User login
Uterine balloon tamponade found safe in postpartum hemorrhage
A new study summarizing and reanalyzing the .
Of 90 studies that reported efficacy data for uterine balloon tamponade (UBT), the procedure had overall success of 85.9% in treating postpartum hemorrhage (PPH). The pooled success rate was highest for women who were treated with a condom UBT, at 90.4%, compared with those treated with a Bakri balloon, at 83.2%, though the one randomized trial that compared the two devices head-to-head found no difference in success rates, wrote Sebastian Suarez, MD, and coauthors.
In all, the investigators looked at 91 studies involving 4,729 women who sustained PPH. The systematic review and meta-analysis included randomized controlled trials (RCTs), nonrandomized studies, and case series in which UBT was used to treat PPH.
Dr. Suarez, of Boston University Medical Center, and colleagues explained that postpartum hemorrhage (PPH) accounts for more maternal mortality and morbidity worldwide than any other complication of pregnancy, with the vast majority of PPH deaths occurring in low- and middle-income countries.
“While treatment of PPH varies depending on the cause, generally less invasive methods should be tried initially,” commented Angela Martin, MD, a maternal-fetal medicine specialist at the University of Kansas, Lawrence,in interview. Dr. Martin, who was not involved in the study, explained that “these options typically include administration of uterotonics or pharmacologic agents, and tamponade of the uterus with intrauterine balloons.” The hope in using less invasive options is that pelvic artery embolization, other surgical techniques, or even hysterectomy can be avoided in the face of the emergency of severe PPH.
One retrospective, nonrandomized study compared UBT plus standard of care with standard of care alone for uterine atony after vaginal delivery. The study found significantly less blood loss (759 mL vs. 1,582 mL) and a 0.22 relative risk of surgical interventions and 0.18 relative risk of blood transfusion for women receiving UBT. However, the authors assessed the evidence for UBT in this study to be of very low quality. Two other RCTs compared UBT and no UBT, and the authors’ meta-analysis of these two studies showed no significant differences between the two groups in risk of maternal death or surgical interventions. The evidence was considered very low quality in these studies as well.
UBT was also examined in uterine atony after Cesarean delivery; a subgroup analysis found overall less efficacy than that in cases of vaginal delivery. Other subgroup analyses found that UBT was more likely to be successful when uterine atony or placenta previa was the cause of PPH, compared with PPH from placenta accreta spectrum or retained products of conception. Also, the overall success of UBT was higher for PPH in vaginal delivery, compared with Cesarean delivery, at 87% versus 81%, regardless of the etiology of hemorrhage.
Looking at safety of UBT, Dr. Suarez and coinvestigators found 39 studies reporting various complications of UBT use for PPH, not all of which reported on all complications. The overall rate for fever or infection was 6.5% in studies reporting on this complication, and endometritis was recorded in 2.3% of participants in studies tracking that complication. Cervical tears, laceration of the lower segment of the vagina, uterine incision rupture or uterine perforation, and acute colonic pseudo-obstruction were all reported in 2% or less of the patients participating in studies that recorded these complications.
The authors excluded studies that included simultaneous use of surgical techniques and UBT and those that involved UBT for hemorrhage after pregnancy loss with a gestation less than 20 weeks’ duration. However, studies were included if UBT was used after surgical procedure failure.
To assess the primary outcome of UBT success rate, the authors used the raw ratio of cases of success divided by the total number of women treated with UBT. For the analysis, successful UBT use was considered to be arrest of PPH bleeding without maternal death or other surgical or radiological interventions after UBT placement, regardless of the definition of “UBT success” used in each study. Similarly, the authors considered “UBT failure” to have occurred in cases of maternal death or when additional surgical or radiological interventions happened after UBT placement.
The authors considered a composite primary outcome measure for the RCT and nonrandomized studies that was made up of maternal death and/or surgical or radiologic interventions.
Secondary outcome measures included UBT’s success rate for individual PPH causes, frequency of surgical and invasive procedures, and maternal outcomes such as death, blood loss, transfusion, ICU admission, and complication rates.
Overall, about half of the studies (n = 48; 53%) were conducted in low- and middle-income countries. Asian countries were the site of 46 studies, or 52% of the total. A quarter were conducted in Europe, and just five studies were conducted in the United States; the remainder were conducted in Africa or Latin America or were multinational studies.
Dr. Martin said that “[the review] findings provide reassurance that UBT can be implemented as a treatment option with a high success rate and low complication rate.” However, she noted, “There was a discrepancy between nonrandomized studies and RCTs on the efficacy and effectiveness of UBT.”
“Two randomized studies concluded there is no benefit to introduction of UBT in management of refractory PPH,” she continued. “The authors point out risk of bias and multiple methodological concerns that likely favored the control group in one effectiveness trial. Lack of benefit may have been due to suboptimal implementation strategies and lack of consistent UBT use.”
Dr. Martin concluded, “Overall, UBT success rates were consistently high across all study types. These findings are reassuring to the practicing clinician. There are many benefits to UBT including ease of use by a variety of health care providers, affordability, and its minimally invasive nature. Now there is evidence that UBT appears safe and has a high rate of success for management of PPH.”
The study’s senior author is a board member of the nonprofit organization Ujenzi Charitable Trust, which received Food and Drug Administration approval for the “Every Second Matters–Uterine Balloon Tamponade” device. Dr. Suarez reported that he had no financial conflicts of interest. The authors reported that there were no external sources of funding for the research. Dr. Martin serves on the editorial board of Ob.Gyn. News.
SOURCE: Suarez S et al. Am J Obstet Gynecol. 2020 Jan 6. doi: 10.1016/j.ajog.2019.11.1287.
A new study summarizing and reanalyzing the .
Of 90 studies that reported efficacy data for uterine balloon tamponade (UBT), the procedure had overall success of 85.9% in treating postpartum hemorrhage (PPH). The pooled success rate was highest for women who were treated with a condom UBT, at 90.4%, compared with those treated with a Bakri balloon, at 83.2%, though the one randomized trial that compared the two devices head-to-head found no difference in success rates, wrote Sebastian Suarez, MD, and coauthors.
In all, the investigators looked at 91 studies involving 4,729 women who sustained PPH. The systematic review and meta-analysis included randomized controlled trials (RCTs), nonrandomized studies, and case series in which UBT was used to treat PPH.
Dr. Suarez, of Boston University Medical Center, and colleagues explained that postpartum hemorrhage (PPH) accounts for more maternal mortality and morbidity worldwide than any other complication of pregnancy, with the vast majority of PPH deaths occurring in low- and middle-income countries.
“While treatment of PPH varies depending on the cause, generally less invasive methods should be tried initially,” commented Angela Martin, MD, a maternal-fetal medicine specialist at the University of Kansas, Lawrence,in interview. Dr. Martin, who was not involved in the study, explained that “these options typically include administration of uterotonics or pharmacologic agents, and tamponade of the uterus with intrauterine balloons.” The hope in using less invasive options is that pelvic artery embolization, other surgical techniques, or even hysterectomy can be avoided in the face of the emergency of severe PPH.
One retrospective, nonrandomized study compared UBT plus standard of care with standard of care alone for uterine atony after vaginal delivery. The study found significantly less blood loss (759 mL vs. 1,582 mL) and a 0.22 relative risk of surgical interventions and 0.18 relative risk of blood transfusion for women receiving UBT. However, the authors assessed the evidence for UBT in this study to be of very low quality. Two other RCTs compared UBT and no UBT, and the authors’ meta-analysis of these two studies showed no significant differences between the two groups in risk of maternal death or surgical interventions. The evidence was considered very low quality in these studies as well.
UBT was also examined in uterine atony after Cesarean delivery; a subgroup analysis found overall less efficacy than that in cases of vaginal delivery. Other subgroup analyses found that UBT was more likely to be successful when uterine atony or placenta previa was the cause of PPH, compared with PPH from placenta accreta spectrum or retained products of conception. Also, the overall success of UBT was higher for PPH in vaginal delivery, compared with Cesarean delivery, at 87% versus 81%, regardless of the etiology of hemorrhage.
Looking at safety of UBT, Dr. Suarez and coinvestigators found 39 studies reporting various complications of UBT use for PPH, not all of which reported on all complications. The overall rate for fever or infection was 6.5% in studies reporting on this complication, and endometritis was recorded in 2.3% of participants in studies tracking that complication. Cervical tears, laceration of the lower segment of the vagina, uterine incision rupture or uterine perforation, and acute colonic pseudo-obstruction were all reported in 2% or less of the patients participating in studies that recorded these complications.
The authors excluded studies that included simultaneous use of surgical techniques and UBT and those that involved UBT for hemorrhage after pregnancy loss with a gestation less than 20 weeks’ duration. However, studies were included if UBT was used after surgical procedure failure.
To assess the primary outcome of UBT success rate, the authors used the raw ratio of cases of success divided by the total number of women treated with UBT. For the analysis, successful UBT use was considered to be arrest of PPH bleeding without maternal death or other surgical or radiological interventions after UBT placement, regardless of the definition of “UBT success” used in each study. Similarly, the authors considered “UBT failure” to have occurred in cases of maternal death or when additional surgical or radiological interventions happened after UBT placement.
The authors considered a composite primary outcome measure for the RCT and nonrandomized studies that was made up of maternal death and/or surgical or radiologic interventions.
Secondary outcome measures included UBT’s success rate for individual PPH causes, frequency of surgical and invasive procedures, and maternal outcomes such as death, blood loss, transfusion, ICU admission, and complication rates.
Overall, about half of the studies (n = 48; 53%) were conducted in low- and middle-income countries. Asian countries were the site of 46 studies, or 52% of the total. A quarter were conducted in Europe, and just five studies were conducted in the United States; the remainder were conducted in Africa or Latin America or were multinational studies.
Dr. Martin said that “[the review] findings provide reassurance that UBT can be implemented as a treatment option with a high success rate and low complication rate.” However, she noted, “There was a discrepancy between nonrandomized studies and RCTs on the efficacy and effectiveness of UBT.”
“Two randomized studies concluded there is no benefit to introduction of UBT in management of refractory PPH,” she continued. “The authors point out risk of bias and multiple methodological concerns that likely favored the control group in one effectiveness trial. Lack of benefit may have been due to suboptimal implementation strategies and lack of consistent UBT use.”
Dr. Martin concluded, “Overall, UBT success rates were consistently high across all study types. These findings are reassuring to the practicing clinician. There are many benefits to UBT including ease of use by a variety of health care providers, affordability, and its minimally invasive nature. Now there is evidence that UBT appears safe and has a high rate of success for management of PPH.”
The study’s senior author is a board member of the nonprofit organization Ujenzi Charitable Trust, which received Food and Drug Administration approval for the “Every Second Matters–Uterine Balloon Tamponade” device. Dr. Suarez reported that he had no financial conflicts of interest. The authors reported that there were no external sources of funding for the research. Dr. Martin serves on the editorial board of Ob.Gyn. News.
SOURCE: Suarez S et al. Am J Obstet Gynecol. 2020 Jan 6. doi: 10.1016/j.ajog.2019.11.1287.
A new study summarizing and reanalyzing the .
Of 90 studies that reported efficacy data for uterine balloon tamponade (UBT), the procedure had overall success of 85.9% in treating postpartum hemorrhage (PPH). The pooled success rate was highest for women who were treated with a condom UBT, at 90.4%, compared with those treated with a Bakri balloon, at 83.2%, though the one randomized trial that compared the two devices head-to-head found no difference in success rates, wrote Sebastian Suarez, MD, and coauthors.
In all, the investigators looked at 91 studies involving 4,729 women who sustained PPH. The systematic review and meta-analysis included randomized controlled trials (RCTs), nonrandomized studies, and case series in which UBT was used to treat PPH.
Dr. Suarez, of Boston University Medical Center, and colleagues explained that postpartum hemorrhage (PPH) accounts for more maternal mortality and morbidity worldwide than any other complication of pregnancy, with the vast majority of PPH deaths occurring in low- and middle-income countries.
“While treatment of PPH varies depending on the cause, generally less invasive methods should be tried initially,” commented Angela Martin, MD, a maternal-fetal medicine specialist at the University of Kansas, Lawrence,in interview. Dr. Martin, who was not involved in the study, explained that “these options typically include administration of uterotonics or pharmacologic agents, and tamponade of the uterus with intrauterine balloons.” The hope in using less invasive options is that pelvic artery embolization, other surgical techniques, or even hysterectomy can be avoided in the face of the emergency of severe PPH.
One retrospective, nonrandomized study compared UBT plus standard of care with standard of care alone for uterine atony after vaginal delivery. The study found significantly less blood loss (759 mL vs. 1,582 mL) and a 0.22 relative risk of surgical interventions and 0.18 relative risk of blood transfusion for women receiving UBT. However, the authors assessed the evidence for UBT in this study to be of very low quality. Two other RCTs compared UBT and no UBT, and the authors’ meta-analysis of these two studies showed no significant differences between the two groups in risk of maternal death or surgical interventions. The evidence was considered very low quality in these studies as well.
UBT was also examined in uterine atony after Cesarean delivery; a subgroup analysis found overall less efficacy than that in cases of vaginal delivery. Other subgroup analyses found that UBT was more likely to be successful when uterine atony or placenta previa was the cause of PPH, compared with PPH from placenta accreta spectrum or retained products of conception. Also, the overall success of UBT was higher for PPH in vaginal delivery, compared with Cesarean delivery, at 87% versus 81%, regardless of the etiology of hemorrhage.
Looking at safety of UBT, Dr. Suarez and coinvestigators found 39 studies reporting various complications of UBT use for PPH, not all of which reported on all complications. The overall rate for fever or infection was 6.5% in studies reporting on this complication, and endometritis was recorded in 2.3% of participants in studies tracking that complication. Cervical tears, laceration of the lower segment of the vagina, uterine incision rupture or uterine perforation, and acute colonic pseudo-obstruction were all reported in 2% or less of the patients participating in studies that recorded these complications.
The authors excluded studies that included simultaneous use of surgical techniques and UBT and those that involved UBT for hemorrhage after pregnancy loss with a gestation less than 20 weeks’ duration. However, studies were included if UBT was used after surgical procedure failure.
To assess the primary outcome of UBT success rate, the authors used the raw ratio of cases of success divided by the total number of women treated with UBT. For the analysis, successful UBT use was considered to be arrest of PPH bleeding without maternal death or other surgical or radiological interventions after UBT placement, regardless of the definition of “UBT success” used in each study. Similarly, the authors considered “UBT failure” to have occurred in cases of maternal death or when additional surgical or radiological interventions happened after UBT placement.
The authors considered a composite primary outcome measure for the RCT and nonrandomized studies that was made up of maternal death and/or surgical or radiologic interventions.
Secondary outcome measures included UBT’s success rate for individual PPH causes, frequency of surgical and invasive procedures, and maternal outcomes such as death, blood loss, transfusion, ICU admission, and complication rates.
Overall, about half of the studies (n = 48; 53%) were conducted in low- and middle-income countries. Asian countries were the site of 46 studies, or 52% of the total. A quarter were conducted in Europe, and just five studies were conducted in the United States; the remainder were conducted in Africa or Latin America or were multinational studies.
Dr. Martin said that “[the review] findings provide reassurance that UBT can be implemented as a treatment option with a high success rate and low complication rate.” However, she noted, “There was a discrepancy between nonrandomized studies and RCTs on the efficacy and effectiveness of UBT.”
“Two randomized studies concluded there is no benefit to introduction of UBT in management of refractory PPH,” she continued. “The authors point out risk of bias and multiple methodological concerns that likely favored the control group in one effectiveness trial. Lack of benefit may have been due to suboptimal implementation strategies and lack of consistent UBT use.”
Dr. Martin concluded, “Overall, UBT success rates were consistently high across all study types. These findings are reassuring to the practicing clinician. There are many benefits to UBT including ease of use by a variety of health care providers, affordability, and its minimally invasive nature. Now there is evidence that UBT appears safe and has a high rate of success for management of PPH.”
The study’s senior author is a board member of the nonprofit organization Ujenzi Charitable Trust, which received Food and Drug Administration approval for the “Every Second Matters–Uterine Balloon Tamponade” device. Dr. Suarez reported that he had no financial conflicts of interest. The authors reported that there were no external sources of funding for the research. Dr. Martin serves on the editorial board of Ob.Gyn. News.
SOURCE: Suarez S et al. Am J Obstet Gynecol. 2020 Jan 6. doi: 10.1016/j.ajog.2019.11.1287.
FROM AMERICAN JOURNAL OF OBSTETRICS AND GYNECOLOGY
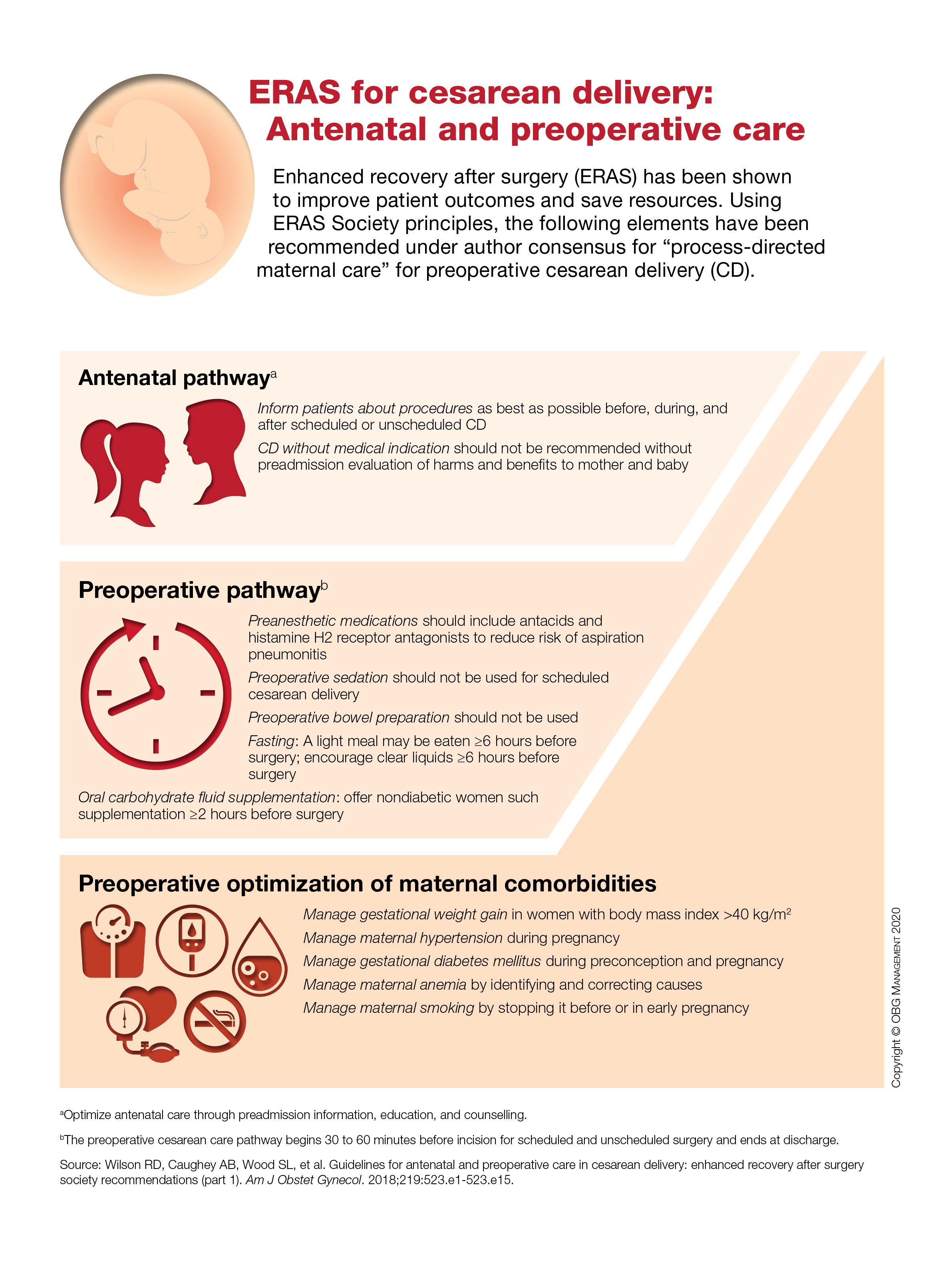
ERAS for cesarean delivery: Antenatal and preoperative care


Product update: Neuromodulation device, cystoscopy simplified, hysteroscopy seal, next immunization frontier
NEW SACRAL NEUROMODULATION DEVICE
FOR MORE INFORMATION, VISIT: https://www.axonics.com/
CERVICAL SEAL FOR HYSTEROSCOPIC DEVICES
For more information, visit: https://gynsurgicalsolutions.com/product/omni-lok/
UNIVERSAL CYSTOSCOPY SIMPLIFIED
FOR MORE INFORMATION, VISIT: https://cystosure.com/
NEXT FRONTIER IN VACCINE IMMUNIZATION
Globally, there are 410,000 cases of GBS every year. GBS is most common in newborns; women who are carriers of the GBS bacteria may pass it on to their newborns during labor and birth. An estimated 10% to 30% of pregnant women carry the GBS bacteria. The disease can manifest as sepsis, pneumonia, and meningitis, with potentially fatal outcomes for some. A maternal vaccine may prevent 231,000 infant and maternal GBS cases, says Pfizer.
According to Pfizer, RSV causes more hospitalizations each year than influenza among young children, with an estimated 33 million cases globally each year in children less than age 5 years.
FOR MORE INFORMATION, VISIT: https://www.pfizer.com/
NEW SACRAL NEUROMODULATION DEVICE
FOR MORE INFORMATION, VISIT: https://www.axonics.com/
CERVICAL SEAL FOR HYSTEROSCOPIC DEVICES
For more information, visit: https://gynsurgicalsolutions.com/product/omni-lok/
UNIVERSAL CYSTOSCOPY SIMPLIFIED
FOR MORE INFORMATION, VISIT: https://cystosure.com/
NEXT FRONTIER IN VACCINE IMMUNIZATION
Globally, there are 410,000 cases of GBS every year. GBS is most common in newborns; women who are carriers of the GBS bacteria may pass it on to their newborns during labor and birth. An estimated 10% to 30% of pregnant women carry the GBS bacteria. The disease can manifest as sepsis, pneumonia, and meningitis, with potentially fatal outcomes for some. A maternal vaccine may prevent 231,000 infant and maternal GBS cases, says Pfizer.
According to Pfizer, RSV causes more hospitalizations each year than influenza among young children, with an estimated 33 million cases globally each year in children less than age 5 years.
FOR MORE INFORMATION, VISIT: https://www.pfizer.com/
NEW SACRAL NEUROMODULATION DEVICE
FOR MORE INFORMATION, VISIT: https://www.axonics.com/
CERVICAL SEAL FOR HYSTEROSCOPIC DEVICES
For more information, visit: https://gynsurgicalsolutions.com/product/omni-lok/
UNIVERSAL CYSTOSCOPY SIMPLIFIED
FOR MORE INFORMATION, VISIT: https://cystosure.com/
NEXT FRONTIER IN VACCINE IMMUNIZATION
Globally, there are 410,000 cases of GBS every year. GBS is most common in newborns; women who are carriers of the GBS bacteria may pass it on to their newborns during labor and birth. An estimated 10% to 30% of pregnant women carry the GBS bacteria. The disease can manifest as sepsis, pneumonia, and meningitis, with potentially fatal outcomes for some. A maternal vaccine may prevent 231,000 infant and maternal GBS cases, says Pfizer.
According to Pfizer, RSV causes more hospitalizations each year than influenza among young children, with an estimated 33 million cases globally each year in children less than age 5 years.
FOR MORE INFORMATION, VISIT: https://www.pfizer.com/
Evidence builds for bariatric surgery’s role in cancer prevention
LAS VEGAS – The ability of bariatric surgery and substantial subsequent weight loss to cut the incidence of a variety of obesity-related cancers and other malignancies received further confirmation in results from two studies reported at a meeting presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery.
In one study, 2,107 adults enrolled in the Longitudinal Assessment of Bariatric Surgery (LABS-2) study showed a statistically significant halving of the cancer incidence during 7 years of follow-up in patients who underwent bariatric surgery and had a reduction of at least 20% in their presurgical body mass index (BMI), compared with patients in the study who underwent bariatric surgery but lost less weight, reported Andrea M. Stroud, MD, a bariatric surgeon at the Oregon Health & Science University, Portland.
In the second study, analysis of about 1.7 million hospitalized U.S. patients in the National Inpatient Sample showed that the incidence of an obesity-related cancer was 21% higher in more than 1.4 million obese individuals (BMI, 35 kg/m2 or greater) with no history of bariatric surgery, compared with nearly 247,000 people in the same database with a history of both obesity and bariatric surgery, said Juliana Henrique, MD, a bariatric surgeon at the Cleveland Clinic Florida in Weston.
The study reported by Dr. Henrique focused specifically on the 13 cancer types identified by the Centers for Disease Control and Prevention as having an incidence that links with overweight and obesity (Morb Mortal Wkly Rep. 2017;66[39]:1052-8), whereas the study presented by Dr. Stroud included all incident cancers during follow-up, but which were predominantly obesity related, with breast cancer – an obesity-related malignancy – having the highest incidence. Overall, 40% of all U.S. cancers in 2014 were obesity related, according to the CDC’s report.
“A number of studies have shown decreases in cancer rates after bariatric surgery, especially female cancers like breast and ovarian,” commented John Scott, MD, director of metabolic and bariatric surgery for Prism Health–Upstate in Greenville, S.C. “These two reports build on that.”
The evidence for weight loss after bariatric surgery as a means to cut the risk of a first or recurrent cancer has become strong enough for some patients to see cancer prophylaxis as a prime reason to undergo the procedure, said surgeons at the meeting.
Bariatric surgery and subsequent weight loss “is a substantial preventive factor for cancer, especially in patients who have obesity and diabetes,” commented Theresa LaMasters, MD, a bariatric surgeon in West Des Moines, Iowa. “It might not just be weight loss. It’s likely a multifactorial effect, including reduced inflammation after bariatric surgery, but weight loss is a component” of the effect, Dr. LaMasters said in an interview. It is now common for her to see patients seeking bariatric surgery because of a family or personal history of cancer. “Patients are trying to reduce their future risk” for cancer with bariatric surgery, she added.
The LABS-2 study enrolled 2,458 patients who were part of the first LABS cohort, LABS-1, but followed them longer term. The data Dr. Stroud reported came from 2,107 of the LABS-2 patients without a history of cancer, no cancer diagnosed in the first year after bariatric surgery, and longer-term follow-up of 7 years. About three-quarters of the patients underwent gastric bypass, with the rest undergoing laparoscopic gastric band placement. Nearly half of those included had diabetes. Their average BMI was 45-50 kg/m2.
Dr. Stroud and associates ran an analysis that divided the populations into tertiles based on percentage of baseline body mass lost at 12 months after surgery and cancer-free survival during the 7 years after the 12-month follow-up. The incidence of cancer was 51% lower in patients who lost 20%-34% of their BMI, compared with those who lost less than 20%, a statistically significant difference, and patients who lost 35% or more of their BMI had a 31% reduced cancer rate, compared with those who lost less than 20%, a difference that was not statistically significant, Dr. Stroud reported. The patients who lost less weight after surgery mostly underwent gastric banding, whereas those who lost more mostly underwent gastric bypass.
The analysis reported by Dr. Henrique used data collected in the U.S. National Inpatient Sample during 2010-2014, which totaled more than 7 million patients hospitalized for cancer, including 1,423,367 with a history of obesity and 246,668 with obesity who had undergone bariatric surgery. Those without bariatric surgery had a 21% higher rate of developing obesity-related cancers after adjustment for many baseline demographic and clinical features, Dr. Henrique said. The cancer protection after bariatric surgery was especially notable in the subset of patients in the sample with a genetic predisposition to developing cancer.
LABS-1 and LABS-2 were funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Stroud and Dr. Henrique had no disclosures.
SOURCES: Stroud AM et al. Obesity Week, Abstract A107; Henrique J et al. Obesity Week, Abstract A108.
LAS VEGAS – The ability of bariatric surgery and substantial subsequent weight loss to cut the incidence of a variety of obesity-related cancers and other malignancies received further confirmation in results from two studies reported at a meeting presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery.
In one study, 2,107 adults enrolled in the Longitudinal Assessment of Bariatric Surgery (LABS-2) study showed a statistically significant halving of the cancer incidence during 7 years of follow-up in patients who underwent bariatric surgery and had a reduction of at least 20% in their presurgical body mass index (BMI), compared with patients in the study who underwent bariatric surgery but lost less weight, reported Andrea M. Stroud, MD, a bariatric surgeon at the Oregon Health & Science University, Portland.
In the second study, analysis of about 1.7 million hospitalized U.S. patients in the National Inpatient Sample showed that the incidence of an obesity-related cancer was 21% higher in more than 1.4 million obese individuals (BMI, 35 kg/m2 or greater) with no history of bariatric surgery, compared with nearly 247,000 people in the same database with a history of both obesity and bariatric surgery, said Juliana Henrique, MD, a bariatric surgeon at the Cleveland Clinic Florida in Weston.
The study reported by Dr. Henrique focused specifically on the 13 cancer types identified by the Centers for Disease Control and Prevention as having an incidence that links with overweight and obesity (Morb Mortal Wkly Rep. 2017;66[39]:1052-8), whereas the study presented by Dr. Stroud included all incident cancers during follow-up, but which were predominantly obesity related, with breast cancer – an obesity-related malignancy – having the highest incidence. Overall, 40% of all U.S. cancers in 2014 were obesity related, according to the CDC’s report.
“A number of studies have shown decreases in cancer rates after bariatric surgery, especially female cancers like breast and ovarian,” commented John Scott, MD, director of metabolic and bariatric surgery for Prism Health–Upstate in Greenville, S.C. “These two reports build on that.”
The evidence for weight loss after bariatric surgery as a means to cut the risk of a first or recurrent cancer has become strong enough for some patients to see cancer prophylaxis as a prime reason to undergo the procedure, said surgeons at the meeting.
Bariatric surgery and subsequent weight loss “is a substantial preventive factor for cancer, especially in patients who have obesity and diabetes,” commented Theresa LaMasters, MD, a bariatric surgeon in West Des Moines, Iowa. “It might not just be weight loss. It’s likely a multifactorial effect, including reduced inflammation after bariatric surgery, but weight loss is a component” of the effect, Dr. LaMasters said in an interview. It is now common for her to see patients seeking bariatric surgery because of a family or personal history of cancer. “Patients are trying to reduce their future risk” for cancer with bariatric surgery, she added.
The LABS-2 study enrolled 2,458 patients who were part of the first LABS cohort, LABS-1, but followed them longer term. The data Dr. Stroud reported came from 2,107 of the LABS-2 patients without a history of cancer, no cancer diagnosed in the first year after bariatric surgery, and longer-term follow-up of 7 years. About three-quarters of the patients underwent gastric bypass, with the rest undergoing laparoscopic gastric band placement. Nearly half of those included had diabetes. Their average BMI was 45-50 kg/m2.
Dr. Stroud and associates ran an analysis that divided the populations into tertiles based on percentage of baseline body mass lost at 12 months after surgery and cancer-free survival during the 7 years after the 12-month follow-up. The incidence of cancer was 51% lower in patients who lost 20%-34% of their BMI, compared with those who lost less than 20%, a statistically significant difference, and patients who lost 35% or more of their BMI had a 31% reduced cancer rate, compared with those who lost less than 20%, a difference that was not statistically significant, Dr. Stroud reported. The patients who lost less weight after surgery mostly underwent gastric banding, whereas those who lost more mostly underwent gastric bypass.
The analysis reported by Dr. Henrique used data collected in the U.S. National Inpatient Sample during 2010-2014, which totaled more than 7 million patients hospitalized for cancer, including 1,423,367 with a history of obesity and 246,668 with obesity who had undergone bariatric surgery. Those without bariatric surgery had a 21% higher rate of developing obesity-related cancers after adjustment for many baseline demographic and clinical features, Dr. Henrique said. The cancer protection after bariatric surgery was especially notable in the subset of patients in the sample with a genetic predisposition to developing cancer.
LABS-1 and LABS-2 were funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Stroud and Dr. Henrique had no disclosures.
SOURCES: Stroud AM et al. Obesity Week, Abstract A107; Henrique J et al. Obesity Week, Abstract A108.
LAS VEGAS – The ability of bariatric surgery and substantial subsequent weight loss to cut the incidence of a variety of obesity-related cancers and other malignancies received further confirmation in results from two studies reported at a meeting presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery.
In one study, 2,107 adults enrolled in the Longitudinal Assessment of Bariatric Surgery (LABS-2) study showed a statistically significant halving of the cancer incidence during 7 years of follow-up in patients who underwent bariatric surgery and had a reduction of at least 20% in their presurgical body mass index (BMI), compared with patients in the study who underwent bariatric surgery but lost less weight, reported Andrea M. Stroud, MD, a bariatric surgeon at the Oregon Health & Science University, Portland.
In the second study, analysis of about 1.7 million hospitalized U.S. patients in the National Inpatient Sample showed that the incidence of an obesity-related cancer was 21% higher in more than 1.4 million obese individuals (BMI, 35 kg/m2 or greater) with no history of bariatric surgery, compared with nearly 247,000 people in the same database with a history of both obesity and bariatric surgery, said Juliana Henrique, MD, a bariatric surgeon at the Cleveland Clinic Florida in Weston.
The study reported by Dr. Henrique focused specifically on the 13 cancer types identified by the Centers for Disease Control and Prevention as having an incidence that links with overweight and obesity (Morb Mortal Wkly Rep. 2017;66[39]:1052-8), whereas the study presented by Dr. Stroud included all incident cancers during follow-up, but which were predominantly obesity related, with breast cancer – an obesity-related malignancy – having the highest incidence. Overall, 40% of all U.S. cancers in 2014 were obesity related, according to the CDC’s report.
“A number of studies have shown decreases in cancer rates after bariatric surgery, especially female cancers like breast and ovarian,” commented John Scott, MD, director of metabolic and bariatric surgery for Prism Health–Upstate in Greenville, S.C. “These two reports build on that.”
The evidence for weight loss after bariatric surgery as a means to cut the risk of a first or recurrent cancer has become strong enough for some patients to see cancer prophylaxis as a prime reason to undergo the procedure, said surgeons at the meeting.
Bariatric surgery and subsequent weight loss “is a substantial preventive factor for cancer, especially in patients who have obesity and diabetes,” commented Theresa LaMasters, MD, a bariatric surgeon in West Des Moines, Iowa. “It might not just be weight loss. It’s likely a multifactorial effect, including reduced inflammation after bariatric surgery, but weight loss is a component” of the effect, Dr. LaMasters said in an interview. It is now common for her to see patients seeking bariatric surgery because of a family or personal history of cancer. “Patients are trying to reduce their future risk” for cancer with bariatric surgery, she added.
The LABS-2 study enrolled 2,458 patients who were part of the first LABS cohort, LABS-1, but followed them longer term. The data Dr. Stroud reported came from 2,107 of the LABS-2 patients without a history of cancer, no cancer diagnosed in the first year after bariatric surgery, and longer-term follow-up of 7 years. About three-quarters of the patients underwent gastric bypass, with the rest undergoing laparoscopic gastric band placement. Nearly half of those included had diabetes. Their average BMI was 45-50 kg/m2.
Dr. Stroud and associates ran an analysis that divided the populations into tertiles based on percentage of baseline body mass lost at 12 months after surgery and cancer-free survival during the 7 years after the 12-month follow-up. The incidence of cancer was 51% lower in patients who lost 20%-34% of their BMI, compared with those who lost less than 20%, a statistically significant difference, and patients who lost 35% or more of their BMI had a 31% reduced cancer rate, compared with those who lost less than 20%, a difference that was not statistically significant, Dr. Stroud reported. The patients who lost less weight after surgery mostly underwent gastric banding, whereas those who lost more mostly underwent gastric bypass.
The analysis reported by Dr. Henrique used data collected in the U.S. National Inpatient Sample during 2010-2014, which totaled more than 7 million patients hospitalized for cancer, including 1,423,367 with a history of obesity and 246,668 with obesity who had undergone bariatric surgery. Those without bariatric surgery had a 21% higher rate of developing obesity-related cancers after adjustment for many baseline demographic and clinical features, Dr. Henrique said. The cancer protection after bariatric surgery was especially notable in the subset of patients in the sample with a genetic predisposition to developing cancer.
LABS-1 and LABS-2 were funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Stroud and Dr. Henrique had no disclosures.
SOURCES: Stroud AM et al. Obesity Week, Abstract A107; Henrique J et al. Obesity Week, Abstract A108.
REPORTING FROM OBESITY WEEK 2019
Should secondary cytoreduction be performed for platinum-sensitive recurrent ovarian cancer?
Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
EXPERT COMMENTARY
Ovarian cancer represents the most lethal gynecologic cancer, with an estimated 14,000 deaths in 2019.1 While the incidence of this disease is low in comparison to uterine cancer, the advanced stage at diagnosis portends poor prognosis. While stage is an independent risk factor for death, it is also a risk for recurrence, with more than 80% of women developing recurrent disease.2-4 Secondary cytoreduction remains an option for patients in which disease recurs; up until now this management option was driven by retrospective data.5
Details of the study
Coleman and colleagues conducted the Gynecologic Oncology Group (GOG) 0213 trial—a phase 3, multicenter, randomized clinical trial that included 485 women with recurrent ovarian cancer. The surgical objective of the trial was to determine whether secondary cytoreduction in operable, platinum-sensitive (PS) patients improved overall survival (OS).
Patients were eligible to participate in the surgical portion of the trial if they had PS measurable disease and had the intention to achieve complete gross resection. Women with ascites, evidence of extraabdominal disease, and “diffuse carcinomatosis” were excluded. The primary and secondary end points were OS and progression-free survival (PFS), respectively.
Results. There were no statistical differences between the surgery and no surgery groups with regard to median OS (50.6 months vs 64.7 months, respectively; hazard ratio [HR], 1.29; 95% confidence interval [CI], 0.97–1.72) or median PFS (18.9 months vs 16.2 months; HR, 0.82; 95% CI, 0.66 to 1.01). When comparing patients in which complete gross resection was achieved (150 patients vs 245 who did not receive surgery), there was only a statistical difference in PFS in favor of the surgical group (22.4 months vs 16.2 months; HR, 0.62; 95% CI, 0.48–0.80).
Of note, 67% of the patients who received surgery (63% intention-to-treat) were debulked to complete gross resection. There were 33% more patients with extraabdominal disease (10% vs 7% of total patients in each group) and 15% more patients with upper abdominal disease (40% vs 33% of total patients in each group) included in the surgical group. Finally, the median time to chemotherapy was 40 days in the surgery group versus 7 days in the no surgery group.
Continue to: Study strengths and weaknesses...
Study strengths and weaknesses
The authors deserve to be commended for this well-designed and laborious trial, which is the first of its kind. The strength of the study is its randomized design producing level I data.
Study weaknesses include lack of reporting of BRCA status and the impact of receiving targeted therapies after the trial was over. It is well established that BRCA-mutated patients have an independent survival advantage, even when taking into account platinum sensitivity.6-8BRCA status of the study population is not specifically addressed in this paper. The authors noted in the first GOG 0213 trial publication, which assessed bevacizumab in the recurrent setting, that BRCA status has an impact on patient outcomes. Subsequently, they state that they do not report BRCA status because “…its independent effect on response to an anti-angiogenesis agent was unknown,” but it clearly would affect survival analysis if unbalanced between groups.9
Similarly, in the introduction to their study, Coleman and colleagues list availability of maintenance therapy, for instance poly ADP (adenosine diphosphate–ribose) polymerase (PARP) inhibitors, as rationale for conducting their trial. They subsequently cite this as a possible reason that the median overall survival was 3 times longer than expected. However, they provide no data on which patients received maintenance therapy, which again could have drastically affected survival outcomes.10 They do report in the supplementary information that, when stratifying those receiving bevacizumab adjuvantly during the trial, the median OS was comparable between the surgical and nonsurgical groups (58.5 months vs 61.7 months).
The authors discuss the presence of patient selection bias as a weakness in the study. Selection bias is evident in this trial (as in many surgical trials) because patients with a limited volume of disease were selected to participate over those with large-volume disease. It is reasonable to conclude that this study is likely selecting patients with less aggressive tumor biology, not only evident by low-volume disease at recurrence but also by the 20.4-month median platinum-free interval in the surgical group, which certainly affects the trial’s validity. Despite being considered PS, the disease biology in a patient with a platinum-free interval of 20.4 months is surely different from the disease biology in a patient with a 6.4-month platinum-free interval; therefore, it is difficult to generalize these data to all PS recurrent ovarian cancer patients. Similarly, other research has suggested strict selection criteria, which was not apparent in this study’s methodology.11 While the number of metastatic sites were relatively equal between the surgery and no surgery groups, there were more patients in the surgical group with extraabdominal disease, which the authors used as an exclusion criterion.
Lastly, the time to treatment commencement in each arm, which was 40 days for the surgical arm and 7 days in the nonsurgical arm, could represent a flaw in this trial. While we expect a difference in duration to account for recovery time, many centers start chemotherapy as soon as 21 days after surgery, which is almost half of the median interval in the surgical group in this trial. While the authors address this by stating that they completed a landmark analysis, no data or information about what time points they used for the analysis are provided. They simply report an interquartile range of 28 to 51 days. It is hard to know what effect this may have had on the outcome.
This is the first randomized clinical trial conducted to assess whether secondary surgical cytoreduction is beneficial in PS recurrent ovarian cancer patients. It provides compelling evidence to critically evaluate whether surgical cytoreduction is appropriate in a similar patient population. However, we would recommend using caution applying these data to patients who have different platinum-free intervals or low-volume disease limited to the pelvis.
The trial is not without flaws, as the authors point out in their discussion, but currently, it is the best evidence afforded to gynecologic oncologists. There are multiple trials currently ongoing, including DESTOP-III, which had similar PFS results as GOG 0213. If consensus is reached with these 2 trials, we believe that secondary cytoreduction will be utilized far less often in patients with recurrent ovarian cancer and a long platinum-free interval, thereby changing the current treatment paradigm for these patients.
MICHAEL D. TOBONI, MD, MPH, AND DAVID G. MUTCH, MD
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34.
- Parmar MK, Ledermann JA, Colombo N, et al. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2.2 trial. Lancet. 2003;361:2099-2106.
- International Collaborative Ovarian Neoplasm Group. Paclitaxel plus carboplatin versus standard chemotherapy with either single-agent carboplatin or cyclophosphamide, doxorubicin, and cisplatin in women with ovarian cancer: the ICON3 randomised trial. Lancet. 2002;360:505-515.
- Mullen MM, Kuroki LM, Thaker PH. Novel treatment options in platinum-sensitive recurrent ovarian cancer: a review. Gynecol Oncol. 2019;152:416-425.
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: ovarian cancer. November 26, 2019. https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf. Accessed December 18, 2019.
- Cass I, Baldwin RL, Varkey T, et al. Improved survival in women with BRCA-associated ovarian carcinoma. Cancer. 2003;97:2187-2195.
- Gallagher DJ, Konner JA, Bell-McGuinn KM, et al. Survival in epithelial ovarian cancer: a multivariate analysis incorporating BRCA mutation status and platinum sensitivity. Ann Oncol. 2011;22:1127-1132.
- Sun C, Li N, Ding D, et al. The role of BRCA status on the prognosis of patients with epithelial ovarian cancer: a systematic review of the literature with a meta-analysis. PLoS One. 2014;9:e95285.
- Coleman RL, Brady MF, Herzog TJ, et al. Bevacizumab and paclitaxel-carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18:779-791.
- Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
- Chi DS, McCaughty K, Diaz JP, et al. Guidelines and selection criteria for secondary cytoreductive surgery in patients with recurrent, platinum-sensitive epithelial ovarian carcinoma. Cancer. 2006;106:1933-1939.
Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
EXPERT COMMENTARY
Ovarian cancer represents the most lethal gynecologic cancer, with an estimated 14,000 deaths in 2019.1 While the incidence of this disease is low in comparison to uterine cancer, the advanced stage at diagnosis portends poor prognosis. While stage is an independent risk factor for death, it is also a risk for recurrence, with more than 80% of women developing recurrent disease.2-4 Secondary cytoreduction remains an option for patients in which disease recurs; up until now this management option was driven by retrospective data.5
Details of the study
Coleman and colleagues conducted the Gynecologic Oncology Group (GOG) 0213 trial—a phase 3, multicenter, randomized clinical trial that included 485 women with recurrent ovarian cancer. The surgical objective of the trial was to determine whether secondary cytoreduction in operable, platinum-sensitive (PS) patients improved overall survival (OS).
Patients were eligible to participate in the surgical portion of the trial if they had PS measurable disease and had the intention to achieve complete gross resection. Women with ascites, evidence of extraabdominal disease, and “diffuse carcinomatosis” were excluded. The primary and secondary end points were OS and progression-free survival (PFS), respectively.
Results. There were no statistical differences between the surgery and no surgery groups with regard to median OS (50.6 months vs 64.7 months, respectively; hazard ratio [HR], 1.29; 95% confidence interval [CI], 0.97–1.72) or median PFS (18.9 months vs 16.2 months; HR, 0.82; 95% CI, 0.66 to 1.01). When comparing patients in which complete gross resection was achieved (150 patients vs 245 who did not receive surgery), there was only a statistical difference in PFS in favor of the surgical group (22.4 months vs 16.2 months; HR, 0.62; 95% CI, 0.48–0.80).
Of note, 67% of the patients who received surgery (63% intention-to-treat) were debulked to complete gross resection. There were 33% more patients with extraabdominal disease (10% vs 7% of total patients in each group) and 15% more patients with upper abdominal disease (40% vs 33% of total patients in each group) included in the surgical group. Finally, the median time to chemotherapy was 40 days in the surgery group versus 7 days in the no surgery group.
Continue to: Study strengths and weaknesses...
Study strengths and weaknesses
The authors deserve to be commended for this well-designed and laborious trial, which is the first of its kind. The strength of the study is its randomized design producing level I data.
Study weaknesses include lack of reporting of BRCA status and the impact of receiving targeted therapies after the trial was over. It is well established that BRCA-mutated patients have an independent survival advantage, even when taking into account platinum sensitivity.6-8BRCA status of the study population is not specifically addressed in this paper. The authors noted in the first GOG 0213 trial publication, which assessed bevacizumab in the recurrent setting, that BRCA status has an impact on patient outcomes. Subsequently, they state that they do not report BRCA status because “…its independent effect on response to an anti-angiogenesis agent was unknown,” but it clearly would affect survival analysis if unbalanced between groups.9
Similarly, in the introduction to their study, Coleman and colleagues list availability of maintenance therapy, for instance poly ADP (adenosine diphosphate–ribose) polymerase (PARP) inhibitors, as rationale for conducting their trial. They subsequently cite this as a possible reason that the median overall survival was 3 times longer than expected. However, they provide no data on which patients received maintenance therapy, which again could have drastically affected survival outcomes.10 They do report in the supplementary information that, when stratifying those receiving bevacizumab adjuvantly during the trial, the median OS was comparable between the surgical and nonsurgical groups (58.5 months vs 61.7 months).
The authors discuss the presence of patient selection bias as a weakness in the study. Selection bias is evident in this trial (as in many surgical trials) because patients with a limited volume of disease were selected to participate over those with large-volume disease. It is reasonable to conclude that this study is likely selecting patients with less aggressive tumor biology, not only evident by low-volume disease at recurrence but also by the 20.4-month median platinum-free interval in the surgical group, which certainly affects the trial’s validity. Despite being considered PS, the disease biology in a patient with a platinum-free interval of 20.4 months is surely different from the disease biology in a patient with a 6.4-month platinum-free interval; therefore, it is difficult to generalize these data to all PS recurrent ovarian cancer patients. Similarly, other research has suggested strict selection criteria, which was not apparent in this study’s methodology.11 While the number of metastatic sites were relatively equal between the surgery and no surgery groups, there were more patients in the surgical group with extraabdominal disease, which the authors used as an exclusion criterion.
Lastly, the time to treatment commencement in each arm, which was 40 days for the surgical arm and 7 days in the nonsurgical arm, could represent a flaw in this trial. While we expect a difference in duration to account for recovery time, many centers start chemotherapy as soon as 21 days after surgery, which is almost half of the median interval in the surgical group in this trial. While the authors address this by stating that they completed a landmark analysis, no data or information about what time points they used for the analysis are provided. They simply report an interquartile range of 28 to 51 days. It is hard to know what effect this may have had on the outcome.
This is the first randomized clinical trial conducted to assess whether secondary surgical cytoreduction is beneficial in PS recurrent ovarian cancer patients. It provides compelling evidence to critically evaluate whether surgical cytoreduction is appropriate in a similar patient population. However, we would recommend using caution applying these data to patients who have different platinum-free intervals or low-volume disease limited to the pelvis.
The trial is not without flaws, as the authors point out in their discussion, but currently, it is the best evidence afforded to gynecologic oncologists. There are multiple trials currently ongoing, including DESTOP-III, which had similar PFS results as GOG 0213. If consensus is reached with these 2 trials, we believe that secondary cytoreduction will be utilized far less often in patients with recurrent ovarian cancer and a long platinum-free interval, thereby changing the current treatment paradigm for these patients.
MICHAEL D. TOBONI, MD, MPH, AND DAVID G. MUTCH, MD
Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
EXPERT COMMENTARY
Ovarian cancer represents the most lethal gynecologic cancer, with an estimated 14,000 deaths in 2019.1 While the incidence of this disease is low in comparison to uterine cancer, the advanced stage at diagnosis portends poor prognosis. While stage is an independent risk factor for death, it is also a risk for recurrence, with more than 80% of women developing recurrent disease.2-4 Secondary cytoreduction remains an option for patients in which disease recurs; up until now this management option was driven by retrospective data.5
Details of the study
Coleman and colleagues conducted the Gynecologic Oncology Group (GOG) 0213 trial—a phase 3, multicenter, randomized clinical trial that included 485 women with recurrent ovarian cancer. The surgical objective of the trial was to determine whether secondary cytoreduction in operable, platinum-sensitive (PS) patients improved overall survival (OS).
Patients were eligible to participate in the surgical portion of the trial if they had PS measurable disease and had the intention to achieve complete gross resection. Women with ascites, evidence of extraabdominal disease, and “diffuse carcinomatosis” were excluded. The primary and secondary end points were OS and progression-free survival (PFS), respectively.
Results. There were no statistical differences between the surgery and no surgery groups with regard to median OS (50.6 months vs 64.7 months, respectively; hazard ratio [HR], 1.29; 95% confidence interval [CI], 0.97–1.72) or median PFS (18.9 months vs 16.2 months; HR, 0.82; 95% CI, 0.66 to 1.01). When comparing patients in which complete gross resection was achieved (150 patients vs 245 who did not receive surgery), there was only a statistical difference in PFS in favor of the surgical group (22.4 months vs 16.2 months; HR, 0.62; 95% CI, 0.48–0.80).
Of note, 67% of the patients who received surgery (63% intention-to-treat) were debulked to complete gross resection. There were 33% more patients with extraabdominal disease (10% vs 7% of total patients in each group) and 15% more patients with upper abdominal disease (40% vs 33% of total patients in each group) included in the surgical group. Finally, the median time to chemotherapy was 40 days in the surgery group versus 7 days in the no surgery group.
Continue to: Study strengths and weaknesses...
Study strengths and weaknesses
The authors deserve to be commended for this well-designed and laborious trial, which is the first of its kind. The strength of the study is its randomized design producing level I data.
Study weaknesses include lack of reporting of BRCA status and the impact of receiving targeted therapies after the trial was over. It is well established that BRCA-mutated patients have an independent survival advantage, even when taking into account platinum sensitivity.6-8BRCA status of the study population is not specifically addressed in this paper. The authors noted in the first GOG 0213 trial publication, which assessed bevacizumab in the recurrent setting, that BRCA status has an impact on patient outcomes. Subsequently, they state that they do not report BRCA status because “…its independent effect on response to an anti-angiogenesis agent was unknown,” but it clearly would affect survival analysis if unbalanced between groups.9
Similarly, in the introduction to their study, Coleman and colleagues list availability of maintenance therapy, for instance poly ADP (adenosine diphosphate–ribose) polymerase (PARP) inhibitors, as rationale for conducting their trial. They subsequently cite this as a possible reason that the median overall survival was 3 times longer than expected. However, they provide no data on which patients received maintenance therapy, which again could have drastically affected survival outcomes.10 They do report in the supplementary information that, when stratifying those receiving bevacizumab adjuvantly during the trial, the median OS was comparable between the surgical and nonsurgical groups (58.5 months vs 61.7 months).
The authors discuss the presence of patient selection bias as a weakness in the study. Selection bias is evident in this trial (as in many surgical trials) because patients with a limited volume of disease were selected to participate over those with large-volume disease. It is reasonable to conclude that this study is likely selecting patients with less aggressive tumor biology, not only evident by low-volume disease at recurrence but also by the 20.4-month median platinum-free interval in the surgical group, which certainly affects the trial’s validity. Despite being considered PS, the disease biology in a patient with a platinum-free interval of 20.4 months is surely different from the disease biology in a patient with a 6.4-month platinum-free interval; therefore, it is difficult to generalize these data to all PS recurrent ovarian cancer patients. Similarly, other research has suggested strict selection criteria, which was not apparent in this study’s methodology.11 While the number of metastatic sites were relatively equal between the surgery and no surgery groups, there were more patients in the surgical group with extraabdominal disease, which the authors used as an exclusion criterion.
Lastly, the time to treatment commencement in each arm, which was 40 days for the surgical arm and 7 days in the nonsurgical arm, could represent a flaw in this trial. While we expect a difference in duration to account for recovery time, many centers start chemotherapy as soon as 21 days after surgery, which is almost half of the median interval in the surgical group in this trial. While the authors address this by stating that they completed a landmark analysis, no data or information about what time points they used for the analysis are provided. They simply report an interquartile range of 28 to 51 days. It is hard to know what effect this may have had on the outcome.
This is the first randomized clinical trial conducted to assess whether secondary surgical cytoreduction is beneficial in PS recurrent ovarian cancer patients. It provides compelling evidence to critically evaluate whether surgical cytoreduction is appropriate in a similar patient population. However, we would recommend using caution applying these data to patients who have different platinum-free intervals or low-volume disease limited to the pelvis.
The trial is not without flaws, as the authors point out in their discussion, but currently, it is the best evidence afforded to gynecologic oncologists. There are multiple trials currently ongoing, including DESTOP-III, which had similar PFS results as GOG 0213. If consensus is reached with these 2 trials, we believe that secondary cytoreduction will be utilized far less often in patients with recurrent ovarian cancer and a long platinum-free interval, thereby changing the current treatment paradigm for these patients.
MICHAEL D. TOBONI, MD, MPH, AND DAVID G. MUTCH, MD
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34.
- Parmar MK, Ledermann JA, Colombo N, et al. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2.2 trial. Lancet. 2003;361:2099-2106.
- International Collaborative Ovarian Neoplasm Group. Paclitaxel plus carboplatin versus standard chemotherapy with either single-agent carboplatin or cyclophosphamide, doxorubicin, and cisplatin in women with ovarian cancer: the ICON3 randomised trial. Lancet. 2002;360:505-515.
- Mullen MM, Kuroki LM, Thaker PH. Novel treatment options in platinum-sensitive recurrent ovarian cancer: a review. Gynecol Oncol. 2019;152:416-425.
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: ovarian cancer. November 26, 2019. https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf. Accessed December 18, 2019.
- Cass I, Baldwin RL, Varkey T, et al. Improved survival in women with BRCA-associated ovarian carcinoma. Cancer. 2003;97:2187-2195.
- Gallagher DJ, Konner JA, Bell-McGuinn KM, et al. Survival in epithelial ovarian cancer: a multivariate analysis incorporating BRCA mutation status and platinum sensitivity. Ann Oncol. 2011;22:1127-1132.
- Sun C, Li N, Ding D, et al. The role of BRCA status on the prognosis of patients with epithelial ovarian cancer: a systematic review of the literature with a meta-analysis. PLoS One. 2014;9:e95285.
- Coleman RL, Brady MF, Herzog TJ, et al. Bevacizumab and paclitaxel-carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18:779-791.
- Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
- Chi DS, McCaughty K, Diaz JP, et al. Guidelines and selection criteria for secondary cytoreductive surgery in patients with recurrent, platinum-sensitive epithelial ovarian carcinoma. Cancer. 2006;106:1933-1939.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34.
- Parmar MK, Ledermann JA, Colombo N, et al. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2.2 trial. Lancet. 2003;361:2099-2106.
- International Collaborative Ovarian Neoplasm Group. Paclitaxel plus carboplatin versus standard chemotherapy with either single-agent carboplatin or cyclophosphamide, doxorubicin, and cisplatin in women with ovarian cancer: the ICON3 randomised trial. Lancet. 2002;360:505-515.
- Mullen MM, Kuroki LM, Thaker PH. Novel treatment options in platinum-sensitive recurrent ovarian cancer: a review. Gynecol Oncol. 2019;152:416-425.
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: ovarian cancer. November 26, 2019. https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf. Accessed December 18, 2019.
- Cass I, Baldwin RL, Varkey T, et al. Improved survival in women with BRCA-associated ovarian carcinoma. Cancer. 2003;97:2187-2195.
- Gallagher DJ, Konner JA, Bell-McGuinn KM, et al. Survival in epithelial ovarian cancer: a multivariate analysis incorporating BRCA mutation status and platinum sensitivity. Ann Oncol. 2011;22:1127-1132.
- Sun C, Li N, Ding D, et al. The role of BRCA status on the prognosis of patients with epithelial ovarian cancer: a systematic review of the literature with a meta-analysis. PLoS One. 2014;9:e95285.
- Coleman RL, Brady MF, Herzog TJ, et al. Bevacizumab and paclitaxel-carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18:779-791.
- Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
- Chi DS, McCaughty K, Diaz JP, et al. Guidelines and selection criteria for secondary cytoreductive surgery in patients with recurrent, platinum-sensitive epithelial ovarian carcinoma. Cancer. 2006;106:1933-1939.
Score predicts bariatric surgery’s benefits for obesity, type 2 diabetes
LAS VEGAS –
The Individualized Diabetes Complications risk score “can provide personalized, evidence-based risk information for patients with type 2 diabetes and obesity about their future cardiovascular disease outcomes and mortality with and without metabolic surgery,” Ali Aminian, MD, said at a meeting presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery.
Although the calculator needs validation in a prospective, randomized study to document its impact on practice, it is now available on two separate websites and as a downloadable app, said Dr. Aminian, a surgeon at the Cleveland Clinic.
The calculator inputs data for 26 distinct, “readily available” demographic and clinical entries, and based on that, estimates the patient’s 10-year risk for all-cause death, diabetic kidney disease, cerebrovascular disease, heart failure, and coronary artery disease if no surgery occurs or after some type of metabolic or bariatric surgery. The calculator does not currently have the ability to individualize predicted risks based on the specific type of metabolic surgery performed, but that is planned as a future refinement of the score.
“We validated the model in the nonsurgical patients, which showed it was very accurate. The next step is to run a randomized trial to see how useful the calculator is” for assisting in patients’ decision making, Dr. Aminian said.
The data for deriving the risk calculator, and for a preliminary validation of it, came from 13,722 patients with obesity (body mass index, 30 kg/m2 or greater) and type 2 diabetes, who were managed at the Cleveland Clinic during 1998-2017, drawn from more than 287,000 such patients in the clinic’s database. The study focused on 2,287 patients who underwent metabolic (bariatric) surgery and 11,435 patients from the same database who did not have surgery and matched by propensity scoring on a 5:1 basis with those who had surgery. The two cohorts this created matched well for age (about 54 years), sex (about two-thirds women), body mass index (about 44 kg/m2), and the prevalence of various comorbidities at baseline.
Dr. Aminian and associates then analyzed the incidence of all-cause mortality and various cardiovascular disease endpoints, as well as nephropathy during follow-up, through December 2018. Patients who had undergone metabolic surgery showed statistically significant reductions in the incidence of each of those events, compared with patients who did not have surgery (JAMA. 2019;322[13]:1271-82).
The investigators used these findings to create their model for calculating a patient’s risk score. For example, to calculate an estimate for the 10-year risk from all-cause mortality, the results showed that the most powerful risk factors were age; baseline body mass index, heart failure, and need for insulin; and smoking status. For the endpoint of nephropathy, the most important factors were estimated glomerular filtration rate at baseline and age. Identified risk factors could account for about 80% of the 10-year risk for all-cause death and for about 75% of the risk for developing nephropathy during 10 years, based on the area-under-the-curve values the model produced.
The risk score may help patients better understand the potential role that metabolic surgery can have in reducing their future event risk, thereby helping them better appreciate the benefit they stand to gain from undergoing surgery, Dr. Aminian said.
The calculator is available at a website maintained by the Cleveland Clinic, at a site of the American Society for Metabolic and Bariatric Surgery, and in app stores, he said.
The work was partially funded by Medtronic. Dr. Aminian has received grants from Medtronic.
SOURCE: Aminian A et al. Obesity Week 2019, Abstract A101.
LAS VEGAS –
The Individualized Diabetes Complications risk score “can provide personalized, evidence-based risk information for patients with type 2 diabetes and obesity about their future cardiovascular disease outcomes and mortality with and without metabolic surgery,” Ali Aminian, MD, said at a meeting presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery.
Although the calculator needs validation in a prospective, randomized study to document its impact on practice, it is now available on two separate websites and as a downloadable app, said Dr. Aminian, a surgeon at the Cleveland Clinic.
The calculator inputs data for 26 distinct, “readily available” demographic and clinical entries, and based on that, estimates the patient’s 10-year risk for all-cause death, diabetic kidney disease, cerebrovascular disease, heart failure, and coronary artery disease if no surgery occurs or after some type of metabolic or bariatric surgery. The calculator does not currently have the ability to individualize predicted risks based on the specific type of metabolic surgery performed, but that is planned as a future refinement of the score.
“We validated the model in the nonsurgical patients, which showed it was very accurate. The next step is to run a randomized trial to see how useful the calculator is” for assisting in patients’ decision making, Dr. Aminian said.
The data for deriving the risk calculator, and for a preliminary validation of it, came from 13,722 patients with obesity (body mass index, 30 kg/m2 or greater) and type 2 diabetes, who were managed at the Cleveland Clinic during 1998-2017, drawn from more than 287,000 such patients in the clinic’s database. The study focused on 2,287 patients who underwent metabolic (bariatric) surgery and 11,435 patients from the same database who did not have surgery and matched by propensity scoring on a 5:1 basis with those who had surgery. The two cohorts this created matched well for age (about 54 years), sex (about two-thirds women), body mass index (about 44 kg/m2), and the prevalence of various comorbidities at baseline.
Dr. Aminian and associates then analyzed the incidence of all-cause mortality and various cardiovascular disease endpoints, as well as nephropathy during follow-up, through December 2018. Patients who had undergone metabolic surgery showed statistically significant reductions in the incidence of each of those events, compared with patients who did not have surgery (JAMA. 2019;322[13]:1271-82).
The investigators used these findings to create their model for calculating a patient’s risk score. For example, to calculate an estimate for the 10-year risk from all-cause mortality, the results showed that the most powerful risk factors were age; baseline body mass index, heart failure, and need for insulin; and smoking status. For the endpoint of nephropathy, the most important factors were estimated glomerular filtration rate at baseline and age. Identified risk factors could account for about 80% of the 10-year risk for all-cause death and for about 75% of the risk for developing nephropathy during 10 years, based on the area-under-the-curve values the model produced.
The risk score may help patients better understand the potential role that metabolic surgery can have in reducing their future event risk, thereby helping them better appreciate the benefit they stand to gain from undergoing surgery, Dr. Aminian said.
The calculator is available at a website maintained by the Cleveland Clinic, at a site of the American Society for Metabolic and Bariatric Surgery, and in app stores, he said.
The work was partially funded by Medtronic. Dr. Aminian has received grants from Medtronic.
SOURCE: Aminian A et al. Obesity Week 2019, Abstract A101.
LAS VEGAS –
The Individualized Diabetes Complications risk score “can provide personalized, evidence-based risk information for patients with type 2 diabetes and obesity about their future cardiovascular disease outcomes and mortality with and without metabolic surgery,” Ali Aminian, MD, said at a meeting presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery.
Although the calculator needs validation in a prospective, randomized study to document its impact on practice, it is now available on two separate websites and as a downloadable app, said Dr. Aminian, a surgeon at the Cleveland Clinic.
The calculator inputs data for 26 distinct, “readily available” demographic and clinical entries, and based on that, estimates the patient’s 10-year risk for all-cause death, diabetic kidney disease, cerebrovascular disease, heart failure, and coronary artery disease if no surgery occurs or after some type of metabolic or bariatric surgery. The calculator does not currently have the ability to individualize predicted risks based on the specific type of metabolic surgery performed, but that is planned as a future refinement of the score.
“We validated the model in the nonsurgical patients, which showed it was very accurate. The next step is to run a randomized trial to see how useful the calculator is” for assisting in patients’ decision making, Dr. Aminian said.
The data for deriving the risk calculator, and for a preliminary validation of it, came from 13,722 patients with obesity (body mass index, 30 kg/m2 or greater) and type 2 diabetes, who were managed at the Cleveland Clinic during 1998-2017, drawn from more than 287,000 such patients in the clinic’s database. The study focused on 2,287 patients who underwent metabolic (bariatric) surgery and 11,435 patients from the same database who did not have surgery and matched by propensity scoring on a 5:1 basis with those who had surgery. The two cohorts this created matched well for age (about 54 years), sex (about two-thirds women), body mass index (about 44 kg/m2), and the prevalence of various comorbidities at baseline.
Dr. Aminian and associates then analyzed the incidence of all-cause mortality and various cardiovascular disease endpoints, as well as nephropathy during follow-up, through December 2018. Patients who had undergone metabolic surgery showed statistically significant reductions in the incidence of each of those events, compared with patients who did not have surgery (JAMA. 2019;322[13]:1271-82).
The investigators used these findings to create their model for calculating a patient’s risk score. For example, to calculate an estimate for the 10-year risk from all-cause mortality, the results showed that the most powerful risk factors were age; baseline body mass index, heart failure, and need for insulin; and smoking status. For the endpoint of nephropathy, the most important factors were estimated glomerular filtration rate at baseline and age. Identified risk factors could account for about 80% of the 10-year risk for all-cause death and for about 75% of the risk for developing nephropathy during 10 years, based on the area-under-the-curve values the model produced.
The risk score may help patients better understand the potential role that metabolic surgery can have in reducing their future event risk, thereby helping them better appreciate the benefit they stand to gain from undergoing surgery, Dr. Aminian said.
The calculator is available at a website maintained by the Cleveland Clinic, at a site of the American Society for Metabolic and Bariatric Surgery, and in app stores, he said.
The work was partially funded by Medtronic. Dr. Aminian has received grants from Medtronic.
SOURCE: Aminian A et al. Obesity Week 2019, Abstract A101.
REPORTING FROM OBESITY WEEK 2019
Is elagolix effective at reducing HMB for women with varying fibroid sizes and types?
Whether or not women experience symptoms from uterine fibroid(s) can be dependent on a fibroid’s size and location. Heavy menstrual bleeding (HMB) is the most common symptom resulting from fibroids, and it occurs in up to one-third of women with fibroids. For fibroids that are large (>10 cm), “bulk” symptoms may occur, including pelvic pressure, urinary urgency or frequency, incontinence, constipation, abdominal protrusion, etc.1
Elagolix, an oral gonadotropin-releasing hormone (GnRH) receptor antagonist, was US Food and Drug Administration–approved in 2018 to treat moderate to severe pain caused by endometriosis. 2 Elagolix is being evaluated in 2 phase 3 randomized, double-blind trials for the additional treatment of HMB associated with uterine fibroids. The results of these studies were presented at the 2019 AAGL meeting on November 12, in Vancouver, Canada.
Phase 3 study details
Premenopausal women aged 18 to 51 years were included in the Elaris UF-1 and UF-2 studies if they had HMB (defined using the alkaline hematin methodology as menstrual blood loss [MBL] >80 mL/cycle) and uterine fibroids as confirmed through ultrasound. Because elagolix suppresses estrogen and progesterone, treatment results in dose- and duration-dependent decreases in bone mineral density (BMD),2 and add-back therapy can lessen these adverse effects. Subsequently, participants were randomly assigned 1:1:2 to placebo, elagolix 300 mg twice daily, or elagolix 300 mg twice daily with add-back therapy (1 mg estradiol/0.5 mg norethidrone acetate [E2/NETA]) once daily. Uterine volume and size and location of uterine fibroid(s) were assessed by ultrasound. Subgroups were defined by baseline FIGO categories, grouped FIGO 0-3, FIGO 4, or FIGO 5-8.3
Over the 6-month studies, 72.2% (95% confidence interval [CI], 67.65–76.73) of the 395 women who received elagolix plus E2/NETA achieved < 80 mL MBL during the final month and ≥ 50% MBL reduction from baseline to the final month. When stratified by FIGO classification, the results were similar for all subgroups: FIGO 0-3, 77.7% (95% CI, 67.21–80.85). Similar results were seen in women with a primary fibroid volume of either greater or less than 36.2 cm3 (median).3
The most frequently reported adverse events among women taking elagolix plus E2/NETA were hot flushes, night sweats, nausea, and headache. Changes in BMD among these women were not significant compared with women taking placebo.3
The Elaris UF-1 and UF-2 studies are funded by AbbVie Inc.
- Al-Hendy A, Myers ER, Stewart E. Uterine fibroids: burden and unmet medical need. Semin Reprod Med. 2017;35:473-480.
- Orilissa [package insert]. North Chicago, IL: AbbVie; August 2019.
- Al-Hendy A, Simon J, Hurtado S, et al. Effect of fibroid location and size on efficacy in elagolix: results from phase 3 clinical trials. Paper presented at: 48th Annual Meeting of the AAGL; November 2019; Vancouver, Canada.
Whether or not women experience symptoms from uterine fibroid(s) can be dependent on a fibroid’s size and location. Heavy menstrual bleeding (HMB) is the most common symptom resulting from fibroids, and it occurs in up to one-third of women with fibroids. For fibroids that are large (>10 cm), “bulk” symptoms may occur, including pelvic pressure, urinary urgency or frequency, incontinence, constipation, abdominal protrusion, etc.1
Elagolix, an oral gonadotropin-releasing hormone (GnRH) receptor antagonist, was US Food and Drug Administration–approved in 2018 to treat moderate to severe pain caused by endometriosis. 2 Elagolix is being evaluated in 2 phase 3 randomized, double-blind trials for the additional treatment of HMB associated with uterine fibroids. The results of these studies were presented at the 2019 AAGL meeting on November 12, in Vancouver, Canada.
Phase 3 study details
Premenopausal women aged 18 to 51 years were included in the Elaris UF-1 and UF-2 studies if they had HMB (defined using the alkaline hematin methodology as menstrual blood loss [MBL] >80 mL/cycle) and uterine fibroids as confirmed through ultrasound. Because elagolix suppresses estrogen and progesterone, treatment results in dose- and duration-dependent decreases in bone mineral density (BMD),2 and add-back therapy can lessen these adverse effects. Subsequently, participants were randomly assigned 1:1:2 to placebo, elagolix 300 mg twice daily, or elagolix 300 mg twice daily with add-back therapy (1 mg estradiol/0.5 mg norethidrone acetate [E2/NETA]) once daily. Uterine volume and size and location of uterine fibroid(s) were assessed by ultrasound. Subgroups were defined by baseline FIGO categories, grouped FIGO 0-3, FIGO 4, or FIGO 5-8.3
Over the 6-month studies, 72.2% (95% confidence interval [CI], 67.65–76.73) of the 395 women who received elagolix plus E2/NETA achieved < 80 mL MBL during the final month and ≥ 50% MBL reduction from baseline to the final month. When stratified by FIGO classification, the results were similar for all subgroups: FIGO 0-3, 77.7% (95% CI, 67.21–80.85). Similar results were seen in women with a primary fibroid volume of either greater or less than 36.2 cm3 (median).3
The most frequently reported adverse events among women taking elagolix plus E2/NETA were hot flushes, night sweats, nausea, and headache. Changes in BMD among these women were not significant compared with women taking placebo.3
The Elaris UF-1 and UF-2 studies are funded by AbbVie Inc.
Whether or not women experience symptoms from uterine fibroid(s) can be dependent on a fibroid’s size and location. Heavy menstrual bleeding (HMB) is the most common symptom resulting from fibroids, and it occurs in up to one-third of women with fibroids. For fibroids that are large (>10 cm), “bulk” symptoms may occur, including pelvic pressure, urinary urgency or frequency, incontinence, constipation, abdominal protrusion, etc.1
Elagolix, an oral gonadotropin-releasing hormone (GnRH) receptor antagonist, was US Food and Drug Administration–approved in 2018 to treat moderate to severe pain caused by endometriosis. 2 Elagolix is being evaluated in 2 phase 3 randomized, double-blind trials for the additional treatment of HMB associated with uterine fibroids. The results of these studies were presented at the 2019 AAGL meeting on November 12, in Vancouver, Canada.
Phase 3 study details
Premenopausal women aged 18 to 51 years were included in the Elaris UF-1 and UF-2 studies if they had HMB (defined using the alkaline hematin methodology as menstrual blood loss [MBL] >80 mL/cycle) and uterine fibroids as confirmed through ultrasound. Because elagolix suppresses estrogen and progesterone, treatment results in dose- and duration-dependent decreases in bone mineral density (BMD),2 and add-back therapy can lessen these adverse effects. Subsequently, participants were randomly assigned 1:1:2 to placebo, elagolix 300 mg twice daily, or elagolix 300 mg twice daily with add-back therapy (1 mg estradiol/0.5 mg norethidrone acetate [E2/NETA]) once daily. Uterine volume and size and location of uterine fibroid(s) were assessed by ultrasound. Subgroups were defined by baseline FIGO categories, grouped FIGO 0-3, FIGO 4, or FIGO 5-8.3
Over the 6-month studies, 72.2% (95% confidence interval [CI], 67.65–76.73) of the 395 women who received elagolix plus E2/NETA achieved < 80 mL MBL during the final month and ≥ 50% MBL reduction from baseline to the final month. When stratified by FIGO classification, the results were similar for all subgroups: FIGO 0-3, 77.7% (95% CI, 67.21–80.85). Similar results were seen in women with a primary fibroid volume of either greater or less than 36.2 cm3 (median).3
The most frequently reported adverse events among women taking elagolix plus E2/NETA were hot flushes, night sweats, nausea, and headache. Changes in BMD among these women were not significant compared with women taking placebo.3
The Elaris UF-1 and UF-2 studies are funded by AbbVie Inc.
- Al-Hendy A, Myers ER, Stewart E. Uterine fibroids: burden and unmet medical need. Semin Reprod Med. 2017;35:473-480.
- Orilissa [package insert]. North Chicago, IL: AbbVie; August 2019.
- Al-Hendy A, Simon J, Hurtado S, et al. Effect of fibroid location and size on efficacy in elagolix: results from phase 3 clinical trials. Paper presented at: 48th Annual Meeting of the AAGL; November 2019; Vancouver, Canada.
- Al-Hendy A, Myers ER, Stewart E. Uterine fibroids: burden and unmet medical need. Semin Reprod Med. 2017;35:473-480.
- Orilissa [package insert]. North Chicago, IL: AbbVie; August 2019.
- Al-Hendy A, Simon J, Hurtado S, et al. Effect of fibroid location and size on efficacy in elagolix: results from phase 3 clinical trials. Paper presented at: 48th Annual Meeting of the AAGL; November 2019; Vancouver, Canada.
Can the office visit interval for routine pessary care be extended safely?
Propst K, Mellen C, O’Sullivan DM, et al. Timing of office-based pessary care: a randomized controlled trial. Obstet Gynecol. 2019 Dec 5. Doi: 10.1097/AOG.0000000000003580.
EXPERT COMMENTARY
Vaginal pessaries are a common and effective approach for managing pelvic organ prolapse (POP) as well as stress urinary incontinence (SUI). Vaginal mucosal erosions, however, may complicate pessary use. The risk for erosions may be associated with the frequency of pessary change, which involves removing the pessary, washing it, and replacing it in the vagina. Existing data do not address the frequency of pessary change. Recently, however, investigators conducted a randomized noninferiority trial to evaluate the effect of pessary visit intervals on the development of vaginal epithelial abnormalities.
Details of the study
At a single US hospital, Propst and colleagues randomly assigned women who used pessaries for POP, SUI, or both to routine pessary care (offices visits every 12 weeks) or to extended interval pessary care (office visits every 24 weeks). The women used ring, incontinence dish, or Gelhorn pessaries, did not change their pessaries on their own, and had no vaginal mucosal abnormalities.
A total of 130 women were randomly assigned, 64 to the routine care group and 66 to the extended interval care group. The mean age was 79 years and 90% were white, 4.6% were black, and 4% were Hispanic. Approximately 74% of the women used vaginal estrogen.
The primary outcome was the rate of vaginal epithelial abnormalities, including epithelial breaks or erosions. The predetermined noninferiority margin was set at 7.5%.
Results. At the 48-week follow-up, the rate of epithelial erosion was 7.4% in the routine care group and 1.7% in the extended interval care group, thus meeting the prespecified criteria for noninferiority of extended interval pessary care.
Women in each care group reported a similar amount of bothersome vaginal discharge. This was reported on a 5-point scale, with higher numbers indicating greater degree of bother. The mean scores were 1.39 in the routine care group and 1.34 in the extended interval care group. No other pessary-related adverse events occurred in either care group.
Study strengths and limitations
This trial provides good evidence that the timing of office pessary care can be extended to 24 weeks without compromising outcomes. However, since nearly three-quarters of the study participants used vaginal estrogen, the results may not be applicable to pessary users who do not use vaginal estrogen.
Many women change their pessary at home as often as weekly or daily. For women who rely on office visits for pessary care, however, the trial by Propst and colleagues provides good quality evidence that pessaries can be changed as infrequently as every 24 weeks without compromising outcomes. An important limitation of these data is that since most study participants used vaginal estrogen, the findings may not apply to pessary use among women who do not use vaginal estrogen.
ANDREW M. KAUNITZ, MD, NCMP
Propst K, Mellen C, O’Sullivan DM, et al. Timing of office-based pessary care: a randomized controlled trial. Obstet Gynecol. 2019 Dec 5. Doi: 10.1097/AOG.0000000000003580.
EXPERT COMMENTARY
Vaginal pessaries are a common and effective approach for managing pelvic organ prolapse (POP) as well as stress urinary incontinence (SUI). Vaginal mucosal erosions, however, may complicate pessary use. The risk for erosions may be associated with the frequency of pessary change, which involves removing the pessary, washing it, and replacing it in the vagina. Existing data do not address the frequency of pessary change. Recently, however, investigators conducted a randomized noninferiority trial to evaluate the effect of pessary visit intervals on the development of vaginal epithelial abnormalities.
Details of the study
At a single US hospital, Propst and colleagues randomly assigned women who used pessaries for POP, SUI, or both to routine pessary care (offices visits every 12 weeks) or to extended interval pessary care (office visits every 24 weeks). The women used ring, incontinence dish, or Gelhorn pessaries, did not change their pessaries on their own, and had no vaginal mucosal abnormalities.
A total of 130 women were randomly assigned, 64 to the routine care group and 66 to the extended interval care group. The mean age was 79 years and 90% were white, 4.6% were black, and 4% were Hispanic. Approximately 74% of the women used vaginal estrogen.
The primary outcome was the rate of vaginal epithelial abnormalities, including epithelial breaks or erosions. The predetermined noninferiority margin was set at 7.5%.
Results. At the 48-week follow-up, the rate of epithelial erosion was 7.4% in the routine care group and 1.7% in the extended interval care group, thus meeting the prespecified criteria for noninferiority of extended interval pessary care.
Women in each care group reported a similar amount of bothersome vaginal discharge. This was reported on a 5-point scale, with higher numbers indicating greater degree of bother. The mean scores were 1.39 in the routine care group and 1.34 in the extended interval care group. No other pessary-related adverse events occurred in either care group.
Study strengths and limitations
This trial provides good evidence that the timing of office pessary care can be extended to 24 weeks without compromising outcomes. However, since nearly three-quarters of the study participants used vaginal estrogen, the results may not be applicable to pessary users who do not use vaginal estrogen.
Many women change their pessary at home as often as weekly or daily. For women who rely on office visits for pessary care, however, the trial by Propst and colleagues provides good quality evidence that pessaries can be changed as infrequently as every 24 weeks without compromising outcomes. An important limitation of these data is that since most study participants used vaginal estrogen, the findings may not apply to pessary use among women who do not use vaginal estrogen.
ANDREW M. KAUNITZ, MD, NCMP
Propst K, Mellen C, O’Sullivan DM, et al. Timing of office-based pessary care: a randomized controlled trial. Obstet Gynecol. 2019 Dec 5. Doi: 10.1097/AOG.0000000000003580.
EXPERT COMMENTARY
Vaginal pessaries are a common and effective approach for managing pelvic organ prolapse (POP) as well as stress urinary incontinence (SUI). Vaginal mucosal erosions, however, may complicate pessary use. The risk for erosions may be associated with the frequency of pessary change, which involves removing the pessary, washing it, and replacing it in the vagina. Existing data do not address the frequency of pessary change. Recently, however, investigators conducted a randomized noninferiority trial to evaluate the effect of pessary visit intervals on the development of vaginal epithelial abnormalities.
Details of the study
At a single US hospital, Propst and colleagues randomly assigned women who used pessaries for POP, SUI, or both to routine pessary care (offices visits every 12 weeks) or to extended interval pessary care (office visits every 24 weeks). The women used ring, incontinence dish, or Gelhorn pessaries, did not change their pessaries on their own, and had no vaginal mucosal abnormalities.
A total of 130 women were randomly assigned, 64 to the routine care group and 66 to the extended interval care group. The mean age was 79 years and 90% were white, 4.6% were black, and 4% were Hispanic. Approximately 74% of the women used vaginal estrogen.
The primary outcome was the rate of vaginal epithelial abnormalities, including epithelial breaks or erosions. The predetermined noninferiority margin was set at 7.5%.
Results. At the 48-week follow-up, the rate of epithelial erosion was 7.4% in the routine care group and 1.7% in the extended interval care group, thus meeting the prespecified criteria for noninferiority of extended interval pessary care.
Women in each care group reported a similar amount of bothersome vaginal discharge. This was reported on a 5-point scale, with higher numbers indicating greater degree of bother. The mean scores were 1.39 in the routine care group and 1.34 in the extended interval care group. No other pessary-related adverse events occurred in either care group.
Study strengths and limitations
This trial provides good evidence that the timing of office pessary care can be extended to 24 weeks without compromising outcomes. However, since nearly three-quarters of the study participants used vaginal estrogen, the results may not be applicable to pessary users who do not use vaginal estrogen.
Many women change their pessary at home as often as weekly or daily. For women who rely on office visits for pessary care, however, the trial by Propst and colleagues provides good quality evidence that pessaries can be changed as infrequently as every 24 weeks without compromising outcomes. An important limitation of these data is that since most study participants used vaginal estrogen, the findings may not apply to pessary use among women who do not use vaginal estrogen.
ANDREW M. KAUNITZ, MD, NCMP
Don’t neglect urinary tract in gynecologic procedures
LAS VEGAS – – but when the time is appropriate, John B. Gebhart, MD, MS, urged.
You don’t need to stop a procedure to fix a bladder injury. Rather, mark the spot with a suture, finish what you are doing, then come back and fix the bladder injury, he advised.
“We need to be thinking [of the]urinary tract all the time in the procedures that we’re doing,” Dr. Gebhart, a urogynecologist and reconstructive pelvic surgeon from the Mayo Clinic, Rochester, Minn, said at the Pelvic Anatomy and Gynecologic Surgery Symposium.
“Can you look at the bladder and see that it’s intact, that ureters are functioning like they should? You don’t need to have the skill set to place stents, but you should be able to look in and know you’re okay leaving the operating room,” he said.
According to Dr. Gebhart, urethral injuries can occur in these procedures: anterior repair, cystoscopy, midurethral sling, and treatment of diverticulitis or Skene’s duct abscess.
He offered these tips about urethral injuries:
- Use catheters, dyes, and urethroscopy to reveal injuries. “Putting in a catheter is great because it helps you identify injury because you can visually see it,” he said. “We can squirt some dye in the urethra and see if it’s leaking out. We can put in a zero-degree scope and do urethroscopy.”
- Consider linking multiple holes in the urethra. “Don’t make individual repairs,” he said. “Connect the holes, making them into one hole that you can fix in one setting.”
- Check your repairs for leakage. “I might take a little indigo carmine or methylene blue in a little [angiocatheter], squirt it down the urethra, and see if I’ve got anything leaking out from my repair site,” he said. “If I do, then I want to go back and repair that so that I’ve got a watertight closure.”
- Consider a catheter after repair. “If you do a repair, you want to place a catheter at the end of the splint in the urethra for 7 to 10 to 14 days to help prevent stricture afterwards.”
Dr. Gebhart also discussed bladder injuries, which he said can occur in anterior repair, cystoscopy, hysterectomy, midurethral slings, sacrocolpopexy, and other procedures.
He offered these tips:
- Use bladder backfilling to detect injury. “We can backfill the bladder with the little methylene blue stain–normal saline to help identify whether you’ve got a leak or an injury,” he said. “[Cystogram] can also be very helpful as well.”
- Don’t stop a hysterectomy to fix a bladder injury. “Mark the hole with a suture, finish the hysterectomy and get it out of the way, then come back and fix the hole in the bladder,” Dr. Gebhart said.
- After repair, drain the bladder with a catheter for 10-14 days. “You’re always better draining through a catheter a little longer than pulling the catheter too soon, putting a stretch on the bladder, and maybe compromising your repair,” he said.
He recommended performing a quick cystogram before pulling the catheter to make sure there’s no leak.
Dr. Gebhart disclosed consultant (Hologic) and advisory board (UroCure) relationships and royalties (UpToDate, Elsevier).
This meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
LAS VEGAS – – but when the time is appropriate, John B. Gebhart, MD, MS, urged.
You don’t need to stop a procedure to fix a bladder injury. Rather, mark the spot with a suture, finish what you are doing, then come back and fix the bladder injury, he advised.
“We need to be thinking [of the]urinary tract all the time in the procedures that we’re doing,” Dr. Gebhart, a urogynecologist and reconstructive pelvic surgeon from the Mayo Clinic, Rochester, Minn, said at the Pelvic Anatomy and Gynecologic Surgery Symposium.
“Can you look at the bladder and see that it’s intact, that ureters are functioning like they should? You don’t need to have the skill set to place stents, but you should be able to look in and know you’re okay leaving the operating room,” he said.
According to Dr. Gebhart, urethral injuries can occur in these procedures: anterior repair, cystoscopy, midurethral sling, and treatment of diverticulitis or Skene’s duct abscess.
He offered these tips about urethral injuries:
- Use catheters, dyes, and urethroscopy to reveal injuries. “Putting in a catheter is great because it helps you identify injury because you can visually see it,” he said. “We can squirt some dye in the urethra and see if it’s leaking out. We can put in a zero-degree scope and do urethroscopy.”
- Consider linking multiple holes in the urethra. “Don’t make individual repairs,” he said. “Connect the holes, making them into one hole that you can fix in one setting.”
- Check your repairs for leakage. “I might take a little indigo carmine or methylene blue in a little [angiocatheter], squirt it down the urethra, and see if I’ve got anything leaking out from my repair site,” he said. “If I do, then I want to go back and repair that so that I’ve got a watertight closure.”
- Consider a catheter after repair. “If you do a repair, you want to place a catheter at the end of the splint in the urethra for 7 to 10 to 14 days to help prevent stricture afterwards.”
Dr. Gebhart also discussed bladder injuries, which he said can occur in anterior repair, cystoscopy, hysterectomy, midurethral slings, sacrocolpopexy, and other procedures.
He offered these tips:
- Use bladder backfilling to detect injury. “We can backfill the bladder with the little methylene blue stain–normal saline to help identify whether you’ve got a leak or an injury,” he said. “[Cystogram] can also be very helpful as well.”
- Don’t stop a hysterectomy to fix a bladder injury. “Mark the hole with a suture, finish the hysterectomy and get it out of the way, then come back and fix the hole in the bladder,” Dr. Gebhart said.
- After repair, drain the bladder with a catheter for 10-14 days. “You’re always better draining through a catheter a little longer than pulling the catheter too soon, putting a stretch on the bladder, and maybe compromising your repair,” he said.
He recommended performing a quick cystogram before pulling the catheter to make sure there’s no leak.
Dr. Gebhart disclosed consultant (Hologic) and advisory board (UroCure) relationships and royalties (UpToDate, Elsevier).
This meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
LAS VEGAS – – but when the time is appropriate, John B. Gebhart, MD, MS, urged.
You don’t need to stop a procedure to fix a bladder injury. Rather, mark the spot with a suture, finish what you are doing, then come back and fix the bladder injury, he advised.
“We need to be thinking [of the]urinary tract all the time in the procedures that we’re doing,” Dr. Gebhart, a urogynecologist and reconstructive pelvic surgeon from the Mayo Clinic, Rochester, Minn, said at the Pelvic Anatomy and Gynecologic Surgery Symposium.
“Can you look at the bladder and see that it’s intact, that ureters are functioning like they should? You don’t need to have the skill set to place stents, but you should be able to look in and know you’re okay leaving the operating room,” he said.
According to Dr. Gebhart, urethral injuries can occur in these procedures: anterior repair, cystoscopy, midurethral sling, and treatment of diverticulitis or Skene’s duct abscess.
He offered these tips about urethral injuries:
- Use catheters, dyes, and urethroscopy to reveal injuries. “Putting in a catheter is great because it helps you identify injury because you can visually see it,” he said. “We can squirt some dye in the urethra and see if it’s leaking out. We can put in a zero-degree scope and do urethroscopy.”
- Consider linking multiple holes in the urethra. “Don’t make individual repairs,” he said. “Connect the holes, making them into one hole that you can fix in one setting.”
- Check your repairs for leakage. “I might take a little indigo carmine or methylene blue in a little [angiocatheter], squirt it down the urethra, and see if I’ve got anything leaking out from my repair site,” he said. “If I do, then I want to go back and repair that so that I’ve got a watertight closure.”
- Consider a catheter after repair. “If you do a repair, you want to place a catheter at the end of the splint in the urethra for 7 to 10 to 14 days to help prevent stricture afterwards.”
Dr. Gebhart also discussed bladder injuries, which he said can occur in anterior repair, cystoscopy, hysterectomy, midurethral slings, sacrocolpopexy, and other procedures.
He offered these tips:
- Use bladder backfilling to detect injury. “We can backfill the bladder with the little methylene blue stain–normal saline to help identify whether you’ve got a leak or an injury,” he said. “[Cystogram] can also be very helpful as well.”
- Don’t stop a hysterectomy to fix a bladder injury. “Mark the hole with a suture, finish the hysterectomy and get it out of the way, then come back and fix the hole in the bladder,” Dr. Gebhart said.
- After repair, drain the bladder with a catheter for 10-14 days. “You’re always better draining through a catheter a little longer than pulling the catheter too soon, putting a stretch on the bladder, and maybe compromising your repair,” he said.
He recommended performing a quick cystogram before pulling the catheter to make sure there’s no leak.
Dr. Gebhart disclosed consultant (Hologic) and advisory board (UroCure) relationships and royalties (UpToDate, Elsevier).
This meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
EXPERT ANALYSIS FROM PAGS 2019
Beware the dangers of nerve injury in vaginal surgery
LAS VEGAS – a pelvic surgeon urged colleagues.
“It’s a very high medical and legal risk. You have to think about the various nerves that can be influenced,” urogynecologist Mickey M. Karram, MD, said at the Pelvic Anatomy and Gynecologic Surgery Symposium.
Dr. Karram, director of urogynecology and reconstructive surgery at the Christ Hospital in Cincinnati and clinical professor of obstetrics and gynecology at the University of Cincinnati, offered these pearls:
- Understand the anatomy of nerves at risk. These include the ilioinguinal nerve, obturator neurovascular bundle, and pudendal nerve.
- Position the patient correctly. The buttocks should be at edge of table, Dr. Karram said, and there should be slight extension and lateral rotation of the thigh. Beware of compression of the lateral knee.
- Avoid compression from stirrups. If you still use candy-cane stirrups, he said, you can get compression along the lateral aspect of the knee. “You can [get] common perineal nerve injuries. You can also get femoral nerve injuries that are stretch injuries and over-extension injuries as well. Just be careful about this.” Dr. Karram said he prefers fin-type stirrups such as the Allen Yellofin brand. Also, he said, avoid compression injuries that result when there are too many people between the patient’s legs and someone leans on the thighs, he said.
- Free the retractor in abdominal procedures. “If you’re operating abdominally and use retractors, free the retractor at times,” he said. Otherwise, “you can get injuries to the genitofemoral nerve and the femoral nerve itself.”
- Beware buttock pain after sacrospinous fixation. “About 15%-20% of the time, you’ll get extreme buttock pain,” Dr. Karram said. “Assuming the buttock pain doesn’t radiate anywhere and doesn’t go down the leg, it’s definitely not a problem. If it goes down the leg, then you have to think about things like deligating pretty quickly.”
Dr. Karram disclosed consulting (Coloplast and Cynosure/Hologic) and speaker (Allergan, Astellas, Coloplast, and Cynosure/Hologic) relationships. He has royalties from Fidelis Medical and LumeNXT.
This meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
LAS VEGAS – a pelvic surgeon urged colleagues.
“It’s a very high medical and legal risk. You have to think about the various nerves that can be influenced,” urogynecologist Mickey M. Karram, MD, said at the Pelvic Anatomy and Gynecologic Surgery Symposium.
Dr. Karram, director of urogynecology and reconstructive surgery at the Christ Hospital in Cincinnati and clinical professor of obstetrics and gynecology at the University of Cincinnati, offered these pearls:
- Understand the anatomy of nerves at risk. These include the ilioinguinal nerve, obturator neurovascular bundle, and pudendal nerve.
- Position the patient correctly. The buttocks should be at edge of table, Dr. Karram said, and there should be slight extension and lateral rotation of the thigh. Beware of compression of the lateral knee.
- Avoid compression from stirrups. If you still use candy-cane stirrups, he said, you can get compression along the lateral aspect of the knee. “You can [get] common perineal nerve injuries. You can also get femoral nerve injuries that are stretch injuries and over-extension injuries as well. Just be careful about this.” Dr. Karram said he prefers fin-type stirrups such as the Allen Yellofin brand. Also, he said, avoid compression injuries that result when there are too many people between the patient’s legs and someone leans on the thighs, he said.
- Free the retractor in abdominal procedures. “If you’re operating abdominally and use retractors, free the retractor at times,” he said. Otherwise, “you can get injuries to the genitofemoral nerve and the femoral nerve itself.”
- Beware buttock pain after sacrospinous fixation. “About 15%-20% of the time, you’ll get extreme buttock pain,” Dr. Karram said. “Assuming the buttock pain doesn’t radiate anywhere and doesn’t go down the leg, it’s definitely not a problem. If it goes down the leg, then you have to think about things like deligating pretty quickly.”
Dr. Karram disclosed consulting (Coloplast and Cynosure/Hologic) and speaker (Allergan, Astellas, Coloplast, and Cynosure/Hologic) relationships. He has royalties from Fidelis Medical and LumeNXT.
This meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
LAS VEGAS – a pelvic surgeon urged colleagues.
“It’s a very high medical and legal risk. You have to think about the various nerves that can be influenced,” urogynecologist Mickey M. Karram, MD, said at the Pelvic Anatomy and Gynecologic Surgery Symposium.
Dr. Karram, director of urogynecology and reconstructive surgery at the Christ Hospital in Cincinnati and clinical professor of obstetrics and gynecology at the University of Cincinnati, offered these pearls:
- Understand the anatomy of nerves at risk. These include the ilioinguinal nerve, obturator neurovascular bundle, and pudendal nerve.
- Position the patient correctly. The buttocks should be at edge of table, Dr. Karram said, and there should be slight extension and lateral rotation of the thigh. Beware of compression of the lateral knee.
- Avoid compression from stirrups. If you still use candy-cane stirrups, he said, you can get compression along the lateral aspect of the knee. “You can [get] common perineal nerve injuries. You can also get femoral nerve injuries that are stretch injuries and over-extension injuries as well. Just be careful about this.” Dr. Karram said he prefers fin-type stirrups such as the Allen Yellofin brand. Also, he said, avoid compression injuries that result when there are too many people between the patient’s legs and someone leans on the thighs, he said.
- Free the retractor in abdominal procedures. “If you’re operating abdominally and use retractors, free the retractor at times,” he said. Otherwise, “you can get injuries to the genitofemoral nerve and the femoral nerve itself.”
- Beware buttock pain after sacrospinous fixation. “About 15%-20% of the time, you’ll get extreme buttock pain,” Dr. Karram said. “Assuming the buttock pain doesn’t radiate anywhere and doesn’t go down the leg, it’s definitely not a problem. If it goes down the leg, then you have to think about things like deligating pretty quickly.”
Dr. Karram disclosed consulting (Coloplast and Cynosure/Hologic) and speaker (Allergan, Astellas, Coloplast, and Cynosure/Hologic) relationships. He has royalties from Fidelis Medical and LumeNXT.
This meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
EXPERT ANALYSIS FROM PAGS 2019