User login
U.S. travelers to Europe need up to date measles immunization
researchers at the Centers for Disease Control and Prevention recommend in a Pediatrics special report.
More than 41,000 measles cases and 37 deaths – primarily due to low immunization coverage – were reported in the World Health Organization European Region in the first 6 months of 2018, the highest incidence since the 1990s. Typical case counts since 2010 have ranged from 5,000 to 24,000 in this region, wrote Kristina M. Angelo, DO, MPH, of the Centers for Disease Control and Prevention Travelers’ Health Branch in Atlanta, and associates.
France, Italy and Greece – all particularly popular countries for U.S. vacationers to visit – have particularly high numbers of cases, as do Georgia, Russia, Serbia and, comprising the majority of cases, Ukraine. Italy, for example, is the 10th most popular destination worldwide for Americans, with an estimated 2.5 million American visitors in 2015.
“The large number of measles infections in the WHO European Region ... is a global concern because the European continent is the most common travel destination worldwide,” but is not perceived as a place with infectious disease risk. So travelers may not consider the need of a pretravel health consultation, including vaccination, they said.
But they need to, Dr. Angelo and associates state, and health care providers should be vigilant about checking for symptoms of measles among those who have recently returned from overseas. Given how highly contagious measles is, unvaccinated and under vaccinated travelers to Europe are susceptible to infection, as are any people they encounter back in the United States if the travelers come home sick.
Measles was eliminated in the United States in 2000, but that status is in jeopardy, CDC officials recently warned. The number of domestic measles cases has exceeded 1,000 just halfway through 2019, the highest count since 1992, nearly a decade before elimination.
“Avoiding international travel with nonimmune infants and performing early vaccination at 6 to 12 months of age per the ACIP [Advisory Committee on Immunization Practices] recommendations if travel is unavoidable are of utmost importance,” Dr. Angelo and colleagues advised. “Other at-risk populations (e.g., immunocompromised individuals and pregnant women), for whom vaccination against the measles virus is contraindicated, may consider alternative destinations or delay travel to measles-endemic destinations or areas with known, ongoing measles outbreaks.”
“Presumptive immunity to measles is defined as 1 or more of the following: birth before 1957, laboratory evidence of immunity or infection, 1 or more doses of a measles containing vaccine administered for preschool-aged children and low-risk adults, or 2 doses of measles vaccine among school-aged children and high-risk adults, including international travelers,” they explained.
In Europe, measles remains endemic in Belgium, Bosnia and Herzegovina, France, Georgia, Germany, Italy, Romania, the Russian Federation, Serbia and the Ukraine, the authors wrote.
“As long as measles remains endemic in other countries, the United States will be challenged by measles importations,” the authors wrote. Yet at least one past study in 2017 revealed a third of U.S. travelers to Europe left the country without being fully vaccinated against measles, most often due to vaccine refusal.
“The reason one-third of travelers to Europe missed an opportunity for measles vaccination remains unclear,” the authors wrote. “It may represent a lack of concern or awareness on the part of travelers and the health care providers about acquiring measles in Europe.”
Dr. Angelo and colleagues also emphasized the importance of returning U.S. travelers seeking health care if they have symptoms of measles, including fever and a rash.
Health care providers should ask all patients about recent international travel, they stated. “If measles is suspected, health care providers should isolate travelers immediately, placing them on airborne precautions until day 4 of the rash.” Providers may consider administering immunoglobulin for unvaccinated and undervaccinated travelers and monitor them for 21 days for development of measles symptoms.
The statement was funded by the CDC. The authors reported no relevant financial disclosures.
SOURCE: Angelo KM et al. Pediatrics. 2019 Jun 17. doi: /10.1542/peds.2019-0414.
researchers at the Centers for Disease Control and Prevention recommend in a Pediatrics special report.
More than 41,000 measles cases and 37 deaths – primarily due to low immunization coverage – were reported in the World Health Organization European Region in the first 6 months of 2018, the highest incidence since the 1990s. Typical case counts since 2010 have ranged from 5,000 to 24,000 in this region, wrote Kristina M. Angelo, DO, MPH, of the Centers for Disease Control and Prevention Travelers’ Health Branch in Atlanta, and associates.
France, Italy and Greece – all particularly popular countries for U.S. vacationers to visit – have particularly high numbers of cases, as do Georgia, Russia, Serbia and, comprising the majority of cases, Ukraine. Italy, for example, is the 10th most popular destination worldwide for Americans, with an estimated 2.5 million American visitors in 2015.
“The large number of measles infections in the WHO European Region ... is a global concern because the European continent is the most common travel destination worldwide,” but is not perceived as a place with infectious disease risk. So travelers may not consider the need of a pretravel health consultation, including vaccination, they said.
But they need to, Dr. Angelo and associates state, and health care providers should be vigilant about checking for symptoms of measles among those who have recently returned from overseas. Given how highly contagious measles is, unvaccinated and under vaccinated travelers to Europe are susceptible to infection, as are any people they encounter back in the United States if the travelers come home sick.
Measles was eliminated in the United States in 2000, but that status is in jeopardy, CDC officials recently warned. The number of domestic measles cases has exceeded 1,000 just halfway through 2019, the highest count since 1992, nearly a decade before elimination.
“Avoiding international travel with nonimmune infants and performing early vaccination at 6 to 12 months of age per the ACIP [Advisory Committee on Immunization Practices] recommendations if travel is unavoidable are of utmost importance,” Dr. Angelo and colleagues advised. “Other at-risk populations (e.g., immunocompromised individuals and pregnant women), for whom vaccination against the measles virus is contraindicated, may consider alternative destinations or delay travel to measles-endemic destinations or areas with known, ongoing measles outbreaks.”
“Presumptive immunity to measles is defined as 1 or more of the following: birth before 1957, laboratory evidence of immunity or infection, 1 or more doses of a measles containing vaccine administered for preschool-aged children and low-risk adults, or 2 doses of measles vaccine among school-aged children and high-risk adults, including international travelers,” they explained.
In Europe, measles remains endemic in Belgium, Bosnia and Herzegovina, France, Georgia, Germany, Italy, Romania, the Russian Federation, Serbia and the Ukraine, the authors wrote.
“As long as measles remains endemic in other countries, the United States will be challenged by measles importations,” the authors wrote. Yet at least one past study in 2017 revealed a third of U.S. travelers to Europe left the country without being fully vaccinated against measles, most often due to vaccine refusal.
“The reason one-third of travelers to Europe missed an opportunity for measles vaccination remains unclear,” the authors wrote. “It may represent a lack of concern or awareness on the part of travelers and the health care providers about acquiring measles in Europe.”
Dr. Angelo and colleagues also emphasized the importance of returning U.S. travelers seeking health care if they have symptoms of measles, including fever and a rash.
Health care providers should ask all patients about recent international travel, they stated. “If measles is suspected, health care providers should isolate travelers immediately, placing them on airborne precautions until day 4 of the rash.” Providers may consider administering immunoglobulin for unvaccinated and undervaccinated travelers and monitor them for 21 days for development of measles symptoms.
The statement was funded by the CDC. The authors reported no relevant financial disclosures.
SOURCE: Angelo KM et al. Pediatrics. 2019 Jun 17. doi: /10.1542/peds.2019-0414.
researchers at the Centers for Disease Control and Prevention recommend in a Pediatrics special report.
More than 41,000 measles cases and 37 deaths – primarily due to low immunization coverage – were reported in the World Health Organization European Region in the first 6 months of 2018, the highest incidence since the 1990s. Typical case counts since 2010 have ranged from 5,000 to 24,000 in this region, wrote Kristina M. Angelo, DO, MPH, of the Centers for Disease Control and Prevention Travelers’ Health Branch in Atlanta, and associates.
France, Italy and Greece – all particularly popular countries for U.S. vacationers to visit – have particularly high numbers of cases, as do Georgia, Russia, Serbia and, comprising the majority of cases, Ukraine. Italy, for example, is the 10th most popular destination worldwide for Americans, with an estimated 2.5 million American visitors in 2015.
“The large number of measles infections in the WHO European Region ... is a global concern because the European continent is the most common travel destination worldwide,” but is not perceived as a place with infectious disease risk. So travelers may not consider the need of a pretravel health consultation, including vaccination, they said.
But they need to, Dr. Angelo and associates state, and health care providers should be vigilant about checking for symptoms of measles among those who have recently returned from overseas. Given how highly contagious measles is, unvaccinated and under vaccinated travelers to Europe are susceptible to infection, as are any people they encounter back in the United States if the travelers come home sick.
Measles was eliminated in the United States in 2000, but that status is in jeopardy, CDC officials recently warned. The number of domestic measles cases has exceeded 1,000 just halfway through 2019, the highest count since 1992, nearly a decade before elimination.
“Avoiding international travel with nonimmune infants and performing early vaccination at 6 to 12 months of age per the ACIP [Advisory Committee on Immunization Practices] recommendations if travel is unavoidable are of utmost importance,” Dr. Angelo and colleagues advised. “Other at-risk populations (e.g., immunocompromised individuals and pregnant women), for whom vaccination against the measles virus is contraindicated, may consider alternative destinations or delay travel to measles-endemic destinations or areas with known, ongoing measles outbreaks.”
“Presumptive immunity to measles is defined as 1 or more of the following: birth before 1957, laboratory evidence of immunity or infection, 1 or more doses of a measles containing vaccine administered for preschool-aged children and low-risk adults, or 2 doses of measles vaccine among school-aged children and high-risk adults, including international travelers,” they explained.
In Europe, measles remains endemic in Belgium, Bosnia and Herzegovina, France, Georgia, Germany, Italy, Romania, the Russian Federation, Serbia and the Ukraine, the authors wrote.
“As long as measles remains endemic in other countries, the United States will be challenged by measles importations,” the authors wrote. Yet at least one past study in 2017 revealed a third of U.S. travelers to Europe left the country without being fully vaccinated against measles, most often due to vaccine refusal.
“The reason one-third of travelers to Europe missed an opportunity for measles vaccination remains unclear,” the authors wrote. “It may represent a lack of concern or awareness on the part of travelers and the health care providers about acquiring measles in Europe.”
Dr. Angelo and colleagues also emphasized the importance of returning U.S. travelers seeking health care if they have symptoms of measles, including fever and a rash.
Health care providers should ask all patients about recent international travel, they stated. “If measles is suspected, health care providers should isolate travelers immediately, placing them on airborne precautions until day 4 of the rash.” Providers may consider administering immunoglobulin for unvaccinated and undervaccinated travelers and monitor them for 21 days for development of measles symptoms.
The statement was funded by the CDC. The authors reported no relevant financial disclosures.
SOURCE: Angelo KM et al. Pediatrics. 2019 Jun 17. doi: /10.1542/peds.2019-0414.
FROM PEDIATRICS
Booster vaccines found largely safe in children on immunosuppressive drugs
MADRID – Administration of live attenuated booster of the MMR vaccine with or without varicella (MMR/V) was not associated with serious adverse events in children on immunosuppressive therapy for a rheumatic disease, according to data presented at the European Congress of Rheumatology.
“The study implies that patients can receive booster vaccinations regardless of age, diagnosis, or therapy,” reported Veronica Bergonzo Moshe, MD, a pediatric rheumatologist at Meir Medical Center, Kfar Saba, Israel.
In the absence of safety data, the vaccination of children with rheumatic diseases taking immunosuppressive therapies has been controversial. Although these children face communicable and sometimes life-threatening diseases without vaccination, many clinicians are not offering this protection because they fear adverse consequences.
Current Paediatric Rheumatology European Society (PReS) guidelines have been equivocal, recommending that vaccines be considered on a “case-by-case basis” in children with a rheumatic disease if they are taking high doses of disease-modifying antirheumatic drugs (DMARDs), glucocorticoids, or any dose of biologics.
“The fear is that a state of immune suppression might decrease response to the vaccine or lead to a flare of the rheumatologic disease,” Dr. Moshe said.
In the retrospective study presented by Dr. Moshe, data were collected on 234 children with rheumatic diseases who received a live attenuated MMR/V booster. The children were drawn from 12 pediatric rheumatology centers in 10 countries.
In this relatively large series, 82% of the children had oligoarticular or polyarticular juvenile idiopathic arthritis (JIA). A range of other rheumatic diseases, including juvenile dermatomyositis, localized scleroderma, and isolated idiopathic uveitis were represented among the remaining patients. All were taking medication, and 48% were in remission.
When broken down by therapy, there were three localized reactions in 110 (2.7%) children who received the booster while on methotrexate. No other adverse events, including disease flare, were observed.
Similarly, six of the seven adverse events observed in 76 (8%) patients who were taking methotrexate plus a tumor necrosis factor (TNF) inhibitor biologic at the time of vaccination were local reactions. Fever was reported in one patient. All of these events were transient.
In the 39 patients taking a TNF inhibitor alone, there was a single case of transient fever. There were no adverse events reported in the three patients vaccinated while on tocilizumab, seven patients while on anakinra, or five patients while on canakinumab.
Following vaccination, there were no signs of symptoms of the diseases that the vaccines are designed to prevent. In the minority of patients who did develop localized reactions or fever in this series, there was no apparent relationship with disease activity, age, or sex when compared to those who did not develop an adverse event.
These retrospective data are not definitive, but they are reassuring, according to Dr. Moshe. A larger prospective study by the PReS vaccination study group is now planned. The issue of leaving children unvaccinated is topical due to the recent outbreaks of measles in the United States.
“We must have clear guidelines on how to deal with the administration of live vaccines in this patient population so that we can provide the safest and most effective practice,” Dr. Moshe said.
These data are a first step.
“This large retrospective study demonstrates that live attenuated booster vaccine is probably safe in children with rheumatic diseases,” said Dr. Moshe, but she deferred to the PReS guidelines in suggesting that the decision to vaccinate still might best be performed on a case-by-case basis.
MADRID – Administration of live attenuated booster of the MMR vaccine with or without varicella (MMR/V) was not associated with serious adverse events in children on immunosuppressive therapy for a rheumatic disease, according to data presented at the European Congress of Rheumatology.
“The study implies that patients can receive booster vaccinations regardless of age, diagnosis, or therapy,” reported Veronica Bergonzo Moshe, MD, a pediatric rheumatologist at Meir Medical Center, Kfar Saba, Israel.
In the absence of safety data, the vaccination of children with rheumatic diseases taking immunosuppressive therapies has been controversial. Although these children face communicable and sometimes life-threatening diseases without vaccination, many clinicians are not offering this protection because they fear adverse consequences.
Current Paediatric Rheumatology European Society (PReS) guidelines have been equivocal, recommending that vaccines be considered on a “case-by-case basis” in children with a rheumatic disease if they are taking high doses of disease-modifying antirheumatic drugs (DMARDs), glucocorticoids, or any dose of biologics.
“The fear is that a state of immune suppression might decrease response to the vaccine or lead to a flare of the rheumatologic disease,” Dr. Moshe said.
In the retrospective study presented by Dr. Moshe, data were collected on 234 children with rheumatic diseases who received a live attenuated MMR/V booster. The children were drawn from 12 pediatric rheumatology centers in 10 countries.
In this relatively large series, 82% of the children had oligoarticular or polyarticular juvenile idiopathic arthritis (JIA). A range of other rheumatic diseases, including juvenile dermatomyositis, localized scleroderma, and isolated idiopathic uveitis were represented among the remaining patients. All were taking medication, and 48% were in remission.
When broken down by therapy, there were three localized reactions in 110 (2.7%) children who received the booster while on methotrexate. No other adverse events, including disease flare, were observed.
Similarly, six of the seven adverse events observed in 76 (8%) patients who were taking methotrexate plus a tumor necrosis factor (TNF) inhibitor biologic at the time of vaccination were local reactions. Fever was reported in one patient. All of these events were transient.
In the 39 patients taking a TNF inhibitor alone, there was a single case of transient fever. There were no adverse events reported in the three patients vaccinated while on tocilizumab, seven patients while on anakinra, or five patients while on canakinumab.
Following vaccination, there were no signs of symptoms of the diseases that the vaccines are designed to prevent. In the minority of patients who did develop localized reactions or fever in this series, there was no apparent relationship with disease activity, age, or sex when compared to those who did not develop an adverse event.
These retrospective data are not definitive, but they are reassuring, according to Dr. Moshe. A larger prospective study by the PReS vaccination study group is now planned. The issue of leaving children unvaccinated is topical due to the recent outbreaks of measles in the United States.
“We must have clear guidelines on how to deal with the administration of live vaccines in this patient population so that we can provide the safest and most effective practice,” Dr. Moshe said.
These data are a first step.
“This large retrospective study demonstrates that live attenuated booster vaccine is probably safe in children with rheumatic diseases,” said Dr. Moshe, but she deferred to the PReS guidelines in suggesting that the decision to vaccinate still might best be performed on a case-by-case basis.
MADRID – Administration of live attenuated booster of the MMR vaccine with or without varicella (MMR/V) was not associated with serious adverse events in children on immunosuppressive therapy for a rheumatic disease, according to data presented at the European Congress of Rheumatology.
“The study implies that patients can receive booster vaccinations regardless of age, diagnosis, or therapy,” reported Veronica Bergonzo Moshe, MD, a pediatric rheumatologist at Meir Medical Center, Kfar Saba, Israel.
In the absence of safety data, the vaccination of children with rheumatic diseases taking immunosuppressive therapies has been controversial. Although these children face communicable and sometimes life-threatening diseases without vaccination, many clinicians are not offering this protection because they fear adverse consequences.
Current Paediatric Rheumatology European Society (PReS) guidelines have been equivocal, recommending that vaccines be considered on a “case-by-case basis” in children with a rheumatic disease if they are taking high doses of disease-modifying antirheumatic drugs (DMARDs), glucocorticoids, or any dose of biologics.
“The fear is that a state of immune suppression might decrease response to the vaccine or lead to a flare of the rheumatologic disease,” Dr. Moshe said.
In the retrospective study presented by Dr. Moshe, data were collected on 234 children with rheumatic diseases who received a live attenuated MMR/V booster. The children were drawn from 12 pediatric rheumatology centers in 10 countries.
In this relatively large series, 82% of the children had oligoarticular or polyarticular juvenile idiopathic arthritis (JIA). A range of other rheumatic diseases, including juvenile dermatomyositis, localized scleroderma, and isolated idiopathic uveitis were represented among the remaining patients. All were taking medication, and 48% were in remission.
When broken down by therapy, there were three localized reactions in 110 (2.7%) children who received the booster while on methotrexate. No other adverse events, including disease flare, were observed.
Similarly, six of the seven adverse events observed in 76 (8%) patients who were taking methotrexate plus a tumor necrosis factor (TNF) inhibitor biologic at the time of vaccination were local reactions. Fever was reported in one patient. All of these events were transient.
In the 39 patients taking a TNF inhibitor alone, there was a single case of transient fever. There were no adverse events reported in the three patients vaccinated while on tocilizumab, seven patients while on anakinra, or five patients while on canakinumab.
Following vaccination, there were no signs of symptoms of the diseases that the vaccines are designed to prevent. In the minority of patients who did develop localized reactions or fever in this series, there was no apparent relationship with disease activity, age, or sex when compared to those who did not develop an adverse event.
These retrospective data are not definitive, but they are reassuring, according to Dr. Moshe. A larger prospective study by the PReS vaccination study group is now planned. The issue of leaving children unvaccinated is topical due to the recent outbreaks of measles in the United States.
“We must have clear guidelines on how to deal with the administration of live vaccines in this patient population so that we can provide the safest and most effective practice,” Dr. Moshe said.
These data are a first step.
“This large retrospective study demonstrates that live attenuated booster vaccine is probably safe in children with rheumatic diseases,” said Dr. Moshe, but she deferred to the PReS guidelines in suggesting that the decision to vaccinate still might best be performed on a case-by-case basis.
REPORTING FROM EULAR 2019 CONGRESS
Get patients vaccinated: Avoid unwelcome international travel souvenirs
Summer officially began June 21, 2019, but many of your patients already may have departed or will soon be headed to international destinations. Reasons for travel are as variable as their destinations and include but are not limited to family vacations, mission trips, study abroad, parental job relocation, and visiting friends and relatives. The majority of the trips are planned at least 3 months in advance; however, for many travelers and their parents, they suddenly get an aha moment and realize there is/are specific vaccines required to obtain a visa or entry to their final destination. Unfortunately, too much emphasis is focused on required vaccines. The well-informed traveler knows that they may be exposed to multiple diseases and many are vaccine preventable.
The accompanying table lists vaccines traditionally considered to be travel vaccines. Several require multiple doses administered over 21-28 days to provide protection. Others such as cholera and yellow fever must be completed at least 10 days prior to departure to be effective. Typhoid has two formulations: The oral and injectable typhoid vaccines should be completed 1 and 2 weeks, respectively, prior to travel. Several vaccines have age limitations. Routine immunization of all infants against hepatitis A was recommended in 2006. Depending on your region, there may be adolescents who have not been immunized. Fortunately, hepatitis A vaccine works immediately.
One of the challenges you face is identifying someone in your area that provides travel medicine advice and immunizations to children and adolescents. Most children and teens travel with their parents, but today many adolescents travel independently with organized groups. Most of the vaccines listed are not routinely administered at your office, yet you most likely will be the first call a parent makes seeking travel advice.
Let me tell you about a few vaccines in particular.
Japanese encephalitis
This is most common cause of encephalitis in Asia and parts of the western Pacific. Risk generally is limited to rural agricultural areas where the causative virus is transmitted by a mosquito. Fatality rates are 20%-30%. Among survivors, 30%-50% have significant neurologic, cognitive, and psychiatric sequelae. Candidates for this vaccine are long-term travelers and short-term travelers with extensive outdoor rural activities.
Meningococcal conjugate vaccines (MCV4)
All travelers to the Hajj Pilgrimage (Aug. 9-14, 2019) and/or Umrah must show proof of immunization. Vaccine must be received at least 10 days prior to and no greater than 5 years prior to arrival to Saudi Arabia. Conjugate vaccine must clearly be documented for validity of 5 years. For all health entry requirements, go to www.moh.gov.sa/en/hajj/pages/healthregulations.aspx.
Measles
The Advisory Committee on Immunization Practices recommends all infants 6-11 months old receive one dose of MMR prior to international travel regardless of the destination. This should be followed by two additional countable doses. All persons at least 12 months of age and born after 1956 should receive two doses of MMR at least 28 days apart prior to international travel.
Rabies
Rabies is a viral disease endemic in more than 150 countries with approximately 60,000 fatal cases worldwide each year. Asia and Africa are the areas with the highest risk of exposure, and dogs are the principal hosts. Human rabies is almost always fatal once symptoms develop. Preexposure vaccine is recommended for persons with prolonged and/or remote travel to countries where rabies immunoglobulin is unavailable and the occurrence of animal rabies is high. Post exposure vaccination on days 0 and 3 still would be required.*
Typhoid
A bacterial infection caused by Salmonella enterica serotype Typhi and Paratyphi manifests with fever, headache, abdominal pain, diarrhea, or constipation. When bacteremia occurs, it usually is referred to as enteric fever. It is acquired by consumption of food/water contaminated with human feces. Highest risk areas include Africa, Southern Asia, and Southeast Asia
Yellow fever
Risk is limited to sub-Saharan Africa and the tropical areas of South America. It is transmitted by the bite of an infected mosquito. The vaccine is required for entry into at least 16 countries. In a country where yellow fever is present, persons transiting through for more than 12 hours to reach their final destination may actually cause a change in the entry requirements for the destination country. For example, travel from the United States to Tanzania requires no yellow fever vaccine while travel from the United States to Nairobi (more than 12 hours) to Tanzania requires yellow fever vaccine for entry into Tanzania. Travel sequence and duration is extremely important. Check the Centers for Disease Control and Prevention yellow fever site and/or the consulate for the most up-to-date yellow fever vaccine requirements.
YF-Vax (yellow fever vaccine) produced by Sanofi Pasteur in the United States currently is unavailable. The company is building a new facility, and vaccine will not be available for the remainder of 2019. To assure vaccine for U.S. travelers, Stamaril, a yellow fever vaccine produced by Sanofi Pasteur in France has been made available at more than 250 sites nationwide. Because Stamaril is offered at a limited number of locations, persons in need of vaccine should not delay seeking it. Because of increased demand related to summer travel, travelers in some areas have reported delays of several weeks in scheduling an appointment. To locate a Stamaril site in your area, go to wwwnc.cdc.gov/travel/page/search-for-stamaril-clinics.
There are several other diseases transmitted by mosquitoes and ticks including malaria, dengue, Zika and rickettsial diseases. Vigilant use of mosquito repellents is a must. Prophylactic medication is available for only malaria and should be initiated prior to exposure. Frequency and duration depends on the medication selected.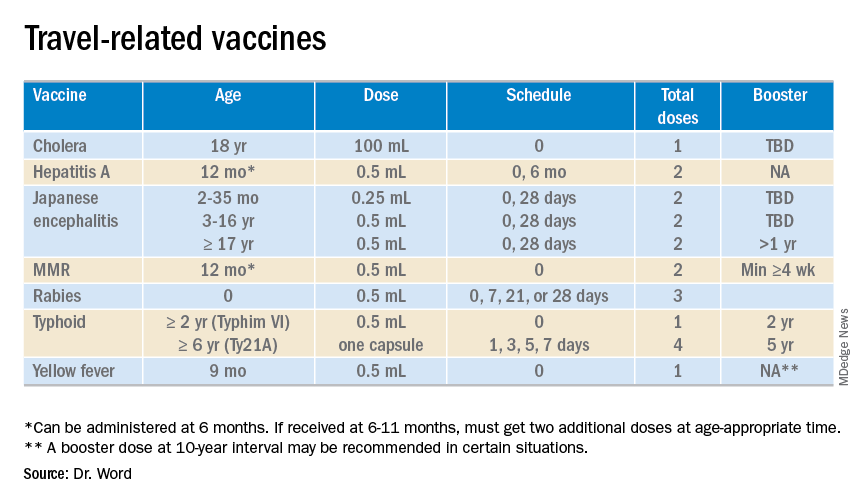
So how do you assist your patients?
Once you’ve identified a travel medicine facility in your area, encourage them to seek pretravel advice 4-6 weeks prior to international travel and make sure their routine immunizations are up to date. Generally, this is not an issue. One challenge is the early administration of MMR. While most practitioners know that early administration for international travel has been recommended for years, many office staff are accustomed to administration at only the 12 month and 4 year visit. When parents call requesting immunization, they often are informed that is it unnecessary and the appointment denied. This is a challenge, especially when coordination of administration of another live vaccine, such as yellow fever, is planned. Familiarizing all members of the health care team with current vaccine recommendations is critical.
For country-specific information, up-to-date travel alerts, and to locate a travel medicine clinic, visit www.cdc.gov/travel.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She had no relevant financial disclosures. Email her at [email protected].
*This article was updated 6/18/2019.
Summer officially began June 21, 2019, but many of your patients already may have departed or will soon be headed to international destinations. Reasons for travel are as variable as their destinations and include but are not limited to family vacations, mission trips, study abroad, parental job relocation, and visiting friends and relatives. The majority of the trips are planned at least 3 months in advance; however, for many travelers and their parents, they suddenly get an aha moment and realize there is/are specific vaccines required to obtain a visa or entry to their final destination. Unfortunately, too much emphasis is focused on required vaccines. The well-informed traveler knows that they may be exposed to multiple diseases and many are vaccine preventable.
The accompanying table lists vaccines traditionally considered to be travel vaccines. Several require multiple doses administered over 21-28 days to provide protection. Others such as cholera and yellow fever must be completed at least 10 days prior to departure to be effective. Typhoid has two formulations: The oral and injectable typhoid vaccines should be completed 1 and 2 weeks, respectively, prior to travel. Several vaccines have age limitations. Routine immunization of all infants against hepatitis A was recommended in 2006. Depending on your region, there may be adolescents who have not been immunized. Fortunately, hepatitis A vaccine works immediately.
One of the challenges you face is identifying someone in your area that provides travel medicine advice and immunizations to children and adolescents. Most children and teens travel with their parents, but today many adolescents travel independently with organized groups. Most of the vaccines listed are not routinely administered at your office, yet you most likely will be the first call a parent makes seeking travel advice.
Let me tell you about a few vaccines in particular.
Japanese encephalitis
This is most common cause of encephalitis in Asia and parts of the western Pacific. Risk generally is limited to rural agricultural areas where the causative virus is transmitted by a mosquito. Fatality rates are 20%-30%. Among survivors, 30%-50% have significant neurologic, cognitive, and psychiatric sequelae. Candidates for this vaccine are long-term travelers and short-term travelers with extensive outdoor rural activities.
Meningococcal conjugate vaccines (MCV4)
All travelers to the Hajj Pilgrimage (Aug. 9-14, 2019) and/or Umrah must show proof of immunization. Vaccine must be received at least 10 days prior to and no greater than 5 years prior to arrival to Saudi Arabia. Conjugate vaccine must clearly be documented for validity of 5 years. For all health entry requirements, go to www.moh.gov.sa/en/hajj/pages/healthregulations.aspx.
Measles
The Advisory Committee on Immunization Practices recommends all infants 6-11 months old receive one dose of MMR prior to international travel regardless of the destination. This should be followed by two additional countable doses. All persons at least 12 months of age and born after 1956 should receive two doses of MMR at least 28 days apart prior to international travel.
Rabies
Rabies is a viral disease endemic in more than 150 countries with approximately 60,000 fatal cases worldwide each year. Asia and Africa are the areas with the highest risk of exposure, and dogs are the principal hosts. Human rabies is almost always fatal once symptoms develop. Preexposure vaccine is recommended for persons with prolonged and/or remote travel to countries where rabies immunoglobulin is unavailable and the occurrence of animal rabies is high. Post exposure vaccination on days 0 and 3 still would be required.*
Typhoid
A bacterial infection caused by Salmonella enterica serotype Typhi and Paratyphi manifests with fever, headache, abdominal pain, diarrhea, or constipation. When bacteremia occurs, it usually is referred to as enteric fever. It is acquired by consumption of food/water contaminated with human feces. Highest risk areas include Africa, Southern Asia, and Southeast Asia
Yellow fever
Risk is limited to sub-Saharan Africa and the tropical areas of South America. It is transmitted by the bite of an infected mosquito. The vaccine is required for entry into at least 16 countries. In a country where yellow fever is present, persons transiting through for more than 12 hours to reach their final destination may actually cause a change in the entry requirements for the destination country. For example, travel from the United States to Tanzania requires no yellow fever vaccine while travel from the United States to Nairobi (more than 12 hours) to Tanzania requires yellow fever vaccine for entry into Tanzania. Travel sequence and duration is extremely important. Check the Centers for Disease Control and Prevention yellow fever site and/or the consulate for the most up-to-date yellow fever vaccine requirements.
YF-Vax (yellow fever vaccine) produced by Sanofi Pasteur in the United States currently is unavailable. The company is building a new facility, and vaccine will not be available for the remainder of 2019. To assure vaccine for U.S. travelers, Stamaril, a yellow fever vaccine produced by Sanofi Pasteur in France has been made available at more than 250 sites nationwide. Because Stamaril is offered at a limited number of locations, persons in need of vaccine should not delay seeking it. Because of increased demand related to summer travel, travelers in some areas have reported delays of several weeks in scheduling an appointment. To locate a Stamaril site in your area, go to wwwnc.cdc.gov/travel/page/search-for-stamaril-clinics.
There are several other diseases transmitted by mosquitoes and ticks including malaria, dengue, Zika and rickettsial diseases. Vigilant use of mosquito repellents is a must. Prophylactic medication is available for only malaria and should be initiated prior to exposure. Frequency and duration depends on the medication selected.
So how do you assist your patients?
Once you’ve identified a travel medicine facility in your area, encourage them to seek pretravel advice 4-6 weeks prior to international travel and make sure their routine immunizations are up to date. Generally, this is not an issue. One challenge is the early administration of MMR. While most practitioners know that early administration for international travel has been recommended for years, many office staff are accustomed to administration at only the 12 month and 4 year visit. When parents call requesting immunization, they often are informed that is it unnecessary and the appointment denied. This is a challenge, especially when coordination of administration of another live vaccine, such as yellow fever, is planned. Familiarizing all members of the health care team with current vaccine recommendations is critical.
For country-specific information, up-to-date travel alerts, and to locate a travel medicine clinic, visit www.cdc.gov/travel.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She had no relevant financial disclosures. Email her at [email protected].
*This article was updated 6/18/2019.
Summer officially began June 21, 2019, but many of your patients already may have departed or will soon be headed to international destinations. Reasons for travel are as variable as their destinations and include but are not limited to family vacations, mission trips, study abroad, parental job relocation, and visiting friends and relatives. The majority of the trips are planned at least 3 months in advance; however, for many travelers and their parents, they suddenly get an aha moment and realize there is/are specific vaccines required to obtain a visa or entry to their final destination. Unfortunately, too much emphasis is focused on required vaccines. The well-informed traveler knows that they may be exposed to multiple diseases and many are vaccine preventable.
The accompanying table lists vaccines traditionally considered to be travel vaccines. Several require multiple doses administered over 21-28 days to provide protection. Others such as cholera and yellow fever must be completed at least 10 days prior to departure to be effective. Typhoid has two formulations: The oral and injectable typhoid vaccines should be completed 1 and 2 weeks, respectively, prior to travel. Several vaccines have age limitations. Routine immunization of all infants against hepatitis A was recommended in 2006. Depending on your region, there may be adolescents who have not been immunized. Fortunately, hepatitis A vaccine works immediately.
One of the challenges you face is identifying someone in your area that provides travel medicine advice and immunizations to children and adolescents. Most children and teens travel with their parents, but today many adolescents travel independently with organized groups. Most of the vaccines listed are not routinely administered at your office, yet you most likely will be the first call a parent makes seeking travel advice.
Let me tell you about a few vaccines in particular.
Japanese encephalitis
This is most common cause of encephalitis in Asia and parts of the western Pacific. Risk generally is limited to rural agricultural areas where the causative virus is transmitted by a mosquito. Fatality rates are 20%-30%. Among survivors, 30%-50% have significant neurologic, cognitive, and psychiatric sequelae. Candidates for this vaccine are long-term travelers and short-term travelers with extensive outdoor rural activities.
Meningococcal conjugate vaccines (MCV4)
All travelers to the Hajj Pilgrimage (Aug. 9-14, 2019) and/or Umrah must show proof of immunization. Vaccine must be received at least 10 days prior to and no greater than 5 years prior to arrival to Saudi Arabia. Conjugate vaccine must clearly be documented for validity of 5 years. For all health entry requirements, go to www.moh.gov.sa/en/hajj/pages/healthregulations.aspx.
Measles
The Advisory Committee on Immunization Practices recommends all infants 6-11 months old receive one dose of MMR prior to international travel regardless of the destination. This should be followed by two additional countable doses. All persons at least 12 months of age and born after 1956 should receive two doses of MMR at least 28 days apart prior to international travel.
Rabies
Rabies is a viral disease endemic in more than 150 countries with approximately 60,000 fatal cases worldwide each year. Asia and Africa are the areas with the highest risk of exposure, and dogs are the principal hosts. Human rabies is almost always fatal once symptoms develop. Preexposure vaccine is recommended for persons with prolonged and/or remote travel to countries where rabies immunoglobulin is unavailable and the occurrence of animal rabies is high. Post exposure vaccination on days 0 and 3 still would be required.*
Typhoid
A bacterial infection caused by Salmonella enterica serotype Typhi and Paratyphi manifests with fever, headache, abdominal pain, diarrhea, or constipation. When bacteremia occurs, it usually is referred to as enteric fever. It is acquired by consumption of food/water contaminated with human feces. Highest risk areas include Africa, Southern Asia, and Southeast Asia
Yellow fever
Risk is limited to sub-Saharan Africa and the tropical areas of South America. It is transmitted by the bite of an infected mosquito. The vaccine is required for entry into at least 16 countries. In a country where yellow fever is present, persons transiting through for more than 12 hours to reach their final destination may actually cause a change in the entry requirements for the destination country. For example, travel from the United States to Tanzania requires no yellow fever vaccine while travel from the United States to Nairobi (more than 12 hours) to Tanzania requires yellow fever vaccine for entry into Tanzania. Travel sequence and duration is extremely important. Check the Centers for Disease Control and Prevention yellow fever site and/or the consulate for the most up-to-date yellow fever vaccine requirements.
YF-Vax (yellow fever vaccine) produced by Sanofi Pasteur in the United States currently is unavailable. The company is building a new facility, and vaccine will not be available for the remainder of 2019. To assure vaccine for U.S. travelers, Stamaril, a yellow fever vaccine produced by Sanofi Pasteur in France has been made available at more than 250 sites nationwide. Because Stamaril is offered at a limited number of locations, persons in need of vaccine should not delay seeking it. Because of increased demand related to summer travel, travelers in some areas have reported delays of several weeks in scheduling an appointment. To locate a Stamaril site in your area, go to wwwnc.cdc.gov/travel/page/search-for-stamaril-clinics.
There are several other diseases transmitted by mosquitoes and ticks including malaria, dengue, Zika and rickettsial diseases. Vigilant use of mosquito repellents is a must. Prophylactic medication is available for only malaria and should be initiated prior to exposure. Frequency and duration depends on the medication selected.
So how do you assist your patients?
Once you’ve identified a travel medicine facility in your area, encourage them to seek pretravel advice 4-6 weeks prior to international travel and make sure their routine immunizations are up to date. Generally, this is not an issue. One challenge is the early administration of MMR. While most practitioners know that early administration for international travel has been recommended for years, many office staff are accustomed to administration at only the 12 month and 4 year visit. When parents call requesting immunization, they often are informed that is it unnecessary and the appointment denied. This is a challenge, especially when coordination of administration of another live vaccine, such as yellow fever, is planned. Familiarizing all members of the health care team with current vaccine recommendations is critical.
For country-specific information, up-to-date travel alerts, and to locate a travel medicine clinic, visit www.cdc.gov/travel.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She had no relevant financial disclosures. Email her at [email protected].
*This article was updated 6/18/2019.
California vaccine exemption proposal gives powerful voices pause
In the past few weeks, Democratic Gov. Gavin Newsom and the members of the Medical Board of California have questioned a bill that would give the California Department of Public Health authority to decide whether a child can skip routine vaccinations.
Anti-vaccine activists have capitalized on these moments, plastering Facebook pages and social media with praise for the officials’ statements.
But those officials are not against vaccinations. In fact, they have made clear they’re committed to vaccines, and to dealing with the problem the bill is supposed to fix — doctors providing kids with medical exemptions for reasons that don’t meet federal standards.
“Having been in public health for a long time, I am a huge supporter of vaccines,” said Michelle Bholat, MD, a family medicine physician in Santa Monica and until recently a member of the medical board, which has oversight over physicians and their licenses.
What concerns her, she said at a late-May meeting of the board, was the measure’s potential effect on doctor-patient relationships and the particulars of who would qualify for a medical exemption.
Pediatrician and State Sen. Richard Pan (D-Sacramento) introduced the bill to address a spike in the number of children who have been granted what he calls “fake” medical exemptions from vaccinations; more than five times as many kids have medical exemptions this past school year than in 2015-16.
SB 276 would give the final say on medical exemption applications to the state public health department, which would be required to follow guidelines established by the Centers for Disease Control and Prevention. Any exemptions provided by doctors would be subject to approval — or denial — by the department.
The only other state that gives control of vaccine exemptions to a public health agency is West Virginia.
The measure passed the state Senate in May and is awaiting consideration in the state Assembly.
The debate over the measure comes as new state data show that the percentage of kindergartners who had all their recommended shots fell for the second straight year, largely due to an increase in medical exemptions written by doctors.
During the past school year, the share of fully vaccinated kindergartners dropped to 94.8%, down from 95.6% in 2016-17, putting the state in potentially dangerous territory — officials recommend 90%-95% coverage for community immunity.
And as vaccination rates dip, measles is spreading nationwide. In the largest outbreak since 1992, more than 1,000 people have been infected across the country this year through June 5, including 51 in California.
Nearly 3 years ago, California enacted a law by Sen. Pan that bars parents from citing personal or religious beliefs to avoid vaccinating their children. Children could be exempted only on medical grounds if the shots were harmful to their health.
That ban improved vaccination rates, though progress has been slipping.
Today, many of the schools that had the highest rates of unvaccinated students before the law took effect still do. Doctors have broad authority to grant medical exemptions from vaccination; some wield that power liberally and sometimes for cash, signing dozens or hundreds of exemptions for children, sometimes in far-off communities.
Sen. Pan’s bill would crack down on this practice and has the strong support of the medical establishment. It was cosponsored by two powerful doctor associations, the American Academy of Pediatrics, California, and the California Medical Association.
“We want to make sure unscrupulous physicians aren’t making medical exemptions for money,” said David Aizuss, MD, president of the California Medical Association. “The idea of the bill is to protect a real personal medical exemption, where kids are on chemotherapy or have an immunological response.”
But it has its critics — and this time, they extend beyond the small but fervent group of people who continue to question the extensive scientific evidence that shows vaccines are safe. And although raising concerns is typical in the legislative process, their criticisms take on outsize importance with a subject as explosive as vaccines.
The biggest name among the new critics is Gov. Newsom, who said he’s worried about interfering with the doctor-patient relationship. “I like doctor-patient relationships. Bureaucratic relationships are more challenging for me,” he said at the state Democratic Party convention in early June.
“I’m a parent; I don’t want someone that the governor of California appointed to make a decision for my family.”
State Sen. Ben Allen (D-Santa Monica), a cosponsor of Sen. Pan’s previous legislation, abstained from voting on the new measure last month, saying he’d made commitments during the previous fight to leave medical exemptions to the discretion of doctors.
Last month, the Medical Board of California offered just lukewarm support, and only to portions of the bill, after listening to 200 members of the public speak against it for more than 2 hours.
The board members called on Sen. Pan to address a variety of concerns, from the potential oversight role the state public health department might play, to the proposed guidelines for medical exemptions.
They agreed on one thing: It should be easier for the board to investigate complaints of questionable medical exemptions. To look into complaints, the board needs to see medical records. To get those records, it generally needs permission from patients or their guardians, something parents who have sought medical exemptions are often unwilling to provide. The bill would give the board access to these records.
One physician, Bob Sears, MD, in Orange County, a well-known opponent to vaccine mandates, was put on probation in 2018 for writing an exemption for a 2-year-old without taking any medical history. Since 2016, at least 173 complaints against physicians for inappropriate exemptions have been filed with the state medical board, with more than 100 currently under investigation, the board said.
Medical exemptions for California kids are clustered in certain communities and schools. In Humboldt County, 5.8% of kindergartners have medical exemptions from shots, according to the new state data. In Nevada County, the rate is 10.6%. All told, nearly one-third of the state’s counties have fallen below 95% immunity from measles.
Dr. Aizuss of the California Medical Association said the organization is working with Gov. Newsom’s office and the medical board, among others, to update the bill so that it will be “workable, effective, and supported by the governor.
“I think that our goal is the same,” he said. “The idea of the bill is to protect ... the sanctity of the true physician-patient relationship, as opposed to a relationship where physicians were granting the medical exemption for a fee, which is not a true physician-patient relationship.”
This story was produced by Kaiser Health News, which publishes California Healthline, an editorially independent service of the California Health Care Foundation. California Healthline reporter Ana B. Ibarra contributed to this report.
In the past few weeks, Democratic Gov. Gavin Newsom and the members of the Medical Board of California have questioned a bill that would give the California Department of Public Health authority to decide whether a child can skip routine vaccinations.
Anti-vaccine activists have capitalized on these moments, plastering Facebook pages and social media with praise for the officials’ statements.
But those officials are not against vaccinations. In fact, they have made clear they’re committed to vaccines, and to dealing with the problem the bill is supposed to fix — doctors providing kids with medical exemptions for reasons that don’t meet federal standards.
“Having been in public health for a long time, I am a huge supporter of vaccines,” said Michelle Bholat, MD, a family medicine physician in Santa Monica and until recently a member of the medical board, which has oversight over physicians and their licenses.
What concerns her, she said at a late-May meeting of the board, was the measure’s potential effect on doctor-patient relationships and the particulars of who would qualify for a medical exemption.
Pediatrician and State Sen. Richard Pan (D-Sacramento) introduced the bill to address a spike in the number of children who have been granted what he calls “fake” medical exemptions from vaccinations; more than five times as many kids have medical exemptions this past school year than in 2015-16.
SB 276 would give the final say on medical exemption applications to the state public health department, which would be required to follow guidelines established by the Centers for Disease Control and Prevention. Any exemptions provided by doctors would be subject to approval — or denial — by the department.
The only other state that gives control of vaccine exemptions to a public health agency is West Virginia.
The measure passed the state Senate in May and is awaiting consideration in the state Assembly.
The debate over the measure comes as new state data show that the percentage of kindergartners who had all their recommended shots fell for the second straight year, largely due to an increase in medical exemptions written by doctors.
During the past school year, the share of fully vaccinated kindergartners dropped to 94.8%, down from 95.6% in 2016-17, putting the state in potentially dangerous territory — officials recommend 90%-95% coverage for community immunity.
And as vaccination rates dip, measles is spreading nationwide. In the largest outbreak since 1992, more than 1,000 people have been infected across the country this year through June 5, including 51 in California.
Nearly 3 years ago, California enacted a law by Sen. Pan that bars parents from citing personal or religious beliefs to avoid vaccinating their children. Children could be exempted only on medical grounds if the shots were harmful to their health.
That ban improved vaccination rates, though progress has been slipping.
Today, many of the schools that had the highest rates of unvaccinated students before the law took effect still do. Doctors have broad authority to grant medical exemptions from vaccination; some wield that power liberally and sometimes for cash, signing dozens or hundreds of exemptions for children, sometimes in far-off communities.
Sen. Pan’s bill would crack down on this practice and has the strong support of the medical establishment. It was cosponsored by two powerful doctor associations, the American Academy of Pediatrics, California, and the California Medical Association.
“We want to make sure unscrupulous physicians aren’t making medical exemptions for money,” said David Aizuss, MD, president of the California Medical Association. “The idea of the bill is to protect a real personal medical exemption, where kids are on chemotherapy or have an immunological response.”
But it has its critics — and this time, they extend beyond the small but fervent group of people who continue to question the extensive scientific evidence that shows vaccines are safe. And although raising concerns is typical in the legislative process, their criticisms take on outsize importance with a subject as explosive as vaccines.
The biggest name among the new critics is Gov. Newsom, who said he’s worried about interfering with the doctor-patient relationship. “I like doctor-patient relationships. Bureaucratic relationships are more challenging for me,” he said at the state Democratic Party convention in early June.
“I’m a parent; I don’t want someone that the governor of California appointed to make a decision for my family.”
State Sen. Ben Allen (D-Santa Monica), a cosponsor of Sen. Pan’s previous legislation, abstained from voting on the new measure last month, saying he’d made commitments during the previous fight to leave medical exemptions to the discretion of doctors.
Last month, the Medical Board of California offered just lukewarm support, and only to portions of the bill, after listening to 200 members of the public speak against it for more than 2 hours.
The board members called on Sen. Pan to address a variety of concerns, from the potential oversight role the state public health department might play, to the proposed guidelines for medical exemptions.
They agreed on one thing: It should be easier for the board to investigate complaints of questionable medical exemptions. To look into complaints, the board needs to see medical records. To get those records, it generally needs permission from patients or their guardians, something parents who have sought medical exemptions are often unwilling to provide. The bill would give the board access to these records.
One physician, Bob Sears, MD, in Orange County, a well-known opponent to vaccine mandates, was put on probation in 2018 for writing an exemption for a 2-year-old without taking any medical history. Since 2016, at least 173 complaints against physicians for inappropriate exemptions have been filed with the state medical board, with more than 100 currently under investigation, the board said.
Medical exemptions for California kids are clustered in certain communities and schools. In Humboldt County, 5.8% of kindergartners have medical exemptions from shots, according to the new state data. In Nevada County, the rate is 10.6%. All told, nearly one-third of the state’s counties have fallen below 95% immunity from measles.
Dr. Aizuss of the California Medical Association said the organization is working with Gov. Newsom’s office and the medical board, among others, to update the bill so that it will be “workable, effective, and supported by the governor.
“I think that our goal is the same,” he said. “The idea of the bill is to protect ... the sanctity of the true physician-patient relationship, as opposed to a relationship where physicians were granting the medical exemption for a fee, which is not a true physician-patient relationship.”
This story was produced by Kaiser Health News, which publishes California Healthline, an editorially independent service of the California Health Care Foundation. California Healthline reporter Ana B. Ibarra contributed to this report.
In the past few weeks, Democratic Gov. Gavin Newsom and the members of the Medical Board of California have questioned a bill that would give the California Department of Public Health authority to decide whether a child can skip routine vaccinations.
Anti-vaccine activists have capitalized on these moments, plastering Facebook pages and social media with praise for the officials’ statements.
But those officials are not against vaccinations. In fact, they have made clear they’re committed to vaccines, and to dealing with the problem the bill is supposed to fix — doctors providing kids with medical exemptions for reasons that don’t meet federal standards.
“Having been in public health for a long time, I am a huge supporter of vaccines,” said Michelle Bholat, MD, a family medicine physician in Santa Monica and until recently a member of the medical board, which has oversight over physicians and their licenses.
What concerns her, she said at a late-May meeting of the board, was the measure’s potential effect on doctor-patient relationships and the particulars of who would qualify for a medical exemption.
Pediatrician and State Sen. Richard Pan (D-Sacramento) introduced the bill to address a spike in the number of children who have been granted what he calls “fake” medical exemptions from vaccinations; more than five times as many kids have medical exemptions this past school year than in 2015-16.
SB 276 would give the final say on medical exemption applications to the state public health department, which would be required to follow guidelines established by the Centers for Disease Control and Prevention. Any exemptions provided by doctors would be subject to approval — or denial — by the department.
The only other state that gives control of vaccine exemptions to a public health agency is West Virginia.
The measure passed the state Senate in May and is awaiting consideration in the state Assembly.
The debate over the measure comes as new state data show that the percentage of kindergartners who had all their recommended shots fell for the second straight year, largely due to an increase in medical exemptions written by doctors.
During the past school year, the share of fully vaccinated kindergartners dropped to 94.8%, down from 95.6% in 2016-17, putting the state in potentially dangerous territory — officials recommend 90%-95% coverage for community immunity.
And as vaccination rates dip, measles is spreading nationwide. In the largest outbreak since 1992, more than 1,000 people have been infected across the country this year through June 5, including 51 in California.
Nearly 3 years ago, California enacted a law by Sen. Pan that bars parents from citing personal or religious beliefs to avoid vaccinating their children. Children could be exempted only on medical grounds if the shots were harmful to their health.
That ban improved vaccination rates, though progress has been slipping.
Today, many of the schools that had the highest rates of unvaccinated students before the law took effect still do. Doctors have broad authority to grant medical exemptions from vaccination; some wield that power liberally and sometimes for cash, signing dozens or hundreds of exemptions for children, sometimes in far-off communities.
Sen. Pan’s bill would crack down on this practice and has the strong support of the medical establishment. It was cosponsored by two powerful doctor associations, the American Academy of Pediatrics, California, and the California Medical Association.
“We want to make sure unscrupulous physicians aren’t making medical exemptions for money,” said David Aizuss, MD, president of the California Medical Association. “The idea of the bill is to protect a real personal medical exemption, where kids are on chemotherapy or have an immunological response.”
But it has its critics — and this time, they extend beyond the small but fervent group of people who continue to question the extensive scientific evidence that shows vaccines are safe. And although raising concerns is typical in the legislative process, their criticisms take on outsize importance with a subject as explosive as vaccines.
The biggest name among the new critics is Gov. Newsom, who said he’s worried about interfering with the doctor-patient relationship. “I like doctor-patient relationships. Bureaucratic relationships are more challenging for me,” he said at the state Democratic Party convention in early June.
“I’m a parent; I don’t want someone that the governor of California appointed to make a decision for my family.”
State Sen. Ben Allen (D-Santa Monica), a cosponsor of Sen. Pan’s previous legislation, abstained from voting on the new measure last month, saying he’d made commitments during the previous fight to leave medical exemptions to the discretion of doctors.
Last month, the Medical Board of California offered just lukewarm support, and only to portions of the bill, after listening to 200 members of the public speak against it for more than 2 hours.
The board members called on Sen. Pan to address a variety of concerns, from the potential oversight role the state public health department might play, to the proposed guidelines for medical exemptions.
They agreed on one thing: It should be easier for the board to investigate complaints of questionable medical exemptions. To look into complaints, the board needs to see medical records. To get those records, it generally needs permission from patients or their guardians, something parents who have sought medical exemptions are often unwilling to provide. The bill would give the board access to these records.
One physician, Bob Sears, MD, in Orange County, a well-known opponent to vaccine mandates, was put on probation in 2018 for writing an exemption for a 2-year-old without taking any medical history. Since 2016, at least 173 complaints against physicians for inappropriate exemptions have been filed with the state medical board, with more than 100 currently under investigation, the board said.
Medical exemptions for California kids are clustered in certain communities and schools. In Humboldt County, 5.8% of kindergartners have medical exemptions from shots, according to the new state data. In Nevada County, the rate is 10.6%. All told, nearly one-third of the state’s counties have fallen below 95% immunity from measles.
Dr. Aizuss of the California Medical Association said the organization is working with Gov. Newsom’s office and the medical board, among others, to update the bill so that it will be “workable, effective, and supported by the governor.
“I think that our goal is the same,” he said. “The idea of the bill is to protect ... the sanctity of the true physician-patient relationship, as opposed to a relationship where physicians were granting the medical exemption for a fee, which is not a true physician-patient relationship.”
This story was produced by Kaiser Health News, which publishes California Healthline, an editorially independent service of the California Health Care Foundation. California Healthline reporter Ana B. Ibarra contributed to this report.
How to have ‘the talk’ with vaccine skeptics
LJUBLJANA, SLOVENIA – An effective strategy in helping vaccine skeptics to come around to accepting immunizations for their children is to pivot the conversation away from vaccine safety and focus instead on the disease itself and its potential consequences, Saad B. Omer, MBBS, PhD, asserted at the annual meeting of the European Society for Paediatric Infectious Diseases.
“Why do we cede ground by focusing too much on the vaccine itself? I call it the disease salience approach,” said Dr. Omer, professor of global health, epidemiology, and pediatrics at Emory University in Atlanta.
It’s a strategy guided by developments in social psychology, persuasion theory, and communication theory. But if applied incorrectly, the disease salience approach can backfire, causing behavioral paralysis and an inability to act, he cautioned.
Dr. Omer explained that it’s a matter of framing.
“Always include a solution to promote self-efficacy and response-efficacy. After you inform parents of disease risks, provide them with actions they can take. Now readdress the vaccine, pointing out that this is the single best way to protect yourself and your baby,” he said. “The lesson is that since vaccines are a social norm, reframe nonvaccination as an active act, rather than vaccination as an active act.”
Don’t attempt to wow parents with statistics on how vaccine complication rates are dwarfed by the disease risk if left unvaccinated, he advised. Studies have shown that‘s generally not effective. What actually works is to provide narratives of disease severity.
“We are excellent linguists, but really, really poor statisticians,” Dr. Omer observed.
Is it ethical to talk to parents about disease risks to influence their behavior? Absolutely, in his view.
“We’re not selling toothpaste. We are in the business of life-saving vaccines. And I would submit that if it’s done correctly it’s entirely ethical to talk about the disease, and sometimes even the severe risks of the disease, instead of the vaccine,” said Dr. Omer.
If parents cite a myth about vaccines, it’s necessary to address it head on without lingering on it. But debunking a myth is tricky because people tend to remember negative information they received earlier.
“If you’re going to debunk a myth, clearly label it as a myth in the headline as you introduce it. State why it’s not true. Replace the myth with the best alternative explanation. Think of it like a blank space where the myth used to reside. That space needs to be filled with an alternative explanation or the myth will come back,” Dr. Omer said.
He is a coauthor of a book titled, ‘The Clinician’s Vaccine Safety Resource Guide: Optimizing Prevention of Vaccine-Preventable Diseases Across the Lifespan.’
LJUBLJANA, SLOVENIA – An effective strategy in helping vaccine skeptics to come around to accepting immunizations for their children is to pivot the conversation away from vaccine safety and focus instead on the disease itself and its potential consequences, Saad B. Omer, MBBS, PhD, asserted at the annual meeting of the European Society for Paediatric Infectious Diseases.
“Why do we cede ground by focusing too much on the vaccine itself? I call it the disease salience approach,” said Dr. Omer, professor of global health, epidemiology, and pediatrics at Emory University in Atlanta.
It’s a strategy guided by developments in social psychology, persuasion theory, and communication theory. But if applied incorrectly, the disease salience approach can backfire, causing behavioral paralysis and an inability to act, he cautioned.
Dr. Omer explained that it’s a matter of framing.
“Always include a solution to promote self-efficacy and response-efficacy. After you inform parents of disease risks, provide them with actions they can take. Now readdress the vaccine, pointing out that this is the single best way to protect yourself and your baby,” he said. “The lesson is that since vaccines are a social norm, reframe nonvaccination as an active act, rather than vaccination as an active act.”
Don’t attempt to wow parents with statistics on how vaccine complication rates are dwarfed by the disease risk if left unvaccinated, he advised. Studies have shown that‘s generally not effective. What actually works is to provide narratives of disease severity.
“We are excellent linguists, but really, really poor statisticians,” Dr. Omer observed.
Is it ethical to talk to parents about disease risks to influence their behavior? Absolutely, in his view.
“We’re not selling toothpaste. We are in the business of life-saving vaccines. And I would submit that if it’s done correctly it’s entirely ethical to talk about the disease, and sometimes even the severe risks of the disease, instead of the vaccine,” said Dr. Omer.
If parents cite a myth about vaccines, it’s necessary to address it head on without lingering on it. But debunking a myth is tricky because people tend to remember negative information they received earlier.
“If you’re going to debunk a myth, clearly label it as a myth in the headline as you introduce it. State why it’s not true. Replace the myth with the best alternative explanation. Think of it like a blank space where the myth used to reside. That space needs to be filled with an alternative explanation or the myth will come back,” Dr. Omer said.
He is a coauthor of a book titled, ‘The Clinician’s Vaccine Safety Resource Guide: Optimizing Prevention of Vaccine-Preventable Diseases Across the Lifespan.’
LJUBLJANA, SLOVENIA – An effective strategy in helping vaccine skeptics to come around to accepting immunizations for their children is to pivot the conversation away from vaccine safety and focus instead on the disease itself and its potential consequences, Saad B. Omer, MBBS, PhD, asserted at the annual meeting of the European Society for Paediatric Infectious Diseases.
“Why do we cede ground by focusing too much on the vaccine itself? I call it the disease salience approach,” said Dr. Omer, professor of global health, epidemiology, and pediatrics at Emory University in Atlanta.
It’s a strategy guided by developments in social psychology, persuasion theory, and communication theory. But if applied incorrectly, the disease salience approach can backfire, causing behavioral paralysis and an inability to act, he cautioned.
Dr. Omer explained that it’s a matter of framing.
“Always include a solution to promote self-efficacy and response-efficacy. After you inform parents of disease risks, provide them with actions they can take. Now readdress the vaccine, pointing out that this is the single best way to protect yourself and your baby,” he said. “The lesson is that since vaccines are a social norm, reframe nonvaccination as an active act, rather than vaccination as an active act.”
Don’t attempt to wow parents with statistics on how vaccine complication rates are dwarfed by the disease risk if left unvaccinated, he advised. Studies have shown that‘s generally not effective. What actually works is to provide narratives of disease severity.
“We are excellent linguists, but really, really poor statisticians,” Dr. Omer observed.
Is it ethical to talk to parents about disease risks to influence their behavior? Absolutely, in his view.
“We’re not selling toothpaste. We are in the business of life-saving vaccines. And I would submit that if it’s done correctly it’s entirely ethical to talk about the disease, and sometimes even the severe risks of the disease, instead of the vaccine,” said Dr. Omer.
If parents cite a myth about vaccines, it’s necessary to address it head on without lingering on it. But debunking a myth is tricky because people tend to remember negative information they received earlier.
“If you’re going to debunk a myth, clearly label it as a myth in the headline as you introduce it. State why it’s not true. Replace the myth with the best alternative explanation. Think of it like a blank space where the myth used to reside. That space needs to be filled with an alternative explanation or the myth will come back,” Dr. Omer said.
He is a coauthor of a book titled, ‘The Clinician’s Vaccine Safety Resource Guide: Optimizing Prevention of Vaccine-Preventable Diseases Across the Lifespan.’
EXPERT ANALYSIS FROM ESPID 2019
Waning pertussis immunity may be linked to acellular vaccine
A large Kaiser Permanente study paints a nuanced picture of the acellular pertussis vaccine, with more cases occurring in fully vaccinated children, but the highest risk of disease occurring among the under- and unvaccinated.
Among nearly half a million children, the unvaccinated were 13 times more likely to develop pertussis than fully vaccinated children, Ousseny Zerbo, PhD, of Kaiser Permanente Northern California in Oakland and colleagues wrote in Pediatrics. But 82% of cases occurred in fully vaccinated children and just 5% in undervaccinated children – and rates increased in both groups the farther they were in time from the last vaccination.
“Within our study population, greater than 80% of pertussis cases occurred among age-appropriately vaccinated children,” the team wrote. “Children who were further away from their last DTaP dose were at increased risk of pertussis, even after controlling for undervaccination. Our results suggest that, in this population, possibly in conjunction with other factors not addressed in this study, suboptimal vaccine efficacy and waning [immunity] played a major role in recent pertussis epidemics.”
The results are consistent with several prior studies, including one finding that the odds of the disease increased by 33% for every additional year after the third or fifth DTaP dose (Pediatrics. 2015;135[2]:331-43).
The current study comprised 469,982 children aged between 3 months and 11 years, who were followed for a mean of 4.6 years. Over the entire study period, there were 738 lab-confirmed pertussis cases. Most of these (515; 70%) occurred in fully vaccinated children. Another 99 (13%) occurred in unvaccinated children, 36 (5%) in undervaccinated children, and 88 (12%) in fully vaccinated plus one dose.
In a multivariate analysis, the risk of pertussis was 13 times higher among the unvaccinated (adjusted hazard ratio, 13) and almost 2 times higher among the undervaccinated (aHR, 1.9), compared with fully vaccinated children. Those who had been fully vaccinated and received a booster had the lowest risk, about half that of fully vaccinated children (aHR, 0.48).
Risk varied according to age, but also was significantly higher among unvaccinated children at each time point. Risk ranged from 4 times higher among those aged 3-5 months to 23 times higher among those aged 19-84 months. Undervaccinated children aged 5-7 months and 19-84 months also were at significantly increased risk for pertussis, compared with fully vaccinated children. Children who were fully vaccinated plus one dose had a significantly reduced risk at 7-19 months and at 19-84 months, compared with the fully vaccinated reference group.
“Across all follow-up and all age groups, VE [vaccine effectiveness] was 86% ... for undervaccinated children, compared with unvaccinated children,” Dr. Zerbo and associates wrote. “VE was even higher for fully vaccinated children [93%] and for those who were fully vaccinated plus one dose [96%].”
But VE waned as time progressed farther from the last DTaP dose. The multivariate model found more than a 100% increased risk for those whose last DTaP was at least 3 years past, compared with less than 1 year past (aHR, 2.58).
The model also found time-bound risk increases among fully vaccinated children, with a more than 300% increased risk among those at least 6 years out from the last DTaP dose, compared with 3 years out (aHR, 4.66).
The results indicate that other factors besides adherence to the recommended vaccine schedule may be at work in recent pertussis outbreaks.
“Although waning immunity is clearly an important factor driving pertussis epidemics in recent years, other factors that we did not evaluate in this study might also contribute to pertussis epidemics individually or in synergy,” Dr. Zerbo and associates wrote. “Results from studies in baboons suggest that the acellular pertussis vaccines are unable to prevent colonization, carriage, and transmission. If this is also true for humans, this could contribute to pertussis epidemics. The causes of recent pertussis epidemics are complex, and we were only able to address some aspects in our study.”
The study was funded by Kaiser Permanente Northern California, the National Institutes of Health, and in part by a National Institute of Allergy and Infectious Diseases grant. One coauthor reported receiving research grant support from Sanofi Pasteur, Novartis, GlaxoSmithKline, Merck, MedImmune, Pfizer, and Dynavax for unrelated studies; the other authors reported no relevant financial disclosures.
SOURCE: Zerbo O et al. Pediatrics. 2019 Jun 10. doi: 10.1542/peds.2018-3466.
Fixing one problem with the pertussis vaccine seemed to have created another, Kathryn M. Edwards, MD, wrote in an accompanying editorial.
The current acellular vaccine was approved in 1997. It was considered a less reactive substitute for the previous whole-cell vaccine, which was associated with injection site pain, swelling, fever, and febrile seizures, Dr. Edwards wrote. “For about a decade, all seemed to be going well with pertussis control. Serological methods were employed to diagnose pertussis infections in adolescents and adults, and polymerase chain reaction methods were devised to more accurately detect pertussis organisms. Thus, the burden of pertussis disease was increasingly appreciated as the diagnostic methods improved.”
But things soon changed. There were pertussis outbreaks, some of them quite large. The increasing disease rates showed that protection conferred by the acellular vaccine waned much more quickly than that conferred by the whole-cell vaccine. “In the current study, Zerbo et al. add to the body of evidence documenting the increase in pertussis risk with time after DTaP vaccination,” she noted.
This has several practical implications, Dr. Edwards wrote.
“First, given the markedly increased risk of pertussis in unvaccinated and undervaccinated children, universal DTaP vaccination should be strongly recommended. Second, the addition of maternal Tdap vaccination administered during pregnancy has been shown to significantly reduce infant disease before primary immunization and should remain the standard,” Dr. Edwards wrote.
More problematic is how to address the waning DTaP immunity now seen. “One option presented [at an international meeting] was a live-attenuated pertussis vaccine administered intranasally that would stimulate local immune responses and prevent colonization with pertussis organisms. This vaccine is currently being studied in adults and might provide a solution for waning immunity seen with DTaP vaccine,” she noted.
Another possibility is adding the live vaccine to the current DTaP, which should, in theory, stimulate more long-lasting immunity. But numerous safety studies in young children would be necessary before adopting such an approach, Dr. Edwards wrote.
Adding more antigens to the acellular vaccine also might work, and investigational vaccines like this are in development.
Studies in animals and humans show that acellular vaccines “generate functionally different T-cell responses than those seen after whole-cell vaccines, with the whole cell vaccines generating more protective T-cell responses. Studies are ongoing to determine if adjuvants can be added to acellular vaccines to modify their T-cell responses to a more protective immune response or whether the T-cell response remains fixed once primed with DTaP vaccine,” she wrote.
Dr. Edwards is a pediatric infectious disease specialist at Vanderbilt University, Nashville, Tenn. She wrote an editorial to accompany Zerbo et al (Pediatrics. 2019. doi: 10.1542/peds.2019-1276). She reported no financial disclosures, and received no funding to write the editorial.
Fixing one problem with the pertussis vaccine seemed to have created another, Kathryn M. Edwards, MD, wrote in an accompanying editorial.
The current acellular vaccine was approved in 1997. It was considered a less reactive substitute for the previous whole-cell vaccine, which was associated with injection site pain, swelling, fever, and febrile seizures, Dr. Edwards wrote. “For about a decade, all seemed to be going well with pertussis control. Serological methods were employed to diagnose pertussis infections in adolescents and adults, and polymerase chain reaction methods were devised to more accurately detect pertussis organisms. Thus, the burden of pertussis disease was increasingly appreciated as the diagnostic methods improved.”
But things soon changed. There were pertussis outbreaks, some of them quite large. The increasing disease rates showed that protection conferred by the acellular vaccine waned much more quickly than that conferred by the whole-cell vaccine. “In the current study, Zerbo et al. add to the body of evidence documenting the increase in pertussis risk with time after DTaP vaccination,” she noted.
This has several practical implications, Dr. Edwards wrote.
“First, given the markedly increased risk of pertussis in unvaccinated and undervaccinated children, universal DTaP vaccination should be strongly recommended. Second, the addition of maternal Tdap vaccination administered during pregnancy has been shown to significantly reduce infant disease before primary immunization and should remain the standard,” Dr. Edwards wrote.
More problematic is how to address the waning DTaP immunity now seen. “One option presented [at an international meeting] was a live-attenuated pertussis vaccine administered intranasally that would stimulate local immune responses and prevent colonization with pertussis organisms. This vaccine is currently being studied in adults and might provide a solution for waning immunity seen with DTaP vaccine,” she noted.
Another possibility is adding the live vaccine to the current DTaP, which should, in theory, stimulate more long-lasting immunity. But numerous safety studies in young children would be necessary before adopting such an approach, Dr. Edwards wrote.
Adding more antigens to the acellular vaccine also might work, and investigational vaccines like this are in development.
Studies in animals and humans show that acellular vaccines “generate functionally different T-cell responses than those seen after whole-cell vaccines, with the whole cell vaccines generating more protective T-cell responses. Studies are ongoing to determine if adjuvants can be added to acellular vaccines to modify their T-cell responses to a more protective immune response or whether the T-cell response remains fixed once primed with DTaP vaccine,” she wrote.
Dr. Edwards is a pediatric infectious disease specialist at Vanderbilt University, Nashville, Tenn. She wrote an editorial to accompany Zerbo et al (Pediatrics. 2019. doi: 10.1542/peds.2019-1276). She reported no financial disclosures, and received no funding to write the editorial.
Fixing one problem with the pertussis vaccine seemed to have created another, Kathryn M. Edwards, MD, wrote in an accompanying editorial.
The current acellular vaccine was approved in 1997. It was considered a less reactive substitute for the previous whole-cell vaccine, which was associated with injection site pain, swelling, fever, and febrile seizures, Dr. Edwards wrote. “For about a decade, all seemed to be going well with pertussis control. Serological methods were employed to diagnose pertussis infections in adolescents and adults, and polymerase chain reaction methods were devised to more accurately detect pertussis organisms. Thus, the burden of pertussis disease was increasingly appreciated as the diagnostic methods improved.”
But things soon changed. There were pertussis outbreaks, some of them quite large. The increasing disease rates showed that protection conferred by the acellular vaccine waned much more quickly than that conferred by the whole-cell vaccine. “In the current study, Zerbo et al. add to the body of evidence documenting the increase in pertussis risk with time after DTaP vaccination,” she noted.
This has several practical implications, Dr. Edwards wrote.
“First, given the markedly increased risk of pertussis in unvaccinated and undervaccinated children, universal DTaP vaccination should be strongly recommended. Second, the addition of maternal Tdap vaccination administered during pregnancy has been shown to significantly reduce infant disease before primary immunization and should remain the standard,” Dr. Edwards wrote.
More problematic is how to address the waning DTaP immunity now seen. “One option presented [at an international meeting] was a live-attenuated pertussis vaccine administered intranasally that would stimulate local immune responses and prevent colonization with pertussis organisms. This vaccine is currently being studied in adults and might provide a solution for waning immunity seen with DTaP vaccine,” she noted.
Another possibility is adding the live vaccine to the current DTaP, which should, in theory, stimulate more long-lasting immunity. But numerous safety studies in young children would be necessary before adopting such an approach, Dr. Edwards wrote.
Adding more antigens to the acellular vaccine also might work, and investigational vaccines like this are in development.
Studies in animals and humans show that acellular vaccines “generate functionally different T-cell responses than those seen after whole-cell vaccines, with the whole cell vaccines generating more protective T-cell responses. Studies are ongoing to determine if adjuvants can be added to acellular vaccines to modify their T-cell responses to a more protective immune response or whether the T-cell response remains fixed once primed with DTaP vaccine,” she wrote.
Dr. Edwards is a pediatric infectious disease specialist at Vanderbilt University, Nashville, Tenn. She wrote an editorial to accompany Zerbo et al (Pediatrics. 2019. doi: 10.1542/peds.2019-1276). She reported no financial disclosures, and received no funding to write the editorial.
A large Kaiser Permanente study paints a nuanced picture of the acellular pertussis vaccine, with more cases occurring in fully vaccinated children, but the highest risk of disease occurring among the under- and unvaccinated.
Among nearly half a million children, the unvaccinated were 13 times more likely to develop pertussis than fully vaccinated children, Ousseny Zerbo, PhD, of Kaiser Permanente Northern California in Oakland and colleagues wrote in Pediatrics. But 82% of cases occurred in fully vaccinated children and just 5% in undervaccinated children – and rates increased in both groups the farther they were in time from the last vaccination.
“Within our study population, greater than 80% of pertussis cases occurred among age-appropriately vaccinated children,” the team wrote. “Children who were further away from their last DTaP dose were at increased risk of pertussis, even after controlling for undervaccination. Our results suggest that, in this population, possibly in conjunction with other factors not addressed in this study, suboptimal vaccine efficacy and waning [immunity] played a major role in recent pertussis epidemics.”
The results are consistent with several prior studies, including one finding that the odds of the disease increased by 33% for every additional year after the third or fifth DTaP dose (Pediatrics. 2015;135[2]:331-43).
The current study comprised 469,982 children aged between 3 months and 11 years, who were followed for a mean of 4.6 years. Over the entire study period, there were 738 lab-confirmed pertussis cases. Most of these (515; 70%) occurred in fully vaccinated children. Another 99 (13%) occurred in unvaccinated children, 36 (5%) in undervaccinated children, and 88 (12%) in fully vaccinated plus one dose.
In a multivariate analysis, the risk of pertussis was 13 times higher among the unvaccinated (adjusted hazard ratio, 13) and almost 2 times higher among the undervaccinated (aHR, 1.9), compared with fully vaccinated children. Those who had been fully vaccinated and received a booster had the lowest risk, about half that of fully vaccinated children (aHR, 0.48).
Risk varied according to age, but also was significantly higher among unvaccinated children at each time point. Risk ranged from 4 times higher among those aged 3-5 months to 23 times higher among those aged 19-84 months. Undervaccinated children aged 5-7 months and 19-84 months also were at significantly increased risk for pertussis, compared with fully vaccinated children. Children who were fully vaccinated plus one dose had a significantly reduced risk at 7-19 months and at 19-84 months, compared with the fully vaccinated reference group.
“Across all follow-up and all age groups, VE [vaccine effectiveness] was 86% ... for undervaccinated children, compared with unvaccinated children,” Dr. Zerbo and associates wrote. “VE was even higher for fully vaccinated children [93%] and for those who were fully vaccinated plus one dose [96%].”
But VE waned as time progressed farther from the last DTaP dose. The multivariate model found more than a 100% increased risk for those whose last DTaP was at least 3 years past, compared with less than 1 year past (aHR, 2.58).
The model also found time-bound risk increases among fully vaccinated children, with a more than 300% increased risk among those at least 6 years out from the last DTaP dose, compared with 3 years out (aHR, 4.66).
The results indicate that other factors besides adherence to the recommended vaccine schedule may be at work in recent pertussis outbreaks.
“Although waning immunity is clearly an important factor driving pertussis epidemics in recent years, other factors that we did not evaluate in this study might also contribute to pertussis epidemics individually or in synergy,” Dr. Zerbo and associates wrote. “Results from studies in baboons suggest that the acellular pertussis vaccines are unable to prevent colonization, carriage, and transmission. If this is also true for humans, this could contribute to pertussis epidemics. The causes of recent pertussis epidemics are complex, and we were only able to address some aspects in our study.”
The study was funded by Kaiser Permanente Northern California, the National Institutes of Health, and in part by a National Institute of Allergy and Infectious Diseases grant. One coauthor reported receiving research grant support from Sanofi Pasteur, Novartis, GlaxoSmithKline, Merck, MedImmune, Pfizer, and Dynavax for unrelated studies; the other authors reported no relevant financial disclosures.
SOURCE: Zerbo O et al. Pediatrics. 2019 Jun 10. doi: 10.1542/peds.2018-3466.
A large Kaiser Permanente study paints a nuanced picture of the acellular pertussis vaccine, with more cases occurring in fully vaccinated children, but the highest risk of disease occurring among the under- and unvaccinated.
Among nearly half a million children, the unvaccinated were 13 times more likely to develop pertussis than fully vaccinated children, Ousseny Zerbo, PhD, of Kaiser Permanente Northern California in Oakland and colleagues wrote in Pediatrics. But 82% of cases occurred in fully vaccinated children and just 5% in undervaccinated children – and rates increased in both groups the farther they were in time from the last vaccination.
“Within our study population, greater than 80% of pertussis cases occurred among age-appropriately vaccinated children,” the team wrote. “Children who were further away from their last DTaP dose were at increased risk of pertussis, even after controlling for undervaccination. Our results suggest that, in this population, possibly in conjunction with other factors not addressed in this study, suboptimal vaccine efficacy and waning [immunity] played a major role in recent pertussis epidemics.”
The results are consistent with several prior studies, including one finding that the odds of the disease increased by 33% for every additional year after the third or fifth DTaP dose (Pediatrics. 2015;135[2]:331-43).
The current study comprised 469,982 children aged between 3 months and 11 years, who were followed for a mean of 4.6 years. Over the entire study period, there were 738 lab-confirmed pertussis cases. Most of these (515; 70%) occurred in fully vaccinated children. Another 99 (13%) occurred in unvaccinated children, 36 (5%) in undervaccinated children, and 88 (12%) in fully vaccinated plus one dose.
In a multivariate analysis, the risk of pertussis was 13 times higher among the unvaccinated (adjusted hazard ratio, 13) and almost 2 times higher among the undervaccinated (aHR, 1.9), compared with fully vaccinated children. Those who had been fully vaccinated and received a booster had the lowest risk, about half that of fully vaccinated children (aHR, 0.48).
Risk varied according to age, but also was significantly higher among unvaccinated children at each time point. Risk ranged from 4 times higher among those aged 3-5 months to 23 times higher among those aged 19-84 months. Undervaccinated children aged 5-7 months and 19-84 months also were at significantly increased risk for pertussis, compared with fully vaccinated children. Children who were fully vaccinated plus one dose had a significantly reduced risk at 7-19 months and at 19-84 months, compared with the fully vaccinated reference group.
“Across all follow-up and all age groups, VE [vaccine effectiveness] was 86% ... for undervaccinated children, compared with unvaccinated children,” Dr. Zerbo and associates wrote. “VE was even higher for fully vaccinated children [93%] and for those who were fully vaccinated plus one dose [96%].”
But VE waned as time progressed farther from the last DTaP dose. The multivariate model found more than a 100% increased risk for those whose last DTaP was at least 3 years past, compared with less than 1 year past (aHR, 2.58).
The model also found time-bound risk increases among fully vaccinated children, with a more than 300% increased risk among those at least 6 years out from the last DTaP dose, compared with 3 years out (aHR, 4.66).
The results indicate that other factors besides adherence to the recommended vaccine schedule may be at work in recent pertussis outbreaks.
“Although waning immunity is clearly an important factor driving pertussis epidemics in recent years, other factors that we did not evaluate in this study might also contribute to pertussis epidemics individually or in synergy,” Dr. Zerbo and associates wrote. “Results from studies in baboons suggest that the acellular pertussis vaccines are unable to prevent colonization, carriage, and transmission. If this is also true for humans, this could contribute to pertussis epidemics. The causes of recent pertussis epidemics are complex, and we were only able to address some aspects in our study.”
The study was funded by Kaiser Permanente Northern California, the National Institutes of Health, and in part by a National Institute of Allergy and Infectious Diseases grant. One coauthor reported receiving research grant support from Sanofi Pasteur, Novartis, GlaxoSmithKline, Merck, MedImmune, Pfizer, and Dynavax for unrelated studies; the other authors reported no relevant financial disclosures.
SOURCE: Zerbo O et al. Pediatrics. 2019 Jun 10. doi: 10.1542/peds.2018-3466.
FROM PEDIATRICS
United States now over 1,000 measles cases this year
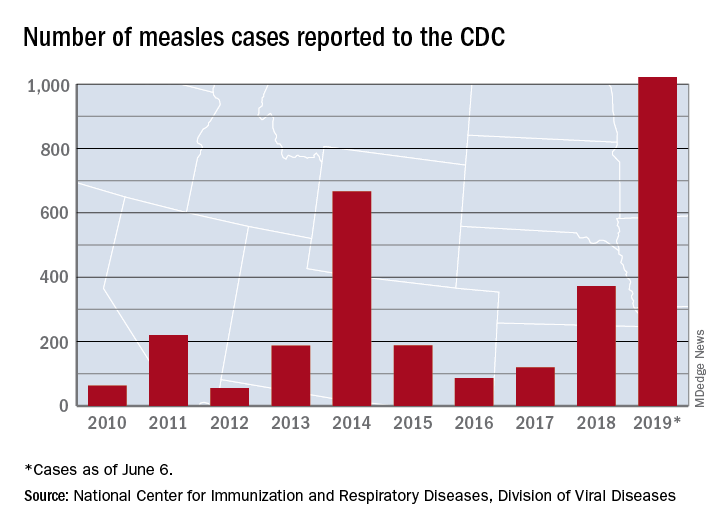
The 41 new cases reported for the week ending June 6 bring the total for the year to 1,022, the CDC reported June 10, and that is more than any year since 1992, when there were 2,237 cases.
Idaho and Virginia reported their first cases of 2019, which makes a total of 28 states with measles cases this year. The Idaho case was reported in Latah County and is the state’s first since 2001. In Virginia, health officials are investigating possible contacts with an infected individual at Dulles International Airport and two other locations on June 2 and 4.
Outbreaks in Georgia, Maryland, and Michigan have ended, while seven others continue in California (Butte, Los Angeles, and Sacramento Counties), New York (Rockland County and New York City), Pennsylvania, and Washington, the CDC said. New York City has the largest outbreak this year with 509 cases through June 3, most of them occurring in Brooklyn.

The 41 new cases reported for the week ending June 6 bring the total for the year to 1,022, the CDC reported June 10, and that is more than any year since 1992, when there were 2,237 cases.
Idaho and Virginia reported their first cases of 2019, which makes a total of 28 states with measles cases this year. The Idaho case was reported in Latah County and is the state’s first since 2001. In Virginia, health officials are investigating possible contacts with an infected individual at Dulles International Airport and two other locations on June 2 and 4.
Outbreaks in Georgia, Maryland, and Michigan have ended, while seven others continue in California (Butte, Los Angeles, and Sacramento Counties), New York (Rockland County and New York City), Pennsylvania, and Washington, the CDC said. New York City has the largest outbreak this year with 509 cases through June 3, most of them occurring in Brooklyn.

The 41 new cases reported for the week ending June 6 bring the total for the year to 1,022, the CDC reported June 10, and that is more than any year since 1992, when there were 2,237 cases.
Idaho and Virginia reported their first cases of 2019, which makes a total of 28 states with measles cases this year. The Idaho case was reported in Latah County and is the state’s first since 2001. In Virginia, health officials are investigating possible contacts with an infected individual at Dulles International Airport and two other locations on June 2 and 4.
Outbreaks in Georgia, Maryland, and Michigan have ended, while seven others continue in California (Butte, Los Angeles, and Sacramento Counties), New York (Rockland County and New York City), Pennsylvania, and Washington, the CDC said. New York City has the largest outbreak this year with 509 cases through June 3, most of them occurring in Brooklyn.
Varicella vaccine delivers doubled benefit to children
than those in unvaccinated children.
The benefit became largely apparent after children received the second vaccination in the recommended series, and persisted throughout childhood, Sheila Weinmann, PhD, of Kaiser Permanente Northern California, Oakland, and colleagues said.*
The analysis included 6.37 million children in the Kaiser Permanente database, 50% of whom were vaccinated for all or some of the study period stretching from 2003 to 2014. Overall, the crude lab-confirmed herpes zoster (HZ) incidence rate was 74/100,000 person-years. When stratified by vaccine status, the crude rate of HZ among vaccinated children was 78% lower than among unvaccinated children (38 vs. 170 cases per 100,000 person years).
Herpes zoster was more common among girls than boys and up to six times more common in immunosuppressed children than in nonimmunosuppressed children.
The authors also found that unvaccinated children benefited from the high rate of vaccination around them. Although the HZ rate was always lower among vaccinated children, the rate among unvaccinated children fell sharply after 2007.
“The trend of decreasing HZ incidence among children who were unvaccinated is likely due to a lack of primary VZV [varicella-zoster virus] infection resulting from herd immunity in a highly vaccinated population,” Dr. Weinmann and her associates said.
There was some variability among age groups, especially among the youngest who were not fully vaccinated.
“In the group aged 1-2 years, the confirmation-adjusted HZ rate among children who were vaccinated was 70% higher than among those who were unvaccinated,” the authors said. In the “older groups, HZ rates were significantly higher in children who were unvaccinated than in those who were vaccinated,” the researchers noted.
The highest incidence was among vaccinated 1-year-olds, who had a 140% higher risk of HZ than did unvaccinated 1-year-olds. But this risk elevation disappeared by age 2 years. For everyone else, aged 2-17 years, the rate of HZ remained significantly lower in vaccinated children.
“Among the small number of children vaccinated at 11 months of age (for whom the vaccine is not recommended), the HZ incidence rate was significantly higher than in children vaccinated at 1 year of age and older. Similarly, children who contract wild-type varicella infection at younger than 1 year of age also have a higher risk of HZ (relative risk, 13.5). The immature adaptive T-cell response in children less than 1 year of age appears less able to contain VZV as a latent infection, compared with older children.
“Our findings for 11-month-olds who were vaccinated should be interpreted with caution because this population included only three cases of HZ and could have included children participating in a prelicensure study with a vaccine formulation different from Varivax,” Dr. Weinmann and her associates said.
Dr. Weinmann and her associates reported no relevant financial disclosures. The study was supported by the Centers for Disease Control and Prevention.
SOURCE: Weinmann S et al. Pediatrics. 2019 Jun 10. doi: 10.1542/peds.2018-2917.
* This article was updated 6/14/2019
The finding of a 78% lower incidence of zoster in varicella-vaccinated children is nothing short of “remarkable,” Anne A Gershon, MD, wrote in an accompanying editorial.
But the benefit could be in jeopardy, as parents question the safety and effectiveness of all vaccines, she wrote.
“That the varicella vaccine prevents not only varicella but zoster as well is an exciting dual benefit from the varicella vaccine, further improving the health of children by immunization,” Dr. Gershon said. “Additional studies will be necessary to show the mechanism for the protection against zoster (viral, immunologic, or both), how long this benefit lasts, and whether additional doses of some form of VZV [varicella-zoster virus] vaccine will be more useful.”
But, she suggested, in a time when cases of clinical varicella are dwindling, so is public awareness of the vaccine’s benefit. Clinical varicella is worse for adults than it is for children.
“Efforts to immunize all children against chickenpox must continue to be made to protect our population from wild-type VZV. Fortunately, antiviral therapy is also available for individuals who are unvaccinated and develop varicella or zoster, but immunization is, as usual, preferable,” Dr. Gershon concluded.
Dr. Gershon, a pediatric infectious disease specialist, is a professor of pediatrics at Columbia University, New York. She wrote a commentary to accompany the article by Weinmann et al. (Pediatrics. 2019 Jun 10. doi: 10.1542/peds.2018-3561). Dr. Gershon had no relevant financial disclosures. The commentary was funded by the National Institutes of Health.
The finding of a 78% lower incidence of zoster in varicella-vaccinated children is nothing short of “remarkable,” Anne A Gershon, MD, wrote in an accompanying editorial.
But the benefit could be in jeopardy, as parents question the safety and effectiveness of all vaccines, she wrote.
“That the varicella vaccine prevents not only varicella but zoster as well is an exciting dual benefit from the varicella vaccine, further improving the health of children by immunization,” Dr. Gershon said. “Additional studies will be necessary to show the mechanism for the protection against zoster (viral, immunologic, or both), how long this benefit lasts, and whether additional doses of some form of VZV [varicella-zoster virus] vaccine will be more useful.”
But, she suggested, in a time when cases of clinical varicella are dwindling, so is public awareness of the vaccine’s benefit. Clinical varicella is worse for adults than it is for children.
“Efforts to immunize all children against chickenpox must continue to be made to protect our population from wild-type VZV. Fortunately, antiviral therapy is also available for individuals who are unvaccinated and develop varicella or zoster, but immunization is, as usual, preferable,” Dr. Gershon concluded.
Dr. Gershon, a pediatric infectious disease specialist, is a professor of pediatrics at Columbia University, New York. She wrote a commentary to accompany the article by Weinmann et al. (Pediatrics. 2019 Jun 10. doi: 10.1542/peds.2018-3561). Dr. Gershon had no relevant financial disclosures. The commentary was funded by the National Institutes of Health.
The finding of a 78% lower incidence of zoster in varicella-vaccinated children is nothing short of “remarkable,” Anne A Gershon, MD, wrote in an accompanying editorial.
But the benefit could be in jeopardy, as parents question the safety and effectiveness of all vaccines, she wrote.
“That the varicella vaccine prevents not only varicella but zoster as well is an exciting dual benefit from the varicella vaccine, further improving the health of children by immunization,” Dr. Gershon said. “Additional studies will be necessary to show the mechanism for the protection against zoster (viral, immunologic, or both), how long this benefit lasts, and whether additional doses of some form of VZV [varicella-zoster virus] vaccine will be more useful.”
But, she suggested, in a time when cases of clinical varicella are dwindling, so is public awareness of the vaccine’s benefit. Clinical varicella is worse for adults than it is for children.
“Efforts to immunize all children against chickenpox must continue to be made to protect our population from wild-type VZV. Fortunately, antiviral therapy is also available for individuals who are unvaccinated and develop varicella or zoster, but immunization is, as usual, preferable,” Dr. Gershon concluded.
Dr. Gershon, a pediatric infectious disease specialist, is a professor of pediatrics at Columbia University, New York. She wrote a commentary to accompany the article by Weinmann et al. (Pediatrics. 2019 Jun 10. doi: 10.1542/peds.2018-3561). Dr. Gershon had no relevant financial disclosures. The commentary was funded by the National Institutes of Health.
than those in unvaccinated children.
The benefit became largely apparent after children received the second vaccination in the recommended series, and persisted throughout childhood, Sheila Weinmann, PhD, of Kaiser Permanente Northern California, Oakland, and colleagues said.*
The analysis included 6.37 million children in the Kaiser Permanente database, 50% of whom were vaccinated for all or some of the study period stretching from 2003 to 2014. Overall, the crude lab-confirmed herpes zoster (HZ) incidence rate was 74/100,000 person-years. When stratified by vaccine status, the crude rate of HZ among vaccinated children was 78% lower than among unvaccinated children (38 vs. 170 cases per 100,000 person years).
Herpes zoster was more common among girls than boys and up to six times more common in immunosuppressed children than in nonimmunosuppressed children.
The authors also found that unvaccinated children benefited from the high rate of vaccination around them. Although the HZ rate was always lower among vaccinated children, the rate among unvaccinated children fell sharply after 2007.
“The trend of decreasing HZ incidence among children who were unvaccinated is likely due to a lack of primary VZV [varicella-zoster virus] infection resulting from herd immunity in a highly vaccinated population,” Dr. Weinmann and her associates said.
There was some variability among age groups, especially among the youngest who were not fully vaccinated.
“In the group aged 1-2 years, the confirmation-adjusted HZ rate among children who were vaccinated was 70% higher than among those who were unvaccinated,” the authors said. In the “older groups, HZ rates were significantly higher in children who were unvaccinated than in those who were vaccinated,” the researchers noted.
The highest incidence was among vaccinated 1-year-olds, who had a 140% higher risk of HZ than did unvaccinated 1-year-olds. But this risk elevation disappeared by age 2 years. For everyone else, aged 2-17 years, the rate of HZ remained significantly lower in vaccinated children.
“Among the small number of children vaccinated at 11 months of age (for whom the vaccine is not recommended), the HZ incidence rate was significantly higher than in children vaccinated at 1 year of age and older. Similarly, children who contract wild-type varicella infection at younger than 1 year of age also have a higher risk of HZ (relative risk, 13.5). The immature adaptive T-cell response in children less than 1 year of age appears less able to contain VZV as a latent infection, compared with older children.
“Our findings for 11-month-olds who were vaccinated should be interpreted with caution because this population included only three cases of HZ and could have included children participating in a prelicensure study with a vaccine formulation different from Varivax,” Dr. Weinmann and her associates said.
Dr. Weinmann and her associates reported no relevant financial disclosures. The study was supported by the Centers for Disease Control and Prevention.
SOURCE: Weinmann S et al. Pediatrics. 2019 Jun 10. doi: 10.1542/peds.2018-2917.
* This article was updated 6/14/2019
than those in unvaccinated children.
The benefit became largely apparent after children received the second vaccination in the recommended series, and persisted throughout childhood, Sheila Weinmann, PhD, of Kaiser Permanente Northern California, Oakland, and colleagues said.*
The analysis included 6.37 million children in the Kaiser Permanente database, 50% of whom were vaccinated for all or some of the study period stretching from 2003 to 2014. Overall, the crude lab-confirmed herpes zoster (HZ) incidence rate was 74/100,000 person-years. When stratified by vaccine status, the crude rate of HZ among vaccinated children was 78% lower than among unvaccinated children (38 vs. 170 cases per 100,000 person years).
Herpes zoster was more common among girls than boys and up to six times more common in immunosuppressed children than in nonimmunosuppressed children.
The authors also found that unvaccinated children benefited from the high rate of vaccination around them. Although the HZ rate was always lower among vaccinated children, the rate among unvaccinated children fell sharply after 2007.
“The trend of decreasing HZ incidence among children who were unvaccinated is likely due to a lack of primary VZV [varicella-zoster virus] infection resulting from herd immunity in a highly vaccinated population,” Dr. Weinmann and her associates said.
There was some variability among age groups, especially among the youngest who were not fully vaccinated.
“In the group aged 1-2 years, the confirmation-adjusted HZ rate among children who were vaccinated was 70% higher than among those who were unvaccinated,” the authors said. In the “older groups, HZ rates were significantly higher in children who were unvaccinated than in those who were vaccinated,” the researchers noted.
The highest incidence was among vaccinated 1-year-olds, who had a 140% higher risk of HZ than did unvaccinated 1-year-olds. But this risk elevation disappeared by age 2 years. For everyone else, aged 2-17 years, the rate of HZ remained significantly lower in vaccinated children.
“Among the small number of children vaccinated at 11 months of age (for whom the vaccine is not recommended), the HZ incidence rate was significantly higher than in children vaccinated at 1 year of age and older. Similarly, children who contract wild-type varicella infection at younger than 1 year of age also have a higher risk of HZ (relative risk, 13.5). The immature adaptive T-cell response in children less than 1 year of age appears less able to contain VZV as a latent infection, compared with older children.
“Our findings for 11-month-olds who were vaccinated should be interpreted with caution because this population included only three cases of HZ and could have included children participating in a prelicensure study with a vaccine formulation different from Varivax,” Dr. Weinmann and her associates said.
Dr. Weinmann and her associates reported no relevant financial disclosures. The study was supported by the Centers for Disease Control and Prevention.
SOURCE: Weinmann S et al. Pediatrics. 2019 Jun 10. doi: 10.1542/peds.2018-2917.
* This article was updated 6/14/2019
FROM PEDIATRICS
Key clinical point: Varicella vaccine is preventing pediatric zoster among children aged 2-17 years.
Major finding: Varicella-vaccinated children have a 78% lower incidence of pediatric zoster than do unvaccinated children.
Study details: The population-based cohort study included more than 6.3 million children.
Disclosures: Dr. Weinmann and her associates reported no relevant financial disclosures. The study was supported by the Centers for Disease Control and Prevention.
Source: Weinmann S et al. Pediatrics. 2019. doi: 10.1542/peds.2018-2917.
Consider measles vaccine booster in HIV-positive patients
LJUBLJANA, SLOVENIA – A “surprisingly low” prevalence of protective antibodies against measles is present in adolescents and adults living with HIV infection despite their prior vaccination against the resurgent disease, Raquel M. Simakawa, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“With the present concern about the global reemergence of measles, we should consider measuring measles antibodies in people living with HIV, especially those who acquired the infection vertically, and then revaccinating those with low titers,” said Dr. Simakawa of the Federal University of São Paolo.
She presented interim findings of an ongoing study of the measles immunologic status of persons living with HIV, which for this analysis included 57 patients who acquired HIV from their mother via vertical transmission and 24 with horizontally acquired HIV. The vertical-transmission group was significantly younger, with a median age of 20 years, compared with 31 years in the horizontal group, who were diagnosed with HIV infection at an average age of 24 years. The vast majority of subjects were on combination antiretroviral therapy. No detectable HIV viral load had been present for a median of 70 months in the vertical group and 25 months in the horizontal group.
Only a mere 7% of the vertical transmission group had protective levels of measles IgG antibodies as measured by enzyme-linked immunosorbent assay, as did 29% of the horizontal group. The likely explanation for the higher rate of protection in the horizontal group, she said, is that they received their routine measles vaccination before they acquired HIV infection, and some of them didn’t lose their protective antibodies during their immune system’s fight against HIV infection.
Session chair Nico G. Hartwig, MD, of Franciscus Hospital in Rotterdam, the Netherlands, posed a question: Given the sky-high rate of measles seronegativity status among the vertically transmitted HIV-positive group – the patient population pediatricians focus on – why bother to measure their measles antibody level? Why not just give them all a measles booster?
Dr. Simakawa replied that that’s worth considering in routine clinical practice now that her study has shown that this group is more vulnerable to measles because of their poor response to immunization. But the study is ongoing, with larger numbers of patients to be enrolled. Also, in the second phase of the study, which will include a control group, measles IgG antibodies will be remeasured 1 month after administration of a new dose of measles vaccine.
She reported having no financial conflicts regarding this study, conducted free of commercial support.
LJUBLJANA, SLOVENIA – A “surprisingly low” prevalence of protective antibodies against measles is present in adolescents and adults living with HIV infection despite their prior vaccination against the resurgent disease, Raquel M. Simakawa, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“With the present concern about the global reemergence of measles, we should consider measuring measles antibodies in people living with HIV, especially those who acquired the infection vertically, and then revaccinating those with low titers,” said Dr. Simakawa of the Federal University of São Paolo.
She presented interim findings of an ongoing study of the measles immunologic status of persons living with HIV, which for this analysis included 57 patients who acquired HIV from their mother via vertical transmission and 24 with horizontally acquired HIV. The vertical-transmission group was significantly younger, with a median age of 20 years, compared with 31 years in the horizontal group, who were diagnosed with HIV infection at an average age of 24 years. The vast majority of subjects were on combination antiretroviral therapy. No detectable HIV viral load had been present for a median of 70 months in the vertical group and 25 months in the horizontal group.
Only a mere 7% of the vertical transmission group had protective levels of measles IgG antibodies as measured by enzyme-linked immunosorbent assay, as did 29% of the horizontal group. The likely explanation for the higher rate of protection in the horizontal group, she said, is that they received their routine measles vaccination before they acquired HIV infection, and some of them didn’t lose their protective antibodies during their immune system’s fight against HIV infection.
Session chair Nico G. Hartwig, MD, of Franciscus Hospital in Rotterdam, the Netherlands, posed a question: Given the sky-high rate of measles seronegativity status among the vertically transmitted HIV-positive group – the patient population pediatricians focus on – why bother to measure their measles antibody level? Why not just give them all a measles booster?
Dr. Simakawa replied that that’s worth considering in routine clinical practice now that her study has shown that this group is more vulnerable to measles because of their poor response to immunization. But the study is ongoing, with larger numbers of patients to be enrolled. Also, in the second phase of the study, which will include a control group, measles IgG antibodies will be remeasured 1 month after administration of a new dose of measles vaccine.
She reported having no financial conflicts regarding this study, conducted free of commercial support.
LJUBLJANA, SLOVENIA – A “surprisingly low” prevalence of protective antibodies against measles is present in adolescents and adults living with HIV infection despite their prior vaccination against the resurgent disease, Raquel M. Simakawa, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“With the present concern about the global reemergence of measles, we should consider measuring measles antibodies in people living with HIV, especially those who acquired the infection vertically, and then revaccinating those with low titers,” said Dr. Simakawa of the Federal University of São Paolo.
She presented interim findings of an ongoing study of the measles immunologic status of persons living with HIV, which for this analysis included 57 patients who acquired HIV from their mother via vertical transmission and 24 with horizontally acquired HIV. The vertical-transmission group was significantly younger, with a median age of 20 years, compared with 31 years in the horizontal group, who were diagnosed with HIV infection at an average age of 24 years. The vast majority of subjects were on combination antiretroviral therapy. No detectable HIV viral load had been present for a median of 70 months in the vertical group and 25 months in the horizontal group.
Only a mere 7% of the vertical transmission group had protective levels of measles IgG antibodies as measured by enzyme-linked immunosorbent assay, as did 29% of the horizontal group. The likely explanation for the higher rate of protection in the horizontal group, she said, is that they received their routine measles vaccination before they acquired HIV infection, and some of them didn’t lose their protective antibodies during their immune system’s fight against HIV infection.
Session chair Nico G. Hartwig, MD, of Franciscus Hospital in Rotterdam, the Netherlands, posed a question: Given the sky-high rate of measles seronegativity status among the vertically transmitted HIV-positive group – the patient population pediatricians focus on – why bother to measure their measles antibody level? Why not just give them all a measles booster?
Dr. Simakawa replied that that’s worth considering in routine clinical practice now that her study has shown that this group is more vulnerable to measles because of their poor response to immunization. But the study is ongoing, with larger numbers of patients to be enrolled. Also, in the second phase of the study, which will include a control group, measles IgG antibodies will be remeasured 1 month after administration of a new dose of measles vaccine.
She reported having no financial conflicts regarding this study, conducted free of commercial support.
REPORTING FROM ESPID 2019
Expanded indication being considered for meningococcal group B vaccine
LJUBLJANA, SLOVENIA – under the agency’s Breakthrough Therapy designation.
Breakthrough Therapy status is reserved for accelerated review of therapies considered to show substantial preliminary promise of effectively targeting a major unmet medical need.
The unmet need here is that there is no meningococcal group B vaccine approved for use in children under age 10 years. Yet infants and children under 5 years of age are at greatest risk of invasive meningococcal B disease, with reported case fatality rates of 8%-9%, Jason D. Maguire, MD, noted at the annual meeting of the European Society for Paediatric Infectious Diseases.
Trumenba has been approved in the United States for patients aged 10-25 years and in the European Union for individuals aged 10 years or older.
Dr. Maguire, of Pfizer’s vaccine clinical research and development program, presented the results of the two phase 2 randomized safety and immunogenicity trials conducted in patients aged 1- 9 years that the company has submitted to the FDA in support of the expanded indication. One study was carried out in 352 1-year-old toddlers, the other in 400 children aged 2-9 years, whose mean age was 4 years. The studies were carried out in Australia, Finland, Poland, and the Czech Republic.
In a pooled analysis of the vaccine’s immunogenicity when administered in a three-dose schedule of 120 mcg at 0, 2, and 6 months to 193 toddlers and 274 of the children aged 2-9 years, robust bactericidal antibody responses were seen against the four major Neisseria meningitidis group B strains that cause invasive disease. In fact, at least a fourfold rise in titers from baseline to 1 month after dose three was documented in the same high proportion of 1- to 9-year-olds as previously seen in the phase 3 trials that led to vaccine licensure in adolescents and young adults.
“These results support that the use of Trumenba, when given to children ages 1 to less than 10 years at the same dose and schedule that is currently approved in adolescents and young adults, can afford a high degree of protective antibody responses that correlate with immunity in this population,” Dr. Maguire said.
The safety and tolerability analysis included all 752 children in the two phase 2 studies, including the 110 toddlers randomized to three 60-mcg doses of the vaccine, although it has subsequently become clear that 120 mcg is the dose that provides the best immunogenicity with an acceptable safety profile, according to the physician.
Across the age groups, local reactions, including redness and swelling, were more common in Trumenba recipients than in controls who received hepatitis A vaccine and saline injections. So were systemic adverse events. Fever – a systemic event of particular interest to parents and clinicians – occurred in 37% of toddlers after vaccination, compared with 25% of 2- to 9-year-olds and 10%-12% of controls. Of note, prophylactic antipyretics weren’t allowed in the study.
“There’s somewhat of an inverse relationship between age and temperature. So as we go down in age, the rate of fever rises. But after each subsequent dose, regardless of age, there’s a reduction in the incidence of fever,” Dr. Maguire observed.
Most fevers were less than 39.0° C. Only 3 of 752 (less than 1%) patients experienced fever in excess of 40.0° C.
Two children withdrew from the study after developing hip synovitis, which was transient. Another withdrew because of prolonged irritability, fatigue, and decreased appetite.
“Although Trumenba had an acceptable safety and tolerability profile in 1- to 9-year-olds, this analysis wasn’t powered enough to detect uncommon adverse events, so we’ll continue to monitor safety for things like synovitis,” he said.
In 10- to 25-year-olds, the meningococcal vaccine can be given concomitantly with other vaccines without interference. There are plans to study concurrent vaccination with MMR and pneumococcal vaccines in 1- to 9-year-olds as well, according to Dr. Maguire.
Pfizer also now is planning clinical trials of the vaccine in infants, another important group currently unprotected against meningococcal group B disease, he added.
Dr. Maguire is an employee of Pfizer, who funded the studies.
LJUBLJANA, SLOVENIA – under the agency’s Breakthrough Therapy designation.
Breakthrough Therapy status is reserved for accelerated review of therapies considered to show substantial preliminary promise of effectively targeting a major unmet medical need.
The unmet need here is that there is no meningococcal group B vaccine approved for use in children under age 10 years. Yet infants and children under 5 years of age are at greatest risk of invasive meningococcal B disease, with reported case fatality rates of 8%-9%, Jason D. Maguire, MD, noted at the annual meeting of the European Society for Paediatric Infectious Diseases.
Trumenba has been approved in the United States for patients aged 10-25 years and in the European Union for individuals aged 10 years or older.
Dr. Maguire, of Pfizer’s vaccine clinical research and development program, presented the results of the two phase 2 randomized safety and immunogenicity trials conducted in patients aged 1- 9 years that the company has submitted to the FDA in support of the expanded indication. One study was carried out in 352 1-year-old toddlers, the other in 400 children aged 2-9 years, whose mean age was 4 years. The studies were carried out in Australia, Finland, Poland, and the Czech Republic.
In a pooled analysis of the vaccine’s immunogenicity when administered in a three-dose schedule of 120 mcg at 0, 2, and 6 months to 193 toddlers and 274 of the children aged 2-9 years, robust bactericidal antibody responses were seen against the four major Neisseria meningitidis group B strains that cause invasive disease. In fact, at least a fourfold rise in titers from baseline to 1 month after dose three was documented in the same high proportion of 1- to 9-year-olds as previously seen in the phase 3 trials that led to vaccine licensure in adolescents and young adults.
“These results support that the use of Trumenba, when given to children ages 1 to less than 10 years at the same dose and schedule that is currently approved in adolescents and young adults, can afford a high degree of protective antibody responses that correlate with immunity in this population,” Dr. Maguire said.
The safety and tolerability analysis included all 752 children in the two phase 2 studies, including the 110 toddlers randomized to three 60-mcg doses of the vaccine, although it has subsequently become clear that 120 mcg is the dose that provides the best immunogenicity with an acceptable safety profile, according to the physician.
Across the age groups, local reactions, including redness and swelling, were more common in Trumenba recipients than in controls who received hepatitis A vaccine and saline injections. So were systemic adverse events. Fever – a systemic event of particular interest to parents and clinicians – occurred in 37% of toddlers after vaccination, compared with 25% of 2- to 9-year-olds and 10%-12% of controls. Of note, prophylactic antipyretics weren’t allowed in the study.
“There’s somewhat of an inverse relationship between age and temperature. So as we go down in age, the rate of fever rises. But after each subsequent dose, regardless of age, there’s a reduction in the incidence of fever,” Dr. Maguire observed.
Most fevers were less than 39.0° C. Only 3 of 752 (less than 1%) patients experienced fever in excess of 40.0° C.
Two children withdrew from the study after developing hip synovitis, which was transient. Another withdrew because of prolonged irritability, fatigue, and decreased appetite.
“Although Trumenba had an acceptable safety and tolerability profile in 1- to 9-year-olds, this analysis wasn’t powered enough to detect uncommon adverse events, so we’ll continue to monitor safety for things like synovitis,” he said.
In 10- to 25-year-olds, the meningococcal vaccine can be given concomitantly with other vaccines without interference. There are plans to study concurrent vaccination with MMR and pneumococcal vaccines in 1- to 9-year-olds as well, according to Dr. Maguire.
Pfizer also now is planning clinical trials of the vaccine in infants, another important group currently unprotected against meningococcal group B disease, he added.
Dr. Maguire is an employee of Pfizer, who funded the studies.
LJUBLJANA, SLOVENIA – under the agency’s Breakthrough Therapy designation.
Breakthrough Therapy status is reserved for accelerated review of therapies considered to show substantial preliminary promise of effectively targeting a major unmet medical need.
The unmet need here is that there is no meningococcal group B vaccine approved for use in children under age 10 years. Yet infants and children under 5 years of age are at greatest risk of invasive meningococcal B disease, with reported case fatality rates of 8%-9%, Jason D. Maguire, MD, noted at the annual meeting of the European Society for Paediatric Infectious Diseases.
Trumenba has been approved in the United States for patients aged 10-25 years and in the European Union for individuals aged 10 years or older.
Dr. Maguire, of Pfizer’s vaccine clinical research and development program, presented the results of the two phase 2 randomized safety and immunogenicity trials conducted in patients aged 1- 9 years that the company has submitted to the FDA in support of the expanded indication. One study was carried out in 352 1-year-old toddlers, the other in 400 children aged 2-9 years, whose mean age was 4 years. The studies were carried out in Australia, Finland, Poland, and the Czech Republic.
In a pooled analysis of the vaccine’s immunogenicity when administered in a three-dose schedule of 120 mcg at 0, 2, and 6 months to 193 toddlers and 274 of the children aged 2-9 years, robust bactericidal antibody responses were seen against the four major Neisseria meningitidis group B strains that cause invasive disease. In fact, at least a fourfold rise in titers from baseline to 1 month after dose three was documented in the same high proportion of 1- to 9-year-olds as previously seen in the phase 3 trials that led to vaccine licensure in adolescents and young adults.
“These results support that the use of Trumenba, when given to children ages 1 to less than 10 years at the same dose and schedule that is currently approved in adolescents and young adults, can afford a high degree of protective antibody responses that correlate with immunity in this population,” Dr. Maguire said.
The safety and tolerability analysis included all 752 children in the two phase 2 studies, including the 110 toddlers randomized to three 60-mcg doses of the vaccine, although it has subsequently become clear that 120 mcg is the dose that provides the best immunogenicity with an acceptable safety profile, according to the physician.
Across the age groups, local reactions, including redness and swelling, were more common in Trumenba recipients than in controls who received hepatitis A vaccine and saline injections. So were systemic adverse events. Fever – a systemic event of particular interest to parents and clinicians – occurred in 37% of toddlers after vaccination, compared with 25% of 2- to 9-year-olds and 10%-12% of controls. Of note, prophylactic antipyretics weren’t allowed in the study.
“There’s somewhat of an inverse relationship between age and temperature. So as we go down in age, the rate of fever rises. But after each subsequent dose, regardless of age, there’s a reduction in the incidence of fever,” Dr. Maguire observed.
Most fevers were less than 39.0° C. Only 3 of 752 (less than 1%) patients experienced fever in excess of 40.0° C.
Two children withdrew from the study after developing hip synovitis, which was transient. Another withdrew because of prolonged irritability, fatigue, and decreased appetite.
“Although Trumenba had an acceptable safety and tolerability profile in 1- to 9-year-olds, this analysis wasn’t powered enough to detect uncommon adverse events, so we’ll continue to monitor safety for things like synovitis,” he said.
In 10- to 25-year-olds, the meningococcal vaccine can be given concomitantly with other vaccines without interference. There are plans to study concurrent vaccination with MMR and pneumococcal vaccines in 1- to 9-year-olds as well, according to Dr. Maguire.
Pfizer also now is planning clinical trials of the vaccine in infants, another important group currently unprotected against meningococcal group B disease, he added.
Dr. Maguire is an employee of Pfizer, who funded the studies.
EXPERT ANALYSIS FROM ESPID 2019
















