User login
Recommended Vaccination Schedule Not Followed in 1 of 4 Children
Roughly one out of every four children in New York state is vaccinated under a schedule that intentionally deviates from the one recommended by the Centers for Disease Control and Prevention, according to a study published in the Journal of Pediatrics.
“The number of children vaccinated off schedule has been increasing in recent years,” said lead investigator Jessica A. Nadeau, Ph.D., of the State University of New York, University at Albany, Rensselaer, and her associates, adding that “The continuing use of alternative schedules has prompted questions regarding the effects of these deviations on undervaccination, disease risk, and the safety and effectiveness of these new vaccine patterns” (J. Pediatr. 2015;166:151-6).
In a retrospective cohort study, Dr. Nadeau and her coinvestigators analyzed data on all children born in New York state, but not in New York City, between Jan. 1, 2009, and Aug. 14, 2011, ultimately ending up with 337,945 children from the New York state Immunization Information System (NYSIIS). Of those, 222,628 (81%) were deemed eligible for inclusion in the study because they had received, starting at age 41 days, at least three vaccinations by age 9 months.
Children were classified into two groups: those who had been vaccinated following the recommended vaccination schedule as outlined by the Centers for Disease Control and Prevention, and those who were vaccinated intentionally following an alternative schedule. Investigators then compared visits for vaccinations made over the course of the study period between both cohorts.
Analysis of the accumulated data revealed that 154,150 subjects (69.3%) were considered to be following the routine vaccination schedule.
Continue for subjects following alternative schedules >>
However, 56,607 subjects (25.4%) were considered to be following alternative schedules. Of those, 20,412 of those subjects (36.1% of subset) were on restrictive schedule only, 16,877 subjects (29.8% of subset) were classified as selective refusals only, and 19,318 subjects (34.1% of subset) were classified as both restrictive and selective refusals of vaccinations.
The remaining 11,871 individuals (5.3%) in the study population were classified as following an “unknown” vaccination schedule.
Children who followed the routine schedule were far more likely to be up to date on their vaccinations than those following alternative schedules: 89.8% vs. 14.8%, respectively (P < .05). The most commonly missed or refused vaccines in the alternative cohort were rotavirus (78.8%), hepatitis B (13.1%), and pneumococcal conjugate vaccine (6.6%).
“Although parents and physicians may view an alternative schedule as the middle ground between the routine schedule and choosing not to vaccinate, intentional deviation from the routine schedule through an alternative schedule leaves children without adequate protection from vaccine preventable disease,” warned Dr. Nadeau and her coauthors, adding that “the reemergence of vaccine-preventable diseases, coupled with increasing proportions of children using an alternative schedule, may set the stage for more widespread outbreaks of vaccine-preventable diseases.”
The authors reported that the study was supported by funding from the Centers for Disease Control and Prevention, but made no other relevant financial disclosures.
Using alternative vaccination schedules, or refusing vaccinations altogether, is certainly a common problem not only in New York state, but also across the country. We’ve lost an understanding, both on the part of the public and on the part of health care providers, as to why we even have schedules. We have them because it’s about trying to gain protection for children as quickly as possible against the diseases they’re most likely to contract after the immunity protection from the mother wanes, which happens even if the mother is still breastfeeding. This idea of getting the vaccinations on schedule – for example, at age 2 months, 4 months, and 6 months – is specifically intended to achieve the goal of immunity by the time that protection from the mother has waned.
Because the schedule has been handed down by the American Academy of Pediatrics and the Centers for Disease Control and Prevention as “recommended” with no detailed explanation why, many pediatricians are unaware of the immunology and all the research that went into creating the schedule. It requires the health care provider’s agreement and the parent’s request to have the results shown in this study, where one in four children intentionally deviated from the routine schedule. If a parent asks me to deviate, I have the knowledge as an immunologist and infectious disease specialist to explain why I don’t think that’s a good idea. And when I do, the parents agree to the routine schedule. But if you’re not informed as to why, then you have other information from sources that are not as well informed, recommending all these alternative vaccination schedules.
We, as health care providers, can never force a parent to do something. There should be discussion between provider and parent to inform the latter of the risks of delaying vaccinations or following alternative schedules. The unfortunate thing is that by not following the recommendations, the parent is making the child more susceptible to contracting the very disease she should have been vaccinated against. I’m aware of a recent case in which an alternative schedule was used, but the child did not achieve full immunity, and as a result, contracted bacterial spinal meningitis and died. Now that parent feels tremendous guilt, and the doctor in charge of caring for the child also feels guilt and wonders if pushing the parent harder to stick to the recommended schedule would have saved the child’s life.
There was also a recent research study that surprised everyone – by Nyhan B et al. (Pediatrics 2014;133:e835-42) – because we’re searching for a way to solve all these problems that we’ve encountered over the last 10 years or so in achieving more complete and more timely vaccination. But the findings of that study were disappointing because the investigators tried several different approaches to achieve that outcome, but none of them worked. And, in some cases, trying to educate parents about certain misconceptions or correcting false information turned out to be counterproductive.
Dr. Michael E. Pichichero is a specialist in pediatric infectious diseases and is the director of the Research Institute at Rochester (N.Y.) General Hospital, as well as a pediatrician at Legacy Pediatrics in Rochester. He was asked to comment on the article by Dr. Nadeau et al. Dr. Pichichero said he had no relevant financial disclosures.
Using alternative vaccination schedules, or refusing vaccinations altogether, is certainly a common problem not only in New York state, but also across the country. We’ve lost an understanding, both on the part of the public and on the part of health care providers, as to why we even have schedules. We have them because it’s about trying to gain protection for children as quickly as possible against the diseases they’re most likely to contract after the immunity protection from the mother wanes, which happens even if the mother is still breastfeeding. This idea of getting the vaccinations on schedule – for example, at age 2 months, 4 months, and 6 months – is specifically intended to achieve the goal of immunity by the time that protection from the mother has waned.
Because the schedule has been handed down by the American Academy of Pediatrics and the Centers for Disease Control and Prevention as “recommended” with no detailed explanation why, many pediatricians are unaware of the immunology and all the research that went into creating the schedule. It requires the health care provider’s agreement and the parent’s request to have the results shown in this study, where one in four children intentionally deviated from the routine schedule. If a parent asks me to deviate, I have the knowledge as an immunologist and infectious disease specialist to explain why I don’t think that’s a good idea. And when I do, the parents agree to the routine schedule. But if you’re not informed as to why, then you have other information from sources that are not as well informed, recommending all these alternative vaccination schedules.
We, as health care providers, can never force a parent to do something. There should be discussion between provider and parent to inform the latter of the risks of delaying vaccinations or following alternative schedules. The unfortunate thing is that by not following the recommendations, the parent is making the child more susceptible to contracting the very disease she should have been vaccinated against. I’m aware of a recent case in which an alternative schedule was used, but the child did not achieve full immunity, and as a result, contracted bacterial spinal meningitis and died. Now that parent feels tremendous guilt, and the doctor in charge of caring for the child also feels guilt and wonders if pushing the parent harder to stick to the recommended schedule would have saved the child’s life.
There was also a recent research study that surprised everyone – by Nyhan B et al. (Pediatrics 2014;133:e835-42) – because we’re searching for a way to solve all these problems that we’ve encountered over the last 10 years or so in achieving more complete and more timely vaccination. But the findings of that study were disappointing because the investigators tried several different approaches to achieve that outcome, but none of them worked. And, in some cases, trying to educate parents about certain misconceptions or correcting false information turned out to be counterproductive.
Dr. Michael E. Pichichero is a specialist in pediatric infectious diseases and is the director of the Research Institute at Rochester (N.Y.) General Hospital, as well as a pediatrician at Legacy Pediatrics in Rochester. He was asked to comment on the article by Dr. Nadeau et al. Dr. Pichichero said he had no relevant financial disclosures.
Using alternative vaccination schedules, or refusing vaccinations altogether, is certainly a common problem not only in New York state, but also across the country. We’ve lost an understanding, both on the part of the public and on the part of health care providers, as to why we even have schedules. We have them because it’s about trying to gain protection for children as quickly as possible against the diseases they’re most likely to contract after the immunity protection from the mother wanes, which happens even if the mother is still breastfeeding. This idea of getting the vaccinations on schedule – for example, at age 2 months, 4 months, and 6 months – is specifically intended to achieve the goal of immunity by the time that protection from the mother has waned.
Because the schedule has been handed down by the American Academy of Pediatrics and the Centers for Disease Control and Prevention as “recommended” with no detailed explanation why, many pediatricians are unaware of the immunology and all the research that went into creating the schedule. It requires the health care provider’s agreement and the parent’s request to have the results shown in this study, where one in four children intentionally deviated from the routine schedule. If a parent asks me to deviate, I have the knowledge as an immunologist and infectious disease specialist to explain why I don’t think that’s a good idea. And when I do, the parents agree to the routine schedule. But if you’re not informed as to why, then you have other information from sources that are not as well informed, recommending all these alternative vaccination schedules.
We, as health care providers, can never force a parent to do something. There should be discussion between provider and parent to inform the latter of the risks of delaying vaccinations or following alternative schedules. The unfortunate thing is that by not following the recommendations, the parent is making the child more susceptible to contracting the very disease she should have been vaccinated against. I’m aware of a recent case in which an alternative schedule was used, but the child did not achieve full immunity, and as a result, contracted bacterial spinal meningitis and died. Now that parent feels tremendous guilt, and the doctor in charge of caring for the child also feels guilt and wonders if pushing the parent harder to stick to the recommended schedule would have saved the child’s life.
There was also a recent research study that surprised everyone – by Nyhan B et al. (Pediatrics 2014;133:e835-42) – because we’re searching for a way to solve all these problems that we’ve encountered over the last 10 years or so in achieving more complete and more timely vaccination. But the findings of that study were disappointing because the investigators tried several different approaches to achieve that outcome, but none of them worked. And, in some cases, trying to educate parents about certain misconceptions or correcting false information turned out to be counterproductive.
Dr. Michael E. Pichichero is a specialist in pediatric infectious diseases and is the director of the Research Institute at Rochester (N.Y.) General Hospital, as well as a pediatrician at Legacy Pediatrics in Rochester. He was asked to comment on the article by Dr. Nadeau et al. Dr. Pichichero said he had no relevant financial disclosures.
Roughly one out of every four children in New York state is vaccinated under a schedule that intentionally deviates from the one recommended by the Centers for Disease Control and Prevention, according to a study published in the Journal of Pediatrics.
“The number of children vaccinated off schedule has been increasing in recent years,” said lead investigator Jessica A. Nadeau, Ph.D., of the State University of New York, University at Albany, Rensselaer, and her associates, adding that “The continuing use of alternative schedules has prompted questions regarding the effects of these deviations on undervaccination, disease risk, and the safety and effectiveness of these new vaccine patterns” (J. Pediatr. 2015;166:151-6).
In a retrospective cohort study, Dr. Nadeau and her coinvestigators analyzed data on all children born in New York state, but not in New York City, between Jan. 1, 2009, and Aug. 14, 2011, ultimately ending up with 337,945 children from the New York state Immunization Information System (NYSIIS). Of those, 222,628 (81%) were deemed eligible for inclusion in the study because they had received, starting at age 41 days, at least three vaccinations by age 9 months.
Children were classified into two groups: those who had been vaccinated following the recommended vaccination schedule as outlined by the Centers for Disease Control and Prevention, and those who were vaccinated intentionally following an alternative schedule. Investigators then compared visits for vaccinations made over the course of the study period between both cohorts.
Analysis of the accumulated data revealed that 154,150 subjects (69.3%) were considered to be following the routine vaccination schedule.
Continue for subjects following alternative schedules >>
However, 56,607 subjects (25.4%) were considered to be following alternative schedules. Of those, 20,412 of those subjects (36.1% of subset) were on restrictive schedule only, 16,877 subjects (29.8% of subset) were classified as selective refusals only, and 19,318 subjects (34.1% of subset) were classified as both restrictive and selective refusals of vaccinations.
The remaining 11,871 individuals (5.3%) in the study population were classified as following an “unknown” vaccination schedule.
Children who followed the routine schedule were far more likely to be up to date on their vaccinations than those following alternative schedules: 89.8% vs. 14.8%, respectively (P < .05). The most commonly missed or refused vaccines in the alternative cohort were rotavirus (78.8%), hepatitis B (13.1%), and pneumococcal conjugate vaccine (6.6%).
“Although parents and physicians may view an alternative schedule as the middle ground between the routine schedule and choosing not to vaccinate, intentional deviation from the routine schedule through an alternative schedule leaves children without adequate protection from vaccine preventable disease,” warned Dr. Nadeau and her coauthors, adding that “the reemergence of vaccine-preventable diseases, coupled with increasing proportions of children using an alternative schedule, may set the stage for more widespread outbreaks of vaccine-preventable diseases.”
The authors reported that the study was supported by funding from the Centers for Disease Control and Prevention, but made no other relevant financial disclosures.
Roughly one out of every four children in New York state is vaccinated under a schedule that intentionally deviates from the one recommended by the Centers for Disease Control and Prevention, according to a study published in the Journal of Pediatrics.
“The number of children vaccinated off schedule has been increasing in recent years,” said lead investigator Jessica A. Nadeau, Ph.D., of the State University of New York, University at Albany, Rensselaer, and her associates, adding that “The continuing use of alternative schedules has prompted questions regarding the effects of these deviations on undervaccination, disease risk, and the safety and effectiveness of these new vaccine patterns” (J. Pediatr. 2015;166:151-6).
In a retrospective cohort study, Dr. Nadeau and her coinvestigators analyzed data on all children born in New York state, but not in New York City, between Jan. 1, 2009, and Aug. 14, 2011, ultimately ending up with 337,945 children from the New York state Immunization Information System (NYSIIS). Of those, 222,628 (81%) were deemed eligible for inclusion in the study because they had received, starting at age 41 days, at least three vaccinations by age 9 months.
Children were classified into two groups: those who had been vaccinated following the recommended vaccination schedule as outlined by the Centers for Disease Control and Prevention, and those who were vaccinated intentionally following an alternative schedule. Investigators then compared visits for vaccinations made over the course of the study period between both cohorts.
Analysis of the accumulated data revealed that 154,150 subjects (69.3%) were considered to be following the routine vaccination schedule.
Continue for subjects following alternative schedules >>
However, 56,607 subjects (25.4%) were considered to be following alternative schedules. Of those, 20,412 of those subjects (36.1% of subset) were on restrictive schedule only, 16,877 subjects (29.8% of subset) were classified as selective refusals only, and 19,318 subjects (34.1% of subset) were classified as both restrictive and selective refusals of vaccinations.
The remaining 11,871 individuals (5.3%) in the study population were classified as following an “unknown” vaccination schedule.
Children who followed the routine schedule were far more likely to be up to date on their vaccinations than those following alternative schedules: 89.8% vs. 14.8%, respectively (P < .05). The most commonly missed or refused vaccines in the alternative cohort were rotavirus (78.8%), hepatitis B (13.1%), and pneumococcal conjugate vaccine (6.6%).
“Although parents and physicians may view an alternative schedule as the middle ground between the routine schedule and choosing not to vaccinate, intentional deviation from the routine schedule through an alternative schedule leaves children without adequate protection from vaccine preventable disease,” warned Dr. Nadeau and her coauthors, adding that “the reemergence of vaccine-preventable diseases, coupled with increasing proportions of children using an alternative schedule, may set the stage for more widespread outbreaks of vaccine-preventable diseases.”
The authors reported that the study was supported by funding from the Centers for Disease Control and Prevention, but made no other relevant financial disclosures.
FROM THE JOURNAL OF PEDIATRICS
Recommended vaccination schedule not followed in 1 of 4 children
Roughly one out of every four children in New York state is vaccinated under a schedule that intentionally deviates from the one recommended by the Centers for Disease Control and Prevention, according to a study published in the Journal of Pediatrics.
“The number of children vaccinated off schedule has been increasing in recent years,” said lead investigator Jessica A. Nadeau, Ph.D., of the State University of New York, University at Albany, Rensselaer, and her associates, adding that “The continuing use of alternative schedules has prompted questions regarding the effects of these deviations on undervaccination, disease risk, and the safety and effectiveness of these new vaccine patterns” (J. Pediatr. 2015;166:151-6).
In a retrospective cohort study, Dr. Nadeau and her coinvestigators analyzed data on all children born in New York state, but not in New York City, between Jan. 1, 2009, and Aug. 14, 2011, ultimately ending up with 337,945 children from the New York state Immunization Information System (NYSIIS). Of those, 222,628 (81%) were deemed eligible for inclusion in the study because they had received, starting at age 41 days, at least three vaccinations by age 9 months.
Children were classified into two groups: those who had been vaccinated following the recommended vaccination schedule as outlined by the Centers for Disease Control and Prevention, and those who were vaccinated intentionally following an alternative schedule. Investigators then compared visits for vaccinations made over the course of the study period between both cohorts.
Analysis of the accumulated data revealed that 154,150 subjects (69.3%) were considered to be following the routine vaccination schedule.
However, 56,607 subjects (25.4%) were considered to be following alternative schedules. Of those, 20,412 of those subjects (36.1% of subset) were on restrictive schedule only, 16,877 subjects (29.8% of subset) were classified as selective refusals only, and 19,318 subjects (34.1% of subset) were classified as both restrictive and selective refusals of vaccinations.
The remaining 11,871 individuals (5.3%) in the study population were classified as following an “unknown” vaccination schedule.
Children who followed the routine schedule were far more likely to be up to date on their vaccinations than those following alternative schedules: 89.8% vs. 14.8%, respectively (P < .05). The most commonly missed or refused vaccines in the alternative cohort were rotavirus (78.8%), hepatitis B (13.1%), and pneumococcal conjugate vaccine (6.6%).
“Although parents and physicians may view an alternative schedule as the middle ground between the routine schedule and choosing not to vaccinate, intentional deviation from the routine schedule through an alternative schedule leaves children without adequate protection from vaccine preventable disease,” warned Dr. Nadeau and her coauthors, adding that “the reemergence of vaccine-preventable diseases, coupled with increasing proportions of children using an alternative schedule, may set the stage for more widespread outbreaks of vaccine-preventable diseases.”
The authors reported that the study was supported by funding from the Centers for Disease Control and Prevention, but made no other relevant financial disclosures.
Using alternative vaccination schedules, or refusing vaccinations altogether, is certainly a common problem not only in New York state, but also across the country. We’ve lost an understanding, both on the part of the public and on the part of health care providers, as to why we even have schedules. We have them because it’s about trying to gain protection for children as quickly as possible against the diseases they’re most likely to contract after the immunity protection from the mother wanes, which happens even if the mother is still breastfeeding. This idea of getting the vaccinations on schedule – for example, at age 2 months, 4 months, and 6 months – is specifically intended to achieve the goal of immunity by the time that protection from the mother has waned.
Because the schedule has been handed down by the American Academy of Pediatrics and the Centers for Disease Control and Prevention as “recommended” with no detailed explanation why, many pediatricians are unaware of the immunology and all the research that went into creating the schedule. It requires the health care provider’s agreement and the parent’s request to have the results shown in this study, where one in four children intentionally deviated from the routine schedule. If a parent asks me to deviate, I have the knowledge as an immunologist and infectious disease specialist to explain why I don’t think that’s a good idea. And when I do, the parents agree to the routine schedule. But if you’re not informed as to why, then you have other information from sources that are not as well informed, recommending all these alternative vaccination schedules.
We, as health care providers, can never force a parent to do something. There should be discussion between provider and parent to inform the latter of the risks of delaying vaccinations or following alternative schedules. The unfortunate thing is that by not following the recommendations, the parent is making the child more susceptible to contracting the very disease she should have been vaccinated against. I’m aware of a recent case in which an alternative schedule was used, but the child did not achieve full immunity, and as a result, contracted bacterial spinal meningitis and died. Now that parent feels tremendous guilt, and the doctor in charge of caring for the child also feels guilt and wonders if pushing the parent harder to stick to the recommended schedule would have saved the child’s life.
There was also a recent research study that surprised everyone – by Nyhan B et al. (Pediatrics 2014;133:e835-42) – because we’re searching for a way to solve all these problems that we’ve encountered over the last 10 years or so in achieving more complete and more timely vaccination. But the findings of that study were disappointing because the investigators tried several different approaches to achieve that outcome, but none of them worked. And, in some cases, trying to educate parents about certain misconceptions or correcting false information turned out to be counterproductive.
Dr. Michael E. Pichichero is a specialist in pediatric infectious diseases and is the director of the Research Institute at Rochester (N.Y.) General Hospital, as well as a pediatrician at Legacy Pediatrics in Rochester. He was asked to comment on the article by Dr. Nadeau et al. Dr. Pichichero said he had no relevant financial disclosures.
Using alternative vaccination schedules, or refusing vaccinations altogether, is certainly a common problem not only in New York state, but also across the country. We’ve lost an understanding, both on the part of the public and on the part of health care providers, as to why we even have schedules. We have them because it’s about trying to gain protection for children as quickly as possible against the diseases they’re most likely to contract after the immunity protection from the mother wanes, which happens even if the mother is still breastfeeding. This idea of getting the vaccinations on schedule – for example, at age 2 months, 4 months, and 6 months – is specifically intended to achieve the goal of immunity by the time that protection from the mother has waned.
Because the schedule has been handed down by the American Academy of Pediatrics and the Centers for Disease Control and Prevention as “recommended” with no detailed explanation why, many pediatricians are unaware of the immunology and all the research that went into creating the schedule. It requires the health care provider’s agreement and the parent’s request to have the results shown in this study, where one in four children intentionally deviated from the routine schedule. If a parent asks me to deviate, I have the knowledge as an immunologist and infectious disease specialist to explain why I don’t think that’s a good idea. And when I do, the parents agree to the routine schedule. But if you’re not informed as to why, then you have other information from sources that are not as well informed, recommending all these alternative vaccination schedules.
We, as health care providers, can never force a parent to do something. There should be discussion between provider and parent to inform the latter of the risks of delaying vaccinations or following alternative schedules. The unfortunate thing is that by not following the recommendations, the parent is making the child more susceptible to contracting the very disease she should have been vaccinated against. I’m aware of a recent case in which an alternative schedule was used, but the child did not achieve full immunity, and as a result, contracted bacterial spinal meningitis and died. Now that parent feels tremendous guilt, and the doctor in charge of caring for the child also feels guilt and wonders if pushing the parent harder to stick to the recommended schedule would have saved the child’s life.
There was also a recent research study that surprised everyone – by Nyhan B et al. (Pediatrics 2014;133:e835-42) – because we’re searching for a way to solve all these problems that we’ve encountered over the last 10 years or so in achieving more complete and more timely vaccination. But the findings of that study were disappointing because the investigators tried several different approaches to achieve that outcome, but none of them worked. And, in some cases, trying to educate parents about certain misconceptions or correcting false information turned out to be counterproductive.
Dr. Michael E. Pichichero is a specialist in pediatric infectious diseases and is the director of the Research Institute at Rochester (N.Y.) General Hospital, as well as a pediatrician at Legacy Pediatrics in Rochester. He was asked to comment on the article by Dr. Nadeau et al. Dr. Pichichero said he had no relevant financial disclosures.
Using alternative vaccination schedules, or refusing vaccinations altogether, is certainly a common problem not only in New York state, but also across the country. We’ve lost an understanding, both on the part of the public and on the part of health care providers, as to why we even have schedules. We have them because it’s about trying to gain protection for children as quickly as possible against the diseases they’re most likely to contract after the immunity protection from the mother wanes, which happens even if the mother is still breastfeeding. This idea of getting the vaccinations on schedule – for example, at age 2 months, 4 months, and 6 months – is specifically intended to achieve the goal of immunity by the time that protection from the mother has waned.
Because the schedule has been handed down by the American Academy of Pediatrics and the Centers for Disease Control and Prevention as “recommended” with no detailed explanation why, many pediatricians are unaware of the immunology and all the research that went into creating the schedule. It requires the health care provider’s agreement and the parent’s request to have the results shown in this study, where one in four children intentionally deviated from the routine schedule. If a parent asks me to deviate, I have the knowledge as an immunologist and infectious disease specialist to explain why I don’t think that’s a good idea. And when I do, the parents agree to the routine schedule. But if you’re not informed as to why, then you have other information from sources that are not as well informed, recommending all these alternative vaccination schedules.
We, as health care providers, can never force a parent to do something. There should be discussion between provider and parent to inform the latter of the risks of delaying vaccinations or following alternative schedules. The unfortunate thing is that by not following the recommendations, the parent is making the child more susceptible to contracting the very disease she should have been vaccinated against. I’m aware of a recent case in which an alternative schedule was used, but the child did not achieve full immunity, and as a result, contracted bacterial spinal meningitis and died. Now that parent feels tremendous guilt, and the doctor in charge of caring for the child also feels guilt and wonders if pushing the parent harder to stick to the recommended schedule would have saved the child’s life.
There was also a recent research study that surprised everyone – by Nyhan B et al. (Pediatrics 2014;133:e835-42) – because we’re searching for a way to solve all these problems that we’ve encountered over the last 10 years or so in achieving more complete and more timely vaccination. But the findings of that study were disappointing because the investigators tried several different approaches to achieve that outcome, but none of them worked. And, in some cases, trying to educate parents about certain misconceptions or correcting false information turned out to be counterproductive.
Dr. Michael E. Pichichero is a specialist in pediatric infectious diseases and is the director of the Research Institute at Rochester (N.Y.) General Hospital, as well as a pediatrician at Legacy Pediatrics in Rochester. He was asked to comment on the article by Dr. Nadeau et al. Dr. Pichichero said he had no relevant financial disclosures.
Roughly one out of every four children in New York state is vaccinated under a schedule that intentionally deviates from the one recommended by the Centers for Disease Control and Prevention, according to a study published in the Journal of Pediatrics.
“The number of children vaccinated off schedule has been increasing in recent years,” said lead investigator Jessica A. Nadeau, Ph.D., of the State University of New York, University at Albany, Rensselaer, and her associates, adding that “The continuing use of alternative schedules has prompted questions regarding the effects of these deviations on undervaccination, disease risk, and the safety and effectiveness of these new vaccine patterns” (J. Pediatr. 2015;166:151-6).
In a retrospective cohort study, Dr. Nadeau and her coinvestigators analyzed data on all children born in New York state, but not in New York City, between Jan. 1, 2009, and Aug. 14, 2011, ultimately ending up with 337,945 children from the New York state Immunization Information System (NYSIIS). Of those, 222,628 (81%) were deemed eligible for inclusion in the study because they had received, starting at age 41 days, at least three vaccinations by age 9 months.
Children were classified into two groups: those who had been vaccinated following the recommended vaccination schedule as outlined by the Centers for Disease Control and Prevention, and those who were vaccinated intentionally following an alternative schedule. Investigators then compared visits for vaccinations made over the course of the study period between both cohorts.
Analysis of the accumulated data revealed that 154,150 subjects (69.3%) were considered to be following the routine vaccination schedule.
However, 56,607 subjects (25.4%) were considered to be following alternative schedules. Of those, 20,412 of those subjects (36.1% of subset) were on restrictive schedule only, 16,877 subjects (29.8% of subset) were classified as selective refusals only, and 19,318 subjects (34.1% of subset) were classified as both restrictive and selective refusals of vaccinations.
The remaining 11,871 individuals (5.3%) in the study population were classified as following an “unknown” vaccination schedule.
Children who followed the routine schedule were far more likely to be up to date on their vaccinations than those following alternative schedules: 89.8% vs. 14.8%, respectively (P < .05). The most commonly missed or refused vaccines in the alternative cohort were rotavirus (78.8%), hepatitis B (13.1%), and pneumococcal conjugate vaccine (6.6%).
“Although parents and physicians may view an alternative schedule as the middle ground between the routine schedule and choosing not to vaccinate, intentional deviation from the routine schedule through an alternative schedule leaves children without adequate protection from vaccine preventable disease,” warned Dr. Nadeau and her coauthors, adding that “the reemergence of vaccine-preventable diseases, coupled with increasing proportions of children using an alternative schedule, may set the stage for more widespread outbreaks of vaccine-preventable diseases.”
The authors reported that the study was supported by funding from the Centers for Disease Control and Prevention, but made no other relevant financial disclosures.
Roughly one out of every four children in New York state is vaccinated under a schedule that intentionally deviates from the one recommended by the Centers for Disease Control and Prevention, according to a study published in the Journal of Pediatrics.
“The number of children vaccinated off schedule has been increasing in recent years,” said lead investigator Jessica A. Nadeau, Ph.D., of the State University of New York, University at Albany, Rensselaer, and her associates, adding that “The continuing use of alternative schedules has prompted questions regarding the effects of these deviations on undervaccination, disease risk, and the safety and effectiveness of these new vaccine patterns” (J. Pediatr. 2015;166:151-6).
In a retrospective cohort study, Dr. Nadeau and her coinvestigators analyzed data on all children born in New York state, but not in New York City, between Jan. 1, 2009, and Aug. 14, 2011, ultimately ending up with 337,945 children from the New York state Immunization Information System (NYSIIS). Of those, 222,628 (81%) were deemed eligible for inclusion in the study because they had received, starting at age 41 days, at least three vaccinations by age 9 months.
Children were classified into two groups: those who had been vaccinated following the recommended vaccination schedule as outlined by the Centers for Disease Control and Prevention, and those who were vaccinated intentionally following an alternative schedule. Investigators then compared visits for vaccinations made over the course of the study period between both cohorts.
Analysis of the accumulated data revealed that 154,150 subjects (69.3%) were considered to be following the routine vaccination schedule.
However, 56,607 subjects (25.4%) were considered to be following alternative schedules. Of those, 20,412 of those subjects (36.1% of subset) were on restrictive schedule only, 16,877 subjects (29.8% of subset) were classified as selective refusals only, and 19,318 subjects (34.1% of subset) were classified as both restrictive and selective refusals of vaccinations.
The remaining 11,871 individuals (5.3%) in the study population were classified as following an “unknown” vaccination schedule.
Children who followed the routine schedule were far more likely to be up to date on their vaccinations than those following alternative schedules: 89.8% vs. 14.8%, respectively (P < .05). The most commonly missed or refused vaccines in the alternative cohort were rotavirus (78.8%), hepatitis B (13.1%), and pneumococcal conjugate vaccine (6.6%).
“Although parents and physicians may view an alternative schedule as the middle ground between the routine schedule and choosing not to vaccinate, intentional deviation from the routine schedule through an alternative schedule leaves children without adequate protection from vaccine preventable disease,” warned Dr. Nadeau and her coauthors, adding that “the reemergence of vaccine-preventable diseases, coupled with increasing proportions of children using an alternative schedule, may set the stage for more widespread outbreaks of vaccine-preventable diseases.”
The authors reported that the study was supported by funding from the Centers for Disease Control and Prevention, but made no other relevant financial disclosures.
FROM THE JOURNAL OF PEDIATRICS
Key clinical point: Almost 25% of children in New York state are vaccinated under a schedule that intentionally deviates from the one recommended by the CDC, possibly leading to increased rates of infection and disease outbreaks.
Major finding: Proportion of children vaccinated following an alternative schedule was 25%; these children were significantly less likely to be up to date at age 9 months (15%), compared with those whose vaccinations adhering to recommended schedule (90%, P < .05).
Data source: Retrospective cohort study of 222,628 children in New York state, outside of New York City.
Disclosures: The authors reported that the study was supported by funding from the Centers for Disease Control and Prevention, but made no other relevant financial disclosures.
Psychopathology and multimorbidity affect prognosis, treatment in substance use disorder
High levels of multimorbidity exist among patients entering treatment for alcohol and other drug use. As a result, clinicians must examine individuals’ psychopathology thoroughly to provide the most complete and effective treatment possible, according to a study published in the Journal of Substance Abuse Treatment.
“The co-occurrence of mental and substance use disorders has important clinical implications: In addition to presenting with a more complex array of problems, clients with co-occurring disorders are at risk of poorer outcomes following treatment,” wrote lead author Karen Urbanoski, Ph.D., of the Centre for Addiction and Mental Health in Toronto, and her associates. “The clustering of multiple disorders within individuals highlights the cumulative burden of psychopathology, constituting one aspect of illness severity,” they added (J. Subst. Abuse Treat. 2015;49:21-6).
To conduct the study, Dr. Urbanoski and her associates recruited 544 adults from three southwest Ontario outpatient addiction treatment clinics. Most of the participants were male (68%), and the average age was 37. In the year before treatment, the most commonly used substances were alcohol (91%), cocaine (63%), cannabis (63%), prescription opioids (36%), and benzodiazepines (27%).
The investigators administered the Psychiatric Diagnostic Screening Questionnaire (PDSQ) for DSM-IV Axis 1 disorders to each patient for completion at treatment admission. Latent class analyses were used to identify and classify subjects who presented with specific patterns of co-occurring mental disorders, with three distinct classes ultimately created for the purposes of this study: no comorbidity (in other words, substance use disorders [SUD] only); co-occurring major depression; and multimorbidity, or a high degree of psychopathology.
The SUD cohort was found to contain 54.6% of patients, while 37.4% fell into the co-occurring major depression classification, and 8% were designated as multimorbidity. Patients who were older had less likelihood of being classified with co-occurring major depression, women were more likely than men to be in the multimorbid class, and individuals who were married or partnered had a lower risk of being in either of the comorbid classes.
“Unlike studies conducted in general population samples, classes characterized by little or no psychopathology or pure internalizing disorders do not apply in this instance, because of the uniform presence of SUD,” wrote the authors. They added: “With the uniform presence of SUD in this sample, one interpretation of these results is that they identify not clusters of disorders, but levels of severity of internalizing symptoms. Most conditions were moderately to highly correlated with the others, and the prevalence of disorders rises consistently across classes.”
Dr. Urbanoski and her associates cited several limitations. One is that the PDSQ is a self-reported questionnaire. In the addition, assessments for all DSM-IV disorders – including bipolar disorder – are not included on the questionnaire. Axis 2 disorders and impulse control disorders are also not included on the PDSQ.
The study was supported by a research award from the Canadian Institutes for Health Research. The authors reported no relevant financial disclosures.
High levels of multimorbidity exist among patients entering treatment for alcohol and other drug use. As a result, clinicians must examine individuals’ psychopathology thoroughly to provide the most complete and effective treatment possible, according to a study published in the Journal of Substance Abuse Treatment.
“The co-occurrence of mental and substance use disorders has important clinical implications: In addition to presenting with a more complex array of problems, clients with co-occurring disorders are at risk of poorer outcomes following treatment,” wrote lead author Karen Urbanoski, Ph.D., of the Centre for Addiction and Mental Health in Toronto, and her associates. “The clustering of multiple disorders within individuals highlights the cumulative burden of psychopathology, constituting one aspect of illness severity,” they added (J. Subst. Abuse Treat. 2015;49:21-6).
To conduct the study, Dr. Urbanoski and her associates recruited 544 adults from three southwest Ontario outpatient addiction treatment clinics. Most of the participants were male (68%), and the average age was 37. In the year before treatment, the most commonly used substances were alcohol (91%), cocaine (63%), cannabis (63%), prescription opioids (36%), and benzodiazepines (27%).
The investigators administered the Psychiatric Diagnostic Screening Questionnaire (PDSQ) for DSM-IV Axis 1 disorders to each patient for completion at treatment admission. Latent class analyses were used to identify and classify subjects who presented with specific patterns of co-occurring mental disorders, with three distinct classes ultimately created for the purposes of this study: no comorbidity (in other words, substance use disorders [SUD] only); co-occurring major depression; and multimorbidity, or a high degree of psychopathology.
The SUD cohort was found to contain 54.6% of patients, while 37.4% fell into the co-occurring major depression classification, and 8% were designated as multimorbidity. Patients who were older had less likelihood of being classified with co-occurring major depression, women were more likely than men to be in the multimorbid class, and individuals who were married or partnered had a lower risk of being in either of the comorbid classes.
“Unlike studies conducted in general population samples, classes characterized by little or no psychopathology or pure internalizing disorders do not apply in this instance, because of the uniform presence of SUD,” wrote the authors. They added: “With the uniform presence of SUD in this sample, one interpretation of these results is that they identify not clusters of disorders, but levels of severity of internalizing symptoms. Most conditions were moderately to highly correlated with the others, and the prevalence of disorders rises consistently across classes.”
Dr. Urbanoski and her associates cited several limitations. One is that the PDSQ is a self-reported questionnaire. In the addition, assessments for all DSM-IV disorders – including bipolar disorder – are not included on the questionnaire. Axis 2 disorders and impulse control disorders are also not included on the PDSQ.
The study was supported by a research award from the Canadian Institutes for Health Research. The authors reported no relevant financial disclosures.
High levels of multimorbidity exist among patients entering treatment for alcohol and other drug use. As a result, clinicians must examine individuals’ psychopathology thoroughly to provide the most complete and effective treatment possible, according to a study published in the Journal of Substance Abuse Treatment.
“The co-occurrence of mental and substance use disorders has important clinical implications: In addition to presenting with a more complex array of problems, clients with co-occurring disorders are at risk of poorer outcomes following treatment,” wrote lead author Karen Urbanoski, Ph.D., of the Centre for Addiction and Mental Health in Toronto, and her associates. “The clustering of multiple disorders within individuals highlights the cumulative burden of psychopathology, constituting one aspect of illness severity,” they added (J. Subst. Abuse Treat. 2015;49:21-6).
To conduct the study, Dr. Urbanoski and her associates recruited 544 adults from three southwest Ontario outpatient addiction treatment clinics. Most of the participants were male (68%), and the average age was 37. In the year before treatment, the most commonly used substances were alcohol (91%), cocaine (63%), cannabis (63%), prescription opioids (36%), and benzodiazepines (27%).
The investigators administered the Psychiatric Diagnostic Screening Questionnaire (PDSQ) for DSM-IV Axis 1 disorders to each patient for completion at treatment admission. Latent class analyses were used to identify and classify subjects who presented with specific patterns of co-occurring mental disorders, with three distinct classes ultimately created for the purposes of this study: no comorbidity (in other words, substance use disorders [SUD] only); co-occurring major depression; and multimorbidity, or a high degree of psychopathology.
The SUD cohort was found to contain 54.6% of patients, while 37.4% fell into the co-occurring major depression classification, and 8% were designated as multimorbidity. Patients who were older had less likelihood of being classified with co-occurring major depression, women were more likely than men to be in the multimorbid class, and individuals who were married or partnered had a lower risk of being in either of the comorbid classes.
“Unlike studies conducted in general population samples, classes characterized by little or no psychopathology or pure internalizing disorders do not apply in this instance, because of the uniform presence of SUD,” wrote the authors. They added: “With the uniform presence of SUD in this sample, one interpretation of these results is that they identify not clusters of disorders, but levels of severity of internalizing symptoms. Most conditions were moderately to highly correlated with the others, and the prevalence of disorders rises consistently across classes.”
Dr. Urbanoski and her associates cited several limitations. One is that the PDSQ is a self-reported questionnaire. In the addition, assessments for all DSM-IV disorders – including bipolar disorder – are not included on the questionnaire. Axis 2 disorders and impulse control disorders are also not included on the PDSQ.
The study was supported by a research award from the Canadian Institutes for Health Research. The authors reported no relevant financial disclosures.
FROM THE JOURNAL OF SUBSTANCE ABUSE TREATMENT
Key clinical point: Identifying patients with substance use disorders and high levels of psychiatric multimorbidity “has relevance for treatment planning and service provision.”
Major finding: Older age was associated with lower risk of co-occurring major depression, women were more likely than men to be classified as multimorbid, and being married or partnered was associated with lower risk of being comorbid.
Data source: Questionnaire-based cohort study of 544 subjects.
Disclosures: The study was supported by a research award from the Canadian Institutes for Health Research. Authors reported no relevant financial disclosures.
Stage IV CRC survival rates up, resection rates down
Although significantly fewer patients with stage IV colorectal cancer have undergone primary tumor resection (PTR) since 1988, overall rates of patient survival have improved over that period, according to the results of a study published in JAMA.
“The role of PTR for asymptomatic patients remains controversial. Some physicians advocate PTR to prevent the development of symptoms associated with an intact primary tumor,” wrote lead author Chung-Yuan Hu, Ph.D., of the University of Texas M.D. Anderson Cancer Center in Houston, and his associates. “Approximately 10%-25% of patients with intact primary tumors will develop symptoms, but there is considerable morbidity (4%-30%) and mortality (2%-10%) associated with noncurative PTR” (JAMA Surg. [doi:10.1001/jamasurg.2014.2253]).
In a retrospective cohort study, Dr. Hu and his colleagues used data on 64,157 patients diagnosed with stage IV colon or rectal cancer between Jan. 1, 1988, and Dec. 31, 2010, from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) CRC registry. The primary outcome measured was difference in PTR rates over the study period. Included subjects had either undergone PTR or not, and investigators calculated rates of PTR and median relative survival for each year. Joinpoint regression analyses were used to determine when a “significant change” in PTR rate had occurred, while logistic regression analyses were used to assess factors associated with PTR.
Findings indicated that 43,273 (67.4%) of the patients analyzed for the study had undergone PTR; overall, between 1988 and 2010, annual PTR rates declined from 74.5% to 57.4%, respectively (P < .001). “Significant change” was noted between 1998 and 2001 and from 2001 to 2010: –0.41% and –2.39%, respectively (P < .001 in both cases). However, median annual survival rates improved substantially from 1988 to 2009, changing from 8.6% to 17.8%, respectively (P < .001).
“Despite the availability of more effective chemotherapeutic options, a considerable number of patients with stage IV CRC continue to undergo PTR,” wrote the authors. “Our findings indicate potential overuse of PTR among these patients and highlight a need to better understand the clinical decisions and outcomes associated with that treatment.”
This study was supported in part by grants from the National Institute of Health’s National Cancer Institute, and the American Society of Clinical Oncology Foundation Career Development Award. Authors reported no financial conflicts of interest.
Although significantly fewer patients with stage IV colorectal cancer have undergone primary tumor resection (PTR) since 1988, overall rates of patient survival have improved over that period, according to the results of a study published in JAMA.
“The role of PTR for asymptomatic patients remains controversial. Some physicians advocate PTR to prevent the development of symptoms associated with an intact primary tumor,” wrote lead author Chung-Yuan Hu, Ph.D., of the University of Texas M.D. Anderson Cancer Center in Houston, and his associates. “Approximately 10%-25% of patients with intact primary tumors will develop symptoms, but there is considerable morbidity (4%-30%) and mortality (2%-10%) associated with noncurative PTR” (JAMA Surg. [doi:10.1001/jamasurg.2014.2253]).
In a retrospective cohort study, Dr. Hu and his colleagues used data on 64,157 patients diagnosed with stage IV colon or rectal cancer between Jan. 1, 1988, and Dec. 31, 2010, from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) CRC registry. The primary outcome measured was difference in PTR rates over the study period. Included subjects had either undergone PTR or not, and investigators calculated rates of PTR and median relative survival for each year. Joinpoint regression analyses were used to determine when a “significant change” in PTR rate had occurred, while logistic regression analyses were used to assess factors associated with PTR.
Findings indicated that 43,273 (67.4%) of the patients analyzed for the study had undergone PTR; overall, between 1988 and 2010, annual PTR rates declined from 74.5% to 57.4%, respectively (P < .001). “Significant change” was noted between 1998 and 2001 and from 2001 to 2010: –0.41% and –2.39%, respectively (P < .001 in both cases). However, median annual survival rates improved substantially from 1988 to 2009, changing from 8.6% to 17.8%, respectively (P < .001).
“Despite the availability of more effective chemotherapeutic options, a considerable number of patients with stage IV CRC continue to undergo PTR,” wrote the authors. “Our findings indicate potential overuse of PTR among these patients and highlight a need to better understand the clinical decisions and outcomes associated with that treatment.”
This study was supported in part by grants from the National Institute of Health’s National Cancer Institute, and the American Society of Clinical Oncology Foundation Career Development Award. Authors reported no financial conflicts of interest.
Although significantly fewer patients with stage IV colorectal cancer have undergone primary tumor resection (PTR) since 1988, overall rates of patient survival have improved over that period, according to the results of a study published in JAMA.
“The role of PTR for asymptomatic patients remains controversial. Some physicians advocate PTR to prevent the development of symptoms associated with an intact primary tumor,” wrote lead author Chung-Yuan Hu, Ph.D., of the University of Texas M.D. Anderson Cancer Center in Houston, and his associates. “Approximately 10%-25% of patients with intact primary tumors will develop symptoms, but there is considerable morbidity (4%-30%) and mortality (2%-10%) associated with noncurative PTR” (JAMA Surg. [doi:10.1001/jamasurg.2014.2253]).
In a retrospective cohort study, Dr. Hu and his colleagues used data on 64,157 patients diagnosed with stage IV colon or rectal cancer between Jan. 1, 1988, and Dec. 31, 2010, from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) CRC registry. The primary outcome measured was difference in PTR rates over the study period. Included subjects had either undergone PTR or not, and investigators calculated rates of PTR and median relative survival for each year. Joinpoint regression analyses were used to determine when a “significant change” in PTR rate had occurred, while logistic regression analyses were used to assess factors associated with PTR.
Findings indicated that 43,273 (67.4%) of the patients analyzed for the study had undergone PTR; overall, between 1988 and 2010, annual PTR rates declined from 74.5% to 57.4%, respectively (P < .001). “Significant change” was noted between 1998 and 2001 and from 2001 to 2010: –0.41% and –2.39%, respectively (P < .001 in both cases). However, median annual survival rates improved substantially from 1988 to 2009, changing from 8.6% to 17.8%, respectively (P < .001).
“Despite the availability of more effective chemotherapeutic options, a considerable number of patients with stage IV CRC continue to undergo PTR,” wrote the authors. “Our findings indicate potential overuse of PTR among these patients and highlight a need to better understand the clinical decisions and outcomes associated with that treatment.”
This study was supported in part by grants from the National Institute of Health’s National Cancer Institute, and the American Society of Clinical Oncology Foundation Career Development Award. Authors reported no financial conflicts of interest.
FROM JAMA SURGERY
Key clinical point: Although rates of primary tumor resection in patients with stage IV colorectal cancer have decreased in recent years, patient survival rates have improved.
Major finding: The annual rate of primary tumor resection decreased from 74.5% in 1988 to 57.4% in 2010, while median relative survival rate improved from 8.6% in 1988 to 17.8% in 2009.
Data source: Retrospective cohort study examining data on 64,157 subjects in the National Cancer Institute’s SEER CRC registry.
Disclosures: Study was supported in part by grants from the National Institute of Health’s National Cancer Institute, and the American Society of Clinical Oncology Foundation Career Development Award. Authors reported no financial conflicts of interest.
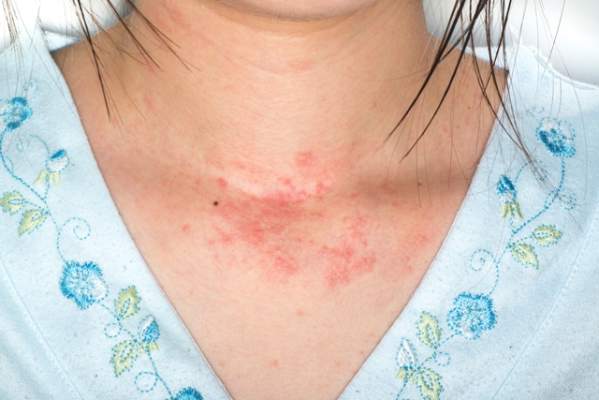
Adults With Eczema Face Increased Risk of Fracture and Bone or Joint Injuries
Eczema in adulthood is directly associated with an increase in incidence of injuries that cause physical limitations, particularly fracture, bone, and joint injuries, according to the results of a prospective study published online in JAMA Dermatology.
“The risk of bone fracture and other injury causing limitation in adults with eczema has been largely unexplored,” noted Dr. Nitin Garg and Dr. Jonathan I. Silverberg of Northwestern University in Chicago.
“Patients with eczema may be at risk of fracture given their use of systemic corticosteroids, which may decrease bone mineral density [and] therefore, studies describing the prevalence of fracture among adults with eczema are needed,” they added (JAMA Dermatol. 2015;151:33-41 [doi:10.1001/jamadermatol.2014.2098]).
Dr. Garg and Dr. Silverberg used data from the 2012 National Health Interview Survey, which was conducted by the National Center for Health Statistics, to create their prospective, questionnaire-based study. The final analysis included 34,500 adults aged 18-85 years. They controlled for factors such as age, sex, race, Hispanic origin, household income, highest level of household education, family structure, and whether or not the subject was born in the United States.
The prevalence of self-reported eczema or skin allergy was 7.2%, the prevalence of any limitation-causing injury was 2.0%, and any limitation-causing injury in the past 12 months among adults was 7.2%. A fracture and bone or joint injury (FBJI) causing limitation was reported by 1.5% of the study population and other types of injury causing limitation were reported by 0.6%. The prevalence of FBJI causing limitation increased gradually with age, to peak in subjects aged 50-69 years, decreasing thereafter.
In a logistic regression analysis, adults with eczema had a 44% increased risk of any injury causing limitation (adjusted odds ratio, 1.44), and a 67% increased risk of FBJI (aOR, 1.67).
Adults with eczema who also reported experiencing fatigue (aOR, 1.59; 95% CI, 1.16-2.19), daytime sleepiness (aOR, 1.81), or insomnia (aOR, 1.74) had higher rates of FBJI than individuals with sleep symptoms but no eczema. Adults with both eczema and psychiatric or behavioral disorders also were more likely to experience FBJI than individuals suffering from eczema alone, or individuals suffering from psychiatric and behavioral disorders, but not eczema.
“Taken together, these data suggest that adult eczema is a previously unrecognized risk factor for fracture and other injury, emphasizing the importance of developing safer and more effective clinical interventions for itch and sleep problems in eczema, as well as preventive measures for injury risk reduction in eczema,” wrote Dr. Garg and Dr. Silverberg. The authors reported no relevant financial disclosures.
Eczema in adulthood is directly associated with an increase in incidence of injuries that cause physical limitations, particularly fracture, bone, and joint injuries, according to the results of a prospective study published online in JAMA Dermatology.
“The risk of bone fracture and other injury causing limitation in adults with eczema has been largely unexplored,” noted Dr. Nitin Garg and Dr. Jonathan I. Silverberg of Northwestern University in Chicago.
“Patients with eczema may be at risk of fracture given their use of systemic corticosteroids, which may decrease bone mineral density [and] therefore, studies describing the prevalence of fracture among adults with eczema are needed,” they added (JAMA Dermatol. 2015;151:33-41 [doi:10.1001/jamadermatol.2014.2098]).
Dr. Garg and Dr. Silverberg used data from the 2012 National Health Interview Survey, which was conducted by the National Center for Health Statistics, to create their prospective, questionnaire-based study. The final analysis included 34,500 adults aged 18-85 years. They controlled for factors such as age, sex, race, Hispanic origin, household income, highest level of household education, family structure, and whether or not the subject was born in the United States.
The prevalence of self-reported eczema or skin allergy was 7.2%, the prevalence of any limitation-causing injury was 2.0%, and any limitation-causing injury in the past 12 months among adults was 7.2%. A fracture and bone or joint injury (FBJI) causing limitation was reported by 1.5% of the study population and other types of injury causing limitation were reported by 0.6%. The prevalence of FBJI causing limitation increased gradually with age, to peak in subjects aged 50-69 years, decreasing thereafter.
In a logistic regression analysis, adults with eczema had a 44% increased risk of any injury causing limitation (adjusted odds ratio, 1.44), and a 67% increased risk of FBJI (aOR, 1.67).
Adults with eczema who also reported experiencing fatigue (aOR, 1.59; 95% CI, 1.16-2.19), daytime sleepiness (aOR, 1.81), or insomnia (aOR, 1.74) had higher rates of FBJI than individuals with sleep symptoms but no eczema. Adults with both eczema and psychiatric or behavioral disorders also were more likely to experience FBJI than individuals suffering from eczema alone, or individuals suffering from psychiatric and behavioral disorders, but not eczema.
“Taken together, these data suggest that adult eczema is a previously unrecognized risk factor for fracture and other injury, emphasizing the importance of developing safer and more effective clinical interventions for itch and sleep problems in eczema, as well as preventive measures for injury risk reduction in eczema,” wrote Dr. Garg and Dr. Silverberg. The authors reported no relevant financial disclosures.
Eczema in adulthood is directly associated with an increase in incidence of injuries that cause physical limitations, particularly fracture, bone, and joint injuries, according to the results of a prospective study published online in JAMA Dermatology.
“The risk of bone fracture and other injury causing limitation in adults with eczema has been largely unexplored,” noted Dr. Nitin Garg and Dr. Jonathan I. Silverberg of Northwestern University in Chicago.
“Patients with eczema may be at risk of fracture given their use of systemic corticosteroids, which may decrease bone mineral density [and] therefore, studies describing the prevalence of fracture among adults with eczema are needed,” they added (JAMA Dermatol. 2015;151:33-41 [doi:10.1001/jamadermatol.2014.2098]).
Dr. Garg and Dr. Silverberg used data from the 2012 National Health Interview Survey, which was conducted by the National Center for Health Statistics, to create their prospective, questionnaire-based study. The final analysis included 34,500 adults aged 18-85 years. They controlled for factors such as age, sex, race, Hispanic origin, household income, highest level of household education, family structure, and whether or not the subject was born in the United States.
The prevalence of self-reported eczema or skin allergy was 7.2%, the prevalence of any limitation-causing injury was 2.0%, and any limitation-causing injury in the past 12 months among adults was 7.2%. A fracture and bone or joint injury (FBJI) causing limitation was reported by 1.5% of the study population and other types of injury causing limitation were reported by 0.6%. The prevalence of FBJI causing limitation increased gradually with age, to peak in subjects aged 50-69 years, decreasing thereafter.
In a logistic regression analysis, adults with eczema had a 44% increased risk of any injury causing limitation (adjusted odds ratio, 1.44), and a 67% increased risk of FBJI (aOR, 1.67).
Adults with eczema who also reported experiencing fatigue (aOR, 1.59; 95% CI, 1.16-2.19), daytime sleepiness (aOR, 1.81), or insomnia (aOR, 1.74) had higher rates of FBJI than individuals with sleep symptoms but no eczema. Adults with both eczema and psychiatric or behavioral disorders also were more likely to experience FBJI than individuals suffering from eczema alone, or individuals suffering from psychiatric and behavioral disorders, but not eczema.
“Taken together, these data suggest that adult eczema is a previously unrecognized risk factor for fracture and other injury, emphasizing the importance of developing safer and more effective clinical interventions for itch and sleep problems in eczema, as well as preventive measures for injury risk reduction in eczema,” wrote Dr. Garg and Dr. Silverberg. The authors reported no relevant financial disclosures.
FROM JAMA DERMATOLOGY
Adults with eczema face increased risk of fracture and bone or joint injuries
Eczema in adulthood is directly associated with an increase in incidence of injuries that cause physical limitations, particularly fracture, bone, and joint injuries, according to the results of a prospective study published online in JAMA Dermatology.
“The risk of bone fracture and other injury causing limitation in adults with eczema has been largely unexplored,” noted Dr. Nitin Garg and Dr. Jonathan I. Silverberg of Northwestern University in Chicago.
“Patients with eczema may be at risk of fracture given their use of systemic corticosteroids, which may decrease bone mineral density [and] therefore, studies describing the prevalence of fracture among adults with eczema are needed,” they added (JAMA Dermatol. 2015;151:33-41 [doi:10.1001/jamadermatol.2014.2098]).
Dr. Garg and Dr. Silverberg used data from the 2012 National Health Interview Survey, which was conducted by the National Center for Health Statistics, to create their prospective, questionnaire-based study. The final analysis included 34,500 adults aged 18-85 years. They controlled for factors such as age, sex, race, Hispanic origin, household income, highest level of household education, family structure, and whether or not the subject was born in the United States.
The prevalence of self-reported eczema or skin allergy was 7.2%, the prevalence of any limitation-causing injury was 2.0%, and any limitation-causing injury in the past 12 months among adults was 7.2%. A fracture and bone or joint injury (FBJI) causing limitation was reported by 1.5% of the study population and other types of injury causing limitation were reported by 0.6%. The prevalence of FBJI causing limitation increased gradually with age, to peak in subjects aged 50-69 years, decreasing thereafter.
In a logistic regression analysis, adults with eczema had a 44% increased risk of any injury causing limitation (adjusted odds ratio, 1.44), and a 67% increased risk of FBJI (aOR, 1.67).
Adults with eczema who also reported experiencing fatigue (aOR, 1.59; 95% CI, 1.16-2.19), daytime sleepiness (aOR, 1.81), or insomnia (aOR, 1.74) had higher rates of FBJI than individuals with sleep symptoms but no eczema. Adults with both eczema and psychiatric or behavioral disorders also were more likely to experience FBJI than individuals suffering from eczema alone, or individuals suffering from psychiatric and behavioral disorders, but not eczema.
“Taken together, these data suggest that adult eczema is a previously unrecognized risk factor for fracture and other injury, emphasizing the importance of developing safer and more effective clinical interventions for itch and sleep problems in eczema, as well as preventive measures for injury risk reduction in eczema,” wrote Dr. Garg and Dr. Silverberg. The authors reported no relevant financial disclosures.
Eczema in adulthood is directly associated with an increase in incidence of injuries that cause physical limitations, particularly fracture, bone, and joint injuries, according to the results of a prospective study published online in JAMA Dermatology.
“The risk of bone fracture and other injury causing limitation in adults with eczema has been largely unexplored,” noted Dr. Nitin Garg and Dr. Jonathan I. Silverberg of Northwestern University in Chicago.
“Patients with eczema may be at risk of fracture given their use of systemic corticosteroids, which may decrease bone mineral density [and] therefore, studies describing the prevalence of fracture among adults with eczema are needed,” they added (JAMA Dermatol. 2015;151:33-41 [doi:10.1001/jamadermatol.2014.2098]).
Dr. Garg and Dr. Silverberg used data from the 2012 National Health Interview Survey, which was conducted by the National Center for Health Statistics, to create their prospective, questionnaire-based study. The final analysis included 34,500 adults aged 18-85 years. They controlled for factors such as age, sex, race, Hispanic origin, household income, highest level of household education, family structure, and whether or not the subject was born in the United States.
The prevalence of self-reported eczema or skin allergy was 7.2%, the prevalence of any limitation-causing injury was 2.0%, and any limitation-causing injury in the past 12 months among adults was 7.2%. A fracture and bone or joint injury (FBJI) causing limitation was reported by 1.5% of the study population and other types of injury causing limitation were reported by 0.6%. The prevalence of FBJI causing limitation increased gradually with age, to peak in subjects aged 50-69 years, decreasing thereafter.
In a logistic regression analysis, adults with eczema had a 44% increased risk of any injury causing limitation (adjusted odds ratio, 1.44), and a 67% increased risk of FBJI (aOR, 1.67).
Adults with eczema who also reported experiencing fatigue (aOR, 1.59; 95% CI, 1.16-2.19), daytime sleepiness (aOR, 1.81), or insomnia (aOR, 1.74) had higher rates of FBJI than individuals with sleep symptoms but no eczema. Adults with both eczema and psychiatric or behavioral disorders also were more likely to experience FBJI than individuals suffering from eczema alone, or individuals suffering from psychiatric and behavioral disorders, but not eczema.
“Taken together, these data suggest that adult eczema is a previously unrecognized risk factor for fracture and other injury, emphasizing the importance of developing safer and more effective clinical interventions for itch and sleep problems in eczema, as well as preventive measures for injury risk reduction in eczema,” wrote Dr. Garg and Dr. Silverberg. The authors reported no relevant financial disclosures.
Eczema in adulthood is directly associated with an increase in incidence of injuries that cause physical limitations, particularly fracture, bone, and joint injuries, according to the results of a prospective study published online in JAMA Dermatology.
“The risk of bone fracture and other injury causing limitation in adults with eczema has been largely unexplored,” noted Dr. Nitin Garg and Dr. Jonathan I. Silverberg of Northwestern University in Chicago.
“Patients with eczema may be at risk of fracture given their use of systemic corticosteroids, which may decrease bone mineral density [and] therefore, studies describing the prevalence of fracture among adults with eczema are needed,” they added (JAMA Dermatol. 2015;151:33-41 [doi:10.1001/jamadermatol.2014.2098]).
Dr. Garg and Dr. Silverberg used data from the 2012 National Health Interview Survey, which was conducted by the National Center for Health Statistics, to create their prospective, questionnaire-based study. The final analysis included 34,500 adults aged 18-85 years. They controlled for factors such as age, sex, race, Hispanic origin, household income, highest level of household education, family structure, and whether or not the subject was born in the United States.
The prevalence of self-reported eczema or skin allergy was 7.2%, the prevalence of any limitation-causing injury was 2.0%, and any limitation-causing injury in the past 12 months among adults was 7.2%. A fracture and bone or joint injury (FBJI) causing limitation was reported by 1.5% of the study population and other types of injury causing limitation were reported by 0.6%. The prevalence of FBJI causing limitation increased gradually with age, to peak in subjects aged 50-69 years, decreasing thereafter.
In a logistic regression analysis, adults with eczema had a 44% increased risk of any injury causing limitation (adjusted odds ratio, 1.44), and a 67% increased risk of FBJI (aOR, 1.67).
Adults with eczema who also reported experiencing fatigue (aOR, 1.59; 95% CI, 1.16-2.19), daytime sleepiness (aOR, 1.81), or insomnia (aOR, 1.74) had higher rates of FBJI than individuals with sleep symptoms but no eczema. Adults with both eczema and psychiatric or behavioral disorders also were more likely to experience FBJI than individuals suffering from eczema alone, or individuals suffering from psychiatric and behavioral disorders, but not eczema.
“Taken together, these data suggest that adult eczema is a previously unrecognized risk factor for fracture and other injury, emphasizing the importance of developing safer and more effective clinical interventions for itch and sleep problems in eczema, as well as preventive measures for injury risk reduction in eczema,” wrote Dr. Garg and Dr. Silverberg. The authors reported no relevant financial disclosures.
FROM JAMA DERMATOLOGY
Key clinical point: Eczema in adulthood is associated with an increased risk of limitation-causing injuries.
Major finding: Adults with eczema had a 67% increased risk of fracture and bone or joint injury (FBJI) causing limitation (aOR 1.67).
Data source: Prospective, questionnaire-based, cohort study of 34,500 adults aged 18-85 years from the 2012 National Health Interview Survey.
Disclosures: The authors reported no financial conflicts of interest.
Psychosocial Factors in Childhood Influence Cardiovascular Health in Adulthood
Cardiovascular health in adulthood is directly related to favorable psychosocial factors in youth, according to the results of a study conducted using a new metrics system developed by the American Heart Association.
“Childhood and youth are important stages of life because cardiovascular diseases are rooted in early life and social determinants of health start to accumulate in childhood,” wrote lead author Laura Pulkki-Råback, Ph.D., of Finland’s University of Helsinki, and her associates (Circulation 2015 [doi:10.1161/CIRCULATIONAHA.113.007104]), adding that “release of the AHA 2020 Impact Goals makes it critical to examine all aspects, including psychosocial factors that may help in [their] attainment,” which includes “expanded emphasis on prevention and greater understanding of the origins of cardiovascular disease.”
Dr. Pulkki-Råback and her coinvestigators enrolled 477 males and 612 females from a nationwide randomized, cohort study, “Cardiovascular Risk in Young Finns.” They were between the ages of 3 and 18 years. Subjects were measured for psychosocial factors – such as socioeconomic factors, emotional family environment, parental health behaviors, stressful events, self-regulatory behavior, and social adjustment – at baseline, and ideal cardiovascular health was measured in adulthood 27 years afterward. Participants were divided into cohorts based on age (young vs. old), and by age and sex. On average, subjects were aged 10 years at baseline and 37 years during the adult measurement phase.
According to the findings of Dr. Pulkki-Råback and associates, subjects’ average score on the ideal cardiovascular health index in adulthood was 2.6. Total favorable psychosocial factors were found to directly associate with ideal cardiovascular health in adulthood: when adjusted for age, sex, and medication use, r = 0.16 (P < .001); adding childhood cardiovascular risk factors, r = 0.15 (P < .001). Multinomial regression analyses that show when favorable psychosocial factors rose by one point, the probability of having 2, 3, 4, 5, or at least 6 ideal cardiovascular health metrics rose by 6%, 14%, 17%, 17%, and 35%, compared with having 1 or fewer ideal cardiovascular health metrics, respectively.
It was discovered that socioeconomic status was associated with healthier behavior of subjects’ parents (r = 0.10, P < .001). Emotional family environment was correlated with healthy behavior of the parents (r = 0.16, P < .001), higher likelihood of self-regulatory behavior as a child (r = 0.12, P < .001), and better social adjustment as a child (r = 0.10, P < .001).
Favorable psychosocial factors score were more strongly associated with leaner body mass index (odds ratio = 1.14, P < .001), not being a smoker (OR = 1.12, P < .001), and better glucose levels (OR = 1.11, P < .001).
“We found that psychosocial factors operated across the whole gradient of ideal cardiovascular health,” wrote the investigators. “There was no evidence for any threshold point after which the effect of psychosocial factors would become unimportant. This may suggest wider scope for prevention than has previously been considered, because all individuals may benefit from improvements in early-life conditions, not only those at the bottom of the gradient. In combination with prior work, this evidence begins to suggest that even improving a single factor would likely result in better future cardiovascular health.”
Funding for the study was provided by grants from the Academy of Finland and numerous other Finnish organizations. The authors reported no conflicts of interest.
Cardiovascular health in adulthood is directly related to favorable psychosocial factors in youth, according to the results of a study conducted using a new metrics system developed by the American Heart Association.
“Childhood and youth are important stages of life because cardiovascular diseases are rooted in early life and social determinants of health start to accumulate in childhood,” wrote lead author Laura Pulkki-Råback, Ph.D., of Finland’s University of Helsinki, and her associates (Circulation 2015 [doi:10.1161/CIRCULATIONAHA.113.007104]), adding that “release of the AHA 2020 Impact Goals makes it critical to examine all aspects, including psychosocial factors that may help in [their] attainment,” which includes “expanded emphasis on prevention and greater understanding of the origins of cardiovascular disease.”
Dr. Pulkki-Råback and her coinvestigators enrolled 477 males and 612 females from a nationwide randomized, cohort study, “Cardiovascular Risk in Young Finns.” They were between the ages of 3 and 18 years. Subjects were measured for psychosocial factors – such as socioeconomic factors, emotional family environment, parental health behaviors, stressful events, self-regulatory behavior, and social adjustment – at baseline, and ideal cardiovascular health was measured in adulthood 27 years afterward. Participants were divided into cohorts based on age (young vs. old), and by age and sex. On average, subjects were aged 10 years at baseline and 37 years during the adult measurement phase.
According to the findings of Dr. Pulkki-Råback and associates, subjects’ average score on the ideal cardiovascular health index in adulthood was 2.6. Total favorable psychosocial factors were found to directly associate with ideal cardiovascular health in adulthood: when adjusted for age, sex, and medication use, r = 0.16 (P < .001); adding childhood cardiovascular risk factors, r = 0.15 (P < .001). Multinomial regression analyses that show when favorable psychosocial factors rose by one point, the probability of having 2, 3, 4, 5, or at least 6 ideal cardiovascular health metrics rose by 6%, 14%, 17%, 17%, and 35%, compared with having 1 or fewer ideal cardiovascular health metrics, respectively.
It was discovered that socioeconomic status was associated with healthier behavior of subjects’ parents (r = 0.10, P < .001). Emotional family environment was correlated with healthy behavior of the parents (r = 0.16, P < .001), higher likelihood of self-regulatory behavior as a child (r = 0.12, P < .001), and better social adjustment as a child (r = 0.10, P < .001).
Favorable psychosocial factors score were more strongly associated with leaner body mass index (odds ratio = 1.14, P < .001), not being a smoker (OR = 1.12, P < .001), and better glucose levels (OR = 1.11, P < .001).
“We found that psychosocial factors operated across the whole gradient of ideal cardiovascular health,” wrote the investigators. “There was no evidence for any threshold point after which the effect of psychosocial factors would become unimportant. This may suggest wider scope for prevention than has previously been considered, because all individuals may benefit from improvements in early-life conditions, not only those at the bottom of the gradient. In combination with prior work, this evidence begins to suggest that even improving a single factor would likely result in better future cardiovascular health.”
Funding for the study was provided by grants from the Academy of Finland and numerous other Finnish organizations. The authors reported no conflicts of interest.
Cardiovascular health in adulthood is directly related to favorable psychosocial factors in youth, according to the results of a study conducted using a new metrics system developed by the American Heart Association.
“Childhood and youth are important stages of life because cardiovascular diseases are rooted in early life and social determinants of health start to accumulate in childhood,” wrote lead author Laura Pulkki-Råback, Ph.D., of Finland’s University of Helsinki, and her associates (Circulation 2015 [doi:10.1161/CIRCULATIONAHA.113.007104]), adding that “release of the AHA 2020 Impact Goals makes it critical to examine all aspects, including psychosocial factors that may help in [their] attainment,” which includes “expanded emphasis on prevention and greater understanding of the origins of cardiovascular disease.”
Dr. Pulkki-Råback and her coinvestigators enrolled 477 males and 612 females from a nationwide randomized, cohort study, “Cardiovascular Risk in Young Finns.” They were between the ages of 3 and 18 years. Subjects were measured for psychosocial factors – such as socioeconomic factors, emotional family environment, parental health behaviors, stressful events, self-regulatory behavior, and social adjustment – at baseline, and ideal cardiovascular health was measured in adulthood 27 years afterward. Participants were divided into cohorts based on age (young vs. old), and by age and sex. On average, subjects were aged 10 years at baseline and 37 years during the adult measurement phase.
According to the findings of Dr. Pulkki-Råback and associates, subjects’ average score on the ideal cardiovascular health index in adulthood was 2.6. Total favorable psychosocial factors were found to directly associate with ideal cardiovascular health in adulthood: when adjusted for age, sex, and medication use, r = 0.16 (P < .001); adding childhood cardiovascular risk factors, r = 0.15 (P < .001). Multinomial regression analyses that show when favorable psychosocial factors rose by one point, the probability of having 2, 3, 4, 5, or at least 6 ideal cardiovascular health metrics rose by 6%, 14%, 17%, 17%, and 35%, compared with having 1 or fewer ideal cardiovascular health metrics, respectively.
It was discovered that socioeconomic status was associated with healthier behavior of subjects’ parents (r = 0.10, P < .001). Emotional family environment was correlated with healthy behavior of the parents (r = 0.16, P < .001), higher likelihood of self-regulatory behavior as a child (r = 0.12, P < .001), and better social adjustment as a child (r = 0.10, P < .001).
Favorable psychosocial factors score were more strongly associated with leaner body mass index (odds ratio = 1.14, P < .001), not being a smoker (OR = 1.12, P < .001), and better glucose levels (OR = 1.11, P < .001).
“We found that psychosocial factors operated across the whole gradient of ideal cardiovascular health,” wrote the investigators. “There was no evidence for any threshold point after which the effect of psychosocial factors would become unimportant. This may suggest wider scope for prevention than has previously been considered, because all individuals may benefit from improvements in early-life conditions, not only those at the bottom of the gradient. In combination with prior work, this evidence begins to suggest that even improving a single factor would likely result in better future cardiovascular health.”
Funding for the study was provided by grants from the Academy of Finland and numerous other Finnish organizations. The authors reported no conflicts of interest.
FROM CIRCULATION
Psychosocial factors in childhood influence cardiovascular health in adulthood
Cardiovascular health in adulthood is directly related to favorable psychosocial factors in youth, according to the results of a study conducted using a new metrics system developed by the American Heart Association.
“Childhood and youth are important stages of life because cardiovascular diseases are rooted in early life and social determinants of health start to accumulate in childhood,” wrote lead author Laura Pulkki-Råback, Ph.D., of Finland’s University of Helsinki, and her associates (Circulation 2015 [doi:10.1161/CIRCULATIONAHA.113.007104]), adding that “release of the AHA 2020 Impact Goals makes it critical to examine all aspects, including psychosocial factors that may help in [their] attainment,” which includes “expanded emphasis on prevention and greater understanding of the origins of cardiovascular disease.”
Dr. Pulkki-Råback and her coinvestigators enrolled 477 males and 612 females from a nationwide randomized, cohort study, “Cardiovascular Risk in Young Finns.” They were between the ages of 3 and 18 years. Subjects were measured for psychosocial factors – such as socioeconomic factors, emotional family environment, parental health behaviors, stressful events, self-regulatory behavior, and social adjustment – at baseline, and ideal cardiovascular health was measured in adulthood 27 years afterward. Participants were divided into cohorts based on age (young vs. old), and by age and sex. On average, subjects were aged 10 years at baseline and 37 years during the adult measurement phase.
According to the findings of Dr. Pulkki-Råback and associates, subjects’ average score on the ideal cardiovascular health index in adulthood was 2.6. Total favorable psychosocial factors were found to directly associate with ideal cardiovascular health in adulthood: when adjusted for age, sex, and medication use, r = 0.16 (P < .001); adding childhood cardiovascular risk factors, r = 0.15 (P < .001). Multinomial regression analyses that show when favorable psychosocial factors rose by one point, the probability of having 2, 3, 4, 5, or at least 6 ideal cardiovascular health metrics rose by 6%, 14%, 17%, 17%, and 35%, compared with having 1 or fewer ideal cardiovascular health metrics, respectively.
It was discovered that socioeconomic status was associated with healthier behavior of subjects’ parents (r = 0.10, P < .001). Emotional family environment was correlated with healthy behavior of the parents (r = 0.16, P < .001), higher likelihood of self-regulatory behavior as a child (r = 0.12, P < .001), and better social adjustment as a child (r = 0.10, P < .001).
Favorable psychosocial factors score were more strongly associated with leaner body mass index (odds ratio = 1.14, P < .001), not being a smoker (OR = 1.12, P < .001), and better glucose levels (OR = 1.11, P < .001).
“We found that psychosocial factors operated across the whole gradient of ideal cardiovascular health,” wrote the investigators. “There was no evidence for any threshold point after which the effect of psychosocial factors would become unimportant. This may suggest wider scope for prevention than has previously been considered, because all individuals may benefit from improvements in early-life conditions, not only those at the bottom of the gradient. In combination with prior work, this evidence begins to suggest that even improving a single factor would likely result in better future cardiovascular health.”
Funding for the study was provided by grants from the Academy of Finland and numerous other Finnish organizations. The authors reported no conflicts of interest.
Cardiovascular health in adulthood is directly related to favorable psychosocial factors in youth, according to the results of a study conducted using a new metrics system developed by the American Heart Association.
“Childhood and youth are important stages of life because cardiovascular diseases are rooted in early life and social determinants of health start to accumulate in childhood,” wrote lead author Laura Pulkki-Råback, Ph.D., of Finland’s University of Helsinki, and her associates (Circulation 2015 [doi:10.1161/CIRCULATIONAHA.113.007104]), adding that “release of the AHA 2020 Impact Goals makes it critical to examine all aspects, including psychosocial factors that may help in [their] attainment,” which includes “expanded emphasis on prevention and greater understanding of the origins of cardiovascular disease.”
Dr. Pulkki-Råback and her coinvestigators enrolled 477 males and 612 females from a nationwide randomized, cohort study, “Cardiovascular Risk in Young Finns.” They were between the ages of 3 and 18 years. Subjects were measured for psychosocial factors – such as socioeconomic factors, emotional family environment, parental health behaviors, stressful events, self-regulatory behavior, and social adjustment – at baseline, and ideal cardiovascular health was measured in adulthood 27 years afterward. Participants were divided into cohorts based on age (young vs. old), and by age and sex. On average, subjects were aged 10 years at baseline and 37 years during the adult measurement phase.
According to the findings of Dr. Pulkki-Råback and associates, subjects’ average score on the ideal cardiovascular health index in adulthood was 2.6. Total favorable psychosocial factors were found to directly associate with ideal cardiovascular health in adulthood: when adjusted for age, sex, and medication use, r = 0.16 (P < .001); adding childhood cardiovascular risk factors, r = 0.15 (P < .001). Multinomial regression analyses that show when favorable psychosocial factors rose by one point, the probability of having 2, 3, 4, 5, or at least 6 ideal cardiovascular health metrics rose by 6%, 14%, 17%, 17%, and 35%, compared with having 1 or fewer ideal cardiovascular health metrics, respectively.
It was discovered that socioeconomic status was associated with healthier behavior of subjects’ parents (r = 0.10, P < .001). Emotional family environment was correlated with healthy behavior of the parents (r = 0.16, P < .001), higher likelihood of self-regulatory behavior as a child (r = 0.12, P < .001), and better social adjustment as a child (r = 0.10, P < .001).
Favorable psychosocial factors score were more strongly associated with leaner body mass index (odds ratio = 1.14, P < .001), not being a smoker (OR = 1.12, P < .001), and better glucose levels (OR = 1.11, P < .001).
“We found that psychosocial factors operated across the whole gradient of ideal cardiovascular health,” wrote the investigators. “There was no evidence for any threshold point after which the effect of psychosocial factors would become unimportant. This may suggest wider scope for prevention than has previously been considered, because all individuals may benefit from improvements in early-life conditions, not only those at the bottom of the gradient. In combination with prior work, this evidence begins to suggest that even improving a single factor would likely result in better future cardiovascular health.”
Funding for the study was provided by grants from the Academy of Finland and numerous other Finnish organizations. The authors reported no conflicts of interest.
Cardiovascular health in adulthood is directly related to favorable psychosocial factors in youth, according to the results of a study conducted using a new metrics system developed by the American Heart Association.
“Childhood and youth are important stages of life because cardiovascular diseases are rooted in early life and social determinants of health start to accumulate in childhood,” wrote lead author Laura Pulkki-Råback, Ph.D., of Finland’s University of Helsinki, and her associates (Circulation 2015 [doi:10.1161/CIRCULATIONAHA.113.007104]), adding that “release of the AHA 2020 Impact Goals makes it critical to examine all aspects, including psychosocial factors that may help in [their] attainment,” which includes “expanded emphasis on prevention and greater understanding of the origins of cardiovascular disease.”
Dr. Pulkki-Råback and her coinvestigators enrolled 477 males and 612 females from a nationwide randomized, cohort study, “Cardiovascular Risk in Young Finns.” They were between the ages of 3 and 18 years. Subjects were measured for psychosocial factors – such as socioeconomic factors, emotional family environment, parental health behaviors, stressful events, self-regulatory behavior, and social adjustment – at baseline, and ideal cardiovascular health was measured in adulthood 27 years afterward. Participants were divided into cohorts based on age (young vs. old), and by age and sex. On average, subjects were aged 10 years at baseline and 37 years during the adult measurement phase.
According to the findings of Dr. Pulkki-Råback and associates, subjects’ average score on the ideal cardiovascular health index in adulthood was 2.6. Total favorable psychosocial factors were found to directly associate with ideal cardiovascular health in adulthood: when adjusted for age, sex, and medication use, r = 0.16 (P < .001); adding childhood cardiovascular risk factors, r = 0.15 (P < .001). Multinomial regression analyses that show when favorable psychosocial factors rose by one point, the probability of having 2, 3, 4, 5, or at least 6 ideal cardiovascular health metrics rose by 6%, 14%, 17%, 17%, and 35%, compared with having 1 or fewer ideal cardiovascular health metrics, respectively.
It was discovered that socioeconomic status was associated with healthier behavior of subjects’ parents (r = 0.10, P < .001). Emotional family environment was correlated with healthy behavior of the parents (r = 0.16, P < .001), higher likelihood of self-regulatory behavior as a child (r = 0.12, P < .001), and better social adjustment as a child (r = 0.10, P < .001).
Favorable psychosocial factors score were more strongly associated with leaner body mass index (odds ratio = 1.14, P < .001), not being a smoker (OR = 1.12, P < .001), and better glucose levels (OR = 1.11, P < .001).
“We found that psychosocial factors operated across the whole gradient of ideal cardiovascular health,” wrote the investigators. “There was no evidence for any threshold point after which the effect of psychosocial factors would become unimportant. This may suggest wider scope for prevention than has previously been considered, because all individuals may benefit from improvements in early-life conditions, not only those at the bottom of the gradient. In combination with prior work, this evidence begins to suggest that even improving a single factor would likely result in better future cardiovascular health.”
Funding for the study was provided by grants from the Academy of Finland and numerous other Finnish organizations. The authors reported no conflicts of interest.
FROM CIRCULATION
Key clinical point: Cardiovascular health in adulthood is directly related to favorable psychosocial factors in youth, according to a new metrics system developed by the American Heart Association.
Major finding: Total favorable psychosocial factors were found to directly associate with ideal cardiovascular health in adulthood: when adjusted for age, sex, and medication use, r = 0.16 (P < .001); adding childhood cardiovascular risk factors, r = 0.15 (P < .001).
Data source: A nationwide randomized, cohort study, “Cardiovascular Risk in Young Finns,” that enrolled 477 males and 612 females, all between the ages of 3 and 18 years. They were reevaluated 27 years later.
Disclosures: Funding for the study was provided by grants from the Academy of Finland and numerous other Finnish organizations. The authors reported no conflicts of interest.
Adalimumab effective in nonpsoriatic peripheral spondyloarthritis
Adalimumab alleviated symptoms and improved physical functions in certain patients with active nonpsoriatic peripheral spondyloarthritis in the first 12 weeks of treatment in the ongoing ABILITY-2 trial.
The findings of the trial, which investigators called the “first global, multicenter, randomized, controlled trial” in patients with nonpsoriatic peripheral spondyloarthritis (SpA), add to the growing list of evidence that tumor necrosis factor inhibitors are effective in treating spondyloarthritis (Arthritis Rheumatol. 2014 Dec. 29 [doi:10.1002/art.39008]).
The randomized, double-blind study examined 165 patients aged at least 18 years with active, nonpsoriatic peripheral SpA. The patients met Assessment of Spondyloarthritis International Society (ASAS) peripheral SpA criteria rather than the “older” European Spondyloarthropathy Study Group (ESSG) criteria and had inadequate response to at least two nonsteroidal anti-inflammatory drugs (NSAIDs) or were intolerant to or had a contraindication for NSAIDs. These subjects were placed into cohorts receiving either 40 mg of adalimumab or a placebo for 12 weeks followed by a 144-week-long open-label period. After randomization, 84 subjects were placed on the adalimumab regimen and the remaining 81 were given a placebo. All patients will enter an open-label, 144-week treatment period on adalimumab, according to lead author Dr. Philip Mease of the University of Washington, Seattle, and his coauthors.
The investigators reported that the groups had similar baseline demographics, including 52%-57% being female and mean ages of 38.5-42.5 years, and disease characteristics, including mean symptom duration of 6.6-7.7 years and time since diagnosis of 3.0-4.2 years.
The investigators used a novel primary outcome measurement that required at least 40% improvement in Patient Global Assessments of Disease Activity (PGA) and Pain (PGA-pain) scores from baseline (with at least 20 mm absolute improvement on a visual analog scale) and at least 40% improvement in one or more of the following areas: swollen joint (SJC) and tender joint counts (TJC); enthesitis count; or dactylitis count. Subjects who achieved the primary endpoint of peripheral SpA response criteria were noted as PSpARC 40; individuals who reached other levels of improvement – 20%, 50%, and 70%, with at least 10, 20, or 30 mm absolute improvement, respectively – were noted as PSpARC 20, PSpARC 50, and PSpARC 70, respectively.
After 12 weeks of treatment, nearly twice as many adalimumab-treated patients achieved PSpARC 40 as did placebo-treated patients (39% [33 of 84] vs. 20% [16 of 81]; P = .006). Also, the proportions of patients who achieved PSpARC 20, PSpARC 50, and PSpARC 70 were higher in subjects on adalimumab than in those receiving the placebo: 56% vs. 37% (P < .05), 34% vs. 11% (P < .001), and 22% vs. 3% (P < .001), respectively.
Significant differences between the two treatments were noted by investigators as early as week 2 of the trial. In subjects who achieved PSpARC 40, investigators recorded a minimum 40% improvement and at least 20-mm improvement in the PGA (54% adalimumab vs. 29% placebo; P < .001) and PGA-pain (54% adalimumab vs. 31% placebo; P = .004), and at least 40% improvement in TJC/SJC (57% adalimumab vs. 30% placebo; P < .001). Adverse effects between both cohorts were not significantly different.
“The safety profile of adalimumab in this patient population was consistent with the well-established safety of adalimumab in multiple immune-mediated diseases, [but] longer-term data are needed to confirm and support the favorable benefit-risk profile of adalimumab in nonpsoriatic peripheral SpA observed in this study,” Dr. Mease and his associates concluded.
In an interview, Dr. Grant Louie of Johns Hopkins Arthritis Center in Baltimore, who was not involved in the study, noted that the results are significant because the investigators used such a “rigorously controlled environment” and a more relevant classification system for diagnosing patients.
“When using the ASAS criteria for classifying peripheral spondyloarthritis, it introduces a little more heterogeneity into the patient population,” said Dr. Louie. “This new system incorporates what used to be referred to as undifferentiated spondyloarthritis as well as patients who have reactive arthritis [and] some with inflammatory bowel disease-associated arthritis, while excluding patients who have psoriatic arthritis and ankylosing spondylitis. So a big advantage is that you can incorporate some of these patients earlier in the process so they can undergo the appropriate treatments.”
However, Dr. Louie explained that he had reservations with how the study measured its primary outcome and how clinicians and researchers will be able to use these data without having an externally validated endpoint.
“The biggest caveat – which the authors themselves do mention in the paper, to their credit – is that they’re introducing a new primary study endpoint,” Dr. Louie said. “This composite score that they’re using, which takes into account factors like tender and swollen joints, has not been validated in any external study. Without knowing if the primary endpoint has validity and reliability, I am a little skeptical.”
Dr. Dafna A. Gladman of the University of Toronto shared some of Dr. Louie’s skepticism of the primary endpoint, but credits the investigators with creating a measure that appears to have “construct validity, as well as sensitivity to change.”
In Dr. Gladman’s editorial (Arthritis Rheumatol. 2014 Dec. 29 [doi:10.1002/art.39014]), she noted that “the development of the measure is not described. Was it developed through a Delphi process? Has it shown face and construct validity in peripheral SpA? Has it shown test retest reliability? What we do know is that this measure distinguished between drug and placebo treated patients, [that] the majority of the response was related to the patient reported outcome which was a prerequisite and to the joint count item, [and] that the individual measures tested in this study showed the same results. One could then conclude that the new measure has construct validity, as well as sensitivity to change and thus would be acceptable as the primary outcome measure in this study.”
The ABILITY-2 study was funded by AbbVie, which markets adalimumab. Two authors are employees of the company. All other authors reported receiving grant/research support, consulting fees, and/or speaking fees from AbbVie and other companies that market biologics.
Adalimumab alleviated symptoms and improved physical functions in certain patients with active nonpsoriatic peripheral spondyloarthritis in the first 12 weeks of treatment in the ongoing ABILITY-2 trial.
The findings of the trial, which investigators called the “first global, multicenter, randomized, controlled trial” in patients with nonpsoriatic peripheral spondyloarthritis (SpA), add to the growing list of evidence that tumor necrosis factor inhibitors are effective in treating spondyloarthritis (Arthritis Rheumatol. 2014 Dec. 29 [doi:10.1002/art.39008]).
The randomized, double-blind study examined 165 patients aged at least 18 years with active, nonpsoriatic peripheral SpA. The patients met Assessment of Spondyloarthritis International Society (ASAS) peripheral SpA criteria rather than the “older” European Spondyloarthropathy Study Group (ESSG) criteria and had inadequate response to at least two nonsteroidal anti-inflammatory drugs (NSAIDs) or were intolerant to or had a contraindication for NSAIDs. These subjects were placed into cohorts receiving either 40 mg of adalimumab or a placebo for 12 weeks followed by a 144-week-long open-label period. After randomization, 84 subjects were placed on the adalimumab regimen and the remaining 81 were given a placebo. All patients will enter an open-label, 144-week treatment period on adalimumab, according to lead author Dr. Philip Mease of the University of Washington, Seattle, and his coauthors.
The investigators reported that the groups had similar baseline demographics, including 52%-57% being female and mean ages of 38.5-42.5 years, and disease characteristics, including mean symptom duration of 6.6-7.7 years and time since diagnosis of 3.0-4.2 years.
The investigators used a novel primary outcome measurement that required at least 40% improvement in Patient Global Assessments of Disease Activity (PGA) and Pain (PGA-pain) scores from baseline (with at least 20 mm absolute improvement on a visual analog scale) and at least 40% improvement in one or more of the following areas: swollen joint (SJC) and tender joint counts (TJC); enthesitis count; or dactylitis count. Subjects who achieved the primary endpoint of peripheral SpA response criteria were noted as PSpARC 40; individuals who reached other levels of improvement – 20%, 50%, and 70%, with at least 10, 20, or 30 mm absolute improvement, respectively – were noted as PSpARC 20, PSpARC 50, and PSpARC 70, respectively.
After 12 weeks of treatment, nearly twice as many adalimumab-treated patients achieved PSpARC 40 as did placebo-treated patients (39% [33 of 84] vs. 20% [16 of 81]; P = .006). Also, the proportions of patients who achieved PSpARC 20, PSpARC 50, and PSpARC 70 were higher in subjects on adalimumab than in those receiving the placebo: 56% vs. 37% (P < .05), 34% vs. 11% (P < .001), and 22% vs. 3% (P < .001), respectively.
Significant differences between the two treatments were noted by investigators as early as week 2 of the trial. In subjects who achieved PSpARC 40, investigators recorded a minimum 40% improvement and at least 20-mm improvement in the PGA (54% adalimumab vs. 29% placebo; P < .001) and PGA-pain (54% adalimumab vs. 31% placebo; P = .004), and at least 40% improvement in TJC/SJC (57% adalimumab vs. 30% placebo; P < .001). Adverse effects between both cohorts were not significantly different.
“The safety profile of adalimumab in this patient population was consistent with the well-established safety of adalimumab in multiple immune-mediated diseases, [but] longer-term data are needed to confirm and support the favorable benefit-risk profile of adalimumab in nonpsoriatic peripheral SpA observed in this study,” Dr. Mease and his associates concluded.
In an interview, Dr. Grant Louie of Johns Hopkins Arthritis Center in Baltimore, who was not involved in the study, noted that the results are significant because the investigators used such a “rigorously controlled environment” and a more relevant classification system for diagnosing patients.
“When using the ASAS criteria for classifying peripheral spondyloarthritis, it introduces a little more heterogeneity into the patient population,” said Dr. Louie. “This new system incorporates what used to be referred to as undifferentiated spondyloarthritis as well as patients who have reactive arthritis [and] some with inflammatory bowel disease-associated arthritis, while excluding patients who have psoriatic arthritis and ankylosing spondylitis. So a big advantage is that you can incorporate some of these patients earlier in the process so they can undergo the appropriate treatments.”
However, Dr. Louie explained that he had reservations with how the study measured its primary outcome and how clinicians and researchers will be able to use these data without having an externally validated endpoint.
“The biggest caveat – which the authors themselves do mention in the paper, to their credit – is that they’re introducing a new primary study endpoint,” Dr. Louie said. “This composite score that they’re using, which takes into account factors like tender and swollen joints, has not been validated in any external study. Without knowing if the primary endpoint has validity and reliability, I am a little skeptical.”
Dr. Dafna A. Gladman of the University of Toronto shared some of Dr. Louie’s skepticism of the primary endpoint, but credits the investigators with creating a measure that appears to have “construct validity, as well as sensitivity to change.”
In Dr. Gladman’s editorial (Arthritis Rheumatol. 2014 Dec. 29 [doi:10.1002/art.39014]), she noted that “the development of the measure is not described. Was it developed through a Delphi process? Has it shown face and construct validity in peripheral SpA? Has it shown test retest reliability? What we do know is that this measure distinguished between drug and placebo treated patients, [that] the majority of the response was related to the patient reported outcome which was a prerequisite and to the joint count item, [and] that the individual measures tested in this study showed the same results. One could then conclude that the new measure has construct validity, as well as sensitivity to change and thus would be acceptable as the primary outcome measure in this study.”
The ABILITY-2 study was funded by AbbVie, which markets adalimumab. Two authors are employees of the company. All other authors reported receiving grant/research support, consulting fees, and/or speaking fees from AbbVie and other companies that market biologics.
Adalimumab alleviated symptoms and improved physical functions in certain patients with active nonpsoriatic peripheral spondyloarthritis in the first 12 weeks of treatment in the ongoing ABILITY-2 trial.
The findings of the trial, which investigators called the “first global, multicenter, randomized, controlled trial” in patients with nonpsoriatic peripheral spondyloarthritis (SpA), add to the growing list of evidence that tumor necrosis factor inhibitors are effective in treating spondyloarthritis (Arthritis Rheumatol. 2014 Dec. 29 [doi:10.1002/art.39008]).
The randomized, double-blind study examined 165 patients aged at least 18 years with active, nonpsoriatic peripheral SpA. The patients met Assessment of Spondyloarthritis International Society (ASAS) peripheral SpA criteria rather than the “older” European Spondyloarthropathy Study Group (ESSG) criteria and had inadequate response to at least two nonsteroidal anti-inflammatory drugs (NSAIDs) or were intolerant to or had a contraindication for NSAIDs. These subjects were placed into cohorts receiving either 40 mg of adalimumab or a placebo for 12 weeks followed by a 144-week-long open-label period. After randomization, 84 subjects were placed on the adalimumab regimen and the remaining 81 were given a placebo. All patients will enter an open-label, 144-week treatment period on adalimumab, according to lead author Dr. Philip Mease of the University of Washington, Seattle, and his coauthors.
The investigators reported that the groups had similar baseline demographics, including 52%-57% being female and mean ages of 38.5-42.5 years, and disease characteristics, including mean symptom duration of 6.6-7.7 years and time since diagnosis of 3.0-4.2 years.
The investigators used a novel primary outcome measurement that required at least 40% improvement in Patient Global Assessments of Disease Activity (PGA) and Pain (PGA-pain) scores from baseline (with at least 20 mm absolute improvement on a visual analog scale) and at least 40% improvement in one or more of the following areas: swollen joint (SJC) and tender joint counts (TJC); enthesitis count; or dactylitis count. Subjects who achieved the primary endpoint of peripheral SpA response criteria were noted as PSpARC 40; individuals who reached other levels of improvement – 20%, 50%, and 70%, with at least 10, 20, or 30 mm absolute improvement, respectively – were noted as PSpARC 20, PSpARC 50, and PSpARC 70, respectively.
After 12 weeks of treatment, nearly twice as many adalimumab-treated patients achieved PSpARC 40 as did placebo-treated patients (39% [33 of 84] vs. 20% [16 of 81]; P = .006). Also, the proportions of patients who achieved PSpARC 20, PSpARC 50, and PSpARC 70 were higher in subjects on adalimumab than in those receiving the placebo: 56% vs. 37% (P < .05), 34% vs. 11% (P < .001), and 22% vs. 3% (P < .001), respectively.
Significant differences between the two treatments were noted by investigators as early as week 2 of the trial. In subjects who achieved PSpARC 40, investigators recorded a minimum 40% improvement and at least 20-mm improvement in the PGA (54% adalimumab vs. 29% placebo; P < .001) and PGA-pain (54% adalimumab vs. 31% placebo; P = .004), and at least 40% improvement in TJC/SJC (57% adalimumab vs. 30% placebo; P < .001). Adverse effects between both cohorts were not significantly different.
“The safety profile of adalimumab in this patient population was consistent with the well-established safety of adalimumab in multiple immune-mediated diseases, [but] longer-term data are needed to confirm and support the favorable benefit-risk profile of adalimumab in nonpsoriatic peripheral SpA observed in this study,” Dr. Mease and his associates concluded.
In an interview, Dr. Grant Louie of Johns Hopkins Arthritis Center in Baltimore, who was not involved in the study, noted that the results are significant because the investigators used such a “rigorously controlled environment” and a more relevant classification system for diagnosing patients.
“When using the ASAS criteria for classifying peripheral spondyloarthritis, it introduces a little more heterogeneity into the patient population,” said Dr. Louie. “This new system incorporates what used to be referred to as undifferentiated spondyloarthritis as well as patients who have reactive arthritis [and] some with inflammatory bowel disease-associated arthritis, while excluding patients who have psoriatic arthritis and ankylosing spondylitis. So a big advantage is that you can incorporate some of these patients earlier in the process so they can undergo the appropriate treatments.”
However, Dr. Louie explained that he had reservations with how the study measured its primary outcome and how clinicians and researchers will be able to use these data without having an externally validated endpoint.
“The biggest caveat – which the authors themselves do mention in the paper, to their credit – is that they’re introducing a new primary study endpoint,” Dr. Louie said. “This composite score that they’re using, which takes into account factors like tender and swollen joints, has not been validated in any external study. Without knowing if the primary endpoint has validity and reliability, I am a little skeptical.”
Dr. Dafna A. Gladman of the University of Toronto shared some of Dr. Louie’s skepticism of the primary endpoint, but credits the investigators with creating a measure that appears to have “construct validity, as well as sensitivity to change.”
In Dr. Gladman’s editorial (Arthritis Rheumatol. 2014 Dec. 29 [doi:10.1002/art.39014]), she noted that “the development of the measure is not described. Was it developed through a Delphi process? Has it shown face and construct validity in peripheral SpA? Has it shown test retest reliability? What we do know is that this measure distinguished between drug and placebo treated patients, [that] the majority of the response was related to the patient reported outcome which was a prerequisite and to the joint count item, [and] that the individual measures tested in this study showed the same results. One could then conclude that the new measure has construct validity, as well as sensitivity to change and thus would be acceptable as the primary outcome measure in this study.”
The ABILITY-2 study was funded by AbbVie, which markets adalimumab. Two authors are employees of the company. All other authors reported receiving grant/research support, consulting fees, and/or speaking fees from AbbVie and other companies that market biologics.
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point: Patients with active nonpsoriatic peripheral spondyloarthritis who have not responded to treatment with NSAIDs might consider treatment with adalimumab.
Major finding: After 12 weeks, significantly more subjects in the adalimumab cohort achieved the primary outcome of at least 40% improvement in peripheral SpA response criteria than did those receiving placebo: 39% vs. 20%, respectively (P = .006).
Data source: The randomized, double-blind ABILITY-2 trial of 165 patients with active nonpsoriatic peripheral spondyloarthritis.
Disclosures: The trial was funded by AbbVie, which markets adalimumab. Two authors are employees of the company. All other authors reported ties with AbbVie and other companies that market biologics.
Efinaconazole Topical Solution Effective Against Early Onychomycosis
Patients with early onychomycosis can be effectively treated with once-daily efinaconazole topical solution, 10%, according to data from more than 1,000 patients.
“Early treatment before the disease progresses to total dystrophic onychomycosis can increase cure rate and may avoid the need for systemic treatments,” wrote study author Dr. Phoebe Rich, a dermatologist in group practice in Portland, Ore.
The study used two identical multicenter, randomized, double-blind, vehicle-controlled trials. Of 1,655 subjects, 1,526 underwent randomization, and subjects were categorized based on disease duration at baseline. A total of 74 subjects had onychomycosis for less than 1 year, 682 for 1-5 years, and 770 for more than 5 years. The primary endpoint was complete cure rate – defined as 0% clinical involvement of target toenail, and both negative results on a potassium hydroxide examination and a fungal culture – after 52 weeks (J. Drugs Dermatol. 2015;14:58-62).
After 52 weeks, 42.6% of patients with a baseline disease duration of less than 1 year had a complete cure with efinaconazole, compared with 16.7% on a vehicle (control). In the 1- to 5-year group, 17.1% of patients with a baseline disease duration had a complete cure with efinaconazole, compared with 4.4% on vehicle (P < .001); in the group of subjects with a disease duration longer than 5 years, 16.2% of patients had a complete cure on efinaconazole, compared with 2.5% on vehicle (P < .001).
Additionally, 28.3% of patients with no other toenails affected at baseline had a complete cure with efinaconazole, compared with 23.7% on vehicle; 18.6% of patients with one to two nontarget toenails affected at baseline had a complete cure with efinaconazole, compared with 4.9% on vehicle (P < .001); and 16.5% of patients with more than two nontarget toenails affected at baseline had a complete cure on efinaconazole, compared with 0.1% on vehicle (P < .001).
“The availability of an effective topical treatment for onychomycosis could encourage earlier treatment and prevent the progression of the disease, where it would become more difficult to treat successfully,” said Dr. Rich, who also cautioned that the study’s 52-week length “may be too brief to evaluate a clinical cure in onychomycosis.”
Dr. Rich has served as a paid consultant to Valeant Pharmaceuticals and was a principal investigator in the pivotal trials with efinaconazole topical solution, 10%.
Patients with early onychomycosis can be effectively treated with once-daily efinaconazole topical solution, 10%, according to data from more than 1,000 patients.
“Early treatment before the disease progresses to total dystrophic onychomycosis can increase cure rate and may avoid the need for systemic treatments,” wrote study author Dr. Phoebe Rich, a dermatologist in group practice in Portland, Ore.
The study used two identical multicenter, randomized, double-blind, vehicle-controlled trials. Of 1,655 subjects, 1,526 underwent randomization, and subjects were categorized based on disease duration at baseline. A total of 74 subjects had onychomycosis for less than 1 year, 682 for 1-5 years, and 770 for more than 5 years. The primary endpoint was complete cure rate – defined as 0% clinical involvement of target toenail, and both negative results on a potassium hydroxide examination and a fungal culture – after 52 weeks (J. Drugs Dermatol. 2015;14:58-62).
After 52 weeks, 42.6% of patients with a baseline disease duration of less than 1 year had a complete cure with efinaconazole, compared with 16.7% on a vehicle (control). In the 1- to 5-year group, 17.1% of patients with a baseline disease duration had a complete cure with efinaconazole, compared with 4.4% on vehicle (P < .001); in the group of subjects with a disease duration longer than 5 years, 16.2% of patients had a complete cure on efinaconazole, compared with 2.5% on vehicle (P < .001).
Additionally, 28.3% of patients with no other toenails affected at baseline had a complete cure with efinaconazole, compared with 23.7% on vehicle; 18.6% of patients with one to two nontarget toenails affected at baseline had a complete cure with efinaconazole, compared with 4.9% on vehicle (P < .001); and 16.5% of patients with more than two nontarget toenails affected at baseline had a complete cure on efinaconazole, compared with 0.1% on vehicle (P < .001).
“The availability of an effective topical treatment for onychomycosis could encourage earlier treatment and prevent the progression of the disease, where it would become more difficult to treat successfully,” said Dr. Rich, who also cautioned that the study’s 52-week length “may be too brief to evaluate a clinical cure in onychomycosis.”
Dr. Rich has served as a paid consultant to Valeant Pharmaceuticals and was a principal investigator in the pivotal trials with efinaconazole topical solution, 10%.
Patients with early onychomycosis can be effectively treated with once-daily efinaconazole topical solution, 10%, according to data from more than 1,000 patients.
“Early treatment before the disease progresses to total dystrophic onychomycosis can increase cure rate and may avoid the need for systemic treatments,” wrote study author Dr. Phoebe Rich, a dermatologist in group practice in Portland, Ore.
The study used two identical multicenter, randomized, double-blind, vehicle-controlled trials. Of 1,655 subjects, 1,526 underwent randomization, and subjects were categorized based on disease duration at baseline. A total of 74 subjects had onychomycosis for less than 1 year, 682 for 1-5 years, and 770 for more than 5 years. The primary endpoint was complete cure rate – defined as 0% clinical involvement of target toenail, and both negative results on a potassium hydroxide examination and a fungal culture – after 52 weeks (J. Drugs Dermatol. 2015;14:58-62).
After 52 weeks, 42.6% of patients with a baseline disease duration of less than 1 year had a complete cure with efinaconazole, compared with 16.7% on a vehicle (control). In the 1- to 5-year group, 17.1% of patients with a baseline disease duration had a complete cure with efinaconazole, compared with 4.4% on vehicle (P < .001); in the group of subjects with a disease duration longer than 5 years, 16.2% of patients had a complete cure on efinaconazole, compared with 2.5% on vehicle (P < .001).
Additionally, 28.3% of patients with no other toenails affected at baseline had a complete cure with efinaconazole, compared with 23.7% on vehicle; 18.6% of patients with one to two nontarget toenails affected at baseline had a complete cure with efinaconazole, compared with 4.9% on vehicle (P < .001); and 16.5% of patients with more than two nontarget toenails affected at baseline had a complete cure on efinaconazole, compared with 0.1% on vehicle (P < .001).
“The availability of an effective topical treatment for onychomycosis could encourage earlier treatment and prevent the progression of the disease, where it would become more difficult to treat successfully,” said Dr. Rich, who also cautioned that the study’s 52-week length “may be too brief to evaluate a clinical cure in onychomycosis.”
Dr. Rich has served as a paid consultant to Valeant Pharmaceuticals and was a principal investigator in the pivotal trials with efinaconazole topical solution, 10%.
FROM THE JOURNAL OF DRUGS IN DERMATOLOGY