User login
The Impact of the Recent Supreme Court Ruling on the Dermatology Recruitment Pipeline
The ruling by the Supreme Court of the United States (SCOTUS) in 20231,2 on the use of race-based criteria in college admissions was met with a range of reactions across the country. Given the implications of this decision on the future makeup of higher education, the downstream effects on medical school admissions, and the possible further impact on graduate medical education programs, we sought to explore the potential impact of the landmark decision from the perspective of dermatology residency program directors and offer insights on this pivotal judgment.
Background on the SCOTUS Ruling
In June 2023, SCOTUS issued its formal decision on 2 court cases brought by the organization Students for Fair Admissions (SFFA) against the University of North Carolina at Chapel Hill1 and Harvard University (Cambridge, Massachusetts)2 that addressed college admissions practices dealing with the use of race as a selection criterion in the application process. The cases alleged that these universities had overly emphasized race in the admissions process and thus were in violation of the Civil Rights Act of 1964 as well as the 14th Amendment.1,2
The SCOTUS justices voted 6 to 3 in favor of the argument presented by the SFFA, determining that the use of race in the college admissions process essentially constituted a form of racial discrimination. The ruling was in contrast to a prior decision in 2003 that centered on law school admissions at the University of Michigan (Ann Arbor, Michigan) in which SCOTUS previously had determined that race could be used as one factor amongst other criteria in the higher education selection process.3 In the 2023 decision siding with SFFA, SCOTUS did acknowledge that it was still acceptable for selection processes to consider “an applicant’s discussion of how race affected his or her life, be it through discrimination, inspiration, or otherwise.”2
Effect on Undergraduate Admissions
Prior to the 2023 ruling, several states had already passed independent laws against the use of affirmative action or race-based selection criteria in the admissions process at public colleges and universities.4 As a result, these institutions would already be conforming to the principles set forth in the SCOTUS ruling and major changes to their undergraduate admissions policies would not be expected; however, a considerable number of colleges and universities—particularly those considered highly selective with applicant acceptance rates that are well below the national average—reported the use of race as a factor in their admissions processes in standardized reporting surveys.5 For these institutions, it is no longer considered acceptable (based on the SCOTUS decision) to use race as a singular factor in admissions or to implement race-conscious decision-making—in which individuals are considered differently based solely on their race—as part of the undergraduate selection process.
In light of these rulings, many institutions have explicitly committed to upholding principles of diversity in their recruitment processes, acknowledging the multifaceted nature of diversity beyond strictly racial terms—including but not limited to socioeconomic diversity, religious diversity, or gender diversity—which is in compliance with the interpretation ruling by the US Department of Education and the US Department of Justice.6 Additionally, select institutions have taken approaches to explicitly include questions on ways in which applicants have overcome obstacles or challenges, allowing an opportunity for individuals who have had such experiences related to race an opportunity to incorporate these elements into their applications. Finally, some institutions have taken a more limited approach, eliminating ways in which race is explicitly addressed in the application and focusing on race-neutral elements of the application in their approach to selection.7
Because the first college admission cycle since the 2023 SCOTUS ruling is still underway, we have yet to witness the full impact of this decision on the current undergraduate admissions landscape.
Effect on Medical School Admissions and Rotations
Although SCOTUS specifically examined the undergraduate admissions process, the ruling on race-conscious admissions also had a profound impact on graduate school admissions including medical school admission processes.1,2,8,9 This is because the language of the majority opinion refers to “university programs” in its ruling, which also has been broadly interpreted to include graduate school programs. As with undergraduate admissions, it has been interpreted by national medical education organizations and institutions that medical schools also cannot consider an applicant’s race or ethnicity as a specific factor in the admissions process.1,2,8,9
Lived individual experiences, including essays that speak to an applicant’s lived experiences and career aspirations related to race, still can be taken into account. In particular, holistic review still can be utilized to evaluate medical school candidates and may play a more integral role in the medical school admissions process now than in the past.8,10,11 After the ruling, Justice Sonia Sotomayor noted that “today’s decision leaves intact holistic college admissions and recruitment efforts that seek to enroll diverse classes without using racial classifications.”1
The ruling asserted that universities may define their mission as they see fit. As a result, the ruling did not affect medical school missions or strategic plans, including those that may aim to diversify the health care workforce.8,10,11 The ruling also did not affect the ability to utilize pathway programs to encourage a career in medicine or recruitment relationships with diverse undergraduate or community-based organizations. Student interest groups also can be involved in the relationship-building or recruitment activities for medical schools.8,10,11 Guidance from the US Department of Education and US Department of Justice noted that institutions may consider race in identifying prospective applicants through recruitment and outreach, “provided that their outreach and recruitment programs do not provide targeted groups of prospective students preference in the admissions process, and provided that all students—whether part of a specifically targeted group or not—enjoy the same opportunity to apply and compete for admission.”12
In regard to pathways programs, slots cannot be reserved and preference cannot be given to applicants who participated in these programs if race was a factor in selecting participants.8 Similarly, medical school away electives related to diversity cannot be reserved for those of a specific race or ethnicity; however, these electives can utilize commitment to stated aims and missions of the rotation, such as a commitment to diversity within medicine, as a basis to selecting candidates.8
The ruling did not address how race or ethnicity is factored into financial aid or scholarship determination. There has been concern in higher education that the legal framework utilized in the SCOTUS decision could affect financial aid and scholarship decisions; therefore, many institutions are proceeding with caution in their approach.8
Effect on Residency Selection
Because the SCOTUS ruling references colleges and universities, not health care employers, it should not affect the residency selection process; however, there is variability in how health care institutions are interpreting the impact of the ruling on residency selection, with some taking a more prescriptive and cautious view on the matter. Additionally, with that said, residency selection is considered an employment practice covered by Title VII of the Civil Rights Act of 1964,13 which already prohibits the consideration of race in hiring decisions.7 Under Title VII, it is unlawful for employers to discriminate against someone because of race, color, religion, sex, or national origin, and it is “unlawful to use policies or practices that seem neutral but have the effect of discriminating against people because of their race, color, religion, sex … or national origin.” Title VII also states that employers cannot “make employment decisions based on stereotypes or assumptions about a person’s abilities, traits, or performance because of their race, color, religion, sex … or national origin.”13
Importantly, Title VII does not imply that employers need to abandon their diversity, equity, or inclusion initiatives, and it does not imply that employers must revoke their mission to improve diversity in the workforce. Title VII does not state that racial information cannot be available. It would be permissible to use racial data to assess recruitment trends, identify inequities, and create programs to eliminate barriers and decrease bias14; for example, if a program identified that, based on their current review system, students who are underrepresented in medicine were disproportionately screened out of the applicant pool or interview group, they may wish to revisit their review process to identify and eliminate possible biases. Programs also may wish to adopt educational programs for reviewers (eg, implicit bias training) or educational content on the potential for bias in commonly used review criteria, such as the US Medical Licensing Examination, clerkship grades, and the Medical Student Performance Evaluation.15 Reviewers can and should consider applications in an individualized and holistic manner in which experiences, traits, skills, and academic metrics are assessed together for compatibility with the values and mission of the training program.16
Future Directions for Dermatology
Beyond the SCOTUS ruling, there have been other shifts in the dermatology residency application process that have affected candidate review. Dermatology programs recently have adopted the use of preference signaling in residency applications. Preliminary data from the Association of American Medical Colleges for the 2024 application cycle indicated that of the 81 programs analyzed, there was a nearly 0% chance of an applicant receiving an interview invitation from a program that they did not signal. The median signal-to-interview conversion rate for the 81 dermatology programs analyzed was 55% for gold signals and 15% for silver signals.17 It can be inferred from these data that programs are using preference signaling as important criteria for consideration of interview invitation. Programs may choose to focus most of their attention on the applicant pool who has signaled them. Because the number and type of signals available is equal among all applicants, we hope that this provides an equitable way for all applicants to garner holistic review from programs that interested them. In addition, there has been a 30% decrease in average applications submitted per dermatology applicant.18 With a substantial decline in applications to dermatology, we hope that reviewers are able to spend more time devoted to comprehensive holistic review.
Although signals are equitable for applicants, their distribution among programs may not be; for example, in a given year, a program might find that all their gold signals came from non–underrepresented in medicine students. We encourage programs to carefully review applicant data to ensure their recruitment process is not inadvertently discriminatory and is in alignment with their goals and mission.
- Students for Fair Admissions, Inc. v University of North Carolina, 567 F. Supp. 3d 580 (M.D.N.C. 2021).
- Students for Fair Admissions, Inc. v President and Fellows of Harvard College, 600 US ___ (2023).
- Grutter v Bollinger, 539 US 306 (2003).
- Saul S. 9 states have banned affirmative action. here’s what that looks like. The New York Times. October 31, 2022. https://www.nytimes.com/2022/10/31/us/politics/affirmative-action-ban-states.html
- Desilver D. Private, selective colleges are most likely to use race, ethnicity as a factor in admissions decisions. Pew Research Center. July 14, 2023. Accessed May 29, 2024. https://www.pewresearch.org/short-reads/2023/07/14/private-selective-colleges-are-most-likely-to-use-race-ethnicity-as-a-factor-in-admissions-decisions/
- US Department of Education. Justice and education departments release resources to advance diversity and opportunity in higher education. August 14, 2023. Accessed May 17, 2024. https://www.ed.gov/news/press-releases/advance-diversity-and-opportunity-higher-education-justice-and-education-departments-release-resources-advance-diversity-and-opportunity-higher-education
- Amponsah MN, Hamid RD. Harvard overhauls college application in wake of affirmative action decision. The Harvard Crimson. August 3, 2023. Accessed May 17, 2024. https://www.thecrimson.com/article/2023/8/3/harvard-admission-essay-change/
- Association of American Medical Colleges. Frequently asked questions: what does the Harvard and UNC decision mean for medical education? August 24, 2023. Accessed May 17, 2024. https://www.aamc.org/media/68771/download?attachment%3Fattachment
- American Medical Association. Affirmative action ends: how Supreme Court ruling impacts medical schools & the health care workforce. July 7, 2023. Accessed May 17, 2024. https://www.ama-assn.org/medical-students/medical-school-life/affirmative-action-ends-how-supreme-court-ruling-impacts
- Association of American Medical Colleges. How can medical schools boost racial diversity in the wake of the recent Supreme Court ruling? July 27, 2023. Accessed May 17, 2024. https://www.aamc.org/news/how-can-medical-schools-boost-racial-diversity-wake-recent-supreme-court-ruling
- Association of American Medical Colleges. Diversity in medical school admissions. Updated March 18, 2024. Accessed May 17, 2024. https://www.aamc.org/about-us/mission-areas/medical-education/diversity-medical-school-admissions
- United States Department of Justice. Questions and answers regarding the Supreme Court’s decision in Students For Fair Admissions, Inc. v. Harvard College and University of North Carolina. August 14, 2023. Accessed May 29, 2024. https://www.justice.gov/d9/2023-08/post-sffa_resource_faq_final_508.pdf
- US Department of Justice. Title VII of the Civil Rights Act of 1964. Accessed May 17, 2024. https://www.justice.gov/crt/laws-we-enforce
- Zheng L. How to effectively—and legally—use racial data for DEI. Harvard Business Review. July 24, 2023. Accessed May 17, 2024. https://hbr.org/2023/07/how-to-effectively-and-legally-use-racial-data-for-dei
- Crites K, Johnson J, Scott N, et al. Increasing diversity in residency training programs. Cureus. 2022;14:E25962. doi:10.7759/cureus.25962
- Association of American Medical Colleges. Holistic principles in resident selection: an introduction. Accessed May 17, 2024. https://www.aamc.org/media/44586/download?attachment
- Association of American Medical Colleges. Exploring the relationship between program signaling & interview invitations across specialties 2024 ERAS® preliminary analysis. December 29, 2023. Accessed May 17, 2024. https://www.aamc.org/media/74811/download?attachment
- Association of American Medical Colleges. Preliminary program signaling data and their impact on residency selection. October 24, 2023. Accessed May 17, 2024. https://www.aamc.org/services/eras-institutions/program-signaling-data#:~:text=Preliminary%20Program%20Signaling%20Data%20and%20Their%20Impact%20on%20Residency%20Selection,-Oct.&text=Program%20signals%20are%20a%20mechanism,whom%20to%20invite%20for%20interview
The ruling by the Supreme Court of the United States (SCOTUS) in 20231,2 on the use of race-based criteria in college admissions was met with a range of reactions across the country. Given the implications of this decision on the future makeup of higher education, the downstream effects on medical school admissions, and the possible further impact on graduate medical education programs, we sought to explore the potential impact of the landmark decision from the perspective of dermatology residency program directors and offer insights on this pivotal judgment.
Background on the SCOTUS Ruling
In June 2023, SCOTUS issued its formal decision on 2 court cases brought by the organization Students for Fair Admissions (SFFA) against the University of North Carolina at Chapel Hill1 and Harvard University (Cambridge, Massachusetts)2 that addressed college admissions practices dealing with the use of race as a selection criterion in the application process. The cases alleged that these universities had overly emphasized race in the admissions process and thus were in violation of the Civil Rights Act of 1964 as well as the 14th Amendment.1,2
The SCOTUS justices voted 6 to 3 in favor of the argument presented by the SFFA, determining that the use of race in the college admissions process essentially constituted a form of racial discrimination. The ruling was in contrast to a prior decision in 2003 that centered on law school admissions at the University of Michigan (Ann Arbor, Michigan) in which SCOTUS previously had determined that race could be used as one factor amongst other criteria in the higher education selection process.3 In the 2023 decision siding with SFFA, SCOTUS did acknowledge that it was still acceptable for selection processes to consider “an applicant’s discussion of how race affected his or her life, be it through discrimination, inspiration, or otherwise.”2
Effect on Undergraduate Admissions
Prior to the 2023 ruling, several states had already passed independent laws against the use of affirmative action or race-based selection criteria in the admissions process at public colleges and universities.4 As a result, these institutions would already be conforming to the principles set forth in the SCOTUS ruling and major changes to their undergraduate admissions policies would not be expected; however, a considerable number of colleges and universities—particularly those considered highly selective with applicant acceptance rates that are well below the national average—reported the use of race as a factor in their admissions processes in standardized reporting surveys.5 For these institutions, it is no longer considered acceptable (based on the SCOTUS decision) to use race as a singular factor in admissions or to implement race-conscious decision-making—in which individuals are considered differently based solely on their race—as part of the undergraduate selection process.
In light of these rulings, many institutions have explicitly committed to upholding principles of diversity in their recruitment processes, acknowledging the multifaceted nature of diversity beyond strictly racial terms—including but not limited to socioeconomic diversity, religious diversity, or gender diversity—which is in compliance with the interpretation ruling by the US Department of Education and the US Department of Justice.6 Additionally, select institutions have taken approaches to explicitly include questions on ways in which applicants have overcome obstacles or challenges, allowing an opportunity for individuals who have had such experiences related to race an opportunity to incorporate these elements into their applications. Finally, some institutions have taken a more limited approach, eliminating ways in which race is explicitly addressed in the application and focusing on race-neutral elements of the application in their approach to selection.7
Because the first college admission cycle since the 2023 SCOTUS ruling is still underway, we have yet to witness the full impact of this decision on the current undergraduate admissions landscape.
Effect on Medical School Admissions and Rotations
Although SCOTUS specifically examined the undergraduate admissions process, the ruling on race-conscious admissions also had a profound impact on graduate school admissions including medical school admission processes.1,2,8,9 This is because the language of the majority opinion refers to “university programs” in its ruling, which also has been broadly interpreted to include graduate school programs. As with undergraduate admissions, it has been interpreted by national medical education organizations and institutions that medical schools also cannot consider an applicant’s race or ethnicity as a specific factor in the admissions process.1,2,8,9
Lived individual experiences, including essays that speak to an applicant’s lived experiences and career aspirations related to race, still can be taken into account. In particular, holistic review still can be utilized to evaluate medical school candidates and may play a more integral role in the medical school admissions process now than in the past.8,10,11 After the ruling, Justice Sonia Sotomayor noted that “today’s decision leaves intact holistic college admissions and recruitment efforts that seek to enroll diverse classes without using racial classifications.”1
The ruling asserted that universities may define their mission as they see fit. As a result, the ruling did not affect medical school missions or strategic plans, including those that may aim to diversify the health care workforce.8,10,11 The ruling also did not affect the ability to utilize pathway programs to encourage a career in medicine or recruitment relationships with diverse undergraduate or community-based organizations. Student interest groups also can be involved in the relationship-building or recruitment activities for medical schools.8,10,11 Guidance from the US Department of Education and US Department of Justice noted that institutions may consider race in identifying prospective applicants through recruitment and outreach, “provided that their outreach and recruitment programs do not provide targeted groups of prospective students preference in the admissions process, and provided that all students—whether part of a specifically targeted group or not—enjoy the same opportunity to apply and compete for admission.”12
In regard to pathways programs, slots cannot be reserved and preference cannot be given to applicants who participated in these programs if race was a factor in selecting participants.8 Similarly, medical school away electives related to diversity cannot be reserved for those of a specific race or ethnicity; however, these electives can utilize commitment to stated aims and missions of the rotation, such as a commitment to diversity within medicine, as a basis to selecting candidates.8
The ruling did not address how race or ethnicity is factored into financial aid or scholarship determination. There has been concern in higher education that the legal framework utilized in the SCOTUS decision could affect financial aid and scholarship decisions; therefore, many institutions are proceeding with caution in their approach.8
Effect on Residency Selection
Because the SCOTUS ruling references colleges and universities, not health care employers, it should not affect the residency selection process; however, there is variability in how health care institutions are interpreting the impact of the ruling on residency selection, with some taking a more prescriptive and cautious view on the matter. Additionally, with that said, residency selection is considered an employment practice covered by Title VII of the Civil Rights Act of 1964,13 which already prohibits the consideration of race in hiring decisions.7 Under Title VII, it is unlawful for employers to discriminate against someone because of race, color, religion, sex, or national origin, and it is “unlawful to use policies or practices that seem neutral but have the effect of discriminating against people because of their race, color, religion, sex … or national origin.” Title VII also states that employers cannot “make employment decisions based on stereotypes or assumptions about a person’s abilities, traits, or performance because of their race, color, religion, sex … or national origin.”13
Importantly, Title VII does not imply that employers need to abandon their diversity, equity, or inclusion initiatives, and it does not imply that employers must revoke their mission to improve diversity in the workforce. Title VII does not state that racial information cannot be available. It would be permissible to use racial data to assess recruitment trends, identify inequities, and create programs to eliminate barriers and decrease bias14; for example, if a program identified that, based on their current review system, students who are underrepresented in medicine were disproportionately screened out of the applicant pool or interview group, they may wish to revisit their review process to identify and eliminate possible biases. Programs also may wish to adopt educational programs for reviewers (eg, implicit bias training) or educational content on the potential for bias in commonly used review criteria, such as the US Medical Licensing Examination, clerkship grades, and the Medical Student Performance Evaluation.15 Reviewers can and should consider applications in an individualized and holistic manner in which experiences, traits, skills, and academic metrics are assessed together for compatibility with the values and mission of the training program.16
Future Directions for Dermatology
Beyond the SCOTUS ruling, there have been other shifts in the dermatology residency application process that have affected candidate review. Dermatology programs recently have adopted the use of preference signaling in residency applications. Preliminary data from the Association of American Medical Colleges for the 2024 application cycle indicated that of the 81 programs analyzed, there was a nearly 0% chance of an applicant receiving an interview invitation from a program that they did not signal. The median signal-to-interview conversion rate for the 81 dermatology programs analyzed was 55% for gold signals and 15% for silver signals.17 It can be inferred from these data that programs are using preference signaling as important criteria for consideration of interview invitation. Programs may choose to focus most of their attention on the applicant pool who has signaled them. Because the number and type of signals available is equal among all applicants, we hope that this provides an equitable way for all applicants to garner holistic review from programs that interested them. In addition, there has been a 30% decrease in average applications submitted per dermatology applicant.18 With a substantial decline in applications to dermatology, we hope that reviewers are able to spend more time devoted to comprehensive holistic review.
Although signals are equitable for applicants, their distribution among programs may not be; for example, in a given year, a program might find that all their gold signals came from non–underrepresented in medicine students. We encourage programs to carefully review applicant data to ensure their recruitment process is not inadvertently discriminatory and is in alignment with their goals and mission.
The ruling by the Supreme Court of the United States (SCOTUS) in 20231,2 on the use of race-based criteria in college admissions was met with a range of reactions across the country. Given the implications of this decision on the future makeup of higher education, the downstream effects on medical school admissions, and the possible further impact on graduate medical education programs, we sought to explore the potential impact of the landmark decision from the perspective of dermatology residency program directors and offer insights on this pivotal judgment.
Background on the SCOTUS Ruling
In June 2023, SCOTUS issued its formal decision on 2 court cases brought by the organization Students for Fair Admissions (SFFA) against the University of North Carolina at Chapel Hill1 and Harvard University (Cambridge, Massachusetts)2 that addressed college admissions practices dealing with the use of race as a selection criterion in the application process. The cases alleged that these universities had overly emphasized race in the admissions process and thus were in violation of the Civil Rights Act of 1964 as well as the 14th Amendment.1,2
The SCOTUS justices voted 6 to 3 in favor of the argument presented by the SFFA, determining that the use of race in the college admissions process essentially constituted a form of racial discrimination. The ruling was in contrast to a prior decision in 2003 that centered on law school admissions at the University of Michigan (Ann Arbor, Michigan) in which SCOTUS previously had determined that race could be used as one factor amongst other criteria in the higher education selection process.3 In the 2023 decision siding with SFFA, SCOTUS did acknowledge that it was still acceptable for selection processes to consider “an applicant’s discussion of how race affected his or her life, be it through discrimination, inspiration, or otherwise.”2
Effect on Undergraduate Admissions
Prior to the 2023 ruling, several states had already passed independent laws against the use of affirmative action or race-based selection criteria in the admissions process at public colleges and universities.4 As a result, these institutions would already be conforming to the principles set forth in the SCOTUS ruling and major changes to their undergraduate admissions policies would not be expected; however, a considerable number of colleges and universities—particularly those considered highly selective with applicant acceptance rates that are well below the national average—reported the use of race as a factor in their admissions processes in standardized reporting surveys.5 For these institutions, it is no longer considered acceptable (based on the SCOTUS decision) to use race as a singular factor in admissions or to implement race-conscious decision-making—in which individuals are considered differently based solely on their race—as part of the undergraduate selection process.
In light of these rulings, many institutions have explicitly committed to upholding principles of diversity in their recruitment processes, acknowledging the multifaceted nature of diversity beyond strictly racial terms—including but not limited to socioeconomic diversity, religious diversity, or gender diversity—which is in compliance with the interpretation ruling by the US Department of Education and the US Department of Justice.6 Additionally, select institutions have taken approaches to explicitly include questions on ways in which applicants have overcome obstacles or challenges, allowing an opportunity for individuals who have had such experiences related to race an opportunity to incorporate these elements into their applications. Finally, some institutions have taken a more limited approach, eliminating ways in which race is explicitly addressed in the application and focusing on race-neutral elements of the application in their approach to selection.7
Because the first college admission cycle since the 2023 SCOTUS ruling is still underway, we have yet to witness the full impact of this decision on the current undergraduate admissions landscape.
Effect on Medical School Admissions and Rotations
Although SCOTUS specifically examined the undergraduate admissions process, the ruling on race-conscious admissions also had a profound impact on graduate school admissions including medical school admission processes.1,2,8,9 This is because the language of the majority opinion refers to “university programs” in its ruling, which also has been broadly interpreted to include graduate school programs. As with undergraduate admissions, it has been interpreted by national medical education organizations and institutions that medical schools also cannot consider an applicant’s race or ethnicity as a specific factor in the admissions process.1,2,8,9
Lived individual experiences, including essays that speak to an applicant’s lived experiences and career aspirations related to race, still can be taken into account. In particular, holistic review still can be utilized to evaluate medical school candidates and may play a more integral role in the medical school admissions process now than in the past.8,10,11 After the ruling, Justice Sonia Sotomayor noted that “today’s decision leaves intact holistic college admissions and recruitment efforts that seek to enroll diverse classes without using racial classifications.”1
The ruling asserted that universities may define their mission as they see fit. As a result, the ruling did not affect medical school missions or strategic plans, including those that may aim to diversify the health care workforce.8,10,11 The ruling also did not affect the ability to utilize pathway programs to encourage a career in medicine or recruitment relationships with diverse undergraduate or community-based organizations. Student interest groups also can be involved in the relationship-building or recruitment activities for medical schools.8,10,11 Guidance from the US Department of Education and US Department of Justice noted that institutions may consider race in identifying prospective applicants through recruitment and outreach, “provided that their outreach and recruitment programs do not provide targeted groups of prospective students preference in the admissions process, and provided that all students—whether part of a specifically targeted group or not—enjoy the same opportunity to apply and compete for admission.”12
In regard to pathways programs, slots cannot be reserved and preference cannot be given to applicants who participated in these programs if race was a factor in selecting participants.8 Similarly, medical school away electives related to diversity cannot be reserved for those of a specific race or ethnicity; however, these electives can utilize commitment to stated aims and missions of the rotation, such as a commitment to diversity within medicine, as a basis to selecting candidates.8
The ruling did not address how race or ethnicity is factored into financial aid or scholarship determination. There has been concern in higher education that the legal framework utilized in the SCOTUS decision could affect financial aid and scholarship decisions; therefore, many institutions are proceeding with caution in their approach.8
Effect on Residency Selection
Because the SCOTUS ruling references colleges and universities, not health care employers, it should not affect the residency selection process; however, there is variability in how health care institutions are interpreting the impact of the ruling on residency selection, with some taking a more prescriptive and cautious view on the matter. Additionally, with that said, residency selection is considered an employment practice covered by Title VII of the Civil Rights Act of 1964,13 which already prohibits the consideration of race in hiring decisions.7 Under Title VII, it is unlawful for employers to discriminate against someone because of race, color, religion, sex, or national origin, and it is “unlawful to use policies or practices that seem neutral but have the effect of discriminating against people because of their race, color, religion, sex … or national origin.” Title VII also states that employers cannot “make employment decisions based on stereotypes or assumptions about a person’s abilities, traits, or performance because of their race, color, religion, sex … or national origin.”13
Importantly, Title VII does not imply that employers need to abandon their diversity, equity, or inclusion initiatives, and it does not imply that employers must revoke their mission to improve diversity in the workforce. Title VII does not state that racial information cannot be available. It would be permissible to use racial data to assess recruitment trends, identify inequities, and create programs to eliminate barriers and decrease bias14; for example, if a program identified that, based on their current review system, students who are underrepresented in medicine were disproportionately screened out of the applicant pool or interview group, they may wish to revisit their review process to identify and eliminate possible biases. Programs also may wish to adopt educational programs for reviewers (eg, implicit bias training) or educational content on the potential for bias in commonly used review criteria, such as the US Medical Licensing Examination, clerkship grades, and the Medical Student Performance Evaluation.15 Reviewers can and should consider applications in an individualized and holistic manner in which experiences, traits, skills, and academic metrics are assessed together for compatibility with the values and mission of the training program.16
Future Directions for Dermatology
Beyond the SCOTUS ruling, there have been other shifts in the dermatology residency application process that have affected candidate review. Dermatology programs recently have adopted the use of preference signaling in residency applications. Preliminary data from the Association of American Medical Colleges for the 2024 application cycle indicated that of the 81 programs analyzed, there was a nearly 0% chance of an applicant receiving an interview invitation from a program that they did not signal. The median signal-to-interview conversion rate for the 81 dermatology programs analyzed was 55% for gold signals and 15% for silver signals.17 It can be inferred from these data that programs are using preference signaling as important criteria for consideration of interview invitation. Programs may choose to focus most of their attention on the applicant pool who has signaled them. Because the number and type of signals available is equal among all applicants, we hope that this provides an equitable way for all applicants to garner holistic review from programs that interested them. In addition, there has been a 30% decrease in average applications submitted per dermatology applicant.18 With a substantial decline in applications to dermatology, we hope that reviewers are able to spend more time devoted to comprehensive holistic review.
Although signals are equitable for applicants, their distribution among programs may not be; for example, in a given year, a program might find that all their gold signals came from non–underrepresented in medicine students. We encourage programs to carefully review applicant data to ensure their recruitment process is not inadvertently discriminatory and is in alignment with their goals and mission.
- Students for Fair Admissions, Inc. v University of North Carolina, 567 F. Supp. 3d 580 (M.D.N.C. 2021).
- Students for Fair Admissions, Inc. v President and Fellows of Harvard College, 600 US ___ (2023).
- Grutter v Bollinger, 539 US 306 (2003).
- Saul S. 9 states have banned affirmative action. here’s what that looks like. The New York Times. October 31, 2022. https://www.nytimes.com/2022/10/31/us/politics/affirmative-action-ban-states.html
- Desilver D. Private, selective colleges are most likely to use race, ethnicity as a factor in admissions decisions. Pew Research Center. July 14, 2023. Accessed May 29, 2024. https://www.pewresearch.org/short-reads/2023/07/14/private-selective-colleges-are-most-likely-to-use-race-ethnicity-as-a-factor-in-admissions-decisions/
- US Department of Education. Justice and education departments release resources to advance diversity and opportunity in higher education. August 14, 2023. Accessed May 17, 2024. https://www.ed.gov/news/press-releases/advance-diversity-and-opportunity-higher-education-justice-and-education-departments-release-resources-advance-diversity-and-opportunity-higher-education
- Amponsah MN, Hamid RD. Harvard overhauls college application in wake of affirmative action decision. The Harvard Crimson. August 3, 2023. Accessed May 17, 2024. https://www.thecrimson.com/article/2023/8/3/harvard-admission-essay-change/
- Association of American Medical Colleges. Frequently asked questions: what does the Harvard and UNC decision mean for medical education? August 24, 2023. Accessed May 17, 2024. https://www.aamc.org/media/68771/download?attachment%3Fattachment
- American Medical Association. Affirmative action ends: how Supreme Court ruling impacts medical schools & the health care workforce. July 7, 2023. Accessed May 17, 2024. https://www.ama-assn.org/medical-students/medical-school-life/affirmative-action-ends-how-supreme-court-ruling-impacts
- Association of American Medical Colleges. How can medical schools boost racial diversity in the wake of the recent Supreme Court ruling? July 27, 2023. Accessed May 17, 2024. https://www.aamc.org/news/how-can-medical-schools-boost-racial-diversity-wake-recent-supreme-court-ruling
- Association of American Medical Colleges. Diversity in medical school admissions. Updated March 18, 2024. Accessed May 17, 2024. https://www.aamc.org/about-us/mission-areas/medical-education/diversity-medical-school-admissions
- United States Department of Justice. Questions and answers regarding the Supreme Court’s decision in Students For Fair Admissions, Inc. v. Harvard College and University of North Carolina. August 14, 2023. Accessed May 29, 2024. https://www.justice.gov/d9/2023-08/post-sffa_resource_faq_final_508.pdf
- US Department of Justice. Title VII of the Civil Rights Act of 1964. Accessed May 17, 2024. https://www.justice.gov/crt/laws-we-enforce
- Zheng L. How to effectively—and legally—use racial data for DEI. Harvard Business Review. July 24, 2023. Accessed May 17, 2024. https://hbr.org/2023/07/how-to-effectively-and-legally-use-racial-data-for-dei
- Crites K, Johnson J, Scott N, et al. Increasing diversity in residency training programs. Cureus. 2022;14:E25962. doi:10.7759/cureus.25962
- Association of American Medical Colleges. Holistic principles in resident selection: an introduction. Accessed May 17, 2024. https://www.aamc.org/media/44586/download?attachment
- Association of American Medical Colleges. Exploring the relationship between program signaling & interview invitations across specialties 2024 ERAS® preliminary analysis. December 29, 2023. Accessed May 17, 2024. https://www.aamc.org/media/74811/download?attachment
- Association of American Medical Colleges. Preliminary program signaling data and their impact on residency selection. October 24, 2023. Accessed May 17, 2024. https://www.aamc.org/services/eras-institutions/program-signaling-data#:~:text=Preliminary%20Program%20Signaling%20Data%20and%20Their%20Impact%20on%20Residency%20Selection,-Oct.&text=Program%20signals%20are%20a%20mechanism,whom%20to%20invite%20for%20interview
- Students for Fair Admissions, Inc. v University of North Carolina, 567 F. Supp. 3d 580 (M.D.N.C. 2021).
- Students for Fair Admissions, Inc. v President and Fellows of Harvard College, 600 US ___ (2023).
- Grutter v Bollinger, 539 US 306 (2003).
- Saul S. 9 states have banned affirmative action. here’s what that looks like. The New York Times. October 31, 2022. https://www.nytimes.com/2022/10/31/us/politics/affirmative-action-ban-states.html
- Desilver D. Private, selective colleges are most likely to use race, ethnicity as a factor in admissions decisions. Pew Research Center. July 14, 2023. Accessed May 29, 2024. https://www.pewresearch.org/short-reads/2023/07/14/private-selective-colleges-are-most-likely-to-use-race-ethnicity-as-a-factor-in-admissions-decisions/
- US Department of Education. Justice and education departments release resources to advance diversity and opportunity in higher education. August 14, 2023. Accessed May 17, 2024. https://www.ed.gov/news/press-releases/advance-diversity-and-opportunity-higher-education-justice-and-education-departments-release-resources-advance-diversity-and-opportunity-higher-education
- Amponsah MN, Hamid RD. Harvard overhauls college application in wake of affirmative action decision. The Harvard Crimson. August 3, 2023. Accessed May 17, 2024. https://www.thecrimson.com/article/2023/8/3/harvard-admission-essay-change/
- Association of American Medical Colleges. Frequently asked questions: what does the Harvard and UNC decision mean for medical education? August 24, 2023. Accessed May 17, 2024. https://www.aamc.org/media/68771/download?attachment%3Fattachment
- American Medical Association. Affirmative action ends: how Supreme Court ruling impacts medical schools & the health care workforce. July 7, 2023. Accessed May 17, 2024. https://www.ama-assn.org/medical-students/medical-school-life/affirmative-action-ends-how-supreme-court-ruling-impacts
- Association of American Medical Colleges. How can medical schools boost racial diversity in the wake of the recent Supreme Court ruling? July 27, 2023. Accessed May 17, 2024. https://www.aamc.org/news/how-can-medical-schools-boost-racial-diversity-wake-recent-supreme-court-ruling
- Association of American Medical Colleges. Diversity in medical school admissions. Updated March 18, 2024. Accessed May 17, 2024. https://www.aamc.org/about-us/mission-areas/medical-education/diversity-medical-school-admissions
- United States Department of Justice. Questions and answers regarding the Supreme Court’s decision in Students For Fair Admissions, Inc. v. Harvard College and University of North Carolina. August 14, 2023. Accessed May 29, 2024. https://www.justice.gov/d9/2023-08/post-sffa_resource_faq_final_508.pdf
- US Department of Justice. Title VII of the Civil Rights Act of 1964. Accessed May 17, 2024. https://www.justice.gov/crt/laws-we-enforce
- Zheng L. How to effectively—and legally—use racial data for DEI. Harvard Business Review. July 24, 2023. Accessed May 17, 2024. https://hbr.org/2023/07/how-to-effectively-and-legally-use-racial-data-for-dei
- Crites K, Johnson J, Scott N, et al. Increasing diversity in residency training programs. Cureus. 2022;14:E25962. doi:10.7759/cureus.25962
- Association of American Medical Colleges. Holistic principles in resident selection: an introduction. Accessed May 17, 2024. https://www.aamc.org/media/44586/download?attachment
- Association of American Medical Colleges. Exploring the relationship between program signaling & interview invitations across specialties 2024 ERAS® preliminary analysis. December 29, 2023. Accessed May 17, 2024. https://www.aamc.org/media/74811/download?attachment
- Association of American Medical Colleges. Preliminary program signaling data and their impact on residency selection. October 24, 2023. Accessed May 17, 2024. https://www.aamc.org/services/eras-institutions/program-signaling-data#:~:text=Preliminary%20Program%20Signaling%20Data%20and%20Their%20Impact%20on%20Residency%20Selection,-Oct.&text=Program%20signals%20are%20a%20mechanism,whom%20to%20invite%20for%20interview
Practice Points
- The 2023 ruling by the Supreme Court of the United States on the use of race-based criteria in college admissions may have implications for the selection of individuals into the dermatology workforce.
- We highlight the impacts of these decisions at the college, medical school, and dermatology residency levels and provide context for future directions in the selection processes for practicing dermatologists.
Understanding the Evaluation and Management Add-on Complexity Code
On January 1, 2024, a new add-on complexity code, G2211, was implemented to the documentation of evaluation and management (E/M) visits.1 Created by the Centers for Medicare & Medicaid Services (CMS), G2211 is defined as “visit complexity inherent to evaluation and management associated with medical care services that serve as the continuing focal point for all needed health care services and/or with medical care services that are part of ongoing care related to a patient’s single, serious, or complex condition.”2 It is an add-on code, meaning that it must be listed with either a new or established outpatient E/M visit.
G2211 originally was introduced in the 2021 Proposed Rule but was delayed via a congressional mandate for 3 years.1 It originally was estimated that this code would be billed with 90% of all office visit claims, accounting for an approximately $3.3 billion increase in physician fee schedule spending; however, this estimate was revised with its reintroduction in the 2024 Final Rule, and it currently is estimated that it will be billed with 38% of all office visit claims.3,4
This add-on code was created to capture the inherent complexity of an E/M visit that is derived from the longitudinal nature of the physician-patient relationship and to better account for the additional resources of these outpatient E/M visits.5 Although these criteria often are met in the setting of an E/M visit within a primary care specialty (eg, family practice, internal medicine, obstetrics/gynecology, pediatrics), this code is not restricted to medical professionals based on specialties. The CMS noted that “the most important information used to determine whether the add-on code could be billed is the relationship between the practitioner and the patient,” specifically if they are fulfilling one of the following roles: “the continuing focal point for all needed health care services” or “ongoing care related to a patient's single, serious and complex condition.”6
Of note, further definitions regarding what constitutes a single, serious or complex condition have not yet been provided by CMS. The code should not be utilized when the relationship with the patient is of a discrete, routine, or time-limited nature. The resulting care should be personalized and should result in a comprehensive, longitudinal, and continuous relationship with the patient and should involve delivery of team-based care that is accessible, coordinated with other practitioners and providers, and integrated with the broader health care landscape.6
Herein, 5 examples are provided of scenarios when G2211 might be utilized as well as when it would not be appropriate to bill for this code.
Example 1
A 48-year-old man (an established patient) with a history of psoriasis and psoriatic arthritis presents to a dermatologist for follow-up. The dermatologist has been managing both conditions for 3 years with methotrexate. The patient’s disease is well controlled at the current visit, and he presents for follow-up of disease activity and laboratory monitoring every 3 months. The dermatologist continues the patient on methotrexate after reviewing the risks, benefits, and adverse effects and orders a complete blood cell count and comprehensive metabolic panel.
Would use of G2211 be appropriate for this visit?—Yes, in this case it would be appropriate to bill for G2211. In this example, the physician is providing longitudinal ongoing medical care related to a patient’s single, serious or complex condition—specifically psoriasis and psoriatic arthritis—via managing methotrexate therapy.
Example 2
Let’s alter the previous example slightly: A 48-year-old man (an established patient) with a history of psoriasis and psoriatic arthritis presents to a dermatologist for follow-up. He is being followed by both a dermatologist and a rheumatologist. The patient is on methotrexate, which was prescribed by the rheumatologist, who also conducts the appropriate laboratory monitoring. The patient’s skin disease currently is well controlled, and the dermatologist discusses this with the patient and advises that he continue to follow up with rheumatology.
Would use of G2211 be appropriate for this visit?—No, in this case it would not be appropriate to utilize G2211. In this example, the dermatologist is providing longitudinal ongoing medical care; however, unlike in the first example, much of the ongoing medical care—in particular the management of the patient’s methotrexate therapy—is being performed by the rheumatologist. Therefore, although these conditions are serious or complex, the dermatologist is not the primary manager of treatment, and it would not be appropriate to bill for G2211.
Example 3
A 35-year-old woman (an established patient) presents to a dermatologist for follow-up of hidradenitis suppurativa. She currently is receiving infliximab infusions that are managed by the dermatologist. At the current presentation, physical examination reveals several persistent active lesions. After discussing possible treatment options, the dermatologist elects to continue infliximab therapy and schedule a deroofing procedure of the persistent areas.
Would use of G2211 be appropriate for this visit?—Yes, in this example it would be appropriate to utilize G2211. The patient has hidradenitis suppurativa, which would be considered a single, serious or complex condition. Additionally, the dermatologist is the primary manager of this condition by prescribing infliximab as well as counseling the patient on the appropriateness of procedural interventions and scheduling for these procedures; the dermatologist also is providing ongoing longitudinal care.
Example 4
Let’s alter the previous example slightly: A 35-year-old woman (an established patient) presents to a dermatologist for follow-up of hidradenitis suppurativa. She currently is receiving infliximab infusions, which are managed by the dermatologist. At the current presentation, physical examination reveals several persistent active lesions. After discussing possible treatment options, the dermatologist elects to perform intralesional triamcinolone injections to active areas during the current visit.
Would use of G2211 be appropriate for this visit?—No, in this case it would not be appropriate to bill for G2211. Similar to Example 3, the dermatologist is treating a single, serious and complex condition and is primarily managing the disease and providing longitudinal care; however, in this case the dermatologist also is performing a minor procedure during the visit: injection of intralesional triamcinolone.
Importantly, G2211 cannot be utilized when modifier -25 is being appended to an outpatient E/M visit. Modifier -25 is defined as a “significant, separately identifiable evaluation and management service by the same physician or other qualified health care professional on the same day of the procedure or other service.”7 Modifier -25 is utilized when a minor procedure is performed by a qualified health care professional on the same day (generally during the same visit) as an E/M visit. Therefore, G2211 cannot be utilized when a minor procedure (eg, a tangential biopsy, punch biopsy, destruction or intralesional injection into skin) is performed during a visit.
Example 5
A 6-year-old girl presents to a dermatologist for a new rash on the trunk that started 5 days after an upper respiratory infection. The dermatologist evaluates the patient and identifies a blanchable macular eruption on the trunk; the patient is diagnosed with a viral exanthem. Because the patient reported associated pruritus, topical triamcinolone is prescribed.
Would use of G2211 be appropriate for this visit?—No, in this case it would not be appropriate to bill for G2211. A viral exanthem would not be considered an ongoing single, serious or complex condition and would be more consistent with a discrete condition; therefore, even though the dermatologist is primarily managing the disease process, it still would not fulfill the criteria necessary to bill for G2211.
Final Thoughts
G2211 is an add-on code created by the CMS that can be utilized in conjunction with an outpatient E/M visit when certain requirements are fulfilled. Specifically, this code can be utilized when the dermatologist is the primary provider of care for a patient’s ongoing single, serious or complex condition or serves as the continuing focal point for all of the patient’s health care needs. Understanding the nuances associated with this code are critical for correct billing.
- Centers for Medicare & Medicaid Services. Calendar Year (CY) 2024 Medicare physician fee schedule final rule. Published November 2, 2023. Accessed April 15, 2024. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2024-medicare-physician-fee-schedule-final-rule
- Centers for Medicare & Medicaid Services. Fact Sheet—Physician Fee Schedule (PFS) payment for office/outpatient evaluation and management (E/M) visits. Published January 11, 2021. Accessed April 15, 2024. https://www.cms.gov/files/document/physician-fee-schedule-pfs-payment-officeoutpatient-evaluation-and-management-em-visits-fact-sheet.pdf
- American Society of Anesthesiologists. Broken Medicare system results in CMS proposing reduced physician payments in 2024. Published July 13, 2023. Accessed April 15, 2024. https://www.asahq.org/advocacy-and-asapac/fda-and-washington-alerts/washington-alerts/2023/07/broken-medicare-system-results-in-cms-proposing-reduced-physician-payments-in-2024
- American Medical Association. CY 2024 Medicare physician payment schedule and quality payment program (QPP) final rule summary. Accessed April 15, 2024. https://www.ama-assn.org/system/files/ama-summary-2024-mfs-proposed-rule.pdf
- Centers for Medicare & Medicaid Services. How to use the office & outpatient evaluation and management visit complexity add-on code G2211. MM13473. MLN Matters. Updated January 18, 2024. Accessed April 15, 2024. https://www.cms.gov/files/document/mm13473-how-use-office-and-outpatient-evaluation-and-management-visit-complexity-add-code-g2211.pdf
- Centers for Medicare & Medicaid Services. CMS manual system. Published January 18, 2024. Accessed April 15, 2024. https://www.cms.gov/files/document/r12461cp.pdf
- American Medical Association. Reporting CPT Modifier 25. CPT Assistant (Online). 2023;33:1-12. Accessed April 15, 2024. https://www.ama-assn.org/system/files/reporting-CPT-modifier-25.pdf
On January 1, 2024, a new add-on complexity code, G2211, was implemented to the documentation of evaluation and management (E/M) visits.1 Created by the Centers for Medicare & Medicaid Services (CMS), G2211 is defined as “visit complexity inherent to evaluation and management associated with medical care services that serve as the continuing focal point for all needed health care services and/or with medical care services that are part of ongoing care related to a patient’s single, serious, or complex condition.”2 It is an add-on code, meaning that it must be listed with either a new or established outpatient E/M visit.
G2211 originally was introduced in the 2021 Proposed Rule but was delayed via a congressional mandate for 3 years.1 It originally was estimated that this code would be billed with 90% of all office visit claims, accounting for an approximately $3.3 billion increase in physician fee schedule spending; however, this estimate was revised with its reintroduction in the 2024 Final Rule, and it currently is estimated that it will be billed with 38% of all office visit claims.3,4
This add-on code was created to capture the inherent complexity of an E/M visit that is derived from the longitudinal nature of the physician-patient relationship and to better account for the additional resources of these outpatient E/M visits.5 Although these criteria often are met in the setting of an E/M visit within a primary care specialty (eg, family practice, internal medicine, obstetrics/gynecology, pediatrics), this code is not restricted to medical professionals based on specialties. The CMS noted that “the most important information used to determine whether the add-on code could be billed is the relationship between the practitioner and the patient,” specifically if they are fulfilling one of the following roles: “the continuing focal point for all needed health care services” or “ongoing care related to a patient's single, serious and complex condition.”6
Of note, further definitions regarding what constitutes a single, serious or complex condition have not yet been provided by CMS. The code should not be utilized when the relationship with the patient is of a discrete, routine, or time-limited nature. The resulting care should be personalized and should result in a comprehensive, longitudinal, and continuous relationship with the patient and should involve delivery of team-based care that is accessible, coordinated with other practitioners and providers, and integrated with the broader health care landscape.6
Herein, 5 examples are provided of scenarios when G2211 might be utilized as well as when it would not be appropriate to bill for this code.
Example 1
A 48-year-old man (an established patient) with a history of psoriasis and psoriatic arthritis presents to a dermatologist for follow-up. The dermatologist has been managing both conditions for 3 years with methotrexate. The patient’s disease is well controlled at the current visit, and he presents for follow-up of disease activity and laboratory monitoring every 3 months. The dermatologist continues the patient on methotrexate after reviewing the risks, benefits, and adverse effects and orders a complete blood cell count and comprehensive metabolic panel.
Would use of G2211 be appropriate for this visit?—Yes, in this case it would be appropriate to bill for G2211. In this example, the physician is providing longitudinal ongoing medical care related to a patient’s single, serious or complex condition—specifically psoriasis and psoriatic arthritis—via managing methotrexate therapy.
Example 2
Let’s alter the previous example slightly: A 48-year-old man (an established patient) with a history of psoriasis and psoriatic arthritis presents to a dermatologist for follow-up. He is being followed by both a dermatologist and a rheumatologist. The patient is on methotrexate, which was prescribed by the rheumatologist, who also conducts the appropriate laboratory monitoring. The patient’s skin disease currently is well controlled, and the dermatologist discusses this with the patient and advises that he continue to follow up with rheumatology.
Would use of G2211 be appropriate for this visit?—No, in this case it would not be appropriate to utilize G2211. In this example, the dermatologist is providing longitudinal ongoing medical care; however, unlike in the first example, much of the ongoing medical care—in particular the management of the patient’s methotrexate therapy—is being performed by the rheumatologist. Therefore, although these conditions are serious or complex, the dermatologist is not the primary manager of treatment, and it would not be appropriate to bill for G2211.
Example 3
A 35-year-old woman (an established patient) presents to a dermatologist for follow-up of hidradenitis suppurativa. She currently is receiving infliximab infusions that are managed by the dermatologist. At the current presentation, physical examination reveals several persistent active lesions. After discussing possible treatment options, the dermatologist elects to continue infliximab therapy and schedule a deroofing procedure of the persistent areas.
Would use of G2211 be appropriate for this visit?—Yes, in this example it would be appropriate to utilize G2211. The patient has hidradenitis suppurativa, which would be considered a single, serious or complex condition. Additionally, the dermatologist is the primary manager of this condition by prescribing infliximab as well as counseling the patient on the appropriateness of procedural interventions and scheduling for these procedures; the dermatologist also is providing ongoing longitudinal care.
Example 4
Let’s alter the previous example slightly: A 35-year-old woman (an established patient) presents to a dermatologist for follow-up of hidradenitis suppurativa. She currently is receiving infliximab infusions, which are managed by the dermatologist. At the current presentation, physical examination reveals several persistent active lesions. After discussing possible treatment options, the dermatologist elects to perform intralesional triamcinolone injections to active areas during the current visit.
Would use of G2211 be appropriate for this visit?—No, in this case it would not be appropriate to bill for G2211. Similar to Example 3, the dermatologist is treating a single, serious and complex condition and is primarily managing the disease and providing longitudinal care; however, in this case the dermatologist also is performing a minor procedure during the visit: injection of intralesional triamcinolone.
Importantly, G2211 cannot be utilized when modifier -25 is being appended to an outpatient E/M visit. Modifier -25 is defined as a “significant, separately identifiable evaluation and management service by the same physician or other qualified health care professional on the same day of the procedure or other service.”7 Modifier -25 is utilized when a minor procedure is performed by a qualified health care professional on the same day (generally during the same visit) as an E/M visit. Therefore, G2211 cannot be utilized when a minor procedure (eg, a tangential biopsy, punch biopsy, destruction or intralesional injection into skin) is performed during a visit.
Example 5
A 6-year-old girl presents to a dermatologist for a new rash on the trunk that started 5 days after an upper respiratory infection. The dermatologist evaluates the patient and identifies a blanchable macular eruption on the trunk; the patient is diagnosed with a viral exanthem. Because the patient reported associated pruritus, topical triamcinolone is prescribed.
Would use of G2211 be appropriate for this visit?—No, in this case it would not be appropriate to bill for G2211. A viral exanthem would not be considered an ongoing single, serious or complex condition and would be more consistent with a discrete condition; therefore, even though the dermatologist is primarily managing the disease process, it still would not fulfill the criteria necessary to bill for G2211.
Final Thoughts
G2211 is an add-on code created by the CMS that can be utilized in conjunction with an outpatient E/M visit when certain requirements are fulfilled. Specifically, this code can be utilized when the dermatologist is the primary provider of care for a patient’s ongoing single, serious or complex condition or serves as the continuing focal point for all of the patient’s health care needs. Understanding the nuances associated with this code are critical for correct billing.
On January 1, 2024, a new add-on complexity code, G2211, was implemented to the documentation of evaluation and management (E/M) visits.1 Created by the Centers for Medicare & Medicaid Services (CMS), G2211 is defined as “visit complexity inherent to evaluation and management associated with medical care services that serve as the continuing focal point for all needed health care services and/or with medical care services that are part of ongoing care related to a patient’s single, serious, or complex condition.”2 It is an add-on code, meaning that it must be listed with either a new or established outpatient E/M visit.
G2211 originally was introduced in the 2021 Proposed Rule but was delayed via a congressional mandate for 3 years.1 It originally was estimated that this code would be billed with 90% of all office visit claims, accounting for an approximately $3.3 billion increase in physician fee schedule spending; however, this estimate was revised with its reintroduction in the 2024 Final Rule, and it currently is estimated that it will be billed with 38% of all office visit claims.3,4
This add-on code was created to capture the inherent complexity of an E/M visit that is derived from the longitudinal nature of the physician-patient relationship and to better account for the additional resources of these outpatient E/M visits.5 Although these criteria often are met in the setting of an E/M visit within a primary care specialty (eg, family practice, internal medicine, obstetrics/gynecology, pediatrics), this code is not restricted to medical professionals based on specialties. The CMS noted that “the most important information used to determine whether the add-on code could be billed is the relationship between the practitioner and the patient,” specifically if they are fulfilling one of the following roles: “the continuing focal point for all needed health care services” or “ongoing care related to a patient's single, serious and complex condition.”6
Of note, further definitions regarding what constitutes a single, serious or complex condition have not yet been provided by CMS. The code should not be utilized when the relationship with the patient is of a discrete, routine, or time-limited nature. The resulting care should be personalized and should result in a comprehensive, longitudinal, and continuous relationship with the patient and should involve delivery of team-based care that is accessible, coordinated with other practitioners and providers, and integrated with the broader health care landscape.6
Herein, 5 examples are provided of scenarios when G2211 might be utilized as well as when it would not be appropriate to bill for this code.
Example 1
A 48-year-old man (an established patient) with a history of psoriasis and psoriatic arthritis presents to a dermatologist for follow-up. The dermatologist has been managing both conditions for 3 years with methotrexate. The patient’s disease is well controlled at the current visit, and he presents for follow-up of disease activity and laboratory monitoring every 3 months. The dermatologist continues the patient on methotrexate after reviewing the risks, benefits, and adverse effects and orders a complete blood cell count and comprehensive metabolic panel.
Would use of G2211 be appropriate for this visit?—Yes, in this case it would be appropriate to bill for G2211. In this example, the physician is providing longitudinal ongoing medical care related to a patient’s single, serious or complex condition—specifically psoriasis and psoriatic arthritis—via managing methotrexate therapy.
Example 2
Let’s alter the previous example slightly: A 48-year-old man (an established patient) with a history of psoriasis and psoriatic arthritis presents to a dermatologist for follow-up. He is being followed by both a dermatologist and a rheumatologist. The patient is on methotrexate, which was prescribed by the rheumatologist, who also conducts the appropriate laboratory monitoring. The patient’s skin disease currently is well controlled, and the dermatologist discusses this with the patient and advises that he continue to follow up with rheumatology.
Would use of G2211 be appropriate for this visit?—No, in this case it would not be appropriate to utilize G2211. In this example, the dermatologist is providing longitudinal ongoing medical care; however, unlike in the first example, much of the ongoing medical care—in particular the management of the patient’s methotrexate therapy—is being performed by the rheumatologist. Therefore, although these conditions are serious or complex, the dermatologist is not the primary manager of treatment, and it would not be appropriate to bill for G2211.
Example 3
A 35-year-old woman (an established patient) presents to a dermatologist for follow-up of hidradenitis suppurativa. She currently is receiving infliximab infusions that are managed by the dermatologist. At the current presentation, physical examination reveals several persistent active lesions. After discussing possible treatment options, the dermatologist elects to continue infliximab therapy and schedule a deroofing procedure of the persistent areas.
Would use of G2211 be appropriate for this visit?—Yes, in this example it would be appropriate to utilize G2211. The patient has hidradenitis suppurativa, which would be considered a single, serious or complex condition. Additionally, the dermatologist is the primary manager of this condition by prescribing infliximab as well as counseling the patient on the appropriateness of procedural interventions and scheduling for these procedures; the dermatologist also is providing ongoing longitudinal care.
Example 4
Let’s alter the previous example slightly: A 35-year-old woman (an established patient) presents to a dermatologist for follow-up of hidradenitis suppurativa. She currently is receiving infliximab infusions, which are managed by the dermatologist. At the current presentation, physical examination reveals several persistent active lesions. After discussing possible treatment options, the dermatologist elects to perform intralesional triamcinolone injections to active areas during the current visit.
Would use of G2211 be appropriate for this visit?—No, in this case it would not be appropriate to bill for G2211. Similar to Example 3, the dermatologist is treating a single, serious and complex condition and is primarily managing the disease and providing longitudinal care; however, in this case the dermatologist also is performing a minor procedure during the visit: injection of intralesional triamcinolone.
Importantly, G2211 cannot be utilized when modifier -25 is being appended to an outpatient E/M visit. Modifier -25 is defined as a “significant, separately identifiable evaluation and management service by the same physician or other qualified health care professional on the same day of the procedure or other service.”7 Modifier -25 is utilized when a minor procedure is performed by a qualified health care professional on the same day (generally during the same visit) as an E/M visit. Therefore, G2211 cannot be utilized when a minor procedure (eg, a tangential biopsy, punch biopsy, destruction or intralesional injection into skin) is performed during a visit.
Example 5
A 6-year-old girl presents to a dermatologist for a new rash on the trunk that started 5 days after an upper respiratory infection. The dermatologist evaluates the patient and identifies a blanchable macular eruption on the trunk; the patient is diagnosed with a viral exanthem. Because the patient reported associated pruritus, topical triamcinolone is prescribed.
Would use of G2211 be appropriate for this visit?—No, in this case it would not be appropriate to bill for G2211. A viral exanthem would not be considered an ongoing single, serious or complex condition and would be more consistent with a discrete condition; therefore, even though the dermatologist is primarily managing the disease process, it still would not fulfill the criteria necessary to bill for G2211.
Final Thoughts
G2211 is an add-on code created by the CMS that can be utilized in conjunction with an outpatient E/M visit when certain requirements are fulfilled. Specifically, this code can be utilized when the dermatologist is the primary provider of care for a patient’s ongoing single, serious or complex condition or serves as the continuing focal point for all of the patient’s health care needs. Understanding the nuances associated with this code are critical for correct billing.
- Centers for Medicare & Medicaid Services. Calendar Year (CY) 2024 Medicare physician fee schedule final rule. Published November 2, 2023. Accessed April 15, 2024. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2024-medicare-physician-fee-schedule-final-rule
- Centers for Medicare & Medicaid Services. Fact Sheet—Physician Fee Schedule (PFS) payment for office/outpatient evaluation and management (E/M) visits. Published January 11, 2021. Accessed April 15, 2024. https://www.cms.gov/files/document/physician-fee-schedule-pfs-payment-officeoutpatient-evaluation-and-management-em-visits-fact-sheet.pdf
- American Society of Anesthesiologists. Broken Medicare system results in CMS proposing reduced physician payments in 2024. Published July 13, 2023. Accessed April 15, 2024. https://www.asahq.org/advocacy-and-asapac/fda-and-washington-alerts/washington-alerts/2023/07/broken-medicare-system-results-in-cms-proposing-reduced-physician-payments-in-2024
- American Medical Association. CY 2024 Medicare physician payment schedule and quality payment program (QPP) final rule summary. Accessed April 15, 2024. https://www.ama-assn.org/system/files/ama-summary-2024-mfs-proposed-rule.pdf
- Centers for Medicare & Medicaid Services. How to use the office & outpatient evaluation and management visit complexity add-on code G2211. MM13473. MLN Matters. Updated January 18, 2024. Accessed April 15, 2024. https://www.cms.gov/files/document/mm13473-how-use-office-and-outpatient-evaluation-and-management-visit-complexity-add-code-g2211.pdf
- Centers for Medicare & Medicaid Services. CMS manual system. Published January 18, 2024. Accessed April 15, 2024. https://www.cms.gov/files/document/r12461cp.pdf
- American Medical Association. Reporting CPT Modifier 25. CPT Assistant (Online). 2023;33:1-12. Accessed April 15, 2024. https://www.ama-assn.org/system/files/reporting-CPT-modifier-25.pdf
- Centers for Medicare & Medicaid Services. Calendar Year (CY) 2024 Medicare physician fee schedule final rule. Published November 2, 2023. Accessed April 15, 2024. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2024-medicare-physician-fee-schedule-final-rule
- Centers for Medicare & Medicaid Services. Fact Sheet—Physician Fee Schedule (PFS) payment for office/outpatient evaluation and management (E/M) visits. Published January 11, 2021. Accessed April 15, 2024. https://www.cms.gov/files/document/physician-fee-schedule-pfs-payment-officeoutpatient-evaluation-and-management-em-visits-fact-sheet.pdf
- American Society of Anesthesiologists. Broken Medicare system results in CMS proposing reduced physician payments in 2024. Published July 13, 2023. Accessed April 15, 2024. https://www.asahq.org/advocacy-and-asapac/fda-and-washington-alerts/washington-alerts/2023/07/broken-medicare-system-results-in-cms-proposing-reduced-physician-payments-in-2024
- American Medical Association. CY 2024 Medicare physician payment schedule and quality payment program (QPP) final rule summary. Accessed April 15, 2024. https://www.ama-assn.org/system/files/ama-summary-2024-mfs-proposed-rule.pdf
- Centers for Medicare & Medicaid Services. How to use the office & outpatient evaluation and management visit complexity add-on code G2211. MM13473. MLN Matters. Updated January 18, 2024. Accessed April 15, 2024. https://www.cms.gov/files/document/mm13473-how-use-office-and-outpatient-evaluation-and-management-visit-complexity-add-code-g2211.pdf
- Centers for Medicare & Medicaid Services. CMS manual system. Published January 18, 2024. Accessed April 15, 2024. https://www.cms.gov/files/document/r12461cp.pdf
- American Medical Association. Reporting CPT Modifier 25. CPT Assistant (Online). 2023;33:1-12. Accessed April 15, 2024. https://www.ama-assn.org/system/files/reporting-CPT-modifier-25.pdf
PRACTICE POINTS
- The add-on code G2211 went into effect on January 1, 2024, and can be applied to outpatient evaluation and management visits that fulfill certain criteria.
- This code should be utilized when one is serving as the continuing focal point for all of the patient's health care needs or providing ongoing medical care related to a patient’s single, serious or complex condition.
Coding the “Spot Check”: Part 2
When the Current Procedural Terminology (CPT) evaluation and management (E/M) reporting rules changed dramatically in January 2021, “bullet counting” became unnecessary and the coding level became based on either the new medical decision making (MDM) table or time spent on all activities relating to the care of the patient on the day of the encounter. 1
To make your documentation more likely to pass audits, explicitly link parts of your documentation to CPT MDM descriptors. Part 1 of this series discussed how to approach the “spot check,” a commonly encountered chief concern (CC) within dermatology, with 2 scenarios presented.2 The American Medical Association3 and American Academy of Dermatology4 have provided education that focuses on how to report a given vignette, but specific examples of documentation with commentary are uncommon. In part 2, we describe how to best code an encounter that includes a “spot check” with other concerns.
Scenario 3: By the Way, Doc
A 34-year-old presents with a new spot on the left cheek that seems to be growing and changing shape rapidly. You examine the patient and discuss treatment options. The documentation reads as follows:
- CC: New spot on left cheek that seems to be growing and changing shape rapidly.
- History: No family history of skin cancer; concerned about scarring, no blood thinner.
- Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy.
- Impression: Rule out melanoma (undiagnosed new problem with uncertain prognosis).
- Plan: Discuss risks, benefits, and alternatives, including biopsy (decision regarding minor surgery with identified patient or procedure risk factors) vs a noninvasive gene expression profiling (GEP) melanoma rule-out test. (Based on the decision you and the patient make, you also would document which option was chosen, so a biopsy would include your standard documentation, and if the GEP is chosen, you would simply state that this was chosen and performed.)
As you turn to leave the room, the patient says:“By the way, Doc, can you do anything about these
How would it be best to approach this scenario? It depends on which treatment option the patient chooses.
If you performed a noninvasive GEP melanoma rule-out test, the CPT reporting does not change with the addition of the new problem, and only the codes 99204 (new patient office or other outpatient visit) or 99214 (established patient office or other outpatient visit) would be reported. This would be because, with the original documentation, the number and complexity of problems would be an “undiagnosed new problem with uncertain prognosis,” which would be moderate complexity (column 1, level 4). There are no data that are reviewed or analyzed, which would be straightforward (column 2, level 2). For risk, the discussion of the biopsy as a diagnostic choice should include possible scarring, bleeding, pain, and infection, which would be best described as a decision regarding minor surgery with identified patient or procedure risk factors, given the identified patient concerns, making this of moderate complexity (column 3, level 4).1
Importantly, even if the procedure is not chosen as the final treatment plan, the discussion regarding the surgery, including the risks, benefits, and alternatives, can still count toward this category in the MDM table. Therefore, in this scenario, documentation would best fit with CPT code 99204 for a new patient or 99214 for an established patient. The addition of the psoriasis diagnosis would not change the level of service but also should include documentation of the psoriasis as medically necessary.
However, if you perform the biopsy, then the documentation above would only allow reporting the biopsy, as the decision to perform a 0- or 10-day global procedure is “bundled” with the procedure if performed on the same date of service. Therefore, with the addition of the psoriasis diagnosis, you would now use a separate E/M code to report the psoriasis. You must append a modifier −25 to the E/M code to certify that you are dealing with a separate and discrete problem with no overlap in physician work.
Clearly you also have an E/M to report. But what level? Is this chronic? Yes, as CPT clearly defines chronic as “[a] problem with an expected duration of at least one year or until the death of the patient.”1,5
But is this stable progressive or showing side effects of treatment? “‘Stable’ for the purposes of categorizing MDM is defined by the specific treatment goals for an individual patient. A patient who is not at his or her treatment goal is not stable, even if the condition has not changed and there is no short-term threat to life or function,” according to the CPT descriptors. Therefore, in this scenario, the documentation would best fit a chronic illness with exacerbation, progression, or side effects of treatment (column 1, level 4), which is of moderate complexity.1
But what about column 3, where we look at risks of testing and treatment? This would depend on the type of treatment given. If an over-the-counter product such as a tar gel is recommended, this is a low risk (column 3, level 3), which would mean this lower value determines the E/M code to be 99213 or 99203 depending on whether this is an established or new patient, respectively. If we treat with a prescription medication such as a topical corticosteroid, we are providing prescription drug management (column 3, level 4), which is moderate risk, and we would use codes 99204 or 99214, assuming we document appropriately. Again, including the CPT terminology of “not at treatment goal” in your impression and “prescription drug management” in your plan tells an auditor what you are thinking and doing.1,5
The Takeaway—Clearly if a GEP is performed, there is a single CPT code used—99204 or 99214. If the biopsy is performed, there would be a biopsy code and an E/M code with a modifier −25 attached to the latter. For the documentation below, a 99204 or 99214 would be the chosen E/M code:
- CC: (1) New spot on left cheek that seems to be growing and changing shape rapidly; (2) Silvery spots on elbows, knees, and buttocks for which patient desires treatment.
- History: No family history of skin cancer; concerned about scarring, no blood thinner. Mom has psoriasis. Tried petroleum jelly on scaly areas but no better.
- Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy. Silver scaly erythematous plaques on elbows, knees, sacrum.
- Impression: (1) Rule out melanoma (undiagnosed new problem with uncertain prognosis); (2) Psoriasis (chronic disease not at treatment goal).
- Plan: (1) Discuss risks, benefits, and alternatives, including biopsy (decision regarding minor surgery with identified patient or procedure risk factors) vs a noninvasive GEP melanoma rule-out test. Patient wants biopsy. Consent, biopsy via shave technique. Lidocaine hydrochloride 1% with epinephrine 1 cc, prepare and drape, aluminum chloride for hemostasis, ointment and bandage applied, care instructions provided; (2) Discuss options. Calcipotriene cream daily; triamcinolone ointment 0.1% twice a day (prescription drug management). Review bathing, avoiding trauma to site, no picking.
Scenario 4: Here for a Total-Body Screening Examination
Medicare does not cover skin cancer screenings as a primary CC. Being worried or knowing someone with melanoma are not CCs that are covered. However, “spot of concern,” “changing mole,” or ”new growth” would be. Conversely, if the patient has a history of skin cancer, actinic keratoses, or other premalignant lesions, and/or is immunosuppressed or has a high-risk genetic syndrome, the visit may be covered if these factors are documented in the note.6
For the diagnosis, the International Classification of Diseases, Tenth Revision, code Z12.83—“encounter for screening for malignant neoplasm of skin”—is not an appropriate primary billing code. However, D48.5—“neoplasm of behavior of skin”—can be, unless there is a specific diagnosis you are able to make (eg, melanocytic nevus, seborrheic keratosis).6
Let’s look at documentation examples:
- CC: 1-year follow-up on basal cell carcinoma (BCC) excision and concern about a new spot on the nose.
- History: Notice new spot on the nose; due for annual follow-up and came early for nose lesion.
- Examination: Left ala with flesh-colored papule dermoscopically banal. Prior left back BCC excision site soft and supple. Total-body examination performed, except perianal and external genitalia, and is unremarkable.
- Impression: Fibrous papule of nose and prior BCC treatment site with no sign of recurrence.
- Plan: Reassure. Annual surveillance in 1 year.
Using what we have previously discussed, this would likely be considered CPT code 99212 (established patient office visit). However, it is important to ensure all concerns and treatment interventions are fully documented. Consider this fuller documentation with bolded additions:
- CC: 1-year follow-up on BCC excision and concern about a new spot on the nose.
- History: Notice new spot on the nose; due for annual follow-up and came early for nose lesion. Also unhappy with generally looking older.
- Examination: Left ala with flesh-colored papule dermoscopically banal. Prior left back BCC excision site soft and supple. Diffuse changes of chronic sun damage. Total-body examination performed, except perianal and external genitalia, and is unremarkable.
- Impression: Fibrous papule of nose and prior BCC treatment site with no sign of recurrence and heliodermatosis/chronic sun damage not at treat-ment goal.
- Plan: Reassure. Annual surveillance in 1 year. Over-the-counter broad-spectrum sun protection factor 30+ sunscreen daily.
This is better but still possibly confusing to an auditor. Consider instead with bolded additions to the changes to the impression:
- CC: 1-year follow-up on BCC excision and concern about a new spot on the nose.
- History: Notice new spot on the nose; due for annual follow-up and came early for nose lesion. Also unhappy with generally looking older.
- Examination: Left ala with flesh-colored papule dermoscopically banal. Prior left back BCC excision site soft and supple. Diffuse changes of chronic sun damage. Total-body examination performed, except perianal and external genitalia, and is unremarkable.
- Impression: Fibrous papule of nose (D22.39)7 and prior BCC treatment site with no sign of recurrence (Z85.828: “personal history of other malignant neoplasm of skin) and heliodermatosis/chronic sun damage not at treatment goal (L57.8: “other skin changes due to chronic exposure to nonionizing radiation”).
- Plan: Reassure. Annual surveillance 1 year. Over-the-counter broad-spectrum sun protection factor 30+ sunscreen daily.
We now have chronic heliodermatitis not at treatment goal, which is moderate (column 1, level 4), and the over-the-counter broad-spectrum sun protection factor 30+ sunscreen (column 1, low) would be best coded as CPT code 99213.
Final Thoughts
“Spot check” encounters are common dermatologic visits, both on their own and in combination with other concerns. With the updated E/M guidelines, it is crucial to clarify and streamline your documentation. In particular, utilize language clearly defining the number and complexity of problems, data to be reviewed and/or analyzed, and appropriate risk stratification to ensure appropriate reimbursement and minimize your difficulties with audits.
- American Medical Association. CPT evaluation and management (E/M) code and guideline changes; 2023. Accessed May 15, 2023. https://www.ama-assn.org/system/files/2023-e-m-descriptors-guidelines.pdf
- Flamm A, Siegel DM. Coding the “spot check”: part 1. Cutis. 2023;111:224-226. doi:10.12788/cutis.0762
- American Medical Association. Evaluation and management (E/M) coding. Accessed May 15, 2023. https://www.ama-assn.org/topics/evaluation-and-management-em-coding
- American Academy of Dermatology Association. Coding resource center. Accessed May 15, 2023. https://www.aad.org/member/practice/coding
- American Medical Association. CPT Professional Edition 2023. American Medical Association; 2022.
- Elizey Coding Solutions, Inc. Dermatology preventive/screening exam visit caution. Updated September 18, 2016. Accessed May 2, 2023. https://www.ellzeycodingsolutions.com/kb_results.asp?ID=9
- 2023 ICD-10-CM diagnosis code D22.39: melanocytic nevi of other parts of the face. Accessed May 2, 2023. https://www.icd10data.com/ICD10CM/Codes/C00-D49/D10-D36/D22-/D22.39
When the Current Procedural Terminology (CPT) evaluation and management (E/M) reporting rules changed dramatically in January 2021, “bullet counting” became unnecessary and the coding level became based on either the new medical decision making (MDM) table or time spent on all activities relating to the care of the patient on the day of the encounter. 1
To make your documentation more likely to pass audits, explicitly link parts of your documentation to CPT MDM descriptors. Part 1 of this series discussed how to approach the “spot check,” a commonly encountered chief concern (CC) within dermatology, with 2 scenarios presented.2 The American Medical Association3 and American Academy of Dermatology4 have provided education that focuses on how to report a given vignette, but specific examples of documentation with commentary are uncommon. In part 2, we describe how to best code an encounter that includes a “spot check” with other concerns.
Scenario 3: By the Way, Doc
A 34-year-old presents with a new spot on the left cheek that seems to be growing and changing shape rapidly. You examine the patient and discuss treatment options. The documentation reads as follows:
- CC: New spot on left cheek that seems to be growing and changing shape rapidly.
- History: No family history of skin cancer; concerned about scarring, no blood thinner.
- Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy.
- Impression: Rule out melanoma (undiagnosed new problem with uncertain prognosis).
- Plan: Discuss risks, benefits, and alternatives, including biopsy (decision regarding minor surgery with identified patient or procedure risk factors) vs a noninvasive gene expression profiling (GEP) melanoma rule-out test. (Based on the decision you and the patient make, you also would document which option was chosen, so a biopsy would include your standard documentation, and if the GEP is chosen, you would simply state that this was chosen and performed.)
As you turn to leave the room, the patient says:“By the way, Doc, can you do anything about these
How would it be best to approach this scenario? It depends on which treatment option the patient chooses.
If you performed a noninvasive GEP melanoma rule-out test, the CPT reporting does not change with the addition of the new problem, and only the codes 99204 (new patient office or other outpatient visit) or 99214 (established patient office or other outpatient visit) would be reported. This would be because, with the original documentation, the number and complexity of problems would be an “undiagnosed new problem with uncertain prognosis,” which would be moderate complexity (column 1, level 4). There are no data that are reviewed or analyzed, which would be straightforward (column 2, level 2). For risk, the discussion of the biopsy as a diagnostic choice should include possible scarring, bleeding, pain, and infection, which would be best described as a decision regarding minor surgery with identified patient or procedure risk factors, given the identified patient concerns, making this of moderate complexity (column 3, level 4).1
Importantly, even if the procedure is not chosen as the final treatment plan, the discussion regarding the surgery, including the risks, benefits, and alternatives, can still count toward this category in the MDM table. Therefore, in this scenario, documentation would best fit with CPT code 99204 for a new patient or 99214 for an established patient. The addition of the psoriasis diagnosis would not change the level of service but also should include documentation of the psoriasis as medically necessary.
However, if you perform the biopsy, then the documentation above would only allow reporting the biopsy, as the decision to perform a 0- or 10-day global procedure is “bundled” with the procedure if performed on the same date of service. Therefore, with the addition of the psoriasis diagnosis, you would now use a separate E/M code to report the psoriasis. You must append a modifier −25 to the E/M code to certify that you are dealing with a separate and discrete problem with no overlap in physician work.
Clearly you also have an E/M to report. But what level? Is this chronic? Yes, as CPT clearly defines chronic as “[a] problem with an expected duration of at least one year or until the death of the patient.”1,5
But is this stable progressive or showing side effects of treatment? “‘Stable’ for the purposes of categorizing MDM is defined by the specific treatment goals for an individual patient. A patient who is not at his or her treatment goal is not stable, even if the condition has not changed and there is no short-term threat to life or function,” according to the CPT descriptors. Therefore, in this scenario, the documentation would best fit a chronic illness with exacerbation, progression, or side effects of treatment (column 1, level 4), which is of moderate complexity.1
But what about column 3, where we look at risks of testing and treatment? This would depend on the type of treatment given. If an over-the-counter product such as a tar gel is recommended, this is a low risk (column 3, level 3), which would mean this lower value determines the E/M code to be 99213 or 99203 depending on whether this is an established or new patient, respectively. If we treat with a prescription medication such as a topical corticosteroid, we are providing prescription drug management (column 3, level 4), which is moderate risk, and we would use codes 99204 or 99214, assuming we document appropriately. Again, including the CPT terminology of “not at treatment goal” in your impression and “prescription drug management” in your plan tells an auditor what you are thinking and doing.1,5
The Takeaway—Clearly if a GEP is performed, there is a single CPT code used—99204 or 99214. If the biopsy is performed, there would be a biopsy code and an E/M code with a modifier −25 attached to the latter. For the documentation below, a 99204 or 99214 would be the chosen E/M code:
- CC: (1) New spot on left cheek that seems to be growing and changing shape rapidly; (2) Silvery spots on elbows, knees, and buttocks for which patient desires treatment.
- History: No family history of skin cancer; concerned about scarring, no blood thinner. Mom has psoriasis. Tried petroleum jelly on scaly areas but no better.
- Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy. Silver scaly erythematous plaques on elbows, knees, sacrum.
- Impression: (1) Rule out melanoma (undiagnosed new problem with uncertain prognosis); (2) Psoriasis (chronic disease not at treatment goal).
- Plan: (1) Discuss risks, benefits, and alternatives, including biopsy (decision regarding minor surgery with identified patient or procedure risk factors) vs a noninvasive GEP melanoma rule-out test. Patient wants biopsy. Consent, biopsy via shave technique. Lidocaine hydrochloride 1% with epinephrine 1 cc, prepare and drape, aluminum chloride for hemostasis, ointment and bandage applied, care instructions provided; (2) Discuss options. Calcipotriene cream daily; triamcinolone ointment 0.1% twice a day (prescription drug management). Review bathing, avoiding trauma to site, no picking.
Scenario 4: Here for a Total-Body Screening Examination
Medicare does not cover skin cancer screenings as a primary CC. Being worried or knowing someone with melanoma are not CCs that are covered. However, “spot of concern,” “changing mole,” or ”new growth” would be. Conversely, if the patient has a history of skin cancer, actinic keratoses, or other premalignant lesions, and/or is immunosuppressed or has a high-risk genetic syndrome, the visit may be covered if these factors are documented in the note.6
For the diagnosis, the International Classification of Diseases, Tenth Revision, code Z12.83—“encounter for screening for malignant neoplasm of skin”—is not an appropriate primary billing code. However, D48.5—“neoplasm of behavior of skin”—can be, unless there is a specific diagnosis you are able to make (eg, melanocytic nevus, seborrheic keratosis).6
Let’s look at documentation examples:
- CC: 1-year follow-up on basal cell carcinoma (BCC) excision and concern about a new spot on the nose.
- History: Notice new spot on the nose; due for annual follow-up and came early for nose lesion.
- Examination: Left ala with flesh-colored papule dermoscopically banal. Prior left back BCC excision site soft and supple. Total-body examination performed, except perianal and external genitalia, and is unremarkable.
- Impression: Fibrous papule of nose and prior BCC treatment site with no sign of recurrence.
- Plan: Reassure. Annual surveillance in 1 year.
Using what we have previously discussed, this would likely be considered CPT code 99212 (established patient office visit). However, it is important to ensure all concerns and treatment interventions are fully documented. Consider this fuller documentation with bolded additions:
- CC: 1-year follow-up on BCC excision and concern about a new spot on the nose.
- History: Notice new spot on the nose; due for annual follow-up and came early for nose lesion. Also unhappy with generally looking older.
- Examination: Left ala with flesh-colored papule dermoscopically banal. Prior left back BCC excision site soft and supple. Diffuse changes of chronic sun damage. Total-body examination performed, except perianal and external genitalia, and is unremarkable.
- Impression: Fibrous papule of nose and prior BCC treatment site with no sign of recurrence and heliodermatosis/chronic sun damage not at treat-ment goal.
- Plan: Reassure. Annual surveillance in 1 year. Over-the-counter broad-spectrum sun protection factor 30+ sunscreen daily.
This is better but still possibly confusing to an auditor. Consider instead with bolded additions to the changes to the impression:
- CC: 1-year follow-up on BCC excision and concern about a new spot on the nose.
- History: Notice new spot on the nose; due for annual follow-up and came early for nose lesion. Also unhappy with generally looking older.
- Examination: Left ala with flesh-colored papule dermoscopically banal. Prior left back BCC excision site soft and supple. Diffuse changes of chronic sun damage. Total-body examination performed, except perianal and external genitalia, and is unremarkable.
- Impression: Fibrous papule of nose (D22.39)7 and prior BCC treatment site with no sign of recurrence (Z85.828: “personal history of other malignant neoplasm of skin) and heliodermatosis/chronic sun damage not at treatment goal (L57.8: “other skin changes due to chronic exposure to nonionizing radiation”).
- Plan: Reassure. Annual surveillance 1 year. Over-the-counter broad-spectrum sun protection factor 30+ sunscreen daily.
We now have chronic heliodermatitis not at treatment goal, which is moderate (column 1, level 4), and the over-the-counter broad-spectrum sun protection factor 30+ sunscreen (column 1, low) would be best coded as CPT code 99213.
Final Thoughts
“Spot check” encounters are common dermatologic visits, both on their own and in combination with other concerns. With the updated E/M guidelines, it is crucial to clarify and streamline your documentation. In particular, utilize language clearly defining the number and complexity of problems, data to be reviewed and/or analyzed, and appropriate risk stratification to ensure appropriate reimbursement and minimize your difficulties with audits.
When the Current Procedural Terminology (CPT) evaluation and management (E/M) reporting rules changed dramatically in January 2021, “bullet counting” became unnecessary and the coding level became based on either the new medical decision making (MDM) table or time spent on all activities relating to the care of the patient on the day of the encounter. 1
To make your documentation more likely to pass audits, explicitly link parts of your documentation to CPT MDM descriptors. Part 1 of this series discussed how to approach the “spot check,” a commonly encountered chief concern (CC) within dermatology, with 2 scenarios presented.2 The American Medical Association3 and American Academy of Dermatology4 have provided education that focuses on how to report a given vignette, but specific examples of documentation with commentary are uncommon. In part 2, we describe how to best code an encounter that includes a “spot check” with other concerns.
Scenario 3: By the Way, Doc
A 34-year-old presents with a new spot on the left cheek that seems to be growing and changing shape rapidly. You examine the patient and discuss treatment options. The documentation reads as follows:
- CC: New spot on left cheek that seems to be growing and changing shape rapidly.
- History: No family history of skin cancer; concerned about scarring, no blood thinner.
- Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy.
- Impression: Rule out melanoma (undiagnosed new problem with uncertain prognosis).
- Plan: Discuss risks, benefits, and alternatives, including biopsy (decision regarding minor surgery with identified patient or procedure risk factors) vs a noninvasive gene expression profiling (GEP) melanoma rule-out test. (Based on the decision you and the patient make, you also would document which option was chosen, so a biopsy would include your standard documentation, and if the GEP is chosen, you would simply state that this was chosen and performed.)
As you turn to leave the room, the patient says:“By the way, Doc, can you do anything about these
How would it be best to approach this scenario? It depends on which treatment option the patient chooses.
If you performed a noninvasive GEP melanoma rule-out test, the CPT reporting does not change with the addition of the new problem, and only the codes 99204 (new patient office or other outpatient visit) or 99214 (established patient office or other outpatient visit) would be reported. This would be because, with the original documentation, the number and complexity of problems would be an “undiagnosed new problem with uncertain prognosis,” which would be moderate complexity (column 1, level 4). There are no data that are reviewed or analyzed, which would be straightforward (column 2, level 2). For risk, the discussion of the biopsy as a diagnostic choice should include possible scarring, bleeding, pain, and infection, which would be best described as a decision regarding minor surgery with identified patient or procedure risk factors, given the identified patient concerns, making this of moderate complexity (column 3, level 4).1
Importantly, even if the procedure is not chosen as the final treatment plan, the discussion regarding the surgery, including the risks, benefits, and alternatives, can still count toward this category in the MDM table. Therefore, in this scenario, documentation would best fit with CPT code 99204 for a new patient or 99214 for an established patient. The addition of the psoriasis diagnosis would not change the level of service but also should include documentation of the psoriasis as medically necessary.
However, if you perform the biopsy, then the documentation above would only allow reporting the biopsy, as the decision to perform a 0- or 10-day global procedure is “bundled” with the procedure if performed on the same date of service. Therefore, with the addition of the psoriasis diagnosis, you would now use a separate E/M code to report the psoriasis. You must append a modifier −25 to the E/M code to certify that you are dealing with a separate and discrete problem with no overlap in physician work.
Clearly you also have an E/M to report. But what level? Is this chronic? Yes, as CPT clearly defines chronic as “[a] problem with an expected duration of at least one year or until the death of the patient.”1,5
But is this stable progressive or showing side effects of treatment? “‘Stable’ for the purposes of categorizing MDM is defined by the specific treatment goals for an individual patient. A patient who is not at his or her treatment goal is not stable, even if the condition has not changed and there is no short-term threat to life or function,” according to the CPT descriptors. Therefore, in this scenario, the documentation would best fit a chronic illness with exacerbation, progression, or side effects of treatment (column 1, level 4), which is of moderate complexity.1
But what about column 3, where we look at risks of testing and treatment? This would depend on the type of treatment given. If an over-the-counter product such as a tar gel is recommended, this is a low risk (column 3, level 3), which would mean this lower value determines the E/M code to be 99213 or 99203 depending on whether this is an established or new patient, respectively. If we treat with a prescription medication such as a topical corticosteroid, we are providing prescription drug management (column 3, level 4), which is moderate risk, and we would use codes 99204 or 99214, assuming we document appropriately. Again, including the CPT terminology of “not at treatment goal” in your impression and “prescription drug management” in your plan tells an auditor what you are thinking and doing.1,5
The Takeaway—Clearly if a GEP is performed, there is a single CPT code used—99204 or 99214. If the biopsy is performed, there would be a biopsy code and an E/M code with a modifier −25 attached to the latter. For the documentation below, a 99204 or 99214 would be the chosen E/M code:
- CC: (1) New spot on left cheek that seems to be growing and changing shape rapidly; (2) Silvery spots on elbows, knees, and buttocks for which patient desires treatment.
- History: No family history of skin cancer; concerned about scarring, no blood thinner. Mom has psoriasis. Tried petroleum jelly on scaly areas but no better.
- Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy. Silver scaly erythematous plaques on elbows, knees, sacrum.
- Impression: (1) Rule out melanoma (undiagnosed new problem with uncertain prognosis); (2) Psoriasis (chronic disease not at treatment goal).
- Plan: (1) Discuss risks, benefits, and alternatives, including biopsy (decision regarding minor surgery with identified patient or procedure risk factors) vs a noninvasive GEP melanoma rule-out test. Patient wants biopsy. Consent, biopsy via shave technique. Lidocaine hydrochloride 1% with epinephrine 1 cc, prepare and drape, aluminum chloride for hemostasis, ointment and bandage applied, care instructions provided; (2) Discuss options. Calcipotriene cream daily; triamcinolone ointment 0.1% twice a day (prescription drug management). Review bathing, avoiding trauma to site, no picking.
Scenario 4: Here for a Total-Body Screening Examination
Medicare does not cover skin cancer screenings as a primary CC. Being worried or knowing someone with melanoma are not CCs that are covered. However, “spot of concern,” “changing mole,” or ”new growth” would be. Conversely, if the patient has a history of skin cancer, actinic keratoses, or other premalignant lesions, and/or is immunosuppressed or has a high-risk genetic syndrome, the visit may be covered if these factors are documented in the note.6
For the diagnosis, the International Classification of Diseases, Tenth Revision, code Z12.83—“encounter for screening for malignant neoplasm of skin”—is not an appropriate primary billing code. However, D48.5—“neoplasm of behavior of skin”—can be, unless there is a specific diagnosis you are able to make (eg, melanocytic nevus, seborrheic keratosis).6
Let’s look at documentation examples:
- CC: 1-year follow-up on basal cell carcinoma (BCC) excision and concern about a new spot on the nose.
- History: Notice new spot on the nose; due for annual follow-up and came early for nose lesion.
- Examination: Left ala with flesh-colored papule dermoscopically banal. Prior left back BCC excision site soft and supple. Total-body examination performed, except perianal and external genitalia, and is unremarkable.
- Impression: Fibrous papule of nose and prior BCC treatment site with no sign of recurrence.
- Plan: Reassure. Annual surveillance in 1 year.
Using what we have previously discussed, this would likely be considered CPT code 99212 (established patient office visit). However, it is important to ensure all concerns and treatment interventions are fully documented. Consider this fuller documentation with bolded additions:
- CC: 1-year follow-up on BCC excision and concern about a new spot on the nose.
- History: Notice new spot on the nose; due for annual follow-up and came early for nose lesion. Also unhappy with generally looking older.
- Examination: Left ala with flesh-colored papule dermoscopically banal. Prior left back BCC excision site soft and supple. Diffuse changes of chronic sun damage. Total-body examination performed, except perianal and external genitalia, and is unremarkable.
- Impression: Fibrous papule of nose and prior BCC treatment site with no sign of recurrence and heliodermatosis/chronic sun damage not at treat-ment goal.
- Plan: Reassure. Annual surveillance in 1 year. Over-the-counter broad-spectrum sun protection factor 30+ sunscreen daily.
This is better but still possibly confusing to an auditor. Consider instead with bolded additions to the changes to the impression:
- CC: 1-year follow-up on BCC excision and concern about a new spot on the nose.
- History: Notice new spot on the nose; due for annual follow-up and came early for nose lesion. Also unhappy with generally looking older.
- Examination: Left ala with flesh-colored papule dermoscopically banal. Prior left back BCC excision site soft and supple. Diffuse changes of chronic sun damage. Total-body examination performed, except perianal and external genitalia, and is unremarkable.
- Impression: Fibrous papule of nose (D22.39)7 and prior BCC treatment site with no sign of recurrence (Z85.828: “personal history of other malignant neoplasm of skin) and heliodermatosis/chronic sun damage not at treatment goal (L57.8: “other skin changes due to chronic exposure to nonionizing radiation”).
- Plan: Reassure. Annual surveillance 1 year. Over-the-counter broad-spectrum sun protection factor 30+ sunscreen daily.
We now have chronic heliodermatitis not at treatment goal, which is moderate (column 1, level 4), and the over-the-counter broad-spectrum sun protection factor 30+ sunscreen (column 1, low) would be best coded as CPT code 99213.
Final Thoughts
“Spot check” encounters are common dermatologic visits, both on their own and in combination with other concerns. With the updated E/M guidelines, it is crucial to clarify and streamline your documentation. In particular, utilize language clearly defining the number and complexity of problems, data to be reviewed and/or analyzed, and appropriate risk stratification to ensure appropriate reimbursement and minimize your difficulties with audits.
- American Medical Association. CPT evaluation and management (E/M) code and guideline changes; 2023. Accessed May 15, 2023. https://www.ama-assn.org/system/files/2023-e-m-descriptors-guidelines.pdf
- Flamm A, Siegel DM. Coding the “spot check”: part 1. Cutis. 2023;111:224-226. doi:10.12788/cutis.0762
- American Medical Association. Evaluation and management (E/M) coding. Accessed May 15, 2023. https://www.ama-assn.org/topics/evaluation-and-management-em-coding
- American Academy of Dermatology Association. Coding resource center. Accessed May 15, 2023. https://www.aad.org/member/practice/coding
- American Medical Association. CPT Professional Edition 2023. American Medical Association; 2022.
- Elizey Coding Solutions, Inc. Dermatology preventive/screening exam visit caution. Updated September 18, 2016. Accessed May 2, 2023. https://www.ellzeycodingsolutions.com/kb_results.asp?ID=9
- 2023 ICD-10-CM diagnosis code D22.39: melanocytic nevi of other parts of the face. Accessed May 2, 2023. https://www.icd10data.com/ICD10CM/Codes/C00-D49/D10-D36/D22-/D22.39
- American Medical Association. CPT evaluation and management (E/M) code and guideline changes; 2023. Accessed May 15, 2023. https://www.ama-assn.org/system/files/2023-e-m-descriptors-guidelines.pdf
- Flamm A, Siegel DM. Coding the “spot check”: part 1. Cutis. 2023;111:224-226. doi:10.12788/cutis.0762
- American Medical Association. Evaluation and management (E/M) coding. Accessed May 15, 2023. https://www.ama-assn.org/topics/evaluation-and-management-em-coding
- American Academy of Dermatology Association. Coding resource center. Accessed May 15, 2023. https://www.aad.org/member/practice/coding
- American Medical Association. CPT Professional Edition 2023. American Medical Association; 2022.
- Elizey Coding Solutions, Inc. Dermatology preventive/screening exam visit caution. Updated September 18, 2016. Accessed May 2, 2023. https://www.ellzeycodingsolutions.com/kb_results.asp?ID=9
- 2023 ICD-10-CM diagnosis code D22.39: melanocytic nevi of other parts of the face. Accessed May 2, 2023. https://www.icd10data.com/ICD10CM/Codes/C00-D49/D10-D36/D22-/D22.39
Practice Points
- Clear documentation that reflects your thought process is an important component of effective coding and billing.
- Include Current Procedural Terminology–defined language within documentation to help ensure appropriate reimbursement and decrease the risk of audits.
Coding the “Spot Check”: Part 1
On January 1, 2021, the Current Procedural Terminology (CPT) evaluation and management (E/M) reporting rules changed dramatically, with “bullet counting” no longer necessary and the coding level now based on either the new medical decision making (MDM) table or time spent on all activities relating to the care of the patient on the day of the encounter.1 This is described in the CPT Professional Edition 2023, a book every practitioner should review annually.2 In particular, every provider should read and reread pages 1 to 14—and beyond if you provide services beyond standard office visits. These changes were made with the intent to simplify the process of documentation and allow a provider to spend more time with patients, though there is still a paucity of data related to whether the new system achieves these aims.
The general rule of reporting work with CPT codes can be simply stated—“Document what you did, do what you documented, and report that which is medically necessary” (David McCafferey, MD, personal communication)—and you should never have any difficulty with audits. Unfortunately, the new system does not let an auditor, who typically lacks a medical degree, audit effectively unless they have a clear understanding of diseases and their stages. Many medical societies, including the American Medical Association3 and American Academy of Dermatology,4 have provided education that focuses on how to report a given vignette, but specific examples of documentation with commentary are uncommon.
To make your documentation more likely to pass audits, explicitly link parts of your documentation to CPT MDM descriptors. We offer scenarios and tips. In part 1 of this series, we discuss how to approach the “spot check,” a commonly encountered chief concern (CC) within dermatology.
A 34-year-old presents with a new spot on the left cheek that seems to be growing and changing shape rapidly. You examine the patient and discuss treatment options. The documentation reads as follows:
• CC: New spot on left cheek that seems to be growing and changing shape rapidly
• History: No family history of skin cancer; concerned about scarring, no blood thinner
• Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy
• Impression: rule out melanoma.
• Plan:
As was the case before 2021, you still need a CC, along with a medically (and medicolegally) appropriate history and physical examination. A diagnostic impression and treatment plan also should be included.
In this situation, reporting is straightforward. There is no separate E/M visit; only the CPT code 11102 for tangential biopsy is reported. An International Classification of Diseases, Tenth Revision code of D48.5 (neoplasm of uncertain behavior of skin) will be included.
Why no E/M code? This is because the biopsy includes preservice and postservice time and work that would be double reported with the E/M. Remember that the preservice work would include any history and physical examination related to the area to be biopsied.
Specifically, preservice work includes:
Inspect and palpate lesion to assess surface size, subcutaneous depth and extension, and whether fixed to underlying structures. Select the most representative and appropriate site to obtain specimen. Examine draining lymph node basins. Discuss need for skin biopsy and biopsy technique options. Describe the tangential biopsy procedure method and expected result and the potential for inconclusive pathology result. Review procedural risks, including bleeding, pain, edema, infection, delayed healing, scarring, and hyper- or hypopigmentation.5
Postservice work includes:
Instruct patient and family on postoperative wound care and dressing changes, as well as problems such as bleeding or pain and restrictions on activities, and follow-up care. Provide prescriptions for pain and antibiotics as necessary. Advise patient and family when results will be available and how they will be communicated. The pathology request form is filled out and signed by the physician. Complete medical record and communicate procedure/results to referring physician as appropriate.5
The Takeaway—Procedure codes include preservice and postservice work. If additional work for the procedure is not documented beyond that, an E/M cannot be included in the encounter.
Scenario 2: What If We Don’t Biopsy?
• CC: New spot on left cheek that seems to be growing and changing shape rapidly.
• History: No family history of skin cancer; concerned about scarring, no blood thinner.
• Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy.
• Impression: rule out melanoma.
• Plan: Review risk, benefits, and alternative options. Schedule biopsy. Discuss unique risk factor of sebaceous peau d’orange skin more prone to contour defects after biopsy.
When determining the coding level for this scenario by MDM, 3 components must be considered: number and complexity of problems addressed at the encounter (column 1), amount and/or complexity of data to be reviewed and analyzed (column 2), and risk of complications and/or morbidity or mortality of patient management (column 3).1 There are no data that are reviewed, so the auditor will assume minimal data to be reviewed and/or analyzed (level 2, row 2 in the MDM table). However, there may be a lot of variation in how an auditor would address the number and complexity of problems (level 1). Consider that you must explicitly state what you are thinking, as an auditor may not know melanoma is a life-threatening diagnosis. From the perspective of the auditor, could this be a:
• Self-limited or minor problem (level 2, or minimal problem in the MDM table)?1
• Stable chronic illness (level 3, or low-level problem)?1
• Undiagnosed new problem with uncertain prognosis (level 4, or moderate level problem)?1
• Acute illness with systemic symptoms (level 4, or moderate level problem)?1
• Acute or chronic illness or injury that poses a threat to life or bodily function (level 5, or high-level problem)?1
• All of the above?
Similarly, there may be variation in how the risk (column 3) would be interpreted in this scenario. The treatment gives no guidance, so the auditor may assume this has a minimal risk of morbidity (level 2) or possibly a low risk of morbidity from additional diagnostic testing or treatment (level 3), as opposed to a moderate risk of morbidity (level 4).1The Takeaway—In the auditor’s mind, this could be a straightforward (CPT codes 99202/99212) or lowlevel (99203/99213) visit as opposed to a moderate-level (99204/99214) visit. From the above documentation, an auditor would not be able to tell what you are thinking, and you can be assured they will not look further into the diagnosis or treatment to learn. That is not their job. So, let us clarify by explicitly stating what you are thinking in the context of the MDM grid.
Modified Scenario 2: A Funny-Looking New Spot With MDM Descriptors to Guide an Auditor
Below are modifications to the documentation for scenario 2 to guide an auditor:
• CC: New spot on left cheek that seems to be growing and changing shape rapidly.
• History: No family history of skin cancer; concerned about scarring, no blood thinner.
• Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy.
• Impression: rule out melanoma
• Plan: Discuss risks, benefits, and alternatives, including biopsy (
In this scenario, the level of MDM is much more clearly documented (as bolded above).
The number and complexity of problems would be an undiagnosed new problem with uncertain prognosis, which would be moderate complexity (column 1, level 4).1 There are no data that are reviewed or analyzed, which would be straightforward (column 2, level 2). For risk, the discussion of the biopsy as part of the diagnostic choices should include discussion of possible scarring, bleeding, pain, and infection, which would be considered best described as a decision regarding minor surgery with identified patient or procedure risk factors, which would make this of moderate complexity (column 3, level 4).1
Importantly, even if the procedure is not chosen as the final treatment plan, the discussion regarding the surgery, including the risks, benefits, and alternatives, can still count toward this category in the MDM table. Therefore, in this scenario with the updated and clarified documentation, this would be reported as CPT code 99204 for a new patient, while an established patient would be 99214.
Scenario 1 Revisited: A Funny-Looking New Spot
Below is scenario 1 with enhanced documentation, now applied to our procedure-only visit.
• CC: New spot on left cheek that seems to be growing and changing shape rapidly.
• History: No family history of skin cancer; concerned about scarring, no blood thinner.
• Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy.
• Impression: rule out melanoma (undiagnosed new problem with uncertain prognosis).
• Plan: Discuss risks, benefits, and alternatives, including biopsy (decision regarding minor surgery with identified patient or procedure risk factors) vs a noninvasive 2 gene expression profiling melanoma rule-out test. Patient wants biopsy. Consent, biopsy via shave technique. Lidocaine hydrochloride 1% with epinephrine, 1 cc, prepare and drape, hemostasis obtained, ointment and bandage applied, and care instructions provided.
This documentation would only allow reporting the biopsy as in Scenario 1, as the decision to perform a 0- or 10-day global procedure is bundled with the procedure if performed on the same date of service.
Final Thoughts
Spot checks are commonly encountered dermatologic visits. With the updated E/M guidelines, clarifying and streamlining your documentation is crucial. In particular, utilizing language that clearly defines number and complexity of problems, amount and/or complexity of data to be reviewed and analyzed, and appropriate risk stratification is crucial to ensuring appropriate reimbursement and minimizing your pain with audits.
- American Medical Association. CPT evaluation and management (E/M) code and guideline changes; 2023. Accessed April 13, 2023. https://www.ama-assn.org/system/files/2023-e-m-descriptors-guidelines.pdf
- American Medical Association. CPT Professional Edition 2023. American Medical Association; 2022.
- American Medical Association. Evaluation and management (E/M) coding. Accessed April 25, 2023. https://www.ama-assn.org/topics/evaluation-and-management-em-coding
- American Academy of Dermatology Association. Coding resource center. Accessed April 13, 2023. https://www.aad.org/member/practice/coding
- American Medical Association. RBVS DataManager Online. Accessed April 13, 2023. https://commerce.ama-assn.org/store/ui/catalog/productDetail?product_id=prod280002&navAction=push
On January 1, 2021, the Current Procedural Terminology (CPT) evaluation and management (E/M) reporting rules changed dramatically, with “bullet counting” no longer necessary and the coding level now based on either the new medical decision making (MDM) table or time spent on all activities relating to the care of the patient on the day of the encounter.1 This is described in the CPT Professional Edition 2023, a book every practitioner should review annually.2 In particular, every provider should read and reread pages 1 to 14—and beyond if you provide services beyond standard office visits. These changes were made with the intent to simplify the process of documentation and allow a provider to spend more time with patients, though there is still a paucity of data related to whether the new system achieves these aims.
The general rule of reporting work with CPT codes can be simply stated—“Document what you did, do what you documented, and report that which is medically necessary” (David McCafferey, MD, personal communication)—and you should never have any difficulty with audits. Unfortunately, the new system does not let an auditor, who typically lacks a medical degree, audit effectively unless they have a clear understanding of diseases and their stages. Many medical societies, including the American Medical Association3 and American Academy of Dermatology,4 have provided education that focuses on how to report a given vignette, but specific examples of documentation with commentary are uncommon.
To make your documentation more likely to pass audits, explicitly link parts of your documentation to CPT MDM descriptors. We offer scenarios and tips. In part 1 of this series, we discuss how to approach the “spot check,” a commonly encountered chief concern (CC) within dermatology.
A 34-year-old presents with a new spot on the left cheek that seems to be growing and changing shape rapidly. You examine the patient and discuss treatment options. The documentation reads as follows:
• CC: New spot on left cheek that seems to be growing and changing shape rapidly
• History: No family history of skin cancer; concerned about scarring, no blood thinner
• Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy
• Impression: rule out melanoma.
• Plan:
As was the case before 2021, you still need a CC, along with a medically (and medicolegally) appropriate history and physical examination. A diagnostic impression and treatment plan also should be included.
In this situation, reporting is straightforward. There is no separate E/M visit; only the CPT code 11102 for tangential biopsy is reported. An International Classification of Diseases, Tenth Revision code of D48.5 (neoplasm of uncertain behavior of skin) will be included.
Why no E/M code? This is because the biopsy includes preservice and postservice time and work that would be double reported with the E/M. Remember that the preservice work would include any history and physical examination related to the area to be biopsied.
Specifically, preservice work includes:
Inspect and palpate lesion to assess surface size, subcutaneous depth and extension, and whether fixed to underlying structures. Select the most representative and appropriate site to obtain specimen. Examine draining lymph node basins. Discuss need for skin biopsy and biopsy technique options. Describe the tangential biopsy procedure method and expected result and the potential for inconclusive pathology result. Review procedural risks, including bleeding, pain, edema, infection, delayed healing, scarring, and hyper- or hypopigmentation.5
Postservice work includes:
Instruct patient and family on postoperative wound care and dressing changes, as well as problems such as bleeding or pain and restrictions on activities, and follow-up care. Provide prescriptions for pain and antibiotics as necessary. Advise patient and family when results will be available and how they will be communicated. The pathology request form is filled out and signed by the physician. Complete medical record and communicate procedure/results to referring physician as appropriate.5
The Takeaway—Procedure codes include preservice and postservice work. If additional work for the procedure is not documented beyond that, an E/M cannot be included in the encounter.
Scenario 2: What If We Don’t Biopsy?
• CC: New spot on left cheek that seems to be growing and changing shape rapidly.
• History: No family history of skin cancer; concerned about scarring, no blood thinner.
• Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy.
• Impression: rule out melanoma.
• Plan: Review risk, benefits, and alternative options. Schedule biopsy. Discuss unique risk factor of sebaceous peau d’orange skin more prone to contour defects after biopsy.
When determining the coding level for this scenario by MDM, 3 components must be considered: number and complexity of problems addressed at the encounter (column 1), amount and/or complexity of data to be reviewed and analyzed (column 2), and risk of complications and/or morbidity or mortality of patient management (column 3).1 There are no data that are reviewed, so the auditor will assume minimal data to be reviewed and/or analyzed (level 2, row 2 in the MDM table). However, there may be a lot of variation in how an auditor would address the number and complexity of problems (level 1). Consider that you must explicitly state what you are thinking, as an auditor may not know melanoma is a life-threatening diagnosis. From the perspective of the auditor, could this be a:
• Self-limited or minor problem (level 2, or minimal problem in the MDM table)?1
• Stable chronic illness (level 3, or low-level problem)?1
• Undiagnosed new problem with uncertain prognosis (level 4, or moderate level problem)?1
• Acute illness with systemic symptoms (level 4, or moderate level problem)?1
• Acute or chronic illness or injury that poses a threat to life or bodily function (level 5, or high-level problem)?1
• All of the above?
Similarly, there may be variation in how the risk (column 3) would be interpreted in this scenario. The treatment gives no guidance, so the auditor may assume this has a minimal risk of morbidity (level 2) or possibly a low risk of morbidity from additional diagnostic testing or treatment (level 3), as opposed to a moderate risk of morbidity (level 4).1The Takeaway—In the auditor’s mind, this could be a straightforward (CPT codes 99202/99212) or lowlevel (99203/99213) visit as opposed to a moderate-level (99204/99214) visit. From the above documentation, an auditor would not be able to tell what you are thinking, and you can be assured they will not look further into the diagnosis or treatment to learn. That is not their job. So, let us clarify by explicitly stating what you are thinking in the context of the MDM grid.
Modified Scenario 2: A Funny-Looking New Spot With MDM Descriptors to Guide an Auditor
Below are modifications to the documentation for scenario 2 to guide an auditor:
• CC: New spot on left cheek that seems to be growing and changing shape rapidly.
• History: No family history of skin cancer; concerned about scarring, no blood thinner.
• Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy.
• Impression: rule out melanoma
• Plan: Discuss risks, benefits, and alternatives, including biopsy (
In this scenario, the level of MDM is much more clearly documented (as bolded above).
The number and complexity of problems would be an undiagnosed new problem with uncertain prognosis, which would be moderate complexity (column 1, level 4).1 There are no data that are reviewed or analyzed, which would be straightforward (column 2, level 2). For risk, the discussion of the biopsy as part of the diagnostic choices should include discussion of possible scarring, bleeding, pain, and infection, which would be considered best described as a decision regarding minor surgery with identified patient or procedure risk factors, which would make this of moderate complexity (column 3, level 4).1
Importantly, even if the procedure is not chosen as the final treatment plan, the discussion regarding the surgery, including the risks, benefits, and alternatives, can still count toward this category in the MDM table. Therefore, in this scenario with the updated and clarified documentation, this would be reported as CPT code 99204 for a new patient, while an established patient would be 99214.
Scenario 1 Revisited: A Funny-Looking New Spot
Below is scenario 1 with enhanced documentation, now applied to our procedure-only visit.
• CC: New spot on left cheek that seems to be growing and changing shape rapidly.
• History: No family history of skin cancer; concerned about scarring, no blood thinner.
• Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy.
• Impression: rule out melanoma (undiagnosed new problem with uncertain prognosis).
• Plan: Discuss risks, benefits, and alternatives, including biopsy (decision regarding minor surgery with identified patient or procedure risk factors) vs a noninvasive 2 gene expression profiling melanoma rule-out test. Patient wants biopsy. Consent, biopsy via shave technique. Lidocaine hydrochloride 1% with epinephrine, 1 cc, prepare and drape, hemostasis obtained, ointment and bandage applied, and care instructions provided.
This documentation would only allow reporting the biopsy as in Scenario 1, as the decision to perform a 0- or 10-day global procedure is bundled with the procedure if performed on the same date of service.
Final Thoughts
Spot checks are commonly encountered dermatologic visits. With the updated E/M guidelines, clarifying and streamlining your documentation is crucial. In particular, utilizing language that clearly defines number and complexity of problems, amount and/or complexity of data to be reviewed and analyzed, and appropriate risk stratification is crucial to ensuring appropriate reimbursement and minimizing your pain with audits.
On January 1, 2021, the Current Procedural Terminology (CPT) evaluation and management (E/M) reporting rules changed dramatically, with “bullet counting” no longer necessary and the coding level now based on either the new medical decision making (MDM) table or time spent on all activities relating to the care of the patient on the day of the encounter.1 This is described in the CPT Professional Edition 2023, a book every practitioner should review annually.2 In particular, every provider should read and reread pages 1 to 14—and beyond if you provide services beyond standard office visits. These changes were made with the intent to simplify the process of documentation and allow a provider to spend more time with patients, though there is still a paucity of data related to whether the new system achieves these aims.
The general rule of reporting work with CPT codes can be simply stated—“Document what you did, do what you documented, and report that which is medically necessary” (David McCafferey, MD, personal communication)—and you should never have any difficulty with audits. Unfortunately, the new system does not let an auditor, who typically lacks a medical degree, audit effectively unless they have a clear understanding of diseases and their stages. Many medical societies, including the American Medical Association3 and American Academy of Dermatology,4 have provided education that focuses on how to report a given vignette, but specific examples of documentation with commentary are uncommon.
To make your documentation more likely to pass audits, explicitly link parts of your documentation to CPT MDM descriptors. We offer scenarios and tips. In part 1 of this series, we discuss how to approach the “spot check,” a commonly encountered chief concern (CC) within dermatology.
A 34-year-old presents with a new spot on the left cheek that seems to be growing and changing shape rapidly. You examine the patient and discuss treatment options. The documentation reads as follows:
• CC: New spot on left cheek that seems to be growing and changing shape rapidly
• History: No family history of skin cancer; concerned about scarring, no blood thinner
• Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy
• Impression: rule out melanoma.
• Plan:
As was the case before 2021, you still need a CC, along with a medically (and medicolegally) appropriate history and physical examination. A diagnostic impression and treatment plan also should be included.
In this situation, reporting is straightforward. There is no separate E/M visit; only the CPT code 11102 for tangential biopsy is reported. An International Classification of Diseases, Tenth Revision code of D48.5 (neoplasm of uncertain behavior of skin) will be included.
Why no E/M code? This is because the biopsy includes preservice and postservice time and work that would be double reported with the E/M. Remember that the preservice work would include any history and physical examination related to the area to be biopsied.
Specifically, preservice work includes:
Inspect and palpate lesion to assess surface size, subcutaneous depth and extension, and whether fixed to underlying structures. Select the most representative and appropriate site to obtain specimen. Examine draining lymph node basins. Discuss need for skin biopsy and biopsy technique options. Describe the tangential biopsy procedure method and expected result and the potential for inconclusive pathology result. Review procedural risks, including bleeding, pain, edema, infection, delayed healing, scarring, and hyper- or hypopigmentation.5
Postservice work includes:
Instruct patient and family on postoperative wound care and dressing changes, as well as problems such as bleeding or pain and restrictions on activities, and follow-up care. Provide prescriptions for pain and antibiotics as necessary. Advise patient and family when results will be available and how they will be communicated. The pathology request form is filled out and signed by the physician. Complete medical record and communicate procedure/results to referring physician as appropriate.5
The Takeaway—Procedure codes include preservice and postservice work. If additional work for the procedure is not documented beyond that, an E/M cannot be included in the encounter.
Scenario 2: What If We Don’t Biopsy?
• CC: New spot on left cheek that seems to be growing and changing shape rapidly.
• History: No family history of skin cancer; concerned about scarring, no blood thinner.
• Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy.
• Impression: rule out melanoma.
• Plan: Review risk, benefits, and alternative options. Schedule biopsy. Discuss unique risk factor of sebaceous peau d’orange skin more prone to contour defects after biopsy.
When determining the coding level for this scenario by MDM, 3 components must be considered: number and complexity of problems addressed at the encounter (column 1), amount and/or complexity of data to be reviewed and analyzed (column 2), and risk of complications and/or morbidity or mortality of patient management (column 3).1 There are no data that are reviewed, so the auditor will assume minimal data to be reviewed and/or analyzed (level 2, row 2 in the MDM table). However, there may be a lot of variation in how an auditor would address the number and complexity of problems (level 1). Consider that you must explicitly state what you are thinking, as an auditor may not know melanoma is a life-threatening diagnosis. From the perspective of the auditor, could this be a:
• Self-limited or minor problem (level 2, or minimal problem in the MDM table)?1
• Stable chronic illness (level 3, or low-level problem)?1
• Undiagnosed new problem with uncertain prognosis (level 4, or moderate level problem)?1
• Acute illness with systemic symptoms (level 4, or moderate level problem)?1
• Acute or chronic illness or injury that poses a threat to life or bodily function (level 5, or high-level problem)?1
• All of the above?
Similarly, there may be variation in how the risk (column 3) would be interpreted in this scenario. The treatment gives no guidance, so the auditor may assume this has a minimal risk of morbidity (level 2) or possibly a low risk of morbidity from additional diagnostic testing or treatment (level 3), as opposed to a moderate risk of morbidity (level 4).1The Takeaway—In the auditor’s mind, this could be a straightforward (CPT codes 99202/99212) or lowlevel (99203/99213) visit as opposed to a moderate-level (99204/99214) visit. From the above documentation, an auditor would not be able to tell what you are thinking, and you can be assured they will not look further into the diagnosis or treatment to learn. That is not their job. So, let us clarify by explicitly stating what you are thinking in the context of the MDM grid.
Modified Scenario 2: A Funny-Looking New Spot With MDM Descriptors to Guide an Auditor
Below are modifications to the documentation for scenario 2 to guide an auditor:
• CC: New spot on left cheek that seems to be growing and changing shape rapidly.
• History: No family history of skin cancer; concerned about scarring, no blood thinner.
• Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy.
• Impression: rule out melanoma
• Plan: Discuss risks, benefits, and alternatives, including biopsy (
In this scenario, the level of MDM is much more clearly documented (as bolded above).
The number and complexity of problems would be an undiagnosed new problem with uncertain prognosis, which would be moderate complexity (column 1, level 4).1 There are no data that are reviewed or analyzed, which would be straightforward (column 2, level 2). For risk, the discussion of the biopsy as part of the diagnostic choices should include discussion of possible scarring, bleeding, pain, and infection, which would be considered best described as a decision regarding minor surgery with identified patient or procedure risk factors, which would make this of moderate complexity (column 3, level 4).1
Importantly, even if the procedure is not chosen as the final treatment plan, the discussion regarding the surgery, including the risks, benefits, and alternatives, can still count toward this category in the MDM table. Therefore, in this scenario with the updated and clarified documentation, this would be reported as CPT code 99204 for a new patient, while an established patient would be 99214.
Scenario 1 Revisited: A Funny-Looking New Spot
Below is scenario 1 with enhanced documentation, now applied to our procedure-only visit.
• CC: New spot on left cheek that seems to be growing and changing shape rapidly.
• History: No family history of skin cancer; concerned about scarring, no blood thinner.
• Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy.
• Impression: rule out melanoma (undiagnosed new problem with uncertain prognosis).
• Plan: Discuss risks, benefits, and alternatives, including biopsy (decision regarding minor surgery with identified patient or procedure risk factors) vs a noninvasive 2 gene expression profiling melanoma rule-out test. Patient wants biopsy. Consent, biopsy via shave technique. Lidocaine hydrochloride 1% with epinephrine, 1 cc, prepare and drape, hemostasis obtained, ointment and bandage applied, and care instructions provided.
This documentation would only allow reporting the biopsy as in Scenario 1, as the decision to perform a 0- or 10-day global procedure is bundled with the procedure if performed on the same date of service.
Final Thoughts
Spot checks are commonly encountered dermatologic visits. With the updated E/M guidelines, clarifying and streamlining your documentation is crucial. In particular, utilizing language that clearly defines number and complexity of problems, amount and/or complexity of data to be reviewed and analyzed, and appropriate risk stratification is crucial to ensuring appropriate reimbursement and minimizing your pain with audits.
- American Medical Association. CPT evaluation and management (E/M) code and guideline changes; 2023. Accessed April 13, 2023. https://www.ama-assn.org/system/files/2023-e-m-descriptors-guidelines.pdf
- American Medical Association. CPT Professional Edition 2023. American Medical Association; 2022.
- American Medical Association. Evaluation and management (E/M) coding. Accessed April 25, 2023. https://www.ama-assn.org/topics/evaluation-and-management-em-coding
- American Academy of Dermatology Association. Coding resource center. Accessed April 13, 2023. https://www.aad.org/member/practice/coding
- American Medical Association. RBVS DataManager Online. Accessed April 13, 2023. https://commerce.ama-assn.org/store/ui/catalog/productDetail?product_id=prod280002&navAction=push
- American Medical Association. CPT evaluation and management (E/M) code and guideline changes; 2023. Accessed April 13, 2023. https://www.ama-assn.org/system/files/2023-e-m-descriptors-guidelines.pdf
- American Medical Association. CPT Professional Edition 2023. American Medical Association; 2022.
- American Medical Association. Evaluation and management (E/M) coding. Accessed April 25, 2023. https://www.ama-assn.org/topics/evaluation-and-management-em-coding
- American Academy of Dermatology Association. Coding resource center. Accessed April 13, 2023. https://www.aad.org/member/practice/coding
- American Medical Association. RBVS DataManager Online. Accessed April 13, 2023. https://commerce.ama-assn.org/store/ui/catalog/productDetail?product_id=prod280002&navAction=push
Practice Points
- Clear documentation that reflects your thought process is an important component of effective coding and billing.
- Include Current Procedural Terminology–defined language within documentation to help ensure appropriate reimbursement and decrease the risk of audits.
How to Effectively Utilize Consultation Codes: 2023 Updates
Consultations and referrals are an important component of many dermatology practices. There are several families of consultation codes that can be utilized based on the setting and format of the patient encounter. In this article, I describe appropriate use of 3 families of consultation codes and recent updates in these areas.
Consultation Definitions
For all of these code sets, the same definition of consultationapplies—namely that the encounter is provided at the request of another physician, other qualified health care professional, or other appropriate source (eg, nonclinical social worker, educator, lawyer, insurance company) for a specific condition or problem. Importantly, a consultation initiated by a patient or family, or both, and not requested by one of the professionals listed above is not reported using a consultation code.1
The consultant’s opinion and any services that were ordered or performed also must be communicated to the requesting provider. The type of communication required varies based on the consultation code set in question.
Outpatient Consultation Codes
Outpatient consultation CPT (Current Procedural Terminology) codes (99241-99245) are a family of codes that can be utilized for evaluation of a new patient or an existing patient with a new problem in the outpatient setting. These codes are not reimbursed by the Centers for Medicare & Medicaid Services, but some private payers do recognize and reimburse for them.2
The consultant’s opinion and any services that were ordered or performed must be communicated by written report to the requesting physician, other qualified health care professional, or other appropriate source. If a consultation is mandated (eg, by a third-party payer), then modifier -32 also should be reported.1 Modifier -32 should not be used for a second request by a patient or a patient’s family.1
This family of codes has been revised in tandem with other evaluation and management (E/M) code sets; changes went into effect January 1, 2023. These updates are part of the ongoing effort to update code wording and structures to reflect guiding principles of the American Medical Association when redesigning E/M codes. These principles include decreasing administrative burden and the need for audits, decreasing unnecessary documentation that is not needed for patient care, and ensuring that payment for E/M is resource based.3 Updated code language and payment structure is found in Table 1.1,2 The main updates to these codes include:
• Code 99241 was deleted. This was in line with removal of 99201 from the outpatient E/M family set.
• Level of service is now based solely on either time on the date of encounter or medical decision-making.
• Definitions regarding medical decision-making are in line with those utilized for outpatient E/M codes.
• If coding by time and the maximum amount of time has been exceeded by 15 or more minutes, prolonged services code 99417 can be utilized.

Inpatient Consultation Codes
Similar to the outpatient consultation codes, the inpatient consultation codes also have been revised as part of E/M updates; revisions went into effect January 1, 2023. Also, as with the outpatient consultation codes, the consultant’s opinion and any services that were ordered or performed must be communicated by written report to the requesting physician, other qualified health care professional, or other appropriate source. If a consultation is mandated (eg, by a third-party payer), then modifier -32 also should be reported.1
When inpatient consultations are performed, 2 code families generally are utilized. For initial consultation, initial inpatient consultation codes (99251-99255) are used; for any follow-up encounters performed while the patient is an inpatient, subsequent inpatient consultation codes (99231-99233) are used. The subsequent code family is the same that is utilized for all subsequent care within the inpatient or observation care setting, regardless of how the care was initiated.1
“Initial service” is when the patient has not received any professional services from either the physician or other qualified health care professional or from another physician or other qualified health care professional ofthe exact same specialty and subspecialty who belongs to the same group practice during the inpatient, observation, or nursing facility admission and stay. “Subsequent service” is when the patient has received professional service(s) from either the physician or other qualified health care professional or from another physician or other qualified health care professional.1 Updated code language and payment structure is found in Table 2.1,2 Major changes include:
• Code 99251 was deleted. This is in line with deletion of a new low-level patient encounter in the outpatient E/M family set and consultation code family set, as noted above.
• Level of service is now based solely on either time on the date of encounter or medical decision-making.
• Definitions regarding medical decision-making are in line with those utilized for outpatient E/M codes.
• If coding by time and the maximum amount of time has been exceeded by 15 or more minutes, prolonged services code 993X0 can be utilized.
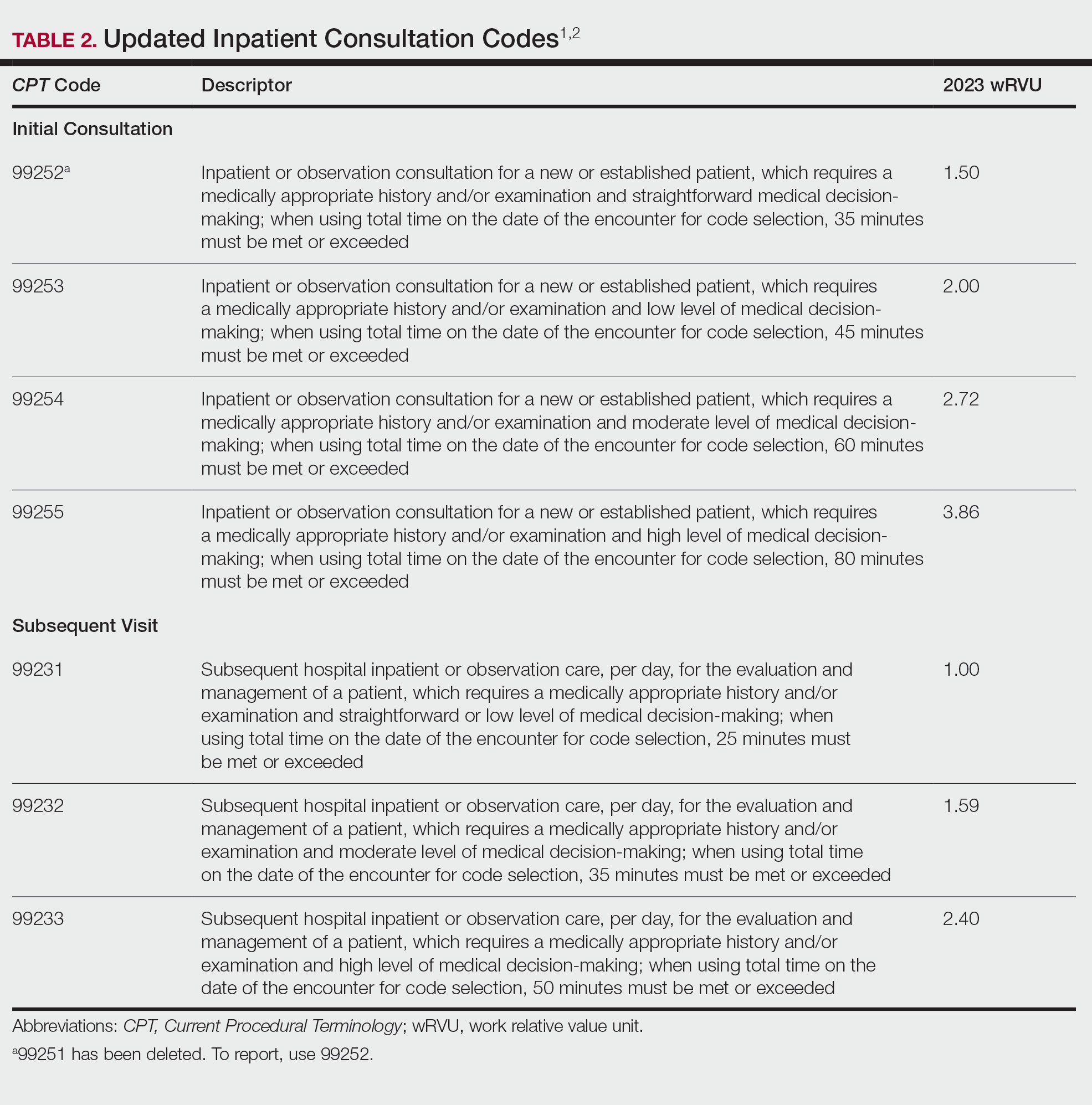
Interprofessional Consultation Codes
An additional code family that can be utilized for consultations is the interprofessional consultation codes. These codes can be utilized when assisting in the diagnosis or management, or both, of a patient without face-to-face contact. These codes are listed in Table 3.2,4 For all of these codes, the consultation is performed by telephone, internet or electronic health record, or a combination of these means. The consultation can be for a new problem or a worsening existing problem. The patient can be a new or established patient to the consultant. Documentation should be performed in the patient’s medical record, including the reason for the request.
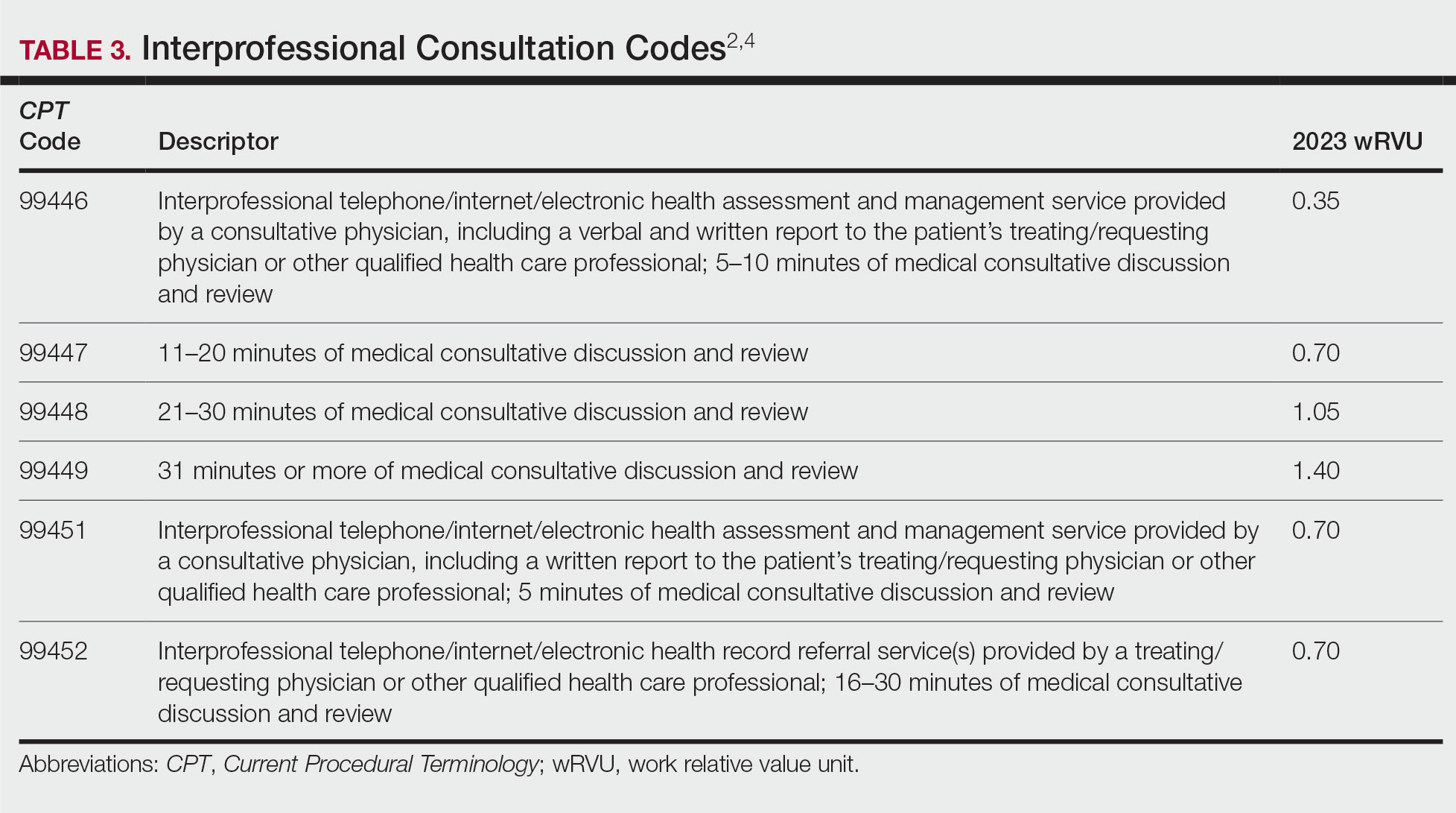
To bill for interprofessional consultation, the consultant should not have seen the patient in a face-to-face encounter within the prior 14 days or see them in the following 14 days. The codes should not be reported more than once in a 7-day period or more than once in a 14-day period in the case of code 99452.4 For codes 99446 to 99449, more than 50% of the time spent by the consulting physician must be devoted to verbal or internet discussion, or both, with the referring physician. For code 99451, service time is based on total review and interprofessional communication time.4 The correct code is chosen based on the following parameters:
• 99446-99449: Describes interprofessional consultation services, which include both a written and a verbal report to the patient’s treating or requesting physician or qualified health care professional. These codes can be utilized by a consulting physician. The correct code is chosen based on time spent by the consulting physician.
• 99451: Describes an interprofessional consultation service, which includes a written report to the patient’s treating or requesting physician or qualified health care professional. This code can be utilized by a consulting physician once 5 minutes of consultative discussion and review has been performed.
• 99452: Describes an interprofessional consultation service provided by the requesting physician. This code can be utilized when a requesting physician spends 16 to 30 minutes in medical consultative discussion and review.
Final Thoughts
Consultation codes can be an important part of a dermatologist’s practice. Differences exist between consultation code sets based on the encounter setting and whether the encounter was performed with or without face-to-face contact. In addition, updates to the E/M inpatient and outpatient consultation codes went into effect January 1, 2023. It is important to understand those changes to correctly bill for these encounters.
- CPT® evaluation and management (E/M) code and guideline changes. American Medical Association. Accessed January 15, 2023. https://www.ama-assn.org/system/files/2023-e-m-descriptors-guidelines.pdf
- RVU23A. US Centers for Medicare and Medicaid Services; January 2023. Accessed January 18, 2023. https://www.cms.gov/medicaremedicare-fee-service-paymentphysicianfeeschedpfs-relative-value-files/rvu23a
- Understanding the landmark E/M office visit changes. American Medical Association. Accessed January 15, 2023. https://www.ama-assn.org/practice-management/cpt/understanding-landmark-em-office-visit-changes
- Synovec MS, Jagmin CL, Hochstetler Z, et al, eds. CPT 2022: Professional Edition. 4th ed. American Medical Association Press; 2021.
Consultations and referrals are an important component of many dermatology practices. There are several families of consultation codes that can be utilized based on the setting and format of the patient encounter. In this article, I describe appropriate use of 3 families of consultation codes and recent updates in these areas.
Consultation Definitions
For all of these code sets, the same definition of consultationapplies—namely that the encounter is provided at the request of another physician, other qualified health care professional, or other appropriate source (eg, nonclinical social worker, educator, lawyer, insurance company) for a specific condition or problem. Importantly, a consultation initiated by a patient or family, or both, and not requested by one of the professionals listed above is not reported using a consultation code.1
The consultant’s opinion and any services that were ordered or performed also must be communicated to the requesting provider. The type of communication required varies based on the consultation code set in question.
Outpatient Consultation Codes
Outpatient consultation CPT (Current Procedural Terminology) codes (99241-99245) are a family of codes that can be utilized for evaluation of a new patient or an existing patient with a new problem in the outpatient setting. These codes are not reimbursed by the Centers for Medicare & Medicaid Services, but some private payers do recognize and reimburse for them.2
The consultant’s opinion and any services that were ordered or performed must be communicated by written report to the requesting physician, other qualified health care professional, or other appropriate source. If a consultation is mandated (eg, by a third-party payer), then modifier -32 also should be reported.1 Modifier -32 should not be used for a second request by a patient or a patient’s family.1
This family of codes has been revised in tandem with other evaluation and management (E/M) code sets; changes went into effect January 1, 2023. These updates are part of the ongoing effort to update code wording and structures to reflect guiding principles of the American Medical Association when redesigning E/M codes. These principles include decreasing administrative burden and the need for audits, decreasing unnecessary documentation that is not needed for patient care, and ensuring that payment for E/M is resource based.3 Updated code language and payment structure is found in Table 1.1,2 The main updates to these codes include:
• Code 99241 was deleted. This was in line with removal of 99201 from the outpatient E/M family set.
• Level of service is now based solely on either time on the date of encounter or medical decision-making.
• Definitions regarding medical decision-making are in line with those utilized for outpatient E/M codes.
• If coding by time and the maximum amount of time has been exceeded by 15 or more minutes, prolonged services code 99417 can be utilized.

Inpatient Consultation Codes
Similar to the outpatient consultation codes, the inpatient consultation codes also have been revised as part of E/M updates; revisions went into effect January 1, 2023. Also, as with the outpatient consultation codes, the consultant’s opinion and any services that were ordered or performed must be communicated by written report to the requesting physician, other qualified health care professional, or other appropriate source. If a consultation is mandated (eg, by a third-party payer), then modifier -32 also should be reported.1
When inpatient consultations are performed, 2 code families generally are utilized. For initial consultation, initial inpatient consultation codes (99251-99255) are used; for any follow-up encounters performed while the patient is an inpatient, subsequent inpatient consultation codes (99231-99233) are used. The subsequent code family is the same that is utilized for all subsequent care within the inpatient or observation care setting, regardless of how the care was initiated.1
“Initial service” is when the patient has not received any professional services from either the physician or other qualified health care professional or from another physician or other qualified health care professional ofthe exact same specialty and subspecialty who belongs to the same group practice during the inpatient, observation, or nursing facility admission and stay. “Subsequent service” is when the patient has received professional service(s) from either the physician or other qualified health care professional or from another physician or other qualified health care professional.1 Updated code language and payment structure is found in Table 2.1,2 Major changes include:
• Code 99251 was deleted. This is in line with deletion of a new low-level patient encounter in the outpatient E/M family set and consultation code family set, as noted above.
• Level of service is now based solely on either time on the date of encounter or medical decision-making.
• Definitions regarding medical decision-making are in line with those utilized for outpatient E/M codes.
• If coding by time and the maximum amount of time has been exceeded by 15 or more minutes, prolonged services code 993X0 can be utilized.

Interprofessional Consultation Codes
An additional code family that can be utilized for consultations is the interprofessional consultation codes. These codes can be utilized when assisting in the diagnosis or management, or both, of a patient without face-to-face contact. These codes are listed in Table 3.2,4 For all of these codes, the consultation is performed by telephone, internet or electronic health record, or a combination of these means. The consultation can be for a new problem or a worsening existing problem. The patient can be a new or established patient to the consultant. Documentation should be performed in the patient’s medical record, including the reason for the request.

To bill for interprofessional consultation, the consultant should not have seen the patient in a face-to-face encounter within the prior 14 days or see them in the following 14 days. The codes should not be reported more than once in a 7-day period or more than once in a 14-day period in the case of code 99452.4 For codes 99446 to 99449, more than 50% of the time spent by the consulting physician must be devoted to verbal or internet discussion, or both, with the referring physician. For code 99451, service time is based on total review and interprofessional communication time.4 The correct code is chosen based on the following parameters:
• 99446-99449: Describes interprofessional consultation services, which include both a written and a verbal report to the patient’s treating or requesting physician or qualified health care professional. These codes can be utilized by a consulting physician. The correct code is chosen based on time spent by the consulting physician.
• 99451: Describes an interprofessional consultation service, which includes a written report to the patient’s treating or requesting physician or qualified health care professional. This code can be utilized by a consulting physician once 5 minutes of consultative discussion and review has been performed.
• 99452: Describes an interprofessional consultation service provided by the requesting physician. This code can be utilized when a requesting physician spends 16 to 30 minutes in medical consultative discussion and review.
Final Thoughts
Consultation codes can be an important part of a dermatologist’s practice. Differences exist between consultation code sets based on the encounter setting and whether the encounter was performed with or without face-to-face contact. In addition, updates to the E/M inpatient and outpatient consultation codes went into effect January 1, 2023. It is important to understand those changes to correctly bill for these encounters.
Consultations and referrals are an important component of many dermatology practices. There are several families of consultation codes that can be utilized based on the setting and format of the patient encounter. In this article, I describe appropriate use of 3 families of consultation codes and recent updates in these areas.
Consultation Definitions
For all of these code sets, the same definition of consultationapplies—namely that the encounter is provided at the request of another physician, other qualified health care professional, or other appropriate source (eg, nonclinical social worker, educator, lawyer, insurance company) for a specific condition or problem. Importantly, a consultation initiated by a patient or family, or both, and not requested by one of the professionals listed above is not reported using a consultation code.1
The consultant’s opinion and any services that were ordered or performed also must be communicated to the requesting provider. The type of communication required varies based on the consultation code set in question.
Outpatient Consultation Codes
Outpatient consultation CPT (Current Procedural Terminology) codes (99241-99245) are a family of codes that can be utilized for evaluation of a new patient or an existing patient with a new problem in the outpatient setting. These codes are not reimbursed by the Centers for Medicare & Medicaid Services, but some private payers do recognize and reimburse for them.2
The consultant’s opinion and any services that were ordered or performed must be communicated by written report to the requesting physician, other qualified health care professional, or other appropriate source. If a consultation is mandated (eg, by a third-party payer), then modifier -32 also should be reported.1 Modifier -32 should not be used for a second request by a patient or a patient’s family.1
This family of codes has been revised in tandem with other evaluation and management (E/M) code sets; changes went into effect January 1, 2023. These updates are part of the ongoing effort to update code wording and structures to reflect guiding principles of the American Medical Association when redesigning E/M codes. These principles include decreasing administrative burden and the need for audits, decreasing unnecessary documentation that is not needed for patient care, and ensuring that payment for E/M is resource based.3 Updated code language and payment structure is found in Table 1.1,2 The main updates to these codes include:
• Code 99241 was deleted. This was in line with removal of 99201 from the outpatient E/M family set.
• Level of service is now based solely on either time on the date of encounter or medical decision-making.
• Definitions regarding medical decision-making are in line with those utilized for outpatient E/M codes.
• If coding by time and the maximum amount of time has been exceeded by 15 or more minutes, prolonged services code 99417 can be utilized.

Inpatient Consultation Codes
Similar to the outpatient consultation codes, the inpatient consultation codes also have been revised as part of E/M updates; revisions went into effect January 1, 2023. Also, as with the outpatient consultation codes, the consultant’s opinion and any services that were ordered or performed must be communicated by written report to the requesting physician, other qualified health care professional, or other appropriate source. If a consultation is mandated (eg, by a third-party payer), then modifier -32 also should be reported.1
When inpatient consultations are performed, 2 code families generally are utilized. For initial consultation, initial inpatient consultation codes (99251-99255) are used; for any follow-up encounters performed while the patient is an inpatient, subsequent inpatient consultation codes (99231-99233) are used. The subsequent code family is the same that is utilized for all subsequent care within the inpatient or observation care setting, regardless of how the care was initiated.1
“Initial service” is when the patient has not received any professional services from either the physician or other qualified health care professional or from another physician or other qualified health care professional ofthe exact same specialty and subspecialty who belongs to the same group practice during the inpatient, observation, or nursing facility admission and stay. “Subsequent service” is when the patient has received professional service(s) from either the physician or other qualified health care professional or from another physician or other qualified health care professional.1 Updated code language and payment structure is found in Table 2.1,2 Major changes include:
• Code 99251 was deleted. This is in line with deletion of a new low-level patient encounter in the outpatient E/M family set and consultation code family set, as noted above.
• Level of service is now based solely on either time on the date of encounter or medical decision-making.
• Definitions regarding medical decision-making are in line with those utilized for outpatient E/M codes.
• If coding by time and the maximum amount of time has been exceeded by 15 or more minutes, prolonged services code 993X0 can be utilized.

Interprofessional Consultation Codes
An additional code family that can be utilized for consultations is the interprofessional consultation codes. These codes can be utilized when assisting in the diagnosis or management, or both, of a patient without face-to-face contact. These codes are listed in Table 3.2,4 For all of these codes, the consultation is performed by telephone, internet or electronic health record, or a combination of these means. The consultation can be for a new problem or a worsening existing problem. The patient can be a new or established patient to the consultant. Documentation should be performed in the patient’s medical record, including the reason for the request.

To bill for interprofessional consultation, the consultant should not have seen the patient in a face-to-face encounter within the prior 14 days or see them in the following 14 days. The codes should not be reported more than once in a 7-day period or more than once in a 14-day period in the case of code 99452.4 For codes 99446 to 99449, more than 50% of the time spent by the consulting physician must be devoted to verbal or internet discussion, or both, with the referring physician. For code 99451, service time is based on total review and interprofessional communication time.4 The correct code is chosen based on the following parameters:
• 99446-99449: Describes interprofessional consultation services, which include both a written and a verbal report to the patient’s treating or requesting physician or qualified health care professional. These codes can be utilized by a consulting physician. The correct code is chosen based on time spent by the consulting physician.
• 99451: Describes an interprofessional consultation service, which includes a written report to the patient’s treating or requesting physician or qualified health care professional. This code can be utilized by a consulting physician once 5 minutes of consultative discussion and review has been performed.
• 99452: Describes an interprofessional consultation service provided by the requesting physician. This code can be utilized when a requesting physician spends 16 to 30 minutes in medical consultative discussion and review.
Final Thoughts
Consultation codes can be an important part of a dermatologist’s practice. Differences exist between consultation code sets based on the encounter setting and whether the encounter was performed with or without face-to-face contact. In addition, updates to the E/M inpatient and outpatient consultation codes went into effect January 1, 2023. It is important to understand those changes to correctly bill for these encounters.
- CPT® evaluation and management (E/M) code and guideline changes. American Medical Association. Accessed January 15, 2023. https://www.ama-assn.org/system/files/2023-e-m-descriptors-guidelines.pdf
- RVU23A. US Centers for Medicare and Medicaid Services; January 2023. Accessed January 18, 2023. https://www.cms.gov/medicaremedicare-fee-service-paymentphysicianfeeschedpfs-relative-value-files/rvu23a
- Understanding the landmark E/M office visit changes. American Medical Association. Accessed January 15, 2023. https://www.ama-assn.org/practice-management/cpt/understanding-landmark-em-office-visit-changes
- Synovec MS, Jagmin CL, Hochstetler Z, et al, eds. CPT 2022: Professional Edition. 4th ed. American Medical Association Press; 2021.
- CPT® evaluation and management (E/M) code and guideline changes. American Medical Association. Accessed January 15, 2023. https://www.ama-assn.org/system/files/2023-e-m-descriptors-guidelines.pdf
- RVU23A. US Centers for Medicare and Medicaid Services; January 2023. Accessed January 18, 2023. https://www.cms.gov/medicaremedicare-fee-service-paymentphysicianfeeschedpfs-relative-value-files/rvu23a
- Understanding the landmark E/M office visit changes. American Medical Association. Accessed January 15, 2023. https://www.ama-assn.org/practice-management/cpt/understanding-landmark-em-office-visit-changes
- Synovec MS, Jagmin CL, Hochstetler Z, et al, eds. CPT 2022: Professional Edition. 4th ed. American Medical Association Press; 2021.
PRACTICE POINTS
- Updates to the inpatient and outpatient consultation codes went into effect January 1, 2023.
- For inpatient and outpatient consultation codes, level of service is now solely based on either time on the date of encounter or medical decision-making.
- Interprofessional consultation codes can be utilized when assisting in the diagnosis and/or management of a patient without face-to-face contact.
The Final Rule for 2022: What’s New and How Changes in the Medicare Physician Fee Schedule and Quality Payment Program Affect Dermatologists
On November 2, 2021, the Centers for Medicare & Medicaid Services (CMS) released its final rule for the 2022 Medicare Physician Fee Schedule (PFS) and the Quality Payment Program (QPP).1,2 These guidelines contain updates that will remarkably impact the field of medicine—and dermatology in particular—in 2022. This article will walk you through some of the updates most relevant to dermatology and how they may affect your practice.
Process for the Final Rule
The CMS releases an annual rule for the PFS and QPP. The interim rule generally is released over the summer with preliminary guidelines for the upcoming payment year. There is then a period of open comment where those affected by these changes, including physicians and medical associations, can submit comments to support what has been proposed or advocate for any changes. This input is then reviewed, and a final rule generally is published in the fall.
For this calendar year, the interim 2022 rule was released on July 13, 2021,3 and included many of guidelines that will be discussed in more detail in this article. Many associations that represent medicine overall and specifically dermatology, including the American Medical Association and the American Academy of Dermatology, submitted comments in response to these proposals.4,5
PFS Conversion Factor
The PFS conversion factor is updated annually to ensure budget neutrality in the setting of changes in relative value units. For 2022, the PFS conversion factor is $34.6062, representing a reduction of approximately $0.29 from the 2021 PFS conversion factor of $34.8931.6 This reduction does not take into account other payment adjustments due to legislative changes.
In combination, these changes previously were estimated to represent an overall payment cut of 10% or higher for dermatology, with those practitioners doing more procedural work or dermatopathology likely being impacted more heavily. However, with the passing of the Protecting Medicare and American Farmers from Sequester Cuts Act, it is estimated that the reductions in payment to dermatology will begin at 0.75% and reach 2.75% in the second half of the year with the phased-in reinstatement of the Medicare sequester.4,5,7
Clinical Labor Pricing Updates
Starting in 2022, the CMS will utilize updated wage rates from the US Bureau of Labor Statistics to revise clinical labor costs over a 4-year period. Clinical labor rates are important, as they are used to calculate practice expense within the PFS. These clinical labor rates were last updated in 2002.8 Median wage data, as opposed to mean data, from the US Bureau of Labor Statistics will be utilized to calculate the updated clinical labor rates.
A multiyear implementation plan was put into place by CMS due to multiple concerns, including that current wage rates are inadequate and may not reflect current labor rate information. Additionally, comments on this proposal voiced concern that updating the supply and equipment pricing without updating the clinical labor pricing could create distortions in the allocation of direct practice expense, which also factored into the implementation of a multiyear plan.8
It is anticipated that specialties that rely primarily on clinical labor will receive the largest increases in these rates and that specialties that rely primarily on supply or equipment items are anticipated to receive the largest reductions relative to other specialties. Dermatology is estimated to have a 0% change during the year 1 transition period; however, it will have an estimated 1% reduction in clinical labor pricing overall once the updates are completed.1 Pathology also is estimated to have a similar overall decrease during this transition period.
Evaluation and Management Visits
The biggest update in this area primarily is related to refining policies for split (shared) evaluation and management (E/M) visits and teaching physician activities. Split E/M visits are defined by the CMS as visits provided in the facility setting by a physician and nonphysician practitioner in the same group, with the visit billed by whomever provides the substantive portion of the visit. For 2022, the term substantive portion will be defined by the CMS as history, physical examination, medical decision-making, or more than half of the total time; for 2023, it will be defined as more than half of the total time spent.3 A split visit also can apply to an E/M visit provided in part by both a teaching physician and resident. Split visits can be reported for new or established patients. For proper reimbursement, the 2 practitioners who performed the services must be documented in the medical record, and the practitioner who provided the substantive portion must sign and date the encounter in the medical record. Additionally, the CMS has indicated the modifier FS must be included on the claim to indicate the split visit.9
For dermatologists who act as teaching physicians, it is important to note that many of the existing CMS policies for billing E/M services are still in place, specifically that if a resident participates in a service in a teaching setting, the teaching physician can bill for the service only if they are present for the key or critical portion of the service. A primary care exception does exist, in which teaching physicians at certain teaching hospital primary care centers can bill for some services performed independently by a resident without the physical presence of the teaching physician; however, this often is not applicable within dermatology.
With updated outpatient E/M guidelines, if time is being selected to bill, only the time that the teaching physician was present can be included to determine the overall E/M level.
Billing for Physician Assistant Services
Currently Medicare can only make payments to the employer or independent contractor of a physician assistant (PA); however, starting January 1, 2022, the CMS has authorized Medicare to make direct payments to PAs for qualifying professional services, in the same manner that nurse practitioners can currently bill. This also will allow PAs to incorporate as a group and bill Medicare for PA services. This stems from a congressional mandate within the Consolidated Appropriations Act of 2021.8 As a result, in states where PAs can practice independently, they can opt out of physician-led care teams and furnish services independently, including dermatologic services.
QPP Updates
Several changes were made to the Merit-Based Incentive Payment System (MIPS). Some of these changes include:
- Increase the MIPS performance threshold to 75 points from 60 points.
- Set the performance threshold at 89 points.
- Reduce the quality performance category weight from 40% to 30% of the final MIPS score.
- Increase the cost performance category weight from 20% to 30% of the final MIPS score.
- The extreme and uncontrollable circumstances application also has been extended to the end of 2022, allowing those remarkably impacted by the COVID-19 public health emergency to request for reweighting on any or all MIPS performance categories.
Cost Measures and MIPS Value Pathways
The melanoma resection cost measure will be implemented in 2022, representing the first dermatology cost measure, which will include the cost to Medicare over a 1-year period for all patient care for the excision of a melanoma. Although cost measures will be part of the MIPS value pathways (MVPs) reporting, dermatology currently is not part of the MVP; however, with the CMS moving forward with an initial set of MVPs that physicians can voluntarily report on in 2023, there is a possibility that dermatology will be asked to be part of the program in the future.10
Final Thoughts
There are many upcoming changes as part of the 2022 final rule, including to the conversion factor, E/M split visits, PA billing, and the QPP. Advocacy in these areas to the CMS and lawmakers, either directly or through dermatologic and other medical societies, is critical to help influence eventual recommendations.
- Medicare Program; CY 2022 payment policies under the Physician Fee Schedule and other changes to part B payment policies; Medicare Shared Savings Program requirements; provider enrollment regulation updates; and provider and supplier prepayment and post-payment medical review requirements. Fed Regist. 2021;86:64996-66031. To be codified at 42 CFR §403, §405, §410, §411, §414, §415, §423, §424, and §425. https://www.federalregister.gov/documents/2021/11/19/2021-23972/medicare-program-cy-2022-payment-policies-under-the-physician-fee-schedule-and-other-changes-to-part
- Centers for Medicare & Medicaid Services. CMS physician payment rule promotes greater access to telehealth services, diabetes prevention programs. Published November 2, 2021. Accessed January 10, 2022. https://www.cms.gov/newsroom/press-releases/cms-physician-payment-rule-promotes-greater-access-telehealth-services-diabetes-prevention-programs
- Centers for Medicare & Medicaid Services. Calendar year (CY) 2022 Medicare Physician Fee Schedule proposed rule. Published July 13, 2021. Accessed January 10, 2022. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2022-medicare-physician-fee-schedule-proposed-rule
- American Academy of Dermatology. Dermatology World Weekly. October 27, 2021. Accessed January 20, 2022. https://www.aad.org/dw/weekly
- O’Reilly KB. 2022 Medicare pay schedule confirms Congress needs to act. American Medical Association website. Published November 10, 2021. Accessed January 10, 2021. https://www.ama-assn.org/practice-management/medicare-medicaid/2022-medicare-pay-schedule-confirms-congress-needs-act
- History of Medicare conversion factors. American Medical Association website. Accessed January 19, 2022. https://www.ama-assn.org/system/files/2021-01/cf-history.pdf
- American Academy of Dermatology. Dermatology World Weekly. December 15, 2021. Accessed January 20, 2022. https://www.aad.org/dw/weekly
- American Medical Association. CY 2022 Medicare Physician Fee Schedule (PFS) and Quality Payment Program (QPP) final rule summary. Accessed January 10, 2021. https://www.ama-assn.org/system/files/2022-pfs-qpp-final-rule.pdf
- Centers for Medicare & Medicaid Services. January 2022 alpha-numeric HCPCS file. Updated December 20, 2021. Accessed January 20, 2022. https://www.cms.gov/Medicare/Coding/HCPCSReleaseCodeSets/HCPCS-Quarterly-Update
- CMS finalizes Medicare payments for 2022. American Academy of Dermatology website. NEED PUB DATE. Accessed January 20, 2022. https://www.aad.org/member/practice/mips/fee-schedule/2022-fee-schedule-final
On November 2, 2021, the Centers for Medicare & Medicaid Services (CMS) released its final rule for the 2022 Medicare Physician Fee Schedule (PFS) and the Quality Payment Program (QPP).1,2 These guidelines contain updates that will remarkably impact the field of medicine—and dermatology in particular—in 2022. This article will walk you through some of the updates most relevant to dermatology and how they may affect your practice.
Process for the Final Rule
The CMS releases an annual rule for the PFS and QPP. The interim rule generally is released over the summer with preliminary guidelines for the upcoming payment year. There is then a period of open comment where those affected by these changes, including physicians and medical associations, can submit comments to support what has been proposed or advocate for any changes. This input is then reviewed, and a final rule generally is published in the fall.
For this calendar year, the interim 2022 rule was released on July 13, 2021,3 and included many of guidelines that will be discussed in more detail in this article. Many associations that represent medicine overall and specifically dermatology, including the American Medical Association and the American Academy of Dermatology, submitted comments in response to these proposals.4,5
PFS Conversion Factor
The PFS conversion factor is updated annually to ensure budget neutrality in the setting of changes in relative value units. For 2022, the PFS conversion factor is $34.6062, representing a reduction of approximately $0.29 from the 2021 PFS conversion factor of $34.8931.6 This reduction does not take into account other payment adjustments due to legislative changes.
In combination, these changes previously were estimated to represent an overall payment cut of 10% or higher for dermatology, with those practitioners doing more procedural work or dermatopathology likely being impacted more heavily. However, with the passing of the Protecting Medicare and American Farmers from Sequester Cuts Act, it is estimated that the reductions in payment to dermatology will begin at 0.75% and reach 2.75% in the second half of the year with the phased-in reinstatement of the Medicare sequester.4,5,7
Clinical Labor Pricing Updates
Starting in 2022, the CMS will utilize updated wage rates from the US Bureau of Labor Statistics to revise clinical labor costs over a 4-year period. Clinical labor rates are important, as they are used to calculate practice expense within the PFS. These clinical labor rates were last updated in 2002.8 Median wage data, as opposed to mean data, from the US Bureau of Labor Statistics will be utilized to calculate the updated clinical labor rates.
A multiyear implementation plan was put into place by CMS due to multiple concerns, including that current wage rates are inadequate and may not reflect current labor rate information. Additionally, comments on this proposal voiced concern that updating the supply and equipment pricing without updating the clinical labor pricing could create distortions in the allocation of direct practice expense, which also factored into the implementation of a multiyear plan.8
It is anticipated that specialties that rely primarily on clinical labor will receive the largest increases in these rates and that specialties that rely primarily on supply or equipment items are anticipated to receive the largest reductions relative to other specialties. Dermatology is estimated to have a 0% change during the year 1 transition period; however, it will have an estimated 1% reduction in clinical labor pricing overall once the updates are completed.1 Pathology also is estimated to have a similar overall decrease during this transition period.
Evaluation and Management Visits
The biggest update in this area primarily is related to refining policies for split (shared) evaluation and management (E/M) visits and teaching physician activities. Split E/M visits are defined by the CMS as visits provided in the facility setting by a physician and nonphysician practitioner in the same group, with the visit billed by whomever provides the substantive portion of the visit. For 2022, the term substantive portion will be defined by the CMS as history, physical examination, medical decision-making, or more than half of the total time; for 2023, it will be defined as more than half of the total time spent.3 A split visit also can apply to an E/M visit provided in part by both a teaching physician and resident. Split visits can be reported for new or established patients. For proper reimbursement, the 2 practitioners who performed the services must be documented in the medical record, and the practitioner who provided the substantive portion must sign and date the encounter in the medical record. Additionally, the CMS has indicated the modifier FS must be included on the claim to indicate the split visit.9
For dermatologists who act as teaching physicians, it is important to note that many of the existing CMS policies for billing E/M services are still in place, specifically that if a resident participates in a service in a teaching setting, the teaching physician can bill for the service only if they are present for the key or critical portion of the service. A primary care exception does exist, in which teaching physicians at certain teaching hospital primary care centers can bill for some services performed independently by a resident without the physical presence of the teaching physician; however, this often is not applicable within dermatology.
With updated outpatient E/M guidelines, if time is being selected to bill, only the time that the teaching physician was present can be included to determine the overall E/M level.
Billing for Physician Assistant Services
Currently Medicare can only make payments to the employer or independent contractor of a physician assistant (PA); however, starting January 1, 2022, the CMS has authorized Medicare to make direct payments to PAs for qualifying professional services, in the same manner that nurse practitioners can currently bill. This also will allow PAs to incorporate as a group and bill Medicare for PA services. This stems from a congressional mandate within the Consolidated Appropriations Act of 2021.8 As a result, in states where PAs can practice independently, they can opt out of physician-led care teams and furnish services independently, including dermatologic services.
QPP Updates
Several changes were made to the Merit-Based Incentive Payment System (MIPS). Some of these changes include:
- Increase the MIPS performance threshold to 75 points from 60 points.
- Set the performance threshold at 89 points.
- Reduce the quality performance category weight from 40% to 30% of the final MIPS score.
- Increase the cost performance category weight from 20% to 30% of the final MIPS score.
- The extreme and uncontrollable circumstances application also has been extended to the end of 2022, allowing those remarkably impacted by the COVID-19 public health emergency to request for reweighting on any or all MIPS performance categories.
Cost Measures and MIPS Value Pathways
The melanoma resection cost measure will be implemented in 2022, representing the first dermatology cost measure, which will include the cost to Medicare over a 1-year period for all patient care for the excision of a melanoma. Although cost measures will be part of the MIPS value pathways (MVPs) reporting, dermatology currently is not part of the MVP; however, with the CMS moving forward with an initial set of MVPs that physicians can voluntarily report on in 2023, there is a possibility that dermatology will be asked to be part of the program in the future.10
Final Thoughts
There are many upcoming changes as part of the 2022 final rule, including to the conversion factor, E/M split visits, PA billing, and the QPP. Advocacy in these areas to the CMS and lawmakers, either directly or through dermatologic and other medical societies, is critical to help influence eventual recommendations.
On November 2, 2021, the Centers for Medicare & Medicaid Services (CMS) released its final rule for the 2022 Medicare Physician Fee Schedule (PFS) and the Quality Payment Program (QPP).1,2 These guidelines contain updates that will remarkably impact the field of medicine—and dermatology in particular—in 2022. This article will walk you through some of the updates most relevant to dermatology and how they may affect your practice.
Process for the Final Rule
The CMS releases an annual rule for the PFS and QPP. The interim rule generally is released over the summer with preliminary guidelines for the upcoming payment year. There is then a period of open comment where those affected by these changes, including physicians and medical associations, can submit comments to support what has been proposed or advocate for any changes. This input is then reviewed, and a final rule generally is published in the fall.
For this calendar year, the interim 2022 rule was released on July 13, 2021,3 and included many of guidelines that will be discussed in more detail in this article. Many associations that represent medicine overall and specifically dermatology, including the American Medical Association and the American Academy of Dermatology, submitted comments in response to these proposals.4,5
PFS Conversion Factor
The PFS conversion factor is updated annually to ensure budget neutrality in the setting of changes in relative value units. For 2022, the PFS conversion factor is $34.6062, representing a reduction of approximately $0.29 from the 2021 PFS conversion factor of $34.8931.6 This reduction does not take into account other payment adjustments due to legislative changes.
In combination, these changes previously were estimated to represent an overall payment cut of 10% or higher for dermatology, with those practitioners doing more procedural work or dermatopathology likely being impacted more heavily. However, with the passing of the Protecting Medicare and American Farmers from Sequester Cuts Act, it is estimated that the reductions in payment to dermatology will begin at 0.75% and reach 2.75% in the second half of the year with the phased-in reinstatement of the Medicare sequester.4,5,7
Clinical Labor Pricing Updates
Starting in 2022, the CMS will utilize updated wage rates from the US Bureau of Labor Statistics to revise clinical labor costs over a 4-year period. Clinical labor rates are important, as they are used to calculate practice expense within the PFS. These clinical labor rates were last updated in 2002.8 Median wage data, as opposed to mean data, from the US Bureau of Labor Statistics will be utilized to calculate the updated clinical labor rates.
A multiyear implementation plan was put into place by CMS due to multiple concerns, including that current wage rates are inadequate and may not reflect current labor rate information. Additionally, comments on this proposal voiced concern that updating the supply and equipment pricing without updating the clinical labor pricing could create distortions in the allocation of direct practice expense, which also factored into the implementation of a multiyear plan.8
It is anticipated that specialties that rely primarily on clinical labor will receive the largest increases in these rates and that specialties that rely primarily on supply or equipment items are anticipated to receive the largest reductions relative to other specialties. Dermatology is estimated to have a 0% change during the year 1 transition period; however, it will have an estimated 1% reduction in clinical labor pricing overall once the updates are completed.1 Pathology also is estimated to have a similar overall decrease during this transition period.
Evaluation and Management Visits
The biggest update in this area primarily is related to refining policies for split (shared) evaluation and management (E/M) visits and teaching physician activities. Split E/M visits are defined by the CMS as visits provided in the facility setting by a physician and nonphysician practitioner in the same group, with the visit billed by whomever provides the substantive portion of the visit. For 2022, the term substantive portion will be defined by the CMS as history, physical examination, medical decision-making, or more than half of the total time; for 2023, it will be defined as more than half of the total time spent.3 A split visit also can apply to an E/M visit provided in part by both a teaching physician and resident. Split visits can be reported for new or established patients. For proper reimbursement, the 2 practitioners who performed the services must be documented in the medical record, and the practitioner who provided the substantive portion must sign and date the encounter in the medical record. Additionally, the CMS has indicated the modifier FS must be included on the claim to indicate the split visit.9
For dermatologists who act as teaching physicians, it is important to note that many of the existing CMS policies for billing E/M services are still in place, specifically that if a resident participates in a service in a teaching setting, the teaching physician can bill for the service only if they are present for the key or critical portion of the service. A primary care exception does exist, in which teaching physicians at certain teaching hospital primary care centers can bill for some services performed independently by a resident without the physical presence of the teaching physician; however, this often is not applicable within dermatology.
With updated outpatient E/M guidelines, if time is being selected to bill, only the time that the teaching physician was present can be included to determine the overall E/M level.
Billing for Physician Assistant Services
Currently Medicare can only make payments to the employer or independent contractor of a physician assistant (PA); however, starting January 1, 2022, the CMS has authorized Medicare to make direct payments to PAs for qualifying professional services, in the same manner that nurse practitioners can currently bill. This also will allow PAs to incorporate as a group and bill Medicare for PA services. This stems from a congressional mandate within the Consolidated Appropriations Act of 2021.8 As a result, in states where PAs can practice independently, they can opt out of physician-led care teams and furnish services independently, including dermatologic services.
QPP Updates
Several changes were made to the Merit-Based Incentive Payment System (MIPS). Some of these changes include:
- Increase the MIPS performance threshold to 75 points from 60 points.
- Set the performance threshold at 89 points.
- Reduce the quality performance category weight from 40% to 30% of the final MIPS score.
- Increase the cost performance category weight from 20% to 30% of the final MIPS score.
- The extreme and uncontrollable circumstances application also has been extended to the end of 2022, allowing those remarkably impacted by the COVID-19 public health emergency to request for reweighting on any or all MIPS performance categories.
Cost Measures and MIPS Value Pathways
The melanoma resection cost measure will be implemented in 2022, representing the first dermatology cost measure, which will include the cost to Medicare over a 1-year period for all patient care for the excision of a melanoma. Although cost measures will be part of the MIPS value pathways (MVPs) reporting, dermatology currently is not part of the MVP; however, with the CMS moving forward with an initial set of MVPs that physicians can voluntarily report on in 2023, there is a possibility that dermatology will be asked to be part of the program in the future.10
Final Thoughts
There are many upcoming changes as part of the 2022 final rule, including to the conversion factor, E/M split visits, PA billing, and the QPP. Advocacy in these areas to the CMS and lawmakers, either directly or through dermatologic and other medical societies, is critical to help influence eventual recommendations.
- Medicare Program; CY 2022 payment policies under the Physician Fee Schedule and other changes to part B payment policies; Medicare Shared Savings Program requirements; provider enrollment regulation updates; and provider and supplier prepayment and post-payment medical review requirements. Fed Regist. 2021;86:64996-66031. To be codified at 42 CFR §403, §405, §410, §411, §414, §415, §423, §424, and §425. https://www.federalregister.gov/documents/2021/11/19/2021-23972/medicare-program-cy-2022-payment-policies-under-the-physician-fee-schedule-and-other-changes-to-part
- Centers for Medicare & Medicaid Services. CMS physician payment rule promotes greater access to telehealth services, diabetes prevention programs. Published November 2, 2021. Accessed January 10, 2022. https://www.cms.gov/newsroom/press-releases/cms-physician-payment-rule-promotes-greater-access-telehealth-services-diabetes-prevention-programs
- Centers for Medicare & Medicaid Services. Calendar year (CY) 2022 Medicare Physician Fee Schedule proposed rule. Published July 13, 2021. Accessed January 10, 2022. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2022-medicare-physician-fee-schedule-proposed-rule
- American Academy of Dermatology. Dermatology World Weekly. October 27, 2021. Accessed January 20, 2022. https://www.aad.org/dw/weekly
- O’Reilly KB. 2022 Medicare pay schedule confirms Congress needs to act. American Medical Association website. Published November 10, 2021. Accessed January 10, 2021. https://www.ama-assn.org/practice-management/medicare-medicaid/2022-medicare-pay-schedule-confirms-congress-needs-act
- History of Medicare conversion factors. American Medical Association website. Accessed January 19, 2022. https://www.ama-assn.org/system/files/2021-01/cf-history.pdf
- American Academy of Dermatology. Dermatology World Weekly. December 15, 2021. Accessed January 20, 2022. https://www.aad.org/dw/weekly
- American Medical Association. CY 2022 Medicare Physician Fee Schedule (PFS) and Quality Payment Program (QPP) final rule summary. Accessed January 10, 2021. https://www.ama-assn.org/system/files/2022-pfs-qpp-final-rule.pdf
- Centers for Medicare & Medicaid Services. January 2022 alpha-numeric HCPCS file. Updated December 20, 2021. Accessed January 20, 2022. https://www.cms.gov/Medicare/Coding/HCPCSReleaseCodeSets/HCPCS-Quarterly-Update
- CMS finalizes Medicare payments for 2022. American Academy of Dermatology website. NEED PUB DATE. Accessed January 20, 2022. https://www.aad.org/member/practice/mips/fee-schedule/2022-fee-schedule-final
- Medicare Program; CY 2022 payment policies under the Physician Fee Schedule and other changes to part B payment policies; Medicare Shared Savings Program requirements; provider enrollment regulation updates; and provider and supplier prepayment and post-payment medical review requirements. Fed Regist. 2021;86:64996-66031. To be codified at 42 CFR §403, §405, §410, §411, §414, §415, §423, §424, and §425. https://www.federalregister.gov/documents/2021/11/19/2021-23972/medicare-program-cy-2022-payment-policies-under-the-physician-fee-schedule-and-other-changes-to-part
- Centers for Medicare & Medicaid Services. CMS physician payment rule promotes greater access to telehealth services, diabetes prevention programs. Published November 2, 2021. Accessed January 10, 2022. https://www.cms.gov/newsroom/press-releases/cms-physician-payment-rule-promotes-greater-access-telehealth-services-diabetes-prevention-programs
- Centers for Medicare & Medicaid Services. Calendar year (CY) 2022 Medicare Physician Fee Schedule proposed rule. Published July 13, 2021. Accessed January 10, 2022. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2022-medicare-physician-fee-schedule-proposed-rule
- American Academy of Dermatology. Dermatology World Weekly. October 27, 2021. Accessed January 20, 2022. https://www.aad.org/dw/weekly
- O’Reilly KB. 2022 Medicare pay schedule confirms Congress needs to act. American Medical Association website. Published November 10, 2021. Accessed January 10, 2021. https://www.ama-assn.org/practice-management/medicare-medicaid/2022-medicare-pay-schedule-confirms-congress-needs-act
- History of Medicare conversion factors. American Medical Association website. Accessed January 19, 2022. https://www.ama-assn.org/system/files/2021-01/cf-history.pdf
- American Academy of Dermatology. Dermatology World Weekly. December 15, 2021. Accessed January 20, 2022. https://www.aad.org/dw/weekly
- American Medical Association. CY 2022 Medicare Physician Fee Schedule (PFS) and Quality Payment Program (QPP) final rule summary. Accessed January 10, 2021. https://www.ama-assn.org/system/files/2022-pfs-qpp-final-rule.pdf
- Centers for Medicare & Medicaid Services. January 2022 alpha-numeric HCPCS file. Updated December 20, 2021. Accessed January 20, 2022. https://www.cms.gov/Medicare/Coding/HCPCSReleaseCodeSets/HCPCS-Quarterly-Update
- CMS finalizes Medicare payments for 2022. American Academy of Dermatology website. NEED PUB DATE. Accessed January 20, 2022. https://www.aad.org/member/practice/mips/fee-schedule/2022-fee-schedule-final
Practice Points
- The Centers for Medicare & Medicaid Services (CMS) 2022 final rule contains multiple updates affecting the practice of dermatology.
- Adjustments to the conversion factor and legislative-level actions have led to changes in reimbursement for many procedures within dermatology and beyond.
- Other notable updates include refining the definition of split evaluation and management visits, clinical labor pricing, and billing for physician assistant services.
- Changes in the Merit-Based Incentive Payment System (MIPS), cost measures, and MIPS value pathways also will impact many dermatology practices.
Advocacy Update: Is Your Practice Equipped to Handle Looming Changes in Dermatopathology?
The proposed 2022 Medicare physician fee schedule and quality payment program (QPP) regulations were released on July 13, 2021.1 Final regulations are expected to be released on or around November 1, 2021, but they may be delayed. Multiple national medical organizations, including the College of American Pathologists (CAP), the American Society of Dermatopathology, the American Academy of Dermatology Association (AADA), and the American Medical Association (AMA) Physicians’ Grassroots Network all work together to engage with the Centers for Medicare & Medicaid Services (CMS) to influence these regulations. Stated advocacy priorities include protecting the value of dermatopathology services, mobilizing dermatopathologists for political action, ensuring dermatopathologists can participate in new payment models, strengthening the profession with advocacy on a state level, and conducting socioeconomic research. Is your practice aware and prepared to handle the changes coming in 2022?
The recent revisions and revaluations of the outpatient evaluation and management (E/M) codes2 resulted in a considerable redistribution of Medicare dollars in 2021, negatively impacting dermatopathologists and other specialties and services due to budget neutrality required by law (Figure). Important steps were taken to mitigate the 2021 Medicare cuts for all non–office-based dermatopathology services (eg, pathology, surgical services, emergency department).1,3 Direct engagement by the CAP, American Society of Dermatopathology, and AADA, along with the AMA Physicians’ Grassroots Network resulted in legislative action on December 27, 2020, which directed Medicare to make a 3.75% positive adjustment to the 2021 physician payments. Additionally, the CMS updated the 2021 physician conversion factor to $34.8931, a 3.3% reduction from the 2020 conversion factor rather than $32.41, or a 10.20% decrease. The 2% payment adjustment (sequestration) through December 21, 2021, also was suspended, and Congress and the Biden administration mandated delayed implementation of the inherent complexity add-on code for E/M services (G2211) until 2024.1,3
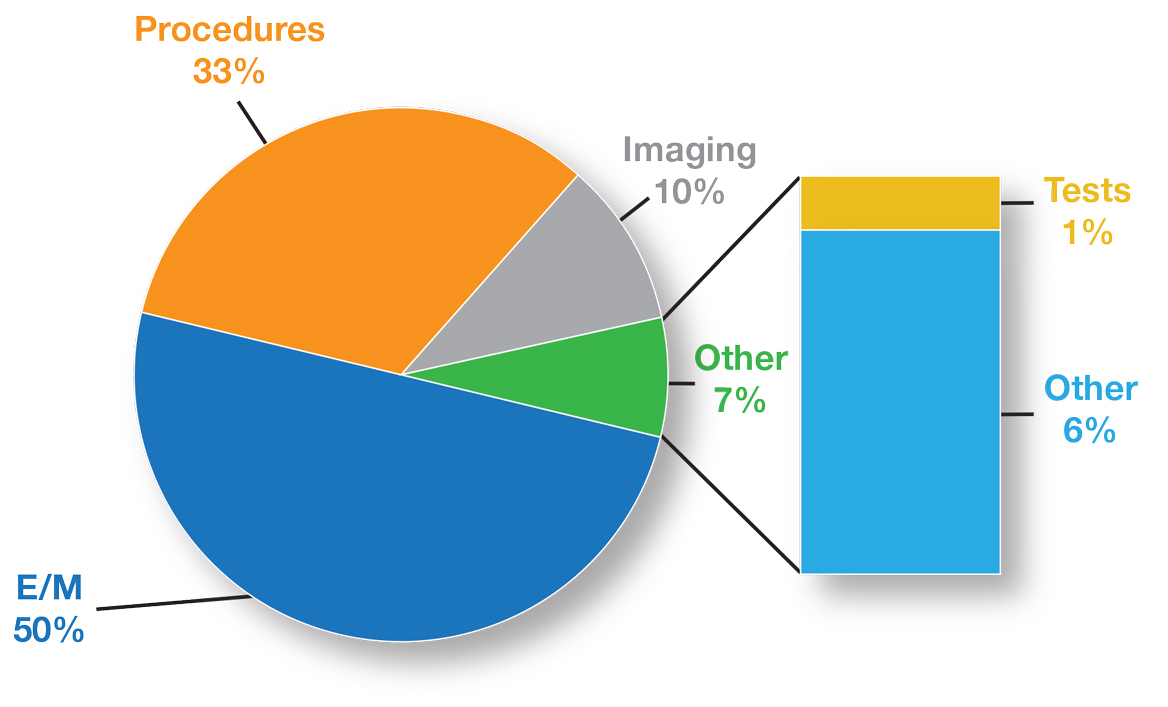
Threat of Medicare Cuts in 2022
Based on dermatopathology utilization data, the overall impact on reimbursement for 2022 represents an approximately 5% decrease from 2021 dermatopathology payments (Table 1).1,4 This represents a 3.75% cut from revaluation of E/M services, and a 1% cut due to changes in practice expense pricing. The estimated change in reimbursement for independent laboratories is a 6% decrease. Advocacy groups have been working to mitigate the 2022 cuts by engaging with Congress and urging them to act before these changes go into effect next year. Keep in mind that approximately half of all pathology Current Procedural Terminology (CPT) codes have been targeted for evaluation by the CMS since 2006.1,4
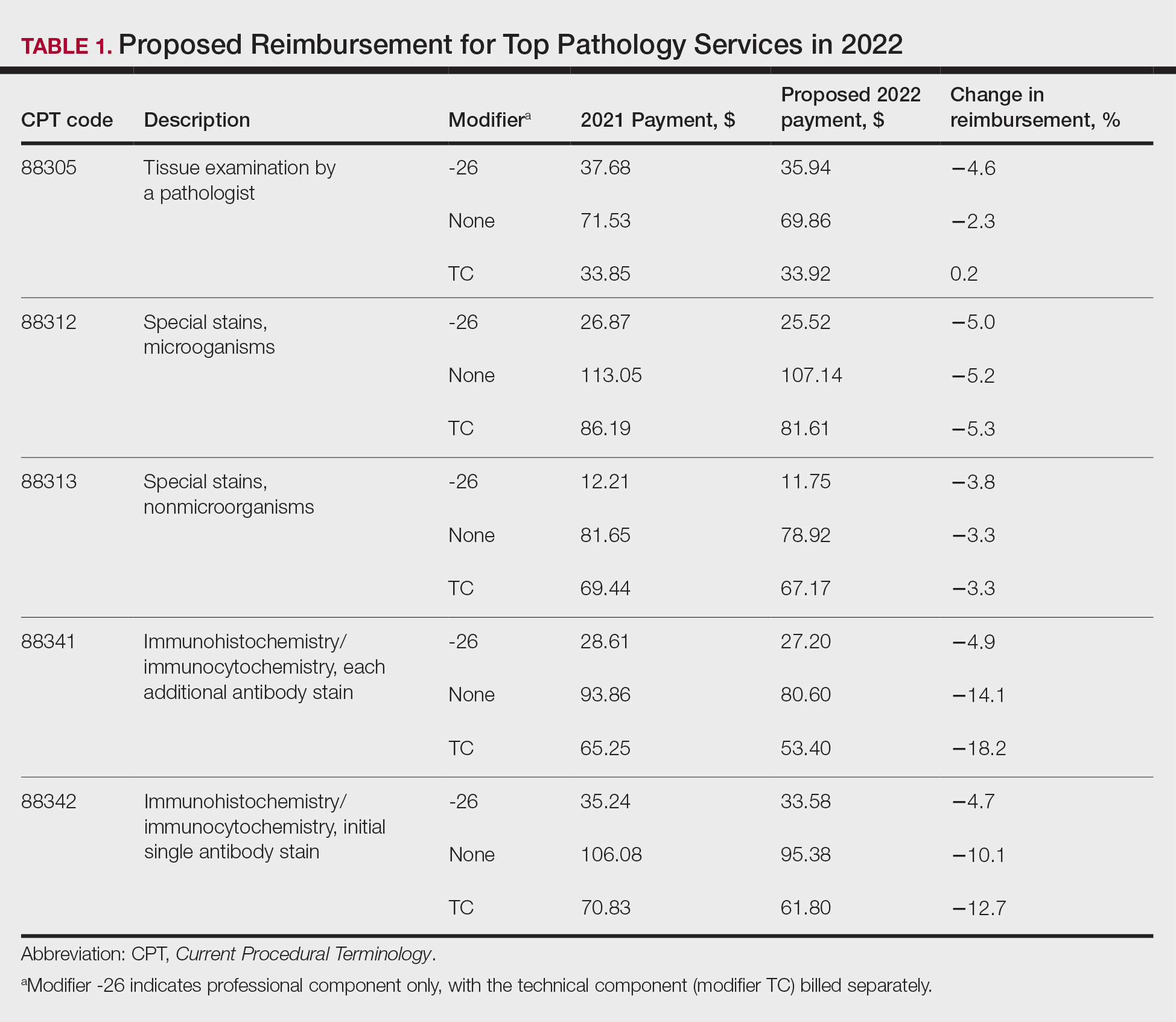
The current clinical pathology consultation services (CPT codes 80500 and 80502) previously were identified as potentially misvalued for review by the AMA Relative Value Scale Update Committee’s (RUC’s) relativity assessment workgroup.4 Consequently, the CAP worked with the AMA’s CPT Editorial Panel to delete codes 80500 and 80502, as well as to modernize and create the 4 new clinical pathology consultation codes: 80XX0, 80XX1, 80XX2, and 80XX3. Then the CAP worked with the RUC to develop physician work and practice expense values for the new clinical pathology consultation codes. Once the fee schedule is finalized, pathologists can begin using the new codes to bill these services in 2022 (Table 2).4
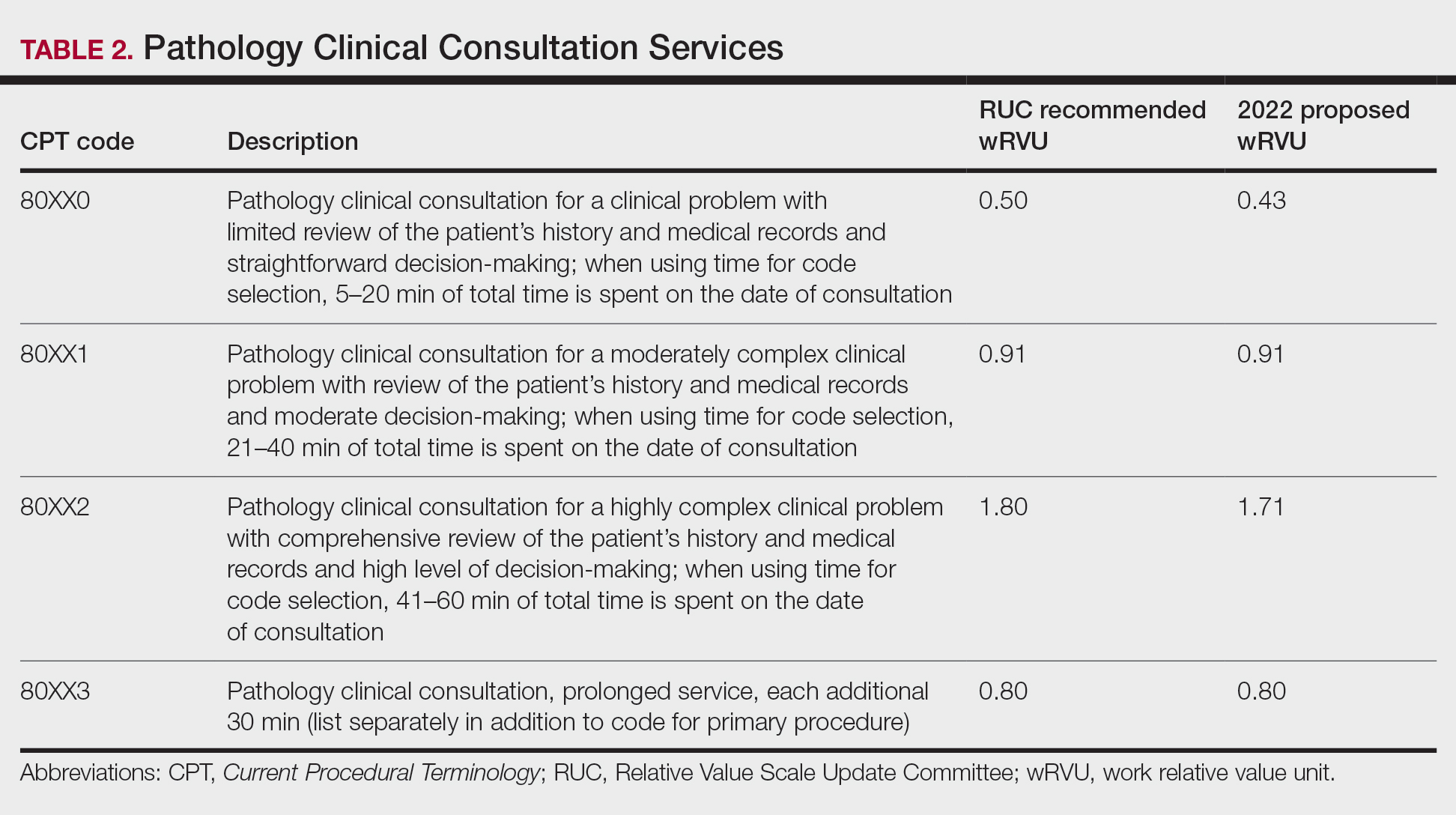
According to CPT, clinical pathology consultation services may be reported when the following criteria have been met: (1) the pathologist renders a clinical pathology consultation at the request of a physician or qualified health care professional at the same or another institution; (2) the pathology clinical consultation request relating to pathology and laboratory findings or other relevant clinical or diagnostic information requiring additional medical interpretative judgment is made; and (3) these codes are not reported in conjunction with codes 88321, 88323, and 88325.4
Proposed 2022 Medicare QPP Requirements
On July 13, 2021, the CMS also published its proposed 2022 QPP proposals that will take effect next year.4 According to the proposed regulation, nearly all dermatopathologists will be required to participate in Medicare’s QPP, either through advanced alternative payment models (APMs) or the Merit-based Incentive Payment System (MIPS). The CAP has long advocated for reducing MIPS reporting burdens for dermatopathologists. In this regulation, the CMS is proposing key program changes that move the program forward but also introduce additional complexities; for example, the CMS will move forward with a new participation pathway called MIPS Value Pathways (MVPs). The CMS proposed 7 specific MVPs that align with certain clinical topics; however, it will not implement these MVPs until the 2023 MIPS performance period.
In 2022, dermatopathologists who are eligible for MIPS will have to take action to avoid penalties that reduce future Medicare Part B payments for their services. Performance in MIPS in 2022 affects Medicare Part B payments in 2024 by an increase of 9% to a decrease of 9%.
In its proposed 2022 QPP regulations, the CMS proposed an increase of the performance threshold from 60 MIPS points to 75 MIPS points. It also proposed an increase of the exceptional Performance Threshold from 85 MIPS points to 89 MIPS points.
The CMS also proposed notable scoring changes for quality measures, including removing the 3-point floor for measures that can be scored against a benchmark. These measures would receive 1 to 10 points. Measures without a benchmark or that do not meet case requirements would earn 0 points, with an exception for small practices. The CMS also proposed removing bonus points for reporting additional outcomes and high-priority measures beyond the 1 that is required, as well as establishing a 5-point floor for the first 2 performance periods for new measures, which is in line with the CAP’s advocacy.
The Pathology Specialty Measure Set will remain the same as the 2021 set containing 6 quality measures, including the AADA-stewarded quality measure #440 (skin cancer: biopsy reporting time—pathologist to clinician). Although the CAP recognizes the importance of prompt turnaround of biopsy reports, it also is working with the CMS and the AADA to mitigate the operational challenges dermatopathologists encounter when using this measure.
Due to advocacy from the CAP, the CMS included a CAP-proposed improvement activity on implementation of a laboratory preparedness plan to support continued or expanded patient care during the COVID-19 pandemic or another public health emergency. This plan should address how the laboratory would maintain or expand access to improve beneficiary health outcomes and reduce health care disparities.
The CAP has actively worked with the CMS to demonstrate the need for more appropriate and alternative measures and improvement activities so that pathologists can more fully participate in MIPS.
Alternative Payment Models—For those dermatopathologists who practice in an APM, the proposed 2022 QPP makes minimal changes to the advanced APM track while adding transition time for accountable care organizations in the Medicare Shared Savings Program to report on certain quality measures and increasing flexibility related to the program’s quality performance standard.
Cures Act 2021: To Do No Harm
The 21st Century Cures Act (Cures Act) was signed into federal law in 2016. The Office of the National Coordinator for Health Information Technology (ONC) laid the groundwork for patients to have easier access to and control of their health information.5 The ONC’s final rule, which went into effect on April 5, 2021, requires that all providers make their office notes, laboratory results, and other diagnostic reports (including dermatopathology reports) available to patients as soon as the physician’s office receives an electronic copy. Penalty for noncompliance has not been determined.
There are information-blocking exceptions, but delaying access to a patient’s report so that a provider can review the result before the patient receives it is not considered an exception.6 The exceptions are situational and must be evaluated by the referring clinician or their employer. Documentation of the exception is critical. The specific facts and circumstances associated with your decision to use an exception will be important to include in your documentation. Information blocking necessary to prevent “harm” to a patient or another person requires a reasonable belief that the practice will substantially reduce the risk of harm.6
The AMA passed a resolution in June 2021 calling for changes to this rule to allow for a delay of pathology results, advocating to the Office for Civil Rights to revise the harm exception to include psychological distress.6 In August 2021, the AADA met with senior officials at the ONC also asking to revise its definition of harm, sharing examples of emotional strain that resulted from receiving results without clinical context.7 California enacted a law requiring a delay before a patient receives the result of a malignant diagnosis, giving the clinician time to contact the patient before they see their report.8
The Cures Act requirements are about patients accessing their health care information. Always consider what is best for the patient and ensure that your policies and procedures reflect this.5
Final Thoughts
It is important to learn and support advocacy priorities and efforts and to join forces to protect your practice. Physician advocacy is no longer an elective pursuit. We need to be involved and engaged through our medical societies to help patients, communities, and ourselves.
- Centers for Medicare & Medicaid Services. Calendar Year (CY) 2022 Medicare Physician Fee Schedule Proposed Rule. Published July 13, 2021. Accessed October 22, 2021. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2022-medicare-physician-fee-schedule-proposed-rule
- Healthcare spending and the Medicare program. Medicare Payment Advisory Commission; July 2020. Accessed October 25, 2021.http://www.medpac.gov/docs/default-source/data-book/july2020_databook_entirereport_sec.pdf
- Frieden J. 2021 Medicare fee schedule includes 10.2% cut in conversion factor. MedPage Today website. Published December 2, 2020. Accessed October 22, 2021. https://www.medpagetoday.com/practicemanagement/reimbursement/89970
- Advocacy. College of American Pathologists website. Accessed October 13, 2021. https://www.cap.org/advocacy
- ONC’s Cures Act Final Rule. The Office of the National Coordinator for Health Information Technology website. Accessed October 13, 2021. https://www.healthit.gov/curesrule/
- Nelson H. Delegates call AMA to advocate for provider info-blocking flexibility. Published June 18, 2021. Accessed October 13, 2021. https://ehrintelligence.com/news/delegates-call-ama-to-advocate-for-provider-info-blocking-flexibility
- Rosamilia LL. Immediate Pathology report release to patients—is the 21st Century Cures Act worse than the disease? American Academy of Dermatology website. Published August 25, 2021. Accessed October 22, 2021. https://www.aad.org/dw/dw-insights-and-inquiries/archive/2021/cures-act-immediate-pathology-report-release-to-patients
- Purington K, Alfreds ST, Pritts J, et al; The National Academy for State Health Policy. Electronic release of clinical laboratory results: a review of state and federal policy. Published January 2010. Accessed October 13, 2021. https://www.nashp.org/wp-content/uploads/2010/02/ElectronicLabResultsExchangePolicy.pdf
The proposed 2022 Medicare physician fee schedule and quality payment program (QPP) regulations were released on July 13, 2021.1 Final regulations are expected to be released on or around November 1, 2021, but they may be delayed. Multiple national medical organizations, including the College of American Pathologists (CAP), the American Society of Dermatopathology, the American Academy of Dermatology Association (AADA), and the American Medical Association (AMA) Physicians’ Grassroots Network all work together to engage with the Centers for Medicare & Medicaid Services (CMS) to influence these regulations. Stated advocacy priorities include protecting the value of dermatopathology services, mobilizing dermatopathologists for political action, ensuring dermatopathologists can participate in new payment models, strengthening the profession with advocacy on a state level, and conducting socioeconomic research. Is your practice aware and prepared to handle the changes coming in 2022?
The recent revisions and revaluations of the outpatient evaluation and management (E/M) codes2 resulted in a considerable redistribution of Medicare dollars in 2021, negatively impacting dermatopathologists and other specialties and services due to budget neutrality required by law (Figure). Important steps were taken to mitigate the 2021 Medicare cuts for all non–office-based dermatopathology services (eg, pathology, surgical services, emergency department).1,3 Direct engagement by the CAP, American Society of Dermatopathology, and AADA, along with the AMA Physicians’ Grassroots Network resulted in legislative action on December 27, 2020, which directed Medicare to make a 3.75% positive adjustment to the 2021 physician payments. Additionally, the CMS updated the 2021 physician conversion factor to $34.8931, a 3.3% reduction from the 2020 conversion factor rather than $32.41, or a 10.20% decrease. The 2% payment adjustment (sequestration) through December 21, 2021, also was suspended, and Congress and the Biden administration mandated delayed implementation of the inherent complexity add-on code for E/M services (G2211) until 2024.1,3

Threat of Medicare Cuts in 2022
Based on dermatopathology utilization data, the overall impact on reimbursement for 2022 represents an approximately 5% decrease from 2021 dermatopathology payments (Table 1).1,4 This represents a 3.75% cut from revaluation of E/M services, and a 1% cut due to changes in practice expense pricing. The estimated change in reimbursement for independent laboratories is a 6% decrease. Advocacy groups have been working to mitigate the 2022 cuts by engaging with Congress and urging them to act before these changes go into effect next year. Keep in mind that approximately half of all pathology Current Procedural Terminology (CPT) codes have been targeted for evaluation by the CMS since 2006.1,4

The current clinical pathology consultation services (CPT codes 80500 and 80502) previously were identified as potentially misvalued for review by the AMA Relative Value Scale Update Committee’s (RUC’s) relativity assessment workgroup.4 Consequently, the CAP worked with the AMA’s CPT Editorial Panel to delete codes 80500 and 80502, as well as to modernize and create the 4 new clinical pathology consultation codes: 80XX0, 80XX1, 80XX2, and 80XX3. Then the CAP worked with the RUC to develop physician work and practice expense values for the new clinical pathology consultation codes. Once the fee schedule is finalized, pathologists can begin using the new codes to bill these services in 2022 (Table 2).4

According to CPT, clinical pathology consultation services may be reported when the following criteria have been met: (1) the pathologist renders a clinical pathology consultation at the request of a physician or qualified health care professional at the same or another institution; (2) the pathology clinical consultation request relating to pathology and laboratory findings or other relevant clinical or diagnostic information requiring additional medical interpretative judgment is made; and (3) these codes are not reported in conjunction with codes 88321, 88323, and 88325.4
Proposed 2022 Medicare QPP Requirements
On July 13, 2021, the CMS also published its proposed 2022 QPP proposals that will take effect next year.4 According to the proposed regulation, nearly all dermatopathologists will be required to participate in Medicare’s QPP, either through advanced alternative payment models (APMs) or the Merit-based Incentive Payment System (MIPS). The CAP has long advocated for reducing MIPS reporting burdens for dermatopathologists. In this regulation, the CMS is proposing key program changes that move the program forward but also introduce additional complexities; for example, the CMS will move forward with a new participation pathway called MIPS Value Pathways (MVPs). The CMS proposed 7 specific MVPs that align with certain clinical topics; however, it will not implement these MVPs until the 2023 MIPS performance period.
In 2022, dermatopathologists who are eligible for MIPS will have to take action to avoid penalties that reduce future Medicare Part B payments for their services. Performance in MIPS in 2022 affects Medicare Part B payments in 2024 by an increase of 9% to a decrease of 9%.
In its proposed 2022 QPP regulations, the CMS proposed an increase of the performance threshold from 60 MIPS points to 75 MIPS points. It also proposed an increase of the exceptional Performance Threshold from 85 MIPS points to 89 MIPS points.
The CMS also proposed notable scoring changes for quality measures, including removing the 3-point floor for measures that can be scored against a benchmark. These measures would receive 1 to 10 points. Measures without a benchmark or that do not meet case requirements would earn 0 points, with an exception for small practices. The CMS also proposed removing bonus points for reporting additional outcomes and high-priority measures beyond the 1 that is required, as well as establishing a 5-point floor for the first 2 performance periods for new measures, which is in line with the CAP’s advocacy.
The Pathology Specialty Measure Set will remain the same as the 2021 set containing 6 quality measures, including the AADA-stewarded quality measure #440 (skin cancer: biopsy reporting time—pathologist to clinician). Although the CAP recognizes the importance of prompt turnaround of biopsy reports, it also is working with the CMS and the AADA to mitigate the operational challenges dermatopathologists encounter when using this measure.
Due to advocacy from the CAP, the CMS included a CAP-proposed improvement activity on implementation of a laboratory preparedness plan to support continued or expanded patient care during the COVID-19 pandemic or another public health emergency. This plan should address how the laboratory would maintain or expand access to improve beneficiary health outcomes and reduce health care disparities.
The CAP has actively worked with the CMS to demonstrate the need for more appropriate and alternative measures and improvement activities so that pathologists can more fully participate in MIPS.
Alternative Payment Models—For those dermatopathologists who practice in an APM, the proposed 2022 QPP makes minimal changes to the advanced APM track while adding transition time for accountable care organizations in the Medicare Shared Savings Program to report on certain quality measures and increasing flexibility related to the program’s quality performance standard.
Cures Act 2021: To Do No Harm
The 21st Century Cures Act (Cures Act) was signed into federal law in 2016. The Office of the National Coordinator for Health Information Technology (ONC) laid the groundwork for patients to have easier access to and control of their health information.5 The ONC’s final rule, which went into effect on April 5, 2021, requires that all providers make their office notes, laboratory results, and other diagnostic reports (including dermatopathology reports) available to patients as soon as the physician’s office receives an electronic copy. Penalty for noncompliance has not been determined.
There are information-blocking exceptions, but delaying access to a patient’s report so that a provider can review the result before the patient receives it is not considered an exception.6 The exceptions are situational and must be evaluated by the referring clinician or their employer. Documentation of the exception is critical. The specific facts and circumstances associated with your decision to use an exception will be important to include in your documentation. Information blocking necessary to prevent “harm” to a patient or another person requires a reasonable belief that the practice will substantially reduce the risk of harm.6
The AMA passed a resolution in June 2021 calling for changes to this rule to allow for a delay of pathology results, advocating to the Office for Civil Rights to revise the harm exception to include psychological distress.6 In August 2021, the AADA met with senior officials at the ONC also asking to revise its definition of harm, sharing examples of emotional strain that resulted from receiving results without clinical context.7 California enacted a law requiring a delay before a patient receives the result of a malignant diagnosis, giving the clinician time to contact the patient before they see their report.8
The Cures Act requirements are about patients accessing their health care information. Always consider what is best for the patient and ensure that your policies and procedures reflect this.5
Final Thoughts
It is important to learn and support advocacy priorities and efforts and to join forces to protect your practice. Physician advocacy is no longer an elective pursuit. We need to be involved and engaged through our medical societies to help patients, communities, and ourselves.
The proposed 2022 Medicare physician fee schedule and quality payment program (QPP) regulations were released on July 13, 2021.1 Final regulations are expected to be released on or around November 1, 2021, but they may be delayed. Multiple national medical organizations, including the College of American Pathologists (CAP), the American Society of Dermatopathology, the American Academy of Dermatology Association (AADA), and the American Medical Association (AMA) Physicians’ Grassroots Network all work together to engage with the Centers for Medicare & Medicaid Services (CMS) to influence these regulations. Stated advocacy priorities include protecting the value of dermatopathology services, mobilizing dermatopathologists for political action, ensuring dermatopathologists can participate in new payment models, strengthening the profession with advocacy on a state level, and conducting socioeconomic research. Is your practice aware and prepared to handle the changes coming in 2022?
The recent revisions and revaluations of the outpatient evaluation and management (E/M) codes2 resulted in a considerable redistribution of Medicare dollars in 2021, negatively impacting dermatopathologists and other specialties and services due to budget neutrality required by law (Figure). Important steps were taken to mitigate the 2021 Medicare cuts for all non–office-based dermatopathology services (eg, pathology, surgical services, emergency department).1,3 Direct engagement by the CAP, American Society of Dermatopathology, and AADA, along with the AMA Physicians’ Grassroots Network resulted in legislative action on December 27, 2020, which directed Medicare to make a 3.75% positive adjustment to the 2021 physician payments. Additionally, the CMS updated the 2021 physician conversion factor to $34.8931, a 3.3% reduction from the 2020 conversion factor rather than $32.41, or a 10.20% decrease. The 2% payment adjustment (sequestration) through December 21, 2021, also was suspended, and Congress and the Biden administration mandated delayed implementation of the inherent complexity add-on code for E/M services (G2211) until 2024.1,3

Threat of Medicare Cuts in 2022
Based on dermatopathology utilization data, the overall impact on reimbursement for 2022 represents an approximately 5% decrease from 2021 dermatopathology payments (Table 1).1,4 This represents a 3.75% cut from revaluation of E/M services, and a 1% cut due to changes in practice expense pricing. The estimated change in reimbursement for independent laboratories is a 6% decrease. Advocacy groups have been working to mitigate the 2022 cuts by engaging with Congress and urging them to act before these changes go into effect next year. Keep in mind that approximately half of all pathology Current Procedural Terminology (CPT) codes have been targeted for evaluation by the CMS since 2006.1,4

The current clinical pathology consultation services (CPT codes 80500 and 80502) previously were identified as potentially misvalued for review by the AMA Relative Value Scale Update Committee’s (RUC’s) relativity assessment workgroup.4 Consequently, the CAP worked with the AMA’s CPT Editorial Panel to delete codes 80500 and 80502, as well as to modernize and create the 4 new clinical pathology consultation codes: 80XX0, 80XX1, 80XX2, and 80XX3. Then the CAP worked with the RUC to develop physician work and practice expense values for the new clinical pathology consultation codes. Once the fee schedule is finalized, pathologists can begin using the new codes to bill these services in 2022 (Table 2).4

According to CPT, clinical pathology consultation services may be reported when the following criteria have been met: (1) the pathologist renders a clinical pathology consultation at the request of a physician or qualified health care professional at the same or another institution; (2) the pathology clinical consultation request relating to pathology and laboratory findings or other relevant clinical or diagnostic information requiring additional medical interpretative judgment is made; and (3) these codes are not reported in conjunction with codes 88321, 88323, and 88325.4
Proposed 2022 Medicare QPP Requirements
On July 13, 2021, the CMS also published its proposed 2022 QPP proposals that will take effect next year.4 According to the proposed regulation, nearly all dermatopathologists will be required to participate in Medicare’s QPP, either through advanced alternative payment models (APMs) or the Merit-based Incentive Payment System (MIPS). The CAP has long advocated for reducing MIPS reporting burdens for dermatopathologists. In this regulation, the CMS is proposing key program changes that move the program forward but also introduce additional complexities; for example, the CMS will move forward with a new participation pathway called MIPS Value Pathways (MVPs). The CMS proposed 7 specific MVPs that align with certain clinical topics; however, it will not implement these MVPs until the 2023 MIPS performance period.
In 2022, dermatopathologists who are eligible for MIPS will have to take action to avoid penalties that reduce future Medicare Part B payments for their services. Performance in MIPS in 2022 affects Medicare Part B payments in 2024 by an increase of 9% to a decrease of 9%.
In its proposed 2022 QPP regulations, the CMS proposed an increase of the performance threshold from 60 MIPS points to 75 MIPS points. It also proposed an increase of the exceptional Performance Threshold from 85 MIPS points to 89 MIPS points.
The CMS also proposed notable scoring changes for quality measures, including removing the 3-point floor for measures that can be scored against a benchmark. These measures would receive 1 to 10 points. Measures without a benchmark or that do not meet case requirements would earn 0 points, with an exception for small practices. The CMS also proposed removing bonus points for reporting additional outcomes and high-priority measures beyond the 1 that is required, as well as establishing a 5-point floor for the first 2 performance periods for new measures, which is in line with the CAP’s advocacy.
The Pathology Specialty Measure Set will remain the same as the 2021 set containing 6 quality measures, including the AADA-stewarded quality measure #440 (skin cancer: biopsy reporting time—pathologist to clinician). Although the CAP recognizes the importance of prompt turnaround of biopsy reports, it also is working with the CMS and the AADA to mitigate the operational challenges dermatopathologists encounter when using this measure.
Due to advocacy from the CAP, the CMS included a CAP-proposed improvement activity on implementation of a laboratory preparedness plan to support continued or expanded patient care during the COVID-19 pandemic or another public health emergency. This plan should address how the laboratory would maintain or expand access to improve beneficiary health outcomes and reduce health care disparities.
The CAP has actively worked with the CMS to demonstrate the need for more appropriate and alternative measures and improvement activities so that pathologists can more fully participate in MIPS.
Alternative Payment Models—For those dermatopathologists who practice in an APM, the proposed 2022 QPP makes minimal changes to the advanced APM track while adding transition time for accountable care organizations in the Medicare Shared Savings Program to report on certain quality measures and increasing flexibility related to the program’s quality performance standard.
Cures Act 2021: To Do No Harm
The 21st Century Cures Act (Cures Act) was signed into federal law in 2016. The Office of the National Coordinator for Health Information Technology (ONC) laid the groundwork for patients to have easier access to and control of their health information.5 The ONC’s final rule, which went into effect on April 5, 2021, requires that all providers make their office notes, laboratory results, and other diagnostic reports (including dermatopathology reports) available to patients as soon as the physician’s office receives an electronic copy. Penalty for noncompliance has not been determined.
There are information-blocking exceptions, but delaying access to a patient’s report so that a provider can review the result before the patient receives it is not considered an exception.6 The exceptions are situational and must be evaluated by the referring clinician or their employer. Documentation of the exception is critical. The specific facts and circumstances associated with your decision to use an exception will be important to include in your documentation. Information blocking necessary to prevent “harm” to a patient or another person requires a reasonable belief that the practice will substantially reduce the risk of harm.6
The AMA passed a resolution in June 2021 calling for changes to this rule to allow for a delay of pathology results, advocating to the Office for Civil Rights to revise the harm exception to include psychological distress.6 In August 2021, the AADA met with senior officials at the ONC also asking to revise its definition of harm, sharing examples of emotional strain that resulted from receiving results without clinical context.7 California enacted a law requiring a delay before a patient receives the result of a malignant diagnosis, giving the clinician time to contact the patient before they see their report.8
The Cures Act requirements are about patients accessing their health care information. Always consider what is best for the patient and ensure that your policies and procedures reflect this.5
Final Thoughts
It is important to learn and support advocacy priorities and efforts and to join forces to protect your practice. Physician advocacy is no longer an elective pursuit. We need to be involved and engaged through our medical societies to help patients, communities, and ourselves.
- Centers for Medicare & Medicaid Services. Calendar Year (CY) 2022 Medicare Physician Fee Schedule Proposed Rule. Published July 13, 2021. Accessed October 22, 2021. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2022-medicare-physician-fee-schedule-proposed-rule
- Healthcare spending and the Medicare program. Medicare Payment Advisory Commission; July 2020. Accessed October 25, 2021.http://www.medpac.gov/docs/default-source/data-book/july2020_databook_entirereport_sec.pdf
- Frieden J. 2021 Medicare fee schedule includes 10.2% cut in conversion factor. MedPage Today website. Published December 2, 2020. Accessed October 22, 2021. https://www.medpagetoday.com/practicemanagement/reimbursement/89970
- Advocacy. College of American Pathologists website. Accessed October 13, 2021. https://www.cap.org/advocacy
- ONC’s Cures Act Final Rule. The Office of the National Coordinator for Health Information Technology website. Accessed October 13, 2021. https://www.healthit.gov/curesrule/
- Nelson H. Delegates call AMA to advocate for provider info-blocking flexibility. Published June 18, 2021. Accessed October 13, 2021. https://ehrintelligence.com/news/delegates-call-ama-to-advocate-for-provider-info-blocking-flexibility
- Rosamilia LL. Immediate Pathology report release to patients—is the 21st Century Cures Act worse than the disease? American Academy of Dermatology website. Published August 25, 2021. Accessed October 22, 2021. https://www.aad.org/dw/dw-insights-and-inquiries/archive/2021/cures-act-immediate-pathology-report-release-to-patients
- Purington K, Alfreds ST, Pritts J, et al; The National Academy for State Health Policy. Electronic release of clinical laboratory results: a review of state and federal policy. Published January 2010. Accessed October 13, 2021. https://www.nashp.org/wp-content/uploads/2010/02/ElectronicLabResultsExchangePolicy.pdf
- Centers for Medicare & Medicaid Services. Calendar Year (CY) 2022 Medicare Physician Fee Schedule Proposed Rule. Published July 13, 2021. Accessed October 22, 2021. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2022-medicare-physician-fee-schedule-proposed-rule
- Healthcare spending and the Medicare program. Medicare Payment Advisory Commission; July 2020. Accessed October 25, 2021.http://www.medpac.gov/docs/default-source/data-book/july2020_databook_entirereport_sec.pdf
- Frieden J. 2021 Medicare fee schedule includes 10.2% cut in conversion factor. MedPage Today website. Published December 2, 2020. Accessed October 22, 2021. https://www.medpagetoday.com/practicemanagement/reimbursement/89970
- Advocacy. College of American Pathologists website. Accessed October 13, 2021. https://www.cap.org/advocacy
- ONC’s Cures Act Final Rule. The Office of the National Coordinator for Health Information Technology website. Accessed October 13, 2021. https://www.healthit.gov/curesrule/
- Nelson H. Delegates call AMA to advocate for provider info-blocking flexibility. Published June 18, 2021. Accessed October 13, 2021. https://ehrintelligence.com/news/delegates-call-ama-to-advocate-for-provider-info-blocking-flexibility
- Rosamilia LL. Immediate Pathology report release to patients—is the 21st Century Cures Act worse than the disease? American Academy of Dermatology website. Published August 25, 2021. Accessed October 22, 2021. https://www.aad.org/dw/dw-insights-and-inquiries/archive/2021/cures-act-immediate-pathology-report-release-to-patients
- Purington K, Alfreds ST, Pritts J, et al; The National Academy for State Health Policy. Electronic release of clinical laboratory results: a review of state and federal policy. Published January 2010. Accessed October 13, 2021. https://www.nashp.org/wp-content/uploads/2010/02/ElectronicLabResultsExchangePolicy.pdf
Practice Points
- A proposed 2022 fee schedule negatively impacting dermatopathology practices has been published by the Centers for Medicare & Medicaid Services (CMS) in July 2021.
- New pathology consultation codes with new payment rates proposed by CMS can be used starting January 1, 2022.
- The 21st Century Cures Act Final Rule has information blocking provisions.
E/M Coding in 2021: The Times (and More) Are A-Changin’
Effective on January 1, 2021, the outpatient evaluation and management (E/M) codes underwent substantial changes, which were the culmination of multiple years of revision and surveying via the American Medical Association (AMA) Relative Value Scale Update Committee and Current Procedural Terminology (RUC-CPT) process to streamline definitions and promote consistency as well as to decrease the administrative burden for all specialties within the house of medicine.1 These updates represent a notable change from the previous documentation requirements for this oft used family of codes. Herein, we break down some of the highlights of the changes and how they may be applied for some commonly used dermatologic diagnoses.
Time Is Time Is Time
Prior to the 2021 revisions, a physician generally could only code for an E/M level by time for a face-to-face encounter dominated by counseling and/or care coordination. With the new updates, any encounter can be coded by total time spent by the physician with the patient1; however, clinical staff time is not included. There also are now clear guidelines of the time ranges corresponding to the level of E/M,1 as noted in Table 1.
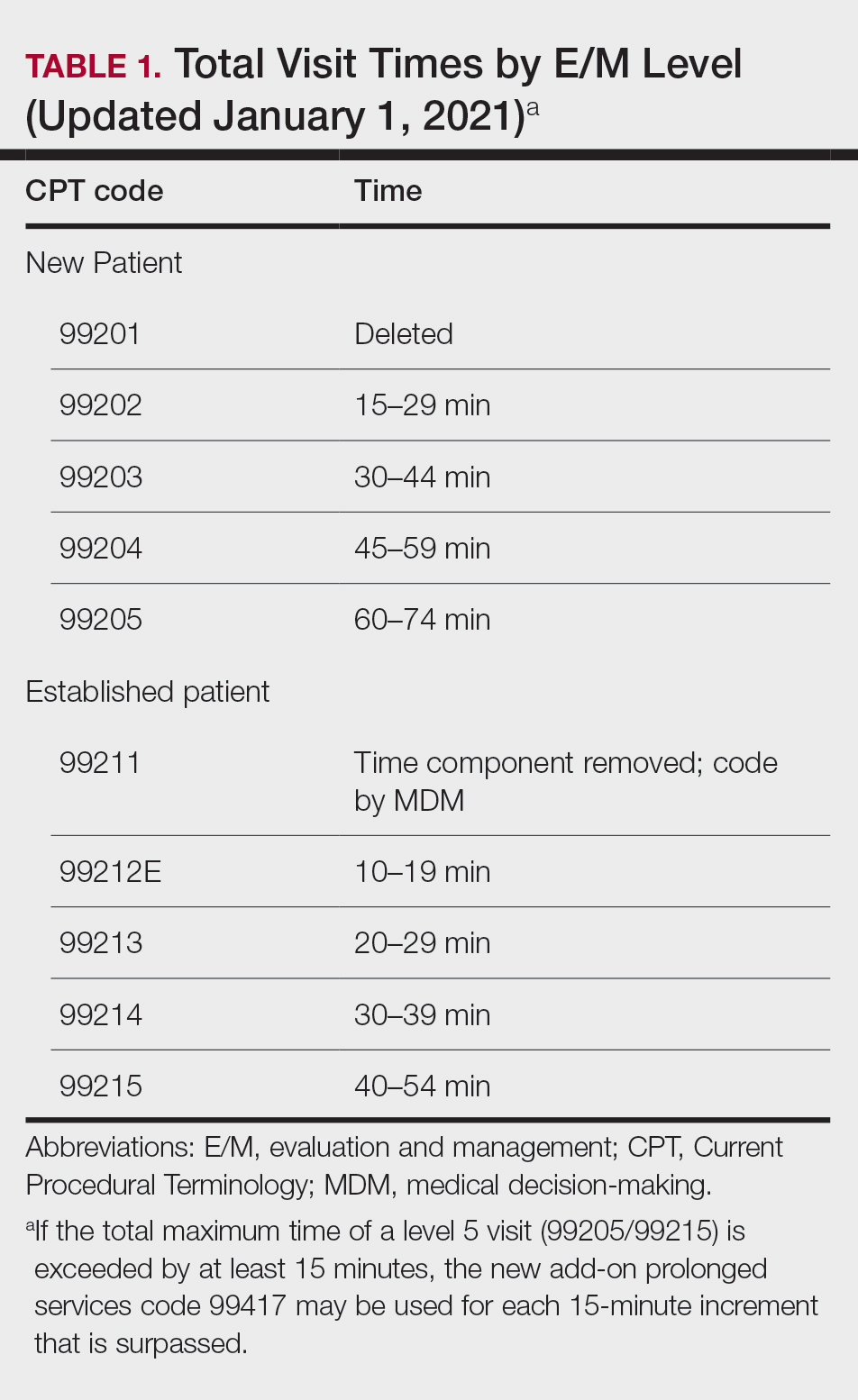
Importantly, time now includes not just face-to-face time with the patient but also any time on the date of the encounter that the physician is involved in the care of the patient when not reported with a separate code. This can include reviewing notes or data before or after the examination, care coordination, ordering laboratory tests, and providing any documentation related to the encounter. Importantly, this applies only when these activities are done on the date of the encounter.
If you work with a nurse practitioner or physician assistant (PA) who assists you and you are the one reporting the service, you cannot double-dip. For example, if your PA spends 10 minutes alone with a patient, you are in the room together for 5 minutes, the PA spends another 10 minutes alone with the patient afterward, and you do chart work for 10 minutes at the end of the day, the total time spent is 35 minutes, not 40 minutes, as you cannot count the time you and the PA spent together twice.
Decisions, Decisions
Evaluation and management coding also can be determined via the level of medical decision-making (MDM). Per the 2021 guidelines, MDM is comprised of 3 categories: (1) number and complexity of problems addressed at the encounter, (2) amount and/or complexity of data to be reviewed or analyzed, and (3) risk of complications and/or morbidity or mortality of patient management.1 To reach a certain overall E/M level, 2 of 3 categories must be met or exceeded. Let’s dive into each of these in a little more detail.
Number and Complexity of Problems Addressed at the Encounter
First, it is important to understand the definition of a problem addressed. Per AMA guidelines, this includes a disease, condition, illness, injury, symptom, sign, finding, complaint, or other matter addressed at the encounter that is evaluated or treated at the encounter by the physician. If the problem is referred to another provider without evaluation or consideration of treatment, it is not considered to be a problem addressed and cannot count toward this first category. An example could be a patient with a lump on the abdomen that you refer to plastic or general surgery for evaluation and treatment.
Once you have determined that you are addressing a problem, you will need to determine the level of complexity of the problem, as outlined in Table 2. Keep in mind that some entities and disease states in dermatology may fit the requirements of more than 1 level of complexity depending on the clinical situation, while there are many entities in dermatology that may not be perfectly captured by any of the levels described. In these situations, clinical judgement is required to determine where the problem would best fit. Importantly, whatever you decide, your documentation should support that decision.
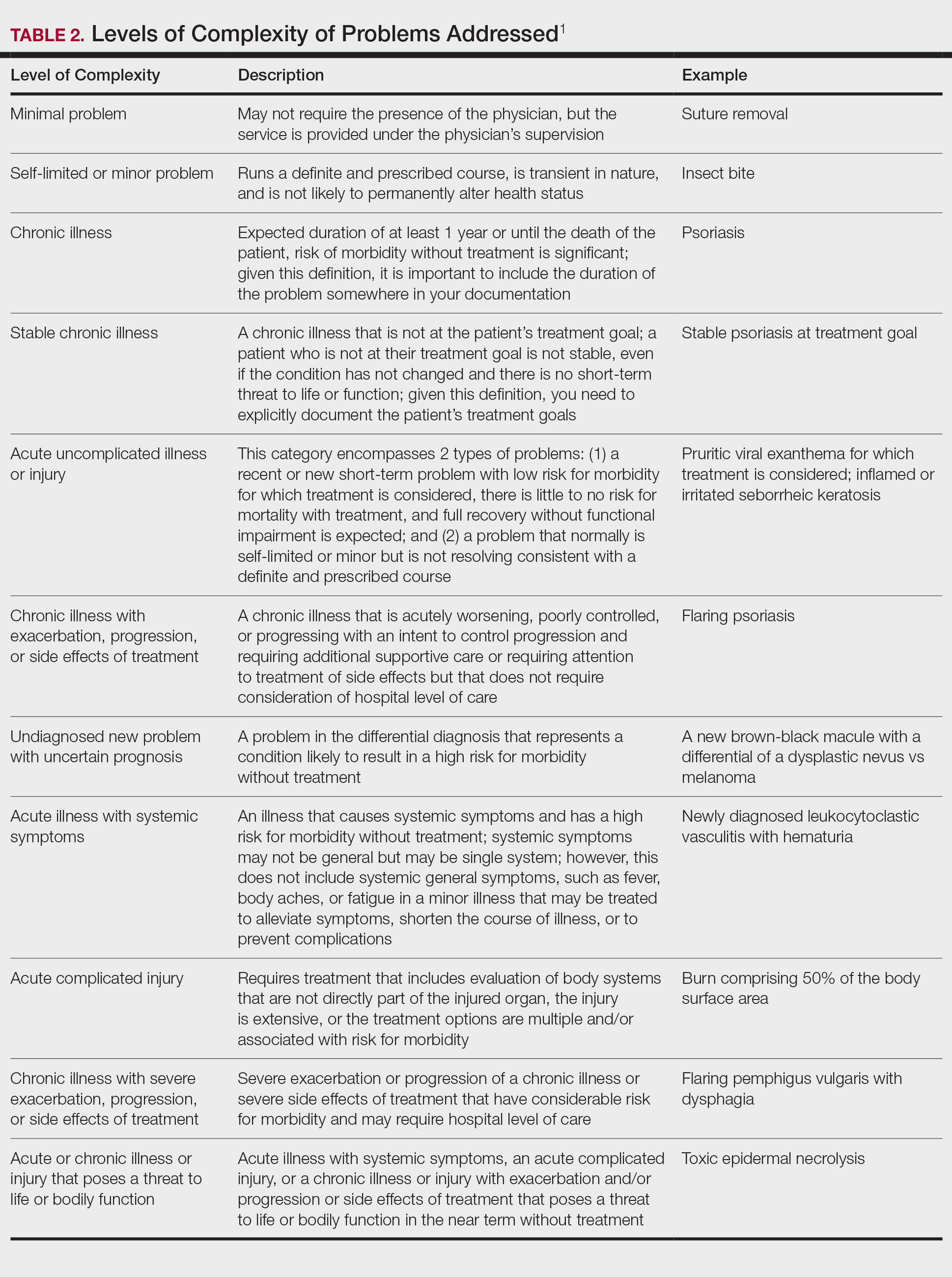
Amount and/or Complexity of Data to Be Reviewed and Analyzed
This category encompasses any external notes reviewed, unique laboratory tests or imaging ordered or reviewed, the need for an independent historian or discussion with external health care providers or appropriate sources, or independent interpretation of tests. Some high-yield definitions in this category are outlined in Table 3.
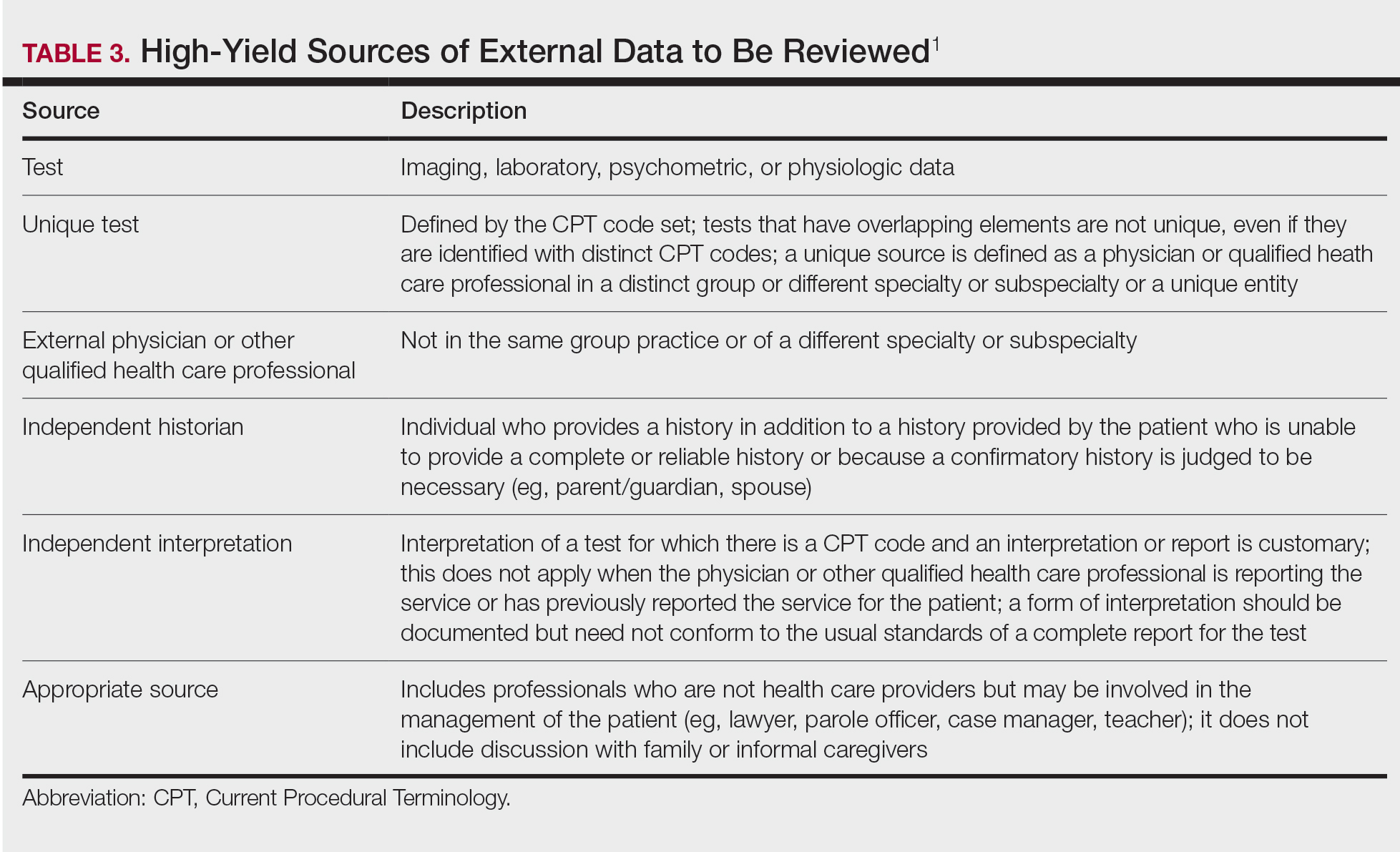
Risk of Complications and/or Morbidity or Mortality of Patient Management
In this category, risk relates to both the patient’s diagnosis and treatment(s). Importantly, for treatment and diagnostic options, these include both the options selected and those considered but not selected. Risk is defined as the probability and/or consequences of an event and is based on the usual behavior and thought processes of a physician in the same specialty. In other words, think of the risk as compared to risk in the setting of other dermatologists diagnosing and/or treating the same condition.
Social determinants of health also play a part in this category and are defined as economic and social conditions that influence the health of individuals and communities. Social determinants of health can be indicated by the specific corresponding International Statistical Classification of Diseases, Tenth Revision code and may need to be included in your billing according to specific institutional or carrier guidelines if they are a factor in your level of MDM.
For the purposes of MDM, risk is stratified into minimal, low, moderate, and high. Some examples for each level are outlined in Table 4.

Putting It All Together
Once you have determined each of the above 3 categories, you can put them together into the MDM chart to ascertain the overall level of MDM. (The official AMA medical decision-making grid is available online [https://www.ama-assn.org/system/files/2019-06/cpt-revised-mdm-grid.pdf]). Keep in mind that 2 of 3 columns in the table must be obtained in that level to reach an overall E/M level; for example, a visit that addresses 2 self-limited or minor problems (level 3) in which no data is reviewed (level 2) and involves prescribing a new medication (level 4), would be an overall level 3 visit.
Final Thoughts
The outpatient E/M guidelines have undergone substantial revisions; therefore, it is crucial to understand the updated definitions to ensure proper billing and documentation. History and physical examination documentation must be medically appropriate but are no longer used to determine overall E/M level; time and MDM are the sole options that can be used. Importantly, try to code as accurately as possible, documenting which problems were both noted and addressed. If you are unsure of a definition within the updated changes and MDM table, referencing the appropriate sources for guidance is recommended.
Although representing a considerable shift, the revaluation of this family of codes and the intended decrease in documentation burden has the ability to be a positive gain for dermatologists. Expect other code families to mirror these changes in the next few years.
- American Medical Association. CPT® Evaluation and management (E/M) office or other outpatient (99202-99215) and prolonged services (99354, 99355, 99356, 99417) code and guideline changes. Accessed May 14, 2021. https://www.ama-assn.org/system/files/2019-06/cpt-office-prolonged-svs-code-changes.pdf
Effective on January 1, 2021, the outpatient evaluation and management (E/M) codes underwent substantial changes, which were the culmination of multiple years of revision and surveying via the American Medical Association (AMA) Relative Value Scale Update Committee and Current Procedural Terminology (RUC-CPT) process to streamline definitions and promote consistency as well as to decrease the administrative burden for all specialties within the house of medicine.1 These updates represent a notable change from the previous documentation requirements for this oft used family of codes. Herein, we break down some of the highlights of the changes and how they may be applied for some commonly used dermatologic diagnoses.
Time Is Time Is Time
Prior to the 2021 revisions, a physician generally could only code for an E/M level by time for a face-to-face encounter dominated by counseling and/or care coordination. With the new updates, any encounter can be coded by total time spent by the physician with the patient1; however, clinical staff time is not included. There also are now clear guidelines of the time ranges corresponding to the level of E/M,1 as noted in Table 1.

Importantly, time now includes not just face-to-face time with the patient but also any time on the date of the encounter that the physician is involved in the care of the patient when not reported with a separate code. This can include reviewing notes or data before or after the examination, care coordination, ordering laboratory tests, and providing any documentation related to the encounter. Importantly, this applies only when these activities are done on the date of the encounter.
If you work with a nurse practitioner or physician assistant (PA) who assists you and you are the one reporting the service, you cannot double-dip. For example, if your PA spends 10 minutes alone with a patient, you are in the room together for 5 minutes, the PA spends another 10 minutes alone with the patient afterward, and you do chart work for 10 minutes at the end of the day, the total time spent is 35 minutes, not 40 minutes, as you cannot count the time you and the PA spent together twice.
Decisions, Decisions
Evaluation and management coding also can be determined via the level of medical decision-making (MDM). Per the 2021 guidelines, MDM is comprised of 3 categories: (1) number and complexity of problems addressed at the encounter, (2) amount and/or complexity of data to be reviewed or analyzed, and (3) risk of complications and/or morbidity or mortality of patient management.1 To reach a certain overall E/M level, 2 of 3 categories must be met or exceeded. Let’s dive into each of these in a little more detail.
Number and Complexity of Problems Addressed at the Encounter
First, it is important to understand the definition of a problem addressed. Per AMA guidelines, this includes a disease, condition, illness, injury, symptom, sign, finding, complaint, or other matter addressed at the encounter that is evaluated or treated at the encounter by the physician. If the problem is referred to another provider without evaluation or consideration of treatment, it is not considered to be a problem addressed and cannot count toward this first category. An example could be a patient with a lump on the abdomen that you refer to plastic or general surgery for evaluation and treatment.
Once you have determined that you are addressing a problem, you will need to determine the level of complexity of the problem, as outlined in Table 2. Keep in mind that some entities and disease states in dermatology may fit the requirements of more than 1 level of complexity depending on the clinical situation, while there are many entities in dermatology that may not be perfectly captured by any of the levels described. In these situations, clinical judgement is required to determine where the problem would best fit. Importantly, whatever you decide, your documentation should support that decision.

Amount and/or Complexity of Data to Be Reviewed and Analyzed
This category encompasses any external notes reviewed, unique laboratory tests or imaging ordered or reviewed, the need for an independent historian or discussion with external health care providers or appropriate sources, or independent interpretation of tests. Some high-yield definitions in this category are outlined in Table 3.

Risk of Complications and/or Morbidity or Mortality of Patient Management
In this category, risk relates to both the patient’s diagnosis and treatment(s). Importantly, for treatment and diagnostic options, these include both the options selected and those considered but not selected. Risk is defined as the probability and/or consequences of an event and is based on the usual behavior and thought processes of a physician in the same specialty. In other words, think of the risk as compared to risk in the setting of other dermatologists diagnosing and/or treating the same condition.
Social determinants of health also play a part in this category and are defined as economic and social conditions that influence the health of individuals and communities. Social determinants of health can be indicated by the specific corresponding International Statistical Classification of Diseases, Tenth Revision code and may need to be included in your billing according to specific institutional or carrier guidelines if they are a factor in your level of MDM.
For the purposes of MDM, risk is stratified into minimal, low, moderate, and high. Some examples for each level are outlined in Table 4.

Putting It All Together
Once you have determined each of the above 3 categories, you can put them together into the MDM chart to ascertain the overall level of MDM. (The official AMA medical decision-making grid is available online [https://www.ama-assn.org/system/files/2019-06/cpt-revised-mdm-grid.pdf]). Keep in mind that 2 of 3 columns in the table must be obtained in that level to reach an overall E/M level; for example, a visit that addresses 2 self-limited or minor problems (level 3) in which no data is reviewed (level 2) and involves prescribing a new medication (level 4), would be an overall level 3 visit.
Final Thoughts
The outpatient E/M guidelines have undergone substantial revisions; therefore, it is crucial to understand the updated definitions to ensure proper billing and documentation. History and physical examination documentation must be medically appropriate but are no longer used to determine overall E/M level; time and MDM are the sole options that can be used. Importantly, try to code as accurately as possible, documenting which problems were both noted and addressed. If you are unsure of a definition within the updated changes and MDM table, referencing the appropriate sources for guidance is recommended.
Although representing a considerable shift, the revaluation of this family of codes and the intended decrease in documentation burden has the ability to be a positive gain for dermatologists. Expect other code families to mirror these changes in the next few years.
Effective on January 1, 2021, the outpatient evaluation and management (E/M) codes underwent substantial changes, which were the culmination of multiple years of revision and surveying via the American Medical Association (AMA) Relative Value Scale Update Committee and Current Procedural Terminology (RUC-CPT) process to streamline definitions and promote consistency as well as to decrease the administrative burden for all specialties within the house of medicine.1 These updates represent a notable change from the previous documentation requirements for this oft used family of codes. Herein, we break down some of the highlights of the changes and how they may be applied for some commonly used dermatologic diagnoses.
Time Is Time Is Time
Prior to the 2021 revisions, a physician generally could only code for an E/M level by time for a face-to-face encounter dominated by counseling and/or care coordination. With the new updates, any encounter can be coded by total time spent by the physician with the patient1; however, clinical staff time is not included. There also are now clear guidelines of the time ranges corresponding to the level of E/M,1 as noted in Table 1.

Importantly, time now includes not just face-to-face time with the patient but also any time on the date of the encounter that the physician is involved in the care of the patient when not reported with a separate code. This can include reviewing notes or data before or after the examination, care coordination, ordering laboratory tests, and providing any documentation related to the encounter. Importantly, this applies only when these activities are done on the date of the encounter.
If you work with a nurse practitioner or physician assistant (PA) who assists you and you are the one reporting the service, you cannot double-dip. For example, if your PA spends 10 minutes alone with a patient, you are in the room together for 5 minutes, the PA spends another 10 minutes alone with the patient afterward, and you do chart work for 10 minutes at the end of the day, the total time spent is 35 minutes, not 40 minutes, as you cannot count the time you and the PA spent together twice.
Decisions, Decisions
Evaluation and management coding also can be determined via the level of medical decision-making (MDM). Per the 2021 guidelines, MDM is comprised of 3 categories: (1) number and complexity of problems addressed at the encounter, (2) amount and/or complexity of data to be reviewed or analyzed, and (3) risk of complications and/or morbidity or mortality of patient management.1 To reach a certain overall E/M level, 2 of 3 categories must be met or exceeded. Let’s dive into each of these in a little more detail.
Number and Complexity of Problems Addressed at the Encounter
First, it is important to understand the definition of a problem addressed. Per AMA guidelines, this includes a disease, condition, illness, injury, symptom, sign, finding, complaint, or other matter addressed at the encounter that is evaluated or treated at the encounter by the physician. If the problem is referred to another provider without evaluation or consideration of treatment, it is not considered to be a problem addressed and cannot count toward this first category. An example could be a patient with a lump on the abdomen that you refer to plastic or general surgery for evaluation and treatment.
Once you have determined that you are addressing a problem, you will need to determine the level of complexity of the problem, as outlined in Table 2. Keep in mind that some entities and disease states in dermatology may fit the requirements of more than 1 level of complexity depending on the clinical situation, while there are many entities in dermatology that may not be perfectly captured by any of the levels described. In these situations, clinical judgement is required to determine where the problem would best fit. Importantly, whatever you decide, your documentation should support that decision.

Amount and/or Complexity of Data to Be Reviewed and Analyzed
This category encompasses any external notes reviewed, unique laboratory tests or imaging ordered or reviewed, the need for an independent historian or discussion with external health care providers or appropriate sources, or independent interpretation of tests. Some high-yield definitions in this category are outlined in Table 3.

Risk of Complications and/or Morbidity or Mortality of Patient Management
In this category, risk relates to both the patient’s diagnosis and treatment(s). Importantly, for treatment and diagnostic options, these include both the options selected and those considered but not selected. Risk is defined as the probability and/or consequences of an event and is based on the usual behavior and thought processes of a physician in the same specialty. In other words, think of the risk as compared to risk in the setting of other dermatologists diagnosing and/or treating the same condition.
Social determinants of health also play a part in this category and are defined as economic and social conditions that influence the health of individuals and communities. Social determinants of health can be indicated by the specific corresponding International Statistical Classification of Diseases, Tenth Revision code and may need to be included in your billing according to specific institutional or carrier guidelines if they are a factor in your level of MDM.
For the purposes of MDM, risk is stratified into minimal, low, moderate, and high. Some examples for each level are outlined in Table 4.

Putting It All Together
Once you have determined each of the above 3 categories, you can put them together into the MDM chart to ascertain the overall level of MDM. (The official AMA medical decision-making grid is available online [https://www.ama-assn.org/system/files/2019-06/cpt-revised-mdm-grid.pdf]). Keep in mind that 2 of 3 columns in the table must be obtained in that level to reach an overall E/M level; for example, a visit that addresses 2 self-limited or minor problems (level 3) in which no data is reviewed (level 2) and involves prescribing a new medication (level 4), would be an overall level 3 visit.
Final Thoughts
The outpatient E/M guidelines have undergone substantial revisions; therefore, it is crucial to understand the updated definitions to ensure proper billing and documentation. History and physical examination documentation must be medically appropriate but are no longer used to determine overall E/M level; time and MDM are the sole options that can be used. Importantly, try to code as accurately as possible, documenting which problems were both noted and addressed. If you are unsure of a definition within the updated changes and MDM table, referencing the appropriate sources for guidance is recommended.
Although representing a considerable shift, the revaluation of this family of codes and the intended decrease in documentation burden has the ability to be a positive gain for dermatologists. Expect other code families to mirror these changes in the next few years.
- American Medical Association. CPT® Evaluation and management (E/M) office or other outpatient (99202-99215) and prolonged services (99354, 99355, 99356, 99417) code and guideline changes. Accessed May 14, 2021. https://www.ama-assn.org/system/files/2019-06/cpt-office-prolonged-svs-code-changes.pdf
- American Medical Association. CPT® Evaluation and management (E/M) office or other outpatient (99202-99215) and prolonged services (99354, 99355, 99356, 99417) code and guideline changes. Accessed May 14, 2021. https://www.ama-assn.org/system/files/2019-06/cpt-office-prolonged-svs-code-changes.pdf
Practice Points
- The outpatient evaluation and management (E/M) codes have undergone substantial changes that took effect January 1, 2021.
- Outpatient E/M visits are now coded based on time or level of medical decision-making (MDM).
- Time now includes all preservice, intraservice, and postservice time the physician spends with the patient on the date of the encounter.
- Many of the key definitions used in order to determine level of MDM have been streamlined and updated.