User login
Most Common Dermatologic Conditions Encountered by Dermatologists and Nondermatologists
Skin diseases are highly prevalent in the United States, affecting an estimated 1 in 3 Americans at any given time.1,2 In 2009 the direct medical costs associated with skin-related diseases, including health services and prescriptions, was approximately $22 billion; the annual total economic burden was estimated to be closer to $96 billion when factoring in the cost of lost productivity and pay for symptom relief.3,4 Effective and efficient management of skin disease is essential to minimizing cost and morbidity. Nondermatologists traditionally have diagnosed the majority of skin diseases.5,6 In particular, primary care physicians commonly manage dermatologic conditions and often are the first health care providers to encounter patients presenting with skin problems. A predicted shortage of dermatologists will likely contribute to an increase in this trend.7,8 Therefore, it is important to adequately prepare nondermatologists to evaluate and treat the skin conditions that they are most likely to encounter in their scope of practice.
Residents, particularly in primary care specialties, often have opportunities to spend 2 to 4 weeks with a dermatologist to learn about skin diseases; however, the skin conditions most often encountered by dermatologists may differ from those most often encountered by physicians in other specialties. For instance, one study demonstrated a disparity between the most common skin problems seen by dermatologists and internists.9 These dissimilarities should be recognized and addressed in curriculum content. The purpose of this study was to identify and compare the 20 most common dermatologic conditions reported by dermatologists versus those reported by nondermatologists (ie, internists, pediatricians, family physicians, emergency medicine physicians, general surgeons, otolaryngologists) from 2001 to 2010. Data also were analyzed to determine the top 20 conditions referred to dermatologists by nondermatologists as a potential indicator for areas of further improvement within medical education. With this knowledge, we hope educational curricula and self-study can be modified to reflect the current epidemiology of cutaneous diseases, thereby improving patient care.
Methods
Data from 2001 to 2010 were extracted from the National Ambulatory Medical Care Survey (NAMCS), which is an ongoing survey conducted by the National Center for Health Statistics. The NAMCS collects descriptive data regarding ambulatory visits to nonfederal office-based physicians in the United States. Participating physicians are instructed to record information about patient visits for a 1-week period, including patient demographics, insurance status, reason for visit, diagnoses, procedures, therapeutics, and referrals made at that time. Data collected for the NAMCS are entered into a multistage probability sample to produce national estimates. Within dermatology, an average of 118 dermatologists are sampled each year, and over the last 10 years, participation rates have ranged from 47% to 77%.
International Classification of Diseases, Ninth Revision, Clinical Modification codes were identified to determine the diagnoses that could be classified as dermatologic conditions. Select infectious and neoplastic disorders of the skin and mucous membrane conditions were included as well as the codes for skin diseases. Nondermatologic diagnoses and V codes were not included in the study. Data for all providers were studied to identify outpatient visits associated with the primary diagnosis of a dermatologic condition. Minor diagnoses that were considered to be subsets of major diagnoses were combined to allow better analysis of the data. For example, all tinea infections (ie, dermatophytosis of various sites, dermatomycosis unspecified) were combined into 1 diagnosis referred to as tinea because the recognition and treatment of this disease does not vary tremendously by anatomic location. Visits to dermatologists that listed nonspecific diagnoses and codes (eg, other postsurgical status [V45.89], neoplasm of uncertain behavior site unspecified [238.9]) were assumed to be for dermatologic problems.
Sampling weights were applied to obtain estimates for the number of each diagnosis made nationally. All data analyses were performed using SAS software and linear regression models were generated using SAS PROC SURVEYREG.
Data were analyzed to determine the dermatologic conditions most commonly encountered by dermatologists and nondermatologists in emergency medicine, family medicine, general surgery, internal medicine, otolaryngology, and pediatrics; these specialties include physicians who are known to commonly diagnose and treat skin diseases.10 Data also were analyzed to determine the most common conditions referred to dermatologists for treatment by nondermatologists from the selected specialties. Permission to conduct this study was obtained from the Wake Forest University institutional review board (Winston-Salem, North Carolina).
Results
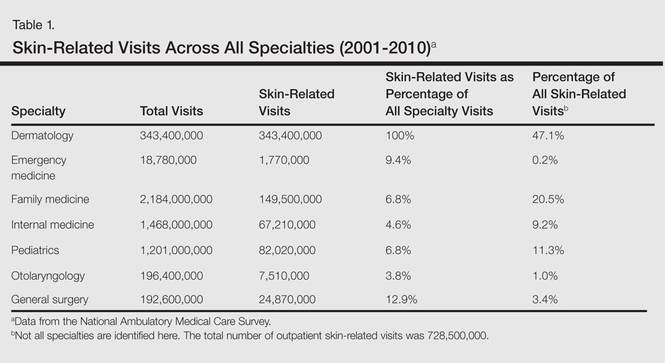
From 2001 to 2010, more than 700 million outpatient visits for skin-related problems were identified, with 676.3 million visits to dermatologists, emergency medicine physicians, family practitioners, general surgeons, internists, otolaryngologists, and pediatricians. More than half (52.9%) of all skin-related visits were addressed by nondermatologists during this time. Among nondermatologists, family practitioners encountered the greatest number of skin diseases (20.5%), followed by pediatricians (11.3%), internists (9.2%), general surgeons (3.4%), otolaryngologists (1.0%), and emergency medicine physicians (0.2%)(Table 1).
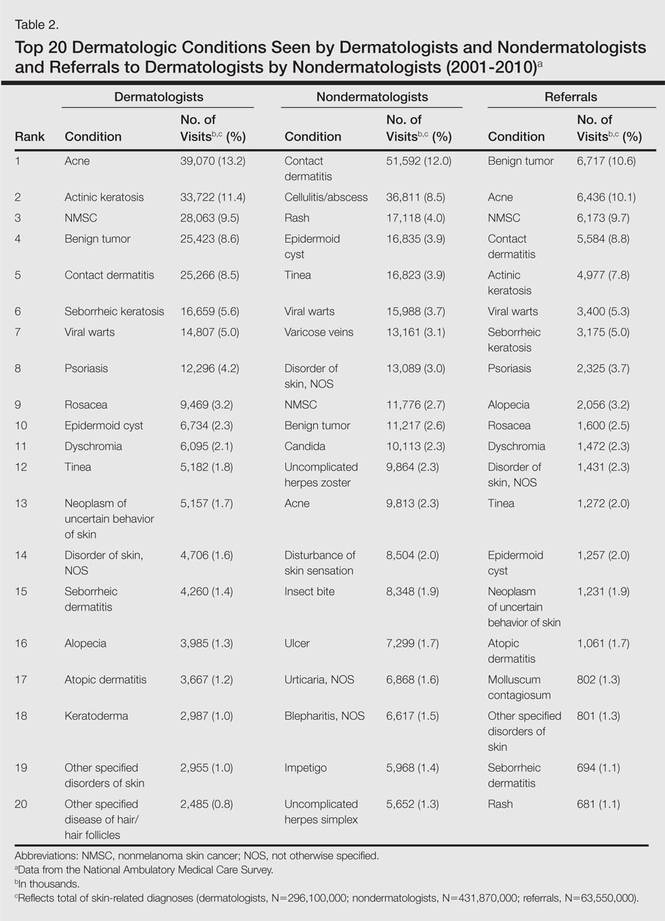
Benign tumors and acne were the most common cutaneous conditions referred to dermatologists by nondermatologists (10.6% and 10.1% of all dermatology referrals, respectively), followed by nonmelanoma skin cancers (9.7%), contact dermatitis (8.8%), and actinic keratosis (7.8%)(Table 2). The top 20 conditions referred to dermatologists accounted for 83.7% of all outpatient referrals to dermatologists.
Among the diseases most frequently reported by nondermatologists, contact dermatitis was the most common (12.0%), with twice the number of visits to nondermatologists for contact dermatitis than to dermatologists (51.6 million vs 25.3 million). In terms of disease categories, infectious skin diseases (ie, bacterial [cellulitis/abscess], viral [warts, herpesvirus], fungal [tinea] and yeast [candida] etiologies) were the most common dermatologic conditions reported by nondermatologists (Table 2).
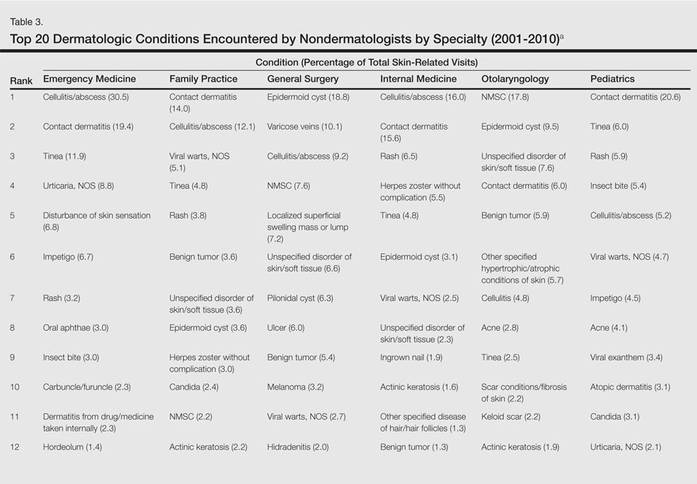
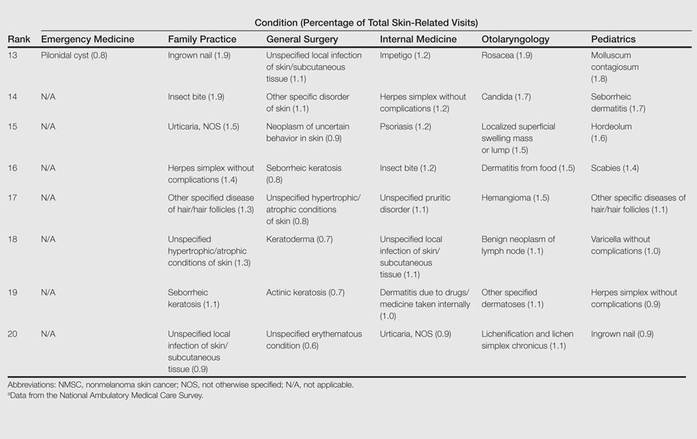
The top 20 dermatologic conditions reported by dermatologists accounted for 85.4% of all diagnoses made by dermatologists. Diseases that were among the top 20 conditions encountered by dermatologists but were not among the top 20 for nondermatologists included actinic keratosis, seborrheic keratosis, atopic dermatitis, psoriasis, alopecia, rosacea, dyschromia, seborrheic dermatitis, follicular disease, and neoplasm of uncertain behavior of skin. Additionally, 5 of the top 20 conditions encountered by dermatologists also were among the top 20 for only 1 individual nondermatologic specialty; these included atopic dermatitis (pediatrics), seborrheic dermatitis (pediatrics), psoriasis (internal medicine), rosacea (otolaryngology), and keratoderma (general surgery). Seborrheic dermatitis, psoriasis, and rosacea also were among the top 20 conditions most commonly referred to dermatologists for treatment by nondermatologists. Table 3 shows the top 20 dermatologic conditions encountered by nondermatologists by comparison.
Comment
According to NAMCS data from 2001 to 2010, visits to nondermatologists accounted for more than half of total outpatient visits for cutaneous diseases in the United States, whereas visits to dermatologists accounted for 47.1%. These findings are consistent with historical data indicating that 30% to 40% of skin-related visits are to dermatologists, and the majority of patients with skin disease are diagnosed by nondermatologists.5,6
Past data indicate that most visits to dermatologists were for evaluation of acne, infections, psoriasis, and neoplasms, whereas most visits to nondermatologists were for evaluation of epidermoid cysts, impetigo, plant dermatitis, cellulitis, and diaper rash.9 Over the last 10 years, acne has been more commonly encountered by nondermatologists, especially pediatricians. Additionally, infectious etiologies have been seen in larger volume by nondermatologists.9 Together, infectious cutaneous conditions make up nearly one-fourth of dermatologic encounters by emergency medicine physicians, internists, and family practitioners but are not within the top 20 diagnoses referred to dermatologists, which suggests that uncomplicated cases of cellulitis, herpes zoster, and other skin-related infections are largely managed by nondermatologists.5,6 Contact dermatitis, often caused by specific allergens such as detergents, solvents, and topical products, was one of the most common reported dermatologic encounters among dermatologists and nondermatologists and also was the fourth most common condition referred to dermatologists by nondermatologists for treatment; however, there may be an element of overuse of the International Classification of Diseases, Ninth Revision code, as any presumed contact dermatitis of unspecified cause can be reported under 692.9 defined as contact dermatitis and other eczema, unspecified cause. The high rate of referrals to dermatologists by nondermatologists may be for patch testing and further management. Additionally, there are no specific codes for allergic or irritant dermatitis, thus these diseases may be lumped together.
Although nearly half of all dermatologic encounters were seen by nondermatologists, dermatologists see a much larger proportion of patients with skin disease than nondermatologists and nondermatologists often have limited exposure to the field of dermatology during residency training. Studies have demonstrated differences in the abilities of dermatologists and nondermatologists to correctly diagnose common cutaneous diseases, which unsurprisingly revealed greater diagnostic accuracy demonstrated by dermatologists.11-16 The increase in acne and skin-related infections reported by nondermatologists is consistent with possible efforts to increase formal training in frequently encountered skin diseases. In one study evaluating the impact of a formal 3-week dermatology curriculum on an internal medicine department, internists demonstrated 100% accuracy in the diagnosis of acne and herpes zoster in contrast to 29% for tinea and 12% for lichen planus.5,6
The current Accreditation Council for Graduate Medical Education guidelines place little emphasis on exposure to dermatology training during residency for internists and pediatricians, as this training is not a required component of these programs.17 Two core problems with current training regarding the evaluation and management of cutaneous disease are minimal exposure to dermatologic conditions in medical school and residency and lack of consensus on the core topics that should be taught to nondermatologists.18 Exposure to dermatologic conditions through rotations in medical school has been shown to increase residents’ self-reported confidence in diagnosing and treating alopecia, cutaneous drug eruptions, warts, acne, rosacea, nonmelanoma skin cancers, sun damage, psoriasis, seborrhea, atopic dermatitis, and contact dermatitis; however, the majority of primary care residents surveyed still felt that this exposure in medical school was inadequate.19
In creating a core curriculum for dermatology training for nondermatologists, it is important to consider the dermatologic conditions that are most frequently encountered by these specialties. Our study revealed that the most commonly encountered dermatologic conditions differ among dermatologists and nondermatologists, with a fair degree of variation even among individual specialties. Failure to recognize these discrepancies has likely contributed to the challenges faced by nondermatologists in the diagnosis and management of dermatologic disease. In this study, contact dermatitis, epidermoid cysts, and skin infections were the most common dermatologic conditions encountered by nondermatologists and also were among the top skin diseases referred to dermatologists by nondermatologists. This finding suggests that nondermatologists are able to identify these conditions but have a tendency to refer approximately 10% of these patients to dermatology for further management. Clinical evaluation and medical management of these cutaneous diseases may be an important area of focus for medical school curricula, as the treatment of these diseases is within the capabilities of the nondermatologist. For example, initial management of dermatitis requires determination of the type of dermatitis (ie, essential, contact, atopic, seborrheic, stasis) and selection of an appropriate topical steroid, with referral to a dermatologist needed for questionable or refractory cases. Although a curriculum cannot be built solely on a list of the top 20 diagnoses provided here, these data may serve as a preliminary platform for medical school dermatology curriculum design. The curriculum also should include serious skin diseases, such as melanoma and severe drug eruptions. Although these conditions are less commonly encountered by nondermatologists, missed diagnosis and/or improper management can be life threatening.
The use of NAMCS data presents a few limitations. For instance, these data only represent outpatient management of skin disease. There is the potential for misdiagnosis and coding errors by the reporting physicians. The volume of data (ie, billions of office visits) prevents verification of diagnostic accuracy. The coding system requires physicians to give a diagnosis but does not provide any means by which to determine the physician’s confidence in that diagnosis. There is no code for “uncertain” or “diagnosis not determined.” Additionally, an “unspecified” diagnosis may reflect uncertainty or may simply imply that no other code accurately described the condition. Despite these limitations, the NAMCS database is a large, nationally representative survey of actual patient visits and represents some of the best data available for a study such as ours.
Conclusion
This study provides an important analysis of the most common outpatient dermatologic conditions encountered by dermatologists and nondermatologists of various specialties and offers a foundation from which to construct curricula for dermatology training tailored to individual specialties based on their needs. In the future, identification of the most common inpatient dermatologic conditions managed by each specialty also may benefit curriculum design.
- Thorpe KE, Florence CS, Joski P. Which medical conditions account for the rise in health care spending? Health Aff (Millwood). 2004;(suppl web exclusives):W4-437-445.
- Johnson ML. Defining the burden of skin disease in the United States—a historical perspective. J Investig Dermatol Symp Proc. 2004;9:108-110.
- Agency for Healthcare Research and Quality. Medical expenditure panel survey. US Department of Health & Human Services Web site. http://meps.ahrq.gov. Accessed November 17, 2014.
- Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490-500.
- Johnson ML. On teaching dermatology to nondermatologists. Arch Dermatol. 1994;130:850-852.
- Ramsay DL, Weary PE. Primary care in dermatology: whose role should it be? J Am Acad Dermatol. 1996;35:1005-1008.
- Kimball AB, Resneck JS Jr. The US dermatology workforce: a specialty remains in shortage. J Am Acad Dermatol. 2008;59:741-745.
- Resneck JS Jr, Kimball AB. Who else is providing care in dermatology practices? trends in the use of nonphysician clinicians. J Am Acad Dermatol. 2008;58:211-216.
- Feldman SR, Fleischer AB Jr, McConnell RC. Most common dermatologic problems identified by internists, 1990-1994. Arch Intern Med. 1998;158:726-730.
- Ahn CS, Davis SA, Debade TS, et al. Noncosmetic skin-related procedures performed in the United States: an analysis of national ambulatory medical care survey data from 1995 to 2010. Dermatol Surg. 2013;39:1912-1921.
- Antic M, Conen D, Itin PH. Teaching effects of dermatological consultations on nondermatologists in the field of internal medicine. a study of 1290 inpatients. Dermatology. 2004;208:32-37.
- Federman DG, Concato J, Kirsner RS. Comparison of dermatologic diagnoses by primary care practitioners and dermatologists. a review of the literature. Arch Fam Med. 1999;8:170-172.
- Fleischer AB Jr, Herbert CR, Feldman SR, et al. Diagnosis of skin disease by nondermatologists. Am J Manag Care. 2000;6:1149-1156.
- Kirsner RS, Federman DG. Lack of correlation between internists’ ability in dermatology and their patterns of treating patients with skin disease. Arch Dermatol. 1996;132:1043-1046.
- McCarthy GM, Lamb GC, Russell TJ, et al. Primary care-based dermatology practice: internists need more training. J Gen Intern Med. 1991;6:52-56.
- Sellheyer K, Bergfeld WF. A retrospective biopsy study of the clinical diagnostic accuracy of common skin diseases by different specialties compared with dermatology. J Am Acad Dermatol. 2005;52:823-830.
- Medical specialties. Accreditation Council for Graduate Medical Education Web site. http://www.acgme.org/acgmeweb/tabid/368ProgramandInstitutionalGuidelines/MedicalAccreditation.aspx. Accessed November 17, 2014.
- McCleskey PE, Gilson RT, DeVillez RL. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.
- Hansra NK, O’Sullivan P, Chen CL, et al. Medical school dermatology curriculum: are we adequately preparing primary care physicians? J Am Acad Dermatol. 2009;61:23-29.
Skin diseases are highly prevalent in the United States, affecting an estimated 1 in 3 Americans at any given time.1,2 In 2009 the direct medical costs associated with skin-related diseases, including health services and prescriptions, was approximately $22 billion; the annual total economic burden was estimated to be closer to $96 billion when factoring in the cost of lost productivity and pay for symptom relief.3,4 Effective and efficient management of skin disease is essential to minimizing cost and morbidity. Nondermatologists traditionally have diagnosed the majority of skin diseases.5,6 In particular, primary care physicians commonly manage dermatologic conditions and often are the first health care providers to encounter patients presenting with skin problems. A predicted shortage of dermatologists will likely contribute to an increase in this trend.7,8 Therefore, it is important to adequately prepare nondermatologists to evaluate and treat the skin conditions that they are most likely to encounter in their scope of practice.
Residents, particularly in primary care specialties, often have opportunities to spend 2 to 4 weeks with a dermatologist to learn about skin diseases; however, the skin conditions most often encountered by dermatologists may differ from those most often encountered by physicians in other specialties. For instance, one study demonstrated a disparity between the most common skin problems seen by dermatologists and internists.9 These dissimilarities should be recognized and addressed in curriculum content. The purpose of this study was to identify and compare the 20 most common dermatologic conditions reported by dermatologists versus those reported by nondermatologists (ie, internists, pediatricians, family physicians, emergency medicine physicians, general surgeons, otolaryngologists) from 2001 to 2010. Data also were analyzed to determine the top 20 conditions referred to dermatologists by nondermatologists as a potential indicator for areas of further improvement within medical education. With this knowledge, we hope educational curricula and self-study can be modified to reflect the current epidemiology of cutaneous diseases, thereby improving patient care.
Methods
Data from 2001 to 2010 were extracted from the National Ambulatory Medical Care Survey (NAMCS), which is an ongoing survey conducted by the National Center for Health Statistics. The NAMCS collects descriptive data regarding ambulatory visits to nonfederal office-based physicians in the United States. Participating physicians are instructed to record information about patient visits for a 1-week period, including patient demographics, insurance status, reason for visit, diagnoses, procedures, therapeutics, and referrals made at that time. Data collected for the NAMCS are entered into a multistage probability sample to produce national estimates. Within dermatology, an average of 118 dermatologists are sampled each year, and over the last 10 years, participation rates have ranged from 47% to 77%.
International Classification of Diseases, Ninth Revision, Clinical Modification codes were identified to determine the diagnoses that could be classified as dermatologic conditions. Select infectious and neoplastic disorders of the skin and mucous membrane conditions were included as well as the codes for skin diseases. Nondermatologic diagnoses and V codes were not included in the study. Data for all providers were studied to identify outpatient visits associated with the primary diagnosis of a dermatologic condition. Minor diagnoses that were considered to be subsets of major diagnoses were combined to allow better analysis of the data. For example, all tinea infections (ie, dermatophytosis of various sites, dermatomycosis unspecified) were combined into 1 diagnosis referred to as tinea because the recognition and treatment of this disease does not vary tremendously by anatomic location. Visits to dermatologists that listed nonspecific diagnoses and codes (eg, other postsurgical status [V45.89], neoplasm of uncertain behavior site unspecified [238.9]) were assumed to be for dermatologic problems.
Sampling weights were applied to obtain estimates for the number of each diagnosis made nationally. All data analyses were performed using SAS software and linear regression models were generated using SAS PROC SURVEYREG.
Data were analyzed to determine the dermatologic conditions most commonly encountered by dermatologists and nondermatologists in emergency medicine, family medicine, general surgery, internal medicine, otolaryngology, and pediatrics; these specialties include physicians who are known to commonly diagnose and treat skin diseases.10 Data also were analyzed to determine the most common conditions referred to dermatologists for treatment by nondermatologists from the selected specialties. Permission to conduct this study was obtained from the Wake Forest University institutional review board (Winston-Salem, North Carolina).
Results
From 2001 to 2010, more than 700 million outpatient visits for skin-related problems were identified, with 676.3 million visits to dermatologists, emergency medicine physicians, family practitioners, general surgeons, internists, otolaryngologists, and pediatricians. More than half (52.9%) of all skin-related visits were addressed by nondermatologists during this time. Among nondermatologists, family practitioners encountered the greatest number of skin diseases (20.5%), followed by pediatricians (11.3%), internists (9.2%), general surgeons (3.4%), otolaryngologists (1.0%), and emergency medicine physicians (0.2%)(Table 1).

Benign tumors and acne were the most common cutaneous conditions referred to dermatologists by nondermatologists (10.6% and 10.1% of all dermatology referrals, respectively), followed by nonmelanoma skin cancers (9.7%), contact dermatitis (8.8%), and actinic keratosis (7.8%)(Table 2). The top 20 conditions referred to dermatologists accounted for 83.7% of all outpatient referrals to dermatologists.
Among the diseases most frequently reported by nondermatologists, contact dermatitis was the most common (12.0%), with twice the number of visits to nondermatologists for contact dermatitis than to dermatologists (51.6 million vs 25.3 million). In terms of disease categories, infectious skin diseases (ie, bacterial [cellulitis/abscess], viral [warts, herpesvirus], fungal [tinea] and yeast [candida] etiologies) were the most common dermatologic conditions reported by nondermatologists (Table 2).

The top 20 dermatologic conditions reported by dermatologists accounted for 85.4% of all diagnoses made by dermatologists. Diseases that were among the top 20 conditions encountered by dermatologists but were not among the top 20 for nondermatologists included actinic keratosis, seborrheic keratosis, atopic dermatitis, psoriasis, alopecia, rosacea, dyschromia, seborrheic dermatitis, follicular disease, and neoplasm of uncertain behavior of skin. Additionally, 5 of the top 20 conditions encountered by dermatologists also were among the top 20 for only 1 individual nondermatologic specialty; these included atopic dermatitis (pediatrics), seborrheic dermatitis (pediatrics), psoriasis (internal medicine), rosacea (otolaryngology), and keratoderma (general surgery). Seborrheic dermatitis, psoriasis, and rosacea also were among the top 20 conditions most commonly referred to dermatologists for treatment by nondermatologists. Table 3 shows the top 20 dermatologic conditions encountered by nondermatologists by comparison.


Comment
According to NAMCS data from 2001 to 2010, visits to nondermatologists accounted for more than half of total outpatient visits for cutaneous diseases in the United States, whereas visits to dermatologists accounted for 47.1%. These findings are consistent with historical data indicating that 30% to 40% of skin-related visits are to dermatologists, and the majority of patients with skin disease are diagnosed by nondermatologists.5,6
Past data indicate that most visits to dermatologists were for evaluation of acne, infections, psoriasis, and neoplasms, whereas most visits to nondermatologists were for evaluation of epidermoid cysts, impetigo, plant dermatitis, cellulitis, and diaper rash.9 Over the last 10 years, acne has been more commonly encountered by nondermatologists, especially pediatricians. Additionally, infectious etiologies have been seen in larger volume by nondermatologists.9 Together, infectious cutaneous conditions make up nearly one-fourth of dermatologic encounters by emergency medicine physicians, internists, and family practitioners but are not within the top 20 diagnoses referred to dermatologists, which suggests that uncomplicated cases of cellulitis, herpes zoster, and other skin-related infections are largely managed by nondermatologists.5,6 Contact dermatitis, often caused by specific allergens such as detergents, solvents, and topical products, was one of the most common reported dermatologic encounters among dermatologists and nondermatologists and also was the fourth most common condition referred to dermatologists by nondermatologists for treatment; however, there may be an element of overuse of the International Classification of Diseases, Ninth Revision code, as any presumed contact dermatitis of unspecified cause can be reported under 692.9 defined as contact dermatitis and other eczema, unspecified cause. The high rate of referrals to dermatologists by nondermatologists may be for patch testing and further management. Additionally, there are no specific codes for allergic or irritant dermatitis, thus these diseases may be lumped together.
Although nearly half of all dermatologic encounters were seen by nondermatologists, dermatologists see a much larger proportion of patients with skin disease than nondermatologists and nondermatologists often have limited exposure to the field of dermatology during residency training. Studies have demonstrated differences in the abilities of dermatologists and nondermatologists to correctly diagnose common cutaneous diseases, which unsurprisingly revealed greater diagnostic accuracy demonstrated by dermatologists.11-16 The increase in acne and skin-related infections reported by nondermatologists is consistent with possible efforts to increase formal training in frequently encountered skin diseases. In one study evaluating the impact of a formal 3-week dermatology curriculum on an internal medicine department, internists demonstrated 100% accuracy in the diagnosis of acne and herpes zoster in contrast to 29% for tinea and 12% for lichen planus.5,6
The current Accreditation Council for Graduate Medical Education guidelines place little emphasis on exposure to dermatology training during residency for internists and pediatricians, as this training is not a required component of these programs.17 Two core problems with current training regarding the evaluation and management of cutaneous disease are minimal exposure to dermatologic conditions in medical school and residency and lack of consensus on the core topics that should be taught to nondermatologists.18 Exposure to dermatologic conditions through rotations in medical school has been shown to increase residents’ self-reported confidence in diagnosing and treating alopecia, cutaneous drug eruptions, warts, acne, rosacea, nonmelanoma skin cancers, sun damage, psoriasis, seborrhea, atopic dermatitis, and contact dermatitis; however, the majority of primary care residents surveyed still felt that this exposure in medical school was inadequate.19
In creating a core curriculum for dermatology training for nondermatologists, it is important to consider the dermatologic conditions that are most frequently encountered by these specialties. Our study revealed that the most commonly encountered dermatologic conditions differ among dermatologists and nondermatologists, with a fair degree of variation even among individual specialties. Failure to recognize these discrepancies has likely contributed to the challenges faced by nondermatologists in the diagnosis and management of dermatologic disease. In this study, contact dermatitis, epidermoid cysts, and skin infections were the most common dermatologic conditions encountered by nondermatologists and also were among the top skin diseases referred to dermatologists by nondermatologists. This finding suggests that nondermatologists are able to identify these conditions but have a tendency to refer approximately 10% of these patients to dermatology for further management. Clinical evaluation and medical management of these cutaneous diseases may be an important area of focus for medical school curricula, as the treatment of these diseases is within the capabilities of the nondermatologist. For example, initial management of dermatitis requires determination of the type of dermatitis (ie, essential, contact, atopic, seborrheic, stasis) and selection of an appropriate topical steroid, with referral to a dermatologist needed for questionable or refractory cases. Although a curriculum cannot be built solely on a list of the top 20 diagnoses provided here, these data may serve as a preliminary platform for medical school dermatology curriculum design. The curriculum also should include serious skin diseases, such as melanoma and severe drug eruptions. Although these conditions are less commonly encountered by nondermatologists, missed diagnosis and/or improper management can be life threatening.
The use of NAMCS data presents a few limitations. For instance, these data only represent outpatient management of skin disease. There is the potential for misdiagnosis and coding errors by the reporting physicians. The volume of data (ie, billions of office visits) prevents verification of diagnostic accuracy. The coding system requires physicians to give a diagnosis but does not provide any means by which to determine the physician’s confidence in that diagnosis. There is no code for “uncertain” or “diagnosis not determined.” Additionally, an “unspecified” diagnosis may reflect uncertainty or may simply imply that no other code accurately described the condition. Despite these limitations, the NAMCS database is a large, nationally representative survey of actual patient visits and represents some of the best data available for a study such as ours.
Conclusion
This study provides an important analysis of the most common outpatient dermatologic conditions encountered by dermatologists and nondermatologists of various specialties and offers a foundation from which to construct curricula for dermatology training tailored to individual specialties based on their needs. In the future, identification of the most common inpatient dermatologic conditions managed by each specialty also may benefit curriculum design.
Skin diseases are highly prevalent in the United States, affecting an estimated 1 in 3 Americans at any given time.1,2 In 2009 the direct medical costs associated with skin-related diseases, including health services and prescriptions, was approximately $22 billion; the annual total economic burden was estimated to be closer to $96 billion when factoring in the cost of lost productivity and pay for symptom relief.3,4 Effective and efficient management of skin disease is essential to minimizing cost and morbidity. Nondermatologists traditionally have diagnosed the majority of skin diseases.5,6 In particular, primary care physicians commonly manage dermatologic conditions and often are the first health care providers to encounter patients presenting with skin problems. A predicted shortage of dermatologists will likely contribute to an increase in this trend.7,8 Therefore, it is important to adequately prepare nondermatologists to evaluate and treat the skin conditions that they are most likely to encounter in their scope of practice.
Residents, particularly in primary care specialties, often have opportunities to spend 2 to 4 weeks with a dermatologist to learn about skin diseases; however, the skin conditions most often encountered by dermatologists may differ from those most often encountered by physicians in other specialties. For instance, one study demonstrated a disparity between the most common skin problems seen by dermatologists and internists.9 These dissimilarities should be recognized and addressed in curriculum content. The purpose of this study was to identify and compare the 20 most common dermatologic conditions reported by dermatologists versus those reported by nondermatologists (ie, internists, pediatricians, family physicians, emergency medicine physicians, general surgeons, otolaryngologists) from 2001 to 2010. Data also were analyzed to determine the top 20 conditions referred to dermatologists by nondermatologists as a potential indicator for areas of further improvement within medical education. With this knowledge, we hope educational curricula and self-study can be modified to reflect the current epidemiology of cutaneous diseases, thereby improving patient care.
Methods
Data from 2001 to 2010 were extracted from the National Ambulatory Medical Care Survey (NAMCS), which is an ongoing survey conducted by the National Center for Health Statistics. The NAMCS collects descriptive data regarding ambulatory visits to nonfederal office-based physicians in the United States. Participating physicians are instructed to record information about patient visits for a 1-week period, including patient demographics, insurance status, reason for visit, diagnoses, procedures, therapeutics, and referrals made at that time. Data collected for the NAMCS are entered into a multistage probability sample to produce national estimates. Within dermatology, an average of 118 dermatologists are sampled each year, and over the last 10 years, participation rates have ranged from 47% to 77%.
International Classification of Diseases, Ninth Revision, Clinical Modification codes were identified to determine the diagnoses that could be classified as dermatologic conditions. Select infectious and neoplastic disorders of the skin and mucous membrane conditions were included as well as the codes for skin diseases. Nondermatologic diagnoses and V codes were not included in the study. Data for all providers were studied to identify outpatient visits associated with the primary diagnosis of a dermatologic condition. Minor diagnoses that were considered to be subsets of major diagnoses were combined to allow better analysis of the data. For example, all tinea infections (ie, dermatophytosis of various sites, dermatomycosis unspecified) were combined into 1 diagnosis referred to as tinea because the recognition and treatment of this disease does not vary tremendously by anatomic location. Visits to dermatologists that listed nonspecific diagnoses and codes (eg, other postsurgical status [V45.89], neoplasm of uncertain behavior site unspecified [238.9]) were assumed to be for dermatologic problems.
Sampling weights were applied to obtain estimates for the number of each diagnosis made nationally. All data analyses were performed using SAS software and linear regression models were generated using SAS PROC SURVEYREG.
Data were analyzed to determine the dermatologic conditions most commonly encountered by dermatologists and nondermatologists in emergency medicine, family medicine, general surgery, internal medicine, otolaryngology, and pediatrics; these specialties include physicians who are known to commonly diagnose and treat skin diseases.10 Data also were analyzed to determine the most common conditions referred to dermatologists for treatment by nondermatologists from the selected specialties. Permission to conduct this study was obtained from the Wake Forest University institutional review board (Winston-Salem, North Carolina).
Results
From 2001 to 2010, more than 700 million outpatient visits for skin-related problems were identified, with 676.3 million visits to dermatologists, emergency medicine physicians, family practitioners, general surgeons, internists, otolaryngologists, and pediatricians. More than half (52.9%) of all skin-related visits were addressed by nondermatologists during this time. Among nondermatologists, family practitioners encountered the greatest number of skin diseases (20.5%), followed by pediatricians (11.3%), internists (9.2%), general surgeons (3.4%), otolaryngologists (1.0%), and emergency medicine physicians (0.2%)(Table 1).

Benign tumors and acne were the most common cutaneous conditions referred to dermatologists by nondermatologists (10.6% and 10.1% of all dermatology referrals, respectively), followed by nonmelanoma skin cancers (9.7%), contact dermatitis (8.8%), and actinic keratosis (7.8%)(Table 2). The top 20 conditions referred to dermatologists accounted for 83.7% of all outpatient referrals to dermatologists.
Among the diseases most frequently reported by nondermatologists, contact dermatitis was the most common (12.0%), with twice the number of visits to nondermatologists for contact dermatitis than to dermatologists (51.6 million vs 25.3 million). In terms of disease categories, infectious skin diseases (ie, bacterial [cellulitis/abscess], viral [warts, herpesvirus], fungal [tinea] and yeast [candida] etiologies) were the most common dermatologic conditions reported by nondermatologists (Table 2).

The top 20 dermatologic conditions reported by dermatologists accounted for 85.4% of all diagnoses made by dermatologists. Diseases that were among the top 20 conditions encountered by dermatologists but were not among the top 20 for nondermatologists included actinic keratosis, seborrheic keratosis, atopic dermatitis, psoriasis, alopecia, rosacea, dyschromia, seborrheic dermatitis, follicular disease, and neoplasm of uncertain behavior of skin. Additionally, 5 of the top 20 conditions encountered by dermatologists also were among the top 20 for only 1 individual nondermatologic specialty; these included atopic dermatitis (pediatrics), seborrheic dermatitis (pediatrics), psoriasis (internal medicine), rosacea (otolaryngology), and keratoderma (general surgery). Seborrheic dermatitis, psoriasis, and rosacea also were among the top 20 conditions most commonly referred to dermatologists for treatment by nondermatologists. Table 3 shows the top 20 dermatologic conditions encountered by nondermatologists by comparison.


Comment
According to NAMCS data from 2001 to 2010, visits to nondermatologists accounted for more than half of total outpatient visits for cutaneous diseases in the United States, whereas visits to dermatologists accounted for 47.1%. These findings are consistent with historical data indicating that 30% to 40% of skin-related visits are to dermatologists, and the majority of patients with skin disease are diagnosed by nondermatologists.5,6
Past data indicate that most visits to dermatologists were for evaluation of acne, infections, psoriasis, and neoplasms, whereas most visits to nondermatologists were for evaluation of epidermoid cysts, impetigo, plant dermatitis, cellulitis, and diaper rash.9 Over the last 10 years, acne has been more commonly encountered by nondermatologists, especially pediatricians. Additionally, infectious etiologies have been seen in larger volume by nondermatologists.9 Together, infectious cutaneous conditions make up nearly one-fourth of dermatologic encounters by emergency medicine physicians, internists, and family practitioners but are not within the top 20 diagnoses referred to dermatologists, which suggests that uncomplicated cases of cellulitis, herpes zoster, and other skin-related infections are largely managed by nondermatologists.5,6 Contact dermatitis, often caused by specific allergens such as detergents, solvents, and topical products, was one of the most common reported dermatologic encounters among dermatologists and nondermatologists and also was the fourth most common condition referred to dermatologists by nondermatologists for treatment; however, there may be an element of overuse of the International Classification of Diseases, Ninth Revision code, as any presumed contact dermatitis of unspecified cause can be reported under 692.9 defined as contact dermatitis and other eczema, unspecified cause. The high rate of referrals to dermatologists by nondermatologists may be for patch testing and further management. Additionally, there are no specific codes for allergic or irritant dermatitis, thus these diseases may be lumped together.
Although nearly half of all dermatologic encounters were seen by nondermatologists, dermatologists see a much larger proportion of patients with skin disease than nondermatologists and nondermatologists often have limited exposure to the field of dermatology during residency training. Studies have demonstrated differences in the abilities of dermatologists and nondermatologists to correctly diagnose common cutaneous diseases, which unsurprisingly revealed greater diagnostic accuracy demonstrated by dermatologists.11-16 The increase in acne and skin-related infections reported by nondermatologists is consistent with possible efforts to increase formal training in frequently encountered skin diseases. In one study evaluating the impact of a formal 3-week dermatology curriculum on an internal medicine department, internists demonstrated 100% accuracy in the diagnosis of acne and herpes zoster in contrast to 29% for tinea and 12% for lichen planus.5,6
The current Accreditation Council for Graduate Medical Education guidelines place little emphasis on exposure to dermatology training during residency for internists and pediatricians, as this training is not a required component of these programs.17 Two core problems with current training regarding the evaluation and management of cutaneous disease are minimal exposure to dermatologic conditions in medical school and residency and lack of consensus on the core topics that should be taught to nondermatologists.18 Exposure to dermatologic conditions through rotations in medical school has been shown to increase residents’ self-reported confidence in diagnosing and treating alopecia, cutaneous drug eruptions, warts, acne, rosacea, nonmelanoma skin cancers, sun damage, psoriasis, seborrhea, atopic dermatitis, and contact dermatitis; however, the majority of primary care residents surveyed still felt that this exposure in medical school was inadequate.19
In creating a core curriculum for dermatology training for nondermatologists, it is important to consider the dermatologic conditions that are most frequently encountered by these specialties. Our study revealed that the most commonly encountered dermatologic conditions differ among dermatologists and nondermatologists, with a fair degree of variation even among individual specialties. Failure to recognize these discrepancies has likely contributed to the challenges faced by nondermatologists in the diagnosis and management of dermatologic disease. In this study, contact dermatitis, epidermoid cysts, and skin infections were the most common dermatologic conditions encountered by nondermatologists and also were among the top skin diseases referred to dermatologists by nondermatologists. This finding suggests that nondermatologists are able to identify these conditions but have a tendency to refer approximately 10% of these patients to dermatology for further management. Clinical evaluation and medical management of these cutaneous diseases may be an important area of focus for medical school curricula, as the treatment of these diseases is within the capabilities of the nondermatologist. For example, initial management of dermatitis requires determination of the type of dermatitis (ie, essential, contact, atopic, seborrheic, stasis) and selection of an appropriate topical steroid, with referral to a dermatologist needed for questionable or refractory cases. Although a curriculum cannot be built solely on a list of the top 20 diagnoses provided here, these data may serve as a preliminary platform for medical school dermatology curriculum design. The curriculum also should include serious skin diseases, such as melanoma and severe drug eruptions. Although these conditions are less commonly encountered by nondermatologists, missed diagnosis and/or improper management can be life threatening.
The use of NAMCS data presents a few limitations. For instance, these data only represent outpatient management of skin disease. There is the potential for misdiagnosis and coding errors by the reporting physicians. The volume of data (ie, billions of office visits) prevents verification of diagnostic accuracy. The coding system requires physicians to give a diagnosis but does not provide any means by which to determine the physician’s confidence in that diagnosis. There is no code for “uncertain” or “diagnosis not determined.” Additionally, an “unspecified” diagnosis may reflect uncertainty or may simply imply that no other code accurately described the condition. Despite these limitations, the NAMCS database is a large, nationally representative survey of actual patient visits and represents some of the best data available for a study such as ours.
Conclusion
This study provides an important analysis of the most common outpatient dermatologic conditions encountered by dermatologists and nondermatologists of various specialties and offers a foundation from which to construct curricula for dermatology training tailored to individual specialties based on their needs. In the future, identification of the most common inpatient dermatologic conditions managed by each specialty also may benefit curriculum design.
- Thorpe KE, Florence CS, Joski P. Which medical conditions account for the rise in health care spending? Health Aff (Millwood). 2004;(suppl web exclusives):W4-437-445.
- Johnson ML. Defining the burden of skin disease in the United States—a historical perspective. J Investig Dermatol Symp Proc. 2004;9:108-110.
- Agency for Healthcare Research and Quality. Medical expenditure panel survey. US Department of Health & Human Services Web site. http://meps.ahrq.gov. Accessed November 17, 2014.
- Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490-500.
- Johnson ML. On teaching dermatology to nondermatologists. Arch Dermatol. 1994;130:850-852.
- Ramsay DL, Weary PE. Primary care in dermatology: whose role should it be? J Am Acad Dermatol. 1996;35:1005-1008.
- Kimball AB, Resneck JS Jr. The US dermatology workforce: a specialty remains in shortage. J Am Acad Dermatol. 2008;59:741-745.
- Resneck JS Jr, Kimball AB. Who else is providing care in dermatology practices? trends in the use of nonphysician clinicians. J Am Acad Dermatol. 2008;58:211-216.
- Feldman SR, Fleischer AB Jr, McConnell RC. Most common dermatologic problems identified by internists, 1990-1994. Arch Intern Med. 1998;158:726-730.
- Ahn CS, Davis SA, Debade TS, et al. Noncosmetic skin-related procedures performed in the United States: an analysis of national ambulatory medical care survey data from 1995 to 2010. Dermatol Surg. 2013;39:1912-1921.
- Antic M, Conen D, Itin PH. Teaching effects of dermatological consultations on nondermatologists in the field of internal medicine. a study of 1290 inpatients. Dermatology. 2004;208:32-37.
- Federman DG, Concato J, Kirsner RS. Comparison of dermatologic diagnoses by primary care practitioners and dermatologists. a review of the literature. Arch Fam Med. 1999;8:170-172.
- Fleischer AB Jr, Herbert CR, Feldman SR, et al. Diagnosis of skin disease by nondermatologists. Am J Manag Care. 2000;6:1149-1156.
- Kirsner RS, Federman DG. Lack of correlation between internists’ ability in dermatology and their patterns of treating patients with skin disease. Arch Dermatol. 1996;132:1043-1046.
- McCarthy GM, Lamb GC, Russell TJ, et al. Primary care-based dermatology practice: internists need more training. J Gen Intern Med. 1991;6:52-56.
- Sellheyer K, Bergfeld WF. A retrospective biopsy study of the clinical diagnostic accuracy of common skin diseases by different specialties compared with dermatology. J Am Acad Dermatol. 2005;52:823-830.
- Medical specialties. Accreditation Council for Graduate Medical Education Web site. http://www.acgme.org/acgmeweb/tabid/368ProgramandInstitutionalGuidelines/MedicalAccreditation.aspx. Accessed November 17, 2014.
- McCleskey PE, Gilson RT, DeVillez RL. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.
- Hansra NK, O’Sullivan P, Chen CL, et al. Medical school dermatology curriculum: are we adequately preparing primary care physicians? J Am Acad Dermatol. 2009;61:23-29.
- Thorpe KE, Florence CS, Joski P. Which medical conditions account for the rise in health care spending? Health Aff (Millwood). 2004;(suppl web exclusives):W4-437-445.
- Johnson ML. Defining the burden of skin disease in the United States—a historical perspective. J Investig Dermatol Symp Proc. 2004;9:108-110.
- Agency for Healthcare Research and Quality. Medical expenditure panel survey. US Department of Health & Human Services Web site. http://meps.ahrq.gov. Accessed November 17, 2014.
- Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490-500.
- Johnson ML. On teaching dermatology to nondermatologists. Arch Dermatol. 1994;130:850-852.
- Ramsay DL, Weary PE. Primary care in dermatology: whose role should it be? J Am Acad Dermatol. 1996;35:1005-1008.
- Kimball AB, Resneck JS Jr. The US dermatology workforce: a specialty remains in shortage. J Am Acad Dermatol. 2008;59:741-745.
- Resneck JS Jr, Kimball AB. Who else is providing care in dermatology practices? trends in the use of nonphysician clinicians. J Am Acad Dermatol. 2008;58:211-216.
- Feldman SR, Fleischer AB Jr, McConnell RC. Most common dermatologic problems identified by internists, 1990-1994. Arch Intern Med. 1998;158:726-730.
- Ahn CS, Davis SA, Debade TS, et al. Noncosmetic skin-related procedures performed in the United States: an analysis of national ambulatory medical care survey data from 1995 to 2010. Dermatol Surg. 2013;39:1912-1921.
- Antic M, Conen D, Itin PH. Teaching effects of dermatological consultations on nondermatologists in the field of internal medicine. a study of 1290 inpatients. Dermatology. 2004;208:32-37.
- Federman DG, Concato J, Kirsner RS. Comparison of dermatologic diagnoses by primary care practitioners and dermatologists. a review of the literature. Arch Fam Med. 1999;8:170-172.
- Fleischer AB Jr, Herbert CR, Feldman SR, et al. Diagnosis of skin disease by nondermatologists. Am J Manag Care. 2000;6:1149-1156.
- Kirsner RS, Federman DG. Lack of correlation between internists’ ability in dermatology and their patterns of treating patients with skin disease. Arch Dermatol. 1996;132:1043-1046.
- McCarthy GM, Lamb GC, Russell TJ, et al. Primary care-based dermatology practice: internists need more training. J Gen Intern Med. 1991;6:52-56.
- Sellheyer K, Bergfeld WF. A retrospective biopsy study of the clinical diagnostic accuracy of common skin diseases by different specialties compared with dermatology. J Am Acad Dermatol. 2005;52:823-830.
- Medical specialties. Accreditation Council for Graduate Medical Education Web site. http://www.acgme.org/acgmeweb/tabid/368ProgramandInstitutionalGuidelines/MedicalAccreditation.aspx. Accessed November 17, 2014.
- McCleskey PE, Gilson RT, DeVillez RL. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.
- Hansra NK, O’Sullivan P, Chen CL, et al. Medical school dermatology curriculum: are we adequately preparing primary care physicians? J Am Acad Dermatol. 2009;61:23-29.
Practice Points
- Approximately half of skin-related visits are to nondermatologists, such as family medicine physicians, pediatricians, and internists.
- Skin conditions that most frequently present to nondermatologists are different from those seen by dermatologists.
- Education efforts in nondermatology specialties should be targeted toward the common skin diseases that present to these specialties to maximize the yield of medical education and improve diagnostic accuracy and patient outcomes.
Flurbiprofen-Induced Unilateral Eyelid Angioedema
To the Editor:
Flurbiprofen, a member of the phenylalkanoic acid derivative group of nonsteroidal anti-inflammatory drugs (NSAIDs), are commonly used to treat fever, inflammation, and pain of arthritis.1 The exact prevalence of allergic reactions to NSAIDs in the general population is not known. Rhinoconjunctivitis, bronchospasm, urticaria, angioedema, and anaphylaxis can occur as an allergic reaction to NSAIDs. Isolated angioedema following NSAID ingestion typically involves the face, particularly the periorbital skin, lips, and mouth.2 These patients may develop urticaria and/or angioedema only after NSAID ingestion, but they do not have underlying chronic urticaria. We report a rare case of isolated unilateral eyelid angioedema with flurbiprofen.
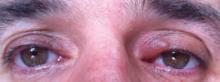
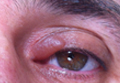
A 39-year-old man presented with the onset of unilateral angioedema of the left upper eyelid that had developed approximately 30 minutes after taking flurbiprofen (100 mg). He reported frequent use of flurbiprofen for headaches. The patient also had a history of taking aspirin, ibuprofen, diclofenac, etodolac, and naproxen sodium as needed for migraines with no prior angioedema. He had no history of chronic urticaria or allergic disease. The patient was treated with oral pheniramine hydrogen maleate and angioedema resolved after 12 hours. Three days later, the patient used flurbiprofen again for a headache. He was readmitted to our clinic with unilateral angioedema of the left upper eyelid (Figure). The symptoms started approximately 30 minutes after taking flurbiprofen. Angioedema resolved within 1 day with oral pheniramine.
Nonsteroidal anti-inflammatory drugs are the most commonly prescribed class of drugs in the world and are the most common cause of all adverse drug reactions.3 Urticaria, angioedema, and anaphylaxis are common adverse reactions to NSAIDs. The prevalence of urticaria and angioedema to NSAIDs has been reported to be 0.1% to 3% worldwide.4
Angioedema is an abrupt localized swelling of the skin and mucous membranes of the face, lips, mouth, throat, larynx, extremities, and genitalia. Angioedema generally develops over minutes to hours and resolves in 24 to 48 hours.5 Angioedema without urticaria is the clinical syndrome that can be caused by an adverse drug reaction. In an Italian review of 2137 reactions, NSAIDs were causative agents in 33.6% of patients with drug-induced angioedema.6 In another study, Leeyaphan et al5 reported that 50% of patients with drug-induced angioedema resulted from NSAIDs, commonly with ibuprofen and diclofenac. Although angioedema is due to inhibition of cyclooxygenase 1, overproduction of leukotrienes, and possibly IgE-mediated reactions to single drugs,7 localized unilateral eyelid angioedema with NSAIDs is rare. The exact mechanism of localized eyelid edema is not known.8 We believe that the unilateral eyelid angioedema in our patient was caused by flurbiprofen use because the reaction recurred when the drug was used again.
1. Roszkowski MT, Swift JQ, Hargreaves KM. Effect of NSAID administration on tissue levels of immunoreactive prostaglandin E2, leukotriene B4, and (S)-flurbiprofen following extraction of impacted third molars. Pain. 1997;73:339-345.
2. Asero R. Multiple sensitivity to NSAID. Allergy. 2000;55:893-894.
3. Nettis E, Colanardi MC, Ferrannini A, et al. Update on sensitivity to nonsteroidal anti-inflammatory drugs. Curr Drug Targets Immune Endocr Metabol Disord. 2001;1:233-240.
4. Kulthanan K, Jiamton S, Boochangkool K, et al. Angioedema: clinical and etiological aspects. Clin Dev Immunol. 2007;2007:26438.
5. Leeyaphan C, Kulthanan K, Jongiarearnprasert K, et al. Drug-induced angioedema without urticaria: prevalence and clinical features [published online ahead of print November 17, 2009]. J Eur Acad Dermatol Venereol. 2010;24:685-691.
6. Cutaneous reactions to analgesic-antipyretics and nonsteroidal anti-inflammatory drugs. analysis of reports to the spontaneous reporting system of the Gruppo Italiano Studi Epidemiologici in Dermatologia. Dermatology. 1993;186:164-169.
7. Stevenson OE, Finch TM. Allergic contact dermatitis from rectified camphor oil in Earex ear drops. Contact Dermatitis. 2003;49:51.
8. Tsuruta D, Oshimo T, Sowa J, et al. Unilateral eyelid angioedema with congestion of the right bulbar conjunctiva due to loxoprofen sodium. Cutis. 2011;87:41-43.
To the Editor:
Flurbiprofen, a member of the phenylalkanoic acid derivative group of nonsteroidal anti-inflammatory drugs (NSAIDs), are commonly used to treat fever, inflammation, and pain of arthritis.1 The exact prevalence of allergic reactions to NSAIDs in the general population is not known. Rhinoconjunctivitis, bronchospasm, urticaria, angioedema, and anaphylaxis can occur as an allergic reaction to NSAIDs. Isolated angioedema following NSAID ingestion typically involves the face, particularly the periorbital skin, lips, and mouth.2 These patients may develop urticaria and/or angioedema only after NSAID ingestion, but they do not have underlying chronic urticaria. We report a rare case of isolated unilateral eyelid angioedema with flurbiprofen.
A 39-year-old man presented with the onset of unilateral angioedema of the left upper eyelid that had developed approximately 30 minutes after taking flurbiprofen (100 mg). He reported frequent use of flurbiprofen for headaches. The patient also had a history of taking aspirin, ibuprofen, diclofenac, etodolac, and naproxen sodium as needed for migraines with no prior angioedema. He had no history of chronic urticaria or allergic disease. The patient was treated with oral pheniramine hydrogen maleate and angioedema resolved after 12 hours. Three days later, the patient used flurbiprofen again for a headache. He was readmitted to our clinic with unilateral angioedema of the left upper eyelid (Figure). The symptoms started approximately 30 minutes after taking flurbiprofen. Angioedema resolved within 1 day with oral pheniramine.
Nonsteroidal anti-inflammatory drugs are the most commonly prescribed class of drugs in the world and are the most common cause of all adverse drug reactions.3 Urticaria, angioedema, and anaphylaxis are common adverse reactions to NSAIDs. The prevalence of urticaria and angioedema to NSAIDs has been reported to be 0.1% to 3% worldwide.4
Angioedema is an abrupt localized swelling of the skin and mucous membranes of the face, lips, mouth, throat, larynx, extremities, and genitalia. Angioedema generally develops over minutes to hours and resolves in 24 to 48 hours.5 Angioedema without urticaria is the clinical syndrome that can be caused by an adverse drug reaction. In an Italian review of 2137 reactions, NSAIDs were causative agents in 33.6% of patients with drug-induced angioedema.6 In another study, Leeyaphan et al5 reported that 50% of patients with drug-induced angioedema resulted from NSAIDs, commonly with ibuprofen and diclofenac. Although angioedema is due to inhibition of cyclooxygenase 1, overproduction of leukotrienes, and possibly IgE-mediated reactions to single drugs,7 localized unilateral eyelid angioedema with NSAIDs is rare. The exact mechanism of localized eyelid edema is not known.8 We believe that the unilateral eyelid angioedema in our patient was caused by flurbiprofen use because the reaction recurred when the drug was used again.
To the Editor:
Flurbiprofen, a member of the phenylalkanoic acid derivative group of nonsteroidal anti-inflammatory drugs (NSAIDs), are commonly used to treat fever, inflammation, and pain of arthritis.1 The exact prevalence of allergic reactions to NSAIDs in the general population is not known. Rhinoconjunctivitis, bronchospasm, urticaria, angioedema, and anaphylaxis can occur as an allergic reaction to NSAIDs. Isolated angioedema following NSAID ingestion typically involves the face, particularly the periorbital skin, lips, and mouth.2 These patients may develop urticaria and/or angioedema only after NSAID ingestion, but they do not have underlying chronic urticaria. We report a rare case of isolated unilateral eyelid angioedema with flurbiprofen.
A 39-year-old man presented with the onset of unilateral angioedema of the left upper eyelid that had developed approximately 30 minutes after taking flurbiprofen (100 mg). He reported frequent use of flurbiprofen for headaches. The patient also had a history of taking aspirin, ibuprofen, diclofenac, etodolac, and naproxen sodium as needed for migraines with no prior angioedema. He had no history of chronic urticaria or allergic disease. The patient was treated with oral pheniramine hydrogen maleate and angioedema resolved after 12 hours. Three days later, the patient used flurbiprofen again for a headache. He was readmitted to our clinic with unilateral angioedema of the left upper eyelid (Figure). The symptoms started approximately 30 minutes after taking flurbiprofen. Angioedema resolved within 1 day with oral pheniramine.
Nonsteroidal anti-inflammatory drugs are the most commonly prescribed class of drugs in the world and are the most common cause of all adverse drug reactions.3 Urticaria, angioedema, and anaphylaxis are common adverse reactions to NSAIDs. The prevalence of urticaria and angioedema to NSAIDs has been reported to be 0.1% to 3% worldwide.4
Angioedema is an abrupt localized swelling of the skin and mucous membranes of the face, lips, mouth, throat, larynx, extremities, and genitalia. Angioedema generally develops over minutes to hours and resolves in 24 to 48 hours.5 Angioedema without urticaria is the clinical syndrome that can be caused by an adverse drug reaction. In an Italian review of 2137 reactions, NSAIDs were causative agents in 33.6% of patients with drug-induced angioedema.6 In another study, Leeyaphan et al5 reported that 50% of patients with drug-induced angioedema resulted from NSAIDs, commonly with ibuprofen and diclofenac. Although angioedema is due to inhibition of cyclooxygenase 1, overproduction of leukotrienes, and possibly IgE-mediated reactions to single drugs,7 localized unilateral eyelid angioedema with NSAIDs is rare. The exact mechanism of localized eyelid edema is not known.8 We believe that the unilateral eyelid angioedema in our patient was caused by flurbiprofen use because the reaction recurred when the drug was used again.
1. Roszkowski MT, Swift JQ, Hargreaves KM. Effect of NSAID administration on tissue levels of immunoreactive prostaglandin E2, leukotriene B4, and (S)-flurbiprofen following extraction of impacted third molars. Pain. 1997;73:339-345.
2. Asero R. Multiple sensitivity to NSAID. Allergy. 2000;55:893-894.
3. Nettis E, Colanardi MC, Ferrannini A, et al. Update on sensitivity to nonsteroidal anti-inflammatory drugs. Curr Drug Targets Immune Endocr Metabol Disord. 2001;1:233-240.
4. Kulthanan K, Jiamton S, Boochangkool K, et al. Angioedema: clinical and etiological aspects. Clin Dev Immunol. 2007;2007:26438.
5. Leeyaphan C, Kulthanan K, Jongiarearnprasert K, et al. Drug-induced angioedema without urticaria: prevalence and clinical features [published online ahead of print November 17, 2009]. J Eur Acad Dermatol Venereol. 2010;24:685-691.
6. Cutaneous reactions to analgesic-antipyretics and nonsteroidal anti-inflammatory drugs. analysis of reports to the spontaneous reporting system of the Gruppo Italiano Studi Epidemiologici in Dermatologia. Dermatology. 1993;186:164-169.
7. Stevenson OE, Finch TM. Allergic contact dermatitis from rectified camphor oil in Earex ear drops. Contact Dermatitis. 2003;49:51.
8. Tsuruta D, Oshimo T, Sowa J, et al. Unilateral eyelid angioedema with congestion of the right bulbar conjunctiva due to loxoprofen sodium. Cutis. 2011;87:41-43.
1. Roszkowski MT, Swift JQ, Hargreaves KM. Effect of NSAID administration on tissue levels of immunoreactive prostaglandin E2, leukotriene B4, and (S)-flurbiprofen following extraction of impacted third molars. Pain. 1997;73:339-345.
2. Asero R. Multiple sensitivity to NSAID. Allergy. 2000;55:893-894.
3. Nettis E, Colanardi MC, Ferrannini A, et al. Update on sensitivity to nonsteroidal anti-inflammatory drugs. Curr Drug Targets Immune Endocr Metabol Disord. 2001;1:233-240.
4. Kulthanan K, Jiamton S, Boochangkool K, et al. Angioedema: clinical and etiological aspects. Clin Dev Immunol. 2007;2007:26438.
5. Leeyaphan C, Kulthanan K, Jongiarearnprasert K, et al. Drug-induced angioedema without urticaria: prevalence and clinical features [published online ahead of print November 17, 2009]. J Eur Acad Dermatol Venereol. 2010;24:685-691.
6. Cutaneous reactions to analgesic-antipyretics and nonsteroidal anti-inflammatory drugs. analysis of reports to the spontaneous reporting system of the Gruppo Italiano Studi Epidemiologici in Dermatologia. Dermatology. 1993;186:164-169.
7. Stevenson OE, Finch TM. Allergic contact dermatitis from rectified camphor oil in Earex ear drops. Contact Dermatitis. 2003;49:51.
8. Tsuruta D, Oshimo T, Sowa J, et al. Unilateral eyelid angioedema with congestion of the right bulbar conjunctiva due to loxoprofen sodium. Cutis. 2011;87:41-43.
Successful Treatment of Schnitzler Syndrome With Canakinumab
To the Editor:
Schnitzler syndrome occurs with a triad of chronic urticaria, recurring fevers, and monoclonal gammopathy. It was recognized as a clinical entity in 1972; now nearly 200 patients are reported in the medical literature.1-3 Flulike symptoms, arthralgia, bone pain, lymphadenopathy, and hepatosplenomegaly also are clinical findings.4,5 The erythrocyte sedimentation rate (ESR) often is markedly elevated, as are other acute phase reactants. Leukocytosis with neutrophilia and IgM and IgG monoclonal gammopathies have been described.4
Schnitzler syndrome shares many clinical characteristics with a subset of autoinflammatory disorders referred to as cryopyrin-associated periodic syndromes (CAPS), which includes familial cold autoinflammatory syndrome and Muckle-Wellssyndrome. These syndromes are associated with mutations in the cold-induced autoinflammatory syndrome 1 gene, CIAS1, which encodes the NALP3 inflammasome, leading to overproduction of IL-1β.5 A gain-of-function mutation in CIAS1 has been described in a patient with Schnitzler syndrome.6
Treatment of urticaria and constitutional symptoms associated with Schnitzler syndrome is challenging. Antihistamines are ineffective, though high-dose systemic glucocorticosteroids control most of the clinical manifestations. Methotrexate sodium, cyclosporine, and tumor necrosis factor antagonists are utilized as glucocorticosteroid-sparing agents. Anakinra, an IL-1 receptor monoclonal antibody that is approved for use in CAPS, has been reported to induce complete resolution of Schnitzler syndrome when administered daily; however, it is not approved by the US Food and Drug Administration for this disorder.7 Canakinumab, an IL-1β monoclonal antibody that is dosed every 8 weeks, was approved by the US Food and Drug Administration in 2009 for the treatment of CAPS. Given the similar clinical characteristics and genetic mutations found in CAPS and Schnitzler syndrome, canakinumab may be an effective treatment of both disorders. We report successful treatment with this monoclonal antibody in 2 patients with Schnitzler syndrome.
A 63-year-old man reported having night sweats and fatigue but had no arthralgia or arthritis. He had a 1-year history of severe urticaria and recurrent fevers (temperature, up to 38.4°C) and he also had type 1 diabetes mellitus, hypothyroidism, and celiac disease. Physical examination revealed an elevated temperature (38.4°C) and generalized urticaria but no evidence of hepatosplenomegaly, adenopathy, or arthritis. Leukocytosis was revealed (white blood cell count, 12,400/μL [reference range, 4500–11,000/μL]) with neutrophilia (88.5% [reference range, 56%]), elevated ESR (81 mm/h [reference range, 0–20 mm/h]), and IgM κ monoclonal gammopathy (0.37 g/L [reference range, 0.4–2.3 g/L]). Clinical examination as well as laboratory and imaging studies did not show evidence of malignancy or autoimmune disease. A skin biopsy identified neutrophilic urticaria without vasculitis. Prednisone 20 mg daily controlled the urticaria and fever, but symptoms recurred within days of glucocorticosteroid withdrawal.
A 47-year-old woman presented with a 7-year history of severe urticaria, fever (temperature, 38.9°C), myalgia, and arthralgia. She had a medical history of allergic rhinitis, gastroesophageal reflux disease, chronic pain syndrome, and depression. Physical examination revealed generalized urticaria with cervical and axillary lymphadenopathy 1 to 2 cm in size but no hepatosplenomegaly or arthritis. Prior evaluations for fever of unknown origin as well as autoimmune and malignant disorders were negative. Skin biopsies reported neutrophilic urticaria without vasculitis, and a lymph node biopsy from the left axilla revealed neutrophilic inflammation. A white blood cell count of 17,800/μL with 61.6% neutrophils, elevated C-reactive protein (153.4 mg/L [reference range, 0.08–3.1 mg/L]) and ESR (90 mm/h), and an IgG λ monoclonal gammopathy were present. She was previously treated with etanercept, methotrexate sodium, golimumab, and adalimumab, with only a partial response. For more than 5 years, prednisone 20 to 50 mg daily was necessary to control her symptoms. Cyclosporine 200 mg twice daily was added as a corticosteroid-sparing drug with partial response.
Both patients were diagnosed with Schnitzler syndrome and were started on canakinumab 150 mg administered subcutaneously in the upper arm every 8 weeks. Resolution of the urticaria and fevers occurred within 2 weeks, and all other medications for the treatment of Schnitzler syndrome were withdrawn without recurrence of symptoms after 3 years. The neutrophil count and acute phase reactants returned within reference range in each patient, but the monoclonal gammopathies remained unchanged. Patient 2 noted worsening of arthralgia after initiation of canakinumab, but long-term corticosteroid withdrawal was considered the cause. Patient 1 has been able to increase the interval of dosing to every 3 to 4 months without recurrence of symptoms. Patient 2 has not tolerated similar changes in dosing interval.
Canakinumab given at 8-week intervals was a safe and effective treatment of Schnitzler syndrome in this open trial of 2 patients. Anakinra also induces remission, but daily dosing is required. Cost may be a notable factor in the choice of therapy, as canakinumab costs substantially more per year than anakinra. Further investigation is required to determine if treatment with canakinumab will result in long-term remission and if less-frequent dosing will provide continued efficacy.
1. Schnitzler L. Lésions urticariennes chroniques permanentes (érythème pétaloïde?). Cas cliniques. nº 46 B. Journee Dermatologique d’Angers. October 1972.
2. Schnitzler L, Schubert B, Boasson M, et al. Urticaire chronique, lésions osseuses, macroglobulinémie IgM: maladie de Waldenstrӧm. Bull Soc Fr Dermatol Syphiligr. 1974;81:363.
3. Simon A, Asli B, Braun-Falco M, et al. Schnitzler’s syndrome: diagnosis, treatment, and follow-up. Allergy. 2013;68:562-568.
4. de Koning HD, Bodar EJ, van der Meer JW, et al. Schnitzler syndrome: beyond the case reports: review and follow-up of 94 patients with an emphasis on prognosis and treatment [published online ahead of print June 21, 2007]. Semin Arthritis Rheum. 2007;37:137-148.
5. Lipsker D, Veran Y, Grunenberger F, et al. The Schnitzler syndrome. four new cases and review of the literature. Medicine (Baltimore). 2001;80:37-44.
6. Loock J, Lamprecht P, Timmann C, et al. Genetic predisposition (NLRP3 V198M mutation) for IL-1-mediated inflammation in a patient with Schnitzler syndrome. J Allergy Clin Immunol. 2010;125:500-502.
7. Ryan JG, de Koning HD, Beck LA, et al. IL-1 blockade in Schnitzler syndrome: ex vivo findings correlate with clinical remission [published online ahead of print October 22, 2007]. J Allergy Clin Immunol. 2008;121:260-262.
To the Editor:
Schnitzler syndrome occurs with a triad of chronic urticaria, recurring fevers, and monoclonal gammopathy. It was recognized as a clinical entity in 1972; now nearly 200 patients are reported in the medical literature.1-3 Flulike symptoms, arthralgia, bone pain, lymphadenopathy, and hepatosplenomegaly also are clinical findings.4,5 The erythrocyte sedimentation rate (ESR) often is markedly elevated, as are other acute phase reactants. Leukocytosis with neutrophilia and IgM and IgG monoclonal gammopathies have been described.4
Schnitzler syndrome shares many clinical characteristics with a subset of autoinflammatory disorders referred to as cryopyrin-associated periodic syndromes (CAPS), which includes familial cold autoinflammatory syndrome and Muckle-Wellssyndrome. These syndromes are associated with mutations in the cold-induced autoinflammatory syndrome 1 gene, CIAS1, which encodes the NALP3 inflammasome, leading to overproduction of IL-1β.5 A gain-of-function mutation in CIAS1 has been described in a patient with Schnitzler syndrome.6
Treatment of urticaria and constitutional symptoms associated with Schnitzler syndrome is challenging. Antihistamines are ineffective, though high-dose systemic glucocorticosteroids control most of the clinical manifestations. Methotrexate sodium, cyclosporine, and tumor necrosis factor antagonists are utilized as glucocorticosteroid-sparing agents. Anakinra, an IL-1 receptor monoclonal antibody that is approved for use in CAPS, has been reported to induce complete resolution of Schnitzler syndrome when administered daily; however, it is not approved by the US Food and Drug Administration for this disorder.7 Canakinumab, an IL-1β monoclonal antibody that is dosed every 8 weeks, was approved by the US Food and Drug Administration in 2009 for the treatment of CAPS. Given the similar clinical characteristics and genetic mutations found in CAPS and Schnitzler syndrome, canakinumab may be an effective treatment of both disorders. We report successful treatment with this monoclonal antibody in 2 patients with Schnitzler syndrome.
A 63-year-old man reported having night sweats and fatigue but had no arthralgia or arthritis. He had a 1-year history of severe urticaria and recurrent fevers (temperature, up to 38.4°C) and he also had type 1 diabetes mellitus, hypothyroidism, and celiac disease. Physical examination revealed an elevated temperature (38.4°C) and generalized urticaria but no evidence of hepatosplenomegaly, adenopathy, or arthritis. Leukocytosis was revealed (white blood cell count, 12,400/μL [reference range, 4500–11,000/μL]) with neutrophilia (88.5% [reference range, 56%]), elevated ESR (81 mm/h [reference range, 0–20 mm/h]), and IgM κ monoclonal gammopathy (0.37 g/L [reference range, 0.4–2.3 g/L]). Clinical examination as well as laboratory and imaging studies did not show evidence of malignancy or autoimmune disease. A skin biopsy identified neutrophilic urticaria without vasculitis. Prednisone 20 mg daily controlled the urticaria and fever, but symptoms recurred within days of glucocorticosteroid withdrawal.
A 47-year-old woman presented with a 7-year history of severe urticaria, fever (temperature, 38.9°C), myalgia, and arthralgia. She had a medical history of allergic rhinitis, gastroesophageal reflux disease, chronic pain syndrome, and depression. Physical examination revealed generalized urticaria with cervical and axillary lymphadenopathy 1 to 2 cm in size but no hepatosplenomegaly or arthritis. Prior evaluations for fever of unknown origin as well as autoimmune and malignant disorders were negative. Skin biopsies reported neutrophilic urticaria without vasculitis, and a lymph node biopsy from the left axilla revealed neutrophilic inflammation. A white blood cell count of 17,800/μL with 61.6% neutrophils, elevated C-reactive protein (153.4 mg/L [reference range, 0.08–3.1 mg/L]) and ESR (90 mm/h), and an IgG λ monoclonal gammopathy were present. She was previously treated with etanercept, methotrexate sodium, golimumab, and adalimumab, with only a partial response. For more than 5 years, prednisone 20 to 50 mg daily was necessary to control her symptoms. Cyclosporine 200 mg twice daily was added as a corticosteroid-sparing drug with partial response.
Both patients were diagnosed with Schnitzler syndrome and were started on canakinumab 150 mg administered subcutaneously in the upper arm every 8 weeks. Resolution of the urticaria and fevers occurred within 2 weeks, and all other medications for the treatment of Schnitzler syndrome were withdrawn without recurrence of symptoms after 3 years. The neutrophil count and acute phase reactants returned within reference range in each patient, but the monoclonal gammopathies remained unchanged. Patient 2 noted worsening of arthralgia after initiation of canakinumab, but long-term corticosteroid withdrawal was considered the cause. Patient 1 has been able to increase the interval of dosing to every 3 to 4 months without recurrence of symptoms. Patient 2 has not tolerated similar changes in dosing interval.
Canakinumab given at 8-week intervals was a safe and effective treatment of Schnitzler syndrome in this open trial of 2 patients. Anakinra also induces remission, but daily dosing is required. Cost may be a notable factor in the choice of therapy, as canakinumab costs substantially more per year than anakinra. Further investigation is required to determine if treatment with canakinumab will result in long-term remission and if less-frequent dosing will provide continued efficacy.
To the Editor:
Schnitzler syndrome occurs with a triad of chronic urticaria, recurring fevers, and monoclonal gammopathy. It was recognized as a clinical entity in 1972; now nearly 200 patients are reported in the medical literature.1-3 Flulike symptoms, arthralgia, bone pain, lymphadenopathy, and hepatosplenomegaly also are clinical findings.4,5 The erythrocyte sedimentation rate (ESR) often is markedly elevated, as are other acute phase reactants. Leukocytosis with neutrophilia and IgM and IgG monoclonal gammopathies have been described.4
Schnitzler syndrome shares many clinical characteristics with a subset of autoinflammatory disorders referred to as cryopyrin-associated periodic syndromes (CAPS), which includes familial cold autoinflammatory syndrome and Muckle-Wellssyndrome. These syndromes are associated with mutations in the cold-induced autoinflammatory syndrome 1 gene, CIAS1, which encodes the NALP3 inflammasome, leading to overproduction of IL-1β.5 A gain-of-function mutation in CIAS1 has been described in a patient with Schnitzler syndrome.6
Treatment of urticaria and constitutional symptoms associated with Schnitzler syndrome is challenging. Antihistamines are ineffective, though high-dose systemic glucocorticosteroids control most of the clinical manifestations. Methotrexate sodium, cyclosporine, and tumor necrosis factor antagonists are utilized as glucocorticosteroid-sparing agents. Anakinra, an IL-1 receptor monoclonal antibody that is approved for use in CAPS, has been reported to induce complete resolution of Schnitzler syndrome when administered daily; however, it is not approved by the US Food and Drug Administration for this disorder.7 Canakinumab, an IL-1β monoclonal antibody that is dosed every 8 weeks, was approved by the US Food and Drug Administration in 2009 for the treatment of CAPS. Given the similar clinical characteristics and genetic mutations found in CAPS and Schnitzler syndrome, canakinumab may be an effective treatment of both disorders. We report successful treatment with this monoclonal antibody in 2 patients with Schnitzler syndrome.
A 63-year-old man reported having night sweats and fatigue but had no arthralgia or arthritis. He had a 1-year history of severe urticaria and recurrent fevers (temperature, up to 38.4°C) and he also had type 1 diabetes mellitus, hypothyroidism, and celiac disease. Physical examination revealed an elevated temperature (38.4°C) and generalized urticaria but no evidence of hepatosplenomegaly, adenopathy, or arthritis. Leukocytosis was revealed (white blood cell count, 12,400/μL [reference range, 4500–11,000/μL]) with neutrophilia (88.5% [reference range, 56%]), elevated ESR (81 mm/h [reference range, 0–20 mm/h]), and IgM κ monoclonal gammopathy (0.37 g/L [reference range, 0.4–2.3 g/L]). Clinical examination as well as laboratory and imaging studies did not show evidence of malignancy or autoimmune disease. A skin biopsy identified neutrophilic urticaria without vasculitis. Prednisone 20 mg daily controlled the urticaria and fever, but symptoms recurred within days of glucocorticosteroid withdrawal.
A 47-year-old woman presented with a 7-year history of severe urticaria, fever (temperature, 38.9°C), myalgia, and arthralgia. She had a medical history of allergic rhinitis, gastroesophageal reflux disease, chronic pain syndrome, and depression. Physical examination revealed generalized urticaria with cervical and axillary lymphadenopathy 1 to 2 cm in size but no hepatosplenomegaly or arthritis. Prior evaluations for fever of unknown origin as well as autoimmune and malignant disorders were negative. Skin biopsies reported neutrophilic urticaria without vasculitis, and a lymph node biopsy from the left axilla revealed neutrophilic inflammation. A white blood cell count of 17,800/μL with 61.6% neutrophils, elevated C-reactive protein (153.4 mg/L [reference range, 0.08–3.1 mg/L]) and ESR (90 mm/h), and an IgG λ monoclonal gammopathy were present. She was previously treated with etanercept, methotrexate sodium, golimumab, and adalimumab, with only a partial response. For more than 5 years, prednisone 20 to 50 mg daily was necessary to control her symptoms. Cyclosporine 200 mg twice daily was added as a corticosteroid-sparing drug with partial response.
Both patients were diagnosed with Schnitzler syndrome and were started on canakinumab 150 mg administered subcutaneously in the upper arm every 8 weeks. Resolution of the urticaria and fevers occurred within 2 weeks, and all other medications for the treatment of Schnitzler syndrome were withdrawn without recurrence of symptoms after 3 years. The neutrophil count and acute phase reactants returned within reference range in each patient, but the monoclonal gammopathies remained unchanged. Patient 2 noted worsening of arthralgia after initiation of canakinumab, but long-term corticosteroid withdrawal was considered the cause. Patient 1 has been able to increase the interval of dosing to every 3 to 4 months without recurrence of symptoms. Patient 2 has not tolerated similar changes in dosing interval.
Canakinumab given at 8-week intervals was a safe and effective treatment of Schnitzler syndrome in this open trial of 2 patients. Anakinra also induces remission, but daily dosing is required. Cost may be a notable factor in the choice of therapy, as canakinumab costs substantially more per year than anakinra. Further investigation is required to determine if treatment with canakinumab will result in long-term remission and if less-frequent dosing will provide continued efficacy.
1. Schnitzler L. Lésions urticariennes chroniques permanentes (érythème pétaloïde?). Cas cliniques. nº 46 B. Journee Dermatologique d’Angers. October 1972.
2. Schnitzler L, Schubert B, Boasson M, et al. Urticaire chronique, lésions osseuses, macroglobulinémie IgM: maladie de Waldenstrӧm. Bull Soc Fr Dermatol Syphiligr. 1974;81:363.
3. Simon A, Asli B, Braun-Falco M, et al. Schnitzler’s syndrome: diagnosis, treatment, and follow-up. Allergy. 2013;68:562-568.
4. de Koning HD, Bodar EJ, van der Meer JW, et al. Schnitzler syndrome: beyond the case reports: review and follow-up of 94 patients with an emphasis on prognosis and treatment [published online ahead of print June 21, 2007]. Semin Arthritis Rheum. 2007;37:137-148.
5. Lipsker D, Veran Y, Grunenberger F, et al. The Schnitzler syndrome. four new cases and review of the literature. Medicine (Baltimore). 2001;80:37-44.
6. Loock J, Lamprecht P, Timmann C, et al. Genetic predisposition (NLRP3 V198M mutation) for IL-1-mediated inflammation in a patient with Schnitzler syndrome. J Allergy Clin Immunol. 2010;125:500-502.
7. Ryan JG, de Koning HD, Beck LA, et al. IL-1 blockade in Schnitzler syndrome: ex vivo findings correlate with clinical remission [published online ahead of print October 22, 2007]. J Allergy Clin Immunol. 2008;121:260-262.
1. Schnitzler L. Lésions urticariennes chroniques permanentes (érythème pétaloïde?). Cas cliniques. nº 46 B. Journee Dermatologique d’Angers. October 1972.
2. Schnitzler L, Schubert B, Boasson M, et al. Urticaire chronique, lésions osseuses, macroglobulinémie IgM: maladie de Waldenstrӧm. Bull Soc Fr Dermatol Syphiligr. 1974;81:363.
3. Simon A, Asli B, Braun-Falco M, et al. Schnitzler’s syndrome: diagnosis, treatment, and follow-up. Allergy. 2013;68:562-568.
4. de Koning HD, Bodar EJ, van der Meer JW, et al. Schnitzler syndrome: beyond the case reports: review and follow-up of 94 patients with an emphasis on prognosis and treatment [published online ahead of print June 21, 2007]. Semin Arthritis Rheum. 2007;37:137-148.
5. Lipsker D, Veran Y, Grunenberger F, et al. The Schnitzler syndrome. four new cases and review of the literature. Medicine (Baltimore). 2001;80:37-44.
6. Loock J, Lamprecht P, Timmann C, et al. Genetic predisposition (NLRP3 V198M mutation) for IL-1-mediated inflammation in a patient with Schnitzler syndrome. J Allergy Clin Immunol. 2010;125:500-502.
7. Ryan JG, de Koning HD, Beck LA, et al. IL-1 blockade in Schnitzler syndrome: ex vivo findings correlate with clinical remission [published online ahead of print October 22, 2007]. J Allergy Clin Immunol. 2008;121:260-262.
Practice Question Answers: Medications in Dermatology, Part 2
1. A 40-year-old woman is diagnosed with systemic lupus erythematosus. You discuss treatment options and decide to start hydroxychloroquine. What laboratory tests and monitoring are required prior to starting this medication?
a. complete blood cell count with differential and glucose-6-phosphate dehydrogenase
b. complete blood cell count with differential and complete metabolic profile
c. ophthalmology evaluation and glucose-6-phosphate dehydrogenase
d. b and c
2. Two months ago you saw a 30-year-old woman with a history of severe atopic dermatitis. She had been using topical steroids with not much improvement. You decided to start a systemic medication. Within 1 month of drug initiation, she called your office to tell you that she is much better but has noticed unwanted hair on her face lately. Which medication is most likely implicated?
a. cyclosporine
b. dapsone
c. hydroxychloroquine
d. methotrexate
3. A 70-year-old man with type 2 diabetes mellitus who drinks 10 cans of beer per week presents to the emergency department with a 3-day history of diffuse tense bullae and pruritus on the legs and trunk. Direct immunofluorescence displayed linear deposition of IgG and C3 at the dermoepidermal junction, confirming your clinical diagnosis. What is the best long-term treatment option for this patient?
a. combination of oral steroids plus methotrexate
b. oral steroids and mycophenolate mofetil
c. oral steroids only
d. topical steroids only
4. A 45-year-old Venezuelan man presents with painful nodules on his bilateral lower legs. A biopsy demonstrates acid-fast bacilli, and a multidrug regimen is initiated for erythema nodosum leprosum. Which of the following is the mechanism of action of the treatment that is US Food and Drug Administration approved for this condition?
a. inhibits chemotaxis
b. inhibits dihydrofolate reductase
c. inhibits tumor necrosis factor α
d. suppresses T-cell function and B-cell antibody production
5. A patient consults her physician because of several side effects from a medication she started 2 weeks ago due to erythematous to violaceous papules on the legs from palpable purpura. She reports diarrhea, abdominal pain, and fatigue. Which medication is she taking?
a. azathioprine
b. colchicine
c. dapsone
d. methotrexate
1. A 40-year-old woman is diagnosed with systemic lupus erythematosus. You discuss treatment options and decide to start hydroxychloroquine. What laboratory tests and monitoring are required prior to starting this medication?
a. complete blood cell count with differential and glucose-6-phosphate dehydrogenase
b. complete blood cell count with differential and complete metabolic profile
c. ophthalmology evaluation and glucose-6-phosphate dehydrogenase
d. b and c
2. Two months ago you saw a 30-year-old woman with a history of severe atopic dermatitis. She had been using topical steroids with not much improvement. You decided to start a systemic medication. Within 1 month of drug initiation, she called your office to tell you that she is much better but has noticed unwanted hair on her face lately. Which medication is most likely implicated?
a. cyclosporine
b. dapsone
c. hydroxychloroquine
d. methotrexate
3. A 70-year-old man with type 2 diabetes mellitus who drinks 10 cans of beer per week presents to the emergency department with a 3-day history of diffuse tense bullae and pruritus on the legs and trunk. Direct immunofluorescence displayed linear deposition of IgG and C3 at the dermoepidermal junction, confirming your clinical diagnosis. What is the best long-term treatment option for this patient?
a. combination of oral steroids plus methotrexate
b. oral steroids and mycophenolate mofetil
c. oral steroids only
d. topical steroids only
4. A 45-year-old Venezuelan man presents with painful nodules on his bilateral lower legs. A biopsy demonstrates acid-fast bacilli, and a multidrug regimen is initiated for erythema nodosum leprosum. Which of the following is the mechanism of action of the treatment that is US Food and Drug Administration approved for this condition?
a. inhibits chemotaxis
b. inhibits dihydrofolate reductase
c. inhibits tumor necrosis factor α
d. suppresses T-cell function and B-cell antibody production
5. A patient consults her physician because of several side effects from a medication she started 2 weeks ago due to erythematous to violaceous papules on the legs from palpable purpura. She reports diarrhea, abdominal pain, and fatigue. Which medication is she taking?
a. azathioprine
b. colchicine
c. dapsone
d. methotrexate
1. A 40-year-old woman is diagnosed with systemic lupus erythematosus. You discuss treatment options and decide to start hydroxychloroquine. What laboratory tests and monitoring are required prior to starting this medication?
a. complete blood cell count with differential and glucose-6-phosphate dehydrogenase
b. complete blood cell count with differential and complete metabolic profile
c. ophthalmology evaluation and glucose-6-phosphate dehydrogenase
d. b and c
2. Two months ago you saw a 30-year-old woman with a history of severe atopic dermatitis. She had been using topical steroids with not much improvement. You decided to start a systemic medication. Within 1 month of drug initiation, she called your office to tell you that she is much better but has noticed unwanted hair on her face lately. Which medication is most likely implicated?
a. cyclosporine
b. dapsone
c. hydroxychloroquine
d. methotrexate
3. A 70-year-old man with type 2 diabetes mellitus who drinks 10 cans of beer per week presents to the emergency department with a 3-day history of diffuse tense bullae and pruritus on the legs and trunk. Direct immunofluorescence displayed linear deposition of IgG and C3 at the dermoepidermal junction, confirming your clinical diagnosis. What is the best long-term treatment option for this patient?
a. combination of oral steroids plus methotrexate
b. oral steroids and mycophenolate mofetil
c. oral steroids only
d. topical steroids only
4. A 45-year-old Venezuelan man presents with painful nodules on his bilateral lower legs. A biopsy demonstrates acid-fast bacilli, and a multidrug regimen is initiated for erythema nodosum leprosum. Which of the following is the mechanism of action of the treatment that is US Food and Drug Administration approved for this condition?
a. inhibits chemotaxis
b. inhibits dihydrofolate reductase
c. inhibits tumor necrosis factor α
d. suppresses T-cell function and B-cell antibody production
5. A patient consults her physician because of several side effects from a medication she started 2 weeks ago due to erythematous to violaceous papules on the legs from palpable purpura. She reports diarrhea, abdominal pain, and fatigue. Which medication is she taking?
a. azathioprine
b. colchicine
c. dapsone
d. methotrexate