-6 and its soluble receptor are both elevated in schizophrenia and are associated with white matter degeneration16,17 and an increase in vCAM1.15 This implies that while neuroinflammation in schizophrenia is occurring, additional leukocytes are being recruited and secreting their own cytokines in a chronic destructive positive feedback loop. Meanwhile, atypical IL-10 levels can no longer maintain balanced levels of inflammatory molecules,16 which leads to reduced control of inflammation.
Genetics and immunohistochemistry suggest that the BBB allows the passage of excess B cells and T cells in schizophrenia. Cytokines from WBCs or the BBB during inflammation recruit these additional infiltrating lymphocytes. In gene-wide association studies, there are several genes in schizophrenia important for B cells and T cells in addition to inflammation that interact in a proinflammatory network.16 These cells are also diffusely found in the white matter18 and hippocampal tissue19 of patients with schizophrenia. Taken together, an increase in adhesion molecules, WBCs, and cytokine crosstalk supports a leaky BBB as an important component of the inflammatory model of schizophrenia.
The role of microglia in BBB dysfunction
The effect of BBB permeability on microglial activation is an important caveat in the current research. Although several reports have linked neuroinflammation to confirmed microglial activation in schizophrenia, there is not enough evidence to claim that the BBB alone is the missing link between these theories. Some research suggests that chronic release of cytokines such as IL-6 from macrophages and T cells could increase migration across the BBB for microglial activation.16,20 However, positron emission tomography has shown mixed results at best. Translocator protein (TSPO) is expressed by microglia that are actively secreting cytokines.21 Researchers tracking TSPO changes in relation to BBB alteration have not seen elevated binding in schizophrenia, change due to stage of disease course, or differentiation from low-grade inflammation.21-24 Moreover, TSPO may be confounded by antipsychotic use25 and microglial expression did not correlate with any changes in adhesion molecules.13 TSPO is not an ideal indicator of microglial activation due to BBB breakdown, but that does not bar the possibility of at least a partial contribution to the development of schizophrenia.
Corsi-Zuelli et al26 created a model that attempts to merge BBB permeability and microglial activation through a different medium—T regulatory cells (TRegs). They write that if TRegs mediate interactions between astrocytes and microglia, their hypofunction would impose a prolonged T cell response. The increased access to a high level of IL-6 and its soluble receptors may keep the TRegs hypofunctional in schizophrenia and promote T cell conversion to inflammatory cell types. Experimentally, TReg induction reversed some psychotic symptoms, and greater TReg expression was associated with fewer negative symptoms.26 In an already insufficient BBB, more access to cytokines and leukocytes would sustain inflammation and microglial secretions.
In addition to the issues described regarding the BBB, the blood-CSF barrier at the choroid plexus may also be insufficient in schizophrenia (Box27-31).
Box
The choroid plexus’ primary role is to make CSF, but it also secretes cytokines and to some extent serves as a barrier. Unlike the blood-brain barrier (BBB), the blood-CSF barrier is composed of endothelial cells with fenestrations as well as tight junctions, which make the blood-CSF barrier overall more permeable.27,28 The most unusual finding regarding the choroid plexus in schizophrenia is size. The choroid plexus is physically larger in patients with schizophrenia, and to a lesser extent, in their first-degree relatives.29 A larger choroid plexus is correlated with more severe cognitive symptoms, increased risk for psychosis via biological stress, and significantly higher interleukin-6 (IL-6).27,29 The increased thickness could be an attempt to compensate for hyperactivity and toxic processes in a permeable environment. More circulating cytokines such as IL-6 and tumor necrosis factor–alpha from microglia can trigger an increase in intercellular adhesion molecule 1, resulting in leukocyte attachment and entry.30 Less claudin-5 at the choroid plexus in schizophrenia implicates similar permissive effects as seen at the BBB.31 Although the contribution of blood-CSF barrier dysfunction to schizophrenia requires further study, reduced barrier function outside the BBB is a viable line of inquiry.
Continue to: Caveats about this research
Caveats about this research
There are 3 important points to note about the current research concerning abnormal BBB permeability:
1. BBB dysfunction may exist only in a subset of people diagnosed with schizophrenia. In most human studies, only some patients with schizophrenia demonstrated alterations that suggested pathological BBB permeability. In addition, even when there is BBB dysfunction, it could be a secondary phenomenon, rather than a primary etiologic process.
2. Patient demographics across studies have not always been adequately described. Potential confounds such as obesity, smoking, or antipsychotic use were not consistently recorded or examined as a possible factor.
3. Currently available biomarkers are not perfect. Cytokine elevation, S100B, and Q-Alb are indirect measures of BBB disruption and are found in other disorders. Therefore, they only support the theory of BBB dysfunction in schizophrenia, rather than prove it. They are also not reliable markers for schizophrenia alone. Researchers have pointed out that these markers and proteins work in concert, which necessitates a network analysis approach.16 More research regarding the details of permeability is required to establish more reliable biomarkers and tailored treatment.
Treatment implications
One of the first treatment directions that comes to mind is managing the gaps in the BBB via tight junctions. Presently, there are no FDA-approved medications for altering tight junction proteins, but researchers are exploring potential agents that can induce claudin-5 and reduce inflammation.14 While we wait for such a medication, patients may benefit from existing anti-inflammatory treatments to control immune infiltration and its products. Various anti-inflammatory agents—including cyclooxygenase inhibitors,
Bottom Line
Recent research has revealed that the blood-brain barrier (BBB) is pathologically permeable in several disease states, including schizophrenia. Better characterization of the leaky BBB in schizophrenia has enormous potential in helping us understand how current theories fit together and could serve as a missing puzzle piece in treating schizophrenia.
Related Resources
- Levine A, Strawn JR. The brain’s Twitter system: neuronal extracellular vesicles. Current Psychiatry. 2022;21(6):9-11, 17-19,27. doi:10.12788/cp.0257
Drug Brand Names
Minocycline • Dynacin, Minocin
1. Li Y, Xia Y, Zhu H, et al. Investigation of neurodevelopmental deficits of 22 q11.2 deletion syndrome with a patient-iPSC-derived blood-brain barrier model. Cells. 2021;10(10):2576. doi:10.3390/cells10102576
2. Kealy J, Greene C, Campbell M. Blood-brain barrier regulation in psychiatric disorders. Neurosci Lett. 2020;726:133664. doi:10.1016/j.neulet.2018.06.033
3. Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16(1):1-13. doi:10.1016/j.nbd.2003.12.016
4. Futtrup J, Margolinsky R, Benros ME, et al. Blood-brain barrier pathology in patients with severe mental disorders: a systematic review and meta-analysis of biomarkers in case-control studies. Brain Behav Immun Health. 2020;6:100102. doi:10.1016/j.bbih.2020.100102
5. Chen S, Tian L, Chen N, et al. Cognitive dysfunction correlates with elevated serum S100B concentration in drug-free acutely relapsed patients with schizophrenia. Psychiatry Res. 2017;247:6-11. doi:10.1016/j.psychres.2016.09.029
6. Wu YF, Sytwu HK, Lung FW. Human aquaporin 4 gene polymorphisms and haplotypes are associated with serum S100B level and negative symptoms of schizophrenia in a southern Chinese Han population. Front Psychiatry. 2018;9:657. doi:10.3389/fpsyt.2018.00657
7. Greene C, Kealy J, Humphries MM, et al. Dose-dependent expression of claudin-5 is a modifying factor in schizophrenia. Mol Psychiatry. 2018;23(11):2156-2166. doi:10.1038/MP.2017.156
8. Greene C, Hanley N, Campbell M. Blood-brain barrier associated tight junction disruption is a hallmark feature of major psychiatric disorders. Transl Psychiatry. 2020;10(1):373. doi:10.1038/s41398-020-01054-3
9. Kakogiannos N, Ferrari L, Giampietro C, et al. JAM-A acts via C/EBP-α to promote claudin-5 expression and enhance endothelial barrier function. Circ Res. 2020:1056-1073. doi:10.1161/CIRCRESAHA.120.316742
10. Erickson MA, Dohi K, Banks WA. Neuroinflammation: a common pathway in CNS diseases as mediated at the blood-brain barrier. Neuroimmunomodulation. 2012;19(2):121-130. doi:10.1159/000330247
11. Ao LY, Yan YY, Zhou L, et al. Immune cells after ischemic stroke onset: roles, migration, and target intervention. J Mol Neurosci. 2018;66(3):342-355. doi:10.1007/s12031-018-1173-4
12. Meixensberger S, Kuzior H, Fiebich BL, et al. Upregulation of sICAM-1 and sVCAM-1 levels in the cerebrospinal fluid of patients with schizophrenia spectrum disorders. Diagnostics (Basel). 2021;11(7):1134. doi:10.3390diagnostics11071134
13. Cai HQ, Catts VS, Webster MJ, et al. Increased macrophages and changed brain endothelial cell gene expression in the frontal cortex of people with schizophrenia displaying inflammation. Mol Psychiatry. 2020;25(4):761-775. doi:10.1038/s41380-018-0235-x
14. Greene C, Hanley N, Reschke CR, et al. Microvascular stabilization via blood-brain barrier regulation prevents seizure activity. Nat Commun. 2022;13(1):2003. doi:10.1038/s41467-022-29657-y
15. García-Juárez M, Camacho-Morales A. Defining the role of anti- and pro-inflammatory outcomes of interleukin-6 in mental health. Neuroscience. 2022;492:32-46. doi:10.1016/j.neuroscience.2022.03.020
16. Pong S, Karmacharya R, Sofman M, et al. The role of brain microvascular endothelial cell and blood-brain barrier dysfunction in schizophrenia. Complex Psychiatry. 2020;6(1-2):30-46. doi:10.1159/000511552
17. Patel A, Zhu Y, Kuzhikandathil EV, et al. Soluble interleukin-6 receptor induces motor stereotypies and co-localizes with gp130 in regions linked to cortico-striato-thalamo-cortical circuits. PLoS One. 2012;7(7): e41623. doi:10.1371/journal.pone.0041623
18. Schlaaff K, Dobrowolny H, Frodl T, et al. Increased densities of T and B lymphocytes indicate neuroinflammation in subgroups of schizophrenia and mood disorder patients. Brain Behav Immun. 2020;88:497-506. doi:10.1016/j.bbi.2020.04.021
19. Busse S, Busse M, Schiltz K, et al. Different distribution patterns of lymphocytes and microglia in the hippocampus of patients with residual versus paranoid schizophrenia: further evidence for disease course-related immune alterations? Brain Behav Immun. 2012;26(8):1273-1279. doi:10.1016/j.bbi.2012.08.005
20. Na KS, Jung HY, Kim YK. The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:277-286. doi:10.1016/j.pnpbp.2012.10.022
21. Conen S, Gregory CJ, Hinz R, et al. Neuroinflammation as measured by positron emission tomography in patients with recent onset and established schizophrenia: implications for immune pathogenesis. Mol Psychiatry. 2021;26(9):5398-5406. doi:10.1038/S41380-020-0829-Y
22. Najjar S, Pahlajani S, De Sanctis V, et al. Neurovascular unit dysfunction and blood-brain barrier hyperpermeability contribute to schizophrenia neurobiology: a theoretical integration of clinical and experimental evidence. Front Psychiatry. 2017;8:83. doi:10.3389/fpsyt.2017.00083
23. Pinjari OF, Dasgupta SK, Okusaga OO. Plasma soluble P-selectin, interleukin-6 and S100B protein in patients with schizophrenia: a pilot study. Psychiatr Q. 2022;93(1):335-345. doi:10.1007/s11126-021-09954-3
24. Di Biase MA, Zalesky A, O’keefe G, et al. PET imaging of putative microglial activation in individuals at ultra-high risk for psychosis, recently diagnosed and chronically ill with schizophrenia. Transl Psychiatry. 2017;7(8):e1225. doi:10.1038/tp.2017.193
25. Holmes SE, Hinz R, Drake RJ, et al. In vivo imaging of brain microglial activity in antipsychotic-free and medicated schizophrenia: a [11C](R)-PK11195 positron emission tomography study. Mol Psychiatry. 2016;21(12):1672-1679. doi:10.1038/mp.2016.180
26. Corsi-Zuelli F, Deakin B, de Lima MHF, et al. T regulatory cells as a potential therapeutic target in psychosis? Current challenges and future perspectives. Brain Behav Immun Health. 2021;17:100330. doi:10.1016/j.bbih.2021.100330
27. Bannai D, Lutz O, Lizano P. Neuroimaging considerations when investigating choroid plexus morphology in idiopathic psychosis. Schizophr Res. 2020;224:19-21. doi:10.1016/j.schres.2020.07.013
28. Hladky SB, Barrand MA. Fluid and ion transfer across the blood-brain and blood-cerebrospinal fluid barriers; a comparative account of mechanisms and roles. Fluids Barriers CNS. 2016;13(1):19. doi:10.1186/s12987-016-0040-3
29. Lizano P, Lutz O, Ling G, et al. Association of choroid plexus enlargement with cognitive, inflammatory, and structural phenotypes across the psychosis spectrum. Am J Psychiatry. 2019;176(7):564-572. doi:10.1176/appi.ajp.2019.18070825
30. Castellani G, Contarini G, Mereu M, et al. Dopamine-mediated immunomodulation affects choroid plexus function. Brain Behav Immun. 2019;81:138-150. doi:10.1016/j.bbi.2019.06.006
31. Bitanihirwe BKY, Lizano P, Woo TW. Deconstructing the functional neuroanatomy of the choroid plexus: an ontogenetic perspective for studying neurodevelopmental and neuropsychiatric disorders. Mol Psychiatry. 2022;1-10. doi:10.1038/s41380-022-01623-6
1. Li Y, Xia Y, Zhu H, et al. Investigation of neurodevelopmental deficits of 22 q11.2 deletion syndrome with a patient-iPSC-derived blood-brain barrier model. Cells. 2021;10(10):2576. doi:10.3390/cells10102576
2. Kealy J, Greene C, Campbell M. Blood-brain barrier regulation in psychiatric disorders. Neurosci Lett. 2020;726:133664. doi:10.1016/j.neulet.2018.06.033
3. Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16(1):1-13. doi:10.1016/j.nbd.2003.12.016
4. Futtrup J, Margolinsky R, Benros ME, et al. Blood-brain barrier pathology in patients with severe mental disorders: a systematic review and meta-analysis of biomarkers in case-control studies. Brain Behav Immun Health. 2020;6:100102. doi:10.1016/j.bbih.2020.100102
5. Chen S, Tian L, Chen N, et al. Cognitive dysfunction correlates with elevated serum S100B concentration in drug-free acutely relapsed patients with schizophrenia. Psychiatry Res. 2017;247:6-11. doi:10.1016/j.psychres.2016.09.029
6. Wu YF, Sytwu HK, Lung FW. Human aquaporin 4 gene polymorphisms and haplotypes are associated with serum S100B level and negative symptoms of schizophrenia in a southern Chinese Han population. Front Psychiatry. 2018;9:657. doi:10.3389/fpsyt.2018.00657
7. Greene C, Kealy J, Humphries MM, et al. Dose-dependent expression of claudin-5 is a modifying factor in schizophrenia. Mol Psychiatry. 2018;23(11):2156-2166. doi:10.1038/MP.2017.156
8. Greene C, Hanley N, Campbell M. Blood-brain barrier associated tight junction disruption is a hallmark feature of major psychiatric disorders. Transl Psychiatry. 2020;10(1):373. doi:10.1038/s41398-020-01054-3
9. Kakogiannos N, Ferrari L, Giampietro C, et al. JAM-A acts via C/EBP-α to promote claudin-5 expression and enhance endothelial barrier function. Circ Res. 2020:1056-1073. doi:10.1161/CIRCRESAHA.120.316742
10. Erickson MA, Dohi K, Banks WA. Neuroinflammation: a common pathway in CNS diseases as mediated at the blood-brain barrier. Neuroimmunomodulation. 2012;19(2):121-130. doi:10.1159/000330247
11. Ao LY, Yan YY, Zhou L, et al. Immune cells after ischemic stroke onset: roles, migration, and target intervention. J Mol Neurosci. 2018;66(3):342-355. doi:10.1007/s12031-018-1173-4
12. Meixensberger S, Kuzior H, Fiebich BL, et al. Upregulation of sICAM-1 and sVCAM-1 levels in the cerebrospinal fluid of patients with schizophrenia spectrum disorders. Diagnostics (Basel). 2021;11(7):1134. doi:10.3390diagnostics11071134
13. Cai HQ, Catts VS, Webster MJ, et al. Increased macrophages and changed brain endothelial cell gene expression in the frontal cortex of people with schizophrenia displaying inflammation. Mol Psychiatry. 2020;25(4):761-775. doi:10.1038/s41380-018-0235-x
14. Greene C, Hanley N, Reschke CR, et al. Microvascular stabilization via blood-brain barrier regulation prevents seizure activity. Nat Commun. 2022;13(1):2003. doi:10.1038/s41467-022-29657-y
15. García-Juárez M, Camacho-Morales A. Defining the role of anti- and pro-inflammatory outcomes of interleukin-6 in mental health. Neuroscience. 2022;492:32-46. doi:10.1016/j.neuroscience.2022.03.020
16. Pong S, Karmacharya R, Sofman M, et al. The role of brain microvascular endothelial cell and blood-brain barrier dysfunction in schizophrenia. Complex Psychiatry. 2020;6(1-2):30-46. doi:10.1159/000511552
17. Patel A, Zhu Y, Kuzhikandathil EV, et al. Soluble interleukin-6 receptor induces motor stereotypies and co-localizes with gp130 in regions linked to cortico-striato-thalamo-cortical circuits. PLoS One. 2012;7(7): e41623. doi:10.1371/journal.pone.0041623
18. Schlaaff K, Dobrowolny H, Frodl T, et al. Increased densities of T and B lymphocytes indicate neuroinflammation in subgroups of schizophrenia and mood disorder patients. Brain Behav Immun. 2020;88:497-506. doi:10.1016/j.bbi.2020.04.021
19. Busse S, Busse M, Schiltz K, et al. Different distribution patterns of lymphocytes and microglia in the hippocampus of patients with residual versus paranoid schizophrenia: further evidence for disease course-related immune alterations? Brain Behav Immun. 2012;26(8):1273-1279. doi:10.1016/j.bbi.2012.08.005
20. Na KS, Jung HY, Kim YK. The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:277-286. doi:10.1016/j.pnpbp.2012.10.022
21. Conen S, Gregory CJ, Hinz R, et al. Neuroinflammation as measured by positron emission tomography in patients with recent onset and established schizophrenia: implications for immune pathogenesis. Mol Psychiatry. 2021;26(9):5398-5406. doi:10.1038/S41380-020-0829-Y
22. Najjar S, Pahlajani S, De Sanctis V, et al. Neurovascular unit dysfunction and blood-brain barrier hyperpermeability contribute to schizophrenia neurobiology: a theoretical integration of clinical and experimental evidence. Front Psychiatry. 2017;8:83. doi:10.3389/fpsyt.2017.00083
23. Pinjari OF, Dasgupta SK, Okusaga OO. Plasma soluble P-selectin, interleukin-6 and S100B protein in patients with schizophrenia: a pilot study. Psychiatr Q. 2022;93(1):335-345. doi:10.1007/s11126-021-09954-3
24. Di Biase MA, Zalesky A, O’keefe G, et al. PET imaging of putative microglial activation in individuals at ultra-high risk for psychosis, recently diagnosed and chronically ill with schizophrenia. Transl Psychiatry. 2017;7(8):e1225. doi:10.1038/tp.2017.193
25. Holmes SE, Hinz R, Drake RJ, et al. In vivo imaging of brain microglial activity in antipsychotic-free and medicated schizophrenia: a [11C](R)-PK11195 positron emission tomography study. Mol Psychiatry. 2016;21(12):1672-1679. doi:10.1038/mp.2016.180
26. Corsi-Zuelli F, Deakin B, de Lima MHF, et al. T regulatory cells as a potential therapeutic target in psychosis? Current challenges and future perspectives. Brain Behav Immun Health. 2021;17:100330. doi:10.1016/j.bbih.2021.100330
27. Bannai D, Lutz O, Lizano P. Neuroimaging considerations when investigating choroid plexus morphology in idiopathic psychosis. Schizophr Res. 2020;224:19-21. doi:10.1016/j.schres.2020.07.013
28. Hladky SB, Barrand MA. Fluid and ion transfer across the blood-brain and blood-cerebrospinal fluid barriers; a comparative account of mechanisms and roles. Fluids Barriers CNS. 2016;13(1):19. doi:10.1186/s12987-016-0040-3
29. Lizano P, Lutz O, Ling G, et al. Association of choroid plexus enlargement with cognitive, inflammatory, and structural phenotypes across the psychosis spectrum. Am J Psychiatry. 2019;176(7):564-572. doi:10.1176/appi.ajp.2019.18070825
30. Castellani G, Contarini G, Mereu M, et al. Dopamine-mediated immunomodulation affects choroid plexus function. Brain Behav Immun. 2019;81:138-150. doi:10.1016/j.bbi.2019.06.006
31. Bitanihirwe BKY, Lizano P, Woo TW. Deconstructing the functional neuroanatomy of the choroid plexus: an ontogenetic perspective for studying neurodevelopmental and neuropsychiatric disorders. Mol Psychiatry. 2022;1-10. doi:10.1038/s41380-022-01623-6
Toward a new open-door model for psychiatric wards
If isolated, patients with mental disorders may end up having higher levels of social impairment. This has led several hospitals in Spain to set up open-door departments that are more accessible.
The purpose of the open-door model is to help remove the stigma from individuals who need to be admitted to a psychiatric ward because they have a mental disorder.
Traditional locked wards
According to the World Health Organization (WHO), in 2019, one in every eight people were living with a mental disorder. Having the least restrictive type of mental health care is one of the 10 basic principles listed in a 1996 reference document from the WHO.
Among people suffering from severe psychiatric disorders, there is a high probability of being involuntarily admitted to a psychiatry ward with locked doors (PWLD). Admission to a PWLD involves the application of a set of measures that restrict the individual’s freedom.
The main argument for keeping the doors locked is that it prevents suicides and self-harm behavior, as well as abscondment. But in recent years, efforts have been made to apply a model called open-door policy psychiatry wards (ODPWs).
Open wards model
Experiments were undertaken in various countries, including the United Kingdom, Australia, Switzerland, and Germany. Investigators found that the new forms of hospitalization led to a reduction in conflictive events; self-harm behavior; restrictive measures, such as seclusion, mechanical restraints, and chemical restraints; as well as forced medication. On the basis of these findings, ODPWs were launched.
According to Ignacio García Cabeza, MD, psychiatrist and coordinator of the department of psychiatry at Gregorio Marañón General University Hospital in Madrid, “The open wards model is founded on the idea of respecting the patient and their autonomy. In addition, it advocates a reduction in coercive measures.
“We wanted our department to be the same as the other departments in the hospital, with patients going in and out, receiving treatment, and being able to have family visits,” he explained. “A patient’s diagnosis should not factor into these things. People with schizophrenia, people with any type of mental disorder, should be able to enjoy this minimally restrictive environment.”
This model also implies fundamental changes in the interaction between health care professionals and patients. The implementation of new nursing care models, among which the Safewards model stands out, is a key element for the success of the project.
Based on a set of tools for preventing and managing conflict, the Safewards model seeks to modify the factors that regulate the relationship between staff and patients. Use of this model brought about a 15% reduction in the rate of conflictive events and a 23% reduction in the rate of coercive interventions, in comparison with a control group.
One of the major debates is about whether every patient should be able to choose this open-door system. For Dr. García Cabeza, the answer is yes, but with one caveat. “There’s a certain group of patients who perhaps need to be in locked wards, who perhaps require greater means of control – patients whose conditions put them at a high risk of suicide or of self-harm behavior or of absconding.”
He had no hesitation in saying that an open-door ward increases the patient’s self-esteem. It helps promote autonomy and a sense of control and of normalcy with respect to a community. “The idea is to get to the point where we’ve got an atmosphere, a climate, that serves to benefit the therapeutic actions that are going to continue to influence the patient’s future progress and their treatment.”
That’s why it’s important to bring about the kind of health care activities that can prevent the patient from experiencing some of the negative psychological effects, such as distrust and feeling removed from normalcy. “In traditional locked wards, the patient feels incapable of making decisions. They feel that they have very little to do with [and have] no say in the decisions made, and a lot of times, this leads to a situation where, after discharge, the patient ends up giving up on the treatments. If we can manage to break this perception held by the patient,” Dr. García Cabeza suggested, “it’s quite likely that we’ll manage to improve the course of their disorder in general.”
What the literature says
The effect of ODPW has been investigated through comparative studies with PWLD and research of the transition from PWLD to ODPW, both from a therapeutic and safety a point of view.
A 15-year observational study published in The Lancet Psychiatry found that, with respect to abscondment, suicide attempts, and suicide, there were no significant differences between hospitals with open-door policies and those without.
A subsequent study that was published in 2017 found that on open wards, any aggressive behavior and restraint or seclusion were less likely than on closed wards.
The Spanish situation
This system is already at work in some Spanish hospitals, among them Inca Comarcal Hospital (Palma de Mallorca), Elda General University Hospital (Alicante), Germans Trias i Pujol Hospital in Badalona, and Gregorio Marañón General University Hospital in Madrid.
“At Gregorio Marañón, we started the experiment just before the pandemic hit. We’re up and running now, but still with some limitations; the patient can go in and out, but not with the flexibility we’d like,” explained Dr. García Cabeza. “An open ward plays a clinical, patient-care role and a symbolic one as well. Locking the doors has a lot to do with the fear felt toward these patients. It’s a stigma that they’ve had to deal with and that they continue to have to deal with. In terms of the symbolic role, there’s also the fear that comes with giving these patients some rights.”
While the experiment at Gregorio Marañón’s psychiatric ward “is still very much in the early stages,” there have been no recorded incidents related to its open-door policy. Dr. García Cabeza is aware of the challenges of such a policy, “starting with assistance when conflictive events arise. Challenges faced by the staff – especially the nursing staff, as they’re the ones who are with the patients 24 hours day – and challenges faced by those in charge of providing care. In all of this, there are new things to learn and be aware of, new ways of understanding and looking at the patient-physician relationship. The fears are still there – they haven’t been done away with. But the way we conduct ourselves should be adjusted, matching how we act toward other patients. Although the differences have to be taken into account, we have to try to normalize, as much as possible, the environment where patients with mental disorders receive treatment.”
Dr. García Cabeza has no doubts. “The most sensible and reasonable decisions need to be made at these sites so as to allow the broadest applicability to cases. Anyone who needs psychiatric hospitalization and who is competent to consent to admission and who voluntarily agrees to be admitted – they can and must be placed in an open ward.”
The hope is that in the future, the number of open wards will increase and the number of locked wards – which have more stigma attached to them – will go down. The involvement of the staff and appropriate institutional support are essential to making this a reality.
This article was translated from Univadis Spain.
If isolated, patients with mental disorders may end up having higher levels of social impairment. This has led several hospitals in Spain to set up open-door departments that are more accessible.
The purpose of the open-door model is to help remove the stigma from individuals who need to be admitted to a psychiatric ward because they have a mental disorder.
Traditional locked wards
According to the World Health Organization (WHO), in 2019, one in every eight people were living with a mental disorder. Having the least restrictive type of mental health care is one of the 10 basic principles listed in a 1996 reference document from the WHO.
Among people suffering from severe psychiatric disorders, there is a high probability of being involuntarily admitted to a psychiatry ward with locked doors (PWLD). Admission to a PWLD involves the application of a set of measures that restrict the individual’s freedom.
The main argument for keeping the doors locked is that it prevents suicides and self-harm behavior, as well as abscondment. But in recent years, efforts have been made to apply a model called open-door policy psychiatry wards (ODPWs).
Open wards model
Experiments were undertaken in various countries, including the United Kingdom, Australia, Switzerland, and Germany. Investigators found that the new forms of hospitalization led to a reduction in conflictive events; self-harm behavior; restrictive measures, such as seclusion, mechanical restraints, and chemical restraints; as well as forced medication. On the basis of these findings, ODPWs were launched.
According to Ignacio García Cabeza, MD, psychiatrist and coordinator of the department of psychiatry at Gregorio Marañón General University Hospital in Madrid, “The open wards model is founded on the idea of respecting the patient and their autonomy. In addition, it advocates a reduction in coercive measures.
“We wanted our department to be the same as the other departments in the hospital, with patients going in and out, receiving treatment, and being able to have family visits,” he explained. “A patient’s diagnosis should not factor into these things. People with schizophrenia, people with any type of mental disorder, should be able to enjoy this minimally restrictive environment.”
This model also implies fundamental changes in the interaction between health care professionals and patients. The implementation of new nursing care models, among which the Safewards model stands out, is a key element for the success of the project.
Based on a set of tools for preventing and managing conflict, the Safewards model seeks to modify the factors that regulate the relationship between staff and patients. Use of this model brought about a 15% reduction in the rate of conflictive events and a 23% reduction in the rate of coercive interventions, in comparison with a control group.
One of the major debates is about whether every patient should be able to choose this open-door system. For Dr. García Cabeza, the answer is yes, but with one caveat. “There’s a certain group of patients who perhaps need to be in locked wards, who perhaps require greater means of control – patients whose conditions put them at a high risk of suicide or of self-harm behavior or of absconding.”
He had no hesitation in saying that an open-door ward increases the patient’s self-esteem. It helps promote autonomy and a sense of control and of normalcy with respect to a community. “The idea is to get to the point where we’ve got an atmosphere, a climate, that serves to benefit the therapeutic actions that are going to continue to influence the patient’s future progress and their treatment.”
That’s why it’s important to bring about the kind of health care activities that can prevent the patient from experiencing some of the negative psychological effects, such as distrust and feeling removed from normalcy. “In traditional locked wards, the patient feels incapable of making decisions. They feel that they have very little to do with [and have] no say in the decisions made, and a lot of times, this leads to a situation where, after discharge, the patient ends up giving up on the treatments. If we can manage to break this perception held by the patient,” Dr. García Cabeza suggested, “it’s quite likely that we’ll manage to improve the course of their disorder in general.”
What the literature says
The effect of ODPW has been investigated through comparative studies with PWLD and research of the transition from PWLD to ODPW, both from a therapeutic and safety a point of view.
A 15-year observational study published in The Lancet Psychiatry found that, with respect to abscondment, suicide attempts, and suicide, there were no significant differences between hospitals with open-door policies and those without.
A subsequent study that was published in 2017 found that on open wards, any aggressive behavior and restraint or seclusion were less likely than on closed wards.
The Spanish situation
This system is already at work in some Spanish hospitals, among them Inca Comarcal Hospital (Palma de Mallorca), Elda General University Hospital (Alicante), Germans Trias i Pujol Hospital in Badalona, and Gregorio Marañón General University Hospital in Madrid.
“At Gregorio Marañón, we started the experiment just before the pandemic hit. We’re up and running now, but still with some limitations; the patient can go in and out, but not with the flexibility we’d like,” explained Dr. García Cabeza. “An open ward plays a clinical, patient-care role and a symbolic one as well. Locking the doors has a lot to do with the fear felt toward these patients. It’s a stigma that they’ve had to deal with and that they continue to have to deal with. In terms of the symbolic role, there’s also the fear that comes with giving these patients some rights.”
While the experiment at Gregorio Marañón’s psychiatric ward “is still very much in the early stages,” there have been no recorded incidents related to its open-door policy. Dr. García Cabeza is aware of the challenges of such a policy, “starting with assistance when conflictive events arise. Challenges faced by the staff – especially the nursing staff, as they’re the ones who are with the patients 24 hours day – and challenges faced by those in charge of providing care. In all of this, there are new things to learn and be aware of, new ways of understanding and looking at the patient-physician relationship. The fears are still there – they haven’t been done away with. But the way we conduct ourselves should be adjusted, matching how we act toward other patients. Although the differences have to be taken into account, we have to try to normalize, as much as possible, the environment where patients with mental disorders receive treatment.”
Dr. García Cabeza has no doubts. “The most sensible and reasonable decisions need to be made at these sites so as to allow the broadest applicability to cases. Anyone who needs psychiatric hospitalization and who is competent to consent to admission and who voluntarily agrees to be admitted – they can and must be placed in an open ward.”
The hope is that in the future, the number of open wards will increase and the number of locked wards – which have more stigma attached to them – will go down. The involvement of the staff and appropriate institutional support are essential to making this a reality.
This article was translated from Univadis Spain.
If isolated, patients with mental disorders may end up having higher levels of social impairment. This has led several hospitals in Spain to set up open-door departments that are more accessible.
The purpose of the open-door model is to help remove the stigma from individuals who need to be admitted to a psychiatric ward because they have a mental disorder.
Traditional locked wards
According to the World Health Organization (WHO), in 2019, one in every eight people were living with a mental disorder. Having the least restrictive type of mental health care is one of the 10 basic principles listed in a 1996 reference document from the WHO.
Among people suffering from severe psychiatric disorders, there is a high probability of being involuntarily admitted to a psychiatry ward with locked doors (PWLD). Admission to a PWLD involves the application of a set of measures that restrict the individual’s freedom.
The main argument for keeping the doors locked is that it prevents suicides and self-harm behavior, as well as abscondment. But in recent years, efforts have been made to apply a model called open-door policy psychiatry wards (ODPWs).
Open wards model
Experiments were undertaken in various countries, including the United Kingdom, Australia, Switzerland, and Germany. Investigators found that the new forms of hospitalization led to a reduction in conflictive events; self-harm behavior; restrictive measures, such as seclusion, mechanical restraints, and chemical restraints; as well as forced medication. On the basis of these findings, ODPWs were launched.
According to Ignacio García Cabeza, MD, psychiatrist and coordinator of the department of psychiatry at Gregorio Marañón General University Hospital in Madrid, “The open wards model is founded on the idea of respecting the patient and their autonomy. In addition, it advocates a reduction in coercive measures.
“We wanted our department to be the same as the other departments in the hospital, with patients going in and out, receiving treatment, and being able to have family visits,” he explained. “A patient’s diagnosis should not factor into these things. People with schizophrenia, people with any type of mental disorder, should be able to enjoy this minimally restrictive environment.”
This model also implies fundamental changes in the interaction between health care professionals and patients. The implementation of new nursing care models, among which the Safewards model stands out, is a key element for the success of the project.
Based on a set of tools for preventing and managing conflict, the Safewards model seeks to modify the factors that regulate the relationship between staff and patients. Use of this model brought about a 15% reduction in the rate of conflictive events and a 23% reduction in the rate of coercive interventions, in comparison with a control group.
One of the major debates is about whether every patient should be able to choose this open-door system. For Dr. García Cabeza, the answer is yes, but with one caveat. “There’s a certain group of patients who perhaps need to be in locked wards, who perhaps require greater means of control – patients whose conditions put them at a high risk of suicide or of self-harm behavior or of absconding.”
He had no hesitation in saying that an open-door ward increases the patient’s self-esteem. It helps promote autonomy and a sense of control and of normalcy with respect to a community. “The idea is to get to the point where we’ve got an atmosphere, a climate, that serves to benefit the therapeutic actions that are going to continue to influence the patient’s future progress and their treatment.”
That’s why it’s important to bring about the kind of health care activities that can prevent the patient from experiencing some of the negative psychological effects, such as distrust and feeling removed from normalcy. “In traditional locked wards, the patient feels incapable of making decisions. They feel that they have very little to do with [and have] no say in the decisions made, and a lot of times, this leads to a situation where, after discharge, the patient ends up giving up on the treatments. If we can manage to break this perception held by the patient,” Dr. García Cabeza suggested, “it’s quite likely that we’ll manage to improve the course of their disorder in general.”
What the literature says
The effect of ODPW has been investigated through comparative studies with PWLD and research of the transition from PWLD to ODPW, both from a therapeutic and safety a point of view.
A 15-year observational study published in The Lancet Psychiatry found that, with respect to abscondment, suicide attempts, and suicide, there were no significant differences between hospitals with open-door policies and those without.
A subsequent study that was published in 2017 found that on open wards, any aggressive behavior and restraint or seclusion were less likely than on closed wards.
The Spanish situation
This system is already at work in some Spanish hospitals, among them Inca Comarcal Hospital (Palma de Mallorca), Elda General University Hospital (Alicante), Germans Trias i Pujol Hospital in Badalona, and Gregorio Marañón General University Hospital in Madrid.
“At Gregorio Marañón, we started the experiment just before the pandemic hit. We’re up and running now, but still with some limitations; the patient can go in and out, but not with the flexibility we’d like,” explained Dr. García Cabeza. “An open ward plays a clinical, patient-care role and a symbolic one as well. Locking the doors has a lot to do with the fear felt toward these patients. It’s a stigma that they’ve had to deal with and that they continue to have to deal with. In terms of the symbolic role, there’s also the fear that comes with giving these patients some rights.”
While the experiment at Gregorio Marañón’s psychiatric ward “is still very much in the early stages,” there have been no recorded incidents related to its open-door policy. Dr. García Cabeza is aware of the challenges of such a policy, “starting with assistance when conflictive events arise. Challenges faced by the staff – especially the nursing staff, as they’re the ones who are with the patients 24 hours day – and challenges faced by those in charge of providing care. In all of this, there are new things to learn and be aware of, new ways of understanding and looking at the patient-physician relationship. The fears are still there – they haven’t been done away with. But the way we conduct ourselves should be adjusted, matching how we act toward other patients. Although the differences have to be taken into account, we have to try to normalize, as much as possible, the environment where patients with mental disorders receive treatment.”
Dr. García Cabeza has no doubts. “The most sensible and reasonable decisions need to be made at these sites so as to allow the broadest applicability to cases. Anyone who needs psychiatric hospitalization and who is competent to consent to admission and who voluntarily agrees to be admitted – they can and must be placed in an open ward.”
The hope is that in the future, the number of open wards will increase and the number of locked wards – which have more stigma attached to them – will go down. The involvement of the staff and appropriate institutional support are essential to making this a reality.
This article was translated from Univadis Spain.
Schizophrenia and postmodernism: A philosophical exercise in treatment
Schizophrenia is defined as having episodes of psychosis: periods of time when one suffers from delusions, hallucinations, disorganized behaviors, disorganized speech, and negative symptoms. The concept of schizophrenia can be simplified as a detachment from reality. Patients who struggle with this illness frame their perceptions with a different set of rules and beliefs than the rest of society. These altered perceptions frequently become the basis of delusions, one of the most recognized symptoms of schizophrenia.
A patient with schizophrenia doesn’t have delusions, as much as having a belief system, which is not recognized by any other. It is not the mismatch between “objective reality” and the held belief, which qualifies the belief as delusional, so much as the mismatch with the beliefs of those around you. Heliocentrism denial, denying the knowledge that the earth rotates around the sun, is incorrect because it is not factual. However, heliocentrism denial is not a delusion because it is incorrect, but because society chooses it to be incorrect.
We’d like to invite the reader to a thought experiment. “Objective reality” can be referred to as “anything that exists as it is independent of any conscious awareness of it.”1 “Consciousness awareness” entails an observer. If we remove the concept of consciousness or observer from existence, how would we then define “objective reality,” as the very definition of “objective reality” points to the existence of an observer. One deduces that there is no way to define “objective reality” without invoking the notion of an observer or of consciousness.
It is our contention that the concept of an “objective reality” is tautological – it answers itself. This philosophical quandary helps explain why a person with schizophrenia may feel alienated by others who do not appreciate their perceived “objective reality.”
Schizophrenia and ‘objective reality’
A patient with schizophrenia enters a psychiatrist’s office and may realize that their belief is not shared by others and society. The schizophrenic patient may understand the concept of delusions as fixed and false beliefs. However, to them, it is everyone else who is delusional. They may attempt to convince you, as their provider, to switch to their side. They may provide you with evidence for their belief system. One could argue that believing them, in response, would be curative. If not only one’s psychiatrist, but society accepted the schizophrenic patient’s belief system, it would no longer be delusional, whether real or not. Objective reality requires the presence of an object, an observer, to grant its value of truth.
In a simplistic way, those were the arguments of postmodernist philosophers. Reality is tainted by its observer, in a similar way that the Heisenberg uncertainty principle teaches that there is a limit to our simultaneous understanding of position and momentum of particles. This perspective may explain why Michel Foucault, PhD, the famous French postmodernist philosopher, was so interested in psychiatry and in particular schizophrenia. Dr. Foucault was deeply concerned with society imposing its beliefs and value system on patients, and positioning itself as the ultimate arbiter of reality. He went on to postulate that the bigger difference between schizophrenic patients and psychiatrists was not who was in the correct plane of reality but who was granted by society to arbitrate the answer. If reality is a subjective construct enforced by a ruling class, who has the power to rule becomes of the utmost importance.
Intersubjectivity theory in psychoanalysis has many of its sensibilities rooted in such thought. It argues against the myth of the isolated mind. Truth, in the context of psychoanalysis, is seen as an emergent product of dialogue between the therapist/patient dyad. It is in line with the ontological shift from a logical-positivist model to the more modern, constructivist framework. In terms of its view of psychosis, “delusional ideas were understood as a form of absolution – a radical decontextualization serving vital and restorative defensive functions.”2
It is an interesting proposition to advance this theory further in contending that it is not the independent consciousness of two entities that create the intersubjective space; but rather that it is the intersubjective space that literally creates the conscious entities. Could it not be said that the subjective relationship is more fundamental than consciousness itself? As Chris Jaenicke, Dipl.-Psych., wrote, “infant research has opened our eyes to the fact that there is no unilateral action.”3
Postmodernism and psychiatry
Postmodernism and its precursor skepticism have significant histories within the field of philosophy. This article will not summarize centuries of philosophical thought. In brief, skepticism is a powerful philosophical tool that can powerfully point out the limitations of human knowledge and certainty.
As a pedagogic jest to trainees, we will often point out that none of us “really knows” our date of birth with absolute certainty. None of us were conscious enough to remember our birth, conscious enough to understand the concept of date or time, and conscious enough to know who participated in it. At a fundamental level, we chose to believe our date of birth. Similarly, while the world could be a fictionalized simulation,4 we chose to believe that it is real because it behaves in a consistent way that permits scientific study. Postmodernism and skepticism are philosophical tools that permit one to question everything but are themselves limited by the real and empiric lives we live.
Psychiatrists are empiricists. We treat real people, who suffer in a very perceptible way, and live in a very tangible world. We frown on the postmodernist perspective and do not spend much or any time studying it as trainees. However, postmodernism, despite its philosophical and practical flaws, and adjacency to antipsychiatry,5 is an essential tool for the psychiatrist. In addition to the standard treatments for schizophrenia, the psychiatrist should attempt to create a bond with someone who is disconnected from the world. Postmodernism provides us with a way of doing so.
A psychiatrist who understands and appreciates postmodernism can show a patient why at some level we cannot refute all delusions. This psychiatrist can subsequently have empathy that some of the core beliefs of a patient may always be left unanswered. The psychiatrist can appreciate that to some degree the reason why the patient’s beliefs are not true is because society has chosen for them not to be true. Additionally, the psychiatrist can acknowledge to the patient that in some ways the correctness of a delusion is less relevant than the power of society to enforce its reality on the patient. This connection in itself is partially curative as it restores the patient’s attachment to society; we now have some plane of reality, the relationship, which is the same.
Psychiatry and philosophy
However, tempting it may be to be satisfied with this approach as an end in itself; this would be dangerous. While gratifying to the patient to be seen and heard, they will over time only become further entrenched in that compromise formation of delusional beliefs. The role of the psychiatrist, once deep and meaningful rapport has been established and solidified, is to point out to the patient the limitations of the delusions’ belief system.
“I empathize that not all your delusions can be disproved. An extension of that thought is that many beliefs can’t be disproved. Society chooses to believe that aliens do not live on earth but at the same time we can’t disprove with absolute certainty that they don’t. We live in a world where attachment to others enriches our lives. If you continue to believe that aliens affect all existence around you, you will disconnect yourself from all of us. I hope that our therapy has shown you the importance of human connection and the sacrifice of your belief system.”
In the modern day, psychiatry has chosen to believe that schizophrenia is a biological disorder that requires treatment with antipsychotics. We choose to believe that this is likely true, and we think that our empirical experience has been consistent with this belief. However, we also think that patients with this illness are salient beings that deserve to have their thoughts examined and addressed in a therapeutic framework that seeks to understand and acknowledge them as worthy and intelligent individuals. Philosophy provides psychiatry with tools on how to do so.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Khalafian practices full time as a general outpatient psychiatrist. He trained at the University of California, San Diego, for his psychiatric residency and currently works as a telepsychiatrist, serving an outpatient clinic population in northern California. Dr. Badre and Dr. Khalafian have no conflicts of interest.
References
1. https://iep.utm.edu/objectiv/.
2. Stolorow, RD. The phenomenology of trauma and the absolutisms of everyday life: A personal journey. Psychoanal Psychol. 1999;16(3):464-8. doi: 10.1037/0736-9735.16.3.464.
3. Jaenicke C. “The Risk of Relatedness: Intersubjectivity Theory in Clinical Practice” Lanham, Md.: Jason Aronson, 2007.
4. Cuthbertson A. “Elon Musk cites Pong as evidence that we are already living in a simulation” The Independent. 2021 Dec 1. https://www.independent.co.uk/space/elon-musk-simulation-pong-video-game-b1972369.html.
5. Foucault M (Howard R, translator). “Madness and Civilization: A History of Insanity in the Age of Reason” New York: Vintage, 1965.
Schizophrenia is defined as having episodes of psychosis: periods of time when one suffers from delusions, hallucinations, disorganized behaviors, disorganized speech, and negative symptoms. The concept of schizophrenia can be simplified as a detachment from reality. Patients who struggle with this illness frame their perceptions with a different set of rules and beliefs than the rest of society. These altered perceptions frequently become the basis of delusions, one of the most recognized symptoms of schizophrenia.
A patient with schizophrenia doesn’t have delusions, as much as having a belief system, which is not recognized by any other. It is not the mismatch between “objective reality” and the held belief, which qualifies the belief as delusional, so much as the mismatch with the beliefs of those around you. Heliocentrism denial, denying the knowledge that the earth rotates around the sun, is incorrect because it is not factual. However, heliocentrism denial is not a delusion because it is incorrect, but because society chooses it to be incorrect.
We’d like to invite the reader to a thought experiment. “Objective reality” can be referred to as “anything that exists as it is independent of any conscious awareness of it.”1 “Consciousness awareness” entails an observer. If we remove the concept of consciousness or observer from existence, how would we then define “objective reality,” as the very definition of “objective reality” points to the existence of an observer. One deduces that there is no way to define “objective reality” without invoking the notion of an observer or of consciousness.
It is our contention that the concept of an “objective reality” is tautological – it answers itself. This philosophical quandary helps explain why a person with schizophrenia may feel alienated by others who do not appreciate their perceived “objective reality.”
Schizophrenia and ‘objective reality’
A patient with schizophrenia enters a psychiatrist’s office and may realize that their belief is not shared by others and society. The schizophrenic patient may understand the concept of delusions as fixed and false beliefs. However, to them, it is everyone else who is delusional. They may attempt to convince you, as their provider, to switch to their side. They may provide you with evidence for their belief system. One could argue that believing them, in response, would be curative. If not only one’s psychiatrist, but society accepted the schizophrenic patient’s belief system, it would no longer be delusional, whether real or not. Objective reality requires the presence of an object, an observer, to grant its value of truth.
In a simplistic way, those were the arguments of postmodernist philosophers. Reality is tainted by its observer, in a similar way that the Heisenberg uncertainty principle teaches that there is a limit to our simultaneous understanding of position and momentum of particles. This perspective may explain why Michel Foucault, PhD, the famous French postmodernist philosopher, was so interested in psychiatry and in particular schizophrenia. Dr. Foucault was deeply concerned with society imposing its beliefs and value system on patients, and positioning itself as the ultimate arbiter of reality. He went on to postulate that the bigger difference between schizophrenic patients and psychiatrists was not who was in the correct plane of reality but who was granted by society to arbitrate the answer. If reality is a subjective construct enforced by a ruling class, who has the power to rule becomes of the utmost importance.
Intersubjectivity theory in psychoanalysis has many of its sensibilities rooted in such thought. It argues against the myth of the isolated mind. Truth, in the context of psychoanalysis, is seen as an emergent product of dialogue between the therapist/patient dyad. It is in line with the ontological shift from a logical-positivist model to the more modern, constructivist framework. In terms of its view of psychosis, “delusional ideas were understood as a form of absolution – a radical decontextualization serving vital and restorative defensive functions.”2
It is an interesting proposition to advance this theory further in contending that it is not the independent consciousness of two entities that create the intersubjective space; but rather that it is the intersubjective space that literally creates the conscious entities. Could it not be said that the subjective relationship is more fundamental than consciousness itself? As Chris Jaenicke, Dipl.-Psych., wrote, “infant research has opened our eyes to the fact that there is no unilateral action.”3
Postmodernism and psychiatry
Postmodernism and its precursor skepticism have significant histories within the field of philosophy. This article will not summarize centuries of philosophical thought. In brief, skepticism is a powerful philosophical tool that can powerfully point out the limitations of human knowledge and certainty.
As a pedagogic jest to trainees, we will often point out that none of us “really knows” our date of birth with absolute certainty. None of us were conscious enough to remember our birth, conscious enough to understand the concept of date or time, and conscious enough to know who participated in it. At a fundamental level, we chose to believe our date of birth. Similarly, while the world could be a fictionalized simulation,4 we chose to believe that it is real because it behaves in a consistent way that permits scientific study. Postmodernism and skepticism are philosophical tools that permit one to question everything but are themselves limited by the real and empiric lives we live.
Psychiatrists are empiricists. We treat real people, who suffer in a very perceptible way, and live in a very tangible world. We frown on the postmodernist perspective and do not spend much or any time studying it as trainees. However, postmodernism, despite its philosophical and practical flaws, and adjacency to antipsychiatry,5 is an essential tool for the psychiatrist. In addition to the standard treatments for schizophrenia, the psychiatrist should attempt to create a bond with someone who is disconnected from the world. Postmodernism provides us with a way of doing so.
A psychiatrist who understands and appreciates postmodernism can show a patient why at some level we cannot refute all delusions. This psychiatrist can subsequently have empathy that some of the core beliefs of a patient may always be left unanswered. The psychiatrist can appreciate that to some degree the reason why the patient’s beliefs are not true is because society has chosen for them not to be true. Additionally, the psychiatrist can acknowledge to the patient that in some ways the correctness of a delusion is less relevant than the power of society to enforce its reality on the patient. This connection in itself is partially curative as it restores the patient’s attachment to society; we now have some plane of reality, the relationship, which is the same.
Psychiatry and philosophy
However, tempting it may be to be satisfied with this approach as an end in itself; this would be dangerous. While gratifying to the patient to be seen and heard, they will over time only become further entrenched in that compromise formation of delusional beliefs. The role of the psychiatrist, once deep and meaningful rapport has been established and solidified, is to point out to the patient the limitations of the delusions’ belief system.
“I empathize that not all your delusions can be disproved. An extension of that thought is that many beliefs can’t be disproved. Society chooses to believe that aliens do not live on earth but at the same time we can’t disprove with absolute certainty that they don’t. We live in a world where attachment to others enriches our lives. If you continue to believe that aliens affect all existence around you, you will disconnect yourself from all of us. I hope that our therapy has shown you the importance of human connection and the sacrifice of your belief system.”
In the modern day, psychiatry has chosen to believe that schizophrenia is a biological disorder that requires treatment with antipsychotics. We choose to believe that this is likely true, and we think that our empirical experience has been consistent with this belief. However, we also think that patients with this illness are salient beings that deserve to have their thoughts examined and addressed in a therapeutic framework that seeks to understand and acknowledge them as worthy and intelligent individuals. Philosophy provides psychiatry with tools on how to do so.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Khalafian practices full time as a general outpatient psychiatrist. He trained at the University of California, San Diego, for his psychiatric residency and currently works as a telepsychiatrist, serving an outpatient clinic population in northern California. Dr. Badre and Dr. Khalafian have no conflicts of interest.
References
1. https://iep.utm.edu/objectiv/.
2. Stolorow, RD. The phenomenology of trauma and the absolutisms of everyday life: A personal journey. Psychoanal Psychol. 1999;16(3):464-8. doi: 10.1037/0736-9735.16.3.464.
3. Jaenicke C. “The Risk of Relatedness: Intersubjectivity Theory in Clinical Practice” Lanham, Md.: Jason Aronson, 2007.
4. Cuthbertson A. “Elon Musk cites Pong as evidence that we are already living in a simulation” The Independent. 2021 Dec 1. https://www.independent.co.uk/space/elon-musk-simulation-pong-video-game-b1972369.html.
5. Foucault M (Howard R, translator). “Madness and Civilization: A History of Insanity in the Age of Reason” New York: Vintage, 1965.
Schizophrenia is defined as having episodes of psychosis: periods of time when one suffers from delusions, hallucinations, disorganized behaviors, disorganized speech, and negative symptoms. The concept of schizophrenia can be simplified as a detachment from reality. Patients who struggle with this illness frame their perceptions with a different set of rules and beliefs than the rest of society. These altered perceptions frequently become the basis of delusions, one of the most recognized symptoms of schizophrenia.
A patient with schizophrenia doesn’t have delusions, as much as having a belief system, which is not recognized by any other. It is not the mismatch between “objective reality” and the held belief, which qualifies the belief as delusional, so much as the mismatch with the beliefs of those around you. Heliocentrism denial, denying the knowledge that the earth rotates around the sun, is incorrect because it is not factual. However, heliocentrism denial is not a delusion because it is incorrect, but because society chooses it to be incorrect.
We’d like to invite the reader to a thought experiment. “Objective reality” can be referred to as “anything that exists as it is independent of any conscious awareness of it.”1 “Consciousness awareness” entails an observer. If we remove the concept of consciousness or observer from existence, how would we then define “objective reality,” as the very definition of “objective reality” points to the existence of an observer. One deduces that there is no way to define “objective reality” without invoking the notion of an observer or of consciousness.
It is our contention that the concept of an “objective reality” is tautological – it answers itself. This philosophical quandary helps explain why a person with schizophrenia may feel alienated by others who do not appreciate their perceived “objective reality.”
Schizophrenia and ‘objective reality’
A patient with schizophrenia enters a psychiatrist’s office and may realize that their belief is not shared by others and society. The schizophrenic patient may understand the concept of delusions as fixed and false beliefs. However, to them, it is everyone else who is delusional. They may attempt to convince you, as their provider, to switch to their side. They may provide you with evidence for their belief system. One could argue that believing them, in response, would be curative. If not only one’s psychiatrist, but society accepted the schizophrenic patient’s belief system, it would no longer be delusional, whether real or not. Objective reality requires the presence of an object, an observer, to grant its value of truth.
In a simplistic way, those were the arguments of postmodernist philosophers. Reality is tainted by its observer, in a similar way that the Heisenberg uncertainty principle teaches that there is a limit to our simultaneous understanding of position and momentum of particles. This perspective may explain why Michel Foucault, PhD, the famous French postmodernist philosopher, was so interested in psychiatry and in particular schizophrenia. Dr. Foucault was deeply concerned with society imposing its beliefs and value system on patients, and positioning itself as the ultimate arbiter of reality. He went on to postulate that the bigger difference between schizophrenic patients and psychiatrists was not who was in the correct plane of reality but who was granted by society to arbitrate the answer. If reality is a subjective construct enforced by a ruling class, who has the power to rule becomes of the utmost importance.
Intersubjectivity theory in psychoanalysis has many of its sensibilities rooted in such thought. It argues against the myth of the isolated mind. Truth, in the context of psychoanalysis, is seen as an emergent product of dialogue between the therapist/patient dyad. It is in line with the ontological shift from a logical-positivist model to the more modern, constructivist framework. In terms of its view of psychosis, “delusional ideas were understood as a form of absolution – a radical decontextualization serving vital and restorative defensive functions.”2
It is an interesting proposition to advance this theory further in contending that it is not the independent consciousness of two entities that create the intersubjective space; but rather that it is the intersubjective space that literally creates the conscious entities. Could it not be said that the subjective relationship is more fundamental than consciousness itself? As Chris Jaenicke, Dipl.-Psych., wrote, “infant research has opened our eyes to the fact that there is no unilateral action.”3
Postmodernism and psychiatry
Postmodernism and its precursor skepticism have significant histories within the field of philosophy. This article will not summarize centuries of philosophical thought. In brief, skepticism is a powerful philosophical tool that can powerfully point out the limitations of human knowledge and certainty.
As a pedagogic jest to trainees, we will often point out that none of us “really knows” our date of birth with absolute certainty. None of us were conscious enough to remember our birth, conscious enough to understand the concept of date or time, and conscious enough to know who participated in it. At a fundamental level, we chose to believe our date of birth. Similarly, while the world could be a fictionalized simulation,4 we chose to believe that it is real because it behaves in a consistent way that permits scientific study. Postmodernism and skepticism are philosophical tools that permit one to question everything but are themselves limited by the real and empiric lives we live.
Psychiatrists are empiricists. We treat real people, who suffer in a very perceptible way, and live in a very tangible world. We frown on the postmodernist perspective and do not spend much or any time studying it as trainees. However, postmodernism, despite its philosophical and practical flaws, and adjacency to antipsychiatry,5 is an essential tool for the psychiatrist. In addition to the standard treatments for schizophrenia, the psychiatrist should attempt to create a bond with someone who is disconnected from the world. Postmodernism provides us with a way of doing so.
A psychiatrist who understands and appreciates postmodernism can show a patient why at some level we cannot refute all delusions. This psychiatrist can subsequently have empathy that some of the core beliefs of a patient may always be left unanswered. The psychiatrist can appreciate that to some degree the reason why the patient’s beliefs are not true is because society has chosen for them not to be true. Additionally, the psychiatrist can acknowledge to the patient that in some ways the correctness of a delusion is less relevant than the power of society to enforce its reality on the patient. This connection in itself is partially curative as it restores the patient’s attachment to society; we now have some plane of reality, the relationship, which is the same.
Psychiatry and philosophy
However, tempting it may be to be satisfied with this approach as an end in itself; this would be dangerous. While gratifying to the patient to be seen and heard, they will over time only become further entrenched in that compromise formation of delusional beliefs. The role of the psychiatrist, once deep and meaningful rapport has been established and solidified, is to point out to the patient the limitations of the delusions’ belief system.
“I empathize that not all your delusions can be disproved. An extension of that thought is that many beliefs can’t be disproved. Society chooses to believe that aliens do not live on earth but at the same time we can’t disprove with absolute certainty that they don’t. We live in a world where attachment to others enriches our lives. If you continue to believe that aliens affect all existence around you, you will disconnect yourself from all of us. I hope that our therapy has shown you the importance of human connection and the sacrifice of your belief system.”
In the modern day, psychiatry has chosen to believe that schizophrenia is a biological disorder that requires treatment with antipsychotics. We choose to believe that this is likely true, and we think that our empirical experience has been consistent with this belief. However, we also think that patients with this illness are salient beings that deserve to have their thoughts examined and addressed in a therapeutic framework that seeks to understand and acknowledge them as worthy and intelligent individuals. Philosophy provides psychiatry with tools on how to do so.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Khalafian practices full time as a general outpatient psychiatrist. He trained at the University of California, San Diego, for his psychiatric residency and currently works as a telepsychiatrist, serving an outpatient clinic population in northern California. Dr. Badre and Dr. Khalafian have no conflicts of interest.
References
1. https://iep.utm.edu/objectiv/.
2. Stolorow, RD. The phenomenology of trauma and the absolutisms of everyday life: A personal journey. Psychoanal Psychol. 1999;16(3):464-8. doi: 10.1037/0736-9735.16.3.464.
3. Jaenicke C. “The Risk of Relatedness: Intersubjectivity Theory in Clinical Practice” Lanham, Md.: Jason Aronson, 2007.
4. Cuthbertson A. “Elon Musk cites Pong as evidence that we are already living in a simulation” The Independent. 2021 Dec 1. https://www.independent.co.uk/space/elon-musk-simulation-pong-video-game-b1972369.html.
5. Foucault M (Howard R, translator). “Madness and Civilization: A History of Insanity in the Age of Reason” New York: Vintage, 1965.









