User login
Transoral fundoplication can be effective against GERD symptoms
Transoral esophagogastric fundoplication can be an effective treatment for patients seeking to alleviate symptoms associated with gastroesophageal reflux disease, particularly in individuals with persistent regurgitation despite prior treatment with proton pump inhibitor therapy, according to the results of a new study published in the February issue of Gastroenterology (doi:10.1053/j.gastro.2014.10.009).
“Gastroesophageal reflux disease (GERD) remains one of the most common conditions for which Americans take daily medication, and PPI use has more than doubled in the last decade,” wrote lead authors Dr. John G. Hunter of Oregon Health & Science University in Portland, and Dr. Peter J. Kahrilas of Northwestern University in Chicago, and their associates. “Despite this, up to 40% of proton pump inhibitor (PPI)–dependent GERD patients have troublesome symptoms of GERD, despite PPI therapy.”
In the Randomized EsophyX vs Sham, Placebo-Controlled Transoral Fundoplication (RESPECT) trial, investigators screened 696 patients who were experiencing “troublesome regurgitation” despite daily PPI treatment. These subjects were evaluated via three validated GERD-specific symptom scales, and were either on or off PPI use at the time of trial commencement. Post trial, patients were blinded to therapy and were reassessed at intervals of 2, 12, and 26 weeks. All patients underwent 48-hour esophageal pH monitoring and esophagogastroduodenoscopy at 66 months after the trial ended.
Regurgitation severity was based on the Montreal definition, which was used to measure efficacy of treatments given as part of the study. The Montreal definition of reflux is described by the authors as “either mucosal damage or troublesome symptoms attributable to reflux.” Those with “least troublesome” regurgitation while on PPIs “underwent barium swallow, esophagogastroduodenoscopy, 48-hour esophageal pH monitoring (off PPIs), and high-resolution esophageal manometry analyses.”
Eighty-seven subjects with GERD and hiatal hernias of at least 2 centimeters were randomly assigned to groups that underwent transoral fundoplication (TF) followed by placebo treatment after 6 months, while 42 subjects, who made up the control group, underwent a “sham surgery” and began regimens of once- or twice-daily omeprazole medication for 6 months.
Results showed that 67% of patients who received TF treatment experienced elimination of adverse regurgitation vs. 45% of those treated with PPI (P = .023). Control of esophageal pH also improved noticeably in patients who received TF treatment versus those who did not (9.3% vs. 6.3% on average, respectively, P < .001), but not in patients who received the “sham surgery” (8.6% preop vs. 8.9% postop on average). Fewer patients who received TF treatment recorded having “no response” after 3 months compared with those in the control group (11% vs. 36%, respectively, P = .004).
“Transoral fundoplication may fill the ‘therapeutic gap’ that exists between PPI and laparoscopic fundoplication,” wrote the authors. “Considering the virtual absence of dysphagia and bloating after TF, which may be problematic with LINX [LINX Reflux Management System], it would appear that TF is an option for patients with troublesome regurgitation, as well as for patients with troublesome GERD symptoms who wish not to take PPI over a protracted period of time.”
Several coauthors disclosed ties with the study sponsor EndoGastric Solutions of Redmond, Wash., as well as individual potential conflicts of interest.
Transoral esophagogastric fundoplication can be an effective treatment for patients seeking to alleviate symptoms associated with gastroesophageal reflux disease, particularly in individuals with persistent regurgitation despite prior treatment with proton pump inhibitor therapy, according to the results of a new study published in the February issue of Gastroenterology (doi:10.1053/j.gastro.2014.10.009).
“Gastroesophageal reflux disease (GERD) remains one of the most common conditions for which Americans take daily medication, and PPI use has more than doubled in the last decade,” wrote lead authors Dr. John G. Hunter of Oregon Health & Science University in Portland, and Dr. Peter J. Kahrilas of Northwestern University in Chicago, and their associates. “Despite this, up to 40% of proton pump inhibitor (PPI)–dependent GERD patients have troublesome symptoms of GERD, despite PPI therapy.”
In the Randomized EsophyX vs Sham, Placebo-Controlled Transoral Fundoplication (RESPECT) trial, investigators screened 696 patients who were experiencing “troublesome regurgitation” despite daily PPI treatment. These subjects were evaluated via three validated GERD-specific symptom scales, and were either on or off PPI use at the time of trial commencement. Post trial, patients were blinded to therapy and were reassessed at intervals of 2, 12, and 26 weeks. All patients underwent 48-hour esophageal pH monitoring and esophagogastroduodenoscopy at 66 months after the trial ended.
Regurgitation severity was based on the Montreal definition, which was used to measure efficacy of treatments given as part of the study. The Montreal definition of reflux is described by the authors as “either mucosal damage or troublesome symptoms attributable to reflux.” Those with “least troublesome” regurgitation while on PPIs “underwent barium swallow, esophagogastroduodenoscopy, 48-hour esophageal pH monitoring (off PPIs), and high-resolution esophageal manometry analyses.”
Eighty-seven subjects with GERD and hiatal hernias of at least 2 centimeters were randomly assigned to groups that underwent transoral fundoplication (TF) followed by placebo treatment after 6 months, while 42 subjects, who made up the control group, underwent a “sham surgery” and began regimens of once- or twice-daily omeprazole medication for 6 months.
Results showed that 67% of patients who received TF treatment experienced elimination of adverse regurgitation vs. 45% of those treated with PPI (P = .023). Control of esophageal pH also improved noticeably in patients who received TF treatment versus those who did not (9.3% vs. 6.3% on average, respectively, P < .001), but not in patients who received the “sham surgery” (8.6% preop vs. 8.9% postop on average). Fewer patients who received TF treatment recorded having “no response” after 3 months compared with those in the control group (11% vs. 36%, respectively, P = .004).
“Transoral fundoplication may fill the ‘therapeutic gap’ that exists between PPI and laparoscopic fundoplication,” wrote the authors. “Considering the virtual absence of dysphagia and bloating after TF, which may be problematic with LINX [LINX Reflux Management System], it would appear that TF is an option for patients with troublesome regurgitation, as well as for patients with troublesome GERD symptoms who wish not to take PPI over a protracted period of time.”
Several coauthors disclosed ties with the study sponsor EndoGastric Solutions of Redmond, Wash., as well as individual potential conflicts of interest.
Transoral esophagogastric fundoplication can be an effective treatment for patients seeking to alleviate symptoms associated with gastroesophageal reflux disease, particularly in individuals with persistent regurgitation despite prior treatment with proton pump inhibitor therapy, according to the results of a new study published in the February issue of Gastroenterology (doi:10.1053/j.gastro.2014.10.009).
“Gastroesophageal reflux disease (GERD) remains one of the most common conditions for which Americans take daily medication, and PPI use has more than doubled in the last decade,” wrote lead authors Dr. John G. Hunter of Oregon Health & Science University in Portland, and Dr. Peter J. Kahrilas of Northwestern University in Chicago, and their associates. “Despite this, up to 40% of proton pump inhibitor (PPI)–dependent GERD patients have troublesome symptoms of GERD, despite PPI therapy.”
In the Randomized EsophyX vs Sham, Placebo-Controlled Transoral Fundoplication (RESPECT) trial, investigators screened 696 patients who were experiencing “troublesome regurgitation” despite daily PPI treatment. These subjects were evaluated via three validated GERD-specific symptom scales, and were either on or off PPI use at the time of trial commencement. Post trial, patients were blinded to therapy and were reassessed at intervals of 2, 12, and 26 weeks. All patients underwent 48-hour esophageal pH monitoring and esophagogastroduodenoscopy at 66 months after the trial ended.
Regurgitation severity was based on the Montreal definition, which was used to measure efficacy of treatments given as part of the study. The Montreal definition of reflux is described by the authors as “either mucosal damage or troublesome symptoms attributable to reflux.” Those with “least troublesome” regurgitation while on PPIs “underwent barium swallow, esophagogastroduodenoscopy, 48-hour esophageal pH monitoring (off PPIs), and high-resolution esophageal manometry analyses.”
Eighty-seven subjects with GERD and hiatal hernias of at least 2 centimeters were randomly assigned to groups that underwent transoral fundoplication (TF) followed by placebo treatment after 6 months, while 42 subjects, who made up the control group, underwent a “sham surgery” and began regimens of once- or twice-daily omeprazole medication for 6 months.
Results showed that 67% of patients who received TF treatment experienced elimination of adverse regurgitation vs. 45% of those treated with PPI (P = .023). Control of esophageal pH also improved noticeably in patients who received TF treatment versus those who did not (9.3% vs. 6.3% on average, respectively, P < .001), but not in patients who received the “sham surgery” (8.6% preop vs. 8.9% postop on average). Fewer patients who received TF treatment recorded having “no response” after 3 months compared with those in the control group (11% vs. 36%, respectively, P = .004).
“Transoral fundoplication may fill the ‘therapeutic gap’ that exists between PPI and laparoscopic fundoplication,” wrote the authors. “Considering the virtual absence of dysphagia and bloating after TF, which may be problematic with LINX [LINX Reflux Management System], it would appear that TF is an option for patients with troublesome regurgitation, as well as for patients with troublesome GERD symptoms who wish not to take PPI over a protracted period of time.”
Several coauthors disclosed ties with the study sponsor EndoGastric Solutions of Redmond, Wash., as well as individual potential conflicts of interest.
FROM GASTROENTEROLOGY
Key clinical point: Transoral esophagogastric fundoplication (TF) is an effective treatment for gastroesophageal reflux disease symptoms, particularly in patients with persistent regurgitation despite proton pump inhibitor therapy (PPI).
Major finding: Of patients who received TF, 67% experienced elimination of adverse regurgitation, compared with 45% of those treated with PPI (P = .023).
Data source: Randomized EsophyX vs Sham, Placebo-Controlled Transoral Fundoplication (RESPECT) trial.
Disclosures: Several coauthors disclosed ties with the study sponsor EndoGastric Solutions of Redmond, Wash., as well as individual potential conflicts of interest.
Postop pancreatectomy complications most deadly in elderly
Although rates of complications following pancreatectomy are relatively similar in patients both above and below the age of 80 years, significantly higher mortality rates occur in the older age group, according to the findings of a new study published online in the Annals of Surgery
“Previous studies have focused solely on mortality after pancreatectomy in older patients or failure to rescue for all patients undergoing pancreatectomy,” wrote lead author Dr. Nina P. Tamirisa of the University of Texas Medical Branch in Galveston, and her associates. “For older patients, it is not clear whether the observed increase in mortality rate is attributed to higher rates of postsurgical complications, higher failure to rescue from these postsurgical complications, or both” (Ann. Surg. 2015 [doi:10.1097/SLA.0000000000001093]).
In this study, “failure to rescue” was calculated as the number of patients who died from complications divided by total number of patients with complications and was understood to measure of a hospital’s ability to recognize and manage postoperative complications.
In a retrospective cohort study, Dr. Tamirisa and her coinvestigators looked at data on 2,805 patients in the American College of Surgeons’ National Surgical Quality Improvement Pancreatectomy Demonstration Project (ACS NSQIP) at 43 high-volume centers around the United States between November 2011 and December 2012. Institutions with fewer than 10 cases were excluded, leaving 2,694 subjects from 37 centers for the study.
Following this, patients were divided into cohorts of those younger than 80 years of age, and those aged 80 years or older, which contained 2,496 and 198 subjects, respectively. Overall and individual cohorts were split roughly evenly between males and females. Postoperative morbidity and in-hospital mortality rates were measured along with rate of “failure to rescue.”
Results indicated that there were no significant differences in the rates of complications between the cohorts: 41.4% in patients under 80 years and 39.4% in patients aged 80 years or older (P = .58). However, in-hospital mortality rates were significant higher in the 80 and older cohort (3.0%) than in the younger group (1.1%) (P = .01).
Overall, unadjusted complication rates in the 37 centers included in the study varied widely from 25.0% to as high as 72.2%, while failure to rescue rates at ranged from 0.0% to 25.0%.
Major complications were seen in 29.3% of patients aged 80 years or older and in 28.5% of patients under 80 years old (P = .79), with perioperative bleeding being the most prevalent. Among patients with postoperative complications, ascites, chronic obstructive pulmonary disease, and diabetes were the comorbidities most highly associated with failure to rescue, along with acute renal failure, septic shock, and postoperative pulmonary complications.
“It is always true that avoiding complications will decrease mortality for all patients undergoing pancreatectomy,” concluded the investigators, adding that it’s crucial for there to be more interventions to facilitate identification and aggressive treatment of complications to decrease mortality in vulnerable older patients.
“Several factors, such as individual surgeon volume, impact patient outcomes and failure to rescue rates are a significant but not the sole contributor to increased mortality rates in older patients undergoing pancreatectomy,” wrote Dr. Tamirisa and her coauthors.
This study was funded by grants from the Cancer Prevention Research Institute of Texas, UTMB Clinical and Translational Science Award, the National Institutes of Health, and the Agency for Healthcare Research & Quality. Coauthor Dr. Bruce L. Hall disclosed being a paid consulting director of the American College of Surgeons’ National Surgical Quality Improvement Program.
Although rates of complications following pancreatectomy are relatively similar in patients both above and below the age of 80 years, significantly higher mortality rates occur in the older age group, according to the findings of a new study published online in the Annals of Surgery
“Previous studies have focused solely on mortality after pancreatectomy in older patients or failure to rescue for all patients undergoing pancreatectomy,” wrote lead author Dr. Nina P. Tamirisa of the University of Texas Medical Branch in Galveston, and her associates. “For older patients, it is not clear whether the observed increase in mortality rate is attributed to higher rates of postsurgical complications, higher failure to rescue from these postsurgical complications, or both” (Ann. Surg. 2015 [doi:10.1097/SLA.0000000000001093]).
In this study, “failure to rescue” was calculated as the number of patients who died from complications divided by total number of patients with complications and was understood to measure of a hospital’s ability to recognize and manage postoperative complications.
In a retrospective cohort study, Dr. Tamirisa and her coinvestigators looked at data on 2,805 patients in the American College of Surgeons’ National Surgical Quality Improvement Pancreatectomy Demonstration Project (ACS NSQIP) at 43 high-volume centers around the United States between November 2011 and December 2012. Institutions with fewer than 10 cases were excluded, leaving 2,694 subjects from 37 centers for the study.
Following this, patients were divided into cohorts of those younger than 80 years of age, and those aged 80 years or older, which contained 2,496 and 198 subjects, respectively. Overall and individual cohorts were split roughly evenly between males and females. Postoperative morbidity and in-hospital mortality rates were measured along with rate of “failure to rescue.”
Results indicated that there were no significant differences in the rates of complications between the cohorts: 41.4% in patients under 80 years and 39.4% in patients aged 80 years or older (P = .58). However, in-hospital mortality rates were significant higher in the 80 and older cohort (3.0%) than in the younger group (1.1%) (P = .01).
Overall, unadjusted complication rates in the 37 centers included in the study varied widely from 25.0% to as high as 72.2%, while failure to rescue rates at ranged from 0.0% to 25.0%.
Major complications were seen in 29.3% of patients aged 80 years or older and in 28.5% of patients under 80 years old (P = .79), with perioperative bleeding being the most prevalent. Among patients with postoperative complications, ascites, chronic obstructive pulmonary disease, and diabetes were the comorbidities most highly associated with failure to rescue, along with acute renal failure, septic shock, and postoperative pulmonary complications.
“It is always true that avoiding complications will decrease mortality for all patients undergoing pancreatectomy,” concluded the investigators, adding that it’s crucial for there to be more interventions to facilitate identification and aggressive treatment of complications to decrease mortality in vulnerable older patients.
“Several factors, such as individual surgeon volume, impact patient outcomes and failure to rescue rates are a significant but not the sole contributor to increased mortality rates in older patients undergoing pancreatectomy,” wrote Dr. Tamirisa and her coauthors.
This study was funded by grants from the Cancer Prevention Research Institute of Texas, UTMB Clinical and Translational Science Award, the National Institutes of Health, and the Agency for Healthcare Research & Quality. Coauthor Dr. Bruce L. Hall disclosed being a paid consulting director of the American College of Surgeons’ National Surgical Quality Improvement Program.
Although rates of complications following pancreatectomy are relatively similar in patients both above and below the age of 80 years, significantly higher mortality rates occur in the older age group, according to the findings of a new study published online in the Annals of Surgery
“Previous studies have focused solely on mortality after pancreatectomy in older patients or failure to rescue for all patients undergoing pancreatectomy,” wrote lead author Dr. Nina P. Tamirisa of the University of Texas Medical Branch in Galveston, and her associates. “For older patients, it is not clear whether the observed increase in mortality rate is attributed to higher rates of postsurgical complications, higher failure to rescue from these postsurgical complications, or both” (Ann. Surg. 2015 [doi:10.1097/SLA.0000000000001093]).
In this study, “failure to rescue” was calculated as the number of patients who died from complications divided by total number of patients with complications and was understood to measure of a hospital’s ability to recognize and manage postoperative complications.
In a retrospective cohort study, Dr. Tamirisa and her coinvestigators looked at data on 2,805 patients in the American College of Surgeons’ National Surgical Quality Improvement Pancreatectomy Demonstration Project (ACS NSQIP) at 43 high-volume centers around the United States between November 2011 and December 2012. Institutions with fewer than 10 cases were excluded, leaving 2,694 subjects from 37 centers for the study.
Following this, patients were divided into cohorts of those younger than 80 years of age, and those aged 80 years or older, which contained 2,496 and 198 subjects, respectively. Overall and individual cohorts were split roughly evenly between males and females. Postoperative morbidity and in-hospital mortality rates were measured along with rate of “failure to rescue.”
Results indicated that there were no significant differences in the rates of complications between the cohorts: 41.4% in patients under 80 years and 39.4% in patients aged 80 years or older (P = .58). However, in-hospital mortality rates were significant higher in the 80 and older cohort (3.0%) than in the younger group (1.1%) (P = .01).
Overall, unadjusted complication rates in the 37 centers included in the study varied widely from 25.0% to as high as 72.2%, while failure to rescue rates at ranged from 0.0% to 25.0%.
Major complications were seen in 29.3% of patients aged 80 years or older and in 28.5% of patients under 80 years old (P = .79), with perioperative bleeding being the most prevalent. Among patients with postoperative complications, ascites, chronic obstructive pulmonary disease, and diabetes were the comorbidities most highly associated with failure to rescue, along with acute renal failure, septic shock, and postoperative pulmonary complications.
“It is always true that avoiding complications will decrease mortality for all patients undergoing pancreatectomy,” concluded the investigators, adding that it’s crucial for there to be more interventions to facilitate identification and aggressive treatment of complications to decrease mortality in vulnerable older patients.
“Several factors, such as individual surgeon volume, impact patient outcomes and failure to rescue rates are a significant but not the sole contributor to increased mortality rates in older patients undergoing pancreatectomy,” wrote Dr. Tamirisa and her coauthors.
This study was funded by grants from the Cancer Prevention Research Institute of Texas, UTMB Clinical and Translational Science Award, the National Institutes of Health, and the Agency for Healthcare Research & Quality. Coauthor Dr. Bruce L. Hall disclosed being a paid consulting director of the American College of Surgeons’ National Surgical Quality Improvement Program.
FROM THE ANNALS OF SURGERY
Key clinical point: Significantly higher rates of mortality are experienced in pancreatectomy patients aged 80 years or older, requiring urgent attention toward minimizing postoperative complications.
Major finding: In-hospital mortality rates were significant higher in the cohort aged 80 years and older (3.0%) than in the younger group (1.1%) (P = .01) even though rates of complications between the two groups were relatively similar.
Data source: Retrospective cohort study of 2,694 patients in the American College of Surgeons’ National Surgical Quality Improvement Pancreatectomy Demonstration Project at 37 high-volume U.S. centers.
Disclosures: Study was funded by grants from the Cancer Prevention Research Institute of Texas, UTMB Clinical and Translational Science Award, the National Institutes of Health, and the Agency for Healthcare Research & Quality. Coauthor Dr. Bruce L. Hall disclosed being a paid consulting director of the American College of Surgeons’ National Surgical Quality Improvement Program.
HCV continuum critical to providing better care in urban areas
By mapping the testing and care of patients with the hepatitis C virus on a continuum, health care providers can get a better picture of the stages at which the disease is being managed effectively and where along the spectrum there may be room for greater improvement, according to a study published in Hepatology.
“This continuum provides a ‘real-life’ snapshot of how this disease is being managed in a major U.S. urban center,” according to the investigators, led by Kendra Viner, Ph.D., of the Philadelphia Department of Public Health (PDPH). “Many patients are lost at each stage, highlighting the need to raise awareness among health care professionals and at-risk populations about appropriate hepatitis testing, referral, support, and care.”
In a retrospective, population-based study, Dr. Viner and her coinvestigators – all of whom are also with PDPH – examined reports of hepatitis filed in the city between January 2010 and December 2013. During this time, 70% of hepatitis reports were from electronic laboratory reporting (ELR), 25% were from fax or phone reports, and the remaining 5% came from “active case findings.” Investigators also used enhanced surveillance data to find hepatitis cases reported during the study period (Hepatology 2014 ([doi:10.1002/hep.27584]).
Using population estimates from the 2010 United States Census, the American Community Survey (ACS), and the National Health and Nutrition Examination Survey (NHANES), the authors were also able to estimate hepatitis C (HCV) seroprevalence in certain demographics. The HCV care continuum (HCV-CoC) was defined as follows: stage 1: HCV Ab screening; stage 2: HCV Ab and RNA testing; stage 3: RNA-confirmation and continuing care; stage 4: RNA-confirmation, care, and HCV treatment.
Based on their estimates, Dr. Viner and her associates postulated that of approximately 1,584,848 Philadelphia residents, 47,207 (2.9%) would have HCV, with test results anticipated for 28,990 (61%) of those individuals. Positive HCV results were received for 13,596 individuals, of whom 6,383 (47%) had a positive HCV RNA test. Of these, 1,745 (27%) were in care, and 956 (15%) had received or were currently receiving treatment.
“These findings elucidate how few HCV-infected residents are successfully mobilized from screening through confirmatory testing and into care and treatment,” wrote Dr. Viner and her coauthors. “Understanding and addressing the specific reasons why patients are lost at each stage is critical if public health and clinical care practitioners hope to affect the outcomes of chronic HCV infection.”
In each category of testing and treatment, men presented nearly twice as often as women: 61% vs. 39%, Ab only; 64% vs. 36%, Ab plus RNA; 64% vs. 36%, Ab plus RNA with no antiviral treatment; and 71% vs. 29%, Ab plus RNA with antiviral treatment, respectively (P < .001). Patients aged 45-64 years presented the most often for both genders (P < .001), and blacks and whites were most common in the study population (P < .001).
The investigators noted that their findings were consistent with national estimates, which say that fewer than half of a city’s population would have HCV infections, and that 3%-5% of cases would be underreported, thus possibly explaining some missing cases in their own data.
“To promote movement through the continuum of HCV care, state and local health departments need to devise ways to improve surveillance and enhance screening and linkage and retention in HCV care services,” concluded the authors. “However, none of this can happen effectively without strong federal support and greater efforts to promote an implementation science agenda that pulls on surveillance data to execute the CDC’s recommendation for routine baby boomer and risk-based screening, and enhance the HCV care continuum,” they added.
The authors reported no financial conflicts of interest.
AGA Resources
AGA provides a HCV Clinical Service Line at www.gastro.org/practice/clinical-service-line/hcv-clinical-service-line.
By mapping the testing and care of patients with the hepatitis C virus on a continuum, health care providers can get a better picture of the stages at which the disease is being managed effectively and where along the spectrum there may be room for greater improvement, according to a study published in Hepatology.
“This continuum provides a ‘real-life’ snapshot of how this disease is being managed in a major U.S. urban center,” according to the investigators, led by Kendra Viner, Ph.D., of the Philadelphia Department of Public Health (PDPH). “Many patients are lost at each stage, highlighting the need to raise awareness among health care professionals and at-risk populations about appropriate hepatitis testing, referral, support, and care.”
In a retrospective, population-based study, Dr. Viner and her coinvestigators – all of whom are also with PDPH – examined reports of hepatitis filed in the city between January 2010 and December 2013. During this time, 70% of hepatitis reports were from electronic laboratory reporting (ELR), 25% were from fax or phone reports, and the remaining 5% came from “active case findings.” Investigators also used enhanced surveillance data to find hepatitis cases reported during the study period (Hepatology 2014 ([doi:10.1002/hep.27584]).
Using population estimates from the 2010 United States Census, the American Community Survey (ACS), and the National Health and Nutrition Examination Survey (NHANES), the authors were also able to estimate hepatitis C (HCV) seroprevalence in certain demographics. The HCV care continuum (HCV-CoC) was defined as follows: stage 1: HCV Ab screening; stage 2: HCV Ab and RNA testing; stage 3: RNA-confirmation and continuing care; stage 4: RNA-confirmation, care, and HCV treatment.
Based on their estimates, Dr. Viner and her associates postulated that of approximately 1,584,848 Philadelphia residents, 47,207 (2.9%) would have HCV, with test results anticipated for 28,990 (61%) of those individuals. Positive HCV results were received for 13,596 individuals, of whom 6,383 (47%) had a positive HCV RNA test. Of these, 1,745 (27%) were in care, and 956 (15%) had received or were currently receiving treatment.
“These findings elucidate how few HCV-infected residents are successfully mobilized from screening through confirmatory testing and into care and treatment,” wrote Dr. Viner and her coauthors. “Understanding and addressing the specific reasons why patients are lost at each stage is critical if public health and clinical care practitioners hope to affect the outcomes of chronic HCV infection.”
In each category of testing and treatment, men presented nearly twice as often as women: 61% vs. 39%, Ab only; 64% vs. 36%, Ab plus RNA; 64% vs. 36%, Ab plus RNA with no antiviral treatment; and 71% vs. 29%, Ab plus RNA with antiviral treatment, respectively (P < .001). Patients aged 45-64 years presented the most often for both genders (P < .001), and blacks and whites were most common in the study population (P < .001).
The investigators noted that their findings were consistent with national estimates, which say that fewer than half of a city’s population would have HCV infections, and that 3%-5% of cases would be underreported, thus possibly explaining some missing cases in their own data.
“To promote movement through the continuum of HCV care, state and local health departments need to devise ways to improve surveillance and enhance screening and linkage and retention in HCV care services,” concluded the authors. “However, none of this can happen effectively without strong federal support and greater efforts to promote an implementation science agenda that pulls on surveillance data to execute the CDC’s recommendation for routine baby boomer and risk-based screening, and enhance the HCV care continuum,” they added.
The authors reported no financial conflicts of interest.
AGA Resources
AGA provides a HCV Clinical Service Line at www.gastro.org/practice/clinical-service-line/hcv-clinical-service-line.
By mapping the testing and care of patients with the hepatitis C virus on a continuum, health care providers can get a better picture of the stages at which the disease is being managed effectively and where along the spectrum there may be room for greater improvement, according to a study published in Hepatology.
“This continuum provides a ‘real-life’ snapshot of how this disease is being managed in a major U.S. urban center,” according to the investigators, led by Kendra Viner, Ph.D., of the Philadelphia Department of Public Health (PDPH). “Many patients are lost at each stage, highlighting the need to raise awareness among health care professionals and at-risk populations about appropriate hepatitis testing, referral, support, and care.”
In a retrospective, population-based study, Dr. Viner and her coinvestigators – all of whom are also with PDPH – examined reports of hepatitis filed in the city between January 2010 and December 2013. During this time, 70% of hepatitis reports were from electronic laboratory reporting (ELR), 25% were from fax or phone reports, and the remaining 5% came from “active case findings.” Investigators also used enhanced surveillance data to find hepatitis cases reported during the study period (Hepatology 2014 ([doi:10.1002/hep.27584]).
Using population estimates from the 2010 United States Census, the American Community Survey (ACS), and the National Health and Nutrition Examination Survey (NHANES), the authors were also able to estimate hepatitis C (HCV) seroprevalence in certain demographics. The HCV care continuum (HCV-CoC) was defined as follows: stage 1: HCV Ab screening; stage 2: HCV Ab and RNA testing; stage 3: RNA-confirmation and continuing care; stage 4: RNA-confirmation, care, and HCV treatment.
Based on their estimates, Dr. Viner and her associates postulated that of approximately 1,584,848 Philadelphia residents, 47,207 (2.9%) would have HCV, with test results anticipated for 28,990 (61%) of those individuals. Positive HCV results were received for 13,596 individuals, of whom 6,383 (47%) had a positive HCV RNA test. Of these, 1,745 (27%) were in care, and 956 (15%) had received or were currently receiving treatment.
“These findings elucidate how few HCV-infected residents are successfully mobilized from screening through confirmatory testing and into care and treatment,” wrote Dr. Viner and her coauthors. “Understanding and addressing the specific reasons why patients are lost at each stage is critical if public health and clinical care practitioners hope to affect the outcomes of chronic HCV infection.”
In each category of testing and treatment, men presented nearly twice as often as women: 61% vs. 39%, Ab only; 64% vs. 36%, Ab plus RNA; 64% vs. 36%, Ab plus RNA with no antiviral treatment; and 71% vs. 29%, Ab plus RNA with antiviral treatment, respectively (P < .001). Patients aged 45-64 years presented the most often for both genders (P < .001), and blacks and whites were most common in the study population (P < .001).
The investigators noted that their findings were consistent with national estimates, which say that fewer than half of a city’s population would have HCV infections, and that 3%-5% of cases would be underreported, thus possibly explaining some missing cases in their own data.
“To promote movement through the continuum of HCV care, state and local health departments need to devise ways to improve surveillance and enhance screening and linkage and retention in HCV care services,” concluded the authors. “However, none of this can happen effectively without strong federal support and greater efforts to promote an implementation science agenda that pulls on surveillance data to execute the CDC’s recommendation for routine baby boomer and risk-based screening, and enhance the HCV care continuum,” they added.
The authors reported no financial conflicts of interest.
AGA Resources
AGA provides a HCV Clinical Service Line at www.gastro.org/practice/clinical-service-line/hcv-clinical-service-line.
FROM HEPATOLOGY
Key clinical point: Education regarding appropriate hepatitis testing, referral, support, and care is critical to mitigating loss of patients at each stage of the hepatitis C virus treatment continuum.
Major finding: Across an HCV care continuum with approximately 1,584,848 Philadelphia residents, 47,207 (2.9%) were estimated to have HCV. Positive HCV results were received for 13,596 individuals, of whom 6,383 (47%) had a positive HCV RNA test. Of these, 1,745 (27%) were in care, and 956 (15%) had received or were currently receiving treatment.
Data source: Retrospective population-based study.
Disclosures: Authors reported no financial conflicts of interest.
Apremilast looks good for psoriasis, psoriatic arthritis
New studies have led to a number of newly approved medications and therapies for patients suffering from psoriasis and psoriatic arthritis, and several more are on the horizon as clinicians and researchers continue finding new ways to effectively treat these conditions.
“This presentation will cover the new agents recently approved and nearing approval over the next 12 months,” Dr. J. Mark Jackson said in an interview. “It will highlight the general efficacy, safety, and dosing of each agent and will also highlight what is on the way.” Dr. Jackson, of the departments of medicine and dermatology at the University of Louisville (Ky.) discussed his findings at the meeting sponsored by Global Academy for Medical Education.
Among the new agents that Dr. Jackson discussed is apremilast (Otezla by Celgene), which has been approved for treatment of both psoriatic arthritis (PsA) and psoriasis (PsO), as of Sept. 23, 2014. The central mechanism of the medication is an intracellular phosphodieterase-4 (PDE-4) inhibitor, and it is administered orally with a titrated dose over the first 2 weeks of treatment from 10 mg once a day to the maintenance dosage of 30 mg twice a day.
“Apremilast is the newest agent approved for PsO and PsA and demonstrates efficacy for both,” explained Dr. Jackson, who also stated that apremilast should warrant further study to test its “utility in the treatment of atopic and endogenous dermatitis, with studies [currently] ongoing.”
Apremilast was tested against a placebo, with doses of apremilast titrated during the first week of administration, in the ESTEEM 1 trial (Study to Evaluate Safety and Effectiveness of Oral Apremilast in Patients With Moderate to Severe Plaque Psoriasis). After 16 weeks, psoriasis area and severity index (PASI) 75, PASI 50, and static Physicians Global Assessment (sPGA) levels were all significantly higher in subjects using apremilast than those randomized into the placebo cohort. “The new interleukin 17 agents have very robust PASI 75 responses and will be a great addition to the market,” said Dr. Jackson.
Similarly robust results were also seen in apremilast used to treat PsA in the PALACE 1 trial (Study of Apremilast to Treat Active Psoriatic Arthritis). In this randomized, double-blind, placebo-controlled study, ACR 20 (arthritis self management), ACR 50, and ACR 70 scores were significantly higher after 24 weeks in subjects given apremilast 30 mg twice daily and apremilast 20 mg twice daily, than in subjects who were on placebo.
Furthermore, Dr. Jackson explained that the aforementioned interleukin-17 agents are “also being investigated for PsA, and it will be interesting to see how they compare in efficacy to the currently approved agents.”
Treatment options that Dr. Jackson spoke about include certolizumab (Cimzia by UCB/Dermira) and golimumab (Simponi by Janssen), both of which are already approved for PsA, and the former of which is in a phase III trial for PsO. Dr. Jackson also discussed several treatments currently in phase III trials, such as secukinumab by Novartis, brodalumab by Amgen and AstraZeneca, and tofacitinib by Pfizer – all of which are for use against both PsO and PsA, the latter being topically applied for PsO – and tildrakizumab by Merck and Sun Pharma for PsO only.
Global Academy and this news organization are owned by the same parent company.
Dr. Jackson said he has received research, honoraria, consulting and/or other support from the following companies: Abbvie, Amgen, Celgene, Galderma, Genentech, Janssen, Lilly, Medicis, Medimetriks, Novartis, Pfizer, Promius, Topica, and TopMD.
New studies have led to a number of newly approved medications and therapies for patients suffering from psoriasis and psoriatic arthritis, and several more are on the horizon as clinicians and researchers continue finding new ways to effectively treat these conditions.
“This presentation will cover the new agents recently approved and nearing approval over the next 12 months,” Dr. J. Mark Jackson said in an interview. “It will highlight the general efficacy, safety, and dosing of each agent and will also highlight what is on the way.” Dr. Jackson, of the departments of medicine and dermatology at the University of Louisville (Ky.) discussed his findings at the meeting sponsored by Global Academy for Medical Education.
Among the new agents that Dr. Jackson discussed is apremilast (Otezla by Celgene), which has been approved for treatment of both psoriatic arthritis (PsA) and psoriasis (PsO), as of Sept. 23, 2014. The central mechanism of the medication is an intracellular phosphodieterase-4 (PDE-4) inhibitor, and it is administered orally with a titrated dose over the first 2 weeks of treatment from 10 mg once a day to the maintenance dosage of 30 mg twice a day.
“Apremilast is the newest agent approved for PsO and PsA and demonstrates efficacy for both,” explained Dr. Jackson, who also stated that apremilast should warrant further study to test its “utility in the treatment of atopic and endogenous dermatitis, with studies [currently] ongoing.”
Apremilast was tested against a placebo, with doses of apremilast titrated during the first week of administration, in the ESTEEM 1 trial (Study to Evaluate Safety and Effectiveness of Oral Apremilast in Patients With Moderate to Severe Plaque Psoriasis). After 16 weeks, psoriasis area and severity index (PASI) 75, PASI 50, and static Physicians Global Assessment (sPGA) levels were all significantly higher in subjects using apremilast than those randomized into the placebo cohort. “The new interleukin 17 agents have very robust PASI 75 responses and will be a great addition to the market,” said Dr. Jackson.
Similarly robust results were also seen in apremilast used to treat PsA in the PALACE 1 trial (Study of Apremilast to Treat Active Psoriatic Arthritis). In this randomized, double-blind, placebo-controlled study, ACR 20 (arthritis self management), ACR 50, and ACR 70 scores were significantly higher after 24 weeks in subjects given apremilast 30 mg twice daily and apremilast 20 mg twice daily, than in subjects who were on placebo.
Furthermore, Dr. Jackson explained that the aforementioned interleukin-17 agents are “also being investigated for PsA, and it will be interesting to see how they compare in efficacy to the currently approved agents.”
Treatment options that Dr. Jackson spoke about include certolizumab (Cimzia by UCB/Dermira) and golimumab (Simponi by Janssen), both of which are already approved for PsA, and the former of which is in a phase III trial for PsO. Dr. Jackson also discussed several treatments currently in phase III trials, such as secukinumab by Novartis, brodalumab by Amgen and AstraZeneca, and tofacitinib by Pfizer – all of which are for use against both PsO and PsA, the latter being topically applied for PsO – and tildrakizumab by Merck and Sun Pharma for PsO only.
Global Academy and this news organization are owned by the same parent company.
Dr. Jackson said he has received research, honoraria, consulting and/or other support from the following companies: Abbvie, Amgen, Celgene, Galderma, Genentech, Janssen, Lilly, Medicis, Medimetriks, Novartis, Pfizer, Promius, Topica, and TopMD.
New studies have led to a number of newly approved medications and therapies for patients suffering from psoriasis and psoriatic arthritis, and several more are on the horizon as clinicians and researchers continue finding new ways to effectively treat these conditions.
“This presentation will cover the new agents recently approved and nearing approval over the next 12 months,” Dr. J. Mark Jackson said in an interview. “It will highlight the general efficacy, safety, and dosing of each agent and will also highlight what is on the way.” Dr. Jackson, of the departments of medicine and dermatology at the University of Louisville (Ky.) discussed his findings at the meeting sponsored by Global Academy for Medical Education.
Among the new agents that Dr. Jackson discussed is apremilast (Otezla by Celgene), which has been approved for treatment of both psoriatic arthritis (PsA) and psoriasis (PsO), as of Sept. 23, 2014. The central mechanism of the medication is an intracellular phosphodieterase-4 (PDE-4) inhibitor, and it is administered orally with a titrated dose over the first 2 weeks of treatment from 10 mg once a day to the maintenance dosage of 30 mg twice a day.
“Apremilast is the newest agent approved for PsO and PsA and demonstrates efficacy for both,” explained Dr. Jackson, who also stated that apremilast should warrant further study to test its “utility in the treatment of atopic and endogenous dermatitis, with studies [currently] ongoing.”
Apremilast was tested against a placebo, with doses of apremilast titrated during the first week of administration, in the ESTEEM 1 trial (Study to Evaluate Safety and Effectiveness of Oral Apremilast in Patients With Moderate to Severe Plaque Psoriasis). After 16 weeks, psoriasis area and severity index (PASI) 75, PASI 50, and static Physicians Global Assessment (sPGA) levels were all significantly higher in subjects using apremilast than those randomized into the placebo cohort. “The new interleukin 17 agents have very robust PASI 75 responses and will be a great addition to the market,” said Dr. Jackson.
Similarly robust results were also seen in apremilast used to treat PsA in the PALACE 1 trial (Study of Apremilast to Treat Active Psoriatic Arthritis). In this randomized, double-blind, placebo-controlled study, ACR 20 (arthritis self management), ACR 50, and ACR 70 scores were significantly higher after 24 weeks in subjects given apremilast 30 mg twice daily and apremilast 20 mg twice daily, than in subjects who were on placebo.
Furthermore, Dr. Jackson explained that the aforementioned interleukin-17 agents are “also being investigated for PsA, and it will be interesting to see how they compare in efficacy to the currently approved agents.”
Treatment options that Dr. Jackson spoke about include certolizumab (Cimzia by UCB/Dermira) and golimumab (Simponi by Janssen), both of which are already approved for PsA, and the former of which is in a phase III trial for PsO. Dr. Jackson also discussed several treatments currently in phase III trials, such as secukinumab by Novartis, brodalumab by Amgen and AstraZeneca, and tofacitinib by Pfizer – all of which are for use against both PsO and PsA, the latter being topically applied for PsO – and tildrakizumab by Merck and Sun Pharma for PsO only.
Global Academy and this news organization are owned by the same parent company.
Dr. Jackson said he has received research, honoraria, consulting and/or other support from the following companies: Abbvie, Amgen, Celgene, Galderma, Genentech, Janssen, Lilly, Medicis, Medimetriks, Novartis, Pfizer, Promius, Topica, and TopMD.
EXPERT ANALYSIS FROM THE CARIBBEAN DERMATOLOGY SYMPOSIUM
CDC: Opioid Use High Among Reproductive Age Women
Nearly 40% of a reproductive age women enrolled in Medicaid and nearly 30% of those with private insurance filled an opioid prescription between 2008 and 2012, rising concerns that opioid use by women during their peak reproductive years may lead to an increase in birth defects, according to a new report from the Centers for Disease Control and Prevention.
“Many women of reproductive age are taking these medicines and may not know they are pregnant and therefore may be unknowingly exposing their unborn child,” Dr. Tom Frieden, CDC director, said in a statement. “That’s why it’s critical for health care professionals to take a thorough health assessment before prescribing these medicines to women of reproductive age.”
Using claims data, CDC researchers analyzed opioid prescriptions for between 400,000 and 800,000 Medicaid-enrolled women aged 15-44 years, and between 4.4 and 6.6 million privately insured women in the same age range for each year during 2008-2012. The findings were published on Jan. 22 in the Morbidity and Mortality Weekly Report (2015;64:37-41).
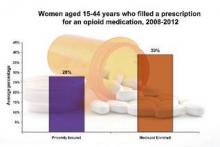
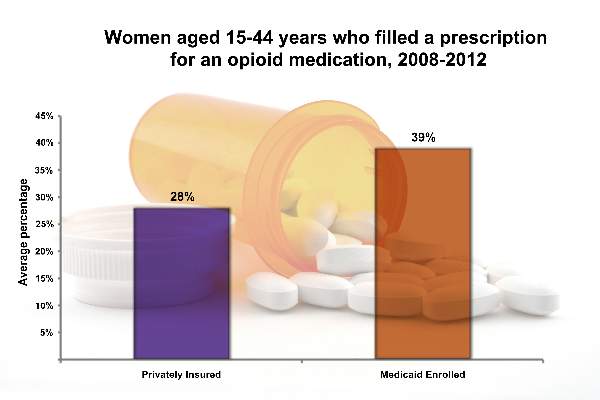
From 2008 to 2012, 39.4% of Medicaid-enrolled women of reproductive age filled an opioid prescription at an outpatient pharmacy, compared with 27.7% among privately-insured women. The year with the highest rate of claims was 2009, with 41.4% of Medicaid-enrolled women and 29.1% of privately insured women filling opioid prescriptions.
The highest subset of opioid prescriptions was in the 30-34 year-old age group for privately insured women (30.9%), and in the 40-44 year-old age group for Medicaid-enrolled women (52.5%).
From 2009 to 2012, there appeared to be a drop in the frequency of opioid use by women of reproductive age, regardless of insurance type, but the researchers urged caution in drawing conclusions about changes over time.
“The apparent decline might indicate improvements in opioid prescribing practices; however, given the potential changes in the composition of the sample used for the privately insured claims data and in the states included in the Medicaid sample each year, this conclusion cannot be drawn from these data,” the researchers wrote.
The most commonly prescribed opioid groups were hydrocodone, codeine, and oxycodone. On average, over the course of the study period, hydrocodone was prescribed 17.5% of the time, followed by codeine (6.9%) and then oxycodone (5.5%) for privately insured women. For women with Medicaid coverage, the rates were higher across the board: 25% for hydrocodone, 9.4% for codeine, and 13.0% for oxycodone.
Nearly 40% of a reproductive age women enrolled in Medicaid and nearly 30% of those with private insurance filled an opioid prescription between 2008 and 2012, rising concerns that opioid use by women during their peak reproductive years may lead to an increase in birth defects, according to a new report from the Centers for Disease Control and Prevention.
“Many women of reproductive age are taking these medicines and may not know they are pregnant and therefore may be unknowingly exposing their unborn child,” Dr. Tom Frieden, CDC director, said in a statement. “That’s why it’s critical for health care professionals to take a thorough health assessment before prescribing these medicines to women of reproductive age.”
Using claims data, CDC researchers analyzed opioid prescriptions for between 400,000 and 800,000 Medicaid-enrolled women aged 15-44 years, and between 4.4 and 6.6 million privately insured women in the same age range for each year during 2008-2012. The findings were published on Jan. 22 in the Morbidity and Mortality Weekly Report (2015;64:37-41).
From 2008 to 2012, 39.4% of Medicaid-enrolled women of reproductive age filled an opioid prescription at an outpatient pharmacy, compared with 27.7% among privately-insured women. The year with the highest rate of claims was 2009, with 41.4% of Medicaid-enrolled women and 29.1% of privately insured women filling opioid prescriptions.
The highest subset of opioid prescriptions was in the 30-34 year-old age group for privately insured women (30.9%), and in the 40-44 year-old age group for Medicaid-enrolled women (52.5%).
From 2009 to 2012, there appeared to be a drop in the frequency of opioid use by women of reproductive age, regardless of insurance type, but the researchers urged caution in drawing conclusions about changes over time.
“The apparent decline might indicate improvements in opioid prescribing practices; however, given the potential changes in the composition of the sample used for the privately insured claims data and in the states included in the Medicaid sample each year, this conclusion cannot be drawn from these data,” the researchers wrote.
The most commonly prescribed opioid groups were hydrocodone, codeine, and oxycodone. On average, over the course of the study period, hydrocodone was prescribed 17.5% of the time, followed by codeine (6.9%) and then oxycodone (5.5%) for privately insured women. For women with Medicaid coverage, the rates were higher across the board: 25% for hydrocodone, 9.4% for codeine, and 13.0% for oxycodone.
Nearly 40% of a reproductive age women enrolled in Medicaid and nearly 30% of those with private insurance filled an opioid prescription between 2008 and 2012, rising concerns that opioid use by women during their peak reproductive years may lead to an increase in birth defects, according to a new report from the Centers for Disease Control and Prevention.
“Many women of reproductive age are taking these medicines and may not know they are pregnant and therefore may be unknowingly exposing their unborn child,” Dr. Tom Frieden, CDC director, said in a statement. “That’s why it’s critical for health care professionals to take a thorough health assessment before prescribing these medicines to women of reproductive age.”
Using claims data, CDC researchers analyzed opioid prescriptions for between 400,000 and 800,000 Medicaid-enrolled women aged 15-44 years, and between 4.4 and 6.6 million privately insured women in the same age range for each year during 2008-2012. The findings were published on Jan. 22 in the Morbidity and Mortality Weekly Report (2015;64:37-41).
From 2008 to 2012, 39.4% of Medicaid-enrolled women of reproductive age filled an opioid prescription at an outpatient pharmacy, compared with 27.7% among privately-insured women. The year with the highest rate of claims was 2009, with 41.4% of Medicaid-enrolled women and 29.1% of privately insured women filling opioid prescriptions.
The highest subset of opioid prescriptions was in the 30-34 year-old age group for privately insured women (30.9%), and in the 40-44 year-old age group for Medicaid-enrolled women (52.5%).
From 2009 to 2012, there appeared to be a drop in the frequency of opioid use by women of reproductive age, regardless of insurance type, but the researchers urged caution in drawing conclusions about changes over time.
“The apparent decline might indicate improvements in opioid prescribing practices; however, given the potential changes in the composition of the sample used for the privately insured claims data and in the states included in the Medicaid sample each year, this conclusion cannot be drawn from these data,” the researchers wrote.
The most commonly prescribed opioid groups were hydrocodone, codeine, and oxycodone. On average, over the course of the study period, hydrocodone was prescribed 17.5% of the time, followed by codeine (6.9%) and then oxycodone (5.5%) for privately insured women. For women with Medicaid coverage, the rates were higher across the board: 25% for hydrocodone, 9.4% for codeine, and 13.0% for oxycodone.
FROM THE MORBIDITY AND MORTALITY WEEKLY REPORT
CDC: Opioid use high among reproductive age women
Nearly 40% of a reproductive age women enrolled in Medicaid and nearly 30% of those with private insurance filled an opioid prescription between 2008 and 2012, rising concerns that opioid use by women during their peak reproductive years may lead to an increase in birth defects, according to a new report from the Centers for Disease Control and Prevention.
“Many women of reproductive age are taking these medicines and may not know they are pregnant and therefore may be unknowingly exposing their unborn child,” Dr. Tom Frieden, CDC director, said in a statement. “That’s why it’s critical for health care professionals to take a thorough health assessment before prescribing these medicines to women of reproductive age.”
Using claims data, CDC researchers analyzed opioid prescriptions for between 400,000 and 800,000 Medicaid-enrolled women aged 15-44 years, and between 4.4 and 6.6 million privately insured women in the same age range for each year during 2008-2012. The findings were published on Jan. 22 in the Morbidity and Mortality Weekly Report (2015;64:37-41).
From 2008 to 2012, 39.4% of Medicaid-enrolled women of reproductive age filled an opioid prescription at an outpatient pharmacy, compared with 27.7% among privately-insured women. The year with the highest rate of claims was 2009, with 41.4% of Medicaid-enrolled women and 29.1% of privately insured women filling opioid prescriptions.
The highest subset of opioid prescriptions was in the 30-34 year-old age group for privately insured women (30.9%), and in the 40-44 year-old age group for Medicaid-enrolled women (52.5%).
From 2009 to 2012, there appeared to be a drop in the frequency of opioid use by women of reproductive age, regardless of insurance type, but the researchers urged caution in drawing conclusions about changes over time.
“The apparent decline might indicate improvements in opioid prescribing practices; however, given the potential changes in the composition of the sample used for the privately insured claims data and in the states included in the Medicaid sample each year, this conclusion cannot be drawn from these data,” the researchers wrote.
The most commonly prescribed opioid groups were hydrocodone, codeine, and oxycodone. On average, over the course of the study period, hydrocodone was prescribed 17.5% of the time, followed by codeine (6.9%) and then oxycodone (5.5%) for privately insured women. For women with Medicaid coverage, the rates were higher across the board: 25% for hydrocodone, 9.4% for codeine, and 13.0% for oxycodone.
Nearly 40% of a reproductive age women enrolled in Medicaid and nearly 30% of those with private insurance filled an opioid prescription between 2008 and 2012, rising concerns that opioid use by women during their peak reproductive years may lead to an increase in birth defects, according to a new report from the Centers for Disease Control and Prevention.
“Many women of reproductive age are taking these medicines and may not know they are pregnant and therefore may be unknowingly exposing their unborn child,” Dr. Tom Frieden, CDC director, said in a statement. “That’s why it’s critical for health care professionals to take a thorough health assessment before prescribing these medicines to women of reproductive age.”
Using claims data, CDC researchers analyzed opioid prescriptions for between 400,000 and 800,000 Medicaid-enrolled women aged 15-44 years, and between 4.4 and 6.6 million privately insured women in the same age range for each year during 2008-2012. The findings were published on Jan. 22 in the Morbidity and Mortality Weekly Report (2015;64:37-41).
From 2008 to 2012, 39.4% of Medicaid-enrolled women of reproductive age filled an opioid prescription at an outpatient pharmacy, compared with 27.7% among privately-insured women. The year with the highest rate of claims was 2009, with 41.4% of Medicaid-enrolled women and 29.1% of privately insured women filling opioid prescriptions.
The highest subset of opioid prescriptions was in the 30-34 year-old age group for privately insured women (30.9%), and in the 40-44 year-old age group for Medicaid-enrolled women (52.5%).
From 2009 to 2012, there appeared to be a drop in the frequency of opioid use by women of reproductive age, regardless of insurance type, but the researchers urged caution in drawing conclusions about changes over time.
“The apparent decline might indicate improvements in opioid prescribing practices; however, given the potential changes in the composition of the sample used for the privately insured claims data and in the states included in the Medicaid sample each year, this conclusion cannot be drawn from these data,” the researchers wrote.
The most commonly prescribed opioid groups were hydrocodone, codeine, and oxycodone. On average, over the course of the study period, hydrocodone was prescribed 17.5% of the time, followed by codeine (6.9%) and then oxycodone (5.5%) for privately insured women. For women with Medicaid coverage, the rates were higher across the board: 25% for hydrocodone, 9.4% for codeine, and 13.0% for oxycodone.
Nearly 40% of a reproductive age women enrolled in Medicaid and nearly 30% of those with private insurance filled an opioid prescription between 2008 and 2012, rising concerns that opioid use by women during their peak reproductive years may lead to an increase in birth defects, according to a new report from the Centers for Disease Control and Prevention.
“Many women of reproductive age are taking these medicines and may not know they are pregnant and therefore may be unknowingly exposing their unborn child,” Dr. Tom Frieden, CDC director, said in a statement. “That’s why it’s critical for health care professionals to take a thorough health assessment before prescribing these medicines to women of reproductive age.”
Using claims data, CDC researchers analyzed opioid prescriptions for between 400,000 and 800,000 Medicaid-enrolled women aged 15-44 years, and between 4.4 and 6.6 million privately insured women in the same age range for each year during 2008-2012. The findings were published on Jan. 22 in the Morbidity and Mortality Weekly Report (2015;64:37-41).
From 2008 to 2012, 39.4% of Medicaid-enrolled women of reproductive age filled an opioid prescription at an outpatient pharmacy, compared with 27.7% among privately-insured women. The year with the highest rate of claims was 2009, with 41.4% of Medicaid-enrolled women and 29.1% of privately insured women filling opioid prescriptions.
The highest subset of opioid prescriptions was in the 30-34 year-old age group for privately insured women (30.9%), and in the 40-44 year-old age group for Medicaid-enrolled women (52.5%).
From 2009 to 2012, there appeared to be a drop in the frequency of opioid use by women of reproductive age, regardless of insurance type, but the researchers urged caution in drawing conclusions about changes over time.
“The apparent decline might indicate improvements in opioid prescribing practices; however, given the potential changes in the composition of the sample used for the privately insured claims data and in the states included in the Medicaid sample each year, this conclusion cannot be drawn from these data,” the researchers wrote.
The most commonly prescribed opioid groups were hydrocodone, codeine, and oxycodone. On average, over the course of the study period, hydrocodone was prescribed 17.5% of the time, followed by codeine (6.9%) and then oxycodone (5.5%) for privately insured women. For women with Medicaid coverage, the rates were higher across the board: 25% for hydrocodone, 9.4% for codeine, and 13.0% for oxycodone.
FROM THE MORBIDITY AND MORTALITY WEEKLY REPORT
Antifungal treatment may cause DNA strain type switching in onychomycosis
Although DNA strain type switches are known to be a natural occurrence in patients with onychomycosis, increases in strain type switching that follow treatment failure could be an antifungal-induced response, according to the results of a study published in the British Journal of Dermatology.
“The dermatophyte Trichophyton rubrum is responsible for the majority (~80%) of [onychomycosis] cases, many of which frequently relapse after successful antifungal treatment,” noted the study authors, led by Dr. Aditya K. Gupta of the University of Toronto. Despite several previous studies of various facets related to onychomycosis, “data outlining onychomycosis infections of T. rubrum with DNA strain type, treatments, outcome and geographical location are still warranted,” they added (Br. J. Dermatol. 2015;172:74-80).
Dr. Gupta and his associates examined 50 adults infected with T. rubrum, determined via analysis of toenail specimens from onychomycosis patients in southwest Ontario. The patients were divided into cohorts based on the treatment they received: oral terbinafine, laser, or placebo (no terbinafine and no laser). Typing of DNA strains was done only in culture-positive samples before and after treatment, leaving a study population of six in the terbinafine group, nine in the laser group, and eight in the placebo group.
Half of the terbinafine subjects were prescribed oral terbinafine 250 mg/day for 12 weeks, while the other three received oral terbinafine 250 mg/day pulse therapy at on/off intervals of 2 weeks up to 12 weeks.
The investigators also used three DNA strains known to be common in Europe for comparison and found that six distinct strains, labeled A-F, accounted for 94% of the T. rubrum strains – these strains corresponded to the European ones. However, three other strains (6% of strains) were found that investigators concluded were native to North America.
Strain type switching occurred in five (83%) of the terbinafine subjects, five (56%) of the laser cohort subjects, and two (25%) of those in the placebo cohort. Roughly half of the type switches noted in the terbinafine cohort were associated with mycological cures and were followed by relapse shortly thereafter. Dr. Gupta and his associates also found that all DNA strains in this cohort were susceptible to terbinafine while in vitro. Strain types in the laser and placebo cohorts did not show any signs of intermittent cures.
The patients were sampled at intervals of 0, 12, 24, 36, 48, 60, and 72 weeks of treatment, and T. rubrum DNA strain types were determined at week 0 (n = 6) and week 48 (n = 1) or 72 (n = 5). Patients in the laser cohort were treated at weeks 0, 8, and 16 and sampled at weeks 0, 8, 16, 24, and 48, with T. rubrum DNA strain types determined at week 0 (n = 9) and week 24 (n = 5) or 48 (n = 4). Finally, placebo patients were sampled at the same regularity as those in the laser cohort, with T. rubrum DNA strain types determined at week 0 (n = 8) and week 24 (n = 1) or 48 (n = 7), they reported.
“The T. rubrum DNA strain type switches observed in ongoing infections among all treatment groups could be attributed to microevolution or coinfections of DNA strains,” the researchers noted. “The presence of coinfecting T. rubrum DNA strains that flux with environmental conditions or local niches could account for the DNA strain type switches observed in all treatment groups, where only the relatively stable types are able to propagate in culture,” they added.
Dr. Gupta and his associates did not disclose any source of funding or any relevant conflicts of interest.
Although DNA strain type switches are known to be a natural occurrence in patients with onychomycosis, increases in strain type switching that follow treatment failure could be an antifungal-induced response, according to the results of a study published in the British Journal of Dermatology.
“The dermatophyte Trichophyton rubrum is responsible for the majority (~80%) of [onychomycosis] cases, many of which frequently relapse after successful antifungal treatment,” noted the study authors, led by Dr. Aditya K. Gupta of the University of Toronto. Despite several previous studies of various facets related to onychomycosis, “data outlining onychomycosis infections of T. rubrum with DNA strain type, treatments, outcome and geographical location are still warranted,” they added (Br. J. Dermatol. 2015;172:74-80).
Dr. Gupta and his associates examined 50 adults infected with T. rubrum, determined via analysis of toenail specimens from onychomycosis patients in southwest Ontario. The patients were divided into cohorts based on the treatment they received: oral terbinafine, laser, or placebo (no terbinafine and no laser). Typing of DNA strains was done only in culture-positive samples before and after treatment, leaving a study population of six in the terbinafine group, nine in the laser group, and eight in the placebo group.
Half of the terbinafine subjects were prescribed oral terbinafine 250 mg/day for 12 weeks, while the other three received oral terbinafine 250 mg/day pulse therapy at on/off intervals of 2 weeks up to 12 weeks.
The investigators also used three DNA strains known to be common in Europe for comparison and found that six distinct strains, labeled A-F, accounted for 94% of the T. rubrum strains – these strains corresponded to the European ones. However, three other strains (6% of strains) were found that investigators concluded were native to North America.
Strain type switching occurred in five (83%) of the terbinafine subjects, five (56%) of the laser cohort subjects, and two (25%) of those in the placebo cohort. Roughly half of the type switches noted in the terbinafine cohort were associated with mycological cures and were followed by relapse shortly thereafter. Dr. Gupta and his associates also found that all DNA strains in this cohort were susceptible to terbinafine while in vitro. Strain types in the laser and placebo cohorts did not show any signs of intermittent cures.
The patients were sampled at intervals of 0, 12, 24, 36, 48, 60, and 72 weeks of treatment, and T. rubrum DNA strain types were determined at week 0 (n = 6) and week 48 (n = 1) or 72 (n = 5). Patients in the laser cohort were treated at weeks 0, 8, and 16 and sampled at weeks 0, 8, 16, 24, and 48, with T. rubrum DNA strain types determined at week 0 (n = 9) and week 24 (n = 5) or 48 (n = 4). Finally, placebo patients were sampled at the same regularity as those in the laser cohort, with T. rubrum DNA strain types determined at week 0 (n = 8) and week 24 (n = 1) or 48 (n = 7), they reported.
“The T. rubrum DNA strain type switches observed in ongoing infections among all treatment groups could be attributed to microevolution or coinfections of DNA strains,” the researchers noted. “The presence of coinfecting T. rubrum DNA strains that flux with environmental conditions or local niches could account for the DNA strain type switches observed in all treatment groups, where only the relatively stable types are able to propagate in culture,” they added.
Dr. Gupta and his associates did not disclose any source of funding or any relevant conflicts of interest.
Although DNA strain type switches are known to be a natural occurrence in patients with onychomycosis, increases in strain type switching that follow treatment failure could be an antifungal-induced response, according to the results of a study published in the British Journal of Dermatology.
“The dermatophyte Trichophyton rubrum is responsible for the majority (~80%) of [onychomycosis] cases, many of which frequently relapse after successful antifungal treatment,” noted the study authors, led by Dr. Aditya K. Gupta of the University of Toronto. Despite several previous studies of various facets related to onychomycosis, “data outlining onychomycosis infections of T. rubrum with DNA strain type, treatments, outcome and geographical location are still warranted,” they added (Br. J. Dermatol. 2015;172:74-80).
Dr. Gupta and his associates examined 50 adults infected with T. rubrum, determined via analysis of toenail specimens from onychomycosis patients in southwest Ontario. The patients were divided into cohorts based on the treatment they received: oral terbinafine, laser, or placebo (no terbinafine and no laser). Typing of DNA strains was done only in culture-positive samples before and after treatment, leaving a study population of six in the terbinafine group, nine in the laser group, and eight in the placebo group.
Half of the terbinafine subjects were prescribed oral terbinafine 250 mg/day for 12 weeks, while the other three received oral terbinafine 250 mg/day pulse therapy at on/off intervals of 2 weeks up to 12 weeks.
The investigators also used three DNA strains known to be common in Europe for comparison and found that six distinct strains, labeled A-F, accounted for 94% of the T. rubrum strains – these strains corresponded to the European ones. However, three other strains (6% of strains) were found that investigators concluded were native to North America.
Strain type switching occurred in five (83%) of the terbinafine subjects, five (56%) of the laser cohort subjects, and two (25%) of those in the placebo cohort. Roughly half of the type switches noted in the terbinafine cohort were associated with mycological cures and were followed by relapse shortly thereafter. Dr. Gupta and his associates also found that all DNA strains in this cohort were susceptible to terbinafine while in vitro. Strain types in the laser and placebo cohorts did not show any signs of intermittent cures.
The patients were sampled at intervals of 0, 12, 24, 36, 48, 60, and 72 weeks of treatment, and T. rubrum DNA strain types were determined at week 0 (n = 6) and week 48 (n = 1) or 72 (n = 5). Patients in the laser cohort were treated at weeks 0, 8, and 16 and sampled at weeks 0, 8, 16, 24, and 48, with T. rubrum DNA strain types determined at week 0 (n = 9) and week 24 (n = 5) or 48 (n = 4). Finally, placebo patients were sampled at the same regularity as those in the laser cohort, with T. rubrum DNA strain types determined at week 0 (n = 8) and week 24 (n = 1) or 48 (n = 7), they reported.
“The T. rubrum DNA strain type switches observed in ongoing infections among all treatment groups could be attributed to microevolution or coinfections of DNA strains,” the researchers noted. “The presence of coinfecting T. rubrum DNA strains that flux with environmental conditions or local niches could account for the DNA strain type switches observed in all treatment groups, where only the relatively stable types are able to propagate in culture,” they added.
Dr. Gupta and his associates did not disclose any source of funding or any relevant conflicts of interest.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Key clinical point: Antifungal treatment of onychomycosis could induce higher rates of DNA strain type switching in certain patients.
Major finding: Strain type switching occurred in 83% of the terbinafine group, 56% of the laser group, and 25% of the placebo group.
Data source: Cohort study of 23 individuals selected from 50 adults with onychomycosis who contributed samples to determine strain types.
Disclosures: The study authors did not disclose any source of funding or any relevant conflicts of interest.
Moderate alcohol consumption can decrease heart failure risk
Although alcohol is known to be a cardiac toxin, consumption of up to seven drinks per week in early to middle age was found to be associated with a decreased risk of future heart failure in a study published by the European Society of Cardiology.
“Although heavy alcohol consumption is associated with impairment in left ventricular function and eventual alcoholic cardiomyopathy with symptomatic HF, moderate alcohol intake could, conversely, lower the risk for HF,” wrote Dr. Alexandra Gonçalves of Brigham and Women’s Hospital, Boston, and her associates. “However, the association between moderate alcohol intake and the risk of heart failure is still controversial, as some studies did not find an association and the cardiovascular mechanisms of potential benefit of alcohol consumption in heart failure are uncertain” (Eur. Heart J. 2014 [doi:10.1093/eurheartj/ehu514]).
From the ongoing, prospective, observational Atherosclerosis Risk in Communities (ARIC) Study, the investigators analyzed 14,629 subjects aged 45-64 years, 55% of whom were female, and 74% of whom were white. Baseline data for all subjects were taken between 1987 and 1989, and no participants reported prevalent heart failure.
Self-reported alcohol consumption was assessed at baseline as the weekly number of drinks, with one drink equaling 14 grams of alcohol, and updated cumulative average alcohol intake was calculated over roughly 9 years. Subjects were placed into one of six groups: former drinkers, abstainers, drinkers of less than 7 drinks per week, drinkers of 7-14 drinks per week, drinkers of 14-21 drinks per week, and those who had 21 or more drinks per week. Using multivariable Cox proportional hazards models, investigators analyzed relationships between alcohol consumption and heart failure and whether those associations were affected in any way by the subjects’ sex.
Results showed that 61% of participants reported consuming no alcohol at all – 19% being former drinkers and 42% abstainers – while 25% of participants said they drank up to 7 drinks per week, 8% between 7 and 14 drinks per week, 3% between 14 and 21 drinks per week, and 3% 21 or more drinks per week. Among former drinkers, there were 376 incident heart failure events in men and 266 in women. Among abstainers, there were 333 for men and 717 for women. Rates per 100 person-years equaled 1.50, 1.12, 1.02, and 0.79, respectively.
For subjects who consumed fewer than seven drinks weekly, there were 281 heart failure incidents among men and 191 among women, for rates per 100 person-years of 0.77 and 0.53, respectively, significantly lower than the rates for abstainers. In the higher drinking categories, no significant differences in heart failure rates, compared with abstainers, were observed.
“We observed that participants who consumed up to 7 drinks/week of alcohol had a lower risk of incident HF compared with abstainers, with a less pronounced association in women than in men,” concluded Dr. Gonçalves and her coinvestigators, adding that they “did not find significant differences between white and black men and women in the risk of HF by alcohol consumption [but] once men and women were stratified by race, the number of cases was relatively small in each category of alcohol intake, limiting our ability to detect small differences by race.”
This study was funded by the National Heart, Lung, and Blood Institute, with support from the Portuguese Foundation for Science and Technology Grant and the Ellison Foundation. The authors declared no relevant financial disclosures.
Although alcohol is known to be a cardiac toxin, consumption of up to seven drinks per week in early to middle age was found to be associated with a decreased risk of future heart failure in a study published by the European Society of Cardiology.
“Although heavy alcohol consumption is associated with impairment in left ventricular function and eventual alcoholic cardiomyopathy with symptomatic HF, moderate alcohol intake could, conversely, lower the risk for HF,” wrote Dr. Alexandra Gonçalves of Brigham and Women’s Hospital, Boston, and her associates. “However, the association between moderate alcohol intake and the risk of heart failure is still controversial, as some studies did not find an association and the cardiovascular mechanisms of potential benefit of alcohol consumption in heart failure are uncertain” (Eur. Heart J. 2014 [doi:10.1093/eurheartj/ehu514]).
From the ongoing, prospective, observational Atherosclerosis Risk in Communities (ARIC) Study, the investigators analyzed 14,629 subjects aged 45-64 years, 55% of whom were female, and 74% of whom were white. Baseline data for all subjects were taken between 1987 and 1989, and no participants reported prevalent heart failure.
Self-reported alcohol consumption was assessed at baseline as the weekly number of drinks, with one drink equaling 14 grams of alcohol, and updated cumulative average alcohol intake was calculated over roughly 9 years. Subjects were placed into one of six groups: former drinkers, abstainers, drinkers of less than 7 drinks per week, drinkers of 7-14 drinks per week, drinkers of 14-21 drinks per week, and those who had 21 or more drinks per week. Using multivariable Cox proportional hazards models, investigators analyzed relationships between alcohol consumption and heart failure and whether those associations were affected in any way by the subjects’ sex.
Results showed that 61% of participants reported consuming no alcohol at all – 19% being former drinkers and 42% abstainers – while 25% of participants said they drank up to 7 drinks per week, 8% between 7 and 14 drinks per week, 3% between 14 and 21 drinks per week, and 3% 21 or more drinks per week. Among former drinkers, there were 376 incident heart failure events in men and 266 in women. Among abstainers, there were 333 for men and 717 for women. Rates per 100 person-years equaled 1.50, 1.12, 1.02, and 0.79, respectively.
For subjects who consumed fewer than seven drinks weekly, there were 281 heart failure incidents among men and 191 among women, for rates per 100 person-years of 0.77 and 0.53, respectively, significantly lower than the rates for abstainers. In the higher drinking categories, no significant differences in heart failure rates, compared with abstainers, were observed.
“We observed that participants who consumed up to 7 drinks/week of alcohol had a lower risk of incident HF compared with abstainers, with a less pronounced association in women than in men,” concluded Dr. Gonçalves and her coinvestigators, adding that they “did not find significant differences between white and black men and women in the risk of HF by alcohol consumption [but] once men and women were stratified by race, the number of cases was relatively small in each category of alcohol intake, limiting our ability to detect small differences by race.”
This study was funded by the National Heart, Lung, and Blood Institute, with support from the Portuguese Foundation for Science and Technology Grant and the Ellison Foundation. The authors declared no relevant financial disclosures.
Although alcohol is known to be a cardiac toxin, consumption of up to seven drinks per week in early to middle age was found to be associated with a decreased risk of future heart failure in a study published by the European Society of Cardiology.
“Although heavy alcohol consumption is associated with impairment in left ventricular function and eventual alcoholic cardiomyopathy with symptomatic HF, moderate alcohol intake could, conversely, lower the risk for HF,” wrote Dr. Alexandra Gonçalves of Brigham and Women’s Hospital, Boston, and her associates. “However, the association between moderate alcohol intake and the risk of heart failure is still controversial, as some studies did not find an association and the cardiovascular mechanisms of potential benefit of alcohol consumption in heart failure are uncertain” (Eur. Heart J. 2014 [doi:10.1093/eurheartj/ehu514]).
From the ongoing, prospective, observational Atherosclerosis Risk in Communities (ARIC) Study, the investigators analyzed 14,629 subjects aged 45-64 years, 55% of whom were female, and 74% of whom were white. Baseline data for all subjects were taken between 1987 and 1989, and no participants reported prevalent heart failure.
Self-reported alcohol consumption was assessed at baseline as the weekly number of drinks, with one drink equaling 14 grams of alcohol, and updated cumulative average alcohol intake was calculated over roughly 9 years. Subjects were placed into one of six groups: former drinkers, abstainers, drinkers of less than 7 drinks per week, drinkers of 7-14 drinks per week, drinkers of 14-21 drinks per week, and those who had 21 or more drinks per week. Using multivariable Cox proportional hazards models, investigators analyzed relationships between alcohol consumption and heart failure and whether those associations were affected in any way by the subjects’ sex.
Results showed that 61% of participants reported consuming no alcohol at all – 19% being former drinkers and 42% abstainers – while 25% of participants said they drank up to 7 drinks per week, 8% between 7 and 14 drinks per week, 3% between 14 and 21 drinks per week, and 3% 21 or more drinks per week. Among former drinkers, there were 376 incident heart failure events in men and 266 in women. Among abstainers, there were 333 for men and 717 for women. Rates per 100 person-years equaled 1.50, 1.12, 1.02, and 0.79, respectively.
For subjects who consumed fewer than seven drinks weekly, there were 281 heart failure incidents among men and 191 among women, for rates per 100 person-years of 0.77 and 0.53, respectively, significantly lower than the rates for abstainers. In the higher drinking categories, no significant differences in heart failure rates, compared with abstainers, were observed.
“We observed that participants who consumed up to 7 drinks/week of alcohol had a lower risk of incident HF compared with abstainers, with a less pronounced association in women than in men,” concluded Dr. Gonçalves and her coinvestigators, adding that they “did not find significant differences between white and black men and women in the risk of HF by alcohol consumption [but] once men and women were stratified by race, the number of cases was relatively small in each category of alcohol intake, limiting our ability to detect small differences by race.”
This study was funded by the National Heart, Lung, and Blood Institute, with support from the Portuguese Foundation for Science and Technology Grant and the Ellison Foundation. The authors declared no relevant financial disclosures.
FROM THE EUROPEAN HEART JOURNAL
Key clinical point: Consumption of up to seven alcoholic beverages per week in early-middle age can lower risk for future heart failure.
Major finding: Among those who consumed up to seven drinks weekly, there were 281 heart failure incidents for men and 191 for women, with rates per 100 person-years of 0.77 and 0.53, respectively (P = .05).
Data source: The biracial, community-based ARIC study, comprising 14,629 adults.
Disclosures: ARIC is funded by the National Heart, Lung, and Blood Institute, with support from the Portuguese Foundation for Science and Technology Grant and the Ellison Foundation. The authors declared no relevant financial disclosures.
IPAH patients’ nonresponse to vasodilator challenge linked to poor recruitment of capillary surface
Patients with idiopathic pulmonary arterial hypertension did not respond to treatment with an acute vasodilator challenge because they were unable to recruit functional capillary surface area to accommodate increased blood flow to the lungs, according to the results of a study published Jan. 19 in Annals of Internal Medicine.
Dr. David Langleben of McGill University, Montreal, and Dr. Stylianos E. Orfanos of Attikon Hospital, Athens, hypothesized that the increased pulmonary vascular resistance (PVR) noted in responders would be based on vascular tone rather than cellular obstruction, and therefore responders should be capable of recruiting functional capillary surface area (CSA) during a vasodilator challenge.
“Despite sharing the baseline hemodynamic profile of IPAH, responders and nonresponders [may] represent different vascular phenotypes and perhaps different diseases,” they wrote.
The investigators recruited 14 patients (12 female, 2 male) with IPAH, none of whom had previously received IPAH-specific therapy or had saline contrast evidence of intracardiac shunting on echocardiography. Each subject underwent a standardized right heart catheterization followed by insertion of a femoral artery sheath. After a 30-minute rest period, investigators measured baseline hemodynamics and pulmonary capillary, endothelium-bound, angiotensin-converting enzyme (PCEB-ACE) metabolic activity.
Subsequently, the researchers performed an acute vasodilator challenge on each patient using epoprostenol (Flolan, GlaxoSmithKline Canada), after which PCEB-ACE metabolic activity and hemodynamics were measured again at the peak epoprostenol dose – determined “individually by maximum hemodynamic response or adverse effects.”
Functional CSA was calculated and normalized to body surface area. Values among nonresponders before the vasodilator challenge and at the peak epoprostenol dose were compared using paired t-tests. Of the 14 subjects, 12 were found to be nonresponders to vasodilator challenge and 2 (1 female, 1 male) did respond to vasodilator challenge treatment. In nonresponders overall, mean pulmonary arterial pressure (mPAP) did not change significantly – 59.5 mm Hg to 58.1 mm Hg – but cardiac output increased and PVR decreased. In the two subjects who responded, cardiac output and PVR increased, but mPAP decreased 43.5 mm Hg to 31.5 mm Hg.
“These data show the inability of nonresponders with IPAH to open occluded arterioles and thus recruit more downstream capillaries to accommodate the observed increased lung blood volumes that should accompany vasodilator administration,” the researchers concluded.
Funding for this study was provided by the William and Ida Pencer Family Foundation, the Dimitrios Banousis Foundation, the Bank of Montreal Center for the Study of Heart Disease in Women, and the Jewish General Hospital Annual Walk for Pulmonary Hypertension, all at Jewish General Hospital. The authors disclosed no conflicts of interest.
Patients with idiopathic pulmonary arterial hypertension did not respond to treatment with an acute vasodilator challenge because they were unable to recruit functional capillary surface area to accommodate increased blood flow to the lungs, according to the results of a study published Jan. 19 in Annals of Internal Medicine.
Dr. David Langleben of McGill University, Montreal, and Dr. Stylianos E. Orfanos of Attikon Hospital, Athens, hypothesized that the increased pulmonary vascular resistance (PVR) noted in responders would be based on vascular tone rather than cellular obstruction, and therefore responders should be capable of recruiting functional capillary surface area (CSA) during a vasodilator challenge.
“Despite sharing the baseline hemodynamic profile of IPAH, responders and nonresponders [may] represent different vascular phenotypes and perhaps different diseases,” they wrote.
The investigators recruited 14 patients (12 female, 2 male) with IPAH, none of whom had previously received IPAH-specific therapy or had saline contrast evidence of intracardiac shunting on echocardiography. Each subject underwent a standardized right heart catheterization followed by insertion of a femoral artery sheath. After a 30-minute rest period, investigators measured baseline hemodynamics and pulmonary capillary, endothelium-bound, angiotensin-converting enzyme (PCEB-ACE) metabolic activity.
Subsequently, the researchers performed an acute vasodilator challenge on each patient using epoprostenol (Flolan, GlaxoSmithKline Canada), after which PCEB-ACE metabolic activity and hemodynamics were measured again at the peak epoprostenol dose – determined “individually by maximum hemodynamic response or adverse effects.”
Functional CSA was calculated and normalized to body surface area. Values among nonresponders before the vasodilator challenge and at the peak epoprostenol dose were compared using paired t-tests. Of the 14 subjects, 12 were found to be nonresponders to vasodilator challenge and 2 (1 female, 1 male) did respond to vasodilator challenge treatment. In nonresponders overall, mean pulmonary arterial pressure (mPAP) did not change significantly – 59.5 mm Hg to 58.1 mm Hg – but cardiac output increased and PVR decreased. In the two subjects who responded, cardiac output and PVR increased, but mPAP decreased 43.5 mm Hg to 31.5 mm Hg.
“These data show the inability of nonresponders with IPAH to open occluded arterioles and thus recruit more downstream capillaries to accommodate the observed increased lung blood volumes that should accompany vasodilator administration,” the researchers concluded.
Funding for this study was provided by the William and Ida Pencer Family Foundation, the Dimitrios Banousis Foundation, the Bank of Montreal Center for the Study of Heart Disease in Women, and the Jewish General Hospital Annual Walk for Pulmonary Hypertension, all at Jewish General Hospital. The authors disclosed no conflicts of interest.
Patients with idiopathic pulmonary arterial hypertension did not respond to treatment with an acute vasodilator challenge because they were unable to recruit functional capillary surface area to accommodate increased blood flow to the lungs, according to the results of a study published Jan. 19 in Annals of Internal Medicine.
Dr. David Langleben of McGill University, Montreal, and Dr. Stylianos E. Orfanos of Attikon Hospital, Athens, hypothesized that the increased pulmonary vascular resistance (PVR) noted in responders would be based on vascular tone rather than cellular obstruction, and therefore responders should be capable of recruiting functional capillary surface area (CSA) during a vasodilator challenge.
“Despite sharing the baseline hemodynamic profile of IPAH, responders and nonresponders [may] represent different vascular phenotypes and perhaps different diseases,” they wrote.
The investigators recruited 14 patients (12 female, 2 male) with IPAH, none of whom had previously received IPAH-specific therapy or had saline contrast evidence of intracardiac shunting on echocardiography. Each subject underwent a standardized right heart catheterization followed by insertion of a femoral artery sheath. After a 30-minute rest period, investigators measured baseline hemodynamics and pulmonary capillary, endothelium-bound, angiotensin-converting enzyme (PCEB-ACE) metabolic activity.
Subsequently, the researchers performed an acute vasodilator challenge on each patient using epoprostenol (Flolan, GlaxoSmithKline Canada), after which PCEB-ACE metabolic activity and hemodynamics were measured again at the peak epoprostenol dose – determined “individually by maximum hemodynamic response or adverse effects.”
Functional CSA was calculated and normalized to body surface area. Values among nonresponders before the vasodilator challenge and at the peak epoprostenol dose were compared using paired t-tests. Of the 14 subjects, 12 were found to be nonresponders to vasodilator challenge and 2 (1 female, 1 male) did respond to vasodilator challenge treatment. In nonresponders overall, mean pulmonary arterial pressure (mPAP) did not change significantly – 59.5 mm Hg to 58.1 mm Hg – but cardiac output increased and PVR decreased. In the two subjects who responded, cardiac output and PVR increased, but mPAP decreased 43.5 mm Hg to 31.5 mm Hg.
“These data show the inability of nonresponders with IPAH to open occluded arterioles and thus recruit more downstream capillaries to accommodate the observed increased lung blood volumes that should accompany vasodilator administration,” the researchers concluded.
Funding for this study was provided by the William and Ida Pencer Family Foundation, the Dimitrios Banousis Foundation, the Bank of Montreal Center for the Study of Heart Disease in Women, and the Jewish General Hospital Annual Walk for Pulmonary Hypertension, all at Jewish General Hospital. The authors disclosed no conflicts of interest.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point: Differential responses by patients with idiopathic pulmonary arterial hypertension (IPAH) to acute vasodilator challenge suggests that responders and nonresponders represent different vascular phenotypes and different diseases.
Major finding: In nonresponders to vasodilator challenge, mPAP increased and PVR decreased, while those who did respond saw both levels decrease; both cohorts experienced an increase in cardiac output.
Data source: Cohort study of 14 individuals with IPAH.
Disclosures: Funding provided by the William and Ida Pencer Family Foundation, the Dimitrios Banousis Foundation, the Bank of Montreal Center for the Study of Heart Disease in Women, and the Jewish General Hospital Annual Walk for Pulmonary Hypertension, all at Jewish General Hospital. The authors disclosed no conflicts of interest.
Incidences of underimmunization, vaccine refusal are clustered geographically
Identification of geographic clusters in which children have higher rates of underimmunization, or whose parents outright refuse vaccination, can help identify similar pockets of vaccine underutilization and help health care providers better focus their approach to providing immunization interventions around the country, according to the findings of a new study published in Pediatrics.
“For optimal public health and patient care, clinicians and health care systems ideally should tailor care to their communities,” wrote Dr. Tracy A. Lieu of Kaiser Permanente Northern California, Oakland, and her associates.
“The ability to identify clusters of underimmunization and vaccine refusal would help providers focus their efforts on the parents in communities with the greatest concerns, as well as identify areas at elevated risk for outbreaks of vaccine-preventable diseases” (Pediatrics 2015 [doi:10.1542/peds.2014-2715]).
In a retrospective cohort study, Dr. Lieu and her coinvestigators looked at children with continuous membership in the Kaiser Permanente Northern California, aged from birth to age 36 months, between Jan. 1, 2000, and December 31, 2009. A total of 154,424 children in 13 northern California counties were selected for inclusion in the study. Children were classified as underimmunized if they missed at least one vaccine by age 36 months, while vaccine refusal was classified using ICD-9-CM codes V6405, V6406, or V6407, as per the International Classification of Diseases, Ninth Revision, Clinical Modification for subjects who refused even one vaccination at any time prior to age 36 months.
Spatial scan analysis of all the accumulated data revealed five statistically significant geographic clusters in which underimmunization was more pronounced, nearly all located in the greater Sacramento and San Francisco metropolitan areas, with underimmunization rates over the course of the study period ranging from 18.1% in northern San Francisco and southern Marin counties to 22.7% in Vallejo (P = .023 and P = .042, respectively).
Across all 13 counties surveyed, rates of underimmunization increased from 8.1% in 2001-2005 to 12.4% in 2010-2012; the peak rate in a single year was 13%, achieved in 2010, while the mean annual increase in underimmunization across all 13 counties was 5.9%. In terms of race/ethnicity, higher percentages of Asian and Hispanic subjects were fully immunized compared with those who were white, black, or of unknown race/ethnicity.
Children in the most statistically significant cluster – the East Bay area, from Richmond to San Leandro – had 1.58 (P < .001) times the rate of underimmunization as displayed by other clusters, with 650 actual underimmunized cases during 2010-2012. Vaccine refusal was also clustered, with rates of 5.5% to 13.5% within clusters, compared with 2.6% outside of them.
Underimmunization of measles, mumps, and rubella and varicella vaccines also clustered in similar geographic areas, a cause for great concern given the recent outbreaks of measles at the Disneyland theme park in Anaheim, Calif. A study published last year, highlights the greater need for health care providers to educate parents about the importance of MMR vaccinations, saying that by doing so, they greatly increase the likelihood of parents agreeing to have their children properly vaccinated (Pediatrics 2014;134:e675-e83). Outbreaks of pertussis also have proved troublesome in recent years, exacerbating the need for greater intervention regarding vaccinating children early and when necessary.
“The existence of clusters may put clinical groups in these locations at a disadvantage in achieving immunization quality benchmarks,” noted Dr. Lieu and her coauthors, adding that “nonmedical exemptions from immunization in California clustered geographically and were associated with clusters of pertussis cases [and] many previous studies have found that vaccine refusal and delay are associated with elevated risk of measles and pertussis outbreaks, as well as elevated individual risks of measles, pertussis, varicella, and pneumococcal infections.”
Funding was provided by a Patient-Centered Outcomes Research Institute Pilot Project contract. Coauthor Dr Klein receives research support from Merck, GlaxoSmithKline, MedImmune, Novartis, Pfizer, Sanofi Pasteur, and Nuron Biotech; no other authors reported any relevant financial disclosures.
This study essentially confirms what we’ve known anecdotally and have had some evidence of before: Underimmunization that results from refusal by concerned parents who do not want their children receiving all the recommended vaccines is not randomly distributed in the population.
These geographic clusters occur not only in California, but in Oregon, Washington, and many other states, but there are predictors for this behavior at the individual and group levels. For example, white, upper-class, higher socioeconomic groups tend to see higher rates; interestingly enough, this paper finds higher underimmunization in areas with more individuals of graduate level or higher education, and also in areas with more poverty. Parents tend to have more and more questions about the safety, efficacy, and need for these vaccinations, and this will often lead to delay or flat-out refusal.
I don’t think any of this is completely new or surprising. The investigators used a new statistical approach for detecting these clusters within the Kaiser database, but there are no real surprises here, and there is certainly no reason to doubt these findings; this is a real phenomenon.
There needs to be more follow-up research in order to bring underimmunization to a minimum; we need to understand more fully what drives this behavior and how we can address and allay people’s concerns, but I wouldn’t be able to speak on that since it’s in the realm of psychology or sociology.
Dr. Arthur L. Reingold is a professor and the head of epidemiology at the University of California, Berkeley, School of Public Health.
This study essentially confirms what we’ve known anecdotally and have had some evidence of before: Underimmunization that results from refusal by concerned parents who do not want their children receiving all the recommended vaccines is not randomly distributed in the population.
These geographic clusters occur not only in California, but in Oregon, Washington, and many other states, but there are predictors for this behavior at the individual and group levels. For example, white, upper-class, higher socioeconomic groups tend to see higher rates; interestingly enough, this paper finds higher underimmunization in areas with more individuals of graduate level or higher education, and also in areas with more poverty. Parents tend to have more and more questions about the safety, efficacy, and need for these vaccinations, and this will often lead to delay or flat-out refusal.
I don’t think any of this is completely new or surprising. The investigators used a new statistical approach for detecting these clusters within the Kaiser database, but there are no real surprises here, and there is certainly no reason to doubt these findings; this is a real phenomenon.
There needs to be more follow-up research in order to bring underimmunization to a minimum; we need to understand more fully what drives this behavior and how we can address and allay people’s concerns, but I wouldn’t be able to speak on that since it’s in the realm of psychology or sociology.
Dr. Arthur L. Reingold is a professor and the head of epidemiology at the University of California, Berkeley, School of Public Health.
This study essentially confirms what we’ve known anecdotally and have had some evidence of before: Underimmunization that results from refusal by concerned parents who do not want their children receiving all the recommended vaccines is not randomly distributed in the population.
These geographic clusters occur not only in California, but in Oregon, Washington, and many other states, but there are predictors for this behavior at the individual and group levels. For example, white, upper-class, higher socioeconomic groups tend to see higher rates; interestingly enough, this paper finds higher underimmunization in areas with more individuals of graduate level or higher education, and also in areas with more poverty. Parents tend to have more and more questions about the safety, efficacy, and need for these vaccinations, and this will often lead to delay or flat-out refusal.
I don’t think any of this is completely new or surprising. The investigators used a new statistical approach for detecting these clusters within the Kaiser database, but there are no real surprises here, and there is certainly no reason to doubt these findings; this is a real phenomenon.
There needs to be more follow-up research in order to bring underimmunization to a minimum; we need to understand more fully what drives this behavior and how we can address and allay people’s concerns, but I wouldn’t be able to speak on that since it’s in the realm of psychology or sociology.
Dr. Arthur L. Reingold is a professor and the head of epidemiology at the University of California, Berkeley, School of Public Health.
Identification of geographic clusters in which children have higher rates of underimmunization, or whose parents outright refuse vaccination, can help identify similar pockets of vaccine underutilization and help health care providers better focus their approach to providing immunization interventions around the country, according to the findings of a new study published in Pediatrics.
“For optimal public health and patient care, clinicians and health care systems ideally should tailor care to their communities,” wrote Dr. Tracy A. Lieu of Kaiser Permanente Northern California, Oakland, and her associates.

“The ability to identify clusters of underimmunization and vaccine refusal would help providers focus their efforts on the parents in communities with the greatest concerns, as well as identify areas at elevated risk for outbreaks of vaccine-preventable diseases” (Pediatrics 2015 [doi:10.1542/peds.2014-2715]).
In a retrospective cohort study, Dr. Lieu and her coinvestigators looked at children with continuous membership in the Kaiser Permanente Northern California, aged from birth to age 36 months, between Jan. 1, 2000, and December 31, 2009. A total of 154,424 children in 13 northern California counties were selected for inclusion in the study. Children were classified as underimmunized if they missed at least one vaccine by age 36 months, while vaccine refusal was classified using ICD-9-CM codes V6405, V6406, or V6407, as per the International Classification of Diseases, Ninth Revision, Clinical Modification for subjects who refused even one vaccination at any time prior to age 36 months.
Spatial scan analysis of all the accumulated data revealed five statistically significant geographic clusters in which underimmunization was more pronounced, nearly all located in the greater Sacramento and San Francisco metropolitan areas, with underimmunization rates over the course of the study period ranging from 18.1% in northern San Francisco and southern Marin counties to 22.7% in Vallejo (P = .023 and P = .042, respectively).
Across all 13 counties surveyed, rates of underimmunization increased from 8.1% in 2001-2005 to 12.4% in 2010-2012; the peak rate in a single year was 13%, achieved in 2010, while the mean annual increase in underimmunization across all 13 counties was 5.9%. In terms of race/ethnicity, higher percentages of Asian and Hispanic subjects were fully immunized compared with those who were white, black, or of unknown race/ethnicity.
Children in the most statistically significant cluster – the East Bay area, from Richmond to San Leandro – had 1.58 (P < .001) times the rate of underimmunization as displayed by other clusters, with 650 actual underimmunized cases during 2010-2012. Vaccine refusal was also clustered, with rates of 5.5% to 13.5% within clusters, compared with 2.6% outside of them.
Underimmunization of measles, mumps, and rubella and varicella vaccines also clustered in similar geographic areas, a cause for great concern given the recent outbreaks of measles at the Disneyland theme park in Anaheim, Calif. A study published last year, highlights the greater need for health care providers to educate parents about the importance of MMR vaccinations, saying that by doing so, they greatly increase the likelihood of parents agreeing to have their children properly vaccinated (Pediatrics 2014;134:e675-e83). Outbreaks of pertussis also have proved troublesome in recent years, exacerbating the need for greater intervention regarding vaccinating children early and when necessary.
“The existence of clusters may put clinical groups in these locations at a disadvantage in achieving immunization quality benchmarks,” noted Dr. Lieu and her coauthors, adding that “nonmedical exemptions from immunization in California clustered geographically and were associated with clusters of pertussis cases [and] many previous studies have found that vaccine refusal and delay are associated with elevated risk of measles and pertussis outbreaks, as well as elevated individual risks of measles, pertussis, varicella, and pneumococcal infections.”
Funding was provided by a Patient-Centered Outcomes Research Institute Pilot Project contract. Coauthor Dr Klein receives research support from Merck, GlaxoSmithKline, MedImmune, Novartis, Pfizer, Sanofi Pasteur, and Nuron Biotech; no other authors reported any relevant financial disclosures.
Identification of geographic clusters in which children have higher rates of underimmunization, or whose parents outright refuse vaccination, can help identify similar pockets of vaccine underutilization and help health care providers better focus their approach to providing immunization interventions around the country, according to the findings of a new study published in Pediatrics.
“For optimal public health and patient care, clinicians and health care systems ideally should tailor care to their communities,” wrote Dr. Tracy A. Lieu of Kaiser Permanente Northern California, Oakland, and her associates.

“The ability to identify clusters of underimmunization and vaccine refusal would help providers focus their efforts on the parents in communities with the greatest concerns, as well as identify areas at elevated risk for outbreaks of vaccine-preventable diseases” (Pediatrics 2015 [doi:10.1542/peds.2014-2715]).
In a retrospective cohort study, Dr. Lieu and her coinvestigators looked at children with continuous membership in the Kaiser Permanente Northern California, aged from birth to age 36 months, between Jan. 1, 2000, and December 31, 2009. A total of 154,424 children in 13 northern California counties were selected for inclusion in the study. Children were classified as underimmunized if they missed at least one vaccine by age 36 months, while vaccine refusal was classified using ICD-9-CM codes V6405, V6406, or V6407, as per the International Classification of Diseases, Ninth Revision, Clinical Modification for subjects who refused even one vaccination at any time prior to age 36 months.
Spatial scan analysis of all the accumulated data revealed five statistically significant geographic clusters in which underimmunization was more pronounced, nearly all located in the greater Sacramento and San Francisco metropolitan areas, with underimmunization rates over the course of the study period ranging from 18.1% in northern San Francisco and southern Marin counties to 22.7% in Vallejo (P = .023 and P = .042, respectively).
Across all 13 counties surveyed, rates of underimmunization increased from 8.1% in 2001-2005 to 12.4% in 2010-2012; the peak rate in a single year was 13%, achieved in 2010, while the mean annual increase in underimmunization across all 13 counties was 5.9%. In terms of race/ethnicity, higher percentages of Asian and Hispanic subjects were fully immunized compared with those who were white, black, or of unknown race/ethnicity.
Children in the most statistically significant cluster – the East Bay area, from Richmond to San Leandro – had 1.58 (P < .001) times the rate of underimmunization as displayed by other clusters, with 650 actual underimmunized cases during 2010-2012. Vaccine refusal was also clustered, with rates of 5.5% to 13.5% within clusters, compared with 2.6% outside of them.
Underimmunization of measles, mumps, and rubella and varicella vaccines also clustered in similar geographic areas, a cause for great concern given the recent outbreaks of measles at the Disneyland theme park in Anaheim, Calif. A study published last year, highlights the greater need for health care providers to educate parents about the importance of MMR vaccinations, saying that by doing so, they greatly increase the likelihood of parents agreeing to have their children properly vaccinated (Pediatrics 2014;134:e675-e83). Outbreaks of pertussis also have proved troublesome in recent years, exacerbating the need for greater intervention regarding vaccinating children early and when necessary.
“The existence of clusters may put clinical groups in these locations at a disadvantage in achieving immunization quality benchmarks,” noted Dr. Lieu and her coauthors, adding that “nonmedical exemptions from immunization in California clustered geographically and were associated with clusters of pertussis cases [and] many previous studies have found that vaccine refusal and delay are associated with elevated risk of measles and pertussis outbreaks, as well as elevated individual risks of measles, pertussis, varicella, and pneumococcal infections.”
Funding was provided by a Patient-Centered Outcomes Research Institute Pilot Project contract. Coauthor Dr Klein receives research support from Merck, GlaxoSmithKline, MedImmune, Novartis, Pfizer, Sanofi Pasteur, and Nuron Biotech; no other authors reported any relevant financial disclosures.
FROM PEDIATRICS
Key clinical point: Underimmunization occurs in geographic clusters, which can be used to predict areas in which underutilization of vaccines occurs and where focused intervention may be necessary.
Major finding: Out of five statistically significant clusters of underimmunization that were identified, underimmunization rates within clusters ranged from 18% to 23%, while the rate outside clusters was 11%.
Data source: Retrospective cohort study of 154,424 children in 13 counties, all of whom have membership in Kaiser Permanente Northern California.
Disclosures: Funding was provided by a Patient-Centered Outcomes Research Institute Pilot Project contract. Coauthor Dr Klein receives research support from Merck, GlaxoSmithKline, MedImmune, Novartis, Pfizer, Sanofi Pasteur, and Nuron Biotech; no other authors reported any relevant financial disclosures.