User login
Staged hemispheric embolization: How to treat hemimegalencephaly within days of birth
BALTIMORE – About one in 4,000 children are born with hemimegalencephaly, meaning one brain hemisphere is abnormally formed and larger than the other.
The abnormal hemisphere causes seizures, and when they become intractable, the standard of care is to remove it as soon as possible; the longer the abnormal hemisphere is left in, the worse children do developmentally, and the less likely hemispherectomy will stop the seizures.
A problem comes up, however, when children become intractable before they’re 3 months old: “Neurosurgeons won’t touch them,” said Taeun Chang, MD, a neonatal neurointensivist at Children’s National Medical Center in Washington.
Newborns’ coagulation systems aren’t fully developed, and the risk of fatal hemorrhage is too high, she explained.
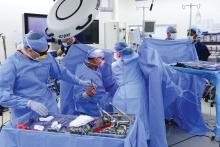
Out of what she said was a sense of “desperation” to address the situation, Dr. Chang has spearheaded a new approach for newborns at Children’s National, serial glue embolization to induce targeted strokes in the affected hemisphere. She reported on the first five cases at the American Epilepsy Society annual meeting.
At this point, “I feel like we’ve pretty much figured out the technique in terms of minimizing the complications. There’s no reason to wait anymore” for surgery as newborns get worse and worse, she said.
The technique
In two or three stages over several days, the major branches of the affected hemisphere’s anterior, middle, and posterior cerebral arteries are embolized. “You have to glue a long area and put in a lot of glue and glue up the secondary branches because [newborns] are so good at forming collaterals,” Dr. Chang said.
Fresh frozen plasma is given before and after each embolization session to boost coagulation proteins. Nicardipine is given during the procedure to prevent vasospasms. The one death in the series, case four, was in an 11-day old girl who vasospasmed, ruptured an artery over the tip of the guidewire, and hemorrhaged.
After the procedure, body temperature is kept at 36° C to prevent fever; sodium is kept high, and ins and outs are matched, to reduce brain edema; and blood pressure is tightly controlled. Children are kept on EEG during embolization and for days afterwards, and seizures, if any, are treated. The next embolization comes after peak swelling has passed in about 48-72 hours.
“The reason we can get away with this without herniation is that newborns’ skulls are soft, and their sutures are open,” so cerebral edema is manageable, Dr. Chang said.
Learning curve and outcomes
“What we learned in the first two cases” – a 23-day-old boy and 49-day-old girl – “was to create effective strokes. That’s not something any of us are taught to do,” she said.
“We were not trying to destroy the whole hemisphere, just the area that was seizing on EEG.” That was a mistake, she said: Adjacent areas began seizing and both children went on to anatomical hemispherectomies and needed shunts.
They are 5 years old now, and both on four seizure medications. The boy is in a wheelchair, fed by a G-tube, and has fewer than 20 words. The girl has a gait trainer, is fed mostly by G-tube, and has more than 50 words.
The third patient had her middle and posterior cerebral arteries embolized beginning when she was 43 days old. She was seizure free when she left the NICU, but eventually had a functional hemispherectomy. She’s 2 years old now, eating by mouth, in a gait trainer, and speaks in one- or two-word sentences. She’s on three seizure medications.
Outcomes have been best for patient five. Her posterior, middle, and anterior cerebral arteries were embolized starting at 14 days. She’s 1 year old now, seizure free on three medications, eating by G-tube and mouth, and has three-five words.
Dr. Chang said that newborns with hemimegalencephaly at Children’s National aren’t lingering as long on failing drug regimens these days. “We go to intervention now that we have this option” after they fail just two or three medications.
Given that the fifth patient, treated at 2 weeks old, is the only one who has been seizure free, she suspects it’s probably best to do embolization sooner rather than later, just as with anatomical hemispherectomy in older children. “We’ve got the sense that even a couple of weeks makes a difference. People need to come to us sooner,” Dr. Chang said.
It’s possible embolization could be a sound alternative to surgery even after 3 months of age. Focal embolization might also be a viable alternative to surgery to knock out epileptogenic lesions in children with tuberous sclerosis. Dr. Chang and her colleagues are interested in those and other possibilities, and plan to continue to develop the approach, she said.
There was no funding, and the investigators didn’t have any relevant disclosures.
SOURCE: Chang T et al. AES 2019, Abstract 1.225.
BALTIMORE – About one in 4,000 children are born with hemimegalencephaly, meaning one brain hemisphere is abnormally formed and larger than the other.
The abnormal hemisphere causes seizures, and when they become intractable, the standard of care is to remove it as soon as possible; the longer the abnormal hemisphere is left in, the worse children do developmentally, and the less likely hemispherectomy will stop the seizures.
A problem comes up, however, when children become intractable before they’re 3 months old: “Neurosurgeons won’t touch them,” said Taeun Chang, MD, a neonatal neurointensivist at Children’s National Medical Center in Washington.
Newborns’ coagulation systems aren’t fully developed, and the risk of fatal hemorrhage is too high, she explained.
Out of what she said was a sense of “desperation” to address the situation, Dr. Chang has spearheaded a new approach for newborns at Children’s National, serial glue embolization to induce targeted strokes in the affected hemisphere. She reported on the first five cases at the American Epilepsy Society annual meeting.
At this point, “I feel like we’ve pretty much figured out the technique in terms of minimizing the complications. There’s no reason to wait anymore” for surgery as newborns get worse and worse, she said.
The technique
In two or three stages over several days, the major branches of the affected hemisphere’s anterior, middle, and posterior cerebral arteries are embolized. “You have to glue a long area and put in a lot of glue and glue up the secondary branches because [newborns] are so good at forming collaterals,” Dr. Chang said.
Fresh frozen plasma is given before and after each embolization session to boost coagulation proteins. Nicardipine is given during the procedure to prevent vasospasms. The one death in the series, case four, was in an 11-day old girl who vasospasmed, ruptured an artery over the tip of the guidewire, and hemorrhaged.
After the procedure, body temperature is kept at 36° C to prevent fever; sodium is kept high, and ins and outs are matched, to reduce brain edema; and blood pressure is tightly controlled. Children are kept on EEG during embolization and for days afterwards, and seizures, if any, are treated. The next embolization comes after peak swelling has passed in about 48-72 hours.
“The reason we can get away with this without herniation is that newborns’ skulls are soft, and their sutures are open,” so cerebral edema is manageable, Dr. Chang said.
Learning curve and outcomes
“What we learned in the first two cases” – a 23-day-old boy and 49-day-old girl – “was to create effective strokes. That’s not something any of us are taught to do,” she said.
“We were not trying to destroy the whole hemisphere, just the area that was seizing on EEG.” That was a mistake, she said: Adjacent areas began seizing and both children went on to anatomical hemispherectomies and needed shunts.
They are 5 years old now, and both on four seizure medications. The boy is in a wheelchair, fed by a G-tube, and has fewer than 20 words. The girl has a gait trainer, is fed mostly by G-tube, and has more than 50 words.
The third patient had her middle and posterior cerebral arteries embolized beginning when she was 43 days old. She was seizure free when she left the NICU, but eventually had a functional hemispherectomy. She’s 2 years old now, eating by mouth, in a gait trainer, and speaks in one- or two-word sentences. She’s on three seizure medications.
Outcomes have been best for patient five. Her posterior, middle, and anterior cerebral arteries were embolized starting at 14 days. She’s 1 year old now, seizure free on three medications, eating by G-tube and mouth, and has three-five words.
Dr. Chang said that newborns with hemimegalencephaly at Children’s National aren’t lingering as long on failing drug regimens these days. “We go to intervention now that we have this option” after they fail just two or three medications.
Given that the fifth patient, treated at 2 weeks old, is the only one who has been seizure free, she suspects it’s probably best to do embolization sooner rather than later, just as with anatomical hemispherectomy in older children. “We’ve got the sense that even a couple of weeks makes a difference. People need to come to us sooner,” Dr. Chang said.
It’s possible embolization could be a sound alternative to surgery even after 3 months of age. Focal embolization might also be a viable alternative to surgery to knock out epileptogenic lesions in children with tuberous sclerosis. Dr. Chang and her colleagues are interested in those and other possibilities, and plan to continue to develop the approach, she said.
There was no funding, and the investigators didn’t have any relevant disclosures.
SOURCE: Chang T et al. AES 2019, Abstract 1.225.
BALTIMORE – About one in 4,000 children are born with hemimegalencephaly, meaning one brain hemisphere is abnormally formed and larger than the other.
The abnormal hemisphere causes seizures, and when they become intractable, the standard of care is to remove it as soon as possible; the longer the abnormal hemisphere is left in, the worse children do developmentally, and the less likely hemispherectomy will stop the seizures.
A problem comes up, however, when children become intractable before they’re 3 months old: “Neurosurgeons won’t touch them,” said Taeun Chang, MD, a neonatal neurointensivist at Children’s National Medical Center in Washington.
Newborns’ coagulation systems aren’t fully developed, and the risk of fatal hemorrhage is too high, she explained.
Out of what she said was a sense of “desperation” to address the situation, Dr. Chang has spearheaded a new approach for newborns at Children’s National, serial glue embolization to induce targeted strokes in the affected hemisphere. She reported on the first five cases at the American Epilepsy Society annual meeting.
At this point, “I feel like we’ve pretty much figured out the technique in terms of minimizing the complications. There’s no reason to wait anymore” for surgery as newborns get worse and worse, she said.
The technique
In two or three stages over several days, the major branches of the affected hemisphere’s anterior, middle, and posterior cerebral arteries are embolized. “You have to glue a long area and put in a lot of glue and glue up the secondary branches because [newborns] are so good at forming collaterals,” Dr. Chang said.
Fresh frozen plasma is given before and after each embolization session to boost coagulation proteins. Nicardipine is given during the procedure to prevent vasospasms. The one death in the series, case four, was in an 11-day old girl who vasospasmed, ruptured an artery over the tip of the guidewire, and hemorrhaged.
After the procedure, body temperature is kept at 36° C to prevent fever; sodium is kept high, and ins and outs are matched, to reduce brain edema; and blood pressure is tightly controlled. Children are kept on EEG during embolization and for days afterwards, and seizures, if any, are treated. The next embolization comes after peak swelling has passed in about 48-72 hours.
“The reason we can get away with this without herniation is that newborns’ skulls are soft, and their sutures are open,” so cerebral edema is manageable, Dr. Chang said.
Learning curve and outcomes
“What we learned in the first two cases” – a 23-day-old boy and 49-day-old girl – “was to create effective strokes. That’s not something any of us are taught to do,” she said.
“We were not trying to destroy the whole hemisphere, just the area that was seizing on EEG.” That was a mistake, she said: Adjacent areas began seizing and both children went on to anatomical hemispherectomies and needed shunts.
They are 5 years old now, and both on four seizure medications. The boy is in a wheelchair, fed by a G-tube, and has fewer than 20 words. The girl has a gait trainer, is fed mostly by G-tube, and has more than 50 words.
The third patient had her middle and posterior cerebral arteries embolized beginning when she was 43 days old. She was seizure free when she left the NICU, but eventually had a functional hemispherectomy. She’s 2 years old now, eating by mouth, in a gait trainer, and speaks in one- or two-word sentences. She’s on three seizure medications.
Outcomes have been best for patient five. Her posterior, middle, and anterior cerebral arteries were embolized starting at 14 days. She’s 1 year old now, seizure free on three medications, eating by G-tube and mouth, and has three-five words.
Dr. Chang said that newborns with hemimegalencephaly at Children’s National aren’t lingering as long on failing drug regimens these days. “We go to intervention now that we have this option” after they fail just two or three medications.
Given that the fifth patient, treated at 2 weeks old, is the only one who has been seizure free, she suspects it’s probably best to do embolization sooner rather than later, just as with anatomical hemispherectomy in older children. “We’ve got the sense that even a couple of weeks makes a difference. People need to come to us sooner,” Dr. Chang said.
It’s possible embolization could be a sound alternative to surgery even after 3 months of age. Focal embolization might also be a viable alternative to surgery to knock out epileptogenic lesions in children with tuberous sclerosis. Dr. Chang and her colleagues are interested in those and other possibilities, and plan to continue to develop the approach, she said.
There was no funding, and the investigators didn’t have any relevant disclosures.
SOURCE: Chang T et al. AES 2019, Abstract 1.225.
REPORTING FROM AES 2019
Performing gender-reaffirming surgery: Guidelines for the general ob.gyn.
According to the DSM-V, gender dysphoria in adolescents and adults “involves a difference between one’s experienced/expressed gender and assigned gender, and significant distress or problems functioning. It lasts at least 6 months,” and several other criteria must be met.1 Many patients with gender dysphoria also identify as transgender. A “transition” or “transitioning” is a process by which individuals come to inhabit their gender identity.2 A gender transition may take many forms, and only some people will choose to include medical assistance in their transition process. Although the scope of this article will not address these concerns, it should be noted that many people in the transgender and gender nonconforming community would object to the concepts of gender dysphoria and gender transition because they rely on a binary model of gender that may exclude individuals that see themselves as something other than “man or woman.”
There are both medical and surgical options for medical assistance in a gender transition. This article will focus on the surgical care of patients assigned female at birth who are seeking masculinizing surgical therapy. Many writers will discuss “gender-affirming” surgery, but we will use the term “gender-reaffirming” surgery because transgender patients have already affirmed their own genders and do not require surgery to inhabit this affirmation. Surgical options might include bilateral mastectomy, hysterectomy, bilateral salpingo-oophorectomy (BSO), metoidioplasty (surgical formation of a neophallus with existing genital tissue), or phalloplasty. There currently is no single surgical subspecialty that encompasses training in all forms of gender-reaffirming surgical therapies. In some areas of the country, centers of excellence have given rise to multidisciplinary teams that combine the skill sets of surgical subspecialists to provide a streamlined approach to gender-reaffirming surgery. Because of the scarcity of these integrated centers, most patients seeking gender-reaffirming surgeries will need to find individual subspecialists whose surgical training focuses on one area of the body. For example, patients seeking all possible surgical options may need a breast surgeon to perform their mastectomy, an ob.gyn. to perform their hysterectomy and BSO, a urologist to perform their metoidioplasty, and a plastic surgeon to perform their phalloplasty. In these scenarios,
There are many reasons why transgender men might desire hysterectomy/BSO as part of their transition. Removal of the uterus and cervix eliminates concerns surrounding menstruation, pregnancy, and cervical cancer screening, all of which may add to their experience of gender dysphoria. Furthermore, removal of the ovaries may simplify long-term hormonal therapy with testosterone by eliminating the need for estrogen suppression. Lastly, a hysterectomy/BSO is a lower-risk and more cost-effective masculinizing surgery, compared with metoidioplasty or phalloplasty.
While the technical aspect of performing a hysterectomy/BSO certainly is within the scope of training for a general ob.gyn., there are several nuances of which providers should be aware when planning gender-reaffirming surgery for a transgender man. During the preoperative planning phase, it is of utmost importance to provide an environment of safety so that the focus of the preop visit is not clouded by communication mishaps between office staff and the patient. These barriers can be avoided by implementing office intake forms that give patients the opportunity to inform the health care team of their chosen name and personal pronouns upon registration for the visit.
A pelvic exam is commonly performed by ob.gyns. to determine surgical approach for a hysterectomy/BSO. When approaching transgender male patients for preoperative pelvic exams, it is important to be mindful of the fact that this type of exam may trigger gender dysphoria. While pelvic exams should be handled in sensitive fashion regardless of a patient’s gender identity, a patient who is a transgender man may benefit from some added steps in discussing the pelvic exam. One approach is to acknowledge that these exams/discussions may be especially triggering of gender dysphoria, and ask if the patient would prefer certain words to be used or not used in reference to their anatomy. As with any patient, the provider should explain the purpose of the examination and offer opportunities for the patient to have some control in the exam such as by assisting with insertion of the speculum or designating a “safe word” that would signal the provider to stop or pause the exam. In some cases, patients may not be able to tolerate the pelvic exam while awake because of the degree of gender dysphoria that the exam would induce. Providers might consider noninvasive imaging studies to help with surgical planning if they find they need more information before scheduling the operation, or they may offer a staged procedure with exam under anesthesia prior to the definitive surgery.
In conclusion, performing a gender-reaffirming hysterectomy/BSO requires thoughtful preparation to ensure a safe surgical environment for this vulnerable population. Care should be taken to plan the operation with a culturally sensitive approach.
Dr. Joyner is an assistant professor at Emory University, and is the director of gynecologic services in the Gender Center at Grady Memorial Hospital, both in Atlanta. Dr. Joyner identifies as a cisgender female and uses she/hers/her as her personal pronouns. Dr. Joey Bahng is a PGY-1 resident physician in Emory University’s gynecology & obstetrics residency program. Dr. Bahng identifies as nonbinary and uses they/them/their as their personal pronouns. Dr. Joyner and Dr. Bahng reported no relevant financial disclosures.
References
1. American Psychiatric Association. What is Gender Dysphoria? https://www.psychiatry.org/patients-families/gender-dysphoria/what-is-gender-dysphoria
2. UCSF Transgender Care. Transition Roadmap. https://transcare.ucsf.edu/transition-roadmap
According to the DSM-V, gender dysphoria in adolescents and adults “involves a difference between one’s experienced/expressed gender and assigned gender, and significant distress or problems functioning. It lasts at least 6 months,” and several other criteria must be met.1 Many patients with gender dysphoria also identify as transgender. A “transition” or “transitioning” is a process by which individuals come to inhabit their gender identity.2 A gender transition may take many forms, and only some people will choose to include medical assistance in their transition process. Although the scope of this article will not address these concerns, it should be noted that many people in the transgender and gender nonconforming community would object to the concepts of gender dysphoria and gender transition because they rely on a binary model of gender that may exclude individuals that see themselves as something other than “man or woman.”
There are both medical and surgical options for medical assistance in a gender transition. This article will focus on the surgical care of patients assigned female at birth who are seeking masculinizing surgical therapy. Many writers will discuss “gender-affirming” surgery, but we will use the term “gender-reaffirming” surgery because transgender patients have already affirmed their own genders and do not require surgery to inhabit this affirmation. Surgical options might include bilateral mastectomy, hysterectomy, bilateral salpingo-oophorectomy (BSO), metoidioplasty (surgical formation of a neophallus with existing genital tissue), or phalloplasty. There currently is no single surgical subspecialty that encompasses training in all forms of gender-reaffirming surgical therapies. In some areas of the country, centers of excellence have given rise to multidisciplinary teams that combine the skill sets of surgical subspecialists to provide a streamlined approach to gender-reaffirming surgery. Because of the scarcity of these integrated centers, most patients seeking gender-reaffirming surgeries will need to find individual subspecialists whose surgical training focuses on one area of the body. For example, patients seeking all possible surgical options may need a breast surgeon to perform their mastectomy, an ob.gyn. to perform their hysterectomy and BSO, a urologist to perform their metoidioplasty, and a plastic surgeon to perform their phalloplasty. In these scenarios,
There are many reasons why transgender men might desire hysterectomy/BSO as part of their transition. Removal of the uterus and cervix eliminates concerns surrounding menstruation, pregnancy, and cervical cancer screening, all of which may add to their experience of gender dysphoria. Furthermore, removal of the ovaries may simplify long-term hormonal therapy with testosterone by eliminating the need for estrogen suppression. Lastly, a hysterectomy/BSO is a lower-risk and more cost-effective masculinizing surgery, compared with metoidioplasty or phalloplasty.
While the technical aspect of performing a hysterectomy/BSO certainly is within the scope of training for a general ob.gyn., there are several nuances of which providers should be aware when planning gender-reaffirming surgery for a transgender man. During the preoperative planning phase, it is of utmost importance to provide an environment of safety so that the focus of the preop visit is not clouded by communication mishaps between office staff and the patient. These barriers can be avoided by implementing office intake forms that give patients the opportunity to inform the health care team of their chosen name and personal pronouns upon registration for the visit.
A pelvic exam is commonly performed by ob.gyns. to determine surgical approach for a hysterectomy/BSO. When approaching transgender male patients for preoperative pelvic exams, it is important to be mindful of the fact that this type of exam may trigger gender dysphoria. While pelvic exams should be handled in sensitive fashion regardless of a patient’s gender identity, a patient who is a transgender man may benefit from some added steps in discussing the pelvic exam. One approach is to acknowledge that these exams/discussions may be especially triggering of gender dysphoria, and ask if the patient would prefer certain words to be used or not used in reference to their anatomy. As with any patient, the provider should explain the purpose of the examination and offer opportunities for the patient to have some control in the exam such as by assisting with insertion of the speculum or designating a “safe word” that would signal the provider to stop or pause the exam. In some cases, patients may not be able to tolerate the pelvic exam while awake because of the degree of gender dysphoria that the exam would induce. Providers might consider noninvasive imaging studies to help with surgical planning if they find they need more information before scheduling the operation, or they may offer a staged procedure with exam under anesthesia prior to the definitive surgery.
In conclusion, performing a gender-reaffirming hysterectomy/BSO requires thoughtful preparation to ensure a safe surgical environment for this vulnerable population. Care should be taken to plan the operation with a culturally sensitive approach.
Dr. Joyner is an assistant professor at Emory University, and is the director of gynecologic services in the Gender Center at Grady Memorial Hospital, both in Atlanta. Dr. Joyner identifies as a cisgender female and uses she/hers/her as her personal pronouns. Dr. Joey Bahng is a PGY-1 resident physician in Emory University’s gynecology & obstetrics residency program. Dr. Bahng identifies as nonbinary and uses they/them/their as their personal pronouns. Dr. Joyner and Dr. Bahng reported no relevant financial disclosures.
References
1. American Psychiatric Association. What is Gender Dysphoria? https://www.psychiatry.org/patients-families/gender-dysphoria/what-is-gender-dysphoria
2. UCSF Transgender Care. Transition Roadmap. https://transcare.ucsf.edu/transition-roadmap
According to the DSM-V, gender dysphoria in adolescents and adults “involves a difference between one’s experienced/expressed gender and assigned gender, and significant distress or problems functioning. It lasts at least 6 months,” and several other criteria must be met.1 Many patients with gender dysphoria also identify as transgender. A “transition” or “transitioning” is a process by which individuals come to inhabit their gender identity.2 A gender transition may take many forms, and only some people will choose to include medical assistance in their transition process. Although the scope of this article will not address these concerns, it should be noted that many people in the transgender and gender nonconforming community would object to the concepts of gender dysphoria and gender transition because they rely on a binary model of gender that may exclude individuals that see themselves as something other than “man or woman.”
There are both medical and surgical options for medical assistance in a gender transition. This article will focus on the surgical care of patients assigned female at birth who are seeking masculinizing surgical therapy. Many writers will discuss “gender-affirming” surgery, but we will use the term “gender-reaffirming” surgery because transgender patients have already affirmed their own genders and do not require surgery to inhabit this affirmation. Surgical options might include bilateral mastectomy, hysterectomy, bilateral salpingo-oophorectomy (BSO), metoidioplasty (surgical formation of a neophallus with existing genital tissue), or phalloplasty. There currently is no single surgical subspecialty that encompasses training in all forms of gender-reaffirming surgical therapies. In some areas of the country, centers of excellence have given rise to multidisciplinary teams that combine the skill sets of surgical subspecialists to provide a streamlined approach to gender-reaffirming surgery. Because of the scarcity of these integrated centers, most patients seeking gender-reaffirming surgeries will need to find individual subspecialists whose surgical training focuses on one area of the body. For example, patients seeking all possible surgical options may need a breast surgeon to perform their mastectomy, an ob.gyn. to perform their hysterectomy and BSO, a urologist to perform their metoidioplasty, and a plastic surgeon to perform their phalloplasty. In these scenarios,
There are many reasons why transgender men might desire hysterectomy/BSO as part of their transition. Removal of the uterus and cervix eliminates concerns surrounding menstruation, pregnancy, and cervical cancer screening, all of which may add to their experience of gender dysphoria. Furthermore, removal of the ovaries may simplify long-term hormonal therapy with testosterone by eliminating the need for estrogen suppression. Lastly, a hysterectomy/BSO is a lower-risk and more cost-effective masculinizing surgery, compared with metoidioplasty or phalloplasty.
While the technical aspect of performing a hysterectomy/BSO certainly is within the scope of training for a general ob.gyn., there are several nuances of which providers should be aware when planning gender-reaffirming surgery for a transgender man. During the preoperative planning phase, it is of utmost importance to provide an environment of safety so that the focus of the preop visit is not clouded by communication mishaps between office staff and the patient. These barriers can be avoided by implementing office intake forms that give patients the opportunity to inform the health care team of their chosen name and personal pronouns upon registration for the visit.
A pelvic exam is commonly performed by ob.gyns. to determine surgical approach for a hysterectomy/BSO. When approaching transgender male patients for preoperative pelvic exams, it is important to be mindful of the fact that this type of exam may trigger gender dysphoria. While pelvic exams should be handled in sensitive fashion regardless of a patient’s gender identity, a patient who is a transgender man may benefit from some added steps in discussing the pelvic exam. One approach is to acknowledge that these exams/discussions may be especially triggering of gender dysphoria, and ask if the patient would prefer certain words to be used or not used in reference to their anatomy. As with any patient, the provider should explain the purpose of the examination and offer opportunities for the patient to have some control in the exam such as by assisting with insertion of the speculum or designating a “safe word” that would signal the provider to stop or pause the exam. In some cases, patients may not be able to tolerate the pelvic exam while awake because of the degree of gender dysphoria that the exam would induce. Providers might consider noninvasive imaging studies to help with surgical planning if they find they need more information before scheduling the operation, or they may offer a staged procedure with exam under anesthesia prior to the definitive surgery.
In conclusion, performing a gender-reaffirming hysterectomy/BSO requires thoughtful preparation to ensure a safe surgical environment for this vulnerable population. Care should be taken to plan the operation with a culturally sensitive approach.
Dr. Joyner is an assistant professor at Emory University, and is the director of gynecologic services in the Gender Center at Grady Memorial Hospital, both in Atlanta. Dr. Joyner identifies as a cisgender female and uses she/hers/her as her personal pronouns. Dr. Joey Bahng is a PGY-1 resident physician in Emory University’s gynecology & obstetrics residency program. Dr. Bahng identifies as nonbinary and uses they/them/their as their personal pronouns. Dr. Joyner and Dr. Bahng reported no relevant financial disclosures.
References
1. American Psychiatric Association. What is Gender Dysphoria? https://www.psychiatry.org/patients-families/gender-dysphoria/what-is-gender-dysphoria
2. UCSF Transgender Care. Transition Roadmap. https://transcare.ucsf.edu/transition-roadmap
Molar pregnancy: The next steps after diagnosis
Molar pregnancy is an uncommon but serious condition that affects young women of reproductive age. The diagnosis and management of molar pregnancy is familiar to most gynecologists. However, in the days and weeks following evacuation of molar pregnancy, clinicians face a critical time period in which they must be vigilant for the development of postmolar gestational trophoblastic neoplasia (GTN). If recognized early and treated appropriately, it almost always can be cured; however, errors or delays in the management of this condition can have catastrophic consequences for patients, including decreasing the likelihood of cure. Here we will review some of the steps and actions that can be taken immediately following the diagnosis of a molar pregnancy to expeditiously identify postmolar GTN and ensure patients are appropriately prepared for further consultation and intervention.
Postmolar GTN includes the diagnoses of invasive mole and choriocarcinoma that contain highly atypical trophoblasts with the capacity for local invasion and metastasis. Typically, the diagnosis is made clinically and not distinguished with histology. While molar pregnancies are a benign condition, invasive moles and choriocarcinoma are malignant conditions in which the molar tissue infiltrates the uterine myometrium, vasculature, and frequently is associated with hematogenous spread with distant metastases. It is a highly chemosensitive disease, and cure with chemotherapy typically is achieved with the ability to preserve fertility if desired even in advanced stage disease.1
After evacuation of a molar pregnancy, gynecologists should be on alert for the development of postmolar GTN if the following known risk factors are present: a history of a prior GTN diagnosis, complete mole on pathology (as opposed to partial mole), serum human chorionic gonadotropin (hCG) levels greater than 100,000 mIU/mL, age greater than 40 years, an enlarged uterus or large ovarian theca lutein cysts, and slow to normalize (more than 2 months) hCG. Symptoms for the development of postmolar GTN include persistent vaginal bleeding after evacuation, a persistently enlarged or enlarging uterine size, and adnexal masses. Ultimately, the diagnosis is made through plateaued or rising serum hCG assessments.2 (See graphic.)
Following the evacuation of a molar pregnancy, hCG levels should be drawn at the same laboratory every 1-2 weeks until normalization and then three consecutive normal values. Once this has been achieved, hCG levels should be tested once at 3 months and again at 6 months. During this 6 month period, patients should use reliable contraception, ideally, and through oral contraceptive pills that suppress the secretion of pituitary hCG if not contraindicated. Should a woman become pregnant during this 6-month surveillance, it becomes impossible to rule out occult postmolar GTN.
Typically after evacuation of a molar pregnancy, there is rapid fall in hCG levels, but this does not occur when the molar pregnancy has become invasive or is associated with choriocarcinoma. In these cases, after an initial drop in hCG levels, there is an observed rise or plateau in levels (as defined in the accompanying table), and this establishes the diagnosis of postmolar GTN. It is common for hCG to fall in fits and starts, rather than have a smooth, consistent diminution, and this can be worrying for gynecologists; however, provided there is a consistent reduction in values in accordance with the stated definitions, observation can continue.
Another source of confusion and concern is an HCG level that fails to completely normalize during observation, yet reaches a very low level. If this is observed, clinicians should consider the diagnosis of quiescent hCG, pituitary hCG, or phantom hCG.3 These can be difficult to distinguish from postmolar GTN, and consultation with a gynecologic oncologist with experience in the diagnosis and management of these rare tumors is helpful to determine if the persistent low levels in hCG require intervention.
Once a clinician has observed a plateau or rise in hCG levels, a gynecologic examination should be performed because the lower genital tract is a common site for metastatic postmolar GTN. If during this evaluation, a suspicious lesion is identified (typically a blue-black, slightly raised, hemorrhagic-appearing lesion), it should not be biopsied, but rather assumed to be a metastatic site. The vasculature of metastatic sites is extremely fragile, and biopsy or disruption can result in catastrophic hemorrhage, even from very small lesions.
In addition to physical examination, several diagnostic studies should be performed which may expedite the triage and management of the case. A pelvic ultrasound should evaluate the endometrial cavity for a new viable pregnancy, and residual molar tissue; sometimes, myometrial invasion consistent with an invasive mole can be appreciated. Chest x-ray or CT scan should be ordered to evaluate for pulmonary metastatic lesions. Additionally, CT scans of the abdomen and pelvis should be ordered, and if lung metastases are present, brain imaging with either MRI or CT scan also should be obtained. These imaging studies will provide the necessary information to stage the GTN (as metastatic or not).
Treatment for postmolar GTN is determined based on further prognostic categorization (“high risk” or “low risk”) in accordance with the WHO classification, which is derived using several prognostic clinical variables including age, antecedent pregnancy, interval from index pregnancy, pretreatment hCG, largest tumor size, sites and number of metastases, and response to previous chemotherapy.4 These assignments are necessary to determine whether single-agent or multiagent chemotherapy should be prescribed.
Laboratory studies are helpful to obtain at this time and include metabolic panels (which can ensure that renal and hepatic function are within normal limits in anticipation of future chemotherapy), and complete blood count ,which can establish viable bone marrow function prior to chemotherapy.
Once postmolar GTN has been diagnosed, it is most appropriate to refer the patient to a gynecologic oncologist with experience in the treatment of these relatively rare malignancies. At that point, the patient will be formally staged, and offered treatment based on these staging results.
Among women with low-risk, nonmetastatic GTN who desire future fertility it is appropriate to offer a repeat dilation and curettage (D&C) procedure rather than immediately proceeding with chemotherapy. Approximately two-thirds of women with low risk disease can avoid chemotherapy with repeat curettage.5 Risk factors for needing chemotherapy after repeat D&C include the presence of trophoblastic disease in the pathology specimen and urinary hCG levels greater than 1,500 mIU/mL at the time of curettage. In my experience, many women appreciate this option to potentially avoid toxic chemotherapy.
For women with low-risk, nonmetastatic postmolar GTN who do not desire future fertility, and hope to avoid chemotherapy, hysterectomy also is a reasonable first option. This can be performed via either minimally invasive, laparotomy, or vaginal route. If performing a minimally invasive procedure in the setting of GTN, there should be caution or avoidance of use of a uterine manipulator because the uterine wall typically is soft and prone to perforation, and bleeding can be significant secondary to disruption of the tumor.
If repeat D&C or hysterectomy are adopted instead of chemotherapy, it is important that patients are very closely monitored post operatively to ensure normalization of their hCG levels (as described above). If it fails to normalize, restaging scans and examinations should be performed, and referral for the appropriate chemotherapy regimen should be initiated without delay.
Postmolar GTN is a serious condition that usually can be cured with chemotherapy or, if appropriate, surgery. and refer to a gynecologic oncologist when criteria are met to ensure that overtreatment is avoided and essential therapy is ensured.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. Lancet Oncol. 2007 Aug;8(8):715-24.
2. J Natl Compr Canc Netw. 2019 Nov 1;17(11):1374-91.
3. Gynecol Oncol. 2009 Mar;112(3):663-72.
4. World Health Organ Tech Rep Ser. 1983;692:7-81.
5. Obstet Gynecol. 2016;128(3):535-42.
Molar pregnancy is an uncommon but serious condition that affects young women of reproductive age. The diagnosis and management of molar pregnancy is familiar to most gynecologists. However, in the days and weeks following evacuation of molar pregnancy, clinicians face a critical time period in which they must be vigilant for the development of postmolar gestational trophoblastic neoplasia (GTN). If recognized early and treated appropriately, it almost always can be cured; however, errors or delays in the management of this condition can have catastrophic consequences for patients, including decreasing the likelihood of cure. Here we will review some of the steps and actions that can be taken immediately following the diagnosis of a molar pregnancy to expeditiously identify postmolar GTN and ensure patients are appropriately prepared for further consultation and intervention.
Postmolar GTN includes the diagnoses of invasive mole and choriocarcinoma that contain highly atypical trophoblasts with the capacity for local invasion and metastasis. Typically, the diagnosis is made clinically and not distinguished with histology. While molar pregnancies are a benign condition, invasive moles and choriocarcinoma are malignant conditions in which the molar tissue infiltrates the uterine myometrium, vasculature, and frequently is associated with hematogenous spread with distant metastases. It is a highly chemosensitive disease, and cure with chemotherapy typically is achieved with the ability to preserve fertility if desired even in advanced stage disease.1
After evacuation of a molar pregnancy, gynecologists should be on alert for the development of postmolar GTN if the following known risk factors are present: a history of a prior GTN diagnosis, complete mole on pathology (as opposed to partial mole), serum human chorionic gonadotropin (hCG) levels greater than 100,000 mIU/mL, age greater than 40 years, an enlarged uterus or large ovarian theca lutein cysts, and slow to normalize (more than 2 months) hCG. Symptoms for the development of postmolar GTN include persistent vaginal bleeding after evacuation, a persistently enlarged or enlarging uterine size, and adnexal masses. Ultimately, the diagnosis is made through plateaued or rising serum hCG assessments.2 (See graphic.)
Following the evacuation of a molar pregnancy, hCG levels should be drawn at the same laboratory every 1-2 weeks until normalization and then three consecutive normal values. Once this has been achieved, hCG levels should be tested once at 3 months and again at 6 months. During this 6 month period, patients should use reliable contraception, ideally, and through oral contraceptive pills that suppress the secretion of pituitary hCG if not contraindicated. Should a woman become pregnant during this 6-month surveillance, it becomes impossible to rule out occult postmolar GTN.
Typically after evacuation of a molar pregnancy, there is rapid fall in hCG levels, but this does not occur when the molar pregnancy has become invasive or is associated with choriocarcinoma. In these cases, after an initial drop in hCG levels, there is an observed rise or plateau in levels (as defined in the accompanying table), and this establishes the diagnosis of postmolar GTN. It is common for hCG to fall in fits and starts, rather than have a smooth, consistent diminution, and this can be worrying for gynecologists; however, provided there is a consistent reduction in values in accordance with the stated definitions, observation can continue.
Another source of confusion and concern is an HCG level that fails to completely normalize during observation, yet reaches a very low level. If this is observed, clinicians should consider the diagnosis of quiescent hCG, pituitary hCG, or phantom hCG.3 These can be difficult to distinguish from postmolar GTN, and consultation with a gynecologic oncologist with experience in the diagnosis and management of these rare tumors is helpful to determine if the persistent low levels in hCG require intervention.
Once a clinician has observed a plateau or rise in hCG levels, a gynecologic examination should be performed because the lower genital tract is a common site for metastatic postmolar GTN. If during this evaluation, a suspicious lesion is identified (typically a blue-black, slightly raised, hemorrhagic-appearing lesion), it should not be biopsied, but rather assumed to be a metastatic site. The vasculature of metastatic sites is extremely fragile, and biopsy or disruption can result in catastrophic hemorrhage, even from very small lesions.
In addition to physical examination, several diagnostic studies should be performed which may expedite the triage and management of the case. A pelvic ultrasound should evaluate the endometrial cavity for a new viable pregnancy, and residual molar tissue; sometimes, myometrial invasion consistent with an invasive mole can be appreciated. Chest x-ray or CT scan should be ordered to evaluate for pulmonary metastatic lesions. Additionally, CT scans of the abdomen and pelvis should be ordered, and if lung metastases are present, brain imaging with either MRI or CT scan also should be obtained. These imaging studies will provide the necessary information to stage the GTN (as metastatic or not).
Treatment for postmolar GTN is determined based on further prognostic categorization (“high risk” or “low risk”) in accordance with the WHO classification, which is derived using several prognostic clinical variables including age, antecedent pregnancy, interval from index pregnancy, pretreatment hCG, largest tumor size, sites and number of metastases, and response to previous chemotherapy.4 These assignments are necessary to determine whether single-agent or multiagent chemotherapy should be prescribed.
Laboratory studies are helpful to obtain at this time and include metabolic panels (which can ensure that renal and hepatic function are within normal limits in anticipation of future chemotherapy), and complete blood count ,which can establish viable bone marrow function prior to chemotherapy.
Once postmolar GTN has been diagnosed, it is most appropriate to refer the patient to a gynecologic oncologist with experience in the treatment of these relatively rare malignancies. At that point, the patient will be formally staged, and offered treatment based on these staging results.
Among women with low-risk, nonmetastatic GTN who desire future fertility it is appropriate to offer a repeat dilation and curettage (D&C) procedure rather than immediately proceeding with chemotherapy. Approximately two-thirds of women with low risk disease can avoid chemotherapy with repeat curettage.5 Risk factors for needing chemotherapy after repeat D&C include the presence of trophoblastic disease in the pathology specimen and urinary hCG levels greater than 1,500 mIU/mL at the time of curettage. In my experience, many women appreciate this option to potentially avoid toxic chemotherapy.
For women with low-risk, nonmetastatic postmolar GTN who do not desire future fertility, and hope to avoid chemotherapy, hysterectomy also is a reasonable first option. This can be performed via either minimally invasive, laparotomy, or vaginal route. If performing a minimally invasive procedure in the setting of GTN, there should be caution or avoidance of use of a uterine manipulator because the uterine wall typically is soft and prone to perforation, and bleeding can be significant secondary to disruption of the tumor.
If repeat D&C or hysterectomy are adopted instead of chemotherapy, it is important that patients are very closely monitored post operatively to ensure normalization of their hCG levels (as described above). If it fails to normalize, restaging scans and examinations should be performed, and referral for the appropriate chemotherapy regimen should be initiated without delay.
Postmolar GTN is a serious condition that usually can be cured with chemotherapy or, if appropriate, surgery. and refer to a gynecologic oncologist when criteria are met to ensure that overtreatment is avoided and essential therapy is ensured.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. Lancet Oncol. 2007 Aug;8(8):715-24.
2. J Natl Compr Canc Netw. 2019 Nov 1;17(11):1374-91.
3. Gynecol Oncol. 2009 Mar;112(3):663-72.
4. World Health Organ Tech Rep Ser. 1983;692:7-81.
5. Obstet Gynecol. 2016;128(3):535-42.
Molar pregnancy is an uncommon but serious condition that affects young women of reproductive age. The diagnosis and management of molar pregnancy is familiar to most gynecologists. However, in the days and weeks following evacuation of molar pregnancy, clinicians face a critical time period in which they must be vigilant for the development of postmolar gestational trophoblastic neoplasia (GTN). If recognized early and treated appropriately, it almost always can be cured; however, errors or delays in the management of this condition can have catastrophic consequences for patients, including decreasing the likelihood of cure. Here we will review some of the steps and actions that can be taken immediately following the diagnosis of a molar pregnancy to expeditiously identify postmolar GTN and ensure patients are appropriately prepared for further consultation and intervention.
Postmolar GTN includes the diagnoses of invasive mole and choriocarcinoma that contain highly atypical trophoblasts with the capacity for local invasion and metastasis. Typically, the diagnosis is made clinically and not distinguished with histology. While molar pregnancies are a benign condition, invasive moles and choriocarcinoma are malignant conditions in which the molar tissue infiltrates the uterine myometrium, vasculature, and frequently is associated with hematogenous spread with distant metastases. It is a highly chemosensitive disease, and cure with chemotherapy typically is achieved with the ability to preserve fertility if desired even in advanced stage disease.1
After evacuation of a molar pregnancy, gynecologists should be on alert for the development of postmolar GTN if the following known risk factors are present: a history of a prior GTN diagnosis, complete mole on pathology (as opposed to partial mole), serum human chorionic gonadotropin (hCG) levels greater than 100,000 mIU/mL, age greater than 40 years, an enlarged uterus or large ovarian theca lutein cysts, and slow to normalize (more than 2 months) hCG. Symptoms for the development of postmolar GTN include persistent vaginal bleeding after evacuation, a persistently enlarged or enlarging uterine size, and adnexal masses. Ultimately, the diagnosis is made through plateaued or rising serum hCG assessments.2 (See graphic.)
Following the evacuation of a molar pregnancy, hCG levels should be drawn at the same laboratory every 1-2 weeks until normalization and then three consecutive normal values. Once this has been achieved, hCG levels should be tested once at 3 months and again at 6 months. During this 6 month period, patients should use reliable contraception, ideally, and through oral contraceptive pills that suppress the secretion of pituitary hCG if not contraindicated. Should a woman become pregnant during this 6-month surveillance, it becomes impossible to rule out occult postmolar GTN.
Typically after evacuation of a molar pregnancy, there is rapid fall in hCG levels, but this does not occur when the molar pregnancy has become invasive or is associated with choriocarcinoma. In these cases, after an initial drop in hCG levels, there is an observed rise or plateau in levels (as defined in the accompanying table), and this establishes the diagnosis of postmolar GTN. It is common for hCG to fall in fits and starts, rather than have a smooth, consistent diminution, and this can be worrying for gynecologists; however, provided there is a consistent reduction in values in accordance with the stated definitions, observation can continue.
Another source of confusion and concern is an HCG level that fails to completely normalize during observation, yet reaches a very low level. If this is observed, clinicians should consider the diagnosis of quiescent hCG, pituitary hCG, or phantom hCG.3 These can be difficult to distinguish from postmolar GTN, and consultation with a gynecologic oncologist with experience in the diagnosis and management of these rare tumors is helpful to determine if the persistent low levels in hCG require intervention.
Once a clinician has observed a plateau or rise in hCG levels, a gynecologic examination should be performed because the lower genital tract is a common site for metastatic postmolar GTN. If during this evaluation, a suspicious lesion is identified (typically a blue-black, slightly raised, hemorrhagic-appearing lesion), it should not be biopsied, but rather assumed to be a metastatic site. The vasculature of metastatic sites is extremely fragile, and biopsy or disruption can result in catastrophic hemorrhage, even from very small lesions.
In addition to physical examination, several diagnostic studies should be performed which may expedite the triage and management of the case. A pelvic ultrasound should evaluate the endometrial cavity for a new viable pregnancy, and residual molar tissue; sometimes, myometrial invasion consistent with an invasive mole can be appreciated. Chest x-ray or CT scan should be ordered to evaluate for pulmonary metastatic lesions. Additionally, CT scans of the abdomen and pelvis should be ordered, and if lung metastases are present, brain imaging with either MRI or CT scan also should be obtained. These imaging studies will provide the necessary information to stage the GTN (as metastatic or not).
Treatment for postmolar GTN is determined based on further prognostic categorization (“high risk” or “low risk”) in accordance with the WHO classification, which is derived using several prognostic clinical variables including age, antecedent pregnancy, interval from index pregnancy, pretreatment hCG, largest tumor size, sites and number of metastases, and response to previous chemotherapy.4 These assignments are necessary to determine whether single-agent or multiagent chemotherapy should be prescribed.
Laboratory studies are helpful to obtain at this time and include metabolic panels (which can ensure that renal and hepatic function are within normal limits in anticipation of future chemotherapy), and complete blood count ,which can establish viable bone marrow function prior to chemotherapy.
Once postmolar GTN has been diagnosed, it is most appropriate to refer the patient to a gynecologic oncologist with experience in the treatment of these relatively rare malignancies. At that point, the patient will be formally staged, and offered treatment based on these staging results.
Among women with low-risk, nonmetastatic GTN who desire future fertility it is appropriate to offer a repeat dilation and curettage (D&C) procedure rather than immediately proceeding with chemotherapy. Approximately two-thirds of women with low risk disease can avoid chemotherapy with repeat curettage.5 Risk factors for needing chemotherapy after repeat D&C include the presence of trophoblastic disease in the pathology specimen and urinary hCG levels greater than 1,500 mIU/mL at the time of curettage. In my experience, many women appreciate this option to potentially avoid toxic chemotherapy.
For women with low-risk, nonmetastatic postmolar GTN who do not desire future fertility, and hope to avoid chemotherapy, hysterectomy also is a reasonable first option. This can be performed via either minimally invasive, laparotomy, or vaginal route. If performing a minimally invasive procedure in the setting of GTN, there should be caution or avoidance of use of a uterine manipulator because the uterine wall typically is soft and prone to perforation, and bleeding can be significant secondary to disruption of the tumor.
If repeat D&C or hysterectomy are adopted instead of chemotherapy, it is important that patients are very closely monitored post operatively to ensure normalization of their hCG levels (as described above). If it fails to normalize, restaging scans and examinations should be performed, and referral for the appropriate chemotherapy regimen should be initiated without delay.
Postmolar GTN is a serious condition that usually can be cured with chemotherapy or, if appropriate, surgery. and refer to a gynecologic oncologist when criteria are met to ensure that overtreatment is avoided and essential therapy is ensured.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. Lancet Oncol. 2007 Aug;8(8):715-24.
2. J Natl Compr Canc Netw. 2019 Nov 1;17(11):1374-91.
3. Gynecol Oncol. 2009 Mar;112(3):663-72.
4. World Health Organ Tech Rep Ser. 1983;692:7-81.
5. Obstet Gynecol. 2016;128(3):535-42.
Bariatric surgery lacks impact on teens’ long-term mental health
Young people treated with bariatric surgery for severe obesity did not experience better mental health in the 5 years following their procedures, Swedish researchers said, and indeed fared worse than their nontreated peers on certain measures.
The results of this study do not necessarily argue “that metabolic and bariatric surgery during adolescence causes mental health problems,” the investigators wrote in the Lancet Child and Adolescent Health, but “it is reasonable to conclude that metabolic and bariatric surgery does not result in a substantial alleviation of mental health problems in adolescents with severe obesity,” and that “long-term mental health support should be required in programs providing adolescent metabolic and bariatric surgery.”
Kajsa Järvholm, PhD, of Skåne University Hospital, in Malmö, Sweden, and colleagues reported results from a prospective nonrandomized study that recruited 81 adolescents in Sweden aged 13-18 years (mean age, 16.5) who had a body mass index of 40 or higher, or BMI of 35 with obesity-related comorbidities and who underwent Roux-en-Y gastric bypass for weight loss. Subjects were matched by age, sex, and BMI to 80 controls (mean age, 15.8 years) who were assigned to conventional nonsurgical treatment. All patients were assessed at 1, 2, and 5 years.
Although mental health treatment, including use of psychiatric drugs, did not differ between the groups at baseline, during the follow-up period the subjects who underwent surgery saw 15% more impatient and outpatient mental health treatment, compared with controls, a significant difference. About a quarter of patients in the surgically treated group required specialized mental health treatment for the first time after their surgeries.
Though the surgical group lost much more weight – mean BMI was 32.3 at 5 years, compared with 41.7 for controls – none of the mental health changes from baseline were significantly associated with percentage change of BMI at 5 years.
The findings from the study are consistent with results from studies in adults in which bariatric surgery improves many health outcomes but does not alter the need for mental health treatment. Although 5 years is a longer follow-up than in previous studies in young patients – a key strength of the study – Dr. Järvholm and colleagues acknowledged some weaknesses, including a nonrandomized design, lack of a comparison group of nonobese youths for mental health measures, a small sample size, and a surgical procedure that is now out of favor in adolescents.
The study was funded by Swedish government and health foundations. Dr. Järvholm disclosed pharmaceutical industry funding not related to the study, and three coauthors also disclosed industry relationships.
SOURCE: Järvholm K et al. Lancet Child Adolesc Health. 2020. doi: 10.1016/s2352-4642(20)30024-9.
Young people treated with bariatric surgery for severe obesity did not experience better mental health in the 5 years following their procedures, Swedish researchers said, and indeed fared worse than their nontreated peers on certain measures.
The results of this study do not necessarily argue “that metabolic and bariatric surgery during adolescence causes mental health problems,” the investigators wrote in the Lancet Child and Adolescent Health, but “it is reasonable to conclude that metabolic and bariatric surgery does not result in a substantial alleviation of mental health problems in adolescents with severe obesity,” and that “long-term mental health support should be required in programs providing adolescent metabolic and bariatric surgery.”
Kajsa Järvholm, PhD, of Skåne University Hospital, in Malmö, Sweden, and colleagues reported results from a prospective nonrandomized study that recruited 81 adolescents in Sweden aged 13-18 years (mean age, 16.5) who had a body mass index of 40 or higher, or BMI of 35 with obesity-related comorbidities and who underwent Roux-en-Y gastric bypass for weight loss. Subjects were matched by age, sex, and BMI to 80 controls (mean age, 15.8 years) who were assigned to conventional nonsurgical treatment. All patients were assessed at 1, 2, and 5 years.
Although mental health treatment, including use of psychiatric drugs, did not differ between the groups at baseline, during the follow-up period the subjects who underwent surgery saw 15% more impatient and outpatient mental health treatment, compared with controls, a significant difference. About a quarter of patients in the surgically treated group required specialized mental health treatment for the first time after their surgeries.
Though the surgical group lost much more weight – mean BMI was 32.3 at 5 years, compared with 41.7 for controls – none of the mental health changes from baseline were significantly associated with percentage change of BMI at 5 years.
The findings from the study are consistent with results from studies in adults in which bariatric surgery improves many health outcomes but does not alter the need for mental health treatment. Although 5 years is a longer follow-up than in previous studies in young patients – a key strength of the study – Dr. Järvholm and colleagues acknowledged some weaknesses, including a nonrandomized design, lack of a comparison group of nonobese youths for mental health measures, a small sample size, and a surgical procedure that is now out of favor in adolescents.
The study was funded by Swedish government and health foundations. Dr. Järvholm disclosed pharmaceutical industry funding not related to the study, and three coauthors also disclosed industry relationships.
SOURCE: Järvholm K et al. Lancet Child Adolesc Health. 2020. doi: 10.1016/s2352-4642(20)30024-9.
Young people treated with bariatric surgery for severe obesity did not experience better mental health in the 5 years following their procedures, Swedish researchers said, and indeed fared worse than their nontreated peers on certain measures.
The results of this study do not necessarily argue “that metabolic and bariatric surgery during adolescence causes mental health problems,” the investigators wrote in the Lancet Child and Adolescent Health, but “it is reasonable to conclude that metabolic and bariatric surgery does not result in a substantial alleviation of mental health problems in adolescents with severe obesity,” and that “long-term mental health support should be required in programs providing adolescent metabolic and bariatric surgery.”
Kajsa Järvholm, PhD, of Skåne University Hospital, in Malmö, Sweden, and colleagues reported results from a prospective nonrandomized study that recruited 81 adolescents in Sweden aged 13-18 years (mean age, 16.5) who had a body mass index of 40 or higher, or BMI of 35 with obesity-related comorbidities and who underwent Roux-en-Y gastric bypass for weight loss. Subjects were matched by age, sex, and BMI to 80 controls (mean age, 15.8 years) who were assigned to conventional nonsurgical treatment. All patients were assessed at 1, 2, and 5 years.
Although mental health treatment, including use of psychiatric drugs, did not differ between the groups at baseline, during the follow-up period the subjects who underwent surgery saw 15% more impatient and outpatient mental health treatment, compared with controls, a significant difference. About a quarter of patients in the surgically treated group required specialized mental health treatment for the first time after their surgeries.
Though the surgical group lost much more weight – mean BMI was 32.3 at 5 years, compared with 41.7 for controls – none of the mental health changes from baseline were significantly associated with percentage change of BMI at 5 years.
The findings from the study are consistent with results from studies in adults in which bariatric surgery improves many health outcomes but does not alter the need for mental health treatment. Although 5 years is a longer follow-up than in previous studies in young patients – a key strength of the study – Dr. Järvholm and colleagues acknowledged some weaknesses, including a nonrandomized design, lack of a comparison group of nonobese youths for mental health measures, a small sample size, and a surgical procedure that is now out of favor in adolescents.
The study was funded by Swedish government and health foundations. Dr. Järvholm disclosed pharmaceutical industry funding not related to the study, and three coauthors also disclosed industry relationships.
SOURCE: Järvholm K et al. Lancet Child Adolesc Health. 2020. doi: 10.1016/s2352-4642(20)30024-9.
FROM THE LANCET CHILD & ADOLESCENT HEALTH
Key clinical point: Bariatric surgery was not associated with improvement in obese adolescents’ long-term mental health, despite significant weight loss.
Major finding: During 5 years of follow up, surgically treated patients experienced 15% more mental health care usage than controls.
Study details: A prospective, nonrandomized study involving 161 adolescents with a BMI of 40 or greater (or 35 with comorbidities).
Disclosures: The Swedish government and Swedish health research foundations sponsored the study.
Source: Järvholm K et al. Lancet Child Adolesc Health. 2020. doi: 10.1016/s2352-4642(20)30024-9.
Catheter cryoablation effective for persistent AFib in pivotal trial
NATIONAL HARBOR, MD. – , setting the stage for the device to become the first to receive U.S. labeling for catheter ablation in this atrial fibrillation population.
The Arctic Front Advance cryoballoon, used on 165 patients with persistent atrial fibrillation (AFib) enrolled in the trial, produced a 55% rate of treatment success, including freedom from recurrent AFib during 12 months of follow-up, and produced one prespecified serious adverse event in the primary safety endpoint.
Both results easily surpassed the prespecified performance goals set by negotiation with the FDA, Hugh Calkins, MD, said at the annual International AF Symposium. The trial design included no control group and instead assessed safety and efficacy against prespecified standards set by the regulatory agency.
The cryoballoon “showed excellent performance. I don’t see how this could possibly be turned down by the FDA,” said Dr. Calkins, professor of medicine and director of the cardiac arrhythmia service at Johns Hopkins Medicine in Baltimore.
Cardiac electrophysiologists have for years routinely performed catheter ablation procedures on patients with persistent AFib even though the devices, based on ablation by radiofrequency or by chilling, have been labeled for use only in treating patients with paroxysmal AFib. Although this off-label use has not resulted in any problems with health insurance coverage, Dr. Calkins said, it has kept manufacturers from marketing their ablation devices for use in persistent AFib patients.
If the reported data result in labeling for the tested cryoballoon for persistant AFib patients, “it will have a big impact,” he predicted. “People have used cryoballoons for ablating persistent AFib for years, but this would put more fuel in the fire, both the [very positive] safety and efficacy data, and getting an FDA label, which is worth a lot,” he said in an interview.
But Dr. Calkins stopped short of anticipating that the results would convince operators experienced and focused on performing radiofrequency ablation to switch to cryo devices for treating persistent AFib patients. “People are pretty stuck in their ways,” he noted, and reports are expected soon from pivotal trials that are now testing various radiofrequency devices, as well as other types of cryo devices, in persistent AFib patients, so the range of device options labeled for this population may soon grow even more.
The STOP Persistent AF trial ran at 25 sites in the United States, Canada, and Japan during March 2017-August 2019, and included 165 adults with symptomatic, persistent AFib who had not responded to at least one antiarrhythmic drug and had a history of AFib episodes lasting at least 7 days but with no episodes persisting for 6 months or longer. The study excluded patients with prior ablation or left atrial surgery, a recent cerebrovascular event, substantially reduced left ventricular function, or substantial left atrial enlargement.
The enrolled patients were an average 65 years of age and 70% were men. Patients had been diagnosed with paroxysmal AFib an average of 5 years before study entry and with persistent AFib a little over 6 months before entry. The most recent AFib episode of enrolled patients averaged about 60 days, on average they had been unsuccessfully treated with just over one antiarrhythmic drug, and on average they had previously undergone about two cardioversions, after which their arrhythmia recurred.
The primary efficacy endpoint – a 55% rate of acute procedural success plus freedom from AFib recurrence during the 9 months following a 90-day blanking period immediately after ablation plus no added or increased antiarrhythmic drugs – significantly exceeded the prespecified performance goal of a 40% rate, Dr. Calkins reported. The study used the standard measure of recurrence as any 30-second or longer AFib episode detected during a weekly ECG telemonitoring session or during 48-hour ambulatory ECG monitoring at 6- and 12-month follow-ups or during in-office 12-lead ECG assessment at 3-, 6-, and 12-month follow-up. Twelve-month follow-up occurred for 145 of the enrolled patients.
The only prespecified primary safety event was one episode of cardiac perforation, which occurred during a repeat procedure. This rate of one safety event among 165 patients (0.6%) fell well within the prespecified safety performance goal of no more than 13%. In addition to this perforation, five additional serious adverse events (3%) occurred that were attributable to the cryoballoon treatment, including two cases of vascular pseudoaneurysm, one puncture-site hematoma, one case of pericarditis, and one episode of atrial tachycardia. Four additional serious adverse events occurred that were attributable to the ablation procedure (one acute cardiac failure, one postprocedure ileus, one respiratory failure, and one urinary tract infection), for an overall serious event rate of 5%.
During follow-up, 13% of patients had a repeat ablation procedure, following an initial ablation limited to pulmonary-vein isolation (PVI). The overall rate of 1-year efficacy, including the relatively low rate of need for redo ablation, “are an impressive endorsement of a PVI-only strategy” for initial ablation, Dr. Calkins said. “I’m a strong believer in PVI only for the first ablation for both paroxysmal and persistent AFib.”
He also noted that the 30-second threshold for scoring recurrent arrhythmia episodes following the 90-day blanking period after ablation was a very conservative measure of treatment failure, but it continues to define recurrence in this and many other current AFib ablation studies because it is the historical criteria for measuring ablation success or failure. It is especially important to maintain this criteria in a study that relied on prespecified performance criteria rather than a control arm for judging efficacy, Dr. Calkins said.
The study also included three quality-of-life measures. Patient scores on the Atrial Fibrillation Effect on Quality-of-Life (AFEQT) questionnaire rose by an average of nearly 26 points from baseline to 12 months, a statistically significant and clinically meaningful increase. Scores on both the physical and mental domains of the Short Form-12 (SF-12) improved from baseline by an average of about five points on each subscale, also statistically significant and clinically meaningful changes. Patients also reported statistically significant and in some cases substantial reductions in the prevalence rates of each of five different AFib symptoms: dizziness, dyspnea, fatigue, palpitations, and rapid heartbeat.
The study was funded by Medtronic, the company that markets the tested cryoballoon (Arctic Front Advance). Dr. Calkins has been a consultant to and has received honoraria from Medtronic, as well as from Abbott, AtriCure, Boehringer Ingelheim, Boston Scientific, and Johnson & Johnson.
NATIONAL HARBOR, MD. – , setting the stage for the device to become the first to receive U.S. labeling for catheter ablation in this atrial fibrillation population.
The Arctic Front Advance cryoballoon, used on 165 patients with persistent atrial fibrillation (AFib) enrolled in the trial, produced a 55% rate of treatment success, including freedom from recurrent AFib during 12 months of follow-up, and produced one prespecified serious adverse event in the primary safety endpoint.
Both results easily surpassed the prespecified performance goals set by negotiation with the FDA, Hugh Calkins, MD, said at the annual International AF Symposium. The trial design included no control group and instead assessed safety and efficacy against prespecified standards set by the regulatory agency.
The cryoballoon “showed excellent performance. I don’t see how this could possibly be turned down by the FDA,” said Dr. Calkins, professor of medicine and director of the cardiac arrhythmia service at Johns Hopkins Medicine in Baltimore.
Cardiac electrophysiologists have for years routinely performed catheter ablation procedures on patients with persistent AFib even though the devices, based on ablation by radiofrequency or by chilling, have been labeled for use only in treating patients with paroxysmal AFib. Although this off-label use has not resulted in any problems with health insurance coverage, Dr. Calkins said, it has kept manufacturers from marketing their ablation devices for use in persistent AFib patients.
If the reported data result in labeling for the tested cryoballoon for persistant AFib patients, “it will have a big impact,” he predicted. “People have used cryoballoons for ablating persistent AFib for years, but this would put more fuel in the fire, both the [very positive] safety and efficacy data, and getting an FDA label, which is worth a lot,” he said in an interview.
But Dr. Calkins stopped short of anticipating that the results would convince operators experienced and focused on performing radiofrequency ablation to switch to cryo devices for treating persistent AFib patients. “People are pretty stuck in their ways,” he noted, and reports are expected soon from pivotal trials that are now testing various radiofrequency devices, as well as other types of cryo devices, in persistent AFib patients, so the range of device options labeled for this population may soon grow even more.
The STOP Persistent AF trial ran at 25 sites in the United States, Canada, and Japan during March 2017-August 2019, and included 165 adults with symptomatic, persistent AFib who had not responded to at least one antiarrhythmic drug and had a history of AFib episodes lasting at least 7 days but with no episodes persisting for 6 months or longer. The study excluded patients with prior ablation or left atrial surgery, a recent cerebrovascular event, substantially reduced left ventricular function, or substantial left atrial enlargement.
The enrolled patients were an average 65 years of age and 70% were men. Patients had been diagnosed with paroxysmal AFib an average of 5 years before study entry and with persistent AFib a little over 6 months before entry. The most recent AFib episode of enrolled patients averaged about 60 days, on average they had been unsuccessfully treated with just over one antiarrhythmic drug, and on average they had previously undergone about two cardioversions, after which their arrhythmia recurred.
The primary efficacy endpoint – a 55% rate of acute procedural success plus freedom from AFib recurrence during the 9 months following a 90-day blanking period immediately after ablation plus no added or increased antiarrhythmic drugs – significantly exceeded the prespecified performance goal of a 40% rate, Dr. Calkins reported. The study used the standard measure of recurrence as any 30-second or longer AFib episode detected during a weekly ECG telemonitoring session or during 48-hour ambulatory ECG monitoring at 6- and 12-month follow-ups or during in-office 12-lead ECG assessment at 3-, 6-, and 12-month follow-up. Twelve-month follow-up occurred for 145 of the enrolled patients.
The only prespecified primary safety event was one episode of cardiac perforation, which occurred during a repeat procedure. This rate of one safety event among 165 patients (0.6%) fell well within the prespecified safety performance goal of no more than 13%. In addition to this perforation, five additional serious adverse events (3%) occurred that were attributable to the cryoballoon treatment, including two cases of vascular pseudoaneurysm, one puncture-site hematoma, one case of pericarditis, and one episode of atrial tachycardia. Four additional serious adverse events occurred that were attributable to the ablation procedure (one acute cardiac failure, one postprocedure ileus, one respiratory failure, and one urinary tract infection), for an overall serious event rate of 5%.
During follow-up, 13% of patients had a repeat ablation procedure, following an initial ablation limited to pulmonary-vein isolation (PVI). The overall rate of 1-year efficacy, including the relatively low rate of need for redo ablation, “are an impressive endorsement of a PVI-only strategy” for initial ablation, Dr. Calkins said. “I’m a strong believer in PVI only for the first ablation for both paroxysmal and persistent AFib.”
He also noted that the 30-second threshold for scoring recurrent arrhythmia episodes following the 90-day blanking period after ablation was a very conservative measure of treatment failure, but it continues to define recurrence in this and many other current AFib ablation studies because it is the historical criteria for measuring ablation success or failure. It is especially important to maintain this criteria in a study that relied on prespecified performance criteria rather than a control arm for judging efficacy, Dr. Calkins said.
The study also included three quality-of-life measures. Patient scores on the Atrial Fibrillation Effect on Quality-of-Life (AFEQT) questionnaire rose by an average of nearly 26 points from baseline to 12 months, a statistically significant and clinically meaningful increase. Scores on both the physical and mental domains of the Short Form-12 (SF-12) improved from baseline by an average of about five points on each subscale, also statistically significant and clinically meaningful changes. Patients also reported statistically significant and in some cases substantial reductions in the prevalence rates of each of five different AFib symptoms: dizziness, dyspnea, fatigue, palpitations, and rapid heartbeat.
The study was funded by Medtronic, the company that markets the tested cryoballoon (Arctic Front Advance). Dr. Calkins has been a consultant to and has received honoraria from Medtronic, as well as from Abbott, AtriCure, Boehringer Ingelheim, Boston Scientific, and Johnson & Johnson.
NATIONAL HARBOR, MD. – , setting the stage for the device to become the first to receive U.S. labeling for catheter ablation in this atrial fibrillation population.
The Arctic Front Advance cryoballoon, used on 165 patients with persistent atrial fibrillation (AFib) enrolled in the trial, produced a 55% rate of treatment success, including freedom from recurrent AFib during 12 months of follow-up, and produced one prespecified serious adverse event in the primary safety endpoint.
Both results easily surpassed the prespecified performance goals set by negotiation with the FDA, Hugh Calkins, MD, said at the annual International AF Symposium. The trial design included no control group and instead assessed safety and efficacy against prespecified standards set by the regulatory agency.
The cryoballoon “showed excellent performance. I don’t see how this could possibly be turned down by the FDA,” said Dr. Calkins, professor of medicine and director of the cardiac arrhythmia service at Johns Hopkins Medicine in Baltimore.
Cardiac electrophysiologists have for years routinely performed catheter ablation procedures on patients with persistent AFib even though the devices, based on ablation by radiofrequency or by chilling, have been labeled for use only in treating patients with paroxysmal AFib. Although this off-label use has not resulted in any problems with health insurance coverage, Dr. Calkins said, it has kept manufacturers from marketing their ablation devices for use in persistent AFib patients.
If the reported data result in labeling for the tested cryoballoon for persistant AFib patients, “it will have a big impact,” he predicted. “People have used cryoballoons for ablating persistent AFib for years, but this would put more fuel in the fire, both the [very positive] safety and efficacy data, and getting an FDA label, which is worth a lot,” he said in an interview.
But Dr. Calkins stopped short of anticipating that the results would convince operators experienced and focused on performing radiofrequency ablation to switch to cryo devices for treating persistent AFib patients. “People are pretty stuck in their ways,” he noted, and reports are expected soon from pivotal trials that are now testing various radiofrequency devices, as well as other types of cryo devices, in persistent AFib patients, so the range of device options labeled for this population may soon grow even more.
The STOP Persistent AF trial ran at 25 sites in the United States, Canada, and Japan during March 2017-August 2019, and included 165 adults with symptomatic, persistent AFib who had not responded to at least one antiarrhythmic drug and had a history of AFib episodes lasting at least 7 days but with no episodes persisting for 6 months or longer. The study excluded patients with prior ablation or left atrial surgery, a recent cerebrovascular event, substantially reduced left ventricular function, or substantial left atrial enlargement.
The enrolled patients were an average 65 years of age and 70% were men. Patients had been diagnosed with paroxysmal AFib an average of 5 years before study entry and with persistent AFib a little over 6 months before entry. The most recent AFib episode of enrolled patients averaged about 60 days, on average they had been unsuccessfully treated with just over one antiarrhythmic drug, and on average they had previously undergone about two cardioversions, after which their arrhythmia recurred.
The primary efficacy endpoint – a 55% rate of acute procedural success plus freedom from AFib recurrence during the 9 months following a 90-day blanking period immediately after ablation plus no added or increased antiarrhythmic drugs – significantly exceeded the prespecified performance goal of a 40% rate, Dr. Calkins reported. The study used the standard measure of recurrence as any 30-second or longer AFib episode detected during a weekly ECG telemonitoring session or during 48-hour ambulatory ECG monitoring at 6- and 12-month follow-ups or during in-office 12-lead ECG assessment at 3-, 6-, and 12-month follow-up. Twelve-month follow-up occurred for 145 of the enrolled patients.
The only prespecified primary safety event was one episode of cardiac perforation, which occurred during a repeat procedure. This rate of one safety event among 165 patients (0.6%) fell well within the prespecified safety performance goal of no more than 13%. In addition to this perforation, five additional serious adverse events (3%) occurred that were attributable to the cryoballoon treatment, including two cases of vascular pseudoaneurysm, one puncture-site hematoma, one case of pericarditis, and one episode of atrial tachycardia. Four additional serious adverse events occurred that were attributable to the ablation procedure (one acute cardiac failure, one postprocedure ileus, one respiratory failure, and one urinary tract infection), for an overall serious event rate of 5%.
During follow-up, 13% of patients had a repeat ablation procedure, following an initial ablation limited to pulmonary-vein isolation (PVI). The overall rate of 1-year efficacy, including the relatively low rate of need for redo ablation, “are an impressive endorsement of a PVI-only strategy” for initial ablation, Dr. Calkins said. “I’m a strong believer in PVI only for the first ablation for both paroxysmal and persistent AFib.”
He also noted that the 30-second threshold for scoring recurrent arrhythmia episodes following the 90-day blanking period after ablation was a very conservative measure of treatment failure, but it continues to define recurrence in this and many other current AFib ablation studies because it is the historical criteria for measuring ablation success or failure. It is especially important to maintain this criteria in a study that relied on prespecified performance criteria rather than a control arm for judging efficacy, Dr. Calkins said.
The study also included three quality-of-life measures. Patient scores on the Atrial Fibrillation Effect on Quality-of-Life (AFEQT) questionnaire rose by an average of nearly 26 points from baseline to 12 months, a statistically significant and clinically meaningful increase. Scores on both the physical and mental domains of the Short Form-12 (SF-12) improved from baseline by an average of about five points on each subscale, also statistically significant and clinically meaningful changes. Patients also reported statistically significant and in some cases substantial reductions in the prevalence rates of each of five different AFib symptoms: dizziness, dyspnea, fatigue, palpitations, and rapid heartbeat.
The study was funded by Medtronic, the company that markets the tested cryoballoon (Arctic Front Advance). Dr. Calkins has been a consultant to and has received honoraria from Medtronic, as well as from Abbott, AtriCure, Boehringer Ingelheim, Boston Scientific, and Johnson & Johnson.
REPORTING FROM THE AF SYMPOSIUM 2020
Key clinical point: Catheter ablation using a cryoballoon was safe and effective for patients with persistent atrial fibrillation in a pivotal trial.
Major finding: The rate of freedom from treatment failure after 12 months was 55%, significantly exceeding the prespecified performance goal of 40%.
Study details: A multicenter, international study with 165 enrolled patients.
Disclosures: The study was funded by Medtronic, the company that markets the tested cryoballoon (Arctic Front Advance). Dr. Calkins has been a consultant to and has received honoraria from Medtronic, as well as from Abbott, AtriCure, Boehringer Ingelheim, Boston Scientific, and Johnson & Johnson.
Hippocampal sparing temporal lobectomy recommended for medically refractory epilepsy
BALTIMORE – according to a review from researchers at Thomas Jefferson University in Philadelphia.
Often, the hippocampus and other mesial structures are removed even if they appear normal. The concern is that even normal looking tissue could harbor epileptogenic elements and leaving them in tact could reduce postoperative seizure control, explained senior investigator and neurologist Michael Sperling, MD, director of the Jefferson Comprehensive Epilepsy Center.
He and his colleagues wanted to see if that was really true, so they compared outcomes in 21 patients who had mesial-sparing lobectomies with 19 patients who had the standard approach. Cases and controls were matched for age, preoperative seizure frequency, side of surgery, and other factors. None of the patients had MTS.
There was no significant difference in postoperative seizure recurrence between the two groups (P = .974). The standard procedure had a slight edge early on, but at 2.5 years, just over 60% of patients in both groups were seizure free. At 5 years, about 50% were seizure free, and almost 40% in both arms at 7.5 years.
About two-thirds of patients in each arm had pre- and postoperative verbal memory testing, with similar duration from surgery to postop evaluation. There was no change among the hippocampus-sparing patients, but a roughly one standard deviation drop in delayed recall and logical memory on the California Verbal Learning Test in the standard group.
Even so, it wasn’t enough to affect employment, which the investigators used as a surrogate for disability; postoperative employment was comparable in both groups. People mostly retained their jobs, and there was no difference in job loss. A few people in each arm actually found jobs after surgery.
The investigators concluded that “it is reasonable to recommend mesial temporal sparing procedure in patients with dominant neocortical temporal lobe epilepsy when the hippocampus appears normal in the MRI. However, as resecting the mesial temporal structures was not associated with a greater chance of becoming unemployed following the surgery, there appears to be no major contraindication to performing an [anterior temporal lobectomy] if clinically warranted.”
The results are reassuring. “My bias walking in was that” seizure recurrence would be worse after hippocampal-sparing surgery. “I was pleased to see that it was about the same. If you want to try to preserve verbal memory and the MRI is normal, you can get away with sparing the mesial temporal structures, and still get a good seizure outcome,” Dr. Sperling said at the annual meeting of the American Epilepsy Society, where the study was presented.
“But if you have to take the hippocampus for whatever reason, the functional consequence of a decline in verbal memory is not severe enough as to be disabling,” which is “one of the big concerns” with temporal lobectomy, he said.
The findings “will make us more likely to recommend mesial-sparing surgery, but at the same time” perhaps not be quite as worried about disability with the standard approach.
Temporal lobe epilepsy with normal mesial structures isn’t very common, which explains the small numbers in the series. It’s possible subtle difference in seizure control and employment outcomes would have been found with a larger series, “but obviously there were no major differences. I think the fundamental questions have been answered to my satisfaction,” Dr. Sperling said.
Overall, “it’s better to operate and try to cure people than to worry that you will make their memory worse when the consequences of having uncontrolled epilepsy is a higher death rate,” he said.
There were about equal numbers of men and women in the review; patients were in their early 30s, on average; and most had left-sided surgery. Just over half in each arm had preoperative tonic-clonic seizures. The mean duration of epilepsy was 14.9 years in the mesial-sparing group, and 8.6 years in the standard arm.
There was no funding for the review, and Dr. Sperling didn’t have any relevant disclosures.
SOURCE: Goldstein L et al. AES 2019. Abstract 1.339.
BALTIMORE – according to a review from researchers at Thomas Jefferson University in Philadelphia.
Often, the hippocampus and other mesial structures are removed even if they appear normal. The concern is that even normal looking tissue could harbor epileptogenic elements and leaving them in tact could reduce postoperative seizure control, explained senior investigator and neurologist Michael Sperling, MD, director of the Jefferson Comprehensive Epilepsy Center.
He and his colleagues wanted to see if that was really true, so they compared outcomes in 21 patients who had mesial-sparing lobectomies with 19 patients who had the standard approach. Cases and controls were matched for age, preoperative seizure frequency, side of surgery, and other factors. None of the patients had MTS.
There was no significant difference in postoperative seizure recurrence between the two groups (P = .974). The standard procedure had a slight edge early on, but at 2.5 years, just over 60% of patients in both groups were seizure free. At 5 years, about 50% were seizure free, and almost 40% in both arms at 7.5 years.
About two-thirds of patients in each arm had pre- and postoperative verbal memory testing, with similar duration from surgery to postop evaluation. There was no change among the hippocampus-sparing patients, but a roughly one standard deviation drop in delayed recall and logical memory on the California Verbal Learning Test in the standard group.
Even so, it wasn’t enough to affect employment, which the investigators used as a surrogate for disability; postoperative employment was comparable in both groups. People mostly retained their jobs, and there was no difference in job loss. A few people in each arm actually found jobs after surgery.
The investigators concluded that “it is reasonable to recommend mesial temporal sparing procedure in patients with dominant neocortical temporal lobe epilepsy when the hippocampus appears normal in the MRI. However, as resecting the mesial temporal structures was not associated with a greater chance of becoming unemployed following the surgery, there appears to be no major contraindication to performing an [anterior temporal lobectomy] if clinically warranted.”
The results are reassuring. “My bias walking in was that” seizure recurrence would be worse after hippocampal-sparing surgery. “I was pleased to see that it was about the same. If you want to try to preserve verbal memory and the MRI is normal, you can get away with sparing the mesial temporal structures, and still get a good seizure outcome,” Dr. Sperling said at the annual meeting of the American Epilepsy Society, where the study was presented.
“But if you have to take the hippocampus for whatever reason, the functional consequence of a decline in verbal memory is not severe enough as to be disabling,” which is “one of the big concerns” with temporal lobectomy, he said.
The findings “will make us more likely to recommend mesial-sparing surgery, but at the same time” perhaps not be quite as worried about disability with the standard approach.
Temporal lobe epilepsy with normal mesial structures isn’t very common, which explains the small numbers in the series. It’s possible subtle difference in seizure control and employment outcomes would have been found with a larger series, “but obviously there were no major differences. I think the fundamental questions have been answered to my satisfaction,” Dr. Sperling said.
Overall, “it’s better to operate and try to cure people than to worry that you will make their memory worse when the consequences of having uncontrolled epilepsy is a higher death rate,” he said.
There were about equal numbers of men and women in the review; patients were in their early 30s, on average; and most had left-sided surgery. Just over half in each arm had preoperative tonic-clonic seizures. The mean duration of epilepsy was 14.9 years in the mesial-sparing group, and 8.6 years in the standard arm.
There was no funding for the review, and Dr. Sperling didn’t have any relevant disclosures.
SOURCE: Goldstein L et al. AES 2019. Abstract 1.339.
BALTIMORE – according to a review from researchers at Thomas Jefferson University in Philadelphia.
Often, the hippocampus and other mesial structures are removed even if they appear normal. The concern is that even normal looking tissue could harbor epileptogenic elements and leaving them in tact could reduce postoperative seizure control, explained senior investigator and neurologist Michael Sperling, MD, director of the Jefferson Comprehensive Epilepsy Center.
He and his colleagues wanted to see if that was really true, so they compared outcomes in 21 patients who had mesial-sparing lobectomies with 19 patients who had the standard approach. Cases and controls were matched for age, preoperative seizure frequency, side of surgery, and other factors. None of the patients had MTS.
There was no significant difference in postoperative seizure recurrence between the two groups (P = .974). The standard procedure had a slight edge early on, but at 2.5 years, just over 60% of patients in both groups were seizure free. At 5 years, about 50% were seizure free, and almost 40% in both arms at 7.5 years.
About two-thirds of patients in each arm had pre- and postoperative verbal memory testing, with similar duration from surgery to postop evaluation. There was no change among the hippocampus-sparing patients, but a roughly one standard deviation drop in delayed recall and logical memory on the California Verbal Learning Test in the standard group.
Even so, it wasn’t enough to affect employment, which the investigators used as a surrogate for disability; postoperative employment was comparable in both groups. People mostly retained their jobs, and there was no difference in job loss. A few people in each arm actually found jobs after surgery.
The investigators concluded that “it is reasonable to recommend mesial temporal sparing procedure in patients with dominant neocortical temporal lobe epilepsy when the hippocampus appears normal in the MRI. However, as resecting the mesial temporal structures was not associated with a greater chance of becoming unemployed following the surgery, there appears to be no major contraindication to performing an [anterior temporal lobectomy] if clinically warranted.”
The results are reassuring. “My bias walking in was that” seizure recurrence would be worse after hippocampal-sparing surgery. “I was pleased to see that it was about the same. If you want to try to preserve verbal memory and the MRI is normal, you can get away with sparing the mesial temporal structures, and still get a good seizure outcome,” Dr. Sperling said at the annual meeting of the American Epilepsy Society, where the study was presented.
“But if you have to take the hippocampus for whatever reason, the functional consequence of a decline in verbal memory is not severe enough as to be disabling,” which is “one of the big concerns” with temporal lobectomy, he said.
The findings “will make us more likely to recommend mesial-sparing surgery, but at the same time” perhaps not be quite as worried about disability with the standard approach.
Temporal lobe epilepsy with normal mesial structures isn’t very common, which explains the small numbers in the series. It’s possible subtle difference in seizure control and employment outcomes would have been found with a larger series, “but obviously there were no major differences. I think the fundamental questions have been answered to my satisfaction,” Dr. Sperling said.
Overall, “it’s better to operate and try to cure people than to worry that you will make their memory worse when the consequences of having uncontrolled epilepsy is a higher death rate,” he said.
There were about equal numbers of men and women in the review; patients were in their early 30s, on average; and most had left-sided surgery. Just over half in each arm had preoperative tonic-clonic seizures. The mean duration of epilepsy was 14.9 years in the mesial-sparing group, and 8.6 years in the standard arm.
There was no funding for the review, and Dr. Sperling didn’t have any relevant disclosures.
SOURCE: Goldstein L et al. AES 2019. Abstract 1.339.
REPORTING FROM AES 2019
Bariatric surgery is most effective early in the diabetes trajectory
LOS ANGELES – In the clinical experience of Kurt GMM Alberti, DPhil, FRCP, FRCPath, The problem is, far fewer people with diabetes are being referred for the surgery than would benefit from it.
At the World Congress on Insulin Resistance, Diabetes, and Cardiovascular Disease, Dr. Alberti said that only about 1% of eligible patients in the United States undergo bariatric surgery, compared with just 0.22% of eligible patients in the United Kingdom.
“Obesity is an increasing problem,” said Dr. Alberti, a senior research investigator in the section of diabetes, endocrinology, and metabolism at Imperial College, London. “The United States is leading the field [in obesity], and it doesn’t seem to be going away. This is in parallel with diabetes. According to the 2019 International Diabetes Federation’s Diabetes Atlas, the diabetes prevalence worldwide is now 463 million. It’s projected to reach 578 million by 2030 and 700 million by 2045. We have a major problem.”
Lifestyle modification with diet and exercise have been the cornerstone of diabetes therapy for more than 100 years, with only modest success. “A range of oral agents have been added [and they] certainly improve glycemic control, but few achieve lasting success, and few achieve remission,” Dr. Alberti said. Findings from DiRECT (Lancet. 2018;391:541-51) and the Look AHEAD (Action for Health in Diabetes) study (N Engl J Med. 2013;369:145-54) have shown dramatic improvements in glycemia, but only in the minority of patients who achieved weight loss of 10 kg or more. “In this group, 80% achieved remission after 2 years in DiRECT,” he said. “It is uncertain whether this can be sustained long term in real life, and how many people will respond. Major community prevention programs are under way in the U.S. and [United Kingdom], but many target individuals with prediabetes. Currently, it is unknown how successful these programs will be.”
The 2018 American Diabetes Association/European Association for the Study of Diabetes clinical guidelines state that bariatric surgery is a recommended treatment option for adults with type 2 diabetes and a body mass index of either 40.0 kg/m2 or higher (or 37.5 kg/m2 or higher in people of Asian ancestry) or 35.0-39.9 kg/m2 (32.5-37.4 kg/m2 in people of Asian ancestry) and who do not achieve durable weight loss and improvement in comorbidities with reasonable nonsurgical methods. According to Dr. Alberti, surgery should be an accepted option in people who have type 2 diabetes and a body mass index of 30 kg/m2 or higher, and priority should be given to adolescents, young adults, and those with a shorter duration of diabetes.
The main suggestive evidence in favor of bariatric surgery having an impact on diabetes comes from the Swedish Obese Subjects Study, which had a median follow-up of 10 years (J Intern Med. 2013;273[3]:219-34). In patients with diabetes at baseline, 30% were still in remission at 15 years after surgery. After 18 years, cumulative microvascular disease had fallen from 42 to 21 per 1,000 person-years, and macrovascular complications had fallen by 25%. “This was not a randomized, controlled trial, however,” Dr. Alberti said. Several of these studies have now been performed, including the STAMPEDE trial (N Engl J Med. 2017;376:641-51) and recent studies led by Geltrude Mingrone, MD, PhD, which show major glycemic benefit (Lancet. 2015;386[9997]:P964-73) with bariatric surgery.
According to Dr. Alberti, laparoscopic adjustable gastric lap band is the easiest bariatric surgery to perform but it has fallen out of favor because of lower diabetes remission rates, compared with the other procedures. Roux-en-Y gastric bypass, sleeve gastrectomy, and biliary pancreatic diversion are all in use. Slightly higher remission rates have been shown with biliary pancreatic diversion. “There have also been consistent improvements in quality of life and cardiovascular risk factors,” he said. “Long-term follow-up is still [needed], but, in general, it seems that diabetic complications are lower than in medically treated control groups, and mortality may also be lower. The real question is, what is the impact on complications? There we have a problem, because we do not have any good randomized, controlled trials covering a 10- to 20-year period, which would give us clear evidence. There is reasonable evidence from cohort studies, though.”
Added benefits of bariatric surgery include improved quality of life, decreased blood pressure, less sleep apnea, improved cardiovascular risk factors, and better musculoskeletal function. For now, though, an unmet need persists. “The problem is that far fewer people are referred for bariatric surgery than would benefit,” Dr. Alberti said. “There are several barriers to greater use of bariatric surgery in those with diabetes. These include physician attitudes, inadequate referrals, patient perceptions, lack of awareness among patients, inadequate insurance coverage, and particularly health system capacity. There is also a lack of sympathy for overweight people in some places.”
Dr. Alberti reported having no financial disclosures.
LOS ANGELES – In the clinical experience of Kurt GMM Alberti, DPhil, FRCP, FRCPath, The problem is, far fewer people with diabetes are being referred for the surgery than would benefit from it.
At the World Congress on Insulin Resistance, Diabetes, and Cardiovascular Disease, Dr. Alberti said that only about 1% of eligible patients in the United States undergo bariatric surgery, compared with just 0.22% of eligible patients in the United Kingdom.
“Obesity is an increasing problem,” said Dr. Alberti, a senior research investigator in the section of diabetes, endocrinology, and metabolism at Imperial College, London. “The United States is leading the field [in obesity], and it doesn’t seem to be going away. This is in parallel with diabetes. According to the 2019 International Diabetes Federation’s Diabetes Atlas, the diabetes prevalence worldwide is now 463 million. It’s projected to reach 578 million by 2030 and 700 million by 2045. We have a major problem.”
Lifestyle modification with diet and exercise have been the cornerstone of diabetes therapy for more than 100 years, with only modest success. “A range of oral agents have been added [and they] certainly improve glycemic control, but few achieve lasting success, and few achieve remission,” Dr. Alberti said. Findings from DiRECT (Lancet. 2018;391:541-51) and the Look AHEAD (Action for Health in Diabetes) study (N Engl J Med. 2013;369:145-54) have shown dramatic improvements in glycemia, but only in the minority of patients who achieved weight loss of 10 kg or more. “In this group, 80% achieved remission after 2 years in DiRECT,” he said. “It is uncertain whether this can be sustained long term in real life, and how many people will respond. Major community prevention programs are under way in the U.S. and [United Kingdom], but many target individuals with prediabetes. Currently, it is unknown how successful these programs will be.”
The 2018 American Diabetes Association/European Association for the Study of Diabetes clinical guidelines state that bariatric surgery is a recommended treatment option for adults with type 2 diabetes and a body mass index of either 40.0 kg/m2 or higher (or 37.5 kg/m2 or higher in people of Asian ancestry) or 35.0-39.9 kg/m2 (32.5-37.4 kg/m2 in people of Asian ancestry) and who do not achieve durable weight loss and improvement in comorbidities with reasonable nonsurgical methods. According to Dr. Alberti, surgery should be an accepted option in people who have type 2 diabetes and a body mass index of 30 kg/m2 or higher, and priority should be given to adolescents, young adults, and those with a shorter duration of diabetes.
The main suggestive evidence in favor of bariatric surgery having an impact on diabetes comes from the Swedish Obese Subjects Study, which had a median follow-up of 10 years (J Intern Med. 2013;273[3]:219-34). In patients with diabetes at baseline, 30% were still in remission at 15 years after surgery. After 18 years, cumulative microvascular disease had fallen from 42 to 21 per 1,000 person-years, and macrovascular complications had fallen by 25%. “This was not a randomized, controlled trial, however,” Dr. Alberti said. Several of these studies have now been performed, including the STAMPEDE trial (N Engl J Med. 2017;376:641-51) and recent studies led by Geltrude Mingrone, MD, PhD, which show major glycemic benefit (Lancet. 2015;386[9997]:P964-73) with bariatric surgery.
According to Dr. Alberti, laparoscopic adjustable gastric lap band is the easiest bariatric surgery to perform but it has fallen out of favor because of lower diabetes remission rates, compared with the other procedures. Roux-en-Y gastric bypass, sleeve gastrectomy, and biliary pancreatic diversion are all in use. Slightly higher remission rates have been shown with biliary pancreatic diversion. “There have also been consistent improvements in quality of life and cardiovascular risk factors,” he said. “Long-term follow-up is still [needed], but, in general, it seems that diabetic complications are lower than in medically treated control groups, and mortality may also be lower. The real question is, what is the impact on complications? There we have a problem, because we do not have any good randomized, controlled trials covering a 10- to 20-year period, which would give us clear evidence. There is reasonable evidence from cohort studies, though.”
Added benefits of bariatric surgery include improved quality of life, decreased blood pressure, less sleep apnea, improved cardiovascular risk factors, and better musculoskeletal function. For now, though, an unmet need persists. “The problem is that far fewer people are referred for bariatric surgery than would benefit,” Dr. Alberti said. “There are several barriers to greater use of bariatric surgery in those with diabetes. These include physician attitudes, inadequate referrals, patient perceptions, lack of awareness among patients, inadequate insurance coverage, and particularly health system capacity. There is also a lack of sympathy for overweight people in some places.”
Dr. Alberti reported having no financial disclosures.
LOS ANGELES – In the clinical experience of Kurt GMM Alberti, DPhil, FRCP, FRCPath, The problem is, far fewer people with diabetes are being referred for the surgery than would benefit from it.
At the World Congress on Insulin Resistance, Diabetes, and Cardiovascular Disease, Dr. Alberti said that only about 1% of eligible patients in the United States undergo bariatric surgery, compared with just 0.22% of eligible patients in the United Kingdom.
“Obesity is an increasing problem,” said Dr. Alberti, a senior research investigator in the section of diabetes, endocrinology, and metabolism at Imperial College, London. “The United States is leading the field [in obesity], and it doesn’t seem to be going away. This is in parallel with diabetes. According to the 2019 International Diabetes Federation’s Diabetes Atlas, the diabetes prevalence worldwide is now 463 million. It’s projected to reach 578 million by 2030 and 700 million by 2045. We have a major problem.”
Lifestyle modification with diet and exercise have been the cornerstone of diabetes therapy for more than 100 years, with only modest success. “A range of oral agents have been added [and they] certainly improve glycemic control, but few achieve lasting success, and few achieve remission,” Dr. Alberti said. Findings from DiRECT (Lancet. 2018;391:541-51) and the Look AHEAD (Action for Health in Diabetes) study (N Engl J Med. 2013;369:145-54) have shown dramatic improvements in glycemia, but only in the minority of patients who achieved weight loss of 10 kg or more. “In this group, 80% achieved remission after 2 years in DiRECT,” he said. “It is uncertain whether this can be sustained long term in real life, and how many people will respond. Major community prevention programs are under way in the U.S. and [United Kingdom], but many target individuals with prediabetes. Currently, it is unknown how successful these programs will be.”
The 2018 American Diabetes Association/European Association for the Study of Diabetes clinical guidelines state that bariatric surgery is a recommended treatment option for adults with type 2 diabetes and a body mass index of either 40.0 kg/m2 or higher (or 37.5 kg/m2 or higher in people of Asian ancestry) or 35.0-39.9 kg/m2 (32.5-37.4 kg/m2 in people of Asian ancestry) and who do not achieve durable weight loss and improvement in comorbidities with reasonable nonsurgical methods. According to Dr. Alberti, surgery should be an accepted option in people who have type 2 diabetes and a body mass index of 30 kg/m2 or higher, and priority should be given to adolescents, young adults, and those with a shorter duration of diabetes.
The main suggestive evidence in favor of bariatric surgery having an impact on diabetes comes from the Swedish Obese Subjects Study, which had a median follow-up of 10 years (J Intern Med. 2013;273[3]:219-34). In patients with diabetes at baseline, 30% were still in remission at 15 years after surgery. After 18 years, cumulative microvascular disease had fallen from 42 to 21 per 1,000 person-years, and macrovascular complications had fallen by 25%. “This was not a randomized, controlled trial, however,” Dr. Alberti said. Several of these studies have now been performed, including the STAMPEDE trial (N Engl J Med. 2017;376:641-51) and recent studies led by Geltrude Mingrone, MD, PhD, which show major glycemic benefit (Lancet. 2015;386[9997]:P964-73) with bariatric surgery.
According to Dr. Alberti, laparoscopic adjustable gastric lap band is the easiest bariatric surgery to perform but it has fallen out of favor because of lower diabetes remission rates, compared with the other procedures. Roux-en-Y gastric bypass, sleeve gastrectomy, and biliary pancreatic diversion are all in use. Slightly higher remission rates have been shown with biliary pancreatic diversion. “There have also been consistent improvements in quality of life and cardiovascular risk factors,” he said. “Long-term follow-up is still [needed], but, in general, it seems that diabetic complications are lower than in medically treated control groups, and mortality may also be lower. The real question is, what is the impact on complications? There we have a problem, because we do not have any good randomized, controlled trials covering a 10- to 20-year period, which would give us clear evidence. There is reasonable evidence from cohort studies, though.”
Added benefits of bariatric surgery include improved quality of life, decreased blood pressure, less sleep apnea, improved cardiovascular risk factors, and better musculoskeletal function. For now, though, an unmet need persists. “The problem is that far fewer people are referred for bariatric surgery than would benefit,” Dr. Alberti said. “There are several barriers to greater use of bariatric surgery in those with diabetes. These include physician attitudes, inadequate referrals, patient perceptions, lack of awareness among patients, inadequate insurance coverage, and particularly health system capacity. There is also a lack of sympathy for overweight people in some places.”
Dr. Alberti reported having no financial disclosures.
EXPERT ANALYSIS FROM WCIRDC 2019
Consider sparing the uterus in prolapse procedures
LAS VEGAS – A female pelvic medicine and reconstructive surgeon urged colleagues to consider uterus-sparing hysteropexies instead of hysterectomies in pelvic organ prolapse repairs.
said Beri M. Ridgeway, MD, of the Cleveland Clinic, at the Pelvic Anatomy and Gynecologic Surgery Symposium. Even so, “in the U.S., gynecologists rarely offer uterine preservation for women who desire repair of their uterovaginal prolapse.”
According to research compiled by Dr. Ridgeway, about 74,000 hysterectomies are performed each year in the United States to treat pelvic organ prolapse. The procedure became standard in the second half of the 20th century, in part to reduce cancer risk.
But attitudes evolved starting in the 1990s “as we have had better cancer screening and more focus on patient sexuality, patient autonomy, and quality of life,” Dr. Ridgeway said.
She offered these reasons to question hysterectomies to treat pelvic organ prolapse repairs:
- It’s not clear whether hysterectomies address the anatomic problems that produce prolapse in the first place. “Prolapse is caused by weakened or damaged tissue – connective tissue, muscles, etc.,” she said in an interview. “The problem is what is supporting the uterus, not the uterus itself.”
- Despite assumptions, women don’t necessarily prefer hysterectomy. Dr. Ridgeway pointed to a 2013 study in which researchers surveyed 213 women with prolapse symptoms about their preferred treatment, assuming that outcomes were the same. The results: 36% preferred uterine preservation, 20% preferred hysterectomy, and 44% reported no strong preference (Am J Obstet Gynecol. 2013 Nov;209[5]:470.e1-6.).
- Hysterectomies hasten menopause.
There has been a perception that uterus removal is appropriate in women who don’t wish to have any more children, Dr. Ridgeway said. “You had your babies, you’re done, you don’t need this anymore.” In fact, “that’s basically not true.”
As she explained, hysterectomy is linked to earlier menopause, and “even losing one ovary pushed patients into menopause significantly earlier.” She pointed to a 2016 Australian study, which found that “women who have a hysterectomy (with ovarian conservation) have a higher risk of hot flushes and night sweats that persist over an extended period” (Maturitas. 2016 Sep;91:1-7).
There’s no consensus on how hysterectomy affects sexual function. However, Dr. Ridgeway noted, it’s clear that pelvic floor disorders disrupt sexual function, and most women see improvement after surgical treatment.
The rate of uterine pathology is low in hysterectomy. Dr. Ridgeway highlighted a 2018 study of 24,076 women who underwent hysterectomy for benign indications. The study reported that “prevalence of occult corpus uteri, cervical, and ovarian malignancy was 1.44%, 0.60%, and 0.19%, respectively, among women undergoing hysterectomy and it varied by patient age and surgical route” (Obstet Gynecol. 2018 Apr;131[4]:642-51).
As an alternative, Dr. Ridgeway pointed to hysteropexy, which can be performed as a vaginal, laparoscopic, robot, or open procedure.
She highlighted a 2018 systematic review of pelvic organ prolapse surgeries that provided a meta-analysis and clinical practice guidelines. It found that “uterine-preserving prolapse surgeries improve operating time, blood loss, and risk of mesh exposure, compared with similar surgical routes with concomitant hysterectomy and do not significantly change short-term prolapse outcomes” (Am J Obstet Gynecol. 2018 Aug;219[2]:129-46.e2).
Dr. Ridgeway reported no relevant disclosures. This meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
LAS VEGAS – A female pelvic medicine and reconstructive surgeon urged colleagues to consider uterus-sparing hysteropexies instead of hysterectomies in pelvic organ prolapse repairs.
said Beri M. Ridgeway, MD, of the Cleveland Clinic, at the Pelvic Anatomy and Gynecologic Surgery Symposium. Even so, “in the U.S., gynecologists rarely offer uterine preservation for women who desire repair of their uterovaginal prolapse.”
According to research compiled by Dr. Ridgeway, about 74,000 hysterectomies are performed each year in the United States to treat pelvic organ prolapse. The procedure became standard in the second half of the 20th century, in part to reduce cancer risk.
But attitudes evolved starting in the 1990s “as we have had better cancer screening and more focus on patient sexuality, patient autonomy, and quality of life,” Dr. Ridgeway said.
She offered these reasons to question hysterectomies to treat pelvic organ prolapse repairs:
- It’s not clear whether hysterectomies address the anatomic problems that produce prolapse in the first place. “Prolapse is caused by weakened or damaged tissue – connective tissue, muscles, etc.,” she said in an interview. “The problem is what is supporting the uterus, not the uterus itself.”
- Despite assumptions, women don’t necessarily prefer hysterectomy. Dr. Ridgeway pointed to a 2013 study in which researchers surveyed 213 women with prolapse symptoms about their preferred treatment, assuming that outcomes were the same. The results: 36% preferred uterine preservation, 20% preferred hysterectomy, and 44% reported no strong preference (Am J Obstet Gynecol. 2013 Nov;209[5]:470.e1-6.).
- Hysterectomies hasten menopause.
There has been a perception that uterus removal is appropriate in women who don’t wish to have any more children, Dr. Ridgeway said. “You had your babies, you’re done, you don’t need this anymore.” In fact, “that’s basically not true.”
As she explained, hysterectomy is linked to earlier menopause, and “even losing one ovary pushed patients into menopause significantly earlier.” She pointed to a 2016 Australian study, which found that “women who have a hysterectomy (with ovarian conservation) have a higher risk of hot flushes and night sweats that persist over an extended period” (Maturitas. 2016 Sep;91:1-7).
There’s no consensus on how hysterectomy affects sexual function. However, Dr. Ridgeway noted, it’s clear that pelvic floor disorders disrupt sexual function, and most women see improvement after surgical treatment.
The rate of uterine pathology is low in hysterectomy. Dr. Ridgeway highlighted a 2018 study of 24,076 women who underwent hysterectomy for benign indications. The study reported that “prevalence of occult corpus uteri, cervical, and ovarian malignancy was 1.44%, 0.60%, and 0.19%, respectively, among women undergoing hysterectomy and it varied by patient age and surgical route” (Obstet Gynecol. 2018 Apr;131[4]:642-51).
As an alternative, Dr. Ridgeway pointed to hysteropexy, which can be performed as a vaginal, laparoscopic, robot, or open procedure.
She highlighted a 2018 systematic review of pelvic organ prolapse surgeries that provided a meta-analysis and clinical practice guidelines. It found that “uterine-preserving prolapse surgeries improve operating time, blood loss, and risk of mesh exposure, compared with similar surgical routes with concomitant hysterectomy and do not significantly change short-term prolapse outcomes” (Am J Obstet Gynecol. 2018 Aug;219[2]:129-46.e2).
Dr. Ridgeway reported no relevant disclosures. This meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
LAS VEGAS – A female pelvic medicine and reconstructive surgeon urged colleagues to consider uterus-sparing hysteropexies instead of hysterectomies in pelvic organ prolapse repairs.
said Beri M. Ridgeway, MD, of the Cleveland Clinic, at the Pelvic Anatomy and Gynecologic Surgery Symposium. Even so, “in the U.S., gynecologists rarely offer uterine preservation for women who desire repair of their uterovaginal prolapse.”
According to research compiled by Dr. Ridgeway, about 74,000 hysterectomies are performed each year in the United States to treat pelvic organ prolapse. The procedure became standard in the second half of the 20th century, in part to reduce cancer risk.
But attitudes evolved starting in the 1990s “as we have had better cancer screening and more focus on patient sexuality, patient autonomy, and quality of life,” Dr. Ridgeway said.
She offered these reasons to question hysterectomies to treat pelvic organ prolapse repairs:
- It’s not clear whether hysterectomies address the anatomic problems that produce prolapse in the first place. “Prolapse is caused by weakened or damaged tissue – connective tissue, muscles, etc.,” she said in an interview. “The problem is what is supporting the uterus, not the uterus itself.”
- Despite assumptions, women don’t necessarily prefer hysterectomy. Dr. Ridgeway pointed to a 2013 study in which researchers surveyed 213 women with prolapse symptoms about their preferred treatment, assuming that outcomes were the same. The results: 36% preferred uterine preservation, 20% preferred hysterectomy, and 44% reported no strong preference (Am J Obstet Gynecol. 2013 Nov;209[5]:470.e1-6.).
- Hysterectomies hasten menopause.
There has been a perception that uterus removal is appropriate in women who don’t wish to have any more children, Dr. Ridgeway said. “You had your babies, you’re done, you don’t need this anymore.” In fact, “that’s basically not true.”
As she explained, hysterectomy is linked to earlier menopause, and “even losing one ovary pushed patients into menopause significantly earlier.” She pointed to a 2016 Australian study, which found that “women who have a hysterectomy (with ovarian conservation) have a higher risk of hot flushes and night sweats that persist over an extended period” (Maturitas. 2016 Sep;91:1-7).
There’s no consensus on how hysterectomy affects sexual function. However, Dr. Ridgeway noted, it’s clear that pelvic floor disorders disrupt sexual function, and most women see improvement after surgical treatment.
The rate of uterine pathology is low in hysterectomy. Dr. Ridgeway highlighted a 2018 study of 24,076 women who underwent hysterectomy for benign indications. The study reported that “prevalence of occult corpus uteri, cervical, and ovarian malignancy was 1.44%, 0.60%, and 0.19%, respectively, among women undergoing hysterectomy and it varied by patient age and surgical route” (Obstet Gynecol. 2018 Apr;131[4]:642-51).
As an alternative, Dr. Ridgeway pointed to hysteropexy, which can be performed as a vaginal, laparoscopic, robot, or open procedure.
She highlighted a 2018 systematic review of pelvic organ prolapse surgeries that provided a meta-analysis and clinical practice guidelines. It found that “uterine-preserving prolapse surgeries improve operating time, blood loss, and risk of mesh exposure, compared with similar surgical routes with concomitant hysterectomy and do not significantly change short-term prolapse outcomes” (Am J Obstet Gynecol. 2018 Aug;219[2]:129-46.e2).
Dr. Ridgeway reported no relevant disclosures. This meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
EXPERT ANALYSIS FROM PAGS 2019
Draft ACR Takayasu’s guidelines: Surgery is the last resort
ATLANTA – One of the goals in soon-to-be-published Takayasu’s arteritis guidelines from the American College of Rheumatology is to wean patients off high-dose steroids once they are in remission.
This recommendation is in opposition to another option – namely, switching these patients to low-dose glucocorticoids. The idea is to prevent long-term side effects, particularly in children. The guidelines also recommend against escalating immunotherapy for asymptomatic increases in inflammatory markers and generally recommend against surgery – stenting in most cases – unless there is threat to life, limb, or organ and also if limb pain is so severe it cramps quality of life and dose escalation doesn’t get the job done. If surgery is planned, patients should be on perioperative steroids if there’s active disease.
It’s draft guidance for now, but it’s probably what the final document will say when it’s published in 2020, according to a presentation at the annual meeting of the American College of Rheumatology by one of the authors, Anisha Dua, MD, an associate professor of rheumatology at Northwestern University, Chicago. She gave a sneak preview at the meeting.
In general, severe, active Takayasu’s calls for high-dose oral steroids in conjunction with a nonsteroid immunosuppressive, such as methotrexate, azathioprine, leflunomide, or mycophenolate mofetil. There’s evidence that dual therapy gives a more durable remission and also reduces the need for steroids.
When that approach doesn’t do the trick, the next step is a tumor necrosis factor (TNF) inhibitor. There’s evidence for infliximab, adalimumab, certolizumab, and etanercept. Dr. Dua noted, “We still can consider” tocilizumab, but it failed to meet its primary endpoint in a randomized trial, and evidence for other biologics is sparse or nonexistent. “TNF inhibitors are the first line” for refractory disease, Dr. Dua said.
The steroid taper comes after 6-12 months of remission. Given their toxicity, “our goal for steroids is zero,” especially in pediatric populations. Even in remission, patients should have a clinical assessment, including inflammatory markers, every 3-12 months.
A rise in C-reactive protein or erythrocyte sedimentation rate, with no new symptoms, might be a reason for more frequent monitoring, but it’s not a reason to escalate immunosuppression. That should be kept in reserve for new vascular lesions, rapid progression on an old one, or worsening of organ or limb ischemia.
“We recommend [escalation] over surgical intervention” because patients often develop collateral circulation that solves the problem; it also gives the disease time to quiet down should the patient eventually go into surgery. Immediate surgery is reserved for organ or life-threatening disease, Dr. Dua said.
“Takayasu’s is different from other vasculitides in the sense that patients often present with certain nonspecific constitutional symptoms,” and there’s not a lot of pathology or histology to work with, “so we do tend to rely on imaging a lot,” Dr. Dua said.
Angiography has fallen out of favor because it’s invasive and exposes patients to radiation, among other problems. The field has moved to noninvasive imaging such as color Doppler ultrasound, CT angiography, magnetic resonance angiography, and PET CT.
“We do recommend regularly scheduled, noninvasive imaging every 6-12 months, in addition to the routine clinical assessment,” except in children with inactive disease; the risk of sedation outweighs the imaging benefit, Dr. Dua said.
In patients with single-vessel cranial or cervical stenosis, without symptoms, “we recommend medical over surgical management because of the risk of surgery. Surgery can be considered for multivessel involvement,” she said.
She and her colleagues also recommend medical management for renal artery stenosis, including antihypertensives and immunotherapy escalation for active disease. Surgery is considered for refractory hypertension or worsening kidney function
Dr. Dua is a primary investigator and adviser for Chemocentryx and an adviser for Novartis and AbbVie.
ATLANTA – One of the goals in soon-to-be-published Takayasu’s arteritis guidelines from the American College of Rheumatology is to wean patients off high-dose steroids once they are in remission.
This recommendation is in opposition to another option – namely, switching these patients to low-dose glucocorticoids. The idea is to prevent long-term side effects, particularly in children. The guidelines also recommend against escalating immunotherapy for asymptomatic increases in inflammatory markers and generally recommend against surgery – stenting in most cases – unless there is threat to life, limb, or organ and also if limb pain is so severe it cramps quality of life and dose escalation doesn’t get the job done. If surgery is planned, patients should be on perioperative steroids if there’s active disease.
It’s draft guidance for now, but it’s probably what the final document will say when it’s published in 2020, according to a presentation at the annual meeting of the American College of Rheumatology by one of the authors, Anisha Dua, MD, an associate professor of rheumatology at Northwestern University, Chicago. She gave a sneak preview at the meeting.
In general, severe, active Takayasu’s calls for high-dose oral steroids in conjunction with a nonsteroid immunosuppressive, such as methotrexate, azathioprine, leflunomide, or mycophenolate mofetil. There’s evidence that dual therapy gives a more durable remission and also reduces the need for steroids.
When that approach doesn’t do the trick, the next step is a tumor necrosis factor (TNF) inhibitor. There’s evidence for infliximab, adalimumab, certolizumab, and etanercept. Dr. Dua noted, “We still can consider” tocilizumab, but it failed to meet its primary endpoint in a randomized trial, and evidence for other biologics is sparse or nonexistent. “TNF inhibitors are the first line” for refractory disease, Dr. Dua said.
The steroid taper comes after 6-12 months of remission. Given their toxicity, “our goal for steroids is zero,” especially in pediatric populations. Even in remission, patients should have a clinical assessment, including inflammatory markers, every 3-12 months.
A rise in C-reactive protein or erythrocyte sedimentation rate, with no new symptoms, might be a reason for more frequent monitoring, but it’s not a reason to escalate immunosuppression. That should be kept in reserve for new vascular lesions, rapid progression on an old one, or worsening of organ or limb ischemia.
“We recommend [escalation] over surgical intervention” because patients often develop collateral circulation that solves the problem; it also gives the disease time to quiet down should the patient eventually go into surgery. Immediate surgery is reserved for organ or life-threatening disease, Dr. Dua said.
“Takayasu’s is different from other vasculitides in the sense that patients often present with certain nonspecific constitutional symptoms,” and there’s not a lot of pathology or histology to work with, “so we do tend to rely on imaging a lot,” Dr. Dua said.
Angiography has fallen out of favor because it’s invasive and exposes patients to radiation, among other problems. The field has moved to noninvasive imaging such as color Doppler ultrasound, CT angiography, magnetic resonance angiography, and PET CT.
“We do recommend regularly scheduled, noninvasive imaging every 6-12 months, in addition to the routine clinical assessment,” except in children with inactive disease; the risk of sedation outweighs the imaging benefit, Dr. Dua said.
In patients with single-vessel cranial or cervical stenosis, without symptoms, “we recommend medical over surgical management because of the risk of surgery. Surgery can be considered for multivessel involvement,” she said.
She and her colleagues also recommend medical management for renal artery stenosis, including antihypertensives and immunotherapy escalation for active disease. Surgery is considered for refractory hypertension or worsening kidney function
Dr. Dua is a primary investigator and adviser for Chemocentryx and an adviser for Novartis and AbbVie.
ATLANTA – One of the goals in soon-to-be-published Takayasu’s arteritis guidelines from the American College of Rheumatology is to wean patients off high-dose steroids once they are in remission.
This recommendation is in opposition to another option – namely, switching these patients to low-dose glucocorticoids. The idea is to prevent long-term side effects, particularly in children. The guidelines also recommend against escalating immunotherapy for asymptomatic increases in inflammatory markers and generally recommend against surgery – stenting in most cases – unless there is threat to life, limb, or organ and also if limb pain is so severe it cramps quality of life and dose escalation doesn’t get the job done. If surgery is planned, patients should be on perioperative steroids if there’s active disease.
It’s draft guidance for now, but it’s probably what the final document will say when it’s published in 2020, according to a presentation at the annual meeting of the American College of Rheumatology by one of the authors, Anisha Dua, MD, an associate professor of rheumatology at Northwestern University, Chicago. She gave a sneak preview at the meeting.
In general, severe, active Takayasu’s calls for high-dose oral steroids in conjunction with a nonsteroid immunosuppressive, such as methotrexate, azathioprine, leflunomide, or mycophenolate mofetil. There’s evidence that dual therapy gives a more durable remission and also reduces the need for steroids.
When that approach doesn’t do the trick, the next step is a tumor necrosis factor (TNF) inhibitor. There’s evidence for infliximab, adalimumab, certolizumab, and etanercept. Dr. Dua noted, “We still can consider” tocilizumab, but it failed to meet its primary endpoint in a randomized trial, and evidence for other biologics is sparse or nonexistent. “TNF inhibitors are the first line” for refractory disease, Dr. Dua said.
The steroid taper comes after 6-12 months of remission. Given their toxicity, “our goal for steroids is zero,” especially in pediatric populations. Even in remission, patients should have a clinical assessment, including inflammatory markers, every 3-12 months.
A rise in C-reactive protein or erythrocyte sedimentation rate, with no new symptoms, might be a reason for more frequent monitoring, but it’s not a reason to escalate immunosuppression. That should be kept in reserve for new vascular lesions, rapid progression on an old one, or worsening of organ or limb ischemia.
“We recommend [escalation] over surgical intervention” because patients often develop collateral circulation that solves the problem; it also gives the disease time to quiet down should the patient eventually go into surgery. Immediate surgery is reserved for organ or life-threatening disease, Dr. Dua said.
“Takayasu’s is different from other vasculitides in the sense that patients often present with certain nonspecific constitutional symptoms,” and there’s not a lot of pathology or histology to work with, “so we do tend to rely on imaging a lot,” Dr. Dua said.
Angiography has fallen out of favor because it’s invasive and exposes patients to radiation, among other problems. The field has moved to noninvasive imaging such as color Doppler ultrasound, CT angiography, magnetic resonance angiography, and PET CT.
“We do recommend regularly scheduled, noninvasive imaging every 6-12 months, in addition to the routine clinical assessment,” except in children with inactive disease; the risk of sedation outweighs the imaging benefit, Dr. Dua said.
In patients with single-vessel cranial or cervical stenosis, without symptoms, “we recommend medical over surgical management because of the risk of surgery. Surgery can be considered for multivessel involvement,” she said.
She and her colleagues also recommend medical management for renal artery stenosis, including antihypertensives and immunotherapy escalation for active disease. Surgery is considered for refractory hypertension or worsening kidney function
Dr. Dua is a primary investigator and adviser for Chemocentryx and an adviser for Novartis and AbbVie.
REPORTING FROM ACR 2019
Beware nerve injuries in laparoscopic surgery
LAS VEGAS – , and it’s not just nerves below the waist that are vulnerable.
But the risk can be lowered through careful positioning that avoids pressure and over-stretching, the two main causes of nerve injuries in these surgeries, Cleveland Clinic obstetrician-gynecologist and surgeon Tommaso Falcone, MD, said at the Pelvic Anatomy and Gynecologic Surgery Symposium.
Dr. Falcone offered tips about avoiding injuries to these nerves:
Brachial plexus. To avoid injury to this nerve, arms should be tucked and pronated or kept at less than a 90-degree angle from the body. Hyperextension injuries can occur when the arm is outstretched too much, said Dr. Falcone, who recommends putting a cushion under the elbow and wrist.
How can you know if an injury has occurred? The patient will typically wake up with a dropped wrist and numbness in the arm, he said, signs suggesting a stretch injury to the brachial plexus.
Ulnar nerve: Improper tucking of the arms can injure the ulnar nerve and cause numbness and weakness in the fourth and fifth fingers. Dr. Falcone recommends keeping the arms pronated if they’re tucked and supinated if they’re on arm boards. Again, he emphasized placing a cushion under the elbow and wrist.
Femoral nerve: Hyperflexion or hyperextension can compress the femoral nerve under the inguinal ligament and cause it to become ischemic, Dr. Falcone said.
Femoral nerve injury can lead to numbness (in the anterior and medial thigh), decreased patellar reflex, weakness of the quadriceps, and loss of knee extension flexibility. Patients may need to use a wheelchair for a time and undergo physical therapy, he said.
Obdurator nerve: Excessive lateral thigh abduction – outward movement – can stretch this nerve, Dr. Falcone said. Avoid excessive abduction by keeping the thigh-to-thigh angle under 90 degrees, he recommended. (This rule also helps to prevent femoral nerve injury.)
Signs of injury can include numbness on the medial aspect of the thigh and weakness in the adductor muscles. Physical therapy can be helpful, he said.
Sciatic nerve: Beware of hyperflexion of the hips followed by sudden straightening of the knees, he said. According to him, this can occur when candy-cane stirrups are used. Signs of injury can include foot drop and numbness (calf, dorsum, sole, and lateral side of the foot).
Peroneal nerve: Prolonged pressure against the knee can injure this nerve and cause foot drop and numbness (over the lower anterior leg and dorsum of the foot).
Dr. Falcone reports honoraria (Journal of Minimally Invasive Gynecology and Up-To-Date) and federal research funds. This meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
LAS VEGAS – , and it’s not just nerves below the waist that are vulnerable.
But the risk can be lowered through careful positioning that avoids pressure and over-stretching, the two main causes of nerve injuries in these surgeries, Cleveland Clinic obstetrician-gynecologist and surgeon Tommaso Falcone, MD, said at the Pelvic Anatomy and Gynecologic Surgery Symposium.
Dr. Falcone offered tips about avoiding injuries to these nerves:
Brachial plexus. To avoid injury to this nerve, arms should be tucked and pronated or kept at less than a 90-degree angle from the body. Hyperextension injuries can occur when the arm is outstretched too much, said Dr. Falcone, who recommends putting a cushion under the elbow and wrist.
How can you know if an injury has occurred? The patient will typically wake up with a dropped wrist and numbness in the arm, he said, signs suggesting a stretch injury to the brachial plexus.
Ulnar nerve: Improper tucking of the arms can injure the ulnar nerve and cause numbness and weakness in the fourth and fifth fingers. Dr. Falcone recommends keeping the arms pronated if they’re tucked and supinated if they’re on arm boards. Again, he emphasized placing a cushion under the elbow and wrist.
Femoral nerve: Hyperflexion or hyperextension can compress the femoral nerve under the inguinal ligament and cause it to become ischemic, Dr. Falcone said.
Femoral nerve injury can lead to numbness (in the anterior and medial thigh), decreased patellar reflex, weakness of the quadriceps, and loss of knee extension flexibility. Patients may need to use a wheelchair for a time and undergo physical therapy, he said.
Obdurator nerve: Excessive lateral thigh abduction – outward movement – can stretch this nerve, Dr. Falcone said. Avoid excessive abduction by keeping the thigh-to-thigh angle under 90 degrees, he recommended. (This rule also helps to prevent femoral nerve injury.)
Signs of injury can include numbness on the medial aspect of the thigh and weakness in the adductor muscles. Physical therapy can be helpful, he said.
Sciatic nerve: Beware of hyperflexion of the hips followed by sudden straightening of the knees, he said. According to him, this can occur when candy-cane stirrups are used. Signs of injury can include foot drop and numbness (calf, dorsum, sole, and lateral side of the foot).
Peroneal nerve: Prolonged pressure against the knee can injure this nerve and cause foot drop and numbness (over the lower anterior leg and dorsum of the foot).
Dr. Falcone reports honoraria (Journal of Minimally Invasive Gynecology and Up-To-Date) and federal research funds. This meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
LAS VEGAS – , and it’s not just nerves below the waist that are vulnerable.
But the risk can be lowered through careful positioning that avoids pressure and over-stretching, the two main causes of nerve injuries in these surgeries, Cleveland Clinic obstetrician-gynecologist and surgeon Tommaso Falcone, MD, said at the Pelvic Anatomy and Gynecologic Surgery Symposium.
Dr. Falcone offered tips about avoiding injuries to these nerves:
Brachial plexus. To avoid injury to this nerve, arms should be tucked and pronated or kept at less than a 90-degree angle from the body. Hyperextension injuries can occur when the arm is outstretched too much, said Dr. Falcone, who recommends putting a cushion under the elbow and wrist.
How can you know if an injury has occurred? The patient will typically wake up with a dropped wrist and numbness in the arm, he said, signs suggesting a stretch injury to the brachial plexus.
Ulnar nerve: Improper tucking of the arms can injure the ulnar nerve and cause numbness and weakness in the fourth and fifth fingers. Dr. Falcone recommends keeping the arms pronated if they’re tucked and supinated if they’re on arm boards. Again, he emphasized placing a cushion under the elbow and wrist.
Femoral nerve: Hyperflexion or hyperextension can compress the femoral nerve under the inguinal ligament and cause it to become ischemic, Dr. Falcone said.
Femoral nerve injury can lead to numbness (in the anterior and medial thigh), decreased patellar reflex, weakness of the quadriceps, and loss of knee extension flexibility. Patients may need to use a wheelchair for a time and undergo physical therapy, he said.
Obdurator nerve: Excessive lateral thigh abduction – outward movement – can stretch this nerve, Dr. Falcone said. Avoid excessive abduction by keeping the thigh-to-thigh angle under 90 degrees, he recommended. (This rule also helps to prevent femoral nerve injury.)
Signs of injury can include numbness on the medial aspect of the thigh and weakness in the adductor muscles. Physical therapy can be helpful, he said.
Sciatic nerve: Beware of hyperflexion of the hips followed by sudden straightening of the knees, he said. According to him, this can occur when candy-cane stirrups are used. Signs of injury can include foot drop and numbness (calf, dorsum, sole, and lateral side of the foot).
Peroneal nerve: Prolonged pressure against the knee can injure this nerve and cause foot drop and numbness (over the lower anterior leg and dorsum of the foot).
Dr. Falcone reports honoraria (Journal of Minimally Invasive Gynecology and Up-To-Date) and federal research funds. This meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
EXPERT ANALYSIS FROM PAGS 2019