User login
Using Patch Testing to Identify Culprit Agents in Suspected Drug Eruptions
Don’t miss these drug reactions
WAILEA, HAWAII – New drugs can mean new drug reactions affecting the skin, notably those associated with hepatitis C therapies and new cancer drugs, according to J. Mark Jackson, MD, of the University of Louisville (Ky.).
When dermatologists recognize the side effects from hepatitis C and cancer drugs, they can monitor patients appropriately and initiate the correct treatments, Dr. Jackson said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/ Skin Disease Education Foundation.
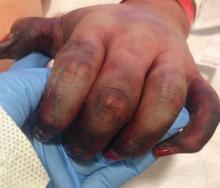
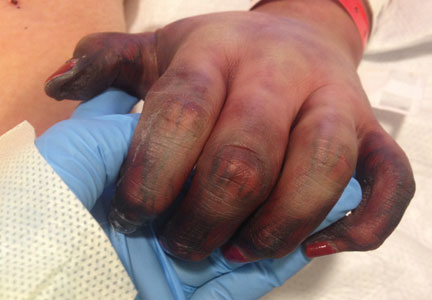
Finally, “telaprevir-related dermatitis, which accounts for 95% of skin events in telaprevir-treated patients, is clinically and histologically eczematous and different from the classic ‘maculopapular’ drug-induced eruptions,” he explained. Some patients develop DRESS syndrome or drug rash with eosinophilia and systemic symptoms, he added.
In addition, four approved hepatitis C antivirals – simeprevir, telaprevir, boceprevir, and sofosbuvir – may cause photosensitivity, Dr. Jackson said. He cited a case of a patient who took simeprevir and developed photodistributed lichenoid eruptions (J Cutan Pathol. 2015 Oct;42[10]:769-73).
New cancer treatments have brought new side effects as well, Dr. Jackson said. Epidermal growth factor receptor inhibitors cause papulopustular and follicular eruptions in many cancer patients, and some of these patients also experience conditions including xerosis cutis, changes to the hair and nails, skin hyperpigmentation, and enhanced radiation dermatitis, he said. Multikinase inhibitors, a common cause of hand-foot syndrome (HFS), are also associated with facial erythema, subungual splinter hemorrhages, and other skin changes, he added.
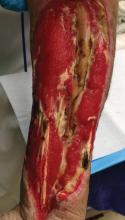
Capecitabine-induced HFS, while not life-threatening, can affect a patient’s quality of life, Dr. Jackson pointed out. “Dose modification of the inciting agent serves as the most effective management of HFS, although a variety of anecdotal reports suggest that other agents may also be efficacious,” he explained.
Dr. Jackson noted one extreme case of a 61-year-old woman with metastatic breast cancer who was treated with capecitabine and developed HFS that led to a pseudomonal superinfection, followed by bacterial sepsis and rapid death. The case suggests that “early adjustment of therapy may prevent adverse outcomes from secondary cutaneous infections while maintaining tumor response,” he noted.
Dr. Jackson disclosed relationships with companies including AbbVie, Amgen, Celgene, Dermira, Galderma, Genentech, Janssen, Lilly, Medimetriks, Merck, Novartis, Pfizer, Promius, and Top MD.
SDEF and this news organization are owned by the same parent company.
WAILEA, HAWAII – New drugs can mean new drug reactions affecting the skin, notably those associated with hepatitis C therapies and new cancer drugs, according to J. Mark Jackson, MD, of the University of Louisville (Ky.).
When dermatologists recognize the side effects from hepatitis C and cancer drugs, they can monitor patients appropriately and initiate the correct treatments, Dr. Jackson said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/ Skin Disease Education Foundation.
Finally, “telaprevir-related dermatitis, which accounts for 95% of skin events in telaprevir-treated patients, is clinically and histologically eczematous and different from the classic ‘maculopapular’ drug-induced eruptions,” he explained. Some patients develop DRESS syndrome or drug rash with eosinophilia and systemic symptoms, he added.
In addition, four approved hepatitis C antivirals – simeprevir, telaprevir, boceprevir, and sofosbuvir – may cause photosensitivity, Dr. Jackson said. He cited a case of a patient who took simeprevir and developed photodistributed lichenoid eruptions (J Cutan Pathol. 2015 Oct;42[10]:769-73).
New cancer treatments have brought new side effects as well, Dr. Jackson said. Epidermal growth factor receptor inhibitors cause papulopustular and follicular eruptions in many cancer patients, and some of these patients also experience conditions including xerosis cutis, changes to the hair and nails, skin hyperpigmentation, and enhanced radiation dermatitis, he said. Multikinase inhibitors, a common cause of hand-foot syndrome (HFS), are also associated with facial erythema, subungual splinter hemorrhages, and other skin changes, he added.
Capecitabine-induced HFS, while not life-threatening, can affect a patient’s quality of life, Dr. Jackson pointed out. “Dose modification of the inciting agent serves as the most effective management of HFS, although a variety of anecdotal reports suggest that other agents may also be efficacious,” he explained.
Dr. Jackson noted one extreme case of a 61-year-old woman with metastatic breast cancer who was treated with capecitabine and developed HFS that led to a pseudomonal superinfection, followed by bacterial sepsis and rapid death. The case suggests that “early adjustment of therapy may prevent adverse outcomes from secondary cutaneous infections while maintaining tumor response,” he noted.
Dr. Jackson disclosed relationships with companies including AbbVie, Amgen, Celgene, Dermira, Galderma, Genentech, Janssen, Lilly, Medimetriks, Merck, Novartis, Pfizer, Promius, and Top MD.
SDEF and this news organization are owned by the same parent company.
WAILEA, HAWAII – New drugs can mean new drug reactions affecting the skin, notably those associated with hepatitis C therapies and new cancer drugs, according to J. Mark Jackson, MD, of the University of Louisville (Ky.).
When dermatologists recognize the side effects from hepatitis C and cancer drugs, they can monitor patients appropriately and initiate the correct treatments, Dr. Jackson said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/ Skin Disease Education Foundation.
Finally, “telaprevir-related dermatitis, which accounts for 95% of skin events in telaprevir-treated patients, is clinically and histologically eczematous and different from the classic ‘maculopapular’ drug-induced eruptions,” he explained. Some patients develop DRESS syndrome or drug rash with eosinophilia and systemic symptoms, he added.
In addition, four approved hepatitis C antivirals – simeprevir, telaprevir, boceprevir, and sofosbuvir – may cause photosensitivity, Dr. Jackson said. He cited a case of a patient who took simeprevir and developed photodistributed lichenoid eruptions (J Cutan Pathol. 2015 Oct;42[10]:769-73).
New cancer treatments have brought new side effects as well, Dr. Jackson said. Epidermal growth factor receptor inhibitors cause papulopustular and follicular eruptions in many cancer patients, and some of these patients also experience conditions including xerosis cutis, changes to the hair and nails, skin hyperpigmentation, and enhanced radiation dermatitis, he said. Multikinase inhibitors, a common cause of hand-foot syndrome (HFS), are also associated with facial erythema, subungual splinter hemorrhages, and other skin changes, he added.
Capecitabine-induced HFS, while not life-threatening, can affect a patient’s quality of life, Dr. Jackson pointed out. “Dose modification of the inciting agent serves as the most effective management of HFS, although a variety of anecdotal reports suggest that other agents may also be efficacious,” he explained.
Dr. Jackson noted one extreme case of a 61-year-old woman with metastatic breast cancer who was treated with capecitabine and developed HFS that led to a pseudomonal superinfection, followed by bacterial sepsis and rapid death. The case suggests that “early adjustment of therapy may prevent adverse outcomes from secondary cutaneous infections while maintaining tumor response,” he noted.
Dr. Jackson disclosed relationships with companies including AbbVie, Amgen, Celgene, Dermira, Galderma, Genentech, Janssen, Lilly, Medimetriks, Merck, Novartis, Pfizer, Promius, and Top MD.
SDEF and this news organization are owned by the same parent company.
AT SDEF HAWAII DERMATOLOGY SEMINAR
Tryptase gene variant linked to GI, joint, and skin symptoms
Researchers have identified a genetic variant associated with inherited elevated basal serum tryptase levels and linked to a distinct group of comorbid multisystem complaints.
These features, including cutaneous flushing, certain chronic pain disorders, autonomic dysfunction, and gastrointestinal dysmotility, have been reported in association with genetic disorders or joint hypermobility syndromes such as Ehlers-Danlos syndrome type III (hypermobility type, EDS III) and often follow a dominant inheritance pattern in affected families, providing a reason to look into a genetic basis for these patient characteristics, according to Jonathan J. Lyons, MD, of the National Institute of Allergy and Infectious Diseases, and his coauthors. The researchers reported their findings Oct. 17 in Nature Genetics.
The researchers recruited 96 individuals from 35 families with a syndrome of elevated basal serum tryptase levels and multiple comorbid symptoms following an autosomal dominant pattern of inheritance.
These symptoms included gastrointestinal dysmotility such as irritable bowel syndrome or chronic gastroesophageal reflux, connective tissue abnormalities such as joint hypermobility, congenital skeletal abnormalities, retained primary dentition, symptoms suggestive of autonomic dysfunction such as postural orthostatic tachycardia syndrome, and elevated composite autonomic symptom scores. Other symptoms included recurrent cutaneous flushing and pruritus – often associated with urticaria and complaints of sleep disruption – and systemic reaction to stinging insects.
Using exome and genome sequencing followed by linkage analysis, researchers identified duplications and triplications within the TPSAB1 gene encoding alpha-tryptase (Nat Genet. 2016 Oct 17. doi: 10.1038/ng.3696). Further analysis found elevated alpha-tryptase/beta-tryptase ratios among affected family members and suggested that multiple copies of the alpha-tryptase sequence were inherited together.
To confirm the finding, researchers examined genetic data from a cohort of healthy unrelated volunteers in the National Human Genome Research Institute ClinSeq cohort, which identified 125 samples with partially enriched duplication of alpha tryptase–encoding sequence using a common haplotype.
Of these, three individuals had single-allele duplications of the alpha tryptase–encoding sequence and also presented with similar symptoms to the original cohort: cutaneous flushing, itching, or hives, systemic venom reactions, irritable bowel syndrome, retained primary dentition, and elevated autonomic symptom scores.
“We have found that this phenotype is most frequently inherited in an autosomal dominant manner and that, when this occurs, it is exclusively associated with increased copy number on a single allele of alpha tryptase–encoding sequence in the TPSAB1 gene, a genetic trait we have termed hereditary alpha-tryptasemia,” the researchers reported. “The families studied in our initial cohort likely represent the most severe phenotypes among individuals affected with hereditary alpha-tryptasemia, owing in part to the lack of detection of triplication of alpha tryptase–encoding sequence in unselected populations, which we have tentatively designated as hereditary alpha-tryptasemia syndrome.”
The authors suggested that part of the clinical presentation of this syndrome included symptoms that may be associated clinically with mast cell mediator release. In the context of elevated basal serum tryptase levels, this might prompt a doctor to investigate for clonal mast cell disease, which would include bone-marrow biopsy.
However, given that such an investigation would be challenging, and given that elevated tryptase levels are not uncommon in the generally population, they suggested tryptase genotyping may be warranted.
The study was supported by the National Institute of Allergy and Infectious Diseases, the ARTrust/the Mastocytosis Society, and the National Human Genome Research Institute. One author declared royalties associated with the tryptase UniCAP assay, and consulting fees from Genentech. Another author declared an advisory position and royalties from private industry.
Researchers have identified a genetic variant associated with inherited elevated basal serum tryptase levels and linked to a distinct group of comorbid multisystem complaints.
These features, including cutaneous flushing, certain chronic pain disorders, autonomic dysfunction, and gastrointestinal dysmotility, have been reported in association with genetic disorders or joint hypermobility syndromes such as Ehlers-Danlos syndrome type III (hypermobility type, EDS III) and often follow a dominant inheritance pattern in affected families, providing a reason to look into a genetic basis for these patient characteristics, according to Jonathan J. Lyons, MD, of the National Institute of Allergy and Infectious Diseases, and his coauthors. The researchers reported their findings Oct. 17 in Nature Genetics.
The researchers recruited 96 individuals from 35 families with a syndrome of elevated basal serum tryptase levels and multiple comorbid symptoms following an autosomal dominant pattern of inheritance.
These symptoms included gastrointestinal dysmotility such as irritable bowel syndrome or chronic gastroesophageal reflux, connective tissue abnormalities such as joint hypermobility, congenital skeletal abnormalities, retained primary dentition, symptoms suggestive of autonomic dysfunction such as postural orthostatic tachycardia syndrome, and elevated composite autonomic symptom scores. Other symptoms included recurrent cutaneous flushing and pruritus – often associated with urticaria and complaints of sleep disruption – and systemic reaction to stinging insects.
Using exome and genome sequencing followed by linkage analysis, researchers identified duplications and triplications within the TPSAB1 gene encoding alpha-tryptase (Nat Genet. 2016 Oct 17. doi: 10.1038/ng.3696). Further analysis found elevated alpha-tryptase/beta-tryptase ratios among affected family members and suggested that multiple copies of the alpha-tryptase sequence were inherited together.
To confirm the finding, researchers examined genetic data from a cohort of healthy unrelated volunteers in the National Human Genome Research Institute ClinSeq cohort, which identified 125 samples with partially enriched duplication of alpha tryptase–encoding sequence using a common haplotype.
Of these, three individuals had single-allele duplications of the alpha tryptase–encoding sequence and also presented with similar symptoms to the original cohort: cutaneous flushing, itching, or hives, systemic venom reactions, irritable bowel syndrome, retained primary dentition, and elevated autonomic symptom scores.
“We have found that this phenotype is most frequently inherited in an autosomal dominant manner and that, when this occurs, it is exclusively associated with increased copy number on a single allele of alpha tryptase–encoding sequence in the TPSAB1 gene, a genetic trait we have termed hereditary alpha-tryptasemia,” the researchers reported. “The families studied in our initial cohort likely represent the most severe phenotypes among individuals affected with hereditary alpha-tryptasemia, owing in part to the lack of detection of triplication of alpha tryptase–encoding sequence in unselected populations, which we have tentatively designated as hereditary alpha-tryptasemia syndrome.”
The authors suggested that part of the clinical presentation of this syndrome included symptoms that may be associated clinically with mast cell mediator release. In the context of elevated basal serum tryptase levels, this might prompt a doctor to investigate for clonal mast cell disease, which would include bone-marrow biopsy.
However, given that such an investigation would be challenging, and given that elevated tryptase levels are not uncommon in the generally population, they suggested tryptase genotyping may be warranted.
The study was supported by the National Institute of Allergy and Infectious Diseases, the ARTrust/the Mastocytosis Society, and the National Human Genome Research Institute. One author declared royalties associated with the tryptase UniCAP assay, and consulting fees from Genentech. Another author declared an advisory position and royalties from private industry.
Researchers have identified a genetic variant associated with inherited elevated basal serum tryptase levels and linked to a distinct group of comorbid multisystem complaints.
These features, including cutaneous flushing, certain chronic pain disorders, autonomic dysfunction, and gastrointestinal dysmotility, have been reported in association with genetic disorders or joint hypermobility syndromes such as Ehlers-Danlos syndrome type III (hypermobility type, EDS III) and often follow a dominant inheritance pattern in affected families, providing a reason to look into a genetic basis for these patient characteristics, according to Jonathan J. Lyons, MD, of the National Institute of Allergy and Infectious Diseases, and his coauthors. The researchers reported their findings Oct. 17 in Nature Genetics.
The researchers recruited 96 individuals from 35 families with a syndrome of elevated basal serum tryptase levels and multiple comorbid symptoms following an autosomal dominant pattern of inheritance.
These symptoms included gastrointestinal dysmotility such as irritable bowel syndrome or chronic gastroesophageal reflux, connective tissue abnormalities such as joint hypermobility, congenital skeletal abnormalities, retained primary dentition, symptoms suggestive of autonomic dysfunction such as postural orthostatic tachycardia syndrome, and elevated composite autonomic symptom scores. Other symptoms included recurrent cutaneous flushing and pruritus – often associated with urticaria and complaints of sleep disruption – and systemic reaction to stinging insects.
Using exome and genome sequencing followed by linkage analysis, researchers identified duplications and triplications within the TPSAB1 gene encoding alpha-tryptase (Nat Genet. 2016 Oct 17. doi: 10.1038/ng.3696). Further analysis found elevated alpha-tryptase/beta-tryptase ratios among affected family members and suggested that multiple copies of the alpha-tryptase sequence were inherited together.
To confirm the finding, researchers examined genetic data from a cohort of healthy unrelated volunteers in the National Human Genome Research Institute ClinSeq cohort, which identified 125 samples with partially enriched duplication of alpha tryptase–encoding sequence using a common haplotype.
Of these, three individuals had single-allele duplications of the alpha tryptase–encoding sequence and also presented with similar symptoms to the original cohort: cutaneous flushing, itching, or hives, systemic venom reactions, irritable bowel syndrome, retained primary dentition, and elevated autonomic symptom scores.
“We have found that this phenotype is most frequently inherited in an autosomal dominant manner and that, when this occurs, it is exclusively associated with increased copy number on a single allele of alpha tryptase–encoding sequence in the TPSAB1 gene, a genetic trait we have termed hereditary alpha-tryptasemia,” the researchers reported. “The families studied in our initial cohort likely represent the most severe phenotypes among individuals affected with hereditary alpha-tryptasemia, owing in part to the lack of detection of triplication of alpha tryptase–encoding sequence in unselected populations, which we have tentatively designated as hereditary alpha-tryptasemia syndrome.”
The authors suggested that part of the clinical presentation of this syndrome included symptoms that may be associated clinically with mast cell mediator release. In the context of elevated basal serum tryptase levels, this might prompt a doctor to investigate for clonal mast cell disease, which would include bone-marrow biopsy.
However, given that such an investigation would be challenging, and given that elevated tryptase levels are not uncommon in the generally population, they suggested tryptase genotyping may be warranted.
The study was supported by the National Institute of Allergy and Infectious Diseases, the ARTrust/the Mastocytosis Society, and the National Human Genome Research Institute. One author declared royalties associated with the tryptase UniCAP assay, and consulting fees from Genentech. Another author declared an advisory position and royalties from private industry.
FROM NATURE GENETICS
Key clinical point:
Major finding: Increased copy number on a single allele of alpha tryptase–encoding sequence in the TPSAB1 gene is associated with elevated basal serum tryptase and a collection of symptoms including irritable bowel syndrome, joint hypermobility, and autonomic dysfunction.
Data source: Study of 96 individuals from 35 families with a syndrome of elevated basal serum tryptase levels and multiple comorbid symptoms.
Disclosures: The study was supported by the National Institute of Allergy and Infectious Diseases, the ARTrust/the Mastocytosis Society, and the National Human Genome Research Institute. One author declared royalties associated with the tryptase UniCAP assay, and consulting fees from Genentech. Another author declared an advisory position and royalties from private industry.
Dermatologists Should Get the Point: Acupuncture for the Treatment of Skin Disorders

Complementary and alternative medicine has a definite adjunctive and even at times primary role in the medical management of patients. Its prevalence in the United States is estimated to be 38% and it is used to treat dermatologic conditions in 6% of patients (Harris et al; Smith et al). Acupuncture, a component of traditional Chinese medicine, has a prevalence of 0.6% to 1.4% and is used to treat 0.6% of dermatologic conditions (Smith et al; Cooper et al).
Acupuncture involves stimulation of specific points usually located along meridians. The source of stimulation on the skin can be elicited using needle points, pressure, or heat. Diseases disturb the body’s vital energy (qi), and stimulation along the appropriate meridian channel achieves balance and cures disease by restoring the normal circulation of the body’s qi.
Ma and Sivamani (J Altern Complement Med. 2015;21:520-529) performed a systematic review of articles indexed for MEDLINE, EMBASE, and the Cochrane Central Register using acupuncture therapy or acupuncture and skin diseases or dermatology as search terms to synthesize the evidence on the use of acupuncture as a primary treatment modality for dermatologic conditions. Twenty-four studies met inclusion criteria; of them, 17 showed statistically significant improvements (P<.05) in outcome measures. Specifically, acupuncture improved the outcome measures in the treatment of several dermatologic conditions including chloasma, dermatitis, facial elasticity, hyperhidrosis, pruritus, and urticaria.
What’s the issue?
Patients often have insight into potential available therapies for their medical problems. Hence, it is not unexpected that individuals with dermatologic conditions may not only be aware of complementary and alternative medicine approaches, such as acupuncture, but also seek dermatologists who can provide them with these possible therapeutic options. Although the frequency and duration of acupuncture treatments may not allow it to be a practical modality for all individuals, this treatment appears to be effective for reducing the severity of itch in patients with atopic dermatitis.
Should dermatologists incorporate acupuncture into their therapeutic armamentarium? Should national dermatology meetings provide courses on acupuncture technique? Should dermatology residency programs add competency in acupuncture management to their curriculum?
Suggested Readings
Cooper KL, Harris PE, Relton C, et al. Prevalence of visits to five types of complementary and alternative medicine practitioners by the general population: a systematic review. Complement Ther Clin Pract. 2013;19:214-220.
Harris PE, Cooper KL, Relton C, et al. Prevalence of complementary and alternative medicine (CAM) used by the general population: a systematic review and update. Int J Clin Pract. 2012;66:924-939.
Smith N, Shin DB, Brauer JA, et al. Use of complementary and alternative medicine among adults with skin disease: results from a national survey. J Am Acad Dermatol. 2009;60:419-425.

Complementary and alternative medicine has a definite adjunctive and even at times primary role in the medical management of patients. Its prevalence in the United States is estimated to be 38% and it is used to treat dermatologic conditions in 6% of patients (Harris et al; Smith et al). Acupuncture, a component of traditional Chinese medicine, has a prevalence of 0.6% to 1.4% and is used to treat 0.6% of dermatologic conditions (Smith et al; Cooper et al).
Acupuncture involves stimulation of specific points usually located along meridians. The source of stimulation on the skin can be elicited using needle points, pressure, or heat. Diseases disturb the body’s vital energy (qi), and stimulation along the appropriate meridian channel achieves balance and cures disease by restoring the normal circulation of the body’s qi.
Ma and Sivamani (J Altern Complement Med. 2015;21:520-529) performed a systematic review of articles indexed for MEDLINE, EMBASE, and the Cochrane Central Register using acupuncture therapy or acupuncture and skin diseases or dermatology as search terms to synthesize the evidence on the use of acupuncture as a primary treatment modality for dermatologic conditions. Twenty-four studies met inclusion criteria; of them, 17 showed statistically significant improvements (P<.05) in outcome measures. Specifically, acupuncture improved the outcome measures in the treatment of several dermatologic conditions including chloasma, dermatitis, facial elasticity, hyperhidrosis, pruritus, and urticaria.
What’s the issue?
Patients often have insight into potential available therapies for their medical problems. Hence, it is not unexpected that individuals with dermatologic conditions may not only be aware of complementary and alternative medicine approaches, such as acupuncture, but also seek dermatologists who can provide them with these possible therapeutic options. Although the frequency and duration of acupuncture treatments may not allow it to be a practical modality for all individuals, this treatment appears to be effective for reducing the severity of itch in patients with atopic dermatitis.
Should dermatologists incorporate acupuncture into their therapeutic armamentarium? Should national dermatology meetings provide courses on acupuncture technique? Should dermatology residency programs add competency in acupuncture management to their curriculum?

Complementary and alternative medicine has a definite adjunctive and even at times primary role in the medical management of patients. Its prevalence in the United States is estimated to be 38% and it is used to treat dermatologic conditions in 6% of patients (Harris et al; Smith et al). Acupuncture, a component of traditional Chinese medicine, has a prevalence of 0.6% to 1.4% and is used to treat 0.6% of dermatologic conditions (Smith et al; Cooper et al).
Acupuncture involves stimulation of specific points usually located along meridians. The source of stimulation on the skin can be elicited using needle points, pressure, or heat. Diseases disturb the body’s vital energy (qi), and stimulation along the appropriate meridian channel achieves balance and cures disease by restoring the normal circulation of the body’s qi.
Ma and Sivamani (J Altern Complement Med. 2015;21:520-529) performed a systematic review of articles indexed for MEDLINE, EMBASE, and the Cochrane Central Register using acupuncture therapy or acupuncture and skin diseases or dermatology as search terms to synthesize the evidence on the use of acupuncture as a primary treatment modality for dermatologic conditions. Twenty-four studies met inclusion criteria; of them, 17 showed statistically significant improvements (P<.05) in outcome measures. Specifically, acupuncture improved the outcome measures in the treatment of several dermatologic conditions including chloasma, dermatitis, facial elasticity, hyperhidrosis, pruritus, and urticaria.
What’s the issue?
Patients often have insight into potential available therapies for their medical problems. Hence, it is not unexpected that individuals with dermatologic conditions may not only be aware of complementary and alternative medicine approaches, such as acupuncture, but also seek dermatologists who can provide them with these possible therapeutic options. Although the frequency and duration of acupuncture treatments may not allow it to be a practical modality for all individuals, this treatment appears to be effective for reducing the severity of itch in patients with atopic dermatitis.
Should dermatologists incorporate acupuncture into their therapeutic armamentarium? Should national dermatology meetings provide courses on acupuncture technique? Should dermatology residency programs add competency in acupuncture management to their curriculum?
Suggested Readings
Cooper KL, Harris PE, Relton C, et al. Prevalence of visits to five types of complementary and alternative medicine practitioners by the general population: a systematic review. Complement Ther Clin Pract. 2013;19:214-220.
Harris PE, Cooper KL, Relton C, et al. Prevalence of complementary and alternative medicine (CAM) used by the general population: a systematic review and update. Int J Clin Pract. 2012;66:924-939.
Smith N, Shin DB, Brauer JA, et al. Use of complementary and alternative medicine among adults with skin disease: results from a national survey. J Am Acad Dermatol. 2009;60:419-425.
Suggested Readings
Cooper KL, Harris PE, Relton C, et al. Prevalence of visits to five types of complementary and alternative medicine practitioners by the general population: a systematic review. Complement Ther Clin Pract. 2013;19:214-220.
Harris PE, Cooper KL, Relton C, et al. Prevalence of complementary and alternative medicine (CAM) used by the general population: a systematic review and update. Int J Clin Pract. 2012;66:924-939.
Smith N, Shin DB, Brauer JA, et al. Use of complementary and alternative medicine among adults with skin disease: results from a national survey. J Am Acad Dermatol. 2009;60:419-425.
Diagnosis and Management of Cold Urticaria
Cold urticaria is a rare condition characterized by a localized or systemic eruption of papules upon exposure of the skin to cold air, liquids, and/or objects. In some cases, angioedema and anaphylaxis can occur. The wheal-and-flare reaction results from a localized or systemic release of histamine, leukotrienes, and various other proinflammatory mast cell mediators. Cold urticaria can be acquired or follow an autosomal-dominant familial transmission pattern. Acquired cold urticaria often presents in young adulthood with a mean duration of 4 to 5 years and remission or improvement of symptoms after 5 years in 50% of cases.1 The familial variant most commonly presents in early childhood and endures throughout the patient’s life.2 Cold urticaria generally is classified as acute or chronic if symptoms persist for more than 6 weeks. Pharmacologic therapies with prophylactic effects that may reduce the intensity of symptoms or inhibit their development include antihistamines, leuko-triene receptor antagonists, biologics, and glucocorticoids. We present the case of a 23-year-old man with cold urticaria that was refractory to initial treatment with H1 antihistamines along with a review of the literature.
Case Report
A 23-year-old man presented to the dermatology clinic for evaluation of recurrent burning, itching, and sometimes development of a painful rash on the face, neck, and arms of 2 years’ duration that typically occurred following exposure to cold, wind, and rain. He also developed symptoms in warm weather when exposed to wind while sweating. His medical history was remarkable for asthma, which was not active. He was not taking any medications and had no known drug or environmental allergies. No other members of his household developed similar symptoms. His only successful means of prevention was to stay indoors, which thereby limited his activities.
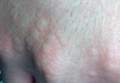
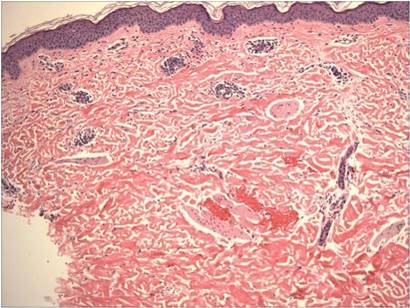
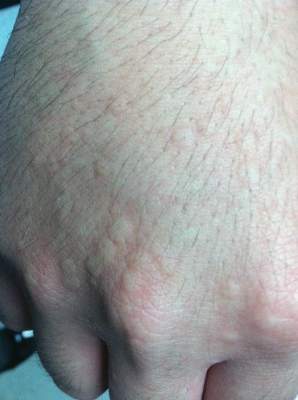
Physical examination of the dorsal hands following an ice cube test revealed numerous 3- to 5-mm urticarial papules with surrounding erythema (Figure).
Following the initial evaluation, the patient was treated unsuccessfully with a mix of first- and second-generation antihistamines in gradually increasing doses to a maximum dose of loratadine 20 mg once daily, cetirizine 20 mg once daily, and hydroxyzine 20 mg once daily. A course of montelukast 10 mg once daily was started in addition to the antihistamines and led to a reduction in the severity of the lesions but not the frequency and did not relieve the burning sensation; the patient subsequently discontinued therapy. Next, a trial of cyclosporine was attempted, but the patient reported that it caused emesis and subsequently discontinued treatment. The patient also did not tolerate prednisone. He eventually decided to treat his symptoms with lifestyle choices only, such as making sure to be well covered in cold temperatures.
Comment
Cold urticaria is a physical urticaria resulting from mast cell degranulation and the subsequent release of histamine and proinflammatory cytokines upon exposure of the skin to cold air, liquid, and/or objects. Symtpoms usually are limited to localized exposed areas of the skin but also can be generalized. Cold urticaria typically manifests as erythematous, pruritic papules and also may be accompanied by deep tissue involvement resulting in angioedema and/or anaphylaxis. Symptoms usually occur within minutes of cold exposure; however, in delayed-type cold urticaria, symptoms may develop 24 to 72 hours later.3 Prevalence is relatively equal in both sexes and is highest among young adults (ie, 18–27 years old), with a greater incidence associated with cold climates.4 In one study, the overall incidence of acquired cold urticaria in Central Europe was estimated to be 0.05%.1
Systemic involvement may occur with extensive cold contact, ranging in severity from generalized urticaria to anaphylaxis and involvement of the cardiovascular, respiratory, and/or gastrointestinal systems.5 Patients who exhibit systemic responses to cold exposure should avoid swimming in cold water, as this may induce anaphylaxis and result in injury or death. In a 2004 study that included 30 children with cold urticaria at a tertiary center in Boston,6 11 (36.7%) participants who underwent cold stimulation testing developed systemic symptoms; 5 (45.5%) participants experienced respiratory distress and 8 (72.7%) experienced a decrease in level of consciousness (eg, faintness, dizziness, hypotension). Aquatic activity was the trigger in all 11 participants except for 1 (9.0%), who experienced systemic symptoms on exposure to cold air. In the same study, 14 (46.7%) participants were diagnosed with asthma and 15 (50%) were diagnosed with allergic rhinitis. Of the 28 participants whose family histories were available for review, 25 (89.3%) had a family history of atopic disease.6 A 2008 Greek study4 of 62 adults with acquired cold urticaria found that 18 (29%) participants had at least 1 serious systemic response resulting in generalized urticaria or angioedema associated with hypotension (eg, dizziness, fainting, disorientation, shock). In both of these studies, a majority of the serious systemic reactions were associated with cold water activities.
Cold urticaria is primarily an idiopathic phenomenon but can be classified as acquired or familial. Acquired cold urticaria may result from primary or secondary causes, which can include cryoglobulinemia, human immunodeficiency virus, syphilis, mononucleosis, rubeola, toxoplasmosis, varicella, hepatitis, and various drugs (eg, penicillin, angiotensin-converting enzyme inhibitors, oral contraceptives).7 Familial causes include cryopyrin-associated periodic syndrome, phospholipase Cγ2 gene–associated antibody deficiency and immune dysregulation, Muckle-Wells syndrome, and neonatal-onset multisystem inflammatory disease.
Typically, cold urticaria is diagnosed using cold stimulation tests such as the ice cube test, in which an ice cube is applied directly to the patient’s skin for 3 to 5 minutes and a response is measured 10 minutes after its removal.8 This test has been shown to have a sensitivity of 83% to 90% and a specificity of 100%.9 Alternatively, cold urticaria may be diagnosed through the use of a Peltier element-based cold-provocation device, which exposes the patient to a variety of temperatures in order for clinicians to determine the threshold upon which there is an observable reaction. With a sensitivity of 93% and specificity of 100%, the accuracy of this test is similar to that of the ice cube test.10 If a patient has a history of serious systemic involvement, any testing that exposes the patient to extensive cold exposure should be used with caution.
Patients should be counseled about potential serious systemic symptoms and the importance of wearing appropriate cold-weather clothing. Avoidance of cold water activities and overexposure to cold weather also should be emphasized. Pharmacologic therapy for prophylaxis typically includes a second-generation H1 antihistamine (eg, cetirizine, loratadine, desloratadine). Since these drugs have been shown to be less sedating than first-generation antihistamines, they are considered a better choice for chronic treatment. At high doses, however, these medications may have a sedative effect; therefore, nighttime use is preferable if possible. The standard dosage is 5 mg to 10 mg daily for oral cetirizine, 10 mg daily for oral loratadine, and 5 mg daily for oral desloratadine; however, up to 4 times the standard dosage of these medications may be required for effective treatment of cold urticaria.11 Given the associated risk of anaphylaxis, patients should be prescribed an epinephrine pen and educated about its appropriate use, including the importance of keeping the pen accessible at all times.
In refractory cases of cold urticaria, an H2 antihistamine (eg, ranitidine) can be used in conjunction with H1 antihistamines.12 Omalizumab, an IgE-mediated treatment, also has been shown to be safe and effective in patients with recalcitrant physical urticaria, including cold urticaria.13,14 One report described the case of a 69-year-old woman with cold urticaria who was unable to leave the house without developing a widespread eruption on the face, trunk, and limbs.15 After undergoing a series of unsuccessful treatments, the patient was started on cyclosporine 125 mg twice daily, which was reduced to 100 mg twice daily after 4 weeks of therapy and then reduced to 75 mg twice daily after 4 months of treatment. One week after therapy was initiated the patient reported that she was able to leave the house, and after 4 weeks of treatment the lesions only developed on the hands and feet. The patient remained in remission with a low-dose therapy of cyclosporine 75 mg twice daily with lesions only occurring on the hands and feet. The low-dose maintenance therapy was associated with minimal adverse effects.15 To our knowledge, there are no known large studies on the efficacy of cyclosporine in the treatment of cold urticaria.
Leukotriene receptor antagonists (eg, montelukast, zafirlukast, zileuton) have been used to treat chronic urticaria. In one report, montelukast was used in a 29-year-old woman with cold urticaria who had initially been treated with cetirizine 30 mg daily, cyproheptadine 4 mg daily, and doxycycline 200 mg daily with minimal to no relief. After treatment with montelukast, she experienced notable and stable improvements in symptoms.16 Hydroxychloroquine also has been shown to be safe and to substantially improve quality of life in patients with idiopathic chronic urticaria.17 Methotrexate (with close patient monitoring for adverse effects) has been reported to benefit some patients whose chronic urticaria was unresponsive to standard treatment.18 Treatment regimens for chronic urticaria have shown variable success in the treatment of cold urticaria and may be considered in cases refractory to treatment with high-dose second-generation H1 antihistamines.
Topical application of capsaicin for 4 to 7 days has been shown to deplete the neuropeptides in sensory fibers that may be involved in cold reactions, although skin irritation may prevent usage.19
Prednisone therapy was used in a small study of 6 patients with acquired cold urticaria.20 Three patients were treated for periods of 3 to 5 days with prednisone 20 mg each morning. Three other patients were given a single dose of prednisone 20 mg or 25 mg in the morning, depending on body weight. Following prednisone therapy, complete or partial pruritus was subjectively improved in all 6 patients. Additionally, significant reductions in venous histamine concentrations at 5 and 10 minutes following cold immersion were noted (P<.05 and P<.025, respectively); however, no significant improvement in either erythema or edema was noted posttreatment following cold immersion.20 Despite these findings, prednisone has not been shown to consistently prevent histamine release. Another report noted the case of a 47-year-old man with cold urticaria who required hypothermic cardiopulmonary bypass. Pretreatment with prednisone 20 mg daily and preoperative hydrocortisone 100 mg intravenously did not prevent histamine release.21
Cold desensitization (ie, exposing progressively larger areas of the patient’s skin to increasingly colder water) may induce tolerance to cold and decrease the temperature threshold at which symptoms develop; however, patients with known serious systemic reactions should be tested with extreme caution and only under the supervision of a clinician.22,23 Tolerance may wane when cold desensitization therapy is stopped.
The prognosis for patients with acquired cold urticaria generally is good. Improvement of symptoms or full remission occurs within 5 to 6 years in 50% of patients.24 Once remission has occurred, patients generally remain symptom free. For other familial variants, symptoms may last a lifetime.
Conclusion
This case report and review of the literature highlights the limitations of cold urticaria and the importance of effective management in improving quality of life in affected patients. Symptoms may limit patients’ ability to work in certain environments, inhibit them from engaging in daily activities, and even prevent them from leaving their homes in colder temperatures. In addition to behavioral modifications, pharmacologic management may provide symptomatic relief. Antihistamines are the first line of treatment in cold urticaria. Second-generation antihistamines, which are more selective for H1 receptors and less sedating, are generally recommended. Up to 4 times the standard dosage of these medications may be required for effective treatment.5 The primary goal of therapy in mild to moderate cases is improvement in quality of life.
- Siebenhaar F, Weller K, Mlynek A, et al. Acquired cold urticaria: clinical picture and update on diagnosis and treatment. Clin Exp Dermatol. 2007;32:241-245.
- Gandhi C, Healy C, Wanderer AA, et al. Familial atypical cold urticaria: description of a new hereditary disease. J Allergy Clin Immunol. 2009;124:1245-1250.
- Bäck O, Larsen A. Delayed cold urticaria. Acta Derm Venereol. 1978;58:369-371.
- Katsarou-Katsari A, Makris M, Lagogianni E, et al. Clinical features and natural history of acquired cold urticaria in a tertiary referral hospital: a 10-year prospective study. J Eur Acad Dermatol Venereol. 2008;22:1405-1411.
- Wanderer AA, Grandel KE, Wasserman SI, et al. Clinical characteristics of cold-induced systemic reactions in acquired cold urticaria syndromes: recommendations for prevention of this complication and a proposal for a diagnostic classification of cold urticaria. J Allergy Clin Immunol. 1986;78(3 Pt 1):417-423.
- Alangari AA, Twarog FJ, Shih MC, et al. Clinical features and anaphylaxis in children with cold urticaria. Pediatrics. 2004;113:e313-e317.
- Wanderer AA, Hoffman HM. The spectrum of acquired and familial cold-induced urticaria/urticaria-like syndromes. Immunol Allergy Clin North Am. 2004;24:259-286.
- Visitsuntorn N, Tuchinda M, Arunyanark N, et al. Ice cube test in children with cold urticaria. Asian Pac J Allergy Immunol. 1992;10:111-115.
- Neittaanmäki H. Cold urticaria. clinical findings in 220 patients. J Am Acad Dermatol. 1985;13:636-644.
- Siebenhaar F, Staubach P, Metz M, et al. Peltier effect-based temperature challenge: an improved method for diagnosing cold urticaria. J Allergy Clin Immunol. 2004;114:1224-1225.
- Siebenhaar F, Degener F, Zuberbier T, et al. High-dose desloratadine decreases wheal volume and improves cold provocation thresholds compared with standard-dose treatment in patients with acquired cold urticaria: a randomized, placebo-controlled, crossover study. J Allergy Clin Immunol. 2009;123:672-679.
- Duc J, Pécoud A. Successful treatment of idiopathic cold urticaria with the association of H1 and H2 antagonists: a case report. Ann Allergy. 1986;56:355-357.
- Metz M, Altrichter S, Ardelean E, et al. Anti-immunoglobulin E treatment of patients with recalcitrant physical urticaria. Int Arch Allergy Immunol. 2011;154:177-180.
- Boyce JA. Successful treatment of cold-induced urticaria/anaphylaxis with anti-IgE. J Allergy Clin Immunol. 2006;117:1415-1418.
- Marsland AM, Beck MH. Cold urticaria responding to systemic cyclosporine. Br J Dermatol. 2003;149:214-215.
- Hani N, Hartmann K, Casper C, et al. Improvement of cold urticaria by treatment with the leukotriene receptor antagonist montelukast. Acta Derm Venereol. 2000;80:229.
- Reeves GE, Boyle MJ, Bonfield J, et al. Impact of hydroxychloroquine therapy on chronic urticaria: chronic autoimmune urticaria study and evaluation. Intern Med J. 2004;34:182-186.
- Perez A, Woods A, Grattan CE. Methotrexate: a useful steroid-sparing agent in recalcitrant chronic urticaria. Br J Dermatol. 2010;162:191-194.
- Tóth-Kása I, Jancsó G, Obál F Jr, et al. Involvement of sensory nerve endings in cold and heat urticaria. J Invest Dermatol. 1983;80:34-36.
- Black AK, Keahey TM, Eady RA, et al. Dissociation of histamine release and clinical improvement following treatment of acquired cold urticaria by prednisone. Br J Clin Pharmacol. 1981;12:327-331.
- Johnston WE, Moss J, Philbin DM, et al. Management of cold urticaria during hypothermic cardiopulmonary bypass. N Engl J Med. 1982;306:219-221.
- Krause K, Zuberbier T, Maurer, M. Modern Approaches to the diagnosis and treatment of cold contact urticaria. Curr Allergy Asthma Rep. 2010;10:273-279.
- von Mackensen YA, Sticherling M. Cold urticaria: tolerance induction with cold baths. Br J Dermatol. 2007;157:835-836.
- Möller A, Henning M, Zuberbier T, et al. Epidemiology and clinical aspects of cold urticaria [article in German]. Hautarzt. 1996;47:510-514.
Cold urticaria is a rare condition characterized by a localized or systemic eruption of papules upon exposure of the skin to cold air, liquids, and/or objects. In some cases, angioedema and anaphylaxis can occur. The wheal-and-flare reaction results from a localized or systemic release of histamine, leukotrienes, and various other proinflammatory mast cell mediators. Cold urticaria can be acquired or follow an autosomal-dominant familial transmission pattern. Acquired cold urticaria often presents in young adulthood with a mean duration of 4 to 5 years and remission or improvement of symptoms after 5 years in 50% of cases.1 The familial variant most commonly presents in early childhood and endures throughout the patient’s life.2 Cold urticaria generally is classified as acute or chronic if symptoms persist for more than 6 weeks. Pharmacologic therapies with prophylactic effects that may reduce the intensity of symptoms or inhibit their development include antihistamines, leuko-triene receptor antagonists, biologics, and glucocorticoids. We present the case of a 23-year-old man with cold urticaria that was refractory to initial treatment with H1 antihistamines along with a review of the literature.
Case Report
A 23-year-old man presented to the dermatology clinic for evaluation of recurrent burning, itching, and sometimes development of a painful rash on the face, neck, and arms of 2 years’ duration that typically occurred following exposure to cold, wind, and rain. He also developed symptoms in warm weather when exposed to wind while sweating. His medical history was remarkable for asthma, which was not active. He was not taking any medications and had no known drug or environmental allergies. No other members of his household developed similar symptoms. His only successful means of prevention was to stay indoors, which thereby limited his activities.
Physical examination of the dorsal hands following an ice cube test revealed numerous 3- to 5-mm urticarial papules with surrounding erythema (Figure).

Following the initial evaluation, the patient was treated unsuccessfully with a mix of first- and second-generation antihistamines in gradually increasing doses to a maximum dose of loratadine 20 mg once daily, cetirizine 20 mg once daily, and hydroxyzine 20 mg once daily. A course of montelukast 10 mg once daily was started in addition to the antihistamines and led to a reduction in the severity of the lesions but not the frequency and did not relieve the burning sensation; the patient subsequently discontinued therapy. Next, a trial of cyclosporine was attempted, but the patient reported that it caused emesis and subsequently discontinued treatment. The patient also did not tolerate prednisone. He eventually decided to treat his symptoms with lifestyle choices only, such as making sure to be well covered in cold temperatures.
Comment
Cold urticaria is a physical urticaria resulting from mast cell degranulation and the subsequent release of histamine and proinflammatory cytokines upon exposure of the skin to cold air, liquid, and/or objects. Symtpoms usually are limited to localized exposed areas of the skin but also can be generalized. Cold urticaria typically manifests as erythematous, pruritic papules and also may be accompanied by deep tissue involvement resulting in angioedema and/or anaphylaxis. Symptoms usually occur within minutes of cold exposure; however, in delayed-type cold urticaria, symptoms may develop 24 to 72 hours later.3 Prevalence is relatively equal in both sexes and is highest among young adults (ie, 18–27 years old), with a greater incidence associated with cold climates.4 In one study, the overall incidence of acquired cold urticaria in Central Europe was estimated to be 0.05%.1
Systemic involvement may occur with extensive cold contact, ranging in severity from generalized urticaria to anaphylaxis and involvement of the cardiovascular, respiratory, and/or gastrointestinal systems.5 Patients who exhibit systemic responses to cold exposure should avoid swimming in cold water, as this may induce anaphylaxis and result in injury or death. In a 2004 study that included 30 children with cold urticaria at a tertiary center in Boston,6 11 (36.7%) participants who underwent cold stimulation testing developed systemic symptoms; 5 (45.5%) participants experienced respiratory distress and 8 (72.7%) experienced a decrease in level of consciousness (eg, faintness, dizziness, hypotension). Aquatic activity was the trigger in all 11 participants except for 1 (9.0%), who experienced systemic symptoms on exposure to cold air. In the same study, 14 (46.7%) participants were diagnosed with asthma and 15 (50%) were diagnosed with allergic rhinitis. Of the 28 participants whose family histories were available for review, 25 (89.3%) had a family history of atopic disease.6 A 2008 Greek study4 of 62 adults with acquired cold urticaria found that 18 (29%) participants had at least 1 serious systemic response resulting in generalized urticaria or angioedema associated with hypotension (eg, dizziness, fainting, disorientation, shock). In both of these studies, a majority of the serious systemic reactions were associated with cold water activities.
Cold urticaria is primarily an idiopathic phenomenon but can be classified as acquired or familial. Acquired cold urticaria may result from primary or secondary causes, which can include cryoglobulinemia, human immunodeficiency virus, syphilis, mononucleosis, rubeola, toxoplasmosis, varicella, hepatitis, and various drugs (eg, penicillin, angiotensin-converting enzyme inhibitors, oral contraceptives).7 Familial causes include cryopyrin-associated periodic syndrome, phospholipase Cγ2 gene–associated antibody deficiency and immune dysregulation, Muckle-Wells syndrome, and neonatal-onset multisystem inflammatory disease.
Typically, cold urticaria is diagnosed using cold stimulation tests such as the ice cube test, in which an ice cube is applied directly to the patient’s skin for 3 to 5 minutes and a response is measured 10 minutes after its removal.8 This test has been shown to have a sensitivity of 83% to 90% and a specificity of 100%.9 Alternatively, cold urticaria may be diagnosed through the use of a Peltier element-based cold-provocation device, which exposes the patient to a variety of temperatures in order for clinicians to determine the threshold upon which there is an observable reaction. With a sensitivity of 93% and specificity of 100%, the accuracy of this test is similar to that of the ice cube test.10 If a patient has a history of serious systemic involvement, any testing that exposes the patient to extensive cold exposure should be used with caution.
Patients should be counseled about potential serious systemic symptoms and the importance of wearing appropriate cold-weather clothing. Avoidance of cold water activities and overexposure to cold weather also should be emphasized. Pharmacologic therapy for prophylaxis typically includes a second-generation H1 antihistamine (eg, cetirizine, loratadine, desloratadine). Since these drugs have been shown to be less sedating than first-generation antihistamines, they are considered a better choice for chronic treatment. At high doses, however, these medications may have a sedative effect; therefore, nighttime use is preferable if possible. The standard dosage is 5 mg to 10 mg daily for oral cetirizine, 10 mg daily for oral loratadine, and 5 mg daily for oral desloratadine; however, up to 4 times the standard dosage of these medications may be required for effective treatment of cold urticaria.11 Given the associated risk of anaphylaxis, patients should be prescribed an epinephrine pen and educated about its appropriate use, including the importance of keeping the pen accessible at all times.
In refractory cases of cold urticaria, an H2 antihistamine (eg, ranitidine) can be used in conjunction with H1 antihistamines.12 Omalizumab, an IgE-mediated treatment, also has been shown to be safe and effective in patients with recalcitrant physical urticaria, including cold urticaria.13,14 One report described the case of a 69-year-old woman with cold urticaria who was unable to leave the house without developing a widespread eruption on the face, trunk, and limbs.15 After undergoing a series of unsuccessful treatments, the patient was started on cyclosporine 125 mg twice daily, which was reduced to 100 mg twice daily after 4 weeks of therapy and then reduced to 75 mg twice daily after 4 months of treatment. One week after therapy was initiated the patient reported that she was able to leave the house, and after 4 weeks of treatment the lesions only developed on the hands and feet. The patient remained in remission with a low-dose therapy of cyclosporine 75 mg twice daily with lesions only occurring on the hands and feet. The low-dose maintenance therapy was associated with minimal adverse effects.15 To our knowledge, there are no known large studies on the efficacy of cyclosporine in the treatment of cold urticaria.
Leukotriene receptor antagonists (eg, montelukast, zafirlukast, zileuton) have been used to treat chronic urticaria. In one report, montelukast was used in a 29-year-old woman with cold urticaria who had initially been treated with cetirizine 30 mg daily, cyproheptadine 4 mg daily, and doxycycline 200 mg daily with minimal to no relief. After treatment with montelukast, she experienced notable and stable improvements in symptoms.16 Hydroxychloroquine also has been shown to be safe and to substantially improve quality of life in patients with idiopathic chronic urticaria.17 Methotrexate (with close patient monitoring for adverse effects) has been reported to benefit some patients whose chronic urticaria was unresponsive to standard treatment.18 Treatment regimens for chronic urticaria have shown variable success in the treatment of cold urticaria and may be considered in cases refractory to treatment with high-dose second-generation H1 antihistamines.
Topical application of capsaicin for 4 to 7 days has been shown to deplete the neuropeptides in sensory fibers that may be involved in cold reactions, although skin irritation may prevent usage.19
Prednisone therapy was used in a small study of 6 patients with acquired cold urticaria.20 Three patients were treated for periods of 3 to 5 days with prednisone 20 mg each morning. Three other patients were given a single dose of prednisone 20 mg or 25 mg in the morning, depending on body weight. Following prednisone therapy, complete or partial pruritus was subjectively improved in all 6 patients. Additionally, significant reductions in venous histamine concentrations at 5 and 10 minutes following cold immersion were noted (P<.05 and P<.025, respectively); however, no significant improvement in either erythema or edema was noted posttreatment following cold immersion.20 Despite these findings, prednisone has not been shown to consistently prevent histamine release. Another report noted the case of a 47-year-old man with cold urticaria who required hypothermic cardiopulmonary bypass. Pretreatment with prednisone 20 mg daily and preoperative hydrocortisone 100 mg intravenously did not prevent histamine release.21
Cold desensitization (ie, exposing progressively larger areas of the patient’s skin to increasingly colder water) may induce tolerance to cold and decrease the temperature threshold at which symptoms develop; however, patients with known serious systemic reactions should be tested with extreme caution and only under the supervision of a clinician.22,23 Tolerance may wane when cold desensitization therapy is stopped.
The prognosis for patients with acquired cold urticaria generally is good. Improvement of symptoms or full remission occurs within 5 to 6 years in 50% of patients.24 Once remission has occurred, patients generally remain symptom free. For other familial variants, symptoms may last a lifetime.
Conclusion
This case report and review of the literature highlights the limitations of cold urticaria and the importance of effective management in improving quality of life in affected patients. Symptoms may limit patients’ ability to work in certain environments, inhibit them from engaging in daily activities, and even prevent them from leaving their homes in colder temperatures. In addition to behavioral modifications, pharmacologic management may provide symptomatic relief. Antihistamines are the first line of treatment in cold urticaria. Second-generation antihistamines, which are more selective for H1 receptors and less sedating, are generally recommended. Up to 4 times the standard dosage of these medications may be required for effective treatment.5 The primary goal of therapy in mild to moderate cases is improvement in quality of life.
Cold urticaria is a rare condition characterized by a localized or systemic eruption of papules upon exposure of the skin to cold air, liquids, and/or objects. In some cases, angioedema and anaphylaxis can occur. The wheal-and-flare reaction results from a localized or systemic release of histamine, leukotrienes, and various other proinflammatory mast cell mediators. Cold urticaria can be acquired or follow an autosomal-dominant familial transmission pattern. Acquired cold urticaria often presents in young adulthood with a mean duration of 4 to 5 years and remission or improvement of symptoms after 5 years in 50% of cases.1 The familial variant most commonly presents in early childhood and endures throughout the patient’s life.2 Cold urticaria generally is classified as acute or chronic if symptoms persist for more than 6 weeks. Pharmacologic therapies with prophylactic effects that may reduce the intensity of symptoms or inhibit their development include antihistamines, leuko-triene receptor antagonists, biologics, and glucocorticoids. We present the case of a 23-year-old man with cold urticaria that was refractory to initial treatment with H1 antihistamines along with a review of the literature.
Case Report
A 23-year-old man presented to the dermatology clinic for evaluation of recurrent burning, itching, and sometimes development of a painful rash on the face, neck, and arms of 2 years’ duration that typically occurred following exposure to cold, wind, and rain. He also developed symptoms in warm weather when exposed to wind while sweating. His medical history was remarkable for asthma, which was not active. He was not taking any medications and had no known drug or environmental allergies. No other members of his household developed similar symptoms. His only successful means of prevention was to stay indoors, which thereby limited his activities.
Physical examination of the dorsal hands following an ice cube test revealed numerous 3- to 5-mm urticarial papules with surrounding erythema (Figure).

Following the initial evaluation, the patient was treated unsuccessfully with a mix of first- and second-generation antihistamines in gradually increasing doses to a maximum dose of loratadine 20 mg once daily, cetirizine 20 mg once daily, and hydroxyzine 20 mg once daily. A course of montelukast 10 mg once daily was started in addition to the antihistamines and led to a reduction in the severity of the lesions but not the frequency and did not relieve the burning sensation; the patient subsequently discontinued therapy. Next, a trial of cyclosporine was attempted, but the patient reported that it caused emesis and subsequently discontinued treatment. The patient also did not tolerate prednisone. He eventually decided to treat his symptoms with lifestyle choices only, such as making sure to be well covered in cold temperatures.
Comment
Cold urticaria is a physical urticaria resulting from mast cell degranulation and the subsequent release of histamine and proinflammatory cytokines upon exposure of the skin to cold air, liquid, and/or objects. Symtpoms usually are limited to localized exposed areas of the skin but also can be generalized. Cold urticaria typically manifests as erythematous, pruritic papules and also may be accompanied by deep tissue involvement resulting in angioedema and/or anaphylaxis. Symptoms usually occur within minutes of cold exposure; however, in delayed-type cold urticaria, symptoms may develop 24 to 72 hours later.3 Prevalence is relatively equal in both sexes and is highest among young adults (ie, 18–27 years old), with a greater incidence associated with cold climates.4 In one study, the overall incidence of acquired cold urticaria in Central Europe was estimated to be 0.05%.1
Systemic involvement may occur with extensive cold contact, ranging in severity from generalized urticaria to anaphylaxis and involvement of the cardiovascular, respiratory, and/or gastrointestinal systems.5 Patients who exhibit systemic responses to cold exposure should avoid swimming in cold water, as this may induce anaphylaxis and result in injury or death. In a 2004 study that included 30 children with cold urticaria at a tertiary center in Boston,6 11 (36.7%) participants who underwent cold stimulation testing developed systemic symptoms; 5 (45.5%) participants experienced respiratory distress and 8 (72.7%) experienced a decrease in level of consciousness (eg, faintness, dizziness, hypotension). Aquatic activity was the trigger in all 11 participants except for 1 (9.0%), who experienced systemic symptoms on exposure to cold air. In the same study, 14 (46.7%) participants were diagnosed with asthma and 15 (50%) were diagnosed with allergic rhinitis. Of the 28 participants whose family histories were available for review, 25 (89.3%) had a family history of atopic disease.6 A 2008 Greek study4 of 62 adults with acquired cold urticaria found that 18 (29%) participants had at least 1 serious systemic response resulting in generalized urticaria or angioedema associated with hypotension (eg, dizziness, fainting, disorientation, shock). In both of these studies, a majority of the serious systemic reactions were associated with cold water activities.
Cold urticaria is primarily an idiopathic phenomenon but can be classified as acquired or familial. Acquired cold urticaria may result from primary or secondary causes, which can include cryoglobulinemia, human immunodeficiency virus, syphilis, mononucleosis, rubeola, toxoplasmosis, varicella, hepatitis, and various drugs (eg, penicillin, angiotensin-converting enzyme inhibitors, oral contraceptives).7 Familial causes include cryopyrin-associated periodic syndrome, phospholipase Cγ2 gene–associated antibody deficiency and immune dysregulation, Muckle-Wells syndrome, and neonatal-onset multisystem inflammatory disease.
Typically, cold urticaria is diagnosed using cold stimulation tests such as the ice cube test, in which an ice cube is applied directly to the patient’s skin for 3 to 5 minutes and a response is measured 10 minutes after its removal.8 This test has been shown to have a sensitivity of 83% to 90% and a specificity of 100%.9 Alternatively, cold urticaria may be diagnosed through the use of a Peltier element-based cold-provocation device, which exposes the patient to a variety of temperatures in order for clinicians to determine the threshold upon which there is an observable reaction. With a sensitivity of 93% and specificity of 100%, the accuracy of this test is similar to that of the ice cube test.10 If a patient has a history of serious systemic involvement, any testing that exposes the patient to extensive cold exposure should be used with caution.
Patients should be counseled about potential serious systemic symptoms and the importance of wearing appropriate cold-weather clothing. Avoidance of cold water activities and overexposure to cold weather also should be emphasized. Pharmacologic therapy for prophylaxis typically includes a second-generation H1 antihistamine (eg, cetirizine, loratadine, desloratadine). Since these drugs have been shown to be less sedating than first-generation antihistamines, they are considered a better choice for chronic treatment. At high doses, however, these medications may have a sedative effect; therefore, nighttime use is preferable if possible. The standard dosage is 5 mg to 10 mg daily for oral cetirizine, 10 mg daily for oral loratadine, and 5 mg daily for oral desloratadine; however, up to 4 times the standard dosage of these medications may be required for effective treatment of cold urticaria.11 Given the associated risk of anaphylaxis, patients should be prescribed an epinephrine pen and educated about its appropriate use, including the importance of keeping the pen accessible at all times.
In refractory cases of cold urticaria, an H2 antihistamine (eg, ranitidine) can be used in conjunction with H1 antihistamines.12 Omalizumab, an IgE-mediated treatment, also has been shown to be safe and effective in patients with recalcitrant physical urticaria, including cold urticaria.13,14 One report described the case of a 69-year-old woman with cold urticaria who was unable to leave the house without developing a widespread eruption on the face, trunk, and limbs.15 After undergoing a series of unsuccessful treatments, the patient was started on cyclosporine 125 mg twice daily, which was reduced to 100 mg twice daily after 4 weeks of therapy and then reduced to 75 mg twice daily after 4 months of treatment. One week after therapy was initiated the patient reported that she was able to leave the house, and after 4 weeks of treatment the lesions only developed on the hands and feet. The patient remained in remission with a low-dose therapy of cyclosporine 75 mg twice daily with lesions only occurring on the hands and feet. The low-dose maintenance therapy was associated with minimal adverse effects.15 To our knowledge, there are no known large studies on the efficacy of cyclosporine in the treatment of cold urticaria.
Leukotriene receptor antagonists (eg, montelukast, zafirlukast, zileuton) have been used to treat chronic urticaria. In one report, montelukast was used in a 29-year-old woman with cold urticaria who had initially been treated with cetirizine 30 mg daily, cyproheptadine 4 mg daily, and doxycycline 200 mg daily with minimal to no relief. After treatment with montelukast, she experienced notable and stable improvements in symptoms.16 Hydroxychloroquine also has been shown to be safe and to substantially improve quality of life in patients with idiopathic chronic urticaria.17 Methotrexate (with close patient monitoring for adverse effects) has been reported to benefit some patients whose chronic urticaria was unresponsive to standard treatment.18 Treatment regimens for chronic urticaria have shown variable success in the treatment of cold urticaria and may be considered in cases refractory to treatment with high-dose second-generation H1 antihistamines.
Topical application of capsaicin for 4 to 7 days has been shown to deplete the neuropeptides in sensory fibers that may be involved in cold reactions, although skin irritation may prevent usage.19
Prednisone therapy was used in a small study of 6 patients with acquired cold urticaria.20 Three patients were treated for periods of 3 to 5 days with prednisone 20 mg each morning. Three other patients were given a single dose of prednisone 20 mg or 25 mg in the morning, depending on body weight. Following prednisone therapy, complete or partial pruritus was subjectively improved in all 6 patients. Additionally, significant reductions in venous histamine concentrations at 5 and 10 minutes following cold immersion were noted (P<.05 and P<.025, respectively); however, no significant improvement in either erythema or edema was noted posttreatment following cold immersion.20 Despite these findings, prednisone has not been shown to consistently prevent histamine release. Another report noted the case of a 47-year-old man with cold urticaria who required hypothermic cardiopulmonary bypass. Pretreatment with prednisone 20 mg daily and preoperative hydrocortisone 100 mg intravenously did not prevent histamine release.21
Cold desensitization (ie, exposing progressively larger areas of the patient’s skin to increasingly colder water) may induce tolerance to cold and decrease the temperature threshold at which symptoms develop; however, patients with known serious systemic reactions should be tested with extreme caution and only under the supervision of a clinician.22,23 Tolerance may wane when cold desensitization therapy is stopped.
The prognosis for patients with acquired cold urticaria generally is good. Improvement of symptoms or full remission occurs within 5 to 6 years in 50% of patients.24 Once remission has occurred, patients generally remain symptom free. For other familial variants, symptoms may last a lifetime.
Conclusion
This case report and review of the literature highlights the limitations of cold urticaria and the importance of effective management in improving quality of life in affected patients. Symptoms may limit patients’ ability to work in certain environments, inhibit them from engaging in daily activities, and even prevent them from leaving their homes in colder temperatures. In addition to behavioral modifications, pharmacologic management may provide symptomatic relief. Antihistamines are the first line of treatment in cold urticaria. Second-generation antihistamines, which are more selective for H1 receptors and less sedating, are generally recommended. Up to 4 times the standard dosage of these medications may be required for effective treatment.5 The primary goal of therapy in mild to moderate cases is improvement in quality of life.
- Siebenhaar F, Weller K, Mlynek A, et al. Acquired cold urticaria: clinical picture and update on diagnosis and treatment. Clin Exp Dermatol. 2007;32:241-245.
- Gandhi C, Healy C, Wanderer AA, et al. Familial atypical cold urticaria: description of a new hereditary disease. J Allergy Clin Immunol. 2009;124:1245-1250.
- Bäck O, Larsen A. Delayed cold urticaria. Acta Derm Venereol. 1978;58:369-371.
- Katsarou-Katsari A, Makris M, Lagogianni E, et al. Clinical features and natural history of acquired cold urticaria in a tertiary referral hospital: a 10-year prospective study. J Eur Acad Dermatol Venereol. 2008;22:1405-1411.
- Wanderer AA, Grandel KE, Wasserman SI, et al. Clinical characteristics of cold-induced systemic reactions in acquired cold urticaria syndromes: recommendations for prevention of this complication and a proposal for a diagnostic classification of cold urticaria. J Allergy Clin Immunol. 1986;78(3 Pt 1):417-423.
- Alangari AA, Twarog FJ, Shih MC, et al. Clinical features and anaphylaxis in children with cold urticaria. Pediatrics. 2004;113:e313-e317.
- Wanderer AA, Hoffman HM. The spectrum of acquired and familial cold-induced urticaria/urticaria-like syndromes. Immunol Allergy Clin North Am. 2004;24:259-286.
- Visitsuntorn N, Tuchinda M, Arunyanark N, et al. Ice cube test in children with cold urticaria. Asian Pac J Allergy Immunol. 1992;10:111-115.
- Neittaanmäki H. Cold urticaria. clinical findings in 220 patients. J Am Acad Dermatol. 1985;13:636-644.
- Siebenhaar F, Staubach P, Metz M, et al. Peltier effect-based temperature challenge: an improved method for diagnosing cold urticaria. J Allergy Clin Immunol. 2004;114:1224-1225.
- Siebenhaar F, Degener F, Zuberbier T, et al. High-dose desloratadine decreases wheal volume and improves cold provocation thresholds compared with standard-dose treatment in patients with acquired cold urticaria: a randomized, placebo-controlled, crossover study. J Allergy Clin Immunol. 2009;123:672-679.
- Duc J, Pécoud A. Successful treatment of idiopathic cold urticaria with the association of H1 and H2 antagonists: a case report. Ann Allergy. 1986;56:355-357.
- Metz M, Altrichter S, Ardelean E, et al. Anti-immunoglobulin E treatment of patients with recalcitrant physical urticaria. Int Arch Allergy Immunol. 2011;154:177-180.
- Boyce JA. Successful treatment of cold-induced urticaria/anaphylaxis with anti-IgE. J Allergy Clin Immunol. 2006;117:1415-1418.
- Marsland AM, Beck MH. Cold urticaria responding to systemic cyclosporine. Br J Dermatol. 2003;149:214-215.
- Hani N, Hartmann K, Casper C, et al. Improvement of cold urticaria by treatment with the leukotriene receptor antagonist montelukast. Acta Derm Venereol. 2000;80:229.
- Reeves GE, Boyle MJ, Bonfield J, et al. Impact of hydroxychloroquine therapy on chronic urticaria: chronic autoimmune urticaria study and evaluation. Intern Med J. 2004;34:182-186.
- Perez A, Woods A, Grattan CE. Methotrexate: a useful steroid-sparing agent in recalcitrant chronic urticaria. Br J Dermatol. 2010;162:191-194.
- Tóth-Kása I, Jancsó G, Obál F Jr, et al. Involvement of sensory nerve endings in cold and heat urticaria. J Invest Dermatol. 1983;80:34-36.
- Black AK, Keahey TM, Eady RA, et al. Dissociation of histamine release and clinical improvement following treatment of acquired cold urticaria by prednisone. Br J Clin Pharmacol. 1981;12:327-331.
- Johnston WE, Moss J, Philbin DM, et al. Management of cold urticaria during hypothermic cardiopulmonary bypass. N Engl J Med. 1982;306:219-221.
- Krause K, Zuberbier T, Maurer, M. Modern Approaches to the diagnosis and treatment of cold contact urticaria. Curr Allergy Asthma Rep. 2010;10:273-279.
- von Mackensen YA, Sticherling M. Cold urticaria: tolerance induction with cold baths. Br J Dermatol. 2007;157:835-836.
- Möller A, Henning M, Zuberbier T, et al. Epidemiology and clinical aspects of cold urticaria [article in German]. Hautarzt. 1996;47:510-514.
- Siebenhaar F, Weller K, Mlynek A, et al. Acquired cold urticaria: clinical picture and update on diagnosis and treatment. Clin Exp Dermatol. 2007;32:241-245.
- Gandhi C, Healy C, Wanderer AA, et al. Familial atypical cold urticaria: description of a new hereditary disease. J Allergy Clin Immunol. 2009;124:1245-1250.
- Bäck O, Larsen A. Delayed cold urticaria. Acta Derm Venereol. 1978;58:369-371.
- Katsarou-Katsari A, Makris M, Lagogianni E, et al. Clinical features and natural history of acquired cold urticaria in a tertiary referral hospital: a 10-year prospective study. J Eur Acad Dermatol Venereol. 2008;22:1405-1411.
- Wanderer AA, Grandel KE, Wasserman SI, et al. Clinical characteristics of cold-induced systemic reactions in acquired cold urticaria syndromes: recommendations for prevention of this complication and a proposal for a diagnostic classification of cold urticaria. J Allergy Clin Immunol. 1986;78(3 Pt 1):417-423.
- Alangari AA, Twarog FJ, Shih MC, et al. Clinical features and anaphylaxis in children with cold urticaria. Pediatrics. 2004;113:e313-e317.
- Wanderer AA, Hoffman HM. The spectrum of acquired and familial cold-induced urticaria/urticaria-like syndromes. Immunol Allergy Clin North Am. 2004;24:259-286.
- Visitsuntorn N, Tuchinda M, Arunyanark N, et al. Ice cube test in children with cold urticaria. Asian Pac J Allergy Immunol. 1992;10:111-115.
- Neittaanmäki H. Cold urticaria. clinical findings in 220 patients. J Am Acad Dermatol. 1985;13:636-644.
- Siebenhaar F, Staubach P, Metz M, et al. Peltier effect-based temperature challenge: an improved method for diagnosing cold urticaria. J Allergy Clin Immunol. 2004;114:1224-1225.
- Siebenhaar F, Degener F, Zuberbier T, et al. High-dose desloratadine decreases wheal volume and improves cold provocation thresholds compared with standard-dose treatment in patients with acquired cold urticaria: a randomized, placebo-controlled, crossover study. J Allergy Clin Immunol. 2009;123:672-679.
- Duc J, Pécoud A. Successful treatment of idiopathic cold urticaria with the association of H1 and H2 antagonists: a case report. Ann Allergy. 1986;56:355-357.
- Metz M, Altrichter S, Ardelean E, et al. Anti-immunoglobulin E treatment of patients with recalcitrant physical urticaria. Int Arch Allergy Immunol. 2011;154:177-180.
- Boyce JA. Successful treatment of cold-induced urticaria/anaphylaxis with anti-IgE. J Allergy Clin Immunol. 2006;117:1415-1418.
- Marsland AM, Beck MH. Cold urticaria responding to systemic cyclosporine. Br J Dermatol. 2003;149:214-215.
- Hani N, Hartmann K, Casper C, et al. Improvement of cold urticaria by treatment with the leukotriene receptor antagonist montelukast. Acta Derm Venereol. 2000;80:229.
- Reeves GE, Boyle MJ, Bonfield J, et al. Impact of hydroxychloroquine therapy on chronic urticaria: chronic autoimmune urticaria study and evaluation. Intern Med J. 2004;34:182-186.
- Perez A, Woods A, Grattan CE. Methotrexate: a useful steroid-sparing agent in recalcitrant chronic urticaria. Br J Dermatol. 2010;162:191-194.
- Tóth-Kása I, Jancsó G, Obál F Jr, et al. Involvement of sensory nerve endings in cold and heat urticaria. J Invest Dermatol. 1983;80:34-36.
- Black AK, Keahey TM, Eady RA, et al. Dissociation of histamine release and clinical improvement following treatment of acquired cold urticaria by prednisone. Br J Clin Pharmacol. 1981;12:327-331.
- Johnston WE, Moss J, Philbin DM, et al. Management of cold urticaria during hypothermic cardiopulmonary bypass. N Engl J Med. 1982;306:219-221.
- Krause K, Zuberbier T, Maurer, M. Modern Approaches to the diagnosis and treatment of cold contact urticaria. Curr Allergy Asthma Rep. 2010;10:273-279.
- von Mackensen YA, Sticherling M. Cold urticaria: tolerance induction with cold baths. Br J Dermatol. 2007;157:835-836.
- Möller A, Henning M, Zuberbier T, et al. Epidemiology and clinical aspects of cold urticaria [article in German]. Hautarzt. 1996;47:510-514.
Practice Points
- Cold urticaria is a physical urticaria characterized by a localized or systemic eruption of papules upon exposure of the skin to cold air, liquids, and/or objects.
- Symptoms of cold urticaria, which range from erythema, pruritus, and hives to angioedema and sometimes anaphylaxis, may be debilitating for patients; therefore, effective treatment is required to improve quality of life.
- First-line treatment for cold urticaria includes second-generation H1 antihistamines at up to 4 times the standard dosage.
Manage Your Dermatology Practice: Answering Patient Questions About Diet
Patients often inquire if their diet has caused a dermatologic condition or if their diet makes it worse. Dr. Gary Goldenberg addresses how diet may impact acne, psoriasis, and urticaria. Ultimately, patient education by the dermatologist is needed to ensure patients are not relying on misinformation on the Internet regarding diets they should consider for their particular condition.
Patients often inquire if their diet has caused a dermatologic condition or if their diet makes it worse. Dr. Gary Goldenberg addresses how diet may impact acne, psoriasis, and urticaria. Ultimately, patient education by the dermatologist is needed to ensure patients are not relying on misinformation on the Internet regarding diets they should consider for their particular condition.
Patients often inquire if their diet has caused a dermatologic condition or if their diet makes it worse. Dr. Gary Goldenberg addresses how diet may impact acne, psoriasis, and urticaria. Ultimately, patient education by the dermatologist is needed to ensure patients are not relying on misinformation on the Internet regarding diets they should consider for their particular condition.
Pruritic Urticarial Papules and Plaques of Pregnancy Occurring Postpartum
The cutaneous effects of pregnancy are variable and numerous. We all have likely seen the pigmentary changes induced by pregnancy as well as both exacerbation and complete resolution of preexisting skin conditions. The dermatoses of pregnancy are classified as a group of inflammatory skin conditions exclusively seen in pregnant women, the most common being pruritic urticarial papules and plaques of pregnancy (PUPPP).1 Also known as polymorphic eruption of pregnancy in Europe, PUPPP was first recognized in 1979 as a distinct entity that manifested as an intense pruritic eruption unique to women in the third trimester of pregnancy.2 The condition usually is self-limited, with the majority of cases spontaneously resolving within 4 to 6 weeks after delivery.3,4 Presentation of PUPPP in the postpartum period is rare.1-4 We report a biopsy-proven case of PUPPP in a 30-year-old woman who presented 2 weeks postpartum with an intensely pruritic generalized eruption. A PubMed search of articles indexed for MEDLINE using the search terms pruritic urticarial papules and plaques of pregnancy or polymorphic eruption of pregnancy and postpartum revealed only 5 reports of PUPPP or polymorphic eruption of pregnancy occurring in the postpartum period, 2 occurring in the United States.5-9
Case Report
A 30-year-old woman who was 2 weeks postpartum presented to our dermatology clinic with an intensely pruritic generalized rash. Within 24 hours of delivery of her first child, the patient developed an itchy rash on the abdomen and was started on oral corticosteroids and antihistamines in the hospital. On discharge, she was instructed to follow up with the dermatology department if the rash did not resolve. After leaving the hospital, she reported that the eruption had progressively spread to the buttocks, legs, and arms, and the itching seemed to be worse despite finishing the course of oral corticosteroids and antihistamines.
The patient’s prenatal course was uneventful. She gained 16 kg during pregnancy, with a prepregnancy weight of 50 kg. A healthy male neonate was delivered at 38 weeks’ gestation without complication. The patient’s medical history was unremarkable. Her current medications included prenatal vitamins, oral prednisone, and loratadine, and she reported no known drug allergies.
On physical examination, the patient was afebrile and her blood pressure was normal. Examination of the skin revealed erythematous papules and urticarial plaques involving the abdominal striae with periumbilical sparing (Figure 1A). Similar lesions were noted on the legs, buttocks, and arms (Figure 1B). The face, palms, and soles were uninvolved. No vesicles or pustules were noted. The oral mucosa was pink, moist, and unremarkable.

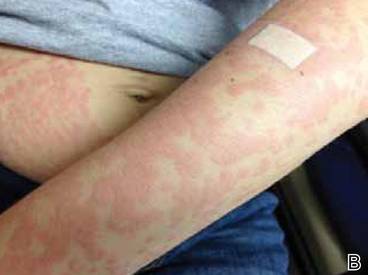
Figure 1. Initial presentation of urticarial plaques involving the abdominal striae with periumbilical sparing (A) and the left arm (B). |
Based on the patient’s clinical presentation, the differential diagnosis included pemphigoid gestationis, a hypersensitivity reaction, cutaneous lupus, cholestasis of pregnancy, and PUPPP. Pruritic urticarial papules and plaques of pregnancy was considered to be unlikely because of the uncharacteristic postpartum presentation of the eruption.
Two 4-mm punch biopsies were performed on the left upper arm and were sent for histopathologic examination and direct immunofluorescence. Laboratory studies including complete blood cell count with differential, complete metabolic panel, antinuclear antibodies, and IgE levels were conducted. The patient was started on triamcinolone cream 0.1% twice daily and her antihistamine was switched from loratadine to cetirizine.
Histopathologic examination revealed a mixed perivascular infiltrate in the superficial dermis consisting of lymphocytes, mast cells, and eosinophils (Figures 2 and 3), which was consistent with a diagnosis of PUPPP. Direct immunofluorescence was negative. Laboratory studies were within reference range and antinuclear antibodies and IgE levels were negative. A diagnosis of postpartum PUPPP was made. Complete resolution of the eruption was experienced by 2-week follow-up (Figures 4A and 4B). The patient noted that her symptoms improved within 2 days of starting topical therapy.
|
|
Comment
Pruritic urticarial papules and plaques of pregnancy complicates 1 of 160 to 1 of 300 pregnancies.1 As seen in our case, the majority of cases of PUPPP are diagnosed in women who are nulliparous or primigravida.10 A study by Aronson et al10 reported that of 57 cases of PUPPP, 24 (42%) patients were primigravida, 16 (28%) were gravida 2, 9 (16%) were gravida 3, 4 (7%) were gravida 4, 3 (5%) were gravida 6, and 1 (2%) was gravida 7. Thirty-nine (68%) patients were nulliparous.10 The average onset of symptoms is approximately 35 weeks’ gestation.9
Classical presentation of PUPPP starts with erythematous papules within the abdominal striae, sparing the periumbilical skin.1 The abdominal striae are most commonly affected, and in some women, it may be the only site affected.10 The lesions then may pro-gress to urticarial plaques involving the extremities, while the face, palms, and soles usually are spared.11 However, clinical manifestations of PUPPP can vary, with reports of targetlike lesions with a surrounding halo resembling erythema multiforme as well as involvement of the face and palmoplantar skin.10-13 Histologic findings are not diagnostic but can help distinguish PUPPP from other pregnancy-associated dermatoses.14 Histologically, PUPPP demonstrates variable epidermal spongiosis and a nonspecific superficial perivascular infiltrate in the dermis composed of lymphocytes with eosinophils or neutrophils, and there may be dermal edema.10,15 Direct immunofluorescence usually is negative in PUPPP; however, 31% of cases have demonstrated deposition of C3 and IgM or IgA, either perivascularly or at the dermoepidermal junction.1,10,15
There are no systemic alterations seen in PUPPP; however, all patients report severe pruritus.12 Pruritic urticarial papules and plaques of pregnancy typically affects women in the third trimester, and delivery is curative in most patients.13 Recurrence of PUPPP usually is not seen with subsequent pregnancies, and the long-term prognosis is excellent.15
The pathogenesis of PUPPP is not well understood and likely is multifactorial. Ohel et al12 found PUPPP to be strongly associated with hypertensive disorders, multiple gestation pregnancies, excessive maternal weight gain, excessive stretching of the abdominal skin, and nulliparity.13 One theory suggests that abdominal skin stretching, if drastic, can damage underlying connective tissue, resulting in the release of antigens that can trigger a reactive inflammatory response.16 The majority of maternal weight gain occurs during the third trimester, which may explain why most cases of PUPPP present in the third trimester.17 Alternative theories have suggested that PUPPP may represent an immunologic response to circulating fetal antigens.18 It is possible, as in our case, that certain nulliparous women who have a healthy weight prior to pregnancy (as determined by a body mass index of 18.5 to 24.9) in combination with excessive weight gain during the third trimester and drastic hormone fluctuations associated with labor and delivery may be at greater risk for developing PUPPP. Another theory may be related to the degree of skin stretching during the third trimester and the abrupt decrease in the stretching of the skin that occurs with delivery.16
Conclusion
Pruritic urticarial papules and plaques of pregnancy can present in a variety of ways, most commonly in the third trimester but also in the postpartum period. When a patient presents in the postpartum period with a pruritic eruption, PUPPP should be included in the differential diagnosis. The pathogenesis of PUPPP is multifactorial and not well understood, and additional research in the field may lead to improved prediction of who may be at risk and what we can do to prevent it.
1. Pomeranz MK. Dermatoses of pregnancy. UpToDate Web site. http://www.uptodate.com/contents/dermatoses-of-pregnancy. Updated December 22, 2014. Accessed May 5, 2015.
2. Lawley TJ, Hertz KC, Wade TR, et al. Pruritic urticarial papules and plaques of pregnancy. JAMA. 1979;241:1696-1699.
3. Kroumpouzos G, Cohen LM. Specific dermatoses of pregnancy: an evidence-based systematic review. Am J Obstet Gynecol. 2003;188:1083-1092.
4. Callen JP, Hanno R. Pruritic urticarial papules and plaques of pregnancy (PUPPP): a clinicopathologic study. J Am Acad Dermatol.1981;5:401-405.
5. Ozcan D, Ozcakmak B, Aydogan FC. J Obstet Gynaecol Res. 2011;37:1158-1161.
6. Journet-Tollhupp J, Tchen T, Remy-Leroux V, et al. Polymorphic eruption of pregnancy and acquired hemophilia A [in French]. Ann Dermatol Venereol. 2010;137:713-717.
7. Buccolo LS, Viera AJ. Pruritic urticarial papules and plaques of pregnancy presenting in the postpartum period: a case report. J Reprod Med. 2005;50:61-63.
8. Kirkup ME, Dunnill MG. Polymorphic eruption of pregnancy developing in the puerrperium. Clin Exp Dermatol. 2002;27:657-660.
9. Yancy KB, Hall RP, Lawley TJ. Pruritic urticarial papules and plaques of pregnancy: clinical experience in twenty-five patients. J Am Acad Dermatol. 1984;10:473-480.
10. Aronson IK, Bond S, Fiedler VC, et al. Pruritic urticarial papules and plaques of pregnancy: clinical and immunopathologic observations in 57 patients. J Am Acad Dermatol. 1998;39:933-939.
11. Roger D, Vaillant L, Fignon A, et al. Specific pruritic dermatoses of pregnancy: a prospective study of 3129 women. Arch Dermatol. 1994;130:734-739.
12. Ohel I, Levy A, Silberstein T, et al. Pregnancy outcome of patients with pruritic urticarial papules and plaques of pregnancy. J Matern Fetal Neonatal Med. 2006;19:305-308.
13. Elling SV, McKenna P, Pawell FC. Pruritic urticarial papules and plaques of pregnancy in twin and triplet pregnancies. J Eur Acad Dermatol Venereol. 2000;14:378-381.
14. Scheinfeld N. Pruritic urticarial papules and plaques of pregnancy wholly abated with one week twice daily application of fluticasone propionate lotion: a case report and review of the literature. Dermatol Online J. 2008;14:4.
15. Shornick JK. Dermatoses of pregnancy. Semin Cutan Med Surg. 1998;17:172-181.
16. Cohen LM, Capeless EL, Krusinski PA, et al. Pruritic urticarial papules and plaques of pregnancy and its relationship to maternal-fetal weight gain and twin pregnancy. Arch Dermatol. 1989;125:1534-1536.
17. Drehmer M, Duncan BB, Kac G, et al. Association of second and third trimester weight gain in pregnancy with maternal and fetal outcomes. PLoS One. 2013;8:e54704.
18. Aractingi S, Berkane N, Bertheau P, et al. Fetal DNA in skin of polymorphic eruptions of pregnancy. Lancet. 1998;352:1898-1901.
The cutaneous effects of pregnancy are variable and numerous. We all have likely seen the pigmentary changes induced by pregnancy as well as both exacerbation and complete resolution of preexisting skin conditions. The dermatoses of pregnancy are classified as a group of inflammatory skin conditions exclusively seen in pregnant women, the most common being pruritic urticarial papules and plaques of pregnancy (PUPPP).1 Also known as polymorphic eruption of pregnancy in Europe, PUPPP was first recognized in 1979 as a distinct entity that manifested as an intense pruritic eruption unique to women in the third trimester of pregnancy.2 The condition usually is self-limited, with the majority of cases spontaneously resolving within 4 to 6 weeks after delivery.3,4 Presentation of PUPPP in the postpartum period is rare.1-4 We report a biopsy-proven case of PUPPP in a 30-year-old woman who presented 2 weeks postpartum with an intensely pruritic generalized eruption. A PubMed search of articles indexed for MEDLINE using the search terms pruritic urticarial papules and plaques of pregnancy or polymorphic eruption of pregnancy and postpartum revealed only 5 reports of PUPPP or polymorphic eruption of pregnancy occurring in the postpartum period, 2 occurring in the United States.5-9
Case Report
A 30-year-old woman who was 2 weeks postpartum presented to our dermatology clinic with an intensely pruritic generalized rash. Within 24 hours of delivery of her first child, the patient developed an itchy rash on the abdomen and was started on oral corticosteroids and antihistamines in the hospital. On discharge, she was instructed to follow up with the dermatology department if the rash did not resolve. After leaving the hospital, she reported that the eruption had progressively spread to the buttocks, legs, and arms, and the itching seemed to be worse despite finishing the course of oral corticosteroids and antihistamines.
The patient’s prenatal course was uneventful. She gained 16 kg during pregnancy, with a prepregnancy weight of 50 kg. A healthy male neonate was delivered at 38 weeks’ gestation without complication. The patient’s medical history was unremarkable. Her current medications included prenatal vitamins, oral prednisone, and loratadine, and she reported no known drug allergies.
On physical examination, the patient was afebrile and her blood pressure was normal. Examination of the skin revealed erythematous papules and urticarial plaques involving the abdominal striae with periumbilical sparing (Figure 1A). Similar lesions were noted on the legs, buttocks, and arms (Figure 1B). The face, palms, and soles were uninvolved. No vesicles or pustules were noted. The oral mucosa was pink, moist, and unremarkable.


Figure 1. Initial presentation of urticarial plaques involving the abdominal striae with periumbilical sparing (A) and the left arm (B). |
Based on the patient’s clinical presentation, the differential diagnosis included pemphigoid gestationis, a hypersensitivity reaction, cutaneous lupus, cholestasis of pregnancy, and PUPPP. Pruritic urticarial papules and plaques of pregnancy was considered to be unlikely because of the uncharacteristic postpartum presentation of the eruption.
Two 4-mm punch biopsies were performed on the left upper arm and were sent for histopathologic examination and direct immunofluorescence. Laboratory studies including complete blood cell count with differential, complete metabolic panel, antinuclear antibodies, and IgE levels were conducted. The patient was started on triamcinolone cream 0.1% twice daily and her antihistamine was switched from loratadine to cetirizine.
Histopathologic examination revealed a mixed perivascular infiltrate in the superficial dermis consisting of lymphocytes, mast cells, and eosinophils (Figures 2 and 3), which was consistent with a diagnosis of PUPPP. Direct immunofluorescence was negative. Laboratory studies were within reference range and antinuclear antibodies and IgE levels were negative. A diagnosis of postpartum PUPPP was made. Complete resolution of the eruption was experienced by 2-week follow-up (Figures 4A and 4B). The patient noted that her symptoms improved within 2 days of starting topical therapy.
|
|
Comment
Pruritic urticarial papules and plaques of pregnancy complicates 1 of 160 to 1 of 300 pregnancies.1 As seen in our case, the majority of cases of PUPPP are diagnosed in women who are nulliparous or primigravida.10 A study by Aronson et al10 reported that of 57 cases of PUPPP, 24 (42%) patients were primigravida, 16 (28%) were gravida 2, 9 (16%) were gravida 3, 4 (7%) were gravida 4, 3 (5%) were gravida 6, and 1 (2%) was gravida 7. Thirty-nine (68%) patients were nulliparous.10 The average onset of symptoms is approximately 35 weeks’ gestation.9
Classical presentation of PUPPP starts with erythematous papules within the abdominal striae, sparing the periumbilical skin.1 The abdominal striae are most commonly affected, and in some women, it may be the only site affected.10 The lesions then may pro-gress to urticarial plaques involving the extremities, while the face, palms, and soles usually are spared.11 However, clinical manifestations of PUPPP can vary, with reports of targetlike lesions with a surrounding halo resembling erythema multiforme as well as involvement of the face and palmoplantar skin.10-13 Histologic findings are not diagnostic but can help distinguish PUPPP from other pregnancy-associated dermatoses.14 Histologically, PUPPP demonstrates variable epidermal spongiosis and a nonspecific superficial perivascular infiltrate in the dermis composed of lymphocytes with eosinophils or neutrophils, and there may be dermal edema.10,15 Direct immunofluorescence usually is negative in PUPPP; however, 31% of cases have demonstrated deposition of C3 and IgM or IgA, either perivascularly or at the dermoepidermal junction.1,10,15
There are no systemic alterations seen in PUPPP; however, all patients report severe pruritus.12 Pruritic urticarial papules and plaques of pregnancy typically affects women in the third trimester, and delivery is curative in most patients.13 Recurrence of PUPPP usually is not seen with subsequent pregnancies, and the long-term prognosis is excellent.15
The pathogenesis of PUPPP is not well understood and likely is multifactorial. Ohel et al12 found PUPPP to be strongly associated with hypertensive disorders, multiple gestation pregnancies, excessive maternal weight gain, excessive stretching of the abdominal skin, and nulliparity.13 One theory suggests that abdominal skin stretching, if drastic, can damage underlying connective tissue, resulting in the release of antigens that can trigger a reactive inflammatory response.16 The majority of maternal weight gain occurs during the third trimester, which may explain why most cases of PUPPP present in the third trimester.17 Alternative theories have suggested that PUPPP may represent an immunologic response to circulating fetal antigens.18 It is possible, as in our case, that certain nulliparous women who have a healthy weight prior to pregnancy (as determined by a body mass index of 18.5 to 24.9) in combination with excessive weight gain during the third trimester and drastic hormone fluctuations associated with labor and delivery may be at greater risk for developing PUPPP. Another theory may be related to the degree of skin stretching during the third trimester and the abrupt decrease in the stretching of the skin that occurs with delivery.16
Conclusion
Pruritic urticarial papules and plaques of pregnancy can present in a variety of ways, most commonly in the third trimester but also in the postpartum period. When a patient presents in the postpartum period with a pruritic eruption, PUPPP should be included in the differential diagnosis. The pathogenesis of PUPPP is multifactorial and not well understood, and additional research in the field may lead to improved prediction of who may be at risk and what we can do to prevent it.
The cutaneous effects of pregnancy are variable and numerous. We all have likely seen the pigmentary changes induced by pregnancy as well as both exacerbation and complete resolution of preexisting skin conditions. The dermatoses of pregnancy are classified as a group of inflammatory skin conditions exclusively seen in pregnant women, the most common being pruritic urticarial papules and plaques of pregnancy (PUPPP).1 Also known as polymorphic eruption of pregnancy in Europe, PUPPP was first recognized in 1979 as a distinct entity that manifested as an intense pruritic eruption unique to women in the third trimester of pregnancy.2 The condition usually is self-limited, with the majority of cases spontaneously resolving within 4 to 6 weeks after delivery.3,4 Presentation of PUPPP in the postpartum period is rare.1-4 We report a biopsy-proven case of PUPPP in a 30-year-old woman who presented 2 weeks postpartum with an intensely pruritic generalized eruption. A PubMed search of articles indexed for MEDLINE using the search terms pruritic urticarial papules and plaques of pregnancy or polymorphic eruption of pregnancy and postpartum revealed only 5 reports of PUPPP or polymorphic eruption of pregnancy occurring in the postpartum period, 2 occurring in the United States.5-9
Case Report
A 30-year-old woman who was 2 weeks postpartum presented to our dermatology clinic with an intensely pruritic generalized rash. Within 24 hours of delivery of her first child, the patient developed an itchy rash on the abdomen and was started on oral corticosteroids and antihistamines in the hospital. On discharge, she was instructed to follow up with the dermatology department if the rash did not resolve. After leaving the hospital, she reported that the eruption had progressively spread to the buttocks, legs, and arms, and the itching seemed to be worse despite finishing the course of oral corticosteroids and antihistamines.
The patient’s prenatal course was uneventful. She gained 16 kg during pregnancy, with a prepregnancy weight of 50 kg. A healthy male neonate was delivered at 38 weeks’ gestation without complication. The patient’s medical history was unremarkable. Her current medications included prenatal vitamins, oral prednisone, and loratadine, and she reported no known drug allergies.
On physical examination, the patient was afebrile and her blood pressure was normal. Examination of the skin revealed erythematous papules and urticarial plaques involving the abdominal striae with periumbilical sparing (Figure 1A). Similar lesions were noted on the legs, buttocks, and arms (Figure 1B). The face, palms, and soles were uninvolved. No vesicles or pustules were noted. The oral mucosa was pink, moist, and unremarkable.


Figure 1. Initial presentation of urticarial plaques involving the abdominal striae with periumbilical sparing (A) and the left arm (B). |
Based on the patient’s clinical presentation, the differential diagnosis included pemphigoid gestationis, a hypersensitivity reaction, cutaneous lupus, cholestasis of pregnancy, and PUPPP. Pruritic urticarial papules and plaques of pregnancy was considered to be unlikely because of the uncharacteristic postpartum presentation of the eruption.
Two 4-mm punch biopsies were performed on the left upper arm and were sent for histopathologic examination and direct immunofluorescence. Laboratory studies including complete blood cell count with differential, complete metabolic panel, antinuclear antibodies, and IgE levels were conducted. The patient was started on triamcinolone cream 0.1% twice daily and her antihistamine was switched from loratadine to cetirizine.
Histopathologic examination revealed a mixed perivascular infiltrate in the superficial dermis consisting of lymphocytes, mast cells, and eosinophils (Figures 2 and 3), which was consistent with a diagnosis of PUPPP. Direct immunofluorescence was negative. Laboratory studies were within reference range and antinuclear antibodies and IgE levels were negative. A diagnosis of postpartum PUPPP was made. Complete resolution of the eruption was experienced by 2-week follow-up (Figures 4A and 4B). The patient noted that her symptoms improved within 2 days of starting topical therapy.
|
|
Comment
Pruritic urticarial papules and plaques of pregnancy complicates 1 of 160 to 1 of 300 pregnancies.1 As seen in our case, the majority of cases of PUPPP are diagnosed in women who are nulliparous or primigravida.10 A study by Aronson et al10 reported that of 57 cases of PUPPP, 24 (42%) patients were primigravida, 16 (28%) were gravida 2, 9 (16%) were gravida 3, 4 (7%) were gravida 4, 3 (5%) were gravida 6, and 1 (2%) was gravida 7. Thirty-nine (68%) patients were nulliparous.10 The average onset of symptoms is approximately 35 weeks’ gestation.9
Classical presentation of PUPPP starts with erythematous papules within the abdominal striae, sparing the periumbilical skin.1 The abdominal striae are most commonly affected, and in some women, it may be the only site affected.10 The lesions then may pro-gress to urticarial plaques involving the extremities, while the face, palms, and soles usually are spared.11 However, clinical manifestations of PUPPP can vary, with reports of targetlike lesions with a surrounding halo resembling erythema multiforme as well as involvement of the face and palmoplantar skin.10-13 Histologic findings are not diagnostic but can help distinguish PUPPP from other pregnancy-associated dermatoses.14 Histologically, PUPPP demonstrates variable epidermal spongiosis and a nonspecific superficial perivascular infiltrate in the dermis composed of lymphocytes with eosinophils or neutrophils, and there may be dermal edema.10,15 Direct immunofluorescence usually is negative in PUPPP; however, 31% of cases have demonstrated deposition of C3 and IgM or IgA, either perivascularly or at the dermoepidermal junction.1,10,15
There are no systemic alterations seen in PUPPP; however, all patients report severe pruritus.12 Pruritic urticarial papules and plaques of pregnancy typically affects women in the third trimester, and delivery is curative in most patients.13 Recurrence of PUPPP usually is not seen with subsequent pregnancies, and the long-term prognosis is excellent.15
The pathogenesis of PUPPP is not well understood and likely is multifactorial. Ohel et al12 found PUPPP to be strongly associated with hypertensive disorders, multiple gestation pregnancies, excessive maternal weight gain, excessive stretching of the abdominal skin, and nulliparity.13 One theory suggests that abdominal skin stretching, if drastic, can damage underlying connective tissue, resulting in the release of antigens that can trigger a reactive inflammatory response.16 The majority of maternal weight gain occurs during the third trimester, which may explain why most cases of PUPPP present in the third trimester.17 Alternative theories have suggested that PUPPP may represent an immunologic response to circulating fetal antigens.18 It is possible, as in our case, that certain nulliparous women who have a healthy weight prior to pregnancy (as determined by a body mass index of 18.5 to 24.9) in combination with excessive weight gain during the third trimester and drastic hormone fluctuations associated with labor and delivery may be at greater risk for developing PUPPP. Another theory may be related to the degree of skin stretching during the third trimester and the abrupt decrease in the stretching of the skin that occurs with delivery.16
Conclusion
Pruritic urticarial papules and plaques of pregnancy can present in a variety of ways, most commonly in the third trimester but also in the postpartum period. When a patient presents in the postpartum period with a pruritic eruption, PUPPP should be included in the differential diagnosis. The pathogenesis of PUPPP is multifactorial and not well understood, and additional research in the field may lead to improved prediction of who may be at risk and what we can do to prevent it.
1. Pomeranz MK. Dermatoses of pregnancy. UpToDate Web site. http://www.uptodate.com/contents/dermatoses-of-pregnancy. Updated December 22, 2014. Accessed May 5, 2015.
2. Lawley TJ, Hertz KC, Wade TR, et al. Pruritic urticarial papules and plaques of pregnancy. JAMA. 1979;241:1696-1699.
3. Kroumpouzos G, Cohen LM. Specific dermatoses of pregnancy: an evidence-based systematic review. Am J Obstet Gynecol. 2003;188:1083-1092.
4. Callen JP, Hanno R. Pruritic urticarial papules and plaques of pregnancy (PUPPP): a clinicopathologic study. J Am Acad Dermatol.1981;5:401-405.
5. Ozcan D, Ozcakmak B, Aydogan FC. J Obstet Gynaecol Res. 2011;37:1158-1161.
6. Journet-Tollhupp J, Tchen T, Remy-Leroux V, et al. Polymorphic eruption of pregnancy and acquired hemophilia A [in French]. Ann Dermatol Venereol. 2010;137:713-717.
7. Buccolo LS, Viera AJ. Pruritic urticarial papules and plaques of pregnancy presenting in the postpartum period: a case report. J Reprod Med. 2005;50:61-63.
8. Kirkup ME, Dunnill MG. Polymorphic eruption of pregnancy developing in the puerrperium. Clin Exp Dermatol. 2002;27:657-660.
9. Yancy KB, Hall RP, Lawley TJ. Pruritic urticarial papules and plaques of pregnancy: clinical experience in twenty-five patients. J Am Acad Dermatol. 1984;10:473-480.
10. Aronson IK, Bond S, Fiedler VC, et al. Pruritic urticarial papules and plaques of pregnancy: clinical and immunopathologic observations in 57 patients. J Am Acad Dermatol. 1998;39:933-939.
11. Roger D, Vaillant L, Fignon A, et al. Specific pruritic dermatoses of pregnancy: a prospective study of 3129 women. Arch Dermatol. 1994;130:734-739.
12. Ohel I, Levy A, Silberstein T, et al. Pregnancy outcome of patients with pruritic urticarial papules and plaques of pregnancy. J Matern Fetal Neonatal Med. 2006;19:305-308.
13. Elling SV, McKenna P, Pawell FC. Pruritic urticarial papules and plaques of pregnancy in twin and triplet pregnancies. J Eur Acad Dermatol Venereol. 2000;14:378-381.
14. Scheinfeld N. Pruritic urticarial papules and plaques of pregnancy wholly abated with one week twice daily application of fluticasone propionate lotion: a case report and review of the literature. Dermatol Online J. 2008;14:4.
15. Shornick JK. Dermatoses of pregnancy. Semin Cutan Med Surg. 1998;17:172-181.
16. Cohen LM, Capeless EL, Krusinski PA, et al. Pruritic urticarial papules and plaques of pregnancy and its relationship to maternal-fetal weight gain and twin pregnancy. Arch Dermatol. 1989;125:1534-1536.
17. Drehmer M, Duncan BB, Kac G, et al. Association of second and third trimester weight gain in pregnancy with maternal and fetal outcomes. PLoS One. 2013;8:e54704.
18. Aractingi S, Berkane N, Bertheau P, et al. Fetal DNA in skin of polymorphic eruptions of pregnancy. Lancet. 1998;352:1898-1901.
1. Pomeranz MK. Dermatoses of pregnancy. UpToDate Web site. http://www.uptodate.com/contents/dermatoses-of-pregnancy. Updated December 22, 2014. Accessed May 5, 2015.
2. Lawley TJ, Hertz KC, Wade TR, et al. Pruritic urticarial papules and plaques of pregnancy. JAMA. 1979;241:1696-1699.
3. Kroumpouzos G, Cohen LM. Specific dermatoses of pregnancy: an evidence-based systematic review. Am J Obstet Gynecol. 2003;188:1083-1092.
4. Callen JP, Hanno R. Pruritic urticarial papules and plaques of pregnancy (PUPPP): a clinicopathologic study. J Am Acad Dermatol.1981;5:401-405.
5. Ozcan D, Ozcakmak B, Aydogan FC. J Obstet Gynaecol Res. 2011;37:1158-1161.
6. Journet-Tollhupp J, Tchen T, Remy-Leroux V, et al. Polymorphic eruption of pregnancy and acquired hemophilia A [in French]. Ann Dermatol Venereol. 2010;137:713-717.
7. Buccolo LS, Viera AJ. Pruritic urticarial papules and plaques of pregnancy presenting in the postpartum period: a case report. J Reprod Med. 2005;50:61-63.
8. Kirkup ME, Dunnill MG. Polymorphic eruption of pregnancy developing in the puerrperium. Clin Exp Dermatol. 2002;27:657-660.
9. Yancy KB, Hall RP, Lawley TJ. Pruritic urticarial papules and plaques of pregnancy: clinical experience in twenty-five patients. J Am Acad Dermatol. 1984;10:473-480.
10. Aronson IK, Bond S, Fiedler VC, et al. Pruritic urticarial papules and plaques of pregnancy: clinical and immunopathologic observations in 57 patients. J Am Acad Dermatol. 1998;39:933-939.
11. Roger D, Vaillant L, Fignon A, et al. Specific pruritic dermatoses of pregnancy: a prospective study of 3129 women. Arch Dermatol. 1994;130:734-739.
12. Ohel I, Levy A, Silberstein T, et al. Pregnancy outcome of patients with pruritic urticarial papules and plaques of pregnancy. J Matern Fetal Neonatal Med. 2006;19:305-308.
13. Elling SV, McKenna P, Pawell FC. Pruritic urticarial papules and plaques of pregnancy in twin and triplet pregnancies. J Eur Acad Dermatol Venereol. 2000;14:378-381.
14. Scheinfeld N. Pruritic urticarial papules and plaques of pregnancy wholly abated with one week twice daily application of fluticasone propionate lotion: a case report and review of the literature. Dermatol Online J. 2008;14:4.
15. Shornick JK. Dermatoses of pregnancy. Semin Cutan Med Surg. 1998;17:172-181.
16. Cohen LM, Capeless EL, Krusinski PA, et al. Pruritic urticarial papules and plaques of pregnancy and its relationship to maternal-fetal weight gain and twin pregnancy. Arch Dermatol. 1989;125:1534-1536.
17. Drehmer M, Duncan BB, Kac G, et al. Association of second and third trimester weight gain in pregnancy with maternal and fetal outcomes. PLoS One. 2013;8:e54704.
18. Aractingi S, Berkane N, Bertheau P, et al. Fetal DNA in skin of polymorphic eruptions of pregnancy. Lancet. 1998;352:1898-1901.
Practice Points
- Pruritic urticarial papules and plaques of pregnancy (PUPPP) is an intensely pruritic eruption that typically affects women during the third trimester of pregnancy.
- Because clinical manifestations can vary, PUPPP should be considered in the differential diagnosis when patients present in the postpartum period with a pruritic eruption.
- Histologic findings are not diagnostic but can help distinguish PUPPP from other pregnancy-associated dermatoses.
Mycoplasma pneumoniae Infection in Adults With Acute and Chronic Urticaria
To the Editor:
Mycoplasma pneumoniae has been implicated as a cause of acute urticaria (AU) in children,1 but its role in adults with AU is unknown. The aim of this retrospective study was to compare the incidence of acute M pneumoniae infection in adults with AU and chronic urticaria (CU).
A chart review was performed on adult patients with AU and CU who presented at a private dermatology practice in Singapore. Acute M pneumoniae infection was diagnosed on the basis of a single indirect microparticle agglutinin assay (MAA) titer of 1:320 or higher. All statistical tests were performed using SPSS version 13.0. P=.05 was regarded as significant. Data from 49 adults with AU and 44 adults with CU were analyzed. The distribution of MAA titers in adults with AU and CU are shown in the Figure. Microparticle agglutinin assay was negative in 10 (20.4%) of 49 adults with AU. Fifteen (30.6%) of 49 adults with AU had evidence of acute M pneumoniae infection, as indicated by an MAA titer of 1:320 or higher. The remaining 24 (49.0%) had evidence of prior infection as indicated by titers above the manufacturer’s cutoff of 1:402 and below our cutoff for acute infection of 1:320 or higher. Microparticle agglutinin assay was negative in 11 (25%) of 44 adults with CU. Three (6.8%) adults with CU were diagnosed with acute M pneumoniae infection and 30 (68.2%) were diagnosed with prior infection. The incidence of acute M pneumoniae infection was 30.6% in adults with AU compared to 6.8% in adults with CU, and the difference was statistically significant (P=.004).
Extrapulmonary complications of M pneumoniae involving practically every organ system have been described and 25% of patients develop cutaneous symptoms3 including AU. In 2007, Kano et al4 reported M pneumoniae infection-induced erythema nodosum, anaphylactoid purpura, and AU in a family of 3. This report was interesting because it showed that M pneumoniae had the ability to elicit different cutaneous reactions depending on the maturity of the adaptive immunity of a host, even among individuals of a common genetic background. A Taiwanese study found that 21 (32%) of 65 children with AU had M pneumoniae infection as determined by a positive Mycoplasma IgM test or an equivocal Mycoplasma IgM coupled with positive cold agglutinin test results.1
In our study, we found serological evidence of acute M pneumoniae infection in 15 (30.6%) of 49 adults with AU compared to 3 (6.8%) of 44 adults with CU (P=.004), suggesting that M pneumoniae also may play a role in the etiology of adult AU. Diagnosis of acute M pneumoniae infection is challenging, as it is often impossible to obtain convalescent serum that will show the 4-fold rise in titer. Single MAA titers of 1:160 or higher have been recommended for diagnosis of acute infection,5 but because of higher background activity in Singapore, we used a higher titer (>1:320). However, in doing so, we could be underestimating the true incidence of acute M pneumoniae infections.
The role of M pneumoniae in CU is uncertain. A Thai study reported that 55% of 38 children with CU had elevated M pneumoniae titers but did not provide details of actual titers or define what they meant by elevated titers.6 The incidence of acute and prior infection in our patients with CU was 6.8% and 68.2%, respectively. Unfortunately, we cannot determine the significance of the 68.2% incidence rate of prior infection in the absence of a normal control population of patients without urticaria. Another limitation of this study is that we compared M pneumoniae infection rate in AU with CU on the assumption that infection is not likely to play a significant role in CU, which may not necessarily be the case. Tests for other etiologic agents, including viruses, also were not performed. Not withstanding these limitations, this study suggests that acute M pneumoniae infection is significantly more common in adults with AU than in adults with CU.
This study showed that M pneumoniae might play a role in the etiology of AU in adults and our findings need to be confirmed by prospective studies. Several more questions must be answered before deciding whether the current practice of treating AU symptomatically without investigation needs to be changed. First, does treatment of M pneumoniae infection have any influence on the course of AU? The fact that AU usually is self-limiting suggests that treatment may not influence the disease course. Second, does treatment of underlying M pneumoniae infection shorten the course of AU? Third, do AU patients with untreated M pneumoniae infection face a higher risk for developing CU? This question is intriguing for the following reasons: (1) 30% to 50% of CU cases are autoimmune in etiology7; (2) antibodies to galactocerebroside that cross-react with glycolipids on M pneumoniae have been detected in patients with M pneumoniae–associated Guillain-Barré syndrome, suggesting a form of molecular mimicry8; and (3) antinuclear antibody also has occasionally been detected in sera of patients with M pneumoniae.9 It would be interesting to test patients with autoimmune and nonautoimmune CU for evidence of M pneumoniae serology infection.
We hope that prospective studies will be conducted in the future to confirm our findings and answer some of the questions raised.
1. Wu CC, Kuo HC, Yu HR, et al. Association of acute urticaria with Mycoplasma pneumoniae infection in hospitalized children. Ann Allergy Asthma Immunol. 2009;103:134-139.
2. Serodia-Myco II [package insert]. Tokyo, Japan: Fujirebo; 2013.
3. Murray HW, Masur H, Senterfit LB, et al. The protean manifestations of Mycoplasma pneumoniae infection in adults. Am J Med. 1975;58:229-242.
4. Kano Y, Mitsuyama Y, Hirahara K, et al. Mycoplasma pneumoniae infection-induced erythema nodosum, anaphylactoid purpura, and acute urticaria in 3 people in a single family. J Am Acad Dermatol. 2007;57(suppl 2):S33-S35.
5. Daxboeck F, Krause R, Wenisch C. Laboratory diagnosis of Mycoplasma pneumoniae infection. Clin Microbiol Infect. 2003;9:263-273.
6. Pongpreuksa S, Boochoo S, Kulthanan K, et al. Chronic urticaria: what is worth doing in pediatric population? J Allergy Clin Immunol. 2004:S134.
7. Grattan CE. Autoimmune urticaria. Immunol Allergy Clin North Am. 2004;24:163-181.
8. Kusunoki S, Shiina M, Kanazawa I. Anti-Gal-C antibodies in GBS subsequent to mycoplasma infection: evidence of molecular mimicry. Neurology. 2001;57:736-738.
9. Arikan S, Ergüven S, Ustaçelebi S, et al. Detection of antinuclear antibody (ANA) in patients with Mycoplasmal Pneumonia. Turk J Med Sci. 1998;28:97-98.
To the Editor:
Mycoplasma pneumoniae has been implicated as a cause of acute urticaria (AU) in children,1 but its role in adults with AU is unknown. The aim of this retrospective study was to compare the incidence of acute M pneumoniae infection in adults with AU and chronic urticaria (CU).
A chart review was performed on adult patients with AU and CU who presented at a private dermatology practice in Singapore. Acute M pneumoniae infection was diagnosed on the basis of a single indirect microparticle agglutinin assay (MAA) titer of 1:320 or higher. All statistical tests were performed using SPSS version 13.0. P=.05 was regarded as significant. Data from 49 adults with AU and 44 adults with CU were analyzed. The distribution of MAA titers in adults with AU and CU are shown in the Figure. Microparticle agglutinin assay was negative in 10 (20.4%) of 49 adults with AU. Fifteen (30.6%) of 49 adults with AU had evidence of acute M pneumoniae infection, as indicated by an MAA titer of 1:320 or higher. The remaining 24 (49.0%) had evidence of prior infection as indicated by titers above the manufacturer’s cutoff of 1:402 and below our cutoff for acute infection of 1:320 or higher. Microparticle agglutinin assay was negative in 11 (25%) of 44 adults with CU. Three (6.8%) adults with CU were diagnosed with acute M pneumoniae infection and 30 (68.2%) were diagnosed with prior infection. The incidence of acute M pneumoniae infection was 30.6% in adults with AU compared to 6.8% in adults with CU, and the difference was statistically significant (P=.004).
Extrapulmonary complications of M pneumoniae involving practically every organ system have been described and 25% of patients develop cutaneous symptoms3 including AU. In 2007, Kano et al4 reported M pneumoniae infection-induced erythema nodosum, anaphylactoid purpura, and AU in a family of 3. This report was interesting because it showed that M pneumoniae had the ability to elicit different cutaneous reactions depending on the maturity of the adaptive immunity of a host, even among individuals of a common genetic background. A Taiwanese study found that 21 (32%) of 65 children with AU had M pneumoniae infection as determined by a positive Mycoplasma IgM test or an equivocal Mycoplasma IgM coupled with positive cold agglutinin test results.1
In our study, we found serological evidence of acute M pneumoniae infection in 15 (30.6%) of 49 adults with AU compared to 3 (6.8%) of 44 adults with CU (P=.004), suggesting that M pneumoniae also may play a role in the etiology of adult AU. Diagnosis of acute M pneumoniae infection is challenging, as it is often impossible to obtain convalescent serum that will show the 4-fold rise in titer. Single MAA titers of 1:160 or higher have been recommended for diagnosis of acute infection,5 but because of higher background activity in Singapore, we used a higher titer (>1:320). However, in doing so, we could be underestimating the true incidence of acute M pneumoniae infections.
The role of M pneumoniae in CU is uncertain. A Thai study reported that 55% of 38 children with CU had elevated M pneumoniae titers but did not provide details of actual titers or define what they meant by elevated titers.6 The incidence of acute and prior infection in our patients with CU was 6.8% and 68.2%, respectively. Unfortunately, we cannot determine the significance of the 68.2% incidence rate of prior infection in the absence of a normal control population of patients without urticaria. Another limitation of this study is that we compared M pneumoniae infection rate in AU with CU on the assumption that infection is not likely to play a significant role in CU, which may not necessarily be the case. Tests for other etiologic agents, including viruses, also were not performed. Not withstanding these limitations, this study suggests that acute M pneumoniae infection is significantly more common in adults with AU than in adults with CU.
This study showed that M pneumoniae might play a role in the etiology of AU in adults and our findings need to be confirmed by prospective studies. Several more questions must be answered before deciding whether the current practice of treating AU symptomatically without investigation needs to be changed. First, does treatment of M pneumoniae infection have any influence on the course of AU? The fact that AU usually is self-limiting suggests that treatment may not influence the disease course. Second, does treatment of underlying M pneumoniae infection shorten the course of AU? Third, do AU patients with untreated M pneumoniae infection face a higher risk for developing CU? This question is intriguing for the following reasons: (1) 30% to 50% of CU cases are autoimmune in etiology7; (2) antibodies to galactocerebroside that cross-react with glycolipids on M pneumoniae have been detected in patients with M pneumoniae–associated Guillain-Barré syndrome, suggesting a form of molecular mimicry8; and (3) antinuclear antibody also has occasionally been detected in sera of patients with M pneumoniae.9 It would be interesting to test patients with autoimmune and nonautoimmune CU for evidence of M pneumoniae serology infection.
We hope that prospective studies will be conducted in the future to confirm our findings and answer some of the questions raised.
To the Editor:
Mycoplasma pneumoniae has been implicated as a cause of acute urticaria (AU) in children,1 but its role in adults with AU is unknown. The aim of this retrospective study was to compare the incidence of acute M pneumoniae infection in adults with AU and chronic urticaria (CU).
A chart review was performed on adult patients with AU and CU who presented at a private dermatology practice in Singapore. Acute M pneumoniae infection was diagnosed on the basis of a single indirect microparticle agglutinin assay (MAA) titer of 1:320 or higher. All statistical tests were performed using SPSS version 13.0. P=.05 was regarded as significant. Data from 49 adults with AU and 44 adults with CU were analyzed. The distribution of MAA titers in adults with AU and CU are shown in the Figure. Microparticle agglutinin assay was negative in 10 (20.4%) of 49 adults with AU. Fifteen (30.6%) of 49 adults with AU had evidence of acute M pneumoniae infection, as indicated by an MAA titer of 1:320 or higher. The remaining 24 (49.0%) had evidence of prior infection as indicated by titers above the manufacturer’s cutoff of 1:402 and below our cutoff for acute infection of 1:320 or higher. Microparticle agglutinin assay was negative in 11 (25%) of 44 adults with CU. Three (6.8%) adults with CU were diagnosed with acute M pneumoniae infection and 30 (68.2%) were diagnosed with prior infection. The incidence of acute M pneumoniae infection was 30.6% in adults with AU compared to 6.8% in adults with CU, and the difference was statistically significant (P=.004).
Extrapulmonary complications of M pneumoniae involving practically every organ system have been described and 25% of patients develop cutaneous symptoms3 including AU. In 2007, Kano et al4 reported M pneumoniae infection-induced erythema nodosum, anaphylactoid purpura, and AU in a family of 3. This report was interesting because it showed that M pneumoniae had the ability to elicit different cutaneous reactions depending on the maturity of the adaptive immunity of a host, even among individuals of a common genetic background. A Taiwanese study found that 21 (32%) of 65 children with AU had M pneumoniae infection as determined by a positive Mycoplasma IgM test or an equivocal Mycoplasma IgM coupled with positive cold agglutinin test results.1
In our study, we found serological evidence of acute M pneumoniae infection in 15 (30.6%) of 49 adults with AU compared to 3 (6.8%) of 44 adults with CU (P=.004), suggesting that M pneumoniae also may play a role in the etiology of adult AU. Diagnosis of acute M pneumoniae infection is challenging, as it is often impossible to obtain convalescent serum that will show the 4-fold rise in titer. Single MAA titers of 1:160 or higher have been recommended for diagnosis of acute infection,5 but because of higher background activity in Singapore, we used a higher titer (>1:320). However, in doing so, we could be underestimating the true incidence of acute M pneumoniae infections.
The role of M pneumoniae in CU is uncertain. A Thai study reported that 55% of 38 children with CU had elevated M pneumoniae titers but did not provide details of actual titers or define what they meant by elevated titers.6 The incidence of acute and prior infection in our patients with CU was 6.8% and 68.2%, respectively. Unfortunately, we cannot determine the significance of the 68.2% incidence rate of prior infection in the absence of a normal control population of patients without urticaria. Another limitation of this study is that we compared M pneumoniae infection rate in AU with CU on the assumption that infection is not likely to play a significant role in CU, which may not necessarily be the case. Tests for other etiologic agents, including viruses, also were not performed. Not withstanding these limitations, this study suggests that acute M pneumoniae infection is significantly more common in adults with AU than in adults with CU.
This study showed that M pneumoniae might play a role in the etiology of AU in adults and our findings need to be confirmed by prospective studies. Several more questions must be answered before deciding whether the current practice of treating AU symptomatically without investigation needs to be changed. First, does treatment of M pneumoniae infection have any influence on the course of AU? The fact that AU usually is self-limiting suggests that treatment may not influence the disease course. Second, does treatment of underlying M pneumoniae infection shorten the course of AU? Third, do AU patients with untreated M pneumoniae infection face a higher risk for developing CU? This question is intriguing for the following reasons: (1) 30% to 50% of CU cases are autoimmune in etiology7; (2) antibodies to galactocerebroside that cross-react with glycolipids on M pneumoniae have been detected in patients with M pneumoniae–associated Guillain-Barré syndrome, suggesting a form of molecular mimicry8; and (3) antinuclear antibody also has occasionally been detected in sera of patients with M pneumoniae.9 It would be interesting to test patients with autoimmune and nonautoimmune CU for evidence of M pneumoniae serology infection.
We hope that prospective studies will be conducted in the future to confirm our findings and answer some of the questions raised.
1. Wu CC, Kuo HC, Yu HR, et al. Association of acute urticaria with Mycoplasma pneumoniae infection in hospitalized children. Ann Allergy Asthma Immunol. 2009;103:134-139.
2. Serodia-Myco II [package insert]. Tokyo, Japan: Fujirebo; 2013.
3. Murray HW, Masur H, Senterfit LB, et al. The protean manifestations of Mycoplasma pneumoniae infection in adults. Am J Med. 1975;58:229-242.
4. Kano Y, Mitsuyama Y, Hirahara K, et al. Mycoplasma pneumoniae infection-induced erythema nodosum, anaphylactoid purpura, and acute urticaria in 3 people in a single family. J Am Acad Dermatol. 2007;57(suppl 2):S33-S35.
5. Daxboeck F, Krause R, Wenisch C. Laboratory diagnosis of Mycoplasma pneumoniae infection. Clin Microbiol Infect. 2003;9:263-273.
6. Pongpreuksa S, Boochoo S, Kulthanan K, et al. Chronic urticaria: what is worth doing in pediatric population? J Allergy Clin Immunol. 2004:S134.
7. Grattan CE. Autoimmune urticaria. Immunol Allergy Clin North Am. 2004;24:163-181.
8. Kusunoki S, Shiina M, Kanazawa I. Anti-Gal-C antibodies in GBS subsequent to mycoplasma infection: evidence of molecular mimicry. Neurology. 2001;57:736-738.
9. Arikan S, Ergüven S, Ustaçelebi S, et al. Detection of antinuclear antibody (ANA) in patients with Mycoplasmal Pneumonia. Turk J Med Sci. 1998;28:97-98.
1. Wu CC, Kuo HC, Yu HR, et al. Association of acute urticaria with Mycoplasma pneumoniae infection in hospitalized children. Ann Allergy Asthma Immunol. 2009;103:134-139.
2. Serodia-Myco II [package insert]. Tokyo, Japan: Fujirebo; 2013.
3. Murray HW, Masur H, Senterfit LB, et al. The protean manifestations of Mycoplasma pneumoniae infection in adults. Am J Med. 1975;58:229-242.
4. Kano Y, Mitsuyama Y, Hirahara K, et al. Mycoplasma pneumoniae infection-induced erythema nodosum, anaphylactoid purpura, and acute urticaria in 3 people in a single family. J Am Acad Dermatol. 2007;57(suppl 2):S33-S35.
5. Daxboeck F, Krause R, Wenisch C. Laboratory diagnosis of Mycoplasma pneumoniae infection. Clin Microbiol Infect. 2003;9:263-273.
6. Pongpreuksa S, Boochoo S, Kulthanan K, et al. Chronic urticaria: what is worth doing in pediatric population? J Allergy Clin Immunol. 2004:S134.
7. Grattan CE. Autoimmune urticaria. Immunol Allergy Clin North Am. 2004;24:163-181.
8. Kusunoki S, Shiina M, Kanazawa I. Anti-Gal-C antibodies in GBS subsequent to mycoplasma infection: evidence of molecular mimicry. Neurology. 2001;57:736-738.
9. Arikan S, Ergüven S, Ustaçelebi S, et al. Detection of antinuclear antibody (ANA) in patients with Mycoplasmal Pneumonia. Turk J Med Sci. 1998;28:97-98.
Cholinergic Urticaria With Anaphylaxis: Hazardous Duty of a Deployed US Marine
Cholinergic urticaria (CU) is a condition that primarily affects young adults. It can severely limit their activity levels and therefore job performance. Rarely, this condition can be associated with anaphylaxis, requiring a high index of suspicion by the clinician to ensure proper evaluation and treatment to prevent future respiratory compromise. We present the case of a 27-year-old US Marine with CU and anaphylaxis confirmed by a water challenge test in a warm bath.
Case Report
An otherwise healthy 27-year-old white man who was a US Marine presented with a concern of hives that appeared during strenuous exercise when he was deployed in Afghanistan approximately 1 year earlier. He initially began to experience urticarial lesions when taking warm showers with concomitant shortness of breath and wheezing. He reported no history of hives or asthma. Despite using diphenhydramine as needed to control symptoms for several months, he noted that the episodes of urticaria occurred with light-headedness, dizziness, or vomiting even with mild physical activity or common daily activities. Symptoms typically would resolve 30 to 90 minutes after he stopped exercising or cooled off. Over the course of approximately 1 year, the patient was prescribed a variety of sedating and nonsedating antihistamines (eg, diphenhydramine, hydroxyzine, doxepin, cetirizine, loratadine, fexofenadine, montelukast) by primary care while deployed, some of which mitigated his symptoms during warm showers and outdoor activities but not during exercise.
After returning from his deployment, the patient was initially referred to the dermatology department. No lesions were noted on physical examination. Based on his history, he was advised to avoid strenuous exercise and activity. During subsequent visits to dermatology an exercise challenge test was considered but not initiated due to lack of facilities to provide appropriate airway monitoring. The allergy department was consulted and the patient also was prescribed leukotriene receptor antagonists in addition to the antihistamines he was already taking. It was decided that a water challenge test in a warm bath would be performed in lieu of an exercise challenge to confirm a diagnosis of CU versus CU with anaphylaxis. If the patient did not have a reaction to the water challenge test, an exercise challenge would be offered.
After stopping treatment with antihistamines and leukotriene receptor antagonists for 1 week, a water challenge test was performed. A heparin lock was placed in the untested left arm for intravenous access. The right arm was immersed in a warm bath for 5 minutes without incident. After confirming no reaction, the arm was immersed for another 5 minutes, after which the patient reported flushing, warmth, and itching with visible 2- to 3-mm urticarial lesions on the back (Figure, A) and chest (Figure, B). The arm was subsequently removed from the water. No lesions were noted on either of the arms. The patient developed a cough after removing the arm from the water and his peak expiratory flow rate dropped from 520 to 440 L/min. After 5 minutes his peak expiratory flow rate recovered to 500 L/min and the coughing subsided. He also reported mild nausea and a headache. He was rapidly cooled with ice to abort any further reaction. An epinephrine autoinjector was on hand but was not used due to rapidly resolving symptoms. The diagnosis of CU with anaphylaxis was confirmed.
Erythematous eruption with 2- to 3-mm urticarial lesions on the back (A) and chest (B). |
Comment
Urticaria is a heterogeneous group of disorders that includes both cholinergic and exercise-induced variants. Cholinergic urticaria affects as many as 11.2% of young adults aged 15 to 35 years, with a peak incidence of 20% between 26 and 28 years of age.1 Clinical presentation consists of wheals (central swelling with peripheral erythema) that are 1 to 5 mm in diameter2 with associated itching/burning that typically resolves within 1 to 24 hours. Cholinergic urticaria is the result of a rise in core body temperature independent of exercise status; it is distinguished from exercise-induced urticaria, which occurs in response to vigorous exercise and is not related to a rise in body temperature.3 In particular, these forms of urticaria can severely impact the lives and careers of young servicemen and servicewomen who are routinely deployed to warm environments.
A provocation test is recommended by Magerl et al4 in patients with a suspected diagnosis of CU. First, an exercise challenge test using a bicycle, treadmill, or similar equipment is recommended, with the patient exercising for 15 minutes beyond the start of sweating. Readings for urticaria are made immediately following the test and 10 minutes later. If the test is positive, a water challenge test in a hot bath (42°C) is then recommended for 15 minutes beyond an increase of 1°C in baseline core body temperature.4 One study demonstrated that 43% (13/30) of patients with CU experienced bronchial hyperresponsiveness on methacholine challenge testing.5 These findings suggest a possible utility in testing CU patients for potential disease-related respiratory compromise. A practical limitation of this study was that it did not examine a link between bronchial hyperresponsiveness and anaphylaxis during cholinergic urticarial flares. An exercise challenge test was not performed in our patient due to a history of wheezing and shortness of breath with exercise; instead we went directly to the water challenge test. We felt that limited immersion in the water (ie, only 1 arm) further minimized the risk for anaphylaxis compared with full-body immersion.
Any activity that raises core body temperature in a patient with CU can induce onset of lesions. One case report described a patient who experienced symptoms while undergoing hemodialysis, which resolved when the dialysate temperature was decreased from the normal 36.5°C to 35°C.6 However, most cases are triggered by daily activities or work. The mainstay of treatment of CU is nonsedating antihistamines. Cetirizine has demonstrated particular efficacy.7 For unresponsive cases, treatments include scopolamine butylbromide8,9; ketotifen10; combinations of cetirizine, montelukast, and propanolol11; and danazol.12
Conclusion
Cholinergic urticaria is mostly prevalent among young adults, with highest incidence in the late 20s. Active duty servicemen and servicewomen are among those who are at the greatest risk for developing CU, especially those deployed to tropical environments. Frequently, CU is associated with bronchial hyperresponsiveness and also can be associated with anaphylaxis, as was seen in our patient. Care must be taken before provocative tests are conducted in these patients and should be done in a controlled environment in which airway compromise can be properly assessed and treated if anaphylaxis were to occur.
1. Zuberbier T, Althaus C, Chantraine-Hess S, et al. Prevalence of cholinergic urticaria in young adults. J Am Acad Dermatol. 1994;31:978-981.
2. Kontou-Fili K, Borici-Mazi R, Kapp A, et al. Physical urticaria: classification and diagnostic guidelines. an EAACI position paper. Allergy. 1997;52:504-513.
3. Zuberbier T, Asero R, Bindslev-Jensen C, et al; Dermatology Section of the European Academy of Allergology and Immunology; Global Allergy and Asthma European Network; European Dermatology Forum; World Allergy Organization. EAACI/GA(2)LEN/EDF/WAO guideline: definition, classification and diagnosis of urticaria. Allergy. 2009;64:1417-1426.
4. Magerl M, Borzova E, Giménez-Arnau A, et al; EAACI/GA2LEN/EDF/UNEV. The definition and diagnostic testing of physical and cholinergic urticarias—EAACI/GA2LEN/EDF/UNEV consensus panel recommendations [published online ahead of print September 30, 2009]. Allergy. 2009;64:1715-1721.
5. Petalas K, Kontou-Fili K, Gratziou C. Bronchial hyperresponsiveness in patients with cholinergic urticaria. Ann Allergy Asthma Immunol. 2009;102:416-421.
6. Morel V, Hauser C. Generalized cholinergic heat urticaria induced by hemodialysis. Kidney Int. 2006;70:230.
7. Zuberbier T, Aberer W, Burtin B, et al. Efficacy of cetirizine in cholinergic urticaria. Acta Derm Venereol. 1995;75:147-149.
8. Tsunemi Y, Ihn H, Saeki H, et al. Cholinergic urticaria successfully treated with scopolamine butylbromide. Int J Dermatol. 2003;42:850.
9. Ujiie H, Shimizu T, Natsuga K, et al. Severe cholinergic urticaria successfully treated with scopolamine butylbromide in addition to antihistamines. Clin Exp Dermatol. 2006;31:588-589.
10. McClean SP, Arreaza EE, Lett-Brown MA, et al. Refractory cholinergic urticaria successfully treated with ketotifen. J Allergy Clin Immunol. 1989;83:738-741.
11. Feinberg JH, Toner CB. Successful treatment of disabling cholinergic urticaria. Mil Med. 2008;173:217-220.
12. La Shell MS, England RW. Severe refractory cholinergic urticaria treated with danazol. J Drugs Dermatol. 2006;5:664-667.
Cholinergic urticaria (CU) is a condition that primarily affects young adults. It can severely limit their activity levels and therefore job performance. Rarely, this condition can be associated with anaphylaxis, requiring a high index of suspicion by the clinician to ensure proper evaluation and treatment to prevent future respiratory compromise. We present the case of a 27-year-old US Marine with CU and anaphylaxis confirmed by a water challenge test in a warm bath.
Case Report
An otherwise healthy 27-year-old white man who was a US Marine presented with a concern of hives that appeared during strenuous exercise when he was deployed in Afghanistan approximately 1 year earlier. He initially began to experience urticarial lesions when taking warm showers with concomitant shortness of breath and wheezing. He reported no history of hives or asthma. Despite using diphenhydramine as needed to control symptoms for several months, he noted that the episodes of urticaria occurred with light-headedness, dizziness, or vomiting even with mild physical activity or common daily activities. Symptoms typically would resolve 30 to 90 minutes after he stopped exercising or cooled off. Over the course of approximately 1 year, the patient was prescribed a variety of sedating and nonsedating antihistamines (eg, diphenhydramine, hydroxyzine, doxepin, cetirizine, loratadine, fexofenadine, montelukast) by primary care while deployed, some of which mitigated his symptoms during warm showers and outdoor activities but not during exercise.
After returning from his deployment, the patient was initially referred to the dermatology department. No lesions were noted on physical examination. Based on his history, he was advised to avoid strenuous exercise and activity. During subsequent visits to dermatology an exercise challenge test was considered but not initiated due to lack of facilities to provide appropriate airway monitoring. The allergy department was consulted and the patient also was prescribed leukotriene receptor antagonists in addition to the antihistamines he was already taking. It was decided that a water challenge test in a warm bath would be performed in lieu of an exercise challenge to confirm a diagnosis of CU versus CU with anaphylaxis. If the patient did not have a reaction to the water challenge test, an exercise challenge would be offered.
After stopping treatment with antihistamines and leukotriene receptor antagonists for 1 week, a water challenge test was performed. A heparin lock was placed in the untested left arm for intravenous access. The right arm was immersed in a warm bath for 5 minutes without incident. After confirming no reaction, the arm was immersed for another 5 minutes, after which the patient reported flushing, warmth, and itching with visible 2- to 3-mm urticarial lesions on the back (Figure, A) and chest (Figure, B). The arm was subsequently removed from the water. No lesions were noted on either of the arms. The patient developed a cough after removing the arm from the water and his peak expiratory flow rate dropped from 520 to 440 L/min. After 5 minutes his peak expiratory flow rate recovered to 500 L/min and the coughing subsided. He also reported mild nausea and a headache. He was rapidly cooled with ice to abort any further reaction. An epinephrine autoinjector was on hand but was not used due to rapidly resolving symptoms. The diagnosis of CU with anaphylaxis was confirmed.


Erythematous eruption with 2- to 3-mm urticarial lesions on the back (A) and chest (B). |
Comment
Urticaria is a heterogeneous group of disorders that includes both cholinergic and exercise-induced variants. Cholinergic urticaria affects as many as 11.2% of young adults aged 15 to 35 years, with a peak incidence of 20% between 26 and 28 years of age.1 Clinical presentation consists of wheals (central swelling with peripheral erythema) that are 1 to 5 mm in diameter2 with associated itching/burning that typically resolves within 1 to 24 hours. Cholinergic urticaria is the result of a rise in core body temperature independent of exercise status; it is distinguished from exercise-induced urticaria, which occurs in response to vigorous exercise and is not related to a rise in body temperature.3 In particular, these forms of urticaria can severely impact the lives and careers of young servicemen and servicewomen who are routinely deployed to warm environments.
A provocation test is recommended by Magerl et al4 in patients with a suspected diagnosis of CU. First, an exercise challenge test using a bicycle, treadmill, or similar equipment is recommended, with the patient exercising for 15 minutes beyond the start of sweating. Readings for urticaria are made immediately following the test and 10 minutes later. If the test is positive, a water challenge test in a hot bath (42°C) is then recommended for 15 minutes beyond an increase of 1°C in baseline core body temperature.4 One study demonstrated that 43% (13/30) of patients with CU experienced bronchial hyperresponsiveness on methacholine challenge testing.5 These findings suggest a possible utility in testing CU patients for potential disease-related respiratory compromise. A practical limitation of this study was that it did not examine a link between bronchial hyperresponsiveness and anaphylaxis during cholinergic urticarial flares. An exercise challenge test was not performed in our patient due to a history of wheezing and shortness of breath with exercise; instead we went directly to the water challenge test. We felt that limited immersion in the water (ie, only 1 arm) further minimized the risk for anaphylaxis compared with full-body immersion.
Any activity that raises core body temperature in a patient with CU can induce onset of lesions. One case report described a patient who experienced symptoms while undergoing hemodialysis, which resolved when the dialysate temperature was decreased from the normal 36.5°C to 35°C.6 However, most cases are triggered by daily activities or work. The mainstay of treatment of CU is nonsedating antihistamines. Cetirizine has demonstrated particular efficacy.7 For unresponsive cases, treatments include scopolamine butylbromide8,9; ketotifen10; combinations of cetirizine, montelukast, and propanolol11; and danazol.12
Conclusion
Cholinergic urticaria is mostly prevalent among young adults, with highest incidence in the late 20s. Active duty servicemen and servicewomen are among those who are at the greatest risk for developing CU, especially those deployed to tropical environments. Frequently, CU is associated with bronchial hyperresponsiveness and also can be associated with anaphylaxis, as was seen in our patient. Care must be taken before provocative tests are conducted in these patients and should be done in a controlled environment in which airway compromise can be properly assessed and treated if anaphylaxis were to occur.
Cholinergic urticaria (CU) is a condition that primarily affects young adults. It can severely limit their activity levels and therefore job performance. Rarely, this condition can be associated with anaphylaxis, requiring a high index of suspicion by the clinician to ensure proper evaluation and treatment to prevent future respiratory compromise. We present the case of a 27-year-old US Marine with CU and anaphylaxis confirmed by a water challenge test in a warm bath.
Case Report
An otherwise healthy 27-year-old white man who was a US Marine presented with a concern of hives that appeared during strenuous exercise when he was deployed in Afghanistan approximately 1 year earlier. He initially began to experience urticarial lesions when taking warm showers with concomitant shortness of breath and wheezing. He reported no history of hives or asthma. Despite using diphenhydramine as needed to control symptoms for several months, he noted that the episodes of urticaria occurred with light-headedness, dizziness, or vomiting even with mild physical activity or common daily activities. Symptoms typically would resolve 30 to 90 minutes after he stopped exercising or cooled off. Over the course of approximately 1 year, the patient was prescribed a variety of sedating and nonsedating antihistamines (eg, diphenhydramine, hydroxyzine, doxepin, cetirizine, loratadine, fexofenadine, montelukast) by primary care while deployed, some of which mitigated his symptoms during warm showers and outdoor activities but not during exercise.
After returning from his deployment, the patient was initially referred to the dermatology department. No lesions were noted on physical examination. Based on his history, he was advised to avoid strenuous exercise and activity. During subsequent visits to dermatology an exercise challenge test was considered but not initiated due to lack of facilities to provide appropriate airway monitoring. The allergy department was consulted and the patient also was prescribed leukotriene receptor antagonists in addition to the antihistamines he was already taking. It was decided that a water challenge test in a warm bath would be performed in lieu of an exercise challenge to confirm a diagnosis of CU versus CU with anaphylaxis. If the patient did not have a reaction to the water challenge test, an exercise challenge would be offered.
After stopping treatment with antihistamines and leukotriene receptor antagonists for 1 week, a water challenge test was performed. A heparin lock was placed in the untested left arm for intravenous access. The right arm was immersed in a warm bath for 5 minutes without incident. After confirming no reaction, the arm was immersed for another 5 minutes, after which the patient reported flushing, warmth, and itching with visible 2- to 3-mm urticarial lesions on the back (Figure, A) and chest (Figure, B). The arm was subsequently removed from the water. No lesions were noted on either of the arms. The patient developed a cough after removing the arm from the water and his peak expiratory flow rate dropped from 520 to 440 L/min. After 5 minutes his peak expiratory flow rate recovered to 500 L/min and the coughing subsided. He also reported mild nausea and a headache. He was rapidly cooled with ice to abort any further reaction. An epinephrine autoinjector was on hand but was not used due to rapidly resolving symptoms. The diagnosis of CU with anaphylaxis was confirmed.


Erythematous eruption with 2- to 3-mm urticarial lesions on the back (A) and chest (B). |
Comment
Urticaria is a heterogeneous group of disorders that includes both cholinergic and exercise-induced variants. Cholinergic urticaria affects as many as 11.2% of young adults aged 15 to 35 years, with a peak incidence of 20% between 26 and 28 years of age.1 Clinical presentation consists of wheals (central swelling with peripheral erythema) that are 1 to 5 mm in diameter2 with associated itching/burning that typically resolves within 1 to 24 hours. Cholinergic urticaria is the result of a rise in core body temperature independent of exercise status; it is distinguished from exercise-induced urticaria, which occurs in response to vigorous exercise and is not related to a rise in body temperature.3 In particular, these forms of urticaria can severely impact the lives and careers of young servicemen and servicewomen who are routinely deployed to warm environments.
A provocation test is recommended by Magerl et al4 in patients with a suspected diagnosis of CU. First, an exercise challenge test using a bicycle, treadmill, or similar equipment is recommended, with the patient exercising for 15 minutes beyond the start of sweating. Readings for urticaria are made immediately following the test and 10 minutes later. If the test is positive, a water challenge test in a hot bath (42°C) is then recommended for 15 minutes beyond an increase of 1°C in baseline core body temperature.4 One study demonstrated that 43% (13/30) of patients with CU experienced bronchial hyperresponsiveness on methacholine challenge testing.5 These findings suggest a possible utility in testing CU patients for potential disease-related respiratory compromise. A practical limitation of this study was that it did not examine a link between bronchial hyperresponsiveness and anaphylaxis during cholinergic urticarial flares. An exercise challenge test was not performed in our patient due to a history of wheezing and shortness of breath with exercise; instead we went directly to the water challenge test. We felt that limited immersion in the water (ie, only 1 arm) further minimized the risk for anaphylaxis compared with full-body immersion.
Any activity that raises core body temperature in a patient with CU can induce onset of lesions. One case report described a patient who experienced symptoms while undergoing hemodialysis, which resolved when the dialysate temperature was decreased from the normal 36.5°C to 35°C.6 However, most cases are triggered by daily activities or work. The mainstay of treatment of CU is nonsedating antihistamines. Cetirizine has demonstrated particular efficacy.7 For unresponsive cases, treatments include scopolamine butylbromide8,9; ketotifen10; combinations of cetirizine, montelukast, and propanolol11; and danazol.12
Conclusion
Cholinergic urticaria is mostly prevalent among young adults, with highest incidence in the late 20s. Active duty servicemen and servicewomen are among those who are at the greatest risk for developing CU, especially those deployed to tropical environments. Frequently, CU is associated with bronchial hyperresponsiveness and also can be associated with anaphylaxis, as was seen in our patient. Care must be taken before provocative tests are conducted in these patients and should be done in a controlled environment in which airway compromise can be properly assessed and treated if anaphylaxis were to occur.
1. Zuberbier T, Althaus C, Chantraine-Hess S, et al. Prevalence of cholinergic urticaria in young adults. J Am Acad Dermatol. 1994;31:978-981.
2. Kontou-Fili K, Borici-Mazi R, Kapp A, et al. Physical urticaria: classification and diagnostic guidelines. an EAACI position paper. Allergy. 1997;52:504-513.
3. Zuberbier T, Asero R, Bindslev-Jensen C, et al; Dermatology Section of the European Academy of Allergology and Immunology; Global Allergy and Asthma European Network; European Dermatology Forum; World Allergy Organization. EAACI/GA(2)LEN/EDF/WAO guideline: definition, classification and diagnosis of urticaria. Allergy. 2009;64:1417-1426.
4. Magerl M, Borzova E, Giménez-Arnau A, et al; EAACI/GA2LEN/EDF/UNEV. The definition and diagnostic testing of physical and cholinergic urticarias—EAACI/GA2LEN/EDF/UNEV consensus panel recommendations [published online ahead of print September 30, 2009]. Allergy. 2009;64:1715-1721.
5. Petalas K, Kontou-Fili K, Gratziou C. Bronchial hyperresponsiveness in patients with cholinergic urticaria. Ann Allergy Asthma Immunol. 2009;102:416-421.
6. Morel V, Hauser C. Generalized cholinergic heat urticaria induced by hemodialysis. Kidney Int. 2006;70:230.
7. Zuberbier T, Aberer W, Burtin B, et al. Efficacy of cetirizine in cholinergic urticaria. Acta Derm Venereol. 1995;75:147-149.
8. Tsunemi Y, Ihn H, Saeki H, et al. Cholinergic urticaria successfully treated with scopolamine butylbromide. Int J Dermatol. 2003;42:850.
9. Ujiie H, Shimizu T, Natsuga K, et al. Severe cholinergic urticaria successfully treated with scopolamine butylbromide in addition to antihistamines. Clin Exp Dermatol. 2006;31:588-589.
10. McClean SP, Arreaza EE, Lett-Brown MA, et al. Refractory cholinergic urticaria successfully treated with ketotifen. J Allergy Clin Immunol. 1989;83:738-741.
11. Feinberg JH, Toner CB. Successful treatment of disabling cholinergic urticaria. Mil Med. 2008;173:217-220.
12. La Shell MS, England RW. Severe refractory cholinergic urticaria treated with danazol. J Drugs Dermatol. 2006;5:664-667.
1. Zuberbier T, Althaus C, Chantraine-Hess S, et al. Prevalence of cholinergic urticaria in young adults. J Am Acad Dermatol. 1994;31:978-981.
2. Kontou-Fili K, Borici-Mazi R, Kapp A, et al. Physical urticaria: classification and diagnostic guidelines. an EAACI position paper. Allergy. 1997;52:504-513.
3. Zuberbier T, Asero R, Bindslev-Jensen C, et al; Dermatology Section of the European Academy of Allergology and Immunology; Global Allergy and Asthma European Network; European Dermatology Forum; World Allergy Organization. EAACI/GA(2)LEN/EDF/WAO guideline: definition, classification and diagnosis of urticaria. Allergy. 2009;64:1417-1426.
4. Magerl M, Borzova E, Giménez-Arnau A, et al; EAACI/GA2LEN/EDF/UNEV. The definition and diagnostic testing of physical and cholinergic urticarias—EAACI/GA2LEN/EDF/UNEV consensus panel recommendations [published online ahead of print September 30, 2009]. Allergy. 2009;64:1715-1721.
5. Petalas K, Kontou-Fili K, Gratziou C. Bronchial hyperresponsiveness in patients with cholinergic urticaria. Ann Allergy Asthma Immunol. 2009;102:416-421.
6. Morel V, Hauser C. Generalized cholinergic heat urticaria induced by hemodialysis. Kidney Int. 2006;70:230.
7. Zuberbier T, Aberer W, Burtin B, et al. Efficacy of cetirizine in cholinergic urticaria. Acta Derm Venereol. 1995;75:147-149.
8. Tsunemi Y, Ihn H, Saeki H, et al. Cholinergic urticaria successfully treated with scopolamine butylbromide. Int J Dermatol. 2003;42:850.
9. Ujiie H, Shimizu T, Natsuga K, et al. Severe cholinergic urticaria successfully treated with scopolamine butylbromide in addition to antihistamines. Clin Exp Dermatol. 2006;31:588-589.
10. McClean SP, Arreaza EE, Lett-Brown MA, et al. Refractory cholinergic urticaria successfully treated with ketotifen. J Allergy Clin Immunol. 1989;83:738-741.
11. Feinberg JH, Toner CB. Successful treatment of disabling cholinergic urticaria. Mil Med. 2008;173:217-220.
12. La Shell MS, England RW. Severe refractory cholinergic urticaria treated with danazol. J Drugs Dermatol. 2006;5:664-667.
Practice Points
- Cholinergic urticaria can be a life-threatening condition and should be diagnosed in a controlled clinical setting where airway can be maintained.
- Cholinergic urticaria can be a profession-limiting condition, affecting people whose work involves exposure to heat or physical activity such as members of the military.
Dermatologic Emergencies
Dermatologic emergency may sound like an oxymoron, but there are many emergencies that dermatology residents may encounter in their careers. In some instances the skin is the primary organ that is affected, while in others cutaneous symptoms and life-threatening signs are important diagnostic clues for what may lie beneath the skin.
As residents who are occasionally on call or on consultation services, it is important for us to recognize dermatologic emergencies quickly because some of these conditions can acutely evolve and become lethal if a diagnosis is not made early in the disease course with the appropriate treatment administered. Dermatologic emergencies can range from severe drug reactions, infections, autoimmune exacerbations, and inflammatory conditions (eg, erythroderma) to environmental insults such as burns (Figure 1) and child abuse.1
Critical Infections
Some dermatologic emergencies are infectious in origin, and although these infections are most commonly bacterial (eg, necrotizing fasciitis), they also can range from viral to fungal (eg, mucormycosis) in nature. Some areas with large populations of immunocompromised patients (eg, human immunodeficiency virus–positive patients, organ transplant recipients) may warrant a high index of suspicion for possible zebras (rare conditions) and opportunistic infections that may quickly escalate to life-threatening situations.
Although few cutaneous manifestations in emergent infections are pathognomonic, they sometimes can be categorized according to the appearance of the primary lesion: erythrodermic (eg, staphylococcal scalded skin syndrome), maculopapular (eg, Lyme disease), purpuric/petechial (eg, Rocky Mountain spotted fever), pustular (eg, disseminated candidiasis), or vesicular (eg, neonatal herpes simplex virus)(Table). On consultations, dermatology residents frequently get called to evaluate hemorrhagic and ischemic lesions in inpatients (Figure 2). Aside from infectious causes, the differential diagnosis may include coagulation abnormalities (eg, concurrent anticoagulant therapies), vasculitides, poisoning, vascular disease, or Stevens-Johnson syndrome and toxic epidermal necrolysis, which can occasionally present with hemorrhagic lesions.1,2
Necrotizing Fasciitis
Dermatology residents may frequently encounter necrotizing fasciitis, either in clinic or on the wards (Figure 3). Recognition of the skin signs in this condition is essential to patient survival. As an intern, I once had an attending teach me that patients with necrotizing fasciitis only have a couple of hours to live. The rapid unfolding of this flesh-eating disease and its high morbidity and mortality has led to recent attention in the press and media.
Although necrotizing fasciitis may be caused by several different bacterial organisms (eg, gram positive, gram negative, polymicrobial), it usually is rapidly progressive, destroying muscle and subcutaneous tissues in a matter of hours.3 Bacteria usually enter through a traumatic or present wound and quickly move along fascial planes, destroying blood vessels and whatever subcutaneous tissues happen to be in the way. Within the first few hours, the involved area that was initially erythematous becomes indurated, woody, extremely painful, and dusky, indicating a lack of circulation to the area. Extensive debridement is required until reaching noninfected tissue that is no longer purulent, necrotic, or woody to the touch. If necrotizing fasciitis is not diagnosed and treated early, patients may lose one or several limbs and death may occur.
Key findings of necrotizing fasciitis include systemic toxicity, localized painful induration, well-defined dusky blue discoloration, and a lack of bleeding or purulent discharge on incision and squeezing of the affected tissue. Crepitation or a crackling sensation can occasionally be felt when palpating the area secondary to gas formation in the tissue, though it is not always present. Patients with necrotizing fasciitis often initially present to dermatology clinics because the first manifestation happens to be in the skin. The role of dermatologists in treating this critical condition may prompt recognition and collaboration with other specialists to reach a viable outcome for the patient.3
Drug Reactions
Cutaneous drug eruptions usually are relatively benign, consisting of a morbilliform eruption often without any other accompanying symptoms. However, sometimes these reactions can present as exfoliative dermatitis or red man syndrome in which patients can develop total body erythema with diffuse scaling and pruritus.4 Aside from drug reactions, other causes of exfoliative dermatitis such as psoriasis, atopic and seborrheic dermatitis, mycosis fungoides, and lymphoma should be ruled out. Other drug eruptions that can be classified as dermatologic emergencies include leukocytoclastic vasculitis, severe urticaria or angioedema, erythema multiforme, or Stevens-Johnson syndrome and toxic epidermal necrolysis.
Severe Acne
If not treated promptly, serious cases of acne can lead to severe scarring and psychologic problems. Acne fulminans is characterized by a rapid eruption of suppurative and large, highly inflamed nodules, plaques, and cysts that result in ragged ulcerations and cicatrization of the chest, back, and occasionally the face. Systemic symptoms of fever, arthralgia, leukocytosis, and myalgia suggest an upregulation of the immune system in affected patients.
Final Comment
In summary, dermatologic emergencies do exist and some may present with characteristic skin findings. In almost all cases, collaboration with other departments such as trauma, burn, internal medicine, rheumatology, and infectious diseases is extremely helpful in diagnosing and treating these medical emergencies. Collaboration can provide insight into how brainstorming through different approaches can lead to a better outcome whether it be solving the cause of a puzzling rash in a patient with multiple comorbidities or surgically removing a bullet from a trauma patient (Figure 4). Recognition of specific cutaneous manifestations and early diagnosis of dermatologic emergencies can be lifesaving.
1. McQueen A, Martin SA, Lio PA. Derm emergencies: detecting early signs of trouble. J Fam Pract. 2012;61:71-78.
2. Bennion S. Dermatologic emergencies. In: Fitzpatrick J, Morelli J, eds. Dermatology Secrets Plus. 4th ed. Philadelphia, PA: Mosby; 2011:442-452.
3. Sarani B, Strong M, Pascual J, et al. Necrotizing fasciitis: current concepts and review of the literature. J Am Coll Surg. 2009;208:279-288.
4. Wolf R, Orion E, Marcos B, et al. Life-threatening acute adverse cutaneous drug reactions. Clin Dermatol. 2005;23:171-181.
Dermatologic emergency may sound like an oxymoron, but there are many emergencies that dermatology residents may encounter in their careers. In some instances the skin is the primary organ that is affected, while in others cutaneous symptoms and life-threatening signs are important diagnostic clues for what may lie beneath the skin.
As residents who are occasionally on call or on consultation services, it is important for us to recognize dermatologic emergencies quickly because some of these conditions can acutely evolve and become lethal if a diagnosis is not made early in the disease course with the appropriate treatment administered. Dermatologic emergencies can range from severe drug reactions, infections, autoimmune exacerbations, and inflammatory conditions (eg, erythroderma) to environmental insults such as burns (Figure 1) and child abuse.1
Critical Infections
Some dermatologic emergencies are infectious in origin, and although these infections are most commonly bacterial (eg, necrotizing fasciitis), they also can range from viral to fungal (eg, mucormycosis) in nature. Some areas with large populations of immunocompromised patients (eg, human immunodeficiency virus–positive patients, organ transplant recipients) may warrant a high index of suspicion for possible zebras (rare conditions) and opportunistic infections that may quickly escalate to life-threatening situations.
Although few cutaneous manifestations in emergent infections are pathognomonic, they sometimes can be categorized according to the appearance of the primary lesion: erythrodermic (eg, staphylococcal scalded skin syndrome), maculopapular (eg, Lyme disease), purpuric/petechial (eg, Rocky Mountain spotted fever), pustular (eg, disseminated candidiasis), or vesicular (eg, neonatal herpes simplex virus)(Table). On consultations, dermatology residents frequently get called to evaluate hemorrhagic and ischemic lesions in inpatients (Figure 2). Aside from infectious causes, the differential diagnosis may include coagulation abnormalities (eg, concurrent anticoagulant therapies), vasculitides, poisoning, vascular disease, or Stevens-Johnson syndrome and toxic epidermal necrolysis, which can occasionally present with hemorrhagic lesions.1,2
Necrotizing Fasciitis
Dermatology residents may frequently encounter necrotizing fasciitis, either in clinic or on the wards (Figure 3). Recognition of the skin signs in this condition is essential to patient survival. As an intern, I once had an attending teach me that patients with necrotizing fasciitis only have a couple of hours to live. The rapid unfolding of this flesh-eating disease and its high morbidity and mortality has led to recent attention in the press and media.
Although necrotizing fasciitis may be caused by several different bacterial organisms (eg, gram positive, gram negative, polymicrobial), it usually is rapidly progressive, destroying muscle and subcutaneous tissues in a matter of hours.3 Bacteria usually enter through a traumatic or present wound and quickly move along fascial planes, destroying blood vessels and whatever subcutaneous tissues happen to be in the way. Within the first few hours, the involved area that was initially erythematous becomes indurated, woody, extremely painful, and dusky, indicating a lack of circulation to the area. Extensive debridement is required until reaching noninfected tissue that is no longer purulent, necrotic, or woody to the touch. If necrotizing fasciitis is not diagnosed and treated early, patients may lose one or several limbs and death may occur.
Key findings of necrotizing fasciitis include systemic toxicity, localized painful induration, well-defined dusky blue discoloration, and a lack of bleeding or purulent discharge on incision and squeezing of the affected tissue. Crepitation or a crackling sensation can occasionally be felt when palpating the area secondary to gas formation in the tissue, though it is not always present. Patients with necrotizing fasciitis often initially present to dermatology clinics because the first manifestation happens to be in the skin. The role of dermatologists in treating this critical condition may prompt recognition and collaboration with other specialists to reach a viable outcome for the patient.3
Drug Reactions
Cutaneous drug eruptions usually are relatively benign, consisting of a morbilliform eruption often without any other accompanying symptoms. However, sometimes these reactions can present as exfoliative dermatitis or red man syndrome in which patients can develop total body erythema with diffuse scaling and pruritus.4 Aside from drug reactions, other causes of exfoliative dermatitis such as psoriasis, atopic and seborrheic dermatitis, mycosis fungoides, and lymphoma should be ruled out. Other drug eruptions that can be classified as dermatologic emergencies include leukocytoclastic vasculitis, severe urticaria or angioedema, erythema multiforme, or Stevens-Johnson syndrome and toxic epidermal necrolysis.
Severe Acne
If not treated promptly, serious cases of acne can lead to severe scarring and psychologic problems. Acne fulminans is characterized by a rapid eruption of suppurative and large, highly inflamed nodules, plaques, and cysts that result in ragged ulcerations and cicatrization of the chest, back, and occasionally the face. Systemic symptoms of fever, arthralgia, leukocytosis, and myalgia suggest an upregulation of the immune system in affected patients.
Final Comment
In summary, dermatologic emergencies do exist and some may present with characteristic skin findings. In almost all cases, collaboration with other departments such as trauma, burn, internal medicine, rheumatology, and infectious diseases is extremely helpful in diagnosing and treating these medical emergencies. Collaboration can provide insight into how brainstorming through different approaches can lead to a better outcome whether it be solving the cause of a puzzling rash in a patient with multiple comorbidities or surgically removing a bullet from a trauma patient (Figure 4). Recognition of specific cutaneous manifestations and early diagnosis of dermatologic emergencies can be lifesaving.
Dermatologic emergency may sound like an oxymoron, but there are many emergencies that dermatology residents may encounter in their careers. In some instances the skin is the primary organ that is affected, while in others cutaneous symptoms and life-threatening signs are important diagnostic clues for what may lie beneath the skin.
As residents who are occasionally on call or on consultation services, it is important for us to recognize dermatologic emergencies quickly because some of these conditions can acutely evolve and become lethal if a diagnosis is not made early in the disease course with the appropriate treatment administered. Dermatologic emergencies can range from severe drug reactions, infections, autoimmune exacerbations, and inflammatory conditions (eg, erythroderma) to environmental insults such as burns (Figure 1) and child abuse.1
Critical Infections
Some dermatologic emergencies are infectious in origin, and although these infections are most commonly bacterial (eg, necrotizing fasciitis), they also can range from viral to fungal (eg, mucormycosis) in nature. Some areas with large populations of immunocompromised patients (eg, human immunodeficiency virus–positive patients, organ transplant recipients) may warrant a high index of suspicion for possible zebras (rare conditions) and opportunistic infections that may quickly escalate to life-threatening situations.
Although few cutaneous manifestations in emergent infections are pathognomonic, they sometimes can be categorized according to the appearance of the primary lesion: erythrodermic (eg, staphylococcal scalded skin syndrome), maculopapular (eg, Lyme disease), purpuric/petechial (eg, Rocky Mountain spotted fever), pustular (eg, disseminated candidiasis), or vesicular (eg, neonatal herpes simplex virus)(Table). On consultations, dermatology residents frequently get called to evaluate hemorrhagic and ischemic lesions in inpatients (Figure 2). Aside from infectious causes, the differential diagnosis may include coagulation abnormalities (eg, concurrent anticoagulant therapies), vasculitides, poisoning, vascular disease, or Stevens-Johnson syndrome and toxic epidermal necrolysis, which can occasionally present with hemorrhagic lesions.1,2
Necrotizing Fasciitis
Dermatology residents may frequently encounter necrotizing fasciitis, either in clinic or on the wards (Figure 3). Recognition of the skin signs in this condition is essential to patient survival. As an intern, I once had an attending teach me that patients with necrotizing fasciitis only have a couple of hours to live. The rapid unfolding of this flesh-eating disease and its high morbidity and mortality has led to recent attention in the press and media.
Although necrotizing fasciitis may be caused by several different bacterial organisms (eg, gram positive, gram negative, polymicrobial), it usually is rapidly progressive, destroying muscle and subcutaneous tissues in a matter of hours.3 Bacteria usually enter through a traumatic or present wound and quickly move along fascial planes, destroying blood vessels and whatever subcutaneous tissues happen to be in the way. Within the first few hours, the involved area that was initially erythematous becomes indurated, woody, extremely painful, and dusky, indicating a lack of circulation to the area. Extensive debridement is required until reaching noninfected tissue that is no longer purulent, necrotic, or woody to the touch. If necrotizing fasciitis is not diagnosed and treated early, patients may lose one or several limbs and death may occur.
Key findings of necrotizing fasciitis include systemic toxicity, localized painful induration, well-defined dusky blue discoloration, and a lack of bleeding or purulent discharge on incision and squeezing of the affected tissue. Crepitation or a crackling sensation can occasionally be felt when palpating the area secondary to gas formation in the tissue, though it is not always present. Patients with necrotizing fasciitis often initially present to dermatology clinics because the first manifestation happens to be in the skin. The role of dermatologists in treating this critical condition may prompt recognition and collaboration with other specialists to reach a viable outcome for the patient.3
Drug Reactions
Cutaneous drug eruptions usually are relatively benign, consisting of a morbilliform eruption often without any other accompanying symptoms. However, sometimes these reactions can present as exfoliative dermatitis or red man syndrome in which patients can develop total body erythema with diffuse scaling and pruritus.4 Aside from drug reactions, other causes of exfoliative dermatitis such as psoriasis, atopic and seborrheic dermatitis, mycosis fungoides, and lymphoma should be ruled out. Other drug eruptions that can be classified as dermatologic emergencies include leukocytoclastic vasculitis, severe urticaria or angioedema, erythema multiforme, or Stevens-Johnson syndrome and toxic epidermal necrolysis.
Severe Acne
If not treated promptly, serious cases of acne can lead to severe scarring and psychologic problems. Acne fulminans is characterized by a rapid eruption of suppurative and large, highly inflamed nodules, plaques, and cysts that result in ragged ulcerations and cicatrization of the chest, back, and occasionally the face. Systemic symptoms of fever, arthralgia, leukocytosis, and myalgia suggest an upregulation of the immune system in affected patients.
Final Comment
In summary, dermatologic emergencies do exist and some may present with characteristic skin findings. In almost all cases, collaboration with other departments such as trauma, burn, internal medicine, rheumatology, and infectious diseases is extremely helpful in diagnosing and treating these medical emergencies. Collaboration can provide insight into how brainstorming through different approaches can lead to a better outcome whether it be solving the cause of a puzzling rash in a patient with multiple comorbidities or surgically removing a bullet from a trauma patient (Figure 4). Recognition of specific cutaneous manifestations and early diagnosis of dermatologic emergencies can be lifesaving.
1. McQueen A, Martin SA, Lio PA. Derm emergencies: detecting early signs of trouble. J Fam Pract. 2012;61:71-78.
2. Bennion S. Dermatologic emergencies. In: Fitzpatrick J, Morelli J, eds. Dermatology Secrets Plus. 4th ed. Philadelphia, PA: Mosby; 2011:442-452.
3. Sarani B, Strong M, Pascual J, et al. Necrotizing fasciitis: current concepts and review of the literature. J Am Coll Surg. 2009;208:279-288.
4. Wolf R, Orion E, Marcos B, et al. Life-threatening acute adverse cutaneous drug reactions. Clin Dermatol. 2005;23:171-181.
1. McQueen A, Martin SA, Lio PA. Derm emergencies: detecting early signs of trouble. J Fam Pract. 2012;61:71-78.
2. Bennion S. Dermatologic emergencies. In: Fitzpatrick J, Morelli J, eds. Dermatology Secrets Plus. 4th ed. Philadelphia, PA: Mosby; 2011:442-452.
3. Sarani B, Strong M, Pascual J, et al. Necrotizing fasciitis: current concepts and review of the literature. J Am Coll Surg. 2009;208:279-288.
4. Wolf R, Orion E, Marcos B, et al. Life-threatening acute adverse cutaneous drug reactions. Clin Dermatol. 2005;23:171-181.