User login
Official Newspaper of the American College of Surgeons
Adjuvant Chemotherapy Boosts Survival in Locoregional Recurrent Breast Cancer
SAN ANTONIO – Adjuvant chemotherapy in women with completely resected locoregional recurrence of breast cancer improved disease-free and overall survival in the randomized CALOR trial.
Indeed, adjuvant chemotherapy reduced the risk of recurrent disease by 41% during 5 years of follow-up in CALOR (Chemotherapy as Adjuvant for Locally Recurrent Breast Cancer) while cutting the risk of all-cause mortality by 59%, Dr. Stefan Aebi reported at the annual San Antonio Breast Cancer Symposium.
Thus, CALOR helps resolve a longstanding controversy regarding the appropriate treatment of patients with isolated local or regional recurrence of breast cancer. But the trial provides only a partial resolution. That’s because while adjuvant chemotherapy had a huge benefit in patients with estrogen receptor–negative locoregional recurrences, in estrogen receptor–positive recurrences it had no significant effect.
"Decisions regarding ER-positive recurrent tumors remain a struggle. But events are very few so far. We consider this analysis premature. We will need longer follow-up for patients with ER-positive recurrences to see if there is a benefit for chemotherapy," said Dr. Aebi, head of the division of medical oncology at Lucerne Canton Hospital in Switzerland.
CALOR included 162 patients with isolated local and/or regional recurrence of breast cancer. After complete excisional surgery, they were randomized to chemotherapy or no chemotherapy. The choice of chemotherapy regimen was left to the patient’s oncologists, with a recommendation from CALOR investigators to use at least two drugs for 3-6 months. Radiation therapy was recommended for all patients, but only about 40% received it.
The 5-year disease-free survival rate – the primary endpoint – was 69% in the chemotherapy group compared with 57% with no adjuvant chemotherapy. This translates to a 41% relative risk reduction (P = .045). The 5-year overall survival rate was 88% in the chemotherapy group vs. 76% in controls, for a 59% reduction in risk (P = .02).
These benefits were driven by the outstanding effectiveness of chemotherapy in patients with ER-negative recurrences. Their 5-year disease-free survival rate was 67% with adjuvant chemotherapy compared with 35% without it, for a 68% reduction in risk (P = .007). Overall survival in the ER-negative recurrence subgroup was 79% with chemotherapy and 69% without. In contrast, the 5-year disease-free survival rate in the ER-positive group was 70% with chemotherapy and 69% without.
In a multivariate analysis controlling for ER status, location of the isolated recurrence, and prior chemotherapy, adjunctive chemotherapy was associated with a 50% reduction in recurrent disease during 5 years of follow-up (P = .01). The only other independent predictor of disease-free survival was time since primary surgery: The risk of recurrent disease during 5 years of follow-up dropped by 9% for each year since primary surgery.
This was a difficult study to conduct. The original plans called for recruitment of nearly 1,000 patients, but enrollment was so slow that Dr. Aebi and coinvestigators had to scale back their ambitions, eventually closing the trial with 162 participants.
"There were many colleagues with preconceived ideas. We had colleagues who just knew that chemotherapy was not needed and others who just knew that it was needed. And if you know, why should you randomize your patients?" he explained.
Dr. Carlos L. Arteaga called CALOR "a very important contribution to what has been an ongoing controversy in this field."
"Some surgeons feel very strongly that resection is enough in treating local recurrences, while many of our medical oncologists feel chemotherapy is also required. I have a lot more impetus to give adjuvant chemotherapy based on the CALOR findings," said Dr. Arteaga, director of the breast cancer program at Vanderbilt-Ingram Cancer Center, Nashville, Tenn.
The CALOR trial was sponsored by several major cancer research organizations. Dr. Aebi reported having no financial conflicts.
SAN ANTONIO – Adjuvant chemotherapy in women with completely resected locoregional recurrence of breast cancer improved disease-free and overall survival in the randomized CALOR trial.
Indeed, adjuvant chemotherapy reduced the risk of recurrent disease by 41% during 5 years of follow-up in CALOR (Chemotherapy as Adjuvant for Locally Recurrent Breast Cancer) while cutting the risk of all-cause mortality by 59%, Dr. Stefan Aebi reported at the annual San Antonio Breast Cancer Symposium.
Thus, CALOR helps resolve a longstanding controversy regarding the appropriate treatment of patients with isolated local or regional recurrence of breast cancer. But the trial provides only a partial resolution. That’s because while adjuvant chemotherapy had a huge benefit in patients with estrogen receptor–negative locoregional recurrences, in estrogen receptor–positive recurrences it had no significant effect.
"Decisions regarding ER-positive recurrent tumors remain a struggle. But events are very few so far. We consider this analysis premature. We will need longer follow-up for patients with ER-positive recurrences to see if there is a benefit for chemotherapy," said Dr. Aebi, head of the division of medical oncology at Lucerne Canton Hospital in Switzerland.
CALOR included 162 patients with isolated local and/or regional recurrence of breast cancer. After complete excisional surgery, they were randomized to chemotherapy or no chemotherapy. The choice of chemotherapy regimen was left to the patient’s oncologists, with a recommendation from CALOR investigators to use at least two drugs for 3-6 months. Radiation therapy was recommended for all patients, but only about 40% received it.
The 5-year disease-free survival rate – the primary endpoint – was 69% in the chemotherapy group compared with 57% with no adjuvant chemotherapy. This translates to a 41% relative risk reduction (P = .045). The 5-year overall survival rate was 88% in the chemotherapy group vs. 76% in controls, for a 59% reduction in risk (P = .02).
These benefits were driven by the outstanding effectiveness of chemotherapy in patients with ER-negative recurrences. Their 5-year disease-free survival rate was 67% with adjuvant chemotherapy compared with 35% without it, for a 68% reduction in risk (P = .007). Overall survival in the ER-negative recurrence subgroup was 79% with chemotherapy and 69% without. In contrast, the 5-year disease-free survival rate in the ER-positive group was 70% with chemotherapy and 69% without.
In a multivariate analysis controlling for ER status, location of the isolated recurrence, and prior chemotherapy, adjunctive chemotherapy was associated with a 50% reduction in recurrent disease during 5 years of follow-up (P = .01). The only other independent predictor of disease-free survival was time since primary surgery: The risk of recurrent disease during 5 years of follow-up dropped by 9% for each year since primary surgery.
This was a difficult study to conduct. The original plans called for recruitment of nearly 1,000 patients, but enrollment was so slow that Dr. Aebi and coinvestigators had to scale back their ambitions, eventually closing the trial with 162 participants.
"There were many colleagues with preconceived ideas. We had colleagues who just knew that chemotherapy was not needed and others who just knew that it was needed. And if you know, why should you randomize your patients?" he explained.
Dr. Carlos L. Arteaga called CALOR "a very important contribution to what has been an ongoing controversy in this field."
"Some surgeons feel very strongly that resection is enough in treating local recurrences, while many of our medical oncologists feel chemotherapy is also required. I have a lot more impetus to give adjuvant chemotherapy based on the CALOR findings," said Dr. Arteaga, director of the breast cancer program at Vanderbilt-Ingram Cancer Center, Nashville, Tenn.
The CALOR trial was sponsored by several major cancer research organizations. Dr. Aebi reported having no financial conflicts.
SAN ANTONIO – Adjuvant chemotherapy in women with completely resected locoregional recurrence of breast cancer improved disease-free and overall survival in the randomized CALOR trial.
Indeed, adjuvant chemotherapy reduced the risk of recurrent disease by 41% during 5 years of follow-up in CALOR (Chemotherapy as Adjuvant for Locally Recurrent Breast Cancer) while cutting the risk of all-cause mortality by 59%, Dr. Stefan Aebi reported at the annual San Antonio Breast Cancer Symposium.
Thus, CALOR helps resolve a longstanding controversy regarding the appropriate treatment of patients with isolated local or regional recurrence of breast cancer. But the trial provides only a partial resolution. That’s because while adjuvant chemotherapy had a huge benefit in patients with estrogen receptor–negative locoregional recurrences, in estrogen receptor–positive recurrences it had no significant effect.
"Decisions regarding ER-positive recurrent tumors remain a struggle. But events are very few so far. We consider this analysis premature. We will need longer follow-up for patients with ER-positive recurrences to see if there is a benefit for chemotherapy," said Dr. Aebi, head of the division of medical oncology at Lucerne Canton Hospital in Switzerland.
CALOR included 162 patients with isolated local and/or regional recurrence of breast cancer. After complete excisional surgery, they were randomized to chemotherapy or no chemotherapy. The choice of chemotherapy regimen was left to the patient’s oncologists, with a recommendation from CALOR investigators to use at least two drugs for 3-6 months. Radiation therapy was recommended for all patients, but only about 40% received it.
The 5-year disease-free survival rate – the primary endpoint – was 69% in the chemotherapy group compared with 57% with no adjuvant chemotherapy. This translates to a 41% relative risk reduction (P = .045). The 5-year overall survival rate was 88% in the chemotherapy group vs. 76% in controls, for a 59% reduction in risk (P = .02).
These benefits were driven by the outstanding effectiveness of chemotherapy in patients with ER-negative recurrences. Their 5-year disease-free survival rate was 67% with adjuvant chemotherapy compared with 35% without it, for a 68% reduction in risk (P = .007). Overall survival in the ER-negative recurrence subgroup was 79% with chemotherapy and 69% without. In contrast, the 5-year disease-free survival rate in the ER-positive group was 70% with chemotherapy and 69% without.
In a multivariate analysis controlling for ER status, location of the isolated recurrence, and prior chemotherapy, adjunctive chemotherapy was associated with a 50% reduction in recurrent disease during 5 years of follow-up (P = .01). The only other independent predictor of disease-free survival was time since primary surgery: The risk of recurrent disease during 5 years of follow-up dropped by 9% for each year since primary surgery.
This was a difficult study to conduct. The original plans called for recruitment of nearly 1,000 patients, but enrollment was so slow that Dr. Aebi and coinvestigators had to scale back their ambitions, eventually closing the trial with 162 participants.
"There were many colleagues with preconceived ideas. We had colleagues who just knew that chemotherapy was not needed and others who just knew that it was needed. And if you know, why should you randomize your patients?" he explained.
Dr. Carlos L. Arteaga called CALOR "a very important contribution to what has been an ongoing controversy in this field."
"Some surgeons feel very strongly that resection is enough in treating local recurrences, while many of our medical oncologists feel chemotherapy is also required. I have a lot more impetus to give adjuvant chemotherapy based on the CALOR findings," said Dr. Arteaga, director of the breast cancer program at Vanderbilt-Ingram Cancer Center, Nashville, Tenn.
The CALOR trial was sponsored by several major cancer research organizations. Dr. Aebi reported having no financial conflicts.
AT THE SAN ANTONIO BREAST CANCER SYMPOSIUM
Major Finding: The 5-year disease-free survival rate after complete surgical removal of isolated local or regional recurrence of breast cancer was 69% in patients randomized to adjuvant chemotherapy compared with 57% in no-chemotherapy controls.
Data Source: The CALOR trial was an international randomized trial involving 162 patients.
Disclosures: The study was sponsored by several major cancer research organizations. The presenter reported having no financial conflicts.
SurgiSIS myringoplasty shortens operative time
WASHINGTON – SurgiSIS, a material derived from porcine small intestinal mucosa, can be safely and effectively used for myringoplasty in children, based on data from a prospective, blinded study of 404 patients.
Patients’ tissue is not always available for tympanic membrane repair, and harvesting the graft may increase intraoperative time, said Dr. Riccardo D’Eredita of Vincenza (Italy) Civil Hospital. SurgiSIS (SIS) "promotes early vessel growth, provides scaffolding for remodeling tissues, and is inexpensive and ready to use." He presented the findings at the annual meeting of the American Academy of Otolaryngology – Head and Neck Surgery Foundation.
The material has been used widely in children, and data from previous studies show that SurgiSIS is gradually replaced by host cells, said Dr. D’Eredita. After 30 days, host cells invade SurgiSIS. After 1 year, SurgiSIS is no longer evident, and has been replaced by the patients’ collagen.
In this study, 404 children underwent tympanic membrane repair in 432 ears; 217 were randomized to myringoplasty with SurgiSIS and 215 were randomized to repair using the patients’ own temporalis fascia.
Overall, the group without SurgiSIS had a 97% rate of stable closures and the group with SurgiSIS had a 95% rate. Surgical time was approximately 15 minutes less for SurgiSIS-treated patients, Dr. D’Eredita said.
The researchers assessed the healing of the tympanic membranes over a 10-year period and found comparable reduction of inflammation in the two groups. There were no adverse reactions in the SIS group.
Dr. D’Eredita had no financial conflicts to disclose.
WASHINGTON – SurgiSIS, a material derived from porcine small intestinal mucosa, can be safely and effectively used for myringoplasty in children, based on data from a prospective, blinded study of 404 patients.
Patients’ tissue is not always available for tympanic membrane repair, and harvesting the graft may increase intraoperative time, said Dr. Riccardo D’Eredita of Vincenza (Italy) Civil Hospital. SurgiSIS (SIS) "promotes early vessel growth, provides scaffolding for remodeling tissues, and is inexpensive and ready to use." He presented the findings at the annual meeting of the American Academy of Otolaryngology – Head and Neck Surgery Foundation.
The material has been used widely in children, and data from previous studies show that SurgiSIS is gradually replaced by host cells, said Dr. D’Eredita. After 30 days, host cells invade SurgiSIS. After 1 year, SurgiSIS is no longer evident, and has been replaced by the patients’ collagen.
In this study, 404 children underwent tympanic membrane repair in 432 ears; 217 were randomized to myringoplasty with SurgiSIS and 215 were randomized to repair using the patients’ own temporalis fascia.
Overall, the group without SurgiSIS had a 97% rate of stable closures and the group with SurgiSIS had a 95% rate. Surgical time was approximately 15 minutes less for SurgiSIS-treated patients, Dr. D’Eredita said.
The researchers assessed the healing of the tympanic membranes over a 10-year period and found comparable reduction of inflammation in the two groups. There were no adverse reactions in the SIS group.
Dr. D’Eredita had no financial conflicts to disclose.
WASHINGTON – SurgiSIS, a material derived from porcine small intestinal mucosa, can be safely and effectively used for myringoplasty in children, based on data from a prospective, blinded study of 404 patients.
Patients’ tissue is not always available for tympanic membrane repair, and harvesting the graft may increase intraoperative time, said Dr. Riccardo D’Eredita of Vincenza (Italy) Civil Hospital. SurgiSIS (SIS) "promotes early vessel growth, provides scaffolding for remodeling tissues, and is inexpensive and ready to use." He presented the findings at the annual meeting of the American Academy of Otolaryngology – Head and Neck Surgery Foundation.
The material has been used widely in children, and data from previous studies show that SurgiSIS is gradually replaced by host cells, said Dr. D’Eredita. After 30 days, host cells invade SurgiSIS. After 1 year, SurgiSIS is no longer evident, and has been replaced by the patients’ collagen.
In this study, 404 children underwent tympanic membrane repair in 432 ears; 217 were randomized to myringoplasty with SurgiSIS and 215 were randomized to repair using the patients’ own temporalis fascia.
Overall, the group without SurgiSIS had a 97% rate of stable closures and the group with SurgiSIS had a 95% rate. Surgical time was approximately 15 minutes less for SurgiSIS-treated patients, Dr. D’Eredita said.
The researchers assessed the healing of the tympanic membranes over a 10-year period and found comparable reduction of inflammation in the two groups. There were no adverse reactions in the SIS group.
Dr. D’Eredita had no financial conflicts to disclose.
AT THE ANNUAL MEETING OF THE AMERICAN ACADEMY OF OTOLARYNGOLOGY - HEAD AND NECK SURGERY FOUNDATION
Major Finding: The number of stable surgical closures was similar in children who had tympanic membrane repair with porcine small intestinal mucosa (212) compared with use of their own tissue (204).
Data Source: The data comprise 432 ears in 404 children.
Disclosures: Dr. D’Eredita had no financial conflicts to disclose.
Facial Nerve Dysfunction Seen in 25% of Pediatric Parotidectomy Patients
WASHINGTON – Facial nerve dysfunction affected 23% of 43 children who had parotidectomies in a single-center study presented at the annual meeting of the American Academy of Otolaryngology–Head and Neck Surgery Foundation.
The findings suggest that facial nerve dysfunction after parotidectomy is common enough in children to merit preoperative counseling, said Dr. James A. Owusu of the University of Minnesota, Minneapolis.
Facial nerve dysfunction rates reported in the literature range from 9% to 60% in adults after parotidectomy, but the condition has not been well studied in children.
Dr. Owusu and his colleagues reviewed the charts of 43 patients younger than age 18 years who underwent parotidectomies at a single tertiary care center between 1999 and 2011. Patients who only had parotid biopsies and those without follow-up data were excluded from the study. The average age of the patients was 4 years, and 58% were girls.
Postoperatively, 33 children (77%) had normal nerve function and 10 (23%) had abnormal nerve function. One patient experienced immediate facial nerve paralysis and nine experienced immediate facial nerve paresis. The marginal mandibular branch was affected in seven patients, the frontal branch in one patient, the buccal branch in one, and both marginal mandibular and frontal branches in one.
The most common diagnosis that led to a parotidectomy was atypical mycobacterium infection (37%), followed by branchial cleft abnormality (19%) and lymphangioma (16%). Nearly all (41) of the children underwent superficial parotidectomy; 2 underwent total parotidectomy.
"Age, gender, and pathologic diagnosis were not predictive of postoperative nerve dysfunction," Dr. Owusu said.
In patients with paresis, full nerve recovery occurred within 1 month for 2 patients, within 2 months for 1 patient, within 6 months for 3 patients, and within 10 months for 2 patients. Final nerve status was not available for 1 patient.
The study was limited by its small size and focus on a single center, Dr. Owusu said.
Dr. Owusu had no financial conflicts to disclose.
WASHINGTON – Facial nerve dysfunction affected 23% of 43 children who had parotidectomies in a single-center study presented at the annual meeting of the American Academy of Otolaryngology–Head and Neck Surgery Foundation.
The findings suggest that facial nerve dysfunction after parotidectomy is common enough in children to merit preoperative counseling, said Dr. James A. Owusu of the University of Minnesota, Minneapolis.
Facial nerve dysfunction rates reported in the literature range from 9% to 60% in adults after parotidectomy, but the condition has not been well studied in children.
Dr. Owusu and his colleagues reviewed the charts of 43 patients younger than age 18 years who underwent parotidectomies at a single tertiary care center between 1999 and 2011. Patients who only had parotid biopsies and those without follow-up data were excluded from the study. The average age of the patients was 4 years, and 58% were girls.
Postoperatively, 33 children (77%) had normal nerve function and 10 (23%) had abnormal nerve function. One patient experienced immediate facial nerve paralysis and nine experienced immediate facial nerve paresis. The marginal mandibular branch was affected in seven patients, the frontal branch in one patient, the buccal branch in one, and both marginal mandibular and frontal branches in one.
The most common diagnosis that led to a parotidectomy was atypical mycobacterium infection (37%), followed by branchial cleft abnormality (19%) and lymphangioma (16%). Nearly all (41) of the children underwent superficial parotidectomy; 2 underwent total parotidectomy.
"Age, gender, and pathologic diagnosis were not predictive of postoperative nerve dysfunction," Dr. Owusu said.
In patients with paresis, full nerve recovery occurred within 1 month for 2 patients, within 2 months for 1 patient, within 6 months for 3 patients, and within 10 months for 2 patients. Final nerve status was not available for 1 patient.
The study was limited by its small size and focus on a single center, Dr. Owusu said.
Dr. Owusu had no financial conflicts to disclose.
WASHINGTON – Facial nerve dysfunction affected 23% of 43 children who had parotidectomies in a single-center study presented at the annual meeting of the American Academy of Otolaryngology–Head and Neck Surgery Foundation.
The findings suggest that facial nerve dysfunction after parotidectomy is common enough in children to merit preoperative counseling, said Dr. James A. Owusu of the University of Minnesota, Minneapolis.
Facial nerve dysfunction rates reported in the literature range from 9% to 60% in adults after parotidectomy, but the condition has not been well studied in children.
Dr. Owusu and his colleagues reviewed the charts of 43 patients younger than age 18 years who underwent parotidectomies at a single tertiary care center between 1999 and 2011. Patients who only had parotid biopsies and those without follow-up data were excluded from the study. The average age of the patients was 4 years, and 58% were girls.
Postoperatively, 33 children (77%) had normal nerve function and 10 (23%) had abnormal nerve function. One patient experienced immediate facial nerve paralysis and nine experienced immediate facial nerve paresis. The marginal mandibular branch was affected in seven patients, the frontal branch in one patient, the buccal branch in one, and both marginal mandibular and frontal branches in one.
The most common diagnosis that led to a parotidectomy was atypical mycobacterium infection (37%), followed by branchial cleft abnormality (19%) and lymphangioma (16%). Nearly all (41) of the children underwent superficial parotidectomy; 2 underwent total parotidectomy.
"Age, gender, and pathologic diagnosis were not predictive of postoperative nerve dysfunction," Dr. Owusu said.
In patients with paresis, full nerve recovery occurred within 1 month for 2 patients, within 2 months for 1 patient, within 6 months for 3 patients, and within 10 months for 2 patients. Final nerve status was not available for 1 patient.
The study was limited by its small size and focus on a single center, Dr. Owusu said.
Dr. Owusu had no financial conflicts to disclose.
AT THE ANNUAL MEETING OF THE AMERICAN ACADEMY OF OTOLARYNGOLOGY - HEAD AND NECK SURGERY FOUNDATION
Major Finding: After parotidectomies, 23% of children experienced facial nerve dysfunction, but most were fully recovered within 6 months.
Data Source: Investigators reviewed the charts of 43 children who underwent parotidectomies at a single center between 1999 and 2011.
Disclosures: Dr. Owusu had no financial conflicts to disclose.
Medicaid or SGR? The Policy & Practice Podcast
The House of Medicine is up in arms about a proposal to use Medicaid parity funds to cover a short term fix to the perennially troublesome Medicare Sustainable Growth Rate (SGR) formula. A coalition of doctors’ organizations is urging lawmakers to find a different way to make up for the 27% cut in Medicare pay slated for Jan. 1.
Under the ACA, Medicaid payment for primary care services would be on par with Medicare pay for two-years, benefiting family physicians, pediatricians, internists and even subspecialists in certain situations. But, even if Congress walks away from this funding proposal, it still will need to come up with a fix for the SGR before New Year’s Day.
Meanwhile, researchers from the Mayo Clinic have found that the call for more primary care physician still falls on deaf ears. In a survey of more than 16,000 third year residents, few reported they planned on becoming general internists. More of these students are headed into subspecialties like cardiology, oncology, or gastroenterology. Why? Experts say it's simple - that's where the money is.
The House of Medicine is up in arms about a proposal to use Medicaid parity funds to cover a short term fix to the perennially troublesome Medicare Sustainable Growth Rate (SGR) formula. A coalition of doctors’ organizations is urging lawmakers to find a different way to make up for the 27% cut in Medicare pay slated for Jan. 1.
Under the ACA, Medicaid payment for primary care services would be on par with Medicare pay for two-years, benefiting family physicians, pediatricians, internists and even subspecialists in certain situations. But, even if Congress walks away from this funding proposal, it still will need to come up with a fix for the SGR before New Year’s Day.
Meanwhile, researchers from the Mayo Clinic have found that the call for more primary care physician still falls on deaf ears. In a survey of more than 16,000 third year residents, few reported they planned on becoming general internists. More of these students are headed into subspecialties like cardiology, oncology, or gastroenterology. Why? Experts say it's simple - that's where the money is.
The House of Medicine is up in arms about a proposal to use Medicaid parity funds to cover a short term fix to the perennially troublesome Medicare Sustainable Growth Rate (SGR) formula. A coalition of doctors’ organizations is urging lawmakers to find a different way to make up for the 27% cut in Medicare pay slated for Jan. 1.
Under the ACA, Medicaid payment for primary care services would be on par with Medicare pay for two-years, benefiting family physicians, pediatricians, internists and even subspecialists in certain situations. But, even if Congress walks away from this funding proposal, it still will need to come up with a fix for the SGR before New Year’s Day.
Meanwhile, researchers from the Mayo Clinic have found that the call for more primary care physician still falls on deaf ears. In a survey of more than 16,000 third year residents, few reported they planned on becoming general internists. More of these students are headed into subspecialties like cardiology, oncology, or gastroenterology. Why? Experts say it's simple - that's where the money is.
Postdischarge Surgery Complications Linked to Certain Procedures, Patient Types
More than 40% of postoperative complications occurred after discharge and were more commonly associated with certain procedures – along with a significantly increased risk of reoperations and death – in a large retrospective study of adults who had undergone general surgery as inpatients.
The results indicate that postdischarge (PD) complications "account for a significant burden of postoperative complications and are an important avenue for quality improvement in inpatient general surgery," concluded Dr. Hadiza S. Kazaure of the department of general surgery, Stanford (Calif.) University, and her coauthors. "More research is needed to develop and explore the utility of a cost-effective and fastidious PD follow-up system for surgical patients," they added, noting that their findings "underscore the need for systematic collection of PD adverse event data to improve postoperative surgical care in the United States" (Arch. Surg. 2012;147:1000-07). The study was based on data from the American College of Surgeons National Surgical Quality Improvement Program (2005-2010) on 551,510 adult patients (mean age, 55 years) who had undergone inpatient surgery that fell into one of 21 types of general surgery procedure categories at U.S. hospitals. The authors analyzed the rates and types of PD complications, as well as associations between the types of surgical procedures, reoperations, and mortality, within 30 days of surgery.
Within 30 days of surgery, nearly 17% of these patients had a complication. Nearly 7% of those complications occurred after discharge, accounting for almost 42% of postoperative complications. Most – 75% – of the PD complications occurred within the first 14 days of discharge, indicating that this period is "a particularly vulnerable time" for patients undergoing general surgery, the authors said.
The highest rates of PD complications were among the patients who underwent a proctectomy (a 14.5% complication rate), followed by those who had an enteric fistula repair (13%) and a pancreatic procedure (11%). Complication rates after discharge were lowest among those undergoing fundoplasty (3%) or an endocrine procedure, which included thyroid, parathyroid, and adrenal procedures (1.5%).
Breast surgery patients accounted for the highest proportion of complications occurring during the PD period. Almost 80% of complications after breast surgery occurred after discharge, compared with 70% after bariatric procedures and 62% after ventral hernia repair. And almost 83% of the PD complications that occurred in surgical patients fell into one of the following 10 categories: colectomy, small bowel procedures, bariatric procedures, ventral hernia repair, appendectomy, cholecystectomy, pancreatic procedures, exploratory laparotomy, breast procedures, and gastrectomy.
Surgical site complications, infections, and thromboembolic events accounted for almost 91% of PD complications after discharge. Patients who had a PD complication were more likely to be older and male, and were more likely to have diabetes, to use steroids, and to have had a complication while in the hospital. Other factors associated with PD complications were an American Society of Anesthesiologist class higher than 3, a mean 35-minute longer operation time, and a mean 3-day longer hospital stay.
Reoperation rates and mortality rates were 18% and 7%, respectively, among those with a PD complication vs. 5% and 2% among those who did not have a complication after discharge – a significant difference.
Since the risk of postdischarge complications was associated with "baseline complexity of illness and potentially modifiable factors such as diabetes, obesity, and use of steroids, our findings could facilitate identification of patients at increased risk and allow for targeted preventive interventions," the authors said.
While they believe this is the largest study of complications occurring after discharge in general surgery patients in the United States, the authors noted some limitations, including possible coding errors and no markers of illness severity.
No conflict of interest disclosures were reported.
Postoperative complication statistics are "increasingly used as quality metrics and will soon carry punitive remunerative consequences from health insurers," Dr. Desmond Winter noted in an accompanying editorial. Advances in reducing surgical morbidity ranging from ether to minimally invasive surgery have aimed to "enhance patient safety, not to satisfy economists," Dr. Winter said. He added that the needs of patients rather than financial penalties should be the primary focus in reducing morbidity and suggested that insurer savings should fund research to continue advancements in surgical care of patients.
Dr. Winter is with the department of surgery, Institute for Clinical Outcomes Research and Education, St. Vincent’s University Hospital, Dublin. He had no conflict of interest to disclose.
Postoperative complication statistics are "increasingly used as quality metrics and will soon carry punitive remunerative consequences from health insurers," Dr. Desmond Winter noted in an accompanying editorial. Advances in reducing surgical morbidity ranging from ether to minimally invasive surgery have aimed to "enhance patient safety, not to satisfy economists," Dr. Winter said. He added that the needs of patients rather than financial penalties should be the primary focus in reducing morbidity and suggested that insurer savings should fund research to continue advancements in surgical care of patients.
Dr. Winter is with the department of surgery, Institute for Clinical Outcomes Research and Education, St. Vincent’s University Hospital, Dublin. He had no conflict of interest to disclose.
Postoperative complication statistics are "increasingly used as quality metrics and will soon carry punitive remunerative consequences from health insurers," Dr. Desmond Winter noted in an accompanying editorial. Advances in reducing surgical morbidity ranging from ether to minimally invasive surgery have aimed to "enhance patient safety, not to satisfy economists," Dr. Winter said. He added that the needs of patients rather than financial penalties should be the primary focus in reducing morbidity and suggested that insurer savings should fund research to continue advancements in surgical care of patients.
Dr. Winter is with the department of surgery, Institute for Clinical Outcomes Research and Education, St. Vincent’s University Hospital, Dublin. He had no conflict of interest to disclose.
More than 40% of postoperative complications occurred after discharge and were more commonly associated with certain procedures – along with a significantly increased risk of reoperations and death – in a large retrospective study of adults who had undergone general surgery as inpatients.
The results indicate that postdischarge (PD) complications "account for a significant burden of postoperative complications and are an important avenue for quality improvement in inpatient general surgery," concluded Dr. Hadiza S. Kazaure of the department of general surgery, Stanford (Calif.) University, and her coauthors. "More research is needed to develop and explore the utility of a cost-effective and fastidious PD follow-up system for surgical patients," they added, noting that their findings "underscore the need for systematic collection of PD adverse event data to improve postoperative surgical care in the United States" (Arch. Surg. 2012;147:1000-07). The study was based on data from the American College of Surgeons National Surgical Quality Improvement Program (2005-2010) on 551,510 adult patients (mean age, 55 years) who had undergone inpatient surgery that fell into one of 21 types of general surgery procedure categories at U.S. hospitals. The authors analyzed the rates and types of PD complications, as well as associations between the types of surgical procedures, reoperations, and mortality, within 30 days of surgery.
Within 30 days of surgery, nearly 17% of these patients had a complication. Nearly 7% of those complications occurred after discharge, accounting for almost 42% of postoperative complications. Most – 75% – of the PD complications occurred within the first 14 days of discharge, indicating that this period is "a particularly vulnerable time" for patients undergoing general surgery, the authors said.
The highest rates of PD complications were among the patients who underwent a proctectomy (a 14.5% complication rate), followed by those who had an enteric fistula repair (13%) and a pancreatic procedure (11%). Complication rates after discharge were lowest among those undergoing fundoplasty (3%) or an endocrine procedure, which included thyroid, parathyroid, and adrenal procedures (1.5%).
Breast surgery patients accounted for the highest proportion of complications occurring during the PD period. Almost 80% of complications after breast surgery occurred after discharge, compared with 70% after bariatric procedures and 62% after ventral hernia repair. And almost 83% of the PD complications that occurred in surgical patients fell into one of the following 10 categories: colectomy, small bowel procedures, bariatric procedures, ventral hernia repair, appendectomy, cholecystectomy, pancreatic procedures, exploratory laparotomy, breast procedures, and gastrectomy.
Surgical site complications, infections, and thromboembolic events accounted for almost 91% of PD complications after discharge. Patients who had a PD complication were more likely to be older and male, and were more likely to have diabetes, to use steroids, and to have had a complication while in the hospital. Other factors associated with PD complications were an American Society of Anesthesiologist class higher than 3, a mean 35-minute longer operation time, and a mean 3-day longer hospital stay.
Reoperation rates and mortality rates were 18% and 7%, respectively, among those with a PD complication vs. 5% and 2% among those who did not have a complication after discharge – a significant difference.
Since the risk of postdischarge complications was associated with "baseline complexity of illness and potentially modifiable factors such as diabetes, obesity, and use of steroids, our findings could facilitate identification of patients at increased risk and allow for targeted preventive interventions," the authors said.
While they believe this is the largest study of complications occurring after discharge in general surgery patients in the United States, the authors noted some limitations, including possible coding errors and no markers of illness severity.
No conflict of interest disclosures were reported.
More than 40% of postoperative complications occurred after discharge and were more commonly associated with certain procedures – along with a significantly increased risk of reoperations and death – in a large retrospective study of adults who had undergone general surgery as inpatients.
The results indicate that postdischarge (PD) complications "account for a significant burden of postoperative complications and are an important avenue for quality improvement in inpatient general surgery," concluded Dr. Hadiza S. Kazaure of the department of general surgery, Stanford (Calif.) University, and her coauthors. "More research is needed to develop and explore the utility of a cost-effective and fastidious PD follow-up system for surgical patients," they added, noting that their findings "underscore the need for systematic collection of PD adverse event data to improve postoperative surgical care in the United States" (Arch. Surg. 2012;147:1000-07). The study was based on data from the American College of Surgeons National Surgical Quality Improvement Program (2005-2010) on 551,510 adult patients (mean age, 55 years) who had undergone inpatient surgery that fell into one of 21 types of general surgery procedure categories at U.S. hospitals. The authors analyzed the rates and types of PD complications, as well as associations between the types of surgical procedures, reoperations, and mortality, within 30 days of surgery.
Within 30 days of surgery, nearly 17% of these patients had a complication. Nearly 7% of those complications occurred after discharge, accounting for almost 42% of postoperative complications. Most – 75% – of the PD complications occurred within the first 14 days of discharge, indicating that this period is "a particularly vulnerable time" for patients undergoing general surgery, the authors said.
The highest rates of PD complications were among the patients who underwent a proctectomy (a 14.5% complication rate), followed by those who had an enteric fistula repair (13%) and a pancreatic procedure (11%). Complication rates after discharge were lowest among those undergoing fundoplasty (3%) or an endocrine procedure, which included thyroid, parathyroid, and adrenal procedures (1.5%).
Breast surgery patients accounted for the highest proportion of complications occurring during the PD period. Almost 80% of complications after breast surgery occurred after discharge, compared with 70% after bariatric procedures and 62% after ventral hernia repair. And almost 83% of the PD complications that occurred in surgical patients fell into one of the following 10 categories: colectomy, small bowel procedures, bariatric procedures, ventral hernia repair, appendectomy, cholecystectomy, pancreatic procedures, exploratory laparotomy, breast procedures, and gastrectomy.
Surgical site complications, infections, and thromboembolic events accounted for almost 91% of PD complications after discharge. Patients who had a PD complication were more likely to be older and male, and were more likely to have diabetes, to use steroids, and to have had a complication while in the hospital. Other factors associated with PD complications were an American Society of Anesthesiologist class higher than 3, a mean 35-minute longer operation time, and a mean 3-day longer hospital stay.
Reoperation rates and mortality rates were 18% and 7%, respectively, among those with a PD complication vs. 5% and 2% among those who did not have a complication after discharge – a significant difference.
Since the risk of postdischarge complications was associated with "baseline complexity of illness and potentially modifiable factors such as diabetes, obesity, and use of steroids, our findings could facilitate identification of patients at increased risk and allow for targeted preventive interventions," the authors said.
While they believe this is the largest study of complications occurring after discharge in general surgery patients in the United States, the authors noted some limitations, including possible coding errors and no markers of illness severity.
No conflict of interest disclosures were reported.
FROM THE ARCHIVES OF SURGERY
Major Finding: A significant proportion – 42% – of postoperative complications after general surgery occurred after discharge from the hospital. Type of surgery and other risk factors for postdischarge complications are potential targets for improving surgical outcomes.
Data Source: This retrospective cohort study analyzed data on 551,000 adults who had one of 21 types of inpatient general surgery procedures at up to 250 hospitals, part of the American College of Surgeons National Surgical Quality Improvement Program from 2005 to 2010.
Disclosures: No conflict of interest disclosures were reported.
Late Leukemia Risk Rises after Breast Cancer Therapy
SAN ANTONIO – The 10-year incidence of leukemia after adjuvant chemotherapy for breast cancer appears to hover around 0.5%, twice as high as previously reported.
A review of more than 20,000 patient records included in the National Comprehensive Cancer Network (NCCN) database found 51 cases of leukemia that developed within 10 years of treatment. Women who had only chemotherapy were at the greatest risk – almost six times more likely to develop the disease than the surgery-only control group, Dr. Antonio Wolff said at that annual San Antonio Breast Cancer Symposium.
"We know that the observed survival benefit with adjuvant therapy does take into account the potential mortality associated with leukemia," said Dr. Wolff of Johns Hopkins Sidney Kimmel Comprehensive Cancer Center, Baltimore. "But often we are in the situation where we make the decision to give chemotherapy ‘just because.’ We need to be very careful here, because some of those patients will potentially derive none of the benefits of chemo, and be at risk of its toxicities."
Dr. Wolff and colleagues examined the 1997-2008 data from eight facilities in the NCCN database – a total of 20,533 women with a first diagnosis of breast cancer. The median follow-up was 5 years, but some data were available for up to 15 years after treatment.
About half of the cohort had stage I disease; in most, the cancer was invasive ductal. Slightly more than half had hormone receptor–positive/HER2 negative disease.
The only baseline characteristic significantly different between the groups was age. Women who developed leukemia were significantly older at the time of their cancer treatment (60 vs. 54 years; P = .02); 78% of those with leukemia were older than 50 years.
Of the 51 cases, 44 were acute myeloid leukemia (AML), with a median onset time of 3.5 years after treatment. One-third of these occurred in women with a family history of breast or ovarian cancer. The median time to onset in the AML cases was 2 years.
None of the tumor characteristics were significantly associated with the development of leukemia. There were no significant differences among those who had breast-conserving surgery, mastectomy, or no surgery; or between those who had no radiation therapy, radiation without surgery, radiation after breast-conserving surgery, or radiation after mastectomy, though larger numbers of patients are needed for some of these smaller subset analyses.
The overall rate of leukemia per 1,000 patient/years was 0.46; in the surgery-only group, the rate was 0.16 per 1,000 patient-years. "Of interest is that the rates in each of the treatment groups were similar to the overall rate," – 0.43 in the radiation-only group, 0.52 in the chemotherapy-only group, and 0.54 in the combination therapy group.
At 5 years, the cumulative incidence of leukemia was 0.05% in the surgery-only group; 0.19% in the radiation-only group; 0.30% in the chemotherapy-only group; and 0.32% in the combination therapy group.
But the onset accelerated over the study period, Dr. Wolff noted, with the bulk of cases developing from years 6 to 10. .By the end of the 10-year period, the incidence was 0.2% in the surgery-only group; 0.44% in the radiation-only group; 0.52% in the chemotherapy group; and 0.51% in the combination group.
In a risk analysis using surgery as the control, the overall hazard ratio of leukemia was 2.7 in the radiation-only group; 5.68 in the chemotherapy only group; and 5.64 in the combination group.
Radiation appeared to confer no significant additional risk when it was combined with chemotherapy, Dr. Wolff added.
The findings are strikingly different than previously identified. The largest study of the association was published in 2003, when researchers from the National Surgical Adjuvant Breast and Bowel Project Operations Center reviewed six trials that examined rates of acute myeloid leukemia and myelodysplastic syndrome after doxorubicin and cyclophosphamide therapy (Clin. Breast Cancer 2003;4:273-9).
The subgroup that received anthracycline/cyclophosphamide in that review had a cumulative 8-year incidence of 0.24%. In Dr. Wolff’s chemotherapy subgroup, the cumulative 8-year incidence was 0.52%.
"It’s very important to keep in mind that the known leukemia latency period for anthracycline is 1-3 years, but that of alkylating drugs like cyclophosphamide is 4-6 years and there are case reports of it developing after 10 years," he said. "We need to be very careful when we talk about survival at 5 years vs. 10 years in exposed patients, because they could be at risk for a decade and, potentially, even longer."
SAN ANTONIO – The 10-year incidence of leukemia after adjuvant chemotherapy for breast cancer appears to hover around 0.5%, twice as high as previously reported.
A review of more than 20,000 patient records included in the National Comprehensive Cancer Network (NCCN) database found 51 cases of leukemia that developed within 10 years of treatment. Women who had only chemotherapy were at the greatest risk – almost six times more likely to develop the disease than the surgery-only control group, Dr. Antonio Wolff said at that annual San Antonio Breast Cancer Symposium.
"We know that the observed survival benefit with adjuvant therapy does take into account the potential mortality associated with leukemia," said Dr. Wolff of Johns Hopkins Sidney Kimmel Comprehensive Cancer Center, Baltimore. "But often we are in the situation where we make the decision to give chemotherapy ‘just because.’ We need to be very careful here, because some of those patients will potentially derive none of the benefits of chemo, and be at risk of its toxicities."
Dr. Wolff and colleagues examined the 1997-2008 data from eight facilities in the NCCN database – a total of 20,533 women with a first diagnosis of breast cancer. The median follow-up was 5 years, but some data were available for up to 15 years after treatment.
About half of the cohort had stage I disease; in most, the cancer was invasive ductal. Slightly more than half had hormone receptor–positive/HER2 negative disease.
The only baseline characteristic significantly different between the groups was age. Women who developed leukemia were significantly older at the time of their cancer treatment (60 vs. 54 years; P = .02); 78% of those with leukemia were older than 50 years.
Of the 51 cases, 44 were acute myeloid leukemia (AML), with a median onset time of 3.5 years after treatment. One-third of these occurred in women with a family history of breast or ovarian cancer. The median time to onset in the AML cases was 2 years.
None of the tumor characteristics were significantly associated with the development of leukemia. There were no significant differences among those who had breast-conserving surgery, mastectomy, or no surgery; or between those who had no radiation therapy, radiation without surgery, radiation after breast-conserving surgery, or radiation after mastectomy, though larger numbers of patients are needed for some of these smaller subset analyses.
The overall rate of leukemia per 1,000 patient/years was 0.46; in the surgery-only group, the rate was 0.16 per 1,000 patient-years. "Of interest is that the rates in each of the treatment groups were similar to the overall rate," – 0.43 in the radiation-only group, 0.52 in the chemotherapy-only group, and 0.54 in the combination therapy group.
At 5 years, the cumulative incidence of leukemia was 0.05% in the surgery-only group; 0.19% in the radiation-only group; 0.30% in the chemotherapy-only group; and 0.32% in the combination therapy group.
But the onset accelerated over the study period, Dr. Wolff noted, with the bulk of cases developing from years 6 to 10. .By the end of the 10-year period, the incidence was 0.2% in the surgery-only group; 0.44% in the radiation-only group; 0.52% in the chemotherapy group; and 0.51% in the combination group.
In a risk analysis using surgery as the control, the overall hazard ratio of leukemia was 2.7 in the radiation-only group; 5.68 in the chemotherapy only group; and 5.64 in the combination group.
Radiation appeared to confer no significant additional risk when it was combined with chemotherapy, Dr. Wolff added.
The findings are strikingly different than previously identified. The largest study of the association was published in 2003, when researchers from the National Surgical Adjuvant Breast and Bowel Project Operations Center reviewed six trials that examined rates of acute myeloid leukemia and myelodysplastic syndrome after doxorubicin and cyclophosphamide therapy (Clin. Breast Cancer 2003;4:273-9).
The subgroup that received anthracycline/cyclophosphamide in that review had a cumulative 8-year incidence of 0.24%. In Dr. Wolff’s chemotherapy subgroup, the cumulative 8-year incidence was 0.52%.
"It’s very important to keep in mind that the known leukemia latency period for anthracycline is 1-3 years, but that of alkylating drugs like cyclophosphamide is 4-6 years and there are case reports of it developing after 10 years," he said. "We need to be very careful when we talk about survival at 5 years vs. 10 years in exposed patients, because they could be at risk for a decade and, potentially, even longer."
SAN ANTONIO – The 10-year incidence of leukemia after adjuvant chemotherapy for breast cancer appears to hover around 0.5%, twice as high as previously reported.
A review of more than 20,000 patient records included in the National Comprehensive Cancer Network (NCCN) database found 51 cases of leukemia that developed within 10 years of treatment. Women who had only chemotherapy were at the greatest risk – almost six times more likely to develop the disease than the surgery-only control group, Dr. Antonio Wolff said at that annual San Antonio Breast Cancer Symposium.
"We know that the observed survival benefit with adjuvant therapy does take into account the potential mortality associated with leukemia," said Dr. Wolff of Johns Hopkins Sidney Kimmel Comprehensive Cancer Center, Baltimore. "But often we are in the situation where we make the decision to give chemotherapy ‘just because.’ We need to be very careful here, because some of those patients will potentially derive none of the benefits of chemo, and be at risk of its toxicities."
Dr. Wolff and colleagues examined the 1997-2008 data from eight facilities in the NCCN database – a total of 20,533 women with a first diagnosis of breast cancer. The median follow-up was 5 years, but some data were available for up to 15 years after treatment.
About half of the cohort had stage I disease; in most, the cancer was invasive ductal. Slightly more than half had hormone receptor–positive/HER2 negative disease.
The only baseline characteristic significantly different between the groups was age. Women who developed leukemia were significantly older at the time of their cancer treatment (60 vs. 54 years; P = .02); 78% of those with leukemia were older than 50 years.
Of the 51 cases, 44 were acute myeloid leukemia (AML), with a median onset time of 3.5 years after treatment. One-third of these occurred in women with a family history of breast or ovarian cancer. The median time to onset in the AML cases was 2 years.
None of the tumor characteristics were significantly associated with the development of leukemia. There were no significant differences among those who had breast-conserving surgery, mastectomy, or no surgery; or between those who had no radiation therapy, radiation without surgery, radiation after breast-conserving surgery, or radiation after mastectomy, though larger numbers of patients are needed for some of these smaller subset analyses.
The overall rate of leukemia per 1,000 patient/years was 0.46; in the surgery-only group, the rate was 0.16 per 1,000 patient-years. "Of interest is that the rates in each of the treatment groups were similar to the overall rate," – 0.43 in the radiation-only group, 0.52 in the chemotherapy-only group, and 0.54 in the combination therapy group.
At 5 years, the cumulative incidence of leukemia was 0.05% in the surgery-only group; 0.19% in the radiation-only group; 0.30% in the chemotherapy-only group; and 0.32% in the combination therapy group.
But the onset accelerated over the study period, Dr. Wolff noted, with the bulk of cases developing from years 6 to 10. .By the end of the 10-year period, the incidence was 0.2% in the surgery-only group; 0.44% in the radiation-only group; 0.52% in the chemotherapy group; and 0.51% in the combination group.
In a risk analysis using surgery as the control, the overall hazard ratio of leukemia was 2.7 in the radiation-only group; 5.68 in the chemotherapy only group; and 5.64 in the combination group.
Radiation appeared to confer no significant additional risk when it was combined with chemotherapy, Dr. Wolff added.
The findings are strikingly different than previously identified. The largest study of the association was published in 2003, when researchers from the National Surgical Adjuvant Breast and Bowel Project Operations Center reviewed six trials that examined rates of acute myeloid leukemia and myelodysplastic syndrome after doxorubicin and cyclophosphamide therapy (Clin. Breast Cancer 2003;4:273-9).
The subgroup that received anthracycline/cyclophosphamide in that review had a cumulative 8-year incidence of 0.24%. In Dr. Wolff’s chemotherapy subgroup, the cumulative 8-year incidence was 0.52%.
"It’s very important to keep in mind that the known leukemia latency period for anthracycline is 1-3 years, but that of alkylating drugs like cyclophosphamide is 4-6 years and there are case reports of it developing after 10 years," he said. "We need to be very careful when we talk about survival at 5 years vs. 10 years in exposed patients, because they could be at risk for a decade and, potentially, even longer."
AT THE ANNUAL SAN ANTONIO BREAST CANCER SYMPOSIUM
Major Finding: The 10-year incidence of leukemia after adjuvant chemotherapy for breast cancer appears to hover around 0.5%, twice as high as previously reported
Data Source: Investigators examined the 1997-2008 data from eight facilities in the NCCN database – a total of 20,533 women with a first diagnosis of breast cancer.
Disclosures: Dr. Wolff had no disclosures.
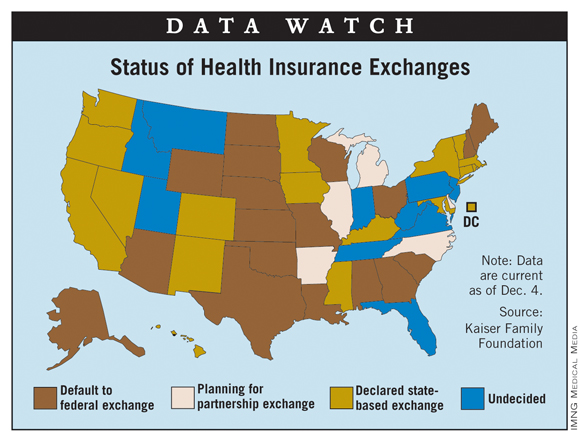
States' Plans for Insurance Exchanges: Latest Info
As of Dec. 4, a total of 17 states and the District of Columbia had indicated that they will establish the state-based insurance exchanges mandated by the Affordable Care Act, according to the Kaiser Family Foundation.
California and New York are the largest states to commit to creating their own exchanges, but Texas and Ohio are among the 18 states that have indicated their intention to default to federally facilitated exchanges. Five states, including Illinois and Michigan, are planning to partner with the federal government to operate their exchanges; 10 states, Florida and Pennsylvania among them, are still undecided, Kaiser reported.

As of Dec. 4, a total of 17 states and the District of Columbia had indicated that they will establish the state-based insurance exchanges mandated by the Affordable Care Act, according to the Kaiser Family Foundation.
California and New York are the largest states to commit to creating their own exchanges, but Texas and Ohio are among the 18 states that have indicated their intention to default to federally facilitated exchanges. Five states, including Illinois and Michigan, are planning to partner with the federal government to operate their exchanges; 10 states, Florida and Pennsylvania among them, are still undecided, Kaiser reported.

As of Dec. 4, a total of 17 states and the District of Columbia had indicated that they will establish the state-based insurance exchanges mandated by the Affordable Care Act, according to the Kaiser Family Foundation.
California and New York are the largest states to commit to creating their own exchanges, but Texas and Ohio are among the 18 states that have indicated their intention to default to federally facilitated exchanges. Five states, including Illinois and Michigan, are planning to partner with the federal government to operate their exchanges; 10 states, Florida and Pennsylvania among them, are still undecided, Kaiser reported.

Less Invasive Biopsy Used Less in Black Breast Cancer Patients
SAN ANTONIO – Black women are significantly less likely to receive a sentinel lymph node biopsy than are white women – and significantly more likely to develop lymphedema, a large database study has determined.
Among more than 31,000 women with invasive breast cancer diagnosed from 2002-2007, 62% of black women underwent the procedure, compared with 74% of white women – a significant difference (P less than .001).
That disparity led to a doubling in the risk of lymphedema in black women, compared with white women, Dr. Dalliah Black said at the San Antonio Breast Cancer Symposium. Axillary sentinel lymph node biopsy (SLNB) is a less-invasive alternative to axillary lymph node dissection (ALND) for breast cancer staging.
The disparity appears to be regional, said Dr. Black of the University of Texas M.D. Anderson Cancer Center in Houston. A preliminary subanalysis of 12 regions in the database found that Louisiana had the lowest rate of sentinel node biopsy among blacks (58%), while Seattle had the highest (89%). Regional differences were related not only to the patient’s socioeconomic status but to the numbers of surgeons available in the region.
Dr. Black used data extracted from the U.S. national Surveillance, Epidemiology, and End Results (SEER) database. The patient group consisted of 31,274 women diagnosed between 2002 and 2007. All patients had invasive breast cancer with no evidence of distant metastasis and underwent a documented axillary surgical procedure. All of the patients had fee-for-service coverage.
Black women composed 6% of the group (1,767). The median age was 74 years; 75% of the patients had a tumor size of 2 cm or smaller. Most (62%) had undergone a lumpectomy, and 73%, a sentinel lymph node biopsy.
The median number of sentinel nodes removed was two, and the median number of axillary nodes, 11.
The rate of sentinel node biopsy increased in both groups over the study period, as the surgery moved from being an alternate management approach to the preferred approach. But the disparity persisted, Dr. Black said. By 2007, the biopsy rate was 70% among black women and 83% among white (P less than .001).
The difference in SLND was also associated with a significantly increased rate of lymphedema. By 5 years after surgery, lymphedema had developed in 18% of black women who had an axillary node biopsy compared with 7% of white women who had a sentinel node biopsy. But when black women had a sentinel node biopsy, their 5-year rate of lymphedema was similar to the rate among white women (9%; P less than .001).
"This shows that if black women had gotten the appropriate surgery, they were not at any increased risk for lymphedema," Dr. Black said.
She intends to reanalyze the groups when the 2010 SEER data is released next spring. "We hope to see the disparity reduced in that analysis, although it may not be," she said in an interview. "If it’s not, we really need to figure out how we can work with national programs to disseminate guidelines and provide reminders to surgeons and multispecialist breast cancer teams – as well as to patients, so they can advocate for themselves. But it’s not only the patient’s responsibility. It’s a two-way street. We need to take some responsibility for this problem."
Dr. Black has no relevant financial relationships to disclose.
SAN ANTONIO – Black women are significantly less likely to receive a sentinel lymph node biopsy than are white women – and significantly more likely to develop lymphedema, a large database study has determined.
Among more than 31,000 women with invasive breast cancer diagnosed from 2002-2007, 62% of black women underwent the procedure, compared with 74% of white women – a significant difference (P less than .001).
That disparity led to a doubling in the risk of lymphedema in black women, compared with white women, Dr. Dalliah Black said at the San Antonio Breast Cancer Symposium. Axillary sentinel lymph node biopsy (SLNB) is a less-invasive alternative to axillary lymph node dissection (ALND) for breast cancer staging.
The disparity appears to be regional, said Dr. Black of the University of Texas M.D. Anderson Cancer Center in Houston. A preliminary subanalysis of 12 regions in the database found that Louisiana had the lowest rate of sentinel node biopsy among blacks (58%), while Seattle had the highest (89%). Regional differences were related not only to the patient’s socioeconomic status but to the numbers of surgeons available in the region.
Dr. Black used data extracted from the U.S. national Surveillance, Epidemiology, and End Results (SEER) database. The patient group consisted of 31,274 women diagnosed between 2002 and 2007. All patients had invasive breast cancer with no evidence of distant metastasis and underwent a documented axillary surgical procedure. All of the patients had fee-for-service coverage.
Black women composed 6% of the group (1,767). The median age was 74 years; 75% of the patients had a tumor size of 2 cm or smaller. Most (62%) had undergone a lumpectomy, and 73%, a sentinel lymph node biopsy.
The median number of sentinel nodes removed was two, and the median number of axillary nodes, 11.
The rate of sentinel node biopsy increased in both groups over the study period, as the surgery moved from being an alternate management approach to the preferred approach. But the disparity persisted, Dr. Black said. By 2007, the biopsy rate was 70% among black women and 83% among white (P less than .001).
The difference in SLND was also associated with a significantly increased rate of lymphedema. By 5 years after surgery, lymphedema had developed in 18% of black women who had an axillary node biopsy compared with 7% of white women who had a sentinel node biopsy. But when black women had a sentinel node biopsy, their 5-year rate of lymphedema was similar to the rate among white women (9%; P less than .001).
"This shows that if black women had gotten the appropriate surgery, they were not at any increased risk for lymphedema," Dr. Black said.
She intends to reanalyze the groups when the 2010 SEER data is released next spring. "We hope to see the disparity reduced in that analysis, although it may not be," she said in an interview. "If it’s not, we really need to figure out how we can work with national programs to disseminate guidelines and provide reminders to surgeons and multispecialist breast cancer teams – as well as to patients, so they can advocate for themselves. But it’s not only the patient’s responsibility. It’s a two-way street. We need to take some responsibility for this problem."
Dr. Black has no relevant financial relationships to disclose.
SAN ANTONIO – Black women are significantly less likely to receive a sentinel lymph node biopsy than are white women – and significantly more likely to develop lymphedema, a large database study has determined.
Among more than 31,000 women with invasive breast cancer diagnosed from 2002-2007, 62% of black women underwent the procedure, compared with 74% of white women – a significant difference (P less than .001).
That disparity led to a doubling in the risk of lymphedema in black women, compared with white women, Dr. Dalliah Black said at the San Antonio Breast Cancer Symposium. Axillary sentinel lymph node biopsy (SLNB) is a less-invasive alternative to axillary lymph node dissection (ALND) for breast cancer staging.
The disparity appears to be regional, said Dr. Black of the University of Texas M.D. Anderson Cancer Center in Houston. A preliminary subanalysis of 12 regions in the database found that Louisiana had the lowest rate of sentinel node biopsy among blacks (58%), while Seattle had the highest (89%). Regional differences were related not only to the patient’s socioeconomic status but to the numbers of surgeons available in the region.
Dr. Black used data extracted from the U.S. national Surveillance, Epidemiology, and End Results (SEER) database. The patient group consisted of 31,274 women diagnosed between 2002 and 2007. All patients had invasive breast cancer with no evidence of distant metastasis and underwent a documented axillary surgical procedure. All of the patients had fee-for-service coverage.
Black women composed 6% of the group (1,767). The median age was 74 years; 75% of the patients had a tumor size of 2 cm or smaller. Most (62%) had undergone a lumpectomy, and 73%, a sentinel lymph node biopsy.
The median number of sentinel nodes removed was two, and the median number of axillary nodes, 11.
The rate of sentinel node biopsy increased in both groups over the study period, as the surgery moved from being an alternate management approach to the preferred approach. But the disparity persisted, Dr. Black said. By 2007, the biopsy rate was 70% among black women and 83% among white (P less than .001).
The difference in SLND was also associated with a significantly increased rate of lymphedema. By 5 years after surgery, lymphedema had developed in 18% of black women who had an axillary node biopsy compared with 7% of white women who had a sentinel node biopsy. But when black women had a sentinel node biopsy, their 5-year rate of lymphedema was similar to the rate among white women (9%; P less than .001).
"This shows that if black women had gotten the appropriate surgery, they were not at any increased risk for lymphedema," Dr. Black said.
She intends to reanalyze the groups when the 2010 SEER data is released next spring. "We hope to see the disparity reduced in that analysis, although it may not be," she said in an interview. "If it’s not, we really need to figure out how we can work with national programs to disseminate guidelines and provide reminders to surgeons and multispecialist breast cancer teams – as well as to patients, so they can advocate for themselves. But it’s not only the patient’s responsibility. It’s a two-way street. We need to take some responsibility for this problem."
Dr. Black has no relevant financial relationships to disclose.
AT THE SAN ANTONIO BREAST CANCER SYMPOSIUM
Major Finding: From 2002 to 2007, significantly fewer black women than white women with invasive breast cancer underwent a sentinel lymph node biopsy (62% vs. 74%)
Data Source: Data were extracted from the national Surveillance, Epidemiology, and End Results database.
Disclosures: Dr. Black has no relevant financial relationships to disclose.
Study Finds Long-Term Benefits of DBS in Refractory Epilepsy
SAN DIEGO – Bilateral stimulation of the anterior nuclei of the thalamus led to a median reduction in partial-onset seizure frequency of 69% after a follow-up period of 5 years in the ongoing multicenter, double-blind, randomized SANTE trial.
"We were surprised that these patients still showed improvement, despite the fact that they had failed so many other treatments, including surgery," Dr. Vicenta Salanova said during a press briefing at the annual meeting of the American Epilepsy Society. "We were happy to see that."
Based on SANTE (Stimulation of the Anterior Nucleus of Thalamus for Epilepsy) trial results to date, deep brain stimulation (DBS) therapy for medically refractory partial and secondarily generalized seizures has been approved in Europe and in Canada, but it remains investigational in the United States. In 2010, the SANTE trial’s sponsor, Medtronic, submitted a premarket approval application for DBS therapy in epilepsy with the Food and Drug Administration, but both parties "are still in discussions," said Dr. Salanova, professor of neurology and director of the Indiana University School of Medicine Comprehensive Epilepsy Program in Indianapolis. "We hope that, based on the results we have shown, this will eventually be approved in the United States."
The researchers reported on 110 participants aged 18-65 years with at least six partial or secondarily generalized seizures per month who had failed treatment with at least three antiepileptic drugs. Patients with an IQ of less than 70 and those who were unable to complete neuropsychological testing or had progressive neurologic lesions were excluded from the analysis. After a baseline period of 3 months, the researchers used a stereotactic technique to implant DBS electrodes in the anterior nuclei of the thalamus bilaterally. One month after implantation, study participants were randomized to stimulation at 5 V or to no stimulation. After 3 months of blinded treatment, all patients received stimulation. Long-term follow-up began at 13 months with stimulation parameters adjusted at the investigators’ discretion. Primary analysis was performed on patients with at least 70 days of seizure diary data.
Dr. Salanova reported that by the end of the blinded treatment phase, the median seizure frequency reduction was 40% in patients who underwent DBS, compared with 14.5% in controls. Over time, patients in the DBS group demonstrated stepwise improvements in the median percent reduction of seizures from baseline, to 41% at 1 year, 56% at 2 years, and 69% at 5 years. Responder rates, defined as a 50% or greater reduction in seizure frequency, also progressively improved over time to 43% at 1 year, 54% at 2 years, and 69% at 5 years.
The researchers also found that over the entire study period 16% of patients were seizure-free for at least 6 months. They observed no unanticipated adverse device effects or symptomatic intracranial hemorrhages. Both the Liverpool Seizure Severity Scale and Quality of Life in Epilepsy-31 inventory also showed statistically significant improvement over baseline by 1 year, which continued to be significant at 5 years (P less than .001).
Medtronic sponsored the study. Dr. Salanova said that she had no relevant financial conflicts to disclose.
SAN DIEGO – Bilateral stimulation of the anterior nuclei of the thalamus led to a median reduction in partial-onset seizure frequency of 69% after a follow-up period of 5 years in the ongoing multicenter, double-blind, randomized SANTE trial.
"We were surprised that these patients still showed improvement, despite the fact that they had failed so many other treatments, including surgery," Dr. Vicenta Salanova said during a press briefing at the annual meeting of the American Epilepsy Society. "We were happy to see that."
Based on SANTE (Stimulation of the Anterior Nucleus of Thalamus for Epilepsy) trial results to date, deep brain stimulation (DBS) therapy for medically refractory partial and secondarily generalized seizures has been approved in Europe and in Canada, but it remains investigational in the United States. In 2010, the SANTE trial’s sponsor, Medtronic, submitted a premarket approval application for DBS therapy in epilepsy with the Food and Drug Administration, but both parties "are still in discussions," said Dr. Salanova, professor of neurology and director of the Indiana University School of Medicine Comprehensive Epilepsy Program in Indianapolis. "We hope that, based on the results we have shown, this will eventually be approved in the United States."
The researchers reported on 110 participants aged 18-65 years with at least six partial or secondarily generalized seizures per month who had failed treatment with at least three antiepileptic drugs. Patients with an IQ of less than 70 and those who were unable to complete neuropsychological testing or had progressive neurologic lesions were excluded from the analysis. After a baseline period of 3 months, the researchers used a stereotactic technique to implant DBS electrodes in the anterior nuclei of the thalamus bilaterally. One month after implantation, study participants were randomized to stimulation at 5 V or to no stimulation. After 3 months of blinded treatment, all patients received stimulation. Long-term follow-up began at 13 months with stimulation parameters adjusted at the investigators’ discretion. Primary analysis was performed on patients with at least 70 days of seizure diary data.
Dr. Salanova reported that by the end of the blinded treatment phase, the median seizure frequency reduction was 40% in patients who underwent DBS, compared with 14.5% in controls. Over time, patients in the DBS group demonstrated stepwise improvements in the median percent reduction of seizures from baseline, to 41% at 1 year, 56% at 2 years, and 69% at 5 years. Responder rates, defined as a 50% or greater reduction in seizure frequency, also progressively improved over time to 43% at 1 year, 54% at 2 years, and 69% at 5 years.
The researchers also found that over the entire study period 16% of patients were seizure-free for at least 6 months. They observed no unanticipated adverse device effects or symptomatic intracranial hemorrhages. Both the Liverpool Seizure Severity Scale and Quality of Life in Epilepsy-31 inventory also showed statistically significant improvement over baseline by 1 year, which continued to be significant at 5 years (P less than .001).
Medtronic sponsored the study. Dr. Salanova said that she had no relevant financial conflicts to disclose.
SAN DIEGO – Bilateral stimulation of the anterior nuclei of the thalamus led to a median reduction in partial-onset seizure frequency of 69% after a follow-up period of 5 years in the ongoing multicenter, double-blind, randomized SANTE trial.
"We were surprised that these patients still showed improvement, despite the fact that they had failed so many other treatments, including surgery," Dr. Vicenta Salanova said during a press briefing at the annual meeting of the American Epilepsy Society. "We were happy to see that."
Based on SANTE (Stimulation of the Anterior Nucleus of Thalamus for Epilepsy) trial results to date, deep brain stimulation (DBS) therapy for medically refractory partial and secondarily generalized seizures has been approved in Europe and in Canada, but it remains investigational in the United States. In 2010, the SANTE trial’s sponsor, Medtronic, submitted a premarket approval application for DBS therapy in epilepsy with the Food and Drug Administration, but both parties "are still in discussions," said Dr. Salanova, professor of neurology and director of the Indiana University School of Medicine Comprehensive Epilepsy Program in Indianapolis. "We hope that, based on the results we have shown, this will eventually be approved in the United States."
The researchers reported on 110 participants aged 18-65 years with at least six partial or secondarily generalized seizures per month who had failed treatment with at least three antiepileptic drugs. Patients with an IQ of less than 70 and those who were unable to complete neuropsychological testing or had progressive neurologic lesions were excluded from the analysis. After a baseline period of 3 months, the researchers used a stereotactic technique to implant DBS electrodes in the anterior nuclei of the thalamus bilaterally. One month after implantation, study participants were randomized to stimulation at 5 V or to no stimulation. After 3 months of blinded treatment, all patients received stimulation. Long-term follow-up began at 13 months with stimulation parameters adjusted at the investigators’ discretion. Primary analysis was performed on patients with at least 70 days of seizure diary data.
Dr. Salanova reported that by the end of the blinded treatment phase, the median seizure frequency reduction was 40% in patients who underwent DBS, compared with 14.5% in controls. Over time, patients in the DBS group demonstrated stepwise improvements in the median percent reduction of seizures from baseline, to 41% at 1 year, 56% at 2 years, and 69% at 5 years. Responder rates, defined as a 50% or greater reduction in seizure frequency, also progressively improved over time to 43% at 1 year, 54% at 2 years, and 69% at 5 years.
The researchers also found that over the entire study period 16% of patients were seizure-free for at least 6 months. They observed no unanticipated adverse device effects or symptomatic intracranial hemorrhages. Both the Liverpool Seizure Severity Scale and Quality of Life in Epilepsy-31 inventory also showed statistically significant improvement over baseline by 1 year, which continued to be significant at 5 years (P less than .001).
Medtronic sponsored the study. Dr. Salanova said that she had no relevant financial conflicts to disclose.
AT THE ANNUAL MEETING OF THE AMERICAN EPILEPSY SOCIETY
Major Finding: Bilateral deep brain stimulation of the anterior nucleus of the thalamus demonstrated stepwise improvements in the median percent reduction of partial-onset seizures from baseline to 41% at 1 year, 56% at 2 years, and 69% at 5 years.
Data Source: This was a 5-year follow-up analysis of 110 patients enrolled in the Stimulation of the Anterior Nucleus of Thalamus for Epilepsy (SANTE) trial, an ongoing multicenter, double-blind, randomized study.
Disclosures: Medtronic sponsored the study. Dr. Salanova said that she had no relevant financial conflicts to disclose.
Rapid Feedback Boosts Adherence to Oncology Quality Measures
SAN DIEGO – A Rapid Quality Reporting System significantly improved oncologists’ adherence to five measures of quality treatment for patients with breast and colon cancer during beta testing involving 64,129 patients at 64 cancer centers.
The system, developed by the American College of Surgeons’ Commission on Cancer, provides next-business-day feedback when centers submit data. Compliance rates climbed as high as 90% by the end of a 5-year period, according to researchers.
Erica J. McNamara and her associates reported the following gains at a symposium on quality care sponsored by the American Society of Clinical Oncology (ASCO):
• The proportion of patients receiving hormone therapy for hormone receptor–positive breast cancer increased from 47% in 2006 to 85% in 2011.
• Treatment with radiation following breast conserving surgery increased from 69% of patients to 90%.
• Use of multi-adjuvant chemotherapy for hormone receptor–negative breast cancer increased from 72% of patients to 90%,
• Treatment with adjuvant chemotherapy for lymph node–positive colon cancer increased from 68% to 86%.
• The proportion of patients with resected colon cancer who had at least 12 regional lymph nodes removed for pathological examination improved from 70% to 90%,
The study gathered data from the National Cancer Database in 2006-2007 for 18,151 patients with breast cancer and 6,369 patients with colon cancer and compared it with data reported to the Rapid Quality Reporting System (RQRS) in 2008-2011 for 31,590 patients with breast cancer and 11,338 patients with colon cancer.
The system monitors the five quality measures using reporting procedures similar to those that hospitals already use to submit patient data to cancer registries. Traditional registries generally report a hospital’s rate of performing quality measures 2 years after data submission, however, while the RQRS allows cancer programs to submit data whenever they want and sends feedback by the next business day, said Ms. McNamara, a quality improvement information analyst for the American College of Surgeons, Chicago.
With as little as a surgical or pathological report, cancer programs can submit a case to the RQRS to get alerts when quality care is not being provided. "What this does is it changes it from looking at retrospective cases to cases that are currently within their first course of therapy," she said in a press conference before the meeting.
Programs participating in the beta-test generally submitted data monthly, and new participants in the RQRS are required to submit data at least quarterly. The RQRS analyzes the data and returns a report in a variety of image formats, such as a year-to-date "dashboard" showing the program’s compliance rates for individual quality measures, and a list of every case submitted and whether the quality measures were applicable to the case or not, or if more information is needed.
The report also includes color-coded "case alerts" with the colors changing to orange and then red as a patient gets closer to the end of the first course of therapy with either no documentation of adjuvant therapy or no documentation that treatment decision has been made to not provide adjuvant therapy. Participants must log in to a password-protected site to view details of the case.
"For each of the adjuvant therapy measures, there’s a specific amount of time that each patient has to receive their adjuvant therapy," Ms. McNamara said. "We find that after about 6-9 months of using RQRS, about a third of programs tell us that they have seen RQRS prevent patients from slipping through the cracks or not receiving timely adjuvant care."
Breakdowns of the data by race, age, and type of insurance showed that quality care significantly improved in all subgroups. Disparities in quality adherence rates between patients of different races, ages, or insurance status were minimized or eliminated with use of the RQRS.
Two factors appeared to produce these improvements. Use of the RQRS improved the coordination of care and led to more complete reporting of adjuvant therapy data, she said.
More than 400 cancer programs now voluntarily use the RQRS. The American College of Surgeons is working on expanding the RQRS to include other measures of quality care for breast cancer and for lung, stomach, and esophageal cancers.
Ms. McNamara reported having no financial disclosures.
| This study is really noteworthy in that the development of this system significantly improved cancer care within a very short amount of time in more than 60 cancer centers nationally. This sort of innovative feedback system provides real-time improvement in care, so it’s very exciting.
Dr. Jyoti D. Patel is a thoracic oncologist at Northwestern University, Chicago, and a member of ASCO’s Cancer Communications Committee. |
|
| This study is really noteworthy in that the development of this system significantly improved cancer care within a very short amount of time in more than 60 cancer centers nationally. This sort of innovative feedback system provides real-time improvement in care, so it’s very exciting.
Dr. Jyoti D. Patel is a thoracic oncologist at Northwestern University, Chicago, and a member of ASCO’s Cancer Communications Committee. |
|
| This study is really noteworthy in that the development of this system significantly improved cancer care within a very short amount of time in more than 60 cancer centers nationally. This sort of innovative feedback system provides real-time improvement in care, so it’s very exciting.
Dr. Jyoti D. Patel is a thoracic oncologist at Northwestern University, Chicago, and a member of ASCO’s Cancer Communications Committee. |
|
SAN DIEGO – A Rapid Quality Reporting System significantly improved oncologists’ adherence to five measures of quality treatment for patients with breast and colon cancer during beta testing involving 64,129 patients at 64 cancer centers.
The system, developed by the American College of Surgeons’ Commission on Cancer, provides next-business-day feedback when centers submit data. Compliance rates climbed as high as 90% by the end of a 5-year period, according to researchers.
Erica J. McNamara and her associates reported the following gains at a symposium on quality care sponsored by the American Society of Clinical Oncology (ASCO):
• The proportion of patients receiving hormone therapy for hormone receptor–positive breast cancer increased from 47% in 2006 to 85% in 2011.
• Treatment with radiation following breast conserving surgery increased from 69% of patients to 90%.
• Use of multi-adjuvant chemotherapy for hormone receptor–negative breast cancer increased from 72% of patients to 90%,
• Treatment with adjuvant chemotherapy for lymph node–positive colon cancer increased from 68% to 86%.
• The proportion of patients with resected colon cancer who had at least 12 regional lymph nodes removed for pathological examination improved from 70% to 90%,
The study gathered data from the National Cancer Database in 2006-2007 for 18,151 patients with breast cancer and 6,369 patients with colon cancer and compared it with data reported to the Rapid Quality Reporting System (RQRS) in 2008-2011 for 31,590 patients with breast cancer and 11,338 patients with colon cancer.
The system monitors the five quality measures using reporting procedures similar to those that hospitals already use to submit patient data to cancer registries. Traditional registries generally report a hospital’s rate of performing quality measures 2 years after data submission, however, while the RQRS allows cancer programs to submit data whenever they want and sends feedback by the next business day, said Ms. McNamara, a quality improvement information analyst for the American College of Surgeons, Chicago.
With as little as a surgical or pathological report, cancer programs can submit a case to the RQRS to get alerts when quality care is not being provided. "What this does is it changes it from looking at retrospective cases to cases that are currently within their first course of therapy," she said in a press conference before the meeting.
Programs participating in the beta-test generally submitted data monthly, and new participants in the RQRS are required to submit data at least quarterly. The RQRS analyzes the data and returns a report in a variety of image formats, such as a year-to-date "dashboard" showing the program’s compliance rates for individual quality measures, and a list of every case submitted and whether the quality measures were applicable to the case or not, or if more information is needed.
The report also includes color-coded "case alerts" with the colors changing to orange and then red as a patient gets closer to the end of the first course of therapy with either no documentation of adjuvant therapy or no documentation that treatment decision has been made to not provide adjuvant therapy. Participants must log in to a password-protected site to view details of the case.
"For each of the adjuvant therapy measures, there’s a specific amount of time that each patient has to receive their adjuvant therapy," Ms. McNamara said. "We find that after about 6-9 months of using RQRS, about a third of programs tell us that they have seen RQRS prevent patients from slipping through the cracks or not receiving timely adjuvant care."
Breakdowns of the data by race, age, and type of insurance showed that quality care significantly improved in all subgroups. Disparities in quality adherence rates between patients of different races, ages, or insurance status were minimized or eliminated with use of the RQRS.
Two factors appeared to produce these improvements. Use of the RQRS improved the coordination of care and led to more complete reporting of adjuvant therapy data, she said.
More than 400 cancer programs now voluntarily use the RQRS. The American College of Surgeons is working on expanding the RQRS to include other measures of quality care for breast cancer and for lung, stomach, and esophageal cancers.
Ms. McNamara reported having no financial disclosures.
SAN DIEGO – A Rapid Quality Reporting System significantly improved oncologists’ adherence to five measures of quality treatment for patients with breast and colon cancer during beta testing involving 64,129 patients at 64 cancer centers.
The system, developed by the American College of Surgeons’ Commission on Cancer, provides next-business-day feedback when centers submit data. Compliance rates climbed as high as 90% by the end of a 5-year period, according to researchers.
Erica J. McNamara and her associates reported the following gains at a symposium on quality care sponsored by the American Society of Clinical Oncology (ASCO):
• The proportion of patients receiving hormone therapy for hormone receptor–positive breast cancer increased from 47% in 2006 to 85% in 2011.
• Treatment with radiation following breast conserving surgery increased from 69% of patients to 90%.
• Use of multi-adjuvant chemotherapy for hormone receptor–negative breast cancer increased from 72% of patients to 90%,
• Treatment with adjuvant chemotherapy for lymph node–positive colon cancer increased from 68% to 86%.
• The proportion of patients with resected colon cancer who had at least 12 regional lymph nodes removed for pathological examination improved from 70% to 90%,
The study gathered data from the National Cancer Database in 2006-2007 for 18,151 patients with breast cancer and 6,369 patients with colon cancer and compared it with data reported to the Rapid Quality Reporting System (RQRS) in 2008-2011 for 31,590 patients with breast cancer and 11,338 patients with colon cancer.
The system monitors the five quality measures using reporting procedures similar to those that hospitals already use to submit patient data to cancer registries. Traditional registries generally report a hospital’s rate of performing quality measures 2 years after data submission, however, while the RQRS allows cancer programs to submit data whenever they want and sends feedback by the next business day, said Ms. McNamara, a quality improvement information analyst for the American College of Surgeons, Chicago.
With as little as a surgical or pathological report, cancer programs can submit a case to the RQRS to get alerts when quality care is not being provided. "What this does is it changes it from looking at retrospective cases to cases that are currently within their first course of therapy," she said in a press conference before the meeting.
Programs participating in the beta-test generally submitted data monthly, and new participants in the RQRS are required to submit data at least quarterly. The RQRS analyzes the data and returns a report in a variety of image formats, such as a year-to-date "dashboard" showing the program’s compliance rates for individual quality measures, and a list of every case submitted and whether the quality measures were applicable to the case or not, or if more information is needed.
The report also includes color-coded "case alerts" with the colors changing to orange and then red as a patient gets closer to the end of the first course of therapy with either no documentation of adjuvant therapy or no documentation that treatment decision has been made to not provide adjuvant therapy. Participants must log in to a password-protected site to view details of the case.
"For each of the adjuvant therapy measures, there’s a specific amount of time that each patient has to receive their adjuvant therapy," Ms. McNamara said. "We find that after about 6-9 months of using RQRS, about a third of programs tell us that they have seen RQRS prevent patients from slipping through the cracks or not receiving timely adjuvant care."
Breakdowns of the data by race, age, and type of insurance showed that quality care significantly improved in all subgroups. Disparities in quality adherence rates between patients of different races, ages, or insurance status were minimized or eliminated with use of the RQRS.
Two factors appeared to produce these improvements. Use of the RQRS improved the coordination of care and led to more complete reporting of adjuvant therapy data, she said.
More than 400 cancer programs now voluntarily use the RQRS. The American College of Surgeons is working on expanding the RQRS to include other measures of quality care for breast cancer and for lung, stomach, and esophageal cancers.
Ms. McNamara reported having no financial disclosures.
AT THE AMERICAN SOCIETY OF CLINICAL ONCOLOGY'S QUALITY CARE SYMPOSIUM
Major Finding: Compliance rates for five quality measures climbed to as high as 90% in centers that used RQRS.
Data Source: Data on 31,590 breast cancer cases and 11,338 colon cancer cases in the RQRS in 3008-2011 were compared with data from the National Cancer Database in 2006-2007 for 18,151 breast cancer cases and 6,369 colon cancer cases.
Disclosures: Ms. McNamara reported having no financial disclosures.