User login
Focus on long-COVID: Perimenopause and post-COVID chronic fatigue
Long COVID (postacute sequelae of SARS-CoV-2 infection, or PASC) is an emerging syndrome that affects 50% to 70% of people who survive COVID-19 for up to 3 months or longer after acute disease.1 It is a multisystem condition that causes dysfunction of respiratory, cardiac, and nervous tissue, at least in part likely due to alterations in cellular energy metabolism and reduced oxygen supply to tissue.3 Patients who have had SARS-CoV-2 infection report persistent symptoms and signs that affect their quality of life. These may include neurocognitive, cardiorespiratory, gastrointestinal, and musculoskeletal symptoms; loss of taste and smell; and constitutional symptoms.2 There is no one test to determine if symptoms are due to COVID-19.3
Acute COVID-19 mortality risk factors include increasing age, chronic comorbidities, and male sex. However, long COVID risk factors are quite different. A meta-analysis and review of 20 articles that met inclusion criteria (n = 13,340 study participants), limited by pooling of crude estimates, found that risk factors were female sex and severity of acute disease.4 A second meta-analysis of 37 studies with 1 preprint found that female sex and comorbidities such as pulmonary disease, diabetes, and obesity were risk factors for long COVID.5 Qualitative analysis of single studies (n = 18 study participants) suggested that older adults can develop more long COVID symptoms than younger adults, but this association between advancing age and long COVID was not supported when data were pooled into a meta-analysis.3 However, both single studies (n = 16 study participants) and the meta-analysis (n = 7 study participants) did support female sex as a risk factor for long COVID, along with single studies suggesting increased risk with medical comorbidities for pulmonary disease, diabetes, and organ transplantation.
Perimenopause
Perimenopause: A temporary disruption to physiologic ovarian steroid hormone production following COVID could acutely exacerbate symptoms of perimenopause and menopause.
JoAnn V. Pinkerton, MD, MSCP
The higher prevalence of long COVID in women younger than 50 years6 supports the overlap that studies have shown between symptoms of long COVID and perimenopause,7 as the median age of natural menopause is 51 years. Thus, health care providers need to differentiate between long COVID and other conditions, such as perimenopause, which share similar symptoms (FIGURE). Perimenopause might be diagnosed as long COVID, or the 2 might affect each other.
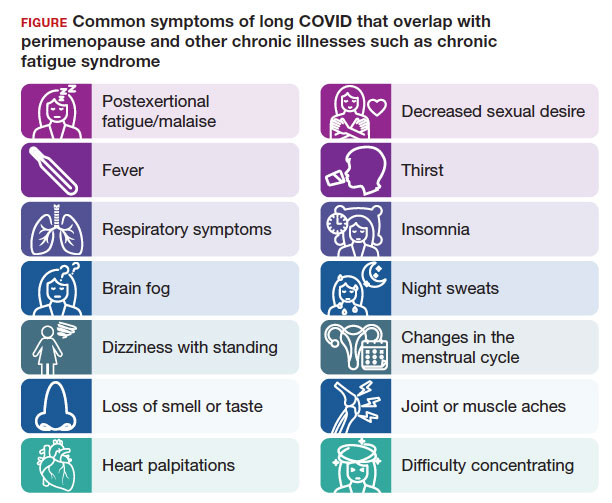
Symptoms of long COVID include fatigue, brain fog, and increased heart rate after recovering from COVID-19 and may continue or increase after an initial infection.8 Common symptoms of perimenopause and menopause, which also could be seen with long COVID, include typical menopausal symptoms such as hot flashes, night sweats, or disrupted sleep; changes in mood including dysthymia, depression, anxiety, or emotional lability; cognitive concerns such as brain fog or decreased concentration; and decreased stamina, fatigue, joint and muscle pains, or more frequent headaches. Therefore, women in their 40s or 50s with persistent symptoms after having COVID-19 without an alternative diagnosis, and who present with menstrual irregularity,9 hot flashes, or night sweats, could be having an exacerbation of perimenopausal symptoms, or they could be experiencing a combination of long COVID and perimenopausal symptoms.
- Consider long COVID, versus perimenopause, or both, in women aged younger than 50 years
- Estradiol, which has been shown to alleviate perimenopausal and menopausal symptoms, also has been shown to have beneficial effects during acute COVID-19 infection
- Hormone therapy could improve symptoms of perimenopause and long COVID if some of the symptoms are due to changes in ovary function
Continue to: Potential pathophysiology...
Potential pathophysiology
Inflammation is likely to be critical in the pathogenesis of postacute sequelae of SARS-CoV-2 infection, or PASC. Individuals with long COVID have elevated inflammatory markers for several months.10 The chronic inflammation associated with long COVID could cause disturbances in the ovary and ovarian hormone production.2,10,11
During perimenopause, the ovary is more sensitive to illnesses such as COVID-19and to stress. The current theory is that COVID-19 affects the ovary with declines in ovarian reserve and ovarian function7 and with potential disruptions to the menstrual cycle, gonadal function, and ovarian sufficiency that lead to issues with menopause or fertility, as well as symptom exacerbation around menstruation.12 Another theory is that SARS-CoV-2 infection affects ovary hormone production, as there is an abundance of angiotensin-converting enzyme-2 receptors on ovarian and endometrial tissue.11 Thus, it makes sense that long COVID could bring on symptoms of perimenopause or menopause more acutely or more severely or lengthen the duration of perimenopausal symptoms.
Perimenopause is the transitional period prior to menopause, when the ovaries gradually produce fewer hormones and is associated with erratic hormonal fluctuations. The length of this transitional period varies from 4 to 10 years. Ethnic variations in the duration of hot flashes have been found, noting that Black and Hispanic women have them for an average of 8 to 10 years (longer), White women for an average of 7 years, and Asian, Japanese, and Chinese women for an average of 5 to 6 years (shorter).17
What should health care providers ask?
Distinguishing perimenopause from long COVID. It is important to try to differentiate between perimenopause and long COVID, and it is possible to have both, with long COVID exacerbating the menopausal symptoms.7,8 Health care providers should be alert to consider perimenopause if women present with shorter or longer cycles (21-40 days), missed periods (particularly 60 days or 2 months), or worsening perimenopausal mood, migraines, insomnia, or hot flashes. Clinicians should actively enquire about all of these symptoms.
Moreover, if a perimenopausal woman reports acutely worsening symptoms after COVID-19, health care providers should address the perimenopausal symptoms and determine whether hormone therapy is appropriate and could improve their symptoms. Women do not need to wait until they go 1 year without a period to be treated with hormone therapy to improve perimenopausal and menopausal symptoms. If women with long COVID have perimenopause or menopause symptoms, they should have access to evidence-based information and discuss menopausal hormone therapy if appropriate. Hormone therapy could improve both perimenopausal symptoms and the long COVID symptoms if some of the symptoms are due to changes in ovary function. Health care providers could consider progesterone or antidepressants during the second half of the cycle (luteal phase) or estrogen combined with progesterone for the entire cycle.18
For health care providers working in long COVID clinics, in addition to asking when symptoms started, what makes symptoms worse, the frequency of symptoms, and which activities are affected, ask about perimenopausal and menopausal symptoms. If a woman has irregular periods, sleep disturbances, fatigue, or mood changes, consider that these could be related to long COVID, perimenopause, or both.8,18 Be able to offer treatment or refer patients to a women’s health specialist who can assess and offer treatment.
A role for vitamin D? A recent retrospective case-matched study found that 6 months after hospital discharge, patients with long COVID had lower levels of 25(OH) vitamin D with the most notable symptom being brain fog.19 Thus, there may be a role for vitamin D supplementation as a preventive strategy in those being discharged after hospitalization. Vitamin D levels and supplementation have not been otherwise evaluated to date.
Lifestyle strategies for women with perimenopause and long COVID
Lifestyle strategies should be encouraged for women during perimenopause and long COVID. This includes good nutrition (avoiding carbs and sweets, particularly before menses), getting at least 7 hours of sleep and using sleep hygiene (regular bedtimes, sleep regimen, no late screens), getting regular exercise 5 days per week, reducing stress, avoiding excess alcohol, and not smoking. All of these factors can help women and their ovarian function during this period of ovarian fluctuations.
The timing of menopause and COVID may coincide with midlife stressors, including relationship issues (separations or divorce), health issues for the individual or their partner, widowhood, parenting challenges (care of young children, struggles with adolescents, grown children returning home), being childless, concerns about aging parents and caregiving responsibilities, as well as midlife career, community, or education issues—all of which make both long COVID and perimenopause more challenging to navigate.
Need for research
There is a need for future research to understand the epidemiologic basis and underlying biological mechanisms of sex differences seen in women with long COVID. Studying the effects of COVID-19 on ovarian function could lead to a better understanding of perimenopause, what causes ovarian failure to speed up, and possibly ways to slow it down8 since there are health risks of early menopause.16
References
- Fernández-de-Las-Peñas C, Palacios-Ceña D, GómezMayordomo V, et al. Defining post-COVID symptoms (postacute COVID, long COVID, persistent post-COVID): an integrative classification. Int J Environ Res Public Health. 2021;18:2621. doi: 10.3390/ijerph18052621
- Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601-615. doi: 10.1038/s41591 -021-01283-z
- Davis HE, McCorkell L, Vogel JM, et al. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21:133-146. doi: 10.1038/s41579-022-00846-2
- Maglietta G, Diodati F, Puntoni M, et al. Prognostic factors for post-COVID-19 syndrome: a systematic review and meta-analysis. J Clin Med. 2022;11:1541. doi: 10.3390 /jcm11061541
- Notarte KI, de Oliveira MHS, Peligro PJ, et al. Age, sex and previous comorbidities as risk factors not associated with SARS-CoV-2 infection for long COVID-19: a systematic review and meta-analysis. J Clin Med. 2022;11:7314. doi: 10.3390 /jcm11247314
- Sigfrid L, Drake TM, Pauley E, et al. Long COVID in adults discharged from UK hospitals after COVID-19: a prospective, multicentre cohort study using the ISARIC WHO Clinical Characterisation Protocol. Lancet Reg Health Eur. 2021;8:100186. doi: 10.1016/j.lanepe.2021.100186
- Pollack B, von Saltza E, McCorkell L, et al. Female reproductive health impacts of long COVID and associated illnesses including ME/CFS, POTS, and connective tissue disorders: a literature review. Front Rehabil Sci. 2023;4:1122673. doi: 10.3389/fresc.2023.1122673
- Stewart S, Newson L, Briggs TA, et al. Long COVID risk - a signal to address sex hormones and women’s health. Lancet Reg Health Eur. 2021;11:100242. doi: 10.1016 /j.lanepe.2021.100242
- Li K, Chen G, Hou H, et al. Analysis of sex hormones and menstruation in COVID-19 women of child-bearing age. Reprod Biomed Online. 2021;42:260-267. doi: 10.1016 /j.rbmo.2020.09.020
- Phetsouphanh C, Darley DR, Wilson DB, et al. Immunological dysfunction persists for 8 months following initial mild-tomoderate SARS-CoV-2 infection. Nat Immunol. 2022;23:210216. doi: 10.1038/s41590-021-01113-x
- Sharp GC, Fraser A, Sawyer G, et al. The COVID-19 pandemic and the menstrual cycle: research gaps and opportunities. Int J Epidemiol. 2022;51:691-700. doi: 10.1093/ije/dyab239
- Ding T, Wang T, Zhang J, et al. Analysis of ovarian injury associated with COVID-19 disease in reproductive-aged women in Wuhan, China: an observational study. Front Med (Lausanne). 2021;8:635255. doi: 10.3389/fmed.2021.635255
- Huang B, Cai Y, Li N, et al. Sex-based clinical and immunological differences in COVID-19. BMC Infect Dis. 2021;21:647. doi: 10.1186/s12879-021-06313-2
- Connor J, Madhavan S, Mokashi M, et al. Health risks and outcomes that disproportionately affect women during the Covid-19 pandemic: a review. Soc Sci Med. 2020;266:113364. doi: 10.1016/j.socscimed.2020.113364
- Mauvais-Jarvis F, Klein SL, Levin ER. Estradiol, progesterone, immunomodulation, and COVID-19 outcomes. Endocrinology. 2020;161:bqaa127. doi:10.1210/endocr/bqaa127
- The 2022 hormone therapy position statement of The North American Menopause Society. Menopause. 2022;29:767-794. doi: 10.1097/GME.0000000000002028
- Avis NE, Crawford SL, Greendale G, et al. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med. 2015;175:531-539. doi:10.1001 /jamainternmed.2014.8063
- Newson L, Lewis R, O’Hara M. Long COVID and menopause - the important role of hormones in long COVID must be considered. Maturitas. 2021;152:74. doi: 10.1016 /j.maturitas.2021.08.026
- di Filippo L, Frara S, Nannipieri F, et al. Low Vitamin D levels are associated with long COVID syndrome in COVID-19 survivors. J Clin Endocrinol Metab. 2023;108:e1106-e1116. doi: 10.1210/clinem/dgad207
Continue to: Chronic fatigue syndrome...
Chronic fatigue syndrome
Chronic fatigue syndrome: A large number of patients have “post-COVID conditions” affecting everyday function, including depression/anxiety, insomnia, and chronic fatigue (with a 3:1 female predominance)
Alexandra Kadl, MD
After 3 years battling acute COVID-19 infections, we encounter now a large number of patients with PASC— also known as “long COVID,” “COVID long-hauler syndrome,” and “post-COVID conditions”—a persistent multisystem syndrome that impacts everyday function.1 As of October 2023, there are more than 100 million COVID-19 survivors reported in the United States; 10% to 85% of COVID survivors2-4 may show lingering, life-altering symptoms after recovery. Common reported symptoms include fatigue, depression/ anxiety, insomnia, and brain fog/difficulty concentrating, which are particularly high in women who often had experienced only mild acute COVID-19 disease and were not even hospitalized. More recently, chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) has been recognized as major component of PASC5 with a 3:1 female predominance.6 Up to 75% of patients with this diagnosis are not able to maintain their jobs and normal life, and up to 25% are so disabled that they are bedbound.6
Diagnosis
Although illnesses resembling CFS have been reported for more than 200 years,7 the diagnosis of CFS/ME remains difficult to make. There is a likely underreporting due to fear of being labeled as malingering when reaching out to health care providers, and there is a reporting bias toward higher socioeconomic groups due to better access to health care. The current criteria for the diagnosis of CFS/ME include the following 3 components8:
- substantial impairment in the ability to function for more than 6 months, accompanied by profound fatigue, not alleviated by rest
- post-exertional malaise (PEM; prolonged, disabling exacerbation of the patient’s baseline symptoms after exercise)
- non-refreshing sleep, PLUS either cognitive impairment or orthostatic intolerance.
Pathophysiology
Originally found to evolve in a small patient population with Epstein-Barr virus infection and Lyme disease, CFS/ME has moved to centerstage after the COVID-19 pandemic. While the diagnosis of COVID-19–related CFS/ME has advanced in the field, a clear mechanistic explanation of why it occurs is still missing. Certain risk factors have been identified for the development of CFS/ME, including female sex, reactivation of herpesviruses, and presence of connective tissue disorders; however, about one-third of patients with CFS/ME do not have identifiable risk factors.9,10 Persistence of viral particles11 and prolonged inflammatory states are speculated to affect the nervous system and mitochondrial function and metabolism. Interestingly, there is no correlation between severity of initial COVID-19 illness and the development of CFS/ME, similar to observations in non–COVID-19–related CFS/ME.
Proposed therapy
There is currently no proven therapy for CFS/ME. At this time, several immunomodulatory, antiviral, and neuromodulator drugs are being tested in clinical trial networks around the world.12 Usual physical therapy with near maximum intensity has been shown to exacerbate symptoms and often results in PEM, which is described as a “crash” or “full collapse” by patients. The time for recovery after such episodes can be several days.13
Instead, the focus should be on addressing “treatable” concomitant symptoms, such as sleep disorders, anxiety and depression, and chronic pain. Lifestyle changes, avoidance of triggers, and exercise without over exertion are currently recommended to avoid incapacitating PEM.
Gaps in knowledge
There is a large knowledge gap regarding the pathophysiology, prevention, and therapy for CFS/ME. Many health care practitioners are not familiar with the disease and have focused on measurable parameters of exercise limitations and fatigue, such as anemias and lung and cardiac impairments, thus treating CFS/ME as a form of deconditioning. Given the large number of patients who recovered from acute COVID-19 that are now disabled due to CFS/ME, a patient-centered research opportunity has arisen. Biomedical/mechanistic research is ongoing, and well-designed clinical trials evaluating pharmacologic intervention as well as tailored exercise programs are needed.
Conclusion
General practitioners and women’s health specialists need to be aware of CFS/ME, especially when managing patients with long COVID. They also need to know that typical physical therapy may worsen symptoms. Furthermore, clinicians should shy away from trial drugs with a theoretical benefit outside of a clinical trial. ●
- Chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) has been recognized as a major component of PASC
- Typical physical therapy has been shown to exacerbate symptoms of CFS/ME
- Treatment should focus on addressing “treatable” concomitant symptoms, lifestyle changes, avoidance of triggers, and exercise without over exertion
References
- Soriano JB, Murthy S, Marshall JC, et al. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 2022;22:e102-e107. doi: 10.1016 /S1473-3099(21)00703-9
- Chen C, Haupert SR, Zimmermann L, et al. Global prevalence of post-coronavirus disease 2019 (COVID-19) condition or long COVID: a meta-analysis and systematic review. J Infect Dis. 2022;226:1593-1607. doi: 10.1093/infdis/jiac136
- Davis HE, McCorkell L, Vogel JM, et al. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21:133-146. doi: 10.1038/s41579-022 -00846-2
- Pavli A, Theodoridou M, Maltezou HC. Post-COVID syndrome: incidence, clinical spectrum, and challenges for primary healthcare professionals. Arch Med Res. 2021;52:575-581. doi: 10.1016/j.arcmed.2021.03.010
- Kedor C, Freitag H, Meyer-Arndt L, et al. A prospective observational study of post-COVID-19 chronic fatigue syndrome following the first pandemic wave in Germany and biomarkers associated with symptom severity. Nat Commun. 2022;13:5104. doi: 10.1038/s41467-022-32507-6
- Bateman L, Bested AC, Bonilla HF, et al. Myalgic encephalomyelitis/chronic fatigue syndrome: essentials of diagnosis and management. Mayo Clin Proc. 2021;96:28612878. doi: 10.1016/j.mayocp.2021.07.004
- Wessely S. History of postviral fatigue syndrome. Br Med Bull. 1991;47:919-941. doi: 10.1093/oxfordjournals.bmb.a072521
- Committee on the Diagnostic Criteria for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome; Board on the Health of Select Populations; Institute of Medicine. Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness. National Academies Press; 2015. doi: 10.17226/19012
- Ceban F, Ling S, Lui LMW, et al. Fatigue and cognitive impairment in post-COVID-19 syndrome: a systematic review and meta-analysis. Brain Behav Immun. 2022;101:93135. doi: 10.1016/j.bbi.2021.12.020
- Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019. doi: 10.1016/j.eclinm.2021.101019
- Hanson MR. The viral origin of myalgic encephalomyelitis/ chronic fatigue syndrome. PLoS Pathog. 2023;19:e1011523. doi: 10.1371/journal.ppat.1011523
- Scheibenbogen C, Bellmann-Strobl JT, Heindrich C, et al. Fighting post-COVID and ME/CFS—development of curative therapies. Front Med (Lausanne). 2023;10:1194754. doi: 10.3389/fmed.2023.1194754
- Stussman B, Williams A, Snow J, et al. Characterization of post-exertional malaise in patients with myalgic encephalomyelitis/chronic fatigue syndrome. Front Neurol. 2020;11:1025. doi: 10.3389/fneur.2020.01025
Long COVID (postacute sequelae of SARS-CoV-2 infection, or PASC) is an emerging syndrome that affects 50% to 70% of people who survive COVID-19 for up to 3 months or longer after acute disease.1 It is a multisystem condition that causes dysfunction of respiratory, cardiac, and nervous tissue, at least in part likely due to alterations in cellular energy metabolism and reduced oxygen supply to tissue.3 Patients who have had SARS-CoV-2 infection report persistent symptoms and signs that affect their quality of life. These may include neurocognitive, cardiorespiratory, gastrointestinal, and musculoskeletal symptoms; loss of taste and smell; and constitutional symptoms.2 There is no one test to determine if symptoms are due to COVID-19.3
Acute COVID-19 mortality risk factors include increasing age, chronic comorbidities, and male sex. However, long COVID risk factors are quite different. A meta-analysis and review of 20 articles that met inclusion criteria (n = 13,340 study participants), limited by pooling of crude estimates, found that risk factors were female sex and severity of acute disease.4 A second meta-analysis of 37 studies with 1 preprint found that female sex and comorbidities such as pulmonary disease, diabetes, and obesity were risk factors for long COVID.5 Qualitative analysis of single studies (n = 18 study participants) suggested that older adults can develop more long COVID symptoms than younger adults, but this association between advancing age and long COVID was not supported when data were pooled into a meta-analysis.3 However, both single studies (n = 16 study participants) and the meta-analysis (n = 7 study participants) did support female sex as a risk factor for long COVID, along with single studies suggesting increased risk with medical comorbidities for pulmonary disease, diabetes, and organ transplantation.
Perimenopause
Perimenopause: A temporary disruption to physiologic ovarian steroid hormone production following COVID could acutely exacerbate symptoms of perimenopause and menopause.
JoAnn V. Pinkerton, MD, MSCP
The higher prevalence of long COVID in women younger than 50 years6 supports the overlap that studies have shown between symptoms of long COVID and perimenopause,7 as the median age of natural menopause is 51 years. Thus, health care providers need to differentiate between long COVID and other conditions, such as perimenopause, which share similar symptoms (FIGURE). Perimenopause might be diagnosed as long COVID, or the 2 might affect each other.

Symptoms of long COVID include fatigue, brain fog, and increased heart rate after recovering from COVID-19 and may continue or increase after an initial infection.8 Common symptoms of perimenopause and menopause, which also could be seen with long COVID, include typical menopausal symptoms such as hot flashes, night sweats, or disrupted sleep; changes in mood including dysthymia, depression, anxiety, or emotional lability; cognitive concerns such as brain fog or decreased concentration; and decreased stamina, fatigue, joint and muscle pains, or more frequent headaches. Therefore, women in their 40s or 50s with persistent symptoms after having COVID-19 without an alternative diagnosis, and who present with menstrual irregularity,9 hot flashes, or night sweats, could be having an exacerbation of perimenopausal symptoms, or they could be experiencing a combination of long COVID and perimenopausal symptoms.
- Consider long COVID, versus perimenopause, or both, in women aged younger than 50 years
- Estradiol, which has been shown to alleviate perimenopausal and menopausal symptoms, also has been shown to have beneficial effects during acute COVID-19 infection
- Hormone therapy could improve symptoms of perimenopause and long COVID if some of the symptoms are due to changes in ovary function
Continue to: Potential pathophysiology...
Potential pathophysiology
Inflammation is likely to be critical in the pathogenesis of postacute sequelae of SARS-CoV-2 infection, or PASC. Individuals with long COVID have elevated inflammatory markers for several months.10 The chronic inflammation associated with long COVID could cause disturbances in the ovary and ovarian hormone production.2,10,11
During perimenopause, the ovary is more sensitive to illnesses such as COVID-19and to stress. The current theory is that COVID-19 affects the ovary with declines in ovarian reserve and ovarian function7 and with potential disruptions to the menstrual cycle, gonadal function, and ovarian sufficiency that lead to issues with menopause or fertility, as well as symptom exacerbation around menstruation.12 Another theory is that SARS-CoV-2 infection affects ovary hormone production, as there is an abundance of angiotensin-converting enzyme-2 receptors on ovarian and endometrial tissue.11 Thus, it makes sense that long COVID could bring on symptoms of perimenopause or menopause more acutely or more severely or lengthen the duration of perimenopausal symptoms.
Perimenopause is the transitional period prior to menopause, when the ovaries gradually produce fewer hormones and is associated with erratic hormonal fluctuations. The length of this transitional period varies from 4 to 10 years. Ethnic variations in the duration of hot flashes have been found, noting that Black and Hispanic women have them for an average of 8 to 10 years (longer), White women for an average of 7 years, and Asian, Japanese, and Chinese women for an average of 5 to 6 years (shorter).17
What should health care providers ask?
Distinguishing perimenopause from long COVID. It is important to try to differentiate between perimenopause and long COVID, and it is possible to have both, with long COVID exacerbating the menopausal symptoms.7,8 Health care providers should be alert to consider perimenopause if women present with shorter or longer cycles (21-40 days), missed periods (particularly 60 days or 2 months), or worsening perimenopausal mood, migraines, insomnia, or hot flashes. Clinicians should actively enquire about all of these symptoms.
Moreover, if a perimenopausal woman reports acutely worsening symptoms after COVID-19, health care providers should address the perimenopausal symptoms and determine whether hormone therapy is appropriate and could improve their symptoms. Women do not need to wait until they go 1 year without a period to be treated with hormone therapy to improve perimenopausal and menopausal symptoms. If women with long COVID have perimenopause or menopause symptoms, they should have access to evidence-based information and discuss menopausal hormone therapy if appropriate. Hormone therapy could improve both perimenopausal symptoms and the long COVID symptoms if some of the symptoms are due to changes in ovary function. Health care providers could consider progesterone or antidepressants during the second half of the cycle (luteal phase) or estrogen combined with progesterone for the entire cycle.18
For health care providers working in long COVID clinics, in addition to asking when symptoms started, what makes symptoms worse, the frequency of symptoms, and which activities are affected, ask about perimenopausal and menopausal symptoms. If a woman has irregular periods, sleep disturbances, fatigue, or mood changes, consider that these could be related to long COVID, perimenopause, or both.8,18 Be able to offer treatment or refer patients to a women’s health specialist who can assess and offer treatment.
A role for vitamin D? A recent retrospective case-matched study found that 6 months after hospital discharge, patients with long COVID had lower levels of 25(OH) vitamin D with the most notable symptom being brain fog.19 Thus, there may be a role for vitamin D supplementation as a preventive strategy in those being discharged after hospitalization. Vitamin D levels and supplementation have not been otherwise evaluated to date.
Lifestyle strategies for women with perimenopause and long COVID
Lifestyle strategies should be encouraged for women during perimenopause and long COVID. This includes good nutrition (avoiding carbs and sweets, particularly before menses), getting at least 7 hours of sleep and using sleep hygiene (regular bedtimes, sleep regimen, no late screens), getting regular exercise 5 days per week, reducing stress, avoiding excess alcohol, and not smoking. All of these factors can help women and their ovarian function during this period of ovarian fluctuations.
The timing of menopause and COVID may coincide with midlife stressors, including relationship issues (separations or divorce), health issues for the individual or their partner, widowhood, parenting challenges (care of young children, struggles with adolescents, grown children returning home), being childless, concerns about aging parents and caregiving responsibilities, as well as midlife career, community, or education issues—all of which make both long COVID and perimenopause more challenging to navigate.
Need for research
There is a need for future research to understand the epidemiologic basis and underlying biological mechanisms of sex differences seen in women with long COVID. Studying the effects of COVID-19 on ovarian function could lead to a better understanding of perimenopause, what causes ovarian failure to speed up, and possibly ways to slow it down8 since there are health risks of early menopause.16
References
- Fernández-de-Las-Peñas C, Palacios-Ceña D, GómezMayordomo V, et al. Defining post-COVID symptoms (postacute COVID, long COVID, persistent post-COVID): an integrative classification. Int J Environ Res Public Health. 2021;18:2621. doi: 10.3390/ijerph18052621
- Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601-615. doi: 10.1038/s41591 -021-01283-z
- Davis HE, McCorkell L, Vogel JM, et al. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21:133-146. doi: 10.1038/s41579-022-00846-2
- Maglietta G, Diodati F, Puntoni M, et al. Prognostic factors for post-COVID-19 syndrome: a systematic review and meta-analysis. J Clin Med. 2022;11:1541. doi: 10.3390 /jcm11061541
- Notarte KI, de Oliveira MHS, Peligro PJ, et al. Age, sex and previous comorbidities as risk factors not associated with SARS-CoV-2 infection for long COVID-19: a systematic review and meta-analysis. J Clin Med. 2022;11:7314. doi: 10.3390 /jcm11247314
- Sigfrid L, Drake TM, Pauley E, et al. Long COVID in adults discharged from UK hospitals after COVID-19: a prospective, multicentre cohort study using the ISARIC WHO Clinical Characterisation Protocol. Lancet Reg Health Eur. 2021;8:100186. doi: 10.1016/j.lanepe.2021.100186
- Pollack B, von Saltza E, McCorkell L, et al. Female reproductive health impacts of long COVID and associated illnesses including ME/CFS, POTS, and connective tissue disorders: a literature review. Front Rehabil Sci. 2023;4:1122673. doi: 10.3389/fresc.2023.1122673
- Stewart S, Newson L, Briggs TA, et al. Long COVID risk - a signal to address sex hormones and women’s health. Lancet Reg Health Eur. 2021;11:100242. doi: 10.1016 /j.lanepe.2021.100242
- Li K, Chen G, Hou H, et al. Analysis of sex hormones and menstruation in COVID-19 women of child-bearing age. Reprod Biomed Online. 2021;42:260-267. doi: 10.1016 /j.rbmo.2020.09.020
- Phetsouphanh C, Darley DR, Wilson DB, et al. Immunological dysfunction persists for 8 months following initial mild-tomoderate SARS-CoV-2 infection. Nat Immunol. 2022;23:210216. doi: 10.1038/s41590-021-01113-x
- Sharp GC, Fraser A, Sawyer G, et al. The COVID-19 pandemic and the menstrual cycle: research gaps and opportunities. Int J Epidemiol. 2022;51:691-700. doi: 10.1093/ije/dyab239
- Ding T, Wang T, Zhang J, et al. Analysis of ovarian injury associated with COVID-19 disease in reproductive-aged women in Wuhan, China: an observational study. Front Med (Lausanne). 2021;8:635255. doi: 10.3389/fmed.2021.635255
- Huang B, Cai Y, Li N, et al. Sex-based clinical and immunological differences in COVID-19. BMC Infect Dis. 2021;21:647. doi: 10.1186/s12879-021-06313-2
- Connor J, Madhavan S, Mokashi M, et al. Health risks and outcomes that disproportionately affect women during the Covid-19 pandemic: a review. Soc Sci Med. 2020;266:113364. doi: 10.1016/j.socscimed.2020.113364
- Mauvais-Jarvis F, Klein SL, Levin ER. Estradiol, progesterone, immunomodulation, and COVID-19 outcomes. Endocrinology. 2020;161:bqaa127. doi:10.1210/endocr/bqaa127
- The 2022 hormone therapy position statement of The North American Menopause Society. Menopause. 2022;29:767-794. doi: 10.1097/GME.0000000000002028
- Avis NE, Crawford SL, Greendale G, et al. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med. 2015;175:531-539. doi:10.1001 /jamainternmed.2014.8063
- Newson L, Lewis R, O’Hara M. Long COVID and menopause - the important role of hormones in long COVID must be considered. Maturitas. 2021;152:74. doi: 10.1016 /j.maturitas.2021.08.026
- di Filippo L, Frara S, Nannipieri F, et al. Low Vitamin D levels are associated with long COVID syndrome in COVID-19 survivors. J Clin Endocrinol Metab. 2023;108:e1106-e1116. doi: 10.1210/clinem/dgad207
Continue to: Chronic fatigue syndrome...
Chronic fatigue syndrome
Chronic fatigue syndrome: A large number of patients have “post-COVID conditions” affecting everyday function, including depression/anxiety, insomnia, and chronic fatigue (with a 3:1 female predominance)
Alexandra Kadl, MD
After 3 years battling acute COVID-19 infections, we encounter now a large number of patients with PASC— also known as “long COVID,” “COVID long-hauler syndrome,” and “post-COVID conditions”—a persistent multisystem syndrome that impacts everyday function.1 As of October 2023, there are more than 100 million COVID-19 survivors reported in the United States; 10% to 85% of COVID survivors2-4 may show lingering, life-altering symptoms after recovery. Common reported symptoms include fatigue, depression/ anxiety, insomnia, and brain fog/difficulty concentrating, which are particularly high in women who often had experienced only mild acute COVID-19 disease and were not even hospitalized. More recently, chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) has been recognized as major component of PASC5 with a 3:1 female predominance.6 Up to 75% of patients with this diagnosis are not able to maintain their jobs and normal life, and up to 25% are so disabled that they are bedbound.6
Diagnosis
Although illnesses resembling CFS have been reported for more than 200 years,7 the diagnosis of CFS/ME remains difficult to make. There is a likely underreporting due to fear of being labeled as malingering when reaching out to health care providers, and there is a reporting bias toward higher socioeconomic groups due to better access to health care. The current criteria for the diagnosis of CFS/ME include the following 3 components8:
- substantial impairment in the ability to function for more than 6 months, accompanied by profound fatigue, not alleviated by rest
- post-exertional malaise (PEM; prolonged, disabling exacerbation of the patient’s baseline symptoms after exercise)
- non-refreshing sleep, PLUS either cognitive impairment or orthostatic intolerance.
Pathophysiology
Originally found to evolve in a small patient population with Epstein-Barr virus infection and Lyme disease, CFS/ME has moved to centerstage after the COVID-19 pandemic. While the diagnosis of COVID-19–related CFS/ME has advanced in the field, a clear mechanistic explanation of why it occurs is still missing. Certain risk factors have been identified for the development of CFS/ME, including female sex, reactivation of herpesviruses, and presence of connective tissue disorders; however, about one-third of patients with CFS/ME do not have identifiable risk factors.9,10 Persistence of viral particles11 and prolonged inflammatory states are speculated to affect the nervous system and mitochondrial function and metabolism. Interestingly, there is no correlation between severity of initial COVID-19 illness and the development of CFS/ME, similar to observations in non–COVID-19–related CFS/ME.
Proposed therapy
There is currently no proven therapy for CFS/ME. At this time, several immunomodulatory, antiviral, and neuromodulator drugs are being tested in clinical trial networks around the world.12 Usual physical therapy with near maximum intensity has been shown to exacerbate symptoms and often results in PEM, which is described as a “crash” or “full collapse” by patients. The time for recovery after such episodes can be several days.13
Instead, the focus should be on addressing “treatable” concomitant symptoms, such as sleep disorders, anxiety and depression, and chronic pain. Lifestyle changes, avoidance of triggers, and exercise without over exertion are currently recommended to avoid incapacitating PEM.
Gaps in knowledge
There is a large knowledge gap regarding the pathophysiology, prevention, and therapy for CFS/ME. Many health care practitioners are not familiar with the disease and have focused on measurable parameters of exercise limitations and fatigue, such as anemias and lung and cardiac impairments, thus treating CFS/ME as a form of deconditioning. Given the large number of patients who recovered from acute COVID-19 that are now disabled due to CFS/ME, a patient-centered research opportunity has arisen. Biomedical/mechanistic research is ongoing, and well-designed clinical trials evaluating pharmacologic intervention as well as tailored exercise programs are needed.
Conclusion
General practitioners and women’s health specialists need to be aware of CFS/ME, especially when managing patients with long COVID. They also need to know that typical physical therapy may worsen symptoms. Furthermore, clinicians should shy away from trial drugs with a theoretical benefit outside of a clinical trial. ●
- Chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) has been recognized as a major component of PASC
- Typical physical therapy has been shown to exacerbate symptoms of CFS/ME
- Treatment should focus on addressing “treatable” concomitant symptoms, lifestyle changes, avoidance of triggers, and exercise without over exertion
References
- Soriano JB, Murthy S, Marshall JC, et al. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 2022;22:e102-e107. doi: 10.1016 /S1473-3099(21)00703-9
- Chen C, Haupert SR, Zimmermann L, et al. Global prevalence of post-coronavirus disease 2019 (COVID-19) condition or long COVID: a meta-analysis and systematic review. J Infect Dis. 2022;226:1593-1607. doi: 10.1093/infdis/jiac136
- Davis HE, McCorkell L, Vogel JM, et al. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21:133-146. doi: 10.1038/s41579-022 -00846-2
- Pavli A, Theodoridou M, Maltezou HC. Post-COVID syndrome: incidence, clinical spectrum, and challenges for primary healthcare professionals. Arch Med Res. 2021;52:575-581. doi: 10.1016/j.arcmed.2021.03.010
- Kedor C, Freitag H, Meyer-Arndt L, et al. A prospective observational study of post-COVID-19 chronic fatigue syndrome following the first pandemic wave in Germany and biomarkers associated with symptom severity. Nat Commun. 2022;13:5104. doi: 10.1038/s41467-022-32507-6
- Bateman L, Bested AC, Bonilla HF, et al. Myalgic encephalomyelitis/chronic fatigue syndrome: essentials of diagnosis and management. Mayo Clin Proc. 2021;96:28612878. doi: 10.1016/j.mayocp.2021.07.004
- Wessely S. History of postviral fatigue syndrome. Br Med Bull. 1991;47:919-941. doi: 10.1093/oxfordjournals.bmb.a072521
- Committee on the Diagnostic Criteria for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome; Board on the Health of Select Populations; Institute of Medicine. Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness. National Academies Press; 2015. doi: 10.17226/19012
- Ceban F, Ling S, Lui LMW, et al. Fatigue and cognitive impairment in post-COVID-19 syndrome: a systematic review and meta-analysis. Brain Behav Immun. 2022;101:93135. doi: 10.1016/j.bbi.2021.12.020
- Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019. doi: 10.1016/j.eclinm.2021.101019
- Hanson MR. The viral origin of myalgic encephalomyelitis/ chronic fatigue syndrome. PLoS Pathog. 2023;19:e1011523. doi: 10.1371/journal.ppat.1011523
- Scheibenbogen C, Bellmann-Strobl JT, Heindrich C, et al. Fighting post-COVID and ME/CFS—development of curative therapies. Front Med (Lausanne). 2023;10:1194754. doi: 10.3389/fmed.2023.1194754
- Stussman B, Williams A, Snow J, et al. Characterization of post-exertional malaise in patients with myalgic encephalomyelitis/chronic fatigue syndrome. Front Neurol. 2020;11:1025. doi: 10.3389/fneur.2020.01025
Long COVID (postacute sequelae of SARS-CoV-2 infection, or PASC) is an emerging syndrome that affects 50% to 70% of people who survive COVID-19 for up to 3 months or longer after acute disease.1 It is a multisystem condition that causes dysfunction of respiratory, cardiac, and nervous tissue, at least in part likely due to alterations in cellular energy metabolism and reduced oxygen supply to tissue.3 Patients who have had SARS-CoV-2 infection report persistent symptoms and signs that affect their quality of life. These may include neurocognitive, cardiorespiratory, gastrointestinal, and musculoskeletal symptoms; loss of taste and smell; and constitutional symptoms.2 There is no one test to determine if symptoms are due to COVID-19.3
Acute COVID-19 mortality risk factors include increasing age, chronic comorbidities, and male sex. However, long COVID risk factors are quite different. A meta-analysis and review of 20 articles that met inclusion criteria (n = 13,340 study participants), limited by pooling of crude estimates, found that risk factors were female sex and severity of acute disease.4 A second meta-analysis of 37 studies with 1 preprint found that female sex and comorbidities such as pulmonary disease, diabetes, and obesity were risk factors for long COVID.5 Qualitative analysis of single studies (n = 18 study participants) suggested that older adults can develop more long COVID symptoms than younger adults, but this association between advancing age and long COVID was not supported when data were pooled into a meta-analysis.3 However, both single studies (n = 16 study participants) and the meta-analysis (n = 7 study participants) did support female sex as a risk factor for long COVID, along with single studies suggesting increased risk with medical comorbidities for pulmonary disease, diabetes, and organ transplantation.
Perimenopause
Perimenopause: A temporary disruption to physiologic ovarian steroid hormone production following COVID could acutely exacerbate symptoms of perimenopause and menopause.
JoAnn V. Pinkerton, MD, MSCP
The higher prevalence of long COVID in women younger than 50 years6 supports the overlap that studies have shown between symptoms of long COVID and perimenopause,7 as the median age of natural menopause is 51 years. Thus, health care providers need to differentiate between long COVID and other conditions, such as perimenopause, which share similar symptoms (FIGURE). Perimenopause might be diagnosed as long COVID, or the 2 might affect each other.

Symptoms of long COVID include fatigue, brain fog, and increased heart rate after recovering from COVID-19 and may continue or increase after an initial infection.8 Common symptoms of perimenopause and menopause, which also could be seen with long COVID, include typical menopausal symptoms such as hot flashes, night sweats, or disrupted sleep; changes in mood including dysthymia, depression, anxiety, or emotional lability; cognitive concerns such as brain fog or decreased concentration; and decreased stamina, fatigue, joint and muscle pains, or more frequent headaches. Therefore, women in their 40s or 50s with persistent symptoms after having COVID-19 without an alternative diagnosis, and who present with menstrual irregularity,9 hot flashes, or night sweats, could be having an exacerbation of perimenopausal symptoms, or they could be experiencing a combination of long COVID and perimenopausal symptoms.
- Consider long COVID, versus perimenopause, or both, in women aged younger than 50 years
- Estradiol, which has been shown to alleviate perimenopausal and menopausal symptoms, also has been shown to have beneficial effects during acute COVID-19 infection
- Hormone therapy could improve symptoms of perimenopause and long COVID if some of the symptoms are due to changes in ovary function
Continue to: Potential pathophysiology...
Potential pathophysiology
Inflammation is likely to be critical in the pathogenesis of postacute sequelae of SARS-CoV-2 infection, or PASC. Individuals with long COVID have elevated inflammatory markers for several months.10 The chronic inflammation associated with long COVID could cause disturbances in the ovary and ovarian hormone production.2,10,11
During perimenopause, the ovary is more sensitive to illnesses such as COVID-19and to stress. The current theory is that COVID-19 affects the ovary with declines in ovarian reserve and ovarian function7 and with potential disruptions to the menstrual cycle, gonadal function, and ovarian sufficiency that lead to issues with menopause or fertility, as well as symptom exacerbation around menstruation.12 Another theory is that SARS-CoV-2 infection affects ovary hormone production, as there is an abundance of angiotensin-converting enzyme-2 receptors on ovarian and endometrial tissue.11 Thus, it makes sense that long COVID could bring on symptoms of perimenopause or menopause more acutely or more severely or lengthen the duration of perimenopausal symptoms.
Perimenopause is the transitional period prior to menopause, when the ovaries gradually produce fewer hormones and is associated with erratic hormonal fluctuations. The length of this transitional period varies from 4 to 10 years. Ethnic variations in the duration of hot flashes have been found, noting that Black and Hispanic women have them for an average of 8 to 10 years (longer), White women for an average of 7 years, and Asian, Japanese, and Chinese women for an average of 5 to 6 years (shorter).17
What should health care providers ask?
Distinguishing perimenopause from long COVID. It is important to try to differentiate between perimenopause and long COVID, and it is possible to have both, with long COVID exacerbating the menopausal symptoms.7,8 Health care providers should be alert to consider perimenopause if women present with shorter or longer cycles (21-40 days), missed periods (particularly 60 days or 2 months), or worsening perimenopausal mood, migraines, insomnia, or hot flashes. Clinicians should actively enquire about all of these symptoms.
Moreover, if a perimenopausal woman reports acutely worsening symptoms after COVID-19, health care providers should address the perimenopausal symptoms and determine whether hormone therapy is appropriate and could improve their symptoms. Women do not need to wait until they go 1 year without a period to be treated with hormone therapy to improve perimenopausal and menopausal symptoms. If women with long COVID have perimenopause or menopause symptoms, they should have access to evidence-based information and discuss menopausal hormone therapy if appropriate. Hormone therapy could improve both perimenopausal symptoms and the long COVID symptoms if some of the symptoms are due to changes in ovary function. Health care providers could consider progesterone or antidepressants during the second half of the cycle (luteal phase) or estrogen combined with progesterone for the entire cycle.18
For health care providers working in long COVID clinics, in addition to asking when symptoms started, what makes symptoms worse, the frequency of symptoms, and which activities are affected, ask about perimenopausal and menopausal symptoms. If a woman has irregular periods, sleep disturbances, fatigue, or mood changes, consider that these could be related to long COVID, perimenopause, or both.8,18 Be able to offer treatment or refer patients to a women’s health specialist who can assess and offer treatment.
A role for vitamin D? A recent retrospective case-matched study found that 6 months after hospital discharge, patients with long COVID had lower levels of 25(OH) vitamin D with the most notable symptom being brain fog.19 Thus, there may be a role for vitamin D supplementation as a preventive strategy in those being discharged after hospitalization. Vitamin D levels and supplementation have not been otherwise evaluated to date.
Lifestyle strategies for women with perimenopause and long COVID
Lifestyle strategies should be encouraged for women during perimenopause and long COVID. This includes good nutrition (avoiding carbs and sweets, particularly before menses), getting at least 7 hours of sleep and using sleep hygiene (regular bedtimes, sleep regimen, no late screens), getting regular exercise 5 days per week, reducing stress, avoiding excess alcohol, and not smoking. All of these factors can help women and their ovarian function during this period of ovarian fluctuations.
The timing of menopause and COVID may coincide with midlife stressors, including relationship issues (separations or divorce), health issues for the individual or their partner, widowhood, parenting challenges (care of young children, struggles with adolescents, grown children returning home), being childless, concerns about aging parents and caregiving responsibilities, as well as midlife career, community, or education issues—all of which make both long COVID and perimenopause more challenging to navigate.
Need for research
There is a need for future research to understand the epidemiologic basis and underlying biological mechanisms of sex differences seen in women with long COVID. Studying the effects of COVID-19 on ovarian function could lead to a better understanding of perimenopause, what causes ovarian failure to speed up, and possibly ways to slow it down8 since there are health risks of early menopause.16
References
- Fernández-de-Las-Peñas C, Palacios-Ceña D, GómezMayordomo V, et al. Defining post-COVID symptoms (postacute COVID, long COVID, persistent post-COVID): an integrative classification. Int J Environ Res Public Health. 2021;18:2621. doi: 10.3390/ijerph18052621
- Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601-615. doi: 10.1038/s41591 -021-01283-z
- Davis HE, McCorkell L, Vogel JM, et al. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21:133-146. doi: 10.1038/s41579-022-00846-2
- Maglietta G, Diodati F, Puntoni M, et al. Prognostic factors for post-COVID-19 syndrome: a systematic review and meta-analysis. J Clin Med. 2022;11:1541. doi: 10.3390 /jcm11061541
- Notarte KI, de Oliveira MHS, Peligro PJ, et al. Age, sex and previous comorbidities as risk factors not associated with SARS-CoV-2 infection for long COVID-19: a systematic review and meta-analysis. J Clin Med. 2022;11:7314. doi: 10.3390 /jcm11247314
- Sigfrid L, Drake TM, Pauley E, et al. Long COVID in adults discharged from UK hospitals after COVID-19: a prospective, multicentre cohort study using the ISARIC WHO Clinical Characterisation Protocol. Lancet Reg Health Eur. 2021;8:100186. doi: 10.1016/j.lanepe.2021.100186
- Pollack B, von Saltza E, McCorkell L, et al. Female reproductive health impacts of long COVID and associated illnesses including ME/CFS, POTS, and connective tissue disorders: a literature review. Front Rehabil Sci. 2023;4:1122673. doi: 10.3389/fresc.2023.1122673
- Stewart S, Newson L, Briggs TA, et al. Long COVID risk - a signal to address sex hormones and women’s health. Lancet Reg Health Eur. 2021;11:100242. doi: 10.1016 /j.lanepe.2021.100242
- Li K, Chen G, Hou H, et al. Analysis of sex hormones and menstruation in COVID-19 women of child-bearing age. Reprod Biomed Online. 2021;42:260-267. doi: 10.1016 /j.rbmo.2020.09.020
- Phetsouphanh C, Darley DR, Wilson DB, et al. Immunological dysfunction persists for 8 months following initial mild-tomoderate SARS-CoV-2 infection. Nat Immunol. 2022;23:210216. doi: 10.1038/s41590-021-01113-x
- Sharp GC, Fraser A, Sawyer G, et al. The COVID-19 pandemic and the menstrual cycle: research gaps and opportunities. Int J Epidemiol. 2022;51:691-700. doi: 10.1093/ije/dyab239
- Ding T, Wang T, Zhang J, et al. Analysis of ovarian injury associated with COVID-19 disease in reproductive-aged women in Wuhan, China: an observational study. Front Med (Lausanne). 2021;8:635255. doi: 10.3389/fmed.2021.635255
- Huang B, Cai Y, Li N, et al. Sex-based clinical and immunological differences in COVID-19. BMC Infect Dis. 2021;21:647. doi: 10.1186/s12879-021-06313-2
- Connor J, Madhavan S, Mokashi M, et al. Health risks and outcomes that disproportionately affect women during the Covid-19 pandemic: a review. Soc Sci Med. 2020;266:113364. doi: 10.1016/j.socscimed.2020.113364
- Mauvais-Jarvis F, Klein SL, Levin ER. Estradiol, progesterone, immunomodulation, and COVID-19 outcomes. Endocrinology. 2020;161:bqaa127. doi:10.1210/endocr/bqaa127
- The 2022 hormone therapy position statement of The North American Menopause Society. Menopause. 2022;29:767-794. doi: 10.1097/GME.0000000000002028
- Avis NE, Crawford SL, Greendale G, et al. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med. 2015;175:531-539. doi:10.1001 /jamainternmed.2014.8063
- Newson L, Lewis R, O’Hara M. Long COVID and menopause - the important role of hormones in long COVID must be considered. Maturitas. 2021;152:74. doi: 10.1016 /j.maturitas.2021.08.026
- di Filippo L, Frara S, Nannipieri F, et al. Low Vitamin D levels are associated with long COVID syndrome in COVID-19 survivors. J Clin Endocrinol Metab. 2023;108:e1106-e1116. doi: 10.1210/clinem/dgad207
Continue to: Chronic fatigue syndrome...
Chronic fatigue syndrome
Chronic fatigue syndrome: A large number of patients have “post-COVID conditions” affecting everyday function, including depression/anxiety, insomnia, and chronic fatigue (with a 3:1 female predominance)
Alexandra Kadl, MD
After 3 years battling acute COVID-19 infections, we encounter now a large number of patients with PASC— also known as “long COVID,” “COVID long-hauler syndrome,” and “post-COVID conditions”—a persistent multisystem syndrome that impacts everyday function.1 As of October 2023, there are more than 100 million COVID-19 survivors reported in the United States; 10% to 85% of COVID survivors2-4 may show lingering, life-altering symptoms after recovery. Common reported symptoms include fatigue, depression/ anxiety, insomnia, and brain fog/difficulty concentrating, which are particularly high in women who often had experienced only mild acute COVID-19 disease and were not even hospitalized. More recently, chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) has been recognized as major component of PASC5 with a 3:1 female predominance.6 Up to 75% of patients with this diagnosis are not able to maintain their jobs and normal life, and up to 25% are so disabled that they are bedbound.6
Diagnosis
Although illnesses resembling CFS have been reported for more than 200 years,7 the diagnosis of CFS/ME remains difficult to make. There is a likely underreporting due to fear of being labeled as malingering when reaching out to health care providers, and there is a reporting bias toward higher socioeconomic groups due to better access to health care. The current criteria for the diagnosis of CFS/ME include the following 3 components8:
- substantial impairment in the ability to function for more than 6 months, accompanied by profound fatigue, not alleviated by rest
- post-exertional malaise (PEM; prolonged, disabling exacerbation of the patient’s baseline symptoms after exercise)
- non-refreshing sleep, PLUS either cognitive impairment or orthostatic intolerance.
Pathophysiology
Originally found to evolve in a small patient population with Epstein-Barr virus infection and Lyme disease, CFS/ME has moved to centerstage after the COVID-19 pandemic. While the diagnosis of COVID-19–related CFS/ME has advanced in the field, a clear mechanistic explanation of why it occurs is still missing. Certain risk factors have been identified for the development of CFS/ME, including female sex, reactivation of herpesviruses, and presence of connective tissue disorders; however, about one-third of patients with CFS/ME do not have identifiable risk factors.9,10 Persistence of viral particles11 and prolonged inflammatory states are speculated to affect the nervous system and mitochondrial function and metabolism. Interestingly, there is no correlation between severity of initial COVID-19 illness and the development of CFS/ME, similar to observations in non–COVID-19–related CFS/ME.
Proposed therapy
There is currently no proven therapy for CFS/ME. At this time, several immunomodulatory, antiviral, and neuromodulator drugs are being tested in clinical trial networks around the world.12 Usual physical therapy with near maximum intensity has been shown to exacerbate symptoms and often results in PEM, which is described as a “crash” or “full collapse” by patients. The time for recovery after such episodes can be several days.13
Instead, the focus should be on addressing “treatable” concomitant symptoms, such as sleep disorders, anxiety and depression, and chronic pain. Lifestyle changes, avoidance of triggers, and exercise without over exertion are currently recommended to avoid incapacitating PEM.
Gaps in knowledge
There is a large knowledge gap regarding the pathophysiology, prevention, and therapy for CFS/ME. Many health care practitioners are not familiar with the disease and have focused on measurable parameters of exercise limitations and fatigue, such as anemias and lung and cardiac impairments, thus treating CFS/ME as a form of deconditioning. Given the large number of patients who recovered from acute COVID-19 that are now disabled due to CFS/ME, a patient-centered research opportunity has arisen. Biomedical/mechanistic research is ongoing, and well-designed clinical trials evaluating pharmacologic intervention as well as tailored exercise programs are needed.
Conclusion
General practitioners and women’s health specialists need to be aware of CFS/ME, especially when managing patients with long COVID. They also need to know that typical physical therapy may worsen symptoms. Furthermore, clinicians should shy away from trial drugs with a theoretical benefit outside of a clinical trial. ●
- Chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) has been recognized as a major component of PASC
- Typical physical therapy has been shown to exacerbate symptoms of CFS/ME
- Treatment should focus on addressing “treatable” concomitant symptoms, lifestyle changes, avoidance of triggers, and exercise without over exertion
References
- Soriano JB, Murthy S, Marshall JC, et al. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 2022;22:e102-e107. doi: 10.1016 /S1473-3099(21)00703-9
- Chen C, Haupert SR, Zimmermann L, et al. Global prevalence of post-coronavirus disease 2019 (COVID-19) condition or long COVID: a meta-analysis and systematic review. J Infect Dis. 2022;226:1593-1607. doi: 10.1093/infdis/jiac136
- Davis HE, McCorkell L, Vogel JM, et al. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21:133-146. doi: 10.1038/s41579-022 -00846-2
- Pavli A, Theodoridou M, Maltezou HC. Post-COVID syndrome: incidence, clinical spectrum, and challenges for primary healthcare professionals. Arch Med Res. 2021;52:575-581. doi: 10.1016/j.arcmed.2021.03.010
- Kedor C, Freitag H, Meyer-Arndt L, et al. A prospective observational study of post-COVID-19 chronic fatigue syndrome following the first pandemic wave in Germany and biomarkers associated with symptom severity. Nat Commun. 2022;13:5104. doi: 10.1038/s41467-022-32507-6
- Bateman L, Bested AC, Bonilla HF, et al. Myalgic encephalomyelitis/chronic fatigue syndrome: essentials of diagnosis and management. Mayo Clin Proc. 2021;96:28612878. doi: 10.1016/j.mayocp.2021.07.004
- Wessely S. History of postviral fatigue syndrome. Br Med Bull. 1991;47:919-941. doi: 10.1093/oxfordjournals.bmb.a072521
- Committee on the Diagnostic Criteria for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome; Board on the Health of Select Populations; Institute of Medicine. Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness. National Academies Press; 2015. doi: 10.17226/19012
- Ceban F, Ling S, Lui LMW, et al. Fatigue and cognitive impairment in post-COVID-19 syndrome: a systematic review and meta-analysis. Brain Behav Immun. 2022;101:93135. doi: 10.1016/j.bbi.2021.12.020
- Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019. doi: 10.1016/j.eclinm.2021.101019
- Hanson MR. The viral origin of myalgic encephalomyelitis/ chronic fatigue syndrome. PLoS Pathog. 2023;19:e1011523. doi: 10.1371/journal.ppat.1011523
- Scheibenbogen C, Bellmann-Strobl JT, Heindrich C, et al. Fighting post-COVID and ME/CFS—development of curative therapies. Front Med (Lausanne). 2023;10:1194754. doi: 10.3389/fmed.2023.1194754
- Stussman B, Williams A, Snow J, et al. Characterization of post-exertional malaise in patients with myalgic encephalomyelitis/chronic fatigue syndrome. Front Neurol. 2020;11:1025. doi: 10.3389/fneur.2020.01025
Time to rethink endometrial ablation: A gyn oncology perspective on the sequelae of an overused procedure
CASE New patient presents with a history of endometrial hyperplasia
A 51-year-old patient (G2P2002) presents to a new gynecologist’s office after moving from a different state. In her medical history, the gynecologist notes that 5 years ago she underwent dilation and curettage and endometrial ablation procedures for heavy menstrual bleeding (HMB). Ultrasonography performed prior to those procedures showed a slightly enlarged uterus, a simple left ovarian cyst, and a non ̶ visualized right ovary. The patient had declined a 2-step procedure due to concerns with anesthesia, and surgical pathology at the time of ablation revealed hyperplasia without atypia. The patient’s medical history was otherwise notable for prediabetes (recent hemoglobin A1c [HbA1c] measurement, 6.0%) and obesity (body mass index, 43 kg/m2). Pertinent family history included her mother’s diagnosis of endometrial cancer at age 36. Given the patient’s diagnosis of endometrial hyperplasia, she was referred to gynecologic oncology, but she ultimately declined hysterectomy, stating that she was happy with the resolution of her abnormal bleeding. At the time of her initial gynecologic oncology consultation, the consultant suggested lifestyle changes to combat prediabetes and obesity to reduce the risk of endometrial cancer, as future signs of cancer, namely bleeding, may be masked by the endometrial ablation. The patient was prescribed metformin given these medical comorbidities.
At today’s appointment, the patient notes continued resolution of bleeding since the procedure. She does, however, note a 6-month history of vasomotor symptoms and one episode of spotting 3 months ago. Three years ago she was diagnosed with type 2 diabetes mellitus, and her current HbA1c is 6.9%. She has gained 10 lb since being diagnosed with endometrial cancer 5 years ago, and she has continued to take metformin.
An in-office endometrial biopsy is unsuccessful due to cervical stenosis. The treating gynecologist orders a transvaginal ultrasound, which reveals a small left ovarian cyst and a thickened endometrium (measuring 10 mm). Concerned that these findings could represent endometrial cancer, the gynecologist refers the patient to gynecologic oncology for further evaluation.
Sequelae and complications following endometrial ablation are often managed by a gynecologic oncologist. Indeed, a 2018 poll of Society of Gynecologic Oncology (SGO) members revealed that 93.8% of respondents had received such a referral, and almost 20% of respondents were managing more than 20 patients with post-ablation complications in their practices.1 These complications, including hematometra, post-ablation tubal sterilization syndrome, other pain syndromes associated with retrograde menstruation, and thickened endometrium with scarring leading to an inability to sample the endometrium to investigate post-ablation bleeding are symptoms and findings that often lead to further surgery, including hysterectomy.2 General gynecologists faced with these complications may refer patients to gynecologic oncology given an inability to sample the post-ablation endometrium or anticipated difficulties with hysterectomy. A recent meta-analysis revealed a 12.4% hysterectomy rate 5 years after endometrial ablation. Among these patients, the incidence of endometrial cancer ranged from 0% to 1.6%.3
In 2023, endometrial cancer incidence continues to increase, as does the incidence of obesity in women of all ages. Endometrial cancer mortality rates are also increasing, and these trends disproportionately affects non-Hispanic Black women.4 As providers and advocates work to narrow these disparities, gynecologic oncologists are simultaneously noting increased referrals for very likely benign conditions.5 Patients referred for post-ablation bleeding are a subset of these, as most patients who undergo endometrial ablation will not develop cancer. Considering the potential bottlenecks created en route to a gynecologic oncology evaluation, it seems prudent to minimize practices, like endometrial ablation, that may directly or indirectly prevent timely referral of patients with cancer to a gynecologic oncologist.
In this review we focus on the current use of endometrial ablation, associated complications, the incidence of treatment failure, and patient selection. Considering these issues in the context of the current endometrial cancer landscape, we posit best practices aimed at optimizing patient outcomes, and empowering general gynecologists to practice cancer prevention and to triage their surgical patients.
- Before performing endometrial ablation, consider whether alternatives such as hysterectomy or insertion of a progestin-containing IUD would be appropriate.
- Clinical management of patients with abnormal bleeding with indications for endometrial ablation should be guidelinedriven.
- Post-ablation bleeding or pain does not inherently require referral to oncology.
- General gynecologists can perform hysterectomy in this setting if appropriate.
- Patients with endometrial hyperplasia at endometrial ablation should be promptly offered hysterectomy. If atypia is not present, this hysterectomy, too, can be performed by a general gynecologist if appropriate, as the chance for malignancy is minimal.
Continue to: Current use of endometrial ablation in the US...
Current use of endometrial ablation in the US
In 2015, more than 500,000 endometrial ablations were performed in the United States.Given the ability to perform in-office ablation, this number is growing and potentially underestimated each year.6 In 2022, the global endometrial ablation market was valued at $3.4 billion, a figure projected to double in 10 years.7 The procedure has evolved as different devices and approaches have developed, offering patients different means to manage bleeding without hysterectomy. The minimally invasive procedure, performed in premenopausal patients with heavy menstrual bleeding (HMB) due to benign causes who have completed childbearing, has been associated with faster recovery times and fewer short-term complications compared with more invasive surgery.8 There are several non-resectoscope ablative devices approved by the US Food and Drug Administration (FDA), and each work to destroy the endometrial lining via thermal or cryoablation. Endometrial ablation can be performed in premenopausal patients with HMB due to benign causes who have completed childbearing.
Recently, promotional literature has begun to report on so-called overuse of hysterectomy, despite decreasing overall hysterectomy rates. This reporting proposes and applies “appropriateness criteria,” accounting for the rate of preoperative counseling regarding alternatives to hysterectomy, as well as the rate of “unsupportive” final pathology.9 The adoption of endometrial ablation and increasing market value of such vendors suggest that this campaign is having its desired effect. From the oncology perspective, we are concerned the pendulum could swing too far away from hysterectomy, a procedure that definitively cures abnormal uterine bleeding, toward endometrial ablation without explicit acknowledgement of the trade-offs involved.
Endometrial ablation complications: Late-onset procedure failure
A number of post-ablation syndromes may present at least 1 month following the procedure. Collectively known as late-onset endometrial ablation failure (LOEAF), these syndromes are characterized by recurrent vaginal bleeding, and/or new cyclic pelvic pain.10 It is difficult to measure the true incidence of LOEAF. Thomassee and colleagues examined a Canadian retrospective cohort of 437 patients who underwent endometrial ablation; 20.8% reported post-ablation pelvic pain after a median 301 days.11 The subsequent need for surgical intervention, often hysterectomy, is a surrogate for LOEAF.
It should be noted that LOEAF is distinct from post-ablation tubal sterilization syndrome (PATSS), which describes cornual menstrual bleeding impeded by the ligated proximal fallopian tube.12 Increased awareness of PATSS, along with the discontinuation of Essure (a permanent hysteroscopic sterilization device) in 2018, has led some surgeons to advocate for concomitant salpingectomy at the time of endometrial ablation.13 The role of opportunistic salpingectomy in primary prevention of epithelial ovarian cancer is well described, and while we strongly support this practice at the time of endometrial ablation, we do not feel that it effectively prevents LOEAF.14
The post-ablation inability to adequately sample the endometrium is also considered a LOEAF. A prospective study of 57 women who underwent endometrial ablation assessed post-ablation sampling feasibility via transvaginal ultrasonography, saline infusion sonohysterography (SIS), and in-office endometrial biopsies. In 23% of the cohort, endometrial sampling failed, and the authors noted decreased reliability of pathologic assessment.15 One systematic review, in which authors examined the incidence of endometrial cancer following endometrial ablation, characterized 38 cases of endometrial cancer and reported a post-ablation endometrial sampling success rate of 89%. This figure was based on a self-selected sample of 18 patients; cases in which endometrial sampling was thought to be impossible were excluded. The study also had a 30% missing data rate and several other biases.16
In the previously mentioned poll of SGO members,1 84% of the surveyed gynecologic oncologists managing post-ablation patients reported that endometrial sampling following endometrial ablation was “moderately” or “extremely” difficult. More than half of the survey respondents believed that hysterectomy was required for accurate diagnosis.1 While we acknowledge the likely sampling bias affecting the survey results, we are not comforted by any data that minimizes this diagnostic challenge.
Appropriate patient selection and contraindications
The ideal candidate for endometrial ablation is a premenopausal patient with HMB who does not desire future fertility. According to the FDA, absolute contraindications include pregnancy or desired fertility, prior ablation, current IUD in place, inadequate preoperative endometrial assessment, known or suspected malignancy, active infection, or unfavorable anatomy.17
What about patients who may be at increased risk for endometrial cancer?
There is a paucity of data regarding the safety of endometrial ablation in patients at increased risk for developing endometrial cancer in the future. The American College of Obstetricians and Gynecologists (ACOG) 2007 practice bulletin on endometrial ablation (no longer accessible online) alludes to this concern and other contraindications,18 but there are no established guidelines. Currently, no ACOG practice bulletin or committee opinion lists relative contraindications to endometrial ablation, long-term complications (except risks associated with future pregnancy), or risk of subsequent hysterectomy. The risk that “it may be harder to detect endometrial cancer after ablation” is noted on ACOG’s web page dedicated to frequently asked questions (FAQs) regarding abnormal uterine bleeding.19 It is not mentioned on their web page dedicated to the FAQs regarding endometrial ablation.20
In the absence of high-quality published data on established contraindications for endometrial ablation, we advocate for the increased awareness of possible relative contraindications—namely well-established risk factors for endometrial cancer (TABLE 1).For example, in a pooled analysis of 24 epidemiologic studies, authors found that the odds of developing endometrial cancer was 7 times higher among patients with a body mass index (BMI) ≥ 40 kg/m2, compared with controls (odds ratio [OR], 7.14; 95% confidence interval [CI], 6.33–8.06).21 Additionally, patients with Lynch syndrome, a history of extended tamoxifen use, or those with a history of chronic anovulation or polycystic ovary syndrome are at increased risk for endometrial cancer.22-24 If the presence of one or more of these factors does not dissuade general gynecologists from performing an endometrial ablation (even armed with a negative preoperative endometrial biopsy), we feel they should at least prompt thoughtful guideline-driven pause.
Continue to: Hysterectomy—A disincentivized option...
Hysterectomy—A disincentivized option
The annual number of hysterectomies performed by general gynecologists has declined over time. One study by Cadish and colleagues revealed that recent residency graduates performed only 3 to 4 annually.25 These numbers partly reflect the decreasing number of hysterectomies performed during residency training. Furthermore, other factors—including the increasing rate of placenta accreta spectrum, the focus on risk stratification of adnexal masses via the ovarian-adnexal reporting and data classification system (O-RADs), and the emphasis on minimally invasive approaches often acquired in subspecialty training—have likely contributed to referral patterns to such specialists as minimally invasive gynecologic surgeons and gynecologic oncologists.26 This trend is self-actualizing, as quality metrics funnel patients to high-volume surgeons, and general gynecologists risk losing hysterectomy privileges.
These factors lend themselves to a growing emphasis on endometrial ablation. Endometrial ablations can be performed in several settings, including in the hospital, in outpatient clinics, and more and more commonly, in ambulatory surgery centers. This increased access to endometrial ablation in the ambulatory surgery setting has corresponded with an annual endometrial ablation market value growth rate of 5% to 7%.27 These rates are likely compounded by payer reimbursement policies that promote endometrial ablation and other alternatives to hysterectomy that are cost savings in the short term.28 While the actual payer models are unavailable to review, they may not consider the costs of LOEAFs, including subsequent hysterectomy up to 5 years after initial ablation procedures. Provocatively, they almost certainly do not consider the costs of delayed care of patients with endometrial cancer vying for gynecologic oncology appointment slots occupied by post-ablation patients.
We urge providers, patients, and advocates to question who benefits from the uptake of ablation procedures: Patients? Payors? Providers? And how will the field of gynecology fare if hysterectomy skills and privileges are supplanted by ablation?
Post-ablation bleeding: Management by the gyn oncologist
Patients with post-ablation bleeding, either immediately or years later, are sometimes referred to a gynecologic oncologist given the possible risk for cancer and need for surgical staging if cancer is found on the hysterectomy specimen. In practice, assuming normal preoperative ultrasonography and no other clinical or radiologic findings suggestive of malignancy (eg, computed tomography findings concerning for metastases, abnormal cervical cytology, etc.), the presence of cancer is extremely unlikely to be determined at the time of surgery. Frozen section is not generally performed on the endometrium; intraoperative evaluation of even the unablated endometrium is notoriously unreliable; and histologic assessment of the ablated endometrium is limited by artifact (FIGURE 1). The abnormalities caused by ablation further impede selection of a representative focus, obfuscating any actionable result.
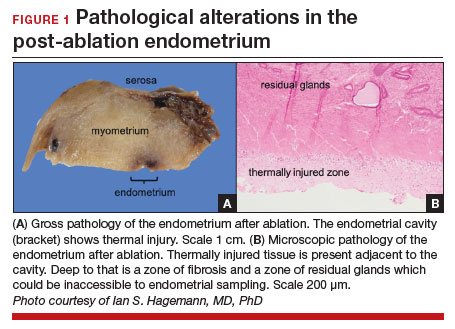
Some surgeons routinely bivalve the excised uterus prior to fixation to assess presence of tumor, tumor size, and the degree of myometrial invasion.29 A combination of factors may compel surgeons to perform lymphadenectomy if not already performed, or if sentinel lymph node mapping was unsuccessful. But this practice has not been studied in patients with post-ablation bleeding, and applying these principles relies on a preoperative diagnosis establishing the presence and grade of a cancer. Furthermore, the utility of frozen section and myometrial assessment to decide whether or not to proceed with lymphadenectomy is less relevant in the era of molecular classification guiding adjuvant therapy. In summary, assuming no pathologic or radiologic findings suggestive of cancer, gynecologic oncologists are unlikely to perform lymphadenectomy at the time of hysterectomy in these post-ablation cases, which therefore can safely be performed by general gynecologists.
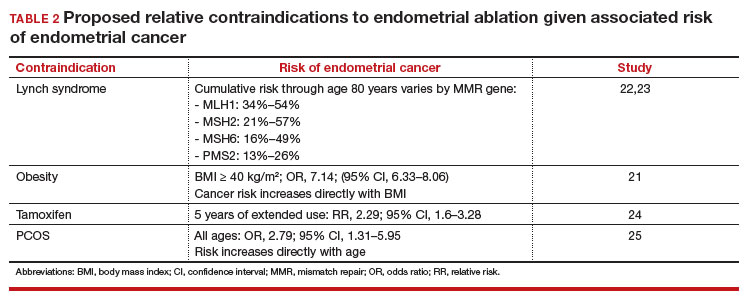
Our recommendations
Consider the LNG-IUD as an alternative to ablation. A recent randomized controlled trial by Beelen and colleagues compared the effectiveness of LNG-releasing IUDs with endometrial ablation in patients with HMB. While the LNG-IUD was inferior to endometrial ablation, quality-of-life measures were similar up to 2 years.31 Realizing that the hysterectomy rate following endometrial ablation increases significantly beyond that time point (2 years), this narrative may be incomplete. A 5- to 10-year follow-up time-frame may be a more helpful gauge of long-term outcomes. This prolonged time-frame also may allow study of the LNG-IUD’s protective effects on the endometrium in the prevention of endometrial hyperplasia and cancer.
Consider hysterectomy. A 2021 Cochrane review revealed that, compared with endometrial ablation, minimally invasive hysterectomy is associated with higher quality-of-life metrics, higher self-reported patient satisfaction, and similar rates of adverse events.32 While patient autonomy is paramount, the developing step-wise approach from endometrial ablation to hysterectomy, and its potential effects on the health care system at a time when endometrial cancer incidence and mortality rates are rising, is troubling.
Postablation, consider hysterectomy by the general gynecologist. Current trends appear to disincentivize general gynecologists from performing hysterectomy either for HMB or LOEAF. We would offer reassurance that they can safely perform this procedure. Referral to oncology may not be necessary since, in the absence of an established diagnosis of cancer, a lymphadenectomy is not typically required. A shift away from referral for these patients can preserve access to oncology for those women, especially minority women, with an explicit need for oncologic care.
In FIGURE 2, we propose a management algorithm for the patient who presents with post–ablation bleeding. We acknowledge that the evidence base for our management recommendations is limited. Still, we hope providers, ACOG, and other guidelines-issuing organizations consider them as they adapt their own practices and recommendations. We believe this is one of many steps needed to improve outcomes for patients with gynecologic cancer, particularly those in marginalized communities disproportionately impacted by current trends.
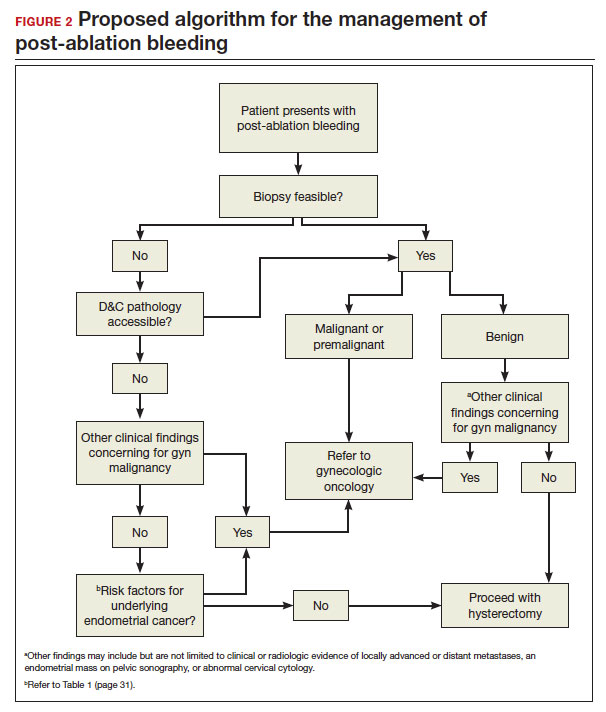
CASE Resolution
After reviewing the relevant documentation and examining the patient, the gynecologic oncology consultant contacts the referring gynecologist. They review the low utility of frozen section and the overall low risk of cancer on the final hysterectomy specimen if the patient were to undergo hysterectomy. The consultant clarifies that there is no other concern for surgical complexity beyond the skill of the referring provider, and they discuss the possibility of referral to a minimally invasive specialist for the surgery.
Ultimately, the patient undergoes uncomplicated laparoscopic hysterectomy performed by the original referring gynecologist. Final pathology reveals inactive endometrium with ablative changes and cornual focus of endometrial hyperplasia without atypia. ●
Acknowledgement
The authors acknowledge Ian Hagemann, MD, PhD, for his review of the manuscript.
- Chen H, Saiz AM, McCausland AM, et al. Experience of gynecologic oncologists regarding endometrial cancer after endometrial ablation. J Clin Oncol. 2018;36:e17566-e.
- McCausland AM, McCausland VM. Long-term complications of endometrial ablation: cause, diagnosis, treatment, and prevention. J Minim Invasive Gynecol. 2007;14:399-406.
- Oderkerk TJ, Beelen P, Bukkems ALA, et al. Risk of hysterectomy after endometrial ablation: a systematic review and meta-analysis. Obstet Gynecol. 2023;142:51-60.
- Clarke MA, Devesa SS, Hammer A, et al. Racial and ethnic differences in hysterectomy-corrected uterine corpus cancer mortality by stage and histologic subtype. JAMA Oncol. 2022;8:895-903.
- Barber EL, Rossi EC, Alexander A, et al. Benign hysterectomy performed by gynecologic oncologists: is selection bias altering our ability to measure surgical quality? Gynecol Oncol. 2018;151:141-144.
- Wortman M. Late-onset endometrial ablation failure. Case Rep Womens Health. 2017;15:11-28.
- Insights FM. Endometrial Ablation Market Outlook.Accessed July 26, 2023. https://www.futuremarketinsights.com/reports/endometrial-ablation -market
- Famuyide A. Endometrial ablation. J Minim Invasive Gynecol. 2018;25:299-307.
- Corona LE, Swenson CW, Sheetz KH, et al. Use of other treatments before hysterectomy for benign conditions in a statewide hospital collaborative. Am J Obstet Gynecol. 2015;212:304.e1-e7.
- Wortman M, Cholkeri A, McCausland AM, et al. Late-onset endometrial ablation failure—etiology, treatment, and prevention. J Minim Invasive Gynecol. 2015;22:323-331.
- Thomassee MS, Curlin H, Yunker A, et al. Predicting pelvic pain after endometrial ablation: which preoperative patient characteristics are associated? J Minim Invasive Gynecol. 2013;20:642-647.
- Townsend DE, McCausland V, McCausland A, et al. Post-ablation-tubal sterilization syndrome. Obstet Gynecol. 1993;82:422-424.
- Greer Polite F, DeAgostino-Kelly M, Marchand GJ. Combination of laparoscopic salpingectomy and endometrial ablation: a potentially underused procedure. J Gynecol Surg. 2021;37:89-91.
- Hanley GE, Pearce CL, Talhouk A, et al. Outcomes from opportunistic salpingectomy for ovarian cancer prevention. JAMA Network Open. 2022;5:e2147343-e.
- Ahonkallio SJ, Liakka AK, Martikainen HK, et al. Feasibility of endometrial assessment after thermal ablation. Eur J Obstet Gynecol Reprod Biol. 2009;147:69-71.
- Tamara JO, Mileen RDvdK, Karlijn MCC, et al. Endometrial cancer after endometrial ablation: a systematic review. Int J Gynecol Cancer. 2022;32:1555.
- US Food and Drug Administration. Endometrial ablation for heavy menstrual bleeding.Accessed July 26, 2023. https://www.fda.gov/medical-devices /surgery-devices/endometrial-ablation-heavy-menstrual-bleeding
- ACOG Practice Bulletin. Clinical management guidelines for obstetriciangynecologists. Number 81, May 2007. Obstet Gynecol. 2007;109:1233-1248.
- The American College of Obstetricians and Gynecologists. Abnormal uterine bleeding frequently asked questions. Accessed July 26, 2023. https://www.acog .org/womens-health/faqs/abnormal-uterine-bleeding
- The American College of Obstetricians and Gynecologists. Endometrial ablation frequently asked questions. Accessed November 28, 2023. https://www.acog. org/womens-health/faqs/endometrial-ablation#:~:text=Can%20I%20still%20 get%20pregnant,should%20not%20have%20this%20procedure
- Setiawan VW, Yang HP, Pike MC, et al. Type I and II endometrial cancers: have they different risk factors? J Clin Oncol. 2013;31:2607-2618.
- National Comprehensive Cancer Network. Lynch Syndrome (Version 2.2023). Accessed November 15, 2023. https://www.nccn.org/professionals /physician_gls/pdf/genetics_colon.pdf
- Bonadona V, Bonaïti B, Olschwang S, et al. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA. 2011;305: 2304-2310.
- Fleming CA, Heneghan HM, O’Brien D, et al. Meta-analysis of the cumulative risk of endometrial malignancy and systematic review of endometrial surveillance in extended tamoxifen therapy. Br J Surg. 2018;105:1098-1106.
- Barry JA, Azizia MM, Hardiman PJ. Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2014;20:748-758.
- Cadish LA, Kropat G, Muffly TM. Hysterectomy volume among recent obstetrics and gynecology residency graduates. Urogynecology. 2021;27.
- Blank SV, Huh WK, Bell M, et al. Doubling down on the future of gynecologic oncology: the SGO future of the profession summit report. Gynecol Oncol. 2023;171:76-82.
- Reports MI. Global endometrial ablation market growth, trends and forecast 2023 to 2028 by types, by application, by regions and by key players like Boston Scientific, Hologic, Olympus, Minerva Surgical. Accessed July 30, 2023. https://www.marketinsightsreports.com/single-report/061612632440/global -endometrial-ablation-market-growth-trends-and-forecast-2023-to-2028-by -types-by-application-by-regions-and-by-key-players-like-boston-scientific -hologic-olympus-minerva-surgical
- London R, Holzman M, Rubin D, et al. Payer cost savings with endometrial ablation therapy. Am J Manag Care. 1999;5:889-897.
- Mariani A, Dowdy SC, Cliby WA, et al. Prospective assessment of lymphatic dissemination in endometrial cancer: a paradigm shift in surgical staging. Gynecol Oncol. 2008;109:11-18.
- Beelen P, van den Brink MJ, Herman MC, et al. Levonorgestrel-releasing intrauterine system versus endometrial ablation for heavy menstrual bleeding. Am J Obstet Gynecol. 2021;224:187.e1-e10.
- Bofill Rodriguez M, Lethaby A, Fergusson RJ. Endometrial resection and ablation versus hysterectomy for heavy menstrual bleeding. Cochrane Database Syst Rev. 2021;2:Cd000329.
CASE New patient presents with a history of endometrial hyperplasia
A 51-year-old patient (G2P2002) presents to a new gynecologist’s office after moving from a different state. In her medical history, the gynecologist notes that 5 years ago she underwent dilation and curettage and endometrial ablation procedures for heavy menstrual bleeding (HMB). Ultrasonography performed prior to those procedures showed a slightly enlarged uterus, a simple left ovarian cyst, and a non ̶ visualized right ovary. The patient had declined a 2-step procedure due to concerns with anesthesia, and surgical pathology at the time of ablation revealed hyperplasia without atypia. The patient’s medical history was otherwise notable for prediabetes (recent hemoglobin A1c [HbA1c] measurement, 6.0%) and obesity (body mass index, 43 kg/m2). Pertinent family history included her mother’s diagnosis of endometrial cancer at age 36. Given the patient’s diagnosis of endometrial hyperplasia, she was referred to gynecologic oncology, but she ultimately declined hysterectomy, stating that she was happy with the resolution of her abnormal bleeding. At the time of her initial gynecologic oncology consultation, the consultant suggested lifestyle changes to combat prediabetes and obesity to reduce the risk of endometrial cancer, as future signs of cancer, namely bleeding, may be masked by the endometrial ablation. The patient was prescribed metformin given these medical comorbidities.
At today’s appointment, the patient notes continued resolution of bleeding since the procedure. She does, however, note a 6-month history of vasomotor symptoms and one episode of spotting 3 months ago. Three years ago she was diagnosed with type 2 diabetes mellitus, and her current HbA1c is 6.9%. She has gained 10 lb since being diagnosed with endometrial cancer 5 years ago, and she has continued to take metformin.
An in-office endometrial biopsy is unsuccessful due to cervical stenosis. The treating gynecologist orders a transvaginal ultrasound, which reveals a small left ovarian cyst and a thickened endometrium (measuring 10 mm). Concerned that these findings could represent endometrial cancer, the gynecologist refers the patient to gynecologic oncology for further evaluation.
Sequelae and complications following endometrial ablation are often managed by a gynecologic oncologist. Indeed, a 2018 poll of Society of Gynecologic Oncology (SGO) members revealed that 93.8% of respondents had received such a referral, and almost 20% of respondents were managing more than 20 patients with post-ablation complications in their practices.1 These complications, including hematometra, post-ablation tubal sterilization syndrome, other pain syndromes associated with retrograde menstruation, and thickened endometrium with scarring leading to an inability to sample the endometrium to investigate post-ablation bleeding are symptoms and findings that often lead to further surgery, including hysterectomy.2 General gynecologists faced with these complications may refer patients to gynecologic oncology given an inability to sample the post-ablation endometrium or anticipated difficulties with hysterectomy. A recent meta-analysis revealed a 12.4% hysterectomy rate 5 years after endometrial ablation. Among these patients, the incidence of endometrial cancer ranged from 0% to 1.6%.3
In 2023, endometrial cancer incidence continues to increase, as does the incidence of obesity in women of all ages. Endometrial cancer mortality rates are also increasing, and these trends disproportionately affects non-Hispanic Black women.4 As providers and advocates work to narrow these disparities, gynecologic oncologists are simultaneously noting increased referrals for very likely benign conditions.5 Patients referred for post-ablation bleeding are a subset of these, as most patients who undergo endometrial ablation will not develop cancer. Considering the potential bottlenecks created en route to a gynecologic oncology evaluation, it seems prudent to minimize practices, like endometrial ablation, that may directly or indirectly prevent timely referral of patients with cancer to a gynecologic oncologist.
In this review we focus on the current use of endometrial ablation, associated complications, the incidence of treatment failure, and patient selection. Considering these issues in the context of the current endometrial cancer landscape, we posit best practices aimed at optimizing patient outcomes, and empowering general gynecologists to practice cancer prevention and to triage their surgical patients.
- Before performing endometrial ablation, consider whether alternatives such as hysterectomy or insertion of a progestin-containing IUD would be appropriate.
- Clinical management of patients with abnormal bleeding with indications for endometrial ablation should be guidelinedriven.
- Post-ablation bleeding or pain does not inherently require referral to oncology.
- General gynecologists can perform hysterectomy in this setting if appropriate.
- Patients with endometrial hyperplasia at endometrial ablation should be promptly offered hysterectomy. If atypia is not present, this hysterectomy, too, can be performed by a general gynecologist if appropriate, as the chance for malignancy is minimal.
Continue to: Current use of endometrial ablation in the US...
Current use of endometrial ablation in the US
In 2015, more than 500,000 endometrial ablations were performed in the United States.Given the ability to perform in-office ablation, this number is growing and potentially underestimated each year.6 In 2022, the global endometrial ablation market was valued at $3.4 billion, a figure projected to double in 10 years.7 The procedure has evolved as different devices and approaches have developed, offering patients different means to manage bleeding without hysterectomy. The minimally invasive procedure, performed in premenopausal patients with heavy menstrual bleeding (HMB) due to benign causes who have completed childbearing, has been associated with faster recovery times and fewer short-term complications compared with more invasive surgery.8 There are several non-resectoscope ablative devices approved by the US Food and Drug Administration (FDA), and each work to destroy the endometrial lining via thermal or cryoablation. Endometrial ablation can be performed in premenopausal patients with HMB due to benign causes who have completed childbearing.
Recently, promotional literature has begun to report on so-called overuse of hysterectomy, despite decreasing overall hysterectomy rates. This reporting proposes and applies “appropriateness criteria,” accounting for the rate of preoperative counseling regarding alternatives to hysterectomy, as well as the rate of “unsupportive” final pathology.9 The adoption of endometrial ablation and increasing market value of such vendors suggest that this campaign is having its desired effect. From the oncology perspective, we are concerned the pendulum could swing too far away from hysterectomy, a procedure that definitively cures abnormal uterine bleeding, toward endometrial ablation without explicit acknowledgement of the trade-offs involved.
Endometrial ablation complications: Late-onset procedure failure
A number of post-ablation syndromes may present at least 1 month following the procedure. Collectively known as late-onset endometrial ablation failure (LOEAF), these syndromes are characterized by recurrent vaginal bleeding, and/or new cyclic pelvic pain.10 It is difficult to measure the true incidence of LOEAF. Thomassee and colleagues examined a Canadian retrospective cohort of 437 patients who underwent endometrial ablation; 20.8% reported post-ablation pelvic pain after a median 301 days.11 The subsequent need for surgical intervention, often hysterectomy, is a surrogate for LOEAF.
It should be noted that LOEAF is distinct from post-ablation tubal sterilization syndrome (PATSS), which describes cornual menstrual bleeding impeded by the ligated proximal fallopian tube.12 Increased awareness of PATSS, along with the discontinuation of Essure (a permanent hysteroscopic sterilization device) in 2018, has led some surgeons to advocate for concomitant salpingectomy at the time of endometrial ablation.13 The role of opportunistic salpingectomy in primary prevention of epithelial ovarian cancer is well described, and while we strongly support this practice at the time of endometrial ablation, we do not feel that it effectively prevents LOEAF.14
The post-ablation inability to adequately sample the endometrium is also considered a LOEAF. A prospective study of 57 women who underwent endometrial ablation assessed post-ablation sampling feasibility via transvaginal ultrasonography, saline infusion sonohysterography (SIS), and in-office endometrial biopsies. In 23% of the cohort, endometrial sampling failed, and the authors noted decreased reliability of pathologic assessment.15 One systematic review, in which authors examined the incidence of endometrial cancer following endometrial ablation, characterized 38 cases of endometrial cancer and reported a post-ablation endometrial sampling success rate of 89%. This figure was based on a self-selected sample of 18 patients; cases in which endometrial sampling was thought to be impossible were excluded. The study also had a 30% missing data rate and several other biases.16
In the previously mentioned poll of SGO members,1 84% of the surveyed gynecologic oncologists managing post-ablation patients reported that endometrial sampling following endometrial ablation was “moderately” or “extremely” difficult. More than half of the survey respondents believed that hysterectomy was required for accurate diagnosis.1 While we acknowledge the likely sampling bias affecting the survey results, we are not comforted by any data that minimizes this diagnostic challenge.
Appropriate patient selection and contraindications
The ideal candidate for endometrial ablation is a premenopausal patient with HMB who does not desire future fertility. According to the FDA, absolute contraindications include pregnancy or desired fertility, prior ablation, current IUD in place, inadequate preoperative endometrial assessment, known or suspected malignancy, active infection, or unfavorable anatomy.17
What about patients who may be at increased risk for endometrial cancer?
There is a paucity of data regarding the safety of endometrial ablation in patients at increased risk for developing endometrial cancer in the future. The American College of Obstetricians and Gynecologists (ACOG) 2007 practice bulletin on endometrial ablation (no longer accessible online) alludes to this concern and other contraindications,18 but there are no established guidelines. Currently, no ACOG practice bulletin or committee opinion lists relative contraindications to endometrial ablation, long-term complications (except risks associated with future pregnancy), or risk of subsequent hysterectomy. The risk that “it may be harder to detect endometrial cancer after ablation” is noted on ACOG’s web page dedicated to frequently asked questions (FAQs) regarding abnormal uterine bleeding.19 It is not mentioned on their web page dedicated to the FAQs regarding endometrial ablation.20
In the absence of high-quality published data on established contraindications for endometrial ablation, we advocate for the increased awareness of possible relative contraindications—namely well-established risk factors for endometrial cancer (TABLE 1).For example, in a pooled analysis of 24 epidemiologic studies, authors found that the odds of developing endometrial cancer was 7 times higher among patients with a body mass index (BMI) ≥ 40 kg/m2, compared with controls (odds ratio [OR], 7.14; 95% confidence interval [CI], 6.33–8.06).21 Additionally, patients with Lynch syndrome, a history of extended tamoxifen use, or those with a history of chronic anovulation or polycystic ovary syndrome are at increased risk for endometrial cancer.22-24 If the presence of one or more of these factors does not dissuade general gynecologists from performing an endometrial ablation (even armed with a negative preoperative endometrial biopsy), we feel they should at least prompt thoughtful guideline-driven pause.

Continue to: Hysterectomy—A disincentivized option...
Hysterectomy—A disincentivized option
The annual number of hysterectomies performed by general gynecologists has declined over time. One study by Cadish and colleagues revealed that recent residency graduates performed only 3 to 4 annually.25 These numbers partly reflect the decreasing number of hysterectomies performed during residency training. Furthermore, other factors—including the increasing rate of placenta accreta spectrum, the focus on risk stratification of adnexal masses via the ovarian-adnexal reporting and data classification system (O-RADs), and the emphasis on minimally invasive approaches often acquired in subspecialty training—have likely contributed to referral patterns to such specialists as minimally invasive gynecologic surgeons and gynecologic oncologists.26 This trend is self-actualizing, as quality metrics funnel patients to high-volume surgeons, and general gynecologists risk losing hysterectomy privileges.
These factors lend themselves to a growing emphasis on endometrial ablation. Endometrial ablations can be performed in several settings, including in the hospital, in outpatient clinics, and more and more commonly, in ambulatory surgery centers. This increased access to endometrial ablation in the ambulatory surgery setting has corresponded with an annual endometrial ablation market value growth rate of 5% to 7%.27 These rates are likely compounded by payer reimbursement policies that promote endometrial ablation and other alternatives to hysterectomy that are cost savings in the short term.28 While the actual payer models are unavailable to review, they may not consider the costs of LOEAFs, including subsequent hysterectomy up to 5 years after initial ablation procedures. Provocatively, they almost certainly do not consider the costs of delayed care of patients with endometrial cancer vying for gynecologic oncology appointment slots occupied by post-ablation patients.
We urge providers, patients, and advocates to question who benefits from the uptake of ablation procedures: Patients? Payors? Providers? And how will the field of gynecology fare if hysterectomy skills and privileges are supplanted by ablation?
Post-ablation bleeding: Management by the gyn oncologist
Patients with post-ablation bleeding, either immediately or years later, are sometimes referred to a gynecologic oncologist given the possible risk for cancer and need for surgical staging if cancer is found on the hysterectomy specimen. In practice, assuming normal preoperative ultrasonography and no other clinical or radiologic findings suggestive of malignancy (eg, computed tomography findings concerning for metastases, abnormal cervical cytology, etc.), the presence of cancer is extremely unlikely to be determined at the time of surgery. Frozen section is not generally performed on the endometrium; intraoperative evaluation of even the unablated endometrium is notoriously unreliable; and histologic assessment of the ablated endometrium is limited by artifact (FIGURE 1). The abnormalities caused by ablation further impede selection of a representative focus, obfuscating any actionable result.

Some surgeons routinely bivalve the excised uterus prior to fixation to assess presence of tumor, tumor size, and the degree of myometrial invasion.29 A combination of factors may compel surgeons to perform lymphadenectomy if not already performed, or if sentinel lymph node mapping was unsuccessful. But this practice has not been studied in patients with post-ablation bleeding, and applying these principles relies on a preoperative diagnosis establishing the presence and grade of a cancer. Furthermore, the utility of frozen section and myometrial assessment to decide whether or not to proceed with lymphadenectomy is less relevant in the era of molecular classification guiding adjuvant therapy. In summary, assuming no pathologic or radiologic findings suggestive of cancer, gynecologic oncologists are unlikely to perform lymphadenectomy at the time of hysterectomy in these post-ablation cases, which therefore can safely be performed by general gynecologists.

Our recommendations
Consider the LNG-IUD as an alternative to ablation. A recent randomized controlled trial by Beelen and colleagues compared the effectiveness of LNG-releasing IUDs with endometrial ablation in patients with HMB. While the LNG-IUD was inferior to endometrial ablation, quality-of-life measures were similar up to 2 years.31 Realizing that the hysterectomy rate following endometrial ablation increases significantly beyond that time point (2 years), this narrative may be incomplete. A 5- to 10-year follow-up time-frame may be a more helpful gauge of long-term outcomes. This prolonged time-frame also may allow study of the LNG-IUD’s protective effects on the endometrium in the prevention of endometrial hyperplasia and cancer.
Consider hysterectomy. A 2021 Cochrane review revealed that, compared with endometrial ablation, minimally invasive hysterectomy is associated with higher quality-of-life metrics, higher self-reported patient satisfaction, and similar rates of adverse events.32 While patient autonomy is paramount, the developing step-wise approach from endometrial ablation to hysterectomy, and its potential effects on the health care system at a time when endometrial cancer incidence and mortality rates are rising, is troubling.
Postablation, consider hysterectomy by the general gynecologist. Current trends appear to disincentivize general gynecologists from performing hysterectomy either for HMB or LOEAF. We would offer reassurance that they can safely perform this procedure. Referral to oncology may not be necessary since, in the absence of an established diagnosis of cancer, a lymphadenectomy is not typically required. A shift away from referral for these patients can preserve access to oncology for those women, especially minority women, with an explicit need for oncologic care.
In FIGURE 2, we propose a management algorithm for the patient who presents with post–ablation bleeding. We acknowledge that the evidence base for our management recommendations is limited. Still, we hope providers, ACOG, and other guidelines-issuing organizations consider them as they adapt their own practices and recommendations. We believe this is one of many steps needed to improve outcomes for patients with gynecologic cancer, particularly those in marginalized communities disproportionately impacted by current trends.

CASE Resolution
After reviewing the relevant documentation and examining the patient, the gynecologic oncology consultant contacts the referring gynecologist. They review the low utility of frozen section and the overall low risk of cancer on the final hysterectomy specimen if the patient were to undergo hysterectomy. The consultant clarifies that there is no other concern for surgical complexity beyond the skill of the referring provider, and they discuss the possibility of referral to a minimally invasive specialist for the surgery.
Ultimately, the patient undergoes uncomplicated laparoscopic hysterectomy performed by the original referring gynecologist. Final pathology reveals inactive endometrium with ablative changes and cornual focus of endometrial hyperplasia without atypia. ●
Acknowledgement
The authors acknowledge Ian Hagemann, MD, PhD, for his review of the manuscript.
CASE New patient presents with a history of endometrial hyperplasia
A 51-year-old patient (G2P2002) presents to a new gynecologist’s office after moving from a different state. In her medical history, the gynecologist notes that 5 years ago she underwent dilation and curettage and endometrial ablation procedures for heavy menstrual bleeding (HMB). Ultrasonography performed prior to those procedures showed a slightly enlarged uterus, a simple left ovarian cyst, and a non ̶ visualized right ovary. The patient had declined a 2-step procedure due to concerns with anesthesia, and surgical pathology at the time of ablation revealed hyperplasia without atypia. The patient’s medical history was otherwise notable for prediabetes (recent hemoglobin A1c [HbA1c] measurement, 6.0%) and obesity (body mass index, 43 kg/m2). Pertinent family history included her mother’s diagnosis of endometrial cancer at age 36. Given the patient’s diagnosis of endometrial hyperplasia, she was referred to gynecologic oncology, but she ultimately declined hysterectomy, stating that she was happy with the resolution of her abnormal bleeding. At the time of her initial gynecologic oncology consultation, the consultant suggested lifestyle changes to combat prediabetes and obesity to reduce the risk of endometrial cancer, as future signs of cancer, namely bleeding, may be masked by the endometrial ablation. The patient was prescribed metformin given these medical comorbidities.
At today’s appointment, the patient notes continued resolution of bleeding since the procedure. She does, however, note a 6-month history of vasomotor symptoms and one episode of spotting 3 months ago. Three years ago she was diagnosed with type 2 diabetes mellitus, and her current HbA1c is 6.9%. She has gained 10 lb since being diagnosed with endometrial cancer 5 years ago, and she has continued to take metformin.
An in-office endometrial biopsy is unsuccessful due to cervical stenosis. The treating gynecologist orders a transvaginal ultrasound, which reveals a small left ovarian cyst and a thickened endometrium (measuring 10 mm). Concerned that these findings could represent endometrial cancer, the gynecologist refers the patient to gynecologic oncology for further evaluation.
Sequelae and complications following endometrial ablation are often managed by a gynecologic oncologist. Indeed, a 2018 poll of Society of Gynecologic Oncology (SGO) members revealed that 93.8% of respondents had received such a referral, and almost 20% of respondents were managing more than 20 patients with post-ablation complications in their practices.1 These complications, including hematometra, post-ablation tubal sterilization syndrome, other pain syndromes associated with retrograde menstruation, and thickened endometrium with scarring leading to an inability to sample the endometrium to investigate post-ablation bleeding are symptoms and findings that often lead to further surgery, including hysterectomy.2 General gynecologists faced with these complications may refer patients to gynecologic oncology given an inability to sample the post-ablation endometrium or anticipated difficulties with hysterectomy. A recent meta-analysis revealed a 12.4% hysterectomy rate 5 years after endometrial ablation. Among these patients, the incidence of endometrial cancer ranged from 0% to 1.6%.3
In 2023, endometrial cancer incidence continues to increase, as does the incidence of obesity in women of all ages. Endometrial cancer mortality rates are also increasing, and these trends disproportionately affects non-Hispanic Black women.4 As providers and advocates work to narrow these disparities, gynecologic oncologists are simultaneously noting increased referrals for very likely benign conditions.5 Patients referred for post-ablation bleeding are a subset of these, as most patients who undergo endometrial ablation will not develop cancer. Considering the potential bottlenecks created en route to a gynecologic oncology evaluation, it seems prudent to minimize practices, like endometrial ablation, that may directly or indirectly prevent timely referral of patients with cancer to a gynecologic oncologist.
In this review we focus on the current use of endometrial ablation, associated complications, the incidence of treatment failure, and patient selection. Considering these issues in the context of the current endometrial cancer landscape, we posit best practices aimed at optimizing patient outcomes, and empowering general gynecologists to practice cancer prevention and to triage their surgical patients.
- Before performing endometrial ablation, consider whether alternatives such as hysterectomy or insertion of a progestin-containing IUD would be appropriate.
- Clinical management of patients with abnormal bleeding with indications for endometrial ablation should be guidelinedriven.
- Post-ablation bleeding or pain does not inherently require referral to oncology.
- General gynecologists can perform hysterectomy in this setting if appropriate.
- Patients with endometrial hyperplasia at endometrial ablation should be promptly offered hysterectomy. If atypia is not present, this hysterectomy, too, can be performed by a general gynecologist if appropriate, as the chance for malignancy is minimal.
Continue to: Current use of endometrial ablation in the US...
Current use of endometrial ablation in the US
In 2015, more than 500,000 endometrial ablations were performed in the United States.Given the ability to perform in-office ablation, this number is growing and potentially underestimated each year.6 In 2022, the global endometrial ablation market was valued at $3.4 billion, a figure projected to double in 10 years.7 The procedure has evolved as different devices and approaches have developed, offering patients different means to manage bleeding without hysterectomy. The minimally invasive procedure, performed in premenopausal patients with heavy menstrual bleeding (HMB) due to benign causes who have completed childbearing, has been associated with faster recovery times and fewer short-term complications compared with more invasive surgery.8 There are several non-resectoscope ablative devices approved by the US Food and Drug Administration (FDA), and each work to destroy the endometrial lining via thermal or cryoablation. Endometrial ablation can be performed in premenopausal patients with HMB due to benign causes who have completed childbearing.
Recently, promotional literature has begun to report on so-called overuse of hysterectomy, despite decreasing overall hysterectomy rates. This reporting proposes and applies “appropriateness criteria,” accounting for the rate of preoperative counseling regarding alternatives to hysterectomy, as well as the rate of “unsupportive” final pathology.9 The adoption of endometrial ablation and increasing market value of such vendors suggest that this campaign is having its desired effect. From the oncology perspective, we are concerned the pendulum could swing too far away from hysterectomy, a procedure that definitively cures abnormal uterine bleeding, toward endometrial ablation without explicit acknowledgement of the trade-offs involved.
Endometrial ablation complications: Late-onset procedure failure
A number of post-ablation syndromes may present at least 1 month following the procedure. Collectively known as late-onset endometrial ablation failure (LOEAF), these syndromes are characterized by recurrent vaginal bleeding, and/or new cyclic pelvic pain.10 It is difficult to measure the true incidence of LOEAF. Thomassee and colleagues examined a Canadian retrospective cohort of 437 patients who underwent endometrial ablation; 20.8% reported post-ablation pelvic pain after a median 301 days.11 The subsequent need for surgical intervention, often hysterectomy, is a surrogate for LOEAF.
It should be noted that LOEAF is distinct from post-ablation tubal sterilization syndrome (PATSS), which describes cornual menstrual bleeding impeded by the ligated proximal fallopian tube.12 Increased awareness of PATSS, along with the discontinuation of Essure (a permanent hysteroscopic sterilization device) in 2018, has led some surgeons to advocate for concomitant salpingectomy at the time of endometrial ablation.13 The role of opportunistic salpingectomy in primary prevention of epithelial ovarian cancer is well described, and while we strongly support this practice at the time of endometrial ablation, we do not feel that it effectively prevents LOEAF.14
The post-ablation inability to adequately sample the endometrium is also considered a LOEAF. A prospective study of 57 women who underwent endometrial ablation assessed post-ablation sampling feasibility via transvaginal ultrasonography, saline infusion sonohysterography (SIS), and in-office endometrial biopsies. In 23% of the cohort, endometrial sampling failed, and the authors noted decreased reliability of pathologic assessment.15 One systematic review, in which authors examined the incidence of endometrial cancer following endometrial ablation, characterized 38 cases of endometrial cancer and reported a post-ablation endometrial sampling success rate of 89%. This figure was based on a self-selected sample of 18 patients; cases in which endometrial sampling was thought to be impossible were excluded. The study also had a 30% missing data rate and several other biases.16
In the previously mentioned poll of SGO members,1 84% of the surveyed gynecologic oncologists managing post-ablation patients reported that endometrial sampling following endometrial ablation was “moderately” or “extremely” difficult. More than half of the survey respondents believed that hysterectomy was required for accurate diagnosis.1 While we acknowledge the likely sampling bias affecting the survey results, we are not comforted by any data that minimizes this diagnostic challenge.
Appropriate patient selection and contraindications
The ideal candidate for endometrial ablation is a premenopausal patient with HMB who does not desire future fertility. According to the FDA, absolute contraindications include pregnancy or desired fertility, prior ablation, current IUD in place, inadequate preoperative endometrial assessment, known or suspected malignancy, active infection, or unfavorable anatomy.17
What about patients who may be at increased risk for endometrial cancer?
There is a paucity of data regarding the safety of endometrial ablation in patients at increased risk for developing endometrial cancer in the future. The American College of Obstetricians and Gynecologists (ACOG) 2007 practice bulletin on endometrial ablation (no longer accessible online) alludes to this concern and other contraindications,18 but there are no established guidelines. Currently, no ACOG practice bulletin or committee opinion lists relative contraindications to endometrial ablation, long-term complications (except risks associated with future pregnancy), or risk of subsequent hysterectomy. The risk that “it may be harder to detect endometrial cancer after ablation” is noted on ACOG’s web page dedicated to frequently asked questions (FAQs) regarding abnormal uterine bleeding.19 It is not mentioned on their web page dedicated to the FAQs regarding endometrial ablation.20
In the absence of high-quality published data on established contraindications for endometrial ablation, we advocate for the increased awareness of possible relative contraindications—namely well-established risk factors for endometrial cancer (TABLE 1).For example, in a pooled analysis of 24 epidemiologic studies, authors found that the odds of developing endometrial cancer was 7 times higher among patients with a body mass index (BMI) ≥ 40 kg/m2, compared with controls (odds ratio [OR], 7.14; 95% confidence interval [CI], 6.33–8.06).21 Additionally, patients with Lynch syndrome, a history of extended tamoxifen use, or those with a history of chronic anovulation or polycystic ovary syndrome are at increased risk for endometrial cancer.22-24 If the presence of one or more of these factors does not dissuade general gynecologists from performing an endometrial ablation (even armed with a negative preoperative endometrial biopsy), we feel they should at least prompt thoughtful guideline-driven pause.

Continue to: Hysterectomy—A disincentivized option...
Hysterectomy—A disincentivized option
The annual number of hysterectomies performed by general gynecologists has declined over time. One study by Cadish and colleagues revealed that recent residency graduates performed only 3 to 4 annually.25 These numbers partly reflect the decreasing number of hysterectomies performed during residency training. Furthermore, other factors—including the increasing rate of placenta accreta spectrum, the focus on risk stratification of adnexal masses via the ovarian-adnexal reporting and data classification system (O-RADs), and the emphasis on minimally invasive approaches often acquired in subspecialty training—have likely contributed to referral patterns to such specialists as minimally invasive gynecologic surgeons and gynecologic oncologists.26 This trend is self-actualizing, as quality metrics funnel patients to high-volume surgeons, and general gynecologists risk losing hysterectomy privileges.
These factors lend themselves to a growing emphasis on endometrial ablation. Endometrial ablations can be performed in several settings, including in the hospital, in outpatient clinics, and more and more commonly, in ambulatory surgery centers. This increased access to endometrial ablation in the ambulatory surgery setting has corresponded with an annual endometrial ablation market value growth rate of 5% to 7%.27 These rates are likely compounded by payer reimbursement policies that promote endometrial ablation and other alternatives to hysterectomy that are cost savings in the short term.28 While the actual payer models are unavailable to review, they may not consider the costs of LOEAFs, including subsequent hysterectomy up to 5 years after initial ablation procedures. Provocatively, they almost certainly do not consider the costs of delayed care of patients with endometrial cancer vying for gynecologic oncology appointment slots occupied by post-ablation patients.
We urge providers, patients, and advocates to question who benefits from the uptake of ablation procedures: Patients? Payors? Providers? And how will the field of gynecology fare if hysterectomy skills and privileges are supplanted by ablation?
Post-ablation bleeding: Management by the gyn oncologist
Patients with post-ablation bleeding, either immediately or years later, are sometimes referred to a gynecologic oncologist given the possible risk for cancer and need for surgical staging if cancer is found on the hysterectomy specimen. In practice, assuming normal preoperative ultrasonography and no other clinical or radiologic findings suggestive of malignancy (eg, computed tomography findings concerning for metastases, abnormal cervical cytology, etc.), the presence of cancer is extremely unlikely to be determined at the time of surgery. Frozen section is not generally performed on the endometrium; intraoperative evaluation of even the unablated endometrium is notoriously unreliable; and histologic assessment of the ablated endometrium is limited by artifact (FIGURE 1). The abnormalities caused by ablation further impede selection of a representative focus, obfuscating any actionable result.

Some surgeons routinely bivalve the excised uterus prior to fixation to assess presence of tumor, tumor size, and the degree of myometrial invasion.29 A combination of factors may compel surgeons to perform lymphadenectomy if not already performed, or if sentinel lymph node mapping was unsuccessful. But this practice has not been studied in patients with post-ablation bleeding, and applying these principles relies on a preoperative diagnosis establishing the presence and grade of a cancer. Furthermore, the utility of frozen section and myometrial assessment to decide whether or not to proceed with lymphadenectomy is less relevant in the era of molecular classification guiding adjuvant therapy. In summary, assuming no pathologic or radiologic findings suggestive of cancer, gynecologic oncologists are unlikely to perform lymphadenectomy at the time of hysterectomy in these post-ablation cases, which therefore can safely be performed by general gynecologists.

Our recommendations
Consider the LNG-IUD as an alternative to ablation. A recent randomized controlled trial by Beelen and colleagues compared the effectiveness of LNG-releasing IUDs with endometrial ablation in patients with HMB. While the LNG-IUD was inferior to endometrial ablation, quality-of-life measures were similar up to 2 years.31 Realizing that the hysterectomy rate following endometrial ablation increases significantly beyond that time point (2 years), this narrative may be incomplete. A 5- to 10-year follow-up time-frame may be a more helpful gauge of long-term outcomes. This prolonged time-frame also may allow study of the LNG-IUD’s protective effects on the endometrium in the prevention of endometrial hyperplasia and cancer.
Consider hysterectomy. A 2021 Cochrane review revealed that, compared with endometrial ablation, minimally invasive hysterectomy is associated with higher quality-of-life metrics, higher self-reported patient satisfaction, and similar rates of adverse events.32 While patient autonomy is paramount, the developing step-wise approach from endometrial ablation to hysterectomy, and its potential effects on the health care system at a time when endometrial cancer incidence and mortality rates are rising, is troubling.
Postablation, consider hysterectomy by the general gynecologist. Current trends appear to disincentivize general gynecologists from performing hysterectomy either for HMB or LOEAF. We would offer reassurance that they can safely perform this procedure. Referral to oncology may not be necessary since, in the absence of an established diagnosis of cancer, a lymphadenectomy is not typically required. A shift away from referral for these patients can preserve access to oncology for those women, especially minority women, with an explicit need for oncologic care.
In FIGURE 2, we propose a management algorithm for the patient who presents with post–ablation bleeding. We acknowledge that the evidence base for our management recommendations is limited. Still, we hope providers, ACOG, and other guidelines-issuing organizations consider them as they adapt their own practices and recommendations. We believe this is one of many steps needed to improve outcomes for patients with gynecologic cancer, particularly those in marginalized communities disproportionately impacted by current trends.

CASE Resolution
After reviewing the relevant documentation and examining the patient, the gynecologic oncology consultant contacts the referring gynecologist. They review the low utility of frozen section and the overall low risk of cancer on the final hysterectomy specimen if the patient were to undergo hysterectomy. The consultant clarifies that there is no other concern for surgical complexity beyond the skill of the referring provider, and they discuss the possibility of referral to a minimally invasive specialist for the surgery.
Ultimately, the patient undergoes uncomplicated laparoscopic hysterectomy performed by the original referring gynecologist. Final pathology reveals inactive endometrium with ablative changes and cornual focus of endometrial hyperplasia without atypia. ●
Acknowledgement
The authors acknowledge Ian Hagemann, MD, PhD, for his review of the manuscript.
- Chen H, Saiz AM, McCausland AM, et al. Experience of gynecologic oncologists regarding endometrial cancer after endometrial ablation. J Clin Oncol. 2018;36:e17566-e.
- McCausland AM, McCausland VM. Long-term complications of endometrial ablation: cause, diagnosis, treatment, and prevention. J Minim Invasive Gynecol. 2007;14:399-406.
- Oderkerk TJ, Beelen P, Bukkems ALA, et al. Risk of hysterectomy after endometrial ablation: a systematic review and meta-analysis. Obstet Gynecol. 2023;142:51-60.
- Clarke MA, Devesa SS, Hammer A, et al. Racial and ethnic differences in hysterectomy-corrected uterine corpus cancer mortality by stage and histologic subtype. JAMA Oncol. 2022;8:895-903.
- Barber EL, Rossi EC, Alexander A, et al. Benign hysterectomy performed by gynecologic oncologists: is selection bias altering our ability to measure surgical quality? Gynecol Oncol. 2018;151:141-144.
- Wortman M. Late-onset endometrial ablation failure. Case Rep Womens Health. 2017;15:11-28.
- Insights FM. Endometrial Ablation Market Outlook.Accessed July 26, 2023. https://www.futuremarketinsights.com/reports/endometrial-ablation -market
- Famuyide A. Endometrial ablation. J Minim Invasive Gynecol. 2018;25:299-307.
- Corona LE, Swenson CW, Sheetz KH, et al. Use of other treatments before hysterectomy for benign conditions in a statewide hospital collaborative. Am J Obstet Gynecol. 2015;212:304.e1-e7.
- Wortman M, Cholkeri A, McCausland AM, et al. Late-onset endometrial ablation failure—etiology, treatment, and prevention. J Minim Invasive Gynecol. 2015;22:323-331.
- Thomassee MS, Curlin H, Yunker A, et al. Predicting pelvic pain after endometrial ablation: which preoperative patient characteristics are associated? J Minim Invasive Gynecol. 2013;20:642-647.
- Townsend DE, McCausland V, McCausland A, et al. Post-ablation-tubal sterilization syndrome. Obstet Gynecol. 1993;82:422-424.
- Greer Polite F, DeAgostino-Kelly M, Marchand GJ. Combination of laparoscopic salpingectomy and endometrial ablation: a potentially underused procedure. J Gynecol Surg. 2021;37:89-91.
- Hanley GE, Pearce CL, Talhouk A, et al. Outcomes from opportunistic salpingectomy for ovarian cancer prevention. JAMA Network Open. 2022;5:e2147343-e.
- Ahonkallio SJ, Liakka AK, Martikainen HK, et al. Feasibility of endometrial assessment after thermal ablation. Eur J Obstet Gynecol Reprod Biol. 2009;147:69-71.
- Tamara JO, Mileen RDvdK, Karlijn MCC, et al. Endometrial cancer after endometrial ablation: a systematic review. Int J Gynecol Cancer. 2022;32:1555.
- US Food and Drug Administration. Endometrial ablation for heavy menstrual bleeding.Accessed July 26, 2023. https://www.fda.gov/medical-devices /surgery-devices/endometrial-ablation-heavy-menstrual-bleeding
- ACOG Practice Bulletin. Clinical management guidelines for obstetriciangynecologists. Number 81, May 2007. Obstet Gynecol. 2007;109:1233-1248.
- The American College of Obstetricians and Gynecologists. Abnormal uterine bleeding frequently asked questions. Accessed July 26, 2023. https://www.acog .org/womens-health/faqs/abnormal-uterine-bleeding
- The American College of Obstetricians and Gynecologists. Endometrial ablation frequently asked questions. Accessed November 28, 2023. https://www.acog. org/womens-health/faqs/endometrial-ablation#:~:text=Can%20I%20still%20 get%20pregnant,should%20not%20have%20this%20procedure
- Setiawan VW, Yang HP, Pike MC, et al. Type I and II endometrial cancers: have they different risk factors? J Clin Oncol. 2013;31:2607-2618.
- National Comprehensive Cancer Network. Lynch Syndrome (Version 2.2023). Accessed November 15, 2023. https://www.nccn.org/professionals /physician_gls/pdf/genetics_colon.pdf
- Bonadona V, Bonaïti B, Olschwang S, et al. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA. 2011;305: 2304-2310.
- Fleming CA, Heneghan HM, O’Brien D, et al. Meta-analysis of the cumulative risk of endometrial malignancy and systematic review of endometrial surveillance in extended tamoxifen therapy. Br J Surg. 2018;105:1098-1106.
- Barry JA, Azizia MM, Hardiman PJ. Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2014;20:748-758.
- Cadish LA, Kropat G, Muffly TM. Hysterectomy volume among recent obstetrics and gynecology residency graduates. Urogynecology. 2021;27.
- Blank SV, Huh WK, Bell M, et al. Doubling down on the future of gynecologic oncology: the SGO future of the profession summit report. Gynecol Oncol. 2023;171:76-82.
- Reports MI. Global endometrial ablation market growth, trends and forecast 2023 to 2028 by types, by application, by regions and by key players like Boston Scientific, Hologic, Olympus, Minerva Surgical. Accessed July 30, 2023. https://www.marketinsightsreports.com/single-report/061612632440/global -endometrial-ablation-market-growth-trends-and-forecast-2023-to-2028-by -types-by-application-by-regions-and-by-key-players-like-boston-scientific -hologic-olympus-minerva-surgical
- London R, Holzman M, Rubin D, et al. Payer cost savings with endometrial ablation therapy. Am J Manag Care. 1999;5:889-897.
- Mariani A, Dowdy SC, Cliby WA, et al. Prospective assessment of lymphatic dissemination in endometrial cancer: a paradigm shift in surgical staging. Gynecol Oncol. 2008;109:11-18.
- Beelen P, van den Brink MJ, Herman MC, et al. Levonorgestrel-releasing intrauterine system versus endometrial ablation for heavy menstrual bleeding. Am J Obstet Gynecol. 2021;224:187.e1-e10.
- Bofill Rodriguez M, Lethaby A, Fergusson RJ. Endometrial resection and ablation versus hysterectomy for heavy menstrual bleeding. Cochrane Database Syst Rev. 2021;2:Cd000329.
- Chen H, Saiz AM, McCausland AM, et al. Experience of gynecologic oncologists regarding endometrial cancer after endometrial ablation. J Clin Oncol. 2018;36:e17566-e.
- McCausland AM, McCausland VM. Long-term complications of endometrial ablation: cause, diagnosis, treatment, and prevention. J Minim Invasive Gynecol. 2007;14:399-406.
- Oderkerk TJ, Beelen P, Bukkems ALA, et al. Risk of hysterectomy after endometrial ablation: a systematic review and meta-analysis. Obstet Gynecol. 2023;142:51-60.
- Clarke MA, Devesa SS, Hammer A, et al. Racial and ethnic differences in hysterectomy-corrected uterine corpus cancer mortality by stage and histologic subtype. JAMA Oncol. 2022;8:895-903.
- Barber EL, Rossi EC, Alexander A, et al. Benign hysterectomy performed by gynecologic oncologists: is selection bias altering our ability to measure surgical quality? Gynecol Oncol. 2018;151:141-144.
- Wortman M. Late-onset endometrial ablation failure. Case Rep Womens Health. 2017;15:11-28.
- Insights FM. Endometrial Ablation Market Outlook.Accessed July 26, 2023. https://www.futuremarketinsights.com/reports/endometrial-ablation -market
- Famuyide A. Endometrial ablation. J Minim Invasive Gynecol. 2018;25:299-307.
- Corona LE, Swenson CW, Sheetz KH, et al. Use of other treatments before hysterectomy for benign conditions in a statewide hospital collaborative. Am J Obstet Gynecol. 2015;212:304.e1-e7.
- Wortman M, Cholkeri A, McCausland AM, et al. Late-onset endometrial ablation failure—etiology, treatment, and prevention. J Minim Invasive Gynecol. 2015;22:323-331.
- Thomassee MS, Curlin H, Yunker A, et al. Predicting pelvic pain after endometrial ablation: which preoperative patient characteristics are associated? J Minim Invasive Gynecol. 2013;20:642-647.
- Townsend DE, McCausland V, McCausland A, et al. Post-ablation-tubal sterilization syndrome. Obstet Gynecol. 1993;82:422-424.
- Greer Polite F, DeAgostino-Kelly M, Marchand GJ. Combination of laparoscopic salpingectomy and endometrial ablation: a potentially underused procedure. J Gynecol Surg. 2021;37:89-91.
- Hanley GE, Pearce CL, Talhouk A, et al. Outcomes from opportunistic salpingectomy for ovarian cancer prevention. JAMA Network Open. 2022;5:e2147343-e.
- Ahonkallio SJ, Liakka AK, Martikainen HK, et al. Feasibility of endometrial assessment after thermal ablation. Eur J Obstet Gynecol Reprod Biol. 2009;147:69-71.
- Tamara JO, Mileen RDvdK, Karlijn MCC, et al. Endometrial cancer after endometrial ablation: a systematic review. Int J Gynecol Cancer. 2022;32:1555.
- US Food and Drug Administration. Endometrial ablation for heavy menstrual bleeding.Accessed July 26, 2023. https://www.fda.gov/medical-devices /surgery-devices/endometrial-ablation-heavy-menstrual-bleeding
- ACOG Practice Bulletin. Clinical management guidelines for obstetriciangynecologists. Number 81, May 2007. Obstet Gynecol. 2007;109:1233-1248.
- The American College of Obstetricians and Gynecologists. Abnormal uterine bleeding frequently asked questions. Accessed July 26, 2023. https://www.acog .org/womens-health/faqs/abnormal-uterine-bleeding
- The American College of Obstetricians and Gynecologists. Endometrial ablation frequently asked questions. Accessed November 28, 2023. https://www.acog. org/womens-health/faqs/endometrial-ablation#:~:text=Can%20I%20still%20 get%20pregnant,should%20not%20have%20this%20procedure
- Setiawan VW, Yang HP, Pike MC, et al. Type I and II endometrial cancers: have they different risk factors? J Clin Oncol. 2013;31:2607-2618.
- National Comprehensive Cancer Network. Lynch Syndrome (Version 2.2023). Accessed November 15, 2023. https://www.nccn.org/professionals /physician_gls/pdf/genetics_colon.pdf
- Bonadona V, Bonaïti B, Olschwang S, et al. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA. 2011;305: 2304-2310.
- Fleming CA, Heneghan HM, O’Brien D, et al. Meta-analysis of the cumulative risk of endometrial malignancy and systematic review of endometrial surveillance in extended tamoxifen therapy. Br J Surg. 2018;105:1098-1106.
- Barry JA, Azizia MM, Hardiman PJ. Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2014;20:748-758.
- Cadish LA, Kropat G, Muffly TM. Hysterectomy volume among recent obstetrics and gynecology residency graduates. Urogynecology. 2021;27.
- Blank SV, Huh WK, Bell M, et al. Doubling down on the future of gynecologic oncology: the SGO future of the profession summit report. Gynecol Oncol. 2023;171:76-82.
- Reports MI. Global endometrial ablation market growth, trends and forecast 2023 to 2028 by types, by application, by regions and by key players like Boston Scientific, Hologic, Olympus, Minerva Surgical. Accessed July 30, 2023. https://www.marketinsightsreports.com/single-report/061612632440/global -endometrial-ablation-market-growth-trends-and-forecast-2023-to-2028-by -types-by-application-by-regions-and-by-key-players-like-boston-scientific -hologic-olympus-minerva-surgical
- London R, Holzman M, Rubin D, et al. Payer cost savings with endometrial ablation therapy. Am J Manag Care. 1999;5:889-897.
- Mariani A, Dowdy SC, Cliby WA, et al. Prospective assessment of lymphatic dissemination in endometrial cancer: a paradigm shift in surgical staging. Gynecol Oncol. 2008;109:11-18.
- Beelen P, van den Brink MJ, Herman MC, et al. Levonorgestrel-releasing intrauterine system versus endometrial ablation for heavy menstrual bleeding. Am J Obstet Gynecol. 2021;224:187.e1-e10.
- Bofill Rodriguez M, Lethaby A, Fergusson RJ. Endometrial resection and ablation versus hysterectomy for heavy menstrual bleeding. Cochrane Database Syst Rev. 2021;2:Cd000329.
Commentary: Bendamustine, PET/CT Biomarkers, and BTKi in B-Cell Lymphoma, December 2023
While chimeric antigen receptor (CAR) T-cell therapy has transformed the management of large B-cell lymphoma (LBCL), the majority of patients will ultimately relapse. Efforts to identify predictors of response remain an active area of investigation. One key variable that has been postulated to influence CAR T-cell outcomes is pretreatment bendamustine exposure. Specifically, there has been concern that the lymphodepleting effects of bendamustine could affect T-cell fitness, thus impairing CAR T-cell response. While consensus guidelines have recommended avoiding bendamustine prior to lymphocyte collection, clear data have been lacking. A recent retrospective, multicenter study, which included patients from seven European sites, reported outcomes based on prior bendamustine exposure (Iacoboni et al). In this study, 439 patients with relapsed or refractory LBCL, who received anti-CD19 commercial CAR T-cell therapy after two or more prior treatment lines of therapy, were included. Of these patients, 80 had received prior bendamustine. The authors found that patients recently exposed to bendamustine (< 9 months), vs bendamustine-naive patients, had a significantly lower overall response rate (40% vs 66%; P = .01), overall survival (OS; adjusted hazard ratio [aHR] 2.11; P < .01), and progression-free survival (PFS; aHR 1.82; P < .01) after CAR T-cell infusion. These differences remained significant after inverse probability treatment weighting and propensity score matching. Of note, the authors did not find that the cumulative dose of bendamustine affected outcomes. The authors also identified that, while the risk for cytokine release syndrome and immune effector cell–associated neurotoxicity syndrome was similar between the groups, hematologic toxicity and severe infections were increased in the bendamustine-exposed patients. These data support the recommendation to avoid bendamustine treatment prior to CAR T-cell apheresis. While treatment regimens such as polatuzumab plus bendamustine and rituximab are available in the relapsed setting for LBCL,1 this regimen should be reserved for post CAR T-cell relapse or for patients not planning to proceed with cellular therapy. The impact of bendamustine exposure on other immune-mediated therapies, such as bispecific antibodies, remains unknown.
Quantitative PET/CT biomarkers have also emerged as predictors of response in diffuse large B-cell lymphoma (DLBCL). A key variable of interest includes total metabolic tumor volume (MTV), which refers to the total volume of tumor with metabolic uptake. While prior studies have demonstrated a correlation of MTV on outcomes following treatment with chemotherapy and CAR T-cell therapy,2,3 the effect of PET/CT biomarkers on outcomes with other novel agents remains poorly described. A recent study by Alderuccio and colleagues explored the predictive power of PET/CT biomarkers on outcomes in a clinical trial cohort of patients treated with the antibody drug conjugate loncastuximab tesirine. This post hoc analysis reviewed the screening PET/CT scans of 138 patients with relapsed or refractory DLBCL treated with two or more prior systemic therapy lines who received loncastuximab tesirine in LOTIS-2<.4 The authors found that an MTV ≥ 96 mL was significantly associated with failure to achieve a complete metabolic response (adjusted odds ratio 5.42; P = .002). Patients with an MTV ≥ 96 mL vs < 96 mL also had a shorter PFS (aHR 2.68; P = .002) and OS (aHR 3.09; P < .0001). In line with prior studies, this analysis demonstrates that baseline MTV has the potential to provide robust risk-stratification and confirms the value of PET/CT biomarkers in DLBCL across treatment types.
This month, the results of the phase 2 TARMAC study, which evaluated treatment with ibrutinib in combination with tisagenlecleucel, were also published. This study included 20 patients with relapsed/refractory mantle cell lymphoma (MCL) who had received one or more prior lines of therapy, including 50% with prior Bruton tyrosine kinase inhibitor (BTKi) exposure. Ibrutinib was initiated prior to leukapheresis and continued through CAR T-cell manufacturing and for at least 6 months post tisagenlecleucel infusion. At 4 months post infusion, the overall and complete response rates were 80% each. Patients without and with prior BTKi exposure had complete response rates of 90% and 70%, respectively. At a median follow-up of 13 months, the estimated 12-month PFS was 75% and OS was 100%. Grades 1-2 and grade 3 cytokine-release syndrome rates were 55% and 20%, respectively, and grade 1-2 immune effector cell–associated neurotoxicity syndrome was seen in 10% of patients. The authors also demonstrated that markers of T-cell exhaustion were decreased in patients with longer ibrutinib exposure prior to leukapheresis. Also of note, the three patients with recent bendamustine therapy did not receive a durable response. Although this is a small study without a control arm, this study provides rationale for the potential advantage of combining BTKi with CAR T-cell therapy, even among patients with prior BTKi exposure.
Additional References
1. Sehn LH, Hertzberg M, Opat S, et al. Polatuzumab vedotin plus bendamustine and rituximab in relapsed/refractory DLBCL: survival update and new extension cohort data. Blood Adv. 2022;6(2):533-543. doi: 10.1182/bloodadvances.2021005794
2. Vercellino L, Cottereau AS, Casasnovas O, et al. High total metabolic tumor volume at baseline predicts survival independent of response to therapy. Blood. 2020;135(16):1396-1405. doi: 10.1182/blood.2019003526
3. Dean EA, Mhaskar RS, Lu H, et al. High metabolic tumor volume is associated with decreased efficacy of axicabtagene ciloleucel in large B-cell lymphoma. Blood Adv. 2020;4(14):3268-3276. doi: 10.1182/bloodadvances.2020001900
4. Caimi PF, Ai W, Alderuccio JP, et al. Loncastuximab tesirine in relapsed or refractory diffuse large B-cell lymphoma (LOTIS-2): a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2021;22(6):790-800. doi:
While chimeric antigen receptor (CAR) T-cell therapy has transformed the management of large B-cell lymphoma (LBCL), the majority of patients will ultimately relapse. Efforts to identify predictors of response remain an active area of investigation. One key variable that has been postulated to influence CAR T-cell outcomes is pretreatment bendamustine exposure. Specifically, there has been concern that the lymphodepleting effects of bendamustine could affect T-cell fitness, thus impairing CAR T-cell response. While consensus guidelines have recommended avoiding bendamustine prior to lymphocyte collection, clear data have been lacking. A recent retrospective, multicenter study, which included patients from seven European sites, reported outcomes based on prior bendamustine exposure (Iacoboni et al). In this study, 439 patients with relapsed or refractory LBCL, who received anti-CD19 commercial CAR T-cell therapy after two or more prior treatment lines of therapy, were included. Of these patients, 80 had received prior bendamustine. The authors found that patients recently exposed to bendamustine (< 9 months), vs bendamustine-naive patients, had a significantly lower overall response rate (40% vs 66%; P = .01), overall survival (OS; adjusted hazard ratio [aHR] 2.11; P < .01), and progression-free survival (PFS; aHR 1.82; P < .01) after CAR T-cell infusion. These differences remained significant after inverse probability treatment weighting and propensity score matching. Of note, the authors did not find that the cumulative dose of bendamustine affected outcomes. The authors also identified that, while the risk for cytokine release syndrome and immune effector cell–associated neurotoxicity syndrome was similar between the groups, hematologic toxicity and severe infections were increased in the bendamustine-exposed patients. These data support the recommendation to avoid bendamustine treatment prior to CAR T-cell apheresis. While treatment regimens such as polatuzumab plus bendamustine and rituximab are available in the relapsed setting for LBCL,1 this regimen should be reserved for post CAR T-cell relapse or for patients not planning to proceed with cellular therapy. The impact of bendamustine exposure on other immune-mediated therapies, such as bispecific antibodies, remains unknown.
Quantitative PET/CT biomarkers have also emerged as predictors of response in diffuse large B-cell lymphoma (DLBCL). A key variable of interest includes total metabolic tumor volume (MTV), which refers to the total volume of tumor with metabolic uptake. While prior studies have demonstrated a correlation of MTV on outcomes following treatment with chemotherapy and CAR T-cell therapy,2,3 the effect of PET/CT biomarkers on outcomes with other novel agents remains poorly described. A recent study by Alderuccio and colleagues explored the predictive power of PET/CT biomarkers on outcomes in a clinical trial cohort of patients treated with the antibody drug conjugate loncastuximab tesirine. This post hoc analysis reviewed the screening PET/CT scans of 138 patients with relapsed or refractory DLBCL treated with two or more prior systemic therapy lines who received loncastuximab tesirine in LOTIS-2<.4 The authors found that an MTV ≥ 96 mL was significantly associated with failure to achieve a complete metabolic response (adjusted odds ratio 5.42; P = .002). Patients with an MTV ≥ 96 mL vs < 96 mL also had a shorter PFS (aHR 2.68; P = .002) and OS (aHR 3.09; P < .0001). In line with prior studies, this analysis demonstrates that baseline MTV has the potential to provide robust risk-stratification and confirms the value of PET/CT biomarkers in DLBCL across treatment types.
This month, the results of the phase 2 TARMAC study, which evaluated treatment with ibrutinib in combination with tisagenlecleucel, were also published. This study included 20 patients with relapsed/refractory mantle cell lymphoma (MCL) who had received one or more prior lines of therapy, including 50% with prior Bruton tyrosine kinase inhibitor (BTKi) exposure. Ibrutinib was initiated prior to leukapheresis and continued through CAR T-cell manufacturing and for at least 6 months post tisagenlecleucel infusion. At 4 months post infusion, the overall and complete response rates were 80% each. Patients without and with prior BTKi exposure had complete response rates of 90% and 70%, respectively. At a median follow-up of 13 months, the estimated 12-month PFS was 75% and OS was 100%. Grades 1-2 and grade 3 cytokine-release syndrome rates were 55% and 20%, respectively, and grade 1-2 immune effector cell–associated neurotoxicity syndrome was seen in 10% of patients. The authors also demonstrated that markers of T-cell exhaustion were decreased in patients with longer ibrutinib exposure prior to leukapheresis. Also of note, the three patients with recent bendamustine therapy did not receive a durable response. Although this is a small study without a control arm, this study provides rationale for the potential advantage of combining BTKi with CAR T-cell therapy, even among patients with prior BTKi exposure.
Additional References
1. Sehn LH, Hertzberg M, Opat S, et al. Polatuzumab vedotin plus bendamustine and rituximab in relapsed/refractory DLBCL: survival update and new extension cohort data. Blood Adv. 2022;6(2):533-543. doi: 10.1182/bloodadvances.2021005794
2. Vercellino L, Cottereau AS, Casasnovas O, et al. High total metabolic tumor volume at baseline predicts survival independent of response to therapy. Blood. 2020;135(16):1396-1405. doi: 10.1182/blood.2019003526
3. Dean EA, Mhaskar RS, Lu H, et al. High metabolic tumor volume is associated with decreased efficacy of axicabtagene ciloleucel in large B-cell lymphoma. Blood Adv. 2020;4(14):3268-3276. doi: 10.1182/bloodadvances.2020001900
4. Caimi PF, Ai W, Alderuccio JP, et al. Loncastuximab tesirine in relapsed or refractory diffuse large B-cell lymphoma (LOTIS-2): a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2021;22(6):790-800. doi:
While chimeric antigen receptor (CAR) T-cell therapy has transformed the management of large B-cell lymphoma (LBCL), the majority of patients will ultimately relapse. Efforts to identify predictors of response remain an active area of investigation. One key variable that has been postulated to influence CAR T-cell outcomes is pretreatment bendamustine exposure. Specifically, there has been concern that the lymphodepleting effects of bendamustine could affect T-cell fitness, thus impairing CAR T-cell response. While consensus guidelines have recommended avoiding bendamustine prior to lymphocyte collection, clear data have been lacking. A recent retrospective, multicenter study, which included patients from seven European sites, reported outcomes based on prior bendamustine exposure (Iacoboni et al). In this study, 439 patients with relapsed or refractory LBCL, who received anti-CD19 commercial CAR T-cell therapy after two or more prior treatment lines of therapy, were included. Of these patients, 80 had received prior bendamustine. The authors found that patients recently exposed to bendamustine (< 9 months), vs bendamustine-naive patients, had a significantly lower overall response rate (40% vs 66%; P = .01), overall survival (OS; adjusted hazard ratio [aHR] 2.11; P < .01), and progression-free survival (PFS; aHR 1.82; P < .01) after CAR T-cell infusion. These differences remained significant after inverse probability treatment weighting and propensity score matching. Of note, the authors did not find that the cumulative dose of bendamustine affected outcomes. The authors also identified that, while the risk for cytokine release syndrome and immune effector cell–associated neurotoxicity syndrome was similar between the groups, hematologic toxicity and severe infections were increased in the bendamustine-exposed patients. These data support the recommendation to avoid bendamustine treatment prior to CAR T-cell apheresis. While treatment regimens such as polatuzumab plus bendamustine and rituximab are available in the relapsed setting for LBCL,1 this regimen should be reserved for post CAR T-cell relapse or for patients not planning to proceed with cellular therapy. The impact of bendamustine exposure on other immune-mediated therapies, such as bispecific antibodies, remains unknown.
Quantitative PET/CT biomarkers have also emerged as predictors of response in diffuse large B-cell lymphoma (DLBCL). A key variable of interest includes total metabolic tumor volume (MTV), which refers to the total volume of tumor with metabolic uptake. While prior studies have demonstrated a correlation of MTV on outcomes following treatment with chemotherapy and CAR T-cell therapy,2,3 the effect of PET/CT biomarkers on outcomes with other novel agents remains poorly described. A recent study by Alderuccio and colleagues explored the predictive power of PET/CT biomarkers on outcomes in a clinical trial cohort of patients treated with the antibody drug conjugate loncastuximab tesirine. This post hoc analysis reviewed the screening PET/CT scans of 138 patients with relapsed or refractory DLBCL treated with two or more prior systemic therapy lines who received loncastuximab tesirine in LOTIS-2<.4 The authors found that an MTV ≥ 96 mL was significantly associated with failure to achieve a complete metabolic response (adjusted odds ratio 5.42; P = .002). Patients with an MTV ≥ 96 mL vs < 96 mL also had a shorter PFS (aHR 2.68; P = .002) and OS (aHR 3.09; P < .0001). In line with prior studies, this analysis demonstrates that baseline MTV has the potential to provide robust risk-stratification and confirms the value of PET/CT biomarkers in DLBCL across treatment types.
This month, the results of the phase 2 TARMAC study, which evaluated treatment with ibrutinib in combination with tisagenlecleucel, were also published. This study included 20 patients with relapsed/refractory mantle cell lymphoma (MCL) who had received one or more prior lines of therapy, including 50% with prior Bruton tyrosine kinase inhibitor (BTKi) exposure. Ibrutinib was initiated prior to leukapheresis and continued through CAR T-cell manufacturing and for at least 6 months post tisagenlecleucel infusion. At 4 months post infusion, the overall and complete response rates were 80% each. Patients without and with prior BTKi exposure had complete response rates of 90% and 70%, respectively. At a median follow-up of 13 months, the estimated 12-month PFS was 75% and OS was 100%. Grades 1-2 and grade 3 cytokine-release syndrome rates were 55% and 20%, respectively, and grade 1-2 immune effector cell–associated neurotoxicity syndrome was seen in 10% of patients. The authors also demonstrated that markers of T-cell exhaustion were decreased in patients with longer ibrutinib exposure prior to leukapheresis. Also of note, the three patients with recent bendamustine therapy did not receive a durable response. Although this is a small study without a control arm, this study provides rationale for the potential advantage of combining BTKi with CAR T-cell therapy, even among patients with prior BTKi exposure.
Additional References
1. Sehn LH, Hertzberg M, Opat S, et al. Polatuzumab vedotin plus bendamustine and rituximab in relapsed/refractory DLBCL: survival update and new extension cohort data. Blood Adv. 2022;6(2):533-543. doi: 10.1182/bloodadvances.2021005794
2. Vercellino L, Cottereau AS, Casasnovas O, et al. High total metabolic tumor volume at baseline predicts survival independent of response to therapy. Blood. 2020;135(16):1396-1405. doi: 10.1182/blood.2019003526
3. Dean EA, Mhaskar RS, Lu H, et al. High metabolic tumor volume is associated with decreased efficacy of axicabtagene ciloleucel in large B-cell lymphoma. Blood Adv. 2020;4(14):3268-3276. doi: 10.1182/bloodadvances.2020001900
4. Caimi PF, Ai W, Alderuccio JP, et al. Loncastuximab tesirine in relapsed or refractory diffuse large B-cell lymphoma (LOTIS-2): a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2021;22(6):790-800. doi:
Commentary: RA and Cancer, and Real-World Medication Studies, December 2023
The association of rheumatoid arthritis (RA) with increased cancer risk compared with the general population has long been known, though the balance between risk related to RA disease activity compared with risk related to immunosuppressive medication has not been clear. This increased risk is seen primarily with lymphoma and lung cancer, and prior research has suggested a risk with biological disease-modifying antirheumatic drugs (bDMARD), such as anti–tumor necrosis factor (TNF) agents. Beydon and colleagues performed a cohort study using a French national claims database; they looked at patients seen for at least 1 year with treatment for RA and compared the incidence of cancer by type. In over 257,000 patients, nearly 24,000 cancer cases were found. The most common cancers were breast, colon, lung, and prostate. All-cancer risk was > 1.2 (standardized incidence ratio) compared with those without cancer, higher in men compared with women, and the risk was increased in patients who received conventional synthetic (cs) DMARD, TNF inhibitors (TNFi), abatacept, and rituximab, but not interleukin (IL)-6 inhibitors or Janus kinase inhibitors (JAKi). Given that the risk was most highly associated with exposure to rituximab, this may show a type of bias rendering the study difficult to interpret, as rituximab is considered "safe" in cancer, and treatments such as csDMARD may have been given because they were not contraindicated in patients with cancer. This renders the study’s other results, such as lower risk with JAKi or higher risk with abatacept, hard to interpret.
Hayashi and colleagues performed a "real-world" comparative study using data from the Japanese observational ANSWER registry database to compare effectiveness of different JAKi over 6 months, a question of high interest given the availability of several JAKi currently. Within the database of over 11,000 participants, only 622 patients were exposed to tofacitinib, baricitinib, peficitinib, or upadacitinib, with 361 included in the final analysis due to missing baseline data (later missing data were imputed). Treatment retention rates were similar among all four JAKi, and discontinuation rates due to adverse events and due to lack of efficacy were similar as well. There was no significant difference in Health Assessment Questionnaire (HAQ), Clinical Disease Activity Index (CDAI), or C-reactive protein after 6 months between the four JAKi. Baricitinib had higher rates of CDAI low disease activity and remission at 6 months when used as a first-line biologic/targeted synthetic (b/ts) DMARD. However, this and other specific findings related to individual JAKi may be affected by the relatively small number of patients included and exposed to each JAKi, and the relatively short duration of follow-up (in terms of drug discontinuation), thus countering the initial premise for the study.
Finally, another important real-world study, by Tageldin and colleagues, looked at tapering therapy in the Rheumatoid Arthritis Medication Tapering (RHEUMTAP) cohort of patients with RA in sustained disease remission or low disease activity for at least 6 months on stable medications (infused bDMARD excluded). This 2-year prospective cohort included reducing frequency, reducing dose, and stopping medication according to predefined regimens. Of 131 patients, 40% underwent tapering, with more flares in the taper group over > 400 days of follow-up; flare rates were much higher in those tapering b/tsDMARD compared with csDMARD. Though limited by small numbers in examining the three different tapering groups, this real-world study provides an important counterpoint to the notion that medication can be tapered easily in RA patients doing well. A more stringent definition or longer duration of disease remission may also affect this finding.
The association of rheumatoid arthritis (RA) with increased cancer risk compared with the general population has long been known, though the balance between risk related to RA disease activity compared with risk related to immunosuppressive medication has not been clear. This increased risk is seen primarily with lymphoma and lung cancer, and prior research has suggested a risk with biological disease-modifying antirheumatic drugs (bDMARD), such as anti–tumor necrosis factor (TNF) agents. Beydon and colleagues performed a cohort study using a French national claims database; they looked at patients seen for at least 1 year with treatment for RA and compared the incidence of cancer by type. In over 257,000 patients, nearly 24,000 cancer cases were found. The most common cancers were breast, colon, lung, and prostate. All-cancer risk was > 1.2 (standardized incidence ratio) compared with those without cancer, higher in men compared with women, and the risk was increased in patients who received conventional synthetic (cs) DMARD, TNF inhibitors (TNFi), abatacept, and rituximab, but not interleukin (IL)-6 inhibitors or Janus kinase inhibitors (JAKi). Given that the risk was most highly associated with exposure to rituximab, this may show a type of bias rendering the study difficult to interpret, as rituximab is considered "safe" in cancer, and treatments such as csDMARD may have been given because they were not contraindicated in patients with cancer. This renders the study’s other results, such as lower risk with JAKi or higher risk with abatacept, hard to interpret.
Hayashi and colleagues performed a "real-world" comparative study using data from the Japanese observational ANSWER registry database to compare effectiveness of different JAKi over 6 months, a question of high interest given the availability of several JAKi currently. Within the database of over 11,000 participants, only 622 patients were exposed to tofacitinib, baricitinib, peficitinib, or upadacitinib, with 361 included in the final analysis due to missing baseline data (later missing data were imputed). Treatment retention rates were similar among all four JAKi, and discontinuation rates due to adverse events and due to lack of efficacy were similar as well. There was no significant difference in Health Assessment Questionnaire (HAQ), Clinical Disease Activity Index (CDAI), or C-reactive protein after 6 months between the four JAKi. Baricitinib had higher rates of CDAI low disease activity and remission at 6 months when used as a first-line biologic/targeted synthetic (b/ts) DMARD. However, this and other specific findings related to individual JAKi may be affected by the relatively small number of patients included and exposed to each JAKi, and the relatively short duration of follow-up (in terms of drug discontinuation), thus countering the initial premise for the study.
Finally, another important real-world study, by Tageldin and colleagues, looked at tapering therapy in the Rheumatoid Arthritis Medication Tapering (RHEUMTAP) cohort of patients with RA in sustained disease remission or low disease activity for at least 6 months on stable medications (infused bDMARD excluded). This 2-year prospective cohort included reducing frequency, reducing dose, and stopping medication according to predefined regimens. Of 131 patients, 40% underwent tapering, with more flares in the taper group over > 400 days of follow-up; flare rates were much higher in those tapering b/tsDMARD compared with csDMARD. Though limited by small numbers in examining the three different tapering groups, this real-world study provides an important counterpoint to the notion that medication can be tapered easily in RA patients doing well. A more stringent definition or longer duration of disease remission may also affect this finding.
The association of rheumatoid arthritis (RA) with increased cancer risk compared with the general population has long been known, though the balance between risk related to RA disease activity compared with risk related to immunosuppressive medication has not been clear. This increased risk is seen primarily with lymphoma and lung cancer, and prior research has suggested a risk with biological disease-modifying antirheumatic drugs (bDMARD), such as anti–tumor necrosis factor (TNF) agents. Beydon and colleagues performed a cohort study using a French national claims database; they looked at patients seen for at least 1 year with treatment for RA and compared the incidence of cancer by type. In over 257,000 patients, nearly 24,000 cancer cases were found. The most common cancers were breast, colon, lung, and prostate. All-cancer risk was > 1.2 (standardized incidence ratio) compared with those without cancer, higher in men compared with women, and the risk was increased in patients who received conventional synthetic (cs) DMARD, TNF inhibitors (TNFi), abatacept, and rituximab, but not interleukin (IL)-6 inhibitors or Janus kinase inhibitors (JAKi). Given that the risk was most highly associated with exposure to rituximab, this may show a type of bias rendering the study difficult to interpret, as rituximab is considered "safe" in cancer, and treatments such as csDMARD may have been given because they were not contraindicated in patients with cancer. This renders the study’s other results, such as lower risk with JAKi or higher risk with abatacept, hard to interpret.
Hayashi and colleagues performed a "real-world" comparative study using data from the Japanese observational ANSWER registry database to compare effectiveness of different JAKi over 6 months, a question of high interest given the availability of several JAKi currently. Within the database of over 11,000 participants, only 622 patients were exposed to tofacitinib, baricitinib, peficitinib, or upadacitinib, with 361 included in the final analysis due to missing baseline data (later missing data were imputed). Treatment retention rates were similar among all four JAKi, and discontinuation rates due to adverse events and due to lack of efficacy were similar as well. There was no significant difference in Health Assessment Questionnaire (HAQ), Clinical Disease Activity Index (CDAI), or C-reactive protein after 6 months between the four JAKi. Baricitinib had higher rates of CDAI low disease activity and remission at 6 months when used as a first-line biologic/targeted synthetic (b/ts) DMARD. However, this and other specific findings related to individual JAKi may be affected by the relatively small number of patients included and exposed to each JAKi, and the relatively short duration of follow-up (in terms of drug discontinuation), thus countering the initial premise for the study.
Finally, another important real-world study, by Tageldin and colleagues, looked at tapering therapy in the Rheumatoid Arthritis Medication Tapering (RHEUMTAP) cohort of patients with RA in sustained disease remission or low disease activity for at least 6 months on stable medications (infused bDMARD excluded). This 2-year prospective cohort included reducing frequency, reducing dose, and stopping medication according to predefined regimens. Of 131 patients, 40% underwent tapering, with more flares in the taper group over > 400 days of follow-up; flare rates were much higher in those tapering b/tsDMARD compared with csDMARD. Though limited by small numbers in examining the three different tapering groups, this real-world study provides an important counterpoint to the notion that medication can be tapered easily in RA patients doing well. A more stringent definition or longer duration of disease remission may also affect this finding.
Commentary: CGRP Monoclonal Antibodies for Migraine, December 2023
Depression is one of the most common comorbidities associated with migraine. Major depressive disorder is both a risk factor for chronic migraine and a condition that one is more likely to develop after being diagnosed with chronic migraine. The study by de Vries Lentsch and colleagues investigated the use of two of the calcitonin gene-related peptide (CGRP) monoclonal antibody (mAb) treatments — erenumab and fremanezumab — compared with a control group of patients with chronic migraine, with an eye on outcomes to measure depression. Of note, reduction in headache frequency (defined as reduction in monthly migraine days) was also investigated as an independent variable.
This was a single-center study performed at the University of Leiden Headache Center. It was not a randomized trial, but all patients were followed with an e-diary and Day 0 vs Day 90 questionnaires that tracked their headache frequency and severity as well as a number of metrics related to depression. Depressive symptoms were assessed using the Hospital Anxiety and Depression Scale (HADS) and the Center for Epidemiological Studies Depression Scale (CES-D). The Headache Impact Test (HIT-6) was used to follow headache-related impact and disability, and the Perceived Stress Scale (PSS) was used to measure the degree of stressful situations the patient was experiencing.
The baseline depression scales between the three groups were 70%, 60%, and 66%, respectively; there were similar baseline levels of migraine frequency and disability as well. Both intervention groups showed a significant decrease in the symptoms of depression, and having a greater level of depression was negatively associated with reduction in monthly migraine days after 3 months. Of note, logistic-regression analysis determined that the reduction in depressive symptoms was independent of the reduction in migraine frequency.
Nearly all headache care providers are faced with challenging situations on a daily basis; often this is due to the comorbidity of mood disorders and high-frequency migraine. A traditional approach has been to provide the patient with a migraine preventive medication in the antidepressant family, such as a tricyclic antidepressant or serotonin and norepinephrine reuptake inhibitor (SNRI). Although these can be helpful, they are less specific for migraine prevention. Many patients are also already taking antidepressant medications, and the addition of a migraine-preventive antidepressant would be contraindicated. This study broadens the possibilities for prevention in these complicated patients and shows that there is benefit in both migraine-related outcomes and markers for depression when using CGRP-based therapy.
The way headache medicine is practiced changed dramatically in 2018 with the advent of CGRP monoclonal antibody (mAb) treatments for migraine. These medications have allowed us to target migraine specifically, whereas all of the preventive medications for migraine prior to 2018 were developed for other conditions and only secondarily helped migraine. These include the antidepressant, antihypertensive, and antiepileptic classes of medications, as well as onabotulinum toxin A, which, although approved for migraine, is not targeting a migraine-specific factor. Moskatel and colleagues sought to better understand the changing patterns of prescribing the nonspecific, or "traditional," migraine preventive medications in light of the advent of CGRP treatment.
This was a retrospective cohort study using aggregated data from the Stanford headache center. The percentage of patients with chronic migraine who had been prescribed one of the 10 most prescribed oral preventive medications or onabotulinum toxin A, or any of the four CGRP mAb, were calculated relative to the total number of patients with chronic migraine who received a prescription for any medication from the clinic during the pre-CGRP mAb years of 2015-2017 and post-approval years of 2019-2021.
The Stanford (STARR) database was filtered, searching for patients living in a California ZIP code with a diagnosis of chronic migraine who were followed from 2015 to 2021. The 10 most common non-CGRP preventive medications were amitriptyline/nortriptyline, valproate, duloxetine, gabapentin, memantine, propranolol, venlafaxine, verapamil, and onabotulinumtoxinA.
Erenumab was noted to initially be the most prescribed CGRP monoclonal antibody medication, but this was overtaken by galcanezumab after the second quarter of 2020 and throughout 2021. There is a statistically significant decrease in the percentage of patients receiving any of the non-CGRP preventive medications since 2018. The most significant decreases were in the tricyclic antidepressant class, as well as valproate, duloxetine, memantine, and onabotulinum toxin A. There was no statistically significant change in venlafaxine or gabapentin prescriptions.
This study highlights the changing face of headache medicine, and having a new class of migraine-specific treatment has significantly affected prescribing patterns. Although there is a statistically significant decrease in the prescribing of these non–migraine-specific preventive medications, they are still often recommended due to step-therapy regulations from insurance formularies, or as part of a polypharmacy regimen that may be more beneficial for a patient. These medications do improve patient outcomes and will remain a mainstay in migraine treatment.
Nearly all patients with migraine are recommended an acute medication to treat migraine attacks abortively; some patients are also recommended preventive therapies if migraine frequency significantly affects their quality of life. The American Headache Society/American Academy of Neurology guidelines for prevention recommend the initiation of a preventive medication at a frequency of 4-5 headache days per month or approximately 1 per week. Lipton and colleagues sought to determine whether there were any efficacy concerns in combining a CGRP mAb for prevention with ubrogepant, an oral CGRP antagonist, for acute treatment.
This was a prospective, open-level observational study assessing pain relief, return to normal function, and treatment satisfaction with patients given 50 or 100 mg of ubrogepant while concomitantly being given a seizure or mAb medication. Patients were allowed to be taking onabotulinumtoxinA as well as a CGRP mAb. The patients in this study were asked to track their headache symptoms using the Migraine Buddy e-diary. Meaningful pain relief was defined as a rating of migraine-related pain with one of the following choices 4 hours after taking the medication: no pain, mild pain, moderate pain, or severe pain. Return to normal function was defined as whether the patient determined they were able to function normally relative to their baseline at specific times post intervention. This was based on a functional disability scale. Treatment satisfaction was determined on the basis of a seven-point rating scale for how satisfied the patient felt with the medication at the end of the trial period.
A total of 245 participants provided at least 30 days of data, with 44.5% of the patients taking erenumab, 35.1% taking galcanezumab, 18.0% taking fremanezumab, and 2.9% taking eptinezumab. Meaningful pain relief was achieved by 61.6% of patients at 2 hours and 80.4% of patients at 4 hours post dose for both the 50-mg and 100-mg dose of ubrogepant. Return to normal function was achieved by 34.7% of patients at 2 hours and 50.5% at 4 hours post dose as well. Patients reported a 72.7% satisfaction level with the medication.
When CGRP acute medications were first approved, there was concern about the use of a mAb together with an oral antagonist. It was thought that CGRP medications would be associated with fewer benefits than when these medications were used alone, due to the belief that only a specific amount of CGRP could be blocked at any specific time. This trial shows that the efficacy of CGRP acute medications is not affected by concomitant use of mAb. Many patients who respond well to CGRP mAb will benefit significantly from the additional abortive use of oral antagonists.
Depression is one of the most common comorbidities associated with migraine. Major depressive disorder is both a risk factor for chronic migraine and a condition that one is more likely to develop after being diagnosed with chronic migraine. The study by de Vries Lentsch and colleagues investigated the use of two of the calcitonin gene-related peptide (CGRP) monoclonal antibody (mAb) treatments — erenumab and fremanezumab — compared with a control group of patients with chronic migraine, with an eye on outcomes to measure depression. Of note, reduction in headache frequency (defined as reduction in monthly migraine days) was also investigated as an independent variable.
This was a single-center study performed at the University of Leiden Headache Center. It was not a randomized trial, but all patients were followed with an e-diary and Day 0 vs Day 90 questionnaires that tracked their headache frequency and severity as well as a number of metrics related to depression. Depressive symptoms were assessed using the Hospital Anxiety and Depression Scale (HADS) and the Center for Epidemiological Studies Depression Scale (CES-D). The Headache Impact Test (HIT-6) was used to follow headache-related impact and disability, and the Perceived Stress Scale (PSS) was used to measure the degree of stressful situations the patient was experiencing.
The baseline depression scales between the three groups were 70%, 60%, and 66%, respectively; there were similar baseline levels of migraine frequency and disability as well. Both intervention groups showed a significant decrease in the symptoms of depression, and having a greater level of depression was negatively associated with reduction in monthly migraine days after 3 months. Of note, logistic-regression analysis determined that the reduction in depressive symptoms was independent of the reduction in migraine frequency.
Nearly all headache care providers are faced with challenging situations on a daily basis; often this is due to the comorbidity of mood disorders and high-frequency migraine. A traditional approach has been to provide the patient with a migraine preventive medication in the antidepressant family, such as a tricyclic antidepressant or serotonin and norepinephrine reuptake inhibitor (SNRI). Although these can be helpful, they are less specific for migraine prevention. Many patients are also already taking antidepressant medications, and the addition of a migraine-preventive antidepressant would be contraindicated. This study broadens the possibilities for prevention in these complicated patients and shows that there is benefit in both migraine-related outcomes and markers for depression when using CGRP-based therapy.
The way headache medicine is practiced changed dramatically in 2018 with the advent of CGRP monoclonal antibody (mAb) treatments for migraine. These medications have allowed us to target migraine specifically, whereas all of the preventive medications for migraine prior to 2018 were developed for other conditions and only secondarily helped migraine. These include the antidepressant, antihypertensive, and antiepileptic classes of medications, as well as onabotulinum toxin A, which, although approved for migraine, is not targeting a migraine-specific factor. Moskatel and colleagues sought to better understand the changing patterns of prescribing the nonspecific, or "traditional," migraine preventive medications in light of the advent of CGRP treatment.
This was a retrospective cohort study using aggregated data from the Stanford headache center. The percentage of patients with chronic migraine who had been prescribed one of the 10 most prescribed oral preventive medications or onabotulinum toxin A, or any of the four CGRP mAb, were calculated relative to the total number of patients with chronic migraine who received a prescription for any medication from the clinic during the pre-CGRP mAb years of 2015-2017 and post-approval years of 2019-2021.
The Stanford (STARR) database was filtered, searching for patients living in a California ZIP code with a diagnosis of chronic migraine who were followed from 2015 to 2021. The 10 most common non-CGRP preventive medications were amitriptyline/nortriptyline, valproate, duloxetine, gabapentin, memantine, propranolol, venlafaxine, verapamil, and onabotulinumtoxinA.
Erenumab was noted to initially be the most prescribed CGRP monoclonal antibody medication, but this was overtaken by galcanezumab after the second quarter of 2020 and throughout 2021. There is a statistically significant decrease in the percentage of patients receiving any of the non-CGRP preventive medications since 2018. The most significant decreases were in the tricyclic antidepressant class, as well as valproate, duloxetine, memantine, and onabotulinum toxin A. There was no statistically significant change in venlafaxine or gabapentin prescriptions.
This study highlights the changing face of headache medicine, and having a new class of migraine-specific treatment has significantly affected prescribing patterns. Although there is a statistically significant decrease in the prescribing of these non–migraine-specific preventive medications, they are still often recommended due to step-therapy regulations from insurance formularies, or as part of a polypharmacy regimen that may be more beneficial for a patient. These medications do improve patient outcomes and will remain a mainstay in migraine treatment.
Nearly all patients with migraine are recommended an acute medication to treat migraine attacks abortively; some patients are also recommended preventive therapies if migraine frequency significantly affects their quality of life. The American Headache Society/American Academy of Neurology guidelines for prevention recommend the initiation of a preventive medication at a frequency of 4-5 headache days per month or approximately 1 per week. Lipton and colleagues sought to determine whether there were any efficacy concerns in combining a CGRP mAb for prevention with ubrogepant, an oral CGRP antagonist, for acute treatment.
This was a prospective, open-level observational study assessing pain relief, return to normal function, and treatment satisfaction with patients given 50 or 100 mg of ubrogepant while concomitantly being given a seizure or mAb medication. Patients were allowed to be taking onabotulinumtoxinA as well as a CGRP mAb. The patients in this study were asked to track their headache symptoms using the Migraine Buddy e-diary. Meaningful pain relief was defined as a rating of migraine-related pain with one of the following choices 4 hours after taking the medication: no pain, mild pain, moderate pain, or severe pain. Return to normal function was defined as whether the patient determined they were able to function normally relative to their baseline at specific times post intervention. This was based on a functional disability scale. Treatment satisfaction was determined on the basis of a seven-point rating scale for how satisfied the patient felt with the medication at the end of the trial period.
A total of 245 participants provided at least 30 days of data, with 44.5% of the patients taking erenumab, 35.1% taking galcanezumab, 18.0% taking fremanezumab, and 2.9% taking eptinezumab. Meaningful pain relief was achieved by 61.6% of patients at 2 hours and 80.4% of patients at 4 hours post dose for both the 50-mg and 100-mg dose of ubrogepant. Return to normal function was achieved by 34.7% of patients at 2 hours and 50.5% at 4 hours post dose as well. Patients reported a 72.7% satisfaction level with the medication.
When CGRP acute medications were first approved, there was concern about the use of a mAb together with an oral antagonist. It was thought that CGRP medications would be associated with fewer benefits than when these medications were used alone, due to the belief that only a specific amount of CGRP could be blocked at any specific time. This trial shows that the efficacy of CGRP acute medications is not affected by concomitant use of mAb. Many patients who respond well to CGRP mAb will benefit significantly from the additional abortive use of oral antagonists.
Depression is one of the most common comorbidities associated with migraine. Major depressive disorder is both a risk factor for chronic migraine and a condition that one is more likely to develop after being diagnosed with chronic migraine. The study by de Vries Lentsch and colleagues investigated the use of two of the calcitonin gene-related peptide (CGRP) monoclonal antibody (mAb) treatments — erenumab and fremanezumab — compared with a control group of patients with chronic migraine, with an eye on outcomes to measure depression. Of note, reduction in headache frequency (defined as reduction in monthly migraine days) was also investigated as an independent variable.
This was a single-center study performed at the University of Leiden Headache Center. It was not a randomized trial, but all patients were followed with an e-diary and Day 0 vs Day 90 questionnaires that tracked their headache frequency and severity as well as a number of metrics related to depression. Depressive symptoms were assessed using the Hospital Anxiety and Depression Scale (HADS) and the Center for Epidemiological Studies Depression Scale (CES-D). The Headache Impact Test (HIT-6) was used to follow headache-related impact and disability, and the Perceived Stress Scale (PSS) was used to measure the degree of stressful situations the patient was experiencing.
The baseline depression scales between the three groups were 70%, 60%, and 66%, respectively; there were similar baseline levels of migraine frequency and disability as well. Both intervention groups showed a significant decrease in the symptoms of depression, and having a greater level of depression was negatively associated with reduction in monthly migraine days after 3 months. Of note, logistic-regression analysis determined that the reduction in depressive symptoms was independent of the reduction in migraine frequency.
Nearly all headache care providers are faced with challenging situations on a daily basis; often this is due to the comorbidity of mood disorders and high-frequency migraine. A traditional approach has been to provide the patient with a migraine preventive medication in the antidepressant family, such as a tricyclic antidepressant or serotonin and norepinephrine reuptake inhibitor (SNRI). Although these can be helpful, they are less specific for migraine prevention. Many patients are also already taking antidepressant medications, and the addition of a migraine-preventive antidepressant would be contraindicated. This study broadens the possibilities for prevention in these complicated patients and shows that there is benefit in both migraine-related outcomes and markers for depression when using CGRP-based therapy.
The way headache medicine is practiced changed dramatically in 2018 with the advent of CGRP monoclonal antibody (mAb) treatments for migraine. These medications have allowed us to target migraine specifically, whereas all of the preventive medications for migraine prior to 2018 were developed for other conditions and only secondarily helped migraine. These include the antidepressant, antihypertensive, and antiepileptic classes of medications, as well as onabotulinum toxin A, which, although approved for migraine, is not targeting a migraine-specific factor. Moskatel and colleagues sought to better understand the changing patterns of prescribing the nonspecific, or "traditional," migraine preventive medications in light of the advent of CGRP treatment.
This was a retrospective cohort study using aggregated data from the Stanford headache center. The percentage of patients with chronic migraine who had been prescribed one of the 10 most prescribed oral preventive medications or onabotulinum toxin A, or any of the four CGRP mAb, were calculated relative to the total number of patients with chronic migraine who received a prescription for any medication from the clinic during the pre-CGRP mAb years of 2015-2017 and post-approval years of 2019-2021.
The Stanford (STARR) database was filtered, searching for patients living in a California ZIP code with a diagnosis of chronic migraine who were followed from 2015 to 2021. The 10 most common non-CGRP preventive medications were amitriptyline/nortriptyline, valproate, duloxetine, gabapentin, memantine, propranolol, venlafaxine, verapamil, and onabotulinumtoxinA.
Erenumab was noted to initially be the most prescribed CGRP monoclonal antibody medication, but this was overtaken by galcanezumab after the second quarter of 2020 and throughout 2021. There is a statistically significant decrease in the percentage of patients receiving any of the non-CGRP preventive medications since 2018. The most significant decreases were in the tricyclic antidepressant class, as well as valproate, duloxetine, memantine, and onabotulinum toxin A. There was no statistically significant change in venlafaxine or gabapentin prescriptions.
This study highlights the changing face of headache medicine, and having a new class of migraine-specific treatment has significantly affected prescribing patterns. Although there is a statistically significant decrease in the prescribing of these non–migraine-specific preventive medications, they are still often recommended due to step-therapy regulations from insurance formularies, or as part of a polypharmacy regimen that may be more beneficial for a patient. These medications do improve patient outcomes and will remain a mainstay in migraine treatment.
Nearly all patients with migraine are recommended an acute medication to treat migraine attacks abortively; some patients are also recommended preventive therapies if migraine frequency significantly affects their quality of life. The American Headache Society/American Academy of Neurology guidelines for prevention recommend the initiation of a preventive medication at a frequency of 4-5 headache days per month or approximately 1 per week. Lipton and colleagues sought to determine whether there were any efficacy concerns in combining a CGRP mAb for prevention with ubrogepant, an oral CGRP antagonist, for acute treatment.
This was a prospective, open-level observational study assessing pain relief, return to normal function, and treatment satisfaction with patients given 50 or 100 mg of ubrogepant while concomitantly being given a seizure or mAb medication. Patients were allowed to be taking onabotulinumtoxinA as well as a CGRP mAb. The patients in this study were asked to track their headache symptoms using the Migraine Buddy e-diary. Meaningful pain relief was defined as a rating of migraine-related pain with one of the following choices 4 hours after taking the medication: no pain, mild pain, moderate pain, or severe pain. Return to normal function was defined as whether the patient determined they were able to function normally relative to their baseline at specific times post intervention. This was based on a functional disability scale. Treatment satisfaction was determined on the basis of a seven-point rating scale for how satisfied the patient felt with the medication at the end of the trial period.
A total of 245 participants provided at least 30 days of data, with 44.5% of the patients taking erenumab, 35.1% taking galcanezumab, 18.0% taking fremanezumab, and 2.9% taking eptinezumab. Meaningful pain relief was achieved by 61.6% of patients at 2 hours and 80.4% of patients at 4 hours post dose for both the 50-mg and 100-mg dose of ubrogepant. Return to normal function was achieved by 34.7% of patients at 2 hours and 50.5% at 4 hours post dose as well. Patients reported a 72.7% satisfaction level with the medication.
When CGRP acute medications were first approved, there was concern about the use of a mAb together with an oral antagonist. It was thought that CGRP medications would be associated with fewer benefits than when these medications were used alone, due to the belief that only a specific amount of CGRP could be blocked at any specific time. This trial shows that the efficacy of CGRP acute medications is not affected by concomitant use of mAb. Many patients who respond well to CGRP mAb will benefit significantly from the additional abortive use of oral antagonists.
LGBTQI+: Special considerations for reproductive health care
CASE A new patient office visit
A new patient is waiting for you in the exam room. You review the chart and see the sex demographic field is blank, and the patient’s name is Alex. As an ObGyn, most of your patients are female, but you have treated your patients’ partners for sexually transmitted infections. As you enter the room, you see 2 androgynously dressed individuals; you introduce yourself and ask,
“What brings you in today, and who is your friend?”
“This is my partner Charlie, and we are worried I have an STD.”
Estimates suggest that between 7% to 12% of the US population identifies as lesbian, gay, bisexual, transgender/non-binary, queer/questioning, intersex, or asexual (LGBTQI+).1 If you practice in an urban area, the odds are quite high that you have encountered an LGBTQI+ person who openly identified as such; if you are in a rural area, you also likely have had an LGBTQI+ patient, but they may not have disclosed this about themselves.2 Maybe you have had training in cultural relevance or are a member of this community and you feel confident in providing quality care to LGBTQI+ patients. Or maybe you think that, as a responsibly practicing health care clinician, you treat all patients the same, so whether or not you know their sexual orientation or gender identity does not impact the care you provide. As the proportion of US adults who identify as LGBTQI+ increases,1 it becomes more important for health care clinicians to understand the challenges these patients face when trying to access health care. To start, let’s review the meaning of LGBTQI+, the history of the community, what it means to be culturally relevant or humble, and how to create a welcoming and safe practice environment.
LGBTQI+ terms and definitions
The first step in providing quality care to LGBTQI+ patients is to understand the terminology associated with sexual orientation, gender identity, and gender expression.3–5
Sexual orientation refers to whom a person is sexually attracted. The term straight/heterosexual suggests a person is sexually attracted to a person of the opposite gender. Lesbian or gay refers to those who are attracted to their same gender. Some people use bisexual (attracted to both the same and opposite gender) and pansexual (attracted to all humans regardless of gender). Still others refer to themselves as queer—people who identify as someone who is not heterosexual or cisgender. A variety of other terms exist to describe one’s sexual attraction. There are also some people who identify as asexual, which suggests they are not sexually attracted to anyone.
Gender identity relates to how one views their own gender. If you were assigned female at birth and identify as a woman, you are cisgender. If you were assigned male at birth and identify as a woman, you may identify as transgender whether or not you have had gender transitioning surgery or have taken hormones. Some people do not identify with the terms male or female and may view themselves as nonbinary. The terms gender queer, gender fluid, gender diverse, and gender non-conforming also may be used to describe various ways that an individual may not identify as male or female. We also can refer to people as “assigned female at birth” or “assigned male at birth”. People with intersex conditions may require taking a unique medical history that includes asking about genetic testing (eg, 46,XX congenital adrenal hyperplasia or 46,XY complete gonadal dysgenesis).
Gender expression refers to how one pre-sents themselves to others through appearance, dress, and behavior. A person may be assigned female at birth, dress in a conventional male fashion, and still identify as a woman. Still others may choose to express their gender in a variety of ways that may not have anything to do with their sexual orientation or gender identity, such as dressing in ways that represent their culture.
People may be fluid in their sexual orientation or gender identity; it may change from day to day, month to month, or even year to year.6,7
*The term LGBTQI+ is not used consistently in the literature. Throughout this article, the terminology used matches that used in the cited reference(s).
Continue to: Health care and the LGBTQI+ community...
Health care and the LGBTQI+ community
The LGBTQI+ community has a history of experiencing societal discrimination and stigma, which stems from medical mistrust often due to a lack of understanding of their medical and psychosocial needs.8,9 A 2019 survey of US LGBTQ adults, found that about 50% of people who identified as transgender reported having negative or discriminatory experiences with a health care clinician.10 About 18% of transgender people anticipated being refused medical care due to their gender identity.10 About 18% of LGBTQ individuals avoid any type of medical care, fearing discrimination.10 Lesbian women are 3 times more likely to have not seen an ObGyn than women who identify as straight.11 Sixty-two percent of lesbian women have biological children and received prenatal care; however, of those, 47% do not receive routine cancer screenings.10,11 Only 45% of age-eligible lesbian women have received at least 1 dose of the HPV vaccine, compared with 60% of straight women.10,11
Due to societal stigma, more than 40% of transgender people have attempted suicide.12 Felt or perceived stigma is also associated with risky health behaviors that contribute to health disparities. LGBTQI+ people are more likely to use substances,13 lesbian women are more likely to be obese,14 and 19% of transgender men are living with HIV/AIDS.15 Rates of unintended pregnancy among lesbian women and transgender men are 28%, compared with 6% in straight women, and 12% in heterosexual teens.15,16
In addition to real or perceived discrimination, there are medical misperceptions among the LGBTQI+ community. For instance, sexual minority women (SMW) are less likely to receive regular screening for cervical cancer. In one survey of more than 400 SMW, about 25% reported not receiving regular screening. SMW may mistakenly believe they do not need Pap testing and pelvic exams because they do not have penile-vaginal intercourse.17,18 Transgender men may not identify with having a cervix, or may perceive ObGyns to be “gendered” toward people who identify as women.18
Embracing cultural humility
Cultural humility expands upon the term cultural competence, with the idea that one can never be fully competent in the culture of another person.19,20 The National Institutes of Health defines cultural humility as “a lifelong process of self-reflection and self-critique whereby the individual not only learns about another’s culture, but one starts with an examination of his/her own beliefs and cultural identities.”21
Having cultural humility is the recognition that, in order to treat your ObGyn patient as a whole person and engage in shared medical decision making in the office setting, you need to know their sexual orientation and gender identity. Treating each patient the same is not providing equitable care (equality does not equal equity) because each patient has different medical and psychosocial needs. Embracing cultural humility is the first step in creating safe and welcoming spaces in the ObGyn office.20
CASE Ways to better introduce yourself
To revisit the case, what options does the clinician have to start off on a best foot to create a safe space for Alex?
- Open with your own preferred pronouns. For instance, for an introduction, consider: “I’m Dr. X, my pronouns are she/her.”
- Don’t assume. Do not make assumptions about the relationship between Alex and the person accompanying them.
4 ways for creating welcoming and affirming spaces in ObGyn
- Make sure your intake form is inclusive. Include a space for pronouns and the patient’s preferred name (which may differ from their legal name). Also allow patients to choose more than 1 sexual orientation and gender identity.20 (An example form is available from the LGBT National Health Education Center: https://www.lgbtqiahealtheducation.org/publication/focus-forms-policy-creating-inclusive-environment-lgbt-patients/.)
- Create a safe environment in the waiting area. Try to ensure that at least 1 bathroom is labeled “All Gender” or “Family.” Gendered bathrooms (eg, Ladies’ or Men’s rooms) are not welcoming. Make sure your non-discrimination policy is displayed and includes sexual orientation and gender identity. Review the patient education and reading materials in your waiting room to ensure they are inclusive. Do they show people with varied gender expression? Do they show same-sex couples or interracial couples?
- Use a trauma-informed approach when taking a sexual history and while conducting a physical exam. Determine if a pelvic exam is necessary at this visit or can it be postponed for another visit, when trust has been established with the patient. Explain each part of the pelvic/vaginal exam prior to conducting and again while performing the exam. Before taking a sexual history, explain why you are asking the questions and be sure to remain neutral with your questioning. For instance, you can say, “It’s important for me to understand your medical history in detail to provide you with the best health care possible.” Instead of asking, “Do you have sex with men, women, or both?” ask, “Do you have sex with people with a penis, vagina, or both? Do you have anal sex?” Recognize that some patients may be in a polyamorous relationship and may have more than 1 committed partner. For sexually active patients consider asking if they have ever exchanged sex for money or other goods, making sure to avoid judgmental body language or wording. Patients who do engage in “survival sex” may benefit from a discussion on pre-exposure prophylaxis to reduce HIV transmission.22
- Provide appropriate counsel based on their feedback.
- Explain their risk for HPV infection and vaccination options.
- Respectfully ask if there is a need for contraception and review options appropriate for their situation.
- Ask about the use of “toys” and provide guidance on sanitation and risk of infection with shared toys.
- Determine current or past hormone use for patients who identify as transgender and nonbinary (although many do not take hormones and have not had gender-affirming procedures, some may be considering these procedures). Be sure to ask these patients if they have had any surgeries or other procedures.
The receipt of gynecologic care can be traumatic for some LGBTQI+ people. Explain to the patient why you are doing everything during your examination and how it might feel. If a pelvic exam is not absolutely necessary that day, perhaps the patient can return another time. For transgender men who have been taking testosterone,vaginal atrophy may be a concern, and you could consider a pediatric speculum.
In summary, the number of people who identify as lesbian, gay, bisexual, transgender/nonbinary, queer/questioning, intersex, or asexual is not insignificant. Many of these patients or their partners may present for ObGyn care at your office. Clinicians need to understand that there is a new language relative to sexual orientation and gender identity. Incorporating cultural humility into one’s practice requires personal introspection and is a first step to creating safe and welcoming spaces in the ObGyn office. ●
- Jones JM. LGBT identification in US ticks up to 7.1%. Gallup News. February 17, 2022. Accessed July 11, 2023. https://news.gallup .com/poll/389792/lgbt-identification-ticks -up.aspx
- Patterson JG, Tree JMJ, and Kamen C. Cultural competency and microaggressions in the provision of care to LGBT patients in rural and Appalachian Tennessee. Patient Educ Couns. 2019;102:2081-2090. doi: 10.1016/j.pec .2019.06.003
- Grasso C, Funk D. Collecting sexual orientation and gender identity (SO/GI) data in electronic health records. The National LGBT Health Education Center. Accessed October 12, 2023. https://fenwayhealth.org/wp-content/uploads /4.-Collecting-SOGI-Data.pdf
- Glossary of terms: LGBTQ. GLAAD website. Accessed October 16, 2023. https://glaad.org /reference/terms.
- LGBTQI+. Social protection and human rights website. Accessed November 2, 2023. https ://socialprotection-humanrights.org/key -issues/disadvantaged-and-vulnerable-groups /lgbtqi/
- Goldberg AE, Manley MH, Ellawala T, et al. Sexuality and sexual identity across the first year of parenthood among male-partnered plurisexual women. Psychol Sex Orientat Gend Divers. 2019;6:75.
- Campbell A, Perales F, Hughes TL, et al. Sexual fluidity and psychological distress: what happens when young women’s sexual identities change? J Health Soc Behav. 2022;63:577-593.
- Gessner M, Bishop MD, Martos A, et al. Sexual minority people’s perspectives of sexual health care: understanding minority stress in sexual health settings. Sex Res Social Policy. 2020;17:607618. doi: 10.1007/s13178-019-00418-9
- Carpenter E. “The health system just wasn’t built for us”: queer cisgender women and gender expansive individuals’ strategies for navigating reproductive health care. Womens Health Issues. 2021;31:478-484. doi: 10.1016 /j.whi.2021.06.004
- Casey LS, Reisner SL, Findling MG, et al. Discrimination in the United States: experiences of lesbian, gay, bisexual, transgender, and queer Americans. Health Serv Res. 2019;54(suppl 2):1454-1466. doi: 10.1111/1475-6773.13229
- Grasso C, Goldhammer H, Brown RJ, et al. Using sexual orientation and gender identity data in electronic health records to assess for disparities in preventive health screening services. Int J Med Inform. 2020:142:104245. doi: 10.1016 /j.ijmedinf.2020.104245
- Austin A, Craig SL, D’Souza S, et al. Suicidality among transgender youth: elucidating the role of interpersonal risk factors. J Interpers Violence. 2022;37:NP2696-NP2718. doi: 10.1177 /0886260520915554. Published correction appears in J Interpers Violence. 2020:8862 60520946128.
- Hibbert MP, Hillis A, Brett CE, et al. A narrative systematic review of sexualised drug use and sexual health outcomes among LGBT people. Int J Drug Policy. 2021;93:103187. doi: 10.1016 /j.drugpo.2021.103187
- Azagba S, Shan L, Latham K. Overweight and obesity among sexual minority adults in the United States. Int J Environ Res Public Health. 2019;16:1828. doi: 10.3390/ijerph16101828
- Klein PW, Psihopaidas D, Xavier J, et al. HIVrelated outcome disparities between transgender women living with HIV and cisgender people living with HIV served by the Health Resources and Services Administration’s Ryan White HIV/ AIDS Program: a retrospective study. PLoS Med. 2020;17:e1003125. doi: 10.1371/journal.pmed .1003125
- Jung C, Hunter A, Saleh M, et al. Breaking the binary: how clinicians can ensure everyone receives high quality reproductive health services. Open Access J Contracept. 2023:14:23-39. doi: 10.2147/OAJC.S368621
- Bustamante G, Reiter PL, McRee AL. Cervical cancer screening among sexual minority women: findings from a national survey. Cancer Causes Control. 2021;32:911-917. doi: 10.1007 /s10552-021-01442-0
- Dhillon N, Oliffe JL, Kelly MT, et al. Bridging barriers to cervical cancer screening in transgender men: a scoping review. Am J Mens Health. 2020;14:1557988320925691. doi: 10.1177/1557988320925691
- Stubbe DE. Practicing cultural competence and cultural humility in the care of diverse patients. Focus (Am Psychiatr Publ). 2020;18:49-51. doi: 10.1176/appi.focus.20190041
- Alpert A, Kamen C, Schabath MB, et al. What exactly are we measuring? Evaluating sexual and gender minority cultural humility training for oncology care clinicians. J Clin Oncol. 2020;38:2605-2609. doi: 10.1200/JCO.19.03300
- Yeager KA, Bauer-Wu S. Cultural humility: essential foundation for clinical researchers. Appl Nurs Res. 2013;26:251-256. doi: 10.1016 /j.apnr.2013.06.008
- Nagle-Yang S, Sachdeva J, Zhao LX, et al. Traumainformed care for obstetric and gynecologic settings. Matern Child Health J. 2022;26:2362-2369.
CASE A new patient office visit
A new patient is waiting for you in the exam room. You review the chart and see the sex demographic field is blank, and the patient’s name is Alex. As an ObGyn, most of your patients are female, but you have treated your patients’ partners for sexually transmitted infections. As you enter the room, you see 2 androgynously dressed individuals; you introduce yourself and ask,
“What brings you in today, and who is your friend?”
“This is my partner Charlie, and we are worried I have an STD.”
Estimates suggest that between 7% to 12% of the US population identifies as lesbian, gay, bisexual, transgender/non-binary, queer/questioning, intersex, or asexual (LGBTQI+).1 If you practice in an urban area, the odds are quite high that you have encountered an LGBTQI+ person who openly identified as such; if you are in a rural area, you also likely have had an LGBTQI+ patient, but they may not have disclosed this about themselves.2 Maybe you have had training in cultural relevance or are a member of this community and you feel confident in providing quality care to LGBTQI+ patients. Or maybe you think that, as a responsibly practicing health care clinician, you treat all patients the same, so whether or not you know their sexual orientation or gender identity does not impact the care you provide. As the proportion of US adults who identify as LGBTQI+ increases,1 it becomes more important for health care clinicians to understand the challenges these patients face when trying to access health care. To start, let’s review the meaning of LGBTQI+, the history of the community, what it means to be culturally relevant or humble, and how to create a welcoming and safe practice environment.
LGBTQI+ terms and definitions
The first step in providing quality care to LGBTQI+ patients is to understand the terminology associated with sexual orientation, gender identity, and gender expression.3–5
Sexual orientation refers to whom a person is sexually attracted. The term straight/heterosexual suggests a person is sexually attracted to a person of the opposite gender. Lesbian or gay refers to those who are attracted to their same gender. Some people use bisexual (attracted to both the same and opposite gender) and pansexual (attracted to all humans regardless of gender). Still others refer to themselves as queer—people who identify as someone who is not heterosexual or cisgender. A variety of other terms exist to describe one’s sexual attraction. There are also some people who identify as asexual, which suggests they are not sexually attracted to anyone.
Gender identity relates to how one views their own gender. If you were assigned female at birth and identify as a woman, you are cisgender. If you were assigned male at birth and identify as a woman, you may identify as transgender whether or not you have had gender transitioning surgery or have taken hormones. Some people do not identify with the terms male or female and may view themselves as nonbinary. The terms gender queer, gender fluid, gender diverse, and gender non-conforming also may be used to describe various ways that an individual may not identify as male or female. We also can refer to people as “assigned female at birth” or “assigned male at birth”. People with intersex conditions may require taking a unique medical history that includes asking about genetic testing (eg, 46,XX congenital adrenal hyperplasia or 46,XY complete gonadal dysgenesis).
Gender expression refers to how one pre-sents themselves to others through appearance, dress, and behavior. A person may be assigned female at birth, dress in a conventional male fashion, and still identify as a woman. Still others may choose to express their gender in a variety of ways that may not have anything to do with their sexual orientation or gender identity, such as dressing in ways that represent their culture.
People may be fluid in their sexual orientation or gender identity; it may change from day to day, month to month, or even year to year.6,7
*The term LGBTQI+ is not used consistently in the literature. Throughout this article, the terminology used matches that used in the cited reference(s).
Continue to: Health care and the LGBTQI+ community...
Health care and the LGBTQI+ community
The LGBTQI+ community has a history of experiencing societal discrimination and stigma, which stems from medical mistrust often due to a lack of understanding of their medical and psychosocial needs.8,9 A 2019 survey of US LGBTQ adults, found that about 50% of people who identified as transgender reported having negative or discriminatory experiences with a health care clinician.10 About 18% of transgender people anticipated being refused medical care due to their gender identity.10 About 18% of LGBTQ individuals avoid any type of medical care, fearing discrimination.10 Lesbian women are 3 times more likely to have not seen an ObGyn than women who identify as straight.11 Sixty-two percent of lesbian women have biological children and received prenatal care; however, of those, 47% do not receive routine cancer screenings.10,11 Only 45% of age-eligible lesbian women have received at least 1 dose of the HPV vaccine, compared with 60% of straight women.10,11
Due to societal stigma, more than 40% of transgender people have attempted suicide.12 Felt or perceived stigma is also associated with risky health behaviors that contribute to health disparities. LGBTQI+ people are more likely to use substances,13 lesbian women are more likely to be obese,14 and 19% of transgender men are living with HIV/AIDS.15 Rates of unintended pregnancy among lesbian women and transgender men are 28%, compared with 6% in straight women, and 12% in heterosexual teens.15,16
In addition to real or perceived discrimination, there are medical misperceptions among the LGBTQI+ community. For instance, sexual minority women (SMW) are less likely to receive regular screening for cervical cancer. In one survey of more than 400 SMW, about 25% reported not receiving regular screening. SMW may mistakenly believe they do not need Pap testing and pelvic exams because they do not have penile-vaginal intercourse.17,18 Transgender men may not identify with having a cervix, or may perceive ObGyns to be “gendered” toward people who identify as women.18
Embracing cultural humility
Cultural humility expands upon the term cultural competence, with the idea that one can never be fully competent in the culture of another person.19,20 The National Institutes of Health defines cultural humility as “a lifelong process of self-reflection and self-critique whereby the individual not only learns about another’s culture, but one starts with an examination of his/her own beliefs and cultural identities.”21
Having cultural humility is the recognition that, in order to treat your ObGyn patient as a whole person and engage in shared medical decision making in the office setting, you need to know their sexual orientation and gender identity. Treating each patient the same is not providing equitable care (equality does not equal equity) because each patient has different medical and psychosocial needs. Embracing cultural humility is the first step in creating safe and welcoming spaces in the ObGyn office.20
CASE Ways to better introduce yourself
To revisit the case, what options does the clinician have to start off on a best foot to create a safe space for Alex?
- Open with your own preferred pronouns. For instance, for an introduction, consider: “I’m Dr. X, my pronouns are she/her.”
- Don’t assume. Do not make assumptions about the relationship between Alex and the person accompanying them.
4 ways for creating welcoming and affirming spaces in ObGyn
- Make sure your intake form is inclusive. Include a space for pronouns and the patient’s preferred name (which may differ from their legal name). Also allow patients to choose more than 1 sexual orientation and gender identity.20 (An example form is available from the LGBT National Health Education Center: https://www.lgbtqiahealtheducation.org/publication/focus-forms-policy-creating-inclusive-environment-lgbt-patients/.)
- Create a safe environment in the waiting area. Try to ensure that at least 1 bathroom is labeled “All Gender” or “Family.” Gendered bathrooms (eg, Ladies’ or Men’s rooms) are not welcoming. Make sure your non-discrimination policy is displayed and includes sexual orientation and gender identity. Review the patient education and reading materials in your waiting room to ensure they are inclusive. Do they show people with varied gender expression? Do they show same-sex couples or interracial couples?
- Use a trauma-informed approach when taking a sexual history and while conducting a physical exam. Determine if a pelvic exam is necessary at this visit or can it be postponed for another visit, when trust has been established with the patient. Explain each part of the pelvic/vaginal exam prior to conducting and again while performing the exam. Before taking a sexual history, explain why you are asking the questions and be sure to remain neutral with your questioning. For instance, you can say, “It’s important for me to understand your medical history in detail to provide you with the best health care possible.” Instead of asking, “Do you have sex with men, women, or both?” ask, “Do you have sex with people with a penis, vagina, or both? Do you have anal sex?” Recognize that some patients may be in a polyamorous relationship and may have more than 1 committed partner. For sexually active patients consider asking if they have ever exchanged sex for money or other goods, making sure to avoid judgmental body language or wording. Patients who do engage in “survival sex” may benefit from a discussion on pre-exposure prophylaxis to reduce HIV transmission.22
- Provide appropriate counsel based on their feedback.
- Explain their risk for HPV infection and vaccination options.
- Respectfully ask if there is a need for contraception and review options appropriate for their situation.
- Ask about the use of “toys” and provide guidance on sanitation and risk of infection with shared toys.
- Determine current or past hormone use for patients who identify as transgender and nonbinary (although many do not take hormones and have not had gender-affirming procedures, some may be considering these procedures). Be sure to ask these patients if they have had any surgeries or other procedures.
The receipt of gynecologic care can be traumatic for some LGBTQI+ people. Explain to the patient why you are doing everything during your examination and how it might feel. If a pelvic exam is not absolutely necessary that day, perhaps the patient can return another time. For transgender men who have been taking testosterone,vaginal atrophy may be a concern, and you could consider a pediatric speculum.
In summary, the number of people who identify as lesbian, gay, bisexual, transgender/nonbinary, queer/questioning, intersex, or asexual is not insignificant. Many of these patients or their partners may present for ObGyn care at your office. Clinicians need to understand that there is a new language relative to sexual orientation and gender identity. Incorporating cultural humility into one’s practice requires personal introspection and is a first step to creating safe and welcoming spaces in the ObGyn office. ●
CASE A new patient office visit
A new patient is waiting for you in the exam room. You review the chart and see the sex demographic field is blank, and the patient’s name is Alex. As an ObGyn, most of your patients are female, but you have treated your patients’ partners for sexually transmitted infections. As you enter the room, you see 2 androgynously dressed individuals; you introduce yourself and ask,
“What brings you in today, and who is your friend?”
“This is my partner Charlie, and we are worried I have an STD.”
Estimates suggest that between 7% to 12% of the US population identifies as lesbian, gay, bisexual, transgender/non-binary, queer/questioning, intersex, or asexual (LGBTQI+).1 If you practice in an urban area, the odds are quite high that you have encountered an LGBTQI+ person who openly identified as such; if you are in a rural area, you also likely have had an LGBTQI+ patient, but they may not have disclosed this about themselves.2 Maybe you have had training in cultural relevance or are a member of this community and you feel confident in providing quality care to LGBTQI+ patients. Or maybe you think that, as a responsibly practicing health care clinician, you treat all patients the same, so whether or not you know their sexual orientation or gender identity does not impact the care you provide. As the proportion of US adults who identify as LGBTQI+ increases,1 it becomes more important for health care clinicians to understand the challenges these patients face when trying to access health care. To start, let’s review the meaning of LGBTQI+, the history of the community, what it means to be culturally relevant or humble, and how to create a welcoming and safe practice environment.
LGBTQI+ terms and definitions
The first step in providing quality care to LGBTQI+ patients is to understand the terminology associated with sexual orientation, gender identity, and gender expression.3–5
Sexual orientation refers to whom a person is sexually attracted. The term straight/heterosexual suggests a person is sexually attracted to a person of the opposite gender. Lesbian or gay refers to those who are attracted to their same gender. Some people use bisexual (attracted to both the same and opposite gender) and pansexual (attracted to all humans regardless of gender). Still others refer to themselves as queer—people who identify as someone who is not heterosexual or cisgender. A variety of other terms exist to describe one’s sexual attraction. There are also some people who identify as asexual, which suggests they are not sexually attracted to anyone.
Gender identity relates to how one views their own gender. If you were assigned female at birth and identify as a woman, you are cisgender. If you were assigned male at birth and identify as a woman, you may identify as transgender whether or not you have had gender transitioning surgery or have taken hormones. Some people do not identify with the terms male or female and may view themselves as nonbinary. The terms gender queer, gender fluid, gender diverse, and gender non-conforming also may be used to describe various ways that an individual may not identify as male or female. We also can refer to people as “assigned female at birth” or “assigned male at birth”. People with intersex conditions may require taking a unique medical history that includes asking about genetic testing (eg, 46,XX congenital adrenal hyperplasia or 46,XY complete gonadal dysgenesis).
Gender expression refers to how one pre-sents themselves to others through appearance, dress, and behavior. A person may be assigned female at birth, dress in a conventional male fashion, and still identify as a woman. Still others may choose to express their gender in a variety of ways that may not have anything to do with their sexual orientation or gender identity, such as dressing in ways that represent their culture.
People may be fluid in their sexual orientation or gender identity; it may change from day to day, month to month, or even year to year.6,7
*The term LGBTQI+ is not used consistently in the literature. Throughout this article, the terminology used matches that used in the cited reference(s).
Continue to: Health care and the LGBTQI+ community...
Health care and the LGBTQI+ community
The LGBTQI+ community has a history of experiencing societal discrimination and stigma, which stems from medical mistrust often due to a lack of understanding of their medical and psychosocial needs.8,9 A 2019 survey of US LGBTQ adults, found that about 50% of people who identified as transgender reported having negative or discriminatory experiences with a health care clinician.10 About 18% of transgender people anticipated being refused medical care due to their gender identity.10 About 18% of LGBTQ individuals avoid any type of medical care, fearing discrimination.10 Lesbian women are 3 times more likely to have not seen an ObGyn than women who identify as straight.11 Sixty-two percent of lesbian women have biological children and received prenatal care; however, of those, 47% do not receive routine cancer screenings.10,11 Only 45% of age-eligible lesbian women have received at least 1 dose of the HPV vaccine, compared with 60% of straight women.10,11
Due to societal stigma, more than 40% of transgender people have attempted suicide.12 Felt or perceived stigma is also associated with risky health behaviors that contribute to health disparities. LGBTQI+ people are more likely to use substances,13 lesbian women are more likely to be obese,14 and 19% of transgender men are living with HIV/AIDS.15 Rates of unintended pregnancy among lesbian women and transgender men are 28%, compared with 6% in straight women, and 12% in heterosexual teens.15,16
In addition to real or perceived discrimination, there are medical misperceptions among the LGBTQI+ community. For instance, sexual minority women (SMW) are less likely to receive regular screening for cervical cancer. In one survey of more than 400 SMW, about 25% reported not receiving regular screening. SMW may mistakenly believe they do not need Pap testing and pelvic exams because they do not have penile-vaginal intercourse.17,18 Transgender men may not identify with having a cervix, or may perceive ObGyns to be “gendered” toward people who identify as women.18
Embracing cultural humility
Cultural humility expands upon the term cultural competence, with the idea that one can never be fully competent in the culture of another person.19,20 The National Institutes of Health defines cultural humility as “a lifelong process of self-reflection and self-critique whereby the individual not only learns about another’s culture, but one starts with an examination of his/her own beliefs and cultural identities.”21
Having cultural humility is the recognition that, in order to treat your ObGyn patient as a whole person and engage in shared medical decision making in the office setting, you need to know their sexual orientation and gender identity. Treating each patient the same is not providing equitable care (equality does not equal equity) because each patient has different medical and psychosocial needs. Embracing cultural humility is the first step in creating safe and welcoming spaces in the ObGyn office.20
CASE Ways to better introduce yourself
To revisit the case, what options does the clinician have to start off on a best foot to create a safe space for Alex?
- Open with your own preferred pronouns. For instance, for an introduction, consider: “I’m Dr. X, my pronouns are she/her.”
- Don’t assume. Do not make assumptions about the relationship between Alex and the person accompanying them.
4 ways for creating welcoming and affirming spaces in ObGyn
- Make sure your intake form is inclusive. Include a space for pronouns and the patient’s preferred name (which may differ from their legal name). Also allow patients to choose more than 1 sexual orientation and gender identity.20 (An example form is available from the LGBT National Health Education Center: https://www.lgbtqiahealtheducation.org/publication/focus-forms-policy-creating-inclusive-environment-lgbt-patients/.)
- Create a safe environment in the waiting area. Try to ensure that at least 1 bathroom is labeled “All Gender” or “Family.” Gendered bathrooms (eg, Ladies’ or Men’s rooms) are not welcoming. Make sure your non-discrimination policy is displayed and includes sexual orientation and gender identity. Review the patient education and reading materials in your waiting room to ensure they are inclusive. Do they show people with varied gender expression? Do they show same-sex couples or interracial couples?
- Use a trauma-informed approach when taking a sexual history and while conducting a physical exam. Determine if a pelvic exam is necessary at this visit or can it be postponed for another visit, when trust has been established with the patient. Explain each part of the pelvic/vaginal exam prior to conducting and again while performing the exam. Before taking a sexual history, explain why you are asking the questions and be sure to remain neutral with your questioning. For instance, you can say, “It’s important for me to understand your medical history in detail to provide you with the best health care possible.” Instead of asking, “Do you have sex with men, women, or both?” ask, “Do you have sex with people with a penis, vagina, or both? Do you have anal sex?” Recognize that some patients may be in a polyamorous relationship and may have more than 1 committed partner. For sexually active patients consider asking if they have ever exchanged sex for money or other goods, making sure to avoid judgmental body language or wording. Patients who do engage in “survival sex” may benefit from a discussion on pre-exposure prophylaxis to reduce HIV transmission.22
- Provide appropriate counsel based on their feedback.
- Explain their risk for HPV infection and vaccination options.
- Respectfully ask if there is a need for contraception and review options appropriate for their situation.
- Ask about the use of “toys” and provide guidance on sanitation and risk of infection with shared toys.
- Determine current or past hormone use for patients who identify as transgender and nonbinary (although many do not take hormones and have not had gender-affirming procedures, some may be considering these procedures). Be sure to ask these patients if they have had any surgeries or other procedures.
The receipt of gynecologic care can be traumatic for some LGBTQI+ people. Explain to the patient why you are doing everything during your examination and how it might feel. If a pelvic exam is not absolutely necessary that day, perhaps the patient can return another time. For transgender men who have been taking testosterone,vaginal atrophy may be a concern, and you could consider a pediatric speculum.
In summary, the number of people who identify as lesbian, gay, bisexual, transgender/nonbinary, queer/questioning, intersex, or asexual is not insignificant. Many of these patients or their partners may present for ObGyn care at your office. Clinicians need to understand that there is a new language relative to sexual orientation and gender identity. Incorporating cultural humility into one’s practice requires personal introspection and is a first step to creating safe and welcoming spaces in the ObGyn office. ●
- Jones JM. LGBT identification in US ticks up to 7.1%. Gallup News. February 17, 2022. Accessed July 11, 2023. https://news.gallup .com/poll/389792/lgbt-identification-ticks -up.aspx
- Patterson JG, Tree JMJ, and Kamen C. Cultural competency and microaggressions in the provision of care to LGBT patients in rural and Appalachian Tennessee. Patient Educ Couns. 2019;102:2081-2090. doi: 10.1016/j.pec .2019.06.003
- Grasso C, Funk D. Collecting sexual orientation and gender identity (SO/GI) data in electronic health records. The National LGBT Health Education Center. Accessed October 12, 2023. https://fenwayhealth.org/wp-content/uploads /4.-Collecting-SOGI-Data.pdf
- Glossary of terms: LGBTQ. GLAAD website. Accessed October 16, 2023. https://glaad.org /reference/terms.
- LGBTQI+. Social protection and human rights website. Accessed November 2, 2023. https ://socialprotection-humanrights.org/key -issues/disadvantaged-and-vulnerable-groups /lgbtqi/
- Goldberg AE, Manley MH, Ellawala T, et al. Sexuality and sexual identity across the first year of parenthood among male-partnered plurisexual women. Psychol Sex Orientat Gend Divers. 2019;6:75.
- Campbell A, Perales F, Hughes TL, et al. Sexual fluidity and psychological distress: what happens when young women’s sexual identities change? J Health Soc Behav. 2022;63:577-593.
- Gessner M, Bishop MD, Martos A, et al. Sexual minority people’s perspectives of sexual health care: understanding minority stress in sexual health settings. Sex Res Social Policy. 2020;17:607618. doi: 10.1007/s13178-019-00418-9
- Carpenter E. “The health system just wasn’t built for us”: queer cisgender women and gender expansive individuals’ strategies for navigating reproductive health care. Womens Health Issues. 2021;31:478-484. doi: 10.1016 /j.whi.2021.06.004
- Casey LS, Reisner SL, Findling MG, et al. Discrimination in the United States: experiences of lesbian, gay, bisexual, transgender, and queer Americans. Health Serv Res. 2019;54(suppl 2):1454-1466. doi: 10.1111/1475-6773.13229
- Grasso C, Goldhammer H, Brown RJ, et al. Using sexual orientation and gender identity data in electronic health records to assess for disparities in preventive health screening services. Int J Med Inform. 2020:142:104245. doi: 10.1016 /j.ijmedinf.2020.104245
- Austin A, Craig SL, D’Souza S, et al. Suicidality among transgender youth: elucidating the role of interpersonal risk factors. J Interpers Violence. 2022;37:NP2696-NP2718. doi: 10.1177 /0886260520915554. Published correction appears in J Interpers Violence. 2020:8862 60520946128.
- Hibbert MP, Hillis A, Brett CE, et al. A narrative systematic review of sexualised drug use and sexual health outcomes among LGBT people. Int J Drug Policy. 2021;93:103187. doi: 10.1016 /j.drugpo.2021.103187
- Azagba S, Shan L, Latham K. Overweight and obesity among sexual minority adults in the United States. Int J Environ Res Public Health. 2019;16:1828. doi: 10.3390/ijerph16101828
- Klein PW, Psihopaidas D, Xavier J, et al. HIVrelated outcome disparities between transgender women living with HIV and cisgender people living with HIV served by the Health Resources and Services Administration’s Ryan White HIV/ AIDS Program: a retrospective study. PLoS Med. 2020;17:e1003125. doi: 10.1371/journal.pmed .1003125
- Jung C, Hunter A, Saleh M, et al. Breaking the binary: how clinicians can ensure everyone receives high quality reproductive health services. Open Access J Contracept. 2023:14:23-39. doi: 10.2147/OAJC.S368621
- Bustamante G, Reiter PL, McRee AL. Cervical cancer screening among sexual minority women: findings from a national survey. Cancer Causes Control. 2021;32:911-917. doi: 10.1007 /s10552-021-01442-0
- Dhillon N, Oliffe JL, Kelly MT, et al. Bridging barriers to cervical cancer screening in transgender men: a scoping review. Am J Mens Health. 2020;14:1557988320925691. doi: 10.1177/1557988320925691
- Stubbe DE. Practicing cultural competence and cultural humility in the care of diverse patients. Focus (Am Psychiatr Publ). 2020;18:49-51. doi: 10.1176/appi.focus.20190041
- Alpert A, Kamen C, Schabath MB, et al. What exactly are we measuring? Evaluating sexual and gender minority cultural humility training for oncology care clinicians. J Clin Oncol. 2020;38:2605-2609. doi: 10.1200/JCO.19.03300
- Yeager KA, Bauer-Wu S. Cultural humility: essential foundation for clinical researchers. Appl Nurs Res. 2013;26:251-256. doi: 10.1016 /j.apnr.2013.06.008
- Nagle-Yang S, Sachdeva J, Zhao LX, et al. Traumainformed care for obstetric and gynecologic settings. Matern Child Health J. 2022;26:2362-2369.
- Jones JM. LGBT identification in US ticks up to 7.1%. Gallup News. February 17, 2022. Accessed July 11, 2023. https://news.gallup .com/poll/389792/lgbt-identification-ticks -up.aspx
- Patterson JG, Tree JMJ, and Kamen C. Cultural competency and microaggressions in the provision of care to LGBT patients in rural and Appalachian Tennessee. Patient Educ Couns. 2019;102:2081-2090. doi: 10.1016/j.pec .2019.06.003
- Grasso C, Funk D. Collecting sexual orientation and gender identity (SO/GI) data in electronic health records. The National LGBT Health Education Center. Accessed October 12, 2023. https://fenwayhealth.org/wp-content/uploads /4.-Collecting-SOGI-Data.pdf
- Glossary of terms: LGBTQ. GLAAD website. Accessed October 16, 2023. https://glaad.org /reference/terms.
- LGBTQI+. Social protection and human rights website. Accessed November 2, 2023. https ://socialprotection-humanrights.org/key -issues/disadvantaged-and-vulnerable-groups /lgbtqi/
- Goldberg AE, Manley MH, Ellawala T, et al. Sexuality and sexual identity across the first year of parenthood among male-partnered plurisexual women. Psychol Sex Orientat Gend Divers. 2019;6:75.
- Campbell A, Perales F, Hughes TL, et al. Sexual fluidity and psychological distress: what happens when young women’s sexual identities change? J Health Soc Behav. 2022;63:577-593.
- Gessner M, Bishop MD, Martos A, et al. Sexual minority people’s perspectives of sexual health care: understanding minority stress in sexual health settings. Sex Res Social Policy. 2020;17:607618. doi: 10.1007/s13178-019-00418-9
- Carpenter E. “The health system just wasn’t built for us”: queer cisgender women and gender expansive individuals’ strategies for navigating reproductive health care. Womens Health Issues. 2021;31:478-484. doi: 10.1016 /j.whi.2021.06.004
- Casey LS, Reisner SL, Findling MG, et al. Discrimination in the United States: experiences of lesbian, gay, bisexual, transgender, and queer Americans. Health Serv Res. 2019;54(suppl 2):1454-1466. doi: 10.1111/1475-6773.13229
- Grasso C, Goldhammer H, Brown RJ, et al. Using sexual orientation and gender identity data in electronic health records to assess for disparities in preventive health screening services. Int J Med Inform. 2020:142:104245. doi: 10.1016 /j.ijmedinf.2020.104245
- Austin A, Craig SL, D’Souza S, et al. Suicidality among transgender youth: elucidating the role of interpersonal risk factors. J Interpers Violence. 2022;37:NP2696-NP2718. doi: 10.1177 /0886260520915554. Published correction appears in J Interpers Violence. 2020:8862 60520946128.
- Hibbert MP, Hillis A, Brett CE, et al. A narrative systematic review of sexualised drug use and sexual health outcomes among LGBT people. Int J Drug Policy. 2021;93:103187. doi: 10.1016 /j.drugpo.2021.103187
- Azagba S, Shan L, Latham K. Overweight and obesity among sexual minority adults in the United States. Int J Environ Res Public Health. 2019;16:1828. doi: 10.3390/ijerph16101828
- Klein PW, Psihopaidas D, Xavier J, et al. HIVrelated outcome disparities between transgender women living with HIV and cisgender people living with HIV served by the Health Resources and Services Administration’s Ryan White HIV/ AIDS Program: a retrospective study. PLoS Med. 2020;17:e1003125. doi: 10.1371/journal.pmed .1003125
- Jung C, Hunter A, Saleh M, et al. Breaking the binary: how clinicians can ensure everyone receives high quality reproductive health services. Open Access J Contracept. 2023:14:23-39. doi: 10.2147/OAJC.S368621
- Bustamante G, Reiter PL, McRee AL. Cervical cancer screening among sexual minority women: findings from a national survey. Cancer Causes Control. 2021;32:911-917. doi: 10.1007 /s10552-021-01442-0
- Dhillon N, Oliffe JL, Kelly MT, et al. Bridging barriers to cervical cancer screening in transgender men: a scoping review. Am J Mens Health. 2020;14:1557988320925691. doi: 10.1177/1557988320925691
- Stubbe DE. Practicing cultural competence and cultural humility in the care of diverse patients. Focus (Am Psychiatr Publ). 2020;18:49-51. doi: 10.1176/appi.focus.20190041
- Alpert A, Kamen C, Schabath MB, et al. What exactly are we measuring? Evaluating sexual and gender minority cultural humility training for oncology care clinicians. J Clin Oncol. 2020;38:2605-2609. doi: 10.1200/JCO.19.03300
- Yeager KA, Bauer-Wu S. Cultural humility: essential foundation for clinical researchers. Appl Nurs Res. 2013;26:251-256. doi: 10.1016 /j.apnr.2013.06.008
- Nagle-Yang S, Sachdeva J, Zhao LX, et al. Traumainformed care for obstetric and gynecologic settings. Matern Child Health J. 2022;26:2362-2369.
Announcement from the publisher
Dear OBG Management Reader:
Frontline Medical Communications Inc has made the difficult decision to discontinue publication of
The online archive of clinical content for
For the latest news and information on obstetrics and gynecology, continue to turn to MDedge ObGyn.
Goodbye to OBG Management
Robert L. Barbieri, MD
OBG
Over 4 decades, the work of the
Our editorial board members are nationally recognized experts in our field and innovators in clinical care. Our editorial members include: Arnold P. Advincula, MD; Linda D. Bradley, MD; Amy L. Garcia, MD; Steven R. Goldstein, MD, MSCP, CCD; Andrew M. Kaunitz, MD, MSCP; Barbara Levy, MD; David G. Mutch, MD; Errol R. Norwitz, MD, PhD, MBA; Jaimey Pauli, MD; JoAnn V. Pinkerton, MD, MSCP; Joseph S. Sanfilippo, MD; and James A. Simon, MD, CCD, IF, MSCP. Prior to his retirement, Dr. John Repke was an important member of our editorial board. Over the past decade our editorial team—Lila O’Connor, Editorial Manager, and Kathy Christie, Senior Medical Content Editor—have ensured that the articles written by our authors are expertly prepared for publication and presentation to our readers.
In clinical practice, we sometimes do not achieve the optimal patient outcomes we desire. Over the past 4 decades, the
Dear OBG Management Reader:
Frontline Medical Communications Inc has made the difficult decision to discontinue publication of
The online archive of clinical content for
For the latest news and information on obstetrics and gynecology, continue to turn to MDedge ObGyn.
Goodbye to OBG Management
Robert L. Barbieri, MD
OBG
Over 4 decades, the work of the
Our editorial board members are nationally recognized experts in our field and innovators in clinical care. Our editorial members include: Arnold P. Advincula, MD; Linda D. Bradley, MD; Amy L. Garcia, MD; Steven R. Goldstein, MD, MSCP, CCD; Andrew M. Kaunitz, MD, MSCP; Barbara Levy, MD; David G. Mutch, MD; Errol R. Norwitz, MD, PhD, MBA; Jaimey Pauli, MD; JoAnn V. Pinkerton, MD, MSCP; Joseph S. Sanfilippo, MD; and James A. Simon, MD, CCD, IF, MSCP. Prior to his retirement, Dr. John Repke was an important member of our editorial board. Over the past decade our editorial team—Lila O’Connor, Editorial Manager, and Kathy Christie, Senior Medical Content Editor—have ensured that the articles written by our authors are expertly prepared for publication and presentation to our readers.
In clinical practice, we sometimes do not achieve the optimal patient outcomes we desire. Over the past 4 decades, the
Dear OBG Management Reader:
Frontline Medical Communications Inc has made the difficult decision to discontinue publication of
The online archive of clinical content for
For the latest news and information on obstetrics and gynecology, continue to turn to MDedge ObGyn.
Goodbye to OBG Management
Robert L. Barbieri, MD
OBG
Over 4 decades, the work of the
Our editorial board members are nationally recognized experts in our field and innovators in clinical care. Our editorial members include: Arnold P. Advincula, MD; Linda D. Bradley, MD; Amy L. Garcia, MD; Steven R. Goldstein, MD, MSCP, CCD; Andrew M. Kaunitz, MD, MSCP; Barbara Levy, MD; David G. Mutch, MD; Errol R. Norwitz, MD, PhD, MBA; Jaimey Pauli, MD; JoAnn V. Pinkerton, MD, MSCP; Joseph S. Sanfilippo, MD; and James A. Simon, MD, CCD, IF, MSCP. Prior to his retirement, Dr. John Repke was an important member of our editorial board. Over the past decade our editorial team—Lila O’Connor, Editorial Manager, and Kathy Christie, Senior Medical Content Editor—have ensured that the articles written by our authors are expertly prepared for publication and presentation to our readers.
In clinical practice, we sometimes do not achieve the optimal patient outcomes we desire. Over the past 4 decades, the
The clinical utility of newly approved angiogenic markers for identifying patients at risk for adverse outcomes due to preeclampsia
In the United States there is an epidemic of hypertensive disorders in pregnancy, with 16% of pregnant people being diagnosed with preeclampsia, gestational hypertension, chronic hypertension, preeclampsia superimposed on chronic hypertension, HELLP, or eclampsia.1 Preeclampsia with severe features increases the maternal risk for stroke, pulmonary edema, kidney injury, abruption, and fetal and maternal death. Preeclampsia also increases the fetal risk for growth restriction, oligohydramnios, and preterm birth.
Angiogenic factors and the pathophysiology of preeclampsia—From bench to bedside
The pathophysiology of preeclampsia is not fully characterized, but a leading theory is that placental ischemia causes increased placental production of anti-angiogenesis factors and a decrease in placental production of pro-angiogenesis factors.2-4 Clinical studies support the theory that preeclampsia is associated with an increase in placental production of anti-angiogenesis factors, including soluble fms-like tyrosine kinase 1 (sFlt-1) and soluble endoglin, and a decrease in the placental production of pro-angiogenesis factors, including placental growth factor (PlGF).5-15
The US Food and Drug Administration (FDA) has recently approved an assay for the measurement of sFlt-1 (Brahms sFlt-1 Kryptor) and PlGF (Brahms sFlt-1 Kryptor) (Thermo Fisher Scientific; Waltham, Massachusetts).16 This editorial focuses on the current and evolving indications for the measurement of sFlt-1 and PlGF in obstetric practice.
FDA approval of a preeclampsia blood test
The FDA approval of the tests to measure sFlt-1 and PlGF is narrowly tailored and focused on using the sFlt-1/PlGF ratio to assess the risk of progression to preeclampsia with severe features within 2 weeks among hospitalized patients with a hypertensive disorder in pregnancy with a singleton pregnancy between 23 weeks 0 days (23w0d) and 34w6d gestation.16 The test is meant to be used in conjunction with other laboratory tests and clinical assessment. The FDA advises that the test results should not be used to diagnose preeclampsia, nor should they be used to determine the timing of delivery or timing of patient discharge.16 The sFlt-1 and PIGF measurements are both reported as pg/mL, and the sFlt-1/PlGF ratio has no units.
The FDA approval is based on clinical studies that demonstrate the effectiveness of the test in predicting the progression of a hypertensive disorder in pregnancy to preeclampsia with severe features within 2 weeks of testing. In one study, the sFlt-1/PlGF ratio was measured in 556 pregnant patients with a singleton pregnancy who were between 23w0d and 34w6d gestation and hospitalized with a hypertensivedisorder in pregnancy without severe features at study enrollment.15 Those patients receiving intravenous heparin were excluded because of the effect of heparin on sFlt-1 levels. Participants’ mean age was 31.7 years, and their mean gestational age was 30w3d. The patients’ mean body mass index (BMI) was 34.2 kg/m2, with mean maximal blood pressure (BP) at enrollment of 159 mm Hg systolic and 95 mm Hg diastolic.
In this cohort, 31% of enrolled patients progressed to preeclampsia with severe features within 2 weeks. At enrollment, the median sFlt-1/PlGF ratio was greater among the patients who progressed to preeclampsia with severe features than among those who did not have progression to preeclampsia with severe features (291 vs 7). An elevated sFlt-1/PlGF ratio (determined to be a ratio ≥ 40) predicted that patients would progress to severe preeclampsiawith severe features—with positive and negative predictive values of 65% and 96%, respectively. Among the subgroup of patients with a history of chronic hypertension, an sFlt-1/PlGF ratio ≥ 40 had positive and negative predictive values of 59% and 94%, respectively. Focusing the analysis on patients who self-reported their race as Black, representing 30% of the cohort, the positive and negative predictive values for a sFlt-1/PlGF ratio ≥ 40 were 66% and 99%, respectively.15
Receiver-operating curve analyses were used to compare the predictive performance of sFlt-1/PlGF measurement versus standard clinical factors and standard laboratory results, including systolic and diastolic BP; levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), and creatinine; and platelet count.15 The area under the curve for predicting progression to preeclampsia with severe features was much greater for the sFlt-1/PlGF test (0.92) than for systolic (0.67) and diastolic BP (0.70), AST level (0.66), ALT level (0.61), creatinine level (0.65), and platelet count (0.57).15 These results demonstrate that measuring sFlt-1/PlGF ratios is a much better way to predict the progression of preeclampsia to severe disease than measuring standard clinical and laboratory results.
Patients with a sFlt-1/PlGF ratio ≥ 40 had higher rates of adverse maternal outcomes including severe hypertension, abruption, stroke, eclampsia, pulmonary edema, thrombocytopenia, low platelets, and/or coagulation disorder, than those patients with a ratio < 40, (16.1% vs 2.8%, respectively; relative risk [RR], 5.8; 95% confidence interval [CI], 2.8 to 12.2).15 Adverse fetal and neonatal outcomes (including fetal death, small for gestational age and early delivery due to progression of disease) were more common among patients with a sFlt-1/PlGF ratio of ≥ 40 (80% vs 26%; RR, 3.1; 95% CI, 2.5–3.8).15 Many other studies support the hypothesis that the sFlt-1/PlGF ratio is predictive of adverse outcomes among patients with hypertensive disorders in pregnancy.6-15
Applying the bottom-line study findings. Patients with a hypertensive disorder in pregnancy and a sFlt-1/PlGF ratio < 40 have a low risk of progressing to preeclampsia with severe features over the following 2 weeks, with a negative predictive value of 96%. The remarkably high negative predictive value of a sFlt-1/PlGF ratio < 40 will help obstetricians generate a care plan that optimizes the use of limited health care resources. Conversely, about two-thirds of patients with a hypertensive disorder in pregnancy and a sFlt-1/PlGF test ≥ 40 will progress to preeclampsia with severe features and may need to prepare for a preterm delivery.
Continue to: Clinical utility of the sFlt-1/PlGF ratio in obstetric triage...
Clinical utility of the sFlt-1/PlGF ratio in obstetric triage
Measurement of the sFlt-1/PlGF ratio may help guide clinical care among patients referred to obstetric triage or admitted to the hospital for the evaluation of suspected preeclampsia. In one study, 402 patients with a singleton pregnancy referred to the hospital for evaluation of suspected preeclampsia, had a standard evaluation plus measurement of an sFlt-1/PlGF ratio.13 The clinicians caring for the patients did not have access to the sFlt-1/PlGF test results. In this cohort, 16% of the patients developed preeclampsia with severe features in the 2 weeks following the initial assessment in triage. In this cohort, a normal sFlt-1/PlGF ratio reliably predicted which patients were not going to develop preeclampsia with severe features over the following 2 weeks, with a negative predictive value of 98%. Among the patients with an elevated sFlt-1/PlGF ratio, however, the positive predictive value of the test was 47% for developing preeclampsia with severe features within the 2 weeks following initial evaluation. Among patients < 34 weeks’ gestation, an elevated sFlt-1/PlGF ratio had a positive predictive value of 65%, and a negative predictive value of 98%. Other studies also have reported that the sFlt-1/PlGF ratio is of value for assessing the risk for progression to preeclampsia with severe features in patients being evaluated for suspected preeclampsia.6,17,18
In obstetric triage, it is difficult to predict the clinical course of patients referred for the evaluation of suspected preeclampsia based on BP measurements or standard laboratory tests. The sFlt-1/PlGF test will help clinicians identify patients at low and high risk of progressing to preeclampsia with severe features.19 Patients with a normal sFlt-1/PlGF test are at low risk of developing preeclampsia with severe features over the following 2 weeks. Patients with an elevated sFlt-1/PlGF test are at higher risk of progressing to preeclampsia with severe features and may warrant more intensive obstetric care. An enhanced care program might include:
- patient education
- remote monitoring of BP or hospitalization
- more frequent assessment of fetal well-being and growth
- administration of glucocorticoids to advance fetal maturity, if indicated by the gestational age.
Twin pregnancy complicated by preeclampsia
Twin pregnancy is associated with a high risk of developing preeclampsia and fetal growth restriction. For patients with a twin pregnancy and a hypertensive disorder in pregnancy, an elevated sFlt-1/PlGF ratio is associated with the need for delivery within 2 weeks and an increased rate of adverse maternal and neonatal outcomes. In a retrospective study involving 164 patients with twin pregnancy first evaluated for suspected preeclampsia at a median gestational age of 33w4d, the sFlt-1/PlGF ratio was positively correlated with progression of preeclampsia without severe features to severe features within 2 weeks.20 In this cohort, at the initial evaluation for suspected preeclampsia, the sFlt-1/PlGF ratio was lower among patients who did not need delivery within 2 weeks compared with those who were delivered within 2 weeks, 24 versus 84 (P<.001). The mean sFlt-1/PlGF ratio was 99 among patients who needed delivery within 1 week following the initial evaluation for suspected preeclampsia. Among patients who delivered within 1 week of presentation, the reasons for delivery were the development of severe hypertension, severe dyspnea, placental abruption, rising levels of serum liver function enzymes, and/or onset of the HELLP syndrome.
An important finding in this study is that a normal
The sFlt-1/PlGF test is a welcome addition to OB care
FDA approval of laboratory tests to measure circulating levels of sFlt-1 and PlGF will advance obstetric practice by identifying patients with a hypertensive disorder in pregnancy who are at low and high risk of developing preeclampsia with severe features within 2 weeks of the test. No laboratory test can replace the clinical judgment of obstetricians who are responsible for balancing the maternal and fetal risks that can occur in the management of a patient with a hypertensive disorder in pregnancy. The
- Ford ND, Cox S, Ko JY, et al. Hypertensive disorders in pregnancy and mortality at delivery hospitalization-United States 2017-2019. Morb Mortal Week Report. 2022;71:585-591.
- Nagamatsu T, Fujii T, Kusumi M, et al. Cytotrophoblasts up-regulate soluble fms-like tyrosine kinase-1 expression under reduced oxygen: an implication for placental vascular development and the pathophysiology of preeclampsia. Endocrinology. 2004;145:4838-4445.
- Rana S, Lemoine E, Granger JP, et al. Preeclampsia: pathophysiology, challenges and perspectives. Circ Res. 2019;124:1094-1112.
- Rana S, Burke SD, Karumanchi SA. Imbalances in circulating angiogenic factors in the pathophysiology of preeclampsia and related disorders. Am J Obstet Gynecol. 2022(2S):S1019-S1034.
- Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672-683.
- Chaiworapongsa T, Romero R, Savasan ZA, et al. Maternal plasma concentrations of angiogenic/ anti-angiogenic factors are of prognostic value in patients presenting to the obstetrical triage area with the suspicion of preeclampsia. J Matern Fetal Neonatal Med. 2011;24:1187-1207.
- Rana S, Powe CE, Salahuddin S, et al. Angiogenic factors and the risk of adverse outcomes in women with suspected preeclampsia. Circulation. 2012;125:911-919.
- Moore AG, Young H, Keller JM, et al. Angiogenic biomarkers for prediction of maternal and neonatal complications in suspected preeclampsia. J Matern Fetal Neonatal Med. 2012;25:2651-2657.
- Verlohren S, Herraiz I, Lapaire O, et al. The sFlt-1/ PlGF ratio in different types of hypertensive pregnancy disorders and its prognostic potential in preeclamptic patients. Am J Obstet Gynecol. 2012;206:58.e1-e8.
- Verlohren S, Herraiz I, Lapaire O, et al. New gestational phase-specific cutoff values for the use of soluble fms-like tyrosine kinase-1/placental growth factor ratio as a diagnostic test for preeclampsia. Hypertension. 2014;63:346-352.
- Zeisler H, Llurba E, Chantraine F, et al. Predictive value of the sFlt-1/PlGF ratio in women with suspected preeclampsia. N Engl J Med. 2016;374:1322.
- Duckworth S, Griffin M, Seed PT, et al. Diagnostic biomarkers in women with suspected preeclampsia in a prospective multicenter study. Obstet Gynecol. 2016;128:245-252.
- Rana S, Salahuddin S, Mueller A, et al. Angiogenic biomarkers in triage and risk for preeclampsia with severe features. Pregnancy Hyertens. 2018;13:100-106.
- Bian X, Biswas A, Huang X, et al. Short-term prediction of adverse outcomes using the sFlt-1/PlGF ratio in Asian women with suspected preeclampsia. Hypertension. 2019;74:164-172.
- Thadhani R, Lemoine E, Rana S, et al. Circulating angiogenic factor levels in hypertensive disorders of pregnancy. N Engl J Med Evidence. 2022. doi 10.1056/EVIDoa2200161.
- US Food and Drug Administration. FDA approval letter for an assay to measure sFlt-1 and PlGF. May 18, 2023. https://www.accessdata.fda.gov/cdrh _docs/pdf22/DEN220027.pdf
- Chaiworapongsa T, Romero R, Korzeniewski SJ, et al. Plasma concentrations of angiogenic/ anti-angiogenic factors have prognostic value in women presenting with suspected preeclampsia to the obstetrical triage area: a prospective study. J Matern Fetal Neonatal Med. 2014;27:132-144.
- Palomaki GE, Haddow JE, Haddow HR, et al. Modeling risk for severe adverse outcomes using angiogenic factor measurements in women with suspected preterm preeclampsia. Prenat Diagn. 2015;35:386-393.
- Verlohren S, Brennecke SP, Galindo A, et al. Clinical interpretation and implementation of the sFlt-1/PlGF ratio in the prediction, diagnosis and management of preeclampsia. Pregnancy Hyper. 2022;27:42-50.
- Binder J, Palmrich P, Pateisky P, et al. The prognostic value of angiogenic markers in twin pregnancies to predict delivery due to maternal complications of preeclampsia. Hypertension. 2020;76:176-183.
- Sapantzoglou I, Rouvali A, Koutras A, et al. sFlt-1, PlGF, the sFlt-1/PlGF ratio and their association with pre-eclampsia in twin pregnancies- a review of the literature. Medicina. 2023;59:1232.
- Satorres E, Martinez-Varea A, Diago-Almela V. sFlt-1/PlGF ratio as a predictor of pregnancy outcomes in twin pregnancies: a systematic review. J Matern Fetal Neonatal Med. 2023;36:2230514.
- Rana S, Hacker MR, Modest AM, et al. Circulating angiogenic factors and risk of adverse maternal and perinatal outcomes in twin pregnancies with suspected preeclampsia. Hypertension. 2012;60:451-458.

In the United States there is an epidemic of hypertensive disorders in pregnancy, with 16% of pregnant people being diagnosed with preeclampsia, gestational hypertension, chronic hypertension, preeclampsia superimposed on chronic hypertension, HELLP, or eclampsia.1 Preeclampsia with severe features increases the maternal risk for stroke, pulmonary edema, kidney injury, abruption, and fetal and maternal death. Preeclampsia also increases the fetal risk for growth restriction, oligohydramnios, and preterm birth.
Angiogenic factors and the pathophysiology of preeclampsia—From bench to bedside
The pathophysiology of preeclampsia is not fully characterized, but a leading theory is that placental ischemia causes increased placental production of anti-angiogenesis factors and a decrease in placental production of pro-angiogenesis factors.2-4 Clinical studies support the theory that preeclampsia is associated with an increase in placental production of anti-angiogenesis factors, including soluble fms-like tyrosine kinase 1 (sFlt-1) and soluble endoglin, and a decrease in the placental production of pro-angiogenesis factors, including placental growth factor (PlGF).5-15
The US Food and Drug Administration (FDA) has recently approved an assay for the measurement of sFlt-1 (Brahms sFlt-1 Kryptor) and PlGF (Brahms sFlt-1 Kryptor) (Thermo Fisher Scientific; Waltham, Massachusetts).16 This editorial focuses on the current and evolving indications for the measurement of sFlt-1 and PlGF in obstetric practice.
FDA approval of a preeclampsia blood test
The FDA approval of the tests to measure sFlt-1 and PlGF is narrowly tailored and focused on using the sFlt-1/PlGF ratio to assess the risk of progression to preeclampsia with severe features within 2 weeks among hospitalized patients with a hypertensive disorder in pregnancy with a singleton pregnancy between 23 weeks 0 days (23w0d) and 34w6d gestation.16 The test is meant to be used in conjunction with other laboratory tests and clinical assessment. The FDA advises that the test results should not be used to diagnose preeclampsia, nor should they be used to determine the timing of delivery or timing of patient discharge.16 The sFlt-1 and PIGF measurements are both reported as pg/mL, and the sFlt-1/PlGF ratio has no units.
The FDA approval is based on clinical studies that demonstrate the effectiveness of the test in predicting the progression of a hypertensive disorder in pregnancy to preeclampsia with severe features within 2 weeks of testing. In one study, the sFlt-1/PlGF ratio was measured in 556 pregnant patients with a singleton pregnancy who were between 23w0d and 34w6d gestation and hospitalized with a hypertensivedisorder in pregnancy without severe features at study enrollment.15 Those patients receiving intravenous heparin were excluded because of the effect of heparin on sFlt-1 levels. Participants’ mean age was 31.7 years, and their mean gestational age was 30w3d. The patients’ mean body mass index (BMI) was 34.2 kg/m2, with mean maximal blood pressure (BP) at enrollment of 159 mm Hg systolic and 95 mm Hg diastolic.
In this cohort, 31% of enrolled patients progressed to preeclampsia with severe features within 2 weeks. At enrollment, the median sFlt-1/PlGF ratio was greater among the patients who progressed to preeclampsia with severe features than among those who did not have progression to preeclampsia with severe features (291 vs 7). An elevated sFlt-1/PlGF ratio (determined to be a ratio ≥ 40) predicted that patients would progress to severe preeclampsiawith severe features—with positive and negative predictive values of 65% and 96%, respectively. Among the subgroup of patients with a history of chronic hypertension, an sFlt-1/PlGF ratio ≥ 40 had positive and negative predictive values of 59% and 94%, respectively. Focusing the analysis on patients who self-reported their race as Black, representing 30% of the cohort, the positive and negative predictive values for a sFlt-1/PlGF ratio ≥ 40 were 66% and 99%, respectively.15
Receiver-operating curve analyses were used to compare the predictive performance of sFlt-1/PlGF measurement versus standard clinical factors and standard laboratory results, including systolic and diastolic BP; levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), and creatinine; and platelet count.15 The area under the curve for predicting progression to preeclampsia with severe features was much greater for the sFlt-1/PlGF test (0.92) than for systolic (0.67) and diastolic BP (0.70), AST level (0.66), ALT level (0.61), creatinine level (0.65), and platelet count (0.57).15 These results demonstrate that measuring sFlt-1/PlGF ratios is a much better way to predict the progression of preeclampsia to severe disease than measuring standard clinical and laboratory results.
Patients with a sFlt-1/PlGF ratio ≥ 40 had higher rates of adverse maternal outcomes including severe hypertension, abruption, stroke, eclampsia, pulmonary edema, thrombocytopenia, low platelets, and/or coagulation disorder, than those patients with a ratio < 40, (16.1% vs 2.8%, respectively; relative risk [RR], 5.8; 95% confidence interval [CI], 2.8 to 12.2).15 Adverse fetal and neonatal outcomes (including fetal death, small for gestational age and early delivery due to progression of disease) were more common among patients with a sFlt-1/PlGF ratio of ≥ 40 (80% vs 26%; RR, 3.1; 95% CI, 2.5–3.8).15 Many other studies support the hypothesis that the sFlt-1/PlGF ratio is predictive of adverse outcomes among patients with hypertensive disorders in pregnancy.6-15
Applying the bottom-line study findings. Patients with a hypertensive disorder in pregnancy and a sFlt-1/PlGF ratio < 40 have a low risk of progressing to preeclampsia with severe features over the following 2 weeks, with a negative predictive value of 96%. The remarkably high negative predictive value of a sFlt-1/PlGF ratio < 40 will help obstetricians generate a care plan that optimizes the use of limited health care resources. Conversely, about two-thirds of patients with a hypertensive disorder in pregnancy and a sFlt-1/PlGF test ≥ 40 will progress to preeclampsia with severe features and may need to prepare for a preterm delivery.
Continue to: Clinical utility of the sFlt-1/PlGF ratio in obstetric triage...
Clinical utility of the sFlt-1/PlGF ratio in obstetric triage
Measurement of the sFlt-1/PlGF ratio may help guide clinical care among patients referred to obstetric triage or admitted to the hospital for the evaluation of suspected preeclampsia. In one study, 402 patients with a singleton pregnancy referred to the hospital for evaluation of suspected preeclampsia, had a standard evaluation plus measurement of an sFlt-1/PlGF ratio.13 The clinicians caring for the patients did not have access to the sFlt-1/PlGF test results. In this cohort, 16% of the patients developed preeclampsia with severe features in the 2 weeks following the initial assessment in triage. In this cohort, a normal sFlt-1/PlGF ratio reliably predicted which patients were not going to develop preeclampsia with severe features over the following 2 weeks, with a negative predictive value of 98%. Among the patients with an elevated sFlt-1/PlGF ratio, however, the positive predictive value of the test was 47% for developing preeclampsia with severe features within the 2 weeks following initial evaluation. Among patients < 34 weeks’ gestation, an elevated sFlt-1/PlGF ratio had a positive predictive value of 65%, and a negative predictive value of 98%. Other studies also have reported that the sFlt-1/PlGF ratio is of value for assessing the risk for progression to preeclampsia with severe features in patients being evaluated for suspected preeclampsia.6,17,18
In obstetric triage, it is difficult to predict the clinical course of patients referred for the evaluation of suspected preeclampsia based on BP measurements or standard laboratory tests. The sFlt-1/PlGF test will help clinicians identify patients at low and high risk of progressing to preeclampsia with severe features.19 Patients with a normal sFlt-1/PlGF test are at low risk of developing preeclampsia with severe features over the following 2 weeks. Patients with an elevated sFlt-1/PlGF test are at higher risk of progressing to preeclampsia with severe features and may warrant more intensive obstetric care. An enhanced care program might include:
- patient education
- remote monitoring of BP or hospitalization
- more frequent assessment of fetal well-being and growth
- administration of glucocorticoids to advance fetal maturity, if indicated by the gestational age.
Twin pregnancy complicated by preeclampsia
Twin pregnancy is associated with a high risk of developing preeclampsia and fetal growth restriction. For patients with a twin pregnancy and a hypertensive disorder in pregnancy, an elevated sFlt-1/PlGF ratio is associated with the need for delivery within 2 weeks and an increased rate of adverse maternal and neonatal outcomes. In a retrospective study involving 164 patients with twin pregnancy first evaluated for suspected preeclampsia at a median gestational age of 33w4d, the sFlt-1/PlGF ratio was positively correlated with progression of preeclampsia without severe features to severe features within 2 weeks.20 In this cohort, at the initial evaluation for suspected preeclampsia, the sFlt-1/PlGF ratio was lower among patients who did not need delivery within 2 weeks compared with those who were delivered within 2 weeks, 24 versus 84 (P<.001). The mean sFlt-1/PlGF ratio was 99 among patients who needed delivery within 1 week following the initial evaluation for suspected preeclampsia. Among patients who delivered within 1 week of presentation, the reasons for delivery were the development of severe hypertension, severe dyspnea, placental abruption, rising levels of serum liver function enzymes, and/or onset of the HELLP syndrome.
An important finding in this study is that a normal
The sFlt-1/PlGF test is a welcome addition to OB care
FDA approval of laboratory tests to measure circulating levels of sFlt-1 and PlGF will advance obstetric practice by identifying patients with a hypertensive disorder in pregnancy who are at low and high risk of developing preeclampsia with severe features within 2 weeks of the test. No laboratory test can replace the clinical judgment of obstetricians who are responsible for balancing the maternal and fetal risks that can occur in the management of a patient with a hypertensive disorder in pregnancy. The

In the United States there is an epidemic of hypertensive disorders in pregnancy, with 16% of pregnant people being diagnosed with preeclampsia, gestational hypertension, chronic hypertension, preeclampsia superimposed on chronic hypertension, HELLP, or eclampsia.1 Preeclampsia with severe features increases the maternal risk for stroke, pulmonary edema, kidney injury, abruption, and fetal and maternal death. Preeclampsia also increases the fetal risk for growth restriction, oligohydramnios, and preterm birth.
Angiogenic factors and the pathophysiology of preeclampsia—From bench to bedside
The pathophysiology of preeclampsia is not fully characterized, but a leading theory is that placental ischemia causes increased placental production of anti-angiogenesis factors and a decrease in placental production of pro-angiogenesis factors.2-4 Clinical studies support the theory that preeclampsia is associated with an increase in placental production of anti-angiogenesis factors, including soluble fms-like tyrosine kinase 1 (sFlt-1) and soluble endoglin, and a decrease in the placental production of pro-angiogenesis factors, including placental growth factor (PlGF).5-15
The US Food and Drug Administration (FDA) has recently approved an assay for the measurement of sFlt-1 (Brahms sFlt-1 Kryptor) and PlGF (Brahms sFlt-1 Kryptor) (Thermo Fisher Scientific; Waltham, Massachusetts).16 This editorial focuses on the current and evolving indications for the measurement of sFlt-1 and PlGF in obstetric practice.
FDA approval of a preeclampsia blood test
The FDA approval of the tests to measure sFlt-1 and PlGF is narrowly tailored and focused on using the sFlt-1/PlGF ratio to assess the risk of progression to preeclampsia with severe features within 2 weeks among hospitalized patients with a hypertensive disorder in pregnancy with a singleton pregnancy between 23 weeks 0 days (23w0d) and 34w6d gestation.16 The test is meant to be used in conjunction with other laboratory tests and clinical assessment. The FDA advises that the test results should not be used to diagnose preeclampsia, nor should they be used to determine the timing of delivery or timing of patient discharge.16 The sFlt-1 and PIGF measurements are both reported as pg/mL, and the sFlt-1/PlGF ratio has no units.
The FDA approval is based on clinical studies that demonstrate the effectiveness of the test in predicting the progression of a hypertensive disorder in pregnancy to preeclampsia with severe features within 2 weeks of testing. In one study, the sFlt-1/PlGF ratio was measured in 556 pregnant patients with a singleton pregnancy who were between 23w0d and 34w6d gestation and hospitalized with a hypertensivedisorder in pregnancy without severe features at study enrollment.15 Those patients receiving intravenous heparin were excluded because of the effect of heparin on sFlt-1 levels. Participants’ mean age was 31.7 years, and their mean gestational age was 30w3d. The patients’ mean body mass index (BMI) was 34.2 kg/m2, with mean maximal blood pressure (BP) at enrollment of 159 mm Hg systolic and 95 mm Hg diastolic.
In this cohort, 31% of enrolled patients progressed to preeclampsia with severe features within 2 weeks. At enrollment, the median sFlt-1/PlGF ratio was greater among the patients who progressed to preeclampsia with severe features than among those who did not have progression to preeclampsia with severe features (291 vs 7). An elevated sFlt-1/PlGF ratio (determined to be a ratio ≥ 40) predicted that patients would progress to severe preeclampsiawith severe features—with positive and negative predictive values of 65% and 96%, respectively. Among the subgroup of patients with a history of chronic hypertension, an sFlt-1/PlGF ratio ≥ 40 had positive and negative predictive values of 59% and 94%, respectively. Focusing the analysis on patients who self-reported their race as Black, representing 30% of the cohort, the positive and negative predictive values for a sFlt-1/PlGF ratio ≥ 40 were 66% and 99%, respectively.15
Receiver-operating curve analyses were used to compare the predictive performance of sFlt-1/PlGF measurement versus standard clinical factors and standard laboratory results, including systolic and diastolic BP; levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), and creatinine; and platelet count.15 The area under the curve for predicting progression to preeclampsia with severe features was much greater for the sFlt-1/PlGF test (0.92) than for systolic (0.67) and diastolic BP (0.70), AST level (0.66), ALT level (0.61), creatinine level (0.65), and platelet count (0.57).15 These results demonstrate that measuring sFlt-1/PlGF ratios is a much better way to predict the progression of preeclampsia to severe disease than measuring standard clinical and laboratory results.
Patients with a sFlt-1/PlGF ratio ≥ 40 had higher rates of adverse maternal outcomes including severe hypertension, abruption, stroke, eclampsia, pulmonary edema, thrombocytopenia, low platelets, and/or coagulation disorder, than those patients with a ratio < 40, (16.1% vs 2.8%, respectively; relative risk [RR], 5.8; 95% confidence interval [CI], 2.8 to 12.2).15 Adverse fetal and neonatal outcomes (including fetal death, small for gestational age and early delivery due to progression of disease) were more common among patients with a sFlt-1/PlGF ratio of ≥ 40 (80% vs 26%; RR, 3.1; 95% CI, 2.5–3.8).15 Many other studies support the hypothesis that the sFlt-1/PlGF ratio is predictive of adverse outcomes among patients with hypertensive disorders in pregnancy.6-15
Applying the bottom-line study findings. Patients with a hypertensive disorder in pregnancy and a sFlt-1/PlGF ratio < 40 have a low risk of progressing to preeclampsia with severe features over the following 2 weeks, with a negative predictive value of 96%. The remarkably high negative predictive value of a sFlt-1/PlGF ratio < 40 will help obstetricians generate a care plan that optimizes the use of limited health care resources. Conversely, about two-thirds of patients with a hypertensive disorder in pregnancy and a sFlt-1/PlGF test ≥ 40 will progress to preeclampsia with severe features and may need to prepare for a preterm delivery.
Continue to: Clinical utility of the sFlt-1/PlGF ratio in obstetric triage...
Clinical utility of the sFlt-1/PlGF ratio in obstetric triage
Measurement of the sFlt-1/PlGF ratio may help guide clinical care among patients referred to obstetric triage or admitted to the hospital for the evaluation of suspected preeclampsia. In one study, 402 patients with a singleton pregnancy referred to the hospital for evaluation of suspected preeclampsia, had a standard evaluation plus measurement of an sFlt-1/PlGF ratio.13 The clinicians caring for the patients did not have access to the sFlt-1/PlGF test results. In this cohort, 16% of the patients developed preeclampsia with severe features in the 2 weeks following the initial assessment in triage. In this cohort, a normal sFlt-1/PlGF ratio reliably predicted which patients were not going to develop preeclampsia with severe features over the following 2 weeks, with a negative predictive value of 98%. Among the patients with an elevated sFlt-1/PlGF ratio, however, the positive predictive value of the test was 47% for developing preeclampsia with severe features within the 2 weeks following initial evaluation. Among patients < 34 weeks’ gestation, an elevated sFlt-1/PlGF ratio had a positive predictive value of 65%, and a negative predictive value of 98%. Other studies also have reported that the sFlt-1/PlGF ratio is of value for assessing the risk for progression to preeclampsia with severe features in patients being evaluated for suspected preeclampsia.6,17,18
In obstetric triage, it is difficult to predict the clinical course of patients referred for the evaluation of suspected preeclampsia based on BP measurements or standard laboratory tests. The sFlt-1/PlGF test will help clinicians identify patients at low and high risk of progressing to preeclampsia with severe features.19 Patients with a normal sFlt-1/PlGF test are at low risk of developing preeclampsia with severe features over the following 2 weeks. Patients with an elevated sFlt-1/PlGF test are at higher risk of progressing to preeclampsia with severe features and may warrant more intensive obstetric care. An enhanced care program might include:
- patient education
- remote monitoring of BP or hospitalization
- more frequent assessment of fetal well-being and growth
- administration of glucocorticoids to advance fetal maturity, if indicated by the gestational age.
Twin pregnancy complicated by preeclampsia
Twin pregnancy is associated with a high risk of developing preeclampsia and fetal growth restriction. For patients with a twin pregnancy and a hypertensive disorder in pregnancy, an elevated sFlt-1/PlGF ratio is associated with the need for delivery within 2 weeks and an increased rate of adverse maternal and neonatal outcomes. In a retrospective study involving 164 patients with twin pregnancy first evaluated for suspected preeclampsia at a median gestational age of 33w4d, the sFlt-1/PlGF ratio was positively correlated with progression of preeclampsia without severe features to severe features within 2 weeks.20 In this cohort, at the initial evaluation for suspected preeclampsia, the sFlt-1/PlGF ratio was lower among patients who did not need delivery within 2 weeks compared with those who were delivered within 2 weeks, 24 versus 84 (P<.001). The mean sFlt-1/PlGF ratio was 99 among patients who needed delivery within 1 week following the initial evaluation for suspected preeclampsia. Among patients who delivered within 1 week of presentation, the reasons for delivery were the development of severe hypertension, severe dyspnea, placental abruption, rising levels of serum liver function enzymes, and/or onset of the HELLP syndrome.
An important finding in this study is that a normal
The sFlt-1/PlGF test is a welcome addition to OB care
FDA approval of laboratory tests to measure circulating levels of sFlt-1 and PlGF will advance obstetric practice by identifying patients with a hypertensive disorder in pregnancy who are at low and high risk of developing preeclampsia with severe features within 2 weeks of the test. No laboratory test can replace the clinical judgment of obstetricians who are responsible for balancing the maternal and fetal risks that can occur in the management of a patient with a hypertensive disorder in pregnancy. The
- Ford ND, Cox S, Ko JY, et al. Hypertensive disorders in pregnancy and mortality at delivery hospitalization-United States 2017-2019. Morb Mortal Week Report. 2022;71:585-591.
- Nagamatsu T, Fujii T, Kusumi M, et al. Cytotrophoblasts up-regulate soluble fms-like tyrosine kinase-1 expression under reduced oxygen: an implication for placental vascular development and the pathophysiology of preeclampsia. Endocrinology. 2004;145:4838-4445.
- Rana S, Lemoine E, Granger JP, et al. Preeclampsia: pathophysiology, challenges and perspectives. Circ Res. 2019;124:1094-1112.
- Rana S, Burke SD, Karumanchi SA. Imbalances in circulating angiogenic factors in the pathophysiology of preeclampsia and related disorders. Am J Obstet Gynecol. 2022(2S):S1019-S1034.
- Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672-683.
- Chaiworapongsa T, Romero R, Savasan ZA, et al. Maternal plasma concentrations of angiogenic/ anti-angiogenic factors are of prognostic value in patients presenting to the obstetrical triage area with the suspicion of preeclampsia. J Matern Fetal Neonatal Med. 2011;24:1187-1207.
- Rana S, Powe CE, Salahuddin S, et al. Angiogenic factors and the risk of adverse outcomes in women with suspected preeclampsia. Circulation. 2012;125:911-919.
- Moore AG, Young H, Keller JM, et al. Angiogenic biomarkers for prediction of maternal and neonatal complications in suspected preeclampsia. J Matern Fetal Neonatal Med. 2012;25:2651-2657.
- Verlohren S, Herraiz I, Lapaire O, et al. The sFlt-1/ PlGF ratio in different types of hypertensive pregnancy disorders and its prognostic potential in preeclamptic patients. Am J Obstet Gynecol. 2012;206:58.e1-e8.
- Verlohren S, Herraiz I, Lapaire O, et al. New gestational phase-specific cutoff values for the use of soluble fms-like tyrosine kinase-1/placental growth factor ratio as a diagnostic test for preeclampsia. Hypertension. 2014;63:346-352.
- Zeisler H, Llurba E, Chantraine F, et al. Predictive value of the sFlt-1/PlGF ratio in women with suspected preeclampsia. N Engl J Med. 2016;374:1322.
- Duckworth S, Griffin M, Seed PT, et al. Diagnostic biomarkers in women with suspected preeclampsia in a prospective multicenter study. Obstet Gynecol. 2016;128:245-252.
- Rana S, Salahuddin S, Mueller A, et al. Angiogenic biomarkers in triage and risk for preeclampsia with severe features. Pregnancy Hyertens. 2018;13:100-106.
- Bian X, Biswas A, Huang X, et al. Short-term prediction of adverse outcomes using the sFlt-1/PlGF ratio in Asian women with suspected preeclampsia. Hypertension. 2019;74:164-172.
- Thadhani R, Lemoine E, Rana S, et al. Circulating angiogenic factor levels in hypertensive disorders of pregnancy. N Engl J Med Evidence. 2022. doi 10.1056/EVIDoa2200161.
- US Food and Drug Administration. FDA approval letter for an assay to measure sFlt-1 and PlGF. May 18, 2023. https://www.accessdata.fda.gov/cdrh _docs/pdf22/DEN220027.pdf
- Chaiworapongsa T, Romero R, Korzeniewski SJ, et al. Plasma concentrations of angiogenic/ anti-angiogenic factors have prognostic value in women presenting with suspected preeclampsia to the obstetrical triage area: a prospective study. J Matern Fetal Neonatal Med. 2014;27:132-144.
- Palomaki GE, Haddow JE, Haddow HR, et al. Modeling risk for severe adverse outcomes using angiogenic factor measurements in women with suspected preterm preeclampsia. Prenat Diagn. 2015;35:386-393.
- Verlohren S, Brennecke SP, Galindo A, et al. Clinical interpretation and implementation of the sFlt-1/PlGF ratio in the prediction, diagnosis and management of preeclampsia. Pregnancy Hyper. 2022;27:42-50.
- Binder J, Palmrich P, Pateisky P, et al. The prognostic value of angiogenic markers in twin pregnancies to predict delivery due to maternal complications of preeclampsia. Hypertension. 2020;76:176-183.
- Sapantzoglou I, Rouvali A, Koutras A, et al. sFlt-1, PlGF, the sFlt-1/PlGF ratio and their association with pre-eclampsia in twin pregnancies- a review of the literature. Medicina. 2023;59:1232.
- Satorres E, Martinez-Varea A, Diago-Almela V. sFlt-1/PlGF ratio as a predictor of pregnancy outcomes in twin pregnancies: a systematic review. J Matern Fetal Neonatal Med. 2023;36:2230514.
- Rana S, Hacker MR, Modest AM, et al. Circulating angiogenic factors and risk of adverse maternal and perinatal outcomes in twin pregnancies with suspected preeclampsia. Hypertension. 2012;60:451-458.
- Ford ND, Cox S, Ko JY, et al. Hypertensive disorders in pregnancy and mortality at delivery hospitalization-United States 2017-2019. Morb Mortal Week Report. 2022;71:585-591.
- Nagamatsu T, Fujii T, Kusumi M, et al. Cytotrophoblasts up-regulate soluble fms-like tyrosine kinase-1 expression under reduced oxygen: an implication for placental vascular development and the pathophysiology of preeclampsia. Endocrinology. 2004;145:4838-4445.
- Rana S, Lemoine E, Granger JP, et al. Preeclampsia: pathophysiology, challenges and perspectives. Circ Res. 2019;124:1094-1112.
- Rana S, Burke SD, Karumanchi SA. Imbalances in circulating angiogenic factors in the pathophysiology of preeclampsia and related disorders. Am J Obstet Gynecol. 2022(2S):S1019-S1034.
- Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672-683.
- Chaiworapongsa T, Romero R, Savasan ZA, et al. Maternal plasma concentrations of angiogenic/ anti-angiogenic factors are of prognostic value in patients presenting to the obstetrical triage area with the suspicion of preeclampsia. J Matern Fetal Neonatal Med. 2011;24:1187-1207.
- Rana S, Powe CE, Salahuddin S, et al. Angiogenic factors and the risk of adverse outcomes in women with suspected preeclampsia. Circulation. 2012;125:911-919.
- Moore AG, Young H, Keller JM, et al. Angiogenic biomarkers for prediction of maternal and neonatal complications in suspected preeclampsia. J Matern Fetal Neonatal Med. 2012;25:2651-2657.
- Verlohren S, Herraiz I, Lapaire O, et al. The sFlt-1/ PlGF ratio in different types of hypertensive pregnancy disorders and its prognostic potential in preeclamptic patients. Am J Obstet Gynecol. 2012;206:58.e1-e8.
- Verlohren S, Herraiz I, Lapaire O, et al. New gestational phase-specific cutoff values for the use of soluble fms-like tyrosine kinase-1/placental growth factor ratio as a diagnostic test for preeclampsia. Hypertension. 2014;63:346-352.
- Zeisler H, Llurba E, Chantraine F, et al. Predictive value of the sFlt-1/PlGF ratio in women with suspected preeclampsia. N Engl J Med. 2016;374:1322.
- Duckworth S, Griffin M, Seed PT, et al. Diagnostic biomarkers in women with suspected preeclampsia in a prospective multicenter study. Obstet Gynecol. 2016;128:245-252.
- Rana S, Salahuddin S, Mueller A, et al. Angiogenic biomarkers in triage and risk for preeclampsia with severe features. Pregnancy Hyertens. 2018;13:100-106.
- Bian X, Biswas A, Huang X, et al. Short-term prediction of adverse outcomes using the sFlt-1/PlGF ratio in Asian women with suspected preeclampsia. Hypertension. 2019;74:164-172.
- Thadhani R, Lemoine E, Rana S, et al. Circulating angiogenic factor levels in hypertensive disorders of pregnancy. N Engl J Med Evidence. 2022. doi 10.1056/EVIDoa2200161.
- US Food and Drug Administration. FDA approval letter for an assay to measure sFlt-1 and PlGF. May 18, 2023. https://www.accessdata.fda.gov/cdrh _docs/pdf22/DEN220027.pdf
- Chaiworapongsa T, Romero R, Korzeniewski SJ, et al. Plasma concentrations of angiogenic/ anti-angiogenic factors have prognostic value in women presenting with suspected preeclampsia to the obstetrical triage area: a prospective study. J Matern Fetal Neonatal Med. 2014;27:132-144.
- Palomaki GE, Haddow JE, Haddow HR, et al. Modeling risk for severe adverse outcomes using angiogenic factor measurements in women with suspected preterm preeclampsia. Prenat Diagn. 2015;35:386-393.
- Verlohren S, Brennecke SP, Galindo A, et al. Clinical interpretation and implementation of the sFlt-1/PlGF ratio in the prediction, diagnosis and management of preeclampsia. Pregnancy Hyper. 2022;27:42-50.
- Binder J, Palmrich P, Pateisky P, et al. The prognostic value of angiogenic markers in twin pregnancies to predict delivery due to maternal complications of preeclampsia. Hypertension. 2020;76:176-183.
- Sapantzoglou I, Rouvali A, Koutras A, et al. sFlt-1, PlGF, the sFlt-1/PlGF ratio and their association with pre-eclampsia in twin pregnancies- a review of the literature. Medicina. 2023;59:1232.
- Satorres E, Martinez-Varea A, Diago-Almela V. sFlt-1/PlGF ratio as a predictor of pregnancy outcomes in twin pregnancies: a systematic review. J Matern Fetal Neonatal Med. 2023;36:2230514.
- Rana S, Hacker MR, Modest AM, et al. Circulating angiogenic factors and risk of adverse maternal and perinatal outcomes in twin pregnancies with suspected preeclampsia. Hypertension. 2012;60:451-458.
Case Q: How can I best remove my patient’s difficult-to-find implant?
Individuals spend close to half of their lives preventing, or planning for, pregnancy. As such, contraception plays a major role in patient-provider interactions. Contraception counseling and management is a common scenario encountered in the general gynecologist’s practice. Luckily, we have 2 evidence-based guidelines developed by the US Centers for Disease Control and Prevention (CDC) that support the provision of contraceptive care:
- US Medical Eligibility for Contraceptive Use (US-MEC),1 which provides guidance on which patients can safely use a method
- US Selected Practice Recommendations for Contraceptive Use (US-SPR),2 which provides method-specific guidance on how to use a method (including how to: initiate or start a method; manage adherence issues, such as a missed pill, etc; and manage common issues like breakthrough bleeding).
Both of these guidelines are updated routinely and are publicly available online or for free, through smartphone applications.
While most contraceptive care is straightforward, there are circumstances that require additional consideration. In the concluding part of this series on contraceptive conundrums, we review 2 clinical cases, existing evidence to guide management decisions, and our recommendations.
CASE 1 Patient presents with hard-to-remove implant
A 44-year-old patient (G2P2) with a new diagnosis of estrogen and progesterone-receptor–positive breast cancer is undergoing her evaluation with her oncologist who recommends removal of her contraceptive implant, which has been in place for 2 years. She presents to your office for removal; however, the device is no longer palpable.
What are your next steps?
Conundrum 1. Should you attempt to remove it?
No, never attempt implant removal if you cannot palpate or localize it. Localization of the implant needs to occur prior to any attempt. However, we recommend checking the contra-lateral arm before sending the patient to obtain imaging, especially if you have no formal documentation regarding in which arm the implant was placed. The next step is identifying what type of implant the patient likely has so you can correctly interpret imaging studies.
Conundrum 2. What type of subdermal contraceptive device is it likely to be?
Currently, the only subdermal contraceptive device available for placement in the United States is the 68-mg etonogestrel implant, marketed with the brand name Nexplanon. This device was initially approved by the US Food and Drug Administration in 2001 and measures 4 cm in length by 2 mm in diameter. It is placed in the medial upper arm, about 8 cm proximal to the medial epicondyle and 3 cm posterior to the sulcus between the biceps and triceps muscles. (The implant should no longer be placed over the bicipital groove.) The implant is impregnated with 15 mg of barium sulfate, making it radiopaque and able to be seen on imaging modalities such as ultrasonography (10–18 mHz high frequency transducer) and x-ray (arm anteroposterior and lateral) for localization in cases in which the device becomes nonpalpable.3
Clinicians also may encounter devices which are no longer marketed in the United States, or which are only available in other countries, and thus should be aware of the appearance and imaging characteristics. It is important to let your imaging team know these characteristics as well:
- From 2006–2010, a 68-mg etonogestrel implant marketed under the name Implanon was available in the United States.4 It has the same dimensions and general placement recommendations as the Nexplanon etonogestrel device but is not able to be seen via imaging.
- A 2-arm, 75-mg levonorgestrel (LNG) device known as Jadelle (or, Norplant II; FIGURE 1) received FDA approval in 1996 and is currently only available overseas.5 It is also placed in the upper, inner arm in a V-shape using a single incision, and has dimensions similar to the etonogestrel implants.
- From 1990– 2002, the 6-rod device known as Norplant was available in the United States. Each rod measured 3.4 cm in length and contained 36 mg of LNG (FIGURE 2).
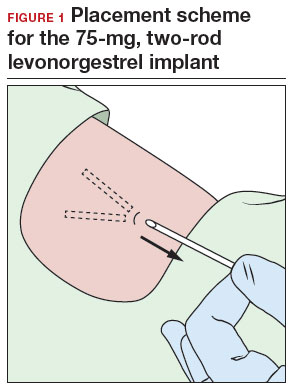
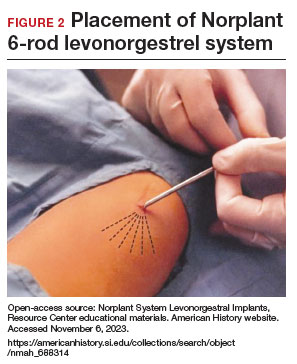
Continue to: How do you approach removal of a deep contraceptive implant?...
How do you approach removal of a deep contraceptive implant?
Clinicians who are not trained in deep or difficult implant removal should refer patients to a trained provider (eg, a complex family planning subspecialist), or if not available, partner with a health care practitioner that has expertise in the anatomy of the upper arm (eg, vascular surgery, orthopedics, or interventional radiology). A resource for finding a nearby trained provider is the Organon Information Center (1-877-467-5266). However, when these services are not readily available, consider the following 3-step approach to complex implant removal.
- Be familiar with the anatomy of the upper arm (FIGURE 3). Nonpalpable implants may be close to or under the biceps or triceps fascia or be near critically important and fragile structures like the neurovascular bundle of the upper arm. Prior to attempting a difficult implant removal, ensure that you are well acquainted with critical structures in the upper arm.
- Locate the device. Prior to attempting removal, localize the device using either x-ray or ultrasonography, depending on local availability. Ultrasound offers the advantage of mapping the location in 3 dimensions, with the ability to map the device with skin markings immediately prior to removal. Typically, a highfrequency transducer (15- or 18-MHz) is used, such as for breast imaging, either in a clinician’s office or in coordination with radiology. If device removal is attempted the same day, the proximal, midportion, and distal aspects of the device should be marked with a skin pen, and it should be noted what position the arm is in when the device is marked (eg, arm flexed at elbow and externally rotated so that the wrist is parallel to the ear).
Rarely, if a device is not seen in the expected extremity, imaging of the contralateral arm or a chest x-ray can be undertaken to rule out mis-documented laterality or a migrated device. Lastly, if no device is seen, and the patient has no memory of device removal, you can obtain the patient’s etonogestrel levels. (Resource: Merck National Service Center, 1-877-888-4231.)
Removal procedure. For nonpalpable implants, strong consideration should be given to performing the procedure with ultrasonography guidance. Rarely, fluoroscopic guidance may be useful for orientation in challenging cases, which may require coordination with other services, such as interventional radiology.
Cleaning and anesthetizing the site is similar to routine removal of a palpable implant. A 2- to 3-mm skin incision is made, either at the distal end of the implant (if one end is amenable to traditional pop-out technique) or over the midportion of the device (if a clinician has experience using the “U” technique).6 The incision should be parallel to the long axis of the implant and not perpendicular, to facilitate extension of the incision if needed during the procedure. Straight or curved hemostat clamps can then be used for blunt dissection of the subcutaneous tissues and to grasp the end of the device. Experienced clinicians may have access to a modified vasectomy clamp (with a
Indications for referral. Typically, referral to a complex family planning specialist or vascular surgeon is required for cases that involve dissection of the muscular fascia or where dissection would be in close proximity to critical neurologic or vascular structures.
CASE 1 Conclusion
Ultrasonography of the patient’s extremity demonstrated a
CASE 2 Patient enquires about immediate IUD insertion
A 28-year-old patient (G1P0) arrives at your clinic for a contraceptive consultation. They report a condom break during intercourse 4 days ago. Prior to that they used condoms consistently with each act of intercourse. They have used combined hormonal contraceptive pills in the past but had difficulty remembering to take them consistently. The patient and their partner have been mutually monogamous for 6 months and have no plans for pregnancy. Last menstrual period was 12 days ago. Their cycles are regular but heavy and painful. They are interested in using a hormonal IUD for contraception and would love to get it today.
- Do not attempt removal of a nonpalpable implant without prior localization via imaging
- Ultrasound-guided removal procedures using a “U” technique are successful for many deep implant removals but require specialized equipment and training
- Referral to a complex family planning specialist or other specialist is highly recommended for implants located below the triceps fascia or close to the nerves and vessels of the upper arm
- Never attempt to remove a nonpalpable implant prior to determining its location via imaging
Continue to: Is same-day IUD an option?...
Is same-day IUD an option?
Yes. This patient needs EC given the recent condom break, but they are still eligible for having an IUD placed today if their pregnancy test is negative and after counseling of the potential risks and benefits. According to the US-SPR it is reasonable to insert an IUD at any time during the cycle as long as you are reasonably certain the patient is not pregnant.7
Options for EC are:
- 1.5-mg oral LNG pill
- 30-mg oral UPA pill
- copper IUD (cu-IUD).
If they are interested in the cu-IUD for long-term contraception, by having a cu-IUD placed they can get both their needs met—EC and an ongoing method of contraception. Any patient receiving EC, whether a pill or an IUD, should be counseled to repeat a home urine pregnancy test in 2 to 4 weeks.
Given the favorable non–contraceptive benefits associated with 52-mg LNG-IUDs, many clinicians and patients have advocated for additional evidence regarding the use of hormonal IUDs alone for EC.
What is the evidence concerning LNG-IUD placement as EC?
The 52-mg LNG-IUD has not been mechanistically proven to work as an EC, but growing evidence exists showing that it is safe for same-day or “quick start” placement even in a population seeking EC—if their pregnancy test result is negative at the time of presentation.
Turok and colleagues performed a noninferiority trial comparing 1-month pregnancy rates after placement of either an LNG-IUD or a cu-IUD for EC.8 This study concluded that the LNG-IUD (which resulted in 1 pregnancy in 317 users; pregnancy rate, 0.3%; 95% confidence interval [CI], 0.01–1.70) is noninferior to cu-IUD (0 pregnancies in 321 users; pregnancy rate, 0%; 95% CI, 0.0–1.1) for EC. Although encouraging, only a small percentage of the study population seeking EC who received an IUD were actually at high risk of pregnancy (eg, they were not mid-cycle or were recently using contraception), which is why it is difficult to determine if the LNG-IUD actually works mechanistically as an EC. More likely, the LNG-IUD helps prevent pregnancy due to its ongoing contraceptive effect.9 Ongoing acts of intercourse post–oral EC initiation without starting a method of contraception is one of the main reasons for EC failure, which is why starting a method immediately is so effective at preventing pregnancy.10
A systematic review conducted by Ramanadhan and colleagues concluded that Turok’s 2021 trial is the only relevant study specific to 52-mg LNG-IUD use as EC, but they also mention that its results are limited in the strength of its conclusions due to biases in randomization, including11:
- the study groups were not balanced in that there was a 10% difference in reported use of contraception at last intercourse, which means that the LNG-IUD group had a lower baseline risk of pregnancy
- and a rare primary outcome (ie, pregnancy, which requires a larger sample size to know if the method works as an EC).
The review authors concluded that more studies are needed to further validate the effectiveness of using the 52-mg LNG-IUD as EC. Thus, for those at highest risk of pregnancy from recent unprotected sex and desiring a 52-mg IUD, it is probably best to continue combining oral EC with a 52-mg LNG-IUD and utilizing the LNG-IUD only as EC on a limited, case-by-case basis.
What we recommend
For anyone with a negative pregnancy test on the day of presentation, the studies mentioned further support the practice of same-day placement of a 52-mg LNG-IUD. However, those seeking EC who are at highest risk for an unplanned pregnancy (ie, the unprotected sex was mid-cycle), we recommend co-administering the LNG-IUD with oral LNG for EC.
CASE 2 Conclusion
After a conversation with the patient about all contraceptive options, through shared decision making the patient decided to take 1.5 mg of oral LNG and have a 52-mg LNG-IUD placed in the office today. They do not wish to be pregnant at this time and would choose termination if they became pregnant. They understood their pregnancy risk and opted to plan a urine pregnancy test at home in 2 weeks with a clear understanding that they should return to clinic immediately if the test is positive. ●
- A copper IUD is the most effective method of emergency contraception (EC).
- 52-mg LNG-IUDs are an emerging consideration for EC, but evidence is still lacking that they work as EC (or whether they just prevent pregnancy after placement for subsequent acts of intercourse). Clinicians should utilize shared decision making and advise patients to repeat a pregnancy test at home in 2 to 4 weeks
- Any patient receiving EC, whether a pill or an IUD, should be counseled to repeat a home urine pregnancy test in 2 to 4 weeks
- Any type of IUD can be placed same day if the clinician is reasonably sure the patient is not pregnant
- It appears safe to co-administer the 52-mg LNG-IUD with oral EC for those seeking emergency contraception but also want to use an LNG-IUD for contraception going forward
- Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. Morb Mortal Wkly Rep. 2016;65:1-66. https://doi .org/10.15585/mmwr .rr6504a1
- Centers for Disease Control and Prevention. National Center for Chronic Disease Prevention and Health Promotion, Division of Reproductive Health. US Selected Practice Recommendations for Contraceptive Use (US-SPR). Accessed October 11, 2023. https://www.cdc.gov/reproductivehealth /contraception/mmwr/spr/summary.html
- Nexplanon [package insert]. Whitehouse Station, NJ: Merck; 2018.
- US Food and Drug Administration. Implanon (etonogestrel implant) 2006. Accessed November 6, 2023. https://www .accessdata.fda.gov/drugsatfda_docs/nda/2006 /021529s000_Lbl.pdf
- US Food and Drug Administration. Jadelle (levonorgestrel implant) 2016. Accessed November 6, 2023. https://www. accessdata.fda.gov/drugsatfda_docs/label/2016/020544s 010lbl.pdf
- Chen MJ, Creinin MD. Removal of a nonpalpable etonogestrel implant with preprocedure ultrasonography and modified vasectomy clamp. Obstet Gynecol. 2015;126:935-938.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. MMWR Recomm Rep Morb Mortal Wkly. 2016;65:1-66. https://doi .org/10.15585/mmwr.rr6504a1
- Turok DK, Gero A, Simmons RG, et al. Levonorgestrel vs. copper intrauterine devices for emergency contraception. N Engl J Med. 2021;384:335-344. https://pubmed.ncbi.nlm .nih.gov/33503342/
- Kaiser JE, Turok DK, Gero A, et al. One-year pregnancy and continuation rates after placement of levonorgestrel or copper intrauterine devices for emergency contraception: a randomized controlled trial. Am J Obstet Gynecol. 2023;228:438.e1-438.e10. https://doi.org/10.1016/j.ajog.2022 .11.1296
- Sander PM, Raymond EG, Weaver MA. Emergency contraceptive use as a marker of future risky sex, pregnancy, and sexually transmitted infection. Am J Obstet Gynecol. 2009;201:146.e1-e6.
- Ramanadhan S, Goldstuck N, Henderson JT, et al. Progestin intrauterine devices versus copper intrauterine devices for emergency contraception. Cochrane Database Syst Rev. 2023;2:CD013744. https://doi.org/10.1002/14651858 .CD013744.pub2
Individuals spend close to half of their lives preventing, or planning for, pregnancy. As such, contraception plays a major role in patient-provider interactions. Contraception counseling and management is a common scenario encountered in the general gynecologist’s practice. Luckily, we have 2 evidence-based guidelines developed by the US Centers for Disease Control and Prevention (CDC) that support the provision of contraceptive care:
- US Medical Eligibility for Contraceptive Use (US-MEC),1 which provides guidance on which patients can safely use a method
- US Selected Practice Recommendations for Contraceptive Use (US-SPR),2 which provides method-specific guidance on how to use a method (including how to: initiate or start a method; manage adherence issues, such as a missed pill, etc; and manage common issues like breakthrough bleeding).
Both of these guidelines are updated routinely and are publicly available online or for free, through smartphone applications.
While most contraceptive care is straightforward, there are circumstances that require additional consideration. In the concluding part of this series on contraceptive conundrums, we review 2 clinical cases, existing evidence to guide management decisions, and our recommendations.
CASE 1 Patient presents with hard-to-remove implant
A 44-year-old patient (G2P2) with a new diagnosis of estrogen and progesterone-receptor–positive breast cancer is undergoing her evaluation with her oncologist who recommends removal of her contraceptive implant, which has been in place for 2 years. She presents to your office for removal; however, the device is no longer palpable.
What are your next steps?
Conundrum 1. Should you attempt to remove it?
No, never attempt implant removal if you cannot palpate or localize it. Localization of the implant needs to occur prior to any attempt. However, we recommend checking the contra-lateral arm before sending the patient to obtain imaging, especially if you have no formal documentation regarding in which arm the implant was placed. The next step is identifying what type of implant the patient likely has so you can correctly interpret imaging studies.
Conundrum 2. What type of subdermal contraceptive device is it likely to be?
Currently, the only subdermal contraceptive device available for placement in the United States is the 68-mg etonogestrel implant, marketed with the brand name Nexplanon. This device was initially approved by the US Food and Drug Administration in 2001 and measures 4 cm in length by 2 mm in diameter. It is placed in the medial upper arm, about 8 cm proximal to the medial epicondyle and 3 cm posterior to the sulcus between the biceps and triceps muscles. (The implant should no longer be placed over the bicipital groove.) The implant is impregnated with 15 mg of barium sulfate, making it radiopaque and able to be seen on imaging modalities such as ultrasonography (10–18 mHz high frequency transducer) and x-ray (arm anteroposterior and lateral) for localization in cases in which the device becomes nonpalpable.3
Clinicians also may encounter devices which are no longer marketed in the United States, or which are only available in other countries, and thus should be aware of the appearance and imaging characteristics. It is important to let your imaging team know these characteristics as well:
- From 2006–2010, a 68-mg etonogestrel implant marketed under the name Implanon was available in the United States.4 It has the same dimensions and general placement recommendations as the Nexplanon etonogestrel device but is not able to be seen via imaging.
- A 2-arm, 75-mg levonorgestrel (LNG) device known as Jadelle (or, Norplant II; FIGURE 1) received FDA approval in 1996 and is currently only available overseas.5 It is also placed in the upper, inner arm in a V-shape using a single incision, and has dimensions similar to the etonogestrel implants.
- From 1990– 2002, the 6-rod device known as Norplant was available in the United States. Each rod measured 3.4 cm in length and contained 36 mg of LNG (FIGURE 2).


Continue to: How do you approach removal of a deep contraceptive implant?...
How do you approach removal of a deep contraceptive implant?
Clinicians who are not trained in deep or difficult implant removal should refer patients to a trained provider (eg, a complex family planning subspecialist), or if not available, partner with a health care practitioner that has expertise in the anatomy of the upper arm (eg, vascular surgery, orthopedics, or interventional radiology). A resource for finding a nearby trained provider is the Organon Information Center (1-877-467-5266). However, when these services are not readily available, consider the following 3-step approach to complex implant removal.
- Be familiar with the anatomy of the upper arm (FIGURE 3). Nonpalpable implants may be close to or under the biceps or triceps fascia or be near critically important and fragile structures like the neurovascular bundle of the upper arm. Prior to attempting a difficult implant removal, ensure that you are well acquainted with critical structures in the upper arm.
- Locate the device. Prior to attempting removal, localize the device using either x-ray or ultrasonography, depending on local availability. Ultrasound offers the advantage of mapping the location in 3 dimensions, with the ability to map the device with skin markings immediately prior to removal. Typically, a highfrequency transducer (15- or 18-MHz) is used, such as for breast imaging, either in a clinician’s office or in coordination with radiology. If device removal is attempted the same day, the proximal, midportion, and distal aspects of the device should be marked with a skin pen, and it should be noted what position the arm is in when the device is marked (eg, arm flexed at elbow and externally rotated so that the wrist is parallel to the ear).

Rarely, if a device is not seen in the expected extremity, imaging of the contralateral arm or a chest x-ray can be undertaken to rule out mis-documented laterality or a migrated device. Lastly, if no device is seen, and the patient has no memory of device removal, you can obtain the patient’s etonogestrel levels. (Resource: Merck National Service Center, 1-877-888-4231.)
Removal procedure. For nonpalpable implants, strong consideration should be given to performing the procedure with ultrasonography guidance. Rarely, fluoroscopic guidance may be useful for orientation in challenging cases, which may require coordination with other services, such as interventional radiology.
Cleaning and anesthetizing the site is similar to routine removal of a palpable implant. A 2- to 3-mm skin incision is made, either at the distal end of the implant (if one end is amenable to traditional pop-out technique) or over the midportion of the device (if a clinician has experience using the “U” technique).6 The incision should be parallel to the long axis of the implant and not perpendicular, to facilitate extension of the incision if needed during the procedure. Straight or curved hemostat clamps can then be used for blunt dissection of the subcutaneous tissues and to grasp the end of the device. Experienced clinicians may have access to a modified vasectomy clamp (with a
Indications for referral. Typically, referral to a complex family planning specialist or vascular surgeon is required for cases that involve dissection of the muscular fascia or where dissection would be in close proximity to critical neurologic or vascular structures.
CASE 1 Conclusion
Ultrasonography of the patient’s extremity demonstrated a
CASE 2 Patient enquires about immediate IUD insertion
A 28-year-old patient (G1P0) arrives at your clinic for a contraceptive consultation. They report a condom break during intercourse 4 days ago. Prior to that they used condoms consistently with each act of intercourse. They have used combined hormonal contraceptive pills in the past but had difficulty remembering to take them consistently. The patient and their partner have been mutually monogamous for 6 months and have no plans for pregnancy. Last menstrual period was 12 days ago. Their cycles are regular but heavy and painful. They are interested in using a hormonal IUD for contraception and would love to get it today.
- Do not attempt removal of a nonpalpable implant without prior localization via imaging
- Ultrasound-guided removal procedures using a “U” technique are successful for many deep implant removals but require specialized equipment and training
- Referral to a complex family planning specialist or other specialist is highly recommended for implants located below the triceps fascia or close to the nerves and vessels of the upper arm
- Never attempt to remove a nonpalpable implant prior to determining its location via imaging
Continue to: Is same-day IUD an option?...
Is same-day IUD an option?
Yes. This patient needs EC given the recent condom break, but they are still eligible for having an IUD placed today if their pregnancy test is negative and after counseling of the potential risks and benefits. According to the US-SPR it is reasonable to insert an IUD at any time during the cycle as long as you are reasonably certain the patient is not pregnant.7
Options for EC are:
- 1.5-mg oral LNG pill
- 30-mg oral UPA pill
- copper IUD (cu-IUD).
If they are interested in the cu-IUD for long-term contraception, by having a cu-IUD placed they can get both their needs met—EC and an ongoing method of contraception. Any patient receiving EC, whether a pill or an IUD, should be counseled to repeat a home urine pregnancy test in 2 to 4 weeks.
Given the favorable non–contraceptive benefits associated with 52-mg LNG-IUDs, many clinicians and patients have advocated for additional evidence regarding the use of hormonal IUDs alone for EC.
What is the evidence concerning LNG-IUD placement as EC?
The 52-mg LNG-IUD has not been mechanistically proven to work as an EC, but growing evidence exists showing that it is safe for same-day or “quick start” placement even in a population seeking EC—if their pregnancy test result is negative at the time of presentation.
Turok and colleagues performed a noninferiority trial comparing 1-month pregnancy rates after placement of either an LNG-IUD or a cu-IUD for EC.8 This study concluded that the LNG-IUD (which resulted in 1 pregnancy in 317 users; pregnancy rate, 0.3%; 95% confidence interval [CI], 0.01–1.70) is noninferior to cu-IUD (0 pregnancies in 321 users; pregnancy rate, 0%; 95% CI, 0.0–1.1) for EC. Although encouraging, only a small percentage of the study population seeking EC who received an IUD were actually at high risk of pregnancy (eg, they were not mid-cycle or were recently using contraception), which is why it is difficult to determine if the LNG-IUD actually works mechanistically as an EC. More likely, the LNG-IUD helps prevent pregnancy due to its ongoing contraceptive effect.9 Ongoing acts of intercourse post–oral EC initiation without starting a method of contraception is one of the main reasons for EC failure, which is why starting a method immediately is so effective at preventing pregnancy.10
A systematic review conducted by Ramanadhan and colleagues concluded that Turok’s 2021 trial is the only relevant study specific to 52-mg LNG-IUD use as EC, but they also mention that its results are limited in the strength of its conclusions due to biases in randomization, including11:
- the study groups were not balanced in that there was a 10% difference in reported use of contraception at last intercourse, which means that the LNG-IUD group had a lower baseline risk of pregnancy
- and a rare primary outcome (ie, pregnancy, which requires a larger sample size to know if the method works as an EC).
The review authors concluded that more studies are needed to further validate the effectiveness of using the 52-mg LNG-IUD as EC. Thus, for those at highest risk of pregnancy from recent unprotected sex and desiring a 52-mg IUD, it is probably best to continue combining oral EC with a 52-mg LNG-IUD and utilizing the LNG-IUD only as EC on a limited, case-by-case basis.
What we recommend
For anyone with a negative pregnancy test on the day of presentation, the studies mentioned further support the practice of same-day placement of a 52-mg LNG-IUD. However, those seeking EC who are at highest risk for an unplanned pregnancy (ie, the unprotected sex was mid-cycle), we recommend co-administering the LNG-IUD with oral LNG for EC.
CASE 2 Conclusion
After a conversation with the patient about all contraceptive options, through shared decision making the patient decided to take 1.5 mg of oral LNG and have a 52-mg LNG-IUD placed in the office today. They do not wish to be pregnant at this time and would choose termination if they became pregnant. They understood their pregnancy risk and opted to plan a urine pregnancy test at home in 2 weeks with a clear understanding that they should return to clinic immediately if the test is positive. ●
- A copper IUD is the most effective method of emergency contraception (EC).
- 52-mg LNG-IUDs are an emerging consideration for EC, but evidence is still lacking that they work as EC (or whether they just prevent pregnancy after placement for subsequent acts of intercourse). Clinicians should utilize shared decision making and advise patients to repeat a pregnancy test at home in 2 to 4 weeks
- Any patient receiving EC, whether a pill or an IUD, should be counseled to repeat a home urine pregnancy test in 2 to 4 weeks
- Any type of IUD can be placed same day if the clinician is reasonably sure the patient is not pregnant
- It appears safe to co-administer the 52-mg LNG-IUD with oral EC for those seeking emergency contraception but also want to use an LNG-IUD for contraception going forward
Individuals spend close to half of their lives preventing, or planning for, pregnancy. As such, contraception plays a major role in patient-provider interactions. Contraception counseling and management is a common scenario encountered in the general gynecologist’s practice. Luckily, we have 2 evidence-based guidelines developed by the US Centers for Disease Control and Prevention (CDC) that support the provision of contraceptive care:
- US Medical Eligibility for Contraceptive Use (US-MEC),1 which provides guidance on which patients can safely use a method
- US Selected Practice Recommendations for Contraceptive Use (US-SPR),2 which provides method-specific guidance on how to use a method (including how to: initiate or start a method; manage adherence issues, such as a missed pill, etc; and manage common issues like breakthrough bleeding).
Both of these guidelines are updated routinely and are publicly available online or for free, through smartphone applications.
While most contraceptive care is straightforward, there are circumstances that require additional consideration. In the concluding part of this series on contraceptive conundrums, we review 2 clinical cases, existing evidence to guide management decisions, and our recommendations.
CASE 1 Patient presents with hard-to-remove implant
A 44-year-old patient (G2P2) with a new diagnosis of estrogen and progesterone-receptor–positive breast cancer is undergoing her evaluation with her oncologist who recommends removal of her contraceptive implant, which has been in place for 2 years. She presents to your office for removal; however, the device is no longer palpable.
What are your next steps?
Conundrum 1. Should you attempt to remove it?
No, never attempt implant removal if you cannot palpate or localize it. Localization of the implant needs to occur prior to any attempt. However, we recommend checking the contra-lateral arm before sending the patient to obtain imaging, especially if you have no formal documentation regarding in which arm the implant was placed. The next step is identifying what type of implant the patient likely has so you can correctly interpret imaging studies.
Conundrum 2. What type of subdermal contraceptive device is it likely to be?
Currently, the only subdermal contraceptive device available for placement in the United States is the 68-mg etonogestrel implant, marketed with the brand name Nexplanon. This device was initially approved by the US Food and Drug Administration in 2001 and measures 4 cm in length by 2 mm in diameter. It is placed in the medial upper arm, about 8 cm proximal to the medial epicondyle and 3 cm posterior to the sulcus between the biceps and triceps muscles. (The implant should no longer be placed over the bicipital groove.) The implant is impregnated with 15 mg of barium sulfate, making it radiopaque and able to be seen on imaging modalities such as ultrasonography (10–18 mHz high frequency transducer) and x-ray (arm anteroposterior and lateral) for localization in cases in which the device becomes nonpalpable.3
Clinicians also may encounter devices which are no longer marketed in the United States, or which are only available in other countries, and thus should be aware of the appearance and imaging characteristics. It is important to let your imaging team know these characteristics as well:
- From 2006–2010, a 68-mg etonogestrel implant marketed under the name Implanon was available in the United States.4 It has the same dimensions and general placement recommendations as the Nexplanon etonogestrel device but is not able to be seen via imaging.
- A 2-arm, 75-mg levonorgestrel (LNG) device known as Jadelle (or, Norplant II; FIGURE 1) received FDA approval in 1996 and is currently only available overseas.5 It is also placed in the upper, inner arm in a V-shape using a single incision, and has dimensions similar to the etonogestrel implants.
- From 1990– 2002, the 6-rod device known as Norplant was available in the United States. Each rod measured 3.4 cm in length and contained 36 mg of LNG (FIGURE 2).


Continue to: How do you approach removal of a deep contraceptive implant?...
How do you approach removal of a deep contraceptive implant?
Clinicians who are not trained in deep or difficult implant removal should refer patients to a trained provider (eg, a complex family planning subspecialist), or if not available, partner with a health care practitioner that has expertise in the anatomy of the upper arm (eg, vascular surgery, orthopedics, or interventional radiology). A resource for finding a nearby trained provider is the Organon Information Center (1-877-467-5266). However, when these services are not readily available, consider the following 3-step approach to complex implant removal.
- Be familiar with the anatomy of the upper arm (FIGURE 3). Nonpalpable implants may be close to or under the biceps or triceps fascia or be near critically important and fragile structures like the neurovascular bundle of the upper arm. Prior to attempting a difficult implant removal, ensure that you are well acquainted with critical structures in the upper arm.
- Locate the device. Prior to attempting removal, localize the device using either x-ray or ultrasonography, depending on local availability. Ultrasound offers the advantage of mapping the location in 3 dimensions, with the ability to map the device with skin markings immediately prior to removal. Typically, a highfrequency transducer (15- or 18-MHz) is used, such as for breast imaging, either in a clinician’s office or in coordination with radiology. If device removal is attempted the same day, the proximal, midportion, and distal aspects of the device should be marked with a skin pen, and it should be noted what position the arm is in when the device is marked (eg, arm flexed at elbow and externally rotated so that the wrist is parallel to the ear).

Rarely, if a device is not seen in the expected extremity, imaging of the contralateral arm or a chest x-ray can be undertaken to rule out mis-documented laterality or a migrated device. Lastly, if no device is seen, and the patient has no memory of device removal, you can obtain the patient’s etonogestrel levels. (Resource: Merck National Service Center, 1-877-888-4231.)
Removal procedure. For nonpalpable implants, strong consideration should be given to performing the procedure with ultrasonography guidance. Rarely, fluoroscopic guidance may be useful for orientation in challenging cases, which may require coordination with other services, such as interventional radiology.
Cleaning and anesthetizing the site is similar to routine removal of a palpable implant. A 2- to 3-mm skin incision is made, either at the distal end of the implant (if one end is amenable to traditional pop-out technique) or over the midportion of the device (if a clinician has experience using the “U” technique).6 The incision should be parallel to the long axis of the implant and not perpendicular, to facilitate extension of the incision if needed during the procedure. Straight or curved hemostat clamps can then be used for blunt dissection of the subcutaneous tissues and to grasp the end of the device. Experienced clinicians may have access to a modified vasectomy clamp (with a
Indications for referral. Typically, referral to a complex family planning specialist or vascular surgeon is required for cases that involve dissection of the muscular fascia or where dissection would be in close proximity to critical neurologic or vascular structures.
CASE 1 Conclusion
Ultrasonography of the patient’s extremity demonstrated a
CASE 2 Patient enquires about immediate IUD insertion
A 28-year-old patient (G1P0) arrives at your clinic for a contraceptive consultation. They report a condom break during intercourse 4 days ago. Prior to that they used condoms consistently with each act of intercourse. They have used combined hormonal contraceptive pills in the past but had difficulty remembering to take them consistently. The patient and their partner have been mutually monogamous for 6 months and have no plans for pregnancy. Last menstrual period was 12 days ago. Their cycles are regular but heavy and painful. They are interested in using a hormonal IUD for contraception and would love to get it today.
- Do not attempt removal of a nonpalpable implant without prior localization via imaging
- Ultrasound-guided removal procedures using a “U” technique are successful for many deep implant removals but require specialized equipment and training
- Referral to a complex family planning specialist or other specialist is highly recommended for implants located below the triceps fascia or close to the nerves and vessels of the upper arm
- Never attempt to remove a nonpalpable implant prior to determining its location via imaging
Continue to: Is same-day IUD an option?...
Is same-day IUD an option?
Yes. This patient needs EC given the recent condom break, but they are still eligible for having an IUD placed today if their pregnancy test is negative and after counseling of the potential risks and benefits. According to the US-SPR it is reasonable to insert an IUD at any time during the cycle as long as you are reasonably certain the patient is not pregnant.7
Options for EC are:
- 1.5-mg oral LNG pill
- 30-mg oral UPA pill
- copper IUD (cu-IUD).
If they are interested in the cu-IUD for long-term contraception, by having a cu-IUD placed they can get both their needs met—EC and an ongoing method of contraception. Any patient receiving EC, whether a pill or an IUD, should be counseled to repeat a home urine pregnancy test in 2 to 4 weeks.
Given the favorable non–contraceptive benefits associated with 52-mg LNG-IUDs, many clinicians and patients have advocated for additional evidence regarding the use of hormonal IUDs alone for EC.
What is the evidence concerning LNG-IUD placement as EC?
The 52-mg LNG-IUD has not been mechanistically proven to work as an EC, but growing evidence exists showing that it is safe for same-day or “quick start” placement even in a population seeking EC—if their pregnancy test result is negative at the time of presentation.
Turok and colleagues performed a noninferiority trial comparing 1-month pregnancy rates after placement of either an LNG-IUD or a cu-IUD for EC.8 This study concluded that the LNG-IUD (which resulted in 1 pregnancy in 317 users; pregnancy rate, 0.3%; 95% confidence interval [CI], 0.01–1.70) is noninferior to cu-IUD (0 pregnancies in 321 users; pregnancy rate, 0%; 95% CI, 0.0–1.1) for EC. Although encouraging, only a small percentage of the study population seeking EC who received an IUD were actually at high risk of pregnancy (eg, they were not mid-cycle or were recently using contraception), which is why it is difficult to determine if the LNG-IUD actually works mechanistically as an EC. More likely, the LNG-IUD helps prevent pregnancy due to its ongoing contraceptive effect.9 Ongoing acts of intercourse post–oral EC initiation without starting a method of contraception is one of the main reasons for EC failure, which is why starting a method immediately is so effective at preventing pregnancy.10
A systematic review conducted by Ramanadhan and colleagues concluded that Turok’s 2021 trial is the only relevant study specific to 52-mg LNG-IUD use as EC, but they also mention that its results are limited in the strength of its conclusions due to biases in randomization, including11:
- the study groups were not balanced in that there was a 10% difference in reported use of contraception at last intercourse, which means that the LNG-IUD group had a lower baseline risk of pregnancy
- and a rare primary outcome (ie, pregnancy, which requires a larger sample size to know if the method works as an EC).
The review authors concluded that more studies are needed to further validate the effectiveness of using the 52-mg LNG-IUD as EC. Thus, for those at highest risk of pregnancy from recent unprotected sex and desiring a 52-mg IUD, it is probably best to continue combining oral EC with a 52-mg LNG-IUD and utilizing the LNG-IUD only as EC on a limited, case-by-case basis.
What we recommend
For anyone with a negative pregnancy test on the day of presentation, the studies mentioned further support the practice of same-day placement of a 52-mg LNG-IUD. However, those seeking EC who are at highest risk for an unplanned pregnancy (ie, the unprotected sex was mid-cycle), we recommend co-administering the LNG-IUD with oral LNG for EC.
CASE 2 Conclusion
After a conversation with the patient about all contraceptive options, through shared decision making the patient decided to take 1.5 mg of oral LNG and have a 52-mg LNG-IUD placed in the office today. They do not wish to be pregnant at this time and would choose termination if they became pregnant. They understood their pregnancy risk and opted to plan a urine pregnancy test at home in 2 weeks with a clear understanding that they should return to clinic immediately if the test is positive. ●
- A copper IUD is the most effective method of emergency contraception (EC).
- 52-mg LNG-IUDs are an emerging consideration for EC, but evidence is still lacking that they work as EC (or whether they just prevent pregnancy after placement for subsequent acts of intercourse). Clinicians should utilize shared decision making and advise patients to repeat a pregnancy test at home in 2 to 4 weeks
- Any patient receiving EC, whether a pill or an IUD, should be counseled to repeat a home urine pregnancy test in 2 to 4 weeks
- Any type of IUD can be placed same day if the clinician is reasonably sure the patient is not pregnant
- It appears safe to co-administer the 52-mg LNG-IUD with oral EC for those seeking emergency contraception but also want to use an LNG-IUD for contraception going forward
- Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. Morb Mortal Wkly Rep. 2016;65:1-66. https://doi .org/10.15585/mmwr .rr6504a1
- Centers for Disease Control and Prevention. National Center for Chronic Disease Prevention and Health Promotion, Division of Reproductive Health. US Selected Practice Recommendations for Contraceptive Use (US-SPR). Accessed October 11, 2023. https://www.cdc.gov/reproductivehealth /contraception/mmwr/spr/summary.html
- Nexplanon [package insert]. Whitehouse Station, NJ: Merck; 2018.
- US Food and Drug Administration. Implanon (etonogestrel implant) 2006. Accessed November 6, 2023. https://www .accessdata.fda.gov/drugsatfda_docs/nda/2006 /021529s000_Lbl.pdf
- US Food and Drug Administration. Jadelle (levonorgestrel implant) 2016. Accessed November 6, 2023. https://www. accessdata.fda.gov/drugsatfda_docs/label/2016/020544s 010lbl.pdf
- Chen MJ, Creinin MD. Removal of a nonpalpable etonogestrel implant with preprocedure ultrasonography and modified vasectomy clamp. Obstet Gynecol. 2015;126:935-938.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. MMWR Recomm Rep Morb Mortal Wkly. 2016;65:1-66. https://doi .org/10.15585/mmwr.rr6504a1
- Turok DK, Gero A, Simmons RG, et al. Levonorgestrel vs. copper intrauterine devices for emergency contraception. N Engl J Med. 2021;384:335-344. https://pubmed.ncbi.nlm .nih.gov/33503342/
- Kaiser JE, Turok DK, Gero A, et al. One-year pregnancy and continuation rates after placement of levonorgestrel or copper intrauterine devices for emergency contraception: a randomized controlled trial. Am J Obstet Gynecol. 2023;228:438.e1-438.e10. https://doi.org/10.1016/j.ajog.2022 .11.1296
- Sander PM, Raymond EG, Weaver MA. Emergency contraceptive use as a marker of future risky sex, pregnancy, and sexually transmitted infection. Am J Obstet Gynecol. 2009;201:146.e1-e6.
- Ramanadhan S, Goldstuck N, Henderson JT, et al. Progestin intrauterine devices versus copper intrauterine devices for emergency contraception. Cochrane Database Syst Rev. 2023;2:CD013744. https://doi.org/10.1002/14651858 .CD013744.pub2
- Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. Morb Mortal Wkly Rep. 2016;65:1-66. https://doi .org/10.15585/mmwr .rr6504a1
- Centers for Disease Control and Prevention. National Center for Chronic Disease Prevention and Health Promotion, Division of Reproductive Health. US Selected Practice Recommendations for Contraceptive Use (US-SPR). Accessed October 11, 2023. https://www.cdc.gov/reproductivehealth /contraception/mmwr/spr/summary.html
- Nexplanon [package insert]. Whitehouse Station, NJ: Merck; 2018.
- US Food and Drug Administration. Implanon (etonogestrel implant) 2006. Accessed November 6, 2023. https://www .accessdata.fda.gov/drugsatfda_docs/nda/2006 /021529s000_Lbl.pdf
- US Food and Drug Administration. Jadelle (levonorgestrel implant) 2016. Accessed November 6, 2023. https://www. accessdata.fda.gov/drugsatfda_docs/label/2016/020544s 010lbl.pdf
- Chen MJ, Creinin MD. Removal of a nonpalpable etonogestrel implant with preprocedure ultrasonography and modified vasectomy clamp. Obstet Gynecol. 2015;126:935-938.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. MMWR Recomm Rep Morb Mortal Wkly. 2016;65:1-66. https://doi .org/10.15585/mmwr.rr6504a1
- Turok DK, Gero A, Simmons RG, et al. Levonorgestrel vs. copper intrauterine devices for emergency contraception. N Engl J Med. 2021;384:335-344. https://pubmed.ncbi.nlm .nih.gov/33503342/
- Kaiser JE, Turok DK, Gero A, et al. One-year pregnancy and continuation rates after placement of levonorgestrel or copper intrauterine devices for emergency contraception: a randomized controlled trial. Am J Obstet Gynecol. 2023;228:438.e1-438.e10. https://doi.org/10.1016/j.ajog.2022 .11.1296
- Sander PM, Raymond EG, Weaver MA. Emergency contraceptive use as a marker of future risky sex, pregnancy, and sexually transmitted infection. Am J Obstet Gynecol. 2009;201:146.e1-e6.
- Ramanadhan S, Goldstuck N, Henderson JT, et al. Progestin intrauterine devices versus copper intrauterine devices for emergency contraception. Cochrane Database Syst Rev. 2023;2:CD013744. https://doi.org/10.1002/14651858 .CD013744.pub2
Quotes to live by: Paving the way to personal and professional success
In the first 2 years of medical school, the most common reasons for unsuccessful performance are a deficiency in cognitive knowledge, inefficient time management, and poor study skills. Thereafter, however, the principal reasons for poor performance in training or practice are personality issues and/or unprofessional behavior.
In this article, I review the attributes expected of a physician and the factors that undermine professionalism. I then offer suggestions for smoothing the pathway for personal and professional success. I crafted these suggestions with the “help” of some unlikely medical philosophers. (Note: Some variations of the cited quotations may exist.) I have tempered their guidance with my own personal experiences as a spouse, parent, and grandparent and my professional experiences over almost 50 years, during which I served as a career military officer, student clerkship director, residency program director, fellowship program director, and associate dean for student affairs. I readily acknowledge that, as major league baseball player Yogi Berra reputedly said, “I made too many wrong mistakes,” and that bad experiences are a tough way to ultimately learn good judgment. I hope these suggestions will help you avoid many of my “wrong mistakes.”
High expectations for the medical professional
“To whom much is given, much shall be required.”
—Luke 12:48
Medicine is a higher calling. It is not the usual type of business, and our patients certainly are not just customers or clients. In the unique moment of personal contact, we are asked to put the interest and well-being of our patient above all else. Our patients rightly have high expectations for what type of person their physician should be. The personal strengths expected of a physician include:
- humility
- honesty—personal and fiscal
- integrity
- strong moral compass
- fairness
- responsible
- diligent
- accountable
- insightful
- wise
- technically competent
- perseverant
- sympathetic
- empathetic
- inspiring.
To exhibit all these characteristics consistently is a herculean task and one that is impossible to fulfill. Many factors conspire to undermine our ability to steadfastly be all that we can be. Among these factors are:
- time constraints
- financial pressures
- physical illness
- emotional illness
- the explosion of information technology and scientific knowledge
- bureaucratic inefficiencies.
Therefore, we need to acknowledge with the philosopher Voltaire that “Perfect is the enemy of good.” We need to set our performance bar at excellence, not perfection. If we expect perfection of ourselves, we are destined to be consistently disappointed.
What follows is a series of well-intentioned and good-natured suggestions for keeping ourselves on an even keel, personally and professionally, and maintaining our compass setting on true north.
Continue to: Practical suggestions...
Practical suggestions
“It may not be that the race always goes to the swift nor the battle to the strong, but that is the way to bet.”
—Damon Runyon, journalist
The message is to study hard, work hard, practice our technical skills, and stay on top of our game. We must commit ourselves to a lifetime of learning.
“Chance favors the prepared mind.”
—Louis Pasteur, scientist
One of the best examples of this adage is Alexander Fleming’s “chance” discovery of the bactericidal effect of a mold growing on a culture plate in his laboratory. This observation led to the development of penicillin, an amazing antibiotic that, over the course of the past century, has saved the lives of literally hundreds of thousands of patients. We need to sustain our scientific curiosity throughout our careers and always remain open to new discoveries. Moreover, we need to maintain our capacity for awe and wonder as we consider the exquisite beauty of the scientific world.
“I have a dream.”
—Martin Luther King Jr, civil rights leader
Like Reverend King, we must aspire to a world where civility, peace, and social justice prevail, a world where we embrace diversity and inclusiveness and eschew prejudice, mean-spiritedness, and narrow-mindedness. We must acknowledge that some truths and moral principles are absolute, not relative.
“Once you learn to quit, it becomes a habit.”
—Vince Lombardi, professional football coach
Our lesson: Never quit. We must be fiercely determined to do the right thing, even in troubled and confusing times.
“A pessimist sees the difficulty in every opportunity; an optimist sees the opportunity in every difficulty.”
—Winston Churchill, British prime minister
Until proven wrong, always think the best of everyone. The bright side is far superior to the dark side. We must strive to consistently have a positive attitude and to be part of the solution to a problem, not the problem itself.
“It’s all such a delicate balance.”
—From “It’s a Delicate Balance” by Tom Dundee, folk singer and songwriter
Our top 3 priorities should always be our own emotional and physical well-being, the well-being and security of our loved ones, and the well-being of our patients. The order of these priorities may change, depending upon circumstances. When urgent patient care demands our presence and we miss a birthday celebration, anniversary dinner, soccer game, or dance recital, we need to make certain that, the next time a conflict arises, we arrange to have a colleague cover our clinical or administrative responsibilities.
We must learn to say no when our plate is too full. Failure to say no inevitably leads to life-work imbalance. It is always flattering to be asked to make a presentation, serve on a committee, or prepare a textbook chapter, and it is natural to be concerned that, if we decline, we will not be invited again. However, that concern is unwarranted. Rather, others will respect us for acknowledging when we are too busy and will be grateful that we did not accept an invitation and then miss important deadlines. Conversely, when we do say yes, we need to honor that commitment in a timely manner.
Continue to: The importance of time...
The importance of time
Perhaps the most common complaints that patients have with respect to their interactions with physicians are that they were forced to wait too long and then felt rushed through their appointment. Therefore:
- We must respect our patients’ time and recognize that their time is as valuable as ours.
- We must schedule our patient appointments appropriately and allow different amounts of time depending upon the complexity of a patient’s condition. We should not consistently overschedule. We need to offer a genuine apology when we keep a patient waiting for more than 15 minutes in the absence of an outright emergency that requires our attention elsewhere.
- When we interact with patients, we should sit down, establish eye-to-eye contact, and never appear hurried.
“You don’t make your character in a crisis; you exhibit it.”
—Oren Arnold, journalist and novelist
In the often-chaotic environment of the operating room or the labor and delivery suite, we must be the calm voice of reason at the center of the storm. We should not yell and make demands of others. We must strive to be unflappable. The other members of the team will be appreciative if they recognize that we have a steady hand on the tiller.
“To do good is noble. To teach others to do good is nobler—and less trouble.”
—Mark Twain, humorist
We need to teach our patients about their condition(s) so that they can assume more responsibility for their own care. We also need to teach our students and colleagues so that they can help us provide the best possible care for our patients. Being a good teacher is inherent in being a good physician. As the famous scientist Albert Einstein said, “If you cannot explain it simply, you do not understand it well enough.”
“It ain’t the things you don’t know that get you. It’s the things you think you know that ain’t so.”
—Artemus Ward, humorist
We must constantly strive to practice evidence-based medicine. We should not be the first to embrace the new or the last to give up the old. In medicine, as opposed to the highway, the best place to be is usually in the middle of the road. However, our commitment to evidence-based medicine cannot be absolute. In fact, no more than half of all our present treatment guidelines are based on level 1 evidence. At times, good old-fashioned common sense tempered by years of sobering experience should carry the day.
“We may be lost, but we’re making good time.”
—Yogi Berra, major league baseball player
In my experience, only the minority of mistakes in medicine result from lack of fundamental knowledge or a deficiency in technical skill. Rather, most result from imprudent haste and/or attempts to multitask. Therefore, our lesson is to slow down, concentrate on one task at a time, complete that task, and then refocus on the next challenge.
“The single greatest problem in communication is the illusion that it has taken place.”
—George Bernard Shaw, playwright
We must be sure that we always “close the loop” in our written and verbal communication so that we can avoid misunderstandings that threaten personal relationships and/or patient safety.
“You raise me up so I can stand on mountains.”
—From “You Raise Me Up” as sung by Josh Groban
All of us need a mentor to raise us up. We must choose our mentors carefully and recognize that we may need different mentors at different stages of our career. As we benefit from effective mentoring, we must pay it forward and be a good mentor to others.
“Worrying is a total waste of time. It accomplishes nothing, changes nothing, and robs you of joy. It is like paying a debt that you don’t owe.”
—Mark Twain, humorist
We have to assiduously cultivate the strength of resilience. We must accept that mistakes inevitably will occur and that perfection in practice is simply not possible, despite our best intentions. We then have to learn from these errors and ensure that they never occur again. We need to apologize for our mistakes and move on. If we carry our last strikeout into our next at bat, we are likely doomed to more misfortune.
“Feeling gratitude and not expressing it is like wrapping a present and not giving it.”
—William Arthur Ward, motivational writer
Our lesson is to be keenly aware of the importance of showing gratitude to those around us. The height of our success will depend directly on the depth of our gratitude. The higher we rise in the hierarchy of the medical profession, the more gracious and kind we need to be.
“Kindness is the language which the deaf can hear and the blind can see.”
—Mark Twain, humorist
“Kindness is the only service that will stand the storm of life and not wash out.”
—Abraham Lincoln, American president
There is never an excuse for rudeness or hubris. We should never teach or conduct business by intimidation. The words please, thank you, and I’m sorry should be front and center in our vocabulary. We must learn not to take ourselves too seriously, to remember that the best part of life is the laughter, and to always strive for grace and humility.
“The secret of the care of the patient is in caring for the patient.”
—Francis Peabody, physician
Patients may quickly forget what we say to them or even what we do for them, but they will never forget how we made them feel. Observe intently, listen carefully, talk less. Most people do not listen with the intent to understand. Rather, they listen with the intent to reply. We need to break this pattern by learning to listen with our heart. In fact, the quieter we become, the more we can hear. There is great symbolism in the fact that we have two ears and only one mouth.
“You got to know when to hold ‘em, know when to fold ‘em.”
—From “The Gambler” as sung by Kenny Rogers
Sometimes the best medicine is no medicine at all, but rather a soft shoulder, an open ear, a kind heart, and a compassionate soul.
“Do small things with great love.”
—Mother Teresa, Catholic missionary
The vast majority of us will not rise to lofty political or administrative positions or ever achieve celebrity status. We are unlikely to win the Nobel Prize and unlikely to find the cure for cancer or preeclampsia. However, we can work diligently to complete each small task with precision so that, like a great artist views his or her work, we, too, will want to sign our name to the patient care plan we have created and implemented.
“Earn this.”
—From Saving Private Ryan, a Steven Spielberg movie
At the end of this movie, the mortally wounded infantry captain (played by Tom Hanks) looks up at Private Ryan (played by Matt Damon) and says, “Earn this,” meaning make sure that you live your life in a way to justify the sacrifices so many made to save you. Like Private Ryan, we have to recognize that our MD degree does not constitute a lifetime entitlement to respect and honor. Rather, we have to practice each day so we continue to earn the respect of our patients, students, and colleagues and, so that, with confidence, we can then say to our patients, “How can I be of help to you?” ●
In the first 2 years of medical school, the most common reasons for unsuccessful performance are a deficiency in cognitive knowledge, inefficient time management, and poor study skills. Thereafter, however, the principal reasons for poor performance in training or practice are personality issues and/or unprofessional behavior.
In this article, I review the attributes expected of a physician and the factors that undermine professionalism. I then offer suggestions for smoothing the pathway for personal and professional success. I crafted these suggestions with the “help” of some unlikely medical philosophers. (Note: Some variations of the cited quotations may exist.) I have tempered their guidance with my own personal experiences as a spouse, parent, and grandparent and my professional experiences over almost 50 years, during which I served as a career military officer, student clerkship director, residency program director, fellowship program director, and associate dean for student affairs. I readily acknowledge that, as major league baseball player Yogi Berra reputedly said, “I made too many wrong mistakes,” and that bad experiences are a tough way to ultimately learn good judgment. I hope these suggestions will help you avoid many of my “wrong mistakes.”
High expectations for the medical professional
“To whom much is given, much shall be required.”
—Luke 12:48
Medicine is a higher calling. It is not the usual type of business, and our patients certainly are not just customers or clients. In the unique moment of personal contact, we are asked to put the interest and well-being of our patient above all else. Our patients rightly have high expectations for what type of person their physician should be. The personal strengths expected of a physician include:
- humility
- honesty—personal and fiscal
- integrity
- strong moral compass
- fairness
- responsible
- diligent
- accountable
- insightful
- wise
- technically competent
- perseverant
- sympathetic
- empathetic
- inspiring.
To exhibit all these characteristics consistently is a herculean task and one that is impossible to fulfill. Many factors conspire to undermine our ability to steadfastly be all that we can be. Among these factors are:
- time constraints
- financial pressures
- physical illness
- emotional illness
- the explosion of information technology and scientific knowledge
- bureaucratic inefficiencies.
Therefore, we need to acknowledge with the philosopher Voltaire that “Perfect is the enemy of good.” We need to set our performance bar at excellence, not perfection. If we expect perfection of ourselves, we are destined to be consistently disappointed.
What follows is a series of well-intentioned and good-natured suggestions for keeping ourselves on an even keel, personally and professionally, and maintaining our compass setting on true north.
Continue to: Practical suggestions...
Practical suggestions
“It may not be that the race always goes to the swift nor the battle to the strong, but that is the way to bet.”
—Damon Runyon, journalist
The message is to study hard, work hard, practice our technical skills, and stay on top of our game. We must commit ourselves to a lifetime of learning.
“Chance favors the prepared mind.”
—Louis Pasteur, scientist
One of the best examples of this adage is Alexander Fleming’s “chance” discovery of the bactericidal effect of a mold growing on a culture plate in his laboratory. This observation led to the development of penicillin, an amazing antibiotic that, over the course of the past century, has saved the lives of literally hundreds of thousands of patients. We need to sustain our scientific curiosity throughout our careers and always remain open to new discoveries. Moreover, we need to maintain our capacity for awe and wonder as we consider the exquisite beauty of the scientific world.
“I have a dream.”
—Martin Luther King Jr, civil rights leader
Like Reverend King, we must aspire to a world where civility, peace, and social justice prevail, a world where we embrace diversity and inclusiveness and eschew prejudice, mean-spiritedness, and narrow-mindedness. We must acknowledge that some truths and moral principles are absolute, not relative.
“Once you learn to quit, it becomes a habit.”
—Vince Lombardi, professional football coach
Our lesson: Never quit. We must be fiercely determined to do the right thing, even in troubled and confusing times.
“A pessimist sees the difficulty in every opportunity; an optimist sees the opportunity in every difficulty.”
—Winston Churchill, British prime minister
Until proven wrong, always think the best of everyone. The bright side is far superior to the dark side. We must strive to consistently have a positive attitude and to be part of the solution to a problem, not the problem itself.
“It’s all such a delicate balance.”
—From “It’s a Delicate Balance” by Tom Dundee, folk singer and songwriter
Our top 3 priorities should always be our own emotional and physical well-being, the well-being and security of our loved ones, and the well-being of our patients. The order of these priorities may change, depending upon circumstances. When urgent patient care demands our presence and we miss a birthday celebration, anniversary dinner, soccer game, or dance recital, we need to make certain that, the next time a conflict arises, we arrange to have a colleague cover our clinical or administrative responsibilities.
We must learn to say no when our plate is too full. Failure to say no inevitably leads to life-work imbalance. It is always flattering to be asked to make a presentation, serve on a committee, or prepare a textbook chapter, and it is natural to be concerned that, if we decline, we will not be invited again. However, that concern is unwarranted. Rather, others will respect us for acknowledging when we are too busy and will be grateful that we did not accept an invitation and then miss important deadlines. Conversely, when we do say yes, we need to honor that commitment in a timely manner.
Continue to: The importance of time...
The importance of time
Perhaps the most common complaints that patients have with respect to their interactions with physicians are that they were forced to wait too long and then felt rushed through their appointment. Therefore:
- We must respect our patients’ time and recognize that their time is as valuable as ours.
- We must schedule our patient appointments appropriately and allow different amounts of time depending upon the complexity of a patient’s condition. We should not consistently overschedule. We need to offer a genuine apology when we keep a patient waiting for more than 15 minutes in the absence of an outright emergency that requires our attention elsewhere.
- When we interact with patients, we should sit down, establish eye-to-eye contact, and never appear hurried.
“You don’t make your character in a crisis; you exhibit it.”
—Oren Arnold, journalist and novelist
In the often-chaotic environment of the operating room or the labor and delivery suite, we must be the calm voice of reason at the center of the storm. We should not yell and make demands of others. We must strive to be unflappable. The other members of the team will be appreciative if they recognize that we have a steady hand on the tiller.
“To do good is noble. To teach others to do good is nobler—and less trouble.”
—Mark Twain, humorist
We need to teach our patients about their condition(s) so that they can assume more responsibility for their own care. We also need to teach our students and colleagues so that they can help us provide the best possible care for our patients. Being a good teacher is inherent in being a good physician. As the famous scientist Albert Einstein said, “If you cannot explain it simply, you do not understand it well enough.”
“It ain’t the things you don’t know that get you. It’s the things you think you know that ain’t so.”
—Artemus Ward, humorist
We must constantly strive to practice evidence-based medicine. We should not be the first to embrace the new or the last to give up the old. In medicine, as opposed to the highway, the best place to be is usually in the middle of the road. However, our commitment to evidence-based medicine cannot be absolute. In fact, no more than half of all our present treatment guidelines are based on level 1 evidence. At times, good old-fashioned common sense tempered by years of sobering experience should carry the day.
“We may be lost, but we’re making good time.”
—Yogi Berra, major league baseball player
In my experience, only the minority of mistakes in medicine result from lack of fundamental knowledge or a deficiency in technical skill. Rather, most result from imprudent haste and/or attempts to multitask. Therefore, our lesson is to slow down, concentrate on one task at a time, complete that task, and then refocus on the next challenge.
“The single greatest problem in communication is the illusion that it has taken place.”
—George Bernard Shaw, playwright
We must be sure that we always “close the loop” in our written and verbal communication so that we can avoid misunderstandings that threaten personal relationships and/or patient safety.
“You raise me up so I can stand on mountains.”
—From “You Raise Me Up” as sung by Josh Groban
All of us need a mentor to raise us up. We must choose our mentors carefully and recognize that we may need different mentors at different stages of our career. As we benefit from effective mentoring, we must pay it forward and be a good mentor to others.
“Worrying is a total waste of time. It accomplishes nothing, changes nothing, and robs you of joy. It is like paying a debt that you don’t owe.”
—Mark Twain, humorist
We have to assiduously cultivate the strength of resilience. We must accept that mistakes inevitably will occur and that perfection in practice is simply not possible, despite our best intentions. We then have to learn from these errors and ensure that they never occur again. We need to apologize for our mistakes and move on. If we carry our last strikeout into our next at bat, we are likely doomed to more misfortune.
“Feeling gratitude and not expressing it is like wrapping a present and not giving it.”
—William Arthur Ward, motivational writer
Our lesson is to be keenly aware of the importance of showing gratitude to those around us. The height of our success will depend directly on the depth of our gratitude. The higher we rise in the hierarchy of the medical profession, the more gracious and kind we need to be.
“Kindness is the language which the deaf can hear and the blind can see.”
—Mark Twain, humorist
“Kindness is the only service that will stand the storm of life and not wash out.”
—Abraham Lincoln, American president
There is never an excuse for rudeness or hubris. We should never teach or conduct business by intimidation. The words please, thank you, and I’m sorry should be front and center in our vocabulary. We must learn not to take ourselves too seriously, to remember that the best part of life is the laughter, and to always strive for grace and humility.
“The secret of the care of the patient is in caring for the patient.”
—Francis Peabody, physician
Patients may quickly forget what we say to them or even what we do for them, but they will never forget how we made them feel. Observe intently, listen carefully, talk less. Most people do not listen with the intent to understand. Rather, they listen with the intent to reply. We need to break this pattern by learning to listen with our heart. In fact, the quieter we become, the more we can hear. There is great symbolism in the fact that we have two ears and only one mouth.
“You got to know when to hold ‘em, know when to fold ‘em.”
—From “The Gambler” as sung by Kenny Rogers
Sometimes the best medicine is no medicine at all, but rather a soft shoulder, an open ear, a kind heart, and a compassionate soul.
“Do small things with great love.”
—Mother Teresa, Catholic missionary
The vast majority of us will not rise to lofty political or administrative positions or ever achieve celebrity status. We are unlikely to win the Nobel Prize and unlikely to find the cure for cancer or preeclampsia. However, we can work diligently to complete each small task with precision so that, like a great artist views his or her work, we, too, will want to sign our name to the patient care plan we have created and implemented.
“Earn this.”
—From Saving Private Ryan, a Steven Spielberg movie
At the end of this movie, the mortally wounded infantry captain (played by Tom Hanks) looks up at Private Ryan (played by Matt Damon) and says, “Earn this,” meaning make sure that you live your life in a way to justify the sacrifices so many made to save you. Like Private Ryan, we have to recognize that our MD degree does not constitute a lifetime entitlement to respect and honor. Rather, we have to practice each day so we continue to earn the respect of our patients, students, and colleagues and, so that, with confidence, we can then say to our patients, “How can I be of help to you?” ●
In the first 2 years of medical school, the most common reasons for unsuccessful performance are a deficiency in cognitive knowledge, inefficient time management, and poor study skills. Thereafter, however, the principal reasons for poor performance in training or practice are personality issues and/or unprofessional behavior.
In this article, I review the attributes expected of a physician and the factors that undermine professionalism. I then offer suggestions for smoothing the pathway for personal and professional success. I crafted these suggestions with the “help” of some unlikely medical philosophers. (Note: Some variations of the cited quotations may exist.) I have tempered their guidance with my own personal experiences as a spouse, parent, and grandparent and my professional experiences over almost 50 years, during which I served as a career military officer, student clerkship director, residency program director, fellowship program director, and associate dean for student affairs. I readily acknowledge that, as major league baseball player Yogi Berra reputedly said, “I made too many wrong mistakes,” and that bad experiences are a tough way to ultimately learn good judgment. I hope these suggestions will help you avoid many of my “wrong mistakes.”
High expectations for the medical professional
“To whom much is given, much shall be required.”
—Luke 12:48
Medicine is a higher calling. It is not the usual type of business, and our patients certainly are not just customers or clients. In the unique moment of personal contact, we are asked to put the interest and well-being of our patient above all else. Our patients rightly have high expectations for what type of person their physician should be. The personal strengths expected of a physician include:
- humility
- honesty—personal and fiscal
- integrity
- strong moral compass
- fairness
- responsible
- diligent
- accountable
- insightful
- wise
- technically competent
- perseverant
- sympathetic
- empathetic
- inspiring.
To exhibit all these characteristics consistently is a herculean task and one that is impossible to fulfill. Many factors conspire to undermine our ability to steadfastly be all that we can be. Among these factors are:
- time constraints
- financial pressures
- physical illness
- emotional illness
- the explosion of information technology and scientific knowledge
- bureaucratic inefficiencies.
Therefore, we need to acknowledge with the philosopher Voltaire that “Perfect is the enemy of good.” We need to set our performance bar at excellence, not perfection. If we expect perfection of ourselves, we are destined to be consistently disappointed.
What follows is a series of well-intentioned and good-natured suggestions for keeping ourselves on an even keel, personally and professionally, and maintaining our compass setting on true north.
Continue to: Practical suggestions...
Practical suggestions
“It may not be that the race always goes to the swift nor the battle to the strong, but that is the way to bet.”
—Damon Runyon, journalist
The message is to study hard, work hard, practice our technical skills, and stay on top of our game. We must commit ourselves to a lifetime of learning.
“Chance favors the prepared mind.”
—Louis Pasteur, scientist
One of the best examples of this adage is Alexander Fleming’s “chance” discovery of the bactericidal effect of a mold growing on a culture plate in his laboratory. This observation led to the development of penicillin, an amazing antibiotic that, over the course of the past century, has saved the lives of literally hundreds of thousands of patients. We need to sustain our scientific curiosity throughout our careers and always remain open to new discoveries. Moreover, we need to maintain our capacity for awe and wonder as we consider the exquisite beauty of the scientific world.
“I have a dream.”
—Martin Luther King Jr, civil rights leader
Like Reverend King, we must aspire to a world where civility, peace, and social justice prevail, a world where we embrace diversity and inclusiveness and eschew prejudice, mean-spiritedness, and narrow-mindedness. We must acknowledge that some truths and moral principles are absolute, not relative.
“Once you learn to quit, it becomes a habit.”
—Vince Lombardi, professional football coach
Our lesson: Never quit. We must be fiercely determined to do the right thing, even in troubled and confusing times.
“A pessimist sees the difficulty in every opportunity; an optimist sees the opportunity in every difficulty.”
—Winston Churchill, British prime minister
Until proven wrong, always think the best of everyone. The bright side is far superior to the dark side. We must strive to consistently have a positive attitude and to be part of the solution to a problem, not the problem itself.
“It’s all such a delicate balance.”
—From “It’s a Delicate Balance” by Tom Dundee, folk singer and songwriter
Our top 3 priorities should always be our own emotional and physical well-being, the well-being and security of our loved ones, and the well-being of our patients. The order of these priorities may change, depending upon circumstances. When urgent patient care demands our presence and we miss a birthday celebration, anniversary dinner, soccer game, or dance recital, we need to make certain that, the next time a conflict arises, we arrange to have a colleague cover our clinical or administrative responsibilities.
We must learn to say no when our plate is too full. Failure to say no inevitably leads to life-work imbalance. It is always flattering to be asked to make a presentation, serve on a committee, or prepare a textbook chapter, and it is natural to be concerned that, if we decline, we will not be invited again. However, that concern is unwarranted. Rather, others will respect us for acknowledging when we are too busy and will be grateful that we did not accept an invitation and then miss important deadlines. Conversely, when we do say yes, we need to honor that commitment in a timely manner.
Continue to: The importance of time...
The importance of time
Perhaps the most common complaints that patients have with respect to their interactions with physicians are that they were forced to wait too long and then felt rushed through their appointment. Therefore:
- We must respect our patients’ time and recognize that their time is as valuable as ours.
- We must schedule our patient appointments appropriately and allow different amounts of time depending upon the complexity of a patient’s condition. We should not consistently overschedule. We need to offer a genuine apology when we keep a patient waiting for more than 15 minutes in the absence of an outright emergency that requires our attention elsewhere.
- When we interact with patients, we should sit down, establish eye-to-eye contact, and never appear hurried.
“You don’t make your character in a crisis; you exhibit it.”
—Oren Arnold, journalist and novelist
In the often-chaotic environment of the operating room or the labor and delivery suite, we must be the calm voice of reason at the center of the storm. We should not yell and make demands of others. We must strive to be unflappable. The other members of the team will be appreciative if they recognize that we have a steady hand on the tiller.
“To do good is noble. To teach others to do good is nobler—and less trouble.”
—Mark Twain, humorist
We need to teach our patients about their condition(s) so that they can assume more responsibility for their own care. We also need to teach our students and colleagues so that they can help us provide the best possible care for our patients. Being a good teacher is inherent in being a good physician. As the famous scientist Albert Einstein said, “If you cannot explain it simply, you do not understand it well enough.”
“It ain’t the things you don’t know that get you. It’s the things you think you know that ain’t so.”
—Artemus Ward, humorist
We must constantly strive to practice evidence-based medicine. We should not be the first to embrace the new or the last to give up the old. In medicine, as opposed to the highway, the best place to be is usually in the middle of the road. However, our commitment to evidence-based medicine cannot be absolute. In fact, no more than half of all our present treatment guidelines are based on level 1 evidence. At times, good old-fashioned common sense tempered by years of sobering experience should carry the day.
“We may be lost, but we’re making good time.”
—Yogi Berra, major league baseball player
In my experience, only the minority of mistakes in medicine result from lack of fundamental knowledge or a deficiency in technical skill. Rather, most result from imprudent haste and/or attempts to multitask. Therefore, our lesson is to slow down, concentrate on one task at a time, complete that task, and then refocus on the next challenge.
“The single greatest problem in communication is the illusion that it has taken place.”
—George Bernard Shaw, playwright
We must be sure that we always “close the loop” in our written and verbal communication so that we can avoid misunderstandings that threaten personal relationships and/or patient safety.
“You raise me up so I can stand on mountains.”
—From “You Raise Me Up” as sung by Josh Groban
All of us need a mentor to raise us up. We must choose our mentors carefully and recognize that we may need different mentors at different stages of our career. As we benefit from effective mentoring, we must pay it forward and be a good mentor to others.
“Worrying is a total waste of time. It accomplishes nothing, changes nothing, and robs you of joy. It is like paying a debt that you don’t owe.”
—Mark Twain, humorist
We have to assiduously cultivate the strength of resilience. We must accept that mistakes inevitably will occur and that perfection in practice is simply not possible, despite our best intentions. We then have to learn from these errors and ensure that they never occur again. We need to apologize for our mistakes and move on. If we carry our last strikeout into our next at bat, we are likely doomed to more misfortune.
“Feeling gratitude and not expressing it is like wrapping a present and not giving it.”
—William Arthur Ward, motivational writer
Our lesson is to be keenly aware of the importance of showing gratitude to those around us. The height of our success will depend directly on the depth of our gratitude. The higher we rise in the hierarchy of the medical profession, the more gracious and kind we need to be.
“Kindness is the language which the deaf can hear and the blind can see.”
—Mark Twain, humorist
“Kindness is the only service that will stand the storm of life and not wash out.”
—Abraham Lincoln, American president
There is never an excuse for rudeness or hubris. We should never teach or conduct business by intimidation. The words please, thank you, and I’m sorry should be front and center in our vocabulary. We must learn not to take ourselves too seriously, to remember that the best part of life is the laughter, and to always strive for grace and humility.
“The secret of the care of the patient is in caring for the patient.”
—Francis Peabody, physician
Patients may quickly forget what we say to them or even what we do for them, but they will never forget how we made them feel. Observe intently, listen carefully, talk less. Most people do not listen with the intent to understand. Rather, they listen with the intent to reply. We need to break this pattern by learning to listen with our heart. In fact, the quieter we become, the more we can hear. There is great symbolism in the fact that we have two ears and only one mouth.
“You got to know when to hold ‘em, know when to fold ‘em.”
—From “The Gambler” as sung by Kenny Rogers
Sometimes the best medicine is no medicine at all, but rather a soft shoulder, an open ear, a kind heart, and a compassionate soul.
“Do small things with great love.”
—Mother Teresa, Catholic missionary
The vast majority of us will not rise to lofty political or administrative positions or ever achieve celebrity status. We are unlikely to win the Nobel Prize and unlikely to find the cure for cancer or preeclampsia. However, we can work diligently to complete each small task with precision so that, like a great artist views his or her work, we, too, will want to sign our name to the patient care plan we have created and implemented.
“Earn this.”
—From Saving Private Ryan, a Steven Spielberg movie
At the end of this movie, the mortally wounded infantry captain (played by Tom Hanks) looks up at Private Ryan (played by Matt Damon) and says, “Earn this,” meaning make sure that you live your life in a way to justify the sacrifices so many made to save you. Like Private Ryan, we have to recognize that our MD degree does not constitute a lifetime entitlement to respect and honor. Rather, we have to practice each day so we continue to earn the respect of our patients, students, and colleagues and, so that, with confidence, we can then say to our patients, “How can I be of help to you?” ●

