User login
Ischemic stroke rates higher in young women than young men
Young women appear to be at a higher risk of ischemic stroke than young men, according to a new systematic review of studies on this topic.
The review included 19 studies that reported on sex-specific stroke incidence among young adults and found that overall, in young adults aged 18-35 years, there were 44% more women with ischemic strokes than men.
This gap narrowed in the age group 35-45 years, for which there was conflicting evidence whether more men or women have ischemic strokes.
“An assertion that young women may be disproportionately at risk of ischemic stroke represents a significant departure from our current scientific understanding and may have important implications about the etiology of ischemic strokes in young adults,” the authors note.
“One of the take-home messages from this study is that stroke happens across the entire age spectrum, including young adults, even if they do not have traditional risk factors,” study coauthor Sharon N. Poisson, MD, associate professor of neurology at the University of Colorado Anschutz Medical Campus, Denver, told this news organization.
“If a young person presents with focal neurological symptoms, the possibility of a stroke should not be discounted just because they may not fit the typical profile of a stroke patient. We need more education of the population that young people – including young women – can have a stroke and that fast action to call emergency services is critical,” she said.
The study was published online Jan. 24 in the journal Stroke as part of a special “Go Red for Women” spotlight issue.
The researchers note that historically it has been believed that men have a higher incidence of stroke in every age group until very old age. However, recent evidence focused on the young adult age group has reported that there are more young women (ages 18-45) with ischemic strokes compared with young men, suggesting that young women may be disproportionately at risk compared with their male counterparts.
Pointing out that a better understanding of these sex differences is important in implementing strategies that can more effectively prevent and treat strokes in this age group, the researchers conducted the current review to synthesize the updated evidence.
They searched PubMed from January 2008 to July 2021 for relevant studies that were population-based and reported stroke incidence by sex or sex-specific incidence rate ratios of young adults age 45 and younger. Statistical synthesis was performed to estimate sex difference by age group (less than or equal to 35, 35-45 and less than or equal to 45 years) and stroke type.
They found 19 relevant studies, including three that reported on overlapping data, with a total of 69,793 young adults (33,775 women and 36,018 men).
Nine studies did not show a statistically significant sex difference among young adults less than or equal to 45 years. Three studies found higher rates of ischemic stroke among men among young adults less than or equal to 30 to 35 years. Four studies showed more women with ischemic strokes among young adults less than or equal to 35 years.
Overall, there was an effect of a significantly higher incidence of ischemic stroke in women younger than age 35 years, with an incidence rate ratio (IRR) of 1.44. In the 35- to 45-year age group, there was a nonsignificant sex difference in the rate of ischemic stroke, with a slight trend toward a higher incidence in women (IRR, 1.08).
“In this study the sex difference was not clear in the 35-45 age group. But in the age group of over 45 years we know that men have a higher risk of stroke than women, which is probably related to a higher level of atherosclerotic risk factors,” Dr. Poisson commented.
“Interpreting data on stroke in young people is challenging, as stroke is not so common in this population,” she said. “Combining multiple studies helps, but this also introduces a lot of variability, so we need to interpret these results with some caution. However, this is certainly intriguing data and suggests that something interesting may be going on in young adults,” she added. “These observations give us an initial clue that we need to look further into this issue.”
The study did not look at the possible mechanisms behind the results, as the current data came from administrative datasets that are limited in terms of the information collected.
But Dr. Poisson noted that the traditional risk factors for stroke are high blood pressure and the usual atherosclerotic factors such as high cholesterol.
“These are normally more common in men than in women, and myocardial infarction is more common in younger men than in younger women. But the observation that young women may have a higher risk of stroke than young men suggests that something different may be going on in the mechanism for stroke.”
She pointed out that women have some unique risk factors for stroke, including oral contraceptive use, pregnancy, and the postpartum period, particularly pre-eclampsia during pregnancy. In addition, migraine, especially migraine with aura, is associated with an increased stroke risk, and migraine is more common in young women than in young men.
“We don’t completely understand the role of these risk factors, but they may contribute to the results that we found,” Dr. Poisson commented. “The role of estrogen in stroke is complicated. While estrogen is generally thought to be protective against atherosclerotic risk factors, it also increases risk of clotting, so high estrogen states like pregnancy increase risk of stroke,” she added.
To better understand what is happening, prospectively collected clinical data on younger patients who have had a stroke are needed. Some such studies are underway, but a concerted effort to do this in a large, multicenter registry would be desirable, Dr. Poisson said.
She noted that the presentation of a stroke in young people would be similar to that in the older population, with the most recent acronym to help recognize stroke symptoms being “BE FAST” – balance, eyes (vision), face (drooping), arm, speech (slurred), time (call emergency services quickly).
Call for more women in clinical trials
In an accompanying commentary, Cheryl Bushnell, MD, professor of neurology at Wake Forest School of Medicine, Winston-Salem, N.C., and Moira Kapral, MD, professor in medicine and health policy at the University of Toronto, say these findings support the need for further study to understand and address the causes and risk factors of stroke in young women.
However, they point out that representation and reporting of women in clinical trials of acute stroke continues to be suboptimal, and they call for improved incorporation of sex and gender into study design, analysis, and interpretation, which they say is critical for producing research that is broadly generalizable and applicable to different populations.
Coauthor Stacey L. Daugherty, MD, is funded by the National Institutes of Health. Dr. Poisson and Dr. Kapral have disclosed no relevant financial relationships. Dr. Bushnell reports ownership interest in Care Directions.
A version of this article first appeared on Medscape.com.
Young women appear to be at a higher risk of ischemic stroke than young men, according to a new systematic review of studies on this topic.
The review included 19 studies that reported on sex-specific stroke incidence among young adults and found that overall, in young adults aged 18-35 years, there were 44% more women with ischemic strokes than men.
This gap narrowed in the age group 35-45 years, for which there was conflicting evidence whether more men or women have ischemic strokes.
“An assertion that young women may be disproportionately at risk of ischemic stroke represents a significant departure from our current scientific understanding and may have important implications about the etiology of ischemic strokes in young adults,” the authors note.
“One of the take-home messages from this study is that stroke happens across the entire age spectrum, including young adults, even if they do not have traditional risk factors,” study coauthor Sharon N. Poisson, MD, associate professor of neurology at the University of Colorado Anschutz Medical Campus, Denver, told this news organization.
“If a young person presents with focal neurological symptoms, the possibility of a stroke should not be discounted just because they may not fit the typical profile of a stroke patient. We need more education of the population that young people – including young women – can have a stroke and that fast action to call emergency services is critical,” she said.
The study was published online Jan. 24 in the journal Stroke as part of a special “Go Red for Women” spotlight issue.
The researchers note that historically it has been believed that men have a higher incidence of stroke in every age group until very old age. However, recent evidence focused on the young adult age group has reported that there are more young women (ages 18-45) with ischemic strokes compared with young men, suggesting that young women may be disproportionately at risk compared with their male counterparts.
Pointing out that a better understanding of these sex differences is important in implementing strategies that can more effectively prevent and treat strokes in this age group, the researchers conducted the current review to synthesize the updated evidence.
They searched PubMed from January 2008 to July 2021 for relevant studies that were population-based and reported stroke incidence by sex or sex-specific incidence rate ratios of young adults age 45 and younger. Statistical synthesis was performed to estimate sex difference by age group (less than or equal to 35, 35-45 and less than or equal to 45 years) and stroke type.
They found 19 relevant studies, including three that reported on overlapping data, with a total of 69,793 young adults (33,775 women and 36,018 men).
Nine studies did not show a statistically significant sex difference among young adults less than or equal to 45 years. Three studies found higher rates of ischemic stroke among men among young adults less than or equal to 30 to 35 years. Four studies showed more women with ischemic strokes among young adults less than or equal to 35 years.
Overall, there was an effect of a significantly higher incidence of ischemic stroke in women younger than age 35 years, with an incidence rate ratio (IRR) of 1.44. In the 35- to 45-year age group, there was a nonsignificant sex difference in the rate of ischemic stroke, with a slight trend toward a higher incidence in women (IRR, 1.08).
“In this study the sex difference was not clear in the 35-45 age group. But in the age group of over 45 years we know that men have a higher risk of stroke than women, which is probably related to a higher level of atherosclerotic risk factors,” Dr. Poisson commented.
“Interpreting data on stroke in young people is challenging, as stroke is not so common in this population,” she said. “Combining multiple studies helps, but this also introduces a lot of variability, so we need to interpret these results with some caution. However, this is certainly intriguing data and suggests that something interesting may be going on in young adults,” she added. “These observations give us an initial clue that we need to look further into this issue.”
The study did not look at the possible mechanisms behind the results, as the current data came from administrative datasets that are limited in terms of the information collected.
But Dr. Poisson noted that the traditional risk factors for stroke are high blood pressure and the usual atherosclerotic factors such as high cholesterol.
“These are normally more common in men than in women, and myocardial infarction is more common in younger men than in younger women. But the observation that young women may have a higher risk of stroke than young men suggests that something different may be going on in the mechanism for stroke.”
She pointed out that women have some unique risk factors for stroke, including oral contraceptive use, pregnancy, and the postpartum period, particularly pre-eclampsia during pregnancy. In addition, migraine, especially migraine with aura, is associated with an increased stroke risk, and migraine is more common in young women than in young men.
“We don’t completely understand the role of these risk factors, but they may contribute to the results that we found,” Dr. Poisson commented. “The role of estrogen in stroke is complicated. While estrogen is generally thought to be protective against atherosclerotic risk factors, it also increases risk of clotting, so high estrogen states like pregnancy increase risk of stroke,” she added.
To better understand what is happening, prospectively collected clinical data on younger patients who have had a stroke are needed. Some such studies are underway, but a concerted effort to do this in a large, multicenter registry would be desirable, Dr. Poisson said.
She noted that the presentation of a stroke in young people would be similar to that in the older population, with the most recent acronym to help recognize stroke symptoms being “BE FAST” – balance, eyes (vision), face (drooping), arm, speech (slurred), time (call emergency services quickly).
Call for more women in clinical trials
In an accompanying commentary, Cheryl Bushnell, MD, professor of neurology at Wake Forest School of Medicine, Winston-Salem, N.C., and Moira Kapral, MD, professor in medicine and health policy at the University of Toronto, say these findings support the need for further study to understand and address the causes and risk factors of stroke in young women.
However, they point out that representation and reporting of women in clinical trials of acute stroke continues to be suboptimal, and they call for improved incorporation of sex and gender into study design, analysis, and interpretation, which they say is critical for producing research that is broadly generalizable and applicable to different populations.
Coauthor Stacey L. Daugherty, MD, is funded by the National Institutes of Health. Dr. Poisson and Dr. Kapral have disclosed no relevant financial relationships. Dr. Bushnell reports ownership interest in Care Directions.
A version of this article first appeared on Medscape.com.
Young women appear to be at a higher risk of ischemic stroke than young men, according to a new systematic review of studies on this topic.
The review included 19 studies that reported on sex-specific stroke incidence among young adults and found that overall, in young adults aged 18-35 years, there were 44% more women with ischemic strokes than men.
This gap narrowed in the age group 35-45 years, for which there was conflicting evidence whether more men or women have ischemic strokes.
“An assertion that young women may be disproportionately at risk of ischemic stroke represents a significant departure from our current scientific understanding and may have important implications about the etiology of ischemic strokes in young adults,” the authors note.
“One of the take-home messages from this study is that stroke happens across the entire age spectrum, including young adults, even if they do not have traditional risk factors,” study coauthor Sharon N. Poisson, MD, associate professor of neurology at the University of Colorado Anschutz Medical Campus, Denver, told this news organization.
“If a young person presents with focal neurological symptoms, the possibility of a stroke should not be discounted just because they may not fit the typical profile of a stroke patient. We need more education of the population that young people – including young women – can have a stroke and that fast action to call emergency services is critical,” she said.
The study was published online Jan. 24 in the journal Stroke as part of a special “Go Red for Women” spotlight issue.
The researchers note that historically it has been believed that men have a higher incidence of stroke in every age group until very old age. However, recent evidence focused on the young adult age group has reported that there are more young women (ages 18-45) with ischemic strokes compared with young men, suggesting that young women may be disproportionately at risk compared with their male counterparts.
Pointing out that a better understanding of these sex differences is important in implementing strategies that can more effectively prevent and treat strokes in this age group, the researchers conducted the current review to synthesize the updated evidence.
They searched PubMed from January 2008 to July 2021 for relevant studies that were population-based and reported stroke incidence by sex or sex-specific incidence rate ratios of young adults age 45 and younger. Statistical synthesis was performed to estimate sex difference by age group (less than or equal to 35, 35-45 and less than or equal to 45 years) and stroke type.
They found 19 relevant studies, including three that reported on overlapping data, with a total of 69,793 young adults (33,775 women and 36,018 men).
Nine studies did not show a statistically significant sex difference among young adults less than or equal to 45 years. Three studies found higher rates of ischemic stroke among men among young adults less than or equal to 30 to 35 years. Four studies showed more women with ischemic strokes among young adults less than or equal to 35 years.
Overall, there was an effect of a significantly higher incidence of ischemic stroke in women younger than age 35 years, with an incidence rate ratio (IRR) of 1.44. In the 35- to 45-year age group, there was a nonsignificant sex difference in the rate of ischemic stroke, with a slight trend toward a higher incidence in women (IRR, 1.08).
“In this study the sex difference was not clear in the 35-45 age group. But in the age group of over 45 years we know that men have a higher risk of stroke than women, which is probably related to a higher level of atherosclerotic risk factors,” Dr. Poisson commented.
“Interpreting data on stroke in young people is challenging, as stroke is not so common in this population,” she said. “Combining multiple studies helps, but this also introduces a lot of variability, so we need to interpret these results with some caution. However, this is certainly intriguing data and suggests that something interesting may be going on in young adults,” she added. “These observations give us an initial clue that we need to look further into this issue.”
The study did not look at the possible mechanisms behind the results, as the current data came from administrative datasets that are limited in terms of the information collected.
But Dr. Poisson noted that the traditional risk factors for stroke are high blood pressure and the usual atherosclerotic factors such as high cholesterol.
“These are normally more common in men than in women, and myocardial infarction is more common in younger men than in younger women. But the observation that young women may have a higher risk of stroke than young men suggests that something different may be going on in the mechanism for stroke.”
She pointed out that women have some unique risk factors for stroke, including oral contraceptive use, pregnancy, and the postpartum period, particularly pre-eclampsia during pregnancy. In addition, migraine, especially migraine with aura, is associated with an increased stroke risk, and migraine is more common in young women than in young men.
“We don’t completely understand the role of these risk factors, but they may contribute to the results that we found,” Dr. Poisson commented. “The role of estrogen in stroke is complicated. While estrogen is generally thought to be protective against atherosclerotic risk factors, it also increases risk of clotting, so high estrogen states like pregnancy increase risk of stroke,” she added.
To better understand what is happening, prospectively collected clinical data on younger patients who have had a stroke are needed. Some such studies are underway, but a concerted effort to do this in a large, multicenter registry would be desirable, Dr. Poisson said.
She noted that the presentation of a stroke in young people would be similar to that in the older population, with the most recent acronym to help recognize stroke symptoms being “BE FAST” – balance, eyes (vision), face (drooping), arm, speech (slurred), time (call emergency services quickly).
Call for more women in clinical trials
In an accompanying commentary, Cheryl Bushnell, MD, professor of neurology at Wake Forest School of Medicine, Winston-Salem, N.C., and Moira Kapral, MD, professor in medicine and health policy at the University of Toronto, say these findings support the need for further study to understand and address the causes and risk factors of stroke in young women.
However, they point out that representation and reporting of women in clinical trials of acute stroke continues to be suboptimal, and they call for improved incorporation of sex and gender into study design, analysis, and interpretation, which they say is critical for producing research that is broadly generalizable and applicable to different populations.
Coauthor Stacey L. Daugherty, MD, is funded by the National Institutes of Health. Dr. Poisson and Dr. Kapral have disclosed no relevant financial relationships. Dr. Bushnell reports ownership interest in Care Directions.
A version of this article first appeared on Medscape.com.
CVS Caremark formulary change freezes out apixaban
Patients looking to refill a prescription for apixaban (Eliquis) through CVS Caremark may be in for a surprise following its decision to exclude the direct oral anticoagulant (DOAC) from its formulary starting Jan. 1.
The move leaves just one DOAC, rivaroxaban (Xarelto), on CVS’ commercial formulary and is being assailed as the latest example of “nonmedical switching” used by health insurers to control costs.
In a letter to CVS Caremark backed by 14 provider and patient organizations, the nonprofit Partnership to Advance Cardiovascular Health (PACH) calls on the pharmacy chain to reverse its “dangerously disruptive” decision to force stable patients at high risk of cardiovascular events to switch anticoagulation, without an apparent option to be grandfathered into the new plan.
PACH president Dharmesh Patel, MD, Stern Cardiovascular Center, Memphis, called the formulary change “reckless and irresponsible, especially because the decision is not based in science and evidence, but on budgets. Patients and their health care providers, not insurance companies, need to be trusted to determine what medication is best,” he said in a statement.
Craig Beavers, PharmD, vice president of Baptist Health Paducah, Kentucky, said that, as chair of the American College of Cardiology’s Cardiovascular Team Section, he and other organizations have met with CVS Caremark medical leadership to advocate for patients and to understand the company’s perspective.
“The underlying driver is cost,” he told this news organization.
Current guidelines recommend DOACs in general for a variety of indications, including to reduce the risk of stroke and embolism in nonvalvular atrial fibrillation and to prevent deep vein thrombosis, but there are select instances where a particular DOAC might be more appropriate, he observed.
“Apixaban may be better for a patient with a history of GI bleeding because there’s less GI bleeding, but the guidelines don’t necessarily spell those things out,” Dr. Beavers said. “That’s where the clinician should advocate for their patient and, unfortunately, they are making their decision strictly based off the guidelines.”
Requests to speak with medical officers at CVS Caremark went unanswered, but its executive director of communications, Christina Peaslee, told this news organization that the formulary decision “maintains clinically appropriate, cost-effective prescription coverage” for its clients and members.
“Both the American Heart Association/American College of Cardiology/Heart Rhythm Society and 2021 CHEST guidelines recommend DOACs over warfarin for treatment of various cardiology conditions such as atrial fibrillation, but neither list a specific agent as preferred – showing that consensus clinical guidelines do not favor one over the other,” she said in an email. “Further, Xarelto has more FDA-approved indications than Eliquis (e.g., Xarelto is approved for a reduction in risk of major CV events in patients with CAD or PAD) in addition to all the same FDA indications as Eliquis.”
Ms. Peaslee pointed out that all formulary changes are evaluated by an external medical expert specializing in the disease state, followed by review and approval by an independent national Pharmacy & Therapeutics Committee.
The decision to exclude apixaban is also limited to a “subset of commercial drug lists,” she said, although specifics on which plans and the number of affected patients were not forthcoming.
The choice of DOAC is a timely question in cardiology, with recent studies suggesting an advantage for apixaban over rivaroxaban in reducing the risk of recurrent venous thromboembolism, as well as reducing the risk of major ischemic or hemorrhagic events in atrial fibrillation.
Ms. Peaslee said CVS Caremark closely monitors medical literature for relevant clinical trial data and that most clients allow reasonable formulary exceptions when justified. “This formulary exceptions process has been successfully used for changes of this type and allows patients to get a medication that is safe and effective, as determined by their prescriber.”
The company will also continue to provide “robust, personalized outreach to the small number of members who will need to switch to an alternative medication,” she added.
Dr. Beavers said negotiations with CVS are still in the early stages, but, in the meantime, the ACC is providing health care providers with tools, such as drug copay cards and electronic prior authorizations, to help ensure patients don’t have gaps in coverage.
In a Jan. 14 news release addressing the formulary change, ACC notes that a patient’s pharmacy can also request a one-time override when trying to fill a nonpreferred DOAC in January to buy time if switching medications with their clinician or requesting a formulary exception.
During discussions with CVS Caremark, it says the ACC and the American Society of Hematology “underscored the negative impacts of this decision on patients currently taking one of the nonpreferred DOACs and on those who have previously tried rivaroxaban and changed medications.”
The groups also highlighted difficulties with other prior authorization programs in terms of the need for dedicated staff and time away from direct patient care.
“The ACC and ASH will continue discussions with CVS Caremark regarding the burden on clinicians and the effect of the formulary decision on patient access,” the release says.
In its letter to CVS, PACH argues that the apixaban exclusion will disproportionately affect historically disadvantaged patients, leaving those who can least afford the change with limited options. Notably, no generic is available for either apixaban or rivaroxaban.
The group also highlights a 2019 national poll, in which nearly 40% of patients who had their medication switched were so frustrated that they stopped their medication altogether.
PACH has an online petition against nonmedical switching, which at press time had garnered 2,126 signatures.
One signee, Jan Griffin, who survived bilateral pulmonary embolisms, writes that she has been on Eliquis [apixaban] successfully since her hospital discharge. “Now, as of midnight, Caremark apparently knows better than my hematologist as to what blood thinner is better for me and will no longer cover my Eliquis prescription. This is criminal, immoral, and unethical. #StopTheSwitch.”
A version of this article first appeared on Medscape.com.
Patients looking to refill a prescription for apixaban (Eliquis) through CVS Caremark may be in for a surprise following its decision to exclude the direct oral anticoagulant (DOAC) from its formulary starting Jan. 1.
The move leaves just one DOAC, rivaroxaban (Xarelto), on CVS’ commercial formulary and is being assailed as the latest example of “nonmedical switching” used by health insurers to control costs.
In a letter to CVS Caremark backed by 14 provider and patient organizations, the nonprofit Partnership to Advance Cardiovascular Health (PACH) calls on the pharmacy chain to reverse its “dangerously disruptive” decision to force stable patients at high risk of cardiovascular events to switch anticoagulation, without an apparent option to be grandfathered into the new plan.
PACH president Dharmesh Patel, MD, Stern Cardiovascular Center, Memphis, called the formulary change “reckless and irresponsible, especially because the decision is not based in science and evidence, but on budgets. Patients and their health care providers, not insurance companies, need to be trusted to determine what medication is best,” he said in a statement.
Craig Beavers, PharmD, vice president of Baptist Health Paducah, Kentucky, said that, as chair of the American College of Cardiology’s Cardiovascular Team Section, he and other organizations have met with CVS Caremark medical leadership to advocate for patients and to understand the company’s perspective.
“The underlying driver is cost,” he told this news organization.
Current guidelines recommend DOACs in general for a variety of indications, including to reduce the risk of stroke and embolism in nonvalvular atrial fibrillation and to prevent deep vein thrombosis, but there are select instances where a particular DOAC might be more appropriate, he observed.
“Apixaban may be better for a patient with a history of GI bleeding because there’s less GI bleeding, but the guidelines don’t necessarily spell those things out,” Dr. Beavers said. “That’s where the clinician should advocate for their patient and, unfortunately, they are making their decision strictly based off the guidelines.”
Requests to speak with medical officers at CVS Caremark went unanswered, but its executive director of communications, Christina Peaslee, told this news organization that the formulary decision “maintains clinically appropriate, cost-effective prescription coverage” for its clients and members.
“Both the American Heart Association/American College of Cardiology/Heart Rhythm Society and 2021 CHEST guidelines recommend DOACs over warfarin for treatment of various cardiology conditions such as atrial fibrillation, but neither list a specific agent as preferred – showing that consensus clinical guidelines do not favor one over the other,” she said in an email. “Further, Xarelto has more FDA-approved indications than Eliquis (e.g., Xarelto is approved for a reduction in risk of major CV events in patients with CAD or PAD) in addition to all the same FDA indications as Eliquis.”
Ms. Peaslee pointed out that all formulary changes are evaluated by an external medical expert specializing in the disease state, followed by review and approval by an independent national Pharmacy & Therapeutics Committee.
The decision to exclude apixaban is also limited to a “subset of commercial drug lists,” she said, although specifics on which plans and the number of affected patients were not forthcoming.
The choice of DOAC is a timely question in cardiology, with recent studies suggesting an advantage for apixaban over rivaroxaban in reducing the risk of recurrent venous thromboembolism, as well as reducing the risk of major ischemic or hemorrhagic events in atrial fibrillation.
Ms. Peaslee said CVS Caremark closely monitors medical literature for relevant clinical trial data and that most clients allow reasonable formulary exceptions when justified. “This formulary exceptions process has been successfully used for changes of this type and allows patients to get a medication that is safe and effective, as determined by their prescriber.”
The company will also continue to provide “robust, personalized outreach to the small number of members who will need to switch to an alternative medication,” she added.
Dr. Beavers said negotiations with CVS are still in the early stages, but, in the meantime, the ACC is providing health care providers with tools, such as drug copay cards and electronic prior authorizations, to help ensure patients don’t have gaps in coverage.
In a Jan. 14 news release addressing the formulary change, ACC notes that a patient’s pharmacy can also request a one-time override when trying to fill a nonpreferred DOAC in January to buy time if switching medications with their clinician or requesting a formulary exception.
During discussions with CVS Caremark, it says the ACC and the American Society of Hematology “underscored the negative impacts of this decision on patients currently taking one of the nonpreferred DOACs and on those who have previously tried rivaroxaban and changed medications.”
The groups also highlighted difficulties with other prior authorization programs in terms of the need for dedicated staff and time away from direct patient care.
“The ACC and ASH will continue discussions with CVS Caremark regarding the burden on clinicians and the effect of the formulary decision on patient access,” the release says.
In its letter to CVS, PACH argues that the apixaban exclusion will disproportionately affect historically disadvantaged patients, leaving those who can least afford the change with limited options. Notably, no generic is available for either apixaban or rivaroxaban.
The group also highlights a 2019 national poll, in which nearly 40% of patients who had their medication switched were so frustrated that they stopped their medication altogether.
PACH has an online petition against nonmedical switching, which at press time had garnered 2,126 signatures.
One signee, Jan Griffin, who survived bilateral pulmonary embolisms, writes that she has been on Eliquis [apixaban] successfully since her hospital discharge. “Now, as of midnight, Caremark apparently knows better than my hematologist as to what blood thinner is better for me and will no longer cover my Eliquis prescription. This is criminal, immoral, and unethical. #StopTheSwitch.”
A version of this article first appeared on Medscape.com.
Patients looking to refill a prescription for apixaban (Eliquis) through CVS Caremark may be in for a surprise following its decision to exclude the direct oral anticoagulant (DOAC) from its formulary starting Jan. 1.
The move leaves just one DOAC, rivaroxaban (Xarelto), on CVS’ commercial formulary and is being assailed as the latest example of “nonmedical switching” used by health insurers to control costs.
In a letter to CVS Caremark backed by 14 provider and patient organizations, the nonprofit Partnership to Advance Cardiovascular Health (PACH) calls on the pharmacy chain to reverse its “dangerously disruptive” decision to force stable patients at high risk of cardiovascular events to switch anticoagulation, without an apparent option to be grandfathered into the new plan.
PACH president Dharmesh Patel, MD, Stern Cardiovascular Center, Memphis, called the formulary change “reckless and irresponsible, especially because the decision is not based in science and evidence, but on budgets. Patients and their health care providers, not insurance companies, need to be trusted to determine what medication is best,” he said in a statement.
Craig Beavers, PharmD, vice president of Baptist Health Paducah, Kentucky, said that, as chair of the American College of Cardiology’s Cardiovascular Team Section, he and other organizations have met with CVS Caremark medical leadership to advocate for patients and to understand the company’s perspective.
“The underlying driver is cost,” he told this news organization.
Current guidelines recommend DOACs in general for a variety of indications, including to reduce the risk of stroke and embolism in nonvalvular atrial fibrillation and to prevent deep vein thrombosis, but there are select instances where a particular DOAC might be more appropriate, he observed.
“Apixaban may be better for a patient with a history of GI bleeding because there’s less GI bleeding, but the guidelines don’t necessarily spell those things out,” Dr. Beavers said. “That’s where the clinician should advocate for their patient and, unfortunately, they are making their decision strictly based off the guidelines.”
Requests to speak with medical officers at CVS Caremark went unanswered, but its executive director of communications, Christina Peaslee, told this news organization that the formulary decision “maintains clinically appropriate, cost-effective prescription coverage” for its clients and members.
“Both the American Heart Association/American College of Cardiology/Heart Rhythm Society and 2021 CHEST guidelines recommend DOACs over warfarin for treatment of various cardiology conditions such as atrial fibrillation, but neither list a specific agent as preferred – showing that consensus clinical guidelines do not favor one over the other,” she said in an email. “Further, Xarelto has more FDA-approved indications than Eliquis (e.g., Xarelto is approved for a reduction in risk of major CV events in patients with CAD or PAD) in addition to all the same FDA indications as Eliquis.”
Ms. Peaslee pointed out that all formulary changes are evaluated by an external medical expert specializing in the disease state, followed by review and approval by an independent national Pharmacy & Therapeutics Committee.
The decision to exclude apixaban is also limited to a “subset of commercial drug lists,” she said, although specifics on which plans and the number of affected patients were not forthcoming.
The choice of DOAC is a timely question in cardiology, with recent studies suggesting an advantage for apixaban over rivaroxaban in reducing the risk of recurrent venous thromboembolism, as well as reducing the risk of major ischemic or hemorrhagic events in atrial fibrillation.
Ms. Peaslee said CVS Caremark closely monitors medical literature for relevant clinical trial data and that most clients allow reasonable formulary exceptions when justified. “This formulary exceptions process has been successfully used for changes of this type and allows patients to get a medication that is safe and effective, as determined by their prescriber.”
The company will also continue to provide “robust, personalized outreach to the small number of members who will need to switch to an alternative medication,” she added.
Dr. Beavers said negotiations with CVS are still in the early stages, but, in the meantime, the ACC is providing health care providers with tools, such as drug copay cards and electronic prior authorizations, to help ensure patients don’t have gaps in coverage.
In a Jan. 14 news release addressing the formulary change, ACC notes that a patient’s pharmacy can also request a one-time override when trying to fill a nonpreferred DOAC in January to buy time if switching medications with their clinician or requesting a formulary exception.
During discussions with CVS Caremark, it says the ACC and the American Society of Hematology “underscored the negative impacts of this decision on patients currently taking one of the nonpreferred DOACs and on those who have previously tried rivaroxaban and changed medications.”
The groups also highlighted difficulties with other prior authorization programs in terms of the need for dedicated staff and time away from direct patient care.
“The ACC and ASH will continue discussions with CVS Caremark regarding the burden on clinicians and the effect of the formulary decision on patient access,” the release says.
In its letter to CVS, PACH argues that the apixaban exclusion will disproportionately affect historically disadvantaged patients, leaving those who can least afford the change with limited options. Notably, no generic is available for either apixaban or rivaroxaban.
The group also highlights a 2019 national poll, in which nearly 40% of patients who had their medication switched were so frustrated that they stopped their medication altogether.
PACH has an online petition against nonmedical switching, which at press time had garnered 2,126 signatures.
One signee, Jan Griffin, who survived bilateral pulmonary embolisms, writes that she has been on Eliquis [apixaban] successfully since her hospital discharge. “Now, as of midnight, Caremark apparently knows better than my hematologist as to what blood thinner is better for me and will no longer cover my Eliquis prescription. This is criminal, immoral, and unethical. #StopTheSwitch.”
A version of this article first appeared on Medscape.com.
A high-risk medical device didn’t meet federal standards. The government paid millions for more
In 2014, when the Food and Drug Administration found serious problems with a life-sustaining heart pump, its warning letter to the manufacturer threatened to notify other federal health agencies about the inspection’s findings.
But for years, no such alert ever went out. Instead, the agency added the warning letter to an online database alongside thousands of others, following its typical procedures, an FDA spokesperson said.
Agencies such as the Centers for Medicare & Medicaid Services and the U.S. Department of Veterans Affairs went on paying to implant the HeartWare Ventricular Assist Device, or HVAD, in new patients even though federal inspectors had found problems with the device linked to patient deaths and injuries.
Taxpayer dollars continued to flow to the original device maker, HeartWare, and then to the company that acquired it in 2016, Medtronic, for 7 years while the issues raised in the warning letter remained unresolved.
If crucial safety information in FDA warning letters doesn’t make it to other arms of the government responsible for deciding which medical devices to pay for, experts said patients are the ones put at risk.
“It’s clearly a breakdown of communication,” said Dr. Rita Redberg, a cardiologist at the University of California, San Francisco, who researches medical device safety and regulation. “It’s not just the money, obviously. It’s people’s lives.”
The FDA acknowledged that it doesn’t directly notify other agencies when it issues warning letters, pointing instead to its online database, which is accessible to both government officials and the public. “The FDA’s decisions are intended to be patient-centric with the health and safety of device users as our highest priority,” the agency spokesperson said in an email.
The HeartWare letter was removed from the public database about 2 years ago, even though the problems remained unresolved and patients were still receiving implants. The database clears out letters that are more than 5 years old.
CMS, which oversees the Medicare and Medicaid programs, would not say why it continued paying for a device that didn’t meet government standards. It directed questions about the HeartWare warning letter to the FDA. “CMS does not have oversight of the manufacturing and related safety assessments of a medical device manufacturer,” a spokesperson said in an email.
The spokesperson noted that CMS requires heart pump patients to have specialized medical teams managing their care, which should monitor FDA communications regarding safety of devices.
CMS doesn’t track data on devices by manufacturer, so it’s essentially impossible to calculate its total spending on HVADs. One 2018 medical journal study found that Medicare and Medicaid paid for more than half the cost of all heart pump implants from 2009 to 2014. If that rate of spending continued, CMS may have spent more than $400 million on implanting HVADs since 2014.
A spokesperson for the VA said his agency was never notified about the HeartWare warning letter. The VA paid HeartWare and Medtronic more than $3 million after the FDA issued the letter in 2014. It offered this explanation for why: “It’s important to note that FDA Warning Letters are notifications issued to manufacturers found to be in significant violation of federal regulations. They are not product recalls.”
In the case of the HVAD, the FDA’s failure to make sure its warning reached beyond the manufacturer may have had life-and-death consequences.
In August, ProPublica reported that federal inspectors continued finding problems at the HVAD’s manufacturing plant for years. Meanwhile, the FDA received thousands of reports of suspicious deaths and injuries and more than a dozen high-risk safety alerts from the manufacturer.
The documents detailed one horrifying device failure after another. A father of four died after his device suddenly failed and his teenage daughter couldn’t resuscitate him. Another patient’s heart tissue was charred after a pump short-circuited and overheated. A teenager died after vomiting blood as his mother struggled to restart a defective pump.
In June, Medtronic ended sales and implants of the device, citing new data that showed patients with HVADs had a higher rate of deaths and strokes than those with a competing heart pump.
Medtronic declined to comment for this story. It has previously said it believed that after the 2014 warning letter the benefits of the HVAD still outweighed the risks for patients with severe heart failure.
Experts said the lack of communication between federal agencies when serious device problems are found is baffling but not surprising. It fits a broader trend of device regulators focusing more on evaluating new products than monitoring the ones already on the market.
“The priority is to get more medical devices out there, paid for and getting used,” said Dr. Joseph Ross, a professor of medicine and public health at Yale University who studies medical device regulation.
Other U.S. health care regulators move more forcefully when providers and suppliers don’t meet the government’s minimum safety requirements for an extended period, putting patients at risk.
Take hospitals. When inspectors find a facility is not meeting safety standards, CMS can issue an immediate jeopardy citation and, if problems aren’t fixed, move to withhold federal payments, which make up substantial portions of most hospitals’ revenues. In the rare cases when hospitals don’t take sufficient action, CMS follows through and revokes funding.
Redberg, the UCSF cardiologist, said the lack of similar action for medical devices offers a clear “opportunity for improvement.” At minimum, the FDA could establish processes to directly inform other agencies when it issues warning letters and finds serious problems with devices being sold in the United States.
“If the agency’s mission is to protect public health, they would want to do these things and move quickly,” she said.
This story was originally published on ProPublica. ProPublica is a nonprofit newsroom that investigates abuses of power. Sign up to receive their biggest stories as soon as they’re published.
In 2014, when the Food and Drug Administration found serious problems with a life-sustaining heart pump, its warning letter to the manufacturer threatened to notify other federal health agencies about the inspection’s findings.
But for years, no such alert ever went out. Instead, the agency added the warning letter to an online database alongside thousands of others, following its typical procedures, an FDA spokesperson said.
Agencies such as the Centers for Medicare & Medicaid Services and the U.S. Department of Veterans Affairs went on paying to implant the HeartWare Ventricular Assist Device, or HVAD, in new patients even though federal inspectors had found problems with the device linked to patient deaths and injuries.
Taxpayer dollars continued to flow to the original device maker, HeartWare, and then to the company that acquired it in 2016, Medtronic, for 7 years while the issues raised in the warning letter remained unresolved.
If crucial safety information in FDA warning letters doesn’t make it to other arms of the government responsible for deciding which medical devices to pay for, experts said patients are the ones put at risk.
“It’s clearly a breakdown of communication,” said Dr. Rita Redberg, a cardiologist at the University of California, San Francisco, who researches medical device safety and regulation. “It’s not just the money, obviously. It’s people’s lives.”
The FDA acknowledged that it doesn’t directly notify other agencies when it issues warning letters, pointing instead to its online database, which is accessible to both government officials and the public. “The FDA’s decisions are intended to be patient-centric with the health and safety of device users as our highest priority,” the agency spokesperson said in an email.
The HeartWare letter was removed from the public database about 2 years ago, even though the problems remained unresolved and patients were still receiving implants. The database clears out letters that are more than 5 years old.
CMS, which oversees the Medicare and Medicaid programs, would not say why it continued paying for a device that didn’t meet government standards. It directed questions about the HeartWare warning letter to the FDA. “CMS does not have oversight of the manufacturing and related safety assessments of a medical device manufacturer,” a spokesperson said in an email.
The spokesperson noted that CMS requires heart pump patients to have specialized medical teams managing their care, which should monitor FDA communications regarding safety of devices.
CMS doesn’t track data on devices by manufacturer, so it’s essentially impossible to calculate its total spending on HVADs. One 2018 medical journal study found that Medicare and Medicaid paid for more than half the cost of all heart pump implants from 2009 to 2014. If that rate of spending continued, CMS may have spent more than $400 million on implanting HVADs since 2014.
A spokesperson for the VA said his agency was never notified about the HeartWare warning letter. The VA paid HeartWare and Medtronic more than $3 million after the FDA issued the letter in 2014. It offered this explanation for why: “It’s important to note that FDA Warning Letters are notifications issued to manufacturers found to be in significant violation of federal regulations. They are not product recalls.”
In the case of the HVAD, the FDA’s failure to make sure its warning reached beyond the manufacturer may have had life-and-death consequences.
In August, ProPublica reported that federal inspectors continued finding problems at the HVAD’s manufacturing plant for years. Meanwhile, the FDA received thousands of reports of suspicious deaths and injuries and more than a dozen high-risk safety alerts from the manufacturer.
The documents detailed one horrifying device failure after another. A father of four died after his device suddenly failed and his teenage daughter couldn’t resuscitate him. Another patient’s heart tissue was charred after a pump short-circuited and overheated. A teenager died after vomiting blood as his mother struggled to restart a defective pump.
In June, Medtronic ended sales and implants of the device, citing new data that showed patients with HVADs had a higher rate of deaths and strokes than those with a competing heart pump.
Medtronic declined to comment for this story. It has previously said it believed that after the 2014 warning letter the benefits of the HVAD still outweighed the risks for patients with severe heart failure.
Experts said the lack of communication between federal agencies when serious device problems are found is baffling but not surprising. It fits a broader trend of device regulators focusing more on evaluating new products than monitoring the ones already on the market.
“The priority is to get more medical devices out there, paid for and getting used,” said Dr. Joseph Ross, a professor of medicine and public health at Yale University who studies medical device regulation.
Other U.S. health care regulators move more forcefully when providers and suppliers don’t meet the government’s minimum safety requirements for an extended period, putting patients at risk.
Take hospitals. When inspectors find a facility is not meeting safety standards, CMS can issue an immediate jeopardy citation and, if problems aren’t fixed, move to withhold federal payments, which make up substantial portions of most hospitals’ revenues. In the rare cases when hospitals don’t take sufficient action, CMS follows through and revokes funding.
Redberg, the UCSF cardiologist, said the lack of similar action for medical devices offers a clear “opportunity for improvement.” At minimum, the FDA could establish processes to directly inform other agencies when it issues warning letters and finds serious problems with devices being sold in the United States.
“If the agency’s mission is to protect public health, they would want to do these things and move quickly,” she said.
This story was originally published on ProPublica. ProPublica is a nonprofit newsroom that investigates abuses of power. Sign up to receive their biggest stories as soon as they’re published.
In 2014, when the Food and Drug Administration found serious problems with a life-sustaining heart pump, its warning letter to the manufacturer threatened to notify other federal health agencies about the inspection’s findings.
But for years, no such alert ever went out. Instead, the agency added the warning letter to an online database alongside thousands of others, following its typical procedures, an FDA spokesperson said.
Agencies such as the Centers for Medicare & Medicaid Services and the U.S. Department of Veterans Affairs went on paying to implant the HeartWare Ventricular Assist Device, or HVAD, in new patients even though federal inspectors had found problems with the device linked to patient deaths and injuries.
Taxpayer dollars continued to flow to the original device maker, HeartWare, and then to the company that acquired it in 2016, Medtronic, for 7 years while the issues raised in the warning letter remained unresolved.
If crucial safety information in FDA warning letters doesn’t make it to other arms of the government responsible for deciding which medical devices to pay for, experts said patients are the ones put at risk.
“It’s clearly a breakdown of communication,” said Dr. Rita Redberg, a cardiologist at the University of California, San Francisco, who researches medical device safety and regulation. “It’s not just the money, obviously. It’s people’s lives.”
The FDA acknowledged that it doesn’t directly notify other agencies when it issues warning letters, pointing instead to its online database, which is accessible to both government officials and the public. “The FDA’s decisions are intended to be patient-centric with the health and safety of device users as our highest priority,” the agency spokesperson said in an email.
The HeartWare letter was removed from the public database about 2 years ago, even though the problems remained unresolved and patients were still receiving implants. The database clears out letters that are more than 5 years old.
CMS, which oversees the Medicare and Medicaid programs, would not say why it continued paying for a device that didn’t meet government standards. It directed questions about the HeartWare warning letter to the FDA. “CMS does not have oversight of the manufacturing and related safety assessments of a medical device manufacturer,” a spokesperson said in an email.
The spokesperson noted that CMS requires heart pump patients to have specialized medical teams managing their care, which should monitor FDA communications regarding safety of devices.
CMS doesn’t track data on devices by manufacturer, so it’s essentially impossible to calculate its total spending on HVADs. One 2018 medical journal study found that Medicare and Medicaid paid for more than half the cost of all heart pump implants from 2009 to 2014. If that rate of spending continued, CMS may have spent more than $400 million on implanting HVADs since 2014.
A spokesperson for the VA said his agency was never notified about the HeartWare warning letter. The VA paid HeartWare and Medtronic more than $3 million after the FDA issued the letter in 2014. It offered this explanation for why: “It’s important to note that FDA Warning Letters are notifications issued to manufacturers found to be in significant violation of federal regulations. They are not product recalls.”
In the case of the HVAD, the FDA’s failure to make sure its warning reached beyond the manufacturer may have had life-and-death consequences.
In August, ProPublica reported that federal inspectors continued finding problems at the HVAD’s manufacturing plant for years. Meanwhile, the FDA received thousands of reports of suspicious deaths and injuries and more than a dozen high-risk safety alerts from the manufacturer.
The documents detailed one horrifying device failure after another. A father of four died after his device suddenly failed and his teenage daughter couldn’t resuscitate him. Another patient’s heart tissue was charred after a pump short-circuited and overheated. A teenager died after vomiting blood as his mother struggled to restart a defective pump.
In June, Medtronic ended sales and implants of the device, citing new data that showed patients with HVADs had a higher rate of deaths and strokes than those with a competing heart pump.
Medtronic declined to comment for this story. It has previously said it believed that after the 2014 warning letter the benefits of the HVAD still outweighed the risks for patients with severe heart failure.
Experts said the lack of communication between federal agencies when serious device problems are found is baffling but not surprising. It fits a broader trend of device regulators focusing more on evaluating new products than monitoring the ones already on the market.
“The priority is to get more medical devices out there, paid for and getting used,” said Dr. Joseph Ross, a professor of medicine and public health at Yale University who studies medical device regulation.
Other U.S. health care regulators move more forcefully when providers and suppliers don’t meet the government’s minimum safety requirements for an extended period, putting patients at risk.
Take hospitals. When inspectors find a facility is not meeting safety standards, CMS can issue an immediate jeopardy citation and, if problems aren’t fixed, move to withhold federal payments, which make up substantial portions of most hospitals’ revenues. In the rare cases when hospitals don’t take sufficient action, CMS follows through and revokes funding.
Redberg, the UCSF cardiologist, said the lack of similar action for medical devices offers a clear “opportunity for improvement.” At minimum, the FDA could establish processes to directly inform other agencies when it issues warning letters and finds serious problems with devices being sold in the United States.
“If the agency’s mission is to protect public health, they would want to do these things and move quickly,” she said.
This story was originally published on ProPublica. ProPublica is a nonprofit newsroom that investigates abuses of power. Sign up to receive their biggest stories as soon as they’re published.
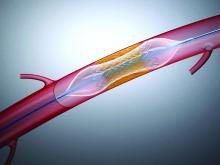
Who benefits most from device PFO closure after a stroke?
It has been well established that device closure has, on average, prevented stroke recurrence in people who’ve had patent foramen ovale–associated stroke, but a meta-analysis has drilled down into clinical trials to advance a potentially practice-changing principle: that, while device closure shows an overall benefit, not all patients derive a benefit and some may actually be harmed by the procedure.
What’s more, the researchers developed a scoring system that helps determine which patients are likely to benefit from device closure.
“What was unknown was how to treat individual patients because the decision to close the patent foramen ovale (PFO) is still preference sensitive because the risk of a recurrent stroke is low, and most of the strokes that recur are not terribly severe,” lead study author David M. Kent, MD, MS, said in an interview.
“On top of this,” he said, “it was still suspected that some of the PFOs, even in trials of well-selected patients, may not be causally related to stroke; the stroke may still have another occult cause, such as paroxysmal atrial fibrillation or aortic arch atheroma.” Dr. Kent is a professor of medicine at Tufts University in Boston and director of the Predictive Analytics and Comparative Effectiveness Center there.
The meta-analysis, conducted by the Systematic, Collaborative, PFO Closure Evaluation (SCOPE) consortium, analyzed data from six randomized clinical trials that compared device closure and medical therapy to medical therapy alone in 3,740 patients who had PFO-associated stroke from 2000 to 2017. It was published in JAMA.
Overall, the rate of recurrent ischemic stroke was less than half that in patients who had device closure, compared with those who were on medical therapy: 0.47% (n = 39 of 1,889) vs. 1.09% (n = 82 of 1,851).
The researchers also applied two tools designed to calculate the probability of recurrent stroke in individual patients: Risk of Paradoxical Embolism (RoPE), an index that assigns a score of 0-10 to stratify cryptogenic stroke patients with PFO by the likelihood that the stroke was associated with their PFO; and the PFO-Associated Stroke Causal Likelihood (PASCAL) classification system, which integrates the RoPE score with physiological and anatomical features – namely, the size of the PFO shunt and the presence of an atrial septal aneurysm.
“We came up with a way to more accurately identify those patients who are likely to get the most benefit from PFO closure based on mathematic modeling that estimates an individual’s probability that the PFO is causally related to the stroke,” Dr. Kent said.
Multivariate analysis determines risk
The study used a multivariate classification system that Dr. Kent had been developing to perform subgroup analyses of the clinical trials. It assigned patients to three different risk groups based on the likelihood that the PFO was causally related to their stroke: PASCAL categories of unlikely, possible, and probable.
The PASCAL unlikely group had a risk of stroke recurrence in the first 2 years of 3.4% (95% confidence interval, 1.1%-5.7%) if they were on medical therapy, and 4.1% (95% CI, 1.7%-6.4%) if they had device closure. In the PASCAL possible group, those risks were 3.6% (95% CI, 2.4%-4.9%) and 1.5% (95% CI, 0.7-2.3%), respectively. For the probable group, device closure represents “a near perfect therapy” with a 90% risk reduction, Dr. Kent said. “Moreover,” he said, “adverse events of device closure, such as atrial fibrillation, appear to be concentrated in those patients who fall into the unlikely classification, who appear to get no benefit.”
The ideal patient for device closure is age 60 years or younger and without vascular risk factors such as hypertension, diabetes, a history of smoking, or a prior stroke, but has high-risk PFO features such as a large shunt or atrial septal aneurysm, Dr. Kent said.
“We think these findings should be practice changing now,” Dr. Kent said.
Faisal M. Merchant, MD, director of cardiac electrophysiology at Emory Healthcare in Atlanta, concurred with that statement. “This is in my mind probably as good as any data we’re going to get on this,” he said in an interview. “The results support what’s been a general gestalt in the clinical world, but [also] really provide an evidence base on how to make decisions.”
He noted that guidelines, including those of the American Academy of Neurology, recommend medical therapy or device closure to prevent recurrent stroke in people who’ve had PFO-associated ischemic stroke. “But they hedge a bit,” he said of the guidelines. “We haven’t had data that’s as robust as this. I think this really solidifies those recommendations.”
He also credited the “unique” study design to extract findings from clinical trials and apply them to personalized medicine. “Clinical trial results give you an average treatment effect of the patients included, but who are ones who really benefit? Who are the ones that don’t benefit? Who are the ones who are harmed?” Dr. Merchant said. “It’s rare that you can parse out this nicely between the people who both benefit and are less likely to be harmed and the people who don’t benefit and are more likely to be harmed.”
The study received funding from the Patient-Centered Outcomes Research Institute. Dr. Kent disclosed relationships with PCORI, W.L. Gore and the Canadian Stroke Consortium. Dr. Merchant has no relevant disclosures.
It has been well established that device closure has, on average, prevented stroke recurrence in people who’ve had patent foramen ovale–associated stroke, but a meta-analysis has drilled down into clinical trials to advance a potentially practice-changing principle: that, while device closure shows an overall benefit, not all patients derive a benefit and some may actually be harmed by the procedure.
What’s more, the researchers developed a scoring system that helps determine which patients are likely to benefit from device closure.
“What was unknown was how to treat individual patients because the decision to close the patent foramen ovale (PFO) is still preference sensitive because the risk of a recurrent stroke is low, and most of the strokes that recur are not terribly severe,” lead study author David M. Kent, MD, MS, said in an interview.
“On top of this,” he said, “it was still suspected that some of the PFOs, even in trials of well-selected patients, may not be causally related to stroke; the stroke may still have another occult cause, such as paroxysmal atrial fibrillation or aortic arch atheroma.” Dr. Kent is a professor of medicine at Tufts University in Boston and director of the Predictive Analytics and Comparative Effectiveness Center there.
The meta-analysis, conducted by the Systematic, Collaborative, PFO Closure Evaluation (SCOPE) consortium, analyzed data from six randomized clinical trials that compared device closure and medical therapy to medical therapy alone in 3,740 patients who had PFO-associated stroke from 2000 to 2017. It was published in JAMA.
Overall, the rate of recurrent ischemic stroke was less than half that in patients who had device closure, compared with those who were on medical therapy: 0.47% (n = 39 of 1,889) vs. 1.09% (n = 82 of 1,851).
The researchers also applied two tools designed to calculate the probability of recurrent stroke in individual patients: Risk of Paradoxical Embolism (RoPE), an index that assigns a score of 0-10 to stratify cryptogenic stroke patients with PFO by the likelihood that the stroke was associated with their PFO; and the PFO-Associated Stroke Causal Likelihood (PASCAL) classification system, which integrates the RoPE score with physiological and anatomical features – namely, the size of the PFO shunt and the presence of an atrial septal aneurysm.
“We came up with a way to more accurately identify those patients who are likely to get the most benefit from PFO closure based on mathematic modeling that estimates an individual’s probability that the PFO is causally related to the stroke,” Dr. Kent said.
Multivariate analysis determines risk
The study used a multivariate classification system that Dr. Kent had been developing to perform subgroup analyses of the clinical trials. It assigned patients to three different risk groups based on the likelihood that the PFO was causally related to their stroke: PASCAL categories of unlikely, possible, and probable.
The PASCAL unlikely group had a risk of stroke recurrence in the first 2 years of 3.4% (95% confidence interval, 1.1%-5.7%) if they were on medical therapy, and 4.1% (95% CI, 1.7%-6.4%) if they had device closure. In the PASCAL possible group, those risks were 3.6% (95% CI, 2.4%-4.9%) and 1.5% (95% CI, 0.7-2.3%), respectively. For the probable group, device closure represents “a near perfect therapy” with a 90% risk reduction, Dr. Kent said. “Moreover,” he said, “adverse events of device closure, such as atrial fibrillation, appear to be concentrated in those patients who fall into the unlikely classification, who appear to get no benefit.”
The ideal patient for device closure is age 60 years or younger and without vascular risk factors such as hypertension, diabetes, a history of smoking, or a prior stroke, but has high-risk PFO features such as a large shunt or atrial septal aneurysm, Dr. Kent said.
“We think these findings should be practice changing now,” Dr. Kent said.
Faisal M. Merchant, MD, director of cardiac electrophysiology at Emory Healthcare in Atlanta, concurred with that statement. “This is in my mind probably as good as any data we’re going to get on this,” he said in an interview. “The results support what’s been a general gestalt in the clinical world, but [also] really provide an evidence base on how to make decisions.”
He noted that guidelines, including those of the American Academy of Neurology, recommend medical therapy or device closure to prevent recurrent stroke in people who’ve had PFO-associated ischemic stroke. “But they hedge a bit,” he said of the guidelines. “We haven’t had data that’s as robust as this. I think this really solidifies those recommendations.”
He also credited the “unique” study design to extract findings from clinical trials and apply them to personalized medicine. “Clinical trial results give you an average treatment effect of the patients included, but who are ones who really benefit? Who are the ones that don’t benefit? Who are the ones who are harmed?” Dr. Merchant said. “It’s rare that you can parse out this nicely between the people who both benefit and are less likely to be harmed and the people who don’t benefit and are more likely to be harmed.”
The study received funding from the Patient-Centered Outcomes Research Institute. Dr. Kent disclosed relationships with PCORI, W.L. Gore and the Canadian Stroke Consortium. Dr. Merchant has no relevant disclosures.
It has been well established that device closure has, on average, prevented stroke recurrence in people who’ve had patent foramen ovale–associated stroke, but a meta-analysis has drilled down into clinical trials to advance a potentially practice-changing principle: that, while device closure shows an overall benefit, not all patients derive a benefit and some may actually be harmed by the procedure.
What’s more, the researchers developed a scoring system that helps determine which patients are likely to benefit from device closure.
“What was unknown was how to treat individual patients because the decision to close the patent foramen ovale (PFO) is still preference sensitive because the risk of a recurrent stroke is low, and most of the strokes that recur are not terribly severe,” lead study author David M. Kent, MD, MS, said in an interview.
“On top of this,” he said, “it was still suspected that some of the PFOs, even in trials of well-selected patients, may not be causally related to stroke; the stroke may still have another occult cause, such as paroxysmal atrial fibrillation or aortic arch atheroma.” Dr. Kent is a professor of medicine at Tufts University in Boston and director of the Predictive Analytics and Comparative Effectiveness Center there.
The meta-analysis, conducted by the Systematic, Collaborative, PFO Closure Evaluation (SCOPE) consortium, analyzed data from six randomized clinical trials that compared device closure and medical therapy to medical therapy alone in 3,740 patients who had PFO-associated stroke from 2000 to 2017. It was published in JAMA.
Overall, the rate of recurrent ischemic stroke was less than half that in patients who had device closure, compared with those who were on medical therapy: 0.47% (n = 39 of 1,889) vs. 1.09% (n = 82 of 1,851).
The researchers also applied two tools designed to calculate the probability of recurrent stroke in individual patients: Risk of Paradoxical Embolism (RoPE), an index that assigns a score of 0-10 to stratify cryptogenic stroke patients with PFO by the likelihood that the stroke was associated with their PFO; and the PFO-Associated Stroke Causal Likelihood (PASCAL) classification system, which integrates the RoPE score with physiological and anatomical features – namely, the size of the PFO shunt and the presence of an atrial septal aneurysm.
“We came up with a way to more accurately identify those patients who are likely to get the most benefit from PFO closure based on mathematic modeling that estimates an individual’s probability that the PFO is causally related to the stroke,” Dr. Kent said.
Multivariate analysis determines risk
The study used a multivariate classification system that Dr. Kent had been developing to perform subgroup analyses of the clinical trials. It assigned patients to three different risk groups based on the likelihood that the PFO was causally related to their stroke: PASCAL categories of unlikely, possible, and probable.
The PASCAL unlikely group had a risk of stroke recurrence in the first 2 years of 3.4% (95% confidence interval, 1.1%-5.7%) if they were on medical therapy, and 4.1% (95% CI, 1.7%-6.4%) if they had device closure. In the PASCAL possible group, those risks were 3.6% (95% CI, 2.4%-4.9%) and 1.5% (95% CI, 0.7-2.3%), respectively. For the probable group, device closure represents “a near perfect therapy” with a 90% risk reduction, Dr. Kent said. “Moreover,” he said, “adverse events of device closure, such as atrial fibrillation, appear to be concentrated in those patients who fall into the unlikely classification, who appear to get no benefit.”
The ideal patient for device closure is age 60 years or younger and without vascular risk factors such as hypertension, diabetes, a history of smoking, or a prior stroke, but has high-risk PFO features such as a large shunt or atrial septal aneurysm, Dr. Kent said.
“We think these findings should be practice changing now,” Dr. Kent said.
Faisal M. Merchant, MD, director of cardiac electrophysiology at Emory Healthcare in Atlanta, concurred with that statement. “This is in my mind probably as good as any data we’re going to get on this,” he said in an interview. “The results support what’s been a general gestalt in the clinical world, but [also] really provide an evidence base on how to make decisions.”
He noted that guidelines, including those of the American Academy of Neurology, recommend medical therapy or device closure to prevent recurrent stroke in people who’ve had PFO-associated ischemic stroke. “But they hedge a bit,” he said of the guidelines. “We haven’t had data that’s as robust as this. I think this really solidifies those recommendations.”
He also credited the “unique” study design to extract findings from clinical trials and apply them to personalized medicine. “Clinical trial results give you an average treatment effect of the patients included, but who are ones who really benefit? Who are the ones that don’t benefit? Who are the ones who are harmed?” Dr. Merchant said. “It’s rare that you can parse out this nicely between the people who both benefit and are less likely to be harmed and the people who don’t benefit and are more likely to be harmed.”
The study received funding from the Patient-Centered Outcomes Research Institute. Dr. Kent disclosed relationships with PCORI, W.L. Gore and the Canadian Stroke Consortium. Dr. Merchant has no relevant disclosures.
FROM JAMA
CDC panel backs mRNA COVID vaccines over J&J because of clot risk
because the Johnson & Johnson shot carries the risk of a rare but potentially fatal side effect that causes blood clots and bleeding in the brain.
In an emergency meeting on December 16, the CDC’s Advisory Committee on Immunization Practices, or ACIP, voted unanimously (15-0) to state a preference for the mRNA vaccines over the Johnson & Johnson shot. The vote came after the panel heard a safety update on cases of thrombosis with thrombocytopenia syndrome, or TTS, a condition that causes large clots that deplete the blood of platelets, resulting in uncontrolled bleeding.
The move brings the United States in line with other wealthy countries. In May, Denmark dropped the Johnson & Johnson shot from its vaccination program because of this risk. Australia and Greece have limited the use of a similar vaccine, made by AstraZeneca, in younger people because of the TTS risk. Both vaccines use the envelope of a different kind of virus, called an adenovirus, to sneak the vaccine instructions into cells. On Dec. 16, health officials said they had determined that TTS was likely due to a class effect, meaning it happens with all adenovirus vector vaccines.
The risk of dying from TTS after a Johnson & Johnson shot is extremely rare. There is an estimated 1 death for every 2 million doses of the vaccine given in the general population. That risk is higher for women ages 30 to 49, rising to about 2 deaths for every 1 million doses given in this age group. There’s no question that the Johnson & Johnson shot has saved many more lives than it has taken, experts said
Still, the committee previously paused the use of the Johnson & Johnson vaccine in April after the first cases of TTS came to light. That pause was lifted just 10 days later, after a new warning was added to the vaccine’s label to raise awareness of the risk.
In updating the safety information on Johnson & Johnson, the panel noted that the warning label had not sufficiently lowered the risk of death from TTS. Doctors seem to be aware of the condition because none of the patients who had developed TTS had been treated with the blood thinner heparin, which can make the syndrome worse. But patients continued to die even after the label was added, the panel noted, because TTS can progress so quickly that doctors simply don’t have time to treat it.
For that reason, and because there are other, safer vaccines available, the panel decided to make what’s called a preferential statement, saying the Pfizer and Moderna mRNA vaccines should be preferred over Johnson & Johnson.
The statement leaves the J&J vaccine on the market and available to patients who are at risk of a severe allergic reaction to the mRNA vaccines. It also means that people can still choose the J&J vaccine if they still want it after being informed about the risks.
About 17 million first doses and 900,000 second doses of the Johnson & Johnson vaccine have been given in the United States. Through the end of August, 54 cases of thrombosis with thrombocytopenia syndrome (TTS) have occurred after the J&J shots in the United States. Nearly half of those were in women ages 30 to 49. There have been nine deaths from TTS after Johnson & Johnson shots.
A version of this article first appeared on WebMD.com.
because the Johnson & Johnson shot carries the risk of a rare but potentially fatal side effect that causes blood clots and bleeding in the brain.
In an emergency meeting on December 16, the CDC’s Advisory Committee on Immunization Practices, or ACIP, voted unanimously (15-0) to state a preference for the mRNA vaccines over the Johnson & Johnson shot. The vote came after the panel heard a safety update on cases of thrombosis with thrombocytopenia syndrome, or TTS, a condition that causes large clots that deplete the blood of platelets, resulting in uncontrolled bleeding.
The move brings the United States in line with other wealthy countries. In May, Denmark dropped the Johnson & Johnson shot from its vaccination program because of this risk. Australia and Greece have limited the use of a similar vaccine, made by AstraZeneca, in younger people because of the TTS risk. Both vaccines use the envelope of a different kind of virus, called an adenovirus, to sneak the vaccine instructions into cells. On Dec. 16, health officials said they had determined that TTS was likely due to a class effect, meaning it happens with all adenovirus vector vaccines.
The risk of dying from TTS after a Johnson & Johnson shot is extremely rare. There is an estimated 1 death for every 2 million doses of the vaccine given in the general population. That risk is higher for women ages 30 to 49, rising to about 2 deaths for every 1 million doses given in this age group. There’s no question that the Johnson & Johnson shot has saved many more lives than it has taken, experts said
Still, the committee previously paused the use of the Johnson & Johnson vaccine in April after the first cases of TTS came to light. That pause was lifted just 10 days later, after a new warning was added to the vaccine’s label to raise awareness of the risk.
In updating the safety information on Johnson & Johnson, the panel noted that the warning label had not sufficiently lowered the risk of death from TTS. Doctors seem to be aware of the condition because none of the patients who had developed TTS had been treated with the blood thinner heparin, which can make the syndrome worse. But patients continued to die even after the label was added, the panel noted, because TTS can progress so quickly that doctors simply don’t have time to treat it.
For that reason, and because there are other, safer vaccines available, the panel decided to make what’s called a preferential statement, saying the Pfizer and Moderna mRNA vaccines should be preferred over Johnson & Johnson.
The statement leaves the J&J vaccine on the market and available to patients who are at risk of a severe allergic reaction to the mRNA vaccines. It also means that people can still choose the J&J vaccine if they still want it after being informed about the risks.
About 17 million first doses and 900,000 second doses of the Johnson & Johnson vaccine have been given in the United States. Through the end of August, 54 cases of thrombosis with thrombocytopenia syndrome (TTS) have occurred after the J&J shots in the United States. Nearly half of those were in women ages 30 to 49. There have been nine deaths from TTS after Johnson & Johnson shots.
A version of this article first appeared on WebMD.com.
because the Johnson & Johnson shot carries the risk of a rare but potentially fatal side effect that causes blood clots and bleeding in the brain.
In an emergency meeting on December 16, the CDC’s Advisory Committee on Immunization Practices, or ACIP, voted unanimously (15-0) to state a preference for the mRNA vaccines over the Johnson & Johnson shot. The vote came after the panel heard a safety update on cases of thrombosis with thrombocytopenia syndrome, or TTS, a condition that causes large clots that deplete the blood of platelets, resulting in uncontrolled bleeding.
The move brings the United States in line with other wealthy countries. In May, Denmark dropped the Johnson & Johnson shot from its vaccination program because of this risk. Australia and Greece have limited the use of a similar vaccine, made by AstraZeneca, in younger people because of the TTS risk. Both vaccines use the envelope of a different kind of virus, called an adenovirus, to sneak the vaccine instructions into cells. On Dec. 16, health officials said they had determined that TTS was likely due to a class effect, meaning it happens with all adenovirus vector vaccines.
The risk of dying from TTS after a Johnson & Johnson shot is extremely rare. There is an estimated 1 death for every 2 million doses of the vaccine given in the general population. That risk is higher for women ages 30 to 49, rising to about 2 deaths for every 1 million doses given in this age group. There’s no question that the Johnson & Johnson shot has saved many more lives than it has taken, experts said
Still, the committee previously paused the use of the Johnson & Johnson vaccine in April after the first cases of TTS came to light. That pause was lifted just 10 days later, after a new warning was added to the vaccine’s label to raise awareness of the risk.
In updating the safety information on Johnson & Johnson, the panel noted that the warning label had not sufficiently lowered the risk of death from TTS. Doctors seem to be aware of the condition because none of the patients who had developed TTS had been treated with the blood thinner heparin, which can make the syndrome worse. But patients continued to die even after the label was added, the panel noted, because TTS can progress so quickly that doctors simply don’t have time to treat it.
For that reason, and because there are other, safer vaccines available, the panel decided to make what’s called a preferential statement, saying the Pfizer and Moderna mRNA vaccines should be preferred over Johnson & Johnson.
The statement leaves the J&J vaccine on the market and available to patients who are at risk of a severe allergic reaction to the mRNA vaccines. It also means that people can still choose the J&J vaccine if they still want it after being informed about the risks.
About 17 million first doses and 900,000 second doses of the Johnson & Johnson vaccine have been given in the United States. Through the end of August, 54 cases of thrombosis with thrombocytopenia syndrome (TTS) have occurred after the J&J shots in the United States. Nearly half of those were in women ages 30 to 49. There have been nine deaths from TTS after Johnson & Johnson shots.
A version of this article first appeared on WebMD.com.
D-dimer thresholds rule out PE in meta-analysis
In a patient suspected to have a PE, “diagnosis is made radiographically, usually with CT pulmonary angiogram, or V/Q scan,” Suman Pal, MD, of the University of New Mexico, Albuquerque, said in an interview.
“Validated clinical decision tools such as Wells’ score or Geneva score may be used to identify patients at low pretest probability of PE who may initially get a D-dimer level check, followed by imaging only if D-dimer level is elevated,” explained Dr. Pal, who was not involved with the new research, which was published in the Annals of Internal Medicine.
According to the authors of the new paper, while current diagnostic strategies in patients with suspected PE include use of a validated clinical decision rule (CDR) and D-dimer testing to rule out PE without imaging tests, the effectiveness of D-dimer tests in older patients, inpatients, cancer patients, and other high-risk groups has not been well-studied.
Lead author of the paper, Milou A.M. Stals, MD, and colleagues said their goal was to evaluate the safety and efficiency of the Wells rule and revised Geneva score in combination with D-dimer tests, and also the YEARS algorithm for D-dimer thresholds, in their paper.
Dr. Stals, of Leiden (the Netherlands) University Medical Center, and the coinvestigators conducted an international systemic review and individual patient data meta-analysis that included 16 studies and 20,553 patients, with all studies having been published between Jan. 1, 1995, and Jan. 1, 2021. Their primary outcomes were the safety and efficiency of each of these three strategies.
In the review, the researchers defined safety as the 3-month incidence of venous thromboembolism after PE was ruled out without imaging at baseline. They defined efficiency as the proportion patients for whom PE was ruled out based on D-dimer thresholds without imaging.
Overall, efficiency was highest in the subset of patients aged younger than 40 years, ranging from 47% to 68% in this group. Efficiency was lowest in patients aged 80 years and older (6.0%-23%), and in patients with cancer (9.6%-26%).
The efficiency was higher when D-dimer thresholds based on pretest probability were used, compared with when fixed or age-adjusted D-dimer thresholds were used.
The key finding was the significant variability in performance of the diagnostic strategies, the researchers said.
“The predicted failure rate was generally highest for strategies incorporating adapted D-dimer thresholds. However, at the same time, predicted overall efficiency was substantially higher with these strategies versus strategies with a fixed D-dimer threshold as well,” they said. Given that the benefits of each of the three diagnostic strategies depends on their correct application, the researchers recommended that an individual hospitalist choose one strategy for their institution.
“Whether clinicians should rely on the Wells rule, the YEARS algorithm, or the revised Geneva score becomes a matter of local preference and experience,” Dr. Stals and colleagues wrote.
The study findings were limited by several factors including between-study differences in scoring predictors and D-dimer assays. Another limitation was that differential verification biases for classifying fatal events and PE may have contributed to overestimation of failure rates of the adapted D-dimer thresholds.
Strengths of the study included its large sample size and original data on pretest probability, and that data support the use of any of the three strategies for ruling out PE in the identified subgroups without the need for imaging tests, the authors wrote.
“Pending the results of ongoing diagnostic randomized trials, physicians and guideline committees should balance the interlink between safety and efficiency of available diagnostic strategies,” they concluded.
Adapted D-dimer benefits some patients
“Clearly, increasing the D-dimer cutoff will lower the number of patients who require radiographic imaging (improved specificity), but this comes with a risk for missing PE (lower sensitivity). Is this risk worth taking?” Daniel J. Brotman, MD, of Johns Hopkins University, Baltimore, asked in an editorial accompanying the new study.
Dr. Brotman was not surprised by the study findings.
“Conditions that predispose to thrombosis through activated hemostasis – such as advanced age, cancer, inflammation, prolonged hospitalization, and trauma – drive D-dimer levels higher independent of the presence or absence of radiographically apparent thrombosis,” he said. However, these patients are unlikely to have normal D-dimer levels regardless of the cutoff used.
Adapted D-dimer cutoffs may benefit some patients, including those with contraindications or limited access to imaging, said Dr. Brotman. D-dimer may be used for risk stratification regardless of PE, since patients with marginally elevated D-dimers have better prognoses than those with higher D-dimer elevations, even if a small PE is missed.
Dr. Brotman wrote that increasing D-dimer cutoffs for high-risk patients in the subgroups analyzed may spare some patients radiographic testing, but doing so carries an increased risk for diagnostic failure. Overall, “the important work by Stals and colleagues offers reassurance that modifying D-dimer thresholds according to age or pretest probability is safe enough for widespread practice, even in high-risk groups.”
Focus on single strategy ‘based on local needs’
“Several validated clinical decision tools, along with age or pretest probability adjusted D-dimer threshold are currently in use as diagnostic strategies for ruling out pulmonary embolism,” Dr. Pal said in an interview.
The current study is important because of limited data on the performance of these strategies in specific subgroups of patients whose risk of PE may differ from the overall patient population, he noted.
“Different diagnostic strategies for PE have a variable performance in patients with differences of age, active cancer, and history of VTE,” said Dr. Pal. “However, in this study, no clear preference for one strategy over others could be established for these subgroups, and clinicians should continue to follow institution-specific guidance.
“A single strategy should be adopted at each institution based on local needs and used as the standard of care until further data are available,” he said.
“The use of D-dimer to rule out PE, either with fixed threshold or age-adjusted thresholds, can be confounded in clinical settings by other comorbid conditions such as sepsis, recent surgery, and more recently, COVID-19,” he said.
“Since the findings of this study do not show a clear benefit of one diagnostic strategy over others in the analyzed subgroups of patients, further prospective head-to-head comparison among the subgroups of interest would be helpful to guide clinical decision making,” Dr. Pal added.
YEARS-specific study supports D-dimer safety and value
A recent paper published in JAMA supported the results of the meta-analysis. In that study, Yonathan Freund, MD, of Sorbonne Université, Paris, and colleagues focused on the YEARS strategy combined with age-adjusted D-dimer thresholds as a way to rule out PE in PERC-positive ED patients.
The authors of this paper randomized 18 EDs to either a protocol of intervention followed by control, or control followed by intervention. The study population included 726 patients in the intervention group and 688 in the control group.
The intervention strategy to rule out PE consisted of assessing the YEARS criteria and D-dimer testing. PE was ruled out in patients with no YEARS criteria and a D-dimer level below 1,000 ng/mL and in patients with one or more YEARS criteria and D-dimers below an age-adjusted threshold (defined as age times 10 ng/mL in patients aged 50 years and older).
The control strategy consisted of D-dimer testing for all patients with the threshold at age-adjusted levels; D-dimers about these levels prompted chest imaging.
Overall, the risk of a missed VTE at 3 months was noninferior between the groups (0.15% in the intervention group and 0.80% in the controls).
“The intervention was associated with a statistically significant reduction in chest imaging use,” the researchers wrote.
This study’s findings were limited by randomization at the center level, rather than the patient level, and the use of imaging on some patients despite negative D-dimer tests, the researchers wrote. However, their findings support those of previous studies and especially support the safety of the intervention, in an emergency medicine setting, as no PEs occurred in patients with a YEARS score of zero who underwent the intervention.
Downsides to applying algorithms to every patient explained
In an editorial accompanying the JAMA study, Marcel Levi, MD, and Nick van Es, MD, of Amsterdam University Medical Center, emphasized the challenges of diagnosing PE given that many patients present with nonspecific clinical manifestations and without typical signs and symptoms. High-resolution CT pulmonary angiography allows for a fast and easy diagnosis in an emergency setting. However, efforts are ongoing to develop alternative strategies that avoid unnecessary scanning for potential PE patients, many of whom have alternative diagnoses such as pulmonary infections, cardiac conditions, pleural disease, or musculoskeletal problems.
On review of the JAMA study using the YEARS rule with adjusted D-dimer thresholds, the editorialists noted that the data were robust and indicated a 10% reduction in chest imaging. They also emphasized the potential to overwhelm busy clinicians with more algorithms.
“Blindly applying algorithms to every patient may be less appropriate or even undesirable in specific situations in which deviation from the rules on clinical grounds is indicated,” but a complex imaging approach may be time consuming and challenging in the acute setting, and a simple algorithm may be safe and efficient in many cases, they wrote. “From a patient perspective, a negative diagnostic algorithm for pulmonary embolism does not diminish the physician’s obligation to consider other diagnoses that explain the symptoms, for which chest CT scans may still be needed and helpful.”
The Annals of Internal Medicine study was supported by the Dutch Research Council. The JAMA study was supported by the French Health Ministry. Dr. Stals, Dr. Freund, Dr. Pal, Dr. Levi, and Dr. van Es had no financial conflicts to disclose.
In a patient suspected to have a PE, “diagnosis is made radiographically, usually with CT pulmonary angiogram, or V/Q scan,” Suman Pal, MD, of the University of New Mexico, Albuquerque, said in an interview.
“Validated clinical decision tools such as Wells’ score or Geneva score may be used to identify patients at low pretest probability of PE who may initially get a D-dimer level check, followed by imaging only if D-dimer level is elevated,” explained Dr. Pal, who was not involved with the new research, which was published in the Annals of Internal Medicine.
According to the authors of the new paper, while current diagnostic strategies in patients with suspected PE include use of a validated clinical decision rule (CDR) and D-dimer testing to rule out PE without imaging tests, the effectiveness of D-dimer tests in older patients, inpatients, cancer patients, and other high-risk groups has not been well-studied.
Lead author of the paper, Milou A.M. Stals, MD, and colleagues said their goal was to evaluate the safety and efficiency of the Wells rule and revised Geneva score in combination with D-dimer tests, and also the YEARS algorithm for D-dimer thresholds, in their paper.
Dr. Stals, of Leiden (the Netherlands) University Medical Center, and the coinvestigators conducted an international systemic review and individual patient data meta-analysis that included 16 studies and 20,553 patients, with all studies having been published between Jan. 1, 1995, and Jan. 1, 2021. Their primary outcomes were the safety and efficiency of each of these three strategies.
In the review, the researchers defined safety as the 3-month incidence of venous thromboembolism after PE was ruled out without imaging at baseline. They defined efficiency as the proportion patients for whom PE was ruled out based on D-dimer thresholds without imaging.
Overall, efficiency was highest in the subset of patients aged younger than 40 years, ranging from 47% to 68% in this group. Efficiency was lowest in patients aged 80 years and older (6.0%-23%), and in patients with cancer (9.6%-26%).
The efficiency was higher when D-dimer thresholds based on pretest probability were used, compared with when fixed or age-adjusted D-dimer thresholds were used.
The key finding was the significant variability in performance of the diagnostic strategies, the researchers said.
“The predicted failure rate was generally highest for strategies incorporating adapted D-dimer thresholds. However, at the same time, predicted overall efficiency was substantially higher with these strategies versus strategies with a fixed D-dimer threshold as well,” they said. Given that the benefits of each of the three diagnostic strategies depends on their correct application, the researchers recommended that an individual hospitalist choose one strategy for their institution.
“Whether clinicians should rely on the Wells rule, the YEARS algorithm, or the revised Geneva score becomes a matter of local preference and experience,” Dr. Stals and colleagues wrote.
The study findings were limited by several factors including between-study differences in scoring predictors and D-dimer assays. Another limitation was that differential verification biases for classifying fatal events and PE may have contributed to overestimation of failure rates of the adapted D-dimer thresholds.
Strengths of the study included its large sample size and original data on pretest probability, and that data support the use of any of the three strategies for ruling out PE in the identified subgroups without the need for imaging tests, the authors wrote.
“Pending the results of ongoing diagnostic randomized trials, physicians and guideline committees should balance the interlink between safety and efficiency of available diagnostic strategies,” they concluded.
Adapted D-dimer benefits some patients
“Clearly, increasing the D-dimer cutoff will lower the number of patients who require radiographic imaging (improved specificity), but this comes with a risk for missing PE (lower sensitivity). Is this risk worth taking?” Daniel J. Brotman, MD, of Johns Hopkins University, Baltimore, asked in an editorial accompanying the new study.
Dr. Brotman was not surprised by the study findings.
“Conditions that predispose to thrombosis through activated hemostasis – such as advanced age, cancer, inflammation, prolonged hospitalization, and trauma – drive D-dimer levels higher independent of the presence or absence of radiographically apparent thrombosis,” he said. However, these patients are unlikely to have normal D-dimer levels regardless of the cutoff used.
Adapted D-dimer cutoffs may benefit some patients, including those with contraindications or limited access to imaging, said Dr. Brotman. D-dimer may be used for risk stratification regardless of PE, since patients with marginally elevated D-dimers have better prognoses than those with higher D-dimer elevations, even if a small PE is missed.
Dr. Brotman wrote that increasing D-dimer cutoffs for high-risk patients in the subgroups analyzed may spare some patients radiographic testing, but doing so carries an increased risk for diagnostic failure. Overall, “the important work by Stals and colleagues offers reassurance that modifying D-dimer thresholds according to age or pretest probability is safe enough for widespread practice, even in high-risk groups.”
Focus on single strategy ‘based on local needs’
“Several validated clinical decision tools, along with age or pretest probability adjusted D-dimer threshold are currently in use as diagnostic strategies for ruling out pulmonary embolism,” Dr. Pal said in an interview.
The current study is important because of limited data on the performance of these strategies in specific subgroups of patients whose risk of PE may differ from the overall patient population, he noted.
“Different diagnostic strategies for PE have a variable performance in patients with differences of age, active cancer, and history of VTE,” said Dr. Pal. “However, in this study, no clear preference for one strategy over others could be established for these subgroups, and clinicians should continue to follow institution-specific guidance.
“A single strategy should be adopted at each institution based on local needs and used as the standard of care until further data are available,” he said.
“The use of D-dimer to rule out PE, either with fixed threshold or age-adjusted thresholds, can be confounded in clinical settings by other comorbid conditions such as sepsis, recent surgery, and more recently, COVID-19,” he said.
“Since the findings of this study do not show a clear benefit of one diagnostic strategy over others in the analyzed subgroups of patients, further prospective head-to-head comparison among the subgroups of interest would be helpful to guide clinical decision making,” Dr. Pal added.
YEARS-specific study supports D-dimer safety and value
A recent paper published in JAMA supported the results of the meta-analysis. In that study, Yonathan Freund, MD, of Sorbonne Université, Paris, and colleagues focused on the YEARS strategy combined with age-adjusted D-dimer thresholds as a way to rule out PE in PERC-positive ED patients.
The authors of this paper randomized 18 EDs to either a protocol of intervention followed by control, or control followed by intervention. The study population included 726 patients in the intervention group and 688 in the control group.
The intervention strategy to rule out PE consisted of assessing the YEARS criteria and D-dimer testing. PE was ruled out in patients with no YEARS criteria and a D-dimer level below 1,000 ng/mL and in patients with one or more YEARS criteria and D-dimers below an age-adjusted threshold (defined as age times 10 ng/mL in patients aged 50 years and older).
The control strategy consisted of D-dimer testing for all patients with the threshold at age-adjusted levels; D-dimers about these levels prompted chest imaging.
Overall, the risk of a missed VTE at 3 months was noninferior between the groups (0.15% in the intervention group and 0.80% in the controls).
“The intervention was associated with a statistically significant reduction in chest imaging use,” the researchers wrote.
This study’s findings were limited by randomization at the center level, rather than the patient level, and the use of imaging on some patients despite negative D-dimer tests, the researchers wrote. However, their findings support those of previous studies and especially support the safety of the intervention, in an emergency medicine setting, as no PEs occurred in patients with a YEARS score of zero who underwent the intervention.
Downsides to applying algorithms to every patient explained
In an editorial accompanying the JAMA study, Marcel Levi, MD, and Nick van Es, MD, of Amsterdam University Medical Center, emphasized the challenges of diagnosing PE given that many patients present with nonspecific clinical manifestations and without typical signs and symptoms. High-resolution CT pulmonary angiography allows for a fast and easy diagnosis in an emergency setting. However, efforts are ongoing to develop alternative strategies that avoid unnecessary scanning for potential PE patients, many of whom have alternative diagnoses such as pulmonary infections, cardiac conditions, pleural disease, or musculoskeletal problems.
On review of the JAMA study using the YEARS rule with adjusted D-dimer thresholds, the editorialists noted that the data were robust and indicated a 10% reduction in chest imaging. They also emphasized the potential to overwhelm busy clinicians with more algorithms.
“Blindly applying algorithms to every patient may be less appropriate or even undesirable in specific situations in which deviation from the rules on clinical grounds is indicated,” but a complex imaging approach may be time consuming and challenging in the acute setting, and a simple algorithm may be safe and efficient in many cases, they wrote. “From a patient perspective, a negative diagnostic algorithm for pulmonary embolism does not diminish the physician’s obligation to consider other diagnoses that explain the symptoms, for which chest CT scans may still be needed and helpful.”
The Annals of Internal Medicine study was supported by the Dutch Research Council. The JAMA study was supported by the French Health Ministry. Dr. Stals, Dr. Freund, Dr. Pal, Dr. Levi, and Dr. van Es had no financial conflicts to disclose.
In a patient suspected to have a PE, “diagnosis is made radiographically, usually with CT pulmonary angiogram, or V/Q scan,” Suman Pal, MD, of the University of New Mexico, Albuquerque, said in an interview.
“Validated clinical decision tools such as Wells’ score or Geneva score may be used to identify patients at low pretest probability of PE who may initially get a D-dimer level check, followed by imaging only if D-dimer level is elevated,” explained Dr. Pal, who was not involved with the new research, which was published in the Annals of Internal Medicine.
According to the authors of the new paper, while current diagnostic strategies in patients with suspected PE include use of a validated clinical decision rule (CDR) and D-dimer testing to rule out PE without imaging tests, the effectiveness of D-dimer tests in older patients, inpatients, cancer patients, and other high-risk groups has not been well-studied.
Lead author of the paper, Milou A.M. Stals, MD, and colleagues said their goal was to evaluate the safety and efficiency of the Wells rule and revised Geneva score in combination with D-dimer tests, and also the YEARS algorithm for D-dimer thresholds, in their paper.
Dr. Stals, of Leiden (the Netherlands) University Medical Center, and the coinvestigators conducted an international systemic review and individual patient data meta-analysis that included 16 studies and 20,553 patients, with all studies having been published between Jan. 1, 1995, and Jan. 1, 2021. Their primary outcomes were the safety and efficiency of each of these three strategies.
In the review, the researchers defined safety as the 3-month incidence of venous thromboembolism after PE was ruled out without imaging at baseline. They defined efficiency as the proportion patients for whom PE was ruled out based on D-dimer thresholds without imaging.
Overall, efficiency was highest in the subset of patients aged younger than 40 years, ranging from 47% to 68% in this group. Efficiency was lowest in patients aged 80 years and older (6.0%-23%), and in patients with cancer (9.6%-26%).
The efficiency was higher when D-dimer thresholds based on pretest probability were used, compared with when fixed or age-adjusted D-dimer thresholds were used.
The key finding was the significant variability in performance of the diagnostic strategies, the researchers said.
“The predicted failure rate was generally highest for strategies incorporating adapted D-dimer thresholds. However, at the same time, predicted overall efficiency was substantially higher with these strategies versus strategies with a fixed D-dimer threshold as well,” they said. Given that the benefits of each of the three diagnostic strategies depends on their correct application, the researchers recommended that an individual hospitalist choose one strategy for their institution.
“Whether clinicians should rely on the Wells rule, the YEARS algorithm, or the revised Geneva score becomes a matter of local preference and experience,” Dr. Stals and colleagues wrote.
The study findings were limited by several factors including between-study differences in scoring predictors and D-dimer assays. Another limitation was that differential verification biases for classifying fatal events and PE may have contributed to overestimation of failure rates of the adapted D-dimer thresholds.
Strengths of the study included its large sample size and original data on pretest probability, and that data support the use of any of the three strategies for ruling out PE in the identified subgroups without the need for imaging tests, the authors wrote.
“Pending the results of ongoing diagnostic randomized trials, physicians and guideline committees should balance the interlink between safety and efficiency of available diagnostic strategies,” they concluded.
Adapted D-dimer benefits some patients
“Clearly, increasing the D-dimer cutoff will lower the number of patients who require radiographic imaging (improved specificity), but this comes with a risk for missing PE (lower sensitivity). Is this risk worth taking?” Daniel J. Brotman, MD, of Johns Hopkins University, Baltimore, asked in an editorial accompanying the new study.
Dr. Brotman was not surprised by the study findings.
“Conditions that predispose to thrombosis through activated hemostasis – such as advanced age, cancer, inflammation, prolonged hospitalization, and trauma – drive D-dimer levels higher independent of the presence or absence of radiographically apparent thrombosis,” he said. However, these patients are unlikely to have normal D-dimer levels regardless of the cutoff used.
Adapted D-dimer cutoffs may benefit some patients, including those with contraindications or limited access to imaging, said Dr. Brotman. D-dimer may be used for risk stratification regardless of PE, since patients with marginally elevated D-dimers have better prognoses than those with higher D-dimer elevations, even if a small PE is missed.
Dr. Brotman wrote that increasing D-dimer cutoffs for high-risk patients in the subgroups analyzed may spare some patients radiographic testing, but doing so carries an increased risk for diagnostic failure. Overall, “the important work by Stals and colleagues offers reassurance that modifying D-dimer thresholds according to age or pretest probability is safe enough for widespread practice, even in high-risk groups.”
Focus on single strategy ‘based on local needs’
“Several validated clinical decision tools, along with age or pretest probability adjusted D-dimer threshold are currently in use as diagnostic strategies for ruling out pulmonary embolism,” Dr. Pal said in an interview.
The current study is important because of limited data on the performance of these strategies in specific subgroups of patients whose risk of PE may differ from the overall patient population, he noted.
“Different diagnostic strategies for PE have a variable performance in patients with differences of age, active cancer, and history of VTE,” said Dr. Pal. “However, in this study, no clear preference for one strategy over others could be established for these subgroups, and clinicians should continue to follow institution-specific guidance.
“A single strategy should be adopted at each institution based on local needs and used as the standard of care until further data are available,” he said.
“The use of D-dimer to rule out PE, either with fixed threshold or age-adjusted thresholds, can be confounded in clinical settings by other comorbid conditions such as sepsis, recent surgery, and more recently, COVID-19,” he said.
“Since the findings of this study do not show a clear benefit of one diagnostic strategy over others in the analyzed subgroups of patients, further prospective head-to-head comparison among the subgroups of interest would be helpful to guide clinical decision making,” Dr. Pal added.
YEARS-specific study supports D-dimer safety and value
A recent paper published in JAMA supported the results of the meta-analysis. In that study, Yonathan Freund, MD, of Sorbonne Université, Paris, and colleagues focused on the YEARS strategy combined with age-adjusted D-dimer thresholds as a way to rule out PE in PERC-positive ED patients.
The authors of this paper randomized 18 EDs to either a protocol of intervention followed by control, or control followed by intervention. The study population included 726 patients in the intervention group and 688 in the control group.
The intervention strategy to rule out PE consisted of assessing the YEARS criteria and D-dimer testing. PE was ruled out in patients with no YEARS criteria and a D-dimer level below 1,000 ng/mL and in patients with one or more YEARS criteria and D-dimers below an age-adjusted threshold (defined as age times 10 ng/mL in patients aged 50 years and older).
The control strategy consisted of D-dimer testing for all patients with the threshold at age-adjusted levels; D-dimers about these levels prompted chest imaging.
Overall, the risk of a missed VTE at 3 months was noninferior between the groups (0.15% in the intervention group and 0.80% in the controls).
“The intervention was associated with a statistically significant reduction in chest imaging use,” the researchers wrote.
This study’s findings were limited by randomization at the center level, rather than the patient level, and the use of imaging on some patients despite negative D-dimer tests, the researchers wrote. However, their findings support those of previous studies and especially support the safety of the intervention, in an emergency medicine setting, as no PEs occurred in patients with a YEARS score of zero who underwent the intervention.
Downsides to applying algorithms to every patient explained
In an editorial accompanying the JAMA study, Marcel Levi, MD, and Nick van Es, MD, of Amsterdam University Medical Center, emphasized the challenges of diagnosing PE given that many patients present with nonspecific clinical manifestations and without typical signs and symptoms. High-resolution CT pulmonary angiography allows for a fast and easy diagnosis in an emergency setting. However, efforts are ongoing to develop alternative strategies that avoid unnecessary scanning for potential PE patients, many of whom have alternative diagnoses such as pulmonary infections, cardiac conditions, pleural disease, or musculoskeletal problems.
On review of the JAMA study using the YEARS rule with adjusted D-dimer thresholds, the editorialists noted that the data were robust and indicated a 10% reduction in chest imaging. They also emphasized the potential to overwhelm busy clinicians with more algorithms.
“Blindly applying algorithms to every patient may be less appropriate or even undesirable in specific situations in which deviation from the rules on clinical grounds is indicated,” but a complex imaging approach may be time consuming and challenging in the acute setting, and a simple algorithm may be safe and efficient in many cases, they wrote. “From a patient perspective, a negative diagnostic algorithm for pulmonary embolism does not diminish the physician’s obligation to consider other diagnoses that explain the symptoms, for which chest CT scans may still be needed and helpful.”
The Annals of Internal Medicine study was supported by the Dutch Research Council. The JAMA study was supported by the French Health Ministry. Dr. Stals, Dr. Freund, Dr. Pal, Dr. Levi, and Dr. van Es had no financial conflicts to disclose.
FROM THE ANNALS OF INTERNAL MEDICINE
ACC, AHA issue new coronary revascularization guideline
Clinicians should approach decisions regarding coronary revascularization based on clinical indications without an eye toward sex, race, or ethnicity, advises a joint clinical practice guideline released Dec. 8 by the American Heart Association and American College of Cardiology.
The new class 1 recommendation leads off the 109-page document and reflects evidence demonstrating that revascularization is equally beneficial for all patients. Still, studies show that women and non-White patients are less likely to receive reperfusion therapy or revascularization.
“This was extremely important to all the committee members because of all of the disparities that have been documented not only in diagnosis but [in] the care provided to underrepresented minorities, women, and other ethnic groups,” said Jennifer S. Lawton, MD, chief of cardiac surgery at Johns Hopkins University, Baltimore, and guideline writing committee chair.
“We wanted to make it clear right at the beginning of the document that these guidelines apply to everyone, and we want it to be known that care should be the same for everyone,” she said in an interview.
The guideline was simultaneously published Dec. 9, 2021, in the journal Circulation and the Journal of the American College of Cardiology.
It updates and consolidates the ACC/AHA 2011 coronary artery bypass surgery (CABG) guideline and the ACC/AHA/Society for Cardiovascular Angiography and Interventions 2011 and 2015 percutaneous coronary intervention (PCI) guidelines.
The new document emphasizes in a class 1 recommendation the importance of the multidisciplinary heart team in patients with coronary artery disease (CAD) where the best treatment strategy is unclear. But it also stresses that treatment decisions should be patient centered – taking into account patient preferences and goals, cultural beliefs, health literacy, and social determinants of cardiovascular health – and made in collaboration with the patient’s support system.
“Oftentimes we recommend a strategy of revascularization that may not be what the patient wants or hasn’t taken into account the patient’s preferences and also the family members,” Dr. Lawson said. “So we felt that was very important.”
Patients should also be provided with available evidence for various treatment options, including risks and benefits of each option, for informed consent. The two new class 1 recommendations are highlighted in a figure illustrating the shared decision-making algorithm that, by design, features a female clinician and Black patient.
“We spent 2 years debating the best revascularization strategies and we’re considered experts in the field – but when we talk to our patients, they really don’t know the benefits and risks,” she said. “In order to translate it to the layperson in basic terms, it’s important to say, ‘If you choose this option, you will likely live longer’ rather than using the jargon.”
DAPT, staged PCI, stable IHD
Among the top 10 take-home messages highlighted by the authors is a 2a recommendation that 1-3 months of dual antiplatelet therapy (DAPT) after PCI with a transition to P2Y12 inhibitor monotherapy is “reasonable” in selected patients to reduce the risk of bleeding events. Previous recommendations called for 6 or 12 months of DAPT.
“We really respect all of the clinical trials that came out showing that a shorter duration of DAPT is not inferior in terms of ischemic events but less bleeding, yet I don’t know how many clinicians are actually just using 3 months of DAPT followed by P2Y12 monotherapy,” guideline committee vice chair Jacqueline Tamis-Holland, MD, professor of medicine, Icahn School of Medicine at Mount Sinai, New York, said in an interview. “So while it’s not a big, glaring giant recommendation, I think it will change a lot of practice.”
Similarly, she suggested that practice may shift as a result of a class 1 recommendation for staged PCI of a significantly stenosed nonculprit artery to reduce the risk for death or MI in selected hemodynamically stable patients presenting with ST-segment elevation MI and multivessel disease. “When you survey physicians, 75% of them do staged PCI but I think there will probably be more of an approach to staged PCI, as opposed to doing multivessel PCI at the time of primary PCI.”
Newer evidence from meta-analyses and the landmark ISCHEMIA trial showing no advantage of CABG over medical therapy in stable ischemic heart disease is reflected in a new class 2b recommendation – downgraded from class 1 in 2011 – that CABG “may be reasonable” to improve survival in stable patients with triple-vessel CAD.
The writing committee concluded that the ability of PCI to improve survival, compared with medical therapy in multivessel CAD “remains uncertain.”
Other recommendations likely to be of interest are that the radial artery is preferred, after the left internal mammary artery, as a surgical revascularization conduit over use of a saphenous vein conduit. Benefits include superior patency, fewer adverse cardiac events, and improved survival, the committee noted.
The radial artery is also recommended (class 1) in patients undergoing PCI who have acute coronary syndromes or stable ischemic heart disease to reduce bleeding and vascular complications compared with a femoral approach.
“Having both new radial recommendations sort of makes a bit of tension because the interventionalist is going to want to use the radial artery, but also the surgeon is too,” observed Dr. Tamis-Holland. “We see that in our own practice, so we try to have a collaborative approach to the patient to say: ‘Maybe do the cardiac cath in the dominant radial and then we can use the nondominant radial for a bypass conduit,’ but using both for each revascularization strategy will benefit the patient.
“So, we just have to remember that we’re going to talk together as a heart team and try to make the best decisions for each patient.”
A version of this article first appeared on Medscape.com.
Clinicians should approach decisions regarding coronary revascularization based on clinical indications without an eye toward sex, race, or ethnicity, advises a joint clinical practice guideline released Dec. 8 by the American Heart Association and American College of Cardiology.
The new class 1 recommendation leads off the 109-page document and reflects evidence demonstrating that revascularization is equally beneficial for all patients. Still, studies show that women and non-White patients are less likely to receive reperfusion therapy or revascularization.
“This was extremely important to all the committee members because of all of the disparities that have been documented not only in diagnosis but [in] the care provided to underrepresented minorities, women, and other ethnic groups,” said Jennifer S. Lawton, MD, chief of cardiac surgery at Johns Hopkins University, Baltimore, and guideline writing committee chair.
“We wanted to make it clear right at the beginning of the document that these guidelines apply to everyone, and we want it to be known that care should be the same for everyone,” she said in an interview.
The guideline was simultaneously published Dec. 9, 2021, in the journal Circulation and the Journal of the American College of Cardiology.
It updates and consolidates the ACC/AHA 2011 coronary artery bypass surgery (CABG) guideline and the ACC/AHA/Society for Cardiovascular Angiography and Interventions 2011 and 2015 percutaneous coronary intervention (PCI) guidelines.
The new document emphasizes in a class 1 recommendation the importance of the multidisciplinary heart team in patients with coronary artery disease (CAD) where the best treatment strategy is unclear. But it also stresses that treatment decisions should be patient centered – taking into account patient preferences and goals, cultural beliefs, health literacy, and social determinants of cardiovascular health – and made in collaboration with the patient’s support system.
“Oftentimes we recommend a strategy of revascularization that may not be what the patient wants or hasn’t taken into account the patient’s preferences and also the family members,” Dr. Lawson said. “So we felt that was very important.”
Patients should also be provided with available evidence for various treatment options, including risks and benefits of each option, for informed consent. The two new class 1 recommendations are highlighted in a figure illustrating the shared decision-making algorithm that, by design, features a female clinician and Black patient.
“We spent 2 years debating the best revascularization strategies and we’re considered experts in the field – but when we talk to our patients, they really don’t know the benefits and risks,” she said. “In order to translate it to the layperson in basic terms, it’s important to say, ‘If you choose this option, you will likely live longer’ rather than using the jargon.”
DAPT, staged PCI, stable IHD
Among the top 10 take-home messages highlighted by the authors is a 2a recommendation that 1-3 months of dual antiplatelet therapy (DAPT) after PCI with a transition to P2Y12 inhibitor monotherapy is “reasonable” in selected patients to reduce the risk of bleeding events. Previous recommendations called for 6 or 12 months of DAPT.
“We really respect all of the clinical trials that came out showing that a shorter duration of DAPT is not inferior in terms of ischemic events but less bleeding, yet I don’t know how many clinicians are actually just using 3 months of DAPT followed by P2Y12 monotherapy,” guideline committee vice chair Jacqueline Tamis-Holland, MD, professor of medicine, Icahn School of Medicine at Mount Sinai, New York, said in an interview. “So while it’s not a big, glaring giant recommendation, I think it will change a lot of practice.”
Similarly, she suggested that practice may shift as a result of a class 1 recommendation for staged PCI of a significantly stenosed nonculprit artery to reduce the risk for death or MI in selected hemodynamically stable patients presenting with ST-segment elevation MI and multivessel disease. “When you survey physicians, 75% of them do staged PCI but I think there will probably be more of an approach to staged PCI, as opposed to doing multivessel PCI at the time of primary PCI.”
Newer evidence from meta-analyses and the landmark ISCHEMIA trial showing no advantage of CABG over medical therapy in stable ischemic heart disease is reflected in a new class 2b recommendation – downgraded from class 1 in 2011 – that CABG “may be reasonable” to improve survival in stable patients with triple-vessel CAD.
The writing committee concluded that the ability of PCI to improve survival, compared with medical therapy in multivessel CAD “remains uncertain.”
Other recommendations likely to be of interest are that the radial artery is preferred, after the left internal mammary artery, as a surgical revascularization conduit over use of a saphenous vein conduit. Benefits include superior patency, fewer adverse cardiac events, and improved survival, the committee noted.
The radial artery is also recommended (class 1) in patients undergoing PCI who have acute coronary syndromes or stable ischemic heart disease to reduce bleeding and vascular complications compared with a femoral approach.
“Having both new radial recommendations sort of makes a bit of tension because the interventionalist is going to want to use the radial artery, but also the surgeon is too,” observed Dr. Tamis-Holland. “We see that in our own practice, so we try to have a collaborative approach to the patient to say: ‘Maybe do the cardiac cath in the dominant radial and then we can use the nondominant radial for a bypass conduit,’ but using both for each revascularization strategy will benefit the patient.
“So, we just have to remember that we’re going to talk together as a heart team and try to make the best decisions for each patient.”
A version of this article first appeared on Medscape.com.
Clinicians should approach decisions regarding coronary revascularization based on clinical indications without an eye toward sex, race, or ethnicity, advises a joint clinical practice guideline released Dec. 8 by the American Heart Association and American College of Cardiology.
The new class 1 recommendation leads off the 109-page document and reflects evidence demonstrating that revascularization is equally beneficial for all patients. Still, studies show that women and non-White patients are less likely to receive reperfusion therapy or revascularization.
“This was extremely important to all the committee members because of all of the disparities that have been documented not only in diagnosis but [in] the care provided to underrepresented minorities, women, and other ethnic groups,” said Jennifer S. Lawton, MD, chief of cardiac surgery at Johns Hopkins University, Baltimore, and guideline writing committee chair.
“We wanted to make it clear right at the beginning of the document that these guidelines apply to everyone, and we want it to be known that care should be the same for everyone,” she said in an interview.
The guideline was simultaneously published Dec. 9, 2021, in the journal Circulation and the Journal of the American College of Cardiology.
It updates and consolidates the ACC/AHA 2011 coronary artery bypass surgery (CABG) guideline and the ACC/AHA/Society for Cardiovascular Angiography and Interventions 2011 and 2015 percutaneous coronary intervention (PCI) guidelines.
The new document emphasizes in a class 1 recommendation the importance of the multidisciplinary heart team in patients with coronary artery disease (CAD) where the best treatment strategy is unclear. But it also stresses that treatment decisions should be patient centered – taking into account patient preferences and goals, cultural beliefs, health literacy, and social determinants of cardiovascular health – and made in collaboration with the patient’s support system.
“Oftentimes we recommend a strategy of revascularization that may not be what the patient wants or hasn’t taken into account the patient’s preferences and also the family members,” Dr. Lawson said. “So we felt that was very important.”
Patients should also be provided with available evidence for various treatment options, including risks and benefits of each option, for informed consent. The two new class 1 recommendations are highlighted in a figure illustrating the shared decision-making algorithm that, by design, features a female clinician and Black patient.
“We spent 2 years debating the best revascularization strategies and we’re considered experts in the field – but when we talk to our patients, they really don’t know the benefits and risks,” she said. “In order to translate it to the layperson in basic terms, it’s important to say, ‘If you choose this option, you will likely live longer’ rather than using the jargon.”
DAPT, staged PCI, stable IHD
Among the top 10 take-home messages highlighted by the authors is a 2a recommendation that 1-3 months of dual antiplatelet therapy (DAPT) after PCI with a transition to P2Y12 inhibitor monotherapy is “reasonable” in selected patients to reduce the risk of bleeding events. Previous recommendations called for 6 or 12 months of DAPT.
“We really respect all of the clinical trials that came out showing that a shorter duration of DAPT is not inferior in terms of ischemic events but less bleeding, yet I don’t know how many clinicians are actually just using 3 months of DAPT followed by P2Y12 monotherapy,” guideline committee vice chair Jacqueline Tamis-Holland, MD, professor of medicine, Icahn School of Medicine at Mount Sinai, New York, said in an interview. “So while it’s not a big, glaring giant recommendation, I think it will change a lot of practice.”
Similarly, she suggested that practice may shift as a result of a class 1 recommendation for staged PCI of a significantly stenosed nonculprit artery to reduce the risk for death or MI in selected hemodynamically stable patients presenting with ST-segment elevation MI and multivessel disease. “When you survey physicians, 75% of them do staged PCI but I think there will probably be more of an approach to staged PCI, as opposed to doing multivessel PCI at the time of primary PCI.”
Newer evidence from meta-analyses and the landmark ISCHEMIA trial showing no advantage of CABG over medical therapy in stable ischemic heart disease is reflected in a new class 2b recommendation – downgraded from class 1 in 2011 – that CABG “may be reasonable” to improve survival in stable patients with triple-vessel CAD.
The writing committee concluded that the ability of PCI to improve survival, compared with medical therapy in multivessel CAD “remains uncertain.”
Other recommendations likely to be of interest are that the radial artery is preferred, after the left internal mammary artery, as a surgical revascularization conduit over use of a saphenous vein conduit. Benefits include superior patency, fewer adverse cardiac events, and improved survival, the committee noted.
The radial artery is also recommended (class 1) in patients undergoing PCI who have acute coronary syndromes or stable ischemic heart disease to reduce bleeding and vascular complications compared with a femoral approach.
“Having both new radial recommendations sort of makes a bit of tension because the interventionalist is going to want to use the radial artery, but also the surgeon is too,” observed Dr. Tamis-Holland. “We see that in our own practice, so we try to have a collaborative approach to the patient to say: ‘Maybe do the cardiac cath in the dominant radial and then we can use the nondominant radial for a bypass conduit,’ but using both for each revascularization strategy will benefit the patient.
“So, we just have to remember that we’re going to talk together as a heart team and try to make the best decisions for each patient.”
A version of this article first appeared on Medscape.com.
Anticoagulant choice in antiphospholipid syndrome–associated thrombosis
Background: DOACs have largely replaced VKAs as first-line therapy for venous thromboembolism in patients with adequate renal function. However, there is concern in APS that DOACs may have higher rates of recurrent thrombosis than VKAs when treating thromboembolism.
Study design: Randomized noninferiority trial.
Setting: Six teaching hospitals in Spain.
Synopsis: Of adults with thrombotic APS, 190 were randomized to receive rivaroxaban or warfarin. Primary outcomes were thrombotic events and major bleeding. Follow-up after 3 years demonstrated new thromboses in 11 patients (11.6%) in the DOAC group and 6 patients (6.3%) in the VKA group (P = .29). Major bleeding occurred in six patients (6.3%) in the DOAC group and seven patients (7.4%) in the VKA group (P = .77). By contrast, stroke occurred in nine patients in the DOAC group while the VKA group had zero events, yielding a significant relative RR of 19.00 (95% CI, 1.12-321.90) for the DOAC group.
The DOAC arm was not proven to be noninferior with respect to the primary outcome of thrombotic events. The higher risk of stroke in this group suggests the need for caution in using DOACs in this population.
Bottom line: DOACs have a higher risk of stroke than VKAs in patients with APS without a significant difference in rate of a major bleed.
Citation: Ordi-Ros J et. al. Rivaroxaban versus vitamin K antagonist in antiphospholipid syndrome. Ann Intern Med. 2019;171(10):685-94. doi: 10.7326/M19-0291.
Dr. Portnoy is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Background: DOACs have largely replaced VKAs as first-line therapy for venous thromboembolism in patients with adequate renal function. However, there is concern in APS that DOACs may have higher rates of recurrent thrombosis than VKAs when treating thromboembolism.
Study design: Randomized noninferiority trial.
Setting: Six teaching hospitals in Spain.
Synopsis: Of adults with thrombotic APS, 190 were randomized to receive rivaroxaban or warfarin. Primary outcomes were thrombotic events and major bleeding. Follow-up after 3 years demonstrated new thromboses in 11 patients (11.6%) in the DOAC group and 6 patients (6.3%) in the VKA group (P = .29). Major bleeding occurred in six patients (6.3%) in the DOAC group and seven patients (7.4%) in the VKA group (P = .77). By contrast, stroke occurred in nine patients in the DOAC group while the VKA group had zero events, yielding a significant relative RR of 19.00 (95% CI, 1.12-321.90) for the DOAC group.
The DOAC arm was not proven to be noninferior with respect to the primary outcome of thrombotic events. The higher risk of stroke in this group suggests the need for caution in using DOACs in this population.
Bottom line: DOACs have a higher risk of stroke than VKAs in patients with APS without a significant difference in rate of a major bleed.
Citation: Ordi-Ros J et. al. Rivaroxaban versus vitamin K antagonist in antiphospholipid syndrome. Ann Intern Med. 2019;171(10):685-94. doi: 10.7326/M19-0291.
Dr. Portnoy is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Background: DOACs have largely replaced VKAs as first-line therapy for venous thromboembolism in patients with adequate renal function. However, there is concern in APS that DOACs may have higher rates of recurrent thrombosis than VKAs when treating thromboembolism.
Study design: Randomized noninferiority trial.
Setting: Six teaching hospitals in Spain.
Synopsis: Of adults with thrombotic APS, 190 were randomized to receive rivaroxaban or warfarin. Primary outcomes were thrombotic events and major bleeding. Follow-up after 3 years demonstrated new thromboses in 11 patients (11.6%) in the DOAC group and 6 patients (6.3%) in the VKA group (P = .29). Major bleeding occurred in six patients (6.3%) in the DOAC group and seven patients (7.4%) in the VKA group (P = .77). By contrast, stroke occurred in nine patients in the DOAC group while the VKA group had zero events, yielding a significant relative RR of 19.00 (95% CI, 1.12-321.90) for the DOAC group.
The DOAC arm was not proven to be noninferior with respect to the primary outcome of thrombotic events. The higher risk of stroke in this group suggests the need for caution in using DOACs in this population.
Bottom line: DOACs have a higher risk of stroke than VKAs in patients with APS without a significant difference in rate of a major bleed.
Citation: Ordi-Ros J et. al. Rivaroxaban versus vitamin K antagonist in antiphospholipid syndrome. Ann Intern Med. 2019;171(10):685-94. doi: 10.7326/M19-0291.
Dr. Portnoy is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Apixaban a reasonable alternative to warfarin in patients with severe renal impairment
Background: Over 6 million Americans are prescribed anticoagulation; however, available anticoagulation options for patients with concomitant renal impairment are limited. Until recently, warfarin was the only recommended option because of a lack of data to support the use of alternative agents in such patients. This study evaluates the safety and effectiveness of apixaban, compared with warfarin, in patients with severe renal dysfunction.
Study design: Multicenter retrospective cohort study.
Setting: Seven hospitals in Michigan between January 2013 and December 2015 and including adult patients with CrCl less than 25 cc/min who were newly initiated on apixaban or warfarin.
Synopsis: Patients in the apixaban group (n=128) had a higher rate of heart failure, atrial fibrillation, stent placement, and hyperlipidemia, while the warfarin group (n=733) had a higher rate of prior venous thromboembolism. The primary outcome was time to first bleeding or thrombotic event. Apixaban was associated with a lower risk of thrombotic or bleeding events, compared with warfarin (HR, 0.47). Post-hoc analysis controlling for patient differences showed similar results. There was no statistical difference in the severity of events or overall mortality. Further subgroup analysis showed that 5 mg B.I.D. dosing was not associated with higher risk of bleeding than 2.5 mg B.I.D.
The main limitation is the retrospective observational design, which may have introduced confounding variables that were not accounted for in the analyses. The study also did not account for patient nonadherence to medication.
Bottom line: Apixaban is a reasonable alternative to warfarin in patients with severe renal impairment.
Citation: Hanni C et al. Outcomes associated with apixaban vs. warfarin in patients with renal dysfunction. Blood Adv. 2020;4(11): 2366-71. doi: 10.1182/bloodadvances.2019000972.
Dr. Narayan is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Background: Over 6 million Americans are prescribed anticoagulation; however, available anticoagulation options for patients with concomitant renal impairment are limited. Until recently, warfarin was the only recommended option because of a lack of data to support the use of alternative agents in such patients. This study evaluates the safety and effectiveness of apixaban, compared with warfarin, in patients with severe renal dysfunction.
Study design: Multicenter retrospective cohort study.
Setting: Seven hospitals in Michigan between January 2013 and December 2015 and including adult patients with CrCl less than 25 cc/min who were newly initiated on apixaban or warfarin.
Synopsis: Patients in the apixaban group (n=128) had a higher rate of heart failure, atrial fibrillation, stent placement, and hyperlipidemia, while the warfarin group (n=733) had a higher rate of prior venous thromboembolism. The primary outcome was time to first bleeding or thrombotic event. Apixaban was associated with a lower risk of thrombotic or bleeding events, compared with warfarin (HR, 0.47). Post-hoc analysis controlling for patient differences showed similar results. There was no statistical difference in the severity of events or overall mortality. Further subgroup analysis showed that 5 mg B.I.D. dosing was not associated with higher risk of bleeding than 2.5 mg B.I.D.
The main limitation is the retrospective observational design, which may have introduced confounding variables that were not accounted for in the analyses. The study also did not account for patient nonadherence to medication.
Bottom line: Apixaban is a reasonable alternative to warfarin in patients with severe renal impairment.
Citation: Hanni C et al. Outcomes associated with apixaban vs. warfarin in patients with renal dysfunction. Blood Adv. 2020;4(11): 2366-71. doi: 10.1182/bloodadvances.2019000972.
Dr. Narayan is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Background: Over 6 million Americans are prescribed anticoagulation; however, available anticoagulation options for patients with concomitant renal impairment are limited. Until recently, warfarin was the only recommended option because of a lack of data to support the use of alternative agents in such patients. This study evaluates the safety and effectiveness of apixaban, compared with warfarin, in patients with severe renal dysfunction.
Study design: Multicenter retrospective cohort study.
Setting: Seven hospitals in Michigan between January 2013 and December 2015 and including adult patients with CrCl less than 25 cc/min who were newly initiated on apixaban or warfarin.
Synopsis: Patients in the apixaban group (n=128) had a higher rate of heart failure, atrial fibrillation, stent placement, and hyperlipidemia, while the warfarin group (n=733) had a higher rate of prior venous thromboembolism. The primary outcome was time to first bleeding or thrombotic event. Apixaban was associated with a lower risk of thrombotic or bleeding events, compared with warfarin (HR, 0.47). Post-hoc analysis controlling for patient differences showed similar results. There was no statistical difference in the severity of events or overall mortality. Further subgroup analysis showed that 5 mg B.I.D. dosing was not associated with higher risk of bleeding than 2.5 mg B.I.D.
The main limitation is the retrospective observational design, which may have introduced confounding variables that were not accounted for in the analyses. The study also did not account for patient nonadherence to medication.
Bottom line: Apixaban is a reasonable alternative to warfarin in patients with severe renal impairment.
Citation: Hanni C et al. Outcomes associated with apixaban vs. warfarin in patients with renal dysfunction. Blood Adv. 2020;4(11): 2366-71. doi: 10.1182/bloodadvances.2019000972.
Dr. Narayan is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Apixaban outmatches rivaroxaban for VTE in study
Recurrent venous thromboembolism (VTE) – a composite of pulmonary embolism and deep vein thrombosis – was the primary effectiveness outcome in the retrospective analysis of new-user data from almost 40,000 patients, which was published in Annals of Internal Medicine. Safety was evaluated through a composite of intracranial and gastrointestinal bleeding.
After a median follow-up of 102 days in the apixaban group and 105 days in the rivaroxaban group, apixaban demonstrated superiority for both primary outcomes.
These real-world findings may guide selection of initial anticoagulant therapy, reported lead author Ghadeer K. Dawwas, PhD, MSc, MBA, of the University of Pennsylvania, Philadelphia, and colleagues.
“Randomized clinical trials comparing apixaban with rivaroxaban in patients with VTE are under way (for example, COBRRA (NCT03266783),” the investigators wrote. “Until the results from these trials become available (The estimated completion date for COBRRA is December 2023.), observational studies that use existing data can provide evidence on the effectiveness and safety of these alternatives to inform clinical practice.”
In the new research, apixaban was associated with a 23% lower rate of recurrent VTE (hazard ratio, 0.77; 95% confidence interval, 0.69-0.87), including a 15% lower rate of deep vein thrombosis and a 41% lower rate of pulmonary embolism. Apixaban was associated with 40% fewer bleeding events (HR, 0.60; 95% CI, 0.53-0.69]), including a 40% lower rate of GI bleeding and a 46% lower rate of intracranial bleeding.
The study involved 37,236 patients with VTE, all of whom were diagnosed in at least one inpatient encounter and initiated direct oral anticoagulant (DOAC) therapy within 30 days, according to Optum’s deidentified Clinformatics Data Mart Database. Patients were evenly split into apixaban and rivaroxaban groups, with 18,618 individuals in each. Propensity score matching was used to minimize differences in baseline characteristics.
Apixaban was associated with an absolute reduction in recurrent VTE of 0.6% and 1.1% over 2 and 6 months, respectively, as well as reductions in bleeding of 1.1% and 1.5% over the same respective time periods.
The investigators noted that these findings were maintained in various sensitivity and subgroup analyses, including a model in which patients with VTE who had transient risk factors were compared with VTE patients exhibiting chronic risk factors.
“These findings suggest that apixaban has superior effectiveness and safety, compared with rivaroxaban and may provide guidance to clinicians and patients regarding selection of an anticoagulant for treatment of VTE,” Dr. Dawwas and colleagues concluded.
Study may have missed some nuance in possible outcomes, according to vascular surgeon
Thomas Wakefield, MD, a vascular surgeon and a professor of surgery at the University of Michigan Health Frankel Cardiovascular Center, Ann Arbor, generally agreed with the investigators’ conclusion, although he noted that DOAC selection may also be influenced by other considerations.
“The results of this study suggest that, when choosing an agent for an individual patient, apixaban does appear to have an advantage over rivaroxaban related to recurrent VTE and bleeding,” Dr. Wakefield said in an interview. “One must keep in mind that these are not the only factors that are considered when choosing an agent and these are not the only two DOACs available. For example, rivaroxaban is given once per day while apixaban is given twice per day, and rivaroxaban has been shown to be successful in the treatment of other thrombotic disorders.”
Dr. Wakefield also pointed out that the study may have missed some nuance in possible outcomes.
“The current study looked at severe outcomes that resulted in inpatient hospitalization, so the generalization to strictly outpatient treatment and less severe outcomes cannot be inferred,” he said.
Damon E. Houghton, MD, of the department of medicine and a consultant in the department of vascular medicine and hematology at Mayo Clinic, Rochester, Minn., called the study a “very nice analysis,” highlighting the large sample size.
“The results are not a reason to abandon rivaroxaban altogether, but do suggest that, when otherwise appropriate for a patient, apixaban should be the first choice,” Dr. Houghton said in a written comment. “Hopefully this analysis will encourage more payers to create financial incentives that facilitate the use of apixaban in more patients.”
Randomized trial needed, says hematologist
Colleen Edwards, MD, of the departments of medicine, hematology, and medical oncology, at the Icahn School of Medicine at Mount Sinai, New York, had a more guarded view of the findings than Dr. Wakefield and Dr. Houghton.
“[The investigators] certainly seem to be doing a lot of statistical gymnastics in this paper,” Dr. Edwards said in an interview. “They used all kinds of surrogates in place of real data that you would get from a randomized trial.”
For example, Dr. Edwards noted the use of prescription refills as a surrogate for medication adherence, and emphasized that inpatient observational data may not reflect outpatient therapy.
“Inpatients are constantly missing their medicines all the time,” she said. “They’re holding it for procedures, they’re NPO, they’re off the floor, so they missed their medicine. So it’s just a very different patient population than the outpatient population, which is where venous thromboembolism is treated now, by and large.”
Although Dr. Edwards suggested that the findings might guide treatment selection “a little bit,” she noted that insurance constraints and costs play a greater role, and ultimately concluded that a randomized trial is needed to materially alter clinical decision-making.
“I think we really have to wait for randomized trial before we abandon our other choices,” she said.
The investigators disclosed relationships with Merck, Celgene, UCB, and others. Dr. Wakefield reported awaiting disclosures. Dr. Houghton and Dr. Edwards reported no relevant conflicts of interest.
Recurrent venous thromboembolism (VTE) – a composite of pulmonary embolism and deep vein thrombosis – was the primary effectiveness outcome in the retrospective analysis of new-user data from almost 40,000 patients, which was published in Annals of Internal Medicine. Safety was evaluated through a composite of intracranial and gastrointestinal bleeding.
After a median follow-up of 102 days in the apixaban group and 105 days in the rivaroxaban group, apixaban demonstrated superiority for both primary outcomes.
These real-world findings may guide selection of initial anticoagulant therapy, reported lead author Ghadeer K. Dawwas, PhD, MSc, MBA, of the University of Pennsylvania, Philadelphia, and colleagues.
“Randomized clinical trials comparing apixaban with rivaroxaban in patients with VTE are under way (for example, COBRRA (NCT03266783),” the investigators wrote. “Until the results from these trials become available (The estimated completion date for COBRRA is December 2023.), observational studies that use existing data can provide evidence on the effectiveness and safety of these alternatives to inform clinical practice.”
In the new research, apixaban was associated with a 23% lower rate of recurrent VTE (hazard ratio, 0.77; 95% confidence interval, 0.69-0.87), including a 15% lower rate of deep vein thrombosis and a 41% lower rate of pulmonary embolism. Apixaban was associated with 40% fewer bleeding events (HR, 0.60; 95% CI, 0.53-0.69]), including a 40% lower rate of GI bleeding and a 46% lower rate of intracranial bleeding.
The study involved 37,236 patients with VTE, all of whom were diagnosed in at least one inpatient encounter and initiated direct oral anticoagulant (DOAC) therapy within 30 days, according to Optum’s deidentified Clinformatics Data Mart Database. Patients were evenly split into apixaban and rivaroxaban groups, with 18,618 individuals in each. Propensity score matching was used to minimize differences in baseline characteristics.
Apixaban was associated with an absolute reduction in recurrent VTE of 0.6% and 1.1% over 2 and 6 months, respectively, as well as reductions in bleeding of 1.1% and 1.5% over the same respective time periods.
The investigators noted that these findings were maintained in various sensitivity and subgroup analyses, including a model in which patients with VTE who had transient risk factors were compared with VTE patients exhibiting chronic risk factors.
“These findings suggest that apixaban has superior effectiveness and safety, compared with rivaroxaban and may provide guidance to clinicians and patients regarding selection of an anticoagulant for treatment of VTE,” Dr. Dawwas and colleagues concluded.
Study may have missed some nuance in possible outcomes, according to vascular surgeon
Thomas Wakefield, MD, a vascular surgeon and a professor of surgery at the University of Michigan Health Frankel Cardiovascular Center, Ann Arbor, generally agreed with the investigators’ conclusion, although he noted that DOAC selection may also be influenced by other considerations.
“The results of this study suggest that, when choosing an agent for an individual patient, apixaban does appear to have an advantage over rivaroxaban related to recurrent VTE and bleeding,” Dr. Wakefield said in an interview. “One must keep in mind that these are not the only factors that are considered when choosing an agent and these are not the only two DOACs available. For example, rivaroxaban is given once per day while apixaban is given twice per day, and rivaroxaban has been shown to be successful in the treatment of other thrombotic disorders.”
Dr. Wakefield also pointed out that the study may have missed some nuance in possible outcomes.
“The current study looked at severe outcomes that resulted in inpatient hospitalization, so the generalization to strictly outpatient treatment and less severe outcomes cannot be inferred,” he said.
Damon E. Houghton, MD, of the department of medicine and a consultant in the department of vascular medicine and hematology at Mayo Clinic, Rochester, Minn., called the study a “very nice analysis,” highlighting the large sample size.
“The results are not a reason to abandon rivaroxaban altogether, but do suggest that, when otherwise appropriate for a patient, apixaban should be the first choice,” Dr. Houghton said in a written comment. “Hopefully this analysis will encourage more payers to create financial incentives that facilitate the use of apixaban in more patients.”
Randomized trial needed, says hematologist
Colleen Edwards, MD, of the departments of medicine, hematology, and medical oncology, at the Icahn School of Medicine at Mount Sinai, New York, had a more guarded view of the findings than Dr. Wakefield and Dr. Houghton.
“[The investigators] certainly seem to be doing a lot of statistical gymnastics in this paper,” Dr. Edwards said in an interview. “They used all kinds of surrogates in place of real data that you would get from a randomized trial.”
For example, Dr. Edwards noted the use of prescription refills as a surrogate for medication adherence, and emphasized that inpatient observational data may not reflect outpatient therapy.
“Inpatients are constantly missing their medicines all the time,” she said. “They’re holding it for procedures, they’re NPO, they’re off the floor, so they missed their medicine. So it’s just a very different patient population than the outpatient population, which is where venous thromboembolism is treated now, by and large.”
Although Dr. Edwards suggested that the findings might guide treatment selection “a little bit,” she noted that insurance constraints and costs play a greater role, and ultimately concluded that a randomized trial is needed to materially alter clinical decision-making.
“I think we really have to wait for randomized trial before we abandon our other choices,” she said.
The investigators disclosed relationships with Merck, Celgene, UCB, and others. Dr. Wakefield reported awaiting disclosures. Dr. Houghton and Dr. Edwards reported no relevant conflicts of interest.
Recurrent venous thromboembolism (VTE) – a composite of pulmonary embolism and deep vein thrombosis – was the primary effectiveness outcome in the retrospective analysis of new-user data from almost 40,000 patients, which was published in Annals of Internal Medicine. Safety was evaluated through a composite of intracranial and gastrointestinal bleeding.
After a median follow-up of 102 days in the apixaban group and 105 days in the rivaroxaban group, apixaban demonstrated superiority for both primary outcomes.
These real-world findings may guide selection of initial anticoagulant therapy, reported lead author Ghadeer K. Dawwas, PhD, MSc, MBA, of the University of Pennsylvania, Philadelphia, and colleagues.
“Randomized clinical trials comparing apixaban with rivaroxaban in patients with VTE are under way (for example, COBRRA (NCT03266783),” the investigators wrote. “Until the results from these trials become available (The estimated completion date for COBRRA is December 2023.), observational studies that use existing data can provide evidence on the effectiveness and safety of these alternatives to inform clinical practice.”
In the new research, apixaban was associated with a 23% lower rate of recurrent VTE (hazard ratio, 0.77; 95% confidence interval, 0.69-0.87), including a 15% lower rate of deep vein thrombosis and a 41% lower rate of pulmonary embolism. Apixaban was associated with 40% fewer bleeding events (HR, 0.60; 95% CI, 0.53-0.69]), including a 40% lower rate of GI bleeding and a 46% lower rate of intracranial bleeding.
The study involved 37,236 patients with VTE, all of whom were diagnosed in at least one inpatient encounter and initiated direct oral anticoagulant (DOAC) therapy within 30 days, according to Optum’s deidentified Clinformatics Data Mart Database. Patients were evenly split into apixaban and rivaroxaban groups, with 18,618 individuals in each. Propensity score matching was used to minimize differences in baseline characteristics.
Apixaban was associated with an absolute reduction in recurrent VTE of 0.6% and 1.1% over 2 and 6 months, respectively, as well as reductions in bleeding of 1.1% and 1.5% over the same respective time periods.
The investigators noted that these findings were maintained in various sensitivity and subgroup analyses, including a model in which patients with VTE who had transient risk factors were compared with VTE patients exhibiting chronic risk factors.
“These findings suggest that apixaban has superior effectiveness and safety, compared with rivaroxaban and may provide guidance to clinicians and patients regarding selection of an anticoagulant for treatment of VTE,” Dr. Dawwas and colleagues concluded.
Study may have missed some nuance in possible outcomes, according to vascular surgeon
Thomas Wakefield, MD, a vascular surgeon and a professor of surgery at the University of Michigan Health Frankel Cardiovascular Center, Ann Arbor, generally agreed with the investigators’ conclusion, although he noted that DOAC selection may also be influenced by other considerations.
“The results of this study suggest that, when choosing an agent for an individual patient, apixaban does appear to have an advantage over rivaroxaban related to recurrent VTE and bleeding,” Dr. Wakefield said in an interview. “One must keep in mind that these are not the only factors that are considered when choosing an agent and these are not the only two DOACs available. For example, rivaroxaban is given once per day while apixaban is given twice per day, and rivaroxaban has been shown to be successful in the treatment of other thrombotic disorders.”
Dr. Wakefield also pointed out that the study may have missed some nuance in possible outcomes.
“The current study looked at severe outcomes that resulted in inpatient hospitalization, so the generalization to strictly outpatient treatment and less severe outcomes cannot be inferred,” he said.
Damon E. Houghton, MD, of the department of medicine and a consultant in the department of vascular medicine and hematology at Mayo Clinic, Rochester, Minn., called the study a “very nice analysis,” highlighting the large sample size.
“The results are not a reason to abandon rivaroxaban altogether, but do suggest that, when otherwise appropriate for a patient, apixaban should be the first choice,” Dr. Houghton said in a written comment. “Hopefully this analysis will encourage more payers to create financial incentives that facilitate the use of apixaban in more patients.”
Randomized trial needed, says hematologist
Colleen Edwards, MD, of the departments of medicine, hematology, and medical oncology, at the Icahn School of Medicine at Mount Sinai, New York, had a more guarded view of the findings than Dr. Wakefield and Dr. Houghton.
“[The investigators] certainly seem to be doing a lot of statistical gymnastics in this paper,” Dr. Edwards said in an interview. “They used all kinds of surrogates in place of real data that you would get from a randomized trial.”
For example, Dr. Edwards noted the use of prescription refills as a surrogate for medication adherence, and emphasized that inpatient observational data may not reflect outpatient therapy.
“Inpatients are constantly missing their medicines all the time,” she said. “They’re holding it for procedures, they’re NPO, they’re off the floor, so they missed their medicine. So it’s just a very different patient population than the outpatient population, which is where venous thromboembolism is treated now, by and large.”
Although Dr. Edwards suggested that the findings might guide treatment selection “a little bit,” she noted that insurance constraints and costs play a greater role, and ultimately concluded that a randomized trial is needed to materially alter clinical decision-making.
“I think we really have to wait for randomized trial before we abandon our other choices,” she said.
The investigators disclosed relationships with Merck, Celgene, UCB, and others. Dr. Wakefield reported awaiting disclosures. Dr. Houghton and Dr. Edwards reported no relevant conflicts of interest.
FROM ANNALS OF INTERNAL MEDICINE