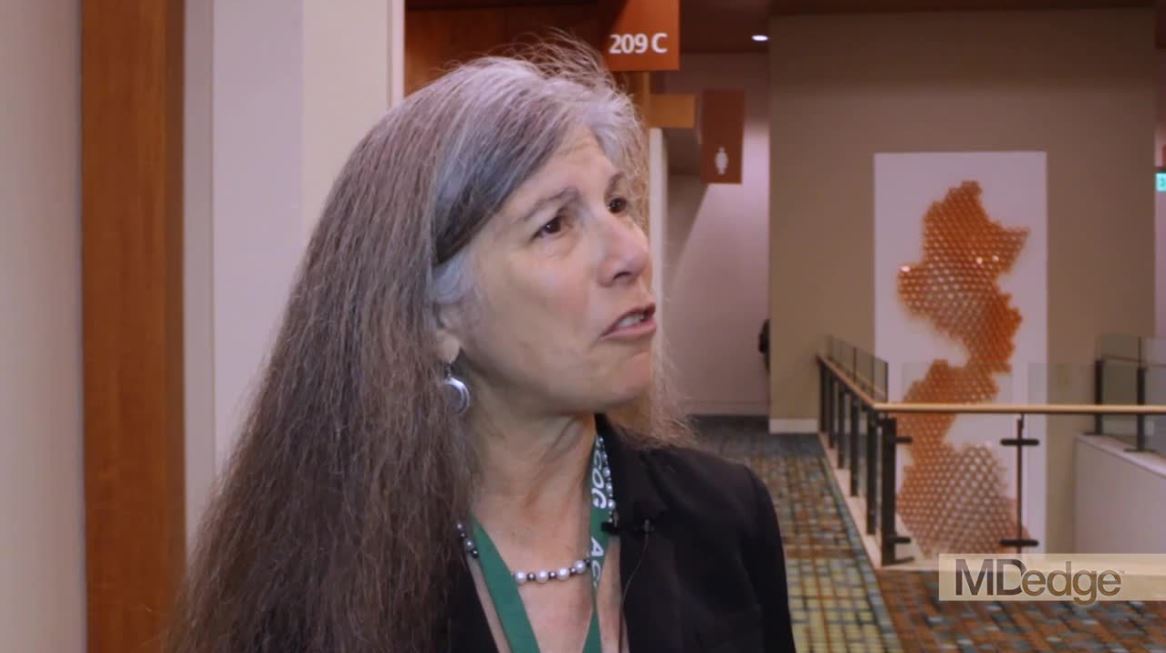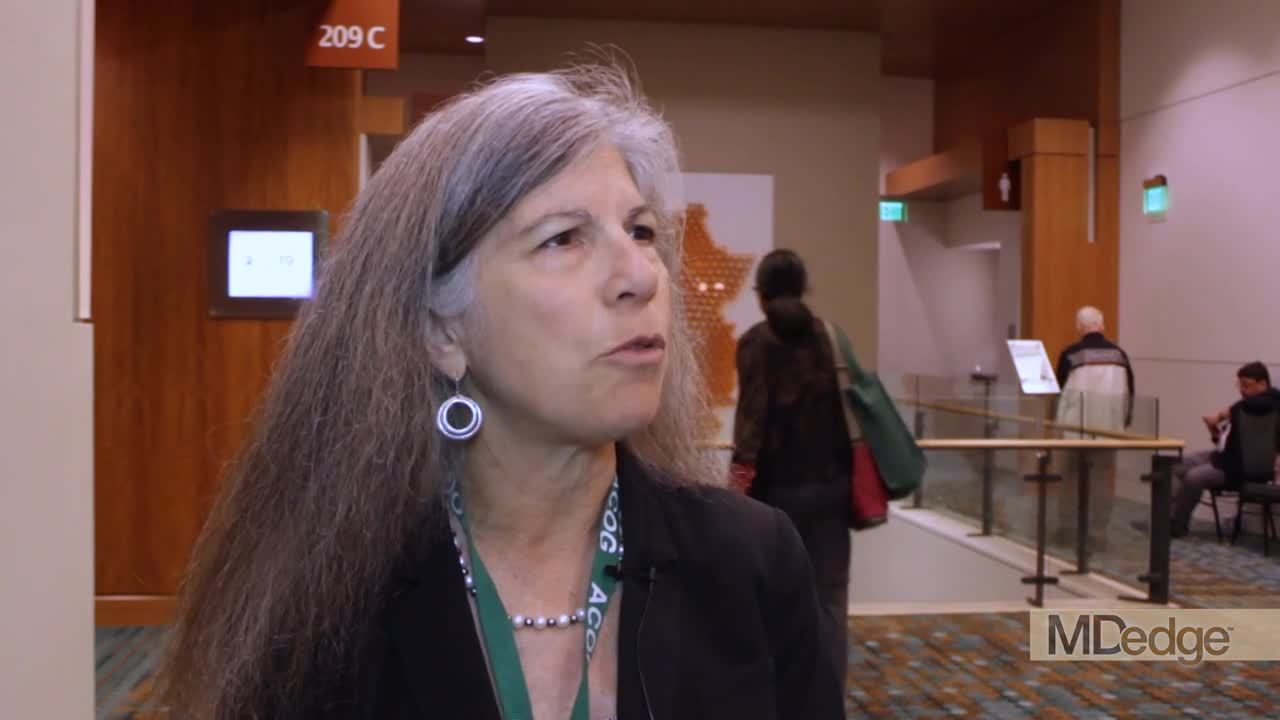User login
Immediate postpartum LARC: ‘Agony and ecstasy’
NASHVILLE, TENN. – according to Eve Espey, MD.
“I think [the rate] is going to settle out at around 15%-20%, but good cost-effectiveness studies show that, even if it were that high, it is still highly cost effective,” she said during an update on contraceptives at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Immediate postpartum long-acting reversible contraception (LARC), including an IUD or implant, may reduce rapid-repeat pregnancy, she added, noting, however, that while Medicaid is covering it in many states, “it turns out that payment models are very cumbersome; they actually don’t work very well.”
At the University of New Mexico (UNM) in Albuquerque, where Dr .Espey is a professor and chair of the department of obstetrics and gynecology and director of the family planning fellowship, immediate postpartum LARC is offered to women with Medicaid coverage, and payment is received in about 97% of cases.
It took about 4 years of persistent effort to make that happen, she said, adding that the UNM Hospital still is the only one in the state offering the service, although efforts are underway to help other hospitals “troubleshoot the issues.”
Another challenge is the lack of private insurance coverage for immediate postpartum LARC, she said.
“I was super enthusiastic about this a few years ago, and I remain super enthusiastic about it, but I think it’s going to take another 5 years or so [for better coverage], and honestly I think what we really need is an inpatient LARC CPT code to make this happen.”
In this video interview, Dr. Espey discusses the “agony and ecstasy” of immediate postpartum LARC, summarizing the main points regarding its benefits and challenges as presented during an “EdTalk” she gave at the meeting.
Dr. Espey reported having no relevant financial disclosures.
NASHVILLE, TENN. – according to Eve Espey, MD.
“I think [the rate] is going to settle out at around 15%-20%, but good cost-effectiveness studies show that, even if it were that high, it is still highly cost effective,” she said during an update on contraceptives at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Immediate postpartum long-acting reversible contraception (LARC), including an IUD or implant, may reduce rapid-repeat pregnancy, she added, noting, however, that while Medicaid is covering it in many states, “it turns out that payment models are very cumbersome; they actually don’t work very well.”
At the University of New Mexico (UNM) in Albuquerque, where Dr .Espey is a professor and chair of the department of obstetrics and gynecology and director of the family planning fellowship, immediate postpartum LARC is offered to women with Medicaid coverage, and payment is received in about 97% of cases.
It took about 4 years of persistent effort to make that happen, she said, adding that the UNM Hospital still is the only one in the state offering the service, although efforts are underway to help other hospitals “troubleshoot the issues.”
Another challenge is the lack of private insurance coverage for immediate postpartum LARC, she said.
“I was super enthusiastic about this a few years ago, and I remain super enthusiastic about it, but I think it’s going to take another 5 years or so [for better coverage], and honestly I think what we really need is an inpatient LARC CPT code to make this happen.”
In this video interview, Dr. Espey discusses the “agony and ecstasy” of immediate postpartum LARC, summarizing the main points regarding its benefits and challenges as presented during an “EdTalk” she gave at the meeting.
Dr. Espey reported having no relevant financial disclosures.
NASHVILLE, TENN. – according to Eve Espey, MD.
“I think [the rate] is going to settle out at around 15%-20%, but good cost-effectiveness studies show that, even if it were that high, it is still highly cost effective,” she said during an update on contraceptives at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Immediate postpartum long-acting reversible contraception (LARC), including an IUD or implant, may reduce rapid-repeat pregnancy, she added, noting, however, that while Medicaid is covering it in many states, “it turns out that payment models are very cumbersome; they actually don’t work very well.”
At the University of New Mexico (UNM) in Albuquerque, where Dr .Espey is a professor and chair of the department of obstetrics and gynecology and director of the family planning fellowship, immediate postpartum LARC is offered to women with Medicaid coverage, and payment is received in about 97% of cases.
It took about 4 years of persistent effort to make that happen, she said, adding that the UNM Hospital still is the only one in the state offering the service, although efforts are underway to help other hospitals “troubleshoot the issues.”
Another challenge is the lack of private insurance coverage for immediate postpartum LARC, she said.
“I was super enthusiastic about this a few years ago, and I remain super enthusiastic about it, but I think it’s going to take another 5 years or so [for better coverage], and honestly I think what we really need is an inpatient LARC CPT code to make this happen.”
In this video interview, Dr. Espey discusses the “agony and ecstasy” of immediate postpartum LARC, summarizing the main points regarding its benefits and challenges as presented during an “EdTalk” she gave at the meeting.
Dr. Espey reported having no relevant financial disclosures.
EXPERT ANALYSIS FROM ACOG 2019
PsA Fast Facts: Symptoms



Sustainable weight loss seen 5 years after endoscopic sleeve gastroplasty

The finding comes from the first long-term analysis of outcomes following endoscopic sleeve gastroplasty, a relatively new, minimally invasive weight-loss procedure that offers patients an alternative to bariatric surgery.
“Endoscopic sleeve gastrectomy is a 1-day outpatient procedure that uses a suturing device attached to an endoscope to create a series of sutures that cinch the stomach like an accordion down to roughly the size of a banana, and leaves no scars,” lead study author Reem Z. Sharaiha, MD, MSc, said during a media briefing in advance of the annual Digestive Disease Week®. “The procedure causes patients to eat less because they feel full faster. This results in weight loss.”
Digestive Disease Week is jointly sponsored by the American Association for the Study of Liver Diseases (AASLD), the American Gastroenterological Association (AGA) Institute, the American Society for Gastrointestinal Endoscopy (ASGE), and the Society for Surgery of the Alimentary Tract (SSAT).
While previous studies have tracked ESG results for 1-2 years, her research team followed 203 patients who underwent the procedure between August 2013 and October 2018. “We felt that a longer-term study was needed to make sure weight loss was sustainable with this method of treatment, because research shows that if you keep weight loss for an extended period of time, you’re more likely to keep it off permanently, which is ultimately what we want for these patients,” said Dr. Sharaiha, who is an attending physician at New York–Presbyterian/Weill Cornell Medicine, New York.
At baseline, the mean age of the 203 patients was 46 years, 67% were female, and their mean body mass index was 39 kg/m2. Dr. Sharaiha and colleagues observed that maximum weight loss was generally achieved by 24 months after the procedure, after which patients tended to regain a small amount of their lost weight. For example, at 1 year, the mean weight loss was 18.1 kg, with a total body weight loss of 15.2% (P less than .0001 for both associations). At 2 years, the mean weight loss was 17.3 kg, with a total body weight loss of 14.5% (P less than .0001 for both associations). At 3 years, the mean weight loss was 20.8 kg, with a total body weight loss of 14.5% (P less than .0001 for both associations). At 5 years, the mean weight loss was 18.7 kg (P = .0003) and the total body weight loss was 14.5% (P = .0002).
Overall, patients gained an average 2.4 kg of weight after achieving their minimum weight after ESG until the end of follow-up. The researchers also found that failure to lose at least 10% of total body weight within the first 3 months after ESG decreased the chance of subsequent significant weight loss by 80%. Fewer than 1% of patients experienced complications, an improvement over surgical procedures.
“Our study showed very sustainable, significant weight loss for our patients between the 1 and 5 year mark,” Dr. Sharaiha said. “Out to 5 years, there was an average 15% total body weight loss. This is significant, because studies have shown that when people lose at least 10% of their body weight, they see improvement in blood pressure, diabetes, and heart outcomes, which are the comorbidities associated with obesity. We hope these findings will help persuade insurance companies that ESG is not experimental, but has value over patients’ lifespans.”
Dr. Sharaiha and colleagues plan to follow the current cohort for the next 10-20 years. “It’s important to show the value of these endoscopic procedures, so we’ll be looking at improvement in comorbidities such as diabetes, high blood pressure, and cholesterol,” she said. “We’re also part of a randomized study that’s currently under way looking at ESG in combination with diet and exercise.”
She reported having no financial disclosures.

The finding comes from the first long-term analysis of outcomes following endoscopic sleeve gastroplasty, a relatively new, minimally invasive weight-loss procedure that offers patients an alternative to bariatric surgery.
“Endoscopic sleeve gastrectomy is a 1-day outpatient procedure that uses a suturing device attached to an endoscope to create a series of sutures that cinch the stomach like an accordion down to roughly the size of a banana, and leaves no scars,” lead study author Reem Z. Sharaiha, MD, MSc, said during a media briefing in advance of the annual Digestive Disease Week®. “The procedure causes patients to eat less because they feel full faster. This results in weight loss.”
Digestive Disease Week is jointly sponsored by the American Association for the Study of Liver Diseases (AASLD), the American Gastroenterological Association (AGA) Institute, the American Society for Gastrointestinal Endoscopy (ASGE), and the Society for Surgery of the Alimentary Tract (SSAT).
While previous studies have tracked ESG results for 1-2 years, her research team followed 203 patients who underwent the procedure between August 2013 and October 2018. “We felt that a longer-term study was needed to make sure weight loss was sustainable with this method of treatment, because research shows that if you keep weight loss for an extended period of time, you’re more likely to keep it off permanently, which is ultimately what we want for these patients,” said Dr. Sharaiha, who is an attending physician at New York–Presbyterian/Weill Cornell Medicine, New York.
At baseline, the mean age of the 203 patients was 46 years, 67% were female, and their mean body mass index was 39 kg/m2. Dr. Sharaiha and colleagues observed that maximum weight loss was generally achieved by 24 months after the procedure, after which patients tended to regain a small amount of their lost weight. For example, at 1 year, the mean weight loss was 18.1 kg, with a total body weight loss of 15.2% (P less than .0001 for both associations). At 2 years, the mean weight loss was 17.3 kg, with a total body weight loss of 14.5% (P less than .0001 for both associations). At 3 years, the mean weight loss was 20.8 kg, with a total body weight loss of 14.5% (P less than .0001 for both associations). At 5 years, the mean weight loss was 18.7 kg (P = .0003) and the total body weight loss was 14.5% (P = .0002).
Overall, patients gained an average 2.4 kg of weight after achieving their minimum weight after ESG until the end of follow-up. The researchers also found that failure to lose at least 10% of total body weight within the first 3 months after ESG decreased the chance of subsequent significant weight loss by 80%. Fewer than 1% of patients experienced complications, an improvement over surgical procedures.
“Our study showed very sustainable, significant weight loss for our patients between the 1 and 5 year mark,” Dr. Sharaiha said. “Out to 5 years, there was an average 15% total body weight loss. This is significant, because studies have shown that when people lose at least 10% of their body weight, they see improvement in blood pressure, diabetes, and heart outcomes, which are the comorbidities associated with obesity. We hope these findings will help persuade insurance companies that ESG is not experimental, but has value over patients’ lifespans.”
Dr. Sharaiha and colleagues plan to follow the current cohort for the next 10-20 years. “It’s important to show the value of these endoscopic procedures, so we’ll be looking at improvement in comorbidities such as diabetes, high blood pressure, and cholesterol,” she said. “We’re also part of a randomized study that’s currently under way looking at ESG in combination with diet and exercise.”
She reported having no financial disclosures.

The finding comes from the first long-term analysis of outcomes following endoscopic sleeve gastroplasty, a relatively new, minimally invasive weight-loss procedure that offers patients an alternative to bariatric surgery.
“Endoscopic sleeve gastrectomy is a 1-day outpatient procedure that uses a suturing device attached to an endoscope to create a series of sutures that cinch the stomach like an accordion down to roughly the size of a banana, and leaves no scars,” lead study author Reem Z. Sharaiha, MD, MSc, said during a media briefing in advance of the annual Digestive Disease Week®. “The procedure causes patients to eat less because they feel full faster. This results in weight loss.”
Digestive Disease Week is jointly sponsored by the American Association for the Study of Liver Diseases (AASLD), the American Gastroenterological Association (AGA) Institute, the American Society for Gastrointestinal Endoscopy (ASGE), and the Society for Surgery of the Alimentary Tract (SSAT).
While previous studies have tracked ESG results for 1-2 years, her research team followed 203 patients who underwent the procedure between August 2013 and October 2018. “We felt that a longer-term study was needed to make sure weight loss was sustainable with this method of treatment, because research shows that if you keep weight loss for an extended period of time, you’re more likely to keep it off permanently, which is ultimately what we want for these patients,” said Dr. Sharaiha, who is an attending physician at New York–Presbyterian/Weill Cornell Medicine, New York.
At baseline, the mean age of the 203 patients was 46 years, 67% were female, and their mean body mass index was 39 kg/m2. Dr. Sharaiha and colleagues observed that maximum weight loss was generally achieved by 24 months after the procedure, after which patients tended to regain a small amount of their lost weight. For example, at 1 year, the mean weight loss was 18.1 kg, with a total body weight loss of 15.2% (P less than .0001 for both associations). At 2 years, the mean weight loss was 17.3 kg, with a total body weight loss of 14.5% (P less than .0001 for both associations). At 3 years, the mean weight loss was 20.8 kg, with a total body weight loss of 14.5% (P less than .0001 for both associations). At 5 years, the mean weight loss was 18.7 kg (P = .0003) and the total body weight loss was 14.5% (P = .0002).
Overall, patients gained an average 2.4 kg of weight after achieving their minimum weight after ESG until the end of follow-up. The researchers also found that failure to lose at least 10% of total body weight within the first 3 months after ESG decreased the chance of subsequent significant weight loss by 80%. Fewer than 1% of patients experienced complications, an improvement over surgical procedures.
“Our study showed very sustainable, significant weight loss for our patients between the 1 and 5 year mark,” Dr. Sharaiha said. “Out to 5 years, there was an average 15% total body weight loss. This is significant, because studies have shown that when people lose at least 10% of their body weight, they see improvement in blood pressure, diabetes, and heart outcomes, which are the comorbidities associated with obesity. We hope these findings will help persuade insurance companies that ESG is not experimental, but has value over patients’ lifespans.”
Dr. Sharaiha and colleagues plan to follow the current cohort for the next 10-20 years. “It’s important to show the value of these endoscopic procedures, so we’ll be looking at improvement in comorbidities such as diabetes, high blood pressure, and cholesterol,” she said. “We’re also part of a randomized study that’s currently under way looking at ESG in combination with diet and exercise.”
She reported having no financial disclosures.
FROM DDW 2019
Key clinical point: Endoscopic sleeve gastroplasty is an effective, minimally invasive weight-loss procedure that results in significant total body weight loss.
Major finding: Between 1 and 5 years after endoscopic sleeve gastroplasty, patients lost 15%-20% of their total body weight.
Study details: A retrospective study of prospectively collected data on 203 patients.
Disclosures: Dr. Sharaiha reported having no financial disclosures.
New risk score predicts cardiac-device infection
SAN FRANCISCO – Researchers have devised a five-item scoring formula to quantify the risk for infection in patients undergoing placement, revision, or removal of a cardiac-rhythm device based on data from nearly 20,000 patients enrolled in a recent infection-prophylaxis trial.
The risk score can help identify patients who might benefit from intensified antibiotic prophylaxis, and it can also help during shared decision making with patients to better understand the risk a patient faces from infection, compared with their predicted device benefit, David H. Birnie, MD, said at the annual scientific sessions of the Heart Rhythm Society.
The new risk score produced a concordance statistic, the area under the receiver-operator characteristic curve, of 0.704. It showed that, although it could use further validation, the score as it currently stands has substantial predictive value, said Dr. Birnie, professor of medicine at the University of Ottawa and deputy chief of cardiology at the University of Ottawa Heart Institute. “It’s certainly better than anything we have now,” he said in a video interview.
Dr. Birnie and his associates used data they collected on baseline characteristics and infection outcomes of the 19,603 patients enrolled in PADIT (Prevention of Arrhythmia Device Infection Trial) who underwent a rhythm-device procedure at 1 of 28 participating Canadian centers. The primary aim of PADIT was to assess the safety and efficacy of an intensified antibiotic-prophylaxis regimen, compared with a standard regimen of a cefazolin infusion just before the procedure. The study’s primary endpoint was the incidence of hospitalization for device infection during 1-year follow-up, and while the intensified prophylactic regimen linked with a 23% relative reduction in the hospitalization rate, compared with standard treatment, the difference was not statistically significant (J Am Coll Cardiol. 2018 Dec 18;72[24]:3098-109).
The researchers analyzed the baseline patient data and the blindly adjudicated infection outcomes and identified five factors that were independently associated with an increased infection rate. They organized the five factors and produced a formula they call the PADIT score (see chart). Those five factors are: prior procedures (the greater the number the greater the risk), age (which unexpectedly had an inverse relationship with infection incidence), depressed renal function, immuno-compromised status, and type of procedure. A patient can potentially score 0-15 points.
Among the PADIT patients a score of 0 correlated with about a 0.3% rate of hospitalization for a device-related infection during 1 year of follow-up, a score of 5 with about a 1.1% rate, a score of 6 with about a 1.8% rate, and a score of seven or more with a 3.4% infection rate over the following year. About 5% of patients had a score of 7 or more, and roughly another 5% had a score of 5 or 6, Dr. Birnie said. At his center, clinicians have begun routinely calculating scores for patients scheduled for an arrhythmia-device procedure, and they are considering routinely administering added antibiotic prophylaxis to patients with a preprocedural score of 6 or higher. They may also use the score to determine whether to use the antibacterial envelope recently reported to prevent cardiac-device infections (N Engl J Med. 2019 May 16;380[20]:1895-905).
“It’s very easy for patients to get to a PADIT score of 7 or higher,” Dr. Birnie noted. As an example, he cited a common patient, an 85-year-old with renal dysfunction who is under consideration for a second replacement of an implantable cardioverter defibrillator. The patient would score 1 point for renal insufficiency, 2 points for the type of device, and 4 points for having a prior history of two devices, and the consequent 3.4% risk for infection might counterbalance the potential benefit this elderly patient could expect from the new device. The score will be very important for targeting treatment, shared decision making, and selection of patients for future intervention trials, he concluded.
“I think this risk score will change practice by giving clinicians a better idea of a patient’s risk for infection,” commented Fred M. Kusumoto, MD, professor of medicine at the Mayo Medical School, Rochester, Minn., and director of heart rhythm services at the Mayo Clinic in Jacksonville, Fla. The PADIT score will help identify patients for whom leaving a device in place is a better option than taking it out because of their infection risk. The risk score could also help improve the cost effectiveness of preventive treatments, such as antibiotic-eluting envelopes, by targeting treatment to higher-risk patients, Dr. Kusumoto said during a press briefing.
SOURCE: Birnie DH. Heart Rhythm 2019, Absract S-LCT02-01.
I like this new infection risk score. It addresses a very clinically relevant issue. It’s important for the electrophysiology community to better understand how to best manage infections related to cardiac rhythm devices and ideally prevent them from happening.
I’m not a big fan of risk scores in general because they can sometimes detract from independent thinking about how to manage a patient. However, it is also helpful to have this type of risk-assessment information when discussing management options with a patient.
The PADIT risk score may also help identify which patients could potentially benefit the most from an antibiotic-eluting envelope when receiving an implanted cardiac-rhythm device. Recently reported results from WRAP-IT showed that routinely using envelopes cut the incidence of major infections by a relative 40%, but in absolute terms, the number needed to treat with the envelop to prevent one major infection was about 200 patients, a big number given the high cost of the envelope (N Engl J Med. 2019 May 16;380[20]:1895-905). It is therefore very interesting to think about using the PADIT risk score to better target an effective but expensive preventive measure like an antibiotic-eluting envelop to patients at the highest risk for infection.
Ulrika Birgersdotter-Green, MD , professor of medicine and director of pacemaker and ICD services at the University of California, San Diego, made these comments as a designated discussant for the report. She has been a consultant to and received honoraria from Abbott, Boston Scientific, and Medtronic.
I like this new infection risk score. It addresses a very clinically relevant issue. It’s important for the electrophysiology community to better understand how to best manage infections related to cardiac rhythm devices and ideally prevent them from happening.
I’m not a big fan of risk scores in general because they can sometimes detract from independent thinking about how to manage a patient. However, it is also helpful to have this type of risk-assessment information when discussing management options with a patient.
The PADIT risk score may also help identify which patients could potentially benefit the most from an antibiotic-eluting envelope when receiving an implanted cardiac-rhythm device. Recently reported results from WRAP-IT showed that routinely using envelopes cut the incidence of major infections by a relative 40%, but in absolute terms, the number needed to treat with the envelop to prevent one major infection was about 200 patients, a big number given the high cost of the envelope (N Engl J Med. 2019 May 16;380[20]:1895-905). It is therefore very interesting to think about using the PADIT risk score to better target an effective but expensive preventive measure like an antibiotic-eluting envelop to patients at the highest risk for infection.
Ulrika Birgersdotter-Green, MD , professor of medicine and director of pacemaker and ICD services at the University of California, San Diego, made these comments as a designated discussant for the report. She has been a consultant to and received honoraria from Abbott, Boston Scientific, and Medtronic.
I like this new infection risk score. It addresses a very clinically relevant issue. It’s important for the electrophysiology community to better understand how to best manage infections related to cardiac rhythm devices and ideally prevent them from happening.
I’m not a big fan of risk scores in general because they can sometimes detract from independent thinking about how to manage a patient. However, it is also helpful to have this type of risk-assessment information when discussing management options with a patient.
The PADIT risk score may also help identify which patients could potentially benefit the most from an antibiotic-eluting envelope when receiving an implanted cardiac-rhythm device. Recently reported results from WRAP-IT showed that routinely using envelopes cut the incidence of major infections by a relative 40%, but in absolute terms, the number needed to treat with the envelop to prevent one major infection was about 200 patients, a big number given the high cost of the envelope (N Engl J Med. 2019 May 16;380[20]:1895-905). It is therefore very interesting to think about using the PADIT risk score to better target an effective but expensive preventive measure like an antibiotic-eluting envelop to patients at the highest risk for infection.
Ulrika Birgersdotter-Green, MD , professor of medicine and director of pacemaker and ICD services at the University of California, San Diego, made these comments as a designated discussant for the report. She has been a consultant to and received honoraria from Abbott, Boston Scientific, and Medtronic.
SAN FRANCISCO – Researchers have devised a five-item scoring formula to quantify the risk for infection in patients undergoing placement, revision, or removal of a cardiac-rhythm device based on data from nearly 20,000 patients enrolled in a recent infection-prophylaxis trial.
The risk score can help identify patients who might benefit from intensified antibiotic prophylaxis, and it can also help during shared decision making with patients to better understand the risk a patient faces from infection, compared with their predicted device benefit, David H. Birnie, MD, said at the annual scientific sessions of the Heart Rhythm Society.
The new risk score produced a concordance statistic, the area under the receiver-operator characteristic curve, of 0.704. It showed that, although it could use further validation, the score as it currently stands has substantial predictive value, said Dr. Birnie, professor of medicine at the University of Ottawa and deputy chief of cardiology at the University of Ottawa Heart Institute. “It’s certainly better than anything we have now,” he said in a video interview.
Dr. Birnie and his associates used data they collected on baseline characteristics and infection outcomes of the 19,603 patients enrolled in PADIT (Prevention of Arrhythmia Device Infection Trial) who underwent a rhythm-device procedure at 1 of 28 participating Canadian centers. The primary aim of PADIT was to assess the safety and efficacy of an intensified antibiotic-prophylaxis regimen, compared with a standard regimen of a cefazolin infusion just before the procedure. The study’s primary endpoint was the incidence of hospitalization for device infection during 1-year follow-up, and while the intensified prophylactic regimen linked with a 23% relative reduction in the hospitalization rate, compared with standard treatment, the difference was not statistically significant (J Am Coll Cardiol. 2018 Dec 18;72[24]:3098-109).
The researchers analyzed the baseline patient data and the blindly adjudicated infection outcomes and identified five factors that were independently associated with an increased infection rate. They organized the five factors and produced a formula they call the PADIT score (see chart). Those five factors are: prior procedures (the greater the number the greater the risk), age (which unexpectedly had an inverse relationship with infection incidence), depressed renal function, immuno-compromised status, and type of procedure. A patient can potentially score 0-15 points.
Among the PADIT patients a score of 0 correlated with about a 0.3% rate of hospitalization for a device-related infection during 1 year of follow-up, a score of 5 with about a 1.1% rate, a score of 6 with about a 1.8% rate, and a score of seven or more with a 3.4% infection rate over the following year. About 5% of patients had a score of 7 or more, and roughly another 5% had a score of 5 or 6, Dr. Birnie said. At his center, clinicians have begun routinely calculating scores for patients scheduled for an arrhythmia-device procedure, and they are considering routinely administering added antibiotic prophylaxis to patients with a preprocedural score of 6 or higher. They may also use the score to determine whether to use the antibacterial envelope recently reported to prevent cardiac-device infections (N Engl J Med. 2019 May 16;380[20]:1895-905).
“It’s very easy for patients to get to a PADIT score of 7 or higher,” Dr. Birnie noted. As an example, he cited a common patient, an 85-year-old with renal dysfunction who is under consideration for a second replacement of an implantable cardioverter defibrillator. The patient would score 1 point for renal insufficiency, 2 points for the type of device, and 4 points for having a prior history of two devices, and the consequent 3.4% risk for infection might counterbalance the potential benefit this elderly patient could expect from the new device. The score will be very important for targeting treatment, shared decision making, and selection of patients for future intervention trials, he concluded.
“I think this risk score will change practice by giving clinicians a better idea of a patient’s risk for infection,” commented Fred M. Kusumoto, MD, professor of medicine at the Mayo Medical School, Rochester, Minn., and director of heart rhythm services at the Mayo Clinic in Jacksonville, Fla. The PADIT score will help identify patients for whom leaving a device in place is a better option than taking it out because of their infection risk. The risk score could also help improve the cost effectiveness of preventive treatments, such as antibiotic-eluting envelopes, by targeting treatment to higher-risk patients, Dr. Kusumoto said during a press briefing.
SOURCE: Birnie DH. Heart Rhythm 2019, Absract S-LCT02-01.
SAN FRANCISCO – Researchers have devised a five-item scoring formula to quantify the risk for infection in patients undergoing placement, revision, or removal of a cardiac-rhythm device based on data from nearly 20,000 patients enrolled in a recent infection-prophylaxis trial.
The risk score can help identify patients who might benefit from intensified antibiotic prophylaxis, and it can also help during shared decision making with patients to better understand the risk a patient faces from infection, compared with their predicted device benefit, David H. Birnie, MD, said at the annual scientific sessions of the Heart Rhythm Society.
The new risk score produced a concordance statistic, the area under the receiver-operator characteristic curve, of 0.704. It showed that, although it could use further validation, the score as it currently stands has substantial predictive value, said Dr. Birnie, professor of medicine at the University of Ottawa and deputy chief of cardiology at the University of Ottawa Heart Institute. “It’s certainly better than anything we have now,” he said in a video interview.
Dr. Birnie and his associates used data they collected on baseline characteristics and infection outcomes of the 19,603 patients enrolled in PADIT (Prevention of Arrhythmia Device Infection Trial) who underwent a rhythm-device procedure at 1 of 28 participating Canadian centers. The primary aim of PADIT was to assess the safety and efficacy of an intensified antibiotic-prophylaxis regimen, compared with a standard regimen of a cefazolin infusion just before the procedure. The study’s primary endpoint was the incidence of hospitalization for device infection during 1-year follow-up, and while the intensified prophylactic regimen linked with a 23% relative reduction in the hospitalization rate, compared with standard treatment, the difference was not statistically significant (J Am Coll Cardiol. 2018 Dec 18;72[24]:3098-109).
The researchers analyzed the baseline patient data and the blindly adjudicated infection outcomes and identified five factors that were independently associated with an increased infection rate. They organized the five factors and produced a formula they call the PADIT score (see chart). Those five factors are: prior procedures (the greater the number the greater the risk), age (which unexpectedly had an inverse relationship with infection incidence), depressed renal function, immuno-compromised status, and type of procedure. A patient can potentially score 0-15 points.
Among the PADIT patients a score of 0 correlated with about a 0.3% rate of hospitalization for a device-related infection during 1 year of follow-up, a score of 5 with about a 1.1% rate, a score of 6 with about a 1.8% rate, and a score of seven or more with a 3.4% infection rate over the following year. About 5% of patients had a score of 7 or more, and roughly another 5% had a score of 5 or 6, Dr. Birnie said. At his center, clinicians have begun routinely calculating scores for patients scheduled for an arrhythmia-device procedure, and they are considering routinely administering added antibiotic prophylaxis to patients with a preprocedural score of 6 or higher. They may also use the score to determine whether to use the antibacterial envelope recently reported to prevent cardiac-device infections (N Engl J Med. 2019 May 16;380[20]:1895-905).
“It’s very easy for patients to get to a PADIT score of 7 or higher,” Dr. Birnie noted. As an example, he cited a common patient, an 85-year-old with renal dysfunction who is under consideration for a second replacement of an implantable cardioverter defibrillator. The patient would score 1 point for renal insufficiency, 2 points for the type of device, and 4 points for having a prior history of two devices, and the consequent 3.4% risk for infection might counterbalance the potential benefit this elderly patient could expect from the new device. The score will be very important for targeting treatment, shared decision making, and selection of patients for future intervention trials, he concluded.
“I think this risk score will change practice by giving clinicians a better idea of a patient’s risk for infection,” commented Fred M. Kusumoto, MD, professor of medicine at the Mayo Medical School, Rochester, Minn., and director of heart rhythm services at the Mayo Clinic in Jacksonville, Fla. The PADIT score will help identify patients for whom leaving a device in place is a better option than taking it out because of their infection risk. The risk score could also help improve the cost effectiveness of preventive treatments, such as antibiotic-eluting envelopes, by targeting treatment to higher-risk patients, Dr. Kusumoto said during a press briefing.
SOURCE: Birnie DH. Heart Rhythm 2019, Absract S-LCT02-01.
REPORTING FROM HEART RHYTHM 2019
Key clinical point: Researchers have devised a five-item scoring formula to predict a patient’s risk for infection from an cardiac rhythm–device procedure.
Major finding: The risk score had an optimism-corrected concordance statistic of 0.704.
Study details: Investigators developed the risk score using data from PADIT, a multicenter, randomized trial with 19,603 patients.
Disclosures: PADIT received no commercial funding. Dr. Birnie had no relevant disclosures.
Source: Birnie DH. Heart Rhythm 2019, Absract S-LCT02-01.
Experimental drug holds promise for the treatment of thyroid eye disease
LOS ANGELES – researchers reported at the annual scientific and clinical congress of the American Association of Clinical Endocrinologists.
“For the first time, there appears to be a medicine that can be given during the active phase of the disease and can actually reverse not just the eyelid swelling and the clinical activity score, but also reduce the eye bulging and double vision and [improve] the [patient’s] quality of life. It could be a watershed moment in the treatment of the disease,” said ophthalmologist Raymond Douglas, MD, PhD, professor of surgery at Cedar-Sinai Medical Center Los Angeles and the study’s coprincipal investigator, in an interview at the meeting.
According to Dr. Douglas, thyroid eye disease, which is also known as Graves’ eye disease, is a severely disabling condition that causes swelling, pain, discomfort, and blindness. “The burden really is quite significant,” he said, with an impact that’s been compared with that of breast cancer in quality-of-life studies.
“Current treatments for thyroid eye disease are rather limited,” he said. “They really encompass just reducing the swelling and the short-term manifestations of the disease. Treatments such as IV steroids and radiation have been shown to not have any effect on long-term manifestations such as eye bulging and double vision.”
Teprotumumab is a fully human monoclonal antibody that targets the insulinlike growth factor I receptor (IGF-IR). It seems to downregulate thyroid eye disease, Dr. Douglas said.
Researchers studied the drug in a randomized, placebo-controlled study: 41 patients were designated to receive eight intravenous infusions of the drug over 21 weeks (10 mg/kg for the first infusion, then 20 mg/kg thereafter). At week 24, 83% of the study group (34 of 41 patients) reached the endpoint of a reduction of eye bulging by at least 2 mm, compared with 10% of patients (4 of 42) in the placebo group, which received infusions of saline solution.
Two millimeters is significant, Dr. Douglas said. “If you noticed someone’s eye was bulging 2 millimeters, you’d say, ‘Hey, I think something is wrong with your eye.’ ”
Initial study results were released in February 2019. New data about secondary endpoints were released at the AACE meeting: Researchers reported that the average reduction in proptosis (eye bulging) was 2.82 mm in the study group, compared with 0.54 mm in the placebo group (P less than .001).
Dr. Douglas said he can “achieve 3 mm of reduction through surgery to drill out the bone between the eye and the brain. [The patients in the study group] were able to achieve almost 3 millimeters of reduction by the drug alone. It’s a rather significant improvement.”
The new data also provided some insight into the timing of clinical improvements. According to Dr. Douglas, “most of the endpoints were met as early as 6 weeks or [after] two infusions of this drug.”
The adverse effects were relatively mild and included muscle spasms, said Dr. Douglas. The side effects seem to be “well tolerated,” he noted, and none led to cessation of therapy.
Participants with potential for motherhood or fatherhood during the trial had to agree to take precautions to avoid becoming pregnant or impregnating a partner.
Dr. Douglas didn’t provide cost information about the drug. However, it seems likely to be expensive. A writer with Seeking Alpha, a stock market analysis site, estimated that the cost could reach “$250,000 or $300,000 per patient per year, which translates to a $3.7 billion to $6 billion market in the United States alone (based on the estimated patient population in the 15,000-20,000 range).”
The drug’s manufacturer, Horizon Therapeutics, expects to apply to the Food and Drug Administration later this year for approval of the drug.
If it is approved, endocrinologists will have an opportunity to partner with eye surgeons to treat thyroid eye disease, Dr. Douglas said. “The crux will be the comanagement with the endocrinologist helping to control the thyroid function and manage some of the side effects of this medication, and the oculoplastic surgeon [working on] diagnosis, appropriate use, and management.”
Horizon Therapeutics funded the study. Dr. Douglas disclosed that he is a consultant with the company.
LOS ANGELES – researchers reported at the annual scientific and clinical congress of the American Association of Clinical Endocrinologists.
“For the first time, there appears to be a medicine that can be given during the active phase of the disease and can actually reverse not just the eyelid swelling and the clinical activity score, but also reduce the eye bulging and double vision and [improve] the [patient’s] quality of life. It could be a watershed moment in the treatment of the disease,” said ophthalmologist Raymond Douglas, MD, PhD, professor of surgery at Cedar-Sinai Medical Center Los Angeles and the study’s coprincipal investigator, in an interview at the meeting.
According to Dr. Douglas, thyroid eye disease, which is also known as Graves’ eye disease, is a severely disabling condition that causes swelling, pain, discomfort, and blindness. “The burden really is quite significant,” he said, with an impact that’s been compared with that of breast cancer in quality-of-life studies.
“Current treatments for thyroid eye disease are rather limited,” he said. “They really encompass just reducing the swelling and the short-term manifestations of the disease. Treatments such as IV steroids and radiation have been shown to not have any effect on long-term manifestations such as eye bulging and double vision.”
Teprotumumab is a fully human monoclonal antibody that targets the insulinlike growth factor I receptor (IGF-IR). It seems to downregulate thyroid eye disease, Dr. Douglas said.
Researchers studied the drug in a randomized, placebo-controlled study: 41 patients were designated to receive eight intravenous infusions of the drug over 21 weeks (10 mg/kg for the first infusion, then 20 mg/kg thereafter). At week 24, 83% of the study group (34 of 41 patients) reached the endpoint of a reduction of eye bulging by at least 2 mm, compared with 10% of patients (4 of 42) in the placebo group, which received infusions of saline solution.
Two millimeters is significant, Dr. Douglas said. “If you noticed someone’s eye was bulging 2 millimeters, you’d say, ‘Hey, I think something is wrong with your eye.’ ”
Initial study results were released in February 2019. New data about secondary endpoints were released at the AACE meeting: Researchers reported that the average reduction in proptosis (eye bulging) was 2.82 mm in the study group, compared with 0.54 mm in the placebo group (P less than .001).
Dr. Douglas said he can “achieve 3 mm of reduction through surgery to drill out the bone between the eye and the brain. [The patients in the study group] were able to achieve almost 3 millimeters of reduction by the drug alone. It’s a rather significant improvement.”
The new data also provided some insight into the timing of clinical improvements. According to Dr. Douglas, “most of the endpoints were met as early as 6 weeks or [after] two infusions of this drug.”
The adverse effects were relatively mild and included muscle spasms, said Dr. Douglas. The side effects seem to be “well tolerated,” he noted, and none led to cessation of therapy.
Participants with potential for motherhood or fatherhood during the trial had to agree to take precautions to avoid becoming pregnant or impregnating a partner.
Dr. Douglas didn’t provide cost information about the drug. However, it seems likely to be expensive. A writer with Seeking Alpha, a stock market analysis site, estimated that the cost could reach “$250,000 or $300,000 per patient per year, which translates to a $3.7 billion to $6 billion market in the United States alone (based on the estimated patient population in the 15,000-20,000 range).”
The drug’s manufacturer, Horizon Therapeutics, expects to apply to the Food and Drug Administration later this year for approval of the drug.
If it is approved, endocrinologists will have an opportunity to partner with eye surgeons to treat thyroid eye disease, Dr. Douglas said. “The crux will be the comanagement with the endocrinologist helping to control the thyroid function and manage some of the side effects of this medication, and the oculoplastic surgeon [working on] diagnosis, appropriate use, and management.”
Horizon Therapeutics funded the study. Dr. Douglas disclosed that he is a consultant with the company.
LOS ANGELES – researchers reported at the annual scientific and clinical congress of the American Association of Clinical Endocrinologists.
“For the first time, there appears to be a medicine that can be given during the active phase of the disease and can actually reverse not just the eyelid swelling and the clinical activity score, but also reduce the eye bulging and double vision and [improve] the [patient’s] quality of life. It could be a watershed moment in the treatment of the disease,” said ophthalmologist Raymond Douglas, MD, PhD, professor of surgery at Cedar-Sinai Medical Center Los Angeles and the study’s coprincipal investigator, in an interview at the meeting.
According to Dr. Douglas, thyroid eye disease, which is also known as Graves’ eye disease, is a severely disabling condition that causes swelling, pain, discomfort, and blindness. “The burden really is quite significant,” he said, with an impact that’s been compared with that of breast cancer in quality-of-life studies.
“Current treatments for thyroid eye disease are rather limited,” he said. “They really encompass just reducing the swelling and the short-term manifestations of the disease. Treatments such as IV steroids and radiation have been shown to not have any effect on long-term manifestations such as eye bulging and double vision.”
Teprotumumab is a fully human monoclonal antibody that targets the insulinlike growth factor I receptor (IGF-IR). It seems to downregulate thyroid eye disease, Dr. Douglas said.
Researchers studied the drug in a randomized, placebo-controlled study: 41 patients were designated to receive eight intravenous infusions of the drug over 21 weeks (10 mg/kg for the first infusion, then 20 mg/kg thereafter). At week 24, 83% of the study group (34 of 41 patients) reached the endpoint of a reduction of eye bulging by at least 2 mm, compared with 10% of patients (4 of 42) in the placebo group, which received infusions of saline solution.
Two millimeters is significant, Dr. Douglas said. “If you noticed someone’s eye was bulging 2 millimeters, you’d say, ‘Hey, I think something is wrong with your eye.’ ”
Initial study results were released in February 2019. New data about secondary endpoints were released at the AACE meeting: Researchers reported that the average reduction in proptosis (eye bulging) was 2.82 mm in the study group, compared with 0.54 mm in the placebo group (P less than .001).
Dr. Douglas said he can “achieve 3 mm of reduction through surgery to drill out the bone between the eye and the brain. [The patients in the study group] were able to achieve almost 3 millimeters of reduction by the drug alone. It’s a rather significant improvement.”
The new data also provided some insight into the timing of clinical improvements. According to Dr. Douglas, “most of the endpoints were met as early as 6 weeks or [after] two infusions of this drug.”
The adverse effects were relatively mild and included muscle spasms, said Dr. Douglas. The side effects seem to be “well tolerated,” he noted, and none led to cessation of therapy.
Participants with potential for motherhood or fatherhood during the trial had to agree to take precautions to avoid becoming pregnant or impregnating a partner.
Dr. Douglas didn’t provide cost information about the drug. However, it seems likely to be expensive. A writer with Seeking Alpha, a stock market analysis site, estimated that the cost could reach “$250,000 or $300,000 per patient per year, which translates to a $3.7 billion to $6 billion market in the United States alone (based on the estimated patient population in the 15,000-20,000 range).”
The drug’s manufacturer, Horizon Therapeutics, expects to apply to the Food and Drug Administration later this year for approval of the drug.
If it is approved, endocrinologists will have an opportunity to partner with eye surgeons to treat thyroid eye disease, Dr. Douglas said. “The crux will be the comanagement with the endocrinologist helping to control the thyroid function and manage some of the side effects of this medication, and the oculoplastic surgeon [working on] diagnosis, appropriate use, and management.”
Horizon Therapeutics funded the study. Dr. Douglas disclosed that he is a consultant with the company.
REPORTING FROM AACE 2019
Renal denervation boosts effectiveness of AFib catheter ablation
SAN FRANCISCO – Adding renal denervation when performing catheter ablation of paroxysmal atrial fibrillation in hypertensive patients substantially reduced their arrhythmia recurrence rate during the subsequent year in a multicenter, randomized trial with 302 patients.
The findings established renal denervation (RDN) as a “reasonable” tool to increase the success of atrial fibrillation (AFib) catheter ablation, Jonathan S. Steinberg, MD, said at the annual scientific sessions of the Heart Rhythm Society.
“The RDN procedure seems remarkably safe and seems to be reliably accomplished when an electrophysiologist does it,” said Dr. Steinberg, director of the Arrhythmia Center of the Summit Medical Group in Montclair, N.J. Given the evidence he reported that performing RDN simultaneously with AFib catheter ablation by pulmonary vein isolation significantly improved freedom from arrhythmia recurrence, this approach “is ready for clinical use at institutions that could mount this kind of program,” he declared.
The rate of freedom from arrhythmia recurrence while off antiarrhythmic drugs during the year following treatment was 57% among 138 patients treated with pulmonary vein isolation only, and 72% in 145 who underwent both pulmonary vein isolation and renal denervation. That’s “a pretty big difference in outcome” with no increased risk and with about 20 added minutes of procedure time, Dr. Steinberg said in a video interview. He acknowledged that, currently, no catheter is approved for U.S. marketing that is specifically designed for renal denervation, but the operators in the study he reported all used conventional radiofrequency ablation catheters with an irrigated tip, a design with U.S. availability.
The ERADICATE-AF (Renal Artery Denervation in Addition to Catheter Ablation to Eliminate Atrial Fibrillation) study randomized 302 patients with paroxysmal AFib and hypertension uncontrolled by medication at three centers in Russia, one in Germany, and one in Poland. Enrolled patients averaged about 60 years of age, about 60% were men, and their average blood pressure was roughly 150/90 mm Hg while on treatment with a median of two antihypertensive drugs, including 100% on either an ACE inhibitor or angiotensin receptor blocker. The study operators performed RDN by placing an average of six lesions in a spiral pattern in each of the patient’s two renal arteries.
The investigators screened for arrhythmia recurrence with 7-day Holter monitoring at 3, 6, 9, and 12 months, with full 12-month follow-up available for 283 patients. After 12 months, blood pressures had declined by an average of 16/11 mm Hg among the patients who underwent RDN, with essentially no change in the patients who had pulmonary vein isolation only. Dr. Steinberg attributed the high success of the renal denervation procedures to the familiarity of the participating electrophysiologist operators with catheter-tip ablations.
“We have gone from treating patients with resistant hypertension to now treating patients with less severe hypertension,” Dr. Steinberg noted, and the next study he is planning will take this approach into patients with paroxysmal AFib but without hypertension, using RDN “solely as an anti-arrhythmic intervention,” he explained.
ERADICATE-AF did not receive commercial funding. Dr. Steinberg has been a consultant to Allergan, AtriCure, Biosense Webster, Corfigo, Medtronic, and Omron. He owns stock in AliveCor and receives salary from National Cardiac and G Medical.
ERADICATE-AF was a well-performed, informative, and provocative study that produced exciting results. I was very impressed that, despite the added complexity of performing an extra procedure, there appeared to be virtually no added risk to patients, with essentially identical complication rates in the two arms of the study. The 15.6% absolute difference in the rate of arrhythmia recurrences means that about six patients need to have renal denervation added to their catheter ablation to prevent one arrhythmia recurrence during 12 months, a pretty remarkable number-needed-to-treat.
Despite the successful outcome, adding renal denervation is not a panacea. These patients still had a 28% rate of recurrent atrial fibrillation during follow-up, and on average they also remained above their goal blood pressure despite the pressure reduction that renal denervation produced. The 43% arrhythmia recurrence rate among the patients who underwent only pulmonary vein isolation was consistent with prior reports on the efficacy of this treatment.
The findings raise the question of whether this approach would also work in AFib patients who are not hypertensive, and we must be cautious about the longer-term safety and durability of this treatment.
Cara N. Pellegrini, MD , is director of cardiac electrophysiology at the San Francisco VA Medical Center. She had no disclosures. She made these comments as designated discussant for ERADICATE-AF.
ERADICATE-AF was a well-performed, informative, and provocative study that produced exciting results. I was very impressed that, despite the added complexity of performing an extra procedure, there appeared to be virtually no added risk to patients, with essentially identical complication rates in the two arms of the study. The 15.6% absolute difference in the rate of arrhythmia recurrences means that about six patients need to have renal denervation added to their catheter ablation to prevent one arrhythmia recurrence during 12 months, a pretty remarkable number-needed-to-treat.
Despite the successful outcome, adding renal denervation is not a panacea. These patients still had a 28% rate of recurrent atrial fibrillation during follow-up, and on average they also remained above their goal blood pressure despite the pressure reduction that renal denervation produced. The 43% arrhythmia recurrence rate among the patients who underwent only pulmonary vein isolation was consistent with prior reports on the efficacy of this treatment.
The findings raise the question of whether this approach would also work in AFib patients who are not hypertensive, and we must be cautious about the longer-term safety and durability of this treatment.
Cara N. Pellegrini, MD , is director of cardiac electrophysiology at the San Francisco VA Medical Center. She had no disclosures. She made these comments as designated discussant for ERADICATE-AF.
ERADICATE-AF was a well-performed, informative, and provocative study that produced exciting results. I was very impressed that, despite the added complexity of performing an extra procedure, there appeared to be virtually no added risk to patients, with essentially identical complication rates in the two arms of the study. The 15.6% absolute difference in the rate of arrhythmia recurrences means that about six patients need to have renal denervation added to their catheter ablation to prevent one arrhythmia recurrence during 12 months, a pretty remarkable number-needed-to-treat.
Despite the successful outcome, adding renal denervation is not a panacea. These patients still had a 28% rate of recurrent atrial fibrillation during follow-up, and on average they also remained above their goal blood pressure despite the pressure reduction that renal denervation produced. The 43% arrhythmia recurrence rate among the patients who underwent only pulmonary vein isolation was consistent with prior reports on the efficacy of this treatment.
The findings raise the question of whether this approach would also work in AFib patients who are not hypertensive, and we must be cautious about the longer-term safety and durability of this treatment.
Cara N. Pellegrini, MD , is director of cardiac electrophysiology at the San Francisco VA Medical Center. She had no disclosures. She made these comments as designated discussant for ERADICATE-AF.
SAN FRANCISCO – Adding renal denervation when performing catheter ablation of paroxysmal atrial fibrillation in hypertensive patients substantially reduced their arrhythmia recurrence rate during the subsequent year in a multicenter, randomized trial with 302 patients.
The findings established renal denervation (RDN) as a “reasonable” tool to increase the success of atrial fibrillation (AFib) catheter ablation, Jonathan S. Steinberg, MD, said at the annual scientific sessions of the Heart Rhythm Society.
“The RDN procedure seems remarkably safe and seems to be reliably accomplished when an electrophysiologist does it,” said Dr. Steinberg, director of the Arrhythmia Center of the Summit Medical Group in Montclair, N.J. Given the evidence he reported that performing RDN simultaneously with AFib catheter ablation by pulmonary vein isolation significantly improved freedom from arrhythmia recurrence, this approach “is ready for clinical use at institutions that could mount this kind of program,” he declared.
The rate of freedom from arrhythmia recurrence while off antiarrhythmic drugs during the year following treatment was 57% among 138 patients treated with pulmonary vein isolation only, and 72% in 145 who underwent both pulmonary vein isolation and renal denervation. That’s “a pretty big difference in outcome” with no increased risk and with about 20 added minutes of procedure time, Dr. Steinberg said in a video interview. He acknowledged that, currently, no catheter is approved for U.S. marketing that is specifically designed for renal denervation, but the operators in the study he reported all used conventional radiofrequency ablation catheters with an irrigated tip, a design with U.S. availability.
The ERADICATE-AF (Renal Artery Denervation in Addition to Catheter Ablation to Eliminate Atrial Fibrillation) study randomized 302 patients with paroxysmal AFib and hypertension uncontrolled by medication at three centers in Russia, one in Germany, and one in Poland. Enrolled patients averaged about 60 years of age, about 60% were men, and their average blood pressure was roughly 150/90 mm Hg while on treatment with a median of two antihypertensive drugs, including 100% on either an ACE inhibitor or angiotensin receptor blocker. The study operators performed RDN by placing an average of six lesions in a spiral pattern in each of the patient’s two renal arteries.
The investigators screened for arrhythmia recurrence with 7-day Holter monitoring at 3, 6, 9, and 12 months, with full 12-month follow-up available for 283 patients. After 12 months, blood pressures had declined by an average of 16/11 mm Hg among the patients who underwent RDN, with essentially no change in the patients who had pulmonary vein isolation only. Dr. Steinberg attributed the high success of the renal denervation procedures to the familiarity of the participating electrophysiologist operators with catheter-tip ablations.
“We have gone from treating patients with resistant hypertension to now treating patients with less severe hypertension,” Dr. Steinberg noted, and the next study he is planning will take this approach into patients with paroxysmal AFib but without hypertension, using RDN “solely as an anti-arrhythmic intervention,” he explained.
ERADICATE-AF did not receive commercial funding. Dr. Steinberg has been a consultant to Allergan, AtriCure, Biosense Webster, Corfigo, Medtronic, and Omron. He owns stock in AliveCor and receives salary from National Cardiac and G Medical.
SAN FRANCISCO – Adding renal denervation when performing catheter ablation of paroxysmal atrial fibrillation in hypertensive patients substantially reduced their arrhythmia recurrence rate during the subsequent year in a multicenter, randomized trial with 302 patients.
The findings established renal denervation (RDN) as a “reasonable” tool to increase the success of atrial fibrillation (AFib) catheter ablation, Jonathan S. Steinberg, MD, said at the annual scientific sessions of the Heart Rhythm Society.
“The RDN procedure seems remarkably safe and seems to be reliably accomplished when an electrophysiologist does it,” said Dr. Steinberg, director of the Arrhythmia Center of the Summit Medical Group in Montclair, N.J. Given the evidence he reported that performing RDN simultaneously with AFib catheter ablation by pulmonary vein isolation significantly improved freedom from arrhythmia recurrence, this approach “is ready for clinical use at institutions that could mount this kind of program,” he declared.
The rate of freedom from arrhythmia recurrence while off antiarrhythmic drugs during the year following treatment was 57% among 138 patients treated with pulmonary vein isolation only, and 72% in 145 who underwent both pulmonary vein isolation and renal denervation. That’s “a pretty big difference in outcome” with no increased risk and with about 20 added minutes of procedure time, Dr. Steinberg said in a video interview. He acknowledged that, currently, no catheter is approved for U.S. marketing that is specifically designed for renal denervation, but the operators in the study he reported all used conventional radiofrequency ablation catheters with an irrigated tip, a design with U.S. availability.
The ERADICATE-AF (Renal Artery Denervation in Addition to Catheter Ablation to Eliminate Atrial Fibrillation) study randomized 302 patients with paroxysmal AFib and hypertension uncontrolled by medication at three centers in Russia, one in Germany, and one in Poland. Enrolled patients averaged about 60 years of age, about 60% were men, and their average blood pressure was roughly 150/90 mm Hg while on treatment with a median of two antihypertensive drugs, including 100% on either an ACE inhibitor or angiotensin receptor blocker. The study operators performed RDN by placing an average of six lesions in a spiral pattern in each of the patient’s two renal arteries.
The investigators screened for arrhythmia recurrence with 7-day Holter monitoring at 3, 6, 9, and 12 months, with full 12-month follow-up available for 283 patients. After 12 months, blood pressures had declined by an average of 16/11 mm Hg among the patients who underwent RDN, with essentially no change in the patients who had pulmonary vein isolation only. Dr. Steinberg attributed the high success of the renal denervation procedures to the familiarity of the participating electrophysiologist operators with catheter-tip ablations.
“We have gone from treating patients with resistant hypertension to now treating patients with less severe hypertension,” Dr. Steinberg noted, and the next study he is planning will take this approach into patients with paroxysmal AFib but without hypertension, using RDN “solely as an anti-arrhythmic intervention,” he explained.
ERADICATE-AF did not receive commercial funding. Dr. Steinberg has been a consultant to Allergan, AtriCure, Biosense Webster, Corfigo, Medtronic, and Omron. He owns stock in AliveCor and receives salary from National Cardiac and G Medical.
REPORTING FROM HEART RHYTHM 2019
Higher AFib ablation volumes linked with better outcomes
SAN FRANCISCO – The number of atrial fibrillation (Afib) catheter ablations a hospital did a year had a substantial, independent effect on patient outcomes in a study of more than 54,000 U.S. catheter ablations performed during 2010-2014.
The results showed that the roughly one-third of studied hospitals with the lowest annual volume of catheter ablations performed, 20 or fewer, had twice the acute complication rate and twice the 30-day in-hospital mortality rate, compared with the hospitals that did 53 or more such procedures annually in patients with atrial fibrillation, Jim W. Cheung, MD, said while presenting a poster at the annual scientific sessions of the Heart Rhythm Society.
The data, taken from 1,738 U.S. hospitals during 2010-2014 and captured in the Nationwide Readmissions Database, also showed that 79% of these hospitals performed 20 or fewer catheter ablations for atrial fibrillation (AFib) annually, with 63% doing 10 or fewer cases per year during the 5 years studied.
The findings raise the question of whether U.S. guidelines for catheter ablation of AFib should specify a minimum case volume for hospital programs, and if so, how high the minimum should be. Volume thresholds are “something to think about,” or a system to designate centers of excellence, Dr. Cheung suggested in a video interview. But interest in setting volume thresholds to better insure competence is often counterbalanced by concerns about patient access, he noted.
The prevailing U.S. guidelines for catheter ablation of AFib are in a 2017 statement from the Heart Rhythm Society and several collaborating groups (J Arrhythm. 2017 Oct;33[5]:369-409). The statement focused on operator volume rather than hospital volume and said that each operator should perform “several” AFib ablation procedures each month, which is generally understood to mean at least 2 per month or at least about 25 annually, commented Hugh Calkins, MD, chair of the panel that wrote the statement and professor of medicine at Johns Hopkins Medicine in Baltimore. The major rationale for setting a suggested minimum of about 25 cases/year came largely from a 2013 report that is cited as the first study to document a volume-outcome relationship for catheter ablation of AFib (Circulation. 2013 Nov 5;128[19]:2104-12), Dr. Calkins noted. “Volume does matter,” he agreed in an interview, but no society or organization monitors hospital or operator volumes, nor takes any steps when volumes are low.
The Nationwide Readmissions Database included 54,599 patients who underwent AFib catheter ablation during 2010-2014. Dr. Cheung and his associates divided these patients into rough tertiles based on the annual procedure volumes of the hospitals that performed these ablations. The 36% of patients treated at hospitals that did 20 or fewer procedures annually were on average older and had more comorbidities than the 31% treated at hospitals in the highest-volume tertile, which performed at least 53 ablations annually. In an analysis that adjusted for these demographic and clinical differences, patients ablated at the lower-volume hospitals had a statistically significant 2.06-fold higher rate of any complication and a 2.24-fold increased rate of in-hospital mortality, either during the index hospitalization or during a 30-day hospital readmission, reported Dr. Cheung, director of clinical electrophysiology research at Weill Cornell Medical College in New York. The increased rate of complications was driven by a fivefold increased rate of cardiac perforations, a greater than doubled periprocedural stroke rate, and a roughly 50% increased rate of vascular complications, compared with the highest-volume hospitals and after adjustment for baseline differences.
Dr. Cheung has been a consultant to Abbott and Biotronik, and has received fellowship support from Biosense Webster, Biotronik, Boston Scientific, Medtronic, and St. Jude. Dr. Calkins disclosed ties to Abbott, Altathera, AtriCure, Boehringer Ingelheim, Boston Scientific, Medtronic, St. Jude, and MRI Interventions.
SOURCE: Cheung JW. HRS 2019, Abstract S-P001-123.
SAN FRANCISCO – The number of atrial fibrillation (Afib) catheter ablations a hospital did a year had a substantial, independent effect on patient outcomes in a study of more than 54,000 U.S. catheter ablations performed during 2010-2014.
The results showed that the roughly one-third of studied hospitals with the lowest annual volume of catheter ablations performed, 20 or fewer, had twice the acute complication rate and twice the 30-day in-hospital mortality rate, compared with the hospitals that did 53 or more such procedures annually in patients with atrial fibrillation, Jim W. Cheung, MD, said while presenting a poster at the annual scientific sessions of the Heart Rhythm Society.
The data, taken from 1,738 U.S. hospitals during 2010-2014 and captured in the Nationwide Readmissions Database, also showed that 79% of these hospitals performed 20 or fewer catheter ablations for atrial fibrillation (AFib) annually, with 63% doing 10 or fewer cases per year during the 5 years studied.
The findings raise the question of whether U.S. guidelines for catheter ablation of AFib should specify a minimum case volume for hospital programs, and if so, how high the minimum should be. Volume thresholds are “something to think about,” or a system to designate centers of excellence, Dr. Cheung suggested in a video interview. But interest in setting volume thresholds to better insure competence is often counterbalanced by concerns about patient access, he noted.
The prevailing U.S. guidelines for catheter ablation of AFib are in a 2017 statement from the Heart Rhythm Society and several collaborating groups (J Arrhythm. 2017 Oct;33[5]:369-409). The statement focused on operator volume rather than hospital volume and said that each operator should perform “several” AFib ablation procedures each month, which is generally understood to mean at least 2 per month or at least about 25 annually, commented Hugh Calkins, MD, chair of the panel that wrote the statement and professor of medicine at Johns Hopkins Medicine in Baltimore. The major rationale for setting a suggested minimum of about 25 cases/year came largely from a 2013 report that is cited as the first study to document a volume-outcome relationship for catheter ablation of AFib (Circulation. 2013 Nov 5;128[19]:2104-12), Dr. Calkins noted. “Volume does matter,” he agreed in an interview, but no society or organization monitors hospital or operator volumes, nor takes any steps when volumes are low.
The Nationwide Readmissions Database included 54,599 patients who underwent AFib catheter ablation during 2010-2014. Dr. Cheung and his associates divided these patients into rough tertiles based on the annual procedure volumes of the hospitals that performed these ablations. The 36% of patients treated at hospitals that did 20 or fewer procedures annually were on average older and had more comorbidities than the 31% treated at hospitals in the highest-volume tertile, which performed at least 53 ablations annually. In an analysis that adjusted for these demographic and clinical differences, patients ablated at the lower-volume hospitals had a statistically significant 2.06-fold higher rate of any complication and a 2.24-fold increased rate of in-hospital mortality, either during the index hospitalization or during a 30-day hospital readmission, reported Dr. Cheung, director of clinical electrophysiology research at Weill Cornell Medical College in New York. The increased rate of complications was driven by a fivefold increased rate of cardiac perforations, a greater than doubled periprocedural stroke rate, and a roughly 50% increased rate of vascular complications, compared with the highest-volume hospitals and after adjustment for baseline differences.
Dr. Cheung has been a consultant to Abbott and Biotronik, and has received fellowship support from Biosense Webster, Biotronik, Boston Scientific, Medtronic, and St. Jude. Dr. Calkins disclosed ties to Abbott, Altathera, AtriCure, Boehringer Ingelheim, Boston Scientific, Medtronic, St. Jude, and MRI Interventions.
SOURCE: Cheung JW. HRS 2019, Abstract S-P001-123.
SAN FRANCISCO – The number of atrial fibrillation (Afib) catheter ablations a hospital did a year had a substantial, independent effect on patient outcomes in a study of more than 54,000 U.S. catheter ablations performed during 2010-2014.
The results showed that the roughly one-third of studied hospitals with the lowest annual volume of catheter ablations performed, 20 or fewer, had twice the acute complication rate and twice the 30-day in-hospital mortality rate, compared with the hospitals that did 53 or more such procedures annually in patients with atrial fibrillation, Jim W. Cheung, MD, said while presenting a poster at the annual scientific sessions of the Heart Rhythm Society.
The data, taken from 1,738 U.S. hospitals during 2010-2014 and captured in the Nationwide Readmissions Database, also showed that 79% of these hospitals performed 20 or fewer catheter ablations for atrial fibrillation (AFib) annually, with 63% doing 10 or fewer cases per year during the 5 years studied.
The findings raise the question of whether U.S. guidelines for catheter ablation of AFib should specify a minimum case volume for hospital programs, and if so, how high the minimum should be. Volume thresholds are “something to think about,” or a system to designate centers of excellence, Dr. Cheung suggested in a video interview. But interest in setting volume thresholds to better insure competence is often counterbalanced by concerns about patient access, he noted.
The prevailing U.S. guidelines for catheter ablation of AFib are in a 2017 statement from the Heart Rhythm Society and several collaborating groups (J Arrhythm. 2017 Oct;33[5]:369-409). The statement focused on operator volume rather than hospital volume and said that each operator should perform “several” AFib ablation procedures each month, which is generally understood to mean at least 2 per month or at least about 25 annually, commented Hugh Calkins, MD, chair of the panel that wrote the statement and professor of medicine at Johns Hopkins Medicine in Baltimore. The major rationale for setting a suggested minimum of about 25 cases/year came largely from a 2013 report that is cited as the first study to document a volume-outcome relationship for catheter ablation of AFib (Circulation. 2013 Nov 5;128[19]:2104-12), Dr. Calkins noted. “Volume does matter,” he agreed in an interview, but no society or organization monitors hospital or operator volumes, nor takes any steps when volumes are low.
The Nationwide Readmissions Database included 54,599 patients who underwent AFib catheter ablation during 2010-2014. Dr. Cheung and his associates divided these patients into rough tertiles based on the annual procedure volumes of the hospitals that performed these ablations. The 36% of patients treated at hospitals that did 20 or fewer procedures annually were on average older and had more comorbidities than the 31% treated at hospitals in the highest-volume tertile, which performed at least 53 ablations annually. In an analysis that adjusted for these demographic and clinical differences, patients ablated at the lower-volume hospitals had a statistically significant 2.06-fold higher rate of any complication and a 2.24-fold increased rate of in-hospital mortality, either during the index hospitalization or during a 30-day hospital readmission, reported Dr. Cheung, director of clinical electrophysiology research at Weill Cornell Medical College in New York. The increased rate of complications was driven by a fivefold increased rate of cardiac perforations, a greater than doubled periprocedural stroke rate, and a roughly 50% increased rate of vascular complications, compared with the highest-volume hospitals and after adjustment for baseline differences.
Dr. Cheung has been a consultant to Abbott and Biotronik, and has received fellowship support from Biosense Webster, Biotronik, Boston Scientific, Medtronic, and St. Jude. Dr. Calkins disclosed ties to Abbott, Altathera, AtriCure, Boehringer Ingelheim, Boston Scientific, Medtronic, St. Jude, and MRI Interventions.
SOURCE: Cheung JW. HRS 2019, Abstract S-P001-123.
REPORTING FROM HEART RHYTHM 2019
Key clinical point: .
Major finding: Hospitals performing 20 or fewer catheter ablations annually had twice as many acute complications as hospitals doing at least 53.
Study details: Analysis of 54,599 atrial fibrillation patients who underwent catheter ablation in the Nationwide Readmissions Database.
Disclosures: Dr. Cheung has been a consultant to Abbott and Biotronik, and has received fellowship support from Biosense Webster, Biotronik, Boston Scientific, Medtronic, and St. Jude.
Source: Cheung JW et al. Heart Rhythm 2019, Abstract S-P001-123.
Experts discuss what’s new in migraine treatment
PHILADELPHIA – At the annual meeting of the American Academy of Neurology, Alan M. Rapoport, MD, clinical professor of neurology at the University of California, Los Angeles, sat down with Stewart J. Tepper, MD, professor of neurology at the Geisel School of Medicine at Dartmouth, Hanover, N.H., to discuss in a video some of the new data presented at the meeting regarding the CGRP monoclonal antibodies, the small molecule receptor antagonists (gepants), and what Dr. Tepper described as “a real shift in paradigm and a watershed moment in migraine.”
The three gepants that are farthest along in clinical trials are ubrogepant, rimegepant, and atogepant. “Reassuring data” was presented, Dr. Tepper said, regarding liver toxicity, which has been a concern with earlier generations of the gepants. The Food and Drug Administration had mandated a close look at liver function with the use of these drugs, which are metabolized in the liver, and, to date, no safety signals have emerged.
The three CGRP monoclonal antibodies that are currently on the market are erenumab (Aimovig), fremanezumab (Ajovy), and galcanezumab (Emgality). Data from numerous open-label extension studies were presented. In general, it seems that “the monoclonal antibodies accumulate greater efficacy over time,” Dr. Tepper said. No safety concerns have emerged from 5 years of clinical trial data. With 250,000 patients on these drugs worldwide, that is “very reassuring,” Dr. Tepper said.
New data also show that the majority of patients with chronic migraine who are taking monoclonal antibodies convert from chronic migraine to episodic migraine. Additionally, new data show that use of monoclonal antibodies “dramatically reduce all migraine medication use,” Dr. Tepper said.
PHILADELPHIA – At the annual meeting of the American Academy of Neurology, Alan M. Rapoport, MD, clinical professor of neurology at the University of California, Los Angeles, sat down with Stewart J. Tepper, MD, professor of neurology at the Geisel School of Medicine at Dartmouth, Hanover, N.H., to discuss in a video some of the new data presented at the meeting regarding the CGRP monoclonal antibodies, the small molecule receptor antagonists (gepants), and what Dr. Tepper described as “a real shift in paradigm and a watershed moment in migraine.”
The three gepants that are farthest along in clinical trials are ubrogepant, rimegepant, and atogepant. “Reassuring data” was presented, Dr. Tepper said, regarding liver toxicity, which has been a concern with earlier generations of the gepants. The Food and Drug Administration had mandated a close look at liver function with the use of these drugs, which are metabolized in the liver, and, to date, no safety signals have emerged.
The three CGRP monoclonal antibodies that are currently on the market are erenumab (Aimovig), fremanezumab (Ajovy), and galcanezumab (Emgality). Data from numerous open-label extension studies were presented. In general, it seems that “the monoclonal antibodies accumulate greater efficacy over time,” Dr. Tepper said. No safety concerns have emerged from 5 years of clinical trial data. With 250,000 patients on these drugs worldwide, that is “very reassuring,” Dr. Tepper said.
New data also show that the majority of patients with chronic migraine who are taking monoclonal antibodies convert from chronic migraine to episodic migraine. Additionally, new data show that use of monoclonal antibodies “dramatically reduce all migraine medication use,” Dr. Tepper said.
PHILADELPHIA – At the annual meeting of the American Academy of Neurology, Alan M. Rapoport, MD, clinical professor of neurology at the University of California, Los Angeles, sat down with Stewart J. Tepper, MD, professor of neurology at the Geisel School of Medicine at Dartmouth, Hanover, N.H., to discuss in a video some of the new data presented at the meeting regarding the CGRP monoclonal antibodies, the small molecule receptor antagonists (gepants), and what Dr. Tepper described as “a real shift in paradigm and a watershed moment in migraine.”
The three gepants that are farthest along in clinical trials are ubrogepant, rimegepant, and atogepant. “Reassuring data” was presented, Dr. Tepper said, regarding liver toxicity, which has been a concern with earlier generations of the gepants. The Food and Drug Administration had mandated a close look at liver function with the use of these drugs, which are metabolized in the liver, and, to date, no safety signals have emerged.
The three CGRP monoclonal antibodies that are currently on the market are erenumab (Aimovig), fremanezumab (Ajovy), and galcanezumab (Emgality). Data from numerous open-label extension studies were presented. In general, it seems that “the monoclonal antibodies accumulate greater efficacy over time,” Dr. Tepper said. No safety concerns have emerged from 5 years of clinical trial data. With 250,000 patients on these drugs worldwide, that is “very reassuring,” Dr. Tepper said.
New data also show that the majority of patients with chronic migraine who are taking monoclonal antibodies convert from chronic migraine to episodic migraine. Additionally, new data show that use of monoclonal antibodies “dramatically reduce all migraine medication use,” Dr. Tepper said.
EXPERT ANALYSIS FROM AAN 2019
2019 Update: Contraceptives and unintended pregnancy rates
NASHVILLE, TENN. – The unintended pregnancy rate is declining after years of hovering at close to 50%.
While the rates among women of color remain high – currently at 58 and 79 per 1,000 women aged 15-44 years for Hispanic and black women, respectively – they have declined from 79 and 92 per 1,000 Hispanic and black women in that age group in 2008, and the overall rate is now at about 45%, Eve Espey, MD, said at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
“Considering the scope and number of women affected by unplanned pregnancy, this is actually a huge public health achievement,” said Dr. Espey, professor and chair of the department of obstetrics & gynecology at the University of New Mexico, Albuquerque.
she noted, adding that “another enormous determinant of this decrease in unintended pregnancy is the use of long-acting reversible contraception [LARC].” About 2% of women used contraceptives in 2002, and now, based on the latest cycle of data from 2015-2017, 16% of women use contraceptives.
In this video interview, Dr. Espey discusses the main points of her talk entitled “Contraceptives: What you need to know in 2019,” including:
- The importance of “following reproductive justice–based principles and counseling” when it comes to prescribing contraceptives.
- The latest data showing that certain LARC methods remain safe and effective beyond their approved duration of use.
- Trends with respect to tubal ligation and salpingectomy.
- The value of the Centers for Disease Control and Prevention’s U.S. Medical Eligibility Criteria (MEC) for evidence-based guidance on selecting contraceptives based on patients’ individual needs.
“[MEC] is something every ob.gyn. should consider using,” she said, noting that access is available through a free app. “As our patients are more complex and have more comorbidities, it’s particularly helpful for matching up patients and their conditions with recommendations for specific contraceptive methods.”
Dr. Espey reported having no financial disclosures.
NASHVILLE, TENN. – The unintended pregnancy rate is declining after years of hovering at close to 50%.
While the rates among women of color remain high – currently at 58 and 79 per 1,000 women aged 15-44 years for Hispanic and black women, respectively – they have declined from 79 and 92 per 1,000 Hispanic and black women in that age group in 2008, and the overall rate is now at about 45%, Eve Espey, MD, said at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
“Considering the scope and number of women affected by unplanned pregnancy, this is actually a huge public health achievement,” said Dr. Espey, professor and chair of the department of obstetrics & gynecology at the University of New Mexico, Albuquerque.
she noted, adding that “another enormous determinant of this decrease in unintended pregnancy is the use of long-acting reversible contraception [LARC].” About 2% of women used contraceptives in 2002, and now, based on the latest cycle of data from 2015-2017, 16% of women use contraceptives.
In this video interview, Dr. Espey discusses the main points of her talk entitled “Contraceptives: What you need to know in 2019,” including:
- The importance of “following reproductive justice–based principles and counseling” when it comes to prescribing contraceptives.
- The latest data showing that certain LARC methods remain safe and effective beyond their approved duration of use.
- Trends with respect to tubal ligation and salpingectomy.
- The value of the Centers for Disease Control and Prevention’s U.S. Medical Eligibility Criteria (MEC) for evidence-based guidance on selecting contraceptives based on patients’ individual needs.
“[MEC] is something every ob.gyn. should consider using,” she said, noting that access is available through a free app. “As our patients are more complex and have more comorbidities, it’s particularly helpful for matching up patients and their conditions with recommendations for specific contraceptive methods.”
Dr. Espey reported having no financial disclosures.
NASHVILLE, TENN. – The unintended pregnancy rate is declining after years of hovering at close to 50%.
While the rates among women of color remain high – currently at 58 and 79 per 1,000 women aged 15-44 years for Hispanic and black women, respectively – they have declined from 79 and 92 per 1,000 Hispanic and black women in that age group in 2008, and the overall rate is now at about 45%, Eve Espey, MD, said at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
“Considering the scope and number of women affected by unplanned pregnancy, this is actually a huge public health achievement,” said Dr. Espey, professor and chair of the department of obstetrics & gynecology at the University of New Mexico, Albuquerque.
she noted, adding that “another enormous determinant of this decrease in unintended pregnancy is the use of long-acting reversible contraception [LARC].” About 2% of women used contraceptives in 2002, and now, based on the latest cycle of data from 2015-2017, 16% of women use contraceptives.
In this video interview, Dr. Espey discusses the main points of her talk entitled “Contraceptives: What you need to know in 2019,” including:
- The importance of “following reproductive justice–based principles and counseling” when it comes to prescribing contraceptives.
- The latest data showing that certain LARC methods remain safe and effective beyond their approved duration of use.
- Trends with respect to tubal ligation and salpingectomy.
- The value of the Centers for Disease Control and Prevention’s U.S. Medical Eligibility Criteria (MEC) for evidence-based guidance on selecting contraceptives based on patients’ individual needs.
“[MEC] is something every ob.gyn. should consider using,” she said, noting that access is available through a free app. “As our patients are more complex and have more comorbidities, it’s particularly helpful for matching up patients and their conditions with recommendations for specific contraceptive methods.”
Dr. Espey reported having no financial disclosures.
EXPERT ANALYSIS FROM ACOG 2019
Consider varying generational needs, preferences in the workplace and with patients
NASHVILLE, TENN. – By 2020, millennials will comprise more than a third of individuals in the workplace, and that has important implications for employment, communication, and education, according to Patrice M. Weiss, MD.
Each generation brings a unique set of experiences and expectations. Millennials – or members of “Generation Y,” who comprised 18% of the workforce in 2018 and 0% in 2008 – tend to prefer flexible work hours and communication via technology, she said during a session on navigating generational differences in the workplace during the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
They, along with members of “Generation X” (generally those born between 1962 and 1981) and “Generation Z” (generally those born from 1987 on), tend to be technology savvy, whereas the “Silent Generation” (generally those born between 1925 and 1942) and older members of the “Baby Boomer Generation” (generally those born between 1943 and 1961), may prefer printed communication and phone calls, said Dr. Weiss, chief medical officer at the Carilion Clinic in Roanoke, Va.
“It’s not good, it’s not bad – it’s just the way things are changing,” she said, adding that it’s important to look at the strengths that each generation brings to the workplace.
Importantly, the she said.
In this video interview, she further discusses how generational differences should be considered in medical practice, and how clinicians can adapt to the changing expectations and different needs of patients from different generations.
“Gen Ys want to communicate with us through technology. They don’t want to pick up the phone and schedule an appointment, they want to be able to go online ... through an app and self-schedule an appointment,” she said. “And they want health care when they want it.
“We as health care providers and health care organizations, we need to meet the needs of each generation ... so what we need to do is really identify what are the needs of all the generations as patients.”
During her presentation, Dr. Weiss further noted that these generational differences present a major challenge with respect to teaching, learning, and communicating.
“Rather than becoming frustrated ... let’s hope that we can ... reach across generations, identify what their strengths are, capitalize on those, and then, as health care providers, be more user and consumer friendly to the generations, particularly the millennials [so] that they have access to us and to information.”
Dr. Weiss said she had no relevant financial disclosures.
NASHVILLE, TENN. – By 2020, millennials will comprise more than a third of individuals in the workplace, and that has important implications for employment, communication, and education, according to Patrice M. Weiss, MD.
Each generation brings a unique set of experiences and expectations. Millennials – or members of “Generation Y,” who comprised 18% of the workforce in 2018 and 0% in 2008 – tend to prefer flexible work hours and communication via technology, she said during a session on navigating generational differences in the workplace during the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
They, along with members of “Generation X” (generally those born between 1962 and 1981) and “Generation Z” (generally those born from 1987 on), tend to be technology savvy, whereas the “Silent Generation” (generally those born between 1925 and 1942) and older members of the “Baby Boomer Generation” (generally those born between 1943 and 1961), may prefer printed communication and phone calls, said Dr. Weiss, chief medical officer at the Carilion Clinic in Roanoke, Va.
“It’s not good, it’s not bad – it’s just the way things are changing,” she said, adding that it’s important to look at the strengths that each generation brings to the workplace.
Importantly, the she said.
In this video interview, she further discusses how generational differences should be considered in medical practice, and how clinicians can adapt to the changing expectations and different needs of patients from different generations.
“Gen Ys want to communicate with us through technology. They don’t want to pick up the phone and schedule an appointment, they want to be able to go online ... through an app and self-schedule an appointment,” she said. “And they want health care when they want it.
“We as health care providers and health care organizations, we need to meet the needs of each generation ... so what we need to do is really identify what are the needs of all the generations as patients.”
During her presentation, Dr. Weiss further noted that these generational differences present a major challenge with respect to teaching, learning, and communicating.
“Rather than becoming frustrated ... let’s hope that we can ... reach across generations, identify what their strengths are, capitalize on those, and then, as health care providers, be more user and consumer friendly to the generations, particularly the millennials [so] that they have access to us and to information.”
Dr. Weiss said she had no relevant financial disclosures.
NASHVILLE, TENN. – By 2020, millennials will comprise more than a third of individuals in the workplace, and that has important implications for employment, communication, and education, according to Patrice M. Weiss, MD.
Each generation brings a unique set of experiences and expectations. Millennials – or members of “Generation Y,” who comprised 18% of the workforce in 2018 and 0% in 2008 – tend to prefer flexible work hours and communication via technology, she said during a session on navigating generational differences in the workplace during the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
They, along with members of “Generation X” (generally those born between 1962 and 1981) and “Generation Z” (generally those born from 1987 on), tend to be technology savvy, whereas the “Silent Generation” (generally those born between 1925 and 1942) and older members of the “Baby Boomer Generation” (generally those born between 1943 and 1961), may prefer printed communication and phone calls, said Dr. Weiss, chief medical officer at the Carilion Clinic in Roanoke, Va.
“It’s not good, it’s not bad – it’s just the way things are changing,” she said, adding that it’s important to look at the strengths that each generation brings to the workplace.
Importantly, the she said.
In this video interview, she further discusses how generational differences should be considered in medical practice, and how clinicians can adapt to the changing expectations and different needs of patients from different generations.
“Gen Ys want to communicate with us through technology. They don’t want to pick up the phone and schedule an appointment, they want to be able to go online ... through an app and self-schedule an appointment,” she said. “And they want health care when they want it.
“We as health care providers and health care organizations, we need to meet the needs of each generation ... so what we need to do is really identify what are the needs of all the generations as patients.”
During her presentation, Dr. Weiss further noted that these generational differences present a major challenge with respect to teaching, learning, and communicating.
“Rather than becoming frustrated ... let’s hope that we can ... reach across generations, identify what their strengths are, capitalize on those, and then, as health care providers, be more user and consumer friendly to the generations, particularly the millennials [so] that they have access to us and to information.”
Dr. Weiss said she had no relevant financial disclosures.
EXPERT ANALYSIS FROM ACOG 2019