User login
Official Newspaper of the American College of Surgeons
Don’t push women into preterm delivery after myomectomy
LAS VEGAS –
The American College of Obstetricians and Gynecologists lists prior myomectomy as a medically-indicated reason for delivery before 39 weeks. The advice reflects a traditional concern that uterine scars will rupture during labor, with potentially devastating consequences for both mother and infant.
Reviews have put the risk at less than 1%, so ob.gyns. have shied away from ACOG’s blanket advice and now use uterine-cavity entry during myomectomy as their talisman for deciding whether or not to offer women vaginal delivery. The assumption is that uterine entry makes rupture more likely, but there’s not much evidence to support that idea, and it’s become clear in recent years that women who have a significant full-thickness insult to uterine integrity – a prior C-section – can usually deliver vaginally with no problem. In short, the uterus seems to have a remarkable ability to heal itself.
Even so, there are still ob.gyns. who pressure women into having premature babies if they’ve had a fibroid removed even without cavity entry. Barring additional indications, that doesn’t happen anymore at Northwestern University, said lead investigator Nathan King, MD, an ob.gyn. resident at the university.
The Northwestern team wanted to clear the fog. What they found adds to “literature that demonstrates the overall low risk of undergoing VTOL [vaginal trial of labor] after a prior myomectomy. We hope providers will feel more comfortable talking to their patients about delivery [options] and the success of VTOL after myomectomy,” Dr. King said at a meeting sponsored by AAGL.*
He and his team analyzed pregnancy outcomes in 112 women who had a live birth after non–cavity-entering myomectomies. Forty-nine women (44%) were allowed to undergo VTOL; 63 others had C-sections, most at term.
Thirty-two VTOL women (65%) had vaginal deliveries, a success rate similar to that of labor after C-section. There was just one uterine rupture in the VTOL group, for an incidence of 2%, which also was comparable to the rupture risk after a low-transverse C-section.
The rupture was discovered after spontaneous vaginal delivery, and an addressed by laparotomy. Both mother and infant were fine.
Adverse events were less likely in the VTOL group, regardless if they ultimately delivered vaginally or by C-section. The lower adverse event rate was driven by fewer postpartum hemorrhages (odds ratio, 0.441, 95% confidence interval, 0.2002-0.9722, P = .042).
There were no demographic difference between women who were allowed to undergo VTOL and those who were not. For most, it was their first delivery.
Women who had their uterine cavities entered during myomectomy weren’t allowed to undergo VTOL at Northwestern, and were not included in the analysis. Also, the study did not include women who became pregnant after myomectomy, but did not have a live delivery. The incidence of uterine rupture among them, if any, was not reported.
There was no external funding for the work, and Dr. King didn’t have any disclosures.
SOURCE: King N et al. 2018 AAGL Global Congress, Abstract 162.
*Correction, 12/11/2018: An earlier version of this story misstated the name of the meeting sponsor. It is AAGL.
LAS VEGAS –
The American College of Obstetricians and Gynecologists lists prior myomectomy as a medically-indicated reason for delivery before 39 weeks. The advice reflects a traditional concern that uterine scars will rupture during labor, with potentially devastating consequences for both mother and infant.
Reviews have put the risk at less than 1%, so ob.gyns. have shied away from ACOG’s blanket advice and now use uterine-cavity entry during myomectomy as their talisman for deciding whether or not to offer women vaginal delivery. The assumption is that uterine entry makes rupture more likely, but there’s not much evidence to support that idea, and it’s become clear in recent years that women who have a significant full-thickness insult to uterine integrity – a prior C-section – can usually deliver vaginally with no problem. In short, the uterus seems to have a remarkable ability to heal itself.
Even so, there are still ob.gyns. who pressure women into having premature babies if they’ve had a fibroid removed even without cavity entry. Barring additional indications, that doesn’t happen anymore at Northwestern University, said lead investigator Nathan King, MD, an ob.gyn. resident at the university.
The Northwestern team wanted to clear the fog. What they found adds to “literature that demonstrates the overall low risk of undergoing VTOL [vaginal trial of labor] after a prior myomectomy. We hope providers will feel more comfortable talking to their patients about delivery [options] and the success of VTOL after myomectomy,” Dr. King said at a meeting sponsored by AAGL.*
He and his team analyzed pregnancy outcomes in 112 women who had a live birth after non–cavity-entering myomectomies. Forty-nine women (44%) were allowed to undergo VTOL; 63 others had C-sections, most at term.
Thirty-two VTOL women (65%) had vaginal deliveries, a success rate similar to that of labor after C-section. There was just one uterine rupture in the VTOL group, for an incidence of 2%, which also was comparable to the rupture risk after a low-transverse C-section.
The rupture was discovered after spontaneous vaginal delivery, and an addressed by laparotomy. Both mother and infant were fine.
Adverse events were less likely in the VTOL group, regardless if they ultimately delivered vaginally or by C-section. The lower adverse event rate was driven by fewer postpartum hemorrhages (odds ratio, 0.441, 95% confidence interval, 0.2002-0.9722, P = .042).
There were no demographic difference between women who were allowed to undergo VTOL and those who were not. For most, it was their first delivery.
Women who had their uterine cavities entered during myomectomy weren’t allowed to undergo VTOL at Northwestern, and were not included in the analysis. Also, the study did not include women who became pregnant after myomectomy, but did not have a live delivery. The incidence of uterine rupture among them, if any, was not reported.
There was no external funding for the work, and Dr. King didn’t have any disclosures.
SOURCE: King N et al. 2018 AAGL Global Congress, Abstract 162.
*Correction, 12/11/2018: An earlier version of this story misstated the name of the meeting sponsor. It is AAGL.
LAS VEGAS –
The American College of Obstetricians and Gynecologists lists prior myomectomy as a medically-indicated reason for delivery before 39 weeks. The advice reflects a traditional concern that uterine scars will rupture during labor, with potentially devastating consequences for both mother and infant.
Reviews have put the risk at less than 1%, so ob.gyns. have shied away from ACOG’s blanket advice and now use uterine-cavity entry during myomectomy as their talisman for deciding whether or not to offer women vaginal delivery. The assumption is that uterine entry makes rupture more likely, but there’s not much evidence to support that idea, and it’s become clear in recent years that women who have a significant full-thickness insult to uterine integrity – a prior C-section – can usually deliver vaginally with no problem. In short, the uterus seems to have a remarkable ability to heal itself.
Even so, there are still ob.gyns. who pressure women into having premature babies if they’ve had a fibroid removed even without cavity entry. Barring additional indications, that doesn’t happen anymore at Northwestern University, said lead investigator Nathan King, MD, an ob.gyn. resident at the university.
The Northwestern team wanted to clear the fog. What they found adds to “literature that demonstrates the overall low risk of undergoing VTOL [vaginal trial of labor] after a prior myomectomy. We hope providers will feel more comfortable talking to their patients about delivery [options] and the success of VTOL after myomectomy,” Dr. King said at a meeting sponsored by AAGL.*
He and his team analyzed pregnancy outcomes in 112 women who had a live birth after non–cavity-entering myomectomies. Forty-nine women (44%) were allowed to undergo VTOL; 63 others had C-sections, most at term.
Thirty-two VTOL women (65%) had vaginal deliveries, a success rate similar to that of labor after C-section. There was just one uterine rupture in the VTOL group, for an incidence of 2%, which also was comparable to the rupture risk after a low-transverse C-section.
The rupture was discovered after spontaneous vaginal delivery, and an addressed by laparotomy. Both mother and infant were fine.
Adverse events were less likely in the VTOL group, regardless if they ultimately delivered vaginally or by C-section. The lower adverse event rate was driven by fewer postpartum hemorrhages (odds ratio, 0.441, 95% confidence interval, 0.2002-0.9722, P = .042).
There were no demographic difference between women who were allowed to undergo VTOL and those who were not. For most, it was their first delivery.
Women who had their uterine cavities entered during myomectomy weren’t allowed to undergo VTOL at Northwestern, and were not included in the analysis. Also, the study did not include women who became pregnant after myomectomy, but did not have a live delivery. The incidence of uterine rupture among them, if any, was not reported.
There was no external funding for the work, and Dr. King didn’t have any disclosures.
SOURCE: King N et al. 2018 AAGL Global Congress, Abstract 162.
*Correction, 12/11/2018: An earlier version of this story misstated the name of the meeting sponsor. It is AAGL.
REPORTING FROM AAGL GLOBAL CONGRESS
Key clinical point: Vaginal trial of labor is safe after myomectomy, at least if the uterine cavity wasn’t entered.
Major finding: Sixty-five percent of women who didn’t have their uterine cavities entered had vaginal deliveries, a success rate similar to labor after C-section.
Study details: Review of 102 pregnancies with live births after myomectomy at Northwestern University, Chicago
Disclosures: There was no external funding, and the lead investigator didn’t have any disclosures.
Source: King N et al. 2018 AAGL Global Congress, Abstract 162.
In Medicare population, carotid revascularization has declined
NEW YORK – The rates of carotid artery revascularization with either endarterectomy or stenting declined precipitously over a recent 15-year period, at least among Medicare fee-for-service beneficiaries, according to data presented at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation.
A reduction in carotid endarterectomies (CEA) largely accounted for the decline during 1999-2014 although there was a cumulative decline in all carotid revascularization procedures when rates of CEA and stenting were combined, according to Brajesh K. Lal, MD, professor of surgery, University of Maryland Medical System, Baltimore.
In 1999, when enthusiasm for CEA appears to have peaked, 81,306 patients received this procedure, but a steady decline was observed until 2014, when 36,325 patients were being treated annually in the Medicare database. When calculated as endarterectomies per 100,000 beneficiaries, the rate declined from 298 to 128 (57%; P less than .001) over this 15-year period.
The number of stenting procedures had not reached its peak in 1999, when 10,416 were performed. Rather, the number performed annually nearly doubled to, 22.865 by 2006. However, it then began to decline and reached 10,208 by 2014, which was slightly fewer than in 1999, according to Dr. Lal.
These trends have been observed even though outcomes are getting better, at least for CEA, according to Dr. Lal. From the same pool of data, there was a 31% (1.1% vs. 1.6%) reduction from 1999 to 2014 in mortality at 30 days following CEA. For a composite of ischemic stroke and all-cause mortality, the rate fell 29.5% (3.1% vs. 4.4%). Both reductions were called statistically significant by Dr. Lal.
The improvements in CEA outcomes were observed even though “the treated patients got sicker when looking at comorbidities and risk factors, particularly hypertension, renal insufficiency, and diabetes,” Dr. Lal said.
Outcomes also improved among patients undergoing carotid stenting in general, although the patterns were described as “more complex.” In general, there was steady improvement on outcomes during 1999-2006, but there was no further gain and some lost ground during 2006-2014. For example, ischemic stroke or death fell from 7.0% in 1999 to 4.8% in 2006, but it had climbed back to 7.0% by 2014 with no net change when the first and last year were compared.
However, with risk adjustment, there was a reduction in in-hospital mortality (1.13% vs. 2.78%) over the study period for patients undergoing carotid stenting, according to Dr. Lal, who said this reached statistical significance. Like the CEA group, there was more comorbidity among those treated with stenting at the end, relative to the early part of the study period.
In the stenting group, patients with symptomatic carotid disease rose from 14.4% in 1999 to 25.9% in 2014. This tracks with Medicare policy, which required patients after 2005 to have symptomatic disease for reimbursement, according to Dr. Lal. Prior to 2005, reimbursement was granted for patients participating in clinical trials only.
The rates of carotid revascularization are not evenly distributed geographically in the United States, according to the Medicare data. Endarterectomy in particular has been more common in the south and Midwest than on either coast. This was true in 1999 and remained so in 2014. The distribution was similar for stenting, although it was also relatively common in the southwest in the early part of the study period.
In the beginning of the study, the increased rate of stenting might have contributed to the decline in endarterectomy, but there are several other factors that are implicated in the observed trends, according to Dr. Lal. He suggested that decreasing reimbursement for the performance of these procedures, better clinical management of risk factors, and advances in medical therapy. He cited a physician survey that showed a growing preference for medical management over invasive procedures in patients with high-grade stenosis and indicated that this last factor might be a particularly important driver of the decline in revascularization referrals for asymptomatic carotid disease.
The degree to which these Medicare data are representative of overall trends in the United States is unclear, but Dr. Lal called for further work to understand the forces that these data suggest are driving the changing patterns of carotid revascularization.
NEW YORK – The rates of carotid artery revascularization with either endarterectomy or stenting declined precipitously over a recent 15-year period, at least among Medicare fee-for-service beneficiaries, according to data presented at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation.
A reduction in carotid endarterectomies (CEA) largely accounted for the decline during 1999-2014 although there was a cumulative decline in all carotid revascularization procedures when rates of CEA and stenting were combined, according to Brajesh K. Lal, MD, professor of surgery, University of Maryland Medical System, Baltimore.
In 1999, when enthusiasm for CEA appears to have peaked, 81,306 patients received this procedure, but a steady decline was observed until 2014, when 36,325 patients were being treated annually in the Medicare database. When calculated as endarterectomies per 100,000 beneficiaries, the rate declined from 298 to 128 (57%; P less than .001) over this 15-year period.
The number of stenting procedures had not reached its peak in 1999, when 10,416 were performed. Rather, the number performed annually nearly doubled to, 22.865 by 2006. However, it then began to decline and reached 10,208 by 2014, which was slightly fewer than in 1999, according to Dr. Lal.
These trends have been observed even though outcomes are getting better, at least for CEA, according to Dr. Lal. From the same pool of data, there was a 31% (1.1% vs. 1.6%) reduction from 1999 to 2014 in mortality at 30 days following CEA. For a composite of ischemic stroke and all-cause mortality, the rate fell 29.5% (3.1% vs. 4.4%). Both reductions were called statistically significant by Dr. Lal.
The improvements in CEA outcomes were observed even though “the treated patients got sicker when looking at comorbidities and risk factors, particularly hypertension, renal insufficiency, and diabetes,” Dr. Lal said.
Outcomes also improved among patients undergoing carotid stenting in general, although the patterns were described as “more complex.” In general, there was steady improvement on outcomes during 1999-2006, but there was no further gain and some lost ground during 2006-2014. For example, ischemic stroke or death fell from 7.0% in 1999 to 4.8% in 2006, but it had climbed back to 7.0% by 2014 with no net change when the first and last year were compared.
However, with risk adjustment, there was a reduction in in-hospital mortality (1.13% vs. 2.78%) over the study period for patients undergoing carotid stenting, according to Dr. Lal, who said this reached statistical significance. Like the CEA group, there was more comorbidity among those treated with stenting at the end, relative to the early part of the study period.
In the stenting group, patients with symptomatic carotid disease rose from 14.4% in 1999 to 25.9% in 2014. This tracks with Medicare policy, which required patients after 2005 to have symptomatic disease for reimbursement, according to Dr. Lal. Prior to 2005, reimbursement was granted for patients participating in clinical trials only.
The rates of carotid revascularization are not evenly distributed geographically in the United States, according to the Medicare data. Endarterectomy in particular has been more common in the south and Midwest than on either coast. This was true in 1999 and remained so in 2014. The distribution was similar for stenting, although it was also relatively common in the southwest in the early part of the study period.
In the beginning of the study, the increased rate of stenting might have contributed to the decline in endarterectomy, but there are several other factors that are implicated in the observed trends, according to Dr. Lal. He suggested that decreasing reimbursement for the performance of these procedures, better clinical management of risk factors, and advances in medical therapy. He cited a physician survey that showed a growing preference for medical management over invasive procedures in patients with high-grade stenosis and indicated that this last factor might be a particularly important driver of the decline in revascularization referrals for asymptomatic carotid disease.
The degree to which these Medicare data are representative of overall trends in the United States is unclear, but Dr. Lal called for further work to understand the forces that these data suggest are driving the changing patterns of carotid revascularization.
NEW YORK – The rates of carotid artery revascularization with either endarterectomy or stenting declined precipitously over a recent 15-year period, at least among Medicare fee-for-service beneficiaries, according to data presented at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation.
A reduction in carotid endarterectomies (CEA) largely accounted for the decline during 1999-2014 although there was a cumulative decline in all carotid revascularization procedures when rates of CEA and stenting were combined, according to Brajesh K. Lal, MD, professor of surgery, University of Maryland Medical System, Baltimore.
In 1999, when enthusiasm for CEA appears to have peaked, 81,306 patients received this procedure, but a steady decline was observed until 2014, when 36,325 patients were being treated annually in the Medicare database. When calculated as endarterectomies per 100,000 beneficiaries, the rate declined from 298 to 128 (57%; P less than .001) over this 15-year period.
The number of stenting procedures had not reached its peak in 1999, when 10,416 were performed. Rather, the number performed annually nearly doubled to, 22.865 by 2006. However, it then began to decline and reached 10,208 by 2014, which was slightly fewer than in 1999, according to Dr. Lal.
These trends have been observed even though outcomes are getting better, at least for CEA, according to Dr. Lal. From the same pool of data, there was a 31% (1.1% vs. 1.6%) reduction from 1999 to 2014 in mortality at 30 days following CEA. For a composite of ischemic stroke and all-cause mortality, the rate fell 29.5% (3.1% vs. 4.4%). Both reductions were called statistically significant by Dr. Lal.
The improvements in CEA outcomes were observed even though “the treated patients got sicker when looking at comorbidities and risk factors, particularly hypertension, renal insufficiency, and diabetes,” Dr. Lal said.
Outcomes also improved among patients undergoing carotid stenting in general, although the patterns were described as “more complex.” In general, there was steady improvement on outcomes during 1999-2006, but there was no further gain and some lost ground during 2006-2014. For example, ischemic stroke or death fell from 7.0% in 1999 to 4.8% in 2006, but it had climbed back to 7.0% by 2014 with no net change when the first and last year were compared.
However, with risk adjustment, there was a reduction in in-hospital mortality (1.13% vs. 2.78%) over the study period for patients undergoing carotid stenting, according to Dr. Lal, who said this reached statistical significance. Like the CEA group, there was more comorbidity among those treated with stenting at the end, relative to the early part of the study period.
In the stenting group, patients with symptomatic carotid disease rose from 14.4% in 1999 to 25.9% in 2014. This tracks with Medicare policy, which required patients after 2005 to have symptomatic disease for reimbursement, according to Dr. Lal. Prior to 2005, reimbursement was granted for patients participating in clinical trials only.
The rates of carotid revascularization are not evenly distributed geographically in the United States, according to the Medicare data. Endarterectomy in particular has been more common in the south and Midwest than on either coast. This was true in 1999 and remained so in 2014. The distribution was similar for stenting, although it was also relatively common in the southwest in the early part of the study period.
In the beginning of the study, the increased rate of stenting might have contributed to the decline in endarterectomy, but there are several other factors that are implicated in the observed trends, according to Dr. Lal. He suggested that decreasing reimbursement for the performance of these procedures, better clinical management of risk factors, and advances in medical therapy. He cited a physician survey that showed a growing preference for medical management over invasive procedures in patients with high-grade stenosis and indicated that this last factor might be a particularly important driver of the decline in revascularization referrals for asymptomatic carotid disease.
The degree to which these Medicare data are representative of overall trends in the United States is unclear, but Dr. Lal called for further work to understand the forces that these data suggest are driving the changing patterns of carotid revascularization.
REPORTING FROM VEITHSYMPOSIUM
Key clinical point:
Major finding: During 1999-2014, the rate of carotid endarterectomy per 100,000 beneficiaries fell from 291 to 128 (57%; P less than .001).
Study details: Retrospective database review.
Disclosures: Dr. Lal reported having no financial conflicts relevant to the study.
Redo carotid endarterectomy is more risky than previously estimated
NEW YORK – It is well known that reoperative carotid endarterectomy can be technically challenging because of the scarring left from the initial procedure, but an analysis of a large database presented at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation also revealed that the risk of complications, particularly stroke, is greater.
When “redo” carotid endarterectomies were compared with the index primary procedure collected in the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) database, the odds ratio for stroke was several times greater (odds ratio, 3.71; P = .002) on univariate analysis, reported Jeffrey J. Siracuse, MD, associate professor of surgery and radiology at Boston University.
Previous single-center reports of redo endarterectomies “showed terrific results, really no perioperative stroke or morbidity, but this is older data from a different era,” said Dr. Siracuse, who undertook this study to determine whether “real-world” data would tell a different story.
In this study, 75,943 primary carotid endarterectomies and 140 redo procedures were identified in the ACS NSQIP database and compared. The redo population had a significantly higher incidence of end-stage renal disease (3.6% vs. 1.1%; P = .004), but history of stroke, whether with deficit (20.8% vs. 15.4%) or without (11.5% vs. 9.1%), was numerically higher among those undergoing a primary procedure even though these differences did not reach statistical significance. Baseline demographics and comorbidities were otherwise similar.
Presumably because of the difficulty of recanalizing scarred tissue, the mean procedure time for redos was longer than that for the primary procedures (137 vs. 49 minutes; P less than .001), but there were no significant differences in the rate of surgical site infections (0.7% vs. 0.3%; P = .482), return to the operating room (3.6% vs. 4%; P = .853), or 30-day readmissions (2.1% vs. 6.9%; P = .810) for the redo and index procedures, respectively.
Although perioperative MI rates were higher in the redo group (2.1%) than in the primary endarterectomy group (0.9%), this difference did not reach statistical significance (P = .125). However, a multivariate analysis associated redo carotid endarterectomy procedures with a nearly threefold increase in risk of a composite of major adverse cardiovascular events when compared on a multivariate analysis (OR, 2.76; P = .007), Dr. Siracuse reported.
For the surgeons considering a redo carotid endarterectomy, these data “inform a risk-benefit analysis,” according to Dr. Siracuse, but he also said that redo procedures still should be considered a viable strategy when considered in the context of other options.
Presenting a case he performed just prior to the VEITHsymposium, Dr. Siracuse displayed CT images that showed internal and common carotids with more than 75% stenosis in an 80-year-old women 7 years after a primary carotid endarterectomy. The tight stenoses and the evidence of substantial intra-arterial debris were concerns, but a decision to perform a redo endarterectomy was reached after other options, including stenting, were considered.
“She did great. She went home and has had no more symptoms,” Dr. Siracuse reported. “The point is you still have to take these [potential redo endarterectomies] on a case-by case basis.”
Dr. Siracuse reported he had no financial relationships relevant to this study.
NEW YORK – It is well known that reoperative carotid endarterectomy can be technically challenging because of the scarring left from the initial procedure, but an analysis of a large database presented at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation also revealed that the risk of complications, particularly stroke, is greater.
When “redo” carotid endarterectomies were compared with the index primary procedure collected in the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) database, the odds ratio for stroke was several times greater (odds ratio, 3.71; P = .002) on univariate analysis, reported Jeffrey J. Siracuse, MD, associate professor of surgery and radiology at Boston University.
Previous single-center reports of redo endarterectomies “showed terrific results, really no perioperative stroke or morbidity, but this is older data from a different era,” said Dr. Siracuse, who undertook this study to determine whether “real-world” data would tell a different story.
In this study, 75,943 primary carotid endarterectomies and 140 redo procedures were identified in the ACS NSQIP database and compared. The redo population had a significantly higher incidence of end-stage renal disease (3.6% vs. 1.1%; P = .004), but history of stroke, whether with deficit (20.8% vs. 15.4%) or without (11.5% vs. 9.1%), was numerically higher among those undergoing a primary procedure even though these differences did not reach statistical significance. Baseline demographics and comorbidities were otherwise similar.
Presumably because of the difficulty of recanalizing scarred tissue, the mean procedure time for redos was longer than that for the primary procedures (137 vs. 49 minutes; P less than .001), but there were no significant differences in the rate of surgical site infections (0.7% vs. 0.3%; P = .482), return to the operating room (3.6% vs. 4%; P = .853), or 30-day readmissions (2.1% vs. 6.9%; P = .810) for the redo and index procedures, respectively.
Although perioperative MI rates were higher in the redo group (2.1%) than in the primary endarterectomy group (0.9%), this difference did not reach statistical significance (P = .125). However, a multivariate analysis associated redo carotid endarterectomy procedures with a nearly threefold increase in risk of a composite of major adverse cardiovascular events when compared on a multivariate analysis (OR, 2.76; P = .007), Dr. Siracuse reported.
For the surgeons considering a redo carotid endarterectomy, these data “inform a risk-benefit analysis,” according to Dr. Siracuse, but he also said that redo procedures still should be considered a viable strategy when considered in the context of other options.
Presenting a case he performed just prior to the VEITHsymposium, Dr. Siracuse displayed CT images that showed internal and common carotids with more than 75% stenosis in an 80-year-old women 7 years after a primary carotid endarterectomy. The tight stenoses and the evidence of substantial intra-arterial debris were concerns, but a decision to perform a redo endarterectomy was reached after other options, including stenting, were considered.
“She did great. She went home and has had no more symptoms,” Dr. Siracuse reported. “The point is you still have to take these [potential redo endarterectomies] on a case-by case basis.”
Dr. Siracuse reported he had no financial relationships relevant to this study.
NEW YORK – It is well known that reoperative carotid endarterectomy can be technically challenging because of the scarring left from the initial procedure, but an analysis of a large database presented at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation also revealed that the risk of complications, particularly stroke, is greater.
When “redo” carotid endarterectomies were compared with the index primary procedure collected in the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) database, the odds ratio for stroke was several times greater (odds ratio, 3.71; P = .002) on univariate analysis, reported Jeffrey J. Siracuse, MD, associate professor of surgery and radiology at Boston University.
Previous single-center reports of redo endarterectomies “showed terrific results, really no perioperative stroke or morbidity, but this is older data from a different era,” said Dr. Siracuse, who undertook this study to determine whether “real-world” data would tell a different story.
In this study, 75,943 primary carotid endarterectomies and 140 redo procedures were identified in the ACS NSQIP database and compared. The redo population had a significantly higher incidence of end-stage renal disease (3.6% vs. 1.1%; P = .004), but history of stroke, whether with deficit (20.8% vs. 15.4%) or without (11.5% vs. 9.1%), was numerically higher among those undergoing a primary procedure even though these differences did not reach statistical significance. Baseline demographics and comorbidities were otherwise similar.
Presumably because of the difficulty of recanalizing scarred tissue, the mean procedure time for redos was longer than that for the primary procedures (137 vs. 49 minutes; P less than .001), but there were no significant differences in the rate of surgical site infections (0.7% vs. 0.3%; P = .482), return to the operating room (3.6% vs. 4%; P = .853), or 30-day readmissions (2.1% vs. 6.9%; P = .810) for the redo and index procedures, respectively.
Although perioperative MI rates were higher in the redo group (2.1%) than in the primary endarterectomy group (0.9%), this difference did not reach statistical significance (P = .125). However, a multivariate analysis associated redo carotid endarterectomy procedures with a nearly threefold increase in risk of a composite of major adverse cardiovascular events when compared on a multivariate analysis (OR, 2.76; P = .007), Dr. Siracuse reported.
For the surgeons considering a redo carotid endarterectomy, these data “inform a risk-benefit analysis,” according to Dr. Siracuse, but he also said that redo procedures still should be considered a viable strategy when considered in the context of other options.
Presenting a case he performed just prior to the VEITHsymposium, Dr. Siracuse displayed CT images that showed internal and common carotids with more than 75% stenosis in an 80-year-old women 7 years after a primary carotid endarterectomy. The tight stenoses and the evidence of substantial intra-arterial debris were concerns, but a decision to perform a redo endarterectomy was reached after other options, including stenting, were considered.
“She did great. She went home and has had no more symptoms,” Dr. Siracuse reported. “The point is you still have to take these [potential redo endarterectomies] on a case-by case basis.”
Dr. Siracuse reported he had no financial relationships relevant to this study.
REPORTING FROM VEITHSYMPOSIUM
Key clinical point:
Major finding: The odds ratio for stroke is 3.71 times higher (P = .002) with redo than with primary carotid endarterectomy.
Study details: Multivariate retrospective database analysis.
Disclosures: Dr. Siracuse reported he had no financial relationships relevant to this study.
Fewer insured may have helped slow health spending growth in 2017
Health care spending as a percentage of gross domestic product remained relatively stable in 2017, despite a slowdown in the growth of spending.
Total health care spending in the United States was $3.5 trillion in 2017, an increase of 3.9% from 2016, according to data released Dec. 6 by the Centers for Medicare & Medicaid Services.
The growth rate was down from that of 2016 (4.8%) but similar to growth rates experienced during 2008-2013, according to the research article in Health Affairs.
“The slower growth in health care spending in 2017 resulted primarily from slower growth in hospital care, physician and clinical services, and retail prescription drugs, with residual use and intensity of these goods and services contributing substantially to the trend,” Anne B. Martin, an economist in the CMS Office of the Actuary’s National Health Statistics Group, and her colleagues wrote.
The report notes that slower growth in the use and intensity of health care goods and services in 2017 “may have been affected by slower growth in overall health insurance enrollment, as the insured share of the population fell from 91.1% in 2016 to 90.9% in 2017.”
Spending on hospital care increased 4.6% to $1.1 trillion in 2017 and accounted for 33% of total health care spending; however, growth was slower than in the previous year (5.6%). Ms. Martin and her colleagues noted that growth in outpatient visits slowed while growth in inpatient days increased at about the same rate and prices in hospital care grew in 2017 to 1.7% from 1.2% in the previous year.
Spending on physician and clinical services grew 4.2% in 2017 to $694.3 billion and accounted for 20% of total health care spending. The growth rate is down from the previous year (5.6%) and a recent peak of 6% in 2015.
“Although spending growth for both physician services and clinical services slowed in 2017, the growth rate for the latter (5.0%) continued to out pace the rate for the former (3.9%), as spending for most types of outpatient care centers contributed to the stronger growth in spending for clinical services,” Ms. Martin and her colleagues reported.
They attributed the slowdown to non-price factors, such as slower growth in the use and intensity of physician and clinical services, although price growth for physician and clinical services increased 0.4% in 2017, up from 0.2% in 2016.
Spending on retail prescription drugs grew 0.4% in 2017 to $333.4 billion and accounted for 10% of total national health spending. It is the slowest growth rate increase since 2012, a year that saw a number of blockbuster drugs lose patent protection. This was down from a growth rate of 2.3% in 2016 and down from recent rates of 12.4% in 2014 and 8.9% in 2015.
“Slower growth in non-price factors, such as the use and mix of retail prescription drugs – and, to a lesser extent, in retail prescription drug prices – contributed to the slower overall growth in retail prescription drug spending in 2017,” according to the authors. Key factors included slower growth in the number of prescriptions dispensed, the continued shift to lower-cost generics, and slower growth in the volume of high-cost drugs, particularly those used to treat hepatitis C. Price decreases in generics and lower increases for existing brand-name drugs also contributed to the lower spending growth in 2017.
Ms. Martin and her colleagues highlighted the slower growth rate in the number of prescriptions (1.8% in 2017, down from 2.3% in 2016) “resulted in large part from a decline in the number of prescriptions dispensed for drugs used to treat pain.”
Medicare spending, which represents 20% of all national health care spending in 2017 ($705.9 billion), grew 4.2%, a slight decline from the 4.3% growth in 2016. Enrollment growth slowed slightly to 2.5% in 2017 from 2.7% in the previous year, while in the same time frame, per-enrollee expenditures increased slightly to 1.7% from 1.6%. Slower growth in fee-for-service Medicare spending was offset by faster growth in spending by Medicare private health plans.
Medicaid spending reached $581.9 billion (17% of national health care spending), and the growth rate slowed for the third straight year, increasing 2.9% in 2017 versus 4.2% in 2016. The slower growth “was influenced by a deceleration in enrollment growth and a reduction in the Medicaid net cost of health insurance as the federal government recovered payments from managed care organizations based on their favorable prior-period experience,” the authors stated. Enrollment growth has been decelerating following a peak of growth of 11.9% in 2014 because of states that elected to expand Medicaid eligibility, which was followed by 3 years of slower growth rates of 4.9%, 3.0% and 2.0% in 2015, 2016, and 2017, respectively. Per-enrollee spending also slowed to 0.9% growth in 2017 from a rate of 1.2% in 2016, attributed to “the decline in government administration and the net cost of insurance.”
SOURCE: Martin A et al. Health Aff. 2018. doi: 10.1377/hlthaff.2018.05085.
Health care spending as a percentage of gross domestic product remained relatively stable in 2017, despite a slowdown in the growth of spending.
Total health care spending in the United States was $3.5 trillion in 2017, an increase of 3.9% from 2016, according to data released Dec. 6 by the Centers for Medicare & Medicaid Services.
The growth rate was down from that of 2016 (4.8%) but similar to growth rates experienced during 2008-2013, according to the research article in Health Affairs.
“The slower growth in health care spending in 2017 resulted primarily from slower growth in hospital care, physician and clinical services, and retail prescription drugs, with residual use and intensity of these goods and services contributing substantially to the trend,” Anne B. Martin, an economist in the CMS Office of the Actuary’s National Health Statistics Group, and her colleagues wrote.
The report notes that slower growth in the use and intensity of health care goods and services in 2017 “may have been affected by slower growth in overall health insurance enrollment, as the insured share of the population fell from 91.1% in 2016 to 90.9% in 2017.”
Spending on hospital care increased 4.6% to $1.1 trillion in 2017 and accounted for 33% of total health care spending; however, growth was slower than in the previous year (5.6%). Ms. Martin and her colleagues noted that growth in outpatient visits slowed while growth in inpatient days increased at about the same rate and prices in hospital care grew in 2017 to 1.7% from 1.2% in the previous year.
Spending on physician and clinical services grew 4.2% in 2017 to $694.3 billion and accounted for 20% of total health care spending. The growth rate is down from the previous year (5.6%) and a recent peak of 6% in 2015.
“Although spending growth for both physician services and clinical services slowed in 2017, the growth rate for the latter (5.0%) continued to out pace the rate for the former (3.9%), as spending for most types of outpatient care centers contributed to the stronger growth in spending for clinical services,” Ms. Martin and her colleagues reported.
They attributed the slowdown to non-price factors, such as slower growth in the use and intensity of physician and clinical services, although price growth for physician and clinical services increased 0.4% in 2017, up from 0.2% in 2016.
Spending on retail prescription drugs grew 0.4% in 2017 to $333.4 billion and accounted for 10% of total national health spending. It is the slowest growth rate increase since 2012, a year that saw a number of blockbuster drugs lose patent protection. This was down from a growth rate of 2.3% in 2016 and down from recent rates of 12.4% in 2014 and 8.9% in 2015.
“Slower growth in non-price factors, such as the use and mix of retail prescription drugs – and, to a lesser extent, in retail prescription drug prices – contributed to the slower overall growth in retail prescription drug spending in 2017,” according to the authors. Key factors included slower growth in the number of prescriptions dispensed, the continued shift to lower-cost generics, and slower growth in the volume of high-cost drugs, particularly those used to treat hepatitis C. Price decreases in generics and lower increases for existing brand-name drugs also contributed to the lower spending growth in 2017.
Ms. Martin and her colleagues highlighted the slower growth rate in the number of prescriptions (1.8% in 2017, down from 2.3% in 2016) “resulted in large part from a decline in the number of prescriptions dispensed for drugs used to treat pain.”
Medicare spending, which represents 20% of all national health care spending in 2017 ($705.9 billion), grew 4.2%, a slight decline from the 4.3% growth in 2016. Enrollment growth slowed slightly to 2.5% in 2017 from 2.7% in the previous year, while in the same time frame, per-enrollee expenditures increased slightly to 1.7% from 1.6%. Slower growth in fee-for-service Medicare spending was offset by faster growth in spending by Medicare private health plans.
Medicaid spending reached $581.9 billion (17% of national health care spending), and the growth rate slowed for the third straight year, increasing 2.9% in 2017 versus 4.2% in 2016. The slower growth “was influenced by a deceleration in enrollment growth and a reduction in the Medicaid net cost of health insurance as the federal government recovered payments from managed care organizations based on their favorable prior-period experience,” the authors stated. Enrollment growth has been decelerating following a peak of growth of 11.9% in 2014 because of states that elected to expand Medicaid eligibility, which was followed by 3 years of slower growth rates of 4.9%, 3.0% and 2.0% in 2015, 2016, and 2017, respectively. Per-enrollee spending also slowed to 0.9% growth in 2017 from a rate of 1.2% in 2016, attributed to “the decline in government administration and the net cost of insurance.”
SOURCE: Martin A et al. Health Aff. 2018. doi: 10.1377/hlthaff.2018.05085.
Health care spending as a percentage of gross domestic product remained relatively stable in 2017, despite a slowdown in the growth of spending.
Total health care spending in the United States was $3.5 trillion in 2017, an increase of 3.9% from 2016, according to data released Dec. 6 by the Centers for Medicare & Medicaid Services.
The growth rate was down from that of 2016 (4.8%) but similar to growth rates experienced during 2008-2013, according to the research article in Health Affairs.
“The slower growth in health care spending in 2017 resulted primarily from slower growth in hospital care, physician and clinical services, and retail prescription drugs, with residual use and intensity of these goods and services contributing substantially to the trend,” Anne B. Martin, an economist in the CMS Office of the Actuary’s National Health Statistics Group, and her colleagues wrote.
The report notes that slower growth in the use and intensity of health care goods and services in 2017 “may have been affected by slower growth in overall health insurance enrollment, as the insured share of the population fell from 91.1% in 2016 to 90.9% in 2017.”
Spending on hospital care increased 4.6% to $1.1 trillion in 2017 and accounted for 33% of total health care spending; however, growth was slower than in the previous year (5.6%). Ms. Martin and her colleagues noted that growth in outpatient visits slowed while growth in inpatient days increased at about the same rate and prices in hospital care grew in 2017 to 1.7% from 1.2% in the previous year.
Spending on physician and clinical services grew 4.2% in 2017 to $694.3 billion and accounted for 20% of total health care spending. The growth rate is down from the previous year (5.6%) and a recent peak of 6% in 2015.
“Although spending growth for both physician services and clinical services slowed in 2017, the growth rate for the latter (5.0%) continued to out pace the rate for the former (3.9%), as spending for most types of outpatient care centers contributed to the stronger growth in spending for clinical services,” Ms. Martin and her colleagues reported.
They attributed the slowdown to non-price factors, such as slower growth in the use and intensity of physician and clinical services, although price growth for physician and clinical services increased 0.4% in 2017, up from 0.2% in 2016.
Spending on retail prescription drugs grew 0.4% in 2017 to $333.4 billion and accounted for 10% of total national health spending. It is the slowest growth rate increase since 2012, a year that saw a number of blockbuster drugs lose patent protection. This was down from a growth rate of 2.3% in 2016 and down from recent rates of 12.4% in 2014 and 8.9% in 2015.
“Slower growth in non-price factors, such as the use and mix of retail prescription drugs – and, to a lesser extent, in retail prescription drug prices – contributed to the slower overall growth in retail prescription drug spending in 2017,” according to the authors. Key factors included slower growth in the number of prescriptions dispensed, the continued shift to lower-cost generics, and slower growth in the volume of high-cost drugs, particularly those used to treat hepatitis C. Price decreases in generics and lower increases for existing brand-name drugs also contributed to the lower spending growth in 2017.
Ms. Martin and her colleagues highlighted the slower growth rate in the number of prescriptions (1.8% in 2017, down from 2.3% in 2016) “resulted in large part from a decline in the number of prescriptions dispensed for drugs used to treat pain.”
Medicare spending, which represents 20% of all national health care spending in 2017 ($705.9 billion), grew 4.2%, a slight decline from the 4.3% growth in 2016. Enrollment growth slowed slightly to 2.5% in 2017 from 2.7% in the previous year, while in the same time frame, per-enrollee expenditures increased slightly to 1.7% from 1.6%. Slower growth in fee-for-service Medicare spending was offset by faster growth in spending by Medicare private health plans.
Medicaid spending reached $581.9 billion (17% of national health care spending), and the growth rate slowed for the third straight year, increasing 2.9% in 2017 versus 4.2% in 2016. The slower growth “was influenced by a deceleration in enrollment growth and a reduction in the Medicaid net cost of health insurance as the federal government recovered payments from managed care organizations based on their favorable prior-period experience,” the authors stated. Enrollment growth has been decelerating following a peak of growth of 11.9% in 2014 because of states that elected to expand Medicaid eligibility, which was followed by 3 years of slower growth rates of 4.9%, 3.0% and 2.0% in 2015, 2016, and 2017, respectively. Per-enrollee spending also slowed to 0.9% growth in 2017 from a rate of 1.2% in 2016, attributed to “the decline in government administration and the net cost of insurance.”
SOURCE: Martin A et al. Health Aff. 2018. doi: 10.1377/hlthaff.2018.05085.
FROM HEALTH AFFAIRS
Key clinical point: National health care spending growth slowed to 3.9% in 2017.
Major finding: The $3.5 trillion in national health care spending represents 17.9% of GDP.
Study details: Annual analysis of national health expenditures conducted by federal actuaries.
Disclosures: Analysis conducted by the Centers for Medicaid & Medicare Services Office of the Actuary; the authors have no relevant financial conflicts of interest.
Source: Martin A et al. Health Affairs. 2018. doi: 10.1377/hlthaff.2018.05085.
Perioperative M&M similar for lobar, sublobar surgeries in early lung cancer
Though lobectomy is the long-held standard of care for people with early stage non–small cell lung cancer, a noninferiority study shows little difference in perioperative morbidity and mortality outcomes when sublobar resections are performed instead.
The study, published online in The Lancet Respiratory Medicine, compared results from 697 functionally and physically fit patients with stage I cancer randomized over a 10-year period to lobar resection (n = 357) or sublobar resection (n = 340). Patients were analyzed for morbidity and mortality outcomes at 30 and 90 days post surgery. Nasser K. Altorki, MD, of Weill Cornell Medicine–New York Presbyterian Hospital, led the study as a post hoc, exploratory analysis of CALGB/Alliance 140503, a multinational phase 3 trial whose primary outcome – still pending – is disease-free survival associated with the two different surgeries.
Dr. Altorki and his colleagues found 30- and 90-day survival to be comparable between surgery types. At 30 days, six patients in the study had died; four in the lobar resection group and two in the sublobar group (1.1% and 0.6%). At 90 days, 10 patients had died, or 1.4% of the cohort; 6 following lobar resection and 4 following sublobar resection. The between-group difference at 30 days was 0.5% (95% confidence interval, –1.1 to 2.3) and at 90 days remained 0.5% (95% CI, –1.5 to 2.6).
Similar rates of serious (grade 3 or worse) adverse advents were seen between surgery groups at 15% and 14%, respectively, and no differences were seen for cardiac or pulmonary complications. In the study, the type of sublobar approach was left to the surgeon’s discretion, and a majority of the sublobar procedures (59%) were found to comprise wedge resections, with the rest segmentectomies. Dr. Altorki and colleagues noted the high rate of wedge resections as striking, because “conventional wisdom … holds that an anatomical segmentectomy, involving individual ligation of segmental vessels and bronchi and wider parenchymal resection, is oncologically superior to nonanatomical wedge resections.” In their analysis the researchers conceded that a three-arm trial allocating patients to lobectomy, segmentectomy, or wedge resection “would have answered more precisely the posited research question,” but said that the sample size needed would have been too large.
The study was funded by the National Cancer Institute. Dr. Altorki reported a research grant from AstraZeneca unrelated to the study; two more coauthors disclosed funding from pharmaceutical or device manufacturers, and an additional 17 coauthors listed no competing interests.
SOURCE: Altorki NK et al. Lancet Respir Med. 2018 Nov 12. doi: 10.1016/S2213-2600(18)30411-9 .
Though lobectomy is the long-held standard of care for people with early stage non–small cell lung cancer, a noninferiority study shows little difference in perioperative morbidity and mortality outcomes when sublobar resections are performed instead.
The study, published online in The Lancet Respiratory Medicine, compared results from 697 functionally and physically fit patients with stage I cancer randomized over a 10-year period to lobar resection (n = 357) or sublobar resection (n = 340). Patients were analyzed for morbidity and mortality outcomes at 30 and 90 days post surgery. Nasser K. Altorki, MD, of Weill Cornell Medicine–New York Presbyterian Hospital, led the study as a post hoc, exploratory analysis of CALGB/Alliance 140503, a multinational phase 3 trial whose primary outcome – still pending – is disease-free survival associated with the two different surgeries.
Dr. Altorki and his colleagues found 30- and 90-day survival to be comparable between surgery types. At 30 days, six patients in the study had died; four in the lobar resection group and two in the sublobar group (1.1% and 0.6%). At 90 days, 10 patients had died, or 1.4% of the cohort; 6 following lobar resection and 4 following sublobar resection. The between-group difference at 30 days was 0.5% (95% confidence interval, –1.1 to 2.3) and at 90 days remained 0.5% (95% CI, –1.5 to 2.6).
Similar rates of serious (grade 3 or worse) adverse advents were seen between surgery groups at 15% and 14%, respectively, and no differences were seen for cardiac or pulmonary complications. In the study, the type of sublobar approach was left to the surgeon’s discretion, and a majority of the sublobar procedures (59%) were found to comprise wedge resections, with the rest segmentectomies. Dr. Altorki and colleagues noted the high rate of wedge resections as striking, because “conventional wisdom … holds that an anatomical segmentectomy, involving individual ligation of segmental vessels and bronchi and wider parenchymal resection, is oncologically superior to nonanatomical wedge resections.” In their analysis the researchers conceded that a three-arm trial allocating patients to lobectomy, segmentectomy, or wedge resection “would have answered more precisely the posited research question,” but said that the sample size needed would have been too large.
The study was funded by the National Cancer Institute. Dr. Altorki reported a research grant from AstraZeneca unrelated to the study; two more coauthors disclosed funding from pharmaceutical or device manufacturers, and an additional 17 coauthors listed no competing interests.
SOURCE: Altorki NK et al. Lancet Respir Med. 2018 Nov 12. doi: 10.1016/S2213-2600(18)30411-9 .
Though lobectomy is the long-held standard of care for people with early stage non–small cell lung cancer, a noninferiority study shows little difference in perioperative morbidity and mortality outcomes when sublobar resections are performed instead.
The study, published online in The Lancet Respiratory Medicine, compared results from 697 functionally and physically fit patients with stage I cancer randomized over a 10-year period to lobar resection (n = 357) or sublobar resection (n = 340). Patients were analyzed for morbidity and mortality outcomes at 30 and 90 days post surgery. Nasser K. Altorki, MD, of Weill Cornell Medicine–New York Presbyterian Hospital, led the study as a post hoc, exploratory analysis of CALGB/Alliance 140503, a multinational phase 3 trial whose primary outcome – still pending – is disease-free survival associated with the two different surgeries.
Dr. Altorki and his colleagues found 30- and 90-day survival to be comparable between surgery types. At 30 days, six patients in the study had died; four in the lobar resection group and two in the sublobar group (1.1% and 0.6%). At 90 days, 10 patients had died, or 1.4% of the cohort; 6 following lobar resection and 4 following sublobar resection. The between-group difference at 30 days was 0.5% (95% confidence interval, –1.1 to 2.3) and at 90 days remained 0.5% (95% CI, –1.5 to 2.6).
Similar rates of serious (grade 3 or worse) adverse advents were seen between surgery groups at 15% and 14%, respectively, and no differences were seen for cardiac or pulmonary complications. In the study, the type of sublobar approach was left to the surgeon’s discretion, and a majority of the sublobar procedures (59%) were found to comprise wedge resections, with the rest segmentectomies. Dr. Altorki and colleagues noted the high rate of wedge resections as striking, because “conventional wisdom … holds that an anatomical segmentectomy, involving individual ligation of segmental vessels and bronchi and wider parenchymal resection, is oncologically superior to nonanatomical wedge resections.” In their analysis the researchers conceded that a three-arm trial allocating patients to lobectomy, segmentectomy, or wedge resection “would have answered more precisely the posited research question,” but said that the sample size needed would have been too large.
The study was funded by the National Cancer Institute. Dr. Altorki reported a research grant from AstraZeneca unrelated to the study; two more coauthors disclosed funding from pharmaceutical or device manufacturers, and an additional 17 coauthors listed no competing interests.
SOURCE: Altorki NK et al. Lancet Respir Med. 2018 Nov 12. doi: 10.1016/S2213-2600(18)30411-9 .
FROM THE LANCET RESPIRATORY MEDICINE
Key clinical point: Patients with 30 and 90 days post surgery.
Major finding: Mortality at 30 days and 90 days was 0.5% for both trial groups and serious adverse advents were similar between groups.
Study details: A post hoc analysis from a multinational trial randomizing about 700 stage I NSCLC patients to lobar or sublobar surgery
Disclosures: National Cancer Institute sponsored the study; three authors including the lead author reported financial ties to manufacturers.
Source: Altorki et al. Lancet Respir Med. 2018 Nov 12. doi: 10.1016/S2213-2600(18)30411-9.
Action on HealthCare.gov picked up during week 5
Activity during week 5 of open enrollment on HealthCare.gov was up by more than 50% over the previous week, but the total number of plans selected for the 2019 coverage year remains lower than it was last year, according to the Centers for Medicare & Medicaid Services.
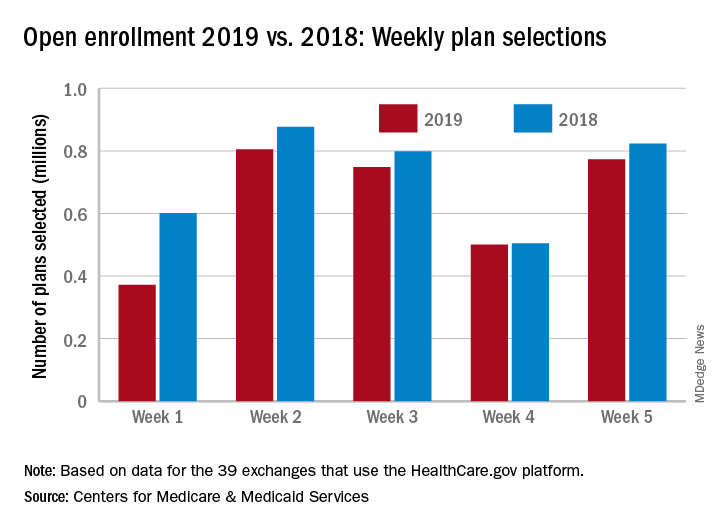
The 773,000 plans selected during week 5 (Nov. 25 – Dec. 1) of the 2019 open enrollment season were an increase of 54% over week 4, CMS data show for the 39 states that use the HealthCare.gov platform, with the cumulative total now at 3.2 million. By comparison, week-5 selections in last year’s open enrollment totaled 823,000, and the cumulative figure was 3.6 million.
The deadline for applying for 2019 coverage on HealthCare.gov is Dec. 15.
Activity during week 5 of open enrollment on HealthCare.gov was up by more than 50% over the previous week, but the total number of plans selected for the 2019 coverage year remains lower than it was last year, according to the Centers for Medicare & Medicaid Services.

The 773,000 plans selected during week 5 (Nov. 25 – Dec. 1) of the 2019 open enrollment season were an increase of 54% over week 4, CMS data show for the 39 states that use the HealthCare.gov platform, with the cumulative total now at 3.2 million. By comparison, week-5 selections in last year’s open enrollment totaled 823,000, and the cumulative figure was 3.6 million.
The deadline for applying for 2019 coverage on HealthCare.gov is Dec. 15.
Activity during week 5 of open enrollment on HealthCare.gov was up by more than 50% over the previous week, but the total number of plans selected for the 2019 coverage year remains lower than it was last year, according to the Centers for Medicare & Medicaid Services.

The 773,000 plans selected during week 5 (Nov. 25 – Dec. 1) of the 2019 open enrollment season were an increase of 54% over week 4, CMS data show for the 39 states that use the HealthCare.gov platform, with the cumulative total now at 3.2 million. By comparison, week-5 selections in last year’s open enrollment totaled 823,000, and the cumulative figure was 3.6 million.
The deadline for applying for 2019 coverage on HealthCare.gov is Dec. 15.
Facing a lawsuit? Take the right steps early
It’s happened. A patient is suing you. Now what? Legal experts warn that a doctor’s first steps after a lawsuit can dramatically impact the outcome of the case – for better or worse.
Below, medical malpractice defense attorneys share the most important do’s and don’ts for physicians after they receive a lawsuit notice. Spoiler: Whatever you do, don’t ignore the summons.
• Do contact your insurer and/or risk manager. Once you receive notice of a lawsuit, the first step is calling your medical malpractice insurer and/or risk manager, said Steven Fitzer, a medical liability defense attorney based in Tacoma, Wash. The insurer and risk manager will take the matter from there and advise your next moves. Resist the urge to disregard the notice and hope that the challenge goes away when the patient is no longer angry, he said. Failing to notify the insurer in a timely manner could be a policy violation and affect current or future coverage.
• Don’t contact the plaintiff/patient or patient’s family. Instinctively, many physicians feel compelled to call the patient and attempt to settle the conflict verbally, particularly if they have had a longstanding relationship, Mr. Fitzer said in an interview. Don’t do it.
“In 42 years, I’ve never come across a physician who successfully talked somebody out of a lawsuit, once it was started,” he said. “It’s a pipe dream.”
Keep in mind that conversations with patients after a lawsuit filing can be used against doctors in court and certain words can easily be misconstrued as admissions of guilt.
• Do secure all medical records pertaining to the case. Obtain and print copies of all information relevant to the patient’s suit, such as history, billing records, letters, and medical chart. Store the data in a secure location in preparation for transferring to the insurer and/or attorney, said Michael Moroney, a medical liability defense attorney based in Teaneck, N.J.
• Don’t access or change the record. It may seem tempting to review the plaintiff’s medical record and fix any errors found. However, accessing the patient’s electronic data can appear as an attempt to manipulate or delete relevant data, said Joshua R. Cohen, a medical liability defense attorney based in New York.
“Avoid accessing [the] EMR or PAC system [and] leaving a digital fingerprint,” he said in an interview. “For example, if a radiologist is sued for an alleged failure to diagnose breast cancer, they should not open that study on their computer as an audit trail will show that. Worse is when they start making measurements after the lawsuit which are now discoverable as part of the lawsuit.”
Leave the record alone and let the attorneys handle the data from here on out, he advised.
• Do discuss the patient case openly with your attorney and risk manager. Honesty about all aspects of a medical case from the start sets the right tone for a positive relationship between doctor and attorney, experts say. Help your attorney understand the medicine so that they can speak intelligently about the details to the court and any retained experts, Mr. Fitzer recommended. If disagreements continually arise among physicians and attorneys, and the match fails, consider speaking to the insurer about a change in attorneys.
• Don’t discuss the case. As Mr. Fitzer puts it, “loose lips sink ships.” Physicians lose confidentiality protections when they talk about lawsuit details with third parties, and those conversations could come back to haunt them. This includes colleagues and staff members in the patient’s care loop, said Catherine Flynn, a medical liability defense attorney based in Teaneck, N.J. The third parties could later be questioned by the plaintiff’s attorney about the case, which could harm your defense.
"It’s like that kid game of telephone where you may something to the nurses and then a year later, they’re deposed, and their recollection is very different,” Ms. Flynn said in an interview. “It turns into something that you did not say.”
Your spouse is the exception. Most states protect conversations among spouses and bar husbands and wives from having to testify against their spouse.
• Do alert staff to the lawsuit and track any document requests. Following a lawsuit notice, inform staff that a claim has been filed by a patient – without going into detail. Be alert to document requests by nonpatients and make sure your attorney is aware of such requests. For example, some plaintiffs hire a private investigator to contact the medical practice and attempt to obtain records, Mr. Moroney said. In other cases, the plaintiff’s attorney or their paralegal tries to get copies of the medical chart or billing records.
• Don’t release any patient data to third parties. Ensure that staff members do not provide any patient information to the plaintiff’s attorney or other third parties, Mr. Moroney said. All relevant records should go through your attorney. No questions about the patient or the circumstances of the complaint should be divulged by the doctor or staff members to any third party, he said.
• Do seek emotional support from family and friends. Facing a lawsuit can be draining, both physically and mentally. Make time for self-care and lean on loved ones when needed, Mr. Fitzer said. Sharing your feelings – without going into detail about the case – can help relieve stress and reduce the emotional strain of being sued.
• Don’t isolate yourself. “This can be an isolating experience,” Mr. Fitzer said. “You need support. You need reinforcements. Take care of yourself and your family – they are your biggest source of support.”
It’s happened. A patient is suing you. Now what? Legal experts warn that a doctor’s first steps after a lawsuit can dramatically impact the outcome of the case – for better or worse.
Below, medical malpractice defense attorneys share the most important do’s and don’ts for physicians after they receive a lawsuit notice. Spoiler: Whatever you do, don’t ignore the summons.
• Do contact your insurer and/or risk manager. Once you receive notice of a lawsuit, the first step is calling your medical malpractice insurer and/or risk manager, said Steven Fitzer, a medical liability defense attorney based in Tacoma, Wash. The insurer and risk manager will take the matter from there and advise your next moves. Resist the urge to disregard the notice and hope that the challenge goes away when the patient is no longer angry, he said. Failing to notify the insurer in a timely manner could be a policy violation and affect current or future coverage.
• Don’t contact the plaintiff/patient or patient’s family. Instinctively, many physicians feel compelled to call the patient and attempt to settle the conflict verbally, particularly if they have had a longstanding relationship, Mr. Fitzer said in an interview. Don’t do it.
“In 42 years, I’ve never come across a physician who successfully talked somebody out of a lawsuit, once it was started,” he said. “It’s a pipe dream.”
Keep in mind that conversations with patients after a lawsuit filing can be used against doctors in court and certain words can easily be misconstrued as admissions of guilt.
• Do secure all medical records pertaining to the case. Obtain and print copies of all information relevant to the patient’s suit, such as history, billing records, letters, and medical chart. Store the data in a secure location in preparation for transferring to the insurer and/or attorney, said Michael Moroney, a medical liability defense attorney based in Teaneck, N.J.
• Don’t access or change the record. It may seem tempting to review the plaintiff’s medical record and fix any errors found. However, accessing the patient’s electronic data can appear as an attempt to manipulate or delete relevant data, said Joshua R. Cohen, a medical liability defense attorney based in New York.
“Avoid accessing [the] EMR or PAC system [and] leaving a digital fingerprint,” he said in an interview. “For example, if a radiologist is sued for an alleged failure to diagnose breast cancer, they should not open that study on their computer as an audit trail will show that. Worse is when they start making measurements after the lawsuit which are now discoverable as part of the lawsuit.”
Leave the record alone and let the attorneys handle the data from here on out, he advised.
• Do discuss the patient case openly with your attorney and risk manager. Honesty about all aspects of a medical case from the start sets the right tone for a positive relationship between doctor and attorney, experts say. Help your attorney understand the medicine so that they can speak intelligently about the details to the court and any retained experts, Mr. Fitzer recommended. If disagreements continually arise among physicians and attorneys, and the match fails, consider speaking to the insurer about a change in attorneys.
• Don’t discuss the case. As Mr. Fitzer puts it, “loose lips sink ships.” Physicians lose confidentiality protections when they talk about lawsuit details with third parties, and those conversations could come back to haunt them. This includes colleagues and staff members in the patient’s care loop, said Catherine Flynn, a medical liability defense attorney based in Teaneck, N.J. The third parties could later be questioned by the plaintiff’s attorney about the case, which could harm your defense.
"It’s like that kid game of telephone where you may something to the nurses and then a year later, they’re deposed, and their recollection is very different,” Ms. Flynn said in an interview. “It turns into something that you did not say.”
Your spouse is the exception. Most states protect conversations among spouses and bar husbands and wives from having to testify against their spouse.
• Do alert staff to the lawsuit and track any document requests. Following a lawsuit notice, inform staff that a claim has been filed by a patient – without going into detail. Be alert to document requests by nonpatients and make sure your attorney is aware of such requests. For example, some plaintiffs hire a private investigator to contact the medical practice and attempt to obtain records, Mr. Moroney said. In other cases, the plaintiff’s attorney or their paralegal tries to get copies of the medical chart or billing records.
• Don’t release any patient data to third parties. Ensure that staff members do not provide any patient information to the plaintiff’s attorney or other third parties, Mr. Moroney said. All relevant records should go through your attorney. No questions about the patient or the circumstances of the complaint should be divulged by the doctor or staff members to any third party, he said.
• Do seek emotional support from family and friends. Facing a lawsuit can be draining, both physically and mentally. Make time for self-care and lean on loved ones when needed, Mr. Fitzer said. Sharing your feelings – without going into detail about the case – can help relieve stress and reduce the emotional strain of being sued.
• Don’t isolate yourself. “This can be an isolating experience,” Mr. Fitzer said. “You need support. You need reinforcements. Take care of yourself and your family – they are your biggest source of support.”
It’s happened. A patient is suing you. Now what? Legal experts warn that a doctor’s first steps after a lawsuit can dramatically impact the outcome of the case – for better or worse.
Below, medical malpractice defense attorneys share the most important do’s and don’ts for physicians after they receive a lawsuit notice. Spoiler: Whatever you do, don’t ignore the summons.
• Do contact your insurer and/or risk manager. Once you receive notice of a lawsuit, the first step is calling your medical malpractice insurer and/or risk manager, said Steven Fitzer, a medical liability defense attorney based in Tacoma, Wash. The insurer and risk manager will take the matter from there and advise your next moves. Resist the urge to disregard the notice and hope that the challenge goes away when the patient is no longer angry, he said. Failing to notify the insurer in a timely manner could be a policy violation and affect current or future coverage.
• Don’t contact the plaintiff/patient or patient’s family. Instinctively, many physicians feel compelled to call the patient and attempt to settle the conflict verbally, particularly if they have had a longstanding relationship, Mr. Fitzer said in an interview. Don’t do it.
“In 42 years, I’ve never come across a physician who successfully talked somebody out of a lawsuit, once it was started,” he said. “It’s a pipe dream.”
Keep in mind that conversations with patients after a lawsuit filing can be used against doctors in court and certain words can easily be misconstrued as admissions of guilt.
• Do secure all medical records pertaining to the case. Obtain and print copies of all information relevant to the patient’s suit, such as history, billing records, letters, and medical chart. Store the data in a secure location in preparation for transferring to the insurer and/or attorney, said Michael Moroney, a medical liability defense attorney based in Teaneck, N.J.
• Don’t access or change the record. It may seem tempting to review the plaintiff’s medical record and fix any errors found. However, accessing the patient’s electronic data can appear as an attempt to manipulate or delete relevant data, said Joshua R. Cohen, a medical liability defense attorney based in New York.
“Avoid accessing [the] EMR or PAC system [and] leaving a digital fingerprint,” he said in an interview. “For example, if a radiologist is sued for an alleged failure to diagnose breast cancer, they should not open that study on their computer as an audit trail will show that. Worse is when they start making measurements after the lawsuit which are now discoverable as part of the lawsuit.”
Leave the record alone and let the attorneys handle the data from here on out, he advised.
• Do discuss the patient case openly with your attorney and risk manager. Honesty about all aspects of a medical case from the start sets the right tone for a positive relationship between doctor and attorney, experts say. Help your attorney understand the medicine so that they can speak intelligently about the details to the court and any retained experts, Mr. Fitzer recommended. If disagreements continually arise among physicians and attorneys, and the match fails, consider speaking to the insurer about a change in attorneys.
• Don’t discuss the case. As Mr. Fitzer puts it, “loose lips sink ships.” Physicians lose confidentiality protections when they talk about lawsuit details with third parties, and those conversations could come back to haunt them. This includes colleagues and staff members in the patient’s care loop, said Catherine Flynn, a medical liability defense attorney based in Teaneck, N.J. The third parties could later be questioned by the plaintiff’s attorney about the case, which could harm your defense.
"It’s like that kid game of telephone where you may something to the nurses and then a year later, they’re deposed, and their recollection is very different,” Ms. Flynn said in an interview. “It turns into something that you did not say.”
Your spouse is the exception. Most states protect conversations among spouses and bar husbands and wives from having to testify against their spouse.
• Do alert staff to the lawsuit and track any document requests. Following a lawsuit notice, inform staff that a claim has been filed by a patient – without going into detail. Be alert to document requests by nonpatients and make sure your attorney is aware of such requests. For example, some plaintiffs hire a private investigator to contact the medical practice and attempt to obtain records, Mr. Moroney said. In other cases, the plaintiff’s attorney or their paralegal tries to get copies of the medical chart or billing records.
• Don’t release any patient data to third parties. Ensure that staff members do not provide any patient information to the plaintiff’s attorney or other third parties, Mr. Moroney said. All relevant records should go through your attorney. No questions about the patient or the circumstances of the complaint should be divulged by the doctor or staff members to any third party, he said.
• Do seek emotional support from family and friends. Facing a lawsuit can be draining, both physically and mentally. Make time for self-care and lean on loved ones when needed, Mr. Fitzer said. Sharing your feelings – without going into detail about the case – can help relieve stress and reduce the emotional strain of being sued.
• Don’t isolate yourself. “This can be an isolating experience,” Mr. Fitzer said. “You need support. You need reinforcements. Take care of yourself and your family – they are your biggest source of support.”
ONC releases draft strategy on reducing EHR burden
A new federal proposal aims to move you away from the keyboard and back face-to-face with your patients.
The draft strategy from the Office of the National Coordinator for Health IT has three aims: to reduce the time and effort to record information in EHRs; to reduce the time and effort required to meet regulatory requirements; and to improve the functionality and ease of use of EHRs.
“This draft strategy includes recommendations that will allow physicians and other clinicians to provide effective care to their patients with a renewed sense of satisfaction for them and their patients,” Andrew Gettinger, MD, chief clinical officer at ONC, and Kate Goodrich, MD, chief medical officer at the Centers for Medicare & Medicaid Services, wrote in a recent blog post. “We are taking one more step toward improving the interoperability and usability of health information by establishing a goal, strategy, and recommendations to reduce regulatory and administrative burdens relating to the use of EHRs.”
To ease documentation burdens, the proposal seeks to “mitigate the EHR-related burden associated with a variety of administrative processes,” the draft strategy notes. “We are considering how reforming certain administrative requirements or optimizing out-of-date requirements for health IT–enabled health care provider work flows can reduce the burden of clinical documentation.”
Specifically, ONC proposes to reduce the overall regulatory burden, leverage data present in the electronic record to reduce the redocumentation, waive certain documentation requirements for participants in advanced alternative payment models (APMs), and promote standardized documentation for ordering and prior authorization.
To improve health IT usability, the draft strategy aims to “address how improvements in the design and use of health IT systems” can reduce burden and calls on clinicians, software developers, and other vendors to collaborate.
To do so, ONC recommends better alignment between EHR design and clinical work flow and making improvements to clinical decision support, as well as improving the presentation of clinical data within EHRs and clinical documentation functionality.
ONC also recommends standardizing basic clinical operations across all EHRs, designing EHR interfaces that are standard to health care delivery, and better integration of the EHR with the exam room.
The draft strategy also includes recommendations to help doctors better understand the financial requirements for successful implementation and optimize the log-in procedures to help reduce burden.
EHR reporting strategies “are designed to address many of the programmatic, technical, and operational challenges raised by stakeholders to reduce EHR-related burden associated with program reporting.”
ONC wants to simplify scoring for the “promoting interoperability” performance category in the Merit-based Incentive Payment System (MIPS) track of the Quality Payment Program and improving other measures of health IT usage; applying additional data standards to make data access, extraction, and integration across multiple systems easier and less costly; and exploring alternate, less burdensome approaches to electronic quality measurement through pilot programs and reporting program incentives.
Finally, public health reporting strategies “look at a set of topics linked to federal, state, local, territorial, and tribal government policies and public health programs, with a specific focus on EPCS [electronic prescribing for controlled substances] and PDMPs [prescription drug monitoring programs]. Where EHR-related burden remains a key barrier to progress in these areas, there are several recommendations for how stakeholders can advance these burden reduction goals related to public health.”
In this area, ONC is recommending increasing adoption of e-prescribing of controlled substances with access to medication history to better inform prescribing of controlled substances, harmonizing reporting requirements across federally funded programs to streamline reporting requirements, and providing better guidance about HIPPA and federal confidentially requirements governing substance abuse disorder to better facilitate the electronic exchange of health information for patient care.
Comments on the report may be submitted electronically through Jan. 28, 2019.
A new federal proposal aims to move you away from the keyboard and back face-to-face with your patients.
The draft strategy from the Office of the National Coordinator for Health IT has three aims: to reduce the time and effort to record information in EHRs; to reduce the time and effort required to meet regulatory requirements; and to improve the functionality and ease of use of EHRs.
“This draft strategy includes recommendations that will allow physicians and other clinicians to provide effective care to their patients with a renewed sense of satisfaction for them and their patients,” Andrew Gettinger, MD, chief clinical officer at ONC, and Kate Goodrich, MD, chief medical officer at the Centers for Medicare & Medicaid Services, wrote in a recent blog post. “We are taking one more step toward improving the interoperability and usability of health information by establishing a goal, strategy, and recommendations to reduce regulatory and administrative burdens relating to the use of EHRs.”
To ease documentation burdens, the proposal seeks to “mitigate the EHR-related burden associated with a variety of administrative processes,” the draft strategy notes. “We are considering how reforming certain administrative requirements or optimizing out-of-date requirements for health IT–enabled health care provider work flows can reduce the burden of clinical documentation.”
Specifically, ONC proposes to reduce the overall regulatory burden, leverage data present in the electronic record to reduce the redocumentation, waive certain documentation requirements for participants in advanced alternative payment models (APMs), and promote standardized documentation for ordering and prior authorization.
To improve health IT usability, the draft strategy aims to “address how improvements in the design and use of health IT systems” can reduce burden and calls on clinicians, software developers, and other vendors to collaborate.
To do so, ONC recommends better alignment between EHR design and clinical work flow and making improvements to clinical decision support, as well as improving the presentation of clinical data within EHRs and clinical documentation functionality.
ONC also recommends standardizing basic clinical operations across all EHRs, designing EHR interfaces that are standard to health care delivery, and better integration of the EHR with the exam room.
The draft strategy also includes recommendations to help doctors better understand the financial requirements for successful implementation and optimize the log-in procedures to help reduce burden.
EHR reporting strategies “are designed to address many of the programmatic, technical, and operational challenges raised by stakeholders to reduce EHR-related burden associated with program reporting.”
ONC wants to simplify scoring for the “promoting interoperability” performance category in the Merit-based Incentive Payment System (MIPS) track of the Quality Payment Program and improving other measures of health IT usage; applying additional data standards to make data access, extraction, and integration across multiple systems easier and less costly; and exploring alternate, less burdensome approaches to electronic quality measurement through pilot programs and reporting program incentives.
Finally, public health reporting strategies “look at a set of topics linked to federal, state, local, territorial, and tribal government policies and public health programs, with a specific focus on EPCS [electronic prescribing for controlled substances] and PDMPs [prescription drug monitoring programs]. Where EHR-related burden remains a key barrier to progress in these areas, there are several recommendations for how stakeholders can advance these burden reduction goals related to public health.”
In this area, ONC is recommending increasing adoption of e-prescribing of controlled substances with access to medication history to better inform prescribing of controlled substances, harmonizing reporting requirements across federally funded programs to streamline reporting requirements, and providing better guidance about HIPPA and federal confidentially requirements governing substance abuse disorder to better facilitate the electronic exchange of health information for patient care.
Comments on the report may be submitted electronically through Jan. 28, 2019.
A new federal proposal aims to move you away from the keyboard and back face-to-face with your patients.
The draft strategy from the Office of the National Coordinator for Health IT has three aims: to reduce the time and effort to record information in EHRs; to reduce the time and effort required to meet regulatory requirements; and to improve the functionality and ease of use of EHRs.
“This draft strategy includes recommendations that will allow physicians and other clinicians to provide effective care to their patients with a renewed sense of satisfaction for them and their patients,” Andrew Gettinger, MD, chief clinical officer at ONC, and Kate Goodrich, MD, chief medical officer at the Centers for Medicare & Medicaid Services, wrote in a recent blog post. “We are taking one more step toward improving the interoperability and usability of health information by establishing a goal, strategy, and recommendations to reduce regulatory and administrative burdens relating to the use of EHRs.”
To ease documentation burdens, the proposal seeks to “mitigate the EHR-related burden associated with a variety of administrative processes,” the draft strategy notes. “We are considering how reforming certain administrative requirements or optimizing out-of-date requirements for health IT–enabled health care provider work flows can reduce the burden of clinical documentation.”
Specifically, ONC proposes to reduce the overall regulatory burden, leverage data present in the electronic record to reduce the redocumentation, waive certain documentation requirements for participants in advanced alternative payment models (APMs), and promote standardized documentation for ordering and prior authorization.
To improve health IT usability, the draft strategy aims to “address how improvements in the design and use of health IT systems” can reduce burden and calls on clinicians, software developers, and other vendors to collaborate.
To do so, ONC recommends better alignment between EHR design and clinical work flow and making improvements to clinical decision support, as well as improving the presentation of clinical data within EHRs and clinical documentation functionality.
ONC also recommends standardizing basic clinical operations across all EHRs, designing EHR interfaces that are standard to health care delivery, and better integration of the EHR with the exam room.
The draft strategy also includes recommendations to help doctors better understand the financial requirements for successful implementation and optimize the log-in procedures to help reduce burden.
EHR reporting strategies “are designed to address many of the programmatic, technical, and operational challenges raised by stakeholders to reduce EHR-related burden associated with program reporting.”
ONC wants to simplify scoring for the “promoting interoperability” performance category in the Merit-based Incentive Payment System (MIPS) track of the Quality Payment Program and improving other measures of health IT usage; applying additional data standards to make data access, extraction, and integration across multiple systems easier and less costly; and exploring alternate, less burdensome approaches to electronic quality measurement through pilot programs and reporting program incentives.
Finally, public health reporting strategies “look at a set of topics linked to federal, state, local, territorial, and tribal government policies and public health programs, with a specific focus on EPCS [electronic prescribing for controlled substances] and PDMPs [prescription drug monitoring programs]. Where EHR-related burden remains a key barrier to progress in these areas, there are several recommendations for how stakeholders can advance these burden reduction goals related to public health.”
In this area, ONC is recommending increasing adoption of e-prescribing of controlled substances with access to medication history to better inform prescribing of controlled substances, harmonizing reporting requirements across federally funded programs to streamline reporting requirements, and providing better guidance about HIPPA and federal confidentially requirements governing substance abuse disorder to better facilitate the electronic exchange of health information for patient care.
Comments on the report may be submitted electronically through Jan. 28, 2019.
Robotic vs. conventional laparoscopic surgery for rectal cancer: No winner yet
Robot-assisted rectal surgery is gaining acceptance but, with some exceptions, outcomes are not significantly improved over the conventional laparoscopic approach, a meta-analysis has found.
Conducted by Katie Jones, MD, and her colleagues at Brighton and Sussex (England) University Hospital NHS Trust, the meta-analysis was designed as a follow-up to ROLARR (isrctn.org ID: ISRCTN80500123), a randomized clinical trial in which robot-assisted and. conventional laparoscopic surgery for rectal cancer were studied for risk of conversion to open surgery. That trial findings showed that robot-assisted laparoscopic surgery did not significantly reduce the risk of conversion. For other outcomes (pathology, complications, bladder, and sexual function), the differences between the two approaches were insignificant. But the two surgical approaches did differ on cost: The robot-assisted operation was significantly more expensive than the conventional laparoscopic procedure.
Dr. Jones and her colleagues analyzed data from ROLARR in the context of 27 other qualifying studies and confirmed many of the ROLARR findings. The 27 case control studies comprised 5,547 patients and had comparable outcomes data.
The outcomes of interest were duration of operation, conversion risk, blood loss, length of stay, oncological outcomes, time to first flatus, reoperation rate, postoperative morbidity, and postoperative mortality.
The investigators found that duration of the operation was longer for the robot-assisted procedure, compared with the conventional laparoscopic approach, though this difference was not statistically significant (z = 1.28, P = .20), and blood loss, morbidity, and mortality were similar between the two groups. Oncological outcomes (risk of positive circumferential resection margins, lymph node yield, and length of distal resection margins) were similar for these two surgical approaches.
In contrast to the ROLARR findings, this meta-analysis found that the risk of conversion favored the robot-assisted procedure (z = 5.51, P = .00001). Hospital stay (z = 2.46, P = 01) and time to first flatus outcomes (z = 3.09, P = .002) favored the robot-assisted procedure. Postop morbidity and mortality and reoperation rate were similar in the two groups.
“Based upon the findings of this largest-ever series on the role of robotic surgery in rectal cancer resection, the [robot-assisted procedure] is certainly a feasible technique and oncologically safe surgical intervention but failed to demonstrate any superiority over [the conventional laparoscopic approach] for many surgical outcomes,” the investigators wrote. “Mere advantage of robotic surgery was noted in only three postoperative outcomes, that is early passage of flatus, lower risk of conversion, and shorter hospitalization.”
Dr. Jones and her colleagues declared they had no conflicts of interest.
SOURCE: Jones K et al. World J Gastroentrol. 2018 Nov 15. doi: 10.4251/wjgo.v10.i11.449.
Robot-assisted rectal surgery is gaining acceptance but, with some exceptions, outcomes are not significantly improved over the conventional laparoscopic approach, a meta-analysis has found.
Conducted by Katie Jones, MD, and her colleagues at Brighton and Sussex (England) University Hospital NHS Trust, the meta-analysis was designed as a follow-up to ROLARR (isrctn.org ID: ISRCTN80500123), a randomized clinical trial in which robot-assisted and. conventional laparoscopic surgery for rectal cancer were studied for risk of conversion to open surgery. That trial findings showed that robot-assisted laparoscopic surgery did not significantly reduce the risk of conversion. For other outcomes (pathology, complications, bladder, and sexual function), the differences between the two approaches were insignificant. But the two surgical approaches did differ on cost: The robot-assisted operation was significantly more expensive than the conventional laparoscopic procedure.
Dr. Jones and her colleagues analyzed data from ROLARR in the context of 27 other qualifying studies and confirmed many of the ROLARR findings. The 27 case control studies comprised 5,547 patients and had comparable outcomes data.
The outcomes of interest were duration of operation, conversion risk, blood loss, length of stay, oncological outcomes, time to first flatus, reoperation rate, postoperative morbidity, and postoperative mortality.
The investigators found that duration of the operation was longer for the robot-assisted procedure, compared with the conventional laparoscopic approach, though this difference was not statistically significant (z = 1.28, P = .20), and blood loss, morbidity, and mortality were similar between the two groups. Oncological outcomes (risk of positive circumferential resection margins, lymph node yield, and length of distal resection margins) were similar for these two surgical approaches.
In contrast to the ROLARR findings, this meta-analysis found that the risk of conversion favored the robot-assisted procedure (z = 5.51, P = .00001). Hospital stay (z = 2.46, P = 01) and time to first flatus outcomes (z = 3.09, P = .002) favored the robot-assisted procedure. Postop morbidity and mortality and reoperation rate were similar in the two groups.
“Based upon the findings of this largest-ever series on the role of robotic surgery in rectal cancer resection, the [robot-assisted procedure] is certainly a feasible technique and oncologically safe surgical intervention but failed to demonstrate any superiority over [the conventional laparoscopic approach] for many surgical outcomes,” the investigators wrote. “Mere advantage of robotic surgery was noted in only three postoperative outcomes, that is early passage of flatus, lower risk of conversion, and shorter hospitalization.”
Dr. Jones and her colleagues declared they had no conflicts of interest.
SOURCE: Jones K et al. World J Gastroentrol. 2018 Nov 15. doi: 10.4251/wjgo.v10.i11.449.
Robot-assisted rectal surgery is gaining acceptance but, with some exceptions, outcomes are not significantly improved over the conventional laparoscopic approach, a meta-analysis has found.
Conducted by Katie Jones, MD, and her colleagues at Brighton and Sussex (England) University Hospital NHS Trust, the meta-analysis was designed as a follow-up to ROLARR (isrctn.org ID: ISRCTN80500123), a randomized clinical trial in which robot-assisted and. conventional laparoscopic surgery for rectal cancer were studied for risk of conversion to open surgery. That trial findings showed that robot-assisted laparoscopic surgery did not significantly reduce the risk of conversion. For other outcomes (pathology, complications, bladder, and sexual function), the differences between the two approaches were insignificant. But the two surgical approaches did differ on cost: The robot-assisted operation was significantly more expensive than the conventional laparoscopic procedure.
Dr. Jones and her colleagues analyzed data from ROLARR in the context of 27 other qualifying studies and confirmed many of the ROLARR findings. The 27 case control studies comprised 5,547 patients and had comparable outcomes data.
The outcomes of interest were duration of operation, conversion risk, blood loss, length of stay, oncological outcomes, time to first flatus, reoperation rate, postoperative morbidity, and postoperative mortality.
The investigators found that duration of the operation was longer for the robot-assisted procedure, compared with the conventional laparoscopic approach, though this difference was not statistically significant (z = 1.28, P = .20), and blood loss, morbidity, and mortality were similar between the two groups. Oncological outcomes (risk of positive circumferential resection margins, lymph node yield, and length of distal resection margins) were similar for these two surgical approaches.
In contrast to the ROLARR findings, this meta-analysis found that the risk of conversion favored the robot-assisted procedure (z = 5.51, P = .00001). Hospital stay (z = 2.46, P = 01) and time to first flatus outcomes (z = 3.09, P = .002) favored the robot-assisted procedure. Postop morbidity and mortality and reoperation rate were similar in the two groups.
“Based upon the findings of this largest-ever series on the role of robotic surgery in rectal cancer resection, the [robot-assisted procedure] is certainly a feasible technique and oncologically safe surgical intervention but failed to demonstrate any superiority over [the conventional laparoscopic approach] for many surgical outcomes,” the investigators wrote. “Mere advantage of robotic surgery was noted in only three postoperative outcomes, that is early passage of flatus, lower risk of conversion, and shorter hospitalization.”
Dr. Jones and her colleagues declared they had no conflicts of interest.
SOURCE: Jones K et al. World J Gastroentrol. 2018 Nov 15. doi: 10.4251/wjgo.v10.i11.449.
FROM WORLD JOURNAL OF GASTROINTESTINAL ONCOLOGY
Key clinical point:
Major finding: Duration of the operation was longer for the robot-assisted procedure, compared with the conventional laparoscopic approach (z = 1.28, P = .20), but blood loss, morbidity, and mortality were similar between the two groups.
Study details: Meta-analysis of 27 studies and one RCT of patients who had robot-assisted laparoscopic surgery or conventional laparoscopic surgery for rectal cancer.
Disclosures: The investigators had no disclosures.
Source: Jones K. World J Gastrointest Oncol. 2018 Nov 15. doi: 10.4251/wjgo.v10.i11.449.
Resection, neurostimulation combo found successful in eloquent cortical regions
NEW ORLEANS – Concurrent surgical resection and implanted strip electrodes eliminated refractory focal seizures in two patients with focal cortical dysplasia and reduced them by 62% in a third patient, according a report presented at the annual meeting of the American Epilepsy Society.
None of the patients had been considered surgical candidates because their seizure foci were in eloquent cortical regions; if fully resected, patients would have experienced marked neurologic deficits. But the combination procedure of flanking the incomplete resected foci with implanted electrodes allowed neurosurgeons to remove less tissue, preserving function while effectively treating previously untreatable seizures, Emily Mirro said at the meeting.
The two-in-one technique makes good surgical sense for these patients, she said in an interview. “If we simply performed the resection and closed without implanting the electrodes, just waiting to see if seizures develop or not, then going back to implant the electrodes, the surgery is riskier and more difficult,” said Ms. Mirro, director of field clinical engineering for NeuroPace, which makes the stimulator system.
At the meeting, she presented three case studies on behalf of primary authors Lawrence Shuer, MD, and Babak Razavi, MD, PhD, both of Stanford (Calif.) University.
The first patient was a 26-year-old with a focal cortical dysplasia in the right parietal region, causing about six seizures each month. At the time of surgery, surgeons flanked the resected region with four cortical strip leads over sensory cortex. The RNS System detected the first postsurgical seizure 1 month afterward. Five months later, the system was enabled at 0.5 milliamps. For the next year, the patient received about 100 stimulations per day, amounting to a total daily stimulation time of about 20 seconds. Electrographic seizures did return, at which point the system increased neurostimulation to about 2,000 per day (a total stimulation time of about 7 minutes per day). At 1.3 years, the patient remains seizure free.
Patient two was a 20-year-old with a left frontal transmantle cortical dysplasia that involved the inferior frontal sulcus. The baseline seizure frequency was about two per day. Surgeons removed the dysplastic area with a 2.0 cm x 0.5 cm resection; the deficit was flanked with two left-front cortical strip leads. In the following 9 days, the patient experienced eight seizures. At 14 days out, the system was enabled at 1 milliamp. This patient became seizure free and remains so at 1.3 years, with about 100 stimulations per day to suppress electrographic abnormalities.
The third patient, also 20 years old, had a left-parietal resection to the margin of the motor cortex. The baseline seizure frequency was up to 150 nocturnal events per month and several seizures during each day as well. The resection was flanked by one strip lead over the motor cortex; one depth lead implanted into it. Immediately after surgery, the patient experienced both electrographic and clinical seizures. The stimulator was enabled a week after surgery at 0.5 milliamps; this was titrated to 3 milliamps over 1.4 years. At last follow-up, the patient had about a 62% reduction in seizure frequency; all are now nocturnal.
None of the patients experienced any peri- or postoperative surgical complications.
Ms. Mirro is an employee of NeuroPace.
SOURCE: Razavi B et al. AES 2018, Abstract 2.315
NEW ORLEANS – Concurrent surgical resection and implanted strip electrodes eliminated refractory focal seizures in two patients with focal cortical dysplasia and reduced them by 62% in a third patient, according a report presented at the annual meeting of the American Epilepsy Society.
None of the patients had been considered surgical candidates because their seizure foci were in eloquent cortical regions; if fully resected, patients would have experienced marked neurologic deficits. But the combination procedure of flanking the incomplete resected foci with implanted electrodes allowed neurosurgeons to remove less tissue, preserving function while effectively treating previously untreatable seizures, Emily Mirro said at the meeting.
The two-in-one technique makes good surgical sense for these patients, she said in an interview. “If we simply performed the resection and closed without implanting the electrodes, just waiting to see if seizures develop or not, then going back to implant the electrodes, the surgery is riskier and more difficult,” said Ms. Mirro, director of field clinical engineering for NeuroPace, which makes the stimulator system.
At the meeting, she presented three case studies on behalf of primary authors Lawrence Shuer, MD, and Babak Razavi, MD, PhD, both of Stanford (Calif.) University.
The first patient was a 26-year-old with a focal cortical dysplasia in the right parietal region, causing about six seizures each month. At the time of surgery, surgeons flanked the resected region with four cortical strip leads over sensory cortex. The RNS System detected the first postsurgical seizure 1 month afterward. Five months later, the system was enabled at 0.5 milliamps. For the next year, the patient received about 100 stimulations per day, amounting to a total daily stimulation time of about 20 seconds. Electrographic seizures did return, at which point the system increased neurostimulation to about 2,000 per day (a total stimulation time of about 7 minutes per day). At 1.3 years, the patient remains seizure free.
Patient two was a 20-year-old with a left frontal transmantle cortical dysplasia that involved the inferior frontal sulcus. The baseline seizure frequency was about two per day. Surgeons removed the dysplastic area with a 2.0 cm x 0.5 cm resection; the deficit was flanked with two left-front cortical strip leads. In the following 9 days, the patient experienced eight seizures. At 14 days out, the system was enabled at 1 milliamp. This patient became seizure free and remains so at 1.3 years, with about 100 stimulations per day to suppress electrographic abnormalities.
The third patient, also 20 years old, had a left-parietal resection to the margin of the motor cortex. The baseline seizure frequency was up to 150 nocturnal events per month and several seizures during each day as well. The resection was flanked by one strip lead over the motor cortex; one depth lead implanted into it. Immediately after surgery, the patient experienced both electrographic and clinical seizures. The stimulator was enabled a week after surgery at 0.5 milliamps; this was titrated to 3 milliamps over 1.4 years. At last follow-up, the patient had about a 62% reduction in seizure frequency; all are now nocturnal.
None of the patients experienced any peri- or postoperative surgical complications.
Ms. Mirro is an employee of NeuroPace.
SOURCE: Razavi B et al. AES 2018, Abstract 2.315
NEW ORLEANS – Concurrent surgical resection and implanted strip electrodes eliminated refractory focal seizures in two patients with focal cortical dysplasia and reduced them by 62% in a third patient, according a report presented at the annual meeting of the American Epilepsy Society.
None of the patients had been considered surgical candidates because their seizure foci were in eloquent cortical regions; if fully resected, patients would have experienced marked neurologic deficits. But the combination procedure of flanking the incomplete resected foci with implanted electrodes allowed neurosurgeons to remove less tissue, preserving function while effectively treating previously untreatable seizures, Emily Mirro said at the meeting.
The two-in-one technique makes good surgical sense for these patients, she said in an interview. “If we simply performed the resection and closed without implanting the electrodes, just waiting to see if seizures develop or not, then going back to implant the electrodes, the surgery is riskier and more difficult,” said Ms. Mirro, director of field clinical engineering for NeuroPace, which makes the stimulator system.
At the meeting, she presented three case studies on behalf of primary authors Lawrence Shuer, MD, and Babak Razavi, MD, PhD, both of Stanford (Calif.) University.
The first patient was a 26-year-old with a focal cortical dysplasia in the right parietal region, causing about six seizures each month. At the time of surgery, surgeons flanked the resected region with four cortical strip leads over sensory cortex. The RNS System detected the first postsurgical seizure 1 month afterward. Five months later, the system was enabled at 0.5 milliamps. For the next year, the patient received about 100 stimulations per day, amounting to a total daily stimulation time of about 20 seconds. Electrographic seizures did return, at which point the system increased neurostimulation to about 2,000 per day (a total stimulation time of about 7 minutes per day). At 1.3 years, the patient remains seizure free.
Patient two was a 20-year-old with a left frontal transmantle cortical dysplasia that involved the inferior frontal sulcus. The baseline seizure frequency was about two per day. Surgeons removed the dysplastic area with a 2.0 cm x 0.5 cm resection; the deficit was flanked with two left-front cortical strip leads. In the following 9 days, the patient experienced eight seizures. At 14 days out, the system was enabled at 1 milliamp. This patient became seizure free and remains so at 1.3 years, with about 100 stimulations per day to suppress electrographic abnormalities.
The third patient, also 20 years old, had a left-parietal resection to the margin of the motor cortex. The baseline seizure frequency was up to 150 nocturnal events per month and several seizures during each day as well. The resection was flanked by one strip lead over the motor cortex; one depth lead implanted into it. Immediately after surgery, the patient experienced both electrographic and clinical seizures. The stimulator was enabled a week after surgery at 0.5 milliamps; this was titrated to 3 milliamps over 1.4 years. At last follow-up, the patient had about a 62% reduction in seizure frequency; all are now nocturnal.
None of the patients experienced any peri- or postoperative surgical complications.
Ms. Mirro is an employee of NeuroPace.
SOURCE: Razavi B et al. AES 2018, Abstract 2.315
REPORTING FROM AES 2018
Key clinical point:
Major finding: Two patients became seizure free and one had a 62% reduction in seizures.
Study details: A three-patient case series.
Disclosures: NeuroPace makes the neurostimulator used in the study. The presenter is an employee of NeuroPace.
Source: Razavi B et al. AES 2018, Abstract 2.315.