User login
Commentary: Babies die as congenital syphilis continues a decade-long surge across the U.S.
The data are shocking: Almost 35,000 U.S. syphilis cases by mid-July 2022 with the highest rates per/100,000 population in Nevada (n = 21), California (n = 19), and Mississippi (n = 16). Excluding Nevada, California, and Oklahoma, rates over 12/100,000 were concentrated in the southernmost U.S. states. Overall, the 2,268 congenital syphilis cases in U.S. children born in 2021 was a 6% increase over 2020, and a 680% increase over 2012. (Note: All 2021 data are not yet available because of public health STI resources being diverted to COVID-19 control.) A telling number is the 166 congenital syphilis deaths in babies born in 2021 – a 1,000% increase over 2012. Another concern is that 50% of U.S. counties reported at least one congenital syphilis case in 2019 – the last time frame from which county-specific data are available.
Syphilis afflicts the underserved and underprivileged more than other demographic groups, particularly when public health budgets are not adequate (funding for public health STI prevention/treatment efforts has lagged for more than a decade), and/or when public health emergencies such as the pandemic divert public health resources away from STI prevention/treatment efforts.
As pediatric care providers, we can help by heightening our vigilance and appropriately testing for and treating syphilis, particularly in newborns/infants, regardless of where we work. And we can advocate for increased public health STI funding allocation whenever possible. It is a smart economic move because it costs nearly 1,000 times more to manage congenital syphilis and its sequelae than to prevent or treat it.
Christopher J. Harrison, MD, is professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. He has no financial conflicts of interest.
The data are shocking: Almost 35,000 U.S. syphilis cases by mid-July 2022 with the highest rates per/100,000 population in Nevada (n = 21), California (n = 19), and Mississippi (n = 16). Excluding Nevada, California, and Oklahoma, rates over 12/100,000 were concentrated in the southernmost U.S. states. Overall, the 2,268 congenital syphilis cases in U.S. children born in 2021 was a 6% increase over 2020, and a 680% increase over 2012. (Note: All 2021 data are not yet available because of public health STI resources being diverted to COVID-19 control.) A telling number is the 166 congenital syphilis deaths in babies born in 2021 – a 1,000% increase over 2012. Another concern is that 50% of U.S. counties reported at least one congenital syphilis case in 2019 – the last time frame from which county-specific data are available.
Syphilis afflicts the underserved and underprivileged more than other demographic groups, particularly when public health budgets are not adequate (funding for public health STI prevention/treatment efforts has lagged for more than a decade), and/or when public health emergencies such as the pandemic divert public health resources away from STI prevention/treatment efforts.
As pediatric care providers, we can help by heightening our vigilance and appropriately testing for and treating syphilis, particularly in newborns/infants, regardless of where we work. And we can advocate for increased public health STI funding allocation whenever possible. It is a smart economic move because it costs nearly 1,000 times more to manage congenital syphilis and its sequelae than to prevent or treat it.
Christopher J. Harrison, MD, is professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. He has no financial conflicts of interest.
The data are shocking: Almost 35,000 U.S. syphilis cases by mid-July 2022 with the highest rates per/100,000 population in Nevada (n = 21), California (n = 19), and Mississippi (n = 16). Excluding Nevada, California, and Oklahoma, rates over 12/100,000 were concentrated in the southernmost U.S. states. Overall, the 2,268 congenital syphilis cases in U.S. children born in 2021 was a 6% increase over 2020, and a 680% increase over 2012. (Note: All 2021 data are not yet available because of public health STI resources being diverted to COVID-19 control.) A telling number is the 166 congenital syphilis deaths in babies born in 2021 – a 1,000% increase over 2012. Another concern is that 50% of U.S. counties reported at least one congenital syphilis case in 2019 – the last time frame from which county-specific data are available.
Syphilis afflicts the underserved and underprivileged more than other demographic groups, particularly when public health budgets are not adequate (funding for public health STI prevention/treatment efforts has lagged for more than a decade), and/or when public health emergencies such as the pandemic divert public health resources away from STI prevention/treatment efforts.
As pediatric care providers, we can help by heightening our vigilance and appropriately testing for and treating syphilis, particularly in newborns/infants, regardless of where we work. And we can advocate for increased public health STI funding allocation whenever possible. It is a smart economic move because it costs nearly 1,000 times more to manage congenital syphilis and its sequelae than to prevent or treat it.
Christopher J. Harrison, MD, is professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. He has no financial conflicts of interest.
Commentary: Meningococcal vaccine shows moderate protective effect against gonorrhea
The data on cross-protection against gonorrhea by outer membrane vesicle (OMV)–based meningococcal B vaccine continue to look encouraging from a recent study in Clinical Infectious Diseases (2022; doi: 10.1093/cid/ciac436). The authors report matched-cohort study data involving over 33,000 teens/young adults followed at Kaiser Permanente Southern California during 2016-2020. Like the studies above, chlamydia-infected patients (n = 26,471) served as negative controls for the 6,641 gonorrhea patients. The researchers compared chances of getting gonorrhea vs. getting chlamydia in light of having previously gotten C4MenB vaccine (OMV-based) or MenACWY vaccine (not OMV-based). The authors reported gonorrhea incidence rates of 2.0/1,000 person-years (95% CI, 1.3–2.8) in 4CMenB vaccinees vs. 5.2 (4.6–5.8) for MenACWY recipients. An adjusted analysis revealed 46% lower gonorrhea rates in 4CMenB vs. MenACWY vaccinees. There was no difference in chlamydia rates.
We await prospective controlled data to validate these observational studies. However, it is intriguing that OMV-based meningococcal vaccine may be a two-fer vaccine with partial cross protection against gonorrhea because of outer membrane protein similarities between the two pathogens. These data seem worth sharing with families who are making decisions about whether to vaccinate their children against B strains of meningococcus whether or not the child has already had conjugate MenACWY.
Christopher J. Harrison, MD, is professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. He has no financial conflicts of interest.
The data on cross-protection against gonorrhea by outer membrane vesicle (OMV)–based meningococcal B vaccine continue to look encouraging from a recent study in Clinical Infectious Diseases (2022; doi: 10.1093/cid/ciac436). The authors report matched-cohort study data involving over 33,000 teens/young adults followed at Kaiser Permanente Southern California during 2016-2020. Like the studies above, chlamydia-infected patients (n = 26,471) served as negative controls for the 6,641 gonorrhea patients. The researchers compared chances of getting gonorrhea vs. getting chlamydia in light of having previously gotten C4MenB vaccine (OMV-based) or MenACWY vaccine (not OMV-based). The authors reported gonorrhea incidence rates of 2.0/1,000 person-years (95% CI, 1.3–2.8) in 4CMenB vaccinees vs. 5.2 (4.6–5.8) for MenACWY recipients. An adjusted analysis revealed 46% lower gonorrhea rates in 4CMenB vs. MenACWY vaccinees. There was no difference in chlamydia rates.
We await prospective controlled data to validate these observational studies. However, it is intriguing that OMV-based meningococcal vaccine may be a two-fer vaccine with partial cross protection against gonorrhea because of outer membrane protein similarities between the two pathogens. These data seem worth sharing with families who are making decisions about whether to vaccinate their children against B strains of meningococcus whether or not the child has already had conjugate MenACWY.
Christopher J. Harrison, MD, is professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. He has no financial conflicts of interest.
The data on cross-protection against gonorrhea by outer membrane vesicle (OMV)–based meningococcal B vaccine continue to look encouraging from a recent study in Clinical Infectious Diseases (2022; doi: 10.1093/cid/ciac436). The authors report matched-cohort study data involving over 33,000 teens/young adults followed at Kaiser Permanente Southern California during 2016-2020. Like the studies above, chlamydia-infected patients (n = 26,471) served as negative controls for the 6,641 gonorrhea patients. The researchers compared chances of getting gonorrhea vs. getting chlamydia in light of having previously gotten C4MenB vaccine (OMV-based) or MenACWY vaccine (not OMV-based). The authors reported gonorrhea incidence rates of 2.0/1,000 person-years (95% CI, 1.3–2.8) in 4CMenB vaccinees vs. 5.2 (4.6–5.8) for MenACWY recipients. An adjusted analysis revealed 46% lower gonorrhea rates in 4CMenB vs. MenACWY vaccinees. There was no difference in chlamydia rates.
We await prospective controlled data to validate these observational studies. However, it is intriguing that OMV-based meningococcal vaccine may be a two-fer vaccine with partial cross protection against gonorrhea because of outer membrane protein similarities between the two pathogens. These data seem worth sharing with families who are making decisions about whether to vaccinate their children against B strains of meningococcus whether or not the child has already had conjugate MenACWY.
Christopher J. Harrison, MD, is professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. He has no financial conflicts of interest.
Commentary: Adolescents are undertested for STIs
Despite current guidelines permitting opt-out STI screening and the ability in most states to treat adolescents 13 years old and older for STIs without parental notification, STI testing rates remain low. The articles about the 2019 survey raise several concerns.
Screening was more common with certain known risky behaviors, so risk-based screening seems prevalent. Of note, one recently reported factor increasing risky behaviors, but not noted above, is homelessness, suggesting it also be a trigger for STI screening (Child Youth Services Rev. 2022;139. doi: 10.1016/j.childyouth.2022.106538). But won’t risk-based screening inevitably lead to undertesting/treating? Are adolescents comfortable/willing to answer even the most carefully crafted, gentle, and simple questions about risky behaviors?
Because STIs are so frequent in adolescents (many asymptomatic), is risk-based screening/testing adequate for common STIs? Could urine-based screening for gonorrhea and chlamydia be useful in any adolescent who has been sexually active or uses the ED for routine care? Syphilis seems different. Screening requires a blood draw and is more difficult to implement, so risk-based testing seems okay. Also, the CDC recommends risk-based screening for syphilis.
Chief complaints at adolescent visits are usually not STI-related, unless symptomatic or visible, e.g., genital warts or herpes. So STI screening (even opt-out) will lengthen visits, perhaps a lot, over what was scheduled – even if only to explain negative results. Multiple visits with screening in the same morning could wreck patient flow. Maybe this doesn’t influence decisions to screen/test, but reexamining our approach can maximize appropriate STI screening/testing.
STIs run in packs, so if you detect one, expand testing to include the others.
Christopher J. Harrison, MD, is professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. He has no financial conflicts of interest.
Despite current guidelines permitting opt-out STI screening and the ability in most states to treat adolescents 13 years old and older for STIs without parental notification, STI testing rates remain low. The articles about the 2019 survey raise several concerns.
Screening was more common with certain known risky behaviors, so risk-based screening seems prevalent. Of note, one recently reported factor increasing risky behaviors, but not noted above, is homelessness, suggesting it also be a trigger for STI screening (Child Youth Services Rev. 2022;139. doi: 10.1016/j.childyouth.2022.106538). But won’t risk-based screening inevitably lead to undertesting/treating? Are adolescents comfortable/willing to answer even the most carefully crafted, gentle, and simple questions about risky behaviors?
Because STIs are so frequent in adolescents (many asymptomatic), is risk-based screening/testing adequate for common STIs? Could urine-based screening for gonorrhea and chlamydia be useful in any adolescent who has been sexually active or uses the ED for routine care? Syphilis seems different. Screening requires a blood draw and is more difficult to implement, so risk-based testing seems okay. Also, the CDC recommends risk-based screening for syphilis.
Chief complaints at adolescent visits are usually not STI-related, unless symptomatic or visible, e.g., genital warts or herpes. So STI screening (even opt-out) will lengthen visits, perhaps a lot, over what was scheduled – even if only to explain negative results. Multiple visits with screening in the same morning could wreck patient flow. Maybe this doesn’t influence decisions to screen/test, but reexamining our approach can maximize appropriate STI screening/testing.
STIs run in packs, so if you detect one, expand testing to include the others.
Christopher J. Harrison, MD, is professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. He has no financial conflicts of interest.
Despite current guidelines permitting opt-out STI screening and the ability in most states to treat adolescents 13 years old and older for STIs without parental notification, STI testing rates remain low. The articles about the 2019 survey raise several concerns.
Screening was more common with certain known risky behaviors, so risk-based screening seems prevalent. Of note, one recently reported factor increasing risky behaviors, but not noted above, is homelessness, suggesting it also be a trigger for STI screening (Child Youth Services Rev. 2022;139. doi: 10.1016/j.childyouth.2022.106538). But won’t risk-based screening inevitably lead to undertesting/treating? Are adolescents comfortable/willing to answer even the most carefully crafted, gentle, and simple questions about risky behaviors?
Because STIs are so frequent in adolescents (many asymptomatic), is risk-based screening/testing adequate for common STIs? Could urine-based screening for gonorrhea and chlamydia be useful in any adolescent who has been sexually active or uses the ED for routine care? Syphilis seems different. Screening requires a blood draw and is more difficult to implement, so risk-based testing seems okay. Also, the CDC recommends risk-based screening for syphilis.
Chief complaints at adolescent visits are usually not STI-related, unless symptomatic or visible, e.g., genital warts or herpes. So STI screening (even opt-out) will lengthen visits, perhaps a lot, over what was scheduled – even if only to explain negative results. Multiple visits with screening in the same morning could wreck patient flow. Maybe this doesn’t influence decisions to screen/test, but reexamining our approach can maximize appropriate STI screening/testing.
STIs run in packs, so if you detect one, expand testing to include the others.
Christopher J. Harrison, MD, is professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. He has no financial conflicts of interest.
Commentary: Norovirus vaccine candidates employ different approaches
Norovirus, as noted above, is now the most common cause of medically attended acute gastroenteritis (AGE) in the United States. Norovirus AGE resembles rotavirus AGE, but a bit heavier on the vomiting. What makes it scary is that it is a low-inoculum infection (as few as 16 virus particles can cause infection), and it survives for prolonged periods in food, 10% chlorinated water, and on environmental surfaces (J Med Virol 2008;80:1468-76); hence, the infamous outbreaks on cruise ships and daycare centers. So a vaccine would be very welcome. The two non-Chinese candidates GI.1/GII.4 vaccines are Takeda’s VLP vaccine and Vaxart’s oral adenovirus vector-based vaccine.
Takeda’s is injectable. If VLP sounds familiar, VLPs make up the FDA-approved HPV vaccine we use. Two doses of various formulations were tested in a recent phase 2 study of 1- to 3- and 4- to 8-year-olds in Finland, Panama, and Colombia with no safety issues identified. The 1- to 3-year-olds responded somewhat better than 4- to 8-year-olds, and titers remained elevated up to day 210 (Vaccine. 2022 Jun 9;40[26]:3588-96).
A recently as yet unpublished phase 1b trial of Vaxart’s vaccine in 55- to 80-year-olds (NCT04854746) showed a dose-dependent response. IgA mucosal cell responses were similar to those in younger adults. Adverse event profiles were similar between vaccinees and placebo recipients.
Progress continues for both vaccines, but we await efficacy trials. We are likely still years from a pediatric vaccine. My sense is that an oral vaccine would be more readily accepted into the pediatric schedule, but how to incorporate it and not cause issues with the rotavirus vaccine will need evaluation.
Christopher J. Harrison, MD, is professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. He has no financial conflicts of interest.
Norovirus, as noted above, is now the most common cause of medically attended acute gastroenteritis (AGE) in the United States. Norovirus AGE resembles rotavirus AGE, but a bit heavier on the vomiting. What makes it scary is that it is a low-inoculum infection (as few as 16 virus particles can cause infection), and it survives for prolonged periods in food, 10% chlorinated water, and on environmental surfaces (J Med Virol 2008;80:1468-76); hence, the infamous outbreaks on cruise ships and daycare centers. So a vaccine would be very welcome. The two non-Chinese candidates GI.1/GII.4 vaccines are Takeda’s VLP vaccine and Vaxart’s oral adenovirus vector-based vaccine.
Takeda’s is injectable. If VLP sounds familiar, VLPs make up the FDA-approved HPV vaccine we use. Two doses of various formulations were tested in a recent phase 2 study of 1- to 3- and 4- to 8-year-olds in Finland, Panama, and Colombia with no safety issues identified. The 1- to 3-year-olds responded somewhat better than 4- to 8-year-olds, and titers remained elevated up to day 210 (Vaccine. 2022 Jun 9;40[26]:3588-96).
A recently as yet unpublished phase 1b trial of Vaxart’s vaccine in 55- to 80-year-olds (NCT04854746) showed a dose-dependent response. IgA mucosal cell responses were similar to those in younger adults. Adverse event profiles were similar between vaccinees and placebo recipients.
Progress continues for both vaccines, but we await efficacy trials. We are likely still years from a pediatric vaccine. My sense is that an oral vaccine would be more readily accepted into the pediatric schedule, but how to incorporate it and not cause issues with the rotavirus vaccine will need evaluation.
Christopher J. Harrison, MD, is professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. He has no financial conflicts of interest.
Norovirus, as noted above, is now the most common cause of medically attended acute gastroenteritis (AGE) in the United States. Norovirus AGE resembles rotavirus AGE, but a bit heavier on the vomiting. What makes it scary is that it is a low-inoculum infection (as few as 16 virus particles can cause infection), and it survives for prolonged periods in food, 10% chlorinated water, and on environmental surfaces (J Med Virol 2008;80:1468-76); hence, the infamous outbreaks on cruise ships and daycare centers. So a vaccine would be very welcome. The two non-Chinese candidates GI.1/GII.4 vaccines are Takeda’s VLP vaccine and Vaxart’s oral adenovirus vector-based vaccine.
Takeda’s is injectable. If VLP sounds familiar, VLPs make up the FDA-approved HPV vaccine we use. Two doses of various formulations were tested in a recent phase 2 study of 1- to 3- and 4- to 8-year-olds in Finland, Panama, and Colombia with no safety issues identified. The 1- to 3-year-olds responded somewhat better than 4- to 8-year-olds, and titers remained elevated up to day 210 (Vaccine. 2022 Jun 9;40[26]:3588-96).
A recently as yet unpublished phase 1b trial of Vaxart’s vaccine in 55- to 80-year-olds (NCT04854746) showed a dose-dependent response. IgA mucosal cell responses were similar to those in younger adults. Adverse event profiles were similar between vaccinees and placebo recipients.
Progress continues for both vaccines, but we await efficacy trials. We are likely still years from a pediatric vaccine. My sense is that an oral vaccine would be more readily accepted into the pediatric schedule, but how to incorporate it and not cause issues with the rotavirus vaccine will need evaluation.
Christopher J. Harrison, MD, is professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. He has no financial conflicts of interest.
Commentary - TB treatment can be shortened for most children: Study
In spring 2022 the World Health Organization did release the new guidelines (module 5) for the shorter (4 month) regimen for 3-month- to 16-year-olds with nonsevere pulmonary tuberculosis (TB) limited to one lobe that is also smear-negative and at least presumed to be due to drug-susceptible M. tuberculosis. This regimen is NOT for children with clinically significant airway obstruction, cavitary disease, miliary TB, complex pleural effusion, or peripheral lymph node involvement. The newly recommended regimen consists of 8 weeks as an “intensive phase” (isoniazid, rifampin, pyrazinamide, and ethambutol, per local guidance) followed by 8 weeks of a “continuation phase” (isoniazid and rifampin only). Of note, the Turkova study had shown nearly identical adverse event and adherence rates – 8% and 94% – for both the short- and traditional-length regimens. The onerous multidrug treatment of uncomplicated TB in most children has become less onerous.
Caveat: The newly recommended 4-month schedule (March 2022) of traditional TB drugs is not to be confused with rifapentine-moxifloxacin–based 4-month regimen recommended by the WHO in June 2021 (CDC added guidance February 2022). This rifapentine-based regimen had been okayed for patients 12 years or older weighing at least 40 kg and also with drug-susceptible pulmonary TB, but no extrapulmonary involvement.
The new shorter regimen shows the value of trials in non-U.S. countries. The careful work in Africa and India has borne fruit that makes things easier for families, providers, and public health organizations.
Christopher J. Harrison, MD, is professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. He has no financial conflicts of interest.
In spring 2022 the World Health Organization did release the new guidelines (module 5) for the shorter (4 month) regimen for 3-month- to 16-year-olds with nonsevere pulmonary tuberculosis (TB) limited to one lobe that is also smear-negative and at least presumed to be due to drug-susceptible M. tuberculosis. This regimen is NOT for children with clinically significant airway obstruction, cavitary disease, miliary TB, complex pleural effusion, or peripheral lymph node involvement. The newly recommended regimen consists of 8 weeks as an “intensive phase” (isoniazid, rifampin, pyrazinamide, and ethambutol, per local guidance) followed by 8 weeks of a “continuation phase” (isoniazid and rifampin only). Of note, the Turkova study had shown nearly identical adverse event and adherence rates – 8% and 94% – for both the short- and traditional-length regimens. The onerous multidrug treatment of uncomplicated TB in most children has become less onerous.
Caveat: The newly recommended 4-month schedule (March 2022) of traditional TB drugs is not to be confused with rifapentine-moxifloxacin–based 4-month regimen recommended by the WHO in June 2021 (CDC added guidance February 2022). This rifapentine-based regimen had been okayed for patients 12 years or older weighing at least 40 kg and also with drug-susceptible pulmonary TB, but no extrapulmonary involvement.
The new shorter regimen shows the value of trials in non-U.S. countries. The careful work in Africa and India has borne fruit that makes things easier for families, providers, and public health organizations.
Christopher J. Harrison, MD, is professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. He has no financial conflicts of interest.
In spring 2022 the World Health Organization did release the new guidelines (module 5) for the shorter (4 month) regimen for 3-month- to 16-year-olds with nonsevere pulmonary tuberculosis (TB) limited to one lobe that is also smear-negative and at least presumed to be due to drug-susceptible M. tuberculosis. This regimen is NOT for children with clinically significant airway obstruction, cavitary disease, miliary TB, complex pleural effusion, or peripheral lymph node involvement. The newly recommended regimen consists of 8 weeks as an “intensive phase” (isoniazid, rifampin, pyrazinamide, and ethambutol, per local guidance) followed by 8 weeks of a “continuation phase” (isoniazid and rifampin only). Of note, the Turkova study had shown nearly identical adverse event and adherence rates – 8% and 94% – for both the short- and traditional-length regimens. The onerous multidrug treatment of uncomplicated TB in most children has become less onerous.
Caveat: The newly recommended 4-month schedule (March 2022) of traditional TB drugs is not to be confused with rifapentine-moxifloxacin–based 4-month regimen recommended by the WHO in June 2021 (CDC added guidance February 2022). This rifapentine-based regimen had been okayed for patients 12 years or older weighing at least 40 kg and also with drug-susceptible pulmonary TB, but no extrapulmonary involvement.
The new shorter regimen shows the value of trials in non-U.S. countries. The careful work in Africa and India has borne fruit that makes things easier for families, providers, and public health organizations.
Christopher J. Harrison, MD, is professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. He has no financial conflicts of interest.
Commentary: Nirsevimab protects healthy infants from RSV
Imagine a time when RSV doesn’t rage through the community each year. That time would result from decades of research that uncovered RSV’s secrets and explained the only partly successful initial vaccines and prophylactic interventions (for example, palivizumab).
Key discovery: The original RSV antigen target, RSV’s fusion (F) protein, is suboptimal despite having been associated with RSV attachment to and breaching of host cell membranes.
Some amazing work showed that having antibodies block the F-protein was like putting up a shield to protect against an arrow only after the arrow had already struck its target. Indeed, the F-protein evolves only after the attachment/breach. The preattachment/breach version (prefusion protein) sits on the virus surface and is like a loaded bow with an arrow in place. The prefusion protein changes configuration when RSV contacts host cells to uncoil and release the “arrow,” creating the entry point for RSV nucleic acids.
Nirsevimab was created to glom onto the prefusion protein and prevent it from uncoiling/releasing its “arrow,” the critical event in RSV infecting a cell. So, it is not surprising that it works better than palivizumab, which targets the fusion protein.
The prefusion protein is also the target of newer vaccine candidates, including one that showed 87% efficacy against RSV challenge in adults (think of mother getting this vaccine and endowing newborns with antiprefusion antibodies; N Engl J Med. 2022;386:2377-86).
The time when RSV is not an annual scourge is closer than ever.
Christopher J. Harrison, MD, is professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. He has no financial conflicts of interest.
Imagine a time when RSV doesn’t rage through the community each year. That time would result from decades of research that uncovered RSV’s secrets and explained the only partly successful initial vaccines and prophylactic interventions (for example, palivizumab).
Key discovery: The original RSV antigen target, RSV’s fusion (F) protein, is suboptimal despite having been associated with RSV attachment to and breaching of host cell membranes.
Some amazing work showed that having antibodies block the F-protein was like putting up a shield to protect against an arrow only after the arrow had already struck its target. Indeed, the F-protein evolves only after the attachment/breach. The preattachment/breach version (prefusion protein) sits on the virus surface and is like a loaded bow with an arrow in place. The prefusion protein changes configuration when RSV contacts host cells to uncoil and release the “arrow,” creating the entry point for RSV nucleic acids.
Nirsevimab was created to glom onto the prefusion protein and prevent it from uncoiling/releasing its “arrow,” the critical event in RSV infecting a cell. So, it is not surprising that it works better than palivizumab, which targets the fusion protein.
The prefusion protein is also the target of newer vaccine candidates, including one that showed 87% efficacy against RSV challenge in adults (think of mother getting this vaccine and endowing newborns with antiprefusion antibodies; N Engl J Med. 2022;386:2377-86).
The time when RSV is not an annual scourge is closer than ever.
Christopher J. Harrison, MD, is professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. He has no financial conflicts of interest.
Imagine a time when RSV doesn’t rage through the community each year. That time would result from decades of research that uncovered RSV’s secrets and explained the only partly successful initial vaccines and prophylactic interventions (for example, palivizumab).
Key discovery: The original RSV antigen target, RSV’s fusion (F) protein, is suboptimal despite having been associated with RSV attachment to and breaching of host cell membranes.
Some amazing work showed that having antibodies block the F-protein was like putting up a shield to protect against an arrow only after the arrow had already struck its target. Indeed, the F-protein evolves only after the attachment/breach. The preattachment/breach version (prefusion protein) sits on the virus surface and is like a loaded bow with an arrow in place. The prefusion protein changes configuration when RSV contacts host cells to uncoil and release the “arrow,” creating the entry point for RSV nucleic acids.
Nirsevimab was created to glom onto the prefusion protein and prevent it from uncoiling/releasing its “arrow,” the critical event in RSV infecting a cell. So, it is not surprising that it works better than palivizumab, which targets the fusion protein.
The prefusion protein is also the target of newer vaccine candidates, including one that showed 87% efficacy against RSV challenge in adults (think of mother getting this vaccine and endowing newborns with antiprefusion antibodies; N Engl J Med. 2022;386:2377-86).
The time when RSV is not an annual scourge is closer than ever.
Christopher J. Harrison, MD, is professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. He has no financial conflicts of interest.
Treatment duration for acute otitis media – so many choices
Twenty years ago, the dilemma in treating acute otitis media (AOM) was which among 10-plus antibiotics to prescribe. A recent column discussed the evolving pathogen distribution in AOM and its effects on antibiotic choices.1 But here we consider treatment duration. Until the past decade, AOM treatment (except azithromycin) involved 10-day courses. But lately, 10-day antibiotic regimens for uncomplicated infections are disappearing. Shorter-course recommendations are the new norm because of the evolving clinical data showing that an appropriately chosen antibiotic (in partnership with host defenses and source control) resolves infection faster than was previously thought. Shorter courses make sense because of fewer adverse effects, less distortion of normal flora, and less likely induction of pathogen resistance. Table 4.12 in the newest 2021-2024 SOID Redbook lists three antibiotic durations for AOM, and actually there are more than that.
Why so many duration options? Clinical data show that not all AOM is alike and short courses work for subsets of AOM because, besides antibiotics, key elements in AOM resolution are host anatomy and immunity. Bacterial AOM results from a combination of refluxed pathogens in the middle ear being trapped when the eustachian tube malfunctions (infection occurs when middle ear plumbing gets stopped up). If the eustachian tube spontaneously drains and the host immune response slows/stops pathogen growth, no antibiotics are needed. Indeed, a sizable proportion of mild/moderate AOM episodes spontaneously resolve, particularly in children over 2 years old. So a high likelihood of spontaneous remission allows an initial 0-days duration option (watchful waiting) or delayed antibiotics (rescue prescriptions) for older children.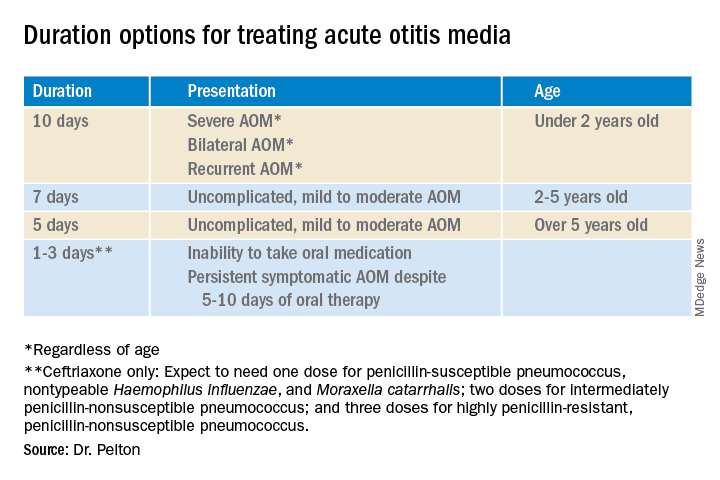
That said, when one chooses to initially prescribe antibiotics for AOM, different durations are recommended. Table 1 has my suggestions.
Data that gave me better microbiological understanding of why oral AOM trials less than 10 days were successful involved purulent AOM drainage from children who had pressure-equalizing (PE) tubes.2 The authors randomized children to either standard-dose amoxicillin-clavulanate or placebo. Of note, 95% of pathogens were susceptible to the antibiotic; 5% were pneumococcus intermediately resistant to penicillin. The authors sampled ear drainage daily for 7 days. Figure 1 shows that cultures remained positive in only around 5% of children by day 3-5 of antibiotics, but viable bacteria persisted through 7 days in over half of placebo recipients. Remember, both groups benefited from a form of source control (drainage of the middle ear via PE tubes). So, if antibiotics can do the job in 3-5 days, why continue antibiotics beyond 5 days?
Anatomy and severity. In children over 5 years old (reasonably mature eustachian tube anatomy) with nonrecurrent (no AOM in past month), nonsevere (no otalgia or high fever) AOM, 5 days is enough. But 2- to 5-year-olds (less mature anatomy) need 7 days and those <2 years old (least mature plumbing) need 10 days. Likewise, severe AOM usually warrants 10 days. Some experts recommend 10 days for bilateral AOM as well.
These age/severity differences make sense because failures are more frequent with:
1. Younger age.3 While not proven, my hypothesis is that “natural” source control (spontaneous internal draining the middle ear into the nasopharynx [NP]) is less frequent in younger children because they have less mature eustachian tube systems. Further, reflux of persisting NP organisms could restart a new AOM episode even if the original pathogen was eliminated by a short 5-day course.
2. Severe AOM. A rationale for longer courses in severe AOM (ear pain, high fever) is that high middle-ear pressures (indicated by degree of tympanic membrane bulging and ear pain) could impede antibiotic penetration, or that high initial bacterial loads (perhaps indicated by systemic fever) require more antibiotic. And finally, return to baseline eustachian tube function may take longer if severe AOM caused enhanced inflammation.
3. Recurrent AOM. (AOM within 1 prior month) – With recurrent AOM, the second “hit” to the eustachian tube may lead to more dysfunction, so a longer antibiotic course may be required to allow more complete source control and more time for more complete functional recovery after a repeated inflammatory injury.
4. Bilateral AOM. Two independent but infected sites mean twice the chance for failure. So, a longer course could allow more time for both sites to undergo “natural” source control.4
More bacteria – more antibiotic? So, is more antibiotic really needed for a higher bacterial load? In vitro this is known as the “inoculum effect,” particularly for beta-lactam drugs, for example, amoxicillin and cephalosporins. Laboratory susceptibility testing is performed with a specifically defined quantity of bacteria (105 bacteria/mL) and the minimum inhibitory concentration (MIC) is the lowest antibiotic concentration that stops bacterial growth. We know that drugs will likely fail if the MIC exceeds the achievable antibiotic concentration at the infection site. But is it as simple as just exceeding the MIC at the infection site? No, pharmacodynamics tell us that overall antibiotic exposure is also important. For example, to be successful, beta-lactam concentrations need to be above the MIC for 40%-50% of the day.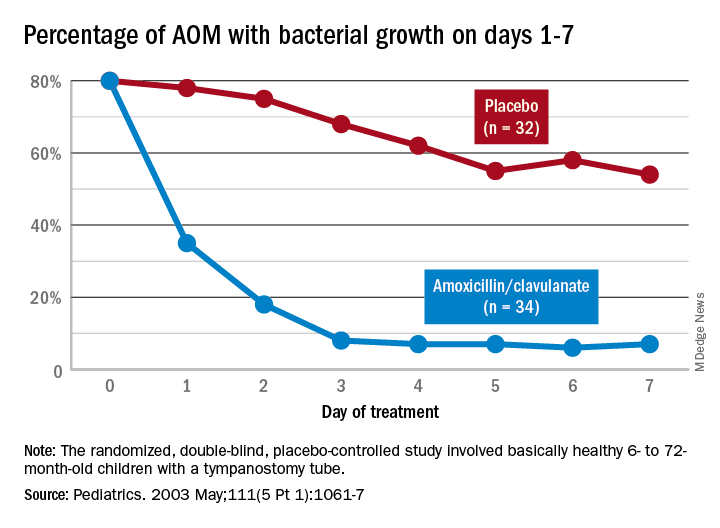
Higher MIC with higher bacterial load. Particularly for beta-lactams, testing with a quantity of bacteria >105/mL produces a higher MIC in vitro. This suggests that clinical failure could occur, even when our in vivo dosing leads to 40%-50% above the “standard” MIC that was obtained from testing the lab standard of 105/mL bacteria, when the infected site’s (middle ear) bacterial load is >105/mL (such higher bacterial loads occur in up to 30% of AOM).5 One way to negate inoculum effect is source control (drain the abscess or debridement), which reduces the bacterial load as well as allowing better antibiotic penetration– both favoring infection resolution. But with suboptimal source control, for example, the middle ear is not drained externally or internally, longer courses (more antibiotic exposure) could aid resolution. Whether the exposure can be administered as higher doses in fewer days or standard doses for more days is debatable but consider that a single parenteral dose of ceftriaxone successfully resolves AOM not attributable to penicillin-nonsusceptible pneumococcus.6Bottom line: Even though the number of potential antibiotics has contracted in the past 20 years, the need to individualize AOM treatment remains important and duration choices are more complex. Indeed, AOM comes in different flavors with patient age, clinical presentation, and episode frequency dictating the choice of duration.
Dr. Christopher J. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics in Kansas City, Mo. Email him at [email protected].
References
1. Pichichero ME. MDedge. 2022 Jan 11.
2. Ruohola A et al. Pediatrics. 2003;111(5):1061-7.
3. Hoberman A et al. N Engl J Med. 2016;375(25):2446-56.
4. Pichichero ME et al. Otolaryngol Head Neck Surg. 2001;124(4):381-7.
5. Harrison CJ et al. Pediatr Infect Dis. 1985;4(6):641-6.
6. Leibovitz E et al. Pediatr Infect Dis. 2000;19(11):1040-5.
Twenty years ago, the dilemma in treating acute otitis media (AOM) was which among 10-plus antibiotics to prescribe. A recent column discussed the evolving pathogen distribution in AOM and its effects on antibiotic choices.1 But here we consider treatment duration. Until the past decade, AOM treatment (except azithromycin) involved 10-day courses. But lately, 10-day antibiotic regimens for uncomplicated infections are disappearing. Shorter-course recommendations are the new norm because of the evolving clinical data showing that an appropriately chosen antibiotic (in partnership with host defenses and source control) resolves infection faster than was previously thought. Shorter courses make sense because of fewer adverse effects, less distortion of normal flora, and less likely induction of pathogen resistance. Table 4.12 in the newest 2021-2024 SOID Redbook lists three antibiotic durations for AOM, and actually there are more than that.
Why so many duration options? Clinical data show that not all AOM is alike and short courses work for subsets of AOM because, besides antibiotics, key elements in AOM resolution are host anatomy and immunity. Bacterial AOM results from a combination of refluxed pathogens in the middle ear being trapped when the eustachian tube malfunctions (infection occurs when middle ear plumbing gets stopped up). If the eustachian tube spontaneously drains and the host immune response slows/stops pathogen growth, no antibiotics are needed. Indeed, a sizable proportion of mild/moderate AOM episodes spontaneously resolve, particularly in children over 2 years old. So a high likelihood of spontaneous remission allows an initial 0-days duration option (watchful waiting) or delayed antibiotics (rescue prescriptions) for older children.
That said, when one chooses to initially prescribe antibiotics for AOM, different durations are recommended. Table 1 has my suggestions.
Data that gave me better microbiological understanding of why oral AOM trials less than 10 days were successful involved purulent AOM drainage from children who had pressure-equalizing (PE) tubes.2 The authors randomized children to either standard-dose amoxicillin-clavulanate or placebo. Of note, 95% of pathogens were susceptible to the antibiotic; 5% were pneumococcus intermediately resistant to penicillin. The authors sampled ear drainage daily for 7 days. Figure 1 shows that cultures remained positive in only around 5% of children by day 3-5 of antibiotics, but viable bacteria persisted through 7 days in over half of placebo recipients. Remember, both groups benefited from a form of source control (drainage of the middle ear via PE tubes). So, if antibiotics can do the job in 3-5 days, why continue antibiotics beyond 5 days?
Anatomy and severity. In children over 5 years old (reasonably mature eustachian tube anatomy) with nonrecurrent (no AOM in past month), nonsevere (no otalgia or high fever) AOM, 5 days is enough. But 2- to 5-year-olds (less mature anatomy) need 7 days and those <2 years old (least mature plumbing) need 10 days. Likewise, severe AOM usually warrants 10 days. Some experts recommend 10 days for bilateral AOM as well.
These age/severity differences make sense because failures are more frequent with:
1. Younger age.3 While not proven, my hypothesis is that “natural” source control (spontaneous internal draining the middle ear into the nasopharynx [NP]) is less frequent in younger children because they have less mature eustachian tube systems. Further, reflux of persisting NP organisms could restart a new AOM episode even if the original pathogen was eliminated by a short 5-day course.
2. Severe AOM. A rationale for longer courses in severe AOM (ear pain, high fever) is that high middle-ear pressures (indicated by degree of tympanic membrane bulging and ear pain) could impede antibiotic penetration, or that high initial bacterial loads (perhaps indicated by systemic fever) require more antibiotic. And finally, return to baseline eustachian tube function may take longer if severe AOM caused enhanced inflammation.
3. Recurrent AOM. (AOM within 1 prior month) – With recurrent AOM, the second “hit” to the eustachian tube may lead to more dysfunction, so a longer antibiotic course may be required to allow more complete source control and more time for more complete functional recovery after a repeated inflammatory injury.
4. Bilateral AOM. Two independent but infected sites mean twice the chance for failure. So, a longer course could allow more time for both sites to undergo “natural” source control.4
More bacteria – more antibiotic? So, is more antibiotic really needed for a higher bacterial load? In vitro this is known as the “inoculum effect,” particularly for beta-lactam drugs, for example, amoxicillin and cephalosporins. Laboratory susceptibility testing is performed with a specifically defined quantity of bacteria (105 bacteria/mL) and the minimum inhibitory concentration (MIC) is the lowest antibiotic concentration that stops bacterial growth. We know that drugs will likely fail if the MIC exceeds the achievable antibiotic concentration at the infection site. But is it as simple as just exceeding the MIC at the infection site? No, pharmacodynamics tell us that overall antibiotic exposure is also important. For example, to be successful, beta-lactam concentrations need to be above the MIC for 40%-50% of the day.
Higher MIC with higher bacterial load. Particularly for beta-lactams, testing with a quantity of bacteria >105/mL produces a higher MIC in vitro. This suggests that clinical failure could occur, even when our in vivo dosing leads to 40%-50% above the “standard” MIC that was obtained from testing the lab standard of 105/mL bacteria, when the infected site’s (middle ear) bacterial load is >105/mL (such higher bacterial loads occur in up to 30% of AOM).5 One way to negate inoculum effect is source control (drain the abscess or debridement), which reduces the bacterial load as well as allowing better antibiotic penetration– both favoring infection resolution. But with suboptimal source control, for example, the middle ear is not drained externally or internally, longer courses (more antibiotic exposure) could aid resolution. Whether the exposure can be administered as higher doses in fewer days or standard doses for more days is debatable but consider that a single parenteral dose of ceftriaxone successfully resolves AOM not attributable to penicillin-nonsusceptible pneumococcus.6Bottom line: Even though the number of potential antibiotics has contracted in the past 20 years, the need to individualize AOM treatment remains important and duration choices are more complex. Indeed, AOM comes in different flavors with patient age, clinical presentation, and episode frequency dictating the choice of duration.
Dr. Christopher J. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics in Kansas City, Mo. Email him at [email protected].
References
1. Pichichero ME. MDedge. 2022 Jan 11.
2. Ruohola A et al. Pediatrics. 2003;111(5):1061-7.
3. Hoberman A et al. N Engl J Med. 2016;375(25):2446-56.
4. Pichichero ME et al. Otolaryngol Head Neck Surg. 2001;124(4):381-7.
5. Harrison CJ et al. Pediatr Infect Dis. 1985;4(6):641-6.
6. Leibovitz E et al. Pediatr Infect Dis. 2000;19(11):1040-5.
Twenty years ago, the dilemma in treating acute otitis media (AOM) was which among 10-plus antibiotics to prescribe. A recent column discussed the evolving pathogen distribution in AOM and its effects on antibiotic choices.1 But here we consider treatment duration. Until the past decade, AOM treatment (except azithromycin) involved 10-day courses. But lately, 10-day antibiotic regimens for uncomplicated infections are disappearing. Shorter-course recommendations are the new norm because of the evolving clinical data showing that an appropriately chosen antibiotic (in partnership with host defenses and source control) resolves infection faster than was previously thought. Shorter courses make sense because of fewer adverse effects, less distortion of normal flora, and less likely induction of pathogen resistance. Table 4.12 in the newest 2021-2024 SOID Redbook lists three antibiotic durations for AOM, and actually there are more than that.
Why so many duration options? Clinical data show that not all AOM is alike and short courses work for subsets of AOM because, besides antibiotics, key elements in AOM resolution are host anatomy and immunity. Bacterial AOM results from a combination of refluxed pathogens in the middle ear being trapped when the eustachian tube malfunctions (infection occurs when middle ear plumbing gets stopped up). If the eustachian tube spontaneously drains and the host immune response slows/stops pathogen growth, no antibiotics are needed. Indeed, a sizable proportion of mild/moderate AOM episodes spontaneously resolve, particularly in children over 2 years old. So a high likelihood of spontaneous remission allows an initial 0-days duration option (watchful waiting) or delayed antibiotics (rescue prescriptions) for older children.
That said, when one chooses to initially prescribe antibiotics for AOM, different durations are recommended. Table 1 has my suggestions.
Data that gave me better microbiological understanding of why oral AOM trials less than 10 days were successful involved purulent AOM drainage from children who had pressure-equalizing (PE) tubes.2 The authors randomized children to either standard-dose amoxicillin-clavulanate or placebo. Of note, 95% of pathogens were susceptible to the antibiotic; 5% were pneumococcus intermediately resistant to penicillin. The authors sampled ear drainage daily for 7 days. Figure 1 shows that cultures remained positive in only around 5% of children by day 3-5 of antibiotics, but viable bacteria persisted through 7 days in over half of placebo recipients. Remember, both groups benefited from a form of source control (drainage of the middle ear via PE tubes). So, if antibiotics can do the job in 3-5 days, why continue antibiotics beyond 5 days?
Anatomy and severity. In children over 5 years old (reasonably mature eustachian tube anatomy) with nonrecurrent (no AOM in past month), nonsevere (no otalgia or high fever) AOM, 5 days is enough. But 2- to 5-year-olds (less mature anatomy) need 7 days and those <2 years old (least mature plumbing) need 10 days. Likewise, severe AOM usually warrants 10 days. Some experts recommend 10 days for bilateral AOM as well.
These age/severity differences make sense because failures are more frequent with:
1. Younger age.3 While not proven, my hypothesis is that “natural” source control (spontaneous internal draining the middle ear into the nasopharynx [NP]) is less frequent in younger children because they have less mature eustachian tube systems. Further, reflux of persisting NP organisms could restart a new AOM episode even if the original pathogen was eliminated by a short 5-day course.
2. Severe AOM. A rationale for longer courses in severe AOM (ear pain, high fever) is that high middle-ear pressures (indicated by degree of tympanic membrane bulging and ear pain) could impede antibiotic penetration, or that high initial bacterial loads (perhaps indicated by systemic fever) require more antibiotic. And finally, return to baseline eustachian tube function may take longer if severe AOM caused enhanced inflammation.
3. Recurrent AOM. (AOM within 1 prior month) – With recurrent AOM, the second “hit” to the eustachian tube may lead to more dysfunction, so a longer antibiotic course may be required to allow more complete source control and more time for more complete functional recovery after a repeated inflammatory injury.
4. Bilateral AOM. Two independent but infected sites mean twice the chance for failure. So, a longer course could allow more time for both sites to undergo “natural” source control.4
More bacteria – more antibiotic? So, is more antibiotic really needed for a higher bacterial load? In vitro this is known as the “inoculum effect,” particularly for beta-lactam drugs, for example, amoxicillin and cephalosporins. Laboratory susceptibility testing is performed with a specifically defined quantity of bacteria (105 bacteria/mL) and the minimum inhibitory concentration (MIC) is the lowest antibiotic concentration that stops bacterial growth. We know that drugs will likely fail if the MIC exceeds the achievable antibiotic concentration at the infection site. But is it as simple as just exceeding the MIC at the infection site? No, pharmacodynamics tell us that overall antibiotic exposure is also important. For example, to be successful, beta-lactam concentrations need to be above the MIC for 40%-50% of the day.
Higher MIC with higher bacterial load. Particularly for beta-lactams, testing with a quantity of bacteria >105/mL produces a higher MIC in vitro. This suggests that clinical failure could occur, even when our in vivo dosing leads to 40%-50% above the “standard” MIC that was obtained from testing the lab standard of 105/mL bacteria, when the infected site’s (middle ear) bacterial load is >105/mL (such higher bacterial loads occur in up to 30% of AOM).5 One way to negate inoculum effect is source control (drain the abscess or debridement), which reduces the bacterial load as well as allowing better antibiotic penetration– both favoring infection resolution. But with suboptimal source control, for example, the middle ear is not drained externally or internally, longer courses (more antibiotic exposure) could aid resolution. Whether the exposure can be administered as higher doses in fewer days or standard doses for more days is debatable but consider that a single parenteral dose of ceftriaxone successfully resolves AOM not attributable to penicillin-nonsusceptible pneumococcus.6Bottom line: Even though the number of potential antibiotics has contracted in the past 20 years, the need to individualize AOM treatment remains important and duration choices are more complex. Indeed, AOM comes in different flavors with patient age, clinical presentation, and episode frequency dictating the choice of duration.
Dr. Christopher J. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics in Kansas City, Mo. Email him at [email protected].
References
1. Pichichero ME. MDedge. 2022 Jan 11.
2. Ruohola A et al. Pediatrics. 2003;111(5):1061-7.
3. Hoberman A et al. N Engl J Med. 2016;375(25):2446-56.
4. Pichichero ME et al. Otolaryngol Head Neck Surg. 2001;124(4):381-7.
5. Harrison CJ et al. Pediatr Infect Dis. 1985;4(6):641-6.
6. Leibovitz E et al. Pediatr Infect Dis. 2000;19(11):1040-5.
Extensive limb swelling after vaccines – including SARS-CoV-2 vaccine
A 19-month-old boy comes to the office with a large firm erythematous swelling of his anterior left thigh that reaches from just below the inguinal crease to the patella. He got his routine immunizations 2 days prior to this visit including the fourth DTaP dose in his left thigh. Clinicians who care for children and who give routine immunizations occasionally see such an adverse effect following immunization (AEFI). These large local reactions have been described for many decades and occur after many vaccines.
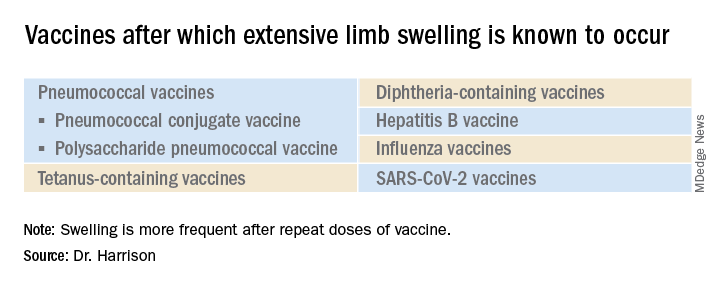
What is extensive limb swelling (ELS)? ELS is defined as erythema/swelling crossing a joint or extending mostly joint to joint. It is a subset of large local AEFIs. ELS is generally firm and often erythematous with varying degrees of pain. ELS is now most frequent after pneumococcal conjugate vaccines (PCV) and DTaP, with a 1%-4% rate after DTaP boosters.1-3 ELS and other large local swelling reactions occur at nearly any age.1 And yet there is still much that is not known about their true pathogenesis. Likewise, there are no accurate predictors of which vaccinees will develop large inflammatory processes at or near the site of immunization.
ELS after standard vaccines
The largest report to date on AEFI of all ages, including ELS, covered 1990-2003.1 Two upfront caveats are: This study evaluated ELS before PCVs were available, and in adults, repeat 23-valent pneumococcal polysaccharide vaccine was the most common cause of ELS in this study, comprising 45% of all adult ELS.
Considering all ages, ELS onset was nearly always greater than 1 hour and was less than 24 hours post vaccine in almost 75% of patients. However, for those aged under 2 years, onset in less than 24 hours was even more frequent (84%). Interestingly, concomitant fever occurred in less than 25% regardless of age. In adults, ELS after tetanus- and diphtheria-containing vaccines occurred mostly in women (75%); whereas for ELS under 8 years of age, males predominated (about 60%). Of note, tetanus- and diphtheria-containing vaccines were the most frequent ELS-inducing vaccines in children, that is, 75% aged under 8 years and 55% for those aged 8-17 years. Focusing on pediatric ELS after DTaP by dose, 33% were after the fourth, 31% after the fifth, 12% after the second, 10% after the first, and 3% after the third dose. In the case above, ELS was after the fourth dose.
Clinicians caring for children know how to manage ELS after DTaP or PCVs. They understand that ELS looks scary and is uncomfortable but is not dangerous and requires no specific treatment. Supportive management, that is, pain reliever, cool compresses, and TLC, are warranted. ELS is not a contraindication to subsequent immunization with the same vaccine. That said, large local reactions or ELS do occur with subsequent doses of that same vaccine at varying rates up to 66% of the time. Management is the same with repeat episodes, and no sequelae are expected. Supportive management only is standard unless one suspects a very rare Arthus reaction. If central necrosis occurs or swelling evolution/resolution is not per expectations, referral to a vaccine expert can sort out if it is an Arthus reaction, in which case, subsequent use of the same vaccine in not recommended.
ELS and SARS-CoV-2 vaccines
With SARS-CoV-2 vaccines now authorized for adolescents and expected in a few months for younger children, large local AEFI reactions related to pediatric SARS-CoV-2 vaccines are expected, given that “COVID arm” is now well described in adults.4 Overall, ELS/large local reactions have been reported more frequently with the Moderna than Pfizer mRNA vaccine.4 In the almost 42% of adults having ELS post first dose, repeat ELS post second dose often appears sooner but also resolves more quickly, with no known sequelae.5
Some biopsies have shown delayed-type hypersensitivity reactions (DTH) (superficial perivascular and perifollicular lymphocytic infiltrates with rare eosinophils and scattered mast cells),6,7 while others show no DTH but these patients have findings of immediate hypersensitivity findings and negative skin testing to the vaccine.8 With regard to sex, Dutch ELS data in White adults reveal 90% occur in females – higher than the 75% female rate after standard vaccines.7 Onset of ELS data show that Pfizer mRNA vaccinees had onset on average at 38 hours (range, <1 hr to 12 days). Boston data mostly in White adults reveal later onset (median, 6 days; range, 2-12 days).4 In contrast, adults of color appear to have later onset (mean, 8 days; range, 4-14 days).9
In addition to the local swelling, patients had concurrent injection-site AEFIs of pain (65%), warmth (63%), and pruritus (26%), plus myalgia (51%), headache (48%), malaise (45%), fatigue (43%), chills (33%), arthralgia (30%), and fever (28%).7
What should we tell families about pediatric ELS before we give SARS-CoV-2 vaccines to children? Clinical pediatric SARS-CoV-2 vaccine trials are smaller “immunologic bridging” studies, not requiring proof of efficacy. So, the precise incidence of pediatric ELS (adult rate is estimated under 1/100,000) may not be known until months after general use. Nevertheless, part of our counseling of families will need to include ELS/large local reactions. Unless new data show otherwise, the spiel that clinicians have developed to counsel about the rare chance of ELS after routine vaccines should also be useful to inform families of the rare chance of ELS post SARS-CoV-2 vaccine.
The bottom line is that the management of pediatric ELS after SARS-CoV-2 vaccines should be the same as after standard vaccines. And remember, whether the reactions are DTH or not, neither immediate local injection-site reactions nor DTH reactions are contraindications to subsequent vaccination unless anaphylaxis or Arthus reaction is suspected.10,11
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Woo EJ and the Vaccine Adverse Event Reporting System Working Group. Clin Infect Dis 2003;37:351-8.
2. Rennels MB et al. Pediatrics 2000;105:e12.
3. Huber BM, Goetschel P. J Pediatr. 2011;158:1033.
4. Blumenthal KG et al. N Engl J Med. 2021;384:1273-7.
5. McMahon DE et al. J Amer Acad Dermatol. 2021;85(1):46-55. 6. Johnston MS et al. JAMA Dermatol. 2021;157(6):716-20 .
7. ELS associated with the administration of Comirnaty®. WHO database Vigilyze (cited 2021 Feb 22). Available from https://vigilyze.who-umc.org/.
8. Baeck M et al. N Engl J Med. 2021 Jun. doi: 10.1056/NEJMc2104751.
9. Samarakoon U et al. N Eng J Med. 2021 Jun 9. doi: 10.1056/NEJMc2108620.
10. Kelso JM et al. J Allergy Clin Immunol. 2012;130:25-43.
11. Zafack JG et al. Pediatrics. 2017;140(3):e20163707.
A 19-month-old boy comes to the office with a large firm erythematous swelling of his anterior left thigh that reaches from just below the inguinal crease to the patella. He got his routine immunizations 2 days prior to this visit including the fourth DTaP dose in his left thigh. Clinicians who care for children and who give routine immunizations occasionally see such an adverse effect following immunization (AEFI). These large local reactions have been described for many decades and occur after many vaccines.

What is extensive limb swelling (ELS)? ELS is defined as erythema/swelling crossing a joint or extending mostly joint to joint. It is a subset of large local AEFIs. ELS is generally firm and often erythematous with varying degrees of pain. ELS is now most frequent after pneumococcal conjugate vaccines (PCV) and DTaP, with a 1%-4% rate after DTaP boosters.1-3 ELS and other large local swelling reactions occur at nearly any age.1 And yet there is still much that is not known about their true pathogenesis. Likewise, there are no accurate predictors of which vaccinees will develop large inflammatory processes at or near the site of immunization.
ELS after standard vaccines
The largest report to date on AEFI of all ages, including ELS, covered 1990-2003.1 Two upfront caveats are: This study evaluated ELS before PCVs were available, and in adults, repeat 23-valent pneumococcal polysaccharide vaccine was the most common cause of ELS in this study, comprising 45% of all adult ELS.
Considering all ages, ELS onset was nearly always greater than 1 hour and was less than 24 hours post vaccine in almost 75% of patients. However, for those aged under 2 years, onset in less than 24 hours was even more frequent (84%). Interestingly, concomitant fever occurred in less than 25% regardless of age. In adults, ELS after tetanus- and diphtheria-containing vaccines occurred mostly in women (75%); whereas for ELS under 8 years of age, males predominated (about 60%). Of note, tetanus- and diphtheria-containing vaccines were the most frequent ELS-inducing vaccines in children, that is, 75% aged under 8 years and 55% for those aged 8-17 years. Focusing on pediatric ELS after DTaP by dose, 33% were after the fourth, 31% after the fifth, 12% after the second, 10% after the first, and 3% after the third dose. In the case above, ELS was after the fourth dose.
Clinicians caring for children know how to manage ELS after DTaP or PCVs. They understand that ELS looks scary and is uncomfortable but is not dangerous and requires no specific treatment. Supportive management, that is, pain reliever, cool compresses, and TLC, are warranted. ELS is not a contraindication to subsequent immunization with the same vaccine. That said, large local reactions or ELS do occur with subsequent doses of that same vaccine at varying rates up to 66% of the time. Management is the same with repeat episodes, and no sequelae are expected. Supportive management only is standard unless one suspects a very rare Arthus reaction. If central necrosis occurs or swelling evolution/resolution is not per expectations, referral to a vaccine expert can sort out if it is an Arthus reaction, in which case, subsequent use of the same vaccine in not recommended.
ELS and SARS-CoV-2 vaccines
With SARS-CoV-2 vaccines now authorized for adolescents and expected in a few months for younger children, large local AEFI reactions related to pediatric SARS-CoV-2 vaccines are expected, given that “COVID arm” is now well described in adults.4 Overall, ELS/large local reactions have been reported more frequently with the Moderna than Pfizer mRNA vaccine.4 In the almost 42% of adults having ELS post first dose, repeat ELS post second dose often appears sooner but also resolves more quickly, with no known sequelae.5
Some biopsies have shown delayed-type hypersensitivity reactions (DTH) (superficial perivascular and perifollicular lymphocytic infiltrates with rare eosinophils and scattered mast cells),6,7 while others show no DTH but these patients have findings of immediate hypersensitivity findings and negative skin testing to the vaccine.8 With regard to sex, Dutch ELS data in White adults reveal 90% occur in females – higher than the 75% female rate after standard vaccines.7 Onset of ELS data show that Pfizer mRNA vaccinees had onset on average at 38 hours (range, <1 hr to 12 days). Boston data mostly in White adults reveal later onset (median, 6 days; range, 2-12 days).4 In contrast, adults of color appear to have later onset (mean, 8 days; range, 4-14 days).9
In addition to the local swelling, patients had concurrent injection-site AEFIs of pain (65%), warmth (63%), and pruritus (26%), plus myalgia (51%), headache (48%), malaise (45%), fatigue (43%), chills (33%), arthralgia (30%), and fever (28%).7
What should we tell families about pediatric ELS before we give SARS-CoV-2 vaccines to children? Clinical pediatric SARS-CoV-2 vaccine trials are smaller “immunologic bridging” studies, not requiring proof of efficacy. So, the precise incidence of pediatric ELS (adult rate is estimated under 1/100,000) may not be known until months after general use. Nevertheless, part of our counseling of families will need to include ELS/large local reactions. Unless new data show otherwise, the spiel that clinicians have developed to counsel about the rare chance of ELS after routine vaccines should also be useful to inform families of the rare chance of ELS post SARS-CoV-2 vaccine.
The bottom line is that the management of pediatric ELS after SARS-CoV-2 vaccines should be the same as after standard vaccines. And remember, whether the reactions are DTH or not, neither immediate local injection-site reactions nor DTH reactions are contraindications to subsequent vaccination unless anaphylaxis or Arthus reaction is suspected.10,11
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Woo EJ and the Vaccine Adverse Event Reporting System Working Group. Clin Infect Dis 2003;37:351-8.
2. Rennels MB et al. Pediatrics 2000;105:e12.
3. Huber BM, Goetschel P. J Pediatr. 2011;158:1033.
4. Blumenthal KG et al. N Engl J Med. 2021;384:1273-7.
5. McMahon DE et al. J Amer Acad Dermatol. 2021;85(1):46-55. 6. Johnston MS et al. JAMA Dermatol. 2021;157(6):716-20 .
7. ELS associated with the administration of Comirnaty®. WHO database Vigilyze (cited 2021 Feb 22). Available from https://vigilyze.who-umc.org/.
8. Baeck M et al. N Engl J Med. 2021 Jun. doi: 10.1056/NEJMc2104751.
9. Samarakoon U et al. N Eng J Med. 2021 Jun 9. doi: 10.1056/NEJMc2108620.
10. Kelso JM et al. J Allergy Clin Immunol. 2012;130:25-43.
11. Zafack JG et al. Pediatrics. 2017;140(3):e20163707.
A 19-month-old boy comes to the office with a large firm erythematous swelling of his anterior left thigh that reaches from just below the inguinal crease to the patella. He got his routine immunizations 2 days prior to this visit including the fourth DTaP dose in his left thigh. Clinicians who care for children and who give routine immunizations occasionally see such an adverse effect following immunization (AEFI). These large local reactions have been described for many decades and occur after many vaccines.

What is extensive limb swelling (ELS)? ELS is defined as erythema/swelling crossing a joint or extending mostly joint to joint. It is a subset of large local AEFIs. ELS is generally firm and often erythematous with varying degrees of pain. ELS is now most frequent after pneumococcal conjugate vaccines (PCV) and DTaP, with a 1%-4% rate after DTaP boosters.1-3 ELS and other large local swelling reactions occur at nearly any age.1 And yet there is still much that is not known about their true pathogenesis. Likewise, there are no accurate predictors of which vaccinees will develop large inflammatory processes at or near the site of immunization.
ELS after standard vaccines
The largest report to date on AEFI of all ages, including ELS, covered 1990-2003.1 Two upfront caveats are: This study evaluated ELS before PCVs were available, and in adults, repeat 23-valent pneumococcal polysaccharide vaccine was the most common cause of ELS in this study, comprising 45% of all adult ELS.
Considering all ages, ELS onset was nearly always greater than 1 hour and was less than 24 hours post vaccine in almost 75% of patients. However, for those aged under 2 years, onset in less than 24 hours was even more frequent (84%). Interestingly, concomitant fever occurred in less than 25% regardless of age. In adults, ELS after tetanus- and diphtheria-containing vaccines occurred mostly in women (75%); whereas for ELS under 8 years of age, males predominated (about 60%). Of note, tetanus- and diphtheria-containing vaccines were the most frequent ELS-inducing vaccines in children, that is, 75% aged under 8 years and 55% for those aged 8-17 years. Focusing on pediatric ELS after DTaP by dose, 33% were after the fourth, 31% after the fifth, 12% after the second, 10% after the first, and 3% after the third dose. In the case above, ELS was after the fourth dose.
Clinicians caring for children know how to manage ELS after DTaP or PCVs. They understand that ELS looks scary and is uncomfortable but is not dangerous and requires no specific treatment. Supportive management, that is, pain reliever, cool compresses, and TLC, are warranted. ELS is not a contraindication to subsequent immunization with the same vaccine. That said, large local reactions or ELS do occur with subsequent doses of that same vaccine at varying rates up to 66% of the time. Management is the same with repeat episodes, and no sequelae are expected. Supportive management only is standard unless one suspects a very rare Arthus reaction. If central necrosis occurs or swelling evolution/resolution is not per expectations, referral to a vaccine expert can sort out if it is an Arthus reaction, in which case, subsequent use of the same vaccine in not recommended.
ELS and SARS-CoV-2 vaccines
With SARS-CoV-2 vaccines now authorized for adolescents and expected in a few months for younger children, large local AEFI reactions related to pediatric SARS-CoV-2 vaccines are expected, given that “COVID arm” is now well described in adults.4 Overall, ELS/large local reactions have been reported more frequently with the Moderna than Pfizer mRNA vaccine.4 In the almost 42% of adults having ELS post first dose, repeat ELS post second dose often appears sooner but also resolves more quickly, with no known sequelae.5
Some biopsies have shown delayed-type hypersensitivity reactions (DTH) (superficial perivascular and perifollicular lymphocytic infiltrates with rare eosinophils and scattered mast cells),6,7 while others show no DTH but these patients have findings of immediate hypersensitivity findings and negative skin testing to the vaccine.8 With regard to sex, Dutch ELS data in White adults reveal 90% occur in females – higher than the 75% female rate after standard vaccines.7 Onset of ELS data show that Pfizer mRNA vaccinees had onset on average at 38 hours (range, <1 hr to 12 days). Boston data mostly in White adults reveal later onset (median, 6 days; range, 2-12 days).4 In contrast, adults of color appear to have later onset (mean, 8 days; range, 4-14 days).9
In addition to the local swelling, patients had concurrent injection-site AEFIs of pain (65%), warmth (63%), and pruritus (26%), plus myalgia (51%), headache (48%), malaise (45%), fatigue (43%), chills (33%), arthralgia (30%), and fever (28%).7
What should we tell families about pediatric ELS before we give SARS-CoV-2 vaccines to children? Clinical pediatric SARS-CoV-2 vaccine trials are smaller “immunologic bridging” studies, not requiring proof of efficacy. So, the precise incidence of pediatric ELS (adult rate is estimated under 1/100,000) may not be known until months after general use. Nevertheless, part of our counseling of families will need to include ELS/large local reactions. Unless new data show otherwise, the spiel that clinicians have developed to counsel about the rare chance of ELS after routine vaccines should also be useful to inform families of the rare chance of ELS post SARS-CoV-2 vaccine.
The bottom line is that the management of pediatric ELS after SARS-CoV-2 vaccines should be the same as after standard vaccines. And remember, whether the reactions are DTH or not, neither immediate local injection-site reactions nor DTH reactions are contraindications to subsequent vaccination unless anaphylaxis or Arthus reaction is suspected.10,11
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Woo EJ and the Vaccine Adverse Event Reporting System Working Group. Clin Infect Dis 2003;37:351-8.
2. Rennels MB et al. Pediatrics 2000;105:e12.
3. Huber BM, Goetschel P. J Pediatr. 2011;158:1033.
4. Blumenthal KG et al. N Engl J Med. 2021;384:1273-7.
5. McMahon DE et al. J Amer Acad Dermatol. 2021;85(1):46-55. 6. Johnston MS et al. JAMA Dermatol. 2021;157(6):716-20 .
7. ELS associated with the administration of Comirnaty®. WHO database Vigilyze (cited 2021 Feb 22). Available from https://vigilyze.who-umc.org/.
8. Baeck M et al. N Engl J Med. 2021 Jun. doi: 10.1056/NEJMc2104751.
9. Samarakoon U et al. N Eng J Med. 2021 Jun 9. doi: 10.1056/NEJMc2108620.
10. Kelso JM et al. J Allergy Clin Immunol. 2012;130:25-43.
11. Zafack JG et al. Pediatrics. 2017;140(3):e20163707.
The lost year – even for common respiratory viruses
In this column in September 2020, you read how common respiratory viruses’ seasons are usually so predictable, each virus arising, peaking, and then dying out in a predictable virus parade (Figure 1).1 Well, the predictable virus seasonal pattern was lost in 2020. Since March of 2020, it is striking how little activity was detected for the usual seasonal viruses in Kansas City after mid-March 2020 (Figure 2).2 So, my concern in September 2020 for possible rampant coinfections of common viruses with or in tandem with SARS-CoV-2 did not pan out. That said, the seasons for non–SARS-CoV-2 viruses did change; I just didn’t expect they would nearly disappear.
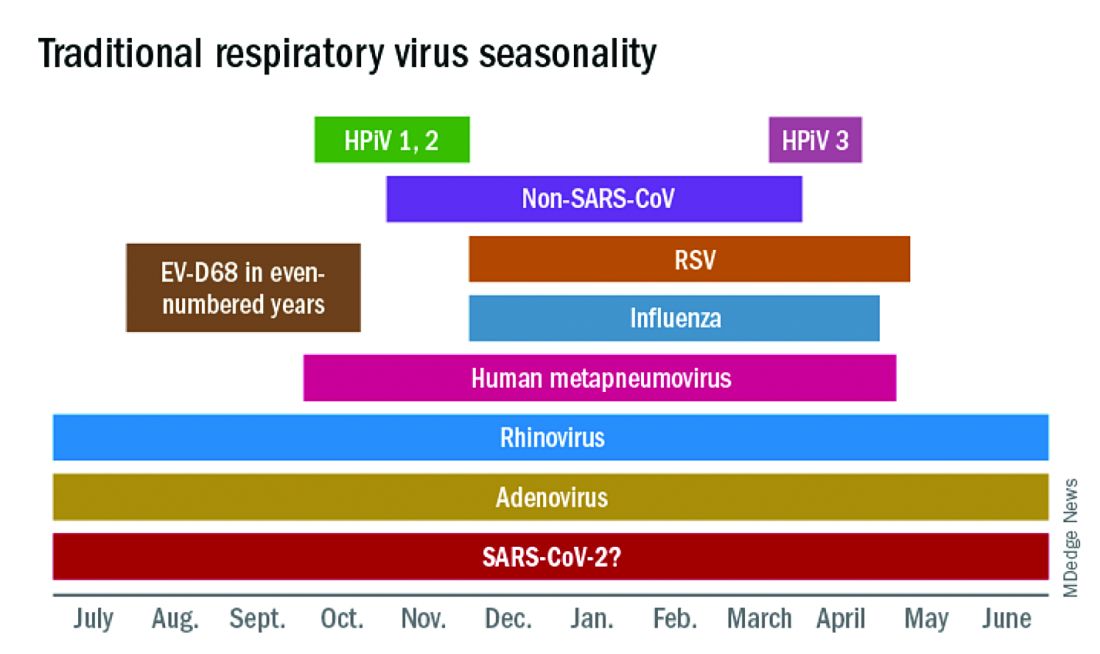
The 2020 winter-spring. In the first quarter (the last part of the overall 2019-2020 respiratory viral season), viral detections were chugging along as usual up to mid-March (Figure 2); influenza, respiratory syncytial virus (RSV), and rhinovirus were the big players.
Influenza. In most years, influenza type B leads off and is quickly replaced by type A only to see B reemerge to end influenza season in March-April. In early 2020, both influenza type A and influenza type B cocirculated nearly equally, but both dropped like a rock in mid-March (Figure 2).2 Neither type has been seen since with the exception of sporadic detections – perhaps being false positives.
RSV. In the usual year in temperate mid-latitudes of the northern hemisphere, RSV season usually starts in early December, peaks in January-March, and declines gradually until the end of RSV season in April (Figure 1). In southern latitudes, RSV is less seasonal, being present most of the year, but peaking in “winter” months.3 But in 2020, RSV also disappeared in mid-March and has yet to reappear.
Other viruses. Small bumps in detection of parainfluenza of varying types usually frame influenza season, one B bump in early autumn and another in April-May. In most years, human metapneumovirus is detected on and off, with worse years at 2- to 3-year intervals. Adenovirus occurs year-round with bumps as children get back to school in autumn. Yet in 2020, almost no parainfluenza, adenovirus, common coronaviruses, or human metapneumovirus were detected in either spring or autumn. This was supposed to be a banner summer-autumn for EV-D68 – but almost none was detected. Interestingly, the cockroach of viruses, rhinovirus, has its usual year (Figure 2).
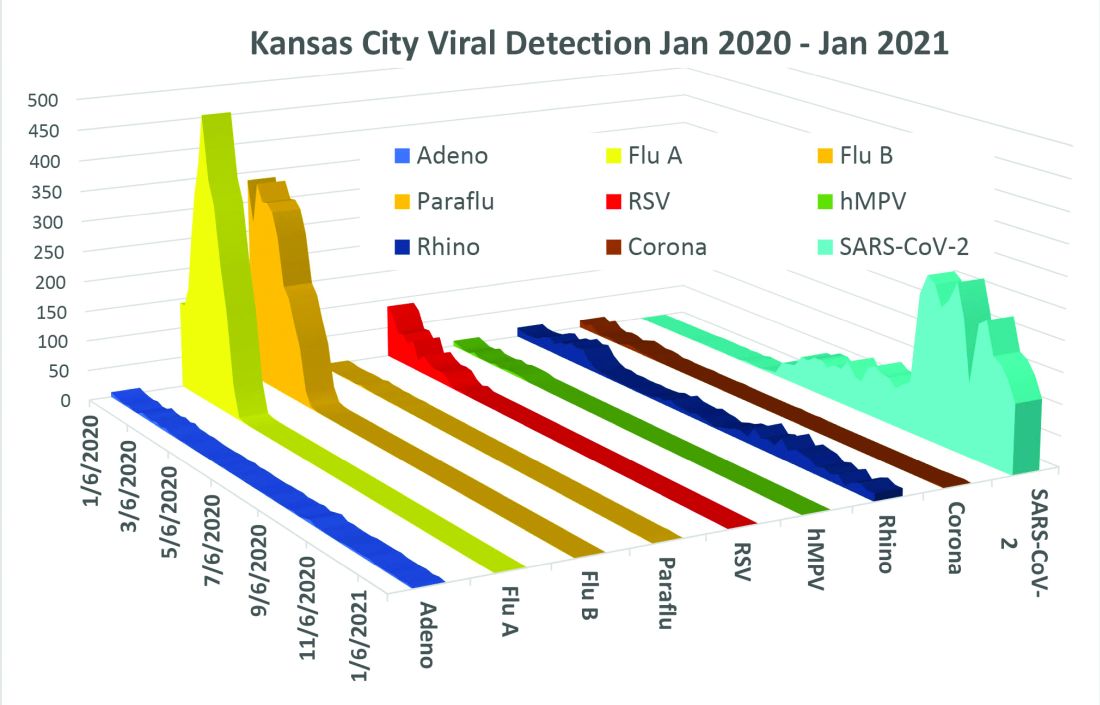
What happened? Intense social mitigation interventions, including social distancing and closing daycares and schools, were likely major factors.4 For influenza, vaccine may have helped but uptake was not remarkably better than most prior years. There may have been “viral competition,”where a new or highly transmissible virus outcompetes less-transmissible viruses with lower affinity for respiratory receptors.5,6 Note that SARS-CoV-2 has very high affinity for the ACE2 receptor and has been highly prevalent. So, SARS-CoV-2 could fit the theoretical mold for a virus that outcompetes others.
Does it matter for the future? Blunted 2019-2020 and nearly absent 2020-2021 respiratory virus season may have set the stage for intense 2021-2022 rebounds for the non–SARS-CoV-2 viruses. We now have two whole and one partial birth cohort with no experience with seasonal respiratory viruses, including EV-D68 (and nonrespiratory viruses too – like norovirus, parechovirus, and other enteroviruses). Most viruses have particularly bad seasons every 2-3 years, thought to be caused by increasing accumulation of susceptible individuals in consecutive birth cohorts until a critical mass of susceptible individuals is achieved. The excess in susceptible individuals means that each contagious case is likely to expose one or more susceptible individuals, enhancing transmission and infection numbers in an ever-extending ripple effect. We have never had this many children aged under 3 years with no immunity to influenza, RSV, etc. So unless mother nature is kind (when has that happened lately?), expect rebound years for seasonal viruses as children return to daycare/schools and as social mitigation becomes less necessary in the waning pandemic.
Options? If you ramped up telehealth visits for the pandemic, that may be a saving grace, i.e., more efficiency so more “visits” can be completed per day, and less potential contact in reception rooms between well and ill children. And if there was ever a time to really intensify efforts to immunize all our pediatric patients, the next two seasons are just that. Adding a bit of a warning to families with young children also seems warranted. If they understand that, while 2021-2022 will be better for SARS-CoV-2, it is likely going to be worse for the other viruses.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Harrison CJ. 2020-2021 respiratory viral season: Onset, presentations, and testing likely to differ in pandemic, Pediatric News: September 17, 2020.
2. Olsen SJ et al. MMWR Morb Mortal Wkly Rep. 2020;69:1305-9.
3. Respiratory Syncytial Virus Surveillance. http://www.floridahealth.gov/diseases-and-conditions/respiratory-syncytial-virus/_documents/2021-w4-rsv-summary.pdf
4. Baker RE et al. PNAS. Dec 2020 117;(48):30547-53.
5. Sema Nickbakhsh et al. PNAS. Dec 2019 116;(52):27142-50.
6. Kirsten M et al. PNAS. Mar 2020 117;(13):6987.
In this column in September 2020, you read how common respiratory viruses’ seasons are usually so predictable, each virus arising, peaking, and then dying out in a predictable virus parade (Figure 1).1 Well, the predictable virus seasonal pattern was lost in 2020. Since March of 2020, it is striking how little activity was detected for the usual seasonal viruses in Kansas City after mid-March 2020 (Figure 2).2 So, my concern in September 2020 for possible rampant coinfections of common viruses with or in tandem with SARS-CoV-2 did not pan out. That said, the seasons for non–SARS-CoV-2 viruses did change; I just didn’t expect they would nearly disappear.

The 2020 winter-spring. In the first quarter (the last part of the overall 2019-2020 respiratory viral season), viral detections were chugging along as usual up to mid-March (Figure 2); influenza, respiratory syncytial virus (RSV), and rhinovirus were the big players.
Influenza. In most years, influenza type B leads off and is quickly replaced by type A only to see B reemerge to end influenza season in March-April. In early 2020, both influenza type A and influenza type B cocirculated nearly equally, but both dropped like a rock in mid-March (Figure 2).2 Neither type has been seen since with the exception of sporadic detections – perhaps being false positives.
RSV. In the usual year in temperate mid-latitudes of the northern hemisphere, RSV season usually starts in early December, peaks in January-March, and declines gradually until the end of RSV season in April (Figure 1). In southern latitudes, RSV is less seasonal, being present most of the year, but peaking in “winter” months.3 But in 2020, RSV also disappeared in mid-March and has yet to reappear.
Other viruses. Small bumps in detection of parainfluenza of varying types usually frame influenza season, one B bump in early autumn and another in April-May. In most years, human metapneumovirus is detected on and off, with worse years at 2- to 3-year intervals. Adenovirus occurs year-round with bumps as children get back to school in autumn. Yet in 2020, almost no parainfluenza, adenovirus, common coronaviruses, or human metapneumovirus were detected in either spring or autumn. This was supposed to be a banner summer-autumn for EV-D68 – but almost none was detected. Interestingly, the cockroach of viruses, rhinovirus, has its usual year (Figure 2).

What happened? Intense social mitigation interventions, including social distancing and closing daycares and schools, were likely major factors.4 For influenza, vaccine may have helped but uptake was not remarkably better than most prior years. There may have been “viral competition,”where a new or highly transmissible virus outcompetes less-transmissible viruses with lower affinity for respiratory receptors.5,6 Note that SARS-CoV-2 has very high affinity for the ACE2 receptor and has been highly prevalent. So, SARS-CoV-2 could fit the theoretical mold for a virus that outcompetes others.
Does it matter for the future? Blunted 2019-2020 and nearly absent 2020-2021 respiratory virus season may have set the stage for intense 2021-2022 rebounds for the non–SARS-CoV-2 viruses. We now have two whole and one partial birth cohort with no experience with seasonal respiratory viruses, including EV-D68 (and nonrespiratory viruses too – like norovirus, parechovirus, and other enteroviruses). Most viruses have particularly bad seasons every 2-3 years, thought to be caused by increasing accumulation of susceptible individuals in consecutive birth cohorts until a critical mass of susceptible individuals is achieved. The excess in susceptible individuals means that each contagious case is likely to expose one or more susceptible individuals, enhancing transmission and infection numbers in an ever-extending ripple effect. We have never had this many children aged under 3 years with no immunity to influenza, RSV, etc. So unless mother nature is kind (when has that happened lately?), expect rebound years for seasonal viruses as children return to daycare/schools and as social mitigation becomes less necessary in the waning pandemic.
Options? If you ramped up telehealth visits for the pandemic, that may be a saving grace, i.e., more efficiency so more “visits” can be completed per day, and less potential contact in reception rooms between well and ill children. And if there was ever a time to really intensify efforts to immunize all our pediatric patients, the next two seasons are just that. Adding a bit of a warning to families with young children also seems warranted. If they understand that, while 2021-2022 will be better for SARS-CoV-2, it is likely going to be worse for the other viruses.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Harrison CJ. 2020-2021 respiratory viral season: Onset, presentations, and testing likely to differ in pandemic, Pediatric News: September 17, 2020.
2. Olsen SJ et al. MMWR Morb Mortal Wkly Rep. 2020;69:1305-9.
3. Respiratory Syncytial Virus Surveillance. http://www.floridahealth.gov/diseases-and-conditions/respiratory-syncytial-virus/_documents/2021-w4-rsv-summary.pdf
4. Baker RE et al. PNAS. Dec 2020 117;(48):30547-53.
5. Sema Nickbakhsh et al. PNAS. Dec 2019 116;(52):27142-50.
6. Kirsten M et al. PNAS. Mar 2020 117;(13):6987.
In this column in September 2020, you read how common respiratory viruses’ seasons are usually so predictable, each virus arising, peaking, and then dying out in a predictable virus parade (Figure 1).1 Well, the predictable virus seasonal pattern was lost in 2020. Since March of 2020, it is striking how little activity was detected for the usual seasonal viruses in Kansas City after mid-March 2020 (Figure 2).2 So, my concern in September 2020 for possible rampant coinfections of common viruses with or in tandem with SARS-CoV-2 did not pan out. That said, the seasons for non–SARS-CoV-2 viruses did change; I just didn’t expect they would nearly disappear.

The 2020 winter-spring. In the first quarter (the last part of the overall 2019-2020 respiratory viral season), viral detections were chugging along as usual up to mid-March (Figure 2); influenza, respiratory syncytial virus (RSV), and rhinovirus were the big players.
Influenza. In most years, influenza type B leads off and is quickly replaced by type A only to see B reemerge to end influenza season in March-April. In early 2020, both influenza type A and influenza type B cocirculated nearly equally, but both dropped like a rock in mid-March (Figure 2).2 Neither type has been seen since with the exception of sporadic detections – perhaps being false positives.
RSV. In the usual year in temperate mid-latitudes of the northern hemisphere, RSV season usually starts in early December, peaks in January-March, and declines gradually until the end of RSV season in April (Figure 1). In southern latitudes, RSV is less seasonal, being present most of the year, but peaking in “winter” months.3 But in 2020, RSV also disappeared in mid-March and has yet to reappear.
Other viruses. Small bumps in detection of parainfluenza of varying types usually frame influenza season, one B bump in early autumn and another in April-May. In most years, human metapneumovirus is detected on and off, with worse years at 2- to 3-year intervals. Adenovirus occurs year-round with bumps as children get back to school in autumn. Yet in 2020, almost no parainfluenza, adenovirus, common coronaviruses, or human metapneumovirus were detected in either spring or autumn. This was supposed to be a banner summer-autumn for EV-D68 – but almost none was detected. Interestingly, the cockroach of viruses, rhinovirus, has its usual year (Figure 2).

What happened? Intense social mitigation interventions, including social distancing and closing daycares and schools, were likely major factors.4 For influenza, vaccine may have helped but uptake was not remarkably better than most prior years. There may have been “viral competition,”where a new or highly transmissible virus outcompetes less-transmissible viruses with lower affinity for respiratory receptors.5,6 Note that SARS-CoV-2 has very high affinity for the ACE2 receptor and has been highly prevalent. So, SARS-CoV-2 could fit the theoretical mold for a virus that outcompetes others.
Does it matter for the future? Blunted 2019-2020 and nearly absent 2020-2021 respiratory virus season may have set the stage for intense 2021-2022 rebounds for the non–SARS-CoV-2 viruses. We now have two whole and one partial birth cohort with no experience with seasonal respiratory viruses, including EV-D68 (and nonrespiratory viruses too – like norovirus, parechovirus, and other enteroviruses). Most viruses have particularly bad seasons every 2-3 years, thought to be caused by increasing accumulation of susceptible individuals in consecutive birth cohorts until a critical mass of susceptible individuals is achieved. The excess in susceptible individuals means that each contagious case is likely to expose one or more susceptible individuals, enhancing transmission and infection numbers in an ever-extending ripple effect. We have never had this many children aged under 3 years with no immunity to influenza, RSV, etc. So unless mother nature is kind (when has that happened lately?), expect rebound years for seasonal viruses as children return to daycare/schools and as social mitigation becomes less necessary in the waning pandemic.
Options? If you ramped up telehealth visits for the pandemic, that may be a saving grace, i.e., more efficiency so more “visits” can be completed per day, and less potential contact in reception rooms between well and ill children. And if there was ever a time to really intensify efforts to immunize all our pediatric patients, the next two seasons are just that. Adding a bit of a warning to families with young children also seems warranted. If they understand that, while 2021-2022 will be better for SARS-CoV-2, it is likely going to be worse for the other viruses.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Harrison CJ. 2020-2021 respiratory viral season: Onset, presentations, and testing likely to differ in pandemic, Pediatric News: September 17, 2020.
2. Olsen SJ et al. MMWR Morb Mortal Wkly Rep. 2020;69:1305-9.
3. Respiratory Syncytial Virus Surveillance. http://www.floridahealth.gov/diseases-and-conditions/respiratory-syncytial-virus/_documents/2021-w4-rsv-summary.pdf
4. Baker RE et al. PNAS. Dec 2020 117;(48):30547-53.
5. Sema Nickbakhsh et al. PNAS. Dec 2019 116;(52):27142-50.
6. Kirsten M et al. PNAS. Mar 2020 117;(13):6987.
2020-2021 respiratory viral season: Onset, presentations, and testing likely to differ in pandemic
Respiratory virus seasons usually follow a fairly well-known pattern. Enterovirus 68 (EV-D68) is a summer-to-early fall virus with biennial peak years. Rhinovirus (HRv) and adenovirus (Adv) occur nearly year-round but may have small upticks in the first month or so that children return to school. Early in the school year, upper respiratory infections from both HRv and Adv and viral sore throats from Adv are common, with conjunctivitis from Adv outbreaks in some years. October to November is human parainfluenza (HPiV) 1 and 2 season, often presenting as croup. Human metapneumovirus infections span October through April. In late November to December, influenza begins, usually with an A type, later transitioning to a B type in February through April. Also in December, respiratory syncytial virus (RSV) starts, characteristically with bronchiolitis presentations, peaking in February to March and tapering off in May. In late March to April, HPiV 3 also appears for 4-6 weeks.

Will 2020-2021 be different?
Summer was remarkably free of expected enterovirus activity, suggesting that the seasonal parade may differ this year. Remember that the 2019-2020 respiratory season suddenly and nearly completely stopped in March because of social distancing and lockdowns needed to address the SARS-CoV-2 pandemic.
The mild influenza season in the southern hemisphere suggests that our influenza season also could be mild. But perhaps not – most southern hemisphere countries that are surveyed for influenza activities had the most intense SARS-CoV-2 mitigations, making the observed mildness potentially related more to social mitigation than less virulent influenza strains. If so, southern hemisphere influenza data may not apply to the United States, where social distancing and masks are ignored or used inconsistently by almost half the population.
Further, the stop-and-go pattern of in-person school/college attendance adds to uncertainties for the usual orderly virus-specific seasonality. The result may be multiple stop-and-go “pop-up” or “mini” outbreaks for any given virus potentially reflected as exaggerated local or regional differences in circulation of various viruses. The erratic seasonality also would increase coinfections, which could present with more severe or different symptoms.
SARS-CoV-2’s potential interaction
Will the relatively mild presentations for most children with SARS-CoV-2 hold up in the setting of coinfections or sequential respiratory viral infections? Could SARS-CoV-2 cause worse/more prolonged symptoms or more sequelae if paired simultaneously or in tandem with a traditional respiratory virus? To date, data on the frequency and severity of SARS-CoV-2 coinfections are conflicting and sparse, but it appears that non-SARS-CoV-2 viruses can be involved in 15%-50% pediatric acute respiratory infections.1,2
However, it may not be important to know about coinfecting viruses other than influenza (can be treated) or SARS-CoV-2 (needs quarantine and contact tracing), unless symptoms are atypical or more severe than usual. For example, a young child with bronchiolitis is most likely infected with RSV, but HPiV, influenza, metapneumovirus, HRv, and even SARS-CoV-2 can cause bronchiolitis. Even so, testing outpatients for RSV or non-influenza is not routine or even clinically helpful. Supportive treatment and restriction from daycare attendance are sufficient management for outpatient ARIs whether presenting as bronchiolitis or not.
Considerations for SARS-CoV-2 testing: Outpatient bronchiolitis
If a child presents with classic bronchiolitis but has above moderate to severe symptoms, is SARS-CoV-2 a consideration? Perhaps, if SARS-CoV-2 acts similarly to non-SARS-CoV-2s.
A recent report from the 30th Multicenter Airway Research Collaboration (MARC-30) surveillance study (2007-2014) of children hospitalized with clinical bronchiolitis evaluated respiratory viruses, including RSV and the four common non-SARS coronaviruses using molecular testing.3 Among 1,880 subjects, a CoV (alpha CoV: NL63 or 229E, or beta CoV: KKU1 or OC43) was detected in 12%. Yet most had only RSV (n = 1,661); 32 had only CoV (n = 32). But note that 219 had both.
Bronchiolitis subjects with CoV were older – median 3.7 (1.4-5.8) vs. 2.8 (1.9-7.2) years – and more likely male than were RSV subjects (68% vs. 58%). OC43 was most frequent followed by equal numbers of HKU1 and NL63, while 229E was the least frequent. Medical utilization and severity did not differ among the CoVs, or between RSV+CoV vs. RSV alone, unless one considered CoV viral load as a variable. ICU use increased when the polymerase chain reaction cycle threshold result indicated a high CoV viral load.
These data suggest CoVs are not infrequent coinfectors with RSV in bronchiolitis – and that SARS-CoV-2 is the same. Therefore, a bronchiolitis presentation doesn’t necessarily take us off the hook for the need to consider SARS-CoV-2 testing, particularly in the somewhat older bronchiolitis patient with more than mild symptoms.
Considerations for SARS-CoV-2 testing: Outpatient influenza-like illness
In 2020-2021, the Centers for Disease Control and Prevention recommends considering empiric antiviral treatment for ILIs (fever plus either cough or sore throat) based upon our clinical judgement, even in non-high-risk children.4
While pediatric COVID-19 illnesses are predominantly asymptomatic or mild, a febrile ARI is also a SARS-CoV-2 compatible presentation. So, if all we use is our clinical judgment, how do we know if the febrile ARI is due to influenza or SARS-CoV-2 or both? At least one study used a highly sensitive and specific molecular influenza test to show that the accuracy of clinically diagnosing influenza in children is not much better than flipping a coin and would lead to potential antiviral overuse.5
So, it seems ideal to test for influenza when possible. Point-of-care (POC) tests are frequently used for outpatients. Eight POC Clinical Laboratory Improvement Amendments (CLIA)–waived kits, some also detecting RSV, are available but most have modest sensitivity (60%-80%) compared with lab-based molecular tests.6 That said, if supplies and kits for one of the POC tests are available to us during these SARS-CoV-2 stressed times (back orders seem more common this year), a positive influenza test in the first 48 hours of symptoms confirms the option to prescribe an antiviral. Yet how will we have confidence that the febrile ARI is not also partly due to SARS-CoV-2? Currently febrile ARIs usually are considered SARS-CoV-2 and the children are sent for SARS-CoV-2 testing. During influenza season, it seems we will need to continue to send febrile outpatients for SARS-CoV-2 testing, even if POC influenza positive, via whatever mechanisms are available as time goes on.
We expect more rapid pediatric testing modalities for SARS-CoV-2 (maybe even saliva tests) to become available over the next months. Indeed, rapid antigen tests and rapid molecular tests are being evaluated in adults and seem destined for CLIA waivers as POC tests, and even home testing kits. Pediatric approvals hopefully also will occur. So, the pathways for SARS-CoV-2 testing available now will likely change over this winter. But be aware that supplies/kits will be prioritized to locations within high need areas and bulk purchase contracts. So POC kits may remain scarce for practices, meaning a reference laboratory still could be the way to go for SARS-CoV-2 for at least the rest of 2020. Reference labs are becoming creative as well; one combined detection of influenza A, influenza B, RSV, and SARS-CoV-2 into one test, and hopes to get approval for swab collection that can be done by families at home and mailed in.
Summary
Expect variations on the traditional parade of seasonal respiratory viruses, with increased numbers of coinfections. Choosing the outpatient who needs influenza testing is the same as in past years, although we have CDC permissive recommendations to prescribe antivirals for any outpatient ILI within the first 48 hours of symptoms. Still, POC testing for influenza remains potentially valuable in the ILI patient. The choice of whether and how to test for SARS-CoV-2 given its potential to be a primary or coinfecting agent in presentations linked more closely to a traditional virus (e.g. RSV bronchiolitis) will be a test of our clinical judgement until more data and easier testing are available. Further complicating coinfection recognition is the fact that many sick visits occur by telehealth and much testing is done at drive-through SARS-CoV-2 testing facilities with no clinician exam. Unless we are liberal in SARS-CoV-2 testing, detecting SARS-CoV-2 coinfections is easier said than done given its usually mild presentation being overshadowed by any coinfecting virus.
But understanding who has SARS-CoV-2, even as a coinfection, still is essential in controlling the pandemic. We will need to be vigilant for evolving approaches to SARS-CoV-2 testing in the context of symptomatic ARI presentations, knowing this will likely remain a moving target for the foreseeable future.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital-Kansas City, Mo. Children’s Mercy Hospital receives grant funding to study two candidate RSV vaccines. The hospital also receives CDC funding under the New Vaccine Surveillance Network for multicenter surveillance of acute respiratory infections, including influenza, RSV, and parainfluenza virus. Email Dr. Harrison at [email protected].
References
1. Pediatrics. 2020;146(1):e20200961.
2. JAMA. 2020 May 26;323(20):2085-6.
3. Pediatrics. 2020. doi: 10.1542/peds.2020-1267.
4. www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm.
5. J. Pediatr. 2020. doi: 10.1016/j.jpeds.2020.08.007.
6. www.cdc.gov/flu/professionals/diagnosis/table-nucleic-acid-detection.html.
Respiratory virus seasons usually follow a fairly well-known pattern. Enterovirus 68 (EV-D68) is a summer-to-early fall virus with biennial peak years. Rhinovirus (HRv) and adenovirus (Adv) occur nearly year-round but may have small upticks in the first month or so that children return to school. Early in the school year, upper respiratory infections from both HRv and Adv and viral sore throats from Adv are common, with conjunctivitis from Adv outbreaks in some years. October to November is human parainfluenza (HPiV) 1 and 2 season, often presenting as croup. Human metapneumovirus infections span October through April. In late November to December, influenza begins, usually with an A type, later transitioning to a B type in February through April. Also in December, respiratory syncytial virus (RSV) starts, characteristically with bronchiolitis presentations, peaking in February to March and tapering off in May. In late March to April, HPiV 3 also appears for 4-6 weeks.

Will 2020-2021 be different?
Summer was remarkably free of expected enterovirus activity, suggesting that the seasonal parade may differ this year. Remember that the 2019-2020 respiratory season suddenly and nearly completely stopped in March because of social distancing and lockdowns needed to address the SARS-CoV-2 pandemic.
The mild influenza season in the southern hemisphere suggests that our influenza season also could be mild. But perhaps not – most southern hemisphere countries that are surveyed for influenza activities had the most intense SARS-CoV-2 mitigations, making the observed mildness potentially related more to social mitigation than less virulent influenza strains. If so, southern hemisphere influenza data may not apply to the United States, where social distancing and masks are ignored or used inconsistently by almost half the population.
Further, the stop-and-go pattern of in-person school/college attendance adds to uncertainties for the usual orderly virus-specific seasonality. The result may be multiple stop-and-go “pop-up” or “mini” outbreaks for any given virus potentially reflected as exaggerated local or regional differences in circulation of various viruses. The erratic seasonality also would increase coinfections, which could present with more severe or different symptoms.
SARS-CoV-2’s potential interaction
Will the relatively mild presentations for most children with SARS-CoV-2 hold up in the setting of coinfections or sequential respiratory viral infections? Could SARS-CoV-2 cause worse/more prolonged symptoms or more sequelae if paired simultaneously or in tandem with a traditional respiratory virus? To date, data on the frequency and severity of SARS-CoV-2 coinfections are conflicting and sparse, but it appears that non-SARS-CoV-2 viruses can be involved in 15%-50% pediatric acute respiratory infections.1,2
However, it may not be important to know about coinfecting viruses other than influenza (can be treated) or SARS-CoV-2 (needs quarantine and contact tracing), unless symptoms are atypical or more severe than usual. For example, a young child with bronchiolitis is most likely infected with RSV, but HPiV, influenza, metapneumovirus, HRv, and even SARS-CoV-2 can cause bronchiolitis. Even so, testing outpatients for RSV or non-influenza is not routine or even clinically helpful. Supportive treatment and restriction from daycare attendance are sufficient management for outpatient ARIs whether presenting as bronchiolitis or not.
Considerations for SARS-CoV-2 testing: Outpatient bronchiolitis
If a child presents with classic bronchiolitis but has above moderate to severe symptoms, is SARS-CoV-2 a consideration? Perhaps, if SARS-CoV-2 acts similarly to non-SARS-CoV-2s.
A recent report from the 30th Multicenter Airway Research Collaboration (MARC-30) surveillance study (2007-2014) of children hospitalized with clinical bronchiolitis evaluated respiratory viruses, including RSV and the four common non-SARS coronaviruses using molecular testing.3 Among 1,880 subjects, a CoV (alpha CoV: NL63 or 229E, or beta CoV: KKU1 or OC43) was detected in 12%. Yet most had only RSV (n = 1,661); 32 had only CoV (n = 32). But note that 219 had both.
Bronchiolitis subjects with CoV were older – median 3.7 (1.4-5.8) vs. 2.8 (1.9-7.2) years – and more likely male than were RSV subjects (68% vs. 58%). OC43 was most frequent followed by equal numbers of HKU1 and NL63, while 229E was the least frequent. Medical utilization and severity did not differ among the CoVs, or between RSV+CoV vs. RSV alone, unless one considered CoV viral load as a variable. ICU use increased when the polymerase chain reaction cycle threshold result indicated a high CoV viral load.
These data suggest CoVs are not infrequent coinfectors with RSV in bronchiolitis – and that SARS-CoV-2 is the same. Therefore, a bronchiolitis presentation doesn’t necessarily take us off the hook for the need to consider SARS-CoV-2 testing, particularly in the somewhat older bronchiolitis patient with more than mild symptoms.
Considerations for SARS-CoV-2 testing: Outpatient influenza-like illness
In 2020-2021, the Centers for Disease Control and Prevention recommends considering empiric antiviral treatment for ILIs (fever plus either cough or sore throat) based upon our clinical judgement, even in non-high-risk children.4
While pediatric COVID-19 illnesses are predominantly asymptomatic or mild, a febrile ARI is also a SARS-CoV-2 compatible presentation. So, if all we use is our clinical judgment, how do we know if the febrile ARI is due to influenza or SARS-CoV-2 or both? At least one study used a highly sensitive and specific molecular influenza test to show that the accuracy of clinically diagnosing influenza in children is not much better than flipping a coin and would lead to potential antiviral overuse.5
So, it seems ideal to test for influenza when possible. Point-of-care (POC) tests are frequently used for outpatients. Eight POC Clinical Laboratory Improvement Amendments (CLIA)–waived kits, some also detecting RSV, are available but most have modest sensitivity (60%-80%) compared with lab-based molecular tests.6 That said, if supplies and kits for one of the POC tests are available to us during these SARS-CoV-2 stressed times (back orders seem more common this year), a positive influenza test in the first 48 hours of symptoms confirms the option to prescribe an antiviral. Yet how will we have confidence that the febrile ARI is not also partly due to SARS-CoV-2? Currently febrile ARIs usually are considered SARS-CoV-2 and the children are sent for SARS-CoV-2 testing. During influenza season, it seems we will need to continue to send febrile outpatients for SARS-CoV-2 testing, even if POC influenza positive, via whatever mechanisms are available as time goes on.
We expect more rapid pediatric testing modalities for SARS-CoV-2 (maybe even saliva tests) to become available over the next months. Indeed, rapid antigen tests and rapid molecular tests are being evaluated in adults and seem destined for CLIA waivers as POC tests, and even home testing kits. Pediatric approvals hopefully also will occur. So, the pathways for SARS-CoV-2 testing available now will likely change over this winter. But be aware that supplies/kits will be prioritized to locations within high need areas and bulk purchase contracts. So POC kits may remain scarce for practices, meaning a reference laboratory still could be the way to go for SARS-CoV-2 for at least the rest of 2020. Reference labs are becoming creative as well; one combined detection of influenza A, influenza B, RSV, and SARS-CoV-2 into one test, and hopes to get approval for swab collection that can be done by families at home and mailed in.
Summary
Expect variations on the traditional parade of seasonal respiratory viruses, with increased numbers of coinfections. Choosing the outpatient who needs influenza testing is the same as in past years, although we have CDC permissive recommendations to prescribe antivirals for any outpatient ILI within the first 48 hours of symptoms. Still, POC testing for influenza remains potentially valuable in the ILI patient. The choice of whether and how to test for SARS-CoV-2 given its potential to be a primary or coinfecting agent in presentations linked more closely to a traditional virus (e.g. RSV bronchiolitis) will be a test of our clinical judgement until more data and easier testing are available. Further complicating coinfection recognition is the fact that many sick visits occur by telehealth and much testing is done at drive-through SARS-CoV-2 testing facilities with no clinician exam. Unless we are liberal in SARS-CoV-2 testing, detecting SARS-CoV-2 coinfections is easier said than done given its usually mild presentation being overshadowed by any coinfecting virus.
But understanding who has SARS-CoV-2, even as a coinfection, still is essential in controlling the pandemic. We will need to be vigilant for evolving approaches to SARS-CoV-2 testing in the context of symptomatic ARI presentations, knowing this will likely remain a moving target for the foreseeable future.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital-Kansas City, Mo. Children’s Mercy Hospital receives grant funding to study two candidate RSV vaccines. The hospital also receives CDC funding under the New Vaccine Surveillance Network for multicenter surveillance of acute respiratory infections, including influenza, RSV, and parainfluenza virus. Email Dr. Harrison at [email protected].
References
1. Pediatrics. 2020;146(1):e20200961.
2. JAMA. 2020 May 26;323(20):2085-6.
3. Pediatrics. 2020. doi: 10.1542/peds.2020-1267.
4. www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm.
5. J. Pediatr. 2020. doi: 10.1016/j.jpeds.2020.08.007.
6. www.cdc.gov/flu/professionals/diagnosis/table-nucleic-acid-detection.html.
Respiratory virus seasons usually follow a fairly well-known pattern. Enterovirus 68 (EV-D68) is a summer-to-early fall virus with biennial peak years. Rhinovirus (HRv) and adenovirus (Adv) occur nearly year-round but may have small upticks in the first month or so that children return to school. Early in the school year, upper respiratory infections from both HRv and Adv and viral sore throats from Adv are common, with conjunctivitis from Adv outbreaks in some years. October to November is human parainfluenza (HPiV) 1 and 2 season, often presenting as croup. Human metapneumovirus infections span October through April. In late November to December, influenza begins, usually with an A type, later transitioning to a B type in February through April. Also in December, respiratory syncytial virus (RSV) starts, characteristically with bronchiolitis presentations, peaking in February to March and tapering off in May. In late March to April, HPiV 3 also appears for 4-6 weeks.

Will 2020-2021 be different?
Summer was remarkably free of expected enterovirus activity, suggesting that the seasonal parade may differ this year. Remember that the 2019-2020 respiratory season suddenly and nearly completely stopped in March because of social distancing and lockdowns needed to address the SARS-CoV-2 pandemic.
The mild influenza season in the southern hemisphere suggests that our influenza season also could be mild. But perhaps not – most southern hemisphere countries that are surveyed for influenza activities had the most intense SARS-CoV-2 mitigations, making the observed mildness potentially related more to social mitigation than less virulent influenza strains. If so, southern hemisphere influenza data may not apply to the United States, where social distancing and masks are ignored or used inconsistently by almost half the population.
Further, the stop-and-go pattern of in-person school/college attendance adds to uncertainties for the usual orderly virus-specific seasonality. The result may be multiple stop-and-go “pop-up” or “mini” outbreaks for any given virus potentially reflected as exaggerated local or regional differences in circulation of various viruses. The erratic seasonality also would increase coinfections, which could present with more severe or different symptoms.
SARS-CoV-2’s potential interaction
Will the relatively mild presentations for most children with SARS-CoV-2 hold up in the setting of coinfections or sequential respiratory viral infections? Could SARS-CoV-2 cause worse/more prolonged symptoms or more sequelae if paired simultaneously or in tandem with a traditional respiratory virus? To date, data on the frequency and severity of SARS-CoV-2 coinfections are conflicting and sparse, but it appears that non-SARS-CoV-2 viruses can be involved in 15%-50% pediatric acute respiratory infections.1,2
However, it may not be important to know about coinfecting viruses other than influenza (can be treated) or SARS-CoV-2 (needs quarantine and contact tracing), unless symptoms are atypical or more severe than usual. For example, a young child with bronchiolitis is most likely infected with RSV, but HPiV, influenza, metapneumovirus, HRv, and even SARS-CoV-2 can cause bronchiolitis. Even so, testing outpatients for RSV or non-influenza is not routine or even clinically helpful. Supportive treatment and restriction from daycare attendance are sufficient management for outpatient ARIs whether presenting as bronchiolitis or not.
Considerations for SARS-CoV-2 testing: Outpatient bronchiolitis
If a child presents with classic bronchiolitis but has above moderate to severe symptoms, is SARS-CoV-2 a consideration? Perhaps, if SARS-CoV-2 acts similarly to non-SARS-CoV-2s.
A recent report from the 30th Multicenter Airway Research Collaboration (MARC-30) surveillance study (2007-2014) of children hospitalized with clinical bronchiolitis evaluated respiratory viruses, including RSV and the four common non-SARS coronaviruses using molecular testing.3 Among 1,880 subjects, a CoV (alpha CoV: NL63 or 229E, or beta CoV: KKU1 or OC43) was detected in 12%. Yet most had only RSV (n = 1,661); 32 had only CoV (n = 32). But note that 219 had both.
Bronchiolitis subjects with CoV were older – median 3.7 (1.4-5.8) vs. 2.8 (1.9-7.2) years – and more likely male than were RSV subjects (68% vs. 58%). OC43 was most frequent followed by equal numbers of HKU1 and NL63, while 229E was the least frequent. Medical utilization and severity did not differ among the CoVs, or between RSV+CoV vs. RSV alone, unless one considered CoV viral load as a variable. ICU use increased when the polymerase chain reaction cycle threshold result indicated a high CoV viral load.
These data suggest CoVs are not infrequent coinfectors with RSV in bronchiolitis – and that SARS-CoV-2 is the same. Therefore, a bronchiolitis presentation doesn’t necessarily take us off the hook for the need to consider SARS-CoV-2 testing, particularly in the somewhat older bronchiolitis patient with more than mild symptoms.
Considerations for SARS-CoV-2 testing: Outpatient influenza-like illness
In 2020-2021, the Centers for Disease Control and Prevention recommends considering empiric antiviral treatment for ILIs (fever plus either cough or sore throat) based upon our clinical judgement, even in non-high-risk children.4
While pediatric COVID-19 illnesses are predominantly asymptomatic or mild, a febrile ARI is also a SARS-CoV-2 compatible presentation. So, if all we use is our clinical judgment, how do we know if the febrile ARI is due to influenza or SARS-CoV-2 or both? At least one study used a highly sensitive and specific molecular influenza test to show that the accuracy of clinically diagnosing influenza in children is not much better than flipping a coin and would lead to potential antiviral overuse.5
So, it seems ideal to test for influenza when possible. Point-of-care (POC) tests are frequently used for outpatients. Eight POC Clinical Laboratory Improvement Amendments (CLIA)–waived kits, some also detecting RSV, are available but most have modest sensitivity (60%-80%) compared with lab-based molecular tests.6 That said, if supplies and kits for one of the POC tests are available to us during these SARS-CoV-2 stressed times (back orders seem more common this year), a positive influenza test in the first 48 hours of symptoms confirms the option to prescribe an antiviral. Yet how will we have confidence that the febrile ARI is not also partly due to SARS-CoV-2? Currently febrile ARIs usually are considered SARS-CoV-2 and the children are sent for SARS-CoV-2 testing. During influenza season, it seems we will need to continue to send febrile outpatients for SARS-CoV-2 testing, even if POC influenza positive, via whatever mechanisms are available as time goes on.
We expect more rapid pediatric testing modalities for SARS-CoV-2 (maybe even saliva tests) to become available over the next months. Indeed, rapid antigen tests and rapid molecular tests are being evaluated in adults and seem destined for CLIA waivers as POC tests, and even home testing kits. Pediatric approvals hopefully also will occur. So, the pathways for SARS-CoV-2 testing available now will likely change over this winter. But be aware that supplies/kits will be prioritized to locations within high need areas and bulk purchase contracts. So POC kits may remain scarce for practices, meaning a reference laboratory still could be the way to go for SARS-CoV-2 for at least the rest of 2020. Reference labs are becoming creative as well; one combined detection of influenza A, influenza B, RSV, and SARS-CoV-2 into one test, and hopes to get approval for swab collection that can be done by families at home and mailed in.
Summary
Expect variations on the traditional parade of seasonal respiratory viruses, with increased numbers of coinfections. Choosing the outpatient who needs influenza testing is the same as in past years, although we have CDC permissive recommendations to prescribe antivirals for any outpatient ILI within the first 48 hours of symptoms. Still, POC testing for influenza remains potentially valuable in the ILI patient. The choice of whether and how to test for SARS-CoV-2 given its potential to be a primary or coinfecting agent in presentations linked more closely to a traditional virus (e.g. RSV bronchiolitis) will be a test of our clinical judgement until more data and easier testing are available. Further complicating coinfection recognition is the fact that many sick visits occur by telehealth and much testing is done at drive-through SARS-CoV-2 testing facilities with no clinician exam. Unless we are liberal in SARS-CoV-2 testing, detecting SARS-CoV-2 coinfections is easier said than done given its usually mild presentation being overshadowed by any coinfecting virus.
But understanding who has SARS-CoV-2, even as a coinfection, still is essential in controlling the pandemic. We will need to be vigilant for evolving approaches to SARS-CoV-2 testing in the context of symptomatic ARI presentations, knowing this will likely remain a moving target for the foreseeable future.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital-Kansas City, Mo. Children’s Mercy Hospital receives grant funding to study two candidate RSV vaccines. The hospital also receives CDC funding under the New Vaccine Surveillance Network for multicenter surveillance of acute respiratory infections, including influenza, RSV, and parainfluenza virus. Email Dr. Harrison at [email protected].
References
1. Pediatrics. 2020;146(1):e20200961.
2. JAMA. 2020 May 26;323(20):2085-6.
3. Pediatrics. 2020. doi: 10.1542/peds.2020-1267.
4. www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm.
5. J. Pediatr. 2020. doi: 10.1016/j.jpeds.2020.08.007.
6. www.cdc.gov/flu/professionals/diagnosis/table-nucleic-acid-detection.html.

