User login
Official Newspaper of the American College of Surgeons
Study supports observation for select cases of porcelain gallbladder
Some adults with porcelain gallbladder may be eligible to forgo prophylactic cholecystectomy, suggest the results of a single-center retrospective study.
Over 1.7 years of median follow-up (range, 0 to 12.7 years), the observational group had no detected gallbladder malignancies and 4% developed adverse events versus 13% in the prophylactic cholecystectomy group (P = .15), wrote Haley DesJardins and her associates at Tufts University, Boston. The report was published in the Journal of the American College of Surgery.
The findings “still raise concern about an association between gallbladder wall calcifications and gallbladder malignancies, and therefore still suggest the need for cholecystectomy in the young, healthy, or symptomatic patient,” the researchers wrote. Nonetheless, surveillance for patients “who are poor surgical candidates is a reasonable approach, with a low risk of malignancy over a limited time frame.”
The investigators suggest that surgeons consider intervention when symptoms and workup points to gallbladder malignancy. But consider avoiding prophylactic cholecystectomy in patients with “limited life expectancy and significant comorbidities,” they emphasized. “Based on the results of this study, the act of prophylactic cholecystectomy for every single patient with gallbladder wall calcifications seems obsolete.”
The study comprised 113 patients with porcelain gallbladder diagnosed between 2004 and 2016. Radiographic reviews identified 70 definite cases and 43 “highly probable” cases. In all, 90 patients started out with observation only, of whom 26% with abdominal pain did not have cholecystectomy because of “significant comorbidities.” Four patients (4.4%) in the observational group subsequently underwent cholecystectomy for biliary colic, as part of liver transplantation, or for prophylactic reasons. None developed complications. In all, 11% developed new gallstones on follow-up imaging and 8% showed progression from focal to diffuse porcelain bladder, the researchers said. None developed gallbladder malignancy during 1.7 years of median follow-up.
Histopathologies of the operative group identified two cases of gallbladder malignancy, of which one was detected on initial imaging. “This patient had a mass at the gallbladder infundibulum extending into the hepatic duct bifurcation,” the researchers explained. “It was not entirely evident whether the resected adenocarcinoma was originating from the gallbladder or from the bile duct. For the purpose of this study, this patient was listed as [having] gallbladder cancer.” The second case consisted of metastatic squamous cell gallbladder carcinoma.
The investigators concluded that “while it is seemingly very reasonable to observe asymptomatic patients with limited life expectancy and significant comorbidities, the decision to proceed with prophylactic cholecystectomy versus observation remains in the hands of the treating physician and patient; especially since absolute criteria or cut-offs cannot be defined at this point.”
No external funding sources were reported. The researchers reported having no conflicts of interest.
SOURCE: DesJardins H et al. J Am Coll Surg. 2018 Apr 22. doi: 10.1016/j.jamcollsurg.2017.11.026.
Some adults with porcelain gallbladder may be eligible to forgo prophylactic cholecystectomy, suggest the results of a single-center retrospective study.
Over 1.7 years of median follow-up (range, 0 to 12.7 years), the observational group had no detected gallbladder malignancies and 4% developed adverse events versus 13% in the prophylactic cholecystectomy group (P = .15), wrote Haley DesJardins and her associates at Tufts University, Boston. The report was published in the Journal of the American College of Surgery.
The findings “still raise concern about an association between gallbladder wall calcifications and gallbladder malignancies, and therefore still suggest the need for cholecystectomy in the young, healthy, or symptomatic patient,” the researchers wrote. Nonetheless, surveillance for patients “who are poor surgical candidates is a reasonable approach, with a low risk of malignancy over a limited time frame.”
The investigators suggest that surgeons consider intervention when symptoms and workup points to gallbladder malignancy. But consider avoiding prophylactic cholecystectomy in patients with “limited life expectancy and significant comorbidities,” they emphasized. “Based on the results of this study, the act of prophylactic cholecystectomy for every single patient with gallbladder wall calcifications seems obsolete.”
The study comprised 113 patients with porcelain gallbladder diagnosed between 2004 and 2016. Radiographic reviews identified 70 definite cases and 43 “highly probable” cases. In all, 90 patients started out with observation only, of whom 26% with abdominal pain did not have cholecystectomy because of “significant comorbidities.” Four patients (4.4%) in the observational group subsequently underwent cholecystectomy for biliary colic, as part of liver transplantation, or for prophylactic reasons. None developed complications. In all, 11% developed new gallstones on follow-up imaging and 8% showed progression from focal to diffuse porcelain bladder, the researchers said. None developed gallbladder malignancy during 1.7 years of median follow-up.
Histopathologies of the operative group identified two cases of gallbladder malignancy, of which one was detected on initial imaging. “This patient had a mass at the gallbladder infundibulum extending into the hepatic duct bifurcation,” the researchers explained. “It was not entirely evident whether the resected adenocarcinoma was originating from the gallbladder or from the bile duct. For the purpose of this study, this patient was listed as [having] gallbladder cancer.” The second case consisted of metastatic squamous cell gallbladder carcinoma.
The investigators concluded that “while it is seemingly very reasonable to observe asymptomatic patients with limited life expectancy and significant comorbidities, the decision to proceed with prophylactic cholecystectomy versus observation remains in the hands of the treating physician and patient; especially since absolute criteria or cut-offs cannot be defined at this point.”
No external funding sources were reported. The researchers reported having no conflicts of interest.
SOURCE: DesJardins H et al. J Am Coll Surg. 2018 Apr 22. doi: 10.1016/j.jamcollsurg.2017.11.026.
Some adults with porcelain gallbladder may be eligible to forgo prophylactic cholecystectomy, suggest the results of a single-center retrospective study.
Over 1.7 years of median follow-up (range, 0 to 12.7 years), the observational group had no detected gallbladder malignancies and 4% developed adverse events versus 13% in the prophylactic cholecystectomy group (P = .15), wrote Haley DesJardins and her associates at Tufts University, Boston. The report was published in the Journal of the American College of Surgery.
The findings “still raise concern about an association between gallbladder wall calcifications and gallbladder malignancies, and therefore still suggest the need for cholecystectomy in the young, healthy, or symptomatic patient,” the researchers wrote. Nonetheless, surveillance for patients “who are poor surgical candidates is a reasonable approach, with a low risk of malignancy over a limited time frame.”
The investigators suggest that surgeons consider intervention when symptoms and workup points to gallbladder malignancy. But consider avoiding prophylactic cholecystectomy in patients with “limited life expectancy and significant comorbidities,” they emphasized. “Based on the results of this study, the act of prophylactic cholecystectomy for every single patient with gallbladder wall calcifications seems obsolete.”
The study comprised 113 patients with porcelain gallbladder diagnosed between 2004 and 2016. Radiographic reviews identified 70 definite cases and 43 “highly probable” cases. In all, 90 patients started out with observation only, of whom 26% with abdominal pain did not have cholecystectomy because of “significant comorbidities.” Four patients (4.4%) in the observational group subsequently underwent cholecystectomy for biliary colic, as part of liver transplantation, or for prophylactic reasons. None developed complications. In all, 11% developed new gallstones on follow-up imaging and 8% showed progression from focal to diffuse porcelain bladder, the researchers said. None developed gallbladder malignancy during 1.7 years of median follow-up.
Histopathologies of the operative group identified two cases of gallbladder malignancy, of which one was detected on initial imaging. “This patient had a mass at the gallbladder infundibulum extending into the hepatic duct bifurcation,” the researchers explained. “It was not entirely evident whether the resected adenocarcinoma was originating from the gallbladder or from the bile duct. For the purpose of this study, this patient was listed as [having] gallbladder cancer.” The second case consisted of metastatic squamous cell gallbladder carcinoma.
The investigators concluded that “while it is seemingly very reasonable to observe asymptomatic patients with limited life expectancy and significant comorbidities, the decision to proceed with prophylactic cholecystectomy versus observation remains in the hands of the treating physician and patient; especially since absolute criteria or cut-offs cannot be defined at this point.”
No external funding sources were reported. The researchers reported having no conflicts of interest.
SOURCE: DesJardins H et al. J Am Coll Surg. 2018 Apr 22. doi: 10.1016/j.jamcollsurg.2017.11.026.
FROM JOURNAL OF THE AMERICAN COLLEGE OF SURGERY
Key clinical point: Observation is an option for select patients with porcelain gallbladder .
Major finding: Rates of adverse events were 4% with observation and 13% with surgery (P = .15).
Study details: Single-center retrospective cohort study of 113 patients.
Disclosures: No external funding sources were reported. The researchers reported having no conflicts of interest.
Source: DesJardins H et al. J Am Coll Surg. 2018 Apr 22. doi: 10.1016/j.jamcollsurg.2017.11.026.
ESBL-B before colorectal surgery ups risk of surgical site infection
MADRID – Patients who are carriers of , despite a standard prophylactic antibiotic regimen.
Surgical site infections (SSIs) occurred in 23% of those who tested positive for the pathogens preoperatively, compared with 10.5% of ESBL-B–negative patients – a significant increased risk of 2.25, Yehuda Carmeli, MD, said at the European Congress of Clinical Microbiology and Infectious Diseases annual congress.
ESBL-B was not the infective pathogen in most infection cases, but being a carrier increased the likelihood of an ESBL-B SSI. ESBL-B was the pathogen in 7.2% of the carriers and 1.6% of the noncarriers. However, investigators are still working to determine if the species present in the wound infection are the same as the ones present at baseline, said Dr. Carmeli of Tel Aviv Medical Center.
All of these results are emerging from the WP4 study, which was carried out in three hospitals in Serbia, Switzerland, and Israel. Designed as a before-and-after trial, it tested the theory that identifying ESBL carriers and targeting presurgical antibiotic prophylaxis could improve their surgical outcomes.
WP4 was one of five studies in the multinational R-GNOSIS project. “Resistance in Gram-Negative Organisms: Studying Intervention Strategies” is a 12-million-euro, 5-year European collaborative research project designed to identify effective interventions for reducing the carriage, infection, and spread of multi-drug resistant Gram-negative bacteria. From 2012 to 2017, WP4 enrolled almost 4,000 adults scheduled to undergo colorectal surgery (excluding appendectomy or minor anorectal procedures).
Several of the studies were reported at ECCMID 2018.
This portion of R-GNOSIS was intended to investigate the relationship between ESBL-B carriage and postoperative surgical site infections among colorectal surgery patients.
The study comprised 3,626 patients who were preoperatively screened for ESBL-B within 2 weeks of colorectal surgery. The ESBL-B carriage rate was 15.3% overall, but ranged from 12% to 20% by site. Of the carriers, 222 were included in this study sample. They were randomly matched with 444 noncarriers.
Anywhere from 2 weeks to 2 days before surgery, all of the patients received a standard prophylactic antibiotic. This was most often an infusion of 1.5 g cefuroxime plus 500 mg metronidazole. Other cephalosporins were allowed at the clinician’s discretion.
Patients were a mean of 62 years old. Nearly half (48%) had cardiovascular disease and about a third had undergone a prior colorectal surgical procedure. Cancer was the surgical indication in about 70%. Other indications were inflammatory bowel disease and diverticular disease.
A multivariate analysis controlled for age, cardiovascular disease, indication for surgery, and whether the procedure included a rectal resection, retention of drain at the surgical site, or stoma. The model also controlled for National Nosocomial Infection Surveillance score, a three-point scale that estimates surgical infection risk. Among this cohort, 48% were at low risk, 43% at moderate risk, and 10% at high risk.
Dr. Carmeli made no financial disclosures.
SOURCE: Carmeli et al, ECCMID 2018, Oral Abstract O1133.
MADRID – Patients who are carriers of , despite a standard prophylactic antibiotic regimen.
Surgical site infections (SSIs) occurred in 23% of those who tested positive for the pathogens preoperatively, compared with 10.5% of ESBL-B–negative patients – a significant increased risk of 2.25, Yehuda Carmeli, MD, said at the European Congress of Clinical Microbiology and Infectious Diseases annual congress.
ESBL-B was not the infective pathogen in most infection cases, but being a carrier increased the likelihood of an ESBL-B SSI. ESBL-B was the pathogen in 7.2% of the carriers and 1.6% of the noncarriers. However, investigators are still working to determine if the species present in the wound infection are the same as the ones present at baseline, said Dr. Carmeli of Tel Aviv Medical Center.
All of these results are emerging from the WP4 study, which was carried out in three hospitals in Serbia, Switzerland, and Israel. Designed as a before-and-after trial, it tested the theory that identifying ESBL carriers and targeting presurgical antibiotic prophylaxis could improve their surgical outcomes.
WP4 was one of five studies in the multinational R-GNOSIS project. “Resistance in Gram-Negative Organisms: Studying Intervention Strategies” is a 12-million-euro, 5-year European collaborative research project designed to identify effective interventions for reducing the carriage, infection, and spread of multi-drug resistant Gram-negative bacteria. From 2012 to 2017, WP4 enrolled almost 4,000 adults scheduled to undergo colorectal surgery (excluding appendectomy or minor anorectal procedures).
Several of the studies were reported at ECCMID 2018.
This portion of R-GNOSIS was intended to investigate the relationship between ESBL-B carriage and postoperative surgical site infections among colorectal surgery patients.
The study comprised 3,626 patients who were preoperatively screened for ESBL-B within 2 weeks of colorectal surgery. The ESBL-B carriage rate was 15.3% overall, but ranged from 12% to 20% by site. Of the carriers, 222 were included in this study sample. They were randomly matched with 444 noncarriers.
Anywhere from 2 weeks to 2 days before surgery, all of the patients received a standard prophylactic antibiotic. This was most often an infusion of 1.5 g cefuroxime plus 500 mg metronidazole. Other cephalosporins were allowed at the clinician’s discretion.
Patients were a mean of 62 years old. Nearly half (48%) had cardiovascular disease and about a third had undergone a prior colorectal surgical procedure. Cancer was the surgical indication in about 70%. Other indications were inflammatory bowel disease and diverticular disease.
A multivariate analysis controlled for age, cardiovascular disease, indication for surgery, and whether the procedure included a rectal resection, retention of drain at the surgical site, or stoma. The model also controlled for National Nosocomial Infection Surveillance score, a three-point scale that estimates surgical infection risk. Among this cohort, 48% were at low risk, 43% at moderate risk, and 10% at high risk.
Dr. Carmeli made no financial disclosures.
SOURCE: Carmeli et al, ECCMID 2018, Oral Abstract O1133.
MADRID – Patients who are carriers of , despite a standard prophylactic antibiotic regimen.
Surgical site infections (SSIs) occurred in 23% of those who tested positive for the pathogens preoperatively, compared with 10.5% of ESBL-B–negative patients – a significant increased risk of 2.25, Yehuda Carmeli, MD, said at the European Congress of Clinical Microbiology and Infectious Diseases annual congress.
ESBL-B was not the infective pathogen in most infection cases, but being a carrier increased the likelihood of an ESBL-B SSI. ESBL-B was the pathogen in 7.2% of the carriers and 1.6% of the noncarriers. However, investigators are still working to determine if the species present in the wound infection are the same as the ones present at baseline, said Dr. Carmeli of Tel Aviv Medical Center.
All of these results are emerging from the WP4 study, which was carried out in three hospitals in Serbia, Switzerland, and Israel. Designed as a before-and-after trial, it tested the theory that identifying ESBL carriers and targeting presurgical antibiotic prophylaxis could improve their surgical outcomes.
WP4 was one of five studies in the multinational R-GNOSIS project. “Resistance in Gram-Negative Organisms: Studying Intervention Strategies” is a 12-million-euro, 5-year European collaborative research project designed to identify effective interventions for reducing the carriage, infection, and spread of multi-drug resistant Gram-negative bacteria. From 2012 to 2017, WP4 enrolled almost 4,000 adults scheduled to undergo colorectal surgery (excluding appendectomy or minor anorectal procedures).
Several of the studies were reported at ECCMID 2018.
This portion of R-GNOSIS was intended to investigate the relationship between ESBL-B carriage and postoperative surgical site infections among colorectal surgery patients.
The study comprised 3,626 patients who were preoperatively screened for ESBL-B within 2 weeks of colorectal surgery. The ESBL-B carriage rate was 15.3% overall, but ranged from 12% to 20% by site. Of the carriers, 222 were included in this study sample. They were randomly matched with 444 noncarriers.
Anywhere from 2 weeks to 2 days before surgery, all of the patients received a standard prophylactic antibiotic. This was most often an infusion of 1.5 g cefuroxime plus 500 mg metronidazole. Other cephalosporins were allowed at the clinician’s discretion.
Patients were a mean of 62 years old. Nearly half (48%) had cardiovascular disease and about a third had undergone a prior colorectal surgical procedure. Cancer was the surgical indication in about 70%. Other indications were inflammatory bowel disease and diverticular disease.
A multivariate analysis controlled for age, cardiovascular disease, indication for surgery, and whether the procedure included a rectal resection, retention of drain at the surgical site, or stoma. The model also controlled for National Nosocomial Infection Surveillance score, a three-point scale that estimates surgical infection risk. Among this cohort, 48% were at low risk, 43% at moderate risk, and 10% at high risk.
Dr. Carmeli made no financial disclosures.
SOURCE: Carmeli et al, ECCMID 2018, Oral Abstract O1133.
REPORTING FROM ECCMID 2018
Key clinical point: ESBL-B colonization increased the risk of surgical site infections after colorectal surgery, despite use of standard preoperative antibiotics.
Major finding: ESBL-B carriage more than doubled the risk of a colorectal surgical site infection by (OR 2.25).
Study details: The prospective study comprised 222 carriers and 444 noncarriers.
Disclosures: The study is part of the R-GNOSIS project, a 12-million-euro, 5-year European collaborative research project designed to identify effective interventions for reducing the carriage, infection, and spread of multi-drug resistant Gram-negative bacteria.
Source: Carmeli Y et al. ECCMID 2018, Oral Abstract O1130.
Two more and counting: Suicide in medical trainees
Like everyone in the arc of social media impact, I was shocked and terribly saddened by the recent suicides of two New York women in medicine – a final-year medical student on May 1 and a second-year resident on May 5. As a specialist in physician health, a former training director, a long-standing member of our institution’s medical student admissions committee, and the ombudsman for our medical students, I am finding these tragedies harder and harder to reconcile. Something isn’t working. But before I get to that, what follows is a bulleted list of some events of the past couple of weeks that may give a context for my statements and have informed my two recommendations.
- May 3, 2018: I give an invited GI grand rounds on stress, burnout, depression, and suicide in physicians. The residents are quiet and say nothing. Faculty members seem only concerned about preventing and eradicating burnout – and not that interested in anything more severe.
- May 5: A psychiatry resident from Melbourne arrives to spend 10 days with me to do an elective in physician health. As in the United States, there is a significant suicide death rate in medical students and residents Down Under. In the afternoon, I present a paper at the annual meeting of the American Academy of Psychodynamic Psychiatry and Psychoanalysis on the use of psychotherapy in treatment-resistant suicidal depression in physicians. There is increasing hope that this essential modality of care will return to the contemporary psychiatrist’s toolbox.
- May 6: At the annual meeting of the American Psychiatric Association in New York, I’m the discussant for powerful heartfelt papers of five psychiatrists (mostly early career psychiatrists and one resident) that talked about living with a psychiatric illness. The audience is huge, and we hear narratives about internal stigma, self-disclosure, external stigma, shunning, bullying, acceptance, rejection, alienation, connection, and love by peers and family. The authenticity and valor of the speakers create an atmosphere of safety, which enables psychiatrists in attendance from all over the world to share their personal stories – some at the microphone, some privately.
- May 7: Again at the APA, I chair and facilitate a workshop on physician suicide. We hear from four speakers, all women, who have lost a loved one to suicide – a husband, a father, a brother, a son – all doctors. Two of the speakers are psychiatrists. The stories are gripping, detailed, and tender. Yes, the atmosphere is very sad, but there is not a pall. We learn how these doctors lived, not just how they died. They all loved medicine; they were creative; they cared deeply; they suffered silently; and with shame, they lost hope. Again, a big audience of psychiatrists, many of whom share their own stories, that they, too, had lost a physician son, wife, or mother to suicide. Some of their deceased family members fell through the cracks and did not receive the life-saving care they deserved; some, fearing assaults to their medical license, hospital privileges, or insurance, refused to see anyone. They died untreated.
- May 8: Still at the APA, a psychiatrist colleague and I collaborate on a clinical case conference. Each of us describes losing a physician patient to suicide. We walk the attendees through the clinical details of assessment, treatment, and the aftermath of their deaths. We talk openly and frankly about our feelings, grief, outreach to colleagues and the family, and our own personal journeys of learning, growth, and healing. The clinician audience members give constructive feedback, and some share their own stories of losing patients to suicide. Like the day before, some psychiatrists are grieving the loss of a physician son or sibling to suicide. As mental health professionals, they suffer from an additional layer of failure and guilt that a loved one died “under their watch.”
- May 8: I rush across the Javits Center to catch the discussant for a concurrent symposium on physician burnout and depression. She foregoes any prepared remarks to share her previous 48 hours with the audience. She is the training director of the program that lost the second-year resident on May 5. She did not learn of the death until 24 hours later. We are all on the edge of our seats as we listen to this grieving, courageous woman, a seasoned psychiatrist and educator, who has been blindsided by this tragedy. She has not slept. She called all of her residents and broke the news personally as best she could. Aided by “After A Suicide: A Toolkit for Residency/Fellowship Programs” (American Foundation for Suicide Prevention), she and her colleagues instituted a plan of action and worked with administration and faculty. Her strength and commitment to the well-being of her trainees is palpable and magnanimous. When the session ends, many of us stand in line to give her a hug. It is a stark reminder of how many lives are affected when someone you know or care about takes his/her own life – and how, in the house of medicine, medical students and residents really are part of an institutional family.
- May 10: I facilitate a meeting of our 12 second-year residents, many of whom knew of or had met the resident who died. Almost everyone speaks, shares their feelings, poses questions, and calls for answers and change. There is disbelief, sadness, confusion, some guilt, and lots of anger. Also a feeling of disillusionment or paradox about the field of psychiatry: “Of all branches of medicine, shouldn’t residents who are struggling with psychiatric issues feel safe, protected, cared for in psychiatry?” There is also a feeling of lip service being paid to personal treatment, as in quoted statements: “By all means, get treatment for your issues, but don’t let it encroach on your duty hours” or “It’s good you’re getting help, but do you still have to go weekly?”
In the immediate aftermath of suicide, feelings run high, as they should. But rather than wait it out – and fearing a return to “business as usual” – let me make only two suggestions:
2. In psychiatry, we need to redouble our efforts in fighting the stigma attached to psychiatric illness in trainees. It is unconscionable that medical students and residents are dying of treatable disorders (I’ve never heard of a doctor dying of cancer who didn’t go to an oncologist at least once), yet too many are not availing themselves of services we provide – even when they’re free of charge or covered by insurance. And are we certain that, when they knock on our doors, we are providing them with state-of-the-art care? Is it possible that unrecognized internal stigma and shame deep within us might make us hesitant to help our trainees in their hour of need? Or cut corners? Or not get a second opinion? Very few psychiatrists on faculty of our medical schools divulge their personal experiences of depression, posttraumatic stress disorders, substance use disorders, and more (with the exception of being in therapy during residency, which is normative and isn’t stigmatized). Coming out is leveling, humane, and respectful – and it shrinks the power differential in the teaching dyad. It might even save a life.
Dr. Myers is a professor of clinical psychiatry at State University of New York, Brooklyn, and the author of “Why Physicians Die by Suicide: Lessons Learned From Their Families and Others Who Cared.”
Like everyone in the arc of social media impact, I was shocked and terribly saddened by the recent suicides of two New York women in medicine – a final-year medical student on May 1 and a second-year resident on May 5. As a specialist in physician health, a former training director, a long-standing member of our institution’s medical student admissions committee, and the ombudsman for our medical students, I am finding these tragedies harder and harder to reconcile. Something isn’t working. But before I get to that, what follows is a bulleted list of some events of the past couple of weeks that may give a context for my statements and have informed my two recommendations.
- May 3, 2018: I give an invited GI grand rounds on stress, burnout, depression, and suicide in physicians. The residents are quiet and say nothing. Faculty members seem only concerned about preventing and eradicating burnout – and not that interested in anything more severe.
- May 5: A psychiatry resident from Melbourne arrives to spend 10 days with me to do an elective in physician health. As in the United States, there is a significant suicide death rate in medical students and residents Down Under. In the afternoon, I present a paper at the annual meeting of the American Academy of Psychodynamic Psychiatry and Psychoanalysis on the use of psychotherapy in treatment-resistant suicidal depression in physicians. There is increasing hope that this essential modality of care will return to the contemporary psychiatrist’s toolbox.
- May 6: At the annual meeting of the American Psychiatric Association in New York, I’m the discussant for powerful heartfelt papers of five psychiatrists (mostly early career psychiatrists and one resident) that talked about living with a psychiatric illness. The audience is huge, and we hear narratives about internal stigma, self-disclosure, external stigma, shunning, bullying, acceptance, rejection, alienation, connection, and love by peers and family. The authenticity and valor of the speakers create an atmosphere of safety, which enables psychiatrists in attendance from all over the world to share their personal stories – some at the microphone, some privately.
- May 7: Again at the APA, I chair and facilitate a workshop on physician suicide. We hear from four speakers, all women, who have lost a loved one to suicide – a husband, a father, a brother, a son – all doctors. Two of the speakers are psychiatrists. The stories are gripping, detailed, and tender. Yes, the atmosphere is very sad, but there is not a pall. We learn how these doctors lived, not just how they died. They all loved medicine; they were creative; they cared deeply; they suffered silently; and with shame, they lost hope. Again, a big audience of psychiatrists, many of whom share their own stories, that they, too, had lost a physician son, wife, or mother to suicide. Some of their deceased family members fell through the cracks and did not receive the life-saving care they deserved; some, fearing assaults to their medical license, hospital privileges, or insurance, refused to see anyone. They died untreated.
- May 8: Still at the APA, a psychiatrist colleague and I collaborate on a clinical case conference. Each of us describes losing a physician patient to suicide. We walk the attendees through the clinical details of assessment, treatment, and the aftermath of their deaths. We talk openly and frankly about our feelings, grief, outreach to colleagues and the family, and our own personal journeys of learning, growth, and healing. The clinician audience members give constructive feedback, and some share their own stories of losing patients to suicide. Like the day before, some psychiatrists are grieving the loss of a physician son or sibling to suicide. As mental health professionals, they suffer from an additional layer of failure and guilt that a loved one died “under their watch.”
- May 8: I rush across the Javits Center to catch the discussant for a concurrent symposium on physician burnout and depression. She foregoes any prepared remarks to share her previous 48 hours with the audience. She is the training director of the program that lost the second-year resident on May 5. She did not learn of the death until 24 hours later. We are all on the edge of our seats as we listen to this grieving, courageous woman, a seasoned psychiatrist and educator, who has been blindsided by this tragedy. She has not slept. She called all of her residents and broke the news personally as best she could. Aided by “After A Suicide: A Toolkit for Residency/Fellowship Programs” (American Foundation for Suicide Prevention), she and her colleagues instituted a plan of action and worked with administration and faculty. Her strength and commitment to the well-being of her trainees is palpable and magnanimous. When the session ends, many of us stand in line to give her a hug. It is a stark reminder of how many lives are affected when someone you know or care about takes his/her own life – and how, in the house of medicine, medical students and residents really are part of an institutional family.
- May 10: I facilitate a meeting of our 12 second-year residents, many of whom knew of or had met the resident who died. Almost everyone speaks, shares their feelings, poses questions, and calls for answers and change. There is disbelief, sadness, confusion, some guilt, and lots of anger. Also a feeling of disillusionment or paradox about the field of psychiatry: “Of all branches of medicine, shouldn’t residents who are struggling with psychiatric issues feel safe, protected, cared for in psychiatry?” There is also a feeling of lip service being paid to personal treatment, as in quoted statements: “By all means, get treatment for your issues, but don’t let it encroach on your duty hours” or “It’s good you’re getting help, but do you still have to go weekly?”
In the immediate aftermath of suicide, feelings run high, as they should. But rather than wait it out – and fearing a return to “business as usual” – let me make only two suggestions:
2. In psychiatry, we need to redouble our efforts in fighting the stigma attached to psychiatric illness in trainees. It is unconscionable that medical students and residents are dying of treatable disorders (I’ve never heard of a doctor dying of cancer who didn’t go to an oncologist at least once), yet too many are not availing themselves of services we provide – even when they’re free of charge or covered by insurance. And are we certain that, when they knock on our doors, we are providing them with state-of-the-art care? Is it possible that unrecognized internal stigma and shame deep within us might make us hesitant to help our trainees in their hour of need? Or cut corners? Or not get a second opinion? Very few psychiatrists on faculty of our medical schools divulge their personal experiences of depression, posttraumatic stress disorders, substance use disorders, and more (with the exception of being in therapy during residency, which is normative and isn’t stigmatized). Coming out is leveling, humane, and respectful – and it shrinks the power differential in the teaching dyad. It might even save a life.
Dr. Myers is a professor of clinical psychiatry at State University of New York, Brooklyn, and the author of “Why Physicians Die by Suicide: Lessons Learned From Their Families and Others Who Cared.”
Like everyone in the arc of social media impact, I was shocked and terribly saddened by the recent suicides of two New York women in medicine – a final-year medical student on May 1 and a second-year resident on May 5. As a specialist in physician health, a former training director, a long-standing member of our institution’s medical student admissions committee, and the ombudsman for our medical students, I am finding these tragedies harder and harder to reconcile. Something isn’t working. But before I get to that, what follows is a bulleted list of some events of the past couple of weeks that may give a context for my statements and have informed my two recommendations.
- May 3, 2018: I give an invited GI grand rounds on stress, burnout, depression, and suicide in physicians. The residents are quiet and say nothing. Faculty members seem only concerned about preventing and eradicating burnout – and not that interested in anything more severe.
- May 5: A psychiatry resident from Melbourne arrives to spend 10 days with me to do an elective in physician health. As in the United States, there is a significant suicide death rate in medical students and residents Down Under. In the afternoon, I present a paper at the annual meeting of the American Academy of Psychodynamic Psychiatry and Psychoanalysis on the use of psychotherapy in treatment-resistant suicidal depression in physicians. There is increasing hope that this essential modality of care will return to the contemporary psychiatrist’s toolbox.
- May 6: At the annual meeting of the American Psychiatric Association in New York, I’m the discussant for powerful heartfelt papers of five psychiatrists (mostly early career psychiatrists and one resident) that talked about living with a psychiatric illness. The audience is huge, and we hear narratives about internal stigma, self-disclosure, external stigma, shunning, bullying, acceptance, rejection, alienation, connection, and love by peers and family. The authenticity and valor of the speakers create an atmosphere of safety, which enables psychiatrists in attendance from all over the world to share their personal stories – some at the microphone, some privately.
- May 7: Again at the APA, I chair and facilitate a workshop on physician suicide. We hear from four speakers, all women, who have lost a loved one to suicide – a husband, a father, a brother, a son – all doctors. Two of the speakers are psychiatrists. The stories are gripping, detailed, and tender. Yes, the atmosphere is very sad, but there is not a pall. We learn how these doctors lived, not just how they died. They all loved medicine; they were creative; they cared deeply; they suffered silently; and with shame, they lost hope. Again, a big audience of psychiatrists, many of whom share their own stories, that they, too, had lost a physician son, wife, or mother to suicide. Some of their deceased family members fell through the cracks and did not receive the life-saving care they deserved; some, fearing assaults to their medical license, hospital privileges, or insurance, refused to see anyone. They died untreated.
- May 8: Still at the APA, a psychiatrist colleague and I collaborate on a clinical case conference. Each of us describes losing a physician patient to suicide. We walk the attendees through the clinical details of assessment, treatment, and the aftermath of their deaths. We talk openly and frankly about our feelings, grief, outreach to colleagues and the family, and our own personal journeys of learning, growth, and healing. The clinician audience members give constructive feedback, and some share their own stories of losing patients to suicide. Like the day before, some psychiatrists are grieving the loss of a physician son or sibling to suicide. As mental health professionals, they suffer from an additional layer of failure and guilt that a loved one died “under their watch.”
- May 8: I rush across the Javits Center to catch the discussant for a concurrent symposium on physician burnout and depression. She foregoes any prepared remarks to share her previous 48 hours with the audience. She is the training director of the program that lost the second-year resident on May 5. She did not learn of the death until 24 hours later. We are all on the edge of our seats as we listen to this grieving, courageous woman, a seasoned psychiatrist and educator, who has been blindsided by this tragedy. She has not slept. She called all of her residents and broke the news personally as best she could. Aided by “After A Suicide: A Toolkit for Residency/Fellowship Programs” (American Foundation for Suicide Prevention), she and her colleagues instituted a plan of action and worked with administration and faculty. Her strength and commitment to the well-being of her trainees is palpable and magnanimous. When the session ends, many of us stand in line to give her a hug. It is a stark reminder of how many lives are affected when someone you know or care about takes his/her own life – and how, in the house of medicine, medical students and residents really are part of an institutional family.
- May 10: I facilitate a meeting of our 12 second-year residents, many of whom knew of or had met the resident who died. Almost everyone speaks, shares their feelings, poses questions, and calls for answers and change. There is disbelief, sadness, confusion, some guilt, and lots of anger. Also a feeling of disillusionment or paradox about the field of psychiatry: “Of all branches of medicine, shouldn’t residents who are struggling with psychiatric issues feel safe, protected, cared for in psychiatry?” There is also a feeling of lip service being paid to personal treatment, as in quoted statements: “By all means, get treatment for your issues, but don’t let it encroach on your duty hours” or “It’s good you’re getting help, but do you still have to go weekly?”
In the immediate aftermath of suicide, feelings run high, as they should. But rather than wait it out – and fearing a return to “business as usual” – let me make only two suggestions:
2. In psychiatry, we need to redouble our efforts in fighting the stigma attached to psychiatric illness in trainees. It is unconscionable that medical students and residents are dying of treatable disorders (I’ve never heard of a doctor dying of cancer who didn’t go to an oncologist at least once), yet too many are not availing themselves of services we provide – even when they’re free of charge or covered by insurance. And are we certain that, when they knock on our doors, we are providing them with state-of-the-art care? Is it possible that unrecognized internal stigma and shame deep within us might make us hesitant to help our trainees in their hour of need? Or cut corners? Or not get a second opinion? Very few psychiatrists on faculty of our medical schools divulge their personal experiences of depression, posttraumatic stress disorders, substance use disorders, and more (with the exception of being in therapy during residency, which is normative and isn’t stigmatized). Coming out is leveling, humane, and respectful – and it shrinks the power differential in the teaching dyad. It might even save a life.
Dr. Myers is a professor of clinical psychiatry at State University of New York, Brooklyn, and the author of “Why Physicians Die by Suicide: Lessons Learned From Their Families and Others Who Cared.”
Surgical specialists are top earners
The five top-earning surgical specialties in 2017 also happened to be the five top-earning specialties, according to a survey by the medical social network Doximity.
Neurosurgery was first among all specialties with an average compensation of $663,000, followed by thoracic surgery ($603,000), orthopedic surgery ($538,000), vascular surgery ($476,000), and plastic surgery ($473,212). Cardiology was just behind plastic surgery at $473,184, which made it the highest-earning nonsurgical specialty, Doximity said in its 2018 Physician Compensation Report, which was based on data from surveys completed by more than 65,000 physicians who practice at least 40 hours a week. Gastroenterologists took in $456,000 and oncologists earned an average of $404,000.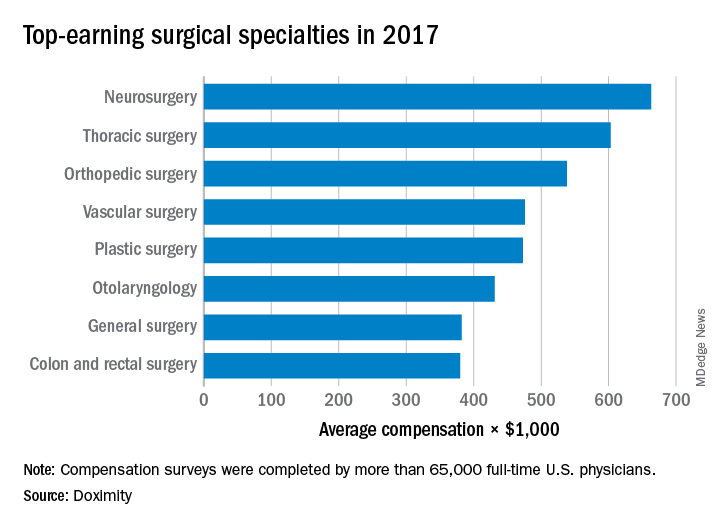
The five top-earning surgical specialties in 2017 also happened to be the five top-earning specialties, according to a survey by the medical social network Doximity.
Neurosurgery was first among all specialties with an average compensation of $663,000, followed by thoracic surgery ($603,000), orthopedic surgery ($538,000), vascular surgery ($476,000), and plastic surgery ($473,212). Cardiology was just behind plastic surgery at $473,184, which made it the highest-earning nonsurgical specialty, Doximity said in its 2018 Physician Compensation Report, which was based on data from surveys completed by more than 65,000 physicians who practice at least 40 hours a week. Gastroenterologists took in $456,000 and oncologists earned an average of $404,000.
The five top-earning surgical specialties in 2017 also happened to be the five top-earning specialties, according to a survey by the medical social network Doximity.
Neurosurgery was first among all specialties with an average compensation of $663,000, followed by thoracic surgery ($603,000), orthopedic surgery ($538,000), vascular surgery ($476,000), and plastic surgery ($473,212). Cardiology was just behind plastic surgery at $473,184, which made it the highest-earning nonsurgical specialty, Doximity said in its 2018 Physician Compensation Report, which was based on data from surveys completed by more than 65,000 physicians who practice at least 40 hours a week. Gastroenterologists took in $456,000 and oncologists earned an average of $404,000.
Surgery may be best option for hip impingement syndrome
LIVERPOOL, ENGLAND – Hip arthroscopic surgery produced better long-term results than did personalized hip physiotherapy for femoroacetabular impingement syndrome in a randomized trial conduced across multiple U.K. centers.
At 12 months, respective International Hip Outcome Tool-33 (iHOT-33) scores were 58.8 and 49.7, a difference of 9.1 points before and 6.8 points after adjustment for potential confounding factors (P = .0093).
“This trial shows that hip arthroscopic surgery and personalized hip therapy both improved hip-related quality of life for patients with FAI [femoroacetabular impingement] syndrome, but that the surgery did indeed produce a greater improvement at our primary time point of 12 months,” she added. Dr. Foster is professor of musculoskeletal health in primary care at Keele University, Newcastle-under-Lyme, England, one of the 23 centers involved in the FASHIoN study in England, Wales, and Scotland.
“FAI is a very common cause of hip and groin pain in young adults, and it’s associated with the development of hip osteoarthritis,” Dr. Foster noted.
There are three types of FAI – pincer, cam, and combined. The pincer type of FAI is where there is “prominence or overcoverage of the rim of the acetabulum,” and the cam type is where there is a “bony prominence of the femoral head-neck junction,” she explained at the meeting sponsored by the Osteoarthritis Research Society International.
The link to OA comes when the femur and acetabulum prematurely connect, usually during activity, causing damage to the labrum and articular cartilage in the long term. Thus, treating FAI is important, not just for relieving patient’s pain and joint stiffness.
Hip arthroscopic surgery has become an established way of treating FAI syndrome – more than 2,400 operations were performed in 2013 in the United Kingdom alone, Dr. Foster observed. The aim of surgery is to try to reshape the hip joint to prevent impingement, and resect, repair, or reconstruct any intra-articular damage that may be present.
“Physiotherapy aims to improve hip muscle control and strength, and to correct the abnormal movement patterns that we see in these patients,” Dr. Foster said. “Through that, we hope to prevent the premature contact that occurs in FAI syndrome and thereby improve symptoms, allowing patients to return to activities and prevent recurrence.”
Working with physiotherapists, physicians, and surgeons, Dr. Foster and her associates have previously developed a “best conservative care” intervention that they call personalized hip therapy (PHT), which involves the delivery and supervision of an individualized exercise program by experienced physiotherapists over a 3- to 6-month period, and which patients repeat at home (PM R. 2013 May;5[5]:418-26).
The aim of the UK FASHIoN trial was to compare the clinical and cost-effectiveness of hip arthroscopy and PHT for FAI syndrome, as there was no robust clinical trial evidence to demonstrate a benefit of one over the other.
A total of 351 adults with hip and groin pain were randomized to either arthroscopic surgery (n = 173) or PHT (n = 178). The mean age of participants was 35 years, with no significant differences between the two treatment groups in terms of baseline demographics or type or duration of hip impingement.
While surgery was better in terms of patient outcomes, the study didn’t demonstrate its cost-effectiveness within the first 12 months, Dr. Foster observed. Cost-effectiveness, together with various other quality-of-life measurements, was a secondary endpoint of the study.
“Longer-term outcomes are required to establish whether improvement is sustained, and whether surgery is cost-effective at the longer time points for our health service,” she said.
Responding to a question about whether any of the patients in the study had radiographic evidence of osteoarthritis, Dr. Foster said that such patients had been excluded from the study.
“One of the hopes of the trial’s team is that, with the long-term follow-up, we might be able to get data at 5 and 10 years on things like hip osteoarthritis in these patients,” she said.
The study was funded by the National Institute for Health Research. Dr. Foster had no financial relationships or commercial interests to disclose.
SOURCE: Griffin DR et al. Osteoarthritis Cartilage. 2018:26(1):S24-25. Abstract 28
LIVERPOOL, ENGLAND – Hip arthroscopic surgery produced better long-term results than did personalized hip physiotherapy for femoroacetabular impingement syndrome in a randomized trial conduced across multiple U.K. centers.
At 12 months, respective International Hip Outcome Tool-33 (iHOT-33) scores were 58.8 and 49.7, a difference of 9.1 points before and 6.8 points after adjustment for potential confounding factors (P = .0093).
“This trial shows that hip arthroscopic surgery and personalized hip therapy both improved hip-related quality of life for patients with FAI [femoroacetabular impingement] syndrome, but that the surgery did indeed produce a greater improvement at our primary time point of 12 months,” she added. Dr. Foster is professor of musculoskeletal health in primary care at Keele University, Newcastle-under-Lyme, England, one of the 23 centers involved in the FASHIoN study in England, Wales, and Scotland.
“FAI is a very common cause of hip and groin pain in young adults, and it’s associated with the development of hip osteoarthritis,” Dr. Foster noted.
There are three types of FAI – pincer, cam, and combined. The pincer type of FAI is where there is “prominence or overcoverage of the rim of the acetabulum,” and the cam type is where there is a “bony prominence of the femoral head-neck junction,” she explained at the meeting sponsored by the Osteoarthritis Research Society International.
The link to OA comes when the femur and acetabulum prematurely connect, usually during activity, causing damage to the labrum and articular cartilage in the long term. Thus, treating FAI is important, not just for relieving patient’s pain and joint stiffness.
Hip arthroscopic surgery has become an established way of treating FAI syndrome – more than 2,400 operations were performed in 2013 in the United Kingdom alone, Dr. Foster observed. The aim of surgery is to try to reshape the hip joint to prevent impingement, and resect, repair, or reconstruct any intra-articular damage that may be present.
“Physiotherapy aims to improve hip muscle control and strength, and to correct the abnormal movement patterns that we see in these patients,” Dr. Foster said. “Through that, we hope to prevent the premature contact that occurs in FAI syndrome and thereby improve symptoms, allowing patients to return to activities and prevent recurrence.”
Working with physiotherapists, physicians, and surgeons, Dr. Foster and her associates have previously developed a “best conservative care” intervention that they call personalized hip therapy (PHT), which involves the delivery and supervision of an individualized exercise program by experienced physiotherapists over a 3- to 6-month period, and which patients repeat at home (PM R. 2013 May;5[5]:418-26).
The aim of the UK FASHIoN trial was to compare the clinical and cost-effectiveness of hip arthroscopy and PHT for FAI syndrome, as there was no robust clinical trial evidence to demonstrate a benefit of one over the other.
A total of 351 adults with hip and groin pain were randomized to either arthroscopic surgery (n = 173) or PHT (n = 178). The mean age of participants was 35 years, with no significant differences between the two treatment groups in terms of baseline demographics or type or duration of hip impingement.
While surgery was better in terms of patient outcomes, the study didn’t demonstrate its cost-effectiveness within the first 12 months, Dr. Foster observed. Cost-effectiveness, together with various other quality-of-life measurements, was a secondary endpoint of the study.
“Longer-term outcomes are required to establish whether improvement is sustained, and whether surgery is cost-effective at the longer time points for our health service,” she said.
Responding to a question about whether any of the patients in the study had radiographic evidence of osteoarthritis, Dr. Foster said that such patients had been excluded from the study.
“One of the hopes of the trial’s team is that, with the long-term follow-up, we might be able to get data at 5 and 10 years on things like hip osteoarthritis in these patients,” she said.
The study was funded by the National Institute for Health Research. Dr. Foster had no financial relationships or commercial interests to disclose.
SOURCE: Griffin DR et al. Osteoarthritis Cartilage. 2018:26(1):S24-25. Abstract 28
LIVERPOOL, ENGLAND – Hip arthroscopic surgery produced better long-term results than did personalized hip physiotherapy for femoroacetabular impingement syndrome in a randomized trial conduced across multiple U.K. centers.
At 12 months, respective International Hip Outcome Tool-33 (iHOT-33) scores were 58.8 and 49.7, a difference of 9.1 points before and 6.8 points after adjustment for potential confounding factors (P = .0093).
“This trial shows that hip arthroscopic surgery and personalized hip therapy both improved hip-related quality of life for patients with FAI [femoroacetabular impingement] syndrome, but that the surgery did indeed produce a greater improvement at our primary time point of 12 months,” she added. Dr. Foster is professor of musculoskeletal health in primary care at Keele University, Newcastle-under-Lyme, England, one of the 23 centers involved in the FASHIoN study in England, Wales, and Scotland.
“FAI is a very common cause of hip and groin pain in young adults, and it’s associated with the development of hip osteoarthritis,” Dr. Foster noted.
There are three types of FAI – pincer, cam, and combined. The pincer type of FAI is where there is “prominence or overcoverage of the rim of the acetabulum,” and the cam type is where there is a “bony prominence of the femoral head-neck junction,” she explained at the meeting sponsored by the Osteoarthritis Research Society International.
The link to OA comes when the femur and acetabulum prematurely connect, usually during activity, causing damage to the labrum and articular cartilage in the long term. Thus, treating FAI is important, not just for relieving patient’s pain and joint stiffness.
Hip arthroscopic surgery has become an established way of treating FAI syndrome – more than 2,400 operations were performed in 2013 in the United Kingdom alone, Dr. Foster observed. The aim of surgery is to try to reshape the hip joint to prevent impingement, and resect, repair, or reconstruct any intra-articular damage that may be present.
“Physiotherapy aims to improve hip muscle control and strength, and to correct the abnormal movement patterns that we see in these patients,” Dr. Foster said. “Through that, we hope to prevent the premature contact that occurs in FAI syndrome and thereby improve symptoms, allowing patients to return to activities and prevent recurrence.”
Working with physiotherapists, physicians, and surgeons, Dr. Foster and her associates have previously developed a “best conservative care” intervention that they call personalized hip therapy (PHT), which involves the delivery and supervision of an individualized exercise program by experienced physiotherapists over a 3- to 6-month period, and which patients repeat at home (PM R. 2013 May;5[5]:418-26).
The aim of the UK FASHIoN trial was to compare the clinical and cost-effectiveness of hip arthroscopy and PHT for FAI syndrome, as there was no robust clinical trial evidence to demonstrate a benefit of one over the other.
A total of 351 adults with hip and groin pain were randomized to either arthroscopic surgery (n = 173) or PHT (n = 178). The mean age of participants was 35 years, with no significant differences between the two treatment groups in terms of baseline demographics or type or duration of hip impingement.
While surgery was better in terms of patient outcomes, the study didn’t demonstrate its cost-effectiveness within the first 12 months, Dr. Foster observed. Cost-effectiveness, together with various other quality-of-life measurements, was a secondary endpoint of the study.
“Longer-term outcomes are required to establish whether improvement is sustained, and whether surgery is cost-effective at the longer time points for our health service,” she said.
Responding to a question about whether any of the patients in the study had radiographic evidence of osteoarthritis, Dr. Foster said that such patients had been excluded from the study.
“One of the hopes of the trial’s team is that, with the long-term follow-up, we might be able to get data at 5 and 10 years on things like hip osteoarthritis in these patients,” she said.
The study was funded by the National Institute for Health Research. Dr. Foster had no financial relationships or commercial interests to disclose.
SOURCE: Griffin DR et al. Osteoarthritis Cartilage. 2018:26(1):S24-25. Abstract 28
REPORTING FROM OARSI 2018
Key clinical point: Hip arthroscopy produced better results at 12 months than did the best conservative care.
Major finding: iHOT-33 scores at 12 months were 58.3 for surgery and 49.7 for personalized hip therapy (P = .0093)
Study details: Multicenter, randomized controlled UK FASHIoN trial of 351 adults with hip and groin pain.
Disclosures: The study was funded by the National Institute for Health Research. Dr. Foster had nothing to disclose.
Source: Griffin DR et al. Osteoarthritis Cartilage. 2018:26(1):S24-25. Abstract 28.
Patients who record office visits
Question: During an office visit, the patient used a smartphone to record his conversation with the doctor. Which of the following statements is best?
A. This is an intrusion into a private and confidential physician-patient encounter and violates laws against eavesdropping and wiretapping.
B. Recordings are rarely made in the doctor’s office.
C. Both parties must consent before the patient or doctor can legally make such a recording.
D. Surreptitious recording by one party is always illegal.
E. All are incorrect.
Answer: E.
Scholars from Dartmouth recently published their viewpoint on this topic in the Aug. 7, 2017, issue of JAMA.1 Many individuals believe that taping or recording a private conversation is per se illegal.
This is a misconception. Although it is a serious felony to violate wiretapping laws, in fact every jurisdiction permits the taping or recording of doctor-patient conversations where there is all-party consent. A majority of states actually allow the recording even if one party has not given his/her consent. This one-party consent rule is the law in 39 states, including Hawaii and New York. On the other hand, 11 states, such as California, Florida, Massachusetts, and Washington, deem such recordings illegal. A listing of the law in the various states can be found in the JAMA article, in which the authors call for “clear policies that facilitate the positive use of digital recordings.”
In a 2011 case against the Cleveland Clinic, a patient died of a cardiac arrest from hyperkalemia 3 days after elective knee surgery.2 The patient’s children had made a covert recording of a meeting with the chief medical officer when discussing the incident. The hospital attempted to bar the use of the recording, claiming that the information was nondiscoverable under the “peer review” privilege.
Both the trial court and the court of appeals disagreed, being unconvinced that such discussions fell within peer review protection. That the recording was made surreptitiously was not raised as an issue, as Ohio is a one-party consent state, i.e., the law permits a patient to legally tape his/her conversations without obtaining prior approval from the doctor.3
There are clear advantages to having a permanent record of a doctor’s professional opinion. The patient can review the information after the visit for a better understanding or for recall purposes, even sharing the information with family members, caregivers, or others, especially where there is a lack of clarity on instructions.4 In the area of informed consent, this is particularly useful for a reminder of medication side effects and potential complications of proposed surgery.
However, many doctors believe that recordings may be disruptive or prove inhibitory to free and open discussions, and they are concerned about their potential use should litigation arises.
Risk managers and malpractice carriers are divided in their views. For example, it has been stated that, “at the Barrow Neurological Institute, in Phoenix, Arizona, where patients are routinely offered video recordings of their visits, clinicians who participate in these recordings receive a 10% reduction in the cost of their medical defense and $1 million extra liability coverage” (P.J. Barr, unpublished data, 2017, as cited in reference 1). Other carriers are not as supportive, discouraging their insureds from allowing recordings to be made.
In the majority of jurisdictions, recordings are legal if consented to by one of the parties. This means that recordings by the patient with/without consent from or with/without knowledge of the doctor are fully legitimate. It also means that the recordings will be admissible into evidence in a courtroom, unless the information is privileged (protected from discovery) or is otherwise irrelevant or unreliable.
On the other hand, in states requiring all-party consent, such recordings are illegal absent across-the-board consent, and they will be inadmissible into evidence. This cardinal difference in state law raises vital implications for both plaintiff and defendant in litigation, because the recordings may contain incriminating or exculpatory information.
Recordings of conversations in the doctor’s office are by no means rare. A survey in the United Kingdom revealed that 15% of the public had secretly recorded a clinic visit, and 11% were aware of someone else doing the same.5 The concerned physician could proactively prohibit all office recordings by posting a “no recording” sign in the waiting room in the name of confidentiality and privacy. And should a physician discover that a patient is covertly recording, risk managers have suggested terminating the visit with a warning that a repeat attempt will result in discharge.
Like it or not, recordings are here to stay, and the omnipresence of modern communications devices such as smartphones, tablets, etc., is likely to increase the prevalence of recordings. A practical approach for practicing physicians is to familiarize themselves with the law in the individual state in which they practice and to improve their communication skills irrespective of whether or not there is a recording.
They may wish to consider the view attributed to Richard Boothman, JD, chief risk officer at the University of Michigan Health System: “Recording should cause any caregiver to mind their professionalism and be disciplined in their remarks to their patients. … I believe it can be a very powerful tool to cement the patient/physician relationship and the patient’s understanding of the clinical messages and information. Physicians are significantly benefited by an informed patient.”6
References
1. JAMA. 2017 Aug 8;318(6):513-4.
2. Smith v. Cleveland Clinic, 197 Ohio App.3d 524, 2011.
3. Ohio Revised Code 2933.52.
4. JAMA. 2015 Apr 28;313(16):1615-6.
5. BMJ Open. 2015 Aug 11;5(8):e008566.
6. “Your office is being recorded.” Medscape, April 3, 2018.
Dr. Tan is emeritus professor of medicine and a former adjunct professor of law at the University of Hawaii, Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. For additional information, readers may contact the author at [email protected].
Question: During an office visit, the patient used a smartphone to record his conversation with the doctor. Which of the following statements is best?
A. This is an intrusion into a private and confidential physician-patient encounter and violates laws against eavesdropping and wiretapping.
B. Recordings are rarely made in the doctor’s office.
C. Both parties must consent before the patient or doctor can legally make such a recording.
D. Surreptitious recording by one party is always illegal.
E. All are incorrect.
Answer: E.
Scholars from Dartmouth recently published their viewpoint on this topic in the Aug. 7, 2017, issue of JAMA.1 Many individuals believe that taping or recording a private conversation is per se illegal.
This is a misconception. Although it is a serious felony to violate wiretapping laws, in fact every jurisdiction permits the taping or recording of doctor-patient conversations where there is all-party consent. A majority of states actually allow the recording even if one party has not given his/her consent. This one-party consent rule is the law in 39 states, including Hawaii and New York. On the other hand, 11 states, such as California, Florida, Massachusetts, and Washington, deem such recordings illegal. A listing of the law in the various states can be found in the JAMA article, in which the authors call for “clear policies that facilitate the positive use of digital recordings.”
In a 2011 case against the Cleveland Clinic, a patient died of a cardiac arrest from hyperkalemia 3 days after elective knee surgery.2 The patient’s children had made a covert recording of a meeting with the chief medical officer when discussing the incident. The hospital attempted to bar the use of the recording, claiming that the information was nondiscoverable under the “peer review” privilege.
Both the trial court and the court of appeals disagreed, being unconvinced that such discussions fell within peer review protection. That the recording was made surreptitiously was not raised as an issue, as Ohio is a one-party consent state, i.e., the law permits a patient to legally tape his/her conversations without obtaining prior approval from the doctor.3
There are clear advantages to having a permanent record of a doctor’s professional opinion. The patient can review the information after the visit for a better understanding or for recall purposes, even sharing the information with family members, caregivers, or others, especially where there is a lack of clarity on instructions.4 In the area of informed consent, this is particularly useful for a reminder of medication side effects and potential complications of proposed surgery.
However, many doctors believe that recordings may be disruptive or prove inhibitory to free and open discussions, and they are concerned about their potential use should litigation arises.
Risk managers and malpractice carriers are divided in their views. For example, it has been stated that, “at the Barrow Neurological Institute, in Phoenix, Arizona, where patients are routinely offered video recordings of their visits, clinicians who participate in these recordings receive a 10% reduction in the cost of their medical defense and $1 million extra liability coverage” (P.J. Barr, unpublished data, 2017, as cited in reference 1). Other carriers are not as supportive, discouraging their insureds from allowing recordings to be made.
In the majority of jurisdictions, recordings are legal if consented to by one of the parties. This means that recordings by the patient with/without consent from or with/without knowledge of the doctor are fully legitimate. It also means that the recordings will be admissible into evidence in a courtroom, unless the information is privileged (protected from discovery) or is otherwise irrelevant or unreliable.
On the other hand, in states requiring all-party consent, such recordings are illegal absent across-the-board consent, and they will be inadmissible into evidence. This cardinal difference in state law raises vital implications for both plaintiff and defendant in litigation, because the recordings may contain incriminating or exculpatory information.
Recordings of conversations in the doctor’s office are by no means rare. A survey in the United Kingdom revealed that 15% of the public had secretly recorded a clinic visit, and 11% were aware of someone else doing the same.5 The concerned physician could proactively prohibit all office recordings by posting a “no recording” sign in the waiting room in the name of confidentiality and privacy. And should a physician discover that a patient is covertly recording, risk managers have suggested terminating the visit with a warning that a repeat attempt will result in discharge.
Like it or not, recordings are here to stay, and the omnipresence of modern communications devices such as smartphones, tablets, etc., is likely to increase the prevalence of recordings. A practical approach for practicing physicians is to familiarize themselves with the law in the individual state in which they practice and to improve their communication skills irrespective of whether or not there is a recording.
They may wish to consider the view attributed to Richard Boothman, JD, chief risk officer at the University of Michigan Health System: “Recording should cause any caregiver to mind their professionalism and be disciplined in their remarks to their patients. … I believe it can be a very powerful tool to cement the patient/physician relationship and the patient’s understanding of the clinical messages and information. Physicians are significantly benefited by an informed patient.”6
References
1. JAMA. 2017 Aug 8;318(6):513-4.
2. Smith v. Cleveland Clinic, 197 Ohio App.3d 524, 2011.
3. Ohio Revised Code 2933.52.
4. JAMA. 2015 Apr 28;313(16):1615-6.
5. BMJ Open. 2015 Aug 11;5(8):e008566.
6. “Your office is being recorded.” Medscape, April 3, 2018.
Dr. Tan is emeritus professor of medicine and a former adjunct professor of law at the University of Hawaii, Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. For additional information, readers may contact the author at [email protected].
Question: During an office visit, the patient used a smartphone to record his conversation with the doctor. Which of the following statements is best?
A. This is an intrusion into a private and confidential physician-patient encounter and violates laws against eavesdropping and wiretapping.
B. Recordings are rarely made in the doctor’s office.
C. Both parties must consent before the patient or doctor can legally make such a recording.
D. Surreptitious recording by one party is always illegal.
E. All are incorrect.
Answer: E.
Scholars from Dartmouth recently published their viewpoint on this topic in the Aug. 7, 2017, issue of JAMA.1 Many individuals believe that taping or recording a private conversation is per se illegal.
This is a misconception. Although it is a serious felony to violate wiretapping laws, in fact every jurisdiction permits the taping or recording of doctor-patient conversations where there is all-party consent. A majority of states actually allow the recording even if one party has not given his/her consent. This one-party consent rule is the law in 39 states, including Hawaii and New York. On the other hand, 11 states, such as California, Florida, Massachusetts, and Washington, deem such recordings illegal. A listing of the law in the various states can be found in the JAMA article, in which the authors call for “clear policies that facilitate the positive use of digital recordings.”
In a 2011 case against the Cleveland Clinic, a patient died of a cardiac arrest from hyperkalemia 3 days after elective knee surgery.2 The patient’s children had made a covert recording of a meeting with the chief medical officer when discussing the incident. The hospital attempted to bar the use of the recording, claiming that the information was nondiscoverable under the “peer review” privilege.
Both the trial court and the court of appeals disagreed, being unconvinced that such discussions fell within peer review protection. That the recording was made surreptitiously was not raised as an issue, as Ohio is a one-party consent state, i.e., the law permits a patient to legally tape his/her conversations without obtaining prior approval from the doctor.3
There are clear advantages to having a permanent record of a doctor’s professional opinion. The patient can review the information after the visit for a better understanding or for recall purposes, even sharing the information with family members, caregivers, or others, especially where there is a lack of clarity on instructions.4 In the area of informed consent, this is particularly useful for a reminder of medication side effects and potential complications of proposed surgery.
However, many doctors believe that recordings may be disruptive or prove inhibitory to free and open discussions, and they are concerned about their potential use should litigation arises.
Risk managers and malpractice carriers are divided in their views. For example, it has been stated that, “at the Barrow Neurological Institute, in Phoenix, Arizona, where patients are routinely offered video recordings of their visits, clinicians who participate in these recordings receive a 10% reduction in the cost of their medical defense and $1 million extra liability coverage” (P.J. Barr, unpublished data, 2017, as cited in reference 1). Other carriers are not as supportive, discouraging their insureds from allowing recordings to be made.
In the majority of jurisdictions, recordings are legal if consented to by one of the parties. This means that recordings by the patient with/without consent from or with/without knowledge of the doctor are fully legitimate. It also means that the recordings will be admissible into evidence in a courtroom, unless the information is privileged (protected from discovery) or is otherwise irrelevant or unreliable.
On the other hand, in states requiring all-party consent, such recordings are illegal absent across-the-board consent, and they will be inadmissible into evidence. This cardinal difference in state law raises vital implications for both plaintiff and defendant in litigation, because the recordings may contain incriminating or exculpatory information.
Recordings of conversations in the doctor’s office are by no means rare. A survey in the United Kingdom revealed that 15% of the public had secretly recorded a clinic visit, and 11% were aware of someone else doing the same.5 The concerned physician could proactively prohibit all office recordings by posting a “no recording” sign in the waiting room in the name of confidentiality and privacy. And should a physician discover that a patient is covertly recording, risk managers have suggested terminating the visit with a warning that a repeat attempt will result in discharge.
Like it or not, recordings are here to stay, and the omnipresence of modern communications devices such as smartphones, tablets, etc., is likely to increase the prevalence of recordings. A practical approach for practicing physicians is to familiarize themselves with the law in the individual state in which they practice and to improve their communication skills irrespective of whether or not there is a recording.
They may wish to consider the view attributed to Richard Boothman, JD, chief risk officer at the University of Michigan Health System: “Recording should cause any caregiver to mind their professionalism and be disciplined in their remarks to their patients. … I believe it can be a very powerful tool to cement the patient/physician relationship and the patient’s understanding of the clinical messages and information. Physicians are significantly benefited by an informed patient.”6
References
1. JAMA. 2017 Aug 8;318(6):513-4.
2. Smith v. Cleveland Clinic, 197 Ohio App.3d 524, 2011.
3. Ohio Revised Code 2933.52.
4. JAMA. 2015 Apr 28;313(16):1615-6.
5. BMJ Open. 2015 Aug 11;5(8):e008566.
6. “Your office is being recorded.” Medscape, April 3, 2018.
Dr. Tan is emeritus professor of medicine and a former adjunct professor of law at the University of Hawaii, Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. For additional information, readers may contact the author at [email protected].
ESBL-resistant bacteria spread in hospital despite strict contact precautions
MADRID – even when staff employed an active surveillance screening protocol to identify every carrier at admission.
The failure of precautions may have root in two thorny issues, said Friederike Maechler, MD, who presented the data at the the European Society of Clinical Microbiology and Infectious Diseases annual congress.
“Adherence to strict contact isolation and hand hygiene is never 100% in a real-life scenario,” said Dr. Maechler, of Charite University Hospital, Berlin. Also, she said, contact isolation can only be effective in a ward if all, or at least most, of the ESBL-E carriers are identified. “Even with an extensive surveillance screening program established, many carriers remained unknown to the health care staff.”
The 25-month study, dubbed R-Gnosis, was conducted in 20 Western European hospitals in Madrid, Berlin, Utrecht, and Geneva. It compared 12 months of contact precaution with standard precaution infection control strategies in medical and surgical non-ICUs.
The entire study hinged on a strict protocol to identify as many ESBL-E carriers as possible. This was done by screening upon admission to the unit, screening once per week during the hospital stay, and screening on discharge. Each patient underwent deep rectal swabs that were cultured on agar and screened for resistance.
The crossover design trial randomized each unit to either contact precautions or standard precautions for 12 months, followed by a 1-month washout period, after which they began the other protocol.
In all, 50,870 patients were entered into the study. By the end, Dr. Maechler had data on 11,367 patients with full screening and follow-up.
Standard precautions did not require a private bedroom, with gloves, gowns, and apron needed for direct contact to body fluids or wounds only, and consistent hand hygiene. Contact precautions required a private bedroom and strict hand hygiene, with gloves, gowns, and aprons used for any patient contact. Study staff monitored compliance with these procedures monthly.
The primary outcome was the ESBL-E acquisition rate per 1,000 patient days. This was defined as a new ESBL-E detection after the patient had a prior negative screen. Dr. Maechler noted that by epidemiological definition, acquisition does not necessarily imply cross-transmission from other patients.
Adherence to the study protocols was good, she said. Adherence to both contact and standard precautions was about 85%, while adherence to hand hygiene was less at around 62%.
Admission ESBL-E screenings revealed that about 12% of the study population was colonized with the strain at admission. The proportion was nearly identical in the contact and standard precaution groups (11.6%, 12.2%).
The incidence density of ward-acquired ESBL-E per 1,000 patient-days at risk was 4.6 in both intervention periods, regardless of the type of precaution taken. Contact precautions appeared to be slightly less effective for Escherichia coli (3.6 per 1,000 patient-days in contact precautions vs. 3.5 in standard), compared with Klebsiella pneumoniae (1.8 vs. 2.2).
A multivariate analysis controlled for screening compliance, colonization pressure, and length of stay, study site, and season of year. It showed that strict contact precautions did not reduce the risk of ward-acquired ESBL-E carriage.
Dr. Maechler had no financial disclosures. The R-Gnosis study was funded by the European Community’s Seventh Framework Programme.
SOURCE: Maechler F et al. ECCMID 2018, Oral Abstract O1130.
MADRID – even when staff employed an active surveillance screening protocol to identify every carrier at admission.
The failure of precautions may have root in two thorny issues, said Friederike Maechler, MD, who presented the data at the the European Society of Clinical Microbiology and Infectious Diseases annual congress.
“Adherence to strict contact isolation and hand hygiene is never 100% in a real-life scenario,” said Dr. Maechler, of Charite University Hospital, Berlin. Also, she said, contact isolation can only be effective in a ward if all, or at least most, of the ESBL-E carriers are identified. “Even with an extensive surveillance screening program established, many carriers remained unknown to the health care staff.”
The 25-month study, dubbed R-Gnosis, was conducted in 20 Western European hospitals in Madrid, Berlin, Utrecht, and Geneva. It compared 12 months of contact precaution with standard precaution infection control strategies in medical and surgical non-ICUs.
The entire study hinged on a strict protocol to identify as many ESBL-E carriers as possible. This was done by screening upon admission to the unit, screening once per week during the hospital stay, and screening on discharge. Each patient underwent deep rectal swabs that were cultured on agar and screened for resistance.
The crossover design trial randomized each unit to either contact precautions or standard precautions for 12 months, followed by a 1-month washout period, after which they began the other protocol.
In all, 50,870 patients were entered into the study. By the end, Dr. Maechler had data on 11,367 patients with full screening and follow-up.
Standard precautions did not require a private bedroom, with gloves, gowns, and apron needed for direct contact to body fluids or wounds only, and consistent hand hygiene. Contact precautions required a private bedroom and strict hand hygiene, with gloves, gowns, and aprons used for any patient contact. Study staff monitored compliance with these procedures monthly.
The primary outcome was the ESBL-E acquisition rate per 1,000 patient days. This was defined as a new ESBL-E detection after the patient had a prior negative screen. Dr. Maechler noted that by epidemiological definition, acquisition does not necessarily imply cross-transmission from other patients.
Adherence to the study protocols was good, she said. Adherence to both contact and standard precautions was about 85%, while adherence to hand hygiene was less at around 62%.
Admission ESBL-E screenings revealed that about 12% of the study population was colonized with the strain at admission. The proportion was nearly identical in the contact and standard precaution groups (11.6%, 12.2%).
The incidence density of ward-acquired ESBL-E per 1,000 patient-days at risk was 4.6 in both intervention periods, regardless of the type of precaution taken. Contact precautions appeared to be slightly less effective for Escherichia coli (3.6 per 1,000 patient-days in contact precautions vs. 3.5 in standard), compared with Klebsiella pneumoniae (1.8 vs. 2.2).
A multivariate analysis controlled for screening compliance, colonization pressure, and length of stay, study site, and season of year. It showed that strict contact precautions did not reduce the risk of ward-acquired ESBL-E carriage.
Dr. Maechler had no financial disclosures. The R-Gnosis study was funded by the European Community’s Seventh Framework Programme.
SOURCE: Maechler F et al. ECCMID 2018, Oral Abstract O1130.
MADRID – even when staff employed an active surveillance screening protocol to identify every carrier at admission.
The failure of precautions may have root in two thorny issues, said Friederike Maechler, MD, who presented the data at the the European Society of Clinical Microbiology and Infectious Diseases annual congress.
“Adherence to strict contact isolation and hand hygiene is never 100% in a real-life scenario,” said Dr. Maechler, of Charite University Hospital, Berlin. Also, she said, contact isolation can only be effective in a ward if all, or at least most, of the ESBL-E carriers are identified. “Even with an extensive surveillance screening program established, many carriers remained unknown to the health care staff.”
The 25-month study, dubbed R-Gnosis, was conducted in 20 Western European hospitals in Madrid, Berlin, Utrecht, and Geneva. It compared 12 months of contact precaution with standard precaution infection control strategies in medical and surgical non-ICUs.
The entire study hinged on a strict protocol to identify as many ESBL-E carriers as possible. This was done by screening upon admission to the unit, screening once per week during the hospital stay, and screening on discharge. Each patient underwent deep rectal swabs that were cultured on agar and screened for resistance.
The crossover design trial randomized each unit to either contact precautions or standard precautions for 12 months, followed by a 1-month washout period, after which they began the other protocol.
In all, 50,870 patients were entered into the study. By the end, Dr. Maechler had data on 11,367 patients with full screening and follow-up.
Standard precautions did not require a private bedroom, with gloves, gowns, and apron needed for direct contact to body fluids or wounds only, and consistent hand hygiene. Contact precautions required a private bedroom and strict hand hygiene, with gloves, gowns, and aprons used for any patient contact. Study staff monitored compliance with these procedures monthly.
The primary outcome was the ESBL-E acquisition rate per 1,000 patient days. This was defined as a new ESBL-E detection after the patient had a prior negative screen. Dr. Maechler noted that by epidemiological definition, acquisition does not necessarily imply cross-transmission from other patients.
Adherence to the study protocols was good, she said. Adherence to both contact and standard precautions was about 85%, while adherence to hand hygiene was less at around 62%.
Admission ESBL-E screenings revealed that about 12% of the study population was colonized with the strain at admission. The proportion was nearly identical in the contact and standard precaution groups (11.6%, 12.2%).
The incidence density of ward-acquired ESBL-E per 1,000 patient-days at risk was 4.6 in both intervention periods, regardless of the type of precaution taken. Contact precautions appeared to be slightly less effective for Escherichia coli (3.6 per 1,000 patient-days in contact precautions vs. 3.5 in standard), compared with Klebsiella pneumoniae (1.8 vs. 2.2).
A multivariate analysis controlled for screening compliance, colonization pressure, and length of stay, study site, and season of year. It showed that strict contact precautions did not reduce the risk of ward-acquired ESBL-E carriage.
Dr. Maechler had no financial disclosures. The R-Gnosis study was funded by the European Community’s Seventh Framework Programme.
SOURCE: Maechler F et al. ECCMID 2018, Oral Abstract O1130.
REPORTING FROM ECCMID 2018
Key clinical point: A protocol of strict contact precautions and hand hygiene was no better than standard contact precautions at preventing the spread of extended-spectrum, beta-lactamase–resistant Enterobacteriaceae.
Major finding: The incidence density of ward-acquired ESBL-E per 1,000 patient-days at risk was 4.6, regardless of precaution.
Study details: The 25-month crossover trial comprised more than 11,000 patients.
Disclosures: Dr. Maechler had no financial disclosures. The R-Gnosis study was funded by the European Community’s Seventh Framework Programme.
Source: Maechler F et al. ECCMID 2018, Oral Abstract O1130.
White House pushes transparency in drug price plan
The Trump administration believes it is a “right that, when you are sitting there with your doctor, you ought to be able to know what your out-of-pocket [cost] is for drug you are going to be prescribed under your precise drug plan. And you ought to have information on what competing drugs are that your doctor is not prescribing and what you would pay out of pocket for that.”
Price transparency is a key theme to the broad package of proposals called American Patients First that the White House released May 11. The plan includes changes that can be made immediately as well as some upon which the administration will seek public comment, according to the plan.
Another tactic the administration is considering for quick action is requiring a drug’s list prices to be included in all direct-to-consumer advertising.
The plan also calls for the banning of gag rules that prevent pharmacists from alerting patients when it would be cheaper to buy a prescribed drug without going through their insurance coverage, as well as moving certain drugs from Medicare Part B to Medicare Part D to improve the government’s ability to negotiations for lower prices.
The overall strategy is laid out across four areas:
- Increasing competition though policy measures that prevent manufacturers from gaming the patent system and promoting innovation and competition among biologics.
- Improving negotiation abilities, including doing more with value-based purchasing; allowing for more substitution, especially in the case of single-source generics; and further examination of the competitive acquisition program for Part B.
- Providing incentives to manufacturers to lower their list prices.
- Lowering out-of-pocket costs.
Currently, there is no incentive to lower drug prices, Mr. Azar noted. He called out pharmacy benefit managers (PBMs) for financially benefiting from both manufacturers and the insurers they represent and said HHS is looking into banning financial compensation to PBMs from the manufacturers. He also said the agency will be releasing a request for information on the feasibility of doing away with rebates as a means of driving manufacturers to lower their prices.
PhRMA, the lobbying group representing pharmaceutical manufacturers, said in a statement that some of the changes related to Part D “could undermine the existing structure of the program that has successfully held down costs and provided seniors with access to comprehensive prescription drug coverage. We also must avoid changes to Medicare Part B that could raise costs for seniors and limit their access to lifesaving treatments.”
The Pharmaceutical Care Management Association, the lobbying group for pharmacy benefit managers, also spoke out against the idea of eliminating rebates, noting in a statement that getting rid of them “and other price concessions would leave patients and payers, including Medicaid and Medicare, at the mercy of drug manufacturer pricing strategies. PBMs have long encouraged manufacturers to offer payers alternative ways to reduce net costs. Simply put, the easiest way to lower costs would be for drug companies to lower their prices.”
The American Medical Association voiced support for the plan.
“The AMA is pleased the Trump administration is moving forward with its effort to address seemingly arbitrary pricing for prescription drugs,” President David Barbe, MD, said in a statement “Physicians see the impact of skyrocketing prices every day as patients are often unable to afford the most medically appropriate medications – even those that have effectively controlled their medical condition for years. No one can understand the logic behind the high and fluctuating prices. We hope the administration can bring some transparency – and relief – to patients.”
During a Rose Garden ceremony to introduce the initiative, President Trump said he is targeting foreign countries that require manufacturers to sell their products well below list prices as a condition of selling in their country, forcing Americans to absorb the research and development costs via higher prices for drugs.
“It’s time to end the global freeloading once and for all,” President Trump said. “I have directed U.S. Trade Representative Bob Lighthizer to make fixing this injustice a top priority with every trading partner.”
One proposal President Trump campaigned on – giving the federal government the power to directly negotiate for drug prices – was not included in the plan.
“I am very disappointed that he has apparently dropped his support for allowing Medicare to negotiate the price of prescription drugs,” Sen. Maggie Hassan (D-N.H.) said in a statement.
Also absent from the plan: a direct negotiation tactic for Part B drugs supported by Mr. Azar during his confirmation hearings.
The Trump administration believes it is a “right that, when you are sitting there with your doctor, you ought to be able to know what your out-of-pocket [cost] is for drug you are going to be prescribed under your precise drug plan. And you ought to have information on what competing drugs are that your doctor is not prescribing and what you would pay out of pocket for that.”
Price transparency is a key theme to the broad package of proposals called American Patients First that the White House released May 11. The plan includes changes that can be made immediately as well as some upon which the administration will seek public comment, according to the plan.
Another tactic the administration is considering for quick action is requiring a drug’s list prices to be included in all direct-to-consumer advertising.
The plan also calls for the banning of gag rules that prevent pharmacists from alerting patients when it would be cheaper to buy a prescribed drug without going through their insurance coverage, as well as moving certain drugs from Medicare Part B to Medicare Part D to improve the government’s ability to negotiations for lower prices.
The overall strategy is laid out across four areas:
- Increasing competition though policy measures that prevent manufacturers from gaming the patent system and promoting innovation and competition among biologics.
- Improving negotiation abilities, including doing more with value-based purchasing; allowing for more substitution, especially in the case of single-source generics; and further examination of the competitive acquisition program for Part B.
- Providing incentives to manufacturers to lower their list prices.
- Lowering out-of-pocket costs.
Currently, there is no incentive to lower drug prices, Mr. Azar noted. He called out pharmacy benefit managers (PBMs) for financially benefiting from both manufacturers and the insurers they represent and said HHS is looking into banning financial compensation to PBMs from the manufacturers. He also said the agency will be releasing a request for information on the feasibility of doing away with rebates as a means of driving manufacturers to lower their prices.
PhRMA, the lobbying group representing pharmaceutical manufacturers, said in a statement that some of the changes related to Part D “could undermine the existing structure of the program that has successfully held down costs and provided seniors with access to comprehensive prescription drug coverage. We also must avoid changes to Medicare Part B that could raise costs for seniors and limit their access to lifesaving treatments.”
The Pharmaceutical Care Management Association, the lobbying group for pharmacy benefit managers, also spoke out against the idea of eliminating rebates, noting in a statement that getting rid of them “and other price concessions would leave patients and payers, including Medicaid and Medicare, at the mercy of drug manufacturer pricing strategies. PBMs have long encouraged manufacturers to offer payers alternative ways to reduce net costs. Simply put, the easiest way to lower costs would be for drug companies to lower their prices.”
The American Medical Association voiced support for the plan.
“The AMA is pleased the Trump administration is moving forward with its effort to address seemingly arbitrary pricing for prescription drugs,” President David Barbe, MD, said in a statement “Physicians see the impact of skyrocketing prices every day as patients are often unable to afford the most medically appropriate medications – even those that have effectively controlled their medical condition for years. No one can understand the logic behind the high and fluctuating prices. We hope the administration can bring some transparency – and relief – to patients.”
During a Rose Garden ceremony to introduce the initiative, President Trump said he is targeting foreign countries that require manufacturers to sell their products well below list prices as a condition of selling in their country, forcing Americans to absorb the research and development costs via higher prices for drugs.
“It’s time to end the global freeloading once and for all,” President Trump said. “I have directed U.S. Trade Representative Bob Lighthizer to make fixing this injustice a top priority with every trading partner.”
One proposal President Trump campaigned on – giving the federal government the power to directly negotiate for drug prices – was not included in the plan.
“I am very disappointed that he has apparently dropped his support for allowing Medicare to negotiate the price of prescription drugs,” Sen. Maggie Hassan (D-N.H.) said in a statement.
Also absent from the plan: a direct negotiation tactic for Part B drugs supported by Mr. Azar during his confirmation hearings.
The Trump administration believes it is a “right that, when you are sitting there with your doctor, you ought to be able to know what your out-of-pocket [cost] is for drug you are going to be prescribed under your precise drug plan. And you ought to have information on what competing drugs are that your doctor is not prescribing and what you would pay out of pocket for that.”
Price transparency is a key theme to the broad package of proposals called American Patients First that the White House released May 11. The plan includes changes that can be made immediately as well as some upon which the administration will seek public comment, according to the plan.
Another tactic the administration is considering for quick action is requiring a drug’s list prices to be included in all direct-to-consumer advertising.
The plan also calls for the banning of gag rules that prevent pharmacists from alerting patients when it would be cheaper to buy a prescribed drug without going through their insurance coverage, as well as moving certain drugs from Medicare Part B to Medicare Part D to improve the government’s ability to negotiations for lower prices.
The overall strategy is laid out across four areas:
- Increasing competition though policy measures that prevent manufacturers from gaming the patent system and promoting innovation and competition among biologics.
- Improving negotiation abilities, including doing more with value-based purchasing; allowing for more substitution, especially in the case of single-source generics; and further examination of the competitive acquisition program for Part B.
- Providing incentives to manufacturers to lower their list prices.
- Lowering out-of-pocket costs.
Currently, there is no incentive to lower drug prices, Mr. Azar noted. He called out pharmacy benefit managers (PBMs) for financially benefiting from both manufacturers and the insurers they represent and said HHS is looking into banning financial compensation to PBMs from the manufacturers. He also said the agency will be releasing a request for information on the feasibility of doing away with rebates as a means of driving manufacturers to lower their prices.
PhRMA, the lobbying group representing pharmaceutical manufacturers, said in a statement that some of the changes related to Part D “could undermine the existing structure of the program that has successfully held down costs and provided seniors with access to comprehensive prescription drug coverage. We also must avoid changes to Medicare Part B that could raise costs for seniors and limit their access to lifesaving treatments.”
The Pharmaceutical Care Management Association, the lobbying group for pharmacy benefit managers, also spoke out against the idea of eliminating rebates, noting in a statement that getting rid of them “and other price concessions would leave patients and payers, including Medicaid and Medicare, at the mercy of drug manufacturer pricing strategies. PBMs have long encouraged manufacturers to offer payers alternative ways to reduce net costs. Simply put, the easiest way to lower costs would be for drug companies to lower their prices.”
The American Medical Association voiced support for the plan.
“The AMA is pleased the Trump administration is moving forward with its effort to address seemingly arbitrary pricing for prescription drugs,” President David Barbe, MD, said in a statement “Physicians see the impact of skyrocketing prices every day as patients are often unable to afford the most medically appropriate medications – even those that have effectively controlled their medical condition for years. No one can understand the logic behind the high and fluctuating prices. We hope the administration can bring some transparency – and relief – to patients.”
During a Rose Garden ceremony to introduce the initiative, President Trump said he is targeting foreign countries that require manufacturers to sell their products well below list prices as a condition of selling in their country, forcing Americans to absorb the research and development costs via higher prices for drugs.
“It’s time to end the global freeloading once and for all,” President Trump said. “I have directed U.S. Trade Representative Bob Lighthizer to make fixing this injustice a top priority with every trading partner.”
One proposal President Trump campaigned on – giving the federal government the power to directly negotiate for drug prices – was not included in the plan.
“I am very disappointed that he has apparently dropped his support for allowing Medicare to negotiate the price of prescription drugs,” Sen. Maggie Hassan (D-N.H.) said in a statement.
Also absent from the plan: a direct negotiation tactic for Part B drugs supported by Mr. Azar during his confirmation hearings.
Gender equity in surgery: It’s complicated
For most of my professional life I have avoided writing about gender inequity in the field of surgery – not because I believe it does not exist, but because the reasons it does exist are manifold and complicated.
To be sure, implicit and explicit bias remain important reasons why only a minority of female medical students still choose surgery as a career in 2018, why female surgeons still earn only 82% of the salary of their male counterparts, and why women occupy only 12% of the chairs in academic surgical departments in the United States. It has long been tempting to lay the blame for those inequities on a stereotypical macho surgical culture that prevailed as the 20th century came to a close. But the factors that perpetuate male/female imbalance in our profession are complicated and run deep in the psyches of both men and women. We are all products of our generations, our culture, upbringing, and environment – men as well as women. Correcting the imbalance requires much more than simply passing laws that require equitable pay or treatment.
I realized how deeply ingrained biases are in all of us while still in my surgical residency in the 1970s. On a rare occasion when neither my husband (also a surgical resident) nor I was on call, we had a dinner party with friends at our house. The telephone rang (no iPhones, just a land line), and I ran to answer our only telephone in another room. It was the hospital operator, who asked, “Is Dr. Deveney there?” Without thinking, I answered, “Just a moment, I’ll get him!” I had taken only three steps away from the phone when it occurred to me what I had just said. I turned back, picked up the phone, and asked, “Which Dr. Deveney were you looking for?”
If even I had been conditioned to think automatically of “doctor” being a man after all of my effort to earn a place in the ranks, was there any hope that equality could be achieved? When I finished my residency and was offered a surgery position at our VA, I asked no questions about salary or other particulars; I was simply grateful that I had been given a job.
That was 1978 – a different time, a different generation. Since then, women have made dramatic progress toward equity in our profession, as in many others. The support, mentoring, and consciousness-raising efforts of the Association of Women Surgeons (AWS) are responsible for much of this progress. The American College of Surgeons was very supportive of the AWS early on and continues to encourage female medical students to choose surgery as a career and to help the advancement of women into leadership roles in surgery. Many surgical residency programs have 50% women in their ranks; half of our residents in Oregon have been women for over a decade. Our program director is a woman, as are 28% of the faculty; not 50%, but definitely progress, since I was the lone woman on the faculty when I arrived in 1987.
Although women occupy only 12% of the surgical chairs in U.S. surgical departments, this number has soared in the past 2 years from 7 in 2016 to 21 this year after languishing in the low single digits since 1987, when the late Olga Jonasson, MD, FACS, became the first female chair at Ohio State University. And yet, women have not reached full equality with men across the United States in surgical training, leadership, or pay. Why not?
Multiple factors have played roles in impeding the progress of women in achieving equality in surgery. Traditional cultural expectations of male and female roles in society affect both genders as they grow up, even when their parents make deliberate efforts to raise their children in as “gender-neutral” a way as possible and encourage their daughters to strive for success to the same degree as their sons. Explicit bias against women remains easier to recognize and combat than implicit or unconscious bias, to which we are all subject.
We have only recently begun to acknowledge and attempt to dispel implicit bias and much work remains to be done if we are to reach a level playing field that is gender neutral. The book, “Why So Slow?” by Virginia Valian (Cambridge, Mass.: The MIT Press, 1998) offered valuable insights that helped me understand why we behave as we do and how our departments and institutions might become more equitable to all. Women tend to underrate their abilities and attribute their success to luck rather than superior performance, to believe that they are less qualified for higher pay or promotion than men are, and to be less assertive than men in negotiations. It will require conscious vigilance and effort by both sexes as well as by institutions themselves to educate all about the criteria necessary for advancement. Institutions need to develop training for all in recognizing and eliminating implicit bias, and in implementing clear and explicit criteria for compensation and promotion, making sure that all faculty are educated to understand what those criteria are.
Dr. Deveney is professor of surgery and vice chair of education in the department of surgery, Oregon Health & Science University, Portland. She is the coeditor of ACS Surgery News.
For most of my professional life I have avoided writing about gender inequity in the field of surgery – not because I believe it does not exist, but because the reasons it does exist are manifold and complicated.
To be sure, implicit and explicit bias remain important reasons why only a minority of female medical students still choose surgery as a career in 2018, why female surgeons still earn only 82% of the salary of their male counterparts, and why women occupy only 12% of the chairs in academic surgical departments in the United States. It has long been tempting to lay the blame for those inequities on a stereotypical macho surgical culture that prevailed as the 20th century came to a close. But the factors that perpetuate male/female imbalance in our profession are complicated and run deep in the psyches of both men and women. We are all products of our generations, our culture, upbringing, and environment – men as well as women. Correcting the imbalance requires much more than simply passing laws that require equitable pay or treatment.
I realized how deeply ingrained biases are in all of us while still in my surgical residency in the 1970s. On a rare occasion when neither my husband (also a surgical resident) nor I was on call, we had a dinner party with friends at our house. The telephone rang (no iPhones, just a land line), and I ran to answer our only telephone in another room. It was the hospital operator, who asked, “Is Dr. Deveney there?” Without thinking, I answered, “Just a moment, I’ll get him!” I had taken only three steps away from the phone when it occurred to me what I had just said. I turned back, picked up the phone, and asked, “Which Dr. Deveney were you looking for?”
If even I had been conditioned to think automatically of “doctor” being a man after all of my effort to earn a place in the ranks, was there any hope that equality could be achieved? When I finished my residency and was offered a surgery position at our VA, I asked no questions about salary or other particulars; I was simply grateful that I had been given a job.
That was 1978 – a different time, a different generation. Since then, women have made dramatic progress toward equity in our profession, as in many others. The support, mentoring, and consciousness-raising efforts of the Association of Women Surgeons (AWS) are responsible for much of this progress. The American College of Surgeons was very supportive of the AWS early on and continues to encourage female medical students to choose surgery as a career and to help the advancement of women into leadership roles in surgery. Many surgical residency programs have 50% women in their ranks; half of our residents in Oregon have been women for over a decade. Our program director is a woman, as are 28% of the faculty; not 50%, but definitely progress, since I was the lone woman on the faculty when I arrived in 1987.
Although women occupy only 12% of the surgical chairs in U.S. surgical departments, this number has soared in the past 2 years from 7 in 2016 to 21 this year after languishing in the low single digits since 1987, when the late Olga Jonasson, MD, FACS, became the first female chair at Ohio State University. And yet, women have not reached full equality with men across the United States in surgical training, leadership, or pay. Why not?
Multiple factors have played roles in impeding the progress of women in achieving equality in surgery. Traditional cultural expectations of male and female roles in society affect both genders as they grow up, even when their parents make deliberate efforts to raise their children in as “gender-neutral” a way as possible and encourage their daughters to strive for success to the same degree as their sons. Explicit bias against women remains easier to recognize and combat than implicit or unconscious bias, to which we are all subject.
We have only recently begun to acknowledge and attempt to dispel implicit bias and much work remains to be done if we are to reach a level playing field that is gender neutral. The book, “Why So Slow?” by Virginia Valian (Cambridge, Mass.: The MIT Press, 1998) offered valuable insights that helped me understand why we behave as we do and how our departments and institutions might become more equitable to all. Women tend to underrate their abilities and attribute their success to luck rather than superior performance, to believe that they are less qualified for higher pay or promotion than men are, and to be less assertive than men in negotiations. It will require conscious vigilance and effort by both sexes as well as by institutions themselves to educate all about the criteria necessary for advancement. Institutions need to develop training for all in recognizing and eliminating implicit bias, and in implementing clear and explicit criteria for compensation and promotion, making sure that all faculty are educated to understand what those criteria are.
Dr. Deveney is professor of surgery and vice chair of education in the department of surgery, Oregon Health & Science University, Portland. She is the coeditor of ACS Surgery News.
For most of my professional life I have avoided writing about gender inequity in the field of surgery – not because I believe it does not exist, but because the reasons it does exist are manifold and complicated.
To be sure, implicit and explicit bias remain important reasons why only a minority of female medical students still choose surgery as a career in 2018, why female surgeons still earn only 82% of the salary of their male counterparts, and why women occupy only 12% of the chairs in academic surgical departments in the United States. It has long been tempting to lay the blame for those inequities on a stereotypical macho surgical culture that prevailed as the 20th century came to a close. But the factors that perpetuate male/female imbalance in our profession are complicated and run deep in the psyches of both men and women. We are all products of our generations, our culture, upbringing, and environment – men as well as women. Correcting the imbalance requires much more than simply passing laws that require equitable pay or treatment.
I realized how deeply ingrained biases are in all of us while still in my surgical residency in the 1970s. On a rare occasion when neither my husband (also a surgical resident) nor I was on call, we had a dinner party with friends at our house. The telephone rang (no iPhones, just a land line), and I ran to answer our only telephone in another room. It was the hospital operator, who asked, “Is Dr. Deveney there?” Without thinking, I answered, “Just a moment, I’ll get him!” I had taken only three steps away from the phone when it occurred to me what I had just said. I turned back, picked up the phone, and asked, “Which Dr. Deveney were you looking for?”
If even I had been conditioned to think automatically of “doctor” being a man after all of my effort to earn a place in the ranks, was there any hope that equality could be achieved? When I finished my residency and was offered a surgery position at our VA, I asked no questions about salary or other particulars; I was simply grateful that I had been given a job.
That was 1978 – a different time, a different generation. Since then, women have made dramatic progress toward equity in our profession, as in many others. The support, mentoring, and consciousness-raising efforts of the Association of Women Surgeons (AWS) are responsible for much of this progress. The American College of Surgeons was very supportive of the AWS early on and continues to encourage female medical students to choose surgery as a career and to help the advancement of women into leadership roles in surgery. Many surgical residency programs have 50% women in their ranks; half of our residents in Oregon have been women for over a decade. Our program director is a woman, as are 28% of the faculty; not 50%, but definitely progress, since I was the lone woman on the faculty when I arrived in 1987.
Although women occupy only 12% of the surgical chairs in U.S. surgical departments, this number has soared in the past 2 years from 7 in 2016 to 21 this year after languishing in the low single digits since 1987, when the late Olga Jonasson, MD, FACS, became the first female chair at Ohio State University. And yet, women have not reached full equality with men across the United States in surgical training, leadership, or pay. Why not?
Multiple factors have played roles in impeding the progress of women in achieving equality in surgery. Traditional cultural expectations of male and female roles in society affect both genders as they grow up, even when their parents make deliberate efforts to raise their children in as “gender-neutral” a way as possible and encourage their daughters to strive for success to the same degree as their sons. Explicit bias against women remains easier to recognize and combat than implicit or unconscious bias, to which we are all subject.
We have only recently begun to acknowledge and attempt to dispel implicit bias and much work remains to be done if we are to reach a level playing field that is gender neutral. The book, “Why So Slow?” by Virginia Valian (Cambridge, Mass.: The MIT Press, 1998) offered valuable insights that helped me understand why we behave as we do and how our departments and institutions might become more equitable to all. Women tend to underrate their abilities and attribute their success to luck rather than superior performance, to believe that they are less qualified for higher pay or promotion than men are, and to be less assertive than men in negotiations. It will require conscious vigilance and effort by both sexes as well as by institutions themselves to educate all about the criteria necessary for advancement. Institutions need to develop training for all in recognizing and eliminating implicit bias, and in implementing clear and explicit criteria for compensation and promotion, making sure that all faculty are educated to understand what those criteria are.
Dr. Deveney is professor of surgery and vice chair of education in the department of surgery, Oregon Health & Science University, Portland. She is the coeditor of ACS Surgery News.
Upping the game of surgical researchers
Many are submitted, but few are chosen.
Concerned about the quality of submitted research papers based on large surgical databases that are not accepted for publication, the editorial board of JAMA Surgery has taken the initiative by giving some pointers to would-be authors. The journal editors have published a 10-point checklist of dos and don’ts to address commonly seen problems with submitted manuscripts. In addition, JAMA Surgery collaborated with the Surgical Outcomes Club to commission a series of practical guides on the most widely used data sets in an effort to improve the quality of surgical database research. The Surgical Outcomes Club is a consortium of surgeons and scientists who work to advance health services and outcomes research in surgery that was launched in 2005 at the American College of Surgeons Clinical Congress.
The authors noted that, although JAMA Surgery receives hundreds of submissions of retrospective studies of large surgical databases each year, most of these studies have flaws in the data analysis or use a hypothesis that the data sets cannot address. Hence, the editors do not send most of them out for peer review. “Of those that are sent out for peer review, many are recommended to be rejected by expert peer reviewers as they find major methodological flaws in the use of these otherwise powerful data sets,” the team wrote.
“Research using data sets can be very powerful as the research can address questions and hypotheses using large populations of people. However, the research can have many weaknesses. First, the research is only as good as the data collection for each data set. Second, the investigator needs to be familiar with the types of research questions and hypotheses that can be addressed with each data set. Third, the statistical methodology used to analyze the data is also imperative,” said Dr. Kibbe in an interview.
The checklist begins with a recommendation that the researchers develop a clear, concise hypothesis using established criteria – either FINER (for feasible, interesting, novel, ethical, relevant) or PICO (patient, population, or problem; intervention, prognostic factor, or exposure; comparison or intervention; outcome); the checklist then goes on to include compliance with institutional review board and data use agreements and to emphasize the importance of a clear take-home message that addresses policy or clinical implications.
The series comprises 11 two-page articles that aim to serve as practical guides for using each of the most widely used surgical data sets, starting with the National Inpatient Sample and ending with the Society of Thoracic Surgeons data set. Each article includes a bulleted list of the data set’s attributes, an explanation of its limitations, a history of the data set, an explanation of how the data is collected and what is unique about the set’s features, and statistical considerations researchers should take into account when analyzing the data.
The series concludes with tips from the statistical editors of JAMA Surgery – Amy H. Kaji, MD, PhD, of Harbor–University of California Los Angeles Medical Center in Torrance; Alfred W. Rademaker, PhD, of Northwestern University, Chicago; and Terry Hyslop, PhD, of Duke University, Durham, N.C. – for performing statistical analysis of large data sets: “With bigger data, random signals may denote statistical significance, and precision may be incorrectly inferred because of narrow confidence intervals,” the statistical editors noted. “While many principles apply to all studies, the importance of these methodological issues is amplified in large, complex data sets.”
However, they noted that large data sets are prone to bias and measurement errors. “It is important to respect and acknowledge the limitations of the data,” the statistical team wrote. They also reprise the introductory editorial’s call for a clear hypothesis and take-home message. “The challenge with Big Data is that it requires a carefully thought-out research question and a transparent analytic strategy,” the statistical editors said.
And there are pitfalls. Dr. Bilimoria noted, “We shouldn’t let the database define our research. We should instead be asking interesting questions and then seeking out a database that fits best to answer the question.” He said one particular problem that comes up often for reviewers is trying to discern how researchers arrived at the population of interest in a study. “A lot of inclusion and exclusions criteria are applied, and unless [the reviewer] can see the decisions that were made in the process, some fairly important biases can be introduced unintentionally. We as reviewers would like to be able to follow that exclusion pathway.”
He said, “A problem we frequently see is that these databases change the variable definitions over time – in fact, change the variables over time. So if researchers aren’t checking to see if that variable was reported the same every year of the study and in the same way, they will get spurious results. Similarly, the number of hospitals reporting is important as well since hospitals come in and out of these data sets.”
In their introductory editorial, the JAMA Surgery team noted that the checklist, practical guides, and statistical tips are a three-pronged approach that authors should consult before submitting their manuscripts. “We hope that by following these simple guides, authors can benefit from the collective wisdom of so many colleagues who have successfully completed similar analyses in the past,” they wrote.
Dr. Bilimoria sees great strengths in database research, such as giving researchers a population-level view of how care is being delivered, insights into the outcomes of care, indications of the effects of policy decisions, and data on rare diseases and operations.
Big Data of all kinds will be increasingly available for researchers. Dr. Kibbe commented that, “In the future, having a comprehensive (not sampling) country- or worldwide electronic medical record that will allow for robust inclusion of all medical data at the individual as well as cohort level will greatly contribute to the era of personalized medicine. In my opinion, this would be a real-time inclusive medical database that would allow for individual as well as population-based prospective studies.”
Dr. Haider receives support from the Henry M. Jackson Foundation of the Department of Defense, the Orthopaedic Research and Education Foundation, and the National Institutes of Health, and nonfinancial research support the Centers for Medicare and Medicaid Services Office of Minority Health. Dr. Bilimoria was the president of the Surgical Outcomes Club from 2016 to 2017.
Many are submitted, but few are chosen.
Concerned about the quality of submitted research papers based on large surgical databases that are not accepted for publication, the editorial board of JAMA Surgery has taken the initiative by giving some pointers to would-be authors. The journal editors have published a 10-point checklist of dos and don’ts to address commonly seen problems with submitted manuscripts. In addition, JAMA Surgery collaborated with the Surgical Outcomes Club to commission a series of practical guides on the most widely used data sets in an effort to improve the quality of surgical database research. The Surgical Outcomes Club is a consortium of surgeons and scientists who work to advance health services and outcomes research in surgery that was launched in 2005 at the American College of Surgeons Clinical Congress.
The authors noted that, although JAMA Surgery receives hundreds of submissions of retrospective studies of large surgical databases each year, most of these studies have flaws in the data analysis or use a hypothesis that the data sets cannot address. Hence, the editors do not send most of them out for peer review. “Of those that are sent out for peer review, many are recommended to be rejected by expert peer reviewers as they find major methodological flaws in the use of these otherwise powerful data sets,” the team wrote.
“Research using data sets can be very powerful as the research can address questions and hypotheses using large populations of people. However, the research can have many weaknesses. First, the research is only as good as the data collection for each data set. Second, the investigator needs to be familiar with the types of research questions and hypotheses that can be addressed with each data set. Third, the statistical methodology used to analyze the data is also imperative,” said Dr. Kibbe in an interview.
The checklist begins with a recommendation that the researchers develop a clear, concise hypothesis using established criteria – either FINER (for feasible, interesting, novel, ethical, relevant) or PICO (patient, population, or problem; intervention, prognostic factor, or exposure; comparison or intervention; outcome); the checklist then goes on to include compliance with institutional review board and data use agreements and to emphasize the importance of a clear take-home message that addresses policy or clinical implications.
The series comprises 11 two-page articles that aim to serve as practical guides for using each of the most widely used surgical data sets, starting with the National Inpatient Sample and ending with the Society of Thoracic Surgeons data set. Each article includes a bulleted list of the data set’s attributes, an explanation of its limitations, a history of the data set, an explanation of how the data is collected and what is unique about the set’s features, and statistical considerations researchers should take into account when analyzing the data.
The series concludes with tips from the statistical editors of JAMA Surgery – Amy H. Kaji, MD, PhD, of Harbor–University of California Los Angeles Medical Center in Torrance; Alfred W. Rademaker, PhD, of Northwestern University, Chicago; and Terry Hyslop, PhD, of Duke University, Durham, N.C. – for performing statistical analysis of large data sets: “With bigger data, random signals may denote statistical significance, and precision may be incorrectly inferred because of narrow confidence intervals,” the statistical editors noted. “While many principles apply to all studies, the importance of these methodological issues is amplified in large, complex data sets.”
However, they noted that large data sets are prone to bias and measurement errors. “It is important to respect and acknowledge the limitations of the data,” the statistical team wrote. They also reprise the introductory editorial’s call for a clear hypothesis and take-home message. “The challenge with Big Data is that it requires a carefully thought-out research question and a transparent analytic strategy,” the statistical editors said.
And there are pitfalls. Dr. Bilimoria noted, “We shouldn’t let the database define our research. We should instead be asking interesting questions and then seeking out a database that fits best to answer the question.” He said one particular problem that comes up often for reviewers is trying to discern how researchers arrived at the population of interest in a study. “A lot of inclusion and exclusions criteria are applied, and unless [the reviewer] can see the decisions that were made in the process, some fairly important biases can be introduced unintentionally. We as reviewers would like to be able to follow that exclusion pathway.”
He said, “A problem we frequently see is that these databases change the variable definitions over time – in fact, change the variables over time. So if researchers aren’t checking to see if that variable was reported the same every year of the study and in the same way, they will get spurious results. Similarly, the number of hospitals reporting is important as well since hospitals come in and out of these data sets.”
In their introductory editorial, the JAMA Surgery team noted that the checklist, practical guides, and statistical tips are a three-pronged approach that authors should consult before submitting their manuscripts. “We hope that by following these simple guides, authors can benefit from the collective wisdom of so many colleagues who have successfully completed similar analyses in the past,” they wrote.
Dr. Bilimoria sees great strengths in database research, such as giving researchers a population-level view of how care is being delivered, insights into the outcomes of care, indications of the effects of policy decisions, and data on rare diseases and operations.
Big Data of all kinds will be increasingly available for researchers. Dr. Kibbe commented that, “In the future, having a comprehensive (not sampling) country- or worldwide electronic medical record that will allow for robust inclusion of all medical data at the individual as well as cohort level will greatly contribute to the era of personalized medicine. In my opinion, this would be a real-time inclusive medical database that would allow for individual as well as population-based prospective studies.”
Dr. Haider receives support from the Henry M. Jackson Foundation of the Department of Defense, the Orthopaedic Research and Education Foundation, and the National Institutes of Health, and nonfinancial research support the Centers for Medicare and Medicaid Services Office of Minority Health. Dr. Bilimoria was the president of the Surgical Outcomes Club from 2016 to 2017.
Many are submitted, but few are chosen.
Concerned about the quality of submitted research papers based on large surgical databases that are not accepted for publication, the editorial board of JAMA Surgery has taken the initiative by giving some pointers to would-be authors. The journal editors have published a 10-point checklist of dos and don’ts to address commonly seen problems with submitted manuscripts. In addition, JAMA Surgery collaborated with the Surgical Outcomes Club to commission a series of practical guides on the most widely used data sets in an effort to improve the quality of surgical database research. The Surgical Outcomes Club is a consortium of surgeons and scientists who work to advance health services and outcomes research in surgery that was launched in 2005 at the American College of Surgeons Clinical Congress.
The authors noted that, although JAMA Surgery receives hundreds of submissions of retrospective studies of large surgical databases each year, most of these studies have flaws in the data analysis or use a hypothesis that the data sets cannot address. Hence, the editors do not send most of them out for peer review. “Of those that are sent out for peer review, many are recommended to be rejected by expert peer reviewers as they find major methodological flaws in the use of these otherwise powerful data sets,” the team wrote.
“Research using data sets can be very powerful as the research can address questions and hypotheses using large populations of people. However, the research can have many weaknesses. First, the research is only as good as the data collection for each data set. Second, the investigator needs to be familiar with the types of research questions and hypotheses that can be addressed with each data set. Third, the statistical methodology used to analyze the data is also imperative,” said Dr. Kibbe in an interview.
The checklist begins with a recommendation that the researchers develop a clear, concise hypothesis using established criteria – either FINER (for feasible, interesting, novel, ethical, relevant) or PICO (patient, population, or problem; intervention, prognostic factor, or exposure; comparison or intervention; outcome); the checklist then goes on to include compliance with institutional review board and data use agreements and to emphasize the importance of a clear take-home message that addresses policy or clinical implications.
The series comprises 11 two-page articles that aim to serve as practical guides for using each of the most widely used surgical data sets, starting with the National Inpatient Sample and ending with the Society of Thoracic Surgeons data set. Each article includes a bulleted list of the data set’s attributes, an explanation of its limitations, a history of the data set, an explanation of how the data is collected and what is unique about the set’s features, and statistical considerations researchers should take into account when analyzing the data.
The series concludes with tips from the statistical editors of JAMA Surgery – Amy H. Kaji, MD, PhD, of Harbor–University of California Los Angeles Medical Center in Torrance; Alfred W. Rademaker, PhD, of Northwestern University, Chicago; and Terry Hyslop, PhD, of Duke University, Durham, N.C. – for performing statistical analysis of large data sets: “With bigger data, random signals may denote statistical significance, and precision may be incorrectly inferred because of narrow confidence intervals,” the statistical editors noted. “While many principles apply to all studies, the importance of these methodological issues is amplified in large, complex data sets.”
However, they noted that large data sets are prone to bias and measurement errors. “It is important to respect and acknowledge the limitations of the data,” the statistical team wrote. They also reprise the introductory editorial’s call for a clear hypothesis and take-home message. “The challenge with Big Data is that it requires a carefully thought-out research question and a transparent analytic strategy,” the statistical editors said.
And there are pitfalls. Dr. Bilimoria noted, “We shouldn’t let the database define our research. We should instead be asking interesting questions and then seeking out a database that fits best to answer the question.” He said one particular problem that comes up often for reviewers is trying to discern how researchers arrived at the population of interest in a study. “A lot of inclusion and exclusions criteria are applied, and unless [the reviewer] can see the decisions that were made in the process, some fairly important biases can be introduced unintentionally. We as reviewers would like to be able to follow that exclusion pathway.”
He said, “A problem we frequently see is that these databases change the variable definitions over time – in fact, change the variables over time. So if researchers aren’t checking to see if that variable was reported the same every year of the study and in the same way, they will get spurious results. Similarly, the number of hospitals reporting is important as well since hospitals come in and out of these data sets.”
In their introductory editorial, the JAMA Surgery team noted that the checklist, practical guides, and statistical tips are a three-pronged approach that authors should consult before submitting their manuscripts. “We hope that by following these simple guides, authors can benefit from the collective wisdom of so many colleagues who have successfully completed similar analyses in the past,” they wrote.
Dr. Bilimoria sees great strengths in database research, such as giving researchers a population-level view of how care is being delivered, insights into the outcomes of care, indications of the effects of policy decisions, and data on rare diseases and operations.
Big Data of all kinds will be increasingly available for researchers. Dr. Kibbe commented that, “In the future, having a comprehensive (not sampling) country- or worldwide electronic medical record that will allow for robust inclusion of all medical data at the individual as well as cohort level will greatly contribute to the era of personalized medicine. In my opinion, this would be a real-time inclusive medical database that would allow for individual as well as population-based prospective studies.”
Dr. Haider receives support from the Henry M. Jackson Foundation of the Department of Defense, the Orthopaedic Research and Education Foundation, and the National Institutes of Health, and nonfinancial research support the Centers for Medicare and Medicaid Services Office of Minority Health. Dr. Bilimoria was the president of the Surgical Outcomes Club from 2016 to 2017.