User login
Early consumption of peanuts can induce tolerance in high-risk children
HOUSTON – The introduction of peanuts at an early age to children who are more highly predisposed to having a peanut allergy can induce tolerance of peanuts and thereby significantly decrease the likelihood of developing a sustained allergy.
This finding from the Learning Early about Peanut Allergy (LEAP) study was presented at the annual meeting of the American Academy of Allergy, Asthma, and Immunology and simultaneously published by the New England Journal of Medicine. Investigators enrolled 640 infants aged 4-11 months between December 2006 and May 6, 2009, all of whom were given skin-prick tests at baseline to determine existing peanut allergies, and randomized them into cohorts that would either consume or avoid peanuts until they reached 60 months of age (N. Engl. J. Med. 2015 Feb. 23 [doi:10.1056/NEJMoa1414850]).
Of the 530 children who had negative skin-prick test results, the prevalence of peanut allergy after 60 months was 13.7% in the avoidance cohort and 1.9% in the consumption cohort (P < .001). Of 98 children who had positive skin-prick test results, 35% of the avoidance cohort had peanut allergy after 60 months, while only 11% of the consumption cohort had the allergy (P = .004). Several children who were initially enrolled and randomized either dropped out or were excluded because of inadequate adherence to treatment or missing data.
Outcomes were measured by placing children in a double-blind, placebo-controlled study that had them consume a cumulative total of 9.4 g of peanut protein to determine whether or not tolerance to peanuts had been effectively induced. All children consumed peanuts by eating peanut butter or a peanut-based snack called Bamba, which was provided for the study at a discounted rate. Children whose skin-prick tests showed wheals of larger than 4 mm in diameter were excluded for presumed existing peanut allergy.
“If you enroll children after 11 months of age, you get about twice the rate of [already allergic] children, with a steady decline as [age] goes down,” corresponding author Dr. Gideon Lack, head of the department of pediatric allergy at King’s College, London, said in a press conference following presentation of the study. “But of children younger than 5 months of age, none of them were peanut allergic, so that would suggest that timing here is key: There is a narrow window of opportunity to intervene if you want to be effective.”
Investigators also noted that children who consumed peanuts had increased levels of IgG4 antibody, while those told to avoid peanuts had higher levels of IgE antibody. They also found that development of peanut allergy was generally associated with subjects who both displayed larger wheals on the skin-prick test and had a lower ratio of IgG4:IgE antibodies. Skin-prick tests that yielded wheals of 1-2 mm in diameter were considered indicative of early risk for peanut allergy.
There was no significant difference in the rates of adverse events or hospitalizations between the consumption and avoidance cohort, but 99% of subjects in each group did experience at least one adverse event: 4,527 in the consumption cohort and 4,287 in the avoidance cohort (P = .02). Infants who consumed peanuts had higher instances of upper respiratory tract infection, viral skin infection, gastroenteritis, urticaria, and conjunctivitis, but events mostly ranged from mild to moderate and their severity was not significantly different from similar incidents reported in the avoidance cohort.
Hospitalization rates also were low, with 52 children in the avoidance cohort and 50 children who consumed peanuts being admitted over the study interval, or 16.2% and 15.7%, respectively (P = .86). Rates of serious adverse effects also were not significantly different between the two cohorts, with 101 children who avoided peanuts and 89 who consumed peanuts experiencing such events (P = .41).
Adequate adherence to treatment for the children randomized into the peanut-avoidance group was defined as eating less than 0.2 g of peanut protein on any single occasion and no more than 0.5 g over the course of a single week in the first 24 months of life. For children in the consumption cohort, adequate adherence meant consuming at least 2 g of peanut protein on at least one occasion in both the first and second years of life, and at least 3 g of peanut protein weekly for at least half the number of weeks for which data was collected.
“Whether or not these benefits can be sustained, we will find out in 1 year,” Dr. Lack said, adding that “all the children who had been consuming peanut have stopped [for] 1 year, and we will see if peanut allergy persists after 1 year of cessation of consumption.” He predicted that the findings would most likely produce a mixed response, with some children relapsing while others maintain sustained tolerance.
The LEAP study was supported by grants from the National Institute of Allergy and Infectious Diseases, Food Allergy and Research Education, the Medical Research Council and Asthma UK, the UK Department of Health’s National Institute for Health Research, the National Peanut Board, and the UK Food Standards Agency. Dr. Lack disclosed financial affiliations with DBV Technologies, and coauthor Dr. Helen Brough disclosed receiving financial support from Fare and Action Medical Research, along with study materials from Stallergenes, Thermo Scientific, and Meridian Foods.
HOUSTON – The introduction of peanuts at an early age to children who are more highly predisposed to having a peanut allergy can induce tolerance of peanuts and thereby significantly decrease the likelihood of developing a sustained allergy.
This finding from the Learning Early about Peanut Allergy (LEAP) study was presented at the annual meeting of the American Academy of Allergy, Asthma, and Immunology and simultaneously published by the New England Journal of Medicine. Investigators enrolled 640 infants aged 4-11 months between December 2006 and May 6, 2009, all of whom were given skin-prick tests at baseline to determine existing peanut allergies, and randomized them into cohorts that would either consume or avoid peanuts until they reached 60 months of age (N. Engl. J. Med. 2015 Feb. 23 [doi:10.1056/NEJMoa1414850]).
Of the 530 children who had negative skin-prick test results, the prevalence of peanut allergy after 60 months was 13.7% in the avoidance cohort and 1.9% in the consumption cohort (P < .001). Of 98 children who had positive skin-prick test results, 35% of the avoidance cohort had peanut allergy after 60 months, while only 11% of the consumption cohort had the allergy (P = .004). Several children who were initially enrolled and randomized either dropped out or were excluded because of inadequate adherence to treatment or missing data.
Outcomes were measured by placing children in a double-blind, placebo-controlled study that had them consume a cumulative total of 9.4 g of peanut protein to determine whether or not tolerance to peanuts had been effectively induced. All children consumed peanuts by eating peanut butter or a peanut-based snack called Bamba, which was provided for the study at a discounted rate. Children whose skin-prick tests showed wheals of larger than 4 mm in diameter were excluded for presumed existing peanut allergy.
“If you enroll children after 11 months of age, you get about twice the rate of [already allergic] children, with a steady decline as [age] goes down,” corresponding author Dr. Gideon Lack, head of the department of pediatric allergy at King’s College, London, said in a press conference following presentation of the study. “But of children younger than 5 months of age, none of them were peanut allergic, so that would suggest that timing here is key: There is a narrow window of opportunity to intervene if you want to be effective.”
Investigators also noted that children who consumed peanuts had increased levels of IgG4 antibody, while those told to avoid peanuts had higher levels of IgE antibody. They also found that development of peanut allergy was generally associated with subjects who both displayed larger wheals on the skin-prick test and had a lower ratio of IgG4:IgE antibodies. Skin-prick tests that yielded wheals of 1-2 mm in diameter were considered indicative of early risk for peanut allergy.
There was no significant difference in the rates of adverse events or hospitalizations between the consumption and avoidance cohort, but 99% of subjects in each group did experience at least one adverse event: 4,527 in the consumption cohort and 4,287 in the avoidance cohort (P = .02). Infants who consumed peanuts had higher instances of upper respiratory tract infection, viral skin infection, gastroenteritis, urticaria, and conjunctivitis, but events mostly ranged from mild to moderate and their severity was not significantly different from similar incidents reported in the avoidance cohort.
Hospitalization rates also were low, with 52 children in the avoidance cohort and 50 children who consumed peanuts being admitted over the study interval, or 16.2% and 15.7%, respectively (P = .86). Rates of serious adverse effects also were not significantly different between the two cohorts, with 101 children who avoided peanuts and 89 who consumed peanuts experiencing such events (P = .41).
Adequate adherence to treatment for the children randomized into the peanut-avoidance group was defined as eating less than 0.2 g of peanut protein on any single occasion and no more than 0.5 g over the course of a single week in the first 24 months of life. For children in the consumption cohort, adequate adherence meant consuming at least 2 g of peanut protein on at least one occasion in both the first and second years of life, and at least 3 g of peanut protein weekly for at least half the number of weeks for which data was collected.
“Whether or not these benefits can be sustained, we will find out in 1 year,” Dr. Lack said, adding that “all the children who had been consuming peanut have stopped [for] 1 year, and we will see if peanut allergy persists after 1 year of cessation of consumption.” He predicted that the findings would most likely produce a mixed response, with some children relapsing while others maintain sustained tolerance.
The LEAP study was supported by grants from the National Institute of Allergy and Infectious Diseases, Food Allergy and Research Education, the Medical Research Council and Asthma UK, the UK Department of Health’s National Institute for Health Research, the National Peanut Board, and the UK Food Standards Agency. Dr. Lack disclosed financial affiliations with DBV Technologies, and coauthor Dr. Helen Brough disclosed receiving financial support from Fare and Action Medical Research, along with study materials from Stallergenes, Thermo Scientific, and Meridian Foods.
HOUSTON – The introduction of peanuts at an early age to children who are more highly predisposed to having a peanut allergy can induce tolerance of peanuts and thereby significantly decrease the likelihood of developing a sustained allergy.
This finding from the Learning Early about Peanut Allergy (LEAP) study was presented at the annual meeting of the American Academy of Allergy, Asthma, and Immunology and simultaneously published by the New England Journal of Medicine. Investigators enrolled 640 infants aged 4-11 months between December 2006 and May 6, 2009, all of whom were given skin-prick tests at baseline to determine existing peanut allergies, and randomized them into cohorts that would either consume or avoid peanuts until they reached 60 months of age (N. Engl. J. Med. 2015 Feb. 23 [doi:10.1056/NEJMoa1414850]).
Of the 530 children who had negative skin-prick test results, the prevalence of peanut allergy after 60 months was 13.7% in the avoidance cohort and 1.9% in the consumption cohort (P < .001). Of 98 children who had positive skin-prick test results, 35% of the avoidance cohort had peanut allergy after 60 months, while only 11% of the consumption cohort had the allergy (P = .004). Several children who were initially enrolled and randomized either dropped out or were excluded because of inadequate adherence to treatment or missing data.
Outcomes were measured by placing children in a double-blind, placebo-controlled study that had them consume a cumulative total of 9.4 g of peanut protein to determine whether or not tolerance to peanuts had been effectively induced. All children consumed peanuts by eating peanut butter or a peanut-based snack called Bamba, which was provided for the study at a discounted rate. Children whose skin-prick tests showed wheals of larger than 4 mm in diameter were excluded for presumed existing peanut allergy.
“If you enroll children after 11 months of age, you get about twice the rate of [already allergic] children, with a steady decline as [age] goes down,” corresponding author Dr. Gideon Lack, head of the department of pediatric allergy at King’s College, London, said in a press conference following presentation of the study. “But of children younger than 5 months of age, none of them were peanut allergic, so that would suggest that timing here is key: There is a narrow window of opportunity to intervene if you want to be effective.”
Investigators also noted that children who consumed peanuts had increased levels of IgG4 antibody, while those told to avoid peanuts had higher levels of IgE antibody. They also found that development of peanut allergy was generally associated with subjects who both displayed larger wheals on the skin-prick test and had a lower ratio of IgG4:IgE antibodies. Skin-prick tests that yielded wheals of 1-2 mm in diameter were considered indicative of early risk for peanut allergy.
There was no significant difference in the rates of adverse events or hospitalizations between the consumption and avoidance cohort, but 99% of subjects in each group did experience at least one adverse event: 4,527 in the consumption cohort and 4,287 in the avoidance cohort (P = .02). Infants who consumed peanuts had higher instances of upper respiratory tract infection, viral skin infection, gastroenteritis, urticaria, and conjunctivitis, but events mostly ranged from mild to moderate and their severity was not significantly different from similar incidents reported in the avoidance cohort.
Hospitalization rates also were low, with 52 children in the avoidance cohort and 50 children who consumed peanuts being admitted over the study interval, or 16.2% and 15.7%, respectively (P = .86). Rates of serious adverse effects also were not significantly different between the two cohorts, with 101 children who avoided peanuts and 89 who consumed peanuts experiencing such events (P = .41).
Adequate adherence to treatment for the children randomized into the peanut-avoidance group was defined as eating less than 0.2 g of peanut protein on any single occasion and no more than 0.5 g over the course of a single week in the first 24 months of life. For children in the consumption cohort, adequate adherence meant consuming at least 2 g of peanut protein on at least one occasion in both the first and second years of life, and at least 3 g of peanut protein weekly for at least half the number of weeks for which data was collected.
“Whether or not these benefits can be sustained, we will find out in 1 year,” Dr. Lack said, adding that “all the children who had been consuming peanut have stopped [for] 1 year, and we will see if peanut allergy persists after 1 year of cessation of consumption.” He predicted that the findings would most likely produce a mixed response, with some children relapsing while others maintain sustained tolerance.
The LEAP study was supported by grants from the National Institute of Allergy and Infectious Diseases, Food Allergy and Research Education, the Medical Research Council and Asthma UK, the UK Department of Health’s National Institute for Health Research, the National Peanut Board, and the UK Food Standards Agency. Dr. Lack disclosed financial affiliations with DBV Technologies, and coauthor Dr. Helen Brough disclosed receiving financial support from Fare and Action Medical Research, along with study materials from Stallergenes, Thermo Scientific, and Meridian Foods.
AT 2015 AAAAI ANNUAL MEETING
Key clinical point: Early introduction of peanuts into the diets of children at high risk for peanut allergy can significantly decrease the possibility of peanut allergy development.
Major finding: Of 530 infants who initially tested negative for peanut allergy, prevalence of said allergy after 60 months was 13.7% in the avoidance cohort and 1.9% in the consumption cohort (P < .001); of the 98 infants who initially tested positive, prevalence after 60 months was 35% in the avoidance cohort and 11% in the consumption cohort (P = .004).
Data source: Randomized cohort study of 640 children enrolled at ages 4-11 months between December 2006 and May 2009.
Disclosures: The LEAP study was funded by several health care agencies in the United States and United Kingdom. Corresponding author Dr. Gideon Lack disclosed financial affiliations with DBV Technologies, and coauthor Dr. Helen Brough disclosed receiving financial support from Food Allergy and Research Education and Action Medical Research, along with study materials from Stallergenes, Thermo Scientific, and Meridian Foods.
Look for adverse events in patients with chronic urticaria
HOUSTON – The risk of adverse events may be cumulative over the lifetime of patients taking oral corticosteroids for urticaria.
Dr. Dennis Ledford, professor of medicine at the University of South Florida, Tampa, and his colleagues examined records of 12,647 patients culled from a commercial claims database between January 2008 and December 2012 who had taken oral corticosteroids for chronic idiopathic or spontaneous urticaria during a 12-month period. More than half (55%) used oral corticosteroids (mean dosage of 367.5 mg) for an average of 16.2 days. At follow-up, patients displayed adverse events at a rate of 27 per 100 patient-years.
Adverse events mostly included skeletal conditions such as osteoporosis and bone fractures, but investigators also noted diabetes, hypertension, lipid disorders, depression, mania, and cataracts, Dr. Ledford said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
More concerning, “there’s a cumulative risk,” Dr. Ledford said in an interview. “The more [prednisone equivalent] you take over your lifetime, the greater the chance is that you’re going to develop the side effects we’ve listed here.”
Using time-sensitive Cox regression models, Dr. Ledford and his colleagues determined that the risk for adverse events went up by 7% for each gram dose of prednisone equivalent to which patients were exposed after adjusting for age, sex, immunomodulator use, and Charlson Comorbidity Index. Only cataracts were not subject to the cumulative effects.
“The message of this fairly large analysis is that there are cumulative side effects to prednisone that may not be evident to the physician or clinician performing day-to-day care of patients,” Dr. Ledford said. “These effects are slow to develop and often present in areas of medicine that the physician treating urticaria would not take care of.”
Patients enrolled in this study had all been diagnosed with urticaria at either of two outpatient clinic visits at least 6 weeks apart in a single calendar year, or had received one diagnosis of urticaria and one of angioedema at two separate outpatient clinics at least 6 weeks apart. Patients were followed for at least 1 year after completion of the initial 12-month study period, until end of enrollment or end of study.
Dr. Ledford stressed the need to use noncorticosteroid therapies when treating chronic urticaria, such as calcineurin inhibitors – which also carry risks of hypertension and cancer – or omalizumab.
The study was funded by Genentech and Novartis Pharma AG which market omalizumab as Xolair. Dr. Ledford disclosed that he is affiliated with Genentech, Novartis Pharma AG, and a number of other pharmaceutical companies.
HOUSTON – The risk of adverse events may be cumulative over the lifetime of patients taking oral corticosteroids for urticaria.
Dr. Dennis Ledford, professor of medicine at the University of South Florida, Tampa, and his colleagues examined records of 12,647 patients culled from a commercial claims database between January 2008 and December 2012 who had taken oral corticosteroids for chronic idiopathic or spontaneous urticaria during a 12-month period. More than half (55%) used oral corticosteroids (mean dosage of 367.5 mg) for an average of 16.2 days. At follow-up, patients displayed adverse events at a rate of 27 per 100 patient-years.
Adverse events mostly included skeletal conditions such as osteoporosis and bone fractures, but investigators also noted diabetes, hypertension, lipid disorders, depression, mania, and cataracts, Dr. Ledford said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
More concerning, “there’s a cumulative risk,” Dr. Ledford said in an interview. “The more [prednisone equivalent] you take over your lifetime, the greater the chance is that you’re going to develop the side effects we’ve listed here.”
Using time-sensitive Cox regression models, Dr. Ledford and his colleagues determined that the risk for adverse events went up by 7% for each gram dose of prednisone equivalent to which patients were exposed after adjusting for age, sex, immunomodulator use, and Charlson Comorbidity Index. Only cataracts were not subject to the cumulative effects.
“The message of this fairly large analysis is that there are cumulative side effects to prednisone that may not be evident to the physician or clinician performing day-to-day care of patients,” Dr. Ledford said. “These effects are slow to develop and often present in areas of medicine that the physician treating urticaria would not take care of.”
Patients enrolled in this study had all been diagnosed with urticaria at either of two outpatient clinic visits at least 6 weeks apart in a single calendar year, or had received one diagnosis of urticaria and one of angioedema at two separate outpatient clinics at least 6 weeks apart. Patients were followed for at least 1 year after completion of the initial 12-month study period, until end of enrollment or end of study.
Dr. Ledford stressed the need to use noncorticosteroid therapies when treating chronic urticaria, such as calcineurin inhibitors – which also carry risks of hypertension and cancer – or omalizumab.
The study was funded by Genentech and Novartis Pharma AG which market omalizumab as Xolair. Dr. Ledford disclosed that he is affiliated with Genentech, Novartis Pharma AG, and a number of other pharmaceutical companies.
HOUSTON – The risk of adverse events may be cumulative over the lifetime of patients taking oral corticosteroids for urticaria.
Dr. Dennis Ledford, professor of medicine at the University of South Florida, Tampa, and his colleagues examined records of 12,647 patients culled from a commercial claims database between January 2008 and December 2012 who had taken oral corticosteroids for chronic idiopathic or spontaneous urticaria during a 12-month period. More than half (55%) used oral corticosteroids (mean dosage of 367.5 mg) for an average of 16.2 days. At follow-up, patients displayed adverse events at a rate of 27 per 100 patient-years.
Adverse events mostly included skeletal conditions such as osteoporosis and bone fractures, but investigators also noted diabetes, hypertension, lipid disorders, depression, mania, and cataracts, Dr. Ledford said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
More concerning, “there’s a cumulative risk,” Dr. Ledford said in an interview. “The more [prednisone equivalent] you take over your lifetime, the greater the chance is that you’re going to develop the side effects we’ve listed here.”
Using time-sensitive Cox regression models, Dr. Ledford and his colleagues determined that the risk for adverse events went up by 7% for each gram dose of prednisone equivalent to which patients were exposed after adjusting for age, sex, immunomodulator use, and Charlson Comorbidity Index. Only cataracts were not subject to the cumulative effects.
“The message of this fairly large analysis is that there are cumulative side effects to prednisone that may not be evident to the physician or clinician performing day-to-day care of patients,” Dr. Ledford said. “These effects are slow to develop and often present in areas of medicine that the physician treating urticaria would not take care of.”
Patients enrolled in this study had all been diagnosed with urticaria at either of two outpatient clinic visits at least 6 weeks apart in a single calendar year, or had received one diagnosis of urticaria and one of angioedema at two separate outpatient clinics at least 6 weeks apart. Patients were followed for at least 1 year after completion of the initial 12-month study period, until end of enrollment or end of study.
Dr. Ledford stressed the need to use noncorticosteroid therapies when treating chronic urticaria, such as calcineurin inhibitors – which also carry risks of hypertension and cancer – or omalizumab.
The study was funded by Genentech and Novartis Pharma AG which market omalizumab as Xolair. Dr. Ledford disclosed that he is affiliated with Genentech, Novartis Pharma AG, and a number of other pharmaceutical companies.
AT 2015 AAAAI ANNUAL MEETING
Key clinical point: The cumulative adverse events of oral corticosteroids may not present to the physician treating the patient for urticaria.
Major finding: The risk for adverse events went up by 7% for each gram dose of prednisone equivalent.
Data source: Retrospective cohort study of 12,647 patients selected from a commercial claims database from 2008 through 2012.
Disclosures: Study funded by Genentech and Novartis Pharma AG; Dr. Ledford is affiliated with Genentech, Novartis Pharma AG, and a number of other pharmaceutical companies.
EXPECT: Omalizumab pregnancy safety data called ‘reassuring’
HOUSTON – Babies born to mothers who took omalizumab just before or during pregnancy exhibited birth defects that are in line with the general population, according to data from the EXPECT registry.
EXPECT (the Xolair Pregnancy Registry) is an ongoing, prospective observational study of pregnant women who received at least 1 dose of omalizumab within 2 months of conception or during their pregnancies.
Dr. Jennifer Namazy of the Scripps Clinic in La Jolla, Calif., presented data from women enrolled between September 2006 and November 2013 at the annual meeting of the American Academy of Allergy, Asthma, and Immunology. The registry aims to enroll 250 women.
Dr. Namazy reported on 186 of the 207 expectant mothers for whom outcomes are recorded. Asthma severity was available for 164. Most (105 or 64%) had severe asthma, while 55 (34%) had moderate asthma, and 4 (2.4%) had mild disease. Each received omalizumab during the first trimester of pregnancy.
A total of 178 births were recorded, 174 of which were live. Of the 170 singletons, 24 (14%) were born at less than 37 weeks’ gestation and of those 3 (13%) were considered small for gestational age (weight less than 10th percentile for gestational age).
Of 140 single-born infants who were carried to full term and also had relevant weight data, 4 (3%) had low birth weight (less than 2.5 kg) and 16 (11%) were considered small for gestational age. Overall, 27 (15%) infants had confirmed congenital anomalies and 11 (6.2%) had a major birth defect.
“This is all very reassuring data,” Dr. Namazy said in an interview. “In terms of the outcomes – major defects and conditional defects – these results are not too unusual, [compared with] the general population. But when we’re looking at infant outcomes, it’s not too different from the severe asthma population.”
She noted, however, that “it’s hard to make large-scale conclusions from this sample size. When you’re talking about congenital malformations, they’re so rare and uncommon that you need a very large population in order to reach any kind of conclusion. But looking at these [data], we’re reassured because there isn’t any consistent information that’s disconcerting; it’s all over the spectrum, and so there’s no significant increase in the rate of birth rate defects that we’re seeing.”
Omalizumab is marketed by Genentech as Xolair. The EXPECT study is funded by Genentech and Novartis Pharma AG. Dr. Namazy is affiliated with Genentech.
HOUSTON – Babies born to mothers who took omalizumab just before or during pregnancy exhibited birth defects that are in line with the general population, according to data from the EXPECT registry.
EXPECT (the Xolair Pregnancy Registry) is an ongoing, prospective observational study of pregnant women who received at least 1 dose of omalizumab within 2 months of conception or during their pregnancies.
Dr. Jennifer Namazy of the Scripps Clinic in La Jolla, Calif., presented data from women enrolled between September 2006 and November 2013 at the annual meeting of the American Academy of Allergy, Asthma, and Immunology. The registry aims to enroll 250 women.
Dr. Namazy reported on 186 of the 207 expectant mothers for whom outcomes are recorded. Asthma severity was available for 164. Most (105 or 64%) had severe asthma, while 55 (34%) had moderate asthma, and 4 (2.4%) had mild disease. Each received omalizumab during the first trimester of pregnancy.
A total of 178 births were recorded, 174 of which were live. Of the 170 singletons, 24 (14%) were born at less than 37 weeks’ gestation and of those 3 (13%) were considered small for gestational age (weight less than 10th percentile for gestational age).
Of 140 single-born infants who were carried to full term and also had relevant weight data, 4 (3%) had low birth weight (less than 2.5 kg) and 16 (11%) were considered small for gestational age. Overall, 27 (15%) infants had confirmed congenital anomalies and 11 (6.2%) had a major birth defect.
“This is all very reassuring data,” Dr. Namazy said in an interview. “In terms of the outcomes – major defects and conditional defects – these results are not too unusual, [compared with] the general population. But when we’re looking at infant outcomes, it’s not too different from the severe asthma population.”
She noted, however, that “it’s hard to make large-scale conclusions from this sample size. When you’re talking about congenital malformations, they’re so rare and uncommon that you need a very large population in order to reach any kind of conclusion. But looking at these [data], we’re reassured because there isn’t any consistent information that’s disconcerting; it’s all over the spectrum, and so there’s no significant increase in the rate of birth rate defects that we’re seeing.”
Omalizumab is marketed by Genentech as Xolair. The EXPECT study is funded by Genentech and Novartis Pharma AG. Dr. Namazy is affiliated with Genentech.
HOUSTON – Babies born to mothers who took omalizumab just before or during pregnancy exhibited birth defects that are in line with the general population, according to data from the EXPECT registry.
EXPECT (the Xolair Pregnancy Registry) is an ongoing, prospective observational study of pregnant women who received at least 1 dose of omalizumab within 2 months of conception or during their pregnancies.
Dr. Jennifer Namazy of the Scripps Clinic in La Jolla, Calif., presented data from women enrolled between September 2006 and November 2013 at the annual meeting of the American Academy of Allergy, Asthma, and Immunology. The registry aims to enroll 250 women.
Dr. Namazy reported on 186 of the 207 expectant mothers for whom outcomes are recorded. Asthma severity was available for 164. Most (105 or 64%) had severe asthma, while 55 (34%) had moderate asthma, and 4 (2.4%) had mild disease. Each received omalizumab during the first trimester of pregnancy.
A total of 178 births were recorded, 174 of which were live. Of the 170 singletons, 24 (14%) were born at less than 37 weeks’ gestation and of those 3 (13%) were considered small for gestational age (weight less than 10th percentile for gestational age).
Of 140 single-born infants who were carried to full term and also had relevant weight data, 4 (3%) had low birth weight (less than 2.5 kg) and 16 (11%) were considered small for gestational age. Overall, 27 (15%) infants had confirmed congenital anomalies and 11 (6.2%) had a major birth defect.
“This is all very reassuring data,” Dr. Namazy said in an interview. “In terms of the outcomes – major defects and conditional defects – these results are not too unusual, [compared with] the general population. But when we’re looking at infant outcomes, it’s not too different from the severe asthma population.”
She noted, however, that “it’s hard to make large-scale conclusions from this sample size. When you’re talking about congenital malformations, they’re so rare and uncommon that you need a very large population in order to reach any kind of conclusion. But looking at these [data], we’re reassured because there isn’t any consistent information that’s disconcerting; it’s all over the spectrum, and so there’s no significant increase in the rate of birth rate defects that we’re seeing.”
Omalizumab is marketed by Genentech as Xolair. The EXPECT study is funded by Genentech and Novartis Pharma AG. Dr. Namazy is affiliated with Genentech.
AT 2015 AAAAI ANNUAL MEETING
Key clinical point: Omalizumab does not appear to increase the risk of birth defects or adverse outcomes in pregnant women and their babies.
Major finding: Of 140 singleton infants carried to full term and for whom relevant weight data was available, 4 (2.9%) had low birth weight and 16 (11.4%) were considered small for gestational age, 27 (15.2%) had congenital anomalies, and 11 (6.2%) had a major birth defect.
Data source: EXPECT trial – an ongoing observational study of 207 prospectively enrolled women.
Disclosures: EXPECT is funded by Genentech and Novartis Pharma AG. Dr. Namazy is affiliated with Genentech.
ECMO alone before lung transplant linked to good survival rates
SAN DIEGO – Extracorporeal membrane oxygenation with spontaneous breathing is the optimal bridging strategy for patients who have rapidly advancing pulmonary disease and are awaiting lung transplantation, based on data from over 18,000 patients who received lung transplants.
In the study, patients on extracorporeal membrane oxygenation (ECMO) alone had outcomes that were comparable to those of patients requiring no invasive support prior to transplantation, Dr. Matthew Schechter of Duke University in Durham, N.C., reported at the annual meeting of the Society of Thoracic Surgeons.
Dr. Schechter and his colleagues analyzed the United Network for Organ Sharing database for all adult patients who underwent lung transplantations between January 2000 and September 2013.
The 18,392 patients selected for study inclusion were divided into cohorts based on the type of preoperative support they received: ECMO with mechanical ventilation; ECMO only; ventilation only; and no support of any kind. Nearly 95% of the patients received no invasive preoperative support. Over 4% received mechanical ventilation alone, less than 1% received ECMO with mechanical ventilation, and about 0.5%) received ECMO only.
By using Kaplan-Meier survival analyses with log-rank testing, Dr. Schechter and his associates were able to compare survival rates for each type of preoperative support. Cox regression models were used to ascertain whether any particular type of preoperative support could definitively be associated with mortality.
At 3 years post transplantation, the survival rates of patients on ECMO alone and of those who received no preoperative support of any kind were comparable at 66% and 65%, respectively. Survival rates at 3 years after transplant were 38% in patients who received ECMO and mechanical ventilation and 52% in patients who received mechanical ventilation alone. The survival advantage in the ECMO only and no support groups was significantly better when compared to the ECMO and mechanical ventilation and the mechanical ventilation alone cohorts (P < .0001).
The findings held up after a multivariate analysis; the hazard ratio was 1.96 (95% confidence interval, 1.36-2.84) for ECMO with mechanical ventilation and 1.52 (95% CI, 1.31-1.78) for mechanical ventilation only (P < .0001 for both).
ECMO alone was not associated with any significant change in survival rate (HR = 1.07; 95% CI, 0.57-2.01; P = .843).
Patients who received just ECMO had the shortest lengths of stay after lung transplant. They also had the lowest rate of acute rejection prior to discharge, although not to an extent that was statistically significant. The incidence of new-onset dialysis was highest in patients who received ECMO with mechanical ventilation.
“ECMO alone may provide a survival advantage over other bridging strategies,” Dr. Schechter concluded. “One advantage of using ECMO only is an avoidance of the risks that come with mechanical ventilation, [which] include generalized muscle atrophy, maladapted muscle fiber remodeling in the diaphragm – which leads to a decrease in the overall durability of this muscle – as well as the induction of the pulmonary and systemic inflammatory risk responses, [all of which] have been shown to affect outcomes following lung transplantation.”
Dr. Schechter explained that patients receiving ECMO without mechanical ventilation can actively rehabilitate themselves post transplantation since nonintubated ECMO patients can participate in physical therapy.
Further study is needed to find an optimal way of assessing patients and determining exactly which ones would be best suited for ECMO with spontaneous breathing support, he said.
Dr, Schechter had no relevant financial disclosures.
SAN DIEGO – Extracorporeal membrane oxygenation with spontaneous breathing is the optimal bridging strategy for patients who have rapidly advancing pulmonary disease and are awaiting lung transplantation, based on data from over 18,000 patients who received lung transplants.
In the study, patients on extracorporeal membrane oxygenation (ECMO) alone had outcomes that were comparable to those of patients requiring no invasive support prior to transplantation, Dr. Matthew Schechter of Duke University in Durham, N.C., reported at the annual meeting of the Society of Thoracic Surgeons.
Dr. Schechter and his colleagues analyzed the United Network for Organ Sharing database for all adult patients who underwent lung transplantations between January 2000 and September 2013.
The 18,392 patients selected for study inclusion were divided into cohorts based on the type of preoperative support they received: ECMO with mechanical ventilation; ECMO only; ventilation only; and no support of any kind. Nearly 95% of the patients received no invasive preoperative support. Over 4% received mechanical ventilation alone, less than 1% received ECMO with mechanical ventilation, and about 0.5%) received ECMO only.
By using Kaplan-Meier survival analyses with log-rank testing, Dr. Schechter and his associates were able to compare survival rates for each type of preoperative support. Cox regression models were used to ascertain whether any particular type of preoperative support could definitively be associated with mortality.
At 3 years post transplantation, the survival rates of patients on ECMO alone and of those who received no preoperative support of any kind were comparable at 66% and 65%, respectively. Survival rates at 3 years after transplant were 38% in patients who received ECMO and mechanical ventilation and 52% in patients who received mechanical ventilation alone. The survival advantage in the ECMO only and no support groups was significantly better when compared to the ECMO and mechanical ventilation and the mechanical ventilation alone cohorts (P < .0001).
The findings held up after a multivariate analysis; the hazard ratio was 1.96 (95% confidence interval, 1.36-2.84) for ECMO with mechanical ventilation and 1.52 (95% CI, 1.31-1.78) for mechanical ventilation only (P < .0001 for both).
ECMO alone was not associated with any significant change in survival rate (HR = 1.07; 95% CI, 0.57-2.01; P = .843).
Patients who received just ECMO had the shortest lengths of stay after lung transplant. They also had the lowest rate of acute rejection prior to discharge, although not to an extent that was statistically significant. The incidence of new-onset dialysis was highest in patients who received ECMO with mechanical ventilation.
“ECMO alone may provide a survival advantage over other bridging strategies,” Dr. Schechter concluded. “One advantage of using ECMO only is an avoidance of the risks that come with mechanical ventilation, [which] include generalized muscle atrophy, maladapted muscle fiber remodeling in the diaphragm – which leads to a decrease in the overall durability of this muscle – as well as the induction of the pulmonary and systemic inflammatory risk responses, [all of which] have been shown to affect outcomes following lung transplantation.”
Dr. Schechter explained that patients receiving ECMO without mechanical ventilation can actively rehabilitate themselves post transplantation since nonintubated ECMO patients can participate in physical therapy.
Further study is needed to find an optimal way of assessing patients and determining exactly which ones would be best suited for ECMO with spontaneous breathing support, he said.
Dr, Schechter had no relevant financial disclosures.
SAN DIEGO – Extracorporeal membrane oxygenation with spontaneous breathing is the optimal bridging strategy for patients who have rapidly advancing pulmonary disease and are awaiting lung transplantation, based on data from over 18,000 patients who received lung transplants.
In the study, patients on extracorporeal membrane oxygenation (ECMO) alone had outcomes that were comparable to those of patients requiring no invasive support prior to transplantation, Dr. Matthew Schechter of Duke University in Durham, N.C., reported at the annual meeting of the Society of Thoracic Surgeons.
Dr. Schechter and his colleagues analyzed the United Network for Organ Sharing database for all adult patients who underwent lung transplantations between January 2000 and September 2013.
The 18,392 patients selected for study inclusion were divided into cohorts based on the type of preoperative support they received: ECMO with mechanical ventilation; ECMO only; ventilation only; and no support of any kind. Nearly 95% of the patients received no invasive preoperative support. Over 4% received mechanical ventilation alone, less than 1% received ECMO with mechanical ventilation, and about 0.5%) received ECMO only.
By using Kaplan-Meier survival analyses with log-rank testing, Dr. Schechter and his associates were able to compare survival rates for each type of preoperative support. Cox regression models were used to ascertain whether any particular type of preoperative support could definitively be associated with mortality.
At 3 years post transplantation, the survival rates of patients on ECMO alone and of those who received no preoperative support of any kind were comparable at 66% and 65%, respectively. Survival rates at 3 years after transplant were 38% in patients who received ECMO and mechanical ventilation and 52% in patients who received mechanical ventilation alone. The survival advantage in the ECMO only and no support groups was significantly better when compared to the ECMO and mechanical ventilation and the mechanical ventilation alone cohorts (P < .0001).
The findings held up after a multivariate analysis; the hazard ratio was 1.96 (95% confidence interval, 1.36-2.84) for ECMO with mechanical ventilation and 1.52 (95% CI, 1.31-1.78) for mechanical ventilation only (P < .0001 for both).
ECMO alone was not associated with any significant change in survival rate (HR = 1.07; 95% CI, 0.57-2.01; P = .843).
Patients who received just ECMO had the shortest lengths of stay after lung transplant. They also had the lowest rate of acute rejection prior to discharge, although not to an extent that was statistically significant. The incidence of new-onset dialysis was highest in patients who received ECMO with mechanical ventilation.
“ECMO alone may provide a survival advantage over other bridging strategies,” Dr. Schechter concluded. “One advantage of using ECMO only is an avoidance of the risks that come with mechanical ventilation, [which] include generalized muscle atrophy, maladapted muscle fiber remodeling in the diaphragm – which leads to a decrease in the overall durability of this muscle – as well as the induction of the pulmonary and systemic inflammatory risk responses, [all of which] have been shown to affect outcomes following lung transplantation.”
Dr. Schechter explained that patients receiving ECMO without mechanical ventilation can actively rehabilitate themselves post transplantation since nonintubated ECMO patients can participate in physical therapy.
Further study is needed to find an optimal way of assessing patients and determining exactly which ones would be best suited for ECMO with spontaneous breathing support, he said.
Dr, Schechter had no relevant financial disclosures.
FROM THE STS ANNUAL MEETING
Key clinical point: ECMO with spontaneous breathing should be considered the preferred bridging strategy for patients who have rapidly advancing pulmonary disease and are awaiting lung transplantations.
Major finding: At 3 years post transplantation, the survival rates of patients on ECMO alone and of those who received no preoperative support of any kind were comparable at 66% and 65%, respectively.
Data source: Retrospective analysis of 18,392 adult patients in the United Network for Organ Sharing database.
Disclosures: Dr. Schechter had no relevant financial disclosures.
Human Liver Cells Can Induce Antiviral Reaction Against Hepatitis C
Immunity against the hepatitis C virus can be induced by cultured primary human liver sinusoidal endothelial cells, according to a study published in the February issue of Gastroenterology (doi:10.1053/j.gastro.2014.10.040).
“We found HLSECs [primary human LSECs] express many of the receptors implicated in HCV [hepatitis C virus] attachment and entry and HLSEC-to-hepatocyte contact was dispensable for uptake,” wrote lead author Dr. Silvia M. Giugliano of the University of Colorado, Denver, and her associates.
In a cohort study, investigators exposed HLSECs and immortalized liver endothelial cells (TMNK-1) to several variants of HCV: full-length transmitted/founder virus, sucrose-purified Japanese fulminant hepatitis–1 (JFH-1), a virus encoding a luciferase reporter, and the HCV-specific pathogen-associated molecular pattern molecules (PAMP, substrate for RIG-I). Cells were analyzed by using polymerase chain reaction (PCR), immunofluorescence, and immunohistochemical assays.
Results showed that HLSECs, regardless of contact between cells, were able to internalize HCV when exposed to it and replicate, though not translate HCV RNA. The HCV RNA “induced consistent and broad transcription of multiple interferons (IFNs) [via] pattern recognition receptors (TLR7 and retinoic acid-inducible gene 1).” Furthermore, supernatants from primary HLSECs transfected with HCV-specific PAMP molecules had larger inductions of IFNs and IFN-stimulated genes in HLSECs.
“Conditioned media from IFN-stimulated HLSECs induced expression of antiviral genes by uninfected primary human hepatocytes,” wrote Dr. Giugliano and her coauthors. “Exosomes, derived from HLSECs following stimulation with either type I or type III IFNs, controlled HCV replication in a dose-dependent manner.”
However, the investigators say that more study is required to understand why HCV is able to present so persistently if the means to combat the virus are so apparent in the immune system.
“These results raise a number of intriguing questions, for example, how the use of exogenous IFN to treat viral hepatitis could be expected to induce additional, previously unrecognized antiviral mechanisms involving HLSECs,” says the study. “Further work is warranted to understand why despite these innate immune responses, HCV is able to establish persistence and fail eradication with IFN-based antiviral therapy.”
The study supported by grants R21-AI103361 and U19 AI 1066328; Dr. Rosen received a Merit Review grant and Dr. Shaw received grant R21-AI 106000. The authors reported no financial conflicts of interest.
Immunity against the hepatitis C virus can be induced by cultured primary human liver sinusoidal endothelial cells, according to a study published in the February issue of Gastroenterology (doi:10.1053/j.gastro.2014.10.040).
“We found HLSECs [primary human LSECs] express many of the receptors implicated in HCV [hepatitis C virus] attachment and entry and HLSEC-to-hepatocyte contact was dispensable for uptake,” wrote lead author Dr. Silvia M. Giugliano of the University of Colorado, Denver, and her associates.
In a cohort study, investigators exposed HLSECs and immortalized liver endothelial cells (TMNK-1) to several variants of HCV: full-length transmitted/founder virus, sucrose-purified Japanese fulminant hepatitis–1 (JFH-1), a virus encoding a luciferase reporter, and the HCV-specific pathogen-associated molecular pattern molecules (PAMP, substrate for RIG-I). Cells were analyzed by using polymerase chain reaction (PCR), immunofluorescence, and immunohistochemical assays.
Results showed that HLSECs, regardless of contact between cells, were able to internalize HCV when exposed to it and replicate, though not translate HCV RNA. The HCV RNA “induced consistent and broad transcription of multiple interferons (IFNs) [via] pattern recognition receptors (TLR7 and retinoic acid-inducible gene 1).” Furthermore, supernatants from primary HLSECs transfected with HCV-specific PAMP molecules had larger inductions of IFNs and IFN-stimulated genes in HLSECs.
“Conditioned media from IFN-stimulated HLSECs induced expression of antiviral genes by uninfected primary human hepatocytes,” wrote Dr. Giugliano and her coauthors. “Exosomes, derived from HLSECs following stimulation with either type I or type III IFNs, controlled HCV replication in a dose-dependent manner.”
However, the investigators say that more study is required to understand why HCV is able to present so persistently if the means to combat the virus are so apparent in the immune system.
“These results raise a number of intriguing questions, for example, how the use of exogenous IFN to treat viral hepatitis could be expected to induce additional, previously unrecognized antiviral mechanisms involving HLSECs,” says the study. “Further work is warranted to understand why despite these innate immune responses, HCV is able to establish persistence and fail eradication with IFN-based antiviral therapy.”
The study supported by grants R21-AI103361 and U19 AI 1066328; Dr. Rosen received a Merit Review grant and Dr. Shaw received grant R21-AI 106000. The authors reported no financial conflicts of interest.
Immunity against the hepatitis C virus can be induced by cultured primary human liver sinusoidal endothelial cells, according to a study published in the February issue of Gastroenterology (doi:10.1053/j.gastro.2014.10.040).
“We found HLSECs [primary human LSECs] express many of the receptors implicated in HCV [hepatitis C virus] attachment and entry and HLSEC-to-hepatocyte contact was dispensable for uptake,” wrote lead author Dr. Silvia M. Giugliano of the University of Colorado, Denver, and her associates.
In a cohort study, investigators exposed HLSECs and immortalized liver endothelial cells (TMNK-1) to several variants of HCV: full-length transmitted/founder virus, sucrose-purified Japanese fulminant hepatitis–1 (JFH-1), a virus encoding a luciferase reporter, and the HCV-specific pathogen-associated molecular pattern molecules (PAMP, substrate for RIG-I). Cells were analyzed by using polymerase chain reaction (PCR), immunofluorescence, and immunohistochemical assays.
Results showed that HLSECs, regardless of contact between cells, were able to internalize HCV when exposed to it and replicate, though not translate HCV RNA. The HCV RNA “induced consistent and broad transcription of multiple interferons (IFNs) [via] pattern recognition receptors (TLR7 and retinoic acid-inducible gene 1).” Furthermore, supernatants from primary HLSECs transfected with HCV-specific PAMP molecules had larger inductions of IFNs and IFN-stimulated genes in HLSECs.
“Conditioned media from IFN-stimulated HLSECs induced expression of antiviral genes by uninfected primary human hepatocytes,” wrote Dr. Giugliano and her coauthors. “Exosomes, derived from HLSECs following stimulation with either type I or type III IFNs, controlled HCV replication in a dose-dependent manner.”
However, the investigators say that more study is required to understand why HCV is able to present so persistently if the means to combat the virus are so apparent in the immune system.
“These results raise a number of intriguing questions, for example, how the use of exogenous IFN to treat viral hepatitis could be expected to induce additional, previously unrecognized antiviral mechanisms involving HLSECs,” says the study. “Further work is warranted to understand why despite these innate immune responses, HCV is able to establish persistence and fail eradication with IFN-based antiviral therapy.”
The study supported by grants R21-AI103361 and U19 AI 1066328; Dr. Rosen received a Merit Review grant and Dr. Shaw received grant R21-AI 106000. The authors reported no financial conflicts of interest.
Secondhand smoke remains problematic in the United States
Exposure to and inhalation of secondhand smoke continue to be a significant problem throughout the United States, with cases of secondhand smoking–related illness and morbidity higher in juvenile and African American populations, among others, Dr. Thomas Frieden, director of the Centers for Disease Control and Prevention, said at a press briefing.
“Secondhand smoke is a Class A carcinogen [and] the Surgeon General has concluded that there is no risk-free level of exposure to secondhand smoke,” Dr. Frieden told reporters ahead of the release of this month’s CDC Vital Signs report (MMWR 2015 Feb.3;64:1-7). “People may not fully appreciate that secondhand smoke is not merely a nuisance – it causes disease and death.”
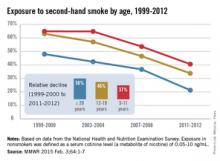
He explained that the Vital Signs report will highlight data from the National Health and Nutrition Examination Survey (NHANES), which was conducted between 1999 and 2012 to examine the health and nutrition of both children and adults across the United States. The study’s findings indicate that 40% of children, or two out of every five, are still exposed to secondhand smoke, and that even though cigarette smoking has decreased while smoke-free laws have increased in recent years, one in four nonsmokers and 58 million people overall are being exposed to secondhand smoke.
Furthermore, certain demographic groups are more likely to suffer exposure to secondhand smoke and, consequently, face several related health problems. In addition to 40.6% of children aged 3-11 years, nearly 70% of African American children in the same age range and 46.8% of all African Americans face a higher risk of secondhand smoke exposure. Mexican Americans and non-Hispanic whites also are highly predisposed to secondhand smoke exposure, with rates of 23.9% and 21.8%, respectively.
Americans living below the poverty line also face a 43% secondhand smoke exposure rate, while 37% of Americans in rental housing also are at risk. In fact, the home is the most significant source of exposure to secondhand smoke for children, although Dr. Frieden credited Americans with doubling the country’s number of smoke-free households over the last 20 years.
“Comprehensive smoke-free laws that prohibit smoking in all indoor areas of bars, restaurants, and workplaces are an important start,” stated Dr. Frieden. “As of today, 26 states and the District of Columbia, plus about 700 communities across the country, have adopted smoke-free laws that cover these three locations [and] we’ve seen tremendous increases in the number of college and university campuses that have gone smoke free.”
According to the CDC, exposure to secondhand smoke has been proven to cause afflictions such as ear infections, respiratory infections, asthma attacks, and sudden infant death syndrome in infants and children, plus stroke, coronary heart disease, heart attack, and lung cancer in nonsmoking adults. Secondhand smoke exposure causes 400 deaths in infants each year and more than 41,000 fatalities among nonsmoking adults per year. CDC figures also state that secondhand smoke exposure costs the United States $5.6 billion annually in lost productivity.
Exposure to and inhalation of secondhand smoke continue to be a significant problem throughout the United States, with cases of secondhand smoking–related illness and morbidity higher in juvenile and African American populations, among others, Dr. Thomas Frieden, director of the Centers for Disease Control and Prevention, said at a press briefing.
“Secondhand smoke is a Class A carcinogen [and] the Surgeon General has concluded that there is no risk-free level of exposure to secondhand smoke,” Dr. Frieden told reporters ahead of the release of this month’s CDC Vital Signs report (MMWR 2015 Feb.3;64:1-7). “People may not fully appreciate that secondhand smoke is not merely a nuisance – it causes disease and death.”
He explained that the Vital Signs report will highlight data from the National Health and Nutrition Examination Survey (NHANES), which was conducted between 1999 and 2012 to examine the health and nutrition of both children and adults across the United States. The study’s findings indicate that 40% of children, or two out of every five, are still exposed to secondhand smoke, and that even though cigarette smoking has decreased while smoke-free laws have increased in recent years, one in four nonsmokers and 58 million people overall are being exposed to secondhand smoke.
Furthermore, certain demographic groups are more likely to suffer exposure to secondhand smoke and, consequently, face several related health problems. In addition to 40.6% of children aged 3-11 years, nearly 70% of African American children in the same age range and 46.8% of all African Americans face a higher risk of secondhand smoke exposure. Mexican Americans and non-Hispanic whites also are highly predisposed to secondhand smoke exposure, with rates of 23.9% and 21.8%, respectively.
Americans living below the poverty line also face a 43% secondhand smoke exposure rate, while 37% of Americans in rental housing also are at risk. In fact, the home is the most significant source of exposure to secondhand smoke for children, although Dr. Frieden credited Americans with doubling the country’s number of smoke-free households over the last 20 years.
“Comprehensive smoke-free laws that prohibit smoking in all indoor areas of bars, restaurants, and workplaces are an important start,” stated Dr. Frieden. “As of today, 26 states and the District of Columbia, plus about 700 communities across the country, have adopted smoke-free laws that cover these three locations [and] we’ve seen tremendous increases in the number of college and university campuses that have gone smoke free.”
According to the CDC, exposure to secondhand smoke has been proven to cause afflictions such as ear infections, respiratory infections, asthma attacks, and sudden infant death syndrome in infants and children, plus stroke, coronary heart disease, heart attack, and lung cancer in nonsmoking adults. Secondhand smoke exposure causes 400 deaths in infants each year and more than 41,000 fatalities among nonsmoking adults per year. CDC figures also state that secondhand smoke exposure costs the United States $5.6 billion annually in lost productivity.
Exposure to and inhalation of secondhand smoke continue to be a significant problem throughout the United States, with cases of secondhand smoking–related illness and morbidity higher in juvenile and African American populations, among others, Dr. Thomas Frieden, director of the Centers for Disease Control and Prevention, said at a press briefing.
“Secondhand smoke is a Class A carcinogen [and] the Surgeon General has concluded that there is no risk-free level of exposure to secondhand smoke,” Dr. Frieden told reporters ahead of the release of this month’s CDC Vital Signs report (MMWR 2015 Feb.3;64:1-7). “People may not fully appreciate that secondhand smoke is not merely a nuisance – it causes disease and death.”
He explained that the Vital Signs report will highlight data from the National Health and Nutrition Examination Survey (NHANES), which was conducted between 1999 and 2012 to examine the health and nutrition of both children and adults across the United States. The study’s findings indicate that 40% of children, or two out of every five, are still exposed to secondhand smoke, and that even though cigarette smoking has decreased while smoke-free laws have increased in recent years, one in four nonsmokers and 58 million people overall are being exposed to secondhand smoke.
Furthermore, certain demographic groups are more likely to suffer exposure to secondhand smoke and, consequently, face several related health problems. In addition to 40.6% of children aged 3-11 years, nearly 70% of African American children in the same age range and 46.8% of all African Americans face a higher risk of secondhand smoke exposure. Mexican Americans and non-Hispanic whites also are highly predisposed to secondhand smoke exposure, with rates of 23.9% and 21.8%, respectively.
Americans living below the poverty line also face a 43% secondhand smoke exposure rate, while 37% of Americans in rental housing also are at risk. In fact, the home is the most significant source of exposure to secondhand smoke for children, although Dr. Frieden credited Americans with doubling the country’s number of smoke-free households over the last 20 years.
“Comprehensive smoke-free laws that prohibit smoking in all indoor areas of bars, restaurants, and workplaces are an important start,” stated Dr. Frieden. “As of today, 26 states and the District of Columbia, plus about 700 communities across the country, have adopted smoke-free laws that cover these three locations [and] we’ve seen tremendous increases in the number of college and university campuses that have gone smoke free.”
According to the CDC, exposure to secondhand smoke has been proven to cause afflictions such as ear infections, respiratory infections, asthma attacks, and sudden infant death syndrome in infants and children, plus stroke, coronary heart disease, heart attack, and lung cancer in nonsmoking adults. Secondhand smoke exposure causes 400 deaths in infants each year and more than 41,000 fatalities among nonsmoking adults per year. CDC figures also state that secondhand smoke exposure costs the United States $5.6 billion annually in lost productivity.
FROM A CDC TELEBRIEFING
Human liver cells can induce antiviral reaction against hepatitis C
Immunity against the hepatitis C virus can be induced by cultured primary human liver sinusoidal endothelial cells, according to a study published in the February issue of Gastroenterology (doi:10.1053/j.gastro.2014.10.040).
“We found HLSECs [primary human LSECs] express many of the receptors implicated in HCV [hepatitis C virus] attachment and entry and HLSEC-to-hepatocyte contact was dispensable for uptake,” wrote lead author Dr. Silvia M. Giugliano of the University of Colorado, Denver, and her associates.
In a cohort study, investigators exposed HLSECs and immortalized liver endothelial cells (TMNK-1) to several variants of HCV: full-length transmitted/founder virus, sucrose-purified Japanese fulminant hepatitis–1 (JFH-1), a virus encoding a luciferase reporter, and the HCV-specific pathogen-associated molecular pattern molecules (PAMP, substrate for RIG-I). Cells were analyzed by using polymerase chain reaction (PCR), immunofluorescence, and immunohistochemical assays.
Results showed that HLSECs, regardless of contact between cells, were able to internalize HCV when exposed to it and replicate, though not translate HCV RNA. The HCV RNA “induced consistent and broad transcription of multiple interferons (IFNs) [via] pattern recognition receptors (TLR7 and retinoic acid-inducible gene 1).” Furthermore, supernatants from primary HLSECs transfected with HCV-specific PAMP molecules had larger inductions of IFNs and IFN-stimulated genes in HLSECs.
“Conditioned media from IFN-stimulated HLSECs induced expression of antiviral genes by uninfected primary human hepatocytes,” wrote Dr. Giugliano and her coauthors. “Exosomes, derived from HLSECs following stimulation with either type I or type III IFNs, controlled HCV replication in a dose-dependent manner.”
However, the investigators say that more study is required to understand why HCV is able to present so persistently if the means to combat the virus are so apparent in the immune system.
“These results raise a number of intriguing questions, for example, how the use of exogenous IFN to treat viral hepatitis could be expected to induce additional, previously unrecognized antiviral mechanisms involving HLSECs,” says the study. “Further work is warranted to understand why despite these innate immune responses, HCV is able to establish persistence and fail eradication with IFN-based antiviral therapy.”
The study supported by grants R21-AI103361 and U19 AI 1066328; Dr. Rosen received a Merit Review grant and Dr. Shaw received grant R21-AI 106000. The authors reported no financial conflicts of interest.
Liver sinusoidal endothelial cells (LSECs) are a large component of the liver immune system but are not very well characterized for their role in diseases like chronic hepatitis C virus (HCV) infection. Dr. Giugliano and her associates tested the antiviral activity of primary human LSECs and an immortalized LSEC cell line (TMNK-1), following exposure to several viral variants. These included naturally

|
| Dr. Arash Grakoui |
occurring founder genotype 1a virus, a JFH-1 infectious clone (cloned originally from a patient with fulminant hepatitis), a modified cell culture of adaptive JFH-1 virus expressing luciferase reporter, and HCV-specific pathogen-associated molecular pattern poly UC RNA that is a substrate for RIG-I. They found LSECs were able to acquire HCV in an LSEC-to-hepatocyte–contact independent manner but that these cells were not permissive for productive infection (replication). Importantly, viral RNA through HCV uptake induced robust transcription of type I and III interferon (IFN) by LSECs via TLR7 and RIG-I pathways. LSEC-derived IFN had antiviral effects on HCV-infected human hepatoma cell line, Huh7.5 cells in vitro, and this effect was mediated through the production of exosomes. The authors concluded that LSECs likely have an important antiviral role in HCV infection through bystander induction of an antiviral state. This study highlights the idea that these cells can function similarly to other innate/phagocytic cells like monocytes/macrophages and furthers the idea that innate activation of LSECs may have protective effects on the antiviral state of hepatocytes. Mechanisms of antiviral immunity by LSECs, whether it occurs through exosomes or IFN, will have a significant impact in the field as the answers will have relevance to numerous facets of liver immunology, including gene transfer, organ transplant, and response to other hepatotropic infections.
Dr. Arash Grakoui is an associate professor jointly appointed in the departments of medicine, division of infectious diseases and Yerkes National Primate Research Center, department of microbiology and immunology at Emory University, Atlanta. He has no conflicts of interest.
Liver sinusoidal endothelial cells (LSECs) are a large component of the liver immune system but are not very well characterized for their role in diseases like chronic hepatitis C virus (HCV) infection. Dr. Giugliano and her associates tested the antiviral activity of primary human LSECs and an immortalized LSEC cell line (TMNK-1), following exposure to several viral variants. These included naturally

|
| Dr. Arash Grakoui |
occurring founder genotype 1a virus, a JFH-1 infectious clone (cloned originally from a patient with fulminant hepatitis), a modified cell culture of adaptive JFH-1 virus expressing luciferase reporter, and HCV-specific pathogen-associated molecular pattern poly UC RNA that is a substrate for RIG-I. They found LSECs were able to acquire HCV in an LSEC-to-hepatocyte–contact independent manner but that these cells were not permissive for productive infection (replication). Importantly, viral RNA through HCV uptake induced robust transcription of type I and III interferon (IFN) by LSECs via TLR7 and RIG-I pathways. LSEC-derived IFN had antiviral effects on HCV-infected human hepatoma cell line, Huh7.5 cells in vitro, and this effect was mediated through the production of exosomes. The authors concluded that LSECs likely have an important antiviral role in HCV infection through bystander induction of an antiviral state. This study highlights the idea that these cells can function similarly to other innate/phagocytic cells like monocytes/macrophages and furthers the idea that innate activation of LSECs may have protective effects on the antiviral state of hepatocytes. Mechanisms of antiviral immunity by LSECs, whether it occurs through exosomes or IFN, will have a significant impact in the field as the answers will have relevance to numerous facets of liver immunology, including gene transfer, organ transplant, and response to other hepatotropic infections.
Dr. Arash Grakoui is an associate professor jointly appointed in the departments of medicine, division of infectious diseases and Yerkes National Primate Research Center, department of microbiology and immunology at Emory University, Atlanta. He has no conflicts of interest.
Liver sinusoidal endothelial cells (LSECs) are a large component of the liver immune system but are not very well characterized for their role in diseases like chronic hepatitis C virus (HCV) infection. Dr. Giugliano and her associates tested the antiviral activity of primary human LSECs and an immortalized LSEC cell line (TMNK-1), following exposure to several viral variants. These included naturally

|
| Dr. Arash Grakoui |
occurring founder genotype 1a virus, a JFH-1 infectious clone (cloned originally from a patient with fulminant hepatitis), a modified cell culture of adaptive JFH-1 virus expressing luciferase reporter, and HCV-specific pathogen-associated molecular pattern poly UC RNA that is a substrate for RIG-I. They found LSECs were able to acquire HCV in an LSEC-to-hepatocyte–contact independent manner but that these cells were not permissive for productive infection (replication). Importantly, viral RNA through HCV uptake induced robust transcription of type I and III interferon (IFN) by LSECs via TLR7 and RIG-I pathways. LSEC-derived IFN had antiviral effects on HCV-infected human hepatoma cell line, Huh7.5 cells in vitro, and this effect was mediated through the production of exosomes. The authors concluded that LSECs likely have an important antiviral role in HCV infection through bystander induction of an antiviral state. This study highlights the idea that these cells can function similarly to other innate/phagocytic cells like monocytes/macrophages and furthers the idea that innate activation of LSECs may have protective effects on the antiviral state of hepatocytes. Mechanisms of antiviral immunity by LSECs, whether it occurs through exosomes or IFN, will have a significant impact in the field as the answers will have relevance to numerous facets of liver immunology, including gene transfer, organ transplant, and response to other hepatotropic infections.
Dr. Arash Grakoui is an associate professor jointly appointed in the departments of medicine, division of infectious diseases and Yerkes National Primate Research Center, department of microbiology and immunology at Emory University, Atlanta. He has no conflicts of interest.
Immunity against the hepatitis C virus can be induced by cultured primary human liver sinusoidal endothelial cells, according to a study published in the February issue of Gastroenterology (doi:10.1053/j.gastro.2014.10.040).
“We found HLSECs [primary human LSECs] express many of the receptors implicated in HCV [hepatitis C virus] attachment and entry and HLSEC-to-hepatocyte contact was dispensable for uptake,” wrote lead author Dr. Silvia M. Giugliano of the University of Colorado, Denver, and her associates.
In a cohort study, investigators exposed HLSECs and immortalized liver endothelial cells (TMNK-1) to several variants of HCV: full-length transmitted/founder virus, sucrose-purified Japanese fulminant hepatitis–1 (JFH-1), a virus encoding a luciferase reporter, and the HCV-specific pathogen-associated molecular pattern molecules (PAMP, substrate for RIG-I). Cells were analyzed by using polymerase chain reaction (PCR), immunofluorescence, and immunohistochemical assays.
Results showed that HLSECs, regardless of contact between cells, were able to internalize HCV when exposed to it and replicate, though not translate HCV RNA. The HCV RNA “induced consistent and broad transcription of multiple interferons (IFNs) [via] pattern recognition receptors (TLR7 and retinoic acid-inducible gene 1).” Furthermore, supernatants from primary HLSECs transfected with HCV-specific PAMP molecules had larger inductions of IFNs and IFN-stimulated genes in HLSECs.
“Conditioned media from IFN-stimulated HLSECs induced expression of antiviral genes by uninfected primary human hepatocytes,” wrote Dr. Giugliano and her coauthors. “Exosomes, derived from HLSECs following stimulation with either type I or type III IFNs, controlled HCV replication in a dose-dependent manner.”
However, the investigators say that more study is required to understand why HCV is able to present so persistently if the means to combat the virus are so apparent in the immune system.
“These results raise a number of intriguing questions, for example, how the use of exogenous IFN to treat viral hepatitis could be expected to induce additional, previously unrecognized antiviral mechanisms involving HLSECs,” says the study. “Further work is warranted to understand why despite these innate immune responses, HCV is able to establish persistence and fail eradication with IFN-based antiviral therapy.”
The study supported by grants R21-AI103361 and U19 AI 1066328; Dr. Rosen received a Merit Review grant and Dr. Shaw received grant R21-AI 106000. The authors reported no financial conflicts of interest.
Immunity against the hepatitis C virus can be induced by cultured primary human liver sinusoidal endothelial cells, according to a study published in the February issue of Gastroenterology (doi:10.1053/j.gastro.2014.10.040).
“We found HLSECs [primary human LSECs] express many of the receptors implicated in HCV [hepatitis C virus] attachment and entry and HLSEC-to-hepatocyte contact was dispensable for uptake,” wrote lead author Dr. Silvia M. Giugliano of the University of Colorado, Denver, and her associates.
In a cohort study, investigators exposed HLSECs and immortalized liver endothelial cells (TMNK-1) to several variants of HCV: full-length transmitted/founder virus, sucrose-purified Japanese fulminant hepatitis–1 (JFH-1), a virus encoding a luciferase reporter, and the HCV-specific pathogen-associated molecular pattern molecules (PAMP, substrate for RIG-I). Cells were analyzed by using polymerase chain reaction (PCR), immunofluorescence, and immunohistochemical assays.
Results showed that HLSECs, regardless of contact between cells, were able to internalize HCV when exposed to it and replicate, though not translate HCV RNA. The HCV RNA “induced consistent and broad transcription of multiple interferons (IFNs) [via] pattern recognition receptors (TLR7 and retinoic acid-inducible gene 1).” Furthermore, supernatants from primary HLSECs transfected with HCV-specific PAMP molecules had larger inductions of IFNs and IFN-stimulated genes in HLSECs.
“Conditioned media from IFN-stimulated HLSECs induced expression of antiviral genes by uninfected primary human hepatocytes,” wrote Dr. Giugliano and her coauthors. “Exosomes, derived from HLSECs following stimulation with either type I or type III IFNs, controlled HCV replication in a dose-dependent manner.”
However, the investigators say that more study is required to understand why HCV is able to present so persistently if the means to combat the virus are so apparent in the immune system.
“These results raise a number of intriguing questions, for example, how the use of exogenous IFN to treat viral hepatitis could be expected to induce additional, previously unrecognized antiviral mechanisms involving HLSECs,” says the study. “Further work is warranted to understand why despite these innate immune responses, HCV is able to establish persistence and fail eradication with IFN-based antiviral therapy.”
The study supported by grants R21-AI103361 and U19 AI 1066328; Dr. Rosen received a Merit Review grant and Dr. Shaw received grant R21-AI 106000. The authors reported no financial conflicts of interest.
FROM GASTROENTEROLOGY
Key clinical point: Cultured primary human liver sinusoidal endothelial cells (HLSECs) induce self-amplifying interferon-mediated responses and release of exosomes with antiviral activity, thus allowing it to mediate immunity against the hepatitis C virus (HCV).
Major finding: HLSECs and CV-specific PAMP molecules induced IFNs and replicated HCV RNA when exposed to various forms of HCV.
Data source: Cohort study
Disclosures: Study supported by grants R21-AI103361 and U19 AI 1066328; Dr. Rosen received a Merit Review grant and Dr. Shaw received grant R21-AI 106000. The authors reported no financial conflicts of interest.
Choose colonoscopy over sigmoidoscopy for screening of proximal advanced serrated lesions
Screening colonoscopy is more effective than sigmoidoscopy for the detection of colorectal cancer by picking up proximal advanced serrated lesions, according to the results of a study published in the February issue of Clinical Gastroenterology and Hepatology.
“Colonoscopy allows the detection and removal of precursor polyps during the same session and is the final common pathway for other screening modalities,” wrote lead author Dr. Charles J. Kahi of Indiana University, Indianapolis, adding that “sigmoidoscopy is an attractive option because it is more straightforward to perform, is less burdensome, and is associated with lower risk for harm than colonoscopy.”
In a retrospective, cross-sectional study, Dr. Kahi and his associates culled data on 1,910 patients who underwent an average-risk screening colonoscopy from August 2005 through April 2012 at Indiana University Hospital and an associated ambulatory surgery center. All patients included were at least 50 years of age, with an average age of 59.3 years ± 8.0 years, and women represented 53.8% of the population (Clin. Gastroenterol. Hepatol. 2015 February [doi:10.1016/j.cgh.2014.07.044]).
All subjects had colonoscopies performed by an endoscopist with “documented high adenoma and serrated polyp detection rates;” when found, tissue samples of all serrated polyps – hyperplastic (HP), sessile serrated adenoma/polyp (SSA/P), or traditional serrated adenoma – were also proximal to the sigmoid colon and serrated polyps larger than 5 mm in the rectum or sigmoid colon were also taken and “reviewed by a gastrointestinal pathologist and reclassified on the basis of World Health Organization [WHO] criteria.”
The WHO classifications for serrated polyps fall into HP, SSA/P with cytologic dysplasia, sessile serrated polyp with cytologic dysplasia (SSA/P-CD) of at least 10 mm, and traditional serrated adenoma (TSA). Advanced conventional adenomatous neoplasia (ACN) was defined as tubular adenoma of at least 10 mm, villous histology, high-grade dysplasia, or cancer. The prevalence of both proximal advanced serrated lesion (ASL) and ACN was calculated based on distal colorectal findings; investigators also performed multivariable logistic regression analysis to determine age-adjusted and sex-adjusted odds of advanced proximal adenomatous and serrated lesions, while “secondary analyses were performed to examine the effect of variable ASL definitions.”
Results indicated that of the 1,910 subjects in the study population, 52 (2.7%) were found to have proximal ASL, while 99 (5.2%) had proximal ACN. Of the 52 individuals with proximal ASL, 27 (52%) had no distal polyps, while 40 (40%) of the 99 subjects with proximal ACN also had no distal polyps. A total of 1,675 patients (87.7%) had no family history of colorectal cancer (CRC); of the remaining 235 patients, 212 (11.1%) had a first-degree relative and 23 (1.2%) had a distant relative with the condition.
Age and type of distal neoplasia, with the exception of nonadvanced serrated lesions, were associated with proximal ACN; however, only patient age was significantly associated with proximal ASL. Investigators found no significant associations between distal polyps and proximal ASL, and in secondary analyses, presence of a distal SSA/P was the lone factor associated with a proximal SSA/P. The authors also found no significant associations with either age or sex.
“These findings are relevant for CRC screening strategies that use sigmoidoscopy as a ‘gateway’ test and triage patients to colonoscopy on the basis of findings at sigmoidoscopy,” the authors note, adding that “for example, a patient with no polyps at sigmoidoscopy who is not referred for a follow-up colonoscopy exclusively on the basis of the estimated risk of advanced ACN could be harboring significant serrated lesions in the proximal colon that would go undetected.”
Funding was provided by a gift from Scott and Kay Schurz of Bloomington, Ind. No financial conflicts of interests were reported.
The findings of this paper confirm prior work linking distal neoplastic findings with conventional proximal advanced neoplasia. For example, those with distal nonadvanced neoplasia are over two times more likely to have advanced proximal conventional neoplasia. More importantly, the study extends our knowledge of the importance of distal neoplastic findings in predicting significant proximal serrated lesions. Interestingly, no strong associations were found. In fact, over half of those with significant proximal serrated neoplasia (i.e., large or those with dysplastic features) had no distal adenomatous marker lesion.

|
| Dr. Douglas Robertson |
The authors contend that the results favor colonoscopy as a primary screening strategy relative to sigmoidoscopy, since many with advanced proximal serrated lesions will have no distal marker lesion to prompt full colonoscopy. Perhaps, but the fact remains that large randomized trials utilizing sigmoidoscopy as a screening tool have uniformly shown marked reductions in colorectal cancer incidence and mortality. The relative importance of this factor (i.e., improved proximal serrated neoplasia detection with colonoscopy) would have to be considered relative to other factors that drive the success of screening programs (e.g., patient compliance, low complication rates) and likely could be fully understood only through direct comparative effectiveness studies.
Even beyond the implications of this study to inform our understanding of these two screening modalities (colonoscopy and sigmoidoscopy) is the contribution to our rapidly growing knowledge about serrated neoplasia. Inarguably, the serrated pathway is an important one in carcinogenesis. This paper is further evidence that what we have previously learned about conventional adenomas cannot directly be applied to serrated lesions. Additional high-quality epidemiologic work like this will be required to understand the important differences.
Dr. Douglas J. Robertson is associate professor of medicine at the Geisel School of Medicine at Dartmouth and the Dartmouth Institute, and chief of Gastroenterology at the VA Medical Center, White River Junction, Vt. He has no conflicts of interest.
The findings of this paper confirm prior work linking distal neoplastic findings with conventional proximal advanced neoplasia. For example, those with distal nonadvanced neoplasia are over two times more likely to have advanced proximal conventional neoplasia. More importantly, the study extends our knowledge of the importance of distal neoplastic findings in predicting significant proximal serrated lesions. Interestingly, no strong associations were found. In fact, over half of those with significant proximal serrated neoplasia (i.e., large or those with dysplastic features) had no distal adenomatous marker lesion.

|
| Dr. Douglas Robertson |
The authors contend that the results favor colonoscopy as a primary screening strategy relative to sigmoidoscopy, since many with advanced proximal serrated lesions will have no distal marker lesion to prompt full colonoscopy. Perhaps, but the fact remains that large randomized trials utilizing sigmoidoscopy as a screening tool have uniformly shown marked reductions in colorectal cancer incidence and mortality. The relative importance of this factor (i.e., improved proximal serrated neoplasia detection with colonoscopy) would have to be considered relative to other factors that drive the success of screening programs (e.g., patient compliance, low complication rates) and likely could be fully understood only through direct comparative effectiveness studies.
Even beyond the implications of this study to inform our understanding of these two screening modalities (colonoscopy and sigmoidoscopy) is the contribution to our rapidly growing knowledge about serrated neoplasia. Inarguably, the serrated pathway is an important one in carcinogenesis. This paper is further evidence that what we have previously learned about conventional adenomas cannot directly be applied to serrated lesions. Additional high-quality epidemiologic work like this will be required to understand the important differences.
Dr. Douglas J. Robertson is associate professor of medicine at the Geisel School of Medicine at Dartmouth and the Dartmouth Institute, and chief of Gastroenterology at the VA Medical Center, White River Junction, Vt. He has no conflicts of interest.
The findings of this paper confirm prior work linking distal neoplastic findings with conventional proximal advanced neoplasia. For example, those with distal nonadvanced neoplasia are over two times more likely to have advanced proximal conventional neoplasia. More importantly, the study extends our knowledge of the importance of distal neoplastic findings in predicting significant proximal serrated lesions. Interestingly, no strong associations were found. In fact, over half of those with significant proximal serrated neoplasia (i.e., large or those with dysplastic features) had no distal adenomatous marker lesion.

|
| Dr. Douglas Robertson |
The authors contend that the results favor colonoscopy as a primary screening strategy relative to sigmoidoscopy, since many with advanced proximal serrated lesions will have no distal marker lesion to prompt full colonoscopy. Perhaps, but the fact remains that large randomized trials utilizing sigmoidoscopy as a screening tool have uniformly shown marked reductions in colorectal cancer incidence and mortality. The relative importance of this factor (i.e., improved proximal serrated neoplasia detection with colonoscopy) would have to be considered relative to other factors that drive the success of screening programs (e.g., patient compliance, low complication rates) and likely could be fully understood only through direct comparative effectiveness studies.
Even beyond the implications of this study to inform our understanding of these two screening modalities (colonoscopy and sigmoidoscopy) is the contribution to our rapidly growing knowledge about serrated neoplasia. Inarguably, the serrated pathway is an important one in carcinogenesis. This paper is further evidence that what we have previously learned about conventional adenomas cannot directly be applied to serrated lesions. Additional high-quality epidemiologic work like this will be required to understand the important differences.
Dr. Douglas J. Robertson is associate professor of medicine at the Geisel School of Medicine at Dartmouth and the Dartmouth Institute, and chief of Gastroenterology at the VA Medical Center, White River Junction, Vt. He has no conflicts of interest.
Screening colonoscopy is more effective than sigmoidoscopy for the detection of colorectal cancer by picking up proximal advanced serrated lesions, according to the results of a study published in the February issue of Clinical Gastroenterology and Hepatology.
“Colonoscopy allows the detection and removal of precursor polyps during the same session and is the final common pathway for other screening modalities,” wrote lead author Dr. Charles J. Kahi of Indiana University, Indianapolis, adding that “sigmoidoscopy is an attractive option because it is more straightforward to perform, is less burdensome, and is associated with lower risk for harm than colonoscopy.”
In a retrospective, cross-sectional study, Dr. Kahi and his associates culled data on 1,910 patients who underwent an average-risk screening colonoscopy from August 2005 through April 2012 at Indiana University Hospital and an associated ambulatory surgery center. All patients included were at least 50 years of age, with an average age of 59.3 years ± 8.0 years, and women represented 53.8% of the population (Clin. Gastroenterol. Hepatol. 2015 February [doi:10.1016/j.cgh.2014.07.044]).
All subjects had colonoscopies performed by an endoscopist with “documented high adenoma and serrated polyp detection rates;” when found, tissue samples of all serrated polyps – hyperplastic (HP), sessile serrated adenoma/polyp (SSA/P), or traditional serrated adenoma – were also proximal to the sigmoid colon and serrated polyps larger than 5 mm in the rectum or sigmoid colon were also taken and “reviewed by a gastrointestinal pathologist and reclassified on the basis of World Health Organization [WHO] criteria.”
The WHO classifications for serrated polyps fall into HP, SSA/P with cytologic dysplasia, sessile serrated polyp with cytologic dysplasia (SSA/P-CD) of at least 10 mm, and traditional serrated adenoma (TSA). Advanced conventional adenomatous neoplasia (ACN) was defined as tubular adenoma of at least 10 mm, villous histology, high-grade dysplasia, or cancer. The prevalence of both proximal advanced serrated lesion (ASL) and ACN was calculated based on distal colorectal findings; investigators also performed multivariable logistic regression analysis to determine age-adjusted and sex-adjusted odds of advanced proximal adenomatous and serrated lesions, while “secondary analyses were performed to examine the effect of variable ASL definitions.”
Results indicated that of the 1,910 subjects in the study population, 52 (2.7%) were found to have proximal ASL, while 99 (5.2%) had proximal ACN. Of the 52 individuals with proximal ASL, 27 (52%) had no distal polyps, while 40 (40%) of the 99 subjects with proximal ACN also had no distal polyps. A total of 1,675 patients (87.7%) had no family history of colorectal cancer (CRC); of the remaining 235 patients, 212 (11.1%) had a first-degree relative and 23 (1.2%) had a distant relative with the condition.
Age and type of distal neoplasia, with the exception of nonadvanced serrated lesions, were associated with proximal ACN; however, only patient age was significantly associated with proximal ASL. Investigators found no significant associations between distal polyps and proximal ASL, and in secondary analyses, presence of a distal SSA/P was the lone factor associated with a proximal SSA/P. The authors also found no significant associations with either age or sex.
“These findings are relevant for CRC screening strategies that use sigmoidoscopy as a ‘gateway’ test and triage patients to colonoscopy on the basis of findings at sigmoidoscopy,” the authors note, adding that “for example, a patient with no polyps at sigmoidoscopy who is not referred for a follow-up colonoscopy exclusively on the basis of the estimated risk of advanced ACN could be harboring significant serrated lesions in the proximal colon that would go undetected.”
Funding was provided by a gift from Scott and Kay Schurz of Bloomington, Ind. No financial conflicts of interests were reported.
Screening colonoscopy is more effective than sigmoidoscopy for the detection of colorectal cancer by picking up proximal advanced serrated lesions, according to the results of a study published in the February issue of Clinical Gastroenterology and Hepatology.
“Colonoscopy allows the detection and removal of precursor polyps during the same session and is the final common pathway for other screening modalities,” wrote lead author Dr. Charles J. Kahi of Indiana University, Indianapolis, adding that “sigmoidoscopy is an attractive option because it is more straightforward to perform, is less burdensome, and is associated with lower risk for harm than colonoscopy.”
In a retrospective, cross-sectional study, Dr. Kahi and his associates culled data on 1,910 patients who underwent an average-risk screening colonoscopy from August 2005 through April 2012 at Indiana University Hospital and an associated ambulatory surgery center. All patients included were at least 50 years of age, with an average age of 59.3 years ± 8.0 years, and women represented 53.8% of the population (Clin. Gastroenterol. Hepatol. 2015 February [doi:10.1016/j.cgh.2014.07.044]).
All subjects had colonoscopies performed by an endoscopist with “documented high adenoma and serrated polyp detection rates;” when found, tissue samples of all serrated polyps – hyperplastic (HP), sessile serrated adenoma/polyp (SSA/P), or traditional serrated adenoma – were also proximal to the sigmoid colon and serrated polyps larger than 5 mm in the rectum or sigmoid colon were also taken and “reviewed by a gastrointestinal pathologist and reclassified on the basis of World Health Organization [WHO] criteria.”
The WHO classifications for serrated polyps fall into HP, SSA/P with cytologic dysplasia, sessile serrated polyp with cytologic dysplasia (SSA/P-CD) of at least 10 mm, and traditional serrated adenoma (TSA). Advanced conventional adenomatous neoplasia (ACN) was defined as tubular adenoma of at least 10 mm, villous histology, high-grade dysplasia, or cancer. The prevalence of both proximal advanced serrated lesion (ASL) and ACN was calculated based on distal colorectal findings; investigators also performed multivariable logistic regression analysis to determine age-adjusted and sex-adjusted odds of advanced proximal adenomatous and serrated lesions, while “secondary analyses were performed to examine the effect of variable ASL definitions.”
Results indicated that of the 1,910 subjects in the study population, 52 (2.7%) were found to have proximal ASL, while 99 (5.2%) had proximal ACN. Of the 52 individuals with proximal ASL, 27 (52%) had no distal polyps, while 40 (40%) of the 99 subjects with proximal ACN also had no distal polyps. A total of 1,675 patients (87.7%) had no family history of colorectal cancer (CRC); of the remaining 235 patients, 212 (11.1%) had a first-degree relative and 23 (1.2%) had a distant relative with the condition.
Age and type of distal neoplasia, with the exception of nonadvanced serrated lesions, were associated with proximal ACN; however, only patient age was significantly associated with proximal ASL. Investigators found no significant associations between distal polyps and proximal ASL, and in secondary analyses, presence of a distal SSA/P was the lone factor associated with a proximal SSA/P. The authors also found no significant associations with either age or sex.
“These findings are relevant for CRC screening strategies that use sigmoidoscopy as a ‘gateway’ test and triage patients to colonoscopy on the basis of findings at sigmoidoscopy,” the authors note, adding that “for example, a patient with no polyps at sigmoidoscopy who is not referred for a follow-up colonoscopy exclusively on the basis of the estimated risk of advanced ACN could be harboring significant serrated lesions in the proximal colon that would go undetected.”
Funding was provided by a gift from Scott and Kay Schurz of Bloomington, Ind. No financial conflicts of interests were reported.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Screening colonoscopy is more effective than sigmoidoscopy for detection of proximal advanced serrated lesions and, consequently, colorectal cancer, particularly in elderly patients.
Major finding: In a population of 1,910 subjects, 52 (2.7%) had proximal ASL, 27 of whom (52%) had no distal polyps. Of the 1,910 population, 99 subjects (5.2%) had proximal advanced conventional adenomatous neoplasia, 40 (40%) of whom had no distal polyps.
Data source: Retrospective, cross-sectional study.
Disclosures: Funding was provided by a gift from Scott and Kay Schurz of Bloomington, Ind. No financial conflicts of interests were reported.
Transoral Fundoplication Can Be Effective Against GERD Symptoms
Transoral esophagogastric fundoplication can be an effective treatment for patients seeking to alleviate symptoms associated with gastroesophageal reflux disease, particularly in individuals with persistent regurgitation despite prior treatment with proton pump inhibitor therapy, according to the results of a new study published in the February issue of Gastroenterology (doi:10.1053/j.gastro.2014.10.009).
“Gastroesophageal reflux disease (GERD) remains one of the most common conditions for which Americans take daily medication, and PPI use has more than doubled in the last decade,” wrote lead authors Dr. John G. Hunter of Oregon Health & Science University in Portland, and Dr. Peter J. Kahrilas of Northwestern University in Chicago, and their associates. “Despite this, up to 40% of proton pump inhibitor (PPI)–dependent GERD patients have troublesome symptoms of GERD, despite PPI therapy.”
In the Randomized EsophyX vs Sham, Placebo-Controlled Transoral Fundoplication (RESPECT) trial, investigators screened 696 patients who were experiencing “troublesome regurgitation” despite daily PPI treatment. These subjects were evaluated via three validated GERD-specific symptom scales, and were either on or off PPI use at the time of trial commencement. Post trial, patients were blinded to therapy and were reassessed at intervals of 2, 12, and 26 weeks. All patients underwent 48-hour esophageal pH monitoring and esophagogastroduodenoscopy at 66 months after the trial ended.
Regurgitation severity was based on the Montreal definition, which was used to measure efficacy of treatments given as part of the study. The Montreal definition of reflux is described by the authors as “either mucosal damage or troublesome symptoms attributable to reflux.” Those with “least troublesome” regurgitation while on PPIs “underwent barium swallow, esophagogastroduodenoscopy, 48-hour esophageal pH monitoring (off PPIs), and high-resolution esophageal manometry analyses.”
Eighty-seven subjects with GERD and hiatal hernias of at least 2 centimeters were randomly assigned to groups that underwent transoral fundoplication (TF) followed by placebo treatment after 6 months, while 42 subjects, who made up the control group, underwent a “sham surgery” and began regimens of once- or twice-daily omeprazole medication for 6 months.
What did results show?
Results showed that 67% of patients who received TF treatment experienced elimination of adverse regurgitation vs. 45% of those treated with PPI (P = .023). Control of esophageal pH also improved noticeably in patients who received TF treatment versus those who did not (9.3% vs. 6.3% on average, respectively, P < .001), but not in patients who received the “sham surgery” (8.6% preop vs. 8.9% postop on average). Fewer patients who received TF treatment recorded having “no response” after 3 months compared with those in the control group (11% vs. 36%, respectively, P = .004).
“Transoral fundoplication may fill the ‘therapeutic gap’ that exists between PPI and laparoscopic fundoplication,” wrote the authors. “Considering the virtual absence of dysphagia and bloating after TF, which may be problematic with LINX [LINX Reflux Management System], it would appear that TF is an option for patients with troublesome regurgitation, as well as for patients with troublesome GERD symptoms who wish not to take PPI over a protracted period of time.”
Several coauthors disclosed ties with the study sponsor EndoGastric Solutions of Redmond, Wash., as well as individual potential conflicts of interest.
Transoral esophagogastric fundoplication can be an effective treatment for patients seeking to alleviate symptoms associated with gastroesophageal reflux disease, particularly in individuals with persistent regurgitation despite prior treatment with proton pump inhibitor therapy, according to the results of a new study published in the February issue of Gastroenterology (doi:10.1053/j.gastro.2014.10.009).
“Gastroesophageal reflux disease (GERD) remains one of the most common conditions for which Americans take daily medication, and PPI use has more than doubled in the last decade,” wrote lead authors Dr. John G. Hunter of Oregon Health & Science University in Portland, and Dr. Peter J. Kahrilas of Northwestern University in Chicago, and their associates. “Despite this, up to 40% of proton pump inhibitor (PPI)–dependent GERD patients have troublesome symptoms of GERD, despite PPI therapy.”
In the Randomized EsophyX vs Sham, Placebo-Controlled Transoral Fundoplication (RESPECT) trial, investigators screened 696 patients who were experiencing “troublesome regurgitation” despite daily PPI treatment. These subjects were evaluated via three validated GERD-specific symptom scales, and were either on or off PPI use at the time of trial commencement. Post trial, patients were blinded to therapy and were reassessed at intervals of 2, 12, and 26 weeks. All patients underwent 48-hour esophageal pH monitoring and esophagogastroduodenoscopy at 66 months after the trial ended.
Regurgitation severity was based on the Montreal definition, which was used to measure efficacy of treatments given as part of the study. The Montreal definition of reflux is described by the authors as “either mucosal damage or troublesome symptoms attributable to reflux.” Those with “least troublesome” regurgitation while on PPIs “underwent barium swallow, esophagogastroduodenoscopy, 48-hour esophageal pH monitoring (off PPIs), and high-resolution esophageal manometry analyses.”
Eighty-seven subjects with GERD and hiatal hernias of at least 2 centimeters were randomly assigned to groups that underwent transoral fundoplication (TF) followed by placebo treatment after 6 months, while 42 subjects, who made up the control group, underwent a “sham surgery” and began regimens of once- or twice-daily omeprazole medication for 6 months.
What did results show?
Results showed that 67% of patients who received TF treatment experienced elimination of adverse regurgitation vs. 45% of those treated with PPI (P = .023). Control of esophageal pH also improved noticeably in patients who received TF treatment versus those who did not (9.3% vs. 6.3% on average, respectively, P < .001), but not in patients who received the “sham surgery” (8.6% preop vs. 8.9% postop on average). Fewer patients who received TF treatment recorded having “no response” after 3 months compared with those in the control group (11% vs. 36%, respectively, P = .004).
“Transoral fundoplication may fill the ‘therapeutic gap’ that exists between PPI and laparoscopic fundoplication,” wrote the authors. “Considering the virtual absence of dysphagia and bloating after TF, which may be problematic with LINX [LINX Reflux Management System], it would appear that TF is an option for patients with troublesome regurgitation, as well as for patients with troublesome GERD symptoms who wish not to take PPI over a protracted period of time.”
Several coauthors disclosed ties with the study sponsor EndoGastric Solutions of Redmond, Wash., as well as individual potential conflicts of interest.
Transoral esophagogastric fundoplication can be an effective treatment for patients seeking to alleviate symptoms associated with gastroesophageal reflux disease, particularly in individuals with persistent regurgitation despite prior treatment with proton pump inhibitor therapy, according to the results of a new study published in the February issue of Gastroenterology (doi:10.1053/j.gastro.2014.10.009).
“Gastroesophageal reflux disease (GERD) remains one of the most common conditions for which Americans take daily medication, and PPI use has more than doubled in the last decade,” wrote lead authors Dr. John G. Hunter of Oregon Health & Science University in Portland, and Dr. Peter J. Kahrilas of Northwestern University in Chicago, and their associates. “Despite this, up to 40% of proton pump inhibitor (PPI)–dependent GERD patients have troublesome symptoms of GERD, despite PPI therapy.”
In the Randomized EsophyX vs Sham, Placebo-Controlled Transoral Fundoplication (RESPECT) trial, investigators screened 696 patients who were experiencing “troublesome regurgitation” despite daily PPI treatment. These subjects were evaluated via three validated GERD-specific symptom scales, and were either on or off PPI use at the time of trial commencement. Post trial, patients were blinded to therapy and were reassessed at intervals of 2, 12, and 26 weeks. All patients underwent 48-hour esophageal pH monitoring and esophagogastroduodenoscopy at 66 months after the trial ended.
Regurgitation severity was based on the Montreal definition, which was used to measure efficacy of treatments given as part of the study. The Montreal definition of reflux is described by the authors as “either mucosal damage or troublesome symptoms attributable to reflux.” Those with “least troublesome” regurgitation while on PPIs “underwent barium swallow, esophagogastroduodenoscopy, 48-hour esophageal pH monitoring (off PPIs), and high-resolution esophageal manometry analyses.”
Eighty-seven subjects with GERD and hiatal hernias of at least 2 centimeters were randomly assigned to groups that underwent transoral fundoplication (TF) followed by placebo treatment after 6 months, while 42 subjects, who made up the control group, underwent a “sham surgery” and began regimens of once- or twice-daily omeprazole medication for 6 months.
What did results show?
Results showed that 67% of patients who received TF treatment experienced elimination of adverse regurgitation vs. 45% of those treated with PPI (P = .023). Control of esophageal pH also improved noticeably in patients who received TF treatment versus those who did not (9.3% vs. 6.3% on average, respectively, P < .001), but not in patients who received the “sham surgery” (8.6% preop vs. 8.9% postop on average). Fewer patients who received TF treatment recorded having “no response” after 3 months compared with those in the control group (11% vs. 36%, respectively, P = .004).
“Transoral fundoplication may fill the ‘therapeutic gap’ that exists between PPI and laparoscopic fundoplication,” wrote the authors. “Considering the virtual absence of dysphagia and bloating after TF, which may be problematic with LINX [LINX Reflux Management System], it would appear that TF is an option for patients with troublesome regurgitation, as well as for patients with troublesome GERD symptoms who wish not to take PPI over a protracted period of time.”
Several coauthors disclosed ties with the study sponsor EndoGastric Solutions of Redmond, Wash., as well as individual potential conflicts of interest.
FROM GASTROENTEROLOGY
Increased prevalence of chronic narcotic use in children with IBD
The prevalence of chronic narcotic use among pediatric patients with inflammatory bowel disease is abnormally high and should be curbed to prevent further adverse effects from taking hold, according to a new study published in the February issue of Clinical Gastroenterology and Hepatology.
“Although pain control is an important aspect of disease management in pediatric IBD [inflammatory bowel disease], little is known about chronic narcotic use in children,” wrote the investigators, led by Dr. Jessie P. Buckley of the University of North Carolina at Chapel Hill. They added that “although children with Crohn’s disease are at increased risk of anxiety and depression, the relationship between narcotic use and psychiatric conditions has not yet been assessed in the pediatric IBD population (Clin. Gastroenterol. Hepatol. 2015 February [doi:10.1016/j.cgh.2014.07.057].”
In a retrospective, cross-sectional study, Dr. Buckley and her associates counted all 4,911,286 patients aged 18 years or younger with continuous health plan enrollment and pharmacy benefits in the MarketScan Commercial Claims and Encounters database between January 1, 2010 and December 31, 2011. Children were defined as having IBD if they had either three or more health care visits for Crohn’s disease or ulcerative colitis during the study’s time frame, or “at least one health care contact for Crohn’s disease or ulcerative colitis and at least one pharmacy claim for any of the following medications: mesalamine, olsalazine, balsalazide, sulfasalazine, 6-mercaptopurine, azathioprine, methotrexate, enteral budesonide, or biologics (infliximab, adalimumab, certolizumab,or natalizumab).”
From this population, the authors used diagnosis codes based on data from the U.S. National Drug Code system, the European Pharmaceutical Market Research Association, and the Pharmaceutical Business Intelligence and Research Group Anatomical Classification System drug classes, along with information on dispensation of IBD medications, to select 4,344 IBD patients, each of whom was subsequently matched with 5 other patients without IBD based on age, sex, and geographic region within the United States (Northeast, North Central, South, or West), yielding 21,720 in this population. Subjects were defined as chronic users if they “had at least three narcotic drug claims during the 2-year study period.” Within the IBD population, 2,737 (63%) of subjects had Crohn’s disease and 1,607 (37%) had ulcerative colitis.
Investigators found that 5.6% (241 individuals) of the 4,344 subjects with IBD were chronic narcotic users, significantly higher than the 2.3% rate (489 subjects) of chronic narcotic use found in the non-IBD population (prevalence odds ratio, 2.59; 95% confidence interval, 2.21–3.04). Furthermore, chronic narcotic use was more prevalent in IBD patients with psychological impairment than those without: 15.7% (POR, 6.8; 95% CI, 4.3–10.6) versus 3.2% (POR, 2.3; 95% CI, 1.9–2.7), respectively. In children with IBD, older age and chronic narcotic use were also more highly associated with “increased health care utilization [and] fracture.”
“Children with IBD had more than twice the prevalence of chronic narcotic use as children without, and associations between IBD status and narcotic use indicate particularly high burden among those with concomitant anxiety or depression,” Dr. Buckley and her associates wrote, adding that “psychiatric diagnoses have also been associated with increased risk of narcotic use among adult patients with IBD. Greater attention to the complex relationships between pain and psychological impairment is warranted because children with IBD are at increased risk of anxiety and depression, compared with their peers, and other cofactors are not easily intervened on (e.g., age, region, fracture).”
The authors disclosed that financial support for this study was provided, at least in part, by GlaxoSmithKline (GSK). Dr. Buckley received funding through a research assistantship at GSK, coauthors Dr. Suzanne F. Cook and Dr. Jeffery K. Allen are employees of GSK, and coauthor Dr. Michael D. Kappelman is a consultant to GSK, among other companies.
The prevalence of chronic narcotic use among pediatric patients with inflammatory bowel disease is abnormally high and should be curbed to prevent further adverse effects from taking hold, according to a new study published in the February issue of Clinical Gastroenterology and Hepatology.
“Although pain control is an important aspect of disease management in pediatric IBD [inflammatory bowel disease], little is known about chronic narcotic use in children,” wrote the investigators, led by Dr. Jessie P. Buckley of the University of North Carolina at Chapel Hill. They added that “although children with Crohn’s disease are at increased risk of anxiety and depression, the relationship between narcotic use and psychiatric conditions has not yet been assessed in the pediatric IBD population (Clin. Gastroenterol. Hepatol. 2015 February [doi:10.1016/j.cgh.2014.07.057].”
In a retrospective, cross-sectional study, Dr. Buckley and her associates counted all 4,911,286 patients aged 18 years or younger with continuous health plan enrollment and pharmacy benefits in the MarketScan Commercial Claims and Encounters database between January 1, 2010 and December 31, 2011. Children were defined as having IBD if they had either three or more health care visits for Crohn’s disease or ulcerative colitis during the study’s time frame, or “at least one health care contact for Crohn’s disease or ulcerative colitis and at least one pharmacy claim for any of the following medications: mesalamine, olsalazine, balsalazide, sulfasalazine, 6-mercaptopurine, azathioprine, methotrexate, enteral budesonide, or biologics (infliximab, adalimumab, certolizumab,or natalizumab).”
From this population, the authors used diagnosis codes based on data from the U.S. National Drug Code system, the European Pharmaceutical Market Research Association, and the Pharmaceutical Business Intelligence and Research Group Anatomical Classification System drug classes, along with information on dispensation of IBD medications, to select 4,344 IBD patients, each of whom was subsequently matched with 5 other patients without IBD based on age, sex, and geographic region within the United States (Northeast, North Central, South, or West), yielding 21,720 in this population. Subjects were defined as chronic users if they “had at least three narcotic drug claims during the 2-year study period.” Within the IBD population, 2,737 (63%) of subjects had Crohn’s disease and 1,607 (37%) had ulcerative colitis.
Investigators found that 5.6% (241 individuals) of the 4,344 subjects with IBD were chronic narcotic users, significantly higher than the 2.3% rate (489 subjects) of chronic narcotic use found in the non-IBD population (prevalence odds ratio, 2.59; 95% confidence interval, 2.21–3.04). Furthermore, chronic narcotic use was more prevalent in IBD patients with psychological impairment than those without: 15.7% (POR, 6.8; 95% CI, 4.3–10.6) versus 3.2% (POR, 2.3; 95% CI, 1.9–2.7), respectively. In children with IBD, older age and chronic narcotic use were also more highly associated with “increased health care utilization [and] fracture.”
“Children with IBD had more than twice the prevalence of chronic narcotic use as children without, and associations between IBD status and narcotic use indicate particularly high burden among those with concomitant anxiety or depression,” Dr. Buckley and her associates wrote, adding that “psychiatric diagnoses have also been associated with increased risk of narcotic use among adult patients with IBD. Greater attention to the complex relationships between pain and psychological impairment is warranted because children with IBD are at increased risk of anxiety and depression, compared with their peers, and other cofactors are not easily intervened on (e.g., age, region, fracture).”
The authors disclosed that financial support for this study was provided, at least in part, by GlaxoSmithKline (GSK). Dr. Buckley received funding through a research assistantship at GSK, coauthors Dr. Suzanne F. Cook and Dr. Jeffery K. Allen are employees of GSK, and coauthor Dr. Michael D. Kappelman is a consultant to GSK, among other companies.
The prevalence of chronic narcotic use among pediatric patients with inflammatory bowel disease is abnormally high and should be curbed to prevent further adverse effects from taking hold, according to a new study published in the February issue of Clinical Gastroenterology and Hepatology.
“Although pain control is an important aspect of disease management in pediatric IBD [inflammatory bowel disease], little is known about chronic narcotic use in children,” wrote the investigators, led by Dr. Jessie P. Buckley of the University of North Carolina at Chapel Hill. They added that “although children with Crohn’s disease are at increased risk of anxiety and depression, the relationship between narcotic use and psychiatric conditions has not yet been assessed in the pediatric IBD population (Clin. Gastroenterol. Hepatol. 2015 February [doi:10.1016/j.cgh.2014.07.057].”
In a retrospective, cross-sectional study, Dr. Buckley and her associates counted all 4,911,286 patients aged 18 years or younger with continuous health plan enrollment and pharmacy benefits in the MarketScan Commercial Claims and Encounters database between January 1, 2010 and December 31, 2011. Children were defined as having IBD if they had either three or more health care visits for Crohn’s disease or ulcerative colitis during the study’s time frame, or “at least one health care contact for Crohn’s disease or ulcerative colitis and at least one pharmacy claim for any of the following medications: mesalamine, olsalazine, balsalazide, sulfasalazine, 6-mercaptopurine, azathioprine, methotrexate, enteral budesonide, or biologics (infliximab, adalimumab, certolizumab,or natalizumab).”
From this population, the authors used diagnosis codes based on data from the U.S. National Drug Code system, the European Pharmaceutical Market Research Association, and the Pharmaceutical Business Intelligence and Research Group Anatomical Classification System drug classes, along with information on dispensation of IBD medications, to select 4,344 IBD patients, each of whom was subsequently matched with 5 other patients without IBD based on age, sex, and geographic region within the United States (Northeast, North Central, South, or West), yielding 21,720 in this population. Subjects were defined as chronic users if they “had at least three narcotic drug claims during the 2-year study period.” Within the IBD population, 2,737 (63%) of subjects had Crohn’s disease and 1,607 (37%) had ulcerative colitis.
Investigators found that 5.6% (241 individuals) of the 4,344 subjects with IBD were chronic narcotic users, significantly higher than the 2.3% rate (489 subjects) of chronic narcotic use found in the non-IBD population (prevalence odds ratio, 2.59; 95% confidence interval, 2.21–3.04). Furthermore, chronic narcotic use was more prevalent in IBD patients with psychological impairment than those without: 15.7% (POR, 6.8; 95% CI, 4.3–10.6) versus 3.2% (POR, 2.3; 95% CI, 1.9–2.7), respectively. In children with IBD, older age and chronic narcotic use were also more highly associated with “increased health care utilization [and] fracture.”
“Children with IBD had more than twice the prevalence of chronic narcotic use as children without, and associations between IBD status and narcotic use indicate particularly high burden among those with concomitant anxiety or depression,” Dr. Buckley and her associates wrote, adding that “psychiatric diagnoses have also been associated with increased risk of narcotic use among adult patients with IBD. Greater attention to the complex relationships between pain and psychological impairment is warranted because children with IBD are at increased risk of anxiety and depression, compared with their peers, and other cofactors are not easily intervened on (e.g., age, region, fracture).”
The authors disclosed that financial support for this study was provided, at least in part, by GlaxoSmithKline (GSK). Dr. Buckley received funding through a research assistantship at GSK, coauthors Dr. Suzanne F. Cook and Dr. Jeffery K. Allen are employees of GSK, and coauthor Dr. Michael D. Kappelman is a consultant to GSK, among other companies.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Chronic narcotic use in pediatric patients with inflammatory bowel disease has become increasingly prevalent.
Major finding: Prevalence of chronic narcotic use was 5.6% among children with IBD vs. 2.3% in the general population (OR, 2.6).
Data source: Retrospective, cross-sectional study.
Disclosures: Support for this study was provided by GlaxoSmithKline; all of the study’s coauthors are either employed by or otherwise affiliated with the company.