User login
VIDEO: CoreValve self-expanding aortic valve beat surgical replacement
WASHINGTON – Transcatheter aortic valve replacement with the CoreValve device showed significantly higher 1-year survival than did surgical replacement, in a randomized trial of patients with severe aortic stenosis and increased risk of death during surgery.
These results, presented at the annual meeting of the American College of Cardiology by Dr. David H. Adams of Mt. Sinai Medical Center, New York, were met with enthusiasm by Dr. Valentin Fuster, the invited discussant at the meeting. "The CoreValve study, to me, is fantastic," Dr. Fuster, also of Mt. Sinai, said in an interview. But he did also urge restraint in placing the device in those for whom it would be inappropriate, especially younger patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WASHINGTON – Transcatheter aortic valve replacement with the CoreValve device showed significantly higher 1-year survival than did surgical replacement, in a randomized trial of patients with severe aortic stenosis and increased risk of death during surgery.
These results, presented at the annual meeting of the American College of Cardiology by Dr. David H. Adams of Mt. Sinai Medical Center, New York, were met with enthusiasm by Dr. Valentin Fuster, the invited discussant at the meeting. "The CoreValve study, to me, is fantastic," Dr. Fuster, also of Mt. Sinai, said in an interview. But he did also urge restraint in placing the device in those for whom it would be inappropriate, especially younger patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WASHINGTON – Transcatheter aortic valve replacement with the CoreValve device showed significantly higher 1-year survival than did surgical replacement, in a randomized trial of patients with severe aortic stenosis and increased risk of death during surgery.
These results, presented at the annual meeting of the American College of Cardiology by Dr. David H. Adams of Mt. Sinai Medical Center, New York, were met with enthusiasm by Dr. Valentin Fuster, the invited discussant at the meeting. "The CoreValve study, to me, is fantastic," Dr. Fuster, also of Mt. Sinai, said in an interview. But he did also urge restraint in placing the device in those for whom it would be inappropriate, especially younger patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
At ACC 14
FDA panel rejects serelaxin approval for heart failure, but urges future study
SILVER SPRING, MD. – A Food and Drug Administration advisory panel voted 11 to 0 that serelaxin should not be approved for the treatment of acute heart failure, but agreed that the data on the drug were promising and that it should be studied further, at a meeting on March 27.
Members of the FDA’s Cardiovascular and Renal Drugs Advisory Committee agreed there was evidence that serelaxin, a recombinant form of human relaxin-2 hormone that is administered in an intravenous infusion over 48 hours, had a positive effect on worsening heart failure in the phase III RELAX-AHF study. But this was not a primary endpoint of the study, and panelists agreed that the study results were hypothesis-generating and were not sufficient to recommend approval. And because only one of the study’s two primary endpoints was positive, the study did not meet the level required for approval of a drug based on a single trial, several panelists pointed out.
"It’s clear that the drug does have an effect on worsening heart failure, but ... given the limitations on how that was not rigorously defined ... we don’t know how much of the effect was on minor heart failure changes, versus major [changes]," said the panel chair, Dr. A. Michael Lincoff, vice chairman for research in the department of cardiovascular medicine at the Cleveland Clinic. "We need better data on understanding the magnitude of benefit in a rigorous fashion, and the data we have here don’t stand alone as a single trial," he added.
Relaxin, which is present in men and women and increases during the first trimester of pregnancy, "primarily stimulates both the rapid and sustained nitric oxide (NO)-mediated vasodilation pathways," and results in vasodilation within minutes of administration, according to the manufacturer, Novartis Pharmaceuticals. The company’s proposed indication for serelaxin is for improving the symptoms of acute heart failure "through reduction of the rate of worsening of heart failure," based on the results of the phase III RELAX-AHF study, an international randomized, placebo-controlled, double-blind study of 1,161 patients with acute heart failure.
The study compared serelaxin started within 16 hours of hospitalization (at a dose of 30 mcg/kg per day for 48 hours) vs. placebo, plus standard therapy, on two primary endpoints that evaluated the drug’s effect on dyspnea: the Visual Analogue Scale (VAS) dyspnea scale and the Likert dyspnea scale. The area under the curve (AUC), representing the change in patient-reported dyspnea from baseline through day 5, significantly increased among those on serelaxin, compared with those on placebo. But the proportion of patients with moderate to marked improvements in dyspnea, based on the 7-point Likert scale at 6, 12, and 24 hours, however, was not significantly different between the two groups (27% among those on serelaxin vs. 26% among those on placebo).
Novartis also presented data to support serelaxin’s effects on worsening heart failure events through day 5, during which time 6.4% of those on serelaxin had a first episode of worsening heart failure or death within 5 days, vs. almost 12% of those on placebo; and less than 1% of those on serelaxin had recurrent worsening heart failure or death with prior event, vs. 2.6% of those on placebo.
In briefing documents provided before the meeting, the FDA stated that it does not recommend approval of serelaxin, citing various reasons, including the claim in the proposed indication that was "somewhat different" from what was evaluated in the study, as well as the "vague" definition of worsening heart failure in the study.
At the meeting, Dr. Melanie Blank, the FDA’s clinical reviewer, pointed out that the study protocol did not include prespecified criteria, such as signs, symptoms, and lab results, for defining worsening heart failure, and it was left to the investigator to determine whether a patient had worsening heart failure. Cases of worsening heart failure ranged from those that required mechanical ventilation to cases that required only a single 20-mg dose of furosemide, she added. Hypotension was the main safety issue in the study, but most cases were managed by reducing the dose and would not preclude approval, according to the FDA.
Because the study was designed to evaluate the effect of treatment on dyspnea, panelist Dr. Stuart Rich, professor of medicine at the University of Chicago, said "this was more a failure of trial design than it was of the drug itself." He added that that like other panelists, he was enthusiastic about the drug’s potential, and "hopefully, this will be a learning study where the hypotheses will be better thought out, tested, and then proven in a subsequent trial."
In a statement issued after the meeting, Tim Wright, global head of development at Novartis, said that the discussion during the meeting "provided important information that we will address with the FDA as it completes its review," and that the company will "continue to drive our robust clinical trial program and build upon the already established body of evidence." In 2013, Novartis started enrolling patients in RELAX-AHF-2, an international, phase III outcomes study of serelaxin in more than 6,300 patients with acute heart failure; results are expected in 2016.
Serelaxin has not been approved elsewhere. The FDA granted serelaxin fast track status in 2009, and in 2013 it was granted breakthrough therapy designation for reduction of cardiovascular mortality. Breakthrough therapy designation is intended to expedite the development and review of drugs for serious or life-threatening conditions.
In January, the Committee for Medicinal Products for Human Use recommended against approving serelaxin for acute heart failure in Europe, citing issues that included no apparent benefit for short-term relief of dyspnea.
The FDA usually follows the recommendations of its advisory panels. Panelists have been cleared of potential conflicts of interest related to the topic of the meeting. Occasionally, a panelist may be given a waiver, but not at this meeting.
SILVER SPRING, MD. – A Food and Drug Administration advisory panel voted 11 to 0 that serelaxin should not be approved for the treatment of acute heart failure, but agreed that the data on the drug were promising and that it should be studied further, at a meeting on March 27.
Members of the FDA’s Cardiovascular and Renal Drugs Advisory Committee agreed there was evidence that serelaxin, a recombinant form of human relaxin-2 hormone that is administered in an intravenous infusion over 48 hours, had a positive effect on worsening heart failure in the phase III RELAX-AHF study. But this was not a primary endpoint of the study, and panelists agreed that the study results were hypothesis-generating and were not sufficient to recommend approval. And because only one of the study’s two primary endpoints was positive, the study did not meet the level required for approval of a drug based on a single trial, several panelists pointed out.
"It’s clear that the drug does have an effect on worsening heart failure, but ... given the limitations on how that was not rigorously defined ... we don’t know how much of the effect was on minor heart failure changes, versus major [changes]," said the panel chair, Dr. A. Michael Lincoff, vice chairman for research in the department of cardiovascular medicine at the Cleveland Clinic. "We need better data on understanding the magnitude of benefit in a rigorous fashion, and the data we have here don’t stand alone as a single trial," he added.
Relaxin, which is present in men and women and increases during the first trimester of pregnancy, "primarily stimulates both the rapid and sustained nitric oxide (NO)-mediated vasodilation pathways," and results in vasodilation within minutes of administration, according to the manufacturer, Novartis Pharmaceuticals. The company’s proposed indication for serelaxin is for improving the symptoms of acute heart failure "through reduction of the rate of worsening of heart failure," based on the results of the phase III RELAX-AHF study, an international randomized, placebo-controlled, double-blind study of 1,161 patients with acute heart failure.
The study compared serelaxin started within 16 hours of hospitalization (at a dose of 30 mcg/kg per day for 48 hours) vs. placebo, plus standard therapy, on two primary endpoints that evaluated the drug’s effect on dyspnea: the Visual Analogue Scale (VAS) dyspnea scale and the Likert dyspnea scale. The area under the curve (AUC), representing the change in patient-reported dyspnea from baseline through day 5, significantly increased among those on serelaxin, compared with those on placebo. But the proportion of patients with moderate to marked improvements in dyspnea, based on the 7-point Likert scale at 6, 12, and 24 hours, however, was not significantly different between the two groups (27% among those on serelaxin vs. 26% among those on placebo).
Novartis also presented data to support serelaxin’s effects on worsening heart failure events through day 5, during which time 6.4% of those on serelaxin had a first episode of worsening heart failure or death within 5 days, vs. almost 12% of those on placebo; and less than 1% of those on serelaxin had recurrent worsening heart failure or death with prior event, vs. 2.6% of those on placebo.
In briefing documents provided before the meeting, the FDA stated that it does not recommend approval of serelaxin, citing various reasons, including the claim in the proposed indication that was "somewhat different" from what was evaluated in the study, as well as the "vague" definition of worsening heart failure in the study.
At the meeting, Dr. Melanie Blank, the FDA’s clinical reviewer, pointed out that the study protocol did not include prespecified criteria, such as signs, symptoms, and lab results, for defining worsening heart failure, and it was left to the investigator to determine whether a patient had worsening heart failure. Cases of worsening heart failure ranged from those that required mechanical ventilation to cases that required only a single 20-mg dose of furosemide, she added. Hypotension was the main safety issue in the study, but most cases were managed by reducing the dose and would not preclude approval, according to the FDA.
Because the study was designed to evaluate the effect of treatment on dyspnea, panelist Dr. Stuart Rich, professor of medicine at the University of Chicago, said "this was more a failure of trial design than it was of the drug itself." He added that that like other panelists, he was enthusiastic about the drug’s potential, and "hopefully, this will be a learning study where the hypotheses will be better thought out, tested, and then proven in a subsequent trial."
In a statement issued after the meeting, Tim Wright, global head of development at Novartis, said that the discussion during the meeting "provided important information that we will address with the FDA as it completes its review," and that the company will "continue to drive our robust clinical trial program and build upon the already established body of evidence." In 2013, Novartis started enrolling patients in RELAX-AHF-2, an international, phase III outcomes study of serelaxin in more than 6,300 patients with acute heart failure; results are expected in 2016.
Serelaxin has not been approved elsewhere. The FDA granted serelaxin fast track status in 2009, and in 2013 it was granted breakthrough therapy designation for reduction of cardiovascular mortality. Breakthrough therapy designation is intended to expedite the development and review of drugs for serious or life-threatening conditions.
In January, the Committee for Medicinal Products for Human Use recommended against approving serelaxin for acute heart failure in Europe, citing issues that included no apparent benefit for short-term relief of dyspnea.
The FDA usually follows the recommendations of its advisory panels. Panelists have been cleared of potential conflicts of interest related to the topic of the meeting. Occasionally, a panelist may be given a waiver, but not at this meeting.
SILVER SPRING, MD. – A Food and Drug Administration advisory panel voted 11 to 0 that serelaxin should not be approved for the treatment of acute heart failure, but agreed that the data on the drug were promising and that it should be studied further, at a meeting on March 27.
Members of the FDA’s Cardiovascular and Renal Drugs Advisory Committee agreed there was evidence that serelaxin, a recombinant form of human relaxin-2 hormone that is administered in an intravenous infusion over 48 hours, had a positive effect on worsening heart failure in the phase III RELAX-AHF study. But this was not a primary endpoint of the study, and panelists agreed that the study results were hypothesis-generating and were not sufficient to recommend approval. And because only one of the study’s two primary endpoints was positive, the study did not meet the level required for approval of a drug based on a single trial, several panelists pointed out.
"It’s clear that the drug does have an effect on worsening heart failure, but ... given the limitations on how that was not rigorously defined ... we don’t know how much of the effect was on minor heart failure changes, versus major [changes]," said the panel chair, Dr. A. Michael Lincoff, vice chairman for research in the department of cardiovascular medicine at the Cleveland Clinic. "We need better data on understanding the magnitude of benefit in a rigorous fashion, and the data we have here don’t stand alone as a single trial," he added.
Relaxin, which is present in men and women and increases during the first trimester of pregnancy, "primarily stimulates both the rapid and sustained nitric oxide (NO)-mediated vasodilation pathways," and results in vasodilation within minutes of administration, according to the manufacturer, Novartis Pharmaceuticals. The company’s proposed indication for serelaxin is for improving the symptoms of acute heart failure "through reduction of the rate of worsening of heart failure," based on the results of the phase III RELAX-AHF study, an international randomized, placebo-controlled, double-blind study of 1,161 patients with acute heart failure.
The study compared serelaxin started within 16 hours of hospitalization (at a dose of 30 mcg/kg per day for 48 hours) vs. placebo, plus standard therapy, on two primary endpoints that evaluated the drug’s effect on dyspnea: the Visual Analogue Scale (VAS) dyspnea scale and the Likert dyspnea scale. The area under the curve (AUC), representing the change in patient-reported dyspnea from baseline through day 5, significantly increased among those on serelaxin, compared with those on placebo. But the proportion of patients with moderate to marked improvements in dyspnea, based on the 7-point Likert scale at 6, 12, and 24 hours, however, was not significantly different between the two groups (27% among those on serelaxin vs. 26% among those on placebo).
Novartis also presented data to support serelaxin’s effects on worsening heart failure events through day 5, during which time 6.4% of those on serelaxin had a first episode of worsening heart failure or death within 5 days, vs. almost 12% of those on placebo; and less than 1% of those on serelaxin had recurrent worsening heart failure or death with prior event, vs. 2.6% of those on placebo.
In briefing documents provided before the meeting, the FDA stated that it does not recommend approval of serelaxin, citing various reasons, including the claim in the proposed indication that was "somewhat different" from what was evaluated in the study, as well as the "vague" definition of worsening heart failure in the study.
At the meeting, Dr. Melanie Blank, the FDA’s clinical reviewer, pointed out that the study protocol did not include prespecified criteria, such as signs, symptoms, and lab results, for defining worsening heart failure, and it was left to the investigator to determine whether a patient had worsening heart failure. Cases of worsening heart failure ranged from those that required mechanical ventilation to cases that required only a single 20-mg dose of furosemide, she added. Hypotension was the main safety issue in the study, but most cases were managed by reducing the dose and would not preclude approval, according to the FDA.
Because the study was designed to evaluate the effect of treatment on dyspnea, panelist Dr. Stuart Rich, professor of medicine at the University of Chicago, said "this was more a failure of trial design than it was of the drug itself." He added that that like other panelists, he was enthusiastic about the drug’s potential, and "hopefully, this will be a learning study where the hypotheses will be better thought out, tested, and then proven in a subsequent trial."
In a statement issued after the meeting, Tim Wright, global head of development at Novartis, said that the discussion during the meeting "provided important information that we will address with the FDA as it completes its review," and that the company will "continue to drive our robust clinical trial program and build upon the already established body of evidence." In 2013, Novartis started enrolling patients in RELAX-AHF-2, an international, phase III outcomes study of serelaxin in more than 6,300 patients with acute heart failure; results are expected in 2016.
Serelaxin has not been approved elsewhere. The FDA granted serelaxin fast track status in 2009, and in 2013 it was granted breakthrough therapy designation for reduction of cardiovascular mortality. Breakthrough therapy designation is intended to expedite the development and review of drugs for serious or life-threatening conditions.
In January, the Committee for Medicinal Products for Human Use recommended against approving serelaxin for acute heart failure in Europe, citing issues that included no apparent benefit for short-term relief of dyspnea.
The FDA usually follows the recommendations of its advisory panels. Panelists have been cleared of potential conflicts of interest related to the topic of the meeting. Occasionally, a panelist may be given a waiver, but not at this meeting.
AT AN FDA ADVISORY COMMITTEE MEETING
Fruit and Vegetable Consumption in Young Women Linked to Later CVD Benefit
A high intake of fruit and vegetables during early adulthood was associated with a lower risk of having calcified arterial plaque 20 years later, a benefit that was only evident in women, in the CARDIA (Coronary Artery Risk Development in Young Adults) study.
Previous studies have also found that women seem to benefit more from a diet that includes a high intake of fruit and vegetables, a disparity that "should be explored further," said Dr. Michael Miedema, a preventive cardiologist at the Minneapolis Heart Institute. He summarized the results of the study during a webcast held prior to the annual meeting of the American College of Cardiology, where he is presenting the data.
The study evaluated the association between the intake of fruit and vegetables in 2,648 people enrolled in the CARDIA study in 1985 and 1986, at an average age of 25 years, and the presence of coronary artery calcium (CAC), measured with electron-beam computed tomography, 20 years later. Participants were excluded if they consumed less than 800 kcal/day or more than 4,500 kcal/day, or if they had missing CAC scores.
Because there are fewer data in young adults, the study addressed the effect of fruit and vegetable intake during young adulthood on later cardiovascular disease risk. Previous studies have found that a diet high in fruit and vegetables is associated with a lower cardiovascular disease risk in middle-aged adults, with risk decreasing further as intake increases, a finding that has been less consistent in men, Dr. Miedema pointed out.
Fruit and vegetable intake was divided into tertiles, based on the number of servings the study participants reported consuming per day: Women in the top third ate 8-9 servings of fruit and vegetables per day, compared with 3-4 servings a day in the bottom third. The amount consumed was slightly lower in men, at 7-8 servings per day in the top third and 2-3 servings per day in the bottom third. Those in the top third had healthier behaviors, with slightly lower cholesterol and blood pressure levels, and were less likely to smoke.
After adjustment for a 2,000-calorie diet, women who consumed 8-9 servings of fruit and vegetables a day as young adults were about 40% less likely to have calcified plaque in their coronary arteries, compared with women who consumed about 3 servings of fruit and vegetables a day as young adults, Dr. Miedema said. This association was independent of age, weight, race, and lifestyle factors that included smoking, amount of exercise, and alcohol consumption.
"But in men, we did not find the same relationship," he said. Although two previous studies have also suggested that men derive fewer benefits from a diet high in fruit and vegetables, only 1,038 men were enrolled in this study, so it may have been underpowered to detect an effect in men, he added.
The results "reinforce the value of establishing healthy behaviors early in adulthood, and as we talk about population-based approaches to trying to reduce coronary vascular disease, it seems that we should probably include a focus on establishing a high intake of fruits and vegetables early in life," Dr. Miedema concluded
As for the gender difference, the message of the study should not be "that fruit and vegetable intake doesn’t matter in men," but that it is possible that a high intake of fruit and vegetables "doesn’t work quite as well in reducing heart disease risk for men as it does for women," he added. There is no biological explanation for the gender difference, which needs further study, he noted.
The CARDIA study, which is evaluating the development of cardiovascular risk factors and cardiovascular disease in healthy black and white adults, is sponsored by the National Institutes of Health. Dr. Miedema had no disclosures.
A high intake of fruit and vegetables during early adulthood was associated with a lower risk of having calcified arterial plaque 20 years later, a benefit that was only evident in women, in the CARDIA (Coronary Artery Risk Development in Young Adults) study.
Previous studies have also found that women seem to benefit more from a diet that includes a high intake of fruit and vegetables, a disparity that "should be explored further," said Dr. Michael Miedema, a preventive cardiologist at the Minneapolis Heart Institute. He summarized the results of the study during a webcast held prior to the annual meeting of the American College of Cardiology, where he is presenting the data.
The study evaluated the association between the intake of fruit and vegetables in 2,648 people enrolled in the CARDIA study in 1985 and 1986, at an average age of 25 years, and the presence of coronary artery calcium (CAC), measured with electron-beam computed tomography, 20 years later. Participants were excluded if they consumed less than 800 kcal/day or more than 4,500 kcal/day, or if they had missing CAC scores.
Because there are fewer data in young adults, the study addressed the effect of fruit and vegetable intake during young adulthood on later cardiovascular disease risk. Previous studies have found that a diet high in fruit and vegetables is associated with a lower cardiovascular disease risk in middle-aged adults, with risk decreasing further as intake increases, a finding that has been less consistent in men, Dr. Miedema pointed out.
Fruit and vegetable intake was divided into tertiles, based on the number of servings the study participants reported consuming per day: Women in the top third ate 8-9 servings of fruit and vegetables per day, compared with 3-4 servings a day in the bottom third. The amount consumed was slightly lower in men, at 7-8 servings per day in the top third and 2-3 servings per day in the bottom third. Those in the top third had healthier behaviors, with slightly lower cholesterol and blood pressure levels, and were less likely to smoke.
After adjustment for a 2,000-calorie diet, women who consumed 8-9 servings of fruit and vegetables a day as young adults were about 40% less likely to have calcified plaque in their coronary arteries, compared with women who consumed about 3 servings of fruit and vegetables a day as young adults, Dr. Miedema said. This association was independent of age, weight, race, and lifestyle factors that included smoking, amount of exercise, and alcohol consumption.
"But in men, we did not find the same relationship," he said. Although two previous studies have also suggested that men derive fewer benefits from a diet high in fruit and vegetables, only 1,038 men were enrolled in this study, so it may have been underpowered to detect an effect in men, he added.
The results "reinforce the value of establishing healthy behaviors early in adulthood, and as we talk about population-based approaches to trying to reduce coronary vascular disease, it seems that we should probably include a focus on establishing a high intake of fruits and vegetables early in life," Dr. Miedema concluded
As for the gender difference, the message of the study should not be "that fruit and vegetable intake doesn’t matter in men," but that it is possible that a high intake of fruit and vegetables "doesn’t work quite as well in reducing heart disease risk for men as it does for women," he added. There is no biological explanation for the gender difference, which needs further study, he noted.
The CARDIA study, which is evaluating the development of cardiovascular risk factors and cardiovascular disease in healthy black and white adults, is sponsored by the National Institutes of Health. Dr. Miedema had no disclosures.
A high intake of fruit and vegetables during early adulthood was associated with a lower risk of having calcified arterial plaque 20 years later, a benefit that was only evident in women, in the CARDIA (Coronary Artery Risk Development in Young Adults) study.
Previous studies have also found that women seem to benefit more from a diet that includes a high intake of fruit and vegetables, a disparity that "should be explored further," said Dr. Michael Miedema, a preventive cardiologist at the Minneapolis Heart Institute. He summarized the results of the study during a webcast held prior to the annual meeting of the American College of Cardiology, where he is presenting the data.
The study evaluated the association between the intake of fruit and vegetables in 2,648 people enrolled in the CARDIA study in 1985 and 1986, at an average age of 25 years, and the presence of coronary artery calcium (CAC), measured with electron-beam computed tomography, 20 years later. Participants were excluded if they consumed less than 800 kcal/day or more than 4,500 kcal/day, or if they had missing CAC scores.
Because there are fewer data in young adults, the study addressed the effect of fruit and vegetable intake during young adulthood on later cardiovascular disease risk. Previous studies have found that a diet high in fruit and vegetables is associated with a lower cardiovascular disease risk in middle-aged adults, with risk decreasing further as intake increases, a finding that has been less consistent in men, Dr. Miedema pointed out.
Fruit and vegetable intake was divided into tertiles, based on the number of servings the study participants reported consuming per day: Women in the top third ate 8-9 servings of fruit and vegetables per day, compared with 3-4 servings a day in the bottom third. The amount consumed was slightly lower in men, at 7-8 servings per day in the top third and 2-3 servings per day in the bottom third. Those in the top third had healthier behaviors, with slightly lower cholesterol and blood pressure levels, and were less likely to smoke.
After adjustment for a 2,000-calorie diet, women who consumed 8-9 servings of fruit and vegetables a day as young adults were about 40% less likely to have calcified plaque in their coronary arteries, compared with women who consumed about 3 servings of fruit and vegetables a day as young adults, Dr. Miedema said. This association was independent of age, weight, race, and lifestyle factors that included smoking, amount of exercise, and alcohol consumption.
"But in men, we did not find the same relationship," he said. Although two previous studies have also suggested that men derive fewer benefits from a diet high in fruit and vegetables, only 1,038 men were enrolled in this study, so it may have been underpowered to detect an effect in men, he added.
The results "reinforce the value of establishing healthy behaviors early in adulthood, and as we talk about population-based approaches to trying to reduce coronary vascular disease, it seems that we should probably include a focus on establishing a high intake of fruits and vegetables early in life," Dr. Miedema concluded
As for the gender difference, the message of the study should not be "that fruit and vegetable intake doesn’t matter in men," but that it is possible that a high intake of fruit and vegetables "doesn’t work quite as well in reducing heart disease risk for men as it does for women," he added. There is no biological explanation for the gender difference, which needs further study, he noted.
The CARDIA study, which is evaluating the development of cardiovascular risk factors and cardiovascular disease in healthy black and white adults, is sponsored by the National Institutes of Health. Dr. Miedema had no disclosures.
FROM ACC 2014
Fruit and vegetable consumption in young women linked to later CVD benefit
A high intake of fruit and vegetables during early adulthood was associated with a lower risk of having calcified arterial plaque 20 years later, a benefit that was only evident in women, in the CARDIA (Coronary Artery Risk Development in Young Adults) study.
Previous studies have also found that women seem to benefit more from a diet that includes a high intake of fruit and vegetables, a disparity that "should be explored further," said Dr. Michael Miedema, a preventive cardiologist at the Minneapolis Heart Institute. He summarized the results of the study during a webcast held prior to the annual meeting of the American College of Cardiology, where he is presenting the data.
The study evaluated the association between the intake of fruit and vegetables in 2,648 people enrolled in the CARDIA study in 1985 and 1986, at an average age of 25 years, and the presence of coronary artery calcium (CAC), measured with electron-beam computed tomography, 20 years later. Participants were excluded if they consumed less than 800 kcal/day or more than 4,500 kcal/day, or if they had missing CAC scores.
Because there are fewer data in young adults, the study addressed the effect of fruit and vegetable intake during young adulthood on later cardiovascular disease risk. Previous studies have found that a diet high in fruit and vegetables is associated with a lower cardiovascular disease risk in middle-aged adults, with risk decreasing further as intake increases, a finding that has been less consistent in men, Dr. Miedema pointed out.
Fruit and vegetable intake was divided into tertiles, based on the number of servings the study participants reported consuming per day: Women in the top third ate 8-9 servings of fruit and vegetables per day, compared with 3-4 servings a day in the bottom third. The amount consumed was slightly lower in men, at 7-8 servings per day in the top third and 2-3 servings per day in the bottom third. Those in the top third had healthier behaviors, with slightly lower cholesterol and blood pressure levels, and were less likely to smoke.
After adjustment for a 2,000-calorie diet, women who consumed 8-9 servings of fruit and vegetables a day as young adults were about 40% less likely to have calcified plaque in their coronary arteries, compared with women who consumed about 3 servings of fruit and vegetables a day as young adults, Dr. Miedema said. This association was independent of age, weight, race, and lifestyle factors that included smoking, amount of exercise, and alcohol consumption.
"But in men, we did not find the same relationship," he said. Although two previous studies have also suggested that men derive fewer benefits from a diet high in fruit and vegetables, only 1,038 men were enrolled in this study, so it may have been underpowered to detect an effect in men, he added.
The results "reinforce the value of establishing healthy behaviors early in adulthood, and as we talk about population-based approaches to trying to reduce coronary vascular disease, it seems that we should probably include a focus on establishing a high intake of fruits and vegetables early in life," Dr. Miedema concluded
As for the gender difference, the message of the study should not be "that fruit and vegetable intake doesn’t matter in men," but that it is possible that a high intake of fruit and vegetables "doesn’t work quite as well in reducing heart disease risk for men as it does for women," he added. There is no biological explanation for the gender difference, which needs further study, he noted.
The CARDIA study, which is evaluating the development of cardiovascular risk factors and cardiovascular disease in healthy black and white adults, is sponsored by the National Institutes of Health. Dr. Miedema had no disclosures.
A high intake of fruit and vegetables during early adulthood was associated with a lower risk of having calcified arterial plaque 20 years later, a benefit that was only evident in women, in the CARDIA (Coronary Artery Risk Development in Young Adults) study.
Previous studies have also found that women seem to benefit more from a diet that includes a high intake of fruit and vegetables, a disparity that "should be explored further," said Dr. Michael Miedema, a preventive cardiologist at the Minneapolis Heart Institute. He summarized the results of the study during a webcast held prior to the annual meeting of the American College of Cardiology, where he is presenting the data.
The study evaluated the association between the intake of fruit and vegetables in 2,648 people enrolled in the CARDIA study in 1985 and 1986, at an average age of 25 years, and the presence of coronary artery calcium (CAC), measured with electron-beam computed tomography, 20 years later. Participants were excluded if they consumed less than 800 kcal/day or more than 4,500 kcal/day, or if they had missing CAC scores.
Because there are fewer data in young adults, the study addressed the effect of fruit and vegetable intake during young adulthood on later cardiovascular disease risk. Previous studies have found that a diet high in fruit and vegetables is associated with a lower cardiovascular disease risk in middle-aged adults, with risk decreasing further as intake increases, a finding that has been less consistent in men, Dr. Miedema pointed out.
Fruit and vegetable intake was divided into tertiles, based on the number of servings the study participants reported consuming per day: Women in the top third ate 8-9 servings of fruit and vegetables per day, compared with 3-4 servings a day in the bottom third. The amount consumed was slightly lower in men, at 7-8 servings per day in the top third and 2-3 servings per day in the bottom third. Those in the top third had healthier behaviors, with slightly lower cholesterol and blood pressure levels, and were less likely to smoke.
After adjustment for a 2,000-calorie diet, women who consumed 8-9 servings of fruit and vegetables a day as young adults were about 40% less likely to have calcified plaque in their coronary arteries, compared with women who consumed about 3 servings of fruit and vegetables a day as young adults, Dr. Miedema said. This association was independent of age, weight, race, and lifestyle factors that included smoking, amount of exercise, and alcohol consumption.
"But in men, we did not find the same relationship," he said. Although two previous studies have also suggested that men derive fewer benefits from a diet high in fruit and vegetables, only 1,038 men were enrolled in this study, so it may have been underpowered to detect an effect in men, he added.
The results "reinforce the value of establishing healthy behaviors early in adulthood, and as we talk about population-based approaches to trying to reduce coronary vascular disease, it seems that we should probably include a focus on establishing a high intake of fruits and vegetables early in life," Dr. Miedema concluded
As for the gender difference, the message of the study should not be "that fruit and vegetable intake doesn’t matter in men," but that it is possible that a high intake of fruit and vegetables "doesn’t work quite as well in reducing heart disease risk for men as it does for women," he added. There is no biological explanation for the gender difference, which needs further study, he noted.
The CARDIA study, which is evaluating the development of cardiovascular risk factors and cardiovascular disease in healthy black and white adults, is sponsored by the National Institutes of Health. Dr. Miedema had no disclosures.
A high intake of fruit and vegetables during early adulthood was associated with a lower risk of having calcified arterial plaque 20 years later, a benefit that was only evident in women, in the CARDIA (Coronary Artery Risk Development in Young Adults) study.
Previous studies have also found that women seem to benefit more from a diet that includes a high intake of fruit and vegetables, a disparity that "should be explored further," said Dr. Michael Miedema, a preventive cardiologist at the Minneapolis Heart Institute. He summarized the results of the study during a webcast held prior to the annual meeting of the American College of Cardiology, where he is presenting the data.
The study evaluated the association between the intake of fruit and vegetables in 2,648 people enrolled in the CARDIA study in 1985 and 1986, at an average age of 25 years, and the presence of coronary artery calcium (CAC), measured with electron-beam computed tomography, 20 years later. Participants were excluded if they consumed less than 800 kcal/day or more than 4,500 kcal/day, or if they had missing CAC scores.
Because there are fewer data in young adults, the study addressed the effect of fruit and vegetable intake during young adulthood on later cardiovascular disease risk. Previous studies have found that a diet high in fruit and vegetables is associated with a lower cardiovascular disease risk in middle-aged adults, with risk decreasing further as intake increases, a finding that has been less consistent in men, Dr. Miedema pointed out.
Fruit and vegetable intake was divided into tertiles, based on the number of servings the study participants reported consuming per day: Women in the top third ate 8-9 servings of fruit and vegetables per day, compared with 3-4 servings a day in the bottom third. The amount consumed was slightly lower in men, at 7-8 servings per day in the top third and 2-3 servings per day in the bottom third. Those in the top third had healthier behaviors, with slightly lower cholesterol and blood pressure levels, and were less likely to smoke.
After adjustment for a 2,000-calorie diet, women who consumed 8-9 servings of fruit and vegetables a day as young adults were about 40% less likely to have calcified plaque in their coronary arteries, compared with women who consumed about 3 servings of fruit and vegetables a day as young adults, Dr. Miedema said. This association was independent of age, weight, race, and lifestyle factors that included smoking, amount of exercise, and alcohol consumption.
"But in men, we did not find the same relationship," he said. Although two previous studies have also suggested that men derive fewer benefits from a diet high in fruit and vegetables, only 1,038 men were enrolled in this study, so it may have been underpowered to detect an effect in men, he added.
The results "reinforce the value of establishing healthy behaviors early in adulthood, and as we talk about population-based approaches to trying to reduce coronary vascular disease, it seems that we should probably include a focus on establishing a high intake of fruits and vegetables early in life," Dr. Miedema concluded
As for the gender difference, the message of the study should not be "that fruit and vegetable intake doesn’t matter in men," but that it is possible that a high intake of fruit and vegetables "doesn’t work quite as well in reducing heart disease risk for men as it does for women," he added. There is no biological explanation for the gender difference, which needs further study, he noted.
The CARDIA study, which is evaluating the development of cardiovascular risk factors and cardiovascular disease in healthy black and white adults, is sponsored by the National Institutes of Health. Dr. Miedema had no disclosures.
FROM ACC 2014
Study links number of live births to future cardiac risk
The number of children a woman has may affect her future risk of cardiovascular disease – and the mechanism may be subclinical atherosclerosis, data from the Dallas Heart Study suggest.
Women who had had two or three live births had the lowest prevalence of subclinical atherosclerosis, compared with women who had four or more children and with women who had no live births or one live birth, depicting "a U-shaped association between the number of children a woman gives birth to and future risk of cardiovascular events," said Dr. Monika Sanghavi, chief cardiology fellow at the University of Texas Southwestern Medical Center, Dallas.
Dr. Sanghavi summarized the study results in a webcast held prior to the annual meeting of the American College of Cardiology, where she is presenting the data.
The number of children women have has been associated with their future cardiovascular disease risk, but the mechanism has not been clearly understood. For this study of 1,644 women enrolled in the Dallas Heart Study the researchers addressed whether the mechanism could be subclinical atherosclerosis by using coronary artery calcium (CAC) scores measured with CT imaging and aortic wall thickness (AWT) measured with magnetic resonance imaging. The average age of the women was 45 years, and 55% were black.
The Dallas Heart Study is a multiethnic population–based sample of Dallas County residents.
CAC scores were positive (greater than 10) in 11% of the women who had 2-3 live births, compared with 27% in those who had at least 4 live births, and 15% of those who had 0-1 live births, all significant differences.
AWTs were abnormal (greater than the 75th percentile for age and gender) in 22% of those with had 2-3 live births, compared with 33% of those who had at least 4 live births and 25% of those who had 0-1 live births. The association persisted after adjustment for cardiovascular disease risk factors, including socioeconomic status, race and education.
"The results support the hypothesis that subclinical atherosclerosis may be the mechanism for this association," Dr. Sanghavi said. As for the two groups identified at risk, she added, "we think that increased risk on either end of the curve likely represents two different processes, such that repeated pregnancies increase risk in a different way than those women who are unable to get pregnant."
These results need to be confirmed in future studies, which will also further explore why women with one or no live births are at increased risk, Dr. Sanghavi said. Those with four or more live births may be at increased risk because of "repeated exposure to an atherogenic milieu," with higher cholesterol levels, insulin resistance and other pregnancy-related changes, or factors not accounted for in the study, she noted.
But women with 0-1 live births may be at increased risk because of an underlying systemic condition such as polycystic ovarian syndrome "or some other inflammatory condition that is preventing them from getting pregnant or carrying multiple pregnancies to term," she added.
Dr. Sanghavi emphasized that the results should not be used to recommend that women should have two or three children.
Dr. Vera Bittner, chair of the ACC Prevention of Cardiovascular Disease Committee, said the results highlight the need to take "a better reproductive history when we’re trying to judge a woman’s cardiovascular risk when they present to our office."
Dr. Sanghavi had no disclosures.
The number of children a woman has may affect her future risk of cardiovascular disease – and the mechanism may be subclinical atherosclerosis, data from the Dallas Heart Study suggest.
Women who had had two or three live births had the lowest prevalence of subclinical atherosclerosis, compared with women who had four or more children and with women who had no live births or one live birth, depicting "a U-shaped association between the number of children a woman gives birth to and future risk of cardiovascular events," said Dr. Monika Sanghavi, chief cardiology fellow at the University of Texas Southwestern Medical Center, Dallas.
Dr. Sanghavi summarized the study results in a webcast held prior to the annual meeting of the American College of Cardiology, where she is presenting the data.
The number of children women have has been associated with their future cardiovascular disease risk, but the mechanism has not been clearly understood. For this study of 1,644 women enrolled in the Dallas Heart Study the researchers addressed whether the mechanism could be subclinical atherosclerosis by using coronary artery calcium (CAC) scores measured with CT imaging and aortic wall thickness (AWT) measured with magnetic resonance imaging. The average age of the women was 45 years, and 55% were black.
The Dallas Heart Study is a multiethnic population–based sample of Dallas County residents.
CAC scores were positive (greater than 10) in 11% of the women who had 2-3 live births, compared with 27% in those who had at least 4 live births, and 15% of those who had 0-1 live births, all significant differences.
AWTs were abnormal (greater than the 75th percentile for age and gender) in 22% of those with had 2-3 live births, compared with 33% of those who had at least 4 live births and 25% of those who had 0-1 live births. The association persisted after adjustment for cardiovascular disease risk factors, including socioeconomic status, race and education.
"The results support the hypothesis that subclinical atherosclerosis may be the mechanism for this association," Dr. Sanghavi said. As for the two groups identified at risk, she added, "we think that increased risk on either end of the curve likely represents two different processes, such that repeated pregnancies increase risk in a different way than those women who are unable to get pregnant."
These results need to be confirmed in future studies, which will also further explore why women with one or no live births are at increased risk, Dr. Sanghavi said. Those with four or more live births may be at increased risk because of "repeated exposure to an atherogenic milieu," with higher cholesterol levels, insulin resistance and other pregnancy-related changes, or factors not accounted for in the study, she noted.
But women with 0-1 live births may be at increased risk because of an underlying systemic condition such as polycystic ovarian syndrome "or some other inflammatory condition that is preventing them from getting pregnant or carrying multiple pregnancies to term," she added.
Dr. Sanghavi emphasized that the results should not be used to recommend that women should have two or three children.
Dr. Vera Bittner, chair of the ACC Prevention of Cardiovascular Disease Committee, said the results highlight the need to take "a better reproductive history when we’re trying to judge a woman’s cardiovascular risk when they present to our office."
Dr. Sanghavi had no disclosures.
The number of children a woman has may affect her future risk of cardiovascular disease – and the mechanism may be subclinical atherosclerosis, data from the Dallas Heart Study suggest.
Women who had had two or three live births had the lowest prevalence of subclinical atherosclerosis, compared with women who had four or more children and with women who had no live births or one live birth, depicting "a U-shaped association between the number of children a woman gives birth to and future risk of cardiovascular events," said Dr. Monika Sanghavi, chief cardiology fellow at the University of Texas Southwestern Medical Center, Dallas.
Dr. Sanghavi summarized the study results in a webcast held prior to the annual meeting of the American College of Cardiology, where she is presenting the data.
The number of children women have has been associated with their future cardiovascular disease risk, but the mechanism has not been clearly understood. For this study of 1,644 women enrolled in the Dallas Heart Study the researchers addressed whether the mechanism could be subclinical atherosclerosis by using coronary artery calcium (CAC) scores measured with CT imaging and aortic wall thickness (AWT) measured with magnetic resonance imaging. The average age of the women was 45 years, and 55% were black.
The Dallas Heart Study is a multiethnic population–based sample of Dallas County residents.
CAC scores were positive (greater than 10) in 11% of the women who had 2-3 live births, compared with 27% in those who had at least 4 live births, and 15% of those who had 0-1 live births, all significant differences.
AWTs were abnormal (greater than the 75th percentile for age and gender) in 22% of those with had 2-3 live births, compared with 33% of those who had at least 4 live births and 25% of those who had 0-1 live births. The association persisted after adjustment for cardiovascular disease risk factors, including socioeconomic status, race and education.
"The results support the hypothesis that subclinical atherosclerosis may be the mechanism for this association," Dr. Sanghavi said. As for the two groups identified at risk, she added, "we think that increased risk on either end of the curve likely represents two different processes, such that repeated pregnancies increase risk in a different way than those women who are unable to get pregnant."
These results need to be confirmed in future studies, which will also further explore why women with one or no live births are at increased risk, Dr. Sanghavi said. Those with four or more live births may be at increased risk because of "repeated exposure to an atherogenic milieu," with higher cholesterol levels, insulin resistance and other pregnancy-related changes, or factors not accounted for in the study, she noted.
But women with 0-1 live births may be at increased risk because of an underlying systemic condition such as polycystic ovarian syndrome "or some other inflammatory condition that is preventing them from getting pregnant or carrying multiple pregnancies to term," she added.
Dr. Sanghavi emphasized that the results should not be used to recommend that women should have two or three children.
Dr. Vera Bittner, chair of the ACC Prevention of Cardiovascular Disease Committee, said the results highlight the need to take "a better reproductive history when we’re trying to judge a woman’s cardiovascular risk when they present to our office."
Dr. Sanghavi had no disclosures.
FROM ACC.14
Melanomas were less invasive at diagnosis in patients with established dermatologist
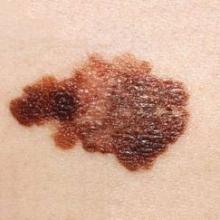
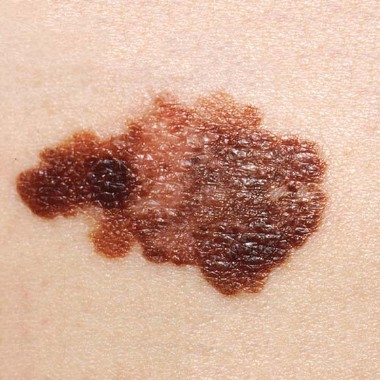
People diagnosed with melanoma were significantly more likely to have melanoma in situ and to have thinner invasive melanomas if they had a regular dermatologist, compared with those who did not have a regular dermatologist, in a retrospective study of 388 patients diagnosed at an academic dermatology department.
These findings were statistically significant for those who had detected the melanomas themselves, but not for those whose dermatologists had detected the melanomas. Self-detected melanomas were in situ in 36 of 61 (59.0%) patients with an established dermatologist vs. 40 of 108 (37.0%) patients without an established dermatologist (P =.006), reported Michelle Cheng and her associates at the University of Pittsburgh.
The time needed to wait to see a dermatologist and the time since the last dermatologic examination were not associated with the invasiveness or the depth of the melanomas at the time of the diagnosis, the investigators said.
These findings "may be explained by a high benefit associated with a first dermatologic visit because of patient education about melanoma detection and/or having a dermatologist to call when the patient finds a suspicious lesion," they wrote (J. Am. Acad. Dermatol. 2014 March [doi:10.1016/j.jaad.2013.10.060]).
The study addressed the uncertainties about the impact of different factors on the invasiveness and depth of melanoma: having had a previous physical exam by a dermatologist before the diagnosis, the recency of that exam, and the time needed to wait for an appointment. The 388 adults (mean age, 55 years) were diagnosed with melanoma at the University of Pittsburgh Medical Center between February 2003 and December 2010. Of these patients, 51% had detected the melanoma themselves and 37% had had a dermatologic exam within the previous year at the university. Of the 388 melanomas diagnosed, 44% (171) were invasive with a mean Breslow depth of 0.96 mm. About 18% (71 patients), had a history of melanoma, and about 37% had seen a dermatologist within the previous year.
Of the 317 with no previous history of melanoma, almost 64% (103 of 162) of those with an established dermatologist were diagnosed with melanoma in situ vs. 44.5% (69 of 155) of those without an established dermatologist, a statistically significant difference (P = .001). The depths of the lesions were also significantly lower among those with an established dermatologist than among those with no dermatologist (median depth, 0.48 mm vs. 0.61 mm, respectively; P = .003).
Among the patients with self-detected melanoma, 41% of those with an established dermatologist had invasive disease, vs. 63% of those who did not have an established dermatologist (P = .006). But among the patients whose melanomas were detected by the dermatologist, 31% of those with an established dermatologist had invasive disease, vs. 40% of those with no established dermatologist, which was not statistically significant (P = .323).
When considering that a skin cancer screen is cost effective, the authors concluded, the results of this study "highlight the value of having even a single dermatologic examination and suggest that educating patients to detect their own melanomas is an important part of improving early detection of melanoma."
All of the patients were from one part of Pennsylvania and were treated at the same medical center, which was a limitation of the study, but the patients were heterogenous and were treated at four dermatology clinics by different attending dermatologists, the authors said. They could not confirm that each patient received the same level of education about melanoma at their visits, but added that most of the dermatologists in the clinics teach the ABCDEs of melanoma and provide counseling in skin self-exams and AAD skin cancer brochures.
The study included clinical research fellowship funding from the Doris Duke Charitable Foundation for one of the authors, UL1/NIH funding for another author, and funding for the statistics consultation from the National Institutes of Health. The authors declared no conflicts of interest.
People diagnosed with melanoma were significantly more likely to have melanoma in situ and to have thinner invasive melanomas if they had a regular dermatologist, compared with those who did not have a regular dermatologist, in a retrospective study of 388 patients diagnosed at an academic dermatology department.
These findings were statistically significant for those who had detected the melanomas themselves, but not for those whose dermatologists had detected the melanomas. Self-detected melanomas were in situ in 36 of 61 (59.0%) patients with an established dermatologist vs. 40 of 108 (37.0%) patients without an established dermatologist (P =.006), reported Michelle Cheng and her associates at the University of Pittsburgh.
The time needed to wait to see a dermatologist and the time since the last dermatologic examination were not associated with the invasiveness or the depth of the melanomas at the time of the diagnosis, the investigators said.
These findings "may be explained by a high benefit associated with a first dermatologic visit because of patient education about melanoma detection and/or having a dermatologist to call when the patient finds a suspicious lesion," they wrote (J. Am. Acad. Dermatol. 2014 March [doi:10.1016/j.jaad.2013.10.060]).
The study addressed the uncertainties about the impact of different factors on the invasiveness and depth of melanoma: having had a previous physical exam by a dermatologist before the diagnosis, the recency of that exam, and the time needed to wait for an appointment. The 388 adults (mean age, 55 years) were diagnosed with melanoma at the University of Pittsburgh Medical Center between February 2003 and December 2010. Of these patients, 51% had detected the melanoma themselves and 37% had had a dermatologic exam within the previous year at the university. Of the 388 melanomas diagnosed, 44% (171) were invasive with a mean Breslow depth of 0.96 mm. About 18% (71 patients), had a history of melanoma, and about 37% had seen a dermatologist within the previous year.
Of the 317 with no previous history of melanoma, almost 64% (103 of 162) of those with an established dermatologist were diagnosed with melanoma in situ vs. 44.5% (69 of 155) of those without an established dermatologist, a statistically significant difference (P = .001). The depths of the lesions were also significantly lower among those with an established dermatologist than among those with no dermatologist (median depth, 0.48 mm vs. 0.61 mm, respectively; P = .003).
Among the patients with self-detected melanoma, 41% of those with an established dermatologist had invasive disease, vs. 63% of those who did not have an established dermatologist (P = .006). But among the patients whose melanomas were detected by the dermatologist, 31% of those with an established dermatologist had invasive disease, vs. 40% of those with no established dermatologist, which was not statistically significant (P = .323).
When considering that a skin cancer screen is cost effective, the authors concluded, the results of this study "highlight the value of having even a single dermatologic examination and suggest that educating patients to detect their own melanomas is an important part of improving early detection of melanoma."
All of the patients were from one part of Pennsylvania and were treated at the same medical center, which was a limitation of the study, but the patients were heterogenous and were treated at four dermatology clinics by different attending dermatologists, the authors said. They could not confirm that each patient received the same level of education about melanoma at their visits, but added that most of the dermatologists in the clinics teach the ABCDEs of melanoma and provide counseling in skin self-exams and AAD skin cancer brochures.
The study included clinical research fellowship funding from the Doris Duke Charitable Foundation for one of the authors, UL1/NIH funding for another author, and funding for the statistics consultation from the National Institutes of Health. The authors declared no conflicts of interest.
People diagnosed with melanoma were significantly more likely to have melanoma in situ and to have thinner invasive melanomas if they had a regular dermatologist, compared with those who did not have a regular dermatologist, in a retrospective study of 388 patients diagnosed at an academic dermatology department.
These findings were statistically significant for those who had detected the melanomas themselves, but not for those whose dermatologists had detected the melanomas. Self-detected melanomas were in situ in 36 of 61 (59.0%) patients with an established dermatologist vs. 40 of 108 (37.0%) patients without an established dermatologist (P =.006), reported Michelle Cheng and her associates at the University of Pittsburgh.
The time needed to wait to see a dermatologist and the time since the last dermatologic examination were not associated with the invasiveness or the depth of the melanomas at the time of the diagnosis, the investigators said.
These findings "may be explained by a high benefit associated with a first dermatologic visit because of patient education about melanoma detection and/or having a dermatologist to call when the patient finds a suspicious lesion," they wrote (J. Am. Acad. Dermatol. 2014 March [doi:10.1016/j.jaad.2013.10.060]).
The study addressed the uncertainties about the impact of different factors on the invasiveness and depth of melanoma: having had a previous physical exam by a dermatologist before the diagnosis, the recency of that exam, and the time needed to wait for an appointment. The 388 adults (mean age, 55 years) were diagnosed with melanoma at the University of Pittsburgh Medical Center between February 2003 and December 2010. Of these patients, 51% had detected the melanoma themselves and 37% had had a dermatologic exam within the previous year at the university. Of the 388 melanomas diagnosed, 44% (171) were invasive with a mean Breslow depth of 0.96 mm. About 18% (71 patients), had a history of melanoma, and about 37% had seen a dermatologist within the previous year.
Of the 317 with no previous history of melanoma, almost 64% (103 of 162) of those with an established dermatologist were diagnosed with melanoma in situ vs. 44.5% (69 of 155) of those without an established dermatologist, a statistically significant difference (P = .001). The depths of the lesions were also significantly lower among those with an established dermatologist than among those with no dermatologist (median depth, 0.48 mm vs. 0.61 mm, respectively; P = .003).
Among the patients with self-detected melanoma, 41% of those with an established dermatologist had invasive disease, vs. 63% of those who did not have an established dermatologist (P = .006). But among the patients whose melanomas were detected by the dermatologist, 31% of those with an established dermatologist had invasive disease, vs. 40% of those with no established dermatologist, which was not statistically significant (P = .323).
When considering that a skin cancer screen is cost effective, the authors concluded, the results of this study "highlight the value of having even a single dermatologic examination and suggest that educating patients to detect their own melanomas is an important part of improving early detection of melanoma."
All of the patients were from one part of Pennsylvania and were treated at the same medical center, which was a limitation of the study, but the patients were heterogenous and were treated at four dermatology clinics by different attending dermatologists, the authors said. They could not confirm that each patient received the same level of education about melanoma at their visits, but added that most of the dermatologists in the clinics teach the ABCDEs of melanoma and provide counseling in skin self-exams and AAD skin cancer brochures.
The study included clinical research fellowship funding from the Doris Duke Charitable Foundation for one of the authors, UL1/NIH funding for another author, and funding for the statistics consultation from the National Institutes of Health. The authors declared no conflicts of interest.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Major finding: In a study of 388 patients diagnosed with melanoma, those who had an established dermatologist were significantly more likely to be diagnosed with melanoma in situ than those with no dermatologist (64% vs. 44.5%; P = .001), and had thinner invasive melanoma-findings (median depth, 0.48 mm vs. 0.61 mm; P = .003).
Data source: A retrospective study of 388 patients with biopsy-confirmed melanomas at an academic medical center, which evaluated the impact of having an established dermatologist and other factors on the depth of the melanoma when diagnosed.
Disclosures: The study included Clinical Research Fellowship funding from the Doris Duke Charitable Foundation for one of the authors, UL1/NIH funding for another author, and funding for the statistics consultation from the National Institutes of Health. The authors declared no conflicts of interest.
Pediatric information added to label of hepatitis B antiviral entecavir
Information on the use of entecavir in children down to age 2 years has been added to the prescribing information for the chronic hepatitis B drug, the Food and Drug Administration has announced.
The changes include a revised indication, which now states that entecavir is indicated for treating chronic hepatitis B virus infection in adults and pediatric patients 2 years of age and older, who have evidence of active viral replication and either evidence of persistent elevations in serum aminotransferases (alanine aminotransferase or aspartate aminotransferase) or histologically active disease. The pediatric indication is based on "clinical trial data in nucleoside-inhibitor treatment–naive and in a limited number of lamivudine-experienced subjects with HbeAg [hepatitis B e antigen]-positive chronic HBV infection and compensated liver disease," according to the revised label.
Previously, the label included the statement that safety and effectiveness of entecavir in pediatric patients under age 16 have not been established. Now, the label states that the efficacy and safety have not been established in patients under age 2, and that the drug has not been studied in this age group because the need to treat children this young is rarely necessary.
Entecavir, a nucleoside analogue, was approved in 2005 and is marketed as Baraclude by Bristol-Myers Squibb. It is administered once-daily orally, in a tablet or solution formulation. The label says that the oral solution should be used for children who weigh up to 30 kg.
The revised label is available at http://packageinserts.bms.com/pi/pi_baraclude.pdf.
Information on the use of entecavir in children down to age 2 years has been added to the prescribing information for the chronic hepatitis B drug, the Food and Drug Administration has announced.
The changes include a revised indication, which now states that entecavir is indicated for treating chronic hepatitis B virus infection in adults and pediatric patients 2 years of age and older, who have evidence of active viral replication and either evidence of persistent elevations in serum aminotransferases (alanine aminotransferase or aspartate aminotransferase) or histologically active disease. The pediatric indication is based on "clinical trial data in nucleoside-inhibitor treatment–naive and in a limited number of lamivudine-experienced subjects with HbeAg [hepatitis B e antigen]-positive chronic HBV infection and compensated liver disease," according to the revised label.
Previously, the label included the statement that safety and effectiveness of entecavir in pediatric patients under age 16 have not been established. Now, the label states that the efficacy and safety have not been established in patients under age 2, and that the drug has not been studied in this age group because the need to treat children this young is rarely necessary.
Entecavir, a nucleoside analogue, was approved in 2005 and is marketed as Baraclude by Bristol-Myers Squibb. It is administered once-daily orally, in a tablet or solution formulation. The label says that the oral solution should be used for children who weigh up to 30 kg.
The revised label is available at http://packageinserts.bms.com/pi/pi_baraclude.pdf.
Information on the use of entecavir in children down to age 2 years has been added to the prescribing information for the chronic hepatitis B drug, the Food and Drug Administration has announced.
The changes include a revised indication, which now states that entecavir is indicated for treating chronic hepatitis B virus infection in adults and pediatric patients 2 years of age and older, who have evidence of active viral replication and either evidence of persistent elevations in serum aminotransferases (alanine aminotransferase or aspartate aminotransferase) or histologically active disease. The pediatric indication is based on "clinical trial data in nucleoside-inhibitor treatment–naive and in a limited number of lamivudine-experienced subjects with HbeAg [hepatitis B e antigen]-positive chronic HBV infection and compensated liver disease," according to the revised label.
Previously, the label included the statement that safety and effectiveness of entecavir in pediatric patients under age 16 have not been established. Now, the label states that the efficacy and safety have not been established in patients under age 2, and that the drug has not been studied in this age group because the need to treat children this young is rarely necessary.
Entecavir, a nucleoside analogue, was approved in 2005 and is marketed as Baraclude by Bristol-Myers Squibb. It is administered once-daily orally, in a tablet or solution formulation. The label says that the oral solution should be used for children who weigh up to 30 kg.
The revised label is available at http://packageinserts.bms.com/pi/pi_baraclude.pdf.
FDA approves PDE-4 inhibitor for treating psoriatic arthritis
Apremilast, an oral phosphodiesterase-4 inhibitor, has been approved for treating adults with active psoriatic arthritis, based on the results of three studies of 1,493 patients, the Food and Drug Administration announced on March 21.
As a postmarketing requirement, the manufacturer will evaluate the effects of exposure to treatment in pregnant women with a pregnancy registry, according to an FDA statement.
In the three studies, the signs and symptoms of psoriatic arthritis improved among patients treated with apremilast, compared with those on placebo. Diarrhea, nausea, and headache were the most common adverse events associated with treatment. Depression was reported more frequently among those treated with apremilast in the studies, according to the FDA.
Results of the phase III studies were reported in 2013 at the American College of Rheumatology annual meeting and at the annual European Congress of Rheumatology.
During treatment, health care professionals are advised to regularly monitor the weight of patients, and "if unexplained or clinically significant weight loss occurs, the weight loss should be evaluated and discontinuation of treatment should be considered," the statement said. Celgene Corporation will market apremilast under the brand name Otezla.
Apremilast, an oral phosphodiesterase-4 inhibitor, has been approved for treating adults with active psoriatic arthritis, based on the results of three studies of 1,493 patients, the Food and Drug Administration announced on March 21.
As a postmarketing requirement, the manufacturer will evaluate the effects of exposure to treatment in pregnant women with a pregnancy registry, according to an FDA statement.
In the three studies, the signs and symptoms of psoriatic arthritis improved among patients treated with apremilast, compared with those on placebo. Diarrhea, nausea, and headache were the most common adverse events associated with treatment. Depression was reported more frequently among those treated with apremilast in the studies, according to the FDA.
Results of the phase III studies were reported in 2013 at the American College of Rheumatology annual meeting and at the annual European Congress of Rheumatology.
During treatment, health care professionals are advised to regularly monitor the weight of patients, and "if unexplained or clinically significant weight loss occurs, the weight loss should be evaluated and discontinuation of treatment should be considered," the statement said. Celgene Corporation will market apremilast under the brand name Otezla.
Apremilast, an oral phosphodiesterase-4 inhibitor, has been approved for treating adults with active psoriatic arthritis, based on the results of three studies of 1,493 patients, the Food and Drug Administration announced on March 21.
As a postmarketing requirement, the manufacturer will evaluate the effects of exposure to treatment in pregnant women with a pregnancy registry, according to an FDA statement.
In the three studies, the signs and symptoms of psoriatic arthritis improved among patients treated with apremilast, compared with those on placebo. Diarrhea, nausea, and headache were the most common adverse events associated with treatment. Depression was reported more frequently among those treated with apremilast in the studies, according to the FDA.
Results of the phase III studies were reported in 2013 at the American College of Rheumatology annual meeting and at the annual European Congress of Rheumatology.
During treatment, health care professionals are advised to regularly monitor the weight of patients, and "if unexplained or clinically significant weight loss occurs, the weight loss should be evaluated and discontinuation of treatment should be considered," the statement said. Celgene Corporation will market apremilast under the brand name Otezla.
TAVR results among highlights of ACC 2014 late-breaking sessions
The results of clinical and registry studies on transcatheter aortic valve replacement are among the presentations slated for the late-breaking clinical trial sessions during the upcoming annual meeting of the American College of Cardiology meeting in Washington.
During an ACC telephone briefing on March 18, one of the meeting cochairs, Dr. Robert J. Siegel, said that results of a study comparing transcatheter placement of the CoreValve to surgical aortic valve replacement in 795 patients with severe aortic stenosis, was one of several studies that will be presented that represent "ongoing paradigm shifts" in the treatment of cardiovascular disease. The patients, whose average age was 82 years, were enrolled at 45 U.S. centers, and were randomized to surgical treatment or treatment with the self-expanding CoreValve system, which was approved by the Food and Drug Administration in January 2014 for patients who are too ill or frail to undergo surgical aortic valve replacement.
TAVR represents a "burgeoning field, and we can expect further expansion of TAVR technology into clinical practice," said Dr. Siegel, director of the Cardiac Noninvasive Laboratory, Cedars-Sinai Medical Center, Los Angeles. Another meeting cochair, Dr. Prediman K. Shah, director of the Oppenheimer Atherosclerosis Research Center and Atherosclerosis Prevention and Treatment Center at Cedars Sinai, predicted that surgical aortic valve replacement will become rare, and that as delivery methods and valve design improve over the next several years, "it’s going to be very hard to tell a patient, if they need an aortic valve, that surgery is their best option."
Results of the STS-ACC transcatheter valve registry, which has enrolled about 7,000 patients to date, will provide complementary data on TAVR, said Dr. Cindy L. Grines, the TCT-ACC-i2 chair, vice president, academic and clinical affairs at Detroit Medical Center Cardiovascular Institute, and professor of medicine Wayne State University, Detroit. This is a "very important" study, with results of 1-year outcomesin patients at 224 different sites, a far larger population than the highly selected population enrolled in clinical trials, presumably with less well-trained operators, and the use of valves other than applications studied in the original trials, she said.
The other studies that Dr. Siegel said represented paradigm shifts in treating cardiovascular disease are the CORP-2 trial, a multicenter study of patients with recurrent pericarditis comparing colchicine to placebo added to standard treatment. The third study is the 3-year follow-up results of the STAMPEDE trial, comparing the effects of bariatric surgery vs. intensive medical therapy on long-term glycemic control and diabetes complications.
Other highlights of the late-breaking clinical trials sessions include:
• Data on the safety and effectiveness of renal artery denervation to treat uncontrolled hypertension in a clinical trial comparing the treatment with medical management in more than 500 patients (SYMPLICITY HTN-3) and in the real world (the Global SYMPLICITY Registry). Dr. Shah said the clinical trial, which had a rigorous design with a sham control, "will provide us some very unique insight" into what the role of renal denervation might be for patients with difficult to control hypertension despite multiple medications." Referring to the manufacturer’s recent announcement that SYMPLICITY HTN-3 did not meet its primary efficacy endpoint, Dr. Shah said there are unanswered questions that will hopefully be addressed in this session, including whether the negative results reflect a problem with the concept of renal denervation or the technique used in the study.
• The results of a European trial addressing whether a highly sensitive troponin test can be used to identify patients who present with chest pain in the emergency department who are at low risk and do not require hospitalization.
One-year follow-up results of the noninvasively or minimally invasively implanted Melody transcatheter pulmonary valve in children and adults with congenital heart disease, who have narrowing of the pulmonic valve, which Dr. Shah said has significant implications fore the increasing population of pediatric and adult survivors of congenital heart disease.
• Two-year outcomes of the NEXT study, which compared the Nobori biodegradable polymer stent with the Xience everolimus-eluting stent, in 3,200 patients. At 1 year, when the polymer was still present, there were no differences, but at 2 years, when the polymer is gone, it will be interesting to see whether outcomes are improved further "making it superior to one of our very popular stents we are using right now," Dr. Grines said.
The results of clinical and registry studies on transcatheter aortic valve replacement are among the presentations slated for the late-breaking clinical trial sessions during the upcoming annual meeting of the American College of Cardiology meeting in Washington.
During an ACC telephone briefing on March 18, one of the meeting cochairs, Dr. Robert J. Siegel, said that results of a study comparing transcatheter placement of the CoreValve to surgical aortic valve replacement in 795 patients with severe aortic stenosis, was one of several studies that will be presented that represent "ongoing paradigm shifts" in the treatment of cardiovascular disease. The patients, whose average age was 82 years, were enrolled at 45 U.S. centers, and were randomized to surgical treatment or treatment with the self-expanding CoreValve system, which was approved by the Food and Drug Administration in January 2014 for patients who are too ill or frail to undergo surgical aortic valve replacement.
TAVR represents a "burgeoning field, and we can expect further expansion of TAVR technology into clinical practice," said Dr. Siegel, director of the Cardiac Noninvasive Laboratory, Cedars-Sinai Medical Center, Los Angeles. Another meeting cochair, Dr. Prediman K. Shah, director of the Oppenheimer Atherosclerosis Research Center and Atherosclerosis Prevention and Treatment Center at Cedars Sinai, predicted that surgical aortic valve replacement will become rare, and that as delivery methods and valve design improve over the next several years, "it’s going to be very hard to tell a patient, if they need an aortic valve, that surgery is their best option."
Results of the STS-ACC transcatheter valve registry, which has enrolled about 7,000 patients to date, will provide complementary data on TAVR, said Dr. Cindy L. Grines, the TCT-ACC-i2 chair, vice president, academic and clinical affairs at Detroit Medical Center Cardiovascular Institute, and professor of medicine Wayne State University, Detroit. This is a "very important" study, with results of 1-year outcomesin patients at 224 different sites, a far larger population than the highly selected population enrolled in clinical trials, presumably with less well-trained operators, and the use of valves other than applications studied in the original trials, she said.
The other studies that Dr. Siegel said represented paradigm shifts in treating cardiovascular disease are the CORP-2 trial, a multicenter study of patients with recurrent pericarditis comparing colchicine to placebo added to standard treatment. The third study is the 3-year follow-up results of the STAMPEDE trial, comparing the effects of bariatric surgery vs. intensive medical therapy on long-term glycemic control and diabetes complications.
Other highlights of the late-breaking clinical trials sessions include:
• Data on the safety and effectiveness of renal artery denervation to treat uncontrolled hypertension in a clinical trial comparing the treatment with medical management in more than 500 patients (SYMPLICITY HTN-3) and in the real world (the Global SYMPLICITY Registry). Dr. Shah said the clinical trial, which had a rigorous design with a sham control, "will provide us some very unique insight" into what the role of renal denervation might be for patients with difficult to control hypertension despite multiple medications." Referring to the manufacturer’s recent announcement that SYMPLICITY HTN-3 did not meet its primary efficacy endpoint, Dr. Shah said there are unanswered questions that will hopefully be addressed in this session, including whether the negative results reflect a problem with the concept of renal denervation or the technique used in the study.
• The results of a European trial addressing whether a highly sensitive troponin test can be used to identify patients who present with chest pain in the emergency department who are at low risk and do not require hospitalization.
One-year follow-up results of the noninvasively or minimally invasively implanted Melody transcatheter pulmonary valve in children and adults with congenital heart disease, who have narrowing of the pulmonic valve, which Dr. Shah said has significant implications fore the increasing population of pediatric and adult survivors of congenital heart disease.
• Two-year outcomes of the NEXT study, which compared the Nobori biodegradable polymer stent with the Xience everolimus-eluting stent, in 3,200 patients. At 1 year, when the polymer was still present, there were no differences, but at 2 years, when the polymer is gone, it will be interesting to see whether outcomes are improved further "making it superior to one of our very popular stents we are using right now," Dr. Grines said.
The results of clinical and registry studies on transcatheter aortic valve replacement are among the presentations slated for the late-breaking clinical trial sessions during the upcoming annual meeting of the American College of Cardiology meeting in Washington.
During an ACC telephone briefing on March 18, one of the meeting cochairs, Dr. Robert J. Siegel, said that results of a study comparing transcatheter placement of the CoreValve to surgical aortic valve replacement in 795 patients with severe aortic stenosis, was one of several studies that will be presented that represent "ongoing paradigm shifts" in the treatment of cardiovascular disease. The patients, whose average age was 82 years, were enrolled at 45 U.S. centers, and were randomized to surgical treatment or treatment with the self-expanding CoreValve system, which was approved by the Food and Drug Administration in January 2014 for patients who are too ill or frail to undergo surgical aortic valve replacement.
TAVR represents a "burgeoning field, and we can expect further expansion of TAVR technology into clinical practice," said Dr. Siegel, director of the Cardiac Noninvasive Laboratory, Cedars-Sinai Medical Center, Los Angeles. Another meeting cochair, Dr. Prediman K. Shah, director of the Oppenheimer Atherosclerosis Research Center and Atherosclerosis Prevention and Treatment Center at Cedars Sinai, predicted that surgical aortic valve replacement will become rare, and that as delivery methods and valve design improve over the next several years, "it’s going to be very hard to tell a patient, if they need an aortic valve, that surgery is their best option."
Results of the STS-ACC transcatheter valve registry, which has enrolled about 7,000 patients to date, will provide complementary data on TAVR, said Dr. Cindy L. Grines, the TCT-ACC-i2 chair, vice president, academic and clinical affairs at Detroit Medical Center Cardiovascular Institute, and professor of medicine Wayne State University, Detroit. This is a "very important" study, with results of 1-year outcomesin patients at 224 different sites, a far larger population than the highly selected population enrolled in clinical trials, presumably with less well-trained operators, and the use of valves other than applications studied in the original trials, she said.
The other studies that Dr. Siegel said represented paradigm shifts in treating cardiovascular disease are the CORP-2 trial, a multicenter study of patients with recurrent pericarditis comparing colchicine to placebo added to standard treatment. The third study is the 3-year follow-up results of the STAMPEDE trial, comparing the effects of bariatric surgery vs. intensive medical therapy on long-term glycemic control and diabetes complications.
Other highlights of the late-breaking clinical trials sessions include:
• Data on the safety and effectiveness of renal artery denervation to treat uncontrolled hypertension in a clinical trial comparing the treatment with medical management in more than 500 patients (SYMPLICITY HTN-3) and in the real world (the Global SYMPLICITY Registry). Dr. Shah said the clinical trial, which had a rigorous design with a sham control, "will provide us some very unique insight" into what the role of renal denervation might be for patients with difficult to control hypertension despite multiple medications." Referring to the manufacturer’s recent announcement that SYMPLICITY HTN-3 did not meet its primary efficacy endpoint, Dr. Shah said there are unanswered questions that will hopefully be addressed in this session, including whether the negative results reflect a problem with the concept of renal denervation or the technique used in the study.
• The results of a European trial addressing whether a highly sensitive troponin test can be used to identify patients who present with chest pain in the emergency department who are at low risk and do not require hospitalization.
One-year follow-up results of the noninvasively or minimally invasively implanted Melody transcatheter pulmonary valve in children and adults with congenital heart disease, who have narrowing of the pulmonic valve, which Dr. Shah said has significant implications fore the increasing population of pediatric and adult survivors of congenital heart disease.
• Two-year outcomes of the NEXT study, which compared the Nobori biodegradable polymer stent with the Xience everolimus-eluting stent, in 3,200 patients. At 1 year, when the polymer was still present, there were no differences, but at 2 years, when the polymer is gone, it will be interesting to see whether outcomes are improved further "making it superior to one of our very popular stents we are using right now," Dr. Grines said.
FDA extends review of pegylated interferon for MS, Biogen says
The Food and Drug Administration review of a pegylated interferon for multiple sclerosis has been extended by 3 months, according to the manufacturer.
A statement issued by Biogen Idec on March 18 said that the agency had extended the Prescription Drug User Fee Act (PDUFA) date for the product’s review by 3 months, "to allow additional time for review of the application." The agency is not requesting additional studies, the statement said.
The product is a subcutaneously administered formulation of pegylated interferon beta-1a for treating relapsing forms of MS. If the agent is approved, Biogen plans to market it as Plegridy.
In the ADVANCE trial of about 1,500 patients with relapsing-remitting MS, treatment with this pegylated interferon administered every 2 weeks was associated with statistically significant advantages for all clinical and radiologic outcomes, compared with placebo, over 1 year.
The product is also being reviewed in Europe for the same indication.
The Food and Drug Administration review of a pegylated interferon for multiple sclerosis has been extended by 3 months, according to the manufacturer.
A statement issued by Biogen Idec on March 18 said that the agency had extended the Prescription Drug User Fee Act (PDUFA) date for the product’s review by 3 months, "to allow additional time for review of the application." The agency is not requesting additional studies, the statement said.
The product is a subcutaneously administered formulation of pegylated interferon beta-1a for treating relapsing forms of MS. If the agent is approved, Biogen plans to market it as Plegridy.
In the ADVANCE trial of about 1,500 patients with relapsing-remitting MS, treatment with this pegylated interferon administered every 2 weeks was associated with statistically significant advantages for all clinical and radiologic outcomes, compared with placebo, over 1 year.
The product is also being reviewed in Europe for the same indication.
The Food and Drug Administration review of a pegylated interferon for multiple sclerosis has been extended by 3 months, according to the manufacturer.
A statement issued by Biogen Idec on March 18 said that the agency had extended the Prescription Drug User Fee Act (PDUFA) date for the product’s review by 3 months, "to allow additional time for review of the application." The agency is not requesting additional studies, the statement said.
The product is a subcutaneously administered formulation of pegylated interferon beta-1a for treating relapsing forms of MS. If the agent is approved, Biogen plans to market it as Plegridy.
In the ADVANCE trial of about 1,500 patients with relapsing-remitting MS, treatment with this pegylated interferon administered every 2 weeks was associated with statistically significant advantages for all clinical and radiologic outcomes, compared with placebo, over 1 year.
The product is also being reviewed in Europe for the same indication.