User login
A COVID-19 Clinical Management Committee to Standardize Care in a 2-Hospital System
From the Department of Medicine (Drs. Meisenberg, Muganlinskaya, Sharma, Amjadi, Arnold, Barnes, Clance, Khalil, Miller, Mooradian, O’Connell, Patel, Press, Samaras, Shanmugam, Tavadze, and Thompson), Department of Pharmacy (Drs. Jiang, Jarawan, Sheth, and Trinh), Department of Nursing (Dr. Ohnmacht), and Department of Women and Children’s Services (Dr. Raji), Luminis Health, Annapolis, MD, and Lanham, MD.
Objective: The COVID-19 pandemic has been a challenge for hospital medical staffs worldwide due to high volumes of patients acutely ill with novel syndromes and prevailing uncertainty regarding optimum supportive and therapeutic interventions. Additionally, the response to this crisis was driven by a plethora of nontraditional information sources, such as email chains, websites, non–peer-reviewed preprints, and press releases. Care patterns became idiosyncratic and often incorporated unproven interventions driven by these nontraditional information sources. This report evaluates the efforts of a health system to create and empower a multidisciplinary committee to develop, implement, and monitor evidence-based, standardized protocols for patients with COVID-19.
Methods: This report describes the composition of the committee, its scope, and its important interactions with the health system pharmacy and therapeutics committee, research teams, and other work groups planning other aspects of COVID-19 management. It illustrates how the committee was used to demonstrate for trainees the process and value of critically examining evidence, even in a chaotic environment.
Results: Data show successful interventions in reducing excessive ordering of certain laboratory tests, reduction of nonrecommended therapies, and rapid uptake of evidence-based or guidelines-supported interventions.
Conclusions: A multidisciplinary committee dedicated solely to planning, implementing, and monitoring standard approaches that eventually became evidence-based decision-making led to an improved focus on treatment options and outcomes for COVID-19 patients. Data presented illustrate the attainable success that is both adaptable and suitable for similar emergencies in the future.
Keywords: COVID-19; clinical management; pharmacy and therapeutics; treatment; therapy.
The COVID-19 pandemic has spread to nearly all countries, carrying with it high morbidity, mortality, and severe impacts on both well-developed and less-well-developed health systems. Media reports of chaos within overwhelmed hospitals have been prominent.1,2 As of January 5, 2022, SARS-CoV-2 has infected more than 295 million people globally and directly caused the death of more than 5.4 million,3 though this number is likely an undercount even in countries with well-developed mortality tracking.4
Throughout the COVID-19 pandemic, hospital-based medical teams have been confronted with a flood of severely ill patients with novel syndromes. Initially, there were no standards for therapy or supportive care except for treatments borrowed from similar syndromes. In the setting of high volumes, high acuity, and public dismay, it is unsurprising that the usual deliberative methods for weighing evidence and initiating interventions were often pushed aside in favor of the solace of active intervention.5 In this milieu of limited evidence, there was a lamentable, if understandable, tendency to seek guidance from “nontraditional” sources,6 including email chains from colleagues, hospital websites, non–peer-reviewed manuscripts, advanced publication by medical journals,7 and nonscientific media presentations. In many localities, practitioners responded in idiosyncratic ways. For example, findings of high cytokine levels in COVID-19,8 along with reports of in-vitro antiviral activity with drugs like hydroxychloroquine against both SARS9 and SARS-CoV-2,10 drove laboratory test ordering and therapeutic interventions, respectively, carving shortcuts into the traditional clinical trial–dependent standards. Clinical trial results eventually emerged.11COVID-19 created a clinical dilemma for hospital medical staffs in terms of how to organize, standardize, and rapidly adapt to a flood of new information. In this report, we describe how 1 health system responded to these challenges by forming a COVID-19 Clinical Management Committee (CCMC) and empowering this interdisciplinary team to review evidence, create and adjust order sets, educate practitioners, oversee care, and collaborate across teams addressing other aspects of the COVID-19 response.
Program Overview
Health System Description
Luminis Health is a health system with 2 acute care hospitals that was formed in 2019 just before the start of the pandemic. Anne Arundel Medical Center (hospital A) is a 385-bed teaching hospital in Annapolis, MD. It has more than 23 000 discharges annually. Patients with COVID-19 were cared for by either an internal medicine teaching service or nonteaching hospitalist services on cohorted nursing units. Doctor’s Community Medical Center, in Lanham, MD (hospital B), is a 206-bed acute care hospital with more than 10 350 annual discharges. COVID-19 patients were cared for by hospitalist groups, initially in noncohorted units with transition to cohorted nursing units after a few months. The medical staffs are generally distinct, with different leadership structures, though the Luminis Health Department of Medicine has oversight responsibilities at both hospitals. More than 47 physicians attended COVID-19 patients at hospital A (with medical residents) and 30 individual physicians at hospital B, respectively, including intensivists. The nursing and pharmacy staffs are distinct, but there is a shared oversight Pharmacy and Therapeutics (P&T) Committee.
The 2 hospitals had distinct electronic medical records (EMR) until January 2021, when hospital B adopted the same EMR as hospital A (Epic).
Mission and Formation of CCMC
In order to coordinate the therapeutic approach across the health system, it was important for the CCMC to be designated by the health system P&T committee as an official subcommittee so that decisions on restrictions of medications and/or new or revised order sets could be rapidly initiated across the system without waiting for the subsequent P&T meetings. The full committee retained oversight of the CCMC. Some P&T members were also on the CCMC.
The committee reviewed new reports in medical journals and prepublication servers and consulted recommendations of professional societies, such as the National Institutes of Health (NIH) COVID-19 guidelines, Infectious Diseases Society of America, Society of Critical Care Medicine, and US Food and Drug Administration (FDA) Emergency Use Authorizations (EUA), among other sources.
Composition of the CCMC
Physician leaders from both hospitals in the following specialties were solicited for participation: critical care, epidemiology, hospital medicine (internal medicine), emergency medicine, infectious diseases, nephrology, women and children’s services, and medical informatics. Specialists in other areas, such as hematology, were invited for topic-specific discussions. Hospital pharmacists with different specialties and nursing leadership were essential contributors. The committee members were expected to use various communication channels to inform frontline clinicians of new care standards and the existence of new order sets, which were embedded in the EMR.
Clinical Research
An important connection for the CCMC was with theCOVID-19 clinical research team. Three members of the research team were also members of the CCMC. All new study proposals for therapeutics were discussed with the CCMC as they were being considered by the research team. In this way, feedback on the feasibility and acceptance of new study opportunities could be discussed with the CCMC. Occasionally, CCMC decisions impacted clinical research accrual strategies. For example, new data from randomized trials about tocilizumab1,2 demonstrated benefits in some subsets of patients and resulted in a recommendation for use by the NIH guideline committee in these populations.1 The CCMC quickly adopted this recommendation, which required a reprioritization of clinical research enrollment for studies testing other immune-modulating agents. This important dialogue was mediated within the CCMC.
Guideline Distribution, Reinforcement, and Platform for Feedback
New guidelines were disseminated to clinicians via daily brief patient huddles held on COVID units, with participation by nursing and pharmacy, and by weekly meetings with hospitalist leaders and frontline hospital physicians. Order sets and guidelines were maintained on the intranet. Adherence was reinforced by unit-based and central pharmacists. Order sets, including admission order sets, could be created only by designated informatics personnel, thus enforcing standardization. Feedback on the utility of the order sets was obtained during the weekly meetings or huddles, as described above. To ensure a sense of transparency, physicians who had interest in commenting on a particular therapy, or who wished to discuss a particular manuscript, news article, or website, were invited to attend CCMC meetings.
Scope of CCMC
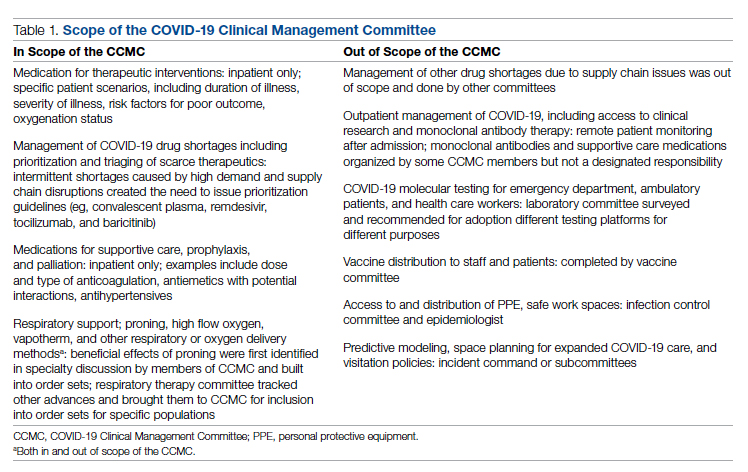
In order to be effective and timely, we limited the scope of our work to the report to the inpatient therapeutic environment, allowing other committees to work on other aspects of the pandemic response. In addition to issuing guidance and creating order sets to direct clinical practice, the CCMC also monitored COVID-19 therapeutic shortages15,16 and advised on prioritization of such treatments as convalescent plasma, remdesivir (prioritization and duration of therapy, 5 vs 10 days), baricitinib, and tocilizumab, depending upon the location of the patient (critical care or not). The CCMC was not involved in the management of non–COVID-19 shortages brought about by supply chain deficiencies.
Table 1 shows some aspects of the health system pandemic-response planning and the committee workforce that undertook that work. Though many items were out of scope for the CCMC, members of the CCMC did participate in the planning work of these other committees and therefore stayed connected to this complementary work.
A Teaching Opportunity About Making Thoughtful Choices
Another important feature of the CCMC was the contributions of residents from both pharmacy and internal medicine. The purpose and operations of the committee were recognized as an opportunity to involve learners in a curriculum based on Kern’s 6-step approach.17 Though the problem identification and general needs assessment were easily defined, the targeted needs assessment, extracted from individual and group interviews with learners and the committee members, pointed at the need to learn how to assess and analyze a rapidly growing body of literature on several relevant clinical aspects of SARS-CoV-2 and COVID-19. To achieve goals and objectives, residents were assigned to present current literature on a particular intervention during a committee meeting, specifically commenting on the merit or deficiencies of the study design, the strength of the data, and applicability to the local context with a recommendation. Prior to the presentations, the residents worked with faculty to identify the best studies or systematic analyses with potential to alter current practices. We thus used the CCMC process as a teaching tool about evidence-based medicine and the dilemma of clinical equipoise. This was imperative, since trainees thrust into the COVID-19 response have often keenly observed a movement away from deliberative decision-making.18 Indeed, including residents in the process of deliberative responses to COVID-19 addresses a recent call to adjust medical education during COVID-19 to “adapt curriculum to current issues in real time.”19
Interventions and Therapies Considered
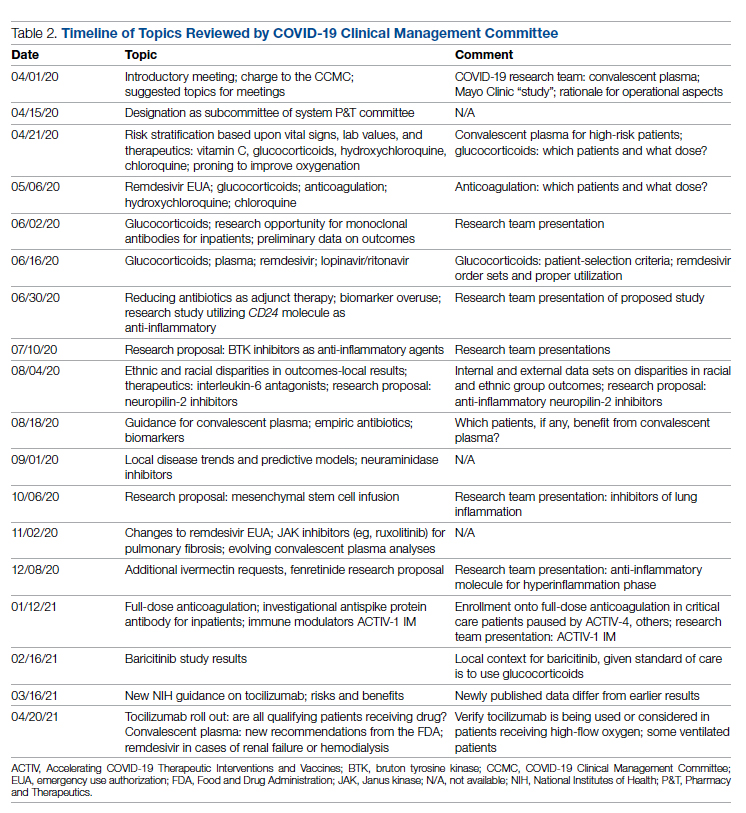
Table 2 shows the topics reviewed by the CCMC. By the time of the first meeting, nonstandardization of care was already a source of concern for clinicians. Dialogue often continued outside of the formal meetings. Many topics were considered more than once as new guidance developed, changes to EUAs occurred, and new data or new publicity arose.
Methods
The Human Protections Administrator determined that this work constituted “quality improvement, and not research” and was therefore exempt from institutional review board review.
Quantitative Analysis
All admitted patients from March 10, 2020, through April 20, 2021, were considered in the quantitative aspects of this report except as noted. Patients diagnosed with COVID-19 were identified by searching our internal data base using diagnostic codes. Patient admissions with the following diagnostic codes were included (prior to April 1, 2020): J12.89, J20.8, J40, J22, J98.8, J80, each with the additional code of B97.29. After April 1, 2020, the guideline for coding COVID-19 was U07.1.
Descriptive statistics were used to measure utilization rates of certain medications and laboratory tests of interest over time. These data were adjusted for number of unique admissions. In a few cases, not all data elements were available from both hospitals due to differences in the EMR.
Case fatality rate was calculated based upon whether the patient died or was admitted to inpatient hospice as a result of COVID-19. Four patients transferred out of hospital A and 18 transferred out of hospital B were censored from case-fatality-rate determination.
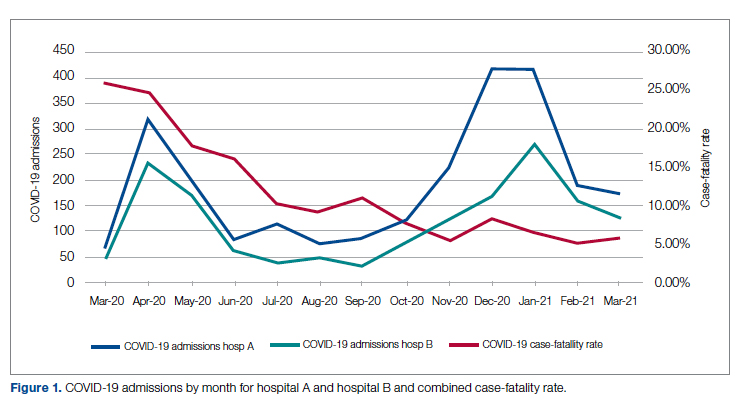
Figure 1 shows the number of admissions for each acute care hospital in the health system and the combined COVID-19 case-fatality rate over time.
Results
A total of 5955 consecutive COVID-19 patients admitted from March 10, 2020, through April 30, 2021, were analyzed. Patients with International Statistical Classification of Diseases, Tenth Revision codes J12.89. J20.8, J40, J22, J98.8, J80, each with the additional code of B97.29 (or the code UO7.1 after April 1, 2020), were included in the analysis. The median age of admitted patients was 65 years (range 19-91 years). Using the NIH classification system for severity,20 the distribution of severity during the first 24 hours after the time of hospital admission was as follows: asymptomatic/presymptomatic, 0.5%; mild illness, 5.3%; moderate illness, 37.1%; severe illness, 55.5%; and critical illness, 1.1%.
The impact of the CCMC can be estimated by looking at care patterns over time. Since the work of the CCMC was aimed at influencing and standardizing physician ordering and therapy choices through order set creation and other forms of oversight, we measured the use of the CCMC-approved order sets at both hospitals and the use of certain laboratory tests and therapies that the CCMC sought either to limit or increase. These counts were adjusted for number of unique COVID-19 admissions. But the limits of the case collection tool meant it also collected cases that were not eligible for some of the interventions. For example, COVID-19 admissions without hypoxemia would not have been eligible for remdesivir or glucocorticoids. When admitted, some patients were already on steroids for other medical indications and did not receive the prescribed dexamethasone dose that we measured in pharmacy databases. Similarly, a few patients were hospitalized for indications unrelated to COVID-19, such as surgery or childbirth, and were found to be SARS-CoV-2-positive on routine screening.
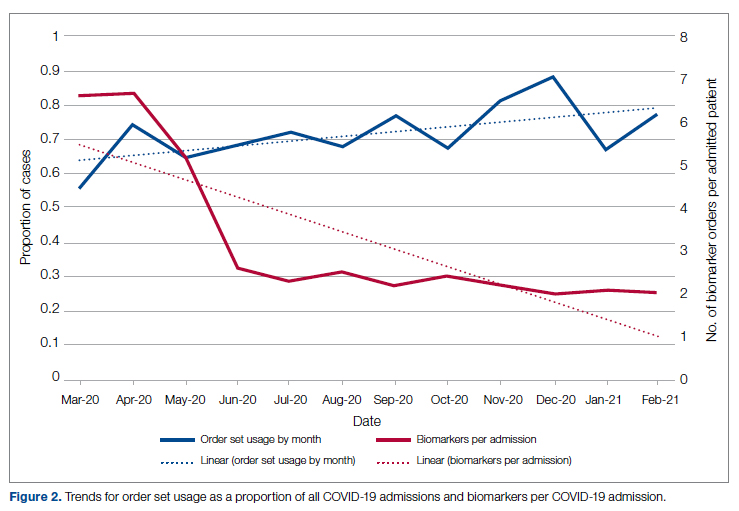
Figure 2 shows the utilization of CCMC-approved standard COVID-19 admission order sets as a proportion of all COVID-19 admissions over time. The trend reveals a modest increase in usage (R2 = 0.34), but these data do not reflect the progressive build of content into order sets over time. One of the goals of the order sets was to standardize and reduce the ordering of certain biomarkers: C-reactive protein, serum ferritin, and D-dimer, which were ordered frequently in many early patients. Orders for these 3 laboratory tests are combined and expressed as an average number of labs per COVID-19 admission in Figure 2. A downward trend, with an R2 value of 0.65, is suggestive of impact from the order sets, though other explanations are possible.
Medication guidance was also a goal of the CCMC, simultaneously discouraging poorly supported interventions and driving uptake of the recommended evidence-based interventions in appropriate patients. Figure 3 shows the utilization pattern for some drugs of interest over the course of the pandemic, specifically the proportion of patients receiving at least 1 dose of medication among all COVID-19 admissions by month. (Data for hospital B was excluded from this analysis because it did not include all admitted patients.)
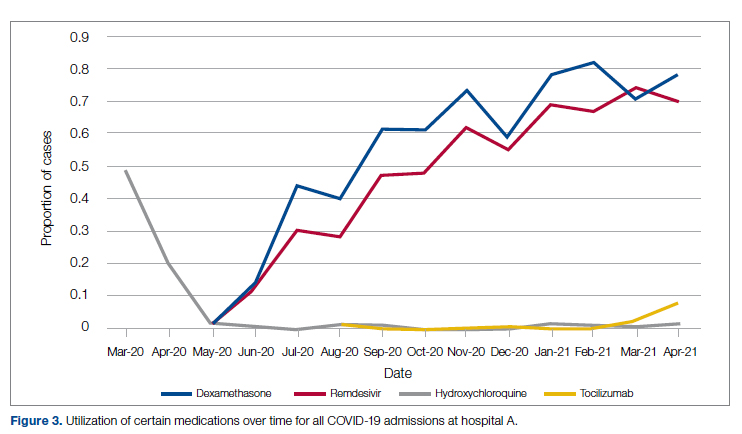
Hydroxychloroquine, which enjoyed a wave of popularity early on during the pandemic, was a target of successful order stewardship through the CCMC. Use of hydroxychloroquine as a COVID-19 therapeutic option after the first 2 months of the pandemic stopped, and subsequent use at low levels likely represented continuation therapy for outpatients who took hydroxychloroquine for rheumatologic indications.
Dexamethasone, as used in the RECOVERY trial,21 had a swift uptake among physicians after it was incorporated into order sets and its use encouraged. Similarly, uptake was immediate for remdesivir when, in May 2020, preliminary reports showed at least some benefits, confirmed by later analysis,22 and it received an FDA EUA.
Our data also show successful stewardship of the interleukin-6 antagonist toclilizumab, which was discouraged early on by the CCMC due to lack of data or negative results. But in March 2021, with new studies releasing data12,13 and new recommendations14 for its use in some subsets of patients with COVID-19, this drug was encouraged in appropriate subsets. A new order set with qualifying indications was prepared by the CCMC and new educational efforts made to encourage its use in appropriate patients.
Ivermectin was nonformulary at the start of the pandemic. This drug enjoyed much publicity from media sources and was promoted by certain physicians and on websites,23 based on in-vitro activity against coronaviruses. Eventually, the World Health Organization24 and the FDA25 found it necessary to issue advisory statements to the public against its use outside of clinical trials. The CCMC had requests from physicians to incorporate ivermectin but declined to add it to the formulary and recommended not approving nonformulary requests due to lack of data. As a result, ivermectin was not used at either hospital.
Discussion
COVID-19 represents many challenges to health systems all over the world. For Luminis Health, the high volume of acutely ill patients with novel syndromes was a particular challenge for the hospital-based care teams. A flood of information from preprints, press releases, preliminary reports, and many other nontraditional sources made deliberative management decisions difficult for individual physicians. Much commentary has appeared around the phenomenon but with less practical advice about how to make day-to-day care decisions at a time of scientific uncertainty and intense pressure to intervene.26,27 The CCMC was designed to overcome the information management dilemma. The need to coordinate, standardize, and oversee care was necessary given the large number of physicians who cared for COVID-19 patients on inpatient services.
It should be noted that creating order sets and issuing guidance is necessary, but not sufficient, to achieve our goals of being updated and consistent. This is especially true with large cadres of health care workers attending COVID-19 patients. Guidelines and recommendations were reinforced by unit-based oversight and stewardship from pharmacy and other leaders who constituted the CCMC.
The reduction in COVID-19 mortality over time experienced in this health care system was not unique and cannot necessarily be attributed to standardization of care. Similar improvements in mortality have been reported at many US hospitals in aggregate.28 Many other factors, including changes in patient characteristics, may be responsible for reduction in mortality over time.
Throughout this report we have relied upon an implicit assumption that standardization of medical therapeutics is desirable and leads to better outcomes as compared with allowing unlimited empiricism by individual physicians, either consultants or hospitalists. Our program represents a single health system with 2 acute care hospitals located 25 miles apart and which thus were similarly impacted by the different phases of the pandemic. Generalizability to health systems either smaller or larger, or in different geographical areas, has not been established. Data limitations have already been discussed.
We did not measure user satisfaction with the program either from physicians or nurses. However, the high rate of compliance suggests general agreement with the content and process.
We cannot definitively ascribe reduction in utilization of some nonrecommended treatments and increased utilization of the recommended therapies to the work of the CCMC. Individual physicians may have made these adjustments on their own or under the influence of other sources.
Finally, it should be noted that the mission to rapidly respond to data from well-conducted trials might be thwarted by too rigid a process or a committee’s lack of a sense of urgency. Organizing a committee and then empowering it to act is no guarantee of success; commitment to the mission is.
Conclusion
COVID-19 represented a challenge to medical staffs everywhere, inundating them with high volumes of acutely ill patients presenting with unfamiliar syndromes. Initial responses were characterized by idiosyncratic management approaches based on nontraditional sources of opinion and influences.
This report describes how a complex medical system brought order and standardization through a deliberative, but urgent, multidisciplinary committee with responsibility for planning, implementing, and monitoring standard approaches that eventually became evidence based. The composition of the committee and its scope of influence, limited to inpatient management, were important elements of success, allowing for better focus on the many treatment decisions. The important connection between the management committee and the system P&T committee, the clinical research effort, and teaching programs in both medicine and pharmacy are offered as exemplars of coordination. The data presented show success in achieving standardized, guideline-directed care. The approach is adoptable and suitable for similar emergencies in the future.
Acknowledgments: The authors thank Gary Scabis, Kip Waite, John Moxley, Angela Clubb, and David Woodley for their assistance in gathering data. We express appreciation and admiration for all our colleagues at the bedside.
Corresponding author: Barry R. Meisenberg, MD, Department of Medicine, Luminis Health, 2001 Medical Pkwy, Annapolis, MD 21401; [email protected].
Financial disclosures: None.
1. Gettleman J, Raj S, Kumar H. India’s health system cracks under the strain as coronavirus cases surge. The New York Times. April 22, 2021. https://www.nytimes.com/2021/04/21/world/asia/india-coronavirus-oxygen.html
2. Rappleye H, Lehren AW, Strickler L, Fitzpatrick S. ‘This system is doomed’: doctors, nurses sound off in NBC News coronavirus survey. NBC News. March 20, 2020. https://www.nbcnews.com/news/us-news/system-doomed-doctors-nurses-sound-nbc-news-coronavirus-survey-n1164841
3. Johns Hopkins Coronavirus Resource Center. Accessed January 5, 2022. https://coronavirus.jhu.edu/map.html
4. Fineberg HV. The toll of COVID-19. JAMA. 2020;324(15):1502-1503. doi:10.1001/jama.2020.20019
5. Meisenberg BR. Medical staffs response to COVID-19 ‘data’: have we misplaced our skeptic’s eye? Am J Med. 2021;134(2):151-152. doi:10.1016/j.amjmed.2020.09.013
6. McMahon JH, Lydeamore MH, Stewardson AJ. Bringing evidence from press release to the clinic in the era of COVID-19. J Antimicrob Chemother. 2021;76(3):547-549. doi:10.1093/jac/dkaa506
7. Rubin EJ, Baden LR, Morrissey S, Campion EW. Medical journals and the 2019-nCoV outbreak. N Engl J Med. 2020;382(9):866. doi:10.1056/NEJMe2001329
8. Liu F, Li L, Xu M, et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. 2020;127:104370. doi:10.1016/j.jcv.2020.104370
9. Vincent MJ, Bergeron E, Benjannet S, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi:10.1186/1743-422X-2-69
10. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269-271. doi:10.1038/s41422-020-0282-0
11. RECOVERY Collaborative Group. Effect of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;383:2030-2040. doi:10.1056/NEJMoa2022926
12. RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): preliminary results of a randomised, controlled, open-label, platform trial [preprint]. February 11, 2021. doi:10.1101/2021.02.11.21249258 https://www.medrxiv.org/content/10.1101/2021.02.11.21249258v1
13. REMAP-CAP Investigators. Interleukin-6 receptor antagonists in critically ill patients with COVID-19. N Engl J Med. 2021;384(16):1491-1502. doi:10.1056/NEJMoa2100433
14. National Institutes of Health. COVID-19 treatment guidelines: interleukin-6 inhibitors. https://www.covid19treatmentguidelines.nih.gov/immunomodulators/interleukin-6-inhibitors/
15. Deana C, Vetrugno L, Tonizzo A, et al. Drug supply during COVID-19 pandemic: remember not to run with your tank empty. Hosp Pharm. 2021;56(5):405-407. doi:10.1177/0018578720931749
16. Choe J, Crane M, Greene J, et al. The Pandemic and the Supply Chain: Addressing Gaps in Pharmaceutical Production and Distribution. Johns Hopkins University, November 2020. https://www.jhsph.edu/research/affiliated-programs/johns-hopkins-drug-access-and-affordability-initiative/publications/Pandemic_Supply_Chain.pdf
17. Kern DE. Overview: a six-step approach to curriculum development. In: Kern DE, Thornton PA, Hughes MT, eds. Curriculum Development for Medical Education: A Six-Step Approach. 3rd ed. Johns Hopkins University Press; 2016.
18. Rice TW, Janz DR. In defense of evidence-based medicine for the treatment of COVID-19 acute respiratory distress syndrome. Ann Am Thorac Soc. 2020;17(7):787-789. doi:10.1513/AnnalsATS.202004-325IP
19. Lucey CR, Johnston SC. The transformational effects of COVID-19 on medical education. JAMA. 2020;324(11):1033-1034. doi:10.1001/jama.2020.14136
20. National Institutes of Health. COVID-19 treatment guidelines: clinical spectrum of SARS-CoV-2 infection. https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/
21. RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693-704. doi:10.1056/NEJMoa2021436
22. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19—final report. N Engl J Med. 2020;383:1813-1826. doi:10.1056/NEJMoa2007764
23. Jiminez D. Ivermectin and Covid-19: how a cheap antiparasitic became political. April 19, 2021. https://www.pharmaceutical-technology.com/features/ivermectin-covid-19-antiparasitic-political/
24. World Health Organization. WHO advises that ivermectin only be used to treat COVID-19 within clinical trials. March 31, 2021. https://www.who.int/news-room/feature-stories/detail/who-advises-that-ivermectin-only-be-used-to-treat-covid-19-within-clinical-trials
25. U.S. Food and Drug Administration. Why you should not use ivermectin to treat or prevent COVID-19. March 5, 2021. https://www.fda.gov/consumers/consumer-updates/why-you-should-not-use-ivermectin-treat-or-prevent-covid-19
26. Seymour CW, McCreary EK, Stegenga J. Sensible medicine-balancing intervention and inaction during the COVID-19 pandemic. JAMA. 2020;324(18):1827-1828. doi:10.1001/jama.2020.20271
27. Flanagin A, Fontanarosa PB, Bauchner H. Preprints involving medical research—do the benefits outweigh the challenges? JAMA. 2020;324(18):1840-1843. doi:10.1001/jama.2020.20674
28. Asch DA, Shells NE, Islam N, et al. Variation in US hospital mortality rates for patients admitted with COVID-19 during the first 6 months of the pandemic. JAMA Intern Med. 2021;181(4):471-478. doi:10.1001/jamainternmed.2020.8193
From the Department of Medicine (Drs. Meisenberg, Muganlinskaya, Sharma, Amjadi, Arnold, Barnes, Clance, Khalil, Miller, Mooradian, O’Connell, Patel, Press, Samaras, Shanmugam, Tavadze, and Thompson), Department of Pharmacy (Drs. Jiang, Jarawan, Sheth, and Trinh), Department of Nursing (Dr. Ohnmacht), and Department of Women and Children’s Services (Dr. Raji), Luminis Health, Annapolis, MD, and Lanham, MD.
Objective: The COVID-19 pandemic has been a challenge for hospital medical staffs worldwide due to high volumes of patients acutely ill with novel syndromes and prevailing uncertainty regarding optimum supportive and therapeutic interventions. Additionally, the response to this crisis was driven by a plethora of nontraditional information sources, such as email chains, websites, non–peer-reviewed preprints, and press releases. Care patterns became idiosyncratic and often incorporated unproven interventions driven by these nontraditional information sources. This report evaluates the efforts of a health system to create and empower a multidisciplinary committee to develop, implement, and monitor evidence-based, standardized protocols for patients with COVID-19.
Methods: This report describes the composition of the committee, its scope, and its important interactions with the health system pharmacy and therapeutics committee, research teams, and other work groups planning other aspects of COVID-19 management. It illustrates how the committee was used to demonstrate for trainees the process and value of critically examining evidence, even in a chaotic environment.
Results: Data show successful interventions in reducing excessive ordering of certain laboratory tests, reduction of nonrecommended therapies, and rapid uptake of evidence-based or guidelines-supported interventions.
Conclusions: A multidisciplinary committee dedicated solely to planning, implementing, and monitoring standard approaches that eventually became evidence-based decision-making led to an improved focus on treatment options and outcomes for COVID-19 patients. Data presented illustrate the attainable success that is both adaptable and suitable for similar emergencies in the future.
Keywords: COVID-19; clinical management; pharmacy and therapeutics; treatment; therapy.
The COVID-19 pandemic has spread to nearly all countries, carrying with it high morbidity, mortality, and severe impacts on both well-developed and less-well-developed health systems. Media reports of chaos within overwhelmed hospitals have been prominent.1,2 As of January 5, 2022, SARS-CoV-2 has infected more than 295 million people globally and directly caused the death of more than 5.4 million,3 though this number is likely an undercount even in countries with well-developed mortality tracking.4
Throughout the COVID-19 pandemic, hospital-based medical teams have been confronted with a flood of severely ill patients with novel syndromes. Initially, there were no standards for therapy or supportive care except for treatments borrowed from similar syndromes. In the setting of high volumes, high acuity, and public dismay, it is unsurprising that the usual deliberative methods for weighing evidence and initiating interventions were often pushed aside in favor of the solace of active intervention.5 In this milieu of limited evidence, there was a lamentable, if understandable, tendency to seek guidance from “nontraditional” sources,6 including email chains from colleagues, hospital websites, non–peer-reviewed manuscripts, advanced publication by medical journals,7 and nonscientific media presentations. In many localities, practitioners responded in idiosyncratic ways. For example, findings of high cytokine levels in COVID-19,8 along with reports of in-vitro antiviral activity with drugs like hydroxychloroquine against both SARS9 and SARS-CoV-2,10 drove laboratory test ordering and therapeutic interventions, respectively, carving shortcuts into the traditional clinical trial–dependent standards. Clinical trial results eventually emerged.11COVID-19 created a clinical dilemma for hospital medical staffs in terms of how to organize, standardize, and rapidly adapt to a flood of new information. In this report, we describe how 1 health system responded to these challenges by forming a COVID-19 Clinical Management Committee (CCMC) and empowering this interdisciplinary team to review evidence, create and adjust order sets, educate practitioners, oversee care, and collaborate across teams addressing other aspects of the COVID-19 response.
Program Overview
Health System Description
Luminis Health is a health system with 2 acute care hospitals that was formed in 2019 just before the start of the pandemic. Anne Arundel Medical Center (hospital A) is a 385-bed teaching hospital in Annapolis, MD. It has more than 23 000 discharges annually. Patients with COVID-19 were cared for by either an internal medicine teaching service or nonteaching hospitalist services on cohorted nursing units. Doctor’s Community Medical Center, in Lanham, MD (hospital B), is a 206-bed acute care hospital with more than 10 350 annual discharges. COVID-19 patients were cared for by hospitalist groups, initially in noncohorted units with transition to cohorted nursing units after a few months. The medical staffs are generally distinct, with different leadership structures, though the Luminis Health Department of Medicine has oversight responsibilities at both hospitals. More than 47 physicians attended COVID-19 patients at hospital A (with medical residents) and 30 individual physicians at hospital B, respectively, including intensivists. The nursing and pharmacy staffs are distinct, but there is a shared oversight Pharmacy and Therapeutics (P&T) Committee.
The 2 hospitals had distinct electronic medical records (EMR) until January 2021, when hospital B adopted the same EMR as hospital A (Epic).
Mission and Formation of CCMC
In order to coordinate the therapeutic approach across the health system, it was important for the CCMC to be designated by the health system P&T committee as an official subcommittee so that decisions on restrictions of medications and/or new or revised order sets could be rapidly initiated across the system without waiting for the subsequent P&T meetings. The full committee retained oversight of the CCMC. Some P&T members were also on the CCMC.
The committee reviewed new reports in medical journals and prepublication servers and consulted recommendations of professional societies, such as the National Institutes of Health (NIH) COVID-19 guidelines, Infectious Diseases Society of America, Society of Critical Care Medicine, and US Food and Drug Administration (FDA) Emergency Use Authorizations (EUA), among other sources.
Composition of the CCMC
Physician leaders from both hospitals in the following specialties were solicited for participation: critical care, epidemiology, hospital medicine (internal medicine), emergency medicine, infectious diseases, nephrology, women and children’s services, and medical informatics. Specialists in other areas, such as hematology, were invited for topic-specific discussions. Hospital pharmacists with different specialties and nursing leadership were essential contributors. The committee members were expected to use various communication channels to inform frontline clinicians of new care standards and the existence of new order sets, which were embedded in the EMR.
Clinical Research
An important connection for the CCMC was with theCOVID-19 clinical research team. Three members of the research team were also members of the CCMC. All new study proposals for therapeutics were discussed with the CCMC as they were being considered by the research team. In this way, feedback on the feasibility and acceptance of new study opportunities could be discussed with the CCMC. Occasionally, CCMC decisions impacted clinical research accrual strategies. For example, new data from randomized trials about tocilizumab1,2 demonstrated benefits in some subsets of patients and resulted in a recommendation for use by the NIH guideline committee in these populations.1 The CCMC quickly adopted this recommendation, which required a reprioritization of clinical research enrollment for studies testing other immune-modulating agents. This important dialogue was mediated within the CCMC.
Guideline Distribution, Reinforcement, and Platform for Feedback
New guidelines were disseminated to clinicians via daily brief patient huddles held on COVID units, with participation by nursing and pharmacy, and by weekly meetings with hospitalist leaders and frontline hospital physicians. Order sets and guidelines were maintained on the intranet. Adherence was reinforced by unit-based and central pharmacists. Order sets, including admission order sets, could be created only by designated informatics personnel, thus enforcing standardization. Feedback on the utility of the order sets was obtained during the weekly meetings or huddles, as described above. To ensure a sense of transparency, physicians who had interest in commenting on a particular therapy, or who wished to discuss a particular manuscript, news article, or website, were invited to attend CCMC meetings.
Scope of CCMC
In order to be effective and timely, we limited the scope of our work to the report to the inpatient therapeutic environment, allowing other committees to work on other aspects of the pandemic response. In addition to issuing guidance and creating order sets to direct clinical practice, the CCMC also monitored COVID-19 therapeutic shortages15,16 and advised on prioritization of such treatments as convalescent plasma, remdesivir (prioritization and duration of therapy, 5 vs 10 days), baricitinib, and tocilizumab, depending upon the location of the patient (critical care or not). The CCMC was not involved in the management of non–COVID-19 shortages brought about by supply chain deficiencies.
Table 1 shows some aspects of the health system pandemic-response planning and the committee workforce that undertook that work. Though many items were out of scope for the CCMC, members of the CCMC did participate in the planning work of these other committees and therefore stayed connected to this complementary work.

A Teaching Opportunity About Making Thoughtful Choices
Another important feature of the CCMC was the contributions of residents from both pharmacy and internal medicine. The purpose and operations of the committee were recognized as an opportunity to involve learners in a curriculum based on Kern’s 6-step approach.17 Though the problem identification and general needs assessment were easily defined, the targeted needs assessment, extracted from individual and group interviews with learners and the committee members, pointed at the need to learn how to assess and analyze a rapidly growing body of literature on several relevant clinical aspects of SARS-CoV-2 and COVID-19. To achieve goals and objectives, residents were assigned to present current literature on a particular intervention during a committee meeting, specifically commenting on the merit or deficiencies of the study design, the strength of the data, and applicability to the local context with a recommendation. Prior to the presentations, the residents worked with faculty to identify the best studies or systematic analyses with potential to alter current practices. We thus used the CCMC process as a teaching tool about evidence-based medicine and the dilemma of clinical equipoise. This was imperative, since trainees thrust into the COVID-19 response have often keenly observed a movement away from deliberative decision-making.18 Indeed, including residents in the process of deliberative responses to COVID-19 addresses a recent call to adjust medical education during COVID-19 to “adapt curriculum to current issues in real time.”19
Interventions and Therapies Considered
Table 2 shows the topics reviewed by the CCMC. By the time of the first meeting, nonstandardization of care was already a source of concern for clinicians. Dialogue often continued outside of the formal meetings. Many topics were considered more than once as new guidance developed, changes to EUAs occurred, and new data or new publicity arose.

Methods
The Human Protections Administrator determined that this work constituted “quality improvement, and not research” and was therefore exempt from institutional review board review.
Quantitative Analysis
All admitted patients from March 10, 2020, through April 20, 2021, were considered in the quantitative aspects of this report except as noted. Patients diagnosed with COVID-19 were identified by searching our internal data base using diagnostic codes. Patient admissions with the following diagnostic codes were included (prior to April 1, 2020): J12.89, J20.8, J40, J22, J98.8, J80, each with the additional code of B97.29. After April 1, 2020, the guideline for coding COVID-19 was U07.1.
Descriptive statistics were used to measure utilization rates of certain medications and laboratory tests of interest over time. These data were adjusted for number of unique admissions. In a few cases, not all data elements were available from both hospitals due to differences in the EMR.
Case fatality rate was calculated based upon whether the patient died or was admitted to inpatient hospice as a result of COVID-19. Four patients transferred out of hospital A and 18 transferred out of hospital B were censored from case-fatality-rate determination.
Figure 1 shows the number of admissions for each acute care hospital in the health system and the combined COVID-19 case-fatality rate over time.

Results
A total of 5955 consecutive COVID-19 patients admitted from March 10, 2020, through April 30, 2021, were analyzed. Patients with International Statistical Classification of Diseases, Tenth Revision codes J12.89. J20.8, J40, J22, J98.8, J80, each with the additional code of B97.29 (or the code UO7.1 after April 1, 2020), were included in the analysis. The median age of admitted patients was 65 years (range 19-91 years). Using the NIH classification system for severity,20 the distribution of severity during the first 24 hours after the time of hospital admission was as follows: asymptomatic/presymptomatic, 0.5%; mild illness, 5.3%; moderate illness, 37.1%; severe illness, 55.5%; and critical illness, 1.1%.
The impact of the CCMC can be estimated by looking at care patterns over time. Since the work of the CCMC was aimed at influencing and standardizing physician ordering and therapy choices through order set creation and other forms of oversight, we measured the use of the CCMC-approved order sets at both hospitals and the use of certain laboratory tests and therapies that the CCMC sought either to limit or increase. These counts were adjusted for number of unique COVID-19 admissions. But the limits of the case collection tool meant it also collected cases that were not eligible for some of the interventions. For example, COVID-19 admissions without hypoxemia would not have been eligible for remdesivir or glucocorticoids. When admitted, some patients were already on steroids for other medical indications and did not receive the prescribed dexamethasone dose that we measured in pharmacy databases. Similarly, a few patients were hospitalized for indications unrelated to COVID-19, such as surgery or childbirth, and were found to be SARS-CoV-2-positive on routine screening.
Figure 2 shows the utilization of CCMC-approved standard COVID-19 admission order sets as a proportion of all COVID-19 admissions over time. The trend reveals a modest increase in usage (R2 = 0.34), but these data do not reflect the progressive build of content into order sets over time. One of the goals of the order sets was to standardize and reduce the ordering of certain biomarkers: C-reactive protein, serum ferritin, and D-dimer, which were ordered frequently in many early patients. Orders for these 3 laboratory tests are combined and expressed as an average number of labs per COVID-19 admission in Figure 2. A downward trend, with an R2 value of 0.65, is suggestive of impact from the order sets, though other explanations are possible.

Medication guidance was also a goal of the CCMC, simultaneously discouraging poorly supported interventions and driving uptake of the recommended evidence-based interventions in appropriate patients. Figure 3 shows the utilization pattern for some drugs of interest over the course of the pandemic, specifically the proportion of patients receiving at least 1 dose of medication among all COVID-19 admissions by month. (Data for hospital B was excluded from this analysis because it did not include all admitted patients.)

Hydroxychloroquine, which enjoyed a wave of popularity early on during the pandemic, was a target of successful order stewardship through the CCMC. Use of hydroxychloroquine as a COVID-19 therapeutic option after the first 2 months of the pandemic stopped, and subsequent use at low levels likely represented continuation therapy for outpatients who took hydroxychloroquine for rheumatologic indications.
Dexamethasone, as used in the RECOVERY trial,21 had a swift uptake among physicians after it was incorporated into order sets and its use encouraged. Similarly, uptake was immediate for remdesivir when, in May 2020, preliminary reports showed at least some benefits, confirmed by later analysis,22 and it received an FDA EUA.
Our data also show successful stewardship of the interleukin-6 antagonist toclilizumab, which was discouraged early on by the CCMC due to lack of data or negative results. But in March 2021, with new studies releasing data12,13 and new recommendations14 for its use in some subsets of patients with COVID-19, this drug was encouraged in appropriate subsets. A new order set with qualifying indications was prepared by the CCMC and new educational efforts made to encourage its use in appropriate patients.
Ivermectin was nonformulary at the start of the pandemic. This drug enjoyed much publicity from media sources and was promoted by certain physicians and on websites,23 based on in-vitro activity against coronaviruses. Eventually, the World Health Organization24 and the FDA25 found it necessary to issue advisory statements to the public against its use outside of clinical trials. The CCMC had requests from physicians to incorporate ivermectin but declined to add it to the formulary and recommended not approving nonformulary requests due to lack of data. As a result, ivermectin was not used at either hospital.
Discussion
COVID-19 represents many challenges to health systems all over the world. For Luminis Health, the high volume of acutely ill patients with novel syndromes was a particular challenge for the hospital-based care teams. A flood of information from preprints, press releases, preliminary reports, and many other nontraditional sources made deliberative management decisions difficult for individual physicians. Much commentary has appeared around the phenomenon but with less practical advice about how to make day-to-day care decisions at a time of scientific uncertainty and intense pressure to intervene.26,27 The CCMC was designed to overcome the information management dilemma. The need to coordinate, standardize, and oversee care was necessary given the large number of physicians who cared for COVID-19 patients on inpatient services.
It should be noted that creating order sets and issuing guidance is necessary, but not sufficient, to achieve our goals of being updated and consistent. This is especially true with large cadres of health care workers attending COVID-19 patients. Guidelines and recommendations were reinforced by unit-based oversight and stewardship from pharmacy and other leaders who constituted the CCMC.
The reduction in COVID-19 mortality over time experienced in this health care system was not unique and cannot necessarily be attributed to standardization of care. Similar improvements in mortality have been reported at many US hospitals in aggregate.28 Many other factors, including changes in patient characteristics, may be responsible for reduction in mortality over time.
Throughout this report we have relied upon an implicit assumption that standardization of medical therapeutics is desirable and leads to better outcomes as compared with allowing unlimited empiricism by individual physicians, either consultants or hospitalists. Our program represents a single health system with 2 acute care hospitals located 25 miles apart and which thus were similarly impacted by the different phases of the pandemic. Generalizability to health systems either smaller or larger, or in different geographical areas, has not been established. Data limitations have already been discussed.
We did not measure user satisfaction with the program either from physicians or nurses. However, the high rate of compliance suggests general agreement with the content and process.
We cannot definitively ascribe reduction in utilization of some nonrecommended treatments and increased utilization of the recommended therapies to the work of the CCMC. Individual physicians may have made these adjustments on their own or under the influence of other sources.
Finally, it should be noted that the mission to rapidly respond to data from well-conducted trials might be thwarted by too rigid a process or a committee’s lack of a sense of urgency. Organizing a committee and then empowering it to act is no guarantee of success; commitment to the mission is.
Conclusion
COVID-19 represented a challenge to medical staffs everywhere, inundating them with high volumes of acutely ill patients presenting with unfamiliar syndromes. Initial responses were characterized by idiosyncratic management approaches based on nontraditional sources of opinion and influences.
This report describes how a complex medical system brought order and standardization through a deliberative, but urgent, multidisciplinary committee with responsibility for planning, implementing, and monitoring standard approaches that eventually became evidence based. The composition of the committee and its scope of influence, limited to inpatient management, were important elements of success, allowing for better focus on the many treatment decisions. The important connection between the management committee and the system P&T committee, the clinical research effort, and teaching programs in both medicine and pharmacy are offered as exemplars of coordination. The data presented show success in achieving standardized, guideline-directed care. The approach is adoptable and suitable for similar emergencies in the future.
Acknowledgments: The authors thank Gary Scabis, Kip Waite, John Moxley, Angela Clubb, and David Woodley for their assistance in gathering data. We express appreciation and admiration for all our colleagues at the bedside.
Corresponding author: Barry R. Meisenberg, MD, Department of Medicine, Luminis Health, 2001 Medical Pkwy, Annapolis, MD 21401; [email protected].
Financial disclosures: None.
From the Department of Medicine (Drs. Meisenberg, Muganlinskaya, Sharma, Amjadi, Arnold, Barnes, Clance, Khalil, Miller, Mooradian, O’Connell, Patel, Press, Samaras, Shanmugam, Tavadze, and Thompson), Department of Pharmacy (Drs. Jiang, Jarawan, Sheth, and Trinh), Department of Nursing (Dr. Ohnmacht), and Department of Women and Children’s Services (Dr. Raji), Luminis Health, Annapolis, MD, and Lanham, MD.
Objective: The COVID-19 pandemic has been a challenge for hospital medical staffs worldwide due to high volumes of patients acutely ill with novel syndromes and prevailing uncertainty regarding optimum supportive and therapeutic interventions. Additionally, the response to this crisis was driven by a plethora of nontraditional information sources, such as email chains, websites, non–peer-reviewed preprints, and press releases. Care patterns became idiosyncratic and often incorporated unproven interventions driven by these nontraditional information sources. This report evaluates the efforts of a health system to create and empower a multidisciplinary committee to develop, implement, and monitor evidence-based, standardized protocols for patients with COVID-19.
Methods: This report describes the composition of the committee, its scope, and its important interactions with the health system pharmacy and therapeutics committee, research teams, and other work groups planning other aspects of COVID-19 management. It illustrates how the committee was used to demonstrate for trainees the process and value of critically examining evidence, even in a chaotic environment.
Results: Data show successful interventions in reducing excessive ordering of certain laboratory tests, reduction of nonrecommended therapies, and rapid uptake of evidence-based or guidelines-supported interventions.
Conclusions: A multidisciplinary committee dedicated solely to planning, implementing, and monitoring standard approaches that eventually became evidence-based decision-making led to an improved focus on treatment options and outcomes for COVID-19 patients. Data presented illustrate the attainable success that is both adaptable and suitable for similar emergencies in the future.
Keywords: COVID-19; clinical management; pharmacy and therapeutics; treatment; therapy.
The COVID-19 pandemic has spread to nearly all countries, carrying with it high morbidity, mortality, and severe impacts on both well-developed and less-well-developed health systems. Media reports of chaos within overwhelmed hospitals have been prominent.1,2 As of January 5, 2022, SARS-CoV-2 has infected more than 295 million people globally and directly caused the death of more than 5.4 million,3 though this number is likely an undercount even in countries with well-developed mortality tracking.4
Throughout the COVID-19 pandemic, hospital-based medical teams have been confronted with a flood of severely ill patients with novel syndromes. Initially, there were no standards for therapy or supportive care except for treatments borrowed from similar syndromes. In the setting of high volumes, high acuity, and public dismay, it is unsurprising that the usual deliberative methods for weighing evidence and initiating interventions were often pushed aside in favor of the solace of active intervention.5 In this milieu of limited evidence, there was a lamentable, if understandable, tendency to seek guidance from “nontraditional” sources,6 including email chains from colleagues, hospital websites, non–peer-reviewed manuscripts, advanced publication by medical journals,7 and nonscientific media presentations. In many localities, practitioners responded in idiosyncratic ways. For example, findings of high cytokine levels in COVID-19,8 along with reports of in-vitro antiviral activity with drugs like hydroxychloroquine against both SARS9 and SARS-CoV-2,10 drove laboratory test ordering and therapeutic interventions, respectively, carving shortcuts into the traditional clinical trial–dependent standards. Clinical trial results eventually emerged.11COVID-19 created a clinical dilemma for hospital medical staffs in terms of how to organize, standardize, and rapidly adapt to a flood of new information. In this report, we describe how 1 health system responded to these challenges by forming a COVID-19 Clinical Management Committee (CCMC) and empowering this interdisciplinary team to review evidence, create and adjust order sets, educate practitioners, oversee care, and collaborate across teams addressing other aspects of the COVID-19 response.
Program Overview
Health System Description
Luminis Health is a health system with 2 acute care hospitals that was formed in 2019 just before the start of the pandemic. Anne Arundel Medical Center (hospital A) is a 385-bed teaching hospital in Annapolis, MD. It has more than 23 000 discharges annually. Patients with COVID-19 were cared for by either an internal medicine teaching service or nonteaching hospitalist services on cohorted nursing units. Doctor’s Community Medical Center, in Lanham, MD (hospital B), is a 206-bed acute care hospital with more than 10 350 annual discharges. COVID-19 patients were cared for by hospitalist groups, initially in noncohorted units with transition to cohorted nursing units after a few months. The medical staffs are generally distinct, with different leadership structures, though the Luminis Health Department of Medicine has oversight responsibilities at both hospitals. More than 47 physicians attended COVID-19 patients at hospital A (with medical residents) and 30 individual physicians at hospital B, respectively, including intensivists. The nursing and pharmacy staffs are distinct, but there is a shared oversight Pharmacy and Therapeutics (P&T) Committee.
The 2 hospitals had distinct electronic medical records (EMR) until January 2021, when hospital B adopted the same EMR as hospital A (Epic).
Mission and Formation of CCMC
In order to coordinate the therapeutic approach across the health system, it was important for the CCMC to be designated by the health system P&T committee as an official subcommittee so that decisions on restrictions of medications and/or new or revised order sets could be rapidly initiated across the system without waiting for the subsequent P&T meetings. The full committee retained oversight of the CCMC. Some P&T members were also on the CCMC.
The committee reviewed new reports in medical journals and prepublication servers and consulted recommendations of professional societies, such as the National Institutes of Health (NIH) COVID-19 guidelines, Infectious Diseases Society of America, Society of Critical Care Medicine, and US Food and Drug Administration (FDA) Emergency Use Authorizations (EUA), among other sources.
Composition of the CCMC
Physician leaders from both hospitals in the following specialties were solicited for participation: critical care, epidemiology, hospital medicine (internal medicine), emergency medicine, infectious diseases, nephrology, women and children’s services, and medical informatics. Specialists in other areas, such as hematology, were invited for topic-specific discussions. Hospital pharmacists with different specialties and nursing leadership were essential contributors. The committee members were expected to use various communication channels to inform frontline clinicians of new care standards and the existence of new order sets, which were embedded in the EMR.
Clinical Research
An important connection for the CCMC was with theCOVID-19 clinical research team. Three members of the research team were also members of the CCMC. All new study proposals for therapeutics were discussed with the CCMC as they were being considered by the research team. In this way, feedback on the feasibility and acceptance of new study opportunities could be discussed with the CCMC. Occasionally, CCMC decisions impacted clinical research accrual strategies. For example, new data from randomized trials about tocilizumab1,2 demonstrated benefits in some subsets of patients and resulted in a recommendation for use by the NIH guideline committee in these populations.1 The CCMC quickly adopted this recommendation, which required a reprioritization of clinical research enrollment for studies testing other immune-modulating agents. This important dialogue was mediated within the CCMC.
Guideline Distribution, Reinforcement, and Platform for Feedback
New guidelines were disseminated to clinicians via daily brief patient huddles held on COVID units, with participation by nursing and pharmacy, and by weekly meetings with hospitalist leaders and frontline hospital physicians. Order sets and guidelines were maintained on the intranet. Adherence was reinforced by unit-based and central pharmacists. Order sets, including admission order sets, could be created only by designated informatics personnel, thus enforcing standardization. Feedback on the utility of the order sets was obtained during the weekly meetings or huddles, as described above. To ensure a sense of transparency, physicians who had interest in commenting on a particular therapy, or who wished to discuss a particular manuscript, news article, or website, were invited to attend CCMC meetings.
Scope of CCMC
In order to be effective and timely, we limited the scope of our work to the report to the inpatient therapeutic environment, allowing other committees to work on other aspects of the pandemic response. In addition to issuing guidance and creating order sets to direct clinical practice, the CCMC also monitored COVID-19 therapeutic shortages15,16 and advised on prioritization of such treatments as convalescent plasma, remdesivir (prioritization and duration of therapy, 5 vs 10 days), baricitinib, and tocilizumab, depending upon the location of the patient (critical care or not). The CCMC was not involved in the management of non–COVID-19 shortages brought about by supply chain deficiencies.
Table 1 shows some aspects of the health system pandemic-response planning and the committee workforce that undertook that work. Though many items were out of scope for the CCMC, members of the CCMC did participate in the planning work of these other committees and therefore stayed connected to this complementary work.

A Teaching Opportunity About Making Thoughtful Choices
Another important feature of the CCMC was the contributions of residents from both pharmacy and internal medicine. The purpose and operations of the committee were recognized as an opportunity to involve learners in a curriculum based on Kern’s 6-step approach.17 Though the problem identification and general needs assessment were easily defined, the targeted needs assessment, extracted from individual and group interviews with learners and the committee members, pointed at the need to learn how to assess and analyze a rapidly growing body of literature on several relevant clinical aspects of SARS-CoV-2 and COVID-19. To achieve goals and objectives, residents were assigned to present current literature on a particular intervention during a committee meeting, specifically commenting on the merit or deficiencies of the study design, the strength of the data, and applicability to the local context with a recommendation. Prior to the presentations, the residents worked with faculty to identify the best studies or systematic analyses with potential to alter current practices. We thus used the CCMC process as a teaching tool about evidence-based medicine and the dilemma of clinical equipoise. This was imperative, since trainees thrust into the COVID-19 response have often keenly observed a movement away from deliberative decision-making.18 Indeed, including residents in the process of deliberative responses to COVID-19 addresses a recent call to adjust medical education during COVID-19 to “adapt curriculum to current issues in real time.”19
Interventions and Therapies Considered
Table 2 shows the topics reviewed by the CCMC. By the time of the first meeting, nonstandardization of care was already a source of concern for clinicians. Dialogue often continued outside of the formal meetings. Many topics were considered more than once as new guidance developed, changes to EUAs occurred, and new data or new publicity arose.

Methods
The Human Protections Administrator determined that this work constituted “quality improvement, and not research” and was therefore exempt from institutional review board review.
Quantitative Analysis
All admitted patients from March 10, 2020, through April 20, 2021, were considered in the quantitative aspects of this report except as noted. Patients diagnosed with COVID-19 were identified by searching our internal data base using diagnostic codes. Patient admissions with the following diagnostic codes were included (prior to April 1, 2020): J12.89, J20.8, J40, J22, J98.8, J80, each with the additional code of B97.29. After April 1, 2020, the guideline for coding COVID-19 was U07.1.
Descriptive statistics were used to measure utilization rates of certain medications and laboratory tests of interest over time. These data were adjusted for number of unique admissions. In a few cases, not all data elements were available from both hospitals due to differences in the EMR.
Case fatality rate was calculated based upon whether the patient died or was admitted to inpatient hospice as a result of COVID-19. Four patients transferred out of hospital A and 18 transferred out of hospital B were censored from case-fatality-rate determination.
Figure 1 shows the number of admissions for each acute care hospital in the health system and the combined COVID-19 case-fatality rate over time.

Results
A total of 5955 consecutive COVID-19 patients admitted from March 10, 2020, through April 30, 2021, were analyzed. Patients with International Statistical Classification of Diseases, Tenth Revision codes J12.89. J20.8, J40, J22, J98.8, J80, each with the additional code of B97.29 (or the code UO7.1 after April 1, 2020), were included in the analysis. The median age of admitted patients was 65 years (range 19-91 years). Using the NIH classification system for severity,20 the distribution of severity during the first 24 hours after the time of hospital admission was as follows: asymptomatic/presymptomatic, 0.5%; mild illness, 5.3%; moderate illness, 37.1%; severe illness, 55.5%; and critical illness, 1.1%.
The impact of the CCMC can be estimated by looking at care patterns over time. Since the work of the CCMC was aimed at influencing and standardizing physician ordering and therapy choices through order set creation and other forms of oversight, we measured the use of the CCMC-approved order sets at both hospitals and the use of certain laboratory tests and therapies that the CCMC sought either to limit or increase. These counts were adjusted for number of unique COVID-19 admissions. But the limits of the case collection tool meant it also collected cases that were not eligible for some of the interventions. For example, COVID-19 admissions without hypoxemia would not have been eligible for remdesivir or glucocorticoids. When admitted, some patients were already on steroids for other medical indications and did not receive the prescribed dexamethasone dose that we measured in pharmacy databases. Similarly, a few patients were hospitalized for indications unrelated to COVID-19, such as surgery or childbirth, and were found to be SARS-CoV-2-positive on routine screening.
Figure 2 shows the utilization of CCMC-approved standard COVID-19 admission order sets as a proportion of all COVID-19 admissions over time. The trend reveals a modest increase in usage (R2 = 0.34), but these data do not reflect the progressive build of content into order sets over time. One of the goals of the order sets was to standardize and reduce the ordering of certain biomarkers: C-reactive protein, serum ferritin, and D-dimer, which were ordered frequently in many early patients. Orders for these 3 laboratory tests are combined and expressed as an average number of labs per COVID-19 admission in Figure 2. A downward trend, with an R2 value of 0.65, is suggestive of impact from the order sets, though other explanations are possible.

Medication guidance was also a goal of the CCMC, simultaneously discouraging poorly supported interventions and driving uptake of the recommended evidence-based interventions in appropriate patients. Figure 3 shows the utilization pattern for some drugs of interest over the course of the pandemic, specifically the proportion of patients receiving at least 1 dose of medication among all COVID-19 admissions by month. (Data for hospital B was excluded from this analysis because it did not include all admitted patients.)

Hydroxychloroquine, which enjoyed a wave of popularity early on during the pandemic, was a target of successful order stewardship through the CCMC. Use of hydroxychloroquine as a COVID-19 therapeutic option after the first 2 months of the pandemic stopped, and subsequent use at low levels likely represented continuation therapy for outpatients who took hydroxychloroquine for rheumatologic indications.
Dexamethasone, as used in the RECOVERY trial,21 had a swift uptake among physicians after it was incorporated into order sets and its use encouraged. Similarly, uptake was immediate for remdesivir when, in May 2020, preliminary reports showed at least some benefits, confirmed by later analysis,22 and it received an FDA EUA.
Our data also show successful stewardship of the interleukin-6 antagonist toclilizumab, which was discouraged early on by the CCMC due to lack of data or negative results. But in March 2021, with new studies releasing data12,13 and new recommendations14 for its use in some subsets of patients with COVID-19, this drug was encouraged in appropriate subsets. A new order set with qualifying indications was prepared by the CCMC and new educational efforts made to encourage its use in appropriate patients.
Ivermectin was nonformulary at the start of the pandemic. This drug enjoyed much publicity from media sources and was promoted by certain physicians and on websites,23 based on in-vitro activity against coronaviruses. Eventually, the World Health Organization24 and the FDA25 found it necessary to issue advisory statements to the public against its use outside of clinical trials. The CCMC had requests from physicians to incorporate ivermectin but declined to add it to the formulary and recommended not approving nonformulary requests due to lack of data. As a result, ivermectin was not used at either hospital.
Discussion
COVID-19 represents many challenges to health systems all over the world. For Luminis Health, the high volume of acutely ill patients with novel syndromes was a particular challenge for the hospital-based care teams. A flood of information from preprints, press releases, preliminary reports, and many other nontraditional sources made deliberative management decisions difficult for individual physicians. Much commentary has appeared around the phenomenon but with less practical advice about how to make day-to-day care decisions at a time of scientific uncertainty and intense pressure to intervene.26,27 The CCMC was designed to overcome the information management dilemma. The need to coordinate, standardize, and oversee care was necessary given the large number of physicians who cared for COVID-19 patients on inpatient services.
It should be noted that creating order sets and issuing guidance is necessary, but not sufficient, to achieve our goals of being updated and consistent. This is especially true with large cadres of health care workers attending COVID-19 patients. Guidelines and recommendations were reinforced by unit-based oversight and stewardship from pharmacy and other leaders who constituted the CCMC.
The reduction in COVID-19 mortality over time experienced in this health care system was not unique and cannot necessarily be attributed to standardization of care. Similar improvements in mortality have been reported at many US hospitals in aggregate.28 Many other factors, including changes in patient characteristics, may be responsible for reduction in mortality over time.
Throughout this report we have relied upon an implicit assumption that standardization of medical therapeutics is desirable and leads to better outcomes as compared with allowing unlimited empiricism by individual physicians, either consultants or hospitalists. Our program represents a single health system with 2 acute care hospitals located 25 miles apart and which thus were similarly impacted by the different phases of the pandemic. Generalizability to health systems either smaller or larger, or in different geographical areas, has not been established. Data limitations have already been discussed.
We did not measure user satisfaction with the program either from physicians or nurses. However, the high rate of compliance suggests general agreement with the content and process.
We cannot definitively ascribe reduction in utilization of some nonrecommended treatments and increased utilization of the recommended therapies to the work of the CCMC. Individual physicians may have made these adjustments on their own or under the influence of other sources.
Finally, it should be noted that the mission to rapidly respond to data from well-conducted trials might be thwarted by too rigid a process or a committee’s lack of a sense of urgency. Organizing a committee and then empowering it to act is no guarantee of success; commitment to the mission is.
Conclusion
COVID-19 represented a challenge to medical staffs everywhere, inundating them with high volumes of acutely ill patients presenting with unfamiliar syndromes. Initial responses were characterized by idiosyncratic management approaches based on nontraditional sources of opinion and influences.
This report describes how a complex medical system brought order and standardization through a deliberative, but urgent, multidisciplinary committee with responsibility for planning, implementing, and monitoring standard approaches that eventually became evidence based. The composition of the committee and its scope of influence, limited to inpatient management, were important elements of success, allowing for better focus on the many treatment decisions. The important connection between the management committee and the system P&T committee, the clinical research effort, and teaching programs in both medicine and pharmacy are offered as exemplars of coordination. The data presented show success in achieving standardized, guideline-directed care. The approach is adoptable and suitable for similar emergencies in the future.
Acknowledgments: The authors thank Gary Scabis, Kip Waite, John Moxley, Angela Clubb, and David Woodley for their assistance in gathering data. We express appreciation and admiration for all our colleagues at the bedside.
Corresponding author: Barry R. Meisenberg, MD, Department of Medicine, Luminis Health, 2001 Medical Pkwy, Annapolis, MD 21401; [email protected].
Financial disclosures: None.
1. Gettleman J, Raj S, Kumar H. India’s health system cracks under the strain as coronavirus cases surge. The New York Times. April 22, 2021. https://www.nytimes.com/2021/04/21/world/asia/india-coronavirus-oxygen.html
2. Rappleye H, Lehren AW, Strickler L, Fitzpatrick S. ‘This system is doomed’: doctors, nurses sound off in NBC News coronavirus survey. NBC News. March 20, 2020. https://www.nbcnews.com/news/us-news/system-doomed-doctors-nurses-sound-nbc-news-coronavirus-survey-n1164841
3. Johns Hopkins Coronavirus Resource Center. Accessed January 5, 2022. https://coronavirus.jhu.edu/map.html
4. Fineberg HV. The toll of COVID-19. JAMA. 2020;324(15):1502-1503. doi:10.1001/jama.2020.20019
5. Meisenberg BR. Medical staffs response to COVID-19 ‘data’: have we misplaced our skeptic’s eye? Am J Med. 2021;134(2):151-152. doi:10.1016/j.amjmed.2020.09.013
6. McMahon JH, Lydeamore MH, Stewardson AJ. Bringing evidence from press release to the clinic in the era of COVID-19. J Antimicrob Chemother. 2021;76(3):547-549. doi:10.1093/jac/dkaa506
7. Rubin EJ, Baden LR, Morrissey S, Campion EW. Medical journals and the 2019-nCoV outbreak. N Engl J Med. 2020;382(9):866. doi:10.1056/NEJMe2001329
8. Liu F, Li L, Xu M, et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. 2020;127:104370. doi:10.1016/j.jcv.2020.104370
9. Vincent MJ, Bergeron E, Benjannet S, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi:10.1186/1743-422X-2-69
10. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269-271. doi:10.1038/s41422-020-0282-0
11. RECOVERY Collaborative Group. Effect of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;383:2030-2040. doi:10.1056/NEJMoa2022926
12. RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): preliminary results of a randomised, controlled, open-label, platform trial [preprint]. February 11, 2021. doi:10.1101/2021.02.11.21249258 https://www.medrxiv.org/content/10.1101/2021.02.11.21249258v1
13. REMAP-CAP Investigators. Interleukin-6 receptor antagonists in critically ill patients with COVID-19. N Engl J Med. 2021;384(16):1491-1502. doi:10.1056/NEJMoa2100433
14. National Institutes of Health. COVID-19 treatment guidelines: interleukin-6 inhibitors. https://www.covid19treatmentguidelines.nih.gov/immunomodulators/interleukin-6-inhibitors/
15. Deana C, Vetrugno L, Tonizzo A, et al. Drug supply during COVID-19 pandemic: remember not to run with your tank empty. Hosp Pharm. 2021;56(5):405-407. doi:10.1177/0018578720931749
16. Choe J, Crane M, Greene J, et al. The Pandemic and the Supply Chain: Addressing Gaps in Pharmaceutical Production and Distribution. Johns Hopkins University, November 2020. https://www.jhsph.edu/research/affiliated-programs/johns-hopkins-drug-access-and-affordability-initiative/publications/Pandemic_Supply_Chain.pdf
17. Kern DE. Overview: a six-step approach to curriculum development. In: Kern DE, Thornton PA, Hughes MT, eds. Curriculum Development for Medical Education: A Six-Step Approach. 3rd ed. Johns Hopkins University Press; 2016.
18. Rice TW, Janz DR. In defense of evidence-based medicine for the treatment of COVID-19 acute respiratory distress syndrome. Ann Am Thorac Soc. 2020;17(7):787-789. doi:10.1513/AnnalsATS.202004-325IP
19. Lucey CR, Johnston SC. The transformational effects of COVID-19 on medical education. JAMA. 2020;324(11):1033-1034. doi:10.1001/jama.2020.14136
20. National Institutes of Health. COVID-19 treatment guidelines: clinical spectrum of SARS-CoV-2 infection. https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/
21. RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693-704. doi:10.1056/NEJMoa2021436
22. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19—final report. N Engl J Med. 2020;383:1813-1826. doi:10.1056/NEJMoa2007764
23. Jiminez D. Ivermectin and Covid-19: how a cheap antiparasitic became political. April 19, 2021. https://www.pharmaceutical-technology.com/features/ivermectin-covid-19-antiparasitic-political/
24. World Health Organization. WHO advises that ivermectin only be used to treat COVID-19 within clinical trials. March 31, 2021. https://www.who.int/news-room/feature-stories/detail/who-advises-that-ivermectin-only-be-used-to-treat-covid-19-within-clinical-trials
25. U.S. Food and Drug Administration. Why you should not use ivermectin to treat or prevent COVID-19. March 5, 2021. https://www.fda.gov/consumers/consumer-updates/why-you-should-not-use-ivermectin-treat-or-prevent-covid-19
26. Seymour CW, McCreary EK, Stegenga J. Sensible medicine-balancing intervention and inaction during the COVID-19 pandemic. JAMA. 2020;324(18):1827-1828. doi:10.1001/jama.2020.20271
27. Flanagin A, Fontanarosa PB, Bauchner H. Preprints involving medical research—do the benefits outweigh the challenges? JAMA. 2020;324(18):1840-1843. doi:10.1001/jama.2020.20674
28. Asch DA, Shells NE, Islam N, et al. Variation in US hospital mortality rates for patients admitted with COVID-19 during the first 6 months of the pandemic. JAMA Intern Med. 2021;181(4):471-478. doi:10.1001/jamainternmed.2020.8193
1. Gettleman J, Raj S, Kumar H. India’s health system cracks under the strain as coronavirus cases surge. The New York Times. April 22, 2021. https://www.nytimes.com/2021/04/21/world/asia/india-coronavirus-oxygen.html
2. Rappleye H, Lehren AW, Strickler L, Fitzpatrick S. ‘This system is doomed’: doctors, nurses sound off in NBC News coronavirus survey. NBC News. March 20, 2020. https://www.nbcnews.com/news/us-news/system-doomed-doctors-nurses-sound-nbc-news-coronavirus-survey-n1164841
3. Johns Hopkins Coronavirus Resource Center. Accessed January 5, 2022. https://coronavirus.jhu.edu/map.html
4. Fineberg HV. The toll of COVID-19. JAMA. 2020;324(15):1502-1503. doi:10.1001/jama.2020.20019
5. Meisenberg BR. Medical staffs response to COVID-19 ‘data’: have we misplaced our skeptic’s eye? Am J Med. 2021;134(2):151-152. doi:10.1016/j.amjmed.2020.09.013
6. McMahon JH, Lydeamore MH, Stewardson AJ. Bringing evidence from press release to the clinic in the era of COVID-19. J Antimicrob Chemother. 2021;76(3):547-549. doi:10.1093/jac/dkaa506
7. Rubin EJ, Baden LR, Morrissey S, Campion EW. Medical journals and the 2019-nCoV outbreak. N Engl J Med. 2020;382(9):866. doi:10.1056/NEJMe2001329
8. Liu F, Li L, Xu M, et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. 2020;127:104370. doi:10.1016/j.jcv.2020.104370
9. Vincent MJ, Bergeron E, Benjannet S, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi:10.1186/1743-422X-2-69
10. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269-271. doi:10.1038/s41422-020-0282-0
11. RECOVERY Collaborative Group. Effect of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;383:2030-2040. doi:10.1056/NEJMoa2022926
12. RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): preliminary results of a randomised, controlled, open-label, platform trial [preprint]. February 11, 2021. doi:10.1101/2021.02.11.21249258 https://www.medrxiv.org/content/10.1101/2021.02.11.21249258v1
13. REMAP-CAP Investigators. Interleukin-6 receptor antagonists in critically ill patients with COVID-19. N Engl J Med. 2021;384(16):1491-1502. doi:10.1056/NEJMoa2100433
14. National Institutes of Health. COVID-19 treatment guidelines: interleukin-6 inhibitors. https://www.covid19treatmentguidelines.nih.gov/immunomodulators/interleukin-6-inhibitors/
15. Deana C, Vetrugno L, Tonizzo A, et al. Drug supply during COVID-19 pandemic: remember not to run with your tank empty. Hosp Pharm. 2021;56(5):405-407. doi:10.1177/0018578720931749
16. Choe J, Crane M, Greene J, et al. The Pandemic and the Supply Chain: Addressing Gaps in Pharmaceutical Production and Distribution. Johns Hopkins University, November 2020. https://www.jhsph.edu/research/affiliated-programs/johns-hopkins-drug-access-and-affordability-initiative/publications/Pandemic_Supply_Chain.pdf
17. Kern DE. Overview: a six-step approach to curriculum development. In: Kern DE, Thornton PA, Hughes MT, eds. Curriculum Development for Medical Education: A Six-Step Approach. 3rd ed. Johns Hopkins University Press; 2016.
18. Rice TW, Janz DR. In defense of evidence-based medicine for the treatment of COVID-19 acute respiratory distress syndrome. Ann Am Thorac Soc. 2020;17(7):787-789. doi:10.1513/AnnalsATS.202004-325IP
19. Lucey CR, Johnston SC. The transformational effects of COVID-19 on medical education. JAMA. 2020;324(11):1033-1034. doi:10.1001/jama.2020.14136
20. National Institutes of Health. COVID-19 treatment guidelines: clinical spectrum of SARS-CoV-2 infection. https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/
21. RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693-704. doi:10.1056/NEJMoa2021436
22. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19—final report. N Engl J Med. 2020;383:1813-1826. doi:10.1056/NEJMoa2007764
23. Jiminez D. Ivermectin and Covid-19: how a cheap antiparasitic became political. April 19, 2021. https://www.pharmaceutical-technology.com/features/ivermectin-covid-19-antiparasitic-political/
24. World Health Organization. WHO advises that ivermectin only be used to treat COVID-19 within clinical trials. March 31, 2021. https://www.who.int/news-room/feature-stories/detail/who-advises-that-ivermectin-only-be-used-to-treat-covid-19-within-clinical-trials
25. U.S. Food and Drug Administration. Why you should not use ivermectin to treat or prevent COVID-19. March 5, 2021. https://www.fda.gov/consumers/consumer-updates/why-you-should-not-use-ivermectin-treat-or-prevent-covid-19
26. Seymour CW, McCreary EK, Stegenga J. Sensible medicine-balancing intervention and inaction during the COVID-19 pandemic. JAMA. 2020;324(18):1827-1828. doi:10.1001/jama.2020.20271
27. Flanagin A, Fontanarosa PB, Bauchner H. Preprints involving medical research—do the benefits outweigh the challenges? JAMA. 2020;324(18):1840-1843. doi:10.1001/jama.2020.20674
28. Asch DA, Shells NE, Islam N, et al. Variation in US hospital mortality rates for patients admitted with COVID-19 during the first 6 months of the pandemic. JAMA Intern Med. 2021;181(4):471-478. doi:10.1001/jamainternmed.2020.8193
Ascites in a 42-year-old woman
A 42-year-old woman is admitted to the hospital with worsening shortness of breath on exertion, poor exercise tolerance, leg edema, and swelling of the abdomen. Her symptoms have been getting worse over the last 4 months. She reports no history of fever, chills, night sweats, bleeding disorder, joint pain, weight loss, or loss of appetite.
She has type 2 diabetes mellitus and hypothyroidism. She had rheumatoid arthritis but said it was “inactive,” not requiring treatment for the last 18 years. Three months ago, she underwent a total hysterectomy and salpingo-oophorectomy for a complex adnexal mass, biopsy of which revealed a benign mucinous ovarian cyst.
Her current medications include furosemide, levothyroxine, and metformin. She is an ex-smoker with a 7 pack-year history. She drinks a glass of wine on social occasions only. Her family history is unremarkable.
On examination, she is not in distress and she has no fever. She has jugular venous distention of 5 cm, tense ascites, and marked edema of the legs, as well as hyperpigmented patches and erythematous plaques over both shins. Neck palpation reveals no lymphadenopathy or thyromegaly.
Her liver and the tip of the spleen are palpable following paracentesis, once ascitic fluid is removed.
The cardiovascular examination is normal. Chest auscultation reveals decreased breath sounds at the right lung base with bibasilar crackles. No focal neurologic deficit is noted on clinical examination.
Laboratory testing at the time of hospital admission (Table 1) includes a hepatitis panel (negative for exposure to hepatitis A, B, and C) and ascitic fluid studies. Chest radiography shows a right pleural effusion. Echocardiography demonstrates moderate pericardial effusion without tamponade; left and right ventricular function is normal. Cardiac magnetic resonance imaging finds no evidence of pericardial constriction or restrictive cardiomyopathy. Pressures are normal on pulmonary artery catheterization.
FINDING THE CAUSE OF ASCITES
1. What is the most likely cause of ascites in this patient?
- Cirrhosis
- Recent abdominal surgery
- Congestive heart failure
- Abdominal malignancy
- Nephrotic syndrome
The serum-ascites albumin gradient—ie, the serum albumin concentration minus the ascitic fluid albumin concentration—helps determine whether ascites is related to portal hypertension.1 A high gradient (ie, above 1.1 g/dL) is seen in cirrhosis, alcoholic hepatitis, congestive heart failure, vascular occlusion syndromes (eg, Budd-Chiari syndrome), and metastatic liver disease.
From the values in Table 1, our patient’s gradient is 0.8 g/dL, which is considered low. However, we cannot completely rule out cirrhosis as the cause of her ascites because she was taking a diuretic, and diuretics can falsely decrease the gradient. Heart failure is unlikely, based on the results of echocardiography and catheterization. In addition, the 24-hour urinary protein concentration is normal, as is alpha-1 antitrypsin secretion in the stool, ruling out protein-losing nephropathy or enteropathy as the cause of her low albumin and ascites.
A high triglyceride content in her ascitic fluid (> 150 mg/dL) is consistent with chylous ascites, which is seen in patients with previous abdominal surgery or with lymphatic obstruction due to malignancy. A high neutrophil count in the ascitic fluid and a negative culture are also consistent with chylous ascites. However, in this patient, recent surgery as the cause of chylous ascites does not explain the systemic features of hepatosplenomegaly, anemia, thrombocytosis, and low albumin. Moreover, her high C-reactive protein value suggests an ongoing inflammatory process, although her erythrocyte sedimentation rate is not significantly elevated.
Therefore, the most likely cause of ascites in this patient is abdominal malignancy.
WHAT SHOULD BE DONE NEXT?
2. Which of the following studies is reasonable in this patient at this point?
- Serum protein electrophoresis
- Computed tomography (CT) of the chest, abdomen, and pelvis
- Liver biopsy
- Cytologic study of the ascitic fluid
All of these studies would be reasonable and in fact were done in this patient.
Serum protein electrophoresis (Table 2) identified a monoclonal protein band in the immunoglobulin G (IgG) kappa region.
Cytologic study of the ascitic fluid was negative for malignant cells.
Chest CT revealed bilateral pleural effusions, pericardial effusion, and bilateral axillary lymphadenopathy. CT of the abdomen and pelvis was normal, except for ascites, and no pelvic tumor was noted.
Liver biopsy was done to look for the source of her unexplained ascites with elevated alkaline phosphatase, as all other investigations so far were normal. It revealed mild centrilobular scarring, but the rest of the parenchymal architecture was normal, with no evidence of bridging fibrosis or nodular regenerative hyperplasia (Figure 1).
Transjugular measurement of the hepatic vein pressure revealed a hepatic vein pressure gradient of 9 mm Hg, indicating mild portal hypertension. Venography showed widely patent hepatic and portal veins. Her high inflammatory marker levels could have been caused by smoldering rheumatoid arthritis; however, since the patient has had no joint symptoms for 18 years, this is very unlikely. It is more likely to be caused by a plasma cell disorder, as suggested by a monoclonal protein on electrophoresis.
WHAT IS THE DIAGNOSIS?
3. What is the most likely diagnosis in our patient?
- Rheumatoid arthritis
- Cryoglobulinemia
- Capillary leak syndrome
- Hematologic malignancy
- Syndrome of polyneuropathy, organomegaly, endocrinopathy, monoclonal protein, and skin changes (POEMS syndrome)
Rheumatoid arthritis can present with hepatosplenomegaly, lymphadenopathy, ascites, and skin rash, particularly if antinuclear antibody and rheumatoid factor are elevated. Ascites is known to occur in association with rheumatoid arthritis in the setting of Felty syndrome or nodular regenerative hyperplasia of the liver.2 However, our patient did not have leukopenia or evidence of regenerative hyperplasia on liver biopsy. Moreover, her rheumatoid arthritis had remained clinically inactive for a long time.
Cryoglobulinemia was possible, given her ascites, neuropathy, and splenomegaly, but her serum hepatic antibody and C4 complement values were normal.3 Also, the appearance of her rash was not typical of cryoglobulinemia.
Capillary leak syndrome was ruled out by the absence of hypotensive episodes, edema of the face or upper extremities, or renal failure.4
Lymphoma was excluded by flow cytometry.
A monoclonal protein on serum electrophoresis may suggest multiple myeloma, but this patient had multisystem involvement including organomegaly, endocrinopathy, and skin abnormalities. Thus, POEMS syndrome is the most likely diagnosis.
4. Which test should be done at this time to confirm the diagnosis of POEMS syndrome?
- Bone marrow biopsy
- Vascular endothelial growth factor testing
- Nerve conduction study
- Complete x-ray bone survey
A test for vascular endothelial growth factor should be done. This growth factor is almost always elevated in POEMS, and a positive test helps confirm the diagnosis of POEMS. Our patient’s level was elevated at 1,664 pg/mL (reference range 31–86).
POEMS is thought to be a variant of plasma cell dyscrasia, and all patients with POEMS have a monoclonal protein on electrophoresis. On this background, multiple myeloma is an important consideration.
Our patient underwent bone marrow biopsy, which revealed mild plasmacytosis (< 10%) (Figure 2). A complete bone survey showed generalized osteopenia without blastic or lytic lesions. To complete the workup for POEMS syndrome, a nerve conduction study was done to look for neuropathy; it showed bilateral sensory motor neuropathy with features of both a demyelinating process and axonal loss.
POEMS SYNDROME
POEMS syndrome is a constellation of features such as organomegaly and endocrine and skin abnormalities in association with neuropathy and a monoclonal protein on electrophoresis.5 In 2003, Dispenzieri et al6 described the major and minor diagnostic criteria based on a retrospective analysis of 99 patients with POEMS syndrome.6 Later, elevated vascular endothelial growth factor was added as a confirmatory diagnostic criterion.7 This growth factor is also an indicator of prognosis in POEMS syndrome, and its level can be used to monitor the response to treatment.7
Our patient met both major criteria for POEMS syndrome, ie, polyneuropathy (based on nerve conduction studies) and a monoclonal protein. Polyneuropathy in POEMS syndrome usually occurs as sensorimotor peripheral neuropathy of insidious onset and is seldom painful. Nerve biopsy study reveals demyelination with features of axonal loss. Interestingly, although our patient had neuropathy as diagnosed by electromyography, she remained clinically asymptomatic.
The monoclonal protein in POEMS syndrome is commonly IgA or IgG. Light chains are always present and are mainly the lambda type; kappa light chains are also reported in rare cases. Our patient had IgG kappa light chains.
Our patient met a number of the minor criteria for POEMS syndrome: ie, organomegaly (hepatosplenomegaly, lymphadenopathy), endocrinopathy (hypothyroidism, diabetes), skin changes (hyperpigmentation and plaques of the lower extremities), edema, pleural effusion, and ascites.
Endocrine disorders in POEMS syndrome
The endocrine abnormalities most often described in POEMS syndrome are hypogonadism, hypothyroidism, and diabetes mellitus. But because hypothyroidism and diabetes are common in the general population, it is debatable whether either of these could constitute the endocrine component of POEMS syndrome. Nevertheless, in three large series,6,7 occurrences of these two disorders were common, although less specific than adrenal or pituitary involvement.
In the analysis by Dispenzieri et al,6 67% of patients had at least one endocrine abnormality. Our patient had no evidence of an adrenal disorder.
Skin, skeletal, and other changes
The skin changes in POEMS syndrome are often nonspecific and include hyperpigmentation, sclerodema-like thickening, and plaques.
Skeletal changes are noted in up to 97% of patients. A skeletal survey in our patient revealed generalized osteopenia as opposed to osteosclerotic lesions, which are common in POEMS syndrome.
Anemia and thrombocytosis (as in our patient) are usually seen in POEMS syndrome and are induced by cytokines.6 POEMS syndrome also leads to increased thrombotic complications from the release of inflammatory cytokines.
Hypoalbuminemia and anasarca including ascites are often seen in POEMS syndrome (prevalence 29% to 89%) and are attributed to cytokine-induced increased vascular permeability. In POEMS syndrome, the serum-ascites albumin gradient is usually less than 1.1 g/dL, as in our patient.
Stepani et al8 reported one case of culture-negative neutrocytic ascites with portal hypertension in POEMS syndrome.8 (Culture-negative neutrocytic ascites is defined as an ascitic fluid polymorphonuclear count greater than 250/mm3 and a negative ascitic fluid culture in the absence of previous antibiotic therapy.) Chylous ascites has not yet been described in POEMS syndrome. However, chylous ascites is predominantly lymphocytic, whereas our patient had neutrocytic ascites.
We concluded that the cause of our patient’s ascites was multifactorial and included previous surgery and POEMS syndrome.
Nonclassic presentation
In addition to its classic presentation, POEMS syndrome is often reported in association with other “unusual features” such as cardiomyopathy, pulmonary hypertension, and cryoglobulinemia.6
So far, very few cases of portal hypertension in POEMS syndrome have been reported. Stepani et al8 described a patient who had POEMS syndrome and portal hypertension with extensive portal fibrosis without cirrhosis on liver biopsy. Inoue et al9 reported a liver biopsy feature consistent with idiopathic portal hypertension, also noting a case with mild fibrosis and few lymphocytic infiltrates in the portal tract.9
The etiopathogenesis of POEMS syndrome is attributed to proangiogenic vascular endothelial growth factor, and other inflammatory cytokines (interleukin 6, interleukin 1 beta, tumor necrosis factor alpha) also play a key role in pulmonary hypertension.10,11 A similar pathogenesis could also contribute to the development of portal hypertension (Figure 3).
CASE CONCLUDED
We started our patient on oral prednisone 60 mg daily for a month, tapered to a maintenance dose of 15 mg to suppress clonal proliferation of plasma cells. Her symptoms improved. Her vascular endothelial growth factor level decreased from 1,664 to 624 pg/mL. She was enrolled in a National Institutes of Health study to evaluate the effect of a potential new immunomodulator treatment for POEMS syndrome.
In conclusion, POEMS syndrome is rare and can present with many atypical features. A high index of suspicion is needed to detect it in a patient who has noncirrhotic portal hypertension with ascites and multisystem involvement.
- Runyon BA, Montano AA, Akriviadis EA, Antillon MR, Irving MA, McHutchison JG. The serum-ascites albumin gradient is superior to the exudate-transudate concept in the differential diagnosis of ascites. Ann Intern Med 1992; 117:215–220.
- Harris M, Rash RM, Dymock IW. Nodular, non-cirrhotic liver associated with portal hypertension in a patient with rheumatoid arthritis. J Clin Pathol 1974; 27:963–966.
- Ramos-Casals M, Stone JH, Cid MC, Bosch X. The cryoglobulinaemias. Lancet 2012; 379:348–360.
- Druey KM, Greipp PR. Narrative review: the systemic capillary leak syndrome. Ann Intern Med 2010; 153:90–98.
- Bardwick PA, Zvaifler NJ, Gill GN, Newman D, Greenway GD, Resnick DL. Plasma cell dyscrasia with polyneuropathy, organomegaly, endocrinopathy, M protein, and skin changes: the POEMS syndrome. Report on two cases and a review of the literature. Medicine (Baltimore) 1980; 59:311–322.
- Dispenzieri A, Kyle RA, Lacy MQ, et al. POEMS syndrome: definitions and long-term outcome. Blood 2003; 101:2496–2506.
- Dispenzieri A. POEMS syndrome. Blood Rev 2007; 21:285–299.
- Stepani P, Courouble Y, Postel P, et al. Portal hypertension and neutrocytic ascites in POEMS syndrome. Gastroenterol Clin Biol 1998; 22:1095–1097. Article in French.
- Inoue R, Nakazawa A, Tsukada N, et al. POEMS syndrome with idiopathic portal hypertension: autopsy case and review of the literature. Pathol Int 2010; 60:316–320.
- Gherardi RK, Bélec L, Soubrier M, et al. Overproduction of proinflammatory cytokines imbalanced by their antagonists in POEMS syndrome. Blood 1996; 87:1458–1465.
- Mukerjee D, Kingdon E, Vanderpump M, Coghlan JG. Pathophysiological insights from a case of reversible pulmonary arterial hypertension. J R Soc Med 2003; 96:403–404.
A 42-year-old woman is admitted to the hospital with worsening shortness of breath on exertion, poor exercise tolerance, leg edema, and swelling of the abdomen. Her symptoms have been getting worse over the last 4 months. She reports no history of fever, chills, night sweats, bleeding disorder, joint pain, weight loss, or loss of appetite.
She has type 2 diabetes mellitus and hypothyroidism. She had rheumatoid arthritis but said it was “inactive,” not requiring treatment for the last 18 years. Three months ago, she underwent a total hysterectomy and salpingo-oophorectomy for a complex adnexal mass, biopsy of which revealed a benign mucinous ovarian cyst.
Her current medications include furosemide, levothyroxine, and metformin. She is an ex-smoker with a 7 pack-year history. She drinks a glass of wine on social occasions only. Her family history is unremarkable.
On examination, she is not in distress and she has no fever. She has jugular venous distention of 5 cm, tense ascites, and marked edema of the legs, as well as hyperpigmented patches and erythematous plaques over both shins. Neck palpation reveals no lymphadenopathy or thyromegaly.
Her liver and the tip of the spleen are palpable following paracentesis, once ascitic fluid is removed.
The cardiovascular examination is normal. Chest auscultation reveals decreased breath sounds at the right lung base with bibasilar crackles. No focal neurologic deficit is noted on clinical examination.
Laboratory testing at the time of hospital admission (Table 1) includes a hepatitis panel (negative for exposure to hepatitis A, B, and C) and ascitic fluid studies. Chest radiography shows a right pleural effusion. Echocardiography demonstrates moderate pericardial effusion without tamponade; left and right ventricular function is normal. Cardiac magnetic resonance imaging finds no evidence of pericardial constriction or restrictive cardiomyopathy. Pressures are normal on pulmonary artery catheterization.
FINDING THE CAUSE OF ASCITES
1. What is the most likely cause of ascites in this patient?
- Cirrhosis
- Recent abdominal surgery
- Congestive heart failure
- Abdominal malignancy
- Nephrotic syndrome
The serum-ascites albumin gradient—ie, the serum albumin concentration minus the ascitic fluid albumin concentration—helps determine whether ascites is related to portal hypertension.1 A high gradient (ie, above 1.1 g/dL) is seen in cirrhosis, alcoholic hepatitis, congestive heart failure, vascular occlusion syndromes (eg, Budd-Chiari syndrome), and metastatic liver disease.
From the values in Table 1, our patient’s gradient is 0.8 g/dL, which is considered low. However, we cannot completely rule out cirrhosis as the cause of her ascites because she was taking a diuretic, and diuretics can falsely decrease the gradient. Heart failure is unlikely, based on the results of echocardiography and catheterization. In addition, the 24-hour urinary protein concentration is normal, as is alpha-1 antitrypsin secretion in the stool, ruling out protein-losing nephropathy or enteropathy as the cause of her low albumin and ascites.
A high triglyceride content in her ascitic fluid (> 150 mg/dL) is consistent with chylous ascites, which is seen in patients with previous abdominal surgery or with lymphatic obstruction due to malignancy. A high neutrophil count in the ascitic fluid and a negative culture are also consistent with chylous ascites. However, in this patient, recent surgery as the cause of chylous ascites does not explain the systemic features of hepatosplenomegaly, anemia, thrombocytosis, and low albumin. Moreover, her high C-reactive protein value suggests an ongoing inflammatory process, although her erythrocyte sedimentation rate is not significantly elevated.
Therefore, the most likely cause of ascites in this patient is abdominal malignancy.
WHAT SHOULD BE DONE NEXT?
2. Which of the following studies is reasonable in this patient at this point?
- Serum protein electrophoresis
- Computed tomography (CT) of the chest, abdomen, and pelvis
- Liver biopsy
- Cytologic study of the ascitic fluid
All of these studies would be reasonable and in fact were done in this patient.
Serum protein electrophoresis (Table 2) identified a monoclonal protein band in the immunoglobulin G (IgG) kappa region.
Cytologic study of the ascitic fluid was negative for malignant cells.
Chest CT revealed bilateral pleural effusions, pericardial effusion, and bilateral axillary lymphadenopathy. CT of the abdomen and pelvis was normal, except for ascites, and no pelvic tumor was noted.
Liver biopsy was done to look for the source of her unexplained ascites with elevated alkaline phosphatase, as all other investigations so far were normal. It revealed mild centrilobular scarring, but the rest of the parenchymal architecture was normal, with no evidence of bridging fibrosis or nodular regenerative hyperplasia (Figure 1).
Transjugular measurement of the hepatic vein pressure revealed a hepatic vein pressure gradient of 9 mm Hg, indicating mild portal hypertension. Venography showed widely patent hepatic and portal veins. Her high inflammatory marker levels could have been caused by smoldering rheumatoid arthritis; however, since the patient has had no joint symptoms for 18 years, this is very unlikely. It is more likely to be caused by a plasma cell disorder, as suggested by a monoclonal protein on electrophoresis.
WHAT IS THE DIAGNOSIS?
3. What is the most likely diagnosis in our patient?
- Rheumatoid arthritis
- Cryoglobulinemia
- Capillary leak syndrome
- Hematologic malignancy
- Syndrome of polyneuropathy, organomegaly, endocrinopathy, monoclonal protein, and skin changes (POEMS syndrome)
Rheumatoid arthritis can present with hepatosplenomegaly, lymphadenopathy, ascites, and skin rash, particularly if antinuclear antibody and rheumatoid factor are elevated. Ascites is known to occur in association with rheumatoid arthritis in the setting of Felty syndrome or nodular regenerative hyperplasia of the liver.2 However, our patient did not have leukopenia or evidence of regenerative hyperplasia on liver biopsy. Moreover, her rheumatoid arthritis had remained clinically inactive for a long time.
Cryoglobulinemia was possible, given her ascites, neuropathy, and splenomegaly, but her serum hepatic antibody and C4 complement values were normal.3 Also, the appearance of her rash was not typical of cryoglobulinemia.
Capillary leak syndrome was ruled out by the absence of hypotensive episodes, edema of the face or upper extremities, or renal failure.4
Lymphoma was excluded by flow cytometry.
A monoclonal protein on serum electrophoresis may suggest multiple myeloma, but this patient had multisystem involvement including organomegaly, endocrinopathy, and skin abnormalities. Thus, POEMS syndrome is the most likely diagnosis.
4. Which test should be done at this time to confirm the diagnosis of POEMS syndrome?
- Bone marrow biopsy
- Vascular endothelial growth factor testing
- Nerve conduction study
- Complete x-ray bone survey
A test for vascular endothelial growth factor should be done. This growth factor is almost always elevated in POEMS, and a positive test helps confirm the diagnosis of POEMS. Our patient’s level was elevated at 1,664 pg/mL (reference range 31–86).
POEMS is thought to be a variant of plasma cell dyscrasia, and all patients with POEMS have a monoclonal protein on electrophoresis. On this background, multiple myeloma is an important consideration.
Our patient underwent bone marrow biopsy, which revealed mild plasmacytosis (< 10%) (Figure 2). A complete bone survey showed generalized osteopenia without blastic or lytic lesions. To complete the workup for POEMS syndrome, a nerve conduction study was done to look for neuropathy; it showed bilateral sensory motor neuropathy with features of both a demyelinating process and axonal loss.
POEMS SYNDROME
POEMS syndrome is a constellation of features such as organomegaly and endocrine and skin abnormalities in association with neuropathy and a monoclonal protein on electrophoresis.5 In 2003, Dispenzieri et al6 described the major and minor diagnostic criteria based on a retrospective analysis of 99 patients with POEMS syndrome.6 Later, elevated vascular endothelial growth factor was added as a confirmatory diagnostic criterion.7 This growth factor is also an indicator of prognosis in POEMS syndrome, and its level can be used to monitor the response to treatment.7
Our patient met both major criteria for POEMS syndrome, ie, polyneuropathy (based on nerve conduction studies) and a monoclonal protein. Polyneuropathy in POEMS syndrome usually occurs as sensorimotor peripheral neuropathy of insidious onset and is seldom painful. Nerve biopsy study reveals demyelination with features of axonal loss. Interestingly, although our patient had neuropathy as diagnosed by electromyography, she remained clinically asymptomatic.
The monoclonal protein in POEMS syndrome is commonly IgA or IgG. Light chains are always present and are mainly the lambda type; kappa light chains are also reported in rare cases. Our patient had IgG kappa light chains.
Our patient met a number of the minor criteria for POEMS syndrome: ie, organomegaly (hepatosplenomegaly, lymphadenopathy), endocrinopathy (hypothyroidism, diabetes), skin changes (hyperpigmentation and plaques of the lower extremities), edema, pleural effusion, and ascites.
Endocrine disorders in POEMS syndrome
The endocrine abnormalities most often described in POEMS syndrome are hypogonadism, hypothyroidism, and diabetes mellitus. But because hypothyroidism and diabetes are common in the general population, it is debatable whether either of these could constitute the endocrine component of POEMS syndrome. Nevertheless, in three large series,6,7 occurrences of these two disorders were common, although less specific than adrenal or pituitary involvement.
In the analysis by Dispenzieri et al,6 67% of patients had at least one endocrine abnormality. Our patient had no evidence of an adrenal disorder.
Skin, skeletal, and other changes
The skin changes in POEMS syndrome are often nonspecific and include hyperpigmentation, sclerodema-like thickening, and plaques.
Skeletal changes are noted in up to 97% of patients. A skeletal survey in our patient revealed generalized osteopenia as opposed to osteosclerotic lesions, which are common in POEMS syndrome.
Anemia and thrombocytosis (as in our patient) are usually seen in POEMS syndrome and are induced by cytokines.6 POEMS syndrome also leads to increased thrombotic complications from the release of inflammatory cytokines.
Hypoalbuminemia and anasarca including ascites are often seen in POEMS syndrome (prevalence 29% to 89%) and are attributed to cytokine-induced increased vascular permeability. In POEMS syndrome, the serum-ascites albumin gradient is usually less than 1.1 g/dL, as in our patient.
Stepani et al8 reported one case of culture-negative neutrocytic ascites with portal hypertension in POEMS syndrome.8 (Culture-negative neutrocytic ascites is defined as an ascitic fluid polymorphonuclear count greater than 250/mm3 and a negative ascitic fluid culture in the absence of previous antibiotic therapy.) Chylous ascites has not yet been described in POEMS syndrome. However, chylous ascites is predominantly lymphocytic, whereas our patient had neutrocytic ascites.
We concluded that the cause of our patient’s ascites was multifactorial and included previous surgery and POEMS syndrome.
Nonclassic presentation
In addition to its classic presentation, POEMS syndrome is often reported in association with other “unusual features” such as cardiomyopathy, pulmonary hypertension, and cryoglobulinemia.6
So far, very few cases of portal hypertension in POEMS syndrome have been reported. Stepani et al8 described a patient who had POEMS syndrome and portal hypertension with extensive portal fibrosis without cirrhosis on liver biopsy. Inoue et al9 reported a liver biopsy feature consistent with idiopathic portal hypertension, also noting a case with mild fibrosis and few lymphocytic infiltrates in the portal tract.9
The etiopathogenesis of POEMS syndrome is attributed to proangiogenic vascular endothelial growth factor, and other inflammatory cytokines (interleukin 6, interleukin 1 beta, tumor necrosis factor alpha) also play a key role in pulmonary hypertension.10,11 A similar pathogenesis could also contribute to the development of portal hypertension (Figure 3).
CASE CONCLUDED
We started our patient on oral prednisone 60 mg daily for a month, tapered to a maintenance dose of 15 mg to suppress clonal proliferation of plasma cells. Her symptoms improved. Her vascular endothelial growth factor level decreased from 1,664 to 624 pg/mL. She was enrolled in a National Institutes of Health study to evaluate the effect of a potential new immunomodulator treatment for POEMS syndrome.
In conclusion, POEMS syndrome is rare and can present with many atypical features. A high index of suspicion is needed to detect it in a patient who has noncirrhotic portal hypertension with ascites and multisystem involvement.
A 42-year-old woman is admitted to the hospital with worsening shortness of breath on exertion, poor exercise tolerance, leg edema, and swelling of the abdomen. Her symptoms have been getting worse over the last 4 months. She reports no history of fever, chills, night sweats, bleeding disorder, joint pain, weight loss, or loss of appetite.
She has type 2 diabetes mellitus and hypothyroidism. She had rheumatoid arthritis but said it was “inactive,” not requiring treatment for the last 18 years. Three months ago, she underwent a total hysterectomy and salpingo-oophorectomy for a complex adnexal mass, biopsy of which revealed a benign mucinous ovarian cyst.
Her current medications include furosemide, levothyroxine, and metformin. She is an ex-smoker with a 7 pack-year history. She drinks a glass of wine on social occasions only. Her family history is unremarkable.
On examination, she is not in distress and she has no fever. She has jugular venous distention of 5 cm, tense ascites, and marked edema of the legs, as well as hyperpigmented patches and erythematous plaques over both shins. Neck palpation reveals no lymphadenopathy or thyromegaly.
Her liver and the tip of the spleen are palpable following paracentesis, once ascitic fluid is removed.
The cardiovascular examination is normal. Chest auscultation reveals decreased breath sounds at the right lung base with bibasilar crackles. No focal neurologic deficit is noted on clinical examination.
Laboratory testing at the time of hospital admission (Table 1) includes a hepatitis panel (negative for exposure to hepatitis A, B, and C) and ascitic fluid studies. Chest radiography shows a right pleural effusion. Echocardiography demonstrates moderate pericardial effusion without tamponade; left and right ventricular function is normal. Cardiac magnetic resonance imaging finds no evidence of pericardial constriction or restrictive cardiomyopathy. Pressures are normal on pulmonary artery catheterization.
FINDING THE CAUSE OF ASCITES
1. What is the most likely cause of ascites in this patient?
- Cirrhosis
- Recent abdominal surgery
- Congestive heart failure
- Abdominal malignancy
- Nephrotic syndrome
The serum-ascites albumin gradient—ie, the serum albumin concentration minus the ascitic fluid albumin concentration—helps determine whether ascites is related to portal hypertension.1 A high gradient (ie, above 1.1 g/dL) is seen in cirrhosis, alcoholic hepatitis, congestive heart failure, vascular occlusion syndromes (eg, Budd-Chiari syndrome), and metastatic liver disease.
From the values in Table 1, our patient’s gradient is 0.8 g/dL, which is considered low. However, we cannot completely rule out cirrhosis as the cause of her ascites because she was taking a diuretic, and diuretics can falsely decrease the gradient. Heart failure is unlikely, based on the results of echocardiography and catheterization. In addition, the 24-hour urinary protein concentration is normal, as is alpha-1 antitrypsin secretion in the stool, ruling out protein-losing nephropathy or enteropathy as the cause of her low albumin and ascites.
A high triglyceride content in her ascitic fluid (> 150 mg/dL) is consistent with chylous ascites, which is seen in patients with previous abdominal surgery or with lymphatic obstruction due to malignancy. A high neutrophil count in the ascitic fluid and a negative culture are also consistent with chylous ascites. However, in this patient, recent surgery as the cause of chylous ascites does not explain the systemic features of hepatosplenomegaly, anemia, thrombocytosis, and low albumin. Moreover, her high C-reactive protein value suggests an ongoing inflammatory process, although her erythrocyte sedimentation rate is not significantly elevated.
Therefore, the most likely cause of ascites in this patient is abdominal malignancy.
WHAT SHOULD BE DONE NEXT?
2. Which of the following studies is reasonable in this patient at this point?
- Serum protein electrophoresis
- Computed tomography (CT) of the chest, abdomen, and pelvis
- Liver biopsy
- Cytologic study of the ascitic fluid
All of these studies would be reasonable and in fact were done in this patient.
Serum protein electrophoresis (Table 2) identified a monoclonal protein band in the immunoglobulin G (IgG) kappa region.
Cytologic study of the ascitic fluid was negative for malignant cells.
Chest CT revealed bilateral pleural effusions, pericardial effusion, and bilateral axillary lymphadenopathy. CT of the abdomen and pelvis was normal, except for ascites, and no pelvic tumor was noted.
Liver biopsy was done to look for the source of her unexplained ascites with elevated alkaline phosphatase, as all other investigations so far were normal. It revealed mild centrilobular scarring, but the rest of the parenchymal architecture was normal, with no evidence of bridging fibrosis or nodular regenerative hyperplasia (Figure 1).
Transjugular measurement of the hepatic vein pressure revealed a hepatic vein pressure gradient of 9 mm Hg, indicating mild portal hypertension. Venography showed widely patent hepatic and portal veins. Her high inflammatory marker levels could have been caused by smoldering rheumatoid arthritis; however, since the patient has had no joint symptoms for 18 years, this is very unlikely. It is more likely to be caused by a plasma cell disorder, as suggested by a monoclonal protein on electrophoresis.
WHAT IS THE DIAGNOSIS?
3. What is the most likely diagnosis in our patient?
- Rheumatoid arthritis
- Cryoglobulinemia
- Capillary leak syndrome
- Hematologic malignancy
- Syndrome of polyneuropathy, organomegaly, endocrinopathy, monoclonal protein, and skin changes (POEMS syndrome)
Rheumatoid arthritis can present with hepatosplenomegaly, lymphadenopathy, ascites, and skin rash, particularly if antinuclear antibody and rheumatoid factor are elevated. Ascites is known to occur in association with rheumatoid arthritis in the setting of Felty syndrome or nodular regenerative hyperplasia of the liver.2 However, our patient did not have leukopenia or evidence of regenerative hyperplasia on liver biopsy. Moreover, her rheumatoid arthritis had remained clinically inactive for a long time.
Cryoglobulinemia was possible, given her ascites, neuropathy, and splenomegaly, but her serum hepatic antibody and C4 complement values were normal.3 Also, the appearance of her rash was not typical of cryoglobulinemia.
Capillary leak syndrome was ruled out by the absence of hypotensive episodes, edema of the face or upper extremities, or renal failure.4
Lymphoma was excluded by flow cytometry.
A monoclonal protein on serum electrophoresis may suggest multiple myeloma, but this patient had multisystem involvement including organomegaly, endocrinopathy, and skin abnormalities. Thus, POEMS syndrome is the most likely diagnosis.
4. Which test should be done at this time to confirm the diagnosis of POEMS syndrome?
- Bone marrow biopsy
- Vascular endothelial growth factor testing
- Nerve conduction study
- Complete x-ray bone survey
A test for vascular endothelial growth factor should be done. This growth factor is almost always elevated in POEMS, and a positive test helps confirm the diagnosis of POEMS. Our patient’s level was elevated at 1,664 pg/mL (reference range 31–86).
POEMS is thought to be a variant of plasma cell dyscrasia, and all patients with POEMS have a monoclonal protein on electrophoresis. On this background, multiple myeloma is an important consideration.
Our patient underwent bone marrow biopsy, which revealed mild plasmacytosis (< 10%) (Figure 2). A complete bone survey showed generalized osteopenia without blastic or lytic lesions. To complete the workup for POEMS syndrome, a nerve conduction study was done to look for neuropathy; it showed bilateral sensory motor neuropathy with features of both a demyelinating process and axonal loss.
POEMS SYNDROME
POEMS syndrome is a constellation of features such as organomegaly and endocrine and skin abnormalities in association with neuropathy and a monoclonal protein on electrophoresis.5 In 2003, Dispenzieri et al6 described the major and minor diagnostic criteria based on a retrospective analysis of 99 patients with POEMS syndrome.6 Later, elevated vascular endothelial growth factor was added as a confirmatory diagnostic criterion.7 This growth factor is also an indicator of prognosis in POEMS syndrome, and its level can be used to monitor the response to treatment.7
Our patient met both major criteria for POEMS syndrome, ie, polyneuropathy (based on nerve conduction studies) and a monoclonal protein. Polyneuropathy in POEMS syndrome usually occurs as sensorimotor peripheral neuropathy of insidious onset and is seldom painful. Nerve biopsy study reveals demyelination with features of axonal loss. Interestingly, although our patient had neuropathy as diagnosed by electromyography, she remained clinically asymptomatic.
The monoclonal protein in POEMS syndrome is commonly IgA or IgG. Light chains are always present and are mainly the lambda type; kappa light chains are also reported in rare cases. Our patient had IgG kappa light chains.
Our patient met a number of the minor criteria for POEMS syndrome: ie, organomegaly (hepatosplenomegaly, lymphadenopathy), endocrinopathy (hypothyroidism, diabetes), skin changes (hyperpigmentation and plaques of the lower extremities), edema, pleural effusion, and ascites.
Endocrine disorders in POEMS syndrome
The endocrine abnormalities most often described in POEMS syndrome are hypogonadism, hypothyroidism, and diabetes mellitus. But because hypothyroidism and diabetes are common in the general population, it is debatable whether either of these could constitute the endocrine component of POEMS syndrome. Nevertheless, in three large series,6,7 occurrences of these two disorders were common, although less specific than adrenal or pituitary involvement.
In the analysis by Dispenzieri et al,6 67% of patients had at least one endocrine abnormality. Our patient had no evidence of an adrenal disorder.
Skin, skeletal, and other changes
The skin changes in POEMS syndrome are often nonspecific and include hyperpigmentation, sclerodema-like thickening, and plaques.
Skeletal changes are noted in up to 97% of patients. A skeletal survey in our patient revealed generalized osteopenia as opposed to osteosclerotic lesions, which are common in POEMS syndrome.
Anemia and thrombocytosis (as in our patient) are usually seen in POEMS syndrome and are induced by cytokines.6 POEMS syndrome also leads to increased thrombotic complications from the release of inflammatory cytokines.
Hypoalbuminemia and anasarca including ascites are often seen in POEMS syndrome (prevalence 29% to 89%) and are attributed to cytokine-induced increased vascular permeability. In POEMS syndrome, the serum-ascites albumin gradient is usually less than 1.1 g/dL, as in our patient.
Stepani et al8 reported one case of culture-negative neutrocytic ascites with portal hypertension in POEMS syndrome.8 (Culture-negative neutrocytic ascites is defined as an ascitic fluid polymorphonuclear count greater than 250/mm3 and a negative ascitic fluid culture in the absence of previous antibiotic therapy.) Chylous ascites has not yet been described in POEMS syndrome. However, chylous ascites is predominantly lymphocytic, whereas our patient had neutrocytic ascites.
We concluded that the cause of our patient’s ascites was multifactorial and included previous surgery and POEMS syndrome.
Nonclassic presentation
In addition to its classic presentation, POEMS syndrome is often reported in association with other “unusual features” such as cardiomyopathy, pulmonary hypertension, and cryoglobulinemia.6
So far, very few cases of portal hypertension in POEMS syndrome have been reported. Stepani et al8 described a patient who had POEMS syndrome and portal hypertension with extensive portal fibrosis without cirrhosis on liver biopsy. Inoue et al9 reported a liver biopsy feature consistent with idiopathic portal hypertension, also noting a case with mild fibrosis and few lymphocytic infiltrates in the portal tract.9
The etiopathogenesis of POEMS syndrome is attributed to proangiogenic vascular endothelial growth factor, and other inflammatory cytokines (interleukin 6, interleukin 1 beta, tumor necrosis factor alpha) also play a key role in pulmonary hypertension.10,11 A similar pathogenesis could also contribute to the development of portal hypertension (Figure 3).
CASE CONCLUDED
We started our patient on oral prednisone 60 mg daily for a month, tapered to a maintenance dose of 15 mg to suppress clonal proliferation of plasma cells. Her symptoms improved. Her vascular endothelial growth factor level decreased from 1,664 to 624 pg/mL. She was enrolled in a National Institutes of Health study to evaluate the effect of a potential new immunomodulator treatment for POEMS syndrome.
In conclusion, POEMS syndrome is rare and can present with many atypical features. A high index of suspicion is needed to detect it in a patient who has noncirrhotic portal hypertension with ascites and multisystem involvement.
- Runyon BA, Montano AA, Akriviadis EA, Antillon MR, Irving MA, McHutchison JG. The serum-ascites albumin gradient is superior to the exudate-transudate concept in the differential diagnosis of ascites. Ann Intern Med 1992; 117:215–220.
- Harris M, Rash RM, Dymock IW. Nodular, non-cirrhotic liver associated with portal hypertension in a patient with rheumatoid arthritis. J Clin Pathol 1974; 27:963–966.
- Ramos-Casals M, Stone JH, Cid MC, Bosch X. The cryoglobulinaemias. Lancet 2012; 379:348–360.
- Druey KM, Greipp PR. Narrative review: the systemic capillary leak syndrome. Ann Intern Med 2010; 153:90–98.
- Bardwick PA, Zvaifler NJ, Gill GN, Newman D, Greenway GD, Resnick DL. Plasma cell dyscrasia with polyneuropathy, organomegaly, endocrinopathy, M protein, and skin changes: the POEMS syndrome. Report on two cases and a review of the literature. Medicine (Baltimore) 1980; 59:311–322.
- Dispenzieri A, Kyle RA, Lacy MQ, et al. POEMS syndrome: definitions and long-term outcome. Blood 2003; 101:2496–2506.
- Dispenzieri A. POEMS syndrome. Blood Rev 2007; 21:285–299.
- Stepani P, Courouble Y, Postel P, et al. Portal hypertension and neutrocytic ascites in POEMS syndrome. Gastroenterol Clin Biol 1998; 22:1095–1097. Article in French.
- Inoue R, Nakazawa A, Tsukada N, et al. POEMS syndrome with idiopathic portal hypertension: autopsy case and review of the literature. Pathol Int 2010; 60:316–320.
- Gherardi RK, Bélec L, Soubrier M, et al. Overproduction of proinflammatory cytokines imbalanced by their antagonists in POEMS syndrome. Blood 1996; 87:1458–1465.
- Mukerjee D, Kingdon E, Vanderpump M, Coghlan JG. Pathophysiological insights from a case of reversible pulmonary arterial hypertension. J R Soc Med 2003; 96:403–404.
- Runyon BA, Montano AA, Akriviadis EA, Antillon MR, Irving MA, McHutchison JG. The serum-ascites albumin gradient is superior to the exudate-transudate concept in the differential diagnosis of ascites. Ann Intern Med 1992; 117:215–220.
- Harris M, Rash RM, Dymock IW. Nodular, non-cirrhotic liver associated with portal hypertension in a patient with rheumatoid arthritis. J Clin Pathol 1974; 27:963–966.
- Ramos-Casals M, Stone JH, Cid MC, Bosch X. The cryoglobulinaemias. Lancet 2012; 379:348–360.
- Druey KM, Greipp PR. Narrative review: the systemic capillary leak syndrome. Ann Intern Med 2010; 153:90–98.
- Bardwick PA, Zvaifler NJ, Gill GN, Newman D, Greenway GD, Resnick DL. Plasma cell dyscrasia with polyneuropathy, organomegaly, endocrinopathy, M protein, and skin changes: the POEMS syndrome. Report on two cases and a review of the literature. Medicine (Baltimore) 1980; 59:311–322.
- Dispenzieri A, Kyle RA, Lacy MQ, et al. POEMS syndrome: definitions and long-term outcome. Blood 2003; 101:2496–2506.
- Dispenzieri A. POEMS syndrome. Blood Rev 2007; 21:285–299.
- Stepani P, Courouble Y, Postel P, et al. Portal hypertension and neutrocytic ascites in POEMS syndrome. Gastroenterol Clin Biol 1998; 22:1095–1097. Article in French.
- Inoue R, Nakazawa A, Tsukada N, et al. POEMS syndrome with idiopathic portal hypertension: autopsy case and review of the literature. Pathol Int 2010; 60:316–320.
- Gherardi RK, Bélec L, Soubrier M, et al. Overproduction of proinflammatory cytokines imbalanced by their antagonists in POEMS syndrome. Blood 1996; 87:1458–1465.
- Mukerjee D, Kingdon E, Vanderpump M, Coghlan JG. Pathophysiological insights from a case of reversible pulmonary arterial hypertension. J R Soc Med 2003; 96:403–404.
Preventing a first episode of esophageal variceal hemorrhage
Variceal hemorrhage is a medical emergency in which up to 20% of patients die.1 Even if the patient survives an initial episode of variceal bleeding, the probability of another episode is high: the rebleeding rate without treatment is 70% within 1 year. The mortality rate with rebleeding is 33%.
With such overwhelming consequences, the best strategy in any patient with cirrhosis and known varices is to try to prevent the first episode of bleeding.
WHO IS AT RISK?
Esophageal varices are present in 30% of patients with compensated cirrhosis and in up to 60% of those with decompensated cirrhosis (ie, with evidence of ascites or encephalopathy).2
The risk of variceal hemorrhage is related to three factors:
- The size of the varices. Varices 5 mm in diameter or smaller have a 7% risk of bleeding in 2 years, while those larger than 5 mm have a 30% risk of bleeding within 2 years.3
- The appearance of the varices. Morphologic features of varices, including red wale signs (red streaks of the mucosa overlying the varix), have been correlated with an increased risk of hemorrhage.
- The severity of liver dysfunction, as assessed by the Child-Pugh classification—an index of liver dysfunction based on serum albumin concentration, bilirubin level, prothrombin time, and the presence of ascites and encephalopathy. A high Child-Pugh score (ie, class B or C), representing decompensated cirrhosis, is associated with an increased risk of bleeding.
HOW VARICES DEVELOP: PORTAL HYPERTENSION
Esophageal varices form as a result of increased portal pressure, the product of increased portal venous inflow and resistance to outflow from the portal venous system. Portal hypertension is a major complication of chronic liver disease. In cirrhosis, architectural distortion of the liver causes an increase in the intrahepatic vascular resistance.
Portal venous inflow depends on mesenteric arteriolar tone, increasing when tone decreases. In cirrhotic patients, the increase in portal pressure results from a combination of increased portal blood flow secondary to splanchnic arteriolar vasodilation and elevated resistance to outflow through distorted hepatic sinusoids.
The potent vasodilator nitric oxide (NO) plays an important role in portal hypertension. In patients with cirrhosis, NO bioavailability is decreased in the intrahepatic circulation due to defects in the posttranslational regulation of endothelial NO synthase.4 This deficiency of NO, along with mechanical factors in the sinusoids, contributes to the increase in intrahepatic resistance. In the systemic and splanchnic circulation, NO bioavailability is increased due to upregulation and posttranslational regulation of endothelial NO synthase, thereby increasing splanchnic vasodilatation and leading to increased portal venous inflow.5 This results in a marked increase in cardiac output and so-called hyperdynamic circulation.
Portal hypertension results in the development of collateral circulation, including venous channels in the esophagus and stomach, by the dilation of preexisting vessels and active angiogenesis. Esophagogastric varices increase in size with the severity of portal hypertension and can rupture when the tension in their walls exceeds a maximal point.
HEPATIC VEIN PRESSURE GRADIENT: A PROXY FOR PORTAL PRESSURE
Ideally, the portal venous pressure should be directly measured. However, since direct measurement is invasive and impractical, the hepatic vein pressure gradient (HVPG) can be measured instead and correlates well with the portal pressure.6
The normal HVPG is 5 mm Hg or less; anything above this value denotes portal hypertension. However, studies have shown that varices may develop but do not bleed if the HVPG is less than 12 mm Hg.8
TWO WAYS TO PREVENT BLEEDING
Bleeding can be prevented either by reducing the portal venous pressure or by obliterating the varices. Portal pressure can be reduced by placing a portosystemic shunt either surgically or percutaneously with radiographic guidance or by giving drugs such as nonselective beta-blockers, nitrates, or a combination of these drugs. Variceal obliteration is typically done by endoscopic methods with either injection of a sclerosant or band ligation.
NONSELECTIVE BETA-BLOCKERS: THE MAINSTAY OF TREATMENT
Nonselective beta-blockers, the most commonly used drugs for preventing first esophageal variceal bleeding, decrease portal pressure by blocking both beta-1 and beta-2 adrenergic receptors.9 Beta-1 blockade decreases portal flow by decreasing the heart rate and cardiac output, while blockade of beta-2 receptors results in unopposed alpha-adrenergic-mediated vasoconstriction.
Selective beta-blockers do not appear to be as useful for primary prophylaxis. More than 2 decades ago, metoprolol (Toprol, Lopressor), a beta-1 selective antagonist, was compared with propranolol (Inderal), a nons-elective agent, in patients with cirrhosis and portal hypertension.10 Although both drugs significantly reduced the heart rate and cardiac output, only those taking propranolol showed a marked fall in portal pressure (mean decrease of 6.8 mm Hg vs 3.8 mm Hg with metoprolol) and a significant reduction in hepatic blood flow. The differences were thought to be related to beta-2 blockade of vasodilator receptors in the splanchnic circulation, which occurs only with nonselective beta-blockers such as propranolol.
The two nonselective beta-blockers most often used to prevent variceal bleeding are nadolol (Corgard) and propranolol. Both have been extensively studied in preventing a first variceal hemorrhage.
Effectiveness of beta-blockers
D’Amico et al11 performed a meta-analysis in 1995, examining nine trials (996 patients total) of the effectiveness of beta-blockers in preventing a first variceal hemorrhage. Seven trials found that bleeding risk was reduced with beta-blockers (significantly in four), one trial found that risk was unchanged, and one trial found that risk was increased—an outlier due to a small sample size. The meta-analysis showed a significant bleeding reduction with the use of a beta-blocker, either including the outlier trial (pooled odds ratio 0.54; 95% confidence interval 0.39–0.74) or excluding it (pooled odds ratio 0.48; 95% confidence interval 0.35–0.66).
Mortality rates were also reduced in seven trials, but the reduction was statistically significant in only one. However, in the pooled estimate, the mortality risk reduction approached statistical significance (pooled odds ratio 0.75; 95% confidence interval 0.57–1.06).
Ideo et al12 gave either nadolol or placebo to 79 patients with cirrhosis and large esophageal varices that had never bled. Nadolol was found to protect against a first variceal hemorrhage: at 2-year follow-up, only 1 of the 30 patients allocated to nadolol had had bleeding, vs 11 of the 49 patients in the placebo group.
Merkel et al13 found that the risk of variceal bleeding was lower in patients who started treatment with beta-blockers when their varices were small (12% at 5 years) than in those who started treatment after a diagnosis of large esophageal varices (22% at 5 years). They concluded that nadolol helps prevent small varices from growing into larger ones.
Response to beta-blockers is not uniform
Although beta-blockers decrease the portal pressure in many cirrhotic patients, the response is not uniform. In a study of 60 cirrhotic patients,14 40% showed no reduction or even a slight increase in HVPG with propranolol. Most patients showed a significant reduction in heart rate (17.5% ± 10%) after receiving 40 mg of propranolol. In the patients whose HVPG did not decrease by at least 10% with 40 mg of propranolol, increasing the dose caused a decrease in HVPG without a further decrease in heart rate. This suggests that 40 mg of propranolol successfully produced beta-1 blockade but that a higher dose was required for effective beta-2 blockade.
Failure to respond in certain patients may be due to a concurrent rise in collateral or hepatic sinusoidal resistance, or both. This was confirmed in a study in portal-hypertensive rats treated with propranolol.15 The reduction in portal blood flow expected was accompanied by a disproportionately small reduction in portal pressure, which was thought to be due to a rise in portal and collateral vascular resistance.
How to tell if beta-blocker treatment is ‘working’
An HVPG ≤ 12 mm Hg? Studies have shown that the most important predictor of efficacy of prophylaxis for variceal bleeding is a decrease in the HVPG to 12 mm Hg or less or a decrease in the initial HVPG of more than 20%.9 Although measuring the HVPG is invasive, expensive, and not routinely done in clinical practice, several studies have investigated the role of measuring hemodynamic response to medication.
Merkel et al16 measured the HVPG in 49 cirrhotic patients with previously nonbleeding varices before starting therapy with beta-blockers with or without nitrates and after 1 to 3 months of treatment. They followed the patients for up to 5 years. The mean HVPG value at baseline was 18.8 mm Hg. At 3 years of follow-up, 7% of those who had responded well to therapy (defined as achieving an HVPG less than 13 mm Hg or a decrease of more than 20%) had experienced a bleeding episode, which was significantly less than the rate (41%) in those who did not meet those hemodynamic end points. No patient reaching an HVPG of 12 mm Hg or less during treatment had variceal bleeding during follow-up.
Groszmann et al17 also prospectively measured the HVPG in patients with cirrhosis and varices, but their patients received either propranolol or placebo. Variceal hemorrhage occurred in 13 patients (11 of 51 in the placebo group and 2 of 51 in the propranolol group), all of whom had an HVPG greater than 12 mm Hg. Again, none of the patients whose HVPG was decreased to 12 mm Hg or less bled from esophageal varices.
Unfortunately, routine HVPG measurement to guide primary prophylaxis is an expensive strategy. Data suggest that measuring the HVPG is cost-effective only when the cost of measuring the HVPG is very low, the risk of variceal bleeding is very high, or the patient is expected to survive at least 3 to 5 years.18
A heart rate of 55 to 60? An alternative to HVPG measurement to monitor the effectiveness of beta-blocker therapy is to follow the heart rate. A 25% reduction from baseline or a heart rate of 55 to 60 beats per minute is the standard goal19,20; yet, at least 40% of patients treated with enough propranolol to decrease the heart rate by 25% do not respond with significant HVPG reductions.14,21
So, although beta-blockade is effective peripherally, it may not reduce HVPG to less than 12 mm Hg or 20% from baseline, and direct HVPG measurement is still the gold standard.
Treatment should be lifelong
Once a patient is started on a beta-blocker to prevent variceal hemorrhage, the treatment should be lifelong.
In 2001, a group of patients (most of them in Child-Pugh class A or B) completing a prospective randomized controlled trial of propranolol for primary prevention of variceal hemorrhage were tapered off propranolol or placebo.22 Of the 49 patients, 9 experienced variceal hemorrhage (6 of 25 former propranolol recipients and 3 of 24 former placebo recipients), and 17 patients died (12 former propranolol and 5 former placebo recipients), suggesting that treatment should be maintained for life.
Therefore, when beta-blocker therapy is discontinued, the risk of variceal hemorrhage returns to what would be expected in an untreated population.
Beta-blockers may not prevent varices
Although many trials have shown that beta-blockers are effective as prophylaxis against a first variceal hemorrhage, there is no evidence that these drugs prevent varices from forming in cirrhotic patients.
Groszmann et al23 treated more than 200 patients who had biopsy-proven cirrhosis and portal hypertension (HVPG > 6 mm Hg) but no varices with timolol (Blocadren), a nonselective beta-blocker, or placebo. At a median follow-up of about 55 months, the groups did not differ significantly in the incidence of primary events (development of varices or variceal hemorrhage) or treatment failures (transplantation or death). Varices developed less frequently among patients with a baseline HVPG of less than 10 mm Hg and among those whose HVPG had decreased by more than 10% at 1 year. In patients whose HVPG increased by more than 10%, varices developed more frequently.
Contraindications, side effects
The major drawbacks to therapy with beta-blockers are their contraindications and side effects.
Contraindications include chronic obstructive lung disease, psychosis, atrioventricular heart blocks, and aortic-valve disease.
Side effects are reported in 15% of patients but severe events are rare.24 Still, an estimated 10% to 20% of patients discontinue treatment because they cannot tolerate it.25 The more common complaints include fatigue, shortness of breath, sexual dysfunction, and sleep disorders.
Dosage
No specific starting dose of beta-blockers is agreed upon, but nadolol 20 to 40 mg once daily or long-acting propranolol 60 mg once daily can be used as initial therapy.25 Once-daily dosing increases the likelihood of compliance.
Since portal pressure progressively declines from 12 noon to 7 PM and then increases throughout the night and back to baseline by 9 AM,26 we recommend that the medication be taken in the evening to counteract increases in portal pressure that occur in the middle of the night.
ENDOSCOPIC VARICEAL LIGATION
Endoscopic variceal ligation has been investigated extensively for use as prophylaxis against first variceal hemorrhage. The procedure involves placing a rubber band around a varix aspirated into a cylinder on the tip of an endoscope.
Effectiveness of ligation
Lay et al,27 in a prospective, randomized trial in 126 cirrhotic patients endoscopically judged to be at high risk of hemorrhage, found that ligation significantly reduced the 2-year cumulative bleeding rate (19% with ligation vs 60% in an untreated control group) and the overall mortality rate (28% vs 58%). The lower risk of bleeding in the ligation group was attributed to a rapid reduction of variceal size; 60% of those in the ligation group had complete eradication of varices and 38% had varices reduced in size.
Imperiale and Chalasani28 performed a meta-analysis in 2001 that included 601 patients in five trials comparing prophylactic ligation with untreated controls and 283 patients in four trials comparing ligation with beta-blocker therapy. Compared with no treatment, ligation reduced the risk of first variceal hemorrhage, bleeding-related death, and death from any cause. Compared with propranolol, ligation reduced the risk of first-time bleeding but had no effect on the death rate.
Schepke et al,29 in a randomized controlled multicenter trial in 152 cirrhotic patients with two or more esophageal varices, found that neither bleeding incidence nor death rate differed significantly between ligation and propranolol.
Lui et al30 followed 172 cirrhotic patients with grade II or III esophageal varices for 6 years and found that ligation was equivalent to propranolol. However, many patients reported side effects with propranolol, and 30% of patients withdrew from propranolol treatment, making ligation a more attractive option.
Khuroo et al31 performed a meta-analysis of eight randomized controlled trials including 596 patients and found that ligation significantly reduced the rates of first gastrointestinal hemorrhage by 31% and of first variceal hemorrhage by 43%. In subgroup analysis, ligation had a significant advantage compared with beta-blockers in trials with patients with a high bleeding risk, ie, trials in which more than 30% of patients were in Child-Pugh class C and more than 50% of the patients had large varices.
Jutabha et al32 performed a multicenter, prospective trial (published in 2005) in 62 patients with high-risk esophageal varices randomized to propranolol or banding. The trial was ended early after an interim analysis showed that the failure rate of propranolol was significantly higher than that of banding (6/31 vs 0/31, P = .0098). Esophageal variceal hemorrhage occurred in 4 (12.9%) of the patients in the propranolol group compared with 0 in the ligation group. Similarly, 4 patients in the propranolol group died, compared with 0 in the ligation group. All the patients in this trial were liver transplant candidates and therefore all had severe liver disease.
In another trial favoring variceal banding over beta-blockers, Psilopoulos et al33 in 2005 followed 60 patients with cirrhosis and esophageal varices with no history of bleeding. Thirty percent of the patients in the propranolol group developed variceal bleeding compared with 6.7% in the ligation group (P = .043).
Lay et al34 followed 100 cirrhotic patients for 2 years and found comparable cumulative bleeding rates with ligation vs propranolol (18% vs 16%, respectively) and also comparable rates of death (28% vs 24%, respectively).
Sarin et al35 investigated the role of propranolol in addition to ligation in the prevention of first hemorrhage in 144 patients. Adding propranolol did not further decrease the incidence of initial bleeding (7% in the combination group vs 11% in the ligation-only group). Survival rates were similar at 20 months: 92% in the combination group vs 85% in the ligation-only group. However, the rate of variceal recurrence was lower with combination therapy: 6% in the combination group vs 15% with ligation alone.
Does esophageal variceal ligation increase gastric varices?
A less researched topic is whether variceal ligation results in gastric hemodynamic changes that increase the size of fundal varices and worsen portal hypertensive gastropathy.
Yuksel et al36 found that 37 of 85 patients had fundal varices before they underwent ligation of esophageal varices, increasing to 46 after the procedure, a statistically significant increment. The severity of portal hypertensive gastropathy also increased.
Further research is required regarding the long-term consequences of these findings.
ANGIOTENSIN II RECEPTOR ANTAGONISTS: ROLE UNKNOWN
Angiotensin II increases portal pressure, and angiotensin II levels are elevated in patients with cirrhosis, suggesting that this hormone plays a role in the pathogenesis of portal hypertension.
Losartan (Cozaar), an angiotensin II receptor antagonist, was found to decrease the HVPG significantly in patients with severe and moderate portal hypertension in a pilot study37 in 1999. However, in two subsequent studies,38,39 losartan only moderately reduced the HVPG and caused hypotension and a reduction in the glomerular filtration rate. The role of angiotensin II receptor blockers in primary prevention of variceal bleeding is still unknown.
SURGICAL PORTAL DECOMPRESSION HAS BEEN ABANDONED
The first method investigated to prevent variceal bleeding was surgical portal decompression.
A meta-analysis of four randomized controlled trials in 302 patients with varices of all sizes compared portocaval shunt surgery and medical therapy.11 Although shunt surgery was very effective in preventing variceal bleeding, the risk of chronic or recurrent encephalopathy was significantly increased (odds ratio 2.0), as was the risk of death (odds ratio 1.6).
These poor results, combined with advances in endoscopic procedures, led to the abandonment of surgical shunting for primary prophylaxis.
TIPS PROCEDURE: NO ROLE AT PRESENT
The transjugular intrahepatic portosystemic shunt (TIPS) procedure is used to treat the main consequences of portal hypertension, including ascites and variceal hemorrhage. The procedure entails accessing the hepatic vein via the right jugular vein and placing a stent to the portal vein, forming a low-resistance channel and allowing blood to return to the systemic circulation.
TIPS placement increases the risk of encephalopathy; liver failure is a rare complication, and procedural complications (ie, shunt dysfunction) also occur. Trials comparing the TIPS procedure with other forms of therapy to prevent first variceal hemorrhages have not been performed.40 Research to improve the outcome of the TIPS procedure is ongoing, but currently this procedure has no role in primary prevention of variceal bleeding.
ENDOSCOPIC SCLEROTHERAPY MAY INCREASE THE RISK OF DEATH
Numerous clinical trials evaluated sclerotherapy as prophylaxis against a first esophageal variceal hemorrhage. The procedure involves injecting a sclerosant in and around varices.
In a large Veterans Administration study,41 sclerotherapy was compared with sham treatment in 281 men with alcoholic liver disease who had documented varices but no history of bleeding. The trial was terminated after 22.5 months because the rate of all-cause mortality was significantly higher in the sclerotherapy group (32.5%) than in the sham therapy group (17.4%). The higher death rate did not persist after the treatment was discontinued, and it was speculated that, although sclerotherapy had reduced new episodes of variceal hemorrhage, the procedure might have caused bleeding from esophageal ulcers, leading to an increased mortality rate in that group.
The PROVA Study Group from Norway and Denmark found similar results when 286 cirrhotic patients were randomized to receive sclerotherapy, propranolol, combination sclerotherapy and propranolol, or no treatment to prevent a first variceal hemorrhage.42 The incidence of variceal bleeding was almost identical in the four groups, but the mortality rate with variceal bleeding was 2.75 times higher in the sclerotherapy groups than in the other groups (P = .002). It was speculated that repeated sclerotherapy sessions might be poorly tolerated by patients in Child-Pugh classes B and C and might have contributed to the precipitation of liver failure and other common complications of cirrhosis.
A meta-analysis by D’Amico et al11 evaluated 19 trials (1,630 patients) comparing sclerotherapy with nonactive treatment. Sclerotherapy tended to be favorable in trials with a high bleeding rate in the control patients and unfavorable in trials with a low bleeding rate. The benefit seen in patients at high risk is consistent with the efficacy of sclerotherapy for preventing rebleeding, whereas the harmful effect in the low-risk patients points towards side effects and complications exceeding the potential benefits.
In general, currently available evidence suggests that the benefits of prophylactic sclerotherapy are marginal, and therefore sclerotherapy is not recommended as primary prophylaxis for variceal hemorrhage.
NITRATES: NO LONGER USED AS MONOTHERAPY
Unlike vasoconstrictors, which decrease portal pressure by decreasing blood flow, vasodilators reduce hepatic pressure by decreasing intrahepatic and portocollateral vascular resistance.43 In addition, larger doses directly affect the arterial circulation, lowering systemic and therefore splanchnic perfusion pressure.44 Unfortunately, the systemic vasodilatory effects of nitrates exacerbate the hyperdynamic state that is characteristic of cirrhosis, thereby limiting their use and tolerability in many patients.
A trial comparing propranolol vs isosorbide mononitrate initially found that the groups did not differ significantly with regard to bleeding rates and 2-year survival rates,45 but a 6-year follow-up found the likelihood of death greater in patients older than 50 years in the nitrate group.46 In an additional study comparing isosorbide mononitrate vs placebo in patients with contraindications to or intolerance of beta-blockers, no difference in the relative risk of first variceal hemorrhage was found between the two groups.47 Therefore, nitrates are no longer used as monotherapy to prevent variceal bleeding.
Combination therapy with beta-blockers plus nitrates is controversial. In a trial in 1996, Merkel et al48 found the cumulative risk of variceal bleeding was 18% at 40 months with nadolol alone vs 7.5% with nadolol plus isosorbide mononitrate. However, in a later trial, Garcia-Pagán et al49 found no significant advantage to combination therapy. The incidence of variceal bleeding at 1 year was 8.3% in the group receiving propranolol plus placebo and 5% in the group receiving propranolol plus isosorbide mononitrate; at 2 years, the rates were 10.6% vs 12.5%.
RECOMMENDATIONS FOR SCREENING AND PROPHYLAXIS
- All patients with cirrhosis should be screened for varices at the time of diagnosis.
- The size of the varices, including small (≤ 5 mm) and large (> 5 mm), and the presence of red wale marks on the varices should be recorded.
- Patients who have no varices on screening endoscopy should be rescreened every 3 years if their liver function is stable or every year if their liver function deteriorates. (Varices grow at a rate proportional to the severity of the liver disease.)
- Patients with portal hypertension but without varices do not need treatment with nonselective beta-blockers. Endoscopy should be performed at the intervals suggested above.
- Those who are found to have small varices on screening endoscopy but who have well-compensated liver disease (Child-Pugh class A) and no red wale marks should be rescreened every other year because the development of large varices is greater in patients with small varices on initial endoscopy than in patients with no varices. Emerging data support the use of beta-blockers to prevent varices from increasing in size.
- Patients who have small varices with red wale signs or who are in Child-Pugh class B or C have an increased risk of bleeding and should be treated with beta-blockers. If beta-blockers are not used, endoscopy should be done every year to look for an increase in variceal size.
- Patients who have large varices without red wale signs or who are in Child-Pugh class B or C should be treated with nonselective beta-blockers. The dose should be adjusted to achieve maximal tolerable decrease in heart rate to a minimum of 55 beats per minute, and treatment should be continued indefinitely.
- Endoscopic variceal ligation is an acceptable alternative to beta-blocker treatment as first-line therapy in those who cannot tolerate beta-blockers or who have contraindications to their use, or in those who have red wale marking or who are in Child-Pugh class B or C.
- D’Amico G, De Franchis R Cooperative Study Group. Upper digestive bleeding in cirrhosis. Post-therapeutic outcome and prognostic indicators. Hepatology 2003; 38:599–612.
- D’Amico G, Luca A. Natural history. Clinicalhaemodynamic correlations. Prediction of the risk of bleeding. Baillieres Clin Gastroenterol 1997; 11:243–256.
- Kamath PS. Esophageal variceal bleeding: primary prophylaxis. Clin Gastroenterol Hepatol 2005; 3:90–93.
- Shah V, Toruner M, Haddad F, et al. Impaired endothelial nitric oxide synthase activity associated with enhanced caveolin binding in experimental cirrhosis in the rat. Gastroenterology 1999; 117:1222–1228.
- Morales-Ruiz M, Jimenez W, Perez-Sala D, et al. Increased nitric oxide synthase expression in arterial vessels of cirrhotic rats with ascites. Hepatology 1996; 24:1481–1486.
- De Franchis R, Dell’Era A, Iannuzzi F. Diagnosis and treatment of portal hypertension. Dig Liver Dis 2004; 36:787–798.
- Groszmann RJ, Wongcharatrawee S. The hepatic venous pressure gradient: anything worth doing should be done right. Hepatology 2004; 39:280–282.
- Garcia-Tsao G, Groszmann RJ, Fisher RL, Conn HO, Atterbury CE, Glickman M. Portal pressure, presence of gastroesophageal varices and variceal bleeding. Hepatology 1985; 5:419–424.
- Bosch J, Masti R, Kravetz D, et al. Effects of propranolol on azygos venous blood flow and hepatic and systemic hemodynamics in cirrhosis. Hepatology 1984; 4:1200–1205.
- Westaby D, Bihari DJ, Gimson AE, Crossley IR, Williams R. Selective and non-selective beta receptor blockade in the reduction of portal pressure in patients with cirrhosis and portal hypertension. Gut 1984; 25:121–124.
- D’Amico G, Pagliaro L, Bosch J. The treatment of portal hypertension: a meta-analytic review. Hepatology 1995; 22:332–354.
- Ideo G, Bellati G, Fesce E, Grimoldi D. Nadolol can prevent the first gastrointestinal bleeding in cirrhotics: a prospective, randomized study. Hepatology 1988; 8:6–9.
- Merkel C, Marin R, Angeli P, et al. Gruppo Triveneto per l’Ipertensione Portale. A placebo-controlled clinical trial of nadolol in the prophylaxis of growth of small esophageal varices in cirrhosis. Gastroenterology 2004; 127:476–484.
- Garcia-Tsao G, Grace ND, Groszmann RJ, et al. Short-term effects of propranolol on portal venous pressure. Hepatology 1986; 6:101–106.
- Kroeger RJ, Groszmann RJ. Increased portal venous resistance hinders portal pressure reduction during the administration of beta-adrenergic blocking agents in a portal hypertensive model. Hepatology 1985; 5:97–101.
- Merkel C, Bolognesi M, Sacerdoti D, et al. The hemodynamic response to medical treatment of portal hypertension as a predictor of clinical effectiveness in the primary prophylaxis of variceal bleeding in cirrhosis. Hepatology 2000; 32:930–934.
- Groszmann RJ, Bosch J, Grace ND, et al. Hemodynamic events in a prospective randomized trial of propranolol versus placebo in the prevention of a first variceal hemorrhage. Gastroenterology 1990; 99:1401–1407.
- Hicken BL, Sharara AI, Abrams GA, Eloubeidi M, Fallon MB, Arguedas MR. Hepatic venous pressure gradient measurements to assess response to primary prophylaxis in patients with cirrhosis: a decision analytical study. Aliment Pharmacol Ther 2003; 17:145–153.
- Grace ND. Diagnosis and treatment of gastrointestinal bleeding secondary to portal hypertension. American College of Gastroenterology Practice Parameters Committee. Am J Gastroenterol 1997; 92:1081–1091.
- Grace ND, Groszmann RJ, Garcia-Tsao G, et al. Portal hypertension and variceal bleeding: an AASLD single topic symposium. Hepatology 1998; 28:868–880.
- Feu F, Garcia-Pagán JC, Bosch J, et al. Relation between portal pressure response to pharmacotherapy and risk of recurrent variceal haemorrhage in patients with cirrhosis. Lancet 1995; 346:1056–1059.
- Abraczinskas DR, Ookubo R, Grace ND, et al. Propranolol for the prevention of first esophageal variceal hemorrhage: a lifetime commitment? Hepatology 2001; 34:1096–1102.
- Groszmann RJ, Garcia-Tsao G, Bosch J, et al Portal Hypertension Collaborative Group. Beta-blockers to prevent gastroesophageal varices in patients with cirrhosis. N Engl J Med 2005; 353:2254–2261.
- Garcia-Pagán JC, Bosch J. Pharmacological prevention of variceal bleeding. New developments. Baillier Clin Gastroenterol 1997; 11:271–287.
- Talwalkar JA, Kamath PS. An evidence-based medicine approach to beta-blocker therapy in patients with cirrhosis. Am J Med 2004; 116:759–766.
- Garcia-Pagan JC, Feu F, Castells A, et al. Circadian variations of portal pressure and variceal hemorrhage in patients with cirrhosis. Hepatology 1994; 19:595–601.
- Lay CS, Tsai YT, Teg CY, et al. Endoscopic variceal ligation in prophylaxis of first variceal bleeding in cirrhotic patients with high-risk esophageal varices. Hepatology 1997; 25:1346–1350.
- Imperiale TF, Chalasani N. A meta-analysis of endoscopic variceal ligation for primary prophylaxis of esophageal variceal bleeding. Hepatology 2001; 33:802–807.
- Schepke M, Kleber G, Nurnberg D, et al. Ligation versus propranolol for the primary prophylaxis of variceal bleeding in cirrhosis. Hepatology 2004; 40:65–72.
- Lui HF, Stanley AJ, Forrest EH, et al. Primary prophylaxis of variceal hemorrhage: a randomized controlled trial comparing band ligation, propranolol, and isosorbide mononitrate. Gastroenterology 2002; 123:735–744.
- Khuroo MS, Khuroo NS, Farahat KL, Khuroo YS, Sofi AA, Dahab ST. Meta-analysis: endoscopic variceal ligation for primary prophylaxis of oesophageal variceal bleeding. Aliment Pharmacol Ther 2005; 21:347–361.
- Jutabha R, Jensen DM, Martin P, Savides T, Han SH, Gornbein J. Randomized study comparing banding and propranolol to prevent initial variceal hemorrhage in cirrhotics with high-risk esophageal varices. Gastroenterology 2005; 128:870–881.
- Psilopoulos D, Galanis P, Goulas S, et al. Endoscopic variceal ligation vs. propranolol for prevention of first variceal bleeding: a randomized controlled trial. Eur J Gastroenterol Hepatol 2005; 17:1111–1117.
- Lay CS, Tsai YT, Lee FY, et al. Endoscopic variceal ligation versus propranolol in prophylaxis of first variceal bleeding in patients with cirrhosis. J Gastroenterol Hepatol 2006; 21:413–419.
- Sarin SK, Wadhawan M, Agarwal SR, Tyagi P, Sharma BC. Endoscopic variceal ligation plus propranolol versus endoscopic variceal ligation alone in primary prophylaxis of variceal bleeding. Am J Gastroenterol 2005; 100:797–804.
- Yuksel O, Koklu S, Arhan M, Yolcu OF, et al. Effects of esophageal variceal eradication on portal hypertensive gastropathy and fundal varices: a retrospective and comparative study. Dig Dis Sci 2006; 51:27–30.
- Schneider AW, Kalk JF, Klein CP. Effect of losartan, an angiotensin II receptor antagonist, on portal pressure in cirrhosis. Hepatology 1999; 29:334–339.
- Schepke M, Werner E, Biecker E, et al. Hemodynamic effects of the angiotensin II receptor antagonist irbesartan in patients with cirrhosis and portal hypertension. Gastroenterology 2001; 121:389–395.
- Gonzalez-Abraldes J, Albillos A, Banares R, et al. Randomized comparison of long-term losartan versus propranolol in lowering portal pressure in cirrhosis. Gastroenterology 2001; 121:382–388.
- Boyer TD, Haskal ZJ American Association for the Study of Liver Diseases. The role of transjugular intrahepatic portosystemic shunt in the management of portal hypertension. Hepatology 2005; 41:386–400.
- Prophylactic sclerotherapy for esophageal varices in men with alcoholic liver disease. A randomized, single-blind, multicenter clinical trial. The Veterans Affairs Cooperative Variceal Sclerotherapy Group. N Engl J Med 1991; 324:1779–1784.
- The PROVA Study Group. Prophylaxis of first hemorrhage from esophageal varices by sclerotherapy, propranolol or both in cirrhotic patients: a randomized multicenter trial. Hepatology 1991; 14:1016–1024.
- Escorsell A, Feu F, Bordas JM, et al. Effects of isosorbide-5-mononitrate on variceal pressure and systemic and splanchnic haemodynamics in patients with cirrhosis. J Hepatol 1996; 24:423–429.
- Hayes PC, Westaby D, Williams R. Effect and mechanism of action of isosorbide-5-mononitrate. Gut 1988; 29:752–755.
- Angelico M, Carli L, Piat C, et al. Isosorbide-5-mononitrate versus propranolol in the prevention of first bleeding in cirrhosis. Gastroenterology 1993; 104:1460–1465.
- Angelico M, Carli L, Piat C, Gentile S, Capocaccia L. Effects of isosorbide-5-mononitrate compared with propranolol on first bleeding and long-term survival in cirrhosis. Gastroenterology 1997; 113:1632–1639.
- Garcia-Pagan JC, Villanueva C, Vila MC, et al. MOVE Group. Mononitrato Varices Esofagicas. Isosorbide mononitrate in the prevention of first variceal bleed in patients who cannot receive beta-blockers. Gastroenterology 2001; 121:908–914.
- Merkel C, Marin R, Enzo E, et al. Randomised trial of nadolol alone or with isosorbide mononitrate for primary prophylaxis of variceal bleeding in cirrhosis. Gruppo-Triveneto per L’ipertensione portale (GTIP). Lancet 1996; 348:1677–1681.
- Garcia-Pagán JC, Morillas R, Banares R, et al Spanish Variceal Bleeding Study Group. Propranolol plus placebo versus propranolol plus isosorbide-5-mononitrate in the prevention of a first variceal bleed: a double-blind RCT. Hepatology 2003; 37:1260–1266.
- Garcia-Tsao G, Sanyal A, Grace N, et al. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology 2007; 46:922–938.
Variceal hemorrhage is a medical emergency in which up to 20% of patients die.1 Even if the patient survives an initial episode of variceal bleeding, the probability of another episode is high: the rebleeding rate without treatment is 70% within 1 year. The mortality rate with rebleeding is 33%.
With such overwhelming consequences, the best strategy in any patient with cirrhosis and known varices is to try to prevent the first episode of bleeding.
WHO IS AT RISK?
Esophageal varices are present in 30% of patients with compensated cirrhosis and in up to 60% of those with decompensated cirrhosis (ie, with evidence of ascites or encephalopathy).2
The risk of variceal hemorrhage is related to three factors:
- The size of the varices. Varices 5 mm in diameter or smaller have a 7% risk of bleeding in 2 years, while those larger than 5 mm have a 30% risk of bleeding within 2 years.3
- The appearance of the varices. Morphologic features of varices, including red wale signs (red streaks of the mucosa overlying the varix), have been correlated with an increased risk of hemorrhage.
- The severity of liver dysfunction, as assessed by the Child-Pugh classification—an index of liver dysfunction based on serum albumin concentration, bilirubin level, prothrombin time, and the presence of ascites and encephalopathy. A high Child-Pugh score (ie, class B or C), representing decompensated cirrhosis, is associated with an increased risk of bleeding.
HOW VARICES DEVELOP: PORTAL HYPERTENSION
Esophageal varices form as a result of increased portal pressure, the product of increased portal venous inflow and resistance to outflow from the portal venous system. Portal hypertension is a major complication of chronic liver disease. In cirrhosis, architectural distortion of the liver causes an increase in the intrahepatic vascular resistance.
Portal venous inflow depends on mesenteric arteriolar tone, increasing when tone decreases. In cirrhotic patients, the increase in portal pressure results from a combination of increased portal blood flow secondary to splanchnic arteriolar vasodilation and elevated resistance to outflow through distorted hepatic sinusoids.
The potent vasodilator nitric oxide (NO) plays an important role in portal hypertension. In patients with cirrhosis, NO bioavailability is decreased in the intrahepatic circulation due to defects in the posttranslational regulation of endothelial NO synthase.4 This deficiency of NO, along with mechanical factors in the sinusoids, contributes to the increase in intrahepatic resistance. In the systemic and splanchnic circulation, NO bioavailability is increased due to upregulation and posttranslational regulation of endothelial NO synthase, thereby increasing splanchnic vasodilatation and leading to increased portal venous inflow.5 This results in a marked increase in cardiac output and so-called hyperdynamic circulation.
Portal hypertension results in the development of collateral circulation, including venous channels in the esophagus and stomach, by the dilation of preexisting vessels and active angiogenesis. Esophagogastric varices increase in size with the severity of portal hypertension and can rupture when the tension in their walls exceeds a maximal point.
HEPATIC VEIN PRESSURE GRADIENT: A PROXY FOR PORTAL PRESSURE
Ideally, the portal venous pressure should be directly measured. However, since direct measurement is invasive and impractical, the hepatic vein pressure gradient (HVPG) can be measured instead and correlates well with the portal pressure.6
The normal HVPG is 5 mm Hg or less; anything above this value denotes portal hypertension. However, studies have shown that varices may develop but do not bleed if the HVPG is less than 12 mm Hg.8
TWO WAYS TO PREVENT BLEEDING
Bleeding can be prevented either by reducing the portal venous pressure or by obliterating the varices. Portal pressure can be reduced by placing a portosystemic shunt either surgically or percutaneously with radiographic guidance or by giving drugs such as nonselective beta-blockers, nitrates, or a combination of these drugs. Variceal obliteration is typically done by endoscopic methods with either injection of a sclerosant or band ligation.
NONSELECTIVE BETA-BLOCKERS: THE MAINSTAY OF TREATMENT
Nonselective beta-blockers, the most commonly used drugs for preventing first esophageal variceal bleeding, decrease portal pressure by blocking both beta-1 and beta-2 adrenergic receptors.9 Beta-1 blockade decreases portal flow by decreasing the heart rate and cardiac output, while blockade of beta-2 receptors results in unopposed alpha-adrenergic-mediated vasoconstriction.
Selective beta-blockers do not appear to be as useful for primary prophylaxis. More than 2 decades ago, metoprolol (Toprol, Lopressor), a beta-1 selective antagonist, was compared with propranolol (Inderal), a nons-elective agent, in patients with cirrhosis and portal hypertension.10 Although both drugs significantly reduced the heart rate and cardiac output, only those taking propranolol showed a marked fall in portal pressure (mean decrease of 6.8 mm Hg vs 3.8 mm Hg with metoprolol) and a significant reduction in hepatic blood flow. The differences were thought to be related to beta-2 blockade of vasodilator receptors in the splanchnic circulation, which occurs only with nonselective beta-blockers such as propranolol.
The two nonselective beta-blockers most often used to prevent variceal bleeding are nadolol (Corgard) and propranolol. Both have been extensively studied in preventing a first variceal hemorrhage.
Effectiveness of beta-blockers
D’Amico et al11 performed a meta-analysis in 1995, examining nine trials (996 patients total) of the effectiveness of beta-blockers in preventing a first variceal hemorrhage. Seven trials found that bleeding risk was reduced with beta-blockers (significantly in four), one trial found that risk was unchanged, and one trial found that risk was increased—an outlier due to a small sample size. The meta-analysis showed a significant bleeding reduction with the use of a beta-blocker, either including the outlier trial (pooled odds ratio 0.54; 95% confidence interval 0.39–0.74) or excluding it (pooled odds ratio 0.48; 95% confidence interval 0.35–0.66).
Mortality rates were also reduced in seven trials, but the reduction was statistically significant in only one. However, in the pooled estimate, the mortality risk reduction approached statistical significance (pooled odds ratio 0.75; 95% confidence interval 0.57–1.06).
Ideo et al12 gave either nadolol or placebo to 79 patients with cirrhosis and large esophageal varices that had never bled. Nadolol was found to protect against a first variceal hemorrhage: at 2-year follow-up, only 1 of the 30 patients allocated to nadolol had had bleeding, vs 11 of the 49 patients in the placebo group.
Merkel et al13 found that the risk of variceal bleeding was lower in patients who started treatment with beta-blockers when their varices were small (12% at 5 years) than in those who started treatment after a diagnosis of large esophageal varices (22% at 5 years). They concluded that nadolol helps prevent small varices from growing into larger ones.
Response to beta-blockers is not uniform
Although beta-blockers decrease the portal pressure in many cirrhotic patients, the response is not uniform. In a study of 60 cirrhotic patients,14 40% showed no reduction or even a slight increase in HVPG with propranolol. Most patients showed a significant reduction in heart rate (17.5% ± 10%) after receiving 40 mg of propranolol. In the patients whose HVPG did not decrease by at least 10% with 40 mg of propranolol, increasing the dose caused a decrease in HVPG without a further decrease in heart rate. This suggests that 40 mg of propranolol successfully produced beta-1 blockade but that a higher dose was required for effective beta-2 blockade.
Failure to respond in certain patients may be due to a concurrent rise in collateral or hepatic sinusoidal resistance, or both. This was confirmed in a study in portal-hypertensive rats treated with propranolol.15 The reduction in portal blood flow expected was accompanied by a disproportionately small reduction in portal pressure, which was thought to be due to a rise in portal and collateral vascular resistance.
How to tell if beta-blocker treatment is ‘working’
An HVPG ≤ 12 mm Hg? Studies have shown that the most important predictor of efficacy of prophylaxis for variceal bleeding is a decrease in the HVPG to 12 mm Hg or less or a decrease in the initial HVPG of more than 20%.9 Although measuring the HVPG is invasive, expensive, and not routinely done in clinical practice, several studies have investigated the role of measuring hemodynamic response to medication.
Merkel et al16 measured the HVPG in 49 cirrhotic patients with previously nonbleeding varices before starting therapy with beta-blockers with or without nitrates and after 1 to 3 months of treatment. They followed the patients for up to 5 years. The mean HVPG value at baseline was 18.8 mm Hg. At 3 years of follow-up, 7% of those who had responded well to therapy (defined as achieving an HVPG less than 13 mm Hg or a decrease of more than 20%) had experienced a bleeding episode, which was significantly less than the rate (41%) in those who did not meet those hemodynamic end points. No patient reaching an HVPG of 12 mm Hg or less during treatment had variceal bleeding during follow-up.
Groszmann et al17 also prospectively measured the HVPG in patients with cirrhosis and varices, but their patients received either propranolol or placebo. Variceal hemorrhage occurred in 13 patients (11 of 51 in the placebo group and 2 of 51 in the propranolol group), all of whom had an HVPG greater than 12 mm Hg. Again, none of the patients whose HVPG was decreased to 12 mm Hg or less bled from esophageal varices.
Unfortunately, routine HVPG measurement to guide primary prophylaxis is an expensive strategy. Data suggest that measuring the HVPG is cost-effective only when the cost of measuring the HVPG is very low, the risk of variceal bleeding is very high, or the patient is expected to survive at least 3 to 5 years.18
A heart rate of 55 to 60? An alternative to HVPG measurement to monitor the effectiveness of beta-blocker therapy is to follow the heart rate. A 25% reduction from baseline or a heart rate of 55 to 60 beats per minute is the standard goal19,20; yet, at least 40% of patients treated with enough propranolol to decrease the heart rate by 25% do not respond with significant HVPG reductions.14,21
So, although beta-blockade is effective peripherally, it may not reduce HVPG to less than 12 mm Hg or 20% from baseline, and direct HVPG measurement is still the gold standard.
Treatment should be lifelong
Once a patient is started on a beta-blocker to prevent variceal hemorrhage, the treatment should be lifelong.
In 2001, a group of patients (most of them in Child-Pugh class A or B) completing a prospective randomized controlled trial of propranolol for primary prevention of variceal hemorrhage were tapered off propranolol or placebo.22 Of the 49 patients, 9 experienced variceal hemorrhage (6 of 25 former propranolol recipients and 3 of 24 former placebo recipients), and 17 patients died (12 former propranolol and 5 former placebo recipients), suggesting that treatment should be maintained for life.
Therefore, when beta-blocker therapy is discontinued, the risk of variceal hemorrhage returns to what would be expected in an untreated population.
Beta-blockers may not prevent varices
Although many trials have shown that beta-blockers are effective as prophylaxis against a first variceal hemorrhage, there is no evidence that these drugs prevent varices from forming in cirrhotic patients.
Groszmann et al23 treated more than 200 patients who had biopsy-proven cirrhosis and portal hypertension (HVPG > 6 mm Hg) but no varices with timolol (Blocadren), a nonselective beta-blocker, or placebo. At a median follow-up of about 55 months, the groups did not differ significantly in the incidence of primary events (development of varices or variceal hemorrhage) or treatment failures (transplantation or death). Varices developed less frequently among patients with a baseline HVPG of less than 10 mm Hg and among those whose HVPG had decreased by more than 10% at 1 year. In patients whose HVPG increased by more than 10%, varices developed more frequently.
Contraindications, side effects
The major drawbacks to therapy with beta-blockers are their contraindications and side effects.
Contraindications include chronic obstructive lung disease, psychosis, atrioventricular heart blocks, and aortic-valve disease.
Side effects are reported in 15% of patients but severe events are rare.24 Still, an estimated 10% to 20% of patients discontinue treatment because they cannot tolerate it.25 The more common complaints include fatigue, shortness of breath, sexual dysfunction, and sleep disorders.
Dosage
No specific starting dose of beta-blockers is agreed upon, but nadolol 20 to 40 mg once daily or long-acting propranolol 60 mg once daily can be used as initial therapy.25 Once-daily dosing increases the likelihood of compliance.
Since portal pressure progressively declines from 12 noon to 7 PM and then increases throughout the night and back to baseline by 9 AM,26 we recommend that the medication be taken in the evening to counteract increases in portal pressure that occur in the middle of the night.
ENDOSCOPIC VARICEAL LIGATION
Endoscopic variceal ligation has been investigated extensively for use as prophylaxis against first variceal hemorrhage. The procedure involves placing a rubber band around a varix aspirated into a cylinder on the tip of an endoscope.
Effectiveness of ligation
Lay et al,27 in a prospective, randomized trial in 126 cirrhotic patients endoscopically judged to be at high risk of hemorrhage, found that ligation significantly reduced the 2-year cumulative bleeding rate (19% with ligation vs 60% in an untreated control group) and the overall mortality rate (28% vs 58%). The lower risk of bleeding in the ligation group was attributed to a rapid reduction of variceal size; 60% of those in the ligation group had complete eradication of varices and 38% had varices reduced in size.
Imperiale and Chalasani28 performed a meta-analysis in 2001 that included 601 patients in five trials comparing prophylactic ligation with untreated controls and 283 patients in four trials comparing ligation with beta-blocker therapy. Compared with no treatment, ligation reduced the risk of first variceal hemorrhage, bleeding-related death, and death from any cause. Compared with propranolol, ligation reduced the risk of first-time bleeding but had no effect on the death rate.
Schepke et al,29 in a randomized controlled multicenter trial in 152 cirrhotic patients with two or more esophageal varices, found that neither bleeding incidence nor death rate differed significantly between ligation and propranolol.
Lui et al30 followed 172 cirrhotic patients with grade II or III esophageal varices for 6 years and found that ligation was equivalent to propranolol. However, many patients reported side effects with propranolol, and 30% of patients withdrew from propranolol treatment, making ligation a more attractive option.
Khuroo et al31 performed a meta-analysis of eight randomized controlled trials including 596 patients and found that ligation significantly reduced the rates of first gastrointestinal hemorrhage by 31% and of first variceal hemorrhage by 43%. In subgroup analysis, ligation had a significant advantage compared with beta-blockers in trials with patients with a high bleeding risk, ie, trials in which more than 30% of patients were in Child-Pugh class C and more than 50% of the patients had large varices.
Jutabha et al32 performed a multicenter, prospective trial (published in 2005) in 62 patients with high-risk esophageal varices randomized to propranolol or banding. The trial was ended early after an interim analysis showed that the failure rate of propranolol was significantly higher than that of banding (6/31 vs 0/31, P = .0098). Esophageal variceal hemorrhage occurred in 4 (12.9%) of the patients in the propranolol group compared with 0 in the ligation group. Similarly, 4 patients in the propranolol group died, compared with 0 in the ligation group. All the patients in this trial were liver transplant candidates and therefore all had severe liver disease.
In another trial favoring variceal banding over beta-blockers, Psilopoulos et al33 in 2005 followed 60 patients with cirrhosis and esophageal varices with no history of bleeding. Thirty percent of the patients in the propranolol group developed variceal bleeding compared with 6.7% in the ligation group (P = .043).
Lay et al34 followed 100 cirrhotic patients for 2 years and found comparable cumulative bleeding rates with ligation vs propranolol (18% vs 16%, respectively) and also comparable rates of death (28% vs 24%, respectively).
Sarin et al35 investigated the role of propranolol in addition to ligation in the prevention of first hemorrhage in 144 patients. Adding propranolol did not further decrease the incidence of initial bleeding (7% in the combination group vs 11% in the ligation-only group). Survival rates were similar at 20 months: 92% in the combination group vs 85% in the ligation-only group. However, the rate of variceal recurrence was lower with combination therapy: 6% in the combination group vs 15% with ligation alone.
Does esophageal variceal ligation increase gastric varices?
A less researched topic is whether variceal ligation results in gastric hemodynamic changes that increase the size of fundal varices and worsen portal hypertensive gastropathy.
Yuksel et al36 found that 37 of 85 patients had fundal varices before they underwent ligation of esophageal varices, increasing to 46 after the procedure, a statistically significant increment. The severity of portal hypertensive gastropathy also increased.
Further research is required regarding the long-term consequences of these findings.
ANGIOTENSIN II RECEPTOR ANTAGONISTS: ROLE UNKNOWN
Angiotensin II increases portal pressure, and angiotensin II levels are elevated in patients with cirrhosis, suggesting that this hormone plays a role in the pathogenesis of portal hypertension.
Losartan (Cozaar), an angiotensin II receptor antagonist, was found to decrease the HVPG significantly in patients with severe and moderate portal hypertension in a pilot study37 in 1999. However, in two subsequent studies,38,39 losartan only moderately reduced the HVPG and caused hypotension and a reduction in the glomerular filtration rate. The role of angiotensin II receptor blockers in primary prevention of variceal bleeding is still unknown.
SURGICAL PORTAL DECOMPRESSION HAS BEEN ABANDONED
The first method investigated to prevent variceal bleeding was surgical portal decompression.
A meta-analysis of four randomized controlled trials in 302 patients with varices of all sizes compared portocaval shunt surgery and medical therapy.11 Although shunt surgery was very effective in preventing variceal bleeding, the risk of chronic or recurrent encephalopathy was significantly increased (odds ratio 2.0), as was the risk of death (odds ratio 1.6).
These poor results, combined with advances in endoscopic procedures, led to the abandonment of surgical shunting for primary prophylaxis.
TIPS PROCEDURE: NO ROLE AT PRESENT
The transjugular intrahepatic portosystemic shunt (TIPS) procedure is used to treat the main consequences of portal hypertension, including ascites and variceal hemorrhage. The procedure entails accessing the hepatic vein via the right jugular vein and placing a stent to the portal vein, forming a low-resistance channel and allowing blood to return to the systemic circulation.
TIPS placement increases the risk of encephalopathy; liver failure is a rare complication, and procedural complications (ie, shunt dysfunction) also occur. Trials comparing the TIPS procedure with other forms of therapy to prevent first variceal hemorrhages have not been performed.40 Research to improve the outcome of the TIPS procedure is ongoing, but currently this procedure has no role in primary prevention of variceal bleeding.
ENDOSCOPIC SCLEROTHERAPY MAY INCREASE THE RISK OF DEATH
Numerous clinical trials evaluated sclerotherapy as prophylaxis against a first esophageal variceal hemorrhage. The procedure involves injecting a sclerosant in and around varices.
In a large Veterans Administration study,41 sclerotherapy was compared with sham treatment in 281 men with alcoholic liver disease who had documented varices but no history of bleeding. The trial was terminated after 22.5 months because the rate of all-cause mortality was significantly higher in the sclerotherapy group (32.5%) than in the sham therapy group (17.4%). The higher death rate did not persist after the treatment was discontinued, and it was speculated that, although sclerotherapy had reduced new episodes of variceal hemorrhage, the procedure might have caused bleeding from esophageal ulcers, leading to an increased mortality rate in that group.
The PROVA Study Group from Norway and Denmark found similar results when 286 cirrhotic patients were randomized to receive sclerotherapy, propranolol, combination sclerotherapy and propranolol, or no treatment to prevent a first variceal hemorrhage.42 The incidence of variceal bleeding was almost identical in the four groups, but the mortality rate with variceal bleeding was 2.75 times higher in the sclerotherapy groups than in the other groups (P = .002). It was speculated that repeated sclerotherapy sessions might be poorly tolerated by patients in Child-Pugh classes B and C and might have contributed to the precipitation of liver failure and other common complications of cirrhosis.
A meta-analysis by D’Amico et al11 evaluated 19 trials (1,630 patients) comparing sclerotherapy with nonactive treatment. Sclerotherapy tended to be favorable in trials with a high bleeding rate in the control patients and unfavorable in trials with a low bleeding rate. The benefit seen in patients at high risk is consistent with the efficacy of sclerotherapy for preventing rebleeding, whereas the harmful effect in the low-risk patients points towards side effects and complications exceeding the potential benefits.
In general, currently available evidence suggests that the benefits of prophylactic sclerotherapy are marginal, and therefore sclerotherapy is not recommended as primary prophylaxis for variceal hemorrhage.
NITRATES: NO LONGER USED AS MONOTHERAPY
Unlike vasoconstrictors, which decrease portal pressure by decreasing blood flow, vasodilators reduce hepatic pressure by decreasing intrahepatic and portocollateral vascular resistance.43 In addition, larger doses directly affect the arterial circulation, lowering systemic and therefore splanchnic perfusion pressure.44 Unfortunately, the systemic vasodilatory effects of nitrates exacerbate the hyperdynamic state that is characteristic of cirrhosis, thereby limiting their use and tolerability in many patients.
A trial comparing propranolol vs isosorbide mononitrate initially found that the groups did not differ significantly with regard to bleeding rates and 2-year survival rates,45 but a 6-year follow-up found the likelihood of death greater in patients older than 50 years in the nitrate group.46 In an additional study comparing isosorbide mononitrate vs placebo in patients with contraindications to or intolerance of beta-blockers, no difference in the relative risk of first variceal hemorrhage was found between the two groups.47 Therefore, nitrates are no longer used as monotherapy to prevent variceal bleeding.
Combination therapy with beta-blockers plus nitrates is controversial. In a trial in 1996, Merkel et al48 found the cumulative risk of variceal bleeding was 18% at 40 months with nadolol alone vs 7.5% with nadolol plus isosorbide mononitrate. However, in a later trial, Garcia-Pagán et al49 found no significant advantage to combination therapy. The incidence of variceal bleeding at 1 year was 8.3% in the group receiving propranolol plus placebo and 5% in the group receiving propranolol plus isosorbide mononitrate; at 2 years, the rates were 10.6% vs 12.5%.
RECOMMENDATIONS FOR SCREENING AND PROPHYLAXIS
- All patients with cirrhosis should be screened for varices at the time of diagnosis.
- The size of the varices, including small (≤ 5 mm) and large (> 5 mm), and the presence of red wale marks on the varices should be recorded.
- Patients who have no varices on screening endoscopy should be rescreened every 3 years if their liver function is stable or every year if their liver function deteriorates. (Varices grow at a rate proportional to the severity of the liver disease.)
- Patients with portal hypertension but without varices do not need treatment with nonselective beta-blockers. Endoscopy should be performed at the intervals suggested above.
- Those who are found to have small varices on screening endoscopy but who have well-compensated liver disease (Child-Pugh class A) and no red wale marks should be rescreened every other year because the development of large varices is greater in patients with small varices on initial endoscopy than in patients with no varices. Emerging data support the use of beta-blockers to prevent varices from increasing in size.
- Patients who have small varices with red wale signs or who are in Child-Pugh class B or C have an increased risk of bleeding and should be treated with beta-blockers. If beta-blockers are not used, endoscopy should be done every year to look for an increase in variceal size.
- Patients who have large varices without red wale signs or who are in Child-Pugh class B or C should be treated with nonselective beta-blockers. The dose should be adjusted to achieve maximal tolerable decrease in heart rate to a minimum of 55 beats per minute, and treatment should be continued indefinitely.
- Endoscopic variceal ligation is an acceptable alternative to beta-blocker treatment as first-line therapy in those who cannot tolerate beta-blockers or who have contraindications to their use, or in those who have red wale marking or who are in Child-Pugh class B or C.
Variceal hemorrhage is a medical emergency in which up to 20% of patients die.1 Even if the patient survives an initial episode of variceal bleeding, the probability of another episode is high: the rebleeding rate without treatment is 70% within 1 year. The mortality rate with rebleeding is 33%.
With such overwhelming consequences, the best strategy in any patient with cirrhosis and known varices is to try to prevent the first episode of bleeding.
WHO IS AT RISK?
Esophageal varices are present in 30% of patients with compensated cirrhosis and in up to 60% of those with decompensated cirrhosis (ie, with evidence of ascites or encephalopathy).2
The risk of variceal hemorrhage is related to three factors:
- The size of the varices. Varices 5 mm in diameter or smaller have a 7% risk of bleeding in 2 years, while those larger than 5 mm have a 30% risk of bleeding within 2 years.3
- The appearance of the varices. Morphologic features of varices, including red wale signs (red streaks of the mucosa overlying the varix), have been correlated with an increased risk of hemorrhage.
- The severity of liver dysfunction, as assessed by the Child-Pugh classification—an index of liver dysfunction based on serum albumin concentration, bilirubin level, prothrombin time, and the presence of ascites and encephalopathy. A high Child-Pugh score (ie, class B or C), representing decompensated cirrhosis, is associated with an increased risk of bleeding.
HOW VARICES DEVELOP: PORTAL HYPERTENSION
Esophageal varices form as a result of increased portal pressure, the product of increased portal venous inflow and resistance to outflow from the portal venous system. Portal hypertension is a major complication of chronic liver disease. In cirrhosis, architectural distortion of the liver causes an increase in the intrahepatic vascular resistance.
Portal venous inflow depends on mesenteric arteriolar tone, increasing when tone decreases. In cirrhotic patients, the increase in portal pressure results from a combination of increased portal blood flow secondary to splanchnic arteriolar vasodilation and elevated resistance to outflow through distorted hepatic sinusoids.
The potent vasodilator nitric oxide (NO) plays an important role in portal hypertension. In patients with cirrhosis, NO bioavailability is decreased in the intrahepatic circulation due to defects in the posttranslational regulation of endothelial NO synthase.4 This deficiency of NO, along with mechanical factors in the sinusoids, contributes to the increase in intrahepatic resistance. In the systemic and splanchnic circulation, NO bioavailability is increased due to upregulation and posttranslational regulation of endothelial NO synthase, thereby increasing splanchnic vasodilatation and leading to increased portal venous inflow.5 This results in a marked increase in cardiac output and so-called hyperdynamic circulation.
Portal hypertension results in the development of collateral circulation, including venous channels in the esophagus and stomach, by the dilation of preexisting vessels and active angiogenesis. Esophagogastric varices increase in size with the severity of portal hypertension and can rupture when the tension in their walls exceeds a maximal point.
HEPATIC VEIN PRESSURE GRADIENT: A PROXY FOR PORTAL PRESSURE
Ideally, the portal venous pressure should be directly measured. However, since direct measurement is invasive and impractical, the hepatic vein pressure gradient (HVPG) can be measured instead and correlates well with the portal pressure.6
The normal HVPG is 5 mm Hg or less; anything above this value denotes portal hypertension. However, studies have shown that varices may develop but do not bleed if the HVPG is less than 12 mm Hg.8
TWO WAYS TO PREVENT BLEEDING
Bleeding can be prevented either by reducing the portal venous pressure or by obliterating the varices. Portal pressure can be reduced by placing a portosystemic shunt either surgically or percutaneously with radiographic guidance or by giving drugs such as nonselective beta-blockers, nitrates, or a combination of these drugs. Variceal obliteration is typically done by endoscopic methods with either injection of a sclerosant or band ligation.
NONSELECTIVE BETA-BLOCKERS: THE MAINSTAY OF TREATMENT
Nonselective beta-blockers, the most commonly used drugs for preventing first esophageal variceal bleeding, decrease portal pressure by blocking both beta-1 and beta-2 adrenergic receptors.9 Beta-1 blockade decreases portal flow by decreasing the heart rate and cardiac output, while blockade of beta-2 receptors results in unopposed alpha-adrenergic-mediated vasoconstriction.
Selective beta-blockers do not appear to be as useful for primary prophylaxis. More than 2 decades ago, metoprolol (Toprol, Lopressor), a beta-1 selective antagonist, was compared with propranolol (Inderal), a nons-elective agent, in patients with cirrhosis and portal hypertension.10 Although both drugs significantly reduced the heart rate and cardiac output, only those taking propranolol showed a marked fall in portal pressure (mean decrease of 6.8 mm Hg vs 3.8 mm Hg with metoprolol) and a significant reduction in hepatic blood flow. The differences were thought to be related to beta-2 blockade of vasodilator receptors in the splanchnic circulation, which occurs only with nonselective beta-blockers such as propranolol.
The two nonselective beta-blockers most often used to prevent variceal bleeding are nadolol (Corgard) and propranolol. Both have been extensively studied in preventing a first variceal hemorrhage.
Effectiveness of beta-blockers
D’Amico et al11 performed a meta-analysis in 1995, examining nine trials (996 patients total) of the effectiveness of beta-blockers in preventing a first variceal hemorrhage. Seven trials found that bleeding risk was reduced with beta-blockers (significantly in four), one trial found that risk was unchanged, and one trial found that risk was increased—an outlier due to a small sample size. The meta-analysis showed a significant bleeding reduction with the use of a beta-blocker, either including the outlier trial (pooled odds ratio 0.54; 95% confidence interval 0.39–0.74) or excluding it (pooled odds ratio 0.48; 95% confidence interval 0.35–0.66).
Mortality rates were also reduced in seven trials, but the reduction was statistically significant in only one. However, in the pooled estimate, the mortality risk reduction approached statistical significance (pooled odds ratio 0.75; 95% confidence interval 0.57–1.06).
Ideo et al12 gave either nadolol or placebo to 79 patients with cirrhosis and large esophageal varices that had never bled. Nadolol was found to protect against a first variceal hemorrhage: at 2-year follow-up, only 1 of the 30 patients allocated to nadolol had had bleeding, vs 11 of the 49 patients in the placebo group.
Merkel et al13 found that the risk of variceal bleeding was lower in patients who started treatment with beta-blockers when their varices were small (12% at 5 years) than in those who started treatment after a diagnosis of large esophageal varices (22% at 5 years). They concluded that nadolol helps prevent small varices from growing into larger ones.
Response to beta-blockers is not uniform
Although beta-blockers decrease the portal pressure in many cirrhotic patients, the response is not uniform. In a study of 60 cirrhotic patients,14 40% showed no reduction or even a slight increase in HVPG with propranolol. Most patients showed a significant reduction in heart rate (17.5% ± 10%) after receiving 40 mg of propranolol. In the patients whose HVPG did not decrease by at least 10% with 40 mg of propranolol, increasing the dose caused a decrease in HVPG without a further decrease in heart rate. This suggests that 40 mg of propranolol successfully produced beta-1 blockade but that a higher dose was required for effective beta-2 blockade.
Failure to respond in certain patients may be due to a concurrent rise in collateral or hepatic sinusoidal resistance, or both. This was confirmed in a study in portal-hypertensive rats treated with propranolol.15 The reduction in portal blood flow expected was accompanied by a disproportionately small reduction in portal pressure, which was thought to be due to a rise in portal and collateral vascular resistance.
How to tell if beta-blocker treatment is ‘working’
An HVPG ≤ 12 mm Hg? Studies have shown that the most important predictor of efficacy of prophylaxis for variceal bleeding is a decrease in the HVPG to 12 mm Hg or less or a decrease in the initial HVPG of more than 20%.9 Although measuring the HVPG is invasive, expensive, and not routinely done in clinical practice, several studies have investigated the role of measuring hemodynamic response to medication.
Merkel et al16 measured the HVPG in 49 cirrhotic patients with previously nonbleeding varices before starting therapy with beta-blockers with or without nitrates and after 1 to 3 months of treatment. They followed the patients for up to 5 years. The mean HVPG value at baseline was 18.8 mm Hg. At 3 years of follow-up, 7% of those who had responded well to therapy (defined as achieving an HVPG less than 13 mm Hg or a decrease of more than 20%) had experienced a bleeding episode, which was significantly less than the rate (41%) in those who did not meet those hemodynamic end points. No patient reaching an HVPG of 12 mm Hg or less during treatment had variceal bleeding during follow-up.
Groszmann et al17 also prospectively measured the HVPG in patients with cirrhosis and varices, but their patients received either propranolol or placebo. Variceal hemorrhage occurred in 13 patients (11 of 51 in the placebo group and 2 of 51 in the propranolol group), all of whom had an HVPG greater than 12 mm Hg. Again, none of the patients whose HVPG was decreased to 12 mm Hg or less bled from esophageal varices.
Unfortunately, routine HVPG measurement to guide primary prophylaxis is an expensive strategy. Data suggest that measuring the HVPG is cost-effective only when the cost of measuring the HVPG is very low, the risk of variceal bleeding is very high, or the patient is expected to survive at least 3 to 5 years.18
A heart rate of 55 to 60? An alternative to HVPG measurement to monitor the effectiveness of beta-blocker therapy is to follow the heart rate. A 25% reduction from baseline or a heart rate of 55 to 60 beats per minute is the standard goal19,20; yet, at least 40% of patients treated with enough propranolol to decrease the heart rate by 25% do not respond with significant HVPG reductions.14,21
So, although beta-blockade is effective peripherally, it may not reduce HVPG to less than 12 mm Hg or 20% from baseline, and direct HVPG measurement is still the gold standard.
Treatment should be lifelong
Once a patient is started on a beta-blocker to prevent variceal hemorrhage, the treatment should be lifelong.
In 2001, a group of patients (most of them in Child-Pugh class A or B) completing a prospective randomized controlled trial of propranolol for primary prevention of variceal hemorrhage were tapered off propranolol or placebo.22 Of the 49 patients, 9 experienced variceal hemorrhage (6 of 25 former propranolol recipients and 3 of 24 former placebo recipients), and 17 patients died (12 former propranolol and 5 former placebo recipients), suggesting that treatment should be maintained for life.
Therefore, when beta-blocker therapy is discontinued, the risk of variceal hemorrhage returns to what would be expected in an untreated population.
Beta-blockers may not prevent varices
Although many trials have shown that beta-blockers are effective as prophylaxis against a first variceal hemorrhage, there is no evidence that these drugs prevent varices from forming in cirrhotic patients.
Groszmann et al23 treated more than 200 patients who had biopsy-proven cirrhosis and portal hypertension (HVPG > 6 mm Hg) but no varices with timolol (Blocadren), a nonselective beta-blocker, or placebo. At a median follow-up of about 55 months, the groups did not differ significantly in the incidence of primary events (development of varices or variceal hemorrhage) or treatment failures (transplantation or death). Varices developed less frequently among patients with a baseline HVPG of less than 10 mm Hg and among those whose HVPG had decreased by more than 10% at 1 year. In patients whose HVPG increased by more than 10%, varices developed more frequently.
Contraindications, side effects
The major drawbacks to therapy with beta-blockers are their contraindications and side effects.
Contraindications include chronic obstructive lung disease, psychosis, atrioventricular heart blocks, and aortic-valve disease.
Side effects are reported in 15% of patients but severe events are rare.24 Still, an estimated 10% to 20% of patients discontinue treatment because they cannot tolerate it.25 The more common complaints include fatigue, shortness of breath, sexual dysfunction, and sleep disorders.
Dosage
No specific starting dose of beta-blockers is agreed upon, but nadolol 20 to 40 mg once daily or long-acting propranolol 60 mg once daily can be used as initial therapy.25 Once-daily dosing increases the likelihood of compliance.
Since portal pressure progressively declines from 12 noon to 7 PM and then increases throughout the night and back to baseline by 9 AM,26 we recommend that the medication be taken in the evening to counteract increases in portal pressure that occur in the middle of the night.
ENDOSCOPIC VARICEAL LIGATION
Endoscopic variceal ligation has been investigated extensively for use as prophylaxis against first variceal hemorrhage. The procedure involves placing a rubber band around a varix aspirated into a cylinder on the tip of an endoscope.
Effectiveness of ligation
Lay et al,27 in a prospective, randomized trial in 126 cirrhotic patients endoscopically judged to be at high risk of hemorrhage, found that ligation significantly reduced the 2-year cumulative bleeding rate (19% with ligation vs 60% in an untreated control group) and the overall mortality rate (28% vs 58%). The lower risk of bleeding in the ligation group was attributed to a rapid reduction of variceal size; 60% of those in the ligation group had complete eradication of varices and 38% had varices reduced in size.
Imperiale and Chalasani28 performed a meta-analysis in 2001 that included 601 patients in five trials comparing prophylactic ligation with untreated controls and 283 patients in four trials comparing ligation with beta-blocker therapy. Compared with no treatment, ligation reduced the risk of first variceal hemorrhage, bleeding-related death, and death from any cause. Compared with propranolol, ligation reduced the risk of first-time bleeding but had no effect on the death rate.
Schepke et al,29 in a randomized controlled multicenter trial in 152 cirrhotic patients with two or more esophageal varices, found that neither bleeding incidence nor death rate differed significantly between ligation and propranolol.
Lui et al30 followed 172 cirrhotic patients with grade II or III esophageal varices for 6 years and found that ligation was equivalent to propranolol. However, many patients reported side effects with propranolol, and 30% of patients withdrew from propranolol treatment, making ligation a more attractive option.
Khuroo et al31 performed a meta-analysis of eight randomized controlled trials including 596 patients and found that ligation significantly reduced the rates of first gastrointestinal hemorrhage by 31% and of first variceal hemorrhage by 43%. In subgroup analysis, ligation had a significant advantage compared with beta-blockers in trials with patients with a high bleeding risk, ie, trials in which more than 30% of patients were in Child-Pugh class C and more than 50% of the patients had large varices.
Jutabha et al32 performed a multicenter, prospective trial (published in 2005) in 62 patients with high-risk esophageal varices randomized to propranolol or banding. The trial was ended early after an interim analysis showed that the failure rate of propranolol was significantly higher than that of banding (6/31 vs 0/31, P = .0098). Esophageal variceal hemorrhage occurred in 4 (12.9%) of the patients in the propranolol group compared with 0 in the ligation group. Similarly, 4 patients in the propranolol group died, compared with 0 in the ligation group. All the patients in this trial were liver transplant candidates and therefore all had severe liver disease.
In another trial favoring variceal banding over beta-blockers, Psilopoulos et al33 in 2005 followed 60 patients with cirrhosis and esophageal varices with no history of bleeding. Thirty percent of the patients in the propranolol group developed variceal bleeding compared with 6.7% in the ligation group (P = .043).
Lay et al34 followed 100 cirrhotic patients for 2 years and found comparable cumulative bleeding rates with ligation vs propranolol (18% vs 16%, respectively) and also comparable rates of death (28% vs 24%, respectively).
Sarin et al35 investigated the role of propranolol in addition to ligation in the prevention of first hemorrhage in 144 patients. Adding propranolol did not further decrease the incidence of initial bleeding (7% in the combination group vs 11% in the ligation-only group). Survival rates were similar at 20 months: 92% in the combination group vs 85% in the ligation-only group. However, the rate of variceal recurrence was lower with combination therapy: 6% in the combination group vs 15% with ligation alone.
Does esophageal variceal ligation increase gastric varices?
A less researched topic is whether variceal ligation results in gastric hemodynamic changes that increase the size of fundal varices and worsen portal hypertensive gastropathy.
Yuksel et al36 found that 37 of 85 patients had fundal varices before they underwent ligation of esophageal varices, increasing to 46 after the procedure, a statistically significant increment. The severity of portal hypertensive gastropathy also increased.
Further research is required regarding the long-term consequences of these findings.
ANGIOTENSIN II RECEPTOR ANTAGONISTS: ROLE UNKNOWN
Angiotensin II increases portal pressure, and angiotensin II levels are elevated in patients with cirrhosis, suggesting that this hormone plays a role in the pathogenesis of portal hypertension.
Losartan (Cozaar), an angiotensin II receptor antagonist, was found to decrease the HVPG significantly in patients with severe and moderate portal hypertension in a pilot study37 in 1999. However, in two subsequent studies,38,39 losartan only moderately reduced the HVPG and caused hypotension and a reduction in the glomerular filtration rate. The role of angiotensin II receptor blockers in primary prevention of variceal bleeding is still unknown.
SURGICAL PORTAL DECOMPRESSION HAS BEEN ABANDONED
The first method investigated to prevent variceal bleeding was surgical portal decompression.
A meta-analysis of four randomized controlled trials in 302 patients with varices of all sizes compared portocaval shunt surgery and medical therapy.11 Although shunt surgery was very effective in preventing variceal bleeding, the risk of chronic or recurrent encephalopathy was significantly increased (odds ratio 2.0), as was the risk of death (odds ratio 1.6).
These poor results, combined with advances in endoscopic procedures, led to the abandonment of surgical shunting for primary prophylaxis.
TIPS PROCEDURE: NO ROLE AT PRESENT
The transjugular intrahepatic portosystemic shunt (TIPS) procedure is used to treat the main consequences of portal hypertension, including ascites and variceal hemorrhage. The procedure entails accessing the hepatic vein via the right jugular vein and placing a stent to the portal vein, forming a low-resistance channel and allowing blood to return to the systemic circulation.
TIPS placement increases the risk of encephalopathy; liver failure is a rare complication, and procedural complications (ie, shunt dysfunction) also occur. Trials comparing the TIPS procedure with other forms of therapy to prevent first variceal hemorrhages have not been performed.40 Research to improve the outcome of the TIPS procedure is ongoing, but currently this procedure has no role in primary prevention of variceal bleeding.
ENDOSCOPIC SCLEROTHERAPY MAY INCREASE THE RISK OF DEATH
Numerous clinical trials evaluated sclerotherapy as prophylaxis against a first esophageal variceal hemorrhage. The procedure involves injecting a sclerosant in and around varices.
In a large Veterans Administration study,41 sclerotherapy was compared with sham treatment in 281 men with alcoholic liver disease who had documented varices but no history of bleeding. The trial was terminated after 22.5 months because the rate of all-cause mortality was significantly higher in the sclerotherapy group (32.5%) than in the sham therapy group (17.4%). The higher death rate did not persist after the treatment was discontinued, and it was speculated that, although sclerotherapy had reduced new episodes of variceal hemorrhage, the procedure might have caused bleeding from esophageal ulcers, leading to an increased mortality rate in that group.
The PROVA Study Group from Norway and Denmark found similar results when 286 cirrhotic patients were randomized to receive sclerotherapy, propranolol, combination sclerotherapy and propranolol, or no treatment to prevent a first variceal hemorrhage.42 The incidence of variceal bleeding was almost identical in the four groups, but the mortality rate with variceal bleeding was 2.75 times higher in the sclerotherapy groups than in the other groups (P = .002). It was speculated that repeated sclerotherapy sessions might be poorly tolerated by patients in Child-Pugh classes B and C and might have contributed to the precipitation of liver failure and other common complications of cirrhosis.
A meta-analysis by D’Amico et al11 evaluated 19 trials (1,630 patients) comparing sclerotherapy with nonactive treatment. Sclerotherapy tended to be favorable in trials with a high bleeding rate in the control patients and unfavorable in trials with a low bleeding rate. The benefit seen in patients at high risk is consistent with the efficacy of sclerotherapy for preventing rebleeding, whereas the harmful effect in the low-risk patients points towards side effects and complications exceeding the potential benefits.
In general, currently available evidence suggests that the benefits of prophylactic sclerotherapy are marginal, and therefore sclerotherapy is not recommended as primary prophylaxis for variceal hemorrhage.
NITRATES: NO LONGER USED AS MONOTHERAPY
Unlike vasoconstrictors, which decrease portal pressure by decreasing blood flow, vasodilators reduce hepatic pressure by decreasing intrahepatic and portocollateral vascular resistance.43 In addition, larger doses directly affect the arterial circulation, lowering systemic and therefore splanchnic perfusion pressure.44 Unfortunately, the systemic vasodilatory effects of nitrates exacerbate the hyperdynamic state that is characteristic of cirrhosis, thereby limiting their use and tolerability in many patients.
A trial comparing propranolol vs isosorbide mononitrate initially found that the groups did not differ significantly with regard to bleeding rates and 2-year survival rates,45 but a 6-year follow-up found the likelihood of death greater in patients older than 50 years in the nitrate group.46 In an additional study comparing isosorbide mononitrate vs placebo in patients with contraindications to or intolerance of beta-blockers, no difference in the relative risk of first variceal hemorrhage was found between the two groups.47 Therefore, nitrates are no longer used as monotherapy to prevent variceal bleeding.
Combination therapy with beta-blockers plus nitrates is controversial. In a trial in 1996, Merkel et al48 found the cumulative risk of variceal bleeding was 18% at 40 months with nadolol alone vs 7.5% with nadolol plus isosorbide mononitrate. However, in a later trial, Garcia-Pagán et al49 found no significant advantage to combination therapy. The incidence of variceal bleeding at 1 year was 8.3% in the group receiving propranolol plus placebo and 5% in the group receiving propranolol plus isosorbide mononitrate; at 2 years, the rates were 10.6% vs 12.5%.
RECOMMENDATIONS FOR SCREENING AND PROPHYLAXIS
- All patients with cirrhosis should be screened for varices at the time of diagnosis.
- The size of the varices, including small (≤ 5 mm) and large (> 5 mm), and the presence of red wale marks on the varices should be recorded.
- Patients who have no varices on screening endoscopy should be rescreened every 3 years if their liver function is stable or every year if their liver function deteriorates. (Varices grow at a rate proportional to the severity of the liver disease.)
- Patients with portal hypertension but without varices do not need treatment with nonselective beta-blockers. Endoscopy should be performed at the intervals suggested above.
- Those who are found to have small varices on screening endoscopy but who have well-compensated liver disease (Child-Pugh class A) and no red wale marks should be rescreened every other year because the development of large varices is greater in patients with small varices on initial endoscopy than in patients with no varices. Emerging data support the use of beta-blockers to prevent varices from increasing in size.
- Patients who have small varices with red wale signs or who are in Child-Pugh class B or C have an increased risk of bleeding and should be treated with beta-blockers. If beta-blockers are not used, endoscopy should be done every year to look for an increase in variceal size.
- Patients who have large varices without red wale signs or who are in Child-Pugh class B or C should be treated with nonselective beta-blockers. The dose should be adjusted to achieve maximal tolerable decrease in heart rate to a minimum of 55 beats per minute, and treatment should be continued indefinitely.
- Endoscopic variceal ligation is an acceptable alternative to beta-blocker treatment as first-line therapy in those who cannot tolerate beta-blockers or who have contraindications to their use, or in those who have red wale marking or who are in Child-Pugh class B or C.
- D’Amico G, De Franchis R Cooperative Study Group. Upper digestive bleeding in cirrhosis. Post-therapeutic outcome and prognostic indicators. Hepatology 2003; 38:599–612.
- D’Amico G, Luca A. Natural history. Clinicalhaemodynamic correlations. Prediction of the risk of bleeding. Baillieres Clin Gastroenterol 1997; 11:243–256.
- Kamath PS. Esophageal variceal bleeding: primary prophylaxis. Clin Gastroenterol Hepatol 2005; 3:90–93.
- Shah V, Toruner M, Haddad F, et al. Impaired endothelial nitric oxide synthase activity associated with enhanced caveolin binding in experimental cirrhosis in the rat. Gastroenterology 1999; 117:1222–1228.
- Morales-Ruiz M, Jimenez W, Perez-Sala D, et al. Increased nitric oxide synthase expression in arterial vessels of cirrhotic rats with ascites. Hepatology 1996; 24:1481–1486.
- De Franchis R, Dell’Era A, Iannuzzi F. Diagnosis and treatment of portal hypertension. Dig Liver Dis 2004; 36:787–798.
- Groszmann RJ, Wongcharatrawee S. The hepatic venous pressure gradient: anything worth doing should be done right. Hepatology 2004; 39:280–282.
- Garcia-Tsao G, Groszmann RJ, Fisher RL, Conn HO, Atterbury CE, Glickman M. Portal pressure, presence of gastroesophageal varices and variceal bleeding. Hepatology 1985; 5:419–424.
- Bosch J, Masti R, Kravetz D, et al. Effects of propranolol on azygos venous blood flow and hepatic and systemic hemodynamics in cirrhosis. Hepatology 1984; 4:1200–1205.
- Westaby D, Bihari DJ, Gimson AE, Crossley IR, Williams R. Selective and non-selective beta receptor blockade in the reduction of portal pressure in patients with cirrhosis and portal hypertension. Gut 1984; 25:121–124.
- D’Amico G, Pagliaro L, Bosch J. The treatment of portal hypertension: a meta-analytic review. Hepatology 1995; 22:332–354.
- Ideo G, Bellati G, Fesce E, Grimoldi D. Nadolol can prevent the first gastrointestinal bleeding in cirrhotics: a prospective, randomized study. Hepatology 1988; 8:6–9.
- Merkel C, Marin R, Angeli P, et al. Gruppo Triveneto per l’Ipertensione Portale. A placebo-controlled clinical trial of nadolol in the prophylaxis of growth of small esophageal varices in cirrhosis. Gastroenterology 2004; 127:476–484.
- Garcia-Tsao G, Grace ND, Groszmann RJ, et al. Short-term effects of propranolol on portal venous pressure. Hepatology 1986; 6:101–106.
- Kroeger RJ, Groszmann RJ. Increased portal venous resistance hinders portal pressure reduction during the administration of beta-adrenergic blocking agents in a portal hypertensive model. Hepatology 1985; 5:97–101.
- Merkel C, Bolognesi M, Sacerdoti D, et al. The hemodynamic response to medical treatment of portal hypertension as a predictor of clinical effectiveness in the primary prophylaxis of variceal bleeding in cirrhosis. Hepatology 2000; 32:930–934.
- Groszmann RJ, Bosch J, Grace ND, et al. Hemodynamic events in a prospective randomized trial of propranolol versus placebo in the prevention of a first variceal hemorrhage. Gastroenterology 1990; 99:1401–1407.
- Hicken BL, Sharara AI, Abrams GA, Eloubeidi M, Fallon MB, Arguedas MR. Hepatic venous pressure gradient measurements to assess response to primary prophylaxis in patients with cirrhosis: a decision analytical study. Aliment Pharmacol Ther 2003; 17:145–153.
- Grace ND. Diagnosis and treatment of gastrointestinal bleeding secondary to portal hypertension. American College of Gastroenterology Practice Parameters Committee. Am J Gastroenterol 1997; 92:1081–1091.
- Grace ND, Groszmann RJ, Garcia-Tsao G, et al. Portal hypertension and variceal bleeding: an AASLD single topic symposium. Hepatology 1998; 28:868–880.
- Feu F, Garcia-Pagán JC, Bosch J, et al. Relation between portal pressure response to pharmacotherapy and risk of recurrent variceal haemorrhage in patients with cirrhosis. Lancet 1995; 346:1056–1059.
- Abraczinskas DR, Ookubo R, Grace ND, et al. Propranolol for the prevention of first esophageal variceal hemorrhage: a lifetime commitment? Hepatology 2001; 34:1096–1102.
- Groszmann RJ, Garcia-Tsao G, Bosch J, et al Portal Hypertension Collaborative Group. Beta-blockers to prevent gastroesophageal varices in patients with cirrhosis. N Engl J Med 2005; 353:2254–2261.
- Garcia-Pagán JC, Bosch J. Pharmacological prevention of variceal bleeding. New developments. Baillier Clin Gastroenterol 1997; 11:271–287.
- Talwalkar JA, Kamath PS. An evidence-based medicine approach to beta-blocker therapy in patients with cirrhosis. Am J Med 2004; 116:759–766.
- Garcia-Pagan JC, Feu F, Castells A, et al. Circadian variations of portal pressure and variceal hemorrhage in patients with cirrhosis. Hepatology 1994; 19:595–601.
- Lay CS, Tsai YT, Teg CY, et al. Endoscopic variceal ligation in prophylaxis of first variceal bleeding in cirrhotic patients with high-risk esophageal varices. Hepatology 1997; 25:1346–1350.
- Imperiale TF, Chalasani N. A meta-analysis of endoscopic variceal ligation for primary prophylaxis of esophageal variceal bleeding. Hepatology 2001; 33:802–807.
- Schepke M, Kleber G, Nurnberg D, et al. Ligation versus propranolol for the primary prophylaxis of variceal bleeding in cirrhosis. Hepatology 2004; 40:65–72.
- Lui HF, Stanley AJ, Forrest EH, et al. Primary prophylaxis of variceal hemorrhage: a randomized controlled trial comparing band ligation, propranolol, and isosorbide mononitrate. Gastroenterology 2002; 123:735–744.
- Khuroo MS, Khuroo NS, Farahat KL, Khuroo YS, Sofi AA, Dahab ST. Meta-analysis: endoscopic variceal ligation for primary prophylaxis of oesophageal variceal bleeding. Aliment Pharmacol Ther 2005; 21:347–361.
- Jutabha R, Jensen DM, Martin P, Savides T, Han SH, Gornbein J. Randomized study comparing banding and propranolol to prevent initial variceal hemorrhage in cirrhotics with high-risk esophageal varices. Gastroenterology 2005; 128:870–881.
- Psilopoulos D, Galanis P, Goulas S, et al. Endoscopic variceal ligation vs. propranolol for prevention of first variceal bleeding: a randomized controlled trial. Eur J Gastroenterol Hepatol 2005; 17:1111–1117.
- Lay CS, Tsai YT, Lee FY, et al. Endoscopic variceal ligation versus propranolol in prophylaxis of first variceal bleeding in patients with cirrhosis. J Gastroenterol Hepatol 2006; 21:413–419.
- Sarin SK, Wadhawan M, Agarwal SR, Tyagi P, Sharma BC. Endoscopic variceal ligation plus propranolol versus endoscopic variceal ligation alone in primary prophylaxis of variceal bleeding. Am J Gastroenterol 2005; 100:797–804.
- Yuksel O, Koklu S, Arhan M, Yolcu OF, et al. Effects of esophageal variceal eradication on portal hypertensive gastropathy and fundal varices: a retrospective and comparative study. Dig Dis Sci 2006; 51:27–30.
- Schneider AW, Kalk JF, Klein CP. Effect of losartan, an angiotensin II receptor antagonist, on portal pressure in cirrhosis. Hepatology 1999; 29:334–339.
- Schepke M, Werner E, Biecker E, et al. Hemodynamic effects of the angiotensin II receptor antagonist irbesartan in patients with cirrhosis and portal hypertension. Gastroenterology 2001; 121:389–395.
- Gonzalez-Abraldes J, Albillos A, Banares R, et al. Randomized comparison of long-term losartan versus propranolol in lowering portal pressure in cirrhosis. Gastroenterology 2001; 121:382–388.
- Boyer TD, Haskal ZJ American Association for the Study of Liver Diseases. The role of transjugular intrahepatic portosystemic shunt in the management of portal hypertension. Hepatology 2005; 41:386–400.
- Prophylactic sclerotherapy for esophageal varices in men with alcoholic liver disease. A randomized, single-blind, multicenter clinical trial. The Veterans Affairs Cooperative Variceal Sclerotherapy Group. N Engl J Med 1991; 324:1779–1784.
- The PROVA Study Group. Prophylaxis of first hemorrhage from esophageal varices by sclerotherapy, propranolol or both in cirrhotic patients: a randomized multicenter trial. Hepatology 1991; 14:1016–1024.
- Escorsell A, Feu F, Bordas JM, et al. Effects of isosorbide-5-mononitrate on variceal pressure and systemic and splanchnic haemodynamics in patients with cirrhosis. J Hepatol 1996; 24:423–429.
- Hayes PC, Westaby D, Williams R. Effect and mechanism of action of isosorbide-5-mononitrate. Gut 1988; 29:752–755.
- Angelico M, Carli L, Piat C, et al. Isosorbide-5-mononitrate versus propranolol in the prevention of first bleeding in cirrhosis. Gastroenterology 1993; 104:1460–1465.
- Angelico M, Carli L, Piat C, Gentile S, Capocaccia L. Effects of isosorbide-5-mononitrate compared with propranolol on first bleeding and long-term survival in cirrhosis. Gastroenterology 1997; 113:1632–1639.
- Garcia-Pagan JC, Villanueva C, Vila MC, et al. MOVE Group. Mononitrato Varices Esofagicas. Isosorbide mononitrate in the prevention of first variceal bleed in patients who cannot receive beta-blockers. Gastroenterology 2001; 121:908–914.
- Merkel C, Marin R, Enzo E, et al. Randomised trial of nadolol alone or with isosorbide mononitrate for primary prophylaxis of variceal bleeding in cirrhosis. Gruppo-Triveneto per L’ipertensione portale (GTIP). Lancet 1996; 348:1677–1681.
- Garcia-Pagán JC, Morillas R, Banares R, et al Spanish Variceal Bleeding Study Group. Propranolol plus placebo versus propranolol plus isosorbide-5-mononitrate in the prevention of a first variceal bleed: a double-blind RCT. Hepatology 2003; 37:1260–1266.
- Garcia-Tsao G, Sanyal A, Grace N, et al. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology 2007; 46:922–938.
- D’Amico G, De Franchis R Cooperative Study Group. Upper digestive bleeding in cirrhosis. Post-therapeutic outcome and prognostic indicators. Hepatology 2003; 38:599–612.
- D’Amico G, Luca A. Natural history. Clinicalhaemodynamic correlations. Prediction of the risk of bleeding. Baillieres Clin Gastroenterol 1997; 11:243–256.
- Kamath PS. Esophageal variceal bleeding: primary prophylaxis. Clin Gastroenterol Hepatol 2005; 3:90–93.
- Shah V, Toruner M, Haddad F, et al. Impaired endothelial nitric oxide synthase activity associated with enhanced caveolin binding in experimental cirrhosis in the rat. Gastroenterology 1999; 117:1222–1228.
- Morales-Ruiz M, Jimenez W, Perez-Sala D, et al. Increased nitric oxide synthase expression in arterial vessels of cirrhotic rats with ascites. Hepatology 1996; 24:1481–1486.
- De Franchis R, Dell’Era A, Iannuzzi F. Diagnosis and treatment of portal hypertension. Dig Liver Dis 2004; 36:787–798.
- Groszmann RJ, Wongcharatrawee S. The hepatic venous pressure gradient: anything worth doing should be done right. Hepatology 2004; 39:280–282.
- Garcia-Tsao G, Groszmann RJ, Fisher RL, Conn HO, Atterbury CE, Glickman M. Portal pressure, presence of gastroesophageal varices and variceal bleeding. Hepatology 1985; 5:419–424.
- Bosch J, Masti R, Kravetz D, et al. Effects of propranolol on azygos venous blood flow and hepatic and systemic hemodynamics in cirrhosis. Hepatology 1984; 4:1200–1205.
- Westaby D, Bihari DJ, Gimson AE, Crossley IR, Williams R. Selective and non-selective beta receptor blockade in the reduction of portal pressure in patients with cirrhosis and portal hypertension. Gut 1984; 25:121–124.
- D’Amico G, Pagliaro L, Bosch J. The treatment of portal hypertension: a meta-analytic review. Hepatology 1995; 22:332–354.
- Ideo G, Bellati G, Fesce E, Grimoldi D. Nadolol can prevent the first gastrointestinal bleeding in cirrhotics: a prospective, randomized study. Hepatology 1988; 8:6–9.
- Merkel C, Marin R, Angeli P, et al. Gruppo Triveneto per l’Ipertensione Portale. A placebo-controlled clinical trial of nadolol in the prophylaxis of growth of small esophageal varices in cirrhosis. Gastroenterology 2004; 127:476–484.
- Garcia-Tsao G, Grace ND, Groszmann RJ, et al. Short-term effects of propranolol on portal venous pressure. Hepatology 1986; 6:101–106.
- Kroeger RJ, Groszmann RJ. Increased portal venous resistance hinders portal pressure reduction during the administration of beta-adrenergic blocking agents in a portal hypertensive model. Hepatology 1985; 5:97–101.
- Merkel C, Bolognesi M, Sacerdoti D, et al. The hemodynamic response to medical treatment of portal hypertension as a predictor of clinical effectiveness in the primary prophylaxis of variceal bleeding in cirrhosis. Hepatology 2000; 32:930–934.
- Groszmann RJ, Bosch J, Grace ND, et al. Hemodynamic events in a prospective randomized trial of propranolol versus placebo in the prevention of a first variceal hemorrhage. Gastroenterology 1990; 99:1401–1407.
- Hicken BL, Sharara AI, Abrams GA, Eloubeidi M, Fallon MB, Arguedas MR. Hepatic venous pressure gradient measurements to assess response to primary prophylaxis in patients with cirrhosis: a decision analytical study. Aliment Pharmacol Ther 2003; 17:145–153.
- Grace ND. Diagnosis and treatment of gastrointestinal bleeding secondary to portal hypertension. American College of Gastroenterology Practice Parameters Committee. Am J Gastroenterol 1997; 92:1081–1091.
- Grace ND, Groszmann RJ, Garcia-Tsao G, et al. Portal hypertension and variceal bleeding: an AASLD single topic symposium. Hepatology 1998; 28:868–880.
- Feu F, Garcia-Pagán JC, Bosch J, et al. Relation between portal pressure response to pharmacotherapy and risk of recurrent variceal haemorrhage in patients with cirrhosis. Lancet 1995; 346:1056–1059.
- Abraczinskas DR, Ookubo R, Grace ND, et al. Propranolol for the prevention of first esophageal variceal hemorrhage: a lifetime commitment? Hepatology 2001; 34:1096–1102.
- Groszmann RJ, Garcia-Tsao G, Bosch J, et al Portal Hypertension Collaborative Group. Beta-blockers to prevent gastroesophageal varices in patients with cirrhosis. N Engl J Med 2005; 353:2254–2261.
- Garcia-Pagán JC, Bosch J. Pharmacological prevention of variceal bleeding. New developments. Baillier Clin Gastroenterol 1997; 11:271–287.
- Talwalkar JA, Kamath PS. An evidence-based medicine approach to beta-blocker therapy in patients with cirrhosis. Am J Med 2004; 116:759–766.
- Garcia-Pagan JC, Feu F, Castells A, et al. Circadian variations of portal pressure and variceal hemorrhage in patients with cirrhosis. Hepatology 1994; 19:595–601.
- Lay CS, Tsai YT, Teg CY, et al. Endoscopic variceal ligation in prophylaxis of first variceal bleeding in cirrhotic patients with high-risk esophageal varices. Hepatology 1997; 25:1346–1350.
- Imperiale TF, Chalasani N. A meta-analysis of endoscopic variceal ligation for primary prophylaxis of esophageal variceal bleeding. Hepatology 2001; 33:802–807.
- Schepke M, Kleber G, Nurnberg D, et al. Ligation versus propranolol for the primary prophylaxis of variceal bleeding in cirrhosis. Hepatology 2004; 40:65–72.
- Lui HF, Stanley AJ, Forrest EH, et al. Primary prophylaxis of variceal hemorrhage: a randomized controlled trial comparing band ligation, propranolol, and isosorbide mononitrate. Gastroenterology 2002; 123:735–744.
- Khuroo MS, Khuroo NS, Farahat KL, Khuroo YS, Sofi AA, Dahab ST. Meta-analysis: endoscopic variceal ligation for primary prophylaxis of oesophageal variceal bleeding. Aliment Pharmacol Ther 2005; 21:347–361.
- Jutabha R, Jensen DM, Martin P, Savides T, Han SH, Gornbein J. Randomized study comparing banding and propranolol to prevent initial variceal hemorrhage in cirrhotics with high-risk esophageal varices. Gastroenterology 2005; 128:870–881.
- Psilopoulos D, Galanis P, Goulas S, et al. Endoscopic variceal ligation vs. propranolol for prevention of first variceal bleeding: a randomized controlled trial. Eur J Gastroenterol Hepatol 2005; 17:1111–1117.
- Lay CS, Tsai YT, Lee FY, et al. Endoscopic variceal ligation versus propranolol in prophylaxis of first variceal bleeding in patients with cirrhosis. J Gastroenterol Hepatol 2006; 21:413–419.
- Sarin SK, Wadhawan M, Agarwal SR, Tyagi P, Sharma BC. Endoscopic variceal ligation plus propranolol versus endoscopic variceal ligation alone in primary prophylaxis of variceal bleeding. Am J Gastroenterol 2005; 100:797–804.
- Yuksel O, Koklu S, Arhan M, Yolcu OF, et al. Effects of esophageal variceal eradication on portal hypertensive gastropathy and fundal varices: a retrospective and comparative study. Dig Dis Sci 2006; 51:27–30.
- Schneider AW, Kalk JF, Klein CP. Effect of losartan, an angiotensin II receptor antagonist, on portal pressure in cirrhosis. Hepatology 1999; 29:334–339.
- Schepke M, Werner E, Biecker E, et al. Hemodynamic effects of the angiotensin II receptor antagonist irbesartan in patients with cirrhosis and portal hypertension. Gastroenterology 2001; 121:389–395.
- Gonzalez-Abraldes J, Albillos A, Banares R, et al. Randomized comparison of long-term losartan versus propranolol in lowering portal pressure in cirrhosis. Gastroenterology 2001; 121:382–388.
- Boyer TD, Haskal ZJ American Association for the Study of Liver Diseases. The role of transjugular intrahepatic portosystemic shunt in the management of portal hypertension. Hepatology 2005; 41:386–400.
- Prophylactic sclerotherapy for esophageal varices in men with alcoholic liver disease. A randomized, single-blind, multicenter clinical trial. The Veterans Affairs Cooperative Variceal Sclerotherapy Group. N Engl J Med 1991; 324:1779–1784.
- The PROVA Study Group. Prophylaxis of first hemorrhage from esophageal varices by sclerotherapy, propranolol or both in cirrhotic patients: a randomized multicenter trial. Hepatology 1991; 14:1016–1024.
- Escorsell A, Feu F, Bordas JM, et al. Effects of isosorbide-5-mononitrate on variceal pressure and systemic and splanchnic haemodynamics in patients with cirrhosis. J Hepatol 1996; 24:423–429.
- Hayes PC, Westaby D, Williams R. Effect and mechanism of action of isosorbide-5-mononitrate. Gut 1988; 29:752–755.
- Angelico M, Carli L, Piat C, et al. Isosorbide-5-mononitrate versus propranolol in the prevention of first bleeding in cirrhosis. Gastroenterology 1993; 104:1460–1465.
- Angelico M, Carli L, Piat C, Gentile S, Capocaccia L. Effects of isosorbide-5-mononitrate compared with propranolol on first bleeding and long-term survival in cirrhosis. Gastroenterology 1997; 113:1632–1639.
- Garcia-Pagan JC, Villanueva C, Vila MC, et al. MOVE Group. Mononitrato Varices Esofagicas. Isosorbide mononitrate in the prevention of first variceal bleed in patients who cannot receive beta-blockers. Gastroenterology 2001; 121:908–914.
- Merkel C, Marin R, Enzo E, et al. Randomised trial of nadolol alone or with isosorbide mononitrate for primary prophylaxis of variceal bleeding in cirrhosis. Gruppo-Triveneto per L’ipertensione portale (GTIP). Lancet 1996; 348:1677–1681.
- Garcia-Pagán JC, Morillas R, Banares R, et al Spanish Variceal Bleeding Study Group. Propranolol plus placebo versus propranolol plus isosorbide-5-mononitrate in the prevention of a first variceal bleed: a double-blind RCT. Hepatology 2003; 37:1260–1266.
- Garcia-Tsao G, Sanyal A, Grace N, et al. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology 2007; 46:922–938.
KEY POINTS
- The hepatic vein pressure gradient (HVPG) correlates well with the portal pressure and is easier to measure. However, whether it is cost-effective to measure the HVPG in clinical practice is controversial.
- Nonselective beta-blockers are the mainstay of treatment; selective beta-blockers do not reduce portal pressure to the same degree and are not recommended for preventing variceal bleeding.
- Endoscopic variceal ligation is an acceptable alternative to beta-blocker therapy for patients who cannot tolerate these drugs and for patients with varices at high risk of bleeding.
- Nitrates are no longer used as monotherapy for preventing variceal hemorrhage, and their use in combination with beta-blockers is controversial. Surgical portal decompression, transjugular intrahepatic portosystemic shunting, and endoscopic sclerotherapy are not recommended.