User login
Obesity doesn’t hamper flu vaccine response in pregnancy
LJUBLJANA, SLOVENIA – ; indeed, it might actually improve their seroconversion rate, Michelle Clarke reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
She presented a prospective cohort study of 90 women vaccinated against influenza during pregnancy, 24 of whom had a BMI of 30 kg/m2 or more. The impetus for the study was the investigators’ understanding that influenza in pregnancy carries an increased risk of severe complications, obesity is a known risk factor for more severe episodes of influenza, and vaccine responses could potentially be adversely affected by obesity, either because of the associated inflammatory state and altered cytokine profile or inadequate vaccine delivery via the intramuscular route. Yet the impact of obesity on vaccine responses in pregnancy has been unclear.
Blood samples obtained before and 1 month after vaccination showed similarly high-titer postvaccination seropositivity rates against influenza B, H3N2, and H1N1 regardless of the women’s weight status. Indeed, the seropositivity rate against all three influenza viruses was higher in the obese subgroup, by a margin of 92%-74%. Also, postvaccination geometric mean antibody titers were significantly higher in the obese group. Particularly impressive was the difference in H1N1 seroconversion, defined as a fourfold increase in titer 28 days after vaccination: 79% versus 55%, noted Ms. Clarke of the University of Adelaide.
Of note, influenza vaccination in the first trimester resulted in a significantly lower seropositive antibody rate than vaccination in the second or third trimesters. The implication is that gestational age at vaccination, regardless of BMI, may be an important determinant of optimal vaccine protection for mothers and their newborns. However, this tentative conclusion requires confirmation in an independent larger sample, because the patient numbers in the study were small: Seropositive antibodies to all three vaccine antigens were documented in just 7 of 12 women (58%) vaccinated in the first trimester, compared with 47 of 53 (89%) vaccinated in the second trimester and 18 of 25 (72%) in the third.
Ms. Clarke reported having no financial conflicts regarding the study, which was supported by the Women’s and Children’s Hospital Research Foundation.
LJUBLJANA, SLOVENIA – ; indeed, it might actually improve their seroconversion rate, Michelle Clarke reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
She presented a prospective cohort study of 90 women vaccinated against influenza during pregnancy, 24 of whom had a BMI of 30 kg/m2 or more. The impetus for the study was the investigators’ understanding that influenza in pregnancy carries an increased risk of severe complications, obesity is a known risk factor for more severe episodes of influenza, and vaccine responses could potentially be adversely affected by obesity, either because of the associated inflammatory state and altered cytokine profile or inadequate vaccine delivery via the intramuscular route. Yet the impact of obesity on vaccine responses in pregnancy has been unclear.
Blood samples obtained before and 1 month after vaccination showed similarly high-titer postvaccination seropositivity rates against influenza B, H3N2, and H1N1 regardless of the women’s weight status. Indeed, the seropositivity rate against all three influenza viruses was higher in the obese subgroup, by a margin of 92%-74%. Also, postvaccination geometric mean antibody titers were significantly higher in the obese group. Particularly impressive was the difference in H1N1 seroconversion, defined as a fourfold increase in titer 28 days after vaccination: 79% versus 55%, noted Ms. Clarke of the University of Adelaide.
Of note, influenza vaccination in the first trimester resulted in a significantly lower seropositive antibody rate than vaccination in the second or third trimesters. The implication is that gestational age at vaccination, regardless of BMI, may be an important determinant of optimal vaccine protection for mothers and their newborns. However, this tentative conclusion requires confirmation in an independent larger sample, because the patient numbers in the study were small: Seropositive antibodies to all three vaccine antigens were documented in just 7 of 12 women (58%) vaccinated in the first trimester, compared with 47 of 53 (89%) vaccinated in the second trimester and 18 of 25 (72%) in the third.
Ms. Clarke reported having no financial conflicts regarding the study, which was supported by the Women’s and Children’s Hospital Research Foundation.
LJUBLJANA, SLOVENIA – ; indeed, it might actually improve their seroconversion rate, Michelle Clarke reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
She presented a prospective cohort study of 90 women vaccinated against influenza during pregnancy, 24 of whom had a BMI of 30 kg/m2 or more. The impetus for the study was the investigators’ understanding that influenza in pregnancy carries an increased risk of severe complications, obesity is a known risk factor for more severe episodes of influenza, and vaccine responses could potentially be adversely affected by obesity, either because of the associated inflammatory state and altered cytokine profile or inadequate vaccine delivery via the intramuscular route. Yet the impact of obesity on vaccine responses in pregnancy has been unclear.
Blood samples obtained before and 1 month after vaccination showed similarly high-titer postvaccination seropositivity rates against influenza B, H3N2, and H1N1 regardless of the women’s weight status. Indeed, the seropositivity rate against all three influenza viruses was higher in the obese subgroup, by a margin of 92%-74%. Also, postvaccination geometric mean antibody titers were significantly higher in the obese group. Particularly impressive was the difference in H1N1 seroconversion, defined as a fourfold increase in titer 28 days after vaccination: 79% versus 55%, noted Ms. Clarke of the University of Adelaide.
Of note, influenza vaccination in the first trimester resulted in a significantly lower seropositive antibody rate than vaccination in the second or third trimesters. The implication is that gestational age at vaccination, regardless of BMI, may be an important determinant of optimal vaccine protection for mothers and their newborns. However, this tentative conclusion requires confirmation in an independent larger sample, because the patient numbers in the study were small: Seropositive antibodies to all three vaccine antigens were documented in just 7 of 12 women (58%) vaccinated in the first trimester, compared with 47 of 53 (89%) vaccinated in the second trimester and 18 of 25 (72%) in the third.
Ms. Clarke reported having no financial conflicts regarding the study, which was supported by the Women’s and Children’s Hospital Research Foundation.
REPORTING FROM ESPID 2019
Key clinical point: High BMI doesn’t impair influenza vaccine responses in pregnant women.
Major finding: Protective antibody levels against all three vaccine antigens were documented 1 month post vaccination in 92% of the obese and 74% of the nonobese mothers.
Study details: This was a prospective observational study of 90 women vaccinated against influenza during pregnancy, 24 of whom were obese.
Disclosures: The study was supported by the University of Adelaide Women’s and Children’s Hospital Research Foundation.
Measles cases now at highest level since 1992
With 971 cases of measles reported after just 5 months of 2019, the United States has hit another dubious milestone by surpassing the 963 cases reported in the preelimination year of 1994, according to the Centers for Disease Control and Prevention.
That leaves 1992, when there were 2,237 cases reported, as the next big obstacle on measles’ current path of distinction, the CDC data show. Only 312 cases were reported in 1993.
“Outbreaks in New York City and Rockland County, New York have continued for nearly 8 months. That loss would be a huge blow for the nation and erase the hard work done by all levels of public health,” the CDC said May 30.
The CDC defines measles elimination as “the absence of continuous disease transmission for 12 months or more in a specific geographic area” and notes that “measles is no longer endemic [constantly present] in the United States.”
“Measles is preventable and the way to end this outbreak is to ensure that all children and adults who can get vaccinated, do get vaccinated. Again, I want to reassure parents that vaccines are safe, they do not cause autism. The greater danger is the disease that vaccination prevents,” CDC director Robert Redfield, MD, said in a statement.
With 971 cases of measles reported after just 5 months of 2019, the United States has hit another dubious milestone by surpassing the 963 cases reported in the preelimination year of 1994, according to the Centers for Disease Control and Prevention.
That leaves 1992, when there were 2,237 cases reported, as the next big obstacle on measles’ current path of distinction, the CDC data show. Only 312 cases were reported in 1993.
“Outbreaks in New York City and Rockland County, New York have continued for nearly 8 months. That loss would be a huge blow for the nation and erase the hard work done by all levels of public health,” the CDC said May 30.
The CDC defines measles elimination as “the absence of continuous disease transmission for 12 months or more in a specific geographic area” and notes that “measles is no longer endemic [constantly present] in the United States.”
“Measles is preventable and the way to end this outbreak is to ensure that all children and adults who can get vaccinated, do get vaccinated. Again, I want to reassure parents that vaccines are safe, they do not cause autism. The greater danger is the disease that vaccination prevents,” CDC director Robert Redfield, MD, said in a statement.
With 971 cases of measles reported after just 5 months of 2019, the United States has hit another dubious milestone by surpassing the 963 cases reported in the preelimination year of 1994, according to the Centers for Disease Control and Prevention.
That leaves 1992, when there were 2,237 cases reported, as the next big obstacle on measles’ current path of distinction, the CDC data show. Only 312 cases were reported in 1993.
“Outbreaks in New York City and Rockland County, New York have continued for nearly 8 months. That loss would be a huge blow for the nation and erase the hard work done by all levels of public health,” the CDC said May 30.
The CDC defines measles elimination as “the absence of continuous disease transmission for 12 months or more in a specific geographic area” and notes that “measles is no longer endemic [constantly present] in the United States.”
“Measles is preventable and the way to end this outbreak is to ensure that all children and adults who can get vaccinated, do get vaccinated. Again, I want to reassure parents that vaccines are safe, they do not cause autism. The greater danger is the disease that vaccination prevents,” CDC director Robert Redfield, MD, said in a statement.
Refrigerator-stable varicella vaccine held safe and effective
profiles when administered concomitantly with MMR vaccine, according to a study in Vaccine.
In this double-blind, controlled, multicenter study, Keith S. Reisinger of Primary Physicians Research, Pittsburgh, and his colleagues randomized 958 subjects aged 12-23 months to receive either 8,000 plaque-forming units (PFU) refrigerated vaccine (n = 320; group 1), 25,000 PFU refrigerated vaccine (n = 315; group 2), or 10,000 PFU frozen vaccine (n = 323; group 3), and subjects in all three groups also received MMR vaccine. The primary endpoint for immunogenicity was percentage of subjects with at least 5 titers of varicella antibody according to glycoprotein enzyme-linked immunosorbent assay among the three groups 6 weeks post vaccination; the primary safety endpoint was incidences of vaccine-related adverse events during days 0-42 post vaccination.
The percentages of subjects meeting the primary endpoint for immunogenicity were comparable among the groups, at 93%, 94%, and 95% for groups 1, 2, and 3, respectively. Results for the safety endpoints also were similar among the groups; for example, rates of injection-site adverse events or vaccine-related injection-site adverse events were 44%, 40%, and 43%, with no statistically significant between-group differences.
The study authors noted that one of the problems with having only frozen formulations is how that limits availability in parts of the world where only refrigeration of 2°C–8°C is available. “Use of a refrigerator-stable formulation of varicella vaccine will allow for increased availability of the product throughout the world and may help to increase vaccination rates against varicella,” they concluded.
Strengths of the study included its head-to-head design. Limitations included how adverse events were based on parental reporting.
Some authors reported relationships, including employment, with Merck & Co, which developed the vaccine in this study and funded the study.
SOURCE: Reisinger KS et al. Vaccine. 2018 Aug 23. doi: 10.1016/j.vaccine.2018.01.089.
profiles when administered concomitantly with MMR vaccine, according to a study in Vaccine.
In this double-blind, controlled, multicenter study, Keith S. Reisinger of Primary Physicians Research, Pittsburgh, and his colleagues randomized 958 subjects aged 12-23 months to receive either 8,000 plaque-forming units (PFU) refrigerated vaccine (n = 320; group 1), 25,000 PFU refrigerated vaccine (n = 315; group 2), or 10,000 PFU frozen vaccine (n = 323; group 3), and subjects in all three groups also received MMR vaccine. The primary endpoint for immunogenicity was percentage of subjects with at least 5 titers of varicella antibody according to glycoprotein enzyme-linked immunosorbent assay among the three groups 6 weeks post vaccination; the primary safety endpoint was incidences of vaccine-related adverse events during days 0-42 post vaccination.
The percentages of subjects meeting the primary endpoint for immunogenicity were comparable among the groups, at 93%, 94%, and 95% for groups 1, 2, and 3, respectively. Results for the safety endpoints also were similar among the groups; for example, rates of injection-site adverse events or vaccine-related injection-site adverse events were 44%, 40%, and 43%, with no statistically significant between-group differences.
The study authors noted that one of the problems with having only frozen formulations is how that limits availability in parts of the world where only refrigeration of 2°C–8°C is available. “Use of a refrigerator-stable formulation of varicella vaccine will allow for increased availability of the product throughout the world and may help to increase vaccination rates against varicella,” they concluded.
Strengths of the study included its head-to-head design. Limitations included how adverse events were based on parental reporting.
Some authors reported relationships, including employment, with Merck & Co, which developed the vaccine in this study and funded the study.
SOURCE: Reisinger KS et al. Vaccine. 2018 Aug 23. doi: 10.1016/j.vaccine.2018.01.089.
profiles when administered concomitantly with MMR vaccine, according to a study in Vaccine.
In this double-blind, controlled, multicenter study, Keith S. Reisinger of Primary Physicians Research, Pittsburgh, and his colleagues randomized 958 subjects aged 12-23 months to receive either 8,000 plaque-forming units (PFU) refrigerated vaccine (n = 320; group 1), 25,000 PFU refrigerated vaccine (n = 315; group 2), or 10,000 PFU frozen vaccine (n = 323; group 3), and subjects in all three groups also received MMR vaccine. The primary endpoint for immunogenicity was percentage of subjects with at least 5 titers of varicella antibody according to glycoprotein enzyme-linked immunosorbent assay among the three groups 6 weeks post vaccination; the primary safety endpoint was incidences of vaccine-related adverse events during days 0-42 post vaccination.
The percentages of subjects meeting the primary endpoint for immunogenicity were comparable among the groups, at 93%, 94%, and 95% for groups 1, 2, and 3, respectively. Results for the safety endpoints also were similar among the groups; for example, rates of injection-site adverse events or vaccine-related injection-site adverse events were 44%, 40%, and 43%, with no statistically significant between-group differences.
The study authors noted that one of the problems with having only frozen formulations is how that limits availability in parts of the world where only refrigeration of 2°C–8°C is available. “Use of a refrigerator-stable formulation of varicella vaccine will allow for increased availability of the product throughout the world and may help to increase vaccination rates against varicella,” they concluded.
Strengths of the study included its head-to-head design. Limitations included how adverse events were based on parental reporting.
Some authors reported relationships, including employment, with Merck & Co, which developed the vaccine in this study and funded the study.
SOURCE: Reisinger KS et al. Vaccine. 2018 Aug 23. doi: 10.1016/j.vaccine.2018.01.089.
HPV vaccine: Is one dose enough?
LJUBLJANA, SLOVENIA – There is good news and bad news about human papillomavirus (HPV) vaccination as a means of preventing cervical cancer.
The bad news is the HPV vaccines are projected to be in short supply, unable to meet global demand until at least 2024. The good news is that – in one study, for 11 years and counting – which would effectively double the existing supply, Aimee R. Kreimer, PhD, said at the annual meeting of the European Society for Paediatric Infectious Diseases.
These data come from post hoc analyses of major phase 3 randomized controlled trials of bivalent HPV vaccine in Costa Rica and quadrivalent vaccine in India. However, these secondary analyses aren’t considered rock solid evidence because the subjects who got a single dose weren’t randomized to that strategy, they simply for one reason or another didn’t receive the recommended additional dose or doses.
“I don’t know if these studies are enough, so several studies have been launched over the past couple of years with an eye toward generating the quality of data that would be sufficient to motivate policy change, if in fact one dose is proven to be effective,” said Dr. Kreimer, a senior scientist at the National Cancer Institute in Bethesda, Md.
The first of these formal randomized, controlled trials – a delayed second-dose study in 9- to 11-year-old U.S. boys and girls – is due to be completed next year. Four other trials ongoing in Africa and Costa Rica, all in females, are expected to report findings in 2022-2025.
Dr. Kreimer is first author of a soon-to-be-published 11-year update from the phase 3 Costa Rica HPV Vaccine Trial, which was launched prior to licensure of the GlaxoSmithKline bivalent HPV vaccine. Previous analyses showed that at both 4 and 7 years of follow-up, a single dose of the vaccine was as effective as two or three in preventing infection with HPV types 16 and 18, which are covered by the vaccine.
“Now the research question has transitioned to, ‘Will one dose be sufficiently durable?’ she explained.
The answer from this study is yes. At 11 years since receipt of the bivalent HPV vaccine, there was no difference in terms of prevalent HPV 16/18 infection between the one-, two-, and three-dose groups. To address the issue of possible selection bias in this post hoc nonrandomized comparison, Dr. Kreimer and her coinvestigators looked at rates of infection with HPV 31 and 45, which aren’t covered by the vaccine. The rates were similar regardless of the number of vaccine doses received 11 years earlier, indicating women in all three dosing groups are at similar risk for acquiring HPV infection, thus bolstering the legitimacy of the conclusion that one dose provides effective long-term protection.
Intriguingly, HPV serum antibody levels in the single-dose group have remained stable for 11 years at a level that’s only about one-quarter of that associated with three doses of the vaccine, albeit an order of magnitude greater than the level induced by natural immunity.
“This really challenges the dogma of the HPV vaccine,” according to Dr. Kreimer. “It suggests that inferior [HPV] antibodies do not necessarily mean inferior protection.”
The explanation for this phenomenon appears to be that HPV subunit vaccine mimics the shell of authentic virions so well that the immune system sees it as dangerous and mounts long-term antibody production. Also, cervical infection by HPV is a relatively slow process, allowing time for vaccine-induced antibodies to interrupt it, she said.
In contrast to the encouraging findings from this post hoc analysis and another from a phase 3 trial of quadrivalent vaccine in India, numerous phase 4 vaccine effectiveness monitoring studies have shown markedly lower vaccine effectiveness for one dose of HPV vaccine. Dr. Kreimer cautioned that this is a flawed conclusion attributable to a methodologic artifact whereby the investigators have lumped together single-dose recipients who were 17 years old or more at the time with those who were younger.
“The problem is that many people who are aged 17-18 years already have HPV infection, so when they are vaccinated it shows up as a vaccine failure. That’s not correct. These are prophylactic HPV vaccines. They’re not meant to help clear an infection,” she noted.
Stepping back, Dr. Kreimer observed that cervical cancer “is really a story of inequality.” Indeed, 90% of cervical cancers occur in low-income countries, where HPV vaccination uptake remains very low even more than a decade after licensure. When modelers project out in the future, they estimate that at current HPV vaccination levels in Sub-Saharan Africa, which has the highest cervical cancer rates in the world, it would take more than 100 years to achieve the World Health Organization goal of eliminating the malignancy.
Asked by an audience member how low a single-dose vaccine effectiveness level she considers acceptable to help reach the goal of eliminating cervical cancer in developing countries, Dr. Kreimer cautioned against the tendency to let ‘perfect’ become the enemy of ‘good.’
“I’ll remind everyone that, in this moment, very few of the target girls in the lower– and upper-lower–income countries are getting any vaccination. So I don’t think it’s a question of whether we should be going from two to one dose, I think it’s really a question of, for those who are at zero doses, how do we get them one dose? And with the HPV vaccine, we’ve even seen suggestions of herd immunity if we have 50% uptake,” she replied.
Dr. Kreimer reported having no financial conflicts regarding her presentation.
LJUBLJANA, SLOVENIA – There is good news and bad news about human papillomavirus (HPV) vaccination as a means of preventing cervical cancer.
The bad news is the HPV vaccines are projected to be in short supply, unable to meet global demand until at least 2024. The good news is that – in one study, for 11 years and counting – which would effectively double the existing supply, Aimee R. Kreimer, PhD, said at the annual meeting of the European Society for Paediatric Infectious Diseases.
These data come from post hoc analyses of major phase 3 randomized controlled trials of bivalent HPV vaccine in Costa Rica and quadrivalent vaccine in India. However, these secondary analyses aren’t considered rock solid evidence because the subjects who got a single dose weren’t randomized to that strategy, they simply for one reason or another didn’t receive the recommended additional dose or doses.
“I don’t know if these studies are enough, so several studies have been launched over the past couple of years with an eye toward generating the quality of data that would be sufficient to motivate policy change, if in fact one dose is proven to be effective,” said Dr. Kreimer, a senior scientist at the National Cancer Institute in Bethesda, Md.
The first of these formal randomized, controlled trials – a delayed second-dose study in 9- to 11-year-old U.S. boys and girls – is due to be completed next year. Four other trials ongoing in Africa and Costa Rica, all in females, are expected to report findings in 2022-2025.
Dr. Kreimer is first author of a soon-to-be-published 11-year update from the phase 3 Costa Rica HPV Vaccine Trial, which was launched prior to licensure of the GlaxoSmithKline bivalent HPV vaccine. Previous analyses showed that at both 4 and 7 years of follow-up, a single dose of the vaccine was as effective as two or three in preventing infection with HPV types 16 and 18, which are covered by the vaccine.
“Now the research question has transitioned to, ‘Will one dose be sufficiently durable?’ she explained.
The answer from this study is yes. At 11 years since receipt of the bivalent HPV vaccine, there was no difference in terms of prevalent HPV 16/18 infection between the one-, two-, and three-dose groups. To address the issue of possible selection bias in this post hoc nonrandomized comparison, Dr. Kreimer and her coinvestigators looked at rates of infection with HPV 31 and 45, which aren’t covered by the vaccine. The rates were similar regardless of the number of vaccine doses received 11 years earlier, indicating women in all three dosing groups are at similar risk for acquiring HPV infection, thus bolstering the legitimacy of the conclusion that one dose provides effective long-term protection.
Intriguingly, HPV serum antibody levels in the single-dose group have remained stable for 11 years at a level that’s only about one-quarter of that associated with three doses of the vaccine, albeit an order of magnitude greater than the level induced by natural immunity.
“This really challenges the dogma of the HPV vaccine,” according to Dr. Kreimer. “It suggests that inferior [HPV] antibodies do not necessarily mean inferior protection.”
The explanation for this phenomenon appears to be that HPV subunit vaccine mimics the shell of authentic virions so well that the immune system sees it as dangerous and mounts long-term antibody production. Also, cervical infection by HPV is a relatively slow process, allowing time for vaccine-induced antibodies to interrupt it, she said.
In contrast to the encouraging findings from this post hoc analysis and another from a phase 3 trial of quadrivalent vaccine in India, numerous phase 4 vaccine effectiveness monitoring studies have shown markedly lower vaccine effectiveness for one dose of HPV vaccine. Dr. Kreimer cautioned that this is a flawed conclusion attributable to a methodologic artifact whereby the investigators have lumped together single-dose recipients who were 17 years old or more at the time with those who were younger.
“The problem is that many people who are aged 17-18 years already have HPV infection, so when they are vaccinated it shows up as a vaccine failure. That’s not correct. These are prophylactic HPV vaccines. They’re not meant to help clear an infection,” she noted.
Stepping back, Dr. Kreimer observed that cervical cancer “is really a story of inequality.” Indeed, 90% of cervical cancers occur in low-income countries, where HPV vaccination uptake remains very low even more than a decade after licensure. When modelers project out in the future, they estimate that at current HPV vaccination levels in Sub-Saharan Africa, which has the highest cervical cancer rates in the world, it would take more than 100 years to achieve the World Health Organization goal of eliminating the malignancy.
Asked by an audience member how low a single-dose vaccine effectiveness level she considers acceptable to help reach the goal of eliminating cervical cancer in developing countries, Dr. Kreimer cautioned against the tendency to let ‘perfect’ become the enemy of ‘good.’
“I’ll remind everyone that, in this moment, very few of the target girls in the lower– and upper-lower–income countries are getting any vaccination. So I don’t think it’s a question of whether we should be going from two to one dose, I think it’s really a question of, for those who are at zero doses, how do we get them one dose? And with the HPV vaccine, we’ve even seen suggestions of herd immunity if we have 50% uptake,” she replied.
Dr. Kreimer reported having no financial conflicts regarding her presentation.
LJUBLJANA, SLOVENIA – There is good news and bad news about human papillomavirus (HPV) vaccination as a means of preventing cervical cancer.
The bad news is the HPV vaccines are projected to be in short supply, unable to meet global demand until at least 2024. The good news is that – in one study, for 11 years and counting – which would effectively double the existing supply, Aimee R. Kreimer, PhD, said at the annual meeting of the European Society for Paediatric Infectious Diseases.
These data come from post hoc analyses of major phase 3 randomized controlled trials of bivalent HPV vaccine in Costa Rica and quadrivalent vaccine in India. However, these secondary analyses aren’t considered rock solid evidence because the subjects who got a single dose weren’t randomized to that strategy, they simply for one reason or another didn’t receive the recommended additional dose or doses.
“I don’t know if these studies are enough, so several studies have been launched over the past couple of years with an eye toward generating the quality of data that would be sufficient to motivate policy change, if in fact one dose is proven to be effective,” said Dr. Kreimer, a senior scientist at the National Cancer Institute in Bethesda, Md.
The first of these formal randomized, controlled trials – a delayed second-dose study in 9- to 11-year-old U.S. boys and girls – is due to be completed next year. Four other trials ongoing in Africa and Costa Rica, all in females, are expected to report findings in 2022-2025.
Dr. Kreimer is first author of a soon-to-be-published 11-year update from the phase 3 Costa Rica HPV Vaccine Trial, which was launched prior to licensure of the GlaxoSmithKline bivalent HPV vaccine. Previous analyses showed that at both 4 and 7 years of follow-up, a single dose of the vaccine was as effective as two or three in preventing infection with HPV types 16 and 18, which are covered by the vaccine.
“Now the research question has transitioned to, ‘Will one dose be sufficiently durable?’ she explained.
The answer from this study is yes. At 11 years since receipt of the bivalent HPV vaccine, there was no difference in terms of prevalent HPV 16/18 infection between the one-, two-, and three-dose groups. To address the issue of possible selection bias in this post hoc nonrandomized comparison, Dr. Kreimer and her coinvestigators looked at rates of infection with HPV 31 and 45, which aren’t covered by the vaccine. The rates were similar regardless of the number of vaccine doses received 11 years earlier, indicating women in all three dosing groups are at similar risk for acquiring HPV infection, thus bolstering the legitimacy of the conclusion that one dose provides effective long-term protection.
Intriguingly, HPV serum antibody levels in the single-dose group have remained stable for 11 years at a level that’s only about one-quarter of that associated with three doses of the vaccine, albeit an order of magnitude greater than the level induced by natural immunity.
“This really challenges the dogma of the HPV vaccine,” according to Dr. Kreimer. “It suggests that inferior [HPV] antibodies do not necessarily mean inferior protection.”
The explanation for this phenomenon appears to be that HPV subunit vaccine mimics the shell of authentic virions so well that the immune system sees it as dangerous and mounts long-term antibody production. Also, cervical infection by HPV is a relatively slow process, allowing time for vaccine-induced antibodies to interrupt it, she said.
In contrast to the encouraging findings from this post hoc analysis and another from a phase 3 trial of quadrivalent vaccine in India, numerous phase 4 vaccine effectiveness monitoring studies have shown markedly lower vaccine effectiveness for one dose of HPV vaccine. Dr. Kreimer cautioned that this is a flawed conclusion attributable to a methodologic artifact whereby the investigators have lumped together single-dose recipients who were 17 years old or more at the time with those who were younger.
“The problem is that many people who are aged 17-18 years already have HPV infection, so when they are vaccinated it shows up as a vaccine failure. That’s not correct. These are prophylactic HPV vaccines. They’re not meant to help clear an infection,” she noted.
Stepping back, Dr. Kreimer observed that cervical cancer “is really a story of inequality.” Indeed, 90% of cervical cancers occur in low-income countries, where HPV vaccination uptake remains very low even more than a decade after licensure. When modelers project out in the future, they estimate that at current HPV vaccination levels in Sub-Saharan Africa, which has the highest cervical cancer rates in the world, it would take more than 100 years to achieve the World Health Organization goal of eliminating the malignancy.
Asked by an audience member how low a single-dose vaccine effectiveness level she considers acceptable to help reach the goal of eliminating cervical cancer in developing countries, Dr. Kreimer cautioned against the tendency to let ‘perfect’ become the enemy of ‘good.’
“I’ll remind everyone that, in this moment, very few of the target girls in the lower– and upper-lower–income countries are getting any vaccination. So I don’t think it’s a question of whether we should be going from two to one dose, I think it’s really a question of, for those who are at zero doses, how do we get them one dose? And with the HPV vaccine, we’ve even seen suggestions of herd immunity if we have 50% uptake,” she replied.
Dr. Kreimer reported having no financial conflicts regarding her presentation.
EXPERT ANALYSIS FROM ESPID 2019
10-valent pneumococcal conjugate vaccine confers similar protection to boys and girls
according to Heta Nieminen, MD, of the National Institute for Health and Welfare in Tampere, Finland, and associates.
For the study, published in Vaccine, the investigators conducted a post hoc analysis of the phase III/IV, cluster-randomized, double-blind FinIP trial, in which more than 30,000 infants received the PHiD-CV10 vaccine or a placebo. Patients were aged less than 7 months when they received their first vaccination, and received two or three primary doses, plus a booster shot after the age of 11 months (Vaccine. 2019 May 20. doi: 10.1016/j.vaccine.2019.05.033).
In term infants, vaccine effectiveness was similar in boys and girls; while the vaccine worked marginally better in girls, the difference was not significant. Infants who received the 2 + 1 schedule had vaccine effectiveness similar to that of those who received the 3 + 1 schedule. In a smaller subanalysis of 1,519 preterm infants, outcomes of pneumonia were more common, but the vaccine seemed to confer protection, although the sample size was not large enough for statistical significance to be reached.
“The point estimates of vaccine effectiveness suggest protection in both sexes, and also among the preterm and low-birth-weight infants. ... There were no significant differences between the 2 + 1 and 3 + 1 schedules in any of the subgroups analyzed. Based on this study, the 2 + 1 or “Nordic” schedule is sufficient also for the risk groups such as the preterm or low-birth-weight infants,” the investigators concluded.
Five study authors are employees of the National Institute for Health and Welfare, which received funding for the study from GlaxoSmithKline. Four coauthors are employees of GlaxoSmithKline; three of them own shares in the company.
according to Heta Nieminen, MD, of the National Institute for Health and Welfare in Tampere, Finland, and associates.
For the study, published in Vaccine, the investigators conducted a post hoc analysis of the phase III/IV, cluster-randomized, double-blind FinIP trial, in which more than 30,000 infants received the PHiD-CV10 vaccine or a placebo. Patients were aged less than 7 months when they received their first vaccination, and received two or three primary doses, plus a booster shot after the age of 11 months (Vaccine. 2019 May 20. doi: 10.1016/j.vaccine.2019.05.033).
In term infants, vaccine effectiveness was similar in boys and girls; while the vaccine worked marginally better in girls, the difference was not significant. Infants who received the 2 + 1 schedule had vaccine effectiveness similar to that of those who received the 3 + 1 schedule. In a smaller subanalysis of 1,519 preterm infants, outcomes of pneumonia were more common, but the vaccine seemed to confer protection, although the sample size was not large enough for statistical significance to be reached.
“The point estimates of vaccine effectiveness suggest protection in both sexes, and also among the preterm and low-birth-weight infants. ... There were no significant differences between the 2 + 1 and 3 + 1 schedules in any of the subgroups analyzed. Based on this study, the 2 + 1 or “Nordic” schedule is sufficient also for the risk groups such as the preterm or low-birth-weight infants,” the investigators concluded.
Five study authors are employees of the National Institute for Health and Welfare, which received funding for the study from GlaxoSmithKline. Four coauthors are employees of GlaxoSmithKline; three of them own shares in the company.
according to Heta Nieminen, MD, of the National Institute for Health and Welfare in Tampere, Finland, and associates.
For the study, published in Vaccine, the investigators conducted a post hoc analysis of the phase III/IV, cluster-randomized, double-blind FinIP trial, in which more than 30,000 infants received the PHiD-CV10 vaccine or a placebo. Patients were aged less than 7 months when they received their first vaccination, and received two or three primary doses, plus a booster shot after the age of 11 months (Vaccine. 2019 May 20. doi: 10.1016/j.vaccine.2019.05.033).
In term infants, vaccine effectiveness was similar in boys and girls; while the vaccine worked marginally better in girls, the difference was not significant. Infants who received the 2 + 1 schedule had vaccine effectiveness similar to that of those who received the 3 + 1 schedule. In a smaller subanalysis of 1,519 preterm infants, outcomes of pneumonia were more common, but the vaccine seemed to confer protection, although the sample size was not large enough for statistical significance to be reached.
“The point estimates of vaccine effectiveness suggest protection in both sexes, and also among the preterm and low-birth-weight infants. ... There were no significant differences between the 2 + 1 and 3 + 1 schedules in any of the subgroups analyzed. Based on this study, the 2 + 1 or “Nordic” schedule is sufficient also for the risk groups such as the preterm or low-birth-weight infants,” the investigators concluded.
Five study authors are employees of the National Institute for Health and Welfare, which received funding for the study from GlaxoSmithKline. Four coauthors are employees of GlaxoSmithKline; three of them own shares in the company.
FROM VACCINE
Measles count for 2019 now over 900 cases
according to the Centers for Disease Control and Prevention.
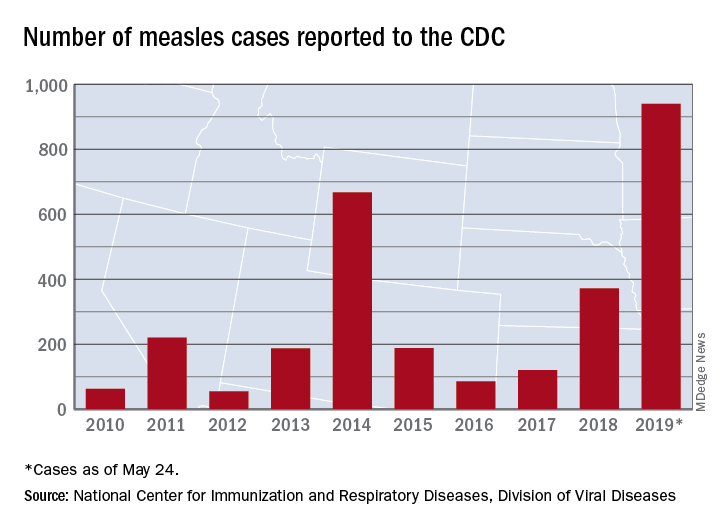
The CDC received reports of 60 new measles cases last week – up from 41 the previous week – bringing the U.S. total to 940 for the year as of May 24. The CDC is currently tracking 10 outbreaks in seven states: California (3), Georgia, Maryland, Michigan, New York (2), Pennsylvania, and Washington.
The Maine Center for Disease Control and Prevention confirmed the state’s first case on May 20. The school-aged child from Somerset County had been vaccinated and is fully recovered from the disease. It’s not yet known where the child was exposed to measles, but sporadic cases are not unexpected, the Maine CDC said.
New Mexico’s first measles case of the year, a 1-year-old in Sierra County, has at least one state lawmaker considering changes to the state’s immunization exemption laws, the Farmington Daily Times reported.
according to the Centers for Disease Control and Prevention.

The CDC received reports of 60 new measles cases last week – up from 41 the previous week – bringing the U.S. total to 940 for the year as of May 24. The CDC is currently tracking 10 outbreaks in seven states: California (3), Georgia, Maryland, Michigan, New York (2), Pennsylvania, and Washington.
The Maine Center for Disease Control and Prevention confirmed the state’s first case on May 20. The school-aged child from Somerset County had been vaccinated and is fully recovered from the disease. It’s not yet known where the child was exposed to measles, but sporadic cases are not unexpected, the Maine CDC said.
New Mexico’s first measles case of the year, a 1-year-old in Sierra County, has at least one state lawmaker considering changes to the state’s immunization exemption laws, the Farmington Daily Times reported.
according to the Centers for Disease Control and Prevention.

The CDC received reports of 60 new measles cases last week – up from 41 the previous week – bringing the U.S. total to 940 for the year as of May 24. The CDC is currently tracking 10 outbreaks in seven states: California (3), Georgia, Maryland, Michigan, New York (2), Pennsylvania, and Washington.
The Maine Center for Disease Control and Prevention confirmed the state’s first case on May 20. The school-aged child from Somerset County had been vaccinated and is fully recovered from the disease. It’s not yet known where the child was exposed to measles, but sporadic cases are not unexpected, the Maine CDC said.
New Mexico’s first measles case of the year, a 1-year-old in Sierra County, has at least one state lawmaker considering changes to the state’s immunization exemption laws, the Farmington Daily Times reported.
Zoster vaccination is underused but looks effective in IBD
For men with inflammatory bowel disease, herpes zoster vaccination was associated with about a 46% decrease in risk of associated infection, according to the results of a retrospective study from the national Veterans Affairs Healthcare System.
Crude rates of herpes zoster infection were 4.09 cases per 1,000 person-years among vaccinated patients versus 6.97 cases per 1,000 person-years among unvaccinated patients, for an adjusted hazard ratio of 0.54 (95% confidence interval, 0.44-0.68), reported Nabeel Khan, MD, of the University of Pennsylvania, Philadelphia, and associates. “This vaccine is therefore effective in patients with IBD, but underused,” they wrote in Clinical Gastroenterology and Hepatology.
Studies have linked IBD with a 1.2- to 1.8-fold increased risk of herpes zoster infection, the researchers noted. Relevant risk factors include older age, disease flare, recent use or high cumulative use of prednisone, and use of thiopurines, either alone or in combination with a tumor necrosis factor (TNF) inhibitor. Although the American College of Gastroenterology recommends that all patients with IBD receive the herpes zoster vaccine by age 50 years, the efficacy of the vaccine in these patients remains unclear.
For their study, Dr. Khan and associates analyzed International Classification of Diseases (ICD) codes and other medical record data from 39,983 veterans with IBD who had not received the herpes zoster vaccine by age 60 years. In all, 97% of patients were male, and 94% were white. Most patients had high rates of health care utilization: Approximately half visited VA clinics or hospitals at least 13 times per year, and another third made 6-12 annual visits.
Despite their many contacts with VA health care systems, only 7,170 (17.9%) patients received the herpes zoster vaccine during 2000-2016, the researchers found. Vaccination rates varied substantially by region – they were highest in the Midwest (35%) and North Atlantic states (29%) but reached only 9% in Montana, Utah, Wyoming, Colorado, Oklahoma, Texas, Arkansas, and Louisiana, collectively.
The crude rate of herpes zoster infection among unvaccinated patients with IBD resembled the incidence reported in prior studies, the researchers said. After researchers accounted for differences in geography, demographics, and health care utilization between vaccinated and unvaccinated veterans with IBD, they found that vaccination was associated with an approximately 46% decrease in the risk of herpes zoster infection.
Very few patients were vaccinated for herpes zoster while on a TNF inhibitor, precluding the ability to study this subgroup. However, the vaccine showed a protective effect (adjusted HR, 0.63) among patients who received thiopurines without a TNF inhibitor. This effect did not reach statistical significance, perhaps because of lack of power, the researchers noted. “Among the 315 patients who were [vaccinated while] on thiopurines, none developed a documented painful or painless vesicular rash within 42 days of herpes zoster vaccination,” they added. One patient developed a painful blister 20 days post vaccination without vesicles or long-term sequelae.
Pfizer provided funding. Dr. Khan disclosed research funding from Pfizer, Luitpold, and Takeda Pharmaceuticals. One coinvestigator disclosed ties to Pfizer, Gilead, Merck, AbbVie, Lilly, Janssen, Johnson & Johnson, UCB, and Nestle Health Science. The remaining researchers disclosed no conflicts.
SOURCE: Khan N et al. Clin Gastroenterol Hepatol. 2018 Oct 13. doi: 10.1016/j.cgh.2018.10.016.
Preventive care is an underemphasized component of IBD management because the primary focus tends to be control of active symptoms. However, as patients are treated with immunosuppression, particularly combinations of therapies and newer mechanisms of action such as the Janus kinase inhibitors, the risk of infections increases, including those that are vaccine preventable including shingles and its related complications.
This study by Khan et al. highlights several important messages for patients and providers. First, in this large older IBD cohort, the vaccination rates were very low at 18% even though more than 80% of patients had more than six annual visits to the VA Health Systems during the study period. These represent multiple missed opportunities to discuss and administer vaccinations. Second, the authors highlighted the vaccine’s efficacy: Persons receiving herpes zoster vaccination had a clearly decreased risk of subsequent infection. While the number of vaccinated patients on immunosuppression was too small to draw conclusions about efficacy, the live attenuated vaccination is contraindicated for immunosuppressed patients. However, the newer recombinant shingles vaccine offers the opportunity to extend the reach of shingles vaccination to include those on immunosuppression. As utilization of the newer vaccine series increases, we will be able to evaluate the efficacy for immunosuppressed IBD patients, although studies from other disease states suggest efficacy. However, vaccinations will never work if they aren’t administered. Counseling patients and providers regarding the importance of vaccinations is a low-risk, efficacious means to decrease infection and associated morbidity.
Christina Ha, MD, AGAF, associate professor of medicine, Inflammatory Bowel Disease Center, division of digestive diseases, Cedars-Sinai Medical Center, Los Angeles. She is a speaker, consultant, or on the advisory board for AbbVie, Janssen, Genentech, Samsung Bioepis, and Takeda. She received grant funding from Pfizer.
Preventive care is an underemphasized component of IBD management because the primary focus tends to be control of active symptoms. However, as patients are treated with immunosuppression, particularly combinations of therapies and newer mechanisms of action such as the Janus kinase inhibitors, the risk of infections increases, including those that are vaccine preventable including shingles and its related complications.
This study by Khan et al. highlights several important messages for patients and providers. First, in this large older IBD cohort, the vaccination rates were very low at 18% even though more than 80% of patients had more than six annual visits to the VA Health Systems during the study period. These represent multiple missed opportunities to discuss and administer vaccinations. Second, the authors highlighted the vaccine’s efficacy: Persons receiving herpes zoster vaccination had a clearly decreased risk of subsequent infection. While the number of vaccinated patients on immunosuppression was too small to draw conclusions about efficacy, the live attenuated vaccination is contraindicated for immunosuppressed patients. However, the newer recombinant shingles vaccine offers the opportunity to extend the reach of shingles vaccination to include those on immunosuppression. As utilization of the newer vaccine series increases, we will be able to evaluate the efficacy for immunosuppressed IBD patients, although studies from other disease states suggest efficacy. However, vaccinations will never work if they aren’t administered. Counseling patients and providers regarding the importance of vaccinations is a low-risk, efficacious means to decrease infection and associated morbidity.
Christina Ha, MD, AGAF, associate professor of medicine, Inflammatory Bowel Disease Center, division of digestive diseases, Cedars-Sinai Medical Center, Los Angeles. She is a speaker, consultant, or on the advisory board for AbbVie, Janssen, Genentech, Samsung Bioepis, and Takeda. She received grant funding from Pfizer.
Preventive care is an underemphasized component of IBD management because the primary focus tends to be control of active symptoms. However, as patients are treated with immunosuppression, particularly combinations of therapies and newer mechanisms of action such as the Janus kinase inhibitors, the risk of infections increases, including those that are vaccine preventable including shingles and its related complications.
This study by Khan et al. highlights several important messages for patients and providers. First, in this large older IBD cohort, the vaccination rates were very low at 18% even though more than 80% of patients had more than six annual visits to the VA Health Systems during the study period. These represent multiple missed opportunities to discuss and administer vaccinations. Second, the authors highlighted the vaccine’s efficacy: Persons receiving herpes zoster vaccination had a clearly decreased risk of subsequent infection. While the number of vaccinated patients on immunosuppression was too small to draw conclusions about efficacy, the live attenuated vaccination is contraindicated for immunosuppressed patients. However, the newer recombinant shingles vaccine offers the opportunity to extend the reach of shingles vaccination to include those on immunosuppression. As utilization of the newer vaccine series increases, we will be able to evaluate the efficacy for immunosuppressed IBD patients, although studies from other disease states suggest efficacy. However, vaccinations will never work if they aren’t administered. Counseling patients and providers regarding the importance of vaccinations is a low-risk, efficacious means to decrease infection and associated morbidity.
Christina Ha, MD, AGAF, associate professor of medicine, Inflammatory Bowel Disease Center, division of digestive diseases, Cedars-Sinai Medical Center, Los Angeles. She is a speaker, consultant, or on the advisory board for AbbVie, Janssen, Genentech, Samsung Bioepis, and Takeda. She received grant funding from Pfizer.
For men with inflammatory bowel disease, herpes zoster vaccination was associated with about a 46% decrease in risk of associated infection, according to the results of a retrospective study from the national Veterans Affairs Healthcare System.
Crude rates of herpes zoster infection were 4.09 cases per 1,000 person-years among vaccinated patients versus 6.97 cases per 1,000 person-years among unvaccinated patients, for an adjusted hazard ratio of 0.54 (95% confidence interval, 0.44-0.68), reported Nabeel Khan, MD, of the University of Pennsylvania, Philadelphia, and associates. “This vaccine is therefore effective in patients with IBD, but underused,” they wrote in Clinical Gastroenterology and Hepatology.
Studies have linked IBD with a 1.2- to 1.8-fold increased risk of herpes zoster infection, the researchers noted. Relevant risk factors include older age, disease flare, recent use or high cumulative use of prednisone, and use of thiopurines, either alone or in combination with a tumor necrosis factor (TNF) inhibitor. Although the American College of Gastroenterology recommends that all patients with IBD receive the herpes zoster vaccine by age 50 years, the efficacy of the vaccine in these patients remains unclear.
For their study, Dr. Khan and associates analyzed International Classification of Diseases (ICD) codes and other medical record data from 39,983 veterans with IBD who had not received the herpes zoster vaccine by age 60 years. In all, 97% of patients were male, and 94% were white. Most patients had high rates of health care utilization: Approximately half visited VA clinics or hospitals at least 13 times per year, and another third made 6-12 annual visits.
Despite their many contacts with VA health care systems, only 7,170 (17.9%) patients received the herpes zoster vaccine during 2000-2016, the researchers found. Vaccination rates varied substantially by region – they were highest in the Midwest (35%) and North Atlantic states (29%) but reached only 9% in Montana, Utah, Wyoming, Colorado, Oklahoma, Texas, Arkansas, and Louisiana, collectively.
The crude rate of herpes zoster infection among unvaccinated patients with IBD resembled the incidence reported in prior studies, the researchers said. After researchers accounted for differences in geography, demographics, and health care utilization between vaccinated and unvaccinated veterans with IBD, they found that vaccination was associated with an approximately 46% decrease in the risk of herpes zoster infection.
Very few patients were vaccinated for herpes zoster while on a TNF inhibitor, precluding the ability to study this subgroup. However, the vaccine showed a protective effect (adjusted HR, 0.63) among patients who received thiopurines without a TNF inhibitor. This effect did not reach statistical significance, perhaps because of lack of power, the researchers noted. “Among the 315 patients who were [vaccinated while] on thiopurines, none developed a documented painful or painless vesicular rash within 42 days of herpes zoster vaccination,” they added. One patient developed a painful blister 20 days post vaccination without vesicles or long-term sequelae.
Pfizer provided funding. Dr. Khan disclosed research funding from Pfizer, Luitpold, and Takeda Pharmaceuticals. One coinvestigator disclosed ties to Pfizer, Gilead, Merck, AbbVie, Lilly, Janssen, Johnson & Johnson, UCB, and Nestle Health Science. The remaining researchers disclosed no conflicts.
SOURCE: Khan N et al. Clin Gastroenterol Hepatol. 2018 Oct 13. doi: 10.1016/j.cgh.2018.10.016.
For men with inflammatory bowel disease, herpes zoster vaccination was associated with about a 46% decrease in risk of associated infection, according to the results of a retrospective study from the national Veterans Affairs Healthcare System.
Crude rates of herpes zoster infection were 4.09 cases per 1,000 person-years among vaccinated patients versus 6.97 cases per 1,000 person-years among unvaccinated patients, for an adjusted hazard ratio of 0.54 (95% confidence interval, 0.44-0.68), reported Nabeel Khan, MD, of the University of Pennsylvania, Philadelphia, and associates. “This vaccine is therefore effective in patients with IBD, but underused,” they wrote in Clinical Gastroenterology and Hepatology.
Studies have linked IBD with a 1.2- to 1.8-fold increased risk of herpes zoster infection, the researchers noted. Relevant risk factors include older age, disease flare, recent use or high cumulative use of prednisone, and use of thiopurines, either alone or in combination with a tumor necrosis factor (TNF) inhibitor. Although the American College of Gastroenterology recommends that all patients with IBD receive the herpes zoster vaccine by age 50 years, the efficacy of the vaccine in these patients remains unclear.
For their study, Dr. Khan and associates analyzed International Classification of Diseases (ICD) codes and other medical record data from 39,983 veterans with IBD who had not received the herpes zoster vaccine by age 60 years. In all, 97% of patients were male, and 94% were white. Most patients had high rates of health care utilization: Approximately half visited VA clinics or hospitals at least 13 times per year, and another third made 6-12 annual visits.
Despite their many contacts with VA health care systems, only 7,170 (17.9%) patients received the herpes zoster vaccine during 2000-2016, the researchers found. Vaccination rates varied substantially by region – they were highest in the Midwest (35%) and North Atlantic states (29%) but reached only 9% in Montana, Utah, Wyoming, Colorado, Oklahoma, Texas, Arkansas, and Louisiana, collectively.
The crude rate of herpes zoster infection among unvaccinated patients with IBD resembled the incidence reported in prior studies, the researchers said. After researchers accounted for differences in geography, demographics, and health care utilization between vaccinated and unvaccinated veterans with IBD, they found that vaccination was associated with an approximately 46% decrease in the risk of herpes zoster infection.
Very few patients were vaccinated for herpes zoster while on a TNF inhibitor, precluding the ability to study this subgroup. However, the vaccine showed a protective effect (adjusted HR, 0.63) among patients who received thiopurines without a TNF inhibitor. This effect did not reach statistical significance, perhaps because of lack of power, the researchers noted. “Among the 315 patients who were [vaccinated while] on thiopurines, none developed a documented painful or painless vesicular rash within 42 days of herpes zoster vaccination,” they added. One patient developed a painful blister 20 days post vaccination without vesicles or long-term sequelae.
Pfizer provided funding. Dr. Khan disclosed research funding from Pfizer, Luitpold, and Takeda Pharmaceuticals. One coinvestigator disclosed ties to Pfizer, Gilead, Merck, AbbVie, Lilly, Janssen, Johnson & Johnson, UCB, and Nestle Health Science. The remaining researchers disclosed no conflicts.
SOURCE: Khan N et al. Clin Gastroenterol Hepatol. 2018 Oct 13. doi: 10.1016/j.cgh.2018.10.016.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
California kindergarten nonvaccination rate on rise again
from immunizations, according to Paul L. Delamater, PhD, of the University of North Carolina at Chapel Hill, and associates.
The investigators focused on vaccination data collected from 2015 – the last year before the passage of SB 277 – to 2017. They also analyzed county-level data collected from 2000 to 2014 to assess demographic behavior. In 2015, the rate of nonvaccination was 7.15%, decreasing to 4.42% in 2016. This decrease was almost entirely caused by a reduction in the rate of conditional entrants, which fell from 4.43% to 1.91%, and in personal belief exceptions, which fell from 2.37% to 0.56%.
While the rates of conditional entrants and personal belief exceptions continued to fall in 2017, other mechanisms allowed the overall rate of kindergarteners not fully up to date on their vaccines to jump to 4.87%. This was fueled by a slight increase in medical exceptions (from 0.51% in 2016 to 0.73% in 2017), and a significant increase in children who were overdue or exempt, which both increased from 0 in 2014 to over 1% by 2017.
“Although the law was successful in reducing the number of students with personal belief exemptions, our analysis reveals that a replacement effect may have stifled a larger increase in students entering kindergarten who are up to date on vaccination. ... Given these findings, policymakers should consider the various options available to increase vaccination coverage or strategies to minimize potential unintended consequences of eliminating nonmedical exemptions such as the replacement effect observed in California,” the investigators reported in Pediatrics (2019, May 21 doi: 10.1542/peds.2018-3301.
One coauthor reported receiving research and consulting support from Pfizer, Merck, and Walgreens; another reported receiving research support from Pfizer, Merck, GlaxoSmithKline, Sanofi Pasteur, Protein Science (now Sanofi Pasteur), Dynavax, and MedImmune.
from immunizations, according to Paul L. Delamater, PhD, of the University of North Carolina at Chapel Hill, and associates.
The investigators focused on vaccination data collected from 2015 – the last year before the passage of SB 277 – to 2017. They also analyzed county-level data collected from 2000 to 2014 to assess demographic behavior. In 2015, the rate of nonvaccination was 7.15%, decreasing to 4.42% in 2016. This decrease was almost entirely caused by a reduction in the rate of conditional entrants, which fell from 4.43% to 1.91%, and in personal belief exceptions, which fell from 2.37% to 0.56%.
While the rates of conditional entrants and personal belief exceptions continued to fall in 2017, other mechanisms allowed the overall rate of kindergarteners not fully up to date on their vaccines to jump to 4.87%. This was fueled by a slight increase in medical exceptions (from 0.51% in 2016 to 0.73% in 2017), and a significant increase in children who were overdue or exempt, which both increased from 0 in 2014 to over 1% by 2017.
“Although the law was successful in reducing the number of students with personal belief exemptions, our analysis reveals that a replacement effect may have stifled a larger increase in students entering kindergarten who are up to date on vaccination. ... Given these findings, policymakers should consider the various options available to increase vaccination coverage or strategies to minimize potential unintended consequences of eliminating nonmedical exemptions such as the replacement effect observed in California,” the investigators reported in Pediatrics (2019, May 21 doi: 10.1542/peds.2018-3301.
One coauthor reported receiving research and consulting support from Pfizer, Merck, and Walgreens; another reported receiving research support from Pfizer, Merck, GlaxoSmithKline, Sanofi Pasteur, Protein Science (now Sanofi Pasteur), Dynavax, and MedImmune.
from immunizations, according to Paul L. Delamater, PhD, of the University of North Carolina at Chapel Hill, and associates.
The investigators focused on vaccination data collected from 2015 – the last year before the passage of SB 277 – to 2017. They also analyzed county-level data collected from 2000 to 2014 to assess demographic behavior. In 2015, the rate of nonvaccination was 7.15%, decreasing to 4.42% in 2016. This decrease was almost entirely caused by a reduction in the rate of conditional entrants, which fell from 4.43% to 1.91%, and in personal belief exceptions, which fell from 2.37% to 0.56%.
While the rates of conditional entrants and personal belief exceptions continued to fall in 2017, other mechanisms allowed the overall rate of kindergarteners not fully up to date on their vaccines to jump to 4.87%. This was fueled by a slight increase in medical exceptions (from 0.51% in 2016 to 0.73% in 2017), and a significant increase in children who were overdue or exempt, which both increased from 0 in 2014 to over 1% by 2017.
“Although the law was successful in reducing the number of students with personal belief exemptions, our analysis reveals that a replacement effect may have stifled a larger increase in students entering kindergarten who are up to date on vaccination. ... Given these findings, policymakers should consider the various options available to increase vaccination coverage or strategies to minimize potential unintended consequences of eliminating nonmedical exemptions such as the replacement effect observed in California,” the investigators reported in Pediatrics (2019, May 21 doi: 10.1542/peds.2018-3301.
One coauthor reported receiving research and consulting support from Pfizer, Merck, and Walgreens; another reported receiving research support from Pfizer, Merck, GlaxoSmithKline, Sanofi Pasteur, Protein Science (now Sanofi Pasteur), Dynavax, and MedImmune.
FROM PEDIATRICS
U.S. measles total sees smallest increase in 2 months
according to the Centers for Disease Control and Prevention.
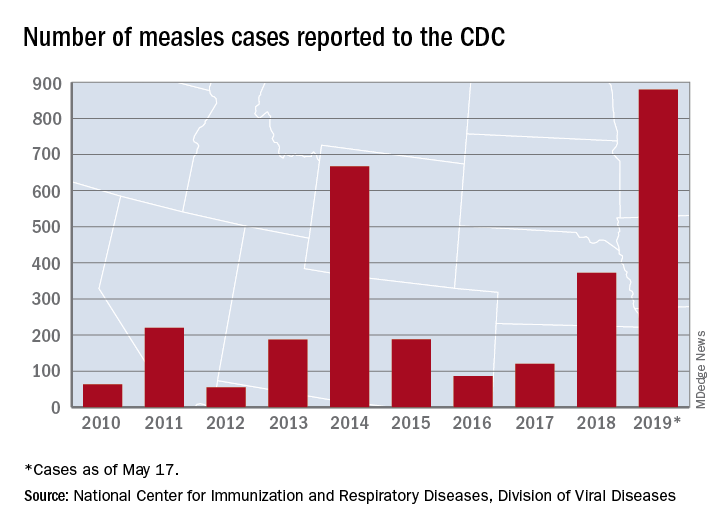
That weekly increase of 41 cases is the smallest since the week ending March 14, when the total rose by 40. The largest 1-week rise of the year came during the week ending April 11, when there were 90 new cases, CDC data show.
A case that has been reported by the media in the last week but not officially through the CDC would make New Mexico the 25th state with a measles case this year. The state’s health department has confirmed measles in a 1-year-old from Sierra County, which is New Mexico’s first case since 2014, the Las Cruces Sun News reported, adding that 4,441 school-aged children had an exemption for vaccination filed with the state in 2018.
Making a return appearance to the CDC’s list of outbreaks is Washington State, which reported six new cases last week in three Puget Sound counties (King, Pierce, and Snohomish). The most likely location and date of exposure was at Seattle-Tacoma International Airport on April 25, the Washington State Department of Health said. In February and March, there were 71 cases in Clark County on the state’s border with Oregon.
The ongoing outbreak in Michigan had been quiet since April, but the state’s Department of Health and Human Services confirmed a measles case in St. Clair County on May 17, bringing the total to 44 for the year. The new case, which is not related to an earlier outbreak that occurred mainly in Oakland County, involves an international traveler visiting Michigan.
according to the Centers for Disease Control and Prevention.

That weekly increase of 41 cases is the smallest since the week ending March 14, when the total rose by 40. The largest 1-week rise of the year came during the week ending April 11, when there were 90 new cases, CDC data show.
A case that has been reported by the media in the last week but not officially through the CDC would make New Mexico the 25th state with a measles case this year. The state’s health department has confirmed measles in a 1-year-old from Sierra County, which is New Mexico’s first case since 2014, the Las Cruces Sun News reported, adding that 4,441 school-aged children had an exemption for vaccination filed with the state in 2018.
Making a return appearance to the CDC’s list of outbreaks is Washington State, which reported six new cases last week in three Puget Sound counties (King, Pierce, and Snohomish). The most likely location and date of exposure was at Seattle-Tacoma International Airport on April 25, the Washington State Department of Health said. In February and March, there were 71 cases in Clark County on the state’s border with Oregon.
The ongoing outbreak in Michigan had been quiet since April, but the state’s Department of Health and Human Services confirmed a measles case in St. Clair County on May 17, bringing the total to 44 for the year. The new case, which is not related to an earlier outbreak that occurred mainly in Oakland County, involves an international traveler visiting Michigan.
according to the Centers for Disease Control and Prevention.

That weekly increase of 41 cases is the smallest since the week ending March 14, when the total rose by 40. The largest 1-week rise of the year came during the week ending April 11, when there were 90 new cases, CDC data show.
A case that has been reported by the media in the last week but not officially through the CDC would make New Mexico the 25th state with a measles case this year. The state’s health department has confirmed measles in a 1-year-old from Sierra County, which is New Mexico’s first case since 2014, the Las Cruces Sun News reported, adding that 4,441 school-aged children had an exemption for vaccination filed with the state in 2018.
Making a return appearance to the CDC’s list of outbreaks is Washington State, which reported six new cases last week in three Puget Sound counties (King, Pierce, and Snohomish). The most likely location and date of exposure was at Seattle-Tacoma International Airport on April 25, the Washington State Department of Health said. In February and March, there were 71 cases in Clark County on the state’s border with Oregon.
The ongoing outbreak in Michigan had been quiet since April, but the state’s Department of Health and Human Services confirmed a measles case in St. Clair County on May 17, bringing the total to 44 for the year. The new case, which is not related to an earlier outbreak that occurred mainly in Oakland County, involves an international traveler visiting Michigan.
Survey: Physicians predict increase in measles deaths
by real-time market insights technology firm InCrowd.
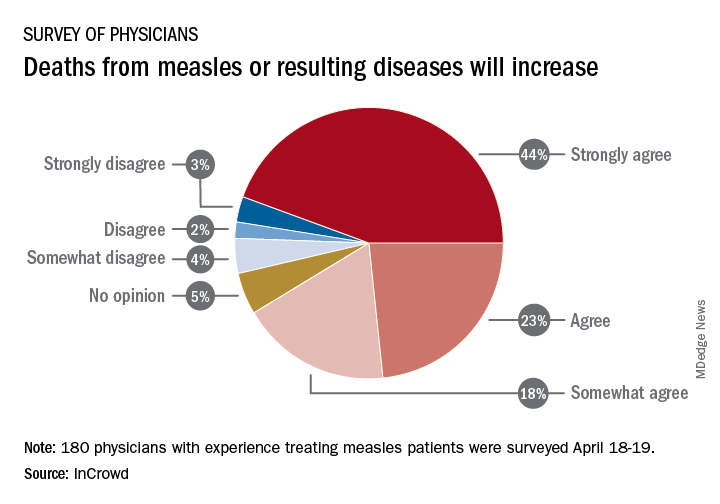
Among the 180 physicians with experience treating measles, 23% agreed and 44% said that they strongly agreed with the statement that measles deaths would increase, and another 18% said that they somewhat agreed. Only 9% expressed some level of disagreement, InCrowd said.
Most of those respondents also believe that summer travel will increase measles outbreaks (29% agreed and 30% strongly agreed) and that more communities will adopt requirements for measles vaccinations (26% and 36%). A majority also said that education about vaccinations will improve (26% agreed and 29% strongly agreed), but almost half of the physicians surveyed also expect vaccination misinformation to get worse (29% and 19%), InCrowd reported.
“With 44% of respondents predicting a high likelihood that deaths caused by measles will increase, the data show the imperative for physicians and patients to keep up the dialogue. … We have a long way to go before declaring victory,” said Diane Hayes, PhD, president and cofounder of InCrowd.
The InCrowd 5-minute microsurvey was conducted on April 18-19, 2019, and included 455 primary care physicians, of whom 40% said that they have treated or knew of colleagues in their facility or community who have treated patients with measles. Of those 180 respondents, 89 were pediatricians and 91 were in other primary care specialties.
by real-time market insights technology firm InCrowd.

Among the 180 physicians with experience treating measles, 23% agreed and 44% said that they strongly agreed with the statement that measles deaths would increase, and another 18% said that they somewhat agreed. Only 9% expressed some level of disagreement, InCrowd said.
Most of those respondents also believe that summer travel will increase measles outbreaks (29% agreed and 30% strongly agreed) and that more communities will adopt requirements for measles vaccinations (26% and 36%). A majority also said that education about vaccinations will improve (26% agreed and 29% strongly agreed), but almost half of the physicians surveyed also expect vaccination misinformation to get worse (29% and 19%), InCrowd reported.
“With 44% of respondents predicting a high likelihood that deaths caused by measles will increase, the data show the imperative for physicians and patients to keep up the dialogue. … We have a long way to go before declaring victory,” said Diane Hayes, PhD, president and cofounder of InCrowd.
The InCrowd 5-minute microsurvey was conducted on April 18-19, 2019, and included 455 primary care physicians, of whom 40% said that they have treated or knew of colleagues in their facility or community who have treated patients with measles. Of those 180 respondents, 89 were pediatricians and 91 were in other primary care specialties.
by real-time market insights technology firm InCrowd.

Among the 180 physicians with experience treating measles, 23% agreed and 44% said that they strongly agreed with the statement that measles deaths would increase, and another 18% said that they somewhat agreed. Only 9% expressed some level of disagreement, InCrowd said.
Most of those respondents also believe that summer travel will increase measles outbreaks (29% agreed and 30% strongly agreed) and that more communities will adopt requirements for measles vaccinations (26% and 36%). A majority also said that education about vaccinations will improve (26% agreed and 29% strongly agreed), but almost half of the physicians surveyed also expect vaccination misinformation to get worse (29% and 19%), InCrowd reported.
“With 44% of respondents predicting a high likelihood that deaths caused by measles will increase, the data show the imperative for physicians and patients to keep up the dialogue. … We have a long way to go before declaring victory,” said Diane Hayes, PhD, president and cofounder of InCrowd.
The InCrowd 5-minute microsurvey was conducted on April 18-19, 2019, and included 455 primary care physicians, of whom 40% said that they have treated or knew of colleagues in their facility or community who have treated patients with measles. Of those 180 respondents, 89 were pediatricians and 91 were in other primary care specialties.