User login
Doug Brunk is a San Diego-based award-winning reporter who began covering health care in 1991. Before joining the company, he wrote for the health sciences division of Columbia University and was an associate editor at Contemporary Long Term Care magazine when it won a Jesse H. Neal Award. His work has been syndicated by the Los Angeles Times and he is the author of two books related to the University of Kentucky Wildcats men's basketball program. Doug has a master’s degree in magazine journalism from the S.I. Newhouse School of Public Communications at Syracuse University. Follow him on Twitter @dougbrunk.
Expert Shares Tips for Optimizing Facelifts
LAS VEGAS – Tripling the concentration of epinephrine in tumescent anesthesia used during facelift procedures has its benefits, according to Joseph Niamtu III, D.M.D.
His preferred solution consists of 1 L normal saline, 1 g lidocaine, and 3 mL epinephrine 1:1,000. "Most clinicians use 1 mL of epinephrine 1:1,000," he said at the annual meeting of the American Academy of Cosmetic Surgery. Using a higher concentration of epinephrine "certainly has faster onset of branching, it’s more robust branching, and the branching lasts longer," said Dr. Niamtu, who has a cosmetic facial surgery practice in Midlothian, Va.
"Over the years, I’ve shaved these procedures down from 4 hours to a little over 2 hours, and this is one of the things that have helped. You get a higher level of pain control, and I’ve not had any disadvantages – no discernible changes in blood pressure or epinephrine-related problems."
Dr. Niamtu also finds that facelift results can be optimized without using bulky dressings. "Patients hate dressings," he said. "They don’t prevent hematoma but they do prevent visualization of the flap. They can constrict the flap and lead to breakdown, and they abrade laser tissue."
Of the 71 facelift procedures he performed in 2011, 45% were done with simultaneous CO2 laser. "The problem was, overnight, the dressings would abrade the freshly lasered skin," he explained. "It’s problematic because the treatment site would take longer to heal and sometimes would start to form a scar." He started decreasing his use of facelift dressings "until I just didn’t use any at all." Now, he said, "it’s certainly easy to promote facelifts [with this approach]. There’s less trepidation when patients know they don’t have to wear these bulky dressings after their surgery."
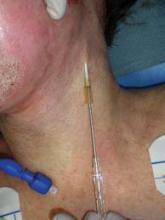
The need for postoperative drains to promote the release of serum, tumescence, and blood products can be another drawback to facelifts. However, Dr. Niamtu devised a solution: a "vent" system that consists of a 14-gauge Angiocath needle placed subcutaneously in the lowest part of the flap, parallel to the sternocleidomastoid muscle, and left overnight on the day of surgery. "These will drain," he said. "Most of these patients are sitting up in a recliner or have their head propped up on pillows the night of their surgery, so they really drain. It’s secured with a mesh elastic gauze and gauze fluffs. We give the patient a lot of these fluffs, because they have to change the dressing overnight, sometimes two or three times. We use these for 24 hours after surgery."
With this approach "I think I’m seeing less bruising, my patients have a tighter neck, and they’re really not bothered by the vent," he said.
In a later interview, Dr. Niamtu said that he is not a fan of short scar facelifts because "I think it is appropriate for only the smallest lifts, and most people – even those in their early 40s – would benefit from a more comprehensive lift. I think there is a trend to do anything to avoid a 4-inch postauricular incision, [but I] feel that without this incision, short scar lifts are flawed.
"It is this posterior incision that allows true tightening of the neck, and eliminating it causes bunching behind the ear as well as shortchanges [the patient] in terms of result and longevity. I am not saying there is never a place for short scar lifts, as there is, but I have seen too much relapse or compromise in results for many patients [who] got a small lift and in reality needed a bigger one."
Dr. Niamtu said that he had no relevant financial conflicts to disclose.
LAS VEGAS – Tripling the concentration of epinephrine in tumescent anesthesia used during facelift procedures has its benefits, according to Joseph Niamtu III, D.M.D.
His preferred solution consists of 1 L normal saline, 1 g lidocaine, and 3 mL epinephrine 1:1,000. "Most clinicians use 1 mL of epinephrine 1:1,000," he said at the annual meeting of the American Academy of Cosmetic Surgery. Using a higher concentration of epinephrine "certainly has faster onset of branching, it’s more robust branching, and the branching lasts longer," said Dr. Niamtu, who has a cosmetic facial surgery practice in Midlothian, Va.
"Over the years, I’ve shaved these procedures down from 4 hours to a little over 2 hours, and this is one of the things that have helped. You get a higher level of pain control, and I’ve not had any disadvantages – no discernible changes in blood pressure or epinephrine-related problems."
Dr. Niamtu also finds that facelift results can be optimized without using bulky dressings. "Patients hate dressings," he said. "They don’t prevent hematoma but they do prevent visualization of the flap. They can constrict the flap and lead to breakdown, and they abrade laser tissue."
Of the 71 facelift procedures he performed in 2011, 45% were done with simultaneous CO2 laser. "The problem was, overnight, the dressings would abrade the freshly lasered skin," he explained. "It’s problematic because the treatment site would take longer to heal and sometimes would start to form a scar." He started decreasing his use of facelift dressings "until I just didn’t use any at all." Now, he said, "it’s certainly easy to promote facelifts [with this approach]. There’s less trepidation when patients know they don’t have to wear these bulky dressings after their surgery."
The need for postoperative drains to promote the release of serum, tumescence, and blood products can be another drawback to facelifts. However, Dr. Niamtu devised a solution: a "vent" system that consists of a 14-gauge Angiocath needle placed subcutaneously in the lowest part of the flap, parallel to the sternocleidomastoid muscle, and left overnight on the day of surgery. "These will drain," he said. "Most of these patients are sitting up in a recliner or have their head propped up on pillows the night of their surgery, so they really drain. It’s secured with a mesh elastic gauze and gauze fluffs. We give the patient a lot of these fluffs, because they have to change the dressing overnight, sometimes two or three times. We use these for 24 hours after surgery."
With this approach "I think I’m seeing less bruising, my patients have a tighter neck, and they’re really not bothered by the vent," he said.
In a later interview, Dr. Niamtu said that he is not a fan of short scar facelifts because "I think it is appropriate for only the smallest lifts, and most people – even those in their early 40s – would benefit from a more comprehensive lift. I think there is a trend to do anything to avoid a 4-inch postauricular incision, [but I] feel that without this incision, short scar lifts are flawed.
"It is this posterior incision that allows true tightening of the neck, and eliminating it causes bunching behind the ear as well as shortchanges [the patient] in terms of result and longevity. I am not saying there is never a place for short scar lifts, as there is, but I have seen too much relapse or compromise in results for many patients [who] got a small lift and in reality needed a bigger one."
Dr. Niamtu said that he had no relevant financial conflicts to disclose.
LAS VEGAS – Tripling the concentration of epinephrine in tumescent anesthesia used during facelift procedures has its benefits, according to Joseph Niamtu III, D.M.D.
His preferred solution consists of 1 L normal saline, 1 g lidocaine, and 3 mL epinephrine 1:1,000. "Most clinicians use 1 mL of epinephrine 1:1,000," he said at the annual meeting of the American Academy of Cosmetic Surgery. Using a higher concentration of epinephrine "certainly has faster onset of branching, it’s more robust branching, and the branching lasts longer," said Dr. Niamtu, who has a cosmetic facial surgery practice in Midlothian, Va.
"Over the years, I’ve shaved these procedures down from 4 hours to a little over 2 hours, and this is one of the things that have helped. You get a higher level of pain control, and I’ve not had any disadvantages – no discernible changes in blood pressure or epinephrine-related problems."
Dr. Niamtu also finds that facelift results can be optimized without using bulky dressings. "Patients hate dressings," he said. "They don’t prevent hematoma but they do prevent visualization of the flap. They can constrict the flap and lead to breakdown, and they abrade laser tissue."
Of the 71 facelift procedures he performed in 2011, 45% were done with simultaneous CO2 laser. "The problem was, overnight, the dressings would abrade the freshly lasered skin," he explained. "It’s problematic because the treatment site would take longer to heal and sometimes would start to form a scar." He started decreasing his use of facelift dressings "until I just didn’t use any at all." Now, he said, "it’s certainly easy to promote facelifts [with this approach]. There’s less trepidation when patients know they don’t have to wear these bulky dressings after their surgery."
The need for postoperative drains to promote the release of serum, tumescence, and blood products can be another drawback to facelifts. However, Dr. Niamtu devised a solution: a "vent" system that consists of a 14-gauge Angiocath needle placed subcutaneously in the lowest part of the flap, parallel to the sternocleidomastoid muscle, and left overnight on the day of surgery. "These will drain," he said. "Most of these patients are sitting up in a recliner or have their head propped up on pillows the night of their surgery, so they really drain. It’s secured with a mesh elastic gauze and gauze fluffs. We give the patient a lot of these fluffs, because they have to change the dressing overnight, sometimes two or three times. We use these for 24 hours after surgery."
With this approach "I think I’m seeing less bruising, my patients have a tighter neck, and they’re really not bothered by the vent," he said.
In a later interview, Dr. Niamtu said that he is not a fan of short scar facelifts because "I think it is appropriate for only the smallest lifts, and most people – even those in their early 40s – would benefit from a more comprehensive lift. I think there is a trend to do anything to avoid a 4-inch postauricular incision, [but I] feel that without this incision, short scar lifts are flawed.
"It is this posterior incision that allows true tightening of the neck, and eliminating it causes bunching behind the ear as well as shortchanges [the patient] in terms of result and longevity. I am not saying there is never a place for short scar lifts, as there is, but I have seen too much relapse or compromise in results for many patients [who] got a small lift and in reality needed a bigger one."
Dr. Niamtu said that he had no relevant financial conflicts to disclose.
EXPERT ANALYSIS FROM THE ANNUAL MEETING OF THE AMERICAN ACADEMY OF COSMETIC SURGERY
Tips to Securing the Postconsult Patient
LAS VEGAS – Taking time to prescreen men and women who inquire about cosmetic surgery procedures can help maximize their chances of choosing your practice over others, and can help you weed out those who aren’t suitable candidates.
"There is no chance in securing a patient without having a consultation," Tracie Lance, director of finance for Southern Surgical Arts, said at the annual meeting of the American Academy of Cosmetic Surgery. "In order to avoid wasting the patient’s time, the physician’s time, and the staff’s time, we do some prescreening."
The first step, she said, is to find a time on the schedule that is most appropriate for the consultation. If someone inquires about breast augmentation, "you probably want to get them in as quickly as possible, to reduce the chances of their going to another practice," said Ms. Lance, also a patient care coordinator for the practice, which has locations in Chattanooga, Tenn., and Calhoun, Ga. If they inquire about a facelift, there’s less of an urgency to get them in right away, she said, because "they’ve probably thought about it a long time before they picked up the phone to call your office. If you respond, ‘We can get you in this afternoon,’ you may not give the best first impression of your office."
A second component of prescreening is gauging the person’s ability to pay for or finance the procedure. "This is nonnegotiable," Ms. Lance said. "In our experience, we have found that it can be unfair to set unrealistic expectations that all patients will qualify for financing. The frustration can be avoided by having patients apply before they come to the consultation." Consider options such as, "We have someone on staff that can help you with financing. Is that something you’re interested in?" and document the reply for the in-person consultation. "Being able to assist our patient in successful financing is a skill that has set our practice above others," she said.
A third component of the prescreening is asking people to complete a health history questionnaire before the in-person consultation. "There might be something like body mass index that [precludes them from] surgery," she noted.
Consultation appointments are complimentary at Southern Surgical Arts, yet a credit card is required to hold the appointment. "We have a 5% no-show rate, which is pretty low compared to the industry standard," Ms. Lance said.
To maximize the patient experience, physicians should be mindful of the appearance of their waiting room and reception area. "People pay attention to detail, so the office should be clean, with minimal clutter," she said. "In terms of patient flow, after our patients check in we try to get them into an exam room as quickly as possible with an iPad that contains before and after photos and patient testimonials."
After candidate patients at Southern Surgical Arts meet with the cosmetic surgeon in the exam room, they move to a dedicated postconsultation room, where the patient coordinator will review the estimated cost of the procedure and answer questions. Establishing a dedicated postconsultation room "is one of the best things we’ve ever done," Ms. Lance said. She described the room as a "warm environment," equipped with "a round table, MacBook Air, a wireless printer, and a phone."
Patients commonly cite fear, financing, timing, and multiple consultations as objections to cosmetic surgery procedures, Ms. Lance said, noting that financing is the biggest obstacle. "If you have a facelift patient, sometimes it’s easier to offer a no-interest plan, as the bill can be around $16,000," she said. "That’s up to each office, but in my experience, the no-interest plan has been extremely successful. Some patients do not use no-interest financing, but I hear facelift patients tell me all the time, ‘Oh my husband will love this,’ because they don’t feel like they’re taking all this money out of savings at once. Payments of $1,100 or $1,200 per month seem easier."
In her experience, patients considering cosmetic surgery for the first time "are nervous and they tell you they’re going to multiple consultations," Ms. Lance added. "I’m not sure that’s always the case. I think that they’re just nervous. It helps to say, ‘we understand that you’re nervous. If you get home and you have questions, don’t hesitate to call us.’ Sometimes it helps for them to hear about another patient’s experience with surgery. We have patients who will do that for us, which is wonderful."
She concluded her remarks by advising patient care coordinators and office staff to offer candidate patients a certain amount of grace and space as they make their decision. "I do not believe in being pushy in a consultation," she said. "I’ve seen that backfire many times; 65% of our patients will give me a deposit on the day that they come in, which is remarkable. But at the same time you have to know when to give someone space. You want to provide the benefits of the surgeons, the benefits of the facility, but not in a pushy way. You want a patient who is just as committed as you are. You want them to be compliant in their care and you want them to follow the rules."
Ms. Lance reported having no relevant financial disclosures.
LAS VEGAS – Taking time to prescreen men and women who inquire about cosmetic surgery procedures can help maximize their chances of choosing your practice over others, and can help you weed out those who aren’t suitable candidates.
"There is no chance in securing a patient without having a consultation," Tracie Lance, director of finance for Southern Surgical Arts, said at the annual meeting of the American Academy of Cosmetic Surgery. "In order to avoid wasting the patient’s time, the physician’s time, and the staff’s time, we do some prescreening."
The first step, she said, is to find a time on the schedule that is most appropriate for the consultation. If someone inquires about breast augmentation, "you probably want to get them in as quickly as possible, to reduce the chances of their going to another practice," said Ms. Lance, also a patient care coordinator for the practice, which has locations in Chattanooga, Tenn., and Calhoun, Ga. If they inquire about a facelift, there’s less of an urgency to get them in right away, she said, because "they’ve probably thought about it a long time before they picked up the phone to call your office. If you respond, ‘We can get you in this afternoon,’ you may not give the best first impression of your office."
A second component of prescreening is gauging the person’s ability to pay for or finance the procedure. "This is nonnegotiable," Ms. Lance said. "In our experience, we have found that it can be unfair to set unrealistic expectations that all patients will qualify for financing. The frustration can be avoided by having patients apply before they come to the consultation." Consider options such as, "We have someone on staff that can help you with financing. Is that something you’re interested in?" and document the reply for the in-person consultation. "Being able to assist our patient in successful financing is a skill that has set our practice above others," she said.
A third component of the prescreening is asking people to complete a health history questionnaire before the in-person consultation. "There might be something like body mass index that [precludes them from] surgery," she noted.
Consultation appointments are complimentary at Southern Surgical Arts, yet a credit card is required to hold the appointment. "We have a 5% no-show rate, which is pretty low compared to the industry standard," Ms. Lance said.
To maximize the patient experience, physicians should be mindful of the appearance of their waiting room and reception area. "People pay attention to detail, so the office should be clean, with minimal clutter," she said. "In terms of patient flow, after our patients check in we try to get them into an exam room as quickly as possible with an iPad that contains before and after photos and patient testimonials."
After candidate patients at Southern Surgical Arts meet with the cosmetic surgeon in the exam room, they move to a dedicated postconsultation room, where the patient coordinator will review the estimated cost of the procedure and answer questions. Establishing a dedicated postconsultation room "is one of the best things we’ve ever done," Ms. Lance said. She described the room as a "warm environment," equipped with "a round table, MacBook Air, a wireless printer, and a phone."
Patients commonly cite fear, financing, timing, and multiple consultations as objections to cosmetic surgery procedures, Ms. Lance said, noting that financing is the biggest obstacle. "If you have a facelift patient, sometimes it’s easier to offer a no-interest plan, as the bill can be around $16,000," she said. "That’s up to each office, but in my experience, the no-interest plan has been extremely successful. Some patients do not use no-interest financing, but I hear facelift patients tell me all the time, ‘Oh my husband will love this,’ because they don’t feel like they’re taking all this money out of savings at once. Payments of $1,100 or $1,200 per month seem easier."
In her experience, patients considering cosmetic surgery for the first time "are nervous and they tell you they’re going to multiple consultations," Ms. Lance added. "I’m not sure that’s always the case. I think that they’re just nervous. It helps to say, ‘we understand that you’re nervous. If you get home and you have questions, don’t hesitate to call us.’ Sometimes it helps for them to hear about another patient’s experience with surgery. We have patients who will do that for us, which is wonderful."
She concluded her remarks by advising patient care coordinators and office staff to offer candidate patients a certain amount of grace and space as they make their decision. "I do not believe in being pushy in a consultation," she said. "I’ve seen that backfire many times; 65% of our patients will give me a deposit on the day that they come in, which is remarkable. But at the same time you have to know when to give someone space. You want to provide the benefits of the surgeons, the benefits of the facility, but not in a pushy way. You want a patient who is just as committed as you are. You want them to be compliant in their care and you want them to follow the rules."
Ms. Lance reported having no relevant financial disclosures.
LAS VEGAS – Taking time to prescreen men and women who inquire about cosmetic surgery procedures can help maximize their chances of choosing your practice over others, and can help you weed out those who aren’t suitable candidates.
"There is no chance in securing a patient without having a consultation," Tracie Lance, director of finance for Southern Surgical Arts, said at the annual meeting of the American Academy of Cosmetic Surgery. "In order to avoid wasting the patient’s time, the physician’s time, and the staff’s time, we do some prescreening."
The first step, she said, is to find a time on the schedule that is most appropriate for the consultation. If someone inquires about breast augmentation, "you probably want to get them in as quickly as possible, to reduce the chances of their going to another practice," said Ms. Lance, also a patient care coordinator for the practice, which has locations in Chattanooga, Tenn., and Calhoun, Ga. If they inquire about a facelift, there’s less of an urgency to get them in right away, she said, because "they’ve probably thought about it a long time before they picked up the phone to call your office. If you respond, ‘We can get you in this afternoon,’ you may not give the best first impression of your office."
A second component of prescreening is gauging the person’s ability to pay for or finance the procedure. "This is nonnegotiable," Ms. Lance said. "In our experience, we have found that it can be unfair to set unrealistic expectations that all patients will qualify for financing. The frustration can be avoided by having patients apply before they come to the consultation." Consider options such as, "We have someone on staff that can help you with financing. Is that something you’re interested in?" and document the reply for the in-person consultation. "Being able to assist our patient in successful financing is a skill that has set our practice above others," she said.
A third component of the prescreening is asking people to complete a health history questionnaire before the in-person consultation. "There might be something like body mass index that [precludes them from] surgery," she noted.
Consultation appointments are complimentary at Southern Surgical Arts, yet a credit card is required to hold the appointment. "We have a 5% no-show rate, which is pretty low compared to the industry standard," Ms. Lance said.
To maximize the patient experience, physicians should be mindful of the appearance of their waiting room and reception area. "People pay attention to detail, so the office should be clean, with minimal clutter," she said. "In terms of patient flow, after our patients check in we try to get them into an exam room as quickly as possible with an iPad that contains before and after photos and patient testimonials."
After candidate patients at Southern Surgical Arts meet with the cosmetic surgeon in the exam room, they move to a dedicated postconsultation room, where the patient coordinator will review the estimated cost of the procedure and answer questions. Establishing a dedicated postconsultation room "is one of the best things we’ve ever done," Ms. Lance said. She described the room as a "warm environment," equipped with "a round table, MacBook Air, a wireless printer, and a phone."
Patients commonly cite fear, financing, timing, and multiple consultations as objections to cosmetic surgery procedures, Ms. Lance said, noting that financing is the biggest obstacle. "If you have a facelift patient, sometimes it’s easier to offer a no-interest plan, as the bill can be around $16,000," she said. "That’s up to each office, but in my experience, the no-interest plan has been extremely successful. Some patients do not use no-interest financing, but I hear facelift patients tell me all the time, ‘Oh my husband will love this,’ because they don’t feel like they’re taking all this money out of savings at once. Payments of $1,100 or $1,200 per month seem easier."
In her experience, patients considering cosmetic surgery for the first time "are nervous and they tell you they’re going to multiple consultations," Ms. Lance added. "I’m not sure that’s always the case. I think that they’re just nervous. It helps to say, ‘we understand that you’re nervous. If you get home and you have questions, don’t hesitate to call us.’ Sometimes it helps for them to hear about another patient’s experience with surgery. We have patients who will do that for us, which is wonderful."
She concluded her remarks by advising patient care coordinators and office staff to offer candidate patients a certain amount of grace and space as they make their decision. "I do not believe in being pushy in a consultation," she said. "I’ve seen that backfire many times; 65% of our patients will give me a deposit on the day that they come in, which is remarkable. But at the same time you have to know when to give someone space. You want to provide the benefits of the surgeons, the benefits of the facility, but not in a pushy way. You want a patient who is just as committed as you are. You want them to be compliant in their care and you want them to follow the rules."
Ms. Lance reported having no relevant financial disclosures.
EXPERT ANALYSIS FROM THE ANNUAL MEETING OF THE AMERICAN ACADEMY OF COSMETIC SURGERY
Fat Transplantation's Mechanism of Action Remains Elusive
LAS VEGAS – Even though transplantation of fat harvested by cannulas has been performed for almost 3 decades, its precise mechanism of action remains elusive.
"One must admit that so far lipostructuring still has to do more with art than with science," Dr. Giovanni Botti said at the annual meeting of the American Academy of Cosmetic Surgery. In many respects, "we haven’t yet come out of the Middle Ages of subjective and empirical opinions."
Despite the relative lack of objective data to support its use, Dr. Botti, a plastic surgeon based in Lake Garda, Italy, has been performing fat transplantation for 25 years. In his opinion, the procedure "can certainly be considered a therapy of first choice in the treatment of soft tissue hypotrophy, as well as for the correction of tropism disorders such as radionecrosis and burns. It is not clear, though, how to obtain consistently positive and long-lasting results."
This begs the question, he continued: If you were to biopsy the area where fat had been injected 1 year earlier, would you be looking at the same fat that was injected, or is it a brand new pad "rebuilt" by the stem cells and modulated by the growing factors present in the grafted material? If the latter hypothesis is true, "how can the stem cells in the grafted fat promote the growth of exactly the wished for amount of fat?" Dr. Botti said. "Why should it take the desired shape? Could the mass on injected tissue serve as a temporary matrix, used by stem cells as a pattern to form their ‘fat net’?"
He speculated that the fat found after 1 year could be composed of fat that was originally injected, as well as stem cells. The stem cells "promote angiogenesis, which would help adipocytes to survive. We can nowadays only make hypotheses that need to be confirmed by further research."
In the meantime, what really matters is achieving the maximum taking rate during fat transplantation, he said. "Very satisfying" results can be achieved in volume restoration and soft tissue regeneration.
The best treatment for the harvested fat prior to injection remains a matter of debate. Recent research suggests that adding stem cells, insulin, the coenzyme Q-10, and platelet rich plasma may favor survival rates, "though no one has yet been able to provide any evidence," he said. "For sure, stem cells can enhance the local blood supply and release growth factors to help the healing process. Thus, theoretically, the graft survival rate is improved. For this reason nowadays regenerative cell enriched fat is increasingly used within various indications."
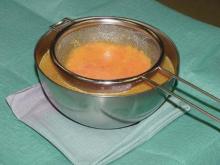
Beginning in 1985, Dr. Botti treated aspirated fat by "washing," decantation, and strainer filtration. Fifteen years later he switched to using a centrifuge, but after about 1 year of using it, "I realized I wasn’t getting any better results than by means of filtration," he said. "I therefore went back to my previous technique."
In 2007, Dr. Botti and his associates carried out a study of 32 patients undergoing fat transplantation in the face. They injected one side of the face with centrifuged fat, and the other side with filtrate fat. The patients were observed at postoperative day 10 and after 2 and 6 months. "We didn’t notice any difference between the side into which filtered fat was injected and [the side] treated with centrifuged fat," he said. "Therefore, we came to the conclusion that the way fat is treated does not affect the taking rate, assuming that the ‘cleaning’ was in all cases delicate and complete. And I am deeply sorry for those, like me, who have spent a few thousand euros to buy a centrifuge."
He noted that Cytori Therapeutics’ PureGraft sterile plastic bag is a promising new tool for preparing fat prior to transplantation. It is a closed system which allows clinicians to manually separate fat tissue from blood, saline, and other materials. "We will be able to judge its effectiveness in a couple of years," he said.
Dr. Botti said that he had no relevant financial disclosures to make.
LAS VEGAS – Even though transplantation of fat harvested by cannulas has been performed for almost 3 decades, its precise mechanism of action remains elusive.
"One must admit that so far lipostructuring still has to do more with art than with science," Dr. Giovanni Botti said at the annual meeting of the American Academy of Cosmetic Surgery. In many respects, "we haven’t yet come out of the Middle Ages of subjective and empirical opinions."
Despite the relative lack of objective data to support its use, Dr. Botti, a plastic surgeon based in Lake Garda, Italy, has been performing fat transplantation for 25 years. In his opinion, the procedure "can certainly be considered a therapy of first choice in the treatment of soft tissue hypotrophy, as well as for the correction of tropism disorders such as radionecrosis and burns. It is not clear, though, how to obtain consistently positive and long-lasting results."
This begs the question, he continued: If you were to biopsy the area where fat had been injected 1 year earlier, would you be looking at the same fat that was injected, or is it a brand new pad "rebuilt" by the stem cells and modulated by the growing factors present in the grafted material? If the latter hypothesis is true, "how can the stem cells in the grafted fat promote the growth of exactly the wished for amount of fat?" Dr. Botti said. "Why should it take the desired shape? Could the mass on injected tissue serve as a temporary matrix, used by stem cells as a pattern to form their ‘fat net’?"
He speculated that the fat found after 1 year could be composed of fat that was originally injected, as well as stem cells. The stem cells "promote angiogenesis, which would help adipocytes to survive. We can nowadays only make hypotheses that need to be confirmed by further research."
In the meantime, what really matters is achieving the maximum taking rate during fat transplantation, he said. "Very satisfying" results can be achieved in volume restoration and soft tissue regeneration.
The best treatment for the harvested fat prior to injection remains a matter of debate. Recent research suggests that adding stem cells, insulin, the coenzyme Q-10, and platelet rich plasma may favor survival rates, "though no one has yet been able to provide any evidence," he said. "For sure, stem cells can enhance the local blood supply and release growth factors to help the healing process. Thus, theoretically, the graft survival rate is improved. For this reason nowadays regenerative cell enriched fat is increasingly used within various indications."
Beginning in 1985, Dr. Botti treated aspirated fat by "washing," decantation, and strainer filtration. Fifteen years later he switched to using a centrifuge, but after about 1 year of using it, "I realized I wasn’t getting any better results than by means of filtration," he said. "I therefore went back to my previous technique."
In 2007, Dr. Botti and his associates carried out a study of 32 patients undergoing fat transplantation in the face. They injected one side of the face with centrifuged fat, and the other side with filtrate fat. The patients were observed at postoperative day 10 and after 2 and 6 months. "We didn’t notice any difference between the side into which filtered fat was injected and [the side] treated with centrifuged fat," he said. "Therefore, we came to the conclusion that the way fat is treated does not affect the taking rate, assuming that the ‘cleaning’ was in all cases delicate and complete. And I am deeply sorry for those, like me, who have spent a few thousand euros to buy a centrifuge."
He noted that Cytori Therapeutics’ PureGraft sterile plastic bag is a promising new tool for preparing fat prior to transplantation. It is a closed system which allows clinicians to manually separate fat tissue from blood, saline, and other materials. "We will be able to judge its effectiveness in a couple of years," he said.
Dr. Botti said that he had no relevant financial disclosures to make.
LAS VEGAS – Even though transplantation of fat harvested by cannulas has been performed for almost 3 decades, its precise mechanism of action remains elusive.
"One must admit that so far lipostructuring still has to do more with art than with science," Dr. Giovanni Botti said at the annual meeting of the American Academy of Cosmetic Surgery. In many respects, "we haven’t yet come out of the Middle Ages of subjective and empirical opinions."
Despite the relative lack of objective data to support its use, Dr. Botti, a plastic surgeon based in Lake Garda, Italy, has been performing fat transplantation for 25 years. In his opinion, the procedure "can certainly be considered a therapy of first choice in the treatment of soft tissue hypotrophy, as well as for the correction of tropism disorders such as radionecrosis and burns. It is not clear, though, how to obtain consistently positive and long-lasting results."
This begs the question, he continued: If you were to biopsy the area where fat had been injected 1 year earlier, would you be looking at the same fat that was injected, or is it a brand new pad "rebuilt" by the stem cells and modulated by the growing factors present in the grafted material? If the latter hypothesis is true, "how can the stem cells in the grafted fat promote the growth of exactly the wished for amount of fat?" Dr. Botti said. "Why should it take the desired shape? Could the mass on injected tissue serve as a temporary matrix, used by stem cells as a pattern to form their ‘fat net’?"
He speculated that the fat found after 1 year could be composed of fat that was originally injected, as well as stem cells. The stem cells "promote angiogenesis, which would help adipocytes to survive. We can nowadays only make hypotheses that need to be confirmed by further research."
In the meantime, what really matters is achieving the maximum taking rate during fat transplantation, he said. "Very satisfying" results can be achieved in volume restoration and soft tissue regeneration.
The best treatment for the harvested fat prior to injection remains a matter of debate. Recent research suggests that adding stem cells, insulin, the coenzyme Q-10, and platelet rich plasma may favor survival rates, "though no one has yet been able to provide any evidence," he said. "For sure, stem cells can enhance the local blood supply and release growth factors to help the healing process. Thus, theoretically, the graft survival rate is improved. For this reason nowadays regenerative cell enriched fat is increasingly used within various indications."
Beginning in 1985, Dr. Botti treated aspirated fat by "washing," decantation, and strainer filtration. Fifteen years later he switched to using a centrifuge, but after about 1 year of using it, "I realized I wasn’t getting any better results than by means of filtration," he said. "I therefore went back to my previous technique."
In 2007, Dr. Botti and his associates carried out a study of 32 patients undergoing fat transplantation in the face. They injected one side of the face with centrifuged fat, and the other side with filtrate fat. The patients were observed at postoperative day 10 and after 2 and 6 months. "We didn’t notice any difference between the side into which filtered fat was injected and [the side] treated with centrifuged fat," he said. "Therefore, we came to the conclusion that the way fat is treated does not affect the taking rate, assuming that the ‘cleaning’ was in all cases delicate and complete. And I am deeply sorry for those, like me, who have spent a few thousand euros to buy a centrifuge."
He noted that Cytori Therapeutics’ PureGraft sterile plastic bag is a promising new tool for preparing fat prior to transplantation. It is a closed system which allows clinicians to manually separate fat tissue from blood, saline, and other materials. "We will be able to judge its effectiveness in a couple of years," he said.
Dr. Botti said that he had no relevant financial disclosures to make.
EXPERT ANALYSIS FROM THE ANNUAL MEETING OF THE AMERICAN ACADEMY OF COSMETIC SURGERY
d-Dimer May Be a Useful Marker of VTE Risk
SAN DIEGO – Obtaining a baseline d-dimer level can identify acutely ill patients at high risk of venous thromboembolism, results from a large analysis have shown.
In fact, the rate of venous thromboembolism (VTE) was 3.5- to 4-fold higher for patients with a baseline d-dimer level more than twice the upper limit of normal, compared with patients whose d-dimer was twice the upper limit of normal or less, Dr. Alexander T. Cohen reported at the annual meeting of the American Society of Hematology.
The findings come from a subset analysis of patients in MAGELLAN, a trial of 8,101 acutely ill, hospitalized adults who were randomized to either oral rivaroxaban (Xarelto) prophylaxis 10 mg once daily for 35 days or to standard enoxaparin (Lovenox) 40 mg once daily for 10 days, followed by placebo. The patients were assessed with ultrasonography on day 10 and day 35. In the overall study population, rivaroxaban met both of its primary outcomes: noninferiority at day 10 vs. enoxaparin and superiority at day 35 vs. enoxaparin, followed by placebo. Rates of clinically relevant bleeding were low in general but were higher for rivaroxaban than for enoxaparin with placebo. MAGELLAN’s primary findings were presented at the 2011 annual meeting of the American College of Cardiology but have not yet been published.
For the current study, outcomes in MAGELLAN were analyzed to investigate the relationship between d-dimer levels and VTE risk, and the effect of rivaroxaban on this risk. Dr. Cohen of the department of surgery at King’s College Hospital, London, and his associates divided patients into two groups: those with d-dimer levels less than or equal to two times the upper limit of normal or less (group 1) and those with d-dimer levels greater than two times the upper limit of normal (group 2).
The primary efficacy outcome was a composite of asymptomatic proximal deep vein thrombosis (DVT), symptomatic DVT, symptomatic nonfatal pulmonary embolism, and VTE-related death. The principal safety outcomes were major and nonmajor clinically relevant bleeding recorded within 2 days after the last intake of study medication. Both outcomes were assessed on day 10 and day 35, and the prespecified net clinical benefit was defined as a composite of the primary efficacy and principal safety outcomes.
The mean age of the patients was 67 years in group 1 and 71 years in group 2. The baseline median d-dimer level was 0.94 mg/L in all patients. Baseline d-dimer level was higher in patients who experienced a primary efficacy outcome event, compared with those who did not have such an event (a median of 1.98 mg/L vs. 0.92 mg/L). Patients in group 2 were about four times as likely to have a VTE by day 10, compared with those in group 1. At day 10, rivaroxaban was noninferior for the primary efficacy outcome, compared with enoxaparin, among patients in group 2.
At day 35, patients in group 2 who were assigned to the rivaroxaban arm had a reduction in their relative risk of the primary efficacy outcome by 29%, compared with those who were assigned to the enoxaparin-placebo arm, a significant difference that translated into an absolute risk reduction of 2.8% (P = .01). No such differences were observed in group 1.
Patients who had a high d-dimer level at baseline were at increased risk of an event occurring between day 11 and day 35, and rivaroxaban reduced that risk, compared with placebo, during this time period, Dr. Cohen said. "Perhaps assessment of d-dimer after 10 days of standard prophylaxis may indicate which patients benefit from rivaroxaban prophylaxis," he noted.
In both d-dimer groups, the rate of clinically relevant bleeding was significantly higher among patients treated with rivaroxaban, compared with those treated with enoxaparin, followed by placebo, across the entire study period. In the net clinical benefit outcomes analysis, the hazard ratio at day 10 was 1.63 for group 1 and 0.96 for group 2, while the hazard ratio at day 35 was 1.71 for group 1 and 1.03 for group 2.
MAGELLAN was funded by Bayer (which licenses rivaroxaban) and Johnson & Johnson (parent company of Janssen Pharmaceuticals, which manufactures rivaroxaban). Dr. Cohen disclosed that he has served as a medical consultant for, and has received honoraria and clinical trial funding from, numerous pharmaceutical companies, including Bayer, Johnson & Johnson, and Sanofi-Aventis.
As the new anticoagulants begin to be prescribed in greater frequency, we are learning more about the potential benefits and negatives of their use. As this presentation outlines, we are also learning more about the disease processes that they treat. The MAGELLAN study suggests that a D-dimer test may predict which acutely ill patients may be at risk for a DVT. Accordingly, we may be able to limit anticoagulation to only those at risk thus obviating the potential for a serious bleeding complication. Since all the new anticoagulants do not have an antidote and are hence essentially irreversible, prevention of bleeding will be extremely important.
Dr. Russell H. Samson is a clinical associate professor of surgery (Vascular) at Florida State University Medical School and attending vascular surgeon, Sarasota Vascular Specialists. He is an associate medical editor of Vascular Specialist.
As the new anticoagulants begin to be prescribed in greater frequency, we are learning more about the potential benefits and negatives of their use. As this presentation outlines, we are also learning more about the disease processes that they treat. The MAGELLAN study suggests that a D-dimer test may predict which acutely ill patients may be at risk for a DVT. Accordingly, we may be able to limit anticoagulation to only those at risk thus obviating the potential for a serious bleeding complication. Since all the new anticoagulants do not have an antidote and are hence essentially irreversible, prevention of bleeding will be extremely important.
Dr. Russell H. Samson is a clinical associate professor of surgery (Vascular) at Florida State University Medical School and attending vascular surgeon, Sarasota Vascular Specialists. He is an associate medical editor of Vascular Specialist.
As the new anticoagulants begin to be prescribed in greater frequency, we are learning more about the potential benefits and negatives of their use. As this presentation outlines, we are also learning more about the disease processes that they treat. The MAGELLAN study suggests that a D-dimer test may predict which acutely ill patients may be at risk for a DVT. Accordingly, we may be able to limit anticoagulation to only those at risk thus obviating the potential for a serious bleeding complication. Since all the new anticoagulants do not have an antidote and are hence essentially irreversible, prevention of bleeding will be extremely important.
Dr. Russell H. Samson is a clinical associate professor of surgery (Vascular) at Florida State University Medical School and attending vascular surgeon, Sarasota Vascular Specialists. He is an associate medical editor of Vascular Specialist.
SAN DIEGO – Obtaining a baseline d-dimer level can identify acutely ill patients at high risk of venous thromboembolism, results from a large analysis have shown.
In fact, the rate of venous thromboembolism (VTE) was 3.5- to 4-fold higher for patients with a baseline d-dimer level more than twice the upper limit of normal, compared with patients whose d-dimer was twice the upper limit of normal or less, Dr. Alexander T. Cohen reported at the annual meeting of the American Society of Hematology.
The findings come from a subset analysis of patients in MAGELLAN, a trial of 8,101 acutely ill, hospitalized adults who were randomized to either oral rivaroxaban (Xarelto) prophylaxis 10 mg once daily for 35 days or to standard enoxaparin (Lovenox) 40 mg once daily for 10 days, followed by placebo. The patients were assessed with ultrasonography on day 10 and day 35. In the overall study population, rivaroxaban met both of its primary outcomes: noninferiority at day 10 vs. enoxaparin and superiority at day 35 vs. enoxaparin, followed by placebo. Rates of clinically relevant bleeding were low in general but were higher for rivaroxaban than for enoxaparin with placebo. MAGELLAN’s primary findings were presented at the 2011 annual meeting of the American College of Cardiology but have not yet been published.
For the current study, outcomes in MAGELLAN were analyzed to investigate the relationship between d-dimer levels and VTE risk, and the effect of rivaroxaban on this risk. Dr. Cohen of the department of surgery at King’s College Hospital, London, and his associates divided patients into two groups: those with d-dimer levels less than or equal to two times the upper limit of normal or less (group 1) and those with d-dimer levels greater than two times the upper limit of normal (group 2).
The primary efficacy outcome was a composite of asymptomatic proximal deep vein thrombosis (DVT), symptomatic DVT, symptomatic nonfatal pulmonary embolism, and VTE-related death. The principal safety outcomes were major and nonmajor clinically relevant bleeding recorded within 2 days after the last intake of study medication. Both outcomes were assessed on day 10 and day 35, and the prespecified net clinical benefit was defined as a composite of the primary efficacy and principal safety outcomes.
The mean age of the patients was 67 years in group 1 and 71 years in group 2. The baseline median d-dimer level was 0.94 mg/L in all patients. Baseline d-dimer level was higher in patients who experienced a primary efficacy outcome event, compared with those who did not have such an event (a median of 1.98 mg/L vs. 0.92 mg/L). Patients in group 2 were about four times as likely to have a VTE by day 10, compared with those in group 1. At day 10, rivaroxaban was noninferior for the primary efficacy outcome, compared with enoxaparin, among patients in group 2.
At day 35, patients in group 2 who were assigned to the rivaroxaban arm had a reduction in their relative risk of the primary efficacy outcome by 29%, compared with those who were assigned to the enoxaparin-placebo arm, a significant difference that translated into an absolute risk reduction of 2.8% (P = .01). No such differences were observed in group 1.
Patients who had a high d-dimer level at baseline were at increased risk of an event occurring between day 11 and day 35, and rivaroxaban reduced that risk, compared with placebo, during this time period, Dr. Cohen said. "Perhaps assessment of d-dimer after 10 days of standard prophylaxis may indicate which patients benefit from rivaroxaban prophylaxis," he noted.
In both d-dimer groups, the rate of clinically relevant bleeding was significantly higher among patients treated with rivaroxaban, compared with those treated with enoxaparin, followed by placebo, across the entire study period. In the net clinical benefit outcomes analysis, the hazard ratio at day 10 was 1.63 for group 1 and 0.96 for group 2, while the hazard ratio at day 35 was 1.71 for group 1 and 1.03 for group 2.
MAGELLAN was funded by Bayer (which licenses rivaroxaban) and Johnson & Johnson (parent company of Janssen Pharmaceuticals, which manufactures rivaroxaban). Dr. Cohen disclosed that he has served as a medical consultant for, and has received honoraria and clinical trial funding from, numerous pharmaceutical companies, including Bayer, Johnson & Johnson, and Sanofi-Aventis.
SAN DIEGO – Obtaining a baseline d-dimer level can identify acutely ill patients at high risk of venous thromboembolism, results from a large analysis have shown.
In fact, the rate of venous thromboembolism (VTE) was 3.5- to 4-fold higher for patients with a baseline d-dimer level more than twice the upper limit of normal, compared with patients whose d-dimer was twice the upper limit of normal or less, Dr. Alexander T. Cohen reported at the annual meeting of the American Society of Hematology.
The findings come from a subset analysis of patients in MAGELLAN, a trial of 8,101 acutely ill, hospitalized adults who were randomized to either oral rivaroxaban (Xarelto) prophylaxis 10 mg once daily for 35 days or to standard enoxaparin (Lovenox) 40 mg once daily for 10 days, followed by placebo. The patients were assessed with ultrasonography on day 10 and day 35. In the overall study population, rivaroxaban met both of its primary outcomes: noninferiority at day 10 vs. enoxaparin and superiority at day 35 vs. enoxaparin, followed by placebo. Rates of clinically relevant bleeding were low in general but were higher for rivaroxaban than for enoxaparin with placebo. MAGELLAN’s primary findings were presented at the 2011 annual meeting of the American College of Cardiology but have not yet been published.
For the current study, outcomes in MAGELLAN were analyzed to investigate the relationship between d-dimer levels and VTE risk, and the effect of rivaroxaban on this risk. Dr. Cohen of the department of surgery at King’s College Hospital, London, and his associates divided patients into two groups: those with d-dimer levels less than or equal to two times the upper limit of normal or less (group 1) and those with d-dimer levels greater than two times the upper limit of normal (group 2).
The primary efficacy outcome was a composite of asymptomatic proximal deep vein thrombosis (DVT), symptomatic DVT, symptomatic nonfatal pulmonary embolism, and VTE-related death. The principal safety outcomes were major and nonmajor clinically relevant bleeding recorded within 2 days after the last intake of study medication. Both outcomes were assessed on day 10 and day 35, and the prespecified net clinical benefit was defined as a composite of the primary efficacy and principal safety outcomes.
The mean age of the patients was 67 years in group 1 and 71 years in group 2. The baseline median d-dimer level was 0.94 mg/L in all patients. Baseline d-dimer level was higher in patients who experienced a primary efficacy outcome event, compared with those who did not have such an event (a median of 1.98 mg/L vs. 0.92 mg/L). Patients in group 2 were about four times as likely to have a VTE by day 10, compared with those in group 1. At day 10, rivaroxaban was noninferior for the primary efficacy outcome, compared with enoxaparin, among patients in group 2.
At day 35, patients in group 2 who were assigned to the rivaroxaban arm had a reduction in their relative risk of the primary efficacy outcome by 29%, compared with those who were assigned to the enoxaparin-placebo arm, a significant difference that translated into an absolute risk reduction of 2.8% (P = .01). No such differences were observed in group 1.
Patients who had a high d-dimer level at baseline were at increased risk of an event occurring between day 11 and day 35, and rivaroxaban reduced that risk, compared with placebo, during this time period, Dr. Cohen said. "Perhaps assessment of d-dimer after 10 days of standard prophylaxis may indicate which patients benefit from rivaroxaban prophylaxis," he noted.
In both d-dimer groups, the rate of clinically relevant bleeding was significantly higher among patients treated with rivaroxaban, compared with those treated with enoxaparin, followed by placebo, across the entire study period. In the net clinical benefit outcomes analysis, the hazard ratio at day 10 was 1.63 for group 1 and 0.96 for group 2, while the hazard ratio at day 35 was 1.71 for group 1 and 1.03 for group 2.
MAGELLAN was funded by Bayer (which licenses rivaroxaban) and Johnson & Johnson (parent company of Janssen Pharmaceuticals, which manufactures rivaroxaban). Dr. Cohen disclosed that he has served as a medical consultant for, and has received honoraria and clinical trial funding from, numerous pharmaceutical companies, including Bayer, Johnson & Johnson, and Sanofi-Aventis.
Major Finding: The rate of VTE was 3.5- to 4-fold higher in patients with a baseline d-dimer more than twice the upper limit of normal, compared with patients whose d-dimer was lower.
Data Source: A study of 8,101 acutely ill, hospitalized adults randomized to either oral rivaroxaban prophylaxis 10 mg once daily for 35 days or to standard enoxaparin 40 mg once daily for 10 days, followed by placebo.
Disclosures: MAGELLAN was funded by Bayer (which licenses rivaroxaban) and Johnson & Johnson (the parent company of Janssen Pharmaceuticals, which manufactures rivaroxaban). Dr. Cohen disclosed that he has served as a medical consultant for, and has received honoraria and clinical trial funding from, numerous pharmaceutical companies, including Bayer, Johnson & Johnson, and Sanofi-Aventis.
Study: Aspirin Reduced Risk of Recurrent Clots
SAN DIEGO – Aspirin reduced the risk of recurrent symptomatic venous thromboembolism by about 40% when given over a 2-year period following 6-12 months of warfarin therapy, with no apparent increase in major bleeding.
The findings, presented during a press briefing at the annual meeting of the American Society of Hematology, suggest that aspirin is a valid alternative to oral anticoagulants in the extended treatment of venous thromboembolism (VTE).
"This has great implications for clinical practice because many patients on anticoagulant treatment after a first-ever VTE are stopped after the initial 6 months of therapy and then they receive nothing for secondary prevention," lead author Dr. Cecilia Becattini, an internist in the stroke unit at the University of Perugia (Italy), said in an interview. "Maybe aspirin is a great opportunity [for VTE prevention] instead of nothing, because warfarin has the complication of major bleeding."
For the multicenter study, known as WARFASA, 402 patients with a first-ever unprovoked VTE who had completed 6-12 months of oral anticoagulant treatment were randomized to receive aspirin 100 mg daily (aspirin group) or placebo for at least 2 years (placebo group).
The primary efficacy outcome was objectively confirmed recurrent symptomatic VTE and VTE-related death. Clinically relevant (major and nonmajor) bleeding were the main safety outcomes. Bleeding was considered major if it was fatal, occurred in a critical organ, or was associated with a decrease in hemoglobin of greater than 2.0 g/dL or led to a transfusion of two units or greater of whole blood or red cells.
The mean age of patients was 64 years and 56% were male. Of the 402 patients, 205 were randomized to the aspirin group while 197 were randomized to the placebo group. During the study period, which was a mean 25 months, Dr. Becattini reported that a VTE recurrence occurred in 28 patients in the aspirin group (6.6% per patient-year) and in 43 patients in the placebo group (11.0% per patient-year). This translated into a hazard ratio of 0.58.
During the on-treatment study period, which was a mean of 22 months, a VTE recurrence occurred in 23 patients in the aspirin group (5.9% per patient-year) and in 39 patients in the placebo group (11% per patient-year). This translated into a hazard ratio of 0.55.
There was one case of major bleeding in each group and three cases of clinically relevant nonmajor bleeding in each group. There were six deaths in the aspirin group (1.4% per patient-year) and five deaths in the placebo group (1.3% per patient-year), a difference that was not statistically significant (HR 1.04).
In an interview, Dr. Charles S. Abrams, professor of medicine at the University of Pennsylvania, Philadelphia, called the study’s findings "intriguing" and said that a larger trial will be needed to confirm the findings. "I’m not sure what the reason for the difference [between the placebo and aspiring groups] is," said Dr. Abrams.
"One possibility is that it’s a relatively small trial. If you look at the incidence of recurrent clots in most circumstances, it’s usually about 8% per year when you stop that anticoagulant."
In the WARFASA trial, he continued, the incidence of recurrent clots in the placebo and aspirin groups "flanked what you would normally expect. It makes you worry that’s some sort of a fluke."
For her part, Dr. Becattini acknowledged that a larger confirmatory trial is needed before the use of aspirin for extended treatment of VTE can be recommended. She said that she discusses the option with patients who are candidates for aspirin therapy. With aspirin, she said, "we can have an alternative to nothing. It is not just an alternative, but it is a safe alternative."
The study was supported by a grant-in-aid from Bayer Pharma to the University of Perugia. Dr. Becattini and her coauthors said they had no relevant conflicts of interest to declare. ☐
SAN DIEGO – Aspirin reduced the risk of recurrent symptomatic venous thromboembolism by about 40% when given over a 2-year period following 6-12 months of warfarin therapy, with no apparent increase in major bleeding.
The findings, presented during a press briefing at the annual meeting of the American Society of Hematology, suggest that aspirin is a valid alternative to oral anticoagulants in the extended treatment of venous thromboembolism (VTE).
"This has great implications for clinical practice because many patients on anticoagulant treatment after a first-ever VTE are stopped after the initial 6 months of therapy and then they receive nothing for secondary prevention," lead author Dr. Cecilia Becattini, an internist in the stroke unit at the University of Perugia (Italy), said in an interview. "Maybe aspirin is a great opportunity [for VTE prevention] instead of nothing, because warfarin has the complication of major bleeding."
For the multicenter study, known as WARFASA, 402 patients with a first-ever unprovoked VTE who had completed 6-12 months of oral anticoagulant treatment were randomized to receive aspirin 100 mg daily (aspirin group) or placebo for at least 2 years (placebo group).
The primary efficacy outcome was objectively confirmed recurrent symptomatic VTE and VTE-related death. Clinically relevant (major and nonmajor) bleeding were the main safety outcomes. Bleeding was considered major if it was fatal, occurred in a critical organ, or was associated with a decrease in hemoglobin of greater than 2.0 g/dL or led to a transfusion of two units or greater of whole blood or red cells.
The mean age of patients was 64 years and 56% were male. Of the 402 patients, 205 were randomized to the aspirin group while 197 were randomized to the placebo group. During the study period, which was a mean 25 months, Dr. Becattini reported that a VTE recurrence occurred in 28 patients in the aspirin group (6.6% per patient-year) and in 43 patients in the placebo group (11.0% per patient-year). This translated into a hazard ratio of 0.58.
During the on-treatment study period, which was a mean of 22 months, a VTE recurrence occurred in 23 patients in the aspirin group (5.9% per patient-year) and in 39 patients in the placebo group (11% per patient-year). This translated into a hazard ratio of 0.55.
There was one case of major bleeding in each group and three cases of clinically relevant nonmajor bleeding in each group. There were six deaths in the aspirin group (1.4% per patient-year) and five deaths in the placebo group (1.3% per patient-year), a difference that was not statistically significant (HR 1.04).
In an interview, Dr. Charles S. Abrams, professor of medicine at the University of Pennsylvania, Philadelphia, called the study’s findings "intriguing" and said that a larger trial will be needed to confirm the findings. "I’m not sure what the reason for the difference [between the placebo and aspiring groups] is," said Dr. Abrams.
"One possibility is that it’s a relatively small trial. If you look at the incidence of recurrent clots in most circumstances, it’s usually about 8% per year when you stop that anticoagulant."
In the WARFASA trial, he continued, the incidence of recurrent clots in the placebo and aspirin groups "flanked what you would normally expect. It makes you worry that’s some sort of a fluke."
For her part, Dr. Becattini acknowledged that a larger confirmatory trial is needed before the use of aspirin for extended treatment of VTE can be recommended. She said that she discusses the option with patients who are candidates for aspirin therapy. With aspirin, she said, "we can have an alternative to nothing. It is not just an alternative, but it is a safe alternative."
The study was supported by a grant-in-aid from Bayer Pharma to the University of Perugia. Dr. Becattini and her coauthors said they had no relevant conflicts of interest to declare. ☐
SAN DIEGO – Aspirin reduced the risk of recurrent symptomatic venous thromboembolism by about 40% when given over a 2-year period following 6-12 months of warfarin therapy, with no apparent increase in major bleeding.
The findings, presented during a press briefing at the annual meeting of the American Society of Hematology, suggest that aspirin is a valid alternative to oral anticoagulants in the extended treatment of venous thromboembolism (VTE).
"This has great implications for clinical practice because many patients on anticoagulant treatment after a first-ever VTE are stopped after the initial 6 months of therapy and then they receive nothing for secondary prevention," lead author Dr. Cecilia Becattini, an internist in the stroke unit at the University of Perugia (Italy), said in an interview. "Maybe aspirin is a great opportunity [for VTE prevention] instead of nothing, because warfarin has the complication of major bleeding."
For the multicenter study, known as WARFASA, 402 patients with a first-ever unprovoked VTE who had completed 6-12 months of oral anticoagulant treatment were randomized to receive aspirin 100 mg daily (aspirin group) or placebo for at least 2 years (placebo group).
The primary efficacy outcome was objectively confirmed recurrent symptomatic VTE and VTE-related death. Clinically relevant (major and nonmajor) bleeding were the main safety outcomes. Bleeding was considered major if it was fatal, occurred in a critical organ, or was associated with a decrease in hemoglobin of greater than 2.0 g/dL or led to a transfusion of two units or greater of whole blood or red cells.
The mean age of patients was 64 years and 56% were male. Of the 402 patients, 205 were randomized to the aspirin group while 197 were randomized to the placebo group. During the study period, which was a mean 25 months, Dr. Becattini reported that a VTE recurrence occurred in 28 patients in the aspirin group (6.6% per patient-year) and in 43 patients in the placebo group (11.0% per patient-year). This translated into a hazard ratio of 0.58.
During the on-treatment study period, which was a mean of 22 months, a VTE recurrence occurred in 23 patients in the aspirin group (5.9% per patient-year) and in 39 patients in the placebo group (11% per patient-year). This translated into a hazard ratio of 0.55.
There was one case of major bleeding in each group and three cases of clinically relevant nonmajor bleeding in each group. There were six deaths in the aspirin group (1.4% per patient-year) and five deaths in the placebo group (1.3% per patient-year), a difference that was not statistically significant (HR 1.04).
In an interview, Dr. Charles S. Abrams, professor of medicine at the University of Pennsylvania, Philadelphia, called the study’s findings "intriguing" and said that a larger trial will be needed to confirm the findings. "I’m not sure what the reason for the difference [between the placebo and aspiring groups] is," said Dr. Abrams.
"One possibility is that it’s a relatively small trial. If you look at the incidence of recurrent clots in most circumstances, it’s usually about 8% per year when you stop that anticoagulant."
In the WARFASA trial, he continued, the incidence of recurrent clots in the placebo and aspirin groups "flanked what you would normally expect. It makes you worry that’s some sort of a fluke."
For her part, Dr. Becattini acknowledged that a larger confirmatory trial is needed before the use of aspirin for extended treatment of VTE can be recommended. She said that she discusses the option with patients who are candidates for aspirin therapy. With aspirin, she said, "we can have an alternative to nothing. It is not just an alternative, but it is a safe alternative."
The study was supported by a grant-in-aid from Bayer Pharma to the University of Perugia. Dr. Becattini and her coauthors said they had no relevant conflicts of interest to declare. ☐
Major Finding: In a population of patients with unprovoked venous thromboembolism who had completed 6-12 months of oral anticoagulant treatment, aspirin reduced the incidence of recurrent VTE by about 40%, compared with placebo.
Data Source: A multicenter study of 402 patients with a mean age of 64 years who were randomized to receive to receive aspirin, 100 mg daily, or placebo for at least 2 years.
Disclosures: The study was supported by a grant-in-aid from Bayer Pharma to the University of Perugia, Italy. Dr. Becattini and her coauthors said they had no relevant conflicts of interest to declare.
Making Your Office 'The Best in Town'
LAS VEGAS – One day, a delivery driver made a routine stop at the Richmond, Va.–based office of cosmetic facial surgeon Joseph Niamtu III, D.M.D., and made memorable remarks to the front office staff.
"He said, ‘I love coming in here,’ " Dr. Niamtu recalled at the annual meeting of the American Academy of Cosmetic Surgery. " 'It’s warm in here, it smells good, and it's a fun atmosphere; is a radiant, welcoming and energized atmosphere, which is very refreshing compared to most other doctor offices I visit.’ Now that’s a compliment. If you don’t feel that you have the best office in town, then you should send your patients to somebody down the street."
During a presentation about the essentials to marketing a cosmetic surgery practice, Dr. Niamtu said that in its purest form, marketing "begins with you and your staff. It’s really about what you say and what you do, and how your office runs. A huge marketing budget cannot compensate for arrogant or rude doctors and staff. The first step in marketing is to treat people better than anybody else."
While he noted that there is no one-size-fits-all approach to marketing, he shared tips that helped him transition from a full-time oral and maxillofacial surgery practice to a full-time facial cosmetic surgery practice in 2004.
• Hire a marketing professional. "Even if you’re just starting out and your marketing budget is $200, you’ve got to have a plan," Dr. Niamtu said. Early each January he meets with his marketing representative to plan events for the entire year – including print ads, television ads, radio spots, and speaking engagements – all while being mindful of his target market, which he described as "women with money and wrinkles. This is planned out for 12 months. I wasn’t doing this 12 years ago." Now, he said, his annual marketing budget is 10% of his production.
• Create a way for people to remember you. Marketing "is creating a brand and a way to stand out from the crowd," he said. "Branding is consistency. We have postage stamps with our logo on it. And I have a trademark: ‘Making Virginia more beautiful ... one face at a time.’ That’s copyrighted, so nobody else can use that."
• Have interactive components on your website. Dr. Niamtu said that three features drive people to his website, www.lovethatface.com: a section called "Ask Dr. Joe," where visitors can leave a question for him, a tab that allows visitors to request a consultation, and a blog that he writes and strives to keep fresh.
"Every day I get 20 or 30 people from all over the world asking about a procedure," he said of the "Ask Dr. Joe" section of his website. "If you do this, you’ve got to be able to answer within a day. It’s weird, but globally, if you talk to somebody you create a bond."
• Include plenty of before and after pictures on your website. When Dr. Niamtu asks new patients why they chose to visit his practice, many tell him it’s because his website contains so many before and after pictures of patients treated by him. "I have more than 6,600 before and after pictures on my website," he said. "Some of my competitors have three Facebook pictures."
Dr. Niamtu said that he had no relevant financial conflicts to disclose.
LAS VEGAS – One day, a delivery driver made a routine stop at the Richmond, Va.–based office of cosmetic facial surgeon Joseph Niamtu III, D.M.D., and made memorable remarks to the front office staff.
"He said, ‘I love coming in here,’ " Dr. Niamtu recalled at the annual meeting of the American Academy of Cosmetic Surgery. " 'It’s warm in here, it smells good, and it's a fun atmosphere; is a radiant, welcoming and energized atmosphere, which is very refreshing compared to most other doctor offices I visit.’ Now that’s a compliment. If you don’t feel that you have the best office in town, then you should send your patients to somebody down the street."
During a presentation about the essentials to marketing a cosmetic surgery practice, Dr. Niamtu said that in its purest form, marketing "begins with you and your staff. It’s really about what you say and what you do, and how your office runs. A huge marketing budget cannot compensate for arrogant or rude doctors and staff. The first step in marketing is to treat people better than anybody else."
While he noted that there is no one-size-fits-all approach to marketing, he shared tips that helped him transition from a full-time oral and maxillofacial surgery practice to a full-time facial cosmetic surgery practice in 2004.
• Hire a marketing professional. "Even if you’re just starting out and your marketing budget is $200, you’ve got to have a plan," Dr. Niamtu said. Early each January he meets with his marketing representative to plan events for the entire year – including print ads, television ads, radio spots, and speaking engagements – all while being mindful of his target market, which he described as "women with money and wrinkles. This is planned out for 12 months. I wasn’t doing this 12 years ago." Now, he said, his annual marketing budget is 10% of his production.
• Create a way for people to remember you. Marketing "is creating a brand and a way to stand out from the crowd," he said. "Branding is consistency. We have postage stamps with our logo on it. And I have a trademark: ‘Making Virginia more beautiful ... one face at a time.’ That’s copyrighted, so nobody else can use that."
• Have interactive components on your website. Dr. Niamtu said that three features drive people to his website, www.lovethatface.com: a section called "Ask Dr. Joe," where visitors can leave a question for him, a tab that allows visitors to request a consultation, and a blog that he writes and strives to keep fresh.
"Every day I get 20 or 30 people from all over the world asking about a procedure," he said of the "Ask Dr. Joe" section of his website. "If you do this, you’ve got to be able to answer within a day. It’s weird, but globally, if you talk to somebody you create a bond."
• Include plenty of before and after pictures on your website. When Dr. Niamtu asks new patients why they chose to visit his practice, many tell him it’s because his website contains so many before and after pictures of patients treated by him. "I have more than 6,600 before and after pictures on my website," he said. "Some of my competitors have three Facebook pictures."
Dr. Niamtu said that he had no relevant financial conflicts to disclose.
LAS VEGAS – One day, a delivery driver made a routine stop at the Richmond, Va.–based office of cosmetic facial surgeon Joseph Niamtu III, D.M.D., and made memorable remarks to the front office staff.
"He said, ‘I love coming in here,’ " Dr. Niamtu recalled at the annual meeting of the American Academy of Cosmetic Surgery. " 'It’s warm in here, it smells good, and it's a fun atmosphere; is a radiant, welcoming and energized atmosphere, which is very refreshing compared to most other doctor offices I visit.’ Now that’s a compliment. If you don’t feel that you have the best office in town, then you should send your patients to somebody down the street."
During a presentation about the essentials to marketing a cosmetic surgery practice, Dr. Niamtu said that in its purest form, marketing "begins with you and your staff. It’s really about what you say and what you do, and how your office runs. A huge marketing budget cannot compensate for arrogant or rude doctors and staff. The first step in marketing is to treat people better than anybody else."
While he noted that there is no one-size-fits-all approach to marketing, he shared tips that helped him transition from a full-time oral and maxillofacial surgery practice to a full-time facial cosmetic surgery practice in 2004.
• Hire a marketing professional. "Even if you’re just starting out and your marketing budget is $200, you’ve got to have a plan," Dr. Niamtu said. Early each January he meets with his marketing representative to plan events for the entire year – including print ads, television ads, radio spots, and speaking engagements – all while being mindful of his target market, which he described as "women with money and wrinkles. This is planned out for 12 months. I wasn’t doing this 12 years ago." Now, he said, his annual marketing budget is 10% of his production.
• Create a way for people to remember you. Marketing "is creating a brand and a way to stand out from the crowd," he said. "Branding is consistency. We have postage stamps with our logo on it. And I have a trademark: ‘Making Virginia more beautiful ... one face at a time.’ That’s copyrighted, so nobody else can use that."
• Have interactive components on your website. Dr. Niamtu said that three features drive people to his website, www.lovethatface.com: a section called "Ask Dr. Joe," where visitors can leave a question for him, a tab that allows visitors to request a consultation, and a blog that he writes and strives to keep fresh.
"Every day I get 20 or 30 people from all over the world asking about a procedure," he said of the "Ask Dr. Joe" section of his website. "If you do this, you’ve got to be able to answer within a day. It’s weird, but globally, if you talk to somebody you create a bond."
• Include plenty of before and after pictures on your website. When Dr. Niamtu asks new patients why they chose to visit his practice, many tell him it’s because his website contains so many before and after pictures of patients treated by him. "I have more than 6,600 before and after pictures on my website," he said. "Some of my competitors have three Facebook pictures."
Dr. Niamtu said that he had no relevant financial conflicts to disclose.
FROM THE ANNUAL MEETING OF THE AMERICAN ACADEMY OF COSMETIC SURGERY
Herbs Are Not Viable Osteoarthritis Treatment
Patients with osteoarthritis who routinely turn to devil’s claw, Indian frankincense, ginger, and other herbal medicines for symptom relief may want to think twice about this practice.
According to a review of these products that appears in the January 2012 issue of Drug and Therapeutics Bulletin, a publication of the London-based BMJ Group, there is little conclusive evidence to justify their widespread use by patients with the disease (Drug Ther. Bull. 2012:50:8-12). A press release about the review points out that few robust studies on the use of herbal medicines for osteoarthritis (OA) have been carried out. "And those that have frequently contain design flaws and limitations, such as variations in the chemical make-up of the same herb, all of which comprise the validity of the findings."
Herbal medicines that are commonly used to treat OA include vegetable extracts of avocado or soybean unsaponifiables (ASUs), cat’s claw, devil’s claw, Indian frankincense, ginger, rosehip, turmeric, and willow bark. According to the review, the best available clinical evidence suggests that ASUs, Indian frankincense, and rosehips may work, "but more robust data are needed." ASUs are available in Europe but not the United States.
"If we did a better job, then patients probably wouldn’t be reaching for herbal medicines."
In an interview, Dr. Roy Altman, professor of medicine at the University of California, Los Angeles, characterized the DTB review as more opinion than an adequate review of the literature. "The reviews of each product were pretty limited; there are longer and more extensive reviews of this topic," said Dr. Altman. Of all the herbals that have been studied as possible agents for OA management, ASUs are supported by the most and best data. "Studies about [ASUs] have been published in several respected journals. The second-best data are with ginger. I don’t think the data with the frankincense and the rosehips are that good, but the rosehips are presently undergoing additional study."
Some herbal medicines may cause adverse reactions in patients taking other medicines and prescription drugs. For example, the chronic use of nettle can interfere with drugs that are used to treat diabetes, lower blood pressure, and depress the central nervous system, whereas willow bark can cause digestive symptoms and renal problems. The review described the use of herbal medicines for OA as "generally under-researched, and information on potentially significant herb-drug interactions is limited."
Although the U.K. Medicines and Healthcare Products Regulatory Agency has approved Traditional Herbal Registrations for several herbal medicinal products containing devil’s claw for rheumatic symptoms, "gthe trial results for this herb are equivocal,"h the review states. "gThere is little conclusive evidence of benefit from other herbs commonly used for symptoms of osteoarthritis, such as cat’s claw, ginger, nettle, turmeric, and willow bark. [Health care] professionals should routinely ask patients with osteoarthritis if they are taking any herbal products."h
Dr. Altman said that he does not currently advocate the use of herbal medicines for OA patients. "We tend to see a lot of patients who ask about frankincense and turmeric," he said. "The major concern we have in this field is not only the approval of these products by some clinical trial basis and the safety of these products, but the verification that the marketed product is consistent from one batch to another. For example, there are about 1,000 different subspecies of ginger. Which of these species are you using, and what time of the year are you harvesting the ginger? The purification process has to be standardized or you’re not going to have the same product from one batch to another. The same problem exists for all of the herbs."
He noted that the review "raises the point that we are not as good at treating OA pain and other problems as we should be. If we did a better job, then patients probably wouldn’t be reaching for herbal medicines."
The review did not include data on glucosamine and chondroitin sulfate, as they are not of herbal origin. Dr. Altman has published research on ginger and has served as a paid consultant in the past with the French company Pharmascience, which manufactures ASUs.
Patients with osteoarthritis who routinely turn to devil’s claw, Indian frankincense, ginger, and other herbal medicines for symptom relief may want to think twice about this practice.
According to a review of these products that appears in the January 2012 issue of Drug and Therapeutics Bulletin, a publication of the London-based BMJ Group, there is little conclusive evidence to justify their widespread use by patients with the disease (Drug Ther. Bull. 2012:50:8-12). A press release about the review points out that few robust studies on the use of herbal medicines for osteoarthritis (OA) have been carried out. "And those that have frequently contain design flaws and limitations, such as variations in the chemical make-up of the same herb, all of which comprise the validity of the findings."
Herbal medicines that are commonly used to treat OA include vegetable extracts of avocado or soybean unsaponifiables (ASUs), cat’s claw, devil’s claw, Indian frankincense, ginger, rosehip, turmeric, and willow bark. According to the review, the best available clinical evidence suggests that ASUs, Indian frankincense, and rosehips may work, "but more robust data are needed." ASUs are available in Europe but not the United States.
"If we did a better job, then patients probably wouldn’t be reaching for herbal medicines."
In an interview, Dr. Roy Altman, professor of medicine at the University of California, Los Angeles, characterized the DTB review as more opinion than an adequate review of the literature. "The reviews of each product were pretty limited; there are longer and more extensive reviews of this topic," said Dr. Altman. Of all the herbals that have been studied as possible agents for OA management, ASUs are supported by the most and best data. "Studies about [ASUs] have been published in several respected journals. The second-best data are with ginger. I don’t think the data with the frankincense and the rosehips are that good, but the rosehips are presently undergoing additional study."
Some herbal medicines may cause adverse reactions in patients taking other medicines and prescription drugs. For example, the chronic use of nettle can interfere with drugs that are used to treat diabetes, lower blood pressure, and depress the central nervous system, whereas willow bark can cause digestive symptoms and renal problems. The review described the use of herbal medicines for OA as "generally under-researched, and information on potentially significant herb-drug interactions is limited."
Although the U.K. Medicines and Healthcare Products Regulatory Agency has approved Traditional Herbal Registrations for several herbal medicinal products containing devil’s claw for rheumatic symptoms, "gthe trial results for this herb are equivocal,"h the review states. "gThere is little conclusive evidence of benefit from other herbs commonly used for symptoms of osteoarthritis, such as cat’s claw, ginger, nettle, turmeric, and willow bark. [Health care] professionals should routinely ask patients with osteoarthritis if they are taking any herbal products."h
Dr. Altman said that he does not currently advocate the use of herbal medicines for OA patients. "We tend to see a lot of patients who ask about frankincense and turmeric," he said. "The major concern we have in this field is not only the approval of these products by some clinical trial basis and the safety of these products, but the verification that the marketed product is consistent from one batch to another. For example, there are about 1,000 different subspecies of ginger. Which of these species are you using, and what time of the year are you harvesting the ginger? The purification process has to be standardized or you’re not going to have the same product from one batch to another. The same problem exists for all of the herbs."
He noted that the review "raises the point that we are not as good at treating OA pain and other problems as we should be. If we did a better job, then patients probably wouldn’t be reaching for herbal medicines."
The review did not include data on glucosamine and chondroitin sulfate, as they are not of herbal origin. Dr. Altman has published research on ginger and has served as a paid consultant in the past with the French company Pharmascience, which manufactures ASUs.
Patients with osteoarthritis who routinely turn to devil’s claw, Indian frankincense, ginger, and other herbal medicines for symptom relief may want to think twice about this practice.
According to a review of these products that appears in the January 2012 issue of Drug and Therapeutics Bulletin, a publication of the London-based BMJ Group, there is little conclusive evidence to justify their widespread use by patients with the disease (Drug Ther. Bull. 2012:50:8-12). A press release about the review points out that few robust studies on the use of herbal medicines for osteoarthritis (OA) have been carried out. "And those that have frequently contain design flaws and limitations, such as variations in the chemical make-up of the same herb, all of which comprise the validity of the findings."
Herbal medicines that are commonly used to treat OA include vegetable extracts of avocado or soybean unsaponifiables (ASUs), cat’s claw, devil’s claw, Indian frankincense, ginger, rosehip, turmeric, and willow bark. According to the review, the best available clinical evidence suggests that ASUs, Indian frankincense, and rosehips may work, "but more robust data are needed." ASUs are available in Europe but not the United States.
"If we did a better job, then patients probably wouldn’t be reaching for herbal medicines."
In an interview, Dr. Roy Altman, professor of medicine at the University of California, Los Angeles, characterized the DTB review as more opinion than an adequate review of the literature. "The reviews of each product were pretty limited; there are longer and more extensive reviews of this topic," said Dr. Altman. Of all the herbals that have been studied as possible agents for OA management, ASUs are supported by the most and best data. "Studies about [ASUs] have been published in several respected journals. The second-best data are with ginger. I don’t think the data with the frankincense and the rosehips are that good, but the rosehips are presently undergoing additional study."
Some herbal medicines may cause adverse reactions in patients taking other medicines and prescription drugs. For example, the chronic use of nettle can interfere with drugs that are used to treat diabetes, lower blood pressure, and depress the central nervous system, whereas willow bark can cause digestive symptoms and renal problems. The review described the use of herbal medicines for OA as "generally under-researched, and information on potentially significant herb-drug interactions is limited."
Although the U.K. Medicines and Healthcare Products Regulatory Agency has approved Traditional Herbal Registrations for several herbal medicinal products containing devil’s claw for rheumatic symptoms, "gthe trial results for this herb are equivocal,"h the review states. "gThere is little conclusive evidence of benefit from other herbs commonly used for symptoms of osteoarthritis, such as cat’s claw, ginger, nettle, turmeric, and willow bark. [Health care] professionals should routinely ask patients with osteoarthritis if they are taking any herbal products."h
Dr. Altman said that he does not currently advocate the use of herbal medicines for OA patients. "We tend to see a lot of patients who ask about frankincense and turmeric," he said. "The major concern we have in this field is not only the approval of these products by some clinical trial basis and the safety of these products, but the verification that the marketed product is consistent from one batch to another. For example, there are about 1,000 different subspecies of ginger. Which of these species are you using, and what time of the year are you harvesting the ginger? The purification process has to be standardized or you’re not going to have the same product from one batch to another. The same problem exists for all of the herbs."
He noted that the review "raises the point that we are not as good at treating OA pain and other problems as we should be. If we did a better job, then patients probably wouldn’t be reaching for herbal medicines."
The review did not include data on glucosamine and chondroitin sulfate, as they are not of herbal origin. Dr. Altman has published research on ginger and has served as a paid consultant in the past with the French company Pharmascience, which manufactures ASUs.
Mindfulness Sessions Eased Arthritis Symptoms
Patients with inflammatory joint diseases who participated in a 10-session mindfulness-based group intervention experienced significant lessening of psychological distress and improvement in self-efficacy, emotional processing, and overall well-being at 6 and 12 months, judging from the results from a randomized controlled trial.
"These lasting improvements indicate that the participants may have incorporated some mindfulness strategies into their daily lives and that these strategies have strengthened their ability to respond to their stressful experiences in a more flexible way," according to Heidi A. Zangi, Ph.D., of the department of rheumatology at Diakonhjemmet Hospital, Oslo, Norway, and her associates (Ann. Rheum. Dis. 2011 Dec. 20 [doi: 10.1136/annrheumdis-2011-200351]).
For the study, which was conducted at three rheumatology departments in Norway between March 2007 and June 2009, Dr. Zangi and her associates randomized 71 adults aged 20-70 years who were diagnosed with an inflammatory rheumatic joint disease at least 1 year earlier to a mindfulness-based group intervention known as the Vitality Training Program (VTP) or to a control group. Patients assigned to the control group received routine care plus a compact disc with mindfulness-based home exercises for individual voluntary use.
The investigators described VTP as an intervention aimed at "strengthening the personal resources of individuals and enhancing their capacity to engage responsibly and satisfactorily in the process of everyday living." Such an approach, they wrote, advocates "the importance of nonjudgmental attention to unwanted thoughts, feelings, and bodily experiences without attempting to avoid or change them."
The VTP consisted of 10 group sessions over a period of 15 weeks, plus a "booster session" that took place about 6 months after the end of the course. Patients in the control group were informed that they would have the opportunity to participate in the VTP after completion of data collection. Patients in both groups received routine medical care throughout the study.
Of 814 patients who were invited to enroll in the study, 71 participated. Of these, 36 were randomized to the VTP arm and 35 to the control arm. The primary outcome measure was psychological distress as measured by the 20-item version of the General Health Questionnaire. In this tool, a sum score can range from 0 (no distress) to 60 (high distress). Co-primary outcomes were self-efficacy, as measured by the pain and symptoms subscales of the Arthritis Self-Efficacy Scale, and emotion-focused coping, as measured by the Emotional Approach Coping Scale.
Secondary outcomes were assessed by numerical rating scales ranging from 0 to 10 (where 10 is very bad) and included pain, fatigue, and patient-reported global assessment of disease activity. All patients were assessed at baseline, at post-treatment, and at 12 months.
The mean age of patients was 54 years, and 79% were women. After adjusting for gender, age, disease duration, and education, the researchers observed significant mean treatment effects in favor of the VTP at post-treatment and at 12 months for the following outcomes: psychological distress (–4.7 and –3.7, respectively), self-efficacy pain (8.2 and 9.1), self-efficacy symptoms (8.8 and 13.1), emotional processing (0.4 and 0.3), fatigue (–0.8, –1.1), self-care ability (1.2 and 1.0), and overall well-being (0.8 and 0.6). No significant group differences were found in emotional expression, pain, or disease activity.
Dr. Zangi and her associates acknowledged certain limitations of the study, including the fact that all outcome measures were patient reported, and that so few of the patients invited to participate chose to do so, "which probably reflects a selection bias in the direction of highly motivated individuals," they wrote. "Consequently, the results cannot be generalized to the whole population of persons with inflammatory arthritis. However, from our clinical experience we know that there are waiting lists for the existing VTP courses, and that the low uptake may partly be explained by unwillingness to participate in a randomized controlled trial."
The study was supported by Diakonhjemmet Hospital. The investigators reported that they did not have any competing financial interests.
Patients with inflammatory joint diseases who participated in a 10-session mindfulness-based group intervention experienced significant lessening of psychological distress and improvement in self-efficacy, emotional processing, and overall well-being at 6 and 12 months, judging from the results from a randomized controlled trial.
"These lasting improvements indicate that the participants may have incorporated some mindfulness strategies into their daily lives and that these strategies have strengthened their ability to respond to their stressful experiences in a more flexible way," according to Heidi A. Zangi, Ph.D., of the department of rheumatology at Diakonhjemmet Hospital, Oslo, Norway, and her associates (Ann. Rheum. Dis. 2011 Dec. 20 [doi: 10.1136/annrheumdis-2011-200351]).
For the study, which was conducted at three rheumatology departments in Norway between March 2007 and June 2009, Dr. Zangi and her associates randomized 71 adults aged 20-70 years who were diagnosed with an inflammatory rheumatic joint disease at least 1 year earlier to a mindfulness-based group intervention known as the Vitality Training Program (VTP) or to a control group. Patients assigned to the control group received routine care plus a compact disc with mindfulness-based home exercises for individual voluntary use.
The investigators described VTP as an intervention aimed at "strengthening the personal resources of individuals and enhancing their capacity to engage responsibly and satisfactorily in the process of everyday living." Such an approach, they wrote, advocates "the importance of nonjudgmental attention to unwanted thoughts, feelings, and bodily experiences without attempting to avoid or change them."
The VTP consisted of 10 group sessions over a period of 15 weeks, plus a "booster session" that took place about 6 months after the end of the course. Patients in the control group were informed that they would have the opportunity to participate in the VTP after completion of data collection. Patients in both groups received routine medical care throughout the study.
Of 814 patients who were invited to enroll in the study, 71 participated. Of these, 36 were randomized to the VTP arm and 35 to the control arm. The primary outcome measure was psychological distress as measured by the 20-item version of the General Health Questionnaire. In this tool, a sum score can range from 0 (no distress) to 60 (high distress). Co-primary outcomes were self-efficacy, as measured by the pain and symptoms subscales of the Arthritis Self-Efficacy Scale, and emotion-focused coping, as measured by the Emotional Approach Coping Scale.
Secondary outcomes were assessed by numerical rating scales ranging from 0 to 10 (where 10 is very bad) and included pain, fatigue, and patient-reported global assessment of disease activity. All patients were assessed at baseline, at post-treatment, and at 12 months.
The mean age of patients was 54 years, and 79% were women. After adjusting for gender, age, disease duration, and education, the researchers observed significant mean treatment effects in favor of the VTP at post-treatment and at 12 months for the following outcomes: psychological distress (–4.7 and –3.7, respectively), self-efficacy pain (8.2 and 9.1), self-efficacy symptoms (8.8 and 13.1), emotional processing (0.4 and 0.3), fatigue (–0.8, –1.1), self-care ability (1.2 and 1.0), and overall well-being (0.8 and 0.6). No significant group differences were found in emotional expression, pain, or disease activity.
Dr. Zangi and her associates acknowledged certain limitations of the study, including the fact that all outcome measures were patient reported, and that so few of the patients invited to participate chose to do so, "which probably reflects a selection bias in the direction of highly motivated individuals," they wrote. "Consequently, the results cannot be generalized to the whole population of persons with inflammatory arthritis. However, from our clinical experience we know that there are waiting lists for the existing VTP courses, and that the low uptake may partly be explained by unwillingness to participate in a randomized controlled trial."
The study was supported by Diakonhjemmet Hospital. The investigators reported that they did not have any competing financial interests.
Patients with inflammatory joint diseases who participated in a 10-session mindfulness-based group intervention experienced significant lessening of psychological distress and improvement in self-efficacy, emotional processing, and overall well-being at 6 and 12 months, judging from the results from a randomized controlled trial.
"These lasting improvements indicate that the participants may have incorporated some mindfulness strategies into their daily lives and that these strategies have strengthened their ability to respond to their stressful experiences in a more flexible way," according to Heidi A. Zangi, Ph.D., of the department of rheumatology at Diakonhjemmet Hospital, Oslo, Norway, and her associates (Ann. Rheum. Dis. 2011 Dec. 20 [doi: 10.1136/annrheumdis-2011-200351]).
For the study, which was conducted at three rheumatology departments in Norway between March 2007 and June 2009, Dr. Zangi and her associates randomized 71 adults aged 20-70 years who were diagnosed with an inflammatory rheumatic joint disease at least 1 year earlier to a mindfulness-based group intervention known as the Vitality Training Program (VTP) or to a control group. Patients assigned to the control group received routine care plus a compact disc with mindfulness-based home exercises for individual voluntary use.
The investigators described VTP as an intervention aimed at "strengthening the personal resources of individuals and enhancing their capacity to engage responsibly and satisfactorily in the process of everyday living." Such an approach, they wrote, advocates "the importance of nonjudgmental attention to unwanted thoughts, feelings, and bodily experiences without attempting to avoid or change them."
The VTP consisted of 10 group sessions over a period of 15 weeks, plus a "booster session" that took place about 6 months after the end of the course. Patients in the control group were informed that they would have the opportunity to participate in the VTP after completion of data collection. Patients in both groups received routine medical care throughout the study.
Of 814 patients who were invited to enroll in the study, 71 participated. Of these, 36 were randomized to the VTP arm and 35 to the control arm. The primary outcome measure was psychological distress as measured by the 20-item version of the General Health Questionnaire. In this tool, a sum score can range from 0 (no distress) to 60 (high distress). Co-primary outcomes were self-efficacy, as measured by the pain and symptoms subscales of the Arthritis Self-Efficacy Scale, and emotion-focused coping, as measured by the Emotional Approach Coping Scale.
Secondary outcomes were assessed by numerical rating scales ranging from 0 to 10 (where 10 is very bad) and included pain, fatigue, and patient-reported global assessment of disease activity. All patients were assessed at baseline, at post-treatment, and at 12 months.
The mean age of patients was 54 years, and 79% were women. After adjusting for gender, age, disease duration, and education, the researchers observed significant mean treatment effects in favor of the VTP at post-treatment and at 12 months for the following outcomes: psychological distress (–4.7 and –3.7, respectively), self-efficacy pain (8.2 and 9.1), self-efficacy symptoms (8.8 and 13.1), emotional processing (0.4 and 0.3), fatigue (–0.8, –1.1), self-care ability (1.2 and 1.0), and overall well-being (0.8 and 0.6). No significant group differences were found in emotional expression, pain, or disease activity.
Dr. Zangi and her associates acknowledged certain limitations of the study, including the fact that all outcome measures were patient reported, and that so few of the patients invited to participate chose to do so, "which probably reflects a selection bias in the direction of highly motivated individuals," they wrote. "Consequently, the results cannot be generalized to the whole population of persons with inflammatory arthritis. However, from our clinical experience we know that there are waiting lists for the existing VTP courses, and that the low uptake may partly be explained by unwillingness to participate in a randomized controlled trial."
The study was supported by Diakonhjemmet Hospital. The investigators reported that they did not have any competing financial interests.
FROM ANNALS OF THE RHEUMATIC DISEASES
Major Finding: Patients with inflammatory rheumatic joint diseases who participated in a 10-session mindfulness-based group intervention experienced significant mean treatment effects in several outcomes at post-treatment and at 12 months, including psychological distress (–4.7 and –3.7, respectively), self-efficacy pain (8.2 and 9.1), and self-efficacy symptoms (8.8 and 13.1).
Data Source: A trial of 71 patients in Norway who were randomized to a mindfulness-based group intervention known as the Vitality Training Program or to a control group. Patients in the control group received routine care plus a compact disc with mindfulness-based home exercises for individual voluntary use.
Disclosures: The study was supported by Diakonhjemmet Hospital. The investigators reported that they did not have any competing financial interests.
Many Elderly AML Patients Not Receiving Chemotherapy
SAN DIEGO – Despite growing use of chemotherapy in elderly patients with acute myeloid leukemia, more than half still did not receive any chemotherapy between 1997 and 2007, according to an analysis of 6,888 cases in a large national database.
Median survival was significantly longer in patients who received chemotherapy than in those given best supportive care only, Yanni F. Yu reported at the annual meeting of the American Society of Hematology. In all, 56% of elderly acute myeloid leukemia (AML) patients did not receive chemotherapy, however, and median survival as well as chemotherapy use declined with advancing age.
"Older patient with AML generally have a poor outcome," said Ms. Yu, manager of health economics and outcomes research at Boehringer Ingelheim Pharmaceuticals. "If they are not eligible for intensive chemotherapy, the median survival is usually less than 3 months," she noted.
Previous studies in the elderly AML population showed that the percentage of chemotherapy treatment increased from 29% in 1991 to 38% in 1999, Ms. Yu said. To determine more current treatment trends in this patient population, she and her associates evaluated data from Medicare patients aged 65 years and older who had a new AML diagnosis between Jan. 1, 1997, and Dec. 31, 2007, recorded in the Surveillance, Epidemiology, and End Results (SEER) cancer registry.
The analysis was limited to fee-for-service Medicare patients who had at least 6 months of pre-AML Medicare Part A and B benefit coverage. The researchers excluded patients who had evidence of another tumor in the SEER registry before the first AML diagnosis and those who had a diagnosis of a solid tumor within 6 months pre-AML in Medicare claims.
Eligible patients were followed from initial AML diagnosis until their date of death or the end of the observation period, which was Dec. 31, 2009.
The researchers evaluated the type of care received, chemotherapy treatment patterns, and mortality and patient survival separately for AML cases diagnosed in three time frames: 1997-1999, 2000-2003, and 2004-2007. They performed multivariate logistic regression to assess predictors of receipt of chemotherapy, including patient demographics, comorbidities, and year of AML diagnosis.
A total of 6,888 patients met the study criteria. Their mean age was 78 years, 48% were women, 88% were white, and 43% received chemotherapy at any point after diagnosis. The use of chemotherapy increased slightly over time, from 40.7% in 1999-2000 to 42.3% in 2000-2003 and 46% in 2004-2007.
More than half of patients (56%) received only best supportive care post diagnosis, although the percentage decreased slightly over time. Among patients receiving best supportive care, rates of hospice care increased from 30.3% in 1997-1999 to 36.4% in 2000-2003 and 42.3% in 2004-2007.
Among patients who received chemotherapy, the use of antibiotics increased substantially over the three time periods (11.1%, 14%, and 29.6%, respectively), as did the use of antifungals (1.3%, 3.1%, and 12.4%), indicating more patients were in need of prophylaxis or treatment for chemotherapy-related infections.
Older AML patients received strikingly less chemotherapy with advancing age. For example, 66.3% of patients aged 65-74 years received chemotherapy, compared with 39.2% of those aged 75-84 years and 14.8% of those aged 85 years and older. The proportions of patients receiving antibiotics and antifungals also decreased with advancing age.
Regression analysis showed a similar association between age and chemotherapy, revealing that the strongest predictor of not receiving chemotherapy was older age, with an odds ratio of 0.12 for being aged 85 years or more and an OR of 0.42 for being aged 75-84 years.
The overall 30-day mortality rate was 20.5%, and the 60-day mortality rate was 42.8%. Median survival among all patients was 2.6 months, and it decreased with advancing age from 4.5 months to 2.4 months to 1.6 months for those aged 65-74 years, 75-84 years, and 85 years and older, respectively.
Furthermore, although nearly all patients had died during follow-up, median survival was much longer in patients receiving chemotherapy than in those receiving best supportive care only. This was true across all age groups, with 8.0 vs. 1.5 months reported in those aged 65-74 years, 5.3 vs. 1.7 months in those 75-84 years, and 3.8 vs. 1.4 months in those 85 years and older.
Ms. Yu acknowledged certain limitations of the study, including the fact that results may not be applicable to younger patients or to those covered by a managed care Medicaid plan.
In addition, "Medicare Part D information was not included since information on prescription claims was available only after 2007," she said. "Also, no information was available on the chemotherapy agents or the doses received in the inpatient or outpatient settings. Patient performance status was also unavailable."
She said that further studies are warranted "to investigate the potential benefit of chemotherapy treatment for elderly patients with AML."
The study was sponsored by Boehringer Ingelheim Pharmaceuticals. Ms. Yu disclosed that she is a full-time employee of the company.
SAN DIEGO – Despite growing use of chemotherapy in elderly patients with acute myeloid leukemia, more than half still did not receive any chemotherapy between 1997 and 2007, according to an analysis of 6,888 cases in a large national database.
Median survival was significantly longer in patients who received chemotherapy than in those given best supportive care only, Yanni F. Yu reported at the annual meeting of the American Society of Hematology. In all, 56% of elderly acute myeloid leukemia (AML) patients did not receive chemotherapy, however, and median survival as well as chemotherapy use declined with advancing age.
"Older patient with AML generally have a poor outcome," said Ms. Yu, manager of health economics and outcomes research at Boehringer Ingelheim Pharmaceuticals. "If they are not eligible for intensive chemotherapy, the median survival is usually less than 3 months," she noted.
Previous studies in the elderly AML population showed that the percentage of chemotherapy treatment increased from 29% in 1991 to 38% in 1999, Ms. Yu said. To determine more current treatment trends in this patient population, she and her associates evaluated data from Medicare patients aged 65 years and older who had a new AML diagnosis between Jan. 1, 1997, and Dec. 31, 2007, recorded in the Surveillance, Epidemiology, and End Results (SEER) cancer registry.
The analysis was limited to fee-for-service Medicare patients who had at least 6 months of pre-AML Medicare Part A and B benefit coverage. The researchers excluded patients who had evidence of another tumor in the SEER registry before the first AML diagnosis and those who had a diagnosis of a solid tumor within 6 months pre-AML in Medicare claims.
Eligible patients were followed from initial AML diagnosis until their date of death or the end of the observation period, which was Dec. 31, 2009.
The researchers evaluated the type of care received, chemotherapy treatment patterns, and mortality and patient survival separately for AML cases diagnosed in three time frames: 1997-1999, 2000-2003, and 2004-2007. They performed multivariate logistic regression to assess predictors of receipt of chemotherapy, including patient demographics, comorbidities, and year of AML diagnosis.
A total of 6,888 patients met the study criteria. Their mean age was 78 years, 48% were women, 88% were white, and 43% received chemotherapy at any point after diagnosis. The use of chemotherapy increased slightly over time, from 40.7% in 1999-2000 to 42.3% in 2000-2003 and 46% in 2004-2007.
More than half of patients (56%) received only best supportive care post diagnosis, although the percentage decreased slightly over time. Among patients receiving best supportive care, rates of hospice care increased from 30.3% in 1997-1999 to 36.4% in 2000-2003 and 42.3% in 2004-2007.
Among patients who received chemotherapy, the use of antibiotics increased substantially over the three time periods (11.1%, 14%, and 29.6%, respectively), as did the use of antifungals (1.3%, 3.1%, and 12.4%), indicating more patients were in need of prophylaxis or treatment for chemotherapy-related infections.
Older AML patients received strikingly less chemotherapy with advancing age. For example, 66.3% of patients aged 65-74 years received chemotherapy, compared with 39.2% of those aged 75-84 years and 14.8% of those aged 85 years and older. The proportions of patients receiving antibiotics and antifungals also decreased with advancing age.
Regression analysis showed a similar association between age and chemotherapy, revealing that the strongest predictor of not receiving chemotherapy was older age, with an odds ratio of 0.12 for being aged 85 years or more and an OR of 0.42 for being aged 75-84 years.
The overall 30-day mortality rate was 20.5%, and the 60-day mortality rate was 42.8%. Median survival among all patients was 2.6 months, and it decreased with advancing age from 4.5 months to 2.4 months to 1.6 months for those aged 65-74 years, 75-84 years, and 85 years and older, respectively.
Furthermore, although nearly all patients had died during follow-up, median survival was much longer in patients receiving chemotherapy than in those receiving best supportive care only. This was true across all age groups, with 8.0 vs. 1.5 months reported in those aged 65-74 years, 5.3 vs. 1.7 months in those 75-84 years, and 3.8 vs. 1.4 months in those 85 years and older.
Ms. Yu acknowledged certain limitations of the study, including the fact that results may not be applicable to younger patients or to those covered by a managed care Medicaid plan.
In addition, "Medicare Part D information was not included since information on prescription claims was available only after 2007," she said. "Also, no information was available on the chemotherapy agents or the doses received in the inpatient or outpatient settings. Patient performance status was also unavailable."
She said that further studies are warranted "to investigate the potential benefit of chemotherapy treatment for elderly patients with AML."
The study was sponsored by Boehringer Ingelheim Pharmaceuticals. Ms. Yu disclosed that she is a full-time employee of the company.
SAN DIEGO – Despite growing use of chemotherapy in elderly patients with acute myeloid leukemia, more than half still did not receive any chemotherapy between 1997 and 2007, according to an analysis of 6,888 cases in a large national database.
Median survival was significantly longer in patients who received chemotherapy than in those given best supportive care only, Yanni F. Yu reported at the annual meeting of the American Society of Hematology. In all, 56% of elderly acute myeloid leukemia (AML) patients did not receive chemotherapy, however, and median survival as well as chemotherapy use declined with advancing age.
"Older patient with AML generally have a poor outcome," said Ms. Yu, manager of health economics and outcomes research at Boehringer Ingelheim Pharmaceuticals. "If they are not eligible for intensive chemotherapy, the median survival is usually less than 3 months," she noted.
Previous studies in the elderly AML population showed that the percentage of chemotherapy treatment increased from 29% in 1991 to 38% in 1999, Ms. Yu said. To determine more current treatment trends in this patient population, she and her associates evaluated data from Medicare patients aged 65 years and older who had a new AML diagnosis between Jan. 1, 1997, and Dec. 31, 2007, recorded in the Surveillance, Epidemiology, and End Results (SEER) cancer registry.
The analysis was limited to fee-for-service Medicare patients who had at least 6 months of pre-AML Medicare Part A and B benefit coverage. The researchers excluded patients who had evidence of another tumor in the SEER registry before the first AML diagnosis and those who had a diagnosis of a solid tumor within 6 months pre-AML in Medicare claims.
Eligible patients were followed from initial AML diagnosis until their date of death or the end of the observation period, which was Dec. 31, 2009.
The researchers evaluated the type of care received, chemotherapy treatment patterns, and mortality and patient survival separately for AML cases diagnosed in three time frames: 1997-1999, 2000-2003, and 2004-2007. They performed multivariate logistic regression to assess predictors of receipt of chemotherapy, including patient demographics, comorbidities, and year of AML diagnosis.
A total of 6,888 patients met the study criteria. Their mean age was 78 years, 48% were women, 88% were white, and 43% received chemotherapy at any point after diagnosis. The use of chemotherapy increased slightly over time, from 40.7% in 1999-2000 to 42.3% in 2000-2003 and 46% in 2004-2007.
More than half of patients (56%) received only best supportive care post diagnosis, although the percentage decreased slightly over time. Among patients receiving best supportive care, rates of hospice care increased from 30.3% in 1997-1999 to 36.4% in 2000-2003 and 42.3% in 2004-2007.
Among patients who received chemotherapy, the use of antibiotics increased substantially over the three time periods (11.1%, 14%, and 29.6%, respectively), as did the use of antifungals (1.3%, 3.1%, and 12.4%), indicating more patients were in need of prophylaxis or treatment for chemotherapy-related infections.
Older AML patients received strikingly less chemotherapy with advancing age. For example, 66.3% of patients aged 65-74 years received chemotherapy, compared with 39.2% of those aged 75-84 years and 14.8% of those aged 85 years and older. The proportions of patients receiving antibiotics and antifungals also decreased with advancing age.
Regression analysis showed a similar association between age and chemotherapy, revealing that the strongest predictor of not receiving chemotherapy was older age, with an odds ratio of 0.12 for being aged 85 years or more and an OR of 0.42 for being aged 75-84 years.
The overall 30-day mortality rate was 20.5%, and the 60-day mortality rate was 42.8%. Median survival among all patients was 2.6 months, and it decreased with advancing age from 4.5 months to 2.4 months to 1.6 months for those aged 65-74 years, 75-84 years, and 85 years and older, respectively.
Furthermore, although nearly all patients had died during follow-up, median survival was much longer in patients receiving chemotherapy than in those receiving best supportive care only. This was true across all age groups, with 8.0 vs. 1.5 months reported in those aged 65-74 years, 5.3 vs. 1.7 months in those 75-84 years, and 3.8 vs. 1.4 months in those 85 years and older.
Ms. Yu acknowledged certain limitations of the study, including the fact that results may not be applicable to younger patients or to those covered by a managed care Medicaid plan.
In addition, "Medicare Part D information was not included since information on prescription claims was available only after 2007," she said. "Also, no information was available on the chemotherapy agents or the doses received in the inpatient or outpatient settings. Patient performance status was also unavailable."
She said that further studies are warranted "to investigate the potential benefit of chemotherapy treatment for elderly patients with AML."
The study was sponsored by Boehringer Ingelheim Pharmaceuticals. Ms. Yu disclosed that she is a full-time employee of the company.
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY OF HEMATOLOGY
Major Finding: More than half of elderly patients (56%) with AML received only best supportive care post diagnosis,
Data Source: A study of 6,888 Medicare patients aged 65 years and older who had a new AML diagnosis between Jan. 1, 1997, and Dec. 31, 2007, in the SEER cancer registry.
Disclosures: The study was sponsored by Boehringer Ingelheim Pharmaceuticals. Ms. Yu disclosed that she is a full-time employee of the company.
New Scoring System Devised for Youth With Hodgkin's Lymphoma
SAN DIEGO – A simple scoring system identified a subset of young patients with Hodgkin’s lymphoma who are predicted to have an event-free survival rate of less than 80%.
The system, known as the Childhood Hodgkin International Prognostic Score (CHIPS), found that four factors were predictive of worse event-free survival: stage IV disease, large mediastinal adenopathy, albumin level of less than 3.5 g/dL, and fever, Dr. Cindy L. Schwartz reported during a poster session at the annual meeting of the American Society of Hematology.
She and her associates with the Children’s Oncology Group evaluated 1,721 patients with intermediate risk Hodgkin’s lymphoma who were younger than age 21 and treated on AHOD0031: a phase III study of dose-intensive therapy.
The current study involved tailoring treatment by early response in 770 patients who were randomized or assigned to receive the same treatment (four cycles of doxorubicin, bleomycin, vincristine, etoposide, prednisone and cyclophosphamide (ABVE-PC) and 21 Gy involved field radiotherapy (IFRT).
According to Dr. Schwartz, director of the division of pediatric hematology/oncology at Hasbro Children’s Hospital, Providence, R.I., rapid early response was defined as a two-dimensional tumor reduction of greater than 60% on CT after two cycles of ABVE-PC. Complete response was defined as a greater than 80% two-dimensional reduction by CT, and resolution of nuclear imaging abnormalities.
Rapid responders who achieved complete response after two additional ABVE-PC treatments were randomized to receive 21 Gy radiation. Slow early responders were randomized to receive dexamethasone, etoposide, cisplatin, and cytarabine (DECA) in addition to the four ABVE-PC treatments and 21 Gy radiation treatment.
Using Cox regression analysis and multivariable predictive modeling, the researchers identified four predictors of event-free survival: stage IV disease (hazard ratio, 1.6), mediastinal adenopathy (HR 1.7), albumin of less than 3.5 g/dL (HR 1.8), and fever (HR 2.5).
Because the hazard ratios were similar, the researchers devised the CHIPS score, which gave one point for each of the four adverse predictors. Using this approach, they determined that the event-free survival rate was 90% for patients with a CHIPS score of 0 or 1, 78% for those with a CHIPS score of 2, and 62% for those with a CHIPS score of 3. (Because the study enrolled only patients with intermediate-risk disease, no one had a CHIPS score of 4.) From this they determined that 22% of an intermediate-risk population can be predicted to have an event-free survival rate of less than 80%.
"Now that we know who’s a good responder to initial chemotherapy, we’re going to try and change their treatment much earlier than we have been able to previously," Dr. Schwartz said in an interview. "The next thing to do is analyze some of the biologic factors that contribute to their response rate."
Studies of CHIPS in additional cohorts of newly diagnosed patients are also planned, she said.
Dr. Schwartz said that she had no relevant financial disclosures to make.
SAN DIEGO – A simple scoring system identified a subset of young patients with Hodgkin’s lymphoma who are predicted to have an event-free survival rate of less than 80%.
The system, known as the Childhood Hodgkin International Prognostic Score (CHIPS), found that four factors were predictive of worse event-free survival: stage IV disease, large mediastinal adenopathy, albumin level of less than 3.5 g/dL, and fever, Dr. Cindy L. Schwartz reported during a poster session at the annual meeting of the American Society of Hematology.
She and her associates with the Children’s Oncology Group evaluated 1,721 patients with intermediate risk Hodgkin’s lymphoma who were younger than age 21 and treated on AHOD0031: a phase III study of dose-intensive therapy.
The current study involved tailoring treatment by early response in 770 patients who were randomized or assigned to receive the same treatment (four cycles of doxorubicin, bleomycin, vincristine, etoposide, prednisone and cyclophosphamide (ABVE-PC) and 21 Gy involved field radiotherapy (IFRT).
According to Dr. Schwartz, director of the division of pediatric hematology/oncology at Hasbro Children’s Hospital, Providence, R.I., rapid early response was defined as a two-dimensional tumor reduction of greater than 60% on CT after two cycles of ABVE-PC. Complete response was defined as a greater than 80% two-dimensional reduction by CT, and resolution of nuclear imaging abnormalities.
Rapid responders who achieved complete response after two additional ABVE-PC treatments were randomized to receive 21 Gy radiation. Slow early responders were randomized to receive dexamethasone, etoposide, cisplatin, and cytarabine (DECA) in addition to the four ABVE-PC treatments and 21 Gy radiation treatment.
Using Cox regression analysis and multivariable predictive modeling, the researchers identified four predictors of event-free survival: stage IV disease (hazard ratio, 1.6), mediastinal adenopathy (HR 1.7), albumin of less than 3.5 g/dL (HR 1.8), and fever (HR 2.5).
Because the hazard ratios were similar, the researchers devised the CHIPS score, which gave one point for each of the four adverse predictors. Using this approach, they determined that the event-free survival rate was 90% for patients with a CHIPS score of 0 or 1, 78% for those with a CHIPS score of 2, and 62% for those with a CHIPS score of 3. (Because the study enrolled only patients with intermediate-risk disease, no one had a CHIPS score of 4.) From this they determined that 22% of an intermediate-risk population can be predicted to have an event-free survival rate of less than 80%.
"Now that we know who’s a good responder to initial chemotherapy, we’re going to try and change their treatment much earlier than we have been able to previously," Dr. Schwartz said in an interview. "The next thing to do is analyze some of the biologic factors that contribute to their response rate."
Studies of CHIPS in additional cohorts of newly diagnosed patients are also planned, she said.
Dr. Schwartz said that she had no relevant financial disclosures to make.
SAN DIEGO – A simple scoring system identified a subset of young patients with Hodgkin’s lymphoma who are predicted to have an event-free survival rate of less than 80%.
The system, known as the Childhood Hodgkin International Prognostic Score (CHIPS), found that four factors were predictive of worse event-free survival: stage IV disease, large mediastinal adenopathy, albumin level of less than 3.5 g/dL, and fever, Dr. Cindy L. Schwartz reported during a poster session at the annual meeting of the American Society of Hematology.
She and her associates with the Children’s Oncology Group evaluated 1,721 patients with intermediate risk Hodgkin’s lymphoma who were younger than age 21 and treated on AHOD0031: a phase III study of dose-intensive therapy.
The current study involved tailoring treatment by early response in 770 patients who were randomized or assigned to receive the same treatment (four cycles of doxorubicin, bleomycin, vincristine, etoposide, prednisone and cyclophosphamide (ABVE-PC) and 21 Gy involved field radiotherapy (IFRT).
According to Dr. Schwartz, director of the division of pediatric hematology/oncology at Hasbro Children’s Hospital, Providence, R.I., rapid early response was defined as a two-dimensional tumor reduction of greater than 60% on CT after two cycles of ABVE-PC. Complete response was defined as a greater than 80% two-dimensional reduction by CT, and resolution of nuclear imaging abnormalities.
Rapid responders who achieved complete response after two additional ABVE-PC treatments were randomized to receive 21 Gy radiation. Slow early responders were randomized to receive dexamethasone, etoposide, cisplatin, and cytarabine (DECA) in addition to the four ABVE-PC treatments and 21 Gy radiation treatment.
Using Cox regression analysis and multivariable predictive modeling, the researchers identified four predictors of event-free survival: stage IV disease (hazard ratio, 1.6), mediastinal adenopathy (HR 1.7), albumin of less than 3.5 g/dL (HR 1.8), and fever (HR 2.5).
Because the hazard ratios were similar, the researchers devised the CHIPS score, which gave one point for each of the four adverse predictors. Using this approach, they determined that the event-free survival rate was 90% for patients with a CHIPS score of 0 or 1, 78% for those with a CHIPS score of 2, and 62% for those with a CHIPS score of 3. (Because the study enrolled only patients with intermediate-risk disease, no one had a CHIPS score of 4.) From this they determined that 22% of an intermediate-risk population can be predicted to have an event-free survival rate of less than 80%.
"Now that we know who’s a good responder to initial chemotherapy, we’re going to try and change their treatment much earlier than we have been able to previously," Dr. Schwartz said in an interview. "The next thing to do is analyze some of the biologic factors that contribute to their response rate."
Studies of CHIPS in additional cohorts of newly diagnosed patients are also planned, she said.
Dr. Schwartz said that she had no relevant financial disclosures to make.
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY OF HEMATOLOGY
Major Finding: Based on new scoring system, 22% of an intermediate-risk population can be predicted to have an event-free survival rate of less than 80%.
Data Source: Application of the Childhood Hodgkin International Prognostic Score (CHIPS) in 770 patients who were randomized or assigned to receive the same treatment.
Disclosures: Dr. Schwartz said that she had no relevant financial conflicts to disclose.