User login
Intraperitoneal chemo superior in low-volume residual ovarian cancer
LOS ANGELES – Patients with resected advanced ovarian cancer and low-volume residual disease fare better in the long term with intraperitoneal chemotherapy instead of intravenous chemotherapy.
A team led by Dr. Devansu Tewari assessed outcomes in 876 women from two key Gynecologic Oncology Group trials: GOG 114 and GOG 172. Combined median follow-up in those trials approached 11 years.
Compared with their peers given intravenous chemotherapy, women given intraperitoneal (IP) chemotherapy had a 16% lower risk of progression or death and a 17% lower risk of death, according to results reported at the annual meeting of the Society of Gynecologic Oncology.
Benefit was evident regardless of the extent of residual disease. Also, each additional cycle of IP chemotherapy reduced the risk of death by 12%.
"A strength of this study is that it is a combined analysis of these two major IP trials that looked at long-term follow-up and showed survival outcomes that are quite significant. The defining difference between the two groups is that one received IP therapy and one did not, as it is very unlikely that IP therapy would have been administered in the recurrent setting," Dr. Tewari commented.
Although more than 7 years have elapsed since the National Cancer Institute recommended consideration of IP chemotherapy for advanced-stage low-volume ovarian cancer, uptake of this therapy has been limited given lingering questions about efficacy, safety, and issues such as the ideal regimen, noted Dr. Tewari, who is director of gynecologic oncology for the Southern California Permanente Medical Group in Orange County, California, and assistant professor of ob.gyn. at the University of California, Irvine.
"We have now updated the results of GOG 172 and GOG 114. But we also acknowledge that in the last 7 years, a lot has changed in the treatment of ovarian cancer in which these advantages may be further enhanced," he noted, for example, through use of bevacizumab (Avastin) and dose-dense therapy.
In particular, oncologists are awaiting results of the recently completed GOG 262 trial (assessing the role of bevacizumab and dose-dense paclitaxel) and the GOG 252 trial (assessing the role of IP carboplatin, bevacizumab, and dose-dense paclitaxel).
"We hope that the results of these studies, combined with the findings before, will bring in the foundation that we need to move forward in terms of laying the groundwork for treating women individually and tailoring their treatment for this cancer specifically for them," Dr. Tewari said.
One session attendee, noting the IP regimens used in the trials studied, asked, "Is it the dose-dense treatment or the IP that actually matters?"
"That’s a very good question. The whole issue with GOG 172 was essentially partial deployment of dose dense because patients [in the IP arm] received [an additional] day 8 treatment, so it has to be acknowledged," Dr. Tewari replied. "I think the answer is going to really come about when we see the findings of GOG 252."
Attendee Dr. Joan Walker of the University of Oklahoma, Oklahoma City, said, "I want to thank the presenters for emphasizing IP chemotherapy with cisplatin, and I think that it still needs to be emphasized."
She noted that the long-term survival gains being seen with IP chemotherapy are "just amazing. And we don’t know why that is, but obviously if GOG 104, 114, and 172 all show the same thing, it can’t necessarily be that the Taxol [paclitaxel] IP is the only contributing factor," she said.
"I think the future is bright for women and their survival, and it may be the bone marrow preservation of cisplatin that’s really causing the long-term effect because we know that patients get treated with multiple agents over and over again," Dr. Walker speculated.
The two trials that Dr. Tewari’s group studied – GOG 114 and GOG 172 – enrolled patients with resected stage III epithelial ovarian or peritoneal carcinoma who had residual disease measuring 1.0 cm or less.
The former trial compared IV carboplatin and paclitaxel with IP cisplatin; the latter trial compared IV paclitaxel with IP cisplatin and paclitaxel. About two-thirds of the women had macroscopic residual disease.
With a median follow-up of 10.7 years, relative to their counterparts given IV chemotherapy, women given IP chemotherapy had better progression-free survival (25 vs. 20 months; hazard ratio, 0.84; P = .03) and overall survival (62 vs. 51 months; hazard ratio, 0.83; P = .048).
The survival benefit of IP over IV chemotherapy was evident among both women with microscopic residual disease (5-year survival, 65% vs. 58%) and women with macroscopic residual disease (44% vs. 35%).
"There has been a lot of debate regarding the role of IP therapy in microscopic and macroscopic residual disease, and we saw an advantage in this cohort in both specific groups," commented Dr. Tewari.
Overall, half of patients given IP chemotherapy completed all six cycles of that chemotherapy. The risk of death fell with each additional cycle of IP chemotherapy (hazard ratio, 0.88, P less than .001).
Dr. Tewari disclosed no conflicts of interest related to the research.
LOS ANGELES – Patients with resected advanced ovarian cancer and low-volume residual disease fare better in the long term with intraperitoneal chemotherapy instead of intravenous chemotherapy.
A team led by Dr. Devansu Tewari assessed outcomes in 876 women from two key Gynecologic Oncology Group trials: GOG 114 and GOG 172. Combined median follow-up in those trials approached 11 years.
Compared with their peers given intravenous chemotherapy, women given intraperitoneal (IP) chemotherapy had a 16% lower risk of progression or death and a 17% lower risk of death, according to results reported at the annual meeting of the Society of Gynecologic Oncology.
Benefit was evident regardless of the extent of residual disease. Also, each additional cycle of IP chemotherapy reduced the risk of death by 12%.
"A strength of this study is that it is a combined analysis of these two major IP trials that looked at long-term follow-up and showed survival outcomes that are quite significant. The defining difference between the two groups is that one received IP therapy and one did not, as it is very unlikely that IP therapy would have been administered in the recurrent setting," Dr. Tewari commented.
Although more than 7 years have elapsed since the National Cancer Institute recommended consideration of IP chemotherapy for advanced-stage low-volume ovarian cancer, uptake of this therapy has been limited given lingering questions about efficacy, safety, and issues such as the ideal regimen, noted Dr. Tewari, who is director of gynecologic oncology for the Southern California Permanente Medical Group in Orange County, California, and assistant professor of ob.gyn. at the University of California, Irvine.
"We have now updated the results of GOG 172 and GOG 114. But we also acknowledge that in the last 7 years, a lot has changed in the treatment of ovarian cancer in which these advantages may be further enhanced," he noted, for example, through use of bevacizumab (Avastin) and dose-dense therapy.
In particular, oncologists are awaiting results of the recently completed GOG 262 trial (assessing the role of bevacizumab and dose-dense paclitaxel) and the GOG 252 trial (assessing the role of IP carboplatin, bevacizumab, and dose-dense paclitaxel).
"We hope that the results of these studies, combined with the findings before, will bring in the foundation that we need to move forward in terms of laying the groundwork for treating women individually and tailoring their treatment for this cancer specifically for them," Dr. Tewari said.
One session attendee, noting the IP regimens used in the trials studied, asked, "Is it the dose-dense treatment or the IP that actually matters?"
"That’s a very good question. The whole issue with GOG 172 was essentially partial deployment of dose dense because patients [in the IP arm] received [an additional] day 8 treatment, so it has to be acknowledged," Dr. Tewari replied. "I think the answer is going to really come about when we see the findings of GOG 252."
Attendee Dr. Joan Walker of the University of Oklahoma, Oklahoma City, said, "I want to thank the presenters for emphasizing IP chemotherapy with cisplatin, and I think that it still needs to be emphasized."
She noted that the long-term survival gains being seen with IP chemotherapy are "just amazing. And we don’t know why that is, but obviously if GOG 104, 114, and 172 all show the same thing, it can’t necessarily be that the Taxol [paclitaxel] IP is the only contributing factor," she said.
"I think the future is bright for women and their survival, and it may be the bone marrow preservation of cisplatin that’s really causing the long-term effect because we know that patients get treated with multiple agents over and over again," Dr. Walker speculated.
The two trials that Dr. Tewari’s group studied – GOG 114 and GOG 172 – enrolled patients with resected stage III epithelial ovarian or peritoneal carcinoma who had residual disease measuring 1.0 cm or less.
The former trial compared IV carboplatin and paclitaxel with IP cisplatin; the latter trial compared IV paclitaxel with IP cisplatin and paclitaxel. About two-thirds of the women had macroscopic residual disease.
With a median follow-up of 10.7 years, relative to their counterparts given IV chemotherapy, women given IP chemotherapy had better progression-free survival (25 vs. 20 months; hazard ratio, 0.84; P = .03) and overall survival (62 vs. 51 months; hazard ratio, 0.83; P = .048).
The survival benefit of IP over IV chemotherapy was evident among both women with microscopic residual disease (5-year survival, 65% vs. 58%) and women with macroscopic residual disease (44% vs. 35%).
"There has been a lot of debate regarding the role of IP therapy in microscopic and macroscopic residual disease, and we saw an advantage in this cohort in both specific groups," commented Dr. Tewari.
Overall, half of patients given IP chemotherapy completed all six cycles of that chemotherapy. The risk of death fell with each additional cycle of IP chemotherapy (hazard ratio, 0.88, P less than .001).
Dr. Tewari disclosed no conflicts of interest related to the research.
LOS ANGELES – Patients with resected advanced ovarian cancer and low-volume residual disease fare better in the long term with intraperitoneal chemotherapy instead of intravenous chemotherapy.
A team led by Dr. Devansu Tewari assessed outcomes in 876 women from two key Gynecologic Oncology Group trials: GOG 114 and GOG 172. Combined median follow-up in those trials approached 11 years.
Compared with their peers given intravenous chemotherapy, women given intraperitoneal (IP) chemotherapy had a 16% lower risk of progression or death and a 17% lower risk of death, according to results reported at the annual meeting of the Society of Gynecologic Oncology.
Benefit was evident regardless of the extent of residual disease. Also, each additional cycle of IP chemotherapy reduced the risk of death by 12%.
"A strength of this study is that it is a combined analysis of these two major IP trials that looked at long-term follow-up and showed survival outcomes that are quite significant. The defining difference between the two groups is that one received IP therapy and one did not, as it is very unlikely that IP therapy would have been administered in the recurrent setting," Dr. Tewari commented.
Although more than 7 years have elapsed since the National Cancer Institute recommended consideration of IP chemotherapy for advanced-stage low-volume ovarian cancer, uptake of this therapy has been limited given lingering questions about efficacy, safety, and issues such as the ideal regimen, noted Dr. Tewari, who is director of gynecologic oncology for the Southern California Permanente Medical Group in Orange County, California, and assistant professor of ob.gyn. at the University of California, Irvine.
"We have now updated the results of GOG 172 and GOG 114. But we also acknowledge that in the last 7 years, a lot has changed in the treatment of ovarian cancer in which these advantages may be further enhanced," he noted, for example, through use of bevacizumab (Avastin) and dose-dense therapy.
In particular, oncologists are awaiting results of the recently completed GOG 262 trial (assessing the role of bevacizumab and dose-dense paclitaxel) and the GOG 252 trial (assessing the role of IP carboplatin, bevacizumab, and dose-dense paclitaxel).
"We hope that the results of these studies, combined with the findings before, will bring in the foundation that we need to move forward in terms of laying the groundwork for treating women individually and tailoring their treatment for this cancer specifically for them," Dr. Tewari said.
One session attendee, noting the IP regimens used in the trials studied, asked, "Is it the dose-dense treatment or the IP that actually matters?"
"That’s a very good question. The whole issue with GOG 172 was essentially partial deployment of dose dense because patients [in the IP arm] received [an additional] day 8 treatment, so it has to be acknowledged," Dr. Tewari replied. "I think the answer is going to really come about when we see the findings of GOG 252."
Attendee Dr. Joan Walker of the University of Oklahoma, Oklahoma City, said, "I want to thank the presenters for emphasizing IP chemotherapy with cisplatin, and I think that it still needs to be emphasized."
She noted that the long-term survival gains being seen with IP chemotherapy are "just amazing. And we don’t know why that is, but obviously if GOG 104, 114, and 172 all show the same thing, it can’t necessarily be that the Taxol [paclitaxel] IP is the only contributing factor," she said.
"I think the future is bright for women and their survival, and it may be the bone marrow preservation of cisplatin that’s really causing the long-term effect because we know that patients get treated with multiple agents over and over again," Dr. Walker speculated.
The two trials that Dr. Tewari’s group studied – GOG 114 and GOG 172 – enrolled patients with resected stage III epithelial ovarian or peritoneal carcinoma who had residual disease measuring 1.0 cm or less.
The former trial compared IV carboplatin and paclitaxel with IP cisplatin; the latter trial compared IV paclitaxel with IP cisplatin and paclitaxel. About two-thirds of the women had macroscopic residual disease.
With a median follow-up of 10.7 years, relative to their counterparts given IV chemotherapy, women given IP chemotherapy had better progression-free survival (25 vs. 20 months; hazard ratio, 0.84; P = .03) and overall survival (62 vs. 51 months; hazard ratio, 0.83; P = .048).
The survival benefit of IP over IV chemotherapy was evident among both women with microscopic residual disease (5-year survival, 65% vs. 58%) and women with macroscopic residual disease (44% vs. 35%).
"There has been a lot of debate regarding the role of IP therapy in microscopic and macroscopic residual disease, and we saw an advantage in this cohort in both specific groups," commented Dr. Tewari.
Overall, half of patients given IP chemotherapy completed all six cycles of that chemotherapy. The risk of death fell with each additional cycle of IP chemotherapy (hazard ratio, 0.88, P less than .001).
Dr. Tewari disclosed no conflicts of interest related to the research.
AT THE ANNUAL MEETING ON WOMEN'S CANCER
Major Finding: With a median follow-up of nearly 11 years, relative to their counterparts given IV chemotherapy, women given IP chemotherapy had better progression-free survival (25 vs. 20 months; hazard ratio, 0.84; P = .03) and overall survival (62 vs. 51 months; hazard ratio, 0.83; P = .048).
Data Source: A combined analysis of the GOG 114 and GOG 172 trials comparing IP vs. IV chemotherapy in 876 patients with resected advanced ovarian cancer and low-volume residual disease.
Disclosures: Dr. Tewari disclosed no relevant conflicts of interest.
Retroperitoneal exploration extends survival in stage IIIc ovarian cancer
LOS ANGELES – Surgically exploring the retroperitoneum for disease may benefit some patients undergoing primary debulking of advanced ovarian cancer, a study reported at the annual meeting of the Society of Gynecologic Oncology has shown.
Investigators analyzed data from Gynecologic Oncology Group (GOG) trial 182, focusing on the 1,876 women who had stage IIIc epithelial ovarian cancer on the basis of intraperitoneal tumor size and who underwent optimal debulking.
Overall, one-third had a retroperitoneal exploration, defined in the study as removal of at least one pelvic or para-aortic lymph node.
Patients who had this procedure were 15% less likely to experience progression or death and 15% less likely to die after other factors were considered, reported lead investigator Dr. Bunja Rungruang, a gynecologic oncologist with Georgia Regents University in Augusta.
In stratified analyses, benefit was seen in the subgroup with minimal gross residual disease but not in the subgroup with microscopic residual disease.
"In this large multi-institutional trial, there is evidence that retroperitoneal exploration at the time of primary debulking surgery of patients with intraperitoneal stage IIIc epithelial ovarian cancer may provide survival benefit," she commented.
"Surgical effort and tumor biology interact to affect patient outcomes," Dr. Rungruang noted. "Retroperitoneal exploration may be a proxy for more thorough surgical effort in these patients, rather than tumor biology alone driving outcomes. Surgeon discretion is a potential factor here as well; it is conceivable that the surgeon’s impression or information about prognosis influences the retroperitoneal exploration decision, based on unmeasured indicators of patient disease burden or vitality.
"Given the small but significant survival differences and the large sample size of this study, it is possible that these survival advantages are to some degree indicative of unmeasured factors or the accuracy of the surgeon’s impression, and not completely about the act of pathologic exploration," she said.
One attendee noted that analyses have suggested that patients who do not have a retroperitoneal exploration fare even more poorly than those who have the procedure and are found to have positive lymph nodes.
"I am concerned that that is because the surgeon thought the prognosis was so bad that they didn’t bother. I don’t know whether you have a sense of whether that conclusion looked appropriate for your analysis of all the tumor burden in that patient," Dr. Rungruang said.
"For some of the patients, it seemed to be a surgeon preference that they didn’t sample the nodes because they felt that the patient was already stage IIIc and called them microscopic optimally debulked, or microscopic optimally debulked without assessing the lymph nodes. Other patients had a much larger surgery, a higher complexity of procedures, and still had a lymph node assessment on top of it. What I can tell from reading the actual operative notes is a lot of [the approach] is based on surgeon preference."
In discussing the lack of additional benefit for exploration in women with microscopic residual disease, Dr. Rungruang explained that "if you have microscopic residual disease, that seems like the best you can do for those patients. I think in the macroscopic residual patients, you see the difference because it is perhaps a proxy for just a more thorough surgical assessment in these patients. Plus, macroscopic residual disease is such a wide spectrum, you can be anywhere from one site of residual disease to miliary disease spread throughout, and that heterogeneity within that residual disease group also accounts for that difference."
Patients enrolled in GOG 182 had advanced epithelial ovarian cancer, primary peritoneal cancer, or fallopian tube cancer, and underwent primary debulking to optimal residual disease (less than 1 cm), followed by randomization to a variety of platinum- and paclitaxel-based adjuvant chemotherapy regimens.
The investigators restricted analyses to the subset whose disease was stage IIIc on the basis of an intraperitoneal tumor measuring at least 2 cm. Overall, 37% of this subset had a retroperitoneal exploration.
The patients undergoing this additional procedure had better median progression-free survival (18.5 vs. 16.0 months, P less than .0001) and overall survival (53.3 vs. 42.8 months, P less than .0001), reported Dr. Rungruang.
When patients were stratified, retroperitoneal exploration was beneficial in those with minimal gross residual disease in terms of both progression-free survival (16.8 vs. 15.1 months, P = .01) and overall survival (44.9 vs. 40.5 months, P = .008). But there was no such benefit in patients who had microscopic residual disease.
In a multivariate analysis, retroperitoneal exploration independently predicted better progression-free survival (hazard ratio, 0.85; P = .004) and overall survival (HR, 0.85; P = .009).
Dr. Rungruang disclosed no relevant financial conflicts.
LOS ANGELES – Surgically exploring the retroperitoneum for disease may benefit some patients undergoing primary debulking of advanced ovarian cancer, a study reported at the annual meeting of the Society of Gynecologic Oncology has shown.
Investigators analyzed data from Gynecologic Oncology Group (GOG) trial 182, focusing on the 1,876 women who had stage IIIc epithelial ovarian cancer on the basis of intraperitoneal tumor size and who underwent optimal debulking.
Overall, one-third had a retroperitoneal exploration, defined in the study as removal of at least one pelvic or para-aortic lymph node.
Patients who had this procedure were 15% less likely to experience progression or death and 15% less likely to die after other factors were considered, reported lead investigator Dr. Bunja Rungruang, a gynecologic oncologist with Georgia Regents University in Augusta.
In stratified analyses, benefit was seen in the subgroup with minimal gross residual disease but not in the subgroup with microscopic residual disease.
"In this large multi-institutional trial, there is evidence that retroperitoneal exploration at the time of primary debulking surgery of patients with intraperitoneal stage IIIc epithelial ovarian cancer may provide survival benefit," she commented.
"Surgical effort and tumor biology interact to affect patient outcomes," Dr. Rungruang noted. "Retroperitoneal exploration may be a proxy for more thorough surgical effort in these patients, rather than tumor biology alone driving outcomes. Surgeon discretion is a potential factor here as well; it is conceivable that the surgeon’s impression or information about prognosis influences the retroperitoneal exploration decision, based on unmeasured indicators of patient disease burden or vitality.
"Given the small but significant survival differences and the large sample size of this study, it is possible that these survival advantages are to some degree indicative of unmeasured factors or the accuracy of the surgeon’s impression, and not completely about the act of pathologic exploration," she said.
One attendee noted that analyses have suggested that patients who do not have a retroperitoneal exploration fare even more poorly than those who have the procedure and are found to have positive lymph nodes.
"I am concerned that that is because the surgeon thought the prognosis was so bad that they didn’t bother. I don’t know whether you have a sense of whether that conclusion looked appropriate for your analysis of all the tumor burden in that patient," Dr. Rungruang said.
"For some of the patients, it seemed to be a surgeon preference that they didn’t sample the nodes because they felt that the patient was already stage IIIc and called them microscopic optimally debulked, or microscopic optimally debulked without assessing the lymph nodes. Other patients had a much larger surgery, a higher complexity of procedures, and still had a lymph node assessment on top of it. What I can tell from reading the actual operative notes is a lot of [the approach] is based on surgeon preference."
In discussing the lack of additional benefit for exploration in women with microscopic residual disease, Dr. Rungruang explained that "if you have microscopic residual disease, that seems like the best you can do for those patients. I think in the macroscopic residual patients, you see the difference because it is perhaps a proxy for just a more thorough surgical assessment in these patients. Plus, macroscopic residual disease is such a wide spectrum, you can be anywhere from one site of residual disease to miliary disease spread throughout, and that heterogeneity within that residual disease group also accounts for that difference."
Patients enrolled in GOG 182 had advanced epithelial ovarian cancer, primary peritoneal cancer, or fallopian tube cancer, and underwent primary debulking to optimal residual disease (less than 1 cm), followed by randomization to a variety of platinum- and paclitaxel-based adjuvant chemotherapy regimens.
The investigators restricted analyses to the subset whose disease was stage IIIc on the basis of an intraperitoneal tumor measuring at least 2 cm. Overall, 37% of this subset had a retroperitoneal exploration.
The patients undergoing this additional procedure had better median progression-free survival (18.5 vs. 16.0 months, P less than .0001) and overall survival (53.3 vs. 42.8 months, P less than .0001), reported Dr. Rungruang.
When patients were stratified, retroperitoneal exploration was beneficial in those with minimal gross residual disease in terms of both progression-free survival (16.8 vs. 15.1 months, P = .01) and overall survival (44.9 vs. 40.5 months, P = .008). But there was no such benefit in patients who had microscopic residual disease.
In a multivariate analysis, retroperitoneal exploration independently predicted better progression-free survival (hazard ratio, 0.85; P = .004) and overall survival (HR, 0.85; P = .009).
Dr. Rungruang disclosed no relevant financial conflicts.
LOS ANGELES – Surgically exploring the retroperitoneum for disease may benefit some patients undergoing primary debulking of advanced ovarian cancer, a study reported at the annual meeting of the Society of Gynecologic Oncology has shown.
Investigators analyzed data from Gynecologic Oncology Group (GOG) trial 182, focusing on the 1,876 women who had stage IIIc epithelial ovarian cancer on the basis of intraperitoneal tumor size and who underwent optimal debulking.
Overall, one-third had a retroperitoneal exploration, defined in the study as removal of at least one pelvic or para-aortic lymph node.
Patients who had this procedure were 15% less likely to experience progression or death and 15% less likely to die after other factors were considered, reported lead investigator Dr. Bunja Rungruang, a gynecologic oncologist with Georgia Regents University in Augusta.
In stratified analyses, benefit was seen in the subgroup with minimal gross residual disease but not in the subgroup with microscopic residual disease.
"In this large multi-institutional trial, there is evidence that retroperitoneal exploration at the time of primary debulking surgery of patients with intraperitoneal stage IIIc epithelial ovarian cancer may provide survival benefit," she commented.
"Surgical effort and tumor biology interact to affect patient outcomes," Dr. Rungruang noted. "Retroperitoneal exploration may be a proxy for more thorough surgical effort in these patients, rather than tumor biology alone driving outcomes. Surgeon discretion is a potential factor here as well; it is conceivable that the surgeon’s impression or information about prognosis influences the retroperitoneal exploration decision, based on unmeasured indicators of patient disease burden or vitality.
"Given the small but significant survival differences and the large sample size of this study, it is possible that these survival advantages are to some degree indicative of unmeasured factors or the accuracy of the surgeon’s impression, and not completely about the act of pathologic exploration," she said.
One attendee noted that analyses have suggested that patients who do not have a retroperitoneal exploration fare even more poorly than those who have the procedure and are found to have positive lymph nodes.
"I am concerned that that is because the surgeon thought the prognosis was so bad that they didn’t bother. I don’t know whether you have a sense of whether that conclusion looked appropriate for your analysis of all the tumor burden in that patient," Dr. Rungruang said.
"For some of the patients, it seemed to be a surgeon preference that they didn’t sample the nodes because they felt that the patient was already stage IIIc and called them microscopic optimally debulked, or microscopic optimally debulked without assessing the lymph nodes. Other patients had a much larger surgery, a higher complexity of procedures, and still had a lymph node assessment on top of it. What I can tell from reading the actual operative notes is a lot of [the approach] is based on surgeon preference."
In discussing the lack of additional benefit for exploration in women with microscopic residual disease, Dr. Rungruang explained that "if you have microscopic residual disease, that seems like the best you can do for those patients. I think in the macroscopic residual patients, you see the difference because it is perhaps a proxy for just a more thorough surgical assessment in these patients. Plus, macroscopic residual disease is such a wide spectrum, you can be anywhere from one site of residual disease to miliary disease spread throughout, and that heterogeneity within that residual disease group also accounts for that difference."
Patients enrolled in GOG 182 had advanced epithelial ovarian cancer, primary peritoneal cancer, or fallopian tube cancer, and underwent primary debulking to optimal residual disease (less than 1 cm), followed by randomization to a variety of platinum- and paclitaxel-based adjuvant chemotherapy regimens.
The investigators restricted analyses to the subset whose disease was stage IIIc on the basis of an intraperitoneal tumor measuring at least 2 cm. Overall, 37% of this subset had a retroperitoneal exploration.
The patients undergoing this additional procedure had better median progression-free survival (18.5 vs. 16.0 months, P less than .0001) and overall survival (53.3 vs. 42.8 months, P less than .0001), reported Dr. Rungruang.
When patients were stratified, retroperitoneal exploration was beneficial in those with minimal gross residual disease in terms of both progression-free survival (16.8 vs. 15.1 months, P = .01) and overall survival (44.9 vs. 40.5 months, P = .008). But there was no such benefit in patients who had microscopic residual disease.
In a multivariate analysis, retroperitoneal exploration independently predicted better progression-free survival (hazard ratio, 0.85; P = .004) and overall survival (HR, 0.85; P = .009).
Dr. Rungruang disclosed no relevant financial conflicts.
AT THE ANNUAL MEETING ON WOMEN'S CANCER
Major finding: Median progression-free survival was better (18.5 vs. 16.0 months, P less than .0001) and overall survival was longer (53.3 vs. 42.8 months, P less than .0001) in patients who had a retroperitoneal exploration.
Data source: A subset analysis of GOG 182 focusing on 1,876 women who had stage IIIc epithelial ovarian cancer and underwent optimal debulking.
Disclosures: Dr. Rungruang disclosed no relevant financial conflicts.
Smaller margins too close for comfort in breast cancer
NATIONAL HARBOR, MD. – Small surgical margins can mean big trouble for patients with breast cancer, said investigators at the annual Society of Surgical Oncology Cancer Symposium.
A retrospective study of outcomes for 2,377 women who underwent either breast-conserving therapy or mastectomy revealed that margins less than 2 mm resulted in a substantial risk of residual disease for all patients, reported Dr. Erin Garvey, a general surgery resident at Mayo Clinic Arizona in Phoenix.
"A policy of re-excision for margins less than 2 mm, coupled with a standardized multidisciplinary approach to breast cancer surgery, results in excellent re-excision and 5-year local recurrence rates. The local recurrence rate is higher, however, for those patients who complete breast-conserving therapy, thus warranting appropriate patient counseling regarding re-excision options and long-term outcome expectations," she said.
In a separate study, investigators from the University of Texas M.D. Anderson Cancer Center, Houston, reported 10-year follow-up data for women who opted for mastectomy to treat ductal carcinoma in situ (DCIS). They found that the incidence of local-regional recurrence (LRR) increased as the surgical margins shrank, and that close margins were the only independent predictor of LRR, reported Dr. Elizabeth FitzSullivan, a surgery fellow at M.D. Anderson.
"However, the local-regional recurrence rate in these patients is so low that routine postmastectomy radiation therapy is not warranted," she said.
No accord on margins
Despite multiple studies and meta-analyses, there is no standard for acceptable margin width in breast cancer, and surveys of both surgeons and radiation oncologists have shown wide variations in preferred margin widths, Dr. Garvey said.
Her group hypothesized that patients with invasive ductal carcinoma without an extensive in situ component who had surgical margins of at least 1 mm would have no evidence of residual disease on re-excision.
To test the idea, they took a retrospective look at records from a prospective database on 2,377 patients who underwent a total of 2,520 procedures from January 2000 through May 2012.
Of this group, 1,498 (63%) underwent lumpectomy, and 180 (12%) required re-excision surgery: 10% who had breast-conserving surgery alone, and 2% whose surgeries were converted to mastectomies. Of the 158 patients who had completed breast-conserving therapy following re-excision, 50 (32%) had residual disease, as did 20 of the 27 patients whose procedures were converted to mastectomies.
Of the 37% (879) who had up-front mastectomies, 2% (19) had re-excision, and of this group, 5 patients had residual disease.
In all, 40% of patients with positive margins had residual disease, compared with 38% of those with margins from 0.1 to 0.9 mm, and 33% for those with margins from 1.0 to 1.9 mm.
In univariate analysis, the presence of residual disease on re-excision did not show any significant association with age, race, menopausal status, width of the closest final margin, hormone receptor status, tumor histology, triple-negative disease, or the presence of angiolymphatic invasion. There was a trend, albeit nonsignificant, toward an association between residual disease and more than one margin narrower than 2 mm, Dr. Garvey noted.
At a median follow-up of 43 months (range, 0-140 months), 5-year local recurrence rates were 1.9% for patients who had breast-conserving therapy, and 1.1% for those who had mastectomy.
Patients who underwent breast-conserving therapy without re-excision had a 5-year local recurrence rate of 1.8%, compared with 4.3% for those who required re-excision, and 0% for those whose procedures were converted to mastectomy.
There was a nonsignificant trend toward higher local recurrence rates for breast-conserving therapy in patients who had re-excisions, which became significant when those patients who had conversion to mastectomy were excluded, with a hazard ratio compared with no re-excision of 2.56 (P = .04).
Narrower margins, larger risk
Dr. FitzSullivan and her M.D. Anderson colleagues reviewed the records of 810 women treated with mastectomy for DCIS from 1996 to 2009. They looked at the final width of histologic margins, defining disease-free margins as those of 3 mm or greater.
In all, 4 patients had positive margins, 59 had margins of 1 mm or smaller, and 35 had margins from 1.1 to 2.9 mm.
In multivariate analysis, independent predictors of close or positive margins were pathologic tumor size of 1.5 cm or greater (odds ratio, 5.11; P = .001), multicentric disease (OR, 5.44; P = .026), and the presence of necrosis (OR, 2.5; P = .003). Neither age, postmenopausal status, skin-sparing mastectomy, nor immediate breast reconstruction were significantly associated with close or positive margins, however.
None of seven patients who underwent postmastectomy radiotherapy had local-regional recurrences. Of the 803 patients who did not receive postsurgery radiation, 10-year LRR rates were 1%, consisting of 7 cases of invasive disease and 1 of DICS. Five patients had surgical management, and the remaining 3 had no further treatment.
When the researchers stratified the local recurrence rates by margin status, they saw that 5% of patients with margins of 1 mm or smaller had LRRs within 10 years, as did 3.6% of those with margins from 1.1 to 2.9 mm, compared with just 0.07% of those with disease-free margins (P less than .001). There was no difference in LRR between the two narrow-margin groups.
Among 546 patients with an intact contralateral breast, the 10-year rate of contralateral breast disease was 6.4%.
On univariate analysis, significant predictors of LRR included margin status (P = .002), multicentric disease (P = .005), and necrosis (P = .005). On multivariate analysis, however, only margin status remained significant, with an HR of 8.0 (P = .006).
Dr. FitzSullivan said that the low rate of LRR of DCIS treated with mastectomy and close surgical margins, compared with the rate of contralateral breast cancer, suggests that routine postmastectomy radiation therapy is not warranted, and should be reserved only for those patients with close or positive surgical margins that cannot be surgically excised.
Each study was internally funded. Dr. Garvey and Dr. FitzSullivan reported having no financial disclosures.
NATIONAL HARBOR, MD. – Small surgical margins can mean big trouble for patients with breast cancer, said investigators at the annual Society of Surgical Oncology Cancer Symposium.
A retrospective study of outcomes for 2,377 women who underwent either breast-conserving therapy or mastectomy revealed that margins less than 2 mm resulted in a substantial risk of residual disease for all patients, reported Dr. Erin Garvey, a general surgery resident at Mayo Clinic Arizona in Phoenix.
"A policy of re-excision for margins less than 2 mm, coupled with a standardized multidisciplinary approach to breast cancer surgery, results in excellent re-excision and 5-year local recurrence rates. The local recurrence rate is higher, however, for those patients who complete breast-conserving therapy, thus warranting appropriate patient counseling regarding re-excision options and long-term outcome expectations," she said.
In a separate study, investigators from the University of Texas M.D. Anderson Cancer Center, Houston, reported 10-year follow-up data for women who opted for mastectomy to treat ductal carcinoma in situ (DCIS). They found that the incidence of local-regional recurrence (LRR) increased as the surgical margins shrank, and that close margins were the only independent predictor of LRR, reported Dr. Elizabeth FitzSullivan, a surgery fellow at M.D. Anderson.
"However, the local-regional recurrence rate in these patients is so low that routine postmastectomy radiation therapy is not warranted," she said.
No accord on margins
Despite multiple studies and meta-analyses, there is no standard for acceptable margin width in breast cancer, and surveys of both surgeons and radiation oncologists have shown wide variations in preferred margin widths, Dr. Garvey said.
Her group hypothesized that patients with invasive ductal carcinoma without an extensive in situ component who had surgical margins of at least 1 mm would have no evidence of residual disease on re-excision.
To test the idea, they took a retrospective look at records from a prospective database on 2,377 patients who underwent a total of 2,520 procedures from January 2000 through May 2012.
Of this group, 1,498 (63%) underwent lumpectomy, and 180 (12%) required re-excision surgery: 10% who had breast-conserving surgery alone, and 2% whose surgeries were converted to mastectomies. Of the 158 patients who had completed breast-conserving therapy following re-excision, 50 (32%) had residual disease, as did 20 of the 27 patients whose procedures were converted to mastectomies.
Of the 37% (879) who had up-front mastectomies, 2% (19) had re-excision, and of this group, 5 patients had residual disease.
In all, 40% of patients with positive margins had residual disease, compared with 38% of those with margins from 0.1 to 0.9 mm, and 33% for those with margins from 1.0 to 1.9 mm.
In univariate analysis, the presence of residual disease on re-excision did not show any significant association with age, race, menopausal status, width of the closest final margin, hormone receptor status, tumor histology, triple-negative disease, or the presence of angiolymphatic invasion. There was a trend, albeit nonsignificant, toward an association between residual disease and more than one margin narrower than 2 mm, Dr. Garvey noted.
At a median follow-up of 43 months (range, 0-140 months), 5-year local recurrence rates were 1.9% for patients who had breast-conserving therapy, and 1.1% for those who had mastectomy.
Patients who underwent breast-conserving therapy without re-excision had a 5-year local recurrence rate of 1.8%, compared with 4.3% for those who required re-excision, and 0% for those whose procedures were converted to mastectomy.
There was a nonsignificant trend toward higher local recurrence rates for breast-conserving therapy in patients who had re-excisions, which became significant when those patients who had conversion to mastectomy were excluded, with a hazard ratio compared with no re-excision of 2.56 (P = .04).
Narrower margins, larger risk
Dr. FitzSullivan and her M.D. Anderson colleagues reviewed the records of 810 women treated with mastectomy for DCIS from 1996 to 2009. They looked at the final width of histologic margins, defining disease-free margins as those of 3 mm or greater.
In all, 4 patients had positive margins, 59 had margins of 1 mm or smaller, and 35 had margins from 1.1 to 2.9 mm.
In multivariate analysis, independent predictors of close or positive margins were pathologic tumor size of 1.5 cm or greater (odds ratio, 5.11; P = .001), multicentric disease (OR, 5.44; P = .026), and the presence of necrosis (OR, 2.5; P = .003). Neither age, postmenopausal status, skin-sparing mastectomy, nor immediate breast reconstruction were significantly associated with close or positive margins, however.
None of seven patients who underwent postmastectomy radiotherapy had local-regional recurrences. Of the 803 patients who did not receive postsurgery radiation, 10-year LRR rates were 1%, consisting of 7 cases of invasive disease and 1 of DICS. Five patients had surgical management, and the remaining 3 had no further treatment.
When the researchers stratified the local recurrence rates by margin status, they saw that 5% of patients with margins of 1 mm or smaller had LRRs within 10 years, as did 3.6% of those with margins from 1.1 to 2.9 mm, compared with just 0.07% of those with disease-free margins (P less than .001). There was no difference in LRR between the two narrow-margin groups.
Among 546 patients with an intact contralateral breast, the 10-year rate of contralateral breast disease was 6.4%.
On univariate analysis, significant predictors of LRR included margin status (P = .002), multicentric disease (P = .005), and necrosis (P = .005). On multivariate analysis, however, only margin status remained significant, with an HR of 8.0 (P = .006).
Dr. FitzSullivan said that the low rate of LRR of DCIS treated with mastectomy and close surgical margins, compared with the rate of contralateral breast cancer, suggests that routine postmastectomy radiation therapy is not warranted, and should be reserved only for those patients with close or positive surgical margins that cannot be surgically excised.
Each study was internally funded. Dr. Garvey and Dr. FitzSullivan reported having no financial disclosures.
NATIONAL HARBOR, MD. – Small surgical margins can mean big trouble for patients with breast cancer, said investigators at the annual Society of Surgical Oncology Cancer Symposium.
A retrospective study of outcomes for 2,377 women who underwent either breast-conserving therapy or mastectomy revealed that margins less than 2 mm resulted in a substantial risk of residual disease for all patients, reported Dr. Erin Garvey, a general surgery resident at Mayo Clinic Arizona in Phoenix.
"A policy of re-excision for margins less than 2 mm, coupled with a standardized multidisciplinary approach to breast cancer surgery, results in excellent re-excision and 5-year local recurrence rates. The local recurrence rate is higher, however, for those patients who complete breast-conserving therapy, thus warranting appropriate patient counseling regarding re-excision options and long-term outcome expectations," she said.
In a separate study, investigators from the University of Texas M.D. Anderson Cancer Center, Houston, reported 10-year follow-up data for women who opted for mastectomy to treat ductal carcinoma in situ (DCIS). They found that the incidence of local-regional recurrence (LRR) increased as the surgical margins shrank, and that close margins were the only independent predictor of LRR, reported Dr. Elizabeth FitzSullivan, a surgery fellow at M.D. Anderson.
"However, the local-regional recurrence rate in these patients is so low that routine postmastectomy radiation therapy is not warranted," she said.
No accord on margins
Despite multiple studies and meta-analyses, there is no standard for acceptable margin width in breast cancer, and surveys of both surgeons and radiation oncologists have shown wide variations in preferred margin widths, Dr. Garvey said.
Her group hypothesized that patients with invasive ductal carcinoma without an extensive in situ component who had surgical margins of at least 1 mm would have no evidence of residual disease on re-excision.
To test the idea, they took a retrospective look at records from a prospective database on 2,377 patients who underwent a total of 2,520 procedures from January 2000 through May 2012.
Of this group, 1,498 (63%) underwent lumpectomy, and 180 (12%) required re-excision surgery: 10% who had breast-conserving surgery alone, and 2% whose surgeries were converted to mastectomies. Of the 158 patients who had completed breast-conserving therapy following re-excision, 50 (32%) had residual disease, as did 20 of the 27 patients whose procedures were converted to mastectomies.
Of the 37% (879) who had up-front mastectomies, 2% (19) had re-excision, and of this group, 5 patients had residual disease.
In all, 40% of patients with positive margins had residual disease, compared with 38% of those with margins from 0.1 to 0.9 mm, and 33% for those with margins from 1.0 to 1.9 mm.
In univariate analysis, the presence of residual disease on re-excision did not show any significant association with age, race, menopausal status, width of the closest final margin, hormone receptor status, tumor histology, triple-negative disease, or the presence of angiolymphatic invasion. There was a trend, albeit nonsignificant, toward an association between residual disease and more than one margin narrower than 2 mm, Dr. Garvey noted.
At a median follow-up of 43 months (range, 0-140 months), 5-year local recurrence rates were 1.9% for patients who had breast-conserving therapy, and 1.1% for those who had mastectomy.
Patients who underwent breast-conserving therapy without re-excision had a 5-year local recurrence rate of 1.8%, compared with 4.3% for those who required re-excision, and 0% for those whose procedures were converted to mastectomy.
There was a nonsignificant trend toward higher local recurrence rates for breast-conserving therapy in patients who had re-excisions, which became significant when those patients who had conversion to mastectomy were excluded, with a hazard ratio compared with no re-excision of 2.56 (P = .04).
Narrower margins, larger risk
Dr. FitzSullivan and her M.D. Anderson colleagues reviewed the records of 810 women treated with mastectomy for DCIS from 1996 to 2009. They looked at the final width of histologic margins, defining disease-free margins as those of 3 mm or greater.
In all, 4 patients had positive margins, 59 had margins of 1 mm or smaller, and 35 had margins from 1.1 to 2.9 mm.
In multivariate analysis, independent predictors of close or positive margins were pathologic tumor size of 1.5 cm or greater (odds ratio, 5.11; P = .001), multicentric disease (OR, 5.44; P = .026), and the presence of necrosis (OR, 2.5; P = .003). Neither age, postmenopausal status, skin-sparing mastectomy, nor immediate breast reconstruction were significantly associated with close or positive margins, however.
None of seven patients who underwent postmastectomy radiotherapy had local-regional recurrences. Of the 803 patients who did not receive postsurgery radiation, 10-year LRR rates were 1%, consisting of 7 cases of invasive disease and 1 of DICS. Five patients had surgical management, and the remaining 3 had no further treatment.
When the researchers stratified the local recurrence rates by margin status, they saw that 5% of patients with margins of 1 mm or smaller had LRRs within 10 years, as did 3.6% of those with margins from 1.1 to 2.9 mm, compared with just 0.07% of those with disease-free margins (P less than .001). There was no difference in LRR between the two narrow-margin groups.
Among 546 patients with an intact contralateral breast, the 10-year rate of contralateral breast disease was 6.4%.
On univariate analysis, significant predictors of LRR included margin status (P = .002), multicentric disease (P = .005), and necrosis (P = .005). On multivariate analysis, however, only margin status remained significant, with an HR of 8.0 (P = .006).
Dr. FitzSullivan said that the low rate of LRR of DCIS treated with mastectomy and close surgical margins, compared with the rate of contralateral breast cancer, suggests that routine postmastectomy radiation therapy is not warranted, and should be reserved only for those patients with close or positive surgical margins that cannot be surgically excised.
Each study was internally funded. Dr. Garvey and Dr. FitzSullivan reported having no financial disclosures.
AT SSO 2013
Major finding: Surgical margin status was associated with an eightfold risk for local-regional recurrence of breast cancer.
Data source: Retrospective studies of data on patients treated for ductal carcinoma in situ or invasive breast cancer.
Disclosures: Each study was internally funded. Dr. Garvey and Dr. FitzSullivan reported having no financial disclosures.
Manage most SEGAs with rapamycin analogs, not surgery
SAN DIEGO – Medical management with sirolimus or everolimus for pediatric patients with tuberous sclerosis complex and subependymal giant cell astrocytomas is more effective and safer than surgery, researchers from the University of Cincinnati and University of California, Los Angeles, have found.
Although the benign tumors have traditionally been left to surgeons, it’s become clear in recent years that rapamycin analogs are effective, too. The question has been "which [approach] is best? Medical management "is known to be pretty mild compared to the surgery," but it’s not curative, explained lead investigator Susanne Yoon, the University of Cincinnati medical student who presented the results at the annual meeting of the American Academy of Neurology.
The team compared outcomes for 23 SEGA (subependymal giant cell astrocytoma) patients who underwent surgery, 81 who took sirolimus or everolimus, and 9 who got both. The surgery patients were diagnosed when they were about 10 years old and were followed for a median of 8.9 years; the medical patients were about 7 years old when diagnosed, and were followed for a median of 2.8 years. Boys made up the majority of both groups.
None of the children who took a rapamycin analog needed surgery; tumors shrank by more than half in 61% (45). The drugs caused infections, weight change, or hyperlipidemia in some, but only 13% (11) needed to stop the drug or go to the hospital because of side effects.
Meanwhile, surgery cured just 39% (9) of the children who got it, sometimes after two or three operations; 61% (14) of those patients had prolonged hospitalizations or were hospitalized due to postoperative complications that included intracranial hemorrhage in 8, hydrocephalus/shunt malfunction in 6, neurologic impairment, and seizures.
"Not only does medical management win in efficacy, but it also wins in the safety issues. Rapalog [rapamycin] therapy, alone or in combination, is becoming a cornerstone of tumor management" in neurocutaneous disorders, said Dr. David H. Viskochil, professor of pediatrics at the University of Utah, Salt Lake City, commenting on the study.
"Of course, there are emergent situations where you’ve just got to go in and get the tumor out; you can’t wait 3 months to see" if drugs work. "But if a child is just starting to show some symptoms and not deteriorating, then you can start with medicine first and see what happens," he said.
"The question is if you got [SEGAs] really early, would surgical cure be much more likely? The studies aren’t quite there yet," he said in an interview.
Ms. Yoon and Dr. Viskochil said they have no disclosures.
SAN DIEGO – Medical management with sirolimus or everolimus for pediatric patients with tuberous sclerosis complex and subependymal giant cell astrocytomas is more effective and safer than surgery, researchers from the University of Cincinnati and University of California, Los Angeles, have found.
Although the benign tumors have traditionally been left to surgeons, it’s become clear in recent years that rapamycin analogs are effective, too. The question has been "which [approach] is best? Medical management "is known to be pretty mild compared to the surgery," but it’s not curative, explained lead investigator Susanne Yoon, the University of Cincinnati medical student who presented the results at the annual meeting of the American Academy of Neurology.
The team compared outcomes for 23 SEGA (subependymal giant cell astrocytoma) patients who underwent surgery, 81 who took sirolimus or everolimus, and 9 who got both. The surgery patients were diagnosed when they were about 10 years old and were followed for a median of 8.9 years; the medical patients were about 7 years old when diagnosed, and were followed for a median of 2.8 years. Boys made up the majority of both groups.
None of the children who took a rapamycin analog needed surgery; tumors shrank by more than half in 61% (45). The drugs caused infections, weight change, or hyperlipidemia in some, but only 13% (11) needed to stop the drug or go to the hospital because of side effects.
Meanwhile, surgery cured just 39% (9) of the children who got it, sometimes after two or three operations; 61% (14) of those patients had prolonged hospitalizations or were hospitalized due to postoperative complications that included intracranial hemorrhage in 8, hydrocephalus/shunt malfunction in 6, neurologic impairment, and seizures.
"Not only does medical management win in efficacy, but it also wins in the safety issues. Rapalog [rapamycin] therapy, alone or in combination, is becoming a cornerstone of tumor management" in neurocutaneous disorders, said Dr. David H. Viskochil, professor of pediatrics at the University of Utah, Salt Lake City, commenting on the study.
"Of course, there are emergent situations where you’ve just got to go in and get the tumor out; you can’t wait 3 months to see" if drugs work. "But if a child is just starting to show some symptoms and not deteriorating, then you can start with medicine first and see what happens," he said.
"The question is if you got [SEGAs] really early, would surgical cure be much more likely? The studies aren’t quite there yet," he said in an interview.
Ms. Yoon and Dr. Viskochil said they have no disclosures.
SAN DIEGO – Medical management with sirolimus or everolimus for pediatric patients with tuberous sclerosis complex and subependymal giant cell astrocytomas is more effective and safer than surgery, researchers from the University of Cincinnati and University of California, Los Angeles, have found.
Although the benign tumors have traditionally been left to surgeons, it’s become clear in recent years that rapamycin analogs are effective, too. The question has been "which [approach] is best? Medical management "is known to be pretty mild compared to the surgery," but it’s not curative, explained lead investigator Susanne Yoon, the University of Cincinnati medical student who presented the results at the annual meeting of the American Academy of Neurology.
The team compared outcomes for 23 SEGA (subependymal giant cell astrocytoma) patients who underwent surgery, 81 who took sirolimus or everolimus, and 9 who got both. The surgery patients were diagnosed when they were about 10 years old and were followed for a median of 8.9 years; the medical patients were about 7 years old when diagnosed, and were followed for a median of 2.8 years. Boys made up the majority of both groups.
None of the children who took a rapamycin analog needed surgery; tumors shrank by more than half in 61% (45). The drugs caused infections, weight change, or hyperlipidemia in some, but only 13% (11) needed to stop the drug or go to the hospital because of side effects.
Meanwhile, surgery cured just 39% (9) of the children who got it, sometimes after two or three operations; 61% (14) of those patients had prolonged hospitalizations or were hospitalized due to postoperative complications that included intracranial hemorrhage in 8, hydrocephalus/shunt malfunction in 6, neurologic impairment, and seizures.
"Not only does medical management win in efficacy, but it also wins in the safety issues. Rapalog [rapamycin] therapy, alone or in combination, is becoming a cornerstone of tumor management" in neurocutaneous disorders, said Dr. David H. Viskochil, professor of pediatrics at the University of Utah, Salt Lake City, commenting on the study.
"Of course, there are emergent situations where you’ve just got to go in and get the tumor out; you can’t wait 3 months to see" if drugs work. "But if a child is just starting to show some symptoms and not deteriorating, then you can start with medicine first and see what happens," he said.
"The question is if you got [SEGAs] really early, would surgical cure be much more likely? The studies aren’t quite there yet," he said in an interview.
Ms. Yoon and Dr. Viskochil said they have no disclosures.
AT THE 2013 AAN ANNUAL MEETING
Major finding: Rapamycin analogs shrink SEGA tumors by more than 50% in a majority of children, and obviate the need for surgery.
Data source: Comparison of surgical and medical treatment of SEGA tumors in 113 children.
Disclosures: Ms. Yoon and Dr. Viskochil said they have no disclosures.
Radical resection trumps local excision in stage I CRC
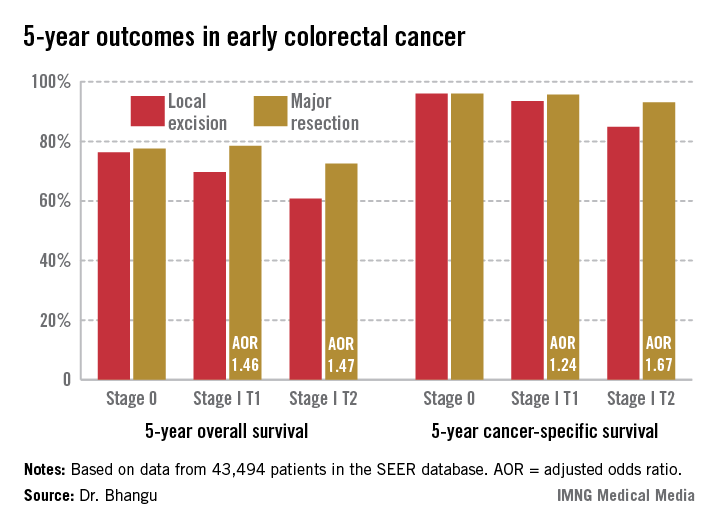
INDIANAPOLIS – Local excision of early invasive stage I colon or rectal carcinoma confers significantly worse 5-year overall and cancer-specific survival than does radical resection, according to analysis of a large national database.
This was true for stage I T1 and T2 disease; that is, for patients with tumor invading the submucosa as well as for those with tumor invading the muscularis propria, Dr. Aneel Bhangu reported at the annual meeting of the American Surgical Association.
In contrast, 5-year survival rates were equivalent with local excision compared with radical resection in patients with stage 0 disease, also known as carcinoma in situ, added Dr. Bhangu of Royal Marsden Hospital, London.
"We recommend that it is safe to perform local excision for stage 0 lesions – that is, carcinoma in situ or severely dysplastic polyps. Refined selection criteria for T1 cancers are required and should be the focus of further research. The use of local excision as a definitive treatment should be carefully considered for patients with T2 colorectal cancer, especially when treating younger, fit patients," he said.
The surgical oncologist presented an analysis of 43,494 patients with surgically treated stage 0 or I adenocarcinoma of the colon or rectum in the Surveillance, Epidemiology, and End Results (SEER) database for 1998-2009. He noted as an aside that the National Cancer Institute’s SEER database is "an open-access and free resource which is the envy of the worldwide oncological community and a shining example of how open-access data can be used by the global community to forward research."
Seventy percent of patients had colonic cancers, 30% rectal. Stage 0 cancer was present in 8.2%, while 91.8% of patients had stage I cancers, 51% of which were T1, 49% T2. Eighteen percent of subjects underwent local excision, while the rest had major resections.
Five-year overall survival was nearly an absolute 8% better in patients with stage I disease treated by radical resection, an advantage that grew even more dramatic in a multivariate analysis adjusted for age and other potential confounders.
Dr. Bhangu observed that these findings take on added import because the number of patients presenting with early colorectal cancer is climbing as a consequence of effective population-based colorectal cancer screening programs. The use of local excision as treatment for such cancers is growing as well. Yet the availability of high-tech tools and techniques for endoscopic local excision of these malignancies has created a dilemma: Such surgery spares the patient from a major operation with all its attendant hazards and morbidity, but when recurrences of these initially small cancers happen they may be inoperable in 10% of cases, and even when they are operable they require extensive visceral resection up to 50% of the time.
Discussant Dr. Genevieve Melton-Meaux of the University of Minnesota, Minneapolis, noted that a surprisingly large percentage of younger patients with stage I disease in this series – that is, patients under age 60 – were treated via local excision.
"We, too, were surprised by this," Dr. Bhangu replied. "If I were to speculate why, I’d say that it may be an issue of clinician equipoise. Some surgeons and endoscopists are true believers in this technology and they may be applying it to a wide scope of patients."
This same lack of equipoise explains the glacially slow recruitment rates into ongoing clinical trials badly needed to establish evidence-based therapy for early-stage colorectal cancer, he added.
He and his coworkers recommended directing future research in this field toward determining which patients with stage I disease are appropriate for local excision. Potentially relevant pathologic markers include depth of invasion and the degree of tumor differentiation. Biomarkers predictive of recurrence also are sorely needed.
Discussant Edward M. Copeland III noted that in light of Dr. Bhangu’s findings it makes sense to offer patients with T2 lesions neoadjuvant radiation and/or chemotherapy.
"You’d probably downstage a lot of them to T0 and you could then locally excise them, doing away with that 8% difference in survival between local excision and major resection you found in the SEER database," said Dr. Copeland, professor and chairman of the department of surgery at the University of Florida, Gainesville.
"I would venture to say if you had a patient with a T2 rectal lesion and offered neoadjuvant therapy followed by local excision, without a major operation, and with virtually zero chance of recurrence, I would take that, personally," he added.
Dr. Bhangu responded that it’s an intriguing notion, but the supporting evidence simply doesn’t exist. However, an ongoing U.K. randomized trial is evaluating just such an approach in patients with stage I T1 and T2 rectal cancer.
"I think this will provide the high-quality evidence that we require to treat these patients with evidence-based principles," he added.
Discussant Dr. Conor P. Delaney praised Dr. Bhangu for presenting "a great study." He placed the findings in perspective.
"It’s important to remember that the disadvantage that you’re showing with local therapy is very similar in size to the benefit that all of medical oncology gives us, with all of the effort that we invest in medical oncology. So this is actually a very significant result," declared Dr. Delaney, professor of surgery and chief of the division of colorectal surgery at Case Western Reserve University, Cleveland.
Dr. Bhangu reported having no financial conflicts.
INDIANAPOLIS – Local excision of early invasive stage I colon or rectal carcinoma confers significantly worse 5-year overall and cancer-specific survival than does radical resection, according to analysis of a large national database.
This was true for stage I T1 and T2 disease; that is, for patients with tumor invading the submucosa as well as for those with tumor invading the muscularis propria, Dr. Aneel Bhangu reported at the annual meeting of the American Surgical Association.
In contrast, 5-year survival rates were equivalent with local excision compared with radical resection in patients with stage 0 disease, also known as carcinoma in situ, added Dr. Bhangu of Royal Marsden Hospital, London.
"We recommend that it is safe to perform local excision for stage 0 lesions – that is, carcinoma in situ or severely dysplastic polyps. Refined selection criteria for T1 cancers are required and should be the focus of further research. The use of local excision as a definitive treatment should be carefully considered for patients with T2 colorectal cancer, especially when treating younger, fit patients," he said.
The surgical oncologist presented an analysis of 43,494 patients with surgically treated stage 0 or I adenocarcinoma of the colon or rectum in the Surveillance, Epidemiology, and End Results (SEER) database for 1998-2009. He noted as an aside that the National Cancer Institute’s SEER database is "an open-access and free resource which is the envy of the worldwide oncological community and a shining example of how open-access data can be used by the global community to forward research."
Seventy percent of patients had colonic cancers, 30% rectal. Stage 0 cancer was present in 8.2%, while 91.8% of patients had stage I cancers, 51% of which were T1, 49% T2. Eighteen percent of subjects underwent local excision, while the rest had major resections.
Five-year overall survival was nearly an absolute 8% better in patients with stage I disease treated by radical resection, an advantage that grew even more dramatic in a multivariate analysis adjusted for age and other potential confounders.
Dr. Bhangu observed that these findings take on added import because the number of patients presenting with early colorectal cancer is climbing as a consequence of effective population-based colorectal cancer screening programs. The use of local excision as treatment for such cancers is growing as well. Yet the availability of high-tech tools and techniques for endoscopic local excision of these malignancies has created a dilemma: Such surgery spares the patient from a major operation with all its attendant hazards and morbidity, but when recurrences of these initially small cancers happen they may be inoperable in 10% of cases, and even when they are operable they require extensive visceral resection up to 50% of the time.
Discussant Dr. Genevieve Melton-Meaux of the University of Minnesota, Minneapolis, noted that a surprisingly large percentage of younger patients with stage I disease in this series – that is, patients under age 60 – were treated via local excision.
"We, too, were surprised by this," Dr. Bhangu replied. "If I were to speculate why, I’d say that it may be an issue of clinician equipoise. Some surgeons and endoscopists are true believers in this technology and they may be applying it to a wide scope of patients."
This same lack of equipoise explains the glacially slow recruitment rates into ongoing clinical trials badly needed to establish evidence-based therapy for early-stage colorectal cancer, he added.
He and his coworkers recommended directing future research in this field toward determining which patients with stage I disease are appropriate for local excision. Potentially relevant pathologic markers include depth of invasion and the degree of tumor differentiation. Biomarkers predictive of recurrence also are sorely needed.
Discussant Edward M. Copeland III noted that in light of Dr. Bhangu’s findings it makes sense to offer patients with T2 lesions neoadjuvant radiation and/or chemotherapy.
"You’d probably downstage a lot of them to T0 and you could then locally excise them, doing away with that 8% difference in survival between local excision and major resection you found in the SEER database," said Dr. Copeland, professor and chairman of the department of surgery at the University of Florida, Gainesville.
"I would venture to say if you had a patient with a T2 rectal lesion and offered neoadjuvant therapy followed by local excision, without a major operation, and with virtually zero chance of recurrence, I would take that, personally," he added.
Dr. Bhangu responded that it’s an intriguing notion, but the supporting evidence simply doesn’t exist. However, an ongoing U.K. randomized trial is evaluating just such an approach in patients with stage I T1 and T2 rectal cancer.
"I think this will provide the high-quality evidence that we require to treat these patients with evidence-based principles," he added.
Discussant Dr. Conor P. Delaney praised Dr. Bhangu for presenting "a great study." He placed the findings in perspective.
"It’s important to remember that the disadvantage that you’re showing with local therapy is very similar in size to the benefit that all of medical oncology gives us, with all of the effort that we invest in medical oncology. So this is actually a very significant result," declared Dr. Delaney, professor of surgery and chief of the division of colorectal surgery at Case Western Reserve University, Cleveland.
Dr. Bhangu reported having no financial conflicts.
INDIANAPOLIS – Local excision of early invasive stage I colon or rectal carcinoma confers significantly worse 5-year overall and cancer-specific survival than does radical resection, according to analysis of a large national database.
This was true for stage I T1 and T2 disease; that is, for patients with tumor invading the submucosa as well as for those with tumor invading the muscularis propria, Dr. Aneel Bhangu reported at the annual meeting of the American Surgical Association.
In contrast, 5-year survival rates were equivalent with local excision compared with radical resection in patients with stage 0 disease, also known as carcinoma in situ, added Dr. Bhangu of Royal Marsden Hospital, London.
"We recommend that it is safe to perform local excision for stage 0 lesions – that is, carcinoma in situ or severely dysplastic polyps. Refined selection criteria for T1 cancers are required and should be the focus of further research. The use of local excision as a definitive treatment should be carefully considered for patients with T2 colorectal cancer, especially when treating younger, fit patients," he said.
The surgical oncologist presented an analysis of 43,494 patients with surgically treated stage 0 or I adenocarcinoma of the colon or rectum in the Surveillance, Epidemiology, and End Results (SEER) database for 1998-2009. He noted as an aside that the National Cancer Institute’s SEER database is "an open-access and free resource which is the envy of the worldwide oncological community and a shining example of how open-access data can be used by the global community to forward research."
Seventy percent of patients had colonic cancers, 30% rectal. Stage 0 cancer was present in 8.2%, while 91.8% of patients had stage I cancers, 51% of which were T1, 49% T2. Eighteen percent of subjects underwent local excision, while the rest had major resections.
Five-year overall survival was nearly an absolute 8% better in patients with stage I disease treated by radical resection, an advantage that grew even more dramatic in a multivariate analysis adjusted for age and other potential confounders.
Dr. Bhangu observed that these findings take on added import because the number of patients presenting with early colorectal cancer is climbing as a consequence of effective population-based colorectal cancer screening programs. The use of local excision as treatment for such cancers is growing as well. Yet the availability of high-tech tools and techniques for endoscopic local excision of these malignancies has created a dilemma: Such surgery spares the patient from a major operation with all its attendant hazards and morbidity, but when recurrences of these initially small cancers happen they may be inoperable in 10% of cases, and even when they are operable they require extensive visceral resection up to 50% of the time.
Discussant Dr. Genevieve Melton-Meaux of the University of Minnesota, Minneapolis, noted that a surprisingly large percentage of younger patients with stage I disease in this series – that is, patients under age 60 – were treated via local excision.
"We, too, were surprised by this," Dr. Bhangu replied. "If I were to speculate why, I’d say that it may be an issue of clinician equipoise. Some surgeons and endoscopists are true believers in this technology and they may be applying it to a wide scope of patients."
This same lack of equipoise explains the glacially slow recruitment rates into ongoing clinical trials badly needed to establish evidence-based therapy for early-stage colorectal cancer, he added.
He and his coworkers recommended directing future research in this field toward determining which patients with stage I disease are appropriate for local excision. Potentially relevant pathologic markers include depth of invasion and the degree of tumor differentiation. Biomarkers predictive of recurrence also are sorely needed.
Discussant Edward M. Copeland III noted that in light of Dr. Bhangu’s findings it makes sense to offer patients with T2 lesions neoadjuvant radiation and/or chemotherapy.
"You’d probably downstage a lot of them to T0 and you could then locally excise them, doing away with that 8% difference in survival between local excision and major resection you found in the SEER database," said Dr. Copeland, professor and chairman of the department of surgery at the University of Florida, Gainesville.
"I would venture to say if you had a patient with a T2 rectal lesion and offered neoadjuvant therapy followed by local excision, without a major operation, and with virtually zero chance of recurrence, I would take that, personally," he added.
Dr. Bhangu responded that it’s an intriguing notion, but the supporting evidence simply doesn’t exist. However, an ongoing U.K. randomized trial is evaluating just such an approach in patients with stage I T1 and T2 rectal cancer.
"I think this will provide the high-quality evidence that we require to treat these patients with evidence-based principles," he added.
Discussant Dr. Conor P. Delaney praised Dr. Bhangu for presenting "a great study." He placed the findings in perspective.
"It’s important to remember that the disadvantage that you’re showing with local therapy is very similar in size to the benefit that all of medical oncology gives us, with all of the effort that we invest in medical oncology. So this is actually a very significant result," declared Dr. Delaney, professor of surgery and chief of the division of colorectal surgery at Case Western Reserve University, Cleveland.
Dr. Bhangu reported having no financial conflicts.
AT THE ASA ANNUAL MEETING
Major Finding: Five-year overall survival in patients with stage I colorectal carcinoma treated via local excision was 68.4% compared to 75.2% with radical resection. Survival rates did not differ between the two surgical strategies in patients with stage 0 cancer.
Data Source: A retrospective analysis of data on nearly 44,000 patients with stage 0 or I colorectal cancer in the SEER database.
Disclosures: The SEER database is sponsored by the National Cancer Institute. The study presenter reported having no financial conflicts.
CA125 level predicts microscopic residual disease in ovarian cancer
LOS ANGELES – Preoperative levels of cancer antigen 125 (CA125) predict surgical and disease outcomes in women with advanced epithelial ovarian cancer who are able to undergo optimal debulking surgery, new data show, and may therefore help guide treatment decisions.
A team led by Dr. Neil S. Horowitz of Brigham and Women’s Hospital and the Dana Farber Cancer Institute in Boston assessed levels of the biomarker among nearly 1,000 women who had stage III or IV disease that was optimally debulked to less than 1 cm of residual disease and who received adjuvant paclitaxel- and platinum-containing chemotherapy.
Results showed that no cutoff value of preoperative CA125 levels clearly separated women in whom microscopic residual disease was achieved surgically from women in whom a greater volume of disease remained, he reported at the annual meeting of the Society of Gynecologic Oncology.
But the probability of achieving microscopic status decreased with increasing CA125 levels. For example, it fell from 33% in women with a level of 500 U/mL to 27% in women with a level of 1,000 U/mL.
"Although a strict CA125 value to predict microscopic residual cannot be made, these data are helpful for counseling patients regarding surgical results and outcome, and should influence decisions regarding primary debulking surgery and possibly use of other ... treatment options like neoadjuvant chemotherapy," Dr. Horowitz said. "Each surgeon and [his or her] patient have to decide for themselves what is an acceptable probability of successful surgical outcome to microscopic residual disease.
Additional study findings showed that women with higher preoperative CA125 levels and women with smaller reductions in CA125 levels between the preoperative period and the pretreatment period, before starting chemotherapy, had significantly worse progression-free and overall survival.
One session attendee asked, "What impact do you think surgical expertise has on the ability to predict the extent of cytoreduction?"
"Most trained gyn oncologists have the ability and training to take somebody to microscopic residual disease. What it takes to get to that place obviously varies from patient to patient and the disease that they have at the time that they present. Ultimately, what has to be decided between the patient and the physician is what’s going to be the best possibility for them and using CA125 as a potential guide, say, taking everything into consideration – age, where their disease is, what their CA125 is, what my comfort level is doing certain procedures being able to get them down to microscopic disease," Dr. Horowitz replied. "So I don’t think it’s a one-size-fits-all [objective] based just on the surgical expertise. Most gyn oncologists are trained adequately to be able to do this. It’s just, because you can do it, the question is, should you be doing it on everybody."
Another attendee said, "In follow-up to that, do you think that the difference in the behavior of the presurgical and the pretreatment CA125 could be confounded, the latter by surgical decisions or surgical intervention?"
"It’s potentially confounded; whether it’s surgical skill or some people would say, I don’t want to use the term honesty, but it may be a reflection of what truly is left behind versus not left behind, if it didn’t really follow the way it should. It may be a window into how accurate our predictions or our estimates of what we left behind really are," Dr. Horowitz explained. "Pretreatment CA125, it is difficult to really get a good understanding of this number because obviously the patients who start with the highest CA125 have the greatest chance to fall ... So it’s a little tricky trying to figure out what to make of that number, whereas patients who start with a lower one, although they didn’t fall with the same percentile, you still may have done just as good a job surgically."
The 998 women studied were from Gynecologic Oncology Group (GOG) trial 182. "This is the largest reported series to evaluate the ability of preoperative CA125 to predict surgical cytoreductive outcomes and survival in a population of women with optimally cytoreduced primary ovarian or peritoneal cancer," Dr. Horowitz noted.
Overall, 33% had microscopic residual disease, while the other 67% had more extensive residual disease but still measuring less than 1 cm.
The median preoperative CA125 level was 346 U/mL in the former group and 870 U/mL in the latter, reported Dr. Horowitz, who disclosed no conflicts of interest related to the research.
"Despite the difference in median preoperative CA125 values, the distributions of the preoperative CA125s in those with microscopic and less than 1 cm residual overlapped almost completely," Dr. Horowitz reported. "This suggests that there is not a preoperative CA125 beyond which one cannot achieve a complete cytoreductive surgery."
However, the higher the preoperative CA125 level, the lower the predicted probability of achieving microscopic residual disease (P less than .01). For example, the probability was 33%, 27%, and 19% for women having a level of 500, 1,000, and 2,500 U/mL, respectively.
"It is important to remember that these curves only reflect data from women who achieved optimal cytoreduction and do not include those with suboptimal primary debulking. Therefore, the estimated predictions and probabilities are likely to decrease when applied to a preoperative population of unknown surgical outcome," he said. "But if one assumes a priori that you can achieve and will achieve optimal cytoreduction, then preoperative CA125 can estimate the likelihood of obtaining either microscopic or less than 1-cm residual."
In adjusted analyses, preoperative CA125 levels and extent of residual disease jointly predicted both progression-free survival (P less than .001) and overall survival (P = .04). For example, median overall survival ranged from 82 months in women having microscopic residual disease and a CA125 level of 35 U/mL to just 39 months in their counterparts with more residual disease and a CA125 level of 1,000 U/mL.
The change from preoperative to pretreatment CA125 levels predicted both progression-free survival (P less than .0001) and overall survival (P less than .0001). For example, median overall survival ranged from 60 months in women having a reduction in levels exceeding 80% to 45 months in their counterparts having stable or increasing levels.
In addition, among the group having a greater than 80% decline in CA125 level, survival was almost twice as long among those achieving microscopic residual disease, at 82 months, as among those with greater residual disease, at 48 months, "suggesting that residual disease rather than change in CA125 is more important to survival," Dr. Horowitz said.
LOS ANGELES – Preoperative levels of cancer antigen 125 (CA125) predict surgical and disease outcomes in women with advanced epithelial ovarian cancer who are able to undergo optimal debulking surgery, new data show, and may therefore help guide treatment decisions.
A team led by Dr. Neil S. Horowitz of Brigham and Women’s Hospital and the Dana Farber Cancer Institute in Boston assessed levels of the biomarker among nearly 1,000 women who had stage III or IV disease that was optimally debulked to less than 1 cm of residual disease and who received adjuvant paclitaxel- and platinum-containing chemotherapy.
Results showed that no cutoff value of preoperative CA125 levels clearly separated women in whom microscopic residual disease was achieved surgically from women in whom a greater volume of disease remained, he reported at the annual meeting of the Society of Gynecologic Oncology.
But the probability of achieving microscopic status decreased with increasing CA125 levels. For example, it fell from 33% in women with a level of 500 U/mL to 27% in women with a level of 1,000 U/mL.
"Although a strict CA125 value to predict microscopic residual cannot be made, these data are helpful for counseling patients regarding surgical results and outcome, and should influence decisions regarding primary debulking surgery and possibly use of other ... treatment options like neoadjuvant chemotherapy," Dr. Horowitz said. "Each surgeon and [his or her] patient have to decide for themselves what is an acceptable probability of successful surgical outcome to microscopic residual disease.
Additional study findings showed that women with higher preoperative CA125 levels and women with smaller reductions in CA125 levels between the preoperative period and the pretreatment period, before starting chemotherapy, had significantly worse progression-free and overall survival.
One session attendee asked, "What impact do you think surgical expertise has on the ability to predict the extent of cytoreduction?"
"Most trained gyn oncologists have the ability and training to take somebody to microscopic residual disease. What it takes to get to that place obviously varies from patient to patient and the disease that they have at the time that they present. Ultimately, what has to be decided between the patient and the physician is what’s going to be the best possibility for them and using CA125 as a potential guide, say, taking everything into consideration – age, where their disease is, what their CA125 is, what my comfort level is doing certain procedures being able to get them down to microscopic disease," Dr. Horowitz replied. "So I don’t think it’s a one-size-fits-all [objective] based just on the surgical expertise. Most gyn oncologists are trained adequately to be able to do this. It’s just, because you can do it, the question is, should you be doing it on everybody."
Another attendee said, "In follow-up to that, do you think that the difference in the behavior of the presurgical and the pretreatment CA125 could be confounded, the latter by surgical decisions or surgical intervention?"
"It’s potentially confounded; whether it’s surgical skill or some people would say, I don’t want to use the term honesty, but it may be a reflection of what truly is left behind versus not left behind, if it didn’t really follow the way it should. It may be a window into how accurate our predictions or our estimates of what we left behind really are," Dr. Horowitz explained. "Pretreatment CA125, it is difficult to really get a good understanding of this number because obviously the patients who start with the highest CA125 have the greatest chance to fall ... So it’s a little tricky trying to figure out what to make of that number, whereas patients who start with a lower one, although they didn’t fall with the same percentile, you still may have done just as good a job surgically."
The 998 women studied were from Gynecologic Oncology Group (GOG) trial 182. "This is the largest reported series to evaluate the ability of preoperative CA125 to predict surgical cytoreductive outcomes and survival in a population of women with optimally cytoreduced primary ovarian or peritoneal cancer," Dr. Horowitz noted.
Overall, 33% had microscopic residual disease, while the other 67% had more extensive residual disease but still measuring less than 1 cm.
The median preoperative CA125 level was 346 U/mL in the former group and 870 U/mL in the latter, reported Dr. Horowitz, who disclosed no conflicts of interest related to the research.
"Despite the difference in median preoperative CA125 values, the distributions of the preoperative CA125s in those with microscopic and less than 1 cm residual overlapped almost completely," Dr. Horowitz reported. "This suggests that there is not a preoperative CA125 beyond which one cannot achieve a complete cytoreductive surgery."
However, the higher the preoperative CA125 level, the lower the predicted probability of achieving microscopic residual disease (P less than .01). For example, the probability was 33%, 27%, and 19% for women having a level of 500, 1,000, and 2,500 U/mL, respectively.
"It is important to remember that these curves only reflect data from women who achieved optimal cytoreduction and do not include those with suboptimal primary debulking. Therefore, the estimated predictions and probabilities are likely to decrease when applied to a preoperative population of unknown surgical outcome," he said. "But if one assumes a priori that you can achieve and will achieve optimal cytoreduction, then preoperative CA125 can estimate the likelihood of obtaining either microscopic or less than 1-cm residual."
In adjusted analyses, preoperative CA125 levels and extent of residual disease jointly predicted both progression-free survival (P less than .001) and overall survival (P = .04). For example, median overall survival ranged from 82 months in women having microscopic residual disease and a CA125 level of 35 U/mL to just 39 months in their counterparts with more residual disease and a CA125 level of 1,000 U/mL.
The change from preoperative to pretreatment CA125 levels predicted both progression-free survival (P less than .0001) and overall survival (P less than .0001). For example, median overall survival ranged from 60 months in women having a reduction in levels exceeding 80% to 45 months in their counterparts having stable or increasing levels.
In addition, among the group having a greater than 80% decline in CA125 level, survival was almost twice as long among those achieving microscopic residual disease, at 82 months, as among those with greater residual disease, at 48 months, "suggesting that residual disease rather than change in CA125 is more important to survival," Dr. Horowitz said.
LOS ANGELES – Preoperative levels of cancer antigen 125 (CA125) predict surgical and disease outcomes in women with advanced epithelial ovarian cancer who are able to undergo optimal debulking surgery, new data show, and may therefore help guide treatment decisions.
A team led by Dr. Neil S. Horowitz of Brigham and Women’s Hospital and the Dana Farber Cancer Institute in Boston assessed levels of the biomarker among nearly 1,000 women who had stage III or IV disease that was optimally debulked to less than 1 cm of residual disease and who received adjuvant paclitaxel- and platinum-containing chemotherapy.
Results showed that no cutoff value of preoperative CA125 levels clearly separated women in whom microscopic residual disease was achieved surgically from women in whom a greater volume of disease remained, he reported at the annual meeting of the Society of Gynecologic Oncology.
But the probability of achieving microscopic status decreased with increasing CA125 levels. For example, it fell from 33% in women with a level of 500 U/mL to 27% in women with a level of 1,000 U/mL.
"Although a strict CA125 value to predict microscopic residual cannot be made, these data are helpful for counseling patients regarding surgical results and outcome, and should influence decisions regarding primary debulking surgery and possibly use of other ... treatment options like neoadjuvant chemotherapy," Dr. Horowitz said. "Each surgeon and [his or her] patient have to decide for themselves what is an acceptable probability of successful surgical outcome to microscopic residual disease.
Additional study findings showed that women with higher preoperative CA125 levels and women with smaller reductions in CA125 levels between the preoperative period and the pretreatment period, before starting chemotherapy, had significantly worse progression-free and overall survival.
One session attendee asked, "What impact do you think surgical expertise has on the ability to predict the extent of cytoreduction?"
"Most trained gyn oncologists have the ability and training to take somebody to microscopic residual disease. What it takes to get to that place obviously varies from patient to patient and the disease that they have at the time that they present. Ultimately, what has to be decided between the patient and the physician is what’s going to be the best possibility for them and using CA125 as a potential guide, say, taking everything into consideration – age, where their disease is, what their CA125 is, what my comfort level is doing certain procedures being able to get them down to microscopic disease," Dr. Horowitz replied. "So I don’t think it’s a one-size-fits-all [objective] based just on the surgical expertise. Most gyn oncologists are trained adequately to be able to do this. It’s just, because you can do it, the question is, should you be doing it on everybody."
Another attendee said, "In follow-up to that, do you think that the difference in the behavior of the presurgical and the pretreatment CA125 could be confounded, the latter by surgical decisions or surgical intervention?"
"It’s potentially confounded; whether it’s surgical skill or some people would say, I don’t want to use the term honesty, but it may be a reflection of what truly is left behind versus not left behind, if it didn’t really follow the way it should. It may be a window into how accurate our predictions or our estimates of what we left behind really are," Dr. Horowitz explained. "Pretreatment CA125, it is difficult to really get a good understanding of this number because obviously the patients who start with the highest CA125 have the greatest chance to fall ... So it’s a little tricky trying to figure out what to make of that number, whereas patients who start with a lower one, although they didn’t fall with the same percentile, you still may have done just as good a job surgically."
The 998 women studied were from Gynecologic Oncology Group (GOG) trial 182. "This is the largest reported series to evaluate the ability of preoperative CA125 to predict surgical cytoreductive outcomes and survival in a population of women with optimally cytoreduced primary ovarian or peritoneal cancer," Dr. Horowitz noted.
Overall, 33% had microscopic residual disease, while the other 67% had more extensive residual disease but still measuring less than 1 cm.
The median preoperative CA125 level was 346 U/mL in the former group and 870 U/mL in the latter, reported Dr. Horowitz, who disclosed no conflicts of interest related to the research.
"Despite the difference in median preoperative CA125 values, the distributions of the preoperative CA125s in those with microscopic and less than 1 cm residual overlapped almost completely," Dr. Horowitz reported. "This suggests that there is not a preoperative CA125 beyond which one cannot achieve a complete cytoreductive surgery."
However, the higher the preoperative CA125 level, the lower the predicted probability of achieving microscopic residual disease (P less than .01). For example, the probability was 33%, 27%, and 19% for women having a level of 500, 1,000, and 2,500 U/mL, respectively.
"It is important to remember that these curves only reflect data from women who achieved optimal cytoreduction and do not include those with suboptimal primary debulking. Therefore, the estimated predictions and probabilities are likely to decrease when applied to a preoperative population of unknown surgical outcome," he said. "But if one assumes a priori that you can achieve and will achieve optimal cytoreduction, then preoperative CA125 can estimate the likelihood of obtaining either microscopic or less than 1-cm residual."
In adjusted analyses, preoperative CA125 levels and extent of residual disease jointly predicted both progression-free survival (P less than .001) and overall survival (P = .04). For example, median overall survival ranged from 82 months in women having microscopic residual disease and a CA125 level of 35 U/mL to just 39 months in their counterparts with more residual disease and a CA125 level of 1,000 U/mL.
The change from preoperative to pretreatment CA125 levels predicted both progression-free survival (P less than .0001) and overall survival (P less than .0001). For example, median overall survival ranged from 60 months in women having a reduction in levels exceeding 80% to 45 months in their counterparts having stable or increasing levels.
In addition, among the group having a greater than 80% decline in CA125 level, survival was almost twice as long among those achieving microscopic residual disease, at 82 months, as among those with greater residual disease, at 48 months, "suggesting that residual disease rather than change in CA125 is more important to survival," Dr. Horowitz said.
AT THE ANNUAL MEETING ON WOMEN'S CANCER
Major finding: The higher the preoperative CA125 level, the lower the predicted probability of achieving microscopic residual disease. Probability was 33%, 27%, and 19% for levels of 500, 1,000, and 2,500 U/mL, respectively.
Data source: An ancillary study among 998 women with optimally debulked advanced epithelial ovarian cancer or primary peritoneal cancer from the GOG 182 trial.
Disclosures: Dr. Horowitz disclosed no relevant conflicts of interest.
Cyberknife therapy offers benefits for recurring gyn cancers
LOS ANGELES – Robotic Cyberknife radiation therapy will likely expand the treatment options for patients whose gynecologic malignancies recur, researchers reported at the annual meeting of the Society of Gynecologic Oncologists.
A total of 50 patients with recurrent gynecologic (mainly ovarian and endometrial) cancers underwent Cyberknife treatment, which allows targeting of high doses of radiation to the tumor while largely sparing adjacent healthy tissue. In this case, patients received an 8-Gy treatment on each of 3 consecutive days.
Results showed that two-thirds of the patients had a complete response, a partial response, or stable disease 6 months after treatment. Higher-severity acute adverse events were uncommon and consisted of single cases of diarrhea and hyperbilirubinemia.
"This is the first phase II clinical trial of robotic radiosurgery in the gynecologic oncology space. Robotic Cyberknife radiation therapy does definitely provide clinical benefit to your patients," commented lead author Dr. Charles Kunos, a radiation oncologist with the Case Comprehensive Cancer Center in Cleveland and the Summa Cancer Institute in Akron, Ohio.
However, the majority of women ultimately had progression in nontargeted areas of disease, and this progression was the cause of death in 40%. Therefore, the investigators have initiated a phase I trial combining Cyberknife therapy with systemic chemotherapy.
One attendee noted, "As I think we all know, many of these patients who have recurrent side-wall disease from gynecologic cancers have a loop of bowel that’s sitting immediately adjacent to the potential target. And we have always felt that you needed to adjust the dose per fraction depending on that in patients with recurrent disease who have been previously irradiated. How do you select your patients for these 8-Gy-fraction treatments?"
"Patient selection is mostly based on target volume and the number of sites that are going to be treated during the course of therapy. ... We find that the 8-Gy dose is extremely well tolerated," he noted. The team is currently performing analyses to determine maximum tissue tolerances and optimally guide patient selection.
Another attendee had a similar question, asking whether any of the lesions treated were located in a previously irradiated field.
"More than 60% of those patients had received prior radiation therapy. Almost all the cervical cancer patients in the trial, as well as the majority of those patients with endometrial cancer, had received prior radiation therapy," Dr. Kunos replied. "The prior radiation therapy is not a barrier for delivering this form of treatment at these doses. Our toxicity rates are extraordinarily low, even in the cases with the prior radiation, so with selection of the patient who has a relatively good performing health status, as well as considering the time of when the previous radiation therapy dose was delivered, I think you can adequately select patients for this type of therapy."
A third attendee posed bigger-picture questions: "Can you tell us a little bit about the cost-effectiveness of the Cyberknife therapy and, given that you have an ongoing phase I trial looking at this in dual-modality [treatment] with chemotherapy, what is the future of this therapeutic approach for gyn malignancies?"
"The NCI [National Cancer Institute] is interested in exploring the radiosurgery platform as a way of testing novel therapeutics as radiation sensitizers, but because the radiation therapy is so exquisitely tuned to the actual cancer target, there is an opportunity to study whether or not there are systemic effects of the novel drugs as well. So I think at some level, the future is relatively bright," Dr. Kunos explained.
"Regarding the cost or the expense of therapy, we are condensing 5, 6, 7 weeks of radiation therapy into 3 days on the trial, so there are considerable savings in that regard to patient travel and other, social aspects of treatment in trying to get patients back and forth for several weeks of therapy in a palliative situation," he continued. "The radiosurgery itself is expensive; that part of the cost structure will probably continue to come down in the future with the way that patients will be managed."
The cohort enrolled in the trial included 25 patients with ovarian cancer, 14 with endometrial cancer, 9 with cervical cancer, and 2 with vulvar cancer. All had received at least one chemotherapy or radiation therapy regimen previously.
The patients had up to four targets of disease to be treated. They underwent implantation of gold seeds for target tracking during treatment, and co-registered computed tomography and positron emission tomography scans were used to generate the radiation therapy plan.
The patients received a total of 24 Gy of radiation delivered in three daily doses of 8 Gy each. The median duration of follow-up was 15 months.
Study results, reported at the meeting and recently published (Front. Oncol. 2012;2:181), showed that 3 months after treatment, 50% of patients had a complete response, 46% had a partial response, and 4% had stable disease, according to Dr. Kunos.
At 6 months, the rate of clinical benefit – capturing patients with a complete response, partial response, or stable disease – was 68%.
Ultimately, 62% of patients had progression in a nontargeted disease site, and 40% died from disease progression.
"Reversible fatigue lasting up to 1 week after treatment was the most noticeable toxicity," Dr. Kunos said. In the first 30 days after treatment, the most common adverse effects were grade 2 fatigue, seen in 16% of patients, and grade 2 nausea, seen in 8%. Only 2% of patients had grade 3 diarrhea, and only 2% had grade 4 hyperbilirubinemia.
Dr. Kunos disclosed that he had no relevant conflicts of interest.
LOS ANGELES – Robotic Cyberknife radiation therapy will likely expand the treatment options for patients whose gynecologic malignancies recur, researchers reported at the annual meeting of the Society of Gynecologic Oncologists.
A total of 50 patients with recurrent gynecologic (mainly ovarian and endometrial) cancers underwent Cyberknife treatment, which allows targeting of high doses of radiation to the tumor while largely sparing adjacent healthy tissue. In this case, patients received an 8-Gy treatment on each of 3 consecutive days.
Results showed that two-thirds of the patients had a complete response, a partial response, or stable disease 6 months after treatment. Higher-severity acute adverse events were uncommon and consisted of single cases of diarrhea and hyperbilirubinemia.
"This is the first phase II clinical trial of robotic radiosurgery in the gynecologic oncology space. Robotic Cyberknife radiation therapy does definitely provide clinical benefit to your patients," commented lead author Dr. Charles Kunos, a radiation oncologist with the Case Comprehensive Cancer Center in Cleveland and the Summa Cancer Institute in Akron, Ohio.
However, the majority of women ultimately had progression in nontargeted areas of disease, and this progression was the cause of death in 40%. Therefore, the investigators have initiated a phase I trial combining Cyberknife therapy with systemic chemotherapy.
One attendee noted, "As I think we all know, many of these patients who have recurrent side-wall disease from gynecologic cancers have a loop of bowel that’s sitting immediately adjacent to the potential target. And we have always felt that you needed to adjust the dose per fraction depending on that in patients with recurrent disease who have been previously irradiated. How do you select your patients for these 8-Gy-fraction treatments?"
"Patient selection is mostly based on target volume and the number of sites that are going to be treated during the course of therapy. ... We find that the 8-Gy dose is extremely well tolerated," he noted. The team is currently performing analyses to determine maximum tissue tolerances and optimally guide patient selection.
Another attendee had a similar question, asking whether any of the lesions treated were located in a previously irradiated field.
"More than 60% of those patients had received prior radiation therapy. Almost all the cervical cancer patients in the trial, as well as the majority of those patients with endometrial cancer, had received prior radiation therapy," Dr. Kunos replied. "The prior radiation therapy is not a barrier for delivering this form of treatment at these doses. Our toxicity rates are extraordinarily low, even in the cases with the prior radiation, so with selection of the patient who has a relatively good performing health status, as well as considering the time of when the previous radiation therapy dose was delivered, I think you can adequately select patients for this type of therapy."
A third attendee posed bigger-picture questions: "Can you tell us a little bit about the cost-effectiveness of the Cyberknife therapy and, given that you have an ongoing phase I trial looking at this in dual-modality [treatment] with chemotherapy, what is the future of this therapeutic approach for gyn malignancies?"
"The NCI [National Cancer Institute] is interested in exploring the radiosurgery platform as a way of testing novel therapeutics as radiation sensitizers, but because the radiation therapy is so exquisitely tuned to the actual cancer target, there is an opportunity to study whether or not there are systemic effects of the novel drugs as well. So I think at some level, the future is relatively bright," Dr. Kunos explained.
"Regarding the cost or the expense of therapy, we are condensing 5, 6, 7 weeks of radiation therapy into 3 days on the trial, so there are considerable savings in that regard to patient travel and other, social aspects of treatment in trying to get patients back and forth for several weeks of therapy in a palliative situation," he continued. "The radiosurgery itself is expensive; that part of the cost structure will probably continue to come down in the future with the way that patients will be managed."
The cohort enrolled in the trial included 25 patients with ovarian cancer, 14 with endometrial cancer, 9 with cervical cancer, and 2 with vulvar cancer. All had received at least one chemotherapy or radiation therapy regimen previously.
The patients had up to four targets of disease to be treated. They underwent implantation of gold seeds for target tracking during treatment, and co-registered computed tomography and positron emission tomography scans were used to generate the radiation therapy plan.
The patients received a total of 24 Gy of radiation delivered in three daily doses of 8 Gy each. The median duration of follow-up was 15 months.
Study results, reported at the meeting and recently published (Front. Oncol. 2012;2:181), showed that 3 months after treatment, 50% of patients had a complete response, 46% had a partial response, and 4% had stable disease, according to Dr. Kunos.
At 6 months, the rate of clinical benefit – capturing patients with a complete response, partial response, or stable disease – was 68%.
Ultimately, 62% of patients had progression in a nontargeted disease site, and 40% died from disease progression.
"Reversible fatigue lasting up to 1 week after treatment was the most noticeable toxicity," Dr. Kunos said. In the first 30 days after treatment, the most common adverse effects were grade 2 fatigue, seen in 16% of patients, and grade 2 nausea, seen in 8%. Only 2% of patients had grade 3 diarrhea, and only 2% had grade 4 hyperbilirubinemia.
Dr. Kunos disclosed that he had no relevant conflicts of interest.
LOS ANGELES – Robotic Cyberknife radiation therapy will likely expand the treatment options for patients whose gynecologic malignancies recur, researchers reported at the annual meeting of the Society of Gynecologic Oncologists.
A total of 50 patients with recurrent gynecologic (mainly ovarian and endometrial) cancers underwent Cyberknife treatment, which allows targeting of high doses of radiation to the tumor while largely sparing adjacent healthy tissue. In this case, patients received an 8-Gy treatment on each of 3 consecutive days.
Results showed that two-thirds of the patients had a complete response, a partial response, or stable disease 6 months after treatment. Higher-severity acute adverse events were uncommon and consisted of single cases of diarrhea and hyperbilirubinemia.
"This is the first phase II clinical trial of robotic radiosurgery in the gynecologic oncology space. Robotic Cyberknife radiation therapy does definitely provide clinical benefit to your patients," commented lead author Dr. Charles Kunos, a radiation oncologist with the Case Comprehensive Cancer Center in Cleveland and the Summa Cancer Institute in Akron, Ohio.
However, the majority of women ultimately had progression in nontargeted areas of disease, and this progression was the cause of death in 40%. Therefore, the investigators have initiated a phase I trial combining Cyberknife therapy with systemic chemotherapy.
One attendee noted, "As I think we all know, many of these patients who have recurrent side-wall disease from gynecologic cancers have a loop of bowel that’s sitting immediately adjacent to the potential target. And we have always felt that you needed to adjust the dose per fraction depending on that in patients with recurrent disease who have been previously irradiated. How do you select your patients for these 8-Gy-fraction treatments?"
"Patient selection is mostly based on target volume and the number of sites that are going to be treated during the course of therapy. ... We find that the 8-Gy dose is extremely well tolerated," he noted. The team is currently performing analyses to determine maximum tissue tolerances and optimally guide patient selection.
Another attendee had a similar question, asking whether any of the lesions treated were located in a previously irradiated field.
"More than 60% of those patients had received prior radiation therapy. Almost all the cervical cancer patients in the trial, as well as the majority of those patients with endometrial cancer, had received prior radiation therapy," Dr. Kunos replied. "The prior radiation therapy is not a barrier for delivering this form of treatment at these doses. Our toxicity rates are extraordinarily low, even in the cases with the prior radiation, so with selection of the patient who has a relatively good performing health status, as well as considering the time of when the previous radiation therapy dose was delivered, I think you can adequately select patients for this type of therapy."
A third attendee posed bigger-picture questions: "Can you tell us a little bit about the cost-effectiveness of the Cyberknife therapy and, given that you have an ongoing phase I trial looking at this in dual-modality [treatment] with chemotherapy, what is the future of this therapeutic approach for gyn malignancies?"
"The NCI [National Cancer Institute] is interested in exploring the radiosurgery platform as a way of testing novel therapeutics as radiation sensitizers, but because the radiation therapy is so exquisitely tuned to the actual cancer target, there is an opportunity to study whether or not there are systemic effects of the novel drugs as well. So I think at some level, the future is relatively bright," Dr. Kunos explained.
"Regarding the cost or the expense of therapy, we are condensing 5, 6, 7 weeks of radiation therapy into 3 days on the trial, so there are considerable savings in that regard to patient travel and other, social aspects of treatment in trying to get patients back and forth for several weeks of therapy in a palliative situation," he continued. "The radiosurgery itself is expensive; that part of the cost structure will probably continue to come down in the future with the way that patients will be managed."
The cohort enrolled in the trial included 25 patients with ovarian cancer, 14 with endometrial cancer, 9 with cervical cancer, and 2 with vulvar cancer. All had received at least one chemotherapy or radiation therapy regimen previously.
The patients had up to four targets of disease to be treated. They underwent implantation of gold seeds for target tracking during treatment, and co-registered computed tomography and positron emission tomography scans were used to generate the radiation therapy plan.
The patients received a total of 24 Gy of radiation delivered in three daily doses of 8 Gy each. The median duration of follow-up was 15 months.
Study results, reported at the meeting and recently published (Front. Oncol. 2012;2:181), showed that 3 months after treatment, 50% of patients had a complete response, 46% had a partial response, and 4% had stable disease, according to Dr. Kunos.
At 6 months, the rate of clinical benefit – capturing patients with a complete response, partial response, or stable disease – was 68%.
Ultimately, 62% of patients had progression in a nontargeted disease site, and 40% died from disease progression.
"Reversible fatigue lasting up to 1 week after treatment was the most noticeable toxicity," Dr. Kunos said. In the first 30 days after treatment, the most common adverse effects were grade 2 fatigue, seen in 16% of patients, and grade 2 nausea, seen in 8%. Only 2% of patients had grade 3 diarrhea, and only 2% had grade 4 hyperbilirubinemia.
Dr. Kunos disclosed that he had no relevant conflicts of interest.
AT THE ANNUAL MEETING ON WOMEN'S CANCER
Major finding: The 6-month clinical benefit rate was 68%. Higher-severity acute adverse events were uncommon.
Data source: A phase II trial among 50 patients with recurrent gynecologic cancers.
Disclosures: Dr. Kunos disclosed that he had no relevant conflicts of interest.
High CRP augurs worse outcomes from palliative surgery for cancer
NATIONAL HARBOR, MD. – Elevated levels of C-reactive protein may be a marker for poor response to palliative surgery, Dr. Andrew M. Blakely said at the annual Society of Surgical Oncology Cancer Symposium.
In a study of 50 patients who underwent elective palliative procedures for various cancer-related symptoms, elevated C-reactive protein (CRP), but not fatigue scores or performance status, was associated with a nearly fourfold risk for worse overall survival, reported Dr. Blakely, a surgery resident at Alpert Medical School, Brown University, Providence, R.I.
Elevated CRP, defined as levels greater than 8 mg/L, was also associated with increased overall and tumor-grade specific complications.
"This is important, because these complications are poorly tolerated by advanced cancer patients, and they serve to greatly diminish the potential benefit of palliative surgery," Dr. Blakely said.
He and colleagues at Brown and the Rhode Island Hospital, also in Providence, looked at potential prognostic factors in patients who chose surgery to relieve symptoms of advanced cancers such as gastrointestinal obstruction, or local-regional control of tumor-related pain or bleeding.
Malignancies treated included gastric, pancreatic, colon, melanoma, sarcomas, breast cancer, hepatobiliary and ovarian cancer, and squamous cell carcinomas.
They found that 44 patients (88%) stated that the surgery "was worth it," and 37 patients (74%) reported symptom resolution or improvement following surgery.
At 1-month follow-up, the complication rate was 44%, and 10% of patients had died. Although there was no association between CRP levels and morbidity or mortality among all patients, 11 of 27 patients with low-grade (grade 1 or 2) complications had elevated CRP (P = .001), as did all 6 patients with high-grade (grade 3 or 4) malignancies (P = .010), and 4 of 5 patients who died (P =.007).
In univariate analysis, factors significantly associated with major complications included elevated CRP (P = .005) and National Cancer Institute (NCI) fatigue score of 1 or greater (P = .001). Factors associated with overall survival were elevated CRP (P less than .0001), and NCI fatigue score (P = .04).
But in multivariate analysis controlling for those factors and for male sex, low albumin levels, significant weight loss, no previous cancer therapy, and hemoglobin less than 10.5 g/dL, only elevated CRP was associated with poor overall survival, with a hazard ratio of 3.6 (P = .0003).
The authors speculate that elevated CRP is a marker for systemic inflammation that may be associated with a higher risk for postoperative complications and for poorer overall survival among patients with advanced cancer.
"Even in this highly selected population, there was 44% morbidity and 10% mortality, illustrating the difficulty in patient selection," Dr. Blakely said.
CRP testing is a simple, low-cost, and objective test that can be easily added on to preoperative evaluation of candidates for palliative surgery, and it may be a useful element in patient selection and counseling, he said.
The study was internally funded. Dr. Blakely reported having no financial disclosures.
NATIONAL HARBOR, MD. – Elevated levels of C-reactive protein may be a marker for poor response to palliative surgery, Dr. Andrew M. Blakely said at the annual Society of Surgical Oncology Cancer Symposium.
In a study of 50 patients who underwent elective palliative procedures for various cancer-related symptoms, elevated C-reactive protein (CRP), but not fatigue scores or performance status, was associated with a nearly fourfold risk for worse overall survival, reported Dr. Blakely, a surgery resident at Alpert Medical School, Brown University, Providence, R.I.
Elevated CRP, defined as levels greater than 8 mg/L, was also associated with increased overall and tumor-grade specific complications.
"This is important, because these complications are poorly tolerated by advanced cancer patients, and they serve to greatly diminish the potential benefit of palliative surgery," Dr. Blakely said.
He and colleagues at Brown and the Rhode Island Hospital, also in Providence, looked at potential prognostic factors in patients who chose surgery to relieve symptoms of advanced cancers such as gastrointestinal obstruction, or local-regional control of tumor-related pain or bleeding.
Malignancies treated included gastric, pancreatic, colon, melanoma, sarcomas, breast cancer, hepatobiliary and ovarian cancer, and squamous cell carcinomas.
They found that 44 patients (88%) stated that the surgery "was worth it," and 37 patients (74%) reported symptom resolution or improvement following surgery.
At 1-month follow-up, the complication rate was 44%, and 10% of patients had died. Although there was no association between CRP levels and morbidity or mortality among all patients, 11 of 27 patients with low-grade (grade 1 or 2) complications had elevated CRP (P = .001), as did all 6 patients with high-grade (grade 3 or 4) malignancies (P = .010), and 4 of 5 patients who died (P =.007).
In univariate analysis, factors significantly associated with major complications included elevated CRP (P = .005) and National Cancer Institute (NCI) fatigue score of 1 or greater (P = .001). Factors associated with overall survival were elevated CRP (P less than .0001), and NCI fatigue score (P = .04).
But in multivariate analysis controlling for those factors and for male sex, low albumin levels, significant weight loss, no previous cancer therapy, and hemoglobin less than 10.5 g/dL, only elevated CRP was associated with poor overall survival, with a hazard ratio of 3.6 (P = .0003).
The authors speculate that elevated CRP is a marker for systemic inflammation that may be associated with a higher risk for postoperative complications and for poorer overall survival among patients with advanced cancer.
"Even in this highly selected population, there was 44% morbidity and 10% mortality, illustrating the difficulty in patient selection," Dr. Blakely said.
CRP testing is a simple, low-cost, and objective test that can be easily added on to preoperative evaluation of candidates for palliative surgery, and it may be a useful element in patient selection and counseling, he said.
The study was internally funded. Dr. Blakely reported having no financial disclosures.
NATIONAL HARBOR, MD. – Elevated levels of C-reactive protein may be a marker for poor response to palliative surgery, Dr. Andrew M. Blakely said at the annual Society of Surgical Oncology Cancer Symposium.
In a study of 50 patients who underwent elective palliative procedures for various cancer-related symptoms, elevated C-reactive protein (CRP), but not fatigue scores or performance status, was associated with a nearly fourfold risk for worse overall survival, reported Dr. Blakely, a surgery resident at Alpert Medical School, Brown University, Providence, R.I.
Elevated CRP, defined as levels greater than 8 mg/L, was also associated with increased overall and tumor-grade specific complications.
"This is important, because these complications are poorly tolerated by advanced cancer patients, and they serve to greatly diminish the potential benefit of palliative surgery," Dr. Blakely said.
He and colleagues at Brown and the Rhode Island Hospital, also in Providence, looked at potential prognostic factors in patients who chose surgery to relieve symptoms of advanced cancers such as gastrointestinal obstruction, or local-regional control of tumor-related pain or bleeding.
Malignancies treated included gastric, pancreatic, colon, melanoma, sarcomas, breast cancer, hepatobiliary and ovarian cancer, and squamous cell carcinomas.
They found that 44 patients (88%) stated that the surgery "was worth it," and 37 patients (74%) reported symptom resolution or improvement following surgery.
At 1-month follow-up, the complication rate was 44%, and 10% of patients had died. Although there was no association between CRP levels and morbidity or mortality among all patients, 11 of 27 patients with low-grade (grade 1 or 2) complications had elevated CRP (P = .001), as did all 6 patients with high-grade (grade 3 or 4) malignancies (P = .010), and 4 of 5 patients who died (P =.007).
In univariate analysis, factors significantly associated with major complications included elevated CRP (P = .005) and National Cancer Institute (NCI) fatigue score of 1 or greater (P = .001). Factors associated with overall survival were elevated CRP (P less than .0001), and NCI fatigue score (P = .04).
But in multivariate analysis controlling for those factors and for male sex, low albumin levels, significant weight loss, no previous cancer therapy, and hemoglobin less than 10.5 g/dL, only elevated CRP was associated with poor overall survival, with a hazard ratio of 3.6 (P = .0003).
The authors speculate that elevated CRP is a marker for systemic inflammation that may be associated with a higher risk for postoperative complications and for poorer overall survival among patients with advanced cancer.
"Even in this highly selected population, there was 44% morbidity and 10% mortality, illustrating the difficulty in patient selection," Dr. Blakely said.
CRP testing is a simple, low-cost, and objective test that can be easily added on to preoperative evaluation of candidates for palliative surgery, and it may be a useful element in patient selection and counseling, he said.
The study was internally funded. Dr. Blakely reported having no financial disclosures.
AT SSO 2013
Major finding: The hazard ratio for worse survival after palliative surgery among advanced cancer patients with elevated C-reactive protein was 3.6 (P = .0003).
Data source: Retrospective review of a prospective single-center database.
Disclosures: The study was internally funded. Dr. Blakely reported having no financial disclosures.
Designer T cells take aim at liver metastases
NATIONAL HARBOR, MD – Designer T cells used in immunotherapy for the treatment of liver metastases are well tolerated and reduced tumor burden in a small phase I study, reported an investigator at the annual Society of Surgical Oncology Cancer Symposium.
But the biggest barrier to the success of the therapy may lie in the liver itself, said Dr. Steven C. Katz, a surgical oncologist at the Roger Williams Cancer Center in Providence, R.I.
"Thinking long term, we’re going to have to address the suppressive forces that exist in the liver. I don’t think this is really going to succeed clinically until we find a way to do that," he said.
Genetically modified, designer T-cell technology allows translational science researchers to manufacture and deliver highly potent, specific agents to invoke a desired antitumor response, rather than trying to boost a general host immune response through, say, a vaccine, Dr. Katz said.
The technology has the potential to act against a variety of tumor types, and has been demonstrated to have activity against chronic lymphocytic leukemia, synovial sarcoma, and melanoma, he said.
The technique involves harvesting T cells from patients, activating them in culture, and then transfecting them with a retrovirus to get them to express a highly specific chimeric antigen receptor (CAR).
Dr. Katz and his colleagues are developing a CAR with an immunoglobulin fragment directed against carcinoembryonic antigen (CEA).
"A T cell that expresses this anti-CEA CAR, when it encounters a CEA-positive tumor, ... is activated to divide [and] produce cytokines and is poised to lyse and kill tumor cells in a highly specific fashion, and we demonstrated this in vitro, as have several other groups," he said.
But getting the antigen to the target – CEA-positive liver metastases – is just part of the challenge, he added. Delivering the CAR into the hepatic arterial circulation appears to be the optimal approach for confining the therapy to the target area, thus avoiding possible complications such as colitis that can occur when anti-CEA is delivered systemically.
A second barrier to the therapy is that the liver contains high numbers of suppressive immune cells such as regulatory T cells and myeloid-derived suppressor cells. Coinfusion of cytokines such as interleukin-2 (IL-2) appears to be a necessary step for preventing intrahepatic suppression of therapy, Dr. Katz said.
The investigators have initiated a phase I safety and intrapatient dose-escalation trial of the technology. Patients with CEA-positive liver metastases are given percutaneous infusions of their modified T cells through a femoral artery puncture directly into the hepatic artery. Embolization of the gastroduodenal and right gastric arteries is performed to prevent or limit extrahepatic infusion of the modified cells.
The patients all have unresectable, histologically confirmed CEA-positive liver metastases from colorectal, hepatobiliary, ampullary, or gastric primary tumors; have no extrahepatic disease beyond the lungs; and have had failure of one or more lines of standard systemic chemotherapy.
The first three patients have been treated in a dose-escalation fashion without cytokines. A second cohort of three patients is receiving both designer T cells and a continuous systemic infusion of IL-2 at a relatively low dose of 75,000 IU/kg per day to suppress the liver’s immune response.
"We know that IL-2 is going to be essential for these T cells to survive and do what we want them to do in vivo," Dr. Katz said.
Of the first six patients enrolled, two have been withdrawn from the study: one who had disease progression within the lungs following a second infusion of designer T cells, and one whose designer T cells were discovered to be infected with West Nile virus.
There have been no serious grade 3 or 4 adverse events to date. Adverse events have included grade 2 fevers, a grade 1 fever, and a rash. There has been no significant elevation over baseline in liver enzymes or bilirubin, Dr. Katz said.
Flow cytometry in early testing has shown that the modified T cells appear to be confined to the normal liver and metastatic sites, and have not been detected in significant numbers in peripheral circulation.
To date, only one patient has been treated with designer T cells and IL-2 support; this patient has thus far had a "very favorable" course, with a 40% decrease in tumor burden, although these results are still too early for clinical conclusions to be drawn.
The investigators are exploring additional strategies to circumvent intrahepatic suppression of the therapy by blocking the action of myeloid-derived suppressor cells.
The study was supported by a Society of Surgical Oncology Clinical Investigator Award, an education grant from Genentech, the National Institutes of Health, and the Rhode Island Foundation. Dr. Katz reported having no relevant financial disclosures.
NATIONAL HARBOR, MD – Designer T cells used in immunotherapy for the treatment of liver metastases are well tolerated and reduced tumor burden in a small phase I study, reported an investigator at the annual Society of Surgical Oncology Cancer Symposium.
But the biggest barrier to the success of the therapy may lie in the liver itself, said Dr. Steven C. Katz, a surgical oncologist at the Roger Williams Cancer Center in Providence, R.I.
"Thinking long term, we’re going to have to address the suppressive forces that exist in the liver. I don’t think this is really going to succeed clinically until we find a way to do that," he said.
Genetically modified, designer T-cell technology allows translational science researchers to manufacture and deliver highly potent, specific agents to invoke a desired antitumor response, rather than trying to boost a general host immune response through, say, a vaccine, Dr. Katz said.
The technology has the potential to act against a variety of tumor types, and has been demonstrated to have activity against chronic lymphocytic leukemia, synovial sarcoma, and melanoma, he said.
The technique involves harvesting T cells from patients, activating them in culture, and then transfecting them with a retrovirus to get them to express a highly specific chimeric antigen receptor (CAR).
Dr. Katz and his colleagues are developing a CAR with an immunoglobulin fragment directed against carcinoembryonic antigen (CEA).
"A T cell that expresses this anti-CEA CAR, when it encounters a CEA-positive tumor, ... is activated to divide [and] produce cytokines and is poised to lyse and kill tumor cells in a highly specific fashion, and we demonstrated this in vitro, as have several other groups," he said.
But getting the antigen to the target – CEA-positive liver metastases – is just part of the challenge, he added. Delivering the CAR into the hepatic arterial circulation appears to be the optimal approach for confining the therapy to the target area, thus avoiding possible complications such as colitis that can occur when anti-CEA is delivered systemically.
A second barrier to the therapy is that the liver contains high numbers of suppressive immune cells such as regulatory T cells and myeloid-derived suppressor cells. Coinfusion of cytokines such as interleukin-2 (IL-2) appears to be a necessary step for preventing intrahepatic suppression of therapy, Dr. Katz said.
The investigators have initiated a phase I safety and intrapatient dose-escalation trial of the technology. Patients with CEA-positive liver metastases are given percutaneous infusions of their modified T cells through a femoral artery puncture directly into the hepatic artery. Embolization of the gastroduodenal and right gastric arteries is performed to prevent or limit extrahepatic infusion of the modified cells.
The patients all have unresectable, histologically confirmed CEA-positive liver metastases from colorectal, hepatobiliary, ampullary, or gastric primary tumors; have no extrahepatic disease beyond the lungs; and have had failure of one or more lines of standard systemic chemotherapy.
The first three patients have been treated in a dose-escalation fashion without cytokines. A second cohort of three patients is receiving both designer T cells and a continuous systemic infusion of IL-2 at a relatively low dose of 75,000 IU/kg per day to suppress the liver’s immune response.
"We know that IL-2 is going to be essential for these T cells to survive and do what we want them to do in vivo," Dr. Katz said.
Of the first six patients enrolled, two have been withdrawn from the study: one who had disease progression within the lungs following a second infusion of designer T cells, and one whose designer T cells were discovered to be infected with West Nile virus.
There have been no serious grade 3 or 4 adverse events to date. Adverse events have included grade 2 fevers, a grade 1 fever, and a rash. There has been no significant elevation over baseline in liver enzymes or bilirubin, Dr. Katz said.
Flow cytometry in early testing has shown that the modified T cells appear to be confined to the normal liver and metastatic sites, and have not been detected in significant numbers in peripheral circulation.
To date, only one patient has been treated with designer T cells and IL-2 support; this patient has thus far had a "very favorable" course, with a 40% decrease in tumor burden, although these results are still too early for clinical conclusions to be drawn.
The investigators are exploring additional strategies to circumvent intrahepatic suppression of the therapy by blocking the action of myeloid-derived suppressor cells.
The study was supported by a Society of Surgical Oncology Clinical Investigator Award, an education grant from Genentech, the National Institutes of Health, and the Rhode Island Foundation. Dr. Katz reported having no relevant financial disclosures.
NATIONAL HARBOR, MD – Designer T cells used in immunotherapy for the treatment of liver metastases are well tolerated and reduced tumor burden in a small phase I study, reported an investigator at the annual Society of Surgical Oncology Cancer Symposium.
But the biggest barrier to the success of the therapy may lie in the liver itself, said Dr. Steven C. Katz, a surgical oncologist at the Roger Williams Cancer Center in Providence, R.I.
"Thinking long term, we’re going to have to address the suppressive forces that exist in the liver. I don’t think this is really going to succeed clinically until we find a way to do that," he said.
Genetically modified, designer T-cell technology allows translational science researchers to manufacture and deliver highly potent, specific agents to invoke a desired antitumor response, rather than trying to boost a general host immune response through, say, a vaccine, Dr. Katz said.
The technology has the potential to act against a variety of tumor types, and has been demonstrated to have activity against chronic lymphocytic leukemia, synovial sarcoma, and melanoma, he said.
The technique involves harvesting T cells from patients, activating them in culture, and then transfecting them with a retrovirus to get them to express a highly specific chimeric antigen receptor (CAR).
Dr. Katz and his colleagues are developing a CAR with an immunoglobulin fragment directed against carcinoembryonic antigen (CEA).
"A T cell that expresses this anti-CEA CAR, when it encounters a CEA-positive tumor, ... is activated to divide [and] produce cytokines and is poised to lyse and kill tumor cells in a highly specific fashion, and we demonstrated this in vitro, as have several other groups," he said.
But getting the antigen to the target – CEA-positive liver metastases – is just part of the challenge, he added. Delivering the CAR into the hepatic arterial circulation appears to be the optimal approach for confining the therapy to the target area, thus avoiding possible complications such as colitis that can occur when anti-CEA is delivered systemically.
A second barrier to the therapy is that the liver contains high numbers of suppressive immune cells such as regulatory T cells and myeloid-derived suppressor cells. Coinfusion of cytokines such as interleukin-2 (IL-2) appears to be a necessary step for preventing intrahepatic suppression of therapy, Dr. Katz said.
The investigators have initiated a phase I safety and intrapatient dose-escalation trial of the technology. Patients with CEA-positive liver metastases are given percutaneous infusions of their modified T cells through a femoral artery puncture directly into the hepatic artery. Embolization of the gastroduodenal and right gastric arteries is performed to prevent or limit extrahepatic infusion of the modified cells.
The patients all have unresectable, histologically confirmed CEA-positive liver metastases from colorectal, hepatobiliary, ampullary, or gastric primary tumors; have no extrahepatic disease beyond the lungs; and have had failure of one or more lines of standard systemic chemotherapy.
The first three patients have been treated in a dose-escalation fashion without cytokines. A second cohort of three patients is receiving both designer T cells and a continuous systemic infusion of IL-2 at a relatively low dose of 75,000 IU/kg per day to suppress the liver’s immune response.
"We know that IL-2 is going to be essential for these T cells to survive and do what we want them to do in vivo," Dr. Katz said.
Of the first six patients enrolled, two have been withdrawn from the study: one who had disease progression within the lungs following a second infusion of designer T cells, and one whose designer T cells were discovered to be infected with West Nile virus.
There have been no serious grade 3 or 4 adverse events to date. Adverse events have included grade 2 fevers, a grade 1 fever, and a rash. There has been no significant elevation over baseline in liver enzymes or bilirubin, Dr. Katz said.
Flow cytometry in early testing has shown that the modified T cells appear to be confined to the normal liver and metastatic sites, and have not been detected in significant numbers in peripheral circulation.
To date, only one patient has been treated with designer T cells and IL-2 support; this patient has thus far had a "very favorable" course, with a 40% decrease in tumor burden, although these results are still too early for clinical conclusions to be drawn.
The investigators are exploring additional strategies to circumvent intrahepatic suppression of the therapy by blocking the action of myeloid-derived suppressor cells.
The study was supported by a Society of Surgical Oncology Clinical Investigator Award, an education grant from Genentech, the National Institutes of Health, and the Rhode Island Foundation. Dr. Katz reported having no relevant financial disclosures.
AT SSO 2013
Major finding: One patient who received designer T cells and interleukin-2 has had a 40% decrease in liver metastases.
Data source: Early report of a phase I safety and dose-escalation trial of T-cell immunotherapy for unresectable liver metastases.
Disclosures: The study was supported by a Society of Surgical Oncology Investigator Award, an educational grant from Genentech, the National Institutes of Health, and the Rhode Island Foundation. Dr. Katz reported having no relevant financial disclosures.
New blood test could identify early pancreatic cancer
An investigational serum biomarker panel accurately identified potentially resectable cases of pancreatic cancer, opening the possibility that a blood test could identify the cancer early and improve its dismal prognosis.
The panel measured four metabolites and had an overall sensitivity of 71% and a specificity of 78% for pancreatic cancer; the specificity for resectable tumors also approached 78%, reported Dr. Masaru Yoshida (Cancer Epidemiol. Biomarkers Prev. 2013 March 29 [doi: 10.1158/1055-9965.EPI-12-1033]).
The panel was more accurate than were any of the currently used biomarkers, including CA19-9 and CEA, said Dr. Yoshida, professor and chief of metabolomics research at Kobe University, Japan.
"This novel diagnostic approach, which is safe and easy to apply as a screening method, is expected to improve the prognosis of patients with pancreatic cancer by detecting their cancers early, when still in a resectable and curable state," Dr. Yoshida said in a press statement.
The researchers created the panel based on study of a cohort of 85 subjects: 43 with pancreatic cancer and 42 healthy controls. First, they pared down 113 potentially useful metabolites into a workable testing panel. Of the 45 candidate metabolites, four of them – xylitol, 1,5-anhydro-D-glucitol, histidine, and inositol – showed the highest variation between the healthy controls and the patients. In the training cohort, the panel of these four markers had a sensitivity of 86% and specificity of 88% for pancreatic cancer.
Next, the researchers evaluated a validation cohort of 42 cancer patients, 41 healthy controls, and 23 patients with chronic pancreatitis. In this cohort, with an area under the curve of 0.76, the panel’s sensitivity for pancreatic cancer was 71% and specificity, 78%. In contrast, the sensitivity of CA19-9 was 69% and specificity 86%; for CEA, those numbers were 36% and 80%, respectively.
In the subset of patients with resectable pancreatic cancer, the panel’s sensitivity was 78%, compared with 56% for CA19-9 and 44% for CEA.
The metabolic panel was also able to identify chronic pancreatitis, with a 17% rate of false positives, compared with a false positive rate of 30% for CA19-9 and 44% for CEA.
A blood panel could address three key problems in the field of pancreatic cancer, Dr. Yoshida and his colleagues said. "The first is the difficulty of detecting pancreatic cancer early in resectable stages. A sensitivity and specificity of about 80% for resectable disease ... should be acceptable for clinical use because most patients with resectable cancer have no symptoms, and blood examinations are useful tools for initial screening examinations."
The second problem is the difficulty in differentiating pancreatic cancer from chronic pancreatitis. "Many gastroenterologists follow up chronic pancreatitis with scheduled CT, magnetic resonance imaging (MRI), and endoscopic ultrasound scans (EUS); tumor marker tests; and endoscopic retrograde cholangiopancreatography (ERCP), but the initial malignant changes are frequently overlooked, and pancreatic tumors can rapidly become unresectable. In addition, unnecessary resections for benign inflammatory lesions are sometimes done because of false-positive results from CA19-9 and/or imaging examinations."
The third clinical problem is the risk of complications that result from pancreatic examinations. Serum samples are easy and safe to obtain, with a low risk of adverse events and, with this panel, a potentially good return of information. A blood test could also be a useful population screening tool, the researchers noted.
The metabolites probably reflect metabolic derangement that arises not only from focal tumorigenesis, but also from systemic reactions to pancreatic disease, Dr. Yoshida and his coinvestigators wrote. Pancreatic cancer causes significant decreases in some amino and fatty acids, probably because the tumor recruits these substances to aid its rapid cellular proliferation.
"Patients with pancreatic disease are also troubled by malnutrition because of pancreatic endocrine and exocrine insufficiency. Therefore, there is a possibility that the decreases in serum metabolite levels also reflect malnutrition."
Finally, they said, the decrease in 1,5-anhydro-D-glucitol indicates the presence of hyperglycemia and recent glycosuria. "These results suggest that glucose tolerance was impaired in these patients because of pancreatic insufficiency."
The authors reported no financial conflicts. The work was supported by grants administered by agencies of the Japanese government.
An investigational serum biomarker panel accurately identified potentially resectable cases of pancreatic cancer, opening the possibility that a blood test could identify the cancer early and improve its dismal prognosis.
The panel measured four metabolites and had an overall sensitivity of 71% and a specificity of 78% for pancreatic cancer; the specificity for resectable tumors also approached 78%, reported Dr. Masaru Yoshida (Cancer Epidemiol. Biomarkers Prev. 2013 March 29 [doi: 10.1158/1055-9965.EPI-12-1033]).
The panel was more accurate than were any of the currently used biomarkers, including CA19-9 and CEA, said Dr. Yoshida, professor and chief of metabolomics research at Kobe University, Japan.
"This novel diagnostic approach, which is safe and easy to apply as a screening method, is expected to improve the prognosis of patients with pancreatic cancer by detecting their cancers early, when still in a resectable and curable state," Dr. Yoshida said in a press statement.
The researchers created the panel based on study of a cohort of 85 subjects: 43 with pancreatic cancer and 42 healthy controls. First, they pared down 113 potentially useful metabolites into a workable testing panel. Of the 45 candidate metabolites, four of them – xylitol, 1,5-anhydro-D-glucitol, histidine, and inositol – showed the highest variation between the healthy controls and the patients. In the training cohort, the panel of these four markers had a sensitivity of 86% and specificity of 88% for pancreatic cancer.
Next, the researchers evaluated a validation cohort of 42 cancer patients, 41 healthy controls, and 23 patients with chronic pancreatitis. In this cohort, with an area under the curve of 0.76, the panel’s sensitivity for pancreatic cancer was 71% and specificity, 78%. In contrast, the sensitivity of CA19-9 was 69% and specificity 86%; for CEA, those numbers were 36% and 80%, respectively.
In the subset of patients with resectable pancreatic cancer, the panel’s sensitivity was 78%, compared with 56% for CA19-9 and 44% for CEA.
The metabolic panel was also able to identify chronic pancreatitis, with a 17% rate of false positives, compared with a false positive rate of 30% for CA19-9 and 44% for CEA.
A blood panel could address three key problems in the field of pancreatic cancer, Dr. Yoshida and his colleagues said. "The first is the difficulty of detecting pancreatic cancer early in resectable stages. A sensitivity and specificity of about 80% for resectable disease ... should be acceptable for clinical use because most patients with resectable cancer have no symptoms, and blood examinations are useful tools for initial screening examinations."
The second problem is the difficulty in differentiating pancreatic cancer from chronic pancreatitis. "Many gastroenterologists follow up chronic pancreatitis with scheduled CT, magnetic resonance imaging (MRI), and endoscopic ultrasound scans (EUS); tumor marker tests; and endoscopic retrograde cholangiopancreatography (ERCP), but the initial malignant changes are frequently overlooked, and pancreatic tumors can rapidly become unresectable. In addition, unnecessary resections for benign inflammatory lesions are sometimes done because of false-positive results from CA19-9 and/or imaging examinations."
The third clinical problem is the risk of complications that result from pancreatic examinations. Serum samples are easy and safe to obtain, with a low risk of adverse events and, with this panel, a potentially good return of information. A blood test could also be a useful population screening tool, the researchers noted.
The metabolites probably reflect metabolic derangement that arises not only from focal tumorigenesis, but also from systemic reactions to pancreatic disease, Dr. Yoshida and his coinvestigators wrote. Pancreatic cancer causes significant decreases in some amino and fatty acids, probably because the tumor recruits these substances to aid its rapid cellular proliferation.
"Patients with pancreatic disease are also troubled by malnutrition because of pancreatic endocrine and exocrine insufficiency. Therefore, there is a possibility that the decreases in serum metabolite levels also reflect malnutrition."
Finally, they said, the decrease in 1,5-anhydro-D-glucitol indicates the presence of hyperglycemia and recent glycosuria. "These results suggest that glucose tolerance was impaired in these patients because of pancreatic insufficiency."
The authors reported no financial conflicts. The work was supported by grants administered by agencies of the Japanese government.
An investigational serum biomarker panel accurately identified potentially resectable cases of pancreatic cancer, opening the possibility that a blood test could identify the cancer early and improve its dismal prognosis.
The panel measured four metabolites and had an overall sensitivity of 71% and a specificity of 78% for pancreatic cancer; the specificity for resectable tumors also approached 78%, reported Dr. Masaru Yoshida (Cancer Epidemiol. Biomarkers Prev. 2013 March 29 [doi: 10.1158/1055-9965.EPI-12-1033]).
The panel was more accurate than were any of the currently used biomarkers, including CA19-9 and CEA, said Dr. Yoshida, professor and chief of metabolomics research at Kobe University, Japan.
"This novel diagnostic approach, which is safe and easy to apply as a screening method, is expected to improve the prognosis of patients with pancreatic cancer by detecting their cancers early, when still in a resectable and curable state," Dr. Yoshida said in a press statement.
The researchers created the panel based on study of a cohort of 85 subjects: 43 with pancreatic cancer and 42 healthy controls. First, they pared down 113 potentially useful metabolites into a workable testing panel. Of the 45 candidate metabolites, four of them – xylitol, 1,5-anhydro-D-glucitol, histidine, and inositol – showed the highest variation between the healthy controls and the patients. In the training cohort, the panel of these four markers had a sensitivity of 86% and specificity of 88% for pancreatic cancer.
Next, the researchers evaluated a validation cohort of 42 cancer patients, 41 healthy controls, and 23 patients with chronic pancreatitis. In this cohort, with an area under the curve of 0.76, the panel’s sensitivity for pancreatic cancer was 71% and specificity, 78%. In contrast, the sensitivity of CA19-9 was 69% and specificity 86%; for CEA, those numbers were 36% and 80%, respectively.
In the subset of patients with resectable pancreatic cancer, the panel’s sensitivity was 78%, compared with 56% for CA19-9 and 44% for CEA.
The metabolic panel was also able to identify chronic pancreatitis, with a 17% rate of false positives, compared with a false positive rate of 30% for CA19-9 and 44% for CEA.
A blood panel could address three key problems in the field of pancreatic cancer, Dr. Yoshida and his colleagues said. "The first is the difficulty of detecting pancreatic cancer early in resectable stages. A sensitivity and specificity of about 80% for resectable disease ... should be acceptable for clinical use because most patients with resectable cancer have no symptoms, and blood examinations are useful tools for initial screening examinations."
The second problem is the difficulty in differentiating pancreatic cancer from chronic pancreatitis. "Many gastroenterologists follow up chronic pancreatitis with scheduled CT, magnetic resonance imaging (MRI), and endoscopic ultrasound scans (EUS); tumor marker tests; and endoscopic retrograde cholangiopancreatography (ERCP), but the initial malignant changes are frequently overlooked, and pancreatic tumors can rapidly become unresectable. In addition, unnecessary resections for benign inflammatory lesions are sometimes done because of false-positive results from CA19-9 and/or imaging examinations."
The third clinical problem is the risk of complications that result from pancreatic examinations. Serum samples are easy and safe to obtain, with a low risk of adverse events and, with this panel, a potentially good return of information. A blood test could also be a useful population screening tool, the researchers noted.
The metabolites probably reflect metabolic derangement that arises not only from focal tumorigenesis, but also from systemic reactions to pancreatic disease, Dr. Yoshida and his coinvestigators wrote. Pancreatic cancer causes significant decreases in some amino and fatty acids, probably because the tumor recruits these substances to aid its rapid cellular proliferation.
"Patients with pancreatic disease are also troubled by malnutrition because of pancreatic endocrine and exocrine insufficiency. Therefore, there is a possibility that the decreases in serum metabolite levels also reflect malnutrition."
Finally, they said, the decrease in 1,5-anhydro-D-glucitol indicates the presence of hyperglycemia and recent glycosuria. "These results suggest that glucose tolerance was impaired in these patients because of pancreatic insufficiency."
The authors reported no financial conflicts. The work was supported by grants administered by agencies of the Japanese government.
FROM CANCER EPIDEMIOLOGY, BIOMARKERS, AND PREVENTION
Major finding: A serum panel of four metabolites had a 78% sensitivity for detecting resectable pancreatic cancers.
Data source: The panel was derived from a test cohort of 85, and a validation cohort of 42 cancer patients, 41 healthy controls, and 23 patients with chronic pancreatitis.
Disclosures: The authors had no financial disclosures. Japanese government agencies funded the study.