User login
Eptinezumab Shows Promise For Treatment-refractory Migraine
Key clinical point: Switching to intravenous eptinezumab may benefit patients with treatment-refractory migraine who have previously failed subcutaneous calcitonin gene-related peptide-receptor (CGRP-R) monoclonal antibodies (mAbs).
Major findings: At 12 and 24 weeks of eptinezumab treatment, 23.1% and 29.7% of patients had ≥30% reduction in monthly migraine days, whereas 15.4% and 21.4% had ≥30% reduction in monthly headache days, respectively. At 21-24 weeks, 38.5% and 52.4% of patients showed significant reductions in the Headache Impact Test and Migraine Disability Assessment scores, respectively. No adverse events were reported during the 24-week treatment period.
Study details: This monocentric retrospective longitudinal cohort study included 41 patients with migraine unresponsive to ≥1 subcutaneous CGRP mAb, who received an initial 100 mg dose of intravenous eptinezumab, followed by 100 or 300 mg after 12 weeks.
Disclosure: This study was supported by the Lundbeck Foundation. Several authors declared receiving personal fees from various sources.
Source: Triller P, Blessing VN, Overeem LH, et al. Efficacy of eptinezumab in non-responders to subcutaneous monoclonal antibodies against CGRP and the CGRP receptor: A retrospective cohort study. Cephalalgia. Published online October 29, 2024. Source
Key clinical point: Switching to intravenous eptinezumab may benefit patients with treatment-refractory migraine who have previously failed subcutaneous calcitonin gene-related peptide-receptor (CGRP-R) monoclonal antibodies (mAbs).
Major findings: At 12 and 24 weeks of eptinezumab treatment, 23.1% and 29.7% of patients had ≥30% reduction in monthly migraine days, whereas 15.4% and 21.4% had ≥30% reduction in monthly headache days, respectively. At 21-24 weeks, 38.5% and 52.4% of patients showed significant reductions in the Headache Impact Test and Migraine Disability Assessment scores, respectively. No adverse events were reported during the 24-week treatment period.
Study details: This monocentric retrospective longitudinal cohort study included 41 patients with migraine unresponsive to ≥1 subcutaneous CGRP mAb, who received an initial 100 mg dose of intravenous eptinezumab, followed by 100 or 300 mg after 12 weeks.
Disclosure: This study was supported by the Lundbeck Foundation. Several authors declared receiving personal fees from various sources.
Source: Triller P, Blessing VN, Overeem LH, et al. Efficacy of eptinezumab in non-responders to subcutaneous monoclonal antibodies against CGRP and the CGRP receptor: A retrospective cohort study. Cephalalgia. Published online October 29, 2024. Source
Key clinical point: Switching to intravenous eptinezumab may benefit patients with treatment-refractory migraine who have previously failed subcutaneous calcitonin gene-related peptide-receptor (CGRP-R) monoclonal antibodies (mAbs).
Major findings: At 12 and 24 weeks of eptinezumab treatment, 23.1% and 29.7% of patients had ≥30% reduction in monthly migraine days, whereas 15.4% and 21.4% had ≥30% reduction in monthly headache days, respectively. At 21-24 weeks, 38.5% and 52.4% of patients showed significant reductions in the Headache Impact Test and Migraine Disability Assessment scores, respectively. No adverse events were reported during the 24-week treatment period.
Study details: This monocentric retrospective longitudinal cohort study included 41 patients with migraine unresponsive to ≥1 subcutaneous CGRP mAb, who received an initial 100 mg dose of intravenous eptinezumab, followed by 100 or 300 mg after 12 weeks.
Disclosure: This study was supported by the Lundbeck Foundation. Several authors declared receiving personal fees from various sources.
Source: Triller P, Blessing VN, Overeem LH, et al. Efficacy of eptinezumab in non-responders to subcutaneous monoclonal antibodies against CGRP and the CGRP receptor: A retrospective cohort study. Cephalalgia. Published online October 29, 2024. Source
Elevated Waist-to-Height Ratio Linked to Increased Migraine Incidence in Young Adults
Key clinical point: An increased waist-to-height ratio (WHtR), indicating central obesity, was associated with an increased incidence of migraine, particularly in individuals aged <60 years.
Major findings: Each unit increase in WHtR was associated with a 70% increase in the incidence of migraines (odds ratio [OR], 1.70; 95% CI, 1.04-2.78). Individuals in the highest WHtR quartile (WHtR values, 0.64-1.01) had a 13% greater incidence of migraines than those in the lowest quartile (WHtR values, 0.35-0.52; OR, 1.13; 95% CI, 0.99-1.28). For individuals aged <60 years, each unit increase in WHtR was associated with an 82% increased risk for migraine (P < .01); however, WHtR was negatively associated with migraine risk among those aged ≥60 years.
Study details: This cross-sectional study analyzed data from the National Health and Nutrition Examination Survey, including 13,344 participants, of whom 2764 had migraines. Disclosure: The study did not receive any funding. The authors declared no conflicts of interest.
Source: Jin J, Zheng Y, Gao T, Lin X, Li S, Huang C. Associations between the waist-to-height ratio index and migraine: A cross-section study of the NHANES 1999–2004. PLoS ONE. Published online October 23, 2024. Source
Key clinical point: An increased waist-to-height ratio (WHtR), indicating central obesity, was associated with an increased incidence of migraine, particularly in individuals aged <60 years.
Major findings: Each unit increase in WHtR was associated with a 70% increase in the incidence of migraines (odds ratio [OR], 1.70; 95% CI, 1.04-2.78). Individuals in the highest WHtR quartile (WHtR values, 0.64-1.01) had a 13% greater incidence of migraines than those in the lowest quartile (WHtR values, 0.35-0.52; OR, 1.13; 95% CI, 0.99-1.28). For individuals aged <60 years, each unit increase in WHtR was associated with an 82% increased risk for migraine (P < .01); however, WHtR was negatively associated with migraine risk among those aged ≥60 years.
Study details: This cross-sectional study analyzed data from the National Health and Nutrition Examination Survey, including 13,344 participants, of whom 2764 had migraines. Disclosure: The study did not receive any funding. The authors declared no conflicts of interest.
Source: Jin J, Zheng Y, Gao T, Lin X, Li S, Huang C. Associations between the waist-to-height ratio index and migraine: A cross-section study of the NHANES 1999–2004. PLoS ONE. Published online October 23, 2024. Source
Key clinical point: An increased waist-to-height ratio (WHtR), indicating central obesity, was associated with an increased incidence of migraine, particularly in individuals aged <60 years.
Major findings: Each unit increase in WHtR was associated with a 70% increase in the incidence of migraines (odds ratio [OR], 1.70; 95% CI, 1.04-2.78). Individuals in the highest WHtR quartile (WHtR values, 0.64-1.01) had a 13% greater incidence of migraines than those in the lowest quartile (WHtR values, 0.35-0.52; OR, 1.13; 95% CI, 0.99-1.28). For individuals aged <60 years, each unit increase in WHtR was associated with an 82% increased risk for migraine (P < .01); however, WHtR was negatively associated with migraine risk among those aged ≥60 years.
Study details: This cross-sectional study analyzed data from the National Health and Nutrition Examination Survey, including 13,344 participants, of whom 2764 had migraines. Disclosure: The study did not receive any funding. The authors declared no conflicts of interest.
Source: Jin J, Zheng Y, Gao T, Lin X, Li S, Huang C. Associations between the waist-to-height ratio index and migraine: A cross-section study of the NHANES 1999–2004. PLoS ONE. Published online October 23, 2024. Source
A Group Approach to Clinical Research Mentorship at a Veterans Affairs Medical Center
A Group Approach to Clinical Research Mentorship at a Veterans Affairs Medical Center
Supporting meaningful research that has a positive impact on the health and quality of life of veterans is a priority of the US Department of Veterans Affairs Office of Research and Development.1 For nearly a century, VA researchers have been conducting high quality studies. To continue this trajectory, it is imperative to attract, train, and retain exceptional investigators while nurturing their development throughout their careers.2
Mentorship is defined as guidance provided by an experienced and trusted party to another (usually junior) individual with the intent of helping the person succeed. It benefits the mentee, mentor, and their institutions.3 Mentorship is crucial for personal and professional development as well as productivity, which may help reduce clinician burnout.4-7 Conversely, a lack of mentorship could have negative effects on work satisfaction and stagnate career progression.8
Mentorship is vital for developing and advancing a VA investigator’s research agenda. Funding, grant writing, and research design were among the most discussed topics in a large comprehensive mentorship program for academic faculty.9 However, there are several known barriers to effective research mentorship; among them include a lack of resources, time constraints, and competing clinical priorities.10,11
Finding time for effective one-on-one research mentoring is difficult within the time constraints of clinical duties; a group mentorship model may help overcome this barrier. Group mentorship can aid in personal and professional development because no single mentor can effectively meet every mentoring need of an individual.12 Group mentorship also allows for the exchange of ideas among individuals with different backgrounds and the ability to utilize the strengths of each member of the group. For example, a member may have methodological expertise, while another may be skilled in grantsmanship. A team of mentors may be more beneficial for both the mentors (eg, establish a more manageable workload) and the mentee (eg, gains a broader perspective of expertise) when compared to having a single mentor.3
Peer mentorship within the group setting may also yield additional benefits. For example, having a supportive peer group may help reduce stress levels and burnout, while also improving overall well-being.3,13 Formal mentorship programs do not frequently discuss concerns such as work-life balance, so including peers as mentors may help fill this void.9 Peer mentorship has also been found to be beneficial in providing mentees with pooled resources and shared learning.12,13 This article describes the components, benefits, impacts, and challenges of a group research mentorship program for VA clinicians interested in conducting VA-relevant research.
Program Description
The VA Clinical Research Mentorship Program was initiated at the VA Ann Arbor Healthcare System (VAAAHS) in October 2015 by the Chief of Medicine to assist VA clinician investigators with developing and submitting VA clinical science and health services research grant applications. The program offers group and one-on-one consultation services through the expertise of 2 experienced investigators/faculty mentors who also serve as program directors, each of whom devote about 3 to 5 hours per month to activities associated with the mentorship program (eg, attending the meeting, reviewing materials sent by mentees, and one-on-one discussions with mentees).
The program also fostered peer-led mentorship. This encourages all attendees to provide feedback during group sessions and communication by mentees outside the group sessions. An experienced project manager serves as program coordinator and contributes about 4 hours per month for activities such as attending, scheduling, and sending reminders for each meeting, distributing handouts, reviewing materials, and answering mentee’s questions via email. A statistician and additional research staff (ie, an epidemiologist and research assistant) do not attend the recurring meetings, but are available for offline consultation as needed. The program runs on a 12-month cycle with regular meetings occurring twice monthly during the 9-month academic period. Resources to support the program, primarily program director(s) and project coordinator effort, are provided by the Chief of Medicine and through the VAAAHS affiliated VA Health Systems Research (formerly Health Services Research & Development) Center of Innovation.
Invitations for new mentees are sent annually. Mentees expressing interest in the program outside of its annual recruitment period are evaluated for inclusion on a rolling basis. Recruitment begins with the program coordinator sending email notifications to all VAAAHS Medicine Service faculty, section chiefs, and division chiefs at the VAAAHS academic affiliate. Recipients are encouraged to distribute the announcement to eligible applicants and refer them to the application materials for entry consideration into the program. The application consists of the applicant’s curriculum vitae and a 1-page summary that includes a description of their research area of interest, how it is relevant to the VA, in addition to an idea for a research study, its potential significance, and proposed methodology. Applicant materials are reviewed by the program coordinator and program directors. The applicants are evaluated using a simple scoring approach that focuses on the applicant’s research area and agenda, past research training, past research productivity, potential for obtaining VA funding, and whether they have sufficient research time.
Program eligibility initially required being a physician with ≥ 1/8 VA appointment from the Medicine Service. However, clinicians with clinical appointments from other VA services are also accepted for participation as needed. Applicants must have previous research experience and have a career goal to obtain external funding for conducting and publishing original research. Those who have previously served as a principal investigator on a funded VA grant proposal are not eligible as new applicants but can remain in the program as peer mentors. The number of annual applicants varies and ranges from 1 to 11; on average, about 90% of applicants receive invitations to join the program.
Sessions
The program holds recurring meetings twice monthly for 1 hour during the 9-month academic year. However, program directors are available year-round, and mentees are encouraged to communicate questions or concerns via email during nonacademic months. Prior to the COVID-19 pandemic, all meetings were held in-person. However, the group pivoted to virtual meetings and continues to utilize this format. The dedicated program coordinator is responsible for coordinating meetings and distributing meeting materials.
Each session is informal, flexible, and supportive. Attendance is not enforced, and mentees are allowed to join meetings as their schedules permit; however, program directors and program coordinator attend each meeting. In advance of each session, the program coordinator sends out a call for agenda items to all active members invited to discuss any research related items. Each mentee presents their ideas to lead the discussion for their portion of the meeting with no defined format required.
A variety of topics are covered including, but not limited to: (1) grant-specific concerns (eg, questions related to specific aim pages, grantsmanship, postsubmission comments from reviewers, or postaward logistics); (2) research procedures (eg, questions related to methodological practices or institutional review board concerns); (3) manuscript or presentation preparation; and (4) careerrelated issues. The program coordinator distributes handouts prior to meetings and mentees may record their presentations. These handouts may include, but are not limited to, specific aims pages, analytical plans, grant solicitations, and PowerPoint presentations. If a resource that can benefit the entire group is mentioned during the meeting, the program coordinator is responsible for distribution.
The program follows a group facilitated discussion format. Program directors facilitate each meeting, but input is encouraged from all attendees. This model allows for mentees to learn from the faculty mentors as well as peer mentees in a simultaneous and efficient fashion. Group discussions foster collective problem solving, peer support, and resource sharing that would not be possible through individualized mentorship. Participants have access to varied expertise during each session which reduces the need to seek specialized help elsewhere. Participants are also encouraged to contact the program directors or research staff for consultation as needed. Some one-on-one consultations have transitioned to a more sustained and ongoing mentorship relationship between a program director and mentee, but most are often brief email exchanges or a single meeting.
Participants
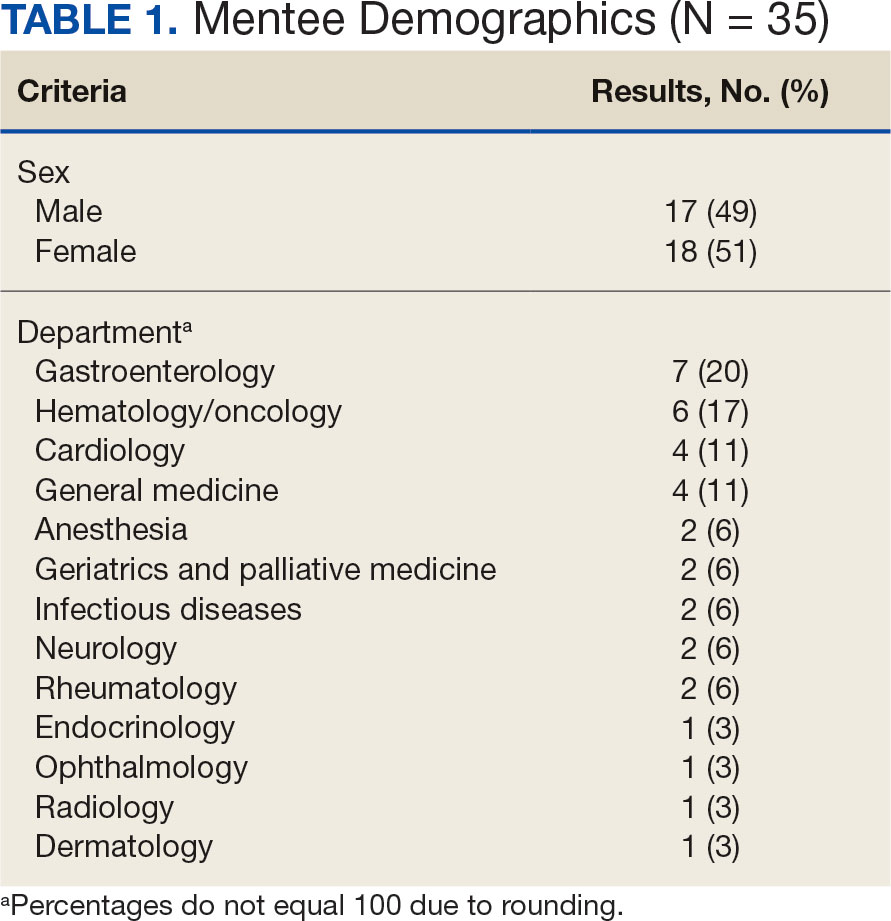
Since its inception in 2015, 35 clinicians have enrolled in the program. The mentees are equally distributed by sex and practice in a variety of disciplines including gastroenterology, hematology/oncology, cardiology, and general medicine (Table 1). Mentees have submitted 33 grant proposals addressing a variety of health care issues to a diverse group of federal and nonfederal funding agencies (Table 2). As of May 15, 2024, 19 (58%) of the submitted applications have been funded.

Many factors contribute to a successfully funded grant application, and several mentees report that participating in the mentorship program was helpful. For example, a mentee became the first lead investigator for a VA Cooperative Studies Program funded at VAAAHS. The VA Cooperative Studies Program, a division of the Office of Research and Development, plans and conducts large multicenter clinical trials and epidemiological studies within the VA via a vast network of clinician investigators, statisticians, and other key research experts.14
Several program mentees have also received VA Clinical Science Research and Development Career Development Awards. The VA Career Development program supports investigators during their early research careers with a goal of retaining talented researchers committed to improving the health and care of veterans.15
Survey Responses
Mentee productivity and updates are tracked through direct mentee input, as requested by the program coordinator. Since 2022, participants could complete an end-of-year survey based on an assessment tool used in a VAAAHS nonresearch mentorship program.16 The survey, distributed to mentees and program directors, requests feedback on logistics (eg, if the meeting was a good use of time and barriers to attendance); perceptions of effectiveness (eg, ability to discuss agenda items, helpfulness with setting and reaching research goals, and quality of mentors’ feedback); and the impact of the mentoring program on work satisfaction and clinician burnout. Respondents are also encouraged to leave open-ended qualitative feedback.
To date the survey has elicited 19 responses. Seventeen (89%) indicated that they agree or strongly agree the meetings were an effective use of their time and 11 (58%) indicated that they were able to discuss all or most of the items they wanted to during the meeting. Sixteen respondents (84%) agreed the program helped them set and achieve their research goals and 14 respondents (74%) agreed the feedback they received during the meeting was specific, actionable, and focused on how to improve their research agenda. Seventeen respondents (89%) agreed the program increased their work satisfaction, while 13 respondents (68%) felt the program reduced levels of clinician burnout.
As attendance was not mandatory, the survey asked participants how often they attended meetings during the past year. Responses were mixed: 4 (21%) respondents attended regularly (12 to 16 times per year) and 8 (42%) attended most sessions (8 to 11 times per year). Noted barriers to attendance included conflicts with patient care activities and conflicts with other high priority meetings.
Mentees also provided qualitive feedback regarding the program. They highlighted the supportive environment, valuable expertise of the mentors, and usefulness of obtaining tailored feedback from the group. “This group is an amazing resource to anyone developing a research career,” a mentee noted, adding that the program directors “fostered an incredibly supportive group where research ideas and methodology can be explored in a nonthreatening and creative environment.”
Conclusions
This mentorship program aims to help aspiring VA clinician investigators develop and submit competitive research grant applications. The addition of the program to the existing robust research environments at VAAAHS and its academic affiliate appears to have contributed to this success, with 58% of applications submitted by program mentees receiving funding.
In addition to funding success, we also found that most participants have a favorable impression of the program. Of the participants who responded to the program evaluation survey, nearly all indicated the program was an effective use of their time. The program also appeared to increase work satisfaction and reduce levels of clinician burnout. Barriers to attendance were also noted, with the most frequent being scheduling conflicts.
This program’s format includes facilitated group discussion as well as peer mentorship. This collaborative structure allows for an efficient and rich learning experience. Feedback from multiple perspectives encourages natural networking and relationship building. Incorporating the collective wisdom of the faculty mentors and peer mentees is beneficial; it not only empowers the mentees but also enriches the experience for the mentors. This program can serve as a model for other VA facilities—or non-VA academic medical centers—to enhance their research programs.
- US Department of Veterans Affairs, Office of Research and Development. Strategic priorities for VA research. Published March 10, 2021. Accessed September 17, 2024. https://www.research.va.gov/about/strategic_priorities.cfm
- US Department of Veterans Affairs, Office of Research and Development. About the Office of Research & Development. Published November 11, 2023. Accessed September 17, 2024. https://www.research.va.gov/about/default.cfm
- Chopra V, Vaughn V, Saint S. The Mentoring Guide: Helping Mentors and Mentees Succeed. Michigan Publishing Services; 2019.
- Gilster SD, Accorinti KL. Mentoring program yields staff satisfaction. Mentoring through the exchange of information across all organizational levels can help administrators retain valuable staff. Provider. 1999;25(10):99-100.
- Ramanan RA, Phillips RS, Davis RB, Silen W, Reede JY. Mentoring in medicine: keys to satisfaction. Am J Med. 2002;112(4):336-341. doi:10.1016/s0002-9343(02)01032-x
- Sambunjak D, Straus SE, Marusi' A. Mentoring in academic medicine: a systematic review. JAMA. 2006;296(9):1103-1115. doi:10.1001/jama.296.9.1103
- Sambunjak D, Straus SE, Marusi' A. A systematic review of qualitative research on the meaning and characteristics of mentoring in academic medicine. J Gen Intern Med. 2010;25(1):72-78. doi:10.1007/s11606-009-1165-8
- Jackson VA, Palepu A, Szalacha L, Caswell C, Carr PL, Inui T. “Having the right chemistry”: a qualitative study of mentoring in academic medicine. Acad Med. 2003;78(3):328-334. doi:10.1097/00001888-200303000-00020
- Feldman MD, Arean PA, Marshall SJ, Lovett M, O’Sullivan P. Does mentoring matter: results from a survey of faculty mentees at a large health sciences university. Med Educ Online. 2010;15:10.3402/meo.v15i0.5063. doi:10.3402/meo.v15i0.5063
- Leary JC, Schainker EG, Leyenaar JK. The unwritten rules of mentorship: facilitators of and barriers to effective mentorship in pediatric hospital medicine. Hosp Pediatr. 2016;6(4):219-225. doi:10.1542/hpeds.2015-0108
- Rustgi AK, Hecht GA. Mentorship in academic medicine. Gastroenterology. 2011;141(3):789-792. doi:10.1053/j.gastro.2011.07.024
- DeCastro R, Sambuco D, Ubel PA, Stewart A, Jagsi R. Mentor networks in academic medicine: moving beyond a dyadic conception of mentoring for junior faculty researchers. Acad Med. 2013;88(4):488-496. doi:10.1097/ACM.0b013e318285d302
- McDaugall M, Beattie RS. Peer mentoring at work: the nature and outcomes of non-hierarchical developmental relationships. Management Learning. 2016;28(4):423-437. doi:10.1177/1350507697284003
- US Department of Veterans Affairs, Office of Rsearch and Development. VA Cooperative Studies Program (CSP). Updated July 2019. Accessed September 17, 2024. https://www.vacsp.research.va.gov
- US Department of Veterans Affairs, Office of Research and Development. Career development program for biomedical laboratory and clinical science R&D services. Published April 17, 2023. Accessed September 17, 2024. https://www.research.va.gov/services/shared_docs/career_dev.cfm
- Houchens N, Kuhn L, Ratz D, Su G, Saint S. Committed to success: a structured mentoring program for clinically-oriented physicians. Mayo Clin Pro Innov Qual Outcomes. 2024;8(4):356-363. doi:10.1016/j.mayocpiqo.2024.05.002
Supporting meaningful research that has a positive impact on the health and quality of life of veterans is a priority of the US Department of Veterans Affairs Office of Research and Development.1 For nearly a century, VA researchers have been conducting high quality studies. To continue this trajectory, it is imperative to attract, train, and retain exceptional investigators while nurturing their development throughout their careers.2
Mentorship is defined as guidance provided by an experienced and trusted party to another (usually junior) individual with the intent of helping the person succeed. It benefits the mentee, mentor, and their institutions.3 Mentorship is crucial for personal and professional development as well as productivity, which may help reduce clinician burnout.4-7 Conversely, a lack of mentorship could have negative effects on work satisfaction and stagnate career progression.8
Mentorship is vital for developing and advancing a VA investigator’s research agenda. Funding, grant writing, and research design were among the most discussed topics in a large comprehensive mentorship program for academic faculty.9 However, there are several known barriers to effective research mentorship; among them include a lack of resources, time constraints, and competing clinical priorities.10,11
Finding time for effective one-on-one research mentoring is difficult within the time constraints of clinical duties; a group mentorship model may help overcome this barrier. Group mentorship can aid in personal and professional development because no single mentor can effectively meet every mentoring need of an individual.12 Group mentorship also allows for the exchange of ideas among individuals with different backgrounds and the ability to utilize the strengths of each member of the group. For example, a member may have methodological expertise, while another may be skilled in grantsmanship. A team of mentors may be more beneficial for both the mentors (eg, establish a more manageable workload) and the mentee (eg, gains a broader perspective of expertise) when compared to having a single mentor.3
Peer mentorship within the group setting may also yield additional benefits. For example, having a supportive peer group may help reduce stress levels and burnout, while also improving overall well-being.3,13 Formal mentorship programs do not frequently discuss concerns such as work-life balance, so including peers as mentors may help fill this void.9 Peer mentorship has also been found to be beneficial in providing mentees with pooled resources and shared learning.12,13 This article describes the components, benefits, impacts, and challenges of a group research mentorship program for VA clinicians interested in conducting VA-relevant research.
Program Description
The VA Clinical Research Mentorship Program was initiated at the VA Ann Arbor Healthcare System (VAAAHS) in October 2015 by the Chief of Medicine to assist VA clinician investigators with developing and submitting VA clinical science and health services research grant applications. The program offers group and one-on-one consultation services through the expertise of 2 experienced investigators/faculty mentors who also serve as program directors, each of whom devote about 3 to 5 hours per month to activities associated with the mentorship program (eg, attending the meeting, reviewing materials sent by mentees, and one-on-one discussions with mentees).
The program also fostered peer-led mentorship. This encourages all attendees to provide feedback during group sessions and communication by mentees outside the group sessions. An experienced project manager serves as program coordinator and contributes about 4 hours per month for activities such as attending, scheduling, and sending reminders for each meeting, distributing handouts, reviewing materials, and answering mentee’s questions via email. A statistician and additional research staff (ie, an epidemiologist and research assistant) do not attend the recurring meetings, but are available for offline consultation as needed. The program runs on a 12-month cycle with regular meetings occurring twice monthly during the 9-month academic period. Resources to support the program, primarily program director(s) and project coordinator effort, are provided by the Chief of Medicine and through the VAAAHS affiliated VA Health Systems Research (formerly Health Services Research & Development) Center of Innovation.
Invitations for new mentees are sent annually. Mentees expressing interest in the program outside of its annual recruitment period are evaluated for inclusion on a rolling basis. Recruitment begins with the program coordinator sending email notifications to all VAAAHS Medicine Service faculty, section chiefs, and division chiefs at the VAAAHS academic affiliate. Recipients are encouraged to distribute the announcement to eligible applicants and refer them to the application materials for entry consideration into the program. The application consists of the applicant’s curriculum vitae and a 1-page summary that includes a description of their research area of interest, how it is relevant to the VA, in addition to an idea for a research study, its potential significance, and proposed methodology. Applicant materials are reviewed by the program coordinator and program directors. The applicants are evaluated using a simple scoring approach that focuses on the applicant’s research area and agenda, past research training, past research productivity, potential for obtaining VA funding, and whether they have sufficient research time.
Program eligibility initially required being a physician with ≥ 1/8 VA appointment from the Medicine Service. However, clinicians with clinical appointments from other VA services are also accepted for participation as needed. Applicants must have previous research experience and have a career goal to obtain external funding for conducting and publishing original research. Those who have previously served as a principal investigator on a funded VA grant proposal are not eligible as new applicants but can remain in the program as peer mentors. The number of annual applicants varies and ranges from 1 to 11; on average, about 90% of applicants receive invitations to join the program.
Sessions
The program holds recurring meetings twice monthly for 1 hour during the 9-month academic year. However, program directors are available year-round, and mentees are encouraged to communicate questions or concerns via email during nonacademic months. Prior to the COVID-19 pandemic, all meetings were held in-person. However, the group pivoted to virtual meetings and continues to utilize this format. The dedicated program coordinator is responsible for coordinating meetings and distributing meeting materials.
Each session is informal, flexible, and supportive. Attendance is not enforced, and mentees are allowed to join meetings as their schedules permit; however, program directors and program coordinator attend each meeting. In advance of each session, the program coordinator sends out a call for agenda items to all active members invited to discuss any research related items. Each mentee presents their ideas to lead the discussion for their portion of the meeting with no defined format required.
A variety of topics are covered including, but not limited to: (1) grant-specific concerns (eg, questions related to specific aim pages, grantsmanship, postsubmission comments from reviewers, or postaward logistics); (2) research procedures (eg, questions related to methodological practices or institutional review board concerns); (3) manuscript or presentation preparation; and (4) careerrelated issues. The program coordinator distributes handouts prior to meetings and mentees may record their presentations. These handouts may include, but are not limited to, specific aims pages, analytical plans, grant solicitations, and PowerPoint presentations. If a resource that can benefit the entire group is mentioned during the meeting, the program coordinator is responsible for distribution.
The program follows a group facilitated discussion format. Program directors facilitate each meeting, but input is encouraged from all attendees. This model allows for mentees to learn from the faculty mentors as well as peer mentees in a simultaneous and efficient fashion. Group discussions foster collective problem solving, peer support, and resource sharing that would not be possible through individualized mentorship. Participants have access to varied expertise during each session which reduces the need to seek specialized help elsewhere. Participants are also encouraged to contact the program directors or research staff for consultation as needed. Some one-on-one consultations have transitioned to a more sustained and ongoing mentorship relationship between a program director and mentee, but most are often brief email exchanges or a single meeting.

Participants
Since its inception in 2015, 35 clinicians have enrolled in the program. The mentees are equally distributed by sex and practice in a variety of disciplines including gastroenterology, hematology/oncology, cardiology, and general medicine (Table 1). Mentees have submitted 33 grant proposals addressing a variety of health care issues to a diverse group of federal and nonfederal funding agencies (Table 2). As of May 15, 2024, 19 (58%) of the submitted applications have been funded.

Many factors contribute to a successfully funded grant application, and several mentees report that participating in the mentorship program was helpful. For example, a mentee became the first lead investigator for a VA Cooperative Studies Program funded at VAAAHS. The VA Cooperative Studies Program, a division of the Office of Research and Development, plans and conducts large multicenter clinical trials and epidemiological studies within the VA via a vast network of clinician investigators, statisticians, and other key research experts.14
Several program mentees have also received VA Clinical Science Research and Development Career Development Awards. The VA Career Development program supports investigators during their early research careers with a goal of retaining talented researchers committed to improving the health and care of veterans.15
Survey Responses
Mentee productivity and updates are tracked through direct mentee input, as requested by the program coordinator. Since 2022, participants could complete an end-of-year survey based on an assessment tool used in a VAAAHS nonresearch mentorship program.16 The survey, distributed to mentees and program directors, requests feedback on logistics (eg, if the meeting was a good use of time and barriers to attendance); perceptions of effectiveness (eg, ability to discuss agenda items, helpfulness with setting and reaching research goals, and quality of mentors’ feedback); and the impact of the mentoring program on work satisfaction and clinician burnout. Respondents are also encouraged to leave open-ended qualitative feedback.
To date the survey has elicited 19 responses. Seventeen (89%) indicated that they agree or strongly agree the meetings were an effective use of their time and 11 (58%) indicated that they were able to discuss all or most of the items they wanted to during the meeting. Sixteen respondents (84%) agreed the program helped them set and achieve their research goals and 14 respondents (74%) agreed the feedback they received during the meeting was specific, actionable, and focused on how to improve their research agenda. Seventeen respondents (89%) agreed the program increased their work satisfaction, while 13 respondents (68%) felt the program reduced levels of clinician burnout.
As attendance was not mandatory, the survey asked participants how often they attended meetings during the past year. Responses were mixed: 4 (21%) respondents attended regularly (12 to 16 times per year) and 8 (42%) attended most sessions (8 to 11 times per year). Noted barriers to attendance included conflicts with patient care activities and conflicts with other high priority meetings.
Mentees also provided qualitive feedback regarding the program. They highlighted the supportive environment, valuable expertise of the mentors, and usefulness of obtaining tailored feedback from the group. “This group is an amazing resource to anyone developing a research career,” a mentee noted, adding that the program directors “fostered an incredibly supportive group where research ideas and methodology can be explored in a nonthreatening and creative environment.”
Conclusions
This mentorship program aims to help aspiring VA clinician investigators develop and submit competitive research grant applications. The addition of the program to the existing robust research environments at VAAAHS and its academic affiliate appears to have contributed to this success, with 58% of applications submitted by program mentees receiving funding.
In addition to funding success, we also found that most participants have a favorable impression of the program. Of the participants who responded to the program evaluation survey, nearly all indicated the program was an effective use of their time. The program also appeared to increase work satisfaction and reduce levels of clinician burnout. Barriers to attendance were also noted, with the most frequent being scheduling conflicts.
This program’s format includes facilitated group discussion as well as peer mentorship. This collaborative structure allows for an efficient and rich learning experience. Feedback from multiple perspectives encourages natural networking and relationship building. Incorporating the collective wisdom of the faculty mentors and peer mentees is beneficial; it not only empowers the mentees but also enriches the experience for the mentors. This program can serve as a model for other VA facilities—or non-VA academic medical centers—to enhance their research programs.
Supporting meaningful research that has a positive impact on the health and quality of life of veterans is a priority of the US Department of Veterans Affairs Office of Research and Development.1 For nearly a century, VA researchers have been conducting high quality studies. To continue this trajectory, it is imperative to attract, train, and retain exceptional investigators while nurturing their development throughout their careers.2
Mentorship is defined as guidance provided by an experienced and trusted party to another (usually junior) individual with the intent of helping the person succeed. It benefits the mentee, mentor, and their institutions.3 Mentorship is crucial for personal and professional development as well as productivity, which may help reduce clinician burnout.4-7 Conversely, a lack of mentorship could have negative effects on work satisfaction and stagnate career progression.8
Mentorship is vital for developing and advancing a VA investigator’s research agenda. Funding, grant writing, and research design were among the most discussed topics in a large comprehensive mentorship program for academic faculty.9 However, there are several known barriers to effective research mentorship; among them include a lack of resources, time constraints, and competing clinical priorities.10,11
Finding time for effective one-on-one research mentoring is difficult within the time constraints of clinical duties; a group mentorship model may help overcome this barrier. Group mentorship can aid in personal and professional development because no single mentor can effectively meet every mentoring need of an individual.12 Group mentorship also allows for the exchange of ideas among individuals with different backgrounds and the ability to utilize the strengths of each member of the group. For example, a member may have methodological expertise, while another may be skilled in grantsmanship. A team of mentors may be more beneficial for both the mentors (eg, establish a more manageable workload) and the mentee (eg, gains a broader perspective of expertise) when compared to having a single mentor.3
Peer mentorship within the group setting may also yield additional benefits. For example, having a supportive peer group may help reduce stress levels and burnout, while also improving overall well-being.3,13 Formal mentorship programs do not frequently discuss concerns such as work-life balance, so including peers as mentors may help fill this void.9 Peer mentorship has also been found to be beneficial in providing mentees with pooled resources and shared learning.12,13 This article describes the components, benefits, impacts, and challenges of a group research mentorship program for VA clinicians interested in conducting VA-relevant research.
Program Description
The VA Clinical Research Mentorship Program was initiated at the VA Ann Arbor Healthcare System (VAAAHS) in October 2015 by the Chief of Medicine to assist VA clinician investigators with developing and submitting VA clinical science and health services research grant applications. The program offers group and one-on-one consultation services through the expertise of 2 experienced investigators/faculty mentors who also serve as program directors, each of whom devote about 3 to 5 hours per month to activities associated with the mentorship program (eg, attending the meeting, reviewing materials sent by mentees, and one-on-one discussions with mentees).
The program also fostered peer-led mentorship. This encourages all attendees to provide feedback during group sessions and communication by mentees outside the group sessions. An experienced project manager serves as program coordinator and contributes about 4 hours per month for activities such as attending, scheduling, and sending reminders for each meeting, distributing handouts, reviewing materials, and answering mentee’s questions via email. A statistician and additional research staff (ie, an epidemiologist and research assistant) do not attend the recurring meetings, but are available for offline consultation as needed. The program runs on a 12-month cycle with regular meetings occurring twice monthly during the 9-month academic period. Resources to support the program, primarily program director(s) and project coordinator effort, are provided by the Chief of Medicine and through the VAAAHS affiliated VA Health Systems Research (formerly Health Services Research & Development) Center of Innovation.
Invitations for new mentees are sent annually. Mentees expressing interest in the program outside of its annual recruitment period are evaluated for inclusion on a rolling basis. Recruitment begins with the program coordinator sending email notifications to all VAAAHS Medicine Service faculty, section chiefs, and division chiefs at the VAAAHS academic affiliate. Recipients are encouraged to distribute the announcement to eligible applicants and refer them to the application materials for entry consideration into the program. The application consists of the applicant’s curriculum vitae and a 1-page summary that includes a description of their research area of interest, how it is relevant to the VA, in addition to an idea for a research study, its potential significance, and proposed methodology. Applicant materials are reviewed by the program coordinator and program directors. The applicants are evaluated using a simple scoring approach that focuses on the applicant’s research area and agenda, past research training, past research productivity, potential for obtaining VA funding, and whether they have sufficient research time.
Program eligibility initially required being a physician with ≥ 1/8 VA appointment from the Medicine Service. However, clinicians with clinical appointments from other VA services are also accepted for participation as needed. Applicants must have previous research experience and have a career goal to obtain external funding for conducting and publishing original research. Those who have previously served as a principal investigator on a funded VA grant proposal are not eligible as new applicants but can remain in the program as peer mentors. The number of annual applicants varies and ranges from 1 to 11; on average, about 90% of applicants receive invitations to join the program.
Sessions
The program holds recurring meetings twice monthly for 1 hour during the 9-month academic year. However, program directors are available year-round, and mentees are encouraged to communicate questions or concerns via email during nonacademic months. Prior to the COVID-19 pandemic, all meetings were held in-person. However, the group pivoted to virtual meetings and continues to utilize this format. The dedicated program coordinator is responsible for coordinating meetings and distributing meeting materials.
Each session is informal, flexible, and supportive. Attendance is not enforced, and mentees are allowed to join meetings as their schedules permit; however, program directors and program coordinator attend each meeting. In advance of each session, the program coordinator sends out a call for agenda items to all active members invited to discuss any research related items. Each mentee presents their ideas to lead the discussion for their portion of the meeting with no defined format required.
A variety of topics are covered including, but not limited to: (1) grant-specific concerns (eg, questions related to specific aim pages, grantsmanship, postsubmission comments from reviewers, or postaward logistics); (2) research procedures (eg, questions related to methodological practices or institutional review board concerns); (3) manuscript or presentation preparation; and (4) careerrelated issues. The program coordinator distributes handouts prior to meetings and mentees may record their presentations. These handouts may include, but are not limited to, specific aims pages, analytical plans, grant solicitations, and PowerPoint presentations. If a resource that can benefit the entire group is mentioned during the meeting, the program coordinator is responsible for distribution.
The program follows a group facilitated discussion format. Program directors facilitate each meeting, but input is encouraged from all attendees. This model allows for mentees to learn from the faculty mentors as well as peer mentees in a simultaneous and efficient fashion. Group discussions foster collective problem solving, peer support, and resource sharing that would not be possible through individualized mentorship. Participants have access to varied expertise during each session which reduces the need to seek specialized help elsewhere. Participants are also encouraged to contact the program directors or research staff for consultation as needed. Some one-on-one consultations have transitioned to a more sustained and ongoing mentorship relationship between a program director and mentee, but most are often brief email exchanges or a single meeting.

Participants
Since its inception in 2015, 35 clinicians have enrolled in the program. The mentees are equally distributed by sex and practice in a variety of disciplines including gastroenterology, hematology/oncology, cardiology, and general medicine (Table 1). Mentees have submitted 33 grant proposals addressing a variety of health care issues to a diverse group of federal and nonfederal funding agencies (Table 2). As of May 15, 2024, 19 (58%) of the submitted applications have been funded.

Many factors contribute to a successfully funded grant application, and several mentees report that participating in the mentorship program was helpful. For example, a mentee became the first lead investigator for a VA Cooperative Studies Program funded at VAAAHS. The VA Cooperative Studies Program, a division of the Office of Research and Development, plans and conducts large multicenter clinical trials and epidemiological studies within the VA via a vast network of clinician investigators, statisticians, and other key research experts.14
Several program mentees have also received VA Clinical Science Research and Development Career Development Awards. The VA Career Development program supports investigators during their early research careers with a goal of retaining talented researchers committed to improving the health and care of veterans.15
Survey Responses
Mentee productivity and updates are tracked through direct mentee input, as requested by the program coordinator. Since 2022, participants could complete an end-of-year survey based on an assessment tool used in a VAAAHS nonresearch mentorship program.16 The survey, distributed to mentees and program directors, requests feedback on logistics (eg, if the meeting was a good use of time and barriers to attendance); perceptions of effectiveness (eg, ability to discuss agenda items, helpfulness with setting and reaching research goals, and quality of mentors’ feedback); and the impact of the mentoring program on work satisfaction and clinician burnout. Respondents are also encouraged to leave open-ended qualitative feedback.
To date the survey has elicited 19 responses. Seventeen (89%) indicated that they agree or strongly agree the meetings were an effective use of their time and 11 (58%) indicated that they were able to discuss all or most of the items they wanted to during the meeting. Sixteen respondents (84%) agreed the program helped them set and achieve their research goals and 14 respondents (74%) agreed the feedback they received during the meeting was specific, actionable, and focused on how to improve their research agenda. Seventeen respondents (89%) agreed the program increased their work satisfaction, while 13 respondents (68%) felt the program reduced levels of clinician burnout.
As attendance was not mandatory, the survey asked participants how often they attended meetings during the past year. Responses were mixed: 4 (21%) respondents attended regularly (12 to 16 times per year) and 8 (42%) attended most sessions (8 to 11 times per year). Noted barriers to attendance included conflicts with patient care activities and conflicts with other high priority meetings.
Mentees also provided qualitive feedback regarding the program. They highlighted the supportive environment, valuable expertise of the mentors, and usefulness of obtaining tailored feedback from the group. “This group is an amazing resource to anyone developing a research career,” a mentee noted, adding that the program directors “fostered an incredibly supportive group where research ideas and methodology can be explored in a nonthreatening and creative environment.”
Conclusions
This mentorship program aims to help aspiring VA clinician investigators develop and submit competitive research grant applications. The addition of the program to the existing robust research environments at VAAAHS and its academic affiliate appears to have contributed to this success, with 58% of applications submitted by program mentees receiving funding.
In addition to funding success, we also found that most participants have a favorable impression of the program. Of the participants who responded to the program evaluation survey, nearly all indicated the program was an effective use of their time. The program also appeared to increase work satisfaction and reduce levels of clinician burnout. Barriers to attendance were also noted, with the most frequent being scheduling conflicts.
This program’s format includes facilitated group discussion as well as peer mentorship. This collaborative structure allows for an efficient and rich learning experience. Feedback from multiple perspectives encourages natural networking and relationship building. Incorporating the collective wisdom of the faculty mentors and peer mentees is beneficial; it not only empowers the mentees but also enriches the experience for the mentors. This program can serve as a model for other VA facilities—or non-VA academic medical centers—to enhance their research programs.
- US Department of Veterans Affairs, Office of Research and Development. Strategic priorities for VA research. Published March 10, 2021. Accessed September 17, 2024. https://www.research.va.gov/about/strategic_priorities.cfm
- US Department of Veterans Affairs, Office of Research and Development. About the Office of Research & Development. Published November 11, 2023. Accessed September 17, 2024. https://www.research.va.gov/about/default.cfm
- Chopra V, Vaughn V, Saint S. The Mentoring Guide: Helping Mentors and Mentees Succeed. Michigan Publishing Services; 2019.
- Gilster SD, Accorinti KL. Mentoring program yields staff satisfaction. Mentoring through the exchange of information across all organizational levels can help administrators retain valuable staff. Provider. 1999;25(10):99-100.
- Ramanan RA, Phillips RS, Davis RB, Silen W, Reede JY. Mentoring in medicine: keys to satisfaction. Am J Med. 2002;112(4):336-341. doi:10.1016/s0002-9343(02)01032-x
- Sambunjak D, Straus SE, Marusi' A. Mentoring in academic medicine: a systematic review. JAMA. 2006;296(9):1103-1115. doi:10.1001/jama.296.9.1103
- Sambunjak D, Straus SE, Marusi' A. A systematic review of qualitative research on the meaning and characteristics of mentoring in academic medicine. J Gen Intern Med. 2010;25(1):72-78. doi:10.1007/s11606-009-1165-8
- Jackson VA, Palepu A, Szalacha L, Caswell C, Carr PL, Inui T. “Having the right chemistry”: a qualitative study of mentoring in academic medicine. Acad Med. 2003;78(3):328-334. doi:10.1097/00001888-200303000-00020
- Feldman MD, Arean PA, Marshall SJ, Lovett M, O’Sullivan P. Does mentoring matter: results from a survey of faculty mentees at a large health sciences university. Med Educ Online. 2010;15:10.3402/meo.v15i0.5063. doi:10.3402/meo.v15i0.5063
- Leary JC, Schainker EG, Leyenaar JK. The unwritten rules of mentorship: facilitators of and barriers to effective mentorship in pediatric hospital medicine. Hosp Pediatr. 2016;6(4):219-225. doi:10.1542/hpeds.2015-0108
- Rustgi AK, Hecht GA. Mentorship in academic medicine. Gastroenterology. 2011;141(3):789-792. doi:10.1053/j.gastro.2011.07.024
- DeCastro R, Sambuco D, Ubel PA, Stewart A, Jagsi R. Mentor networks in academic medicine: moving beyond a dyadic conception of mentoring for junior faculty researchers. Acad Med. 2013;88(4):488-496. doi:10.1097/ACM.0b013e318285d302
- McDaugall M, Beattie RS. Peer mentoring at work: the nature and outcomes of non-hierarchical developmental relationships. Management Learning. 2016;28(4):423-437. doi:10.1177/1350507697284003
- US Department of Veterans Affairs, Office of Rsearch and Development. VA Cooperative Studies Program (CSP). Updated July 2019. Accessed September 17, 2024. https://www.vacsp.research.va.gov
- US Department of Veterans Affairs, Office of Research and Development. Career development program for biomedical laboratory and clinical science R&D services. Published April 17, 2023. Accessed September 17, 2024. https://www.research.va.gov/services/shared_docs/career_dev.cfm
- Houchens N, Kuhn L, Ratz D, Su G, Saint S. Committed to success: a structured mentoring program for clinically-oriented physicians. Mayo Clin Pro Innov Qual Outcomes. 2024;8(4):356-363. doi:10.1016/j.mayocpiqo.2024.05.002
- US Department of Veterans Affairs, Office of Research and Development. Strategic priorities for VA research. Published March 10, 2021. Accessed September 17, 2024. https://www.research.va.gov/about/strategic_priorities.cfm
- US Department of Veterans Affairs, Office of Research and Development. About the Office of Research & Development. Published November 11, 2023. Accessed September 17, 2024. https://www.research.va.gov/about/default.cfm
- Chopra V, Vaughn V, Saint S. The Mentoring Guide: Helping Mentors and Mentees Succeed. Michigan Publishing Services; 2019.
- Gilster SD, Accorinti KL. Mentoring program yields staff satisfaction. Mentoring through the exchange of information across all organizational levels can help administrators retain valuable staff. Provider. 1999;25(10):99-100.
- Ramanan RA, Phillips RS, Davis RB, Silen W, Reede JY. Mentoring in medicine: keys to satisfaction. Am J Med. 2002;112(4):336-341. doi:10.1016/s0002-9343(02)01032-x
- Sambunjak D, Straus SE, Marusi' A. Mentoring in academic medicine: a systematic review. JAMA. 2006;296(9):1103-1115. doi:10.1001/jama.296.9.1103
- Sambunjak D, Straus SE, Marusi' A. A systematic review of qualitative research on the meaning and characteristics of mentoring in academic medicine. J Gen Intern Med. 2010;25(1):72-78. doi:10.1007/s11606-009-1165-8
- Jackson VA, Palepu A, Szalacha L, Caswell C, Carr PL, Inui T. “Having the right chemistry”: a qualitative study of mentoring in academic medicine. Acad Med. 2003;78(3):328-334. doi:10.1097/00001888-200303000-00020
- Feldman MD, Arean PA, Marshall SJ, Lovett M, O’Sullivan P. Does mentoring matter: results from a survey of faculty mentees at a large health sciences university. Med Educ Online. 2010;15:10.3402/meo.v15i0.5063. doi:10.3402/meo.v15i0.5063
- Leary JC, Schainker EG, Leyenaar JK. The unwritten rules of mentorship: facilitators of and barriers to effective mentorship in pediatric hospital medicine. Hosp Pediatr. 2016;6(4):219-225. doi:10.1542/hpeds.2015-0108
- Rustgi AK, Hecht GA. Mentorship in academic medicine. Gastroenterology. 2011;141(3):789-792. doi:10.1053/j.gastro.2011.07.024
- DeCastro R, Sambuco D, Ubel PA, Stewart A, Jagsi R. Mentor networks in academic medicine: moving beyond a dyadic conception of mentoring for junior faculty researchers. Acad Med. 2013;88(4):488-496. doi:10.1097/ACM.0b013e318285d302
- McDaugall M, Beattie RS. Peer mentoring at work: the nature and outcomes of non-hierarchical developmental relationships. Management Learning. 2016;28(4):423-437. doi:10.1177/1350507697284003
- US Department of Veterans Affairs, Office of Rsearch and Development. VA Cooperative Studies Program (CSP). Updated July 2019. Accessed September 17, 2024. https://www.vacsp.research.va.gov
- US Department of Veterans Affairs, Office of Research and Development. Career development program for biomedical laboratory and clinical science R&D services. Published April 17, 2023. Accessed September 17, 2024. https://www.research.va.gov/services/shared_docs/career_dev.cfm
- Houchens N, Kuhn L, Ratz D, Su G, Saint S. Committed to success: a structured mentoring program for clinically-oriented physicians. Mayo Clin Pro Innov Qual Outcomes. 2024;8(4):356-363. doi:10.1016/j.mayocpiqo.2024.05.002
A Group Approach to Clinical Research Mentorship at a Veterans Affairs Medical Center
A Group Approach to Clinical Research Mentorship at a Veterans Affairs Medical Center
Satisfaction With Department of Veterans Affairs Prosthetics and Support Services as Reported by Women and Men Veterans
Satisfaction With Department of Veterans Affairs Prosthetics and Support Services as Reported by Women and Men Veterans
Limb loss is a significant and growing concern in the United States. Nearly 2 million Americans are living with limb loss, and up to 185,000 people undergo amputations annually.1-4 Of these patients, about 35% are women.5 The Veterans Health Administration (VHA) provides about 10% of US amputations.6-8 Between 2015 and 2019, the number of prosthetic devices provided to female veterans increased from 3.3 million to 4.6 million.5,9,10
Previous research identified disparities in prosthetic care between men and women, both within and outside the VHA. These disparities include slower prosthesis prescription and receipt among women, in addition to differences in self-reported mobility, satisfaction, rates of prosthesis rejection, and challenges related to prosthesis appearance and fit.5,10,11 Recent studies suggest women tend to have worse outcomes following amputation, and are underrepresented in amputation research.12,13 However, these disparities are poorly described in a large, national sample. Because women represent a growing portion of patients with limb loss in the VHA, understanding their needs is critical.14
The Johnny Isakson and David P. Roe, MD Veterans Health Care and Benefits Improvement Act of 2020 was enacted, in part, to improve the care provided to women veterans.15 The law required the VHA to conduct a survey of ≥ 50,000 veterans to assess the satisfaction of women veterans with prostheses provided by the VHA. To comply with this legislation and understand how women veterans rate their prostheses and related care in the VHA, the US Department of Veterans Affairs (VA) Center for Collaborative Evaluation (VACE) conducted a large national survey of veterans with limb loss that oversampled women veterans. This article describes the survey results, including characteristics of female veterans with limb loss receiving care from the VHA, assesses their satisfaction with prostheses and prosthetic care, and highlights where their responses differ from those of male veterans.
Methods
We conducted a cross-sectional, mixedmode survey of eligible amputees in the VHA Support Service Capital Assets Amputee Data Cube. We identified a cohort of veterans with any major amputation (above the ankle or wrist) or partial hand or foot amputation who received VHA care between October 1, 2019, and September 30, 2020. The final cohort yielded 46,646 potentially eligible veterans. Thirty-three had invalid contact information, leaving 46,613 veterans who were asked to participate, including 1356 women.
Survey
We created a survey instrument de novo that included questions from validated instruments, including the Trinity Amputation Prosthesis and Experience Scales to assess prosthetic device satisfaction, the Prosthesis Evaluation Questionnaire to assess quality of life (QOL) satisfaction, and the Orthotics Prosthetics Users Survey to assess prosthesis-related care satisfaction. 16-18 Additional questions were incorporated from a survey of veterans with upper limb amputation to assess the importance of cosmetic considerations related to the prosthesis and comfort with prosthesis use in intimate relationships.19 Questions were also included to assess amputation type, year of amputation, if a prosthesis was currently used, reasons for ceasing use of a prosthesis, reasons for never using a prosthesis, the types of prostheses used, intensity of prosthesis use, satisfaction with time required to receive a prosthetic limb, and if the prosthesis reflected the veteran’s selfidentified gender. Veterans were asked to answer questions based on their most recent amputation.
We tested the survey using cognitive interviews with 6 veterans to refine the survey and better understand how veterans interpreted the questions. Pilot testers completed the survey and participated in individual interviews with experienced interviewers (CL and RRK) to describe how they selected their responses.20 This feedback was used to refine the survey. The online survey was programmed using Qualtrics Software and manually translated into Spanish.
Given the multimodal design, surveys were distributed by email, text message, and US Postal Service (USPS). Surveys were emailed to all veterans for whom a valid email address was available. If emails were undeliverable, veterans were contacted via text message or the USPS. Surveys were distributed by text message to all veterans without an email address but with a cellphone number. We were unable to consistently identify invalid numbers among all text message recipients. Invitations with a survey URL and QR code were sent via USPS to veterans who had no valid email address or cellphone number. Targeted efforts were made to increase the response rate for women. A random sample of 200 women who had not completed the survey 2 weeks prior to the closing date (15% of women in sample) was selected to receive personal phone calls. Another random sample of 400 women was selected to receive personalized outreach emails. The survey data were confidential, and responses could not be traced to identifying information.
Data Analyses
We conducted a descriptive analysis, including percentages and means for responses to variables focused on describing amputation characteristics, prosthesis characteristics, and QOL. All data, including missing values, were used to document the percentage of respondents for each question. Removing missing data from the denominator when calculating percentages could introduce bias to the analysis because we cannot be certain data are missing at random. Missing variables were removed to avoid underinflation of mean scores.
We compared responses across 2 groups: individuals who self-identified as men and individuals who self-identified as women. For each question, we assessed whether each of these groups differed significantly from the remaining sample. For example, we examined whether the percentage of men who answered affirmatively to a question was significantly higher or lower than that of individuals not identifying as male, and whether the percentage of women who answered affirmatively was significantly higher or lower than that of individuals not identifying as female. We utilized x2 tests to determine significant differences for percentage calculations and t tests to determine significant differences in means across gender.
Since conducting multiple comparisons within a dataset may result in inflating statistical significance (type 1 errors), we used a more conservative estimate of statistical significance (α = 0.01) and high significance (α = 0.001). This study was deemed quality improvement by the VHA Rehabilitation and Prosthetic Services (12RPS) and acknowledged by the VA Research Office at Eastern Colorado Health Care System and was not subject to institutional review board review.
Results
Surveys were distributed to 46,613 veterans and were completed by 4981 respondents for a 10.7% overall response rate. Survey respondents were generally similar to the eligible population invited to participate, but the proportion of women who completed the survey was higher than the proportion of women eligible to participate (2.0% of eligible population vs 16.7% of respondents), likely due to specific efforts to target women. Survey respondents were slightly younger than the general population (67.3 years vs 68.7 years), less likely to be male (97.1% vs 83.3%), showed similar representation of Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) veterans (4.4% vs 4.1%), and were less likely to have diabetes (58.0% vs 52.7% had diabetes) (Table 1).
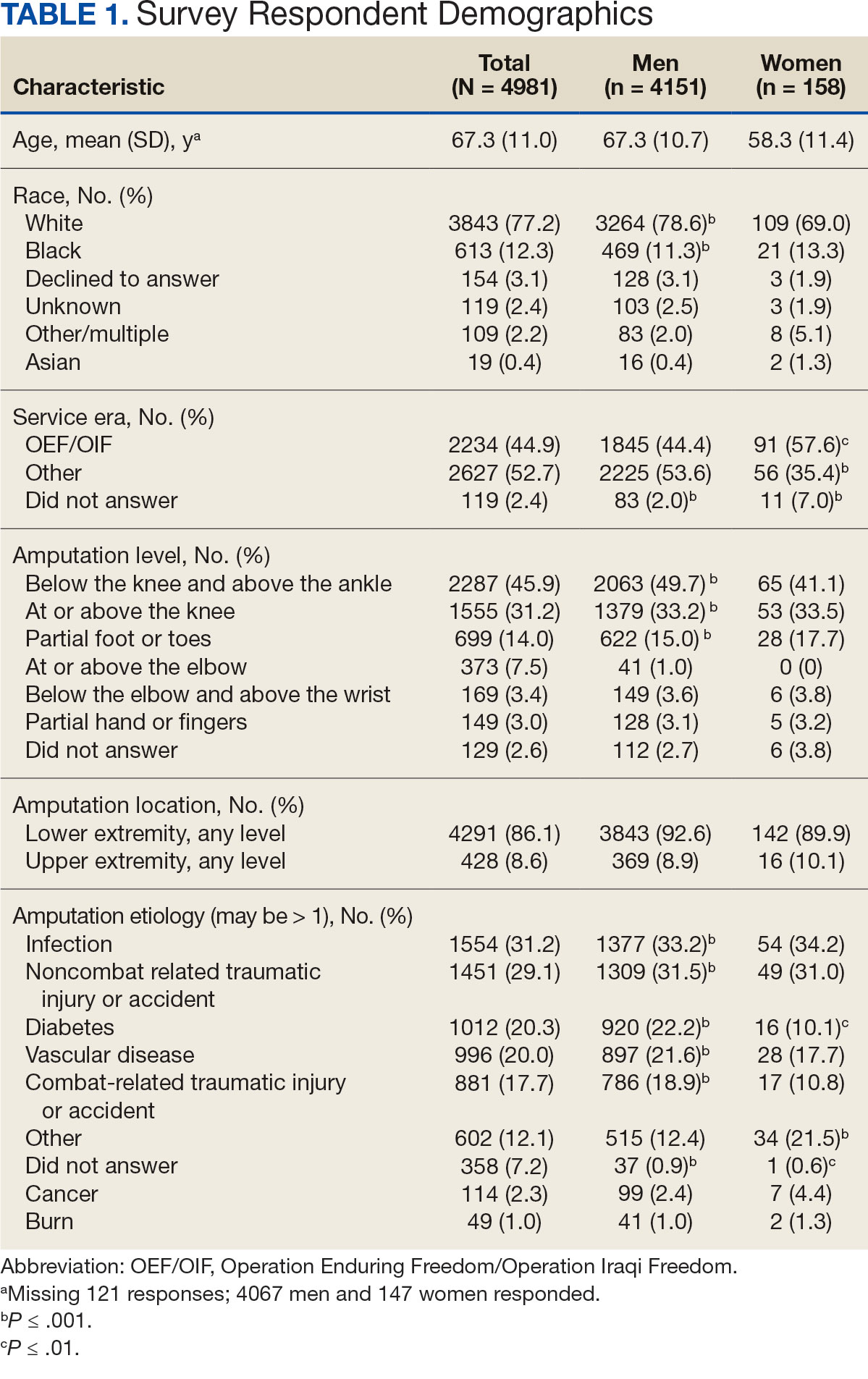
The mean age of male respondents was 67.3 years, while the mean age of female respondents was 58.3 years. The majority of respondents were male (83.3%) and White (77.2%). Female respondents were less likely to have diabetes (35.4% of women vs 53.5% of men) and less likely to report that their most recent amputation resulted from diabetes (10.1% of women vs 22.2% of men). Women respondents were more likely to report an amputation due to other causes, such as adverse results of surgery, neurologic disease, suicide attempt, blood clots, tumors, rheumatoid arthritis, and revisions of previous amputations. Most women respondents did not serve during the OEF or OIF eras. The most common amputation site for women respondents was lower limb, either below the knee and above the ankle or above the knee.
Most participants use an everyday prosthesis, but women were more likely to report using a sports-specific prosthesis (Table 2). Overall, most respondents report using a prosthesis (87.7%); however, women were more likely to report not using a prosthesis (19.4% of women vs 11.1% of men; P ≤ .01). Additionally, a lower proportion of women report using a prosthesis for < 12 hours per day (30.6% of women vs 46.4% of men; P ≤ .01) or using a prosthesis every day (54.8% of women vs 74.6% of men; P ≤ .001).
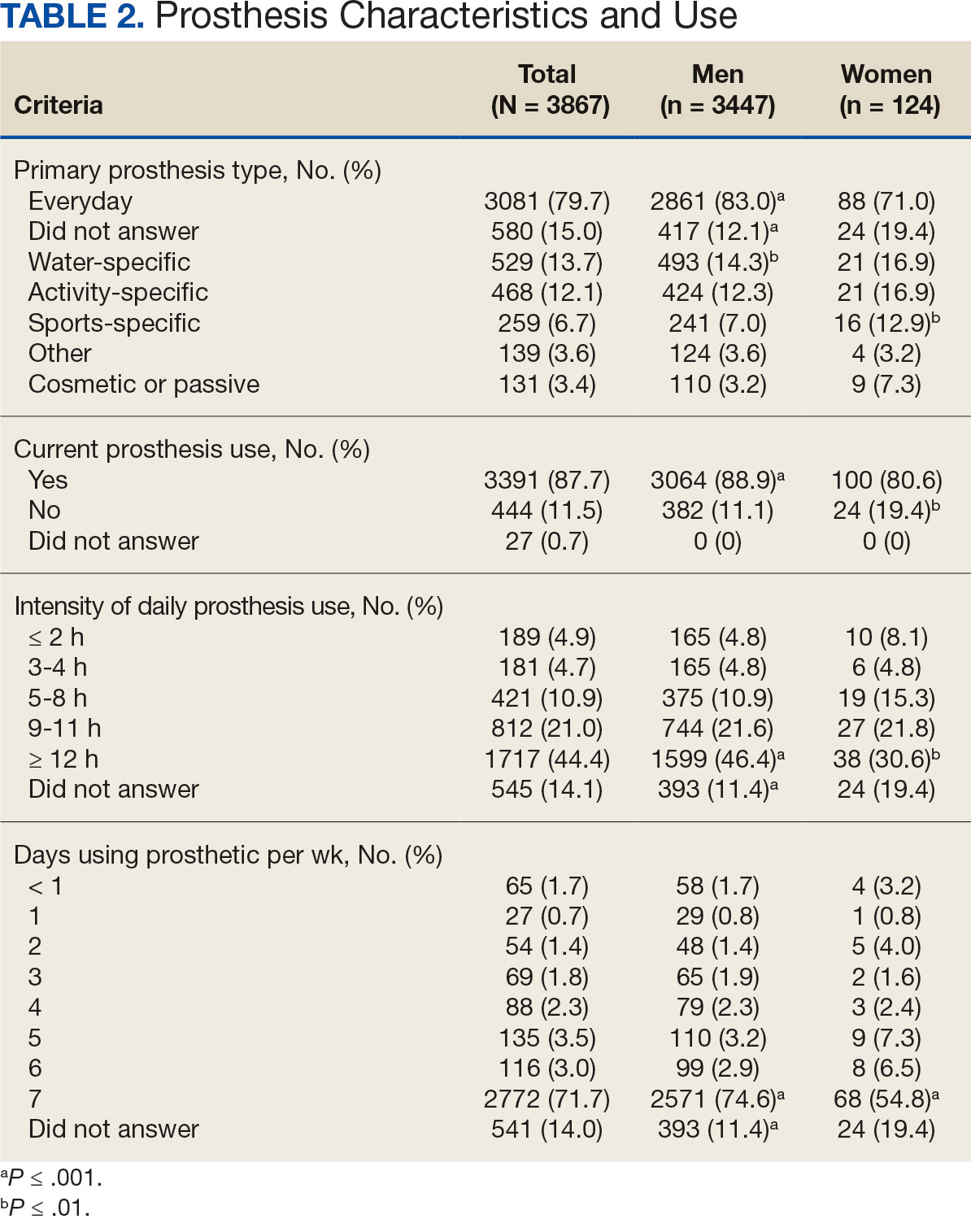
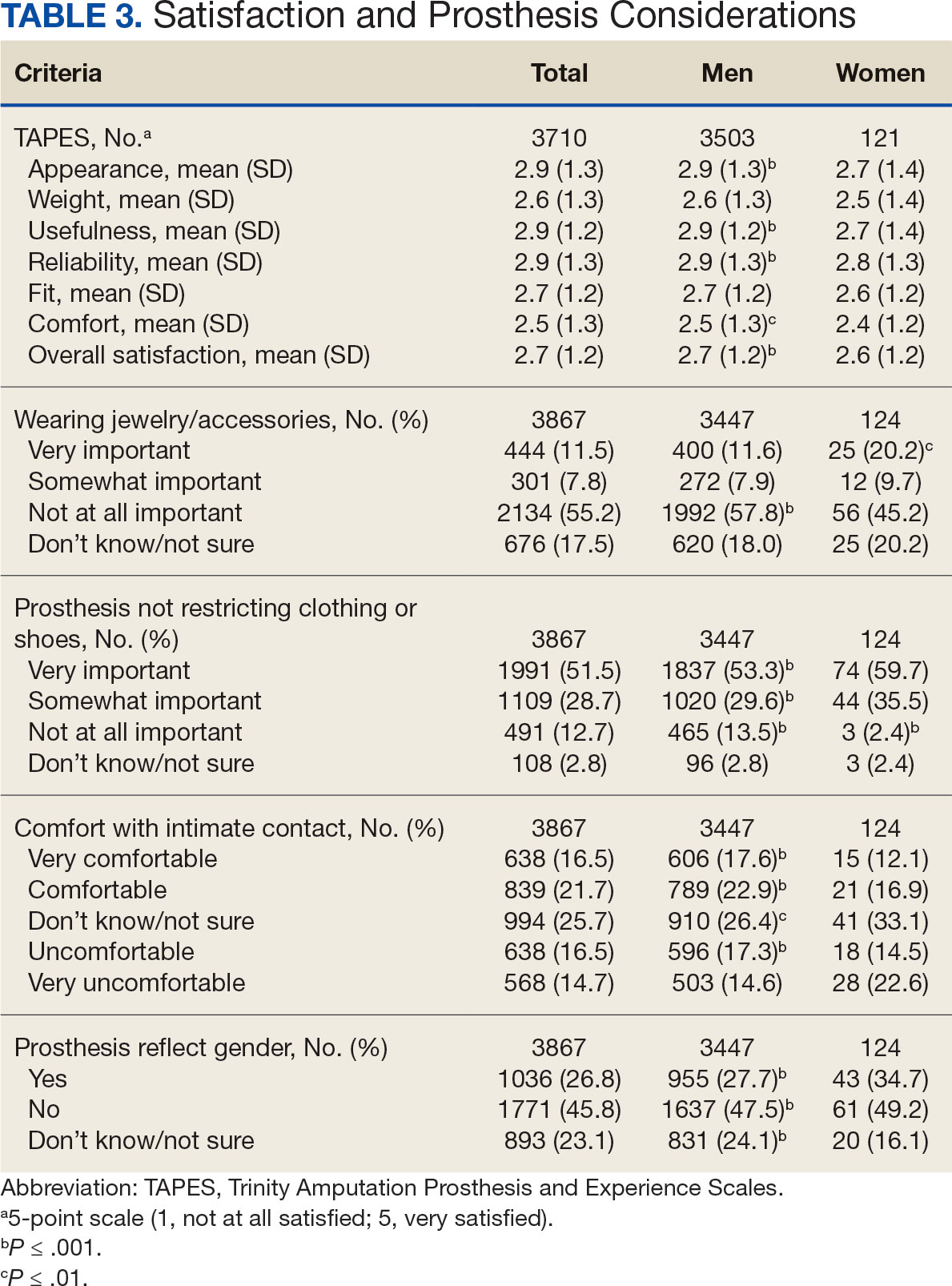
In the overall sample, the mean satisfaction score with a prosthesis was 2.7 on a 5-point scale, and women had slightly lower overall satisfaction scores (2.6 for women vs 2.7 for men; P ≤ .001) (Table 3). Women also had lower satisfaction scores related to appearance, usefulness, reliability, and comfort. Women were more likely to indicate that it was very important to be able to wear jewelry and accessories (20.2% of women vs 11.6% of men; P ≤ .01), while men were less likely to indicate that it was somewhat or very important that the prosthesis not restrict clothing or shoes (95.2% of women vs 82.9% of men; P ≤ .001). Men were more likely than women to report being comfortable or very comfortable using their prosthesis in intimate contact: 40.5% vs 29.0%, respectively (P ≤ .001).
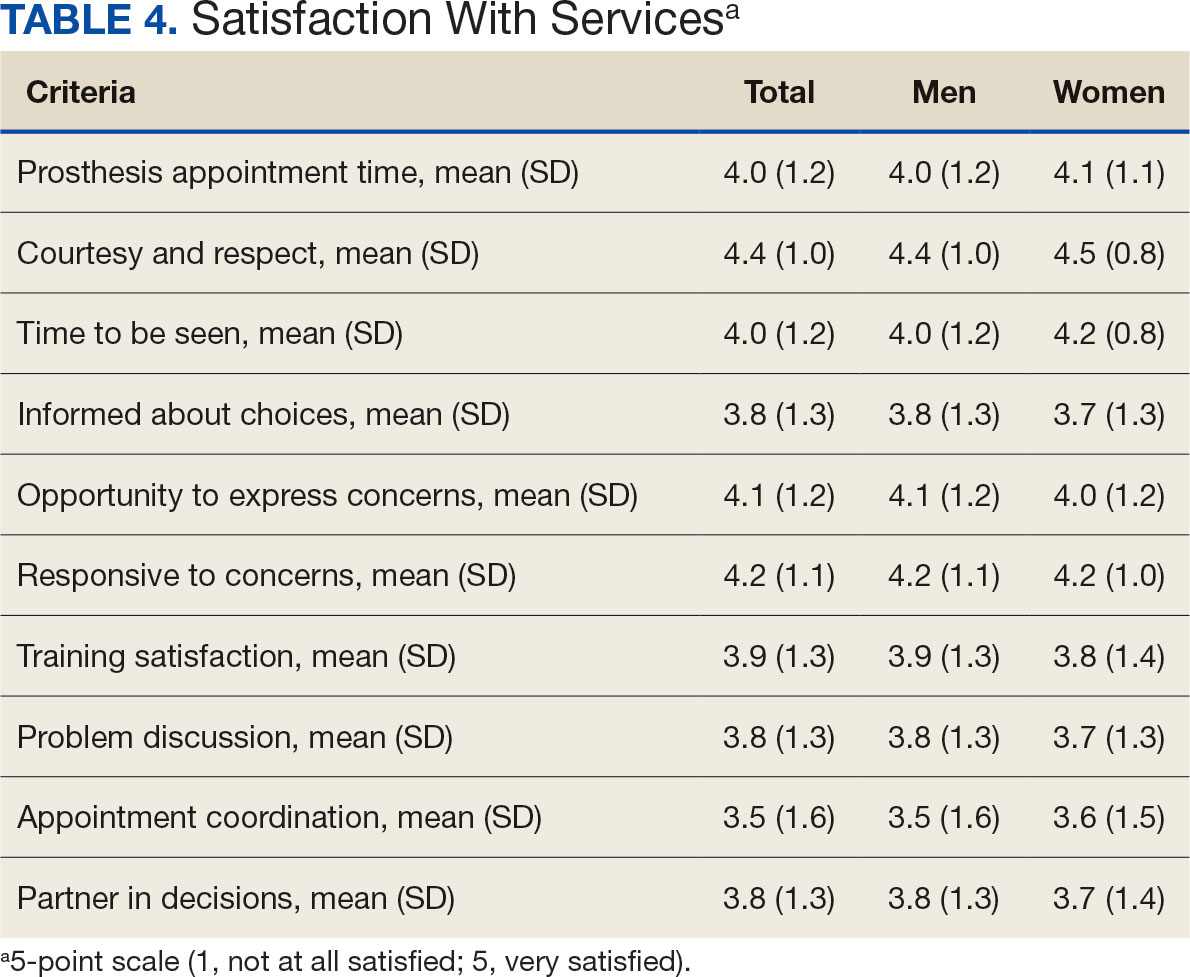
Overall, participants reported high satisfaction with appointment times, wait times, courteous treatment, opportunities to express concerns, and staff responsiveness. Men were slightly more likely than women to be satisfied with training (P ≤ 0.001) and problem discussion (P ≤ 0.01) (Table 4). There were no statistically significant differences in satisfaction or QOL ratings between women and men. The overall sample rated both QOL and satisfaction with QOL 6.7 on a 10-point scale.
Discussion
The goal of this study was to characterize the experience of veterans with limb loss receiving care in the VHA and assess their satisfaction with prostheses and prosthetic care. We received responses from nearly 5000 veterans, 158 of whom were women. Women veteran respondents were slightly younger and less likely to have an amputation due to diabetes. We did not observe significant differences in amputation level between men and women but women were less likely to use a prosthesis, reported lower intensity of prosthesis use, and were less satisfied with certain aspects of their prostheses. Women may also be less satisfied with prosthesis training and problem discussion. However, we found no differences in QOL ratings between men and women.
Findings indicating women were more likely to report not using a prosthesis and that a lower proportion of women report using a prosthesis for > 12 hours a day or every day are consistent with previous research. 21,22 Interestingly, women were more likely to report using a sports-specific prosthesis. This is notable because prior research suggests that individuals with amputations may avoid participating in sports and exercise, and a lack of access to sports-specific prostheses may inhibit physical activity.23,24 Women in this sample were slightly less satisfied with their prostheses overall and reported lower satisfaction scores regarding appearance, usefulness, reliability, and comfort, consistent with previous findings.25
A lower percentage of women in this sample reported being comfortable or very comfortable using their prosthesis during intimate contact. Previous research on prosthesis satisfaction suggests individuals who rate prosthesis satisfaction lower also report lower body image across genders. 26 While women in this sample did not rate their prosthesis satisfaction lower than men, they did report lower intensity of prosthesis use, suggesting potential issues with their prostheses this survey did not evaluate. Women indicated the importance of prostheses not restricting jewelry, accessories, clothing, or shoes. These results have significant clinical and social implications. A recent qualitative study emphasizes that women veterans feel prostheses are primarily designed for men and may not work well with their physiological needs.9 Research focused on limbs better suited to women’s bodies could result in better fitting sockets, lightweight limbs, or less bulky designs. Additional research has also explored the difficulties in accommodating a range of footwear for patients with lower limb amputation. One study found that varying footwear heights affect the function of adjustable prosthetic feet in ways that may not be optimal.27
Ratings of satisfaction with prosthesisrelated services between men and women in this sample are consistent with a recent study showing that women veterans do not have significant differences in satisfaction with prosthesis-related services.28 However, this study focused specifically on lower limb amputations, while the respondents of this study include those with both upper and lower limb amputations. Importantly, our findings that women are less likely to be satisfied with prosthesis training and problem discussions support recent qualitative findings in which women expressed a desire to work with prosthetists who listen to them, take their concerns seriously, and seek solutions that fit their needs. We did not observe a difference in QOL ratings between men and women in the sample despite lower satisfaction among women with some elements of prosthesis-related services. Previous research suggests many factors impact QOL after amputation, most notably time since amputation.16,29
Limitations
This survey was deployed in a short timeline that did not allow for careful sample selection or implementing strategies to increase response rate. Additionally, the study was conducted among veterans receiving care in the VHA, and findings may not be generalizable to limb loss in other settings. Finally, the discrepancy in number of respondents who identified as men vs women made it difficult to compare differences between the 2 groups.
Conclusions
This is the largest sample of survey respondents of veterans with limb loss to date. While the findings suggest veterans are generally satisfied with prosthetic-related services overall, they also highlight several areas for improvement with services or prostheses. Given that most veterans with limb loss are men, there is a significant discrepancy between the number of women and men respondents. Additional studies with more comparable numbers of men and women have found similar ratings of satisfaction with prostheses and services.28 Further research specifically focused on improving the experiences of women should focus on better characterizing their experiences and identifying how they differ from those of male veterans. For example, understanding how to engage female veterans with limb loss in prosthesis training and problem discussions may improve their experience with their care teams and improve their use of prostheses. Understanding experiences and needs that are specific to women could lead to the development of processes, resources, or devices that are tailored to the unique requirements of women with limb loss.
- Ziegler-Graham K, MacKenzie EJ, Ephraim PL, Travison TG, Brookmeyer R. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil. 2008;89(3):422-429. doi:10.1016/j.apmr.2007.11.005
- Dillingham TR, Pezzin LE, MacKenzie EJ. Limb amputation and limb deficiency: epidemiology and recent trends in the united states. South Med J. 2002;95(8):875-883. doi:10.1097/00007611-200208000-00018
- Dillingham TR, Pezzin LE, Shore AD. Reamputation, mortality, and health care costs among persons with dysvascular lower-limb amputations. Arch Phys Med Rehabil. 2005;86(3):480-486. doi:10.1016/j.apmr.2004.06.072
- Centers for Disease Control and Prevention. Ambulatory and inpatient procedures in the United States. Accessed September 30, 2024. https://www.cdc.gov/nchs/pressroom/98facts/ambulat.htm
- Ljung J, Iacangelo A. Identifying and acknowledging a sex gap in lower-limb prosthetics. JPO. 2024;36(1):e18-e24. doi:10.1097/JPO.0000000000000470
- Feinglass J, Brown JL, LoSasso A, et al. Rates of lower-extremity amputation and arterial reconstruction in the united states, 1979 to 1996. Am J Public Health. 1999;89(8):1222- 1227. doi:10.2105/ajph.89.8.1222
- Mayfield JA, Reiber GE, Maynard C, Czerniecki JM, Caps MT, Sangeorzan BJ. Trends in lower limb amputation in the Veterans Health Administration, 1989-1998. J Rehabil Res Dev. 2000;37(1):23-30.
- Feinglass J, Pearce WH, Martin GJ, et al. Postoperative and late survival outcomes after major amputation: findings from the department of veterans affairs national surgical quality improvement program. Surgery. 2001;130(1):21-29. doi:10.1067/msy.2001.115359
- Lehavot K, Young JP, Thomas RM, et al. Voices of women veterans with lower limb prostheses: a qualitative study. J Gen Intern Med. 2022;37(3):799-805. doi:10.1007/s11606-022-07572-8
- US Government Accountability Office. COVID-19: Opportunities to improve federal response. GAO-21-60. Published November 12, 2020. Accessed September 30, 2024. https://www.gao.gov/products/gao-21-60
- Littman AJ, Peterson AC, Korpak A, et al. Differences in prosthetic prescription between men and women veterans after transtibial or transfemoral lowerextremity amputation: a longitudinal cohort study. Arch Phys Med Rehabil. 2023;104(8)1274-1281. doi:10.1016/j.amjsurg.2023.02.011
- Cimino SR, Vijayakumar A, MacKay C, Mayo AL, Hitzig SL, Guilcher SJT. Sex and gender differences in quality of life and related domains for individuals with adult acquired lower-limb amputation: a scoping review. Disabil Rehabil. 2022 Oct 23;44(22):6899-6925. doi:10.1080/09638288.2021.1974106
- DadeMatthews OO, Roper JA, Vazquez A, Shannon DM, Sefton JM. Prosthetic device and service satisfaction, quality of life, and functional performance in lower limb prosthesis clients. Prosthet Orthot Int. 2024;48(4):422-430. doi:10.1097/PXR.0000000000000285
- Hamilton AB, Schwarz EB, Thomas HN, Goldstein KM. Moving women veterans’ health research forward: a special supplement. J Gen Intern Med. 2022;37(Suppl3):665– 667. doi:10.1007/s11606-022-07606-1
- US Congress. Public Law 116-315: An Act to Improve the Lives of Veterans, S 5108 (2) (F). 116th Congress; 2021. Accessed September 30, 2024. https://www.congress.gov/116/plaws/publ315/PLAW-116publ315.pdf
- Gallagher P, MacLachlan M. The Trinity amputation and prosthesis experience scales and quality of life in people with lower-limb amputation. Arch Phys Med Rehabil. 2004;85(5):730-736. doi:10.1016/j.apmr.2003.07.009
- Legro MW, Reiber GD, Smith DG, del Aguila M, Larsen J, Boone D. Prosthesis evaluation questionnaire for persons with lower limb amputations: assessing prosthesis-related quality of life. Arch Phys Med Rehabil. 1998;79(8):931-938. doi:10.1016/s0003-9993(98)90090-9
- Legro MW, Reiber GD, Smith DG, del Aguila M, Larsen J, Boone D. Prosthesis evaluation questionnaire for persons with lower limb amputations: assessing prosthesis-related quality of life. Arch Phys Med Rehabil. 1998;79(8):931-938. doi:10.1016/s0003-9993(98)90090-9
- Heinemann AW, Bode RK, O’Reilly C. Development and measurement properties of the orthotics and prosthetics users’ survey (OPUS): a comprehensive set of clinical outcome instruments. Prosthet Orthot Int. 2003;27(3):191-206. doi:10.1080/03093640308726682
- Resnik LJ, Borgia ML, Clark MA. A national survey of prosthesis use in veterans with major upper limb amputation: comparisons by gender. PM R. 2020;12(11):1086-1098. doi:10.1002/pmrj.12351
- Collins D. Pretesting survey instruments: an overview of cognitive methods. Qual Life Res. 2003;12(3):229-238. doi:10.1023/a:1023254226592
- Østlie K, Lesjø IM, Franklin RJ, Garfelt B, Skjeldal OH, Magnus P. Prosthesis rejection in acquired major upper-limb amputees: a population-based survey. Disabil Rehabil Assist Technol. 2012;7(4):294-303. doi:10.3109/17483107.2011.635405
- Pezzin LE, Dillingham TR, MacKenzie EJ, Ephraim P, Rossbach P. Use and satisfaction with prosthetic limb devices and related services. Arch Phys Med Rehabil. 2004;85(5):723-729. doi:10.1016/j.apmr.2003.06.002
- Deans S, Burns D, McGarry A, Murray K, Mutrie N. Motivations and barriers to prosthesis users participation in physical activity, exercise and sport: a review of the literature. Prosthet Orthot Int. 2012;36(3):260-269. doi:10.1177/0309364612437905
- McDonald CL, Kahn A, Hafner BJ, Morgan SJ. Prevalence of secondary prosthesis use in lower limb prosthesis users. Disabil Rehabil. 2023;46(5):1016-1022. doi:10.1080/09638288.2023.2182919
- Baars EC, Schrier E, Dijkstra PU, Geertzen JHB. Prosthesis satisfaction in lower limb amputees: a systematic review of associated factors and questionnaires. Medicine (Baltimore). 2018;97(39):e12296. doi:10.1097/MD.0000000000012296
- Murray CD, Fox J. Body image and prosthesis satisfaction in the lower limb amputee. Disabil Rehabil. 2002;24(17):925–931. doi:10.1080/09638280210150014
- Major MJ, Quinlan J, Hansen AH, Esposito ER. Effects of women’s footwear on the mechanical function of heel-height accommodating prosthetic feet. PLoS One. 2022;17(1). doi:10.1371/journal.pone.0262910.
- Kuo PB, Lehavot K, Thomas RM, et al. Gender differences in prosthesis-related outcomes among veterans: results of a national survey of U.S. veterans. PM R. 2024;16(3):239- 249. doi:10.1002/pmrj.13028
- Asano M, Rushton P, Miller WC, Deathe BA. Predictors of quality of life among individuals who have a lower limb amputation. Prosthet Orthot Int. 2008;32(2):231-243. doi:10.1080/03093640802024955
Limb loss is a significant and growing concern in the United States. Nearly 2 million Americans are living with limb loss, and up to 185,000 people undergo amputations annually.1-4 Of these patients, about 35% are women.5 The Veterans Health Administration (VHA) provides about 10% of US amputations.6-8 Between 2015 and 2019, the number of prosthetic devices provided to female veterans increased from 3.3 million to 4.6 million.5,9,10
Previous research identified disparities in prosthetic care between men and women, both within and outside the VHA. These disparities include slower prosthesis prescription and receipt among women, in addition to differences in self-reported mobility, satisfaction, rates of prosthesis rejection, and challenges related to prosthesis appearance and fit.5,10,11 Recent studies suggest women tend to have worse outcomes following amputation, and are underrepresented in amputation research.12,13 However, these disparities are poorly described in a large, national sample. Because women represent a growing portion of patients with limb loss in the VHA, understanding their needs is critical.14
The Johnny Isakson and David P. Roe, MD Veterans Health Care and Benefits Improvement Act of 2020 was enacted, in part, to improve the care provided to women veterans.15 The law required the VHA to conduct a survey of ≥ 50,000 veterans to assess the satisfaction of women veterans with prostheses provided by the VHA. To comply with this legislation and understand how women veterans rate their prostheses and related care in the VHA, the US Department of Veterans Affairs (VA) Center for Collaborative Evaluation (VACE) conducted a large national survey of veterans with limb loss that oversampled women veterans. This article describes the survey results, including characteristics of female veterans with limb loss receiving care from the VHA, assesses their satisfaction with prostheses and prosthetic care, and highlights where their responses differ from those of male veterans.
Methods
We conducted a cross-sectional, mixedmode survey of eligible amputees in the VHA Support Service Capital Assets Amputee Data Cube. We identified a cohort of veterans with any major amputation (above the ankle or wrist) or partial hand or foot amputation who received VHA care between October 1, 2019, and September 30, 2020. The final cohort yielded 46,646 potentially eligible veterans. Thirty-three had invalid contact information, leaving 46,613 veterans who were asked to participate, including 1356 women.
Survey
We created a survey instrument de novo that included questions from validated instruments, including the Trinity Amputation Prosthesis and Experience Scales to assess prosthetic device satisfaction, the Prosthesis Evaluation Questionnaire to assess quality of life (QOL) satisfaction, and the Orthotics Prosthetics Users Survey to assess prosthesis-related care satisfaction. 16-18 Additional questions were incorporated from a survey of veterans with upper limb amputation to assess the importance of cosmetic considerations related to the prosthesis and comfort with prosthesis use in intimate relationships.19 Questions were also included to assess amputation type, year of amputation, if a prosthesis was currently used, reasons for ceasing use of a prosthesis, reasons for never using a prosthesis, the types of prostheses used, intensity of prosthesis use, satisfaction with time required to receive a prosthetic limb, and if the prosthesis reflected the veteran’s selfidentified gender. Veterans were asked to answer questions based on their most recent amputation.
We tested the survey using cognitive interviews with 6 veterans to refine the survey and better understand how veterans interpreted the questions. Pilot testers completed the survey and participated in individual interviews with experienced interviewers (CL and RRK) to describe how they selected their responses.20 This feedback was used to refine the survey. The online survey was programmed using Qualtrics Software and manually translated into Spanish.
Given the multimodal design, surveys were distributed by email, text message, and US Postal Service (USPS). Surveys were emailed to all veterans for whom a valid email address was available. If emails were undeliverable, veterans were contacted via text message or the USPS. Surveys were distributed by text message to all veterans without an email address but with a cellphone number. We were unable to consistently identify invalid numbers among all text message recipients. Invitations with a survey URL and QR code were sent via USPS to veterans who had no valid email address or cellphone number. Targeted efforts were made to increase the response rate for women. A random sample of 200 women who had not completed the survey 2 weeks prior to the closing date (15% of women in sample) was selected to receive personal phone calls. Another random sample of 400 women was selected to receive personalized outreach emails. The survey data were confidential, and responses could not be traced to identifying information.
Data Analyses
We conducted a descriptive analysis, including percentages and means for responses to variables focused on describing amputation characteristics, prosthesis characteristics, and QOL. All data, including missing values, were used to document the percentage of respondents for each question. Removing missing data from the denominator when calculating percentages could introduce bias to the analysis because we cannot be certain data are missing at random. Missing variables were removed to avoid underinflation of mean scores.
We compared responses across 2 groups: individuals who self-identified as men and individuals who self-identified as women. For each question, we assessed whether each of these groups differed significantly from the remaining sample. For example, we examined whether the percentage of men who answered affirmatively to a question was significantly higher or lower than that of individuals not identifying as male, and whether the percentage of women who answered affirmatively was significantly higher or lower than that of individuals not identifying as female. We utilized x2 tests to determine significant differences for percentage calculations and t tests to determine significant differences in means across gender.
Since conducting multiple comparisons within a dataset may result in inflating statistical significance (type 1 errors), we used a more conservative estimate of statistical significance (α = 0.01) and high significance (α = 0.001). This study was deemed quality improvement by the VHA Rehabilitation and Prosthetic Services (12RPS) and acknowledged by the VA Research Office at Eastern Colorado Health Care System and was not subject to institutional review board review.
Results
Surveys were distributed to 46,613 veterans and were completed by 4981 respondents for a 10.7% overall response rate. Survey respondents were generally similar to the eligible population invited to participate, but the proportion of women who completed the survey was higher than the proportion of women eligible to participate (2.0% of eligible population vs 16.7% of respondents), likely due to specific efforts to target women. Survey respondents were slightly younger than the general population (67.3 years vs 68.7 years), less likely to be male (97.1% vs 83.3%), showed similar representation of Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) veterans (4.4% vs 4.1%), and were less likely to have diabetes (58.0% vs 52.7% had diabetes) (Table 1).

The mean age of male respondents was 67.3 years, while the mean age of female respondents was 58.3 years. The majority of respondents were male (83.3%) and White (77.2%). Female respondents were less likely to have diabetes (35.4% of women vs 53.5% of men) and less likely to report that their most recent amputation resulted from diabetes (10.1% of women vs 22.2% of men). Women respondents were more likely to report an amputation due to other causes, such as adverse results of surgery, neurologic disease, suicide attempt, blood clots, tumors, rheumatoid arthritis, and revisions of previous amputations. Most women respondents did not serve during the OEF or OIF eras. The most common amputation site for women respondents was lower limb, either below the knee and above the ankle or above the knee.
Most participants use an everyday prosthesis, but women were more likely to report using a sports-specific prosthesis (Table 2). Overall, most respondents report using a prosthesis (87.7%); however, women were more likely to report not using a prosthesis (19.4% of women vs 11.1% of men; P ≤ .01). Additionally, a lower proportion of women report using a prosthesis for < 12 hours per day (30.6% of women vs 46.4% of men; P ≤ .01) or using a prosthesis every day (54.8% of women vs 74.6% of men; P ≤ .001).

In the overall sample, the mean satisfaction score with a prosthesis was 2.7 on a 5-point scale, and women had slightly lower overall satisfaction scores (2.6 for women vs 2.7 for men; P ≤ .001) (Table 3). Women also had lower satisfaction scores related to appearance, usefulness, reliability, and comfort. Women were more likely to indicate that it was very important to be able to wear jewelry and accessories (20.2% of women vs 11.6% of men; P ≤ .01), while men were less likely to indicate that it was somewhat or very important that the prosthesis not restrict clothing or shoes (95.2% of women vs 82.9% of men; P ≤ .001). Men were more likely than women to report being comfortable or very comfortable using their prosthesis in intimate contact: 40.5% vs 29.0%, respectively (P ≤ .001).

Overall, participants reported high satisfaction with appointment times, wait times, courteous treatment, opportunities to express concerns, and staff responsiveness. Men were slightly more likely than women to be satisfied with training (P ≤ 0.001) and problem discussion (P ≤ 0.01) (Table 4). There were no statistically significant differences in satisfaction or QOL ratings between women and men. The overall sample rated both QOL and satisfaction with QOL 6.7 on a 10-point scale.

Discussion
The goal of this study was to characterize the experience of veterans with limb loss receiving care in the VHA and assess their satisfaction with prostheses and prosthetic care. We received responses from nearly 5000 veterans, 158 of whom were women. Women veteran respondents were slightly younger and less likely to have an amputation due to diabetes. We did not observe significant differences in amputation level between men and women but women were less likely to use a prosthesis, reported lower intensity of prosthesis use, and were less satisfied with certain aspects of their prostheses. Women may also be less satisfied with prosthesis training and problem discussion. However, we found no differences in QOL ratings between men and women.
Findings indicating women were more likely to report not using a prosthesis and that a lower proportion of women report using a prosthesis for > 12 hours a day or every day are consistent with previous research. 21,22 Interestingly, women were more likely to report using a sports-specific prosthesis. This is notable because prior research suggests that individuals with amputations may avoid participating in sports and exercise, and a lack of access to sports-specific prostheses may inhibit physical activity.23,24 Women in this sample were slightly less satisfied with their prostheses overall and reported lower satisfaction scores regarding appearance, usefulness, reliability, and comfort, consistent with previous findings.25
A lower percentage of women in this sample reported being comfortable or very comfortable using their prosthesis during intimate contact. Previous research on prosthesis satisfaction suggests individuals who rate prosthesis satisfaction lower also report lower body image across genders. 26 While women in this sample did not rate their prosthesis satisfaction lower than men, they did report lower intensity of prosthesis use, suggesting potential issues with their prostheses this survey did not evaluate. Women indicated the importance of prostheses not restricting jewelry, accessories, clothing, or shoes. These results have significant clinical and social implications. A recent qualitative study emphasizes that women veterans feel prostheses are primarily designed for men and may not work well with their physiological needs.9 Research focused on limbs better suited to women’s bodies could result in better fitting sockets, lightweight limbs, or less bulky designs. Additional research has also explored the difficulties in accommodating a range of footwear for patients with lower limb amputation. One study found that varying footwear heights affect the function of adjustable prosthetic feet in ways that may not be optimal.27
Ratings of satisfaction with prosthesisrelated services between men and women in this sample are consistent with a recent study showing that women veterans do not have significant differences in satisfaction with prosthesis-related services.28 However, this study focused specifically on lower limb amputations, while the respondents of this study include those with both upper and lower limb amputations. Importantly, our findings that women are less likely to be satisfied with prosthesis training and problem discussions support recent qualitative findings in which women expressed a desire to work with prosthetists who listen to them, take their concerns seriously, and seek solutions that fit their needs. We did not observe a difference in QOL ratings between men and women in the sample despite lower satisfaction among women with some elements of prosthesis-related services. Previous research suggests many factors impact QOL after amputation, most notably time since amputation.16,29
Limitations
This survey was deployed in a short timeline that did not allow for careful sample selection or implementing strategies to increase response rate. Additionally, the study was conducted among veterans receiving care in the VHA, and findings may not be generalizable to limb loss in other settings. Finally, the discrepancy in number of respondents who identified as men vs women made it difficult to compare differences between the 2 groups.
Conclusions
This is the largest sample of survey respondents of veterans with limb loss to date. While the findings suggest veterans are generally satisfied with prosthetic-related services overall, they also highlight several areas for improvement with services or prostheses. Given that most veterans with limb loss are men, there is a significant discrepancy between the number of women and men respondents. Additional studies with more comparable numbers of men and women have found similar ratings of satisfaction with prostheses and services.28 Further research specifically focused on improving the experiences of women should focus on better characterizing their experiences and identifying how they differ from those of male veterans. For example, understanding how to engage female veterans with limb loss in prosthesis training and problem discussions may improve their experience with their care teams and improve their use of prostheses. Understanding experiences and needs that are specific to women could lead to the development of processes, resources, or devices that are tailored to the unique requirements of women with limb loss.
Limb loss is a significant and growing concern in the United States. Nearly 2 million Americans are living with limb loss, and up to 185,000 people undergo amputations annually.1-4 Of these patients, about 35% are women.5 The Veterans Health Administration (VHA) provides about 10% of US amputations.6-8 Between 2015 and 2019, the number of prosthetic devices provided to female veterans increased from 3.3 million to 4.6 million.5,9,10
Previous research identified disparities in prosthetic care between men and women, both within and outside the VHA. These disparities include slower prosthesis prescription and receipt among women, in addition to differences in self-reported mobility, satisfaction, rates of prosthesis rejection, and challenges related to prosthesis appearance and fit.5,10,11 Recent studies suggest women tend to have worse outcomes following amputation, and are underrepresented in amputation research.12,13 However, these disparities are poorly described in a large, national sample. Because women represent a growing portion of patients with limb loss in the VHA, understanding their needs is critical.14
The Johnny Isakson and David P. Roe, MD Veterans Health Care and Benefits Improvement Act of 2020 was enacted, in part, to improve the care provided to women veterans.15 The law required the VHA to conduct a survey of ≥ 50,000 veterans to assess the satisfaction of women veterans with prostheses provided by the VHA. To comply with this legislation and understand how women veterans rate their prostheses and related care in the VHA, the US Department of Veterans Affairs (VA) Center for Collaborative Evaluation (VACE) conducted a large national survey of veterans with limb loss that oversampled women veterans. This article describes the survey results, including characteristics of female veterans with limb loss receiving care from the VHA, assesses their satisfaction with prostheses and prosthetic care, and highlights where their responses differ from those of male veterans.
Methods
We conducted a cross-sectional, mixedmode survey of eligible amputees in the VHA Support Service Capital Assets Amputee Data Cube. We identified a cohort of veterans with any major amputation (above the ankle or wrist) or partial hand or foot amputation who received VHA care between October 1, 2019, and September 30, 2020. The final cohort yielded 46,646 potentially eligible veterans. Thirty-three had invalid contact information, leaving 46,613 veterans who were asked to participate, including 1356 women.
Survey
We created a survey instrument de novo that included questions from validated instruments, including the Trinity Amputation Prosthesis and Experience Scales to assess prosthetic device satisfaction, the Prosthesis Evaluation Questionnaire to assess quality of life (QOL) satisfaction, and the Orthotics Prosthetics Users Survey to assess prosthesis-related care satisfaction. 16-18 Additional questions were incorporated from a survey of veterans with upper limb amputation to assess the importance of cosmetic considerations related to the prosthesis and comfort with prosthesis use in intimate relationships.19 Questions were also included to assess amputation type, year of amputation, if a prosthesis was currently used, reasons for ceasing use of a prosthesis, reasons for never using a prosthesis, the types of prostheses used, intensity of prosthesis use, satisfaction with time required to receive a prosthetic limb, and if the prosthesis reflected the veteran’s selfidentified gender. Veterans were asked to answer questions based on their most recent amputation.
We tested the survey using cognitive interviews with 6 veterans to refine the survey and better understand how veterans interpreted the questions. Pilot testers completed the survey and participated in individual interviews with experienced interviewers (CL and RRK) to describe how they selected their responses.20 This feedback was used to refine the survey. The online survey was programmed using Qualtrics Software and manually translated into Spanish.
Given the multimodal design, surveys were distributed by email, text message, and US Postal Service (USPS). Surveys were emailed to all veterans for whom a valid email address was available. If emails were undeliverable, veterans were contacted via text message or the USPS. Surveys were distributed by text message to all veterans without an email address but with a cellphone number. We were unable to consistently identify invalid numbers among all text message recipients. Invitations with a survey URL and QR code were sent via USPS to veterans who had no valid email address or cellphone number. Targeted efforts were made to increase the response rate for women. A random sample of 200 women who had not completed the survey 2 weeks prior to the closing date (15% of women in sample) was selected to receive personal phone calls. Another random sample of 400 women was selected to receive personalized outreach emails. The survey data were confidential, and responses could not be traced to identifying information.
Data Analyses
We conducted a descriptive analysis, including percentages and means for responses to variables focused on describing amputation characteristics, prosthesis characteristics, and QOL. All data, including missing values, were used to document the percentage of respondents for each question. Removing missing data from the denominator when calculating percentages could introduce bias to the analysis because we cannot be certain data are missing at random. Missing variables were removed to avoid underinflation of mean scores.
We compared responses across 2 groups: individuals who self-identified as men and individuals who self-identified as women. For each question, we assessed whether each of these groups differed significantly from the remaining sample. For example, we examined whether the percentage of men who answered affirmatively to a question was significantly higher or lower than that of individuals not identifying as male, and whether the percentage of women who answered affirmatively was significantly higher or lower than that of individuals not identifying as female. We utilized x2 tests to determine significant differences for percentage calculations and t tests to determine significant differences in means across gender.
Since conducting multiple comparisons within a dataset may result in inflating statistical significance (type 1 errors), we used a more conservative estimate of statistical significance (α = 0.01) and high significance (α = 0.001). This study was deemed quality improvement by the VHA Rehabilitation and Prosthetic Services (12RPS) and acknowledged by the VA Research Office at Eastern Colorado Health Care System and was not subject to institutional review board review.
Results
Surveys were distributed to 46,613 veterans and were completed by 4981 respondents for a 10.7% overall response rate. Survey respondents were generally similar to the eligible population invited to participate, but the proportion of women who completed the survey was higher than the proportion of women eligible to participate (2.0% of eligible population vs 16.7% of respondents), likely due to specific efforts to target women. Survey respondents were slightly younger than the general population (67.3 years vs 68.7 years), less likely to be male (97.1% vs 83.3%), showed similar representation of Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) veterans (4.4% vs 4.1%), and were less likely to have diabetes (58.0% vs 52.7% had diabetes) (Table 1).

The mean age of male respondents was 67.3 years, while the mean age of female respondents was 58.3 years. The majority of respondents were male (83.3%) and White (77.2%). Female respondents were less likely to have diabetes (35.4% of women vs 53.5% of men) and less likely to report that their most recent amputation resulted from diabetes (10.1% of women vs 22.2% of men). Women respondents were more likely to report an amputation due to other causes, such as adverse results of surgery, neurologic disease, suicide attempt, blood clots, tumors, rheumatoid arthritis, and revisions of previous amputations. Most women respondents did not serve during the OEF or OIF eras. The most common amputation site for women respondents was lower limb, either below the knee and above the ankle or above the knee.
Most participants use an everyday prosthesis, but women were more likely to report using a sports-specific prosthesis (Table 2). Overall, most respondents report using a prosthesis (87.7%); however, women were more likely to report not using a prosthesis (19.4% of women vs 11.1% of men; P ≤ .01). Additionally, a lower proportion of women report using a prosthesis for < 12 hours per day (30.6% of women vs 46.4% of men; P ≤ .01) or using a prosthesis every day (54.8% of women vs 74.6% of men; P ≤ .001).

In the overall sample, the mean satisfaction score with a prosthesis was 2.7 on a 5-point scale, and women had slightly lower overall satisfaction scores (2.6 for women vs 2.7 for men; P ≤ .001) (Table 3). Women also had lower satisfaction scores related to appearance, usefulness, reliability, and comfort. Women were more likely to indicate that it was very important to be able to wear jewelry and accessories (20.2% of women vs 11.6% of men; P ≤ .01), while men were less likely to indicate that it was somewhat or very important that the prosthesis not restrict clothing or shoes (95.2% of women vs 82.9% of men; P ≤ .001). Men were more likely than women to report being comfortable or very comfortable using their prosthesis in intimate contact: 40.5% vs 29.0%, respectively (P ≤ .001).

Overall, participants reported high satisfaction with appointment times, wait times, courteous treatment, opportunities to express concerns, and staff responsiveness. Men were slightly more likely than women to be satisfied with training (P ≤ 0.001) and problem discussion (P ≤ 0.01) (Table 4). There were no statistically significant differences in satisfaction or QOL ratings between women and men. The overall sample rated both QOL and satisfaction with QOL 6.7 on a 10-point scale.

Discussion
The goal of this study was to characterize the experience of veterans with limb loss receiving care in the VHA and assess their satisfaction with prostheses and prosthetic care. We received responses from nearly 5000 veterans, 158 of whom were women. Women veteran respondents were slightly younger and less likely to have an amputation due to diabetes. We did not observe significant differences in amputation level between men and women but women were less likely to use a prosthesis, reported lower intensity of prosthesis use, and were less satisfied with certain aspects of their prostheses. Women may also be less satisfied with prosthesis training and problem discussion. However, we found no differences in QOL ratings between men and women.
Findings indicating women were more likely to report not using a prosthesis and that a lower proportion of women report using a prosthesis for > 12 hours a day or every day are consistent with previous research. 21,22 Interestingly, women were more likely to report using a sports-specific prosthesis. This is notable because prior research suggests that individuals with amputations may avoid participating in sports and exercise, and a lack of access to sports-specific prostheses may inhibit physical activity.23,24 Women in this sample were slightly less satisfied with their prostheses overall and reported lower satisfaction scores regarding appearance, usefulness, reliability, and comfort, consistent with previous findings.25
A lower percentage of women in this sample reported being comfortable or very comfortable using their prosthesis during intimate contact. Previous research on prosthesis satisfaction suggests individuals who rate prosthesis satisfaction lower also report lower body image across genders. 26 While women in this sample did not rate their prosthesis satisfaction lower than men, they did report lower intensity of prosthesis use, suggesting potential issues with their prostheses this survey did not evaluate. Women indicated the importance of prostheses not restricting jewelry, accessories, clothing, or shoes. These results have significant clinical and social implications. A recent qualitative study emphasizes that women veterans feel prostheses are primarily designed for men and may not work well with their physiological needs.9 Research focused on limbs better suited to women’s bodies could result in better fitting sockets, lightweight limbs, or less bulky designs. Additional research has also explored the difficulties in accommodating a range of footwear for patients with lower limb amputation. One study found that varying footwear heights affect the function of adjustable prosthetic feet in ways that may not be optimal.27
Ratings of satisfaction with prosthesisrelated services between men and women in this sample are consistent with a recent study showing that women veterans do not have significant differences in satisfaction with prosthesis-related services.28 However, this study focused specifically on lower limb amputations, while the respondents of this study include those with both upper and lower limb amputations. Importantly, our findings that women are less likely to be satisfied with prosthesis training and problem discussions support recent qualitative findings in which women expressed a desire to work with prosthetists who listen to them, take their concerns seriously, and seek solutions that fit their needs. We did not observe a difference in QOL ratings between men and women in the sample despite lower satisfaction among women with some elements of prosthesis-related services. Previous research suggests many factors impact QOL after amputation, most notably time since amputation.16,29
Limitations
This survey was deployed in a short timeline that did not allow for careful sample selection or implementing strategies to increase response rate. Additionally, the study was conducted among veterans receiving care in the VHA, and findings may not be generalizable to limb loss in other settings. Finally, the discrepancy in number of respondents who identified as men vs women made it difficult to compare differences between the 2 groups.
Conclusions
This is the largest sample of survey respondents of veterans with limb loss to date. While the findings suggest veterans are generally satisfied with prosthetic-related services overall, they also highlight several areas for improvement with services or prostheses. Given that most veterans with limb loss are men, there is a significant discrepancy between the number of women and men respondents. Additional studies with more comparable numbers of men and women have found similar ratings of satisfaction with prostheses and services.28 Further research specifically focused on improving the experiences of women should focus on better characterizing their experiences and identifying how they differ from those of male veterans. For example, understanding how to engage female veterans with limb loss in prosthesis training and problem discussions may improve their experience with their care teams and improve their use of prostheses. Understanding experiences and needs that are specific to women could lead to the development of processes, resources, or devices that are tailored to the unique requirements of women with limb loss.
- Ziegler-Graham K, MacKenzie EJ, Ephraim PL, Travison TG, Brookmeyer R. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil. 2008;89(3):422-429. doi:10.1016/j.apmr.2007.11.005
- Dillingham TR, Pezzin LE, MacKenzie EJ. Limb amputation and limb deficiency: epidemiology and recent trends in the united states. South Med J. 2002;95(8):875-883. doi:10.1097/00007611-200208000-00018
- Dillingham TR, Pezzin LE, Shore AD. Reamputation, mortality, and health care costs among persons with dysvascular lower-limb amputations. Arch Phys Med Rehabil. 2005;86(3):480-486. doi:10.1016/j.apmr.2004.06.072
- Centers for Disease Control and Prevention. Ambulatory and inpatient procedures in the United States. Accessed September 30, 2024. https://www.cdc.gov/nchs/pressroom/98facts/ambulat.htm
- Ljung J, Iacangelo A. Identifying and acknowledging a sex gap in lower-limb prosthetics. JPO. 2024;36(1):e18-e24. doi:10.1097/JPO.0000000000000470
- Feinglass J, Brown JL, LoSasso A, et al. Rates of lower-extremity amputation and arterial reconstruction in the united states, 1979 to 1996. Am J Public Health. 1999;89(8):1222- 1227. doi:10.2105/ajph.89.8.1222
- Mayfield JA, Reiber GE, Maynard C, Czerniecki JM, Caps MT, Sangeorzan BJ. Trends in lower limb amputation in the Veterans Health Administration, 1989-1998. J Rehabil Res Dev. 2000;37(1):23-30.
- Feinglass J, Pearce WH, Martin GJ, et al. Postoperative and late survival outcomes after major amputation: findings from the department of veterans affairs national surgical quality improvement program. Surgery. 2001;130(1):21-29. doi:10.1067/msy.2001.115359
- Lehavot K, Young JP, Thomas RM, et al. Voices of women veterans with lower limb prostheses: a qualitative study. J Gen Intern Med. 2022;37(3):799-805. doi:10.1007/s11606-022-07572-8
- US Government Accountability Office. COVID-19: Opportunities to improve federal response. GAO-21-60. Published November 12, 2020. Accessed September 30, 2024. https://www.gao.gov/products/gao-21-60
- Littman AJ, Peterson AC, Korpak A, et al. Differences in prosthetic prescription between men and women veterans after transtibial or transfemoral lowerextremity amputation: a longitudinal cohort study. Arch Phys Med Rehabil. 2023;104(8)1274-1281. doi:10.1016/j.amjsurg.2023.02.011
- Cimino SR, Vijayakumar A, MacKay C, Mayo AL, Hitzig SL, Guilcher SJT. Sex and gender differences in quality of life and related domains for individuals with adult acquired lower-limb amputation: a scoping review. Disabil Rehabil. 2022 Oct 23;44(22):6899-6925. doi:10.1080/09638288.2021.1974106
- DadeMatthews OO, Roper JA, Vazquez A, Shannon DM, Sefton JM. Prosthetic device and service satisfaction, quality of life, and functional performance in lower limb prosthesis clients. Prosthet Orthot Int. 2024;48(4):422-430. doi:10.1097/PXR.0000000000000285
- Hamilton AB, Schwarz EB, Thomas HN, Goldstein KM. Moving women veterans’ health research forward: a special supplement. J Gen Intern Med. 2022;37(Suppl3):665– 667. doi:10.1007/s11606-022-07606-1
- US Congress. Public Law 116-315: An Act to Improve the Lives of Veterans, S 5108 (2) (F). 116th Congress; 2021. Accessed September 30, 2024. https://www.congress.gov/116/plaws/publ315/PLAW-116publ315.pdf
- Gallagher P, MacLachlan M. The Trinity amputation and prosthesis experience scales and quality of life in people with lower-limb amputation. Arch Phys Med Rehabil. 2004;85(5):730-736. doi:10.1016/j.apmr.2003.07.009
- Legro MW, Reiber GD, Smith DG, del Aguila M, Larsen J, Boone D. Prosthesis evaluation questionnaire for persons with lower limb amputations: assessing prosthesis-related quality of life. Arch Phys Med Rehabil. 1998;79(8):931-938. doi:10.1016/s0003-9993(98)90090-9
- Legro MW, Reiber GD, Smith DG, del Aguila M, Larsen J, Boone D. Prosthesis evaluation questionnaire for persons with lower limb amputations: assessing prosthesis-related quality of life. Arch Phys Med Rehabil. 1998;79(8):931-938. doi:10.1016/s0003-9993(98)90090-9
- Heinemann AW, Bode RK, O’Reilly C. Development and measurement properties of the orthotics and prosthetics users’ survey (OPUS): a comprehensive set of clinical outcome instruments. Prosthet Orthot Int. 2003;27(3):191-206. doi:10.1080/03093640308726682
- Resnik LJ, Borgia ML, Clark MA. A national survey of prosthesis use in veterans with major upper limb amputation: comparisons by gender. PM R. 2020;12(11):1086-1098. doi:10.1002/pmrj.12351
- Collins D. Pretesting survey instruments: an overview of cognitive methods. Qual Life Res. 2003;12(3):229-238. doi:10.1023/a:1023254226592
- Østlie K, Lesjø IM, Franklin RJ, Garfelt B, Skjeldal OH, Magnus P. Prosthesis rejection in acquired major upper-limb amputees: a population-based survey. Disabil Rehabil Assist Technol. 2012;7(4):294-303. doi:10.3109/17483107.2011.635405
- Pezzin LE, Dillingham TR, MacKenzie EJ, Ephraim P, Rossbach P. Use and satisfaction with prosthetic limb devices and related services. Arch Phys Med Rehabil. 2004;85(5):723-729. doi:10.1016/j.apmr.2003.06.002
- Deans S, Burns D, McGarry A, Murray K, Mutrie N. Motivations and barriers to prosthesis users participation in physical activity, exercise and sport: a review of the literature. Prosthet Orthot Int. 2012;36(3):260-269. doi:10.1177/0309364612437905
- McDonald CL, Kahn A, Hafner BJ, Morgan SJ. Prevalence of secondary prosthesis use in lower limb prosthesis users. Disabil Rehabil. 2023;46(5):1016-1022. doi:10.1080/09638288.2023.2182919
- Baars EC, Schrier E, Dijkstra PU, Geertzen JHB. Prosthesis satisfaction in lower limb amputees: a systematic review of associated factors and questionnaires. Medicine (Baltimore). 2018;97(39):e12296. doi:10.1097/MD.0000000000012296
- Murray CD, Fox J. Body image and prosthesis satisfaction in the lower limb amputee. Disabil Rehabil. 2002;24(17):925–931. doi:10.1080/09638280210150014
- Major MJ, Quinlan J, Hansen AH, Esposito ER. Effects of women’s footwear on the mechanical function of heel-height accommodating prosthetic feet. PLoS One. 2022;17(1). doi:10.1371/journal.pone.0262910.
- Kuo PB, Lehavot K, Thomas RM, et al. Gender differences in prosthesis-related outcomes among veterans: results of a national survey of U.S. veterans. PM R. 2024;16(3):239- 249. doi:10.1002/pmrj.13028
- Asano M, Rushton P, Miller WC, Deathe BA. Predictors of quality of life among individuals who have a lower limb amputation. Prosthet Orthot Int. 2008;32(2):231-243. doi:10.1080/03093640802024955
- Ziegler-Graham K, MacKenzie EJ, Ephraim PL, Travison TG, Brookmeyer R. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil. 2008;89(3):422-429. doi:10.1016/j.apmr.2007.11.005
- Dillingham TR, Pezzin LE, MacKenzie EJ. Limb amputation and limb deficiency: epidemiology and recent trends in the united states. South Med J. 2002;95(8):875-883. doi:10.1097/00007611-200208000-00018
- Dillingham TR, Pezzin LE, Shore AD. Reamputation, mortality, and health care costs among persons with dysvascular lower-limb amputations. Arch Phys Med Rehabil. 2005;86(3):480-486. doi:10.1016/j.apmr.2004.06.072
- Centers for Disease Control and Prevention. Ambulatory and inpatient procedures in the United States. Accessed September 30, 2024. https://www.cdc.gov/nchs/pressroom/98facts/ambulat.htm
- Ljung J, Iacangelo A. Identifying and acknowledging a sex gap in lower-limb prosthetics. JPO. 2024;36(1):e18-e24. doi:10.1097/JPO.0000000000000470
- Feinglass J, Brown JL, LoSasso A, et al. Rates of lower-extremity amputation and arterial reconstruction in the united states, 1979 to 1996. Am J Public Health. 1999;89(8):1222- 1227. doi:10.2105/ajph.89.8.1222
- Mayfield JA, Reiber GE, Maynard C, Czerniecki JM, Caps MT, Sangeorzan BJ. Trends in lower limb amputation in the Veterans Health Administration, 1989-1998. J Rehabil Res Dev. 2000;37(1):23-30.
- Feinglass J, Pearce WH, Martin GJ, et al. Postoperative and late survival outcomes after major amputation: findings from the department of veterans affairs national surgical quality improvement program. Surgery. 2001;130(1):21-29. doi:10.1067/msy.2001.115359
- Lehavot K, Young JP, Thomas RM, et al. Voices of women veterans with lower limb prostheses: a qualitative study. J Gen Intern Med. 2022;37(3):799-805. doi:10.1007/s11606-022-07572-8
- US Government Accountability Office. COVID-19: Opportunities to improve federal response. GAO-21-60. Published November 12, 2020. Accessed September 30, 2024. https://www.gao.gov/products/gao-21-60
- Littman AJ, Peterson AC, Korpak A, et al. Differences in prosthetic prescription between men and women veterans after transtibial or transfemoral lowerextremity amputation: a longitudinal cohort study. Arch Phys Med Rehabil. 2023;104(8)1274-1281. doi:10.1016/j.amjsurg.2023.02.011
- Cimino SR, Vijayakumar A, MacKay C, Mayo AL, Hitzig SL, Guilcher SJT. Sex and gender differences in quality of life and related domains for individuals with adult acquired lower-limb amputation: a scoping review. Disabil Rehabil. 2022 Oct 23;44(22):6899-6925. doi:10.1080/09638288.2021.1974106
- DadeMatthews OO, Roper JA, Vazquez A, Shannon DM, Sefton JM. Prosthetic device and service satisfaction, quality of life, and functional performance in lower limb prosthesis clients. Prosthet Orthot Int. 2024;48(4):422-430. doi:10.1097/PXR.0000000000000285
- Hamilton AB, Schwarz EB, Thomas HN, Goldstein KM. Moving women veterans’ health research forward: a special supplement. J Gen Intern Med. 2022;37(Suppl3):665– 667. doi:10.1007/s11606-022-07606-1
- US Congress. Public Law 116-315: An Act to Improve the Lives of Veterans, S 5108 (2) (F). 116th Congress; 2021. Accessed September 30, 2024. https://www.congress.gov/116/plaws/publ315/PLAW-116publ315.pdf
- Gallagher P, MacLachlan M. The Trinity amputation and prosthesis experience scales and quality of life in people with lower-limb amputation. Arch Phys Med Rehabil. 2004;85(5):730-736. doi:10.1016/j.apmr.2003.07.009
- Legro MW, Reiber GD, Smith DG, del Aguila M, Larsen J, Boone D. Prosthesis evaluation questionnaire for persons with lower limb amputations: assessing prosthesis-related quality of life. Arch Phys Med Rehabil. 1998;79(8):931-938. doi:10.1016/s0003-9993(98)90090-9
- Legro MW, Reiber GD, Smith DG, del Aguila M, Larsen J, Boone D. Prosthesis evaluation questionnaire for persons with lower limb amputations: assessing prosthesis-related quality of life. Arch Phys Med Rehabil. 1998;79(8):931-938. doi:10.1016/s0003-9993(98)90090-9
- Heinemann AW, Bode RK, O’Reilly C. Development and measurement properties of the orthotics and prosthetics users’ survey (OPUS): a comprehensive set of clinical outcome instruments. Prosthet Orthot Int. 2003;27(3):191-206. doi:10.1080/03093640308726682
- Resnik LJ, Borgia ML, Clark MA. A national survey of prosthesis use in veterans with major upper limb amputation: comparisons by gender. PM R. 2020;12(11):1086-1098. doi:10.1002/pmrj.12351
- Collins D. Pretesting survey instruments: an overview of cognitive methods. Qual Life Res. 2003;12(3):229-238. doi:10.1023/a:1023254226592
- Østlie K, Lesjø IM, Franklin RJ, Garfelt B, Skjeldal OH, Magnus P. Prosthesis rejection in acquired major upper-limb amputees: a population-based survey. Disabil Rehabil Assist Technol. 2012;7(4):294-303. doi:10.3109/17483107.2011.635405
- Pezzin LE, Dillingham TR, MacKenzie EJ, Ephraim P, Rossbach P. Use and satisfaction with prosthetic limb devices and related services. Arch Phys Med Rehabil. 2004;85(5):723-729. doi:10.1016/j.apmr.2003.06.002
- Deans S, Burns D, McGarry A, Murray K, Mutrie N. Motivations and barriers to prosthesis users participation in physical activity, exercise and sport: a review of the literature. Prosthet Orthot Int. 2012;36(3):260-269. doi:10.1177/0309364612437905
- McDonald CL, Kahn A, Hafner BJ, Morgan SJ. Prevalence of secondary prosthesis use in lower limb prosthesis users. Disabil Rehabil. 2023;46(5):1016-1022. doi:10.1080/09638288.2023.2182919
- Baars EC, Schrier E, Dijkstra PU, Geertzen JHB. Prosthesis satisfaction in lower limb amputees: a systematic review of associated factors and questionnaires. Medicine (Baltimore). 2018;97(39):e12296. doi:10.1097/MD.0000000000012296
- Murray CD, Fox J. Body image and prosthesis satisfaction in the lower limb amputee. Disabil Rehabil. 2002;24(17):925–931. doi:10.1080/09638280210150014
- Major MJ, Quinlan J, Hansen AH, Esposito ER. Effects of women’s footwear on the mechanical function of heel-height accommodating prosthetic feet. PLoS One. 2022;17(1). doi:10.1371/journal.pone.0262910.
- Kuo PB, Lehavot K, Thomas RM, et al. Gender differences in prosthesis-related outcomes among veterans: results of a national survey of U.S. veterans. PM R. 2024;16(3):239- 249. doi:10.1002/pmrj.13028
- Asano M, Rushton P, Miller WC, Deathe BA. Predictors of quality of life among individuals who have a lower limb amputation. Prosthet Orthot Int. 2008;32(2):231-243. doi:10.1080/03093640802024955
Satisfaction With Department of Veterans Affairs Prosthetics and Support Services as Reported by Women and Men Veterans
Satisfaction With Department of Veterans Affairs Prosthetics and Support Services as Reported by Women and Men Veterans
The Veteran’s Canon Under Fire
The Veteran’s Canon Under Fire
As Veterans Day approaches, stores and restaurants will offer discounts and free meals to veterans. Children will write thank you letters, and citizens nationwide will raise flags to honor and thank veterans. We can never repay those who lost their life, health, or livelihood in defense of the nation. Since the American Revolution, and in gratitude for that incalculable debt, the US government, on behalf of the American public, has seen fit to grant a host of benefits and services to those who wore the uniform.2,3 Among the best known are health care, burial services, compensation and pensions, home loans, and the GI Bill.
Less recognized yet arguably essential for the fair and consistent provision of these entitlements is a legal principle: the veteran’s canon. A canon is a system of rules or maxims used to interpret legal instruments, such as statutes. They are not rules but serve as a “principle that guides the interpretation of the text.”4 Since I am not a lawyer, I will undoubtedly oversimplify this legal principle, but I hope to get enough right to explain why the veteran’s canon should matter to federal health care professionals.
At its core, the veteran’s canon means that when the US Department of Veterans Affairs (VA) and a veteran have a legal dispute about VA benefits, the courts will give deference to the veteran. Underscoring that any ambiguity in the statute is resolved in the veteran’s favor, the canon is known in legal circles as the Gardner deference. This is a reference to a 1994 case in which a Korean War veteran underwent surgery in a VA facility for a herniated disc he alleged caused pain and weakness in his left lower extremity.5 Gardner argued that federal statutes 38 USC § 1151 underlying corresponding VA regulation 38 CFR § 3.358(c)(3) granted disability benefits to veterans injured during VA treatment. The VA denied the disability claim, contending the regulation restricted compensation to veterans whose injury was the fault of the VA; thus, the disability had to have been the result of negligent treatment or an unforeseen therapeutic accident.5
The case wound its way through various appeals boards and courts until the Supreme Court of the United States (SCOTUS) ruled that the statute’s context left no ambiguity, and that any care provided under VA auspices was covered under the statute. What is important for this column is that the justices opined that had ambiguity been present, it would have legally necessitated, “applying the rule that interpretive doubt is to be resolved in the veteran’s favor.”5 In Gardner’s case, the courts reaffirmed nearly 80 years of judicial precedent upholding the veteran’s canon.
Thirty years later, Rudisill v McDonough again questioned the veteran’s canon.6 Educational benefits, namely the GI Bill, were the issue in this case. Rudisill served during 3 different periods in the US Army, totaling 8 years. Two educational programs overlapped during Rudisill’s tenure in the military: the Montgomery GI Bill and Post-9/11 Veterans Educational Assistance Act. Rudisill had used a portion of his Montgomery benefits to fund his undergraduate education and now wished to use the more extensive Post-9/11 assistance to finance his graduate degree. Rudisill and the VA disagreed about when his combined benefits would be capped, either at 36 or 48 months. After working its way through appeals courts, SCOTUS was again called upon for judgment.
The justices found that Rudisill qualified under both programs and could use them in any order he wished up to the cap. The majority found no ambiguity in the statute; however, if interpretation was required, the majority of justices indicated that the veteran’s canon would have supported Rudisill. While this sounds like good news for veterans, 2 justices authored a dissenting opinion that questioned the constitutional grounding of the veteran’s canon, noting that the “canon appears to have developed almost by accident.”6 The minority opinion suggested that when the veteran’s canon allocates resources to pay for specific veteran benefits, other interests and groups are deprived of those same resources, resulting in potential inequity.7
The potential ethical import and clinical impact of striking down the veteran’s canon is serious. It is especially concerning given that in a recent case, the SCOTUS ruling struck down another legal interpretation that also benefited the VA and ultimately veterans: the Chevron deference.8 This precedent held that when a legal dispute arises about the meaning of a specific federal agency regulation or policy, the courts should defer to the federal agency’s presumably superior understanding of the matter. The principle places the locus of decision-making with the subject-matter experts of the respective agency rather than the courts.
Ironically, given the legislative purposes of both interpretive principles, their overturning would likely introduce much more uncertainty, variation, and unpredictability in cases involving veteran benefits. This is bad news for both veterans and the VA. Veterans might not prevail as often in court when they have a reasonable claim, leading to more aggressive challenges. In response, the VA would have a heavier and more costly burden of administrative proof to defend sound decisions.9 Recently, the VA has tried to reduce the backlog of claims. The inability to have legal recourse to Chevron or Gardener could result in even more delay in adjudicating veterans’ claims that enable them to access benefits and services, already an object of congressional pressure.10
Courts will continue to debate the issue with another judicial test of the canon on the current SCOTUS docket (Bufkin v McDonough).11 The veteran’s canon was put in place to equalize the power differential between the VA and the veteran: in administrative language, to make it more likely than not that the veteran would prevail when regulations were ambiguous. There are many legal and political rationales for veteran’s canon, including enabling veterans to file claims for service-connected illnesses. The veteran’s cannon helped Vietnam War-era veterans receive VA care while researchers were still studying the sequela of Agent Orange exposure. 12 The legislative purpose of the veteran’s canon is the same as that of all VA benefits and services commemorated on Veterans Day. As expressed by SCOTUS justices in the wake of World War II, the benefit statutes should be “liberally construed for the benefit of those who left private life to serve their country in its hour of greatest need.”13
- Henderson v Shinseki, 562 US. 428, 440-441 (2011).
- US Department of Veterans Affairs, National Veteran Outreach Office. The difference between Veterans Day and Memorial Day. October 30, 2023. Accessed October 21, 2024. https://news.va.gov/125549/difference-between-veterans-day-memorial-day/
- US Department of Veterans Affairs. VA history summary. Updated August 6, 2024. Accessed October 21, 2024. https://department.va.gov/history/history-overview
- Cornell Law School, Legal Information Institute. Canons of construction. Updated March 2022. Accessed October 21, 2024. https://www.law.cornell.edu/wex/canons_of_construction
- Brown v Gardner, 513 US 115 (1994).
- Rudisill v McDonough, 601 US __ (2024).
- Hoover J. Justices will decide if vets are getting the ‘benefit of the doubt’. National Law Journal. April 30, 2024. Accessed October 21, 2024. https://www.law.com/nationallawjournal/2024/04/30/justices-will-decide-if-vets-are-getting-the-benefit-of-the-doubt/
- Relentless, Inc. v Department of Commerce Docket # 22-219, January 17, 2024.
- Kime P. Two veterans will argue to Supreme Court that VA disability claims aren’t getting, ‘benefit of the doubt’. Military. com. October 15, 2024. Accessed October 21, 2024. https:// www.military.com/daily-news/2024/10/15/supreme-court-hears-case-questioning-vas-commitment-favoring-veterans-benefits-decisions.html
- Rehagen J. SCOTUS’s chevron deference ruling: how it could hurt veterans and the VA. Veteran.com. Updated July 9, 2024. Accessed October 21, 2024. https://veteran.com/scotus-chevron-deference-impact-va-veteran/
- Hersey LF. Lawmakers urge VA to reduce backlog, wait times on veterans claims for benefits. Stars & Stripes. June 27, 2024. Accessed October 21, 2024. https://www.stripes.com/veterans/2024-06-27/veterans-benefits-claims-backlog-pact-act-14315042.html
- Harper CJ. Give veterans the benefit of the doubt: Chevron, Auer, and the veteran’s canon. Harvard J Law Public Policy. 2019; 42(3):931-969. https://journals.law.harvard.edu/jlpp/wp-content/uploads/sites/90/2019/06/42_3-Full-Issue.pdf
- Fishgold v Sullivan Drydock & Repair Corp, 328 US 275, 285 (1946).
As Veterans Day approaches, stores and restaurants will offer discounts and free meals to veterans. Children will write thank you letters, and citizens nationwide will raise flags to honor and thank veterans. We can never repay those who lost their life, health, or livelihood in defense of the nation. Since the American Revolution, and in gratitude for that incalculable debt, the US government, on behalf of the American public, has seen fit to grant a host of benefits and services to those who wore the uniform.2,3 Among the best known are health care, burial services, compensation and pensions, home loans, and the GI Bill.
Less recognized yet arguably essential for the fair and consistent provision of these entitlements is a legal principle: the veteran’s canon. A canon is a system of rules or maxims used to interpret legal instruments, such as statutes. They are not rules but serve as a “principle that guides the interpretation of the text.”4 Since I am not a lawyer, I will undoubtedly oversimplify this legal principle, but I hope to get enough right to explain why the veteran’s canon should matter to federal health care professionals.
At its core, the veteran’s canon means that when the US Department of Veterans Affairs (VA) and a veteran have a legal dispute about VA benefits, the courts will give deference to the veteran. Underscoring that any ambiguity in the statute is resolved in the veteran’s favor, the canon is known in legal circles as the Gardner deference. This is a reference to a 1994 case in which a Korean War veteran underwent surgery in a VA facility for a herniated disc he alleged caused pain and weakness in his left lower extremity.5 Gardner argued that federal statutes 38 USC § 1151 underlying corresponding VA regulation 38 CFR § 3.358(c)(3) granted disability benefits to veterans injured during VA treatment. The VA denied the disability claim, contending the regulation restricted compensation to veterans whose injury was the fault of the VA; thus, the disability had to have been the result of negligent treatment or an unforeseen therapeutic accident.5
The case wound its way through various appeals boards and courts until the Supreme Court of the United States (SCOTUS) ruled that the statute’s context left no ambiguity, and that any care provided under VA auspices was covered under the statute. What is important for this column is that the justices opined that had ambiguity been present, it would have legally necessitated, “applying the rule that interpretive doubt is to be resolved in the veteran’s favor.”5 In Gardner’s case, the courts reaffirmed nearly 80 years of judicial precedent upholding the veteran’s canon.
Thirty years later, Rudisill v McDonough again questioned the veteran’s canon.6 Educational benefits, namely the GI Bill, were the issue in this case. Rudisill served during 3 different periods in the US Army, totaling 8 years. Two educational programs overlapped during Rudisill’s tenure in the military: the Montgomery GI Bill and Post-9/11 Veterans Educational Assistance Act. Rudisill had used a portion of his Montgomery benefits to fund his undergraduate education and now wished to use the more extensive Post-9/11 assistance to finance his graduate degree. Rudisill and the VA disagreed about when his combined benefits would be capped, either at 36 or 48 months. After working its way through appeals courts, SCOTUS was again called upon for judgment.
The justices found that Rudisill qualified under both programs and could use them in any order he wished up to the cap. The majority found no ambiguity in the statute; however, if interpretation was required, the majority of justices indicated that the veteran’s canon would have supported Rudisill. While this sounds like good news for veterans, 2 justices authored a dissenting opinion that questioned the constitutional grounding of the veteran’s canon, noting that the “canon appears to have developed almost by accident.”6 The minority opinion suggested that when the veteran’s canon allocates resources to pay for specific veteran benefits, other interests and groups are deprived of those same resources, resulting in potential inequity.7
The potential ethical import and clinical impact of striking down the veteran’s canon is serious. It is especially concerning given that in a recent case, the SCOTUS ruling struck down another legal interpretation that also benefited the VA and ultimately veterans: the Chevron deference.8 This precedent held that when a legal dispute arises about the meaning of a specific federal agency regulation or policy, the courts should defer to the federal agency’s presumably superior understanding of the matter. The principle places the locus of decision-making with the subject-matter experts of the respective agency rather than the courts.
Ironically, given the legislative purposes of both interpretive principles, their overturning would likely introduce much more uncertainty, variation, and unpredictability in cases involving veteran benefits. This is bad news for both veterans and the VA. Veterans might not prevail as often in court when they have a reasonable claim, leading to more aggressive challenges. In response, the VA would have a heavier and more costly burden of administrative proof to defend sound decisions.9 Recently, the VA has tried to reduce the backlog of claims. The inability to have legal recourse to Chevron or Gardener could result in even more delay in adjudicating veterans’ claims that enable them to access benefits and services, already an object of congressional pressure.10
Courts will continue to debate the issue with another judicial test of the canon on the current SCOTUS docket (Bufkin v McDonough).11 The veteran’s canon was put in place to equalize the power differential between the VA and the veteran: in administrative language, to make it more likely than not that the veteran would prevail when regulations were ambiguous. There are many legal and political rationales for veteran’s canon, including enabling veterans to file claims for service-connected illnesses. The veteran’s cannon helped Vietnam War-era veterans receive VA care while researchers were still studying the sequela of Agent Orange exposure. 12 The legislative purpose of the veteran’s canon is the same as that of all VA benefits and services commemorated on Veterans Day. As expressed by SCOTUS justices in the wake of World War II, the benefit statutes should be “liberally construed for the benefit of those who left private life to serve their country in its hour of greatest need.”13
As Veterans Day approaches, stores and restaurants will offer discounts and free meals to veterans. Children will write thank you letters, and citizens nationwide will raise flags to honor and thank veterans. We can never repay those who lost their life, health, or livelihood in defense of the nation. Since the American Revolution, and in gratitude for that incalculable debt, the US government, on behalf of the American public, has seen fit to grant a host of benefits and services to those who wore the uniform.2,3 Among the best known are health care, burial services, compensation and pensions, home loans, and the GI Bill.
Less recognized yet arguably essential for the fair and consistent provision of these entitlements is a legal principle: the veteran’s canon. A canon is a system of rules or maxims used to interpret legal instruments, such as statutes. They are not rules but serve as a “principle that guides the interpretation of the text.”4 Since I am not a lawyer, I will undoubtedly oversimplify this legal principle, but I hope to get enough right to explain why the veteran’s canon should matter to federal health care professionals.
At its core, the veteran’s canon means that when the US Department of Veterans Affairs (VA) and a veteran have a legal dispute about VA benefits, the courts will give deference to the veteran. Underscoring that any ambiguity in the statute is resolved in the veteran’s favor, the canon is known in legal circles as the Gardner deference. This is a reference to a 1994 case in which a Korean War veteran underwent surgery in a VA facility for a herniated disc he alleged caused pain and weakness in his left lower extremity.5 Gardner argued that federal statutes 38 USC § 1151 underlying corresponding VA regulation 38 CFR § 3.358(c)(3) granted disability benefits to veterans injured during VA treatment. The VA denied the disability claim, contending the regulation restricted compensation to veterans whose injury was the fault of the VA; thus, the disability had to have been the result of negligent treatment or an unforeseen therapeutic accident.5
The case wound its way through various appeals boards and courts until the Supreme Court of the United States (SCOTUS) ruled that the statute’s context left no ambiguity, and that any care provided under VA auspices was covered under the statute. What is important for this column is that the justices opined that had ambiguity been present, it would have legally necessitated, “applying the rule that interpretive doubt is to be resolved in the veteran’s favor.”5 In Gardner’s case, the courts reaffirmed nearly 80 years of judicial precedent upholding the veteran’s canon.
Thirty years later, Rudisill v McDonough again questioned the veteran’s canon.6 Educational benefits, namely the GI Bill, were the issue in this case. Rudisill served during 3 different periods in the US Army, totaling 8 years. Two educational programs overlapped during Rudisill’s tenure in the military: the Montgomery GI Bill and Post-9/11 Veterans Educational Assistance Act. Rudisill had used a portion of his Montgomery benefits to fund his undergraduate education and now wished to use the more extensive Post-9/11 assistance to finance his graduate degree. Rudisill and the VA disagreed about when his combined benefits would be capped, either at 36 or 48 months. After working its way through appeals courts, SCOTUS was again called upon for judgment.
The justices found that Rudisill qualified under both programs and could use them in any order he wished up to the cap. The majority found no ambiguity in the statute; however, if interpretation was required, the majority of justices indicated that the veteran’s canon would have supported Rudisill. While this sounds like good news for veterans, 2 justices authored a dissenting opinion that questioned the constitutional grounding of the veteran’s canon, noting that the “canon appears to have developed almost by accident.”6 The minority opinion suggested that when the veteran’s canon allocates resources to pay for specific veteran benefits, other interests and groups are deprived of those same resources, resulting in potential inequity.7
The potential ethical import and clinical impact of striking down the veteran’s canon is serious. It is especially concerning given that in a recent case, the SCOTUS ruling struck down another legal interpretation that also benefited the VA and ultimately veterans: the Chevron deference.8 This precedent held that when a legal dispute arises about the meaning of a specific federal agency regulation or policy, the courts should defer to the federal agency’s presumably superior understanding of the matter. The principle places the locus of decision-making with the subject-matter experts of the respective agency rather than the courts.
Ironically, given the legislative purposes of both interpretive principles, their overturning would likely introduce much more uncertainty, variation, and unpredictability in cases involving veteran benefits. This is bad news for both veterans and the VA. Veterans might not prevail as often in court when they have a reasonable claim, leading to more aggressive challenges. In response, the VA would have a heavier and more costly burden of administrative proof to defend sound decisions.9 Recently, the VA has tried to reduce the backlog of claims. The inability to have legal recourse to Chevron or Gardener could result in even more delay in adjudicating veterans’ claims that enable them to access benefits and services, already an object of congressional pressure.10
Courts will continue to debate the issue with another judicial test of the canon on the current SCOTUS docket (Bufkin v McDonough).11 The veteran’s canon was put in place to equalize the power differential between the VA and the veteran: in administrative language, to make it more likely than not that the veteran would prevail when regulations were ambiguous. There are many legal and political rationales for veteran’s canon, including enabling veterans to file claims for service-connected illnesses. The veteran’s cannon helped Vietnam War-era veterans receive VA care while researchers were still studying the sequela of Agent Orange exposure. 12 The legislative purpose of the veteran’s canon is the same as that of all VA benefits and services commemorated on Veterans Day. As expressed by SCOTUS justices in the wake of World War II, the benefit statutes should be “liberally construed for the benefit of those who left private life to serve their country in its hour of greatest need.”13
- Henderson v Shinseki, 562 US. 428, 440-441 (2011).
- US Department of Veterans Affairs, National Veteran Outreach Office. The difference between Veterans Day and Memorial Day. October 30, 2023. Accessed October 21, 2024. https://news.va.gov/125549/difference-between-veterans-day-memorial-day/
- US Department of Veterans Affairs. VA history summary. Updated August 6, 2024. Accessed October 21, 2024. https://department.va.gov/history/history-overview
- Cornell Law School, Legal Information Institute. Canons of construction. Updated March 2022. Accessed October 21, 2024. https://www.law.cornell.edu/wex/canons_of_construction
- Brown v Gardner, 513 US 115 (1994).
- Rudisill v McDonough, 601 US __ (2024).
- Hoover J. Justices will decide if vets are getting the ‘benefit of the doubt’. National Law Journal. April 30, 2024. Accessed October 21, 2024. https://www.law.com/nationallawjournal/2024/04/30/justices-will-decide-if-vets-are-getting-the-benefit-of-the-doubt/
- Relentless, Inc. v Department of Commerce Docket # 22-219, January 17, 2024.
- Kime P. Two veterans will argue to Supreme Court that VA disability claims aren’t getting, ‘benefit of the doubt’. Military. com. October 15, 2024. Accessed October 21, 2024. https:// www.military.com/daily-news/2024/10/15/supreme-court-hears-case-questioning-vas-commitment-favoring-veterans-benefits-decisions.html
- Rehagen J. SCOTUS’s chevron deference ruling: how it could hurt veterans and the VA. Veteran.com. Updated July 9, 2024. Accessed October 21, 2024. https://veteran.com/scotus-chevron-deference-impact-va-veteran/
- Hersey LF. Lawmakers urge VA to reduce backlog, wait times on veterans claims for benefits. Stars & Stripes. June 27, 2024. Accessed October 21, 2024. https://www.stripes.com/veterans/2024-06-27/veterans-benefits-claims-backlog-pact-act-14315042.html
- Harper CJ. Give veterans the benefit of the doubt: Chevron, Auer, and the veteran’s canon. Harvard J Law Public Policy. 2019; 42(3):931-969. https://journals.law.harvard.edu/jlpp/wp-content/uploads/sites/90/2019/06/42_3-Full-Issue.pdf
- Fishgold v Sullivan Drydock & Repair Corp, 328 US 275, 285 (1946).
- Henderson v Shinseki, 562 US. 428, 440-441 (2011).
- US Department of Veterans Affairs, National Veteran Outreach Office. The difference between Veterans Day and Memorial Day. October 30, 2023. Accessed October 21, 2024. https://news.va.gov/125549/difference-between-veterans-day-memorial-day/
- US Department of Veterans Affairs. VA history summary. Updated August 6, 2024. Accessed October 21, 2024. https://department.va.gov/history/history-overview
- Cornell Law School, Legal Information Institute. Canons of construction. Updated March 2022. Accessed October 21, 2024. https://www.law.cornell.edu/wex/canons_of_construction
- Brown v Gardner, 513 US 115 (1994).
- Rudisill v McDonough, 601 US __ (2024).
- Hoover J. Justices will decide if vets are getting the ‘benefit of the doubt’. National Law Journal. April 30, 2024. Accessed October 21, 2024. https://www.law.com/nationallawjournal/2024/04/30/justices-will-decide-if-vets-are-getting-the-benefit-of-the-doubt/
- Relentless, Inc. v Department of Commerce Docket # 22-219, January 17, 2024.
- Kime P. Two veterans will argue to Supreme Court that VA disability claims aren’t getting, ‘benefit of the doubt’. Military. com. October 15, 2024. Accessed October 21, 2024. https:// www.military.com/daily-news/2024/10/15/supreme-court-hears-case-questioning-vas-commitment-favoring-veterans-benefits-decisions.html
- Rehagen J. SCOTUS’s chevron deference ruling: how it could hurt veterans and the VA. Veteran.com. Updated July 9, 2024. Accessed October 21, 2024. https://veteran.com/scotus-chevron-deference-impact-va-veteran/
- Hersey LF. Lawmakers urge VA to reduce backlog, wait times on veterans claims for benefits. Stars & Stripes. June 27, 2024. Accessed October 21, 2024. https://www.stripes.com/veterans/2024-06-27/veterans-benefits-claims-backlog-pact-act-14315042.html
- Harper CJ. Give veterans the benefit of the doubt: Chevron, Auer, and the veteran’s canon. Harvard J Law Public Policy. 2019; 42(3):931-969. https://journals.law.harvard.edu/jlpp/wp-content/uploads/sites/90/2019/06/42_3-Full-Issue.pdf
- Fishgold v Sullivan Drydock & Repair Corp, 328 US 275, 285 (1946).
The Veteran’s Canon Under Fire
The Veteran’s Canon Under Fire
Effect of Alirocumab Monotherapy vs Ezetimibe Plus Statin Therapy on LDL-C Lowering in Veterans With History of ASCVD
Atherosclerotic cardiovascular disease (ASCVD) is a significant cause of morbidity and mortality in the United States. ASCVD involves the buildup of cholesterol plaque in arteries and includes acute coronary syndrome, peripheral arterial disease, and events such as myocardial infarction and stroke.1 Cardiovascular disease (CVD) risk factors include high cholesterol levels, elevated blood pressure, insulin resistance, elevated blood glucose levels, smoking, poor dietary habits, and a sedentary lifestyle.2
According to the Centers for Disease Control and Prevention, about 86 million adults aged ≥ 20 years have total cholesterol levels > 200 mg/dL. More than half (54.5%) who could benefit are currently taking cholesterol-lowering medications.3 Controlling high cholesterol in American adults, especially veterans, is essential for reducing CVD morbidity and mortality.
The 2018 American College of Cardiology/American Heart Association (ACC/AHA) guideline recommends a low-density lipoprotein cholesterol (LDL-C) target goal of < 70 mg/dL for patients at high risk for ASCVD. Very high-risk ASCVD includes a history of multiple major ASCVD events or 1 major ASCVD event and multiple high-risk conditions (eg, age ≥ 65 years, smoking, or diabetes).4 Major ASCVD events include recent acute coronary syndrome (within the past 12 months), a history of myocardial infarction or ischemic stroke, and symptomatic peripheral artery disease.
The ACC/AHA guideline suggests that if the LDL-C level remains ≥ 70 mg/dL, adding ezetimibe (a dietary cholesterol absorption inhibitor) to maximally tolerated statin therapy is reasonable. If LDL-C levels remain ≥ 70 mg/dL, adding a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor, such as alirocumab, is reasonable.4 The US Departments of Veterans Affairs/US Department of Defense guidelines recommend using maximally tolerated statins and ezetimibe before PCSK9 inhibitors due to established long-term safety and reduction in CVD events.
Generic statins and ezetimibe are administered orally and widely available. In contrast, PCSK9 inhibitors have unknown long-term safety profiles, require subcutaneous injection once or twice monthly, and are significantly more expensive. They also require patient education on proper use while providing comparable or lesser relative risk reductions.2
These 3 classes of medication vary in their mechanisms of action to reduce LDL.5,6 Ezetimibe and several statin medications are included on the Veterans Affairs Sioux Falls Health Care System (VASFHCS) formulary and do not require review prior to prescribing. Alirocumab is available at VASFHCS but is restricted to patients with a history of ASCVD or a diagnosis of familial hypercholesterolemia, and who are receiving maximally tolerated statin and ezetimibe therapy but require further LDL-C lowering to reduce their ASCVD risk.
Studies have found ezetimibe monotherapy reduces LDL-C in patients with dyslipidemia by 18% after 12 weeks.7 One found that the percentage reduction in LDL-C was significantly greater (P < .001) with all doses of ezetimibe plus simvastatin (46% to 59%) compared with either atorvastatin 10 mg (37%) or simvastatin 20 mg (38%) monotherapy after 6 weeks.8
Although alirocumab can be added to other lipid therapies, most VASFHCS patients are prescribed alirocumab monotherapy. In the ODYSSEY CHOICE II study, patients were randomly assigned to receive either a placebo or alirocumab 150 mg every 4 weeks or alirocumab 75 mg every 2 weeks. The primary efficacy endpoint was LDL-C percentage change from baseline to week 24. In the alirocumab 150 mg every 4 weeks and 75 mg every 2 weeks groups, the least-squares mean LDL-C changes from baseline to week 24 were 51.7% and 53.5%, respectively, compared to a 4.7% increase in the placebo group (both groups P < .001 vs placebo). The authors also reported that alirocumab 150 mg every 4 weeks as monotherapy demonstrated a 47.4% reduction in LDL-C levels from baseline in a phase 1 study.9Although alirocumab monotherapy and ezetimibe plus statin therapy have been shown to effectively decrease LDL-C independently, a direct comparison of alirocumab monotherapy vs ezetimibe plus statin therapy has not been assessed, to our knowledge. Understanding the differences in effectiveness and safety between these 2 regimens will be valuable for clinicians when selecting a medication regimen for veterans with a history of ASCVD.
METHODS
This retrospective, single-center chart review used VASFHCS Computerized Patient Record System (CPRS) and Joint Longitudinal Viewer (JLV) records to compare patients with a history of ASCVD events who were treated with alirocumab monotherapy or ezetimibe plus statin. The 2 groups were randomized in a 1:3 ratio. The primary endpoint was achieving LDL-C < 70 mg/dL after 4 to 12 weeks, 13 to 24 weeks, and 25 to 52 weeks. Secondary endpoints included the mean percentage change from baseline in total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), LDL-C, and triglycerides (TG) over 52 weeks. The incidence of ASCVD events during this period was also assessed. If LDL-C < 70 mg/dL was achieved > 1 time during each time frame, only 1 incident was counted for analysis. Safety was assessed based on the incidence of any adverse event (AE) that led to treatment discontinuation.
Patients were identified by screening the prescription fill history between October 1, 2019, and December 31, 2022. The 52-week data collection period was counted from the first available fill date. Additionally, the prior authorization drug request file from January 1, 2017, to December 31, 2022, was used to obtain a list of patients prescribed alirocumab. Patients were included if they were veterans aged ≥ 18 years and had a history of an ASCVD event, had a alirocumab monotherapy or ezetimibe plus statin prescription between October 1, 2019, and December 31, 2022, or had an approved prior authorization drug request for alirocumab between January 1, 2017, and December 31, 2022. Patients missing a baseline or follow-up lipid panel and those with concurrent use of alirocumab and ezetimibe and/or statin were excluded.
Baseline characteristics collected for patients included age, sex, race, weight, body mass index, lipid parameters (LDL-C, TC, HDL-C, and TG), dosing of each type of statin before adding ezetimibe, and use of any other antihyperlipidemic medication. We also collected histories of hypertension, hyperlipidemia, diabetes, chronic kidney disease, congestive heart failure, and smoking or tobacco use status. The baseline lipid panel was the most recent lipid panel documented before starting alirocumab or ezetimibe plus statin therapy. Follow-up lipid panel values were gathered at 4 to 12 weeks, 13 to 24 weeks, and 25 to 52 weeks following initiation of either therapy.
High-, moderate-, and low-intensity dosing of statin therapy and alirocumab dosing (75 mg every 2 weeks, 150 mg every 2 weeks, or 300 mg every 4 weeks) were recorded at the specified intervals. However, no patients in this study received the latter dosing regimen. ASCVD events and safety endpoints were recorded based on a review of clinical notes over the 52 weeks following the first available start date.
Statistical Analysis
The primary endpoint of achieving the LDL-C < 70 mg/dL goal from baseline to 4 to 12 weeks, 13 to 24 weeks, and 25 to 52 weeks after initiation was compared between alirocumab monotherapy and ezetimibe plus statin therapy using the χ² test. Mean percentage change from baseline in TC, HDL-C, LDL-C, and TG were compared using the independent t test. P < .05 was considered statistically significant. Incidence of ASCVD events and the safety endpoint (incidence of AEs leading to treatment discontinuation) were also compared using the χ² test. Continuous baseline characteristics were reported mean (SD) and nominal baseline characteristics were reported as a percentage.
RESULTS
There were 80 participants in this study: 20 in the alirocumab monotherapy group and 60 in the ezetimibe plus statin therapy group. More than 100 patients did not meet the prespecified inclusion criteria and were excluded. Mean (SD) age was 75 (8) years in the alirocumab group and 74 (8) years in the ezetimibe plus statin group. There was no significant differences in mean (SD) weight or mean (SD) body mass index. All study participants identified as White and male except for 2 patients in the ezetimibe plus statin therapy group whose race was not documented. Differences in lipid parameters were observed between groups, with mean baseline LDL-C, HDL-C, and TC higher in the alirocumab monotherapy group than in the ezetimibe plus statin therapy group, with significant differences in LDL-C and TC (Table 1).
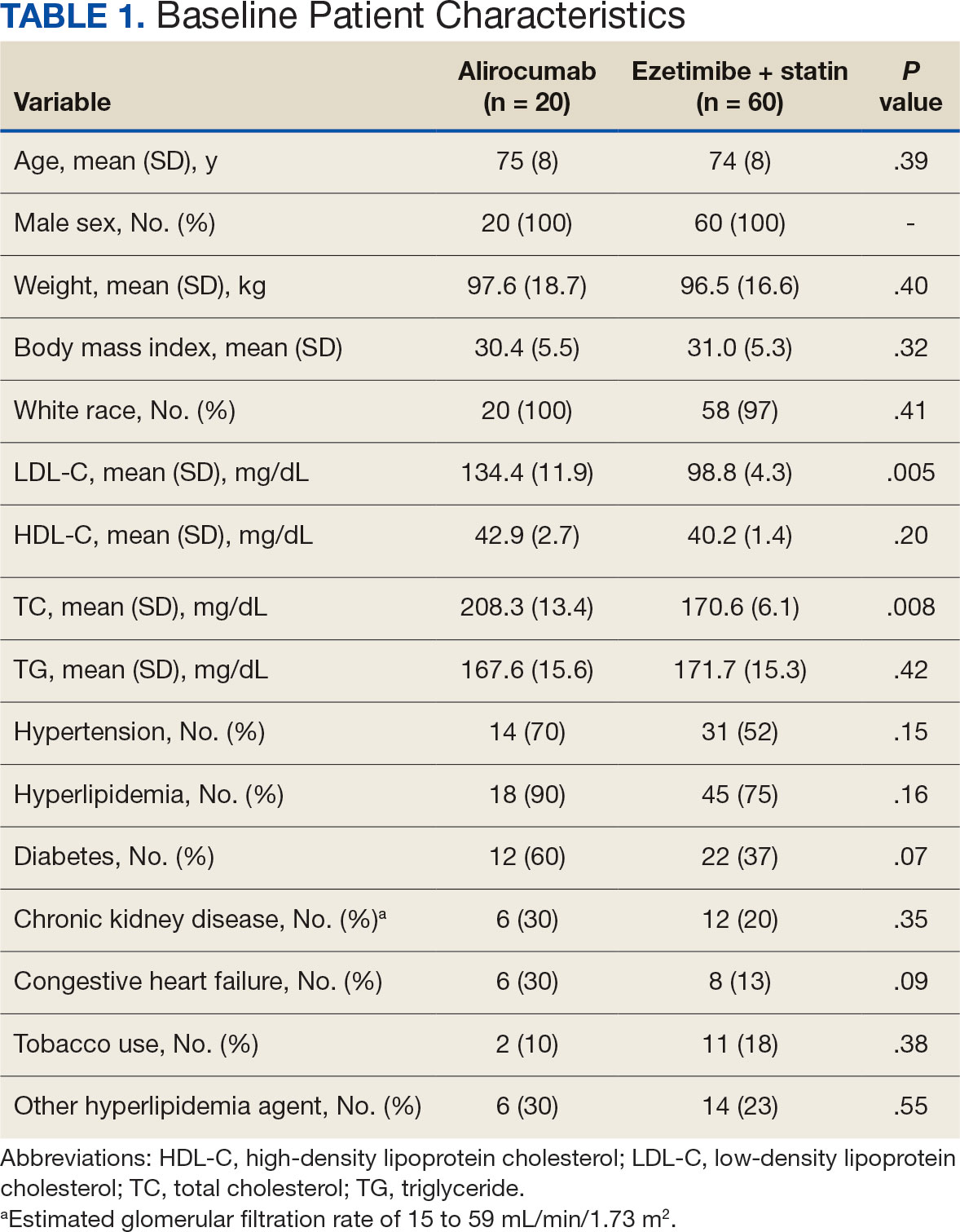
Fourteen patients (70%) in the alirocumab monotherapy group had hypertension, compared with 31 (52%) in the ezetimibe plus statin therapy group. In both groups, most patients had previously been diagnosed with hyperlipidemia. More patients (60%) in the alirocumab group had diabetes than in the ezetimibe plus statin therapy group (37%). The alirocumab monotherapy group also had a higher percentage of patients with diagnoses of congestive heart failure and used other antihyperlipidemic medications than in the ezetimibe plus statin therapy group. Five patients (25%) in the alirocumab monotherapy group and 12 patients (20%) in the ezetimibe plus statin therapy group took fish oil. In the ezetimibe plus statin therapy group, 2 patients (3%) took gemfibrozil, and 2 patients (3%) took fenofibrate. Six (30%) patients in the alirocumab monotherapy group and 12 (20%) patients in the ezetimibe plus statin therapy group had chronic kidney disease. Although the majority of patients in each group did not use tobacco products, there were more tobacco users in the ezetimibe plus statin therapy group.
In the alirocumab monotherapy group, 15 patients (75%) were prescribed 75 mg every 2 weeks and 5 patients (25%) were prescribed 150 mg every 2 weeks. In the ezetimibe plus statin therapy group, 59 patients (98%) were prescribed ezetimibe 10 mg/d (Table 2). Forty-three patients (72%) were prescribed a high-intensity statin 10 received moderate-intensity (17%) and 7 received low-intensity statin (12%). Most patients were prescribed rosuvastatin (45%), followed by atorvastatin (42%), pravastatin (10%), and simvastatin (3%).
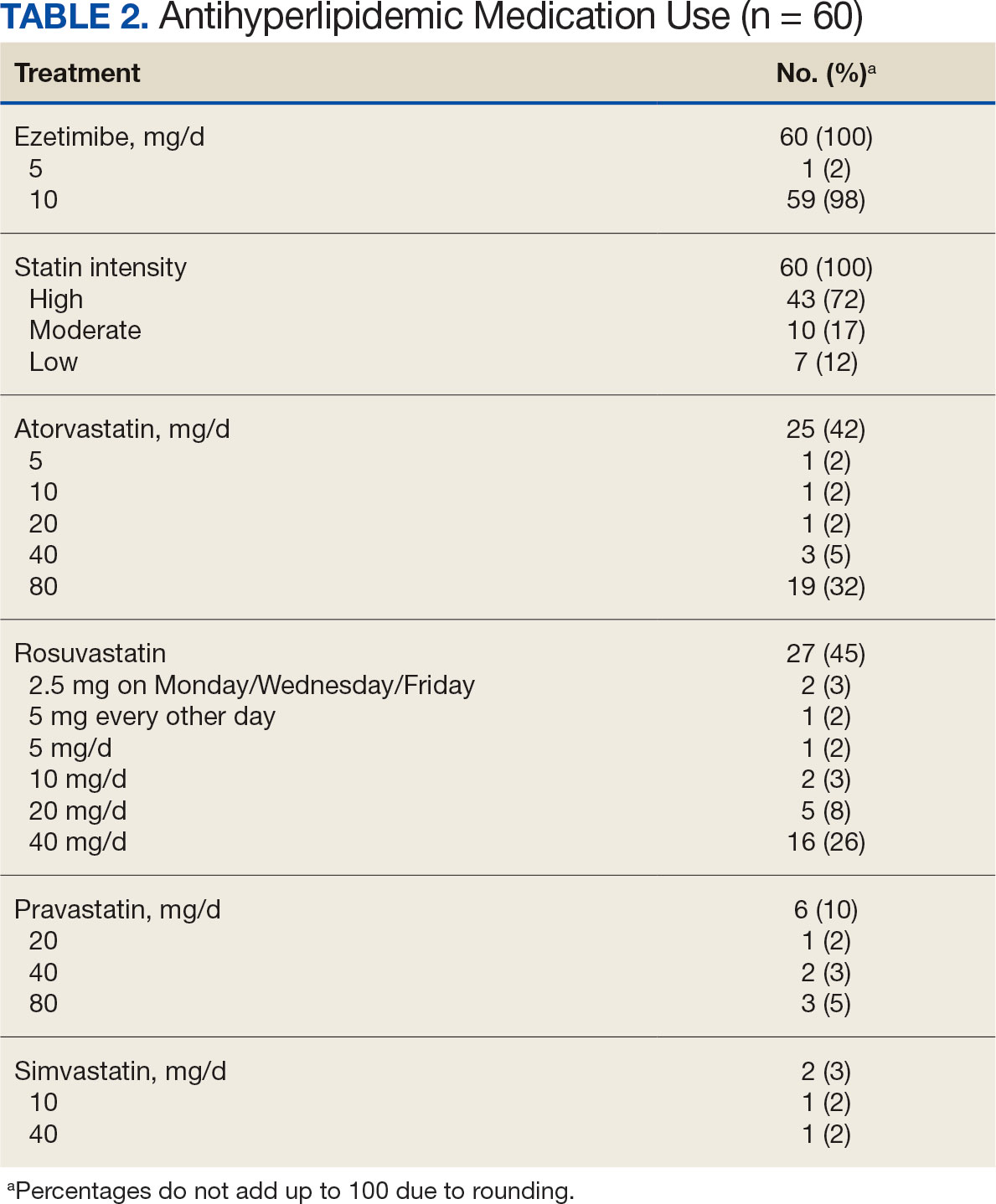
Primary Endpoint
During the 52-week study, more patients met the LDL-C goal of < 70 mg/dL in the alirocumab monotherapy group (70%) than in the ezetimibe plus statin therapy group (57%); however, the difference was not significant (P = .29). Of the patients prescribed alirocumab monotherapy who achieved LDL-C < 70 mg/dL, 15% achieved this goal in 4 to 12 weeks, 40% in 13 to 24 weeks, and 45% in 25 to 52 weeks. In the ezetimibe plus statin therapy group, 28% of patients achieved LDL-C < 70 mg/dL in 4 to 12 weeks, 31% in 13 to 24 weeks, and 41% in 25 to 52 weeks (Table 3).
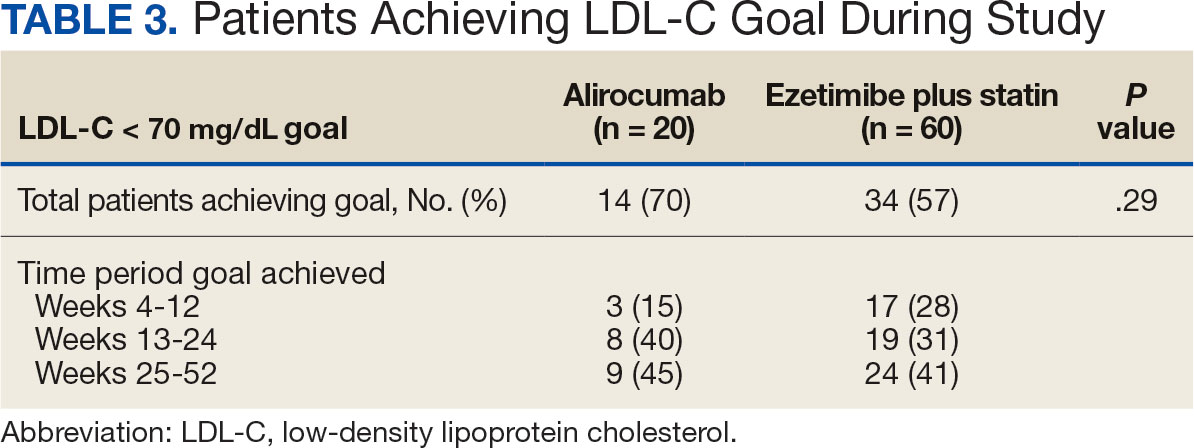
Secondary Endpoints
During weeks 4 to 52 of treatment, the mean percentage change decreased in LDL-C (37.7% vs 21.4%; P = .01), TC (24.7% vs 12.5%; P = .01), and TG (0.9% vs 7.0%; P = .28) in the alirocumab monotherapy group and the ezetimibe plus statin therapy group, respectively (Table 4). The mean percentage change increased in HDL-C by 3.6% in the alirocumab monotherapy group and 1.8% in the ezetimibe plus statin therapy group (P = .36). During the study, ASCVD events occurred in 1 patient (5%) in the alirocumab monotherapy group and 3 patients (5%) in the ezetimibe plus statin therapy group (P = .99). The patient in the alirocumab monotherapy group had unstable angina 1 month after taking alirocumab. One patient in the ezetimibe plus statin therapy group had coronary artery disease and 2 patients had coronary heart disease that required stents during the 52-week period. There was 1 patient in each group who reported an AE that led to treatment discontinuation (P = .41). One patient stopped alirocumab after a trial of 2 months due to intolerance, but no specific AE was reported in the CPRS. In the ezetimibe plus statin therapy group, 1 patient requested to discontinue ezetimibe after a trial of 3 months without a specific reason noted in the medical record.
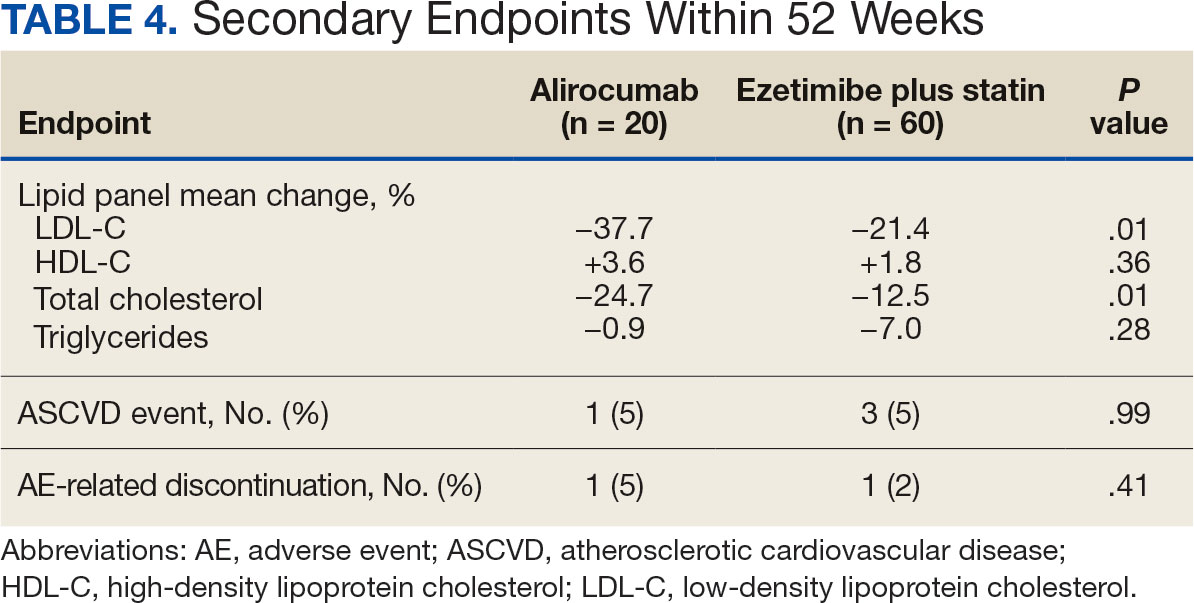
DISCUSSION
This study found no statistically significant difference in the incidence of reaching an LDL-C goal of < 70 mg/dL after alirocumab monotherapy initiation compared with ezetimibe plus statin therapy. This occurred despite baseline LDL-C being lower in the ezetimibe plus statin therapy group, which required a smaller reduction in LDL-C to reach the primary goal. Most patients on alirocumab monotherapy were prescribed a lower initial dose of 75 mg every 2 weeks. Of those patients, 30% did not achieve the LDL-C goal < 70 mg/dL. Thus, a higher dose may have led to more patients achieving the LDL-C goal.
Secondary endpoints, including mean percentage change in HDL-C and TG and incidence of ASCVD events during 52 weeks of treatment, were not statistically significant. The mean percentage increase in HDL-C was negligible in both groups, while the mean percentage reduction in TG favored the ezetimibe plus statin therapy group. In the ezetimibe plus statin therapy group, patients who also took fenofibrate experienced a significant reduction in TG while none of the patients in the alirocumab group were prescribed fenofibrate. Although the alirocumab monotherapy group had a statistically significant greater reduction in LDL-C and TC compared with those prescribed ezetimibe plus statin, the mean baseline LDL-C and TC were significantly greater in the alirocumab monotherapy group, which could contribute to higher reductions in LDL-C and TC after alirocumab monotherapy.Based on the available literature, we expected greater reductions in LDL-C in both study groups compared with statin therapy alone.8,9 However, it was unclear whether the LDL-C and TC reductions were clinically significant.
Limitations
The study design did not permit randomization prior to the treatments, restricting our ability to account for some confounding factors, such as diet, exercise, other antihyperlipidemic medication, and medication adherence, which may have affected LDL-C, HDL-C, TG, and TC levels. Differences in baseline characteristics—particularly major risk factors, such as hypertension, diabetes, and tobacco use—also could have confounding affect on lipid levels and ASCVD events. Additionally, patients prescribed alirocumab monotherapy may have switched from statin or ezetimibe therapy, and the washout period was not reviewed or recorded, which could have affected the lipid panel results.
The small sample size of this study also may have limited the ability to detect significant differences between groups. A direct comparison of alirocumab monotherapy vs ezetimibe plus statin therapy has not been performed, making it difficult to prospectively evaluate what sample size would be needed to power this study. A posthoc analysis was used to calculate power, which was found to be only 17%. Many patients were excluded due to a lack of laboratory results within the study period, contributing to the small sample size.
Another limitation was the reliance on documentation in CPRS and JLV. For example, having documentation of the specific AEs for the 2 patients who discontinued alirocumab or ezetimibe could have helped determine the severity of the AEs. Several patients were followed by non-VA clinicians, which could have contributed to limited documentation in the CPRS and JLV. It is difficult to draw any conclusions regarding ASCVD events and AEs that led to treatment discontinuation between alirocumab monotherapy and ezetimibe plus statin therapy based on the results of this retrospective study due to the limited number of events within the 52-week period.
CONCLUSIONS
This study found that there was no statistically significant difference in LDL-C reduction to < 70 mg/dL between alirocumab monotherapy and ezetimibe plus statin therapy in a small population of veterans with ASCVD, with a higher percentage of participants in both groups achieving that goal in 25 to 52 weeks. There also was no significant difference in percentage change in HDL-C or TG or in incidence of ASCVD events and AEs leading to treatment discontinuation. However, there was a statistically significant difference in percentage reduction for LDL-C and TC during 52 weeks of alirocumab monotherapy vs ezetimibe plus statin therapy.
Although there was no significant difference in LDL-C reduction to < 70 mg/dL, targeting this goal in patients with ASCVD is still clinically warranted. This study does not support a change in current VA criteria for use of alirocumab or a change in current guidelines for secondary prevention of ASCVD. Still, this study does indicate that the efficacy of alirocumab monotherapy is similar to that of ezetimibe plus statin therapy in patients with a history of ASCVD and may be useful in clinical settings when an alternative to ezetimibe plus statin therapy is needed. Alirocumab also may be more effective in lowering LDL-C and TC than ezetimibe plus statin therapy in veterans with ASCVD and could be added to statin therapy or ezetimibe when additional LDL-C or TC reduction is needed.
Lucchi T. Dyslipidemia and prevention of atherosclerotic cardiovascular disease in the elderly. Minerva Med. 2021;112:804-816. doi:10.23736/S0026-4806.21.07347-X
The Management of Dyslipidemia for Cardiovascular Risk Reduction Work Group. VA/DoD Clinical Practice Guideline for the Management of Dyslipidemia for Cardiovascular Risk Reduction. Version 4.0. June 2020. Accessed September 5, 2024. https://www.healthquality.va.gov/guidelines/CD/lipids/VADoDDyslipidemiaCPG5087212020.pdf
Centers for Disease Control and Prevention. High Cholesterol Facts. May 15, 2024. Accessed October 3, 2024. https://www.cdc.gov/cholesterol/data-research/facts-stats/index.html
Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082-e1143. doi:10.1161/CIR.0000000000000625
Vavlukis M, Vavlukis A. Statins alone or in combination with ezetimibe or PCSK9 inhibitors in atherosclerotic cardiovascular disease protection. IntechOpen. January 24, 2019. doi:10.5772/intechopen.82520
Alirocumab. Prescribing information. Regeneron Pharmaceuticals, Inc.; 2024. Accessed September 5, 2024. https://www.regeneron.com/downloads/praluent_pi.pdf
Pandor A, Ara RM, Tumur I, et al. Ezetimibe monotherapy for cholesterol lowering in 2,722 people: systematic review and meta-analysis of randomized controlled trials. J Intern Med. 2009;265(5):568-580. doi:10.1111/j.1365-2796.2008.02062.x
McKenney J, Ballantyne CM, Feldman TA, et al. LDL-C goal attainment with ezetimibe plus simvastatin coadministration vs atorvastatin or simvastatin monotherapy in patients at high risk of CHD. MedGenMed. 2005;7(3):3.
Stroes E, Guyton JR, Lepor N, et al. Efficacy and safety of alirocumab 150 mg every 4 weeks in patients with hypercholesterolemia not on statin therapy: the ODYSSEY CHOICE II study. J Am Heart Assoc. 2016;5(9):e003421. doi:10.1161/JAHA.116.003421
Atherosclerotic cardiovascular disease (ASCVD) is a significant cause of morbidity and mortality in the United States. ASCVD involves the buildup of cholesterol plaque in arteries and includes acute coronary syndrome, peripheral arterial disease, and events such as myocardial infarction and stroke.1 Cardiovascular disease (CVD) risk factors include high cholesterol levels, elevated blood pressure, insulin resistance, elevated blood glucose levels, smoking, poor dietary habits, and a sedentary lifestyle.2
According to the Centers for Disease Control and Prevention, about 86 million adults aged ≥ 20 years have total cholesterol levels > 200 mg/dL. More than half (54.5%) who could benefit are currently taking cholesterol-lowering medications.3 Controlling high cholesterol in American adults, especially veterans, is essential for reducing CVD morbidity and mortality.
The 2018 American College of Cardiology/American Heart Association (ACC/AHA) guideline recommends a low-density lipoprotein cholesterol (LDL-C) target goal of < 70 mg/dL for patients at high risk for ASCVD. Very high-risk ASCVD includes a history of multiple major ASCVD events or 1 major ASCVD event and multiple high-risk conditions (eg, age ≥ 65 years, smoking, or diabetes).4 Major ASCVD events include recent acute coronary syndrome (within the past 12 months), a history of myocardial infarction or ischemic stroke, and symptomatic peripheral artery disease.
The ACC/AHA guideline suggests that if the LDL-C level remains ≥ 70 mg/dL, adding ezetimibe (a dietary cholesterol absorption inhibitor) to maximally tolerated statin therapy is reasonable. If LDL-C levels remain ≥ 70 mg/dL, adding a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor, such as alirocumab, is reasonable.4 The US Departments of Veterans Affairs/US Department of Defense guidelines recommend using maximally tolerated statins and ezetimibe before PCSK9 inhibitors due to established long-term safety and reduction in CVD events.
Generic statins and ezetimibe are administered orally and widely available. In contrast, PCSK9 inhibitors have unknown long-term safety profiles, require subcutaneous injection once or twice monthly, and are significantly more expensive. They also require patient education on proper use while providing comparable or lesser relative risk reductions.2
These 3 classes of medication vary in their mechanisms of action to reduce LDL.5,6 Ezetimibe and several statin medications are included on the Veterans Affairs Sioux Falls Health Care System (VASFHCS) formulary and do not require review prior to prescribing. Alirocumab is available at VASFHCS but is restricted to patients with a history of ASCVD or a diagnosis of familial hypercholesterolemia, and who are receiving maximally tolerated statin and ezetimibe therapy but require further LDL-C lowering to reduce their ASCVD risk.
Studies have found ezetimibe monotherapy reduces LDL-C in patients with dyslipidemia by 18% after 12 weeks.7 One found that the percentage reduction in LDL-C was significantly greater (P < .001) with all doses of ezetimibe plus simvastatin (46% to 59%) compared with either atorvastatin 10 mg (37%) or simvastatin 20 mg (38%) monotherapy after 6 weeks.8
Although alirocumab can be added to other lipid therapies, most VASFHCS patients are prescribed alirocumab monotherapy. In the ODYSSEY CHOICE II study, patients were randomly assigned to receive either a placebo or alirocumab 150 mg every 4 weeks or alirocumab 75 mg every 2 weeks. The primary efficacy endpoint was LDL-C percentage change from baseline to week 24. In the alirocumab 150 mg every 4 weeks and 75 mg every 2 weeks groups, the least-squares mean LDL-C changes from baseline to week 24 were 51.7% and 53.5%, respectively, compared to a 4.7% increase in the placebo group (both groups P < .001 vs placebo). The authors also reported that alirocumab 150 mg every 4 weeks as monotherapy demonstrated a 47.4% reduction in LDL-C levels from baseline in a phase 1 study.9Although alirocumab monotherapy and ezetimibe plus statin therapy have been shown to effectively decrease LDL-C independently, a direct comparison of alirocumab monotherapy vs ezetimibe plus statin therapy has not been assessed, to our knowledge. Understanding the differences in effectiveness and safety between these 2 regimens will be valuable for clinicians when selecting a medication regimen for veterans with a history of ASCVD.
METHODS
This retrospective, single-center chart review used VASFHCS Computerized Patient Record System (CPRS) and Joint Longitudinal Viewer (JLV) records to compare patients with a history of ASCVD events who were treated with alirocumab monotherapy or ezetimibe plus statin. The 2 groups were randomized in a 1:3 ratio. The primary endpoint was achieving LDL-C < 70 mg/dL after 4 to 12 weeks, 13 to 24 weeks, and 25 to 52 weeks. Secondary endpoints included the mean percentage change from baseline in total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), LDL-C, and triglycerides (TG) over 52 weeks. The incidence of ASCVD events during this period was also assessed. If LDL-C < 70 mg/dL was achieved > 1 time during each time frame, only 1 incident was counted for analysis. Safety was assessed based on the incidence of any adverse event (AE) that led to treatment discontinuation.
Patients were identified by screening the prescription fill history between October 1, 2019, and December 31, 2022. The 52-week data collection period was counted from the first available fill date. Additionally, the prior authorization drug request file from January 1, 2017, to December 31, 2022, was used to obtain a list of patients prescribed alirocumab. Patients were included if they were veterans aged ≥ 18 years and had a history of an ASCVD event, had a alirocumab monotherapy or ezetimibe plus statin prescription between October 1, 2019, and December 31, 2022, or had an approved prior authorization drug request for alirocumab between January 1, 2017, and December 31, 2022. Patients missing a baseline or follow-up lipid panel and those with concurrent use of alirocumab and ezetimibe and/or statin were excluded.
Baseline characteristics collected for patients included age, sex, race, weight, body mass index, lipid parameters (LDL-C, TC, HDL-C, and TG), dosing of each type of statin before adding ezetimibe, and use of any other antihyperlipidemic medication. We also collected histories of hypertension, hyperlipidemia, diabetes, chronic kidney disease, congestive heart failure, and smoking or tobacco use status. The baseline lipid panel was the most recent lipid panel documented before starting alirocumab or ezetimibe plus statin therapy. Follow-up lipid panel values were gathered at 4 to 12 weeks, 13 to 24 weeks, and 25 to 52 weeks following initiation of either therapy.
High-, moderate-, and low-intensity dosing of statin therapy and alirocumab dosing (75 mg every 2 weeks, 150 mg every 2 weeks, or 300 mg every 4 weeks) were recorded at the specified intervals. However, no patients in this study received the latter dosing regimen. ASCVD events and safety endpoints were recorded based on a review of clinical notes over the 52 weeks following the first available start date.
Statistical Analysis
The primary endpoint of achieving the LDL-C < 70 mg/dL goal from baseline to 4 to 12 weeks, 13 to 24 weeks, and 25 to 52 weeks after initiation was compared between alirocumab monotherapy and ezetimibe plus statin therapy using the χ² test. Mean percentage change from baseline in TC, HDL-C, LDL-C, and TG were compared using the independent t test. P < .05 was considered statistically significant. Incidence of ASCVD events and the safety endpoint (incidence of AEs leading to treatment discontinuation) were also compared using the χ² test. Continuous baseline characteristics were reported mean (SD) and nominal baseline characteristics were reported as a percentage.
RESULTS
There were 80 participants in this study: 20 in the alirocumab monotherapy group and 60 in the ezetimibe plus statin therapy group. More than 100 patients did not meet the prespecified inclusion criteria and were excluded. Mean (SD) age was 75 (8) years in the alirocumab group and 74 (8) years in the ezetimibe plus statin group. There was no significant differences in mean (SD) weight or mean (SD) body mass index. All study participants identified as White and male except for 2 patients in the ezetimibe plus statin therapy group whose race was not documented. Differences in lipid parameters were observed between groups, with mean baseline LDL-C, HDL-C, and TC higher in the alirocumab monotherapy group than in the ezetimibe plus statin therapy group, with significant differences in LDL-C and TC (Table 1).

Fourteen patients (70%) in the alirocumab monotherapy group had hypertension, compared with 31 (52%) in the ezetimibe plus statin therapy group. In both groups, most patients had previously been diagnosed with hyperlipidemia. More patients (60%) in the alirocumab group had diabetes than in the ezetimibe plus statin therapy group (37%). The alirocumab monotherapy group also had a higher percentage of patients with diagnoses of congestive heart failure and used other antihyperlipidemic medications than in the ezetimibe plus statin therapy group. Five patients (25%) in the alirocumab monotherapy group and 12 patients (20%) in the ezetimibe plus statin therapy group took fish oil. In the ezetimibe plus statin therapy group, 2 patients (3%) took gemfibrozil, and 2 patients (3%) took fenofibrate. Six (30%) patients in the alirocumab monotherapy group and 12 (20%) patients in the ezetimibe plus statin therapy group had chronic kidney disease. Although the majority of patients in each group did not use tobacco products, there were more tobacco users in the ezetimibe plus statin therapy group.
In the alirocumab monotherapy group, 15 patients (75%) were prescribed 75 mg every 2 weeks and 5 patients (25%) were prescribed 150 mg every 2 weeks. In the ezetimibe plus statin therapy group, 59 patients (98%) were prescribed ezetimibe 10 mg/d (Table 2). Forty-three patients (72%) were prescribed a high-intensity statin 10 received moderate-intensity (17%) and 7 received low-intensity statin (12%). Most patients were prescribed rosuvastatin (45%), followed by atorvastatin (42%), pravastatin (10%), and simvastatin (3%).

Primary Endpoint
During the 52-week study, more patients met the LDL-C goal of < 70 mg/dL in the alirocumab monotherapy group (70%) than in the ezetimibe plus statin therapy group (57%); however, the difference was not significant (P = .29). Of the patients prescribed alirocumab monotherapy who achieved LDL-C < 70 mg/dL, 15% achieved this goal in 4 to 12 weeks, 40% in 13 to 24 weeks, and 45% in 25 to 52 weeks. In the ezetimibe plus statin therapy group, 28% of patients achieved LDL-C < 70 mg/dL in 4 to 12 weeks, 31% in 13 to 24 weeks, and 41% in 25 to 52 weeks (Table 3).

Secondary Endpoints
During weeks 4 to 52 of treatment, the mean percentage change decreased in LDL-C (37.7% vs 21.4%; P = .01), TC (24.7% vs 12.5%; P = .01), and TG (0.9% vs 7.0%; P = .28) in the alirocumab monotherapy group and the ezetimibe plus statin therapy group, respectively (Table 4). The mean percentage change increased in HDL-C by 3.6% in the alirocumab monotherapy group and 1.8% in the ezetimibe plus statin therapy group (P = .36). During the study, ASCVD events occurred in 1 patient (5%) in the alirocumab monotherapy group and 3 patients (5%) in the ezetimibe plus statin therapy group (P = .99). The patient in the alirocumab monotherapy group had unstable angina 1 month after taking alirocumab. One patient in the ezetimibe plus statin therapy group had coronary artery disease and 2 patients had coronary heart disease that required stents during the 52-week period. There was 1 patient in each group who reported an AE that led to treatment discontinuation (P = .41). One patient stopped alirocumab after a trial of 2 months due to intolerance, but no specific AE was reported in the CPRS. In the ezetimibe plus statin therapy group, 1 patient requested to discontinue ezetimibe after a trial of 3 months without a specific reason noted in the medical record.

DISCUSSION
This study found no statistically significant difference in the incidence of reaching an LDL-C goal of < 70 mg/dL after alirocumab monotherapy initiation compared with ezetimibe plus statin therapy. This occurred despite baseline LDL-C being lower in the ezetimibe plus statin therapy group, which required a smaller reduction in LDL-C to reach the primary goal. Most patients on alirocumab monotherapy were prescribed a lower initial dose of 75 mg every 2 weeks. Of those patients, 30% did not achieve the LDL-C goal < 70 mg/dL. Thus, a higher dose may have led to more patients achieving the LDL-C goal.
Secondary endpoints, including mean percentage change in HDL-C and TG and incidence of ASCVD events during 52 weeks of treatment, were not statistically significant. The mean percentage increase in HDL-C was negligible in both groups, while the mean percentage reduction in TG favored the ezetimibe plus statin therapy group. In the ezetimibe plus statin therapy group, patients who also took fenofibrate experienced a significant reduction in TG while none of the patients in the alirocumab group were prescribed fenofibrate. Although the alirocumab monotherapy group had a statistically significant greater reduction in LDL-C and TC compared with those prescribed ezetimibe plus statin, the mean baseline LDL-C and TC were significantly greater in the alirocumab monotherapy group, which could contribute to higher reductions in LDL-C and TC after alirocumab monotherapy.Based on the available literature, we expected greater reductions in LDL-C in both study groups compared with statin therapy alone.8,9 However, it was unclear whether the LDL-C and TC reductions were clinically significant.
Limitations
The study design did not permit randomization prior to the treatments, restricting our ability to account for some confounding factors, such as diet, exercise, other antihyperlipidemic medication, and medication adherence, which may have affected LDL-C, HDL-C, TG, and TC levels. Differences in baseline characteristics—particularly major risk factors, such as hypertension, diabetes, and tobacco use—also could have confounding affect on lipid levels and ASCVD events. Additionally, patients prescribed alirocumab monotherapy may have switched from statin or ezetimibe therapy, and the washout period was not reviewed or recorded, which could have affected the lipid panel results.
The small sample size of this study also may have limited the ability to detect significant differences between groups. A direct comparison of alirocumab monotherapy vs ezetimibe plus statin therapy has not been performed, making it difficult to prospectively evaluate what sample size would be needed to power this study. A posthoc analysis was used to calculate power, which was found to be only 17%. Many patients were excluded due to a lack of laboratory results within the study period, contributing to the small sample size.
Another limitation was the reliance on documentation in CPRS and JLV. For example, having documentation of the specific AEs for the 2 patients who discontinued alirocumab or ezetimibe could have helped determine the severity of the AEs. Several patients were followed by non-VA clinicians, which could have contributed to limited documentation in the CPRS and JLV. It is difficult to draw any conclusions regarding ASCVD events and AEs that led to treatment discontinuation between alirocumab monotherapy and ezetimibe plus statin therapy based on the results of this retrospective study due to the limited number of events within the 52-week period.
CONCLUSIONS
This study found that there was no statistically significant difference in LDL-C reduction to < 70 mg/dL between alirocumab monotherapy and ezetimibe plus statin therapy in a small population of veterans with ASCVD, with a higher percentage of participants in both groups achieving that goal in 25 to 52 weeks. There also was no significant difference in percentage change in HDL-C or TG or in incidence of ASCVD events and AEs leading to treatment discontinuation. However, there was a statistically significant difference in percentage reduction for LDL-C and TC during 52 weeks of alirocumab monotherapy vs ezetimibe plus statin therapy.
Although there was no significant difference in LDL-C reduction to < 70 mg/dL, targeting this goal in patients with ASCVD is still clinically warranted. This study does not support a change in current VA criteria for use of alirocumab or a change in current guidelines for secondary prevention of ASCVD. Still, this study does indicate that the efficacy of alirocumab monotherapy is similar to that of ezetimibe plus statin therapy in patients with a history of ASCVD and may be useful in clinical settings when an alternative to ezetimibe plus statin therapy is needed. Alirocumab also may be more effective in lowering LDL-C and TC than ezetimibe plus statin therapy in veterans with ASCVD and could be added to statin therapy or ezetimibe when additional LDL-C or TC reduction is needed.
Atherosclerotic cardiovascular disease (ASCVD) is a significant cause of morbidity and mortality in the United States. ASCVD involves the buildup of cholesterol plaque in arteries and includes acute coronary syndrome, peripheral arterial disease, and events such as myocardial infarction and stroke.1 Cardiovascular disease (CVD) risk factors include high cholesterol levels, elevated blood pressure, insulin resistance, elevated blood glucose levels, smoking, poor dietary habits, and a sedentary lifestyle.2
According to the Centers for Disease Control and Prevention, about 86 million adults aged ≥ 20 years have total cholesterol levels > 200 mg/dL. More than half (54.5%) who could benefit are currently taking cholesterol-lowering medications.3 Controlling high cholesterol in American adults, especially veterans, is essential for reducing CVD morbidity and mortality.
The 2018 American College of Cardiology/American Heart Association (ACC/AHA) guideline recommends a low-density lipoprotein cholesterol (LDL-C) target goal of < 70 mg/dL for patients at high risk for ASCVD. Very high-risk ASCVD includes a history of multiple major ASCVD events or 1 major ASCVD event and multiple high-risk conditions (eg, age ≥ 65 years, smoking, or diabetes).4 Major ASCVD events include recent acute coronary syndrome (within the past 12 months), a history of myocardial infarction or ischemic stroke, and symptomatic peripheral artery disease.
The ACC/AHA guideline suggests that if the LDL-C level remains ≥ 70 mg/dL, adding ezetimibe (a dietary cholesterol absorption inhibitor) to maximally tolerated statin therapy is reasonable. If LDL-C levels remain ≥ 70 mg/dL, adding a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor, such as alirocumab, is reasonable.4 The US Departments of Veterans Affairs/US Department of Defense guidelines recommend using maximally tolerated statins and ezetimibe before PCSK9 inhibitors due to established long-term safety and reduction in CVD events.
Generic statins and ezetimibe are administered orally and widely available. In contrast, PCSK9 inhibitors have unknown long-term safety profiles, require subcutaneous injection once or twice monthly, and are significantly more expensive. They also require patient education on proper use while providing comparable or lesser relative risk reductions.2
These 3 classes of medication vary in their mechanisms of action to reduce LDL.5,6 Ezetimibe and several statin medications are included on the Veterans Affairs Sioux Falls Health Care System (VASFHCS) formulary and do not require review prior to prescribing. Alirocumab is available at VASFHCS but is restricted to patients with a history of ASCVD or a diagnosis of familial hypercholesterolemia, and who are receiving maximally tolerated statin and ezetimibe therapy but require further LDL-C lowering to reduce their ASCVD risk.
Studies have found ezetimibe monotherapy reduces LDL-C in patients with dyslipidemia by 18% after 12 weeks.7 One found that the percentage reduction in LDL-C was significantly greater (P < .001) with all doses of ezetimibe plus simvastatin (46% to 59%) compared with either atorvastatin 10 mg (37%) or simvastatin 20 mg (38%) monotherapy after 6 weeks.8
Although alirocumab can be added to other lipid therapies, most VASFHCS patients are prescribed alirocumab monotherapy. In the ODYSSEY CHOICE II study, patients were randomly assigned to receive either a placebo or alirocumab 150 mg every 4 weeks or alirocumab 75 mg every 2 weeks. The primary efficacy endpoint was LDL-C percentage change from baseline to week 24. In the alirocumab 150 mg every 4 weeks and 75 mg every 2 weeks groups, the least-squares mean LDL-C changes from baseline to week 24 were 51.7% and 53.5%, respectively, compared to a 4.7% increase in the placebo group (both groups P < .001 vs placebo). The authors also reported that alirocumab 150 mg every 4 weeks as monotherapy demonstrated a 47.4% reduction in LDL-C levels from baseline in a phase 1 study.9Although alirocumab monotherapy and ezetimibe plus statin therapy have been shown to effectively decrease LDL-C independently, a direct comparison of alirocumab monotherapy vs ezetimibe plus statin therapy has not been assessed, to our knowledge. Understanding the differences in effectiveness and safety between these 2 regimens will be valuable for clinicians when selecting a medication regimen for veterans with a history of ASCVD.
METHODS
This retrospective, single-center chart review used VASFHCS Computerized Patient Record System (CPRS) and Joint Longitudinal Viewer (JLV) records to compare patients with a history of ASCVD events who were treated with alirocumab monotherapy or ezetimibe plus statin. The 2 groups were randomized in a 1:3 ratio. The primary endpoint was achieving LDL-C < 70 mg/dL after 4 to 12 weeks, 13 to 24 weeks, and 25 to 52 weeks. Secondary endpoints included the mean percentage change from baseline in total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), LDL-C, and triglycerides (TG) over 52 weeks. The incidence of ASCVD events during this period was also assessed. If LDL-C < 70 mg/dL was achieved > 1 time during each time frame, only 1 incident was counted for analysis. Safety was assessed based on the incidence of any adverse event (AE) that led to treatment discontinuation.
Patients were identified by screening the prescription fill history between October 1, 2019, and December 31, 2022. The 52-week data collection period was counted from the first available fill date. Additionally, the prior authorization drug request file from January 1, 2017, to December 31, 2022, was used to obtain a list of patients prescribed alirocumab. Patients were included if they were veterans aged ≥ 18 years and had a history of an ASCVD event, had a alirocumab monotherapy or ezetimibe plus statin prescription between October 1, 2019, and December 31, 2022, or had an approved prior authorization drug request for alirocumab between January 1, 2017, and December 31, 2022. Patients missing a baseline or follow-up lipid panel and those with concurrent use of alirocumab and ezetimibe and/or statin were excluded.
Baseline characteristics collected for patients included age, sex, race, weight, body mass index, lipid parameters (LDL-C, TC, HDL-C, and TG), dosing of each type of statin before adding ezetimibe, and use of any other antihyperlipidemic medication. We also collected histories of hypertension, hyperlipidemia, diabetes, chronic kidney disease, congestive heart failure, and smoking or tobacco use status. The baseline lipid panel was the most recent lipid panel documented before starting alirocumab or ezetimibe plus statin therapy. Follow-up lipid panel values were gathered at 4 to 12 weeks, 13 to 24 weeks, and 25 to 52 weeks following initiation of either therapy.
High-, moderate-, and low-intensity dosing of statin therapy and alirocumab dosing (75 mg every 2 weeks, 150 mg every 2 weeks, or 300 mg every 4 weeks) were recorded at the specified intervals. However, no patients in this study received the latter dosing regimen. ASCVD events and safety endpoints were recorded based on a review of clinical notes over the 52 weeks following the first available start date.
Statistical Analysis
The primary endpoint of achieving the LDL-C < 70 mg/dL goal from baseline to 4 to 12 weeks, 13 to 24 weeks, and 25 to 52 weeks after initiation was compared between alirocumab monotherapy and ezetimibe plus statin therapy using the χ² test. Mean percentage change from baseline in TC, HDL-C, LDL-C, and TG were compared using the independent t test. P < .05 was considered statistically significant. Incidence of ASCVD events and the safety endpoint (incidence of AEs leading to treatment discontinuation) were also compared using the χ² test. Continuous baseline characteristics were reported mean (SD) and nominal baseline characteristics were reported as a percentage.
RESULTS
There were 80 participants in this study: 20 in the alirocumab monotherapy group and 60 in the ezetimibe plus statin therapy group. More than 100 patients did not meet the prespecified inclusion criteria and were excluded. Mean (SD) age was 75 (8) years in the alirocumab group and 74 (8) years in the ezetimibe plus statin group. There was no significant differences in mean (SD) weight or mean (SD) body mass index. All study participants identified as White and male except for 2 patients in the ezetimibe plus statin therapy group whose race was not documented. Differences in lipid parameters were observed between groups, with mean baseline LDL-C, HDL-C, and TC higher in the alirocumab monotherapy group than in the ezetimibe plus statin therapy group, with significant differences in LDL-C and TC (Table 1).

Fourteen patients (70%) in the alirocumab monotherapy group had hypertension, compared with 31 (52%) in the ezetimibe plus statin therapy group. In both groups, most patients had previously been diagnosed with hyperlipidemia. More patients (60%) in the alirocumab group had diabetes than in the ezetimibe plus statin therapy group (37%). The alirocumab monotherapy group also had a higher percentage of patients with diagnoses of congestive heart failure and used other antihyperlipidemic medications than in the ezetimibe plus statin therapy group. Five patients (25%) in the alirocumab monotherapy group and 12 patients (20%) in the ezetimibe plus statin therapy group took fish oil. In the ezetimibe plus statin therapy group, 2 patients (3%) took gemfibrozil, and 2 patients (3%) took fenofibrate. Six (30%) patients in the alirocumab monotherapy group and 12 (20%) patients in the ezetimibe plus statin therapy group had chronic kidney disease. Although the majority of patients in each group did not use tobacco products, there were more tobacco users in the ezetimibe plus statin therapy group.
In the alirocumab monotherapy group, 15 patients (75%) were prescribed 75 mg every 2 weeks and 5 patients (25%) were prescribed 150 mg every 2 weeks. In the ezetimibe plus statin therapy group, 59 patients (98%) were prescribed ezetimibe 10 mg/d (Table 2). Forty-three patients (72%) were prescribed a high-intensity statin 10 received moderate-intensity (17%) and 7 received low-intensity statin (12%). Most patients were prescribed rosuvastatin (45%), followed by atorvastatin (42%), pravastatin (10%), and simvastatin (3%).

Primary Endpoint
During the 52-week study, more patients met the LDL-C goal of < 70 mg/dL in the alirocumab monotherapy group (70%) than in the ezetimibe plus statin therapy group (57%); however, the difference was not significant (P = .29). Of the patients prescribed alirocumab monotherapy who achieved LDL-C < 70 mg/dL, 15% achieved this goal in 4 to 12 weeks, 40% in 13 to 24 weeks, and 45% in 25 to 52 weeks. In the ezetimibe plus statin therapy group, 28% of patients achieved LDL-C < 70 mg/dL in 4 to 12 weeks, 31% in 13 to 24 weeks, and 41% in 25 to 52 weeks (Table 3).

Secondary Endpoints
During weeks 4 to 52 of treatment, the mean percentage change decreased in LDL-C (37.7% vs 21.4%; P = .01), TC (24.7% vs 12.5%; P = .01), and TG (0.9% vs 7.0%; P = .28) in the alirocumab monotherapy group and the ezetimibe plus statin therapy group, respectively (Table 4). The mean percentage change increased in HDL-C by 3.6% in the alirocumab monotherapy group and 1.8% in the ezetimibe plus statin therapy group (P = .36). During the study, ASCVD events occurred in 1 patient (5%) in the alirocumab monotherapy group and 3 patients (5%) in the ezetimibe plus statin therapy group (P = .99). The patient in the alirocumab monotherapy group had unstable angina 1 month after taking alirocumab. One patient in the ezetimibe plus statin therapy group had coronary artery disease and 2 patients had coronary heart disease that required stents during the 52-week period. There was 1 patient in each group who reported an AE that led to treatment discontinuation (P = .41). One patient stopped alirocumab after a trial of 2 months due to intolerance, but no specific AE was reported in the CPRS. In the ezetimibe plus statin therapy group, 1 patient requested to discontinue ezetimibe after a trial of 3 months without a specific reason noted in the medical record.

DISCUSSION
This study found no statistically significant difference in the incidence of reaching an LDL-C goal of < 70 mg/dL after alirocumab monotherapy initiation compared with ezetimibe plus statin therapy. This occurred despite baseline LDL-C being lower in the ezetimibe plus statin therapy group, which required a smaller reduction in LDL-C to reach the primary goal. Most patients on alirocumab monotherapy were prescribed a lower initial dose of 75 mg every 2 weeks. Of those patients, 30% did not achieve the LDL-C goal < 70 mg/dL. Thus, a higher dose may have led to more patients achieving the LDL-C goal.
Secondary endpoints, including mean percentage change in HDL-C and TG and incidence of ASCVD events during 52 weeks of treatment, were not statistically significant. The mean percentage increase in HDL-C was negligible in both groups, while the mean percentage reduction in TG favored the ezetimibe plus statin therapy group. In the ezetimibe plus statin therapy group, patients who also took fenofibrate experienced a significant reduction in TG while none of the patients in the alirocumab group were prescribed fenofibrate. Although the alirocumab monotherapy group had a statistically significant greater reduction in LDL-C and TC compared with those prescribed ezetimibe plus statin, the mean baseline LDL-C and TC were significantly greater in the alirocumab monotherapy group, which could contribute to higher reductions in LDL-C and TC after alirocumab monotherapy.Based on the available literature, we expected greater reductions in LDL-C in both study groups compared with statin therapy alone.8,9 However, it was unclear whether the LDL-C and TC reductions were clinically significant.
Limitations
The study design did not permit randomization prior to the treatments, restricting our ability to account for some confounding factors, such as diet, exercise, other antihyperlipidemic medication, and medication adherence, which may have affected LDL-C, HDL-C, TG, and TC levels. Differences in baseline characteristics—particularly major risk factors, such as hypertension, diabetes, and tobacco use—also could have confounding affect on lipid levels and ASCVD events. Additionally, patients prescribed alirocumab monotherapy may have switched from statin or ezetimibe therapy, and the washout period was not reviewed or recorded, which could have affected the lipid panel results.
The small sample size of this study also may have limited the ability to detect significant differences between groups. A direct comparison of alirocumab monotherapy vs ezetimibe plus statin therapy has not been performed, making it difficult to prospectively evaluate what sample size would be needed to power this study. A posthoc analysis was used to calculate power, which was found to be only 17%. Many patients were excluded due to a lack of laboratory results within the study period, contributing to the small sample size.
Another limitation was the reliance on documentation in CPRS and JLV. For example, having documentation of the specific AEs for the 2 patients who discontinued alirocumab or ezetimibe could have helped determine the severity of the AEs. Several patients were followed by non-VA clinicians, which could have contributed to limited documentation in the CPRS and JLV. It is difficult to draw any conclusions regarding ASCVD events and AEs that led to treatment discontinuation between alirocumab monotherapy and ezetimibe plus statin therapy based on the results of this retrospective study due to the limited number of events within the 52-week period.
CONCLUSIONS
This study found that there was no statistically significant difference in LDL-C reduction to < 70 mg/dL between alirocumab monotherapy and ezetimibe plus statin therapy in a small population of veterans with ASCVD, with a higher percentage of participants in both groups achieving that goal in 25 to 52 weeks. There also was no significant difference in percentage change in HDL-C or TG or in incidence of ASCVD events and AEs leading to treatment discontinuation. However, there was a statistically significant difference in percentage reduction for LDL-C and TC during 52 weeks of alirocumab monotherapy vs ezetimibe plus statin therapy.
Although there was no significant difference in LDL-C reduction to < 70 mg/dL, targeting this goal in patients with ASCVD is still clinically warranted. This study does not support a change in current VA criteria for use of alirocumab or a change in current guidelines for secondary prevention of ASCVD. Still, this study does indicate that the efficacy of alirocumab monotherapy is similar to that of ezetimibe plus statin therapy in patients with a history of ASCVD and may be useful in clinical settings when an alternative to ezetimibe plus statin therapy is needed. Alirocumab also may be more effective in lowering LDL-C and TC than ezetimibe plus statin therapy in veterans with ASCVD and could be added to statin therapy or ezetimibe when additional LDL-C or TC reduction is needed.
Lucchi T. Dyslipidemia and prevention of atherosclerotic cardiovascular disease in the elderly. Minerva Med. 2021;112:804-816. doi:10.23736/S0026-4806.21.07347-X
The Management of Dyslipidemia for Cardiovascular Risk Reduction Work Group. VA/DoD Clinical Practice Guideline for the Management of Dyslipidemia for Cardiovascular Risk Reduction. Version 4.0. June 2020. Accessed September 5, 2024. https://www.healthquality.va.gov/guidelines/CD/lipids/VADoDDyslipidemiaCPG5087212020.pdf
Centers for Disease Control and Prevention. High Cholesterol Facts. May 15, 2024. Accessed October 3, 2024. https://www.cdc.gov/cholesterol/data-research/facts-stats/index.html
Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082-e1143. doi:10.1161/CIR.0000000000000625
Vavlukis M, Vavlukis A. Statins alone or in combination with ezetimibe or PCSK9 inhibitors in atherosclerotic cardiovascular disease protection. IntechOpen. January 24, 2019. doi:10.5772/intechopen.82520
Alirocumab. Prescribing information. Regeneron Pharmaceuticals, Inc.; 2024. Accessed September 5, 2024. https://www.regeneron.com/downloads/praluent_pi.pdf
Pandor A, Ara RM, Tumur I, et al. Ezetimibe monotherapy for cholesterol lowering in 2,722 people: systematic review and meta-analysis of randomized controlled trials. J Intern Med. 2009;265(5):568-580. doi:10.1111/j.1365-2796.2008.02062.x
McKenney J, Ballantyne CM, Feldman TA, et al. LDL-C goal attainment with ezetimibe plus simvastatin coadministration vs atorvastatin or simvastatin monotherapy in patients at high risk of CHD. MedGenMed. 2005;7(3):3.
Stroes E, Guyton JR, Lepor N, et al. Efficacy and safety of alirocumab 150 mg every 4 weeks in patients with hypercholesterolemia not on statin therapy: the ODYSSEY CHOICE II study. J Am Heart Assoc. 2016;5(9):e003421. doi:10.1161/JAHA.116.003421
Lucchi T. Dyslipidemia and prevention of atherosclerotic cardiovascular disease in the elderly. Minerva Med. 2021;112:804-816. doi:10.23736/S0026-4806.21.07347-X
The Management of Dyslipidemia for Cardiovascular Risk Reduction Work Group. VA/DoD Clinical Practice Guideline for the Management of Dyslipidemia for Cardiovascular Risk Reduction. Version 4.0. June 2020. Accessed September 5, 2024. https://www.healthquality.va.gov/guidelines/CD/lipids/VADoDDyslipidemiaCPG5087212020.pdf
Centers for Disease Control and Prevention. High Cholesterol Facts. May 15, 2024. Accessed October 3, 2024. https://www.cdc.gov/cholesterol/data-research/facts-stats/index.html
Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082-e1143. doi:10.1161/CIR.0000000000000625
Vavlukis M, Vavlukis A. Statins alone or in combination with ezetimibe or PCSK9 inhibitors in atherosclerotic cardiovascular disease protection. IntechOpen. January 24, 2019. doi:10.5772/intechopen.82520
Alirocumab. Prescribing information. Regeneron Pharmaceuticals, Inc.; 2024. Accessed September 5, 2024. https://www.regeneron.com/downloads/praluent_pi.pdf
Pandor A, Ara RM, Tumur I, et al. Ezetimibe monotherapy for cholesterol lowering in 2,722 people: systematic review and meta-analysis of randomized controlled trials. J Intern Med. 2009;265(5):568-580. doi:10.1111/j.1365-2796.2008.02062.x
McKenney J, Ballantyne CM, Feldman TA, et al. LDL-C goal attainment with ezetimibe plus simvastatin coadministration vs atorvastatin or simvastatin monotherapy in patients at high risk of CHD. MedGenMed. 2005;7(3):3.
Stroes E, Guyton JR, Lepor N, et al. Efficacy and safety of alirocumab 150 mg every 4 weeks in patients with hypercholesterolemia not on statin therapy: the ODYSSEY CHOICE II study. J Am Heart Assoc. 2016;5(9):e003421. doi:10.1161/JAHA.116.003421
Impact and Recovery of VHA Epilepsy Care Services During the COVID-19 Pandemic
The COVID-19 pandemic affected diverse workplaces globally, leading to temporary and permanent changes across the health care landscape. Included among the impacted areas of care were epilepsy and electroencephalogram (EEG) clinicians and services. Surveys among epilepsy specialists and neurophysiologists conducted at the onset of the pandemic to evaluate working conditions include analyses from the American Epilepsy Society (AES), the National Association of Epilepsy Centers (NAEC), the International League Against Epilepsy, and an Italian national survey.1-4 These investigations revealed reductions in epilepsy monitoring unit (EMU) admissions (23% decline), epilepsy surgery (6% decline), inpatient EEG (22% of respondents reported decline), and patients having difficulty accessing epilepsy professionals (28% of respondents reported decline) or obtaining medications (20% of respondents reported decline).1-3
While such research provided evidence for changes to epilepsy care in 2020, there are limited data on subsequent adaptations during the pandemic. These studies did not incorporate data on the spread of COVID-19 or administrative workload numbers to analyze service delivery beyond self reports. This study aimed to address this gap in the literature by highlighting results from longitudinal national surveys conducted at the Epilepsy Centers of Excellence (ECoE), a specialty care service within the Veterans Health Administration (VHA), which annually serves > 9 million veterans.5 The ECoE represents epileptologists and neurophysiologists across the United States at the 17 primary facilities that were established at the time of this survey (2 ECoEs have been added since survey completion) in 4 geographical regions and for which other regional facilities refer patients for diagnostic services or specialty care.6
National surveys were conducted among the ECoE directors regarding adaptations made from May 2020 to June 2022 to provide a comprehensive account of limitations they experienced and how adjustments have been made to improve patient care. Survey responses were compared to administrative workload numbers and COVID-19 spread data from the Centers for Disease Control and Prevention (CDC) to provide a comprehensive analysis of performance during the pandemic.
METHODS
Data were collected as part of a quality improvement initiative by the VHA ECoE; institutional review board approval was not required. An 18-item survey covering 5 broad domains was sent to ECoE directors 4 separate times to accumulate data from 4 time periods: May to June 2020 (T1); December 2020 to February 2021 (T2); July to August 2021 (T3); and June to July 2022 (T4). These periods correspond to the following phases of the pandemic: T1, onset of pandemic; T2, vaccine availability; T3, Delta variant predominant; T4, Omicron variant predominant.
Data on the spread of COVID-19 were collected from the CDC archived dataset, US COVID-19 County Level of Community Transmission Historical Changes (Table 1).7 Administrative workload (patient counts) for EEG, EMU, and outpatient clinics were extracted from VHA administrative databases for the participating sites for the months prior to each survey: T1, April 2020; T2, November 2020; T3, June 2021; and T4, May 2022 (Table 2).
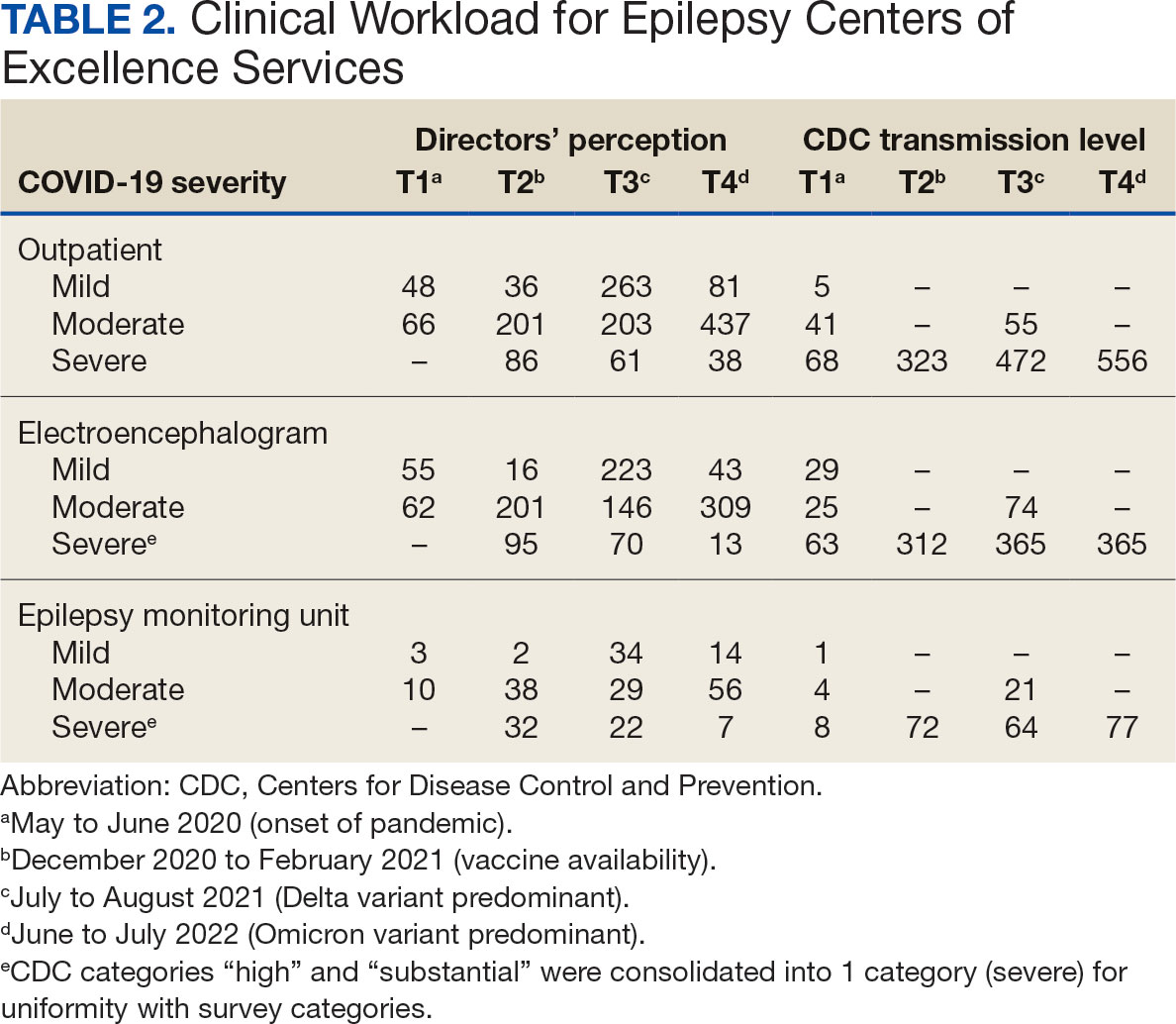
Survey Structure and Content
The survey was developed by the ECoE and was not validated prior to its use due to the time-sensitive nature of gathering information during the pandemic. The first survey (T1) was an emailed spreadsheet with open-ended questions to gauge availability of services (ie, outpatient clinic, EEG, EMU), assess whether safety precautions were being introduced, and understand whether national or local guidelines were thought to be helpful. Responses from this and subsequent surveys were standardized into yes/no and multiple choice formats. Subsequent surveys were administered online using a Research Electronic Data Capture tool.8,9
Availability of outpatient epilepsy services across the 4 time periods were categorized as unlimited (in-person with no restrictions), limited (in-person with restrictions), planned (not currently performed but scheduled for the near future), and unavailable (no in-person services offered) (eAppendices 1-6, available in article PDF).
Statistical Analyses
Analyses were performed to compare survey responses to workload and CDC data on COVID-19 community spread. The following associations were examined: (1) CDC COVID-19 spread vs respondents’ perception of spread; (2) respondents’ perception of spread vs availability of services; (3) CDC COVID-19 spread vs availability of services; (4) respondents’ perception of spread vs workload; and (5) CDC COVID-19 spread vs workload. Availability of services was dichotomized for analyses, with limited or fully available services classified as available. As services were mostly open at T3 regardless of the spread of the virus, and the CDC COVID-19 spread classification for all sites was severe or high at T2 and T4, corresponding associations were not tested at these time points. For associations 1 through 3, Fisher exact tests were used; for associations 4 and 5, Mann-Whitney U tests (where the COVID-19 spread fell into 2 categories) and Kruskal-Wallis tests (for 3 categories of COVID-19 spread) were performed. All tests were 2-tailed and performed at 0.05 error rate. Bonferroni corrections were applied to adjust P values for multiple hypotheses tests.
RESULTS
From the 17 sites invited, responses at each time point were obtained from 13 (T1),17 (T2), 15 (T3), and 16 (T4) centers. There was no significant association between self-reported COVID-19 spread and CDC classification of COVID spread. There were no associations between COVID-19 community spread (respondent reported or CDC severity level) and outpatient clinic availability (self-reported or workload captured). At T3, a positive association was found between the CDC spread level and workload (P = .008), but this was not significant after Bonferroni correction (P = .06).
EEG availability surpassed EMU availability at all time points, although EMU services made some recovery at T3 and T4. No associations were found between COVID-19 community spread (self-reported or CDC severity level) and outpatient EEG or EMU availability (self-reported or workload captured). At T3, there was a positive association between EEG workload and CDC COVID-19 severity level (P = .04), but this was not significant after Bonferroni correction (P = .30).
For outpatient EEG, staff and patient mask use were universally implemented by T2, while the use of full personal protective equipment (PPE) occurred at a subset of sites (T2, 6/17 [35%]; T3, 3/15 [20%]; T4: 4/16 [25%]). COVID-19 testing was rarely implemented prior to outpatient EEG (T1, 0 sites; T2, 1 site; T3, 1 site; T4, 0 sites). Within the EMU, safety precautions including COVID-19 testing, patient mask usage, staff mask usage, and aerosolization demonstrated a sustained majority usage across the 4 surveys.
National and Local Guidelines
The open-ended survey at T1 asked site directors, “Should there be national recommendations on how EEGs and related procedures should be done during the pandemic or should this be left to local conditions?” Responses were mixed, with 5 respondents desiring a national standard, 4 respondents favoring a local response, and 4 respondents believing a national standard should be in place but with modifications based on local outbreak levels and needs.
Surveys performed at T2 through T4 asked, “Which of the following do you feel was/will be helpful in adapting to COVID-19–related changes?” Overall, there was substantial agreement that guidelines were helpful. Most sites anticipated permanent changes in enhanced safety precautions and telehealth.
DISCUSSION
This longitudinal study across 4 time points describes how epilepsy services within the VHA and ECoE adapted to the COVID-19 pandemic. The first survey, conducted 2 months after COVID-19 was declared a pandemic, allowed a comparison with other concurrent US national surveys.1,2,10 The subsequent surveys describe longitudinal adaptations to balance patient and staff safety with service availability and is a unique feature of the current report. Results demonstrate flexibility and adaptability by the ECoEs surveyed, which surprisingly did not show significant associations between CDC COVID-19 spread data and administrative workload data.
Trends in Availability of Services
The most significant impact of COVID-19 restrictions was during T1. There were no significant relationships between service availability/workload and objective CDC COVID-19 spread levels or subjective self-reported COVID-19 spread. Respondents’ perceptions of local COVID-19 spread showed no association with CDC COVID-19 spread data. It appears that subjective perception of spread may be unreliable and factors other than actual or perceived COVID-19 spread were likely driving patterns for service availability.
In-person outpatient visits were most impacted at T1, similar to other civilian surveys, with only 1 site reporting in-person outpatient visits without limitations.1,2 These numbers significantly changed by T2, with all sites offering either limited or unlimited in-person visits. While the surveys did not evaluate factors leading to this rapid recovery, it may be related to the availability of COVID-19 vaccinations within the VHA during this time.11 The US Department of Veterans Affairs was the first federal agency to mandate employee vaccination.12 By the most recent time point (T4), all responding sites offered outpatient visits. Outpatient EEGs followed a similar trend, with T1 being the most restrictive and full, unrestricted outpatient EEGs available by T3.
Fiscal year (FY) trends from ECoE annual reports suggest that encounters slowly recovered over the course of the pandemic. In FY 2019 there were 13,143 outpatient encounters and 6394 EEGs, which dropped to 8097 outpatient encounters and 4432 EEGs in FY 2020 before rising to 8489 outpatient encounters and 5604 EEGs in FY 2021 and 9772 outpatient encounters and 5062 EEGs in FY 2022. Thus, while clinicians described availability of services, patients may have remained hesitant or were otherwise unable to fulfill in-person appointments. The increased availability of home EEG (145 encounter days in 2021 and 436 encounter days in 2022) may be filling this gap.
In contrast to outpatient clinics and EEG, EMU availability showed relatively slower reimplementation. In the last survey, about 30% of sites were still not offering EMU or had limited services. Early trends regarding reduced staffing and patient reluctance for elective admission cited in other surveys may have also affected EMU availability within the VHA.2,13 Consistent with trends in availability, ECoE annual report data suggest EMU patient participation was about one-half of prepandemic rates: 3069 encounters in FY 2019 dropped to 1614 encounters in 2020. By 2021, rates were about two-thirds of prepandemic rates with 2058 encounters in 2021 and 2101 encounters in 2022.
Early survey results (T1) from this study echo trends from other surveys. In the AES survey (April to June 2020), about a quarter of respondents (22%) reported doing fewer EEG studies than usual. The Italian national survey (April 2020) revealed reduced presurgical evaluations (81%), ambulatory EEG (78%), standard EEG (5%) and long-term EEG (32%).4 In the NAEC survey (end of 2020)—which roughly corresponded to T2—outpatient EEGs were still < 75% of pre-COVID levels in one-half of the centers.
National and Local Guidelines
Both national and local guidelines were perceived as useful by most respondents, with national guidelines being more beneficial. This aligns with the NAEC survey, where there was a perceived need for detailed recommendations for PPE and COVID-19 testing of patients, visitors, and staff. Based on national and local guidelines, ECoE implemented safety procedures, as reflected in responses. Staff masking procedures appeared to be the most widely adopted for all services, while the use of full PPE waned as the pandemic progressed. COVID-19 testing was rarely used for routine outpatient visits but common in EMU admissions. This is similar to a survey conducted by the American Academy of Neurology which found full PPE implementation intermittently in outpatient settings and more frequently in inpatient settings.14
Telehealth Attitudes
While most sites anticipated permanent implementation of safety precautions and telehealth, the latter was consistently reported as more likely to be sustained. The VHA had a large and well-developed system of telehealth services that considerably predated the pandemic.15,16 Through this established infrastructure, remote services were quickly increased across the VHA.17-19 This telehealth structure was supplemented by the ability of VHA clinicians to practice across state lines, following a 2018 federal rule.20 The AES survey noted the VHA ECoE's longstanding experience with telehealth as a model for telemedicine use in providing direct patient care, remote EEG analysis, and clinician-to-clinician consultation.1
Trends in the number of telehealth patients seen, observed through patterns in ECoE annual reports are consistent with positive views toward this method of service provision. Specifically, these annual reports capture trends in Video Telehealth Clinic (local station), Video Telehealth Clinic (different station), Home Video Telehealth, Telephone Clinic, and eConsults. Though video telehealth at in-person stations had a precipitous drop in 2020 that continued to wane in subsequent years (898 encounters in 2019; 455 encounters in 2020; 90 encounters in 2021; 88 encounters in 2022), use of home video telehealth rose over time (143 encounters in 2019; 1003 encounters in 2020; 3206 encounters in 2021; 3315 encounters in 2022). Use of telephone services rose drastically in 2020 but has since become a less frequently used service method (2636 in 2019; 5923 in 2020; 5319 in 2021; 3704 in 2022).
Limitations
While the survey encouraged a high response rate, this limited its scope and interpretability. While the availability of services was evaluated, the underlying reasons were not queried. Follow-up questions about barriers to reopening may have allowed for a better understanding of why some services, such as EMU, continued to operate suboptimally later in the pandemic. Similarly, asking about unique strategies or barriers for telehealth would have allowed for a better understanding of its current and future use. We hypothesize that staffing changes during the pandemic may have influenced the availability of services, but changes to staffing were not assessed via the survey and were not readily available via other sources (eg, ECoE annual reports) at the time of publication. An additional limitation is the lack of comparable surveys in the literature for time points T2 to T4, as most analogous surveys were performed early in 2020.
Conclusions
This longitudinal study performed at 4 time points during the COVID-19 pandemic is the first to offer a comprehensive picture of changes to epilepsy and EEG services over time, given that other similar surveys lacked follow-up. Results reveal a significant limitation of services at VHA ECoE shortly after the onset of the pandemic, with return to near-complete operational status 2 years later. While safety precautions and telehealth are predicted to continue, telehealth is perceived as a more permanent change in services.
Albert DVF, Das RR, Acharya JN, et al. The impact of COVID-19 on epilepsy care: a survey of the American Epilepsy Society membership. Epilepsy Curr. 2020;20(5):316-324. doi:10.1177/1535759720956994
Ahrens SM, Ostendorf AP, Lado FA, et al. Impact of the COVID-19 pandemic on epilepsy center practice in the United States. Neurology. 2022;98(19):e1893-e1901. doi:10.1212/WNL.0000000000200285
Cross JH, Kwon CS, Asadi-Pooya AA, et al. Epilepsy care during the COVID-19 pandemic. Epilepsia. 2021;62(10):2322-2332. doi:10.1111/epi.17045
Assenza G, Lanzone J, Ricci L, et al. Electroencephalography at the time of Covid-19 pandemic in Italy. Neurol Sci. 2020;41(8):1999-2004. doi:10.1007/s10072-020-04546-8
US Department of Veterans Affairs. National Center for Veterans Analysis and Statistics. Veteran population. Updated September 7, 2022. Accessed October 25, 2024. https://www.va.gov/vetdata/veteran_population.asp
US Department of Veterans Affairs, Veterans Health Administration. Epilepsy Centers of Excellence (ECoE). Annual report fiscal year 2019. Accessed October 25, 2024. https://www.epilepsy.va.gov/docs/FY19AnnualReport-VHAEpilepsyCentersofExcellence.pdf
Centers for Disease Control and Prevention. United States COVID-19 county level of community transmission historical changes – ARCHIVED. Updated February 20, 2024. Accessed October 25, 2024. https://data.cdc.gov/Public-Health-Surveillance/United-States-COVID-19-County-Level-of-Community-T/nra9-vzzn
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. doi:10.1016/j.jbi.2008.08.010
Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi:10.1016/j.jbi.2019.103208
World Health Organization. Rolling updates on coronavirus disease (COVID-19). Updated July 31, 2020. Accessed October 25, 2024. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen
US Department of Veterans Affairs. VA announces initial plans for COVID-19 vaccine distribution. News release. December 10, 2020. Accessed October 25, 2024. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5580
Steinhauer J. V.A. Issues Vaccine Mandate for Health Care Workers, a First for a Federal Agency. The New York Times. August 16, 2021. Accessed October 25, 2024. https://www.nytimes.com/2021/07/26/us/politics/veterans-affairs-coronavirus-covid-19.html
Zafar SF, Khozein RJ, LaRoche SM, Westover MB, Gilmore EJ. Impact of the COVID-19 pandemic on continuous EEG utilization. J Clin Neurophysiol. 2022;39(7):567-574. doi:10.1097/WNP.0000000000000802
Qureshi AI, Rheaume C, Huang W, et al. COVID-19 exposure during neurology practice. Neurologist. 2021;26(6):225-230. doi:10.1097/NRL.0000000000000346
Darkins A, Cruise C, Armstrong M, Peters J, Finn M. Enhancing access of combat-wounded veterans to specialist rehabilitation services: the VA Polytrauma Telehealth Network. Arch Phys Med Rehabil. 2008;89(1):182-187. doi:10.1016/j.apmr.2007.07.027
Darkins A, Ryan P, Kobb R, et al. Care coordination/home telehealth: the systematic implementation of health informatics, home telehealth, and disease management to support the care of veteran patients with chronic conditions. Telemed J E Health. 2008;14(10):1118-1126. doi:10.1089/tmj.2008.0021
Gentry MT, Puspitasari AJ, McKean AJ, et al. Clinician satisfaction with rapid adoption and implementation of telehealth services during the COVID-19 pandemic. Telemed J E Health. 2021;27(12):1385-1392. doi:10.1089/tmj.2020.0575
Connolly SL, Stolzmann KL, Heyworth L, et al. Patient and provider predictors of telemental health use prior to and during the COVID-19 pandemic within the Department of Veterans Affairs. Am Psychol. 2022;77(2):249-261. doi:10.1037/amp0000895
Shelton CJ, Kim A, Hassan AM, Bhat A, Barnello J, Castro CA. System-wide implementation of telehealth to support military veterans and their families in response to COVID-19: a paradigm shift. J Mil Veteran Fam Health. 2020;6(S2):50-57. doi:10.3138/jmvfh-CO19-0003
VA expands telehealth by allowing health care providers to treat patients across state lines. News release. US Dept of Veterans Affairs. May 11, 2018. Accessed October 25, 2024. https://news.va.gov/press-room/va-expands-telehealth-by-allowing-health-care-providers-to-treat-patients-across-state-lines/
The COVID-19 pandemic affected diverse workplaces globally, leading to temporary and permanent changes across the health care landscape. Included among the impacted areas of care were epilepsy and electroencephalogram (EEG) clinicians and services. Surveys among epilepsy specialists and neurophysiologists conducted at the onset of the pandemic to evaluate working conditions include analyses from the American Epilepsy Society (AES), the National Association of Epilepsy Centers (NAEC), the International League Against Epilepsy, and an Italian national survey.1-4 These investigations revealed reductions in epilepsy monitoring unit (EMU) admissions (23% decline), epilepsy surgery (6% decline), inpatient EEG (22% of respondents reported decline), and patients having difficulty accessing epilepsy professionals (28% of respondents reported decline) or obtaining medications (20% of respondents reported decline).1-3
While such research provided evidence for changes to epilepsy care in 2020, there are limited data on subsequent adaptations during the pandemic. These studies did not incorporate data on the spread of COVID-19 or administrative workload numbers to analyze service delivery beyond self reports. This study aimed to address this gap in the literature by highlighting results from longitudinal national surveys conducted at the Epilepsy Centers of Excellence (ECoE), a specialty care service within the Veterans Health Administration (VHA), which annually serves > 9 million veterans.5 The ECoE represents epileptologists and neurophysiologists across the United States at the 17 primary facilities that were established at the time of this survey (2 ECoEs have been added since survey completion) in 4 geographical regions and for which other regional facilities refer patients for diagnostic services or specialty care.6
National surveys were conducted among the ECoE directors regarding adaptations made from May 2020 to June 2022 to provide a comprehensive account of limitations they experienced and how adjustments have been made to improve patient care. Survey responses were compared to administrative workload numbers and COVID-19 spread data from the Centers for Disease Control and Prevention (CDC) to provide a comprehensive analysis of performance during the pandemic.
METHODS
Data were collected as part of a quality improvement initiative by the VHA ECoE; institutional review board approval was not required. An 18-item survey covering 5 broad domains was sent to ECoE directors 4 separate times to accumulate data from 4 time periods: May to June 2020 (T1); December 2020 to February 2021 (T2); July to August 2021 (T3); and June to July 2022 (T4). These periods correspond to the following phases of the pandemic: T1, onset of pandemic; T2, vaccine availability; T3, Delta variant predominant; T4, Omicron variant predominant.

Data on the spread of COVID-19 were collected from the CDC archived dataset, US COVID-19 County Level of Community Transmission Historical Changes (Table 1).7 Administrative workload (patient counts) for EEG, EMU, and outpatient clinics were extracted from VHA administrative databases for the participating sites for the months prior to each survey: T1, April 2020; T2, November 2020; T3, June 2021; and T4, May 2022 (Table 2).

Survey Structure and Content
The survey was developed by the ECoE and was not validated prior to its use due to the time-sensitive nature of gathering information during the pandemic. The first survey (T1) was an emailed spreadsheet with open-ended questions to gauge availability of services (ie, outpatient clinic, EEG, EMU), assess whether safety precautions were being introduced, and understand whether national or local guidelines were thought to be helpful. Responses from this and subsequent surveys were standardized into yes/no and multiple choice formats. Subsequent surveys were administered online using a Research Electronic Data Capture tool.8,9
Availability of outpatient epilepsy services across the 4 time periods were categorized as unlimited (in-person with no restrictions), limited (in-person with restrictions), planned (not currently performed but scheduled for the near future), and unavailable (no in-person services offered) (eAppendices 1-6, available in article PDF).
Statistical Analyses
Analyses were performed to compare survey responses to workload and CDC data on COVID-19 community spread. The following associations were examined: (1) CDC COVID-19 spread vs respondents’ perception of spread; (2) respondents’ perception of spread vs availability of services; (3) CDC COVID-19 spread vs availability of services; (4) respondents’ perception of spread vs workload; and (5) CDC COVID-19 spread vs workload. Availability of services was dichotomized for analyses, with limited or fully available services classified as available. As services were mostly open at T3 regardless of the spread of the virus, and the CDC COVID-19 spread classification for all sites was severe or high at T2 and T4, corresponding associations were not tested at these time points. For associations 1 through 3, Fisher exact tests were used; for associations 4 and 5, Mann-Whitney U tests (where the COVID-19 spread fell into 2 categories) and Kruskal-Wallis tests (for 3 categories of COVID-19 spread) were performed. All tests were 2-tailed and performed at 0.05 error rate. Bonferroni corrections were applied to adjust P values for multiple hypotheses tests.
RESULTS
From the 17 sites invited, responses at each time point were obtained from 13 (T1),17 (T2), 15 (T3), and 16 (T4) centers. There was no significant association between self-reported COVID-19 spread and CDC classification of COVID spread. There were no associations between COVID-19 community spread (respondent reported or CDC severity level) and outpatient clinic availability (self-reported or workload captured). At T3, a positive association was found between the CDC spread level and workload (P = .008), but this was not significant after Bonferroni correction (P = .06).
EEG availability surpassed EMU availability at all time points, although EMU services made some recovery at T3 and T4. No associations were found between COVID-19 community spread (self-reported or CDC severity level) and outpatient EEG or EMU availability (self-reported or workload captured). At T3, there was a positive association between EEG workload and CDC COVID-19 severity level (P = .04), but this was not significant after Bonferroni correction (P = .30).
For outpatient EEG, staff and patient mask use were universally implemented by T2, while the use of full personal protective equipment (PPE) occurred at a subset of sites (T2, 6/17 [35%]; T3, 3/15 [20%]; T4: 4/16 [25%]). COVID-19 testing was rarely implemented prior to outpatient EEG (T1, 0 sites; T2, 1 site; T3, 1 site; T4, 0 sites). Within the EMU, safety precautions including COVID-19 testing, patient mask usage, staff mask usage, and aerosolization demonstrated a sustained majority usage across the 4 surveys.
National and Local Guidelines
The open-ended survey at T1 asked site directors, “Should there be national recommendations on how EEGs and related procedures should be done during the pandemic or should this be left to local conditions?” Responses were mixed, with 5 respondents desiring a national standard, 4 respondents favoring a local response, and 4 respondents believing a national standard should be in place but with modifications based on local outbreak levels and needs.
Surveys performed at T2 through T4 asked, “Which of the following do you feel was/will be helpful in adapting to COVID-19–related changes?” Overall, there was substantial agreement that guidelines were helpful. Most sites anticipated permanent changes in enhanced safety precautions and telehealth.
DISCUSSION
This longitudinal study across 4 time points describes how epilepsy services within the VHA and ECoE adapted to the COVID-19 pandemic. The first survey, conducted 2 months after COVID-19 was declared a pandemic, allowed a comparison with other concurrent US national surveys.1,2,10 The subsequent surveys describe longitudinal adaptations to balance patient and staff safety with service availability and is a unique feature of the current report. Results demonstrate flexibility and adaptability by the ECoEs surveyed, which surprisingly did not show significant associations between CDC COVID-19 spread data and administrative workload data.
Trends in Availability of Services
The most significant impact of COVID-19 restrictions was during T1. There were no significant relationships between service availability/workload and objective CDC COVID-19 spread levels or subjective self-reported COVID-19 spread. Respondents’ perceptions of local COVID-19 spread showed no association with CDC COVID-19 spread data. It appears that subjective perception of spread may be unreliable and factors other than actual or perceived COVID-19 spread were likely driving patterns for service availability.
In-person outpatient visits were most impacted at T1, similar to other civilian surveys, with only 1 site reporting in-person outpatient visits without limitations.1,2 These numbers significantly changed by T2, with all sites offering either limited or unlimited in-person visits. While the surveys did not evaluate factors leading to this rapid recovery, it may be related to the availability of COVID-19 vaccinations within the VHA during this time.11 The US Department of Veterans Affairs was the first federal agency to mandate employee vaccination.12 By the most recent time point (T4), all responding sites offered outpatient visits. Outpatient EEGs followed a similar trend, with T1 being the most restrictive and full, unrestricted outpatient EEGs available by T3.
Fiscal year (FY) trends from ECoE annual reports suggest that encounters slowly recovered over the course of the pandemic. In FY 2019 there were 13,143 outpatient encounters and 6394 EEGs, which dropped to 8097 outpatient encounters and 4432 EEGs in FY 2020 before rising to 8489 outpatient encounters and 5604 EEGs in FY 2021 and 9772 outpatient encounters and 5062 EEGs in FY 2022. Thus, while clinicians described availability of services, patients may have remained hesitant or were otherwise unable to fulfill in-person appointments. The increased availability of home EEG (145 encounter days in 2021 and 436 encounter days in 2022) may be filling this gap.
In contrast to outpatient clinics and EEG, EMU availability showed relatively slower reimplementation. In the last survey, about 30% of sites were still not offering EMU or had limited services. Early trends regarding reduced staffing and patient reluctance for elective admission cited in other surveys may have also affected EMU availability within the VHA.2,13 Consistent with trends in availability, ECoE annual report data suggest EMU patient participation was about one-half of prepandemic rates: 3069 encounters in FY 2019 dropped to 1614 encounters in 2020. By 2021, rates were about two-thirds of prepandemic rates with 2058 encounters in 2021 and 2101 encounters in 2022.
Early survey results (T1) from this study echo trends from other surveys. In the AES survey (April to June 2020), about a quarter of respondents (22%) reported doing fewer EEG studies than usual. The Italian national survey (April 2020) revealed reduced presurgical evaluations (81%), ambulatory EEG (78%), standard EEG (5%) and long-term EEG (32%).4 In the NAEC survey (end of 2020)—which roughly corresponded to T2—outpatient EEGs were still < 75% of pre-COVID levels in one-half of the centers.
National and Local Guidelines
Both national and local guidelines were perceived as useful by most respondents, with national guidelines being more beneficial. This aligns with the NAEC survey, where there was a perceived need for detailed recommendations for PPE and COVID-19 testing of patients, visitors, and staff. Based on national and local guidelines, ECoE implemented safety procedures, as reflected in responses. Staff masking procedures appeared to be the most widely adopted for all services, while the use of full PPE waned as the pandemic progressed. COVID-19 testing was rarely used for routine outpatient visits but common in EMU admissions. This is similar to a survey conducted by the American Academy of Neurology which found full PPE implementation intermittently in outpatient settings and more frequently in inpatient settings.14
Telehealth Attitudes
While most sites anticipated permanent implementation of safety precautions and telehealth, the latter was consistently reported as more likely to be sustained. The VHA had a large and well-developed system of telehealth services that considerably predated the pandemic.15,16 Through this established infrastructure, remote services were quickly increased across the VHA.17-19 This telehealth structure was supplemented by the ability of VHA clinicians to practice across state lines, following a 2018 federal rule.20 The AES survey noted the VHA ECoE's longstanding experience with telehealth as a model for telemedicine use in providing direct patient care, remote EEG analysis, and clinician-to-clinician consultation.1
Trends in the number of telehealth patients seen, observed through patterns in ECoE annual reports are consistent with positive views toward this method of service provision. Specifically, these annual reports capture trends in Video Telehealth Clinic (local station), Video Telehealth Clinic (different station), Home Video Telehealth, Telephone Clinic, and eConsults. Though video telehealth at in-person stations had a precipitous drop in 2020 that continued to wane in subsequent years (898 encounters in 2019; 455 encounters in 2020; 90 encounters in 2021; 88 encounters in 2022), use of home video telehealth rose over time (143 encounters in 2019; 1003 encounters in 2020; 3206 encounters in 2021; 3315 encounters in 2022). Use of telephone services rose drastically in 2020 but has since become a less frequently used service method (2636 in 2019; 5923 in 2020; 5319 in 2021; 3704 in 2022).
Limitations
While the survey encouraged a high response rate, this limited its scope and interpretability. While the availability of services was evaluated, the underlying reasons were not queried. Follow-up questions about barriers to reopening may have allowed for a better understanding of why some services, such as EMU, continued to operate suboptimally later in the pandemic. Similarly, asking about unique strategies or barriers for telehealth would have allowed for a better understanding of its current and future use. We hypothesize that staffing changes during the pandemic may have influenced the availability of services, but changes to staffing were not assessed via the survey and were not readily available via other sources (eg, ECoE annual reports) at the time of publication. An additional limitation is the lack of comparable surveys in the literature for time points T2 to T4, as most analogous surveys were performed early in 2020.
Conclusions
This longitudinal study performed at 4 time points during the COVID-19 pandemic is the first to offer a comprehensive picture of changes to epilepsy and EEG services over time, given that other similar surveys lacked follow-up. Results reveal a significant limitation of services at VHA ECoE shortly after the onset of the pandemic, with return to near-complete operational status 2 years later. While safety precautions and telehealth are predicted to continue, telehealth is perceived as a more permanent change in services.
The COVID-19 pandemic affected diverse workplaces globally, leading to temporary and permanent changes across the health care landscape. Included among the impacted areas of care were epilepsy and electroencephalogram (EEG) clinicians and services. Surveys among epilepsy specialists and neurophysiologists conducted at the onset of the pandemic to evaluate working conditions include analyses from the American Epilepsy Society (AES), the National Association of Epilepsy Centers (NAEC), the International League Against Epilepsy, and an Italian national survey.1-4 These investigations revealed reductions in epilepsy monitoring unit (EMU) admissions (23% decline), epilepsy surgery (6% decline), inpatient EEG (22% of respondents reported decline), and patients having difficulty accessing epilepsy professionals (28% of respondents reported decline) or obtaining medications (20% of respondents reported decline).1-3
While such research provided evidence for changes to epilepsy care in 2020, there are limited data on subsequent adaptations during the pandemic. These studies did not incorporate data on the spread of COVID-19 or administrative workload numbers to analyze service delivery beyond self reports. This study aimed to address this gap in the literature by highlighting results from longitudinal national surveys conducted at the Epilepsy Centers of Excellence (ECoE), a specialty care service within the Veterans Health Administration (VHA), which annually serves > 9 million veterans.5 The ECoE represents epileptologists and neurophysiologists across the United States at the 17 primary facilities that were established at the time of this survey (2 ECoEs have been added since survey completion) in 4 geographical regions and for which other regional facilities refer patients for diagnostic services or specialty care.6
National surveys were conducted among the ECoE directors regarding adaptations made from May 2020 to June 2022 to provide a comprehensive account of limitations they experienced and how adjustments have been made to improve patient care. Survey responses were compared to administrative workload numbers and COVID-19 spread data from the Centers for Disease Control and Prevention (CDC) to provide a comprehensive analysis of performance during the pandemic.
METHODS
Data were collected as part of a quality improvement initiative by the VHA ECoE; institutional review board approval was not required. An 18-item survey covering 5 broad domains was sent to ECoE directors 4 separate times to accumulate data from 4 time periods: May to June 2020 (T1); December 2020 to February 2021 (T2); July to August 2021 (T3); and June to July 2022 (T4). These periods correspond to the following phases of the pandemic: T1, onset of pandemic; T2, vaccine availability; T3, Delta variant predominant; T4, Omicron variant predominant.

Data on the spread of COVID-19 were collected from the CDC archived dataset, US COVID-19 County Level of Community Transmission Historical Changes (Table 1).7 Administrative workload (patient counts) for EEG, EMU, and outpatient clinics were extracted from VHA administrative databases for the participating sites for the months prior to each survey: T1, April 2020; T2, November 2020; T3, June 2021; and T4, May 2022 (Table 2).

Survey Structure and Content
The survey was developed by the ECoE and was not validated prior to its use due to the time-sensitive nature of gathering information during the pandemic. The first survey (T1) was an emailed spreadsheet with open-ended questions to gauge availability of services (ie, outpatient clinic, EEG, EMU), assess whether safety precautions were being introduced, and understand whether national or local guidelines were thought to be helpful. Responses from this and subsequent surveys were standardized into yes/no and multiple choice formats. Subsequent surveys were administered online using a Research Electronic Data Capture tool.8,9
Availability of outpatient epilepsy services across the 4 time periods were categorized as unlimited (in-person with no restrictions), limited (in-person with restrictions), planned (not currently performed but scheduled for the near future), and unavailable (no in-person services offered) (eAppendices 1-6, available in article PDF).
Statistical Analyses
Analyses were performed to compare survey responses to workload and CDC data on COVID-19 community spread. The following associations were examined: (1) CDC COVID-19 spread vs respondents’ perception of spread; (2) respondents’ perception of spread vs availability of services; (3) CDC COVID-19 spread vs availability of services; (4) respondents’ perception of spread vs workload; and (5) CDC COVID-19 spread vs workload. Availability of services was dichotomized for analyses, with limited or fully available services classified as available. As services were mostly open at T3 regardless of the spread of the virus, and the CDC COVID-19 spread classification for all sites was severe or high at T2 and T4, corresponding associations were not tested at these time points. For associations 1 through 3, Fisher exact tests were used; for associations 4 and 5, Mann-Whitney U tests (where the COVID-19 spread fell into 2 categories) and Kruskal-Wallis tests (for 3 categories of COVID-19 spread) were performed. All tests were 2-tailed and performed at 0.05 error rate. Bonferroni corrections were applied to adjust P values for multiple hypotheses tests.
RESULTS
From the 17 sites invited, responses at each time point were obtained from 13 (T1),17 (T2), 15 (T3), and 16 (T4) centers. There was no significant association between self-reported COVID-19 spread and CDC classification of COVID spread. There were no associations between COVID-19 community spread (respondent reported or CDC severity level) and outpatient clinic availability (self-reported or workload captured). At T3, a positive association was found between the CDC spread level and workload (P = .008), but this was not significant after Bonferroni correction (P = .06).
EEG availability surpassed EMU availability at all time points, although EMU services made some recovery at T3 and T4. No associations were found between COVID-19 community spread (self-reported or CDC severity level) and outpatient EEG or EMU availability (self-reported or workload captured). At T3, there was a positive association between EEG workload and CDC COVID-19 severity level (P = .04), but this was not significant after Bonferroni correction (P = .30).
For outpatient EEG, staff and patient mask use were universally implemented by T2, while the use of full personal protective equipment (PPE) occurred at a subset of sites (T2, 6/17 [35%]; T3, 3/15 [20%]; T4: 4/16 [25%]). COVID-19 testing was rarely implemented prior to outpatient EEG (T1, 0 sites; T2, 1 site; T3, 1 site; T4, 0 sites). Within the EMU, safety precautions including COVID-19 testing, patient mask usage, staff mask usage, and aerosolization demonstrated a sustained majority usage across the 4 surveys.
National and Local Guidelines
The open-ended survey at T1 asked site directors, “Should there be national recommendations on how EEGs and related procedures should be done during the pandemic or should this be left to local conditions?” Responses were mixed, with 5 respondents desiring a national standard, 4 respondents favoring a local response, and 4 respondents believing a national standard should be in place but with modifications based on local outbreak levels and needs.
Surveys performed at T2 through T4 asked, “Which of the following do you feel was/will be helpful in adapting to COVID-19–related changes?” Overall, there was substantial agreement that guidelines were helpful. Most sites anticipated permanent changes in enhanced safety precautions and telehealth.
DISCUSSION
This longitudinal study across 4 time points describes how epilepsy services within the VHA and ECoE adapted to the COVID-19 pandemic. The first survey, conducted 2 months after COVID-19 was declared a pandemic, allowed a comparison with other concurrent US national surveys.1,2,10 The subsequent surveys describe longitudinal adaptations to balance patient and staff safety with service availability and is a unique feature of the current report. Results demonstrate flexibility and adaptability by the ECoEs surveyed, which surprisingly did not show significant associations between CDC COVID-19 spread data and administrative workload data.
Trends in Availability of Services
The most significant impact of COVID-19 restrictions was during T1. There were no significant relationships between service availability/workload and objective CDC COVID-19 spread levels or subjective self-reported COVID-19 spread. Respondents’ perceptions of local COVID-19 spread showed no association with CDC COVID-19 spread data. It appears that subjective perception of spread may be unreliable and factors other than actual or perceived COVID-19 spread were likely driving patterns for service availability.
In-person outpatient visits were most impacted at T1, similar to other civilian surveys, with only 1 site reporting in-person outpatient visits without limitations.1,2 These numbers significantly changed by T2, with all sites offering either limited or unlimited in-person visits. While the surveys did not evaluate factors leading to this rapid recovery, it may be related to the availability of COVID-19 vaccinations within the VHA during this time.11 The US Department of Veterans Affairs was the first federal agency to mandate employee vaccination.12 By the most recent time point (T4), all responding sites offered outpatient visits. Outpatient EEGs followed a similar trend, with T1 being the most restrictive and full, unrestricted outpatient EEGs available by T3.
Fiscal year (FY) trends from ECoE annual reports suggest that encounters slowly recovered over the course of the pandemic. In FY 2019 there were 13,143 outpatient encounters and 6394 EEGs, which dropped to 8097 outpatient encounters and 4432 EEGs in FY 2020 before rising to 8489 outpatient encounters and 5604 EEGs in FY 2021 and 9772 outpatient encounters and 5062 EEGs in FY 2022. Thus, while clinicians described availability of services, patients may have remained hesitant or were otherwise unable to fulfill in-person appointments. The increased availability of home EEG (145 encounter days in 2021 and 436 encounter days in 2022) may be filling this gap.
In contrast to outpatient clinics and EEG, EMU availability showed relatively slower reimplementation. In the last survey, about 30% of sites were still not offering EMU or had limited services. Early trends regarding reduced staffing and patient reluctance for elective admission cited in other surveys may have also affected EMU availability within the VHA.2,13 Consistent with trends in availability, ECoE annual report data suggest EMU patient participation was about one-half of prepandemic rates: 3069 encounters in FY 2019 dropped to 1614 encounters in 2020. By 2021, rates were about two-thirds of prepandemic rates with 2058 encounters in 2021 and 2101 encounters in 2022.
Early survey results (T1) from this study echo trends from other surveys. In the AES survey (April to June 2020), about a quarter of respondents (22%) reported doing fewer EEG studies than usual. The Italian national survey (April 2020) revealed reduced presurgical evaluations (81%), ambulatory EEG (78%), standard EEG (5%) and long-term EEG (32%).4 In the NAEC survey (end of 2020)—which roughly corresponded to T2—outpatient EEGs were still < 75% of pre-COVID levels in one-half of the centers.
National and Local Guidelines
Both national and local guidelines were perceived as useful by most respondents, with national guidelines being more beneficial. This aligns with the NAEC survey, where there was a perceived need for detailed recommendations for PPE and COVID-19 testing of patients, visitors, and staff. Based on national and local guidelines, ECoE implemented safety procedures, as reflected in responses. Staff masking procedures appeared to be the most widely adopted for all services, while the use of full PPE waned as the pandemic progressed. COVID-19 testing was rarely used for routine outpatient visits but common in EMU admissions. This is similar to a survey conducted by the American Academy of Neurology which found full PPE implementation intermittently in outpatient settings and more frequently in inpatient settings.14
Telehealth Attitudes
While most sites anticipated permanent implementation of safety precautions and telehealth, the latter was consistently reported as more likely to be sustained. The VHA had a large and well-developed system of telehealth services that considerably predated the pandemic.15,16 Through this established infrastructure, remote services were quickly increased across the VHA.17-19 This telehealth structure was supplemented by the ability of VHA clinicians to practice across state lines, following a 2018 federal rule.20 The AES survey noted the VHA ECoE's longstanding experience with telehealth as a model for telemedicine use in providing direct patient care, remote EEG analysis, and clinician-to-clinician consultation.1
Trends in the number of telehealth patients seen, observed through patterns in ECoE annual reports are consistent with positive views toward this method of service provision. Specifically, these annual reports capture trends in Video Telehealth Clinic (local station), Video Telehealth Clinic (different station), Home Video Telehealth, Telephone Clinic, and eConsults. Though video telehealth at in-person stations had a precipitous drop in 2020 that continued to wane in subsequent years (898 encounters in 2019; 455 encounters in 2020; 90 encounters in 2021; 88 encounters in 2022), use of home video telehealth rose over time (143 encounters in 2019; 1003 encounters in 2020; 3206 encounters in 2021; 3315 encounters in 2022). Use of telephone services rose drastically in 2020 but has since become a less frequently used service method (2636 in 2019; 5923 in 2020; 5319 in 2021; 3704 in 2022).
Limitations
While the survey encouraged a high response rate, this limited its scope and interpretability. While the availability of services was evaluated, the underlying reasons were not queried. Follow-up questions about barriers to reopening may have allowed for a better understanding of why some services, such as EMU, continued to operate suboptimally later in the pandemic. Similarly, asking about unique strategies or barriers for telehealth would have allowed for a better understanding of its current and future use. We hypothesize that staffing changes during the pandemic may have influenced the availability of services, but changes to staffing were not assessed via the survey and were not readily available via other sources (eg, ECoE annual reports) at the time of publication. An additional limitation is the lack of comparable surveys in the literature for time points T2 to T4, as most analogous surveys were performed early in 2020.
Conclusions
This longitudinal study performed at 4 time points during the COVID-19 pandemic is the first to offer a comprehensive picture of changes to epilepsy and EEG services over time, given that other similar surveys lacked follow-up. Results reveal a significant limitation of services at VHA ECoE shortly after the onset of the pandemic, with return to near-complete operational status 2 years later. While safety precautions and telehealth are predicted to continue, telehealth is perceived as a more permanent change in services.
Albert DVF, Das RR, Acharya JN, et al. The impact of COVID-19 on epilepsy care: a survey of the American Epilepsy Society membership. Epilepsy Curr. 2020;20(5):316-324. doi:10.1177/1535759720956994
Ahrens SM, Ostendorf AP, Lado FA, et al. Impact of the COVID-19 pandemic on epilepsy center practice in the United States. Neurology. 2022;98(19):e1893-e1901. doi:10.1212/WNL.0000000000200285
Cross JH, Kwon CS, Asadi-Pooya AA, et al. Epilepsy care during the COVID-19 pandemic. Epilepsia. 2021;62(10):2322-2332. doi:10.1111/epi.17045
Assenza G, Lanzone J, Ricci L, et al. Electroencephalography at the time of Covid-19 pandemic in Italy. Neurol Sci. 2020;41(8):1999-2004. doi:10.1007/s10072-020-04546-8
US Department of Veterans Affairs. National Center for Veterans Analysis and Statistics. Veteran population. Updated September 7, 2022. Accessed October 25, 2024. https://www.va.gov/vetdata/veteran_population.asp
US Department of Veterans Affairs, Veterans Health Administration. Epilepsy Centers of Excellence (ECoE). Annual report fiscal year 2019. Accessed October 25, 2024. https://www.epilepsy.va.gov/docs/FY19AnnualReport-VHAEpilepsyCentersofExcellence.pdf
Centers for Disease Control and Prevention. United States COVID-19 county level of community transmission historical changes – ARCHIVED. Updated February 20, 2024. Accessed October 25, 2024. https://data.cdc.gov/Public-Health-Surveillance/United-States-COVID-19-County-Level-of-Community-T/nra9-vzzn
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. doi:10.1016/j.jbi.2008.08.010
Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi:10.1016/j.jbi.2019.103208
World Health Organization. Rolling updates on coronavirus disease (COVID-19). Updated July 31, 2020. Accessed October 25, 2024. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen
US Department of Veterans Affairs. VA announces initial plans for COVID-19 vaccine distribution. News release. December 10, 2020. Accessed October 25, 2024. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5580
Steinhauer J. V.A. Issues Vaccine Mandate for Health Care Workers, a First for a Federal Agency. The New York Times. August 16, 2021. Accessed October 25, 2024. https://www.nytimes.com/2021/07/26/us/politics/veterans-affairs-coronavirus-covid-19.html
Zafar SF, Khozein RJ, LaRoche SM, Westover MB, Gilmore EJ. Impact of the COVID-19 pandemic on continuous EEG utilization. J Clin Neurophysiol. 2022;39(7):567-574. doi:10.1097/WNP.0000000000000802
Qureshi AI, Rheaume C, Huang W, et al. COVID-19 exposure during neurology practice. Neurologist. 2021;26(6):225-230. doi:10.1097/NRL.0000000000000346
Darkins A, Cruise C, Armstrong M, Peters J, Finn M. Enhancing access of combat-wounded veterans to specialist rehabilitation services: the VA Polytrauma Telehealth Network. Arch Phys Med Rehabil. 2008;89(1):182-187. doi:10.1016/j.apmr.2007.07.027
Darkins A, Ryan P, Kobb R, et al. Care coordination/home telehealth: the systematic implementation of health informatics, home telehealth, and disease management to support the care of veteran patients with chronic conditions. Telemed J E Health. 2008;14(10):1118-1126. doi:10.1089/tmj.2008.0021
Gentry MT, Puspitasari AJ, McKean AJ, et al. Clinician satisfaction with rapid adoption and implementation of telehealth services during the COVID-19 pandemic. Telemed J E Health. 2021;27(12):1385-1392. doi:10.1089/tmj.2020.0575
Connolly SL, Stolzmann KL, Heyworth L, et al. Patient and provider predictors of telemental health use prior to and during the COVID-19 pandemic within the Department of Veterans Affairs. Am Psychol. 2022;77(2):249-261. doi:10.1037/amp0000895
Shelton CJ, Kim A, Hassan AM, Bhat A, Barnello J, Castro CA. System-wide implementation of telehealth to support military veterans and their families in response to COVID-19: a paradigm shift. J Mil Veteran Fam Health. 2020;6(S2):50-57. doi:10.3138/jmvfh-CO19-0003
VA expands telehealth by allowing health care providers to treat patients across state lines. News release. US Dept of Veterans Affairs. May 11, 2018. Accessed October 25, 2024. https://news.va.gov/press-room/va-expands-telehealth-by-allowing-health-care-providers-to-treat-patients-across-state-lines/
Albert DVF, Das RR, Acharya JN, et al. The impact of COVID-19 on epilepsy care: a survey of the American Epilepsy Society membership. Epilepsy Curr. 2020;20(5):316-324. doi:10.1177/1535759720956994
Ahrens SM, Ostendorf AP, Lado FA, et al. Impact of the COVID-19 pandemic on epilepsy center practice in the United States. Neurology. 2022;98(19):e1893-e1901. doi:10.1212/WNL.0000000000200285
Cross JH, Kwon CS, Asadi-Pooya AA, et al. Epilepsy care during the COVID-19 pandemic. Epilepsia. 2021;62(10):2322-2332. doi:10.1111/epi.17045
Assenza G, Lanzone J, Ricci L, et al. Electroencephalography at the time of Covid-19 pandemic in Italy. Neurol Sci. 2020;41(8):1999-2004. doi:10.1007/s10072-020-04546-8
US Department of Veterans Affairs. National Center for Veterans Analysis and Statistics. Veteran population. Updated September 7, 2022. Accessed October 25, 2024. https://www.va.gov/vetdata/veteran_population.asp
US Department of Veterans Affairs, Veterans Health Administration. Epilepsy Centers of Excellence (ECoE). Annual report fiscal year 2019. Accessed October 25, 2024. https://www.epilepsy.va.gov/docs/FY19AnnualReport-VHAEpilepsyCentersofExcellence.pdf
Centers for Disease Control and Prevention. United States COVID-19 county level of community transmission historical changes – ARCHIVED. Updated February 20, 2024. Accessed October 25, 2024. https://data.cdc.gov/Public-Health-Surveillance/United-States-COVID-19-County-Level-of-Community-T/nra9-vzzn
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. doi:10.1016/j.jbi.2008.08.010
Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi:10.1016/j.jbi.2019.103208
World Health Organization. Rolling updates on coronavirus disease (COVID-19). Updated July 31, 2020. Accessed October 25, 2024. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen
US Department of Veterans Affairs. VA announces initial plans for COVID-19 vaccine distribution. News release. December 10, 2020. Accessed October 25, 2024. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5580
Steinhauer J. V.A. Issues Vaccine Mandate for Health Care Workers, a First for a Federal Agency. The New York Times. August 16, 2021. Accessed October 25, 2024. https://www.nytimes.com/2021/07/26/us/politics/veterans-affairs-coronavirus-covid-19.html
Zafar SF, Khozein RJ, LaRoche SM, Westover MB, Gilmore EJ. Impact of the COVID-19 pandemic on continuous EEG utilization. J Clin Neurophysiol. 2022;39(7):567-574. doi:10.1097/WNP.0000000000000802
Qureshi AI, Rheaume C, Huang W, et al. COVID-19 exposure during neurology practice. Neurologist. 2021;26(6):225-230. doi:10.1097/NRL.0000000000000346
Darkins A, Cruise C, Armstrong M, Peters J, Finn M. Enhancing access of combat-wounded veterans to specialist rehabilitation services: the VA Polytrauma Telehealth Network. Arch Phys Med Rehabil. 2008;89(1):182-187. doi:10.1016/j.apmr.2007.07.027
Darkins A, Ryan P, Kobb R, et al. Care coordination/home telehealth: the systematic implementation of health informatics, home telehealth, and disease management to support the care of veteran patients with chronic conditions. Telemed J E Health. 2008;14(10):1118-1126. doi:10.1089/tmj.2008.0021
Gentry MT, Puspitasari AJ, McKean AJ, et al. Clinician satisfaction with rapid adoption and implementation of telehealth services during the COVID-19 pandemic. Telemed J E Health. 2021;27(12):1385-1392. doi:10.1089/tmj.2020.0575
Connolly SL, Stolzmann KL, Heyworth L, et al. Patient and provider predictors of telemental health use prior to and during the COVID-19 pandemic within the Department of Veterans Affairs. Am Psychol. 2022;77(2):249-261. doi:10.1037/amp0000895
Shelton CJ, Kim A, Hassan AM, Bhat A, Barnello J, Castro CA. System-wide implementation of telehealth to support military veterans and their families in response to COVID-19: a paradigm shift. J Mil Veteran Fam Health. 2020;6(S2):50-57. doi:10.3138/jmvfh-CO19-0003
VA expands telehealth by allowing health care providers to treat patients across state lines. News release. US Dept of Veterans Affairs. May 11, 2018. Accessed October 25, 2024. https://news.va.gov/press-room/va-expands-telehealth-by-allowing-health-care-providers-to-treat-patients-across-state-lines/
What’s Eating You? Hookworm and Cutaneous Larva Migrans
What’s Eating You? Hookworm and
Cutaneous Larva Migrans
It is estimated that the prevalence of human hookworm infection is approximately 450 million individuals worldwide, representing a substantial global disease burden.1 The annual global public health burden ranges from approximately 2 million to 4 million disability-adjusted life-years and $10 billion to $140 billion in hookwormrelated costs.2 In this article, we discuss the lifecycle, transmission, and disease burden of cutaneous larva migrans (CLM) as well as prevention and treatment strategies.
Background
The Ancylostomatidae nematode family comprises at least 68 known species of hookworm that infect more than 110 different species of mammals.3 Many of these parasites are able to infect more than 1 primary host species, but from a disease perspective they can be classified as either anthropophilic, with humans as the intended host, or zoonotic, with humans as an incidental host. It is important to make this distinction because, though the lifecycles and biology of hookworm species generally are similar, the manifestations of incidental human infection from zoonotic hookworms are different from those of anthropophilic hookworms. Of the anthropophilic species, Necator americanus and Ancylostoma duodenale predominate. In the instance of zoonotic hookworm, dog-infecting A caninum and cat- and doginfecting A braziliense and Uncinaria stenocephala are common causes of incidental human disease.3
The life cycle of Ancylostomatidae organisms is astounding. Through millions of years of co-evolution with mammals,4 these parasitic worms have developed perhaps one of the most circuitous paths to propagate themselves in the natural world. Hookworms start their arduous journey as eggs deposited in soil, sand, and ground vegetation from the feces of infected animals.5 Approximately 1 day after the eggs are deposited, they hatch and begin the larval stage, during which they become infective 1 to 5 weeks later. At this point, the larvae become sensitive to their environment, responding to rising temperatures, increasing carbon dioxide levels, and vibrations in the soil—all of which suggest the presence of a potential host and contribute to a concordant increase in undulatory movement of the larvae.5,6 Here, the most vulnerable tissues include the uncovered soles, palms, and buttocks of host mammals that come into contact with contaminated soil. In an undulating fashion and guided by temperature cues, the larvae locate the skin of the host and utilize a mixture of enzymes including hyaluronidases, metalloprotease, and other proteases to penetrate the epidermis.7 Anthropophilic hookworms such as N americanus and A duodenale will enter the circulatory system; from there, the hookworms migrate through the right-sided cardiopulmonary circuit and eventually ascend into the pulmonary vasculature.8 They then penetrate the lung capillary beds and parenchyma to reach the alveoli, ascend the respiratory tree, and, with the help of the mucociliary escalator, reach the esophagus, where they are swallowed by the host. In the gastrointestinal tract, adult hookworms consume host blood, mate, and lay eggs over a period of approximately 1 to 3 years if left untreated.9 Eggs are laid into the lower gastrointestinal tract, and the journey begins again in feces contacting ground or soil.
Geographic Distribution
Hookworms are found in almost all regions of the world, with species-specific distributions that highlight tropical and subtropical regions. Necator americanus and A duodenale are the most common hookworm species, with the former found predominantly in Southeast Asia and Latin America and the latter in Asia-Pacific regions.10 The highest prevalence of hookworms is in Southeast Asia followed by Sub-Saharan Africa, and the unique climate and soil composition of a region help determine the best environments for specific species of hookworm to thrive.11 In addition, socioeconomics and social determinants of health play a big role in the spread of hookworms, as hygiene practices (eg, wearing clean shoes and clothing, bathing), infrastructure (eg, clean water and streets), and anthelmintic campaigns help reduce transmission.12 Soil-transmitted helminths were once endemic to the southeastern United States, with some reports of approximately 40% of individuals infected in the south in the early 1900s.13 Anthelmintic campaigns such as water, sanitation, and hygiene programs as well as deworming of humans and livestock have proven effective in reducing the prevalence of helminth disease in industrialized nations.13,14 However, zoonotic infections remain a problem in these regions, and in some parts of the United States more than 40% of sampled cats and dogs harbored species such as A braziliense.15
Clinical Manifestation
Initial hookworm infection often goes unnoticed because symptoms can range in severity, but it is characterized by transient ground itch—a local pruritic, erythematous, and papular eruption that develops in response to epidermal penetration.16 Because the larvae must traverse the host from skin to target organs for reproduction over several weeks, iron-deficiency anemia will manifest much later than signs of the initial penetration. In the case of incidental infection from zoonotic Ancylostomatidae organisms, the misguided larvae result in CLM, an often intensely pruritic skin condition that will self-resolve in 2 to 8 weeks with eventual death of the larvae.5
Diagnosis and Pathology of Disease
Zoonotic Hookworm—The major presenting sign of zoonotic hookworm infection is CLM. The diagnosis of CLM usually is made clinically, as the larvae themselves are 0.5 mm thick to 10 mm long (Figure 1) and usually extend several centimeters beyond the dermal lesion, with dermoscopy having limited utility.17 Patients may begin to experience itching as little as 1 hour after hookworm penetration of the skin.18 Once in contact with the skin, the hookworms’ hyaluronidases and proteases are capable of breaking through the epidermis, but zoonotic hookworms typically are unable to penetrate the basal layer of the human epidermis and remain entombed between the stratum granulosum and stratum corneum. With the exception of rare cases of direct or indirect pulmonary involvement resulting in Löffler syndrome,19 the larvae will die within weeks to months, and symptoms will subsequently resolve.
Although the infection generally is self-limiting, the dermatologic manifestations of CLM can be severe and warrant intervention. The lesions start as small reddish papules at the site of penetration (Figure 2), then the hallmark elevated, migrating, serpiginous, urticarial rash develops (Figure 3). Cutaneous larva migrans generally manifests unilaterally and is both erythematous and intensely pruritic. As the larvae migrate, they leave behind 1- to 5-cm tunneled creeping eruptions in their wake. The lesions, which can manifest with pain or be painless, may develop eczematous, bullous, follicular, or impetiginized appearances.20 Atypical manifestations include folliculitis and urticarial plaques.17
cutaneous larval migrans on the palm.
Anthropophilic Hookworm—The lifecycles of N americanus and A duodenale are completed in human infection. Dermatologic manifestations are transient with the development of ground itch at the site of epidermal penetration. The hookworms employ collagenases that allow penetration of the basal layer of the skin, and eosinophilia develops as the parasites travel from the skin to the small intestine. Once attached to the gastrointestinal lumen, blood meals and proteolytic enzymes result in iron-deficiency anemia in the host and may lead to weakness, fatigue, and low birth weights in pregnant patients. With prolonged infection or heavy parasitic burden, patients can develop hypoproteinemia, anasarca, and yellowing of the skin known as chlorosis.11 A clinical diagnosis can be made by examining patient stool samples for eggs, and definitive characterization can be made using molecular tools such as polymerase chain reaction.21,22
Common to hookworm infections is the immune reaction, which promotes inflammation with localized eosinophilia and mastocytosis.11 In a clinical biopsy specimen of gut—usually obtained through esophagogastroduodenoscopy— T-helper (Th) 2–type immune (IL-4, IL-5, IL-9 and IL-13), regulatory Th10 (IL-10 and transcription growth factor β), and some evidence of Th1 (interferon gamma and IL-2) cytokines are present, but little evidence of Th17-type immune response was found.23 It is believed that in zoonotic infections, antiparasitic IgE from basophils are somewhat successful at trapping the helminths in the epidermis, but in the anthropophilic species, IgE and Th2 responses are ineffective at clearing the parasite from the gut, and the defeated immune system transitions to a host-tolerance approach of limiting infection.11 It is now believed that this natural armistice can be manipulated into a potential therapy against autoimmune and inflammatory conditions. Intentional infection with zoonotic whipworm or hookworm has been proposed as a mechanism of switching Th1 and Th2 responses to host-tolerant mechanisms in conditions such as Crohn disease and celiac disease,24 and it has even been hypothesized that prior hookworm infection may reduce the chance of developing allergic conditions such as eczema.25
Treatment and Prevention
The World Health Organization and Centers for Disease Control and Prevention recommend a single oral dose of 400 mg albendazole for adults or 10 to 15 mg/kg in children for CLM. A single dose of ivermectin at 12 mg in adults or 150 μg/kg in children can be used as an alternative where albendazole is not available.11 Topical applications of thiabendazole 10% to 15% under occlusion or 3 times daily for 15 days without occlusion also can manage CLM, and pruritus can be treated with topical corticosteroids for symptomatic relief. Oral albendazole 400 mg twice daily or mebendazole 100 mg twice daily for 3 days or a single 500-mg dose, as well as 11 mg/kg (up to a maximum of 1 g) oral pyrantel pamoate once daily for 3 days can be used to treat intestinal hookworm infection, though it should be avoided in pregnancy. Iron deficiency should be managed with supplementation.11
Prevention of hookworm infection is focused around 2 broad public health efforts: mass drug administration programs and the water, sanitation, and hygiene program. In mass drug administration, treatments such as benzimidazoles are given in mass to communities affected by endemic hookworm as a single dose to reduce the burden of disease. Together, these strategies effectively eliminated hookworms in many developed nations, but areas of resurgence are beginning to surface worldwide. With changes in climate, emerging drug resistance, and socioeconomic disparities, particularly affecting the southeast, a resurgence of hookworm has occurred in the United States.26 One recent study demonstrated that almost one-third (19/55) of children sampled in an impoverished area of rural Alabama had hookworm eggs in their stool.27 Furthermore, pets serve not only as zoonotic reservoirs for CLM recurrence but also as vehicles for the evolution of drug-resistant strains, leading some to call for a ban of animals from beaches and playgrounds as well as tightly controlled veterinary programs.5,28 Ubiquitous benzimidazole use in livestock has led to bendazole-resistant strains, and it is likely that with continued and poorly adherent drug use, more zoonotic and anthropophilic drug-resistant strains of hookworm will emerge.29,30
Conclusion
The burden of hookworm infection and CLM is substantial in parts of the United States. Dermatologists play a critical role in the recognition and management of hookworm infection for both treatment of affected patients and the subsequent prevention of its spread. As drug-resistant strains evolve, clinicians, public health officials, and scientists need to continue to work together to prevent and treat hookworm infection.
- Vos T, Abajobir AA, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211-1259.
- Bartsch SM, Hotez PJ, Asti L, et al. The global economic and health burden of human hookworm infection. PLoS Negl Trop Dis. 2016;10:E0004922.
- Seguel M, Gottdenker N. The diversity and impact of hookworm infections in wildlife. Int J Parasitol Parasites Wildl. 2017;6:177-194.
- Adams BJ, Peat SM, Dillman AR. Phylogeny and evolution. In: Entomopathogenic Nematodes: Systematics, Phylogeny and Bacterial Symbionts. Brill; 2010:693-733.
- Heukelbach J, Feldmeier H. Epidemiological and clinical characteristics of hookworm-related cutaneous larva migrans. Lancet Infect Dis. 2008;8:302-309.
- Haas W, Haberl B, Idris I, et al. Infective larvae of the human hookworms Necator americanus and Ancylostoma duodenale differ in their orientation behaviour when crawling on surfaces. Parasitol Res. 2005;95:25-29.
- Hotez P, Narasimhan S, Haggerty J, et al. Hyaluronidase from infective Ancylostoma hookworm larvae and its possible function as a virulence factor in tissue invasion and in cutaneous larva migrans. Infect Immun. 1992;60:1018-1023.
- Brooker S, Bethony J, Hotez PJ. Human hookworm infection in the 21st century. Adv Parasitol. 2004;58:197-288.
- Hoagland K, Schad G. Necator americanus and Ancylostoma duodenale: life history parameters and epidemiological implications of two sympatric hookworms of humans. Exp Parasitol. 1978;44:36-49.
- Clements ACA, Alene KA. Global distribution of human hookworm species and differences in their morbidity effects: a systematic review. Lancet Microbe. 2022;3:E72-E79.
- Loukas A, Hotez PJ, Diemert D, et al. Hookworm infection. Nat Rev Dis Primers. 2016;2:1-18.
- Gazzinelli A, Correa-Oliveira R, Yang GJ, et al. A research agenda for helminth diseases of humans: social ecology, environmental determinants, and health systems. PLoS Negl Trop Dis. 2012;6:E1603.
- Starr MC, Montgomery SP. Soil-transmitted helminthiasis in the United States: a systematic review—1940-2010. Am J Trop Med Hyg. 2011;85:680-684.
- Strunz EC, Addiss DG, Stocks ME, et al. Water, sanitation, hygiene, and soil-transmitted helminth infection: a systematic review and metaanalysis. PLoS Med. 2014;11:E1001620.
- Liotta JL, Youn H, Aksel S, et al. Prevalence of Ancylostoma braziliense in dogs from Alachua and Marion Counties, Florida, United States. J Parasitol. 2012;98:1039-1040.
- Hotez PJ, Brooker S, Bethony JM, et al. Hookworm infection. N Engl J Med. 2004;351:799-807.
- Prickett KA, Ferringer TC. What’s eating you? cutaneous larva migrans. Cutis. 2015;95:126-128.
- Feldmeier H, Schuster A. Mini review: hookworm-related cutaneous larva migrans. Eur J Clin Microbiol Infect Dis. 2012;31:915-918.
- Tan SK, Liu TT. Cutaneous larva migrans complicated by Löffler syndrome. Arch Dermatol. 2010;146:210-212.
- Eksomtramage T, Aiempanakit K. Bullous and pustular cutaneous larva migrans: two case reports and a literature review. IDCases. 2018;12:130-132.
- Utzinger J, Rinaldi L, Lohourignon LK, et al. FLOTAC: a new sensitive technique for the diagnosis of hookworm infections in humans. Trans R Soc Trop Med Hyg. 2008;102:84-90.
- Chidambaram M, Parija SC, Toi PC, et al. Evaluation of the utility of conventional polymerase chain reaction for detection and species differentiation in human hookworm infections. Trop Parasitol. 2017;7:111-116.
- Gaze S, McSorley HJ, Daveson J, et al. Characterising the mucosal and systemic immune responses to experimental human hookworm infection. PLoS Pathog. 2012;8:E1002520.
- Croese J, O’Neil J, Masson J, et al. A proof of concept study establishing Necator americanus in Crohn’s patients and reservoir donors. Gut. 2006;55:136-137.
- Mpairwe H, Amoah AS. Parasites and allergy: observations from Africa. Parasite Immunol. 2019;41:E12589.
- Albonico M, Savioli L. Hookworm: a neglected resurgent infection. Editorial. BMJ. 2017;359:j4813.
- McKenna ML, McAtee S, Bryan PE, et al. Human intestinal parasite burden and poor sanitation in rural Alabama. Am J Trop Med Hyg. 2017;97:1623-1628.
- Traversa D. Pet roundworms and hookworms: a continuing need for global worming. Parasit Vectors. 2012;5:1-19.
- Geerts S, Gryseels B. Drug resistance in human helminths: current situation and lessons from livestock. Clin Microbiol Rev. 2000;13:207-222.
- Jimenez Castro PD, Howell SB, Schaefer JJ, et al. Multiple drug resistance in the canine hookworm Ancylostoma caninum: an emerging threat? Parasit Vectors. 2019;12:1-15.
It is estimated that the prevalence of human hookworm infection is approximately 450 million individuals worldwide, representing a substantial global disease burden.1 The annual global public health burden ranges from approximately 2 million to 4 million disability-adjusted life-years and $10 billion to $140 billion in hookwormrelated costs.2 In this article, we discuss the lifecycle, transmission, and disease burden of cutaneous larva migrans (CLM) as well as prevention and treatment strategies.
Background
The Ancylostomatidae nematode family comprises at least 68 known species of hookworm that infect more than 110 different species of mammals.3 Many of these parasites are able to infect more than 1 primary host species, but from a disease perspective they can be classified as either anthropophilic, with humans as the intended host, or zoonotic, with humans as an incidental host. It is important to make this distinction because, though the lifecycles and biology of hookworm species generally are similar, the manifestations of incidental human infection from zoonotic hookworms are different from those of anthropophilic hookworms. Of the anthropophilic species, Necator americanus and Ancylostoma duodenale predominate. In the instance of zoonotic hookworm, dog-infecting A caninum and cat- and doginfecting A braziliense and Uncinaria stenocephala are common causes of incidental human disease.3
The life cycle of Ancylostomatidae organisms is astounding. Through millions of years of co-evolution with mammals,4 these parasitic worms have developed perhaps one of the most circuitous paths to propagate themselves in the natural world. Hookworms start their arduous journey as eggs deposited in soil, sand, and ground vegetation from the feces of infected animals.5 Approximately 1 day after the eggs are deposited, they hatch and begin the larval stage, during which they become infective 1 to 5 weeks later. At this point, the larvae become sensitive to their environment, responding to rising temperatures, increasing carbon dioxide levels, and vibrations in the soil—all of which suggest the presence of a potential host and contribute to a concordant increase in undulatory movement of the larvae.5,6 Here, the most vulnerable tissues include the uncovered soles, palms, and buttocks of host mammals that come into contact with contaminated soil. In an undulating fashion and guided by temperature cues, the larvae locate the skin of the host and utilize a mixture of enzymes including hyaluronidases, metalloprotease, and other proteases to penetrate the epidermis.7 Anthropophilic hookworms such as N americanus and A duodenale will enter the circulatory system; from there, the hookworms migrate through the right-sided cardiopulmonary circuit and eventually ascend into the pulmonary vasculature.8 They then penetrate the lung capillary beds and parenchyma to reach the alveoli, ascend the respiratory tree, and, with the help of the mucociliary escalator, reach the esophagus, where they are swallowed by the host. In the gastrointestinal tract, adult hookworms consume host blood, mate, and lay eggs over a period of approximately 1 to 3 years if left untreated.9 Eggs are laid into the lower gastrointestinal tract, and the journey begins again in feces contacting ground or soil.
Geographic Distribution
Hookworms are found in almost all regions of the world, with species-specific distributions that highlight tropical and subtropical regions. Necator americanus and A duodenale are the most common hookworm species, with the former found predominantly in Southeast Asia and Latin America and the latter in Asia-Pacific regions.10 The highest prevalence of hookworms is in Southeast Asia followed by Sub-Saharan Africa, and the unique climate and soil composition of a region help determine the best environments for specific species of hookworm to thrive.11 In addition, socioeconomics and social determinants of health play a big role in the spread of hookworms, as hygiene practices (eg, wearing clean shoes and clothing, bathing), infrastructure (eg, clean water and streets), and anthelmintic campaigns help reduce transmission.12 Soil-transmitted helminths were once endemic to the southeastern United States, with some reports of approximately 40% of individuals infected in the south in the early 1900s.13 Anthelmintic campaigns such as water, sanitation, and hygiene programs as well as deworming of humans and livestock have proven effective in reducing the prevalence of helminth disease in industrialized nations.13,14 However, zoonotic infections remain a problem in these regions, and in some parts of the United States more than 40% of sampled cats and dogs harbored species such as A braziliense.15
Clinical Manifestation
Initial hookworm infection often goes unnoticed because symptoms can range in severity, but it is characterized by transient ground itch—a local pruritic, erythematous, and papular eruption that develops in response to epidermal penetration.16 Because the larvae must traverse the host from skin to target organs for reproduction over several weeks, iron-deficiency anemia will manifest much later than signs of the initial penetration. In the case of incidental infection from zoonotic Ancylostomatidae organisms, the misguided larvae result in CLM, an often intensely pruritic skin condition that will self-resolve in 2 to 8 weeks with eventual death of the larvae.5
Diagnosis and Pathology of Disease
Zoonotic Hookworm—The major presenting sign of zoonotic hookworm infection is CLM. The diagnosis of CLM usually is made clinically, as the larvae themselves are 0.5 mm thick to 10 mm long (Figure 1) and usually extend several centimeters beyond the dermal lesion, with dermoscopy having limited utility.17 Patients may begin to experience itching as little as 1 hour after hookworm penetration of the skin.18 Once in contact with the skin, the hookworms’ hyaluronidases and proteases are capable of breaking through the epidermis, but zoonotic hookworms typically are unable to penetrate the basal layer of the human epidermis and remain entombed between the stratum granulosum and stratum corneum. With the exception of rare cases of direct or indirect pulmonary involvement resulting in Löffler syndrome,19 the larvae will die within weeks to months, and symptoms will subsequently resolve.
Although the infection generally is self-limiting, the dermatologic manifestations of CLM can be severe and warrant intervention. The lesions start as small reddish papules at the site of penetration (Figure 2), then the hallmark elevated, migrating, serpiginous, urticarial rash develops (Figure 3). Cutaneous larva migrans generally manifests unilaterally and is both erythematous and intensely pruritic. As the larvae migrate, they leave behind 1- to 5-cm tunneled creeping eruptions in their wake. The lesions, which can manifest with pain or be painless, may develop eczematous, bullous, follicular, or impetiginized appearances.20 Atypical manifestations include folliculitis and urticarial plaques.17
cutaneous larval migrans on the palm.
Anthropophilic Hookworm—The lifecycles of N americanus and A duodenale are completed in human infection. Dermatologic manifestations are transient with the development of ground itch at the site of epidermal penetration. The hookworms employ collagenases that allow penetration of the basal layer of the skin, and eosinophilia develops as the parasites travel from the skin to the small intestine. Once attached to the gastrointestinal lumen, blood meals and proteolytic enzymes result in iron-deficiency anemia in the host and may lead to weakness, fatigue, and low birth weights in pregnant patients. With prolonged infection or heavy parasitic burden, patients can develop hypoproteinemia, anasarca, and yellowing of the skin known as chlorosis.11 A clinical diagnosis can be made by examining patient stool samples for eggs, and definitive characterization can be made using molecular tools such as polymerase chain reaction.21,22
Common to hookworm infections is the immune reaction, which promotes inflammation with localized eosinophilia and mastocytosis.11 In a clinical biopsy specimen of gut—usually obtained through esophagogastroduodenoscopy— T-helper (Th) 2–type immune (IL-4, IL-5, IL-9 and IL-13), regulatory Th10 (IL-10 and transcription growth factor β), and some evidence of Th1 (interferon gamma and IL-2) cytokines are present, but little evidence of Th17-type immune response was found.23 It is believed that in zoonotic infections, antiparasitic IgE from basophils are somewhat successful at trapping the helminths in the epidermis, but in the anthropophilic species, IgE and Th2 responses are ineffective at clearing the parasite from the gut, and the defeated immune system transitions to a host-tolerance approach of limiting infection.11 It is now believed that this natural armistice can be manipulated into a potential therapy against autoimmune and inflammatory conditions. Intentional infection with zoonotic whipworm or hookworm has been proposed as a mechanism of switching Th1 and Th2 responses to host-tolerant mechanisms in conditions such as Crohn disease and celiac disease,24 and it has even been hypothesized that prior hookworm infection may reduce the chance of developing allergic conditions such as eczema.25
Treatment and Prevention
The World Health Organization and Centers for Disease Control and Prevention recommend a single oral dose of 400 mg albendazole for adults or 10 to 15 mg/kg in children for CLM. A single dose of ivermectin at 12 mg in adults or 150 μg/kg in children can be used as an alternative where albendazole is not available.11 Topical applications of thiabendazole 10% to 15% under occlusion or 3 times daily for 15 days without occlusion also can manage CLM, and pruritus can be treated with topical corticosteroids for symptomatic relief. Oral albendazole 400 mg twice daily or mebendazole 100 mg twice daily for 3 days or a single 500-mg dose, as well as 11 mg/kg (up to a maximum of 1 g) oral pyrantel pamoate once daily for 3 days can be used to treat intestinal hookworm infection, though it should be avoided in pregnancy. Iron deficiency should be managed with supplementation.11
Prevention of hookworm infection is focused around 2 broad public health efforts: mass drug administration programs and the water, sanitation, and hygiene program. In mass drug administration, treatments such as benzimidazoles are given in mass to communities affected by endemic hookworm as a single dose to reduce the burden of disease. Together, these strategies effectively eliminated hookworms in many developed nations, but areas of resurgence are beginning to surface worldwide. With changes in climate, emerging drug resistance, and socioeconomic disparities, particularly affecting the southeast, a resurgence of hookworm has occurred in the United States.26 One recent study demonstrated that almost one-third (19/55) of children sampled in an impoverished area of rural Alabama had hookworm eggs in their stool.27 Furthermore, pets serve not only as zoonotic reservoirs for CLM recurrence but also as vehicles for the evolution of drug-resistant strains, leading some to call for a ban of animals from beaches and playgrounds as well as tightly controlled veterinary programs.5,28 Ubiquitous benzimidazole use in livestock has led to bendazole-resistant strains, and it is likely that with continued and poorly adherent drug use, more zoonotic and anthropophilic drug-resistant strains of hookworm will emerge.29,30
Conclusion
The burden of hookworm infection and CLM is substantial in parts of the United States. Dermatologists play a critical role in the recognition and management of hookworm infection for both treatment of affected patients and the subsequent prevention of its spread. As drug-resistant strains evolve, clinicians, public health officials, and scientists need to continue to work together to prevent and treat hookworm infection.
It is estimated that the prevalence of human hookworm infection is approximately 450 million individuals worldwide, representing a substantial global disease burden.1 The annual global public health burden ranges from approximately 2 million to 4 million disability-adjusted life-years and $10 billion to $140 billion in hookwormrelated costs.2 In this article, we discuss the lifecycle, transmission, and disease burden of cutaneous larva migrans (CLM) as well as prevention and treatment strategies.
Background
The Ancylostomatidae nematode family comprises at least 68 known species of hookworm that infect more than 110 different species of mammals.3 Many of these parasites are able to infect more than 1 primary host species, but from a disease perspective they can be classified as either anthropophilic, with humans as the intended host, or zoonotic, with humans as an incidental host. It is important to make this distinction because, though the lifecycles and biology of hookworm species generally are similar, the manifestations of incidental human infection from zoonotic hookworms are different from those of anthropophilic hookworms. Of the anthropophilic species, Necator americanus and Ancylostoma duodenale predominate. In the instance of zoonotic hookworm, dog-infecting A caninum and cat- and doginfecting A braziliense and Uncinaria stenocephala are common causes of incidental human disease.3
The life cycle of Ancylostomatidae organisms is astounding. Through millions of years of co-evolution with mammals,4 these parasitic worms have developed perhaps one of the most circuitous paths to propagate themselves in the natural world. Hookworms start their arduous journey as eggs deposited in soil, sand, and ground vegetation from the feces of infected animals.5 Approximately 1 day after the eggs are deposited, they hatch and begin the larval stage, during which they become infective 1 to 5 weeks later. At this point, the larvae become sensitive to their environment, responding to rising temperatures, increasing carbon dioxide levels, and vibrations in the soil—all of which suggest the presence of a potential host and contribute to a concordant increase in undulatory movement of the larvae.5,6 Here, the most vulnerable tissues include the uncovered soles, palms, and buttocks of host mammals that come into contact with contaminated soil. In an undulating fashion and guided by temperature cues, the larvae locate the skin of the host and utilize a mixture of enzymes including hyaluronidases, metalloprotease, and other proteases to penetrate the epidermis.7 Anthropophilic hookworms such as N americanus and A duodenale will enter the circulatory system; from there, the hookworms migrate through the right-sided cardiopulmonary circuit and eventually ascend into the pulmonary vasculature.8 They then penetrate the lung capillary beds and parenchyma to reach the alveoli, ascend the respiratory tree, and, with the help of the mucociliary escalator, reach the esophagus, where they are swallowed by the host. In the gastrointestinal tract, adult hookworms consume host blood, mate, and lay eggs over a period of approximately 1 to 3 years if left untreated.9 Eggs are laid into the lower gastrointestinal tract, and the journey begins again in feces contacting ground or soil.
Geographic Distribution
Hookworms are found in almost all regions of the world, with species-specific distributions that highlight tropical and subtropical regions. Necator americanus and A duodenale are the most common hookworm species, with the former found predominantly in Southeast Asia and Latin America and the latter in Asia-Pacific regions.10 The highest prevalence of hookworms is in Southeast Asia followed by Sub-Saharan Africa, and the unique climate and soil composition of a region help determine the best environments for specific species of hookworm to thrive.11 In addition, socioeconomics and social determinants of health play a big role in the spread of hookworms, as hygiene practices (eg, wearing clean shoes and clothing, bathing), infrastructure (eg, clean water and streets), and anthelmintic campaigns help reduce transmission.12 Soil-transmitted helminths were once endemic to the southeastern United States, with some reports of approximately 40% of individuals infected in the south in the early 1900s.13 Anthelmintic campaigns such as water, sanitation, and hygiene programs as well as deworming of humans and livestock have proven effective in reducing the prevalence of helminth disease in industrialized nations.13,14 However, zoonotic infections remain a problem in these regions, and in some parts of the United States more than 40% of sampled cats and dogs harbored species such as A braziliense.15
Clinical Manifestation
Initial hookworm infection often goes unnoticed because symptoms can range in severity, but it is characterized by transient ground itch—a local pruritic, erythematous, and papular eruption that develops in response to epidermal penetration.16 Because the larvae must traverse the host from skin to target organs for reproduction over several weeks, iron-deficiency anemia will manifest much later than signs of the initial penetration. In the case of incidental infection from zoonotic Ancylostomatidae organisms, the misguided larvae result in CLM, an often intensely pruritic skin condition that will self-resolve in 2 to 8 weeks with eventual death of the larvae.5
Diagnosis and Pathology of Disease
Zoonotic Hookworm—The major presenting sign of zoonotic hookworm infection is CLM. The diagnosis of CLM usually is made clinically, as the larvae themselves are 0.5 mm thick to 10 mm long (Figure 1) and usually extend several centimeters beyond the dermal lesion, with dermoscopy having limited utility.17 Patients may begin to experience itching as little as 1 hour after hookworm penetration of the skin.18 Once in contact with the skin, the hookworms’ hyaluronidases and proteases are capable of breaking through the epidermis, but zoonotic hookworms typically are unable to penetrate the basal layer of the human epidermis and remain entombed between the stratum granulosum and stratum corneum. With the exception of rare cases of direct or indirect pulmonary involvement resulting in Löffler syndrome,19 the larvae will die within weeks to months, and symptoms will subsequently resolve.
Although the infection generally is self-limiting, the dermatologic manifestations of CLM can be severe and warrant intervention. The lesions start as small reddish papules at the site of penetration (Figure 2), then the hallmark elevated, migrating, serpiginous, urticarial rash develops (Figure 3). Cutaneous larva migrans generally manifests unilaterally and is both erythematous and intensely pruritic. As the larvae migrate, they leave behind 1- to 5-cm tunneled creeping eruptions in their wake. The lesions, which can manifest with pain or be painless, may develop eczematous, bullous, follicular, or impetiginized appearances.20 Atypical manifestations include folliculitis and urticarial plaques.17
cutaneous larval migrans on the palm.
Anthropophilic Hookworm—The lifecycles of N americanus and A duodenale are completed in human infection. Dermatologic manifestations are transient with the development of ground itch at the site of epidermal penetration. The hookworms employ collagenases that allow penetration of the basal layer of the skin, and eosinophilia develops as the parasites travel from the skin to the small intestine. Once attached to the gastrointestinal lumen, blood meals and proteolytic enzymes result in iron-deficiency anemia in the host and may lead to weakness, fatigue, and low birth weights in pregnant patients. With prolonged infection or heavy parasitic burden, patients can develop hypoproteinemia, anasarca, and yellowing of the skin known as chlorosis.11 A clinical diagnosis can be made by examining patient stool samples for eggs, and definitive characterization can be made using molecular tools such as polymerase chain reaction.21,22
Common to hookworm infections is the immune reaction, which promotes inflammation with localized eosinophilia and mastocytosis.11 In a clinical biopsy specimen of gut—usually obtained through esophagogastroduodenoscopy— T-helper (Th) 2–type immune (IL-4, IL-5, IL-9 and IL-13), regulatory Th10 (IL-10 and transcription growth factor β), and some evidence of Th1 (interferon gamma and IL-2) cytokines are present, but little evidence of Th17-type immune response was found.23 It is believed that in zoonotic infections, antiparasitic IgE from basophils are somewhat successful at trapping the helminths in the epidermis, but in the anthropophilic species, IgE and Th2 responses are ineffective at clearing the parasite from the gut, and the defeated immune system transitions to a host-tolerance approach of limiting infection.11 It is now believed that this natural armistice can be manipulated into a potential therapy against autoimmune and inflammatory conditions. Intentional infection with zoonotic whipworm or hookworm has been proposed as a mechanism of switching Th1 and Th2 responses to host-tolerant mechanisms in conditions such as Crohn disease and celiac disease,24 and it has even been hypothesized that prior hookworm infection may reduce the chance of developing allergic conditions such as eczema.25
Treatment and Prevention
The World Health Organization and Centers for Disease Control and Prevention recommend a single oral dose of 400 mg albendazole for adults or 10 to 15 mg/kg in children for CLM. A single dose of ivermectin at 12 mg in adults or 150 μg/kg in children can be used as an alternative where albendazole is not available.11 Topical applications of thiabendazole 10% to 15% under occlusion or 3 times daily for 15 days without occlusion also can manage CLM, and pruritus can be treated with topical corticosteroids for symptomatic relief. Oral albendazole 400 mg twice daily or mebendazole 100 mg twice daily for 3 days or a single 500-mg dose, as well as 11 mg/kg (up to a maximum of 1 g) oral pyrantel pamoate once daily for 3 days can be used to treat intestinal hookworm infection, though it should be avoided in pregnancy. Iron deficiency should be managed with supplementation.11
Prevention of hookworm infection is focused around 2 broad public health efforts: mass drug administration programs and the water, sanitation, and hygiene program. In mass drug administration, treatments such as benzimidazoles are given in mass to communities affected by endemic hookworm as a single dose to reduce the burden of disease. Together, these strategies effectively eliminated hookworms in many developed nations, but areas of resurgence are beginning to surface worldwide. With changes in climate, emerging drug resistance, and socioeconomic disparities, particularly affecting the southeast, a resurgence of hookworm has occurred in the United States.26 One recent study demonstrated that almost one-third (19/55) of children sampled in an impoverished area of rural Alabama had hookworm eggs in their stool.27 Furthermore, pets serve not only as zoonotic reservoirs for CLM recurrence but also as vehicles for the evolution of drug-resistant strains, leading some to call for a ban of animals from beaches and playgrounds as well as tightly controlled veterinary programs.5,28 Ubiquitous benzimidazole use in livestock has led to bendazole-resistant strains, and it is likely that with continued and poorly adherent drug use, more zoonotic and anthropophilic drug-resistant strains of hookworm will emerge.29,30
Conclusion
The burden of hookworm infection and CLM is substantial in parts of the United States. Dermatologists play a critical role in the recognition and management of hookworm infection for both treatment of affected patients and the subsequent prevention of its spread. As drug-resistant strains evolve, clinicians, public health officials, and scientists need to continue to work together to prevent and treat hookworm infection.
- Vos T, Abajobir AA, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211-1259.
- Bartsch SM, Hotez PJ, Asti L, et al. The global economic and health burden of human hookworm infection. PLoS Negl Trop Dis. 2016;10:E0004922.
- Seguel M, Gottdenker N. The diversity and impact of hookworm infections in wildlife. Int J Parasitol Parasites Wildl. 2017;6:177-194.
- Adams BJ, Peat SM, Dillman AR. Phylogeny and evolution. In: Entomopathogenic Nematodes: Systematics, Phylogeny and Bacterial Symbionts. Brill; 2010:693-733.
- Heukelbach J, Feldmeier H. Epidemiological and clinical characteristics of hookworm-related cutaneous larva migrans. Lancet Infect Dis. 2008;8:302-309.
- Haas W, Haberl B, Idris I, et al. Infective larvae of the human hookworms Necator americanus and Ancylostoma duodenale differ in their orientation behaviour when crawling on surfaces. Parasitol Res. 2005;95:25-29.
- Hotez P, Narasimhan S, Haggerty J, et al. Hyaluronidase from infective Ancylostoma hookworm larvae and its possible function as a virulence factor in tissue invasion and in cutaneous larva migrans. Infect Immun. 1992;60:1018-1023.
- Brooker S, Bethony J, Hotez PJ. Human hookworm infection in the 21st century. Adv Parasitol. 2004;58:197-288.
- Hoagland K, Schad G. Necator americanus and Ancylostoma duodenale: life history parameters and epidemiological implications of two sympatric hookworms of humans. Exp Parasitol. 1978;44:36-49.
- Clements ACA, Alene KA. Global distribution of human hookworm species and differences in their morbidity effects: a systematic review. Lancet Microbe. 2022;3:E72-E79.
- Loukas A, Hotez PJ, Diemert D, et al. Hookworm infection. Nat Rev Dis Primers. 2016;2:1-18.
- Gazzinelli A, Correa-Oliveira R, Yang GJ, et al. A research agenda for helminth diseases of humans: social ecology, environmental determinants, and health systems. PLoS Negl Trop Dis. 2012;6:E1603.
- Starr MC, Montgomery SP. Soil-transmitted helminthiasis in the United States: a systematic review—1940-2010. Am J Trop Med Hyg. 2011;85:680-684.
- Strunz EC, Addiss DG, Stocks ME, et al. Water, sanitation, hygiene, and soil-transmitted helminth infection: a systematic review and metaanalysis. PLoS Med. 2014;11:E1001620.
- Liotta JL, Youn H, Aksel S, et al. Prevalence of Ancylostoma braziliense in dogs from Alachua and Marion Counties, Florida, United States. J Parasitol. 2012;98:1039-1040.
- Hotez PJ, Brooker S, Bethony JM, et al. Hookworm infection. N Engl J Med. 2004;351:799-807.
- Prickett KA, Ferringer TC. What’s eating you? cutaneous larva migrans. Cutis. 2015;95:126-128.
- Feldmeier H, Schuster A. Mini review: hookworm-related cutaneous larva migrans. Eur J Clin Microbiol Infect Dis. 2012;31:915-918.
- Tan SK, Liu TT. Cutaneous larva migrans complicated by Löffler syndrome. Arch Dermatol. 2010;146:210-212.
- Eksomtramage T, Aiempanakit K. Bullous and pustular cutaneous larva migrans: two case reports and a literature review. IDCases. 2018;12:130-132.
- Utzinger J, Rinaldi L, Lohourignon LK, et al. FLOTAC: a new sensitive technique for the diagnosis of hookworm infections in humans. Trans R Soc Trop Med Hyg. 2008;102:84-90.
- Chidambaram M, Parija SC, Toi PC, et al. Evaluation of the utility of conventional polymerase chain reaction for detection and species differentiation in human hookworm infections. Trop Parasitol. 2017;7:111-116.
- Gaze S, McSorley HJ, Daveson J, et al. Characterising the mucosal and systemic immune responses to experimental human hookworm infection. PLoS Pathog. 2012;8:E1002520.
- Croese J, O’Neil J, Masson J, et al. A proof of concept study establishing Necator americanus in Crohn’s patients and reservoir donors. Gut. 2006;55:136-137.
- Mpairwe H, Amoah AS. Parasites and allergy: observations from Africa. Parasite Immunol. 2019;41:E12589.
- Albonico M, Savioli L. Hookworm: a neglected resurgent infection. Editorial. BMJ. 2017;359:j4813.
- McKenna ML, McAtee S, Bryan PE, et al. Human intestinal parasite burden and poor sanitation in rural Alabama. Am J Trop Med Hyg. 2017;97:1623-1628.
- Traversa D. Pet roundworms and hookworms: a continuing need for global worming. Parasit Vectors. 2012;5:1-19.
- Geerts S, Gryseels B. Drug resistance in human helminths: current situation and lessons from livestock. Clin Microbiol Rev. 2000;13:207-222.
- Jimenez Castro PD, Howell SB, Schaefer JJ, et al. Multiple drug resistance in the canine hookworm Ancylostoma caninum: an emerging threat? Parasit Vectors. 2019;12:1-15.
- Vos T, Abajobir AA, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211-1259.
- Bartsch SM, Hotez PJ, Asti L, et al. The global economic and health burden of human hookworm infection. PLoS Negl Trop Dis. 2016;10:E0004922.
- Seguel M, Gottdenker N. The diversity and impact of hookworm infections in wildlife. Int J Parasitol Parasites Wildl. 2017;6:177-194.
- Adams BJ, Peat SM, Dillman AR. Phylogeny and evolution. In: Entomopathogenic Nematodes: Systematics, Phylogeny and Bacterial Symbionts. Brill; 2010:693-733.
- Heukelbach J, Feldmeier H. Epidemiological and clinical characteristics of hookworm-related cutaneous larva migrans. Lancet Infect Dis. 2008;8:302-309.
- Haas W, Haberl B, Idris I, et al. Infective larvae of the human hookworms Necator americanus and Ancylostoma duodenale differ in their orientation behaviour when crawling on surfaces. Parasitol Res. 2005;95:25-29.
- Hotez P, Narasimhan S, Haggerty J, et al. Hyaluronidase from infective Ancylostoma hookworm larvae and its possible function as a virulence factor in tissue invasion and in cutaneous larva migrans. Infect Immun. 1992;60:1018-1023.
- Brooker S, Bethony J, Hotez PJ. Human hookworm infection in the 21st century. Adv Parasitol. 2004;58:197-288.
- Hoagland K, Schad G. Necator americanus and Ancylostoma duodenale: life history parameters and epidemiological implications of two sympatric hookworms of humans. Exp Parasitol. 1978;44:36-49.
- Clements ACA, Alene KA. Global distribution of human hookworm species and differences in their morbidity effects: a systematic review. Lancet Microbe. 2022;3:E72-E79.
- Loukas A, Hotez PJ, Diemert D, et al. Hookworm infection. Nat Rev Dis Primers. 2016;2:1-18.
- Gazzinelli A, Correa-Oliveira R, Yang GJ, et al. A research agenda for helminth diseases of humans: social ecology, environmental determinants, and health systems. PLoS Negl Trop Dis. 2012;6:E1603.
- Starr MC, Montgomery SP. Soil-transmitted helminthiasis in the United States: a systematic review—1940-2010. Am J Trop Med Hyg. 2011;85:680-684.
- Strunz EC, Addiss DG, Stocks ME, et al. Water, sanitation, hygiene, and soil-transmitted helminth infection: a systematic review and metaanalysis. PLoS Med. 2014;11:E1001620.
- Liotta JL, Youn H, Aksel S, et al. Prevalence of Ancylostoma braziliense in dogs from Alachua and Marion Counties, Florida, United States. J Parasitol. 2012;98:1039-1040.
- Hotez PJ, Brooker S, Bethony JM, et al. Hookworm infection. N Engl J Med. 2004;351:799-807.
- Prickett KA, Ferringer TC. What’s eating you? cutaneous larva migrans. Cutis. 2015;95:126-128.
- Feldmeier H, Schuster A. Mini review: hookworm-related cutaneous larva migrans. Eur J Clin Microbiol Infect Dis. 2012;31:915-918.
- Tan SK, Liu TT. Cutaneous larva migrans complicated by Löffler syndrome. Arch Dermatol. 2010;146:210-212.
- Eksomtramage T, Aiempanakit K. Bullous and pustular cutaneous larva migrans: two case reports and a literature review. IDCases. 2018;12:130-132.
- Utzinger J, Rinaldi L, Lohourignon LK, et al. FLOTAC: a new sensitive technique for the diagnosis of hookworm infections in humans. Trans R Soc Trop Med Hyg. 2008;102:84-90.
- Chidambaram M, Parija SC, Toi PC, et al. Evaluation of the utility of conventional polymerase chain reaction for detection and species differentiation in human hookworm infections. Trop Parasitol. 2017;7:111-116.
- Gaze S, McSorley HJ, Daveson J, et al. Characterising the mucosal and systemic immune responses to experimental human hookworm infection. PLoS Pathog. 2012;8:E1002520.
- Croese J, O’Neil J, Masson J, et al. A proof of concept study establishing Necator americanus in Crohn’s patients and reservoir donors. Gut. 2006;55:136-137.
- Mpairwe H, Amoah AS. Parasites and allergy: observations from Africa. Parasite Immunol. 2019;41:E12589.
- Albonico M, Savioli L. Hookworm: a neglected resurgent infection. Editorial. BMJ. 2017;359:j4813.
- McKenna ML, McAtee S, Bryan PE, et al. Human intestinal parasite burden and poor sanitation in rural Alabama. Am J Trop Med Hyg. 2017;97:1623-1628.
- Traversa D. Pet roundworms and hookworms: a continuing need for global worming. Parasit Vectors. 2012;5:1-19.
- Geerts S, Gryseels B. Drug resistance in human helminths: current situation and lessons from livestock. Clin Microbiol Rev. 2000;13:207-222.
- Jimenez Castro PD, Howell SB, Schaefer JJ, et al. Multiple drug resistance in the canine hookworm Ancylostoma caninum: an emerging threat? Parasit Vectors. 2019;12:1-15.
What’s Eating You? Hookworm and
Cutaneous Larva Migrans
What’s Eating You? Hookworm and
Cutaneous Larva Migrans
PRACTICE POINTS
- Anthropophilic hookworm infection should be considered with evidence of either transient ground itch or iron-deficient anemia in individuals who go barefoot, permitting ground-to-skin transmission.
- Zoonotic hookworm infection manifests as cutaneous larva migrans, an elevated serpiginous rash that, while usually self-resolving, can be intensely pruritic and should be treated accordingly.
- Considered a neglected tropical disease, hookworm infection still represents an enormous global disease burden. In addition to ongoing afflicted regions, hookworms are making a resurgence in developed nations, and drug-resistant strains have evolved.
Comparing Patient Care Models at a Local Free Clinic vs an Insurance- Based University Medical Center
Comparing Patient Care Models at a Local Free Clinic vs an Insurance- Based University Medical Center
Approximately 25% of Americans have at least one skin condition, and 20% are estimated to develop skin cancer during their lifetime.1,2 However, 40% of the US population lives in areas underserved by dermatologists. 3 The severity and mortality of skin cancers such as melanoma and mycosis fungoides have been positively associated with minoritized race, lack of health insurance, and unstable housing status.4-6 Patients who receive health care at free clinics often are of a racial or ethnic minoritized social group, are uninsured, and/or lack stable housing; this underserved group also includes recent immigrants to the United States who have limited English proficiency (LEP).7 Only 25% of free clinics offer specialty care services such as dermatology.7,8
Of the 42 free clinics and Federally Qualified Health Centers in Pittsburgh, Pennsylvania, the Birmingham Free Clinic (BFC) is one of the few that offers specialty care services including dermatology.9 Founded in 1994, the BFC serves as a safety net for Pittsburgh’s medically underserved population, offering primary and acute care, medication access, and social services. From January 2020 to May 2022, the BFC offered 27 dermatology clinics that provided approximately 100 people with comprehensive care including full-body skin examinations, dermatologic diagnoses and treatments, minor procedures, and dermatopathology services.
In this study, we compared the BFC dermatology patient care model with that of the dermatology department at the University of Pittsburgh Medical Center (UPMC), an insurance-based tertiary referral health care system in western Pennsylvania. By analyzing the demographics, dermatologic diagnoses, and management strategies of both the BFC and UPMC, we gained an understanding of how these patient care models differ and how they can be improved to care for diverse patient populations.
Methods
A retrospective chart review of dermatology patients seen in person at the BFC and UPMC during the period from January 2020 to May 2022 was performed. The UPMC group included patients seen by 3 general dermatologists (including A.J.J.) at matched time points. Data were collected from patients’ first in-person visit during the study period. Variables of interest included patient age, sex, race, ethnicity, primary language, zip code, health insurance status, distance to clinic (estimated using Google Maps to calculate the shortest driving distance from the patient’s zip code to the clinic), history of skin cancer, dermatologic diagnoses, and management strategies. These variables were not collected for patients who cancelled or noshowed their first in-person appointments. All patient charts and notes corresponding to the date and visit of interest were accessed through the electronic medical record (EMR). Patient data were de-identified and stored in a password-protected spreadsheet. Comparisons between the BFC and UPMC patient populations were performed using X2 tests of independence, Fisher exact tests, and Mann-Whitney U tests via SPSS software (IBM). Statistical significance was set at P<.05.
Results
Patient Characteristics—Our analysis included 76 initial appointments at the BFC and 322 at UPMC (Table 1). The mean age for patients at the BFC and UPMC was 39.6 years and 47.8 years, respectively (P=.001). Males accounted for 39 (51.3%) and 112 (34.8%) of BFC and UPMC patients, respectively (P=.008); 2 (0.6%) patients from UPMC were transgender. Of the BFC and UPMC patients, 44.7% (34/76) and 0.9% (3/322) were Hispanic, respectively (P<.001). With regard to race, 52.6% (40/76) of BFC patients were White, 19.7% (15/76) were Black, 6.6% (5/76) were Asian/Pacific Islander (Chinese, 1.3% [1/76]; other Asian, 5.3% [4/76]), and 21.1% (16/76) were American Indian/other/unspecified (American Indian, 1.3% [1/76]; other, 13.2% [10/76]; unspecified, 6.6% [5/76]). At UPMC, 61.2% (197/322) of patients were White, 28.0% (90/322) were Black, 5.3% (17/322) were Asian/Pacific Islander (Chinese, 1.2% [4/322]; Indian [Asian], 1.9% [6/322]; Japanese, 0.3% [1/322]; other Asian, 1.6% [5/322]; other Asian/American Indian, 0.3% [1/322]), and 5.6% (18/322) were American Indian/other/ unspecified (American Indian, 0.3% [1/322]; other, 0.3% [1/322]; unspecified, 5.0% [16/322]). Overall, the BFC patient population was more ethnically and racially diverse than that of UPMC (P<.001).
Forty-six percent (35/76) of BFC patients and 4.3% (14/322) of UPMC patients had LEP (P<.001). Primary languages among BFC patients were 53.9% (41/76) English, 40.8% (31/76) Spanish, and 5.2% (4/76) other/ unspecified (Chinese, 1.3% [1/76]; Indonesian, 2.6% [2/76]; unspecified, 1.3% [1/76]). Primary languages among UPMC patients were 95.7% (308/322) English and 4.3% (14/322) other/unspecified (Chinese, 0.6% [2/322]; Nepali, 0.6% [2/322]; Pali, 0.3% [1/322]; Russian, 0.3% [1/322]; unspecified, 2.5% [8/322]). There were notable differences in insurance status at the BFC vs UPMC (P<.001), with more UPMC patients having private insurance (52.8% [170/322] vs 11.8% [9/76]) and more BFC patients being uninsured (52.8% [51/76] vs 1.9% [6/322]). There was no significant difference in distance to clinic between the 2 groups (P=.183). More UPMC patients had a history of skin cancer (P=.003). More patients at the BFC were no-shows for their appointments (P<.001), and UPMC patients more frequently canceled their appointments (P<.001).
Dermatologic Diagnoses—The most commonly diagnosed dermatologic conditions at the BFC were dermatitis (23.7% [18/76]), neoplasm of uncertain behavior (15.8% [12/76]), alopecia (11.8% [9/76]), and acne (10.5% [8/76]) (Table 2). The most commonly diagnosed conditions at UPMC were nevi (26.4% [85/322]), dermatitis (22.7% [73/322]), seborrheic keratosis (21.7% [70/322]), and skin cancer screening (21.4% [70/322]). Neoplasm of uncertain behavior was more common in BFC vs UPMC patients (P=.040), while UPMC patients were more frequently diagnosed with nevi (P<.001), seborrheic keratosis (P<.001), and skin cancer screening (P<.001). There was no significant difference between the incidence of skin cancer diagnoses in the BFC (1.3% [1/76]) and UPMC (0.6% [2/76]) patient populations (P=.471). Among the biopsied neoplasms, there was also no significant difference in malignant (BFC, 50.0% [5/10]; UPMC, 32.0% [8/25]) and benign (BFC, 50.0% [5/10]; UPMC, 36.0% [9/25]) neoplasms diagnosed at each clinic (P=.444).
Management Strategies—Systemic antibiotics were more frequently prescribed (P<.001) and laboratory testing/ imaging were more frequently ordered (P=.005) at the BFC vs UPMC (Table 3). Patients at the BFC also more frequently required emergency insurance (P=.036). Patients at UPMC were more frequently recommended sunscreen (P=.003) and received education about skin cancer signs by review of the ABCDEs of melanoma (P<.001), sun-protective behaviors (P=.001), and skin examination frequency (P<.001). Notes in the EMR for UPMC patients more frequently specified patient followup instructions (P<.001).
Comment
As of 2020, the city of Pittsburgh had an estimated population of nearly 303,000 based on US Census data.10 Its population is predominantly White (62.7%) followed by Black/African American (22.8%) and Asian (6.5%); 5.9% identify as 2 or more races. Approximately 3.8% identify as Hispanic or Latino. More than 11% of the Pittsburgh population aged 5 years and older speaks a language other than English as their primary language, including Spanish (2.3%), other Indo-European languages (3.9%), and Asian and Pacific Island languages (3.5%).11 More than 5% of the Pittsburgh population does not have health insurance.12
The BFC is located in Pittsburgh’s South Side area, while one of UPMC’s primary dermatology clinics is located in the Oakland district; however, most patients who seek care at these clinics live outside these areas. Our study results indicated that the BFC and UPMC serve distinct groups of people within the Pittsburgh population. The BFC patient population was younger with a higher percentage of patients who were male, Hispanic, racially diverse, and with LEP compared with the UPMC patient population. In this clinical setting, the BFC health care team engages with people from diverse backgrounds and requires greater interpreter and medical support services.
The BFC largely is supported by volunteers, UPMC, grants, and philanthropy. Dermatology clinics are staffed by paid and volunteer team members. Paid team members include 1 nurse and 1 access lead who operates the front desk and registration. Volunteer team members include 1 board-certified dermatologist from UPMC (A.J.J.), or an affiliate clinic and 1 or 2 of each of the following: UPMC dermatology residents, medical or undergraduate students from the University of Pittsburgh, AmeriCorps national service members, and student or community medical interpreters. The onsite pharmacy is run by volunteer faculty, resident, and student pharmacists from the University of Pittsburgh. Dermatology clinics are half-day clinics that occur monthly. Volunteers for each clinic are recruited approximately 1 month in advance.
Dermatology patients at the BFC are referred from the BFC general medicine clinic and nearby Federally Qualified Health Center s for simple to complex medical and surgical dermatologic skin conditions. Each BFC dermatology clinic schedules an average of 7 patients per clinic and places other patients on a wait-list unless more urgent triage is needed. Patients are notified when they are scheduled via phone or text message, and they receive a reminder call or text 1 or 2 days prior to their appointment that also asks them to confirm attendance. Patients with LEP are called with an interpreter and also may receive text reminders that can be translated using Google Translate. Patients are instructed to notify the BFC if they need to cancel or reschedule their appointment. At the end of each visit, patients are given an after-visit summary that lists follow-up instructions, medications prescribed during the visit, and upcoming appointments. The BFC offers bus tickets to help patients get to their appointments. In rare cases, the BFC may pay for a car service to drive patients to and from the clinic.
Dermatology clinics at UPMC use scheduling and self-scheduling systems through which patients can make appointments at a location of their choice with any available board-certified dermatologist or physician assistant. Patients receive a reminder phone call 3 days prior to their appointment instructing them to call the office if they are unable to keep their appointment. Patients signed up for the online portal also receive a reminder message and an option to confirm or cancel their appointment. Patients with cell phone numbers in the UPMC system receive a text message approximately 2 days prior to their appointment that allows them to preregister and pay their copayment in advance. They receive another text 20 minutes prior to their appointment with an option for contactless check-in. At the conclusion of their visit, patients can schedule a follow-up appointment and receive a printed copy of their after-visit summary that provides information about follow-up instructions, prescribed medications, and upcoming visits. They may alternatively access this summary via the online patient portal. Patients are not provided transportation to UPMC clinics, but they are offered parking validation.
Among the most common dermatologic diagnoses for each group, BFC patients presented for treatment of more acute dermatologic conditions, while UPMC patients presented for more benign and preventive-care conditions. This difference may be attributable to the BFC’s referral and triage system, wherein patients with more urgent problems are given scheduling priority. This patient care model contrasts with UPMC’s scheduling process in which no known formal triage system is utilized. Interestingly, there was no difference in skin cancer incidence despite a higher percentage of preventive skin cancer screenings at UPMC.
Patients at the BFC more often required emergency insurance for surgical interventions, which is consistent with the higher percentage of uninsured individuals in this population. Patients at UPMC more frequently were recommended sunscreen and were educated about skin cancer, sun protection, and skin examination, in part due to this group’s more extensive history of skin cancer and frequent presentation for skin cancer screenings. At the same time, educational materials for skin care at both the BFC and UPMC are populated into the EMR in English, whereas materials in other languages are less readily available.
Our retrospective study had several limitations. Demographic information that relied on clinic-dependent intake questionnaires may be limited due to variable intake processes and patients opting out of self-reporting. By comparing patient populations between 2 clinics, confounding variables such as location and hours of operation may impact the patient demographics recorded at the BFC vs UPMC. Resources and staff availability may affect the management strategies and follow-up care offered by each clinic. Our study period also was unique in that COVID-19 may have affected resources, staffing, scheduling, and logistics at both clinics.
Based on the aforementioned differences between the BFC and UPMC patient characteristics, care models should be strategically designed to support the needs of diverse populations. The BFC patient care model appropriately focuses on communication skills with patients with LEP by using interpreter services. Providing more skin care education and follow-up instructions in patients’ primary languages will help them develop a better understanding of their skin conditions. Another key asset of the BFC patient care model is its provision of social services such as transportation and insurance assistance.
To improve the UPMC patient care model, providing patients with bus tickets and car services may potentially reduce appointment cancellations. Using interpreter services to call and text appointment reminders, as well as interpreter resources to facilitate patient visits and patient instructions, also can mitigate language barriers for patients with LEP. Implementing a triage system into the UPMC scheduling system may help patients with more urgent skin conditions to be seen in a timely manner.
Other investigators have analyzed costs of care and proven the value of dermatologic services at free clinics to guide allocation of supplies and resources, demonstrating an area for future investigation at the BFC.13 A cost analysis of care provided at the BFC compared to UPMC could inform us about the value of the BFC’s services.
Conclusion
The dermatology clinics at the BFC and UPMC have distinct demographics, diagnoses, and management strategies to provide an inclusive patient care model. The services provided by both clinics are necessary to ensure that people in Pittsburgh have access to dermatologic care regardless of social barriers (eg, lack of health insurance, LEP). To achieve greater accessibility and health equity, dermatologic care at the BFC and UPMC can be improved by strengthening communication with people with LEP, providing skin care education, and offering social and scheduling services.
- Lim HW, Collins SAB, Resneck JS, et al. The burden of skin disease in the United States. J Am Acad Dermatol. 2017;76:958-972.e2. doi:10.1016/j.jaad.2016.12.043
- American Academy of Dermatology. Skin cancer. Accessed October 7, 2024. https://www.aad.org/media/stats-skin-cancer
- Suneja T, Smith ED, Chen GJ, et al. Waiting times to see a dermatologist are perceived as too long by dermatologists: implications for the dermatology workforce. Arch Dermatol. 2001;137:1303-1307. doi:10.1001/archderm.137.10.1303
- Grossberg AL, Carranza D, Lamp K, et al. Dermatologic care in the homeless and underserved populations: observations from the Venice Family Clinic. Cutis. 2012;89:25-32.
- Amini A, Rusthoven CG, Waxweiler TV, et al. Association of health insurance with outcomes in adults ages 18 to 64 years with melanoma in the United States. J Am Acad Dermatol. 2016;74:309-316. doi:10.1016/j.jaad.2015.09.054
- Su C, Nguyen KA, Bai HX, et al. Racial disparity in mycosis fungoides: an analysis of 4495 cases from the US National Cancer Database. J Am Acad Dermatol. 2017;77:497-502.e2. doi:10.1016/j.jaad .2017.04.1137
- Darnell JS. Free clinics in the United States: a nationwide survey. Arch Intern Med. 2010;170:946-953. doi:10.1001/archinternmed .2010.107
- Madray V, Ginjupalli S, Hashmi O, et al. Access to dermatology services at free medical clinics: a nationwide cross-sectional survey. J Am Acad Dermatol. 2019;81:245-246. doi:10.1016/j.jaad.2018.12.011
- Pennsylvania free and income-based clinics. Accessed October 7, 2024. https://www.freeclinics.com/sta/pennsylvania
- United States Census Bureau. Decennial census. P1: race. Accessed October 7, 2024. https://data.census.gov/table/DECENNIALPL2020.P1?g=160XX00US4261000
- United States Census Bureau. American community survey. S1601: language spoken at home. Accessed October 7, 2024. https://data.census.gov/table/ACSST5Y2020S1601?g=160XX00US4261000
- United States Census Bureau. S2701: selected characteristics of health insurance coverage in the United States. Accessed October 7, 2024. https://data.census.gov/table/ACSST5Y2020.S2701?g=160XX00US4261000
- Lin CP, Loy S, Boothe WD, et al. Value of Dermatology Nights at a student-run free clinic. Proc (Bayl Univ Med Cent). 2020;34:260-261. doi:10.1080/08998280.2020.1834771
Approximately 25% of Americans have at least one skin condition, and 20% are estimated to develop skin cancer during their lifetime.1,2 However, 40% of the US population lives in areas underserved by dermatologists. 3 The severity and mortality of skin cancers such as melanoma and mycosis fungoides have been positively associated with minoritized race, lack of health insurance, and unstable housing status.4-6 Patients who receive health care at free clinics often are of a racial or ethnic minoritized social group, are uninsured, and/or lack stable housing; this underserved group also includes recent immigrants to the United States who have limited English proficiency (LEP).7 Only 25% of free clinics offer specialty care services such as dermatology.7,8
Of the 42 free clinics and Federally Qualified Health Centers in Pittsburgh, Pennsylvania, the Birmingham Free Clinic (BFC) is one of the few that offers specialty care services including dermatology.9 Founded in 1994, the BFC serves as a safety net for Pittsburgh’s medically underserved population, offering primary and acute care, medication access, and social services. From January 2020 to May 2022, the BFC offered 27 dermatology clinics that provided approximately 100 people with comprehensive care including full-body skin examinations, dermatologic diagnoses and treatments, minor procedures, and dermatopathology services.
In this study, we compared the BFC dermatology patient care model with that of the dermatology department at the University of Pittsburgh Medical Center (UPMC), an insurance-based tertiary referral health care system in western Pennsylvania. By analyzing the demographics, dermatologic diagnoses, and management strategies of both the BFC and UPMC, we gained an understanding of how these patient care models differ and how they can be improved to care for diverse patient populations.
Methods
A retrospective chart review of dermatology patients seen in person at the BFC and UPMC during the period from January 2020 to May 2022 was performed. The UPMC group included patients seen by 3 general dermatologists (including A.J.J.) at matched time points. Data were collected from patients’ first in-person visit during the study period. Variables of interest included patient age, sex, race, ethnicity, primary language, zip code, health insurance status, distance to clinic (estimated using Google Maps to calculate the shortest driving distance from the patient’s zip code to the clinic), history of skin cancer, dermatologic diagnoses, and management strategies. These variables were not collected for patients who cancelled or noshowed their first in-person appointments. All patient charts and notes corresponding to the date and visit of interest were accessed through the electronic medical record (EMR). Patient data were de-identified and stored in a password-protected spreadsheet. Comparisons between the BFC and UPMC patient populations were performed using X2 tests of independence, Fisher exact tests, and Mann-Whitney U tests via SPSS software (IBM). Statistical significance was set at P<.05.
Results
Patient Characteristics—Our analysis included 76 initial appointments at the BFC and 322 at UPMC (Table 1). The mean age for patients at the BFC and UPMC was 39.6 years and 47.8 years, respectively (P=.001). Males accounted for 39 (51.3%) and 112 (34.8%) of BFC and UPMC patients, respectively (P=.008); 2 (0.6%) patients from UPMC were transgender. Of the BFC and UPMC patients, 44.7% (34/76) and 0.9% (3/322) were Hispanic, respectively (P<.001). With regard to race, 52.6% (40/76) of BFC patients were White, 19.7% (15/76) were Black, 6.6% (5/76) were Asian/Pacific Islander (Chinese, 1.3% [1/76]; other Asian, 5.3% [4/76]), and 21.1% (16/76) were American Indian/other/unspecified (American Indian, 1.3% [1/76]; other, 13.2% [10/76]; unspecified, 6.6% [5/76]). At UPMC, 61.2% (197/322) of patients were White, 28.0% (90/322) were Black, 5.3% (17/322) were Asian/Pacific Islander (Chinese, 1.2% [4/322]; Indian [Asian], 1.9% [6/322]; Japanese, 0.3% [1/322]; other Asian, 1.6% [5/322]; other Asian/American Indian, 0.3% [1/322]), and 5.6% (18/322) were American Indian/other/ unspecified (American Indian, 0.3% [1/322]; other, 0.3% [1/322]; unspecified, 5.0% [16/322]). Overall, the BFC patient population was more ethnically and racially diverse than that of UPMC (P<.001).
Forty-six percent (35/76) of BFC patients and 4.3% (14/322) of UPMC patients had LEP (P<.001). Primary languages among BFC patients were 53.9% (41/76) English, 40.8% (31/76) Spanish, and 5.2% (4/76) other/ unspecified (Chinese, 1.3% [1/76]; Indonesian, 2.6% [2/76]; unspecified, 1.3% [1/76]). Primary languages among UPMC patients were 95.7% (308/322) English and 4.3% (14/322) other/unspecified (Chinese, 0.6% [2/322]; Nepali, 0.6% [2/322]; Pali, 0.3% [1/322]; Russian, 0.3% [1/322]; unspecified, 2.5% [8/322]). There were notable differences in insurance status at the BFC vs UPMC (P<.001), with more UPMC patients having private insurance (52.8% [170/322] vs 11.8% [9/76]) and more BFC patients being uninsured (52.8% [51/76] vs 1.9% [6/322]). There was no significant difference in distance to clinic between the 2 groups (P=.183). More UPMC patients had a history of skin cancer (P=.003). More patients at the BFC were no-shows for their appointments (P<.001), and UPMC patients more frequently canceled their appointments (P<.001).
Dermatologic Diagnoses—The most commonly diagnosed dermatologic conditions at the BFC were dermatitis (23.7% [18/76]), neoplasm of uncertain behavior (15.8% [12/76]), alopecia (11.8% [9/76]), and acne (10.5% [8/76]) (Table 2). The most commonly diagnosed conditions at UPMC were nevi (26.4% [85/322]), dermatitis (22.7% [73/322]), seborrheic keratosis (21.7% [70/322]), and skin cancer screening (21.4% [70/322]). Neoplasm of uncertain behavior was more common in BFC vs UPMC patients (P=.040), while UPMC patients were more frequently diagnosed with nevi (P<.001), seborrheic keratosis (P<.001), and skin cancer screening (P<.001). There was no significant difference between the incidence of skin cancer diagnoses in the BFC (1.3% [1/76]) and UPMC (0.6% [2/76]) patient populations (P=.471). Among the biopsied neoplasms, there was also no significant difference in malignant (BFC, 50.0% [5/10]; UPMC, 32.0% [8/25]) and benign (BFC, 50.0% [5/10]; UPMC, 36.0% [9/25]) neoplasms diagnosed at each clinic (P=.444).
Management Strategies—Systemic antibiotics were more frequently prescribed (P<.001) and laboratory testing/ imaging were more frequently ordered (P=.005) at the BFC vs UPMC (Table 3). Patients at the BFC also more frequently required emergency insurance (P=.036). Patients at UPMC were more frequently recommended sunscreen (P=.003) and received education about skin cancer signs by review of the ABCDEs of melanoma (P<.001), sun-protective behaviors (P=.001), and skin examination frequency (P<.001). Notes in the EMR for UPMC patients more frequently specified patient followup instructions (P<.001).
Comment
As of 2020, the city of Pittsburgh had an estimated population of nearly 303,000 based on US Census data.10 Its population is predominantly White (62.7%) followed by Black/African American (22.8%) and Asian (6.5%); 5.9% identify as 2 or more races. Approximately 3.8% identify as Hispanic or Latino. More than 11% of the Pittsburgh population aged 5 years and older speaks a language other than English as their primary language, including Spanish (2.3%), other Indo-European languages (3.9%), and Asian and Pacific Island languages (3.5%).11 More than 5% of the Pittsburgh population does not have health insurance.12
The BFC is located in Pittsburgh’s South Side area, while one of UPMC’s primary dermatology clinics is located in the Oakland district; however, most patients who seek care at these clinics live outside these areas. Our study results indicated that the BFC and UPMC serve distinct groups of people within the Pittsburgh population. The BFC patient population was younger with a higher percentage of patients who were male, Hispanic, racially diverse, and with LEP compared with the UPMC patient population. In this clinical setting, the BFC health care team engages with people from diverse backgrounds and requires greater interpreter and medical support services.
The BFC largely is supported by volunteers, UPMC, grants, and philanthropy. Dermatology clinics are staffed by paid and volunteer team members. Paid team members include 1 nurse and 1 access lead who operates the front desk and registration. Volunteer team members include 1 board-certified dermatologist from UPMC (A.J.J.), or an affiliate clinic and 1 or 2 of each of the following: UPMC dermatology residents, medical or undergraduate students from the University of Pittsburgh, AmeriCorps national service members, and student or community medical interpreters. The onsite pharmacy is run by volunteer faculty, resident, and student pharmacists from the University of Pittsburgh. Dermatology clinics are half-day clinics that occur monthly. Volunteers for each clinic are recruited approximately 1 month in advance.
Dermatology patients at the BFC are referred from the BFC general medicine clinic and nearby Federally Qualified Health Center s for simple to complex medical and surgical dermatologic skin conditions. Each BFC dermatology clinic schedules an average of 7 patients per clinic and places other patients on a wait-list unless more urgent triage is needed. Patients are notified when they are scheduled via phone or text message, and they receive a reminder call or text 1 or 2 days prior to their appointment that also asks them to confirm attendance. Patients with LEP are called with an interpreter and also may receive text reminders that can be translated using Google Translate. Patients are instructed to notify the BFC if they need to cancel or reschedule their appointment. At the end of each visit, patients are given an after-visit summary that lists follow-up instructions, medications prescribed during the visit, and upcoming appointments. The BFC offers bus tickets to help patients get to their appointments. In rare cases, the BFC may pay for a car service to drive patients to and from the clinic.
Dermatology clinics at UPMC use scheduling and self-scheduling systems through which patients can make appointments at a location of their choice with any available board-certified dermatologist or physician assistant. Patients receive a reminder phone call 3 days prior to their appointment instructing them to call the office if they are unable to keep their appointment. Patients signed up for the online portal also receive a reminder message and an option to confirm or cancel their appointment. Patients with cell phone numbers in the UPMC system receive a text message approximately 2 days prior to their appointment that allows them to preregister and pay their copayment in advance. They receive another text 20 minutes prior to their appointment with an option for contactless check-in. At the conclusion of their visit, patients can schedule a follow-up appointment and receive a printed copy of their after-visit summary that provides information about follow-up instructions, prescribed medications, and upcoming visits. They may alternatively access this summary via the online patient portal. Patients are not provided transportation to UPMC clinics, but they are offered parking validation.
Among the most common dermatologic diagnoses for each group, BFC patients presented for treatment of more acute dermatologic conditions, while UPMC patients presented for more benign and preventive-care conditions. This difference may be attributable to the BFC’s referral and triage system, wherein patients with more urgent problems are given scheduling priority. This patient care model contrasts with UPMC’s scheduling process in which no known formal triage system is utilized. Interestingly, there was no difference in skin cancer incidence despite a higher percentage of preventive skin cancer screenings at UPMC.
Patients at the BFC more often required emergency insurance for surgical interventions, which is consistent with the higher percentage of uninsured individuals in this population. Patients at UPMC more frequently were recommended sunscreen and were educated about skin cancer, sun protection, and skin examination, in part due to this group’s more extensive history of skin cancer and frequent presentation for skin cancer screenings. At the same time, educational materials for skin care at both the BFC and UPMC are populated into the EMR in English, whereas materials in other languages are less readily available.
Our retrospective study had several limitations. Demographic information that relied on clinic-dependent intake questionnaires may be limited due to variable intake processes and patients opting out of self-reporting. By comparing patient populations between 2 clinics, confounding variables such as location and hours of operation may impact the patient demographics recorded at the BFC vs UPMC. Resources and staff availability may affect the management strategies and follow-up care offered by each clinic. Our study period also was unique in that COVID-19 may have affected resources, staffing, scheduling, and logistics at both clinics.
Based on the aforementioned differences between the BFC and UPMC patient characteristics, care models should be strategically designed to support the needs of diverse populations. The BFC patient care model appropriately focuses on communication skills with patients with LEP by using interpreter services. Providing more skin care education and follow-up instructions in patients’ primary languages will help them develop a better understanding of their skin conditions. Another key asset of the BFC patient care model is its provision of social services such as transportation and insurance assistance.
To improve the UPMC patient care model, providing patients with bus tickets and car services may potentially reduce appointment cancellations. Using interpreter services to call and text appointment reminders, as well as interpreter resources to facilitate patient visits and patient instructions, also can mitigate language barriers for patients with LEP. Implementing a triage system into the UPMC scheduling system may help patients with more urgent skin conditions to be seen in a timely manner.
Other investigators have analyzed costs of care and proven the value of dermatologic services at free clinics to guide allocation of supplies and resources, demonstrating an area for future investigation at the BFC.13 A cost analysis of care provided at the BFC compared to UPMC could inform us about the value of the BFC’s services.
Conclusion
The dermatology clinics at the BFC and UPMC have distinct demographics, diagnoses, and management strategies to provide an inclusive patient care model. The services provided by both clinics are necessary to ensure that people in Pittsburgh have access to dermatologic care regardless of social barriers (eg, lack of health insurance, LEP). To achieve greater accessibility and health equity, dermatologic care at the BFC and UPMC can be improved by strengthening communication with people with LEP, providing skin care education, and offering social and scheduling services.
Approximately 25% of Americans have at least one skin condition, and 20% are estimated to develop skin cancer during their lifetime.1,2 However, 40% of the US population lives in areas underserved by dermatologists. 3 The severity and mortality of skin cancers such as melanoma and mycosis fungoides have been positively associated with minoritized race, lack of health insurance, and unstable housing status.4-6 Patients who receive health care at free clinics often are of a racial or ethnic minoritized social group, are uninsured, and/or lack stable housing; this underserved group also includes recent immigrants to the United States who have limited English proficiency (LEP).7 Only 25% of free clinics offer specialty care services such as dermatology.7,8
Of the 42 free clinics and Federally Qualified Health Centers in Pittsburgh, Pennsylvania, the Birmingham Free Clinic (BFC) is one of the few that offers specialty care services including dermatology.9 Founded in 1994, the BFC serves as a safety net for Pittsburgh’s medically underserved population, offering primary and acute care, medication access, and social services. From January 2020 to May 2022, the BFC offered 27 dermatology clinics that provided approximately 100 people with comprehensive care including full-body skin examinations, dermatologic diagnoses and treatments, minor procedures, and dermatopathology services.
In this study, we compared the BFC dermatology patient care model with that of the dermatology department at the University of Pittsburgh Medical Center (UPMC), an insurance-based tertiary referral health care system in western Pennsylvania. By analyzing the demographics, dermatologic diagnoses, and management strategies of both the BFC and UPMC, we gained an understanding of how these patient care models differ and how they can be improved to care for diverse patient populations.
Methods
A retrospective chart review of dermatology patients seen in person at the BFC and UPMC during the period from January 2020 to May 2022 was performed. The UPMC group included patients seen by 3 general dermatologists (including A.J.J.) at matched time points. Data were collected from patients’ first in-person visit during the study period. Variables of interest included patient age, sex, race, ethnicity, primary language, zip code, health insurance status, distance to clinic (estimated using Google Maps to calculate the shortest driving distance from the patient’s zip code to the clinic), history of skin cancer, dermatologic diagnoses, and management strategies. These variables were not collected for patients who cancelled or noshowed their first in-person appointments. All patient charts and notes corresponding to the date and visit of interest were accessed through the electronic medical record (EMR). Patient data were de-identified and stored in a password-protected spreadsheet. Comparisons between the BFC and UPMC patient populations were performed using X2 tests of independence, Fisher exact tests, and Mann-Whitney U tests via SPSS software (IBM). Statistical significance was set at P<.05.
Results
Patient Characteristics—Our analysis included 76 initial appointments at the BFC and 322 at UPMC (Table 1). The mean age for patients at the BFC and UPMC was 39.6 years and 47.8 years, respectively (P=.001). Males accounted for 39 (51.3%) and 112 (34.8%) of BFC and UPMC patients, respectively (P=.008); 2 (0.6%) patients from UPMC were transgender. Of the BFC and UPMC patients, 44.7% (34/76) and 0.9% (3/322) were Hispanic, respectively (P<.001). With regard to race, 52.6% (40/76) of BFC patients were White, 19.7% (15/76) were Black, 6.6% (5/76) were Asian/Pacific Islander (Chinese, 1.3% [1/76]; other Asian, 5.3% [4/76]), and 21.1% (16/76) were American Indian/other/unspecified (American Indian, 1.3% [1/76]; other, 13.2% [10/76]; unspecified, 6.6% [5/76]). At UPMC, 61.2% (197/322) of patients were White, 28.0% (90/322) were Black, 5.3% (17/322) were Asian/Pacific Islander (Chinese, 1.2% [4/322]; Indian [Asian], 1.9% [6/322]; Japanese, 0.3% [1/322]; other Asian, 1.6% [5/322]; other Asian/American Indian, 0.3% [1/322]), and 5.6% (18/322) were American Indian/other/ unspecified (American Indian, 0.3% [1/322]; other, 0.3% [1/322]; unspecified, 5.0% [16/322]). Overall, the BFC patient population was more ethnically and racially diverse than that of UPMC (P<.001).
Forty-six percent (35/76) of BFC patients and 4.3% (14/322) of UPMC patients had LEP (P<.001). Primary languages among BFC patients were 53.9% (41/76) English, 40.8% (31/76) Spanish, and 5.2% (4/76) other/ unspecified (Chinese, 1.3% [1/76]; Indonesian, 2.6% [2/76]; unspecified, 1.3% [1/76]). Primary languages among UPMC patients were 95.7% (308/322) English and 4.3% (14/322) other/unspecified (Chinese, 0.6% [2/322]; Nepali, 0.6% [2/322]; Pali, 0.3% [1/322]; Russian, 0.3% [1/322]; unspecified, 2.5% [8/322]). There were notable differences in insurance status at the BFC vs UPMC (P<.001), with more UPMC patients having private insurance (52.8% [170/322] vs 11.8% [9/76]) and more BFC patients being uninsured (52.8% [51/76] vs 1.9% [6/322]). There was no significant difference in distance to clinic between the 2 groups (P=.183). More UPMC patients had a history of skin cancer (P=.003). More patients at the BFC were no-shows for their appointments (P<.001), and UPMC patients more frequently canceled their appointments (P<.001).
Dermatologic Diagnoses—The most commonly diagnosed dermatologic conditions at the BFC were dermatitis (23.7% [18/76]), neoplasm of uncertain behavior (15.8% [12/76]), alopecia (11.8% [9/76]), and acne (10.5% [8/76]) (Table 2). The most commonly diagnosed conditions at UPMC were nevi (26.4% [85/322]), dermatitis (22.7% [73/322]), seborrheic keratosis (21.7% [70/322]), and skin cancer screening (21.4% [70/322]). Neoplasm of uncertain behavior was more common in BFC vs UPMC patients (P=.040), while UPMC patients were more frequently diagnosed with nevi (P<.001), seborrheic keratosis (P<.001), and skin cancer screening (P<.001). There was no significant difference between the incidence of skin cancer diagnoses in the BFC (1.3% [1/76]) and UPMC (0.6% [2/76]) patient populations (P=.471). Among the biopsied neoplasms, there was also no significant difference in malignant (BFC, 50.0% [5/10]; UPMC, 32.0% [8/25]) and benign (BFC, 50.0% [5/10]; UPMC, 36.0% [9/25]) neoplasms diagnosed at each clinic (P=.444).
Management Strategies—Systemic antibiotics were more frequently prescribed (P<.001) and laboratory testing/ imaging were more frequently ordered (P=.005) at the BFC vs UPMC (Table 3). Patients at the BFC also more frequently required emergency insurance (P=.036). Patients at UPMC were more frequently recommended sunscreen (P=.003) and received education about skin cancer signs by review of the ABCDEs of melanoma (P<.001), sun-protective behaviors (P=.001), and skin examination frequency (P<.001). Notes in the EMR for UPMC patients more frequently specified patient followup instructions (P<.001).
Comment
As of 2020, the city of Pittsburgh had an estimated population of nearly 303,000 based on US Census data.10 Its population is predominantly White (62.7%) followed by Black/African American (22.8%) and Asian (6.5%); 5.9% identify as 2 or more races. Approximately 3.8% identify as Hispanic or Latino. More than 11% of the Pittsburgh population aged 5 years and older speaks a language other than English as their primary language, including Spanish (2.3%), other Indo-European languages (3.9%), and Asian and Pacific Island languages (3.5%).11 More than 5% of the Pittsburgh population does not have health insurance.12
The BFC is located in Pittsburgh’s South Side area, while one of UPMC’s primary dermatology clinics is located in the Oakland district; however, most patients who seek care at these clinics live outside these areas. Our study results indicated that the BFC and UPMC serve distinct groups of people within the Pittsburgh population. The BFC patient population was younger with a higher percentage of patients who were male, Hispanic, racially diverse, and with LEP compared with the UPMC patient population. In this clinical setting, the BFC health care team engages with people from diverse backgrounds and requires greater interpreter and medical support services.
The BFC largely is supported by volunteers, UPMC, grants, and philanthropy. Dermatology clinics are staffed by paid and volunteer team members. Paid team members include 1 nurse and 1 access lead who operates the front desk and registration. Volunteer team members include 1 board-certified dermatologist from UPMC (A.J.J.), or an affiliate clinic and 1 or 2 of each of the following: UPMC dermatology residents, medical or undergraduate students from the University of Pittsburgh, AmeriCorps national service members, and student or community medical interpreters. The onsite pharmacy is run by volunteer faculty, resident, and student pharmacists from the University of Pittsburgh. Dermatology clinics are half-day clinics that occur monthly. Volunteers for each clinic are recruited approximately 1 month in advance.
Dermatology patients at the BFC are referred from the BFC general medicine clinic and nearby Federally Qualified Health Center s for simple to complex medical and surgical dermatologic skin conditions. Each BFC dermatology clinic schedules an average of 7 patients per clinic and places other patients on a wait-list unless more urgent triage is needed. Patients are notified when they are scheduled via phone or text message, and they receive a reminder call or text 1 or 2 days prior to their appointment that also asks them to confirm attendance. Patients with LEP are called with an interpreter and also may receive text reminders that can be translated using Google Translate. Patients are instructed to notify the BFC if they need to cancel or reschedule their appointment. At the end of each visit, patients are given an after-visit summary that lists follow-up instructions, medications prescribed during the visit, and upcoming appointments. The BFC offers bus tickets to help patients get to their appointments. In rare cases, the BFC may pay for a car service to drive patients to and from the clinic.
Dermatology clinics at UPMC use scheduling and self-scheduling systems through which patients can make appointments at a location of their choice with any available board-certified dermatologist or physician assistant. Patients receive a reminder phone call 3 days prior to their appointment instructing them to call the office if they are unable to keep their appointment. Patients signed up for the online portal also receive a reminder message and an option to confirm or cancel their appointment. Patients with cell phone numbers in the UPMC system receive a text message approximately 2 days prior to their appointment that allows them to preregister and pay their copayment in advance. They receive another text 20 minutes prior to their appointment with an option for contactless check-in. At the conclusion of their visit, patients can schedule a follow-up appointment and receive a printed copy of their after-visit summary that provides information about follow-up instructions, prescribed medications, and upcoming visits. They may alternatively access this summary via the online patient portal. Patients are not provided transportation to UPMC clinics, but they are offered parking validation.
Among the most common dermatologic diagnoses for each group, BFC patients presented for treatment of more acute dermatologic conditions, while UPMC patients presented for more benign and preventive-care conditions. This difference may be attributable to the BFC’s referral and triage system, wherein patients with more urgent problems are given scheduling priority. This patient care model contrasts with UPMC’s scheduling process in which no known formal triage system is utilized. Interestingly, there was no difference in skin cancer incidence despite a higher percentage of preventive skin cancer screenings at UPMC.
Patients at the BFC more often required emergency insurance for surgical interventions, which is consistent with the higher percentage of uninsured individuals in this population. Patients at UPMC more frequently were recommended sunscreen and were educated about skin cancer, sun protection, and skin examination, in part due to this group’s more extensive history of skin cancer and frequent presentation for skin cancer screenings. At the same time, educational materials for skin care at both the BFC and UPMC are populated into the EMR in English, whereas materials in other languages are less readily available.
Our retrospective study had several limitations. Demographic information that relied on clinic-dependent intake questionnaires may be limited due to variable intake processes and patients opting out of self-reporting. By comparing patient populations between 2 clinics, confounding variables such as location and hours of operation may impact the patient demographics recorded at the BFC vs UPMC. Resources and staff availability may affect the management strategies and follow-up care offered by each clinic. Our study period also was unique in that COVID-19 may have affected resources, staffing, scheduling, and logistics at both clinics.
Based on the aforementioned differences between the BFC and UPMC patient characteristics, care models should be strategically designed to support the needs of diverse populations. The BFC patient care model appropriately focuses on communication skills with patients with LEP by using interpreter services. Providing more skin care education and follow-up instructions in patients’ primary languages will help them develop a better understanding of their skin conditions. Another key asset of the BFC patient care model is its provision of social services such as transportation and insurance assistance.
To improve the UPMC patient care model, providing patients with bus tickets and car services may potentially reduce appointment cancellations. Using interpreter services to call and text appointment reminders, as well as interpreter resources to facilitate patient visits and patient instructions, also can mitigate language barriers for patients with LEP. Implementing a triage system into the UPMC scheduling system may help patients with more urgent skin conditions to be seen in a timely manner.
Other investigators have analyzed costs of care and proven the value of dermatologic services at free clinics to guide allocation of supplies and resources, demonstrating an area for future investigation at the BFC.13 A cost analysis of care provided at the BFC compared to UPMC could inform us about the value of the BFC’s services.
Conclusion
The dermatology clinics at the BFC and UPMC have distinct demographics, diagnoses, and management strategies to provide an inclusive patient care model. The services provided by both clinics are necessary to ensure that people in Pittsburgh have access to dermatologic care regardless of social barriers (eg, lack of health insurance, LEP). To achieve greater accessibility and health equity, dermatologic care at the BFC and UPMC can be improved by strengthening communication with people with LEP, providing skin care education, and offering social and scheduling services.
- Lim HW, Collins SAB, Resneck JS, et al. The burden of skin disease in the United States. J Am Acad Dermatol. 2017;76:958-972.e2. doi:10.1016/j.jaad.2016.12.043
- American Academy of Dermatology. Skin cancer. Accessed October 7, 2024. https://www.aad.org/media/stats-skin-cancer
- Suneja T, Smith ED, Chen GJ, et al. Waiting times to see a dermatologist are perceived as too long by dermatologists: implications for the dermatology workforce. Arch Dermatol. 2001;137:1303-1307. doi:10.1001/archderm.137.10.1303
- Grossberg AL, Carranza D, Lamp K, et al. Dermatologic care in the homeless and underserved populations: observations from the Venice Family Clinic. Cutis. 2012;89:25-32.
- Amini A, Rusthoven CG, Waxweiler TV, et al. Association of health insurance with outcomes in adults ages 18 to 64 years with melanoma in the United States. J Am Acad Dermatol. 2016;74:309-316. doi:10.1016/j.jaad.2015.09.054
- Su C, Nguyen KA, Bai HX, et al. Racial disparity in mycosis fungoides: an analysis of 4495 cases from the US National Cancer Database. J Am Acad Dermatol. 2017;77:497-502.e2. doi:10.1016/j.jaad .2017.04.1137
- Darnell JS. Free clinics in the United States: a nationwide survey. Arch Intern Med. 2010;170:946-953. doi:10.1001/archinternmed .2010.107
- Madray V, Ginjupalli S, Hashmi O, et al. Access to dermatology services at free medical clinics: a nationwide cross-sectional survey. J Am Acad Dermatol. 2019;81:245-246. doi:10.1016/j.jaad.2018.12.011
- Pennsylvania free and income-based clinics. Accessed October 7, 2024. https://www.freeclinics.com/sta/pennsylvania
- United States Census Bureau. Decennial census. P1: race. Accessed October 7, 2024. https://data.census.gov/table/DECENNIALPL2020.P1?g=160XX00US4261000
- United States Census Bureau. American community survey. S1601: language spoken at home. Accessed October 7, 2024. https://data.census.gov/table/ACSST5Y2020S1601?g=160XX00US4261000
- United States Census Bureau. S2701: selected characteristics of health insurance coverage in the United States. Accessed October 7, 2024. https://data.census.gov/table/ACSST5Y2020.S2701?g=160XX00US4261000
- Lin CP, Loy S, Boothe WD, et al. Value of Dermatology Nights at a student-run free clinic. Proc (Bayl Univ Med Cent). 2020;34:260-261. doi:10.1080/08998280.2020.1834771
- Lim HW, Collins SAB, Resneck JS, et al. The burden of skin disease in the United States. J Am Acad Dermatol. 2017;76:958-972.e2. doi:10.1016/j.jaad.2016.12.043
- American Academy of Dermatology. Skin cancer. Accessed October 7, 2024. https://www.aad.org/media/stats-skin-cancer
- Suneja T, Smith ED, Chen GJ, et al. Waiting times to see a dermatologist are perceived as too long by dermatologists: implications for the dermatology workforce. Arch Dermatol. 2001;137:1303-1307. doi:10.1001/archderm.137.10.1303
- Grossberg AL, Carranza D, Lamp K, et al. Dermatologic care in the homeless and underserved populations: observations from the Venice Family Clinic. Cutis. 2012;89:25-32.
- Amini A, Rusthoven CG, Waxweiler TV, et al. Association of health insurance with outcomes in adults ages 18 to 64 years with melanoma in the United States. J Am Acad Dermatol. 2016;74:309-316. doi:10.1016/j.jaad.2015.09.054
- Su C, Nguyen KA, Bai HX, et al. Racial disparity in mycosis fungoides: an analysis of 4495 cases from the US National Cancer Database. J Am Acad Dermatol. 2017;77:497-502.e2. doi:10.1016/j.jaad .2017.04.1137
- Darnell JS. Free clinics in the United States: a nationwide survey. Arch Intern Med. 2010;170:946-953. doi:10.1001/archinternmed .2010.107
- Madray V, Ginjupalli S, Hashmi O, et al. Access to dermatology services at free medical clinics: a nationwide cross-sectional survey. J Am Acad Dermatol. 2019;81:245-246. doi:10.1016/j.jaad.2018.12.011
- Pennsylvania free and income-based clinics. Accessed October 7, 2024. https://www.freeclinics.com/sta/pennsylvania
- United States Census Bureau. Decennial census. P1: race. Accessed October 7, 2024. https://data.census.gov/table/DECENNIALPL2020.P1?g=160XX00US4261000
- United States Census Bureau. American community survey. S1601: language spoken at home. Accessed October 7, 2024. https://data.census.gov/table/ACSST5Y2020S1601?g=160XX00US4261000
- United States Census Bureau. S2701: selected characteristics of health insurance coverage in the United States. Accessed October 7, 2024. https://data.census.gov/table/ACSST5Y2020.S2701?g=160XX00US4261000
- Lin CP, Loy S, Boothe WD, et al. Value of Dermatology Nights at a student-run free clinic. Proc (Bayl Univ Med Cent). 2020;34:260-261. doi:10.1080/08998280.2020.1834771
Comparing Patient Care Models at a Local Free Clinic vs an Insurance- Based University Medical Center
Comparing Patient Care Models at a Local Free Clinic vs an Insurance- Based University Medical Center
PRACTICE POINTS
- Both free clinics and insurance-based health care systems serve dermatology patients with diverse characteristics, necessitating inclusive health care models.
- Dermatologic care can be improved at both free and insurance-based clinics by strengthening communication with individuals with limited English proficiency, providing skin care education, and offering social and scheduling services such as transportation, insurance assistance, and triage.
A Special Supplement on Hot Topics in Primary Care 2024

Hot Topics in Primary Care 2024 is a resource that explores the newest developments in primary care topics that impact your daily clinical practice.
Click on the link below to access the entire supplement.

- Case Studies in Continuous Glucose Monitoring
- Detection and Diagnosis of Early Symptomatic
Alzheimer’s Disease in Primary Care - Elevating the Importance of Asthma Care in the United States
- Hypercortisolism is More Common Than You Think –
Here’s How to Find It - Improving COPD Management at Transitions of Care
- Improving Patient-Centric COPD Management
- The Role of Finerenone in Optimizing Cardiovascular-
Kidney-Metabolic Health: Everything PCPs Should Know - What Primary Care Clinicians Need to Know About Once-Weekly Insulins
This supplement offers the opportunity to earn a total of 3 continuing medical education (CME) credits. Credit is awarded for the successful completion of the evaluation after reading the article. The links can be found within the supplement on the first page of each article where CME credits are offered.
Click here to read the 2024 Hot Topics in Primary Care

Hot Topics in Primary Care 2024 is a resource that explores the newest developments in primary care topics that impact your daily clinical practice.
Click on the link below to access the entire supplement.

- Case Studies in Continuous Glucose Monitoring
- Detection and Diagnosis of Early Symptomatic
Alzheimer’s Disease in Primary Care - Elevating the Importance of Asthma Care in the United States
- Hypercortisolism is More Common Than You Think –
Here’s How to Find It - Improving COPD Management at Transitions of Care
- Improving Patient-Centric COPD Management
- The Role of Finerenone in Optimizing Cardiovascular-
Kidney-Metabolic Health: Everything PCPs Should Know - What Primary Care Clinicians Need to Know About Once-Weekly Insulins
This supplement offers the opportunity to earn a total of 3 continuing medical education (CME) credits. Credit is awarded for the successful completion of the evaluation after reading the article. The links can be found within the supplement on the first page of each article where CME credits are offered.
Click here to read the 2024 Hot Topics in Primary Care

Hot Topics in Primary Care 2024 is a resource that explores the newest developments in primary care topics that impact your daily clinical practice.
Click on the link below to access the entire supplement.

- Case Studies in Continuous Glucose Monitoring
- Detection and Diagnosis of Early Symptomatic
Alzheimer’s Disease in Primary Care - Elevating the Importance of Asthma Care in the United States
- Hypercortisolism is More Common Than You Think –
Here’s How to Find It - Improving COPD Management at Transitions of Care
- Improving Patient-Centric COPD Management
- The Role of Finerenone in Optimizing Cardiovascular-
Kidney-Metabolic Health: Everything PCPs Should Know - What Primary Care Clinicians Need to Know About Once-Weekly Insulins
This supplement offers the opportunity to earn a total of 3 continuing medical education (CME) credits. Credit is awarded for the successful completion of the evaluation after reading the article. The links can be found within the supplement on the first page of each article where CME credits are offered.