User login
Catherine Cooper Nellist is editor of Pediatric News and Ob. Gyn. News. She has more than 30 years of experience reporting, writing, and editing stories about clinical medicine and the U.S. health care industry. Prior to taking the helm of these award-winning publications, Catherine covered major medical research meetings throughout the United States and Canada, and had been editor of Clinical Psychiatry News, and Dermatology News. She joined the company in 1984 after graduating magna cum laude from Dickinson College, Carlisle, Pa., with a BA in English.
Ob.Gyn. News welcomes Dr. Trolice to the board
Dr. Trolice is director of Fertility CARE: The IVF Center in Winter Park, Fla., and associate professor of obstetrics and gynecology at the University of Central Florida, Orlando, responsible for the medical education of ob.gyn. residents and medical students, as well as medical endocrinology fellows. He is past president of the Florida Society of Reproductive Endocrinology & Infertility (REI) and past division director of REI at Winnie Palmer Hospital, part of Orlando Health.
He is double board-certified in REI and ob.gyn., maintains annual recertification, and has been awarded the American Medical Association’s “Physicians’ Recognition Award” annually. He holds the unique distinction of being a fellow in all three American colleges: ob.gyn., surgeons, and endocrinology. His colleagues select him as a “Top Doctor in America” annually, one among the top 5% of doctors in the United States. In 2018, he was awarded the “Social Responsibility Award” by the National Polycystic Ovary Syndrome Association. For 10 years, his foundation, Fertile Dreams, organized seminars to increase fertility awareness and granted national scholarships for those unable to afford in vitro fertilization (IVF) treatment.
Dr. Trolice serves on committees for the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology. He has conducted scientific studies with resultant numerous publications and been appointed a reviewer in many leading medical journals and textbooks. He has lectured at numerous physician and patient seminars around the country. In addition, he is interviewed regularly on TV news/talk shows, radio, podcasts, print/online magazines, and newspapers on reproductive health topics. In the fall of 2018, he launched a podcast entitled “Fertility Health” featuring discussions with national experts on pertinent infertility and reproductive medicine topics. His current book is on the infertility journey, to be published by Harvard Common Press in mid 2019.
Dr. Trolice is director of Fertility CARE: The IVF Center in Winter Park, Fla., and associate professor of obstetrics and gynecology at the University of Central Florida, Orlando, responsible for the medical education of ob.gyn. residents and medical students, as well as medical endocrinology fellows. He is past president of the Florida Society of Reproductive Endocrinology & Infertility (REI) and past division director of REI at Winnie Palmer Hospital, part of Orlando Health.
He is double board-certified in REI and ob.gyn., maintains annual recertification, and has been awarded the American Medical Association’s “Physicians’ Recognition Award” annually. He holds the unique distinction of being a fellow in all three American colleges: ob.gyn., surgeons, and endocrinology. His colleagues select him as a “Top Doctor in America” annually, one among the top 5% of doctors in the United States. In 2018, he was awarded the “Social Responsibility Award” by the National Polycystic Ovary Syndrome Association. For 10 years, his foundation, Fertile Dreams, organized seminars to increase fertility awareness and granted national scholarships for those unable to afford in vitro fertilization (IVF) treatment.
Dr. Trolice serves on committees for the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology. He has conducted scientific studies with resultant numerous publications and been appointed a reviewer in many leading medical journals and textbooks. He has lectured at numerous physician and patient seminars around the country. In addition, he is interviewed regularly on TV news/talk shows, radio, podcasts, print/online magazines, and newspapers on reproductive health topics. In the fall of 2018, he launched a podcast entitled “Fertility Health” featuring discussions with national experts on pertinent infertility and reproductive medicine topics. His current book is on the infertility journey, to be published by Harvard Common Press in mid 2019.
Dr. Trolice is director of Fertility CARE: The IVF Center in Winter Park, Fla., and associate professor of obstetrics and gynecology at the University of Central Florida, Orlando, responsible for the medical education of ob.gyn. residents and medical students, as well as medical endocrinology fellows. He is past president of the Florida Society of Reproductive Endocrinology & Infertility (REI) and past division director of REI at Winnie Palmer Hospital, part of Orlando Health.
He is double board-certified in REI and ob.gyn., maintains annual recertification, and has been awarded the American Medical Association’s “Physicians’ Recognition Award” annually. He holds the unique distinction of being a fellow in all three American colleges: ob.gyn., surgeons, and endocrinology. His colleagues select him as a “Top Doctor in America” annually, one among the top 5% of doctors in the United States. In 2018, he was awarded the “Social Responsibility Award” by the National Polycystic Ovary Syndrome Association. For 10 years, his foundation, Fertile Dreams, organized seminars to increase fertility awareness and granted national scholarships for those unable to afford in vitro fertilization (IVF) treatment.
Dr. Trolice serves on committees for the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology. He has conducted scientific studies with resultant numerous publications and been appointed a reviewer in many leading medical journals and textbooks. He has lectured at numerous physician and patient seminars around the country. In addition, he is interviewed regularly on TV news/talk shows, radio, podcasts, print/online magazines, and newspapers on reproductive health topics. In the fall of 2018, he launched a podcast entitled “Fertility Health” featuring discussions with national experts on pertinent infertility and reproductive medicine topics. His current book is on the infertility journey, to be published by Harvard Common Press in mid 2019.
Ob.Gyn. News welcomes Dr. Badell to the Board
Dr. Badell is an assistant professor of gynecology and obstetrics in the division of maternal-fetal medicine at Emory University in Atlanta. She also is director of the Perinatal Center at Emory University Hospital Midtown.
Dr. Badell has been a primary author or coauthor of 35 articles published and accepted in refereed medical publications on topics including Zika infection during pregnancy, HIV infection during pregnancy, use of complementary and alternative medication in obstetrics and gynecology, botulism during pregnancy, maternal and fetal risk associated with assisted reproductive technology, and perinatal outcomes among women with congenital heart disease. She also has written several book chapters, and she currently is involved in research involving pediatric and maternal HIV/AIDS, as well as chronic hypertension during pregnancy.
Dr. Badell serves on a number of committees at Emory for the department of obstetrics and gynecology, including the clinical competency committee and program evaluation committee, as well as similar committees for the maternal-fetal medicine fellowship program. She also is a member of the Emory University Hospital Midtown’s quality enhancement committee.
Dr. Badell graduated with distinction from the University of Rochester (N.Y.), did a clinical research fellowship in obstetrics and gynecology at Baylor College of Medicine in Houston, and a fellowship in maternal-fetal medicine at Emory University. Dr. Badell has won too many honors and awards to mention, but a few of them are membership in Phi Beta Kappa; an outstanding clinician award in maternal-fetal medicine; an excellence in teaching, maternal-fetal medicine award; and a faculty recognition “Hidden Gem” award.
Dr. Badell is an assistant professor of gynecology and obstetrics in the division of maternal-fetal medicine at Emory University in Atlanta. She also is director of the Perinatal Center at Emory University Hospital Midtown.
Dr. Badell has been a primary author or coauthor of 35 articles published and accepted in refereed medical publications on topics including Zika infection during pregnancy, HIV infection during pregnancy, use of complementary and alternative medication in obstetrics and gynecology, botulism during pregnancy, maternal and fetal risk associated with assisted reproductive technology, and perinatal outcomes among women with congenital heart disease. She also has written several book chapters, and she currently is involved in research involving pediatric and maternal HIV/AIDS, as well as chronic hypertension during pregnancy.
Dr. Badell serves on a number of committees at Emory for the department of obstetrics and gynecology, including the clinical competency committee and program evaluation committee, as well as similar committees for the maternal-fetal medicine fellowship program. She also is a member of the Emory University Hospital Midtown’s quality enhancement committee.
Dr. Badell graduated with distinction from the University of Rochester (N.Y.), did a clinical research fellowship in obstetrics and gynecology at Baylor College of Medicine in Houston, and a fellowship in maternal-fetal medicine at Emory University. Dr. Badell has won too many honors and awards to mention, but a few of them are membership in Phi Beta Kappa; an outstanding clinician award in maternal-fetal medicine; an excellence in teaching, maternal-fetal medicine award; and a faculty recognition “Hidden Gem” award.
Dr. Badell is an assistant professor of gynecology and obstetrics in the division of maternal-fetal medicine at Emory University in Atlanta. She also is director of the Perinatal Center at Emory University Hospital Midtown.
Dr. Badell has been a primary author or coauthor of 35 articles published and accepted in refereed medical publications on topics including Zika infection during pregnancy, HIV infection during pregnancy, use of complementary and alternative medication in obstetrics and gynecology, botulism during pregnancy, maternal and fetal risk associated with assisted reproductive technology, and perinatal outcomes among women with congenital heart disease. She also has written several book chapters, and she currently is involved in research involving pediatric and maternal HIV/AIDS, as well as chronic hypertension during pregnancy.
Dr. Badell serves on a number of committees at Emory for the department of obstetrics and gynecology, including the clinical competency committee and program evaluation committee, as well as similar committees for the maternal-fetal medicine fellowship program. She also is a member of the Emory University Hospital Midtown’s quality enhancement committee.
Dr. Badell graduated with distinction from the University of Rochester (N.Y.), did a clinical research fellowship in obstetrics and gynecology at Baylor College of Medicine in Houston, and a fellowship in maternal-fetal medicine at Emory University. Dr. Badell has won too many honors and awards to mention, but a few of them are membership in Phi Beta Kappa; an outstanding clinician award in maternal-fetal medicine; an excellence in teaching, maternal-fetal medicine award; and a faculty recognition “Hidden Gem” award.
New NIH consortium aims to coordinate pediatric research programs
across its institutes and centers.
Almost all of the 27 institutes and centers of the NIH fund at least some kind of child health research, totaling more than $4 billion in the 2017 fiscal year, according to an NIH statement. “The new consortium aims to harmonize these activities, explore gaps and opportunities in the overall pediatric research portfolio, and set priorities.”
Research funded by NIH “has resulted in tremendous advances against diseases and conditions that affect child health and well-being, including asthma, cancer, autism, obesity, and intellectual and developmental disabilities,” explained Diana W. Bianchi, MD, in the statement. Dr. Bianchi is director of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the lead NIH institute for the consortium.
The new consortium, which will be led by the NICHD director, will meet several times a year.
across its institutes and centers.
Almost all of the 27 institutes and centers of the NIH fund at least some kind of child health research, totaling more than $4 billion in the 2017 fiscal year, according to an NIH statement. “The new consortium aims to harmonize these activities, explore gaps and opportunities in the overall pediatric research portfolio, and set priorities.”
Research funded by NIH “has resulted in tremendous advances against diseases and conditions that affect child health and well-being, including asthma, cancer, autism, obesity, and intellectual and developmental disabilities,” explained Diana W. Bianchi, MD, in the statement. Dr. Bianchi is director of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the lead NIH institute for the consortium.
The new consortium, which will be led by the NICHD director, will meet several times a year.
across its institutes and centers.
Almost all of the 27 institutes and centers of the NIH fund at least some kind of child health research, totaling more than $4 billion in the 2017 fiscal year, according to an NIH statement. “The new consortium aims to harmonize these activities, explore gaps and opportunities in the overall pediatric research portfolio, and set priorities.”
Research funded by NIH “has resulted in tremendous advances against diseases and conditions that affect child health and well-being, including asthma, cancer, autism, obesity, and intellectual and developmental disabilities,” explained Diana W. Bianchi, MD, in the statement. Dr. Bianchi is director of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the lead NIH institute for the consortium.
The new consortium, which will be led by the NICHD director, will meet several times a year.
Psoriasis therapy with biologics not linked to increased cancer risk
Biologic treatments were not associated with an increased risk of cancer among patients with psoriasis in the medium term, in a study that analyzed data from patient registries.
“Cumulative length of exposure to biologics was not associated with the risk of developing cancers, even after controlling for the effect of age, gender, location,” as well as for previous exposure to methotrexate, cyclosporine, and phototherapy; duration of psoriasis; and comorbidities, reported Ignacio García-Doval, MD, of the Fundación Academia Española de Dermatología y Venereología, Madrid, and his associates.
The pooled adjusted odds ratio of cancer per year of biologic exposure was 1.02 (95% confidence interval, 0.92-1.13), demonstrating no significantly increased risk of cancer per cumulative year of biologic exposure for psoriasis therapy, Dr. García-Doval and his associates reported in the study, published in the British Journal of Dermatology. This was true even when broken down within the registries for comparison, and when analyzed by type of cancers, such as squamous cell carcinoma and basal cell carcinoma.
A limitation of the study was inadequate power to detect and compare risk between individual biologics, they said. Also, “as our data describe limited follow-up and latencies, it is still possible that a risk after longer periods of exposure and latencies exists.”
Most of the authors had numerous financial disclosures related to pharmaceutical companies. Psonet was supported with funds from the European Association of Venereology and Dermatology and the Italian Drug Agency. Funding for the individual registries includes support from pharmaceutical companies.
SOURCE: García-Doval I et al. Br J Dermatol. 2018 May 3. doi: 10.1111/bjd.16715.
Biologic treatments were not associated with an increased risk of cancer among patients with psoriasis in the medium term, in a study that analyzed data from patient registries.
“Cumulative length of exposure to biologics was not associated with the risk of developing cancers, even after controlling for the effect of age, gender, location,” as well as for previous exposure to methotrexate, cyclosporine, and phototherapy; duration of psoriasis; and comorbidities, reported Ignacio García-Doval, MD, of the Fundación Academia Española de Dermatología y Venereología, Madrid, and his associates.
The pooled adjusted odds ratio of cancer per year of biologic exposure was 1.02 (95% confidence interval, 0.92-1.13), demonstrating no significantly increased risk of cancer per cumulative year of biologic exposure for psoriasis therapy, Dr. García-Doval and his associates reported in the study, published in the British Journal of Dermatology. This was true even when broken down within the registries for comparison, and when analyzed by type of cancers, such as squamous cell carcinoma and basal cell carcinoma.
A limitation of the study was inadequate power to detect and compare risk between individual biologics, they said. Also, “as our data describe limited follow-up and latencies, it is still possible that a risk after longer periods of exposure and latencies exists.”
Most of the authors had numerous financial disclosures related to pharmaceutical companies. Psonet was supported with funds from the European Association of Venereology and Dermatology and the Italian Drug Agency. Funding for the individual registries includes support from pharmaceutical companies.
SOURCE: García-Doval I et al. Br J Dermatol. 2018 May 3. doi: 10.1111/bjd.16715.
Biologic treatments were not associated with an increased risk of cancer among patients with psoriasis in the medium term, in a study that analyzed data from patient registries.
“Cumulative length of exposure to biologics was not associated with the risk of developing cancers, even after controlling for the effect of age, gender, location,” as well as for previous exposure to methotrexate, cyclosporine, and phototherapy; duration of psoriasis; and comorbidities, reported Ignacio García-Doval, MD, of the Fundación Academia Española de Dermatología y Venereología, Madrid, and his associates.
The pooled adjusted odds ratio of cancer per year of biologic exposure was 1.02 (95% confidence interval, 0.92-1.13), demonstrating no significantly increased risk of cancer per cumulative year of biologic exposure for psoriasis therapy, Dr. García-Doval and his associates reported in the study, published in the British Journal of Dermatology. This was true even when broken down within the registries for comparison, and when analyzed by type of cancers, such as squamous cell carcinoma and basal cell carcinoma.
A limitation of the study was inadequate power to detect and compare risk between individual biologics, they said. Also, “as our data describe limited follow-up and latencies, it is still possible that a risk after longer periods of exposure and latencies exists.”
Most of the authors had numerous financial disclosures related to pharmaceutical companies. Psonet was supported with funds from the European Association of Venereology and Dermatology and the Italian Drug Agency. Funding for the individual registries includes support from pharmaceutical companies.
SOURCE: García-Doval I et al. Br J Dermatol. 2018 May 3. doi: 10.1111/bjd.16715.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Key clinical point:
Major finding: The pooled adjusted odds ratio of cancer per year of biologic exposure was 1.02.
Study details: Patient data were drawn from four national databases within Psonet, which included 579 cancer cases and 2,671 matched controls.
Disclosures: Most of the authors had numerous financial disclosures related to pharmaceutical companies. Psonet was supported with funds from the European Association of Venereology and Dermatology and the Italian Drug Agency. Funding for the individual registries includes support from pharmaceutical companies.
Source: García-Doval I et al. Br J Dermatol. 2018 May 3. doi: 10.1111/bjd.16715.
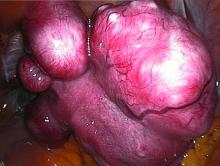
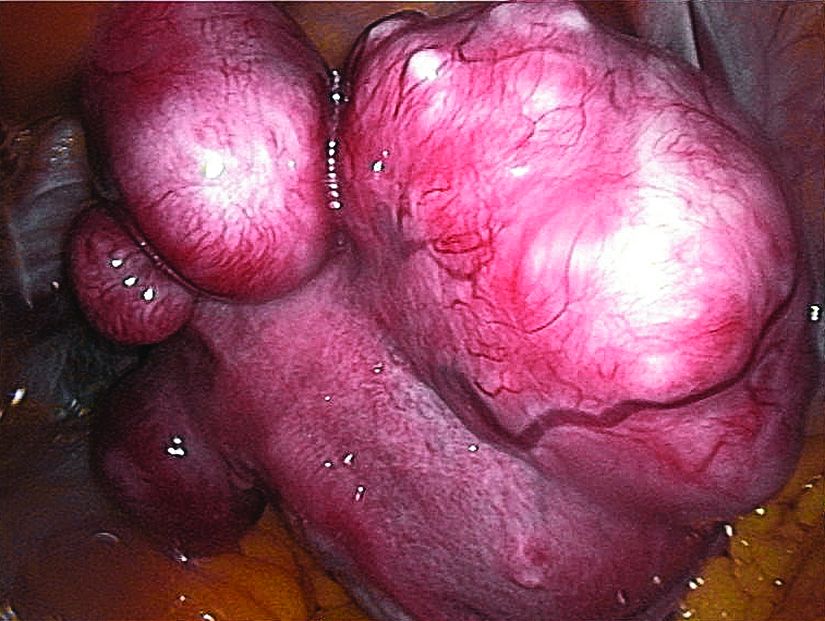
Uterine fibroids registry looks to provide comparative treatment data
Early findings from a uterine fibroids registry suggest more than half of enrolled women chose an alternative to hysterectomy, underscoring the need to study the efficacy of these uterine-sparing treatment options, said Elizabeth A. Stewart, MD, of the Mayo Clinic, Rochester, Minn., and her associates.
There is very little evidence on the comparative efficacy of uterine fibroid treatment options so the COMPARE-UF (Comparing Options for Management: Patient-Centered Results for Uterine Fibroids) registry was initiated for women who choose procedural therapy for symptomatic uterine fibroids. Data at the nine clinical centers – representing rural and urban populations – are being collected regarding hysterectomy, myomectomy (abdominal, hysteroscopic, vaginal, and laparoscopic/robotic), endometrial ablation, radiofrequency fibroid ablation, uterine artery embolization, magnetic resonance–guided focused ultrasound, and therapeutic use of progestin-releasing intrauterine devices.
A total of 16% of the women were under age 35, and 40% were aged 40 years or younger. “The fact that a sizable proportion of women are under age 40 suggests that with long-term follow-up, data on reproductive outcomes can be obtained as these women seek pregnancy,” Dr. Stewart and her associates noted.
Although African American women make up only 13% of the U.S. population, they constitute 42% of enrollment in COMPARE-UF, the investigators reported in the American Journal of Obstetrics and Gynecology. African American women chose similar or greater numbers of each type of myomectomy and uterine embolization as white women chose.
The study was supported by a grant from the Agency for Healthcare Research and Quality. The registry is supported by AHRQ and the Patient-Centered Outcomes Research Institute. Dr. Stewart reported personal fees from AbbVie, Allergan, Astellas, Pharma, Bayer, Gynesonics, and Myovant Sciences. Several researchers reported grants from pharmaceutical companies outside this study, and the remainder of the investigators reported no relevant financial disclosures.
SOURCE: Stewart EA et al. Am J Obstet Gynecol. 2018 May 8. doi: 10.1016/j.ajog.2018.05.004.
Early findings from a uterine fibroids registry suggest more than half of enrolled women chose an alternative to hysterectomy, underscoring the need to study the efficacy of these uterine-sparing treatment options, said Elizabeth A. Stewart, MD, of the Mayo Clinic, Rochester, Minn., and her associates.
There is very little evidence on the comparative efficacy of uterine fibroid treatment options so the COMPARE-UF (Comparing Options for Management: Patient-Centered Results for Uterine Fibroids) registry was initiated for women who choose procedural therapy for symptomatic uterine fibroids. Data at the nine clinical centers – representing rural and urban populations – are being collected regarding hysterectomy, myomectomy (abdominal, hysteroscopic, vaginal, and laparoscopic/robotic), endometrial ablation, radiofrequency fibroid ablation, uterine artery embolization, magnetic resonance–guided focused ultrasound, and therapeutic use of progestin-releasing intrauterine devices.
A total of 16% of the women were under age 35, and 40% were aged 40 years or younger. “The fact that a sizable proportion of women are under age 40 suggests that with long-term follow-up, data on reproductive outcomes can be obtained as these women seek pregnancy,” Dr. Stewart and her associates noted.
Although African American women make up only 13% of the U.S. population, they constitute 42% of enrollment in COMPARE-UF, the investigators reported in the American Journal of Obstetrics and Gynecology. African American women chose similar or greater numbers of each type of myomectomy and uterine embolization as white women chose.
The study was supported by a grant from the Agency for Healthcare Research and Quality. The registry is supported by AHRQ and the Patient-Centered Outcomes Research Institute. Dr. Stewart reported personal fees from AbbVie, Allergan, Astellas, Pharma, Bayer, Gynesonics, and Myovant Sciences. Several researchers reported grants from pharmaceutical companies outside this study, and the remainder of the investigators reported no relevant financial disclosures.
SOURCE: Stewart EA et al. Am J Obstet Gynecol. 2018 May 8. doi: 10.1016/j.ajog.2018.05.004.
Early findings from a uterine fibroids registry suggest more than half of enrolled women chose an alternative to hysterectomy, underscoring the need to study the efficacy of these uterine-sparing treatment options, said Elizabeth A. Stewart, MD, of the Mayo Clinic, Rochester, Minn., and her associates.
There is very little evidence on the comparative efficacy of uterine fibroid treatment options so the COMPARE-UF (Comparing Options for Management: Patient-Centered Results for Uterine Fibroids) registry was initiated for women who choose procedural therapy for symptomatic uterine fibroids. Data at the nine clinical centers – representing rural and urban populations – are being collected regarding hysterectomy, myomectomy (abdominal, hysteroscopic, vaginal, and laparoscopic/robotic), endometrial ablation, radiofrequency fibroid ablation, uterine artery embolization, magnetic resonance–guided focused ultrasound, and therapeutic use of progestin-releasing intrauterine devices.
A total of 16% of the women were under age 35, and 40% were aged 40 years or younger. “The fact that a sizable proportion of women are under age 40 suggests that with long-term follow-up, data on reproductive outcomes can be obtained as these women seek pregnancy,” Dr. Stewart and her associates noted.
Although African American women make up only 13% of the U.S. population, they constitute 42% of enrollment in COMPARE-UF, the investigators reported in the American Journal of Obstetrics and Gynecology. African American women chose similar or greater numbers of each type of myomectomy and uterine embolization as white women chose.
The study was supported by a grant from the Agency for Healthcare Research and Quality. The registry is supported by AHRQ and the Patient-Centered Outcomes Research Institute. Dr. Stewart reported personal fees from AbbVie, Allergan, Astellas, Pharma, Bayer, Gynesonics, and Myovant Sciences. Several researchers reported grants from pharmaceutical companies outside this study, and the remainder of the investigators reported no relevant financial disclosures.
SOURCE: Stewart EA et al. Am J Obstet Gynecol. 2018 May 8. doi: 10.1016/j.ajog.2018.05.004.
FROM THE AMERICAN JOURNAL OF OBSTETRICS AND GYNECOLOGY
Key clinical point:
Major finding: Of the initial 2,031 women enrolled in the registry, hysterectomy was chosen by 38%, and myomectomies were chosen by 46%.
Study details: Initial results from the COMPARE-UF registry.
Disclosures: The study was supported by a grant from the Agency for Healthcare Research and Quality. Dr. Stewart reported personal fees from AbbVie, Allergan, Astellas, Pharma, Bayer, Gynesonics, and Myovant Sciences. Several researchers reported grants from pharmaceutical companies outside this study, and the remainder of the investigators reported no relevant financial disclosures.
Source: Stewart EA et al. Am J Obstet Gynecol. 2018 May 8. doi: 10.1016/j.ajog.2018.05.004.
Colchicine looks promising for treating prurigo pigmentosa in case report
said Isa An, MD of Dicle University, Diyarbakır, Turkey, and associates.
They described a 16-year-old Turkish girl who presented with an itchy rash on her back of 4 weeks’ duration. Treatment with oral antihistamines, and topical and systemic steroids were ineffective. On physical examination, there were erythematous macules, sporadic excoriated papules, and reticulate hyperpigmentation on her back. Complete blood count and liver function tests were normal.
Histopathologic examination showed “infrequent necrotic keratinocytes with marked acanthosis and spongiosis on the epidermis, increased basal layer pigmentation, and perivascular lymphocytic infiltrate on the upper dermis.” Because of these findings, a diagnosis of prurigo pigmentosa was made, they wrote in Pediatric Dermatology.
Treatment with colchicine 1.5 g/day was started, and while the lesions resolved in the second week of treatment, the reticulate hyperpigmentation remained, Dr. An and her associates reported. The reticulate hyperpigmentation did not regress during the first month of treatment. At 6-month follow-up, there were no more symptoms of itch or recurrence of the original lesions. The reticulate hyperpigmentation was not treated.
A rare inflammatory skin disease seen principally in young Japanese women, prurigo pigmentosa has been reported in both men and women of other ethnicities, they said. Generally, macrolide antibiotics, such as clarithromycin, dapsone, and isotretinoin have been used effectively to treat prurigo pigmentosa. Minocycline and doxycycline are considered to be effective in treating this skin disease because of their anti-inflammatory effects and because they have “been shown to inhibit the synthesis of cytokines and chemokines that regulate leukocyte differentiation and activation.” Leukocyte differentiation and activation “are key pathologic features of prurigo pigmentosa,” the authors added.
Colchicine, a neutral, liposoluble tricyclic alkaloid, “exerts an anti-inflammatory effect by inhibiting neutrophil chemotaxis,” said Dr. An and her associates, which is why it is thought to be a potentially effective drug for treating prurigo pigmentosa, as shown in this case study. “Further studies are required to verify whether colchicine is an effective treatment option,” for prurigo pigmentosa, they added.
SOURCE: An I et al. Pediatr Dermatol. 2018 Apr 11. doi: 10.1111/pde.13480.
said Isa An, MD of Dicle University, Diyarbakır, Turkey, and associates.
They described a 16-year-old Turkish girl who presented with an itchy rash on her back of 4 weeks’ duration. Treatment with oral antihistamines, and topical and systemic steroids were ineffective. On physical examination, there were erythematous macules, sporadic excoriated papules, and reticulate hyperpigmentation on her back. Complete blood count and liver function tests were normal.
Histopathologic examination showed “infrequent necrotic keratinocytes with marked acanthosis and spongiosis on the epidermis, increased basal layer pigmentation, and perivascular lymphocytic infiltrate on the upper dermis.” Because of these findings, a diagnosis of prurigo pigmentosa was made, they wrote in Pediatric Dermatology.
Treatment with colchicine 1.5 g/day was started, and while the lesions resolved in the second week of treatment, the reticulate hyperpigmentation remained, Dr. An and her associates reported. The reticulate hyperpigmentation did not regress during the first month of treatment. At 6-month follow-up, there were no more symptoms of itch or recurrence of the original lesions. The reticulate hyperpigmentation was not treated.
A rare inflammatory skin disease seen principally in young Japanese women, prurigo pigmentosa has been reported in both men and women of other ethnicities, they said. Generally, macrolide antibiotics, such as clarithromycin, dapsone, and isotretinoin have been used effectively to treat prurigo pigmentosa. Minocycline and doxycycline are considered to be effective in treating this skin disease because of their anti-inflammatory effects and because they have “been shown to inhibit the synthesis of cytokines and chemokines that regulate leukocyte differentiation and activation.” Leukocyte differentiation and activation “are key pathologic features of prurigo pigmentosa,” the authors added.
Colchicine, a neutral, liposoluble tricyclic alkaloid, “exerts an anti-inflammatory effect by inhibiting neutrophil chemotaxis,” said Dr. An and her associates, which is why it is thought to be a potentially effective drug for treating prurigo pigmentosa, as shown in this case study. “Further studies are required to verify whether colchicine is an effective treatment option,” for prurigo pigmentosa, they added.
SOURCE: An I et al. Pediatr Dermatol. 2018 Apr 11. doi: 10.1111/pde.13480.
said Isa An, MD of Dicle University, Diyarbakır, Turkey, and associates.
They described a 16-year-old Turkish girl who presented with an itchy rash on her back of 4 weeks’ duration. Treatment with oral antihistamines, and topical and systemic steroids were ineffective. On physical examination, there were erythematous macules, sporadic excoriated papules, and reticulate hyperpigmentation on her back. Complete blood count and liver function tests were normal.
Histopathologic examination showed “infrequent necrotic keratinocytes with marked acanthosis and spongiosis on the epidermis, increased basal layer pigmentation, and perivascular lymphocytic infiltrate on the upper dermis.” Because of these findings, a diagnosis of prurigo pigmentosa was made, they wrote in Pediatric Dermatology.
Treatment with colchicine 1.5 g/day was started, and while the lesions resolved in the second week of treatment, the reticulate hyperpigmentation remained, Dr. An and her associates reported. The reticulate hyperpigmentation did not regress during the first month of treatment. At 6-month follow-up, there were no more symptoms of itch or recurrence of the original lesions. The reticulate hyperpigmentation was not treated.
A rare inflammatory skin disease seen principally in young Japanese women, prurigo pigmentosa has been reported in both men and women of other ethnicities, they said. Generally, macrolide antibiotics, such as clarithromycin, dapsone, and isotretinoin have been used effectively to treat prurigo pigmentosa. Minocycline and doxycycline are considered to be effective in treating this skin disease because of their anti-inflammatory effects and because they have “been shown to inhibit the synthesis of cytokines and chemokines that regulate leukocyte differentiation and activation.” Leukocyte differentiation and activation “are key pathologic features of prurigo pigmentosa,” the authors added.
Colchicine, a neutral, liposoluble tricyclic alkaloid, “exerts an anti-inflammatory effect by inhibiting neutrophil chemotaxis,” said Dr. An and her associates, which is why it is thought to be a potentially effective drug for treating prurigo pigmentosa, as shown in this case study. “Further studies are required to verify whether colchicine is an effective treatment option,” for prurigo pigmentosa, they added.
SOURCE: An I et al. Pediatr Dermatol. 2018 Apr 11. doi: 10.1111/pde.13480.
FROM PEDIATRIC DERMATOLOGY
Ideal sun protection practices by parents low in Toronto
according to Marcus G. Tan, MD, of the University of Toronto, and his associates.
Too much sun exposure as a child is a known risk factor for skin cancer, and “it has been estimated that approximately half of cumulative ultraviolet exposure by age 60 occurs before age 20.” So it is important that children be protected from excessive sun exposure, the investigators wrote, underscoring the reason for their study of parental sun protection by Canadian parents in Toronto, which has a multiracial population.
Parental sun protection efforts were considered ideal if they used sun protection every day; on sunny days all year round; or during the summer, using at least SPF 30, and reapplying sunscreen at least every 2 hours. Parental efforts would be considered nonideal if they did not meet any of these three criteria.
Of 183 parents, 17% used ideal sun protection for their children; 28% were in the lighter-skinned group (Fitzpatrick phototype I-III) and 5% in the darker-skinned group (Fitzpatrick phototype IV-V), The greater likelihood of using ideal sun protection among those in the lighter-skinned group was statistically significant (odds ratio, 7.4; P less than .001). As each child grew 1 year older, parents were 31% less likely to use ideal sun protection (OR, 0.69; P = .007).
Parents whose children were in the lighter-skinned group were “more likely to believe that sun exposure was harmful” for their children (OR, 17.2), and “to perceive value in sun protection,” (OR, 11.4) the researchers said. The parents whose children were in the darker-skinned group were more likely to consider sun protection as having drawbacks (OR, 9.2), and to believe that darker skin tone provides more sun protection (OR, 12.4). Neither of the two groups was more likely to consider that sun exposure provided health benefits or that it improved appearance.
Parents whose children were in the lighter-skinned group reported significantly more frequent use of sunscreen (OR, 3.6), sunglasses (OR, 2.3), and sun suits (OR, 2.7). The parents of the children in this group reported similar or higher rates of use of hats, long-sleeved clothing, and umbrellas; seeking shade; and staying indoors between 12 pm and 2 pm, but these rates were not statistically significantly different from those reported by the parents of children in the darker-skinned group, the investigators said.
“Our study suggests that more targeted education guidelines are necessary to ensure sun safety for all children, especially in a multiracial population,” said Dr. Tan and his colleagues. “Dermatologists and primary care physicians are in ideal positions to initiate personalized efforts to improve overall rates of sun protection.”
Dr. Tan reported no relevant financial disclosures. Dr. Miriam Weinstein has worked as an advisory board member for Johnson & Johnson, which manufactures sunscreen; and Dr. Shudeshna Nag consults for Valeant, which manufactures sunscreen.
SOURCE: Tan MG et al. Pediatr Dermatol. 2018 Feb 13. doi: 10.1111/pde.13433.
according to Marcus G. Tan, MD, of the University of Toronto, and his associates.
Too much sun exposure as a child is a known risk factor for skin cancer, and “it has been estimated that approximately half of cumulative ultraviolet exposure by age 60 occurs before age 20.” So it is important that children be protected from excessive sun exposure, the investigators wrote, underscoring the reason for their study of parental sun protection by Canadian parents in Toronto, which has a multiracial population.
Parental sun protection efforts were considered ideal if they used sun protection every day; on sunny days all year round; or during the summer, using at least SPF 30, and reapplying sunscreen at least every 2 hours. Parental efforts would be considered nonideal if they did not meet any of these three criteria.
Of 183 parents, 17% used ideal sun protection for their children; 28% were in the lighter-skinned group (Fitzpatrick phototype I-III) and 5% in the darker-skinned group (Fitzpatrick phototype IV-V), The greater likelihood of using ideal sun protection among those in the lighter-skinned group was statistically significant (odds ratio, 7.4; P less than .001). As each child grew 1 year older, parents were 31% less likely to use ideal sun protection (OR, 0.69; P = .007).
Parents whose children were in the lighter-skinned group were “more likely to believe that sun exposure was harmful” for their children (OR, 17.2), and “to perceive value in sun protection,” (OR, 11.4) the researchers said. The parents whose children were in the darker-skinned group were more likely to consider sun protection as having drawbacks (OR, 9.2), and to believe that darker skin tone provides more sun protection (OR, 12.4). Neither of the two groups was more likely to consider that sun exposure provided health benefits or that it improved appearance.
Parents whose children were in the lighter-skinned group reported significantly more frequent use of sunscreen (OR, 3.6), sunglasses (OR, 2.3), and sun suits (OR, 2.7). The parents of the children in this group reported similar or higher rates of use of hats, long-sleeved clothing, and umbrellas; seeking shade; and staying indoors between 12 pm and 2 pm, but these rates were not statistically significantly different from those reported by the parents of children in the darker-skinned group, the investigators said.
“Our study suggests that more targeted education guidelines are necessary to ensure sun safety for all children, especially in a multiracial population,” said Dr. Tan and his colleagues. “Dermatologists and primary care physicians are in ideal positions to initiate personalized efforts to improve overall rates of sun protection.”
Dr. Tan reported no relevant financial disclosures. Dr. Miriam Weinstein has worked as an advisory board member for Johnson & Johnson, which manufactures sunscreen; and Dr. Shudeshna Nag consults for Valeant, which manufactures sunscreen.
SOURCE: Tan MG et al. Pediatr Dermatol. 2018 Feb 13. doi: 10.1111/pde.13433.
according to Marcus G. Tan, MD, of the University of Toronto, and his associates.
Too much sun exposure as a child is a known risk factor for skin cancer, and “it has been estimated that approximately half of cumulative ultraviolet exposure by age 60 occurs before age 20.” So it is important that children be protected from excessive sun exposure, the investigators wrote, underscoring the reason for their study of parental sun protection by Canadian parents in Toronto, which has a multiracial population.
Parental sun protection efforts were considered ideal if they used sun protection every day; on sunny days all year round; or during the summer, using at least SPF 30, and reapplying sunscreen at least every 2 hours. Parental efforts would be considered nonideal if they did not meet any of these three criteria.
Of 183 parents, 17% used ideal sun protection for their children; 28% were in the lighter-skinned group (Fitzpatrick phototype I-III) and 5% in the darker-skinned group (Fitzpatrick phototype IV-V), The greater likelihood of using ideal sun protection among those in the lighter-skinned group was statistically significant (odds ratio, 7.4; P less than .001). As each child grew 1 year older, parents were 31% less likely to use ideal sun protection (OR, 0.69; P = .007).
Parents whose children were in the lighter-skinned group were “more likely to believe that sun exposure was harmful” for their children (OR, 17.2), and “to perceive value in sun protection,” (OR, 11.4) the researchers said. The parents whose children were in the darker-skinned group were more likely to consider sun protection as having drawbacks (OR, 9.2), and to believe that darker skin tone provides more sun protection (OR, 12.4). Neither of the two groups was more likely to consider that sun exposure provided health benefits or that it improved appearance.
Parents whose children were in the lighter-skinned group reported significantly more frequent use of sunscreen (OR, 3.6), sunglasses (OR, 2.3), and sun suits (OR, 2.7). The parents of the children in this group reported similar or higher rates of use of hats, long-sleeved clothing, and umbrellas; seeking shade; and staying indoors between 12 pm and 2 pm, but these rates were not statistically significantly different from those reported by the parents of children in the darker-skinned group, the investigators said.
“Our study suggests that more targeted education guidelines are necessary to ensure sun safety for all children, especially in a multiracial population,” said Dr. Tan and his colleagues. “Dermatologists and primary care physicians are in ideal positions to initiate personalized efforts to improve overall rates of sun protection.”
Dr. Tan reported no relevant financial disclosures. Dr. Miriam Weinstein has worked as an advisory board member for Johnson & Johnson, which manufactures sunscreen; and Dr. Shudeshna Nag consults for Valeant, which manufactures sunscreen.
SOURCE: Tan MG et al. Pediatr Dermatol. 2018 Feb 13. doi: 10.1111/pde.13433.
FROM PEDIATRIC DERMATOLOGY
Key clinical point: More targeted education of parents regarding sun protection practices may be needed.
Major finding: Of 183 parents, 17% used ideal sun protection; 28% were in the lighter-skinned group and 5% in the darker-skinned group (odds ratio, 7.4; P less than .001).
Study details: The parents of 183 children aged from 6 months to 6 years in Toronto completed a questionnaire about their sun protection practices.
Disclosures: Dr. Marcus G. Tan reported no relevant financial disclosures. Dr.Miriam Weinstein has worked as an advisory board member for Johnson & Johnson, which manufactures sunscreen; and Dr. Shudeshna Nag consults for Valeant, which manufactures sunscreen.
Source: Tan MG et al. Pediatr Dermatol. 2018 Feb 13. doi: 10.1111/pde.13433.
Always get culture in symptomatic children with neurogenic bladder
In the symptomatic child with neurogenic bladder at risk for urinary tract infection (UTI), urine culture should be performed regardless of the results of urinalysis, recommended Catherine S. Forster, MD, of Cincinnati Children’s Hospital Medical Center, and her associates.
In a general pediatric population, studies have found that certain uropathogens – such as Enterococcus species, Klebsiella species, and Pseudomonas aeruginosa – are less likely to be associated with pyuria than Escherichia coli.
According to the guidelines of the Infectious Disease Society of America guidelines for the diagnosis of catheter-associated UTI, pyuria is not considered diagnostic of UTI in patients who require CIC.
Children with neurogenic bladder requiring CIC often have bacteriuria and often undergo urinalyses to determine if empirical antibiotics are warranted until urine culture results are available. “Although timely initiation of antibiotics can prevent the progression of infection and decrease the risk of renal scars, unnecessary antimicrobial agents contribute to the emergence of bacterial resistance,” Dr. Forster and her associates said.
So the researchers designed a study to find out if the presence of pyuria was associated with particular uropathogens in children with neurogenic bladders.
In an analysis of 2,420 urinalysis and urine culture results from EHRs between Jan.1, 2008, and Dec. 31, 2014, for patients aged 18 years and younger with neurogenic bladders requiring CIC, the most frequently isolated uropathogen was E. coli (37%), followed by Enterococcus species (14%), Klebsiella species (11%), and various other uropathogen species (38%).
In children needing CIC for neurogenic bladder, growth of Enterococcus species on urine culture was linked with lower odds of both microscopic pyuria (0.44) and leukocyte esterase (0.45).
“With these results, we suggest that the current markers of UTI evidenced on urinalysis and urine microscopy are insufficient for predicting bacteriuria in this population,” Dr. Forster and her colleagues said, leading to their recommendation to perform a culture in symptomatic children with neurogenic bladders irrespective of urinalysis results.
Dr. Forster received a research grant from the National Institutes of Health. The investigators said they had no relevant conflicts of interest.
SOURCE: Forster CS et al. Pediatrics. 2018;141(5):e20173006.
In the symptomatic child with neurogenic bladder at risk for urinary tract infection (UTI), urine culture should be performed regardless of the results of urinalysis, recommended Catherine S. Forster, MD, of Cincinnati Children’s Hospital Medical Center, and her associates.
In a general pediatric population, studies have found that certain uropathogens – such as Enterococcus species, Klebsiella species, and Pseudomonas aeruginosa – are less likely to be associated with pyuria than Escherichia coli.
According to the guidelines of the Infectious Disease Society of America guidelines for the diagnosis of catheter-associated UTI, pyuria is not considered diagnostic of UTI in patients who require CIC.
Children with neurogenic bladder requiring CIC often have bacteriuria and often undergo urinalyses to determine if empirical antibiotics are warranted until urine culture results are available. “Although timely initiation of antibiotics can prevent the progression of infection and decrease the risk of renal scars, unnecessary antimicrobial agents contribute to the emergence of bacterial resistance,” Dr. Forster and her associates said.
So the researchers designed a study to find out if the presence of pyuria was associated with particular uropathogens in children with neurogenic bladders.
In an analysis of 2,420 urinalysis and urine culture results from EHRs between Jan.1, 2008, and Dec. 31, 2014, for patients aged 18 years and younger with neurogenic bladders requiring CIC, the most frequently isolated uropathogen was E. coli (37%), followed by Enterococcus species (14%), Klebsiella species (11%), and various other uropathogen species (38%).
In children needing CIC for neurogenic bladder, growth of Enterococcus species on urine culture was linked with lower odds of both microscopic pyuria (0.44) and leukocyte esterase (0.45).
“With these results, we suggest that the current markers of UTI evidenced on urinalysis and urine microscopy are insufficient for predicting bacteriuria in this population,” Dr. Forster and her colleagues said, leading to their recommendation to perform a culture in symptomatic children with neurogenic bladders irrespective of urinalysis results.
Dr. Forster received a research grant from the National Institutes of Health. The investigators said they had no relevant conflicts of interest.
SOURCE: Forster CS et al. Pediatrics. 2018;141(5):e20173006.
In the symptomatic child with neurogenic bladder at risk for urinary tract infection (UTI), urine culture should be performed regardless of the results of urinalysis, recommended Catherine S. Forster, MD, of Cincinnati Children’s Hospital Medical Center, and her associates.
In a general pediatric population, studies have found that certain uropathogens – such as Enterococcus species, Klebsiella species, and Pseudomonas aeruginosa – are less likely to be associated with pyuria than Escherichia coli.
According to the guidelines of the Infectious Disease Society of America guidelines for the diagnosis of catheter-associated UTI, pyuria is not considered diagnostic of UTI in patients who require CIC.
Children with neurogenic bladder requiring CIC often have bacteriuria and often undergo urinalyses to determine if empirical antibiotics are warranted until urine culture results are available. “Although timely initiation of antibiotics can prevent the progression of infection and decrease the risk of renal scars, unnecessary antimicrobial agents contribute to the emergence of bacterial resistance,” Dr. Forster and her associates said.
So the researchers designed a study to find out if the presence of pyuria was associated with particular uropathogens in children with neurogenic bladders.
In an analysis of 2,420 urinalysis and urine culture results from EHRs between Jan.1, 2008, and Dec. 31, 2014, for patients aged 18 years and younger with neurogenic bladders requiring CIC, the most frequently isolated uropathogen was E. coli (37%), followed by Enterococcus species (14%), Klebsiella species (11%), and various other uropathogen species (38%).
In children needing CIC for neurogenic bladder, growth of Enterococcus species on urine culture was linked with lower odds of both microscopic pyuria (0.44) and leukocyte esterase (0.45).
“With these results, we suggest that the current markers of UTI evidenced on urinalysis and urine microscopy are insufficient for predicting bacteriuria in this population,” Dr. Forster and her colleagues said, leading to their recommendation to perform a culture in symptomatic children with neurogenic bladders irrespective of urinalysis results.
Dr. Forster received a research grant from the National Institutes of Health. The investigators said they had no relevant conflicts of interest.
SOURCE: Forster CS et al. Pediatrics. 2018;141(5):e20173006.
FROM PEDIATRICS
Key clinical point:
Major finding: In children needing CIC for neurogenic bladder, growth of Enterococcus species on urine culture was linked with lower odds of both microscopic pyuria (0.44) and leukocyte esterase (0.45).
Study details: Assessment of 2,420 urinalyses and urine cultures in children with neurogenic bladders.
Disclosures: Dr. Forster received a research grant from the National Institutes of Health. The investigators said they had no relevant conflicts of interest.
Source: Forster CS et al. Pediatrics. 2018;141(5):e20173006.
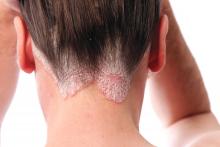
Alopecia areata has female predominance, more severe types common in boys
reported Iris Wohlmuth-Wieser, MD, of the department of dermatology, MD Anderson Cancer Center, Houston, and her associates, who conducted a large review of U.S. registry data.
Although it is the third most common dermatosis in children, there are not much data on AA in children, so the researchers used information from the National Alopecia Areata Registry, which was established in 2000. First, interested patients and parents were contacted and asked to fill out a web-based screening questionnaire. In the second phase, they were asked to fill out a more extensive survey and visit one of five U.S. sites for a clinical exam by a dermatologist.
Of the 2,218 children and teens who completed the initial questionnaire, the mean age at the time of the survey was 10 years, and their mean age of onset of AA was 6 years. The female to male ratio was 1.5:1; boys were significantly more likely to have severe types of AA (P = .009). Most patients (70%) were white, followed by mixed ethnicity (11%), Hispanic (3%), and then other ethnicities. About 3% of patients said they had a sibling with AA, 14% said that another first-degree relative had AA, and 8% said that at least three first-degree relatives had AA.
In terms of the degree of hair loss, 45% lost all scalp hair, 31% lost all body hair, and 14% lost all nails.
Concomitant diseases were reported by 47% of the responders, with atopic dermatitis, asthma, hay fever, and allergies the most common.
Of the 643 children and teens who completed a more detailed questionnaire and underwent clinical examination, 63% were female; 26% had at least one relative with AA and 8% had at least three first-degree relatives with AA. Almost 4% had congenital AA.
At the physical exam, there were data on the amount of hair loss in 617 children: Of these children, 37% had lost all scalp hair and 19% had lost up to three-quarters of their scalp hair. In 618 children (in whom information on body hair loss was obtained at the physical exam), 72% lost all or some of their body hair. Information on nails was available in 609 children; in this group, 44% had some nail involvement. More detailed information was available in 290 children; in this group, findings included pitting in 86%, dystrophy in 10%, onycholysis in 2%, ridging in 1%, and onychomycosis in 1%.
Commenting on the 25 children who presented with congenital AA, the authors wrote that this is “an extremely rare and infrequently reported form of AA.
“This is an interesting and important finding, because AA has traditionally been described as an acquired disease,” they added.
In their cohort overall, 25% had a family history of AA, with 8% having more than three first-degree relatives with AA. The researchers said that the percentage of children with AA and a positive family history ranges from 8% to 52% in the literature.
“The predominant presentation of AA types in our cohort (61.4%) was severe hair loss (76%-100% of scalp hair loss). This is comparable with a European study reporting a prevalence of 65%. Other studies on childhood AA conducted in Asian and Arab populations observed mainly mild cases,” they wrote.
Nail involvement, often reported with AA, was evident in 39% of patients who completed the questionnaire online and in 44% on physical exam. This agreed with the 26%-40% involvement reported in the literature.
There were no conflicts of interest or funding information reported.
SOURCE: Wohlmuth-Wieser I et al. Pediatr Dermatol. 2018 Jan 15. doi: 10.1111/pde.13387.
reported Iris Wohlmuth-Wieser, MD, of the department of dermatology, MD Anderson Cancer Center, Houston, and her associates, who conducted a large review of U.S. registry data.
Although it is the third most common dermatosis in children, there are not much data on AA in children, so the researchers used information from the National Alopecia Areata Registry, which was established in 2000. First, interested patients and parents were contacted and asked to fill out a web-based screening questionnaire. In the second phase, they were asked to fill out a more extensive survey and visit one of five U.S. sites for a clinical exam by a dermatologist.
Of the 2,218 children and teens who completed the initial questionnaire, the mean age at the time of the survey was 10 years, and their mean age of onset of AA was 6 years. The female to male ratio was 1.5:1; boys were significantly more likely to have severe types of AA (P = .009). Most patients (70%) were white, followed by mixed ethnicity (11%), Hispanic (3%), and then other ethnicities. About 3% of patients said they had a sibling with AA, 14% said that another first-degree relative had AA, and 8% said that at least three first-degree relatives had AA.
In terms of the degree of hair loss, 45% lost all scalp hair, 31% lost all body hair, and 14% lost all nails.
Concomitant diseases were reported by 47% of the responders, with atopic dermatitis, asthma, hay fever, and allergies the most common.
Of the 643 children and teens who completed a more detailed questionnaire and underwent clinical examination, 63% were female; 26% had at least one relative with AA and 8% had at least three first-degree relatives with AA. Almost 4% had congenital AA.
At the physical exam, there were data on the amount of hair loss in 617 children: Of these children, 37% had lost all scalp hair and 19% had lost up to three-quarters of their scalp hair. In 618 children (in whom information on body hair loss was obtained at the physical exam), 72% lost all or some of their body hair. Information on nails was available in 609 children; in this group, 44% had some nail involvement. More detailed information was available in 290 children; in this group, findings included pitting in 86%, dystrophy in 10%, onycholysis in 2%, ridging in 1%, and onychomycosis in 1%.
Commenting on the 25 children who presented with congenital AA, the authors wrote that this is “an extremely rare and infrequently reported form of AA.
“This is an interesting and important finding, because AA has traditionally been described as an acquired disease,” they added.
In their cohort overall, 25% had a family history of AA, with 8% having more than three first-degree relatives with AA. The researchers said that the percentage of children with AA and a positive family history ranges from 8% to 52% in the literature.
“The predominant presentation of AA types in our cohort (61.4%) was severe hair loss (76%-100% of scalp hair loss). This is comparable with a European study reporting a prevalence of 65%. Other studies on childhood AA conducted in Asian and Arab populations observed mainly mild cases,” they wrote.
Nail involvement, often reported with AA, was evident in 39% of patients who completed the questionnaire online and in 44% on physical exam. This agreed with the 26%-40% involvement reported in the literature.
There were no conflicts of interest or funding information reported.
SOURCE: Wohlmuth-Wieser I et al. Pediatr Dermatol. 2018 Jan 15. doi: 10.1111/pde.13387.
reported Iris Wohlmuth-Wieser, MD, of the department of dermatology, MD Anderson Cancer Center, Houston, and her associates, who conducted a large review of U.S. registry data.
Although it is the third most common dermatosis in children, there are not much data on AA in children, so the researchers used information from the National Alopecia Areata Registry, which was established in 2000. First, interested patients and parents were contacted and asked to fill out a web-based screening questionnaire. In the second phase, they were asked to fill out a more extensive survey and visit one of five U.S. sites for a clinical exam by a dermatologist.
Of the 2,218 children and teens who completed the initial questionnaire, the mean age at the time of the survey was 10 years, and their mean age of onset of AA was 6 years. The female to male ratio was 1.5:1; boys were significantly more likely to have severe types of AA (P = .009). Most patients (70%) were white, followed by mixed ethnicity (11%), Hispanic (3%), and then other ethnicities. About 3% of patients said they had a sibling with AA, 14% said that another first-degree relative had AA, and 8% said that at least three first-degree relatives had AA.
In terms of the degree of hair loss, 45% lost all scalp hair, 31% lost all body hair, and 14% lost all nails.
Concomitant diseases were reported by 47% of the responders, with atopic dermatitis, asthma, hay fever, and allergies the most common.
Of the 643 children and teens who completed a more detailed questionnaire and underwent clinical examination, 63% were female; 26% had at least one relative with AA and 8% had at least three first-degree relatives with AA. Almost 4% had congenital AA.
At the physical exam, there were data on the amount of hair loss in 617 children: Of these children, 37% had lost all scalp hair and 19% had lost up to three-quarters of their scalp hair. In 618 children (in whom information on body hair loss was obtained at the physical exam), 72% lost all or some of their body hair. Information on nails was available in 609 children; in this group, 44% had some nail involvement. More detailed information was available in 290 children; in this group, findings included pitting in 86%, dystrophy in 10%, onycholysis in 2%, ridging in 1%, and onychomycosis in 1%.
Commenting on the 25 children who presented with congenital AA, the authors wrote that this is “an extremely rare and infrequently reported form of AA.
“This is an interesting and important finding, because AA has traditionally been described as an acquired disease,” they added.
In their cohort overall, 25% had a family history of AA, with 8% having more than three first-degree relatives with AA. The researchers said that the percentage of children with AA and a positive family history ranges from 8% to 52% in the literature.
“The predominant presentation of AA types in our cohort (61.4%) was severe hair loss (76%-100% of scalp hair loss). This is comparable with a European study reporting a prevalence of 65%. Other studies on childhood AA conducted in Asian and Arab populations observed mainly mild cases,” they wrote.
Nail involvement, often reported with AA, was evident in 39% of patients who completed the questionnaire online and in 44% on physical exam. This agreed with the 26%-40% involvement reported in the literature.
There were no conflicts of interest or funding information reported.
SOURCE: Wohlmuth-Wieser I et al. Pediatr Dermatol. 2018 Jan 15. doi: 10.1111/pde.13387.
FROM PEDIATRIC DERMATOLOGY
Key clinical point: The predominant presentation is total hair loss and nail involvement is common.
Major finding: The female to male ratio was 1.5:1; the boys were significantly more likely to have severe types of AA (P = .009).
Study details: National Alopecia Areata Registry registrants under age 18 years were asked to complete a survey.
Disclosures: There were no conflicts of interest or funding information reported.
Source: Wohlmuth-Wieser I et al. Pediatr Dermatol. 2018. doi: 10.1111/pde.13387.
Pilot study: Topical anticholinergic improved axillary hyperhidrosis in teens, young adults
Nicholas V. Nguyen, MD, of the division of dermatology, Akron Children’s Hospital, Ohio, and his associates reported.
Ten patients aged 13-24 years with moderate to severe axillary hyperhidrosis started the study and were treated with 1 g of oxybutynin 3% gel applied to each axilla every morning for 4 weeks. Of the seven patients who completed the study, four had a two-point reduction in the Hyperhidrosis Disease Severity Scale (HDSS) at week 1 and all seven achieved that endpoint at week 4. Of the five patients who also had hyperhidrosis of the palms, four had a two-point reduction in the HDSS at weeks 1 and 4; the remaining patient reported no change. Of the five patients who also had plantar hyperhidrosis, two reported a reduction at weeks 1 and 4, two reported no change, and one had no change at week 1 and experienced worse hyperhidrosis at week 4.
Safety data were available in the seven patients who completed the study and in two others, one lost to follow-up after the first week, and one patient who dropped out of the study because of a severe adverse event. Three patients reported application site irritation, but it was mild to moderate and did not require any intervention. Two patients reported xerostomia, which resolved by itself, and one reported constipation and blurry vision unrelated to the study drug.
One patient developed pyelonephritis on the sixth day of treatment, possibly related to the study drug. “This patient had a history of kidney transplantation, immunosuppression, and recurrent urinary tract infections. For these reasons, the drug should be used with caution in patients with a history of urinary retention or recurrent urinary tract infections,” Dr. Nguyen and his colleagues said.
The manufacturer discontinued the 3% formulation during enrollment, because of business reasons. A large, randomized, placebo-controlled, double-blind trial to address unanswered questions is warranted, the researchers noted. Oxybutynin 10% gel, approved for treating overactive bladder, is still commercially available.
The study was supported by a Society for Pediatric Dermatology pilot project grant and a Colorado Clinical and Translational Sciences Institute grant.
SOURCE: Nguyen NV et al. Pediatr Dermatol. 2018 Jan 15. doi: 10.1111/pde.13404.
Nicholas V. Nguyen, MD, of the division of dermatology, Akron Children’s Hospital, Ohio, and his associates reported.
Ten patients aged 13-24 years with moderate to severe axillary hyperhidrosis started the study and were treated with 1 g of oxybutynin 3% gel applied to each axilla every morning for 4 weeks. Of the seven patients who completed the study, four had a two-point reduction in the Hyperhidrosis Disease Severity Scale (HDSS) at week 1 and all seven achieved that endpoint at week 4. Of the five patients who also had hyperhidrosis of the palms, four had a two-point reduction in the HDSS at weeks 1 and 4; the remaining patient reported no change. Of the five patients who also had plantar hyperhidrosis, two reported a reduction at weeks 1 and 4, two reported no change, and one had no change at week 1 and experienced worse hyperhidrosis at week 4.
Safety data were available in the seven patients who completed the study and in two others, one lost to follow-up after the first week, and one patient who dropped out of the study because of a severe adverse event. Three patients reported application site irritation, but it was mild to moderate and did not require any intervention. Two patients reported xerostomia, which resolved by itself, and one reported constipation and blurry vision unrelated to the study drug.
One patient developed pyelonephritis on the sixth day of treatment, possibly related to the study drug. “This patient had a history of kidney transplantation, immunosuppression, and recurrent urinary tract infections. For these reasons, the drug should be used with caution in patients with a history of urinary retention or recurrent urinary tract infections,” Dr. Nguyen and his colleagues said.
The manufacturer discontinued the 3% formulation during enrollment, because of business reasons. A large, randomized, placebo-controlled, double-blind trial to address unanswered questions is warranted, the researchers noted. Oxybutynin 10% gel, approved for treating overactive bladder, is still commercially available.
The study was supported by a Society for Pediatric Dermatology pilot project grant and a Colorado Clinical and Translational Sciences Institute grant.
SOURCE: Nguyen NV et al. Pediatr Dermatol. 2018 Jan 15. doi: 10.1111/pde.13404.
Nicholas V. Nguyen, MD, of the division of dermatology, Akron Children’s Hospital, Ohio, and his associates reported.
Ten patients aged 13-24 years with moderate to severe axillary hyperhidrosis started the study and were treated with 1 g of oxybutynin 3% gel applied to each axilla every morning for 4 weeks. Of the seven patients who completed the study, four had a two-point reduction in the Hyperhidrosis Disease Severity Scale (HDSS) at week 1 and all seven achieved that endpoint at week 4. Of the five patients who also had hyperhidrosis of the palms, four had a two-point reduction in the HDSS at weeks 1 and 4; the remaining patient reported no change. Of the five patients who also had plantar hyperhidrosis, two reported a reduction at weeks 1 and 4, two reported no change, and one had no change at week 1 and experienced worse hyperhidrosis at week 4.
Safety data were available in the seven patients who completed the study and in two others, one lost to follow-up after the first week, and one patient who dropped out of the study because of a severe adverse event. Three patients reported application site irritation, but it was mild to moderate and did not require any intervention. Two patients reported xerostomia, which resolved by itself, and one reported constipation and blurry vision unrelated to the study drug.
One patient developed pyelonephritis on the sixth day of treatment, possibly related to the study drug. “This patient had a history of kidney transplantation, immunosuppression, and recurrent urinary tract infections. For these reasons, the drug should be used with caution in patients with a history of urinary retention or recurrent urinary tract infections,” Dr. Nguyen and his colleagues said.
The manufacturer discontinued the 3% formulation during enrollment, because of business reasons. A large, randomized, placebo-controlled, double-blind trial to address unanswered questions is warranted, the researchers noted. Oxybutynin 10% gel, approved for treating overactive bladder, is still commercially available.
The study was supported by a Society for Pediatric Dermatology pilot project grant and a Colorado Clinical and Translational Sciences Institute grant.
SOURCE: Nguyen NV et al. Pediatr Dermatol. 2018 Jan 15. doi: 10.1111/pde.13404.
FROM PEDIATRIC DERMATOLOGY