User login
Official Newspaper of the American College of Surgeons
IDSA, SHEA release inpatient antibiotic stewardship guidelines
The Infectious Diseases Society of America (IDSA) and the Society for Healthcare Epidemiology of America (SHEA) have jointly released evidence-based guidelines for implementing an inpatient antibiotic stewardship program.
The guidelines, published April 13 online in Clinical Infectious Diseases, address the optimal use of antibiotics in inpatient populations, and were prepared by a multidisciplinary expert panel of the IDSA and the SHEA, which included representation from the specialties of internal medicine, emergency medicine, microbiology, critical care, surgery, epidemiology, pharmacy, and adult and pediatric infectious diseases.
Antibiotic stewardship has been defined by IDSA, SHEA, and the Pediatric Infectious Diseases Society as “coordinated interventions designed to improve and measure the appropriate use of [antibiotic] agents by promoting the selection of the optimal [antibiotic] drug regimen including dosing, duration of therapy, and route of administration.” The new guidelines discuss a broad range of possible interventions, but the authors emphasize the need “for each site to assess its clinical needs and available resources and individualize its [antibiotic stewardship program] with that assessment in mind.”
The process used in the development of the guidelines included a systematic weighting of the strength of recommendation and quality of evidence using the GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) system, according to Dr. Tamar F. Barlam of the section of infectious diseases at Boston University, and her colleagues.
“The benefits of antibiotic stewardship include improved patient outcomes, reduced adverse events including Clostridium difficile infection, improvement in rates of antibiotic susceptibilities to targeted antibiotics, and optimization of resource utilization across the continuum of care,” Dr. Barlam and her coauthors wrote.
A complete list of any potential conflicts of interest for the multiple coauthors is provided with the full stewardship guidelines, which can be reviewed in Clinical Infectious Diseases (doi: 10.1093/cid/ciw118).
On Twitter @richpizzi
The Infectious Diseases Society of America (IDSA) and the Society for Healthcare Epidemiology of America (SHEA) have jointly released evidence-based guidelines for implementing an inpatient antibiotic stewardship program.
The guidelines, published April 13 online in Clinical Infectious Diseases, address the optimal use of antibiotics in inpatient populations, and were prepared by a multidisciplinary expert panel of the IDSA and the SHEA, which included representation from the specialties of internal medicine, emergency medicine, microbiology, critical care, surgery, epidemiology, pharmacy, and adult and pediatric infectious diseases.
Antibiotic stewardship has been defined by IDSA, SHEA, and the Pediatric Infectious Diseases Society as “coordinated interventions designed to improve and measure the appropriate use of [antibiotic] agents by promoting the selection of the optimal [antibiotic] drug regimen including dosing, duration of therapy, and route of administration.” The new guidelines discuss a broad range of possible interventions, but the authors emphasize the need “for each site to assess its clinical needs and available resources and individualize its [antibiotic stewardship program] with that assessment in mind.”
The process used in the development of the guidelines included a systematic weighting of the strength of recommendation and quality of evidence using the GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) system, according to Dr. Tamar F. Barlam of the section of infectious diseases at Boston University, and her colleagues.
“The benefits of antibiotic stewardship include improved patient outcomes, reduced adverse events including Clostridium difficile infection, improvement in rates of antibiotic susceptibilities to targeted antibiotics, and optimization of resource utilization across the continuum of care,” Dr. Barlam and her coauthors wrote.
A complete list of any potential conflicts of interest for the multiple coauthors is provided with the full stewardship guidelines, which can be reviewed in Clinical Infectious Diseases (doi: 10.1093/cid/ciw118).
On Twitter @richpizzi
The Infectious Diseases Society of America (IDSA) and the Society for Healthcare Epidemiology of America (SHEA) have jointly released evidence-based guidelines for implementing an inpatient antibiotic stewardship program.
The guidelines, published April 13 online in Clinical Infectious Diseases, address the optimal use of antibiotics in inpatient populations, and were prepared by a multidisciplinary expert panel of the IDSA and the SHEA, which included representation from the specialties of internal medicine, emergency medicine, microbiology, critical care, surgery, epidemiology, pharmacy, and adult and pediatric infectious diseases.
Antibiotic stewardship has been defined by IDSA, SHEA, and the Pediatric Infectious Diseases Society as “coordinated interventions designed to improve and measure the appropriate use of [antibiotic] agents by promoting the selection of the optimal [antibiotic] drug regimen including dosing, duration of therapy, and route of administration.” The new guidelines discuss a broad range of possible interventions, but the authors emphasize the need “for each site to assess its clinical needs and available resources and individualize its [antibiotic stewardship program] with that assessment in mind.”
The process used in the development of the guidelines included a systematic weighting of the strength of recommendation and quality of evidence using the GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) system, according to Dr. Tamar F. Barlam of the section of infectious diseases at Boston University, and her colleagues.
“The benefits of antibiotic stewardship include improved patient outcomes, reduced adverse events including Clostridium difficile infection, improvement in rates of antibiotic susceptibilities to targeted antibiotics, and optimization of resource utilization across the continuum of care,” Dr. Barlam and her coauthors wrote.
A complete list of any potential conflicts of interest for the multiple coauthors is provided with the full stewardship guidelines, which can be reviewed in Clinical Infectious Diseases (doi: 10.1093/cid/ciw118).
On Twitter @richpizzi
FROM CLINICAL INFECTIOUS DISEASES
Benefit of lumbar fusion for spinal stenosis found to be small to nonexistent
The benefit of adding lumbar fusion surgery to decompression surgery for spinal stenosis was nonexistent in one large clinical trial and very modest in another, according to separate reports published online April 13 in the New England Journal of Medicine.
Both studies indicated that, given the considerable cost and the potential complications associated with lumbar fusion, it may not be worthwhile to add it to decompression surgery for spinal stenosis. “The goal of surgery in lumbar spinal stenosis is to improve walking distance and to relieve pain by decompression of the nerve roots. The addition of instrumented fusion – ‘just to be sure’ – for the treatment of the most frequent forms of lumbar spinal stenosis does not create any added value for patients and might be regarded as an overcautious and unnecessary treatment,” Dr. Wilco C. Peul and Dr. Wouter A. Moojen said in an editorial accompanying the two reports.
Surgical decompression of spinal stenosis using laminectomy is increasingly being supplemented with lumbar fusion, which is thought to firm up spinal instability and minimize the risk of future deformity. In the United States, approximately half of patients who have surgery for spinal stenosis undergo fusion procedures. Of those who also show degenerative spondylolisthesis on preoperative imaging studies, 96% undergo fusion procedures because many spine surgeons see this as a sign of instability and a mandatory indication for fusion. However, the evidence supporting the use of fusion plus decompression, as opposed to decompression alone, is weak, according to the investigators who conducted the Swedish Spinal Stenosis Study. The other study in the New England Journal of Medicine, the Spinal Laminectomy Versus Instrumented Pedicle Screw (SLIP) trial, was conducted in the United States.
Both of those clinical trials were performed to shed further light on the issue.
In the Swedish Spinal Stenosis Study, the investigators assessed outcomes in 247 patients aged 50-80 years who were treated at seven Swedish hospitals over the course of 6 years. This open-label, superiority trial randomly assigned 124 patients to decompression surgery alone and 123 to decompression plus fusion. The primary outcome measure was score on the Oswestry Disability Index (ODI), which measures disability and quality of life in patients with low-back pain, 2 years after surgery. The ODI scale runs from 0 to 100, with higher scores indicating more severe disability, said Dr. Peter Försth of the department of surgical sciences at Uppsala (Sweden) University and the Stockholm Spine Center and his associates.
At 2 years, there was no significant difference between the two study groups; the decompression-only group had a mean ODI score of 24, and the fusion group had a mean score of 27. The ODI scores in both groups had improved from baseline to a similar degree: by 17 points with decompression alone and by 15 points with fusion. In addition, fusion surgery was not superior to decompression alone regardless of whether patients had any degree of spondylolisthesis and regardless of whether they had severe spondylolisthesis involving a vertebral slip of 7.4 mm or more, the investigators reported (N Engl J Med. 2016 April 13. doi: 10.1056/NEJMoa1513721).The two study groups also showed no significant differences in secondary outcome measures, including performance on the 6-minute walk test and subjective patient assessment of improvement in walking ability. Moreover, these results persisted in the 144 patients who were assessed at 5-year follow-up.
In contrast, decompression alone was associated with fewer complications than decompression plus fusion. Postoperative wound infection developed in only 4% of the decompression-only group, compared with 10% of the fusion group. Although this study wasn’t adequately powered to draw firm conclusions regarding complications, a previous analysis of registry data reported that adding fusion surgery to decompression surgery doubles the risk of severe adverse events in older patients, Dr. Försth and his associates said.
Decompression alone also was markedly less expensive than fusion surgery. Mean direct costs were $6,800 higher for fusion than for decompression alone, because of the additional operating time needed, the extended hospital stay, and the cost of the implant.
In the SLIP trial, the researchers compared outcomes in 66 patients aged 50-80 years who all had spinal stenosis with grade 1 degenerative spondylolisthesis. The participants were randomly assigned to undergo decompression alone (35 patients) or decompression plus fusion (31 patients) at five U.S. medical centers, said Dr. Zoher Ghogawala of the Alan and Jacqueline B. Stuart Spine Research Center in the department of neurosurgery at Lahey Hospital and Medical Center, Burlington, Mass., and his associates.
The primary outcome measure was the physical-component summary score on the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36) 2 years after surgery. This scale also runs from 0 to 100, but higher scores indicate better physical health. Five points was prespecified as the minimal clinically important difference on the SF-36.
At 2 years, patients in the fusion group showed a small but significant advantage of 5.7 points on the SF-36, with a mean score of 15.2, compared with patients in the decompression-only group (mean score, 9.5). However, the ODI scores, a secondary outcome measure in this study, were not significantly different between the two study groups, Dr. Ghogawala and his associates reported (N Engl J Med. 2016 April 13. doi: 10.1056/NEJMoa1508788).Surgical complications, blood loss, and length of stay all were significantly greater with fusion than with decompression alone.
Dr. Försth’s study was supported by Uppsala University, Uppsala County Council, the Stockholm Spine Center, and Johnson & Johnson. Two of his associates reported ties to Medtronic and Quantify Research. Dr. Ghogawala’s study was supported by the Jean and David Wallace Foundation, the Greenwich Lumbar Stenosis SLIP Study Fund. His associates reported ties to numerous industry sources.
Both of these studies clearly demonstrated that for most patients, stenosis surgery should be limited to decompression when no overt instability is present. Dr. Ghogawala and his colleagues correctly concluded that the modest difference in SF-36 score in favor of fusion doesn’t justify that procedure’s higher cost and complication rate.
Fusion surgery is no longer best practice and should be restricted to patients who have proven spinal instability; vertebral destruction caused by trauma, tumors, infections, or spinal deformities; or possibly neuroforamen stenosis with compressed exiting nerves due to postsurgical disk collapse.
Dr. Wilco C. Peul is at Leiden (the Netherlands) University Medical Center and at Medical Center Haaglanden, the Hague. Dr. Wouter A. Moojen is at Medical Center Haaglanden. Dr. Peul reported receiving grants from ZonMW, Paradigm Spine, Medtronic, Eurospine Foundation, and CVZ. Dr. Moojen reported having no relevant financial disclosures. Dr. Peul and Dr. Moojen made these remarks in an editorial accompanying the reports on the Swedish Spinal Stenosis Study and the Spinal Laminectomy Versus Instrumented Pedicle Screw trial (N Engl J Med. 2016 April 13. doi: 10.1056/NEJMe1600955).
Both of these studies clearly demonstrated that for most patients, stenosis surgery should be limited to decompression when no overt instability is present. Dr. Ghogawala and his colleagues correctly concluded that the modest difference in SF-36 score in favor of fusion doesn’t justify that procedure’s higher cost and complication rate.
Fusion surgery is no longer best practice and should be restricted to patients who have proven spinal instability; vertebral destruction caused by trauma, tumors, infections, or spinal deformities; or possibly neuroforamen stenosis with compressed exiting nerves due to postsurgical disk collapse.
Dr. Wilco C. Peul is at Leiden (the Netherlands) University Medical Center and at Medical Center Haaglanden, the Hague. Dr. Wouter A. Moojen is at Medical Center Haaglanden. Dr. Peul reported receiving grants from ZonMW, Paradigm Spine, Medtronic, Eurospine Foundation, and CVZ. Dr. Moojen reported having no relevant financial disclosures. Dr. Peul and Dr. Moojen made these remarks in an editorial accompanying the reports on the Swedish Spinal Stenosis Study and the Spinal Laminectomy Versus Instrumented Pedicle Screw trial (N Engl J Med. 2016 April 13. doi: 10.1056/NEJMe1600955).
Both of these studies clearly demonstrated that for most patients, stenosis surgery should be limited to decompression when no overt instability is present. Dr. Ghogawala and his colleagues correctly concluded that the modest difference in SF-36 score in favor of fusion doesn’t justify that procedure’s higher cost and complication rate.
Fusion surgery is no longer best practice and should be restricted to patients who have proven spinal instability; vertebral destruction caused by trauma, tumors, infections, or spinal deformities; or possibly neuroforamen stenosis with compressed exiting nerves due to postsurgical disk collapse.
Dr. Wilco C. Peul is at Leiden (the Netherlands) University Medical Center and at Medical Center Haaglanden, the Hague. Dr. Wouter A. Moojen is at Medical Center Haaglanden. Dr. Peul reported receiving grants from ZonMW, Paradigm Spine, Medtronic, Eurospine Foundation, and CVZ. Dr. Moojen reported having no relevant financial disclosures. Dr. Peul and Dr. Moojen made these remarks in an editorial accompanying the reports on the Swedish Spinal Stenosis Study and the Spinal Laminectomy Versus Instrumented Pedicle Screw trial (N Engl J Med. 2016 April 13. doi: 10.1056/NEJMe1600955).
The benefit of adding lumbar fusion surgery to decompression surgery for spinal stenosis was nonexistent in one large clinical trial and very modest in another, according to separate reports published online April 13 in the New England Journal of Medicine.
Both studies indicated that, given the considerable cost and the potential complications associated with lumbar fusion, it may not be worthwhile to add it to decompression surgery for spinal stenosis. “The goal of surgery in lumbar spinal stenosis is to improve walking distance and to relieve pain by decompression of the nerve roots. The addition of instrumented fusion – ‘just to be sure’ – for the treatment of the most frequent forms of lumbar spinal stenosis does not create any added value for patients and might be regarded as an overcautious and unnecessary treatment,” Dr. Wilco C. Peul and Dr. Wouter A. Moojen said in an editorial accompanying the two reports.
Surgical decompression of spinal stenosis using laminectomy is increasingly being supplemented with lumbar fusion, which is thought to firm up spinal instability and minimize the risk of future deformity. In the United States, approximately half of patients who have surgery for spinal stenosis undergo fusion procedures. Of those who also show degenerative spondylolisthesis on preoperative imaging studies, 96% undergo fusion procedures because many spine surgeons see this as a sign of instability and a mandatory indication for fusion. However, the evidence supporting the use of fusion plus decompression, as opposed to decompression alone, is weak, according to the investigators who conducted the Swedish Spinal Stenosis Study. The other study in the New England Journal of Medicine, the Spinal Laminectomy Versus Instrumented Pedicle Screw (SLIP) trial, was conducted in the United States.
Both of those clinical trials were performed to shed further light on the issue.
In the Swedish Spinal Stenosis Study, the investigators assessed outcomes in 247 patients aged 50-80 years who were treated at seven Swedish hospitals over the course of 6 years. This open-label, superiority trial randomly assigned 124 patients to decompression surgery alone and 123 to decompression plus fusion. The primary outcome measure was score on the Oswestry Disability Index (ODI), which measures disability and quality of life in patients with low-back pain, 2 years after surgery. The ODI scale runs from 0 to 100, with higher scores indicating more severe disability, said Dr. Peter Försth of the department of surgical sciences at Uppsala (Sweden) University and the Stockholm Spine Center and his associates.
At 2 years, there was no significant difference between the two study groups; the decompression-only group had a mean ODI score of 24, and the fusion group had a mean score of 27. The ODI scores in both groups had improved from baseline to a similar degree: by 17 points with decompression alone and by 15 points with fusion. In addition, fusion surgery was not superior to decompression alone regardless of whether patients had any degree of spondylolisthesis and regardless of whether they had severe spondylolisthesis involving a vertebral slip of 7.4 mm or more, the investigators reported (N Engl J Med. 2016 April 13. doi: 10.1056/NEJMoa1513721).The two study groups also showed no significant differences in secondary outcome measures, including performance on the 6-minute walk test and subjective patient assessment of improvement in walking ability. Moreover, these results persisted in the 144 patients who were assessed at 5-year follow-up.
In contrast, decompression alone was associated with fewer complications than decompression plus fusion. Postoperative wound infection developed in only 4% of the decompression-only group, compared with 10% of the fusion group. Although this study wasn’t adequately powered to draw firm conclusions regarding complications, a previous analysis of registry data reported that adding fusion surgery to decompression surgery doubles the risk of severe adverse events in older patients, Dr. Försth and his associates said.
Decompression alone also was markedly less expensive than fusion surgery. Mean direct costs were $6,800 higher for fusion than for decompression alone, because of the additional operating time needed, the extended hospital stay, and the cost of the implant.
In the SLIP trial, the researchers compared outcomes in 66 patients aged 50-80 years who all had spinal stenosis with grade 1 degenerative spondylolisthesis. The participants were randomly assigned to undergo decompression alone (35 patients) or decompression plus fusion (31 patients) at five U.S. medical centers, said Dr. Zoher Ghogawala of the Alan and Jacqueline B. Stuart Spine Research Center in the department of neurosurgery at Lahey Hospital and Medical Center, Burlington, Mass., and his associates.
The primary outcome measure was the physical-component summary score on the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36) 2 years after surgery. This scale also runs from 0 to 100, but higher scores indicate better physical health. Five points was prespecified as the minimal clinically important difference on the SF-36.
At 2 years, patients in the fusion group showed a small but significant advantage of 5.7 points on the SF-36, with a mean score of 15.2, compared with patients in the decompression-only group (mean score, 9.5). However, the ODI scores, a secondary outcome measure in this study, were not significantly different between the two study groups, Dr. Ghogawala and his associates reported (N Engl J Med. 2016 April 13. doi: 10.1056/NEJMoa1508788).Surgical complications, blood loss, and length of stay all were significantly greater with fusion than with decompression alone.
Dr. Försth’s study was supported by Uppsala University, Uppsala County Council, the Stockholm Spine Center, and Johnson & Johnson. Two of his associates reported ties to Medtronic and Quantify Research. Dr. Ghogawala’s study was supported by the Jean and David Wallace Foundation, the Greenwich Lumbar Stenosis SLIP Study Fund. His associates reported ties to numerous industry sources.
The benefit of adding lumbar fusion surgery to decompression surgery for spinal stenosis was nonexistent in one large clinical trial and very modest in another, according to separate reports published online April 13 in the New England Journal of Medicine.
Both studies indicated that, given the considerable cost and the potential complications associated with lumbar fusion, it may not be worthwhile to add it to decompression surgery for spinal stenosis. “The goal of surgery in lumbar spinal stenosis is to improve walking distance and to relieve pain by decompression of the nerve roots. The addition of instrumented fusion – ‘just to be sure’ – for the treatment of the most frequent forms of lumbar spinal stenosis does not create any added value for patients and might be regarded as an overcautious and unnecessary treatment,” Dr. Wilco C. Peul and Dr. Wouter A. Moojen said in an editorial accompanying the two reports.
Surgical decompression of spinal stenosis using laminectomy is increasingly being supplemented with lumbar fusion, which is thought to firm up spinal instability and minimize the risk of future deformity. In the United States, approximately half of patients who have surgery for spinal stenosis undergo fusion procedures. Of those who also show degenerative spondylolisthesis on preoperative imaging studies, 96% undergo fusion procedures because many spine surgeons see this as a sign of instability and a mandatory indication for fusion. However, the evidence supporting the use of fusion plus decompression, as opposed to decompression alone, is weak, according to the investigators who conducted the Swedish Spinal Stenosis Study. The other study in the New England Journal of Medicine, the Spinal Laminectomy Versus Instrumented Pedicle Screw (SLIP) trial, was conducted in the United States.
Both of those clinical trials were performed to shed further light on the issue.
In the Swedish Spinal Stenosis Study, the investigators assessed outcomes in 247 patients aged 50-80 years who were treated at seven Swedish hospitals over the course of 6 years. This open-label, superiority trial randomly assigned 124 patients to decompression surgery alone and 123 to decompression plus fusion. The primary outcome measure was score on the Oswestry Disability Index (ODI), which measures disability and quality of life in patients with low-back pain, 2 years after surgery. The ODI scale runs from 0 to 100, with higher scores indicating more severe disability, said Dr. Peter Försth of the department of surgical sciences at Uppsala (Sweden) University and the Stockholm Spine Center and his associates.
At 2 years, there was no significant difference between the two study groups; the decompression-only group had a mean ODI score of 24, and the fusion group had a mean score of 27. The ODI scores in both groups had improved from baseline to a similar degree: by 17 points with decompression alone and by 15 points with fusion. In addition, fusion surgery was not superior to decompression alone regardless of whether patients had any degree of spondylolisthesis and regardless of whether they had severe spondylolisthesis involving a vertebral slip of 7.4 mm or more, the investigators reported (N Engl J Med. 2016 April 13. doi: 10.1056/NEJMoa1513721).The two study groups also showed no significant differences in secondary outcome measures, including performance on the 6-minute walk test and subjective patient assessment of improvement in walking ability. Moreover, these results persisted in the 144 patients who were assessed at 5-year follow-up.
In contrast, decompression alone was associated with fewer complications than decompression plus fusion. Postoperative wound infection developed in only 4% of the decompression-only group, compared with 10% of the fusion group. Although this study wasn’t adequately powered to draw firm conclusions regarding complications, a previous analysis of registry data reported that adding fusion surgery to decompression surgery doubles the risk of severe adverse events in older patients, Dr. Försth and his associates said.
Decompression alone also was markedly less expensive than fusion surgery. Mean direct costs were $6,800 higher for fusion than for decompression alone, because of the additional operating time needed, the extended hospital stay, and the cost of the implant.
In the SLIP trial, the researchers compared outcomes in 66 patients aged 50-80 years who all had spinal stenosis with grade 1 degenerative spondylolisthesis. The participants were randomly assigned to undergo decompression alone (35 patients) or decompression plus fusion (31 patients) at five U.S. medical centers, said Dr. Zoher Ghogawala of the Alan and Jacqueline B. Stuart Spine Research Center in the department of neurosurgery at Lahey Hospital and Medical Center, Burlington, Mass., and his associates.
The primary outcome measure was the physical-component summary score on the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36) 2 years after surgery. This scale also runs from 0 to 100, but higher scores indicate better physical health. Five points was prespecified as the minimal clinically important difference on the SF-36.
At 2 years, patients in the fusion group showed a small but significant advantage of 5.7 points on the SF-36, with a mean score of 15.2, compared with patients in the decompression-only group (mean score, 9.5). However, the ODI scores, a secondary outcome measure in this study, were not significantly different between the two study groups, Dr. Ghogawala and his associates reported (N Engl J Med. 2016 April 13. doi: 10.1056/NEJMoa1508788).Surgical complications, blood loss, and length of stay all were significantly greater with fusion than with decompression alone.
Dr. Försth’s study was supported by Uppsala University, Uppsala County Council, the Stockholm Spine Center, and Johnson & Johnson. Two of his associates reported ties to Medtronic and Quantify Research. Dr. Ghogawala’s study was supported by the Jean and David Wallace Foundation, the Greenwich Lumbar Stenosis SLIP Study Fund. His associates reported ties to numerous industry sources.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: The benefit of adding lumbar fusion surgery to decompression surgery for spinal stenosis was nonexistent in one large trial and very modest in another.
Major finding: At 2 years in the Swedish Spinal Stenosis Study, there was no significant difference between the two study groups; the decompression-only group had a mean Oswestry Disability Index score of 24, and the fusion group had a mean score of 27.
Data source: Two multicenter, randomized trials involving 247 patients and 66 patients, comparing decompression surgery alone with decompression plus fusion.
Disclosures: Dr. Försth’s study was supported by Uppsala University, Uppsala County Council, Stockholm Spine Center, and Johnson & Johnson. Two of his associates reported ties to Medtronic and Quantify Research. Dr. Ghogawala’s study was supported by the Jean and David Wallace Foundation, the Greenwich Lumbar Stenosis SLIP Study Fund. His associates reported ties to numerous industry sources.
VIDEO: Stenting to improve quality of life in esophageal cancer
PHILADELPHIA – An esophageal stent can improve quality of life for patients with advanced esophageal cancer, according to Dr. Sushil Ahlawat, director of endoscopy at Rutgers University, New Brunswick, N.J.
“An esophageal stent can be an important modality for palliating patients’ dysphagia, [which] can happen because the tumor is obstructing the esophagus,” says Dr. Ahlawat in this video. He also discusses how this minimally invasive procedure can support those undergoing chemoradiation therapy or surgery for esophageal cancer.
The video was recorded at this year’s meeting, held by Global Academy for Medical Education and Rutgers, the State University of New Jersey. Global Academy for Medical Education and this news organization are owned by the same company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @whitneymcknight
PHILADELPHIA – An esophageal stent can improve quality of life for patients with advanced esophageal cancer, according to Dr. Sushil Ahlawat, director of endoscopy at Rutgers University, New Brunswick, N.J.
“An esophageal stent can be an important modality for palliating patients’ dysphagia, [which] can happen because the tumor is obstructing the esophagus,” says Dr. Ahlawat in this video. He also discusses how this minimally invasive procedure can support those undergoing chemoradiation therapy or surgery for esophageal cancer.
The video was recorded at this year’s meeting, held by Global Academy for Medical Education and Rutgers, the State University of New Jersey. Global Academy for Medical Education and this news organization are owned by the same company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @whitneymcknight
PHILADELPHIA – An esophageal stent can improve quality of life for patients with advanced esophageal cancer, according to Dr. Sushil Ahlawat, director of endoscopy at Rutgers University, New Brunswick, N.J.
“An esophageal stent can be an important modality for palliating patients’ dysphagia, [which] can happen because the tumor is obstructing the esophagus,” says Dr. Ahlawat in this video. He also discusses how this minimally invasive procedure can support those undergoing chemoradiation therapy or surgery for esophageal cancer.
The video was recorded at this year’s meeting, held by Global Academy for Medical Education and Rutgers, the State University of New Jersey. Global Academy for Medical Education and this news organization are owned by the same company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @whitneymcknight
EXPERT ANALYSIS FROM DIGESTIVE DISEASES: NEW ADVANCES
Study finds inappropriate oophorectomy at time of hysterectomy
Among premenopausal California women undergoing nonradical hysterectomies over a 7-year period, more than one-third underwent concurrent oophorectomies for no apparent reason.
“Regardless of what our national guidelines are telling us to do, we’re still not doing a good enough job of educating our patients and providing guideline-driven care,” Dr. Amandeep S. Mahal said in an interview prior to the annual scientific meeting of the Society of Gynecologic Surgeons.
Emerging evidence suggests that premenopausal oophorectomy is associated with worsened long-term health outcomes, including increased mortality and risk of cardiovascular events, said Dr. Mahal, a second-year fellow in the department of obstetrics and gynecology at Stanford (Calif.) University Hospital. The current recommendation by the American College of Obstetricians and Gynecologists (ACOG) is that “strong consideration should be given to retaining normal ovaries in premenopausal women who are not at increased genetic risk of ovarian cancer. However, given the risk of ovarian cancer in postmenopausal women, ovarian removal at the time of hysterectomy should be considered for these women” (Obstet. Gynecol. 2008;111[1]:231-41).
In an effort to determine the rate of potentially unnecessary oophorectomies being performed in premenopausal women for benign indications, the researchers reviewed 259,294 inpatient, nonradical hysterectomies performed in California hospitals between 2005 and 2011. Women younger than age 50 were categorized as premenopausal. The records were obtained from California’s Office of Statewide Health Planning patient discharge database, which includes all non–federal hospital discharges. Each discharge contains a primary diagnosis as well as up to 19 secondary procedure codes and 24 secondary diagnosis codes. Dr. Mahal and his associates considered oophorectomies as appropriate if a supporting ICD-9 code such as “ovarian cyst” or “endometriosis” was linked to it, and inappropriate if no such codes were linked.
Of the 259,294 benign hysterectomies performed during the study period, 37% included concomitant removal of all ovaries, and 53% of the oophorectomies were performed in premenopausal women. Of the oophorectomies in premenopausal women, 37% were deemed to be “inappropriate” based on the documented reason for removal. The researchers observed that the total number of premenopausal hysterectomies with oophorectomy decreased from 10,166 per year in 2004 to 4,672 per year in 2011, but the percentage of oophorectomies deemed to be inappropriate remained stable, in the range of 36%-38%.
“We were very diligent and went through every possible diagnosis we could think of that would give you a reason to remove ovaries,” Dr. Mahal said. “Even being exhaustive in that manner, we could not find a reason why for more than one in three women who underwent oophorectomy prior to natural menopause.”
Logistic regression analysis revealed Hispanic and black race as the only demographic factors associated with an increased odds of inappropriate oophorectomy at the time of hysterectomy (P less than .001). Hospital characteristics and type of insurance did not account for any observed differences.
Even if premenopausal women have no risk factors for ovarian cancer in the future, undergoing an oophorectomy “is a decision they should make with their physician,” Dr. Mahal said. “One of the things we don’t know [about this study] is how many patients had a conversation with their doctor, understood the risks, and decided ‘it’s worth it for me to go ahead and remove the ovaries at the time of the hysterectomy.’ ”
The meeting was jointly sponsored by the American College of Surgeons.
Dr. Mahal reported having no financial disclosures.
Among premenopausal California women undergoing nonradical hysterectomies over a 7-year period, more than one-third underwent concurrent oophorectomies for no apparent reason.
“Regardless of what our national guidelines are telling us to do, we’re still not doing a good enough job of educating our patients and providing guideline-driven care,” Dr. Amandeep S. Mahal said in an interview prior to the annual scientific meeting of the Society of Gynecologic Surgeons.
Emerging evidence suggests that premenopausal oophorectomy is associated with worsened long-term health outcomes, including increased mortality and risk of cardiovascular events, said Dr. Mahal, a second-year fellow in the department of obstetrics and gynecology at Stanford (Calif.) University Hospital. The current recommendation by the American College of Obstetricians and Gynecologists (ACOG) is that “strong consideration should be given to retaining normal ovaries in premenopausal women who are not at increased genetic risk of ovarian cancer. However, given the risk of ovarian cancer in postmenopausal women, ovarian removal at the time of hysterectomy should be considered for these women” (Obstet. Gynecol. 2008;111[1]:231-41).
In an effort to determine the rate of potentially unnecessary oophorectomies being performed in premenopausal women for benign indications, the researchers reviewed 259,294 inpatient, nonradical hysterectomies performed in California hospitals between 2005 and 2011. Women younger than age 50 were categorized as premenopausal. The records were obtained from California’s Office of Statewide Health Planning patient discharge database, which includes all non–federal hospital discharges. Each discharge contains a primary diagnosis as well as up to 19 secondary procedure codes and 24 secondary diagnosis codes. Dr. Mahal and his associates considered oophorectomies as appropriate if a supporting ICD-9 code such as “ovarian cyst” or “endometriosis” was linked to it, and inappropriate if no such codes were linked.
Of the 259,294 benign hysterectomies performed during the study period, 37% included concomitant removal of all ovaries, and 53% of the oophorectomies were performed in premenopausal women. Of the oophorectomies in premenopausal women, 37% were deemed to be “inappropriate” based on the documented reason for removal. The researchers observed that the total number of premenopausal hysterectomies with oophorectomy decreased from 10,166 per year in 2004 to 4,672 per year in 2011, but the percentage of oophorectomies deemed to be inappropriate remained stable, in the range of 36%-38%.
“We were very diligent and went through every possible diagnosis we could think of that would give you a reason to remove ovaries,” Dr. Mahal said. “Even being exhaustive in that manner, we could not find a reason why for more than one in three women who underwent oophorectomy prior to natural menopause.”
Logistic regression analysis revealed Hispanic and black race as the only demographic factors associated with an increased odds of inappropriate oophorectomy at the time of hysterectomy (P less than .001). Hospital characteristics and type of insurance did not account for any observed differences.
Even if premenopausal women have no risk factors for ovarian cancer in the future, undergoing an oophorectomy “is a decision they should make with their physician,” Dr. Mahal said. “One of the things we don’t know [about this study] is how many patients had a conversation with their doctor, understood the risks, and decided ‘it’s worth it for me to go ahead and remove the ovaries at the time of the hysterectomy.’ ”
The meeting was jointly sponsored by the American College of Surgeons.
Dr. Mahal reported having no financial disclosures.
Among premenopausal California women undergoing nonradical hysterectomies over a 7-year period, more than one-third underwent concurrent oophorectomies for no apparent reason.
“Regardless of what our national guidelines are telling us to do, we’re still not doing a good enough job of educating our patients and providing guideline-driven care,” Dr. Amandeep S. Mahal said in an interview prior to the annual scientific meeting of the Society of Gynecologic Surgeons.
Emerging evidence suggests that premenopausal oophorectomy is associated with worsened long-term health outcomes, including increased mortality and risk of cardiovascular events, said Dr. Mahal, a second-year fellow in the department of obstetrics and gynecology at Stanford (Calif.) University Hospital. The current recommendation by the American College of Obstetricians and Gynecologists (ACOG) is that “strong consideration should be given to retaining normal ovaries in premenopausal women who are not at increased genetic risk of ovarian cancer. However, given the risk of ovarian cancer in postmenopausal women, ovarian removal at the time of hysterectomy should be considered for these women” (Obstet. Gynecol. 2008;111[1]:231-41).
In an effort to determine the rate of potentially unnecessary oophorectomies being performed in premenopausal women for benign indications, the researchers reviewed 259,294 inpatient, nonradical hysterectomies performed in California hospitals between 2005 and 2011. Women younger than age 50 were categorized as premenopausal. The records were obtained from California’s Office of Statewide Health Planning patient discharge database, which includes all non–federal hospital discharges. Each discharge contains a primary diagnosis as well as up to 19 secondary procedure codes and 24 secondary diagnosis codes. Dr. Mahal and his associates considered oophorectomies as appropriate if a supporting ICD-9 code such as “ovarian cyst” or “endometriosis” was linked to it, and inappropriate if no such codes were linked.
Of the 259,294 benign hysterectomies performed during the study period, 37% included concomitant removal of all ovaries, and 53% of the oophorectomies were performed in premenopausal women. Of the oophorectomies in premenopausal women, 37% were deemed to be “inappropriate” based on the documented reason for removal. The researchers observed that the total number of premenopausal hysterectomies with oophorectomy decreased from 10,166 per year in 2004 to 4,672 per year in 2011, but the percentage of oophorectomies deemed to be inappropriate remained stable, in the range of 36%-38%.
“We were very diligent and went through every possible diagnosis we could think of that would give you a reason to remove ovaries,” Dr. Mahal said. “Even being exhaustive in that manner, we could not find a reason why for more than one in three women who underwent oophorectomy prior to natural menopause.”
Logistic regression analysis revealed Hispanic and black race as the only demographic factors associated with an increased odds of inappropriate oophorectomy at the time of hysterectomy (P less than .001). Hospital characteristics and type of insurance did not account for any observed differences.
Even if premenopausal women have no risk factors for ovarian cancer in the future, undergoing an oophorectomy “is a decision they should make with their physician,” Dr. Mahal said. “One of the things we don’t know [about this study] is how many patients had a conversation with their doctor, understood the risks, and decided ‘it’s worth it for me to go ahead and remove the ovaries at the time of the hysterectomy.’ ”
The meeting was jointly sponsored by the American College of Surgeons.
Dr. Mahal reported having no financial disclosures.
FROM SGS 2016
Key clinical point: More than one in three women underwent oophorectomy prior to natural menopause for no apparent reason.
Major finding: Of the oophorectomies in premenopausal women, 37% were deemed to be “inappropriate” based on the documented reason for removal.
Data source: A review of 259,294 inpatient, nonradical hysterectomies performed in California hospitals between 2005 and 2011.
Disclosures: Dr. Mahal reported having no financial disclosures.
Angina rates similar across metal stents
WASHINGTON – In patients with coronary artery disease, there is no increased risk of angina 1 year after percutaneous coronary intervention for bare metal stents relative to drug-eluting metal stents, according to data presented at Cardiovascular Research Technologies 2016.
“Angina pectoris in the first year after PCI [percutaneous coronary intervention] is remarkably common, affecting 32.3% of patients,” but “metallic stent type is not independently associated with the occurrence of angina,” reported Dr. Michael A. Gaglia Jr., an interventional cardiologist at MedStar Heart and Vascular Institute in Washington.
This conclusion was based on a study in which 8,804 patients who underwent PCI with metal stents were questioned about angina and its severity. The incidence of angina was compared for bare-metal stents relative to five drug-eluting metal stents: Cypher (sirolimus-eluting, Johnson & Johnson), Taxus Express2 (paclitaxel, Boston Scientific), Xience V (everolimus, Abbott Vascular), Promus Element (everolimus, Boston Scientific), and Resolute Integrity (zotarolimus, Medtronic).
For nearly 3 months, the cumulative incidence of angina remained tightly grouped at 5% or less across stent types. Incidence rates began climbing slowly through the first 9 months of follow-up and then more steeply at about 10 months. When depicted graphically, the incidence of angina appeared higher after placement of the Cypher stent, which was discontinued in 2011, but multivariate analysis found “no significant association between stent type and angina at 1 year after PCI,” Dr. Gaglia reported.
Although risk of angina was not correlated with type of metal stent, angina was highly correlated with risk of a major adverse cardiovascular event (MACE). When angina severity was stratified by the Canadian Cardiovascular Society system, MACE, defined as a composite of all-cause mortality, target vessel revascularization, and Q-wave myocardial infarction, occurred in 6.8% of those without angina, 10.0% of those with class 1 or 2 angina, and 19.7% of those with class 3 or 4 angina (P less than .001 for this trend) over the course of follow-up.
Independent of stent type, angina was more common in patients with a history of severe angina prior to PCI, a prior PCI, or prior coronary artery bypass grafting. A reduced likelihood of angina was independently associated with older age, male sex, a presentation of acute coronary syndrome, and a longer stented length.
Other studies have also shown that angina after PCI is associated with an increased risk of MACE relative to the absence of ischemia, but the contribution of this study is that it is the first set of data to suggest that drug eluting stents provide no advantage over bare metal stents for controlling angina, according to the authors. In the graphic representation of angina incidence for different stent types over 1-year of follow-up, four of the six lines, including the line representing bare metal stents, were essentially superimposable. In addition to the line representing angina incidence in those receiving the Cypher stent, the line representing angina incidence on the Promus Element stent climbed higher at 9 months relative to the remaining four stent types, but this line had rejoined the others at 12 months.
“Metallic coronary stents alter vessel geometry, shear stress, and hemodynamics. Stents also vary in design and architecture,” Dr. Gaglia observed. Although protection from angina is one of the major indications for the placement of stents, Dr. Gaglia emphasized that data comparing different metallic stents in regards to the incidence of angina pectoris at long-term follow-up have until this study “been lacking.”
The meeting was sponsored by the Cardiovascular Research Institute at Washington Hospital Center. Dr. Gaglia reports no relevant financial relationships. Abbott Vascular funded the study.
WASHINGTON – In patients with coronary artery disease, there is no increased risk of angina 1 year after percutaneous coronary intervention for bare metal stents relative to drug-eluting metal stents, according to data presented at Cardiovascular Research Technologies 2016.
“Angina pectoris in the first year after PCI [percutaneous coronary intervention] is remarkably common, affecting 32.3% of patients,” but “metallic stent type is not independently associated with the occurrence of angina,” reported Dr. Michael A. Gaglia Jr., an interventional cardiologist at MedStar Heart and Vascular Institute in Washington.
This conclusion was based on a study in which 8,804 patients who underwent PCI with metal stents were questioned about angina and its severity. The incidence of angina was compared for bare-metal stents relative to five drug-eluting metal stents: Cypher (sirolimus-eluting, Johnson & Johnson), Taxus Express2 (paclitaxel, Boston Scientific), Xience V (everolimus, Abbott Vascular), Promus Element (everolimus, Boston Scientific), and Resolute Integrity (zotarolimus, Medtronic).
For nearly 3 months, the cumulative incidence of angina remained tightly grouped at 5% or less across stent types. Incidence rates began climbing slowly through the first 9 months of follow-up and then more steeply at about 10 months. When depicted graphically, the incidence of angina appeared higher after placement of the Cypher stent, which was discontinued in 2011, but multivariate analysis found “no significant association between stent type and angina at 1 year after PCI,” Dr. Gaglia reported.
Although risk of angina was not correlated with type of metal stent, angina was highly correlated with risk of a major adverse cardiovascular event (MACE). When angina severity was stratified by the Canadian Cardiovascular Society system, MACE, defined as a composite of all-cause mortality, target vessel revascularization, and Q-wave myocardial infarction, occurred in 6.8% of those without angina, 10.0% of those with class 1 or 2 angina, and 19.7% of those with class 3 or 4 angina (P less than .001 for this trend) over the course of follow-up.
Independent of stent type, angina was more common in patients with a history of severe angina prior to PCI, a prior PCI, or prior coronary artery bypass grafting. A reduced likelihood of angina was independently associated with older age, male sex, a presentation of acute coronary syndrome, and a longer stented length.
Other studies have also shown that angina after PCI is associated with an increased risk of MACE relative to the absence of ischemia, but the contribution of this study is that it is the first set of data to suggest that drug eluting stents provide no advantage over bare metal stents for controlling angina, according to the authors. In the graphic representation of angina incidence for different stent types over 1-year of follow-up, four of the six lines, including the line representing bare metal stents, were essentially superimposable. In addition to the line representing angina incidence in those receiving the Cypher stent, the line representing angina incidence on the Promus Element stent climbed higher at 9 months relative to the remaining four stent types, but this line had rejoined the others at 12 months.
“Metallic coronary stents alter vessel geometry, shear stress, and hemodynamics. Stents also vary in design and architecture,” Dr. Gaglia observed. Although protection from angina is one of the major indications for the placement of stents, Dr. Gaglia emphasized that data comparing different metallic stents in regards to the incidence of angina pectoris at long-term follow-up have until this study “been lacking.”
The meeting was sponsored by the Cardiovascular Research Institute at Washington Hospital Center. Dr. Gaglia reports no relevant financial relationships. Abbott Vascular funded the study.
WASHINGTON – In patients with coronary artery disease, there is no increased risk of angina 1 year after percutaneous coronary intervention for bare metal stents relative to drug-eluting metal stents, according to data presented at Cardiovascular Research Technologies 2016.
“Angina pectoris in the first year after PCI [percutaneous coronary intervention] is remarkably common, affecting 32.3% of patients,” but “metallic stent type is not independently associated with the occurrence of angina,” reported Dr. Michael A. Gaglia Jr., an interventional cardiologist at MedStar Heart and Vascular Institute in Washington.
This conclusion was based on a study in which 8,804 patients who underwent PCI with metal stents were questioned about angina and its severity. The incidence of angina was compared for bare-metal stents relative to five drug-eluting metal stents: Cypher (sirolimus-eluting, Johnson & Johnson), Taxus Express2 (paclitaxel, Boston Scientific), Xience V (everolimus, Abbott Vascular), Promus Element (everolimus, Boston Scientific), and Resolute Integrity (zotarolimus, Medtronic).
For nearly 3 months, the cumulative incidence of angina remained tightly grouped at 5% or less across stent types. Incidence rates began climbing slowly through the first 9 months of follow-up and then more steeply at about 10 months. When depicted graphically, the incidence of angina appeared higher after placement of the Cypher stent, which was discontinued in 2011, but multivariate analysis found “no significant association between stent type and angina at 1 year after PCI,” Dr. Gaglia reported.
Although risk of angina was not correlated with type of metal stent, angina was highly correlated with risk of a major adverse cardiovascular event (MACE). When angina severity was stratified by the Canadian Cardiovascular Society system, MACE, defined as a composite of all-cause mortality, target vessel revascularization, and Q-wave myocardial infarction, occurred in 6.8% of those without angina, 10.0% of those with class 1 or 2 angina, and 19.7% of those with class 3 or 4 angina (P less than .001 for this trend) over the course of follow-up.
Independent of stent type, angina was more common in patients with a history of severe angina prior to PCI, a prior PCI, or prior coronary artery bypass grafting. A reduced likelihood of angina was independently associated with older age, male sex, a presentation of acute coronary syndrome, and a longer stented length.
Other studies have also shown that angina after PCI is associated with an increased risk of MACE relative to the absence of ischemia, but the contribution of this study is that it is the first set of data to suggest that drug eluting stents provide no advantage over bare metal stents for controlling angina, according to the authors. In the graphic representation of angina incidence for different stent types over 1-year of follow-up, four of the six lines, including the line representing bare metal stents, were essentially superimposable. In addition to the line representing angina incidence in those receiving the Cypher stent, the line representing angina incidence on the Promus Element stent climbed higher at 9 months relative to the remaining four stent types, but this line had rejoined the others at 12 months.
“Metallic coronary stents alter vessel geometry, shear stress, and hemodynamics. Stents also vary in design and architecture,” Dr. Gaglia observed. Although protection from angina is one of the major indications for the placement of stents, Dr. Gaglia emphasized that data comparing different metallic stents in regards to the incidence of angina pectoris at long-term follow-up have until this study “been lacking.”
The meeting was sponsored by the Cardiovascular Research Institute at Washington Hospital Center. Dr. Gaglia reports no relevant financial relationships. Abbott Vascular funded the study.
AT CARDIOVASCULAR RESEARCH TECHNOLOGIES 2016
Key clinical point: One year after stent placement, angina rates are no higher with bare metal stents than with drug-eluting metal stents.
Major finding: One year after stenting, the incidence of angina was 32.3%.
Data source: Observational study with 8,804 patients.
Disclosures: Dr. Gaglia reports no relevant financial relationships. Abbott Vascular funded the study.
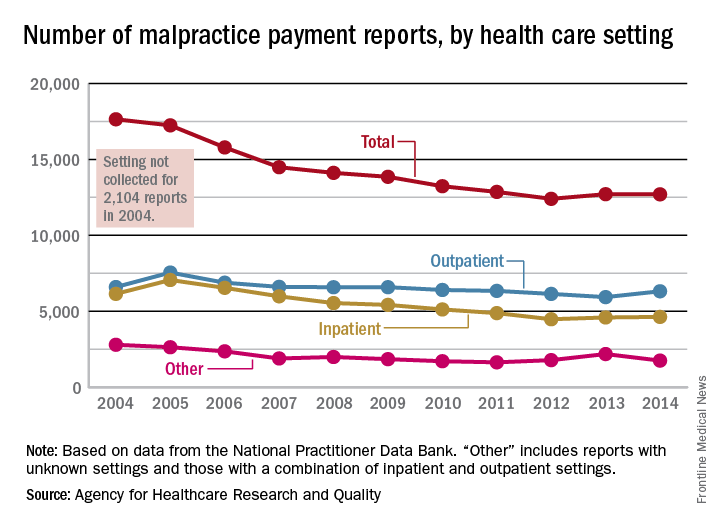
Number of malpractice payments down 28% since 2004
The annual number of medical malpractice payment reports fell 28% from 2004 to 2014, according to the Agency for Healthcare Research and Quality.
The total number of medical malpractice payment reports (MMPRs) for 2014 was 12,699, a decrease of 28% since 2004, when there were 17,641. The total had gone down every year until a slight increase in 2013, but the number held steady in 2014, the AHRQ reported in the Chartbook on Patient Safety.
Since 2004, MMPRs related to inpatient settings have been dropping slightly faster than outpatient-related MMPRs, with the exception, again, of 2013, when the number of inpatient MMPRs went up while the outpatient total dropped. Both types went up in 2014, but the category of “other” – reports related to unknown settings and those from a combination of the two – dropped in 2014 to keep the overall number from going up again, data from the National Practitioner Data Bank show.
Looking at the types of allegation leading to MMPRs, treatment was highest, accounting for 27.4% of the total from 2004 to 2014, with diagnosis right behind at 27.1%, followed by surgery at 23.5% and obstetrics at 6.7%. Medication-related cases represented 5.3% of all MMPRs over that period, the AHRQ noted.
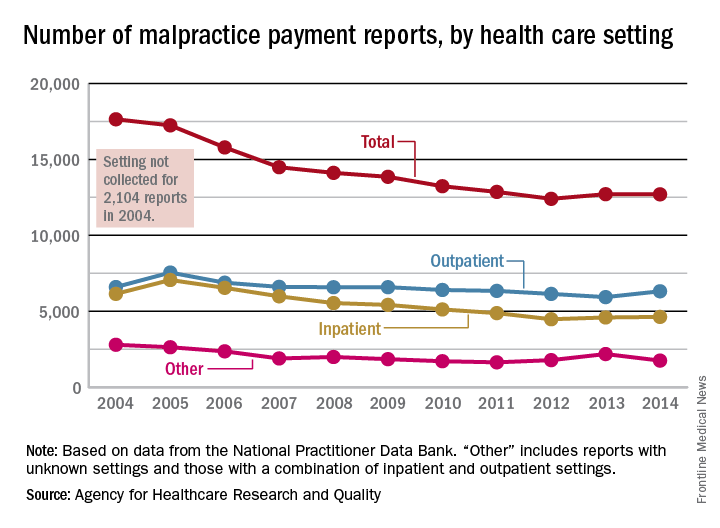
The annual number of medical malpractice payment reports fell 28% from 2004 to 2014, according to the Agency for Healthcare Research and Quality.
The total number of medical malpractice payment reports (MMPRs) for 2014 was 12,699, a decrease of 28% since 2004, when there were 17,641. The total had gone down every year until a slight increase in 2013, but the number held steady in 2014, the AHRQ reported in the Chartbook on Patient Safety.

Since 2004, MMPRs related to inpatient settings have been dropping slightly faster than outpatient-related MMPRs, with the exception, again, of 2013, when the number of inpatient MMPRs went up while the outpatient total dropped. Both types went up in 2014, but the category of “other” – reports related to unknown settings and those from a combination of the two – dropped in 2014 to keep the overall number from going up again, data from the National Practitioner Data Bank show.
Looking at the types of allegation leading to MMPRs, treatment was highest, accounting for 27.4% of the total from 2004 to 2014, with diagnosis right behind at 27.1%, followed by surgery at 23.5% and obstetrics at 6.7%. Medication-related cases represented 5.3% of all MMPRs over that period, the AHRQ noted.
The annual number of medical malpractice payment reports fell 28% from 2004 to 2014, according to the Agency for Healthcare Research and Quality.
The total number of medical malpractice payment reports (MMPRs) for 2014 was 12,699, a decrease of 28% since 2004, when there were 17,641. The total had gone down every year until a slight increase in 2013, but the number held steady in 2014, the AHRQ reported in the Chartbook on Patient Safety.

Since 2004, MMPRs related to inpatient settings have been dropping slightly faster than outpatient-related MMPRs, with the exception, again, of 2013, when the number of inpatient MMPRs went up while the outpatient total dropped. Both types went up in 2014, but the category of “other” – reports related to unknown settings and those from a combination of the two – dropped in 2014 to keep the overall number from going up again, data from the National Practitioner Data Bank show.
Looking at the types of allegation leading to MMPRs, treatment was highest, accounting for 27.4% of the total from 2004 to 2014, with diagnosis right behind at 27.1%, followed by surgery at 23.5% and obstetrics at 6.7%. Medication-related cases represented 5.3% of all MMPRs over that period, the AHRQ noted.
Number of malpractice payments down 28% since 2004
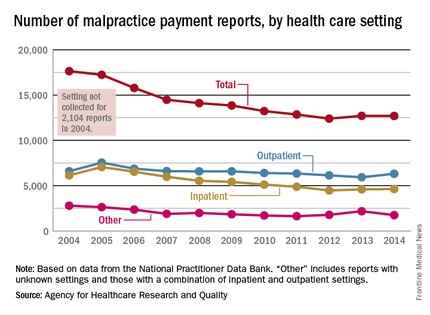
The annual number of medical malpractice payment reports fell 28% from 2004 to 2014, according to the Agency for Healthcare Research and Quality.
The total number of medical malpractice payment reports (MMPRs) for 2014 was 12,699, a decrease of 28% since 2004, when there were 17,641. The total had gone down every year until a slight increase in 2013, but the number held steady in 2014, the AHRQ reported in the Chartbook on Patient Safety.

Since 2004, MMPRs related to inpatient settings have been dropping slightly faster than outpatient-related MMPRs, with the exception, again, of 2013, when the number of inpatient MMPRs went up while the outpatient total dropped. Both types went up in 2014, but the category of “other” – reports related to unknown settings and those from a combination of the two – dropped in 2014 to keep the overall number from going up again, data from the National Practitioner Data Bank show.
Looking at the types of allegation leading to MMPRs, treatment was highest, accounting for 27.4% of the total from 2004 to 2014, with diagnosis right behind at 27.1%, followed by surgery at 23.5% and obstetrics at 6.7%. Medication-related cases represented 5.3% of all MMPRs over that period, the AHRQ noted.
The annual number of medical malpractice payment reports fell 28% from 2004 to 2014, according to the Agency for Healthcare Research and Quality.
The total number of medical malpractice payment reports (MMPRs) for 2014 was 12,699, a decrease of 28% since 2004, when there were 17,641. The total had gone down every year until a slight increase in 2013, but the number held steady in 2014, the AHRQ reported in the Chartbook on Patient Safety.

Since 2004, MMPRs related to inpatient settings have been dropping slightly faster than outpatient-related MMPRs, with the exception, again, of 2013, when the number of inpatient MMPRs went up while the outpatient total dropped. Both types went up in 2014, but the category of “other” – reports related to unknown settings and those from a combination of the two – dropped in 2014 to keep the overall number from going up again, data from the National Practitioner Data Bank show.
Looking at the types of allegation leading to MMPRs, treatment was highest, accounting for 27.4% of the total from 2004 to 2014, with diagnosis right behind at 27.1%, followed by surgery at 23.5% and obstetrics at 6.7%. Medication-related cases represented 5.3% of all MMPRs over that period, the AHRQ noted.
The annual number of medical malpractice payment reports fell 28% from 2004 to 2014, according to the Agency for Healthcare Research and Quality.
The total number of medical malpractice payment reports (MMPRs) for 2014 was 12,699, a decrease of 28% since 2004, when there were 17,641. The total had gone down every year until a slight increase in 2013, but the number held steady in 2014, the AHRQ reported in the Chartbook on Patient Safety.

Since 2004, MMPRs related to inpatient settings have been dropping slightly faster than outpatient-related MMPRs, with the exception, again, of 2013, when the number of inpatient MMPRs went up while the outpatient total dropped. Both types went up in 2014, but the category of “other” – reports related to unknown settings and those from a combination of the two – dropped in 2014 to keep the overall number from going up again, data from the National Practitioner Data Bank show.
Looking at the types of allegation leading to MMPRs, treatment was highest, accounting for 27.4% of the total from 2004 to 2014, with diagnosis right behind at 27.1%, followed by surgery at 23.5% and obstetrics at 6.7%. Medication-related cases represented 5.3% of all MMPRs over that period, the AHRQ noted.
Prostate cancer’s future seen in molecular tests
HOLLYWOOD, FLA. – Current evidence suggests that molecular tests for prostate cancer are prognostic and can help clinicians and patients with difficult treatment decisions. In the not-too-distant future, gene tests could also guide choice of therapies.
“I think that the largest impact is going to come in areas of both the greatest treatment uncertainty and areas where we can be predictive about the response to treatment,” said Dr. Ashley Ross, a urologic oncologist and pathologist at the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Baltimore.
Multigene panels may soon be able help identify which patients might benefit more from radical prostatectomy or radiation therapy, whether radiation therapy effects could be enhanced with the addition of androgen-deprivation therapy, and whether early use of docetaxel might add therapeutic benefit, he said at the annual conference of the National Comprehensive Cancer Network.
The 2016 iteration of the NCCN guidelines for the treatment of prostate cancer include a note stating that “men with clinically localized disease may consider the use of tumor-based molecular assays. Retrospective case cohort studies have shown that molecular assays performed on biopsy or prostatectomy specimens provide prognostic information independent of NCCN risk groups.”
The use of molecular assays may inform treatment decisions by helping to predict the likelihood of death if a patient is managed conservatively, risks for biochemical progression after radical prostatectomy or external beam therapy, and the likelihood that a patient could develop metastatic disease after radical prostatectomy or salvage radiotherapy, the guidelines say.
Dr. Ross reviewed the molecular biology of localized prostate cancer and the benefits and risks of currently available molecular tests.
“We’ve had an increased ability to get molecular information or genomic information from very limited amounts of routinely-stored pathologic tissue, and that’s resulted in the generation of many molecular-based tissue tests in prostate cancer. With the emergence of those tests and a lot of aggressive marketing, there has been a lot of confusion for patients and providers about whether we should use them or not and in what context,” he said.
Prostate cancer is genomically complex, even in the localized stage, with copy number alterations, deletions, and amplifications; chromosomal rearrangements; and point mutations, he said.
One of the best characterized genomic events is the early loss of the tumor suppressor gene PTEN (phosphatase and tensin homolog). This gene works within the PI3 kinase (PI3K)/AKT pathway. PI3K pathway mutations have been identified in up to 40% of all primary prostate cancers and 100% of mutations, Dr. Ross explained.
Loss of PTEN itself has been detected in about 15%-40% of primary prostate cancers and 50% of metastases, and the loss correlates with disease stage and tumor grade.
The NCCN guidelines list six available tissue-based tests for prostate cancer prognosis, including tests based on general cancer features such as cell-cycle proliferation, and those based on specific molecular features of cancer.
An example of the general type of test is the Ki-67 immunohistochemistry (IHC) test, which looks for a cellular marker of proliferation, and has been shown to have independent prognostic significance after radiation therapy or radical prostatectomy. This test is not currently recommended by the Medicare Molecular Diagnostic Services (MolDx) program, however.
Another general-type test is the Polaris quantitative reverse-transcriptase polymerase chain reaction (RT-PCR) panel, which tests for 31 cell-cycle-related genes and 15 “housekeeping” controls. This test is recommended for post-biopsy evaluation of men with very-low-risk or low-risk prostate cancer who at the time of diagnosis have at least 10 years of life expectancy. It has been shown to independently predict prostate cancer–specific mortality, biochemical failure/recurrence, and metastasis, the guidelines say.
Tests based on molecular features include:
• PTEN/ERG, an IHC or fluorescent in situ hybridization test that has been shown to predict prostate cancer–specific mortality, upgrading to Gleason pattern 4 on radical prostatectomy, and biochemical recurrence (not MolDx recommended).
• Decipher, a whole-transcriptome 1.4M RNA expression oligonucleotide microarray shown to predict biochemical failure, metastasis, and prostate cancer–specific mortality (recommended for postradical prostatectomy for patients with pT2 tumors with positive margins, and pT3 disease, and rising PSA above nadir);
• Oncotype DX, an RT-PCR assay for 12 prostate cancer genes and five housekeeping controls (recommended for post-biopsy evaluation of men with very-low-risk or low-risk prostate cancer who at the time of diagnosis have at least 10 years of life expectancy).
• ProMark, multiplex immunofluorescent staining of eight proteins, which has been shown to independently predict non–organ-confined pT3 disease or Gleason pattern 4 disease on radical prostatectomy (not reviewed).
Dr. Ross said that in his practice, he generally does not order molecular testing for surveillance of men older than 65 who have very-low-risk disease. For men with low-risk disease, however, molecular testing may help in clinical decision making to predict upgrading or disease progression.
“There’s limited data from surveillance populations, but these tests can be used in this context with retrospective data available, realizing that in most cases the tests will be confirmative, or another way of thinking about it is ‘noninformative,’ so there are some considerations about cost in that context,” he said. For men with intermediate or high-risk disease, however, currently available tests are not good at predicting what an individual patient’s response would be to a specific type of therapy, whether surgery, radiation, androgen deprivation, chemotherapy, or a combination.
“This is an area where predictive biomarkers would be very informative. There is ongoing research, and I think this is an area of potentially large advancement in how we risk-stratify our patients,” Dr. Ross said.
HOLLYWOOD, FLA. – Current evidence suggests that molecular tests for prostate cancer are prognostic and can help clinicians and patients with difficult treatment decisions. In the not-too-distant future, gene tests could also guide choice of therapies.
“I think that the largest impact is going to come in areas of both the greatest treatment uncertainty and areas where we can be predictive about the response to treatment,” said Dr. Ashley Ross, a urologic oncologist and pathologist at the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Baltimore.
Multigene panels may soon be able help identify which patients might benefit more from radical prostatectomy or radiation therapy, whether radiation therapy effects could be enhanced with the addition of androgen-deprivation therapy, and whether early use of docetaxel might add therapeutic benefit, he said at the annual conference of the National Comprehensive Cancer Network.
The 2016 iteration of the NCCN guidelines for the treatment of prostate cancer include a note stating that “men with clinically localized disease may consider the use of tumor-based molecular assays. Retrospective case cohort studies have shown that molecular assays performed on biopsy or prostatectomy specimens provide prognostic information independent of NCCN risk groups.”
The use of molecular assays may inform treatment decisions by helping to predict the likelihood of death if a patient is managed conservatively, risks for biochemical progression after radical prostatectomy or external beam therapy, and the likelihood that a patient could develop metastatic disease after radical prostatectomy or salvage radiotherapy, the guidelines say.
Dr. Ross reviewed the molecular biology of localized prostate cancer and the benefits and risks of currently available molecular tests.
“We’ve had an increased ability to get molecular information or genomic information from very limited amounts of routinely-stored pathologic tissue, and that’s resulted in the generation of many molecular-based tissue tests in prostate cancer. With the emergence of those tests and a lot of aggressive marketing, there has been a lot of confusion for patients and providers about whether we should use them or not and in what context,” he said.
Prostate cancer is genomically complex, even in the localized stage, with copy number alterations, deletions, and amplifications; chromosomal rearrangements; and point mutations, he said.
One of the best characterized genomic events is the early loss of the tumor suppressor gene PTEN (phosphatase and tensin homolog). This gene works within the PI3 kinase (PI3K)/AKT pathway. PI3K pathway mutations have been identified in up to 40% of all primary prostate cancers and 100% of mutations, Dr. Ross explained.
Loss of PTEN itself has been detected in about 15%-40% of primary prostate cancers and 50% of metastases, and the loss correlates with disease stage and tumor grade.
The NCCN guidelines list six available tissue-based tests for prostate cancer prognosis, including tests based on general cancer features such as cell-cycle proliferation, and those based on specific molecular features of cancer.
An example of the general type of test is the Ki-67 immunohistochemistry (IHC) test, which looks for a cellular marker of proliferation, and has been shown to have independent prognostic significance after radiation therapy or radical prostatectomy. This test is not currently recommended by the Medicare Molecular Diagnostic Services (MolDx) program, however.
Another general-type test is the Polaris quantitative reverse-transcriptase polymerase chain reaction (RT-PCR) panel, which tests for 31 cell-cycle-related genes and 15 “housekeeping” controls. This test is recommended for post-biopsy evaluation of men with very-low-risk or low-risk prostate cancer who at the time of diagnosis have at least 10 years of life expectancy. It has been shown to independently predict prostate cancer–specific mortality, biochemical failure/recurrence, and metastasis, the guidelines say.
Tests based on molecular features include:
• PTEN/ERG, an IHC or fluorescent in situ hybridization test that has been shown to predict prostate cancer–specific mortality, upgrading to Gleason pattern 4 on radical prostatectomy, and biochemical recurrence (not MolDx recommended).
• Decipher, a whole-transcriptome 1.4M RNA expression oligonucleotide microarray shown to predict biochemical failure, metastasis, and prostate cancer–specific mortality (recommended for postradical prostatectomy for patients with pT2 tumors with positive margins, and pT3 disease, and rising PSA above nadir);
• Oncotype DX, an RT-PCR assay for 12 prostate cancer genes and five housekeeping controls (recommended for post-biopsy evaluation of men with very-low-risk or low-risk prostate cancer who at the time of diagnosis have at least 10 years of life expectancy).
• ProMark, multiplex immunofluorescent staining of eight proteins, which has been shown to independently predict non–organ-confined pT3 disease or Gleason pattern 4 disease on radical prostatectomy (not reviewed).
Dr. Ross said that in his practice, he generally does not order molecular testing for surveillance of men older than 65 who have very-low-risk disease. For men with low-risk disease, however, molecular testing may help in clinical decision making to predict upgrading or disease progression.
“There’s limited data from surveillance populations, but these tests can be used in this context with retrospective data available, realizing that in most cases the tests will be confirmative, or another way of thinking about it is ‘noninformative,’ so there are some considerations about cost in that context,” he said. For men with intermediate or high-risk disease, however, currently available tests are not good at predicting what an individual patient’s response would be to a specific type of therapy, whether surgery, radiation, androgen deprivation, chemotherapy, or a combination.
“This is an area where predictive biomarkers would be very informative. There is ongoing research, and I think this is an area of potentially large advancement in how we risk-stratify our patients,” Dr. Ross said.
HOLLYWOOD, FLA. – Current evidence suggests that molecular tests for prostate cancer are prognostic and can help clinicians and patients with difficult treatment decisions. In the not-too-distant future, gene tests could also guide choice of therapies.
“I think that the largest impact is going to come in areas of both the greatest treatment uncertainty and areas where we can be predictive about the response to treatment,” said Dr. Ashley Ross, a urologic oncologist and pathologist at the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Baltimore.
Multigene panels may soon be able help identify which patients might benefit more from radical prostatectomy or radiation therapy, whether radiation therapy effects could be enhanced with the addition of androgen-deprivation therapy, and whether early use of docetaxel might add therapeutic benefit, he said at the annual conference of the National Comprehensive Cancer Network.
The 2016 iteration of the NCCN guidelines for the treatment of prostate cancer include a note stating that “men with clinically localized disease may consider the use of tumor-based molecular assays. Retrospective case cohort studies have shown that molecular assays performed on biopsy or prostatectomy specimens provide prognostic information independent of NCCN risk groups.”
The use of molecular assays may inform treatment decisions by helping to predict the likelihood of death if a patient is managed conservatively, risks for biochemical progression after radical prostatectomy or external beam therapy, and the likelihood that a patient could develop metastatic disease after radical prostatectomy or salvage radiotherapy, the guidelines say.
Dr. Ross reviewed the molecular biology of localized prostate cancer and the benefits and risks of currently available molecular tests.
“We’ve had an increased ability to get molecular information or genomic information from very limited amounts of routinely-stored pathologic tissue, and that’s resulted in the generation of many molecular-based tissue tests in prostate cancer. With the emergence of those tests and a lot of aggressive marketing, there has been a lot of confusion for patients and providers about whether we should use them or not and in what context,” he said.
Prostate cancer is genomically complex, even in the localized stage, with copy number alterations, deletions, and amplifications; chromosomal rearrangements; and point mutations, he said.
One of the best characterized genomic events is the early loss of the tumor suppressor gene PTEN (phosphatase and tensin homolog). This gene works within the PI3 kinase (PI3K)/AKT pathway. PI3K pathway mutations have been identified in up to 40% of all primary prostate cancers and 100% of mutations, Dr. Ross explained.
Loss of PTEN itself has been detected in about 15%-40% of primary prostate cancers and 50% of metastases, and the loss correlates with disease stage and tumor grade.
The NCCN guidelines list six available tissue-based tests for prostate cancer prognosis, including tests based on general cancer features such as cell-cycle proliferation, and those based on specific molecular features of cancer.
An example of the general type of test is the Ki-67 immunohistochemistry (IHC) test, which looks for a cellular marker of proliferation, and has been shown to have independent prognostic significance after radiation therapy or radical prostatectomy. This test is not currently recommended by the Medicare Molecular Diagnostic Services (MolDx) program, however.
Another general-type test is the Polaris quantitative reverse-transcriptase polymerase chain reaction (RT-PCR) panel, which tests for 31 cell-cycle-related genes and 15 “housekeeping” controls. This test is recommended for post-biopsy evaluation of men with very-low-risk or low-risk prostate cancer who at the time of diagnosis have at least 10 years of life expectancy. It has been shown to independently predict prostate cancer–specific mortality, biochemical failure/recurrence, and metastasis, the guidelines say.
Tests based on molecular features include:
• PTEN/ERG, an IHC or fluorescent in situ hybridization test that has been shown to predict prostate cancer–specific mortality, upgrading to Gleason pattern 4 on radical prostatectomy, and biochemical recurrence (not MolDx recommended).
• Decipher, a whole-transcriptome 1.4M RNA expression oligonucleotide microarray shown to predict biochemical failure, metastasis, and prostate cancer–specific mortality (recommended for postradical prostatectomy for patients with pT2 tumors with positive margins, and pT3 disease, and rising PSA above nadir);
• Oncotype DX, an RT-PCR assay for 12 prostate cancer genes and five housekeeping controls (recommended for post-biopsy evaluation of men with very-low-risk or low-risk prostate cancer who at the time of diagnosis have at least 10 years of life expectancy).
• ProMark, multiplex immunofluorescent staining of eight proteins, which has been shown to independently predict non–organ-confined pT3 disease or Gleason pattern 4 disease on radical prostatectomy (not reviewed).
Dr. Ross said that in his practice, he generally does not order molecular testing for surveillance of men older than 65 who have very-low-risk disease. For men with low-risk disease, however, molecular testing may help in clinical decision making to predict upgrading or disease progression.
“There’s limited data from surveillance populations, but these tests can be used in this context with retrospective data available, realizing that in most cases the tests will be confirmative, or another way of thinking about it is ‘noninformative,’ so there are some considerations about cost in that context,” he said. For men with intermediate or high-risk disease, however, currently available tests are not good at predicting what an individual patient’s response would be to a specific type of therapy, whether surgery, radiation, androgen deprivation, chemotherapy, or a combination.
“This is an area where predictive biomarkers would be very informative. There is ongoing research, and I think this is an area of potentially large advancement in how we risk-stratify our patients,” Dr. Ross said.
EXPERT ANALYSIS FROM THE NCCN ANNUAL CONFERENCE
Prostate cancer’s future seen in molecular tests
HOLLYWOOD, FLA. – Current evidence suggests that molecular tests for prostate cancer are prognostic and can help clinicians and patients with difficult treatment decisions. In the not-too-distant future, gene tests could also guide choice of therapies.
“I think that the largest impact is going to come in areas of both the greatest treatment uncertainty and areas where we can be predictive about the response to treatment,” said Dr. Ashley Ross, a urologic oncologist and pathologist at the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Baltimore.
Multigene panels may soon be able help identify which patients might benefit more from radical prostatectomy or radiation therapy, whether radiation therapy effects could be enhanced with the addition of androgen-deprivation therapy, and whether early use of docetaxel might add therapeutic benefit, he said at the annual conference of the National Comprehensive Cancer Network.
The 2016 iteration of the NCCN guidelines for the treatment of prostate cancer include a note stating that “men with clinically localized disease may consider the use of tumor-based molecular assays. Retrospective case cohort studies have shown that molecular assays performed on biopsy or prostatectomy specimens provide prognostic information independent of NCCN risk groups.”
The use of molecular assays may inform treatment decisions by helping to predict the likelihood of death if a patient is managed conservatively, risks for biochemical progression after radical prostatectomy or external beam therapy, and the likelihood that a patient could develop metastatic disease after radical prostatectomy or salvage radiotherapy, the guidelines say.
Dr. Ross reviewed the molecular biology of localized prostate cancer and the benefits and risks of currently available molecular tests.
“We’ve had an increased ability to get molecular information or genomic information from very limited amounts of routinely-stored pathologic tissue, and that’s resulted in the generation of many molecular-based tissue tests in prostate cancer. With the emergence of those tests and a lot of aggressive marketing, there has been a lot of confusion for patients and providers about whether we should use them or not and in what context,” he said.
Prostate cancer is genomically complex, even in the localized stage, with copy number alterations, deletions, and amplifications; chromosomal rearrangements; and point mutations, he said.
One of the best characterized genomic events is the early loss of the tumor suppressor gene PTEN (phosphatase and tensin homolog). This gene works within the PI3 kinase (PI3K)/AKT pathway. PI3K pathway mutations have been identified in up to 40% of all primary prostate cancers and 100% of mutations, Dr. Ross explained.
Loss of PTEN itself has been detected in about 15%-40% of primary prostate cancers and 50% of metastases, and the loss correlates with disease stage and tumor grade.
The NCCN guidelines list six available tissue-based tests for prostate cancer prognosis, including tests based on general cancer features such as cell-cycle proliferation, and those based on specific molecular features of cancer.
An example of the general type of test is the Ki-67 immunohistochemistry (IHC) test, which looks for a cellular marker of proliferation, and has been shown to have independent prognostic significance after radiation therapy or radical prostatectomy. This test is not currently recommended by the Medicare Molecular Diagnostic Services (MolDx) program, however.
Another general-type test is the Polaris quantitative reverse-transcriptase polymerase chain reaction (RT-PCR) panel, which tests for 31 cell-cycle-related genes and 15 “housekeeping” controls. This test is recommended for post-biopsy evaluation of men with very-low-risk or low-risk prostate cancer who at the time of diagnosis have at least 10 years of life expectancy. It has been shown to independently predict prostate cancer–specific mortality, biochemical failure/recurrence, and metastasis, the guidelines say.
Tests based on molecular features include:
• PTEN/ERG, an IHC or fluorescent in situ hybridization test that has been shown to predict prostate cancer–specific mortality, upgrading to Gleason pattern 4 on radical prostatectomy, and biochemical recurrence (not MolDx recommended).
• Decipher, a whole-transcriptome 1.4M RNA expression oligonucleotide microarray shown to predict biochemical failure, metastasis, and prostate cancer–specific mortality (recommended for postradical prostatectomy for patients with pT2 tumors with positive margins, and pT3 disease, and rising PSA above nadir);
• Oncotype DX, an RT-PCR assay for 12 prostate cancer genes and five housekeeping controls (recommended for post-biopsy evaluation of men with very-low-risk or low-risk prostate cancer who at the time of diagnosis have at least 10 years of life expectancy).
• ProMark, multiplex immunofluorescent staining of eight proteins, which has been shown to independently predict non–organ-confined pT3 disease or Gleason pattern 4 disease on radical prostatectomy (not reviewed).
Dr. Ross said that in his practice, he generally does not order molecular testing for surveillance of men older than 65 who have very-low-risk disease. For men with low-risk disease, however, molecular testing may help in clinical decision making to predict upgrading or disease progression.
“There’s limited data from surveillance populations, but these tests can be used in this context with retrospective data available, realizing that in most cases the tests will be confirmative, or another way of thinking about it is ‘noninformative,’ so there are some considerations about cost in that context,” he said. For men with intermediate or high-risk disease, however, currently available tests are not good at predicting what an individual patient’s response would be to a specific type of therapy, whether surgery, radiation, androgen deprivation, chemotherapy, or a combination.
“This is an area where predictive biomarkers would be very informative. There is ongoing research, and I think this is an area of potentially large advancement in how we risk-stratify our patients,” Dr. Ross said.
HOLLYWOOD, FLA. – Current evidence suggests that molecular tests for prostate cancer are prognostic and can help clinicians and patients with difficult treatment decisions. In the not-too-distant future, gene tests could also guide choice of therapies.
“I think that the largest impact is going to come in areas of both the greatest treatment uncertainty and areas where we can be predictive about the response to treatment,” said Dr. Ashley Ross, a urologic oncologist and pathologist at the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Baltimore.
Multigene panels may soon be able help identify which patients might benefit more from radical prostatectomy or radiation therapy, whether radiation therapy effects could be enhanced with the addition of androgen-deprivation therapy, and whether early use of docetaxel might add therapeutic benefit, he said at the annual conference of the National Comprehensive Cancer Network.
The 2016 iteration of the NCCN guidelines for the treatment of prostate cancer include a note stating that “men with clinically localized disease may consider the use of tumor-based molecular assays. Retrospective case cohort studies have shown that molecular assays performed on biopsy or prostatectomy specimens provide prognostic information independent of NCCN risk groups.”
The use of molecular assays may inform treatment decisions by helping to predict the likelihood of death if a patient is managed conservatively, risks for biochemical progression after radical prostatectomy or external beam therapy, and the likelihood that a patient could develop metastatic disease after radical prostatectomy or salvage radiotherapy, the guidelines say.
Dr. Ross reviewed the molecular biology of localized prostate cancer and the benefits and risks of currently available molecular tests.
“We’ve had an increased ability to get molecular information or genomic information from very limited amounts of routinely-stored pathologic tissue, and that’s resulted in the generation of many molecular-based tissue tests in prostate cancer. With the emergence of those tests and a lot of aggressive marketing, there has been a lot of confusion for patients and providers about whether we should use them or not and in what context,” he said.
Prostate cancer is genomically complex, even in the localized stage, with copy number alterations, deletions, and amplifications; chromosomal rearrangements; and point mutations, he said.
One of the best characterized genomic events is the early loss of the tumor suppressor gene PTEN (phosphatase and tensin homolog). This gene works within the PI3 kinase (PI3K)/AKT pathway. PI3K pathway mutations have been identified in up to 40% of all primary prostate cancers and 100% of mutations, Dr. Ross explained.
Loss of PTEN itself has been detected in about 15%-40% of primary prostate cancers and 50% of metastases, and the loss correlates with disease stage and tumor grade.
The NCCN guidelines list six available tissue-based tests for prostate cancer prognosis, including tests based on general cancer features such as cell-cycle proliferation, and those based on specific molecular features of cancer.
An example of the general type of test is the Ki-67 immunohistochemistry (IHC) test, which looks for a cellular marker of proliferation, and has been shown to have independent prognostic significance after radiation therapy or radical prostatectomy. This test is not currently recommended by the Medicare Molecular Diagnostic Services (MolDx) program, however.
Another general-type test is the Polaris quantitative reverse-transcriptase polymerase chain reaction (RT-PCR) panel, which tests for 31 cell-cycle-related genes and 15 “housekeeping” controls. This test is recommended for post-biopsy evaluation of men with very-low-risk or low-risk prostate cancer who at the time of diagnosis have at least 10 years of life expectancy. It has been shown to independently predict prostate cancer–specific mortality, biochemical failure/recurrence, and metastasis, the guidelines say.
Tests based on molecular features include:
• PTEN/ERG, an IHC or fluorescent in situ hybridization test that has been shown to predict prostate cancer–specific mortality, upgrading to Gleason pattern 4 on radical prostatectomy, and biochemical recurrence (not MolDx recommended).
• Decipher, a whole-transcriptome 1.4M RNA expression oligonucleotide microarray shown to predict biochemical failure, metastasis, and prostate cancer–specific mortality (recommended for postradical prostatectomy for patients with pT2 tumors with positive margins, and pT3 disease, and rising PSA above nadir);
• Oncotype DX, an RT-PCR assay for 12 prostate cancer genes and five housekeeping controls (recommended for post-biopsy evaluation of men with very-low-risk or low-risk prostate cancer who at the time of diagnosis have at least 10 years of life expectancy).
• ProMark, multiplex immunofluorescent staining of eight proteins, which has been shown to independently predict non–organ-confined pT3 disease or Gleason pattern 4 disease on radical prostatectomy (not reviewed).
Dr. Ross said that in his practice, he generally does not order molecular testing for surveillance of men older than 65 who have very-low-risk disease. For men with low-risk disease, however, molecular testing may help in clinical decision making to predict upgrading or disease progression.
“There’s limited data from surveillance populations, but these tests can be used in this context with retrospective data available, realizing that in most cases the tests will be confirmative, or another way of thinking about it is ‘noninformative,’ so there are some considerations about cost in that context,” he said. For men with intermediate or high-risk disease, however, currently available tests are not good at predicting what an individual patient’s response would be to a specific type of therapy, whether surgery, radiation, androgen deprivation, chemotherapy, or a combination.
“This is an area where predictive biomarkers would be very informative. There is ongoing research, and I think this is an area of potentially large advancement in how we risk-stratify our patients,” Dr. Ross said.
HOLLYWOOD, FLA. – Current evidence suggests that molecular tests for prostate cancer are prognostic and can help clinicians and patients with difficult treatment decisions. In the not-too-distant future, gene tests could also guide choice of therapies.
“I think that the largest impact is going to come in areas of both the greatest treatment uncertainty and areas where we can be predictive about the response to treatment,” said Dr. Ashley Ross, a urologic oncologist and pathologist at the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Baltimore.
Multigene panels may soon be able help identify which patients might benefit more from radical prostatectomy or radiation therapy, whether radiation therapy effects could be enhanced with the addition of androgen-deprivation therapy, and whether early use of docetaxel might add therapeutic benefit, he said at the annual conference of the National Comprehensive Cancer Network.
The 2016 iteration of the NCCN guidelines for the treatment of prostate cancer include a note stating that “men with clinically localized disease may consider the use of tumor-based molecular assays. Retrospective case cohort studies have shown that molecular assays performed on biopsy or prostatectomy specimens provide prognostic information independent of NCCN risk groups.”
The use of molecular assays may inform treatment decisions by helping to predict the likelihood of death if a patient is managed conservatively, risks for biochemical progression after radical prostatectomy or external beam therapy, and the likelihood that a patient could develop metastatic disease after radical prostatectomy or salvage radiotherapy, the guidelines say.
Dr. Ross reviewed the molecular biology of localized prostate cancer and the benefits and risks of currently available molecular tests.
“We’ve had an increased ability to get molecular information or genomic information from very limited amounts of routinely-stored pathologic tissue, and that’s resulted in the generation of many molecular-based tissue tests in prostate cancer. With the emergence of those tests and a lot of aggressive marketing, there has been a lot of confusion for patients and providers about whether we should use them or not and in what context,” he said.
Prostate cancer is genomically complex, even in the localized stage, with copy number alterations, deletions, and amplifications; chromosomal rearrangements; and point mutations, he said.
One of the best characterized genomic events is the early loss of the tumor suppressor gene PTEN (phosphatase and tensin homolog). This gene works within the PI3 kinase (PI3K)/AKT pathway. PI3K pathway mutations have been identified in up to 40% of all primary prostate cancers and 100% of mutations, Dr. Ross explained.
Loss of PTEN itself has been detected in about 15%-40% of primary prostate cancers and 50% of metastases, and the loss correlates with disease stage and tumor grade.
The NCCN guidelines list six available tissue-based tests for prostate cancer prognosis, including tests based on general cancer features such as cell-cycle proliferation, and those based on specific molecular features of cancer.
An example of the general type of test is the Ki-67 immunohistochemistry (IHC) test, which looks for a cellular marker of proliferation, and has been shown to have independent prognostic significance after radiation therapy or radical prostatectomy. This test is not currently recommended by the Medicare Molecular Diagnostic Services (MolDx) program, however.
Another general-type test is the Polaris quantitative reverse-transcriptase polymerase chain reaction (RT-PCR) panel, which tests for 31 cell-cycle-related genes and 15 “housekeeping” controls. This test is recommended for post-biopsy evaluation of men with very-low-risk or low-risk prostate cancer who at the time of diagnosis have at least 10 years of life expectancy. It has been shown to independently predict prostate cancer–specific mortality, biochemical failure/recurrence, and metastasis, the guidelines say.
Tests based on molecular features include:
• PTEN/ERG, an IHC or fluorescent in situ hybridization test that has been shown to predict prostate cancer–specific mortality, upgrading to Gleason pattern 4 on radical prostatectomy, and biochemical recurrence (not MolDx recommended).
• Decipher, a whole-transcriptome 1.4M RNA expression oligonucleotide microarray shown to predict biochemical failure, metastasis, and prostate cancer–specific mortality (recommended for postradical prostatectomy for patients with pT2 tumors with positive margins, and pT3 disease, and rising PSA above nadir);
• Oncotype DX, an RT-PCR assay for 12 prostate cancer genes and five housekeeping controls (recommended for post-biopsy evaluation of men with very-low-risk or low-risk prostate cancer who at the time of diagnosis have at least 10 years of life expectancy).
• ProMark, multiplex immunofluorescent staining of eight proteins, which has been shown to independently predict non–organ-confined pT3 disease or Gleason pattern 4 disease on radical prostatectomy (not reviewed).
Dr. Ross said that in his practice, he generally does not order molecular testing for surveillance of men older than 65 who have very-low-risk disease. For men with low-risk disease, however, molecular testing may help in clinical decision making to predict upgrading or disease progression.
“There’s limited data from surveillance populations, but these tests can be used in this context with retrospective data available, realizing that in most cases the tests will be confirmative, or another way of thinking about it is ‘noninformative,’ so there are some considerations about cost in that context,” he said. For men with intermediate or high-risk disease, however, currently available tests are not good at predicting what an individual patient’s response would be to a specific type of therapy, whether surgery, radiation, androgen deprivation, chemotherapy, or a combination.
“This is an area where predictive biomarkers would be very informative. There is ongoing research, and I think this is an area of potentially large advancement in how we risk-stratify our patients,” Dr. Ross said.
EXPERT ANALYSIS FROM THE NCCN ANNUAL CONFERENCE
Surgical issues top reasons for readmissions after hysterectomy
INDIAN WELLS, CALIF. – Unplanned, 30-day readmissions after hysterectomy for benign indications mainly occur because of surgical complications, regardless of approach, with the most common issue being surgical site infections.
Additionally, there is an increased vulnerability to readmission shortly after discharge, especially within the first 15 days.
Those are the key findings from an analysis of the American College of Surgeons National Surgical Quality Improvement Project (ACS NSQIP) database participant user file for 2012 and 2013, presented by Dr. Courtney Penn at the annual scientific meeting of the Society of Gynecologic Surgeons.
“A seminal article in 2009 found that one in five Medicare patients are readmitted within 30 days, and unplanned readmissions account for 17% of total hospital payments from Medicare, or $17.4 billion annually,” said Dr. Penn, the lead study author and a resident in the department of obstetrics and gynecology at the University of Michigan, Ann Arbor. “Thus, addressing the problem of hospital readmissions is viewed as a golden opportunity to reduce healthcare costs and improve patient care quality. Despite this national focus on hospital readmissions, little is known about readmissions after hysterectomy.”
In an effort to characterize the most common reasons for unplanned 30-day readmissions following hysterectomy, and to characterize the timing of readmissions, the researchers retrospectively evaluated data from the ACS NSQIP database participant user file for 2012 and 2013. After using the International Classification of Diseases, Ninth Revision, Clinical Modification to identify common readmission diagnoses, they divided reasons for readmission into several categories: surgical site infection, surgical injury, non-infectious wound complications, gastrointestinal, genitourinary, venous thromboembolic, pain, medical, and “other” reasons. Results were stratified based on surgical approach.
Dr. Penn reported results from 40,580 patients who underwent hysterectomies at hospitals that participated in the ACS NSQIP. The overall, unadjusted readmission rate following hysterectomy was 2.8%, and was highest among those who underwent the procedure by abdominal approach (3.7%), followed by those who underwent the procedure by laparoscopic and vaginal approaches (2.6% vs. 2.1%, respectively).
After adjusting for potential confounding factors such as age, race, BMI, and operative time, readmissions were not significantly more likely when performed laparoscopically than with the vaginal approach. However, readmissions were significantly more likely when hysterectomy was performed via the open abdominal route, compared with the vaginal approach.
When categorizing reasons for reasons for readmission, traditional surgical complications, including surgical site infection, visceral entities, and non-infectious wound complications, were more common reasons for readmission than traditional medical complications, such as venous thromboembolism, myocardial infarction, and pulmonary edema. Slightly more than half of all readmissions (52%) were surgical in nature, compared with 9% that were attributable to traditional medical complications.
“This trend held true regardless of surgical approach, whether vaginal, laparoscopic, or abdominal,” Dr. Penn said.
Surgical site infections were the most common primary readmission diagnosis overall. “It was the underlying reason for readmission in approximately one-third of total readmissions,” she said. It was also the most common reason for readmission diagnosis for each surgical approach: 37% of abdominal, 28% of laparoscopic, and 33% of vaginal hysterectomy readmissions had a surgical site infection as the primary readmission diagnosis.
The researchers observed a few differences on reasons for readmission based on surgical approach. For example, surgical injury – such as hematoma and visceral injury – was higher after laparoscopic and vaginal hysterectomy, compared with that observed for abdominal cases (odds ratio, 2.4 and 2.8, respectively). Additionally, the proportion of readmissions related to gastrointestinal complications was higher after abdominal hysterectomies, compared with that observed among laparoscopic and vaginal cases (OR, 2.4 and 2.8, respectively).
For all surgical approaches, there was an increased likelihood of unplanned readmission within the first 15 days of discharge. In fact, 82% of all readmissions occurred within the first 15 days after discharge.
“We found that all major readmissions categories had a median time to readmission within the first 10 days after discharge, and the median time to readmission varied based on readmission diagnosis,” Dr. Penn said at the meeting, which was jointly sponsored by the American College of Surgeons. “Pain-related reasons for readmission had the shortest time to readmission, with a median of 3 days, and non-infectious wound complications had the longest time to readmission, with a median of 10 days.”
She acknowledged certain limitations of the study including the retrospective design, the database’s over-representation of urban and academic medical centers, as well the study’s reliance on one readmission diagnosis to capture the principal cause of readmission, “when the true reason for readmission may be multifactorial.”
Dr. Penn reported having no financial disclosures.
INDIAN WELLS, CALIF. – Unplanned, 30-day readmissions after hysterectomy for benign indications mainly occur because of surgical complications, regardless of approach, with the most common issue being surgical site infections.
Additionally, there is an increased vulnerability to readmission shortly after discharge, especially within the first 15 days.
Those are the key findings from an analysis of the American College of Surgeons National Surgical Quality Improvement Project (ACS NSQIP) database participant user file for 2012 and 2013, presented by Dr. Courtney Penn at the annual scientific meeting of the Society of Gynecologic Surgeons.
“A seminal article in 2009 found that one in five Medicare patients are readmitted within 30 days, and unplanned readmissions account for 17% of total hospital payments from Medicare, or $17.4 billion annually,” said Dr. Penn, the lead study author and a resident in the department of obstetrics and gynecology at the University of Michigan, Ann Arbor. “Thus, addressing the problem of hospital readmissions is viewed as a golden opportunity to reduce healthcare costs and improve patient care quality. Despite this national focus on hospital readmissions, little is known about readmissions after hysterectomy.”
In an effort to characterize the most common reasons for unplanned 30-day readmissions following hysterectomy, and to characterize the timing of readmissions, the researchers retrospectively evaluated data from the ACS NSQIP database participant user file for 2012 and 2013. After using the International Classification of Diseases, Ninth Revision, Clinical Modification to identify common readmission diagnoses, they divided reasons for readmission into several categories: surgical site infection, surgical injury, non-infectious wound complications, gastrointestinal, genitourinary, venous thromboembolic, pain, medical, and “other” reasons. Results were stratified based on surgical approach.
Dr. Penn reported results from 40,580 patients who underwent hysterectomies at hospitals that participated in the ACS NSQIP. The overall, unadjusted readmission rate following hysterectomy was 2.8%, and was highest among those who underwent the procedure by abdominal approach (3.7%), followed by those who underwent the procedure by laparoscopic and vaginal approaches (2.6% vs. 2.1%, respectively).
After adjusting for potential confounding factors such as age, race, BMI, and operative time, readmissions were not significantly more likely when performed laparoscopically than with the vaginal approach. However, readmissions were significantly more likely when hysterectomy was performed via the open abdominal route, compared with the vaginal approach.
When categorizing reasons for reasons for readmission, traditional surgical complications, including surgical site infection, visceral entities, and non-infectious wound complications, were more common reasons for readmission than traditional medical complications, such as venous thromboembolism, myocardial infarction, and pulmonary edema. Slightly more than half of all readmissions (52%) were surgical in nature, compared with 9% that were attributable to traditional medical complications.
“This trend held true regardless of surgical approach, whether vaginal, laparoscopic, or abdominal,” Dr. Penn said.
Surgical site infections were the most common primary readmission diagnosis overall. “It was the underlying reason for readmission in approximately one-third of total readmissions,” she said. It was also the most common reason for readmission diagnosis for each surgical approach: 37% of abdominal, 28% of laparoscopic, and 33% of vaginal hysterectomy readmissions had a surgical site infection as the primary readmission diagnosis.
The researchers observed a few differences on reasons for readmission based on surgical approach. For example, surgical injury – such as hematoma and visceral injury – was higher after laparoscopic and vaginal hysterectomy, compared with that observed for abdominal cases (odds ratio, 2.4 and 2.8, respectively). Additionally, the proportion of readmissions related to gastrointestinal complications was higher after abdominal hysterectomies, compared with that observed among laparoscopic and vaginal cases (OR, 2.4 and 2.8, respectively).
For all surgical approaches, there was an increased likelihood of unplanned readmission within the first 15 days of discharge. In fact, 82% of all readmissions occurred within the first 15 days after discharge.
“We found that all major readmissions categories had a median time to readmission within the first 10 days after discharge, and the median time to readmission varied based on readmission diagnosis,” Dr. Penn said at the meeting, which was jointly sponsored by the American College of Surgeons. “Pain-related reasons for readmission had the shortest time to readmission, with a median of 3 days, and non-infectious wound complications had the longest time to readmission, with a median of 10 days.”
She acknowledged certain limitations of the study including the retrospective design, the database’s over-representation of urban and academic medical centers, as well the study’s reliance on one readmission diagnosis to capture the principal cause of readmission, “when the true reason for readmission may be multifactorial.”
Dr. Penn reported having no financial disclosures.
INDIAN WELLS, CALIF. – Unplanned, 30-day readmissions after hysterectomy for benign indications mainly occur because of surgical complications, regardless of approach, with the most common issue being surgical site infections.
Additionally, there is an increased vulnerability to readmission shortly after discharge, especially within the first 15 days.
Those are the key findings from an analysis of the American College of Surgeons National Surgical Quality Improvement Project (ACS NSQIP) database participant user file for 2012 and 2013, presented by Dr. Courtney Penn at the annual scientific meeting of the Society of Gynecologic Surgeons.
“A seminal article in 2009 found that one in five Medicare patients are readmitted within 30 days, and unplanned readmissions account for 17% of total hospital payments from Medicare, or $17.4 billion annually,” said Dr. Penn, the lead study author and a resident in the department of obstetrics and gynecology at the University of Michigan, Ann Arbor. “Thus, addressing the problem of hospital readmissions is viewed as a golden opportunity to reduce healthcare costs and improve patient care quality. Despite this national focus on hospital readmissions, little is known about readmissions after hysterectomy.”
In an effort to characterize the most common reasons for unplanned 30-day readmissions following hysterectomy, and to characterize the timing of readmissions, the researchers retrospectively evaluated data from the ACS NSQIP database participant user file for 2012 and 2013. After using the International Classification of Diseases, Ninth Revision, Clinical Modification to identify common readmission diagnoses, they divided reasons for readmission into several categories: surgical site infection, surgical injury, non-infectious wound complications, gastrointestinal, genitourinary, venous thromboembolic, pain, medical, and “other” reasons. Results were stratified based on surgical approach.
Dr. Penn reported results from 40,580 patients who underwent hysterectomies at hospitals that participated in the ACS NSQIP. The overall, unadjusted readmission rate following hysterectomy was 2.8%, and was highest among those who underwent the procedure by abdominal approach (3.7%), followed by those who underwent the procedure by laparoscopic and vaginal approaches (2.6% vs. 2.1%, respectively).
After adjusting for potential confounding factors such as age, race, BMI, and operative time, readmissions were not significantly more likely when performed laparoscopically than with the vaginal approach. However, readmissions were significantly more likely when hysterectomy was performed via the open abdominal route, compared with the vaginal approach.
When categorizing reasons for reasons for readmission, traditional surgical complications, including surgical site infection, visceral entities, and non-infectious wound complications, were more common reasons for readmission than traditional medical complications, such as venous thromboembolism, myocardial infarction, and pulmonary edema. Slightly more than half of all readmissions (52%) were surgical in nature, compared with 9% that were attributable to traditional medical complications.
“This trend held true regardless of surgical approach, whether vaginal, laparoscopic, or abdominal,” Dr. Penn said.
Surgical site infections were the most common primary readmission diagnosis overall. “It was the underlying reason for readmission in approximately one-third of total readmissions,” she said. It was also the most common reason for readmission diagnosis for each surgical approach: 37% of abdominal, 28% of laparoscopic, and 33% of vaginal hysterectomy readmissions had a surgical site infection as the primary readmission diagnosis.
The researchers observed a few differences on reasons for readmission based on surgical approach. For example, surgical injury – such as hematoma and visceral injury – was higher after laparoscopic and vaginal hysterectomy, compared with that observed for abdominal cases (odds ratio, 2.4 and 2.8, respectively). Additionally, the proportion of readmissions related to gastrointestinal complications was higher after abdominal hysterectomies, compared with that observed among laparoscopic and vaginal cases (OR, 2.4 and 2.8, respectively).
For all surgical approaches, there was an increased likelihood of unplanned readmission within the first 15 days of discharge. In fact, 82% of all readmissions occurred within the first 15 days after discharge.
“We found that all major readmissions categories had a median time to readmission within the first 10 days after discharge, and the median time to readmission varied based on readmission diagnosis,” Dr. Penn said at the meeting, which was jointly sponsored by the American College of Surgeons. “Pain-related reasons for readmission had the shortest time to readmission, with a median of 3 days, and non-infectious wound complications had the longest time to readmission, with a median of 10 days.”
She acknowledged certain limitations of the study including the retrospective design, the database’s over-representation of urban and academic medical centers, as well the study’s reliance on one readmission diagnosis to capture the principal cause of readmission, “when the true reason for readmission may be multifactorial.”
Dr. Penn reported having no financial disclosures.
AT SGS 2016
Key clinical point: More than half of readmissions following hysterectomy were for surgical reasons.
Major finding: Slightly more than half of all readmissions (52%) were attributed to surgical complications, compared with 9% for medical complications.
Data source: A retrospective review of 40,580 patients who underwent hysterectomies at hospitals nationwide.
Disclosures: Dr. Penn reported having no financial disclosures.