User login
Official Newspaper of the American College of Surgeons
Feds aim to clarify regs on copay free contraception
The Obama administration issued a proposed a rule Feb. 1 clarifying how certain religious organizations could provide copayment free contraceptive coverage under the Affordable Care Act.
The proposed rule comes after months of controversy and several lawsuits from institutions that opposed having to provide contraceptive services based on their religious beliefs. During a press briefing Feb. 1, federal officials would not comment on whether the lawsuits prompted the policy change.
"Today, the administration is taking the next step in providing women across the nation with coverage of recommended preventive care at no cost, while respecting religious concerns," Health and Human Services Secretary Kathleen Sebelius said in a statement. "We will continue to work with faith-based organizations, women’s organizations, insurers, and others to achieve these goals."
The Family Research Council came out in strong opposition to the proposed regulation, saying that it did nothing to change the current policy and that it did not protect women’s health.
"The accounting gimmicks HHS is now proposing under the latest [proposed] regulation fail to satisfy the religious freedom protections that exist in other current laws and in the First Amendment of the U.S. Constitution," Anna Higgins, director of FRC’s Center for Human Dignity, said in a statement.
Meanwhile, organizations such as ACLU and Planned Parenthood Federation of America voiced support for the proposed rule.
"This policy delivers on the promise of women having access to birth control without co-pays no matter where they work," said Cecile Richards, president of Planned Parenthood Federation of America, in a statement. "This policy makes it clear that your boss does not get to decide whether you can have birth control."
Under the proposed rule, nonprofit religious organization, including nonprofit religious hospitals and higher education institutions, can receive an accommodation that would provide their employees enrolled in the health plans they sponsor with separate contraceptive coverage, with no copays, and at no cost to the organization.
Religious organizations would provide a notice to their insurer or third-party administrator, and in turn, the insurer or third-party administrator would provide the enrollees with copay-free contraceptive coverage through separate individual health insurance policies.
HHS officials said that they have not estimated the cost of the proposal, but it will be subsidized mostly by user fees that insurers will have to pay to participate in insurance exchanges. They said they didn’t expect the regulation to affect federal funds, because preventive services would lead to overall cost reduction.
Under the proposed rule, religious employers are not required to provide copay-free coverage for contraceptives or other services that violate their beliefs. HHS officials clarified the definition of "religious employer" by using a tax code primarily including churches, other houses of worship, and their affiliated organizations.
The proposed rule is open for public comment through April 8 this year. Comments can be given at www.regulations.gov.
On Twitter @NaseemSMiller
The Obama administration issued a proposed a rule Feb. 1 clarifying how certain religious organizations could provide copayment free contraceptive coverage under the Affordable Care Act.
The proposed rule comes after months of controversy and several lawsuits from institutions that opposed having to provide contraceptive services based on their religious beliefs. During a press briefing Feb. 1, federal officials would not comment on whether the lawsuits prompted the policy change.
"Today, the administration is taking the next step in providing women across the nation with coverage of recommended preventive care at no cost, while respecting religious concerns," Health and Human Services Secretary Kathleen Sebelius said in a statement. "We will continue to work with faith-based organizations, women’s organizations, insurers, and others to achieve these goals."
The Family Research Council came out in strong opposition to the proposed regulation, saying that it did nothing to change the current policy and that it did not protect women’s health.
"The accounting gimmicks HHS is now proposing under the latest [proposed] regulation fail to satisfy the religious freedom protections that exist in other current laws and in the First Amendment of the U.S. Constitution," Anna Higgins, director of FRC’s Center for Human Dignity, said in a statement.
Meanwhile, organizations such as ACLU and Planned Parenthood Federation of America voiced support for the proposed rule.
"This policy delivers on the promise of women having access to birth control without co-pays no matter where they work," said Cecile Richards, president of Planned Parenthood Federation of America, in a statement. "This policy makes it clear that your boss does not get to decide whether you can have birth control."
Under the proposed rule, nonprofit religious organization, including nonprofit religious hospitals and higher education institutions, can receive an accommodation that would provide their employees enrolled in the health plans they sponsor with separate contraceptive coverage, with no copays, and at no cost to the organization.
Religious organizations would provide a notice to their insurer or third-party administrator, and in turn, the insurer or third-party administrator would provide the enrollees with copay-free contraceptive coverage through separate individual health insurance policies.
HHS officials said that they have not estimated the cost of the proposal, but it will be subsidized mostly by user fees that insurers will have to pay to participate in insurance exchanges. They said they didn’t expect the regulation to affect federal funds, because preventive services would lead to overall cost reduction.
Under the proposed rule, religious employers are not required to provide copay-free coverage for contraceptives or other services that violate their beliefs. HHS officials clarified the definition of "religious employer" by using a tax code primarily including churches, other houses of worship, and their affiliated organizations.
The proposed rule is open for public comment through April 8 this year. Comments can be given at www.regulations.gov.
On Twitter @NaseemSMiller
The Obama administration issued a proposed a rule Feb. 1 clarifying how certain religious organizations could provide copayment free contraceptive coverage under the Affordable Care Act.
The proposed rule comes after months of controversy and several lawsuits from institutions that opposed having to provide contraceptive services based on their religious beliefs. During a press briefing Feb. 1, federal officials would not comment on whether the lawsuits prompted the policy change.
"Today, the administration is taking the next step in providing women across the nation with coverage of recommended preventive care at no cost, while respecting religious concerns," Health and Human Services Secretary Kathleen Sebelius said in a statement. "We will continue to work with faith-based organizations, women’s organizations, insurers, and others to achieve these goals."
The Family Research Council came out in strong opposition to the proposed regulation, saying that it did nothing to change the current policy and that it did not protect women’s health.
"The accounting gimmicks HHS is now proposing under the latest [proposed] regulation fail to satisfy the religious freedom protections that exist in other current laws and in the First Amendment of the U.S. Constitution," Anna Higgins, director of FRC’s Center for Human Dignity, said in a statement.
Meanwhile, organizations such as ACLU and Planned Parenthood Federation of America voiced support for the proposed rule.
"This policy delivers on the promise of women having access to birth control without co-pays no matter where they work," said Cecile Richards, president of Planned Parenthood Federation of America, in a statement. "This policy makes it clear that your boss does not get to decide whether you can have birth control."
Under the proposed rule, nonprofit religious organization, including nonprofit religious hospitals and higher education institutions, can receive an accommodation that would provide their employees enrolled in the health plans they sponsor with separate contraceptive coverage, with no copays, and at no cost to the organization.
Religious organizations would provide a notice to their insurer or third-party administrator, and in turn, the insurer or third-party administrator would provide the enrollees with copay-free contraceptive coverage through separate individual health insurance policies.
HHS officials said that they have not estimated the cost of the proposal, but it will be subsidized mostly by user fees that insurers will have to pay to participate in insurance exchanges. They said they didn’t expect the regulation to affect federal funds, because preventive services would lead to overall cost reduction.
Under the proposed rule, religious employers are not required to provide copay-free coverage for contraceptives or other services that violate their beliefs. HHS officials clarified the definition of "religious employer" by using a tax code primarily including churches, other houses of worship, and their affiliated organizations.
The proposed rule is open for public comment through April 8 this year. Comments can be given at www.regulations.gov.
On Twitter @NaseemSMiller
Implementing Health Reform: Boosting clinical trial participation
Following the lead of Medicare and several states, the Affordable Care Act guarantees insurance coverage for individuals participating in clinical trials for the treatment of cancer and other life-threatening diseases.
Under the 2010 health law, health plans offering individual or group coverage cannot bar participation in clinical trials and cannot discriminate against patients who take part in trials. Health plans must cover the routine patient costs associated with participation in certain clinical trials. The plans do not, however, need to cover the investigational drug or device or services provided solely to satisfy data collection and analysis needs.
The policy applies to all phase I-IV clinical trials that are conducted for the prevention, detection, or treatment of cancer or other life-threatening diseases, including federally funded trials, investigational new drug applications reviewed by the Food and Drug Administration, or drug trials that are exempt from having an investigational new drug application.
The new federal policy, which takes effect on Jan. 1, 2014, sets a minimum standard of coverage and permits more expansive state coverage laws to continue.
Dr. Sandra M. Swain, president of the American Society of Clinical Oncology (ASCO), is an expert in the field of inflammatory breast cancer treatment and has led more than 20 clinical trials. She explained how the policy change is likely to impact clinical trial participation.
Question: How many states already mandate coverage of clinical trials and do the laws vary?
Dr. Swain: Twenty-nine states and the District of Columbia have laws and six states have voluntary agreements with insurers to provide coverage. The laws vary tremendously. The laws and agreements do not cover plans for self-insured, large-employer plans (or so-called ERISA plans) because they are regulated by federal, not state law.
Question: Will this new federal policy follow Medicare’s example and covering the treatment of complications in clinical trials?
Dr. Swain: The ACA statute does not specifically mention coverage of complications. ASCO led a coalition of 19 cancer organizations in advocating for the Centers for Medicare and Medicaid Services (CMS) – the federal agency in charge of drafting the implementing regulations – to require in those regulations that insurers cover complications. The coalition submitted proposed regulatory language on this and a number of other issues and met with CMS. We are waiting on the draft regulations.
Question: Is the current lack of insurance coverage for clinical trials a significant barrier to participation?
Dr. Swain: Our members have cited this as a major concern. Health plans do not always deny coverage, but they often don’t make coverage explicit and there is a lot of paperwork and time delays. This can make it difficult for patients to enroll in trials in a timely manner. Some patients also choose not to consider trial participation when they learn that their health plan may not provide coverage. An analysis from Johns Hopkins University, Baltimore, provides the most recent data.
Question: Since this doesn’t apply to grandfathered health plans, how much of an impact is it likely to have?
Dr. Swain: When the Office of Management and Budget released a rule on grandfathered plans in June 2010, it also estimated how many plans would relinquish their grandfathered status by 2013. The conservative estimate is 39% while the high-end estimate is 69%. As time goes on, the number of plans that lose their status will increase, thereby also increasing the effect of the provision.
Question: What will the impact be on cancer research and patients?
Dr. Swain: We’re hoping it will help make it easier to participate in clinical trials. Perhaps our outreach to ASCO members and patients about the provision will increase awareness. Anything that makes it easier to participate in research will ultimately help bring new treatments to our patients.
Question: What will need to be addressed when the Department of Health and Human Services issues regulations on this provision?
Dr. Swain: The statutory language about which trials are covered is very clear. Federally funded trials (including those funded by Cooperative Groups and National Cancer Institute–designated oncology centers) for the prevention, detection, or treatment of cancer are covered – including all phases of trials (I-IV). In addition, these same types of trials that are privately sponsored are covered if they are regulated by the FDA under an investigational new drug (INDA) application or if they meet requirements to be INDA exempt. We are working with the federal government to make the coverage process as timely and straightforward as possible. We developed a standard form that could be sent to any insurer to confirm that a trial meets the coverage requirements. We are hopeful that the federal government will promote use of this type of streamlined process. It is crucial that we help patients obtain a clear coverage answer as quickly as possible.
Dr. Swain is the president of ASCO and the medical director of the Washington Cancer Institute at the MedStar Washington Hospital Center.
Following the lead of Medicare and several states, the Affordable Care Act guarantees insurance coverage for individuals participating in clinical trials for the treatment of cancer and other life-threatening diseases.
Under the 2010 health law, health plans offering individual or group coverage cannot bar participation in clinical trials and cannot discriminate against patients who take part in trials. Health plans must cover the routine patient costs associated with participation in certain clinical trials. The plans do not, however, need to cover the investigational drug or device or services provided solely to satisfy data collection and analysis needs.
The policy applies to all phase I-IV clinical trials that are conducted for the prevention, detection, or treatment of cancer or other life-threatening diseases, including federally funded trials, investigational new drug applications reviewed by the Food and Drug Administration, or drug trials that are exempt from having an investigational new drug application.
The new federal policy, which takes effect on Jan. 1, 2014, sets a minimum standard of coverage and permits more expansive state coverage laws to continue.
Dr. Sandra M. Swain, president of the American Society of Clinical Oncology (ASCO), is an expert in the field of inflammatory breast cancer treatment and has led more than 20 clinical trials. She explained how the policy change is likely to impact clinical trial participation.
Question: How many states already mandate coverage of clinical trials and do the laws vary?
Dr. Swain: Twenty-nine states and the District of Columbia have laws and six states have voluntary agreements with insurers to provide coverage. The laws vary tremendously. The laws and agreements do not cover plans for self-insured, large-employer plans (or so-called ERISA plans) because they are regulated by federal, not state law.
Question: Will this new federal policy follow Medicare’s example and covering the treatment of complications in clinical trials?
Dr. Swain: The ACA statute does not specifically mention coverage of complications. ASCO led a coalition of 19 cancer organizations in advocating for the Centers for Medicare and Medicaid Services (CMS) – the federal agency in charge of drafting the implementing regulations – to require in those regulations that insurers cover complications. The coalition submitted proposed regulatory language on this and a number of other issues and met with CMS. We are waiting on the draft regulations.
Question: Is the current lack of insurance coverage for clinical trials a significant barrier to participation?
Dr. Swain: Our members have cited this as a major concern. Health plans do not always deny coverage, but they often don’t make coverage explicit and there is a lot of paperwork and time delays. This can make it difficult for patients to enroll in trials in a timely manner. Some patients also choose not to consider trial participation when they learn that their health plan may not provide coverage. An analysis from Johns Hopkins University, Baltimore, provides the most recent data.
Question: Since this doesn’t apply to grandfathered health plans, how much of an impact is it likely to have?
Dr. Swain: When the Office of Management and Budget released a rule on grandfathered plans in June 2010, it also estimated how many plans would relinquish their grandfathered status by 2013. The conservative estimate is 39% while the high-end estimate is 69%. As time goes on, the number of plans that lose their status will increase, thereby also increasing the effect of the provision.
Question: What will the impact be on cancer research and patients?
Dr. Swain: We’re hoping it will help make it easier to participate in clinical trials. Perhaps our outreach to ASCO members and patients about the provision will increase awareness. Anything that makes it easier to participate in research will ultimately help bring new treatments to our patients.
Question: What will need to be addressed when the Department of Health and Human Services issues regulations on this provision?
Dr. Swain: The statutory language about which trials are covered is very clear. Federally funded trials (including those funded by Cooperative Groups and National Cancer Institute–designated oncology centers) for the prevention, detection, or treatment of cancer are covered – including all phases of trials (I-IV). In addition, these same types of trials that are privately sponsored are covered if they are regulated by the FDA under an investigational new drug (INDA) application or if they meet requirements to be INDA exempt. We are working with the federal government to make the coverage process as timely and straightforward as possible. We developed a standard form that could be sent to any insurer to confirm that a trial meets the coverage requirements. We are hopeful that the federal government will promote use of this type of streamlined process. It is crucial that we help patients obtain a clear coverage answer as quickly as possible.
Dr. Swain is the president of ASCO and the medical director of the Washington Cancer Institute at the MedStar Washington Hospital Center.
Following the lead of Medicare and several states, the Affordable Care Act guarantees insurance coverage for individuals participating in clinical trials for the treatment of cancer and other life-threatening diseases.
Under the 2010 health law, health plans offering individual or group coverage cannot bar participation in clinical trials and cannot discriminate against patients who take part in trials. Health plans must cover the routine patient costs associated with participation in certain clinical trials. The plans do not, however, need to cover the investigational drug or device or services provided solely to satisfy data collection and analysis needs.
The policy applies to all phase I-IV clinical trials that are conducted for the prevention, detection, or treatment of cancer or other life-threatening diseases, including federally funded trials, investigational new drug applications reviewed by the Food and Drug Administration, or drug trials that are exempt from having an investigational new drug application.
The new federal policy, which takes effect on Jan. 1, 2014, sets a minimum standard of coverage and permits more expansive state coverage laws to continue.
Dr. Sandra M. Swain, president of the American Society of Clinical Oncology (ASCO), is an expert in the field of inflammatory breast cancer treatment and has led more than 20 clinical trials. She explained how the policy change is likely to impact clinical trial participation.
Question: How many states already mandate coverage of clinical trials and do the laws vary?
Dr. Swain: Twenty-nine states and the District of Columbia have laws and six states have voluntary agreements with insurers to provide coverage. The laws vary tremendously. The laws and agreements do not cover plans for self-insured, large-employer plans (or so-called ERISA plans) because they are regulated by federal, not state law.
Question: Will this new federal policy follow Medicare’s example and covering the treatment of complications in clinical trials?
Dr. Swain: The ACA statute does not specifically mention coverage of complications. ASCO led a coalition of 19 cancer organizations in advocating for the Centers for Medicare and Medicaid Services (CMS) – the federal agency in charge of drafting the implementing regulations – to require in those regulations that insurers cover complications. The coalition submitted proposed regulatory language on this and a number of other issues and met with CMS. We are waiting on the draft regulations.
Question: Is the current lack of insurance coverage for clinical trials a significant barrier to participation?
Dr. Swain: Our members have cited this as a major concern. Health plans do not always deny coverage, but they often don’t make coverage explicit and there is a lot of paperwork and time delays. This can make it difficult for patients to enroll in trials in a timely manner. Some patients also choose not to consider trial participation when they learn that their health plan may not provide coverage. An analysis from Johns Hopkins University, Baltimore, provides the most recent data.
Question: Since this doesn’t apply to grandfathered health plans, how much of an impact is it likely to have?
Dr. Swain: When the Office of Management and Budget released a rule on grandfathered plans in June 2010, it also estimated how many plans would relinquish their grandfathered status by 2013. The conservative estimate is 39% while the high-end estimate is 69%. As time goes on, the number of plans that lose their status will increase, thereby also increasing the effect of the provision.
Question: What will the impact be on cancer research and patients?
Dr. Swain: We’re hoping it will help make it easier to participate in clinical trials. Perhaps our outreach to ASCO members and patients about the provision will increase awareness. Anything that makes it easier to participate in research will ultimately help bring new treatments to our patients.
Question: What will need to be addressed when the Department of Health and Human Services issues regulations on this provision?
Dr. Swain: The statutory language about which trials are covered is very clear. Federally funded trials (including those funded by Cooperative Groups and National Cancer Institute–designated oncology centers) for the prevention, detection, or treatment of cancer are covered – including all phases of trials (I-IV). In addition, these same types of trials that are privately sponsored are covered if they are regulated by the FDA under an investigational new drug (INDA) application or if they meet requirements to be INDA exempt. We are working with the federal government to make the coverage process as timely and straightforward as possible. We developed a standard form that could be sent to any insurer to confirm that a trial meets the coverage requirements. We are hopeful that the federal government will promote use of this type of streamlined process. It is crucial that we help patients obtain a clear coverage answer as quickly as possible.
Dr. Swain is the president of ASCO and the medical director of the Washington Cancer Institute at the MedStar Washington Hospital Center.
Lasers expand options for vascular lesion treatment
LAS VEGAS – The 595-nm pulsed dye laser, which allows for the application of 8 micropulses instead of a single pulse is one go-to device for treating vascular lesions, according to Dr. Melanie Palm.
"This allows me to use higher fluences without some of that eggplant purple discoloration or purpura that I would get if I used higher fluences in earlier generations of this laser," Dr. Palm said at the annual meeting of the American Academy of Cosmetic Surgery.
For example, when treating nasal telangiectasias, Dr. Palm said she sets the parameters to a fluence of 13-15 J/cm2, a pulse width of 40 milliseconds, and a spot size of 7 mm. "Using this new platform, I don’t get any of the purpura that you would expect with the more traditional 585-nm pulsed dye laser," said Dr. Palm, a dermatologist in Solana Beach, Calif.
Dr. Palm said she also has used the 595-nm pulsed dye laser (PDL) to treat rosacea, cherry angiomas, venous lakes, vascular malformations, postinflammatory erythema, striae distensae, scars, and purpura. "I will often combine treatments," she continued. For scars, she may combine 5-fluorouracil and intralesional Kenalog (triamcinolone), and immediately treat with the 595-nm PDL set to a fluence of 8 J/cm2, a pulse width of 10 milliseconds, and a spot size of 7 mm. For recalcitrant warts, she will often try intralesional bleomycin combined with the 595-nm PDL set to a fluence of 1-15 J/cm2, a pulse width of 1.5 milliseconds, and a spot size of 7 mm. "If the 595-nm PDL is the only laser in your office, you can use it to treat solar lentigines and other pigmentary disorders with some success," Dr. Palm said. "I also use it a lot for posttreatment bruising."
Intense pulsed light (IPL) is another technology Dr. Palm said she uses to treat vascular lesions. When discussing this technology with her patients, "I set the expectation that this is going to involve multiple treatments," Dr. Palm said. "I’ll often show them right after treatment that the vessels have gone into vasospasm. They have disappeared, but they will come back, and it will be several weeks before they see improvement."
Dr. Palm said she typically uses lidocaine cream as a numbing agent to improve patient comfort prior to IPL procedures. "But if patients want a stronger numbing agent, I mix lidocaine with tetracaine, which has a tendency to cause flushing," she said. "You can also use a hair dryer to aggravate erythema on the face prior to treatment."
Dr. Palm said she often uses the 515-nm filter with IPL energy applied in triple pulses to treat facial erythema. For facial telangiectasias, she typically uses the 560-nm filter with IPL energy applied in double pulses. "For stubborn spots, I switch to a smaller treatment hand piece, which creates higher fluence," she said.
Dr. Palm said she advises clinicians to be aggressive in treating postoperative scars. "If I see some redness, I’ll often treat as early as 1 month after treatment, using either a PDL or an IPL," she said. If she uses a PDL, she sets it to a fluence of 7-10 J/cm2, a pulse width of 10 milliseconds, and a spot size of 7 mm. If she uses an IPL, she employs a 560-nm filter, and sets the device to a fluence of 16-18 J/cm2 and a pulse width of 4 milliseconds.
To treat postprocedural bruising, Dr. Palm said she may use a PDL set to a fluence of 6 J/cm2, a pulse width of 6 milliseconds, and a spot size of 10 mm. If she opts to treat the bruising with an IPL, she employs a 560-nm filter and sets the device to a fluence of 13-15 J/cm2 and a pulse width of 4 milliseconds, and applies it in a double-pulse fashion. "You want to titrate the fluence inversely to the degree of bruising," Dr. Palm advised. "If you have an intense bruise, you want to decrease the fluence. If it’s a light bruise, you want to use higher fluences," she said. "I typically use a single pulse. You want to avoid pulse stacking because you can make the bruising worse. I don’t just treat where the bruise is. I treat within a centimeter around the bruised area as well."
Dr. Palm also discussed her experience using the Q-switched Nd:YAG double-frequency 532-nm laser as "a peel" to treat facial redness. "It’s usually a single-pass treatment that uses a double-frequency 1,064 Nd:YAG platform," she said. "I typically use an 8-mm hand piece set to a fluence of 3.5-5 J/cm2. Results are usually apparent within one to two treatments," she noted.
Dr. Palm disclosed that she is a speaker for Valeant, Medicis, and Lumenis. She is also a consultant for Lutronic.
LAS VEGAS – The 595-nm pulsed dye laser, which allows for the application of 8 micropulses instead of a single pulse is one go-to device for treating vascular lesions, according to Dr. Melanie Palm.
"This allows me to use higher fluences without some of that eggplant purple discoloration or purpura that I would get if I used higher fluences in earlier generations of this laser," Dr. Palm said at the annual meeting of the American Academy of Cosmetic Surgery.
For example, when treating nasal telangiectasias, Dr. Palm said she sets the parameters to a fluence of 13-15 J/cm2, a pulse width of 40 milliseconds, and a spot size of 7 mm. "Using this new platform, I don’t get any of the purpura that you would expect with the more traditional 585-nm pulsed dye laser," said Dr. Palm, a dermatologist in Solana Beach, Calif.
Dr. Palm said she also has used the 595-nm pulsed dye laser (PDL) to treat rosacea, cherry angiomas, venous lakes, vascular malformations, postinflammatory erythema, striae distensae, scars, and purpura. "I will often combine treatments," she continued. For scars, she may combine 5-fluorouracil and intralesional Kenalog (triamcinolone), and immediately treat with the 595-nm PDL set to a fluence of 8 J/cm2, a pulse width of 10 milliseconds, and a spot size of 7 mm. For recalcitrant warts, she will often try intralesional bleomycin combined with the 595-nm PDL set to a fluence of 1-15 J/cm2, a pulse width of 1.5 milliseconds, and a spot size of 7 mm. "If the 595-nm PDL is the only laser in your office, you can use it to treat solar lentigines and other pigmentary disorders with some success," Dr. Palm said. "I also use it a lot for posttreatment bruising."
Intense pulsed light (IPL) is another technology Dr. Palm said she uses to treat vascular lesions. When discussing this technology with her patients, "I set the expectation that this is going to involve multiple treatments," Dr. Palm said. "I’ll often show them right after treatment that the vessels have gone into vasospasm. They have disappeared, but they will come back, and it will be several weeks before they see improvement."
Dr. Palm said she typically uses lidocaine cream as a numbing agent to improve patient comfort prior to IPL procedures. "But if patients want a stronger numbing agent, I mix lidocaine with tetracaine, which has a tendency to cause flushing," she said. "You can also use a hair dryer to aggravate erythema on the face prior to treatment."
Dr. Palm said she often uses the 515-nm filter with IPL energy applied in triple pulses to treat facial erythema. For facial telangiectasias, she typically uses the 560-nm filter with IPL energy applied in double pulses. "For stubborn spots, I switch to a smaller treatment hand piece, which creates higher fluence," she said.
Dr. Palm said she advises clinicians to be aggressive in treating postoperative scars. "If I see some redness, I’ll often treat as early as 1 month after treatment, using either a PDL or an IPL," she said. If she uses a PDL, she sets it to a fluence of 7-10 J/cm2, a pulse width of 10 milliseconds, and a spot size of 7 mm. If she uses an IPL, she employs a 560-nm filter, and sets the device to a fluence of 16-18 J/cm2 and a pulse width of 4 milliseconds.
To treat postprocedural bruising, Dr. Palm said she may use a PDL set to a fluence of 6 J/cm2, a pulse width of 6 milliseconds, and a spot size of 10 mm. If she opts to treat the bruising with an IPL, she employs a 560-nm filter and sets the device to a fluence of 13-15 J/cm2 and a pulse width of 4 milliseconds, and applies it in a double-pulse fashion. "You want to titrate the fluence inversely to the degree of bruising," Dr. Palm advised. "If you have an intense bruise, you want to decrease the fluence. If it’s a light bruise, you want to use higher fluences," she said. "I typically use a single pulse. You want to avoid pulse stacking because you can make the bruising worse. I don’t just treat where the bruise is. I treat within a centimeter around the bruised area as well."
Dr. Palm also discussed her experience using the Q-switched Nd:YAG double-frequency 532-nm laser as "a peel" to treat facial redness. "It’s usually a single-pass treatment that uses a double-frequency 1,064 Nd:YAG platform," she said. "I typically use an 8-mm hand piece set to a fluence of 3.5-5 J/cm2. Results are usually apparent within one to two treatments," she noted.
Dr. Palm disclosed that she is a speaker for Valeant, Medicis, and Lumenis. She is also a consultant for Lutronic.
LAS VEGAS – The 595-nm pulsed dye laser, which allows for the application of 8 micropulses instead of a single pulse is one go-to device for treating vascular lesions, according to Dr. Melanie Palm.
"This allows me to use higher fluences without some of that eggplant purple discoloration or purpura that I would get if I used higher fluences in earlier generations of this laser," Dr. Palm said at the annual meeting of the American Academy of Cosmetic Surgery.
For example, when treating nasal telangiectasias, Dr. Palm said she sets the parameters to a fluence of 13-15 J/cm2, a pulse width of 40 milliseconds, and a spot size of 7 mm. "Using this new platform, I don’t get any of the purpura that you would expect with the more traditional 585-nm pulsed dye laser," said Dr. Palm, a dermatologist in Solana Beach, Calif.
Dr. Palm said she also has used the 595-nm pulsed dye laser (PDL) to treat rosacea, cherry angiomas, venous lakes, vascular malformations, postinflammatory erythema, striae distensae, scars, and purpura. "I will often combine treatments," she continued. For scars, she may combine 5-fluorouracil and intralesional Kenalog (triamcinolone), and immediately treat with the 595-nm PDL set to a fluence of 8 J/cm2, a pulse width of 10 milliseconds, and a spot size of 7 mm. For recalcitrant warts, she will often try intralesional bleomycin combined with the 595-nm PDL set to a fluence of 1-15 J/cm2, a pulse width of 1.5 milliseconds, and a spot size of 7 mm. "If the 595-nm PDL is the only laser in your office, you can use it to treat solar lentigines and other pigmentary disorders with some success," Dr. Palm said. "I also use it a lot for posttreatment bruising."
Intense pulsed light (IPL) is another technology Dr. Palm said she uses to treat vascular lesions. When discussing this technology with her patients, "I set the expectation that this is going to involve multiple treatments," Dr. Palm said. "I’ll often show them right after treatment that the vessels have gone into vasospasm. They have disappeared, but they will come back, and it will be several weeks before they see improvement."
Dr. Palm said she typically uses lidocaine cream as a numbing agent to improve patient comfort prior to IPL procedures. "But if patients want a stronger numbing agent, I mix lidocaine with tetracaine, which has a tendency to cause flushing," she said. "You can also use a hair dryer to aggravate erythema on the face prior to treatment."
Dr. Palm said she often uses the 515-nm filter with IPL energy applied in triple pulses to treat facial erythema. For facial telangiectasias, she typically uses the 560-nm filter with IPL energy applied in double pulses. "For stubborn spots, I switch to a smaller treatment hand piece, which creates higher fluence," she said.
Dr. Palm said she advises clinicians to be aggressive in treating postoperative scars. "If I see some redness, I’ll often treat as early as 1 month after treatment, using either a PDL or an IPL," she said. If she uses a PDL, she sets it to a fluence of 7-10 J/cm2, a pulse width of 10 milliseconds, and a spot size of 7 mm. If she uses an IPL, she employs a 560-nm filter, and sets the device to a fluence of 16-18 J/cm2 and a pulse width of 4 milliseconds.
To treat postprocedural bruising, Dr. Palm said she may use a PDL set to a fluence of 6 J/cm2, a pulse width of 6 milliseconds, and a spot size of 10 mm. If she opts to treat the bruising with an IPL, she employs a 560-nm filter and sets the device to a fluence of 13-15 J/cm2 and a pulse width of 4 milliseconds, and applies it in a double-pulse fashion. "You want to titrate the fluence inversely to the degree of bruising," Dr. Palm advised. "If you have an intense bruise, you want to decrease the fluence. If it’s a light bruise, you want to use higher fluences," she said. "I typically use a single pulse. You want to avoid pulse stacking because you can make the bruising worse. I don’t just treat where the bruise is. I treat within a centimeter around the bruised area as well."
Dr. Palm also discussed her experience using the Q-switched Nd:YAG double-frequency 532-nm laser as "a peel" to treat facial redness. "It’s usually a single-pass treatment that uses a double-frequency 1,064 Nd:YAG platform," she said. "I typically use an 8-mm hand piece set to a fluence of 3.5-5 J/cm2. Results are usually apparent within one to two treatments," she noted.
Dr. Palm disclosed that she is a speaker for Valeant, Medicis, and Lumenis. She is also a consultant for Lutronic.
EXPERT ANALYSIS FROM THE ANNUAL MEETING OF THE AMERICAN ACADEMY OF COSMETIC SURGERY
The problem with 'futility'
This morning while attending our department’s weekly Surgical Morbidity and Mortality conference, I was struck by how similar the case sounded to so many others that we have discussed in the past. An elderly patient with multiple comorbidities was found to have evidence of an acute abdomen. Unfortunately, the patient was intubated at the time of the surgical consultation, and it was unclear what his wishes would have been. Here was an apparent surgical problem in a very-high-risk patient.
The patient’s family, the medicine team, and the surgery team had several discussions and all agreed that the patient’s condition was very serious and that he would likely die without surgery. In addition, the surgical team felt that the chances for survival even with an exploratory laparotomy were extremely low. After much discussion, the decision was made to operate on the patient. He survived the operation only to have a gradual decline in his condition such that he developed multisystem organ failure. The resident presenting the case noted that eventually the surgical team was convinced that "further treatments were futile" and "after discussing the patient’s condition with the family, the decision was made to withdraw aggressive treatment." The patient was made comfortable and died a short time later.
As the discussion at the M&M conference showed, there were many surgeons present who felt that the outcome was expected and even a few who questioned whether the patient should even have had surgery. These are important issues, but what struck me most was the use of the term "futility" in reference to this patient’s care.
In recent years, there has been significant analysis within the medical ethics literature of the concept of futility. Futility in this context is difficult to define. Moreover, it appears some doctors determine a treatment to be futile as a means of pulling back control from the patient or surrogate who may be asking for a course of action. In other words, if we accept the importance of respecting patient autonomy and if patients/surrogates want a particular treatment, doctors often have difficulty saying "no" unless they define the treatment as futile. Since it is widely accepted that physicians need not offer futile treatments, defining a treatment as futile may be a way to limit the choices for patients/surrogates to consider or request.
In line with much of this literature, I have previously argued that we should "strike the term ["futility"] from our professional lexicon" (World J. Surg. 2009;33:1338-40). However, despite the chorus of suggestions that futility is a problematic concept when it comes to caring for patients, it continues to be used in discussions of actual patient care. I have concluded that it is impossible to eliminate the term "futility." In contrast, perhaps a better approach would be to realize that calling a certain set of treatments "futile" actually provides very little information to the people with whom we are talking. When we say a treatment would be an exercise in futility, we are really saying that in our best medical judgment the likelihood of success is very low. In addition, calling something futile suggests that a careful weighing of burdens and benefits of a particular treatment has been undertaken, and the doctor believes that the burdens so clearly outweigh the benefits that the treatment should not be offered to the patient. Therefore, rather than removing "futility" from our discussions with patients and each other, we should strive to realize how little the term actually conveys to our patients/surrogates.
When we use the term "futile" to describe a treatment, we are saying it just does not make sense in a specific case. The problem is that what a patient/surrogate considers to be the burdens and benefits might differ from what the medical team sees. For example, if an operation has virtually no chance of curing a patient, it might be considered futile. However, if the patient’s primary goal is palliation of certain symptoms for even a few days, then the operation should perhaps be viewed as "potentially beneficial" relative to a particular goal rather than "futile."
Surgeons should remember that the weighing of burdens and benefits requires more than medical knowledge. As such, every time the concept of "futility" is raised in the context of caring for a specific patient, the medical team should carefully explain to the patient/surrogate the benefits and burdens are that are being considered. Since it seems impossible for us to eliminate "futility" from our clinical discussions, let us instead use the term as a reminder to communicate the details and implications of a course of action. In this manner, a surgeon’s assessment of futility might prove an opportunity for further discussions rather than a statement of a definitive conclusion.
Dr. Peter Angelos is an ACS Fellow, the Linda Kohler Anderson Professor of Surgery and Surgical Ethics, chief of endocrine surgery, and associate director, MacLean Center for Clinical Medical Ethics, University of Chicago.
This morning while attending our department’s weekly Surgical Morbidity and Mortality conference, I was struck by how similar the case sounded to so many others that we have discussed in the past. An elderly patient with multiple comorbidities was found to have evidence of an acute abdomen. Unfortunately, the patient was intubated at the time of the surgical consultation, and it was unclear what his wishes would have been. Here was an apparent surgical problem in a very-high-risk patient.
The patient’s family, the medicine team, and the surgery team had several discussions and all agreed that the patient’s condition was very serious and that he would likely die without surgery. In addition, the surgical team felt that the chances for survival even with an exploratory laparotomy were extremely low. After much discussion, the decision was made to operate on the patient. He survived the operation only to have a gradual decline in his condition such that he developed multisystem organ failure. The resident presenting the case noted that eventually the surgical team was convinced that "further treatments were futile" and "after discussing the patient’s condition with the family, the decision was made to withdraw aggressive treatment." The patient was made comfortable and died a short time later.
As the discussion at the M&M conference showed, there were many surgeons present who felt that the outcome was expected and even a few who questioned whether the patient should even have had surgery. These are important issues, but what struck me most was the use of the term "futility" in reference to this patient’s care.
In recent years, there has been significant analysis within the medical ethics literature of the concept of futility. Futility in this context is difficult to define. Moreover, it appears some doctors determine a treatment to be futile as a means of pulling back control from the patient or surrogate who may be asking for a course of action. In other words, if we accept the importance of respecting patient autonomy and if patients/surrogates want a particular treatment, doctors often have difficulty saying "no" unless they define the treatment as futile. Since it is widely accepted that physicians need not offer futile treatments, defining a treatment as futile may be a way to limit the choices for patients/surrogates to consider or request.
In line with much of this literature, I have previously argued that we should "strike the term ["futility"] from our professional lexicon" (World J. Surg. 2009;33:1338-40). However, despite the chorus of suggestions that futility is a problematic concept when it comes to caring for patients, it continues to be used in discussions of actual patient care. I have concluded that it is impossible to eliminate the term "futility." In contrast, perhaps a better approach would be to realize that calling a certain set of treatments "futile" actually provides very little information to the people with whom we are talking. When we say a treatment would be an exercise in futility, we are really saying that in our best medical judgment the likelihood of success is very low. In addition, calling something futile suggests that a careful weighing of burdens and benefits of a particular treatment has been undertaken, and the doctor believes that the burdens so clearly outweigh the benefits that the treatment should not be offered to the patient. Therefore, rather than removing "futility" from our discussions with patients and each other, we should strive to realize how little the term actually conveys to our patients/surrogates.
When we use the term "futile" to describe a treatment, we are saying it just does not make sense in a specific case. The problem is that what a patient/surrogate considers to be the burdens and benefits might differ from what the medical team sees. For example, if an operation has virtually no chance of curing a patient, it might be considered futile. However, if the patient’s primary goal is palliation of certain symptoms for even a few days, then the operation should perhaps be viewed as "potentially beneficial" relative to a particular goal rather than "futile."
Surgeons should remember that the weighing of burdens and benefits requires more than medical knowledge. As such, every time the concept of "futility" is raised in the context of caring for a specific patient, the medical team should carefully explain to the patient/surrogate the benefits and burdens are that are being considered. Since it seems impossible for us to eliminate "futility" from our clinical discussions, let us instead use the term as a reminder to communicate the details and implications of a course of action. In this manner, a surgeon’s assessment of futility might prove an opportunity for further discussions rather than a statement of a definitive conclusion.
Dr. Peter Angelos is an ACS Fellow, the Linda Kohler Anderson Professor of Surgery and Surgical Ethics, chief of endocrine surgery, and associate director, MacLean Center for Clinical Medical Ethics, University of Chicago.
This morning while attending our department’s weekly Surgical Morbidity and Mortality conference, I was struck by how similar the case sounded to so many others that we have discussed in the past. An elderly patient with multiple comorbidities was found to have evidence of an acute abdomen. Unfortunately, the patient was intubated at the time of the surgical consultation, and it was unclear what his wishes would have been. Here was an apparent surgical problem in a very-high-risk patient.
The patient’s family, the medicine team, and the surgery team had several discussions and all agreed that the patient’s condition was very serious and that he would likely die without surgery. In addition, the surgical team felt that the chances for survival even with an exploratory laparotomy were extremely low. After much discussion, the decision was made to operate on the patient. He survived the operation only to have a gradual decline in his condition such that he developed multisystem organ failure. The resident presenting the case noted that eventually the surgical team was convinced that "further treatments were futile" and "after discussing the patient’s condition with the family, the decision was made to withdraw aggressive treatment." The patient was made comfortable and died a short time later.
As the discussion at the M&M conference showed, there were many surgeons present who felt that the outcome was expected and even a few who questioned whether the patient should even have had surgery. These are important issues, but what struck me most was the use of the term "futility" in reference to this patient’s care.
In recent years, there has been significant analysis within the medical ethics literature of the concept of futility. Futility in this context is difficult to define. Moreover, it appears some doctors determine a treatment to be futile as a means of pulling back control from the patient or surrogate who may be asking for a course of action. In other words, if we accept the importance of respecting patient autonomy and if patients/surrogates want a particular treatment, doctors often have difficulty saying "no" unless they define the treatment as futile. Since it is widely accepted that physicians need not offer futile treatments, defining a treatment as futile may be a way to limit the choices for patients/surrogates to consider or request.
In line with much of this literature, I have previously argued that we should "strike the term ["futility"] from our professional lexicon" (World J. Surg. 2009;33:1338-40). However, despite the chorus of suggestions that futility is a problematic concept when it comes to caring for patients, it continues to be used in discussions of actual patient care. I have concluded that it is impossible to eliminate the term "futility." In contrast, perhaps a better approach would be to realize that calling a certain set of treatments "futile" actually provides very little information to the people with whom we are talking. When we say a treatment would be an exercise in futility, we are really saying that in our best medical judgment the likelihood of success is very low. In addition, calling something futile suggests that a careful weighing of burdens and benefits of a particular treatment has been undertaken, and the doctor believes that the burdens so clearly outweigh the benefits that the treatment should not be offered to the patient. Therefore, rather than removing "futility" from our discussions with patients and each other, we should strive to realize how little the term actually conveys to our patients/surrogates.
When we use the term "futile" to describe a treatment, we are saying it just does not make sense in a specific case. The problem is that what a patient/surrogate considers to be the burdens and benefits might differ from what the medical team sees. For example, if an operation has virtually no chance of curing a patient, it might be considered futile. However, if the patient’s primary goal is palliation of certain symptoms for even a few days, then the operation should perhaps be viewed as "potentially beneficial" relative to a particular goal rather than "futile."
Surgeons should remember that the weighing of burdens and benefits requires more than medical knowledge. As such, every time the concept of "futility" is raised in the context of caring for a specific patient, the medical team should carefully explain to the patient/surrogate the benefits and burdens are that are being considered. Since it seems impossible for us to eliminate "futility" from our clinical discussions, let us instead use the term as a reminder to communicate the details and implications of a course of action. In this manner, a surgeon’s assessment of futility might prove an opportunity for further discussions rather than a statement of a definitive conclusion.
Dr. Peter Angelos is an ACS Fellow, the Linda Kohler Anderson Professor of Surgery and Surgical Ethics, chief of endocrine surgery, and associate director, MacLean Center for Clinical Medical Ethics, University of Chicago.
Feds get specific on ACA individual mandate rules
After years of legal wrangling and a showdown in front of the Supreme Court, the federal government has finally begun to implement the Affordable Care Act’s controversial individual insurance mandate.
Starting on Jan. 1, 2014, Americans will have a choice: Buy basic health insurance, qualify for an exemption, or pay a penalty when filing federal income taxes, according to proposed regulations issued Jan. 30 by the Treasury Department and the Health and Human Services Department.
Individuals will be able to meet the requirement to for "minimum essential coverage" through a government-sponsored program, an employer-sponsored plan, an individual health plan, or a grandfathered health plan. HHS also is working on regulations to designate other coverage options, according to the proposed regulation.
Individuals will not have to pay a penalty if they can’t find affordable insurance or if they spend less than 3 consecutive months without coverage. The federal government will offer "hardship" exemptions for individuals who would be eligible for Medicaid under the expansion outlined in the Affordable Care Act (ACA) and who live in states that are not expanding eligibility.
Under the Treasury department proposed regulation, individuals would be considered covered for a month as long as they were covered for a single day in that month.
The federal government also proposes to grant exemptions for people with religious objections, members of Indian tribes, taxpayers with income below the income tax filing threshold, members of a health care sharing ministry, and the incarcerated.
The proposed rules are aimed at ensuring that only a "limited group of taxpayers who choose to spend a substantial period of time without coverage despite having ready access to affordable coverage" will have to pay the penalty, according to an HHS fact sheet.
Based on data from the Congressional Budget Office, HHS officials estimate that less than 2% of Americans actually will be required to pay a penalty.
Comments on the proposed regulation from the Treasury Department are due by May 2. Comments on the HHS proposal are due by March 18. Both proposed regulations reference a public meeting on the proposals to be held on May 29.
After years of legal wrangling and a showdown in front of the Supreme Court, the federal government has finally begun to implement the Affordable Care Act’s controversial individual insurance mandate.
Starting on Jan. 1, 2014, Americans will have a choice: Buy basic health insurance, qualify for an exemption, or pay a penalty when filing federal income taxes, according to proposed regulations issued Jan. 30 by the Treasury Department and the Health and Human Services Department.
Individuals will be able to meet the requirement to for "minimum essential coverage" through a government-sponsored program, an employer-sponsored plan, an individual health plan, or a grandfathered health plan. HHS also is working on regulations to designate other coverage options, according to the proposed regulation.
Individuals will not have to pay a penalty if they can’t find affordable insurance or if they spend less than 3 consecutive months without coverage. The federal government will offer "hardship" exemptions for individuals who would be eligible for Medicaid under the expansion outlined in the Affordable Care Act (ACA) and who live in states that are not expanding eligibility.
Under the Treasury department proposed regulation, individuals would be considered covered for a month as long as they were covered for a single day in that month.
The federal government also proposes to grant exemptions for people with religious objections, members of Indian tribes, taxpayers with income below the income tax filing threshold, members of a health care sharing ministry, and the incarcerated.
The proposed rules are aimed at ensuring that only a "limited group of taxpayers who choose to spend a substantial period of time without coverage despite having ready access to affordable coverage" will have to pay the penalty, according to an HHS fact sheet.
Based on data from the Congressional Budget Office, HHS officials estimate that less than 2% of Americans actually will be required to pay a penalty.
Comments on the proposed regulation from the Treasury Department are due by May 2. Comments on the HHS proposal are due by March 18. Both proposed regulations reference a public meeting on the proposals to be held on May 29.
After years of legal wrangling and a showdown in front of the Supreme Court, the federal government has finally begun to implement the Affordable Care Act’s controversial individual insurance mandate.
Starting on Jan. 1, 2014, Americans will have a choice: Buy basic health insurance, qualify for an exemption, or pay a penalty when filing federal income taxes, according to proposed regulations issued Jan. 30 by the Treasury Department and the Health and Human Services Department.
Individuals will be able to meet the requirement to for "minimum essential coverage" through a government-sponsored program, an employer-sponsored plan, an individual health plan, or a grandfathered health plan. HHS also is working on regulations to designate other coverage options, according to the proposed regulation.
Individuals will not have to pay a penalty if they can’t find affordable insurance or if they spend less than 3 consecutive months without coverage. The federal government will offer "hardship" exemptions for individuals who would be eligible for Medicaid under the expansion outlined in the Affordable Care Act (ACA) and who live in states that are not expanding eligibility.
Under the Treasury department proposed regulation, individuals would be considered covered for a month as long as they were covered for a single day in that month.
The federal government also proposes to grant exemptions for people with religious objections, members of Indian tribes, taxpayers with income below the income tax filing threshold, members of a health care sharing ministry, and the incarcerated.
The proposed rules are aimed at ensuring that only a "limited group of taxpayers who choose to spend a substantial period of time without coverage despite having ready access to affordable coverage" will have to pay the penalty, according to an HHS fact sheet.
Based on data from the Congressional Budget Office, HHS officials estimate that less than 2% of Americans actually will be required to pay a penalty.
Comments on the proposed regulation from the Treasury Department are due by May 2. Comments on the HHS proposal are due by March 18. Both proposed regulations reference a public meeting on the proposals to be held on May 29.
Surgeon, respect the levator muscle
LAS VEGAS – Knowing and respecting the anatomy of the levator muscle can help clinicians steer clear of complications from blepharoplasty and manage ptosis, according to Dr. Marc S. Cohen.
"It’s very helpful if you have a good understanding of how to find the levator muscle during eyelid surgery," said Dr. Cohen, an ophthalmic plastic surgeon at the Wills Eye Institute, Philadelphia. "In order to do this, you need to understand the relationship between the levator and the other eyelid structures."
The levator muscle elevates the eyelid and helps form the eyelid crease. It also creates the margin contour. As the levator muscle approaches the eyelid, it changes direction from vertically oriented to horizontally oriented. The muscle then advances inferiorly toward the eyelid margin, "and for the final centimeter or so, it becomes a fibrous aponeurosis, which attaches to the tarsus posteriorly," said Dr. Cohen, who also has a private cosmetic surgery practice. Behind the levator muscle are Müller’s muscle and the conjunctiva.
Whether a surgeon performs blepharoplasty with a CO2 laser, a blade, cautery, or radiofrequency, the first structure encountered posteriorly is the orbicularis oculi muscle, which closes the eyelid. "It’s highly vascular, and is the site where most of the bleeding occurs during blepharoplasty," Dr. Cohen said at the annual meeting of the American Academy of Cosmetic Surgery.
The next layer contains the orbital septum. "It’s important to understand that the septum does not travel all the way to the eyelid margin," he added. "The septum starts at the orbital rim and attaches to the levator muscle. This layer really has two structures: the septum and the levator. Behind the septum are the eyelid fat pads."
In a dissection above and behind in the eyelid, the septum and the fat precede the levator muscle. However, in the inferior eyelid, the levator is just deep to the orbicularis muscle. Beneath the fat, the levator muscle moves posteriorly into the orbit; this causes it to narrow.
"Lateral to the muscle at this point is the lacrimal gland, but medially is just orbital fat," Dr. Cohen said. Upon reaching the orbicularis muscle, the goal is to protect the levator muscle. "The levator muscle is protected by septum fat superiorly, whereas more inferiorly the levator fuses with the orbicularis, so this is a danger zone," Dr. Cohen said. "Laterally is the lacrimal gland and supramedially is the safest point, because there you have the fat, and nothing else to really worry about superficially. So what you do is press on the globe through the eyelid, have the fat prolapse forward, and dissect there."
Reattaching the levator muscle can be tricky in the context of levator resection ptosis surgery, said Dr. Cohen. "Where you make the attachment is going to affect the contour postoperatively," he said. "Grasp the tarsus and pull it upward to see if you have obtained a natural curve. If you grasp it at the wrong point, you’ll have a curve that’s not aesthetically pleasing," he cautioned.
"When you get the right point, that is where you are going to put the sutures to reattach the levator. A double-armed 6-0 suture is passed in a horizontal mattress fashion, partial thickness, through the tarsus. The suture is then passed in a posterior to anterior direction, which shortens the levator muscle."
Placement of the suture determines how much the muscle shortens. "The suture is then temporarily tied, and the patient is asked to open their eyes to assess the height and the contour," Dr. Cohen said. "If you need to adjust height vertically, you can move the suture vertically on the levator muscle. If there’s a problem with the contour, you change the fixation point to the tarsus. Then the suture is permanently tied and the skin is closed."
Dr. Cohen warned about the risk of complications from blepharoplasty in patients with active Graves’ disease, a common autoimmune condition that can cause hyperthyroidism and fibrosis of the extraocular tissues. The severe form of Graves’ disease can cause eyelid retraction, difficulty closing the eyes, double vision, and anterior displacement of the globes. "Many patients present with much more subtle findings," he noted. "For example, fibrosis of the levator with lid retraction is a common presentation in women aged 40-60 – the same demographic that tends to have blepharoplasty. It’s often subtle and underdiagnosed."
Patients with undiagnosed Graves’ disease prior to a blepharoplasty "can develop signs and symptoms which are indistinguishable from the complications of blepharoplasty," Dr. Cohen said. "You need to make the diagnosis before surgery and make sure the disease has stabilized before you do any surgery. That happens on average in about 18 months but is variable."
Dr. Cohen disclosed that he is a member of the advisory board for Allergan and that he is a speaker for Allergan and Medicis.
LAS VEGAS – Knowing and respecting the anatomy of the levator muscle can help clinicians steer clear of complications from blepharoplasty and manage ptosis, according to Dr. Marc S. Cohen.
"It’s very helpful if you have a good understanding of how to find the levator muscle during eyelid surgery," said Dr. Cohen, an ophthalmic plastic surgeon at the Wills Eye Institute, Philadelphia. "In order to do this, you need to understand the relationship between the levator and the other eyelid structures."
The levator muscle elevates the eyelid and helps form the eyelid crease. It also creates the margin contour. As the levator muscle approaches the eyelid, it changes direction from vertically oriented to horizontally oriented. The muscle then advances inferiorly toward the eyelid margin, "and for the final centimeter or so, it becomes a fibrous aponeurosis, which attaches to the tarsus posteriorly," said Dr. Cohen, who also has a private cosmetic surgery practice. Behind the levator muscle are Müller’s muscle and the conjunctiva.
Whether a surgeon performs blepharoplasty with a CO2 laser, a blade, cautery, or radiofrequency, the first structure encountered posteriorly is the orbicularis oculi muscle, which closes the eyelid. "It’s highly vascular, and is the site where most of the bleeding occurs during blepharoplasty," Dr. Cohen said at the annual meeting of the American Academy of Cosmetic Surgery.
The next layer contains the orbital septum. "It’s important to understand that the septum does not travel all the way to the eyelid margin," he added. "The septum starts at the orbital rim and attaches to the levator muscle. This layer really has two structures: the septum and the levator. Behind the septum are the eyelid fat pads."
In a dissection above and behind in the eyelid, the septum and the fat precede the levator muscle. However, in the inferior eyelid, the levator is just deep to the orbicularis muscle. Beneath the fat, the levator muscle moves posteriorly into the orbit; this causes it to narrow.
"Lateral to the muscle at this point is the lacrimal gland, but medially is just orbital fat," Dr. Cohen said. Upon reaching the orbicularis muscle, the goal is to protect the levator muscle. "The levator muscle is protected by septum fat superiorly, whereas more inferiorly the levator fuses with the orbicularis, so this is a danger zone," Dr. Cohen said. "Laterally is the lacrimal gland and supramedially is the safest point, because there you have the fat, and nothing else to really worry about superficially. So what you do is press on the globe through the eyelid, have the fat prolapse forward, and dissect there."
Reattaching the levator muscle can be tricky in the context of levator resection ptosis surgery, said Dr. Cohen. "Where you make the attachment is going to affect the contour postoperatively," he said. "Grasp the tarsus and pull it upward to see if you have obtained a natural curve. If you grasp it at the wrong point, you’ll have a curve that’s not aesthetically pleasing," he cautioned.
"When you get the right point, that is where you are going to put the sutures to reattach the levator. A double-armed 6-0 suture is passed in a horizontal mattress fashion, partial thickness, through the tarsus. The suture is then passed in a posterior to anterior direction, which shortens the levator muscle."
Placement of the suture determines how much the muscle shortens. "The suture is then temporarily tied, and the patient is asked to open their eyes to assess the height and the contour," Dr. Cohen said. "If you need to adjust height vertically, you can move the suture vertically on the levator muscle. If there’s a problem with the contour, you change the fixation point to the tarsus. Then the suture is permanently tied and the skin is closed."
Dr. Cohen warned about the risk of complications from blepharoplasty in patients with active Graves’ disease, a common autoimmune condition that can cause hyperthyroidism and fibrosis of the extraocular tissues. The severe form of Graves’ disease can cause eyelid retraction, difficulty closing the eyes, double vision, and anterior displacement of the globes. "Many patients present with much more subtle findings," he noted. "For example, fibrosis of the levator with lid retraction is a common presentation in women aged 40-60 – the same demographic that tends to have blepharoplasty. It’s often subtle and underdiagnosed."
Patients with undiagnosed Graves’ disease prior to a blepharoplasty "can develop signs and symptoms which are indistinguishable from the complications of blepharoplasty," Dr. Cohen said. "You need to make the diagnosis before surgery and make sure the disease has stabilized before you do any surgery. That happens on average in about 18 months but is variable."
Dr. Cohen disclosed that he is a member of the advisory board for Allergan and that he is a speaker for Allergan and Medicis.
LAS VEGAS – Knowing and respecting the anatomy of the levator muscle can help clinicians steer clear of complications from blepharoplasty and manage ptosis, according to Dr. Marc S. Cohen.
"It’s very helpful if you have a good understanding of how to find the levator muscle during eyelid surgery," said Dr. Cohen, an ophthalmic plastic surgeon at the Wills Eye Institute, Philadelphia. "In order to do this, you need to understand the relationship between the levator and the other eyelid structures."
The levator muscle elevates the eyelid and helps form the eyelid crease. It also creates the margin contour. As the levator muscle approaches the eyelid, it changes direction from vertically oriented to horizontally oriented. The muscle then advances inferiorly toward the eyelid margin, "and for the final centimeter or so, it becomes a fibrous aponeurosis, which attaches to the tarsus posteriorly," said Dr. Cohen, who also has a private cosmetic surgery practice. Behind the levator muscle are Müller’s muscle and the conjunctiva.
Whether a surgeon performs blepharoplasty with a CO2 laser, a blade, cautery, or radiofrequency, the first structure encountered posteriorly is the orbicularis oculi muscle, which closes the eyelid. "It’s highly vascular, and is the site where most of the bleeding occurs during blepharoplasty," Dr. Cohen said at the annual meeting of the American Academy of Cosmetic Surgery.
The next layer contains the orbital septum. "It’s important to understand that the septum does not travel all the way to the eyelid margin," he added. "The septum starts at the orbital rim and attaches to the levator muscle. This layer really has two structures: the septum and the levator. Behind the septum are the eyelid fat pads."
In a dissection above and behind in the eyelid, the septum and the fat precede the levator muscle. However, in the inferior eyelid, the levator is just deep to the orbicularis muscle. Beneath the fat, the levator muscle moves posteriorly into the orbit; this causes it to narrow.
"Lateral to the muscle at this point is the lacrimal gland, but medially is just orbital fat," Dr. Cohen said. Upon reaching the orbicularis muscle, the goal is to protect the levator muscle. "The levator muscle is protected by septum fat superiorly, whereas more inferiorly the levator fuses with the orbicularis, so this is a danger zone," Dr. Cohen said. "Laterally is the lacrimal gland and supramedially is the safest point, because there you have the fat, and nothing else to really worry about superficially. So what you do is press on the globe through the eyelid, have the fat prolapse forward, and dissect there."
Reattaching the levator muscle can be tricky in the context of levator resection ptosis surgery, said Dr. Cohen. "Where you make the attachment is going to affect the contour postoperatively," he said. "Grasp the tarsus and pull it upward to see if you have obtained a natural curve. If you grasp it at the wrong point, you’ll have a curve that’s not aesthetically pleasing," he cautioned.
"When you get the right point, that is where you are going to put the sutures to reattach the levator. A double-armed 6-0 suture is passed in a horizontal mattress fashion, partial thickness, through the tarsus. The suture is then passed in a posterior to anterior direction, which shortens the levator muscle."
Placement of the suture determines how much the muscle shortens. "The suture is then temporarily tied, and the patient is asked to open their eyes to assess the height and the contour," Dr. Cohen said. "If you need to adjust height vertically, you can move the suture vertically on the levator muscle. If there’s a problem with the contour, you change the fixation point to the tarsus. Then the suture is permanently tied and the skin is closed."
Dr. Cohen warned about the risk of complications from blepharoplasty in patients with active Graves’ disease, a common autoimmune condition that can cause hyperthyroidism and fibrosis of the extraocular tissues. The severe form of Graves’ disease can cause eyelid retraction, difficulty closing the eyes, double vision, and anterior displacement of the globes. "Many patients present with much more subtle findings," he noted. "For example, fibrosis of the levator with lid retraction is a common presentation in women aged 40-60 – the same demographic that tends to have blepharoplasty. It’s often subtle and underdiagnosed."
Patients with undiagnosed Graves’ disease prior to a blepharoplasty "can develop signs and symptoms which are indistinguishable from the complications of blepharoplasty," Dr. Cohen said. "You need to make the diagnosis before surgery and make sure the disease has stabilized before you do any surgery. That happens on average in about 18 months but is variable."
Dr. Cohen disclosed that he is a member of the advisory board for Allergan and that he is a speaker for Allergan and Medicis.
EXPERT ANALYSIS FROM THE ANNUAL MEETING OF THE AMERICAN ACADEMY OF COSMETIC SURGERY
Robotic surgery called 'in the destiny of humanity'
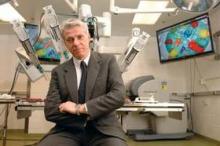
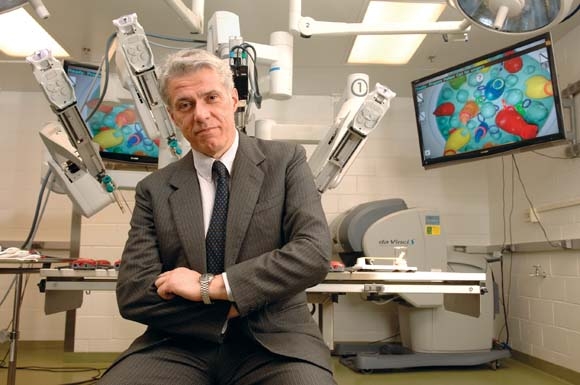
The way Dr. Pier Cristoforo Giulianotti sees it, robots will play an increasingly prominent role in the future of surgery. He should know. Dr. Giulianotti, the Lloyd Nyhus Professor of Surgery and chief, division of minimally invasive, general, and robotic surgery at the University of Illinois at Chicago, pioneered robotic lung resection in 2002 and was the first surgeon to perform a robotic Whipple procedure in 2001.
"In my opinion there is no way back from robotic surgery," he said in an interview. "It’s in the destiny of humanity, for the simple reason: to perform more precisely and to overcome our limitations – not only manual limitations, the ability to control movements at a microscopic level, for example – but also diagnostic limitations. The computer and the artificial intelligence of the future will integrate our senses and our mechanical abilities, so we will be able to perform more precisely on selected targets."
Currently, most abdominal procedures performed at the University of Illinois Medical Center are done robotically, including the Whipple procedure, splenectomy, total gastrectomy, lung lobectomy, colorectal surgery, thyroidectomy, adrenalectomy, esophagectomy, major hepatectomies, and common bile duct procedures. The robot "has enormous benefits for the patients," Dr. Giulianotti said. "Open procedures involve a longer postoperative stay and more complications, and it takes more time to enter adjuvant oncological treatment after surgery, and there is less blood loss."
In Dr. Giulianotti’s published experience of 134 robotic-assisted laparoscopic pancreatic surgery procedures, morbidity was 26%, mortality was 2.2%, the conversion rate was 10.4%, and the fistula rate was 20.9%. The majority were grade A fistulas not requiring any treatment (Surg. Endosc. 2010; 24:1646-57). He said that robotic surgery "enables difficult technical maneuvers to be performed that facilitate the success of pancreatic minimally invasive surgery. The results in this series demonstrate feasibility and safety with clinical outcomes."
In a separate study, investigators who compared 32 open vs. 28 laparoscopic vs. 17 robotic distal pancreatectomies found that all three procedures resulted in a similar cost, while the robotic group had a shorter hospital stay, a higher rate of spleen-preserving cases, and an increased operative time, compared with patients in the other two groups (Surgery 2010;148:814-23).
Pelvic indications that are becoming the gold standard for robotic surgery include robotic prostatectomy and resection for rectal cancer. "When you have a very deep and complex surgical field like the pelvis, and you need to do a radical resection for cancer while at the same time sparing nerves that can be important for urinary function, the robot combines three-dimensional vision plus improved functionality of the instruments, allowing for a more precise dissection," Dr. Giulianotti said.
Dr. Leela M. Prasad, chief of the division of colon and rectal surgery at the University of Illinois at Chicago, described robotic-assisted surgery for rectal cancer as "far superior to anything we know right now," especially in overweight males. According to combined results from three published studies, circumferential margin positivity ranged from 0 to 7%, which, Dr. Prasad said, is significantly lower than the average number of positive margins reported following open and laparoscopic rectal surgery procedures (14% and 16%, respectively) (Ann. Surg. Oncol. 2007;14:3168-73; Surg. Oncol. 2009;16:1480-7; Dis. Colon Rectum 2009;52:1824-30).
At the present time, however, in his clinical experience there are no significant differences in outcomes between robotic-assisted and laparoscopically assisted open surgery for colon cancer. "We looked at our data, and it did not make any difference robotically or laparoscopically in terms of length of stay, lymph node harvest, or survival," he said. A recent study from Korean investigators supports those findings (Surg. Endosc. 2012 Dec. 13).
Dr. Giulianotti estimated that there are more than 1,600 robotic surgery systems in the United States. Some of them are devoted to urologic or gynecologic applications, "but in the majority of hospitals there is a growing tendency to use the robot as part of a multidisciplinary program," he said. "That means at the same institution different surgical teams on different days are using the robot for different indications. Of the 1,600 systems out there, I would estimate that about half are used for general surgery."
While hospital administrators have warmed up to the idea of initiating robotic surgery programs at their institutions in recent years, Dr. Giulianotti and Dr. Prasad both called for a culture change in terms of how surgeons and medical educators think about the robot’s place in clinical medicine. "There is a cultural resistance in accepting big changes in surgery," Dr. Giulianotti said. "Academic medical institutions can play a key role here. They need to start teaching robotics in a mandatory way by offering training opportunities in a lab. We also believe that simulation has a big future in this kind of training."
The economic investment required to launch a robotic surgery program is another challenge. "Some hospitals are only concerned about the cost," he said. "When you are doing the same procedure laparoscopically, the cost is probably about 30% less. In the future I hope we can better impact the overall economic aspect of surgical procedures. That means speeding up the postoperative and outpatient treatment of some pathologies. We are already doing Nissen fundoplication and gallbladder removal with robotic surgery, and patients are being discharged a few hours after the surgery."
Dr. Prasad said the field will be poised to further advance when makers of robotic surgery devices and instruments improve on existing technology. "I am using the same robot and instruments as I was using 3 years ago," he said. "I think we need new technologies – including a smaller robot to cut down the costs and speed up the operations. This will make it easier for patients."
To launch a successful robotic surgery program in this day and age, "you need a good team of nurses and surgeons who are dedicated to doing robotic surgery," Dr. Prasad concluded. "It is dangerous in the hands of a surgeon who is not qualified."
Dr. Giulianotti said that he had no relevant financial conflicts to disclose. Dr. Prasad disclosed that he has received honoraria from Intuitive Surgical, Ethicon Endo-Surgery, and Covidien.
I would like to congratulate Dr. Pier Cristoforo Giulianotti for his work to date demonstrating the effectiveness of robotic pancreatic surgery. Clearly the “robot,” as it is affectionately termed, is here to stay.
It has clear applications in all surgical subspecialties and is being embraced by surgeons, administrators, and patients as an important tool. It is a platform that will facilitate future developments that are certain to change the way we practice our craft.
Dr. John Sweeney |
Given the focus being placed on the value of health care deliveredin the United States, it is incumbent upon surgeons to continuously evaluate the quality and costs of each intervention that we offer to our patients. To many, this type of evaluative process might seem to come up short when it comes to robotic applications for many minimally invasive procedures. Quality in most studies has been equivalent, while the costs associated with this expensive technology can be very significant. Because this new piece of technology is the first step in a new direction, we must continue to place the time, effort and cost into refining, enhancing and improving it’s applications as the technology stands today. However unless we are able to accomplish this goal, I fear we fall into the trap of “using a Cadillac for a golf cart” which is something that the U.S. health care system cannot afford nor sustain.
Dr. John Sweeney, is an ACS Fellow and the W. Dean Warren Distinguished Chair in Surgery at Emory University, Atlanta.
I would like to congratulate Dr. Pier Cristoforo Giulianotti for his work to date demonstrating the effectiveness of robotic pancreatic surgery. Clearly the “robot,” as it is affectionately termed, is here to stay.
It has clear applications in all surgical subspecialties and is being embraced by surgeons, administrators, and patients as an important tool. It is a platform that will facilitate future developments that are certain to change the way we practice our craft.
Dr. John Sweeney |
Given the focus being placed on the value of health care deliveredin the United States, it is incumbent upon surgeons to continuously evaluate the quality and costs of each intervention that we offer to our patients. To many, this type of evaluative process might seem to come up short when it comes to robotic applications for many minimally invasive procedures. Quality in most studies has been equivalent, while the costs associated with this expensive technology can be very significant. Because this new piece of technology is the first step in a new direction, we must continue to place the time, effort and cost into refining, enhancing and improving it’s applications as the technology stands today. However unless we are able to accomplish this goal, I fear we fall into the trap of “using a Cadillac for a golf cart” which is something that the U.S. health care system cannot afford nor sustain.
Dr. John Sweeney, is an ACS Fellow and the W. Dean Warren Distinguished Chair in Surgery at Emory University, Atlanta.
I would like to congratulate Dr. Pier Cristoforo Giulianotti for his work to date demonstrating the effectiveness of robotic pancreatic surgery. Clearly the “robot,” as it is affectionately termed, is here to stay.
It has clear applications in all surgical subspecialties and is being embraced by surgeons, administrators, and patients as an important tool. It is a platform that will facilitate future developments that are certain to change the way we practice our craft.
Dr. John Sweeney |
Given the focus being placed on the value of health care deliveredin the United States, it is incumbent upon surgeons to continuously evaluate the quality and costs of each intervention that we offer to our patients. To many, this type of evaluative process might seem to come up short when it comes to robotic applications for many minimally invasive procedures. Quality in most studies has been equivalent, while the costs associated with this expensive technology can be very significant. Because this new piece of technology is the first step in a new direction, we must continue to place the time, effort and cost into refining, enhancing and improving it’s applications as the technology stands today. However unless we are able to accomplish this goal, I fear we fall into the trap of “using a Cadillac for a golf cart” which is something that the U.S. health care system cannot afford nor sustain.
Dr. John Sweeney, is an ACS Fellow and the W. Dean Warren Distinguished Chair in Surgery at Emory University, Atlanta.
The way Dr. Pier Cristoforo Giulianotti sees it, robots will play an increasingly prominent role in the future of surgery. He should know. Dr. Giulianotti, the Lloyd Nyhus Professor of Surgery and chief, division of minimally invasive, general, and robotic surgery at the University of Illinois at Chicago, pioneered robotic lung resection in 2002 and was the first surgeon to perform a robotic Whipple procedure in 2001.
"In my opinion there is no way back from robotic surgery," he said in an interview. "It’s in the destiny of humanity, for the simple reason: to perform more precisely and to overcome our limitations – not only manual limitations, the ability to control movements at a microscopic level, for example – but also diagnostic limitations. The computer and the artificial intelligence of the future will integrate our senses and our mechanical abilities, so we will be able to perform more precisely on selected targets."
Currently, most abdominal procedures performed at the University of Illinois Medical Center are done robotically, including the Whipple procedure, splenectomy, total gastrectomy, lung lobectomy, colorectal surgery, thyroidectomy, adrenalectomy, esophagectomy, major hepatectomies, and common bile duct procedures. The robot "has enormous benefits for the patients," Dr. Giulianotti said. "Open procedures involve a longer postoperative stay and more complications, and it takes more time to enter adjuvant oncological treatment after surgery, and there is less blood loss."
In Dr. Giulianotti’s published experience of 134 robotic-assisted laparoscopic pancreatic surgery procedures, morbidity was 26%, mortality was 2.2%, the conversion rate was 10.4%, and the fistula rate was 20.9%. The majority were grade A fistulas not requiring any treatment (Surg. Endosc. 2010; 24:1646-57). He said that robotic surgery "enables difficult technical maneuvers to be performed that facilitate the success of pancreatic minimally invasive surgery. The results in this series demonstrate feasibility and safety with clinical outcomes."
In a separate study, investigators who compared 32 open vs. 28 laparoscopic vs. 17 robotic distal pancreatectomies found that all three procedures resulted in a similar cost, while the robotic group had a shorter hospital stay, a higher rate of spleen-preserving cases, and an increased operative time, compared with patients in the other two groups (Surgery 2010;148:814-23).
Pelvic indications that are becoming the gold standard for robotic surgery include robotic prostatectomy and resection for rectal cancer. "When you have a very deep and complex surgical field like the pelvis, and you need to do a radical resection for cancer while at the same time sparing nerves that can be important for urinary function, the robot combines three-dimensional vision plus improved functionality of the instruments, allowing for a more precise dissection," Dr. Giulianotti said.
Dr. Leela M. Prasad, chief of the division of colon and rectal surgery at the University of Illinois at Chicago, described robotic-assisted surgery for rectal cancer as "far superior to anything we know right now," especially in overweight males. According to combined results from three published studies, circumferential margin positivity ranged from 0 to 7%, which, Dr. Prasad said, is significantly lower than the average number of positive margins reported following open and laparoscopic rectal surgery procedures (14% and 16%, respectively) (Ann. Surg. Oncol. 2007;14:3168-73; Surg. Oncol. 2009;16:1480-7; Dis. Colon Rectum 2009;52:1824-30).
At the present time, however, in his clinical experience there are no significant differences in outcomes between robotic-assisted and laparoscopically assisted open surgery for colon cancer. "We looked at our data, and it did not make any difference robotically or laparoscopically in terms of length of stay, lymph node harvest, or survival," he said. A recent study from Korean investigators supports those findings (Surg. Endosc. 2012 Dec. 13).
Dr. Giulianotti estimated that there are more than 1,600 robotic surgery systems in the United States. Some of them are devoted to urologic or gynecologic applications, "but in the majority of hospitals there is a growing tendency to use the robot as part of a multidisciplinary program," he said. "That means at the same institution different surgical teams on different days are using the robot for different indications. Of the 1,600 systems out there, I would estimate that about half are used for general surgery."
While hospital administrators have warmed up to the idea of initiating robotic surgery programs at their institutions in recent years, Dr. Giulianotti and Dr. Prasad both called for a culture change in terms of how surgeons and medical educators think about the robot’s place in clinical medicine. "There is a cultural resistance in accepting big changes in surgery," Dr. Giulianotti said. "Academic medical institutions can play a key role here. They need to start teaching robotics in a mandatory way by offering training opportunities in a lab. We also believe that simulation has a big future in this kind of training."
The economic investment required to launch a robotic surgery program is another challenge. "Some hospitals are only concerned about the cost," he said. "When you are doing the same procedure laparoscopically, the cost is probably about 30% less. In the future I hope we can better impact the overall economic aspect of surgical procedures. That means speeding up the postoperative and outpatient treatment of some pathologies. We are already doing Nissen fundoplication and gallbladder removal with robotic surgery, and patients are being discharged a few hours after the surgery."
Dr. Prasad said the field will be poised to further advance when makers of robotic surgery devices and instruments improve on existing technology. "I am using the same robot and instruments as I was using 3 years ago," he said. "I think we need new technologies – including a smaller robot to cut down the costs and speed up the operations. This will make it easier for patients."
To launch a successful robotic surgery program in this day and age, "you need a good team of nurses and surgeons who are dedicated to doing robotic surgery," Dr. Prasad concluded. "It is dangerous in the hands of a surgeon who is not qualified."
Dr. Giulianotti said that he had no relevant financial conflicts to disclose. Dr. Prasad disclosed that he has received honoraria from Intuitive Surgical, Ethicon Endo-Surgery, and Covidien.
The way Dr. Pier Cristoforo Giulianotti sees it, robots will play an increasingly prominent role in the future of surgery. He should know. Dr. Giulianotti, the Lloyd Nyhus Professor of Surgery and chief, division of minimally invasive, general, and robotic surgery at the University of Illinois at Chicago, pioneered robotic lung resection in 2002 and was the first surgeon to perform a robotic Whipple procedure in 2001.
"In my opinion there is no way back from robotic surgery," he said in an interview. "It’s in the destiny of humanity, for the simple reason: to perform more precisely and to overcome our limitations – not only manual limitations, the ability to control movements at a microscopic level, for example – but also diagnostic limitations. The computer and the artificial intelligence of the future will integrate our senses and our mechanical abilities, so we will be able to perform more precisely on selected targets."
Currently, most abdominal procedures performed at the University of Illinois Medical Center are done robotically, including the Whipple procedure, splenectomy, total gastrectomy, lung lobectomy, colorectal surgery, thyroidectomy, adrenalectomy, esophagectomy, major hepatectomies, and common bile duct procedures. The robot "has enormous benefits for the patients," Dr. Giulianotti said. "Open procedures involve a longer postoperative stay and more complications, and it takes more time to enter adjuvant oncological treatment after surgery, and there is less blood loss."
In Dr. Giulianotti’s published experience of 134 robotic-assisted laparoscopic pancreatic surgery procedures, morbidity was 26%, mortality was 2.2%, the conversion rate was 10.4%, and the fistula rate was 20.9%. The majority were grade A fistulas not requiring any treatment (Surg. Endosc. 2010; 24:1646-57). He said that robotic surgery "enables difficult technical maneuvers to be performed that facilitate the success of pancreatic minimally invasive surgery. The results in this series demonstrate feasibility and safety with clinical outcomes."
In a separate study, investigators who compared 32 open vs. 28 laparoscopic vs. 17 robotic distal pancreatectomies found that all three procedures resulted in a similar cost, while the robotic group had a shorter hospital stay, a higher rate of spleen-preserving cases, and an increased operative time, compared with patients in the other two groups (Surgery 2010;148:814-23).
Pelvic indications that are becoming the gold standard for robotic surgery include robotic prostatectomy and resection for rectal cancer. "When you have a very deep and complex surgical field like the pelvis, and you need to do a radical resection for cancer while at the same time sparing nerves that can be important for urinary function, the robot combines three-dimensional vision plus improved functionality of the instruments, allowing for a more precise dissection," Dr. Giulianotti said.
Dr. Leela M. Prasad, chief of the division of colon and rectal surgery at the University of Illinois at Chicago, described robotic-assisted surgery for rectal cancer as "far superior to anything we know right now," especially in overweight males. According to combined results from three published studies, circumferential margin positivity ranged from 0 to 7%, which, Dr. Prasad said, is significantly lower than the average number of positive margins reported following open and laparoscopic rectal surgery procedures (14% and 16%, respectively) (Ann. Surg. Oncol. 2007;14:3168-73; Surg. Oncol. 2009;16:1480-7; Dis. Colon Rectum 2009;52:1824-30).
At the present time, however, in his clinical experience there are no significant differences in outcomes between robotic-assisted and laparoscopically assisted open surgery for colon cancer. "We looked at our data, and it did not make any difference robotically or laparoscopically in terms of length of stay, lymph node harvest, or survival," he said. A recent study from Korean investigators supports those findings (Surg. Endosc. 2012 Dec. 13).
Dr. Giulianotti estimated that there are more than 1,600 robotic surgery systems in the United States. Some of them are devoted to urologic or gynecologic applications, "but in the majority of hospitals there is a growing tendency to use the robot as part of a multidisciplinary program," he said. "That means at the same institution different surgical teams on different days are using the robot for different indications. Of the 1,600 systems out there, I would estimate that about half are used for general surgery."
While hospital administrators have warmed up to the idea of initiating robotic surgery programs at their institutions in recent years, Dr. Giulianotti and Dr. Prasad both called for a culture change in terms of how surgeons and medical educators think about the robot’s place in clinical medicine. "There is a cultural resistance in accepting big changes in surgery," Dr. Giulianotti said. "Academic medical institutions can play a key role here. They need to start teaching robotics in a mandatory way by offering training opportunities in a lab. We also believe that simulation has a big future in this kind of training."
The economic investment required to launch a robotic surgery program is another challenge. "Some hospitals are only concerned about the cost," he said. "When you are doing the same procedure laparoscopically, the cost is probably about 30% less. In the future I hope we can better impact the overall economic aspect of surgical procedures. That means speeding up the postoperative and outpatient treatment of some pathologies. We are already doing Nissen fundoplication and gallbladder removal with robotic surgery, and patients are being discharged a few hours after the surgery."
Dr. Prasad said the field will be poised to further advance when makers of robotic surgery devices and instruments improve on existing technology. "I am using the same robot and instruments as I was using 3 years ago," he said. "I think we need new technologies – including a smaller robot to cut down the costs and speed up the operations. This will make it easier for patients."
To launch a successful robotic surgery program in this day and age, "you need a good team of nurses and surgeons who are dedicated to doing robotic surgery," Dr. Prasad concluded. "It is dangerous in the hands of a surgeon who is not qualified."
Dr. Giulianotti said that he had no relevant financial conflicts to disclose. Dr. Prasad disclosed that he has received honoraria from Intuitive Surgical, Ethicon Endo-Surgery, and Covidien.
Anticoagulant dabigatran ups the required dose of heparin
SAN JUAN, P.R. – The new oral anticoagulant dabigatran is the cardiologist’s darling but the intensivist’s headache and the trauma surgeon’s nightmare, suggested investigators here.
Dabigatran (Pradaxa) is a direct thrombin inhibitor approved in the United States for the reduction of risk from stroke and systemic embolism in patients with nonvalvular atrial fibrillation. Unlike Coumadin/warfarin, dabigatran’s effects are not reversible.
Pharmacists at the Scripps Mercy Hospital in San Diego evaluated prescribing patterns for dabigatran among patients in their hospital and found that 13% received it for off-label indications, a practice that has the potential for patient harm, they said at the annual meeting of the Society of Critical Care Medicine.
Of 38 patients prescribed dabigatran during their hospital stay, 33 received it for the Food and Drug Administration–approved indication but 5 (13%) received it for other, unspecified indications, reported Dr. Trevor Perry and Dr. Harminder Sikand, both clinical pharmacists at Scripps. "Prescribing was equally divided between house staff, hospitalists, and cardiologists," researchers reported (Crit. Care Med. 2012 [doi:10.1097/01.ccm.0000424500.73199.04]).
The incidence of gastrointestinal bleeding with the drug in their study was 10.5%, higher than the 6.1% rate for any gastrointestinal bleeding stated in the package insert, Dr. Perry said in an interview.
In addition, pharmacists needed to correct the dabigatran dose in 24% of patients, and dabigatran had noticeable effects on clotting parameters, with 74% of patients having an activated partial thromboplastin time (aPTT) above the upper limit of normal, and 64% of patients having an international normalized ratio (INR) above the upper limit.
This finding suggests that in these patients, the clotting assays "may be useful to determine medication adherence but not to determine the level of anticoagulation," the authors wrote in a poster presentation.
"Clinicians need to be aware of the appropriate indication for use and renal dosing of dabigatran to prevent patient harm, as only 87% of patients were prescribed dabigatran for the FDA-labeled indication," they noted.
Major heparin boost needed
In a separate study, Dr. Thomas Edrich from the department of anesthesiology, perioperative and pain medicine at Brigham & Women’s Hospital in Boston and his colleagues found that for patients scheduled for catheter-based atrial ablation procedures, those who were on dabigatran required an approximately 50% greater dose of heparin to achieve full anticoagulation for the procedure than did patients on warfarin (Crit. Care Med. 2012 [doi:10.1097/01.ccm.0000425177.10736.a4]).
Patients who had been on warfarin until 12 hours before the procedure required about 3,000-4,000 IU of heparin/hr to achieve an activated clotting time of 350 seconds, compared with about 6,500-9,000 IU/hr in patients on dabigatran, Dr. Edrich said. They studied retrospective data for 36 patients on dabigatran, 100 patients on warfarin (53 with an INR above 2.0), and 29 patients on no anticoagulation.
"The interesting finding here is that if you’ve been on dabigatran, you’re going to need twice as much heparin," he said in a poster discussion session.
Patients like it, surgeons don’t
Although patients like the convenience of oral dosing without the need for regular INR monitoring with the new anticoagulants, often they are not told that convenience may come at a very high price if there is no effective therapy to reverse the anticoagulation effect, commented Dr. Christine Toevs, a critical care surgeon at the West Penn Allegheny Health System in Pittsburgh.
"The problem is that patients aren\'t informed enough to make that choice. They understand that they’re not getting their labs drawn once a week or twice a week, and they understand that’s not a cost that they are paying. But if they fall and they have a head injury, that is a life lost, and we cannot stop it," she said during a debate on the costs of new medications.
Dr. Perry’s and Dr. Edrich’s studies were internally funded; they reported having no financial disclosures. Dr. Toevs reported having no financial disclosures.
SAN JUAN, P.R. – The new oral anticoagulant dabigatran is the cardiologist’s darling but the intensivist’s headache and the trauma surgeon’s nightmare, suggested investigators here.
Dabigatran (Pradaxa) is a direct thrombin inhibitor approved in the United States for the reduction of risk from stroke and systemic embolism in patients with nonvalvular atrial fibrillation. Unlike Coumadin/warfarin, dabigatran’s effects are not reversible.
Pharmacists at the Scripps Mercy Hospital in San Diego evaluated prescribing patterns for dabigatran among patients in their hospital and found that 13% received it for off-label indications, a practice that has the potential for patient harm, they said at the annual meeting of the Society of Critical Care Medicine.
Of 38 patients prescribed dabigatran during their hospital stay, 33 received it for the Food and Drug Administration–approved indication but 5 (13%) received it for other, unspecified indications, reported Dr. Trevor Perry and Dr. Harminder Sikand, both clinical pharmacists at Scripps. "Prescribing was equally divided between house staff, hospitalists, and cardiologists," researchers reported (Crit. Care Med. 2012 [doi:10.1097/01.ccm.0000424500.73199.04]).
The incidence of gastrointestinal bleeding with the drug in their study was 10.5%, higher than the 6.1% rate for any gastrointestinal bleeding stated in the package insert, Dr. Perry said in an interview.
In addition, pharmacists needed to correct the dabigatran dose in 24% of patients, and dabigatran had noticeable effects on clotting parameters, with 74% of patients having an activated partial thromboplastin time (aPTT) above the upper limit of normal, and 64% of patients having an international normalized ratio (INR) above the upper limit.
This finding suggests that in these patients, the clotting assays "may be useful to determine medication adherence but not to determine the level of anticoagulation," the authors wrote in a poster presentation.
"Clinicians need to be aware of the appropriate indication for use and renal dosing of dabigatran to prevent patient harm, as only 87% of patients were prescribed dabigatran for the FDA-labeled indication," they noted.
Major heparin boost needed
In a separate study, Dr. Thomas Edrich from the department of anesthesiology, perioperative and pain medicine at Brigham & Women’s Hospital in Boston and his colleagues found that for patients scheduled for catheter-based atrial ablation procedures, those who were on dabigatran required an approximately 50% greater dose of heparin to achieve full anticoagulation for the procedure than did patients on warfarin (Crit. Care Med. 2012 [doi:10.1097/01.ccm.0000425177.10736.a4]).
Patients who had been on warfarin until 12 hours before the procedure required about 3,000-4,000 IU of heparin/hr to achieve an activated clotting time of 350 seconds, compared with about 6,500-9,000 IU/hr in patients on dabigatran, Dr. Edrich said. They studied retrospective data for 36 patients on dabigatran, 100 patients on warfarin (53 with an INR above 2.0), and 29 patients on no anticoagulation.
"The interesting finding here is that if you’ve been on dabigatran, you’re going to need twice as much heparin," he said in a poster discussion session.
Patients like it, surgeons don’t
Although patients like the convenience of oral dosing without the need for regular INR monitoring with the new anticoagulants, often they are not told that convenience may come at a very high price if there is no effective therapy to reverse the anticoagulation effect, commented Dr. Christine Toevs, a critical care surgeon at the West Penn Allegheny Health System in Pittsburgh.
"The problem is that patients aren\'t informed enough to make that choice. They understand that they’re not getting their labs drawn once a week or twice a week, and they understand that’s not a cost that they are paying. But if they fall and they have a head injury, that is a life lost, and we cannot stop it," she said during a debate on the costs of new medications.
Dr. Perry’s and Dr. Edrich’s studies were internally funded; they reported having no financial disclosures. Dr. Toevs reported having no financial disclosures.
SAN JUAN, P.R. – The new oral anticoagulant dabigatran is the cardiologist’s darling but the intensivist’s headache and the trauma surgeon’s nightmare, suggested investigators here.
Dabigatran (Pradaxa) is a direct thrombin inhibitor approved in the United States for the reduction of risk from stroke and systemic embolism in patients with nonvalvular atrial fibrillation. Unlike Coumadin/warfarin, dabigatran’s effects are not reversible.
Pharmacists at the Scripps Mercy Hospital in San Diego evaluated prescribing patterns for dabigatran among patients in their hospital and found that 13% received it for off-label indications, a practice that has the potential for patient harm, they said at the annual meeting of the Society of Critical Care Medicine.
Of 38 patients prescribed dabigatran during their hospital stay, 33 received it for the Food and Drug Administration–approved indication but 5 (13%) received it for other, unspecified indications, reported Dr. Trevor Perry and Dr. Harminder Sikand, both clinical pharmacists at Scripps. "Prescribing was equally divided between house staff, hospitalists, and cardiologists," researchers reported (Crit. Care Med. 2012 [doi:10.1097/01.ccm.0000424500.73199.04]).
The incidence of gastrointestinal bleeding with the drug in their study was 10.5%, higher than the 6.1% rate for any gastrointestinal bleeding stated in the package insert, Dr. Perry said in an interview.
In addition, pharmacists needed to correct the dabigatran dose in 24% of patients, and dabigatran had noticeable effects on clotting parameters, with 74% of patients having an activated partial thromboplastin time (aPTT) above the upper limit of normal, and 64% of patients having an international normalized ratio (INR) above the upper limit.
This finding suggests that in these patients, the clotting assays "may be useful to determine medication adherence but not to determine the level of anticoagulation," the authors wrote in a poster presentation.
"Clinicians need to be aware of the appropriate indication for use and renal dosing of dabigatran to prevent patient harm, as only 87% of patients were prescribed dabigatran for the FDA-labeled indication," they noted.
Major heparin boost needed
In a separate study, Dr. Thomas Edrich from the department of anesthesiology, perioperative and pain medicine at Brigham & Women’s Hospital in Boston and his colleagues found that for patients scheduled for catheter-based atrial ablation procedures, those who were on dabigatran required an approximately 50% greater dose of heparin to achieve full anticoagulation for the procedure than did patients on warfarin (Crit. Care Med. 2012 [doi:10.1097/01.ccm.0000425177.10736.a4]).
Patients who had been on warfarin until 12 hours before the procedure required about 3,000-4,000 IU of heparin/hr to achieve an activated clotting time of 350 seconds, compared with about 6,500-9,000 IU/hr in patients on dabigatran, Dr. Edrich said. They studied retrospective data for 36 patients on dabigatran, 100 patients on warfarin (53 with an INR above 2.0), and 29 patients on no anticoagulation.
"The interesting finding here is that if you’ve been on dabigatran, you’re going to need twice as much heparin," he said in a poster discussion session.
Patients like it, surgeons don’t
Although patients like the convenience of oral dosing without the need for regular INR monitoring with the new anticoagulants, often they are not told that convenience may come at a very high price if there is no effective therapy to reverse the anticoagulation effect, commented Dr. Christine Toevs, a critical care surgeon at the West Penn Allegheny Health System in Pittsburgh.
"The problem is that patients aren\'t informed enough to make that choice. They understand that they’re not getting their labs drawn once a week or twice a week, and they understand that’s not a cost that they are paying. But if they fall and they have a head injury, that is a life lost, and we cannot stop it," she said during a debate on the costs of new medications.
Dr. Perry’s and Dr. Edrich’s studies were internally funded; they reported having no financial disclosures. Dr. Toevs reported having no financial disclosures.
AT THE ANNUAL MEETING OF THE SOCIETY OF CRITICAL CARE MEDICINE
Major finding: Of 38 patients prescribed dabigatran during their hospital stay, 5 (13%) received it for unlabeled indications.
Data source: A prospective drug evaluation study of 38 patients; a retrospective study of 189 patients scheduled for catheter ablation of atrial fibrillation.
Disclosures: Dr. Perry's and Dr. Edrich's studies were internally funded; they reported having no financial disclosures. Dr. Toevs reported having no financial disclosures.
ACS trauma verification ups survival at level II centers
SCOTTSDALE, AZ. – Level II trauma centers appeared to benefit most from attaining American College of Surgeons trauma verification, a retrospective analysis of a large national sample suggests.
Attaining ACS trauma consultation/verification was an independent predictor of patient survival in level II trauma centers (adjusted odds ratio, 1.26; P less than .01), but not in level I trauma centers (OR, 1.0; P = .84), which already performed well as a group.
Level II centers with state designation only were 3.5 times more likely to be poor performers based on survival outcomes than were level II centers that had attained ACS verification, Dr. Joshua Brown reported at the annual meeting of the Eastern Association for the Surgery of Trauma.
"Promoting ACS verification among level II trauma centers may represent an opportunity for improved outcomes in select trauma centers," he said.
A rigorous process that demands significant financial and personnel resources, ACS verification has been shown in single-center reports to improve outcomes. Yet less than 25% of level I and II trauma centers pursue ACS verification if already designated by their state as a trauma center, said Dr. Brown, a general surgery resident at the University of Pittsburgh Medical Center.
To compare mortality at ACS verified and state designated centers in a national sample, the investigators identified 900,274 patients, at least 16 years old, in the National Trauma Data Bank 2007-2008. Their mean age was 42 years, mean Injury Severity Score was 9, and 76% were transported from the trauma scene. The patients were admitted to 189 level I (65% ACS verified) and 185 level II trauma centers (67% ACS verified).
A mortality prediction model was constructed based on published Trauma Quality Improvement Program (TQIP) methodology, and multiple imputation used for missing data. The model had an area under the curve of 0.92, indicating excellent discrimination to predict death, Dr. Brown said.
Level I ACS centers had a significantly lower median observed-to-expected (O/E) mortality ratio than did level I state centers, (0.95 vs. 1.02; P less than .01).
No difference was observed in median O/E mortality ratio between level II ACS and state centers (0.94 vs. 0.87; P = .30), he said.
As noted earlier, however, level II state centers had more than three times the poor performers – high O/E ratio outliers, defined by an O/E and 90% confidence interval greater than 1.0 – than level II ACS centers (13% vs. 4%; OR 3.59; P = .03).
The proportion of good performers – low O/E outliers, defined by an O/E and C.I. less than 1.0 – was similar at state vs. ACS level II centers (21% vs. 23%;P = .57).
At level I trauma centers, no significant differences were observed between ACS-verified and state-verified centers in the proportion of high outliers (15% vs. 24%; P = .16) or low outliers (28% vs. 16%; P = .1), Dr. Brown reported.
Dr. Keith Clancy of York (Penn.) Hospital, who was invited to discuss the study, said, "It was a well-written, well-designed paper on a very important topic that will provide some pause for states that may inappropriately believe that simply by incorporating ACS standards into their state trauma standards they can automatically achieve the same outcomes as ACS verification."
Dr. Brown and Dr. Clancy reported no financial disclosures.
SCOTTSDALE, AZ. – Level II trauma centers appeared to benefit most from attaining American College of Surgeons trauma verification, a retrospective analysis of a large national sample suggests.
Attaining ACS trauma consultation/verification was an independent predictor of patient survival in level II trauma centers (adjusted odds ratio, 1.26; P less than .01), but not in level I trauma centers (OR, 1.0; P = .84), which already performed well as a group.
Level II centers with state designation only were 3.5 times more likely to be poor performers based on survival outcomes than were level II centers that had attained ACS verification, Dr. Joshua Brown reported at the annual meeting of the Eastern Association for the Surgery of Trauma.
"Promoting ACS verification among level II trauma centers may represent an opportunity for improved outcomes in select trauma centers," he said.
A rigorous process that demands significant financial and personnel resources, ACS verification has been shown in single-center reports to improve outcomes. Yet less than 25% of level I and II trauma centers pursue ACS verification if already designated by their state as a trauma center, said Dr. Brown, a general surgery resident at the University of Pittsburgh Medical Center.
To compare mortality at ACS verified and state designated centers in a national sample, the investigators identified 900,274 patients, at least 16 years old, in the National Trauma Data Bank 2007-2008. Their mean age was 42 years, mean Injury Severity Score was 9, and 76% were transported from the trauma scene. The patients were admitted to 189 level I (65% ACS verified) and 185 level II trauma centers (67% ACS verified).
A mortality prediction model was constructed based on published Trauma Quality Improvement Program (TQIP) methodology, and multiple imputation used for missing data. The model had an area under the curve of 0.92, indicating excellent discrimination to predict death, Dr. Brown said.
Level I ACS centers had a significantly lower median observed-to-expected (O/E) mortality ratio than did level I state centers, (0.95 vs. 1.02; P less than .01).
No difference was observed in median O/E mortality ratio between level II ACS and state centers (0.94 vs. 0.87; P = .30), he said.
As noted earlier, however, level II state centers had more than three times the poor performers – high O/E ratio outliers, defined by an O/E and 90% confidence interval greater than 1.0 – than level II ACS centers (13% vs. 4%; OR 3.59; P = .03).
The proportion of good performers – low O/E outliers, defined by an O/E and C.I. less than 1.0 – was similar at state vs. ACS level II centers (21% vs. 23%;P = .57).
At level I trauma centers, no significant differences were observed between ACS-verified and state-verified centers in the proportion of high outliers (15% vs. 24%; P = .16) or low outliers (28% vs. 16%; P = .1), Dr. Brown reported.
Dr. Keith Clancy of York (Penn.) Hospital, who was invited to discuss the study, said, "It was a well-written, well-designed paper on a very important topic that will provide some pause for states that may inappropriately believe that simply by incorporating ACS standards into their state trauma standards they can automatically achieve the same outcomes as ACS verification."
Dr. Brown and Dr. Clancy reported no financial disclosures.
SCOTTSDALE, AZ. – Level II trauma centers appeared to benefit most from attaining American College of Surgeons trauma verification, a retrospective analysis of a large national sample suggests.
Attaining ACS trauma consultation/verification was an independent predictor of patient survival in level II trauma centers (adjusted odds ratio, 1.26; P less than .01), but not in level I trauma centers (OR, 1.0; P = .84), which already performed well as a group.
Level II centers with state designation only were 3.5 times more likely to be poor performers based on survival outcomes than were level II centers that had attained ACS verification, Dr. Joshua Brown reported at the annual meeting of the Eastern Association for the Surgery of Trauma.
"Promoting ACS verification among level II trauma centers may represent an opportunity for improved outcomes in select trauma centers," he said.
A rigorous process that demands significant financial and personnel resources, ACS verification has been shown in single-center reports to improve outcomes. Yet less than 25% of level I and II trauma centers pursue ACS verification if already designated by their state as a trauma center, said Dr. Brown, a general surgery resident at the University of Pittsburgh Medical Center.
To compare mortality at ACS verified and state designated centers in a national sample, the investigators identified 900,274 patients, at least 16 years old, in the National Trauma Data Bank 2007-2008. Their mean age was 42 years, mean Injury Severity Score was 9, and 76% were transported from the trauma scene. The patients were admitted to 189 level I (65% ACS verified) and 185 level II trauma centers (67% ACS verified).
A mortality prediction model was constructed based on published Trauma Quality Improvement Program (TQIP) methodology, and multiple imputation used for missing data. The model had an area under the curve of 0.92, indicating excellent discrimination to predict death, Dr. Brown said.
Level I ACS centers had a significantly lower median observed-to-expected (O/E) mortality ratio than did level I state centers, (0.95 vs. 1.02; P less than .01).
No difference was observed in median O/E mortality ratio between level II ACS and state centers (0.94 vs. 0.87; P = .30), he said.
As noted earlier, however, level II state centers had more than three times the poor performers – high O/E ratio outliers, defined by an O/E and 90% confidence interval greater than 1.0 – than level II ACS centers (13% vs. 4%; OR 3.59; P = .03).
The proportion of good performers – low O/E outliers, defined by an O/E and C.I. less than 1.0 – was similar at state vs. ACS level II centers (21% vs. 23%;P = .57).
At level I trauma centers, no significant differences were observed between ACS-verified and state-verified centers in the proportion of high outliers (15% vs. 24%; P = .16) or low outliers (28% vs. 16%; P = .1), Dr. Brown reported.
Dr. Keith Clancy of York (Penn.) Hospital, who was invited to discuss the study, said, "It was a well-written, well-designed paper on a very important topic that will provide some pause for states that may inappropriately believe that simply by incorporating ACS standards into their state trauma standards they can automatically achieve the same outcomes as ACS verification."
Dr. Brown and Dr. Clancy reported no financial disclosures.
AT THE ANNUAL MEETING OF THE EASTERN ASSOCIATION FOR THE SURGERY OF TRAUMA
Major Finding: ACS trauma verification was a significant independent predictor of survival at level II trauma centers (adjusted OR, 1.26; P less than .01), but not at level I centers (OR, 1.0; P = .84).
Data Source: Retrospective analysis of 900,274 patients in the National Trauma Data Bank.
Disclosures: Dr. Brown and Dr. Clancy reported no disclosures.
New tool predicts late recurrence in breast cancer
SAN ANTONIO – A novel multigene signature known as the breast cancer index markedly outperformed the widely used Oncotype DX Recurrence Score and IHC4 tools in predicting late recurrences of estrogen receptor-positive/lymph node-negative breast cancer, Dr. Dennis C. Sgroi reported at the annual San Antonio Breast Cancer Symposium.
In a large clinical study, all three tools demonstrated significant prognostic value for early distant recurrences – that is, breast cancers recurring within 5 years of diagnosis. But only the breast cancer index (BCI) had sustained power for predicting distant recurrences arising during years 5-10. Both Oncotype DX and the IHC4 – a test based upon a tumor’s estrogen receptor, progesterone receptor, HER2, and Ki-67 status – lost their prognostic ability at about the 5-year mark, observed Dr. Sgroi of Massachusetts General Hospital, Boston.
More than half of all recurrences of estrogen receptor–positive early-stage breast cancer take place after 5 years of adjuvant tamoxifen or aromatase inhibitor therapy. Thus, the BCI fills a major need: a biomarker capable of identifying those estrogen receptor–positive breast cancer patients at increased risk for recurrence 5-10 years after their diagnosis.
The BCI includes the ratio of HOXB13-to-IL17BR gene expression and a five-gene molecular grade index shown to be predictive beyond tumor grade of distance metastasis. Dr. Sgroi and coinvestigators have previously established that these two biomarkers are complementary in their power to predict recurrences (Clin. Cancer Res. 2008;14:2601-8).
At the San Antonio symposium, Dr. Sgroi presented an analysis of the comparative prognostic performance of the BCI, the Oncotype DX recurrence score, and the IHC4 as applied to baseline tumor samples from 665 hormone receptor-positive participants in the randomized, multicenter TransATAC (Arimedex, Tamoxifen, Alone or Together) trial. The primary endpoint was distant recurrence.
The baseline BCI score differentiated three risk groups over the course of 10 years of follow-up in TransATAC: 58% of women fell into the low-risk group with a 4.2% risk of distant recurrence within 10 years, 25% had an intermediate score with an 18% risk, and 17% had a high score with a 30% risk.
In the first 5 years of follow-up, patients with a low- or intermediate-risk BCI had a distant recurrence risk of 1.3% and 5.6%, respectively. Thus, the BCI identified 83% of study participants as having, on average, a 5-year distant recurrence risk of less than 5%. In contrast, women with a high BCI score had a 5-year recurrence risk of 18%.
Among patients who remained disease free at 5 years, the 61% with a low baseline BCI score had a 3.5% rate of distant recurrence during years 5-10. Those with an intermediate or high BCI had a 13.4% rate.
In a multivariate analysis adjusted for the clinical treatment score – an algorithm based upon nodal status, tumor size and grade, age, and treatment (J. Clin. Oncol. 2011;29:4273-8) – the BCI, IHC4, and Oncotype DX displayed similar prognostic performance for distant recurrences in years 0-5. But the latter two tests lost their predictive capability in years 5-10.
Estrogen receptor–positive/node-negative patients have traditionally been considered at low risk. But, as shown in the TransATAC analysis, applying the BCI to primary tumor specimens from such patients enables physicians to identify two groups at the time of diagnosis: those who are at low risk for recurrence within the next 5 years and who are adequately treated with endocrine therapy alone; and a high-risk subgroup who should be considered for chemotherapy or some other additional therapy along with endocrine therapy, Dr. Sgroi concluded.
Moreover, the BCI score at diagnosis also permits identification of two distinct groups among patients remaining disease free at 5 years of follow up: those at low risk of late recurrence and who don’t need subsequent therapy; and those at roughly a threefold greater risk of late recurrence and are therefore candidates for further systemic adjuvant therapy.
"What that additional treatment should be is something we don’t know at this point. It might be extended adjuvant hormonal therapy alone or in combination with another therapeutic agent," he added.
In response to an audience question, Dr. Sgroi admitted that the exact biologic role of the genes incorporated in the BCI is "still a bit of a mystery," although it’s clear that they’re not solely involved in proliferation.
The TransATAC study was funded by AstraZeneca. Dr. Sgroi’s BCI work is supported by the National Institutes of Health, the U.S. Department of Defense, the Avon Foundation, and the Susan G. Komen for the Cure.
SAN ANTONIO – A novel multigene signature known as the breast cancer index markedly outperformed the widely used Oncotype DX Recurrence Score and IHC4 tools in predicting late recurrences of estrogen receptor-positive/lymph node-negative breast cancer, Dr. Dennis C. Sgroi reported at the annual San Antonio Breast Cancer Symposium.
In a large clinical study, all three tools demonstrated significant prognostic value for early distant recurrences – that is, breast cancers recurring within 5 years of diagnosis. But only the breast cancer index (BCI) had sustained power for predicting distant recurrences arising during years 5-10. Both Oncotype DX and the IHC4 – a test based upon a tumor’s estrogen receptor, progesterone receptor, HER2, and Ki-67 status – lost their prognostic ability at about the 5-year mark, observed Dr. Sgroi of Massachusetts General Hospital, Boston.
More than half of all recurrences of estrogen receptor–positive early-stage breast cancer take place after 5 years of adjuvant tamoxifen or aromatase inhibitor therapy. Thus, the BCI fills a major need: a biomarker capable of identifying those estrogen receptor–positive breast cancer patients at increased risk for recurrence 5-10 years after their diagnosis.
The BCI includes the ratio of HOXB13-to-IL17BR gene expression and a five-gene molecular grade index shown to be predictive beyond tumor grade of distance metastasis. Dr. Sgroi and coinvestigators have previously established that these two biomarkers are complementary in their power to predict recurrences (Clin. Cancer Res. 2008;14:2601-8).
At the San Antonio symposium, Dr. Sgroi presented an analysis of the comparative prognostic performance of the BCI, the Oncotype DX recurrence score, and the IHC4 as applied to baseline tumor samples from 665 hormone receptor-positive participants in the randomized, multicenter TransATAC (Arimedex, Tamoxifen, Alone or Together) trial. The primary endpoint was distant recurrence.
The baseline BCI score differentiated three risk groups over the course of 10 years of follow-up in TransATAC: 58% of women fell into the low-risk group with a 4.2% risk of distant recurrence within 10 years, 25% had an intermediate score with an 18% risk, and 17% had a high score with a 30% risk.
In the first 5 years of follow-up, patients with a low- or intermediate-risk BCI had a distant recurrence risk of 1.3% and 5.6%, respectively. Thus, the BCI identified 83% of study participants as having, on average, a 5-year distant recurrence risk of less than 5%. In contrast, women with a high BCI score had a 5-year recurrence risk of 18%.
Among patients who remained disease free at 5 years, the 61% with a low baseline BCI score had a 3.5% rate of distant recurrence during years 5-10. Those with an intermediate or high BCI had a 13.4% rate.
In a multivariate analysis adjusted for the clinical treatment score – an algorithm based upon nodal status, tumor size and grade, age, and treatment (J. Clin. Oncol. 2011;29:4273-8) – the BCI, IHC4, and Oncotype DX displayed similar prognostic performance for distant recurrences in years 0-5. But the latter two tests lost their predictive capability in years 5-10.
Estrogen receptor–positive/node-negative patients have traditionally been considered at low risk. But, as shown in the TransATAC analysis, applying the BCI to primary tumor specimens from such patients enables physicians to identify two groups at the time of diagnosis: those who are at low risk for recurrence within the next 5 years and who are adequately treated with endocrine therapy alone; and a high-risk subgroup who should be considered for chemotherapy or some other additional therapy along with endocrine therapy, Dr. Sgroi concluded.
Moreover, the BCI score at diagnosis also permits identification of two distinct groups among patients remaining disease free at 5 years of follow up: those at low risk of late recurrence and who don’t need subsequent therapy; and those at roughly a threefold greater risk of late recurrence and are therefore candidates for further systemic adjuvant therapy.
"What that additional treatment should be is something we don’t know at this point. It might be extended adjuvant hormonal therapy alone or in combination with another therapeutic agent," he added.
In response to an audience question, Dr. Sgroi admitted that the exact biologic role of the genes incorporated in the BCI is "still a bit of a mystery," although it’s clear that they’re not solely involved in proliferation.
The TransATAC study was funded by AstraZeneca. Dr. Sgroi’s BCI work is supported by the National Institutes of Health, the U.S. Department of Defense, the Avon Foundation, and the Susan G. Komen for the Cure.
SAN ANTONIO – A novel multigene signature known as the breast cancer index markedly outperformed the widely used Oncotype DX Recurrence Score and IHC4 tools in predicting late recurrences of estrogen receptor-positive/lymph node-negative breast cancer, Dr. Dennis C. Sgroi reported at the annual San Antonio Breast Cancer Symposium.
In a large clinical study, all three tools demonstrated significant prognostic value for early distant recurrences – that is, breast cancers recurring within 5 years of diagnosis. But only the breast cancer index (BCI) had sustained power for predicting distant recurrences arising during years 5-10. Both Oncotype DX and the IHC4 – a test based upon a tumor’s estrogen receptor, progesterone receptor, HER2, and Ki-67 status – lost their prognostic ability at about the 5-year mark, observed Dr. Sgroi of Massachusetts General Hospital, Boston.
More than half of all recurrences of estrogen receptor–positive early-stage breast cancer take place after 5 years of adjuvant tamoxifen or aromatase inhibitor therapy. Thus, the BCI fills a major need: a biomarker capable of identifying those estrogen receptor–positive breast cancer patients at increased risk for recurrence 5-10 years after their diagnosis.
The BCI includes the ratio of HOXB13-to-IL17BR gene expression and a five-gene molecular grade index shown to be predictive beyond tumor grade of distance metastasis. Dr. Sgroi and coinvestigators have previously established that these two biomarkers are complementary in their power to predict recurrences (Clin. Cancer Res. 2008;14:2601-8).
At the San Antonio symposium, Dr. Sgroi presented an analysis of the comparative prognostic performance of the BCI, the Oncotype DX recurrence score, and the IHC4 as applied to baseline tumor samples from 665 hormone receptor-positive participants in the randomized, multicenter TransATAC (Arimedex, Tamoxifen, Alone or Together) trial. The primary endpoint was distant recurrence.
The baseline BCI score differentiated three risk groups over the course of 10 years of follow-up in TransATAC: 58% of women fell into the low-risk group with a 4.2% risk of distant recurrence within 10 years, 25% had an intermediate score with an 18% risk, and 17% had a high score with a 30% risk.
In the first 5 years of follow-up, patients with a low- or intermediate-risk BCI had a distant recurrence risk of 1.3% and 5.6%, respectively. Thus, the BCI identified 83% of study participants as having, on average, a 5-year distant recurrence risk of less than 5%. In contrast, women with a high BCI score had a 5-year recurrence risk of 18%.
Among patients who remained disease free at 5 years, the 61% with a low baseline BCI score had a 3.5% rate of distant recurrence during years 5-10. Those with an intermediate or high BCI had a 13.4% rate.
In a multivariate analysis adjusted for the clinical treatment score – an algorithm based upon nodal status, tumor size and grade, age, and treatment (J. Clin. Oncol. 2011;29:4273-8) – the BCI, IHC4, and Oncotype DX displayed similar prognostic performance for distant recurrences in years 0-5. But the latter two tests lost their predictive capability in years 5-10.
Estrogen receptor–positive/node-negative patients have traditionally been considered at low risk. But, as shown in the TransATAC analysis, applying the BCI to primary tumor specimens from such patients enables physicians to identify two groups at the time of diagnosis: those who are at low risk for recurrence within the next 5 years and who are adequately treated with endocrine therapy alone; and a high-risk subgroup who should be considered for chemotherapy or some other additional therapy along with endocrine therapy, Dr. Sgroi concluded.
Moreover, the BCI score at diagnosis also permits identification of two distinct groups among patients remaining disease free at 5 years of follow up: those at low risk of late recurrence and who don’t need subsequent therapy; and those at roughly a threefold greater risk of late recurrence and are therefore candidates for further systemic adjuvant therapy.
"What that additional treatment should be is something we don’t know at this point. It might be extended adjuvant hormonal therapy alone or in combination with another therapeutic agent," he added.
In response to an audience question, Dr. Sgroi admitted that the exact biologic role of the genes incorporated in the BCI is "still a bit of a mystery," although it’s clear that they’re not solely involved in proliferation.
The TransATAC study was funded by AstraZeneca. Dr. Sgroi’s BCI work is supported by the National Institutes of Health, the U.S. Department of Defense, the Avon Foundation, and the Susan G. Komen for the Cure.
AT THE ANNUAL SAN ANTONIO BREAST CANCER SYMPOSIUM
Major Finding: Among patients who remained disease free at 5 years, the 61% with a low baseline BCI score had a 3.5% rate of distant recurrence during years 5-10. Those with an intermediate or high BCI had a 13.4% rate.
Data Source: This study compared the prognostic performance of three biomarker-based tools in predicting the risk at 5-10 years of distant recurrence of breast cancer in TransATAC study participants.
Disclosures: The TransATAC study was funded by AstraZeneca. Dr. Sgroi’s BCI work is supported by the National Institutes of Health, the U.S. Department of Defense, the Avon Foundation, and the Susan G. Komen for the Cure.