User login
FDA approves first live vaccine for smallpox, monkeypox prevention
The Food and Drug Administration has approved Jynneos, a live, nonreplicating vaccine based on the vaccinia virus, for smallpox and monkeypox, becoming the first FDA-approved vaccine for the prevention of monkeypox disease.
FDA approval for Jynneos for smallpox is based on results from a clinical trial that compared Jynneos with ACAM2000, a previously FDA-approved smallpox vaccine, in about 400 healthy adults aged 18-42 years. Adults who received Jynneos had a noninferior immune response to those who received ACAM2000. In addition, safety was assessed in 7,800 people who received at least one vaccine dose, with the most commonly reported side effects including pain, redness, swelling, itching, firmness at the injection site, muscle pain, headache, and fatigue.
The effectiveness of Jynneos to prevent monkeypox – a disease similar to but somewhat milder than smallpox caused by the non–U.S.-native monkeypox virus – was inferred from antibody responses of participants in the smallpox clinical trial and from studies on nonhuman primates that showed protection from the monkeypox virus after being vaccinated with Jynneos.
“Routine [smallpox] vaccination of the American public was stopped in 1972 after the disease was eradicated in the U.S. and, as a result, a large proportion of the U.S., as well as the global population has no immunity,” said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research. “Although naturally occurring smallpox disease is no longer a global threat, the intentional release of this highly contagious virus could have a devastating effect.”
This vaccine is also part of the Strategic National Stockpile, the nation’s largest supply of potentially lifesaving pharmaceuticals and medical supplies for use in a public health emergency, according to the announcement.
Find the full press release on the FDA website.
The Food and Drug Administration has approved Jynneos, a live, nonreplicating vaccine based on the vaccinia virus, for smallpox and monkeypox, becoming the first FDA-approved vaccine for the prevention of monkeypox disease.
FDA approval for Jynneos for smallpox is based on results from a clinical trial that compared Jynneos with ACAM2000, a previously FDA-approved smallpox vaccine, in about 400 healthy adults aged 18-42 years. Adults who received Jynneos had a noninferior immune response to those who received ACAM2000. In addition, safety was assessed in 7,800 people who received at least one vaccine dose, with the most commonly reported side effects including pain, redness, swelling, itching, firmness at the injection site, muscle pain, headache, and fatigue.
The effectiveness of Jynneos to prevent monkeypox – a disease similar to but somewhat milder than smallpox caused by the non–U.S.-native monkeypox virus – was inferred from antibody responses of participants in the smallpox clinical trial and from studies on nonhuman primates that showed protection from the monkeypox virus after being vaccinated with Jynneos.
“Routine [smallpox] vaccination of the American public was stopped in 1972 after the disease was eradicated in the U.S. and, as a result, a large proportion of the U.S., as well as the global population has no immunity,” said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research. “Although naturally occurring smallpox disease is no longer a global threat, the intentional release of this highly contagious virus could have a devastating effect.”
This vaccine is also part of the Strategic National Stockpile, the nation’s largest supply of potentially lifesaving pharmaceuticals and medical supplies for use in a public health emergency, according to the announcement.
Find the full press release on the FDA website.
The Food and Drug Administration has approved Jynneos, a live, nonreplicating vaccine based on the vaccinia virus, for smallpox and monkeypox, becoming the first FDA-approved vaccine for the prevention of monkeypox disease.
FDA approval for Jynneos for smallpox is based on results from a clinical trial that compared Jynneos with ACAM2000, a previously FDA-approved smallpox vaccine, in about 400 healthy adults aged 18-42 years. Adults who received Jynneos had a noninferior immune response to those who received ACAM2000. In addition, safety was assessed in 7,800 people who received at least one vaccine dose, with the most commonly reported side effects including pain, redness, swelling, itching, firmness at the injection site, muscle pain, headache, and fatigue.
The effectiveness of Jynneos to prevent monkeypox – a disease similar to but somewhat milder than smallpox caused by the non–U.S.-native monkeypox virus – was inferred from antibody responses of participants in the smallpox clinical trial and from studies on nonhuman primates that showed protection from the monkeypox virus after being vaccinated with Jynneos.
“Routine [smallpox] vaccination of the American public was stopped in 1972 after the disease was eradicated in the U.S. and, as a result, a large proportion of the U.S., as well as the global population has no immunity,” said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research. “Although naturally occurring smallpox disease is no longer a global threat, the intentional release of this highly contagious virus could have a devastating effect.”
This vaccine is also part of the Strategic National Stockpile, the nation’s largest supply of potentially lifesaving pharmaceuticals and medical supplies for use in a public health emergency, according to the announcement.
Find the full press release on the FDA website.
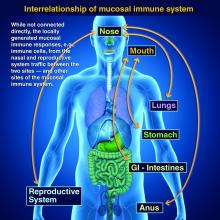
Taking vaccines to the next level via mucosal immunity
Vaccines are marvelous, and there are many well documented success stories, including rotavirus (RV) vaccines, where a live vaccine is administered to the gastrointestinal mucosa via oral drops. Antigens presented at the mucosal/epithelial surface not only induce systemic serum IgG – as do injectable vaccines – but also induce secretory IgA (sIgA), which is most helpful in diseases that directly affect the mucosa.

Mucosal vs. systemic immunity
Antibody being present on mucosal surfaces (point of initial pathogen contact) has a chance to neutralize the pathogen before it gains a foothold. Pathogen-specific mucosal lymphoid elements (e.g. in Peyer’s patches in the gut) also appear critical for optimal protection.1 The presence of both mucosal immune elements means that infection is severely limited or at times entirely prevented. So virus entering the GI tract causes minimal to no gut lining injury. Hence, there is no or mostly reduced vomiting/diarrhea. A downside of mucosally-administered live vaccines is that preexisting antibody to the vaccine antigens can reduce or block vaccine virus replication in the vaccinee, blunting or preventing protection. Note: Preexisting antibody also affects injectable live vaccines, such as the measles vaccine, similarly.
Classic injectable live or nonlive vaccines provide their most potent protection via systemic cellular responses antibody and/or antibodies in serum and extracellular fluid (ECF) where IgG and IgM are in highest concentrations. So even successful injectable vaccines still allow mucosal infection to start but then intercept further spread and prevent most of the downstream damage (think pertussis) or neutralize an infection-generated toxin (pertussis or tetanus). It usually is only after infection-induced damage occurs that systemic IgG and IgM gain better access to respiratory epithelial surfaces, but still only at a fraction of circulating concentrations. Indeed, pertussis vaccine–induced systemic immunity allows the pathogen to attack and replicate in/on host surface cells, causing toxin release and variable amounts of local mucosal injury/inflammation before vaccine-induced systemic immunity gains adequate access to the pathogen and/or to its toxin which may enter systemic circulation.
Live attenuated influenza vaccine (LAIV) induces mucosal immunity
Another “standard” vaccine that induces mucosal immunity – LAIV – was developed to improve on protection afforded by injectable influenza vaccines (IIVs), but LAIV has had hiccups in the United States. One example is several years of negligible protection against H1N1 disease. As long as LAIV’s vaccine strain had reasonably matched the circulating strains, LAIV worked at least as well as injectable influenza vaccine, and even offered some cross-protection against mildly mismatched strains. But after a number of years of LAIV use, vaccine effectiveness in the United States vs. H1N1 strains appeared to fade due to previously undetected but significant changes in the circulating H1N1 strain. The lesson is that mucosal immunity’s advantages are lost if too much change occurs in the pathogen target for sIgA and mucosally-associated lymphoid tissue cells (MALT)).
Other vaccines likely need to induce mucosal immunity
Protection at the mucosal level will likely be needed for success against norovirus, parainfluenza, respiratory syncytial virus (RSV), Neisseria gonorrhea, and chlamydia. Another helpful aspect of mucosal immunity is that immune cells and sIgA not only reside on the mucosa where the antigen was originally presented, but there is also a reasonable chance that these components will traffic to other mucosal surfaces.2
So intranasal vaccine could be expected to protect distant mucosal surfaces (urogenital, GI, and respiratory), leading to vaccine-induced systemic antibody plus mucosal immunity (sIGA and MALT responses) at each site.
Let’s look at a novel “two-site” chlamydia vaccine
Recently a phase 1 chlamydia vaccine that used a novel two-pronged administration site/schedule was successful at inducing both mucosal and systemic immunity in a proof-of-concept study – achieving the best of both worlds.3 This may be a template for vaccines in years to come. British investigators studied 50 healthy women aged 19-45 years in a double-blind, parallel, randomized, placebo-controlled trial that used a recombinant chlamydia protein subunit antigen (CTH522). The vaccine schedule involved three injectable priming doses followed soon thereafter by two intranasal boosting doses. There were three groups:
1. CTH522 adjuvanted with CAF01 liposomes (CTH522:CAF01).
2. CTH522 adjuvanted with aluminum hydroxide (CTH522:AH).
3. Placebo (saline).
The intramuscular (IM) priming schedule was 0, 1, and 4 months. The intranasal vaccine booster doses or placebo were given at 4.5 and 5 months. No related serious adverse reactions occurred. For injectable dosing, the most frequent adverse event was mild local injection-site reactions in all subjects in both vaccine groups vs. in 60% of placebo recipients (P = .053). The adjuvants were the likely cause for local reactions. Intranasal doses had local reactions in 47% of both vaccine groups and 60% of placebo recipients; P = 1.000).
Both vaccines produced systemic IgG seroconversion (including neutralizing antibody) plus small amounts of IgG in the nasal cavity and genital tract in all vaccine recipients; no placebo recipient seroconverted. Interestingly, liposomally-adjuvanted vaccine produced a more rapid systemic IgG response and higher serum titers than the alum-adjuvanted vaccine. Likewise, the IM liposomal vaccine also induced higher but still small mucosal IgG antibody responses (P = .0091). Intranasal IM-induced IgG titers were not boosted by later intranasal vaccine dosing.
Subjects getting liposomal vaccine (but not alum vaccine or placebo) boosters had detectable sIgA titers in both nasal and genital tract secretions. Liposomal vaccine recipients also had fivefold to sixfold higher median titers than alum vaccine recipients after the priming dose, and these higher titers persisted to the end of the study. All liposomal vaccine recipients developed antichlamydial cell-mediated responses vs. 57% alum-adjuvanted vaccine recipients. (P = .01). So both use of two-site dosing and the liposomal adjuvant appeared critical to better responses.
In summary
While this candidate vaccine has hurdles to overcome before coming into routine use, the proof-of-principle that a combination injectable-intranasal vaccine schedule can induce robust systemic and mucosal immunity when given with an appropriate adjuvant is very promising. Adding more vaccines to the schedule then becomes an issue, but that is one of those “good” problems we can deal with later.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital-Kansas City, Mo. Children’s Mercy Hospital receives grant funding to study two candidate RSV vaccines, receives funding from GlaxoSmithKline for studies on pneumococcal and rotavirus vaccines, and from Pfizer for a study on pneumococcal vaccine on which Dr. Harrison is a sub-investigator. The hospital also receives Centers for Disease Control and Prevention funding under the New Vaccine Surveillance Network for multicenter surveillance of acute respiratory infections, including influenza, RSV, and parainfluenza virus, and also for rotavirus. Email Dr. Harrison at [email protected].
References
1. PLOS Biology. 2012 Sep 1. doi: 10.1371/journal.pbio.1001397.
2. Mucosal Immunity in the Human Female Reproductive Tract in “Mucosal Immunology,” 4th ed., Volume 2 (Cambridge, MA: Academic Press, 2015, pp. 2097-124).
3. Lancet Infect Dis. 2019. doi: 10.1016/S1473-3099(19)30279-8.
Vaccines are marvelous, and there are many well documented success stories, including rotavirus (RV) vaccines, where a live vaccine is administered to the gastrointestinal mucosa via oral drops. Antigens presented at the mucosal/epithelial surface not only induce systemic serum IgG – as do injectable vaccines – but also induce secretory IgA (sIgA), which is most helpful in diseases that directly affect the mucosa.

Mucosal vs. systemic immunity
Antibody being present on mucosal surfaces (point of initial pathogen contact) has a chance to neutralize the pathogen before it gains a foothold. Pathogen-specific mucosal lymphoid elements (e.g. in Peyer’s patches in the gut) also appear critical for optimal protection.1 The presence of both mucosal immune elements means that infection is severely limited or at times entirely prevented. So virus entering the GI tract causes minimal to no gut lining injury. Hence, there is no or mostly reduced vomiting/diarrhea. A downside of mucosally-administered live vaccines is that preexisting antibody to the vaccine antigens can reduce or block vaccine virus replication in the vaccinee, blunting or preventing protection. Note: Preexisting antibody also affects injectable live vaccines, such as the measles vaccine, similarly.
Classic injectable live or nonlive vaccines provide their most potent protection via systemic cellular responses antibody and/or antibodies in serum and extracellular fluid (ECF) where IgG and IgM are in highest concentrations. So even successful injectable vaccines still allow mucosal infection to start but then intercept further spread and prevent most of the downstream damage (think pertussis) or neutralize an infection-generated toxin (pertussis or tetanus). It usually is only after infection-induced damage occurs that systemic IgG and IgM gain better access to respiratory epithelial surfaces, but still only at a fraction of circulating concentrations. Indeed, pertussis vaccine–induced systemic immunity allows the pathogen to attack and replicate in/on host surface cells, causing toxin release and variable amounts of local mucosal injury/inflammation before vaccine-induced systemic immunity gains adequate access to the pathogen and/or to its toxin which may enter systemic circulation.
Live attenuated influenza vaccine (LAIV) induces mucosal immunity
Another “standard” vaccine that induces mucosal immunity – LAIV – was developed to improve on protection afforded by injectable influenza vaccines (IIVs), but LAIV has had hiccups in the United States. One example is several years of negligible protection against H1N1 disease. As long as LAIV’s vaccine strain had reasonably matched the circulating strains, LAIV worked at least as well as injectable influenza vaccine, and even offered some cross-protection against mildly mismatched strains. But after a number of years of LAIV use, vaccine effectiveness in the United States vs. H1N1 strains appeared to fade due to previously undetected but significant changes in the circulating H1N1 strain. The lesson is that mucosal immunity’s advantages are lost if too much change occurs in the pathogen target for sIgA and mucosally-associated lymphoid tissue cells (MALT)).
Other vaccines likely need to induce mucosal immunity
Protection at the mucosal level will likely be needed for success against norovirus, parainfluenza, respiratory syncytial virus (RSV), Neisseria gonorrhea, and chlamydia. Another helpful aspect of mucosal immunity is that immune cells and sIgA not only reside on the mucosa where the antigen was originally presented, but there is also a reasonable chance that these components will traffic to other mucosal surfaces.2
So intranasal vaccine could be expected to protect distant mucosal surfaces (urogenital, GI, and respiratory), leading to vaccine-induced systemic antibody plus mucosal immunity (sIGA and MALT responses) at each site.
Let’s look at a novel “two-site” chlamydia vaccine
Recently a phase 1 chlamydia vaccine that used a novel two-pronged administration site/schedule was successful at inducing both mucosal and systemic immunity in a proof-of-concept study – achieving the best of both worlds.3 This may be a template for vaccines in years to come. British investigators studied 50 healthy women aged 19-45 years in a double-blind, parallel, randomized, placebo-controlled trial that used a recombinant chlamydia protein subunit antigen (CTH522). The vaccine schedule involved three injectable priming doses followed soon thereafter by two intranasal boosting doses. There were three groups:
1. CTH522 adjuvanted with CAF01 liposomes (CTH522:CAF01).
2. CTH522 adjuvanted with aluminum hydroxide (CTH522:AH).
3. Placebo (saline).
The intramuscular (IM) priming schedule was 0, 1, and 4 months. The intranasal vaccine booster doses or placebo were given at 4.5 and 5 months. No related serious adverse reactions occurred. For injectable dosing, the most frequent adverse event was mild local injection-site reactions in all subjects in both vaccine groups vs. in 60% of placebo recipients (P = .053). The adjuvants were the likely cause for local reactions. Intranasal doses had local reactions in 47% of both vaccine groups and 60% of placebo recipients; P = 1.000).
Both vaccines produced systemic IgG seroconversion (including neutralizing antibody) plus small amounts of IgG in the nasal cavity and genital tract in all vaccine recipients; no placebo recipient seroconverted. Interestingly, liposomally-adjuvanted vaccine produced a more rapid systemic IgG response and higher serum titers than the alum-adjuvanted vaccine. Likewise, the IM liposomal vaccine also induced higher but still small mucosal IgG antibody responses (P = .0091). Intranasal IM-induced IgG titers were not boosted by later intranasal vaccine dosing.
Subjects getting liposomal vaccine (but not alum vaccine or placebo) boosters had detectable sIgA titers in both nasal and genital tract secretions. Liposomal vaccine recipients also had fivefold to sixfold higher median titers than alum vaccine recipients after the priming dose, and these higher titers persisted to the end of the study. All liposomal vaccine recipients developed antichlamydial cell-mediated responses vs. 57% alum-adjuvanted vaccine recipients. (P = .01). So both use of two-site dosing and the liposomal adjuvant appeared critical to better responses.
In summary
While this candidate vaccine has hurdles to overcome before coming into routine use, the proof-of-principle that a combination injectable-intranasal vaccine schedule can induce robust systemic and mucosal immunity when given with an appropriate adjuvant is very promising. Adding more vaccines to the schedule then becomes an issue, but that is one of those “good” problems we can deal with later.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital-Kansas City, Mo. Children’s Mercy Hospital receives grant funding to study two candidate RSV vaccines, receives funding from GlaxoSmithKline for studies on pneumococcal and rotavirus vaccines, and from Pfizer for a study on pneumococcal vaccine on which Dr. Harrison is a sub-investigator. The hospital also receives Centers for Disease Control and Prevention funding under the New Vaccine Surveillance Network for multicenter surveillance of acute respiratory infections, including influenza, RSV, and parainfluenza virus, and also for rotavirus. Email Dr. Harrison at [email protected].
References
1. PLOS Biology. 2012 Sep 1. doi: 10.1371/journal.pbio.1001397.
2. Mucosal Immunity in the Human Female Reproductive Tract in “Mucosal Immunology,” 4th ed., Volume 2 (Cambridge, MA: Academic Press, 2015, pp. 2097-124).
3. Lancet Infect Dis. 2019. doi: 10.1016/S1473-3099(19)30279-8.
Vaccines are marvelous, and there are many well documented success stories, including rotavirus (RV) vaccines, where a live vaccine is administered to the gastrointestinal mucosa via oral drops. Antigens presented at the mucosal/epithelial surface not only induce systemic serum IgG – as do injectable vaccines – but also induce secretory IgA (sIgA), which is most helpful in diseases that directly affect the mucosa.

Mucosal vs. systemic immunity
Antibody being present on mucosal surfaces (point of initial pathogen contact) has a chance to neutralize the pathogen before it gains a foothold. Pathogen-specific mucosal lymphoid elements (e.g. in Peyer’s patches in the gut) also appear critical for optimal protection.1 The presence of both mucosal immune elements means that infection is severely limited or at times entirely prevented. So virus entering the GI tract causes minimal to no gut lining injury. Hence, there is no or mostly reduced vomiting/diarrhea. A downside of mucosally-administered live vaccines is that preexisting antibody to the vaccine antigens can reduce or block vaccine virus replication in the vaccinee, blunting or preventing protection. Note: Preexisting antibody also affects injectable live vaccines, such as the measles vaccine, similarly.
Classic injectable live or nonlive vaccines provide their most potent protection via systemic cellular responses antibody and/or antibodies in serum and extracellular fluid (ECF) where IgG and IgM are in highest concentrations. So even successful injectable vaccines still allow mucosal infection to start but then intercept further spread and prevent most of the downstream damage (think pertussis) or neutralize an infection-generated toxin (pertussis or tetanus). It usually is only after infection-induced damage occurs that systemic IgG and IgM gain better access to respiratory epithelial surfaces, but still only at a fraction of circulating concentrations. Indeed, pertussis vaccine–induced systemic immunity allows the pathogen to attack and replicate in/on host surface cells, causing toxin release and variable amounts of local mucosal injury/inflammation before vaccine-induced systemic immunity gains adequate access to the pathogen and/or to its toxin which may enter systemic circulation.
Live attenuated influenza vaccine (LAIV) induces mucosal immunity
Another “standard” vaccine that induces mucosal immunity – LAIV – was developed to improve on protection afforded by injectable influenza vaccines (IIVs), but LAIV has had hiccups in the United States. One example is several years of negligible protection against H1N1 disease. As long as LAIV’s vaccine strain had reasonably matched the circulating strains, LAIV worked at least as well as injectable influenza vaccine, and even offered some cross-protection against mildly mismatched strains. But after a number of years of LAIV use, vaccine effectiveness in the United States vs. H1N1 strains appeared to fade due to previously undetected but significant changes in the circulating H1N1 strain. The lesson is that mucosal immunity’s advantages are lost if too much change occurs in the pathogen target for sIgA and mucosally-associated lymphoid tissue cells (MALT)).
Other vaccines likely need to induce mucosal immunity
Protection at the mucosal level will likely be needed for success against norovirus, parainfluenza, respiratory syncytial virus (RSV), Neisseria gonorrhea, and chlamydia. Another helpful aspect of mucosal immunity is that immune cells and sIgA not only reside on the mucosa where the antigen was originally presented, but there is also a reasonable chance that these components will traffic to other mucosal surfaces.2
So intranasal vaccine could be expected to protect distant mucosal surfaces (urogenital, GI, and respiratory), leading to vaccine-induced systemic antibody plus mucosal immunity (sIGA and MALT responses) at each site.
Let’s look at a novel “two-site” chlamydia vaccine
Recently a phase 1 chlamydia vaccine that used a novel two-pronged administration site/schedule was successful at inducing both mucosal and systemic immunity in a proof-of-concept study – achieving the best of both worlds.3 This may be a template for vaccines in years to come. British investigators studied 50 healthy women aged 19-45 years in a double-blind, parallel, randomized, placebo-controlled trial that used a recombinant chlamydia protein subunit antigen (CTH522). The vaccine schedule involved three injectable priming doses followed soon thereafter by two intranasal boosting doses. There were three groups:
1. CTH522 adjuvanted with CAF01 liposomes (CTH522:CAF01).
2. CTH522 adjuvanted with aluminum hydroxide (CTH522:AH).
3. Placebo (saline).
The intramuscular (IM) priming schedule was 0, 1, and 4 months. The intranasal vaccine booster doses or placebo were given at 4.5 and 5 months. No related serious adverse reactions occurred. For injectable dosing, the most frequent adverse event was mild local injection-site reactions in all subjects in both vaccine groups vs. in 60% of placebo recipients (P = .053). The adjuvants were the likely cause for local reactions. Intranasal doses had local reactions in 47% of both vaccine groups and 60% of placebo recipients; P = 1.000).
Both vaccines produced systemic IgG seroconversion (including neutralizing antibody) plus small amounts of IgG in the nasal cavity and genital tract in all vaccine recipients; no placebo recipient seroconverted. Interestingly, liposomally-adjuvanted vaccine produced a more rapid systemic IgG response and higher serum titers than the alum-adjuvanted vaccine. Likewise, the IM liposomal vaccine also induced higher but still small mucosal IgG antibody responses (P = .0091). Intranasal IM-induced IgG titers were not boosted by later intranasal vaccine dosing.
Subjects getting liposomal vaccine (but not alum vaccine or placebo) boosters had detectable sIgA titers in both nasal and genital tract secretions. Liposomal vaccine recipients also had fivefold to sixfold higher median titers than alum vaccine recipients after the priming dose, and these higher titers persisted to the end of the study. All liposomal vaccine recipients developed antichlamydial cell-mediated responses vs. 57% alum-adjuvanted vaccine recipients. (P = .01). So both use of two-site dosing and the liposomal adjuvant appeared critical to better responses.
In summary
While this candidate vaccine has hurdles to overcome before coming into routine use, the proof-of-principle that a combination injectable-intranasal vaccine schedule can induce robust systemic and mucosal immunity when given with an appropriate adjuvant is very promising. Adding more vaccines to the schedule then becomes an issue, but that is one of those “good” problems we can deal with later.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital-Kansas City, Mo. Children’s Mercy Hospital receives grant funding to study two candidate RSV vaccines, receives funding from GlaxoSmithKline for studies on pneumococcal and rotavirus vaccines, and from Pfizer for a study on pneumococcal vaccine on which Dr. Harrison is a sub-investigator. The hospital also receives Centers for Disease Control and Prevention funding under the New Vaccine Surveillance Network for multicenter surveillance of acute respiratory infections, including influenza, RSV, and parainfluenza virus, and also for rotavirus. Email Dr. Harrison at [email protected].
References
1. PLOS Biology. 2012 Sep 1. doi: 10.1371/journal.pbio.1001397.
2. Mucosal Immunity in the Human Female Reproductive Tract in “Mucosal Immunology,” 4th ed., Volume 2 (Cambridge, MA: Academic Press, 2015, pp. 2097-124).
3. Lancet Infect Dis. 2019. doi: 10.1016/S1473-3099(19)30279-8.
New engineered HIV-1 vaccine candidate shows improved immunogenicity in early trial
ALVAC-HIV vaccine showed immunogenicity across several HIV clades in an early trial involving 100 healthy patients at low risk of HIV infection, according to a study by Glenda E. Gray, MBBCH, FCPaed, of the University of the Witwatersrand, Johannesburg, South Africa, and colleagues that was published online in the Sep. 18 issue of Science Translational Medicine.
ALVAC-HIV (vCP1521) is a live attenuated recombinant canarypox-derived virus that expresses gene products from the HIV-1 gp120 (92TH023/clade E), Gag (clade B), and Pro (clade B) that is cultured in chicken embryo fibroblast cells.
Four injections of ALVAC-HIV were given at months 0, 1, 3, and 6. At months 3 and 6, two booster injections were given of AIDSVAX/BE, a bivalent HIV glycoprotein 120 (gp120) that was previously studied in the RV144 trial. The HVTN 097 trial examined primary immunogenicity endpoints including the frequency and magnitude of IgG and IgG3 antibody binding, measured in serum specimens obtained at baseline, at a peak time point (2 weeks after second ALVAC/AIDSVAX vaccination), a durability time point (6 months after second ALVAC/AIDSVAX vaccination), and the response rates and magnitudes of CD4+ and CD8+ T-cell responses at the baseline, peak, and durability time points. One hundred healthy adults at low risk for HIV infection were randomized in 3:1:1 ratio to group T1 (HIV vaccines, tetanus vaccine, and hepatitis B vaccine), group T2 (HIV vaccine only), and the placebo group T3 (tetanus vaccine and hepatitis B vaccine). There were no meaningful differences in HIV immune responses between the HIV vaccine recipients with or without the tetanus and hepatitis B vaccines, so the researchers pooled the data from groups T1 and T2 in their analysis.
At the peak immunogenicity time point, the vaccine schedule predominantly induced CD4+ T cells directed to HIV-1 Env; this was measured by expression of interleukin-2 and/or interferon-gamma. The Env-specific CD4+ T-cell response rate was significantly higher in HVTN 097 vaccine recipients than it was in those in the RV144 trial (51.9% vs. 36.4%; P = .043). The HVTN 097 trial also showed significantly higher response rates for CD40L(59.3% for HVTN 097 vs. 33.7% for RV144; P less than .001) and for interferon-gamma (42.6% in HVTN 097 vs. 19.5% in RV144; P = .001).
However, durability at 6 months after the second vaccine injection remained an issue, with the frequency of circulating Env-specific CD4+ T-cell responses among vaccine recipients declining significantly; the response rate dropped from 70.8% to 36.1%.
“These data may indicate that cross-clade immune responses, especially to non-neutralizing epitopes correlated with decreased HIV-1 risk, can be achieved for a globally effective vaccine by using unique HIV Env strains,” Dr. Gray and associates concluded.
The authors declared that they had no competing interests.
SOURCE: Gray GE et al. Sci. Transl. Med. 2019 Sep 18. doi: 10.1126/scitranslmed.aax1880..
ALVAC-HIV vaccine showed immunogenicity across several HIV clades in an early trial involving 100 healthy patients at low risk of HIV infection, according to a study by Glenda E. Gray, MBBCH, FCPaed, of the University of the Witwatersrand, Johannesburg, South Africa, and colleagues that was published online in the Sep. 18 issue of Science Translational Medicine.
ALVAC-HIV (vCP1521) is a live attenuated recombinant canarypox-derived virus that expresses gene products from the HIV-1 gp120 (92TH023/clade E), Gag (clade B), and Pro (clade B) that is cultured in chicken embryo fibroblast cells.
Four injections of ALVAC-HIV were given at months 0, 1, 3, and 6. At months 3 and 6, two booster injections were given of AIDSVAX/BE, a bivalent HIV glycoprotein 120 (gp120) that was previously studied in the RV144 trial. The HVTN 097 trial examined primary immunogenicity endpoints including the frequency and magnitude of IgG and IgG3 antibody binding, measured in serum specimens obtained at baseline, at a peak time point (2 weeks after second ALVAC/AIDSVAX vaccination), a durability time point (6 months after second ALVAC/AIDSVAX vaccination), and the response rates and magnitudes of CD4+ and CD8+ T-cell responses at the baseline, peak, and durability time points. One hundred healthy adults at low risk for HIV infection were randomized in 3:1:1 ratio to group T1 (HIV vaccines, tetanus vaccine, and hepatitis B vaccine), group T2 (HIV vaccine only), and the placebo group T3 (tetanus vaccine and hepatitis B vaccine). There were no meaningful differences in HIV immune responses between the HIV vaccine recipients with or without the tetanus and hepatitis B vaccines, so the researchers pooled the data from groups T1 and T2 in their analysis.
At the peak immunogenicity time point, the vaccine schedule predominantly induced CD4+ T cells directed to HIV-1 Env; this was measured by expression of interleukin-2 and/or interferon-gamma. The Env-specific CD4+ T-cell response rate was significantly higher in HVTN 097 vaccine recipients than it was in those in the RV144 trial (51.9% vs. 36.4%; P = .043). The HVTN 097 trial also showed significantly higher response rates for CD40L(59.3% for HVTN 097 vs. 33.7% for RV144; P less than .001) and for interferon-gamma (42.6% in HVTN 097 vs. 19.5% in RV144; P = .001).
However, durability at 6 months after the second vaccine injection remained an issue, with the frequency of circulating Env-specific CD4+ T-cell responses among vaccine recipients declining significantly; the response rate dropped from 70.8% to 36.1%.
“These data may indicate that cross-clade immune responses, especially to non-neutralizing epitopes correlated with decreased HIV-1 risk, can be achieved for a globally effective vaccine by using unique HIV Env strains,” Dr. Gray and associates concluded.
The authors declared that they had no competing interests.
SOURCE: Gray GE et al. Sci. Transl. Med. 2019 Sep 18. doi: 10.1126/scitranslmed.aax1880..
ALVAC-HIV vaccine showed immunogenicity across several HIV clades in an early trial involving 100 healthy patients at low risk of HIV infection, according to a study by Glenda E. Gray, MBBCH, FCPaed, of the University of the Witwatersrand, Johannesburg, South Africa, and colleagues that was published online in the Sep. 18 issue of Science Translational Medicine.
ALVAC-HIV (vCP1521) is a live attenuated recombinant canarypox-derived virus that expresses gene products from the HIV-1 gp120 (92TH023/clade E), Gag (clade B), and Pro (clade B) that is cultured in chicken embryo fibroblast cells.
Four injections of ALVAC-HIV were given at months 0, 1, 3, and 6. At months 3 and 6, two booster injections were given of AIDSVAX/BE, a bivalent HIV glycoprotein 120 (gp120) that was previously studied in the RV144 trial. The HVTN 097 trial examined primary immunogenicity endpoints including the frequency and magnitude of IgG and IgG3 antibody binding, measured in serum specimens obtained at baseline, at a peak time point (2 weeks after second ALVAC/AIDSVAX vaccination), a durability time point (6 months after second ALVAC/AIDSVAX vaccination), and the response rates and magnitudes of CD4+ and CD8+ T-cell responses at the baseline, peak, and durability time points. One hundred healthy adults at low risk for HIV infection were randomized in 3:1:1 ratio to group T1 (HIV vaccines, tetanus vaccine, and hepatitis B vaccine), group T2 (HIV vaccine only), and the placebo group T3 (tetanus vaccine and hepatitis B vaccine). There were no meaningful differences in HIV immune responses between the HIV vaccine recipients with or without the tetanus and hepatitis B vaccines, so the researchers pooled the data from groups T1 and T2 in their analysis.
At the peak immunogenicity time point, the vaccine schedule predominantly induced CD4+ T cells directed to HIV-1 Env; this was measured by expression of interleukin-2 and/or interferon-gamma. The Env-specific CD4+ T-cell response rate was significantly higher in HVTN 097 vaccine recipients than it was in those in the RV144 trial (51.9% vs. 36.4%; P = .043). The HVTN 097 trial also showed significantly higher response rates for CD40L(59.3% for HVTN 097 vs. 33.7% for RV144; P less than .001) and for interferon-gamma (42.6% in HVTN 097 vs. 19.5% in RV144; P = .001).
However, durability at 6 months after the second vaccine injection remained an issue, with the frequency of circulating Env-specific CD4+ T-cell responses among vaccine recipients declining significantly; the response rate dropped from 70.8% to 36.1%.
“These data may indicate that cross-clade immune responses, especially to non-neutralizing epitopes correlated with decreased HIV-1 risk, can be achieved for a globally effective vaccine by using unique HIV Env strains,” Dr. Gray and associates concluded.
The authors declared that they had no competing interests.
SOURCE: Gray GE et al. Sci. Transl. Med. 2019 Sep 18. doi: 10.1126/scitranslmed.aax1880..
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: “These data may indicate that cross-clade immune responses ... can be achieved for a globally effective vaccine by using unique HIV Env strains.”
Major finding: At the peak immunogenicity time point, the vaccine schedule predominantly induced CD4+ T cells directed to HIV-1 Env .
Study details: A phase 1b randomized, double-blind, placebo-controlled trial to assess the safety and immunogenicity of the ALVAC-HIV vaccine in 100 healthy patients at low risk of HIV infection.
Disclosures: The study was supported by the National Institute of Allergy and Infectious Diseases and other global health agencies. The authors declared that they had no competing interests.
Source: Gray GE et al. Sci Transl Med. 2019 Sep 18. doi: 10.1126/scitranslmed.aax1880.
Australia’s rotavirus outbreak wasn’t caused by vaccine effectiveness decline
In 2017, the Australian state of New South Wales experienced an outbreak of rotavirus gastroenteritis in children despite a high level of rotavirus immunization. In a new study, researchers reported evidence that suggests a decline in vaccine effectiveness (VE) isn’t the cause, although they found that VE declines over time as children age.
“More analysis is required to investigate how novel or unusual strains ... interact with rotavirus vaccines and whether antigenic changes affect VE and challenge vaccination programs,” the study authors wrote in Pediatrics.
Researchers led by Julia E. Maguire, BSc, MSci(Epi), of Australia’s National Center for Immunization Research and the Australian National University, Canberra, launched the analysis in the wake of a 2017 outbreak of 2,319 rotavirus cases in New South Wales, a 210% increase over the rate in 2016. (The state, the largest in Australia, has about 7.5 million residents.)
The study authors tracked VE from 2010 to 2017 by analyzing 9,517 rotavirus cases in the state (50% male; median age, 5 years). Half weren’t eligible for rotavirus immunization because of their age; of the rest, 31% weren’t vaccinated.
Ms. Maguire and associates found that “In our study, two doses of RV1 [the Rotarix vaccine] was 73.7% effective in protecting children aged 6 months to 9 years against laboratory-confirmed rotavirus over our 8-year study period. Somewhat surprisingly in the 2017 outbreak year, a high two-dose VE of 88.4% in those aged 6-11 months was also observed.”
They added that “the median age of rotavirus cases has increased in Australia over the last 8 years from 3.9 years in 2010 to 7.1 years in 2017. Adults and older children born before the availability of vaccination in Australia are unimmunized and may have been less likely to have repeated subclinical infections because of reductions in virus circulation overall, resulting in less immune boosting.”
Going forward, the study authors wrote that “investigation of population-level VE in relation to rotavirus genotype data should continue in a range of settings to improve our understanding of rotavirus vaccines and the impact they have on disease across the age spectrum over time.”
In an accompanying commentary, Benjamin Lee, MD, and E. Ross Colgate, PhD, of the University of Vermont, Burlington, wrote that Australia’s adoption of rotavirus immunization in 2017 “with state-level implementation of either Rotarix or RotaTeq ... enabled a fascinating natural experiment of VE and strain selection.”
Pressure from vaccines “potentially enables the emergence of novel strains,” they wrote. “Despite this, large-scale strain replacement has not been demonstrated in rotaviruses, in contrast to the development of pneumococcal serotype replacement that was seen after pneumococcal conjugate vaccine introduction. Similarly, there has been no evidence of widespread vaccine escape due to antigenic drift or shift, as occurs with another important segmented RNA virus, influenza A.”
As Dr. Lee and Dr. Colgate noted, 100 million children worldwide remain unvaccinated against rotavirus, and more than 128,000 die because of rotavirus-associated gastroenteritis each year. “Improving vaccine access and coverage and solving the riddle of [oral rotavirus vaccine] underperformance in low-income countries are urgent priorities, which may ultimately require next-generation oral and/or parenteral vaccines, a number of which are under development and in clinical trials. In addition, because the emergence of novel strains of disease-causing pathogens is always a possibility, vigilance in rotavirus surveillance, including genotype assessment, should remain a priority for public health programs.”
The study was funded by Australia’s National Center for Immunization Research and Surveillance, which receives government funding. The Australian Rotavirus Surveillance Program is supported by government funding and the vaccine companies Commonwealth Serum Laboratories and GlaxoSmithKline. Ms. Maguire is supported by an Australian Government Research Training Program Scholarship. One author is director of the Australian Rotavirus Surveillance Program, which received funding as above. The other study authors and the commentary authors reported no relevant financial disclosures.
SOURCES: Maguire JE et al. Pediatrics. 2019 Sep 17. doi: 10.1542/peds.2019-1024; Lee B, Colgate ER. Pediatrics. 2019 Sep 17. doi: 10.1542/peds.2019-2426.
In 2017, the Australian state of New South Wales experienced an outbreak of rotavirus gastroenteritis in children despite a high level of rotavirus immunization. In a new study, researchers reported evidence that suggests a decline in vaccine effectiveness (VE) isn’t the cause, although they found that VE declines over time as children age.
“More analysis is required to investigate how novel or unusual strains ... interact with rotavirus vaccines and whether antigenic changes affect VE and challenge vaccination programs,” the study authors wrote in Pediatrics.
Researchers led by Julia E. Maguire, BSc, MSci(Epi), of Australia’s National Center for Immunization Research and the Australian National University, Canberra, launched the analysis in the wake of a 2017 outbreak of 2,319 rotavirus cases in New South Wales, a 210% increase over the rate in 2016. (The state, the largest in Australia, has about 7.5 million residents.)
The study authors tracked VE from 2010 to 2017 by analyzing 9,517 rotavirus cases in the state (50% male; median age, 5 years). Half weren’t eligible for rotavirus immunization because of their age; of the rest, 31% weren’t vaccinated.
Ms. Maguire and associates found that “In our study, two doses of RV1 [the Rotarix vaccine] was 73.7% effective in protecting children aged 6 months to 9 years against laboratory-confirmed rotavirus over our 8-year study period. Somewhat surprisingly in the 2017 outbreak year, a high two-dose VE of 88.4% in those aged 6-11 months was also observed.”
They added that “the median age of rotavirus cases has increased in Australia over the last 8 years from 3.9 years in 2010 to 7.1 years in 2017. Adults and older children born before the availability of vaccination in Australia are unimmunized and may have been less likely to have repeated subclinical infections because of reductions in virus circulation overall, resulting in less immune boosting.”
Going forward, the study authors wrote that “investigation of population-level VE in relation to rotavirus genotype data should continue in a range of settings to improve our understanding of rotavirus vaccines and the impact they have on disease across the age spectrum over time.”
In an accompanying commentary, Benjamin Lee, MD, and E. Ross Colgate, PhD, of the University of Vermont, Burlington, wrote that Australia’s adoption of rotavirus immunization in 2017 “with state-level implementation of either Rotarix or RotaTeq ... enabled a fascinating natural experiment of VE and strain selection.”
Pressure from vaccines “potentially enables the emergence of novel strains,” they wrote. “Despite this, large-scale strain replacement has not been demonstrated in rotaviruses, in contrast to the development of pneumococcal serotype replacement that was seen after pneumococcal conjugate vaccine introduction. Similarly, there has been no evidence of widespread vaccine escape due to antigenic drift or shift, as occurs with another important segmented RNA virus, influenza A.”
As Dr. Lee and Dr. Colgate noted, 100 million children worldwide remain unvaccinated against rotavirus, and more than 128,000 die because of rotavirus-associated gastroenteritis each year. “Improving vaccine access and coverage and solving the riddle of [oral rotavirus vaccine] underperformance in low-income countries are urgent priorities, which may ultimately require next-generation oral and/or parenteral vaccines, a number of which are under development and in clinical trials. In addition, because the emergence of novel strains of disease-causing pathogens is always a possibility, vigilance in rotavirus surveillance, including genotype assessment, should remain a priority for public health programs.”
The study was funded by Australia’s National Center for Immunization Research and Surveillance, which receives government funding. The Australian Rotavirus Surveillance Program is supported by government funding and the vaccine companies Commonwealth Serum Laboratories and GlaxoSmithKline. Ms. Maguire is supported by an Australian Government Research Training Program Scholarship. One author is director of the Australian Rotavirus Surveillance Program, which received funding as above. The other study authors and the commentary authors reported no relevant financial disclosures.
SOURCES: Maguire JE et al. Pediatrics. 2019 Sep 17. doi: 10.1542/peds.2019-1024; Lee B, Colgate ER. Pediatrics. 2019 Sep 17. doi: 10.1542/peds.2019-2426.
In 2017, the Australian state of New South Wales experienced an outbreak of rotavirus gastroenteritis in children despite a high level of rotavirus immunization. In a new study, researchers reported evidence that suggests a decline in vaccine effectiveness (VE) isn’t the cause, although they found that VE declines over time as children age.
“More analysis is required to investigate how novel or unusual strains ... interact with rotavirus vaccines and whether antigenic changes affect VE and challenge vaccination programs,” the study authors wrote in Pediatrics.
Researchers led by Julia E. Maguire, BSc, MSci(Epi), of Australia’s National Center for Immunization Research and the Australian National University, Canberra, launched the analysis in the wake of a 2017 outbreak of 2,319 rotavirus cases in New South Wales, a 210% increase over the rate in 2016. (The state, the largest in Australia, has about 7.5 million residents.)
The study authors tracked VE from 2010 to 2017 by analyzing 9,517 rotavirus cases in the state (50% male; median age, 5 years). Half weren’t eligible for rotavirus immunization because of their age; of the rest, 31% weren’t vaccinated.
Ms. Maguire and associates found that “In our study, two doses of RV1 [the Rotarix vaccine] was 73.7% effective in protecting children aged 6 months to 9 years against laboratory-confirmed rotavirus over our 8-year study period. Somewhat surprisingly in the 2017 outbreak year, a high two-dose VE of 88.4% in those aged 6-11 months was also observed.”
They added that “the median age of rotavirus cases has increased in Australia over the last 8 years from 3.9 years in 2010 to 7.1 years in 2017. Adults and older children born before the availability of vaccination in Australia are unimmunized and may have been less likely to have repeated subclinical infections because of reductions in virus circulation overall, resulting in less immune boosting.”
Going forward, the study authors wrote that “investigation of population-level VE in relation to rotavirus genotype data should continue in a range of settings to improve our understanding of rotavirus vaccines and the impact they have on disease across the age spectrum over time.”
In an accompanying commentary, Benjamin Lee, MD, and E. Ross Colgate, PhD, of the University of Vermont, Burlington, wrote that Australia’s adoption of rotavirus immunization in 2017 “with state-level implementation of either Rotarix or RotaTeq ... enabled a fascinating natural experiment of VE and strain selection.”
Pressure from vaccines “potentially enables the emergence of novel strains,” they wrote. “Despite this, large-scale strain replacement has not been demonstrated in rotaviruses, in contrast to the development of pneumococcal serotype replacement that was seen after pneumococcal conjugate vaccine introduction. Similarly, there has been no evidence of widespread vaccine escape due to antigenic drift or shift, as occurs with another important segmented RNA virus, influenza A.”
As Dr. Lee and Dr. Colgate noted, 100 million children worldwide remain unvaccinated against rotavirus, and more than 128,000 die because of rotavirus-associated gastroenteritis each year. “Improving vaccine access and coverage and solving the riddle of [oral rotavirus vaccine] underperformance in low-income countries are urgent priorities, which may ultimately require next-generation oral and/or parenteral vaccines, a number of which are under development and in clinical trials. In addition, because the emergence of novel strains of disease-causing pathogens is always a possibility, vigilance in rotavirus surveillance, including genotype assessment, should remain a priority for public health programs.”
The study was funded by Australia’s National Center for Immunization Research and Surveillance, which receives government funding. The Australian Rotavirus Surveillance Program is supported by government funding and the vaccine companies Commonwealth Serum Laboratories and GlaxoSmithKline. Ms. Maguire is supported by an Australian Government Research Training Program Scholarship. One author is director of the Australian Rotavirus Surveillance Program, which received funding as above. The other study authors and the commentary authors reported no relevant financial disclosures.
SOURCES: Maguire JE et al. Pediatrics. 2019 Sep 17. doi: 10.1542/peds.2019-1024; Lee B, Colgate ER. Pediatrics. 2019 Sep 17. doi: 10.1542/peds.2019-2426.
FROM PEDIATRICS
Measles cases continue to decline
according to the Centers for Disease Control and Prevention.

A total of 24 measles cases were confirmed in August, and the total for the year is now 1,241 cases in 31 states. Only seven of those cases were added during the most recent reporting week, which ended Sept. 5, but five were older cases that had just been reported, the CDC said Sept. 9.
With the ending of the measles outbreak in New York, announced Sept. 3, the largest of the three remaining active outbreaks in the country is in Rockland County, N.Y., just north of the city, which has reported 312 cases since it began in 2018.
The two other outbreaks are located in El Paso, Tex., where six cases have been reported so far, and Wyoming County in western New York State, where five cases have occurred.
according to the Centers for Disease Control and Prevention.

A total of 24 measles cases were confirmed in August, and the total for the year is now 1,241 cases in 31 states. Only seven of those cases were added during the most recent reporting week, which ended Sept. 5, but five were older cases that had just been reported, the CDC said Sept. 9.
With the ending of the measles outbreak in New York, announced Sept. 3, the largest of the three remaining active outbreaks in the country is in Rockland County, N.Y., just north of the city, which has reported 312 cases since it began in 2018.
The two other outbreaks are located in El Paso, Tex., where six cases have been reported so far, and Wyoming County in western New York State, where five cases have occurred.
according to the Centers for Disease Control and Prevention.

A total of 24 measles cases were confirmed in August, and the total for the year is now 1,241 cases in 31 states. Only seven of those cases were added during the most recent reporting week, which ended Sept. 5, but five were older cases that had just been reported, the CDC said Sept. 9.
With the ending of the measles outbreak in New York, announced Sept. 3, the largest of the three remaining active outbreaks in the country is in Rockland County, N.Y., just north of the city, which has reported 312 cases since it began in 2018.
The two other outbreaks are located in El Paso, Tex., where six cases have been reported so far, and Wyoming County in western New York State, where five cases have occurred.
Measles outbreak in New York City has ended
The measles outbreak in New York City, the largest in the nation this year, has officially ended, Mayor Bill de Blasio and city health officials announced Sept. 3.
“Ending the measles outbreak required extensive collaboration with community organizations and Jewish leaders. They helped encourage vaccinations and achieve record immunization levels in parts of Brooklyn,” Mayor de Blasio said in a written statement. “As we head back to school this week, we just remain vigilant. To keep our children and communities safe, I urge all New Yorkers to get vaccinated. It’s the best defense we have.”
A measles outbreak is considered to be over when 42 days, or two incubation periods, have elapsed since the last affected persons in the area were no longer infectious. “That time period has now passed for the people most recently infected with measles and reported,” the city health department said in the statement.
Since the outbreak began in October of last year, 654 individuals were diagnosed with measles in the five boroughs of New York, although 72% occurred in the Williamsburg neighborhood of Brooklyn. according to the health department. The majority of affected people were under 18 years of age (80%), and most were either unvaccinated (73%) or incompletely vaccinated (7%).
The end of the measles outbreak also brings an end to the public health emergency that was declared on April 9 for parts of Brooklyn, the statement noted.
“Vaccination coverage has increased significantly since the emergency order, which has been supported by community-led efforts. We are grateful to the New Yorkers who shared the truth about vaccines and protected the health of their friends and neighbors through this outbreak,” city health commissioner Dr. Oxiris Barbot said in the statement.
The measles outbreak in New York City, the largest in the nation this year, has officially ended, Mayor Bill de Blasio and city health officials announced Sept. 3.
“Ending the measles outbreak required extensive collaboration with community organizations and Jewish leaders. They helped encourage vaccinations and achieve record immunization levels in parts of Brooklyn,” Mayor de Blasio said in a written statement. “As we head back to school this week, we just remain vigilant. To keep our children and communities safe, I urge all New Yorkers to get vaccinated. It’s the best defense we have.”
A measles outbreak is considered to be over when 42 days, or two incubation periods, have elapsed since the last affected persons in the area were no longer infectious. “That time period has now passed for the people most recently infected with measles and reported,” the city health department said in the statement.
Since the outbreak began in October of last year, 654 individuals were diagnosed with measles in the five boroughs of New York, although 72% occurred in the Williamsburg neighborhood of Brooklyn. according to the health department. The majority of affected people were under 18 years of age (80%), and most were either unvaccinated (73%) or incompletely vaccinated (7%).
The end of the measles outbreak also brings an end to the public health emergency that was declared on April 9 for parts of Brooklyn, the statement noted.
“Vaccination coverage has increased significantly since the emergency order, which has been supported by community-led efforts. We are grateful to the New Yorkers who shared the truth about vaccines and protected the health of their friends and neighbors through this outbreak,” city health commissioner Dr. Oxiris Barbot said in the statement.
The measles outbreak in New York City, the largest in the nation this year, has officially ended, Mayor Bill de Blasio and city health officials announced Sept. 3.
“Ending the measles outbreak required extensive collaboration with community organizations and Jewish leaders. They helped encourage vaccinations and achieve record immunization levels in parts of Brooklyn,” Mayor de Blasio said in a written statement. “As we head back to school this week, we just remain vigilant. To keep our children and communities safe, I urge all New Yorkers to get vaccinated. It’s the best defense we have.”
A measles outbreak is considered to be over when 42 days, or two incubation periods, have elapsed since the last affected persons in the area were no longer infectious. “That time period has now passed for the people most recently infected with measles and reported,” the city health department said in the statement.
Since the outbreak began in October of last year, 654 individuals were diagnosed with measles in the five boroughs of New York, although 72% occurred in the Williamsburg neighborhood of Brooklyn. according to the health department. The majority of affected people were under 18 years of age (80%), and most were either unvaccinated (73%) or incompletely vaccinated (7%).
The end of the measles outbreak also brings an end to the public health emergency that was declared on April 9 for parts of Brooklyn, the statement noted.
“Vaccination coverage has increased significantly since the emergency order, which has been supported by community-led efforts. We are grateful to the New Yorkers who shared the truth about vaccines and protected the health of their friends and neighbors through this outbreak,” city health commissioner Dr. Oxiris Barbot said in the statement.
ACIP issues 2 new recs on HPV vaccination
References
1. Markowitz L. Overview and background (HPV). CDC Web site. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-02/HPV-2-Markowitz-508.pdf. Presented February 27, 2019. Accessed August 1, 2019.
2. Brisson M, Laprise J-F. Cost-effectiveness of extending HPV vaccination above age 26 years in the U.S. CDC Web site. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-02/HPV-3-Brisson-508.pdf. Presented February 2019. Accessed August 1, 2019.
3. Markowitz L. Recommendations for mid-adult HPV vaccination work group considerations. CDC Web site. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-02/HPV-7-Markowitz-508.pdf, Presented February 27, 2019. Accessed August 1, 2019.
4. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702.
References
1. Markowitz L. Overview and background (HPV). CDC Web site. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-02/HPV-2-Markowitz-508.pdf. Presented February 27, 2019. Accessed August 1, 2019.
2. Brisson M, Laprise J-F. Cost-effectiveness of extending HPV vaccination above age 26 years in the U.S. CDC Web site. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-02/HPV-3-Brisson-508.pdf. Presented February 2019. Accessed August 1, 2019.
3. Markowitz L. Recommendations for mid-adult HPV vaccination work group considerations. CDC Web site. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-02/HPV-7-Markowitz-508.pdf, Presented February 27, 2019. Accessed August 1, 2019.
4. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702.
References
1. Markowitz L. Overview and background (HPV). CDC Web site. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-02/HPV-2-Markowitz-508.pdf. Presented February 27, 2019. Accessed August 1, 2019.
2. Brisson M, Laprise J-F. Cost-effectiveness of extending HPV vaccination above age 26 years in the U.S. CDC Web site. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-02/HPV-3-Brisson-508.pdf. Presented February 2019. Accessed August 1, 2019.
3. Markowitz L. Recommendations for mid-adult HPV vaccination work group considerations. CDC Web site. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-02/HPV-7-Markowitz-508.pdf, Presented February 27, 2019. Accessed August 1, 2019.
4. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702.
Decision making regarding vaccines varies among accepters, deniers, partial accepters
Parents who accepted, denied, or partially accepted participation in the Dutch National Immunization Program reached their decisions through different methods, according to Kim A.G.J. Romijnders of the National Institute for Public Health and the Environment in Bilthoven, the Netherlands, and associates.
For the study published in Vaccine, the investigators conducted a series of 12 focus groups: 3 with accepters (n = 19), 3 with deniers (n =12), and 6 with partial accepters (n =24); in the partial accepters groups, there were three groups with parents delaying vaccination and three with parents refusing some vaccinations. Three-quarters of participants were women, the average age was 39 years, and 96% had at least university education. Parents were asked about their knowledge, attitudes, deliberation, and information needs regarding childhood vaccination.
Vaccine accepters regarded the decision to vaccinate their children as self-evident, but deniers and partial accepters reported conducting extensive deliberation on the pros and cons of vaccination. Deniers and partial accepters, in general, perceived fewer risks of vaccine-preventable diseases, more risks of vaccine side effects, less social support from their environment, less trust in child welfare centers, and provided less information than accepters.
The investigators noted that This process alienated them from their child vaccine provider, with trust being lost when the provider either refused or was unable to answer questions. Partial accepters also reported a lack of social support from friends, family, and providers regarding partial vaccine acceptance.
“The findings can facilitate informed decision making among parents by promoting an open dialogue at the [child welfare center], and improving the type and form of information presented. An open dialogue between parents and [child vaccine providers] may increase deliberation among parents, strengthen positive attitudes, prevent misperceptions, and resolve decisional conflict,” the investigators concluded.
The study was supported by the Dutch National Institute for Public Health and the Environment; the authors reported no conflicts of interest.
SOURCE: Romijnders KAGJ et al. Vaccine. 2019 Aug 2. doi: 10.1016/j.vaccine.2019.07.060.
Parents who accepted, denied, or partially accepted participation in the Dutch National Immunization Program reached their decisions through different methods, according to Kim A.G.J. Romijnders of the National Institute for Public Health and the Environment in Bilthoven, the Netherlands, and associates.
For the study published in Vaccine, the investigators conducted a series of 12 focus groups: 3 with accepters (n = 19), 3 with deniers (n =12), and 6 with partial accepters (n =24); in the partial accepters groups, there were three groups with parents delaying vaccination and three with parents refusing some vaccinations. Three-quarters of participants were women, the average age was 39 years, and 96% had at least university education. Parents were asked about their knowledge, attitudes, deliberation, and information needs regarding childhood vaccination.
Vaccine accepters regarded the decision to vaccinate their children as self-evident, but deniers and partial accepters reported conducting extensive deliberation on the pros and cons of vaccination. Deniers and partial accepters, in general, perceived fewer risks of vaccine-preventable diseases, more risks of vaccine side effects, less social support from their environment, less trust in child welfare centers, and provided less information than accepters.
The investigators noted that This process alienated them from their child vaccine provider, with trust being lost when the provider either refused or was unable to answer questions. Partial accepters also reported a lack of social support from friends, family, and providers regarding partial vaccine acceptance.
“The findings can facilitate informed decision making among parents by promoting an open dialogue at the [child welfare center], and improving the type and form of information presented. An open dialogue between parents and [child vaccine providers] may increase deliberation among parents, strengthen positive attitudes, prevent misperceptions, and resolve decisional conflict,” the investigators concluded.
The study was supported by the Dutch National Institute for Public Health and the Environment; the authors reported no conflicts of interest.
SOURCE: Romijnders KAGJ et al. Vaccine. 2019 Aug 2. doi: 10.1016/j.vaccine.2019.07.060.
Parents who accepted, denied, or partially accepted participation in the Dutch National Immunization Program reached their decisions through different methods, according to Kim A.G.J. Romijnders of the National Institute for Public Health and the Environment in Bilthoven, the Netherlands, and associates.
For the study published in Vaccine, the investigators conducted a series of 12 focus groups: 3 with accepters (n = 19), 3 with deniers (n =12), and 6 with partial accepters (n =24); in the partial accepters groups, there were three groups with parents delaying vaccination and three with parents refusing some vaccinations. Three-quarters of participants were women, the average age was 39 years, and 96% had at least university education. Parents were asked about their knowledge, attitudes, deliberation, and information needs regarding childhood vaccination.
Vaccine accepters regarded the decision to vaccinate their children as self-evident, but deniers and partial accepters reported conducting extensive deliberation on the pros and cons of vaccination. Deniers and partial accepters, in general, perceived fewer risks of vaccine-preventable diseases, more risks of vaccine side effects, less social support from their environment, less trust in child welfare centers, and provided less information than accepters.
The investigators noted that This process alienated them from their child vaccine provider, with trust being lost when the provider either refused or was unable to answer questions. Partial accepters also reported a lack of social support from friends, family, and providers regarding partial vaccine acceptance.
“The findings can facilitate informed decision making among parents by promoting an open dialogue at the [child welfare center], and improving the type and form of information presented. An open dialogue between parents and [child vaccine providers] may increase deliberation among parents, strengthen positive attitudes, prevent misperceptions, and resolve decisional conflict,” the investigators concluded.
The study was supported by the Dutch National Institute for Public Health and the Environment; the authors reported no conflicts of interest.
SOURCE: Romijnders KAGJ et al. Vaccine. 2019 Aug 2. doi: 10.1016/j.vaccine.2019.07.060.
FROM VACCINE
MenB vaccination coverage higher in those receiving MenB-4C
While meningococcal group B (MenB) vaccination remains suboptimal in the United States, completion was significantly higher for the MenB-4C (Bexsero) vaccine, compared with the other vaccine option, MenB-FHbp (Trumenba), according to Elizabeth Packnett of IBM Watson Health in Bethesda, Md., and associates.
In a study published in Vaccine, the investigators retrospectively analyzed 65,205 (36,118 received MenB-4C; 29,087 received MenB-FHbp) commercially insured individuals from the MarketScan Commercial Claims and Encounters Database during Jan. 1, 2015–Feb. 28, 2018, as well as 13,535 (10,153 received MenB-4C; 3,382 received MenB-FHbp) Medicaid-covered individuals from the Medicaid Multi-State Database during Jan. 1, 2015–Dec. 31, 2017.
The rate of vaccine completion in the MarketScan database within 15 months of initiation was 63% for MenB-4C and 52% for MenB-FHbp, and dosing schedule adherence was 62% for MenB-4C and 18% for MenB-FHbp. The median time to completion among those who finished vaccination was 68 days for MenB-4C versus 258 days for MenB-FHbp.
In the Medicaid database, the rate of vaccine completion within 15 months of initiation was 49% for MenB-4C and 31% for MenB-FHbp; dosing schedule adherence was 48% and 8%, respectively. Median time to vaccine completion was 88 days for MenB-4C versus 309 days for MenB-FHbp.
“The observations of improved completion and schedule adherence rates for MenB-4C, compared with MenB-FHbp, were consistent across both the commercial and Medicaid populations, and persisted after adjusting for individual factors in multivariable analyses, suggesting that the results were not skewed by population differences in demographic or other characteristics,” the investigators noted, adding that the significant difference in completion and schedule adherence between vaccines likely reflects the MenB-4C flexible dosing schedule.
The study was funded by GlaxoSmithKline, the maker of MenB-4C, and four coauthors reported being employed by the company. Five coauthors were employed by IBM Watson Health, which conducted the study.
SOURCE: Packnett E et al. Vaccine. 2019 Aug 20. doi: 10.1016/j.vaccine.2019.06.065.
While meningococcal group B (MenB) vaccination remains suboptimal in the United States, completion was significantly higher for the MenB-4C (Bexsero) vaccine, compared with the other vaccine option, MenB-FHbp (Trumenba), according to Elizabeth Packnett of IBM Watson Health in Bethesda, Md., and associates.
In a study published in Vaccine, the investigators retrospectively analyzed 65,205 (36,118 received MenB-4C; 29,087 received MenB-FHbp) commercially insured individuals from the MarketScan Commercial Claims and Encounters Database during Jan. 1, 2015–Feb. 28, 2018, as well as 13,535 (10,153 received MenB-4C; 3,382 received MenB-FHbp) Medicaid-covered individuals from the Medicaid Multi-State Database during Jan. 1, 2015–Dec. 31, 2017.
The rate of vaccine completion in the MarketScan database within 15 months of initiation was 63% for MenB-4C and 52% for MenB-FHbp, and dosing schedule adherence was 62% for MenB-4C and 18% for MenB-FHbp. The median time to completion among those who finished vaccination was 68 days for MenB-4C versus 258 days for MenB-FHbp.
In the Medicaid database, the rate of vaccine completion within 15 months of initiation was 49% for MenB-4C and 31% for MenB-FHbp; dosing schedule adherence was 48% and 8%, respectively. Median time to vaccine completion was 88 days for MenB-4C versus 309 days for MenB-FHbp.
“The observations of improved completion and schedule adherence rates for MenB-4C, compared with MenB-FHbp, were consistent across both the commercial and Medicaid populations, and persisted after adjusting for individual factors in multivariable analyses, suggesting that the results were not skewed by population differences in demographic or other characteristics,” the investigators noted, adding that the significant difference in completion and schedule adherence between vaccines likely reflects the MenB-4C flexible dosing schedule.
The study was funded by GlaxoSmithKline, the maker of MenB-4C, and four coauthors reported being employed by the company. Five coauthors were employed by IBM Watson Health, which conducted the study.
SOURCE: Packnett E et al. Vaccine. 2019 Aug 20. doi: 10.1016/j.vaccine.2019.06.065.
While meningococcal group B (MenB) vaccination remains suboptimal in the United States, completion was significantly higher for the MenB-4C (Bexsero) vaccine, compared with the other vaccine option, MenB-FHbp (Trumenba), according to Elizabeth Packnett of IBM Watson Health in Bethesda, Md., and associates.
In a study published in Vaccine, the investigators retrospectively analyzed 65,205 (36,118 received MenB-4C; 29,087 received MenB-FHbp) commercially insured individuals from the MarketScan Commercial Claims and Encounters Database during Jan. 1, 2015–Feb. 28, 2018, as well as 13,535 (10,153 received MenB-4C; 3,382 received MenB-FHbp) Medicaid-covered individuals from the Medicaid Multi-State Database during Jan. 1, 2015–Dec. 31, 2017.
The rate of vaccine completion in the MarketScan database within 15 months of initiation was 63% for MenB-4C and 52% for MenB-FHbp, and dosing schedule adherence was 62% for MenB-4C and 18% for MenB-FHbp. The median time to completion among those who finished vaccination was 68 days for MenB-4C versus 258 days for MenB-FHbp.
In the Medicaid database, the rate of vaccine completion within 15 months of initiation was 49% for MenB-4C and 31% for MenB-FHbp; dosing schedule adherence was 48% and 8%, respectively. Median time to vaccine completion was 88 days for MenB-4C versus 309 days for MenB-FHbp.
“The observations of improved completion and schedule adherence rates for MenB-4C, compared with MenB-FHbp, were consistent across both the commercial and Medicaid populations, and persisted after adjusting for individual factors in multivariable analyses, suggesting that the results were not skewed by population differences in demographic or other characteristics,” the investigators noted, adding that the significant difference in completion and schedule adherence between vaccines likely reflects the MenB-4C flexible dosing schedule.
The study was funded by GlaxoSmithKline, the maker of MenB-4C, and four coauthors reported being employed by the company. Five coauthors were employed by IBM Watson Health, which conducted the study.
SOURCE: Packnett E et al. Vaccine. 2019 Aug 20. doi: 10.1016/j.vaccine.2019.06.065.
FROM VACCINE
AAN guideline encourages vaccinations for MS patients
according to an American Academy of Neurology practice guideline.
A summary of the guideline on vaccine-preventable infections and immunization in MS was published online Aug. 28 in Neurology. The new effort updates a 2002 guideline on this topic and incorporates new evidence, vaccines, and disease-modifying therapies (DMTs). The guideline was endorsed by the Consortium of Multiple Sclerosis Centers and by the Multiple Sclerosis Association of America.
To create the guideline, lead author Mauricio F. Farez, MD, of the Raúl Carrea Institute for Neurological Research (FLENI) in Buenos Aires and colleagues on the 17-member guideline panel performed a systematic review of the evidence and reached consensus on recommendations using a modified Delphi voting process. The review included randomized, controlled trials; cohort studies; and case-control studies published between 1990 and March 2018.
“Immunosuppressive or immunomodulating agents used to treat MS may suppress or modulate normal immune function. These drugs may increase susceptibility to infections and may reduce vaccine effectiveness because of a decreased ability to mount an immune response,” the authors said.
Based on its review of the evidence, principles of care, and inferences, the authors made the following eight recommendations:
- Clinicians should discuss with patients the evidence regarding immunization in MS (Level B). In addition, clinicians should examine patients’ opinions, preferences, and questions regarding immunizations (Level B).
- Clinicians should recommend that patients with MS follow all local vaccine standards in the absence of specific contraindications (Level B).
- Clinicians should consider local risks of vaccine-preventable diseases when counseling patients (Level B).
- Clinicians should recommend that patients with MS receive the influenza vaccination if there is no specific contraindication, such as a previous severe reaction (Level B).
- When treatment with an immunosuppressive or immunomodulating agent is considered, clinicians should counsel patients about infection risks associated with the specific medication and the treatment-specific vaccination guidance in the medication’s prescribing instructions (Level B). In addition, physicians should assess patients’ vaccination status before prescribing immunosuppressive or immunomodulating therapy and vaccinate patients according to local regulatory standards and treatment-specific infectious risks at least 4-6 weeks before initiating therapy, as advised by the prescribing information (Level B). Furthermore, clinicians may discuss the advantages of vaccination soon after MS diagnosis, regardless of initial therapeutic plans, to prevent delays should immunosuppressive or immunomodulating therapies be initiated in the future (Level C, based on variation in patient preferences).
- Clinicians must screen for certain infections (such as hepatitis, tuberculosis, and varicella zoster virus) according to a medication’s prescribing information before starting immunosuppressive or immunomodulating treatment (Level A) and should treat patients who have latent infections before MS treatment according to the medication prescribing information (Level B, based on feasibility and cost relative to benefit). Further, in high-risk populations or in countries with a high burden of infectious disease, clinicians must screen for latent infections before starting immunosuppressive or immunomodulating medications, even when such screening is not specifically mentioned in the prescribing information (Level A). Clinicians should consult infectious disease or other specialists about treating patients with latent infection before starting immunosuppressive or immunomodulating medications (Level B).
- Clinicians should recommend against live-attenuated vaccines in people with MS who receive immunosuppressive or immunomodulating therapies or have recently discontinued these therapies (Level B, based on importance of outcomes). When the risk of infection is high, clinicians may recommend live-attenuated vaccines if killed vaccines are unavailable (Level C, based on variation in patient preferences, benefit relative to harm, and importance of outcomes).
- If a patient with MS is experiencing a relapse, clinicians should delay vaccination until the relapse has clinically resolved or is no longer active, often many weeks after relapse onset (Level B).
Personal and population-level benefits
“There is no evidence that vaccination increases the risk of MS exacerbation, although the literature is sparse,” the authors said. “In addition to conferring personal benefits, vaccination of the MS patient population contributes to the well-established phenomenon of herd immunity for the communities in which patients with MS live,” the authors wrote.
Because influenza infection has known risks of exacerbation and morbidity, whereas influenza vaccine has no identified risks of exacerbation, “benefits of influenza vaccination outweigh the risks in most scenarios, although patients with MS receiving some [immunosuppressive or immunomodulating] treatments (fingolimod [Gilenya], glatiramer acetate [Copaxone], and mitoxantrone) may have a reduced response to influenza vaccination,” the authors said. Studies in patients with diseases other than MS suggest that rituximab (Rituxan) also may be associated with reduced influenza vaccine responsiveness.
Immunosuppressive or immunomodulatory medications including alemtuzumab (Lemtrada), dimethyl fumarate (Tecfidera), fingolimod, mitoxantrone, natalizumab (Tysabri), ocrelizumab (Ocrevus), rituximab, and teriflunomide (Aubagio) have been associated with severe occurrences or recurrences of vaccine-preventable infections, and many package inserts approved by the Food and Drug Administration provide guidance regarding immunization with live vaccines and treatment.
Prescribing information for alemtuzumab, fingolimod, ocrelizumab, and teriflunomide recommends against the use of live vaccines during and immediately preceding treatment. Furthermore, the prescribing information recommends waiting 2-6 months after treatment to immunize with live vaccines, depending on the half-life of the specific therapy.
“The guideline panel identified no evidence that vaccines increase the risk of relapse or worsen relapse severity, but studies are limited,” Dr. Farez and colleagues wrote. “Experts remain concerned that vaccines may worsen relapse severity if given to patients who are actively experiencing an MS relapse.” In addition, use of glucocorticoids may raise concerns about the safety of live-virus vaccines. “Immunization is not typically an urgent need and, in most cases, can be temporarily delayed without a marked increase in infection risk,” the guideline says.
Few high-quality studies
Data were lacking or insufficient to assess whether most vaccine-preventable diseases increase the risk of MS exacerbations. “It is probable that individuals with active MS exacerbations have higher odds of varicella zoster virus viral DNA present in peripheral blood mononuclear cells than individuals with MS in remission,” the guideline says.
Human papillomavirus, pertussis, and tetanus toxoid vaccinations probably are associated with a lower likelihood of a subsequent MS diagnosis, and smallpox vaccination is possibly associated with a lower likelihood of a subsequent MS diagnosis, the review found.
Studies included in the systematic review did not address whether live-attenuated vaccines are as effective in patients with MS as they are in the general population. With regard to the effectiveness of inactivated vaccines, patients with MS possibly are less likely to have a sufficient response to influenza vaccination, compared with controls.
The systematic review “found few high-quality studies to inform recommendations,” the authors said. “As more [immunosuppressive or immunomodulating] agents are developed to manage chronic diseases such as MS, long-term prospective cohort studies are required to evaluate both the safety and effectiveness of immunizations in MS.”
Dr. Farez has received funding for travel from Teva Argentina, Novartis Argentina, and Merck Serono Argentina and has received research support from Biogen. Coauthors’ disclosures included financial ties to pharmaceutical companies.
SOURCE: Farez M et al. Neurology. 2019 Aug 28. doi: 10.1212/WNL.0000000000008157.
according to an American Academy of Neurology practice guideline.
A summary of the guideline on vaccine-preventable infections and immunization in MS was published online Aug. 28 in Neurology. The new effort updates a 2002 guideline on this topic and incorporates new evidence, vaccines, and disease-modifying therapies (DMTs). The guideline was endorsed by the Consortium of Multiple Sclerosis Centers and by the Multiple Sclerosis Association of America.
To create the guideline, lead author Mauricio F. Farez, MD, of the Raúl Carrea Institute for Neurological Research (FLENI) in Buenos Aires and colleagues on the 17-member guideline panel performed a systematic review of the evidence and reached consensus on recommendations using a modified Delphi voting process. The review included randomized, controlled trials; cohort studies; and case-control studies published between 1990 and March 2018.
“Immunosuppressive or immunomodulating agents used to treat MS may suppress or modulate normal immune function. These drugs may increase susceptibility to infections and may reduce vaccine effectiveness because of a decreased ability to mount an immune response,” the authors said.
Based on its review of the evidence, principles of care, and inferences, the authors made the following eight recommendations:
- Clinicians should discuss with patients the evidence regarding immunization in MS (Level B). In addition, clinicians should examine patients’ opinions, preferences, and questions regarding immunizations (Level B).
- Clinicians should recommend that patients with MS follow all local vaccine standards in the absence of specific contraindications (Level B).
- Clinicians should consider local risks of vaccine-preventable diseases when counseling patients (Level B).
- Clinicians should recommend that patients with MS receive the influenza vaccination if there is no specific contraindication, such as a previous severe reaction (Level B).
- When treatment with an immunosuppressive or immunomodulating agent is considered, clinicians should counsel patients about infection risks associated with the specific medication and the treatment-specific vaccination guidance in the medication’s prescribing instructions (Level B). In addition, physicians should assess patients’ vaccination status before prescribing immunosuppressive or immunomodulating therapy and vaccinate patients according to local regulatory standards and treatment-specific infectious risks at least 4-6 weeks before initiating therapy, as advised by the prescribing information (Level B). Furthermore, clinicians may discuss the advantages of vaccination soon after MS diagnosis, regardless of initial therapeutic plans, to prevent delays should immunosuppressive or immunomodulating therapies be initiated in the future (Level C, based on variation in patient preferences).
- Clinicians must screen for certain infections (such as hepatitis, tuberculosis, and varicella zoster virus) according to a medication’s prescribing information before starting immunosuppressive or immunomodulating treatment (Level A) and should treat patients who have latent infections before MS treatment according to the medication prescribing information (Level B, based on feasibility and cost relative to benefit). Further, in high-risk populations or in countries with a high burden of infectious disease, clinicians must screen for latent infections before starting immunosuppressive or immunomodulating medications, even when such screening is not specifically mentioned in the prescribing information (Level A). Clinicians should consult infectious disease or other specialists about treating patients with latent infection before starting immunosuppressive or immunomodulating medications (Level B).
- Clinicians should recommend against live-attenuated vaccines in people with MS who receive immunosuppressive or immunomodulating therapies or have recently discontinued these therapies (Level B, based on importance of outcomes). When the risk of infection is high, clinicians may recommend live-attenuated vaccines if killed vaccines are unavailable (Level C, based on variation in patient preferences, benefit relative to harm, and importance of outcomes).
- If a patient with MS is experiencing a relapse, clinicians should delay vaccination until the relapse has clinically resolved or is no longer active, often many weeks after relapse onset (Level B).
Personal and population-level benefits
“There is no evidence that vaccination increases the risk of MS exacerbation, although the literature is sparse,” the authors said. “In addition to conferring personal benefits, vaccination of the MS patient population contributes to the well-established phenomenon of herd immunity for the communities in which patients with MS live,” the authors wrote.
Because influenza infection has known risks of exacerbation and morbidity, whereas influenza vaccine has no identified risks of exacerbation, “benefits of influenza vaccination outweigh the risks in most scenarios, although patients with MS receiving some [immunosuppressive or immunomodulating] treatments (fingolimod [Gilenya], glatiramer acetate [Copaxone], and mitoxantrone) may have a reduced response to influenza vaccination,” the authors said. Studies in patients with diseases other than MS suggest that rituximab (Rituxan) also may be associated with reduced influenza vaccine responsiveness.
Immunosuppressive or immunomodulatory medications including alemtuzumab (Lemtrada), dimethyl fumarate (Tecfidera), fingolimod, mitoxantrone, natalizumab (Tysabri), ocrelizumab (Ocrevus), rituximab, and teriflunomide (Aubagio) have been associated with severe occurrences or recurrences of vaccine-preventable infections, and many package inserts approved by the Food and Drug Administration provide guidance regarding immunization with live vaccines and treatment.
Prescribing information for alemtuzumab, fingolimod, ocrelizumab, and teriflunomide recommends against the use of live vaccines during and immediately preceding treatment. Furthermore, the prescribing information recommends waiting 2-6 months after treatment to immunize with live vaccines, depending on the half-life of the specific therapy.
“The guideline panel identified no evidence that vaccines increase the risk of relapse or worsen relapse severity, but studies are limited,” Dr. Farez and colleagues wrote. “Experts remain concerned that vaccines may worsen relapse severity if given to patients who are actively experiencing an MS relapse.” In addition, use of glucocorticoids may raise concerns about the safety of live-virus vaccines. “Immunization is not typically an urgent need and, in most cases, can be temporarily delayed without a marked increase in infection risk,” the guideline says.
Few high-quality studies
Data were lacking or insufficient to assess whether most vaccine-preventable diseases increase the risk of MS exacerbations. “It is probable that individuals with active MS exacerbations have higher odds of varicella zoster virus viral DNA present in peripheral blood mononuclear cells than individuals with MS in remission,” the guideline says.
Human papillomavirus, pertussis, and tetanus toxoid vaccinations probably are associated with a lower likelihood of a subsequent MS diagnosis, and smallpox vaccination is possibly associated with a lower likelihood of a subsequent MS diagnosis, the review found.
Studies included in the systematic review did not address whether live-attenuated vaccines are as effective in patients with MS as they are in the general population. With regard to the effectiveness of inactivated vaccines, patients with MS possibly are less likely to have a sufficient response to influenza vaccination, compared with controls.
The systematic review “found few high-quality studies to inform recommendations,” the authors said. “As more [immunosuppressive or immunomodulating] agents are developed to manage chronic diseases such as MS, long-term prospective cohort studies are required to evaluate both the safety and effectiveness of immunizations in MS.”
Dr. Farez has received funding for travel from Teva Argentina, Novartis Argentina, and Merck Serono Argentina and has received research support from Biogen. Coauthors’ disclosures included financial ties to pharmaceutical companies.
SOURCE: Farez M et al. Neurology. 2019 Aug 28. doi: 10.1212/WNL.0000000000008157.
according to an American Academy of Neurology practice guideline.
A summary of the guideline on vaccine-preventable infections and immunization in MS was published online Aug. 28 in Neurology. The new effort updates a 2002 guideline on this topic and incorporates new evidence, vaccines, and disease-modifying therapies (DMTs). The guideline was endorsed by the Consortium of Multiple Sclerosis Centers and by the Multiple Sclerosis Association of America.
To create the guideline, lead author Mauricio F. Farez, MD, of the Raúl Carrea Institute for Neurological Research (FLENI) in Buenos Aires and colleagues on the 17-member guideline panel performed a systematic review of the evidence and reached consensus on recommendations using a modified Delphi voting process. The review included randomized, controlled trials; cohort studies; and case-control studies published between 1990 and March 2018.
“Immunosuppressive or immunomodulating agents used to treat MS may suppress or modulate normal immune function. These drugs may increase susceptibility to infections and may reduce vaccine effectiveness because of a decreased ability to mount an immune response,” the authors said.
Based on its review of the evidence, principles of care, and inferences, the authors made the following eight recommendations:
- Clinicians should discuss with patients the evidence regarding immunization in MS (Level B). In addition, clinicians should examine patients’ opinions, preferences, and questions regarding immunizations (Level B).
- Clinicians should recommend that patients with MS follow all local vaccine standards in the absence of specific contraindications (Level B).
- Clinicians should consider local risks of vaccine-preventable diseases when counseling patients (Level B).
- Clinicians should recommend that patients with MS receive the influenza vaccination if there is no specific contraindication, such as a previous severe reaction (Level B).
- When treatment with an immunosuppressive or immunomodulating agent is considered, clinicians should counsel patients about infection risks associated with the specific medication and the treatment-specific vaccination guidance in the medication’s prescribing instructions (Level B). In addition, physicians should assess patients’ vaccination status before prescribing immunosuppressive or immunomodulating therapy and vaccinate patients according to local regulatory standards and treatment-specific infectious risks at least 4-6 weeks before initiating therapy, as advised by the prescribing information (Level B). Furthermore, clinicians may discuss the advantages of vaccination soon after MS diagnosis, regardless of initial therapeutic plans, to prevent delays should immunosuppressive or immunomodulating therapies be initiated in the future (Level C, based on variation in patient preferences).
- Clinicians must screen for certain infections (such as hepatitis, tuberculosis, and varicella zoster virus) according to a medication’s prescribing information before starting immunosuppressive or immunomodulating treatment (Level A) and should treat patients who have latent infections before MS treatment according to the medication prescribing information (Level B, based on feasibility and cost relative to benefit). Further, in high-risk populations or in countries with a high burden of infectious disease, clinicians must screen for latent infections before starting immunosuppressive or immunomodulating medications, even when such screening is not specifically mentioned in the prescribing information (Level A). Clinicians should consult infectious disease or other specialists about treating patients with latent infection before starting immunosuppressive or immunomodulating medications (Level B).
- Clinicians should recommend against live-attenuated vaccines in people with MS who receive immunosuppressive or immunomodulating therapies or have recently discontinued these therapies (Level B, based on importance of outcomes). When the risk of infection is high, clinicians may recommend live-attenuated vaccines if killed vaccines are unavailable (Level C, based on variation in patient preferences, benefit relative to harm, and importance of outcomes).
- If a patient with MS is experiencing a relapse, clinicians should delay vaccination until the relapse has clinically resolved or is no longer active, often many weeks after relapse onset (Level B).
Personal and population-level benefits
“There is no evidence that vaccination increases the risk of MS exacerbation, although the literature is sparse,” the authors said. “In addition to conferring personal benefits, vaccination of the MS patient population contributes to the well-established phenomenon of herd immunity for the communities in which patients with MS live,” the authors wrote.
Because influenza infection has known risks of exacerbation and morbidity, whereas influenza vaccine has no identified risks of exacerbation, “benefits of influenza vaccination outweigh the risks in most scenarios, although patients with MS receiving some [immunosuppressive or immunomodulating] treatments (fingolimod [Gilenya], glatiramer acetate [Copaxone], and mitoxantrone) may have a reduced response to influenza vaccination,” the authors said. Studies in patients with diseases other than MS suggest that rituximab (Rituxan) also may be associated with reduced influenza vaccine responsiveness.
Immunosuppressive or immunomodulatory medications including alemtuzumab (Lemtrada), dimethyl fumarate (Tecfidera), fingolimod, mitoxantrone, natalizumab (Tysabri), ocrelizumab (Ocrevus), rituximab, and teriflunomide (Aubagio) have been associated with severe occurrences or recurrences of vaccine-preventable infections, and many package inserts approved by the Food and Drug Administration provide guidance regarding immunization with live vaccines and treatment.
Prescribing information for alemtuzumab, fingolimod, ocrelizumab, and teriflunomide recommends against the use of live vaccines during and immediately preceding treatment. Furthermore, the prescribing information recommends waiting 2-6 months after treatment to immunize with live vaccines, depending on the half-life of the specific therapy.
“The guideline panel identified no evidence that vaccines increase the risk of relapse or worsen relapse severity, but studies are limited,” Dr. Farez and colleagues wrote. “Experts remain concerned that vaccines may worsen relapse severity if given to patients who are actively experiencing an MS relapse.” In addition, use of glucocorticoids may raise concerns about the safety of live-virus vaccines. “Immunization is not typically an urgent need and, in most cases, can be temporarily delayed without a marked increase in infection risk,” the guideline says.
Few high-quality studies
Data were lacking or insufficient to assess whether most vaccine-preventable diseases increase the risk of MS exacerbations. “It is probable that individuals with active MS exacerbations have higher odds of varicella zoster virus viral DNA present in peripheral blood mononuclear cells than individuals with MS in remission,” the guideline says.
Human papillomavirus, pertussis, and tetanus toxoid vaccinations probably are associated with a lower likelihood of a subsequent MS diagnosis, and smallpox vaccination is possibly associated with a lower likelihood of a subsequent MS diagnosis, the review found.
Studies included in the systematic review did not address whether live-attenuated vaccines are as effective in patients with MS as they are in the general population. With regard to the effectiveness of inactivated vaccines, patients with MS possibly are less likely to have a sufficient response to influenza vaccination, compared with controls.
The systematic review “found few high-quality studies to inform recommendations,” the authors said. “As more [immunosuppressive or immunomodulating] agents are developed to manage chronic diseases such as MS, long-term prospective cohort studies are required to evaluate both the safety and effectiveness of immunizations in MS.”
Dr. Farez has received funding for travel from Teva Argentina, Novartis Argentina, and Merck Serono Argentina and has received research support from Biogen. Coauthors’ disclosures included financial ties to pharmaceutical companies.
SOURCE: Farez M et al. Neurology. 2019 Aug 28. doi: 10.1212/WNL.0000000000008157.
FROM NEUROLOGY