User login
Adequate Midlife Protein, Especially From Plants, Tied to Healthy Aging
Intake of protein, especially from plants, in middle age is associated with higher odds of healthy aging and positive mental and physical health status in older women, a recent analysis of the Nurses’ Health Study (NHS) data suggests.
The study is said to be the first to examine the long-term impact of midlife protein consumption on later health status.
Writing in the American Journal of Clinical Nutrition, a team led by Andres V. Ardisson Korat, DSc, a nutritional epidemiologist at the USDA Human Nutrition Research Center on Aging at Tufts University in Boston, Massachusetts, found the following midlife protein–related odds ratios (ORs) for later healthy aging measured at ages 70-93.
For each 3% energy increment from various protein sources:
- 1.05 (95% confidence interval, 1.01-1.10) for total protein
- 1.07 (1.02-1.11) for animal protein
- 1.14 (1.06-1.23) for dairy protein
- 1.38 (1.24-1.54) for plant protein
In substitution analyses, significant positive associations were observed for the isocaloric replacement of animal or dairy protein, carbohydrate, or fat with plant protein — with increased ORs for healthy aging of 1.22-1.58 for each 3% of energy replacement.
On the measure of physical function, for example, replacing calories from all macronutrient variables with equivalent calories from plant protein was associated with 20%-60% higher odds of having no physical function limitations. Plant protein was also associated with higher odds for good mental status.
“Other studies have looked at protein intake in older adults, but we felt midlife was a more relevant etiological window,” Dr. Ardisson Korat said in an interview. “Our findings generally align, however, with those of protein intake in older populations, which have shown that protein can reduce the risk of frailty.”
He added that the benefits of protein, especially from plant sources, would likely apply to men as well and increasing plant protein intake is not difficult. “If you want a snack during the day, eat a handful of nuts instead of potato chips,” he advised. And eating several meals a week featuring beans, peas, lentils, tofu, whole grains, or seeds is an easy way to boost dietary plant protein, which comes with health-promoting soluble and insoluble fiber as well as antioxidant and anti-inflammatory polyphenols and other phytochemicals.
Conversely, plant but not animal protein consumption in older adulthood was linked to a lower risk of frailty in a previous NHS trial.
Higher plant protein intake was associated with a better probability of achieving healthy aging defined by changes in functional impairments, self-reported health/vitality, mental health, and use of health services in the Spanish Seniors-Estudio Sobre Nutricion y Riesgo Cardiovascular.
In contrast, animal protein intake in middle adulthood has been linked to an increased risk of premature death from chronic diseases driven by cardiovascular disease mortality.
The present findings are consistent with those observed for protein intakes in older adulthood, Dr. Ardisson Korat said.
“This study underscores the health advantages for midlife adults consuming adequate dietary protein — particularly plant protein — as one component of pursuing a healthy lifestyle,” said Douglas R. Dirschl, MD, chair of orthopedic surgery at Baylor College of Medicine in Houston, Texas. Most Americans consume adequate amounts of protein, but according to Dr. Dirschl, who treats many older patients for osteoporotic fractures and other musculoskeletal conditions, many US diets are subpar in this nutrient.
While protein is essential for bone and muscle formation and maintenance, “a surprising number of Americans are protein deficient, even those who seem hale and are overweight,” he said.
Dietary Recommendations for Midlife Patients
Physicians should therefore advise midlife patients to meet or perhaps modestly exceed the recommended dietary allowance (RDA) for protein of 0.8 g/kg per day and to make plant protein a substantial component of daily dietary protein intake, Dr. Dirschl said.
Luke D. Kim, MD, MEd, a geriatrician at the Cleveland Clinic in Cleveland, Ohio, noted that patients with lower socioeconomic status or with difficulty in day-to-day functioning are likely to have suboptimal protein intake. Such patients may need encouragement to eat more protein. “But we should keep in mind that showing a higher associated odds ratio of better health with increased protein take does not mean causality,” he said.
According to Rachel L. Amdur, MD, an internist at Northwestern Medicine in Chicago, Illinois, the long-term follow-up data from the NHS are uniquely helpful. “Middle-aged persons may think they no longer need much dietary protein and need to be reminded. Sometimes eating carbohydrates is just easier,” she said in an interview. Physicians need to asses and counsel patients on nutrition at all stages of life. “As I tell my patients, it’s best to think of your future self now.”
In agreement is Louis J. Morledge, MD, an internist at Northwell Health in New York City. “I firmly counsel my patients about adequate and often increased protein intake in middle life. But this is always within a larger framework of overall nutritional health.” He added that middle-aged persons often find themselves “stuck in food ruts,” and one of his clinical focuses is to advise patients about the importance of healthier food choices so they can better adjust to mental, emotional, physical, and skeletal changes as they age.
Study Details
The NHS analysis drew on prospective data from 48,762 nurses under age 60 in 1984. Total protein, animal protein, dairy protein, and plant protein were derived from validated food-frequency questionnaires.
Adjusting for lifestyle, demographics, and health status, the investigators identified 3721 (7.6% of cohort) eligible participants. The mean age of participants at baseline was 48.6 years; 38.6% had body mass indexes (BMI; in kg/m2) greater than 25; 22.9% were current smokers; and 88.2% were married.
Healthy aging was defined as freedom from 11 major chronic diseases, good mental health, and no impairments in cognitive or physical function, as assessed in the 2014 or 2016 NHS participant questionnaires. Diseases/treatments included cancer, type 2 diabetes, myocardial infarction, coronary artery bypass graft or coronary angioplasty, congestive heart failure, stroke, kidney failure, chronic obstructive pulmonary disease, Parkinson disease, multiple sclerosis, and amyotrophic lateral sclerosis.
Mean total protein consumption as a percentage of energy was 18.3% (standard deviation 3%), slightly higher than the average 16% in the US diet. Of this, 13.3% derived from animals, 3.6% from dairy products, and 4.9% from plants.
Total protein intake was positively associated with higher education levels, being physically active, higher BMI, and a baseline history of hypertension and hypercholesterolemia. Conversely, total protein intake was inversely associated with intakes of total carbohydrates, nuts, alcohol, and sugar-sweetened beverages.
The associations between protein intake and healthy aging are complex and not fully understood, the authors stated.
Effects of Protein Intake
In studies of older adult populations lower protein intake has been associated with lean mass loss. Animal protein supplementation studies in older adults have shown lean mass gains potentially related to amino acid composition.
In terms of mechanisms, evidence suggests that protein-related activation of the rapamycin complex 1 pathway may play a role, the authors suggested. The activity of this signaling pathway decreases with age.
Rapamycin, a compound used to prevent organ transplant rejection, has been associated with delayed aging. In the body, dietary protein and exercise activate this pathway, thereby stimulating muscle protein synthesis and possibly improving physical function.
As for the differential associations of plant and animal protein on the chronic disease domain of the healthy aging phenotype, Dr. Ardisson Korat and coauthors said plant protein has been associated with favorable levels of important risk factors for cardiometabolic diseases, such as reduced LDL cholesterol, lower blood pressure, and insulin sensitivity, as well as decreased levels of proinflammatory markers.
Conversely, total and animal protein intakes have been positively associated with concentrations of insulin-like growth factor 1, which is implicated in the growth of malignant cells in breast and prostate tissue.
This study is the first step in evaluating the long-term health effect of protein intake in midlife, the relevant development window for most chronic conditions, the NHS study authors said. More research is needed, however, to corroborate the study findings in other populations and identify underlying mechanisms.
This study was supported by the USDA Agricultural Research Service and the National Institutes of Health. The authors reported no conflicts of interest. The commentators disclosed no relevant competing interests.
Intake of protein, especially from plants, in middle age is associated with higher odds of healthy aging and positive mental and physical health status in older women, a recent analysis of the Nurses’ Health Study (NHS) data suggests.
The study is said to be the first to examine the long-term impact of midlife protein consumption on later health status.
Writing in the American Journal of Clinical Nutrition, a team led by Andres V. Ardisson Korat, DSc, a nutritional epidemiologist at the USDA Human Nutrition Research Center on Aging at Tufts University in Boston, Massachusetts, found the following midlife protein–related odds ratios (ORs) for later healthy aging measured at ages 70-93.
For each 3% energy increment from various protein sources:
- 1.05 (95% confidence interval, 1.01-1.10) for total protein
- 1.07 (1.02-1.11) for animal protein
- 1.14 (1.06-1.23) for dairy protein
- 1.38 (1.24-1.54) for plant protein
In substitution analyses, significant positive associations were observed for the isocaloric replacement of animal or dairy protein, carbohydrate, or fat with plant protein — with increased ORs for healthy aging of 1.22-1.58 for each 3% of energy replacement.
On the measure of physical function, for example, replacing calories from all macronutrient variables with equivalent calories from plant protein was associated with 20%-60% higher odds of having no physical function limitations. Plant protein was also associated with higher odds for good mental status.
“Other studies have looked at protein intake in older adults, but we felt midlife was a more relevant etiological window,” Dr. Ardisson Korat said in an interview. “Our findings generally align, however, with those of protein intake in older populations, which have shown that protein can reduce the risk of frailty.”
He added that the benefits of protein, especially from plant sources, would likely apply to men as well and increasing plant protein intake is not difficult. “If you want a snack during the day, eat a handful of nuts instead of potato chips,” he advised. And eating several meals a week featuring beans, peas, lentils, tofu, whole grains, or seeds is an easy way to boost dietary plant protein, which comes with health-promoting soluble and insoluble fiber as well as antioxidant and anti-inflammatory polyphenols and other phytochemicals.
Conversely, plant but not animal protein consumption in older adulthood was linked to a lower risk of frailty in a previous NHS trial.
Higher plant protein intake was associated with a better probability of achieving healthy aging defined by changes in functional impairments, self-reported health/vitality, mental health, and use of health services in the Spanish Seniors-Estudio Sobre Nutricion y Riesgo Cardiovascular.
In contrast, animal protein intake in middle adulthood has been linked to an increased risk of premature death from chronic diseases driven by cardiovascular disease mortality.
The present findings are consistent with those observed for protein intakes in older adulthood, Dr. Ardisson Korat said.
“This study underscores the health advantages for midlife adults consuming adequate dietary protein — particularly plant protein — as one component of pursuing a healthy lifestyle,” said Douglas R. Dirschl, MD, chair of orthopedic surgery at Baylor College of Medicine in Houston, Texas. Most Americans consume adequate amounts of protein, but according to Dr. Dirschl, who treats many older patients for osteoporotic fractures and other musculoskeletal conditions, many US diets are subpar in this nutrient.
While protein is essential for bone and muscle formation and maintenance, “a surprising number of Americans are protein deficient, even those who seem hale and are overweight,” he said.
Dietary Recommendations for Midlife Patients
Physicians should therefore advise midlife patients to meet or perhaps modestly exceed the recommended dietary allowance (RDA) for protein of 0.8 g/kg per day and to make plant protein a substantial component of daily dietary protein intake, Dr. Dirschl said.
Luke D. Kim, MD, MEd, a geriatrician at the Cleveland Clinic in Cleveland, Ohio, noted that patients with lower socioeconomic status or with difficulty in day-to-day functioning are likely to have suboptimal protein intake. Such patients may need encouragement to eat more protein. “But we should keep in mind that showing a higher associated odds ratio of better health with increased protein take does not mean causality,” he said.
According to Rachel L. Amdur, MD, an internist at Northwestern Medicine in Chicago, Illinois, the long-term follow-up data from the NHS are uniquely helpful. “Middle-aged persons may think they no longer need much dietary protein and need to be reminded. Sometimes eating carbohydrates is just easier,” she said in an interview. Physicians need to asses and counsel patients on nutrition at all stages of life. “As I tell my patients, it’s best to think of your future self now.”
In agreement is Louis J. Morledge, MD, an internist at Northwell Health in New York City. “I firmly counsel my patients about adequate and often increased protein intake in middle life. But this is always within a larger framework of overall nutritional health.” He added that middle-aged persons often find themselves “stuck in food ruts,” and one of his clinical focuses is to advise patients about the importance of healthier food choices so they can better adjust to mental, emotional, physical, and skeletal changes as they age.
Study Details
The NHS analysis drew on prospective data from 48,762 nurses under age 60 in 1984. Total protein, animal protein, dairy protein, and plant protein were derived from validated food-frequency questionnaires.
Adjusting for lifestyle, demographics, and health status, the investigators identified 3721 (7.6% of cohort) eligible participants. The mean age of participants at baseline was 48.6 years; 38.6% had body mass indexes (BMI; in kg/m2) greater than 25; 22.9% were current smokers; and 88.2% were married.
Healthy aging was defined as freedom from 11 major chronic diseases, good mental health, and no impairments in cognitive or physical function, as assessed in the 2014 or 2016 NHS participant questionnaires. Diseases/treatments included cancer, type 2 diabetes, myocardial infarction, coronary artery bypass graft or coronary angioplasty, congestive heart failure, stroke, kidney failure, chronic obstructive pulmonary disease, Parkinson disease, multiple sclerosis, and amyotrophic lateral sclerosis.
Mean total protein consumption as a percentage of energy was 18.3% (standard deviation 3%), slightly higher than the average 16% in the US diet. Of this, 13.3% derived from animals, 3.6% from dairy products, and 4.9% from plants.
Total protein intake was positively associated with higher education levels, being physically active, higher BMI, and a baseline history of hypertension and hypercholesterolemia. Conversely, total protein intake was inversely associated with intakes of total carbohydrates, nuts, alcohol, and sugar-sweetened beverages.
The associations between protein intake and healthy aging are complex and not fully understood, the authors stated.
Effects of Protein Intake
In studies of older adult populations lower protein intake has been associated with lean mass loss. Animal protein supplementation studies in older adults have shown lean mass gains potentially related to amino acid composition.
In terms of mechanisms, evidence suggests that protein-related activation of the rapamycin complex 1 pathway may play a role, the authors suggested. The activity of this signaling pathway decreases with age.
Rapamycin, a compound used to prevent organ transplant rejection, has been associated with delayed aging. In the body, dietary protein and exercise activate this pathway, thereby stimulating muscle protein synthesis and possibly improving physical function.
As for the differential associations of plant and animal protein on the chronic disease domain of the healthy aging phenotype, Dr. Ardisson Korat and coauthors said plant protein has been associated with favorable levels of important risk factors for cardiometabolic diseases, such as reduced LDL cholesterol, lower blood pressure, and insulin sensitivity, as well as decreased levels of proinflammatory markers.
Conversely, total and animal protein intakes have been positively associated with concentrations of insulin-like growth factor 1, which is implicated in the growth of malignant cells in breast and prostate tissue.
This study is the first step in evaluating the long-term health effect of protein intake in midlife, the relevant development window for most chronic conditions, the NHS study authors said. More research is needed, however, to corroborate the study findings in other populations and identify underlying mechanisms.
This study was supported by the USDA Agricultural Research Service and the National Institutes of Health. The authors reported no conflicts of interest. The commentators disclosed no relevant competing interests.
Intake of protein, especially from plants, in middle age is associated with higher odds of healthy aging and positive mental and physical health status in older women, a recent analysis of the Nurses’ Health Study (NHS) data suggests.
The study is said to be the first to examine the long-term impact of midlife protein consumption on later health status.
Writing in the American Journal of Clinical Nutrition, a team led by Andres V. Ardisson Korat, DSc, a nutritional epidemiologist at the USDA Human Nutrition Research Center on Aging at Tufts University in Boston, Massachusetts, found the following midlife protein–related odds ratios (ORs) for later healthy aging measured at ages 70-93.
For each 3% energy increment from various protein sources:
- 1.05 (95% confidence interval, 1.01-1.10) for total protein
- 1.07 (1.02-1.11) for animal protein
- 1.14 (1.06-1.23) for dairy protein
- 1.38 (1.24-1.54) for plant protein
In substitution analyses, significant positive associations were observed for the isocaloric replacement of animal or dairy protein, carbohydrate, or fat with plant protein — with increased ORs for healthy aging of 1.22-1.58 for each 3% of energy replacement.
On the measure of physical function, for example, replacing calories from all macronutrient variables with equivalent calories from plant protein was associated with 20%-60% higher odds of having no physical function limitations. Plant protein was also associated with higher odds for good mental status.
“Other studies have looked at protein intake in older adults, but we felt midlife was a more relevant etiological window,” Dr. Ardisson Korat said in an interview. “Our findings generally align, however, with those of protein intake in older populations, which have shown that protein can reduce the risk of frailty.”
He added that the benefits of protein, especially from plant sources, would likely apply to men as well and increasing plant protein intake is not difficult. “If you want a snack during the day, eat a handful of nuts instead of potato chips,” he advised. And eating several meals a week featuring beans, peas, lentils, tofu, whole grains, or seeds is an easy way to boost dietary plant protein, which comes with health-promoting soluble and insoluble fiber as well as antioxidant and anti-inflammatory polyphenols and other phytochemicals.
Conversely, plant but not animal protein consumption in older adulthood was linked to a lower risk of frailty in a previous NHS trial.
Higher plant protein intake was associated with a better probability of achieving healthy aging defined by changes in functional impairments, self-reported health/vitality, mental health, and use of health services in the Spanish Seniors-Estudio Sobre Nutricion y Riesgo Cardiovascular.
In contrast, animal protein intake in middle adulthood has been linked to an increased risk of premature death from chronic diseases driven by cardiovascular disease mortality.
The present findings are consistent with those observed for protein intakes in older adulthood, Dr. Ardisson Korat said.
“This study underscores the health advantages for midlife adults consuming adequate dietary protein — particularly plant protein — as one component of pursuing a healthy lifestyle,” said Douglas R. Dirschl, MD, chair of orthopedic surgery at Baylor College of Medicine in Houston, Texas. Most Americans consume adequate amounts of protein, but according to Dr. Dirschl, who treats many older patients for osteoporotic fractures and other musculoskeletal conditions, many US diets are subpar in this nutrient.
While protein is essential for bone and muscle formation and maintenance, “a surprising number of Americans are protein deficient, even those who seem hale and are overweight,” he said.
Dietary Recommendations for Midlife Patients
Physicians should therefore advise midlife patients to meet or perhaps modestly exceed the recommended dietary allowance (RDA) for protein of 0.8 g/kg per day and to make plant protein a substantial component of daily dietary protein intake, Dr. Dirschl said.
Luke D. Kim, MD, MEd, a geriatrician at the Cleveland Clinic in Cleveland, Ohio, noted that patients with lower socioeconomic status or with difficulty in day-to-day functioning are likely to have suboptimal protein intake. Such patients may need encouragement to eat more protein. “But we should keep in mind that showing a higher associated odds ratio of better health with increased protein take does not mean causality,” he said.
According to Rachel L. Amdur, MD, an internist at Northwestern Medicine in Chicago, Illinois, the long-term follow-up data from the NHS are uniquely helpful. “Middle-aged persons may think they no longer need much dietary protein and need to be reminded. Sometimes eating carbohydrates is just easier,” she said in an interview. Physicians need to asses and counsel patients on nutrition at all stages of life. “As I tell my patients, it’s best to think of your future self now.”
In agreement is Louis J. Morledge, MD, an internist at Northwell Health in New York City. “I firmly counsel my patients about adequate and often increased protein intake in middle life. But this is always within a larger framework of overall nutritional health.” He added that middle-aged persons often find themselves “stuck in food ruts,” and one of his clinical focuses is to advise patients about the importance of healthier food choices so they can better adjust to mental, emotional, physical, and skeletal changes as they age.
Study Details
The NHS analysis drew on prospective data from 48,762 nurses under age 60 in 1984. Total protein, animal protein, dairy protein, and plant protein were derived from validated food-frequency questionnaires.
Adjusting for lifestyle, demographics, and health status, the investigators identified 3721 (7.6% of cohort) eligible participants. The mean age of participants at baseline was 48.6 years; 38.6% had body mass indexes (BMI; in kg/m2) greater than 25; 22.9% were current smokers; and 88.2% were married.
Healthy aging was defined as freedom from 11 major chronic diseases, good mental health, and no impairments in cognitive or physical function, as assessed in the 2014 or 2016 NHS participant questionnaires. Diseases/treatments included cancer, type 2 diabetes, myocardial infarction, coronary artery bypass graft or coronary angioplasty, congestive heart failure, stroke, kidney failure, chronic obstructive pulmonary disease, Parkinson disease, multiple sclerosis, and amyotrophic lateral sclerosis.
Mean total protein consumption as a percentage of energy was 18.3% (standard deviation 3%), slightly higher than the average 16% in the US diet. Of this, 13.3% derived from animals, 3.6% from dairy products, and 4.9% from plants.
Total protein intake was positively associated with higher education levels, being physically active, higher BMI, and a baseline history of hypertension and hypercholesterolemia. Conversely, total protein intake was inversely associated with intakes of total carbohydrates, nuts, alcohol, and sugar-sweetened beverages.
The associations between protein intake and healthy aging are complex and not fully understood, the authors stated.
Effects of Protein Intake
In studies of older adult populations lower protein intake has been associated with lean mass loss. Animal protein supplementation studies in older adults have shown lean mass gains potentially related to amino acid composition.
In terms of mechanisms, evidence suggests that protein-related activation of the rapamycin complex 1 pathway may play a role, the authors suggested. The activity of this signaling pathway decreases with age.
Rapamycin, a compound used to prevent organ transplant rejection, has been associated with delayed aging. In the body, dietary protein and exercise activate this pathway, thereby stimulating muscle protein synthesis and possibly improving physical function.
As for the differential associations of plant and animal protein on the chronic disease domain of the healthy aging phenotype, Dr. Ardisson Korat and coauthors said plant protein has been associated with favorable levels of important risk factors for cardiometabolic diseases, such as reduced LDL cholesterol, lower blood pressure, and insulin sensitivity, as well as decreased levels of proinflammatory markers.
Conversely, total and animal protein intakes have been positively associated with concentrations of insulin-like growth factor 1, which is implicated in the growth of malignant cells in breast and prostate tissue.
This study is the first step in evaluating the long-term health effect of protein intake in midlife, the relevant development window for most chronic conditions, the NHS study authors said. More research is needed, however, to corroborate the study findings in other populations and identify underlying mechanisms.
This study was supported by the USDA Agricultural Research Service and the National Institutes of Health. The authors reported no conflicts of interest. The commentators disclosed no relevant competing interests.
Magnesium Sulfate for Fetal Neuroprotection in Preterm Birth
Introduction: The Many Lanes of Research on Magnesium Sulfate
The research that improves human health in the most expedient and most impactful ways is multitiered, with basic or fundamental research, translational research, interventional studies, and retrospective research often occurring simultaneously. There should be no “single lane” of research and one type of research does not preclude the other.
Too often, we fall short in one of these lanes. While we have achieved many moonshots in obstetrics and maternal-fetal medicine, we have tended not to place a high priority on basic research, which can provide a strong understanding of the biology of major diseases and conditions affecting women and their offspring. When conducted with proper commitment and funding, such research can lead to biologically directed therapy.
Within our specialty, research on how we can effectively prevent preterm birth, prematurity, and preeclampsia has taken a long road, with various types of therapies being tried, but none being overwhelmingly effective — with an ongoing need for more basic or fundamental research. Nevertheless, we can benefit and gain great insights from retrospective and interventional studies associated with clinical therapies used to treat premature labor and preeclampsia when these therapies have an unanticipated and important secondary benefit.
This month our Master Class is focused on the neuroprotection of prematurity. Magnesium sulfate is a valuable tool for the treatment of both premature labor and preeclampsia, and more recently, also for neuroprotection of the fetus. Interestingly, this use stemmed from researchers looking retrospectively at outcomes in women who received the compound for other reasons. It took many years for researchers to prove its neuroprotective value through interventional trials, while researchers simultaneously strove to understand on a basic biologic level how magnesium sulfate works to prevent outcomes such as cerebral palsy.
Basic research underway today continues to improve our understanding of its precise mechanisms of action. Combined with other tiers of research — including more interventional studies and more translational research — we can improve its utility for the neuroprotection of prematurity. Alternatively, ongoing research may lead to different, even more effective treatments.
Our guest author is Irina Burd, MD, PhD, Sylvan Freiman, MD Endowed Professor and Chair of the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine.* Dr. Burd is also a physician-scientist. She recounts the important story of magnesium sulfate and what is currently known about its biologic plausibility in neuroprotection — including through her own studies – as well as what may be coming in the future.
E. Albert Reece, MD, PhD, MBA, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president; currently the endowed professor and director of the Center for Advanced Research Training and Innovation (CARTI), and senior scientist in the Center for Birth Defects Research. Dr. Reece reported no relevant disclosures. He is the medical editor of this column. Contact him at [email protected].
Magnesium Sulfate for Fetal Neuroprotection in Preterm Birth
Without a doubt, magnesium sulfate (MgSO4) given before anticipated preterm birth reduces the risk of cerebral palsy. It is a valuable tool for fetal neuroprotection at a time when there are no proven alternatives. Yet without the persistent research that occurred over more than 20 years, it may not have won the endorsement of the American College of Obstetrics and Gynecologists in 2010 and worked its way into routine practice.
Its history is worthy of reflection. It took years of observational trials (not all of which showed neuroprotective effects), six randomized controlled trials (none of which met their primary endpoint), three meta-analyses, and a Cochrane Database Systematic Review to arrive at the conclusion that antenatal magnesium sulfate therapy given to women at risk of preterm birth has definitive neuroprotective benefit.
This history also holds lessons for our specialty given the dearth of drugs approved for use in pregnancy and the recent withdrawal from the market of Makena — one of only nine drugs to ever be approved by the Food and Drug Administration for use in pregnancy — after a second trial showed lack of benefit in preventing recurrent preterm birth. The story of MgSO4 tells us it’s acceptable to have major stumbling blocks: At one point, MgSO4 was considered to be not only not helpful, but harmful, causing neonatal death. Further research disproved this initial finding.
Moreover, the MgSO4 story is one that remains unfinished, as my laboratory and other researchers work to better understand its biologic plausibility and to discover additional neuroprotective agents for anticipated preterm birth that may further reduce the risk of cerebral palsy. This leading cause of chronic childhood disability is estimated by the United Cerebral Palsy Foundation to affect approximately 800,000 people in the United States.
Origins and Biologic Plausibility
The MgSO4 story is rooted in the late seventeenth century discovery by physician Nehemiah Grew that the compound was the key component of the then-famous medicinal spring waters in Epsom, England.1 MgSO4 was first used for eclampsia in 1906,2 and was first reported in the American literature for eclampsia in 1925.3 In 1959, its effect as a tocolytic agent was reported.4
More than 30 years later, in 1995, an observational study coauthored by Karin B. Nelson, MD, and Judith K. Grether, PhD of the National Institutes of Health, showed a reduced risk of cerebral palsy in very-low-birth-weight infants (VLBW).5 The report marked a turning point in research interest on neuroprotection for anticipated preterm birth.
The precise molecular mechanisms of action of MgSO4 for neuroprotection are still not well understood. However, research findings from the University of Maryland and other institutions have provided biologic plausibility for its use to prevent cerebral palsy. Our current thinking is that it involves the prevention of periventricular white matter injury and/or the prevention of oxidative stress and a neuronal injury mechanism called excitotoxicity.
Periventricular white matter injury involving injury to preoligodendrocytes before 32 weeks’ gestation is the most prevalent injury seen in cerebral palsy; preoligodendrocytes are precursors of myelinating oligodendrocytes, which constitute a major glial population in the white matter. Our research in a mouse model demonstrated that the intrauterine inflammation frequently associated with preterm birth can lead to neuronal injury as well as white matter damage, and that MgSO4 may ameliorate both.6,7
Excitotoxicity results from excessive stimulation of N-methyl-D-aspartate (NMDA) glutamatergic receptors on preoligodendrocytes and a rush of calcium through the voltage-gated channels. This calcium influx leads to the production of nitric oxide, oxidative stress, and subsequent mitochondrial damage and cell death. As a bivalent ion, MgSO4 sits in the voltage-gated channels of the NMDA receptors and reduces glutamatergic signaling, thus serving as a calcium antagonist and modulating calcium influx (See Figure).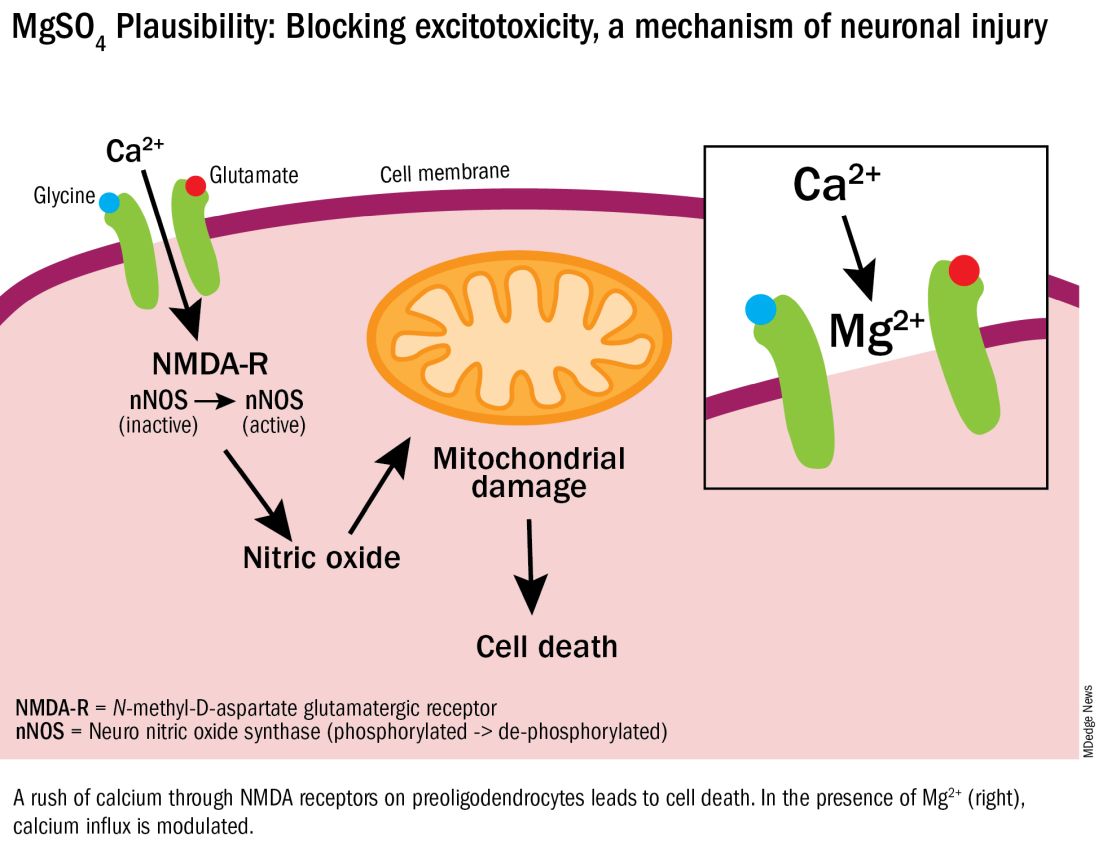
In vitro research in our laboratory has also shown that MgSO4 may dampen inflammatory reactions driven by intrauterine infections, which, like preterm birth, increase the risk of cerebral palsy and adverse neurodevelopmental outcomes.8 MgSO4 appears to do so by blocking the voltage-gated P2X7 receptor in umbilical vein endothelial cells, thus blocking endothelial secretion of the proinflammatory cytokine interleukin (IL)–1beta. Much more research is needed to determine whether MgSO4 could help prevent cerebral palsy through this mechanism.
The Long Route of Research
The 1995 Nelson-Grether study compared VLBW (< 1500 g) infants who survived and developed moderate/severe cerebral palsy within 3 years to randomly selected VLBW controls with respect to whether their mothers had received MgSO4 to prevent seizures in preeclampsia or as a tocolytic agent.5 In a population of more than 155,000 children born between 1983 and 1985, in utero exposure to MgSO4 was reported in 7.1% of 42 VLBW infants with cerebral palsy and 36% of 75 VLBW controls (odds ratio [OR], 0.14; 95% CI, 0.05-0.51). In women without preeclampsia the OR increased to 0.25.
This motivating study had been preceded by several observational studies showing that infants born to women with preeclampsia who received MgSO4 had significantly lower risks of developing intraventricular hemorrhage (IVH) and germinal matrix hemorrhage (GMH). In one of these studies, published in 1992, Karl C. Kuban, MD, and coauthors reported that “maternal receipt of magnesium sulfate was associated with diminished risk of GMH-IVH even in those babies born to mothers who apparently did not have preeclampsia.”9
In the several years following the 1995 Nelson-Grether study, several other case-control/observational studies were reported, with conflicting conclusions, and investigators around the world began designing and conducting needed randomized controlled trials.
The six published randomized controlled trials looking at MgSO4 and neuroprotection varied in their inclusion and exclusion criteria, their recruitment and enrollment style, the gestational ages for MgSO4 administration, loading and maintenance doses, how cerebral palsy or neuroprotection was assessed, and other factors (See Table for RCT characteristics and main outcomes).10-14 One of the trials aimed primarily at evaluating the efficacy of MgSO4 for preventing preeclampsia.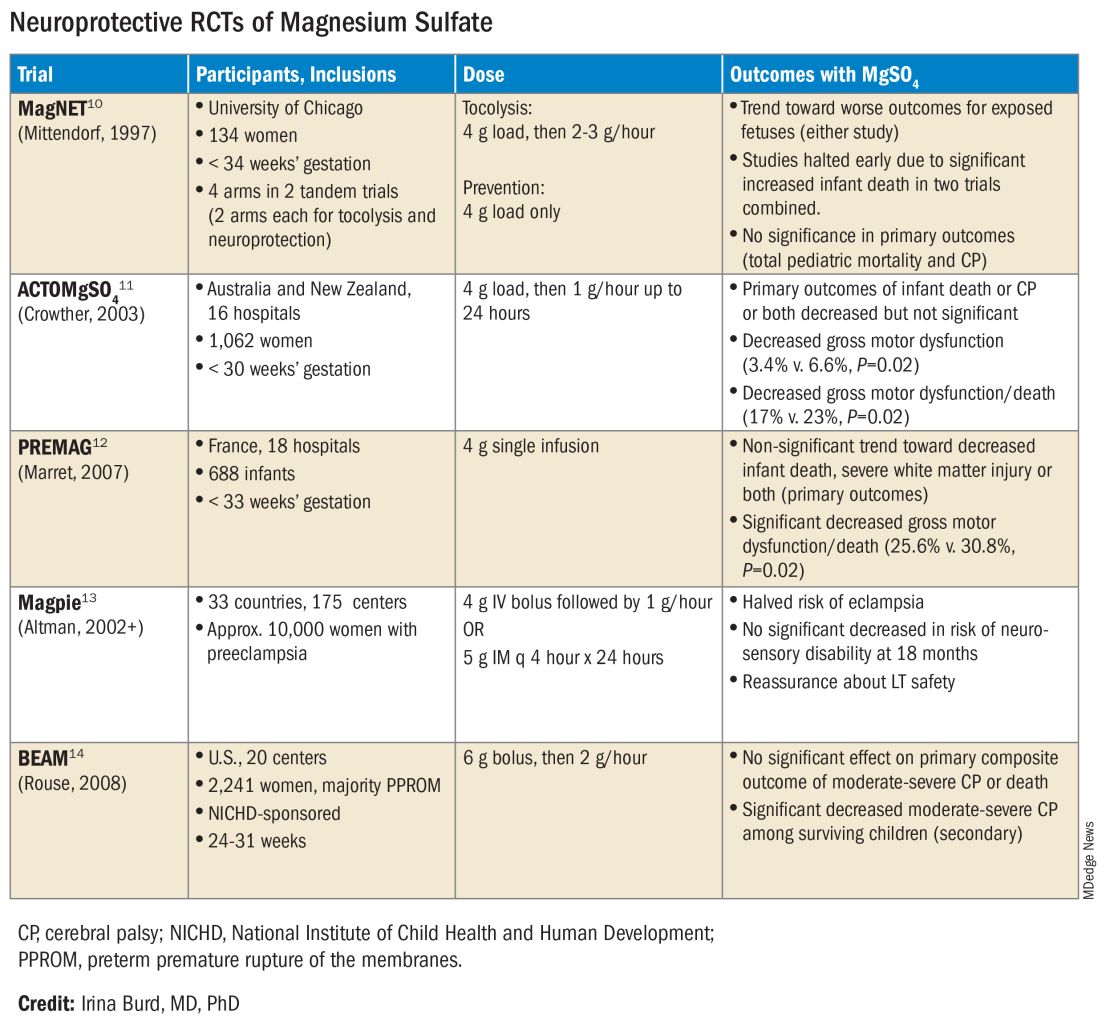
Again, none of the randomized controlled trials demonstrated statistical significance for their primary outcomes or concluded that there was a significant neuroprotective effect for cerebral palsy. Rather, most suggested benefit through secondary analyses. Moreover, as mentioned earlier, research that proceeded after the first published randomized controlled trial — the Magnesium and Neurologic Endpoints (MAGnet) trial — was suspended early when an interim analysis showed a significantly increased risk of mortality in MgSO4-exposed fetuses. All told, it wasn’t until researchers obtained unpublished data and conducted meta-analyses and systematic reviews that a significant effect of MgSO4 on cerebral palsy could be seen.
The three systematic reviews and the Cochrane review, each of which used slightly different methodologies, were published in rapid succession in 2009. One review calculated a relative risk of cerebral palsy of 0.71 (95% CI, 0.55-0.91) — and a relative risk for the combined outcome of death and cerebral palsy at 0.85 (95% CI, 0.74-0.98) — when women at risk of preterm birth were given MgSO4.15 The number needed to treat (NNT) to prevent one case of cerebral palsy was 63, investigators determined, and the NNT to prevent one case of cerebral palsy or infant death was 44.
Another review estimated the NNT for prevention of one case of cerebral palsy at 52 when MgSO4 is given at less than 34 weeks’ gestation, and similarly concluded that MgSO4 is associated with a significantly “reduced risk of moderate/severe CP and substantial gross motor dysfunction without any statistically significant effect on the risk of total pediatric mortality.”16
A third review, from the National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network (MFMU), estimated an NNT of 46 to prevent one case of cerebral palsy in infants exposed to MgSO4 before 30 weeks, and an NNT of 56 when exposure occurs before 32-34 weeks.17
The Cochrane Review, meanwhile, reported a relative reduction in the risk of cerebral palsy of 0.68 (95% CI, 0.54-0.87) when antenatal MgSO4 is given at less than 37 weeks’ gestation, as well as a significant reduction in the rate of substantial gross motor dysfunction (RR, 0.61; 95% CI, 0.44-0.85).18 The NNT to avoid one case of cerebral palsy, researchers reported, was 63.
Moving Forward
The NNTs calculated in these reviews — ranging from 44 to 63 — are convincing, and are comparable with evidence-based medicine data for prevention of other common diseases.19 For instance, the NNT for a life saved when aspirin is given immediately after a heart attack is 42. Statins given for 5 years in people with known heart disease have an NNT of 83 to save one life, an NNT of 39 to prevent one nonfatal heart attack, and an NNT of 125 to prevent one stroke. For oral anticoagulants used in nonvalvular atrial fibrillation for primary stroke prevention, the NNTs to prevent one stroke, and one death, are 22 and 42, respectively.19
In its 2010 Committee Opinion on Magnesium Sulfate Before Anticipated Preterm Birth for Neuroprotection (reaffirmed in 2020), the American College of Obstetricians and Gynecologists left it to institutions to develop their own guidelines “regarding inclusion criteria, treatment regimens, concurrent tocolysis, and monitoring in accordance with one of the larger trials.”20
Not surprisingly, most if not all hospitals have chosen a higher dose of MgSO4 administered up to 31 weeks’ gestation in keeping with the protocols employed in the NICHD-sponsored BEAM trial (See Table).
The hope moving forward is to expand treatment options for neuroprotection in cases of imminent preterm birth. Researchers have been assessing the ability of melatonin to provide neuroprotection in cases of growth restriction and neonatal asphyxia. Melatonin has anti-inflammatory and antioxidant properties and is known to mediate neuronal generation and synaptic plasticity.21
N-acetyl-L-cysteine is another potential neuroprotective agent. It acts as an antioxidant, a precursor to glutathione, and a modulator of the glutamate system and has been studied as a neuroprotective agent in cases of maternal chorioamnionitis.21 Both melatonin and N-acetyl-L-cysteine are regarded as safe in pregnancy, but much more clinical study is needed to prove their neuroprotective potential when given shortly before birth or earlier.
Dr. Burd is the Sylvan Freiman, MD Endowed Professor and Chair of the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, Baltimore. She has no conflicts of interest.
References
1. Clio Med. 1984;19(1-2):1-21.
2. Medicinsk Rev. (Bergen) 1906;32:264-272.
3. Am J Obstet Gynecol. 1996;174(4):1390-1391.
4. Am J Obstet Gynecol. 1959;78(1):27-32.
5. Pediatrics. 1995;95(2):263-269.
6. Am J Obstet Gynecol. 2009;201(3):279.e1-279.e8.
7. Am J Obstet Gynecol. 2010;202(3):292.e1-292.e9.
8. Pediatr Res. 2020;87(3):463-471.
9. J Child Neurol. 1992;7(1):70-76.
10. Lancet. 1997;350:1517-1518.
11. JAMA. 2003;290:2669-2676.
12. BJOG. 2007;114(3):310-318.
13. Lancet. 2002;359(9321):1877-1890.
14. N Engl J Med. 2008;359:895-905.
15. Obstet Gynecol. 2009;113(6):1327-1333.
16. Am J Obstet Gynecol. 2009;200(6):595-609.
17. Obstet Gynecol 2009;114:354-364.
18. Cochrane Database Syst Rev. 2009 Jan 21:(1):CD004661.
19. www.thennt.com.
20. Obstet Gynecol. 2010;115:669-671.
21. Front Synaptic Neurosci. 2012;13:680899.
*This story was corrected on June 10, 2024.
Introduction: The Many Lanes of Research on Magnesium Sulfate
The research that improves human health in the most expedient and most impactful ways is multitiered, with basic or fundamental research, translational research, interventional studies, and retrospective research often occurring simultaneously. There should be no “single lane” of research and one type of research does not preclude the other.
Too often, we fall short in one of these lanes. While we have achieved many moonshots in obstetrics and maternal-fetal medicine, we have tended not to place a high priority on basic research, which can provide a strong understanding of the biology of major diseases and conditions affecting women and their offspring. When conducted with proper commitment and funding, such research can lead to biologically directed therapy.
Within our specialty, research on how we can effectively prevent preterm birth, prematurity, and preeclampsia has taken a long road, with various types of therapies being tried, but none being overwhelmingly effective — with an ongoing need for more basic or fundamental research. Nevertheless, we can benefit and gain great insights from retrospective and interventional studies associated with clinical therapies used to treat premature labor and preeclampsia when these therapies have an unanticipated and important secondary benefit.
This month our Master Class is focused on the neuroprotection of prematurity. Magnesium sulfate is a valuable tool for the treatment of both premature labor and preeclampsia, and more recently, also for neuroprotection of the fetus. Interestingly, this use stemmed from researchers looking retrospectively at outcomes in women who received the compound for other reasons. It took many years for researchers to prove its neuroprotective value through interventional trials, while researchers simultaneously strove to understand on a basic biologic level how magnesium sulfate works to prevent outcomes such as cerebral palsy.
Basic research underway today continues to improve our understanding of its precise mechanisms of action. Combined with other tiers of research — including more interventional studies and more translational research — we can improve its utility for the neuroprotection of prematurity. Alternatively, ongoing research may lead to different, even more effective treatments.
Our guest author is Irina Burd, MD, PhD, Sylvan Freiman, MD Endowed Professor and Chair of the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine.* Dr. Burd is also a physician-scientist. She recounts the important story of magnesium sulfate and what is currently known about its biologic plausibility in neuroprotection — including through her own studies – as well as what may be coming in the future.
E. Albert Reece, MD, PhD, MBA, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president; currently the endowed professor and director of the Center for Advanced Research Training and Innovation (CARTI), and senior scientist in the Center for Birth Defects Research. Dr. Reece reported no relevant disclosures. He is the medical editor of this column. Contact him at [email protected].
Magnesium Sulfate for Fetal Neuroprotection in Preterm Birth
Without a doubt, magnesium sulfate (MgSO4) given before anticipated preterm birth reduces the risk of cerebral palsy. It is a valuable tool for fetal neuroprotection at a time when there are no proven alternatives. Yet without the persistent research that occurred over more than 20 years, it may not have won the endorsement of the American College of Obstetrics and Gynecologists in 2010 and worked its way into routine practice.
Its history is worthy of reflection. It took years of observational trials (not all of which showed neuroprotective effects), six randomized controlled trials (none of which met their primary endpoint), three meta-analyses, and a Cochrane Database Systematic Review to arrive at the conclusion that antenatal magnesium sulfate therapy given to women at risk of preterm birth has definitive neuroprotective benefit.
This history also holds lessons for our specialty given the dearth of drugs approved for use in pregnancy and the recent withdrawal from the market of Makena — one of only nine drugs to ever be approved by the Food and Drug Administration for use in pregnancy — after a second trial showed lack of benefit in preventing recurrent preterm birth. The story of MgSO4 tells us it’s acceptable to have major stumbling blocks: At one point, MgSO4 was considered to be not only not helpful, but harmful, causing neonatal death. Further research disproved this initial finding.
Moreover, the MgSO4 story is one that remains unfinished, as my laboratory and other researchers work to better understand its biologic plausibility and to discover additional neuroprotective agents for anticipated preterm birth that may further reduce the risk of cerebral palsy. This leading cause of chronic childhood disability is estimated by the United Cerebral Palsy Foundation to affect approximately 800,000 people in the United States.
Origins and Biologic Plausibility
The MgSO4 story is rooted in the late seventeenth century discovery by physician Nehemiah Grew that the compound was the key component of the then-famous medicinal spring waters in Epsom, England.1 MgSO4 was first used for eclampsia in 1906,2 and was first reported in the American literature for eclampsia in 1925.3 In 1959, its effect as a tocolytic agent was reported.4
More than 30 years later, in 1995, an observational study coauthored by Karin B. Nelson, MD, and Judith K. Grether, PhD of the National Institutes of Health, showed a reduced risk of cerebral palsy in very-low-birth-weight infants (VLBW).5 The report marked a turning point in research interest on neuroprotection for anticipated preterm birth.
The precise molecular mechanisms of action of MgSO4 for neuroprotection are still not well understood. However, research findings from the University of Maryland and other institutions have provided biologic plausibility for its use to prevent cerebral palsy. Our current thinking is that it involves the prevention of periventricular white matter injury and/or the prevention of oxidative stress and a neuronal injury mechanism called excitotoxicity.
Periventricular white matter injury involving injury to preoligodendrocytes before 32 weeks’ gestation is the most prevalent injury seen in cerebral palsy; preoligodendrocytes are precursors of myelinating oligodendrocytes, which constitute a major glial population in the white matter. Our research in a mouse model demonstrated that the intrauterine inflammation frequently associated with preterm birth can lead to neuronal injury as well as white matter damage, and that MgSO4 may ameliorate both.6,7
Excitotoxicity results from excessive stimulation of N-methyl-D-aspartate (NMDA) glutamatergic receptors on preoligodendrocytes and a rush of calcium through the voltage-gated channels. This calcium influx leads to the production of nitric oxide, oxidative stress, and subsequent mitochondrial damage and cell death. As a bivalent ion, MgSO4 sits in the voltage-gated channels of the NMDA receptors and reduces glutamatergic signaling, thus serving as a calcium antagonist and modulating calcium influx (See Figure).
In vitro research in our laboratory has also shown that MgSO4 may dampen inflammatory reactions driven by intrauterine infections, which, like preterm birth, increase the risk of cerebral palsy and adverse neurodevelopmental outcomes.8 MgSO4 appears to do so by blocking the voltage-gated P2X7 receptor in umbilical vein endothelial cells, thus blocking endothelial secretion of the proinflammatory cytokine interleukin (IL)–1beta. Much more research is needed to determine whether MgSO4 could help prevent cerebral palsy through this mechanism.
The Long Route of Research
The 1995 Nelson-Grether study compared VLBW (< 1500 g) infants who survived and developed moderate/severe cerebral palsy within 3 years to randomly selected VLBW controls with respect to whether their mothers had received MgSO4 to prevent seizures in preeclampsia or as a tocolytic agent.5 In a population of more than 155,000 children born between 1983 and 1985, in utero exposure to MgSO4 was reported in 7.1% of 42 VLBW infants with cerebral palsy and 36% of 75 VLBW controls (odds ratio [OR], 0.14; 95% CI, 0.05-0.51). In women without preeclampsia the OR increased to 0.25.
This motivating study had been preceded by several observational studies showing that infants born to women with preeclampsia who received MgSO4 had significantly lower risks of developing intraventricular hemorrhage (IVH) and germinal matrix hemorrhage (GMH). In one of these studies, published in 1992, Karl C. Kuban, MD, and coauthors reported that “maternal receipt of magnesium sulfate was associated with diminished risk of GMH-IVH even in those babies born to mothers who apparently did not have preeclampsia.”9
In the several years following the 1995 Nelson-Grether study, several other case-control/observational studies were reported, with conflicting conclusions, and investigators around the world began designing and conducting needed randomized controlled trials.
The six published randomized controlled trials looking at MgSO4 and neuroprotection varied in their inclusion and exclusion criteria, their recruitment and enrollment style, the gestational ages for MgSO4 administration, loading and maintenance doses, how cerebral palsy or neuroprotection was assessed, and other factors (See Table for RCT characteristics and main outcomes).10-14 One of the trials aimed primarily at evaluating the efficacy of MgSO4 for preventing preeclampsia.
Again, none of the randomized controlled trials demonstrated statistical significance for their primary outcomes or concluded that there was a significant neuroprotective effect for cerebral palsy. Rather, most suggested benefit through secondary analyses. Moreover, as mentioned earlier, research that proceeded after the first published randomized controlled trial — the Magnesium and Neurologic Endpoints (MAGnet) trial — was suspended early when an interim analysis showed a significantly increased risk of mortality in MgSO4-exposed fetuses. All told, it wasn’t until researchers obtained unpublished data and conducted meta-analyses and systematic reviews that a significant effect of MgSO4 on cerebral palsy could be seen.
The three systematic reviews and the Cochrane review, each of which used slightly different methodologies, were published in rapid succession in 2009. One review calculated a relative risk of cerebral palsy of 0.71 (95% CI, 0.55-0.91) — and a relative risk for the combined outcome of death and cerebral palsy at 0.85 (95% CI, 0.74-0.98) — when women at risk of preterm birth were given MgSO4.15 The number needed to treat (NNT) to prevent one case of cerebral palsy was 63, investigators determined, and the NNT to prevent one case of cerebral palsy or infant death was 44.
Another review estimated the NNT for prevention of one case of cerebral palsy at 52 when MgSO4 is given at less than 34 weeks’ gestation, and similarly concluded that MgSO4 is associated with a significantly “reduced risk of moderate/severe CP and substantial gross motor dysfunction without any statistically significant effect on the risk of total pediatric mortality.”16
A third review, from the National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network (MFMU), estimated an NNT of 46 to prevent one case of cerebral palsy in infants exposed to MgSO4 before 30 weeks, and an NNT of 56 when exposure occurs before 32-34 weeks.17
The Cochrane Review, meanwhile, reported a relative reduction in the risk of cerebral palsy of 0.68 (95% CI, 0.54-0.87) when antenatal MgSO4 is given at less than 37 weeks’ gestation, as well as a significant reduction in the rate of substantial gross motor dysfunction (RR, 0.61; 95% CI, 0.44-0.85).18 The NNT to avoid one case of cerebral palsy, researchers reported, was 63.
Moving Forward
The NNTs calculated in these reviews — ranging from 44 to 63 — are convincing, and are comparable with evidence-based medicine data for prevention of other common diseases.19 For instance, the NNT for a life saved when aspirin is given immediately after a heart attack is 42. Statins given for 5 years in people with known heart disease have an NNT of 83 to save one life, an NNT of 39 to prevent one nonfatal heart attack, and an NNT of 125 to prevent one stroke. For oral anticoagulants used in nonvalvular atrial fibrillation for primary stroke prevention, the NNTs to prevent one stroke, and one death, are 22 and 42, respectively.19
In its 2010 Committee Opinion on Magnesium Sulfate Before Anticipated Preterm Birth for Neuroprotection (reaffirmed in 2020), the American College of Obstetricians and Gynecologists left it to institutions to develop their own guidelines “regarding inclusion criteria, treatment regimens, concurrent tocolysis, and monitoring in accordance with one of the larger trials.”20
Not surprisingly, most if not all hospitals have chosen a higher dose of MgSO4 administered up to 31 weeks’ gestation in keeping with the protocols employed in the NICHD-sponsored BEAM trial (See Table).
The hope moving forward is to expand treatment options for neuroprotection in cases of imminent preterm birth. Researchers have been assessing the ability of melatonin to provide neuroprotection in cases of growth restriction and neonatal asphyxia. Melatonin has anti-inflammatory and antioxidant properties and is known to mediate neuronal generation and synaptic plasticity.21
N-acetyl-L-cysteine is another potential neuroprotective agent. It acts as an antioxidant, a precursor to glutathione, and a modulator of the glutamate system and has been studied as a neuroprotective agent in cases of maternal chorioamnionitis.21 Both melatonin and N-acetyl-L-cysteine are regarded as safe in pregnancy, but much more clinical study is needed to prove their neuroprotective potential when given shortly before birth or earlier.
Dr. Burd is the Sylvan Freiman, MD Endowed Professor and Chair of the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, Baltimore. She has no conflicts of interest.
References
1. Clio Med. 1984;19(1-2):1-21.
2. Medicinsk Rev. (Bergen) 1906;32:264-272.
3. Am J Obstet Gynecol. 1996;174(4):1390-1391.
4. Am J Obstet Gynecol. 1959;78(1):27-32.
5. Pediatrics. 1995;95(2):263-269.
6. Am J Obstet Gynecol. 2009;201(3):279.e1-279.e8.
7. Am J Obstet Gynecol. 2010;202(3):292.e1-292.e9.
8. Pediatr Res. 2020;87(3):463-471.
9. J Child Neurol. 1992;7(1):70-76.
10. Lancet. 1997;350:1517-1518.
11. JAMA. 2003;290:2669-2676.
12. BJOG. 2007;114(3):310-318.
13. Lancet. 2002;359(9321):1877-1890.
14. N Engl J Med. 2008;359:895-905.
15. Obstet Gynecol. 2009;113(6):1327-1333.
16. Am J Obstet Gynecol. 2009;200(6):595-609.
17. Obstet Gynecol 2009;114:354-364.
18. Cochrane Database Syst Rev. 2009 Jan 21:(1):CD004661.
19. www.thennt.com.
20. Obstet Gynecol. 2010;115:669-671.
21. Front Synaptic Neurosci. 2012;13:680899.
*This story was corrected on June 10, 2024.
Introduction: The Many Lanes of Research on Magnesium Sulfate
The research that improves human health in the most expedient and most impactful ways is multitiered, with basic or fundamental research, translational research, interventional studies, and retrospective research often occurring simultaneously. There should be no “single lane” of research and one type of research does not preclude the other.
Too often, we fall short in one of these lanes. While we have achieved many moonshots in obstetrics and maternal-fetal medicine, we have tended not to place a high priority on basic research, which can provide a strong understanding of the biology of major diseases and conditions affecting women and their offspring. When conducted with proper commitment and funding, such research can lead to biologically directed therapy.
Within our specialty, research on how we can effectively prevent preterm birth, prematurity, and preeclampsia has taken a long road, with various types of therapies being tried, but none being overwhelmingly effective — with an ongoing need for more basic or fundamental research. Nevertheless, we can benefit and gain great insights from retrospective and interventional studies associated with clinical therapies used to treat premature labor and preeclampsia when these therapies have an unanticipated and important secondary benefit.
This month our Master Class is focused on the neuroprotection of prematurity. Magnesium sulfate is a valuable tool for the treatment of both premature labor and preeclampsia, and more recently, also for neuroprotection of the fetus. Interestingly, this use stemmed from researchers looking retrospectively at outcomes in women who received the compound for other reasons. It took many years for researchers to prove its neuroprotective value through interventional trials, while researchers simultaneously strove to understand on a basic biologic level how magnesium sulfate works to prevent outcomes such as cerebral palsy.
Basic research underway today continues to improve our understanding of its precise mechanisms of action. Combined with other tiers of research — including more interventional studies and more translational research — we can improve its utility for the neuroprotection of prematurity. Alternatively, ongoing research may lead to different, even more effective treatments.
Our guest author is Irina Burd, MD, PhD, Sylvan Freiman, MD Endowed Professor and Chair of the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine.* Dr. Burd is also a physician-scientist. She recounts the important story of magnesium sulfate and what is currently known about its biologic plausibility in neuroprotection — including through her own studies – as well as what may be coming in the future.
E. Albert Reece, MD, PhD, MBA, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president; currently the endowed professor and director of the Center for Advanced Research Training and Innovation (CARTI), and senior scientist in the Center for Birth Defects Research. Dr. Reece reported no relevant disclosures. He is the medical editor of this column. Contact him at [email protected].
Magnesium Sulfate for Fetal Neuroprotection in Preterm Birth
Without a doubt, magnesium sulfate (MgSO4) given before anticipated preterm birth reduces the risk of cerebral palsy. It is a valuable tool for fetal neuroprotection at a time when there are no proven alternatives. Yet without the persistent research that occurred over more than 20 years, it may not have won the endorsement of the American College of Obstetrics and Gynecologists in 2010 and worked its way into routine practice.
Its history is worthy of reflection. It took years of observational trials (not all of which showed neuroprotective effects), six randomized controlled trials (none of which met their primary endpoint), three meta-analyses, and a Cochrane Database Systematic Review to arrive at the conclusion that antenatal magnesium sulfate therapy given to women at risk of preterm birth has definitive neuroprotective benefit.
This history also holds lessons for our specialty given the dearth of drugs approved for use in pregnancy and the recent withdrawal from the market of Makena — one of only nine drugs to ever be approved by the Food and Drug Administration for use in pregnancy — after a second trial showed lack of benefit in preventing recurrent preterm birth. The story of MgSO4 tells us it’s acceptable to have major stumbling blocks: At one point, MgSO4 was considered to be not only not helpful, but harmful, causing neonatal death. Further research disproved this initial finding.
Moreover, the MgSO4 story is one that remains unfinished, as my laboratory and other researchers work to better understand its biologic plausibility and to discover additional neuroprotective agents for anticipated preterm birth that may further reduce the risk of cerebral palsy. This leading cause of chronic childhood disability is estimated by the United Cerebral Palsy Foundation to affect approximately 800,000 people in the United States.
Origins and Biologic Plausibility
The MgSO4 story is rooted in the late seventeenth century discovery by physician Nehemiah Grew that the compound was the key component of the then-famous medicinal spring waters in Epsom, England.1 MgSO4 was first used for eclampsia in 1906,2 and was first reported in the American literature for eclampsia in 1925.3 In 1959, its effect as a tocolytic agent was reported.4
More than 30 years later, in 1995, an observational study coauthored by Karin B. Nelson, MD, and Judith K. Grether, PhD of the National Institutes of Health, showed a reduced risk of cerebral palsy in very-low-birth-weight infants (VLBW).5 The report marked a turning point in research interest on neuroprotection for anticipated preterm birth.
The precise molecular mechanisms of action of MgSO4 for neuroprotection are still not well understood. However, research findings from the University of Maryland and other institutions have provided biologic plausibility for its use to prevent cerebral palsy. Our current thinking is that it involves the prevention of periventricular white matter injury and/or the prevention of oxidative stress and a neuronal injury mechanism called excitotoxicity.
Periventricular white matter injury involving injury to preoligodendrocytes before 32 weeks’ gestation is the most prevalent injury seen in cerebral palsy; preoligodendrocytes are precursors of myelinating oligodendrocytes, which constitute a major glial population in the white matter. Our research in a mouse model demonstrated that the intrauterine inflammation frequently associated with preterm birth can lead to neuronal injury as well as white matter damage, and that MgSO4 may ameliorate both.6,7
Excitotoxicity results from excessive stimulation of N-methyl-D-aspartate (NMDA) glutamatergic receptors on preoligodendrocytes and a rush of calcium through the voltage-gated channels. This calcium influx leads to the production of nitric oxide, oxidative stress, and subsequent mitochondrial damage and cell death. As a bivalent ion, MgSO4 sits in the voltage-gated channels of the NMDA receptors and reduces glutamatergic signaling, thus serving as a calcium antagonist and modulating calcium influx (See Figure).
In vitro research in our laboratory has also shown that MgSO4 may dampen inflammatory reactions driven by intrauterine infections, which, like preterm birth, increase the risk of cerebral palsy and adverse neurodevelopmental outcomes.8 MgSO4 appears to do so by blocking the voltage-gated P2X7 receptor in umbilical vein endothelial cells, thus blocking endothelial secretion of the proinflammatory cytokine interleukin (IL)–1beta. Much more research is needed to determine whether MgSO4 could help prevent cerebral palsy through this mechanism.
The Long Route of Research
The 1995 Nelson-Grether study compared VLBW (< 1500 g) infants who survived and developed moderate/severe cerebral palsy within 3 years to randomly selected VLBW controls with respect to whether their mothers had received MgSO4 to prevent seizures in preeclampsia or as a tocolytic agent.5 In a population of more than 155,000 children born between 1983 and 1985, in utero exposure to MgSO4 was reported in 7.1% of 42 VLBW infants with cerebral palsy and 36% of 75 VLBW controls (odds ratio [OR], 0.14; 95% CI, 0.05-0.51). In women without preeclampsia the OR increased to 0.25.
This motivating study had been preceded by several observational studies showing that infants born to women with preeclampsia who received MgSO4 had significantly lower risks of developing intraventricular hemorrhage (IVH) and germinal matrix hemorrhage (GMH). In one of these studies, published in 1992, Karl C. Kuban, MD, and coauthors reported that “maternal receipt of magnesium sulfate was associated with diminished risk of GMH-IVH even in those babies born to mothers who apparently did not have preeclampsia.”9
In the several years following the 1995 Nelson-Grether study, several other case-control/observational studies were reported, with conflicting conclusions, and investigators around the world began designing and conducting needed randomized controlled trials.
The six published randomized controlled trials looking at MgSO4 and neuroprotection varied in their inclusion and exclusion criteria, their recruitment and enrollment style, the gestational ages for MgSO4 administration, loading and maintenance doses, how cerebral palsy or neuroprotection was assessed, and other factors (See Table for RCT characteristics and main outcomes).10-14 One of the trials aimed primarily at evaluating the efficacy of MgSO4 for preventing preeclampsia.
Again, none of the randomized controlled trials demonstrated statistical significance for their primary outcomes or concluded that there was a significant neuroprotective effect for cerebral palsy. Rather, most suggested benefit through secondary analyses. Moreover, as mentioned earlier, research that proceeded after the first published randomized controlled trial — the Magnesium and Neurologic Endpoints (MAGnet) trial — was suspended early when an interim analysis showed a significantly increased risk of mortality in MgSO4-exposed fetuses. All told, it wasn’t until researchers obtained unpublished data and conducted meta-analyses and systematic reviews that a significant effect of MgSO4 on cerebral palsy could be seen.
The three systematic reviews and the Cochrane review, each of which used slightly different methodologies, were published in rapid succession in 2009. One review calculated a relative risk of cerebral palsy of 0.71 (95% CI, 0.55-0.91) — and a relative risk for the combined outcome of death and cerebral palsy at 0.85 (95% CI, 0.74-0.98) — when women at risk of preterm birth were given MgSO4.15 The number needed to treat (NNT) to prevent one case of cerebral palsy was 63, investigators determined, and the NNT to prevent one case of cerebral palsy or infant death was 44.
Another review estimated the NNT for prevention of one case of cerebral palsy at 52 when MgSO4 is given at less than 34 weeks’ gestation, and similarly concluded that MgSO4 is associated with a significantly “reduced risk of moderate/severe CP and substantial gross motor dysfunction without any statistically significant effect on the risk of total pediatric mortality.”16
A third review, from the National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network (MFMU), estimated an NNT of 46 to prevent one case of cerebral palsy in infants exposed to MgSO4 before 30 weeks, and an NNT of 56 when exposure occurs before 32-34 weeks.17
The Cochrane Review, meanwhile, reported a relative reduction in the risk of cerebral palsy of 0.68 (95% CI, 0.54-0.87) when antenatal MgSO4 is given at less than 37 weeks’ gestation, as well as a significant reduction in the rate of substantial gross motor dysfunction (RR, 0.61; 95% CI, 0.44-0.85).18 The NNT to avoid one case of cerebral palsy, researchers reported, was 63.
Moving Forward
The NNTs calculated in these reviews — ranging from 44 to 63 — are convincing, and are comparable with evidence-based medicine data for prevention of other common diseases.19 For instance, the NNT for a life saved when aspirin is given immediately after a heart attack is 42. Statins given for 5 years in people with known heart disease have an NNT of 83 to save one life, an NNT of 39 to prevent one nonfatal heart attack, and an NNT of 125 to prevent one stroke. For oral anticoagulants used in nonvalvular atrial fibrillation for primary stroke prevention, the NNTs to prevent one stroke, and one death, are 22 and 42, respectively.19
In its 2010 Committee Opinion on Magnesium Sulfate Before Anticipated Preterm Birth for Neuroprotection (reaffirmed in 2020), the American College of Obstetricians and Gynecologists left it to institutions to develop their own guidelines “regarding inclusion criteria, treatment regimens, concurrent tocolysis, and monitoring in accordance with one of the larger trials.”20
Not surprisingly, most if not all hospitals have chosen a higher dose of MgSO4 administered up to 31 weeks’ gestation in keeping with the protocols employed in the NICHD-sponsored BEAM trial (See Table).
The hope moving forward is to expand treatment options for neuroprotection in cases of imminent preterm birth. Researchers have been assessing the ability of melatonin to provide neuroprotection in cases of growth restriction and neonatal asphyxia. Melatonin has anti-inflammatory and antioxidant properties and is known to mediate neuronal generation and synaptic plasticity.21
N-acetyl-L-cysteine is another potential neuroprotective agent. It acts as an antioxidant, a precursor to glutathione, and a modulator of the glutamate system and has been studied as a neuroprotective agent in cases of maternal chorioamnionitis.21 Both melatonin and N-acetyl-L-cysteine are regarded as safe in pregnancy, but much more clinical study is needed to prove their neuroprotective potential when given shortly before birth or earlier.
Dr. Burd is the Sylvan Freiman, MD Endowed Professor and Chair of the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, Baltimore. She has no conflicts of interest.
References
1. Clio Med. 1984;19(1-2):1-21.
2. Medicinsk Rev. (Bergen) 1906;32:264-272.
3. Am J Obstet Gynecol. 1996;174(4):1390-1391.
4. Am J Obstet Gynecol. 1959;78(1):27-32.
5. Pediatrics. 1995;95(2):263-269.
6. Am J Obstet Gynecol. 2009;201(3):279.e1-279.e8.
7. Am J Obstet Gynecol. 2010;202(3):292.e1-292.e9.
8. Pediatr Res. 2020;87(3):463-471.
9. J Child Neurol. 1992;7(1):70-76.
10. Lancet. 1997;350:1517-1518.
11. JAMA. 2003;290:2669-2676.
12. BJOG. 2007;114(3):310-318.
13. Lancet. 2002;359(9321):1877-1890.
14. N Engl J Med. 2008;359:895-905.
15. Obstet Gynecol. 2009;113(6):1327-1333.
16. Am J Obstet Gynecol. 2009;200(6):595-609.
17. Obstet Gynecol 2009;114:354-364.
18. Cochrane Database Syst Rev. 2009 Jan 21:(1):CD004661.
19. www.thennt.com.
20. Obstet Gynecol. 2010;115:669-671.
21. Front Synaptic Neurosci. 2012;13:680899.
*This story was corrected on June 10, 2024.
Gestational Diabetes May Double Chronic Kidney Disease Risk
TOPLINE:
Previous gestational diabetes mellitus (GDM) nearly doubles future chronic kidney disease (CKD) risk, irrespective of subsequent diabetes and hypertension, a study showed.
METHODOLOGY:
- A nationwide, cohort study was based on data from the Danish Medical Birth Register and included 697,622 women who gave birth between 1997 and 2018.
- Of all study participants, 3.4% reported GDM in at least one pregnancy, and 12.8% of women with GDM received insulin, a proxy for a more severe metabolic dysfunction.
- The women were followed up for a median of 11.9 years.
- Researchers studied CKD and acute kidney disease as the outcomes of interest, the mediating effects of subsequent diabetes and hypertension on future CKD, and how GDM severity affected later risk for kidney disease.
TAKEAWAY:
- Women with GDM showed significantly higher CKD risk than those without GDM (adjusted hazard ratio [aHR], 1.92; 95% CI, 1.67-2.21).
- Women who received insulin during pregnancy due to severe metabolic dysfunction but did not develop subsequent diabetes had a proportionally higher risk for CKD (aHR, 2.35; 95% CI, 1.39-3.97).
- Women with GDM who went on to develop diabetes or hypertension faced even higher risks for CKD, suggesting that preventing diabetes and hypertension after GDM may reduce the development of CKD.
- GDM did not affect the risk for acute kidney disease (aHR, 1.08; 95% CI, 0.90-1.29).
IN PRACTICE:
“Women with severe metabolic dysfunction during pregnancy constitute a high-risk group regarding future CKD,” the authors wrote. “The significantly elevated CKD risk was observed from 2 years after pregnancy and beyond.”
SOURCE:
The study, with first author Maria Hornstrup Christensen, of Odense University Hospital, Odense, Denmark, was published online on December 15 in Diabetes Care.
LIMITATIONS:
GDM may be underdiagnosed, and undiagnosed diabetes may be misclassified as GDM. The proxies of GDM and insulin treatment may not have captured the increasing severity of metabolic dysfunction. The prevalence of insulin treatment was lower than expected, perhaps due to the practice of providing a patient’s first insulin pen without a prescription and perhaps not recording it in a patient’s health record.
DISCLOSURES:
This work received financial support from the University of Southern Denmark, the Region of Southern Denmark, and the Danish Diabetes Academy, which is funded by the Novo Nordisk Foundation. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
Previous gestational diabetes mellitus (GDM) nearly doubles future chronic kidney disease (CKD) risk, irrespective of subsequent diabetes and hypertension, a study showed.
METHODOLOGY:
- A nationwide, cohort study was based on data from the Danish Medical Birth Register and included 697,622 women who gave birth between 1997 and 2018.
- Of all study participants, 3.4% reported GDM in at least one pregnancy, and 12.8% of women with GDM received insulin, a proxy for a more severe metabolic dysfunction.
- The women were followed up for a median of 11.9 years.
- Researchers studied CKD and acute kidney disease as the outcomes of interest, the mediating effects of subsequent diabetes and hypertension on future CKD, and how GDM severity affected later risk for kidney disease.
TAKEAWAY:
- Women with GDM showed significantly higher CKD risk than those without GDM (adjusted hazard ratio [aHR], 1.92; 95% CI, 1.67-2.21).
- Women who received insulin during pregnancy due to severe metabolic dysfunction but did not develop subsequent diabetes had a proportionally higher risk for CKD (aHR, 2.35; 95% CI, 1.39-3.97).
- Women with GDM who went on to develop diabetes or hypertension faced even higher risks for CKD, suggesting that preventing diabetes and hypertension after GDM may reduce the development of CKD.
- GDM did not affect the risk for acute kidney disease (aHR, 1.08; 95% CI, 0.90-1.29).
IN PRACTICE:
“Women with severe metabolic dysfunction during pregnancy constitute a high-risk group regarding future CKD,” the authors wrote. “The significantly elevated CKD risk was observed from 2 years after pregnancy and beyond.”
SOURCE:
The study, with first author Maria Hornstrup Christensen, of Odense University Hospital, Odense, Denmark, was published online on December 15 in Diabetes Care.
LIMITATIONS:
GDM may be underdiagnosed, and undiagnosed diabetes may be misclassified as GDM. The proxies of GDM and insulin treatment may not have captured the increasing severity of metabolic dysfunction. The prevalence of insulin treatment was lower than expected, perhaps due to the practice of providing a patient’s first insulin pen without a prescription and perhaps not recording it in a patient’s health record.
DISCLOSURES:
This work received financial support from the University of Southern Denmark, the Region of Southern Denmark, and the Danish Diabetes Academy, which is funded by the Novo Nordisk Foundation. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
Previous gestational diabetes mellitus (GDM) nearly doubles future chronic kidney disease (CKD) risk, irrespective of subsequent diabetes and hypertension, a study showed.
METHODOLOGY:
- A nationwide, cohort study was based on data from the Danish Medical Birth Register and included 697,622 women who gave birth between 1997 and 2018.
- Of all study participants, 3.4% reported GDM in at least one pregnancy, and 12.8% of women with GDM received insulin, a proxy for a more severe metabolic dysfunction.
- The women were followed up for a median of 11.9 years.
- Researchers studied CKD and acute kidney disease as the outcomes of interest, the mediating effects of subsequent diabetes and hypertension on future CKD, and how GDM severity affected later risk for kidney disease.
TAKEAWAY:
- Women with GDM showed significantly higher CKD risk than those without GDM (adjusted hazard ratio [aHR], 1.92; 95% CI, 1.67-2.21).
- Women who received insulin during pregnancy due to severe metabolic dysfunction but did not develop subsequent diabetes had a proportionally higher risk for CKD (aHR, 2.35; 95% CI, 1.39-3.97).
- Women with GDM who went on to develop diabetes or hypertension faced even higher risks for CKD, suggesting that preventing diabetes and hypertension after GDM may reduce the development of CKD.
- GDM did not affect the risk for acute kidney disease (aHR, 1.08; 95% CI, 0.90-1.29).
IN PRACTICE:
“Women with severe metabolic dysfunction during pregnancy constitute a high-risk group regarding future CKD,” the authors wrote. “The significantly elevated CKD risk was observed from 2 years after pregnancy and beyond.”
SOURCE:
The study, with first author Maria Hornstrup Christensen, of Odense University Hospital, Odense, Denmark, was published online on December 15 in Diabetes Care.
LIMITATIONS:
GDM may be underdiagnosed, and undiagnosed diabetes may be misclassified as GDM. The proxies of GDM and insulin treatment may not have captured the increasing severity of metabolic dysfunction. The prevalence of insulin treatment was lower than expected, perhaps due to the practice of providing a patient’s first insulin pen without a prescription and perhaps not recording it in a patient’s health record.
DISCLOSURES:
This work received financial support from the University of Southern Denmark, the Region of Southern Denmark, and the Danish Diabetes Academy, which is funded by the Novo Nordisk Foundation. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
Autoimmune Diseases and Perinatal Depression May Share Two-Way Link
Women with autoimmune disease are more likely to have perinatal depression (PND), according to findings from a new study that also suggested the reverse relationship is true: Women with a history of PND have a higher risk of developing autoimmune disease.
The research, published online on January 9, 2024, in Molecular Psychiatry, was led by Emma Bränn, PhD, Institute of Environmental Medicine, Karolinska Institute, Stockholm, Sweden.
The researchers used data from the Swedish Medical Birth Register and identified all women who had given birth in Sweden between 2001 and 2013. Out of the group of approximately 815,000 women and 1.3 million pregnancies, just more than 55,000 women had been diagnosed with depression during their pregnancy or within a year after delivery.
The researchers then compared the incidence of 41 autoimmune diseases in women who had and did not have PND. They controlled for factors including genetic makeup and childhood environment.
Results indicated that women with autoimmune disease were 30% more likely to have PND (odds ratio, 1.30; 95% CI, 1.25-1.35). Conversely, women with PND were 30% more likely than women with no PND to develop an autoimmune disease (hazard ratio, 1.30; 95% CI, 1.25-1.36).
A sibling comparison helped confirm the results by controlling for some shared genetic and early life environmental factors related to the household in which sisters grew up.
Potential Shared Biological Mechanisms
The association was independent of psychiatric comorbidities, suggesting there may be shared biological mechanisms.
Dr. Bränn told this news organization that the research team wanted to do the study because previous research has shown involvement of the immune system in depression, with similarities in both the symptoms of immune system–activated diseases and depression and the molecular pathways activated by the immune system.
“Adding on top of the tremendous changes in the immune system that we see in the body of the woman during the perinatal period, we hypothesized that autoimmune diseases could be associated to perinatal depression,” she said. “This had also been shown in some previous literature but not to the extent as what we have investigated in this paper.”
She said their results help make a case for counseling women at several points in healthcare interactions — before and after conception and childbirth — and in rheumatology visits to inform women with autoimmune diseases who are contemplating motherhood of the association with developing PND. The results may also demonstrate a need for monitoring women in these groups for depression or autoimmune disease.
Fred Miller, MD, PhD, retired Scientist Emeritus of the Environmental Autoimmunity Group at the National Institute of Environmental Health Sciences, who was not part of the study, said the results seem plausible as they build on early work that demonstrated selected associations between autoimmune conditions and mental illness.
“These associations may be the result of shared genetic and environmental risk factors, including stress, hormonal changes, medications, and the proinflammatory states that can lead to both,” he said.
The novelty, he said, is in the relatively strong associations of PND with autoimmune disease overall and with specific autoimmune diseases.
Strong Link Found With Multiple Sclerosis (MS)
According to the paper, a significant positive bidirectional link was found for autoimmune thyroid disease, psoriasis, MS, ulcerative colitis, and celiac disease.
Researchers found a particularly strong association — double the risk in both directions — between PND and MS.
Dr. Miller said though it is unclear from this study why the association of PND with MS was stronger than with other autoimmune diseases, people with MS are known to be at a high risk for depression in general. That may come from greater shared genetic and environmental risk factors, he added.
Additionally, MS is one of the more common autoimmune diseases, he noted, so the population is larger for study.
He said he was surprised the researchers didn’t investigate medication use because medications used in depression have immunologic effects and medications used in autoimmune diseases could have effects on mental conditions.
The study has implications for clinicians in a wide variety of specialties, Dr. Miller noted.
“It suggests that caregivers be more alert to the signs of developing autoimmune disease in women with perinatal depression and to the signs of developing perinatal depression in those with autoimmune disease,” Dr. Miller said, “so that appropriate screening, diagnostics, and interventions may be undertaken.”
The researchers say they will continue to examine the long-term effects of depression during pregnancy and in the year after childbirth.
“Depression during this sensitive period can have serious consequences for both the mother and the baby,” Dr. Bränn said. “We hope that our results will help decision-makers to steer funding toward maternal healthcare so that more women can get help and support in time.”
The study was financed by Karolinska Institute, Forte (the Swedish Research Council for Health, Working Life and Welfare), the Swedish Research Council, and the Icelandic Research Fund.
The researchers and Dr. Miller reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Women with autoimmune disease are more likely to have perinatal depression (PND), according to findings from a new study that also suggested the reverse relationship is true: Women with a history of PND have a higher risk of developing autoimmune disease.
The research, published online on January 9, 2024, in Molecular Psychiatry, was led by Emma Bränn, PhD, Institute of Environmental Medicine, Karolinska Institute, Stockholm, Sweden.
The researchers used data from the Swedish Medical Birth Register and identified all women who had given birth in Sweden between 2001 and 2013. Out of the group of approximately 815,000 women and 1.3 million pregnancies, just more than 55,000 women had been diagnosed with depression during their pregnancy or within a year after delivery.
The researchers then compared the incidence of 41 autoimmune diseases in women who had and did not have PND. They controlled for factors including genetic makeup and childhood environment.
Results indicated that women with autoimmune disease were 30% more likely to have PND (odds ratio, 1.30; 95% CI, 1.25-1.35). Conversely, women with PND were 30% more likely than women with no PND to develop an autoimmune disease (hazard ratio, 1.30; 95% CI, 1.25-1.36).
A sibling comparison helped confirm the results by controlling for some shared genetic and early life environmental factors related to the household in which sisters grew up.
Potential Shared Biological Mechanisms
The association was independent of psychiatric comorbidities, suggesting there may be shared biological mechanisms.
Dr. Bränn told this news organization that the research team wanted to do the study because previous research has shown involvement of the immune system in depression, with similarities in both the symptoms of immune system–activated diseases and depression and the molecular pathways activated by the immune system.
“Adding on top of the tremendous changes in the immune system that we see in the body of the woman during the perinatal period, we hypothesized that autoimmune diseases could be associated to perinatal depression,” she said. “This had also been shown in some previous literature but not to the extent as what we have investigated in this paper.”
She said their results help make a case for counseling women at several points in healthcare interactions — before and after conception and childbirth — and in rheumatology visits to inform women with autoimmune diseases who are contemplating motherhood of the association with developing PND. The results may also demonstrate a need for monitoring women in these groups for depression or autoimmune disease.
Fred Miller, MD, PhD, retired Scientist Emeritus of the Environmental Autoimmunity Group at the National Institute of Environmental Health Sciences, who was not part of the study, said the results seem plausible as they build on early work that demonstrated selected associations between autoimmune conditions and mental illness.
“These associations may be the result of shared genetic and environmental risk factors, including stress, hormonal changes, medications, and the proinflammatory states that can lead to both,” he said.
The novelty, he said, is in the relatively strong associations of PND with autoimmune disease overall and with specific autoimmune diseases.
Strong Link Found With Multiple Sclerosis (MS)
According to the paper, a significant positive bidirectional link was found for autoimmune thyroid disease, psoriasis, MS, ulcerative colitis, and celiac disease.
Researchers found a particularly strong association — double the risk in both directions — between PND and MS.
Dr. Miller said though it is unclear from this study why the association of PND with MS was stronger than with other autoimmune diseases, people with MS are known to be at a high risk for depression in general. That may come from greater shared genetic and environmental risk factors, he added.
Additionally, MS is one of the more common autoimmune diseases, he noted, so the population is larger for study.
He said he was surprised the researchers didn’t investigate medication use because medications used in depression have immunologic effects and medications used in autoimmune diseases could have effects on mental conditions.
The study has implications for clinicians in a wide variety of specialties, Dr. Miller noted.
“It suggests that caregivers be more alert to the signs of developing autoimmune disease in women with perinatal depression and to the signs of developing perinatal depression in those with autoimmune disease,” Dr. Miller said, “so that appropriate screening, diagnostics, and interventions may be undertaken.”
The researchers say they will continue to examine the long-term effects of depression during pregnancy and in the year after childbirth.
“Depression during this sensitive period can have serious consequences for both the mother and the baby,” Dr. Bränn said. “We hope that our results will help decision-makers to steer funding toward maternal healthcare so that more women can get help and support in time.”
The study was financed by Karolinska Institute, Forte (the Swedish Research Council for Health, Working Life and Welfare), the Swedish Research Council, and the Icelandic Research Fund.
The researchers and Dr. Miller reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Women with autoimmune disease are more likely to have perinatal depression (PND), according to findings from a new study that also suggested the reverse relationship is true: Women with a history of PND have a higher risk of developing autoimmune disease.
The research, published online on January 9, 2024, in Molecular Psychiatry, was led by Emma Bränn, PhD, Institute of Environmental Medicine, Karolinska Institute, Stockholm, Sweden.
The researchers used data from the Swedish Medical Birth Register and identified all women who had given birth in Sweden between 2001 and 2013. Out of the group of approximately 815,000 women and 1.3 million pregnancies, just more than 55,000 women had been diagnosed with depression during their pregnancy or within a year after delivery.
The researchers then compared the incidence of 41 autoimmune diseases in women who had and did not have PND. They controlled for factors including genetic makeup and childhood environment.
Results indicated that women with autoimmune disease were 30% more likely to have PND (odds ratio, 1.30; 95% CI, 1.25-1.35). Conversely, women with PND were 30% more likely than women with no PND to develop an autoimmune disease (hazard ratio, 1.30; 95% CI, 1.25-1.36).
A sibling comparison helped confirm the results by controlling for some shared genetic and early life environmental factors related to the household in which sisters grew up.
Potential Shared Biological Mechanisms
The association was independent of psychiatric comorbidities, suggesting there may be shared biological mechanisms.
Dr. Bränn told this news organization that the research team wanted to do the study because previous research has shown involvement of the immune system in depression, with similarities in both the symptoms of immune system–activated diseases and depression and the molecular pathways activated by the immune system.
“Adding on top of the tremendous changes in the immune system that we see in the body of the woman during the perinatal period, we hypothesized that autoimmune diseases could be associated to perinatal depression,” she said. “This had also been shown in some previous literature but not to the extent as what we have investigated in this paper.”
She said their results help make a case for counseling women at several points in healthcare interactions — before and after conception and childbirth — and in rheumatology visits to inform women with autoimmune diseases who are contemplating motherhood of the association with developing PND. The results may also demonstrate a need for monitoring women in these groups for depression or autoimmune disease.
Fred Miller, MD, PhD, retired Scientist Emeritus of the Environmental Autoimmunity Group at the National Institute of Environmental Health Sciences, who was not part of the study, said the results seem plausible as they build on early work that demonstrated selected associations between autoimmune conditions and mental illness.
“These associations may be the result of shared genetic and environmental risk factors, including stress, hormonal changes, medications, and the proinflammatory states that can lead to both,” he said.
The novelty, he said, is in the relatively strong associations of PND with autoimmune disease overall and with specific autoimmune diseases.
Strong Link Found With Multiple Sclerosis (MS)
According to the paper, a significant positive bidirectional link was found for autoimmune thyroid disease, psoriasis, MS, ulcerative colitis, and celiac disease.
Researchers found a particularly strong association — double the risk in both directions — between PND and MS.
Dr. Miller said though it is unclear from this study why the association of PND with MS was stronger than with other autoimmune diseases, people with MS are known to be at a high risk for depression in general. That may come from greater shared genetic and environmental risk factors, he added.
Additionally, MS is one of the more common autoimmune diseases, he noted, so the population is larger for study.
He said he was surprised the researchers didn’t investigate medication use because medications used in depression have immunologic effects and medications used in autoimmune diseases could have effects on mental conditions.
The study has implications for clinicians in a wide variety of specialties, Dr. Miller noted.
“It suggests that caregivers be more alert to the signs of developing autoimmune disease in women with perinatal depression and to the signs of developing perinatal depression in those with autoimmune disease,” Dr. Miller said, “so that appropriate screening, diagnostics, and interventions may be undertaken.”
The researchers say they will continue to examine the long-term effects of depression during pregnancy and in the year after childbirth.
“Depression during this sensitive period can have serious consequences for both the mother and the baby,” Dr. Bränn said. “We hope that our results will help decision-makers to steer funding toward maternal healthcare so that more women can get help and support in time.”
The study was financed by Karolinska Institute, Forte (the Swedish Research Council for Health, Working Life and Welfare), the Swedish Research Council, and the Icelandic Research Fund.
The researchers and Dr. Miller reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM MOLECULAR PSYCHIATRY
SUDs rates highest in head, neck, and gastric cancer survivors
.
The association between cancer and substance use is well known, but data on the prevalence of different substance use disorders (SUDs) in different types of cancer are limited, Katie F. Jones, PhD, of the VA Boston Healthcare System, and colleagues, wrote in their paper.
“Substance use and use disorders are on the rise in general and among older adults, who represent the majority of people diagnosed with cancer, and SUDs have significant potential to complicate cancer care and negatively impact cancer outcomes,” corresponding author Devon K. Check, PhD, of Duke University, Durham, N.C., said in an interview. “We thought it was important to understand whether SUDs are more common with certain types of cancer. We can use that information to guide resources toward populations where interventions to integrate SUD treatment and cancer treatment are most needed,” he said. “In addition, because different SUDs (opioid use disorder, alcohol use disorder) might complicate cancer treatment in different ways and necessitate different types of interventions, we thought it was important to understand the distribution of specific disorders,” he explained.
In the cross-sectional study published in JAMA Oncology, the researchers reviewed data from 6,101 adult cancer survivors who participated in the National Survey of Drug Use and Health (NSDUH) between 2015 and 2020.
The study population included survivors of solid tumor cancers. SUD was defined as meeting at least one of four criteria for substance abuse or at least 3 of 6 criteria for dependence based on the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) criteria.
Overall, 3.83% of the participants met criteria for SUD. Survivors of head and neck cancers and survivors of gastric and esophageal cancers had the highest rates of SUDs (approximately 9%), followed by cervical cancer and melanoma survivors (approximately 6%).
Alcohol use disorder was the most common SUD both overall (2.8%) and among survivors of head and neck cancers, cervical cancers, and melanoma.
Cannabis use disorder was the most prevalent SUD among esophageal and gastric cancer survivors (approximately 9%).
The prevalence of SUDs overall and within the past year (active) was approximately 4%, but the prevalence of active SUDs was significantly higher for those with head and neck cancers and cervical cancer (18.73% and 15.70%, respectively). However, the distribution of specific SUDs was different in the newly diagnosed patients. Sedative use disorder took the top spot as the most common SUD for head and neck cancer survivors (9.81%), while alcohol use disorder was the most common SUD among cervical cancer survivors (10.49%).
Limitations and Implications
The findings were limited by several factors, including the nature of the study population and the data source, said Dr. Check.
“The average prevalence of SUD (or the prevalence across cancer types) was lower than we might have expected,” but the results make sense given the mainly older and female study population, he said. SUDs are less common among older adults compared with younger adults and among women compared with men, and the study’s data source (NSDUH) has been shown in other research to underestimate the prevalence of opioid use disorder, he added.
“Otherwise, the study findings were generally consistent with what we would expect,” Dr. Check said in an interview. “For example, alcohol use disorder is the most common SUD in the general U.S. population, and that was true for our study population of cancer survivors as well. In addition, SUD prevalence was higher in cancers such as cervical cancer and head and neck cancers that are causally linked to alcohol and/or tobacco use,” he said.
Integrated care is needed
“Among people diagnosed with certain types of cancers, including cervical and head and neck cancers, the estimated prevalence of SUD is similar to those [with] medical comorbidities such as diabetes and cardiopulmonary conditions,” said Dr. Check. “Within the field, there is an increasing emphasis on ensuring that people diagnosed with cancer have access to integrated care for their comorbid medical conditions. Similar efforts for people who concurrently manage cancer and SUD are largely absent but critically needed; these efforts should prioritize cancer populations where SUD prevalence is high,” he said.
Looking ahead, “We need to understand more about the specific challenges that arise at the intersection of cancer and SUD so we can design interventions and programs to better support both patients who concurrently manage cancer and SUD and the clinicians who care for them,” Dr. Check added.
Recognize risk factors
“It is very important to study overall substance use disorders in patients with cancer, because understanding the risks of developing these issues after treatment helps us develop approaches to best support these patients following their cancer therapies,” Henry S. Park, MD, a radiation oncologist at Yale University, New Haven, Connecticut, said in an interview.
The current study findings “are generally consistent with my experience and intuition, but it is still helpful to see the actual data,” said Dr. Park, who was not involved in the study. “This may be partially because of the baseline elevated risk of preexisting SUDs for certain patients from the higher-prevalence disease sites. However, it may also be related to the intense side effects that survivors of some types of cancers, such as head and neck cancer, gastroesophageal cancer, and cervical cancer, may experience soon after treatment, and even chronically long after treatment,” he said.
Individualize risk assessment
“Ultimately, clinicians should be aware that not all patients with cancer are the same, and that the majority do not necessarily develop SUDs,” Dr. Park said in an interview. “We should be careful to treat symptoms appropriately, and not withhold therapies purely because of an elevated risk of developing SUDs. However, there are some patients who are at higher risk of SUDs who will need extra support and care from physicians, advanced practice providers, nutritionists, social workers, psychologists, dietitians, and survivorship clinics, both in the short-term and long-term,” he emphasized.
As for additional research, “more work needs to be done on which particular patients within each disease subset are most likely to develop SUDs,” said Dr. Park. “Most importantly, once we identify our high-risk group as reliably as possible, we will have to study interventions that rely on supporting and partnering with patients to decrease the risk of developing SUDs as much as possible, while adequately treating residual symptoms and quality-of-life effects following cancer treatment,” he said.
The study received no outside funding. Dr. Check disclosed grants from Duke University during the study period and grants from the National Institutes of Health and AstraZeneca unrelated to the current study. Dr. Park had no financial conflicts to disclose.
.
The association between cancer and substance use is well known, but data on the prevalence of different substance use disorders (SUDs) in different types of cancer are limited, Katie F. Jones, PhD, of the VA Boston Healthcare System, and colleagues, wrote in their paper.
“Substance use and use disorders are on the rise in general and among older adults, who represent the majority of people diagnosed with cancer, and SUDs have significant potential to complicate cancer care and negatively impact cancer outcomes,” corresponding author Devon K. Check, PhD, of Duke University, Durham, N.C., said in an interview. “We thought it was important to understand whether SUDs are more common with certain types of cancer. We can use that information to guide resources toward populations where interventions to integrate SUD treatment and cancer treatment are most needed,” he said. “In addition, because different SUDs (opioid use disorder, alcohol use disorder) might complicate cancer treatment in different ways and necessitate different types of interventions, we thought it was important to understand the distribution of specific disorders,” he explained.
In the cross-sectional study published in JAMA Oncology, the researchers reviewed data from 6,101 adult cancer survivors who participated in the National Survey of Drug Use and Health (NSDUH) between 2015 and 2020.
The study population included survivors of solid tumor cancers. SUD was defined as meeting at least one of four criteria for substance abuse or at least 3 of 6 criteria for dependence based on the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) criteria.
Overall, 3.83% of the participants met criteria for SUD. Survivors of head and neck cancers and survivors of gastric and esophageal cancers had the highest rates of SUDs (approximately 9%), followed by cervical cancer and melanoma survivors (approximately 6%).
Alcohol use disorder was the most common SUD both overall (2.8%) and among survivors of head and neck cancers, cervical cancers, and melanoma.
Cannabis use disorder was the most prevalent SUD among esophageal and gastric cancer survivors (approximately 9%).
The prevalence of SUDs overall and within the past year (active) was approximately 4%, but the prevalence of active SUDs was significantly higher for those with head and neck cancers and cervical cancer (18.73% and 15.70%, respectively). However, the distribution of specific SUDs was different in the newly diagnosed patients. Sedative use disorder took the top spot as the most common SUD for head and neck cancer survivors (9.81%), while alcohol use disorder was the most common SUD among cervical cancer survivors (10.49%).
Limitations and Implications
The findings were limited by several factors, including the nature of the study population and the data source, said Dr. Check.
“The average prevalence of SUD (or the prevalence across cancer types) was lower than we might have expected,” but the results make sense given the mainly older and female study population, he said. SUDs are less common among older adults compared with younger adults and among women compared with men, and the study’s data source (NSDUH) has been shown in other research to underestimate the prevalence of opioid use disorder, he added.
“Otherwise, the study findings were generally consistent with what we would expect,” Dr. Check said in an interview. “For example, alcohol use disorder is the most common SUD in the general U.S. population, and that was true for our study population of cancer survivors as well. In addition, SUD prevalence was higher in cancers such as cervical cancer and head and neck cancers that are causally linked to alcohol and/or tobacco use,” he said.
Integrated care is needed
“Among people diagnosed with certain types of cancers, including cervical and head and neck cancers, the estimated prevalence of SUD is similar to those [with] medical comorbidities such as diabetes and cardiopulmonary conditions,” said Dr. Check. “Within the field, there is an increasing emphasis on ensuring that people diagnosed with cancer have access to integrated care for their comorbid medical conditions. Similar efforts for people who concurrently manage cancer and SUD are largely absent but critically needed; these efforts should prioritize cancer populations where SUD prevalence is high,” he said.
Looking ahead, “We need to understand more about the specific challenges that arise at the intersection of cancer and SUD so we can design interventions and programs to better support both patients who concurrently manage cancer and SUD and the clinicians who care for them,” Dr. Check added.
Recognize risk factors
“It is very important to study overall substance use disorders in patients with cancer, because understanding the risks of developing these issues after treatment helps us develop approaches to best support these patients following their cancer therapies,” Henry S. Park, MD, a radiation oncologist at Yale University, New Haven, Connecticut, said in an interview.
The current study findings “are generally consistent with my experience and intuition, but it is still helpful to see the actual data,” said Dr. Park, who was not involved in the study. “This may be partially because of the baseline elevated risk of preexisting SUDs for certain patients from the higher-prevalence disease sites. However, it may also be related to the intense side effects that survivors of some types of cancers, such as head and neck cancer, gastroesophageal cancer, and cervical cancer, may experience soon after treatment, and even chronically long after treatment,” he said.
Individualize risk assessment
“Ultimately, clinicians should be aware that not all patients with cancer are the same, and that the majority do not necessarily develop SUDs,” Dr. Park said in an interview. “We should be careful to treat symptoms appropriately, and not withhold therapies purely because of an elevated risk of developing SUDs. However, there are some patients who are at higher risk of SUDs who will need extra support and care from physicians, advanced practice providers, nutritionists, social workers, psychologists, dietitians, and survivorship clinics, both in the short-term and long-term,” he emphasized.
As for additional research, “more work needs to be done on which particular patients within each disease subset are most likely to develop SUDs,” said Dr. Park. “Most importantly, once we identify our high-risk group as reliably as possible, we will have to study interventions that rely on supporting and partnering with patients to decrease the risk of developing SUDs as much as possible, while adequately treating residual symptoms and quality-of-life effects following cancer treatment,” he said.
The study received no outside funding. Dr. Check disclosed grants from Duke University during the study period and grants from the National Institutes of Health and AstraZeneca unrelated to the current study. Dr. Park had no financial conflicts to disclose.
.
The association between cancer and substance use is well known, but data on the prevalence of different substance use disorders (SUDs) in different types of cancer are limited, Katie F. Jones, PhD, of the VA Boston Healthcare System, and colleagues, wrote in their paper.
“Substance use and use disorders are on the rise in general and among older adults, who represent the majority of people diagnosed with cancer, and SUDs have significant potential to complicate cancer care and negatively impact cancer outcomes,” corresponding author Devon K. Check, PhD, of Duke University, Durham, N.C., said in an interview. “We thought it was important to understand whether SUDs are more common with certain types of cancer. We can use that information to guide resources toward populations where interventions to integrate SUD treatment and cancer treatment are most needed,” he said. “In addition, because different SUDs (opioid use disorder, alcohol use disorder) might complicate cancer treatment in different ways and necessitate different types of interventions, we thought it was important to understand the distribution of specific disorders,” he explained.
In the cross-sectional study published in JAMA Oncology, the researchers reviewed data from 6,101 adult cancer survivors who participated in the National Survey of Drug Use and Health (NSDUH) between 2015 and 2020.
The study population included survivors of solid tumor cancers. SUD was defined as meeting at least one of four criteria for substance abuse or at least 3 of 6 criteria for dependence based on the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) criteria.
Overall, 3.83% of the participants met criteria for SUD. Survivors of head and neck cancers and survivors of gastric and esophageal cancers had the highest rates of SUDs (approximately 9%), followed by cervical cancer and melanoma survivors (approximately 6%).
Alcohol use disorder was the most common SUD both overall (2.8%) and among survivors of head and neck cancers, cervical cancers, and melanoma.
Cannabis use disorder was the most prevalent SUD among esophageal and gastric cancer survivors (approximately 9%).
The prevalence of SUDs overall and within the past year (active) was approximately 4%, but the prevalence of active SUDs was significantly higher for those with head and neck cancers and cervical cancer (18.73% and 15.70%, respectively). However, the distribution of specific SUDs was different in the newly diagnosed patients. Sedative use disorder took the top spot as the most common SUD for head and neck cancer survivors (9.81%), while alcohol use disorder was the most common SUD among cervical cancer survivors (10.49%).
Limitations and Implications
The findings were limited by several factors, including the nature of the study population and the data source, said Dr. Check.
“The average prevalence of SUD (or the prevalence across cancer types) was lower than we might have expected,” but the results make sense given the mainly older and female study population, he said. SUDs are less common among older adults compared with younger adults and among women compared with men, and the study’s data source (NSDUH) has been shown in other research to underestimate the prevalence of opioid use disorder, he added.
“Otherwise, the study findings were generally consistent with what we would expect,” Dr. Check said in an interview. “For example, alcohol use disorder is the most common SUD in the general U.S. population, and that was true for our study population of cancer survivors as well. In addition, SUD prevalence was higher in cancers such as cervical cancer and head and neck cancers that are causally linked to alcohol and/or tobacco use,” he said.
Integrated care is needed
“Among people diagnosed with certain types of cancers, including cervical and head and neck cancers, the estimated prevalence of SUD is similar to those [with] medical comorbidities such as diabetes and cardiopulmonary conditions,” said Dr. Check. “Within the field, there is an increasing emphasis on ensuring that people diagnosed with cancer have access to integrated care for their comorbid medical conditions. Similar efforts for people who concurrently manage cancer and SUD are largely absent but critically needed; these efforts should prioritize cancer populations where SUD prevalence is high,” he said.
Looking ahead, “We need to understand more about the specific challenges that arise at the intersection of cancer and SUD so we can design interventions and programs to better support both patients who concurrently manage cancer and SUD and the clinicians who care for them,” Dr. Check added.
Recognize risk factors
“It is very important to study overall substance use disorders in patients with cancer, because understanding the risks of developing these issues after treatment helps us develop approaches to best support these patients following their cancer therapies,” Henry S. Park, MD, a radiation oncologist at Yale University, New Haven, Connecticut, said in an interview.
The current study findings “are generally consistent with my experience and intuition, but it is still helpful to see the actual data,” said Dr. Park, who was not involved in the study. “This may be partially because of the baseline elevated risk of preexisting SUDs for certain patients from the higher-prevalence disease sites. However, it may also be related to the intense side effects that survivors of some types of cancers, such as head and neck cancer, gastroesophageal cancer, and cervical cancer, may experience soon after treatment, and even chronically long after treatment,” he said.
Individualize risk assessment
“Ultimately, clinicians should be aware that not all patients with cancer are the same, and that the majority do not necessarily develop SUDs,” Dr. Park said in an interview. “We should be careful to treat symptoms appropriately, and not withhold therapies purely because of an elevated risk of developing SUDs. However, there are some patients who are at higher risk of SUDs who will need extra support and care from physicians, advanced practice providers, nutritionists, social workers, psychologists, dietitians, and survivorship clinics, both in the short-term and long-term,” he emphasized.
As for additional research, “more work needs to be done on which particular patients within each disease subset are most likely to develop SUDs,” said Dr. Park. “Most importantly, once we identify our high-risk group as reliably as possible, we will have to study interventions that rely on supporting and partnering with patients to decrease the risk of developing SUDs as much as possible, while adequately treating residual symptoms and quality-of-life effects following cancer treatment,” he said.
The study received no outside funding. Dr. Check disclosed grants from Duke University during the study period and grants from the National Institutes of Health and AstraZeneca unrelated to the current study. Dr. Park had no financial conflicts to disclose.
FROM JAMA ONCOLOGY
A 27-year-old Haitian woman presented with a painful umbilical mass which had been growing in size for 5 months
Endometriosis is defined as the presence of endometrial tissue outside of the uterine cavity, commonly occurring in women of reproductive age. The condition usually affects the adnexa (ovaries, Fallopian tubes, and associated ligaments and connective tissue) but can also be seen in extrapelvic structures.
Cutaneous endometriosis is an uncommon subtype that accounts for 1% of endometriosis cases and occurs when endometrial tissue is found on the surface of the skin. It is divided into primary and secondary cutaneous endometriosis. The that may lead to seeding of endometrial tissue on the skin. In the case of our patient, it appears that her laparoscopic procedure 2 years ago was the cause of endometrial seeding in the umbilicus.
Clinically, the condition may present with a palpable mass, cyclic pain, and bloody discharge from the affected area. Due to the rarity of cutaneous endometriosis, it may be hard to distinguish from other diagnoses such as keloids, dermatofibromas, hernias, or cutaneous metastasis of cancers (Sister Mary Joseph nodules).
The definitive diagnosis can be made by biopsy and histopathological assessment showing a mixture of endometrial glands and stromal tissue. Imaging studies such as computed tomography (CT) scan and magnetic resonance imaging (MRI) are helpful in excluding more common diagnoses such as hernia or cutaneous metastasis. In this patient, the mass was surgically excised. Histopathological assessment established the diagnosis of cutaneous endometriosis.
Treatment options include surgical excision and medical therapy. Medical therapy entails the use of hormonal agents such as gonadotropin-releasing hormone agonists, danazol (a pituitary gonadotropin inhibitor), and oral contraceptives, which reduce the cyclical proliferation of endothelial tissue. These agents can be used preoperatively to reduce the size of the cutaneous mass before surgical excision, or as an alternative treatment for patients who wish to avoid surgery. The rate of recurrence is observed to be higher with medical therapy rather than surgical treatment.
The case and photo were submitted by Mina Ahmed, MBBS, Brooke Resh Sateesh MD, and Nathan Uebelhoer MD, of San Diego Family Dermatology, San Diego, California. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Gonzalez RH et al. Am J Case Rep. 2021;22:e932493-1–e932493-4.
2. Raffi L et al. Int J Womens Dermatol. 2019 Dec;5(5):384-386.
3. Sharma A, Apostol R. Cutaneous endometriosis. Treasure Island, Fla: Statpearls Publishing, 2023.
Endometriosis is defined as the presence of endometrial tissue outside of the uterine cavity, commonly occurring in women of reproductive age. The condition usually affects the adnexa (ovaries, Fallopian tubes, and associated ligaments and connective tissue) but can also be seen in extrapelvic structures.
Cutaneous endometriosis is an uncommon subtype that accounts for 1% of endometriosis cases and occurs when endometrial tissue is found on the surface of the skin. It is divided into primary and secondary cutaneous endometriosis. The that may lead to seeding of endometrial tissue on the skin. In the case of our patient, it appears that her laparoscopic procedure 2 years ago was the cause of endometrial seeding in the umbilicus.
Clinically, the condition may present with a palpable mass, cyclic pain, and bloody discharge from the affected area. Due to the rarity of cutaneous endometriosis, it may be hard to distinguish from other diagnoses such as keloids, dermatofibromas, hernias, or cutaneous metastasis of cancers (Sister Mary Joseph nodules).
The definitive diagnosis can be made by biopsy and histopathological assessment showing a mixture of endometrial glands and stromal tissue. Imaging studies such as computed tomography (CT) scan and magnetic resonance imaging (MRI) are helpful in excluding more common diagnoses such as hernia or cutaneous metastasis. In this patient, the mass was surgically excised. Histopathological assessment established the diagnosis of cutaneous endometriosis.
Treatment options include surgical excision and medical therapy. Medical therapy entails the use of hormonal agents such as gonadotropin-releasing hormone agonists, danazol (a pituitary gonadotropin inhibitor), and oral contraceptives, which reduce the cyclical proliferation of endothelial tissue. These agents can be used preoperatively to reduce the size of the cutaneous mass before surgical excision, or as an alternative treatment for patients who wish to avoid surgery. The rate of recurrence is observed to be higher with medical therapy rather than surgical treatment.
The case and photo were submitted by Mina Ahmed, MBBS, Brooke Resh Sateesh MD, and Nathan Uebelhoer MD, of San Diego Family Dermatology, San Diego, California. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Gonzalez RH et al. Am J Case Rep. 2021;22:e932493-1–e932493-4.
2. Raffi L et al. Int J Womens Dermatol. 2019 Dec;5(5):384-386.
3. Sharma A, Apostol R. Cutaneous endometriosis. Treasure Island, Fla: Statpearls Publishing, 2023.
Endometriosis is defined as the presence of endometrial tissue outside of the uterine cavity, commonly occurring in women of reproductive age. The condition usually affects the adnexa (ovaries, Fallopian tubes, and associated ligaments and connective tissue) but can also be seen in extrapelvic structures.
Cutaneous endometriosis is an uncommon subtype that accounts for 1% of endometriosis cases and occurs when endometrial tissue is found on the surface of the skin. It is divided into primary and secondary cutaneous endometriosis. The that may lead to seeding of endometrial tissue on the skin. In the case of our patient, it appears that her laparoscopic procedure 2 years ago was the cause of endometrial seeding in the umbilicus.
Clinically, the condition may present with a palpable mass, cyclic pain, and bloody discharge from the affected area. Due to the rarity of cutaneous endometriosis, it may be hard to distinguish from other diagnoses such as keloids, dermatofibromas, hernias, or cutaneous metastasis of cancers (Sister Mary Joseph nodules).
The definitive diagnosis can be made by biopsy and histopathological assessment showing a mixture of endometrial glands and stromal tissue. Imaging studies such as computed tomography (CT) scan and magnetic resonance imaging (MRI) are helpful in excluding more common diagnoses such as hernia or cutaneous metastasis. In this patient, the mass was surgically excised. Histopathological assessment established the diagnosis of cutaneous endometriosis.
Treatment options include surgical excision and medical therapy. Medical therapy entails the use of hormonal agents such as gonadotropin-releasing hormone agonists, danazol (a pituitary gonadotropin inhibitor), and oral contraceptives, which reduce the cyclical proliferation of endothelial tissue. These agents can be used preoperatively to reduce the size of the cutaneous mass before surgical excision, or as an alternative treatment for patients who wish to avoid surgery. The rate of recurrence is observed to be higher with medical therapy rather than surgical treatment.
The case and photo were submitted by Mina Ahmed, MBBS, Brooke Resh Sateesh MD, and Nathan Uebelhoer MD, of San Diego Family Dermatology, San Diego, California. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Gonzalez RH et al. Am J Case Rep. 2021;22:e932493-1–e932493-4.
2. Raffi L et al. Int J Womens Dermatol. 2019 Dec;5(5):384-386.
3. Sharma A, Apostol R. Cutaneous endometriosis. Treasure Island, Fla: Statpearls Publishing, 2023.
Gestational Diabetes Treatment Moves Forward With Uncertainty And Hope
FAIRFAX, VIRGINIA — , but researchers at the biennial meeting of the Diabetes in Pregnancy Study Group of North America expressed hope for more clarity in the near future and the ability to someday individualize treatment to account for what is increasingly viewed as a heterogeneous condition.
Until studies in 2015 and 2018 cast doubt on glyburide, “we used to have 80% [of our GDM patients] on glyburide, and 20% on insulin,” Maisa Feghali, MD, of the University of Pittsburgh, said during a discussion period. “Now we have 95% on insulin and 5% on oral hypoglycemics. I rely on insulin because I don’t have a better option, and I rely on research efforts [underway to provide better options]” in the future.
The American College of Obstetricians and Gynecologists recommends insulin as the preferred first-line pharmacologic therapy for GDM when pharmacologic therapy is needed, with metformin as an option when patients decline or cannot safely use insulin. Glyburide, ACOG said in its 2018 practice bulletin on GDM (Obstet Gynecol. 2018;131[2]:e49-64), should not be recommended as a first-line pharmacologic therapy.
The Society of Maternal-Fetal Medicine, on the other hand, has accepted metformin as a “reasonable and safe” first-line alternative to insulin — while recognizing that half of women will still require insulin to achieve glycemic control — and does not rule out consideration of glyburide. In its 2018 statement on the pharmacologic treatment of GDM, the society said that the evidence of benefit of one oral agent over another remains limited.
“When you have dueling guidelines, it means the data are not that clear,” George Saade, MD, professor and chair of obstetrics and gynecology at the Eastern Virginia School of Medicine, Norfolk, said in a presentation on GDM. An upcoming $12 million multicenter study to be led by the Ohio State University College of Medicine — coined the DECIDE trial — should provide clarity, he said.
The trial, funded by the Patient-Centered Outcomes Research Institute, which funds comparative clinical effectiveness research designed to be broadly applicable to practice, will enroll and randomize over 1500 pregnant individuals with GDM to either oral metformin or insulin and will follow mothers and children until 2 years after delivery.
The study’s primary and secondary hypotheses, respectively, are that metformin is not inferior to insulin in reducing a composite adverse neonatal outcome (large for gestational age, neonatal hypoglycemia and/or hyperbilirubemia) and that metformin does not result in increased child body mass index at 2 years, compared with insulin. It will also look at patient-reported factors associated with metformin use compared to insulin use — factors that “are important ... to enable clinical implementation of study findings,” said Dr. Saade, who played a role in designing the study over the past several years.
The study will take a pragmatic, real-world approach by ensuring racial and ethnic, socioeconomic, urban and rural, and geographic diversity at both large academic and community-based sites across the United States.
The trial, to be led by Mark Landon, MD, and Kartik Venkatesh, MD, PhD, of Ohio State University, will be the first large trial in the United States to both directly compare the ability of oral hypoglycemics and insulin to prevent GDM-associated pregnancy complications, and to follow children for 2 years, Dr. Saade said. “Prior research was either outside the United States, not randomized, not adequately powered, or had no long-term child follow-up,” he added after the meeting.
The State Of Knowledge About Oral Hypoglycemics
The trial was envisioned several years ago as a three-arm comparative trial including the sulfonylurea glyburide, but data published in recent years has increasingly “not favored” glyburide, and many providers “have stopped using it,” Dr. Saade said during and after the meeting. At this point, “it would not be useful to include it” in a pragmatic trial, he said.
Glyburide became the number one agent after a seminal trial published in 2000 (N Engl J Med. 2000;343:1134-8) showed equivalent glycemic control in about 400 women with GDM who were randomized to receive insulin or glyburide. While the trial was not powered to evaluate other outcomes, there were no significant differences in neonatal complications.
In 2015, a large retrospective population-based study (JAMA Pediatr. 2015;169[5]:452-8) of more than 9,000 women with GDM showed higher risks of neonatal intensive care admission, neonatal hypoglycemia, and large-for-gestational age with glyburide compared with insulin. “It prompted a pause in thinking,” Dr. Saade recalled at the DPSG meeting. After that, several meta-analyses/systematic reviews compared the two treatments, showing varying and sometimes conflicting degrees of difference in neonatal outcomes.
In 2018, a French noninferiority randomized controlled trial (JAMA 2018;319[17]:1773-80) did not show that glyburide is not inferior to insulin in the prevention of perinatal outcomes (macrosomia, neonatal hypoglycemia, and hyperbilirubinemia). “If you add this trial to the systematic reviews, it would probably would shift more in favor of insulin,” Dr. Saade said, noting that the trial’s supplementary data included a higher rate of maternal hypoglycemia with glyburide. “I feel personally now, with all the data, that glyburide is inferior to insulin.”
A 2021 network meta-analysis (BMC Endocr Disord. 2021;21:199) that looked at glycemic control and neonatal outcomes in GDM treated with glyburide, metformin, or insulin, also offers valuable insight, Dr. Saade said. The meta-analysis used a Bayesian framework and presents results as a ranking estimated probability of a treatment being the best or worst — or in between — for different outcomes (glycemic control and neonatal outcomes), which “is one of the best ways to look at data these days,” he said.
“It tells us how likely [it is for one agent] to be better than others. Will it work most of the time? More than 60% of the time?” Dr. Saade explained. For example, the analysis “tell us that for large for gestational age, glyburide has a 94% chance of being the worst, metformin has an 80% change of being the best, and insulin a 76% chance of being in between.”
Overall, the 2021 analysis suggests that “glyburide is the most likely to be worst in most outcomes and that there is equipoise between metformin and insulin,” he said.
Meta-analyses of pharmacologic treatment of GDM have been challenged, he said, by inconsistent reporting in trials of GDM diagnostic criteria, severity of hyperglycemia, and small sample sizes (and wide confidence intervals). Criteria for supplemental insulin are also often “unclear” in trials, Dr. Saade said, as is involvement of social determinants of health and the “care package” enveloping pharmacologic interventions.
Dr. Saade, Dr. Landon, and other researchers have also lamented over the years that there is limited long-term follow-up of exposed offspring.
The Challenge of Heterogeneity
In another presentation on GDM, Maisa Feghali, MD, MS, emphasized that GDM is a heterogeneous condition, with clinical hyperglycemia not capturing individual variation in underlying physiologic processes. A 2016 study (Diabetes Care. 2016;39[6]:1052-5) assessing insulin sensitivity and secretion in 800-plus women at 24-30 weeks’ gestation found that about 50% of those with GDM had predominant insulin resistance, 30% had predominant insulin secretion deficit, and 20% were mixed.
Those with predominant insulin resistance had higher BMI, higher fasting glucose, larger infants, and greater risk of GDM-associated adverse outcomes, “suggesting that the risk is not universal or equivalent,” said Dr. Feghali, assistant professor in the department of obstetrics, gynecology and reproductive sciences at the University of Pittsburgh and the UPCM Magee-Women’s Hospital.
A 2019 multicenter European study (Diabetologia. 2019;62[11]:2118-28) found an even higher proportion of GDM involving predominant insulin resistance and, similarly, a greater risk of adverse pregnancy outcomes in these women than in insulin-sensitive women with GDM, “again suggesting that there’s probably some benefit to looking deeper at physiology to understand individual risk,” she said.
Research published decades ago showed that insulin sensitivity decreases by over 50% during pregnancy, and “what we’ve come to recognize is there [can be] insulin secretion deficiency that’s not able to surmount or overcome the insulin resistance that develops during advanced gestation,” she said. “We need to think not at the population level but at the individual level.”
Dr. Feghali is leading the MATCh-GDM (Metabolic Analysis for Treatment Choice in GDM) study, which has been randomizing women to receive either usual, unmatched treatment or treatment matched to GDM mechanism — metformin for predominant insulin resistance, glyburide, or insulin for predominant insulin secretion defects, and one of the three for combined mechanisms. Data are not available yet.
There is still more to be learned about the pharmacologic effects of oral hypoglycemics, she noted, pointing to a 2020 study (Clin Pharmacol Ther. 2020;107[6]:1362-72) that randomized women to glyburide, metformin, or glyburide/metformin combination therapy and measured insulin sensitivity, beta-cell responsivity, and disposition index. (The latter describes the overall metabolic state and is a product of insulin sensitivity and total beta-cell responsivity.)
“Somewhat surprisingly, they found metformin performed better than glyburide,” shifting the overall disposition index closer to normal, Dr. Feghali said. “But not surprisingly, they found the combination worked best.”
Total beta-cell responsivity occurred in 56% of the glyburide group and 74% of the combination group. Improvements in insulin sensitivity occurred in 84% of the metformin group and 74% of the combination group. Surprisingly, there was “a decrease in first-phase insulin secretion” with glyburide, noted Dr. Feghali — a finding that means “the glyburide story has turned out to be a little more complicated.” With metformin, there was a positive change in insulin secretion as well as insulin sensitivity.
The authors’ conclusion, she noted, “is that there’s potential in thinking about metformin first, as the primary treatment, and then adding glyburide after that.”
Future Use Of Incretin Mimetics, and Intensive Targets in Overweight/Obesity
Dr. Feghali wonders whether incretin hormone mimetics — such as glucagonlike peptide–1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP) — could play a future role in GDM treatment, helping to increase insulin secretion.
She is currently recruiting for a pilot study on the pharmacokinetics and pharmacodynamics in GDM of exenatide, a FDA-approved GLP-1 agonist that has been shown not to cross the placenta and that should, research suggests, lower the risk of maternal hypoglycemia and limit the risk of excessive fetal growth, “overcoming some of the concerns we have with glyburide,” Dr. Feghali said.
A recent study of the gut-generated incretin response during an oral glucose tolerance test in pregnant women with and without GDM showed that post-load GLP-1 and GIP were higher in women with GDM, and that the GLP-1 secretion was associated with insulin secretion only in those with GDM (J Clin Endocrinol Metab. 2022;107(6):e2425-30). “In those with normal OGTT, insulin secretion was independent of GLP-1,” she said. “This study suggests there’s a potential role for incretin mimetics in GDM.”
Also regarding the individualization of GDM treatment, patients who are overweight or obese in the prepregnancy setting and have gestational diabetes represent a different phenotype, she noted, with higher fasting and postprandial blood glucose compared to normal-weight counterparts despite higher doses of medication.
“After controlling for gestational weight gain and glycemic control, we see there’s an independent effect of prepregnancy obesity specifically for an increased risk of macrosomia, preterm birth, and hypertensive disorders of pregnancy,” said Dr. Feghali, referring to a 2015 retrospective study of GDM and obesity (Obstet Gynecol. 2015;126:316-25). “It suggests that we might think about redrawing the line, not on diagnosis and screening but on treatment.”
The randomized, controlled Intensive Glycemic Targets in Overweight and Obese Women with Gestational Diabetes Mellitus (iGDM) trial, is now recruiting at multiple centers, including at Dr. Feghali’s University of Pittsburgh, and will investigate the effect of intensive glycemic targets (fasting < 90 mg/dL, 1-hour postprandial < 120 mg/dL) versus standard glycemic targets (fasting < 95 mg/dL, 1-hour postprandial < 140 mg/dL), she said.
In another presentation on GDM, Monica Longo, MD, PhD, of the Inova Health System in Fairfax, Va., said researchers are also looking at whether nutritional supplements such as myo-inositol can reduce the risk of adverse pregnancy outcomes in GDM, and whether probiotics can improve insulin sensitivity in some patients.
Data on newer insulin analogs in pregnancy are lacking, she noted. “Preliminary data has shown no malformations in infants, but there is some increase in hypoglycemia-related admissions to the NICU,” she said. “It’s worth it [to research more].”
FAIRFAX, VIRGINIA — , but researchers at the biennial meeting of the Diabetes in Pregnancy Study Group of North America expressed hope for more clarity in the near future and the ability to someday individualize treatment to account for what is increasingly viewed as a heterogeneous condition.
Until studies in 2015 and 2018 cast doubt on glyburide, “we used to have 80% [of our GDM patients] on glyburide, and 20% on insulin,” Maisa Feghali, MD, of the University of Pittsburgh, said during a discussion period. “Now we have 95% on insulin and 5% on oral hypoglycemics. I rely on insulin because I don’t have a better option, and I rely on research efforts [underway to provide better options]” in the future.
The American College of Obstetricians and Gynecologists recommends insulin as the preferred first-line pharmacologic therapy for GDM when pharmacologic therapy is needed, with metformin as an option when patients decline or cannot safely use insulin. Glyburide, ACOG said in its 2018 practice bulletin on GDM (Obstet Gynecol. 2018;131[2]:e49-64), should not be recommended as a first-line pharmacologic therapy.
The Society of Maternal-Fetal Medicine, on the other hand, has accepted metformin as a “reasonable and safe” first-line alternative to insulin — while recognizing that half of women will still require insulin to achieve glycemic control — and does not rule out consideration of glyburide. In its 2018 statement on the pharmacologic treatment of GDM, the society said that the evidence of benefit of one oral agent over another remains limited.
“When you have dueling guidelines, it means the data are not that clear,” George Saade, MD, professor and chair of obstetrics and gynecology at the Eastern Virginia School of Medicine, Norfolk, said in a presentation on GDM. An upcoming $12 million multicenter study to be led by the Ohio State University College of Medicine — coined the DECIDE trial — should provide clarity, he said.
The trial, funded by the Patient-Centered Outcomes Research Institute, which funds comparative clinical effectiveness research designed to be broadly applicable to practice, will enroll and randomize over 1500 pregnant individuals with GDM to either oral metformin or insulin and will follow mothers and children until 2 years after delivery.
The study’s primary and secondary hypotheses, respectively, are that metformin is not inferior to insulin in reducing a composite adverse neonatal outcome (large for gestational age, neonatal hypoglycemia and/or hyperbilirubemia) and that metformin does not result in increased child body mass index at 2 years, compared with insulin. It will also look at patient-reported factors associated with metformin use compared to insulin use — factors that “are important ... to enable clinical implementation of study findings,” said Dr. Saade, who played a role in designing the study over the past several years.
The study will take a pragmatic, real-world approach by ensuring racial and ethnic, socioeconomic, urban and rural, and geographic diversity at both large academic and community-based sites across the United States.
The trial, to be led by Mark Landon, MD, and Kartik Venkatesh, MD, PhD, of Ohio State University, will be the first large trial in the United States to both directly compare the ability of oral hypoglycemics and insulin to prevent GDM-associated pregnancy complications, and to follow children for 2 years, Dr. Saade said. “Prior research was either outside the United States, not randomized, not adequately powered, or had no long-term child follow-up,” he added after the meeting.
The State Of Knowledge About Oral Hypoglycemics
The trial was envisioned several years ago as a three-arm comparative trial including the sulfonylurea glyburide, but data published in recent years has increasingly “not favored” glyburide, and many providers “have stopped using it,” Dr. Saade said during and after the meeting. At this point, “it would not be useful to include it” in a pragmatic trial, he said.
Glyburide became the number one agent after a seminal trial published in 2000 (N Engl J Med. 2000;343:1134-8) showed equivalent glycemic control in about 400 women with GDM who were randomized to receive insulin or glyburide. While the trial was not powered to evaluate other outcomes, there were no significant differences in neonatal complications.
In 2015, a large retrospective population-based study (JAMA Pediatr. 2015;169[5]:452-8) of more than 9,000 women with GDM showed higher risks of neonatal intensive care admission, neonatal hypoglycemia, and large-for-gestational age with glyburide compared with insulin. “It prompted a pause in thinking,” Dr. Saade recalled at the DPSG meeting. After that, several meta-analyses/systematic reviews compared the two treatments, showing varying and sometimes conflicting degrees of difference in neonatal outcomes.
In 2018, a French noninferiority randomized controlled trial (JAMA 2018;319[17]:1773-80) did not show that glyburide is not inferior to insulin in the prevention of perinatal outcomes (macrosomia, neonatal hypoglycemia, and hyperbilirubinemia). “If you add this trial to the systematic reviews, it would probably would shift more in favor of insulin,” Dr. Saade said, noting that the trial’s supplementary data included a higher rate of maternal hypoglycemia with glyburide. “I feel personally now, with all the data, that glyburide is inferior to insulin.”
A 2021 network meta-analysis (BMC Endocr Disord. 2021;21:199) that looked at glycemic control and neonatal outcomes in GDM treated with glyburide, metformin, or insulin, also offers valuable insight, Dr. Saade said. The meta-analysis used a Bayesian framework and presents results as a ranking estimated probability of a treatment being the best or worst — or in between — for different outcomes (glycemic control and neonatal outcomes), which “is one of the best ways to look at data these days,” he said.
“It tells us how likely [it is for one agent] to be better than others. Will it work most of the time? More than 60% of the time?” Dr. Saade explained. For example, the analysis “tell us that for large for gestational age, glyburide has a 94% chance of being the worst, metformin has an 80% change of being the best, and insulin a 76% chance of being in between.”
Overall, the 2021 analysis suggests that “glyburide is the most likely to be worst in most outcomes and that there is equipoise between metformin and insulin,” he said.
Meta-analyses of pharmacologic treatment of GDM have been challenged, he said, by inconsistent reporting in trials of GDM diagnostic criteria, severity of hyperglycemia, and small sample sizes (and wide confidence intervals). Criteria for supplemental insulin are also often “unclear” in trials, Dr. Saade said, as is involvement of social determinants of health and the “care package” enveloping pharmacologic interventions.
Dr. Saade, Dr. Landon, and other researchers have also lamented over the years that there is limited long-term follow-up of exposed offspring.
The Challenge of Heterogeneity
In another presentation on GDM, Maisa Feghali, MD, MS, emphasized that GDM is a heterogeneous condition, with clinical hyperglycemia not capturing individual variation in underlying physiologic processes. A 2016 study (Diabetes Care. 2016;39[6]:1052-5) assessing insulin sensitivity and secretion in 800-plus women at 24-30 weeks’ gestation found that about 50% of those with GDM had predominant insulin resistance, 30% had predominant insulin secretion deficit, and 20% were mixed.
Those with predominant insulin resistance had higher BMI, higher fasting glucose, larger infants, and greater risk of GDM-associated adverse outcomes, “suggesting that the risk is not universal or equivalent,” said Dr. Feghali, assistant professor in the department of obstetrics, gynecology and reproductive sciences at the University of Pittsburgh and the UPCM Magee-Women’s Hospital.
A 2019 multicenter European study (Diabetologia. 2019;62[11]:2118-28) found an even higher proportion of GDM involving predominant insulin resistance and, similarly, a greater risk of adverse pregnancy outcomes in these women than in insulin-sensitive women with GDM, “again suggesting that there’s probably some benefit to looking deeper at physiology to understand individual risk,” she said.
Research published decades ago showed that insulin sensitivity decreases by over 50% during pregnancy, and “what we’ve come to recognize is there [can be] insulin secretion deficiency that’s not able to surmount or overcome the insulin resistance that develops during advanced gestation,” she said. “We need to think not at the population level but at the individual level.”
Dr. Feghali is leading the MATCh-GDM (Metabolic Analysis for Treatment Choice in GDM) study, which has been randomizing women to receive either usual, unmatched treatment or treatment matched to GDM mechanism — metformin for predominant insulin resistance, glyburide, or insulin for predominant insulin secretion defects, and one of the three for combined mechanisms. Data are not available yet.
There is still more to be learned about the pharmacologic effects of oral hypoglycemics, she noted, pointing to a 2020 study (Clin Pharmacol Ther. 2020;107[6]:1362-72) that randomized women to glyburide, metformin, or glyburide/metformin combination therapy and measured insulin sensitivity, beta-cell responsivity, and disposition index. (The latter describes the overall metabolic state and is a product of insulin sensitivity and total beta-cell responsivity.)
“Somewhat surprisingly, they found metformin performed better than glyburide,” shifting the overall disposition index closer to normal, Dr. Feghali said. “But not surprisingly, they found the combination worked best.”
Total beta-cell responsivity occurred in 56% of the glyburide group and 74% of the combination group. Improvements in insulin sensitivity occurred in 84% of the metformin group and 74% of the combination group. Surprisingly, there was “a decrease in first-phase insulin secretion” with glyburide, noted Dr. Feghali — a finding that means “the glyburide story has turned out to be a little more complicated.” With metformin, there was a positive change in insulin secretion as well as insulin sensitivity.
The authors’ conclusion, she noted, “is that there’s potential in thinking about metformin first, as the primary treatment, and then adding glyburide after that.”
Future Use Of Incretin Mimetics, and Intensive Targets in Overweight/Obesity
Dr. Feghali wonders whether incretin hormone mimetics — such as glucagonlike peptide–1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP) — could play a future role in GDM treatment, helping to increase insulin secretion.
She is currently recruiting for a pilot study on the pharmacokinetics and pharmacodynamics in GDM of exenatide, a FDA-approved GLP-1 agonist that has been shown not to cross the placenta and that should, research suggests, lower the risk of maternal hypoglycemia and limit the risk of excessive fetal growth, “overcoming some of the concerns we have with glyburide,” Dr. Feghali said.
A recent study of the gut-generated incretin response during an oral glucose tolerance test in pregnant women with and without GDM showed that post-load GLP-1 and GIP were higher in women with GDM, and that the GLP-1 secretion was associated with insulin secretion only in those with GDM (J Clin Endocrinol Metab. 2022;107(6):e2425-30). “In those with normal OGTT, insulin secretion was independent of GLP-1,” she said. “This study suggests there’s a potential role for incretin mimetics in GDM.”
Also regarding the individualization of GDM treatment, patients who are overweight or obese in the prepregnancy setting and have gestational diabetes represent a different phenotype, she noted, with higher fasting and postprandial blood glucose compared to normal-weight counterparts despite higher doses of medication.
“After controlling for gestational weight gain and glycemic control, we see there’s an independent effect of prepregnancy obesity specifically for an increased risk of macrosomia, preterm birth, and hypertensive disorders of pregnancy,” said Dr. Feghali, referring to a 2015 retrospective study of GDM and obesity (Obstet Gynecol. 2015;126:316-25). “It suggests that we might think about redrawing the line, not on diagnosis and screening but on treatment.”
The randomized, controlled Intensive Glycemic Targets in Overweight and Obese Women with Gestational Diabetes Mellitus (iGDM) trial, is now recruiting at multiple centers, including at Dr. Feghali’s University of Pittsburgh, and will investigate the effect of intensive glycemic targets (fasting < 90 mg/dL, 1-hour postprandial < 120 mg/dL) versus standard glycemic targets (fasting < 95 mg/dL, 1-hour postprandial < 140 mg/dL), she said.
In another presentation on GDM, Monica Longo, MD, PhD, of the Inova Health System in Fairfax, Va., said researchers are also looking at whether nutritional supplements such as myo-inositol can reduce the risk of adverse pregnancy outcomes in GDM, and whether probiotics can improve insulin sensitivity in some patients.
Data on newer insulin analogs in pregnancy are lacking, she noted. “Preliminary data has shown no malformations in infants, but there is some increase in hypoglycemia-related admissions to the NICU,” she said. “It’s worth it [to research more].”
FAIRFAX, VIRGINIA — , but researchers at the biennial meeting of the Diabetes in Pregnancy Study Group of North America expressed hope for more clarity in the near future and the ability to someday individualize treatment to account for what is increasingly viewed as a heterogeneous condition.
Until studies in 2015 and 2018 cast doubt on glyburide, “we used to have 80% [of our GDM patients] on glyburide, and 20% on insulin,” Maisa Feghali, MD, of the University of Pittsburgh, said during a discussion period. “Now we have 95% on insulin and 5% on oral hypoglycemics. I rely on insulin because I don’t have a better option, and I rely on research efforts [underway to provide better options]” in the future.
The American College of Obstetricians and Gynecologists recommends insulin as the preferred first-line pharmacologic therapy for GDM when pharmacologic therapy is needed, with metformin as an option when patients decline or cannot safely use insulin. Glyburide, ACOG said in its 2018 practice bulletin on GDM (Obstet Gynecol. 2018;131[2]:e49-64), should not be recommended as a first-line pharmacologic therapy.
The Society of Maternal-Fetal Medicine, on the other hand, has accepted metformin as a “reasonable and safe” first-line alternative to insulin — while recognizing that half of women will still require insulin to achieve glycemic control — and does not rule out consideration of glyburide. In its 2018 statement on the pharmacologic treatment of GDM, the society said that the evidence of benefit of one oral agent over another remains limited.
“When you have dueling guidelines, it means the data are not that clear,” George Saade, MD, professor and chair of obstetrics and gynecology at the Eastern Virginia School of Medicine, Norfolk, said in a presentation on GDM. An upcoming $12 million multicenter study to be led by the Ohio State University College of Medicine — coined the DECIDE trial — should provide clarity, he said.
The trial, funded by the Patient-Centered Outcomes Research Institute, which funds comparative clinical effectiveness research designed to be broadly applicable to practice, will enroll and randomize over 1500 pregnant individuals with GDM to either oral metformin or insulin and will follow mothers and children until 2 years after delivery.
The study’s primary and secondary hypotheses, respectively, are that metformin is not inferior to insulin in reducing a composite adverse neonatal outcome (large for gestational age, neonatal hypoglycemia and/or hyperbilirubemia) and that metformin does not result in increased child body mass index at 2 years, compared with insulin. It will also look at patient-reported factors associated with metformin use compared to insulin use — factors that “are important ... to enable clinical implementation of study findings,” said Dr. Saade, who played a role in designing the study over the past several years.
The study will take a pragmatic, real-world approach by ensuring racial and ethnic, socioeconomic, urban and rural, and geographic diversity at both large academic and community-based sites across the United States.
The trial, to be led by Mark Landon, MD, and Kartik Venkatesh, MD, PhD, of Ohio State University, will be the first large trial in the United States to both directly compare the ability of oral hypoglycemics and insulin to prevent GDM-associated pregnancy complications, and to follow children for 2 years, Dr. Saade said. “Prior research was either outside the United States, not randomized, not adequately powered, or had no long-term child follow-up,” he added after the meeting.
The State Of Knowledge About Oral Hypoglycemics
The trial was envisioned several years ago as a three-arm comparative trial including the sulfonylurea glyburide, but data published in recent years has increasingly “not favored” glyburide, and many providers “have stopped using it,” Dr. Saade said during and after the meeting. At this point, “it would not be useful to include it” in a pragmatic trial, he said.
Glyburide became the number one agent after a seminal trial published in 2000 (N Engl J Med. 2000;343:1134-8) showed equivalent glycemic control in about 400 women with GDM who were randomized to receive insulin or glyburide. While the trial was not powered to evaluate other outcomes, there were no significant differences in neonatal complications.
In 2015, a large retrospective population-based study (JAMA Pediatr. 2015;169[5]:452-8) of more than 9,000 women with GDM showed higher risks of neonatal intensive care admission, neonatal hypoglycemia, and large-for-gestational age with glyburide compared with insulin. “It prompted a pause in thinking,” Dr. Saade recalled at the DPSG meeting. After that, several meta-analyses/systematic reviews compared the two treatments, showing varying and sometimes conflicting degrees of difference in neonatal outcomes.
In 2018, a French noninferiority randomized controlled trial (JAMA 2018;319[17]:1773-80) did not show that glyburide is not inferior to insulin in the prevention of perinatal outcomes (macrosomia, neonatal hypoglycemia, and hyperbilirubinemia). “If you add this trial to the systematic reviews, it would probably would shift more in favor of insulin,” Dr. Saade said, noting that the trial’s supplementary data included a higher rate of maternal hypoglycemia with glyburide. “I feel personally now, with all the data, that glyburide is inferior to insulin.”
A 2021 network meta-analysis (BMC Endocr Disord. 2021;21:199) that looked at glycemic control and neonatal outcomes in GDM treated with glyburide, metformin, or insulin, also offers valuable insight, Dr. Saade said. The meta-analysis used a Bayesian framework and presents results as a ranking estimated probability of a treatment being the best or worst — or in between — for different outcomes (glycemic control and neonatal outcomes), which “is one of the best ways to look at data these days,” he said.
“It tells us how likely [it is for one agent] to be better than others. Will it work most of the time? More than 60% of the time?” Dr. Saade explained. For example, the analysis “tell us that for large for gestational age, glyburide has a 94% chance of being the worst, metformin has an 80% change of being the best, and insulin a 76% chance of being in between.”
Overall, the 2021 analysis suggests that “glyburide is the most likely to be worst in most outcomes and that there is equipoise between metformin and insulin,” he said.
Meta-analyses of pharmacologic treatment of GDM have been challenged, he said, by inconsistent reporting in trials of GDM diagnostic criteria, severity of hyperglycemia, and small sample sizes (and wide confidence intervals). Criteria for supplemental insulin are also often “unclear” in trials, Dr. Saade said, as is involvement of social determinants of health and the “care package” enveloping pharmacologic interventions.
Dr. Saade, Dr. Landon, and other researchers have also lamented over the years that there is limited long-term follow-up of exposed offspring.
The Challenge of Heterogeneity
In another presentation on GDM, Maisa Feghali, MD, MS, emphasized that GDM is a heterogeneous condition, with clinical hyperglycemia not capturing individual variation in underlying physiologic processes. A 2016 study (Diabetes Care. 2016;39[6]:1052-5) assessing insulin sensitivity and secretion in 800-plus women at 24-30 weeks’ gestation found that about 50% of those with GDM had predominant insulin resistance, 30% had predominant insulin secretion deficit, and 20% were mixed.
Those with predominant insulin resistance had higher BMI, higher fasting glucose, larger infants, and greater risk of GDM-associated adverse outcomes, “suggesting that the risk is not universal or equivalent,” said Dr. Feghali, assistant professor in the department of obstetrics, gynecology and reproductive sciences at the University of Pittsburgh and the UPCM Magee-Women’s Hospital.
A 2019 multicenter European study (Diabetologia. 2019;62[11]:2118-28) found an even higher proportion of GDM involving predominant insulin resistance and, similarly, a greater risk of adverse pregnancy outcomes in these women than in insulin-sensitive women with GDM, “again suggesting that there’s probably some benefit to looking deeper at physiology to understand individual risk,” she said.
Research published decades ago showed that insulin sensitivity decreases by over 50% during pregnancy, and “what we’ve come to recognize is there [can be] insulin secretion deficiency that’s not able to surmount or overcome the insulin resistance that develops during advanced gestation,” she said. “We need to think not at the population level but at the individual level.”
Dr. Feghali is leading the MATCh-GDM (Metabolic Analysis for Treatment Choice in GDM) study, which has been randomizing women to receive either usual, unmatched treatment or treatment matched to GDM mechanism — metformin for predominant insulin resistance, glyburide, or insulin for predominant insulin secretion defects, and one of the three for combined mechanisms. Data are not available yet.
There is still more to be learned about the pharmacologic effects of oral hypoglycemics, she noted, pointing to a 2020 study (Clin Pharmacol Ther. 2020;107[6]:1362-72) that randomized women to glyburide, metformin, or glyburide/metformin combination therapy and measured insulin sensitivity, beta-cell responsivity, and disposition index. (The latter describes the overall metabolic state and is a product of insulin sensitivity and total beta-cell responsivity.)
“Somewhat surprisingly, they found metformin performed better than glyburide,” shifting the overall disposition index closer to normal, Dr. Feghali said. “But not surprisingly, they found the combination worked best.”
Total beta-cell responsivity occurred in 56% of the glyburide group and 74% of the combination group. Improvements in insulin sensitivity occurred in 84% of the metformin group and 74% of the combination group. Surprisingly, there was “a decrease in first-phase insulin secretion” with glyburide, noted Dr. Feghali — a finding that means “the glyburide story has turned out to be a little more complicated.” With metformin, there was a positive change in insulin secretion as well as insulin sensitivity.
The authors’ conclusion, she noted, “is that there’s potential in thinking about metformin first, as the primary treatment, and then adding glyburide after that.”
Future Use Of Incretin Mimetics, and Intensive Targets in Overweight/Obesity
Dr. Feghali wonders whether incretin hormone mimetics — such as glucagonlike peptide–1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP) — could play a future role in GDM treatment, helping to increase insulin secretion.
She is currently recruiting for a pilot study on the pharmacokinetics and pharmacodynamics in GDM of exenatide, a FDA-approved GLP-1 agonist that has been shown not to cross the placenta and that should, research suggests, lower the risk of maternal hypoglycemia and limit the risk of excessive fetal growth, “overcoming some of the concerns we have with glyburide,” Dr. Feghali said.
A recent study of the gut-generated incretin response during an oral glucose tolerance test in pregnant women with and without GDM showed that post-load GLP-1 and GIP were higher in women with GDM, and that the GLP-1 secretion was associated with insulin secretion only in those with GDM (J Clin Endocrinol Metab. 2022;107(6):e2425-30). “In those with normal OGTT, insulin secretion was independent of GLP-1,” she said. “This study suggests there’s a potential role for incretin mimetics in GDM.”
Also regarding the individualization of GDM treatment, patients who are overweight or obese in the prepregnancy setting and have gestational diabetes represent a different phenotype, she noted, with higher fasting and postprandial blood glucose compared to normal-weight counterparts despite higher doses of medication.
“After controlling for gestational weight gain and glycemic control, we see there’s an independent effect of prepregnancy obesity specifically for an increased risk of macrosomia, preterm birth, and hypertensive disorders of pregnancy,” said Dr. Feghali, referring to a 2015 retrospective study of GDM and obesity (Obstet Gynecol. 2015;126:316-25). “It suggests that we might think about redrawing the line, not on diagnosis and screening but on treatment.”
The randomized, controlled Intensive Glycemic Targets in Overweight and Obese Women with Gestational Diabetes Mellitus (iGDM) trial, is now recruiting at multiple centers, including at Dr. Feghali’s University of Pittsburgh, and will investigate the effect of intensive glycemic targets (fasting < 90 mg/dL, 1-hour postprandial < 120 mg/dL) versus standard glycemic targets (fasting < 95 mg/dL, 1-hour postprandial < 140 mg/dL), she said.
In another presentation on GDM, Monica Longo, MD, PhD, of the Inova Health System in Fairfax, Va., said researchers are also looking at whether nutritional supplements such as myo-inositol can reduce the risk of adverse pregnancy outcomes in GDM, and whether probiotics can improve insulin sensitivity in some patients.
Data on newer insulin analogs in pregnancy are lacking, she noted. “Preliminary data has shown no malformations in infants, but there is some increase in hypoglycemia-related admissions to the NICU,” she said. “It’s worth it [to research more].”
FROM DPSG-NA 2023
The Knowns and Unknowns About Delivery Timing in Diabetes
FAIRFAX, VIRGINIA — The lack of data on optimal timing of delivery for pregnancies complicated by diabetes remains a major challenge in obstetrics — one with considerable implications given the high and rising prevalence of pregestational and gestational diabetes, Katherine Laughon Grantz, MD, MS, of the National Institute of Child Health and Human Development, said at the biennial meeting of the Diabetes in Pregnancy Study Group of North America.
“While 39-40 weeks might be ideal for low-risk pregnancies, the optimal timing for pregnancies with complications [like diabetes] is unknown,” said Dr. Grantz, a senior investigator in the NICHD’s epidemiology branch.
The percentage of mothers with gestational diabetes mellitus (GDM) increased from 6% in 2016 to 8% in 2021, according to the most recent data from the National Vital Statistics System of the Centers for Disease Control and Prevention (MMWR Morb Mortal Wkly Rep. 2023;72:16). Meanwhile, the prevalence of prepregnancy obesity, which raises the risk of gestational and type 2 diabetes, was 29% in 2019; this represents an 11% increase from 2015 (NCHS Data Brief. 2020;392:1-8) and has occurred across all maternal ages, races, ethnic groups, and educational levels, she said.
“The reason clinicians deliver pregnancies with diabetes earlier is because there’s a decreased risk of macrosomia, shoulder dystocia, and stillbirth. And these risks need to be balanced with the increased risk of neonatal morbidity and mortality associated with earlier delivery,” said Dr. Grantz, who noted during her talk that delivery timing also appears to influence long-term neurodevelopmental outcomes. “Yet despite [diabetes in pregnancy] being so common, there is complete uncertainty about when to deliver.”
ACOG Recommendations, Randomized Trials (New And Old)
The American College of Obstetricians and Gynecologists, in a Committee Opinion on Medically Indicated Late-Preterm and Early-Term Deliveries, published in collaboration with the Society of Maternal-Fetal Medicine, offers recommendations based on the type of diabetes and the level of control. For instance, the suggested delivery timing for well-controlled GDM is full term (39 0/7 to 40 6/7 weeks of gestation), while the recommendation for poorly controlled diabetes is individualized late preterm/early term management (Obstet Gynecol. 2021;138:e35-9).
In defining and evaluating control, she noted, “the clinical focus is on glucose, but there are likely other important parameters that are not taken into account ... which [could be] important when considering the timing of delivery.” Potentially important factors include estimated fetal weight, fetal growth velocity, lipids, and amino acids, she said.
ACOG’s recommendations are based mainly on retrospective data, Dr. Grantz said. Only two randomized controlled trials have investigated the timing of delivery in the context of diabetes, and both focused on cesarean section and were “generally underpowered to study neonatal outcomes,” she said.
The first RCT, published in 1993, enrolled 200 women with uncomplicated insulin-requiring diabetes (187 with GDM and 13 with pregestational diabetes) at 38 weeks of gestation, and compared active induction of labor within 5 days to expectant management. There was no significant difference in the cesarean delivery rate (the primary outcome), but rates of macrosomia and large for gestational age were higher in the expectant management group (27% vs. 15%, P = .05, and 23% vs. 10%, P = .02, respectively). Shoulder dystocia occurred in three deliveries, each of which was expectantly managed (Am J Obstet Gynecol. 1993;169[3]:611-5). Notably, the study included “only women with excellent glucose control,” Dr. Grantz said.
The second RCT, published in 2017 by a group in Italy, enrolled 425 patients with GDM (diagnosed by the International Association of Diabetes and Pregnancy Study Groups criteria) between week 38 and week 39 of gestation and similarly randomized them to induction of labor or expectant management. No difference in cesarean delivery was found (BJOG. 2017;124[4]:669-77). Induction of labor was associated with a higher risk of hyperbilirubinemia, and there was a trend toward a decreased risk of macrosomia, but again, the study was underpowered to detect differences in most outcomes, she said. (The study also was stopped early because of an inability to recruit, she noted.)
Dr. Grantz is currently recruiting for a randomized trial aimed at determining the optimal time between 37 and 39 weeks to initiate delivery — the time when neonatal morbidity and perinatal mortality risk is the lowest – for uncontrolled GDM-complicated pregnancies. The trial is designed to recruit up to 3,450 pregnant women with uncontrolled GDM and randomize the timing of their delivery (NCT05515744).
Those who are eligible for the study but do not consent to participate in randomization for delivery will be asked about chart review only (an estimated additional 3,000). The SPAN TIME study will also assess newborn development and behavior outcomes, as well as anthropometric measures, as secondary outcomes. An exploratory analysis will look for clinical, nonclinical or biochemical factors that could be helpful in optimizing delivery timing.
What Retrospective Studies Reveal
Factors that may influence the timing of delivery include the duration of neonatal exposure to hyperglycemia/hyperinsulinemia (pregestational vs. gestational diabetes), the level of diabetes control, and comorbidities (e.g. maternal renal disease or chronic hypertension). However, research “investigating how these factors influence morbidity and the timing of delivery is limited,” said Dr. Grantz.
Overall, it has been difficult through retrospective studies, she said, to investigate neonatal morbidity in diabetic pregnancies and tease apart the relative effects of diabetes as a precursor for early delivery and prematurity itself. Among the studies suggesting an independent risk of diabetes is a retrospective study focusing on neonatal respiratory morbidity — “one of the most common adverse outcomes associated with diabetes.”
The study, an analysis of the Consortium on Safe Labor study (an electronic medical record study of more than 220,000 singleton pregnancies), stratified morbidity by the probability of delivering at term (≥ 37 weeks). GDM and pregestational diabetes complicated 5.1% and 1.5% of the pregnancies, respectively, and were found to be associated with increased risks of neonatal respiratory morbidity compared to women without diabetes — regardless of the probability of delivering at term.
However, these associations were stronger with a higher probability of delivering at term, which suggests that the neonatal respiratory morbidity associated with diabetes is not fully explained by a greater propensity for prematurity (Am J Perinatol. 2017;34[11]:1160-8).
In addition, the rates of all neonatal respiratory morbidities and mortality were higher for pregestational diabetes compared with gestational diabetes, said Dr. Grantz, a senior author of the study. (Morbidities included neonatal intensive care unit admission, transient tachypnea of newborn, apnea, respiratory distress syndrome, mechanical ventilation, and stillbirth.)
The pathophysiology of diabetes and neonatal respiratory morbidity is “not fully known,” she said. It is believed that fetal hyperinsulinemia may cause delayed pulmonary maturation and there is evidence from animal studies that insulin decreases the incorporation of glucose and fatty acids into phospholipid phosphatidylglycerol. Indirect effects stem from the physiologic immaturity of earlier delivery and a higher cesarean delivery rate in pregnancies complicated by diabetes, Dr. Grantz said.
Among other retrospective studies was a population-based study from Canada (2004-2014), published in 2020, of large numbers of women with all types of diabetes and a comparison group of over 2.5 million without diabetes. For maternal morbidity/mortality, there were no significant differences by gestational age between iatrogenic delivery and expectant management among any form of diabetes. But for neonatal morbidity and mortality, the study found differences.
In women with gestational diabetes, iatrogenic delivery was associated with increased risk of neonatal morbidity/mortality at 36 and 37 weeks’ gestation and with decreased risk at weeks 38-40. Increased risk with iatrogenic delivery was also found for women with type 1 and type 2 diabetes at weeks 36 and 37 (Acta Obstet Gynecol Scand. 2020;99[3]:341-9).
Another retrospective study using California vital statistics (1997-2006) examined rates of stillbirth and infant death in women with GDM by gestational age at delivery (Am J Obstet Gynecol. 2012;206[4]:309.e1-e7). The 190,000-plus women with GDM had elevated risk of stillbirth at each gestational age compared to those without GDM, but “the [excess] risk for GDM was lowest at 38 weeks and again at 40 weeks,” Dr. Grantz said. The investigators concluded, she said, “that the risk of expectant management exceeded that of delivery at 38 weeks and beyond.”
Dr. Grantz reported no disclosures.
FAIRFAX, VIRGINIA — The lack of data on optimal timing of delivery for pregnancies complicated by diabetes remains a major challenge in obstetrics — one with considerable implications given the high and rising prevalence of pregestational and gestational diabetes, Katherine Laughon Grantz, MD, MS, of the National Institute of Child Health and Human Development, said at the biennial meeting of the Diabetes in Pregnancy Study Group of North America.
“While 39-40 weeks might be ideal for low-risk pregnancies, the optimal timing for pregnancies with complications [like diabetes] is unknown,” said Dr. Grantz, a senior investigator in the NICHD’s epidemiology branch.
The percentage of mothers with gestational diabetes mellitus (GDM) increased from 6% in 2016 to 8% in 2021, according to the most recent data from the National Vital Statistics System of the Centers for Disease Control and Prevention (MMWR Morb Mortal Wkly Rep. 2023;72:16). Meanwhile, the prevalence of prepregnancy obesity, which raises the risk of gestational and type 2 diabetes, was 29% in 2019; this represents an 11% increase from 2015 (NCHS Data Brief. 2020;392:1-8) and has occurred across all maternal ages, races, ethnic groups, and educational levels, she said.
“The reason clinicians deliver pregnancies with diabetes earlier is because there’s a decreased risk of macrosomia, shoulder dystocia, and stillbirth. And these risks need to be balanced with the increased risk of neonatal morbidity and mortality associated with earlier delivery,” said Dr. Grantz, who noted during her talk that delivery timing also appears to influence long-term neurodevelopmental outcomes. “Yet despite [diabetes in pregnancy] being so common, there is complete uncertainty about when to deliver.”
ACOG Recommendations, Randomized Trials (New And Old)
The American College of Obstetricians and Gynecologists, in a Committee Opinion on Medically Indicated Late-Preterm and Early-Term Deliveries, published in collaboration with the Society of Maternal-Fetal Medicine, offers recommendations based on the type of diabetes and the level of control. For instance, the suggested delivery timing for well-controlled GDM is full term (39 0/7 to 40 6/7 weeks of gestation), while the recommendation for poorly controlled diabetes is individualized late preterm/early term management (Obstet Gynecol. 2021;138:e35-9).
In defining and evaluating control, she noted, “the clinical focus is on glucose, but there are likely other important parameters that are not taken into account ... which [could be] important when considering the timing of delivery.” Potentially important factors include estimated fetal weight, fetal growth velocity, lipids, and amino acids, she said.
ACOG’s recommendations are based mainly on retrospective data, Dr. Grantz said. Only two randomized controlled trials have investigated the timing of delivery in the context of diabetes, and both focused on cesarean section and were “generally underpowered to study neonatal outcomes,” she said.
The first RCT, published in 1993, enrolled 200 women with uncomplicated insulin-requiring diabetes (187 with GDM and 13 with pregestational diabetes) at 38 weeks of gestation, and compared active induction of labor within 5 days to expectant management. There was no significant difference in the cesarean delivery rate (the primary outcome), but rates of macrosomia and large for gestational age were higher in the expectant management group (27% vs. 15%, P = .05, and 23% vs. 10%, P = .02, respectively). Shoulder dystocia occurred in three deliveries, each of which was expectantly managed (Am J Obstet Gynecol. 1993;169[3]:611-5). Notably, the study included “only women with excellent glucose control,” Dr. Grantz said.
The second RCT, published in 2017 by a group in Italy, enrolled 425 patients with GDM (diagnosed by the International Association of Diabetes and Pregnancy Study Groups criteria) between week 38 and week 39 of gestation and similarly randomized them to induction of labor or expectant management. No difference in cesarean delivery was found (BJOG. 2017;124[4]:669-77). Induction of labor was associated with a higher risk of hyperbilirubinemia, and there was a trend toward a decreased risk of macrosomia, but again, the study was underpowered to detect differences in most outcomes, she said. (The study also was stopped early because of an inability to recruit, she noted.)
Dr. Grantz is currently recruiting for a randomized trial aimed at determining the optimal time between 37 and 39 weeks to initiate delivery — the time when neonatal morbidity and perinatal mortality risk is the lowest – for uncontrolled GDM-complicated pregnancies. The trial is designed to recruit up to 3,450 pregnant women with uncontrolled GDM and randomize the timing of their delivery (NCT05515744).
Those who are eligible for the study but do not consent to participate in randomization for delivery will be asked about chart review only (an estimated additional 3,000). The SPAN TIME study will also assess newborn development and behavior outcomes, as well as anthropometric measures, as secondary outcomes. An exploratory analysis will look for clinical, nonclinical or biochemical factors that could be helpful in optimizing delivery timing.
What Retrospective Studies Reveal
Factors that may influence the timing of delivery include the duration of neonatal exposure to hyperglycemia/hyperinsulinemia (pregestational vs. gestational diabetes), the level of diabetes control, and comorbidities (e.g. maternal renal disease or chronic hypertension). However, research “investigating how these factors influence morbidity and the timing of delivery is limited,” said Dr. Grantz.
Overall, it has been difficult through retrospective studies, she said, to investigate neonatal morbidity in diabetic pregnancies and tease apart the relative effects of diabetes as a precursor for early delivery and prematurity itself. Among the studies suggesting an independent risk of diabetes is a retrospective study focusing on neonatal respiratory morbidity — “one of the most common adverse outcomes associated with diabetes.”
The study, an analysis of the Consortium on Safe Labor study (an electronic medical record study of more than 220,000 singleton pregnancies), stratified morbidity by the probability of delivering at term (≥ 37 weeks). GDM and pregestational diabetes complicated 5.1% and 1.5% of the pregnancies, respectively, and were found to be associated with increased risks of neonatal respiratory morbidity compared to women without diabetes — regardless of the probability of delivering at term.
However, these associations were stronger with a higher probability of delivering at term, which suggests that the neonatal respiratory morbidity associated with diabetes is not fully explained by a greater propensity for prematurity (Am J Perinatol. 2017;34[11]:1160-8).
In addition, the rates of all neonatal respiratory morbidities and mortality were higher for pregestational diabetes compared with gestational diabetes, said Dr. Grantz, a senior author of the study. (Morbidities included neonatal intensive care unit admission, transient tachypnea of newborn, apnea, respiratory distress syndrome, mechanical ventilation, and stillbirth.)
The pathophysiology of diabetes and neonatal respiratory morbidity is “not fully known,” she said. It is believed that fetal hyperinsulinemia may cause delayed pulmonary maturation and there is evidence from animal studies that insulin decreases the incorporation of glucose and fatty acids into phospholipid phosphatidylglycerol. Indirect effects stem from the physiologic immaturity of earlier delivery and a higher cesarean delivery rate in pregnancies complicated by diabetes, Dr. Grantz said.
Among other retrospective studies was a population-based study from Canada (2004-2014), published in 2020, of large numbers of women with all types of diabetes and a comparison group of over 2.5 million without diabetes. For maternal morbidity/mortality, there were no significant differences by gestational age between iatrogenic delivery and expectant management among any form of diabetes. But for neonatal morbidity and mortality, the study found differences.
In women with gestational diabetes, iatrogenic delivery was associated with increased risk of neonatal morbidity/mortality at 36 and 37 weeks’ gestation and with decreased risk at weeks 38-40. Increased risk with iatrogenic delivery was also found for women with type 1 and type 2 diabetes at weeks 36 and 37 (Acta Obstet Gynecol Scand. 2020;99[3]:341-9).
Another retrospective study using California vital statistics (1997-2006) examined rates of stillbirth and infant death in women with GDM by gestational age at delivery (Am J Obstet Gynecol. 2012;206[4]:309.e1-e7). The 190,000-plus women with GDM had elevated risk of stillbirth at each gestational age compared to those without GDM, but “the [excess] risk for GDM was lowest at 38 weeks and again at 40 weeks,” Dr. Grantz said. The investigators concluded, she said, “that the risk of expectant management exceeded that of delivery at 38 weeks and beyond.”
Dr. Grantz reported no disclosures.
FAIRFAX, VIRGINIA — The lack of data on optimal timing of delivery for pregnancies complicated by diabetes remains a major challenge in obstetrics — one with considerable implications given the high and rising prevalence of pregestational and gestational diabetes, Katherine Laughon Grantz, MD, MS, of the National Institute of Child Health and Human Development, said at the biennial meeting of the Diabetes in Pregnancy Study Group of North America.
“While 39-40 weeks might be ideal for low-risk pregnancies, the optimal timing for pregnancies with complications [like diabetes] is unknown,” said Dr. Grantz, a senior investigator in the NICHD’s epidemiology branch.
The percentage of mothers with gestational diabetes mellitus (GDM) increased from 6% in 2016 to 8% in 2021, according to the most recent data from the National Vital Statistics System of the Centers for Disease Control and Prevention (MMWR Morb Mortal Wkly Rep. 2023;72:16). Meanwhile, the prevalence of prepregnancy obesity, which raises the risk of gestational and type 2 diabetes, was 29% in 2019; this represents an 11% increase from 2015 (NCHS Data Brief. 2020;392:1-8) and has occurred across all maternal ages, races, ethnic groups, and educational levels, she said.
“The reason clinicians deliver pregnancies with diabetes earlier is because there’s a decreased risk of macrosomia, shoulder dystocia, and stillbirth. And these risks need to be balanced with the increased risk of neonatal morbidity and mortality associated with earlier delivery,” said Dr. Grantz, who noted during her talk that delivery timing also appears to influence long-term neurodevelopmental outcomes. “Yet despite [diabetes in pregnancy] being so common, there is complete uncertainty about when to deliver.”
ACOG Recommendations, Randomized Trials (New And Old)
The American College of Obstetricians and Gynecologists, in a Committee Opinion on Medically Indicated Late-Preterm and Early-Term Deliveries, published in collaboration with the Society of Maternal-Fetal Medicine, offers recommendations based on the type of diabetes and the level of control. For instance, the suggested delivery timing for well-controlled GDM is full term (39 0/7 to 40 6/7 weeks of gestation), while the recommendation for poorly controlled diabetes is individualized late preterm/early term management (Obstet Gynecol. 2021;138:e35-9).
In defining and evaluating control, she noted, “the clinical focus is on glucose, but there are likely other important parameters that are not taken into account ... which [could be] important when considering the timing of delivery.” Potentially important factors include estimated fetal weight, fetal growth velocity, lipids, and amino acids, she said.
ACOG’s recommendations are based mainly on retrospective data, Dr. Grantz said. Only two randomized controlled trials have investigated the timing of delivery in the context of diabetes, and both focused on cesarean section and were “generally underpowered to study neonatal outcomes,” she said.
The first RCT, published in 1993, enrolled 200 women with uncomplicated insulin-requiring diabetes (187 with GDM and 13 with pregestational diabetes) at 38 weeks of gestation, and compared active induction of labor within 5 days to expectant management. There was no significant difference in the cesarean delivery rate (the primary outcome), but rates of macrosomia and large for gestational age were higher in the expectant management group (27% vs. 15%, P = .05, and 23% vs. 10%, P = .02, respectively). Shoulder dystocia occurred in three deliveries, each of which was expectantly managed (Am J Obstet Gynecol. 1993;169[3]:611-5). Notably, the study included “only women with excellent glucose control,” Dr. Grantz said.
The second RCT, published in 2017 by a group in Italy, enrolled 425 patients with GDM (diagnosed by the International Association of Diabetes and Pregnancy Study Groups criteria) between week 38 and week 39 of gestation and similarly randomized them to induction of labor or expectant management. No difference in cesarean delivery was found (BJOG. 2017;124[4]:669-77). Induction of labor was associated with a higher risk of hyperbilirubinemia, and there was a trend toward a decreased risk of macrosomia, but again, the study was underpowered to detect differences in most outcomes, she said. (The study also was stopped early because of an inability to recruit, she noted.)
Dr. Grantz is currently recruiting for a randomized trial aimed at determining the optimal time between 37 and 39 weeks to initiate delivery — the time when neonatal morbidity and perinatal mortality risk is the lowest – for uncontrolled GDM-complicated pregnancies. The trial is designed to recruit up to 3,450 pregnant women with uncontrolled GDM and randomize the timing of their delivery (NCT05515744).
Those who are eligible for the study but do not consent to participate in randomization for delivery will be asked about chart review only (an estimated additional 3,000). The SPAN TIME study will also assess newborn development and behavior outcomes, as well as anthropometric measures, as secondary outcomes. An exploratory analysis will look for clinical, nonclinical or biochemical factors that could be helpful in optimizing delivery timing.
What Retrospective Studies Reveal
Factors that may influence the timing of delivery include the duration of neonatal exposure to hyperglycemia/hyperinsulinemia (pregestational vs. gestational diabetes), the level of diabetes control, and comorbidities (e.g. maternal renal disease or chronic hypertension). However, research “investigating how these factors influence morbidity and the timing of delivery is limited,” said Dr. Grantz.
Overall, it has been difficult through retrospective studies, she said, to investigate neonatal morbidity in diabetic pregnancies and tease apart the relative effects of diabetes as a precursor for early delivery and prematurity itself. Among the studies suggesting an independent risk of diabetes is a retrospective study focusing on neonatal respiratory morbidity — “one of the most common adverse outcomes associated with diabetes.”
The study, an analysis of the Consortium on Safe Labor study (an electronic medical record study of more than 220,000 singleton pregnancies), stratified morbidity by the probability of delivering at term (≥ 37 weeks). GDM and pregestational diabetes complicated 5.1% and 1.5% of the pregnancies, respectively, and were found to be associated with increased risks of neonatal respiratory morbidity compared to women without diabetes — regardless of the probability of delivering at term.
However, these associations were stronger with a higher probability of delivering at term, which suggests that the neonatal respiratory morbidity associated with diabetes is not fully explained by a greater propensity for prematurity (Am J Perinatol. 2017;34[11]:1160-8).
In addition, the rates of all neonatal respiratory morbidities and mortality were higher for pregestational diabetes compared with gestational diabetes, said Dr. Grantz, a senior author of the study. (Morbidities included neonatal intensive care unit admission, transient tachypnea of newborn, apnea, respiratory distress syndrome, mechanical ventilation, and stillbirth.)
The pathophysiology of diabetes and neonatal respiratory morbidity is “not fully known,” she said. It is believed that fetal hyperinsulinemia may cause delayed pulmonary maturation and there is evidence from animal studies that insulin decreases the incorporation of glucose and fatty acids into phospholipid phosphatidylglycerol. Indirect effects stem from the physiologic immaturity of earlier delivery and a higher cesarean delivery rate in pregnancies complicated by diabetes, Dr. Grantz said.
Among other retrospective studies was a population-based study from Canada (2004-2014), published in 2020, of large numbers of women with all types of diabetes and a comparison group of over 2.5 million without diabetes. For maternal morbidity/mortality, there were no significant differences by gestational age between iatrogenic delivery and expectant management among any form of diabetes. But for neonatal morbidity and mortality, the study found differences.
In women with gestational diabetes, iatrogenic delivery was associated with increased risk of neonatal morbidity/mortality at 36 and 37 weeks’ gestation and with decreased risk at weeks 38-40. Increased risk with iatrogenic delivery was also found for women with type 1 and type 2 diabetes at weeks 36 and 37 (Acta Obstet Gynecol Scand. 2020;99[3]:341-9).
Another retrospective study using California vital statistics (1997-2006) examined rates of stillbirth and infant death in women with GDM by gestational age at delivery (Am J Obstet Gynecol. 2012;206[4]:309.e1-e7). The 190,000-plus women with GDM had elevated risk of stillbirth at each gestational age compared to those without GDM, but “the [excess] risk for GDM was lowest at 38 weeks and again at 40 weeks,” Dr. Grantz said. The investigators concluded, she said, “that the risk of expectant management exceeded that of delivery at 38 weeks and beyond.”
Dr. Grantz reported no disclosures.
FROM DPSG-NA 2023
Clinical Exams Fall Short in Second Breast Cancer Detection
TOPLINE:
METHODOLOGY:
- National Comprehensive Cancer Network guidelines recommend DCIS surveillance with a physical exam every 6-12 months for 5 years and then annually with a mammogram every 12 months. Research, however, suggested clinical breast exams only detect 15% of second breast cancers.
- A retrospective cohort study of 1550 female members of Kaiser Permanente Northern California diagnosed with unilateral DCIS between January 1, 2008, and January 1, 2011, who were followed until 2021.
- Patients who developed a second breast cancer within 10 years of follow-up were identified from the electronic health records. The detection methods were categorized into three groups: Patient-detected, physician-detected, and imaging-detected.
TAKEAWAY:
- During follow-up, 11.5% of women developed a second breast cancer with a median time to diagnosis of 57 months. Among patients with second breast cancers, 43.0% were ipsilateral, 54.8% were contralateral, and 2.2% presented with distant metastases.
- Overall, patients had a median of five mammograms between years 1 and 6 of surveillance and a median of seven clinic visits with most providers completing a clinical examination during the visit.
- Second breast cancers were detected through imaging in 74.3% of cases compared with 20.1% detected by patients and only 2.2% detected by physicians during physical exams. The remaining 3.4% were detected incidentally from plastic surgery procedures unrelated to oncologic surveillance.
- Mammogram detected 99.2% of cases (132 of 133 cases) identified by imaging.
IN PRACTICE:
“Our findings highlight the importance of mammogram screening and patient education regarding self-detection and can inform future NCCN recommendations for DCIS survivorship care,” the authors concluded, adding that “decreasing the need for in-person breast examinations could allow for other effective methods of survivorship monitoring.”
SOURCE:
This study, led by Bethany T. Waites of Kaiser Permanente San Francisco Medical Center, San Francisco, California, was published online on December 28 in the Journal of the National Comprehensive Cancer Network.
LIMITATIONS:
The retrospective design may have introduced selection bias or confounding. The study’s follow-up period until 2021, including the initial 18 months of the COVID-19 pandemic, may have affected surveillance patterns.
DISCLOSURES:
This study was supported by the Kaiser Permanente Northern California Graduate Medical Education program. The authors declared no relevant financial relationships.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- National Comprehensive Cancer Network guidelines recommend DCIS surveillance with a physical exam every 6-12 months for 5 years and then annually with a mammogram every 12 months. Research, however, suggested clinical breast exams only detect 15% of second breast cancers.
- A retrospective cohort study of 1550 female members of Kaiser Permanente Northern California diagnosed with unilateral DCIS between January 1, 2008, and January 1, 2011, who were followed until 2021.
- Patients who developed a second breast cancer within 10 years of follow-up were identified from the electronic health records. The detection methods were categorized into three groups: Patient-detected, physician-detected, and imaging-detected.
TAKEAWAY:
- During follow-up, 11.5% of women developed a second breast cancer with a median time to diagnosis of 57 months. Among patients with second breast cancers, 43.0% were ipsilateral, 54.8% were contralateral, and 2.2% presented with distant metastases.
- Overall, patients had a median of five mammograms between years 1 and 6 of surveillance and a median of seven clinic visits with most providers completing a clinical examination during the visit.
- Second breast cancers were detected through imaging in 74.3% of cases compared with 20.1% detected by patients and only 2.2% detected by physicians during physical exams. The remaining 3.4% were detected incidentally from plastic surgery procedures unrelated to oncologic surveillance.
- Mammogram detected 99.2% of cases (132 of 133 cases) identified by imaging.
IN PRACTICE:
“Our findings highlight the importance of mammogram screening and patient education regarding self-detection and can inform future NCCN recommendations for DCIS survivorship care,” the authors concluded, adding that “decreasing the need for in-person breast examinations could allow for other effective methods of survivorship monitoring.”
SOURCE:
This study, led by Bethany T. Waites of Kaiser Permanente San Francisco Medical Center, San Francisco, California, was published online on December 28 in the Journal of the National Comprehensive Cancer Network.
LIMITATIONS:
The retrospective design may have introduced selection bias or confounding. The study’s follow-up period until 2021, including the initial 18 months of the COVID-19 pandemic, may have affected surveillance patterns.
DISCLOSURES:
This study was supported by the Kaiser Permanente Northern California Graduate Medical Education program. The authors declared no relevant financial relationships.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- National Comprehensive Cancer Network guidelines recommend DCIS surveillance with a physical exam every 6-12 months for 5 years and then annually with a mammogram every 12 months. Research, however, suggested clinical breast exams only detect 15% of second breast cancers.
- A retrospective cohort study of 1550 female members of Kaiser Permanente Northern California diagnosed with unilateral DCIS between January 1, 2008, and January 1, 2011, who were followed until 2021.
- Patients who developed a second breast cancer within 10 years of follow-up were identified from the electronic health records. The detection methods were categorized into three groups: Patient-detected, physician-detected, and imaging-detected.
TAKEAWAY:
- During follow-up, 11.5% of women developed a second breast cancer with a median time to diagnosis of 57 months. Among patients with second breast cancers, 43.0% were ipsilateral, 54.8% were contralateral, and 2.2% presented with distant metastases.
- Overall, patients had a median of five mammograms between years 1 and 6 of surveillance and a median of seven clinic visits with most providers completing a clinical examination during the visit.
- Second breast cancers were detected through imaging in 74.3% of cases compared with 20.1% detected by patients and only 2.2% detected by physicians during physical exams. The remaining 3.4% were detected incidentally from plastic surgery procedures unrelated to oncologic surveillance.
- Mammogram detected 99.2% of cases (132 of 133 cases) identified by imaging.
IN PRACTICE:
“Our findings highlight the importance of mammogram screening and patient education regarding self-detection and can inform future NCCN recommendations for DCIS survivorship care,” the authors concluded, adding that “decreasing the need for in-person breast examinations could allow for other effective methods of survivorship monitoring.”
SOURCE:
This study, led by Bethany T. Waites of Kaiser Permanente San Francisco Medical Center, San Francisco, California, was published online on December 28 in the Journal of the National Comprehensive Cancer Network.
LIMITATIONS:
The retrospective design may have introduced selection bias or confounding. The study’s follow-up period until 2021, including the initial 18 months of the COVID-19 pandemic, may have affected surveillance patterns.
DISCLOSURES:
This study was supported by the Kaiser Permanente Northern California Graduate Medical Education program. The authors declared no relevant financial relationships.
A version of this article appeared on Medscape.com.
Impact of Pregnancy on Rosacea Unpredictable, Study Suggests
TOPLINE:
Among women diagnosed with rosacea, the impact of pregnancy on the disease is unpredictable.
METHODOLOGY:
- Researchers conducted a telephone survey of 39 women with a diagnosis of rosacea in the electronic medical records prior to the onset of pregnancy who had been admitted to Oregon Health & Science University for labor and delivery from June 27, 2015, to June 27, 2020.
- Patient global assessment of clear (0), mild (1), moderate (2), or severe (3) rosacea was rated across five timepoints: 1-3 months preconception; first, second, and third trimesters; and 6 weeks postpartum.
TAKEAWAY:
- The mean age of the survey participants was 35.5 years, the mean gestational age at delivery was 39.4 weeks, and most had singleton pregnancies.
- All but one study participant (97.4%) reported symptoms of erythematotelangiectatic rosacea, while 26 (67%) reported symptoms of papulopustular rosacea.
- Nearly half of the participants (19, 48.7%) said their rosacea worsened during pregnancy, 13 (33.3%) reported no change in rosacea severity during pregnancy, and 7 (17.9%) reported that their rosacea improved during pregnancy.
- Before conceiving, the mean rosacea severity score among participants was mild (1.10; 95% CI, 0.92-1.29) and did not change significantly over time, a reflection of individual variations. In addition, 83.3% of participants did not use prescription rosacea treatments prior to pregnancy, and 89.6% did not use them during pregnancy.
IN PRACTICE:
“Rosacea, like acne, lacks a predictable group effect, and instead, each individual may have a different response to the physiologic changes of pregnancy,” the authors concluded.
SOURCE:
Genevieve Benedetti, MD, MPP, of the Department of Dermatology at Oregon Health & Science University, Portland, Oregon, led the research, published as a research letter in the International Journal of Women’s Dermatology.
LIMITATIONS:
The small sample size, single-center design, and overall prevalence of mild disease limit the ability to detect change.
DISCLOSURES:
The researchers reported having no disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
Among women diagnosed with rosacea, the impact of pregnancy on the disease is unpredictable.
METHODOLOGY:
- Researchers conducted a telephone survey of 39 women with a diagnosis of rosacea in the electronic medical records prior to the onset of pregnancy who had been admitted to Oregon Health & Science University for labor and delivery from June 27, 2015, to June 27, 2020.
- Patient global assessment of clear (0), mild (1), moderate (2), or severe (3) rosacea was rated across five timepoints: 1-3 months preconception; first, second, and third trimesters; and 6 weeks postpartum.
TAKEAWAY:
- The mean age of the survey participants was 35.5 years, the mean gestational age at delivery was 39.4 weeks, and most had singleton pregnancies.
- All but one study participant (97.4%) reported symptoms of erythematotelangiectatic rosacea, while 26 (67%) reported symptoms of papulopustular rosacea.
- Nearly half of the participants (19, 48.7%) said their rosacea worsened during pregnancy, 13 (33.3%) reported no change in rosacea severity during pregnancy, and 7 (17.9%) reported that their rosacea improved during pregnancy.
- Before conceiving, the mean rosacea severity score among participants was mild (1.10; 95% CI, 0.92-1.29) and did not change significantly over time, a reflection of individual variations. In addition, 83.3% of participants did not use prescription rosacea treatments prior to pregnancy, and 89.6% did not use them during pregnancy.
IN PRACTICE:
“Rosacea, like acne, lacks a predictable group effect, and instead, each individual may have a different response to the physiologic changes of pregnancy,” the authors concluded.
SOURCE:
Genevieve Benedetti, MD, MPP, of the Department of Dermatology at Oregon Health & Science University, Portland, Oregon, led the research, published as a research letter in the International Journal of Women’s Dermatology.
LIMITATIONS:
The small sample size, single-center design, and overall prevalence of mild disease limit the ability to detect change.
DISCLOSURES:
The researchers reported having no disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
Among women diagnosed with rosacea, the impact of pregnancy on the disease is unpredictable.
METHODOLOGY:
- Researchers conducted a telephone survey of 39 women with a diagnosis of rosacea in the electronic medical records prior to the onset of pregnancy who had been admitted to Oregon Health & Science University for labor and delivery from June 27, 2015, to June 27, 2020.
- Patient global assessment of clear (0), mild (1), moderate (2), or severe (3) rosacea was rated across five timepoints: 1-3 months preconception; first, second, and third trimesters; and 6 weeks postpartum.
TAKEAWAY:
- The mean age of the survey participants was 35.5 years, the mean gestational age at delivery was 39.4 weeks, and most had singleton pregnancies.
- All but one study participant (97.4%) reported symptoms of erythematotelangiectatic rosacea, while 26 (67%) reported symptoms of papulopustular rosacea.
- Nearly half of the participants (19, 48.7%) said their rosacea worsened during pregnancy, 13 (33.3%) reported no change in rosacea severity during pregnancy, and 7 (17.9%) reported that their rosacea improved during pregnancy.
- Before conceiving, the mean rosacea severity score among participants was mild (1.10; 95% CI, 0.92-1.29) and did not change significantly over time, a reflection of individual variations. In addition, 83.3% of participants did not use prescription rosacea treatments prior to pregnancy, and 89.6% did not use them during pregnancy.
IN PRACTICE:
“Rosacea, like acne, lacks a predictable group effect, and instead, each individual may have a different response to the physiologic changes of pregnancy,” the authors concluded.
SOURCE:
Genevieve Benedetti, MD, MPP, of the Department of Dermatology at Oregon Health & Science University, Portland, Oregon, led the research, published as a research letter in the International Journal of Women’s Dermatology.
LIMITATIONS:
The small sample size, single-center design, and overall prevalence of mild disease limit the ability to detect change.
DISCLOSURES:
The researchers reported having no disclosures.
A version of this article appeared on Medscape.com.