User login
Hold the central lymph node dissection for small parathyroid tumors?
BOSTON – Central lymph node dissection may not be necessary in patients with small parathyroid carcinomas, results of a data review suggest.
Among 405 U.S. patients treated in the last two decades for parathyroid carcinomas, disease-specific mortality and degree of local tumor invasion did not differ depending on nodal involvement, reported Dr. Kun-Tai Hsu, an associate research specialist in the surgery department at the University of Wisconsin, Madison.
Patients with tumors greater than 3 cm in diameter and distal metastasis had worse disease-specific mortality, and larger tumors were significantly more likely to be associated with positive lymph nodes, Dr. Hsu reported at the annual meeting of the American Association of Endocrine Surgeons.
The findings raise the question of whether central lymph node dissection is necessary for all patients with parathyroid carcinomas, but "our conclusions may not translate directly into definitive recommendations," said Dr. Hsu, who advised further study of whether patients with tumors larger than 3 cm may benefit from lymph node dissection.
Parathyroid carcinomas are rare cancers, accounting for 0.005% of all malignancies and less than 1% of primary hyperparathyroidism. The current standard of therapy is en bloc removal of the parathyroid tumor and ipsilateral lobectomy, isthmusectomy, and central lymph node dissection.
Over at least 5 years of follow-up, neither tumor size nor lymph node status was significantly predictive of outcomes in an earlier retrospective study of 286 patients treated in the 1980s and 1990s.
In addition, a 2006 study using data from the Surveillance, Epidemiology and End Results (SEER) database showed a significant increase in the incidence of this malignancy from 1988 through 2003. The authors identified younger age, female sex, more recent diagnosis, and absence of distant metastases as favorable prognostic factors (Cancer 2007;109:1736-41).
What the previous studies could not answer, however, was whether lymph node metastases were associated with worse disease-specific mortality and whether central lymph node dissection might improve survival in patients with parathyroid carcinomas, Dr. Hsu noted.
He and his colleagues queried SEER for disease-specific survival outcomes and lymph-node status of all patients treated in the United States for parathyroid carcinomas from 1988 through 2010.
They identified 212 female and 193 male patients. Among all patients, 112 (27.7%) had tumors 3 cm or greater, and 12 (3%) had positive lymph nodes. Median follow-up was 68 months.
In a multivariate analysis of disease-specific mortality predictors adjusted for sex and age only, tumors 3 cm or greater and the presence of metastasis were predictive of worse survival, with respective hazard ratios of 5.35 (P = .01) and 45.1 (P less than .01).
Similarly, an analysis adjusted for sex, age, and year of diagnosis showed that tumor size but not age or sex significantly predicted lymph node metastasis (HR, 19.48; P = .02)
A comparison of outcomes between patients with and without lymph node examinations found no differences in disease-specific mortality by sex, age, year of diagnosis, tumor size, local invasion, or metastasis. Significant predictors of disease-specific mortality in this analysis included surgery type (parathyroidectomy, en bloc excision, or debulking), the use of radiation, and white race.
Dr. Hsu noted that the study was limited by its retrospective design, lack of information on other significant clinical variables, lack of detailed follow-up data, and the possibility of misclassification or miscoding of cases.
He acknowledged that uncertainty about the diagnosis is a drawback to SEER-based studies, but that given the rarity of the disease, SEER data are the most reliable source of information.
The study was supported by the Wisconsin Surgical Outcomes Research Program. Dr. Hsu reported having no relevant disclosures. Study coauthor Dr. Rebecca Sippel is a member of this publication’s editorial advisory board.
The accuracy of diagnoses is questionable among patients in this study. A diagnosis of parathyroid carcinoma is based on several factors, including the presence of local invasion, lymph node metastases, or systemic metastases, but only a relatively small percentage of patients in the study had local invasion, suggesting that the diagnosis was based on examination of microscopic tumor features in the absence of local invasion.
An alternative explanation for the lack of a disease-specific mortality difference by lymph node status is that you may have included patients who really don’t have parathyroid cancer.
Dr. Christopher McHenry is vice-chair of surgery at MetroHealth in Cleveland. He had no relevant disclosures.
The accuracy of diagnoses is questionable among patients in this study. A diagnosis of parathyroid carcinoma is based on several factors, including the presence of local invasion, lymph node metastases, or systemic metastases, but only a relatively small percentage of patients in the study had local invasion, suggesting that the diagnosis was based on examination of microscopic tumor features in the absence of local invasion.
An alternative explanation for the lack of a disease-specific mortality difference by lymph node status is that you may have included patients who really don’t have parathyroid cancer.
Dr. Christopher McHenry is vice-chair of surgery at MetroHealth in Cleveland. He had no relevant disclosures.
The accuracy of diagnoses is questionable among patients in this study. A diagnosis of parathyroid carcinoma is based on several factors, including the presence of local invasion, lymph node metastases, or systemic metastases, but only a relatively small percentage of patients in the study had local invasion, suggesting that the diagnosis was based on examination of microscopic tumor features in the absence of local invasion.
An alternative explanation for the lack of a disease-specific mortality difference by lymph node status is that you may have included patients who really don’t have parathyroid cancer.
Dr. Christopher McHenry is vice-chair of surgery at MetroHealth in Cleveland. He had no relevant disclosures.
BOSTON – Central lymph node dissection may not be necessary in patients with small parathyroid carcinomas, results of a data review suggest.
Among 405 U.S. patients treated in the last two decades for parathyroid carcinomas, disease-specific mortality and degree of local tumor invasion did not differ depending on nodal involvement, reported Dr. Kun-Tai Hsu, an associate research specialist in the surgery department at the University of Wisconsin, Madison.
Patients with tumors greater than 3 cm in diameter and distal metastasis had worse disease-specific mortality, and larger tumors were significantly more likely to be associated with positive lymph nodes, Dr. Hsu reported at the annual meeting of the American Association of Endocrine Surgeons.
The findings raise the question of whether central lymph node dissection is necessary for all patients with parathyroid carcinomas, but "our conclusions may not translate directly into definitive recommendations," said Dr. Hsu, who advised further study of whether patients with tumors larger than 3 cm may benefit from lymph node dissection.
Parathyroid carcinomas are rare cancers, accounting for 0.005% of all malignancies and less than 1% of primary hyperparathyroidism. The current standard of therapy is en bloc removal of the parathyroid tumor and ipsilateral lobectomy, isthmusectomy, and central lymph node dissection.
Over at least 5 years of follow-up, neither tumor size nor lymph node status was significantly predictive of outcomes in an earlier retrospective study of 286 patients treated in the 1980s and 1990s.
In addition, a 2006 study using data from the Surveillance, Epidemiology and End Results (SEER) database showed a significant increase in the incidence of this malignancy from 1988 through 2003. The authors identified younger age, female sex, more recent diagnosis, and absence of distant metastases as favorable prognostic factors (Cancer 2007;109:1736-41).
What the previous studies could not answer, however, was whether lymph node metastases were associated with worse disease-specific mortality and whether central lymph node dissection might improve survival in patients with parathyroid carcinomas, Dr. Hsu noted.
He and his colleagues queried SEER for disease-specific survival outcomes and lymph-node status of all patients treated in the United States for parathyroid carcinomas from 1988 through 2010.
They identified 212 female and 193 male patients. Among all patients, 112 (27.7%) had tumors 3 cm or greater, and 12 (3%) had positive lymph nodes. Median follow-up was 68 months.
In a multivariate analysis of disease-specific mortality predictors adjusted for sex and age only, tumors 3 cm or greater and the presence of metastasis were predictive of worse survival, with respective hazard ratios of 5.35 (P = .01) and 45.1 (P less than .01).
Similarly, an analysis adjusted for sex, age, and year of diagnosis showed that tumor size but not age or sex significantly predicted lymph node metastasis (HR, 19.48; P = .02)
A comparison of outcomes between patients with and without lymph node examinations found no differences in disease-specific mortality by sex, age, year of diagnosis, tumor size, local invasion, or metastasis. Significant predictors of disease-specific mortality in this analysis included surgery type (parathyroidectomy, en bloc excision, or debulking), the use of radiation, and white race.
Dr. Hsu noted that the study was limited by its retrospective design, lack of information on other significant clinical variables, lack of detailed follow-up data, and the possibility of misclassification or miscoding of cases.
He acknowledged that uncertainty about the diagnosis is a drawback to SEER-based studies, but that given the rarity of the disease, SEER data are the most reliable source of information.
The study was supported by the Wisconsin Surgical Outcomes Research Program. Dr. Hsu reported having no relevant disclosures. Study coauthor Dr. Rebecca Sippel is a member of this publication’s editorial advisory board.
BOSTON – Central lymph node dissection may not be necessary in patients with small parathyroid carcinomas, results of a data review suggest.
Among 405 U.S. patients treated in the last two decades for parathyroid carcinomas, disease-specific mortality and degree of local tumor invasion did not differ depending on nodal involvement, reported Dr. Kun-Tai Hsu, an associate research specialist in the surgery department at the University of Wisconsin, Madison.
Patients with tumors greater than 3 cm in diameter and distal metastasis had worse disease-specific mortality, and larger tumors were significantly more likely to be associated with positive lymph nodes, Dr. Hsu reported at the annual meeting of the American Association of Endocrine Surgeons.
The findings raise the question of whether central lymph node dissection is necessary for all patients with parathyroid carcinomas, but "our conclusions may not translate directly into definitive recommendations," said Dr. Hsu, who advised further study of whether patients with tumors larger than 3 cm may benefit from lymph node dissection.
Parathyroid carcinomas are rare cancers, accounting for 0.005% of all malignancies and less than 1% of primary hyperparathyroidism. The current standard of therapy is en bloc removal of the parathyroid tumor and ipsilateral lobectomy, isthmusectomy, and central lymph node dissection.
Over at least 5 years of follow-up, neither tumor size nor lymph node status was significantly predictive of outcomes in an earlier retrospective study of 286 patients treated in the 1980s and 1990s.
In addition, a 2006 study using data from the Surveillance, Epidemiology and End Results (SEER) database showed a significant increase in the incidence of this malignancy from 1988 through 2003. The authors identified younger age, female sex, more recent diagnosis, and absence of distant metastases as favorable prognostic factors (Cancer 2007;109:1736-41).
What the previous studies could not answer, however, was whether lymph node metastases were associated with worse disease-specific mortality and whether central lymph node dissection might improve survival in patients with parathyroid carcinomas, Dr. Hsu noted.
He and his colleagues queried SEER for disease-specific survival outcomes and lymph-node status of all patients treated in the United States for parathyroid carcinomas from 1988 through 2010.
They identified 212 female and 193 male patients. Among all patients, 112 (27.7%) had tumors 3 cm or greater, and 12 (3%) had positive lymph nodes. Median follow-up was 68 months.
In a multivariate analysis of disease-specific mortality predictors adjusted for sex and age only, tumors 3 cm or greater and the presence of metastasis were predictive of worse survival, with respective hazard ratios of 5.35 (P = .01) and 45.1 (P less than .01).
Similarly, an analysis adjusted for sex, age, and year of diagnosis showed that tumor size but not age or sex significantly predicted lymph node metastasis (HR, 19.48; P = .02)
A comparison of outcomes between patients with and without lymph node examinations found no differences in disease-specific mortality by sex, age, year of diagnosis, tumor size, local invasion, or metastasis. Significant predictors of disease-specific mortality in this analysis included surgery type (parathyroidectomy, en bloc excision, or debulking), the use of radiation, and white race.
Dr. Hsu noted that the study was limited by its retrospective design, lack of information on other significant clinical variables, lack of detailed follow-up data, and the possibility of misclassification or miscoding of cases.
He acknowledged that uncertainty about the diagnosis is a drawback to SEER-based studies, but that given the rarity of the disease, SEER data are the most reliable source of information.
The study was supported by the Wisconsin Surgical Outcomes Research Program. Dr. Hsu reported having no relevant disclosures. Study coauthor Dr. Rebecca Sippel is a member of this publication’s editorial advisory board.
AT AAES 2014
Key clinical point: Central lymph node dissection may not be needed for patients with small parathyroid tumors.
Major finding: In a multivariate analysis, tumors 3 cm or greater and the presence of metastasis were predictive of worse survival, with respective hazard ratios of 5.35 (P = .01) and 45.1 (P less than .01).
Data source: Retrospective study of SEER data on 405 patients.
Disclosures: The study was supported by the Wisconsin Surgical Outcomes Research Program. Dr. Hsu reported having no relevant disclosures.
Big gains shown in ED pediatric readiness
DALLAS – Emergency departments have substantially improved their readiness to receive pediatric patients since a 2009 multispecialty call to action was triggered by documented major deficiencies in preparedness, the results of a comprehensive new survey indicate.
"We’ve seen significant improvement in all ED patient volume classes in terms of median pediatric readiness scores. The data are pretty exciting. It says we’re moving in the right direction," Dr. Marianne Gausche-Hill said in presenting the survey findings at the annual meeting of the Society for Academic Emergency Medicine.

The key outcome measure in the national survey was a weighted ED pediatric readiness score based upon the extent to which an ED was compliant with the major recommendations of the 2009 joint American Academy of Pediatrics/American College of Emergency Physicians/Emergency Nurses Association guidelines for the care of children in EDs (Pediatrics 2009;124:1233-44.
The guidelines lay out in concrete terms what ED pediatric readiness entails in terms of specialized equipment, medications, policies and protocols, safety issues, staff, and professional education.
Lack of recommended equipment needed to handle pediatric emergencies, such as laryngeal mask airways for children, was a major problem identified in the first survey. Indeed, at that time only 6% of EDs had all the recommended equipment and supplies. In contrast, the new survey showed that today EDs have a median of 91% of all recommended equipment; in higher-volume EDs that figure rises to virtually 100%, according to Dr. Gausche-Hill.
Overall, the median ED pediatric readiness score in the new survey was 69 on a 0-100 scale, up sharply from a median of 55 in an earlier nationwide survey, conducted in 2003 and published in 2007, for which Dr. Gausche-Hill also was the lead author (Pediatrics 2007;120:1229-37).
A test score of 55 gets a big red F in any classroom not grading on the curve. So this disappointing performance was an impetus for the 2009 joint guidelines, of which Dr. Gausche-Hill was a lead coauthor. The guidelines were endorsed by nearly two dozen organizations, including the American Academy of Family Physicians and the American College of Surgeons.
A theme emphasized in the joint guidelines is that all EDs need to be prepared to meet the unique needs of pediatric patients; 90% of all ED visits by patients under age 15 are to nonchildren’s hospitals, and roughly one-third of these visits are to EDs in rural and remote areas, which generally scored poorly in the initial national survey.
Another impetus for issuing the guidelines was recognition that pediatric ED visits are increasing even as the number of EDs nationwide is declining, worsening overcrowding, explained Dr. Gausche-Hill, professor of emergency medicine at the University of California, Los Angeles, and director of emergency medical services at Harbor-UCLA Medical Center.
She and her coinvestigators in the large coalition known as the National Pediatric Readiness Project sent the survey to more than 5,000 U.S. EDs. The response rate was an impressive 83%, despite the fact that the 55-question survey addressed 189 items and took a full hour to complete. Dr. Gausche-Hill attributed this remarkable response rate to on-site advocates’ passion for improved ED pediatric preparedness. Another factor: survey respondents received immediate feedback about how their hospital’s ED score compared with the average score of other hospitals with similar patient volume, as well as information about the top three things their hospital needs to do to reach a preparedness score of at least 80, which is the coalition’s short-term goal.
In analyzing the ED scores, the single most effective way most hospitals can improve their ED pediatric readiness score is for the hospital ED medical director to appoint a physician and/or a nurse pediatric emergency care coordinator. Hospitals with a coordinator scored higher, and the 42% of hospitals with both a physician and a nurse pediatric emergency care coordinator scored highest of all. Hospitals with at least one pediatric emergency care coordinator were more than fivefold more likely to have a quality improvement plan in place for ED pediatric patients, as recommended in a 2006 Institute of Medicine report that described the nation’s ED pediatric readiness as uneven.
In the new survey, 82% of EDs reported one or more barriers to compliance with the pediatric readiness guidelines. These barriers will be the focus of future quality improvement initiatives.
What’s next? Dr. Gausche-Hill said the coalition is reaching out to health care corporate groups in an effort to convince them to make changes in their hospitals.
"We’ve seen the Hospital Corporation of America make a huge initiative and change their average readiness score from 66 to 91 in the intervention hospitals. Also, Kaiser Permanente has initiated a readiness initiative," she said.
The plan is to keep the survey’s internet portal open so that hospitals can enter updated data and show continuous quality improvement. In addition, the coalition’s website – www.pediatricreadiness.org – includes tools to improve readiness.
Dr. Gausche-Hill reported having no financial conflicts regarding this project.
DALLAS – Emergency departments have substantially improved their readiness to receive pediatric patients since a 2009 multispecialty call to action was triggered by documented major deficiencies in preparedness, the results of a comprehensive new survey indicate.
"We’ve seen significant improvement in all ED patient volume classes in terms of median pediatric readiness scores. The data are pretty exciting. It says we’re moving in the right direction," Dr. Marianne Gausche-Hill said in presenting the survey findings at the annual meeting of the Society for Academic Emergency Medicine.

The key outcome measure in the national survey was a weighted ED pediatric readiness score based upon the extent to which an ED was compliant with the major recommendations of the 2009 joint American Academy of Pediatrics/American College of Emergency Physicians/Emergency Nurses Association guidelines for the care of children in EDs (Pediatrics 2009;124:1233-44.
The guidelines lay out in concrete terms what ED pediatric readiness entails in terms of specialized equipment, medications, policies and protocols, safety issues, staff, and professional education.
Lack of recommended equipment needed to handle pediatric emergencies, such as laryngeal mask airways for children, was a major problem identified in the first survey. Indeed, at that time only 6% of EDs had all the recommended equipment and supplies. In contrast, the new survey showed that today EDs have a median of 91% of all recommended equipment; in higher-volume EDs that figure rises to virtually 100%, according to Dr. Gausche-Hill.
Overall, the median ED pediatric readiness score in the new survey was 69 on a 0-100 scale, up sharply from a median of 55 in an earlier nationwide survey, conducted in 2003 and published in 2007, for which Dr. Gausche-Hill also was the lead author (Pediatrics 2007;120:1229-37).
A test score of 55 gets a big red F in any classroom not grading on the curve. So this disappointing performance was an impetus for the 2009 joint guidelines, of which Dr. Gausche-Hill was a lead coauthor. The guidelines were endorsed by nearly two dozen organizations, including the American Academy of Family Physicians and the American College of Surgeons.
A theme emphasized in the joint guidelines is that all EDs need to be prepared to meet the unique needs of pediatric patients; 90% of all ED visits by patients under age 15 are to nonchildren’s hospitals, and roughly one-third of these visits are to EDs in rural and remote areas, which generally scored poorly in the initial national survey.
Another impetus for issuing the guidelines was recognition that pediatric ED visits are increasing even as the number of EDs nationwide is declining, worsening overcrowding, explained Dr. Gausche-Hill, professor of emergency medicine at the University of California, Los Angeles, and director of emergency medical services at Harbor-UCLA Medical Center.
She and her coinvestigators in the large coalition known as the National Pediatric Readiness Project sent the survey to more than 5,000 U.S. EDs. The response rate was an impressive 83%, despite the fact that the 55-question survey addressed 189 items and took a full hour to complete. Dr. Gausche-Hill attributed this remarkable response rate to on-site advocates’ passion for improved ED pediatric preparedness. Another factor: survey respondents received immediate feedback about how their hospital’s ED score compared with the average score of other hospitals with similar patient volume, as well as information about the top three things their hospital needs to do to reach a preparedness score of at least 80, which is the coalition’s short-term goal.
In analyzing the ED scores, the single most effective way most hospitals can improve their ED pediatric readiness score is for the hospital ED medical director to appoint a physician and/or a nurse pediatric emergency care coordinator. Hospitals with a coordinator scored higher, and the 42% of hospitals with both a physician and a nurse pediatric emergency care coordinator scored highest of all. Hospitals with at least one pediatric emergency care coordinator were more than fivefold more likely to have a quality improvement plan in place for ED pediatric patients, as recommended in a 2006 Institute of Medicine report that described the nation’s ED pediatric readiness as uneven.
In the new survey, 82% of EDs reported one or more barriers to compliance with the pediatric readiness guidelines. These barriers will be the focus of future quality improvement initiatives.
What’s next? Dr. Gausche-Hill said the coalition is reaching out to health care corporate groups in an effort to convince them to make changes in their hospitals.
"We’ve seen the Hospital Corporation of America make a huge initiative and change their average readiness score from 66 to 91 in the intervention hospitals. Also, Kaiser Permanente has initiated a readiness initiative," she said.
The plan is to keep the survey’s internet portal open so that hospitals can enter updated data and show continuous quality improvement. In addition, the coalition’s website – www.pediatricreadiness.org – includes tools to improve readiness.
Dr. Gausche-Hill reported having no financial conflicts regarding this project.
DALLAS – Emergency departments have substantially improved their readiness to receive pediatric patients since a 2009 multispecialty call to action was triggered by documented major deficiencies in preparedness, the results of a comprehensive new survey indicate.
"We’ve seen significant improvement in all ED patient volume classes in terms of median pediatric readiness scores. The data are pretty exciting. It says we’re moving in the right direction," Dr. Marianne Gausche-Hill said in presenting the survey findings at the annual meeting of the Society for Academic Emergency Medicine.

The key outcome measure in the national survey was a weighted ED pediatric readiness score based upon the extent to which an ED was compliant with the major recommendations of the 2009 joint American Academy of Pediatrics/American College of Emergency Physicians/Emergency Nurses Association guidelines for the care of children in EDs (Pediatrics 2009;124:1233-44.
The guidelines lay out in concrete terms what ED pediatric readiness entails in terms of specialized equipment, medications, policies and protocols, safety issues, staff, and professional education.
Lack of recommended equipment needed to handle pediatric emergencies, such as laryngeal mask airways for children, was a major problem identified in the first survey. Indeed, at that time only 6% of EDs had all the recommended equipment and supplies. In contrast, the new survey showed that today EDs have a median of 91% of all recommended equipment; in higher-volume EDs that figure rises to virtually 100%, according to Dr. Gausche-Hill.
Overall, the median ED pediatric readiness score in the new survey was 69 on a 0-100 scale, up sharply from a median of 55 in an earlier nationwide survey, conducted in 2003 and published in 2007, for which Dr. Gausche-Hill also was the lead author (Pediatrics 2007;120:1229-37).
A test score of 55 gets a big red F in any classroom not grading on the curve. So this disappointing performance was an impetus for the 2009 joint guidelines, of which Dr. Gausche-Hill was a lead coauthor. The guidelines were endorsed by nearly two dozen organizations, including the American Academy of Family Physicians and the American College of Surgeons.
A theme emphasized in the joint guidelines is that all EDs need to be prepared to meet the unique needs of pediatric patients; 90% of all ED visits by patients under age 15 are to nonchildren’s hospitals, and roughly one-third of these visits are to EDs in rural and remote areas, which generally scored poorly in the initial national survey.
Another impetus for issuing the guidelines was recognition that pediatric ED visits are increasing even as the number of EDs nationwide is declining, worsening overcrowding, explained Dr. Gausche-Hill, professor of emergency medicine at the University of California, Los Angeles, and director of emergency medical services at Harbor-UCLA Medical Center.
She and her coinvestigators in the large coalition known as the National Pediatric Readiness Project sent the survey to more than 5,000 U.S. EDs. The response rate was an impressive 83%, despite the fact that the 55-question survey addressed 189 items and took a full hour to complete. Dr. Gausche-Hill attributed this remarkable response rate to on-site advocates’ passion for improved ED pediatric preparedness. Another factor: survey respondents received immediate feedback about how their hospital’s ED score compared with the average score of other hospitals with similar patient volume, as well as information about the top three things their hospital needs to do to reach a preparedness score of at least 80, which is the coalition’s short-term goal.
In analyzing the ED scores, the single most effective way most hospitals can improve their ED pediatric readiness score is for the hospital ED medical director to appoint a physician and/or a nurse pediatric emergency care coordinator. Hospitals with a coordinator scored higher, and the 42% of hospitals with both a physician and a nurse pediatric emergency care coordinator scored highest of all. Hospitals with at least one pediatric emergency care coordinator were more than fivefold more likely to have a quality improvement plan in place for ED pediatric patients, as recommended in a 2006 Institute of Medicine report that described the nation’s ED pediatric readiness as uneven.
In the new survey, 82% of EDs reported one or more barriers to compliance with the pediatric readiness guidelines. These barriers will be the focus of future quality improvement initiatives.
What’s next? Dr. Gausche-Hill said the coalition is reaching out to health care corporate groups in an effort to convince them to make changes in their hospitals.
"We’ve seen the Hospital Corporation of America make a huge initiative and change their average readiness score from 66 to 91 in the intervention hospitals. Also, Kaiser Permanente has initiated a readiness initiative," she said.
The plan is to keep the survey’s internet portal open so that hospitals can enter updated data and show continuous quality improvement. In addition, the coalition’s website – www.pediatricreadiness.org – includes tools to improve readiness.
Dr. Gausche-Hill reported having no financial conflicts regarding this project.
AT SAEM 2014
Key clinical point: The nation’s hospital emergency departments are markedly better prepared to treat pediatric patients than they were 7 years ago.
Major finding: The median ED pediatric readiness score in a new national survey was 69 on a 0-100 scale, as compared to 55 in a survey published 7 years ago.
Data source: This detailed survey was sent to all of the more than 5,000 EDs in the U.S. and its territories. The response rate was 83%.
Disclosures: The National Pediatric Readiness Project is supported primarily by funding from federal agencies. The study presenter reported having no financial conflicts.
A third of follicular thyroid lesions of undetermined significance were malignant
CHICAGO – At least in some institutions, about a third of follicular thyroid lesions of undetermined significance are malignant, reported researchers from the University of Wisconsin, Madison.
That rate is a higher proportion than the 5%-15% rate estimated by the Bethesda System, a national standard for reporting thyroid cytopathology, said Dr. Juan Carlos Jaume at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
"The message here is to be aware of your local institutional rates for cancer in FLUS, because following [the Bethesda System] may mislead you and your patient" when deciding on a course of action, be it surgery, repeat biopsy, or observation, he said. "Other institutions [should be encouraged to] do similar analyses to generate more accurate local guidelines for management of FLUS," noted Dr. Jaume, senior author of the study.
Of 1,420 nodules assessed over 2 years at the thyroid clinic at the University of Wisconsin, Madison, 134 (9.4%) were reported as follicular lesions of undetermined significance (FLUS) on fine-needle aspiration. Eighty patients opted for surgery; pathology revealed that 27 (34%) actually had differentiated thyroid cancer. Cancer also was found in four more patients, but at sites different from the original fine-needle aspiration. Most of the cancers (27) were papillary, 3 were follicular, and 1 was a Hurthle cell tumor, the investigators reported.
It’s unlikely the findings were due to selection bias, with patients who were more likely to have cancer opting for surgery. More than half of the patients chose surgery after discussing risks and benefits with their providers, not because of tumor progression. Almost all the others opted for surgery because of compression symptoms or because they had a nodule larger than 4 cm.
Even if there was a bias, "the highest expectation [with Bethesda] is 15%; our rate was 34%," a large difference, Dr. Jaume said. "As soon as we had the rate available, we conveyed the information" to providers so they could more accurately counsel patients. "I think eventually we will see an increase in the number of patients deciding on surgery."
The team performed the study because providers at the university had been relying on the Bethesda estimate to guide patients, but had a hunch that their local FLUS cancer rates were higher.
The majority of the 134 FLUS patients who opted against surgery chose ultrasound monitoring. Among the 22 who chose repeat fine-needle aspiration, half were rediagnosed with benign cytology, 5 were again diagnosed with FLUS, and most of the rest were lost to follow-up.
Among the 80 surgical patients, pathology was benign in 47 and parathyroid tissue was present in 1 biopsy. Records were unavailable for the final patient.
Some cytopathologists tend to call thyroid lesions FLUS more frequently than others; possibly, that predilection has something to do with the discordance in reported cancer rates, Dr. Jaume said.
Ultimately, the solution will be genetic analysis of fine-needle aspiration samples. There is a commercial product on the market, but "we are not using [it] in our institution because the negative predictive value is high, but the positive predictive value is low," he said.
The investigators had no relevant disclosures and had no outside funding for their work.
*Correction, 8/25/2014: An earlier version of this article misspelled Dr. Jaume's name.
CHICAGO – At least in some institutions, about a third of follicular thyroid lesions of undetermined significance are malignant, reported researchers from the University of Wisconsin, Madison.
That rate is a higher proportion than the 5%-15% rate estimated by the Bethesda System, a national standard for reporting thyroid cytopathology, said Dr. Juan Carlos Jaume at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
"The message here is to be aware of your local institutional rates for cancer in FLUS, because following [the Bethesda System] may mislead you and your patient" when deciding on a course of action, be it surgery, repeat biopsy, or observation, he said. "Other institutions [should be encouraged to] do similar analyses to generate more accurate local guidelines for management of FLUS," noted Dr. Jaume, senior author of the study.
Of 1,420 nodules assessed over 2 years at the thyroid clinic at the University of Wisconsin, Madison, 134 (9.4%) were reported as follicular lesions of undetermined significance (FLUS) on fine-needle aspiration. Eighty patients opted for surgery; pathology revealed that 27 (34%) actually had differentiated thyroid cancer. Cancer also was found in four more patients, but at sites different from the original fine-needle aspiration. Most of the cancers (27) were papillary, 3 were follicular, and 1 was a Hurthle cell tumor, the investigators reported.
It’s unlikely the findings were due to selection bias, with patients who were more likely to have cancer opting for surgery. More than half of the patients chose surgery after discussing risks and benefits with their providers, not because of tumor progression. Almost all the others opted for surgery because of compression symptoms or because they had a nodule larger than 4 cm.
Even if there was a bias, "the highest expectation [with Bethesda] is 15%; our rate was 34%," a large difference, Dr. Jaume said. "As soon as we had the rate available, we conveyed the information" to providers so they could more accurately counsel patients. "I think eventually we will see an increase in the number of patients deciding on surgery."
The team performed the study because providers at the university had been relying on the Bethesda estimate to guide patients, but had a hunch that their local FLUS cancer rates were higher.
The majority of the 134 FLUS patients who opted against surgery chose ultrasound monitoring. Among the 22 who chose repeat fine-needle aspiration, half were rediagnosed with benign cytology, 5 were again diagnosed with FLUS, and most of the rest were lost to follow-up.
Among the 80 surgical patients, pathology was benign in 47 and parathyroid tissue was present in 1 biopsy. Records were unavailable for the final patient.
Some cytopathologists tend to call thyroid lesions FLUS more frequently than others; possibly, that predilection has something to do with the discordance in reported cancer rates, Dr. Jaume said.
Ultimately, the solution will be genetic analysis of fine-needle aspiration samples. There is a commercial product on the market, but "we are not using [it] in our institution because the negative predictive value is high, but the positive predictive value is low," he said.
The investigators had no relevant disclosures and had no outside funding for their work.
*Correction, 8/25/2014: An earlier version of this article misspelled Dr. Jaume's name.
CHICAGO – At least in some institutions, about a third of follicular thyroid lesions of undetermined significance are malignant, reported researchers from the University of Wisconsin, Madison.
That rate is a higher proportion than the 5%-15% rate estimated by the Bethesda System, a national standard for reporting thyroid cytopathology, said Dr. Juan Carlos Jaume at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
"The message here is to be aware of your local institutional rates for cancer in FLUS, because following [the Bethesda System] may mislead you and your patient" when deciding on a course of action, be it surgery, repeat biopsy, or observation, he said. "Other institutions [should be encouraged to] do similar analyses to generate more accurate local guidelines for management of FLUS," noted Dr. Jaume, senior author of the study.
Of 1,420 nodules assessed over 2 years at the thyroid clinic at the University of Wisconsin, Madison, 134 (9.4%) were reported as follicular lesions of undetermined significance (FLUS) on fine-needle aspiration. Eighty patients opted for surgery; pathology revealed that 27 (34%) actually had differentiated thyroid cancer. Cancer also was found in four more patients, but at sites different from the original fine-needle aspiration. Most of the cancers (27) were papillary, 3 were follicular, and 1 was a Hurthle cell tumor, the investigators reported.
It’s unlikely the findings were due to selection bias, with patients who were more likely to have cancer opting for surgery. More than half of the patients chose surgery after discussing risks and benefits with their providers, not because of tumor progression. Almost all the others opted for surgery because of compression symptoms or because they had a nodule larger than 4 cm.
Even if there was a bias, "the highest expectation [with Bethesda] is 15%; our rate was 34%," a large difference, Dr. Jaume said. "As soon as we had the rate available, we conveyed the information" to providers so they could more accurately counsel patients. "I think eventually we will see an increase in the number of patients deciding on surgery."
The team performed the study because providers at the university had been relying on the Bethesda estimate to guide patients, but had a hunch that their local FLUS cancer rates were higher.
The majority of the 134 FLUS patients who opted against surgery chose ultrasound monitoring. Among the 22 who chose repeat fine-needle aspiration, half were rediagnosed with benign cytology, 5 were again diagnosed with FLUS, and most of the rest were lost to follow-up.
Among the 80 surgical patients, pathology was benign in 47 and parathyroid tissue was present in 1 biopsy. Records were unavailable for the final patient.
Some cytopathologists tend to call thyroid lesions FLUS more frequently than others; possibly, that predilection has something to do with the discordance in reported cancer rates, Dr. Jaume said.
Ultimately, the solution will be genetic analysis of fine-needle aspiration samples. There is a commercial product on the market, but "we are not using [it] in our institution because the negative predictive value is high, but the positive predictive value is low," he said.
The investigators had no relevant disclosures and had no outside funding for their work.
*Correction, 8/25/2014: An earlier version of this article misspelled Dr. Jaume's name.
AT ICE/ENDO 2014
Key clinical point: National estimates of FLUS malignancy may not apply to your institution.
Major finding: Among 80 patients diagnosed with FLUS who opted for surgery, the lesion turned out to be differentiated thyroid cancer in 27 (34%).
Data source: Retrospective study of outcomes for 134 FLUS nodules.
Disclosures: The investigators had no disclosures and had no outside funding for their study.
Medical consultation rates for surgical cases vary
The use of inpatient medical consultations for hospitalized surgical patients was found to vary by hospital, but consultations didn’t appear to have much of an impact on risk-adjusted 30-day mortality rates, a study found.
Rates of medical consultations varied from 50% to 91% among 91,684 patients undergoing colectomy and from 36% to 90% among 339,319 patients undergoing total hip replacements, a retrospective study found.
The variation was most dramatic for patients undergoing colectomy who did not have complications, among whom rates of inpatient medical consultation ranged from 47% to 79% between hospitals, Dr. Lena M. Chen and her associates reported. For patients undergoing colectomy who did have complications, 90%-95% received medical consultations.
Similarly, variation in the use of medical consultation for patients getting total hip replacement was wider for those without complications (36%-87%), compared with patients with complications (89%-94%).
The results highlight the fact that there’s no consensus on when and how to best provide medical consultation for hospitalized surgical patients. "Wide variation in medical consultation use – particularly among patients without complications – suggests that understanding when medical consultations provide value will be important as hospitals seek to increase their efficiency under bundled payments," wrote Dr. Chen of the University of Michigan, Ann Arbor (JAMA Intern. Med. 2014 Aug. 4 [doi:10.1001/jamainternmed.2014.3376]).
She and her associates analyzed Medicare claims data and American Hospital Association data on patients aged 65-99 years who underwent colectomy at 930 hospitals or total hip replacement at 1,589 hospitals in 2007-2010. These are 2 of the top 10 procedures performed on Medicare patients, and total hip replacement is included in the Centers for Medicare & Medicaid Services bundled payment demonstration project, the authors noted.
At least one medical consultant saw 69% of patients undergoing colectomy and 63% of patients getting a total hip replacement. Among patients who got consultations, colectomy patients saw consultants a median of nine times, and hip replacement patients saw consultants a median of three times.
Colectomy patients most often saw general medicine consultants (50%), followed by cardiologists (28%), oncologists (25%), or gastroenterologists (22%). Among patients receiving total hip replacement, 53% had a general medicine consultation, and the most common specialist consultations were for physical medicine and rehabilitation (11%) or cardiology (8%).
Approximately a third of hip replacement patients were "comanaged" by surgeons and medical consultants, defined by records of a claim for evaluation and management by a medicine physician on at least 70% of inpatient days.
It seems logical to assume that extra care from nonsurgical physicians should improve outcomes for some surgical patients, but an exploratory analysis of the data found that risk-adjusted 30-day mortality rates were not significantly different between hospitals with the greatest or least use of medical consultations, Dr. Chen reported. The 30-day mortality rate for colectomy patients was 5% at hospitals in the lowest quintile of medical consultations and 6% at hospitals in the highest quintile. The results for total hip replacement were similar.
Greater use of medical consultation was associated with a significantly greater likelihood of having at least one postoperative complication, affecting 24% of colectomy patients at hospitals in the lowest quintile of consultations and 28% at hospitals in the highest quintile. The results for total hip replacement were similar.
The National Institute of Aging and a University of Michigan McCubed grant funded the study. Dr. Chen reported having no relevant financial disclosures. One of her coinvestigators owns stock in ArborMetrix, a company that analyzes hospital quality and cost efficiency.
On Twitter @sherryboschert
Dr. Chen’s findings that rates of medical consultation varied widely for surgical patients without complications complement a 2010 study by Dr. Gulshan Sharma and his associates that found 35% of patients hospitalized for a common surgical procedure were comanaged by medicine physicians (Arch. Intern. Med. 2010;170:363-8).
"I agree [with Dr. Chen] that understanding the ‘value’ of medical consultation is an important next step, especially in low-risk patients undergoing elective surgery," Dr. Sharma commented in an article accompanying Dr. Chen’s study (JAMA Intern. Med. 2014 Aug. 4 [doi:10.1001/jamainternmed.2014.1499]).
Comanagement may benefit patients by increasing the use of evidence-based treatments or reducing the time to surgery, postoperative complications, the need for ICU care, the length of stay, and readmission rates, among other possibilities. But there are possible downsides, too, including potential confusion among caregivers, more complicated decision making, lack of "ownership" of problems, or added costs, he noted.
Hospitals with the greatest use of medical consultation in Dr. Chen’s study had higher risk-adjusted rates of 30-day mortality and complications, compared with hospitals with the least use of medical consultation (though the difference in mortality was not statistically significant). "Is there a potential harm associated with medical consultation?" Dr. Sharma asked.
"There is no one fit for all" hospitals, he wrote. Institutional data on quality and cost should drive decisions on the routine use of medical consultation. The practice of mandating comanagement of all surgical patients "should be discouraged," Dr. Sharma said. "During preoperative evaluation, patients with comorbid conditions and those at significant risk of postoperative complications should be considered for medical comanagement."
Dr. Sharma is director of the division of pulmonary critical care and sleep medicine at the University of Texas Medical Branch, Galveston. He reported having no relevant financial disclosures.
Dr. Chen’s findings that rates of medical consultation varied widely for surgical patients without complications complement a 2010 study by Dr. Gulshan Sharma and his associates that found 35% of patients hospitalized for a common surgical procedure were comanaged by medicine physicians (Arch. Intern. Med. 2010;170:363-8).
"I agree [with Dr. Chen] that understanding the ‘value’ of medical consultation is an important next step, especially in low-risk patients undergoing elective surgery," Dr. Sharma commented in an article accompanying Dr. Chen’s study (JAMA Intern. Med. 2014 Aug. 4 [doi:10.1001/jamainternmed.2014.1499]).
Comanagement may benefit patients by increasing the use of evidence-based treatments or reducing the time to surgery, postoperative complications, the need for ICU care, the length of stay, and readmission rates, among other possibilities. But there are possible downsides, too, including potential confusion among caregivers, more complicated decision making, lack of "ownership" of problems, or added costs, he noted.
Hospitals with the greatest use of medical consultation in Dr. Chen’s study had higher risk-adjusted rates of 30-day mortality and complications, compared with hospitals with the least use of medical consultation (though the difference in mortality was not statistically significant). "Is there a potential harm associated with medical consultation?" Dr. Sharma asked.
"There is no one fit for all" hospitals, he wrote. Institutional data on quality and cost should drive decisions on the routine use of medical consultation. The practice of mandating comanagement of all surgical patients "should be discouraged," Dr. Sharma said. "During preoperative evaluation, patients with comorbid conditions and those at significant risk of postoperative complications should be considered for medical comanagement."
Dr. Sharma is director of the division of pulmonary critical care and sleep medicine at the University of Texas Medical Branch, Galveston. He reported having no relevant financial disclosures.
Dr. Chen’s findings that rates of medical consultation varied widely for surgical patients without complications complement a 2010 study by Dr. Gulshan Sharma and his associates that found 35% of patients hospitalized for a common surgical procedure were comanaged by medicine physicians (Arch. Intern. Med. 2010;170:363-8).
"I agree [with Dr. Chen] that understanding the ‘value’ of medical consultation is an important next step, especially in low-risk patients undergoing elective surgery," Dr. Sharma commented in an article accompanying Dr. Chen’s study (JAMA Intern. Med. 2014 Aug. 4 [doi:10.1001/jamainternmed.2014.1499]).
Comanagement may benefit patients by increasing the use of evidence-based treatments or reducing the time to surgery, postoperative complications, the need for ICU care, the length of stay, and readmission rates, among other possibilities. But there are possible downsides, too, including potential confusion among caregivers, more complicated decision making, lack of "ownership" of problems, or added costs, he noted.
Hospitals with the greatest use of medical consultation in Dr. Chen’s study had higher risk-adjusted rates of 30-day mortality and complications, compared with hospitals with the least use of medical consultation (though the difference in mortality was not statistically significant). "Is there a potential harm associated with medical consultation?" Dr. Sharma asked.
"There is no one fit for all" hospitals, he wrote. Institutional data on quality and cost should drive decisions on the routine use of medical consultation. The practice of mandating comanagement of all surgical patients "should be discouraged," Dr. Sharma said. "During preoperative evaluation, patients with comorbid conditions and those at significant risk of postoperative complications should be considered for medical comanagement."
Dr. Sharma is director of the division of pulmonary critical care and sleep medicine at the University of Texas Medical Branch, Galveston. He reported having no relevant financial disclosures.
The use of inpatient medical consultations for hospitalized surgical patients was found to vary by hospital, but consultations didn’t appear to have much of an impact on risk-adjusted 30-day mortality rates, a study found.
Rates of medical consultations varied from 50% to 91% among 91,684 patients undergoing colectomy and from 36% to 90% among 339,319 patients undergoing total hip replacements, a retrospective study found.
The variation was most dramatic for patients undergoing colectomy who did not have complications, among whom rates of inpatient medical consultation ranged from 47% to 79% between hospitals, Dr. Lena M. Chen and her associates reported. For patients undergoing colectomy who did have complications, 90%-95% received medical consultations.
Similarly, variation in the use of medical consultation for patients getting total hip replacement was wider for those without complications (36%-87%), compared with patients with complications (89%-94%).
The results highlight the fact that there’s no consensus on when and how to best provide medical consultation for hospitalized surgical patients. "Wide variation in medical consultation use – particularly among patients without complications – suggests that understanding when medical consultations provide value will be important as hospitals seek to increase their efficiency under bundled payments," wrote Dr. Chen of the University of Michigan, Ann Arbor (JAMA Intern. Med. 2014 Aug. 4 [doi:10.1001/jamainternmed.2014.3376]).
She and her associates analyzed Medicare claims data and American Hospital Association data on patients aged 65-99 years who underwent colectomy at 930 hospitals or total hip replacement at 1,589 hospitals in 2007-2010. These are 2 of the top 10 procedures performed on Medicare patients, and total hip replacement is included in the Centers for Medicare & Medicaid Services bundled payment demonstration project, the authors noted.
At least one medical consultant saw 69% of patients undergoing colectomy and 63% of patients getting a total hip replacement. Among patients who got consultations, colectomy patients saw consultants a median of nine times, and hip replacement patients saw consultants a median of three times.
Colectomy patients most often saw general medicine consultants (50%), followed by cardiologists (28%), oncologists (25%), or gastroenterologists (22%). Among patients receiving total hip replacement, 53% had a general medicine consultation, and the most common specialist consultations were for physical medicine and rehabilitation (11%) or cardiology (8%).
Approximately a third of hip replacement patients were "comanaged" by surgeons and medical consultants, defined by records of a claim for evaluation and management by a medicine physician on at least 70% of inpatient days.
It seems logical to assume that extra care from nonsurgical physicians should improve outcomes for some surgical patients, but an exploratory analysis of the data found that risk-adjusted 30-day mortality rates were not significantly different between hospitals with the greatest or least use of medical consultations, Dr. Chen reported. The 30-day mortality rate for colectomy patients was 5% at hospitals in the lowest quintile of medical consultations and 6% at hospitals in the highest quintile. The results for total hip replacement were similar.
Greater use of medical consultation was associated with a significantly greater likelihood of having at least one postoperative complication, affecting 24% of colectomy patients at hospitals in the lowest quintile of consultations and 28% at hospitals in the highest quintile. The results for total hip replacement were similar.
The National Institute of Aging and a University of Michigan McCubed grant funded the study. Dr. Chen reported having no relevant financial disclosures. One of her coinvestigators owns stock in ArborMetrix, a company that analyzes hospital quality and cost efficiency.
On Twitter @sherryboschert
The use of inpatient medical consultations for hospitalized surgical patients was found to vary by hospital, but consultations didn’t appear to have much of an impact on risk-adjusted 30-day mortality rates, a study found.
Rates of medical consultations varied from 50% to 91% among 91,684 patients undergoing colectomy and from 36% to 90% among 339,319 patients undergoing total hip replacements, a retrospective study found.
The variation was most dramatic for patients undergoing colectomy who did not have complications, among whom rates of inpatient medical consultation ranged from 47% to 79% between hospitals, Dr. Lena M. Chen and her associates reported. For patients undergoing colectomy who did have complications, 90%-95% received medical consultations.
Similarly, variation in the use of medical consultation for patients getting total hip replacement was wider for those without complications (36%-87%), compared with patients with complications (89%-94%).
The results highlight the fact that there’s no consensus on when and how to best provide medical consultation for hospitalized surgical patients. "Wide variation in medical consultation use – particularly among patients without complications – suggests that understanding when medical consultations provide value will be important as hospitals seek to increase their efficiency under bundled payments," wrote Dr. Chen of the University of Michigan, Ann Arbor (JAMA Intern. Med. 2014 Aug. 4 [doi:10.1001/jamainternmed.2014.3376]).
She and her associates analyzed Medicare claims data and American Hospital Association data on patients aged 65-99 years who underwent colectomy at 930 hospitals or total hip replacement at 1,589 hospitals in 2007-2010. These are 2 of the top 10 procedures performed on Medicare patients, and total hip replacement is included in the Centers for Medicare & Medicaid Services bundled payment demonstration project, the authors noted.
At least one medical consultant saw 69% of patients undergoing colectomy and 63% of patients getting a total hip replacement. Among patients who got consultations, colectomy patients saw consultants a median of nine times, and hip replacement patients saw consultants a median of three times.
Colectomy patients most often saw general medicine consultants (50%), followed by cardiologists (28%), oncologists (25%), or gastroenterologists (22%). Among patients receiving total hip replacement, 53% had a general medicine consultation, and the most common specialist consultations were for physical medicine and rehabilitation (11%) or cardiology (8%).
Approximately a third of hip replacement patients were "comanaged" by surgeons and medical consultants, defined by records of a claim for evaluation and management by a medicine physician on at least 70% of inpatient days.
It seems logical to assume that extra care from nonsurgical physicians should improve outcomes for some surgical patients, but an exploratory analysis of the data found that risk-adjusted 30-day mortality rates were not significantly different between hospitals with the greatest or least use of medical consultations, Dr. Chen reported. The 30-day mortality rate for colectomy patients was 5% at hospitals in the lowest quintile of medical consultations and 6% at hospitals in the highest quintile. The results for total hip replacement were similar.
Greater use of medical consultation was associated with a significantly greater likelihood of having at least one postoperative complication, affecting 24% of colectomy patients at hospitals in the lowest quintile of consultations and 28% at hospitals in the highest quintile. The results for total hip replacement were similar.
The National Institute of Aging and a University of Michigan McCubed grant funded the study. Dr. Chen reported having no relevant financial disclosures. One of her coinvestigators owns stock in ArborMetrix, a company that analyzes hospital quality and cost efficiency.
On Twitter @sherryboschert
FROM JAMA INTERNAL MEDICINE
Key clinical point: In the era of bundled payments for episodes of care, consider when and how medical consultation for surgical patients is helpful.
Major finding: Use of medical consultations ranged from 50%-91% for colectomies and 36%-90% for total hip replacements.
Data source: A retrospective study of Medicare data on 431,003 older adults undergoing colectomy or total hip replacement in 2007-2010.
Disclosures: Dr. Chen reported having no financial disclosures. One of her associates owns stock in ArborMetrix, a company that analyzes hospital quality and cost efficiency.
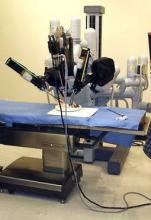
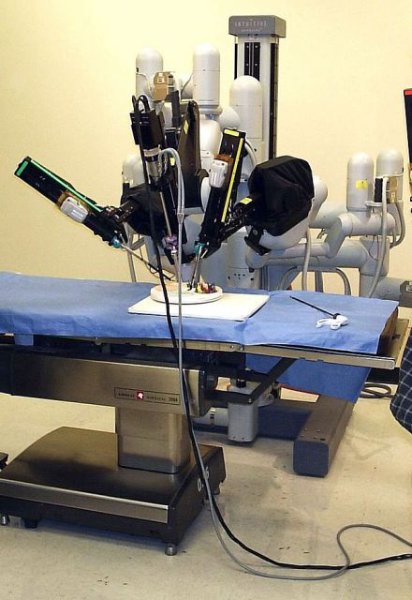
Robot-assisted radical cystectomy doesn’t cut complications
Compared with open radical cystectomy, robot-assisted laparoscopic radical cystectomy did not reduce the number or severity of complications among patients with bladder cancer enrolled in a single-center randomized clinical trial, according to a letter to the editor in the New England Journal of Medicine.
Retrospective studies have suggested that the robot-assisted laparoscopic approach reduces the complication rate and shortens the length of hospitalization in patients with bladder cancer, many of whom are older, are smokers, and have coexisting conditions. This trial was designed to compare that approach against open radical cystectomy, with both procedures using extracorporeal urinary diversion, in 118 patients who had stage Ta-3N0-3M0 bladder cancer, said Dr. Bernard H. Bochner and his associates at Memorial Sloan Kettering Cancer Center, New York.
The primary outcome – the rate of complications of grade 2-5 within 90 days of surgery—was 62% (37 of 60 patients) with robot-assisted laparoscopic surgery, which was not significantly different from the 66% rate (38 of 58 patients) with open surgery. Similarly, the rates of high-grade complications were not significantly different (22% vs 21%), and the mean length of hospitalization was identical (8 days) in both groups. The amount of intraoperative blood loss was smaller with the robot-assisted surgery (mean difference, 159 cc), but the duration of surgery was shorter with open surgery (mean difference, 127 minutes).
"Because the trial was performed by experienced surgeons at a single, high-volume referral center, the results may not be generalizable to all clinical settings. Nonetheless, these results highlight the need for randomized trials to inform the benefits and risks of new surgical technologies before widespread implementation," Dr. Bochner and his associates said (N. Engl. J. Med. 2014;371:389-90).
This work was supported by the Sidney Kimmel Center for Prostate and Urologic Cancers at Memorial Sloan Kettering Cancer Center, Pin Down Bladder Cancer, and the Michael A. and Zena Wiener Research and Therapeutics Program in Bladder Cancer. Dr. Bochner and his associates reported no financial conflicts of interest.
Compared with open radical cystectomy, robot-assisted laparoscopic radical cystectomy did not reduce the number or severity of complications among patients with bladder cancer enrolled in a single-center randomized clinical trial, according to a letter to the editor in the New England Journal of Medicine.
Retrospective studies have suggested that the robot-assisted laparoscopic approach reduces the complication rate and shortens the length of hospitalization in patients with bladder cancer, many of whom are older, are smokers, and have coexisting conditions. This trial was designed to compare that approach against open radical cystectomy, with both procedures using extracorporeal urinary diversion, in 118 patients who had stage Ta-3N0-3M0 bladder cancer, said Dr. Bernard H. Bochner and his associates at Memorial Sloan Kettering Cancer Center, New York.
The primary outcome – the rate of complications of grade 2-5 within 90 days of surgery—was 62% (37 of 60 patients) with robot-assisted laparoscopic surgery, which was not significantly different from the 66% rate (38 of 58 patients) with open surgery. Similarly, the rates of high-grade complications were not significantly different (22% vs 21%), and the mean length of hospitalization was identical (8 days) in both groups. The amount of intraoperative blood loss was smaller with the robot-assisted surgery (mean difference, 159 cc), but the duration of surgery was shorter with open surgery (mean difference, 127 minutes).
"Because the trial was performed by experienced surgeons at a single, high-volume referral center, the results may not be generalizable to all clinical settings. Nonetheless, these results highlight the need for randomized trials to inform the benefits and risks of new surgical technologies before widespread implementation," Dr. Bochner and his associates said (N. Engl. J. Med. 2014;371:389-90).
This work was supported by the Sidney Kimmel Center for Prostate and Urologic Cancers at Memorial Sloan Kettering Cancer Center, Pin Down Bladder Cancer, and the Michael A. and Zena Wiener Research and Therapeutics Program in Bladder Cancer. Dr. Bochner and his associates reported no financial conflicts of interest.
Compared with open radical cystectomy, robot-assisted laparoscopic radical cystectomy did not reduce the number or severity of complications among patients with bladder cancer enrolled in a single-center randomized clinical trial, according to a letter to the editor in the New England Journal of Medicine.
Retrospective studies have suggested that the robot-assisted laparoscopic approach reduces the complication rate and shortens the length of hospitalization in patients with bladder cancer, many of whom are older, are smokers, and have coexisting conditions. This trial was designed to compare that approach against open radical cystectomy, with both procedures using extracorporeal urinary diversion, in 118 patients who had stage Ta-3N0-3M0 bladder cancer, said Dr. Bernard H. Bochner and his associates at Memorial Sloan Kettering Cancer Center, New York.
The primary outcome – the rate of complications of grade 2-5 within 90 days of surgery—was 62% (37 of 60 patients) with robot-assisted laparoscopic surgery, which was not significantly different from the 66% rate (38 of 58 patients) with open surgery. Similarly, the rates of high-grade complications were not significantly different (22% vs 21%), and the mean length of hospitalization was identical (8 days) in both groups. The amount of intraoperative blood loss was smaller with the robot-assisted surgery (mean difference, 159 cc), but the duration of surgery was shorter with open surgery (mean difference, 127 minutes).
"Because the trial was performed by experienced surgeons at a single, high-volume referral center, the results may not be generalizable to all clinical settings. Nonetheless, these results highlight the need for randomized trials to inform the benefits and risks of new surgical technologies before widespread implementation," Dr. Bochner and his associates said (N. Engl. J. Med. 2014;371:389-90).
This work was supported by the Sidney Kimmel Center for Prostate and Urologic Cancers at Memorial Sloan Kettering Cancer Center, Pin Down Bladder Cancer, and the Michael A. and Zena Wiener Research and Therapeutics Program in Bladder Cancer. Dr. Bochner and his associates reported no financial conflicts of interest.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Decisions about surgical approach to bladder cancer surgery should not be based on the assumption that robot-assisted laparoscopic radical cystectomy will necessarily result in fewer complications.
Major finding: The primary outcome – the rate of complications of grade 2-5 within 90 days of surgery – was 62% (37 of 60 patients) with robot-assisted laparoscopic surgery, which was not significantly different from the 66% rate (38 of 58 patients) with open surgery.
Data source: A 3-year single-center randomized, controlled clinical trial involving 60 patients who underwent robot-assisted laparoscopic radical cystectomy and 58 who underwent open radical cystectomy to treat bladder cancer.
Disclosures: This work was supported by the Sidney Kimmel Center for Prostate and Urologic Cancers at Memorial Sloan-Kettering Cancer Center, Pin Down Bladder Cancer, and the Michael A. and Zena Wiener Research and Therapeutics Program in Bladder Cancer. Dr. Bochner and his associates reported no financial conflicts of interest.
Survival benefit from contralateral prophylactic mastectomy small
The absolute 20-year survival benefit from contralateral prophylactic mastectomy stands at less than 1%, regardless of age, estrogen receptor status, and cancer stage, a decision analysis demonstrated.
"Long-term survival in women with unilateral breast cancer treated with or without CPM depends upon several factors, including mortality of the primary breast cancer, risk of CBC [contralateral breast cancer], stage and mortality of the CBC, and the individual patient’s overall life expectancy," wrote Dr. Pamela R. Portschy of the University of Minnesota, Minneapolis.
The report was published July 16 in the Journal of the National Cancer Institute.
"Prospective randomized trials comparing CPM with no CPM are not feasible. Retrospective studies evaluating a potential survival benefit with CPM are limited by short follow-up, potential selection bias, and lack of important clinical information," noted Dr. Portschy and her associates.
They limited their analysis to women with stage I and II breast cancer without BRCA mutations. They developed a Markov model to simulate survival outcomes among those who did and did not have contralateral prophylactic mastectomy (CPM), and they used published studies to estimate probabilities for developing CBC, dying from CBC, dying from primary breast cancer, and age-specific mortality rates. Data were extracted from numerous sources including Surveillance, Epidemiology, and End Results (SEER), the Early Breast Cancer Trialists’ Collaborative Group, and the Oregon State Cancer Registry.
The researchers estimated the 20-year overall survival and life expectancy, but not quality of life or cost, and their analysis considered variation in age, estrogen receptor status, and cancer stage (J. Natl. Cancer Inst. 2014 July 16 [doi:10.1093/jnci/dju160]).
The predicted life expectancy gain from CPM ranged from .13 to .59 years for women with stage I breast cancer, and .08 to .29 years for those with stage II breast cancer. CPM conferred a life expectancy benefit among younger women and among those who had stage I and estrogen receptor–positive disease. "The potential benefit of CPM was consistently lower for patients with stage II breast cancer because of the worse prognosis associated with the primary breast cancer," the researchers wrote. "Similarly, the potential benefits of CPM are more modest for older women because they have relatively fewer years remaining of [life expectancy]."
Dr. Portschy and her associates could not identify any cohort of women that had a greater than 1% absolute survival difference at 20 years. In fact, the predicted 20-year survival differences ranged from .56 to .94% for women with stage I breast cancer and .36 to .61% for those with stage II breast cancer.
The researchers acknowledged limitations of the study, including the fact that the results "do not apply to BRCA gene mutation carriers with unilateral breast cancer who have a cumulative 10-year risk of CBC of approximately 30% to 40%," they wrote. "The outcomes of this analysis were limited to overall and disease-specific survival; we did not evaluate other important outcomes such as surgical complications and quality of life. Also, we assumed the mortality of CBC was the same as the mortality of the index cancer reported by SEER."
They also noted that survival is not the only potential benefit of a cancer risk reduction strategy. "Effects on cancer-related anxiety, cosmesis, and self-image are also important in the decision-making process," they wrote. "For some women, the negative impact of CPM on quality of life may outweigh a potential survival benefit. For others who are very anxious about CBC, CPM may result in a psychological benefit even if survival benefits are minimal."
They concluded that the survival estimates from their Markov model "may be useful for physicians and breast cancer patients to arrive at evidence-based informed decisions regarding CPM. Moreover, the use of accurate and easily understood decision aids may reverse some of the mastectomy trends recently observed in the United States."
The researchers stated that they had no relevant financial conflicts to disclose.
The decision of whether or not to undergo a contralateral prophylactic mastectomy after being treated for breast cancer is a difficult one for many women. The goal of such aggressive therapy is to lower the likelihood of a second primary carcinoma. The downsides are operative risk, impairment of the woman’s self-image, and short-term and long-term morbidities.
This is a well done analysis from an experienced group of investigators and is based on the currently available data. Given the JNCI audience, we shall refrain from niggling points about modeling. Rather, we will stick to the big picture and clinical implications. Although the survival benefit from CPM is small as demonstrated in this model, it is greater than zero, which suggests that for some patients even that small gain may be enough to make it a not unreasonable choice.
From a societal perspective, which was not addressed by Portschy et al., the associated costs of CPM, including the procedure, its complications, reconstruction, and perhaps psychotherapy, may outweigh the modest benefit CPM provides. The small denominator of the cost-effectiveness ratio, were one to be calculated, would imply that the ratio would be very high, making CPM a suboptimal use of health care dollars. Further, we suspect that adding quality of life to the analysis would diminish the benefit and well might turn it into a net harm, in particular for patients with high concern for negative impact of CPM on cosmesis, self image, and morbidity. However, in a fraction of patients who are very troubled by a 0.7% risk of a second, contralateral cancer, CPM might provide an acceptable benefit. The balance between harm and benefit depends on the patient’s preferences and highlights the importance of capturing the patient’s values and expectations before considering CPM.
Of course, these conclusions are based on analysis of women who are at average risk for a contralateral second primary. In women at substantially higher risk (based either on family history or genetics), the benefit of CPM might be far greater, and CPM might be a good choice for the patient or for society.
Dr. Stephen G. Pauker and Dr. Mohamed Alseiari are with the division of clinical decision making in the department of medicine at Tufts Medical Center, Boston. They reported no relevant financial conflicts. This was excerpted from an editorial (J. Natl. Cancer Inst. 2014 July 16 [doi:10.1093/jnci/dju175]).
The decision of whether or not to undergo a contralateral prophylactic mastectomy after being treated for breast cancer is a difficult one for many women. The goal of such aggressive therapy is to lower the likelihood of a second primary carcinoma. The downsides are operative risk, impairment of the woman’s self-image, and short-term and long-term morbidities.
This is a well done analysis from an experienced group of investigators and is based on the currently available data. Given the JNCI audience, we shall refrain from niggling points about modeling. Rather, we will stick to the big picture and clinical implications. Although the survival benefit from CPM is small as demonstrated in this model, it is greater than zero, which suggests that for some patients even that small gain may be enough to make it a not unreasonable choice.
From a societal perspective, which was not addressed by Portschy et al., the associated costs of CPM, including the procedure, its complications, reconstruction, and perhaps psychotherapy, may outweigh the modest benefit CPM provides. The small denominator of the cost-effectiveness ratio, were one to be calculated, would imply that the ratio would be very high, making CPM a suboptimal use of health care dollars. Further, we suspect that adding quality of life to the analysis would diminish the benefit and well might turn it into a net harm, in particular for patients with high concern for negative impact of CPM on cosmesis, self image, and morbidity. However, in a fraction of patients who are very troubled by a 0.7% risk of a second, contralateral cancer, CPM might provide an acceptable benefit. The balance between harm and benefit depends on the patient’s preferences and highlights the importance of capturing the patient’s values and expectations before considering CPM.
Of course, these conclusions are based on analysis of women who are at average risk for a contralateral second primary. In women at substantially higher risk (based either on family history or genetics), the benefit of CPM might be far greater, and CPM might be a good choice for the patient or for society.
Dr. Stephen G. Pauker and Dr. Mohamed Alseiari are with the division of clinical decision making in the department of medicine at Tufts Medical Center, Boston. They reported no relevant financial conflicts. This was excerpted from an editorial (J. Natl. Cancer Inst. 2014 July 16 [doi:10.1093/jnci/dju175]).
The decision of whether or not to undergo a contralateral prophylactic mastectomy after being treated for breast cancer is a difficult one for many women. The goal of such aggressive therapy is to lower the likelihood of a second primary carcinoma. The downsides are operative risk, impairment of the woman’s self-image, and short-term and long-term morbidities.
This is a well done analysis from an experienced group of investigators and is based on the currently available data. Given the JNCI audience, we shall refrain from niggling points about modeling. Rather, we will stick to the big picture and clinical implications. Although the survival benefit from CPM is small as demonstrated in this model, it is greater than zero, which suggests that for some patients even that small gain may be enough to make it a not unreasonable choice.
From a societal perspective, which was not addressed by Portschy et al., the associated costs of CPM, including the procedure, its complications, reconstruction, and perhaps psychotherapy, may outweigh the modest benefit CPM provides. The small denominator of the cost-effectiveness ratio, were one to be calculated, would imply that the ratio would be very high, making CPM a suboptimal use of health care dollars. Further, we suspect that adding quality of life to the analysis would diminish the benefit and well might turn it into a net harm, in particular for patients with high concern for negative impact of CPM on cosmesis, self image, and morbidity. However, in a fraction of patients who are very troubled by a 0.7% risk of a second, contralateral cancer, CPM might provide an acceptable benefit. The balance between harm and benefit depends on the patient’s preferences and highlights the importance of capturing the patient’s values and expectations before considering CPM.
Of course, these conclusions are based on analysis of women who are at average risk for a contralateral second primary. In women at substantially higher risk (based either on family history or genetics), the benefit of CPM might be far greater, and CPM might be a good choice for the patient or for society.
Dr. Stephen G. Pauker and Dr. Mohamed Alseiari are with the division of clinical decision making in the department of medicine at Tufts Medical Center, Boston. They reported no relevant financial conflicts. This was excerpted from an editorial (J. Natl. Cancer Inst. 2014 July 16 [doi:10.1093/jnci/dju175]).
The absolute 20-year survival benefit from contralateral prophylactic mastectomy stands at less than 1%, regardless of age, estrogen receptor status, and cancer stage, a decision analysis demonstrated.
"Long-term survival in women with unilateral breast cancer treated with or without CPM depends upon several factors, including mortality of the primary breast cancer, risk of CBC [contralateral breast cancer], stage and mortality of the CBC, and the individual patient’s overall life expectancy," wrote Dr. Pamela R. Portschy of the University of Minnesota, Minneapolis.
The report was published July 16 in the Journal of the National Cancer Institute.
"Prospective randomized trials comparing CPM with no CPM are not feasible. Retrospective studies evaluating a potential survival benefit with CPM are limited by short follow-up, potential selection bias, and lack of important clinical information," noted Dr. Portschy and her associates.
They limited their analysis to women with stage I and II breast cancer without BRCA mutations. They developed a Markov model to simulate survival outcomes among those who did and did not have contralateral prophylactic mastectomy (CPM), and they used published studies to estimate probabilities for developing CBC, dying from CBC, dying from primary breast cancer, and age-specific mortality rates. Data were extracted from numerous sources including Surveillance, Epidemiology, and End Results (SEER), the Early Breast Cancer Trialists’ Collaborative Group, and the Oregon State Cancer Registry.
The researchers estimated the 20-year overall survival and life expectancy, but not quality of life or cost, and their analysis considered variation in age, estrogen receptor status, and cancer stage (J. Natl. Cancer Inst. 2014 July 16 [doi:10.1093/jnci/dju160]).
The predicted life expectancy gain from CPM ranged from .13 to .59 years for women with stage I breast cancer, and .08 to .29 years for those with stage II breast cancer. CPM conferred a life expectancy benefit among younger women and among those who had stage I and estrogen receptor–positive disease. "The potential benefit of CPM was consistently lower for patients with stage II breast cancer because of the worse prognosis associated with the primary breast cancer," the researchers wrote. "Similarly, the potential benefits of CPM are more modest for older women because they have relatively fewer years remaining of [life expectancy]."
Dr. Portschy and her associates could not identify any cohort of women that had a greater than 1% absolute survival difference at 20 years. In fact, the predicted 20-year survival differences ranged from .56 to .94% for women with stage I breast cancer and .36 to .61% for those with stage II breast cancer.
The researchers acknowledged limitations of the study, including the fact that the results "do not apply to BRCA gene mutation carriers with unilateral breast cancer who have a cumulative 10-year risk of CBC of approximately 30% to 40%," they wrote. "The outcomes of this analysis were limited to overall and disease-specific survival; we did not evaluate other important outcomes such as surgical complications and quality of life. Also, we assumed the mortality of CBC was the same as the mortality of the index cancer reported by SEER."
They also noted that survival is not the only potential benefit of a cancer risk reduction strategy. "Effects on cancer-related anxiety, cosmesis, and self-image are also important in the decision-making process," they wrote. "For some women, the negative impact of CPM on quality of life may outweigh a potential survival benefit. For others who are very anxious about CBC, CPM may result in a psychological benefit even if survival benefits are minimal."
They concluded that the survival estimates from their Markov model "may be useful for physicians and breast cancer patients to arrive at evidence-based informed decisions regarding CPM. Moreover, the use of accurate and easily understood decision aids may reverse some of the mastectomy trends recently observed in the United States."
The researchers stated that they had no relevant financial conflicts to disclose.
The absolute 20-year survival benefit from contralateral prophylactic mastectomy stands at less than 1%, regardless of age, estrogen receptor status, and cancer stage, a decision analysis demonstrated.
"Long-term survival in women with unilateral breast cancer treated with or without CPM depends upon several factors, including mortality of the primary breast cancer, risk of CBC [contralateral breast cancer], stage and mortality of the CBC, and the individual patient’s overall life expectancy," wrote Dr. Pamela R. Portschy of the University of Minnesota, Minneapolis.
The report was published July 16 in the Journal of the National Cancer Institute.
"Prospective randomized trials comparing CPM with no CPM are not feasible. Retrospective studies evaluating a potential survival benefit with CPM are limited by short follow-up, potential selection bias, and lack of important clinical information," noted Dr. Portschy and her associates.
They limited their analysis to women with stage I and II breast cancer without BRCA mutations. They developed a Markov model to simulate survival outcomes among those who did and did not have contralateral prophylactic mastectomy (CPM), and they used published studies to estimate probabilities for developing CBC, dying from CBC, dying from primary breast cancer, and age-specific mortality rates. Data were extracted from numerous sources including Surveillance, Epidemiology, and End Results (SEER), the Early Breast Cancer Trialists’ Collaborative Group, and the Oregon State Cancer Registry.
The researchers estimated the 20-year overall survival and life expectancy, but not quality of life or cost, and their analysis considered variation in age, estrogen receptor status, and cancer stage (J. Natl. Cancer Inst. 2014 July 16 [doi:10.1093/jnci/dju160]).
The predicted life expectancy gain from CPM ranged from .13 to .59 years for women with stage I breast cancer, and .08 to .29 years for those with stage II breast cancer. CPM conferred a life expectancy benefit among younger women and among those who had stage I and estrogen receptor–positive disease. "The potential benefit of CPM was consistently lower for patients with stage II breast cancer because of the worse prognosis associated with the primary breast cancer," the researchers wrote. "Similarly, the potential benefits of CPM are more modest for older women because they have relatively fewer years remaining of [life expectancy]."
Dr. Portschy and her associates could not identify any cohort of women that had a greater than 1% absolute survival difference at 20 years. In fact, the predicted 20-year survival differences ranged from .56 to .94% for women with stage I breast cancer and .36 to .61% for those with stage II breast cancer.
The researchers acknowledged limitations of the study, including the fact that the results "do not apply to BRCA gene mutation carriers with unilateral breast cancer who have a cumulative 10-year risk of CBC of approximately 30% to 40%," they wrote. "The outcomes of this analysis were limited to overall and disease-specific survival; we did not evaluate other important outcomes such as surgical complications and quality of life. Also, we assumed the mortality of CBC was the same as the mortality of the index cancer reported by SEER."
They also noted that survival is not the only potential benefit of a cancer risk reduction strategy. "Effects on cancer-related anxiety, cosmesis, and self-image are also important in the decision-making process," they wrote. "For some women, the negative impact of CPM on quality of life may outweigh a potential survival benefit. For others who are very anxious about CBC, CPM may result in a psychological benefit even if survival benefits are minimal."
They concluded that the survival estimates from their Markov model "may be useful for physicians and breast cancer patients to arrive at evidence-based informed decisions regarding CPM. Moreover, the use of accurate and easily understood decision aids may reverse some of the mastectomy trends recently observed in the United States."
The researchers stated that they had no relevant financial conflicts to disclose.
FROM THE JOURNAL OF THE NATIONAL CANCER INSTITUTE
Key clinical point: The long-term survival benefit of contralateral prophylactic mastectomy is small.
Major Finding: The absolute 20-year survival benefit from contralateral prophylactic mastectomy was less than 1% among all age groups, regardless of estrogen receptor status and cancer stage.
Data Source: Results from a Markov model designed to simulate 20-year survival outcomes among those who did and did not have CPM, with considerations for variation in age, estrogen receptor status, and cancer stage.
Disclosures: The researchers disclosed no relevant financial conflicts.
Hospital use of minimally invasive surgery shows disparity in surgical care nationwide
The use of minimally invasive surgery for appendectomy, colectomy, hysterectomy, and lung lobectomy varies widely in the United States, even though the complication rates were lower from each procedure than with open surgery, results from a large retrospective study demonstrated.
"This study has important implications for quality improvement," researchers led by Dr. Martin A. Makary, professor of surgery at Johns Hopkins University, Baltimore, wrote. "Based on our findings, many hospitals have an opportunity to decrease surgical complications by increasing utilization of minimally invasive surgery."
To investigate the levels of variation in the use of minimally invasive surgery across the United States, Dr. Makary and his associates used the National Inpatient Sample database, which is administered by the Agency for Healthcare Research & Quality, to evaluate hospitalizations at hospitals that performed at least 10 of these procedures in 2010. The sample included 1,051 hospitals in 45 states, and was limited to appendectomy, colectomy, hysterectomy, and lung lobectomy. The researchers used a propensity score model to calculate the predicted proportion of minimally invasive operations for each hospital based on patient characteristics. For each procedure, they categorized hospitals as low, medium, or high based on their actual to predicted proportion of minimally invasive surgery use (BMJ 2014;349:g4198).
On average, the use of minimally invasive surgery by the hospitals sampled was 71% for appendectomy, 28% for colectomy, 13% for hysterectomy, and 32% for lung lobectomy. Overall surgical complications for minimally invasive surgery, compared with open surgery, were, respectively, for appendectomy: 3.94% vs. 7.90% (P less than .001); colectomy: 13.8% vs. 35.8% (P less than .001); hysterectomy: 4.69% vs. 6.64% (P less than .001); and lung lobectomy: 17.1% vs. 25.4% (P less than .05). "In our analysis using Agency for Healthcare Research & Quality patient safety indicators for surgical care, we noted fewer wound, infectious, thrombotic, pulmonary, and mortality complications associated with minimally invasive surgery," the researchers wrote. "Based on our findings, increased hospital utilization of minimally invasive surgery at many U.S. hospitals represents a tremendous opportunity to prevent surgical site infection events."
The use of minimally invasive surgery was highly variable among the sampled hospitals. In fact, some never used minimally invasive surgery for some of the four procedures, while others used minimally invasive surgery for more than 75% of these procedures. Factors associated with the use of minimally invasive surgery were urban location, large hospital size, teaching hospital, and, for certain procedures, the hospital being located in the Midwest, South, or West.
"This [regional] disparity may be due to the broad range of surgical services some surgeons in rural areas are required to provide, and a scarcity of surgical specialists in such areas with advanced skills in minimally invasive surgery. Alternatively, the disparity may be a function of a lack of patient awareness about surgical options, decreased competition for patients, or a lack of minimally invasive surgery equipment, staff, or support in rural areas," the researchers wrote.
The findings of underutilization of minimally invasive surgery may also have something to do with a training gap.
"One reason that hospitals may be underperforming minimally invasive surgery is variability in appropriate training in residency and fellowship," Dr. Makary and his associates wrote. "One strategy that hospitals may consider in managing surgeons who cannot or choose not to acquire skills for performing minimally invasive surgery is to create a division of labor where patients who are not candidates for minimally invasive surgery are cared for by these surgeons. Increased standardization of competencies in minimally invasive surgery in surgical residency is needed to tackle wide variations in training."
The researchers acknowledged certain limitations of the study, including the fact that administrative claims data "can have incomplete coding, particularly of preexisting conditions," they wrote. "Another limitation is the lack of information available in the database for physician factors, such as laparoscopic training and experience that may influence the choice of procedure."
The researchers stated that they had no relevant financial conflicts to disclose.
The use of minimally invasive surgery for appendectomy, colectomy, hysterectomy, and lung lobectomy varies widely in the United States, even though the complication rates were lower from each procedure than with open surgery, results from a large retrospective study demonstrated.
"This study has important implications for quality improvement," researchers led by Dr. Martin A. Makary, professor of surgery at Johns Hopkins University, Baltimore, wrote. "Based on our findings, many hospitals have an opportunity to decrease surgical complications by increasing utilization of minimally invasive surgery."
To investigate the levels of variation in the use of minimally invasive surgery across the United States, Dr. Makary and his associates used the National Inpatient Sample database, which is administered by the Agency for Healthcare Research & Quality, to evaluate hospitalizations at hospitals that performed at least 10 of these procedures in 2010. The sample included 1,051 hospitals in 45 states, and was limited to appendectomy, colectomy, hysterectomy, and lung lobectomy. The researchers used a propensity score model to calculate the predicted proportion of minimally invasive operations for each hospital based on patient characteristics. For each procedure, they categorized hospitals as low, medium, or high based on their actual to predicted proportion of minimally invasive surgery use (BMJ 2014;349:g4198).
On average, the use of minimally invasive surgery by the hospitals sampled was 71% for appendectomy, 28% for colectomy, 13% for hysterectomy, and 32% for lung lobectomy. Overall surgical complications for minimally invasive surgery, compared with open surgery, were, respectively, for appendectomy: 3.94% vs. 7.90% (P less than .001); colectomy: 13.8% vs. 35.8% (P less than .001); hysterectomy: 4.69% vs. 6.64% (P less than .001); and lung lobectomy: 17.1% vs. 25.4% (P less than .05). "In our analysis using Agency for Healthcare Research & Quality patient safety indicators for surgical care, we noted fewer wound, infectious, thrombotic, pulmonary, and mortality complications associated with minimally invasive surgery," the researchers wrote. "Based on our findings, increased hospital utilization of minimally invasive surgery at many U.S. hospitals represents a tremendous opportunity to prevent surgical site infection events."
The use of minimally invasive surgery was highly variable among the sampled hospitals. In fact, some never used minimally invasive surgery for some of the four procedures, while others used minimally invasive surgery for more than 75% of these procedures. Factors associated with the use of minimally invasive surgery were urban location, large hospital size, teaching hospital, and, for certain procedures, the hospital being located in the Midwest, South, or West.
"This [regional] disparity may be due to the broad range of surgical services some surgeons in rural areas are required to provide, and a scarcity of surgical specialists in such areas with advanced skills in minimally invasive surgery. Alternatively, the disparity may be a function of a lack of patient awareness about surgical options, decreased competition for patients, or a lack of minimally invasive surgery equipment, staff, or support in rural areas," the researchers wrote.
The findings of underutilization of minimally invasive surgery may also have something to do with a training gap.
"One reason that hospitals may be underperforming minimally invasive surgery is variability in appropriate training in residency and fellowship," Dr. Makary and his associates wrote. "One strategy that hospitals may consider in managing surgeons who cannot or choose not to acquire skills for performing minimally invasive surgery is to create a division of labor where patients who are not candidates for minimally invasive surgery are cared for by these surgeons. Increased standardization of competencies in minimally invasive surgery in surgical residency is needed to tackle wide variations in training."
The researchers acknowledged certain limitations of the study, including the fact that administrative claims data "can have incomplete coding, particularly of preexisting conditions," they wrote. "Another limitation is the lack of information available in the database for physician factors, such as laparoscopic training and experience that may influence the choice of procedure."
The researchers stated that they had no relevant financial conflicts to disclose.
The use of minimally invasive surgery for appendectomy, colectomy, hysterectomy, and lung lobectomy varies widely in the United States, even though the complication rates were lower from each procedure than with open surgery, results from a large retrospective study demonstrated.
"This study has important implications for quality improvement," researchers led by Dr. Martin A. Makary, professor of surgery at Johns Hopkins University, Baltimore, wrote. "Based on our findings, many hospitals have an opportunity to decrease surgical complications by increasing utilization of minimally invasive surgery."
To investigate the levels of variation in the use of minimally invasive surgery across the United States, Dr. Makary and his associates used the National Inpatient Sample database, which is administered by the Agency for Healthcare Research & Quality, to evaluate hospitalizations at hospitals that performed at least 10 of these procedures in 2010. The sample included 1,051 hospitals in 45 states, and was limited to appendectomy, colectomy, hysterectomy, and lung lobectomy. The researchers used a propensity score model to calculate the predicted proportion of minimally invasive operations for each hospital based on patient characteristics. For each procedure, they categorized hospitals as low, medium, or high based on their actual to predicted proportion of minimally invasive surgery use (BMJ 2014;349:g4198).
On average, the use of minimally invasive surgery by the hospitals sampled was 71% for appendectomy, 28% for colectomy, 13% for hysterectomy, and 32% for lung lobectomy. Overall surgical complications for minimally invasive surgery, compared with open surgery, were, respectively, for appendectomy: 3.94% vs. 7.90% (P less than .001); colectomy: 13.8% vs. 35.8% (P less than .001); hysterectomy: 4.69% vs. 6.64% (P less than .001); and lung lobectomy: 17.1% vs. 25.4% (P less than .05). "In our analysis using Agency for Healthcare Research & Quality patient safety indicators for surgical care, we noted fewer wound, infectious, thrombotic, pulmonary, and mortality complications associated with minimally invasive surgery," the researchers wrote. "Based on our findings, increased hospital utilization of minimally invasive surgery at many U.S. hospitals represents a tremendous opportunity to prevent surgical site infection events."
The use of minimally invasive surgery was highly variable among the sampled hospitals. In fact, some never used minimally invasive surgery for some of the four procedures, while others used minimally invasive surgery for more than 75% of these procedures. Factors associated with the use of minimally invasive surgery were urban location, large hospital size, teaching hospital, and, for certain procedures, the hospital being located in the Midwest, South, or West.
"This [regional] disparity may be due to the broad range of surgical services some surgeons in rural areas are required to provide, and a scarcity of surgical specialists in such areas with advanced skills in minimally invasive surgery. Alternatively, the disparity may be a function of a lack of patient awareness about surgical options, decreased competition for patients, or a lack of minimally invasive surgery equipment, staff, or support in rural areas," the researchers wrote.
The findings of underutilization of minimally invasive surgery may also have something to do with a training gap.
"One reason that hospitals may be underperforming minimally invasive surgery is variability in appropriate training in residency and fellowship," Dr. Makary and his associates wrote. "One strategy that hospitals may consider in managing surgeons who cannot or choose not to acquire skills for performing minimally invasive surgery is to create a division of labor where patients who are not candidates for minimally invasive surgery are cared for by these surgeons. Increased standardization of competencies in minimally invasive surgery in surgical residency is needed to tackle wide variations in training."
The researchers acknowledged certain limitations of the study, including the fact that administrative claims data "can have incomplete coding, particularly of preexisting conditions," they wrote. "Another limitation is the lack of information available in the database for physician factors, such as laparoscopic training and experience that may influence the choice of procedure."
The researchers stated that they had no relevant financial conflicts to disclose.
FROM THE BRITISH MEDICAL JOURNAL
Key clinical point: Hospital use of minimally invasive surgical procedures appears to vary widely in the United States.
Major Finding:. The use of minimally invasive surgery by the hospitals sampled was 71% for appendectomy, 28% for colectomy, 13% for hysterectomy, and 32% for lung lobectomy.
Data Source: An analysis of data from the National Inpatient Sample in 2010 that included 1,051 hospitals in 45 states, and was limited to appendectomy, colectomy, hysterectomy, and lung lobectomy.
Disclosures: The authors stated that they had no relevant financial conflicts to disclose.
Helping breast cancer patients analyze risk
Dr. Sarah Hawley and her coinvestigators are to be applauded for generating insightful data regarding factors and concerns that motivate a woman to undergo contralateral prophylactic mastectomy in the setting of unilateral breast cancer (JAMA Surgery 2014 May 21 [doi:10.1001/jamasurg.2013.5689]).
Hawley et al. found that fear of recurrence was one of the strongest factors leading women to choose contralateral prophylactic mastectomy (CPM). This finding clearly demonstrates that we need to do a better job of explaining and defining the significance of (i) breast cancer local recurrence; (ii) breast cancer distant recurrence; and (iii) the development of a new/second primary breast cancer. Since cross-metastasis of a primary breast cancer to the contralateral breast is an extremely rare event, and since distant metastasis from the initial primary breast cancer tends to determine survival rates, CPM by definition will influence the incidence of only the third pattern. Furthermore, since the risk of experiencing a new contralateral malignancy is less than 1% per year for the general population of breast cancer patients, only a minority of these women will actually become bilateral breast cancer patients. Fear of recurrence is therefore a totally inappropriate reason for patients to pursue CPM, and the reasonableness of CPM to reduce the risk of a contralateral new primary breast cancer is debatable.
It can be reasonably stated that prophylactic surgery by definition is never a medically indicated necessity. Furthermore, despite the fact that a personal history of breast cancer is indeed a risk factor for developing a second primary cancer in the contralateral breast, numerous studies have demonstrated equivalent survival rates for women with unilateral breast cancer, compared with those diagnosed with bilateral/metachronous breast cancer (Cancer 2001;91:1845-53; Am. J. Clin. Oncol. 1997;20:541-5). Survival tends to be driven by the stage and effectiveness of treatment for the first cancer. By virtue of its earlier presentation, it is likely that the initially diagnosed cancer has established itself as the faster-growing malignancy with a lead time advantage in establishing distant organ micrometastatic disease; furthermore, patients with a unilateral breast cancer diagnosis are generally undergoing diligent surveillance and a contralateral malignancy is more often detected at an early stage.
Messages to our patients
It is essential for those of us who manage breast cancer to clearly emphasize several messages to our newly diagnosed breast cancer patients: First, although unilateral breast cancer increases the likelihood of developing a second primary tumor, it is certainly not inevitable, and in fact, the majority of patients are not destined to develop contralateral disease. Second, reducing the risk of being diagnosed with a contralateral breast cancer does not mitigate the mortality risk associated with the first cancer. And, finally, prophylactic mastectomy is the most aggressive and effective strategy for reducing the incidence of primary breast cancer (by approximately 90%), but it does not confer complete protection, as microscopic foci of breast tissue may be left behind in the mastectomy skin flaps, along the pectoralis, or in the axilla.
The messages above are critical: Our patients must understand that the priority is to address the known cancer. In this regard, appropriately selected patients should be encouraged to strongly consider breast-conserving surgery whenever feasible, as this low-morbidity treatment is equivalent to mastectomy from the perspective of overall survival. The question of CPM is most relevant for those patients that are ineligible for breast conservation or patients unwilling to undergo lumpectomy and breast radiation.
If a mastectomy for the cancerous breast is planned, we must then address the questions that routinely arise regarding bilateral surgery. In our efforts to clarify the reality of what CPM can and cannot achieve, we must also avoid being too dogmatic and paternalistic with our patients. There are clearly specific scenarios, as delineated in Dr. Hawley’s work, where the risk of a second primary breast cancer is likely to be considered excessive by most women, and where the decision to pursue CPM may be easier. Examples of such cases would be women known to harbor BRCA mutations or women with suspected hereditary susceptibility based on a strong family history of breast and/or ovarian cancer. The risk of a new contralateral breast cancer can be in the range of 4%-5% per year in cases of hereditary disease, compared with the general population of women with sporadic breast cancer, where the risk ranges from 0.25% to 1% per year.
Conveying an understanding of risk
Patients must understand that the risk to the contralateral breast is predominantly expressed in the future – the likelihood of having a clinically occult, incidentally detected cancer identified in the contralateral mastectomy specimen is only 6%, as demonstrated most recently by King et al. (Ann. Surg. 2011;254:2-7), and with ductal carcinoma in situ accounting for the high majority of these lesions.
Defining the threshold for the amount of risk that an individual woman finds to be acceptable, however, can be a very difficult and personal decision. Even after a patient comes to understand that CPM is unlikely to provide a survival advantage, she may continue to request bilateral surgery purely for the risk-reducing benefits, and out of a desire to minimize her chances of having to repeat the breast cancer diagnosis and treatment experience. In some cases this choice will be influenced by reconstruction factors. A woman may be motivated to pursue bilateral surgery if she has an adequate volume of abdominal tissue because of the fact that the autogenous TRAM (transverse rectus abdominis myocutaneous) flap can be harvested only once. In other cases the decision is influenced by body habitus, for example, a woman with large pendulous breasts who is not interested in breast reconstruction may decide that she is more comfortable with a symmetrically flat chest wall in order to avoid chest wall imbalance and the inconvenience of finding/wearing a prosthesis that matches the remaining breast.
As breast cancer surgeons we should openly discuss these issues with our patients and present viable alternatives when feasible, such as reduction mammoplasty for the large-breasted patient. Ultimately, however, the patient must decide the surgical approach that provides her with the optimal sense of treatment satisfaction, quality of life, and comfort.
Discussion strategies
In my own practice I have found two discussion strategies to be particularly useful in guiding patients through the decision about CPM.
The first approach is relevant for women who are lumpectomy candidates, but who express a "reflex" interest in bilateral mastectomy while they are still in the emotional fog of processing the new cancer diagnosis. For these women it is obviously important to stress the survival equivalence of mastectomy and breast-conserving surgery, and this is also a great opportunity to educate patients about the potential axillary surgery advantages of breast conservation. The American College of Surgeons Oncology Group Z11 trial (JAMA 2011;305:69-75) has provided strong evidence supporting the safety of avoiding an axillary lymph node dissection (ALND) in women with sentinel lymph node (SLN) metastatic disease if the primary breast cancer is managed by lumpectomy and breast radiation.
At this point in time, we do not have comparably strong data to justify avoiding the ALND in the setting of mastectomy patients with SLN metastatic disease. The mastectomy patient with SLN metastasis is usually committed to undergo the completion axillary lymph node dissection specifically so that definitive decisions can be made regarding the need for postmastectomy radiation, and many of these patients become ineligible for immediate reconstruction because of this possible radiation. I therefore accentuate the advantage of at least initiating treatment with lumpectomy and sentinel lymph node biopsy. The patient preserves all of her surgical options with the benefit of having more staging information. If she is found to have SLN metastatic disease then she is in a better position to avoid the ALND with lumpectomy and radiation, and the option of future mastectomy and immediate reconstruction would still be available to her in the future (after completing all of her cancer treatment and healing from her radiation); if the SLN is negative, she can either continue with the breast-conservation treatment plan or she can pursue mastectomy (with or without immediate breast reconstruction, since prophylactic mamillary radiation therapy is not likely to be indicated for node-negative disease).
The second approach is relevant to the patient requiring mastectomy but for whom delayed reconstruction is planned because of medical issues or anticipated postmastectomy radiation. I encourage these patients to at least consider deferring the decision for the CPM until they return for the delayed reconstruction of the cancerous mastectomy, because at that time they can undergo the prophylactic mastectomy with the cosmetic advantages of immediate reconstruction.
Cost considerations
From the public health and population-based breast cancer burden perspectives as well as for individual patients, there are additional issues to be factored into the CPM discussion. It is a basic reality that cost is relevant when it comes to sorting out the net benefit of particular medical interventions, especially those that are prophylactic. Interestingly, a cost analysis study by Zendejas et al. (J. Clin. Oncol. 2011;29:2993-3000) from the Mayo Clinic demonstrated that CPM is actually cost effective, compared with surveillance for patients diagnosed when they are younger than 70 years of age.
The Women’s Health and Cancer Rights Act was implemented in 1999, mandating insurance coverage for breast reconstruction after mastectomy performed for cancer. This legislation promoted more widespread acceptance (and reimbursement) for contralateral mastectomy/reconstruction, but patients should nonetheless be proactive about confirming that their individual policy will indeed cover the expenses of prophylactic surgery. Furthermore, we must continue to monitor outcomes in women who choose to undergo CPM, as advances in breast cancer therapies may influence the survival benefits of this surgical approach. Indeed, selected retrospective studies have recently demonstrated that patients undergoing CPM have an improved survival, compared with those focusing on unilateral breast cancer surgery (Ann. Surg. Oncol. 2010;17:2702-9; J. Natl. Cancer Inst. 2010;102:401-9; J. Clin. Oncol. 2005;23:4275-86; Am. J. Surg. 2000;180:439-45). These results suggest a survival advantage associated with avoidance of a contralateral breast cancer, in contrast to the historical data alluded to above, regarding survival equivalence for patients with unilateral compared to metachronous bilateral breast cancer. As adjuvant systemic therapies for breast cancer continue to improve in effectiveness and ability to completely eliminate distant organ micrometastases, it is likely that we will continue to increase the pool of women who are essentially "cured" of the first cancer. This in turn could potentially increase the longevity threat of a second/metachronous cancer though a renewed metastatic risk. Nonetheless, data on possible survival advantages of CPM have not yet matured to the point where it can be recommended as a medically "indicated" procedure.
Our breast cancer patients face an abundance of very legitimate fears related to the morbidity and mortality risks of the actual cancer as well as the adverse effects and toxicities of treatment for that cancer. Fortunately, we can assure them that for the majority of cases these treatments will be effective and their longevity will be protected. It is therefore understandable that the desire to avoid repeating this particular life experience may be strong. We have an obligation to explain the advantages and disadvantages, as well as the alternatives to CPM, with sensitivity and patience. We must also strive to make sure that our patients do not make premature decisions without understanding the consequences. Last, but certainly not least, we are ethically bound to offer only those treatments that we feel are medically reasonable and safe as well as oncologically sound. But we must also remember that the decision to pursue treatment and the choice between the options that we offer are ultimately rights that belong to the patient.
Dr. Newman in an ACS Fellow, professor of surgery, and director of the Breast Care Center and Multidisciplinary Breast Fellowship Program, University of Michigan Comprehensive Cancer Center, Ann Arbor.
Dr. Sarah Hawley and her coinvestigators are to be applauded for generating insightful data regarding factors and concerns that motivate a woman to undergo contralateral prophylactic mastectomy in the setting of unilateral breast cancer (JAMA Surgery 2014 May 21 [doi:10.1001/jamasurg.2013.5689]).
Hawley et al. found that fear of recurrence was one of the strongest factors leading women to choose contralateral prophylactic mastectomy (CPM). This finding clearly demonstrates that we need to do a better job of explaining and defining the significance of (i) breast cancer local recurrence; (ii) breast cancer distant recurrence; and (iii) the development of a new/second primary breast cancer. Since cross-metastasis of a primary breast cancer to the contralateral breast is an extremely rare event, and since distant metastasis from the initial primary breast cancer tends to determine survival rates, CPM by definition will influence the incidence of only the third pattern. Furthermore, since the risk of experiencing a new contralateral malignancy is less than 1% per year for the general population of breast cancer patients, only a minority of these women will actually become bilateral breast cancer patients. Fear of recurrence is therefore a totally inappropriate reason for patients to pursue CPM, and the reasonableness of CPM to reduce the risk of a contralateral new primary breast cancer is debatable.
It can be reasonably stated that prophylactic surgery by definition is never a medically indicated necessity. Furthermore, despite the fact that a personal history of breast cancer is indeed a risk factor for developing a second primary cancer in the contralateral breast, numerous studies have demonstrated equivalent survival rates for women with unilateral breast cancer, compared with those diagnosed with bilateral/metachronous breast cancer (Cancer 2001;91:1845-53; Am. J. Clin. Oncol. 1997;20:541-5). Survival tends to be driven by the stage and effectiveness of treatment for the first cancer. By virtue of its earlier presentation, it is likely that the initially diagnosed cancer has established itself as the faster-growing malignancy with a lead time advantage in establishing distant organ micrometastatic disease; furthermore, patients with a unilateral breast cancer diagnosis are generally undergoing diligent surveillance and a contralateral malignancy is more often detected at an early stage.
Messages to our patients
It is essential for those of us who manage breast cancer to clearly emphasize several messages to our newly diagnosed breast cancer patients: First, although unilateral breast cancer increases the likelihood of developing a second primary tumor, it is certainly not inevitable, and in fact, the majority of patients are not destined to develop contralateral disease. Second, reducing the risk of being diagnosed with a contralateral breast cancer does not mitigate the mortality risk associated with the first cancer. And, finally, prophylactic mastectomy is the most aggressive and effective strategy for reducing the incidence of primary breast cancer (by approximately 90%), but it does not confer complete protection, as microscopic foci of breast tissue may be left behind in the mastectomy skin flaps, along the pectoralis, or in the axilla.
The messages above are critical: Our patients must understand that the priority is to address the known cancer. In this regard, appropriately selected patients should be encouraged to strongly consider breast-conserving surgery whenever feasible, as this low-morbidity treatment is equivalent to mastectomy from the perspective of overall survival. The question of CPM is most relevant for those patients that are ineligible for breast conservation or patients unwilling to undergo lumpectomy and breast radiation.
If a mastectomy for the cancerous breast is planned, we must then address the questions that routinely arise regarding bilateral surgery. In our efforts to clarify the reality of what CPM can and cannot achieve, we must also avoid being too dogmatic and paternalistic with our patients. There are clearly specific scenarios, as delineated in Dr. Hawley’s work, where the risk of a second primary breast cancer is likely to be considered excessive by most women, and where the decision to pursue CPM may be easier. Examples of such cases would be women known to harbor BRCA mutations or women with suspected hereditary susceptibility based on a strong family history of breast and/or ovarian cancer. The risk of a new contralateral breast cancer can be in the range of 4%-5% per year in cases of hereditary disease, compared with the general population of women with sporadic breast cancer, where the risk ranges from 0.25% to 1% per year.
Conveying an understanding of risk
Patients must understand that the risk to the contralateral breast is predominantly expressed in the future – the likelihood of having a clinically occult, incidentally detected cancer identified in the contralateral mastectomy specimen is only 6%, as demonstrated most recently by King et al. (Ann. Surg. 2011;254:2-7), and with ductal carcinoma in situ accounting for the high majority of these lesions.
Defining the threshold for the amount of risk that an individual woman finds to be acceptable, however, can be a very difficult and personal decision. Even after a patient comes to understand that CPM is unlikely to provide a survival advantage, she may continue to request bilateral surgery purely for the risk-reducing benefits, and out of a desire to minimize her chances of having to repeat the breast cancer diagnosis and treatment experience. In some cases this choice will be influenced by reconstruction factors. A woman may be motivated to pursue bilateral surgery if she has an adequate volume of abdominal tissue because of the fact that the autogenous TRAM (transverse rectus abdominis myocutaneous) flap can be harvested only once. In other cases the decision is influenced by body habitus, for example, a woman with large pendulous breasts who is not interested in breast reconstruction may decide that she is more comfortable with a symmetrically flat chest wall in order to avoid chest wall imbalance and the inconvenience of finding/wearing a prosthesis that matches the remaining breast.
As breast cancer surgeons we should openly discuss these issues with our patients and present viable alternatives when feasible, such as reduction mammoplasty for the large-breasted patient. Ultimately, however, the patient must decide the surgical approach that provides her with the optimal sense of treatment satisfaction, quality of life, and comfort.
Discussion strategies
In my own practice I have found two discussion strategies to be particularly useful in guiding patients through the decision about CPM.
The first approach is relevant for women who are lumpectomy candidates, but who express a "reflex" interest in bilateral mastectomy while they are still in the emotional fog of processing the new cancer diagnosis. For these women it is obviously important to stress the survival equivalence of mastectomy and breast-conserving surgery, and this is also a great opportunity to educate patients about the potential axillary surgery advantages of breast conservation. The American College of Surgeons Oncology Group Z11 trial (JAMA 2011;305:69-75) has provided strong evidence supporting the safety of avoiding an axillary lymph node dissection (ALND) in women with sentinel lymph node (SLN) metastatic disease if the primary breast cancer is managed by lumpectomy and breast radiation.
At this point in time, we do not have comparably strong data to justify avoiding the ALND in the setting of mastectomy patients with SLN metastatic disease. The mastectomy patient with SLN metastasis is usually committed to undergo the completion axillary lymph node dissection specifically so that definitive decisions can be made regarding the need for postmastectomy radiation, and many of these patients become ineligible for immediate reconstruction because of this possible radiation. I therefore accentuate the advantage of at least initiating treatment with lumpectomy and sentinel lymph node biopsy. The patient preserves all of her surgical options with the benefit of having more staging information. If she is found to have SLN metastatic disease then she is in a better position to avoid the ALND with lumpectomy and radiation, and the option of future mastectomy and immediate reconstruction would still be available to her in the future (after completing all of her cancer treatment and healing from her radiation); if the SLN is negative, she can either continue with the breast-conservation treatment plan or she can pursue mastectomy (with or without immediate breast reconstruction, since prophylactic mamillary radiation therapy is not likely to be indicated for node-negative disease).
The second approach is relevant to the patient requiring mastectomy but for whom delayed reconstruction is planned because of medical issues or anticipated postmastectomy radiation. I encourage these patients to at least consider deferring the decision for the CPM until they return for the delayed reconstruction of the cancerous mastectomy, because at that time they can undergo the prophylactic mastectomy with the cosmetic advantages of immediate reconstruction.
Cost considerations
From the public health and population-based breast cancer burden perspectives as well as for individual patients, there are additional issues to be factored into the CPM discussion. It is a basic reality that cost is relevant when it comes to sorting out the net benefit of particular medical interventions, especially those that are prophylactic. Interestingly, a cost analysis study by Zendejas et al. (J. Clin. Oncol. 2011;29:2993-3000) from the Mayo Clinic demonstrated that CPM is actually cost effective, compared with surveillance for patients diagnosed when they are younger than 70 years of age.
The Women’s Health and Cancer Rights Act was implemented in 1999, mandating insurance coverage for breast reconstruction after mastectomy performed for cancer. This legislation promoted more widespread acceptance (and reimbursement) for contralateral mastectomy/reconstruction, but patients should nonetheless be proactive about confirming that their individual policy will indeed cover the expenses of prophylactic surgery. Furthermore, we must continue to monitor outcomes in women who choose to undergo CPM, as advances in breast cancer therapies may influence the survival benefits of this surgical approach. Indeed, selected retrospective studies have recently demonstrated that patients undergoing CPM have an improved survival, compared with those focusing on unilateral breast cancer surgery (Ann. Surg. Oncol. 2010;17:2702-9; J. Natl. Cancer Inst. 2010;102:401-9; J. Clin. Oncol. 2005;23:4275-86; Am. J. Surg. 2000;180:439-45). These results suggest a survival advantage associated with avoidance of a contralateral breast cancer, in contrast to the historical data alluded to above, regarding survival equivalence for patients with unilateral compared to metachronous bilateral breast cancer. As adjuvant systemic therapies for breast cancer continue to improve in effectiveness and ability to completely eliminate distant organ micrometastases, it is likely that we will continue to increase the pool of women who are essentially "cured" of the first cancer. This in turn could potentially increase the longevity threat of a second/metachronous cancer though a renewed metastatic risk. Nonetheless, data on possible survival advantages of CPM have not yet matured to the point where it can be recommended as a medically "indicated" procedure.
Our breast cancer patients face an abundance of very legitimate fears related to the morbidity and mortality risks of the actual cancer as well as the adverse effects and toxicities of treatment for that cancer. Fortunately, we can assure them that for the majority of cases these treatments will be effective and their longevity will be protected. It is therefore understandable that the desire to avoid repeating this particular life experience may be strong. We have an obligation to explain the advantages and disadvantages, as well as the alternatives to CPM, with sensitivity and patience. We must also strive to make sure that our patients do not make premature decisions without understanding the consequences. Last, but certainly not least, we are ethically bound to offer only those treatments that we feel are medically reasonable and safe as well as oncologically sound. But we must also remember that the decision to pursue treatment and the choice between the options that we offer are ultimately rights that belong to the patient.
Dr. Newman in an ACS Fellow, professor of surgery, and director of the Breast Care Center and Multidisciplinary Breast Fellowship Program, University of Michigan Comprehensive Cancer Center, Ann Arbor.
Dr. Sarah Hawley and her coinvestigators are to be applauded for generating insightful data regarding factors and concerns that motivate a woman to undergo contralateral prophylactic mastectomy in the setting of unilateral breast cancer (JAMA Surgery 2014 May 21 [doi:10.1001/jamasurg.2013.5689]).
Hawley et al. found that fear of recurrence was one of the strongest factors leading women to choose contralateral prophylactic mastectomy (CPM). This finding clearly demonstrates that we need to do a better job of explaining and defining the significance of (i) breast cancer local recurrence; (ii) breast cancer distant recurrence; and (iii) the development of a new/second primary breast cancer. Since cross-metastasis of a primary breast cancer to the contralateral breast is an extremely rare event, and since distant metastasis from the initial primary breast cancer tends to determine survival rates, CPM by definition will influence the incidence of only the third pattern. Furthermore, since the risk of experiencing a new contralateral malignancy is less than 1% per year for the general population of breast cancer patients, only a minority of these women will actually become bilateral breast cancer patients. Fear of recurrence is therefore a totally inappropriate reason for patients to pursue CPM, and the reasonableness of CPM to reduce the risk of a contralateral new primary breast cancer is debatable.
It can be reasonably stated that prophylactic surgery by definition is never a medically indicated necessity. Furthermore, despite the fact that a personal history of breast cancer is indeed a risk factor for developing a second primary cancer in the contralateral breast, numerous studies have demonstrated equivalent survival rates for women with unilateral breast cancer, compared with those diagnosed with bilateral/metachronous breast cancer (Cancer 2001;91:1845-53; Am. J. Clin. Oncol. 1997;20:541-5). Survival tends to be driven by the stage and effectiveness of treatment for the first cancer. By virtue of its earlier presentation, it is likely that the initially diagnosed cancer has established itself as the faster-growing malignancy with a lead time advantage in establishing distant organ micrometastatic disease; furthermore, patients with a unilateral breast cancer diagnosis are generally undergoing diligent surveillance and a contralateral malignancy is more often detected at an early stage.
Messages to our patients
It is essential for those of us who manage breast cancer to clearly emphasize several messages to our newly diagnosed breast cancer patients: First, although unilateral breast cancer increases the likelihood of developing a second primary tumor, it is certainly not inevitable, and in fact, the majority of patients are not destined to develop contralateral disease. Second, reducing the risk of being diagnosed with a contralateral breast cancer does not mitigate the mortality risk associated with the first cancer. And, finally, prophylactic mastectomy is the most aggressive and effective strategy for reducing the incidence of primary breast cancer (by approximately 90%), but it does not confer complete protection, as microscopic foci of breast tissue may be left behind in the mastectomy skin flaps, along the pectoralis, or in the axilla.
The messages above are critical: Our patients must understand that the priority is to address the known cancer. In this regard, appropriately selected patients should be encouraged to strongly consider breast-conserving surgery whenever feasible, as this low-morbidity treatment is equivalent to mastectomy from the perspective of overall survival. The question of CPM is most relevant for those patients that are ineligible for breast conservation or patients unwilling to undergo lumpectomy and breast radiation.
If a mastectomy for the cancerous breast is planned, we must then address the questions that routinely arise regarding bilateral surgery. In our efforts to clarify the reality of what CPM can and cannot achieve, we must also avoid being too dogmatic and paternalistic with our patients. There are clearly specific scenarios, as delineated in Dr. Hawley’s work, where the risk of a second primary breast cancer is likely to be considered excessive by most women, and where the decision to pursue CPM may be easier. Examples of such cases would be women known to harbor BRCA mutations or women with suspected hereditary susceptibility based on a strong family history of breast and/or ovarian cancer. The risk of a new contralateral breast cancer can be in the range of 4%-5% per year in cases of hereditary disease, compared with the general population of women with sporadic breast cancer, where the risk ranges from 0.25% to 1% per year.
Conveying an understanding of risk
Patients must understand that the risk to the contralateral breast is predominantly expressed in the future – the likelihood of having a clinically occult, incidentally detected cancer identified in the contralateral mastectomy specimen is only 6%, as demonstrated most recently by King et al. (Ann. Surg. 2011;254:2-7), and with ductal carcinoma in situ accounting for the high majority of these lesions.
Defining the threshold for the amount of risk that an individual woman finds to be acceptable, however, can be a very difficult and personal decision. Even after a patient comes to understand that CPM is unlikely to provide a survival advantage, she may continue to request bilateral surgery purely for the risk-reducing benefits, and out of a desire to minimize her chances of having to repeat the breast cancer diagnosis and treatment experience. In some cases this choice will be influenced by reconstruction factors. A woman may be motivated to pursue bilateral surgery if she has an adequate volume of abdominal tissue because of the fact that the autogenous TRAM (transverse rectus abdominis myocutaneous) flap can be harvested only once. In other cases the decision is influenced by body habitus, for example, a woman with large pendulous breasts who is not interested in breast reconstruction may decide that she is more comfortable with a symmetrically flat chest wall in order to avoid chest wall imbalance and the inconvenience of finding/wearing a prosthesis that matches the remaining breast.
As breast cancer surgeons we should openly discuss these issues with our patients and present viable alternatives when feasible, such as reduction mammoplasty for the large-breasted patient. Ultimately, however, the patient must decide the surgical approach that provides her with the optimal sense of treatment satisfaction, quality of life, and comfort.
Discussion strategies
In my own practice I have found two discussion strategies to be particularly useful in guiding patients through the decision about CPM.
The first approach is relevant for women who are lumpectomy candidates, but who express a "reflex" interest in bilateral mastectomy while they are still in the emotional fog of processing the new cancer diagnosis. For these women it is obviously important to stress the survival equivalence of mastectomy and breast-conserving surgery, and this is also a great opportunity to educate patients about the potential axillary surgery advantages of breast conservation. The American College of Surgeons Oncology Group Z11 trial (JAMA 2011;305:69-75) has provided strong evidence supporting the safety of avoiding an axillary lymph node dissection (ALND) in women with sentinel lymph node (SLN) metastatic disease if the primary breast cancer is managed by lumpectomy and breast radiation.
At this point in time, we do not have comparably strong data to justify avoiding the ALND in the setting of mastectomy patients with SLN metastatic disease. The mastectomy patient with SLN metastasis is usually committed to undergo the completion axillary lymph node dissection specifically so that definitive decisions can be made regarding the need for postmastectomy radiation, and many of these patients become ineligible for immediate reconstruction because of this possible radiation. I therefore accentuate the advantage of at least initiating treatment with lumpectomy and sentinel lymph node biopsy. The patient preserves all of her surgical options with the benefit of having more staging information. If she is found to have SLN metastatic disease then she is in a better position to avoid the ALND with lumpectomy and radiation, and the option of future mastectomy and immediate reconstruction would still be available to her in the future (after completing all of her cancer treatment and healing from her radiation); if the SLN is negative, she can either continue with the breast-conservation treatment plan or she can pursue mastectomy (with or without immediate breast reconstruction, since prophylactic mamillary radiation therapy is not likely to be indicated for node-negative disease).
The second approach is relevant to the patient requiring mastectomy but for whom delayed reconstruction is planned because of medical issues or anticipated postmastectomy radiation. I encourage these patients to at least consider deferring the decision for the CPM until they return for the delayed reconstruction of the cancerous mastectomy, because at that time they can undergo the prophylactic mastectomy with the cosmetic advantages of immediate reconstruction.
Cost considerations
From the public health and population-based breast cancer burden perspectives as well as for individual patients, there are additional issues to be factored into the CPM discussion. It is a basic reality that cost is relevant when it comes to sorting out the net benefit of particular medical interventions, especially those that are prophylactic. Interestingly, a cost analysis study by Zendejas et al. (J. Clin. Oncol. 2011;29:2993-3000) from the Mayo Clinic demonstrated that CPM is actually cost effective, compared with surveillance for patients diagnosed when they are younger than 70 years of age.
The Women’s Health and Cancer Rights Act was implemented in 1999, mandating insurance coverage for breast reconstruction after mastectomy performed for cancer. This legislation promoted more widespread acceptance (and reimbursement) for contralateral mastectomy/reconstruction, but patients should nonetheless be proactive about confirming that their individual policy will indeed cover the expenses of prophylactic surgery. Furthermore, we must continue to monitor outcomes in women who choose to undergo CPM, as advances in breast cancer therapies may influence the survival benefits of this surgical approach. Indeed, selected retrospective studies have recently demonstrated that patients undergoing CPM have an improved survival, compared with those focusing on unilateral breast cancer surgery (Ann. Surg. Oncol. 2010;17:2702-9; J. Natl. Cancer Inst. 2010;102:401-9; J. Clin. Oncol. 2005;23:4275-86; Am. J. Surg. 2000;180:439-45). These results suggest a survival advantage associated with avoidance of a contralateral breast cancer, in contrast to the historical data alluded to above, regarding survival equivalence for patients with unilateral compared to metachronous bilateral breast cancer. As adjuvant systemic therapies for breast cancer continue to improve in effectiveness and ability to completely eliminate distant organ micrometastases, it is likely that we will continue to increase the pool of women who are essentially "cured" of the first cancer. This in turn could potentially increase the longevity threat of a second/metachronous cancer though a renewed metastatic risk. Nonetheless, data on possible survival advantages of CPM have not yet matured to the point where it can be recommended as a medically "indicated" procedure.
Our breast cancer patients face an abundance of very legitimate fears related to the morbidity and mortality risks of the actual cancer as well as the adverse effects and toxicities of treatment for that cancer. Fortunately, we can assure them that for the majority of cases these treatments will be effective and their longevity will be protected. It is therefore understandable that the desire to avoid repeating this particular life experience may be strong. We have an obligation to explain the advantages and disadvantages, as well as the alternatives to CPM, with sensitivity and patience. We must also strive to make sure that our patients do not make premature decisions without understanding the consequences. Last, but certainly not least, we are ethically bound to offer only those treatments that we feel are medically reasonable and safe as well as oncologically sound. But we must also remember that the decision to pursue treatment and the choice between the options that we offer are ultimately rights that belong to the patient.
Dr. Newman in an ACS Fellow, professor of surgery, and director of the Breast Care Center and Multidisciplinary Breast Fellowship Program, University of Michigan Comprehensive Cancer Center, Ann Arbor.
Neoadjuvant chemoradiotherapy fails to boost survival
For patients with early-stage esophageal cancer, undergoing chemotherapy and radiotherapy before surgical excision failed to improve the rate of curative resection and, most importantly, failed to boost survival in a phase III clinical trial, according to a report published online June 30 in the Journal of Clinical Oncology.
Unfortunately this treatment strategy also tripled postoperative mortality, making the risk-benefit ratio even more lopsided for this patient population, said Dr. Christophe Mariette of the department of digestive and oncologic surgery, University Hospital Claude Huriez-Regional University Hospital Center, Lille (France), and his associates.
Clinical trials examining neoadjuvant chemoradiotherapy for esophageal cancer have produced conflicting results, with some showing that the approach is effective, in some cases doubling median survival, while others showed no benefit. Most such studies have been limited by small sample sizes, heterogeneity of tumor types, variations in radiation doses and chemotherapy regiments, and differences in preoperative staging techniques and the adequacy of surgical resections. Moreover, the number of study participants with early-stage esophageal cancer has been very small because most patients already have more advanced disease at presentation, the investigators noted.
For their study, Dr. Mariette and his associates confined the cohort to patients younger than 75 years with treatment-naive esophageal adenocarcinoma or squamous-cell carcinoma judged to be stage I or II using thoracoabdominal CT and endoscopic ultrasound; additional preoperative assessments using PET scanning, cervical ultrasound, or radionuclide bone scanning were optional. It required 9 years to enroll 195 patients at 30 French medical centers. These study participants were randomly assigned to receive either neoadjuvant chemotherapy plus radiotherapy before potentially curative surgery (98 subjects) or potentially curative surgery alone (97 subjects).
In the intervention group, radiotherapy involved a total dose of 45 Gy delivered in 25 fractions over the course of 5 weeks. Chemotherapy was administered during the same time period and involved two cycles of fluorouracil and cisplatin infusions. All patients in this group were clinically reevaluated 2-4 weeks after completing this regimen, and surgery was performed soon afterward.
Surgery comprised a transthoracic esophagectomy with extended two-field lymphadenectomy and either high intrathoracic anastomosis (for tumors with an infracarinal proximal margin) or cervical anastomosis (for tumors with a proximal margin above the carina).
Median follow-up was 7.8 years. There were 125 deaths: 62.4% of the intervention group died, as did 66% of the surgery-only group, a nonsignificant difference, the investigators said (J. Clin. Oncol. 2014 June 30 [doi:10.1200/JCO.2013.53.6532]).
Median overall survival was 31.8 months in the intervention group and 41.2 months in the surgery-only group, a nonsignificant difference. Similarly, 3-year overall survival was 47.5% and 5-year overall survival was 41.1% in the intervention group, compared with 53% and 33.8%, respectively, in the surgery-only group, which were also nonsignificant differences.
The rate of curative resection also was not significantly different between the intervention group (93.8%) and the surgery-only group (92.1%), indicating that reducing the tumor with chemotherapy and radiotherapy had no beneficial effect in these early-stage cancers. Previous studies have demonstrated that such downsizing is effective in more advanced esophageal cancers, Dr. Mariette and his associates noted.
Postoperative mortality was more than threefold higher among patients who underwent preoperative chemoradiotherapy (11.1%) than in the surgery-only group (3.4%). The causes of postoperative death included aortic rupture, uncontrollable chylothorax, anastomotic leak, gastric conduit necrosis, mesenteric and lower limb ischemia, and acute RDS in the intervention group, compared with pneumonia and acute RDS in the surgery-only group.
These findings suggest that preoperative chemoradiotherapy "is not the appropriate neoadjuvant therapeutic strategy for stage I or II esophageal cancer," the investigators said.
Since patients with early-stage esophageal cancer don’t appear to benefit from preoperative neoadjuvant chemoradiotherapy, perhaps it is time to consider a different approach: definitive rather than neoadjuvant chemoradiotherapy as the first-line treatment, said Dr. Brian G. Czito, Dr. Manisha Palta, and Dr. Christopher G. Willett.
Some medical centers have already adopted this approach for patients with potentially curable esophageal cancer, reserving surgery as salvage treatment. Compared with surgery as first-line treatment, definitive chemoradiotherapy is associated with a lower rate of treatment-related mortality and similar survival outcomes, they noted.
Dr. Czito, Dr. Palta, and Dr. Willett are in the department of radiation oncology at Duke Cancer Institute, Durham, N.C. They reported no financial conflicts of interest. These remarks were taken from their editorial accompanying Dr. Mariette’s report (J. Clin. Oncol. 2014 June 30 [doi:10.1200/JCO.2013.53.6532]).
Since patients with early-stage esophageal cancer don’t appear to benefit from preoperative neoadjuvant chemoradiotherapy, perhaps it is time to consider a different approach: definitive rather than neoadjuvant chemoradiotherapy as the first-line treatment, said Dr. Brian G. Czito, Dr. Manisha Palta, and Dr. Christopher G. Willett.
Some medical centers have already adopted this approach for patients with potentially curable esophageal cancer, reserving surgery as salvage treatment. Compared with surgery as first-line treatment, definitive chemoradiotherapy is associated with a lower rate of treatment-related mortality and similar survival outcomes, they noted.
Dr. Czito, Dr. Palta, and Dr. Willett are in the department of radiation oncology at Duke Cancer Institute, Durham, N.C. They reported no financial conflicts of interest. These remarks were taken from their editorial accompanying Dr. Mariette’s report (J. Clin. Oncol. 2014 June 30 [doi:10.1200/JCO.2013.53.6532]).
Since patients with early-stage esophageal cancer don’t appear to benefit from preoperative neoadjuvant chemoradiotherapy, perhaps it is time to consider a different approach: definitive rather than neoadjuvant chemoradiotherapy as the first-line treatment, said Dr. Brian G. Czito, Dr. Manisha Palta, and Dr. Christopher G. Willett.
Some medical centers have already adopted this approach for patients with potentially curable esophageal cancer, reserving surgery as salvage treatment. Compared with surgery as first-line treatment, definitive chemoradiotherapy is associated with a lower rate of treatment-related mortality and similar survival outcomes, they noted.
Dr. Czito, Dr. Palta, and Dr. Willett are in the department of radiation oncology at Duke Cancer Institute, Durham, N.C. They reported no financial conflicts of interest. These remarks were taken from their editorial accompanying Dr. Mariette’s report (J. Clin. Oncol. 2014 June 30 [doi:10.1200/JCO.2013.53.6532]).
For patients with early-stage esophageal cancer, undergoing chemotherapy and radiotherapy before surgical excision failed to improve the rate of curative resection and, most importantly, failed to boost survival in a phase III clinical trial, according to a report published online June 30 in the Journal of Clinical Oncology.
Unfortunately this treatment strategy also tripled postoperative mortality, making the risk-benefit ratio even more lopsided for this patient population, said Dr. Christophe Mariette of the department of digestive and oncologic surgery, University Hospital Claude Huriez-Regional University Hospital Center, Lille (France), and his associates.
Clinical trials examining neoadjuvant chemoradiotherapy for esophageal cancer have produced conflicting results, with some showing that the approach is effective, in some cases doubling median survival, while others showed no benefit. Most such studies have been limited by small sample sizes, heterogeneity of tumor types, variations in radiation doses and chemotherapy regiments, and differences in preoperative staging techniques and the adequacy of surgical resections. Moreover, the number of study participants with early-stage esophageal cancer has been very small because most patients already have more advanced disease at presentation, the investigators noted.
For their study, Dr. Mariette and his associates confined the cohort to patients younger than 75 years with treatment-naive esophageal adenocarcinoma or squamous-cell carcinoma judged to be stage I or II using thoracoabdominal CT and endoscopic ultrasound; additional preoperative assessments using PET scanning, cervical ultrasound, or radionuclide bone scanning were optional. It required 9 years to enroll 195 patients at 30 French medical centers. These study participants were randomly assigned to receive either neoadjuvant chemotherapy plus radiotherapy before potentially curative surgery (98 subjects) or potentially curative surgery alone (97 subjects).
In the intervention group, radiotherapy involved a total dose of 45 Gy delivered in 25 fractions over the course of 5 weeks. Chemotherapy was administered during the same time period and involved two cycles of fluorouracil and cisplatin infusions. All patients in this group were clinically reevaluated 2-4 weeks after completing this regimen, and surgery was performed soon afterward.
Surgery comprised a transthoracic esophagectomy with extended two-field lymphadenectomy and either high intrathoracic anastomosis (for tumors with an infracarinal proximal margin) or cervical anastomosis (for tumors with a proximal margin above the carina).
Median follow-up was 7.8 years. There were 125 deaths: 62.4% of the intervention group died, as did 66% of the surgery-only group, a nonsignificant difference, the investigators said (J. Clin. Oncol. 2014 June 30 [doi:10.1200/JCO.2013.53.6532]).
Median overall survival was 31.8 months in the intervention group and 41.2 months in the surgery-only group, a nonsignificant difference. Similarly, 3-year overall survival was 47.5% and 5-year overall survival was 41.1% in the intervention group, compared with 53% and 33.8%, respectively, in the surgery-only group, which were also nonsignificant differences.
The rate of curative resection also was not significantly different between the intervention group (93.8%) and the surgery-only group (92.1%), indicating that reducing the tumor with chemotherapy and radiotherapy had no beneficial effect in these early-stage cancers. Previous studies have demonstrated that such downsizing is effective in more advanced esophageal cancers, Dr. Mariette and his associates noted.
Postoperative mortality was more than threefold higher among patients who underwent preoperative chemoradiotherapy (11.1%) than in the surgery-only group (3.4%). The causes of postoperative death included aortic rupture, uncontrollable chylothorax, anastomotic leak, gastric conduit necrosis, mesenteric and lower limb ischemia, and acute RDS in the intervention group, compared with pneumonia and acute RDS in the surgery-only group.
These findings suggest that preoperative chemoradiotherapy "is not the appropriate neoadjuvant therapeutic strategy for stage I or II esophageal cancer," the investigators said.
For patients with early-stage esophageal cancer, undergoing chemotherapy and radiotherapy before surgical excision failed to improve the rate of curative resection and, most importantly, failed to boost survival in a phase III clinical trial, according to a report published online June 30 in the Journal of Clinical Oncology.
Unfortunately this treatment strategy also tripled postoperative mortality, making the risk-benefit ratio even more lopsided for this patient population, said Dr. Christophe Mariette of the department of digestive and oncologic surgery, University Hospital Claude Huriez-Regional University Hospital Center, Lille (France), and his associates.
Clinical trials examining neoadjuvant chemoradiotherapy for esophageal cancer have produced conflicting results, with some showing that the approach is effective, in some cases doubling median survival, while others showed no benefit. Most such studies have been limited by small sample sizes, heterogeneity of tumor types, variations in radiation doses and chemotherapy regiments, and differences in preoperative staging techniques and the adequacy of surgical resections. Moreover, the number of study participants with early-stage esophageal cancer has been very small because most patients already have more advanced disease at presentation, the investigators noted.
For their study, Dr. Mariette and his associates confined the cohort to patients younger than 75 years with treatment-naive esophageal adenocarcinoma or squamous-cell carcinoma judged to be stage I or II using thoracoabdominal CT and endoscopic ultrasound; additional preoperative assessments using PET scanning, cervical ultrasound, or radionuclide bone scanning were optional. It required 9 years to enroll 195 patients at 30 French medical centers. These study participants were randomly assigned to receive either neoadjuvant chemotherapy plus radiotherapy before potentially curative surgery (98 subjects) or potentially curative surgery alone (97 subjects).
In the intervention group, radiotherapy involved a total dose of 45 Gy delivered in 25 fractions over the course of 5 weeks. Chemotherapy was administered during the same time period and involved two cycles of fluorouracil and cisplatin infusions. All patients in this group were clinically reevaluated 2-4 weeks after completing this regimen, and surgery was performed soon afterward.
Surgery comprised a transthoracic esophagectomy with extended two-field lymphadenectomy and either high intrathoracic anastomosis (for tumors with an infracarinal proximal margin) or cervical anastomosis (for tumors with a proximal margin above the carina).
Median follow-up was 7.8 years. There were 125 deaths: 62.4% of the intervention group died, as did 66% of the surgery-only group, a nonsignificant difference, the investigators said (J. Clin. Oncol. 2014 June 30 [doi:10.1200/JCO.2013.53.6532]).
Median overall survival was 31.8 months in the intervention group and 41.2 months in the surgery-only group, a nonsignificant difference. Similarly, 3-year overall survival was 47.5% and 5-year overall survival was 41.1% in the intervention group, compared with 53% and 33.8%, respectively, in the surgery-only group, which were also nonsignificant differences.
The rate of curative resection also was not significantly different between the intervention group (93.8%) and the surgery-only group (92.1%), indicating that reducing the tumor with chemotherapy and radiotherapy had no beneficial effect in these early-stage cancers. Previous studies have demonstrated that such downsizing is effective in more advanced esophageal cancers, Dr. Mariette and his associates noted.
Postoperative mortality was more than threefold higher among patients who underwent preoperative chemoradiotherapy (11.1%) than in the surgery-only group (3.4%). The causes of postoperative death included aortic rupture, uncontrollable chylothorax, anastomotic leak, gastric conduit necrosis, mesenteric and lower limb ischemia, and acute RDS in the intervention group, compared with pneumonia and acute RDS in the surgery-only group.
These findings suggest that preoperative chemoradiotherapy "is not the appropriate neoadjuvant therapeutic strategy for stage I or II esophageal cancer," the investigators said.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Don’t postpone surgery for esophageal cancer to perform chemoradiotherapy.
Major finding: Median overall survival was 31.8 months in the intervention group and 41.2 months in the surgery-only group; 3-year overall survival was 47.5% and 5-year overall survival was 41.1% in the intervention group, compared with 53% and 33.8%, respectively, in the surgery-only group. All these differences are nonsignificant.
Data source: A multicenter randomized phase III clinical trial involving 98 patients treated with neoadjuvant chemoradiotherapy and 97 treated with surgery alone for early-stage esophageal cancer, who were followed for a median of approximately 8 years.
Disclosures: This study was supported by the French National Cancer Institute’s Programme Hospitalier pour la Recherche Clinque and Lille University Hospital; it received no commercial support. Dr. Mariette reported no financial conflicts of interest; one of his associates reported ties to Roche and Merck.
Adding tomosynthesis improved mammography’s cancer detection
Adding tomosynthesis to screening digital mammography decreases the "recall" rate by 15% while increasing the cancer detection rate by 29%, according to a report published online June 25 in JAMA.
Researchers compared the performance of screening digital mammography alone (281,187 cases) against the performance after tomosynthesis was added to mammography (173,663 cases) at 13 sites in geographically diverse regions across the country. The work was funded primarily by Hologic, maker of the only Food and Drug Administration–approved tomosynthesis equipment at the time of this retrospective study, said Dr. Sarah M. Friedewald of the Caldwell Breast Center, Advocate Lutheran General Hospital, Park Ridge, Ill., and her associates.
The "recall" rate – the proportion of patients requiring additional imaging based on the results of their screening mammography – declined from 107 per 1,000 screens with digital mammography alone to 91 per 1,000 screens when tomosynthesis was added, a relative reduction of 15%. At the same time, the cancer detection rate rose from 4.2 to 5.4 per 1,000 scans, a relative increase of 29%. After tomosynthesis was introduced, the detection rate of invasive cancers rose from 2.9 to 4.1 per 1,000 scans, while that of ductal carcinoma in situ remained unchanged at 1.4 per 1,000, the investigators said (JAMA 2014 June 25 [doi:10.1001/jama.2014.6095]).
It is important to note that this study did not assess clinical outcomes, so it remains to be seen whether these improvements in screening mammography translate into clinically relevant improvements in breast cancer mortality. In addition, this study was limited in that it was retrospective and nonrandomized, they wrote.
This study was funded by tomosynthesis equipment maker Hologic and the National Cancer Institute. Dr. Friedewald and her associates also reported other ties to Hologic.
Tomosynthesis likely represents an advance over digital mammography for breast cancer screening, but only appropriately powered, prospective, randomized, multicenter clinical trials involving all four currently available tomosynthesis systems will answer this question definitively, said Dr. Etta D. Pisano and Martin J. Yaffe, Ph.D.
Despite the promising results of Friedewald et al., it is still uncertain whether women should specifically ask for tomosynthesis or whether screening centers should convert to tomosynthesis. People who believe screening mammography saves lives without undue false-positive results and overdiagnosis will likely advocate the addition of tomosynthesis; those who question the utility of screening mammography will not likely change their views after seeing these results, Dr. Pisano and Dr. Yaffe said.
Dr. Pisano is at the Medical University of South Carolina, Charleston. Dr. Yaffe is at the Ontario Institute for Cancer Research at the University of Toronto. Dr. Pisano reported ties to FujiFilm, Koning Corp., Siemens, Philips, and NextRay Inc.; Dr. Yaffe reported ties to GE Healthcare and Mammographic Physics Inc. These remarks were taken from their editorial accompanying Dr. Friedewald’s report (JAMA 2014;311:2488-9).
Tomosynthesis likely represents an advance over digital mammography for breast cancer screening, but only appropriately powered, prospective, randomized, multicenter clinical trials involving all four currently available tomosynthesis systems will answer this question definitively, said Dr. Etta D. Pisano and Martin J. Yaffe, Ph.D.
Despite the promising results of Friedewald et al., it is still uncertain whether women should specifically ask for tomosynthesis or whether screening centers should convert to tomosynthesis. People who believe screening mammography saves lives without undue false-positive results and overdiagnosis will likely advocate the addition of tomosynthesis; those who question the utility of screening mammography will not likely change their views after seeing these results, Dr. Pisano and Dr. Yaffe said.
Dr. Pisano is at the Medical University of South Carolina, Charleston. Dr. Yaffe is at the Ontario Institute for Cancer Research at the University of Toronto. Dr. Pisano reported ties to FujiFilm, Koning Corp., Siemens, Philips, and NextRay Inc.; Dr. Yaffe reported ties to GE Healthcare and Mammographic Physics Inc. These remarks were taken from their editorial accompanying Dr. Friedewald’s report (JAMA 2014;311:2488-9).
Tomosynthesis likely represents an advance over digital mammography for breast cancer screening, but only appropriately powered, prospective, randomized, multicenter clinical trials involving all four currently available tomosynthesis systems will answer this question definitively, said Dr. Etta D. Pisano and Martin J. Yaffe, Ph.D.
Despite the promising results of Friedewald et al., it is still uncertain whether women should specifically ask for tomosynthesis or whether screening centers should convert to tomosynthesis. People who believe screening mammography saves lives without undue false-positive results and overdiagnosis will likely advocate the addition of tomosynthesis; those who question the utility of screening mammography will not likely change their views after seeing these results, Dr. Pisano and Dr. Yaffe said.
Dr. Pisano is at the Medical University of South Carolina, Charleston. Dr. Yaffe is at the Ontario Institute for Cancer Research at the University of Toronto. Dr. Pisano reported ties to FujiFilm, Koning Corp., Siemens, Philips, and NextRay Inc.; Dr. Yaffe reported ties to GE Healthcare and Mammographic Physics Inc. These remarks were taken from their editorial accompanying Dr. Friedewald’s report (JAMA 2014;311:2488-9).
Adding tomosynthesis to screening digital mammography decreases the "recall" rate by 15% while increasing the cancer detection rate by 29%, according to a report published online June 25 in JAMA.
Researchers compared the performance of screening digital mammography alone (281,187 cases) against the performance after tomosynthesis was added to mammography (173,663 cases) at 13 sites in geographically diverse regions across the country. The work was funded primarily by Hologic, maker of the only Food and Drug Administration–approved tomosynthesis equipment at the time of this retrospective study, said Dr. Sarah M. Friedewald of the Caldwell Breast Center, Advocate Lutheran General Hospital, Park Ridge, Ill., and her associates.
The "recall" rate – the proportion of patients requiring additional imaging based on the results of their screening mammography – declined from 107 per 1,000 screens with digital mammography alone to 91 per 1,000 screens when tomosynthesis was added, a relative reduction of 15%. At the same time, the cancer detection rate rose from 4.2 to 5.4 per 1,000 scans, a relative increase of 29%. After tomosynthesis was introduced, the detection rate of invasive cancers rose from 2.9 to 4.1 per 1,000 scans, while that of ductal carcinoma in situ remained unchanged at 1.4 per 1,000, the investigators said (JAMA 2014 June 25 [doi:10.1001/jama.2014.6095]).
It is important to note that this study did not assess clinical outcomes, so it remains to be seen whether these improvements in screening mammography translate into clinically relevant improvements in breast cancer mortality. In addition, this study was limited in that it was retrospective and nonrandomized, they wrote.
This study was funded by tomosynthesis equipment maker Hologic and the National Cancer Institute. Dr. Friedewald and her associates also reported other ties to Hologic.
Adding tomosynthesis to screening digital mammography decreases the "recall" rate by 15% while increasing the cancer detection rate by 29%, according to a report published online June 25 in JAMA.
Researchers compared the performance of screening digital mammography alone (281,187 cases) against the performance after tomosynthesis was added to mammography (173,663 cases) at 13 sites in geographically diverse regions across the country. The work was funded primarily by Hologic, maker of the only Food and Drug Administration–approved tomosynthesis equipment at the time of this retrospective study, said Dr. Sarah M. Friedewald of the Caldwell Breast Center, Advocate Lutheran General Hospital, Park Ridge, Ill., and her associates.
The "recall" rate – the proportion of patients requiring additional imaging based on the results of their screening mammography – declined from 107 per 1,000 screens with digital mammography alone to 91 per 1,000 screens when tomosynthesis was added, a relative reduction of 15%. At the same time, the cancer detection rate rose from 4.2 to 5.4 per 1,000 scans, a relative increase of 29%. After tomosynthesis was introduced, the detection rate of invasive cancers rose from 2.9 to 4.1 per 1,000 scans, while that of ductal carcinoma in situ remained unchanged at 1.4 per 1,000, the investigators said (JAMA 2014 June 25 [doi:10.1001/jama.2014.6095]).
It is important to note that this study did not assess clinical outcomes, so it remains to be seen whether these improvements in screening mammography translate into clinically relevant improvements in breast cancer mortality. In addition, this study was limited in that it was retrospective and nonrandomized, they wrote.
This study was funded by tomosynthesis equipment maker Hologic and the National Cancer Institute. Dr. Friedewald and her associates also reported other ties to Hologic.
FROM JAMA
Key clinical point: Tomosynthesis may reduce the need for repeat imaging to detect breast cancer.
Major finding: The "recall" rate declined from 107 per 1,000 screens with digital mammography alone to 91 per 1,000 screens when tomosynthesis was added, a relative reduction of 15%; at the same time, the cancer detection rate rose from 4.2 to 5.4 per 1,000 scans, a relative increase of 29%.
Data source: A retrospective multicenter analysis of screening digital mammography’s performance before (281,187 scans) and after (173,663 scans) the introduction of tomosynthesis to improve its accuracy.
Disclosures: This study was funded by Hologic and the National Cancer Institute. Dr. Friedewald and her associates also reported other ties to Hologic, which makes tomosynthesis equipment.