User login
PERT alerts improve pulmonary embolism outcomes
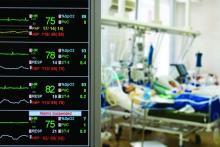
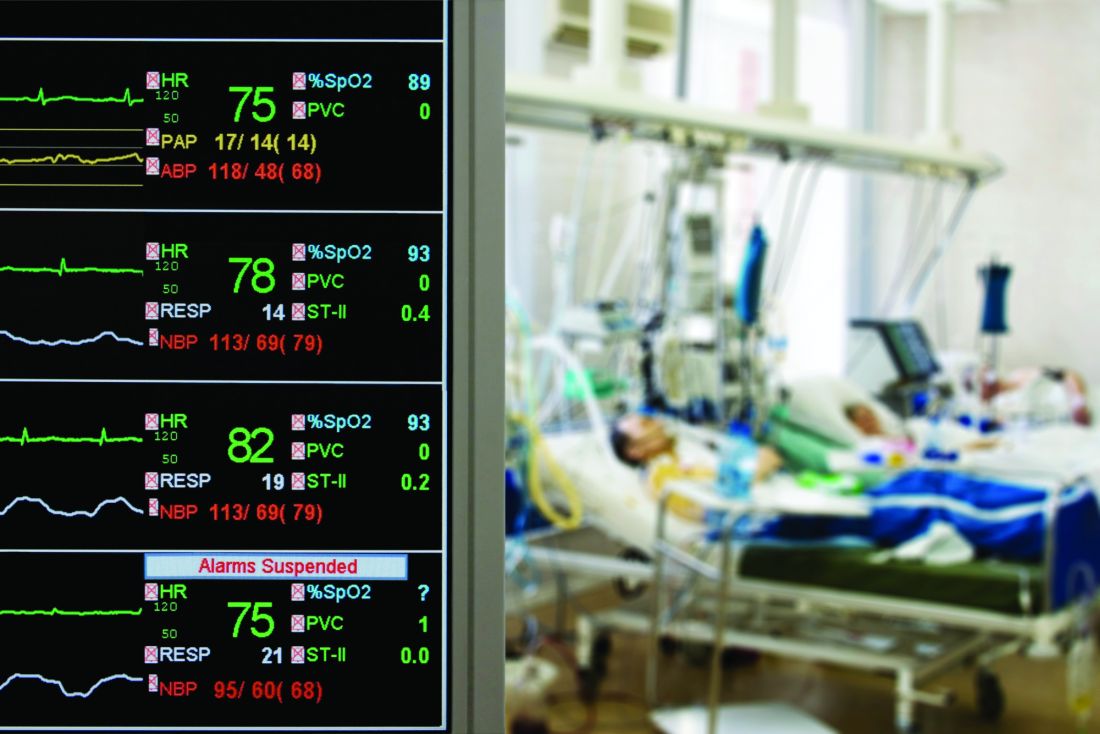
SAN DIEGO – One year after implementation, a at the Christiana Medical Center in Delaware. An analysis of data collected showed reductions in ICU stays, early death, and overall hospital length of stay.
Such patients pose a challenge to clinicians because some will go on to develop more serious pulmonary embolism (PE), yet aggressive treatment options carry their own risk. Existing guidelines, such as those by the European Society of Cardiology, recommend conservative treatment of these patients, with more aggressive measures if conditions don’t quickly improve.
However, about 12% of patients on conservative therapy die, or about 100,000 per year. Those patients who go on to have bad outcomes “are obviously intermediate high risk or high risk. This is the patient population that we’re interested in [addressing through PERT]. These aren’t really sick patients. The blood pressure is normal, but they have the risk based on comorbidities or the clot burden to do poorly over the next day or two,” said Michael Benninghoff, DO, section chief of medical critical care and director of respiratory therapy at Christiana Medical Center, Wilmington, Del. Dr. Benninghoff presented the study at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
The PERT concept was developed by physicians at Massachusetts General Hospital. It establishes clinical criteria that, if met, prompt a PERT alert, which in turn triggers a meeting between the initiating provider, a pulmonary intensivist, and a vascular and interventional radiology physician within 15 minutes to review the case and make rapid clinical decisions.
A PERT alert requires either a CT diagnosis of PE or a VQ scan showing a high probability of PE, combined with one of three additional criteria: elevated B-type (brain) natriuretic peptide (BNP) and troponin; echocardiographic evidence of right ventricular dysfunction; or clinical instability as indicated by heart rate over 110 beats per minute, systolic blood pressure below 100 mm Hg, or oxygen saturation lower than 90%.
The PERT program caught Dr. Benninghoff’s attention because of institutional experience with patients deteriorating on conservative treatment, but also because the treatment of submassive PE with RV dysfunction is quite scattered. “We were seeing really conservative to really aggressive treatment. I don’t think we’ve had the data to support treating a patient whose blood pressure is normal but they have signs of right ventricular dysfunction, whether it’s echocardiographic, radiographic, or laboratory evidence of myocardial necrosis. I don’t think we have a group conscience as providers as far as how aggressive to be with those patients,” said Dr. Benninghoff.
To examine the efficacy of the PERT program after 1 year, Dr. Benninghoff’s team reviewed all PE cases from 2016 (pre-PERT, n = 717) and 2017 (post-PERT, n = 752). The mortality index declined 30%, from 1.13 to 0.79, while the percentage of early death declined 52%, from 2.51 to 1.20. The mean number of ICU days fell from 5.01 to 4.40.
When the team restricted the analysis to PE lysis patients (n = 27 in 2016; n = 33 in 2017), the mean length of ICU stay dropped from 66.1 hours to 58.8 hours, and fewer patients were transferred from a lower level of care to the ICU (6 vs. 3).
“We think we have shown that just by talking in real time, forcing physicians to communicate – it certainly doesn’t hurt, that it probably helps with ICU utilization and perhaps even mortality,” said Dr. Benninghoff.
The results are far from definitive, and much more work needs to be done to determine how best to manage patients with submassive PEs and RV dysfunction. Dr. Benninghoff doesn’t have the answers, but he’s hopeful that the PERT program can eventually provide some. “Probably the most important thing is we’re giving back to the medical community by enrolling in the consortium, putting our data in the hands of the Boston research institute, and seeing what comes of it. Hopefully in 5 years we will have a standard of care based on the work we’re doing now,” he said.
The study was funded internally. Dr. Benninghoff declared no conflicts of interest.
SOURCE: Benninghoff M. Critical Care Congress 2019, Abstract 490.
SAN DIEGO – One year after implementation, a at the Christiana Medical Center in Delaware. An analysis of data collected showed reductions in ICU stays, early death, and overall hospital length of stay.
Such patients pose a challenge to clinicians because some will go on to develop more serious pulmonary embolism (PE), yet aggressive treatment options carry their own risk. Existing guidelines, such as those by the European Society of Cardiology, recommend conservative treatment of these patients, with more aggressive measures if conditions don’t quickly improve.
However, about 12% of patients on conservative therapy die, or about 100,000 per year. Those patients who go on to have bad outcomes “are obviously intermediate high risk or high risk. This is the patient population that we’re interested in [addressing through PERT]. These aren’t really sick patients. The blood pressure is normal, but they have the risk based on comorbidities or the clot burden to do poorly over the next day or two,” said Michael Benninghoff, DO, section chief of medical critical care and director of respiratory therapy at Christiana Medical Center, Wilmington, Del. Dr. Benninghoff presented the study at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
The PERT concept was developed by physicians at Massachusetts General Hospital. It establishes clinical criteria that, if met, prompt a PERT alert, which in turn triggers a meeting between the initiating provider, a pulmonary intensivist, and a vascular and interventional radiology physician within 15 minutes to review the case and make rapid clinical decisions.
A PERT alert requires either a CT diagnosis of PE or a VQ scan showing a high probability of PE, combined with one of three additional criteria: elevated B-type (brain) natriuretic peptide (BNP) and troponin; echocardiographic evidence of right ventricular dysfunction; or clinical instability as indicated by heart rate over 110 beats per minute, systolic blood pressure below 100 mm Hg, or oxygen saturation lower than 90%.
The PERT program caught Dr. Benninghoff’s attention because of institutional experience with patients deteriorating on conservative treatment, but also because the treatment of submassive PE with RV dysfunction is quite scattered. “We were seeing really conservative to really aggressive treatment. I don’t think we’ve had the data to support treating a patient whose blood pressure is normal but they have signs of right ventricular dysfunction, whether it’s echocardiographic, radiographic, or laboratory evidence of myocardial necrosis. I don’t think we have a group conscience as providers as far as how aggressive to be with those patients,” said Dr. Benninghoff.
To examine the efficacy of the PERT program after 1 year, Dr. Benninghoff’s team reviewed all PE cases from 2016 (pre-PERT, n = 717) and 2017 (post-PERT, n = 752). The mortality index declined 30%, from 1.13 to 0.79, while the percentage of early death declined 52%, from 2.51 to 1.20. The mean number of ICU days fell from 5.01 to 4.40.
When the team restricted the analysis to PE lysis patients (n = 27 in 2016; n = 33 in 2017), the mean length of ICU stay dropped from 66.1 hours to 58.8 hours, and fewer patients were transferred from a lower level of care to the ICU (6 vs. 3).
“We think we have shown that just by talking in real time, forcing physicians to communicate – it certainly doesn’t hurt, that it probably helps with ICU utilization and perhaps even mortality,” said Dr. Benninghoff.
The results are far from definitive, and much more work needs to be done to determine how best to manage patients with submassive PEs and RV dysfunction. Dr. Benninghoff doesn’t have the answers, but he’s hopeful that the PERT program can eventually provide some. “Probably the most important thing is we’re giving back to the medical community by enrolling in the consortium, putting our data in the hands of the Boston research institute, and seeing what comes of it. Hopefully in 5 years we will have a standard of care based on the work we’re doing now,” he said.
The study was funded internally. Dr. Benninghoff declared no conflicts of interest.
SOURCE: Benninghoff M. Critical Care Congress 2019, Abstract 490.
SAN DIEGO – One year after implementation, a at the Christiana Medical Center in Delaware. An analysis of data collected showed reductions in ICU stays, early death, and overall hospital length of stay.
Such patients pose a challenge to clinicians because some will go on to develop more serious pulmonary embolism (PE), yet aggressive treatment options carry their own risk. Existing guidelines, such as those by the European Society of Cardiology, recommend conservative treatment of these patients, with more aggressive measures if conditions don’t quickly improve.
However, about 12% of patients on conservative therapy die, or about 100,000 per year. Those patients who go on to have bad outcomes “are obviously intermediate high risk or high risk. This is the patient population that we’re interested in [addressing through PERT]. These aren’t really sick patients. The blood pressure is normal, but they have the risk based on comorbidities or the clot burden to do poorly over the next day or two,” said Michael Benninghoff, DO, section chief of medical critical care and director of respiratory therapy at Christiana Medical Center, Wilmington, Del. Dr. Benninghoff presented the study at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
The PERT concept was developed by physicians at Massachusetts General Hospital. It establishes clinical criteria that, if met, prompt a PERT alert, which in turn triggers a meeting between the initiating provider, a pulmonary intensivist, and a vascular and interventional radiology physician within 15 minutes to review the case and make rapid clinical decisions.
A PERT alert requires either a CT diagnosis of PE or a VQ scan showing a high probability of PE, combined with one of three additional criteria: elevated B-type (brain) natriuretic peptide (BNP) and troponin; echocardiographic evidence of right ventricular dysfunction; or clinical instability as indicated by heart rate over 110 beats per minute, systolic blood pressure below 100 mm Hg, or oxygen saturation lower than 90%.
The PERT program caught Dr. Benninghoff’s attention because of institutional experience with patients deteriorating on conservative treatment, but also because the treatment of submassive PE with RV dysfunction is quite scattered. “We were seeing really conservative to really aggressive treatment. I don’t think we’ve had the data to support treating a patient whose blood pressure is normal but they have signs of right ventricular dysfunction, whether it’s echocardiographic, radiographic, or laboratory evidence of myocardial necrosis. I don’t think we have a group conscience as providers as far as how aggressive to be with those patients,” said Dr. Benninghoff.
To examine the efficacy of the PERT program after 1 year, Dr. Benninghoff’s team reviewed all PE cases from 2016 (pre-PERT, n = 717) and 2017 (post-PERT, n = 752). The mortality index declined 30%, from 1.13 to 0.79, while the percentage of early death declined 52%, from 2.51 to 1.20. The mean number of ICU days fell from 5.01 to 4.40.
When the team restricted the analysis to PE lysis patients (n = 27 in 2016; n = 33 in 2017), the mean length of ICU stay dropped from 66.1 hours to 58.8 hours, and fewer patients were transferred from a lower level of care to the ICU (6 vs. 3).
“We think we have shown that just by talking in real time, forcing physicians to communicate – it certainly doesn’t hurt, that it probably helps with ICU utilization and perhaps even mortality,” said Dr. Benninghoff.
The results are far from definitive, and much more work needs to be done to determine how best to manage patients with submassive PEs and RV dysfunction. Dr. Benninghoff doesn’t have the answers, but he’s hopeful that the PERT program can eventually provide some. “Probably the most important thing is we’re giving back to the medical community by enrolling in the consortium, putting our data in the hands of the Boston research institute, and seeing what comes of it. Hopefully in 5 years we will have a standard of care based on the work we’re doing now,” he said.
The study was funded internally. Dr. Benninghoff declared no conflicts of interest.
SOURCE: Benninghoff M. Critical Care Congress 2019, Abstract 490.
REPORTING FROM CCC48
ICYMI: Andexanet alfa reduces anti–factor Xa activity from apixaban, rivaroxaban
Patients with acute major bleeding associated with factor Xa inhibitor usage who received andexanet alfa experienced a significant decrease in anti–factor Xa activity, with more than three-quarters of patients experiencing good or excellent hemostatic efficiency after 12 hours. That finding emerged from the multicenter, prospective, open-label, single-group ANNEXA-4 trial published in the New England Journal of Medicine (2019 Feb 11. doi: 10.1056/NEJMoa1814051).
We reported this story at the annual meeting of the American College of Cardiology before it was published in the journal. Find our coverage at the link below.
Patients with acute major bleeding associated with factor Xa inhibitor usage who received andexanet alfa experienced a significant decrease in anti–factor Xa activity, with more than three-quarters of patients experiencing good or excellent hemostatic efficiency after 12 hours. That finding emerged from the multicenter, prospective, open-label, single-group ANNEXA-4 trial published in the New England Journal of Medicine (2019 Feb 11. doi: 10.1056/NEJMoa1814051).
We reported this story at the annual meeting of the American College of Cardiology before it was published in the journal. Find our coverage at the link below.
Patients with acute major bleeding associated with factor Xa inhibitor usage who received andexanet alfa experienced a significant decrease in anti–factor Xa activity, with more than three-quarters of patients experiencing good or excellent hemostatic efficiency after 12 hours. That finding emerged from the multicenter, prospective, open-label, single-group ANNEXA-4 trial published in the New England Journal of Medicine (2019 Feb 11. doi: 10.1056/NEJMoa1814051).
We reported this story at the annual meeting of the American College of Cardiology before it was published in the journal. Find our coverage at the link below.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Venous thromboembolism risk elevated in ankylosing spondylitis patients
Newly diagnosed ankylosing spondylitis (AS) patients are at increased risk for venous thromboembolism (VTE), especially during the first year after diagnosis, according to a population-based study of 7,190 cases.
In a study published in Annals of the Rheumatic Diseases, the researchers identified 7,190 incident cases of AS among adults using a health care database of residents of British Columbia and matched them for age, sex, and entry time into the cohort with 71,900 healthy individuals from the general population over a mean follow-up time of 6.2 years.
The incidence rate of VTE overall per 1,000 person-years was 1.56 among AS patients, compared with 0.77 in a control cohort from the general population. The incidence rates for DVT were 1.06 in AS patients and 0.50 in controls; incidence rates for PE were 0.79 in AS patients and 0.40 in controls.
The adjusted hazard ratios for VTE overall and DVT were similar and statistically significant in AS patients at 1.53 and 1.62, respectively, versus controls. But the adjusted hazard ratio of 1.36 for PE did not reach statistical significance. The adjusted risks of VTE overall, PE, and DVT were highest in the first year of diagnosis, reaching twofold greater risk for all, but none of the risks were statistically significant.
More research is needed to better identify subsets of AS patients at increased risk for VTE, and to assess whether treatment of inflammation can mitigate this risk, but in the meantime clinicians should be alert to the possibility of life-threatening complications from DVT and PE in their AS patients, especially soon after diagnosis, the researchers said.
The findings are supported by the study’s large sample size but are also limited by several factors, including the observational nature of the study and an inability to account for use of NSAIDs, the researchers noted.
“These results call for awareness of this complication, increased vigilance, and preventive intervention by controlling the inflammatory process or by anticoagulation in a high-risk AS population,” they concluded.
The study was supported in part by grants from the Canadian Arthritis Network, the Arthritis Society of Canada, the British Columbia Lupus Society, and the Canadian Institutes for Health Research. The researchers had no financial conflicts to disclose.
SOURCE: Aviña-Zubieta JA et al. Ann Rheum Dis. 2019 Feb 8. doi: 10.1136/annrheumdis-2018-214388.
Newly diagnosed ankylosing spondylitis (AS) patients are at increased risk for venous thromboembolism (VTE), especially during the first year after diagnosis, according to a population-based study of 7,190 cases.
In a study published in Annals of the Rheumatic Diseases, the researchers identified 7,190 incident cases of AS among adults using a health care database of residents of British Columbia and matched them for age, sex, and entry time into the cohort with 71,900 healthy individuals from the general population over a mean follow-up time of 6.2 years.
The incidence rate of VTE overall per 1,000 person-years was 1.56 among AS patients, compared with 0.77 in a control cohort from the general population. The incidence rates for DVT were 1.06 in AS patients and 0.50 in controls; incidence rates for PE were 0.79 in AS patients and 0.40 in controls.
The adjusted hazard ratios for VTE overall and DVT were similar and statistically significant in AS patients at 1.53 and 1.62, respectively, versus controls. But the adjusted hazard ratio of 1.36 for PE did not reach statistical significance. The adjusted risks of VTE overall, PE, and DVT were highest in the first year of diagnosis, reaching twofold greater risk for all, but none of the risks were statistically significant.
More research is needed to better identify subsets of AS patients at increased risk for VTE, and to assess whether treatment of inflammation can mitigate this risk, but in the meantime clinicians should be alert to the possibility of life-threatening complications from DVT and PE in their AS patients, especially soon after diagnosis, the researchers said.
The findings are supported by the study’s large sample size but are also limited by several factors, including the observational nature of the study and an inability to account for use of NSAIDs, the researchers noted.
“These results call for awareness of this complication, increased vigilance, and preventive intervention by controlling the inflammatory process or by anticoagulation in a high-risk AS population,” they concluded.
The study was supported in part by grants from the Canadian Arthritis Network, the Arthritis Society of Canada, the British Columbia Lupus Society, and the Canadian Institutes for Health Research. The researchers had no financial conflicts to disclose.
SOURCE: Aviña-Zubieta JA et al. Ann Rheum Dis. 2019 Feb 8. doi: 10.1136/annrheumdis-2018-214388.
Newly diagnosed ankylosing spondylitis (AS) patients are at increased risk for venous thromboembolism (VTE), especially during the first year after diagnosis, according to a population-based study of 7,190 cases.
In a study published in Annals of the Rheumatic Diseases, the researchers identified 7,190 incident cases of AS among adults using a health care database of residents of British Columbia and matched them for age, sex, and entry time into the cohort with 71,900 healthy individuals from the general population over a mean follow-up time of 6.2 years.
The incidence rate of VTE overall per 1,000 person-years was 1.56 among AS patients, compared with 0.77 in a control cohort from the general population. The incidence rates for DVT were 1.06 in AS patients and 0.50 in controls; incidence rates for PE were 0.79 in AS patients and 0.40 in controls.
The adjusted hazard ratios for VTE overall and DVT were similar and statistically significant in AS patients at 1.53 and 1.62, respectively, versus controls. But the adjusted hazard ratio of 1.36 for PE did not reach statistical significance. The adjusted risks of VTE overall, PE, and DVT were highest in the first year of diagnosis, reaching twofold greater risk for all, but none of the risks were statistically significant.
More research is needed to better identify subsets of AS patients at increased risk for VTE, and to assess whether treatment of inflammation can mitigate this risk, but in the meantime clinicians should be alert to the possibility of life-threatening complications from DVT and PE in their AS patients, especially soon after diagnosis, the researchers said.
The findings are supported by the study’s large sample size but are also limited by several factors, including the observational nature of the study and an inability to account for use of NSAIDs, the researchers noted.
“These results call for awareness of this complication, increased vigilance, and preventive intervention by controlling the inflammatory process or by anticoagulation in a high-risk AS population,” they concluded.
The study was supported in part by grants from the Canadian Arthritis Network, the Arthritis Society of Canada, the British Columbia Lupus Society, and the Canadian Institutes for Health Research. The researchers had no financial conflicts to disclose.
SOURCE: Aviña-Zubieta JA et al. Ann Rheum Dis. 2019 Feb 8. doi: 10.1136/annrheumdis-2018-214388.
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point: Newly diagnosed AS patients demonstrated increased risk of venous thromboembolism, including deep vein thrombosis and pulmonary embolism, compared with controls.
Major finding: The relative risk for deep vein thrombosis was 63% higher for AS patients versus controls, but a 39% higher risk of pulmonary embolism did not reach statistical significance.
Study details: A population-based study including 7,190 incident AS cases and 71,900 matched controls from a health care database of residents of British Columbia.
Disclosures: The study was supported in part by grants from the Canadian Arthritis Network, the Arthritis Society of Canada, the British Columbia Lupus Society, and the Canadian Institutes for Health Research. The researchers had no financial conflicts to disclose.
Source: Aviña-Zubieta JA et al. Ann Rheum Dis. 2019 Feb 8. doi: 10.1136/annrheumdis-2018-214388.
Biomarkers predict VTE risk with menopausal oral hormone therapy
CHICAGO – An elevated baseline D-dimer level is helpful to women and their physicians in clarifying decision making about oral hormone therapy for troublesome menopausal symptoms, Mary Cushman, MD, said at the American Heart Association scientific sessions.
She was lead investigator in a nested case-control study embedded in the landmark Women’s Health Initiative (WHI), which showed that participants who had a baseline D-dimer greater than 0.54 mg/L – putting them in the top 25% – and were randomized to oral menopausal hormone therapy had a 5-year incidence of venous thromboembolism (VTE) of 6%. That’s 500% higher than in women with a lower D-dimer randomized to placebo.
“The number needed to test for D-dimer in advance of prescribing in order to prevent one VTE over 5 years of hormone therapy was only 33. So this is potentially something in the toolbox you can use in counseling women about oral hormone therapy,” said Dr. Cushman, professor of medicine and pathology and medical director of the thrombosis and hemostasis program at the University of Vermont, Burlington.
The biomarker study included 1,082 WHI participants aged 50-79 years randomized to oral conjugated equine estrogen with or without medroxyprogesterone acetate or to placebo, 215 of whom experienced VTE during a mean 4.1 years of follow-up. Levels of a variety of biomarkers obtained at baseline were assessed in terms of their associated risk of future VTE. The biomarkers included C-reactive protein and procoagulant, anticoagulant, and fibrinolytic factors.
In a logistic regression analysis adjusted for age, race, body mass index, and hysterectomy, the strongest association with VTE was a high D-dimer. That 500% increased risk of VTE with hormone therapy in women with a D-dimer greater than 0.54 mg/L was comparable in magnitude with the risk Dr. Cushman and her coinvestigators previously reported for the combination of factor V Leiden and hormone therapy.
Dr. Cushman and her associates also took a first step towards developing a multibiomarker risk score. They found that WHI participants randomized to hormone therapy who had abnormal baseline values for any three or more of eight biomarkers had a 1,450% greater risk of future VTE than women with zero or one abnormal biomarker who were assigned to placebo. The eight-biomarker panel described in the recently published study comprised D-dimer, factor V Leiden, protein C, total protein S, free protein S, antithrombin, plasmin-antiplasmin complex, and fragment 1.2. However, the investigators indicated the risk score needs further study before it’s ready for adoption in clinical practice (Res Pract Thromb Haemost. 2018 Apr 17;2[2]:310-9).
Dr. Cushman noted that, although the main findings of the WHI have largely resulted in abandonment of menopausal hormone therapy for disease prevention, many women still want to take oral hormone therapy for relief of bothersome menopausal symptoms. She tries to steer them instead to safer nonoral formulations. Transdermal estrogen replacement has no associated risk of VTE and doesn’t activate anticoagulation. Neither does vaginal estradiol.
In offering what she called “the 30,000-foot view of the impact of venous thrombosis on women’s health,” Dr. Cushman noted that VTE is the third-most common vascular disease in the United States, with up to 900,000 cases per year. The lifetime risk in women after age 45 is 8.4%. Half of VTEs are provoked and therefore potentially preventable, with common triggers being surgery, cancer, pregnancy, trauma, and immobilization, especially during travel.
In addition, a retrospective study conducted in the Worcester, Mass., area showed that 1-month mortality after VTE remained static in the 5%-10% range during 1999-2009.
“This is a fatal disease, even though we treat it as an outpatient quite a lot,” Dr. Cushman observed.
Common nonfatal complications of VTE include major bleeding in 5%-10% of cases, a recurrence rate of 5%-10% annually, a 20%-40% of the burdensome and not infrequently disabling condition known as postthrombotic syndrome, and a 3%-4% incidence of chronic thromboembolic pulmonary hypertension. Yet despite the seriousness of VTE, awareness about VTE is poor among both patients and physicians, and appropriate prophylaxis is underutilized, she said.
The key to improved primary prevention of VTE, Dr. Cushman continued, is greater attention to modifiable behavioral risk factors, along with more use of prophylactic medication when needed.
The traditional cardiovascular risk factors, like hypertension, smoking, and hyperlipidemia, aren’t relevant to VTE risk. But obesity and sedentary lifestyle have come to be recognized as important modifiable risk factors. In one study of more than 30,000 Americans, the risk of VTE was shown to be reduced by 40% in individuals who exercised at least four times per week, compared with the physically inactive.
And in an analysis led by Dr. Cushman of nearly 21,000 participants over age 45 years with 12.6 years of follow-up in the Longitudinal Investigation of Thromboembolism Etiology (LITE), the investigators found that greater levels of all body size measures – not just body mass index, but calf circumference, waist-hip ratio, hip circumference, and others – were associated with increased VTE risk. These associations weren’t affected by levels of circulating biomarkers for inflammation or hypercoagulability, suggesting that it’s obesity per se, with its associated adverse impact on blood flow caused by physical factors, that explains the mechanism underlying obesity as a risk factor for VTE (Thromb Res. 2016 Aug;144:127-32).
At the meeting’s opening ceremonies, AHA President Ivor Benjamin, MD, of the Medical College of Wisconsin, Milwaukee, presented Dr. Cushman with the AHA Population Research Prize. She was honored for her “critically acclaimed research utilizing biomarker assessments in population studies to elucidate pathways of disease etiology for the three most common vascular diseases – coronary heart disease, stroke, and venous thromboembolism – as well as their risk factors,” said Dr. Benjamin.
Dr. Cushman reported having no financial conflicts regarding her D-dimer study, which was funded by the National Institutes of Health.
CHICAGO – An elevated baseline D-dimer level is helpful to women and their physicians in clarifying decision making about oral hormone therapy for troublesome menopausal symptoms, Mary Cushman, MD, said at the American Heart Association scientific sessions.
She was lead investigator in a nested case-control study embedded in the landmark Women’s Health Initiative (WHI), which showed that participants who had a baseline D-dimer greater than 0.54 mg/L – putting them in the top 25% – and were randomized to oral menopausal hormone therapy had a 5-year incidence of venous thromboembolism (VTE) of 6%. That’s 500% higher than in women with a lower D-dimer randomized to placebo.
“The number needed to test for D-dimer in advance of prescribing in order to prevent one VTE over 5 years of hormone therapy was only 33. So this is potentially something in the toolbox you can use in counseling women about oral hormone therapy,” said Dr. Cushman, professor of medicine and pathology and medical director of the thrombosis and hemostasis program at the University of Vermont, Burlington.
The biomarker study included 1,082 WHI participants aged 50-79 years randomized to oral conjugated equine estrogen with or without medroxyprogesterone acetate or to placebo, 215 of whom experienced VTE during a mean 4.1 years of follow-up. Levels of a variety of biomarkers obtained at baseline were assessed in terms of their associated risk of future VTE. The biomarkers included C-reactive protein and procoagulant, anticoagulant, and fibrinolytic factors.
In a logistic regression analysis adjusted for age, race, body mass index, and hysterectomy, the strongest association with VTE was a high D-dimer. That 500% increased risk of VTE with hormone therapy in women with a D-dimer greater than 0.54 mg/L was comparable in magnitude with the risk Dr. Cushman and her coinvestigators previously reported for the combination of factor V Leiden and hormone therapy.
Dr. Cushman and her associates also took a first step towards developing a multibiomarker risk score. They found that WHI participants randomized to hormone therapy who had abnormal baseline values for any three or more of eight biomarkers had a 1,450% greater risk of future VTE than women with zero or one abnormal biomarker who were assigned to placebo. The eight-biomarker panel described in the recently published study comprised D-dimer, factor V Leiden, protein C, total protein S, free protein S, antithrombin, plasmin-antiplasmin complex, and fragment 1.2. However, the investigators indicated the risk score needs further study before it’s ready for adoption in clinical practice (Res Pract Thromb Haemost. 2018 Apr 17;2[2]:310-9).
Dr. Cushman noted that, although the main findings of the WHI have largely resulted in abandonment of menopausal hormone therapy for disease prevention, many women still want to take oral hormone therapy for relief of bothersome menopausal symptoms. She tries to steer them instead to safer nonoral formulations. Transdermal estrogen replacement has no associated risk of VTE and doesn’t activate anticoagulation. Neither does vaginal estradiol.
In offering what she called “the 30,000-foot view of the impact of venous thrombosis on women’s health,” Dr. Cushman noted that VTE is the third-most common vascular disease in the United States, with up to 900,000 cases per year. The lifetime risk in women after age 45 is 8.4%. Half of VTEs are provoked and therefore potentially preventable, with common triggers being surgery, cancer, pregnancy, trauma, and immobilization, especially during travel.
In addition, a retrospective study conducted in the Worcester, Mass., area showed that 1-month mortality after VTE remained static in the 5%-10% range during 1999-2009.
“This is a fatal disease, even though we treat it as an outpatient quite a lot,” Dr. Cushman observed.
Common nonfatal complications of VTE include major bleeding in 5%-10% of cases, a recurrence rate of 5%-10% annually, a 20%-40% of the burdensome and not infrequently disabling condition known as postthrombotic syndrome, and a 3%-4% incidence of chronic thromboembolic pulmonary hypertension. Yet despite the seriousness of VTE, awareness about VTE is poor among both patients and physicians, and appropriate prophylaxis is underutilized, she said.
The key to improved primary prevention of VTE, Dr. Cushman continued, is greater attention to modifiable behavioral risk factors, along with more use of prophylactic medication when needed.
The traditional cardiovascular risk factors, like hypertension, smoking, and hyperlipidemia, aren’t relevant to VTE risk. But obesity and sedentary lifestyle have come to be recognized as important modifiable risk factors. In one study of more than 30,000 Americans, the risk of VTE was shown to be reduced by 40% in individuals who exercised at least four times per week, compared with the physically inactive.
And in an analysis led by Dr. Cushman of nearly 21,000 participants over age 45 years with 12.6 years of follow-up in the Longitudinal Investigation of Thromboembolism Etiology (LITE), the investigators found that greater levels of all body size measures – not just body mass index, but calf circumference, waist-hip ratio, hip circumference, and others – were associated with increased VTE risk. These associations weren’t affected by levels of circulating biomarkers for inflammation or hypercoagulability, suggesting that it’s obesity per se, with its associated adverse impact on blood flow caused by physical factors, that explains the mechanism underlying obesity as a risk factor for VTE (Thromb Res. 2016 Aug;144:127-32).
At the meeting’s opening ceremonies, AHA President Ivor Benjamin, MD, of the Medical College of Wisconsin, Milwaukee, presented Dr. Cushman with the AHA Population Research Prize. She was honored for her “critically acclaimed research utilizing biomarker assessments in population studies to elucidate pathways of disease etiology for the three most common vascular diseases – coronary heart disease, stroke, and venous thromboembolism – as well as their risk factors,” said Dr. Benjamin.
Dr. Cushman reported having no financial conflicts regarding her D-dimer study, which was funded by the National Institutes of Health.
CHICAGO – An elevated baseline D-dimer level is helpful to women and their physicians in clarifying decision making about oral hormone therapy for troublesome menopausal symptoms, Mary Cushman, MD, said at the American Heart Association scientific sessions.
She was lead investigator in a nested case-control study embedded in the landmark Women’s Health Initiative (WHI), which showed that participants who had a baseline D-dimer greater than 0.54 mg/L – putting them in the top 25% – and were randomized to oral menopausal hormone therapy had a 5-year incidence of venous thromboembolism (VTE) of 6%. That’s 500% higher than in women with a lower D-dimer randomized to placebo.
“The number needed to test for D-dimer in advance of prescribing in order to prevent one VTE over 5 years of hormone therapy was only 33. So this is potentially something in the toolbox you can use in counseling women about oral hormone therapy,” said Dr. Cushman, professor of medicine and pathology and medical director of the thrombosis and hemostasis program at the University of Vermont, Burlington.
The biomarker study included 1,082 WHI participants aged 50-79 years randomized to oral conjugated equine estrogen with or without medroxyprogesterone acetate or to placebo, 215 of whom experienced VTE during a mean 4.1 years of follow-up. Levels of a variety of biomarkers obtained at baseline were assessed in terms of their associated risk of future VTE. The biomarkers included C-reactive protein and procoagulant, anticoagulant, and fibrinolytic factors.
In a logistic regression analysis adjusted for age, race, body mass index, and hysterectomy, the strongest association with VTE was a high D-dimer. That 500% increased risk of VTE with hormone therapy in women with a D-dimer greater than 0.54 mg/L was comparable in magnitude with the risk Dr. Cushman and her coinvestigators previously reported for the combination of factor V Leiden and hormone therapy.
Dr. Cushman and her associates also took a first step towards developing a multibiomarker risk score. They found that WHI participants randomized to hormone therapy who had abnormal baseline values for any three or more of eight biomarkers had a 1,450% greater risk of future VTE than women with zero or one abnormal biomarker who were assigned to placebo. The eight-biomarker panel described in the recently published study comprised D-dimer, factor V Leiden, protein C, total protein S, free protein S, antithrombin, plasmin-antiplasmin complex, and fragment 1.2. However, the investigators indicated the risk score needs further study before it’s ready for adoption in clinical practice (Res Pract Thromb Haemost. 2018 Apr 17;2[2]:310-9).
Dr. Cushman noted that, although the main findings of the WHI have largely resulted in abandonment of menopausal hormone therapy for disease prevention, many women still want to take oral hormone therapy for relief of bothersome menopausal symptoms. She tries to steer them instead to safer nonoral formulations. Transdermal estrogen replacement has no associated risk of VTE and doesn’t activate anticoagulation. Neither does vaginal estradiol.
In offering what she called “the 30,000-foot view of the impact of venous thrombosis on women’s health,” Dr. Cushman noted that VTE is the third-most common vascular disease in the United States, with up to 900,000 cases per year. The lifetime risk in women after age 45 is 8.4%. Half of VTEs are provoked and therefore potentially preventable, with common triggers being surgery, cancer, pregnancy, trauma, and immobilization, especially during travel.
In addition, a retrospective study conducted in the Worcester, Mass., area showed that 1-month mortality after VTE remained static in the 5%-10% range during 1999-2009.
“This is a fatal disease, even though we treat it as an outpatient quite a lot,” Dr. Cushman observed.
Common nonfatal complications of VTE include major bleeding in 5%-10% of cases, a recurrence rate of 5%-10% annually, a 20%-40% of the burdensome and not infrequently disabling condition known as postthrombotic syndrome, and a 3%-4% incidence of chronic thromboembolic pulmonary hypertension. Yet despite the seriousness of VTE, awareness about VTE is poor among both patients and physicians, and appropriate prophylaxis is underutilized, she said.
The key to improved primary prevention of VTE, Dr. Cushman continued, is greater attention to modifiable behavioral risk factors, along with more use of prophylactic medication when needed.
The traditional cardiovascular risk factors, like hypertension, smoking, and hyperlipidemia, aren’t relevant to VTE risk. But obesity and sedentary lifestyle have come to be recognized as important modifiable risk factors. In one study of more than 30,000 Americans, the risk of VTE was shown to be reduced by 40% in individuals who exercised at least four times per week, compared with the physically inactive.
And in an analysis led by Dr. Cushman of nearly 21,000 participants over age 45 years with 12.6 years of follow-up in the Longitudinal Investigation of Thromboembolism Etiology (LITE), the investigators found that greater levels of all body size measures – not just body mass index, but calf circumference, waist-hip ratio, hip circumference, and others – were associated with increased VTE risk. These associations weren’t affected by levels of circulating biomarkers for inflammation or hypercoagulability, suggesting that it’s obesity per se, with its associated adverse impact on blood flow caused by physical factors, that explains the mechanism underlying obesity as a risk factor for VTE (Thromb Res. 2016 Aug;144:127-32).
At the meeting’s opening ceremonies, AHA President Ivor Benjamin, MD, of the Medical College of Wisconsin, Milwaukee, presented Dr. Cushman with the AHA Population Research Prize. She was honored for her “critically acclaimed research utilizing biomarker assessments in population studies to elucidate pathways of disease etiology for the three most common vascular diseases – coronary heart disease, stroke, and venous thromboembolism – as well as their risk factors,” said Dr. Benjamin.
Dr. Cushman reported having no financial conflicts regarding her D-dimer study, which was funded by the National Institutes of Health.
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: Women in the top 25% for D-dimer level before going on menopausal hormone therapy had a 6% incidence of venous thromboembolism over 5 years.
Study details: This was a nested case-control study focused on identifying biomarkers for venous thromboembolism risk which included 1,082 participants in the Women’s Health Initiative randomized to menopausal hormone therapy or placebo.
Disclosures: The presenter reported having no financial conflicts regarding the study, which was funded by the National Institutes of Health.
Immunotherapy’s cardiac effects require early monitoring, management
WASHINGTON – Unquestionably, immunotherapy is revolutionizing the care of patients with various solid tumors and hematologic malignancies.
But it’s equally true that there’s no such thing as either a free lunch or a cancer therapy free of side effects, whether it’s increased risk for heart failure associated with anthracycline-based chemotherapy, or inflammatory conditions, arrhythmias, and thromboembolic events associated with immune checkpoint inhibitors, said R. Frank Cornell, MD, of Vanderbilt University Medical Center in Nashville, Tenn.
“Early awareness and intervention is critical for improved outcomes, and a multidisciplinary approach between oncology, cardiology, the clinic nurse, and other health care providers is critical in managing these patients with these complicated therapies,” he said at the American College of Cardiology’s Advancing the Cardiovascular Care of the Oncology Patient meeting.
Checkpoint inhibitors and the heart
Toxicities associated with immune checkpoint inhibitors such as the programmed death 1/ligand 1 (PD-1/PD-L1) inhibitors nivolumab (Opdivo) and pembrolizumab (Keytruda) and the cytotoxic T-lymphocyte antigen 4 antibody ipilimumab (Yervoy) tend to mimic autoimmune conditions, Dr. Cornell said.
Cardiovascular events associated with these agents, while uncommon, include myocarditis, pericarditis, arrhythmias, impaired ventricular function with heart failure, vasculitis, and venous thromboembolism, he said, citing an American Society of Clinical Oncology (ASCO) clinical practice guideline (J Clin Oncol 2018;36[17]:1714-68).
Dr. Cornell described the case of a 63-year-old woman with disseminated metastatic melanoma who presented to the emergency department 10 days after starting on combination therapy with ipilimumab and nivolumab. She had developed shortness of breath, pleuritic chest pain, and a mild cough for 1 or 2 days.
Her cardiac laboratory markers had been normal at baseline, but were markedly elevated on presentation, and electrocardiograms showed complete heart block and subsequent ventricular tachycardia.
The patient was started on high-dose prednisone, but she died in hospital, and an autopsy showed that the cause of death was infiltration into the myocardium of CD3-positive and CD8-positive T lymphocytes.
“So how do we manage this? This is a good opportunity, I think, for further cardiology and oncology collaboration to develop more robust guidelines for what we can do to best prevent this,” Dr. Cornell said.
Patients started on the ipilimumab/nivolumab combination should be tested weekly for cardiac troponin, creatine kinase (CK) and CK-muscle/brain (CK-MB) weekly for the first 3-4 weeks of therapy. Therapy should be stopped if troponin levels continue to rise, and the patient should be started on high-dose steroids, he said.
The role of other anti-inflammatory agents such as infliximab (Remicade and biosimilars) is unclear and needs further study, he added.
Dr. Cornell cited a 2018 letter to The Lancet by Javid J. Moslehi, MD, and colleagues from Vanderbilt describing an increase in reports of fatal myocarditis among patients treated with checkpoint inhibitors.
“We highlight the high mortality rate with severe immune checkpoint inhibitor–related myocarditis, which is more frequent with combination PD-1 and CTLA-4 blockade, but can also occur with monotherapy. Myocarditis was observed across immune checkpoint inhibitor regimens, although it remains too early to determine whether the incidence differs between use of anti-PD1 and anti-PD-L1 drugs. Furthermore, this condition occurs early on during therapy and across cancer types,” they wrote.
Most of the patients had no preexisting cardiovascular disease, and most were not taking medications for hypertension, cardiovascular disease, or diabetes.
CAR-T cells and cardiac disease
The primary cardiac complications associated with CAR-T cell therapy are related to the cytokine release syndrome (CRS), a condition marked by progressive elevation in inflammatory cytokines that in turn leads to marked elevations in C-reactive protein (CRP), interferon gamma, tumor necrosis factor al, and release of pro-inflammatory cytokines including interleukin (IL) 6, IL-10, IL-12, and IL-1 beta.
In rare instances, CRS can lead to disseminated intravascular coagulation (DIC), capillary leak syndrome, and a hemophagocytic lymphohistiocytosis-like (HLH) syndrome, Dr. Cornell said.
Package inserts for the two Food and Drug Administration–approved CAR-T cell products, axicabtagene ciloleucel (Yescarta) and tisagenlecleucel (Kymriah) show that each was associated in clinical trials with a high incidence of CRS.
Among patients treated with axicabtagene ciloleucel, 94% developed CRS, which was grade 3 or greater in severity in 13%. The median time to onset was 2 days, and the median duration was 7 days. Cardiovascular adverse events included grade 3 or greater tachycardia in 2%, arrhythmias in 7%, edema in 1%, dyspnea in 3%, pleural effusion in 2%, hypotension in 15%, hypertension in 6%, and thrombosis in 1%.
Among patients treated with tisagenlecleucel, 79% treated for B-cell acute lymphoblastic leukemia (B-ALL) and 74% treated for diffuse large B cell lymphoma (DLBCL) developed CRS, which was grade 3 or greater in 49% and 23% of patients, respectively. The median time to onset was 3 days, and the median duration of CRS was 8 days.
Cardiovascular adverse events of grade 3 or greater among these patients included tachycardia in 4%, fluid overload in 7%, edema in 1%, dyspnea in 12%, pulmonary edema in 4%, hypotension in 22%, and hypertension in 6%.
Risk factors for CRS include high pre-infusion tumor burden, active infections, and concurrent inflammatory processes, Dr. Cornell said.
Prevention of cardiovascular complications of CAR-T cell therapy requires management of CRS. Patients with grade 2 or greater CRS should receive the anti-IL-6 agent tocilizumab (Actemra) 8 mg/kg intravenously over 1 hour to a maximum dose of 800 mg. Tocilizumab infusions can be repeated every 8 hours as needed if the patient is not responsive to intravenous fluids or increasing supplement oxygen, but should be limited to a maximum of three doses over 24 hours, and a maximum total of four doses.
Patients with grade 3 CRS should also receive intravenous methylprednisolone 1 mg/kg twice daily or the equivalent amount of dexamethasone, with corticosteroids continued until the severity of CRS is grade 1 or less, then tapered over 3 days,
Patients with grade 4 CRS should also receive IV methylprednisolone 1,000 mg per day for 3 days, and if symptoms improve, continue management as per grade 3, Dr. Cornell said.
Dr. Cornell reported having nothing to disclose.
WASHINGTON – Unquestionably, immunotherapy is revolutionizing the care of patients with various solid tumors and hematologic malignancies.
But it’s equally true that there’s no such thing as either a free lunch or a cancer therapy free of side effects, whether it’s increased risk for heart failure associated with anthracycline-based chemotherapy, or inflammatory conditions, arrhythmias, and thromboembolic events associated with immune checkpoint inhibitors, said R. Frank Cornell, MD, of Vanderbilt University Medical Center in Nashville, Tenn.
“Early awareness and intervention is critical for improved outcomes, and a multidisciplinary approach between oncology, cardiology, the clinic nurse, and other health care providers is critical in managing these patients with these complicated therapies,” he said at the American College of Cardiology’s Advancing the Cardiovascular Care of the Oncology Patient meeting.
Checkpoint inhibitors and the heart
Toxicities associated with immune checkpoint inhibitors such as the programmed death 1/ligand 1 (PD-1/PD-L1) inhibitors nivolumab (Opdivo) and pembrolizumab (Keytruda) and the cytotoxic T-lymphocyte antigen 4 antibody ipilimumab (Yervoy) tend to mimic autoimmune conditions, Dr. Cornell said.
Cardiovascular events associated with these agents, while uncommon, include myocarditis, pericarditis, arrhythmias, impaired ventricular function with heart failure, vasculitis, and venous thromboembolism, he said, citing an American Society of Clinical Oncology (ASCO) clinical practice guideline (J Clin Oncol 2018;36[17]:1714-68).
Dr. Cornell described the case of a 63-year-old woman with disseminated metastatic melanoma who presented to the emergency department 10 days after starting on combination therapy with ipilimumab and nivolumab. She had developed shortness of breath, pleuritic chest pain, and a mild cough for 1 or 2 days.
Her cardiac laboratory markers had been normal at baseline, but were markedly elevated on presentation, and electrocardiograms showed complete heart block and subsequent ventricular tachycardia.
The patient was started on high-dose prednisone, but she died in hospital, and an autopsy showed that the cause of death was infiltration into the myocardium of CD3-positive and CD8-positive T lymphocytes.
“So how do we manage this? This is a good opportunity, I think, for further cardiology and oncology collaboration to develop more robust guidelines for what we can do to best prevent this,” Dr. Cornell said.
Patients started on the ipilimumab/nivolumab combination should be tested weekly for cardiac troponin, creatine kinase (CK) and CK-muscle/brain (CK-MB) weekly for the first 3-4 weeks of therapy. Therapy should be stopped if troponin levels continue to rise, and the patient should be started on high-dose steroids, he said.
The role of other anti-inflammatory agents such as infliximab (Remicade and biosimilars) is unclear and needs further study, he added.
Dr. Cornell cited a 2018 letter to The Lancet by Javid J. Moslehi, MD, and colleagues from Vanderbilt describing an increase in reports of fatal myocarditis among patients treated with checkpoint inhibitors.
“We highlight the high mortality rate with severe immune checkpoint inhibitor–related myocarditis, which is more frequent with combination PD-1 and CTLA-4 blockade, but can also occur with monotherapy. Myocarditis was observed across immune checkpoint inhibitor regimens, although it remains too early to determine whether the incidence differs between use of anti-PD1 and anti-PD-L1 drugs. Furthermore, this condition occurs early on during therapy and across cancer types,” they wrote.
Most of the patients had no preexisting cardiovascular disease, and most were not taking medications for hypertension, cardiovascular disease, or diabetes.
CAR-T cells and cardiac disease
The primary cardiac complications associated with CAR-T cell therapy are related to the cytokine release syndrome (CRS), a condition marked by progressive elevation in inflammatory cytokines that in turn leads to marked elevations in C-reactive protein (CRP), interferon gamma, tumor necrosis factor al, and release of pro-inflammatory cytokines including interleukin (IL) 6, IL-10, IL-12, and IL-1 beta.
In rare instances, CRS can lead to disseminated intravascular coagulation (DIC), capillary leak syndrome, and a hemophagocytic lymphohistiocytosis-like (HLH) syndrome, Dr. Cornell said.
Package inserts for the two Food and Drug Administration–approved CAR-T cell products, axicabtagene ciloleucel (Yescarta) and tisagenlecleucel (Kymriah) show that each was associated in clinical trials with a high incidence of CRS.
Among patients treated with axicabtagene ciloleucel, 94% developed CRS, which was grade 3 or greater in severity in 13%. The median time to onset was 2 days, and the median duration was 7 days. Cardiovascular adverse events included grade 3 or greater tachycardia in 2%, arrhythmias in 7%, edema in 1%, dyspnea in 3%, pleural effusion in 2%, hypotension in 15%, hypertension in 6%, and thrombosis in 1%.
Among patients treated with tisagenlecleucel, 79% treated for B-cell acute lymphoblastic leukemia (B-ALL) and 74% treated for diffuse large B cell lymphoma (DLBCL) developed CRS, which was grade 3 or greater in 49% and 23% of patients, respectively. The median time to onset was 3 days, and the median duration of CRS was 8 days.
Cardiovascular adverse events of grade 3 or greater among these patients included tachycardia in 4%, fluid overload in 7%, edema in 1%, dyspnea in 12%, pulmonary edema in 4%, hypotension in 22%, and hypertension in 6%.
Risk factors for CRS include high pre-infusion tumor burden, active infections, and concurrent inflammatory processes, Dr. Cornell said.
Prevention of cardiovascular complications of CAR-T cell therapy requires management of CRS. Patients with grade 2 or greater CRS should receive the anti-IL-6 agent tocilizumab (Actemra) 8 mg/kg intravenously over 1 hour to a maximum dose of 800 mg. Tocilizumab infusions can be repeated every 8 hours as needed if the patient is not responsive to intravenous fluids or increasing supplement oxygen, but should be limited to a maximum of three doses over 24 hours, and a maximum total of four doses.
Patients with grade 3 CRS should also receive intravenous methylprednisolone 1 mg/kg twice daily or the equivalent amount of dexamethasone, with corticosteroids continued until the severity of CRS is grade 1 or less, then tapered over 3 days,
Patients with grade 4 CRS should also receive IV methylprednisolone 1,000 mg per day for 3 days, and if symptoms improve, continue management as per grade 3, Dr. Cornell said.
Dr. Cornell reported having nothing to disclose.
WASHINGTON – Unquestionably, immunotherapy is revolutionizing the care of patients with various solid tumors and hematologic malignancies.
But it’s equally true that there’s no such thing as either a free lunch or a cancer therapy free of side effects, whether it’s increased risk for heart failure associated with anthracycline-based chemotherapy, or inflammatory conditions, arrhythmias, and thromboembolic events associated with immune checkpoint inhibitors, said R. Frank Cornell, MD, of Vanderbilt University Medical Center in Nashville, Tenn.
“Early awareness and intervention is critical for improved outcomes, and a multidisciplinary approach between oncology, cardiology, the clinic nurse, and other health care providers is critical in managing these patients with these complicated therapies,” he said at the American College of Cardiology’s Advancing the Cardiovascular Care of the Oncology Patient meeting.
Checkpoint inhibitors and the heart
Toxicities associated with immune checkpoint inhibitors such as the programmed death 1/ligand 1 (PD-1/PD-L1) inhibitors nivolumab (Opdivo) and pembrolizumab (Keytruda) and the cytotoxic T-lymphocyte antigen 4 antibody ipilimumab (Yervoy) tend to mimic autoimmune conditions, Dr. Cornell said.
Cardiovascular events associated with these agents, while uncommon, include myocarditis, pericarditis, arrhythmias, impaired ventricular function with heart failure, vasculitis, and venous thromboembolism, he said, citing an American Society of Clinical Oncology (ASCO) clinical practice guideline (J Clin Oncol 2018;36[17]:1714-68).
Dr. Cornell described the case of a 63-year-old woman with disseminated metastatic melanoma who presented to the emergency department 10 days after starting on combination therapy with ipilimumab and nivolumab. She had developed shortness of breath, pleuritic chest pain, and a mild cough for 1 or 2 days.
Her cardiac laboratory markers had been normal at baseline, but were markedly elevated on presentation, and electrocardiograms showed complete heart block and subsequent ventricular tachycardia.
The patient was started on high-dose prednisone, but she died in hospital, and an autopsy showed that the cause of death was infiltration into the myocardium of CD3-positive and CD8-positive T lymphocytes.
“So how do we manage this? This is a good opportunity, I think, for further cardiology and oncology collaboration to develop more robust guidelines for what we can do to best prevent this,” Dr. Cornell said.
Patients started on the ipilimumab/nivolumab combination should be tested weekly for cardiac troponin, creatine kinase (CK) and CK-muscle/brain (CK-MB) weekly for the first 3-4 weeks of therapy. Therapy should be stopped if troponin levels continue to rise, and the patient should be started on high-dose steroids, he said.
The role of other anti-inflammatory agents such as infliximab (Remicade and biosimilars) is unclear and needs further study, he added.
Dr. Cornell cited a 2018 letter to The Lancet by Javid J. Moslehi, MD, and colleagues from Vanderbilt describing an increase in reports of fatal myocarditis among patients treated with checkpoint inhibitors.
“We highlight the high mortality rate with severe immune checkpoint inhibitor–related myocarditis, which is more frequent with combination PD-1 and CTLA-4 blockade, but can also occur with monotherapy. Myocarditis was observed across immune checkpoint inhibitor regimens, although it remains too early to determine whether the incidence differs between use of anti-PD1 and anti-PD-L1 drugs. Furthermore, this condition occurs early on during therapy and across cancer types,” they wrote.
Most of the patients had no preexisting cardiovascular disease, and most were not taking medications for hypertension, cardiovascular disease, or diabetes.
CAR-T cells and cardiac disease
The primary cardiac complications associated with CAR-T cell therapy are related to the cytokine release syndrome (CRS), a condition marked by progressive elevation in inflammatory cytokines that in turn leads to marked elevations in C-reactive protein (CRP), interferon gamma, tumor necrosis factor al, and release of pro-inflammatory cytokines including interleukin (IL) 6, IL-10, IL-12, and IL-1 beta.
In rare instances, CRS can lead to disseminated intravascular coagulation (DIC), capillary leak syndrome, and a hemophagocytic lymphohistiocytosis-like (HLH) syndrome, Dr. Cornell said.
Package inserts for the two Food and Drug Administration–approved CAR-T cell products, axicabtagene ciloleucel (Yescarta) and tisagenlecleucel (Kymriah) show that each was associated in clinical trials with a high incidence of CRS.
Among patients treated with axicabtagene ciloleucel, 94% developed CRS, which was grade 3 or greater in severity in 13%. The median time to onset was 2 days, and the median duration was 7 days. Cardiovascular adverse events included grade 3 or greater tachycardia in 2%, arrhythmias in 7%, edema in 1%, dyspnea in 3%, pleural effusion in 2%, hypotension in 15%, hypertension in 6%, and thrombosis in 1%.
Among patients treated with tisagenlecleucel, 79% treated for B-cell acute lymphoblastic leukemia (B-ALL) and 74% treated for diffuse large B cell lymphoma (DLBCL) developed CRS, which was grade 3 or greater in 49% and 23% of patients, respectively. The median time to onset was 3 days, and the median duration of CRS was 8 days.
Cardiovascular adverse events of grade 3 or greater among these patients included tachycardia in 4%, fluid overload in 7%, edema in 1%, dyspnea in 12%, pulmonary edema in 4%, hypotension in 22%, and hypertension in 6%.
Risk factors for CRS include high pre-infusion tumor burden, active infections, and concurrent inflammatory processes, Dr. Cornell said.
Prevention of cardiovascular complications of CAR-T cell therapy requires management of CRS. Patients with grade 2 or greater CRS should receive the anti-IL-6 agent tocilizumab (Actemra) 8 mg/kg intravenously over 1 hour to a maximum dose of 800 mg. Tocilizumab infusions can be repeated every 8 hours as needed if the patient is not responsive to intravenous fluids or increasing supplement oxygen, but should be limited to a maximum of three doses over 24 hours, and a maximum total of four doses.
Patients with grade 3 CRS should also receive intravenous methylprednisolone 1 mg/kg twice daily or the equivalent amount of dexamethasone, with corticosteroids continued until the severity of CRS is grade 1 or less, then tapered over 3 days,
Patients with grade 4 CRS should also receive IV methylprednisolone 1,000 mg per day for 3 days, and if symptoms improve, continue management as per grade 3, Dr. Cornell said.
Dr. Cornell reported having nothing to disclose.
REPORTING FROM ACC CARDIO-ONCOLOGY
Key clinical point: Monitor for cardiac symptoms and treat or interrupt immunotherapy as needed.
Major finding: Immune checkpoint inhibitors and CAR T-cell therapies are associated with distinct cardiovascular adverse events.
Study details: Review of strategies for managing the cardiovascular consequences of cancer immunotherapies.
Disclosures: Dr. Cornell reported having nothing to disclose.
When is it safe to resume anticoagulation in my patient with hemorrhagic stroke?
Balancing risk is critical to decision making
Department of Medicine, Massachusetts General Hospital, Boston
Case
A 75 year-old woman with a history of hypertension, diabetes mellitus, heart failure and nonvalvular atrial fibrillation (CHA2DS2-VASc score, 8) on anticoagulation is admitted with weakness and dysarthria. Exam is notable for hypertension and right-sided hemiparesis. CT of the head shows an intraparenchymal hemorrhage in the left putamen. Her anticoagulation is reversed and blood pressure well controlled. She is discharged 12 days later.
Brief overview of the issue
Intracranial hemorrhage (ICH) is the second most common cause of stroke and is associated with high morbidity and mortality.1 It is estimated that 10%-15% of spontaneous ICH cases occur in patients on therapeutic anticoagulation for atrial fibrillation.2 As our population ages and more people develop atrial fibrillation, anticoagulation for primary or secondary prevention of embolic stroke also will likely increase, placing more people at risk for ICH. Even stringently controlled therapeutic international normalized ratios (INRs) between 2 and 3 may double the risk of ICH.3
Patients with ICH require close monitoring and treatment, including blood pressure control, reversal of anticoagulation, reduction of intracranial pressure and, at times, neurosurgery.4 Although anticoagulation is discontinued and reversed at the onset of ICH, no clear consensus exists as to when it is safe to resume it. Although anticoagulation decreases the risk of stroke/thromboembolism, it may also increase the amount of bleeding associated with the initial ICH or lead to its recurrence.
Factors that may contribute to rebleeding include uncontrolled hypertension, advanced age, time to resumption of anticoagulation, and lobar location of ICH (i.e., in cerebral cortex and/or underlying white matter).5 Traditionally, lobar ICH has high incidence of cerebral amyloid angiopathy and has been associated with higher bleeding rates than has deep ICH (i.e., involving the thalami, basal ganglia, cerebellum, or brainstem) where cerebral amyloid angiopathy is rare and ICH is usually from hypertensive vessel disease. However, in patients with active thromboembolic disease, high-risk atrial fibrillation, and mechanical valves, withholding anticoagulation could place them at high risk of stroke.
Two questions should be addressed in the case presented: Is it safe to restart therapeutic anticoagulation; and if so, what is the optimal time interval between ICH and reinitiation of anticoagulation?
Overview of the data
There is limited guidance from major professional societies regarding the reinitiation of anticoagulation and the optimal timing of safely resuming anticoagulation in patients with prior ICH.
Current European Stroke Organization guidelines provide no specific recommendations for anticoagulation resumption after ICH.7 The American Heart Association/American Stroke Association guideline has a class IIA (weak) recommendation to avoid anticoagulation in spontaneous lobar ICH and a class IIB (very weak) recommendation to consider resuming anticoagulation in nonlobar ICH on a case-by-case basis.4
Two recent meta-analyses have examined outcomes of resuming anticoagulation after ICH. In a meta-analysis of 5,300 patients with nonlobar ICH involving eight retrospective studies, Murthy et al. evaluated the risk of thromboembolic events (described as a composite outcome of MI and stroke) and the risk of recurrent ICH.8 They reported that resumption of therapeutic anticoagulation was associated with a decrease in the rate of thromboembolic events (6.7% vs. 17.6%; risk ratio, 0.35; 95% confidence interval, 0.25-0.45) with no significant change in the rate of repeat ICH (8.7% vs. 7.8%).
A second meta-analysis of three retrospective trials conducted by Biffi et al. examined anticoagulation resumption in 1,012 patients with ICH solely in the setting of thromboprophylaxis for nonvalvular atrial fibrillation.9 Reinitiation of anticoagulation after ICH was associated with decreased mortality (hazard ratio, 0.27; 95% CI, 0.19-0.40; P less than .0001), improved functional outcome (HR, 4.15; 95% CI, 2.92-5.90; P less than .0001), and reduction in all-cause stroke recurrence (HR 0.47; 95% CI, 0.36-0.64; P less than .0001). There was no significant difference in the rate of recurrent ICH when anticoagulation was resumed. Despite the notion that patients with cerebral amyloid angiopathy are at high risk of rebleeding, this positive association still held irrespective of lobar vs. nonlobar location of ICH.
Collectively, these studies suggest that resumption of anticoagulation may be effective in decreasing the rates of thromboembolism, as well as provide a functional and mortality benefit without increasing the risk of rebleeding, irrespective of the location of the bleed.
Less is known about the optimal timing of resumption of therapeutic anticoagulation, with data ranging from 72 hours to 30 weeks.10 The American Heart Association/American Stroke Association has a class IIB (very weak) recommendation to avoid anticoagulation for at least 4 weeks in patients without mechanical heart valves.4 The median time to resumption of therapeutic anticoagulation in aforementioned meta-analyses ranged from 10 to 44 days.8,9
A recent observational study of 2,619 ICH survivors explored the relationship between the timing of reinitiation of anticoagulation and the incidence of thrombotic events (defined as ischemic stroke or death because of MI or systemic arterial thromboembolism) and hemorrhagic events (defined as recurrent ICH or bleeding event leading to death) occurring at least 28 days after initial ICH in patients with atrial fibrillation.11
A decrease in thrombotic events was demonstrated if anticoagulation was started 4-16 weeks after ICH. However, when anticoagulation was started more than 16 weeks after ICH, no benefit was seen. Additionally, there was no significant difference in hemorrhagic events between men and women who resumed anticoagulation. In patients with high venous thromboembolism risk based on CHA2DS2-VASc score, resumption of anticoagulation was associated with a decreased predicted incidence of vascular death and nonfatal stroke, with the greatest benefit observed when anticoagulation was started at 7-8 weeks after ICH.
Unfortunately, published literature to date on anticoagulation after ICH is based entirely on retrospective studies – not randomized, controlled studies – making it more likely that anticoagulation would have been resumed in healthier patients, not those left debilitated by the ICH.
Furthermore, information on the location and size of the hemorrhages – which may serve as another confounding factor – often has not been reported. This is important since patients with smaller hemorrhages in less precarious areas also may be more likely to have resumption of anticoagulation. Another limitation of the current literature is that warfarin is the most common anticoagulant studied, with few studies involving the increasingly prescribed newer direct oral anticoagulants. It is also important to stress that a causal relationship between use of anticoagulants and certain outcomes or adverse effects following ICH may be more difficult to invoke in the absence of randomized controlled study designs.
Application of the data to our patient
Resumption of anticoagulation in our patient with ICH requires balancing the risk of hemorrhage expansion and recurrent ICH with the risk of thromboembolic disease.
Our patient is at higher risk of bleeding because of her advanced age, but adequate control of her blood pressure and nonlobar location of her ICH in the basal ganglia also may decrease her risk of recurrent ICH. Her high CHA2DS2-VASc score places her at high risk of thromboembolic event and stroke, making it more likely for reinitiation of anticoagulation to confer a mortality benefit.
Based on AHA guidelines,4 we should wait at least 4 weeks, or possibly wait until weeks 7-8 after ICH when the greatest benefit may be expected based on prediction models.11
Bottom line
It would likely be safe to resume anticoagulation 4-8 weeks after ICH in our patient.
Dr. Gibson, Dr. Restrepo, Dr. Sasidhara, and Dr. Manian are hospitalists at Massachusetts General Hospital, Boston.
References
1. An SJ et al. Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: An update. J Stroke. 2017 Jan;19:3-10.
2. Horstmann S et al. Intracerebral hemorrhage during anticoagulation with vitamin K antagonists: a consecutive observational study. J Neurol. 2013 Aug;260:2046-51.
3. Rosand J et al. The effect of warfarin and intensity of anticoagulation on outcome of intracerebral hemorrhage. Arch Intern Med. 2004 Apr 26;164:880-4.
4. Hemphill JC et al. Guidelines for the management of spontaneous intracerebral hemorrhage. Stroke. 2015 Jul;46:2032-60.
5. Aguillar MI et al. Update in intracerebral hemorrhage. Neurohospitalist. 2011;1:148-59.
6. Hill MD et al. Rate of stroke recurrence in patients with primary intracerebral hemorrhage. Stroke. 2000;31:123-7.
7. Steiner T et al. European Stroke Organization (ESO) guidelines for the management of spontaneous cerebral hemorrhage. Int J Stroke. 2014;9:840-55.
8. Murthy SB et al. Restarting anticoagulation therapy after intracranial hemorrhage: A systematic review and meta-analysis. Stroke. 2017 Jun;48:1594-600.
9. Biffi A et al. Oral anticoagulation and functional outcome after intracerebral hemorrhage. Ann Neurol. 2017 Nov;82:755-65.
10. Witt DM. What to do after the bleed: Resuming anticoagulation after major bleeding. Hematology Am Soc Hematol Educ Program. 2016 Dec 2;206:620-4.
11. Pennlert J et al. Optimal timing of anticoagulant treatment after intracerebral hemorrhage in patients with atrial fibrillation. Stroke. 2017 Feb;48:314-20.
Key Points
- Robust scientific data on when to resume anticoagulation after ICH does not exist.
- Retrospective studies have shown that anticoagulation resumption after 4-8 weeks decreases the risk of thromboembolic events, decreases mortality, and improves functional status following ICH with no significant change in the risk of its recurrence.
- Prospective, randomized controlled trials are needed to explore risks/benefits of anticoagulation resumption and better define its optimal timing in relation to ICH.
Quiz
Which of the following is false regarding ICH?
A. Lobar ICHs are usually associated with cerebral amyloid angiopathy which are prone to bleeding.
B. Randomized, controlled studies have helped guide the decision as to when to resume anticoagulation in patients with ICH.
C. Current guidelines suggest deferring therapeutic anticoagulation for at least 4 weeks following ICH.
D. Resumption of anticoagulation after 4-8 weeks does not lead to increased risk of rebleeding in patients with prior ICH.
The false answer is B: Current recommendations regarding resumption of anticoagulation in patients with ICH are based solely on retrospective observational studies; there are no randomized, control trials to date.
A is true: In contrast to hypertensive vessel disease associated with deep ICH, lobar hemorrhages are usually associated with cerebral amyloid angiopathy, which are more prone to bleeding.
C is true: The AHA/ASA has a class IIB recommendation to avoid anticoagulation for at least 4 weeks after ICH in patients without mechanical heart valves.
D is true: Several studies have shown that resumption of anticoagulation 4-8 weeks after ICH does not increase the risk of rebleeding.
Balancing risk is critical to decision making
Balancing risk is critical to decision making
Department of Medicine, Massachusetts General Hospital, Boston
Case
A 75 year-old woman with a history of hypertension, diabetes mellitus, heart failure and nonvalvular atrial fibrillation (CHA2DS2-VASc score, 8) on anticoagulation is admitted with weakness and dysarthria. Exam is notable for hypertension and right-sided hemiparesis. CT of the head shows an intraparenchymal hemorrhage in the left putamen. Her anticoagulation is reversed and blood pressure well controlled. She is discharged 12 days later.
Brief overview of the issue
Intracranial hemorrhage (ICH) is the second most common cause of stroke and is associated with high morbidity and mortality.1 It is estimated that 10%-15% of spontaneous ICH cases occur in patients on therapeutic anticoagulation for atrial fibrillation.2 As our population ages and more people develop atrial fibrillation, anticoagulation for primary or secondary prevention of embolic stroke also will likely increase, placing more people at risk for ICH. Even stringently controlled therapeutic international normalized ratios (INRs) between 2 and 3 may double the risk of ICH.3
Patients with ICH require close monitoring and treatment, including blood pressure control, reversal of anticoagulation, reduction of intracranial pressure and, at times, neurosurgery.4 Although anticoagulation is discontinued and reversed at the onset of ICH, no clear consensus exists as to when it is safe to resume it. Although anticoagulation decreases the risk of stroke/thromboembolism, it may also increase the amount of bleeding associated with the initial ICH or lead to its recurrence.
Factors that may contribute to rebleeding include uncontrolled hypertension, advanced age, time to resumption of anticoagulation, and lobar location of ICH (i.e., in cerebral cortex and/or underlying white matter).5 Traditionally, lobar ICH has high incidence of cerebral amyloid angiopathy and has been associated with higher bleeding rates than has deep ICH (i.e., involving the thalami, basal ganglia, cerebellum, or brainstem) where cerebral amyloid angiopathy is rare and ICH is usually from hypertensive vessel disease. However, in patients with active thromboembolic disease, high-risk atrial fibrillation, and mechanical valves, withholding anticoagulation could place them at high risk of stroke.
Two questions should be addressed in the case presented: Is it safe to restart therapeutic anticoagulation; and if so, what is the optimal time interval between ICH and reinitiation of anticoagulation?
Overview of the data
There is limited guidance from major professional societies regarding the reinitiation of anticoagulation and the optimal timing of safely resuming anticoagulation in patients with prior ICH.
Current European Stroke Organization guidelines provide no specific recommendations for anticoagulation resumption after ICH.7 The American Heart Association/American Stroke Association guideline has a class IIA (weak) recommendation to avoid anticoagulation in spontaneous lobar ICH and a class IIB (very weak) recommendation to consider resuming anticoagulation in nonlobar ICH on a case-by-case basis.4
Two recent meta-analyses have examined outcomes of resuming anticoagulation after ICH. In a meta-analysis of 5,300 patients with nonlobar ICH involving eight retrospective studies, Murthy et al. evaluated the risk of thromboembolic events (described as a composite outcome of MI and stroke) and the risk of recurrent ICH.8 They reported that resumption of therapeutic anticoagulation was associated with a decrease in the rate of thromboembolic events (6.7% vs. 17.6%; risk ratio, 0.35; 95% confidence interval, 0.25-0.45) with no significant change in the rate of repeat ICH (8.7% vs. 7.8%).
A second meta-analysis of three retrospective trials conducted by Biffi et al. examined anticoagulation resumption in 1,012 patients with ICH solely in the setting of thromboprophylaxis for nonvalvular atrial fibrillation.9 Reinitiation of anticoagulation after ICH was associated with decreased mortality (hazard ratio, 0.27; 95% CI, 0.19-0.40; P less than .0001), improved functional outcome (HR, 4.15; 95% CI, 2.92-5.90; P less than .0001), and reduction in all-cause stroke recurrence (HR 0.47; 95% CI, 0.36-0.64; P less than .0001). There was no significant difference in the rate of recurrent ICH when anticoagulation was resumed. Despite the notion that patients with cerebral amyloid angiopathy are at high risk of rebleeding, this positive association still held irrespective of lobar vs. nonlobar location of ICH.
Collectively, these studies suggest that resumption of anticoagulation may be effective in decreasing the rates of thromboembolism, as well as provide a functional and mortality benefit without increasing the risk of rebleeding, irrespective of the location of the bleed.
Less is known about the optimal timing of resumption of therapeutic anticoagulation, with data ranging from 72 hours to 30 weeks.10 The American Heart Association/American Stroke Association has a class IIB (very weak) recommendation to avoid anticoagulation for at least 4 weeks in patients without mechanical heart valves.4 The median time to resumption of therapeutic anticoagulation in aforementioned meta-analyses ranged from 10 to 44 days.8,9
A recent observational study of 2,619 ICH survivors explored the relationship between the timing of reinitiation of anticoagulation and the incidence of thrombotic events (defined as ischemic stroke or death because of MI or systemic arterial thromboembolism) and hemorrhagic events (defined as recurrent ICH or bleeding event leading to death) occurring at least 28 days after initial ICH in patients with atrial fibrillation.11
A decrease in thrombotic events was demonstrated if anticoagulation was started 4-16 weeks after ICH. However, when anticoagulation was started more than 16 weeks after ICH, no benefit was seen. Additionally, there was no significant difference in hemorrhagic events between men and women who resumed anticoagulation. In patients with high venous thromboembolism risk based on CHA2DS2-VASc score, resumption of anticoagulation was associated with a decreased predicted incidence of vascular death and nonfatal stroke, with the greatest benefit observed when anticoagulation was started at 7-8 weeks after ICH.
Unfortunately, published literature to date on anticoagulation after ICH is based entirely on retrospective studies – not randomized, controlled studies – making it more likely that anticoagulation would have been resumed in healthier patients, not those left debilitated by the ICH.
Furthermore, information on the location and size of the hemorrhages – which may serve as another confounding factor – often has not been reported. This is important since patients with smaller hemorrhages in less precarious areas also may be more likely to have resumption of anticoagulation. Another limitation of the current literature is that warfarin is the most common anticoagulant studied, with few studies involving the increasingly prescribed newer direct oral anticoagulants. It is also important to stress that a causal relationship between use of anticoagulants and certain outcomes or adverse effects following ICH may be more difficult to invoke in the absence of randomized controlled study designs.
Application of the data to our patient
Resumption of anticoagulation in our patient with ICH requires balancing the risk of hemorrhage expansion and recurrent ICH with the risk of thromboembolic disease.
Our patient is at higher risk of bleeding because of her advanced age, but adequate control of her blood pressure and nonlobar location of her ICH in the basal ganglia also may decrease her risk of recurrent ICH. Her high CHA2DS2-VASc score places her at high risk of thromboembolic event and stroke, making it more likely for reinitiation of anticoagulation to confer a mortality benefit.
Based on AHA guidelines,4 we should wait at least 4 weeks, or possibly wait until weeks 7-8 after ICH when the greatest benefit may be expected based on prediction models.11
Bottom line
It would likely be safe to resume anticoagulation 4-8 weeks after ICH in our patient.
Dr. Gibson, Dr. Restrepo, Dr. Sasidhara, and Dr. Manian are hospitalists at Massachusetts General Hospital, Boston.
References
1. An SJ et al. Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: An update. J Stroke. 2017 Jan;19:3-10.
2. Horstmann S et al. Intracerebral hemorrhage during anticoagulation with vitamin K antagonists: a consecutive observational study. J Neurol. 2013 Aug;260:2046-51.
3. Rosand J et al. The effect of warfarin and intensity of anticoagulation on outcome of intracerebral hemorrhage. Arch Intern Med. 2004 Apr 26;164:880-4.
4. Hemphill JC et al. Guidelines for the management of spontaneous intracerebral hemorrhage. Stroke. 2015 Jul;46:2032-60.
5. Aguillar MI et al. Update in intracerebral hemorrhage. Neurohospitalist. 2011;1:148-59.
6. Hill MD et al. Rate of stroke recurrence in patients with primary intracerebral hemorrhage. Stroke. 2000;31:123-7.
7. Steiner T et al. European Stroke Organization (ESO) guidelines for the management of spontaneous cerebral hemorrhage. Int J Stroke. 2014;9:840-55.
8. Murthy SB et al. Restarting anticoagulation therapy after intracranial hemorrhage: A systematic review and meta-analysis. Stroke. 2017 Jun;48:1594-600.
9. Biffi A et al. Oral anticoagulation and functional outcome after intracerebral hemorrhage. Ann Neurol. 2017 Nov;82:755-65.
10. Witt DM. What to do after the bleed: Resuming anticoagulation after major bleeding. Hematology Am Soc Hematol Educ Program. 2016 Dec 2;206:620-4.
11. Pennlert J et al. Optimal timing of anticoagulant treatment after intracerebral hemorrhage in patients with atrial fibrillation. Stroke. 2017 Feb;48:314-20.
Key Points
- Robust scientific data on when to resume anticoagulation after ICH does not exist.
- Retrospective studies have shown that anticoagulation resumption after 4-8 weeks decreases the risk of thromboembolic events, decreases mortality, and improves functional status following ICH with no significant change in the risk of its recurrence.
- Prospective, randomized controlled trials are needed to explore risks/benefits of anticoagulation resumption and better define its optimal timing in relation to ICH.
Quiz
Which of the following is false regarding ICH?
A. Lobar ICHs are usually associated with cerebral amyloid angiopathy which are prone to bleeding.
B. Randomized, controlled studies have helped guide the decision as to when to resume anticoagulation in patients with ICH.
C. Current guidelines suggest deferring therapeutic anticoagulation for at least 4 weeks following ICH.
D. Resumption of anticoagulation after 4-8 weeks does not lead to increased risk of rebleeding in patients with prior ICH.
The false answer is B: Current recommendations regarding resumption of anticoagulation in patients with ICH are based solely on retrospective observational studies; there are no randomized, control trials to date.
A is true: In contrast to hypertensive vessel disease associated with deep ICH, lobar hemorrhages are usually associated with cerebral amyloid angiopathy, which are more prone to bleeding.
C is true: The AHA/ASA has a class IIB recommendation to avoid anticoagulation for at least 4 weeks after ICH in patients without mechanical heart valves.
D is true: Several studies have shown that resumption of anticoagulation 4-8 weeks after ICH does not increase the risk of rebleeding.
Department of Medicine, Massachusetts General Hospital, Boston
Case
A 75 year-old woman with a history of hypertension, diabetes mellitus, heart failure and nonvalvular atrial fibrillation (CHA2DS2-VASc score, 8) on anticoagulation is admitted with weakness and dysarthria. Exam is notable for hypertension and right-sided hemiparesis. CT of the head shows an intraparenchymal hemorrhage in the left putamen. Her anticoagulation is reversed and blood pressure well controlled. She is discharged 12 days later.
Brief overview of the issue
Intracranial hemorrhage (ICH) is the second most common cause of stroke and is associated with high morbidity and mortality.1 It is estimated that 10%-15% of spontaneous ICH cases occur in patients on therapeutic anticoagulation for atrial fibrillation.2 As our population ages and more people develop atrial fibrillation, anticoagulation for primary or secondary prevention of embolic stroke also will likely increase, placing more people at risk for ICH. Even stringently controlled therapeutic international normalized ratios (INRs) between 2 and 3 may double the risk of ICH.3
Patients with ICH require close monitoring and treatment, including blood pressure control, reversal of anticoagulation, reduction of intracranial pressure and, at times, neurosurgery.4 Although anticoagulation is discontinued and reversed at the onset of ICH, no clear consensus exists as to when it is safe to resume it. Although anticoagulation decreases the risk of stroke/thromboembolism, it may also increase the amount of bleeding associated with the initial ICH or lead to its recurrence.
Factors that may contribute to rebleeding include uncontrolled hypertension, advanced age, time to resumption of anticoagulation, and lobar location of ICH (i.e., in cerebral cortex and/or underlying white matter).5 Traditionally, lobar ICH has high incidence of cerebral amyloid angiopathy and has been associated with higher bleeding rates than has deep ICH (i.e., involving the thalami, basal ganglia, cerebellum, or brainstem) where cerebral amyloid angiopathy is rare and ICH is usually from hypertensive vessel disease. However, in patients with active thromboembolic disease, high-risk atrial fibrillation, and mechanical valves, withholding anticoagulation could place them at high risk of stroke.
Two questions should be addressed in the case presented: Is it safe to restart therapeutic anticoagulation; and if so, what is the optimal time interval between ICH and reinitiation of anticoagulation?
Overview of the data
There is limited guidance from major professional societies regarding the reinitiation of anticoagulation and the optimal timing of safely resuming anticoagulation in patients with prior ICH.
Current European Stroke Organization guidelines provide no specific recommendations for anticoagulation resumption after ICH.7 The American Heart Association/American Stroke Association guideline has a class IIA (weak) recommendation to avoid anticoagulation in spontaneous lobar ICH and a class IIB (very weak) recommendation to consider resuming anticoagulation in nonlobar ICH on a case-by-case basis.4
Two recent meta-analyses have examined outcomes of resuming anticoagulation after ICH. In a meta-analysis of 5,300 patients with nonlobar ICH involving eight retrospective studies, Murthy et al. evaluated the risk of thromboembolic events (described as a composite outcome of MI and stroke) and the risk of recurrent ICH.8 They reported that resumption of therapeutic anticoagulation was associated with a decrease in the rate of thromboembolic events (6.7% vs. 17.6%; risk ratio, 0.35; 95% confidence interval, 0.25-0.45) with no significant change in the rate of repeat ICH (8.7% vs. 7.8%).
A second meta-analysis of three retrospective trials conducted by Biffi et al. examined anticoagulation resumption in 1,012 patients with ICH solely in the setting of thromboprophylaxis for nonvalvular atrial fibrillation.9 Reinitiation of anticoagulation after ICH was associated with decreased mortality (hazard ratio, 0.27; 95% CI, 0.19-0.40; P less than .0001), improved functional outcome (HR, 4.15; 95% CI, 2.92-5.90; P less than .0001), and reduction in all-cause stroke recurrence (HR 0.47; 95% CI, 0.36-0.64; P less than .0001). There was no significant difference in the rate of recurrent ICH when anticoagulation was resumed. Despite the notion that patients with cerebral amyloid angiopathy are at high risk of rebleeding, this positive association still held irrespective of lobar vs. nonlobar location of ICH.
Collectively, these studies suggest that resumption of anticoagulation may be effective in decreasing the rates of thromboembolism, as well as provide a functional and mortality benefit without increasing the risk of rebleeding, irrespective of the location of the bleed.
Less is known about the optimal timing of resumption of therapeutic anticoagulation, with data ranging from 72 hours to 30 weeks.10 The American Heart Association/American Stroke Association has a class IIB (very weak) recommendation to avoid anticoagulation for at least 4 weeks in patients without mechanical heart valves.4 The median time to resumption of therapeutic anticoagulation in aforementioned meta-analyses ranged from 10 to 44 days.8,9
A recent observational study of 2,619 ICH survivors explored the relationship between the timing of reinitiation of anticoagulation and the incidence of thrombotic events (defined as ischemic stroke or death because of MI or systemic arterial thromboembolism) and hemorrhagic events (defined as recurrent ICH or bleeding event leading to death) occurring at least 28 days after initial ICH in patients with atrial fibrillation.11
A decrease in thrombotic events was demonstrated if anticoagulation was started 4-16 weeks after ICH. However, when anticoagulation was started more than 16 weeks after ICH, no benefit was seen. Additionally, there was no significant difference in hemorrhagic events between men and women who resumed anticoagulation. In patients with high venous thromboembolism risk based on CHA2DS2-VASc score, resumption of anticoagulation was associated with a decreased predicted incidence of vascular death and nonfatal stroke, with the greatest benefit observed when anticoagulation was started at 7-8 weeks after ICH.
Unfortunately, published literature to date on anticoagulation after ICH is based entirely on retrospective studies – not randomized, controlled studies – making it more likely that anticoagulation would have been resumed in healthier patients, not those left debilitated by the ICH.
Furthermore, information on the location and size of the hemorrhages – which may serve as another confounding factor – often has not been reported. This is important since patients with smaller hemorrhages in less precarious areas also may be more likely to have resumption of anticoagulation. Another limitation of the current literature is that warfarin is the most common anticoagulant studied, with few studies involving the increasingly prescribed newer direct oral anticoagulants. It is also important to stress that a causal relationship between use of anticoagulants and certain outcomes or adverse effects following ICH may be more difficult to invoke in the absence of randomized controlled study designs.
Application of the data to our patient
Resumption of anticoagulation in our patient with ICH requires balancing the risk of hemorrhage expansion and recurrent ICH with the risk of thromboembolic disease.
Our patient is at higher risk of bleeding because of her advanced age, but adequate control of her blood pressure and nonlobar location of her ICH in the basal ganglia also may decrease her risk of recurrent ICH. Her high CHA2DS2-VASc score places her at high risk of thromboembolic event and stroke, making it more likely for reinitiation of anticoagulation to confer a mortality benefit.
Based on AHA guidelines,4 we should wait at least 4 weeks, or possibly wait until weeks 7-8 after ICH when the greatest benefit may be expected based on prediction models.11
Bottom line
It would likely be safe to resume anticoagulation 4-8 weeks after ICH in our patient.
Dr. Gibson, Dr. Restrepo, Dr. Sasidhara, and Dr. Manian are hospitalists at Massachusetts General Hospital, Boston.
References
1. An SJ et al. Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: An update. J Stroke. 2017 Jan;19:3-10.
2. Horstmann S et al. Intracerebral hemorrhage during anticoagulation with vitamin K antagonists: a consecutive observational study. J Neurol. 2013 Aug;260:2046-51.
3. Rosand J et al. The effect of warfarin and intensity of anticoagulation on outcome of intracerebral hemorrhage. Arch Intern Med. 2004 Apr 26;164:880-4.
4. Hemphill JC et al. Guidelines for the management of spontaneous intracerebral hemorrhage. Stroke. 2015 Jul;46:2032-60.
5. Aguillar MI et al. Update in intracerebral hemorrhage. Neurohospitalist. 2011;1:148-59.
6. Hill MD et al. Rate of stroke recurrence in patients with primary intracerebral hemorrhage. Stroke. 2000;31:123-7.
7. Steiner T et al. European Stroke Organization (ESO) guidelines for the management of spontaneous cerebral hemorrhage. Int J Stroke. 2014;9:840-55.
8. Murthy SB et al. Restarting anticoagulation therapy after intracranial hemorrhage: A systematic review and meta-analysis. Stroke. 2017 Jun;48:1594-600.
9. Biffi A et al. Oral anticoagulation and functional outcome after intracerebral hemorrhage. Ann Neurol. 2017 Nov;82:755-65.
10. Witt DM. What to do after the bleed: Resuming anticoagulation after major bleeding. Hematology Am Soc Hematol Educ Program. 2016 Dec 2;206:620-4.
11. Pennlert J et al. Optimal timing of anticoagulant treatment after intracerebral hemorrhage in patients with atrial fibrillation. Stroke. 2017 Feb;48:314-20.
Key Points
- Robust scientific data on when to resume anticoagulation after ICH does not exist.
- Retrospective studies have shown that anticoagulation resumption after 4-8 weeks decreases the risk of thromboembolic events, decreases mortality, and improves functional status following ICH with no significant change in the risk of its recurrence.
- Prospective, randomized controlled trials are needed to explore risks/benefits of anticoagulation resumption and better define its optimal timing in relation to ICH.
Quiz
Which of the following is false regarding ICH?
A. Lobar ICHs are usually associated with cerebral amyloid angiopathy which are prone to bleeding.
B. Randomized, controlled studies have helped guide the decision as to when to resume anticoagulation in patients with ICH.
C. Current guidelines suggest deferring therapeutic anticoagulation for at least 4 weeks following ICH.
D. Resumption of anticoagulation after 4-8 weeks does not lead to increased risk of rebleeding in patients with prior ICH.
The false answer is B: Current recommendations regarding resumption of anticoagulation in patients with ICH are based solely on retrospective observational studies; there are no randomized, control trials to date.
A is true: In contrast to hypertensive vessel disease associated with deep ICH, lobar hemorrhages are usually associated with cerebral amyloid angiopathy, which are more prone to bleeding.
C is true: The AHA/ASA has a class IIB recommendation to avoid anticoagulation for at least 4 weeks after ICH in patients without mechanical heart valves.
D is true: Several studies have shown that resumption of anticoagulation 4-8 weeks after ICH does not increase the risk of rebleeding.
New recall for CoaguChek test strips issued
According to a release, the Food and Drug Administration has identified this recall as Class I, which is the most serious type of recall and indicates that “use of these devices may cause serious injuries or death.”
These strips are used by patients taking warfarin to help determine the patients’ international normalized ratio, which doctors and patients then use to decide whether the dose is appropriate. Roche Diagnostics, the strips’ manufacturer, issued a recall in September 2018; the test strips distributed by Terrific Care and Medex, however, were not labeled or authorized for sale in the United States and were therefore not included in that original recall. According to the release, the strips in this recall, which was initiated Dec. 21, 2018, were purchased by Terrific Care and Medex from an unknown source and then distributed in the United States. On Jan. 28, 2019, Terrific Care sent an Urgent Medical Device Recall Notification Letter to customers.
The full recall is described on the FDA website.
According to a release, the Food and Drug Administration has identified this recall as Class I, which is the most serious type of recall and indicates that “use of these devices may cause serious injuries or death.”
These strips are used by patients taking warfarin to help determine the patients’ international normalized ratio, which doctors and patients then use to decide whether the dose is appropriate. Roche Diagnostics, the strips’ manufacturer, issued a recall in September 2018; the test strips distributed by Terrific Care and Medex, however, were not labeled or authorized for sale in the United States and were therefore not included in that original recall. According to the release, the strips in this recall, which was initiated Dec. 21, 2018, were purchased by Terrific Care and Medex from an unknown source and then distributed in the United States. On Jan. 28, 2019, Terrific Care sent an Urgent Medical Device Recall Notification Letter to customers.
The full recall is described on the FDA website.
According to a release, the Food and Drug Administration has identified this recall as Class I, which is the most serious type of recall and indicates that “use of these devices may cause serious injuries or death.”
These strips are used by patients taking warfarin to help determine the patients’ international normalized ratio, which doctors and patients then use to decide whether the dose is appropriate. Roche Diagnostics, the strips’ manufacturer, issued a recall in September 2018; the test strips distributed by Terrific Care and Medex, however, were not labeled or authorized for sale in the United States and were therefore not included in that original recall. According to the release, the strips in this recall, which was initiated Dec. 21, 2018, were purchased by Terrific Care and Medex from an unknown source and then distributed in the United States. On Jan. 28, 2019, Terrific Care sent an Urgent Medical Device Recall Notification Letter to customers.
The full recall is described on the FDA website.
Impaired clot lysis associated with mild bleeding symptoms
Patients with self-reported mild bleeding symptoms may have impaired clot lysis, according to investigators. This finding is remarkable because it contrasts with known bleeding disorders, such as hemophilia, which are associated with enhanced clot lysis, reported lead author Minka J.A. Vries, MD, of the Cardiovascular Research Institute Maastricht (CARIM) at Maastricht (the Netherlands) University and her colleagues.
The observational study, which included 335 patients undergoing elective surgery at Maastricht University Medical Center, was conducted to better understand lysis capacity, which is challenging to assess in a clinical setting. Although the Euglobulin Lysis Time (ELT) is often used in the clinic, it cannot determine the influence of hemostatic proteins or formation of a fibrin clot under physiological conditions.
“In the more recently developed lysis assays,” the investigators wrote in Thrombosis Research, “the turbidity lysis assay and the tissue plasminogen activator–rotational thromboelastometry (tPA-ROTEM) [assay], all plasma proteins are present and fibrin is formed under more physiological conditions for the measurement of fibrinolysis.” These two tests were used in the present study.
Of the 335 adult patients, 240 had self-reported mild bleeding symptoms, and 95 did not. Patients with bleeding disorders, thrombocytopenia, or anemia were excluded, as were pregnant women and those taking blood thinners or NSAIDs. Along with assessing time parameters of fibrinolysis, clot-associated proteins were measured for possible imbalances.
“We hypothesized that clot lysis capacity is enhanced in patients with mild bleeding symptoms,” the investigators wrote, based on other bleeding disorders. Surprisingly, the results told a different story.
After adjusting for sex, BMI, and age, patients with bleeding symptoms had lower tPA-ROTEM lysis speed (beta −0.35; P = .007) and longer tPA-ROTEM lysis time (beta 0.29; P = .022) than did patients without bleeding symptoms. The investigators found that tPA-ROTEM measurements depended on factor II, factor XII, alpha2-antiplasmin, plasminogen, thrombin activatable fibrinolysis inhibitor (TAFI), and plasminogen activator inhibitor–1 (PAI-1) level. In contrast, turbidity lysis assay measurements were not significantly different between groups. This latter assay was influenced by alpha2-antiplasmin, TAFI, and PAI-1.
“We did not find evidence for systemic hyperfibrinolytic capacity in patients reporting mild bleeding symptoms in comparison to patients not reporting bleeding symptoms,” the investigators concluded. “tPA-ROTEM even suggested a slower clot lysis in these patients. Though this may appear counterintuitive, our results are in line with two papers assessing systemic clot lysis in mild bleeders.”
While this phenomenon gains supporting evidence, it remains poorly understood.
“We have no good explanation for these findings,” the investigators noted.
This study was funded by the Sint Annadal Foundation Maastricht, Maastricht University Medical Centre, CTMM INCOAG Maastricht, Cardiovascular Research Institute Maastricht, and the British Heart Foundation. No conflicts of interest were reported.
SOURCE: Vries MJA et al. Thromb Res. 2018 Dec 4. doi: 10.1016/j.thromres.2018.12.004.
Patients with self-reported mild bleeding symptoms may have impaired clot lysis, according to investigators. This finding is remarkable because it contrasts with known bleeding disorders, such as hemophilia, which are associated with enhanced clot lysis, reported lead author Minka J.A. Vries, MD, of the Cardiovascular Research Institute Maastricht (CARIM) at Maastricht (the Netherlands) University and her colleagues.
The observational study, which included 335 patients undergoing elective surgery at Maastricht University Medical Center, was conducted to better understand lysis capacity, which is challenging to assess in a clinical setting. Although the Euglobulin Lysis Time (ELT) is often used in the clinic, it cannot determine the influence of hemostatic proteins or formation of a fibrin clot under physiological conditions.
“In the more recently developed lysis assays,” the investigators wrote in Thrombosis Research, “the turbidity lysis assay and the tissue plasminogen activator–rotational thromboelastometry (tPA-ROTEM) [assay], all plasma proteins are present and fibrin is formed under more physiological conditions for the measurement of fibrinolysis.” These two tests were used in the present study.
Of the 335 adult patients, 240 had self-reported mild bleeding symptoms, and 95 did not. Patients with bleeding disorders, thrombocytopenia, or anemia were excluded, as were pregnant women and those taking blood thinners or NSAIDs. Along with assessing time parameters of fibrinolysis, clot-associated proteins were measured for possible imbalances.
“We hypothesized that clot lysis capacity is enhanced in patients with mild bleeding symptoms,” the investigators wrote, based on other bleeding disorders. Surprisingly, the results told a different story.
After adjusting for sex, BMI, and age, patients with bleeding symptoms had lower tPA-ROTEM lysis speed (beta −0.35; P = .007) and longer tPA-ROTEM lysis time (beta 0.29; P = .022) than did patients without bleeding symptoms. The investigators found that tPA-ROTEM measurements depended on factor II, factor XII, alpha2-antiplasmin, plasminogen, thrombin activatable fibrinolysis inhibitor (TAFI), and plasminogen activator inhibitor–1 (PAI-1) level. In contrast, turbidity lysis assay measurements were not significantly different between groups. This latter assay was influenced by alpha2-antiplasmin, TAFI, and PAI-1.
“We did not find evidence for systemic hyperfibrinolytic capacity in patients reporting mild bleeding symptoms in comparison to patients not reporting bleeding symptoms,” the investigators concluded. “tPA-ROTEM even suggested a slower clot lysis in these patients. Though this may appear counterintuitive, our results are in line with two papers assessing systemic clot lysis in mild bleeders.”
While this phenomenon gains supporting evidence, it remains poorly understood.
“We have no good explanation for these findings,” the investigators noted.
This study was funded by the Sint Annadal Foundation Maastricht, Maastricht University Medical Centre, CTMM INCOAG Maastricht, Cardiovascular Research Institute Maastricht, and the British Heart Foundation. No conflicts of interest were reported.
SOURCE: Vries MJA et al. Thromb Res. 2018 Dec 4. doi: 10.1016/j.thromres.2018.12.004.
Patients with self-reported mild bleeding symptoms may have impaired clot lysis, according to investigators. This finding is remarkable because it contrasts with known bleeding disorders, such as hemophilia, which are associated with enhanced clot lysis, reported lead author Minka J.A. Vries, MD, of the Cardiovascular Research Institute Maastricht (CARIM) at Maastricht (the Netherlands) University and her colleagues.
The observational study, which included 335 patients undergoing elective surgery at Maastricht University Medical Center, was conducted to better understand lysis capacity, which is challenging to assess in a clinical setting. Although the Euglobulin Lysis Time (ELT) is often used in the clinic, it cannot determine the influence of hemostatic proteins or formation of a fibrin clot under physiological conditions.
“In the more recently developed lysis assays,” the investigators wrote in Thrombosis Research, “the turbidity lysis assay and the tissue plasminogen activator–rotational thromboelastometry (tPA-ROTEM) [assay], all plasma proteins are present and fibrin is formed under more physiological conditions for the measurement of fibrinolysis.” These two tests were used in the present study.
Of the 335 adult patients, 240 had self-reported mild bleeding symptoms, and 95 did not. Patients with bleeding disorders, thrombocytopenia, or anemia were excluded, as were pregnant women and those taking blood thinners or NSAIDs. Along with assessing time parameters of fibrinolysis, clot-associated proteins were measured for possible imbalances.
“We hypothesized that clot lysis capacity is enhanced in patients with mild bleeding symptoms,” the investigators wrote, based on other bleeding disorders. Surprisingly, the results told a different story.
After adjusting for sex, BMI, and age, patients with bleeding symptoms had lower tPA-ROTEM lysis speed (beta −0.35; P = .007) and longer tPA-ROTEM lysis time (beta 0.29; P = .022) than did patients without bleeding symptoms. The investigators found that tPA-ROTEM measurements depended on factor II, factor XII, alpha2-antiplasmin, plasminogen, thrombin activatable fibrinolysis inhibitor (TAFI), and plasminogen activator inhibitor–1 (PAI-1) level. In contrast, turbidity lysis assay measurements were not significantly different between groups. This latter assay was influenced by alpha2-antiplasmin, TAFI, and PAI-1.
“We did not find evidence for systemic hyperfibrinolytic capacity in patients reporting mild bleeding symptoms in comparison to patients not reporting bleeding symptoms,” the investigators concluded. “tPA-ROTEM even suggested a slower clot lysis in these patients. Though this may appear counterintuitive, our results are in line with two papers assessing systemic clot lysis in mild bleeders.”
While this phenomenon gains supporting evidence, it remains poorly understood.
“We have no good explanation for these findings,” the investigators noted.
This study was funded by the Sint Annadal Foundation Maastricht, Maastricht University Medical Centre, CTMM INCOAG Maastricht, Cardiovascular Research Institute Maastricht, and the British Heart Foundation. No conflicts of interest were reported.
SOURCE: Vries MJA et al. Thromb Res. 2018 Dec 4. doi: 10.1016/j.thromres.2018.12.004.
FROM THROMBOSIS RESEARCH
Key clinical point: Patients with self-reported mild bleeding symptoms may have impaired clot lysis, in contrast with known bleeding disorders.
Major finding: Patients with mild bleeding had longer whole blood tissue plasminogen activator-rotational thromboelastometry lysis times (P = .022) than did patients without symptoms.
Study details: An observational study of 335 adult patients undergoing elective surgery.
Disclosures: This study was funded by the Sint Annadal Foundation, Maastricht University Medical Center, CTMM INCOAG Maastricht, Cardiovascular Research Institute Maastricht, and the British Heart Foundation. No conflicts of interest were reported.
Source: Vries MJA et al. Thromb Res. 2018 Dec 4. doi: 10.1016/j.thromres.2018.12.004.
COPD linked to higher in-hospital death rates in patients with PAD
A growing body of evidence suggests that, along with other vascular beds, smoking and chronic obstructive pulmonary disease (COPD) affect the arteries of the lower limbs in terms of the development of peripheral arterial disease (PAD), reported Karsten Keller, MD, of the Johannes Gutenberg-University Mainz (Germany) and his colleagues.
This provided the rationale for their large database analysis of inpatients with concomitant COPD and PAD. They found that the additional presence of COPD was associated with increased in-hospital mortality in patients with PAD.
“Our data suggest that COPD increased the mortality of PAD patients by the factor 1.2-fold,” they wrote in Respiratory Medicine. “Unexpectedly, this increase was not driven by [myocardial infarction] as the life-threatening acute presentation of [coronary artery disease], but rather related to an increased risk for [pulmonary embolism] and a higher coprevalence of cancer.”
Dr. Keller and his colleagues inspected the German inpatient national database based on ICD codes. They identified 5,611,827 adult inpatients (64.8% men) diagnosed with PAD between January 2005 and December 2015, and of those, 13.6% also were coded for COPD. Overall, 277,894 PAD patients (5.0%) died in the hospital, Dr. Keller and his colleagues wrote.
The all-cause, in-hospital mortality was significantly higher in PAD patients with COPD, compared with those without COPD (6.5% vs. 4.7%, respectively; P less than .001), and cardiovascular events comprising pulmonary embolism (PE), deep vein thrombosis (DVT), and myocardial infarction (MI) occurred more often in coprevalence with PAD and COPD than in PAD without COPD.
In PAD patients, COPD was an independent predictor of in-hospital death (odds ratio, 1.16; 95% confidence interval, 1.15-1.17; P less than .001) as well as an independent predictor for PE (OR, 1.44; 95% CI, 1.40-1.49; P less than .001).
Overall, PAD patients with COPD were of similar age as (73 years), but stayed slightly longer in the hospital than (9 vs. 8 days), those without COPD. PAD patients without COPD revealed more often cardiovascular risk factors like essential arterial hypertension and diabetes, but the prevalence of cardiovascular diseases such as coronary artery disease and heart failure were more often found in PAD patients with COPD. In addition, cancer and renal insufficiency also were more common in PAD patients with COPD, according to the authors.
“Remarkably, PAD patients with COPD showed more frequently lower PAD stages than those without COPD. Especially, PAD stage IV was more prevalent in PAD patients without COPD (19.6% vs. 13.8%; P less than 0.001),” the authors stated. In addition, amputations were more often performed in PAD patients without COPD.
Dr. Keller and his colleagues had the following conclusions regarding the clinical implications of their study: “I) PAD patients with long-standing tobacco use might benefit from COPD screening and treatment. II) PAD patients with additional COPD should be monitored more intensively, and the treatment for COPD should be optimized. III) COPD increases the risk for PE, and it is critical not to overlook this life-threatening disease. IV) MI and PE are important causes of in-hospital death in PAD patients with and without COPD.”
The German Federal Ministry of Education and Research funded the study, and the authors reported having no conflicts.
SOURCE: Keller K et al. Respir Med. 2019 Feb;147:1-6.
A growing body of evidence suggests that, along with other vascular beds, smoking and chronic obstructive pulmonary disease (COPD) affect the arteries of the lower limbs in terms of the development of peripheral arterial disease (PAD), reported Karsten Keller, MD, of the Johannes Gutenberg-University Mainz (Germany) and his colleagues.
This provided the rationale for their large database analysis of inpatients with concomitant COPD and PAD. They found that the additional presence of COPD was associated with increased in-hospital mortality in patients with PAD.
“Our data suggest that COPD increased the mortality of PAD patients by the factor 1.2-fold,” they wrote in Respiratory Medicine. “Unexpectedly, this increase was not driven by [myocardial infarction] as the life-threatening acute presentation of [coronary artery disease], but rather related to an increased risk for [pulmonary embolism] and a higher coprevalence of cancer.”
Dr. Keller and his colleagues inspected the German inpatient national database based on ICD codes. They identified 5,611,827 adult inpatients (64.8% men) diagnosed with PAD between January 2005 and December 2015, and of those, 13.6% also were coded for COPD. Overall, 277,894 PAD patients (5.0%) died in the hospital, Dr. Keller and his colleagues wrote.
The all-cause, in-hospital mortality was significantly higher in PAD patients with COPD, compared with those without COPD (6.5% vs. 4.7%, respectively; P less than .001), and cardiovascular events comprising pulmonary embolism (PE), deep vein thrombosis (DVT), and myocardial infarction (MI) occurred more often in coprevalence with PAD and COPD than in PAD without COPD.
In PAD patients, COPD was an independent predictor of in-hospital death (odds ratio, 1.16; 95% confidence interval, 1.15-1.17; P less than .001) as well as an independent predictor for PE (OR, 1.44; 95% CI, 1.40-1.49; P less than .001).
Overall, PAD patients with COPD were of similar age as (73 years), but stayed slightly longer in the hospital than (9 vs. 8 days), those without COPD. PAD patients without COPD revealed more often cardiovascular risk factors like essential arterial hypertension and diabetes, but the prevalence of cardiovascular diseases such as coronary artery disease and heart failure were more often found in PAD patients with COPD. In addition, cancer and renal insufficiency also were more common in PAD patients with COPD, according to the authors.
“Remarkably, PAD patients with COPD showed more frequently lower PAD stages than those without COPD. Especially, PAD stage IV was more prevalent in PAD patients without COPD (19.6% vs. 13.8%; P less than 0.001),” the authors stated. In addition, amputations were more often performed in PAD patients without COPD.
Dr. Keller and his colleagues had the following conclusions regarding the clinical implications of their study: “I) PAD patients with long-standing tobacco use might benefit from COPD screening and treatment. II) PAD patients with additional COPD should be monitored more intensively, and the treatment for COPD should be optimized. III) COPD increases the risk for PE, and it is critical not to overlook this life-threatening disease. IV) MI and PE are important causes of in-hospital death in PAD patients with and without COPD.”
The German Federal Ministry of Education and Research funded the study, and the authors reported having no conflicts.
SOURCE: Keller K et al. Respir Med. 2019 Feb;147:1-6.
A growing body of evidence suggests that, along with other vascular beds, smoking and chronic obstructive pulmonary disease (COPD) affect the arteries of the lower limbs in terms of the development of peripheral arterial disease (PAD), reported Karsten Keller, MD, of the Johannes Gutenberg-University Mainz (Germany) and his colleagues.
This provided the rationale for their large database analysis of inpatients with concomitant COPD and PAD. They found that the additional presence of COPD was associated with increased in-hospital mortality in patients with PAD.
“Our data suggest that COPD increased the mortality of PAD patients by the factor 1.2-fold,” they wrote in Respiratory Medicine. “Unexpectedly, this increase was not driven by [myocardial infarction] as the life-threatening acute presentation of [coronary artery disease], but rather related to an increased risk for [pulmonary embolism] and a higher coprevalence of cancer.”
Dr. Keller and his colleagues inspected the German inpatient national database based on ICD codes. They identified 5,611,827 adult inpatients (64.8% men) diagnosed with PAD between January 2005 and December 2015, and of those, 13.6% also were coded for COPD. Overall, 277,894 PAD patients (5.0%) died in the hospital, Dr. Keller and his colleagues wrote.
The all-cause, in-hospital mortality was significantly higher in PAD patients with COPD, compared with those without COPD (6.5% vs. 4.7%, respectively; P less than .001), and cardiovascular events comprising pulmonary embolism (PE), deep vein thrombosis (DVT), and myocardial infarction (MI) occurred more often in coprevalence with PAD and COPD than in PAD without COPD.
In PAD patients, COPD was an independent predictor of in-hospital death (odds ratio, 1.16; 95% confidence interval, 1.15-1.17; P less than .001) as well as an independent predictor for PE (OR, 1.44; 95% CI, 1.40-1.49; P less than .001).
Overall, PAD patients with COPD were of similar age as (73 years), but stayed slightly longer in the hospital than (9 vs. 8 days), those without COPD. PAD patients without COPD revealed more often cardiovascular risk factors like essential arterial hypertension and diabetes, but the prevalence of cardiovascular diseases such as coronary artery disease and heart failure were more often found in PAD patients with COPD. In addition, cancer and renal insufficiency also were more common in PAD patients with COPD, according to the authors.
“Remarkably, PAD patients with COPD showed more frequently lower PAD stages than those without COPD. Especially, PAD stage IV was more prevalent in PAD patients without COPD (19.6% vs. 13.8%; P less than 0.001),” the authors stated. In addition, amputations were more often performed in PAD patients without COPD.
Dr. Keller and his colleagues had the following conclusions regarding the clinical implications of their study: “I) PAD patients with long-standing tobacco use might benefit from COPD screening and treatment. II) PAD patients with additional COPD should be monitored more intensively, and the treatment for COPD should be optimized. III) COPD increases the risk for PE, and it is critical not to overlook this life-threatening disease. IV) MI and PE are important causes of in-hospital death in PAD patients with and without COPD.”
The German Federal Ministry of Education and Research funded the study, and the authors reported having no conflicts.
SOURCE: Keller K et al. Respir Med. 2019 Feb;147:1-6.
FROM RESPIRATORY MEDICINE
Key clinical point:
Major finding: All-cause, in-hospital mortality was significantly higher in PAD patients with COPD, compared with those without (6.5% vs. 4.7%; P less than 0.001).
Study details: Database analysis of 5.6 million German PAD inpatients stratified for COPD.
Disclosures: The German Federal Ministry of Education and Research funded the study, and the authors reported having no conflicts.
Source: Keller K et al. Respir Med. 2019 Feb;147:1-6.
CTPA may not rule out VTE in high-risk patients
Clinical question: Does a negative computed tomography pulmonary angiography rule out venous thromboembolism (VTE)?
Background: Computed tomography pulmonary angiography (CTPA) is the most common diagnostic modality used to diagnose pulmonary embolism (PE) and has a high negative predictive value in patients with a low 3-month risk of VTE. In patients with higher pretest probability of PE, it is unknown whether CTPA is sufficient to rule out VTE.
Study design: Meta-analysis.
Setting: Published prospective outcome studies of patients with suspected PE using CTPA as a diagnostic strategy.
Synopsis: The authors reviewed 3,143 publications from MEDLINE, EMBASE, and the Cochrane Library and identified 22 prospective outcome studies to include in their meta-analysis. A VTE was diagnosed in 3,923 out of 11,872 participants (33%) using CTPA. Of the 7,863 patients with a negative CTPA, 148 patients had an acute VTE confirmed by venous ultrasound, ventilation/perfusion scan, or angiography, and 74 patients experienced VTE during a 3-month follow-up period, yielding an overall proportion of 2.4% of patients (95% confidence interval, 1.3%-3.8%).
Subgroup analysis showed that cumulative occurrence of VTE was related to pretest prevalence. In the subgroup of patients with a VTE prevalence greater than 40%, VTE was observed in 8.1% of patients with a negative CTPA (95% CI, 3.4%-14.5%).
Bottom line: CTPA may be insufficient to rule out VTE in patients with a high pretest probability of PE.
Citation: Belzile D et al. Outcomes following a negative computed tomography pulmonary angiography according to pulmonary embolism prevalence: a meta-analysisof the management outcome studies. J Thromb Haemost. 2018 Jun;16(6):1107-20.
Dr. Jenkins is assistant professor of medicine and an academic hospitalist, University of Utah, Salt Lake City.
Clinical question: Does a negative computed tomography pulmonary angiography rule out venous thromboembolism (VTE)?
Background: Computed tomography pulmonary angiography (CTPA) is the most common diagnostic modality used to diagnose pulmonary embolism (PE) and has a high negative predictive value in patients with a low 3-month risk of VTE. In patients with higher pretest probability of PE, it is unknown whether CTPA is sufficient to rule out VTE.
Study design: Meta-analysis.
Setting: Published prospective outcome studies of patients with suspected PE using CTPA as a diagnostic strategy.
Synopsis: The authors reviewed 3,143 publications from MEDLINE, EMBASE, and the Cochrane Library and identified 22 prospective outcome studies to include in their meta-analysis. A VTE was diagnosed in 3,923 out of 11,872 participants (33%) using CTPA. Of the 7,863 patients with a negative CTPA, 148 patients had an acute VTE confirmed by venous ultrasound, ventilation/perfusion scan, or angiography, and 74 patients experienced VTE during a 3-month follow-up period, yielding an overall proportion of 2.4% of patients (95% confidence interval, 1.3%-3.8%).
Subgroup analysis showed that cumulative occurrence of VTE was related to pretest prevalence. In the subgroup of patients with a VTE prevalence greater than 40%, VTE was observed in 8.1% of patients with a negative CTPA (95% CI, 3.4%-14.5%).
Bottom line: CTPA may be insufficient to rule out VTE in patients with a high pretest probability of PE.
Citation: Belzile D et al. Outcomes following a negative computed tomography pulmonary angiography according to pulmonary embolism prevalence: a meta-analysisof the management outcome studies. J Thromb Haemost. 2018 Jun;16(6):1107-20.
Dr. Jenkins is assistant professor of medicine and an academic hospitalist, University of Utah, Salt Lake City.
Clinical question: Does a negative computed tomography pulmonary angiography rule out venous thromboembolism (VTE)?
Background: Computed tomography pulmonary angiography (CTPA) is the most common diagnostic modality used to diagnose pulmonary embolism (PE) and has a high negative predictive value in patients with a low 3-month risk of VTE. In patients with higher pretest probability of PE, it is unknown whether CTPA is sufficient to rule out VTE.
Study design: Meta-analysis.
Setting: Published prospective outcome studies of patients with suspected PE using CTPA as a diagnostic strategy.
Synopsis: The authors reviewed 3,143 publications from MEDLINE, EMBASE, and the Cochrane Library and identified 22 prospective outcome studies to include in their meta-analysis. A VTE was diagnosed in 3,923 out of 11,872 participants (33%) using CTPA. Of the 7,863 patients with a negative CTPA, 148 patients had an acute VTE confirmed by venous ultrasound, ventilation/perfusion scan, or angiography, and 74 patients experienced VTE during a 3-month follow-up period, yielding an overall proportion of 2.4% of patients (95% confidence interval, 1.3%-3.8%).
Subgroup analysis showed that cumulative occurrence of VTE was related to pretest prevalence. In the subgroup of patients with a VTE prevalence greater than 40%, VTE was observed in 8.1% of patients with a negative CTPA (95% CI, 3.4%-14.5%).
Bottom line: CTPA may be insufficient to rule out VTE in patients with a high pretest probability of PE.
Citation: Belzile D et al. Outcomes following a negative computed tomography pulmonary angiography according to pulmonary embolism prevalence: a meta-analysisof the management outcome studies. J Thromb Haemost. 2018 Jun;16(6):1107-20.
Dr. Jenkins is assistant professor of medicine and an academic hospitalist, University of Utah, Salt Lake City.