User login
Official Newspaper of the American College of Surgeons
GOP Doctors Caucus seeks lower MIPS threshold
The House GOP Doctors Caucus is pushing officials at the Centers for Medicare & Medicaid to lower the exclusion threshold for participation in the Merit-Based Incentive Payment System (MIPS).
In a letter to CMS Administrator Seema Verma, the lawmakers noted that about 60% of health care providers are excluded from MIPS – one track of the agency’s Quality Payment Program – mostly because of the high participation threshold set by the agency.
Since the program provides incentive payments to doctors by shifting Medicare Part B payments, the low participation is also lowering the payments available for high-level performance.
“The most notable ramification of the current threshold has been lower maximum positive updates on how MIPS ultimately adjusts Part B payments,” the lawmakers wrote. “For example, high performers are estimated to receive an aggregate payment adjustment in 2019 of 1.1% – based on their 2017 performance – even though adjustments of up to 4% are authorized.”
This payment trend “fails to incentivize meaningful participation in MIPS,” they wrote.
The current MIPS threshold excludes any physician or practice that generates $90,000 or less in Part B billing or sees 200 or fewer Medicare patients. The agency had initially set the threshold lower, at $30,000 or less in billing or 100 patients but created a larger exemption based on feedback from many physician groups.
The five members of the House GOP Doctors Caucus who signed on to the letter are Phil Roe (Tenn.), Andy Harris (Md.), Earl “Buddy” Carter (Ga.), Larry Bucshon (Ind.), and Scott DesJarlais (Tenn.).
The House GOP Doctors Caucus is pushing officials at the Centers for Medicare & Medicaid to lower the exclusion threshold for participation in the Merit-Based Incentive Payment System (MIPS).
In a letter to CMS Administrator Seema Verma, the lawmakers noted that about 60% of health care providers are excluded from MIPS – one track of the agency’s Quality Payment Program – mostly because of the high participation threshold set by the agency.
Since the program provides incentive payments to doctors by shifting Medicare Part B payments, the low participation is also lowering the payments available for high-level performance.
“The most notable ramification of the current threshold has been lower maximum positive updates on how MIPS ultimately adjusts Part B payments,” the lawmakers wrote. “For example, high performers are estimated to receive an aggregate payment adjustment in 2019 of 1.1% – based on their 2017 performance – even though adjustments of up to 4% are authorized.”
This payment trend “fails to incentivize meaningful participation in MIPS,” they wrote.
The current MIPS threshold excludes any physician or practice that generates $90,000 or less in Part B billing or sees 200 or fewer Medicare patients. The agency had initially set the threshold lower, at $30,000 or less in billing or 100 patients but created a larger exemption based on feedback from many physician groups.
The five members of the House GOP Doctors Caucus who signed on to the letter are Phil Roe (Tenn.), Andy Harris (Md.), Earl “Buddy” Carter (Ga.), Larry Bucshon (Ind.), and Scott DesJarlais (Tenn.).
The House GOP Doctors Caucus is pushing officials at the Centers for Medicare & Medicaid to lower the exclusion threshold for participation in the Merit-Based Incentive Payment System (MIPS).
In a letter to CMS Administrator Seema Verma, the lawmakers noted that about 60% of health care providers are excluded from MIPS – one track of the agency’s Quality Payment Program – mostly because of the high participation threshold set by the agency.
Since the program provides incentive payments to doctors by shifting Medicare Part B payments, the low participation is also lowering the payments available for high-level performance.
“The most notable ramification of the current threshold has been lower maximum positive updates on how MIPS ultimately adjusts Part B payments,” the lawmakers wrote. “For example, high performers are estimated to receive an aggregate payment adjustment in 2019 of 1.1% – based on their 2017 performance – even though adjustments of up to 4% are authorized.”
This payment trend “fails to incentivize meaningful participation in MIPS,” they wrote.
The current MIPS threshold excludes any physician or practice that generates $90,000 or less in Part B billing or sees 200 or fewer Medicare patients. The agency had initially set the threshold lower, at $30,000 or less in billing or 100 patients but created a larger exemption based on feedback from many physician groups.
The five members of the House GOP Doctors Caucus who signed on to the letter are Phil Roe (Tenn.), Andy Harris (Md.), Earl “Buddy” Carter (Ga.), Larry Bucshon (Ind.), and Scott DesJarlais (Tenn.).
Clinical trial: Robotic versus laparoscopic ventral hernia repair
The Robotic Versus Laparoscopic Ventral Hernia Repair study is an interventional trial recruiting patients undergoing elective ventral hernia repair appropriate for minimally invasive surgery.
The trial will compare outcomes of laparoscopic and robotic approaches to ventral hernia repair. compared with the laparoscopic approach, and has been endorsed by the American Hernia Society. However, this evidence is based on database and cohort studies, and more randomized, controlled trials are needed to assess the true effects of the robotic approach.
Patients will be included if they are scheduled for a ventral hernia repair that has been deemed appropriate for minimally invasive surgery. Exclusion criteria include being unlikely to survive for 2 years post surgery, being unlikely to follow up, having advanced chronic obstructive pulmonary disease or congestive heart failure, having a history of open abdomen or extensive lysis of adhesions, having ascites caused by cirrhosis or malignancy, having an active infection, and having a hernia defect size larger than 12 cm.
The primary outcome measure is total number of days spent in the hospital. Secondary outcomes include rates of surgical site infection, rates of surgical site occurrence, rates of hernia reoccurrence, patient-centered outcomes collected using HerQLes (a hernia quality of life measuring instrument), patient-centered outcomes collected using the EQ-5D questionnaire, and cost from a health care perspective.
The primary completion date is April 30, 2020, and the study completion date is April 30, 2023. About 120 people are expected to be recruited.
Find more information on the study page at Clinicaltrials.gov.
The Robotic Versus Laparoscopic Ventral Hernia Repair study is an interventional trial recruiting patients undergoing elective ventral hernia repair appropriate for minimally invasive surgery.
The trial will compare outcomes of laparoscopic and robotic approaches to ventral hernia repair. compared with the laparoscopic approach, and has been endorsed by the American Hernia Society. However, this evidence is based on database and cohort studies, and more randomized, controlled trials are needed to assess the true effects of the robotic approach.
Patients will be included if they are scheduled for a ventral hernia repair that has been deemed appropriate for minimally invasive surgery. Exclusion criteria include being unlikely to survive for 2 years post surgery, being unlikely to follow up, having advanced chronic obstructive pulmonary disease or congestive heart failure, having a history of open abdomen or extensive lysis of adhesions, having ascites caused by cirrhosis or malignancy, having an active infection, and having a hernia defect size larger than 12 cm.
The primary outcome measure is total number of days spent in the hospital. Secondary outcomes include rates of surgical site infection, rates of surgical site occurrence, rates of hernia reoccurrence, patient-centered outcomes collected using HerQLes (a hernia quality of life measuring instrument), patient-centered outcomes collected using the EQ-5D questionnaire, and cost from a health care perspective.
The primary completion date is April 30, 2020, and the study completion date is April 30, 2023. About 120 people are expected to be recruited.
Find more information on the study page at Clinicaltrials.gov.
The Robotic Versus Laparoscopic Ventral Hernia Repair study is an interventional trial recruiting patients undergoing elective ventral hernia repair appropriate for minimally invasive surgery.
The trial will compare outcomes of laparoscopic and robotic approaches to ventral hernia repair. compared with the laparoscopic approach, and has been endorsed by the American Hernia Society. However, this evidence is based on database and cohort studies, and more randomized, controlled trials are needed to assess the true effects of the robotic approach.
Patients will be included if they are scheduled for a ventral hernia repair that has been deemed appropriate for minimally invasive surgery. Exclusion criteria include being unlikely to survive for 2 years post surgery, being unlikely to follow up, having advanced chronic obstructive pulmonary disease or congestive heart failure, having a history of open abdomen or extensive lysis of adhesions, having ascites caused by cirrhosis or malignancy, having an active infection, and having a hernia defect size larger than 12 cm.
The primary outcome measure is total number of days spent in the hospital. Secondary outcomes include rates of surgical site infection, rates of surgical site occurrence, rates of hernia reoccurrence, patient-centered outcomes collected using HerQLes (a hernia quality of life measuring instrument), patient-centered outcomes collected using the EQ-5D questionnaire, and cost from a health care perspective.
The primary completion date is April 30, 2020, and the study completion date is April 30, 2023. About 120 people are expected to be recruited.
Find more information on the study page at Clinicaltrials.gov.
Despite U.S. court’s ruling, Medicaid work requirements advance in other states
.
The decision by Judge James Boasberg immediately blocked Kentucky from enacting the provision in Campbell County, which had been set to start Sunday and roll out statewide later this year. Within 36 hours, Kentucky Gov. Matt Bevin, a Republican, eliminated vision and dental benefits to nearly 500,000 Medicaid enrollees, saying the state could no longer afford it.
Meanwhile, Arkansas, New Hampshire and Indiana are moving ahead with the implementation of their versions of a Medicaid work requirement. It is not clear how or if Boasberg’s ruling invalidating the Trump administration’s approval of Kentucky’s plan affects these states.
Arkansas is implementing its requirement this summer while New Hampshire and Indiana plan to phase in their rules beginning In January.
Virginia health officials say they still plan to seek federal permission to enact a work requirement but it isn’t needed in order to expand Medicaid eligibility on Jan.1.
Virginia lawmakers approved Medicaid expansion in June with the condition the state apply for federal permission to include the new mandate.
“We remain focused on the work necessary to ensure that new health coverage for Virginia adults is available beginning on January 1, 2019,” Dr. Jennifer Lee, director of the Virginia Department of Medical Assistance Services, said in a statement. “Developing a waiver is a separate and ongoing process, as described in the final state budget.”
Virginia Medicaid is in discussions with the U.S. Centers for Medicare & Medicaid Services about its waiver, which has not yet been submitted.
Many Republican lawmakers in Virginia voted to expand Medicaid only after it was assured new enrollees would have to work or do volunteer service.
Dr. Scott Garrett, a Virginia House member from Lynchburg, Va., said he was under the impression the bill signed in June by Virginia Gov. Ralph Northam, a Democrat, meant the Medicaid expansion would begin only with a work requirement in place.
“The intent of the General Assembly ... was that you could not do one absent the other,” he said.
Garrett, a Republican, said he long opposed plans to add 400,000 adults to Medicaid because of cost concerns. He said requiring these enrollees to work or do volunteer service would make them healthier and improve their well-being.
Patricia Boozang, senior managing director for Manatt Health, a consulting firm, said she is not surprised states are moving ahead with work requirement plans regardless of the court ruling, which was specific to Kentucky.
She said the decision would cause the Trump administration to review the pending applications more closely so they could withstand a judicial review.
The federal court said Health and Human Services Secretary Alex Azar did not adequately take into account how many people would lose coverage for the work requirement and did not prove such a provision would improve enrollees’ health.
“It’s going to be challenging for them to make the case that health and well-being is going to be improved by the [work requirement] waiver,” she said.
In Arkansas, some Medicaid enrollees face a Thursday deadline to register their status – that they worked, did volunteer service in June or meet one of the state’s many exemptions.
“The ruling does not have an immediate effect on Arkansas’ work requirement,” said spokeswoman Marci Manley.
Advocates for low-income people are weighing whether to file lawsuits to stop the work requirement in other states that have won federal approval.
“We have ... partnerships with state legal advocates in these states and are exploring enforcement and litigation options with them,” said Jane Perkins, legal director of the National Health Law Program, which filed the suit on behalf of Medicaid enrollees in Kentucky to block the work requirements.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
.
The decision by Judge James Boasberg immediately blocked Kentucky from enacting the provision in Campbell County, which had been set to start Sunday and roll out statewide later this year. Within 36 hours, Kentucky Gov. Matt Bevin, a Republican, eliminated vision and dental benefits to nearly 500,000 Medicaid enrollees, saying the state could no longer afford it.
Meanwhile, Arkansas, New Hampshire and Indiana are moving ahead with the implementation of their versions of a Medicaid work requirement. It is not clear how or if Boasberg’s ruling invalidating the Trump administration’s approval of Kentucky’s plan affects these states.
Arkansas is implementing its requirement this summer while New Hampshire and Indiana plan to phase in their rules beginning In January.
Virginia health officials say they still plan to seek federal permission to enact a work requirement but it isn’t needed in order to expand Medicaid eligibility on Jan.1.
Virginia lawmakers approved Medicaid expansion in June with the condition the state apply for federal permission to include the new mandate.
“We remain focused on the work necessary to ensure that new health coverage for Virginia adults is available beginning on January 1, 2019,” Dr. Jennifer Lee, director of the Virginia Department of Medical Assistance Services, said in a statement. “Developing a waiver is a separate and ongoing process, as described in the final state budget.”
Virginia Medicaid is in discussions with the U.S. Centers for Medicare & Medicaid Services about its waiver, which has not yet been submitted.
Many Republican lawmakers in Virginia voted to expand Medicaid only after it was assured new enrollees would have to work or do volunteer service.
Dr. Scott Garrett, a Virginia House member from Lynchburg, Va., said he was under the impression the bill signed in June by Virginia Gov. Ralph Northam, a Democrat, meant the Medicaid expansion would begin only with a work requirement in place.
“The intent of the General Assembly ... was that you could not do one absent the other,” he said.
Garrett, a Republican, said he long opposed plans to add 400,000 adults to Medicaid because of cost concerns. He said requiring these enrollees to work or do volunteer service would make them healthier and improve their well-being.
Patricia Boozang, senior managing director for Manatt Health, a consulting firm, said she is not surprised states are moving ahead with work requirement plans regardless of the court ruling, which was specific to Kentucky.
She said the decision would cause the Trump administration to review the pending applications more closely so they could withstand a judicial review.
The federal court said Health and Human Services Secretary Alex Azar did not adequately take into account how many people would lose coverage for the work requirement and did not prove such a provision would improve enrollees’ health.
“It’s going to be challenging for them to make the case that health and well-being is going to be improved by the [work requirement] waiver,” she said.
In Arkansas, some Medicaid enrollees face a Thursday deadline to register their status – that they worked, did volunteer service in June or meet one of the state’s many exemptions.
“The ruling does not have an immediate effect on Arkansas’ work requirement,” said spokeswoman Marci Manley.
Advocates for low-income people are weighing whether to file lawsuits to stop the work requirement in other states that have won federal approval.
“We have ... partnerships with state legal advocates in these states and are exploring enforcement and litigation options with them,” said Jane Perkins, legal director of the National Health Law Program, which filed the suit on behalf of Medicaid enrollees in Kentucky to block the work requirements.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
.
The decision by Judge James Boasberg immediately blocked Kentucky from enacting the provision in Campbell County, which had been set to start Sunday and roll out statewide later this year. Within 36 hours, Kentucky Gov. Matt Bevin, a Republican, eliminated vision and dental benefits to nearly 500,000 Medicaid enrollees, saying the state could no longer afford it.
Meanwhile, Arkansas, New Hampshire and Indiana are moving ahead with the implementation of their versions of a Medicaid work requirement. It is not clear how or if Boasberg’s ruling invalidating the Trump administration’s approval of Kentucky’s plan affects these states.
Arkansas is implementing its requirement this summer while New Hampshire and Indiana plan to phase in their rules beginning In January.
Virginia health officials say they still plan to seek federal permission to enact a work requirement but it isn’t needed in order to expand Medicaid eligibility on Jan.1.
Virginia lawmakers approved Medicaid expansion in June with the condition the state apply for federal permission to include the new mandate.
“We remain focused on the work necessary to ensure that new health coverage for Virginia adults is available beginning on January 1, 2019,” Dr. Jennifer Lee, director of the Virginia Department of Medical Assistance Services, said in a statement. “Developing a waiver is a separate and ongoing process, as described in the final state budget.”
Virginia Medicaid is in discussions with the U.S. Centers for Medicare & Medicaid Services about its waiver, which has not yet been submitted.
Many Republican lawmakers in Virginia voted to expand Medicaid only after it was assured new enrollees would have to work or do volunteer service.
Dr. Scott Garrett, a Virginia House member from Lynchburg, Va., said he was under the impression the bill signed in June by Virginia Gov. Ralph Northam, a Democrat, meant the Medicaid expansion would begin only with a work requirement in place.
“The intent of the General Assembly ... was that you could not do one absent the other,” he said.
Garrett, a Republican, said he long opposed plans to add 400,000 adults to Medicaid because of cost concerns. He said requiring these enrollees to work or do volunteer service would make them healthier and improve their well-being.
Patricia Boozang, senior managing director for Manatt Health, a consulting firm, said she is not surprised states are moving ahead with work requirement plans regardless of the court ruling, which was specific to Kentucky.
She said the decision would cause the Trump administration to review the pending applications more closely so they could withstand a judicial review.
The federal court said Health and Human Services Secretary Alex Azar did not adequately take into account how many people would lose coverage for the work requirement and did not prove such a provision would improve enrollees’ health.
“It’s going to be challenging for them to make the case that health and well-being is going to be improved by the [work requirement] waiver,” she said.
In Arkansas, some Medicaid enrollees face a Thursday deadline to register their status – that they worked, did volunteer service in June or meet one of the state’s many exemptions.
“The ruling does not have an immediate effect on Arkansas’ work requirement,” said spokeswoman Marci Manley.
Advocates for low-income people are weighing whether to file lawsuits to stop the work requirement in other states that have won federal approval.
“We have ... partnerships with state legal advocates in these states and are exploring enforcement and litigation options with them,” said Jane Perkins, legal director of the National Health Law Program, which filed the suit on behalf of Medicaid enrollees in Kentucky to block the work requirements.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Emerging CPAP options show sustained benefits
BALTIMORE – Emerging developments for the treatment of obstructive sleep apnea have shown promise in providing options beyond continuous-positive airway pressure, investigators reported at the annual meeting of the Associated Professional Sleep Societies. These developments include a single-use nasopharyngeal airway stent, upper-airway stimulation using a pacemaker-like device, a negative-pressure device that opens the airway, and an artificial intelligence approach that can predict outcomes of oral appliance therapy.
Nasopharyngeal airway stent
Clete A. Kushida, MD, of Stanford (Calif.) University reported on recent results of a trial of the nasopharyngeal airway stent (NAS) single-use disposable insert (Seven Dreamers Laboratories, Tokyo). This device consists of a flexible semi-rigid silicone rubber tube 120-145 mm in length and coated with a hydrophilic gel. The patient inserts the distal end of the tube into the nostril, and it positions itself within the nasopharynx and retropalatal oropharynx to open the airway. A clip attaches to the exterior septum to keep the device in place. The patient removes the NAS in the morning. The device is commercially available in Japan and some European countries.
Dr. Kushida reported on two posters that were presented at Sleep 2018. The first by the Osaka University Graduate School of Dentistry in Japan investigated the predictability of NAS efficacy in patients with a velopharynx that was narrower than the hypopharynx (Sleep. 2018;41 (suppl 1):A207: doi.org/10.1093/sleep/zsy061.554). The study showed that 11 responders had a narrow velopharynx while 18 nonresponders had a narrow hypopharynx. Response was defined as 50% or greater reduction of apnea-hypopnea index (AHI) from baseline. “The success rate of NAS for the patients with narrowing of the velopharynx is 83.3%,” Dr. Kushida said.
He also reported on a study he led of NAS in patients with obstructive sleep apnea and healthy controls (Sleep. 2018;41 (suppl 1):A207: doi.org/10.1093/sleep/zsy061.555). The trial was conducted at Stanford and Tokyo sleep medical centers, with healthy controls at the Tokyo site only. AHI improved in all three obstructive sleep apnea (OSA) groups, but most significantly in those with moderate (n = 23) and severe (n = 21) OSA: 7.2 (P = .0038) and 11.7 (P = .0069), respectively. In the Stanford cohort, 2 of 32 patients originally enrolled dropped out because they found the NAS uncomfortable; none dropped out of the Tokyo cohort.
“NAS can be effective in treatment of snoring and OSA, in particular those with moderate/severe OSA, with significant improvement in mean obstructive apnea index,” Dr. Kushida said. He also noted the device is more effective in patients with narrowing of the oropharynx/velopharynx rather than the hypopharynx.
Dr. Kushida reported he had a financial relationship with the Seven Dreamers Laboratories.
Continuous negative external pressure
Jerrold A. Kram, MD, reported on a device that employs continuous negative external pressure – known as cNEP – that uses a silicone collar covering the front of the throat and a pump that applies suction to keep the airway open from the outside. He cited a small 2015 study out of Japan that found the device was effective in keeping the pharyngeal airway open in nonobese women (J Appl Physiol. 2015;118:912-20). Another small U.S. study found cNEP reduces respiratory impairment during screening colonoscopy (Endoscopy. 2016;48:584-7). Sommetrics, which has patented the technology, is developing a version of the product for obstructive sleep apnea, Dr. Kram said. The company already has a Food and Drug Administration–approved product to treat apneas that people experience when under mild to moderate sedation, such as a colonoscopy. It is approved for sale in Canada, but not in the United States.
“It gives us another tool in the box,” Dr. Kram said. “It’s very small and portable, easy to take on an airplane with you, and it reduces the chance for claustrophobia” that comes with continuous-positive airway pressure (CPAP), said Dr. Kram. There are no tubes or masks and no humidifier to deal with.
Dr. Kram conducted a small study of 15 OSA patients using cNEP last year. Among nine excellent responders, the lowest AHI was 1.5 on average, from an average baseline of 43.9; among four partial responders, the lowest observed AHI averaged 11.75 (J Clin Sleep Med. 2017;13:1009-12).
The cNEP device is also the subject of a home study of patients with OSA in Canada, Dr. Kram said. Unpublished results indicate that 46% (27/59) of patients had an initial response rate to either -25 or -30 characteristic moment waveform (cmw) of negative airway pressure. For those who completed 3 weeks of treatment, the response rate was 64% (16/25). “Seventy-six percent of subjects felt their overall experience with cNEP was better than with CPAP,” Dr. Kram said.
However, studies have noted some minor issues with cNEP, Dr. Kram said. They include mild skin irritation, choking sensation if not properly placed, limited size availability, and absence of efficacy data. Dr. Kram said a U.S. pivotal trial is scheduled to start soon.
Dr. Kram disclosed he is a paid adviser for Sommetrics.
Oral appliance therapy
Oral appliance therapy (OAT) has been very effective in some patients and has been endorsed by the American Academy of Sleep Medicine since 2006, but it has been underutilized predominantly for two reasons, noted John Remmers, MD, because fitting the device requires a trial-and-error approach that can discourage patients; and the therapeutic success rate is 50%-60%.
To predict which patients are likely to succeed with OAT, Dr. Remmers and his colleagues at Zephyr Sleep Technologies have developed an artificial intelligence (AI) platform that uses a feedback-controlled mandibular positioner, a mouthpiece-like device that opens the airway during sleep. Dr. Remmers is chief medical officer and cofounder of Zephyr Sleep Technologies, based in Calgary, Alta.
While imaging has been used in awake patients to fit OAT devices, Dr. Remmers noted a number of shortcomings with this modality. “Sleep apnea results from an anatomic problem – i.e., structural encroachment on the pharyngeal airway, which is neurally compensated when the patient is awake,” he said. “Because of this, we need to do the test when the patient is asleep.”
The AI platform with feedback-controlled mandibular positioner uses temporary dental trays to create impressions for the mouthpiece the patient uses during sleep. The mouthpiece connects to a remote-controlled device that makes real-time adjustments in the mandibular position without disturbing the patient’s sleep. “The computer identifies respiratory events all night long, adjusting the position of the mandible,” he said in explaining the AI component of the device. “Just think of it as similar to autotitration with CPAP.”
Dr. Remmers reported results of a phase 2 study of the platform presented as a poster reporting on a study of 101 patients with OSA participants. The study reported sensitivity of 86% and specificity of 92% in predicting success with the feedback-controlled mandibular positioner, he said.
“The sensitivity and overall predictive accuracy of the AI-based approach was greater than an intuitive approach using a pretreatment, temporary appliance, indicating that feedback-controlled mandibular positioning test outperformed the intuitive approach,” he said. The device has been approved by Health Canada and is awaiting clearance in the United States.
Upper airway stimulation
Upper airway stimulation (UAS) is emerging as a new class of therapy, said Patrick J. Strollo Jr., MD, of the University of Pittsburgh. The therapy involves an impulse generator (IPG) similar to a pacemaker that is implanted in the left side of the chest and connected to a stimulation lead secured to the distal hypoglossal nerve in the neck. The UAS system also incorporates a sensing lead that is implanted between the intercostal muscles and attached to the IPG allowing for phasic stimulation of the genioglossal muscle. The patient uses a remote control to turn the device on at night and off in the morning.
Dr. Strollo was lead author of the Stimulation Therapy for Apnea Reduction (STAR) trial (N Engl J Med. 2014:370;139-49), a prospective multicenter trial with a randomized therapy withdrawal arm. In 126 participants, he said, the median AHI score declined 68% in 12 months, from 29.3 at baseline to 9 (P less than .0001).
He provided updated results that showed 80% of patients continued to use the device after 5 years (Otolaryngol Head Neck Surg. 2018; doi: 10.1177/0194599818762383). Median AHI at 5 years was 6.9, Dr. Strollo said, and median Epworth Sleepiness Scale scores declined from 11.6 at baseline to 6.9 at 5 years.
Another postapproval study of UAS, the ADHERE registry, has enrolled 348 patients at 10 centers with a goal of 2,500, Dr. Strollo said (Otolaryngol Head Neck Surg. 2018: doi: 10.1177/0194599818764896). Twelve-month study results have shown reductions in AHI and Epworth Sleepiness Scale scores comparable to previous reports. ADHERE also reported that 92% of patients were satisfied with UAS.
The latest innovation for UAS is the ability to download data from the implant at office visits so the physician can review patient adherence patterns, along with energy levels and settings for sensing and stimulation, Dr. Strollo said.
“Upper airway stimulation is an additional tool in the management of properly selected, at-risk apnea patients who do not accept or adhere to positive pressure therapy,” Dr. Strollo said. “The STAR trial has provided robust evidence that upper airway stimulation is safe and effective in participants with moderate to severe OSA, and the treatment effect is maintained beyond the 12-month endpoint.”
Dr. Strollo disclosed a financial relationship with Inspire Medical Systems, manufacturer of the UAS device.
BALTIMORE – Emerging developments for the treatment of obstructive sleep apnea have shown promise in providing options beyond continuous-positive airway pressure, investigators reported at the annual meeting of the Associated Professional Sleep Societies. These developments include a single-use nasopharyngeal airway stent, upper-airway stimulation using a pacemaker-like device, a negative-pressure device that opens the airway, and an artificial intelligence approach that can predict outcomes of oral appliance therapy.
Nasopharyngeal airway stent
Clete A. Kushida, MD, of Stanford (Calif.) University reported on recent results of a trial of the nasopharyngeal airway stent (NAS) single-use disposable insert (Seven Dreamers Laboratories, Tokyo). This device consists of a flexible semi-rigid silicone rubber tube 120-145 mm in length and coated with a hydrophilic gel. The patient inserts the distal end of the tube into the nostril, and it positions itself within the nasopharynx and retropalatal oropharynx to open the airway. A clip attaches to the exterior septum to keep the device in place. The patient removes the NAS in the morning. The device is commercially available in Japan and some European countries.
Dr. Kushida reported on two posters that were presented at Sleep 2018. The first by the Osaka University Graduate School of Dentistry in Japan investigated the predictability of NAS efficacy in patients with a velopharynx that was narrower than the hypopharynx (Sleep. 2018;41 (suppl 1):A207: doi.org/10.1093/sleep/zsy061.554). The study showed that 11 responders had a narrow velopharynx while 18 nonresponders had a narrow hypopharynx. Response was defined as 50% or greater reduction of apnea-hypopnea index (AHI) from baseline. “The success rate of NAS for the patients with narrowing of the velopharynx is 83.3%,” Dr. Kushida said.
He also reported on a study he led of NAS in patients with obstructive sleep apnea and healthy controls (Sleep. 2018;41 (suppl 1):A207: doi.org/10.1093/sleep/zsy061.555). The trial was conducted at Stanford and Tokyo sleep medical centers, with healthy controls at the Tokyo site only. AHI improved in all three obstructive sleep apnea (OSA) groups, but most significantly in those with moderate (n = 23) and severe (n = 21) OSA: 7.2 (P = .0038) and 11.7 (P = .0069), respectively. In the Stanford cohort, 2 of 32 patients originally enrolled dropped out because they found the NAS uncomfortable; none dropped out of the Tokyo cohort.
“NAS can be effective in treatment of snoring and OSA, in particular those with moderate/severe OSA, with significant improvement in mean obstructive apnea index,” Dr. Kushida said. He also noted the device is more effective in patients with narrowing of the oropharynx/velopharynx rather than the hypopharynx.
Dr. Kushida reported he had a financial relationship with the Seven Dreamers Laboratories.
Continuous negative external pressure
Jerrold A. Kram, MD, reported on a device that employs continuous negative external pressure – known as cNEP – that uses a silicone collar covering the front of the throat and a pump that applies suction to keep the airway open from the outside. He cited a small 2015 study out of Japan that found the device was effective in keeping the pharyngeal airway open in nonobese women (J Appl Physiol. 2015;118:912-20). Another small U.S. study found cNEP reduces respiratory impairment during screening colonoscopy (Endoscopy. 2016;48:584-7). Sommetrics, which has patented the technology, is developing a version of the product for obstructive sleep apnea, Dr. Kram said. The company already has a Food and Drug Administration–approved product to treat apneas that people experience when under mild to moderate sedation, such as a colonoscopy. It is approved for sale in Canada, but not in the United States.
“It gives us another tool in the box,” Dr. Kram said. “It’s very small and portable, easy to take on an airplane with you, and it reduces the chance for claustrophobia” that comes with continuous-positive airway pressure (CPAP), said Dr. Kram. There are no tubes or masks and no humidifier to deal with.
Dr. Kram conducted a small study of 15 OSA patients using cNEP last year. Among nine excellent responders, the lowest AHI was 1.5 on average, from an average baseline of 43.9; among four partial responders, the lowest observed AHI averaged 11.75 (J Clin Sleep Med. 2017;13:1009-12).
The cNEP device is also the subject of a home study of patients with OSA in Canada, Dr. Kram said. Unpublished results indicate that 46% (27/59) of patients had an initial response rate to either -25 or -30 characteristic moment waveform (cmw) of negative airway pressure. For those who completed 3 weeks of treatment, the response rate was 64% (16/25). “Seventy-six percent of subjects felt their overall experience with cNEP was better than with CPAP,” Dr. Kram said.
However, studies have noted some minor issues with cNEP, Dr. Kram said. They include mild skin irritation, choking sensation if not properly placed, limited size availability, and absence of efficacy data. Dr. Kram said a U.S. pivotal trial is scheduled to start soon.
Dr. Kram disclosed he is a paid adviser for Sommetrics.
Oral appliance therapy
Oral appliance therapy (OAT) has been very effective in some patients and has been endorsed by the American Academy of Sleep Medicine since 2006, but it has been underutilized predominantly for two reasons, noted John Remmers, MD, because fitting the device requires a trial-and-error approach that can discourage patients; and the therapeutic success rate is 50%-60%.
To predict which patients are likely to succeed with OAT, Dr. Remmers and his colleagues at Zephyr Sleep Technologies have developed an artificial intelligence (AI) platform that uses a feedback-controlled mandibular positioner, a mouthpiece-like device that opens the airway during sleep. Dr. Remmers is chief medical officer and cofounder of Zephyr Sleep Technologies, based in Calgary, Alta.
While imaging has been used in awake patients to fit OAT devices, Dr. Remmers noted a number of shortcomings with this modality. “Sleep apnea results from an anatomic problem – i.e., structural encroachment on the pharyngeal airway, which is neurally compensated when the patient is awake,” he said. “Because of this, we need to do the test when the patient is asleep.”
The AI platform with feedback-controlled mandibular positioner uses temporary dental trays to create impressions for the mouthpiece the patient uses during sleep. The mouthpiece connects to a remote-controlled device that makes real-time adjustments in the mandibular position without disturbing the patient’s sleep. “The computer identifies respiratory events all night long, adjusting the position of the mandible,” he said in explaining the AI component of the device. “Just think of it as similar to autotitration with CPAP.”
Dr. Remmers reported results of a phase 2 study of the platform presented as a poster reporting on a study of 101 patients with OSA participants. The study reported sensitivity of 86% and specificity of 92% in predicting success with the feedback-controlled mandibular positioner, he said.
“The sensitivity and overall predictive accuracy of the AI-based approach was greater than an intuitive approach using a pretreatment, temporary appliance, indicating that feedback-controlled mandibular positioning test outperformed the intuitive approach,” he said. The device has been approved by Health Canada and is awaiting clearance in the United States.
Upper airway stimulation
Upper airway stimulation (UAS) is emerging as a new class of therapy, said Patrick J. Strollo Jr., MD, of the University of Pittsburgh. The therapy involves an impulse generator (IPG) similar to a pacemaker that is implanted in the left side of the chest and connected to a stimulation lead secured to the distal hypoglossal nerve in the neck. The UAS system also incorporates a sensing lead that is implanted between the intercostal muscles and attached to the IPG allowing for phasic stimulation of the genioglossal muscle. The patient uses a remote control to turn the device on at night and off in the morning.
Dr. Strollo was lead author of the Stimulation Therapy for Apnea Reduction (STAR) trial (N Engl J Med. 2014:370;139-49), a prospective multicenter trial with a randomized therapy withdrawal arm. In 126 participants, he said, the median AHI score declined 68% in 12 months, from 29.3 at baseline to 9 (P less than .0001).
He provided updated results that showed 80% of patients continued to use the device after 5 years (Otolaryngol Head Neck Surg. 2018; doi: 10.1177/0194599818762383). Median AHI at 5 years was 6.9, Dr. Strollo said, and median Epworth Sleepiness Scale scores declined from 11.6 at baseline to 6.9 at 5 years.
Another postapproval study of UAS, the ADHERE registry, has enrolled 348 patients at 10 centers with a goal of 2,500, Dr. Strollo said (Otolaryngol Head Neck Surg. 2018: doi: 10.1177/0194599818764896). Twelve-month study results have shown reductions in AHI and Epworth Sleepiness Scale scores comparable to previous reports. ADHERE also reported that 92% of patients were satisfied with UAS.
The latest innovation for UAS is the ability to download data from the implant at office visits so the physician can review patient adherence patterns, along with energy levels and settings for sensing and stimulation, Dr. Strollo said.
“Upper airway stimulation is an additional tool in the management of properly selected, at-risk apnea patients who do not accept or adhere to positive pressure therapy,” Dr. Strollo said. “The STAR trial has provided robust evidence that upper airway stimulation is safe and effective in participants with moderate to severe OSA, and the treatment effect is maintained beyond the 12-month endpoint.”
Dr. Strollo disclosed a financial relationship with Inspire Medical Systems, manufacturer of the UAS device.
BALTIMORE – Emerging developments for the treatment of obstructive sleep apnea have shown promise in providing options beyond continuous-positive airway pressure, investigators reported at the annual meeting of the Associated Professional Sleep Societies. These developments include a single-use nasopharyngeal airway stent, upper-airway stimulation using a pacemaker-like device, a negative-pressure device that opens the airway, and an artificial intelligence approach that can predict outcomes of oral appliance therapy.
Nasopharyngeal airway stent
Clete A. Kushida, MD, of Stanford (Calif.) University reported on recent results of a trial of the nasopharyngeal airway stent (NAS) single-use disposable insert (Seven Dreamers Laboratories, Tokyo). This device consists of a flexible semi-rigid silicone rubber tube 120-145 mm in length and coated with a hydrophilic gel. The patient inserts the distal end of the tube into the nostril, and it positions itself within the nasopharynx and retropalatal oropharynx to open the airway. A clip attaches to the exterior septum to keep the device in place. The patient removes the NAS in the morning. The device is commercially available in Japan and some European countries.
Dr. Kushida reported on two posters that were presented at Sleep 2018. The first by the Osaka University Graduate School of Dentistry in Japan investigated the predictability of NAS efficacy in patients with a velopharynx that was narrower than the hypopharynx (Sleep. 2018;41 (suppl 1):A207: doi.org/10.1093/sleep/zsy061.554). The study showed that 11 responders had a narrow velopharynx while 18 nonresponders had a narrow hypopharynx. Response was defined as 50% or greater reduction of apnea-hypopnea index (AHI) from baseline. “The success rate of NAS for the patients with narrowing of the velopharynx is 83.3%,” Dr. Kushida said.
He also reported on a study he led of NAS in patients with obstructive sleep apnea and healthy controls (Sleep. 2018;41 (suppl 1):A207: doi.org/10.1093/sleep/zsy061.555). The trial was conducted at Stanford and Tokyo sleep medical centers, with healthy controls at the Tokyo site only. AHI improved in all three obstructive sleep apnea (OSA) groups, but most significantly in those with moderate (n = 23) and severe (n = 21) OSA: 7.2 (P = .0038) and 11.7 (P = .0069), respectively. In the Stanford cohort, 2 of 32 patients originally enrolled dropped out because they found the NAS uncomfortable; none dropped out of the Tokyo cohort.
“NAS can be effective in treatment of snoring and OSA, in particular those with moderate/severe OSA, with significant improvement in mean obstructive apnea index,” Dr. Kushida said. He also noted the device is more effective in patients with narrowing of the oropharynx/velopharynx rather than the hypopharynx.
Dr. Kushida reported he had a financial relationship with the Seven Dreamers Laboratories.
Continuous negative external pressure
Jerrold A. Kram, MD, reported on a device that employs continuous negative external pressure – known as cNEP – that uses a silicone collar covering the front of the throat and a pump that applies suction to keep the airway open from the outside. He cited a small 2015 study out of Japan that found the device was effective in keeping the pharyngeal airway open in nonobese women (J Appl Physiol. 2015;118:912-20). Another small U.S. study found cNEP reduces respiratory impairment during screening colonoscopy (Endoscopy. 2016;48:584-7). Sommetrics, which has patented the technology, is developing a version of the product for obstructive sleep apnea, Dr. Kram said. The company already has a Food and Drug Administration–approved product to treat apneas that people experience when under mild to moderate sedation, such as a colonoscopy. It is approved for sale in Canada, but not in the United States.
“It gives us another tool in the box,” Dr. Kram said. “It’s very small and portable, easy to take on an airplane with you, and it reduces the chance for claustrophobia” that comes with continuous-positive airway pressure (CPAP), said Dr. Kram. There are no tubes or masks and no humidifier to deal with.
Dr. Kram conducted a small study of 15 OSA patients using cNEP last year. Among nine excellent responders, the lowest AHI was 1.5 on average, from an average baseline of 43.9; among four partial responders, the lowest observed AHI averaged 11.75 (J Clin Sleep Med. 2017;13:1009-12).
The cNEP device is also the subject of a home study of patients with OSA in Canada, Dr. Kram said. Unpublished results indicate that 46% (27/59) of patients had an initial response rate to either -25 or -30 characteristic moment waveform (cmw) of negative airway pressure. For those who completed 3 weeks of treatment, the response rate was 64% (16/25). “Seventy-six percent of subjects felt their overall experience with cNEP was better than with CPAP,” Dr. Kram said.
However, studies have noted some minor issues with cNEP, Dr. Kram said. They include mild skin irritation, choking sensation if not properly placed, limited size availability, and absence of efficacy data. Dr. Kram said a U.S. pivotal trial is scheduled to start soon.
Dr. Kram disclosed he is a paid adviser for Sommetrics.
Oral appliance therapy
Oral appliance therapy (OAT) has been very effective in some patients and has been endorsed by the American Academy of Sleep Medicine since 2006, but it has been underutilized predominantly for two reasons, noted John Remmers, MD, because fitting the device requires a trial-and-error approach that can discourage patients; and the therapeutic success rate is 50%-60%.
To predict which patients are likely to succeed with OAT, Dr. Remmers and his colleagues at Zephyr Sleep Technologies have developed an artificial intelligence (AI) platform that uses a feedback-controlled mandibular positioner, a mouthpiece-like device that opens the airway during sleep. Dr. Remmers is chief medical officer and cofounder of Zephyr Sleep Technologies, based in Calgary, Alta.
While imaging has been used in awake patients to fit OAT devices, Dr. Remmers noted a number of shortcomings with this modality. “Sleep apnea results from an anatomic problem – i.e., structural encroachment on the pharyngeal airway, which is neurally compensated when the patient is awake,” he said. “Because of this, we need to do the test when the patient is asleep.”
The AI platform with feedback-controlled mandibular positioner uses temporary dental trays to create impressions for the mouthpiece the patient uses during sleep. The mouthpiece connects to a remote-controlled device that makes real-time adjustments in the mandibular position without disturbing the patient’s sleep. “The computer identifies respiratory events all night long, adjusting the position of the mandible,” he said in explaining the AI component of the device. “Just think of it as similar to autotitration with CPAP.”
Dr. Remmers reported results of a phase 2 study of the platform presented as a poster reporting on a study of 101 patients with OSA participants. The study reported sensitivity of 86% and specificity of 92% in predicting success with the feedback-controlled mandibular positioner, he said.
“The sensitivity and overall predictive accuracy of the AI-based approach was greater than an intuitive approach using a pretreatment, temporary appliance, indicating that feedback-controlled mandibular positioning test outperformed the intuitive approach,” he said. The device has been approved by Health Canada and is awaiting clearance in the United States.
Upper airway stimulation
Upper airway stimulation (UAS) is emerging as a new class of therapy, said Patrick J. Strollo Jr., MD, of the University of Pittsburgh. The therapy involves an impulse generator (IPG) similar to a pacemaker that is implanted in the left side of the chest and connected to a stimulation lead secured to the distal hypoglossal nerve in the neck. The UAS system also incorporates a sensing lead that is implanted between the intercostal muscles and attached to the IPG allowing for phasic stimulation of the genioglossal muscle. The patient uses a remote control to turn the device on at night and off in the morning.
Dr. Strollo was lead author of the Stimulation Therapy for Apnea Reduction (STAR) trial (N Engl J Med. 2014:370;139-49), a prospective multicenter trial with a randomized therapy withdrawal arm. In 126 participants, he said, the median AHI score declined 68% in 12 months, from 29.3 at baseline to 9 (P less than .0001).
He provided updated results that showed 80% of patients continued to use the device after 5 years (Otolaryngol Head Neck Surg. 2018; doi: 10.1177/0194599818762383). Median AHI at 5 years was 6.9, Dr. Strollo said, and median Epworth Sleepiness Scale scores declined from 11.6 at baseline to 6.9 at 5 years.
Another postapproval study of UAS, the ADHERE registry, has enrolled 348 patients at 10 centers with a goal of 2,500, Dr. Strollo said (Otolaryngol Head Neck Surg. 2018: doi: 10.1177/0194599818764896). Twelve-month study results have shown reductions in AHI and Epworth Sleepiness Scale scores comparable to previous reports. ADHERE also reported that 92% of patients were satisfied with UAS.
The latest innovation for UAS is the ability to download data from the implant at office visits so the physician can review patient adherence patterns, along with energy levels and settings for sensing and stimulation, Dr. Strollo said.
“Upper airway stimulation is an additional tool in the management of properly selected, at-risk apnea patients who do not accept or adhere to positive pressure therapy,” Dr. Strollo said. “The STAR trial has provided robust evidence that upper airway stimulation is safe and effective in participants with moderate to severe OSA, and the treatment effect is maintained beyond the 12-month endpoint.”
Dr. Strollo disclosed a financial relationship with Inspire Medical Systems, manufacturer of the UAS device.
REPORTING FROM SLEEP 2018
Key clinical point: Alternative treatments have shown sustained improvement in sleep apnea symptoms.
Major finding: The negative-pressure device improved oxygen flow in OSA to 64% vs. 25% for continuous-positive airway pressure.
Data source: Multiple abstracts presented at Sleep 2018 and published studies, including ADHERE study of 326 patients and a multicenter study of 430 patients using the upper airway stimulation device; a trial of 67 patients using the nasopharyngeal airway stent; and 101 patients in the artificial intelligence study.
Disclosures: Dr. Kushida disclosed a relationship with Seven Dreamers Laboratories. Dr. Kram is a paid adviser for Sommetrics. Dr. Remmers is founder of Zephyr Sleep Technologies. Dr. Strollo is an investigator in the STAR trial, supported by Inspire Medical Systems.
Methamphetamine use climbing among opioid users
SAN DIEGO – As the deadly opioid epidemic continues, a new study suggests that a fast-rising number of users are turning to another drug of abuse – methamphetamine. In some cases, a researcher says, their co-use is reminiscent of the fad for “speedball” mixtures of cocaine and heroin.
During 2011-2017, the percentage of surveyed opioid users seeking treatment who reported also using methamphetamine over the past month skyrocketed from 19% to 34%, researchers reported at the 2018 annual meeting of the College on Problems of Drug Dependence.
Use of crystal meth specifically went up by 82% and the use of prescription stimulants rose by 15%. By contrast, use of marijuana went up by just 6%, while the use of muscle relaxants and prescription sleep drugs fell by more than half.
The findings matter, because the use of multiple illicit drugs is even more dangerous than one alone, said study coauthor and doctoral candidate Matthew S. Ellis, of Washington University in St. Louis, in an interview. “Illicit opioids carry their own serious risks such as unknown purity, not knowing if heroin is laced with fentanyl, or inexperience of users who are used to clearly marked prescription pills. Add in a secondary drug, also often used in non-oral ways, and your risk for overdose is going to significantly increase.”
The rising use of methamphetamine, which comes in such forms as crystal meth, has been overshadowed by news about the opioid epidemic. Still, as a 2018 Lancet report put it, “while the opioid crisis has exploded, the lull in the methamphetamine epidemic has quietly and swiftly reversed course, now accounting for 11% of the total number of overdose deaths.”
In regard to co-use of opioids and methamphetamines, the report said, “in states including Wisconsin and Oregon, new patterns suggest they are beginning to overlap as increasing numbers of people use both drugs” (Lancet. 2018 Feb. 24;391[10122]:713).
Meanwhile, the New York Times published a story in February 2018 headlined “Meth, the forgotten killer, is back. And it’s everywhere.” It noted that meth overdose deaths in Oregon outpace those from opioids and added: “At the United States border, agents are seizing 10-20 times the amounts they did a decade ago. Methamphetamine, experts say, has never been purer, cheaper, or more lethal.”
Overall, there’s little known about co-use of opioids and methamphetamines, said study lead author Mr. Ellis. “The reason for this is that opioid use patterns and populations of users have drastically changed in the past 20 years, and continue to do so,” he said. “Methamphetamine is becoming increasingly available at the same time that heroin and illicit fentanyl are as well. Reports suggest that the United States has shifted from a market of home-grown methamphetamine to that manufactured and sent from other countries, creating a broader market than previously seen.”
For the new study, Mr. Ellis and his colleagues examined statistics from a U.S. surveillance program of opioid users entering substance abuse programs. They focused on 13,521 participants in 47 states during 2011-2017.
Of 12 drug classes examined, only co-use of methamphetamine rose significantly over the 6-year period, Mr. Ellis said.
Among demographic and geographic groups, the researchers saw the largest increases in co-use of the two drugs in the West, Northeast, and Midwest regions, in rural and suburban areas, among groups aged 18-44 years, and among whites.
Why is co-use among opioid users increasing? “We have begun to do some qualitative work with a number of participants suggesting they use opioids and methamphetamine to balance each other out,” Mr. Ellis said. “So an addict can use opioids, but if they need to go to work, they can reinvigorate themselves with methamphetamine.”
Mr. Ellis said “this is not necessarily a new trend,” noting that the co-use of the drugs is akin to the “speedball” – a mixture of cocaine and heroin designed to blend their opposite modes of action.
However, Mr. Ellis said, “ The increases in production and spread of illicit opioids and methamphetamine into an existing market of those previously using prescription opioids was a perfect storm for these two drugs to be a problem, both separately and together.”
He said researchers also are finding that “if methamphetamine is the only thing an opioid addict can find, they will use it to stave off withdrawals as well.”
Indeed, National Public Radio reported in June 2018 that “as opioids are becoming harder to obtain, more and more users are turning to cheap methamphetamine” in Ohio’s tiny Vinton County, near Columbus.
Moving forward, Mr. Ellis said, “we cannot treat substance use in a silo of a single drug. If we attempt to treat opioid abusers by simply treating their opioid abuse – and not other drugs – then we have less of a chance of success. More of a focus needs to be put on the fact that the vast majority of opioid abusers are polysubstance users.”
The study is funded by the RADARS (Researched Abuse, Diversion and Addiction-Related Surveillance) System, an independent, nonprofit postmarketing surveillance system supported by subscription fees from pharmaceutical manufacturers that use RADARS data to track medication use and meet regulatory obligations. The study authors report no relevant disclosures.
SAN DIEGO – As the deadly opioid epidemic continues, a new study suggests that a fast-rising number of users are turning to another drug of abuse – methamphetamine. In some cases, a researcher says, their co-use is reminiscent of the fad for “speedball” mixtures of cocaine and heroin.
During 2011-2017, the percentage of surveyed opioid users seeking treatment who reported also using methamphetamine over the past month skyrocketed from 19% to 34%, researchers reported at the 2018 annual meeting of the College on Problems of Drug Dependence.
Use of crystal meth specifically went up by 82% and the use of prescription stimulants rose by 15%. By contrast, use of marijuana went up by just 6%, while the use of muscle relaxants and prescription sleep drugs fell by more than half.
The findings matter, because the use of multiple illicit drugs is even more dangerous than one alone, said study coauthor and doctoral candidate Matthew S. Ellis, of Washington University in St. Louis, in an interview. “Illicit opioids carry their own serious risks such as unknown purity, not knowing if heroin is laced with fentanyl, or inexperience of users who are used to clearly marked prescription pills. Add in a secondary drug, also often used in non-oral ways, and your risk for overdose is going to significantly increase.”
The rising use of methamphetamine, which comes in such forms as crystal meth, has been overshadowed by news about the opioid epidemic. Still, as a 2018 Lancet report put it, “while the opioid crisis has exploded, the lull in the methamphetamine epidemic has quietly and swiftly reversed course, now accounting for 11% of the total number of overdose deaths.”
In regard to co-use of opioids and methamphetamines, the report said, “in states including Wisconsin and Oregon, new patterns suggest they are beginning to overlap as increasing numbers of people use both drugs” (Lancet. 2018 Feb. 24;391[10122]:713).
Meanwhile, the New York Times published a story in February 2018 headlined “Meth, the forgotten killer, is back. And it’s everywhere.” It noted that meth overdose deaths in Oregon outpace those from opioids and added: “At the United States border, agents are seizing 10-20 times the amounts they did a decade ago. Methamphetamine, experts say, has never been purer, cheaper, or more lethal.”
Overall, there’s little known about co-use of opioids and methamphetamines, said study lead author Mr. Ellis. “The reason for this is that opioid use patterns and populations of users have drastically changed in the past 20 years, and continue to do so,” he said. “Methamphetamine is becoming increasingly available at the same time that heroin and illicit fentanyl are as well. Reports suggest that the United States has shifted from a market of home-grown methamphetamine to that manufactured and sent from other countries, creating a broader market than previously seen.”
For the new study, Mr. Ellis and his colleagues examined statistics from a U.S. surveillance program of opioid users entering substance abuse programs. They focused on 13,521 participants in 47 states during 2011-2017.
Of 12 drug classes examined, only co-use of methamphetamine rose significantly over the 6-year period, Mr. Ellis said.
Among demographic and geographic groups, the researchers saw the largest increases in co-use of the two drugs in the West, Northeast, and Midwest regions, in rural and suburban areas, among groups aged 18-44 years, and among whites.
Why is co-use among opioid users increasing? “We have begun to do some qualitative work with a number of participants suggesting they use opioids and methamphetamine to balance each other out,” Mr. Ellis said. “So an addict can use opioids, but if they need to go to work, they can reinvigorate themselves with methamphetamine.”
Mr. Ellis said “this is not necessarily a new trend,” noting that the co-use of the drugs is akin to the “speedball” – a mixture of cocaine and heroin designed to blend their opposite modes of action.
However, Mr. Ellis said, “ The increases in production and spread of illicit opioids and methamphetamine into an existing market of those previously using prescription opioids was a perfect storm for these two drugs to be a problem, both separately and together.”
He said researchers also are finding that “if methamphetamine is the only thing an opioid addict can find, they will use it to stave off withdrawals as well.”
Indeed, National Public Radio reported in June 2018 that “as opioids are becoming harder to obtain, more and more users are turning to cheap methamphetamine” in Ohio’s tiny Vinton County, near Columbus.
Moving forward, Mr. Ellis said, “we cannot treat substance use in a silo of a single drug. If we attempt to treat opioid abusers by simply treating their opioid abuse – and not other drugs – then we have less of a chance of success. More of a focus needs to be put on the fact that the vast majority of opioid abusers are polysubstance users.”
The study is funded by the RADARS (Researched Abuse, Diversion and Addiction-Related Surveillance) System, an independent, nonprofit postmarketing surveillance system supported by subscription fees from pharmaceutical manufacturers that use RADARS data to track medication use and meet regulatory obligations. The study authors report no relevant disclosures.
SAN DIEGO – As the deadly opioid epidemic continues, a new study suggests that a fast-rising number of users are turning to another drug of abuse – methamphetamine. In some cases, a researcher says, their co-use is reminiscent of the fad for “speedball” mixtures of cocaine and heroin.
During 2011-2017, the percentage of surveyed opioid users seeking treatment who reported also using methamphetamine over the past month skyrocketed from 19% to 34%, researchers reported at the 2018 annual meeting of the College on Problems of Drug Dependence.
Use of crystal meth specifically went up by 82% and the use of prescription stimulants rose by 15%. By contrast, use of marijuana went up by just 6%, while the use of muscle relaxants and prescription sleep drugs fell by more than half.
The findings matter, because the use of multiple illicit drugs is even more dangerous than one alone, said study coauthor and doctoral candidate Matthew S. Ellis, of Washington University in St. Louis, in an interview. “Illicit opioids carry their own serious risks such as unknown purity, not knowing if heroin is laced with fentanyl, or inexperience of users who are used to clearly marked prescription pills. Add in a secondary drug, also often used in non-oral ways, and your risk for overdose is going to significantly increase.”
The rising use of methamphetamine, which comes in such forms as crystal meth, has been overshadowed by news about the opioid epidemic. Still, as a 2018 Lancet report put it, “while the opioid crisis has exploded, the lull in the methamphetamine epidemic has quietly and swiftly reversed course, now accounting for 11% of the total number of overdose deaths.”
In regard to co-use of opioids and methamphetamines, the report said, “in states including Wisconsin and Oregon, new patterns suggest they are beginning to overlap as increasing numbers of people use both drugs” (Lancet. 2018 Feb. 24;391[10122]:713).
Meanwhile, the New York Times published a story in February 2018 headlined “Meth, the forgotten killer, is back. And it’s everywhere.” It noted that meth overdose deaths in Oregon outpace those from opioids and added: “At the United States border, agents are seizing 10-20 times the amounts they did a decade ago. Methamphetamine, experts say, has never been purer, cheaper, or more lethal.”
Overall, there’s little known about co-use of opioids and methamphetamines, said study lead author Mr. Ellis. “The reason for this is that opioid use patterns and populations of users have drastically changed in the past 20 years, and continue to do so,” he said. “Methamphetamine is becoming increasingly available at the same time that heroin and illicit fentanyl are as well. Reports suggest that the United States has shifted from a market of home-grown methamphetamine to that manufactured and sent from other countries, creating a broader market than previously seen.”
For the new study, Mr. Ellis and his colleagues examined statistics from a U.S. surveillance program of opioid users entering substance abuse programs. They focused on 13,521 participants in 47 states during 2011-2017.
Of 12 drug classes examined, only co-use of methamphetamine rose significantly over the 6-year period, Mr. Ellis said.
Among demographic and geographic groups, the researchers saw the largest increases in co-use of the two drugs in the West, Northeast, and Midwest regions, in rural and suburban areas, among groups aged 18-44 years, and among whites.
Why is co-use among opioid users increasing? “We have begun to do some qualitative work with a number of participants suggesting they use opioids and methamphetamine to balance each other out,” Mr. Ellis said. “So an addict can use opioids, but if they need to go to work, they can reinvigorate themselves with methamphetamine.”
Mr. Ellis said “this is not necessarily a new trend,” noting that the co-use of the drugs is akin to the “speedball” – a mixture of cocaine and heroin designed to blend their opposite modes of action.
However, Mr. Ellis said, “ The increases in production and spread of illicit opioids and methamphetamine into an existing market of those previously using prescription opioids was a perfect storm for these two drugs to be a problem, both separately and together.”
He said researchers also are finding that “if methamphetamine is the only thing an opioid addict can find, they will use it to stave off withdrawals as well.”
Indeed, National Public Radio reported in June 2018 that “as opioids are becoming harder to obtain, more and more users are turning to cheap methamphetamine” in Ohio’s tiny Vinton County, near Columbus.
Moving forward, Mr. Ellis said, “we cannot treat substance use in a silo of a single drug. If we attempt to treat opioid abusers by simply treating their opioid abuse – and not other drugs – then we have less of a chance of success. More of a focus needs to be put on the fact that the vast majority of opioid abusers are polysubstance users.”
The study is funded by the RADARS (Researched Abuse, Diversion and Addiction-Related Surveillance) System, an independent, nonprofit postmarketing surveillance system supported by subscription fees from pharmaceutical manufacturers that use RADARS data to track medication use and meet regulatory obligations. The study authors report no relevant disclosures.
REPORTING FROM CPDD 2018
Key clinical point: The percentage of opioid users who also use methamphetamine is on the rise.
Major finding: During 2011-2017, the percentage of opioid users reporting methamphetamine use over the past month grew from 19% to 34%.
Study details: Analysis of 2011-2017 data from 13,521 opioid-using participants entering substance abuse programs.
Disclosures: The study is funded by the RADARS System, an independent, nonprofit postmarketing surveillance system supported by subscription fees from pharmaceutical manufacturers that use RADARS data to track medication use and meet regulatory obligations. The study authors report no relevant disclosures.
Cost led to missed care for 4.5% of Americans in 2017
The percentage of Americans who went without medical care due to cost rose to 4.5% in 2017, reversing a 6-year trend, the National Center for Health Statistics reported.
The rate was 4.4% in 2016, which represented a slowdown in what had been steady decline over the previous 5 years, according to data from the National Health Interview Survey. Declining rates corresponded with the implementation of early provisions of the Affordable Care Act in 2010.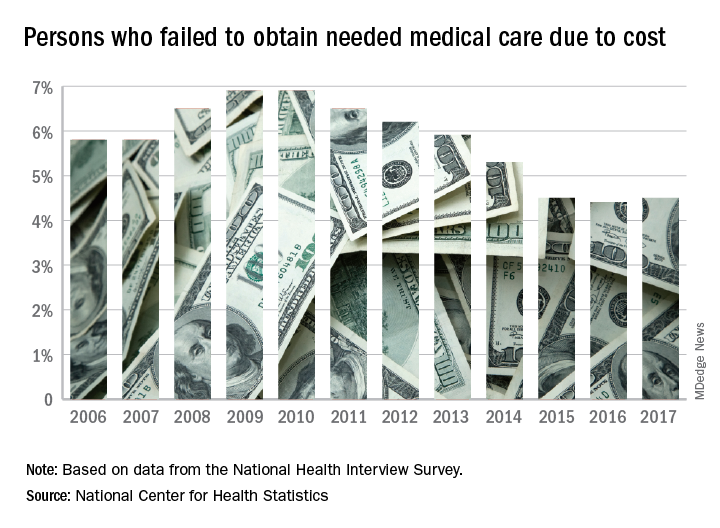
The 2017 rate varied considerably by age group. Not surprisingly, more working-age people – those aged 18-64 years – reported that they did not seek medical care at some point in the previous 12 months due to cost (6.1%). The rate was 1.2% for those under 18 years and 2.7% for the Medicare eligible – those aged 65 years and older.
For 2016, the rates were 6.2% for those aged 18-64 years, 1.2% for the under-18 group, and 2.1% for the 65+ group, the data show.
Among females of all ages in 2017, 4.8% failed to get needed care at some point in the previous year, compared with 4.1% of men. Those numbers were unchanged from 2016 but down from 4.9% for females in 2015 and up from 4.0% for males that year, the NCHS said.
In 2017, the rate also varied by race/ethnicity – 4.1% for whites, 5.3% for Hispanics, 6.1% for blacks – and by location – 4.1% for large metropolitan areas, 4.9% for small metro areas, and 5.5% for rural locales, according to the early release of survey data.
The percentage of Americans who went without medical care due to cost rose to 4.5% in 2017, reversing a 6-year trend, the National Center for Health Statistics reported.
The rate was 4.4% in 2016, which represented a slowdown in what had been steady decline over the previous 5 years, according to data from the National Health Interview Survey. Declining rates corresponded with the implementation of early provisions of the Affordable Care Act in 2010.
The 2017 rate varied considerably by age group. Not surprisingly, more working-age people – those aged 18-64 years – reported that they did not seek medical care at some point in the previous 12 months due to cost (6.1%). The rate was 1.2% for those under 18 years and 2.7% for the Medicare eligible – those aged 65 years and older.
For 2016, the rates were 6.2% for those aged 18-64 years, 1.2% for the under-18 group, and 2.1% for the 65+ group, the data show.
Among females of all ages in 2017, 4.8% failed to get needed care at some point in the previous year, compared with 4.1% of men. Those numbers were unchanged from 2016 but down from 4.9% for females in 2015 and up from 4.0% for males that year, the NCHS said.
In 2017, the rate also varied by race/ethnicity – 4.1% for whites, 5.3% for Hispanics, 6.1% for blacks – and by location – 4.1% for large metropolitan areas, 4.9% for small metro areas, and 5.5% for rural locales, according to the early release of survey data.
The percentage of Americans who went without medical care due to cost rose to 4.5% in 2017, reversing a 6-year trend, the National Center for Health Statistics reported.
The rate was 4.4% in 2016, which represented a slowdown in what had been steady decline over the previous 5 years, according to data from the National Health Interview Survey. Declining rates corresponded with the implementation of early provisions of the Affordable Care Act in 2010.
The 2017 rate varied considerably by age group. Not surprisingly, more working-age people – those aged 18-64 years – reported that they did not seek medical care at some point in the previous 12 months due to cost (6.1%). The rate was 1.2% for those under 18 years and 2.7% for the Medicare eligible – those aged 65 years and older.
For 2016, the rates were 6.2% for those aged 18-64 years, 1.2% for the under-18 group, and 2.1% for the 65+ group, the data show.
Among females of all ages in 2017, 4.8% failed to get needed care at some point in the previous year, compared with 4.1% of men. Those numbers were unchanged from 2016 but down from 4.9% for females in 2015 and up from 4.0% for males that year, the NCHS said.
In 2017, the rate also varied by race/ethnicity – 4.1% for whites, 5.3% for Hispanics, 6.1% for blacks – and by location – 4.1% for large metropolitan areas, 4.9% for small metro areas, and 5.5% for rural locales, according to the early release of survey data.
Resident debt burden may cloud professional future
Surgical trainees have a large, potentially unmanageable debt burden and are in need of long-term financial education to help better navigate the growing cost of medical education, according to new research.
“Surgical residents are highly leveraged financially and have minimal financial training,” Sarah E. Tevis, MD, of the University of Texas MD Anderson Cancer Center, Houston, and her colleagues wrote in a study in the Journal of the American College of Surgeons. “This places residents in a volatile financial situation as they complete their training and start accumulating debt liabilities, such as mortgages and child care, in the face of tremendous amounts of educational and other debt liabilities.”
Studies of resident debt load typically account for medical education debt, but not for other debts such as undergraduate loans, consumer debt, and mortgages. Residents’ actual debt burden may be considerably higher than has been reported.
The researchers sent surveys to all surgical residents at the University of Wisconsin, Madison, in 2015, with 105 responding (an 80% response rate). Of those responding, 38% reported having more than $200,000 in educational debt, and 82% had a moderate- or high-risk debt-to-asset ratio.
“We found that surgical residents are dangerously overleveraged, with 70% of residents found to have high debt-to-asset ratios,” Dr. Tevis and her colleagues wrote, with the addition of mortgages and vehicle debt on top of educational debt being the key factors of moving residents into the high-risk debt-to-asset category.
The debt-to-asset ratio was calculated as the sum of student loan debt + nonstudent loan debt + credit card balance + mortgage debt + vehicle debt divided by the value of home and other real estate + value of household vehicles + amount in savings + value of retirement investment. A debt-to-asset ratio of 0.5 to 0.9 was considered moderate risk, with a ratio greater than or equal to 0.9 considered high risk.
The debt-to-income ratio was calculated as the sum of student loan debt + nonstudent loan debt + credit card balance + mortgage debt + vehicle debt divided by total household income. A high-risk ratio defined as being greater than 0.4, the line at which surgical trainees might be restricted from obtaining a traditional mortgage.
Total household income included personal income, domestic partner income, military income, and any income from moonlighting, rental properties, and other sources of revenue. Assets included home and second home purchase prices when applicable, value of vehicles, amount in savings, value of retirement accounts, and value of investments. Contributors to debt included student loan, nonstudent loan, mortgage, vehicle financing, and credit card balances.
Salary data for U.S. residents, which strictly tracked U.S. inflation, was calculated over a 15-year period (2000-2015) using data from the Association of American Medical Colleges for comparison.
In examining debt-to-income, researchers found that “83% of residents have a high-risk debt-to-income ratio [greater than 0.4],” the authors wrote. “We found that the majority of residents were classified in the high financial risk exposure cohort when comprehensive total debt liabilities were considered. In this group of highly leveraged residents, over 80% of residents were dangerously unable to manage regular monthly liabilities with their current level of income.”
No statistically significant association between sex, residency year, residency program, or who manages finances and risk debt-to-asset ratios were found in this study.
The authors noted that, although this study did not look at the psychological impact of significant debt load and lack of training on how to manage finances, these factors have been shown in other studies to correlate with resident burnout and psychological stress.
Bruce A. Harms, MD, FACS, coauthor of the study said in an interview, “We are in an evolving era in surgery and in health care in general and financial resources are being stretched. We don’t know for sure that the rising educational debt and overall debt burden as residents enter their prime years will drive the next generation of physicians to certain career choices. It may even perhaps drive a given fully trained young surgeon away from a practice that is more exposed to an underserved population. Excessive financial debt induced stress may influence a resident’s decision on what they do with their skill set but to what degree is largely unknown.”
Dr. Harms added that residents may assume that when they eventually enter practice, they will have a pathway and the means to deal with educational debt. “They would be correct in that starting salaries are keeping pace with inflation. However, in many instances, they are also entering a time in their lives when they will be taking on additional debt in the form of home mortgage, family, and child care costs. I believe, in most instances, residents are focused almost totally on their residency training and many other financial considerations take a back seat and ‘we’ll deal with our debt problem in the future’ attitude. Residents for the most part don’t have the financial means and resources to deal with debt anyway during the course of their lengthy residency training. The exception would be having a secondary income from a spouse or partner that would allow for a more robust debt-attrition strategy. Also, residents are likely not focused on or considering a strategy for the best return on investment of their time, additional expense, and career delay from their prolonged pathway to becoming a fully trained surgeon.”
The bottom line is that basic financial educational is not included in core surgical training even though most surgical residents would like some degree of financial education. That is the basic problem and shortcoming of existing residency training programs, Dr. Tevis and her colleagues wrote.
Given the financial burdens that education and other factors are placing on surgical residents, Dr. Tevis and her colleagues proposed “that formal training in the business of medicine and personal finance for surgical residents be strongly considered at the training program level or in partnership with other organizations, such as the American College of Surgeons, in an effort to improve the financial status of residents and prepare them for their careers, both personally and professionally.”
Dr. Harms noted, “It is probable that in most cases, educational loan debt principal is not being paid down to any significant degree given the current residency salary structure. We can only hope that residents are given some degree of good information on strategies for managing educational loan debt, which may include federally sponsored loan repayment programs such as [those offered] through NIH-sponsored research or federal loan forgiveness programs that residents may qualify for. In most cases, federal loan forgiveness programs require a minimum monthly payment that is calculated based upon current income. As an absolute minimum, interest payments should be made as additional interest debt will add significantly to the overall debt burden as interest will continue to accrue.”
Getting that financial training early could have significant benefits on the back end. The study authors noted that salary data from the Association of American Medical Colleges showed assistant professor salaries mirrored inflation metrics, but even better, surgeon salaries continued to exceed inflation-indexed targets and continued upward trends even through recession periods.
“Therefore, the financial pathway, built on increases in surgeon starting salaries exceeding annual inflation, presently still exists for deleveraging of critical debt exposure if personal finances are optimally managed,” the authors stated.
The study did have its limitations. It did not include certain variable expenses such as utilities, food, and other shopping habits, although that may have been captured as the survey asked respondents to list other “major” sources of income and debt. It also was limited to surgical residents at a single institution and may not be applicable to other specialties or geographic locations. It did not assess whether residents with mortgage payments were able to make educational loan payments beyond the minimum.
The investigators reported no conflicts.
SOURCE: Tevis SE et al. J Am Coll Surg. 2018 May 31. doi: 10.1016/j.jamcollsurg.2018.05.002.
Surgical trainees have a large, potentially unmanageable debt burden and are in need of long-term financial education to help better navigate the growing cost of medical education, according to new research.
“Surgical residents are highly leveraged financially and have minimal financial training,” Sarah E. Tevis, MD, of the University of Texas MD Anderson Cancer Center, Houston, and her colleagues wrote in a study in the Journal of the American College of Surgeons. “This places residents in a volatile financial situation as they complete their training and start accumulating debt liabilities, such as mortgages and child care, in the face of tremendous amounts of educational and other debt liabilities.”
Studies of resident debt load typically account for medical education debt, but not for other debts such as undergraduate loans, consumer debt, and mortgages. Residents’ actual debt burden may be considerably higher than has been reported.
The researchers sent surveys to all surgical residents at the University of Wisconsin, Madison, in 2015, with 105 responding (an 80% response rate). Of those responding, 38% reported having more than $200,000 in educational debt, and 82% had a moderate- or high-risk debt-to-asset ratio.
“We found that surgical residents are dangerously overleveraged, with 70% of residents found to have high debt-to-asset ratios,” Dr. Tevis and her colleagues wrote, with the addition of mortgages and vehicle debt on top of educational debt being the key factors of moving residents into the high-risk debt-to-asset category.
The debt-to-asset ratio was calculated as the sum of student loan debt + nonstudent loan debt + credit card balance + mortgage debt + vehicle debt divided by the value of home and other real estate + value of household vehicles + amount in savings + value of retirement investment. A debt-to-asset ratio of 0.5 to 0.9 was considered moderate risk, with a ratio greater than or equal to 0.9 considered high risk.
The debt-to-income ratio was calculated as the sum of student loan debt + nonstudent loan debt + credit card balance + mortgage debt + vehicle debt divided by total household income. A high-risk ratio defined as being greater than 0.4, the line at which surgical trainees might be restricted from obtaining a traditional mortgage.
Total household income included personal income, domestic partner income, military income, and any income from moonlighting, rental properties, and other sources of revenue. Assets included home and second home purchase prices when applicable, value of vehicles, amount in savings, value of retirement accounts, and value of investments. Contributors to debt included student loan, nonstudent loan, mortgage, vehicle financing, and credit card balances.
Salary data for U.S. residents, which strictly tracked U.S. inflation, was calculated over a 15-year period (2000-2015) using data from the Association of American Medical Colleges for comparison.
In examining debt-to-income, researchers found that “83% of residents have a high-risk debt-to-income ratio [greater than 0.4],” the authors wrote. “We found that the majority of residents were classified in the high financial risk exposure cohort when comprehensive total debt liabilities were considered. In this group of highly leveraged residents, over 80% of residents were dangerously unable to manage regular monthly liabilities with their current level of income.”
No statistically significant association between sex, residency year, residency program, or who manages finances and risk debt-to-asset ratios were found in this study.
The authors noted that, although this study did not look at the psychological impact of significant debt load and lack of training on how to manage finances, these factors have been shown in other studies to correlate with resident burnout and psychological stress.
Bruce A. Harms, MD, FACS, coauthor of the study said in an interview, “We are in an evolving era in surgery and in health care in general and financial resources are being stretched. We don’t know for sure that the rising educational debt and overall debt burden as residents enter their prime years will drive the next generation of physicians to certain career choices. It may even perhaps drive a given fully trained young surgeon away from a practice that is more exposed to an underserved population. Excessive financial debt induced stress may influence a resident’s decision on what they do with their skill set but to what degree is largely unknown.”
Dr. Harms added that residents may assume that when they eventually enter practice, they will have a pathway and the means to deal with educational debt. “They would be correct in that starting salaries are keeping pace with inflation. However, in many instances, they are also entering a time in their lives when they will be taking on additional debt in the form of home mortgage, family, and child care costs. I believe, in most instances, residents are focused almost totally on their residency training and many other financial considerations take a back seat and ‘we’ll deal with our debt problem in the future’ attitude. Residents for the most part don’t have the financial means and resources to deal with debt anyway during the course of their lengthy residency training. The exception would be having a secondary income from a spouse or partner that would allow for a more robust debt-attrition strategy. Also, residents are likely not focused on or considering a strategy for the best return on investment of their time, additional expense, and career delay from their prolonged pathway to becoming a fully trained surgeon.”
The bottom line is that basic financial educational is not included in core surgical training even though most surgical residents would like some degree of financial education. That is the basic problem and shortcoming of existing residency training programs, Dr. Tevis and her colleagues wrote.
Given the financial burdens that education and other factors are placing on surgical residents, Dr. Tevis and her colleagues proposed “that formal training in the business of medicine and personal finance for surgical residents be strongly considered at the training program level or in partnership with other organizations, such as the American College of Surgeons, in an effort to improve the financial status of residents and prepare them for their careers, both personally and professionally.”
Dr. Harms noted, “It is probable that in most cases, educational loan debt principal is not being paid down to any significant degree given the current residency salary structure. We can only hope that residents are given some degree of good information on strategies for managing educational loan debt, which may include federally sponsored loan repayment programs such as [those offered] through NIH-sponsored research or federal loan forgiveness programs that residents may qualify for. In most cases, federal loan forgiveness programs require a minimum monthly payment that is calculated based upon current income. As an absolute minimum, interest payments should be made as additional interest debt will add significantly to the overall debt burden as interest will continue to accrue.”
Getting that financial training early could have significant benefits on the back end. The study authors noted that salary data from the Association of American Medical Colleges showed assistant professor salaries mirrored inflation metrics, but even better, surgeon salaries continued to exceed inflation-indexed targets and continued upward trends even through recession periods.
“Therefore, the financial pathway, built on increases in surgeon starting salaries exceeding annual inflation, presently still exists for deleveraging of critical debt exposure if personal finances are optimally managed,” the authors stated.
The study did have its limitations. It did not include certain variable expenses such as utilities, food, and other shopping habits, although that may have been captured as the survey asked respondents to list other “major” sources of income and debt. It also was limited to surgical residents at a single institution and may not be applicable to other specialties or geographic locations. It did not assess whether residents with mortgage payments were able to make educational loan payments beyond the minimum.
The investigators reported no conflicts.
SOURCE: Tevis SE et al. J Am Coll Surg. 2018 May 31. doi: 10.1016/j.jamcollsurg.2018.05.002.
Surgical trainees have a large, potentially unmanageable debt burden and are in need of long-term financial education to help better navigate the growing cost of medical education, according to new research.
“Surgical residents are highly leveraged financially and have minimal financial training,” Sarah E. Tevis, MD, of the University of Texas MD Anderson Cancer Center, Houston, and her colleagues wrote in a study in the Journal of the American College of Surgeons. “This places residents in a volatile financial situation as they complete their training and start accumulating debt liabilities, such as mortgages and child care, in the face of tremendous amounts of educational and other debt liabilities.”
Studies of resident debt load typically account for medical education debt, but not for other debts such as undergraduate loans, consumer debt, and mortgages. Residents’ actual debt burden may be considerably higher than has been reported.
The researchers sent surveys to all surgical residents at the University of Wisconsin, Madison, in 2015, with 105 responding (an 80% response rate). Of those responding, 38% reported having more than $200,000 in educational debt, and 82% had a moderate- or high-risk debt-to-asset ratio.
“We found that surgical residents are dangerously overleveraged, with 70% of residents found to have high debt-to-asset ratios,” Dr. Tevis and her colleagues wrote, with the addition of mortgages and vehicle debt on top of educational debt being the key factors of moving residents into the high-risk debt-to-asset category.
The debt-to-asset ratio was calculated as the sum of student loan debt + nonstudent loan debt + credit card balance + mortgage debt + vehicle debt divided by the value of home and other real estate + value of household vehicles + amount in savings + value of retirement investment. A debt-to-asset ratio of 0.5 to 0.9 was considered moderate risk, with a ratio greater than or equal to 0.9 considered high risk.
The debt-to-income ratio was calculated as the sum of student loan debt + nonstudent loan debt + credit card balance + mortgage debt + vehicle debt divided by total household income. A high-risk ratio defined as being greater than 0.4, the line at which surgical trainees might be restricted from obtaining a traditional mortgage.
Total household income included personal income, domestic partner income, military income, and any income from moonlighting, rental properties, and other sources of revenue. Assets included home and second home purchase prices when applicable, value of vehicles, amount in savings, value of retirement accounts, and value of investments. Contributors to debt included student loan, nonstudent loan, mortgage, vehicle financing, and credit card balances.
Salary data for U.S. residents, which strictly tracked U.S. inflation, was calculated over a 15-year period (2000-2015) using data from the Association of American Medical Colleges for comparison.
In examining debt-to-income, researchers found that “83% of residents have a high-risk debt-to-income ratio [greater than 0.4],” the authors wrote. “We found that the majority of residents were classified in the high financial risk exposure cohort when comprehensive total debt liabilities were considered. In this group of highly leveraged residents, over 80% of residents were dangerously unable to manage regular monthly liabilities with their current level of income.”
No statistically significant association between sex, residency year, residency program, or who manages finances and risk debt-to-asset ratios were found in this study.
The authors noted that, although this study did not look at the psychological impact of significant debt load and lack of training on how to manage finances, these factors have been shown in other studies to correlate with resident burnout and psychological stress.
Bruce A. Harms, MD, FACS, coauthor of the study said in an interview, “We are in an evolving era in surgery and in health care in general and financial resources are being stretched. We don’t know for sure that the rising educational debt and overall debt burden as residents enter their prime years will drive the next generation of physicians to certain career choices. It may even perhaps drive a given fully trained young surgeon away from a practice that is more exposed to an underserved population. Excessive financial debt induced stress may influence a resident’s decision on what they do with their skill set but to what degree is largely unknown.”
Dr. Harms added that residents may assume that when they eventually enter practice, they will have a pathway and the means to deal with educational debt. “They would be correct in that starting salaries are keeping pace with inflation. However, in many instances, they are also entering a time in their lives when they will be taking on additional debt in the form of home mortgage, family, and child care costs. I believe, in most instances, residents are focused almost totally on their residency training and many other financial considerations take a back seat and ‘we’ll deal with our debt problem in the future’ attitude. Residents for the most part don’t have the financial means and resources to deal with debt anyway during the course of their lengthy residency training. The exception would be having a secondary income from a spouse or partner that would allow for a more robust debt-attrition strategy. Also, residents are likely not focused on or considering a strategy for the best return on investment of their time, additional expense, and career delay from their prolonged pathway to becoming a fully trained surgeon.”
The bottom line is that basic financial educational is not included in core surgical training even though most surgical residents would like some degree of financial education. That is the basic problem and shortcoming of existing residency training programs, Dr. Tevis and her colleagues wrote.
Given the financial burdens that education and other factors are placing on surgical residents, Dr. Tevis and her colleagues proposed “that formal training in the business of medicine and personal finance for surgical residents be strongly considered at the training program level or in partnership with other organizations, such as the American College of Surgeons, in an effort to improve the financial status of residents and prepare them for their careers, both personally and professionally.”
Dr. Harms noted, “It is probable that in most cases, educational loan debt principal is not being paid down to any significant degree given the current residency salary structure. We can only hope that residents are given some degree of good information on strategies for managing educational loan debt, which may include federally sponsored loan repayment programs such as [those offered] through NIH-sponsored research or federal loan forgiveness programs that residents may qualify for. In most cases, federal loan forgiveness programs require a minimum monthly payment that is calculated based upon current income. As an absolute minimum, interest payments should be made as additional interest debt will add significantly to the overall debt burden as interest will continue to accrue.”
Getting that financial training early could have significant benefits on the back end. The study authors noted that salary data from the Association of American Medical Colleges showed assistant professor salaries mirrored inflation metrics, but even better, surgeon salaries continued to exceed inflation-indexed targets and continued upward trends even through recession periods.
“Therefore, the financial pathway, built on increases in surgeon starting salaries exceeding annual inflation, presently still exists for deleveraging of critical debt exposure if personal finances are optimally managed,” the authors stated.
The study did have its limitations. It did not include certain variable expenses such as utilities, food, and other shopping habits, although that may have been captured as the survey asked respondents to list other “major” sources of income and debt. It also was limited to surgical residents at a single institution and may not be applicable to other specialties or geographic locations. It did not assess whether residents with mortgage payments were able to make educational loan payments beyond the minimum.
The investigators reported no conflicts.
SOURCE: Tevis SE et al. J Am Coll Surg. 2018 May 31. doi: 10.1016/j.jamcollsurg.2018.05.002.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF SURGEONS
Key clinical point: Residents’ actual debt may be considerably higher than has been reported.
Major finding: More than one-third of surgical residents responding to a survey reported more than $200,000 in educational debt.
Study details: An analysis of responses to a survey from 105 surgical residents at the University of Wisconsin.
Disclosures: The study authors reported no disclosures.
Source: Tevis SE et al. J Am Coll Surg. 2018 May 31. doi: 10.1016/j.jamcollsurg.2018.05.002.
Medicare hospital deaths decline, hospice usage increases
Since 2000, Medicare beneficiaries have become less likely to die in hospitals, and more likely to die in their homes or in community health care facilities.
A review of Medicare records also determined that there was a decline in health care transitions in the final 3 days of life for these patients, Joan M. Teno, MD, and her colleagues wrote in JAMA.
It is not possible to identify a specific reason for the shift, wrote Dr. Teno, professor of medicine at the Oregon Health & Science University, Portland. Between the study years of 2000 and 2015, there were several large efforts to improve care at the end of life.
“Since 2009, programs ranging from ensuring informed patient decision making to enhanced care coordination have had the goal of improving care at the end of life. Specific interventions have included promoting conversations about the goals of care, continued growth of hospice services and palliative care, and the debate and passage of the Affordable Care Act … It is difficult to disentangle efforts such as public education, promotion of advance directives through the Patient Self- Determination Act, increased access to hospice and palliative care services, financial incentives of payment policies, and other secular changes.”
The study mined data from the Centers for Medicare & Medicaid Services, and examined end-of-life outcomes among two Medicare groups: Medicare fee-for-service recipients (1,361,870) during 2009-2015, and Medicare Advantage recipients (871,845), comparing 2011 and 2015. The mean age of both cohorts was 82 years.
Outcomes included site of death and “potentially burdensome transitions,” during the last days of life. These were defined as three or more hospitalizations in the previous 3 months, or two or more hospitalizations for pneumonia, urinary tract infection, dehydration, or sepsis during the last 120 days of life. Prolonged mechanical ventilation also was deemed potentially burdensome.
Among fee-for-service recipients, deaths in acute care hospitals declined from 32.6% to19.8%. Deaths in nursing homes remained steady, at 27.2% and 24.9%. Many of these deaths (42.9%) were preceded by a stay in an ICU. There was a transient increase in end-of-life ICU use, around 2009, but by 2015, the percentage was down to 29%, compared with 65.2% in 2000.
Transitions between a nursing home and hospital in the last 90 days of life were 0.49/person in 2000 and 0.33/person in 2015. Hospitalizations for infection or dehydration fell from 14.6% to12.2%. Hospitalization with prolonged ventilation fell from 3.1% to 2.5%.
Dying in hospice care increased from 21.6% to 50.4%, and people were taking advantage of hospice services longer: the proportion using short-term services (3 days or less) fell from 9.8% to 7.7%.
Among Medicare Advantage recipients, the numbers were somewhat different. More than 50% of recipients entered hospice care in both 2011 and 2015; in both years, 8% had services for more than 3 days. About 27% had ICU care in the last days of life, in both years. Compared to fee-for-service recipients, fewer Medicare Advantage patients were in nursing homes at the time of death, and that number declined from 2011 to 2015 (37.7% to 33.2%).
In each year, about 10% of these patients had a hospitalization for dehydration or infection, and 3% had a stay requiring prolonged mechanical ventilation in each year. The mean number of health care transitions remained steady, at 0.23 and 0.21 per person each year.
Dr. Teno had no financial disclosures.
SOURCE: Teno JM et al. JAMA. 2018 Jun 25. doi: 10.1001/jama.2018.8981.
Since 2000, Medicare beneficiaries have become less likely to die in hospitals, and more likely to die in their homes or in community health care facilities.
A review of Medicare records also determined that there was a decline in health care transitions in the final 3 days of life for these patients, Joan M. Teno, MD, and her colleagues wrote in JAMA.
It is not possible to identify a specific reason for the shift, wrote Dr. Teno, professor of medicine at the Oregon Health & Science University, Portland. Between the study years of 2000 and 2015, there were several large efforts to improve care at the end of life.
“Since 2009, programs ranging from ensuring informed patient decision making to enhanced care coordination have had the goal of improving care at the end of life. Specific interventions have included promoting conversations about the goals of care, continued growth of hospice services and palliative care, and the debate and passage of the Affordable Care Act … It is difficult to disentangle efforts such as public education, promotion of advance directives through the Patient Self- Determination Act, increased access to hospice and palliative care services, financial incentives of payment policies, and other secular changes.”
The study mined data from the Centers for Medicare & Medicaid Services, and examined end-of-life outcomes among two Medicare groups: Medicare fee-for-service recipients (1,361,870) during 2009-2015, and Medicare Advantage recipients (871,845), comparing 2011 and 2015. The mean age of both cohorts was 82 years.
Outcomes included site of death and “potentially burdensome transitions,” during the last days of life. These were defined as three or more hospitalizations in the previous 3 months, or two or more hospitalizations for pneumonia, urinary tract infection, dehydration, or sepsis during the last 120 days of life. Prolonged mechanical ventilation also was deemed potentially burdensome.
Among fee-for-service recipients, deaths in acute care hospitals declined from 32.6% to19.8%. Deaths in nursing homes remained steady, at 27.2% and 24.9%. Many of these deaths (42.9%) were preceded by a stay in an ICU. There was a transient increase in end-of-life ICU use, around 2009, but by 2015, the percentage was down to 29%, compared with 65.2% in 2000.
Transitions between a nursing home and hospital in the last 90 days of life were 0.49/person in 2000 and 0.33/person in 2015. Hospitalizations for infection or dehydration fell from 14.6% to12.2%. Hospitalization with prolonged ventilation fell from 3.1% to 2.5%.
Dying in hospice care increased from 21.6% to 50.4%, and people were taking advantage of hospice services longer: the proportion using short-term services (3 days or less) fell from 9.8% to 7.7%.
Among Medicare Advantage recipients, the numbers were somewhat different. More than 50% of recipients entered hospice care in both 2011 and 2015; in both years, 8% had services for more than 3 days. About 27% had ICU care in the last days of life, in both years. Compared to fee-for-service recipients, fewer Medicare Advantage patients were in nursing homes at the time of death, and that number declined from 2011 to 2015 (37.7% to 33.2%).
In each year, about 10% of these patients had a hospitalization for dehydration or infection, and 3% had a stay requiring prolonged mechanical ventilation in each year. The mean number of health care transitions remained steady, at 0.23 and 0.21 per person each year.
Dr. Teno had no financial disclosures.
SOURCE: Teno JM et al. JAMA. 2018 Jun 25. doi: 10.1001/jama.2018.8981.
Since 2000, Medicare beneficiaries have become less likely to die in hospitals, and more likely to die in their homes or in community health care facilities.
A review of Medicare records also determined that there was a decline in health care transitions in the final 3 days of life for these patients, Joan M. Teno, MD, and her colleagues wrote in JAMA.
It is not possible to identify a specific reason for the shift, wrote Dr. Teno, professor of medicine at the Oregon Health & Science University, Portland. Between the study years of 2000 and 2015, there were several large efforts to improve care at the end of life.
“Since 2009, programs ranging from ensuring informed patient decision making to enhanced care coordination have had the goal of improving care at the end of life. Specific interventions have included promoting conversations about the goals of care, continued growth of hospice services and palliative care, and the debate and passage of the Affordable Care Act … It is difficult to disentangle efforts such as public education, promotion of advance directives through the Patient Self- Determination Act, increased access to hospice and palliative care services, financial incentives of payment policies, and other secular changes.”
The study mined data from the Centers for Medicare & Medicaid Services, and examined end-of-life outcomes among two Medicare groups: Medicare fee-for-service recipients (1,361,870) during 2009-2015, and Medicare Advantage recipients (871,845), comparing 2011 and 2015. The mean age of both cohorts was 82 years.
Outcomes included site of death and “potentially burdensome transitions,” during the last days of life. These were defined as three or more hospitalizations in the previous 3 months, or two or more hospitalizations for pneumonia, urinary tract infection, dehydration, or sepsis during the last 120 days of life. Prolonged mechanical ventilation also was deemed potentially burdensome.
Among fee-for-service recipients, deaths in acute care hospitals declined from 32.6% to19.8%. Deaths in nursing homes remained steady, at 27.2% and 24.9%. Many of these deaths (42.9%) were preceded by a stay in an ICU. There was a transient increase in end-of-life ICU use, around 2009, but by 2015, the percentage was down to 29%, compared with 65.2% in 2000.
Transitions between a nursing home and hospital in the last 90 days of life were 0.49/person in 2000 and 0.33/person in 2015. Hospitalizations for infection or dehydration fell from 14.6% to12.2%. Hospitalization with prolonged ventilation fell from 3.1% to 2.5%.
Dying in hospice care increased from 21.6% to 50.4%, and people were taking advantage of hospice services longer: the proportion using short-term services (3 days or less) fell from 9.8% to 7.7%.
Among Medicare Advantage recipients, the numbers were somewhat different. More than 50% of recipients entered hospice care in both 2011 and 2015; in both years, 8% had services for more than 3 days. About 27% had ICU care in the last days of life, in both years. Compared to fee-for-service recipients, fewer Medicare Advantage patients were in nursing homes at the time of death, and that number declined from 2011 to 2015 (37.7% to 33.2%).
In each year, about 10% of these patients had a hospitalization for dehydration or infection, and 3% had a stay requiring prolonged mechanical ventilation in each year. The mean number of health care transitions remained steady, at 0.23 and 0.21 per person each year.
Dr. Teno had no financial disclosures.
SOURCE: Teno JM et al. JAMA. 2018 Jun 25. doi: 10.1001/jama.2018.8981.
FROM JAMA
Key clinical point: During 2000-2015, Medicare recipients became less likely to die in hospitals.
Major finding:
Study details: The retrospective study comprised more than 2.3 million Medicare recipients.
Disclosures: Dr. Teno had no financial disclosures.
Source: Teno JM et al. JAMA. 2018 Jun 25. doi: 10.1001/jama.2018.8981.
Accidental bowel injury occurs in 2% of hernia repairs
occurring in only about 2% of these cases, a database review has determined. But patients who experience this kind of injury have a significantly longer length of stay and are at increased risk for fistulas, sepsis, reoperations and readmissions, and even death, David M. Krpata, MD, of the Cleveland Clinic and his colleagues wrote in Surgery.
“When these events occur, the surgeon must decide whether to repair primarily or resect the bowel, proceed with definitive hernia repair with mesh, or abort the procedure and repair primarily the hernia defect,” they wrote. The lack of published studies on this injury prompted the research team to look into prevalence and outcomes in order to offer some data to guide surgical decision making.
The research team examined surgical outcomes among 5,916 patients who underwent a ventral hernia repair during 2013-2017 and were included in the Americas Hernia Society Quality Collaborative, a national hernia surgery database. The database included information from the records of 180 surgeons.
The multivariate analysis controlled for sex, race, elective case, wound status, hernia width, immunosuppressants, subcutaneous flaps, myofascial release, drains, smoking, body mass index, age, diabetes, laparoscopic surgery, mesh type, and concomitant procedure.
Among the cohort, there were 110 full-thickness bowel injuries (1.9%). Three patients also had a bladder injury. Most of the enterotomies were small-bowel injuries (85%); the rest were colon injuries. The majority of patients (64%) underwent a primary repair; 36% required bowel resection. Injuries were most common among patients with larger hernia defects, recurrent repairs, mesh or active infection, a history of abdominal wound infection, and older age.
Patients with the accidental enterotomies were less likely to get a mesh repair (85% vs. 94%). When they did, their surgeons were less likely to use a permanent synthetic barrier–coated mesh and more likely to use biologic mesh, absorbable mesh, and/or uncoated synthetic mesh. But the investigators wrote: “Further data are necessary to address specifically what is the most appropriate mesh to utilize (if any) after an inadvertent enterotomy has occurred and which compartment within the abdominal wall is safest.”
In the fully adjusted analysis, injured patients were no more likely to experience surgical site infections, but they were significantly more likely to develop an enterocutaneous fistula (4% vs. 1%), sepsis (2% vs. 1%), and to die (3% vs 1%) after a bowel injury. They also had a significantly longer length of stay (7 vs. 4 days), and more reoperations (6% vs. 3%). Major wound complication was the most common reason for reoperation (43%) and readmission (58%).
The limitations of this study mostly reflect variables not captured by the database. For example, the tenacity of adhesions and the duration of adhesiolysis are not accounted for. The investigators noted that “patients with an enterotomy likely had more tenacious adhesions, given that an operative time greater than 2 hours had a greater association with an enterotomy (91% vs. 71%).” These patients were more likely to be older and have COPD. In addition, unrecognized enterotomies were not accounted for in the data, but inclusion of those injuries would likely have meant worse outcomes.
“Although definitive hernia repair with mesh can be safely performed, surgeons should consider multiple factors, including type of mesh and location of mesh in the abdominal wall, before proceeding with definitive repair in any case of an enterotomy,” Dr. Krpata and his coauthors concluded.
The investigators reported no financial disclosures.
SOURCE: Krpata et al. Surg. 2018. doi: 10.1016/j.surg.2018.04.003
occurring in only about 2% of these cases, a database review has determined. But patients who experience this kind of injury have a significantly longer length of stay and are at increased risk for fistulas, sepsis, reoperations and readmissions, and even death, David M. Krpata, MD, of the Cleveland Clinic and his colleagues wrote in Surgery.
“When these events occur, the surgeon must decide whether to repair primarily or resect the bowel, proceed with definitive hernia repair with mesh, or abort the procedure and repair primarily the hernia defect,” they wrote. The lack of published studies on this injury prompted the research team to look into prevalence and outcomes in order to offer some data to guide surgical decision making.
The research team examined surgical outcomes among 5,916 patients who underwent a ventral hernia repair during 2013-2017 and were included in the Americas Hernia Society Quality Collaborative, a national hernia surgery database. The database included information from the records of 180 surgeons.
The multivariate analysis controlled for sex, race, elective case, wound status, hernia width, immunosuppressants, subcutaneous flaps, myofascial release, drains, smoking, body mass index, age, diabetes, laparoscopic surgery, mesh type, and concomitant procedure.
Among the cohort, there were 110 full-thickness bowel injuries (1.9%). Three patients also had a bladder injury. Most of the enterotomies were small-bowel injuries (85%); the rest were colon injuries. The majority of patients (64%) underwent a primary repair; 36% required bowel resection. Injuries were most common among patients with larger hernia defects, recurrent repairs, mesh or active infection, a history of abdominal wound infection, and older age.
Patients with the accidental enterotomies were less likely to get a mesh repair (85% vs. 94%). When they did, their surgeons were less likely to use a permanent synthetic barrier–coated mesh and more likely to use biologic mesh, absorbable mesh, and/or uncoated synthetic mesh. But the investigators wrote: “Further data are necessary to address specifically what is the most appropriate mesh to utilize (if any) after an inadvertent enterotomy has occurred and which compartment within the abdominal wall is safest.”
In the fully adjusted analysis, injured patients were no more likely to experience surgical site infections, but they were significantly more likely to develop an enterocutaneous fistula (4% vs. 1%), sepsis (2% vs. 1%), and to die (3% vs 1%) after a bowel injury. They also had a significantly longer length of stay (7 vs. 4 days), and more reoperations (6% vs. 3%). Major wound complication was the most common reason for reoperation (43%) and readmission (58%).
The limitations of this study mostly reflect variables not captured by the database. For example, the tenacity of adhesions and the duration of adhesiolysis are not accounted for. The investigators noted that “patients with an enterotomy likely had more tenacious adhesions, given that an operative time greater than 2 hours had a greater association with an enterotomy (91% vs. 71%).” These patients were more likely to be older and have COPD. In addition, unrecognized enterotomies were not accounted for in the data, but inclusion of those injuries would likely have meant worse outcomes.
“Although definitive hernia repair with mesh can be safely performed, surgeons should consider multiple factors, including type of mesh and location of mesh in the abdominal wall, before proceeding with definitive repair in any case of an enterotomy,” Dr. Krpata and his coauthors concluded.
The investigators reported no financial disclosures.
SOURCE: Krpata et al. Surg. 2018. doi: 10.1016/j.surg.2018.04.003
occurring in only about 2% of these cases, a database review has determined. But patients who experience this kind of injury have a significantly longer length of stay and are at increased risk for fistulas, sepsis, reoperations and readmissions, and even death, David M. Krpata, MD, of the Cleveland Clinic and his colleagues wrote in Surgery.
“When these events occur, the surgeon must decide whether to repair primarily or resect the bowel, proceed with definitive hernia repair with mesh, or abort the procedure and repair primarily the hernia defect,” they wrote. The lack of published studies on this injury prompted the research team to look into prevalence and outcomes in order to offer some data to guide surgical decision making.
The research team examined surgical outcomes among 5,916 patients who underwent a ventral hernia repair during 2013-2017 and were included in the Americas Hernia Society Quality Collaborative, a national hernia surgery database. The database included information from the records of 180 surgeons.
The multivariate analysis controlled for sex, race, elective case, wound status, hernia width, immunosuppressants, subcutaneous flaps, myofascial release, drains, smoking, body mass index, age, diabetes, laparoscopic surgery, mesh type, and concomitant procedure.
Among the cohort, there were 110 full-thickness bowel injuries (1.9%). Three patients also had a bladder injury. Most of the enterotomies were small-bowel injuries (85%); the rest were colon injuries. The majority of patients (64%) underwent a primary repair; 36% required bowel resection. Injuries were most common among patients with larger hernia defects, recurrent repairs, mesh or active infection, a history of abdominal wound infection, and older age.
Patients with the accidental enterotomies were less likely to get a mesh repair (85% vs. 94%). When they did, their surgeons were less likely to use a permanent synthetic barrier–coated mesh and more likely to use biologic mesh, absorbable mesh, and/or uncoated synthetic mesh. But the investigators wrote: “Further data are necessary to address specifically what is the most appropriate mesh to utilize (if any) after an inadvertent enterotomy has occurred and which compartment within the abdominal wall is safest.”
In the fully adjusted analysis, injured patients were no more likely to experience surgical site infections, but they were significantly more likely to develop an enterocutaneous fistula (4% vs. 1%), sepsis (2% vs. 1%), and to die (3% vs 1%) after a bowel injury. They also had a significantly longer length of stay (7 vs. 4 days), and more reoperations (6% vs. 3%). Major wound complication was the most common reason for reoperation (43%) and readmission (58%).
The limitations of this study mostly reflect variables not captured by the database. For example, the tenacity of adhesions and the duration of adhesiolysis are not accounted for. The investigators noted that “patients with an enterotomy likely had more tenacious adhesions, given that an operative time greater than 2 hours had a greater association with an enterotomy (91% vs. 71%).” These patients were more likely to be older and have COPD. In addition, unrecognized enterotomies were not accounted for in the data, but inclusion of those injuries would likely have meant worse outcomes.
“Although definitive hernia repair with mesh can be safely performed, surgeons should consider multiple factors, including type of mesh and location of mesh in the abdominal wall, before proceeding with definitive repair in any case of an enterotomy,” Dr. Krpata and his coauthors concluded.
The investigators reported no financial disclosures.
SOURCE: Krpata et al. Surg. 2018. doi: 10.1016/j.surg.2018.04.003
FROM SURGERY
Key clinical point: Accidental bowel injuries during ventral hernia increase risk for longer hospital stays, fistula, sepsis, and readmissions.
Major finding: The overall rate of accidental enterotomy during ventral hernia repair was 2%.
Study details: The database review included 5,916 hernia repair patients.
Disclosures: None of the authors reported any financial disclosures.
Source: Krpata D et al. Surg 2018; doi.org/10.1016/j.surg.2018.04.003
Trump administration proposes changes to HHS, FDA
The Trump administration seeks to reorganize several federal agencies as part of a sweeping reform proposal, issued June 21.
“Government in the 21st century is fundamentally a services business, and modern information technology should be at the heart of the U.S. government service delivery model,” according to the administration’s reform proposal. “And yet, today’s Executive branch is still aligned to the stove-piped organizational constructs of the 20th century, which in many cases have grown inefficient and out of date. Consequently, the public and our workforce are frustrated with government’s ability to deliver its mission in an effective, efficient, and secure way.”
Under the proposal, nutrition assistance programs currently run out of the U.S. Department of Agriculture (USDA) including the Supplemental Nutrition Assistance Program (SNAP) and the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) would move to the Department of Health and Human Services, which would be rebranded the Department of Health and Public Welfare.
Moving these programs “would allow for better and easier coordination across programs that serve similar populations, ensuring consistent policies and a single point of administration for the major public assistance programs,” according to the proposal. “This single point of administration would lead to reduced duplication in state reporting requirements and other administrative burdens, and a more streamlined process for issuing guidance, writing regulations, and approving waivers.”
Food oversight functions would move from the Food and Drug Administration to the USDA; FDA would be rebranded the Federal Drug Administration and focus on drugs, devices, biologics, tobacco, dietary supplements, and cosmetics.
The administration also proposed to created a Council on Public Assistance comprised of “all federal agencies that administer public benefits, with a statutory authority to set cross-cutting program policies, including uniform work requirements.”
Other functions of the council would include approving service plans and waivers by states under Welfare-to-Work projects; resolving disputes when multiple agencies disagree on a particular policy; and recommending policy changes to eliminate barriers at the federal, state, and local level to getting welfare beneficiaries to work.
The proposal also calls for a restructuring of the National Institutes of Health “to ensure operations are effective and efficient,” with no detail provided. It would also place the Agency for Healthcare Research and Quality under the auspices of NIH.
The Strategic National Stockpile would be managed by the Assistant Secretary for Preparedness and Response “to consolidate strategic decision making around the development and procurement of medical countermeasures, and streamline operational decisions during responses to public health and other emergencies to improve responsiveness.”
The Trump administration seeks to reorganize several federal agencies as part of a sweeping reform proposal, issued June 21.
“Government in the 21st century is fundamentally a services business, and modern information technology should be at the heart of the U.S. government service delivery model,” according to the administration’s reform proposal. “And yet, today’s Executive branch is still aligned to the stove-piped organizational constructs of the 20th century, which in many cases have grown inefficient and out of date. Consequently, the public and our workforce are frustrated with government’s ability to deliver its mission in an effective, efficient, and secure way.”
Under the proposal, nutrition assistance programs currently run out of the U.S. Department of Agriculture (USDA) including the Supplemental Nutrition Assistance Program (SNAP) and the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) would move to the Department of Health and Human Services, which would be rebranded the Department of Health and Public Welfare.
Moving these programs “would allow for better and easier coordination across programs that serve similar populations, ensuring consistent policies and a single point of administration for the major public assistance programs,” according to the proposal. “This single point of administration would lead to reduced duplication in state reporting requirements and other administrative burdens, and a more streamlined process for issuing guidance, writing regulations, and approving waivers.”
Food oversight functions would move from the Food and Drug Administration to the USDA; FDA would be rebranded the Federal Drug Administration and focus on drugs, devices, biologics, tobacco, dietary supplements, and cosmetics.
The administration also proposed to created a Council on Public Assistance comprised of “all federal agencies that administer public benefits, with a statutory authority to set cross-cutting program policies, including uniform work requirements.”
Other functions of the council would include approving service plans and waivers by states under Welfare-to-Work projects; resolving disputes when multiple agencies disagree on a particular policy; and recommending policy changes to eliminate barriers at the federal, state, and local level to getting welfare beneficiaries to work.
The proposal also calls for a restructuring of the National Institutes of Health “to ensure operations are effective and efficient,” with no detail provided. It would also place the Agency for Healthcare Research and Quality under the auspices of NIH.
The Strategic National Stockpile would be managed by the Assistant Secretary for Preparedness and Response “to consolidate strategic decision making around the development and procurement of medical countermeasures, and streamline operational decisions during responses to public health and other emergencies to improve responsiveness.”
The Trump administration seeks to reorganize several federal agencies as part of a sweeping reform proposal, issued June 21.
“Government in the 21st century is fundamentally a services business, and modern information technology should be at the heart of the U.S. government service delivery model,” according to the administration’s reform proposal. “And yet, today’s Executive branch is still aligned to the stove-piped organizational constructs of the 20th century, which in many cases have grown inefficient and out of date. Consequently, the public and our workforce are frustrated with government’s ability to deliver its mission in an effective, efficient, and secure way.”
Under the proposal, nutrition assistance programs currently run out of the U.S. Department of Agriculture (USDA) including the Supplemental Nutrition Assistance Program (SNAP) and the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) would move to the Department of Health and Human Services, which would be rebranded the Department of Health and Public Welfare.
Moving these programs “would allow for better and easier coordination across programs that serve similar populations, ensuring consistent policies and a single point of administration for the major public assistance programs,” according to the proposal. “This single point of administration would lead to reduced duplication in state reporting requirements and other administrative burdens, and a more streamlined process for issuing guidance, writing regulations, and approving waivers.”
Food oversight functions would move from the Food and Drug Administration to the USDA; FDA would be rebranded the Federal Drug Administration and focus on drugs, devices, biologics, tobacco, dietary supplements, and cosmetics.
The administration also proposed to created a Council on Public Assistance comprised of “all federal agencies that administer public benefits, with a statutory authority to set cross-cutting program policies, including uniform work requirements.”
Other functions of the council would include approving service plans and waivers by states under Welfare-to-Work projects; resolving disputes when multiple agencies disagree on a particular policy; and recommending policy changes to eliminate barriers at the federal, state, and local level to getting welfare beneficiaries to work.
The proposal also calls for a restructuring of the National Institutes of Health “to ensure operations are effective and efficient,” with no detail provided. It would also place the Agency for Healthcare Research and Quality under the auspices of NIH.
The Strategic National Stockpile would be managed by the Assistant Secretary for Preparedness and Response “to consolidate strategic decision making around the development and procurement of medical countermeasures, and streamline operational decisions during responses to public health and other emergencies to improve responsiveness.”














