User login
Can We Successfully Adapt to Changes in Direction and Support for Acne?
Can We Successfully Adapt to Changes in Direction and Support for Acne?
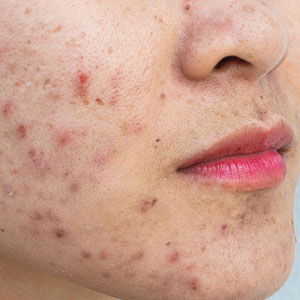
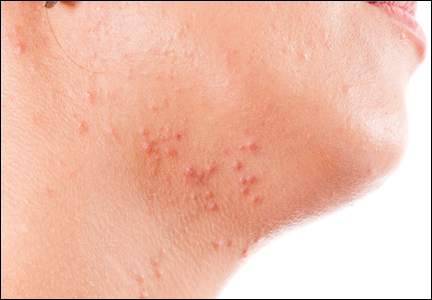
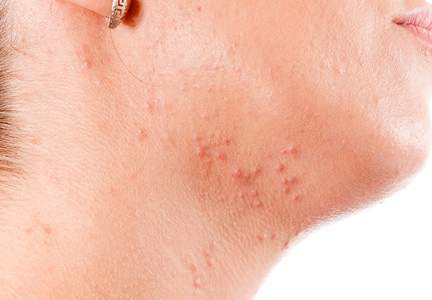
How did I develop a strong interest in acne and rosacea? Interest on a personal level was with me throughout my adolescence and post-teen years as I suffered with very severe facial acne from ages 13 through 23 (1967-1977). I was sometimes called “pizza face” in high school, and biweekly trips to a dermatology office that always had a packed waiting room were of little help that I could appreciate visibly. Six straight years of extractions, intralesional injections, draining of fluctuant cysts, UVC light treatments, oral tetracycline, irritating topical formulations of benzoyl peroxide and tretinoin, and topical sulfacetamide-sulfur products resulted in minimal improvement. However, maybe all of this did something to what was happening underneath the skin surface, as I have no residual acne scars. I do recall vividly that I walked the halls in high school and college consistently affected by a very red face from the topical agents and smelling like rotten eggs from the topical sulfur application. I fortunately handled it well emotionally and socially, for which I am very thankful. Many people affected with acne do not.
In dermatology, I have always had a strong interest in pathophysiology and therapeutics, rooted I am sure in my background as a pharmacist. Although I was always interested in acne therapy, I was fully captivated by a presentation given by Dr. Jim Leyden many years ago at a small meeting in Myrtle Beach, South Carolina. He brought the subject of acne to life in a way that more than grabbed my complete attention and ignited an interest in learning everything I could about it. Over time, I was fortunate enough to work alongside Dr. Leyden and many other household names in acne at meetings and publications to further education on one of the most common disease states seen in ambulatory dermatology practices worldwide. The rest is history, leading to almost 4 decades of work in acne on many levels in dermatology, all being efforts that I am grateful for.
What I have observed to date is that we have had few revolutionary advances in acne therapy, the major one being oral isotretinoin, which was first brought to market in 1982. We are still utilizing many of the same therapeutic agents that I used back when I was treated for acne. A few new topical compounds have emerged, such as dapsone and clascoterone, and a narrow-spectrum tetracycline agent, sarecycline, also was developed. These agents do represent important advances with some specific benefits. There have been many major improvements in drug delivery formulations, including several vehicle technologies that allow augmented skin tolerability, increased efficacy, and improved stability, allowing for combination therapy products containing 2 or 3 active ingredients. A recent example is the first triple-combination topical acne therapy with excellent supporting data on speed of onset, efficacy, and safety.1
Technological advances also have aided in the development of modified- or extended-release formulations of oral antibiotics, such as doxycycline and minocycline, which allow for reduced adverse effects and lower daily dosages. Lidose formulations of isotretinoin have circumvented the need for concurrent ingestion of a high-fat meal to facilitate its absorption in the gastrointestinal tract (as required with conventional formulations). Many hours also have been spent on delivery devices and vehicles such as pumps, foams, and aqueous-based gels. Let us not forget the efforts and myriad products directed at skin care, cosmeceuticals, and physical devices (lasers and lights) for acne. Regardless of the above, we have not seen the monumental therapeutic and research revolution for acne that we have experienced more recently with biologic agents, Janus kinase inhibitors, and other modes of action for many common disease states such as atopic dermatitis, psoriasis, alopecia areata, vitiligo, hidradenitis suppurativa, prurigo nodularis, and chronic spontaneous urticaria.
Unfortunately, the slow development of advances in treatments for acne has been compounded further by the widespread availability of generic equivalents of most topical and oral therapies along with several over-the- counter topical medications. The expanded skin care and cosmeceutical product world has further diluted the perceived value of topical prescription therapies for acne. The marked difficulty in achieving and sustaining total clearance of acne, with the exception of many individuals treated with oral isotretinoin, results in many patients searching for other options, often through sources beyond dermatology practices (eg, the internet). While some of these sources may provide valid suggestions, they often are not truly substantiated by valid clinical research and are not formally regulated by the US Food and Drug Administration.
All of the above, in addition to the barriers to medication coverage put in place by third-party organizations such as pharmacy benefit managers, have contributed to the extreme slowdown in the development of new prescription therapies for acne. What this leads me to believe is that until there is a true meeting of the minds of all stakeholders on policies that facilitate access to both established and newly available acne therapies, there will be an enduring diminished incentive to support the development of newer acne treatments that will continue to spiral progressively downward. Some research on acne will always continue, such as the search for an acne vaccine and cutaneous microbiome alterations that are in progress.2,3 However, I do not see much happening in the foreseeable future. I am not inherently a pessimist or a “prophet of doom,” so I sincerely hope I am wrong.
- Stein Gold L, Baldwin H, Kircik LH, et al. Efficacy and safety of a fixed-dose clindamycin phosphate 1.2%, benzoyl peroxide 3.1%, and adapalene 0.15% gel for moderate-to-severe acne: a randomized phase II study of the first triple-combination drug. Am J Clin Dermatol. 2022;23:93-104. doi:10.1007/s40257-021-00650-3
- Keshari S, Kumar M, Balasubramaniam A, et al. Prospects of acne vaccines targeting secreted virulence factors of Cutibacterium acnes. Expert Rev Vaccines. 2019;18:433-437. doi:10.1080/14760584
- Dreno B, Dekio I, Baldwin H, et al. Acne microbiome: from phyla to phylotypes. J Eur Acad Dermatol Venereol. 2024;38:657- 664. doi:10.1111/jdv.19540 .2019.1593830
How did I develop a strong interest in acne and rosacea? Interest on a personal level was with me throughout my adolescence and post-teen years as I suffered with very severe facial acne from ages 13 through 23 (1967-1977). I was sometimes called “pizza face” in high school, and biweekly trips to a dermatology office that always had a packed waiting room were of little help that I could appreciate visibly. Six straight years of extractions, intralesional injections, draining of fluctuant cysts, UVC light treatments, oral tetracycline, irritating topical formulations of benzoyl peroxide and tretinoin, and topical sulfacetamide-sulfur products resulted in minimal improvement. However, maybe all of this did something to what was happening underneath the skin surface, as I have no residual acne scars. I do recall vividly that I walked the halls in high school and college consistently affected by a very red face from the topical agents and smelling like rotten eggs from the topical sulfur application. I fortunately handled it well emotionally and socially, for which I am very thankful. Many people affected with acne do not.
In dermatology, I have always had a strong interest in pathophysiology and therapeutics, rooted I am sure in my background as a pharmacist. Although I was always interested in acne therapy, I was fully captivated by a presentation given by Dr. Jim Leyden many years ago at a small meeting in Myrtle Beach, South Carolina. He brought the subject of acne to life in a way that more than grabbed my complete attention and ignited an interest in learning everything I could about it. Over time, I was fortunate enough to work alongside Dr. Leyden and many other household names in acne at meetings and publications to further education on one of the most common disease states seen in ambulatory dermatology practices worldwide. The rest is history, leading to almost 4 decades of work in acne on many levels in dermatology, all being efforts that I am grateful for.
What I have observed to date is that we have had few revolutionary advances in acne therapy, the major one being oral isotretinoin, which was first brought to market in 1982. We are still utilizing many of the same therapeutic agents that I used back when I was treated for acne. A few new topical compounds have emerged, such as dapsone and clascoterone, and a narrow-spectrum tetracycline agent, sarecycline, also was developed. These agents do represent important advances with some specific benefits. There have been many major improvements in drug delivery formulations, including several vehicle technologies that allow augmented skin tolerability, increased efficacy, and improved stability, allowing for combination therapy products containing 2 or 3 active ingredients. A recent example is the first triple-combination topical acne therapy with excellent supporting data on speed of onset, efficacy, and safety.1
Technological advances also have aided in the development of modified- or extended-release formulations of oral antibiotics, such as doxycycline and minocycline, which allow for reduced adverse effects and lower daily dosages. Lidose formulations of isotretinoin have circumvented the need for concurrent ingestion of a high-fat meal to facilitate its absorption in the gastrointestinal tract (as required with conventional formulations). Many hours also have been spent on delivery devices and vehicles such as pumps, foams, and aqueous-based gels. Let us not forget the efforts and myriad products directed at skin care, cosmeceuticals, and physical devices (lasers and lights) for acne. Regardless of the above, we have not seen the monumental therapeutic and research revolution for acne that we have experienced more recently with biologic agents, Janus kinase inhibitors, and other modes of action for many common disease states such as atopic dermatitis, psoriasis, alopecia areata, vitiligo, hidradenitis suppurativa, prurigo nodularis, and chronic spontaneous urticaria.
Unfortunately, the slow development of advances in treatments for acne has been compounded further by the widespread availability of generic equivalents of most topical and oral therapies along with several over-the- counter topical medications. The expanded skin care and cosmeceutical product world has further diluted the perceived value of topical prescription therapies for acne. The marked difficulty in achieving and sustaining total clearance of acne, with the exception of many individuals treated with oral isotretinoin, results in many patients searching for other options, often through sources beyond dermatology practices (eg, the internet). While some of these sources may provide valid suggestions, they often are not truly substantiated by valid clinical research and are not formally regulated by the US Food and Drug Administration.
All of the above, in addition to the barriers to medication coverage put in place by third-party organizations such as pharmacy benefit managers, have contributed to the extreme slowdown in the development of new prescription therapies for acne. What this leads me to believe is that until there is a true meeting of the minds of all stakeholders on policies that facilitate access to both established and newly available acne therapies, there will be an enduring diminished incentive to support the development of newer acne treatments that will continue to spiral progressively downward. Some research on acne will always continue, such as the search for an acne vaccine and cutaneous microbiome alterations that are in progress.2,3 However, I do not see much happening in the foreseeable future. I am not inherently a pessimist or a “prophet of doom,” so I sincerely hope I am wrong.
How did I develop a strong interest in acne and rosacea? Interest on a personal level was with me throughout my adolescence and post-teen years as I suffered with very severe facial acne from ages 13 through 23 (1967-1977). I was sometimes called “pizza face” in high school, and biweekly trips to a dermatology office that always had a packed waiting room were of little help that I could appreciate visibly. Six straight years of extractions, intralesional injections, draining of fluctuant cysts, UVC light treatments, oral tetracycline, irritating topical formulations of benzoyl peroxide and tretinoin, and topical sulfacetamide-sulfur products resulted in minimal improvement. However, maybe all of this did something to what was happening underneath the skin surface, as I have no residual acne scars. I do recall vividly that I walked the halls in high school and college consistently affected by a very red face from the topical agents and smelling like rotten eggs from the topical sulfur application. I fortunately handled it well emotionally and socially, for which I am very thankful. Many people affected with acne do not.
In dermatology, I have always had a strong interest in pathophysiology and therapeutics, rooted I am sure in my background as a pharmacist. Although I was always interested in acne therapy, I was fully captivated by a presentation given by Dr. Jim Leyden many years ago at a small meeting in Myrtle Beach, South Carolina. He brought the subject of acne to life in a way that more than grabbed my complete attention and ignited an interest in learning everything I could about it. Over time, I was fortunate enough to work alongside Dr. Leyden and many other household names in acne at meetings and publications to further education on one of the most common disease states seen in ambulatory dermatology practices worldwide. The rest is history, leading to almost 4 decades of work in acne on many levels in dermatology, all being efforts that I am grateful for.
What I have observed to date is that we have had few revolutionary advances in acne therapy, the major one being oral isotretinoin, which was first brought to market in 1982. We are still utilizing many of the same therapeutic agents that I used back when I was treated for acne. A few new topical compounds have emerged, such as dapsone and clascoterone, and a narrow-spectrum tetracycline agent, sarecycline, also was developed. These agents do represent important advances with some specific benefits. There have been many major improvements in drug delivery formulations, including several vehicle technologies that allow augmented skin tolerability, increased efficacy, and improved stability, allowing for combination therapy products containing 2 or 3 active ingredients. A recent example is the first triple-combination topical acne therapy with excellent supporting data on speed of onset, efficacy, and safety.1
Technological advances also have aided in the development of modified- or extended-release formulations of oral antibiotics, such as doxycycline and minocycline, which allow for reduced adverse effects and lower daily dosages. Lidose formulations of isotretinoin have circumvented the need for concurrent ingestion of a high-fat meal to facilitate its absorption in the gastrointestinal tract (as required with conventional formulations). Many hours also have been spent on delivery devices and vehicles such as pumps, foams, and aqueous-based gels. Let us not forget the efforts and myriad products directed at skin care, cosmeceuticals, and physical devices (lasers and lights) for acne. Regardless of the above, we have not seen the monumental therapeutic and research revolution for acne that we have experienced more recently with biologic agents, Janus kinase inhibitors, and other modes of action for many common disease states such as atopic dermatitis, psoriasis, alopecia areata, vitiligo, hidradenitis suppurativa, prurigo nodularis, and chronic spontaneous urticaria.
Unfortunately, the slow development of advances in treatments for acne has been compounded further by the widespread availability of generic equivalents of most topical and oral therapies along with several over-the- counter topical medications. The expanded skin care and cosmeceutical product world has further diluted the perceived value of topical prescription therapies for acne. The marked difficulty in achieving and sustaining total clearance of acne, with the exception of many individuals treated with oral isotretinoin, results in many patients searching for other options, often through sources beyond dermatology practices (eg, the internet). While some of these sources may provide valid suggestions, they often are not truly substantiated by valid clinical research and are not formally regulated by the US Food and Drug Administration.
All of the above, in addition to the barriers to medication coverage put in place by third-party organizations such as pharmacy benefit managers, have contributed to the extreme slowdown in the development of new prescription therapies for acne. What this leads me to believe is that until there is a true meeting of the minds of all stakeholders on policies that facilitate access to both established and newly available acne therapies, there will be an enduring diminished incentive to support the development of newer acne treatments that will continue to spiral progressively downward. Some research on acne will always continue, such as the search for an acne vaccine and cutaneous microbiome alterations that are in progress.2,3 However, I do not see much happening in the foreseeable future. I am not inherently a pessimist or a “prophet of doom,” so I sincerely hope I am wrong.
- Stein Gold L, Baldwin H, Kircik LH, et al. Efficacy and safety of a fixed-dose clindamycin phosphate 1.2%, benzoyl peroxide 3.1%, and adapalene 0.15% gel for moderate-to-severe acne: a randomized phase II study of the first triple-combination drug. Am J Clin Dermatol. 2022;23:93-104. doi:10.1007/s40257-021-00650-3
- Keshari S, Kumar M, Balasubramaniam A, et al. Prospects of acne vaccines targeting secreted virulence factors of Cutibacterium acnes. Expert Rev Vaccines. 2019;18:433-437. doi:10.1080/14760584
- Dreno B, Dekio I, Baldwin H, et al. Acne microbiome: from phyla to phylotypes. J Eur Acad Dermatol Venereol. 2024;38:657- 664. doi:10.1111/jdv.19540 .2019.1593830
- Stein Gold L, Baldwin H, Kircik LH, et al. Efficacy and safety of a fixed-dose clindamycin phosphate 1.2%, benzoyl peroxide 3.1%, and adapalene 0.15% gel for moderate-to-severe acne: a randomized phase II study of the first triple-combination drug. Am J Clin Dermatol. 2022;23:93-104. doi:10.1007/s40257-021-00650-3
- Keshari S, Kumar M, Balasubramaniam A, et al. Prospects of acne vaccines targeting secreted virulence factors of Cutibacterium acnes. Expert Rev Vaccines. 2019;18:433-437. doi:10.1080/14760584
- Dreno B, Dekio I, Baldwin H, et al. Acne microbiome: from phyla to phylotypes. J Eur Acad Dermatol Venereol. 2024;38:657- 664. doi:10.1111/jdv.19540 .2019.1593830
Can We Successfully Adapt to Changes in Direction and Support for Acne?
Can We Successfully Adapt to Changes in Direction and Support for Acne?
Benzoyl Peroxide, Benzene, and Lots of Unanswered Questions: Where Are We Now?
March 2024 proved to be a busy month for benzoyl peroxide in the media! We are now at almost 4 months since Valisure, an independent analytical laboratory located in Connecticut, filed a Citizen Petition on benzene in benzoyl peroxide drug products with the US Food and Drug Administration (FDA) on March 5, 2024.1 This petition was filed shortly before the annual meeting of the American Academy of Dermatology was held in San Diego, California, creating quite a stir of concern in the dermatology world. Further information on the degradation of benzoyl peroxide with production of benzene was published in the medical literature in March 2024.2 As benzene is recognized as a human carcinogen, manufacturing regulations exist to assure that it does not appear in topical products either through contamination or degradation over the course of a product’s shelf-life.3
As anticipated, several opinions and commentaries appeared quickly, both on video and in various articles. The American Acne & Rosacea Society (AARS) released a statement on this issue on March 20, 2024.4 The safety of the public is the overarching primary concern. This AARS statement does include some general suggestions related to benzoyl peroxide use based on the best assessment to date while awaiting further guidance from the FDA on this issue. Benzoyl peroxide is approved for use by the FDA as an over-the-counter (OTC) topical product for acne and also is in several FDA-approved prescription topical products.5,6
The following reflects my personal viewpoint as both a dermatologist and a grandfather who has grandchildren who use acne products. My views are not necessarily those of AARS. Since early March 2024, I have read several documents and spoken to several dermatologists, scientists, and formulators with knowledge in this area, including contacts at Valisure. I was hoping to get to some reasonable definitive answer but have not been able to achieve this to my full satisfaction. There are many opinions and concerns, and each one makes sense based on the vantage point of the presenter. However, several unanswered questions remain related to what testing and data are currently required of companies to gain FDA approval of a benzoyl peroxide product, including:
- assessment of stability and degradation products (including benzene),
- validation of testing methods,
- the issue of benzoyl peroxide stability in commercial products, and
- the relevant magnitude of resultant benzene exposures, especially as we are all exposed to benzene from several sources each day.
I am certain that companies with benzoyl peroxide products will evaluate their already-approved products and also do further testing. However, in this situation, which impacts millions of people on so many levels, I feel there needs to be an organized approach to evaluate and resolve the issue, otherwise the likelihood of continued confusion and uncertainty is high. As the FDA is the approval body, I am hoping it will provide definitive guidance within a reasonable timeline so that clinicians, patients, and manufacturers of benzoyl peroxide can proceed with full confidence. Right now, we all remain in a state of limbo. It is time for less talk and more definitive action to sort out this issue.
- Valisure Citizen Petition on Benzene in Benzoyl Peroxide Products. March 5, 2024. Accessed June 5, 2024. https://assets-global.website-files.com/6215052733f8bb8fea016220/65e8560962ed23f744902a7b_Valisure%20Citizen%20Petition%20on%20Benzene%20in%20Benzoyl%20Peroxide%20Drug%20Products.pdf
- Kucera K, Zenzola N, Hudspeth A, et al. Benzoyl peroxide drug products form benzene. Environ Health Perspect. 2024;132:37702. doi:10.1289/EHP13984
- US Food and Drug Administration. Reformulating drug products that contain carbomers manufactured with benzene. December 2023. Accessed June 12, 2024. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/reformulating-drug-products-contain-carbomers-manufactured-benzene
- American Acne & Rosacea Society. Response Statement from the AARS to the Valisure Citizen Petition on Benzene in Benzoyl Peroxide Drug Products. March 20, 2024. Accessed June 12, 2024. https://www.einpresswire.com/article/697481595/response-statement-from-the-aars-to-the-valisure-citizen-petition-on-benzene-in-benzoyl-peroxide-drug-products
- Department of Health and Human Services. Classification of benzoyl peroxide as safe and effective and revision of labeling to drug facts format; topical acne drug products for over-the-counter human use; Final Rule. Fed Registr. 2010;75:9767-9777.
- US Food and Drug Administration. Topical acne drug products for over-the-counter human use—revision of labeling and classification of benzoyl peroxide as safe and effective. June 2011. Accessed June 12, 2024. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/topical-acne-drug-products-over-counter-human-use-revision-labeling-and-classification-benzoyl
March 2024 proved to be a busy month for benzoyl peroxide in the media! We are now at almost 4 months since Valisure, an independent analytical laboratory located in Connecticut, filed a Citizen Petition on benzene in benzoyl peroxide drug products with the US Food and Drug Administration (FDA) on March 5, 2024.1 This petition was filed shortly before the annual meeting of the American Academy of Dermatology was held in San Diego, California, creating quite a stir of concern in the dermatology world. Further information on the degradation of benzoyl peroxide with production of benzene was published in the medical literature in March 2024.2 As benzene is recognized as a human carcinogen, manufacturing regulations exist to assure that it does not appear in topical products either through contamination or degradation over the course of a product’s shelf-life.3
As anticipated, several opinions and commentaries appeared quickly, both on video and in various articles. The American Acne & Rosacea Society (AARS) released a statement on this issue on March 20, 2024.4 The safety of the public is the overarching primary concern. This AARS statement does include some general suggestions related to benzoyl peroxide use based on the best assessment to date while awaiting further guidance from the FDA on this issue. Benzoyl peroxide is approved for use by the FDA as an over-the-counter (OTC) topical product for acne and also is in several FDA-approved prescription topical products.5,6
The following reflects my personal viewpoint as both a dermatologist and a grandfather who has grandchildren who use acne products. My views are not necessarily those of AARS. Since early March 2024, I have read several documents and spoken to several dermatologists, scientists, and formulators with knowledge in this area, including contacts at Valisure. I was hoping to get to some reasonable definitive answer but have not been able to achieve this to my full satisfaction. There are many opinions and concerns, and each one makes sense based on the vantage point of the presenter. However, several unanswered questions remain related to what testing and data are currently required of companies to gain FDA approval of a benzoyl peroxide product, including:
- assessment of stability and degradation products (including benzene),
- validation of testing methods,
- the issue of benzoyl peroxide stability in commercial products, and
- the relevant magnitude of resultant benzene exposures, especially as we are all exposed to benzene from several sources each day.
I am certain that companies with benzoyl peroxide products will evaluate their already-approved products and also do further testing. However, in this situation, which impacts millions of people on so many levels, I feel there needs to be an organized approach to evaluate and resolve the issue, otherwise the likelihood of continued confusion and uncertainty is high. As the FDA is the approval body, I am hoping it will provide definitive guidance within a reasonable timeline so that clinicians, patients, and manufacturers of benzoyl peroxide can proceed with full confidence. Right now, we all remain in a state of limbo. It is time for less talk and more definitive action to sort out this issue.
March 2024 proved to be a busy month for benzoyl peroxide in the media! We are now at almost 4 months since Valisure, an independent analytical laboratory located in Connecticut, filed a Citizen Petition on benzene in benzoyl peroxide drug products with the US Food and Drug Administration (FDA) on March 5, 2024.1 This petition was filed shortly before the annual meeting of the American Academy of Dermatology was held in San Diego, California, creating quite a stir of concern in the dermatology world. Further information on the degradation of benzoyl peroxide with production of benzene was published in the medical literature in March 2024.2 As benzene is recognized as a human carcinogen, manufacturing regulations exist to assure that it does not appear in topical products either through contamination or degradation over the course of a product’s shelf-life.3
As anticipated, several opinions and commentaries appeared quickly, both on video and in various articles. The American Acne & Rosacea Society (AARS) released a statement on this issue on March 20, 2024.4 The safety of the public is the overarching primary concern. This AARS statement does include some general suggestions related to benzoyl peroxide use based on the best assessment to date while awaiting further guidance from the FDA on this issue. Benzoyl peroxide is approved for use by the FDA as an over-the-counter (OTC) topical product for acne and also is in several FDA-approved prescription topical products.5,6
The following reflects my personal viewpoint as both a dermatologist and a grandfather who has grandchildren who use acne products. My views are not necessarily those of AARS. Since early March 2024, I have read several documents and spoken to several dermatologists, scientists, and formulators with knowledge in this area, including contacts at Valisure. I was hoping to get to some reasonable definitive answer but have not been able to achieve this to my full satisfaction. There are many opinions and concerns, and each one makes sense based on the vantage point of the presenter. However, several unanswered questions remain related to what testing and data are currently required of companies to gain FDA approval of a benzoyl peroxide product, including:
- assessment of stability and degradation products (including benzene),
- validation of testing methods,
- the issue of benzoyl peroxide stability in commercial products, and
- the relevant magnitude of resultant benzene exposures, especially as we are all exposed to benzene from several sources each day.
I am certain that companies with benzoyl peroxide products will evaluate their already-approved products and also do further testing. However, in this situation, which impacts millions of people on so many levels, I feel there needs to be an organized approach to evaluate and resolve the issue, otherwise the likelihood of continued confusion and uncertainty is high. As the FDA is the approval body, I am hoping it will provide definitive guidance within a reasonable timeline so that clinicians, patients, and manufacturers of benzoyl peroxide can proceed with full confidence. Right now, we all remain in a state of limbo. It is time for less talk and more definitive action to sort out this issue.
- Valisure Citizen Petition on Benzene in Benzoyl Peroxide Products. March 5, 2024. Accessed June 5, 2024. https://assets-global.website-files.com/6215052733f8bb8fea016220/65e8560962ed23f744902a7b_Valisure%20Citizen%20Petition%20on%20Benzene%20in%20Benzoyl%20Peroxide%20Drug%20Products.pdf
- Kucera K, Zenzola N, Hudspeth A, et al. Benzoyl peroxide drug products form benzene. Environ Health Perspect. 2024;132:37702. doi:10.1289/EHP13984
- US Food and Drug Administration. Reformulating drug products that contain carbomers manufactured with benzene. December 2023. Accessed June 12, 2024. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/reformulating-drug-products-contain-carbomers-manufactured-benzene
- American Acne & Rosacea Society. Response Statement from the AARS to the Valisure Citizen Petition on Benzene in Benzoyl Peroxide Drug Products. March 20, 2024. Accessed June 12, 2024. https://www.einpresswire.com/article/697481595/response-statement-from-the-aars-to-the-valisure-citizen-petition-on-benzene-in-benzoyl-peroxide-drug-products
- Department of Health and Human Services. Classification of benzoyl peroxide as safe and effective and revision of labeling to drug facts format; topical acne drug products for over-the-counter human use; Final Rule. Fed Registr. 2010;75:9767-9777.
- US Food and Drug Administration. Topical acne drug products for over-the-counter human use—revision of labeling and classification of benzoyl peroxide as safe and effective. June 2011. Accessed June 12, 2024. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/topical-acne-drug-products-over-counter-human-use-revision-labeling-and-classification-benzoyl
- Valisure Citizen Petition on Benzene in Benzoyl Peroxide Products. March 5, 2024. Accessed June 5, 2024. https://assets-global.website-files.com/6215052733f8bb8fea016220/65e8560962ed23f744902a7b_Valisure%20Citizen%20Petition%20on%20Benzene%20in%20Benzoyl%20Peroxide%20Drug%20Products.pdf
- Kucera K, Zenzola N, Hudspeth A, et al. Benzoyl peroxide drug products form benzene. Environ Health Perspect. 2024;132:37702. doi:10.1289/EHP13984
- US Food and Drug Administration. Reformulating drug products that contain carbomers manufactured with benzene. December 2023. Accessed June 12, 2024. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/reformulating-drug-products-contain-carbomers-manufactured-benzene
- American Acne & Rosacea Society. Response Statement from the AARS to the Valisure Citizen Petition on Benzene in Benzoyl Peroxide Drug Products. March 20, 2024. Accessed June 12, 2024. https://www.einpresswire.com/article/697481595/response-statement-from-the-aars-to-the-valisure-citizen-petition-on-benzene-in-benzoyl-peroxide-drug-products
- Department of Health and Human Services. Classification of benzoyl peroxide as safe and effective and revision of labeling to drug facts format; topical acne drug products for over-the-counter human use; Final Rule. Fed Registr. 2010;75:9767-9777.
- US Food and Drug Administration. Topical acne drug products for over-the-counter human use—revision of labeling and classification of benzoyl peroxide as safe and effective. June 2011. Accessed June 12, 2024. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/topical-acne-drug-products-over-counter-human-use-revision-labeling-and-classification-benzoyl
The Growing Pains of Changing Times for Acne and Rosacea Pathophysiology: Where Will It All End Up?
It is interesting to observe the changes in dermatology that have occurred over the last 1 to 2 decades, especially as major advances in basic science research techniques have rapidly expanded our current understanding of the pathophysiology of many disease states—psoriasis, psoriatic arthritis, atopic dermatitis, alopecia areata, vitiligo, hidradenitis suppurativa, and lichen planus.1 Although acne vulgaris (AV) and rosacea do not make front-page news quite as often as some of these other aforementioned disease states in the pathophysiology arena, advances still have been made in understanding the pathophysiology, albeit slower and often less popularized in dermatology publications and other forms of media.2-4
If one looks at our fundamental understanding of AV, most of the discussion over multiple decades has been driven by new treatments and in some cases new formulations and packaging differences with topical agents. Although we understood that adrenarche, a subsequent increase in androgen synthesis, and the ensuing sebocyte development with formation of sebum were prerequisites for the development of AV, the absence of therapeutic options to address these vital components of AV—especially US Food and Drug Administration (FDA)–approved therapies—resulted in limited discussion about this specific area.5 Rather, the discussion was dominated by the notable role of Propionibacterium acnes (now called Cutibacterium acnes) in AV pathophysiology, as we had therapies such as benzoyl peroxide and antibiotics that improved AV in direct correlation with reductions in P acnes.6 This was soon coupled with an advanced understanding of how to reduce follicular hyperkeratinization with the development of topical tretinoin, followed by 3 other topical retinoids over time—adapalene, tazarotene, and trifarotene. Over subsequent years, slowly emerging basic science developments and collective data reviews added to our understanding of AV and how different therapies appear to work, including the role of toll-like receptors, anti-inflammatory properties of tetracyclines, and inflammasomes.7-9 Without a doubt, the availability of oral isotretinoin revolutionized AV therapy, especially in patients with severe refractory disease, with advanced formulations allowing for optimization of sustained remission without the need for high dietary fat intake.10-12
Progress in the pathophysiology of rosacea has been slower to develop, with the first true discussion of specific clinical presentations published after the new millennium.13 This was followed by more advanced basic science and clinical research, which led to an improved ability to understand modes of action of various therapies and to correlate treatment selection with specific visible manifestations of rosacea, including incorporation of physical devices.14-16 A newer perspective on evaluation and management of rosacea moved away from the “buckets” of rosacea subtypes to phenotypes observed at the time of clinical presentation.17,18
I could elaborate on research advancements with both diseases, but the bottom line is that information, developments, and current perspectives change over time. Keeping up is a challenge for all who study and practice dermatology. It is human nature to revert to what we already believe and do, which sometimes remains valid and other times is quite outdated and truly replaced by more optimal approaches. With AV and rosacea, progress is much slower in availability of newer agents. With AV, new agents have included topical dapsone, oral sarecycline, and topical clascoterone, with the latter being the first FDA-approved topical agent to mitigate the effects of androgens and sebum in both males and females. For rosacea, the 2 most recent FDA-approved therapies are minocycline foam and microencapsulated benzoyl peroxide. All of these therapies are proven to be effective for the modes of action and skin manifestations they specifically manage. Over the upcoming year, we are hoping to see the first triple-combination topical product come to market for AV, which will prompt our minds to consider if and how 3 established agents can work together to further augment treatment efficacy with favorable tolerability and safety.
Where will all of this end up? It is hard to say. We still have several other areas to tackle with both disease states, including establishing a well-substantiated understanding of the pathophysiologic role of the microbiome, sorting out the role of antibiotic use due to concerns about bacterial resistance, integration of FDA-approved physical devices in AV, and data on both diet and optimized skin care, to name a few.19-21
There is a lot on the plate to accomplish and digest. I have remained very involved in this subject matter for almost 3 decades and am still feeling the growing pains. Fortunately, the satisfaction of being part of a process so important to the lives of millions of patients makes this worth every moment. Stay tuned—more valuable information is to come.
- Wu J, Fang Z, Liu T, et al. Maximizing the utility of transcriptomics data in inflammatory skin diseases. Front Immunol. 2021;12:761890.
- Firlej E, Kowalska W, Szymaszek K, et al. The role of skin immune system in acne. J Clin Med. 2022;11:1579.
- Mias C, Mengeaud V, Bessou-Touya S, et al. Recent advances in understanding inflammatory acne: deciphering the relationship between Cutibacterium acnes and Th17 inflammatory pathway. J Eur Acad Dermatol Venereol. 2023;(37 suppl 2):3-11.
- Buddenkotte J, Steinhoff M. Recent advances in understanding and managing rosacea. F1000Res. 2018;7:F1000 Faculty Rev-1885. doi:10.12688/f1000research.16537.1
- Platsidaki E, Dessinioti C. Recent advances in understanding Propionibacterium acnes (Cutibacterium acnes) in acne. F1000Res. 2018;7:F1000 Faculty Rev-1953. doi:10.12688/f1000research.15659.1
- Leyden JJ. The evolving role of Propionibacterium acnes in acne. Semin Cutan Med Surg. 2001;20:139-143.
- Kim J. Review of the innate immune response in acne vulgaris: activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. Dermatology. 2005;211:193-198.
- Del Rosso JQ, Webster G, Weiss JS, et al. Nonantibiotic properties of tetracyclines in rosacea and their clinical implications. J Clin Aesthet Dermatol. 2021;14:14-21.
- Zhu W, Wang HL, Bu XL, et al. A narrative review of research progress on the role of NLRP3 inflammasome in acne vulgaris. Ann Transl Med. 2022;10:645.
- Leyden JJ, Del Rosso JQ, Baum EW. The use of isotretinoin in the treatment of acne vulgaris: clinical considerations and future directions. J Clin Aesthet Dermatol. 2014;7(2 suppl):S3-S21.
- Webster GF, Leyden JJ, Gross JA. Comparative pharmacokinetic profiles of a novel isotretinoin formulation (isotretinoin-Lidose) and the innovator isotretinoin formulation: a randomized, treatment, crossover study. J Am Acad Dermatol. 2013;69:762-767.
- Del Rosso JQ, Stein Gold L, Seagal J, et al. An open-label, phase IV study evaluating Lidose-isotretinoin administered without food in patients with severe recalcitrant nodular acne: low relapse rates observed over the 104-week post-treatment period. J Clin Aesthet Dermatol. 2019;12:13-18.
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Steinhoff M, Buddenkotte J, Aubert J, et al. Clinical, cellular, and molecular aspects in the pathophysiology of rosacea. J Investig Dermatol Symp Proc. 2011;15:2-11.
- Yamasaki K, Gallo RL. The molecular pathology of rosacea. J Dermatol Sci. 2009;55:77-81.
- Tanghetti E, Del Rosso JQ, Thiboutot D, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 4: a status report on physical modalities and devices. Cutis. 2014;93:71-76.
- Del Rosso JQ, Gallo RL, Tanghetti E, et al. An evaluation of potential correlations between pathophysiologic mechanisms, clinical manifestations, and management of rosacea. Cutis. 2013;91(3 suppl):1-8.
- Schaller M, Almeida LMC, Bewley A, et al. Recommendations for rosacea diagnosis, classification and management: update from the global ROSacea COnsensus 2019 panel. Br J Dermatol. 2020;182:1269-1276.
- Xu H, Li H. Acne, the skin microbiome, and antibiotic treatment. Am J Clin Dermatol. 2019;20:335-344.
- Daou H, Paradiso M, Hennessy K. Rosacea and the microbiome: a systematic review. Dermatol Ther (Heidelb). 2021;11:1-12.
- Kayiran MA, Karadag AS, Al-Khuzaei S, et al. Antibiotic resistance in acne: mechanisms, complications and management. Am J Clin Dermatol. 2020;21:813-819.
It is interesting to observe the changes in dermatology that have occurred over the last 1 to 2 decades, especially as major advances in basic science research techniques have rapidly expanded our current understanding of the pathophysiology of many disease states—psoriasis, psoriatic arthritis, atopic dermatitis, alopecia areata, vitiligo, hidradenitis suppurativa, and lichen planus.1 Although acne vulgaris (AV) and rosacea do not make front-page news quite as often as some of these other aforementioned disease states in the pathophysiology arena, advances still have been made in understanding the pathophysiology, albeit slower and often less popularized in dermatology publications and other forms of media.2-4
If one looks at our fundamental understanding of AV, most of the discussion over multiple decades has been driven by new treatments and in some cases new formulations and packaging differences with topical agents. Although we understood that adrenarche, a subsequent increase in androgen synthesis, and the ensuing sebocyte development with formation of sebum were prerequisites for the development of AV, the absence of therapeutic options to address these vital components of AV—especially US Food and Drug Administration (FDA)–approved therapies—resulted in limited discussion about this specific area.5 Rather, the discussion was dominated by the notable role of Propionibacterium acnes (now called Cutibacterium acnes) in AV pathophysiology, as we had therapies such as benzoyl peroxide and antibiotics that improved AV in direct correlation with reductions in P acnes.6 This was soon coupled with an advanced understanding of how to reduce follicular hyperkeratinization with the development of topical tretinoin, followed by 3 other topical retinoids over time—adapalene, tazarotene, and trifarotene. Over subsequent years, slowly emerging basic science developments and collective data reviews added to our understanding of AV and how different therapies appear to work, including the role of toll-like receptors, anti-inflammatory properties of tetracyclines, and inflammasomes.7-9 Without a doubt, the availability of oral isotretinoin revolutionized AV therapy, especially in patients with severe refractory disease, with advanced formulations allowing for optimization of sustained remission without the need for high dietary fat intake.10-12
Progress in the pathophysiology of rosacea has been slower to develop, with the first true discussion of specific clinical presentations published after the new millennium.13 This was followed by more advanced basic science and clinical research, which led to an improved ability to understand modes of action of various therapies and to correlate treatment selection with specific visible manifestations of rosacea, including incorporation of physical devices.14-16 A newer perspective on evaluation and management of rosacea moved away from the “buckets” of rosacea subtypes to phenotypes observed at the time of clinical presentation.17,18
I could elaborate on research advancements with both diseases, but the bottom line is that information, developments, and current perspectives change over time. Keeping up is a challenge for all who study and practice dermatology. It is human nature to revert to what we already believe and do, which sometimes remains valid and other times is quite outdated and truly replaced by more optimal approaches. With AV and rosacea, progress is much slower in availability of newer agents. With AV, new agents have included topical dapsone, oral sarecycline, and topical clascoterone, with the latter being the first FDA-approved topical agent to mitigate the effects of androgens and sebum in both males and females. For rosacea, the 2 most recent FDA-approved therapies are minocycline foam and microencapsulated benzoyl peroxide. All of these therapies are proven to be effective for the modes of action and skin manifestations they specifically manage. Over the upcoming year, we are hoping to see the first triple-combination topical product come to market for AV, which will prompt our minds to consider if and how 3 established agents can work together to further augment treatment efficacy with favorable tolerability and safety.
Where will all of this end up? It is hard to say. We still have several other areas to tackle with both disease states, including establishing a well-substantiated understanding of the pathophysiologic role of the microbiome, sorting out the role of antibiotic use due to concerns about bacterial resistance, integration of FDA-approved physical devices in AV, and data on both diet and optimized skin care, to name a few.19-21
There is a lot on the plate to accomplish and digest. I have remained very involved in this subject matter for almost 3 decades and am still feeling the growing pains. Fortunately, the satisfaction of being part of a process so important to the lives of millions of patients makes this worth every moment. Stay tuned—more valuable information is to come.
It is interesting to observe the changes in dermatology that have occurred over the last 1 to 2 decades, especially as major advances in basic science research techniques have rapidly expanded our current understanding of the pathophysiology of many disease states—psoriasis, psoriatic arthritis, atopic dermatitis, alopecia areata, vitiligo, hidradenitis suppurativa, and lichen planus.1 Although acne vulgaris (AV) and rosacea do not make front-page news quite as often as some of these other aforementioned disease states in the pathophysiology arena, advances still have been made in understanding the pathophysiology, albeit slower and often less popularized in dermatology publications and other forms of media.2-4
If one looks at our fundamental understanding of AV, most of the discussion over multiple decades has been driven by new treatments and in some cases new formulations and packaging differences with topical agents. Although we understood that adrenarche, a subsequent increase in androgen synthesis, and the ensuing sebocyte development with formation of sebum were prerequisites for the development of AV, the absence of therapeutic options to address these vital components of AV—especially US Food and Drug Administration (FDA)–approved therapies—resulted in limited discussion about this specific area.5 Rather, the discussion was dominated by the notable role of Propionibacterium acnes (now called Cutibacterium acnes) in AV pathophysiology, as we had therapies such as benzoyl peroxide and antibiotics that improved AV in direct correlation with reductions in P acnes.6 This was soon coupled with an advanced understanding of how to reduce follicular hyperkeratinization with the development of topical tretinoin, followed by 3 other topical retinoids over time—adapalene, tazarotene, and trifarotene. Over subsequent years, slowly emerging basic science developments and collective data reviews added to our understanding of AV and how different therapies appear to work, including the role of toll-like receptors, anti-inflammatory properties of tetracyclines, and inflammasomes.7-9 Without a doubt, the availability of oral isotretinoin revolutionized AV therapy, especially in patients with severe refractory disease, with advanced formulations allowing for optimization of sustained remission without the need for high dietary fat intake.10-12
Progress in the pathophysiology of rosacea has been slower to develop, with the first true discussion of specific clinical presentations published after the new millennium.13 This was followed by more advanced basic science and clinical research, which led to an improved ability to understand modes of action of various therapies and to correlate treatment selection with specific visible manifestations of rosacea, including incorporation of physical devices.14-16 A newer perspective on evaluation and management of rosacea moved away from the “buckets” of rosacea subtypes to phenotypes observed at the time of clinical presentation.17,18
I could elaborate on research advancements with both diseases, but the bottom line is that information, developments, and current perspectives change over time. Keeping up is a challenge for all who study and practice dermatology. It is human nature to revert to what we already believe and do, which sometimes remains valid and other times is quite outdated and truly replaced by more optimal approaches. With AV and rosacea, progress is much slower in availability of newer agents. With AV, new agents have included topical dapsone, oral sarecycline, and topical clascoterone, with the latter being the first FDA-approved topical agent to mitigate the effects of androgens and sebum in both males and females. For rosacea, the 2 most recent FDA-approved therapies are minocycline foam and microencapsulated benzoyl peroxide. All of these therapies are proven to be effective for the modes of action and skin manifestations they specifically manage. Over the upcoming year, we are hoping to see the first triple-combination topical product come to market for AV, which will prompt our minds to consider if and how 3 established agents can work together to further augment treatment efficacy with favorable tolerability and safety.
Where will all of this end up? It is hard to say. We still have several other areas to tackle with both disease states, including establishing a well-substantiated understanding of the pathophysiologic role of the microbiome, sorting out the role of antibiotic use due to concerns about bacterial resistance, integration of FDA-approved physical devices in AV, and data on both diet and optimized skin care, to name a few.19-21
There is a lot on the plate to accomplish and digest. I have remained very involved in this subject matter for almost 3 decades and am still feeling the growing pains. Fortunately, the satisfaction of being part of a process so important to the lives of millions of patients makes this worth every moment. Stay tuned—more valuable information is to come.
- Wu J, Fang Z, Liu T, et al. Maximizing the utility of transcriptomics data in inflammatory skin diseases. Front Immunol. 2021;12:761890.
- Firlej E, Kowalska W, Szymaszek K, et al. The role of skin immune system in acne. J Clin Med. 2022;11:1579.
- Mias C, Mengeaud V, Bessou-Touya S, et al. Recent advances in understanding inflammatory acne: deciphering the relationship between Cutibacterium acnes and Th17 inflammatory pathway. J Eur Acad Dermatol Venereol. 2023;(37 suppl 2):3-11.
- Buddenkotte J, Steinhoff M. Recent advances in understanding and managing rosacea. F1000Res. 2018;7:F1000 Faculty Rev-1885. doi:10.12688/f1000research.16537.1
- Platsidaki E, Dessinioti C. Recent advances in understanding Propionibacterium acnes (Cutibacterium acnes) in acne. F1000Res. 2018;7:F1000 Faculty Rev-1953. doi:10.12688/f1000research.15659.1
- Leyden JJ. The evolving role of Propionibacterium acnes in acne. Semin Cutan Med Surg. 2001;20:139-143.
- Kim J. Review of the innate immune response in acne vulgaris: activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. Dermatology. 2005;211:193-198.
- Del Rosso JQ, Webster G, Weiss JS, et al. Nonantibiotic properties of tetracyclines in rosacea and their clinical implications. J Clin Aesthet Dermatol. 2021;14:14-21.
- Zhu W, Wang HL, Bu XL, et al. A narrative review of research progress on the role of NLRP3 inflammasome in acne vulgaris. Ann Transl Med. 2022;10:645.
- Leyden JJ, Del Rosso JQ, Baum EW. The use of isotretinoin in the treatment of acne vulgaris: clinical considerations and future directions. J Clin Aesthet Dermatol. 2014;7(2 suppl):S3-S21.
- Webster GF, Leyden JJ, Gross JA. Comparative pharmacokinetic profiles of a novel isotretinoin formulation (isotretinoin-Lidose) and the innovator isotretinoin formulation: a randomized, treatment, crossover study. J Am Acad Dermatol. 2013;69:762-767.
- Del Rosso JQ, Stein Gold L, Seagal J, et al. An open-label, phase IV study evaluating Lidose-isotretinoin administered without food in patients with severe recalcitrant nodular acne: low relapse rates observed over the 104-week post-treatment period. J Clin Aesthet Dermatol. 2019;12:13-18.
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Steinhoff M, Buddenkotte J, Aubert J, et al. Clinical, cellular, and molecular aspects in the pathophysiology of rosacea. J Investig Dermatol Symp Proc. 2011;15:2-11.
- Yamasaki K, Gallo RL. The molecular pathology of rosacea. J Dermatol Sci. 2009;55:77-81.
- Tanghetti E, Del Rosso JQ, Thiboutot D, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 4: a status report on physical modalities and devices. Cutis. 2014;93:71-76.
- Del Rosso JQ, Gallo RL, Tanghetti E, et al. An evaluation of potential correlations between pathophysiologic mechanisms, clinical manifestations, and management of rosacea. Cutis. 2013;91(3 suppl):1-8.
- Schaller M, Almeida LMC, Bewley A, et al. Recommendations for rosacea diagnosis, classification and management: update from the global ROSacea COnsensus 2019 panel. Br J Dermatol. 2020;182:1269-1276.
- Xu H, Li H. Acne, the skin microbiome, and antibiotic treatment. Am J Clin Dermatol. 2019;20:335-344.
- Daou H, Paradiso M, Hennessy K. Rosacea and the microbiome: a systematic review. Dermatol Ther (Heidelb). 2021;11:1-12.
- Kayiran MA, Karadag AS, Al-Khuzaei S, et al. Antibiotic resistance in acne: mechanisms, complications and management. Am J Clin Dermatol. 2020;21:813-819.
- Wu J, Fang Z, Liu T, et al. Maximizing the utility of transcriptomics data in inflammatory skin diseases. Front Immunol. 2021;12:761890.
- Firlej E, Kowalska W, Szymaszek K, et al. The role of skin immune system in acne. J Clin Med. 2022;11:1579.
- Mias C, Mengeaud V, Bessou-Touya S, et al. Recent advances in understanding inflammatory acne: deciphering the relationship between Cutibacterium acnes and Th17 inflammatory pathway. J Eur Acad Dermatol Venereol. 2023;(37 suppl 2):3-11.
- Buddenkotte J, Steinhoff M. Recent advances in understanding and managing rosacea. F1000Res. 2018;7:F1000 Faculty Rev-1885. doi:10.12688/f1000research.16537.1
- Platsidaki E, Dessinioti C. Recent advances in understanding Propionibacterium acnes (Cutibacterium acnes) in acne. F1000Res. 2018;7:F1000 Faculty Rev-1953. doi:10.12688/f1000research.15659.1
- Leyden JJ. The evolving role of Propionibacterium acnes in acne. Semin Cutan Med Surg. 2001;20:139-143.
- Kim J. Review of the innate immune response in acne vulgaris: activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. Dermatology. 2005;211:193-198.
- Del Rosso JQ, Webster G, Weiss JS, et al. Nonantibiotic properties of tetracyclines in rosacea and their clinical implications. J Clin Aesthet Dermatol. 2021;14:14-21.
- Zhu W, Wang HL, Bu XL, et al. A narrative review of research progress on the role of NLRP3 inflammasome in acne vulgaris. Ann Transl Med. 2022;10:645.
- Leyden JJ, Del Rosso JQ, Baum EW. The use of isotretinoin in the treatment of acne vulgaris: clinical considerations and future directions. J Clin Aesthet Dermatol. 2014;7(2 suppl):S3-S21.
- Webster GF, Leyden JJ, Gross JA. Comparative pharmacokinetic profiles of a novel isotretinoin formulation (isotretinoin-Lidose) and the innovator isotretinoin formulation: a randomized, treatment, crossover study. J Am Acad Dermatol. 2013;69:762-767.
- Del Rosso JQ, Stein Gold L, Seagal J, et al. An open-label, phase IV study evaluating Lidose-isotretinoin administered without food in patients with severe recalcitrant nodular acne: low relapse rates observed over the 104-week post-treatment period. J Clin Aesthet Dermatol. 2019;12:13-18.
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Steinhoff M, Buddenkotte J, Aubert J, et al. Clinical, cellular, and molecular aspects in the pathophysiology of rosacea. J Investig Dermatol Symp Proc. 2011;15:2-11.
- Yamasaki K, Gallo RL. The molecular pathology of rosacea. J Dermatol Sci. 2009;55:77-81.
- Tanghetti E, Del Rosso JQ, Thiboutot D, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 4: a status report on physical modalities and devices. Cutis. 2014;93:71-76.
- Del Rosso JQ, Gallo RL, Tanghetti E, et al. An evaluation of potential correlations between pathophysiologic mechanisms, clinical manifestations, and management of rosacea. Cutis. 2013;91(3 suppl):1-8.
- Schaller M, Almeida LMC, Bewley A, et al. Recommendations for rosacea diagnosis, classification and management: update from the global ROSacea COnsensus 2019 panel. Br J Dermatol. 2020;182:1269-1276.
- Xu H, Li H. Acne, the skin microbiome, and antibiotic treatment. Am J Clin Dermatol. 2019;20:335-344.
- Daou H, Paradiso M, Hennessy K. Rosacea and the microbiome: a systematic review. Dermatol Ther (Heidelb). 2021;11:1-12.
- Kayiran MA, Karadag AS, Al-Khuzaei S, et al. Antibiotic resistance in acne: mechanisms, complications and management. Am J Clin Dermatol. 2020;21:813-819.
Adapting to Changes in Acne Management: Take One Step at a Time
After most dermatology residents graduate from their programs, they go out into practice and will often carry with them what they learned from their teachers, especially clinicians. Everyone else in their dermatology residency programs approaches disease management and the use of different therapies in the same way, right?
It does not take very long before these same dermatology residents realize that things are different in real-world clinical practice in many ways. Most clinicians develop a range of fairly predictable patterns in how they approach and treat common skin disorders such as acne, rosacea, psoriasis, atopic dermatitis/eczema, and seborrheic dermatitis. These patterns often include what testing is performed at baseline and at follow-up.
Recently, I have been giving thought to how clinicians—myself included—change their approaches to management of specific skin diseases over time, especially as new information and therapies emerge. Are we fast adopters, or are we slow adopters? How much evidence do we need to see before we consider adjusting our approach? Is the needle moving too fast or not fast enough?
I would like to use an example that relates to acne treatment, especially as this is one of the most common skin disorders encountered in outpatient dermatologic practice. Despite lack of US Food and Drug Administration (FDA) approval for use in acne, oral spironolactone commonly is used in females, especially adults, with acne vulgaris and has a long history as an acceptable approach in dermatology.1 Because spironolactone is a potassium-sparing diuretic, one question that commonly arises is: Do we monitor serum potassium levels at baseline and periodically during treatment with spironolactone? There has never been a definitive consensus on which approach to take. However, there has been evidence to suggest that such monitoring is not necessary in young healthy women due to a negligible risk for clinically relevant hyperkalemia.2,3
In fact, the suggestion that there is a very low risk for clinically significant hyperkalemia in healthy young women treated with spironolactone is accurate based on population-based studies. Nevertheless, the clinician is faced with confirming the patient is in fact healthy rather than assuming this is the case due to her “young” age. In addition, it is important to exclude potential drug-drug interactions that can increase the risk for hyperkalemia when coadministered with spironolactone and also to exclude an unknown underlying decrease in renal function.1 At the end of the day, I support the continued research that is being done to evaluate questions that can challenge the recycled dogma on how we manage patients, and I do not fault those who follow what they believe to be new cogent evidence. However, in the case of oral spironolactone use, I also could never fault a clinician for monitoring renal function and electrolytes including serum potassium levels in a female patient treated for acne, especially with a drug that has the known potential to cause hyperkalemia in certain clinical situations and is not FDA approved for the indication of acne (ie, the guidance that accompanies the level of investigation needed for such FDA approval is missing). The clinical judgment of the clinician who is responsible for the individual patient trumps the results from population-based studies completed thus far. Ultimately, it is the responsibility of that clinician to assure the safety of their patient in a manner that they are comfortable with.
It takes time to make changes in our approaches to patient management, and in the majority of cases, that is rightfully so. There are several potential limitations to how certain data are collected, and a reasonable verification of results over time is what tends to change behavior patterns. Ultimately, the common goal is to do what is in the best interest of our patients. No one can argue successfully against that.
- Kim GK, Del Rosso JQ. Oral spironolactone in post-teenage female patients with acne vulgaris: practical considerations for the clinician based on current data and clinical experience. J Clin Aesthet Dermatol. 2012;5:37-50.
- Plovanich M, Weng QY, Arash Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944.
- Barbieri JS, Margolis DJ, Mostaghimi A. Temporal trends and clinician variability in potassium monitoring of healthy young women treated for acne with spironolactone. JAMA Dermatol. 2021;157:296-300.
After most dermatology residents graduate from their programs, they go out into practice and will often carry with them what they learned from their teachers, especially clinicians. Everyone else in their dermatology residency programs approaches disease management and the use of different therapies in the same way, right?
It does not take very long before these same dermatology residents realize that things are different in real-world clinical practice in many ways. Most clinicians develop a range of fairly predictable patterns in how they approach and treat common skin disorders such as acne, rosacea, psoriasis, atopic dermatitis/eczema, and seborrheic dermatitis. These patterns often include what testing is performed at baseline and at follow-up.
Recently, I have been giving thought to how clinicians—myself included—change their approaches to management of specific skin diseases over time, especially as new information and therapies emerge. Are we fast adopters, or are we slow adopters? How much evidence do we need to see before we consider adjusting our approach? Is the needle moving too fast or not fast enough?
I would like to use an example that relates to acne treatment, especially as this is one of the most common skin disorders encountered in outpatient dermatologic practice. Despite lack of US Food and Drug Administration (FDA) approval for use in acne, oral spironolactone commonly is used in females, especially adults, with acne vulgaris and has a long history as an acceptable approach in dermatology.1 Because spironolactone is a potassium-sparing diuretic, one question that commonly arises is: Do we monitor serum potassium levels at baseline and periodically during treatment with spironolactone? There has never been a definitive consensus on which approach to take. However, there has been evidence to suggest that such monitoring is not necessary in young healthy women due to a negligible risk for clinically relevant hyperkalemia.2,3
In fact, the suggestion that there is a very low risk for clinically significant hyperkalemia in healthy young women treated with spironolactone is accurate based on population-based studies. Nevertheless, the clinician is faced with confirming the patient is in fact healthy rather than assuming this is the case due to her “young” age. In addition, it is important to exclude potential drug-drug interactions that can increase the risk for hyperkalemia when coadministered with spironolactone and also to exclude an unknown underlying decrease in renal function.1 At the end of the day, I support the continued research that is being done to evaluate questions that can challenge the recycled dogma on how we manage patients, and I do not fault those who follow what they believe to be new cogent evidence. However, in the case of oral spironolactone use, I also could never fault a clinician for monitoring renal function and electrolytes including serum potassium levels in a female patient treated for acne, especially with a drug that has the known potential to cause hyperkalemia in certain clinical situations and is not FDA approved for the indication of acne (ie, the guidance that accompanies the level of investigation needed for such FDA approval is missing). The clinical judgment of the clinician who is responsible for the individual patient trumps the results from population-based studies completed thus far. Ultimately, it is the responsibility of that clinician to assure the safety of their patient in a manner that they are comfortable with.
It takes time to make changes in our approaches to patient management, and in the majority of cases, that is rightfully so. There are several potential limitations to how certain data are collected, and a reasonable verification of results over time is what tends to change behavior patterns. Ultimately, the common goal is to do what is in the best interest of our patients. No one can argue successfully against that.
After most dermatology residents graduate from their programs, they go out into practice and will often carry with them what they learned from their teachers, especially clinicians. Everyone else in their dermatology residency programs approaches disease management and the use of different therapies in the same way, right?
It does not take very long before these same dermatology residents realize that things are different in real-world clinical practice in many ways. Most clinicians develop a range of fairly predictable patterns in how they approach and treat common skin disorders such as acne, rosacea, psoriasis, atopic dermatitis/eczema, and seborrheic dermatitis. These patterns often include what testing is performed at baseline and at follow-up.
Recently, I have been giving thought to how clinicians—myself included—change their approaches to management of specific skin diseases over time, especially as new information and therapies emerge. Are we fast adopters, or are we slow adopters? How much evidence do we need to see before we consider adjusting our approach? Is the needle moving too fast or not fast enough?
I would like to use an example that relates to acne treatment, especially as this is one of the most common skin disorders encountered in outpatient dermatologic practice. Despite lack of US Food and Drug Administration (FDA) approval for use in acne, oral spironolactone commonly is used in females, especially adults, with acne vulgaris and has a long history as an acceptable approach in dermatology.1 Because spironolactone is a potassium-sparing diuretic, one question that commonly arises is: Do we monitor serum potassium levels at baseline and periodically during treatment with spironolactone? There has never been a definitive consensus on which approach to take. However, there has been evidence to suggest that such monitoring is not necessary in young healthy women due to a negligible risk for clinically relevant hyperkalemia.2,3
In fact, the suggestion that there is a very low risk for clinically significant hyperkalemia in healthy young women treated with spironolactone is accurate based on population-based studies. Nevertheless, the clinician is faced with confirming the patient is in fact healthy rather than assuming this is the case due to her “young” age. In addition, it is important to exclude potential drug-drug interactions that can increase the risk for hyperkalemia when coadministered with spironolactone and also to exclude an unknown underlying decrease in renal function.1 At the end of the day, I support the continued research that is being done to evaluate questions that can challenge the recycled dogma on how we manage patients, and I do not fault those who follow what they believe to be new cogent evidence. However, in the case of oral spironolactone use, I also could never fault a clinician for monitoring renal function and electrolytes including serum potassium levels in a female patient treated for acne, especially with a drug that has the known potential to cause hyperkalemia in certain clinical situations and is not FDA approved for the indication of acne (ie, the guidance that accompanies the level of investigation needed for such FDA approval is missing). The clinical judgment of the clinician who is responsible for the individual patient trumps the results from population-based studies completed thus far. Ultimately, it is the responsibility of that clinician to assure the safety of their patient in a manner that they are comfortable with.
It takes time to make changes in our approaches to patient management, and in the majority of cases, that is rightfully so. There are several potential limitations to how certain data are collected, and a reasonable verification of results over time is what tends to change behavior patterns. Ultimately, the common goal is to do what is in the best interest of our patients. No one can argue successfully against that.
- Kim GK, Del Rosso JQ. Oral spironolactone in post-teenage female patients with acne vulgaris: practical considerations for the clinician based on current data and clinical experience. J Clin Aesthet Dermatol. 2012;5:37-50.
- Plovanich M, Weng QY, Arash Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944.
- Barbieri JS, Margolis DJ, Mostaghimi A. Temporal trends and clinician variability in potassium monitoring of healthy young women treated for acne with spironolactone. JAMA Dermatol. 2021;157:296-300.
- Kim GK, Del Rosso JQ. Oral spironolactone in post-teenage female patients with acne vulgaris: practical considerations for the clinician based on current data and clinical experience. J Clin Aesthet Dermatol. 2012;5:37-50.
- Plovanich M, Weng QY, Arash Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944.
- Barbieri JS, Margolis DJ, Mostaghimi A. Temporal trends and clinician variability in potassium monitoring of healthy young women treated for acne with spironolactone. JAMA Dermatol. 2021;157:296-300.
Just Like Rock and Roll, Topical Medications for Psoriasis Are Here to Stay
When I finished my dermatology training in 1986, the only moving parts in the skin that I recall were keratinocytes moving upward from the basal layer of the epidermis until they were desquamated 4 or 5 weeks later and hairs growing within their follicles until they were shed. Now we are learning about countless cytokines, chemokines, interleukins, antibodies, receptors, enzymes, and cell types, as well as their associated pathways, at an endless pace. Every day I am looking in my inbox to sign up for the “Cytokine of the Month” club! Despite the challenges of sorting through what is relevant clinically, it is a very exciting time. Coupled with this myriad of fundamental science is the emergence of newer therapies that are more directly targeting specific disease states and dramatically changing the lives of patients. We see prominent examples of these therapeutic results every day in patients we treat, especially with psoriasis and atopic dermatitis. Importantly, there also is hope for patients with notoriously refractory skin disorders, such as hidradenitis suppurativa, alopecia areata, and vitiligo, as newer therapies are being thoroughly studied in clinical trials.
Despite the best advances in therapy that we currently have available and those anticipated in the foreseeable future, patients with chronic dermatoses such as psoriasis and atopic dermatitis still require prolonged constant or frequently used intermittent therapies to adequately control their disease. Fortunately, as dermatologists we understand the importance of proper skin care and topical medications as well as how to incorporate them in the management plan. To date, specifically with psoriasis, we have a variety of brand and generic topical corticosteroids, calcipotriene (vitamin D analogue), and tazarotene (retinoid), as well as combination formulations, in our toolbox to help manage localized areas of involvement.1 This includes both patients with more limited psoriasis and those responding favorably to systemic therapy but who still develop some new or persistent areas of localized psoriatic lesions. New data with the brand formulation of calcipotriene–betamethasone dipropionate (Cal-BDP) foam applied once daily shows that after adequate control is achieved, continued application to the affected sites twice weekly is superior to vehicle in preventing relapse of psoriasis.2 A highly cosmetically acceptable Cal-BDP cream incorporating a unique vehicle technology has been US Food and Drug Administration (FDA) approved for once-daily use for plaque psoriasis, overcoming the compatibility difficulties encountered in combining both active ingredients in an aqueous-based formulation and also optimizing the delivery of the active ingredients into the skin. This Cal-BDP cream demonstrated efficacy superior to a brand Cal-BDP suspension, rapid reduction in pruritus, and favorable tolerability and safety.3 Another combination formulation that is FDA approved for plaque psoriasis with once-daily application that has been shown to be effective and safe is halobetasol propionate–tazarotene lotion. This formulation contains lower concentrations of both active ingredients than those normally used in a barrier-friendly polymeric emulsion vehicle, allowing for augmented delivery of both active ingredients into the skin than with the individual agents applied separately and sequentially.4,5 In the best of circumstances, most patients with psoriasis still require use of topical therapy and appreciate its availability. Just like on any menu, it is good to have multiple good options.
What else does this psoriasis management story need? A pipeline! I am happy to tell you that with topical therapy, 2 nonsteroidal agents are under development with completion of phase 2 and phase 3 trials submitted to the FDA to evaluate for approval for psoriasis. They are tapinarof cream, an aryl hydrocarbon receptor agonist, and roflumilast cream, a phosphodiesterase 4 (PDE4) inhibitor. Both of these modes of action involve intracellular pathways that are highly conserved in humans and are ubiquitously present in structural and hematopoietic cells.
Topical application of tapinarof cream once daily has been shown to be effective and safe for plaque psoriasis, is well tolerated with some reports of folliculitis observed that did not typically interfere with use, exhibits a remittive effect in patients achieving clearance on therapy, and is devoid of any systemic safety signals with both short-term and long-term use.6-8 It also is currently under evaluation for atopic dermatitis. Topical roflumilast cream once daily has been shown to be effective and safe for plaque psoriasis as well as intertriginous psoriasis; is well tolerated including negligible rates of skin tolerability reactions such as stinging and burning; and is devoid of systemic safety signals, including those often observed with oral PDE4 inhibitor therapy (apremilast).9,10 In addition, roflumilast has been shown to be more inherently potent in PDE4 inhibition activity than crisaborole and apremilast.11 Roflumilast cream also is being studied for atopic dermatitis and a foam formulation is being evaluated for seborrheic dermatitis. Importantly, both tapinarof and roflumilast are not corticosteroids and are not associated with adverse effects observed with topical corticosteroid therapy, such as atrophy, striae, telangiectasia, and hypothalamic-pituitary-adrenal axis suppression. This provides a sense of comfort for clinicians and patients, as potential side effects associated with more prolonged topical corticosteroid therapy are common and lingering concerns.
To summarize, topical therapy for psoriasis is here to stay, just like all the rock and roll we have more access to than ever through expanded modern-day radio access and several music streaming sources, most of which are on demand. Also available to us are some viable current options, including a few newer brand formulations. New nonsteroidal agents with favorable data thus far are on the horizon, providing their own inherent efficacy and safety, which appear to be advantageous thus far. As the late Ric Ocasek of the Cars sang, “Let the good times roll.”
- Lebwohl MG, Van de Kerkhof PCM. Psoriasis. In: Lebwohl MG, Heymann WR, Berth-Jones J, et al, eds. Treatment of Skin Disease: Comprehensive Therapeutic Strategies. 4th ed. Elsevier Saunders; 2014:640-650.
- Lebwohl M, Kircik L, Lacour JP, et al. Twice-weekly topical calcipotriene/betamethasone dipropionate foam as proactive management of plaque psoriasis increases time in remission and is well tolerated over 52 weeks (PSO-LONG trial). J Am Acad Dermatol. 2021;84:1269-1277.
- Wynzora (calcipotriene and betamethasone dipropionate) cream, for topical use. Package insert. EPI Health, LLC; 2020.
- Ramachandran V, Bertus B, Bashyam AM, et al. Treating psoriasis with halobetasol propionate and tazarotene combination: a review of phase II and III clinical trials. Ann Pharmacother. 2020;54:872-878.
- Lebwohl MG, Tanghetti EA, Stein Gold L, et al. Fixed-combination halobetasol propionate and tazarotene in the treatment of psoriasis: narrative review of mechanisms of action and therapeutic benefits. Dermatol Ther (Heidelb). 2021;11:1157-1174.
- Bissonnette R, Stein Gold L, Rubenstein DS, et al. Tapinarof in the treatment of psoriasis: a review of the unique mechanism of action of a novel therapeutic aryl hydrocarbon receptor-modulating agent. J Am Acad Dermatol. 2021;84:1059-1067.
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229.
- Jett JE, McLaughlin M, Lee MS, et al. Tapinarof cream 1% for extensive plaque psoriasis: a maximal use trial on safety, tolerability, and pharmacokinetics [published online October 28, 2021]. Am J Clin Dermatol. doi:10.100/s40257-021-00641-4
- Lebwohl MG, Papp KA, Stein Gold L, et al. Trial of roflumilast cream for chronic plaque psoriasis. N Engl J Med. 2020;383:229-239.
- Papp KA, Gooderham M, Droege M, et al. Roflumilast cream improves signs and symptoms of plaque psoriasis: results from a phase 1/2a randomized, controlled study. J Drugs Dermatol. 2020;19:734-740.
- Dong C, Virtucio C, Zemska O, et al. Treatment of skin inflammation with benzoxaborole phosphodiesterase inhibitors: selectivity, cellular activity, and effect on cytokines associated with skin inflammation and skin architecture changes. J Pharmacol Exp Ther. 2016;358:413-422.
When I finished my dermatology training in 1986, the only moving parts in the skin that I recall were keratinocytes moving upward from the basal layer of the epidermis until they were desquamated 4 or 5 weeks later and hairs growing within their follicles until they were shed. Now we are learning about countless cytokines, chemokines, interleukins, antibodies, receptors, enzymes, and cell types, as well as their associated pathways, at an endless pace. Every day I am looking in my inbox to sign up for the “Cytokine of the Month” club! Despite the challenges of sorting through what is relevant clinically, it is a very exciting time. Coupled with this myriad of fundamental science is the emergence of newer therapies that are more directly targeting specific disease states and dramatically changing the lives of patients. We see prominent examples of these therapeutic results every day in patients we treat, especially with psoriasis and atopic dermatitis. Importantly, there also is hope for patients with notoriously refractory skin disorders, such as hidradenitis suppurativa, alopecia areata, and vitiligo, as newer therapies are being thoroughly studied in clinical trials.
Despite the best advances in therapy that we currently have available and those anticipated in the foreseeable future, patients with chronic dermatoses such as psoriasis and atopic dermatitis still require prolonged constant or frequently used intermittent therapies to adequately control their disease. Fortunately, as dermatologists we understand the importance of proper skin care and topical medications as well as how to incorporate them in the management plan. To date, specifically with psoriasis, we have a variety of brand and generic topical corticosteroids, calcipotriene (vitamin D analogue), and tazarotene (retinoid), as well as combination formulations, in our toolbox to help manage localized areas of involvement.1 This includes both patients with more limited psoriasis and those responding favorably to systemic therapy but who still develop some new or persistent areas of localized psoriatic lesions. New data with the brand formulation of calcipotriene–betamethasone dipropionate (Cal-BDP) foam applied once daily shows that after adequate control is achieved, continued application to the affected sites twice weekly is superior to vehicle in preventing relapse of psoriasis.2 A highly cosmetically acceptable Cal-BDP cream incorporating a unique vehicle technology has been US Food and Drug Administration (FDA) approved for once-daily use for plaque psoriasis, overcoming the compatibility difficulties encountered in combining both active ingredients in an aqueous-based formulation and also optimizing the delivery of the active ingredients into the skin. This Cal-BDP cream demonstrated efficacy superior to a brand Cal-BDP suspension, rapid reduction in pruritus, and favorable tolerability and safety.3 Another combination formulation that is FDA approved for plaque psoriasis with once-daily application that has been shown to be effective and safe is halobetasol propionate–tazarotene lotion. This formulation contains lower concentrations of both active ingredients than those normally used in a barrier-friendly polymeric emulsion vehicle, allowing for augmented delivery of both active ingredients into the skin than with the individual agents applied separately and sequentially.4,5 In the best of circumstances, most patients with psoriasis still require use of topical therapy and appreciate its availability. Just like on any menu, it is good to have multiple good options.
What else does this psoriasis management story need? A pipeline! I am happy to tell you that with topical therapy, 2 nonsteroidal agents are under development with completion of phase 2 and phase 3 trials submitted to the FDA to evaluate for approval for psoriasis. They are tapinarof cream, an aryl hydrocarbon receptor agonist, and roflumilast cream, a phosphodiesterase 4 (PDE4) inhibitor. Both of these modes of action involve intracellular pathways that are highly conserved in humans and are ubiquitously present in structural and hematopoietic cells.
Topical application of tapinarof cream once daily has been shown to be effective and safe for plaque psoriasis, is well tolerated with some reports of folliculitis observed that did not typically interfere with use, exhibits a remittive effect in patients achieving clearance on therapy, and is devoid of any systemic safety signals with both short-term and long-term use.6-8 It also is currently under evaluation for atopic dermatitis. Topical roflumilast cream once daily has been shown to be effective and safe for plaque psoriasis as well as intertriginous psoriasis; is well tolerated including negligible rates of skin tolerability reactions such as stinging and burning; and is devoid of systemic safety signals, including those often observed with oral PDE4 inhibitor therapy (apremilast).9,10 In addition, roflumilast has been shown to be more inherently potent in PDE4 inhibition activity than crisaborole and apremilast.11 Roflumilast cream also is being studied for atopic dermatitis and a foam formulation is being evaluated for seborrheic dermatitis. Importantly, both tapinarof and roflumilast are not corticosteroids and are not associated with adverse effects observed with topical corticosteroid therapy, such as atrophy, striae, telangiectasia, and hypothalamic-pituitary-adrenal axis suppression. This provides a sense of comfort for clinicians and patients, as potential side effects associated with more prolonged topical corticosteroid therapy are common and lingering concerns.
To summarize, topical therapy for psoriasis is here to stay, just like all the rock and roll we have more access to than ever through expanded modern-day radio access and several music streaming sources, most of which are on demand. Also available to us are some viable current options, including a few newer brand formulations. New nonsteroidal agents with favorable data thus far are on the horizon, providing their own inherent efficacy and safety, which appear to be advantageous thus far. As the late Ric Ocasek of the Cars sang, “Let the good times roll.”
When I finished my dermatology training in 1986, the only moving parts in the skin that I recall were keratinocytes moving upward from the basal layer of the epidermis until they were desquamated 4 or 5 weeks later and hairs growing within their follicles until they were shed. Now we are learning about countless cytokines, chemokines, interleukins, antibodies, receptors, enzymes, and cell types, as well as their associated pathways, at an endless pace. Every day I am looking in my inbox to sign up for the “Cytokine of the Month” club! Despite the challenges of sorting through what is relevant clinically, it is a very exciting time. Coupled with this myriad of fundamental science is the emergence of newer therapies that are more directly targeting specific disease states and dramatically changing the lives of patients. We see prominent examples of these therapeutic results every day in patients we treat, especially with psoriasis and atopic dermatitis. Importantly, there also is hope for patients with notoriously refractory skin disorders, such as hidradenitis suppurativa, alopecia areata, and vitiligo, as newer therapies are being thoroughly studied in clinical trials.
Despite the best advances in therapy that we currently have available and those anticipated in the foreseeable future, patients with chronic dermatoses such as psoriasis and atopic dermatitis still require prolonged constant or frequently used intermittent therapies to adequately control their disease. Fortunately, as dermatologists we understand the importance of proper skin care and topical medications as well as how to incorporate them in the management plan. To date, specifically with psoriasis, we have a variety of brand and generic topical corticosteroids, calcipotriene (vitamin D analogue), and tazarotene (retinoid), as well as combination formulations, in our toolbox to help manage localized areas of involvement.1 This includes both patients with more limited psoriasis and those responding favorably to systemic therapy but who still develop some new or persistent areas of localized psoriatic lesions. New data with the brand formulation of calcipotriene–betamethasone dipropionate (Cal-BDP) foam applied once daily shows that after adequate control is achieved, continued application to the affected sites twice weekly is superior to vehicle in preventing relapse of psoriasis.2 A highly cosmetically acceptable Cal-BDP cream incorporating a unique vehicle technology has been US Food and Drug Administration (FDA) approved for once-daily use for plaque psoriasis, overcoming the compatibility difficulties encountered in combining both active ingredients in an aqueous-based formulation and also optimizing the delivery of the active ingredients into the skin. This Cal-BDP cream demonstrated efficacy superior to a brand Cal-BDP suspension, rapid reduction in pruritus, and favorable tolerability and safety.3 Another combination formulation that is FDA approved for plaque psoriasis with once-daily application that has been shown to be effective and safe is halobetasol propionate–tazarotene lotion. This formulation contains lower concentrations of both active ingredients than those normally used in a barrier-friendly polymeric emulsion vehicle, allowing for augmented delivery of both active ingredients into the skin than with the individual agents applied separately and sequentially.4,5 In the best of circumstances, most patients with psoriasis still require use of topical therapy and appreciate its availability. Just like on any menu, it is good to have multiple good options.
What else does this psoriasis management story need? A pipeline! I am happy to tell you that with topical therapy, 2 nonsteroidal agents are under development with completion of phase 2 and phase 3 trials submitted to the FDA to evaluate for approval for psoriasis. They are tapinarof cream, an aryl hydrocarbon receptor agonist, and roflumilast cream, a phosphodiesterase 4 (PDE4) inhibitor. Both of these modes of action involve intracellular pathways that are highly conserved in humans and are ubiquitously present in structural and hematopoietic cells.
Topical application of tapinarof cream once daily has been shown to be effective and safe for plaque psoriasis, is well tolerated with some reports of folliculitis observed that did not typically interfere with use, exhibits a remittive effect in patients achieving clearance on therapy, and is devoid of any systemic safety signals with both short-term and long-term use.6-8 It also is currently under evaluation for atopic dermatitis. Topical roflumilast cream once daily has been shown to be effective and safe for plaque psoriasis as well as intertriginous psoriasis; is well tolerated including negligible rates of skin tolerability reactions such as stinging and burning; and is devoid of systemic safety signals, including those often observed with oral PDE4 inhibitor therapy (apremilast).9,10 In addition, roflumilast has been shown to be more inherently potent in PDE4 inhibition activity than crisaborole and apremilast.11 Roflumilast cream also is being studied for atopic dermatitis and a foam formulation is being evaluated for seborrheic dermatitis. Importantly, both tapinarof and roflumilast are not corticosteroids and are not associated with adverse effects observed with topical corticosteroid therapy, such as atrophy, striae, telangiectasia, and hypothalamic-pituitary-adrenal axis suppression. This provides a sense of comfort for clinicians and patients, as potential side effects associated with more prolonged topical corticosteroid therapy are common and lingering concerns.
To summarize, topical therapy for psoriasis is here to stay, just like all the rock and roll we have more access to than ever through expanded modern-day radio access and several music streaming sources, most of which are on demand. Also available to us are some viable current options, including a few newer brand formulations. New nonsteroidal agents with favorable data thus far are on the horizon, providing their own inherent efficacy and safety, which appear to be advantageous thus far. As the late Ric Ocasek of the Cars sang, “Let the good times roll.”
- Lebwohl MG, Van de Kerkhof PCM. Psoriasis. In: Lebwohl MG, Heymann WR, Berth-Jones J, et al, eds. Treatment of Skin Disease: Comprehensive Therapeutic Strategies. 4th ed. Elsevier Saunders; 2014:640-650.
- Lebwohl M, Kircik L, Lacour JP, et al. Twice-weekly topical calcipotriene/betamethasone dipropionate foam as proactive management of plaque psoriasis increases time in remission and is well tolerated over 52 weeks (PSO-LONG trial). J Am Acad Dermatol. 2021;84:1269-1277.
- Wynzora (calcipotriene and betamethasone dipropionate) cream, for topical use. Package insert. EPI Health, LLC; 2020.
- Ramachandran V, Bertus B, Bashyam AM, et al. Treating psoriasis with halobetasol propionate and tazarotene combination: a review of phase II and III clinical trials. Ann Pharmacother. 2020;54:872-878.
- Lebwohl MG, Tanghetti EA, Stein Gold L, et al. Fixed-combination halobetasol propionate and tazarotene in the treatment of psoriasis: narrative review of mechanisms of action and therapeutic benefits. Dermatol Ther (Heidelb). 2021;11:1157-1174.
- Bissonnette R, Stein Gold L, Rubenstein DS, et al. Tapinarof in the treatment of psoriasis: a review of the unique mechanism of action of a novel therapeutic aryl hydrocarbon receptor-modulating agent. J Am Acad Dermatol. 2021;84:1059-1067.
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229.
- Jett JE, McLaughlin M, Lee MS, et al. Tapinarof cream 1% for extensive plaque psoriasis: a maximal use trial on safety, tolerability, and pharmacokinetics [published online October 28, 2021]. Am J Clin Dermatol. doi:10.100/s40257-021-00641-4
- Lebwohl MG, Papp KA, Stein Gold L, et al. Trial of roflumilast cream for chronic plaque psoriasis. N Engl J Med. 2020;383:229-239.
- Papp KA, Gooderham M, Droege M, et al. Roflumilast cream improves signs and symptoms of plaque psoriasis: results from a phase 1/2a randomized, controlled study. J Drugs Dermatol. 2020;19:734-740.
- Dong C, Virtucio C, Zemska O, et al. Treatment of skin inflammation with benzoxaborole phosphodiesterase inhibitors: selectivity, cellular activity, and effect on cytokines associated with skin inflammation and skin architecture changes. J Pharmacol Exp Ther. 2016;358:413-422.
- Lebwohl MG, Van de Kerkhof PCM. Psoriasis. In: Lebwohl MG, Heymann WR, Berth-Jones J, et al, eds. Treatment of Skin Disease: Comprehensive Therapeutic Strategies. 4th ed. Elsevier Saunders; 2014:640-650.
- Lebwohl M, Kircik L, Lacour JP, et al. Twice-weekly topical calcipotriene/betamethasone dipropionate foam as proactive management of plaque psoriasis increases time in remission and is well tolerated over 52 weeks (PSO-LONG trial). J Am Acad Dermatol. 2021;84:1269-1277.
- Wynzora (calcipotriene and betamethasone dipropionate) cream, for topical use. Package insert. EPI Health, LLC; 2020.
- Ramachandran V, Bertus B, Bashyam AM, et al. Treating psoriasis with halobetasol propionate and tazarotene combination: a review of phase II and III clinical trials. Ann Pharmacother. 2020;54:872-878.
- Lebwohl MG, Tanghetti EA, Stein Gold L, et al. Fixed-combination halobetasol propionate and tazarotene in the treatment of psoriasis: narrative review of mechanisms of action and therapeutic benefits. Dermatol Ther (Heidelb). 2021;11:1157-1174.
- Bissonnette R, Stein Gold L, Rubenstein DS, et al. Tapinarof in the treatment of psoriasis: a review of the unique mechanism of action of a novel therapeutic aryl hydrocarbon receptor-modulating agent. J Am Acad Dermatol. 2021;84:1059-1067.
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229.
- Jett JE, McLaughlin M, Lee MS, et al. Tapinarof cream 1% for extensive plaque psoriasis: a maximal use trial on safety, tolerability, and pharmacokinetics [published online October 28, 2021]. Am J Clin Dermatol. doi:10.100/s40257-021-00641-4
- Lebwohl MG, Papp KA, Stein Gold L, et al. Trial of roflumilast cream for chronic plaque psoriasis. N Engl J Med. 2020;383:229-239.
- Papp KA, Gooderham M, Droege M, et al. Roflumilast cream improves signs and symptoms of plaque psoriasis: results from a phase 1/2a randomized, controlled study. J Drugs Dermatol. 2020;19:734-740.
- Dong C, Virtucio C, Zemska O, et al. Treatment of skin inflammation with benzoxaborole phosphodiesterase inhibitors: selectivity, cellular activity, and effect on cytokines associated with skin inflammation and skin architecture changes. J Pharmacol Exp Ther. 2016;358:413-422.
Why Is It That the Biggest Resistance With Fighting the Battle Against Bacterial Resistance Seems to Fall on Dermatology Clinicians?
This discussion focuses on antibiotic resistance in acne therapy but also includes general principles related to this subject. “Seeing is believing” is a concept we have all heard many times, and we generally can all agree with and relate to what this is saying to us. However, it is harder to get a consensus of agreement on concepts that are happening beneath the surface but are not visibly apparent. Antibiotic resistance is a concept that falls into this latter category, especially in acne treatment. Many clinicians are not convinced antibiotic resistance is clinically relevant, exclaiming “I just do not see it in my practice.” The problem is—especially in the case of acne where oral tetracycline agents commonly are prescribed—how does the clinician “see” antibiotic resistance? Clinicians do not obtain bacterial cultures or perform sensitivity testing as they might do when evaluating a suspected cutaneous infection such as folliculitis, an inflamed postsurgical wound, a purulent leg ulcer, or an abscess. Additionally, if the selected therapy is not as effective as anticipated, it may be attributed to the patient needing another type of treatment or something “stronger,” or maybe they are not fully compliant. In fact, a very possible reason for inadequate therapeutic response may be that the predominant Cutibacterium acnes strains in a particular case are proinflammatory, and many of the strains are not highly sensitive to the chosen antibiotic.1
In the United States, antibiotic resistance in C acnes is most prevalent with erythromycin, followed by clindamycin, tetracycline, doxycycline, and minocycline, respectively.2 The relative patterns of antibiotic resistance in specific geographic regions correlate with the magnitude of specific antibiotic use, and that consistent reduction in use of a given antibiotic in a community can reverse the prevalence of resistance to that antibiotic progressively over time.3 Combination therapy approaches to mitigate emergence of resistant bacteria during acne treatment with an exit plan explained up-front with the patient are important to reduce prolonged use or repeated cycles of antibiotic use and in some cases to circumvent antibiotic use and incorporate a different therapeutic approach.1-3 Interestingly, in a retrospective chart review of acne patients who were eventually treated with oral isotretinoin at dermatology practices within a major university health system, approximately two-thirds received oral antibiotics for 6 months or longer and one-third for 1 year or longer.4 It is easy for all of us to have good intentions; however, in reality it is not always easy, practical, or in the best interest of the patient to stringently enforce recommendations that are determined not to be the best option at that time. Patients get a vote, too, as long as they are fully informed of benefits vs risks.
The concern about emergence of less-sensitive bacteria during acne antibiotic treatment is not limited to discussion of C acnes resistance. Use of both oral and topical antibiotics creates “ecologic mischief,” which is the emergence of less-sensitive strains of other bacteria exposed to the antibiotic—both commensal and opportunistic—especially at anatomic sites such as the skin, nasopharyngeal region, and gastrointestinal and genitourinary tracts.5-7 Application of topical erythromycin to the face can induce erythromycin-resistant bacteria such as staphylococci on the face as well as at remote sites such as the nares (nasal vestibule) and the back.6 Antibiotics used to treat acne, predominantly oral tetracyclines, showed positive oropharyngeal cultures for Streptococcus pyogenes in 33% (13/39) of treated patients; among these positive cultures, 85% (11/13) were resistant to at least one tetracycline antibiotic.7 Importantly, the streptococcal colonization of the oropharynx in individuals taking an oral antibiotic for acne may not induce a clinically apparent pharyngitis in that individual, but that person can carry and spread that streptococcal pathogen to others. In either case, the dermatology clinician, even if he/she suspects the connection related to antibiotic selection pressure and resistance, would not “see” the antibiotic resistance, as the individuals who develop a “sore throat” or strep throat do not seek care for this problem through a dermatology office.
The first formally organized and independent group in dermatology to address antibiotic use and resistance issues was the Scientific Panel on Antibiotic Use in Dermatology, which I put together in 2004 with James J. Leyden, MD (Philadelphia, Pennsylvania) and Guy F. Webster, MD (Hockessin, Delaware), and was comprised mostly of interested dermatologists with contributions from microbiologists and infectious disease specialists. A series of meetings, publications, and presentations have emerged from this group, which now falls under the auspices of the American Acne & Rosacea Society. Through the efforts of these organizations and other groups and companies with a strong interest in combating antibiotic resistance, we continue to see slow but steady progress in enlightening dermatology clinicians to think about if and when antibiotic therapy is needed and for how long. The subject of when antibiotics are not necessary also has been addressed, including both oral and topical antibiotics in many common scenarios encountered in dermatology practice.8 Examples include incision and drainage of an inflamed epidermal cyst without antibiotic therapy and use of white petrolatum instead of a topical antibiotic after most dermatologic procedures such as biopsies, tangential procedures, and closures after excisional procedures. Overall, the potential for topical antibiotics containing bacitracin and/or neomycin to induce allergic contact dermatitis is higher than the risk for postoperative wound infection. A major reason to avoid facilitating the emergence of antibiotic-resistant bacteria is that these organisms are efficient in packaging their resistance genes along with those from other bacteria, thus creating multidrug-resistant bacterial strains. This situation creates bigger challenges with trying to select effective therapies.
A cross-sectional analysis of antibiotics prescribed by dermatologists from January 1, 2008, to December 31, 2016, performed via a large commercial prescription claims database showed that among almost 1 million courses of oral antibiotics prescribed by approximately 12,000 unique dermatology prescribers, overall antibiotic prescribing decreased 36.6%, reflecting a drop of 1.23 courses per 100 visits, with much of the reduction occurring among extended antibiotic courses for acne and rosacea.9 Dermatology clinicians appear to be increasing their consideration of treatment alternatives such as oral spironolactone in adult female patients or earlier transition to oral isotretinoin therapy before starting another cycle with the same or a different oral antibiotic. Some have increased the use of physical device therapies. Importantly, we do not want to throw out the baby with the bathwater. Oral antibiotics remain important agents for treatment of moderate to severe inflammatory acne and in rosacea when subantibiotic-dose doxycycline is not accessible or is not effective after an adequate trial of therapy. Last but not least, a full-court press with an optimal topical regimen is the foundation of acne therapy, as monotherapy with an oral antibiotic for acne is considered dermatologic heresy and for good reason.
- Del Rosso JQ, Leyden JJ. Status report on antibiotic resistance: implications for the dermatologist. Dermatol Clin. 2007;25:127-132.
- Leyden JJ, Del Rosso JQ, Webster GF. Clinical considerations in the treatment of acne vulgaris and other inflammatory skin disorders: a status report. Dermatol Clin. 2009;27:1-15.
- Del Rosso JQ, Zeichner JA. The clinical relevance of antibiotic resistance: thirteen principles that every dermatologist needs to consider when prescribing antibiotic therapy. Dermatol Clin. 2016;34:167-173.
- Nagler AR, Milam EC, Orlow SJ. The use of oral antibiotics before isotretinoin therapy in patients with acne. J Am Acad Dermatol. 2016;74:273-279.
- Del Rosso JQ, Kim GK. Topical antibiotics: therapeutic value or ecologic mischief? Dermatol Ther. 2009;22:398-406.
- Bowe WP, Leyden JJ. Clinical implications of antibiotic resistance: risk of systemic infection from Staphylococcus and Streptococcus. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, England: Informa Healthcare; 2011:125-133.
- Levy RM, Huang EY, Roling D, et al. Effect of antibiotics on the oropharyngeal flora in patients with acne. Arch Dermatol. 2003;139:467-471.
- Hirschmann JV. When antibiotics are unnecessary. Dermatol Clin. 2009;27:75-83.
- Barbieri JS, Bhate K, Hartnett KP, et al. Trends in oral antibiotic prescription in dermatology, 2008 to 2016. JAMA Dermatol. 2019;155:290-297.
This discussion focuses on antibiotic resistance in acne therapy but also includes general principles related to this subject. “Seeing is believing” is a concept we have all heard many times, and we generally can all agree with and relate to what this is saying to us. However, it is harder to get a consensus of agreement on concepts that are happening beneath the surface but are not visibly apparent. Antibiotic resistance is a concept that falls into this latter category, especially in acne treatment. Many clinicians are not convinced antibiotic resistance is clinically relevant, exclaiming “I just do not see it in my practice.” The problem is—especially in the case of acne where oral tetracycline agents commonly are prescribed—how does the clinician “see” antibiotic resistance? Clinicians do not obtain bacterial cultures or perform sensitivity testing as they might do when evaluating a suspected cutaneous infection such as folliculitis, an inflamed postsurgical wound, a purulent leg ulcer, or an abscess. Additionally, if the selected therapy is not as effective as anticipated, it may be attributed to the patient needing another type of treatment or something “stronger,” or maybe they are not fully compliant. In fact, a very possible reason for inadequate therapeutic response may be that the predominant Cutibacterium acnes strains in a particular case are proinflammatory, and many of the strains are not highly sensitive to the chosen antibiotic.1
In the United States, antibiotic resistance in C acnes is most prevalent with erythromycin, followed by clindamycin, tetracycline, doxycycline, and minocycline, respectively.2 The relative patterns of antibiotic resistance in specific geographic regions correlate with the magnitude of specific antibiotic use, and that consistent reduction in use of a given antibiotic in a community can reverse the prevalence of resistance to that antibiotic progressively over time.3 Combination therapy approaches to mitigate emergence of resistant bacteria during acne treatment with an exit plan explained up-front with the patient are important to reduce prolonged use or repeated cycles of antibiotic use and in some cases to circumvent antibiotic use and incorporate a different therapeutic approach.1-3 Interestingly, in a retrospective chart review of acne patients who were eventually treated with oral isotretinoin at dermatology practices within a major university health system, approximately two-thirds received oral antibiotics for 6 months or longer and one-third for 1 year or longer.4 It is easy for all of us to have good intentions; however, in reality it is not always easy, practical, or in the best interest of the patient to stringently enforce recommendations that are determined not to be the best option at that time. Patients get a vote, too, as long as they are fully informed of benefits vs risks.
The concern about emergence of less-sensitive bacteria during acne antibiotic treatment is not limited to discussion of C acnes resistance. Use of both oral and topical antibiotics creates “ecologic mischief,” which is the emergence of less-sensitive strains of other bacteria exposed to the antibiotic—both commensal and opportunistic—especially at anatomic sites such as the skin, nasopharyngeal region, and gastrointestinal and genitourinary tracts.5-7 Application of topical erythromycin to the face can induce erythromycin-resistant bacteria such as staphylococci on the face as well as at remote sites such as the nares (nasal vestibule) and the back.6 Antibiotics used to treat acne, predominantly oral tetracyclines, showed positive oropharyngeal cultures for Streptococcus pyogenes in 33% (13/39) of treated patients; among these positive cultures, 85% (11/13) were resistant to at least one tetracycline antibiotic.7 Importantly, the streptococcal colonization of the oropharynx in individuals taking an oral antibiotic for acne may not induce a clinically apparent pharyngitis in that individual, but that person can carry and spread that streptococcal pathogen to others. In either case, the dermatology clinician, even if he/she suspects the connection related to antibiotic selection pressure and resistance, would not “see” the antibiotic resistance, as the individuals who develop a “sore throat” or strep throat do not seek care for this problem through a dermatology office.
The first formally organized and independent group in dermatology to address antibiotic use and resistance issues was the Scientific Panel on Antibiotic Use in Dermatology, which I put together in 2004 with James J. Leyden, MD (Philadelphia, Pennsylvania) and Guy F. Webster, MD (Hockessin, Delaware), and was comprised mostly of interested dermatologists with contributions from microbiologists and infectious disease specialists. A series of meetings, publications, and presentations have emerged from this group, which now falls under the auspices of the American Acne & Rosacea Society. Through the efforts of these organizations and other groups and companies with a strong interest in combating antibiotic resistance, we continue to see slow but steady progress in enlightening dermatology clinicians to think about if and when antibiotic therapy is needed and for how long. The subject of when antibiotics are not necessary also has been addressed, including both oral and topical antibiotics in many common scenarios encountered in dermatology practice.8 Examples include incision and drainage of an inflamed epidermal cyst without antibiotic therapy and use of white petrolatum instead of a topical antibiotic after most dermatologic procedures such as biopsies, tangential procedures, and closures after excisional procedures. Overall, the potential for topical antibiotics containing bacitracin and/or neomycin to induce allergic contact dermatitis is higher than the risk for postoperative wound infection. A major reason to avoid facilitating the emergence of antibiotic-resistant bacteria is that these organisms are efficient in packaging their resistance genes along with those from other bacteria, thus creating multidrug-resistant bacterial strains. This situation creates bigger challenges with trying to select effective therapies.
A cross-sectional analysis of antibiotics prescribed by dermatologists from January 1, 2008, to December 31, 2016, performed via a large commercial prescription claims database showed that among almost 1 million courses of oral antibiotics prescribed by approximately 12,000 unique dermatology prescribers, overall antibiotic prescribing decreased 36.6%, reflecting a drop of 1.23 courses per 100 visits, with much of the reduction occurring among extended antibiotic courses for acne and rosacea.9 Dermatology clinicians appear to be increasing their consideration of treatment alternatives such as oral spironolactone in adult female patients or earlier transition to oral isotretinoin therapy before starting another cycle with the same or a different oral antibiotic. Some have increased the use of physical device therapies. Importantly, we do not want to throw out the baby with the bathwater. Oral antibiotics remain important agents for treatment of moderate to severe inflammatory acne and in rosacea when subantibiotic-dose doxycycline is not accessible or is not effective after an adequate trial of therapy. Last but not least, a full-court press with an optimal topical regimen is the foundation of acne therapy, as monotherapy with an oral antibiotic for acne is considered dermatologic heresy and for good reason.
This discussion focuses on antibiotic resistance in acne therapy but also includes general principles related to this subject. “Seeing is believing” is a concept we have all heard many times, and we generally can all agree with and relate to what this is saying to us. However, it is harder to get a consensus of agreement on concepts that are happening beneath the surface but are not visibly apparent. Antibiotic resistance is a concept that falls into this latter category, especially in acne treatment. Many clinicians are not convinced antibiotic resistance is clinically relevant, exclaiming “I just do not see it in my practice.” The problem is—especially in the case of acne where oral tetracycline agents commonly are prescribed—how does the clinician “see” antibiotic resistance? Clinicians do not obtain bacterial cultures or perform sensitivity testing as they might do when evaluating a suspected cutaneous infection such as folliculitis, an inflamed postsurgical wound, a purulent leg ulcer, or an abscess. Additionally, if the selected therapy is not as effective as anticipated, it may be attributed to the patient needing another type of treatment or something “stronger,” or maybe they are not fully compliant. In fact, a very possible reason for inadequate therapeutic response may be that the predominant Cutibacterium acnes strains in a particular case are proinflammatory, and many of the strains are not highly sensitive to the chosen antibiotic.1
In the United States, antibiotic resistance in C acnes is most prevalent with erythromycin, followed by clindamycin, tetracycline, doxycycline, and minocycline, respectively.2 The relative patterns of antibiotic resistance in specific geographic regions correlate with the magnitude of specific antibiotic use, and that consistent reduction in use of a given antibiotic in a community can reverse the prevalence of resistance to that antibiotic progressively over time.3 Combination therapy approaches to mitigate emergence of resistant bacteria during acne treatment with an exit plan explained up-front with the patient are important to reduce prolonged use or repeated cycles of antibiotic use and in some cases to circumvent antibiotic use and incorporate a different therapeutic approach.1-3 Interestingly, in a retrospective chart review of acne patients who were eventually treated with oral isotretinoin at dermatology practices within a major university health system, approximately two-thirds received oral antibiotics for 6 months or longer and one-third for 1 year or longer.4 It is easy for all of us to have good intentions; however, in reality it is not always easy, practical, or in the best interest of the patient to stringently enforce recommendations that are determined not to be the best option at that time. Patients get a vote, too, as long as they are fully informed of benefits vs risks.
The concern about emergence of less-sensitive bacteria during acne antibiotic treatment is not limited to discussion of C acnes resistance. Use of both oral and topical antibiotics creates “ecologic mischief,” which is the emergence of less-sensitive strains of other bacteria exposed to the antibiotic—both commensal and opportunistic—especially at anatomic sites such as the skin, nasopharyngeal region, and gastrointestinal and genitourinary tracts.5-7 Application of topical erythromycin to the face can induce erythromycin-resistant bacteria such as staphylococci on the face as well as at remote sites such as the nares (nasal vestibule) and the back.6 Antibiotics used to treat acne, predominantly oral tetracyclines, showed positive oropharyngeal cultures for Streptococcus pyogenes in 33% (13/39) of treated patients; among these positive cultures, 85% (11/13) were resistant to at least one tetracycline antibiotic.7 Importantly, the streptococcal colonization of the oropharynx in individuals taking an oral antibiotic for acne may not induce a clinically apparent pharyngitis in that individual, but that person can carry and spread that streptococcal pathogen to others. In either case, the dermatology clinician, even if he/she suspects the connection related to antibiotic selection pressure and resistance, would not “see” the antibiotic resistance, as the individuals who develop a “sore throat” or strep throat do not seek care for this problem through a dermatology office.
The first formally organized and independent group in dermatology to address antibiotic use and resistance issues was the Scientific Panel on Antibiotic Use in Dermatology, which I put together in 2004 with James J. Leyden, MD (Philadelphia, Pennsylvania) and Guy F. Webster, MD (Hockessin, Delaware), and was comprised mostly of interested dermatologists with contributions from microbiologists and infectious disease specialists. A series of meetings, publications, and presentations have emerged from this group, which now falls under the auspices of the American Acne & Rosacea Society. Through the efforts of these organizations and other groups and companies with a strong interest in combating antibiotic resistance, we continue to see slow but steady progress in enlightening dermatology clinicians to think about if and when antibiotic therapy is needed and for how long. The subject of when antibiotics are not necessary also has been addressed, including both oral and topical antibiotics in many common scenarios encountered in dermatology practice.8 Examples include incision and drainage of an inflamed epidermal cyst without antibiotic therapy and use of white petrolatum instead of a topical antibiotic after most dermatologic procedures such as biopsies, tangential procedures, and closures after excisional procedures. Overall, the potential for topical antibiotics containing bacitracin and/or neomycin to induce allergic contact dermatitis is higher than the risk for postoperative wound infection. A major reason to avoid facilitating the emergence of antibiotic-resistant bacteria is that these organisms are efficient in packaging their resistance genes along with those from other bacteria, thus creating multidrug-resistant bacterial strains. This situation creates bigger challenges with trying to select effective therapies.
A cross-sectional analysis of antibiotics prescribed by dermatologists from January 1, 2008, to December 31, 2016, performed via a large commercial prescription claims database showed that among almost 1 million courses of oral antibiotics prescribed by approximately 12,000 unique dermatology prescribers, overall antibiotic prescribing decreased 36.6%, reflecting a drop of 1.23 courses per 100 visits, with much of the reduction occurring among extended antibiotic courses for acne and rosacea.9 Dermatology clinicians appear to be increasing their consideration of treatment alternatives such as oral spironolactone in adult female patients or earlier transition to oral isotretinoin therapy before starting another cycle with the same or a different oral antibiotic. Some have increased the use of physical device therapies. Importantly, we do not want to throw out the baby with the bathwater. Oral antibiotics remain important agents for treatment of moderate to severe inflammatory acne and in rosacea when subantibiotic-dose doxycycline is not accessible or is not effective after an adequate trial of therapy. Last but not least, a full-court press with an optimal topical regimen is the foundation of acne therapy, as monotherapy with an oral antibiotic for acne is considered dermatologic heresy and for good reason.
- Del Rosso JQ, Leyden JJ. Status report on antibiotic resistance: implications for the dermatologist. Dermatol Clin. 2007;25:127-132.
- Leyden JJ, Del Rosso JQ, Webster GF. Clinical considerations in the treatment of acne vulgaris and other inflammatory skin disorders: a status report. Dermatol Clin. 2009;27:1-15.
- Del Rosso JQ, Zeichner JA. The clinical relevance of antibiotic resistance: thirteen principles that every dermatologist needs to consider when prescribing antibiotic therapy. Dermatol Clin. 2016;34:167-173.
- Nagler AR, Milam EC, Orlow SJ. The use of oral antibiotics before isotretinoin therapy in patients with acne. J Am Acad Dermatol. 2016;74:273-279.
- Del Rosso JQ, Kim GK. Topical antibiotics: therapeutic value or ecologic mischief? Dermatol Ther. 2009;22:398-406.
- Bowe WP, Leyden JJ. Clinical implications of antibiotic resistance: risk of systemic infection from Staphylococcus and Streptococcus. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, England: Informa Healthcare; 2011:125-133.
- Levy RM, Huang EY, Roling D, et al. Effect of antibiotics on the oropharyngeal flora in patients with acne. Arch Dermatol. 2003;139:467-471.
- Hirschmann JV. When antibiotics are unnecessary. Dermatol Clin. 2009;27:75-83.
- Barbieri JS, Bhate K, Hartnett KP, et al. Trends in oral antibiotic prescription in dermatology, 2008 to 2016. JAMA Dermatol. 2019;155:290-297.
- Del Rosso JQ, Leyden JJ. Status report on antibiotic resistance: implications for the dermatologist. Dermatol Clin. 2007;25:127-132.
- Leyden JJ, Del Rosso JQ, Webster GF. Clinical considerations in the treatment of acne vulgaris and other inflammatory skin disorders: a status report. Dermatol Clin. 2009;27:1-15.
- Del Rosso JQ, Zeichner JA. The clinical relevance of antibiotic resistance: thirteen principles that every dermatologist needs to consider when prescribing antibiotic therapy. Dermatol Clin. 2016;34:167-173.
- Nagler AR, Milam EC, Orlow SJ. The use of oral antibiotics before isotretinoin therapy in patients with acne. J Am Acad Dermatol. 2016;74:273-279.
- Del Rosso JQ, Kim GK. Topical antibiotics: therapeutic value or ecologic mischief? Dermatol Ther. 2009;22:398-406.
- Bowe WP, Leyden JJ. Clinical implications of antibiotic resistance: risk of systemic infection from Staphylococcus and Streptococcus. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, England: Informa Healthcare; 2011:125-133.
- Levy RM, Huang EY, Roling D, et al. Effect of antibiotics on the oropharyngeal flora in patients with acne. Arch Dermatol. 2003;139:467-471.
- Hirschmann JV. When antibiotics are unnecessary. Dermatol Clin. 2009;27:75-83.
- Barbieri JS, Bhate K, Hartnett KP, et al. Trends in oral antibiotic prescription in dermatology, 2008 to 2016. JAMA Dermatol. 2019;155:290-297.
Clindamycin Phosphate–Tretinoin Combination Gel Revisited: Status Report on a Specific Formulation Used for Acne Treatment
Topical management of acne vulgaris (AV) incorporates a variety of agents with diverse modes of action (MOAs), including retinoids and antibiotics.1-3 The first topical retinoid developed for acne therapy was tretinoin, available in the United States since 1971.2,4 Topical retinoids, including tretinoin, exhibit multiple pharmacologic effects that are believed to correlate with efficacy for acne treatment,1,2,4,5 such as the reduction of inflammatory and comedonal lesions and contribution to dermal matrix remodeling.1,2,4-9 The predominant topical antibiotic used for acne treatment, often in combination with benzoyl peroxide (BP) and/or a topical retinoid, is clindamycin. Clindamycin is a lincosamide antibiotic that is closely related to erythromycin, a member of the macrolide antibiotic category.1,3,10 Available data support that over time topical clindamycin has sustained greater efficacy in reducing AV lesions than topical erythromycin; the latter also has been shown to exhibit a greater prevalence of Propionibacterium acnes resistance than clindamycin.1,3,10-12
Combination gel formulations of clindamycin phosphate 1.2%–tretinoin 0.025% (CP-Tret) are approved by the US Food and Drug Administration and available in the United States for once-daily treatment of AV in patients 12 years of age and older.13-15 Large-scale randomized controlled trials (RCTs) have demonstrated both efficacy and safety for these formulations.16,17 This article reviews important considerations related to both individual active ingredients (clindamycin phosphate [CP] and tretinoin [Tret]), formulation characteristics, and data from pivotal RCTs with a CP-Tret gel that has more recently been reintroduced into the US marketplace for acne therapy (Veltin, Aqua Pharmaceuticals).
What is the rationale behind combining CP and Tret in a single combination formulation?
Clindamycin is a lincosamide antibiotic that has been used for the treatment of AV for approximately 5 decades.1,3,10,17 The main MOA of clindamycin in the treatment of AV is believed to be reduction of P acnes; however, anti-inflammatory effects maypotentially play some role in AV lesion reduction.3,10,12,17-19 Multiple RCTs completed over approximately 3 decades and inclusive of more than 2000 participants treated topically with clindamycin as monotherapy have shown that the efficacy of this agent in reducing AV lesions has remained consistent overall,3,20-24 unlike topical erythromycin, which did not sustain its efficacy over a similar comparative time period.20 Importantly, these data are based on RCTs designed to evaluate the efficacy and safety of individual agents, including topical clindamycin; however, topical antibiotic therapy is not recommended as monotherapy for AV treatment due to emergence of antibiotic-resistant bacterial strains.1,3,11,12,25-28 Although the prevalence of resistant strains of P acnes is lower in the United States and many other countries for clindamycin versus erythromycin, the magnitude of clindamycin-resistant P acnes strains increases and response to clindamycin therapy may decrease when this agent is used alone.12,25-27,29,30 Therefore, it is recommended that a BP formulation that exhibits the ability to adequately reduce P acnes counts be used concurrently with antibiotic therapy for AV to reduce the emergence and proliferation of antibiotic-resistant P acnes organisms; short-contact BP therapy using a high-concentration (9.8%) emollient foam formulation and sufficient contact time (ie, 2 minutes) prior to washing off also has been shown to markedly reduce truncal P acnes organism counts.1,3,10-12,25-33 The Table depicts the major characteristics of clindamycin related to its use for treatment of AV.
Tretinoin has been used extensively for the treatment of AV since its introduction in the United States in 1971.1,2,4,5 The proposed MOAs of topical retinoids, including tretinoin, based on available data include a variety of pharmacologic effects such as inhibition of follicular hyperkeratosis (decreased microcomedone formation), modulation of keratinocyte differentiation, anti-inflammatory properties, and inhibition of dermal matrix degradation (Figure).1,2,4,5,14,34,35 Topical retinoids, including tretinoin, have been shown to reduce both inflammatory and comedonal acne lesions, likely due to multiple MOAs, and are devoid of antibiotic properties.2,4-8,16 Available data support that topical combination therapy for AV with a retinoid and a topical antimicrobial agent augments the therapeutic benefit as compared to use of either agent alone.1-4,12,15,16,28,31,32
The rationale for incorporating both clindamycin and tretinoin together into one topical formulation includes combining different MOAs that appear to correlate with suppression of AV lesion formation and to improve patient adherence through once-daily application of a single topical product.16,31,32,36 Importantly, formulation researchers were able to combine CP-Tret into a specific aqueous gel formulation that maintained the stability of both active ingredients and proved to be effective and safe in preliminary studies completed in participants with AV.16,23,37-39 This aqueous formulation incorporated a limited number of excipients with low to negligible potential for cutaneous irritation or allergenicity, including anhydrous citric acid (chelating agent, preservative, emulsifier, acidulent), butylated hydroxytoluene (antioxidant), carbomer homopolymer type C (thickening agent, dispersing agent, biocompatible gel matrix), edetate disodium (chelating agent), laureth 4 (emulsifier, dissolution agent), methylparaben (preservative), propylene glycol (low-concentration humectant), purified water (diluent), and tromethamine (buffer, permeability enhancer).14
What are the clinical data evaluating the efficacy and tolerability/safety of the specific aqueous-based gel formulation of CP-Tret?
An aqueous-based gel formulation (referred to in the literature as a hydrogel) of CP-Tret is devoid of alcohol and contains the excipients described above.14 This formulation was shown to be efficacious, well tolerated, and safe in smaller clinical studies of participants with AV.23,37-39 Two large-scale phase 3 studies were completed (N=2219), powered to compare the efficacy and tolerability/safety of CP-Tret hydrogel (n=634) versus CP hydrogel (n=635), Tret hydrogel (n=635), and vehicle hydrogel (n=315) in participants with facial AV. All 4 study drug formulations in both studies—CP-Tret, CP, Tret, vehicle—used the same hydrogel vehicle, hereafter referred to simply as gel.16
In both trials, participants 12 years of age and older with AV were randomized to active drug groups versus vehicle (2:2:2:1 randomization), each applied once daily at bedtime for 12 weeks.16 The baseline demographics among all 4 study groups were well matched, with approximately two-thirds of white participants and one-third Asian (2%–3%), black (19%–21%), or Hispanic (9%–10%). Approximately half of enrolled participants were 16 years of age or younger (mean age [range], 19.0–20.2 years). Enrolled participants in each study group presented at baseline predominantly with facial AV of mild (grade 2 [20%–23% of enrolled participants]) or moderate (grade 3 [60%–62% of enrolled participants]) severity based on a protocol-mandated, 6-point investigator static global assessment scale. Investigator static global assessment scores and acne lesion counts, including noninflammatory (comedonal), inflammatory (papules, pustules), and total AV lesions, were evaluated at baseline and weeks 2, 4, 8, and 12 (end of study [EOS]). Among the 4 study groups at baseline, the range of mean lesion counts was 27.7 to 29.3 for noninflammatory lesions, 26.0 to 26.4 for inflammatory lesions, and 76.4 to 78.3 for total lesions. All enrolled participants met protocol-mandated, standardized, inclusion, exclusion, and prestudy washout period criteria.16
The primary efficacy end points determined based on intention-to-treat analysis were the percentage reduction in all 3 lesion counts at EOS compared to baseline and the proportion of participants who achieved scores of clear (grade 0) or almost clear (grade 1) at EOS. The secondary end point parameter was time to 50% reduction in total lesion counts.16
The study efficacy outcomes were as follows: The mean percentage reduction in inflammatory lesions at EOS versus baseline was significantly higher in the CP-Tret group than in each of the other 3 groups (CP-Tret, 53.4%; CP, 47.5%; Tret, 43.3%; vehicle, 30.3%)(P<.005).16 The mean percentage reduction in noninflammatory lesions at EOS versus baseline was significantly higher in the CP-Tret group than in each of the other 3 groups (CP-Tret, 45.2%; CP, 31.6%; Tret, 37.9%; vehicle, 18.5%)(P≤.0004). The mean percentage reduction in total AV lesions at EOS versus baseline was significantly higher in the CP-Tret group than in each of the other 3 groups (CP-Tret, 48.7%; CP, 38.3%; Tret, 40.3%; vehicle, 23.2%)(P≤.0001). The median time to 50% reduction in total AV lesion counts was significantly faster with CP-Tret (8 weeks) compared to the other 3 groups (CP, 12 weeks [P<.0001]; Tret, 12 weeks [P<.001]; vehicle, not reached by EOS [P<.0001]). The consistency of results, methodologies, and overall study characteristics between the 2 phase 3 RCTs allowed for accurate pooling of data.16
Tolerability and safety assessments were completed at each visit for all enrolled participants. No adverse events (AEs) were noted in approximately 90% of enrolled participants.16 The most common AEs noted over the course of the study were mild to moderate application-site reactions (eg, dryness, erythema, burning, pruritus, desquamation), mostly correlated with the 2 groups containing tretinoin—CP-Tret and Tret—which is not unanticipated with topical retinoid use; 1.2% of these participants withdrew from the study due to such application-site AEs. No serious AEs or systemic safety signals emerged during the study.16
What summarizing statements can be made about CP-Tret gel from these study results that may be helpful to clinicians treating patients with AV?
The gel formulation of CP-Tret incorporates active ingredients that target different pathophysiologic cascades in AV, providing antimicrobial, anti-inflammatory, and anticomedonal effects.
Applied once daily, CP-Tret gel demonstrated the ability to achieve complete or near-complete clearance of comedonal and papulopustular facial AV lesions of mild to moderate severity in approximately 40% of participants within 12 weeks of use in 2 large-scale RCTs.16 The ability to achieve a median 50% reduction in total lesions by 8 weeks of use provides relevant information for patients regarding reasonable expectations with therapy.
The favorable cutaneous tolerability profile and low number of AEs demonstrated with CP-Tret gel are major considerations, especially as skin tolerability reactions can impede patient adherence with treatment. Any issues that interfere with achieving a favorable therapeutic outcome can lead to patients giving up with their therapy.
The large number of patients with skin of color treated with CP-Tret gel (n=209) in the 2 phase 3 RCTs is important, as the spectrum of racial origins, skin types, and skin colors seen in dermatology practices is diversifying across the United States. Both clinicians and patients with skin of color are often concerned about the sequelae of medication-induced skin irritation, which can lead to ensuing dyschromia.
Concerns related to potential development of clindamycin-resistant P acnes with CP-Tret gel may be addressed by concurrent use of BP, including with leave-on or short-contact therapy.
Although phase 3 RCTs evaluate therapeutic agents as monotherapy, in real world clinical practice, combination topical regimens using different individual products are common to optimize therapeutic outcomes. Advantages of the CP-Tret gel formulation, if a clinician desires to use it along with another topical product, are once-daily use and the low risk for cutaneous irritation.
- Gollnick H, Cunliffe W, Berson D, et al. Management of acne: a report from the Global Alliance to improve outcomes in acne. J Am Acad Dermatol. 2003;49(suppl 1):S1-S37.
- Hui AM, Shalita AR. Topical retinoids. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:86-94.
- Del Rosso JQ. Topical antibiotics. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:95-104.
- Sami N. Topical retinoids. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2013:505-517.
- Baldwin HE, Nighland M, Kendall C, et al. 40 years of topical tretinoin use in review. J Drugs Dermatol. 2013;12:638-642.
- Retin-A Micro [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals; 2015.
- Tazorac [package insert]. Irvine, CA: Allergan, Inc; 2014.
- Differin [package insert]. Fort Worth, TX: Galderma Laboratories, LP; 2011.
- Kang S. The mechanism of action of topical retinoids. Cutis. 2005;75(suppl 2):10-13; discussion 13.
- Motaparthi K, Hsu S. Topical antibacterial agents. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2013:445-459.
- Leyden JJ. The evolving role of Propionibacterium acnes in acne. Semin Cutan Med Surg. 2001;20:139-143.
- Leyden JJ, Del Rosso JQ, Webster GF. Clinical considerations in the treatment of acne vulgaris and other inflammatory skin disorders: focus on antibiotic resistance. Cutis. 2007;79(suppl 6):9-25.
- Ziana [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals; 2016.
- Veltin [package insert]. Exton, PA: Aqua Pharmaceuticals; 2015.
- Ochsendorf F. Clindamycin phosphate 1.2%/tretinoin 0.025%: a novel fixed-dose combination treatment for acne vulgaris. J Eur Acad Dermatol Venereol. 2015;29(suppl 5):8-13.
- Leyden JJ, Krochmal L, Yaroshinsky A. Two randomized, double-blind, controlled trials of 2219 subjects to compare the combination clindamycin/tretinoin hydrogel with each agent alone and vehicle for the treatment of acne vulgaris. J Am Acad Dermatol. 2006;54:73-81.
- Del Rosso JQ. Topical and oral antibiotics for acne vulgaris. Semin Cutan Med Surg. 2016;35:57-61.
- Leyden JJ. Open-label evaluation of topical antimicrobial and anti-acne preparations for effectiveness versus Propionibacterium acnes in vivo. Cutis. 1992;49(suppl 6A):8-11.
- Del Rosso JQ, Schmidt NF. A review of the anti-inflammatory properties of clindamycin in the treatment of acne vulgaris. Cutis. 2010;85:15-24.
- Simonart T, Dramaix M. Treatment of acne with topical antibiotics: lessons from clinical studies. Br J Dermatol. 2005;153:395-403.
- Schlessinger J, Menter A, Gold M, et al. Clinical safety and efficacy studies of a novel formulation combining 1.2% clindamycin phosphate and 0.025% tretinoin for the treatment of acne vulgaris. J Drugs Dermatol. 2007;6:607-615.
- Thiboutot D, Zaenglein A, Weiss J, et al. An aqueous gel fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 2.5% for the once-daily treatment of moderate to severe acne vulgaris: assessment of efficacy and safety in 2813 patients. J Am Acad Dermatol. 2008;59:792-800.
- Zouboulis CC, Derumeaux L, Decroix J, et al. A multicentre, single-blind, randomized comparison of a fixed clindamycin phosphate/tretinoin gel formulation (Velac) applied once daily and a clindamycin lotion formulation (Dalacin T) applied twice daily in the topical treatment of acne vulgaris. Br J Dermatol. 2000;143:498-505.
- Del Rosso JQ. Topical therapy for acne in women: is there a role for clindamycin phosphate-benzoyl peroxide gel? Cutis. 2014;94:177-182.
- Del Rosso JQ, Zeichner JA. The clinical relevance of antibiotic resistance: thirteen principles that every dermatologist needs to consider when prescribing antibiotic therapy. Dermatol Clin. 2016;34:167-173.
- Leyden JJ. Antibiotic resistance in the topical treatment of acne vulgaris. Cutis. 2004;73(6 suppl):6-10.
- Del Rosso JQ, Webster GF, Rosen T, et al. Status report from the Scientific Panel on Antibiotic Use in Dermatology of the American Acne and Rosacea Society: part 1: antibiotic prescribing patterns, sources of antibiotic exposure, antibiotic consumption and emergence of antibiotic resistance, impact of alterations in antibiotic prescribing, and clinical sequelae of antibiotic use. J Clin Aesthet Dermatol. 2016;9:18-24.
- Layton AM. Top ten list of clinical pearls in the treatment of acne vulgaris. Dermatol Clin. 2016;34:147-157.
- Leyden JJ. In vivo antibacterial effects of tretinoin-clindamycin and clindamycin alone on Propionibacterium acnes with varying clindamycin minimum inhibitory. J Drugs Dermatol. 2012;11:1434-1438.
- Cunliffe WJ, Holland KT, Bojar R, et al. A randomized, double-blind comparison of a clindamycin phosphate/benzoyl peroxide gel formulation and a matching clindamycin gel with respect to microbiologic activity and clinical efficacy in the topical treatment of acne vulgaris. Clin Ther. 2002;24:1117-1133.
- Villasenor J, Berson DS, Kroshinsky D. Combination therapy. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:105-112.
- Feneran A, Kaufman WS, Dabade TS, et al. Retinoid plus antimicrobial combination treatments for acne. Clin Cosmet Investig Dermatol. 2011;4:79-92.
- Leyden JJ, Del Rosso JQ. The effect of benzoyl peroxide 9.8% emollient foam on reduction of Propionibacterium acnes on the back using a short contact therapy approach. J Drugs Dermatol. 2012;11:830-833.
- Bikowski JB. Mechanisms of the comedolytic and anti-inflammatory properties of topical retinoids. J Drugs Dermatol. 2005;4:41-47.
- Del Rosso JQ. Retinoic acid receptors and topical acne therapy: establishing the link between gene expression and drug efficacy. Cutis. 2002;70:127-129.
- Zaghloul SS, Cunliffe WJ, Goodfield MJ. Objective assessment of compliance with treatments in acne. Br J Dermatol. 2005;152:1015-1021.
- Richter JR, Bousema MT, DeBoulle KLVM, et al. Efficacy of fixed clindamycin 1.2%, tretinoin 0.025% gel formulation (Velac) in topical control of facial acne lesions. J Dermatol Treat. 1998;9:81-90.
- Richter JR, Fӧrstrӧm LR, Kiistala UO, et al. Efficacy of fixed 1.2% clindamycin phosphate, 0.025% tretinoin gel formulation (Velac) and a proprietary 0.025% tretinoin gel formulation (Aberela) in the topical control of facial acne. J Eur Acad Dermatol Venereol. 1998;11:227-233.
- Cambazard F. Clinical efficacy of Velac, a new tretinoin and clindamycin gel in acne vulgaris. J Eur Acad Dermatol Venereol. 1998;11(suppl 1):S20-S27; discussion S28-S29.
Topical management of acne vulgaris (AV) incorporates a variety of agents with diverse modes of action (MOAs), including retinoids and antibiotics.1-3 The first topical retinoid developed for acne therapy was tretinoin, available in the United States since 1971.2,4 Topical retinoids, including tretinoin, exhibit multiple pharmacologic effects that are believed to correlate with efficacy for acne treatment,1,2,4,5 such as the reduction of inflammatory and comedonal lesions and contribution to dermal matrix remodeling.1,2,4-9 The predominant topical antibiotic used for acne treatment, often in combination with benzoyl peroxide (BP) and/or a topical retinoid, is clindamycin. Clindamycin is a lincosamide antibiotic that is closely related to erythromycin, a member of the macrolide antibiotic category.1,3,10 Available data support that over time topical clindamycin has sustained greater efficacy in reducing AV lesions than topical erythromycin; the latter also has been shown to exhibit a greater prevalence of Propionibacterium acnes resistance than clindamycin.1,3,10-12
Combination gel formulations of clindamycin phosphate 1.2%–tretinoin 0.025% (CP-Tret) are approved by the US Food and Drug Administration and available in the United States for once-daily treatment of AV in patients 12 years of age and older.13-15 Large-scale randomized controlled trials (RCTs) have demonstrated both efficacy and safety for these formulations.16,17 This article reviews important considerations related to both individual active ingredients (clindamycin phosphate [CP] and tretinoin [Tret]), formulation characteristics, and data from pivotal RCTs with a CP-Tret gel that has more recently been reintroduced into the US marketplace for acne therapy (Veltin, Aqua Pharmaceuticals).
What is the rationale behind combining CP and Tret in a single combination formulation?
Clindamycin is a lincosamide antibiotic that has been used for the treatment of AV for approximately 5 decades.1,3,10,17 The main MOA of clindamycin in the treatment of AV is believed to be reduction of P acnes; however, anti-inflammatory effects maypotentially play some role in AV lesion reduction.3,10,12,17-19 Multiple RCTs completed over approximately 3 decades and inclusive of more than 2000 participants treated topically with clindamycin as monotherapy have shown that the efficacy of this agent in reducing AV lesions has remained consistent overall,3,20-24 unlike topical erythromycin, which did not sustain its efficacy over a similar comparative time period.20 Importantly, these data are based on RCTs designed to evaluate the efficacy and safety of individual agents, including topical clindamycin; however, topical antibiotic therapy is not recommended as monotherapy for AV treatment due to emergence of antibiotic-resistant bacterial strains.1,3,11,12,25-28 Although the prevalence of resistant strains of P acnes is lower in the United States and many other countries for clindamycin versus erythromycin, the magnitude of clindamycin-resistant P acnes strains increases and response to clindamycin therapy may decrease when this agent is used alone.12,25-27,29,30 Therefore, it is recommended that a BP formulation that exhibits the ability to adequately reduce P acnes counts be used concurrently with antibiotic therapy for AV to reduce the emergence and proliferation of antibiotic-resistant P acnes organisms; short-contact BP therapy using a high-concentration (9.8%) emollient foam formulation and sufficient contact time (ie, 2 minutes) prior to washing off also has been shown to markedly reduce truncal P acnes organism counts.1,3,10-12,25-33 The Table depicts the major characteristics of clindamycin related to its use for treatment of AV.

Tretinoin has been used extensively for the treatment of AV since its introduction in the United States in 1971.1,2,4,5 The proposed MOAs of topical retinoids, including tretinoin, based on available data include a variety of pharmacologic effects such as inhibition of follicular hyperkeratosis (decreased microcomedone formation), modulation of keratinocyte differentiation, anti-inflammatory properties, and inhibition of dermal matrix degradation (Figure).1,2,4,5,14,34,35 Topical retinoids, including tretinoin, have been shown to reduce both inflammatory and comedonal acne lesions, likely due to multiple MOAs, and are devoid of antibiotic properties.2,4-8,16 Available data support that topical combination therapy for AV with a retinoid and a topical antimicrobial agent augments the therapeutic benefit as compared to use of either agent alone.1-4,12,15,16,28,31,32
The rationale for incorporating both clindamycin and tretinoin together into one topical formulation includes combining different MOAs that appear to correlate with suppression of AV lesion formation and to improve patient adherence through once-daily application of a single topical product.16,31,32,36 Importantly, formulation researchers were able to combine CP-Tret into a specific aqueous gel formulation that maintained the stability of both active ingredients and proved to be effective and safe in preliminary studies completed in participants with AV.16,23,37-39 This aqueous formulation incorporated a limited number of excipients with low to negligible potential for cutaneous irritation or allergenicity, including anhydrous citric acid (chelating agent, preservative, emulsifier, acidulent), butylated hydroxytoluene (antioxidant), carbomer homopolymer type C (thickening agent, dispersing agent, biocompatible gel matrix), edetate disodium (chelating agent), laureth 4 (emulsifier, dissolution agent), methylparaben (preservative), propylene glycol (low-concentration humectant), purified water (diluent), and tromethamine (buffer, permeability enhancer).14

What are the clinical data evaluating the efficacy and tolerability/safety of the specific aqueous-based gel formulation of CP-Tret?
An aqueous-based gel formulation (referred to in the literature as a hydrogel) of CP-Tret is devoid of alcohol and contains the excipients described above.14 This formulation was shown to be efficacious, well tolerated, and safe in smaller clinical studies of participants with AV.23,37-39 Two large-scale phase 3 studies were completed (N=2219), powered to compare the efficacy and tolerability/safety of CP-Tret hydrogel (n=634) versus CP hydrogel (n=635), Tret hydrogel (n=635), and vehicle hydrogel (n=315) in participants with facial AV. All 4 study drug formulations in both studies—CP-Tret, CP, Tret, vehicle—used the same hydrogel vehicle, hereafter referred to simply as gel.16
In both trials, participants 12 years of age and older with AV were randomized to active drug groups versus vehicle (2:2:2:1 randomization), each applied once daily at bedtime for 12 weeks.16 The baseline demographics among all 4 study groups were well matched, with approximately two-thirds of white participants and one-third Asian (2%–3%), black (19%–21%), or Hispanic (9%–10%). Approximately half of enrolled participants were 16 years of age or younger (mean age [range], 19.0–20.2 years). Enrolled participants in each study group presented at baseline predominantly with facial AV of mild (grade 2 [20%–23% of enrolled participants]) or moderate (grade 3 [60%–62% of enrolled participants]) severity based on a protocol-mandated, 6-point investigator static global assessment scale. Investigator static global assessment scores and acne lesion counts, including noninflammatory (comedonal), inflammatory (papules, pustules), and total AV lesions, were evaluated at baseline and weeks 2, 4, 8, and 12 (end of study [EOS]). Among the 4 study groups at baseline, the range of mean lesion counts was 27.7 to 29.3 for noninflammatory lesions, 26.0 to 26.4 for inflammatory lesions, and 76.4 to 78.3 for total lesions. All enrolled participants met protocol-mandated, standardized, inclusion, exclusion, and prestudy washout period criteria.16
The primary efficacy end points determined based on intention-to-treat analysis were the percentage reduction in all 3 lesion counts at EOS compared to baseline and the proportion of participants who achieved scores of clear (grade 0) or almost clear (grade 1) at EOS. The secondary end point parameter was time to 50% reduction in total lesion counts.16
The study efficacy outcomes were as follows: The mean percentage reduction in inflammatory lesions at EOS versus baseline was significantly higher in the CP-Tret group than in each of the other 3 groups (CP-Tret, 53.4%; CP, 47.5%; Tret, 43.3%; vehicle, 30.3%)(P<.005).16 The mean percentage reduction in noninflammatory lesions at EOS versus baseline was significantly higher in the CP-Tret group than in each of the other 3 groups (CP-Tret, 45.2%; CP, 31.6%; Tret, 37.9%; vehicle, 18.5%)(P≤.0004). The mean percentage reduction in total AV lesions at EOS versus baseline was significantly higher in the CP-Tret group than in each of the other 3 groups (CP-Tret, 48.7%; CP, 38.3%; Tret, 40.3%; vehicle, 23.2%)(P≤.0001). The median time to 50% reduction in total AV lesion counts was significantly faster with CP-Tret (8 weeks) compared to the other 3 groups (CP, 12 weeks [P<.0001]; Tret, 12 weeks [P<.001]; vehicle, not reached by EOS [P<.0001]). The consistency of results, methodologies, and overall study characteristics between the 2 phase 3 RCTs allowed for accurate pooling of data.16
Tolerability and safety assessments were completed at each visit for all enrolled participants. No adverse events (AEs) were noted in approximately 90% of enrolled participants.16 The most common AEs noted over the course of the study were mild to moderate application-site reactions (eg, dryness, erythema, burning, pruritus, desquamation), mostly correlated with the 2 groups containing tretinoin—CP-Tret and Tret—which is not unanticipated with topical retinoid use; 1.2% of these participants withdrew from the study due to such application-site AEs. No serious AEs or systemic safety signals emerged during the study.16
What summarizing statements can be made about CP-Tret gel from these study results that may be helpful to clinicians treating patients with AV?
The gel formulation of CP-Tret incorporates active ingredients that target different pathophysiologic cascades in AV, providing antimicrobial, anti-inflammatory, and anticomedonal effects.
Applied once daily, CP-Tret gel demonstrated the ability to achieve complete or near-complete clearance of comedonal and papulopustular facial AV lesions of mild to moderate severity in approximately 40% of participants within 12 weeks of use in 2 large-scale RCTs.16 The ability to achieve a median 50% reduction in total lesions by 8 weeks of use provides relevant information for patients regarding reasonable expectations with therapy.
The favorable cutaneous tolerability profile and low number of AEs demonstrated with CP-Tret gel are major considerations, especially as skin tolerability reactions can impede patient adherence with treatment. Any issues that interfere with achieving a favorable therapeutic outcome can lead to patients giving up with their therapy.
The large number of patients with skin of color treated with CP-Tret gel (n=209) in the 2 phase 3 RCTs is important, as the spectrum of racial origins, skin types, and skin colors seen in dermatology practices is diversifying across the United States. Both clinicians and patients with skin of color are often concerned about the sequelae of medication-induced skin irritation, which can lead to ensuing dyschromia.
Concerns related to potential development of clindamycin-resistant P acnes with CP-Tret gel may be addressed by concurrent use of BP, including with leave-on or short-contact therapy.
Although phase 3 RCTs evaluate therapeutic agents as monotherapy, in real world clinical practice, combination topical regimens using different individual products are common to optimize therapeutic outcomes. Advantages of the CP-Tret gel formulation, if a clinician desires to use it along with another topical product, are once-daily use and the low risk for cutaneous irritation.
Topical management of acne vulgaris (AV) incorporates a variety of agents with diverse modes of action (MOAs), including retinoids and antibiotics.1-3 The first topical retinoid developed for acne therapy was tretinoin, available in the United States since 1971.2,4 Topical retinoids, including tretinoin, exhibit multiple pharmacologic effects that are believed to correlate with efficacy for acne treatment,1,2,4,5 such as the reduction of inflammatory and comedonal lesions and contribution to dermal matrix remodeling.1,2,4-9 The predominant topical antibiotic used for acne treatment, often in combination with benzoyl peroxide (BP) and/or a topical retinoid, is clindamycin. Clindamycin is a lincosamide antibiotic that is closely related to erythromycin, a member of the macrolide antibiotic category.1,3,10 Available data support that over time topical clindamycin has sustained greater efficacy in reducing AV lesions than topical erythromycin; the latter also has been shown to exhibit a greater prevalence of Propionibacterium acnes resistance than clindamycin.1,3,10-12
Combination gel formulations of clindamycin phosphate 1.2%–tretinoin 0.025% (CP-Tret) are approved by the US Food and Drug Administration and available in the United States for once-daily treatment of AV in patients 12 years of age and older.13-15 Large-scale randomized controlled trials (RCTs) have demonstrated both efficacy and safety for these formulations.16,17 This article reviews important considerations related to both individual active ingredients (clindamycin phosphate [CP] and tretinoin [Tret]), formulation characteristics, and data from pivotal RCTs with a CP-Tret gel that has more recently been reintroduced into the US marketplace for acne therapy (Veltin, Aqua Pharmaceuticals).
What is the rationale behind combining CP and Tret in a single combination formulation?
Clindamycin is a lincosamide antibiotic that has been used for the treatment of AV for approximately 5 decades.1,3,10,17 The main MOA of clindamycin in the treatment of AV is believed to be reduction of P acnes; however, anti-inflammatory effects maypotentially play some role in AV lesion reduction.3,10,12,17-19 Multiple RCTs completed over approximately 3 decades and inclusive of more than 2000 participants treated topically with clindamycin as monotherapy have shown that the efficacy of this agent in reducing AV lesions has remained consistent overall,3,20-24 unlike topical erythromycin, which did not sustain its efficacy over a similar comparative time period.20 Importantly, these data are based on RCTs designed to evaluate the efficacy and safety of individual agents, including topical clindamycin; however, topical antibiotic therapy is not recommended as monotherapy for AV treatment due to emergence of antibiotic-resistant bacterial strains.1,3,11,12,25-28 Although the prevalence of resistant strains of P acnes is lower in the United States and many other countries for clindamycin versus erythromycin, the magnitude of clindamycin-resistant P acnes strains increases and response to clindamycin therapy may decrease when this agent is used alone.12,25-27,29,30 Therefore, it is recommended that a BP formulation that exhibits the ability to adequately reduce P acnes counts be used concurrently with antibiotic therapy for AV to reduce the emergence and proliferation of antibiotic-resistant P acnes organisms; short-contact BP therapy using a high-concentration (9.8%) emollient foam formulation and sufficient contact time (ie, 2 minutes) prior to washing off also has been shown to markedly reduce truncal P acnes organism counts.1,3,10-12,25-33 The Table depicts the major characteristics of clindamycin related to its use for treatment of AV.

Tretinoin has been used extensively for the treatment of AV since its introduction in the United States in 1971.1,2,4,5 The proposed MOAs of topical retinoids, including tretinoin, based on available data include a variety of pharmacologic effects such as inhibition of follicular hyperkeratosis (decreased microcomedone formation), modulation of keratinocyte differentiation, anti-inflammatory properties, and inhibition of dermal matrix degradation (Figure).1,2,4,5,14,34,35 Topical retinoids, including tretinoin, have been shown to reduce both inflammatory and comedonal acne lesions, likely due to multiple MOAs, and are devoid of antibiotic properties.2,4-8,16 Available data support that topical combination therapy for AV with a retinoid and a topical antimicrobial agent augments the therapeutic benefit as compared to use of either agent alone.1-4,12,15,16,28,31,32
The rationale for incorporating both clindamycin and tretinoin together into one topical formulation includes combining different MOAs that appear to correlate with suppression of AV lesion formation and to improve patient adherence through once-daily application of a single topical product.16,31,32,36 Importantly, formulation researchers were able to combine CP-Tret into a specific aqueous gel formulation that maintained the stability of both active ingredients and proved to be effective and safe in preliminary studies completed in participants with AV.16,23,37-39 This aqueous formulation incorporated a limited number of excipients with low to negligible potential for cutaneous irritation or allergenicity, including anhydrous citric acid (chelating agent, preservative, emulsifier, acidulent), butylated hydroxytoluene (antioxidant), carbomer homopolymer type C (thickening agent, dispersing agent, biocompatible gel matrix), edetate disodium (chelating agent), laureth 4 (emulsifier, dissolution agent), methylparaben (preservative), propylene glycol (low-concentration humectant), purified water (diluent), and tromethamine (buffer, permeability enhancer).14

What are the clinical data evaluating the efficacy and tolerability/safety of the specific aqueous-based gel formulation of CP-Tret?
An aqueous-based gel formulation (referred to in the literature as a hydrogel) of CP-Tret is devoid of alcohol and contains the excipients described above.14 This formulation was shown to be efficacious, well tolerated, and safe in smaller clinical studies of participants with AV.23,37-39 Two large-scale phase 3 studies were completed (N=2219), powered to compare the efficacy and tolerability/safety of CP-Tret hydrogel (n=634) versus CP hydrogel (n=635), Tret hydrogel (n=635), and vehicle hydrogel (n=315) in participants with facial AV. All 4 study drug formulations in both studies—CP-Tret, CP, Tret, vehicle—used the same hydrogel vehicle, hereafter referred to simply as gel.16
In both trials, participants 12 years of age and older with AV were randomized to active drug groups versus vehicle (2:2:2:1 randomization), each applied once daily at bedtime for 12 weeks.16 The baseline demographics among all 4 study groups were well matched, with approximately two-thirds of white participants and one-third Asian (2%–3%), black (19%–21%), or Hispanic (9%–10%). Approximately half of enrolled participants were 16 years of age or younger (mean age [range], 19.0–20.2 years). Enrolled participants in each study group presented at baseline predominantly with facial AV of mild (grade 2 [20%–23% of enrolled participants]) or moderate (grade 3 [60%–62% of enrolled participants]) severity based on a protocol-mandated, 6-point investigator static global assessment scale. Investigator static global assessment scores and acne lesion counts, including noninflammatory (comedonal), inflammatory (papules, pustules), and total AV lesions, were evaluated at baseline and weeks 2, 4, 8, and 12 (end of study [EOS]). Among the 4 study groups at baseline, the range of mean lesion counts was 27.7 to 29.3 for noninflammatory lesions, 26.0 to 26.4 for inflammatory lesions, and 76.4 to 78.3 for total lesions. All enrolled participants met protocol-mandated, standardized, inclusion, exclusion, and prestudy washout period criteria.16
The primary efficacy end points determined based on intention-to-treat analysis were the percentage reduction in all 3 lesion counts at EOS compared to baseline and the proportion of participants who achieved scores of clear (grade 0) or almost clear (grade 1) at EOS. The secondary end point parameter was time to 50% reduction in total lesion counts.16
The study efficacy outcomes were as follows: The mean percentage reduction in inflammatory lesions at EOS versus baseline was significantly higher in the CP-Tret group than in each of the other 3 groups (CP-Tret, 53.4%; CP, 47.5%; Tret, 43.3%; vehicle, 30.3%)(P<.005).16 The mean percentage reduction in noninflammatory lesions at EOS versus baseline was significantly higher in the CP-Tret group than in each of the other 3 groups (CP-Tret, 45.2%; CP, 31.6%; Tret, 37.9%; vehicle, 18.5%)(P≤.0004). The mean percentage reduction in total AV lesions at EOS versus baseline was significantly higher in the CP-Tret group than in each of the other 3 groups (CP-Tret, 48.7%; CP, 38.3%; Tret, 40.3%; vehicle, 23.2%)(P≤.0001). The median time to 50% reduction in total AV lesion counts was significantly faster with CP-Tret (8 weeks) compared to the other 3 groups (CP, 12 weeks [P<.0001]; Tret, 12 weeks [P<.001]; vehicle, not reached by EOS [P<.0001]). The consistency of results, methodologies, and overall study characteristics between the 2 phase 3 RCTs allowed for accurate pooling of data.16
Tolerability and safety assessments were completed at each visit for all enrolled participants. No adverse events (AEs) were noted in approximately 90% of enrolled participants.16 The most common AEs noted over the course of the study were mild to moderate application-site reactions (eg, dryness, erythema, burning, pruritus, desquamation), mostly correlated with the 2 groups containing tretinoin—CP-Tret and Tret—which is not unanticipated with topical retinoid use; 1.2% of these participants withdrew from the study due to such application-site AEs. No serious AEs or systemic safety signals emerged during the study.16
What summarizing statements can be made about CP-Tret gel from these study results that may be helpful to clinicians treating patients with AV?
The gel formulation of CP-Tret incorporates active ingredients that target different pathophysiologic cascades in AV, providing antimicrobial, anti-inflammatory, and anticomedonal effects.
Applied once daily, CP-Tret gel demonstrated the ability to achieve complete or near-complete clearance of comedonal and papulopustular facial AV lesions of mild to moderate severity in approximately 40% of participants within 12 weeks of use in 2 large-scale RCTs.16 The ability to achieve a median 50% reduction in total lesions by 8 weeks of use provides relevant information for patients regarding reasonable expectations with therapy.
The favorable cutaneous tolerability profile and low number of AEs demonstrated with CP-Tret gel are major considerations, especially as skin tolerability reactions can impede patient adherence with treatment. Any issues that interfere with achieving a favorable therapeutic outcome can lead to patients giving up with their therapy.
The large number of patients with skin of color treated with CP-Tret gel (n=209) in the 2 phase 3 RCTs is important, as the spectrum of racial origins, skin types, and skin colors seen in dermatology practices is diversifying across the United States. Both clinicians and patients with skin of color are often concerned about the sequelae of medication-induced skin irritation, which can lead to ensuing dyschromia.
Concerns related to potential development of clindamycin-resistant P acnes with CP-Tret gel may be addressed by concurrent use of BP, including with leave-on or short-contact therapy.
Although phase 3 RCTs evaluate therapeutic agents as monotherapy, in real world clinical practice, combination topical regimens using different individual products are common to optimize therapeutic outcomes. Advantages of the CP-Tret gel formulation, if a clinician desires to use it along with another topical product, are once-daily use and the low risk for cutaneous irritation.
- Gollnick H, Cunliffe W, Berson D, et al. Management of acne: a report from the Global Alliance to improve outcomes in acne. J Am Acad Dermatol. 2003;49(suppl 1):S1-S37.
- Hui AM, Shalita AR. Topical retinoids. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:86-94.
- Del Rosso JQ. Topical antibiotics. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:95-104.
- Sami N. Topical retinoids. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2013:505-517.
- Baldwin HE, Nighland M, Kendall C, et al. 40 years of topical tretinoin use in review. J Drugs Dermatol. 2013;12:638-642.
- Retin-A Micro [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals; 2015.
- Tazorac [package insert]. Irvine, CA: Allergan, Inc; 2014.
- Differin [package insert]. Fort Worth, TX: Galderma Laboratories, LP; 2011.
- Kang S. The mechanism of action of topical retinoids. Cutis. 2005;75(suppl 2):10-13; discussion 13.
- Motaparthi K, Hsu S. Topical antibacterial agents. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2013:445-459.
- Leyden JJ. The evolving role of Propionibacterium acnes in acne. Semin Cutan Med Surg. 2001;20:139-143.
- Leyden JJ, Del Rosso JQ, Webster GF. Clinical considerations in the treatment of acne vulgaris and other inflammatory skin disorders: focus on antibiotic resistance. Cutis. 2007;79(suppl 6):9-25.
- Ziana [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals; 2016.
- Veltin [package insert]. Exton, PA: Aqua Pharmaceuticals; 2015.
- Ochsendorf F. Clindamycin phosphate 1.2%/tretinoin 0.025%: a novel fixed-dose combination treatment for acne vulgaris. J Eur Acad Dermatol Venereol. 2015;29(suppl 5):8-13.
- Leyden JJ, Krochmal L, Yaroshinsky A. Two randomized, double-blind, controlled trials of 2219 subjects to compare the combination clindamycin/tretinoin hydrogel with each agent alone and vehicle for the treatment of acne vulgaris. J Am Acad Dermatol. 2006;54:73-81.
- Del Rosso JQ. Topical and oral antibiotics for acne vulgaris. Semin Cutan Med Surg. 2016;35:57-61.
- Leyden JJ. Open-label evaluation of topical antimicrobial and anti-acne preparations for effectiveness versus Propionibacterium acnes in vivo. Cutis. 1992;49(suppl 6A):8-11.
- Del Rosso JQ, Schmidt NF. A review of the anti-inflammatory properties of clindamycin in the treatment of acne vulgaris. Cutis. 2010;85:15-24.
- Simonart T, Dramaix M. Treatment of acne with topical antibiotics: lessons from clinical studies. Br J Dermatol. 2005;153:395-403.
- Schlessinger J, Menter A, Gold M, et al. Clinical safety and efficacy studies of a novel formulation combining 1.2% clindamycin phosphate and 0.025% tretinoin for the treatment of acne vulgaris. J Drugs Dermatol. 2007;6:607-615.
- Thiboutot D, Zaenglein A, Weiss J, et al. An aqueous gel fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 2.5% for the once-daily treatment of moderate to severe acne vulgaris: assessment of efficacy and safety in 2813 patients. J Am Acad Dermatol. 2008;59:792-800.
- Zouboulis CC, Derumeaux L, Decroix J, et al. A multicentre, single-blind, randomized comparison of a fixed clindamycin phosphate/tretinoin gel formulation (Velac) applied once daily and a clindamycin lotion formulation (Dalacin T) applied twice daily in the topical treatment of acne vulgaris. Br J Dermatol. 2000;143:498-505.
- Del Rosso JQ. Topical therapy for acne in women: is there a role for clindamycin phosphate-benzoyl peroxide gel? Cutis. 2014;94:177-182.
- Del Rosso JQ, Zeichner JA. The clinical relevance of antibiotic resistance: thirteen principles that every dermatologist needs to consider when prescribing antibiotic therapy. Dermatol Clin. 2016;34:167-173.
- Leyden JJ. Antibiotic resistance in the topical treatment of acne vulgaris. Cutis. 2004;73(6 suppl):6-10.
- Del Rosso JQ, Webster GF, Rosen T, et al. Status report from the Scientific Panel on Antibiotic Use in Dermatology of the American Acne and Rosacea Society: part 1: antibiotic prescribing patterns, sources of antibiotic exposure, antibiotic consumption and emergence of antibiotic resistance, impact of alterations in antibiotic prescribing, and clinical sequelae of antibiotic use. J Clin Aesthet Dermatol. 2016;9:18-24.
- Layton AM. Top ten list of clinical pearls in the treatment of acne vulgaris. Dermatol Clin. 2016;34:147-157.
- Leyden JJ. In vivo antibacterial effects of tretinoin-clindamycin and clindamycin alone on Propionibacterium acnes with varying clindamycin minimum inhibitory. J Drugs Dermatol. 2012;11:1434-1438.
- Cunliffe WJ, Holland KT, Bojar R, et al. A randomized, double-blind comparison of a clindamycin phosphate/benzoyl peroxide gel formulation and a matching clindamycin gel with respect to microbiologic activity and clinical efficacy in the topical treatment of acne vulgaris. Clin Ther. 2002;24:1117-1133.
- Villasenor J, Berson DS, Kroshinsky D. Combination therapy. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:105-112.
- Feneran A, Kaufman WS, Dabade TS, et al. Retinoid plus antimicrobial combination treatments for acne. Clin Cosmet Investig Dermatol. 2011;4:79-92.
- Leyden JJ, Del Rosso JQ. The effect of benzoyl peroxide 9.8% emollient foam on reduction of Propionibacterium acnes on the back using a short contact therapy approach. J Drugs Dermatol. 2012;11:830-833.
- Bikowski JB. Mechanisms of the comedolytic and anti-inflammatory properties of topical retinoids. J Drugs Dermatol. 2005;4:41-47.
- Del Rosso JQ. Retinoic acid receptors and topical acne therapy: establishing the link between gene expression and drug efficacy. Cutis. 2002;70:127-129.
- Zaghloul SS, Cunliffe WJ, Goodfield MJ. Objective assessment of compliance with treatments in acne. Br J Dermatol. 2005;152:1015-1021.
- Richter JR, Bousema MT, DeBoulle KLVM, et al. Efficacy of fixed clindamycin 1.2%, tretinoin 0.025% gel formulation (Velac) in topical control of facial acne lesions. J Dermatol Treat. 1998;9:81-90.
- Richter JR, Fӧrstrӧm LR, Kiistala UO, et al. Efficacy of fixed 1.2% clindamycin phosphate, 0.025% tretinoin gel formulation (Velac) and a proprietary 0.025% tretinoin gel formulation (Aberela) in the topical control of facial acne. J Eur Acad Dermatol Venereol. 1998;11:227-233.
- Cambazard F. Clinical efficacy of Velac, a new tretinoin and clindamycin gel in acne vulgaris. J Eur Acad Dermatol Venereol. 1998;11(suppl 1):S20-S27; discussion S28-S29.
- Gollnick H, Cunliffe W, Berson D, et al. Management of acne: a report from the Global Alliance to improve outcomes in acne. J Am Acad Dermatol. 2003;49(suppl 1):S1-S37.
- Hui AM, Shalita AR. Topical retinoids. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:86-94.
- Del Rosso JQ. Topical antibiotics. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:95-104.
- Sami N. Topical retinoids. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2013:505-517.
- Baldwin HE, Nighland M, Kendall C, et al. 40 years of topical tretinoin use in review. J Drugs Dermatol. 2013;12:638-642.
- Retin-A Micro [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals; 2015.
- Tazorac [package insert]. Irvine, CA: Allergan, Inc; 2014.
- Differin [package insert]. Fort Worth, TX: Galderma Laboratories, LP; 2011.
- Kang S. The mechanism of action of topical retinoids. Cutis. 2005;75(suppl 2):10-13; discussion 13.
- Motaparthi K, Hsu S. Topical antibacterial agents. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2013:445-459.
- Leyden JJ. The evolving role of Propionibacterium acnes in acne. Semin Cutan Med Surg. 2001;20:139-143.
- Leyden JJ, Del Rosso JQ, Webster GF. Clinical considerations in the treatment of acne vulgaris and other inflammatory skin disorders: focus on antibiotic resistance. Cutis. 2007;79(suppl 6):9-25.
- Ziana [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals; 2016.
- Veltin [package insert]. Exton, PA: Aqua Pharmaceuticals; 2015.
- Ochsendorf F. Clindamycin phosphate 1.2%/tretinoin 0.025%: a novel fixed-dose combination treatment for acne vulgaris. J Eur Acad Dermatol Venereol. 2015;29(suppl 5):8-13.
- Leyden JJ, Krochmal L, Yaroshinsky A. Two randomized, double-blind, controlled trials of 2219 subjects to compare the combination clindamycin/tretinoin hydrogel with each agent alone and vehicle for the treatment of acne vulgaris. J Am Acad Dermatol. 2006;54:73-81.
- Del Rosso JQ. Topical and oral antibiotics for acne vulgaris. Semin Cutan Med Surg. 2016;35:57-61.
- Leyden JJ. Open-label evaluation of topical antimicrobial and anti-acne preparations for effectiveness versus Propionibacterium acnes in vivo. Cutis. 1992;49(suppl 6A):8-11.
- Del Rosso JQ, Schmidt NF. A review of the anti-inflammatory properties of clindamycin in the treatment of acne vulgaris. Cutis. 2010;85:15-24.
- Simonart T, Dramaix M. Treatment of acne with topical antibiotics: lessons from clinical studies. Br J Dermatol. 2005;153:395-403.
- Schlessinger J, Menter A, Gold M, et al. Clinical safety and efficacy studies of a novel formulation combining 1.2% clindamycin phosphate and 0.025% tretinoin for the treatment of acne vulgaris. J Drugs Dermatol. 2007;6:607-615.
- Thiboutot D, Zaenglein A, Weiss J, et al. An aqueous gel fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 2.5% for the once-daily treatment of moderate to severe acne vulgaris: assessment of efficacy and safety in 2813 patients. J Am Acad Dermatol. 2008;59:792-800.
- Zouboulis CC, Derumeaux L, Decroix J, et al. A multicentre, single-blind, randomized comparison of a fixed clindamycin phosphate/tretinoin gel formulation (Velac) applied once daily and a clindamycin lotion formulation (Dalacin T) applied twice daily in the topical treatment of acne vulgaris. Br J Dermatol. 2000;143:498-505.
- Del Rosso JQ. Topical therapy for acne in women: is there a role for clindamycin phosphate-benzoyl peroxide gel? Cutis. 2014;94:177-182.
- Del Rosso JQ, Zeichner JA. The clinical relevance of antibiotic resistance: thirteen principles that every dermatologist needs to consider when prescribing antibiotic therapy. Dermatol Clin. 2016;34:167-173.
- Leyden JJ. Antibiotic resistance in the topical treatment of acne vulgaris. Cutis. 2004;73(6 suppl):6-10.
- Del Rosso JQ, Webster GF, Rosen T, et al. Status report from the Scientific Panel on Antibiotic Use in Dermatology of the American Acne and Rosacea Society: part 1: antibiotic prescribing patterns, sources of antibiotic exposure, antibiotic consumption and emergence of antibiotic resistance, impact of alterations in antibiotic prescribing, and clinical sequelae of antibiotic use. J Clin Aesthet Dermatol. 2016;9:18-24.
- Layton AM. Top ten list of clinical pearls in the treatment of acne vulgaris. Dermatol Clin. 2016;34:147-157.
- Leyden JJ. In vivo antibacterial effects of tretinoin-clindamycin and clindamycin alone on Propionibacterium acnes with varying clindamycin minimum inhibitory. J Drugs Dermatol. 2012;11:1434-1438.
- Cunliffe WJ, Holland KT, Bojar R, et al. A randomized, double-blind comparison of a clindamycin phosphate/benzoyl peroxide gel formulation and a matching clindamycin gel with respect to microbiologic activity and clinical efficacy in the topical treatment of acne vulgaris. Clin Ther. 2002;24:1117-1133.
- Villasenor J, Berson DS, Kroshinsky D. Combination therapy. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:105-112.
- Feneran A, Kaufman WS, Dabade TS, et al. Retinoid plus antimicrobial combination treatments for acne. Clin Cosmet Investig Dermatol. 2011;4:79-92.
- Leyden JJ, Del Rosso JQ. The effect of benzoyl peroxide 9.8% emollient foam on reduction of Propionibacterium acnes on the back using a short contact therapy approach. J Drugs Dermatol. 2012;11:830-833.
- Bikowski JB. Mechanisms of the comedolytic and anti-inflammatory properties of topical retinoids. J Drugs Dermatol. 2005;4:41-47.
- Del Rosso JQ. Retinoic acid receptors and topical acne therapy: establishing the link between gene expression and drug efficacy. Cutis. 2002;70:127-129.
- Zaghloul SS, Cunliffe WJ, Goodfield MJ. Objective assessment of compliance with treatments in acne. Br J Dermatol. 2005;152:1015-1021.
- Richter JR, Bousema MT, DeBoulle KLVM, et al. Efficacy of fixed clindamycin 1.2%, tretinoin 0.025% gel formulation (Velac) in topical control of facial acne lesions. J Dermatol Treat. 1998;9:81-90.
- Richter JR, Fӧrstrӧm LR, Kiistala UO, et al. Efficacy of fixed 1.2% clindamycin phosphate, 0.025% tretinoin gel formulation (Velac) and a proprietary 0.025% tretinoin gel formulation (Aberela) in the topical control of facial acne. J Eur Acad Dermatol Venereol. 1998;11:227-233.
- Cambazard F. Clinical efficacy of Velac, a new tretinoin and clindamycin gel in acne vulgaris. J Eur Acad Dermatol Venereol. 1998;11(suppl 1):S20-S27; discussion S28-S29.
Practice Points
- Clindamycin phosphate (CP)–tretinoin (Tret) formulated in an aqueous gel is effective based on clinical trials of the management of acne vulgaris (AV).
- The favorable tolerability of CP-Tret gel is advantageous, as topical agents often are used in combination with other therapies to treat AV, especially with a benzoyl peroxide–containing product.
- The availability of 2 active agents in 1 formulation is likely to optimize compliance.
When Do Efficacy Outcomes in Clinical Trials Correlate With Clinical Relevance? Analysis of Clindamycin Phosphate 1.2%–Benzoyl Peroxide 3.75% Gel in Moderate to Severe Acne Vulgaris
Acne vulgaris (AV) is a common skin disease that usually presents in adolescence and can persist into adulthood. Some cases may start in adulthood, especially in women. Acne vulgaris remains a challenge to treat successfully, both in teenagers and adults. Unrealistic expectations that therapy will rapidly clear and sustain clearance of AV completely can lead to incomplete adherence or complete cessation of treatment.1-4 Local tolerability reactions also may decrease adherence to topical medications. Suboptimal adherence to medications for AV is one of the major reasons for treatment failure.5 Acne vulgaris can strongly influence psychological well-being and self-esteem.6 In general, severe AV causes more psychological distress, but the adverse emotional impact of AV can be independent of its severity.7
An effective relationship between the patient and his/her physician and staff is believed to be important in setting realistic expectations, optimizing adherence, and achieving a positive therapeutic outcome. One component related to setting reasonable expectations is the discussion about when the patient may begin to visibly perceive that the treatment regimen is working. This article evaluates the time course of a clinically meaningful response using pivotal trial data with clindamycin phosphate 1.2%–benzoyl peroxide 3.75% (clindamycin-BP 3.75%) gel for treatment of AV.
Are data available that evaluate the time course of a clinically relevant response to treatment of AV?
Unfortunately, data on what might be perceived as a clinically meaningful improvement in AV and how long it might take to achieve this treatment effect are limited. A meta-analysis of more than 4000 patients with moderate to severe AV suggested that a 10% to 20% difference in acne lesion counts from baseline as compared to a subsequent designated time point was clinically relevant.8 A review of 24 comparative studies of patients with mild to moderate AV used a primary outcome parameter of a 25% reduction in mean inflammatory lesion count to evaluate time to onset of action (TOA) to achieve a clinically meaningful benefit.9 This outcome was based on a previously identified threshold of clinical relevance and the authors’ clinical experience in a patient population with milder AV. In this same analysis, a difference of greater than 4 days between the active group and the vehicle group was considered to be relevant to the patient.9
A faster onset of visible improvement as perceived by the patient should be more desirable and is likely to improve treatment adherence, as long as it is not counterbalanced by an increase in adverse events.
What is meant by TOA?
Time to onset of action refers to the duration required to achieve a 25% mean lesion count reduction from baseline, which is believed to correlate with the time point at which many patients would be able to perceive visible improvement when viewing their full face. Therefore, TOA represents an attempt to correlate data that is quantitative (based on lesion count reduction) with what is likely to be the average time that a patient may qualitatively observe an initial visible improvement in their AV. This concept may be useful as a tool when communicating with AV patients but should not be used in a way that will overpromise and underdeliver; rather, it is a guide for discussion with the patient and with a parent or guardian when applicable.
Consistent with the comparative AV study analysis that evaluated TOA, a linear course of lesion reductions between the provided time intervals was assumed. In this linear model, the TOA was calculated using the 2 extracted lesion count values between which the 25% lesion reduction was achieved as well as their corresponding given time points.9 Differences between the results in the active and vehicle study arms were calculated for a number of determinants.
How was pivotal trial data with clindamycin-BP 3.75% gel used to assess TOA?
A total of 498 patients with moderate to severe AV were randomized (1:1) to receive clindamycin-BP 3.75% gel or vehicle in a multicenter, double-blind, controlled, 12-week, 2-arm study.10 Before randomization, patients were stratified by acne severity based on a static Evaluator’s Global Severity Score (EGSS) ranging from 0 (clear) to 5 (very severe). Specifically, moderate AV (EGSS of 3) was described as predominantly noninflammatory lesions with evidence of multiple inflammatory lesions; several to many comedones, papules, and pustules; and no more than 1 small nodulocystic lesion. Severe AV (EGSS of 4) was characterized by inflammatory lesions; numerous comedones, papules, and pustules; and possibly a few nodulocystic lesions.10
Male and female patients aged 12 to 40 years with moderate to severe AV—defined as 20 to 40 inflammatory lesions (papules, pustules, nodules), 20 to 100 noninflammatory lesions (comedones), and no more than 2 nodules—were included in the study. Standard washout periods were required for patients using prior prescription and over-the-counter acne treatments.10
Efficacy evaluations included inflammatory and noninflammatory lesion counts and EGSS at screening, baseline, and during treatment (weeks 4, 8, and 12).10 Primary efficacy end points included absolute change in mean inflammatory and noninflammatory lesion counts and the proportion of patients who achieved at least a 2-grade reduction in EGSS from baseline to week 12 (treatment success at end of study). Secondary efficacy end points included mean percentage change from baseline to week 12 in inflammatory and noninflammatory lesion counts and the proportion of patients who considered themselves clear or almost clear at week 12.10
After 12 weeks of daily treatment, inflammatory and noninflammatory lesion counts decreased by a mean of 60.4% and 51.8%, respectively, with clindamycin-BP 3.75% gel compared to 31.3% and 27.6%, respectively, with vehicle (both P<.001). At weeks 4, 8, and 12, the difference in inflammatory and noninflammatory lesion counts for the active treatment was 17.4%, 24.8%, and 29.1%, respectively, and 8.1%, 19.8%, and 24.2%, respectively, for vehicle.10
Treatment success (at least a 2-grade improvement in EGSS) was achieved by 9.1% of patients using clindamycin-BP 3.75% gel compared to 4.6% using vehicle by week 4. Additionally, 6.3% of patients considered their AV as clear or almost clear compared to 3.5% with vehicle at week 2 (Figure 1).10
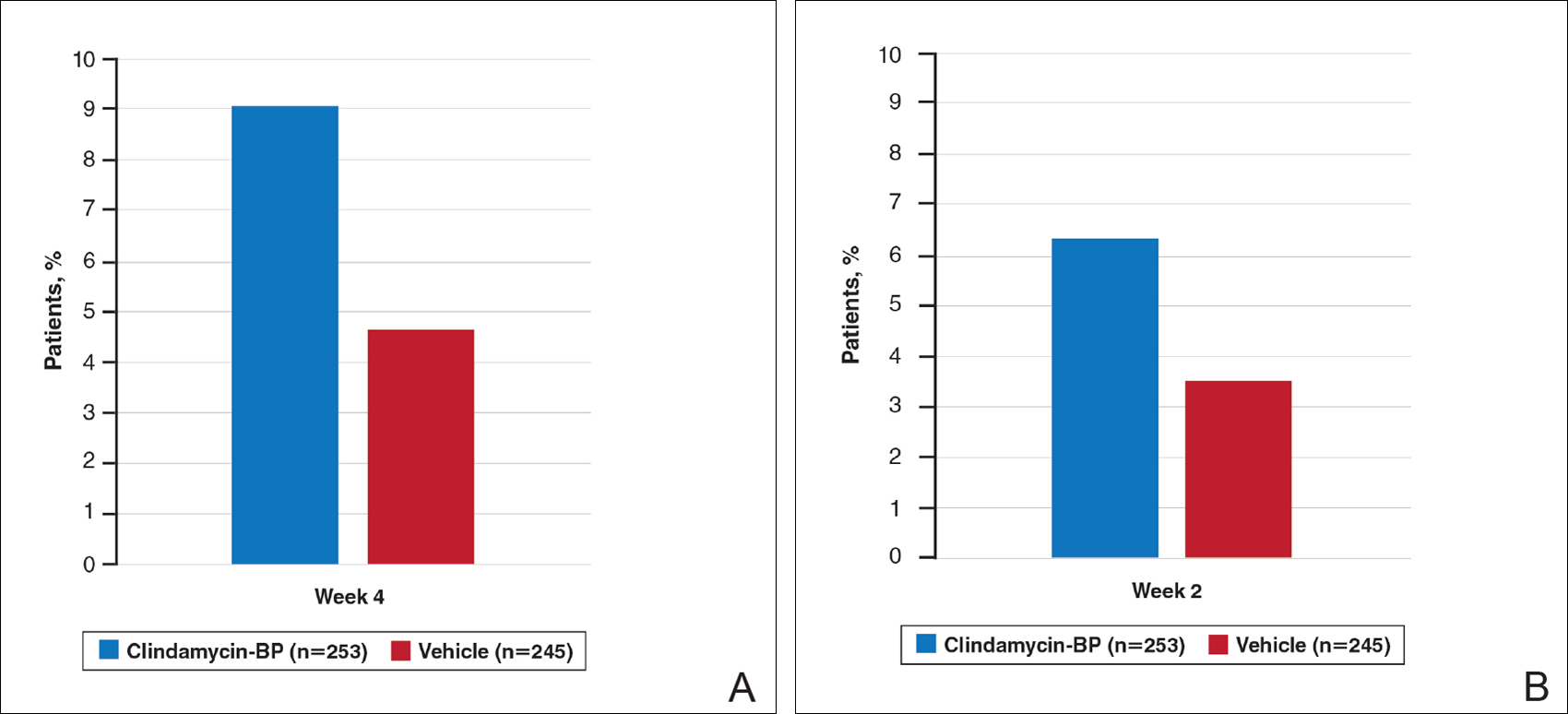
This analysis represents the first attempt to evaluate and report TOA results with clindamycin-BP 3.75% gel. Time to onset of action for inflammatory lesions treated with clindamycin-BP 3.75% gel was calculated as 2.5 weeks versus 6.2 weeks for vehicle (Figure 2A). Time to onset of action for noninflammatory lesions was 3.7 weeks with clindamycin-BP 3.75% gel versus 8.6 weeks with vehicle (Figure 2B). The difference in TOA between the active and vehicle study groups was 3.7 weeks and 4.9 weeks, respectively. In addition, among actively treated patients, TOA was shorter in females (2.1 weeks) than in males (2.6 weeks) and in moderate AV (2.5 weeks) compared to severe AV (3.0 weeks).
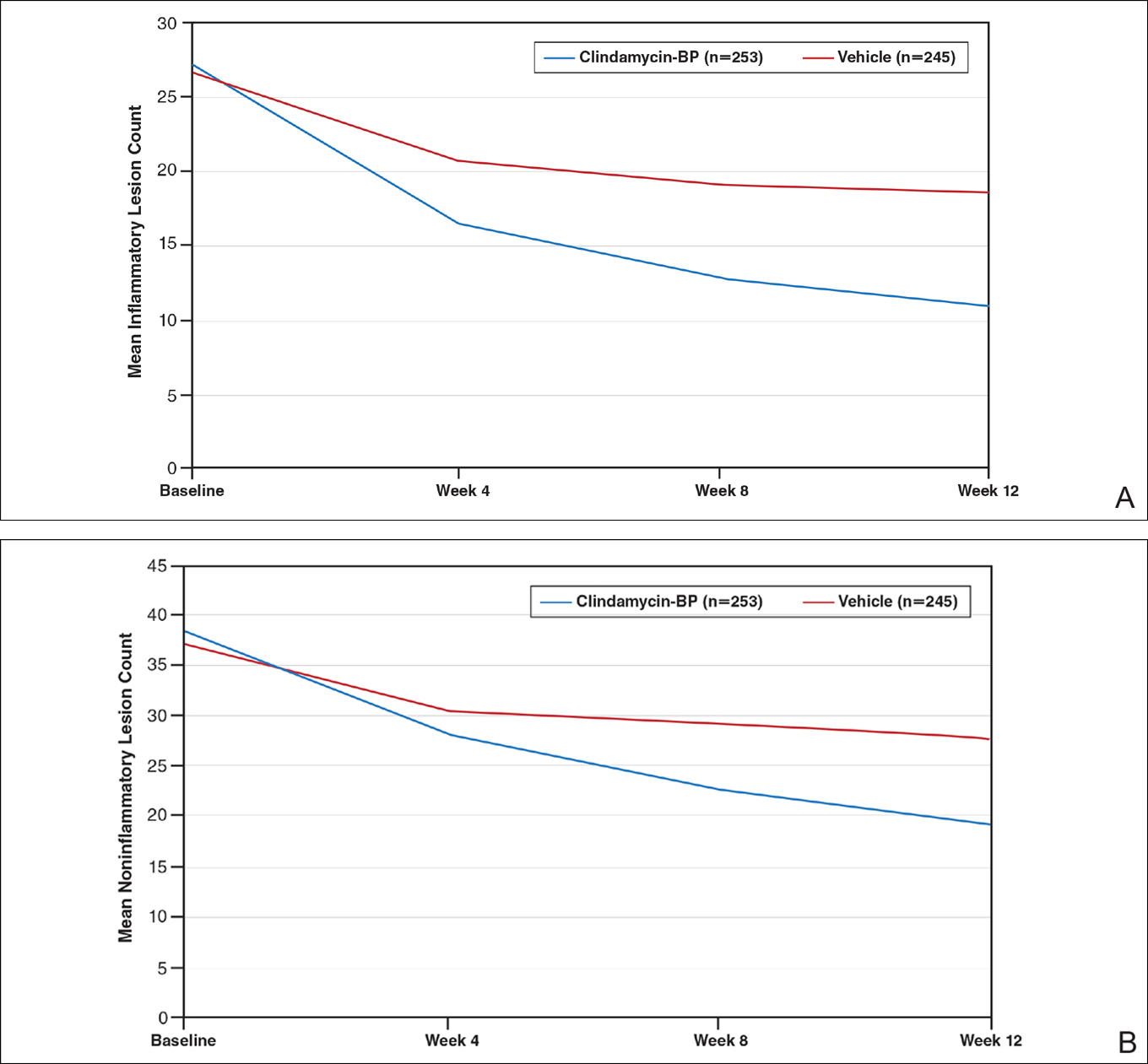
Comment
Differences in lesion counts between clindamycin-BP 3.75% gel and vehicle suggest a clinically relevant benefit in favor of active treatment with both inflammatory and noninflammatory lesions. Nearly twice as many patients were rated as treatment successes using EGSS by week 4 or clear or almost clear as early as week 2 compared to the vehicle group.10 However, these data are suggested as an overall guide but do not provide adequate guidance on when visible improvement may start to be evident in a given patient.
The analysis reported here shows a TOA of 2.5 weeks with clindamycin-BP 3.75% gel for inflammatory lesions, approximately 4 weeks faster than with the vehicle. In most cases, a reduction in inflammatory lesions is more likely to have a greater impact on patient perception of TOA. Unless a patient is aware or focused enough to actively distinguish visibly between inflammatory and noninflammatory (comedonal) AV lesions, their eye is more likely to be drawn initially to reduction in inflammatory lesions, which are erythematous and more visible at a greater viewing distance. Although noninflammatory AV lesions usually require closer inspection to visualize them (especially closed comedones), they are often slower to respond to treatment. Analysis of the pivotal trial data reports a longer TOA with clindamycin-BP 3.75% gel for noninflammatory lesions (3.7 weeks) versus inflammatory lesions (2.5 weeks).
As expected, TOA was shorter in patients with moderate AV than severe AV (2.5 weeks vs 3.0 weeks). Time to onset of action also was shorter in females overall. It is unclear why we see gender differences in acne studies. A number of reasons have been suggested, including differences in AV pathophysiology and/or treatment adherence.11,12 Greater efficacy of clindamycin-BP 3.75% gel in females compared with males has already been reported, and better overall efficacy leading to a shorter TOA has been noted by others.13
There are limitations with this analysis. First, it is not possible to assess the contributions from each of the monads to the efficacy of clindamycin-BP 3.75% gel or TOA. Also, the data extraction method used assumes a linear progression model during the provided time points and was used to provide some comparison with calculations for other combination products.9 Although no strong deviations from the linear model are likely, calculations of TOA using other methodologies may give different results. The definition of a clinically meaningful benefit, defined here as a 25% reduction in the mean lesion count, has been used as a guide, but it has not been validated in clinical practice. It also is important to recognize that the initial visible perception of improvement of AV is likely to differ based on interpatient variability; that is, how different individuals perceive improvement. It also may be affected by differences in baseline severity of AV among different patients. Additionally, the TOA reflects an average duration of time, so it should not be described to patients as a suggestion of when they will definitely see visible improvement in their AV.
Conclusion
Unrealistic expectations of acne therapy or poor tolerability can lead to low adherence and poor clinical outcomes.1-4 The data on TOA reported here suggests that a clinically meaningful benefit with clindamycin-BP 3.75% gel may be seen in some patients within 2 to 3 weeks and maybe sooner in females or those with milder disease; however, longer durations may be required in some patients. This information can help clinicians and their staff in providing reasonable expectations and stress the importance of encouraging patients about the need to adhere to treatment.
Acknowledgments
The author thanks Brian Bulley, MSc (Inergy Limited, Lindfield, West Sussex, United Kingdom), for publication support. Valeant Pharmaceuticals North America, LLC, funded Inergy’s activities pertaining to this analysis. The author did not receive funding or any form of compensation for authorship of this publication.
- Krakowski AC, Stendardo S, Eichenfield LF. Practical considerations in acne treatment and the clinical impact of topical combination therapy. Pediatr Dermatol. 2008;25(suppl 1):1-14.
- Yentzer BA, Ade RA, Fountain JM, et al. Simplifying regimens promotes greater adherence and outcomes with topical acne medications: a randomized controlled trial. Cutis. 2010;86:103-108.
- Zaghloul SS, Cunliffe WJ, Goodfield MJ. Objective assessment of compliance with treatments in acne. Br J Dermatol. 2005;152:1015-1021.
- Snyder S, Crandell I, Davis SA, et al. Medical adherence to acne therapy: a systematic review. Am J Clin Dermatol. 2014;15:87-94.
- Miyachi Y, Hayashi N, Furukawa F, et al. Acne management in Japan: study of patient adherence. Dermatology. 2011;223:174-181.
- Zauli S, Caracciolo S, Borghi A, et al. Which factors influence quality of life in acne patients? J Eur Acad Dermatol Venereol. 2014;28:46-50.
- Mulder MM, Sigurdsson V, van Zuuren EJ, et al. Psychosocial impact of acne vulgaris. evaluation of the relation between a change in clinical acne severity and psychosocial state. Dermatology. 2001;203:124-130.
- Gerlinger C, Stadtler G, Gotzelmann R, et al. A noninferiority margin for acne lesion counts. Drug Inf J. 2008;42:607-615.
- Jacobs A, Starke G, Rosumeck S, et al. Systematic review on the rapidity of the onset of action of topical treatments in the therapy of mild-to-moderate acne vulgaris. Br J Dermatol. 2014;170:557-564.
- Pariser DM, Rich P, Cook-Bolden FE, et al. An aqueous gel fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 3.75% for the once-daily treatment of moderate to severe acne vulgaris. J Drugs Dermatol. 2014;13:611-617.
- Tanghetti E, Harper JC, Oefelein MG. The efficacy and tolerability of dapsone 5% gel in female vs male patients with facial acne vulgaris: gender as a clinically relevant outcome variable. J Drugs Dermatol. 2012;11:1417-1421.
- Lott R, Taylor SL, O’Neill JL, et al. Medication adherence among acne patients: a review. J Cosmet Dermatol. 2010;9:160-166.
- Harper JC. The efficacy and tolerability of a fixed combination clindamycin (1.2%) and benzoyl peroxide (3.75%) aqueous gel in patients with facial acne vulgaris: gender as a clinically relevant outcome variable. J Drugs Dermatol. 2015;14:381-384.
Acne vulgaris (AV) is a common skin disease that usually presents in adolescence and can persist into adulthood. Some cases may start in adulthood, especially in women. Acne vulgaris remains a challenge to treat successfully, both in teenagers and adults. Unrealistic expectations that therapy will rapidly clear and sustain clearance of AV completely can lead to incomplete adherence or complete cessation of treatment.1-4 Local tolerability reactions also may decrease adherence to topical medications. Suboptimal adherence to medications for AV is one of the major reasons for treatment failure.5 Acne vulgaris can strongly influence psychological well-being and self-esteem.6 In general, severe AV causes more psychological distress, but the adverse emotional impact of AV can be independent of its severity.7
An effective relationship between the patient and his/her physician and staff is believed to be important in setting realistic expectations, optimizing adherence, and achieving a positive therapeutic outcome. One component related to setting reasonable expectations is the discussion about when the patient may begin to visibly perceive that the treatment regimen is working. This article evaluates the time course of a clinically meaningful response using pivotal trial data with clindamycin phosphate 1.2%–benzoyl peroxide 3.75% (clindamycin-BP 3.75%) gel for treatment of AV.
Are data available that evaluate the time course of a clinically relevant response to treatment of AV?
Unfortunately, data on what might be perceived as a clinically meaningful improvement in AV and how long it might take to achieve this treatment effect are limited. A meta-analysis of more than 4000 patients with moderate to severe AV suggested that a 10% to 20% difference in acne lesion counts from baseline as compared to a subsequent designated time point was clinically relevant.8 A review of 24 comparative studies of patients with mild to moderate AV used a primary outcome parameter of a 25% reduction in mean inflammatory lesion count to evaluate time to onset of action (TOA) to achieve a clinically meaningful benefit.9 This outcome was based on a previously identified threshold of clinical relevance and the authors’ clinical experience in a patient population with milder AV. In this same analysis, a difference of greater than 4 days between the active group and the vehicle group was considered to be relevant to the patient.9
A faster onset of visible improvement as perceived by the patient should be more desirable and is likely to improve treatment adherence, as long as it is not counterbalanced by an increase in adverse events.
What is meant by TOA?
Time to onset of action refers to the duration required to achieve a 25% mean lesion count reduction from baseline, which is believed to correlate with the time point at which many patients would be able to perceive visible improvement when viewing their full face. Therefore, TOA represents an attempt to correlate data that is quantitative (based on lesion count reduction) with what is likely to be the average time that a patient may qualitatively observe an initial visible improvement in their AV. This concept may be useful as a tool when communicating with AV patients but should not be used in a way that will overpromise and underdeliver; rather, it is a guide for discussion with the patient and with a parent or guardian when applicable.
Consistent with the comparative AV study analysis that evaluated TOA, a linear course of lesion reductions between the provided time intervals was assumed. In this linear model, the TOA was calculated using the 2 extracted lesion count values between which the 25% lesion reduction was achieved as well as their corresponding given time points.9 Differences between the results in the active and vehicle study arms were calculated for a number of determinants.
How was pivotal trial data with clindamycin-BP 3.75% gel used to assess TOA?
A total of 498 patients with moderate to severe AV were randomized (1:1) to receive clindamycin-BP 3.75% gel or vehicle in a multicenter, double-blind, controlled, 12-week, 2-arm study.10 Before randomization, patients were stratified by acne severity based on a static Evaluator’s Global Severity Score (EGSS) ranging from 0 (clear) to 5 (very severe). Specifically, moderate AV (EGSS of 3) was described as predominantly noninflammatory lesions with evidence of multiple inflammatory lesions; several to many comedones, papules, and pustules; and no more than 1 small nodulocystic lesion. Severe AV (EGSS of 4) was characterized by inflammatory lesions; numerous comedones, papules, and pustules; and possibly a few nodulocystic lesions.10
Male and female patients aged 12 to 40 years with moderate to severe AV—defined as 20 to 40 inflammatory lesions (papules, pustules, nodules), 20 to 100 noninflammatory lesions (comedones), and no more than 2 nodules—were included in the study. Standard washout periods were required for patients using prior prescription and over-the-counter acne treatments.10
Efficacy evaluations included inflammatory and noninflammatory lesion counts and EGSS at screening, baseline, and during treatment (weeks 4, 8, and 12).10 Primary efficacy end points included absolute change in mean inflammatory and noninflammatory lesion counts and the proportion of patients who achieved at least a 2-grade reduction in EGSS from baseline to week 12 (treatment success at end of study). Secondary efficacy end points included mean percentage change from baseline to week 12 in inflammatory and noninflammatory lesion counts and the proportion of patients who considered themselves clear or almost clear at week 12.10
After 12 weeks of daily treatment, inflammatory and noninflammatory lesion counts decreased by a mean of 60.4% and 51.8%, respectively, with clindamycin-BP 3.75% gel compared to 31.3% and 27.6%, respectively, with vehicle (both P<.001). At weeks 4, 8, and 12, the difference in inflammatory and noninflammatory lesion counts for the active treatment was 17.4%, 24.8%, and 29.1%, respectively, and 8.1%, 19.8%, and 24.2%, respectively, for vehicle.10
Treatment success (at least a 2-grade improvement in EGSS) was achieved by 9.1% of patients using clindamycin-BP 3.75% gel compared to 4.6% using vehicle by week 4. Additionally, 6.3% of patients considered their AV as clear or almost clear compared to 3.5% with vehicle at week 2 (Figure 1).10

This analysis represents the first attempt to evaluate and report TOA results with clindamycin-BP 3.75% gel. Time to onset of action for inflammatory lesions treated with clindamycin-BP 3.75% gel was calculated as 2.5 weeks versus 6.2 weeks for vehicle (Figure 2A). Time to onset of action for noninflammatory lesions was 3.7 weeks with clindamycin-BP 3.75% gel versus 8.6 weeks with vehicle (Figure 2B). The difference in TOA between the active and vehicle study groups was 3.7 weeks and 4.9 weeks, respectively. In addition, among actively treated patients, TOA was shorter in females (2.1 weeks) than in males (2.6 weeks) and in moderate AV (2.5 weeks) compared to severe AV (3.0 weeks).

Comment
Differences in lesion counts between clindamycin-BP 3.75% gel and vehicle suggest a clinically relevant benefit in favor of active treatment with both inflammatory and noninflammatory lesions. Nearly twice as many patients were rated as treatment successes using EGSS by week 4 or clear or almost clear as early as week 2 compared to the vehicle group.10 However, these data are suggested as an overall guide but do not provide adequate guidance on when visible improvement may start to be evident in a given patient.
The analysis reported here shows a TOA of 2.5 weeks with clindamycin-BP 3.75% gel for inflammatory lesions, approximately 4 weeks faster than with the vehicle. In most cases, a reduction in inflammatory lesions is more likely to have a greater impact on patient perception of TOA. Unless a patient is aware or focused enough to actively distinguish visibly between inflammatory and noninflammatory (comedonal) AV lesions, their eye is more likely to be drawn initially to reduction in inflammatory lesions, which are erythematous and more visible at a greater viewing distance. Although noninflammatory AV lesions usually require closer inspection to visualize them (especially closed comedones), they are often slower to respond to treatment. Analysis of the pivotal trial data reports a longer TOA with clindamycin-BP 3.75% gel for noninflammatory lesions (3.7 weeks) versus inflammatory lesions (2.5 weeks).
As expected, TOA was shorter in patients with moderate AV than severe AV (2.5 weeks vs 3.0 weeks). Time to onset of action also was shorter in females overall. It is unclear why we see gender differences in acne studies. A number of reasons have been suggested, including differences in AV pathophysiology and/or treatment adherence.11,12 Greater efficacy of clindamycin-BP 3.75% gel in females compared with males has already been reported, and better overall efficacy leading to a shorter TOA has been noted by others.13
There are limitations with this analysis. First, it is not possible to assess the contributions from each of the monads to the efficacy of clindamycin-BP 3.75% gel or TOA. Also, the data extraction method used assumes a linear progression model during the provided time points and was used to provide some comparison with calculations for other combination products.9 Although no strong deviations from the linear model are likely, calculations of TOA using other methodologies may give different results. The definition of a clinically meaningful benefit, defined here as a 25% reduction in the mean lesion count, has been used as a guide, but it has not been validated in clinical practice. It also is important to recognize that the initial visible perception of improvement of AV is likely to differ based on interpatient variability; that is, how different individuals perceive improvement. It also may be affected by differences in baseline severity of AV among different patients. Additionally, the TOA reflects an average duration of time, so it should not be described to patients as a suggestion of when they will definitely see visible improvement in their AV.
Conclusion
Unrealistic expectations of acne therapy or poor tolerability can lead to low adherence and poor clinical outcomes.1-4 The data on TOA reported here suggests that a clinically meaningful benefit with clindamycin-BP 3.75% gel may be seen in some patients within 2 to 3 weeks and maybe sooner in females or those with milder disease; however, longer durations may be required in some patients. This information can help clinicians and their staff in providing reasonable expectations and stress the importance of encouraging patients about the need to adhere to treatment.
Acknowledgments
The author thanks Brian Bulley, MSc (Inergy Limited, Lindfield, West Sussex, United Kingdom), for publication support. Valeant Pharmaceuticals North America, LLC, funded Inergy’s activities pertaining to this analysis. The author did not receive funding or any form of compensation for authorship of this publication.
Acne vulgaris (AV) is a common skin disease that usually presents in adolescence and can persist into adulthood. Some cases may start in adulthood, especially in women. Acne vulgaris remains a challenge to treat successfully, both in teenagers and adults. Unrealistic expectations that therapy will rapidly clear and sustain clearance of AV completely can lead to incomplete adherence or complete cessation of treatment.1-4 Local tolerability reactions also may decrease adherence to topical medications. Suboptimal adherence to medications for AV is one of the major reasons for treatment failure.5 Acne vulgaris can strongly influence psychological well-being and self-esteem.6 In general, severe AV causes more psychological distress, but the adverse emotional impact of AV can be independent of its severity.7
An effective relationship between the patient and his/her physician and staff is believed to be important in setting realistic expectations, optimizing adherence, and achieving a positive therapeutic outcome. One component related to setting reasonable expectations is the discussion about when the patient may begin to visibly perceive that the treatment regimen is working. This article evaluates the time course of a clinically meaningful response using pivotal trial data with clindamycin phosphate 1.2%–benzoyl peroxide 3.75% (clindamycin-BP 3.75%) gel for treatment of AV.
Are data available that evaluate the time course of a clinically relevant response to treatment of AV?
Unfortunately, data on what might be perceived as a clinically meaningful improvement in AV and how long it might take to achieve this treatment effect are limited. A meta-analysis of more than 4000 patients with moderate to severe AV suggested that a 10% to 20% difference in acne lesion counts from baseline as compared to a subsequent designated time point was clinically relevant.8 A review of 24 comparative studies of patients with mild to moderate AV used a primary outcome parameter of a 25% reduction in mean inflammatory lesion count to evaluate time to onset of action (TOA) to achieve a clinically meaningful benefit.9 This outcome was based on a previously identified threshold of clinical relevance and the authors’ clinical experience in a patient population with milder AV. In this same analysis, a difference of greater than 4 days between the active group and the vehicle group was considered to be relevant to the patient.9
A faster onset of visible improvement as perceived by the patient should be more desirable and is likely to improve treatment adherence, as long as it is not counterbalanced by an increase in adverse events.
What is meant by TOA?
Time to onset of action refers to the duration required to achieve a 25% mean lesion count reduction from baseline, which is believed to correlate with the time point at which many patients would be able to perceive visible improvement when viewing their full face. Therefore, TOA represents an attempt to correlate data that is quantitative (based on lesion count reduction) with what is likely to be the average time that a patient may qualitatively observe an initial visible improvement in their AV. This concept may be useful as a tool when communicating with AV patients but should not be used in a way that will overpromise and underdeliver; rather, it is a guide for discussion with the patient and with a parent or guardian when applicable.
Consistent with the comparative AV study analysis that evaluated TOA, a linear course of lesion reductions between the provided time intervals was assumed. In this linear model, the TOA was calculated using the 2 extracted lesion count values between which the 25% lesion reduction was achieved as well as their corresponding given time points.9 Differences between the results in the active and vehicle study arms were calculated for a number of determinants.
How was pivotal trial data with clindamycin-BP 3.75% gel used to assess TOA?
A total of 498 patients with moderate to severe AV were randomized (1:1) to receive clindamycin-BP 3.75% gel or vehicle in a multicenter, double-blind, controlled, 12-week, 2-arm study.10 Before randomization, patients were stratified by acne severity based on a static Evaluator’s Global Severity Score (EGSS) ranging from 0 (clear) to 5 (very severe). Specifically, moderate AV (EGSS of 3) was described as predominantly noninflammatory lesions with evidence of multiple inflammatory lesions; several to many comedones, papules, and pustules; and no more than 1 small nodulocystic lesion. Severe AV (EGSS of 4) was characterized by inflammatory lesions; numerous comedones, papules, and pustules; and possibly a few nodulocystic lesions.10
Male and female patients aged 12 to 40 years with moderate to severe AV—defined as 20 to 40 inflammatory lesions (papules, pustules, nodules), 20 to 100 noninflammatory lesions (comedones), and no more than 2 nodules—were included in the study. Standard washout periods were required for patients using prior prescription and over-the-counter acne treatments.10
Efficacy evaluations included inflammatory and noninflammatory lesion counts and EGSS at screening, baseline, and during treatment (weeks 4, 8, and 12).10 Primary efficacy end points included absolute change in mean inflammatory and noninflammatory lesion counts and the proportion of patients who achieved at least a 2-grade reduction in EGSS from baseline to week 12 (treatment success at end of study). Secondary efficacy end points included mean percentage change from baseline to week 12 in inflammatory and noninflammatory lesion counts and the proportion of patients who considered themselves clear or almost clear at week 12.10
After 12 weeks of daily treatment, inflammatory and noninflammatory lesion counts decreased by a mean of 60.4% and 51.8%, respectively, with clindamycin-BP 3.75% gel compared to 31.3% and 27.6%, respectively, with vehicle (both P<.001). At weeks 4, 8, and 12, the difference in inflammatory and noninflammatory lesion counts for the active treatment was 17.4%, 24.8%, and 29.1%, respectively, and 8.1%, 19.8%, and 24.2%, respectively, for vehicle.10
Treatment success (at least a 2-grade improvement in EGSS) was achieved by 9.1% of patients using clindamycin-BP 3.75% gel compared to 4.6% using vehicle by week 4. Additionally, 6.3% of patients considered their AV as clear or almost clear compared to 3.5% with vehicle at week 2 (Figure 1).10

This analysis represents the first attempt to evaluate and report TOA results with clindamycin-BP 3.75% gel. Time to onset of action for inflammatory lesions treated with clindamycin-BP 3.75% gel was calculated as 2.5 weeks versus 6.2 weeks for vehicle (Figure 2A). Time to onset of action for noninflammatory lesions was 3.7 weeks with clindamycin-BP 3.75% gel versus 8.6 weeks with vehicle (Figure 2B). The difference in TOA between the active and vehicle study groups was 3.7 weeks and 4.9 weeks, respectively. In addition, among actively treated patients, TOA was shorter in females (2.1 weeks) than in males (2.6 weeks) and in moderate AV (2.5 weeks) compared to severe AV (3.0 weeks).

Comment
Differences in lesion counts between clindamycin-BP 3.75% gel and vehicle suggest a clinically relevant benefit in favor of active treatment with both inflammatory and noninflammatory lesions. Nearly twice as many patients were rated as treatment successes using EGSS by week 4 or clear or almost clear as early as week 2 compared to the vehicle group.10 However, these data are suggested as an overall guide but do not provide adequate guidance on when visible improvement may start to be evident in a given patient.
The analysis reported here shows a TOA of 2.5 weeks with clindamycin-BP 3.75% gel for inflammatory lesions, approximately 4 weeks faster than with the vehicle. In most cases, a reduction in inflammatory lesions is more likely to have a greater impact on patient perception of TOA. Unless a patient is aware or focused enough to actively distinguish visibly between inflammatory and noninflammatory (comedonal) AV lesions, their eye is more likely to be drawn initially to reduction in inflammatory lesions, which are erythematous and more visible at a greater viewing distance. Although noninflammatory AV lesions usually require closer inspection to visualize them (especially closed comedones), they are often slower to respond to treatment. Analysis of the pivotal trial data reports a longer TOA with clindamycin-BP 3.75% gel for noninflammatory lesions (3.7 weeks) versus inflammatory lesions (2.5 weeks).
As expected, TOA was shorter in patients with moderate AV than severe AV (2.5 weeks vs 3.0 weeks). Time to onset of action also was shorter in females overall. It is unclear why we see gender differences in acne studies. A number of reasons have been suggested, including differences in AV pathophysiology and/or treatment adherence.11,12 Greater efficacy of clindamycin-BP 3.75% gel in females compared with males has already been reported, and better overall efficacy leading to a shorter TOA has been noted by others.13
There are limitations with this analysis. First, it is not possible to assess the contributions from each of the monads to the efficacy of clindamycin-BP 3.75% gel or TOA. Also, the data extraction method used assumes a linear progression model during the provided time points and was used to provide some comparison with calculations for other combination products.9 Although no strong deviations from the linear model are likely, calculations of TOA using other methodologies may give different results. The definition of a clinically meaningful benefit, defined here as a 25% reduction in the mean lesion count, has been used as a guide, but it has not been validated in clinical practice. It also is important to recognize that the initial visible perception of improvement of AV is likely to differ based on interpatient variability; that is, how different individuals perceive improvement. It also may be affected by differences in baseline severity of AV among different patients. Additionally, the TOA reflects an average duration of time, so it should not be described to patients as a suggestion of when they will definitely see visible improvement in their AV.
Conclusion
Unrealistic expectations of acne therapy or poor tolerability can lead to low adherence and poor clinical outcomes.1-4 The data on TOA reported here suggests that a clinically meaningful benefit with clindamycin-BP 3.75% gel may be seen in some patients within 2 to 3 weeks and maybe sooner in females or those with milder disease; however, longer durations may be required in some patients. This information can help clinicians and their staff in providing reasonable expectations and stress the importance of encouraging patients about the need to adhere to treatment.
Acknowledgments
The author thanks Brian Bulley, MSc (Inergy Limited, Lindfield, West Sussex, United Kingdom), for publication support. Valeant Pharmaceuticals North America, LLC, funded Inergy’s activities pertaining to this analysis. The author did not receive funding or any form of compensation for authorship of this publication.
- Krakowski AC, Stendardo S, Eichenfield LF. Practical considerations in acne treatment and the clinical impact of topical combination therapy. Pediatr Dermatol. 2008;25(suppl 1):1-14.
- Yentzer BA, Ade RA, Fountain JM, et al. Simplifying regimens promotes greater adherence and outcomes with topical acne medications: a randomized controlled trial. Cutis. 2010;86:103-108.
- Zaghloul SS, Cunliffe WJ, Goodfield MJ. Objective assessment of compliance with treatments in acne. Br J Dermatol. 2005;152:1015-1021.
- Snyder S, Crandell I, Davis SA, et al. Medical adherence to acne therapy: a systematic review. Am J Clin Dermatol. 2014;15:87-94.
- Miyachi Y, Hayashi N, Furukawa F, et al. Acne management in Japan: study of patient adherence. Dermatology. 2011;223:174-181.
- Zauli S, Caracciolo S, Borghi A, et al. Which factors influence quality of life in acne patients? J Eur Acad Dermatol Venereol. 2014;28:46-50.
- Mulder MM, Sigurdsson V, van Zuuren EJ, et al. Psychosocial impact of acne vulgaris. evaluation of the relation between a change in clinical acne severity and psychosocial state. Dermatology. 2001;203:124-130.
- Gerlinger C, Stadtler G, Gotzelmann R, et al. A noninferiority margin for acne lesion counts. Drug Inf J. 2008;42:607-615.
- Jacobs A, Starke G, Rosumeck S, et al. Systematic review on the rapidity of the onset of action of topical treatments in the therapy of mild-to-moderate acne vulgaris. Br J Dermatol. 2014;170:557-564.
- Pariser DM, Rich P, Cook-Bolden FE, et al. An aqueous gel fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 3.75% for the once-daily treatment of moderate to severe acne vulgaris. J Drugs Dermatol. 2014;13:611-617.
- Tanghetti E, Harper JC, Oefelein MG. The efficacy and tolerability of dapsone 5% gel in female vs male patients with facial acne vulgaris: gender as a clinically relevant outcome variable. J Drugs Dermatol. 2012;11:1417-1421.
- Lott R, Taylor SL, O’Neill JL, et al. Medication adherence among acne patients: a review. J Cosmet Dermatol. 2010;9:160-166.
- Harper JC. The efficacy and tolerability of a fixed combination clindamycin (1.2%) and benzoyl peroxide (3.75%) aqueous gel in patients with facial acne vulgaris: gender as a clinically relevant outcome variable. J Drugs Dermatol. 2015;14:381-384.
- Krakowski AC, Stendardo S, Eichenfield LF. Practical considerations in acne treatment and the clinical impact of topical combination therapy. Pediatr Dermatol. 2008;25(suppl 1):1-14.
- Yentzer BA, Ade RA, Fountain JM, et al. Simplifying regimens promotes greater adherence and outcomes with topical acne medications: a randomized controlled trial. Cutis. 2010;86:103-108.
- Zaghloul SS, Cunliffe WJ, Goodfield MJ. Objective assessment of compliance with treatments in acne. Br J Dermatol. 2005;152:1015-1021.
- Snyder S, Crandell I, Davis SA, et al. Medical adherence to acne therapy: a systematic review. Am J Clin Dermatol. 2014;15:87-94.
- Miyachi Y, Hayashi N, Furukawa F, et al. Acne management in Japan: study of patient adherence. Dermatology. 2011;223:174-181.
- Zauli S, Caracciolo S, Borghi A, et al. Which factors influence quality of life in acne patients? J Eur Acad Dermatol Venereol. 2014;28:46-50.
- Mulder MM, Sigurdsson V, van Zuuren EJ, et al. Psychosocial impact of acne vulgaris. evaluation of the relation between a change in clinical acne severity and psychosocial state. Dermatology. 2001;203:124-130.
- Gerlinger C, Stadtler G, Gotzelmann R, et al. A noninferiority margin for acne lesion counts. Drug Inf J. 2008;42:607-615.
- Jacobs A, Starke G, Rosumeck S, et al. Systematic review on the rapidity of the onset of action of topical treatments in the therapy of mild-to-moderate acne vulgaris. Br J Dermatol. 2014;170:557-564.
- Pariser DM, Rich P, Cook-Bolden FE, et al. An aqueous gel fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 3.75% for the once-daily treatment of moderate to severe acne vulgaris. J Drugs Dermatol. 2014;13:611-617.
- Tanghetti E, Harper JC, Oefelein MG. The efficacy and tolerability of dapsone 5% gel in female vs male patients with facial acne vulgaris: gender as a clinically relevant outcome variable. J Drugs Dermatol. 2012;11:1417-1421.
- Lott R, Taylor SL, O’Neill JL, et al. Medication adherence among acne patients: a review. J Cosmet Dermatol. 2010;9:160-166.
- Harper JC. The efficacy and tolerability of a fixed combination clindamycin (1.2%) and benzoyl peroxide (3.75%) aqueous gel in patients with facial acne vulgaris: gender as a clinically relevant outcome variable. J Drugs Dermatol. 2015;14:381-384.
Practice Points
- Time to onset of action (TOA) refers to how long it takes after starting a therapy for a patient to perceive visible improvement.
- Time to onset of action has been determined based on data to date to correlate overall with a 25% lesion reduction.
- The TOA for clindamycin phosphate 1.2%–benzoyl peroxide 3.75% gel applied once daily based on analysis of pivotal trial data is 3 weeks or less depending on the severity of acne vulgaris at baseline.
Status Report From the American Acne & Rosacea Society on Medical Management of Acne in Adult Women, Part 3: Oral Therapies
Selection of oral agents for treatment of AV in adult women is dependent on multiple factors including the patient’s age, medication history, child-bearing potential, clinical presentation, and treatment preference following a discussion of the anticipated benefits versus potential risks.1,2 In patients with the mixed inflammatory and comedonal clinical pattern of AV, oral antibiotics can be used concurrently with topical therapies when moderate to severe inflammatory lesions are noted.3,4 However, many adult women who had AV as teenagers have already utilized oral antibiotic therapies in the past and often are interested in alternative options, express concerns regarding antibiotic resistance, report a history of antibiotic-associated yeast infections or other side effects, and/or encounter issues related to drug-drug interactions.3,5-8 Oral hormonal therapies such as combination oral contraceptives (COCs) or spironolactone often are utilized to treat adult women with AV, sometimes in combination with each other or other agents. Combination oral contraceptives appear to be especially effective in the management of the U-shaped clinical pattern or predominantly inflammatory, late-onset AV.1,5,9,10 Potential warnings, contraindications, adverse effects, and drug-drug interactions are important to keep in mind when considering the use of oral hormonal therapies.8-10 Oral isotretinoin, which should be prescribed with strict adherence to the iPLEDGE™ program (https://www.ipledgeprogram.com/), remains a viable option for cases of severe nodular AV and selected cases of refractory inflammatory AV, especially when scarring and/or marked psychosocial distress are noted.1,2,5,11 Although it is recognized that adult women with AV typically present with either a mixed inflammatory and comedonal or U-shaped clinical pattern predominantly involving the lower face and anterolateral neck, the available data do not adequately differentiate the relative responsiveness of these clinical patterns to specific therapeutic agents.
Combination Oral Contraceptives
Combination oral contraceptives are commonly used to treat AV in adult women, including those without and those with measurable androgen excess (eg, polycystic ovary syndrome [PCOS]). Combination oral contraceptives contain ethinyl estradiol and a progestational agent (eg, progestin); the latter varies in terms of its nonselective receptor interactions and the relative magnitude or absence of androgenic effects.10,12,13 Although some COCs are approved by the US Food and Drug Administration (FDA) for AV, there is little data available to determine the comparative efficacy among these and other COCs.10,14 When choosing a COC for treatment of AV, it is best to select an agent whose effectiveness is supported by evidence from clinical studies.10,15
Mechanisms of Action
The reported mechanisms of action for COCs include inhibition of ovarian androgen production and ovulation through gonadotropin suppression; upregulated synthesis of sex hormone–binding globulin, which decreases free testosterone levels through receptor binding; and inhibition of 5α-reductase (by some progestins), which reduces conversion of testosterone to dihydrotestosterone, the active derivative that induces androgenic effects at peripheral target tissues.10,13,16,17
Therapeutic Benefits
Use of COCs to treat AV in adult women who do not have measurable androgen excess is most rational in patients who also desire a method of contraception. Multiple monotherapy studies have demonstrated the efficacy of COCs in the treatment of AV on the face and trunk.4,10,12,15,17,18 It may take a minimum of 3 monthly cycles of use before acne lesion counts begin to appreciably decrease.12,15,19-21 Initiating COC therapy during menstruation ensures the absence of pregnancy. Combination oral contraceptives may be used with other topical and oral therapies for AV.2,3,9,10 Potential ancillary benefits of COCs include normalization of the menstrual cycle; reduced premenstrual dysphoric disorder symptoms; and reduced risk of endometrial cancer (approximately 50%), ovarian cancer (approximately 40%), and colorectal cancer.22-24
Risks and Contraindications
It is important to consider the potential risks associated with the use of COCs, especially in women with AV who are not seeking a method of contraception. Side effects of COCs can include nausea, breast tenderness, breakthrough bleeding, and weight gain.25,26 Potential adverse associations of COCs are described in the Table. The major potential vascular associations include venous thromboembolism, myocardial infarction, and cerebrovascular accident, all of which are influenced by concurrent factors such as a history of smoking, age (≥35 years), and hypertension.27-32 It is recommended that blood pressure be measured before initiating COC therapy as part of the general examination.33
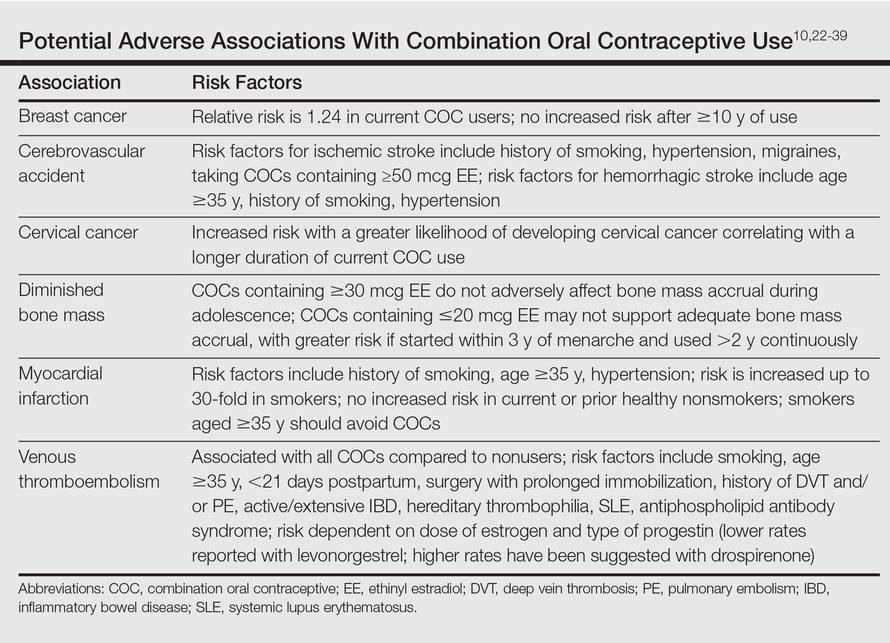
The potential increase in breast cancer risk appears to be low, while the cervical cancer risk is reported to increase relative to the duration of use.34-37 This latter observation may be due to the greater likelihood of unprotected sex in women using a COC and exposure to multiple sexual partners in some cases, which may increase the likelihood of oncogenic human papillomavirus infection of the cervix. If a dermatologist elects to prescribe a COC to treat AV, it has been suggested that the patient also consult with her general practitioner or gynecologist to undergo pelvic and breast examinations and a Papanicolaou test.33 The recommendation for initial screening for cervical cancer is within 3 years of initiation of sexual intercourse or by 21 years of age, whichever is first.33,38,39
Combination oral contraceptives are not ideal for all adult women with AV. Absolute contraindications are pregnancy and history of thromboembolic, cardiac, or hepatic disease; in women aged 35 years and older who smoke, relative contraindications include hypertension, diabetes, migraines, breastfeeding, and current breast or liver cancer.33 In adult women with AV who have relative contra-indications but are likely to benefit from the use of a COC when other options are limited or not viable, consultation with a gynecologist is prudent. Other than rifamycin antibiotics (eg, rifampin) and griseofulvin, there is no definitive evidence that oral antibiotics (eg, tetracycline) or oral antifungal agents reduce the contraceptive efficacy of COCs, although cautions remain in print within some approved package inserts.8
Spironolactone
Available since 1957, spironolactone is an oral aldos-terone antagonist and potassium-sparing diuretic used to treat hypertension and congestive heart failure.9 Recognition of its antiandrogenic effects led to its use in dermatology to treat certain dermatologic disorders in women (eg, hirsutism, alopecia, AV).1,4,5,9,10 Spironolactone is not approved for AV by the FDA; therefore, available data from multiple independent studies and retrospective analyses that have been collectively reviewed support its efficacy when used as both monotherapy or in combination with other agents in adult women with AV, especially those with a U-shaped pattern and/or late-onset AV.9,40-43
Mechanism of Action
Spironolactone inhibits sebaceous gland activity through peripheral androgen receptor blockade, inhibition of 5α-reductase, decrease in androgen production, and increase in sex hormone–binding globulin.9,10,40
Therapeutic Benefits
Good to excellent improvement of AV in women, many of whom are postadolescent, has ranged from 66% to 100% in published reports9,40-43; however, inclusion and exclusion criteria, dosing regimens, and concomitant therapies were not usually controlled. Spironolactone has been used to treat AV in adult women as monotherapy or in combination with topical agents, oral antibiotics, and COCs.9,40-42 Additionally, dose-ranging studies have not been completed with spironolactone for AV.9,40 The suggested dose range is 50 mg to 200 mg daily; however, it usually is best to start at 50 mg daily and increase to 100 mg daily if clinical response is not adequate after 2 to 3 months. The gastrointestinal (GI) absorption of spironolactone is increased when ingested with a high-fat meal.9,10
Once effective control of AV is achieved, it is optimal to use the lowest dose needed to continue reasonable suppression of new AV lesions. There is no defined end point for spironolactone use in AV, with or without concurrent PCOS, as many adult women usually continue treatment with low-dose therapy because they experience marked flaring shortly after the drug is stopped.9
Risks and Contraindications
Side effects associated with spironolactone are dose related and include increased diuresis, migraines, menstrual irregularities, breast tenderness, gynecomastia, fatigue, and dizziness.9,10,40-44 Side effects (particularly menstrual irregularities and breast tenderness) are more common at doses higher than 100 mg daily, especially when used as monotherapy without concurrent use of a COC.9,40
Spironolactone-associated hyperkalemia is most clinically relevant in patients on higher doses (eg, 100–200 mg daily), in those with renal impairment and/or congestive heart failure, and when used concurrently with certain other medications. In any patient on spironolactone, the risk of clinically relevant hyperkalemia may be increased by coingestion of potassium supplements, potassium-based salt substitutes, potassium-sparing diuretics (eg, amiloride, triamterene); aldosterone antagonists and angiotensin-converting enzyme inhibitors (eg, lisinopril, benazepril); angiotensin II receptor blockers (eg, losartan, valsartan); and tri-methoprim (with or without sulfamethoxazole).8,9,40,45 Spironolactone may also increase serum levels of lithium or digoxin.9,40,45,46 For management of AV, it is best that spironolactone be avoided in patients taking any of these medications.9
In healthy adult women with AV who are not on medications or supplements that interact adversely with spironolactone, there is no definitive recommendation regarding monitoring of serum potassium levels during treatment with spironolactone, and it has been suggested that monitoring serum potassium levels in this subgroup is not necessary.47 However, each clinician is advised to choose whether or not they wish to obtain baseline and/or periodic serum potassium levels when prescribing spironolactone for AV based on their degree of comfort and the patient’s history. Baseline and periodic blood testing to evaluate serum electrolytes and renal function are reasonable, especially as adult women with AV are usually treated with spironolactone over a prolonged period of time.9
The FDA black box warning for spironolactone states that it is tumorigenic in chronic toxicity studies in rats and refers to exposures 25- to 100-fold higher than those administered to humans.9,48 Although continued vigilance is warranted, evaluation of large populations of women treated with spironolactone do not suggest an association with increased risk of breast cancer.49,50
Spironolactone is a category C drug and thus should be avoided during pregnancy, primarily due to animal data suggesting risks of hypospadias and feminization in male fetuses.9 Importantly, there is an absence of reports linking exposure during pregnancy with congenital defects in humans, including in 2 known cases of high-dose exposures for maternal Bartter syndrome.9
The active metabolite, canrenone, is known to be present in breast milk at 0.2% of the maternal daily dose, but breastfeeding is generally believed to be safe with spironolactone based on evidence to date.9
Oral Antibiotics
Oral antibiotic therapy may be used in combination with a topical regimen to treat AV in adult women, keeping in mind some important caveats.1-7 For instance, monotherapy with oral antibiotics should be avoided, and concomitant use of benzoyl peroxide is suggested to reduce emergence of antibiotic-resistant Propionibacterium acnes strains.3,4 A therapeutic exit plan also is suggested when prescribing oral antibiotics to limit treatment to 3 to 4 months, if possible, to help mitigate the emergence of antibiotic-resistant bacteria (eg, staphylococci and streptococci).3-5,51
Tetracyclines, especially doxycycline and minocycline, are the most commonly prescribed agents. Doxycycline use warrants patient education on measures to limit the risks of esophageal and GI side effects and phototoxicity; enteric-coated and small tablet formulations have been shown to reduce GI side effects, especially when administered with food.3,52-55 In addition to vestibular side effects and hyperpigmentation, minocycline may be associated with rare but potentially severe adverse reactions such as drug hypersensitivity syndrome, autoimmune hepatitis, and lupus-like syndrome, which are reported more commonly in women.5,52,54 Vestibular side effects have been shown to decrease with use of extended-release tablets with weight-based dosing.53
Oral Isotretinoin
Oral isotretinoin is well established as highly effective for treatment of severe, recalcitrant AV, including nodular acne on the face and trunk.4,56 Currently available oral isotretinoins are branded generic formulations based on the pharmacokinetic profile of the original brand (Accutane [Roche Pharmaceuticals]) and with the use of Lidose Technology (Absorica [Cipher Pharmaceuticals]), which substantially increases GI absorption of isotretinoin in the absence of ingestion with a high-calorie, high-fat meal.57 The short- and long-term efficacy, dosing regimens, safety considerations, and serious teratogenic risks for oral isotretinoin are well published.4,56-58 Importantly, oral isotretinoin must be prescribed with strict adherence to the federally mandated iPLEDGE risk management program.
Low-dose oral isotretinoin therapy (<0.5 mg/kg–1 mg/kg daily) administered over several months longer than conventional regimens (ie, 16–20 weeks) has been suggested with demonstrated efficacy.57 However, this approach is not optimal due to the lack of established sustained clearance of AV after discontinuation of therapy and the greater potential for exposure to isotretinoin during pregnancy. Recurrences of AV do occur after completion of isotretinoin therapy, especially if cumulative systemic exposure to the drug during the initial course of treatment was inadequate.56,57
Oral isotretinoin has been shown to be effective in AV in adult women with or without PCOS with 0.5 mg/kg to 1 mg/kg daily and a total cumulative exposure of 120 mg/kg to 150 mg/kg.59 In one study, the presence of PCOS and greater number of nodules at baseline were predictive of a higher risk of relapse during the second year posttreatment.59
Conclusion
All oral therapies that are used to treat AV in adult women warrant individual consideration of possible benefits versus risks. Careful attention to possible side effects, patient-related risk factors, and potential drug-drug interactions is important. End points of therapy are not well established, with the exception of oral isotretinoin therapy. Clinicians must use their judgment in each case along with obtaining feedback from patients regarding the selection of therapy after a discussion of the available options.
- Holzmann R, Shakery K. Postadolescent acne in females. Skin Pharmacol Physiol. 2014;27(suppl 1):3-8.
- Villasenor J, Berson DS, Kroshinsky D. Treatment guidelines in adult women. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:198-207.
- Del Rosso JQ, Kim G. Optimizing use of oral antibiotics in acne vulgaris. Dermatol Clin. 2009;27:33-42.
- Gollnick H, Cunliffe W, Berson D, et al. Management of acne: report from a Global Alliance to Improve Outcomes in Acne. J Am Acad Dermatol. 2003;49(suppl 1):S1-S37.
- Fisk WA, Lev-Tov HA, Sivamani RK. Epidemiology and management of acne in adult women. Curr Derm Rep. 2014;3:29-39.
- Del Rosso JQ, Leyden JJ. Status report on antibiotic resistance: implications for the dermatologist. Dermatol Clin. 2007;25:127-132.
- Bowe WP, Leyden JJ. Clinical implications of antibiotic resistance: risk of systemic infection from Staphylococcus and Streptococcus. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:125-133.
- Del Rosso JQ. Oral antibiotic drug interactions of clinical significance to dermatologists. Dermatol Clin. 2009;27:91-94.
- Kim GK, Del Rosso JQ. Oral spironolactone in post-teenage female patients with acne vulgaris: practical considerations for the clinician based on current data and clinical experience. J Clin Aesthet Dermatol. 2012;5:37-50.
- Keri J, Berson DS, Thiboutot DM. Hormonal treatment of acne in women. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:146-155.
- American Academy of Dermatology. Position statement on isotretinoin. AAD Web site. https://www.aad.org /Forms/Policies/Uploads/PS/PS-Isotretinoin.pdf. Updated November 13, 2010. Accessed October 28, 2015.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. June 2012;7:CD004425.
- Sitruk-Ware R. Pharmacology of different progestogens: the special case of drospirenone. Climacteric. 2005;8 (suppl 3):4-12.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for the treatment of acne. Cochrane Database Syst Rev. July 2012;7:CD004425.
- Thiboutot D, Archer DF, Lemay A, et al. A randomized, controlled trial of a low-dose contraceptive containing 20 microg of ethinyl estradiol and 100 microg of levonogestrel for acne treatment. Fertil Steril. 2001;76:461-468.
- Koulianos GT. Treatment of acne with oral contraceptives: criteria for pill selection. Cutis. 2000;66:281-286.
- Rabe T, Kowald A, Ortmann J, et al. Inhibition of skin 5-alpha reductase by oral contraceptive progestins in vitro. Gynecol Endocrinol. 2000;14:223-230.
- Palli MB, Reyes-Habito CM, Lima XT, et al. A single-center, randomized double-blind, parallel-group study to examine the safety and efficacy of 3mg drospirenone/0.02mg ethinyl estradiol compared with placebo in the treatment of moderate truncal acne vulgaris. J Drugs Dermatol. 2013;12:633-637.
- Koltun W, Maloney JM, Marr J, et al. Treatment of moderate acne vulgaris using a combined oral contraceptive containing ethinylestradiol 20 μg plus drospirenone 3 mg administered in a 24/4 regimen: a pooled analysis. Eur J Obstet Gynecol Reprod Biol. 2011;155:171-175.
- Maloney JM, Dietze P, Watson D, et al. A randomized controlled trial of a low-dose combined oral contraceptive containing 3 mg drospirenone plus 20 μg ethinylestradiol in the treatment of acne vulgaris: lesion counts, investigator ratings and subject self-assessment. J Drugs Dermatol. 2009;8:837-844.
- Lucky AW, Koltun W, Thiboutot D, et al. A combined oral contraceptive containing 3-mg drospirenone/20-μg ethinyl estradiol in the treatment of acne vulgaris: a randomized, double-blind, placebo-controlled study evaluating lesion counts and participant self-assessment. Cutis. 2008;82:143-150.
- Burkman R, Schlesselman JJ, Zieman M. Safety concerns and health benefits associated with oral contraception. Am J Obstet Gynecol. 2004;190(suppl 4):S5-S22.
- Maguire K, Westhoff C. The state of hormonal contraception today: established and emerging noncontraceptive health benefits. Am J Obstet Gynecol. 2011;205 (suppl 4):S4-S8.
- Weiss NS, Sayvetz TA. Incidence of endometrial cancer in relation to the use of oral contraceptives. N Engl J Med. 1980;302:551-554.
- Tyler KH, Zirwas MJ. Contraception and the dermatologist. J Am Acad Dermatol. 2013;68:1022-1029.
- Gallo MF, Lopez LM, Grimes DA, et al. Combination contraceptives: effects on weight. Cochrane Database Syst Rev. 2008;4:CD003987.
- de Bastos M, Stegeman BH, Rosendaal FR, et al. Combined oral contraceptives: venous thrombosis. Cochrane Database Syst Rev. 2014;3:CD010813.
- Raymond EG, Burke AE, Espey E. Combined hormonal contraceptives and venous thromboembolism: putting the risks into perspective. Obstet Gynecol. 2012;119:1039-1044.
- Jick SS, Hernandez RK. Risk of non-fatal venous thromboembolism in women using oral contraceptives containing drospirenone compared with women using oral contraceptives containing levonorgestrel: case-control study using United States claims data. BMJ. 2011;342:d2151.
- US Food and Drug Administration Office of Surveillance and Epidemiology. Combined hormonal contraceptives (CHCs) and the risk of cardiovascular disease endpoints. US Food and Drug Administration Web site. http://www.fda.gov/downloads/Drugs /Drug Safety/UCM277384.pdf. Accessed October 28, 2015.
- The American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. Risk of venous thromboembolism among users of drospirenone-containing oral contraceptive pills. Obstet Gynecol. 2012;120:1239-1242.
- World Health Organization. Cardiovascular Disease and Steroid Hormone Contraception: Report of a WHO Scientific Group. Geneva, Switzerland: World Health Organization; 1998. Technical Report Series 877.
- Frangos JE, Alavian CN, Kimball AB. Acne and oral contraceptives: update on women’s health screening guidelines. J Am Acad Dermatol. 2008;58:781-786.
- Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53 297 women with breast cancer and 100 239 women without breast cancer from 54 epidemiological studies. Lancet. 1996;347:1713-1727.
- Gierisch JM, Coeytaux RR, Urrutia RP, et al. Oral contraceptive use and risk of breast, cervical, colorectal, and endometrial cancers: a systematic review. Cancer Epidemiol Biomarkers Prev. 2013;22:1931-1943.
- International Collaboration of Epidemiological Studies of Cervical Cancer. Cervical cancer and hormonal contraceptives: collaborative reanalysis of individual data for 16 573 women with cervical cancer and 35 509 women without cervical cancer from 24 epidemiological studies. Lancet. 2007;370:1609-1621.
- Agostino H, Di Meglio G. Low-dose oral contraceptives in adolescents: how low can you go? J Pediatr Adolesc Gynecol. 2010;23:195-201.
- Buzney E, Sheu J, Buzney C, et al. Polycystic ovary syndrome: a review for dermatologists: part II. Treatment. J Am Acad Dermatol. 2014;71:859.e1-859.e15.
- Stewart FH, Harper CC, Ellertson CE, et al. Clinical breast and pelvic examination requirements for hormonal contraception: current practice vs evidence. JAMA. 2001;285:2232-2239.
- Sawaya ME, Somani N. Antiandrogens and androgen inhibitors. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelpha, PA: Saunders; 2013:361-374.
- Muhlemann MF, Carter GD, Cream JJ, et al. Oral spironolactone: an effective treatment for acne vulgaris in women. Br J Dermatol. 1986;115:227-232.
- Shaw JC. Low-dose adjunctive spironolactone in the treatment of acne in women: a retrospective analysis of 85 consecutively treated patients. J Am Acad Dermatol. 2000;43:498-502.
- Sato K, Matsumoto D, Iizuka F, et al. Anti-androgenic therapy using oral spironolactone for acne vulgaris in Asians. Aesth Plast Surg. 2006;30:689-694.
- Shaw JC, White LE. Long-term safety of spironolactone in acne: results of an 8-year follow-up study. J Cutan Med Surg. 2002;6:541-545.
- Stockley I. Antihypertensive drug interactions. In: Stockley I, ed. Drug Interactions. 5th ed. London, United Kingdom: Pharmaceutical Press; 1999:335-347.
- Antoniou T, Gomes T, Mamdani MM, et al. Trimethoprim-sulfamethoxazole induced hyperkalaemia in elderly patients receiving spironolactone: nested case-control study. BMJ. 2011;343:d5228.
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944.
- Aldactone [package insert]. New York, NY: Pfizer Inc; 2008.
- Biggar RJ, Andersen EW, Wohlfahrt J, et al. Spironolactone use and the risk of breast and gynecologic cancers. Cancer Epidemiol. 2013;37:870-875.
- Mackenzie IS, Macdonald TM, Thompson A, et al. Spironolactone and risk of incident breast cancer in women older than 55 years: retrospective, matched cohort study. BMJ. 2012;345:e4447.
- Dreno B, Thiboutot D, Gollnick H, et al. Antibiotic stewardship in dermatology: limiting antibiotic use in acne. Eur J Dermatol. 2014;24:330-334.
- Kim S, Michaels BD, Kim GK, et al. Systemic antibacterial agents. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelpha, PA: Saunders; 2013:61-97.
- Leyden JJ, Del Rosso JQ. Oral antibiotic therapy for acne vulgaris: pharmacokinetic and pharmacodynamics perspectives. J Clin Aesthet Dermatol. 2011;4:40-47.
- Del Rosso JQ. Oral antibiotics. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:113-124.
- Del Rosso JQ. Oral doxycycline in the management of acne vulgaris: current perspectives on clinical use and recent findings with a new double-scored small tablet formulation. J Clin Aesthet Dermatol. 2015;8:19-26.
- Osofsky MG, Strauss JS. Isotretinoin. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:134-145.
- Leyden JJ, Del Rosso JQ, Baum EW. The use of isotretinoin in the treatment of acne vulgaris: clinical considerations and future directions. J Clin Aesthet Dermatol. 2014;7(suppl 2):S3-S21.
- Patton TJ, Ferris LK. Systemic retinoids. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelpha, PA: Saunders; 2013:252-268.
- Cakir GA, Erdogan FG, Gurler A. Isotretinoin treatment in nodulocystic acne with and without polycystic ovary syndrome: efficacy and determinants of relapse. Int J Dermatol. 2013;52:371-376.
Selection of oral agents for treatment of AV in adult women is dependent on multiple factors including the patient’s age, medication history, child-bearing potential, clinical presentation, and treatment preference following a discussion of the anticipated benefits versus potential risks.1,2 In patients with the mixed inflammatory and comedonal clinical pattern of AV, oral antibiotics can be used concurrently with topical therapies when moderate to severe inflammatory lesions are noted.3,4 However, many adult women who had AV as teenagers have already utilized oral antibiotic therapies in the past and often are interested in alternative options, express concerns regarding antibiotic resistance, report a history of antibiotic-associated yeast infections or other side effects, and/or encounter issues related to drug-drug interactions.3,5-8 Oral hormonal therapies such as combination oral contraceptives (COCs) or spironolactone often are utilized to treat adult women with AV, sometimes in combination with each other or other agents. Combination oral contraceptives appear to be especially effective in the management of the U-shaped clinical pattern or predominantly inflammatory, late-onset AV.1,5,9,10 Potential warnings, contraindications, adverse effects, and drug-drug interactions are important to keep in mind when considering the use of oral hormonal therapies.8-10 Oral isotretinoin, which should be prescribed with strict adherence to the iPLEDGE™ program (https://www.ipledgeprogram.com/), remains a viable option for cases of severe nodular AV and selected cases of refractory inflammatory AV, especially when scarring and/or marked psychosocial distress are noted.1,2,5,11 Although it is recognized that adult women with AV typically present with either a mixed inflammatory and comedonal or U-shaped clinical pattern predominantly involving the lower face and anterolateral neck, the available data do not adequately differentiate the relative responsiveness of these clinical patterns to specific therapeutic agents.
Combination Oral Contraceptives
Combination oral contraceptives are commonly used to treat AV in adult women, including those without and those with measurable androgen excess (eg, polycystic ovary syndrome [PCOS]). Combination oral contraceptives contain ethinyl estradiol and a progestational agent (eg, progestin); the latter varies in terms of its nonselective receptor interactions and the relative magnitude or absence of androgenic effects.10,12,13 Although some COCs are approved by the US Food and Drug Administration (FDA) for AV, there is little data available to determine the comparative efficacy among these and other COCs.10,14 When choosing a COC for treatment of AV, it is best to select an agent whose effectiveness is supported by evidence from clinical studies.10,15
Mechanisms of Action
The reported mechanisms of action for COCs include inhibition of ovarian androgen production and ovulation through gonadotropin suppression; upregulated synthesis of sex hormone–binding globulin, which decreases free testosterone levels through receptor binding; and inhibition of 5α-reductase (by some progestins), which reduces conversion of testosterone to dihydrotestosterone, the active derivative that induces androgenic effects at peripheral target tissues.10,13,16,17
Therapeutic Benefits
Use of COCs to treat AV in adult women who do not have measurable androgen excess is most rational in patients who also desire a method of contraception. Multiple monotherapy studies have demonstrated the efficacy of COCs in the treatment of AV on the face and trunk.4,10,12,15,17,18 It may take a minimum of 3 monthly cycles of use before acne lesion counts begin to appreciably decrease.12,15,19-21 Initiating COC therapy during menstruation ensures the absence of pregnancy. Combination oral contraceptives may be used with other topical and oral therapies for AV.2,3,9,10 Potential ancillary benefits of COCs include normalization of the menstrual cycle; reduced premenstrual dysphoric disorder symptoms; and reduced risk of endometrial cancer (approximately 50%), ovarian cancer (approximately 40%), and colorectal cancer.22-24
Risks and Contraindications
It is important to consider the potential risks associated with the use of COCs, especially in women with AV who are not seeking a method of contraception. Side effects of COCs can include nausea, breast tenderness, breakthrough bleeding, and weight gain.25,26 Potential adverse associations of COCs are described in the Table. The major potential vascular associations include venous thromboembolism, myocardial infarction, and cerebrovascular accident, all of which are influenced by concurrent factors such as a history of smoking, age (≥35 years), and hypertension.27-32 It is recommended that blood pressure be measured before initiating COC therapy as part of the general examination.33

The potential increase in breast cancer risk appears to be low, while the cervical cancer risk is reported to increase relative to the duration of use.34-37 This latter observation may be due to the greater likelihood of unprotected sex in women using a COC and exposure to multiple sexual partners in some cases, which may increase the likelihood of oncogenic human papillomavirus infection of the cervix. If a dermatologist elects to prescribe a COC to treat AV, it has been suggested that the patient also consult with her general practitioner or gynecologist to undergo pelvic and breast examinations and a Papanicolaou test.33 The recommendation for initial screening for cervical cancer is within 3 years of initiation of sexual intercourse or by 21 years of age, whichever is first.33,38,39
Combination oral contraceptives are not ideal for all adult women with AV. Absolute contraindications are pregnancy and history of thromboembolic, cardiac, or hepatic disease; in women aged 35 years and older who smoke, relative contraindications include hypertension, diabetes, migraines, breastfeeding, and current breast or liver cancer.33 In adult women with AV who have relative contra-indications but are likely to benefit from the use of a COC when other options are limited or not viable, consultation with a gynecologist is prudent. Other than rifamycin antibiotics (eg, rifampin) and griseofulvin, there is no definitive evidence that oral antibiotics (eg, tetracycline) or oral antifungal agents reduce the contraceptive efficacy of COCs, although cautions remain in print within some approved package inserts.8
Spironolactone
Available since 1957, spironolactone is an oral aldos-terone antagonist and potassium-sparing diuretic used to treat hypertension and congestive heart failure.9 Recognition of its antiandrogenic effects led to its use in dermatology to treat certain dermatologic disorders in women (eg, hirsutism, alopecia, AV).1,4,5,9,10 Spironolactone is not approved for AV by the FDA; therefore, available data from multiple independent studies and retrospective analyses that have been collectively reviewed support its efficacy when used as both monotherapy or in combination with other agents in adult women with AV, especially those with a U-shaped pattern and/or late-onset AV.9,40-43
Mechanism of Action
Spironolactone inhibits sebaceous gland activity through peripheral androgen receptor blockade, inhibition of 5α-reductase, decrease in androgen production, and increase in sex hormone–binding globulin.9,10,40
Therapeutic Benefits
Good to excellent improvement of AV in women, many of whom are postadolescent, has ranged from 66% to 100% in published reports9,40-43; however, inclusion and exclusion criteria, dosing regimens, and concomitant therapies were not usually controlled. Spironolactone has been used to treat AV in adult women as monotherapy or in combination with topical agents, oral antibiotics, and COCs.9,40-42 Additionally, dose-ranging studies have not been completed with spironolactone for AV.9,40 The suggested dose range is 50 mg to 200 mg daily; however, it usually is best to start at 50 mg daily and increase to 100 mg daily if clinical response is not adequate after 2 to 3 months. The gastrointestinal (GI) absorption of spironolactone is increased when ingested with a high-fat meal.9,10
Once effective control of AV is achieved, it is optimal to use the lowest dose needed to continue reasonable suppression of new AV lesions. There is no defined end point for spironolactone use in AV, with or without concurrent PCOS, as many adult women usually continue treatment with low-dose therapy because they experience marked flaring shortly after the drug is stopped.9
Risks and Contraindications
Side effects associated with spironolactone are dose related and include increased diuresis, migraines, menstrual irregularities, breast tenderness, gynecomastia, fatigue, and dizziness.9,10,40-44 Side effects (particularly menstrual irregularities and breast tenderness) are more common at doses higher than 100 mg daily, especially when used as monotherapy without concurrent use of a COC.9,40
Spironolactone-associated hyperkalemia is most clinically relevant in patients on higher doses (eg, 100–200 mg daily), in those with renal impairment and/or congestive heart failure, and when used concurrently with certain other medications. In any patient on spironolactone, the risk of clinically relevant hyperkalemia may be increased by coingestion of potassium supplements, potassium-based salt substitutes, potassium-sparing diuretics (eg, amiloride, triamterene); aldosterone antagonists and angiotensin-converting enzyme inhibitors (eg, lisinopril, benazepril); angiotensin II receptor blockers (eg, losartan, valsartan); and tri-methoprim (with or without sulfamethoxazole).8,9,40,45 Spironolactone may also increase serum levels of lithium or digoxin.9,40,45,46 For management of AV, it is best that spironolactone be avoided in patients taking any of these medications.9
In healthy adult women with AV who are not on medications or supplements that interact adversely with spironolactone, there is no definitive recommendation regarding monitoring of serum potassium levels during treatment with spironolactone, and it has been suggested that monitoring serum potassium levels in this subgroup is not necessary.47 However, each clinician is advised to choose whether or not they wish to obtain baseline and/or periodic serum potassium levels when prescribing spironolactone for AV based on their degree of comfort and the patient’s history. Baseline and periodic blood testing to evaluate serum electrolytes and renal function are reasonable, especially as adult women with AV are usually treated with spironolactone over a prolonged period of time.9
The FDA black box warning for spironolactone states that it is tumorigenic in chronic toxicity studies in rats and refers to exposures 25- to 100-fold higher than those administered to humans.9,48 Although continued vigilance is warranted, evaluation of large populations of women treated with spironolactone do not suggest an association with increased risk of breast cancer.49,50
Spironolactone is a category C drug and thus should be avoided during pregnancy, primarily due to animal data suggesting risks of hypospadias and feminization in male fetuses.9 Importantly, there is an absence of reports linking exposure during pregnancy with congenital defects in humans, including in 2 known cases of high-dose exposures for maternal Bartter syndrome.9
The active metabolite, canrenone, is known to be present in breast milk at 0.2% of the maternal daily dose, but breastfeeding is generally believed to be safe with spironolactone based on evidence to date.9
Oral Antibiotics
Oral antibiotic therapy may be used in combination with a topical regimen to treat AV in adult women, keeping in mind some important caveats.1-7 For instance, monotherapy with oral antibiotics should be avoided, and concomitant use of benzoyl peroxide is suggested to reduce emergence of antibiotic-resistant Propionibacterium acnes strains.3,4 A therapeutic exit plan also is suggested when prescribing oral antibiotics to limit treatment to 3 to 4 months, if possible, to help mitigate the emergence of antibiotic-resistant bacteria (eg, staphylococci and streptococci).3-5,51
Tetracyclines, especially doxycycline and minocycline, are the most commonly prescribed agents. Doxycycline use warrants patient education on measures to limit the risks of esophageal and GI side effects and phototoxicity; enteric-coated and small tablet formulations have been shown to reduce GI side effects, especially when administered with food.3,52-55 In addition to vestibular side effects and hyperpigmentation, minocycline may be associated with rare but potentially severe adverse reactions such as drug hypersensitivity syndrome, autoimmune hepatitis, and lupus-like syndrome, which are reported more commonly in women.5,52,54 Vestibular side effects have been shown to decrease with use of extended-release tablets with weight-based dosing.53
Oral Isotretinoin
Oral isotretinoin is well established as highly effective for treatment of severe, recalcitrant AV, including nodular acne on the face and trunk.4,56 Currently available oral isotretinoins are branded generic formulations based on the pharmacokinetic profile of the original brand (Accutane [Roche Pharmaceuticals]) and with the use of Lidose Technology (Absorica [Cipher Pharmaceuticals]), which substantially increases GI absorption of isotretinoin in the absence of ingestion with a high-calorie, high-fat meal.57 The short- and long-term efficacy, dosing regimens, safety considerations, and serious teratogenic risks for oral isotretinoin are well published.4,56-58 Importantly, oral isotretinoin must be prescribed with strict adherence to the federally mandated iPLEDGE risk management program.
Low-dose oral isotretinoin therapy (<0.5 mg/kg–1 mg/kg daily) administered over several months longer than conventional regimens (ie, 16–20 weeks) has been suggested with demonstrated efficacy.57 However, this approach is not optimal due to the lack of established sustained clearance of AV after discontinuation of therapy and the greater potential for exposure to isotretinoin during pregnancy. Recurrences of AV do occur after completion of isotretinoin therapy, especially if cumulative systemic exposure to the drug during the initial course of treatment was inadequate.56,57
Oral isotretinoin has been shown to be effective in AV in adult women with or without PCOS with 0.5 mg/kg to 1 mg/kg daily and a total cumulative exposure of 120 mg/kg to 150 mg/kg.59 In one study, the presence of PCOS and greater number of nodules at baseline were predictive of a higher risk of relapse during the second year posttreatment.59
Conclusion
All oral therapies that are used to treat AV in adult women warrant individual consideration of possible benefits versus risks. Careful attention to possible side effects, patient-related risk factors, and potential drug-drug interactions is important. End points of therapy are not well established, with the exception of oral isotretinoin therapy. Clinicians must use their judgment in each case along with obtaining feedback from patients regarding the selection of therapy after a discussion of the available options.
Selection of oral agents for treatment of AV in adult women is dependent on multiple factors including the patient’s age, medication history, child-bearing potential, clinical presentation, and treatment preference following a discussion of the anticipated benefits versus potential risks.1,2 In patients with the mixed inflammatory and comedonal clinical pattern of AV, oral antibiotics can be used concurrently with topical therapies when moderate to severe inflammatory lesions are noted.3,4 However, many adult women who had AV as teenagers have already utilized oral antibiotic therapies in the past and often are interested in alternative options, express concerns regarding antibiotic resistance, report a history of antibiotic-associated yeast infections or other side effects, and/or encounter issues related to drug-drug interactions.3,5-8 Oral hormonal therapies such as combination oral contraceptives (COCs) or spironolactone often are utilized to treat adult women with AV, sometimes in combination with each other or other agents. Combination oral contraceptives appear to be especially effective in the management of the U-shaped clinical pattern or predominantly inflammatory, late-onset AV.1,5,9,10 Potential warnings, contraindications, adverse effects, and drug-drug interactions are important to keep in mind when considering the use of oral hormonal therapies.8-10 Oral isotretinoin, which should be prescribed with strict adherence to the iPLEDGE™ program (https://www.ipledgeprogram.com/), remains a viable option for cases of severe nodular AV and selected cases of refractory inflammatory AV, especially when scarring and/or marked psychosocial distress are noted.1,2,5,11 Although it is recognized that adult women with AV typically present with either a mixed inflammatory and comedonal or U-shaped clinical pattern predominantly involving the lower face and anterolateral neck, the available data do not adequately differentiate the relative responsiveness of these clinical patterns to specific therapeutic agents.
Combination Oral Contraceptives
Combination oral contraceptives are commonly used to treat AV in adult women, including those without and those with measurable androgen excess (eg, polycystic ovary syndrome [PCOS]). Combination oral contraceptives contain ethinyl estradiol and a progestational agent (eg, progestin); the latter varies in terms of its nonselective receptor interactions and the relative magnitude or absence of androgenic effects.10,12,13 Although some COCs are approved by the US Food and Drug Administration (FDA) for AV, there is little data available to determine the comparative efficacy among these and other COCs.10,14 When choosing a COC for treatment of AV, it is best to select an agent whose effectiveness is supported by evidence from clinical studies.10,15
Mechanisms of Action
The reported mechanisms of action for COCs include inhibition of ovarian androgen production and ovulation through gonadotropin suppression; upregulated synthesis of sex hormone–binding globulin, which decreases free testosterone levels through receptor binding; and inhibition of 5α-reductase (by some progestins), which reduces conversion of testosterone to dihydrotestosterone, the active derivative that induces androgenic effects at peripheral target tissues.10,13,16,17
Therapeutic Benefits
Use of COCs to treat AV in adult women who do not have measurable androgen excess is most rational in patients who also desire a method of contraception. Multiple monotherapy studies have demonstrated the efficacy of COCs in the treatment of AV on the face and trunk.4,10,12,15,17,18 It may take a minimum of 3 monthly cycles of use before acne lesion counts begin to appreciably decrease.12,15,19-21 Initiating COC therapy during menstruation ensures the absence of pregnancy. Combination oral contraceptives may be used with other topical and oral therapies for AV.2,3,9,10 Potential ancillary benefits of COCs include normalization of the menstrual cycle; reduced premenstrual dysphoric disorder symptoms; and reduced risk of endometrial cancer (approximately 50%), ovarian cancer (approximately 40%), and colorectal cancer.22-24
Risks and Contraindications
It is important to consider the potential risks associated with the use of COCs, especially in women with AV who are not seeking a method of contraception. Side effects of COCs can include nausea, breast tenderness, breakthrough bleeding, and weight gain.25,26 Potential adverse associations of COCs are described in the Table. The major potential vascular associations include venous thromboembolism, myocardial infarction, and cerebrovascular accident, all of which are influenced by concurrent factors such as a history of smoking, age (≥35 years), and hypertension.27-32 It is recommended that blood pressure be measured before initiating COC therapy as part of the general examination.33

The potential increase in breast cancer risk appears to be low, while the cervical cancer risk is reported to increase relative to the duration of use.34-37 This latter observation may be due to the greater likelihood of unprotected sex in women using a COC and exposure to multiple sexual partners in some cases, which may increase the likelihood of oncogenic human papillomavirus infection of the cervix. If a dermatologist elects to prescribe a COC to treat AV, it has been suggested that the patient also consult with her general practitioner or gynecologist to undergo pelvic and breast examinations and a Papanicolaou test.33 The recommendation for initial screening for cervical cancer is within 3 years of initiation of sexual intercourse or by 21 years of age, whichever is first.33,38,39
Combination oral contraceptives are not ideal for all adult women with AV. Absolute contraindications are pregnancy and history of thromboembolic, cardiac, or hepatic disease; in women aged 35 years and older who smoke, relative contraindications include hypertension, diabetes, migraines, breastfeeding, and current breast or liver cancer.33 In adult women with AV who have relative contra-indications but are likely to benefit from the use of a COC when other options are limited or not viable, consultation with a gynecologist is prudent. Other than rifamycin antibiotics (eg, rifampin) and griseofulvin, there is no definitive evidence that oral antibiotics (eg, tetracycline) or oral antifungal agents reduce the contraceptive efficacy of COCs, although cautions remain in print within some approved package inserts.8
Spironolactone
Available since 1957, spironolactone is an oral aldos-terone antagonist and potassium-sparing diuretic used to treat hypertension and congestive heart failure.9 Recognition of its antiandrogenic effects led to its use in dermatology to treat certain dermatologic disorders in women (eg, hirsutism, alopecia, AV).1,4,5,9,10 Spironolactone is not approved for AV by the FDA; therefore, available data from multiple independent studies and retrospective analyses that have been collectively reviewed support its efficacy when used as both monotherapy or in combination with other agents in adult women with AV, especially those with a U-shaped pattern and/or late-onset AV.9,40-43
Mechanism of Action
Spironolactone inhibits sebaceous gland activity through peripheral androgen receptor blockade, inhibition of 5α-reductase, decrease in androgen production, and increase in sex hormone–binding globulin.9,10,40
Therapeutic Benefits
Good to excellent improvement of AV in women, many of whom are postadolescent, has ranged from 66% to 100% in published reports9,40-43; however, inclusion and exclusion criteria, dosing regimens, and concomitant therapies were not usually controlled. Spironolactone has been used to treat AV in adult women as monotherapy or in combination with topical agents, oral antibiotics, and COCs.9,40-42 Additionally, dose-ranging studies have not been completed with spironolactone for AV.9,40 The suggested dose range is 50 mg to 200 mg daily; however, it usually is best to start at 50 mg daily and increase to 100 mg daily if clinical response is not adequate after 2 to 3 months. The gastrointestinal (GI) absorption of spironolactone is increased when ingested with a high-fat meal.9,10
Once effective control of AV is achieved, it is optimal to use the lowest dose needed to continue reasonable suppression of new AV lesions. There is no defined end point for spironolactone use in AV, with or without concurrent PCOS, as many adult women usually continue treatment with low-dose therapy because they experience marked flaring shortly after the drug is stopped.9
Risks and Contraindications
Side effects associated with spironolactone are dose related and include increased diuresis, migraines, menstrual irregularities, breast tenderness, gynecomastia, fatigue, and dizziness.9,10,40-44 Side effects (particularly menstrual irregularities and breast tenderness) are more common at doses higher than 100 mg daily, especially when used as monotherapy without concurrent use of a COC.9,40
Spironolactone-associated hyperkalemia is most clinically relevant in patients on higher doses (eg, 100–200 mg daily), in those with renal impairment and/or congestive heart failure, and when used concurrently with certain other medications. In any patient on spironolactone, the risk of clinically relevant hyperkalemia may be increased by coingestion of potassium supplements, potassium-based salt substitutes, potassium-sparing diuretics (eg, amiloride, triamterene); aldosterone antagonists and angiotensin-converting enzyme inhibitors (eg, lisinopril, benazepril); angiotensin II receptor blockers (eg, losartan, valsartan); and tri-methoprim (with or without sulfamethoxazole).8,9,40,45 Spironolactone may also increase serum levels of lithium or digoxin.9,40,45,46 For management of AV, it is best that spironolactone be avoided in patients taking any of these medications.9
In healthy adult women with AV who are not on medications or supplements that interact adversely with spironolactone, there is no definitive recommendation regarding monitoring of serum potassium levels during treatment with spironolactone, and it has been suggested that monitoring serum potassium levels in this subgroup is not necessary.47 However, each clinician is advised to choose whether or not they wish to obtain baseline and/or periodic serum potassium levels when prescribing spironolactone for AV based on their degree of comfort and the patient’s history. Baseline and periodic blood testing to evaluate serum electrolytes and renal function are reasonable, especially as adult women with AV are usually treated with spironolactone over a prolonged period of time.9
The FDA black box warning for spironolactone states that it is tumorigenic in chronic toxicity studies in rats and refers to exposures 25- to 100-fold higher than those administered to humans.9,48 Although continued vigilance is warranted, evaluation of large populations of women treated with spironolactone do not suggest an association with increased risk of breast cancer.49,50
Spironolactone is a category C drug and thus should be avoided during pregnancy, primarily due to animal data suggesting risks of hypospadias and feminization in male fetuses.9 Importantly, there is an absence of reports linking exposure during pregnancy with congenital defects in humans, including in 2 known cases of high-dose exposures for maternal Bartter syndrome.9
The active metabolite, canrenone, is known to be present in breast milk at 0.2% of the maternal daily dose, but breastfeeding is generally believed to be safe with spironolactone based on evidence to date.9
Oral Antibiotics
Oral antibiotic therapy may be used in combination with a topical regimen to treat AV in adult women, keeping in mind some important caveats.1-7 For instance, monotherapy with oral antibiotics should be avoided, and concomitant use of benzoyl peroxide is suggested to reduce emergence of antibiotic-resistant Propionibacterium acnes strains.3,4 A therapeutic exit plan also is suggested when prescribing oral antibiotics to limit treatment to 3 to 4 months, if possible, to help mitigate the emergence of antibiotic-resistant bacteria (eg, staphylococci and streptococci).3-5,51
Tetracyclines, especially doxycycline and minocycline, are the most commonly prescribed agents. Doxycycline use warrants patient education on measures to limit the risks of esophageal and GI side effects and phototoxicity; enteric-coated and small tablet formulations have been shown to reduce GI side effects, especially when administered with food.3,52-55 In addition to vestibular side effects and hyperpigmentation, minocycline may be associated with rare but potentially severe adverse reactions such as drug hypersensitivity syndrome, autoimmune hepatitis, and lupus-like syndrome, which are reported more commonly in women.5,52,54 Vestibular side effects have been shown to decrease with use of extended-release tablets with weight-based dosing.53
Oral Isotretinoin
Oral isotretinoin is well established as highly effective for treatment of severe, recalcitrant AV, including nodular acne on the face and trunk.4,56 Currently available oral isotretinoins are branded generic formulations based on the pharmacokinetic profile of the original brand (Accutane [Roche Pharmaceuticals]) and with the use of Lidose Technology (Absorica [Cipher Pharmaceuticals]), which substantially increases GI absorption of isotretinoin in the absence of ingestion with a high-calorie, high-fat meal.57 The short- and long-term efficacy, dosing regimens, safety considerations, and serious teratogenic risks for oral isotretinoin are well published.4,56-58 Importantly, oral isotretinoin must be prescribed with strict adherence to the federally mandated iPLEDGE risk management program.
Low-dose oral isotretinoin therapy (<0.5 mg/kg–1 mg/kg daily) administered over several months longer than conventional regimens (ie, 16–20 weeks) has been suggested with demonstrated efficacy.57 However, this approach is not optimal due to the lack of established sustained clearance of AV after discontinuation of therapy and the greater potential for exposure to isotretinoin during pregnancy. Recurrences of AV do occur after completion of isotretinoin therapy, especially if cumulative systemic exposure to the drug during the initial course of treatment was inadequate.56,57
Oral isotretinoin has been shown to be effective in AV in adult women with or without PCOS with 0.5 mg/kg to 1 mg/kg daily and a total cumulative exposure of 120 mg/kg to 150 mg/kg.59 In one study, the presence of PCOS and greater number of nodules at baseline were predictive of a higher risk of relapse during the second year posttreatment.59
Conclusion
All oral therapies that are used to treat AV in adult women warrant individual consideration of possible benefits versus risks. Careful attention to possible side effects, patient-related risk factors, and potential drug-drug interactions is important. End points of therapy are not well established, with the exception of oral isotretinoin therapy. Clinicians must use their judgment in each case along with obtaining feedback from patients regarding the selection of therapy after a discussion of the available options.
- Holzmann R, Shakery K. Postadolescent acne in females. Skin Pharmacol Physiol. 2014;27(suppl 1):3-8.
- Villasenor J, Berson DS, Kroshinsky D. Treatment guidelines in adult women. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:198-207.
- Del Rosso JQ, Kim G. Optimizing use of oral antibiotics in acne vulgaris. Dermatol Clin. 2009;27:33-42.
- Gollnick H, Cunliffe W, Berson D, et al. Management of acne: report from a Global Alliance to Improve Outcomes in Acne. J Am Acad Dermatol. 2003;49(suppl 1):S1-S37.
- Fisk WA, Lev-Tov HA, Sivamani RK. Epidemiology and management of acne in adult women. Curr Derm Rep. 2014;3:29-39.
- Del Rosso JQ, Leyden JJ. Status report on antibiotic resistance: implications for the dermatologist. Dermatol Clin. 2007;25:127-132.
- Bowe WP, Leyden JJ. Clinical implications of antibiotic resistance: risk of systemic infection from Staphylococcus and Streptococcus. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:125-133.
- Del Rosso JQ. Oral antibiotic drug interactions of clinical significance to dermatologists. Dermatol Clin. 2009;27:91-94.
- Kim GK, Del Rosso JQ. Oral spironolactone in post-teenage female patients with acne vulgaris: practical considerations for the clinician based on current data and clinical experience. J Clin Aesthet Dermatol. 2012;5:37-50.
- Keri J, Berson DS, Thiboutot DM. Hormonal treatment of acne in women. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:146-155.
- American Academy of Dermatology. Position statement on isotretinoin. AAD Web site. https://www.aad.org /Forms/Policies/Uploads/PS/PS-Isotretinoin.pdf. Updated November 13, 2010. Accessed October 28, 2015.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. June 2012;7:CD004425.
- Sitruk-Ware R. Pharmacology of different progestogens: the special case of drospirenone. Climacteric. 2005;8 (suppl 3):4-12.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for the treatment of acne. Cochrane Database Syst Rev. July 2012;7:CD004425.
- Thiboutot D, Archer DF, Lemay A, et al. A randomized, controlled trial of a low-dose contraceptive containing 20 microg of ethinyl estradiol and 100 microg of levonogestrel for acne treatment. Fertil Steril. 2001;76:461-468.
- Koulianos GT. Treatment of acne with oral contraceptives: criteria for pill selection. Cutis. 2000;66:281-286.
- Rabe T, Kowald A, Ortmann J, et al. Inhibition of skin 5-alpha reductase by oral contraceptive progestins in vitro. Gynecol Endocrinol. 2000;14:223-230.
- Palli MB, Reyes-Habito CM, Lima XT, et al. A single-center, randomized double-blind, parallel-group study to examine the safety and efficacy of 3mg drospirenone/0.02mg ethinyl estradiol compared with placebo in the treatment of moderate truncal acne vulgaris. J Drugs Dermatol. 2013;12:633-637.
- Koltun W, Maloney JM, Marr J, et al. Treatment of moderate acne vulgaris using a combined oral contraceptive containing ethinylestradiol 20 μg plus drospirenone 3 mg administered in a 24/4 regimen: a pooled analysis. Eur J Obstet Gynecol Reprod Biol. 2011;155:171-175.
- Maloney JM, Dietze P, Watson D, et al. A randomized controlled trial of a low-dose combined oral contraceptive containing 3 mg drospirenone plus 20 μg ethinylestradiol in the treatment of acne vulgaris: lesion counts, investigator ratings and subject self-assessment. J Drugs Dermatol. 2009;8:837-844.
- Lucky AW, Koltun W, Thiboutot D, et al. A combined oral contraceptive containing 3-mg drospirenone/20-μg ethinyl estradiol in the treatment of acne vulgaris: a randomized, double-blind, placebo-controlled study evaluating lesion counts and participant self-assessment. Cutis. 2008;82:143-150.
- Burkman R, Schlesselman JJ, Zieman M. Safety concerns and health benefits associated with oral contraception. Am J Obstet Gynecol. 2004;190(suppl 4):S5-S22.
- Maguire K, Westhoff C. The state of hormonal contraception today: established and emerging noncontraceptive health benefits. Am J Obstet Gynecol. 2011;205 (suppl 4):S4-S8.
- Weiss NS, Sayvetz TA. Incidence of endometrial cancer in relation to the use of oral contraceptives. N Engl J Med. 1980;302:551-554.
- Tyler KH, Zirwas MJ. Contraception and the dermatologist. J Am Acad Dermatol. 2013;68:1022-1029.
- Gallo MF, Lopez LM, Grimes DA, et al. Combination contraceptives: effects on weight. Cochrane Database Syst Rev. 2008;4:CD003987.
- de Bastos M, Stegeman BH, Rosendaal FR, et al. Combined oral contraceptives: venous thrombosis. Cochrane Database Syst Rev. 2014;3:CD010813.
- Raymond EG, Burke AE, Espey E. Combined hormonal contraceptives and venous thromboembolism: putting the risks into perspective. Obstet Gynecol. 2012;119:1039-1044.
- Jick SS, Hernandez RK. Risk of non-fatal venous thromboembolism in women using oral contraceptives containing drospirenone compared with women using oral contraceptives containing levonorgestrel: case-control study using United States claims data. BMJ. 2011;342:d2151.
- US Food and Drug Administration Office of Surveillance and Epidemiology. Combined hormonal contraceptives (CHCs) and the risk of cardiovascular disease endpoints. US Food and Drug Administration Web site. http://www.fda.gov/downloads/Drugs /Drug Safety/UCM277384.pdf. Accessed October 28, 2015.
- The American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. Risk of venous thromboembolism among users of drospirenone-containing oral contraceptive pills. Obstet Gynecol. 2012;120:1239-1242.
- World Health Organization. Cardiovascular Disease and Steroid Hormone Contraception: Report of a WHO Scientific Group. Geneva, Switzerland: World Health Organization; 1998. Technical Report Series 877.
- Frangos JE, Alavian CN, Kimball AB. Acne and oral contraceptives: update on women’s health screening guidelines. J Am Acad Dermatol. 2008;58:781-786.
- Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53 297 women with breast cancer and 100 239 women without breast cancer from 54 epidemiological studies. Lancet. 1996;347:1713-1727.
- Gierisch JM, Coeytaux RR, Urrutia RP, et al. Oral contraceptive use and risk of breast, cervical, colorectal, and endometrial cancers: a systematic review. Cancer Epidemiol Biomarkers Prev. 2013;22:1931-1943.
- International Collaboration of Epidemiological Studies of Cervical Cancer. Cervical cancer and hormonal contraceptives: collaborative reanalysis of individual data for 16 573 women with cervical cancer and 35 509 women without cervical cancer from 24 epidemiological studies. Lancet. 2007;370:1609-1621.
- Agostino H, Di Meglio G. Low-dose oral contraceptives in adolescents: how low can you go? J Pediatr Adolesc Gynecol. 2010;23:195-201.
- Buzney E, Sheu J, Buzney C, et al. Polycystic ovary syndrome: a review for dermatologists: part II. Treatment. J Am Acad Dermatol. 2014;71:859.e1-859.e15.
- Stewart FH, Harper CC, Ellertson CE, et al. Clinical breast and pelvic examination requirements for hormonal contraception: current practice vs evidence. JAMA. 2001;285:2232-2239.
- Sawaya ME, Somani N. Antiandrogens and androgen inhibitors. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelpha, PA: Saunders; 2013:361-374.
- Muhlemann MF, Carter GD, Cream JJ, et al. Oral spironolactone: an effective treatment for acne vulgaris in women. Br J Dermatol. 1986;115:227-232.
- Shaw JC. Low-dose adjunctive spironolactone in the treatment of acne in women: a retrospective analysis of 85 consecutively treated patients. J Am Acad Dermatol. 2000;43:498-502.
- Sato K, Matsumoto D, Iizuka F, et al. Anti-androgenic therapy using oral spironolactone for acne vulgaris in Asians. Aesth Plast Surg. 2006;30:689-694.
- Shaw JC, White LE. Long-term safety of spironolactone in acne: results of an 8-year follow-up study. J Cutan Med Surg. 2002;6:541-545.
- Stockley I. Antihypertensive drug interactions. In: Stockley I, ed. Drug Interactions. 5th ed. London, United Kingdom: Pharmaceutical Press; 1999:335-347.
- Antoniou T, Gomes T, Mamdani MM, et al. Trimethoprim-sulfamethoxazole induced hyperkalaemia in elderly patients receiving spironolactone: nested case-control study. BMJ. 2011;343:d5228.
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944.
- Aldactone [package insert]. New York, NY: Pfizer Inc; 2008.
- Biggar RJ, Andersen EW, Wohlfahrt J, et al. Spironolactone use and the risk of breast and gynecologic cancers. Cancer Epidemiol. 2013;37:870-875.
- Mackenzie IS, Macdonald TM, Thompson A, et al. Spironolactone and risk of incident breast cancer in women older than 55 years: retrospective, matched cohort study. BMJ. 2012;345:e4447.
- Dreno B, Thiboutot D, Gollnick H, et al. Antibiotic stewardship in dermatology: limiting antibiotic use in acne. Eur J Dermatol. 2014;24:330-334.
- Kim S, Michaels BD, Kim GK, et al. Systemic antibacterial agents. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelpha, PA: Saunders; 2013:61-97.
- Leyden JJ, Del Rosso JQ. Oral antibiotic therapy for acne vulgaris: pharmacokinetic and pharmacodynamics perspectives. J Clin Aesthet Dermatol. 2011;4:40-47.
- Del Rosso JQ. Oral antibiotics. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:113-124.
- Del Rosso JQ. Oral doxycycline in the management of acne vulgaris: current perspectives on clinical use and recent findings with a new double-scored small tablet formulation. J Clin Aesthet Dermatol. 2015;8:19-26.
- Osofsky MG, Strauss JS. Isotretinoin. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:134-145.
- Leyden JJ, Del Rosso JQ, Baum EW. The use of isotretinoin in the treatment of acne vulgaris: clinical considerations and future directions. J Clin Aesthet Dermatol. 2014;7(suppl 2):S3-S21.
- Patton TJ, Ferris LK. Systemic retinoids. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelpha, PA: Saunders; 2013:252-268.
- Cakir GA, Erdogan FG, Gurler A. Isotretinoin treatment in nodulocystic acne with and without polycystic ovary syndrome: efficacy and determinants of relapse. Int J Dermatol. 2013;52:371-376.
- Holzmann R, Shakery K. Postadolescent acne in females. Skin Pharmacol Physiol. 2014;27(suppl 1):3-8.
- Villasenor J, Berson DS, Kroshinsky D. Treatment guidelines in adult women. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:198-207.
- Del Rosso JQ, Kim G. Optimizing use of oral antibiotics in acne vulgaris. Dermatol Clin. 2009;27:33-42.
- Gollnick H, Cunliffe W, Berson D, et al. Management of acne: report from a Global Alliance to Improve Outcomes in Acne. J Am Acad Dermatol. 2003;49(suppl 1):S1-S37.
- Fisk WA, Lev-Tov HA, Sivamani RK. Epidemiology and management of acne in adult women. Curr Derm Rep. 2014;3:29-39.
- Del Rosso JQ, Leyden JJ. Status report on antibiotic resistance: implications for the dermatologist. Dermatol Clin. 2007;25:127-132.
- Bowe WP, Leyden JJ. Clinical implications of antibiotic resistance: risk of systemic infection from Staphylococcus and Streptococcus. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:125-133.
- Del Rosso JQ. Oral antibiotic drug interactions of clinical significance to dermatologists. Dermatol Clin. 2009;27:91-94.
- Kim GK, Del Rosso JQ. Oral spironolactone in post-teenage female patients with acne vulgaris: practical considerations for the clinician based on current data and clinical experience. J Clin Aesthet Dermatol. 2012;5:37-50.
- Keri J, Berson DS, Thiboutot DM. Hormonal treatment of acne in women. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:146-155.
- American Academy of Dermatology. Position statement on isotretinoin. AAD Web site. https://www.aad.org /Forms/Policies/Uploads/PS/PS-Isotretinoin.pdf. Updated November 13, 2010. Accessed October 28, 2015.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. June 2012;7:CD004425.
- Sitruk-Ware R. Pharmacology of different progestogens: the special case of drospirenone. Climacteric. 2005;8 (suppl 3):4-12.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for the treatment of acne. Cochrane Database Syst Rev. July 2012;7:CD004425.
- Thiboutot D, Archer DF, Lemay A, et al. A randomized, controlled trial of a low-dose contraceptive containing 20 microg of ethinyl estradiol and 100 microg of levonogestrel for acne treatment. Fertil Steril. 2001;76:461-468.
- Koulianos GT. Treatment of acne with oral contraceptives: criteria for pill selection. Cutis. 2000;66:281-286.
- Rabe T, Kowald A, Ortmann J, et al. Inhibition of skin 5-alpha reductase by oral contraceptive progestins in vitro. Gynecol Endocrinol. 2000;14:223-230.
- Palli MB, Reyes-Habito CM, Lima XT, et al. A single-center, randomized double-blind, parallel-group study to examine the safety and efficacy of 3mg drospirenone/0.02mg ethinyl estradiol compared with placebo in the treatment of moderate truncal acne vulgaris. J Drugs Dermatol. 2013;12:633-637.
- Koltun W, Maloney JM, Marr J, et al. Treatment of moderate acne vulgaris using a combined oral contraceptive containing ethinylestradiol 20 μg plus drospirenone 3 mg administered in a 24/4 regimen: a pooled analysis. Eur J Obstet Gynecol Reprod Biol. 2011;155:171-175.
- Maloney JM, Dietze P, Watson D, et al. A randomized controlled trial of a low-dose combined oral contraceptive containing 3 mg drospirenone plus 20 μg ethinylestradiol in the treatment of acne vulgaris: lesion counts, investigator ratings and subject self-assessment. J Drugs Dermatol. 2009;8:837-844.
- Lucky AW, Koltun W, Thiboutot D, et al. A combined oral contraceptive containing 3-mg drospirenone/20-μg ethinyl estradiol in the treatment of acne vulgaris: a randomized, double-blind, placebo-controlled study evaluating lesion counts and participant self-assessment. Cutis. 2008;82:143-150.
- Burkman R, Schlesselman JJ, Zieman M. Safety concerns and health benefits associated with oral contraception. Am J Obstet Gynecol. 2004;190(suppl 4):S5-S22.
- Maguire K, Westhoff C. The state of hormonal contraception today: established and emerging noncontraceptive health benefits. Am J Obstet Gynecol. 2011;205 (suppl 4):S4-S8.
- Weiss NS, Sayvetz TA. Incidence of endometrial cancer in relation to the use of oral contraceptives. N Engl J Med. 1980;302:551-554.
- Tyler KH, Zirwas MJ. Contraception and the dermatologist. J Am Acad Dermatol. 2013;68:1022-1029.
- Gallo MF, Lopez LM, Grimes DA, et al. Combination contraceptives: effects on weight. Cochrane Database Syst Rev. 2008;4:CD003987.
- de Bastos M, Stegeman BH, Rosendaal FR, et al. Combined oral contraceptives: venous thrombosis. Cochrane Database Syst Rev. 2014;3:CD010813.
- Raymond EG, Burke AE, Espey E. Combined hormonal contraceptives and venous thromboembolism: putting the risks into perspective. Obstet Gynecol. 2012;119:1039-1044.
- Jick SS, Hernandez RK. Risk of non-fatal venous thromboembolism in women using oral contraceptives containing drospirenone compared with women using oral contraceptives containing levonorgestrel: case-control study using United States claims data. BMJ. 2011;342:d2151.
- US Food and Drug Administration Office of Surveillance and Epidemiology. Combined hormonal contraceptives (CHCs) and the risk of cardiovascular disease endpoints. US Food and Drug Administration Web site. http://www.fda.gov/downloads/Drugs /Drug Safety/UCM277384.pdf. Accessed October 28, 2015.
- The American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. Risk of venous thromboembolism among users of drospirenone-containing oral contraceptive pills. Obstet Gynecol. 2012;120:1239-1242.
- World Health Organization. Cardiovascular Disease and Steroid Hormone Contraception: Report of a WHO Scientific Group. Geneva, Switzerland: World Health Organization; 1998. Technical Report Series 877.
- Frangos JE, Alavian CN, Kimball AB. Acne and oral contraceptives: update on women’s health screening guidelines. J Am Acad Dermatol. 2008;58:781-786.
- Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53 297 women with breast cancer and 100 239 women without breast cancer from 54 epidemiological studies. Lancet. 1996;347:1713-1727.
- Gierisch JM, Coeytaux RR, Urrutia RP, et al. Oral contraceptive use and risk of breast, cervical, colorectal, and endometrial cancers: a systematic review. Cancer Epidemiol Biomarkers Prev. 2013;22:1931-1943.
- International Collaboration of Epidemiological Studies of Cervical Cancer. Cervical cancer and hormonal contraceptives: collaborative reanalysis of individual data for 16 573 women with cervical cancer and 35 509 women without cervical cancer from 24 epidemiological studies. Lancet. 2007;370:1609-1621.
- Agostino H, Di Meglio G. Low-dose oral contraceptives in adolescents: how low can you go? J Pediatr Adolesc Gynecol. 2010;23:195-201.
- Buzney E, Sheu J, Buzney C, et al. Polycystic ovary syndrome: a review for dermatologists: part II. Treatment. J Am Acad Dermatol. 2014;71:859.e1-859.e15.
- Stewart FH, Harper CC, Ellertson CE, et al. Clinical breast and pelvic examination requirements for hormonal contraception: current practice vs evidence. JAMA. 2001;285:2232-2239.
- Sawaya ME, Somani N. Antiandrogens and androgen inhibitors. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelpha, PA: Saunders; 2013:361-374.
- Muhlemann MF, Carter GD, Cream JJ, et al. Oral spironolactone: an effective treatment for acne vulgaris in women. Br J Dermatol. 1986;115:227-232.
- Shaw JC. Low-dose adjunctive spironolactone in the treatment of acne in women: a retrospective analysis of 85 consecutively treated patients. J Am Acad Dermatol. 2000;43:498-502.
- Sato K, Matsumoto D, Iizuka F, et al. Anti-androgenic therapy using oral spironolactone for acne vulgaris in Asians. Aesth Plast Surg. 2006;30:689-694.
- Shaw JC, White LE. Long-term safety of spironolactone in acne: results of an 8-year follow-up study. J Cutan Med Surg. 2002;6:541-545.
- Stockley I. Antihypertensive drug interactions. In: Stockley I, ed. Drug Interactions. 5th ed. London, United Kingdom: Pharmaceutical Press; 1999:335-347.
- Antoniou T, Gomes T, Mamdani MM, et al. Trimethoprim-sulfamethoxazole induced hyperkalaemia in elderly patients receiving spironolactone: nested case-control study. BMJ. 2011;343:d5228.
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944.
- Aldactone [package insert]. New York, NY: Pfizer Inc; 2008.
- Biggar RJ, Andersen EW, Wohlfahrt J, et al. Spironolactone use and the risk of breast and gynecologic cancers. Cancer Epidemiol. 2013;37:870-875.
- Mackenzie IS, Macdonald TM, Thompson A, et al. Spironolactone and risk of incident breast cancer in women older than 55 years: retrospective, matched cohort study. BMJ. 2012;345:e4447.
- Dreno B, Thiboutot D, Gollnick H, et al. Antibiotic stewardship in dermatology: limiting antibiotic use in acne. Eur J Dermatol. 2014;24:330-334.
- Kim S, Michaels BD, Kim GK, et al. Systemic antibacterial agents. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelpha, PA: Saunders; 2013:61-97.
- Leyden JJ, Del Rosso JQ. Oral antibiotic therapy for acne vulgaris: pharmacokinetic and pharmacodynamics perspectives. J Clin Aesthet Dermatol. 2011;4:40-47.
- Del Rosso JQ. Oral antibiotics. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:113-124.
- Del Rosso JQ. Oral doxycycline in the management of acne vulgaris: current perspectives on clinical use and recent findings with a new double-scored small tablet formulation. J Clin Aesthet Dermatol. 2015;8:19-26.
- Osofsky MG, Strauss JS. Isotretinoin. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:134-145.
- Leyden JJ, Del Rosso JQ, Baum EW. The use of isotretinoin in the treatment of acne vulgaris: clinical considerations and future directions. J Clin Aesthet Dermatol. 2014;7(suppl 2):S3-S21.
- Patton TJ, Ferris LK. Systemic retinoids. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelpha, PA: Saunders; 2013:252-268.
- Cakir GA, Erdogan FG, Gurler A. Isotretinoin treatment in nodulocystic acne with and without polycystic ovary syndrome: efficacy and determinants of relapse. Int J Dermatol. 2013;52:371-376.
Practice Points
- Use of combination oral contraceptives to treat acne vulgaris (AV) in adult women who do not have measurable androgen excess is most rational in patients who also desire a method of contraception.
- Spironolactone is widely accepted as an oral agent that can be effective in treating adult women with AV and may be used in combination with other therapies.
- Monotherapy with oral antibiotics should be avoided in the treatment of adult women with AV, and concomitant use of benzoyl peroxide is suggested to reduce emergence of antibiotic-resistant Propionibacterium acnes strains.
- Oral isotretinoin use in adult women with AV warrants strict adherence to pregnancy prevention measures and requirements set forth by the federally mandated iPLEDGE™ risk management program.
Status Report From the American Acne & Rosacea Society on Medical Management of Acne in Adult Women, Part 1: Overview, Clinical Characteristics, and Laboratory Evaluation
It was not long ago that acne vulgaris (AV) was commonly considered to be a skin disease that affected teenagers with little attention given to preadolescent and postadolescent AV. This perspective has changed, with more attention being given to AV across a broad range of affected age groups, including preadolescent, adolescent, and postadolescent subgroups.1-5 Earlier onset of adrenarche has led to earlier development of AV in many young girls, with a higher range of dehydroepiandrosterone sulfate (DHEAS) levels observed overall in those with AV as compared to a normal age-matched population.3,4 At the other end of the age spectrum, AV is a common phenomenon in adult females, with at least half of women estimated to exhibit some form of AV.1,2,5-8 Based on a large survey of females and males (N=1013), the prevalence of AV in adult females has been reported to be 50.9%, 35.2%, 26.3%, and 15.3% among women aged 20 to 29 years, 30 to 39 years, 40 to 49 years, and 50 years and older, respectively.2 Acne vulgaris that persists beyond adolescence into adulthood is termed persistent acne, or early-onset acne, and the development of AV in women 25 years and older who have not previously been affected by AV has been termed late-onset acne.6,8,9 Publications on the management of AV in adult women have focused primarily on systemic hormonal therapies; however, topical therapies more recently have received greater attention in this subpopulation9-12 and will be discussed in part 2 of this series. Because data on AV in women are limited primarily to involvement of the face and neck region, this article does not address truncal AV unless otherwise specified. Table 1 depicts factors that can influence the management of AV in adult women.
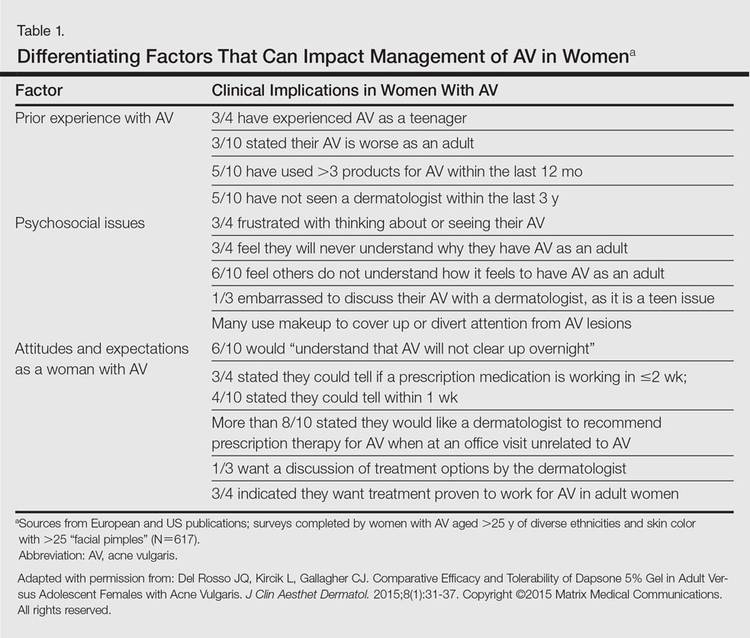
Visible Patterns and Considerations for Clinical Evaluation
Clinical Patterns
Although epidemiologic and demographic data are limited in the subpopulation of women with AV, it is reported that females account for up to 82% of adults with AV, with approximately 75% presenting with AV that is clinically similar to their disease course in adolescence.2,5,13 Among those women with persistent AV, some state that their AV is worse compared to adolescence, while others report it is not as severe. The pattern of AV often is similar to that seen in adolescence, presenting as mixed comedonal and inflammatory papular/pustular lesions diffusely distributed on the face; in other cases, a more selectively distributed U-shaped pattern is noted, characterized predominantly by inflammatory papules and/or nodules involving the lower cheeks and jawline margin, with lesions also commonly noted on the anterior and lateral neck.5,8,9,13-16 A U-shaped pattern is believed to be more common in late-onset AV, often with persistence into the mid-40s.1,15,17 It is important to emphasize the need for additional studies on the demographics and clinical characteristics of AV in adult females, especially correlations between onset, age, and clinical patterns of AV.
An international, prospective, observational study assessed the clinical characteristics of AV in adults (aged ≥25 years) at a dermatology visit for acne (N=374).16 Participants who were under management for their AV showed severity grades of mild (clear/almost clear) in 47.3% of cases. Involvement of multiple facial sites—cheeks, forehead, mandibular region, and temples—was noted in 89.8% of women, often with both inflammatory and comedonal lesions, which is a pattern similar to adolescent AV. Inflammatory lesions alone were observed in 6.4% of women, 17.1% had comedonal AV only, and truncal AV was present in 48.4%.16 Additional well-designed studies are needed to determine if this study reflects an accurate qualitative and quantitative depiction of the spectrum of AV in adult females.
Mandibular Pattern
In the observational study of AV in adults, AV localized to the mandibular area was noted in only 11.2% of participants.16 Women with localized mandibular AV were more likely than women without localized AV to be employed, noted greater daily stress levels, and tended to report more psychologically stressful jobs. Interestingly, the subgroup with mandibular acne alone was much less likely to exhibit a global severity grade of moderate or higher (7.1% vs 50.1%), truncal acne (19.0% vs 51.9%), postinflammatory hyperpigmentation (23.8% vs 51.9%), and erythema (19.0% vs 48.4%), suggesting a unique subset of AV presentation.16
Ethnicity/Skin Color
Women of all ethnicities and skin types may be affected by AV.1,18-20 Earlier age of onset of AV has been suggested in white women; however, earlier onset of adrenarche may be more frequent in black girls, which supports an earlier age of onset of AV in this subpopulation.15-17 Women with skin of color usually express greater concern with persistent dyschromia at sites where lesions have resolved, and presence of acne scars is a concern among women regardless of skin color, ethnicity, or race.18,20-22
Scarring
Acne scarring has been noted to affect up to three-fourths of adult women in one report17 and often is stated by patients to be a cause of concern and frustration.1,5,17
Perimenstrual Flaring
Flaring associated with menses is commonly reported in adult females with AV, with 56%, 17%, and 3% of women in one study (n=230) reporting worsening before, during, or after menses, respectively.21
External Factors
Comedogenic products used for skin care, cover-up makeup, or hair care may be important to consider in selected cases as potential etiologic or exacerbating factors in adult females with AV; they also may be used in the management of AV.23-25 Adult females often are perplexed and frustrated by the presence of AV after their teenaged years and anxiously wonder about or search for the potential causes. Many women use cosmetic products to cover up facial AV.5,23-25 Therefore, even if skin care or personal hygiene products or makeup are not believed to be an etiologic factor, many patients appreciate that their dermatologist addressed skin care and cosmetics as a component of AV management and provided appropriate recommendations.5,13
Ingestion of dietary supplements containing whey protein have been associated with precipitation of AV.26,27 Diets with specific content characteristics have been implicated as potential etiologic or exacerbating factors for AV; however, data are limited and specific recommendations remain elusive at present. Individual cases may warrant consideration of dietary factors, especially when treatment resistance is noted.28 Importantly, progestin-only contraceptives (ie, injectables, intrauterine devices) also can exacerbate or induce AV.29
Hyperandrogenism
Although most adult females with AV are reported to have normal serum androgen levels when tested, it is important to explore potential signs and symptoms that are suggestive of underlying hyperandrogenism through both the patient’s history and physical examination.9-11,21,29-33 Some investigators have suggested that underlying peripheral hyperandrogenism is the leading cause of AV in adult females, with or without concurrent polycystic ovarian syndrome (PCOS), though it is believed that most women with AV exhibit normal results when undergoing laboratory testing for androgen excess.10,11,21,29,30 Nevertheless, it is important to consider the possibility of underlying causes of androgen excess (Table 2), the most common being PCOS and late-onset congenital adrenal hyperplasia; an androgen-secreting tumor is less common.11,29-33 It is suggested that screening for underlying endocrinopathy should be conducted in women presenting with (1) AV recalcitrant to conventional treatment, (2) sudden emergence of severe AV, (3) concurrent signs/symptoms of androgen excess, and/or (4) AV relapse shortly after isotretinoin therapy.7,11,16,33

Hirsutism and acanthosis nigricans have been reported to be more reliable predictors of hyperandrogenism than androgenic alopecia.21 Although it may be subtle in some cases, acanthosis nigricans is harder to camouflage, so the clinician can usually detect it if a thorough physical examination is performed. However, a patient may not voluntarily report to the clinician and their staff that she has hair removed, so despite a thorough examination, the clinician may not detect hirsutism. Therefore, it is important to inquire directly about the presence of hairs (pigmented terminal vs “peach fuzz” hairs), their anatomic location, and any hair removal practices the patient has used. The absence of androgenic alopecia does not exclude underlying hyperandrogenism; however, its presence, especially in younger women, may serve as a clinical marker for underlying hyperandrogenism.5 Some women may camouflage more subtle alopecia through hairstyling, but obtaining this history usually is not problematic, as most women are distressed by any degree of hair loss.
Laboratory Evaluation—A relatively straightforward approach to the workup of androgen excess includes assessment of serum DHEAS, free testosterone, and total testosterone levels.10,30 Elevation of serum DHEAS levels indicates an adrenal source of androgen production. Elevation of testosterone is associated with excess androgens produced by the ovaries. Modest elevations of DHEAS are most commonly associated with late-onset congenital adrenal hyperplasia that may not have been previously diagnosed. Modest elevation of testosterone is most commonly associated with PCOS, which also can be accompanied by an elevated luteinizing hormone:follicle-stimulating hormone ratio of 2.5:1 to 3:1.10,30 Marked elevations of DHEAS or testosterone can be indicative of adrenal or ovarian tumors, respectively.30
In some cases, a woman might have elevated DHEAS and testosterone levels. A 17-hydroxyprogesterone test can help discriminate between an adrenal or ovarian source of androgen excess in these cases, as elevated 17-hydroxyprogesterone levels indicate that the androgens are coming from the adrenal gland.10,30
It is important that laboratory evaluation be performed when ovulation is not occurring. Blood tests can be drawn just prior to or during menses. It is important that a woman is not taking an oral contraceptive at the time of testing, which can mask an underlying endocrine abnormality.10,11,29,30 Generally, testing can be performed at least 4 to 6 weeks after stopping the oral contraceptive.
Psychosocial Impact
Facial AV exhibits a broad range of adverse psychological and social effects on many adult females.2,5,13,18 It can be associated with depression, anxiety, psychological stress, and suicidal ideation; therefore, thorough screening for these comorbidities may be warranted in some patients.2,18
Conclusion
The epidemiology, clinical presentation, and clinical and laboratory evaluation of AV in adult females was reviewed in part 1 of this 3-part series. It is important for the clinician to assess the clinical presentation, psychosocial effects, and the possibility of underlying causes of androgen excess. In part 2, skin care and topical management of AV in adult females will be discussed.
1. Perkins AC, Maglione J, Hillebrand GG, et al. Acne vulgaris in women: prevalence across the life span. J Womens Health (Larchmt). 2012;21: 223-230.
2. Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-59.
3. Lucky AW, Biro FM, Huster GA, et al. Acne vulgaris in premenarchal girls. an early sign of puberty associated with rising levels of dehydroepiandrosterone. Arch Dermatol. 1994;130:308-314.
4. Mancini AJ, Baldwin HE, Eichenfield LF, et al. Acne life cycle: the spectrum of pediatric disease. Semin Cutan Med Surg. 2011;30(suppl 3):S2-S5.
5. Tanghetti EA, Kawata AK, Daniels SR, et al. Understanding the burden of adult female acne. J Clin Aesthet Dermatol. 2014;7:22-30.
6. Goulden V, Stables GI, Cunliffe WJ. Prevalence of facial acne in adults. J Am Acad Dermatol. 1999;41: 577-580.
7. Marks R. Acne and its management beyond the age of 35 years. Am J Clin Dermatol. 2004;5:459-462.
8. Preneau S, Dreno B. Female acne—a different subtype of teenager acne? J Eur Acad Dermatol Venereol. 2012;26:277-282.
9. Kim GK, Del Rosso JQ. Oral spironolactone in post-teenage female patients with acne vulgaris: practical considerations for the clinician based on current data and clinical experience. J Clin Aesthet Dermatol. 2012;5:37-50.
10. Thiboutot D, Chen W. Update and future of hormonal therapy in acne. Dermatology. 2003;206:57-67.
11. Villasenor J, Berson D, Kroshinsky D. Treatment guidelines in adult women. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:198-207.
12. Del Rosso JQ, Zeichner J. What’s new in the medicine cabinet? a panoramic review of clinically relevant information for the busy dermatologist. J Clin Aesthet Dermatol. 2014;7:26-30.
13. Del Rosso JQ, Kircik L, Gallagher CJ. Comparative efficacy and tolerability of dapsone 5% gel in adult versus adolescent females with acne vulgaris. J Clin Aesthet Dermatol. 2015;8:31-37.
14. Dreno B, Layton A, Zouboulis CC, et al. Adult female acne: a new paradigm. J Eur Acad Dermatol Venereol. 2013;27:1063-1070.
15. Choi CW, Lee DH, Kim HS, et al. The clinical features of late onset acne compared with early onset acne in women. J Eur Acad Dermatol Venereol. 2011;25:454-461.
16. Dréno B, Thiboutot D, Layton AM, et al; Global Alliance to Improve Outcomes in Acne. Large-scale international study enhances understanding of an emerging acne population: adult females. J Eur Acad Dermatol Venereol. 2015;29:1096-1106.
17. Kane A, Niang SO, Diagne AC, et al. Epidemiologic, clinical, and therapeutic features of acne in Dakar, Senegal. Int J Dermatol. 2007;46(suppl 1):36-38.
18. Callender VD, Alexis AF, Daniels SR, et al. Racial differences in clinical characteristics, perceptions and behaviors, and psychosocial impact of adult female acne. J Clin Aesthet Dermatol. 2014;7:19-31.
19. Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
20. Rendon MI, Rodriguez DA, Kawata AK, et al. Acne treatment patterns, expectations, and satisfaction among adult females of different races/ethnicities. Clin Cosmet Investig Dermatol. 2015;8:231-238.
21. Khunger N, Kumar C. A clinico-epidemiological study of adult acne: is it different from adolescent acne? Indian J Dermatol Venereol Leprol. 2012;78: 335-341.
22. Alexis AF. Acne vulgaris in skin of color: understanding nuances and optimizing treatment outcomes. J Drugs Dermatol. 2014;13(suppl 6):S61-S65.
23. Dall’oglio F, Tedeschi A, Fabbrocini G, et al. Cosmetics for acne: indications and recommendations for an evidence-based approach. G Ital Dermatol Venereol. 2015;150:1-11.
24. Draelos Z. Facial cosmetics for acne patients. In: Draelos Z. Cosmetics in Dermatology. 2nd Ed. New York, NY: Churchill Livingstone Inc; 1995:15-28.
25. Cunliffe WJ. Acne. London, United Kingdom: Martin Dunitz Ltd; 1989.
26. Simonart T. Acne and whey protein supplementation among bodybuilders. Dermatology. 2012;225:256-258.
27. Silverberg NB. Whey protein precipitating moderate to severe acne flares in 5 teenaged athletes. Cutis. 2012;90:70-72.
28. Bronsnick T, Murzaku EC, Rao BK. Diet in dermatology: part I. atopic dermatitis, acne, and nonmelanoma skin cancer. J Am Acad Dermatol. 2014;71:1039.
29. Keri J, Berson DS, Thiboutot DM. Hormonal treatment of acne in women. In: Shalita AR, Del Rosso J, Webster G, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:146-155.
30. Thiboutot D. Hormones and acne: pathophysiology, clinical evaluation and therapies. Sem Cutan Med Surg. 2001;20:144-153.
31. Borgia F, Cannavò S, Guarneri F, et al. Correlation between endocrinological parameters and acne severity in adult women. Acta Derm Venereol. 2004;84:201-204.
32. Clark CM, Rudolph J, Gerber DA, et al. Dermatologic manifestation of hyperandrogenism: a retrospective chart review. Skinmed. 2014;12:84-88.
33. Zeichner JA. Evaluating and treating the adult female patient with acne. J Drugs Dermatol. 2013;12:1416-1427.
It was not long ago that acne vulgaris (AV) was commonly considered to be a skin disease that affected teenagers with little attention given to preadolescent and postadolescent AV. This perspective has changed, with more attention being given to AV across a broad range of affected age groups, including preadolescent, adolescent, and postadolescent subgroups.1-5 Earlier onset of adrenarche has led to earlier development of AV in many young girls, with a higher range of dehydroepiandrosterone sulfate (DHEAS) levels observed overall in those with AV as compared to a normal age-matched population.3,4 At the other end of the age spectrum, AV is a common phenomenon in adult females, with at least half of women estimated to exhibit some form of AV.1,2,5-8 Based on a large survey of females and males (N=1013), the prevalence of AV in adult females has been reported to be 50.9%, 35.2%, 26.3%, and 15.3% among women aged 20 to 29 years, 30 to 39 years, 40 to 49 years, and 50 years and older, respectively.2 Acne vulgaris that persists beyond adolescence into adulthood is termed persistent acne, or early-onset acne, and the development of AV in women 25 years and older who have not previously been affected by AV has been termed late-onset acne.6,8,9 Publications on the management of AV in adult women have focused primarily on systemic hormonal therapies; however, topical therapies more recently have received greater attention in this subpopulation9-12 and will be discussed in part 2 of this series. Because data on AV in women are limited primarily to involvement of the face and neck region, this article does not address truncal AV unless otherwise specified. Table 1 depicts factors that can influence the management of AV in adult women.

Visible Patterns and Considerations for Clinical Evaluation
Clinical Patterns
Although epidemiologic and demographic data are limited in the subpopulation of women with AV, it is reported that females account for up to 82% of adults with AV, with approximately 75% presenting with AV that is clinically similar to their disease course in adolescence.2,5,13 Among those women with persistent AV, some state that their AV is worse compared to adolescence, while others report it is not as severe. The pattern of AV often is similar to that seen in adolescence, presenting as mixed comedonal and inflammatory papular/pustular lesions diffusely distributed on the face; in other cases, a more selectively distributed U-shaped pattern is noted, characterized predominantly by inflammatory papules and/or nodules involving the lower cheeks and jawline margin, with lesions also commonly noted on the anterior and lateral neck.5,8,9,13-16 A U-shaped pattern is believed to be more common in late-onset AV, often with persistence into the mid-40s.1,15,17 It is important to emphasize the need for additional studies on the demographics and clinical characteristics of AV in adult females, especially correlations between onset, age, and clinical patterns of AV.
An international, prospective, observational study assessed the clinical characteristics of AV in adults (aged ≥25 years) at a dermatology visit for acne (N=374).16 Participants who were under management for their AV showed severity grades of mild (clear/almost clear) in 47.3% of cases. Involvement of multiple facial sites—cheeks, forehead, mandibular region, and temples—was noted in 89.8% of women, often with both inflammatory and comedonal lesions, which is a pattern similar to adolescent AV. Inflammatory lesions alone were observed in 6.4% of women, 17.1% had comedonal AV only, and truncal AV was present in 48.4%.16 Additional well-designed studies are needed to determine if this study reflects an accurate qualitative and quantitative depiction of the spectrum of AV in adult females.
Mandibular Pattern
In the observational study of AV in adults, AV localized to the mandibular area was noted in only 11.2% of participants.16 Women with localized mandibular AV were more likely than women without localized AV to be employed, noted greater daily stress levels, and tended to report more psychologically stressful jobs. Interestingly, the subgroup with mandibular acne alone was much less likely to exhibit a global severity grade of moderate or higher (7.1% vs 50.1%), truncal acne (19.0% vs 51.9%), postinflammatory hyperpigmentation (23.8% vs 51.9%), and erythema (19.0% vs 48.4%), suggesting a unique subset of AV presentation.16
Ethnicity/Skin Color
Women of all ethnicities and skin types may be affected by AV.1,18-20 Earlier age of onset of AV has been suggested in white women; however, earlier onset of adrenarche may be more frequent in black girls, which supports an earlier age of onset of AV in this subpopulation.15-17 Women with skin of color usually express greater concern with persistent dyschromia at sites where lesions have resolved, and presence of acne scars is a concern among women regardless of skin color, ethnicity, or race.18,20-22
Scarring
Acne scarring has been noted to affect up to three-fourths of adult women in one report17 and often is stated by patients to be a cause of concern and frustration.1,5,17
Perimenstrual Flaring
Flaring associated with menses is commonly reported in adult females with AV, with 56%, 17%, and 3% of women in one study (n=230) reporting worsening before, during, or after menses, respectively.21
External Factors
Comedogenic products used for skin care, cover-up makeup, or hair care may be important to consider in selected cases as potential etiologic or exacerbating factors in adult females with AV; they also may be used in the management of AV.23-25 Adult females often are perplexed and frustrated by the presence of AV after their teenaged years and anxiously wonder about or search for the potential causes. Many women use cosmetic products to cover up facial AV.5,23-25 Therefore, even if skin care or personal hygiene products or makeup are not believed to be an etiologic factor, many patients appreciate that their dermatologist addressed skin care and cosmetics as a component of AV management and provided appropriate recommendations.5,13
Ingestion of dietary supplements containing whey protein have been associated with precipitation of AV.26,27 Diets with specific content characteristics have been implicated as potential etiologic or exacerbating factors for AV; however, data are limited and specific recommendations remain elusive at present. Individual cases may warrant consideration of dietary factors, especially when treatment resistance is noted.28 Importantly, progestin-only contraceptives (ie, injectables, intrauterine devices) also can exacerbate or induce AV.29
Hyperandrogenism
Although most adult females with AV are reported to have normal serum androgen levels when tested, it is important to explore potential signs and symptoms that are suggestive of underlying hyperandrogenism through both the patient’s history and physical examination.9-11,21,29-33 Some investigators have suggested that underlying peripheral hyperandrogenism is the leading cause of AV in adult females, with or without concurrent polycystic ovarian syndrome (PCOS), though it is believed that most women with AV exhibit normal results when undergoing laboratory testing for androgen excess.10,11,21,29,30 Nevertheless, it is important to consider the possibility of underlying causes of androgen excess (Table 2), the most common being PCOS and late-onset congenital adrenal hyperplasia; an androgen-secreting tumor is less common.11,29-33 It is suggested that screening for underlying endocrinopathy should be conducted in women presenting with (1) AV recalcitrant to conventional treatment, (2) sudden emergence of severe AV, (3) concurrent signs/symptoms of androgen excess, and/or (4) AV relapse shortly after isotretinoin therapy.7,11,16,33

Hirsutism and acanthosis nigricans have been reported to be more reliable predictors of hyperandrogenism than androgenic alopecia.21 Although it may be subtle in some cases, acanthosis nigricans is harder to camouflage, so the clinician can usually detect it if a thorough physical examination is performed. However, a patient may not voluntarily report to the clinician and their staff that she has hair removed, so despite a thorough examination, the clinician may not detect hirsutism. Therefore, it is important to inquire directly about the presence of hairs (pigmented terminal vs “peach fuzz” hairs), their anatomic location, and any hair removal practices the patient has used. The absence of androgenic alopecia does not exclude underlying hyperandrogenism; however, its presence, especially in younger women, may serve as a clinical marker for underlying hyperandrogenism.5 Some women may camouflage more subtle alopecia through hairstyling, but obtaining this history usually is not problematic, as most women are distressed by any degree of hair loss.
Laboratory Evaluation—A relatively straightforward approach to the workup of androgen excess includes assessment of serum DHEAS, free testosterone, and total testosterone levels.10,30 Elevation of serum DHEAS levels indicates an adrenal source of androgen production. Elevation of testosterone is associated with excess androgens produced by the ovaries. Modest elevations of DHEAS are most commonly associated with late-onset congenital adrenal hyperplasia that may not have been previously diagnosed. Modest elevation of testosterone is most commonly associated with PCOS, which also can be accompanied by an elevated luteinizing hormone:follicle-stimulating hormone ratio of 2.5:1 to 3:1.10,30 Marked elevations of DHEAS or testosterone can be indicative of adrenal or ovarian tumors, respectively.30
In some cases, a woman might have elevated DHEAS and testosterone levels. A 17-hydroxyprogesterone test can help discriminate between an adrenal or ovarian source of androgen excess in these cases, as elevated 17-hydroxyprogesterone levels indicate that the androgens are coming from the adrenal gland.10,30
It is important that laboratory evaluation be performed when ovulation is not occurring. Blood tests can be drawn just prior to or during menses. It is important that a woman is not taking an oral contraceptive at the time of testing, which can mask an underlying endocrine abnormality.10,11,29,30 Generally, testing can be performed at least 4 to 6 weeks after stopping the oral contraceptive.
Psychosocial Impact
Facial AV exhibits a broad range of adverse psychological and social effects on many adult females.2,5,13,18 It can be associated with depression, anxiety, psychological stress, and suicidal ideation; therefore, thorough screening for these comorbidities may be warranted in some patients.2,18
Conclusion
The epidemiology, clinical presentation, and clinical and laboratory evaluation of AV in adult females was reviewed in part 1 of this 3-part series. It is important for the clinician to assess the clinical presentation, psychosocial effects, and the possibility of underlying causes of androgen excess. In part 2, skin care and topical management of AV in adult females will be discussed.
It was not long ago that acne vulgaris (AV) was commonly considered to be a skin disease that affected teenagers with little attention given to preadolescent and postadolescent AV. This perspective has changed, with more attention being given to AV across a broad range of affected age groups, including preadolescent, adolescent, and postadolescent subgroups.1-5 Earlier onset of adrenarche has led to earlier development of AV in many young girls, with a higher range of dehydroepiandrosterone sulfate (DHEAS) levels observed overall in those with AV as compared to a normal age-matched population.3,4 At the other end of the age spectrum, AV is a common phenomenon in adult females, with at least half of women estimated to exhibit some form of AV.1,2,5-8 Based on a large survey of females and males (N=1013), the prevalence of AV in adult females has been reported to be 50.9%, 35.2%, 26.3%, and 15.3% among women aged 20 to 29 years, 30 to 39 years, 40 to 49 years, and 50 years and older, respectively.2 Acne vulgaris that persists beyond adolescence into adulthood is termed persistent acne, or early-onset acne, and the development of AV in women 25 years and older who have not previously been affected by AV has been termed late-onset acne.6,8,9 Publications on the management of AV in adult women have focused primarily on systemic hormonal therapies; however, topical therapies more recently have received greater attention in this subpopulation9-12 and will be discussed in part 2 of this series. Because data on AV in women are limited primarily to involvement of the face and neck region, this article does not address truncal AV unless otherwise specified. Table 1 depicts factors that can influence the management of AV in adult women.

Visible Patterns and Considerations for Clinical Evaluation
Clinical Patterns
Although epidemiologic and demographic data are limited in the subpopulation of women with AV, it is reported that females account for up to 82% of adults with AV, with approximately 75% presenting with AV that is clinically similar to their disease course in adolescence.2,5,13 Among those women with persistent AV, some state that their AV is worse compared to adolescence, while others report it is not as severe. The pattern of AV often is similar to that seen in adolescence, presenting as mixed comedonal and inflammatory papular/pustular lesions diffusely distributed on the face; in other cases, a more selectively distributed U-shaped pattern is noted, characterized predominantly by inflammatory papules and/or nodules involving the lower cheeks and jawline margin, with lesions also commonly noted on the anterior and lateral neck.5,8,9,13-16 A U-shaped pattern is believed to be more common in late-onset AV, often with persistence into the mid-40s.1,15,17 It is important to emphasize the need for additional studies on the demographics and clinical characteristics of AV in adult females, especially correlations between onset, age, and clinical patterns of AV.
An international, prospective, observational study assessed the clinical characteristics of AV in adults (aged ≥25 years) at a dermatology visit for acne (N=374).16 Participants who were under management for their AV showed severity grades of mild (clear/almost clear) in 47.3% of cases. Involvement of multiple facial sites—cheeks, forehead, mandibular region, and temples—was noted in 89.8% of women, often with both inflammatory and comedonal lesions, which is a pattern similar to adolescent AV. Inflammatory lesions alone were observed in 6.4% of women, 17.1% had comedonal AV only, and truncal AV was present in 48.4%.16 Additional well-designed studies are needed to determine if this study reflects an accurate qualitative and quantitative depiction of the spectrum of AV in adult females.
Mandibular Pattern
In the observational study of AV in adults, AV localized to the mandibular area was noted in only 11.2% of participants.16 Women with localized mandibular AV were more likely than women without localized AV to be employed, noted greater daily stress levels, and tended to report more psychologically stressful jobs. Interestingly, the subgroup with mandibular acne alone was much less likely to exhibit a global severity grade of moderate or higher (7.1% vs 50.1%), truncal acne (19.0% vs 51.9%), postinflammatory hyperpigmentation (23.8% vs 51.9%), and erythema (19.0% vs 48.4%), suggesting a unique subset of AV presentation.16
Ethnicity/Skin Color
Women of all ethnicities and skin types may be affected by AV.1,18-20 Earlier age of onset of AV has been suggested in white women; however, earlier onset of adrenarche may be more frequent in black girls, which supports an earlier age of onset of AV in this subpopulation.15-17 Women with skin of color usually express greater concern with persistent dyschromia at sites where lesions have resolved, and presence of acne scars is a concern among women regardless of skin color, ethnicity, or race.18,20-22
Scarring
Acne scarring has been noted to affect up to three-fourths of adult women in one report17 and often is stated by patients to be a cause of concern and frustration.1,5,17
Perimenstrual Flaring
Flaring associated with menses is commonly reported in adult females with AV, with 56%, 17%, and 3% of women in one study (n=230) reporting worsening before, during, or after menses, respectively.21
External Factors
Comedogenic products used for skin care, cover-up makeup, or hair care may be important to consider in selected cases as potential etiologic or exacerbating factors in adult females with AV; they also may be used in the management of AV.23-25 Adult females often are perplexed and frustrated by the presence of AV after their teenaged years and anxiously wonder about or search for the potential causes. Many women use cosmetic products to cover up facial AV.5,23-25 Therefore, even if skin care or personal hygiene products or makeup are not believed to be an etiologic factor, many patients appreciate that their dermatologist addressed skin care and cosmetics as a component of AV management and provided appropriate recommendations.5,13
Ingestion of dietary supplements containing whey protein have been associated with precipitation of AV.26,27 Diets with specific content characteristics have been implicated as potential etiologic or exacerbating factors for AV; however, data are limited and specific recommendations remain elusive at present. Individual cases may warrant consideration of dietary factors, especially when treatment resistance is noted.28 Importantly, progestin-only contraceptives (ie, injectables, intrauterine devices) also can exacerbate or induce AV.29
Hyperandrogenism
Although most adult females with AV are reported to have normal serum androgen levels when tested, it is important to explore potential signs and symptoms that are suggestive of underlying hyperandrogenism through both the patient’s history and physical examination.9-11,21,29-33 Some investigators have suggested that underlying peripheral hyperandrogenism is the leading cause of AV in adult females, with or without concurrent polycystic ovarian syndrome (PCOS), though it is believed that most women with AV exhibit normal results when undergoing laboratory testing for androgen excess.10,11,21,29,30 Nevertheless, it is important to consider the possibility of underlying causes of androgen excess (Table 2), the most common being PCOS and late-onset congenital adrenal hyperplasia; an androgen-secreting tumor is less common.11,29-33 It is suggested that screening for underlying endocrinopathy should be conducted in women presenting with (1) AV recalcitrant to conventional treatment, (2) sudden emergence of severe AV, (3) concurrent signs/symptoms of androgen excess, and/or (4) AV relapse shortly after isotretinoin therapy.7,11,16,33

Hirsutism and acanthosis nigricans have been reported to be more reliable predictors of hyperandrogenism than androgenic alopecia.21 Although it may be subtle in some cases, acanthosis nigricans is harder to camouflage, so the clinician can usually detect it if a thorough physical examination is performed. However, a patient may not voluntarily report to the clinician and their staff that she has hair removed, so despite a thorough examination, the clinician may not detect hirsutism. Therefore, it is important to inquire directly about the presence of hairs (pigmented terminal vs “peach fuzz” hairs), their anatomic location, and any hair removal practices the patient has used. The absence of androgenic alopecia does not exclude underlying hyperandrogenism; however, its presence, especially in younger women, may serve as a clinical marker for underlying hyperandrogenism.5 Some women may camouflage more subtle alopecia through hairstyling, but obtaining this history usually is not problematic, as most women are distressed by any degree of hair loss.
Laboratory Evaluation—A relatively straightforward approach to the workup of androgen excess includes assessment of serum DHEAS, free testosterone, and total testosterone levels.10,30 Elevation of serum DHEAS levels indicates an adrenal source of androgen production. Elevation of testosterone is associated with excess androgens produced by the ovaries. Modest elevations of DHEAS are most commonly associated with late-onset congenital adrenal hyperplasia that may not have been previously diagnosed. Modest elevation of testosterone is most commonly associated with PCOS, which also can be accompanied by an elevated luteinizing hormone:follicle-stimulating hormone ratio of 2.5:1 to 3:1.10,30 Marked elevations of DHEAS or testosterone can be indicative of adrenal or ovarian tumors, respectively.30
In some cases, a woman might have elevated DHEAS and testosterone levels. A 17-hydroxyprogesterone test can help discriminate between an adrenal or ovarian source of androgen excess in these cases, as elevated 17-hydroxyprogesterone levels indicate that the androgens are coming from the adrenal gland.10,30
It is important that laboratory evaluation be performed when ovulation is not occurring. Blood tests can be drawn just prior to or during menses. It is important that a woman is not taking an oral contraceptive at the time of testing, which can mask an underlying endocrine abnormality.10,11,29,30 Generally, testing can be performed at least 4 to 6 weeks after stopping the oral contraceptive.
Psychosocial Impact
Facial AV exhibits a broad range of adverse psychological and social effects on many adult females.2,5,13,18 It can be associated with depression, anxiety, psychological stress, and suicidal ideation; therefore, thorough screening for these comorbidities may be warranted in some patients.2,18
Conclusion
The epidemiology, clinical presentation, and clinical and laboratory evaluation of AV in adult females was reviewed in part 1 of this 3-part series. It is important for the clinician to assess the clinical presentation, psychosocial effects, and the possibility of underlying causes of androgen excess. In part 2, skin care and topical management of AV in adult females will be discussed.
1. Perkins AC, Maglione J, Hillebrand GG, et al. Acne vulgaris in women: prevalence across the life span. J Womens Health (Larchmt). 2012;21: 223-230.
2. Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-59.
3. Lucky AW, Biro FM, Huster GA, et al. Acne vulgaris in premenarchal girls. an early sign of puberty associated with rising levels of dehydroepiandrosterone. Arch Dermatol. 1994;130:308-314.
4. Mancini AJ, Baldwin HE, Eichenfield LF, et al. Acne life cycle: the spectrum of pediatric disease. Semin Cutan Med Surg. 2011;30(suppl 3):S2-S5.
5. Tanghetti EA, Kawata AK, Daniels SR, et al. Understanding the burden of adult female acne. J Clin Aesthet Dermatol. 2014;7:22-30.
6. Goulden V, Stables GI, Cunliffe WJ. Prevalence of facial acne in adults. J Am Acad Dermatol. 1999;41: 577-580.
7. Marks R. Acne and its management beyond the age of 35 years. Am J Clin Dermatol. 2004;5:459-462.
8. Preneau S, Dreno B. Female acne—a different subtype of teenager acne? J Eur Acad Dermatol Venereol. 2012;26:277-282.
9. Kim GK, Del Rosso JQ. Oral spironolactone in post-teenage female patients with acne vulgaris: practical considerations for the clinician based on current data and clinical experience. J Clin Aesthet Dermatol. 2012;5:37-50.
10. Thiboutot D, Chen W. Update and future of hormonal therapy in acne. Dermatology. 2003;206:57-67.
11. Villasenor J, Berson D, Kroshinsky D. Treatment guidelines in adult women. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:198-207.
12. Del Rosso JQ, Zeichner J. What’s new in the medicine cabinet? a panoramic review of clinically relevant information for the busy dermatologist. J Clin Aesthet Dermatol. 2014;7:26-30.
13. Del Rosso JQ, Kircik L, Gallagher CJ. Comparative efficacy and tolerability of dapsone 5% gel in adult versus adolescent females with acne vulgaris. J Clin Aesthet Dermatol. 2015;8:31-37.
14. Dreno B, Layton A, Zouboulis CC, et al. Adult female acne: a new paradigm. J Eur Acad Dermatol Venereol. 2013;27:1063-1070.
15. Choi CW, Lee DH, Kim HS, et al. The clinical features of late onset acne compared with early onset acne in women. J Eur Acad Dermatol Venereol. 2011;25:454-461.
16. Dréno B, Thiboutot D, Layton AM, et al; Global Alliance to Improve Outcomes in Acne. Large-scale international study enhances understanding of an emerging acne population: adult females. J Eur Acad Dermatol Venereol. 2015;29:1096-1106.
17. Kane A, Niang SO, Diagne AC, et al. Epidemiologic, clinical, and therapeutic features of acne in Dakar, Senegal. Int J Dermatol. 2007;46(suppl 1):36-38.
18. Callender VD, Alexis AF, Daniels SR, et al. Racial differences in clinical characteristics, perceptions and behaviors, and psychosocial impact of adult female acne. J Clin Aesthet Dermatol. 2014;7:19-31.
19. Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
20. Rendon MI, Rodriguez DA, Kawata AK, et al. Acne treatment patterns, expectations, and satisfaction among adult females of different races/ethnicities. Clin Cosmet Investig Dermatol. 2015;8:231-238.
21. Khunger N, Kumar C. A clinico-epidemiological study of adult acne: is it different from adolescent acne? Indian J Dermatol Venereol Leprol. 2012;78: 335-341.
22. Alexis AF. Acne vulgaris in skin of color: understanding nuances and optimizing treatment outcomes. J Drugs Dermatol. 2014;13(suppl 6):S61-S65.
23. Dall’oglio F, Tedeschi A, Fabbrocini G, et al. Cosmetics for acne: indications and recommendations for an evidence-based approach. G Ital Dermatol Venereol. 2015;150:1-11.
24. Draelos Z. Facial cosmetics for acne patients. In: Draelos Z. Cosmetics in Dermatology. 2nd Ed. New York, NY: Churchill Livingstone Inc; 1995:15-28.
25. Cunliffe WJ. Acne. London, United Kingdom: Martin Dunitz Ltd; 1989.
26. Simonart T. Acne and whey protein supplementation among bodybuilders. Dermatology. 2012;225:256-258.
27. Silverberg NB. Whey protein precipitating moderate to severe acne flares in 5 teenaged athletes. Cutis. 2012;90:70-72.
28. Bronsnick T, Murzaku EC, Rao BK. Diet in dermatology: part I. atopic dermatitis, acne, and nonmelanoma skin cancer. J Am Acad Dermatol. 2014;71:1039.
29. Keri J, Berson DS, Thiboutot DM. Hormonal treatment of acne in women. In: Shalita AR, Del Rosso J, Webster G, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:146-155.
30. Thiboutot D. Hormones and acne: pathophysiology, clinical evaluation and therapies. Sem Cutan Med Surg. 2001;20:144-153.
31. Borgia F, Cannavò S, Guarneri F, et al. Correlation between endocrinological parameters and acne severity in adult women. Acta Derm Venereol. 2004;84:201-204.
32. Clark CM, Rudolph J, Gerber DA, et al. Dermatologic manifestation of hyperandrogenism: a retrospective chart review. Skinmed. 2014;12:84-88.
33. Zeichner JA. Evaluating and treating the adult female patient with acne. J Drugs Dermatol. 2013;12:1416-1427.
1. Perkins AC, Maglione J, Hillebrand GG, et al. Acne vulgaris in women: prevalence across the life span. J Womens Health (Larchmt). 2012;21: 223-230.
2. Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-59.
3. Lucky AW, Biro FM, Huster GA, et al. Acne vulgaris in premenarchal girls. an early sign of puberty associated with rising levels of dehydroepiandrosterone. Arch Dermatol. 1994;130:308-314.
4. Mancini AJ, Baldwin HE, Eichenfield LF, et al. Acne life cycle: the spectrum of pediatric disease. Semin Cutan Med Surg. 2011;30(suppl 3):S2-S5.
5. Tanghetti EA, Kawata AK, Daniels SR, et al. Understanding the burden of adult female acne. J Clin Aesthet Dermatol. 2014;7:22-30.
6. Goulden V, Stables GI, Cunliffe WJ. Prevalence of facial acne in adults. J Am Acad Dermatol. 1999;41: 577-580.
7. Marks R. Acne and its management beyond the age of 35 years. Am J Clin Dermatol. 2004;5:459-462.
8. Preneau S, Dreno B. Female acne—a different subtype of teenager acne? J Eur Acad Dermatol Venereol. 2012;26:277-282.
9. Kim GK, Del Rosso JQ. Oral spironolactone in post-teenage female patients with acne vulgaris: practical considerations for the clinician based on current data and clinical experience. J Clin Aesthet Dermatol. 2012;5:37-50.
10. Thiboutot D, Chen W. Update and future of hormonal therapy in acne. Dermatology. 2003;206:57-67.
11. Villasenor J, Berson D, Kroshinsky D. Treatment guidelines in adult women. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:198-207.
12. Del Rosso JQ, Zeichner J. What’s new in the medicine cabinet? a panoramic review of clinically relevant information for the busy dermatologist. J Clin Aesthet Dermatol. 2014;7:26-30.
13. Del Rosso JQ, Kircik L, Gallagher CJ. Comparative efficacy and tolerability of dapsone 5% gel in adult versus adolescent females with acne vulgaris. J Clin Aesthet Dermatol. 2015;8:31-37.
14. Dreno B, Layton A, Zouboulis CC, et al. Adult female acne: a new paradigm. J Eur Acad Dermatol Venereol. 2013;27:1063-1070.
15. Choi CW, Lee DH, Kim HS, et al. The clinical features of late onset acne compared with early onset acne in women. J Eur Acad Dermatol Venereol. 2011;25:454-461.
16. Dréno B, Thiboutot D, Layton AM, et al; Global Alliance to Improve Outcomes in Acne. Large-scale international study enhances understanding of an emerging acne population: adult females. J Eur Acad Dermatol Venereol. 2015;29:1096-1106.
17. Kane A, Niang SO, Diagne AC, et al. Epidemiologic, clinical, and therapeutic features of acne in Dakar, Senegal. Int J Dermatol. 2007;46(suppl 1):36-38.
18. Callender VD, Alexis AF, Daniels SR, et al. Racial differences in clinical characteristics, perceptions and behaviors, and psychosocial impact of adult female acne. J Clin Aesthet Dermatol. 2014;7:19-31.
19. Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
20. Rendon MI, Rodriguez DA, Kawata AK, et al. Acne treatment patterns, expectations, and satisfaction among adult females of different races/ethnicities. Clin Cosmet Investig Dermatol. 2015;8:231-238.
21. Khunger N, Kumar C. A clinico-epidemiological study of adult acne: is it different from adolescent acne? Indian J Dermatol Venereol Leprol. 2012;78: 335-341.
22. Alexis AF. Acne vulgaris in skin of color: understanding nuances and optimizing treatment outcomes. J Drugs Dermatol. 2014;13(suppl 6):S61-S65.
23. Dall’oglio F, Tedeschi A, Fabbrocini G, et al. Cosmetics for acne: indications and recommendations for an evidence-based approach. G Ital Dermatol Venereol. 2015;150:1-11.
24. Draelos Z. Facial cosmetics for acne patients. In: Draelos Z. Cosmetics in Dermatology. 2nd Ed. New York, NY: Churchill Livingstone Inc; 1995:15-28.
25. Cunliffe WJ. Acne. London, United Kingdom: Martin Dunitz Ltd; 1989.
26. Simonart T. Acne and whey protein supplementation among bodybuilders. Dermatology. 2012;225:256-258.
27. Silverberg NB. Whey protein precipitating moderate to severe acne flares in 5 teenaged athletes. Cutis. 2012;90:70-72.
28. Bronsnick T, Murzaku EC, Rao BK. Diet in dermatology: part I. atopic dermatitis, acne, and nonmelanoma skin cancer. J Am Acad Dermatol. 2014;71:1039.
29. Keri J, Berson DS, Thiboutot DM. Hormonal treatment of acne in women. In: Shalita AR, Del Rosso J, Webster G, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:146-155.
30. Thiboutot D. Hormones and acne: pathophysiology, clinical evaluation and therapies. Sem Cutan Med Surg. 2001;20:144-153.
31. Borgia F, Cannavò S, Guarneri F, et al. Correlation between endocrinological parameters and acne severity in adult women. Acta Derm Venereol. 2004;84:201-204.
32. Clark CM, Rudolph J, Gerber DA, et al. Dermatologic manifestation of hyperandrogenism: a retrospective chart review. Skinmed. 2014;12:84-88.
33. Zeichner JA. Evaluating and treating the adult female patient with acne. J Drugs Dermatol. 2013;12:1416-1427.
Practice Points
- Acne in adult women is common and may persist beyond the adolescent years or may be late in onset with emergence usually during the early to mid-20s.
- Adult women with acne often are frustrated, as they perceive it as a disorder of teenagers and are perplexed by its presence later in life. They often are distressed by unpredictable flares as well as difficulty with covering lesions and associated dyschromia and scarring.
- Clinical patterns of acne in adult women are mixed inflammatory and comedonal facial acne or a U-shaped pattern of inflammatory lesions involving the lower face and neck.
- Laboratory testing is not considered mandatory in all cases. The clinician is encouraged to carefully evaluate each case and determine if further evaluation to detect a cause of androgen excess is warranted.