User login
Jeff Evans has been editor of Rheumatology News/MDedge Rheumatology and the EULAR Congress News since 2013. He started at Frontline Medical Communications in 2001 and was a reporter for 8 years before serving as editor of Clinical Neurology News and World Neurology, and briefly as editor of GI & Hepatology News. He graduated cum laude from Cornell University (New York) with a BA in biological sciences, concentrating in neurobiology and behavior.
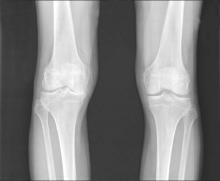
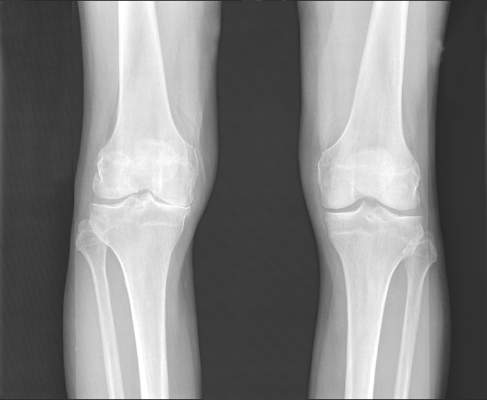
Lateral wedge insoles provide minimal biomechanical help in knee OA
Lateral wedge insoles worn by people with medial knee osteoarthritis (OA) provide a limited amount of immediate biomechanical improvement during walking and may be best suited to people who have biomechanical phenotypes that would benefit the most, according to findings from a systematic review and meta-analysis of studies examining the intraindividual effects of the insoles.
“This review is ... the most definitive, up-to-date and comprehensive analysis on this issue to clarify the effects of lateral wedge insoles on biomechanical risk factors for knee OA progression,” wrote lead investigator John Arnold, Ph.D., of the University of South Australia, Adelaide, and his colleagues (Arthritis Care Res. 2015 Nov 25. doi: 10.1002/acr.22797).
The investigators reviewed 18 studies with a total of 534 participants and found small, but statistically significant reductions in estimates of knee joint loading based on the surrogate measures of external knee adduction moment (EKAM) and the knee adduction angular impulse (KAAI).
Most studies (14) tested full-length insoles, and the remaining four allowed a customized amount based on comfort and/or pain level. Another two used heel wedges, and two others tested both. The inclination angle of the insoles was most commonly 5 degrees, but ranged from 4 to 11 degrees. Some studies used a concomitant medial arch support; these were of a generic design in four studies and were made to order in another three. The lateral wedge insoles were compared against flat insoles, the patients’ own footwear, or standardized footwear.
The pooled effect sizes of both the first and second peak EKAM reductions were small, with standard mean differences of –0.20 to –0.25. For the first EKAM, the effect sizes did not vary according to whether studies used flat insoles or shoes only as comparators, whereas for the eight studies that reported second EKAM outcomes, there was a larger pooled effect size for comparisons against shoe-only than for one study that made flat insole comparisons. The pooled estimate for the standard mean difference in nine studies that reported KAAI was –0.14.
There was only weak evidence for publication bias in all the comparisons for the surrogate measures, and most had a low level of statistical heterogeneity between the outcomes of the studies.
The investigators noted that this meta-analysis of surrogate measures for knee joint loading does not take cumulative loading into account, so that even though the reduction in peak EKAM and KAAI was small, it may amount “to a large cumulative effect imparted on the knee over the course of the day. This should be considered when interpreting the findings of this review and future research on load modifying interventions in knee osteoarthritis.” They said that while EKAM has been associated with OA progression, KAAI has been thought to be a better measure of the duration and magnitude of loading in knee OA and has been associated with medial tibiofemoral cartilage loss over 1-2 years.
“Prescription [for lateral wedge insoles] based on biomechanical response and use of insoles only in individuals who show reductions in knee joint loading (biomechanical phenotypes) appears more appropriate to increase the likelihood of a favorable long-term response regarding the attenuation of structural changes. This would limit their application and benefit to a smaller number of individuals, but is still likely to be significant considering the overall prevalence of knee OA and projected rise due to population aging and rising obesity levels,” the authors concluded.
The investigators had no outside funding source for their systematic review. One of the authors may receive royalties from Salford Insole, a manufacturer of lateral wedge insoles.
Lateral wedge insoles worn by people with medial knee osteoarthritis (OA) provide a limited amount of immediate biomechanical improvement during walking and may be best suited to people who have biomechanical phenotypes that would benefit the most, according to findings from a systematic review and meta-analysis of studies examining the intraindividual effects of the insoles.
“This review is ... the most definitive, up-to-date and comprehensive analysis on this issue to clarify the effects of lateral wedge insoles on biomechanical risk factors for knee OA progression,” wrote lead investigator John Arnold, Ph.D., of the University of South Australia, Adelaide, and his colleagues (Arthritis Care Res. 2015 Nov 25. doi: 10.1002/acr.22797).
The investigators reviewed 18 studies with a total of 534 participants and found small, but statistically significant reductions in estimates of knee joint loading based on the surrogate measures of external knee adduction moment (EKAM) and the knee adduction angular impulse (KAAI).
Most studies (14) tested full-length insoles, and the remaining four allowed a customized amount based on comfort and/or pain level. Another two used heel wedges, and two others tested both. The inclination angle of the insoles was most commonly 5 degrees, but ranged from 4 to 11 degrees. Some studies used a concomitant medial arch support; these were of a generic design in four studies and were made to order in another three. The lateral wedge insoles were compared against flat insoles, the patients’ own footwear, or standardized footwear.
The pooled effect sizes of both the first and second peak EKAM reductions were small, with standard mean differences of –0.20 to –0.25. For the first EKAM, the effect sizes did not vary according to whether studies used flat insoles or shoes only as comparators, whereas for the eight studies that reported second EKAM outcomes, there was a larger pooled effect size for comparisons against shoe-only than for one study that made flat insole comparisons. The pooled estimate for the standard mean difference in nine studies that reported KAAI was –0.14.
There was only weak evidence for publication bias in all the comparisons for the surrogate measures, and most had a low level of statistical heterogeneity between the outcomes of the studies.
The investigators noted that this meta-analysis of surrogate measures for knee joint loading does not take cumulative loading into account, so that even though the reduction in peak EKAM and KAAI was small, it may amount “to a large cumulative effect imparted on the knee over the course of the day. This should be considered when interpreting the findings of this review and future research on load modifying interventions in knee osteoarthritis.” They said that while EKAM has been associated with OA progression, KAAI has been thought to be a better measure of the duration and magnitude of loading in knee OA and has been associated with medial tibiofemoral cartilage loss over 1-2 years.
“Prescription [for lateral wedge insoles] based on biomechanical response and use of insoles only in individuals who show reductions in knee joint loading (biomechanical phenotypes) appears more appropriate to increase the likelihood of a favorable long-term response regarding the attenuation of structural changes. This would limit their application and benefit to a smaller number of individuals, but is still likely to be significant considering the overall prevalence of knee OA and projected rise due to population aging and rising obesity levels,” the authors concluded.
The investigators had no outside funding source for their systematic review. One of the authors may receive royalties from Salford Insole, a manufacturer of lateral wedge insoles.
Lateral wedge insoles worn by people with medial knee osteoarthritis (OA) provide a limited amount of immediate biomechanical improvement during walking and may be best suited to people who have biomechanical phenotypes that would benefit the most, according to findings from a systematic review and meta-analysis of studies examining the intraindividual effects of the insoles.
“This review is ... the most definitive, up-to-date and comprehensive analysis on this issue to clarify the effects of lateral wedge insoles on biomechanical risk factors for knee OA progression,” wrote lead investigator John Arnold, Ph.D., of the University of South Australia, Adelaide, and his colleagues (Arthritis Care Res. 2015 Nov 25. doi: 10.1002/acr.22797).
The investigators reviewed 18 studies with a total of 534 participants and found small, but statistically significant reductions in estimates of knee joint loading based on the surrogate measures of external knee adduction moment (EKAM) and the knee adduction angular impulse (KAAI).
Most studies (14) tested full-length insoles, and the remaining four allowed a customized amount based on comfort and/or pain level. Another two used heel wedges, and two others tested both. The inclination angle of the insoles was most commonly 5 degrees, but ranged from 4 to 11 degrees. Some studies used a concomitant medial arch support; these were of a generic design in four studies and were made to order in another three. The lateral wedge insoles were compared against flat insoles, the patients’ own footwear, or standardized footwear.
The pooled effect sizes of both the first and second peak EKAM reductions were small, with standard mean differences of –0.20 to –0.25. For the first EKAM, the effect sizes did not vary according to whether studies used flat insoles or shoes only as comparators, whereas for the eight studies that reported second EKAM outcomes, there was a larger pooled effect size for comparisons against shoe-only than for one study that made flat insole comparisons. The pooled estimate for the standard mean difference in nine studies that reported KAAI was –0.14.
There was only weak evidence for publication bias in all the comparisons for the surrogate measures, and most had a low level of statistical heterogeneity between the outcomes of the studies.
The investigators noted that this meta-analysis of surrogate measures for knee joint loading does not take cumulative loading into account, so that even though the reduction in peak EKAM and KAAI was small, it may amount “to a large cumulative effect imparted on the knee over the course of the day. This should be considered when interpreting the findings of this review and future research on load modifying interventions in knee osteoarthritis.” They said that while EKAM has been associated with OA progression, KAAI has been thought to be a better measure of the duration and magnitude of loading in knee OA and has been associated with medial tibiofemoral cartilage loss over 1-2 years.
“Prescription [for lateral wedge insoles] based on biomechanical response and use of insoles only in individuals who show reductions in knee joint loading (biomechanical phenotypes) appears more appropriate to increase the likelihood of a favorable long-term response regarding the attenuation of structural changes. This would limit their application and benefit to a smaller number of individuals, but is still likely to be significant considering the overall prevalence of knee OA and projected rise due to population aging and rising obesity levels,” the authors concluded.
The investigators had no outside funding source for their systematic review. One of the authors may receive royalties from Salford Insole, a manufacturer of lateral wedge insoles.
FROM ARTHRITIS CARE & RESEARCH
Key clinical point: Make sure that patients with medial knee OA have an appropriate biomechanical phenotype to use lateral wedge insoles.
Major finding: The pooled effect sizes of both the first and second peak external knee adduction moment reductions were small, with standard mean differences of –0.20 to –0.25.
Data source: A systematic review and meta-analysis of 18 studies involving 534 patients with medial knee OA.
Disclosures: The investigators had no outside funding source for their systematic review. One of the authors may receive royalties from Salford Insole, a manufacturer of lateral wedge insoles.
BRCA1 may have an impact in Alzheimer’s disease
Low levels of the BRCA1 protein in brain cells may be associated with dementia caused by Alzheimer’s disease, according to new evidence from the brains of deceased patients with Alzheimer’s disease or mild cognitive impairment, as well as experimental mouse models of the disease.
Elsa Suberbielle, D.V.M., Ph.D., of Gladstone Institute of Neurological Disease, San Francisco, and her colleagues found low levels of BRCA1 protein, a DNA repair enzyme, in the brains of deceased Alzheimer’s and mild cognitive impairment patients, compared with controls, as well as in mouse models of Alzheimer’s. Mutations in BRCA1 are associated with ovarian and breast cancers.
Further experiments in wild-type mice showed that experimental lowering of BRCA1 levels in the dentate gyrus caused neuronal dysfunction and shrinkage, as well as impairments in synaptic plasticity, excessive neuronal excitability, spatial learning and memory deficits, and increased DNA damage. The addition of beta-amyloid oligomers to cultures of neurons also lowered BRCA1 protein levels in the cells. Low levels of BRCA1 did not appear to cause these deficits through increased neuronal apoptosis or loss.
Additional research will be necessary to determine whether BRCA1 mutations that lead to cancer also affect brain function and whether the neuronal dysfunction caused by low BRCA1 levels results from faulty repair of double-stranded DNA breaks or from reductions in other functions performed by BRCA1, the investigators said.
The full paper is published in Nature Communications (2015 Nov 30. doi: 10.1038/ncomms9897).
Low levels of the BRCA1 protein in brain cells may be associated with dementia caused by Alzheimer’s disease, according to new evidence from the brains of deceased patients with Alzheimer’s disease or mild cognitive impairment, as well as experimental mouse models of the disease.
Elsa Suberbielle, D.V.M., Ph.D., of Gladstone Institute of Neurological Disease, San Francisco, and her colleagues found low levels of BRCA1 protein, a DNA repair enzyme, in the brains of deceased Alzheimer’s and mild cognitive impairment patients, compared with controls, as well as in mouse models of Alzheimer’s. Mutations in BRCA1 are associated with ovarian and breast cancers.
Further experiments in wild-type mice showed that experimental lowering of BRCA1 levels in the dentate gyrus caused neuronal dysfunction and shrinkage, as well as impairments in synaptic plasticity, excessive neuronal excitability, spatial learning and memory deficits, and increased DNA damage. The addition of beta-amyloid oligomers to cultures of neurons also lowered BRCA1 protein levels in the cells. Low levels of BRCA1 did not appear to cause these deficits through increased neuronal apoptosis or loss.
Additional research will be necessary to determine whether BRCA1 mutations that lead to cancer also affect brain function and whether the neuronal dysfunction caused by low BRCA1 levels results from faulty repair of double-stranded DNA breaks or from reductions in other functions performed by BRCA1, the investigators said.
The full paper is published in Nature Communications (2015 Nov 30. doi: 10.1038/ncomms9897).
Low levels of the BRCA1 protein in brain cells may be associated with dementia caused by Alzheimer’s disease, according to new evidence from the brains of deceased patients with Alzheimer’s disease or mild cognitive impairment, as well as experimental mouse models of the disease.
Elsa Suberbielle, D.V.M., Ph.D., of Gladstone Institute of Neurological Disease, San Francisco, and her colleagues found low levels of BRCA1 protein, a DNA repair enzyme, in the brains of deceased Alzheimer’s and mild cognitive impairment patients, compared with controls, as well as in mouse models of Alzheimer’s. Mutations in BRCA1 are associated with ovarian and breast cancers.
Further experiments in wild-type mice showed that experimental lowering of BRCA1 levels in the dentate gyrus caused neuronal dysfunction and shrinkage, as well as impairments in synaptic plasticity, excessive neuronal excitability, spatial learning and memory deficits, and increased DNA damage. The addition of beta-amyloid oligomers to cultures of neurons also lowered BRCA1 protein levels in the cells. Low levels of BRCA1 did not appear to cause these deficits through increased neuronal apoptosis or loss.
Additional research will be necessary to determine whether BRCA1 mutations that lead to cancer also affect brain function and whether the neuronal dysfunction caused by low BRCA1 levels results from faulty repair of double-stranded DNA breaks or from reductions in other functions performed by BRCA1, the investigators said.
The full paper is published in Nature Communications (2015 Nov 30. doi: 10.1038/ncomms9897).
FROM NATURE COMMUNICATIONS
State laws, regulatory concerns complicate biosimilars landscape
SAN FRANCISCO – A growing number of states are passing laws that set the parameters for how originator biologics may be replaced with biosimilars, and not all of them are helpful to physicians trying to maintain some knowledge and control over how prescriptions for biologics are dispensed, according to speakers at the annual meeting of the American College of Rheumatology.
These laws – now passed by a total of 19 states and Puerto Rico, including 12 in 2015 – address a requirement built into the Biologics Price Competition and Innovation Act of 2009 (part of the Affordable Care Act) that introduced the term “interchangeability” into the biosimilars approval pathway, stating that an approved biosimilar “may be substituted for the reference product without the intervention of the health care provider who prescribed the reference product.” The law also gives 1 year of exclusive marketing rights to the first biosimilar approved as being interchangeable with the reference product.
Although regulations on how the Food and Drug Administration will deem a biosimilar to be interchangeable have yet to be drafted, it’s important for physicians to play an active role in shaping the laws that address interchangeability, said Dr. J. Eugene Huffstutter, a rheumatologist in private practice in Hixson, Tenn., and clinical assistant professor of medicine at the University of Tennessee, Chattanooga.
Dr. Huffstutter encouraged rheumatologists and other physicians to work with their local state medical associations to contact their state legislators to explain to them the need for strict laws on how biosimilars can be dispensed. He suggested that good state legislation on biosimilars should contain provisions stating that:
• Only FDA-approved interchangeable biosimilars can be substituted.
• No substitution can be made with “dispense as written” prescription.
• The prescribing physician must be notified of substitution within 1-5 days by electronic record or fax.
• The pharmacy must maintain a record of the dispensed drug.
Organizations including the ACR, the Coalition of State Rheumatology Organizations, patient groups, electronic prescribers, state medical societies, and pharmaceutical companies (for the most part) support strong state laws on biosimilars, while the pharmacy lobby and insurance companies have opposed them, Dr. Huffstutter said.
The 19 states with biosimilars laws include California, Colorado, Delaware, Florida, Georgia, Idaho, Illinois, Indiana, Louisiana, Massachusetts, New Jersey, North Carolina, North Dakota, Oregon, Tennessee, Texas, Utah, Virginia, and Washington, along with Puerto Rico. However, the laws in Oregon and Virginia will sunset in 2016. Another seven states have 2015 or current session legislation on biosimilar substitution: Hawaii, Maryland, Michigan, Mississippi, Oklahoma, Pennsylvania, and Vermont. States with bills that failed or were not acted upon at adjournment include Arizona, Arkansas, Nevada, and Rhode Island, according to information provided to Dr. Huffstutter by the Coalition.
State laws may differ on substitution notification, he noted. In Tennessee, the law (H.B. 572) states that notification should occur in “a reasonable period of time” instead of a defined period. “What may be reasonable for one person may not be reasonable for another. I really think 5 days is the outside time, or 5 business days, because if you’re thinking about some of the biosimilars that are coming down the pike, they’ll be given on a weekly basis, so you’ll at least know before [patients] get their second shot what they’re receiving,” Dr. Huffstutter noted.
Idaho is unique in that it impaneled a board of pharmacy regulation on biosimilar substitution rather than a separate law. It states that “A pharmacist may substitute an interchangeable biosimilar product for a prescribed biological product if the biosimilar has been determined by the FDA to be interchangeable and published in the Purple Book; the prescriber does not indicate by any means that the prescribed biological product must be dispensed; and the name of the drug and the manufacturer or the NDC [National Drug Code] number is documented in the patient medical record.”
The situation may become trickier as more biosimilars are approved, noted Dr. Huffstutter, who is a member of the ACR’s Government Affairs Committee and is a liaison to the Committee on Rheumatologic Care. It could potentially be a problem when a patient is taking a biosimilar for a particular originator biologic and then other biosimilars for that originator join the marketplace. A patient could potentially be switched from one biosimilar to another when a prescription is filled if the company marketing the second biosimilar happens to win a competitive bid with an insurance company, he said.
The filgrastim biosimilar called Zarxio is the only biosimilar currently approved in the United States, but the FDA has received two applications for biosimilars for inflammatory diseases: one from Celltrion for infliximab biosimilar Remsima (August 2014) and one from Sandoz for an etanercept biosimilar (October 2015). In addition to two infliximab biosimilars that have been approved in other countries, one etanercept biosimilar that has been approved in South Korea, and one adalimumab biosimilar approved in India, there are many others in development to treat inflammatory diseases as of July 2015, including 12 for adalimumab, 9 for etanercept, 5 for infliximab, 2 for tocilizumab, and 7 for rituximab, according to Dr. Jonathan Kay, professor of medicine and director of clinical research in the division of rheumatology at the University of Massachusetts, Worcester.
Unresolved regulatory concerns
In separate presentations at the meeting, Dr. Kay described many of the nuances that define biosimilars, which go by different terminology in other countries, such as “follow-on biologic” in Japan, “subsequent-entry biological” in Canada, and “similar biological medicinal product” in the European Union. They are defined as a legitimate copy of an off-patent biopharmaceutical that has undergone rigorous analytical comparison and clinical assessment, in comparison to its reference product, and are approved by a regulatory agency according to a specific pathway for biosimilar evaluation. These differ from biomimetics, which are developed as a replica of a biopharmaceutical but have not been developed, assessed, or approved according to regulatory guidelines for biosimilars.
The FDA takes a “totality of the evidence” approach to establish biosimilarity. The biosimilar pathway for approval is different from the originator pathway by relying more on analytical and preclinical studies and less on the clinical pharmacology and clinical trial data to support biosimilarity.
However, the agency has not yet published final guidance for all steps in demonstrating biosimilarity, particularly for clinical pharmacology data. Draft guidance says the biosimilar must be analyzed and assigned to an assessment grade: not similar, similar, highly similar, and highly similar with fingerprintlike similarity.
During production, both originator and biosimilar biopharmaceuticals can have protein-folding variants, misfolding, aggregation, enzymatic cleavage, and degradation that can lead to inactivation of the protein or increased immunogenicity. Even so, most biosimilars are not identical to the originator because of post-translational modifications, but they do not matter as long as they are not clinically meaningful. Even originator products can drift over time because small changes in manufacturing processes can lead to gradual changes in the molecule, Dr. Kay said.
Extrapolation of indications from the originator to a biosimilar is another area of concern, Dr. Kay said. The FDA will consider the extrapolation of data from a clinical trial of a biosimilar conducted in one disease to support approval for additional indications for which the originator product is already licensed, but not across indications with different mechanisms of action, such as between rheumatoid arthritis and non-Hodgkin’s lymphoma for rituximab. But for which inflammatory diseases should a biosimilar be studied to provide adequate information for extrapolation of indications? Dr. Kay asked. This situation brings about questions of whether dermatologists would be comfortable using a biosimilar that has been studied for rheumatoid arthritis to treat psoriasis or gastroenterologists using a drug with data only from psoriatic arthritis to treat Crohn’s disease or ulcerative colitis.
Both the Coalition of State Rheumatology Organizations and the ACR oppose extrapolation of indications from the clinical trial data of a biosimilar in one disease to support approval of additional indications for which the originator product is already licensed, but Dr. Kay thought that “extrapolation of indications makes perfect sense” because it won’t be possible to review a biosimilar for indications not already approved for the originator and it’s natural to extrapolate to already approved indications once the protein is shown to function nearly identically to the originator.
Ideally, the nomenclature system for biosimilars should clearly identify each product to improve pharmacovigilance and to differentiate between products that have not been determined to be interchangeable, Dr. Kay said. In August, the FDA announced draft guidance proposing that all biopharmaceutical products (originator and biosimilars) would have a nonproprietary name that includes a suffix of four lowercase letters that is devoid of meaning. For example, all products that share a core name such as replicamab would be named replicamab-cznm, replicamab-hixf, and so on, Dr. Kay said. Some people have voiced concern that it would be better for names to have some meaning, such as using an abbreviated name for the developing company along with the nonproprietary name of the originator product, but others noted that method could be problematic when companies merge or are acquired by others.
Dr. Huffstutter disclosed relationships with Janssen, UCB, Lilly, Pfizer, Genentech, Bristol-Myers Squibb, and Celgene. Dr. Kay has received grant and research support from many pharmaceutical companies and has served as a consultant or on an advisory board for many pharmaceutical companies, including those developing biosimilars.
SAN FRANCISCO – A growing number of states are passing laws that set the parameters for how originator biologics may be replaced with biosimilars, and not all of them are helpful to physicians trying to maintain some knowledge and control over how prescriptions for biologics are dispensed, according to speakers at the annual meeting of the American College of Rheumatology.
These laws – now passed by a total of 19 states and Puerto Rico, including 12 in 2015 – address a requirement built into the Biologics Price Competition and Innovation Act of 2009 (part of the Affordable Care Act) that introduced the term “interchangeability” into the biosimilars approval pathway, stating that an approved biosimilar “may be substituted for the reference product without the intervention of the health care provider who prescribed the reference product.” The law also gives 1 year of exclusive marketing rights to the first biosimilar approved as being interchangeable with the reference product.
Although regulations on how the Food and Drug Administration will deem a biosimilar to be interchangeable have yet to be drafted, it’s important for physicians to play an active role in shaping the laws that address interchangeability, said Dr. J. Eugene Huffstutter, a rheumatologist in private practice in Hixson, Tenn., and clinical assistant professor of medicine at the University of Tennessee, Chattanooga.
Dr. Huffstutter encouraged rheumatologists and other physicians to work with their local state medical associations to contact their state legislators to explain to them the need for strict laws on how biosimilars can be dispensed. He suggested that good state legislation on biosimilars should contain provisions stating that:
• Only FDA-approved interchangeable biosimilars can be substituted.
• No substitution can be made with “dispense as written” prescription.
• The prescribing physician must be notified of substitution within 1-5 days by electronic record or fax.
• The pharmacy must maintain a record of the dispensed drug.
Organizations including the ACR, the Coalition of State Rheumatology Organizations, patient groups, electronic prescribers, state medical societies, and pharmaceutical companies (for the most part) support strong state laws on biosimilars, while the pharmacy lobby and insurance companies have opposed them, Dr. Huffstutter said.
The 19 states with biosimilars laws include California, Colorado, Delaware, Florida, Georgia, Idaho, Illinois, Indiana, Louisiana, Massachusetts, New Jersey, North Carolina, North Dakota, Oregon, Tennessee, Texas, Utah, Virginia, and Washington, along with Puerto Rico. However, the laws in Oregon and Virginia will sunset in 2016. Another seven states have 2015 or current session legislation on biosimilar substitution: Hawaii, Maryland, Michigan, Mississippi, Oklahoma, Pennsylvania, and Vermont. States with bills that failed or were not acted upon at adjournment include Arizona, Arkansas, Nevada, and Rhode Island, according to information provided to Dr. Huffstutter by the Coalition.
State laws may differ on substitution notification, he noted. In Tennessee, the law (H.B. 572) states that notification should occur in “a reasonable period of time” instead of a defined period. “What may be reasonable for one person may not be reasonable for another. I really think 5 days is the outside time, or 5 business days, because if you’re thinking about some of the biosimilars that are coming down the pike, they’ll be given on a weekly basis, so you’ll at least know before [patients] get their second shot what they’re receiving,” Dr. Huffstutter noted.
Idaho is unique in that it impaneled a board of pharmacy regulation on biosimilar substitution rather than a separate law. It states that “A pharmacist may substitute an interchangeable biosimilar product for a prescribed biological product if the biosimilar has been determined by the FDA to be interchangeable and published in the Purple Book; the prescriber does not indicate by any means that the prescribed biological product must be dispensed; and the name of the drug and the manufacturer or the NDC [National Drug Code] number is documented in the patient medical record.”
The situation may become trickier as more biosimilars are approved, noted Dr. Huffstutter, who is a member of the ACR’s Government Affairs Committee and is a liaison to the Committee on Rheumatologic Care. It could potentially be a problem when a patient is taking a biosimilar for a particular originator biologic and then other biosimilars for that originator join the marketplace. A patient could potentially be switched from one biosimilar to another when a prescription is filled if the company marketing the second biosimilar happens to win a competitive bid with an insurance company, he said.
The filgrastim biosimilar called Zarxio is the only biosimilar currently approved in the United States, but the FDA has received two applications for biosimilars for inflammatory diseases: one from Celltrion for infliximab biosimilar Remsima (August 2014) and one from Sandoz for an etanercept biosimilar (October 2015). In addition to two infliximab biosimilars that have been approved in other countries, one etanercept biosimilar that has been approved in South Korea, and one adalimumab biosimilar approved in India, there are many others in development to treat inflammatory diseases as of July 2015, including 12 for adalimumab, 9 for etanercept, 5 for infliximab, 2 for tocilizumab, and 7 for rituximab, according to Dr. Jonathan Kay, professor of medicine and director of clinical research in the division of rheumatology at the University of Massachusetts, Worcester.
Unresolved regulatory concerns
In separate presentations at the meeting, Dr. Kay described many of the nuances that define biosimilars, which go by different terminology in other countries, such as “follow-on biologic” in Japan, “subsequent-entry biological” in Canada, and “similar biological medicinal product” in the European Union. They are defined as a legitimate copy of an off-patent biopharmaceutical that has undergone rigorous analytical comparison and clinical assessment, in comparison to its reference product, and are approved by a regulatory agency according to a specific pathway for biosimilar evaluation. These differ from biomimetics, which are developed as a replica of a biopharmaceutical but have not been developed, assessed, or approved according to regulatory guidelines for biosimilars.
The FDA takes a “totality of the evidence” approach to establish biosimilarity. The biosimilar pathway for approval is different from the originator pathway by relying more on analytical and preclinical studies and less on the clinical pharmacology and clinical trial data to support biosimilarity.
However, the agency has not yet published final guidance for all steps in demonstrating biosimilarity, particularly for clinical pharmacology data. Draft guidance says the biosimilar must be analyzed and assigned to an assessment grade: not similar, similar, highly similar, and highly similar with fingerprintlike similarity.
During production, both originator and biosimilar biopharmaceuticals can have protein-folding variants, misfolding, aggregation, enzymatic cleavage, and degradation that can lead to inactivation of the protein or increased immunogenicity. Even so, most biosimilars are not identical to the originator because of post-translational modifications, but they do not matter as long as they are not clinically meaningful. Even originator products can drift over time because small changes in manufacturing processes can lead to gradual changes in the molecule, Dr. Kay said.
Extrapolation of indications from the originator to a biosimilar is another area of concern, Dr. Kay said. The FDA will consider the extrapolation of data from a clinical trial of a biosimilar conducted in one disease to support approval for additional indications for which the originator product is already licensed, but not across indications with different mechanisms of action, such as between rheumatoid arthritis and non-Hodgkin’s lymphoma for rituximab. But for which inflammatory diseases should a biosimilar be studied to provide adequate information for extrapolation of indications? Dr. Kay asked. This situation brings about questions of whether dermatologists would be comfortable using a biosimilar that has been studied for rheumatoid arthritis to treat psoriasis or gastroenterologists using a drug with data only from psoriatic arthritis to treat Crohn’s disease or ulcerative colitis.
Both the Coalition of State Rheumatology Organizations and the ACR oppose extrapolation of indications from the clinical trial data of a biosimilar in one disease to support approval of additional indications for which the originator product is already licensed, but Dr. Kay thought that “extrapolation of indications makes perfect sense” because it won’t be possible to review a biosimilar for indications not already approved for the originator and it’s natural to extrapolate to already approved indications once the protein is shown to function nearly identically to the originator.
Ideally, the nomenclature system for biosimilars should clearly identify each product to improve pharmacovigilance and to differentiate between products that have not been determined to be interchangeable, Dr. Kay said. In August, the FDA announced draft guidance proposing that all biopharmaceutical products (originator and biosimilars) would have a nonproprietary name that includes a suffix of four lowercase letters that is devoid of meaning. For example, all products that share a core name such as replicamab would be named replicamab-cznm, replicamab-hixf, and so on, Dr. Kay said. Some people have voiced concern that it would be better for names to have some meaning, such as using an abbreviated name for the developing company along with the nonproprietary name of the originator product, but others noted that method could be problematic when companies merge or are acquired by others.
Dr. Huffstutter disclosed relationships with Janssen, UCB, Lilly, Pfizer, Genentech, Bristol-Myers Squibb, and Celgene. Dr. Kay has received grant and research support from many pharmaceutical companies and has served as a consultant or on an advisory board for many pharmaceutical companies, including those developing biosimilars.
SAN FRANCISCO – A growing number of states are passing laws that set the parameters for how originator biologics may be replaced with biosimilars, and not all of them are helpful to physicians trying to maintain some knowledge and control over how prescriptions for biologics are dispensed, according to speakers at the annual meeting of the American College of Rheumatology.
These laws – now passed by a total of 19 states and Puerto Rico, including 12 in 2015 – address a requirement built into the Biologics Price Competition and Innovation Act of 2009 (part of the Affordable Care Act) that introduced the term “interchangeability” into the biosimilars approval pathway, stating that an approved biosimilar “may be substituted for the reference product without the intervention of the health care provider who prescribed the reference product.” The law also gives 1 year of exclusive marketing rights to the first biosimilar approved as being interchangeable with the reference product.
Although regulations on how the Food and Drug Administration will deem a biosimilar to be interchangeable have yet to be drafted, it’s important for physicians to play an active role in shaping the laws that address interchangeability, said Dr. J. Eugene Huffstutter, a rheumatologist in private practice in Hixson, Tenn., and clinical assistant professor of medicine at the University of Tennessee, Chattanooga.
Dr. Huffstutter encouraged rheumatologists and other physicians to work with their local state medical associations to contact their state legislators to explain to them the need for strict laws on how biosimilars can be dispensed. He suggested that good state legislation on biosimilars should contain provisions stating that:
• Only FDA-approved interchangeable biosimilars can be substituted.
• No substitution can be made with “dispense as written” prescription.
• The prescribing physician must be notified of substitution within 1-5 days by electronic record or fax.
• The pharmacy must maintain a record of the dispensed drug.
Organizations including the ACR, the Coalition of State Rheumatology Organizations, patient groups, electronic prescribers, state medical societies, and pharmaceutical companies (for the most part) support strong state laws on biosimilars, while the pharmacy lobby and insurance companies have opposed them, Dr. Huffstutter said.
The 19 states with biosimilars laws include California, Colorado, Delaware, Florida, Georgia, Idaho, Illinois, Indiana, Louisiana, Massachusetts, New Jersey, North Carolina, North Dakota, Oregon, Tennessee, Texas, Utah, Virginia, and Washington, along with Puerto Rico. However, the laws in Oregon and Virginia will sunset in 2016. Another seven states have 2015 or current session legislation on biosimilar substitution: Hawaii, Maryland, Michigan, Mississippi, Oklahoma, Pennsylvania, and Vermont. States with bills that failed or were not acted upon at adjournment include Arizona, Arkansas, Nevada, and Rhode Island, according to information provided to Dr. Huffstutter by the Coalition.
State laws may differ on substitution notification, he noted. In Tennessee, the law (H.B. 572) states that notification should occur in “a reasonable period of time” instead of a defined period. “What may be reasonable for one person may not be reasonable for another. I really think 5 days is the outside time, or 5 business days, because if you’re thinking about some of the biosimilars that are coming down the pike, they’ll be given on a weekly basis, so you’ll at least know before [patients] get their second shot what they’re receiving,” Dr. Huffstutter noted.
Idaho is unique in that it impaneled a board of pharmacy regulation on biosimilar substitution rather than a separate law. It states that “A pharmacist may substitute an interchangeable biosimilar product for a prescribed biological product if the biosimilar has been determined by the FDA to be interchangeable and published in the Purple Book; the prescriber does not indicate by any means that the prescribed biological product must be dispensed; and the name of the drug and the manufacturer or the NDC [National Drug Code] number is documented in the patient medical record.”
The situation may become trickier as more biosimilars are approved, noted Dr. Huffstutter, who is a member of the ACR’s Government Affairs Committee and is a liaison to the Committee on Rheumatologic Care. It could potentially be a problem when a patient is taking a biosimilar for a particular originator biologic and then other biosimilars for that originator join the marketplace. A patient could potentially be switched from one biosimilar to another when a prescription is filled if the company marketing the second biosimilar happens to win a competitive bid with an insurance company, he said.
The filgrastim biosimilar called Zarxio is the only biosimilar currently approved in the United States, but the FDA has received two applications for biosimilars for inflammatory diseases: one from Celltrion for infliximab biosimilar Remsima (August 2014) and one from Sandoz for an etanercept biosimilar (October 2015). In addition to two infliximab biosimilars that have been approved in other countries, one etanercept biosimilar that has been approved in South Korea, and one adalimumab biosimilar approved in India, there are many others in development to treat inflammatory diseases as of July 2015, including 12 for adalimumab, 9 for etanercept, 5 for infliximab, 2 for tocilizumab, and 7 for rituximab, according to Dr. Jonathan Kay, professor of medicine and director of clinical research in the division of rheumatology at the University of Massachusetts, Worcester.
Unresolved regulatory concerns
In separate presentations at the meeting, Dr. Kay described many of the nuances that define biosimilars, which go by different terminology in other countries, such as “follow-on biologic” in Japan, “subsequent-entry biological” in Canada, and “similar biological medicinal product” in the European Union. They are defined as a legitimate copy of an off-patent biopharmaceutical that has undergone rigorous analytical comparison and clinical assessment, in comparison to its reference product, and are approved by a regulatory agency according to a specific pathway for biosimilar evaluation. These differ from biomimetics, which are developed as a replica of a biopharmaceutical but have not been developed, assessed, or approved according to regulatory guidelines for biosimilars.
The FDA takes a “totality of the evidence” approach to establish biosimilarity. The biosimilar pathway for approval is different from the originator pathway by relying more on analytical and preclinical studies and less on the clinical pharmacology and clinical trial data to support biosimilarity.
However, the agency has not yet published final guidance for all steps in demonstrating biosimilarity, particularly for clinical pharmacology data. Draft guidance says the biosimilar must be analyzed and assigned to an assessment grade: not similar, similar, highly similar, and highly similar with fingerprintlike similarity.
During production, both originator and biosimilar biopharmaceuticals can have protein-folding variants, misfolding, aggregation, enzymatic cleavage, and degradation that can lead to inactivation of the protein or increased immunogenicity. Even so, most biosimilars are not identical to the originator because of post-translational modifications, but they do not matter as long as they are not clinically meaningful. Even originator products can drift over time because small changes in manufacturing processes can lead to gradual changes in the molecule, Dr. Kay said.
Extrapolation of indications from the originator to a biosimilar is another area of concern, Dr. Kay said. The FDA will consider the extrapolation of data from a clinical trial of a biosimilar conducted in one disease to support approval for additional indications for which the originator product is already licensed, but not across indications with different mechanisms of action, such as between rheumatoid arthritis and non-Hodgkin’s lymphoma for rituximab. But for which inflammatory diseases should a biosimilar be studied to provide adequate information for extrapolation of indications? Dr. Kay asked. This situation brings about questions of whether dermatologists would be comfortable using a biosimilar that has been studied for rheumatoid arthritis to treat psoriasis or gastroenterologists using a drug with data only from psoriatic arthritis to treat Crohn’s disease or ulcerative colitis.
Both the Coalition of State Rheumatology Organizations and the ACR oppose extrapolation of indications from the clinical trial data of a biosimilar in one disease to support approval of additional indications for which the originator product is already licensed, but Dr. Kay thought that “extrapolation of indications makes perfect sense” because it won’t be possible to review a biosimilar for indications not already approved for the originator and it’s natural to extrapolate to already approved indications once the protein is shown to function nearly identically to the originator.
Ideally, the nomenclature system for biosimilars should clearly identify each product to improve pharmacovigilance and to differentiate between products that have not been determined to be interchangeable, Dr. Kay said. In August, the FDA announced draft guidance proposing that all biopharmaceutical products (originator and biosimilars) would have a nonproprietary name that includes a suffix of four lowercase letters that is devoid of meaning. For example, all products that share a core name such as replicamab would be named replicamab-cznm, replicamab-hixf, and so on, Dr. Kay said. Some people have voiced concern that it would be better for names to have some meaning, such as using an abbreviated name for the developing company along with the nonproprietary name of the originator product, but others noted that method could be problematic when companies merge or are acquired by others.
Dr. Huffstutter disclosed relationships with Janssen, UCB, Lilly, Pfizer, Genentech, Bristol-Myers Squibb, and Celgene. Dr. Kay has received grant and research support from many pharmaceutical companies and has served as a consultant or on an advisory board for many pharmaceutical companies, including those developing biosimilars.
EXPERT ANALYSIS FROM THE ACR ANNUAL MEETING
Pseudoatrophy with interferon beta-1a may warrant MRI delay in MS patients
Treatment of relapsing-remitting multiple sclerosis with interferon beta-1a produces a decline in brain volume over the first 3 months and follows a course consistent with pseudoatrophy that is likely caused by “hydrodynamic changes related to resolution of inflammation, edema, and cellular infiltration rather than the loss of actual brain tissue,” according to Michael G. Dwyer, Ph.D., of the State University of New York at Buffalo, and his coauthors.
The investigators’ open-label trial, which involved 23 relapsing-remitting multiple sclerosis (RRMS) patients given interferon (IFN) beta-1a 44 mcg (Rebif) subcutaneously three times a week, and 15 healthy controls, is the first to study the effect of subcutaneous IFN beta-1a on early changes in brain volume and its relationship to changes in inflammatory markers (BMC Neurol. 2015 Nov 11;15:232. doi:10.1186/s12883-015-0488-9).
Dr. Dwyer and his colleagues did not find a loss in white matter as reported by previous studies investigating pseudoatrophy in the beginning of treatment for RRMS, but instead found that the atrophy was largely driven by gray matter loss. They observed significant mean brain volume loss of –0.95% and gray matter loss of –1.52% in the first 3 months in RRMS patients, compared with controls, which was significantly correlated with decreased pro-inflammatory IL-17 F–expressing CD4-positive or CD8-positive T cells. Change in white matter volume experienced by IFN beta-1a–treated patients, compared with controls, did not differ at 3 months (–0.41%), from 3 to 6 months (0.30%), or from baseline to 6 months (–0.21%). The decrease in mean brain volume, however, slowed to just –0.08% from month 3 to 6, and was –0.91% from baseline to 6 months. Gray matter loss was not significant in RRMS patients from 3 to 6 months (–0.46%), but was significant from baseline to 6 months (–1.66%).
The lack of significant changes in white matter volume may have been affected, the investigators said, by a lack of statistical power to detect its change and the susceptibility of cortical gray matter measurement to “partial volume errors owing to its thin and convoluted nature.”
The findings are “supportive of an early anti-inflammatory therapeutic effect for IFN beta-1a,” the investigators wrote, and also send a message to use caution in applying MRI to measure disease progression via brain atrophy by having patients with RRMS with active disease who are starting on disease-modifying drug therapy obtain a baseline for MRI measures of brain volume “only after several months of therapy, once the pseudoatrophy effect has run its course, rather than at the beginning of therapy.”
EMD Serono and Pfizer sponsored the study. Nine of the 13 authors had financial disclosures with companies that market MS drugs, including EMD Serono and Pfizer.
Treatment of relapsing-remitting multiple sclerosis with interferon beta-1a produces a decline in brain volume over the first 3 months and follows a course consistent with pseudoatrophy that is likely caused by “hydrodynamic changes related to resolution of inflammation, edema, and cellular infiltration rather than the loss of actual brain tissue,” according to Michael G. Dwyer, Ph.D., of the State University of New York at Buffalo, and his coauthors.
The investigators’ open-label trial, which involved 23 relapsing-remitting multiple sclerosis (RRMS) patients given interferon (IFN) beta-1a 44 mcg (Rebif) subcutaneously three times a week, and 15 healthy controls, is the first to study the effect of subcutaneous IFN beta-1a on early changes in brain volume and its relationship to changes in inflammatory markers (BMC Neurol. 2015 Nov 11;15:232. doi:10.1186/s12883-015-0488-9).
Dr. Dwyer and his colleagues did not find a loss in white matter as reported by previous studies investigating pseudoatrophy in the beginning of treatment for RRMS, but instead found that the atrophy was largely driven by gray matter loss. They observed significant mean brain volume loss of –0.95% and gray matter loss of –1.52% in the first 3 months in RRMS patients, compared with controls, which was significantly correlated with decreased pro-inflammatory IL-17 F–expressing CD4-positive or CD8-positive T cells. Change in white matter volume experienced by IFN beta-1a–treated patients, compared with controls, did not differ at 3 months (–0.41%), from 3 to 6 months (0.30%), or from baseline to 6 months (–0.21%). The decrease in mean brain volume, however, slowed to just –0.08% from month 3 to 6, and was –0.91% from baseline to 6 months. Gray matter loss was not significant in RRMS patients from 3 to 6 months (–0.46%), but was significant from baseline to 6 months (–1.66%).
The lack of significant changes in white matter volume may have been affected, the investigators said, by a lack of statistical power to detect its change and the susceptibility of cortical gray matter measurement to “partial volume errors owing to its thin and convoluted nature.”
The findings are “supportive of an early anti-inflammatory therapeutic effect for IFN beta-1a,” the investigators wrote, and also send a message to use caution in applying MRI to measure disease progression via brain atrophy by having patients with RRMS with active disease who are starting on disease-modifying drug therapy obtain a baseline for MRI measures of brain volume “only after several months of therapy, once the pseudoatrophy effect has run its course, rather than at the beginning of therapy.”
EMD Serono and Pfizer sponsored the study. Nine of the 13 authors had financial disclosures with companies that market MS drugs, including EMD Serono and Pfizer.
Treatment of relapsing-remitting multiple sclerosis with interferon beta-1a produces a decline in brain volume over the first 3 months and follows a course consistent with pseudoatrophy that is likely caused by “hydrodynamic changes related to resolution of inflammation, edema, and cellular infiltration rather than the loss of actual brain tissue,” according to Michael G. Dwyer, Ph.D., of the State University of New York at Buffalo, and his coauthors.
The investigators’ open-label trial, which involved 23 relapsing-remitting multiple sclerosis (RRMS) patients given interferon (IFN) beta-1a 44 mcg (Rebif) subcutaneously three times a week, and 15 healthy controls, is the first to study the effect of subcutaneous IFN beta-1a on early changes in brain volume and its relationship to changes in inflammatory markers (BMC Neurol. 2015 Nov 11;15:232. doi:10.1186/s12883-015-0488-9).
Dr. Dwyer and his colleagues did not find a loss in white matter as reported by previous studies investigating pseudoatrophy in the beginning of treatment for RRMS, but instead found that the atrophy was largely driven by gray matter loss. They observed significant mean brain volume loss of –0.95% and gray matter loss of –1.52% in the first 3 months in RRMS patients, compared with controls, which was significantly correlated with decreased pro-inflammatory IL-17 F–expressing CD4-positive or CD8-positive T cells. Change in white matter volume experienced by IFN beta-1a–treated patients, compared with controls, did not differ at 3 months (–0.41%), from 3 to 6 months (0.30%), or from baseline to 6 months (–0.21%). The decrease in mean brain volume, however, slowed to just –0.08% from month 3 to 6, and was –0.91% from baseline to 6 months. Gray matter loss was not significant in RRMS patients from 3 to 6 months (–0.46%), but was significant from baseline to 6 months (–1.66%).
The lack of significant changes in white matter volume may have been affected, the investigators said, by a lack of statistical power to detect its change and the susceptibility of cortical gray matter measurement to “partial volume errors owing to its thin and convoluted nature.”
The findings are “supportive of an early anti-inflammatory therapeutic effect for IFN beta-1a,” the investigators wrote, and also send a message to use caution in applying MRI to measure disease progression via brain atrophy by having patients with RRMS with active disease who are starting on disease-modifying drug therapy obtain a baseline for MRI measures of brain volume “only after several months of therapy, once the pseudoatrophy effect has run its course, rather than at the beginning of therapy.”
EMD Serono and Pfizer sponsored the study. Nine of the 13 authors had financial disclosures with companies that market MS drugs, including EMD Serono and Pfizer.
FROM BMC NEUROLOGY
Key clinical point: Initial treatment of patients with relapsing-remitting multiple sclerosis with subcutaneous interferon beta-1a may cause a pseudoatrophy of the brain over the first 3 months of treatment that may be related to the resolution of inflammation.
Major finding: RRMS patients had a significant mean brain volume loss of –0.95% and gray matter loss of –1.52% in the first 3 months, compared with controls, but losses were no longer significant during 3-6 months.
Data source: An open-label study of 23 RRMS patients given interferon (IFN) beta-1a 44 mcg (Rebif) subcutaneously three times a week, and 15 healthy controls.
Disclosures: EMD Serono and Pfizer sponsored the study. Nine of the 13 authors had financial disclosures with companies that market MS drugs, including EMD Serono and Pfizer.
VIDEO: Trial points to using non-TNF biologic when first anti-TNF drug fails
SAN FRANCISCO – Rheumatoid arthritis patients who did not respond to the first tumor necrosis factor inhibitor they tried had a higher rate of good or moderate response when they switched to a non–TNF inhibitor biologic, rather than another anti-TNF agent, after 48 weeks in the first randomized trial to compare the two strategies.
The results of this French open-label study, called Rotation or Change, provides evidence to guide the choice of a second biologic in patients who fail first biologic treatment with an anti-TNF agent. The trial also confirms findings reported in observational registry studies, principal investigator Dr. Jacques-Eric Gottenberg said in an interview at the annual meeting of the American College of Rheumatology.
But in contrast to the main study results, a post hoc analysis suggested that a second TNF inhibitor may be just as effective as a non–TNF inhibitor biologic in patients who develop antidrug antibodies to the first TNF inhibitor, said Dr. Gottenberg of the department of rheumatology at Strasbourg (France) University Hospital.
He and his colleagues aimed to mimic what happens in daily practice by allowing participating rheumatologists at French academic medical centers to choose which drug each patient received after randomization. During 2009-2012, 292 patients were randomized to any of four TNF inhibitors (adalimumab, certolizumab pegol, etanercept, or infliximab) or any of three non–TNF inhibitor biologics (abatacept, rituximab, or tocilizumab). (Golimumab was not available in France when the trial began in 2009.)
At 12 weeks, 48% of participants who switched to another TNF inhibitor had good or moderate responses based on European League Against Rheumatism criteria, and this rose to 52% at 24 weeks and then dropped to 43% at 48 weeks. At those time points, good or moderate response occurred in an additional 16%-18% of participants who switched to a non–TNF inhibitor biologic: 64% at 12 weeks, 70% at 24 weeks, and 60% at 48 weeks.
In the post hoc analysis of 278 patients who were tested for antidrug antibodies, 20 patients with ADAs who had been randomized to a non–TNF targeted biologic had a response at 24 weeks that was similar to 12 patients with ADAs who had been randomized to a second TNF inhibitor.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN FRANCISCO – Rheumatoid arthritis patients who did not respond to the first tumor necrosis factor inhibitor they tried had a higher rate of good or moderate response when they switched to a non–TNF inhibitor biologic, rather than another anti-TNF agent, after 48 weeks in the first randomized trial to compare the two strategies.
The results of this French open-label study, called Rotation or Change, provides evidence to guide the choice of a second biologic in patients who fail first biologic treatment with an anti-TNF agent. The trial also confirms findings reported in observational registry studies, principal investigator Dr. Jacques-Eric Gottenberg said in an interview at the annual meeting of the American College of Rheumatology.
But in contrast to the main study results, a post hoc analysis suggested that a second TNF inhibitor may be just as effective as a non–TNF inhibitor biologic in patients who develop antidrug antibodies to the first TNF inhibitor, said Dr. Gottenberg of the department of rheumatology at Strasbourg (France) University Hospital.
He and his colleagues aimed to mimic what happens in daily practice by allowing participating rheumatologists at French academic medical centers to choose which drug each patient received after randomization. During 2009-2012, 292 patients were randomized to any of four TNF inhibitors (adalimumab, certolizumab pegol, etanercept, or infliximab) or any of three non–TNF inhibitor biologics (abatacept, rituximab, or tocilizumab). (Golimumab was not available in France when the trial began in 2009.)
At 12 weeks, 48% of participants who switched to another TNF inhibitor had good or moderate responses based on European League Against Rheumatism criteria, and this rose to 52% at 24 weeks and then dropped to 43% at 48 weeks. At those time points, good or moderate response occurred in an additional 16%-18% of participants who switched to a non–TNF inhibitor biologic: 64% at 12 weeks, 70% at 24 weeks, and 60% at 48 weeks.
In the post hoc analysis of 278 patients who were tested for antidrug antibodies, 20 patients with ADAs who had been randomized to a non–TNF targeted biologic had a response at 24 weeks that was similar to 12 patients with ADAs who had been randomized to a second TNF inhibitor.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN FRANCISCO – Rheumatoid arthritis patients who did not respond to the first tumor necrosis factor inhibitor they tried had a higher rate of good or moderate response when they switched to a non–TNF inhibitor biologic, rather than another anti-TNF agent, after 48 weeks in the first randomized trial to compare the two strategies.
The results of this French open-label study, called Rotation or Change, provides evidence to guide the choice of a second biologic in patients who fail first biologic treatment with an anti-TNF agent. The trial also confirms findings reported in observational registry studies, principal investigator Dr. Jacques-Eric Gottenberg said in an interview at the annual meeting of the American College of Rheumatology.
But in contrast to the main study results, a post hoc analysis suggested that a second TNF inhibitor may be just as effective as a non–TNF inhibitor biologic in patients who develop antidrug antibodies to the first TNF inhibitor, said Dr. Gottenberg of the department of rheumatology at Strasbourg (France) University Hospital.
He and his colleagues aimed to mimic what happens in daily practice by allowing participating rheumatologists at French academic medical centers to choose which drug each patient received after randomization. During 2009-2012, 292 patients were randomized to any of four TNF inhibitors (adalimumab, certolizumab pegol, etanercept, or infliximab) or any of three non–TNF inhibitor biologics (abatacept, rituximab, or tocilizumab). (Golimumab was not available in France when the trial began in 2009.)
At 12 weeks, 48% of participants who switched to another TNF inhibitor had good or moderate responses based on European League Against Rheumatism criteria, and this rose to 52% at 24 weeks and then dropped to 43% at 48 weeks. At those time points, good or moderate response occurred in an additional 16%-18% of participants who switched to a non–TNF inhibitor biologic: 64% at 12 weeks, 70% at 24 weeks, and 60% at 48 weeks.
In the post hoc analysis of 278 patients who were tested for antidrug antibodies, 20 patients with ADAs who had been randomized to a non–TNF targeted biologic had a response at 24 weeks that was similar to 12 patients with ADAs who had been randomized to a second TNF inhibitor.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE ACR ANNUAL MEETING
Whet your appetite: ACR 2015 loads a full plate of pediatric rheumatology
As rheumatologists gather in San Francisco this week for the annual meeting of the American College of Rheumatology Nov. 6-11, those who see pediatric patients will have a chance to sharpen their skills and broaden their knowledge base by attending some of the many sessions geared specifically for them.
Here’s a day-by-day look at highlights of what’s on the menu for pediatric rheumatologists:
Sunday, Nov. 8
8:30-10:00 a.m. Clots in Children: Where They Come From and What to Do With Them. Attendees will learn all about antiphospholipid antibodies and lupus anticoagulants in children; how and when to perform diagnostic imaging of patients with central nervous system vasculopathy; and how to decide if, when, and how to treat these conditions.
2:30-4:00 p.m. Pediatric Rheumatology: Clinical and Therapeutic Aspects I – Juvenile Idiopathic Arthritis. This abstract session on JIA features studies about cancer risk; increased risk of infection and other adverse events when one or more biologics are added to methotrexate; findings from a double-blind, placebo-controlled trial of probiotics on enthesitis related disease; the relevance of the duration of inactive disease when determining when to withdraw methotrexate; results from the Childhood Arthritis and Rheumatology Research Alliance’s Systemic JIA Consensus Treatment Plans pilot study; and the efficacy of canakinumab in patients with systemic JIA previously treated with biologics.
4:30-6:00 p.m. Pediatric Lupus Transitional Care: An Interactive Experience. Three speakers will share the different perspectives of the emerging-adult, young-adult, and adolescent patients on their transition experiences through multimedia.
Monday, Nov. 9
8:30-10:00 a.m. Nuts and Bolts of Macrophage Activation Syndrome. Learn more about recognizing, diagnosing, and treating the condition – even in adult patients – by applying new diagnostic criteria in patients with and without ongoing biologic therapy and knowing when and how to use targeted anticytokine therapy.
2:30-4:00 p.m. Pediatric Rheumatology: Clinical and Therapeutic Aspects II – Pediatric Systemic Lupus Erythematosus. The six studies presented in this abstract session cover a wide range of topics in juvenile SLE, including a systematic classification of the subtypes of pancreatitis in childhood-onset SLE; the sensitivity and specificity of cell-bound complement activation products in the differential diagnosis of childhood-onset lupus; axonal dysfunction’s relationship with neuropsychiatric manifestations and disease activity; risk factors for morbidity and long-term outcome of infants with cardiac neonatal lupus; cross-validating an automated cognitive performance test that screens for neurocognitive problems; and assessing the effects of vitamin D supplementation on bone microarchitecture.
Tuesday, Nov. 10
6:15-7:30 a.m. Childhood Arthritis & Rheumatology Research Alliance (CARRA). The organization is having a membership breakfast meeting at the San Francisco Marriott Marquis.
9:00-10:00 a.m. Pediatric Rheumatology Year in Review and Awards. This session provides a succinct update on what is newest and most important in basic science, translational, and clinical research in pediatric rheumatology in the past year.
11:00 a.m.-12:00 p.m. Controversies in Kawasaki Disease: Implications for Long-Term Outcomes. This session will inform attendees of how progress made in understanding the pathogenesis of Kawasaki disease has assisted researchers in finding novel biomarkers and treatment to improve long-term outcomes.
4:30-6:00 p.m. Pediatric Rheumatology: Clinical and Therapeutic Aspects IV – Imaging and Novel Clinical Interventions. This session contains abstract presentations on new MRI methods for detecting progression of erosion in JIA by focusing on bony depressions and for assessing whole-body disease damage in juvenile dermatomyositis, as well as a brain biopsy study of the histopathology and defining characteristics of patients with childhood primary small vessel CNS vasculitis. Another three studies of JIA patients examine the efficacy of tocilizumab in a small case series of patients with refractory uveitis, a pilot study of mesenchymal stromal cell treatment, how often patients meet the varying clinical definitions of inactive disease or minimal disease activity.
Wednesday, Nov. 11
7:30-8:30 a.m. New Adult and Juvenile Myositis Response Criteria. Learn how to apply the new response criteria in both clinical and research settings in this session. The new criteria use cut points to define minimal, moderate, and major levels of treatment response and are based on absolute changes in the measurements of improvement, rather than relative changes.
9:00-10:30 a.m. Chronic Nonbacterial Osteomyelitis (CNO): From the Bedside to the Bench. Presenters will offer a broad overview of the clinical features and management of this sterile, inflammatory bone disorder of unknown etiology, as well as growing knowledge of the genetics of the disease. Evidence will be presented that suggests the microbiome may play a role in the clinical manifestations of the disease.
As rheumatologists gather in San Francisco this week for the annual meeting of the American College of Rheumatology Nov. 6-11, those who see pediatric patients will have a chance to sharpen their skills and broaden their knowledge base by attending some of the many sessions geared specifically for them.
Here’s a day-by-day look at highlights of what’s on the menu for pediatric rheumatologists:
Sunday, Nov. 8
8:30-10:00 a.m. Clots in Children: Where They Come From and What to Do With Them. Attendees will learn all about antiphospholipid antibodies and lupus anticoagulants in children; how and when to perform diagnostic imaging of patients with central nervous system vasculopathy; and how to decide if, when, and how to treat these conditions.
2:30-4:00 p.m. Pediatric Rheumatology: Clinical and Therapeutic Aspects I – Juvenile Idiopathic Arthritis. This abstract session on JIA features studies about cancer risk; increased risk of infection and other adverse events when one or more biologics are added to methotrexate; findings from a double-blind, placebo-controlled trial of probiotics on enthesitis related disease; the relevance of the duration of inactive disease when determining when to withdraw methotrexate; results from the Childhood Arthritis and Rheumatology Research Alliance’s Systemic JIA Consensus Treatment Plans pilot study; and the efficacy of canakinumab in patients with systemic JIA previously treated with biologics.
4:30-6:00 p.m. Pediatric Lupus Transitional Care: An Interactive Experience. Three speakers will share the different perspectives of the emerging-adult, young-adult, and adolescent patients on their transition experiences through multimedia.
Monday, Nov. 9
8:30-10:00 a.m. Nuts and Bolts of Macrophage Activation Syndrome. Learn more about recognizing, diagnosing, and treating the condition – even in adult patients – by applying new diagnostic criteria in patients with and without ongoing biologic therapy and knowing when and how to use targeted anticytokine therapy.
2:30-4:00 p.m. Pediatric Rheumatology: Clinical and Therapeutic Aspects II – Pediatric Systemic Lupus Erythematosus. The six studies presented in this abstract session cover a wide range of topics in juvenile SLE, including a systematic classification of the subtypes of pancreatitis in childhood-onset SLE; the sensitivity and specificity of cell-bound complement activation products in the differential diagnosis of childhood-onset lupus; axonal dysfunction’s relationship with neuropsychiatric manifestations and disease activity; risk factors for morbidity and long-term outcome of infants with cardiac neonatal lupus; cross-validating an automated cognitive performance test that screens for neurocognitive problems; and assessing the effects of vitamin D supplementation on bone microarchitecture.
Tuesday, Nov. 10
6:15-7:30 a.m. Childhood Arthritis & Rheumatology Research Alliance (CARRA). The organization is having a membership breakfast meeting at the San Francisco Marriott Marquis.
9:00-10:00 a.m. Pediatric Rheumatology Year in Review and Awards. This session provides a succinct update on what is newest and most important in basic science, translational, and clinical research in pediatric rheumatology in the past year.
11:00 a.m.-12:00 p.m. Controversies in Kawasaki Disease: Implications for Long-Term Outcomes. This session will inform attendees of how progress made in understanding the pathogenesis of Kawasaki disease has assisted researchers in finding novel biomarkers and treatment to improve long-term outcomes.
4:30-6:00 p.m. Pediatric Rheumatology: Clinical and Therapeutic Aspects IV – Imaging and Novel Clinical Interventions. This session contains abstract presentations on new MRI methods for detecting progression of erosion in JIA by focusing on bony depressions and for assessing whole-body disease damage in juvenile dermatomyositis, as well as a brain biopsy study of the histopathology and defining characteristics of patients with childhood primary small vessel CNS vasculitis. Another three studies of JIA patients examine the efficacy of tocilizumab in a small case series of patients with refractory uveitis, a pilot study of mesenchymal stromal cell treatment, how often patients meet the varying clinical definitions of inactive disease or minimal disease activity.
Wednesday, Nov. 11
7:30-8:30 a.m. New Adult and Juvenile Myositis Response Criteria. Learn how to apply the new response criteria in both clinical and research settings in this session. The new criteria use cut points to define minimal, moderate, and major levels of treatment response and are based on absolute changes in the measurements of improvement, rather than relative changes.
9:00-10:30 a.m. Chronic Nonbacterial Osteomyelitis (CNO): From the Bedside to the Bench. Presenters will offer a broad overview of the clinical features and management of this sterile, inflammatory bone disorder of unknown etiology, as well as growing knowledge of the genetics of the disease. Evidence will be presented that suggests the microbiome may play a role in the clinical manifestations of the disease.
As rheumatologists gather in San Francisco this week for the annual meeting of the American College of Rheumatology Nov. 6-11, those who see pediatric patients will have a chance to sharpen their skills and broaden their knowledge base by attending some of the many sessions geared specifically for them.
Here’s a day-by-day look at highlights of what’s on the menu for pediatric rheumatologists:
Sunday, Nov. 8
8:30-10:00 a.m. Clots in Children: Where They Come From and What to Do With Them. Attendees will learn all about antiphospholipid antibodies and lupus anticoagulants in children; how and when to perform diagnostic imaging of patients with central nervous system vasculopathy; and how to decide if, when, and how to treat these conditions.
2:30-4:00 p.m. Pediatric Rheumatology: Clinical and Therapeutic Aspects I – Juvenile Idiopathic Arthritis. This abstract session on JIA features studies about cancer risk; increased risk of infection and other adverse events when one or more biologics are added to methotrexate; findings from a double-blind, placebo-controlled trial of probiotics on enthesitis related disease; the relevance of the duration of inactive disease when determining when to withdraw methotrexate; results from the Childhood Arthritis and Rheumatology Research Alliance’s Systemic JIA Consensus Treatment Plans pilot study; and the efficacy of canakinumab in patients with systemic JIA previously treated with biologics.
4:30-6:00 p.m. Pediatric Lupus Transitional Care: An Interactive Experience. Three speakers will share the different perspectives of the emerging-adult, young-adult, and adolescent patients on their transition experiences through multimedia.
Monday, Nov. 9
8:30-10:00 a.m. Nuts and Bolts of Macrophage Activation Syndrome. Learn more about recognizing, diagnosing, and treating the condition – even in adult patients – by applying new diagnostic criteria in patients with and without ongoing biologic therapy and knowing when and how to use targeted anticytokine therapy.
2:30-4:00 p.m. Pediatric Rheumatology: Clinical and Therapeutic Aspects II – Pediatric Systemic Lupus Erythematosus. The six studies presented in this abstract session cover a wide range of topics in juvenile SLE, including a systematic classification of the subtypes of pancreatitis in childhood-onset SLE; the sensitivity and specificity of cell-bound complement activation products in the differential diagnosis of childhood-onset lupus; axonal dysfunction’s relationship with neuropsychiatric manifestations and disease activity; risk factors for morbidity and long-term outcome of infants with cardiac neonatal lupus; cross-validating an automated cognitive performance test that screens for neurocognitive problems; and assessing the effects of vitamin D supplementation on bone microarchitecture.
Tuesday, Nov. 10
6:15-7:30 a.m. Childhood Arthritis & Rheumatology Research Alliance (CARRA). The organization is having a membership breakfast meeting at the San Francisco Marriott Marquis.
9:00-10:00 a.m. Pediatric Rheumatology Year in Review and Awards. This session provides a succinct update on what is newest and most important in basic science, translational, and clinical research in pediatric rheumatology in the past year.
11:00 a.m.-12:00 p.m. Controversies in Kawasaki Disease: Implications for Long-Term Outcomes. This session will inform attendees of how progress made in understanding the pathogenesis of Kawasaki disease has assisted researchers in finding novel biomarkers and treatment to improve long-term outcomes.
4:30-6:00 p.m. Pediatric Rheumatology: Clinical and Therapeutic Aspects IV – Imaging and Novel Clinical Interventions. This session contains abstract presentations on new MRI methods for detecting progression of erosion in JIA by focusing on bony depressions and for assessing whole-body disease damage in juvenile dermatomyositis, as well as a brain biopsy study of the histopathology and defining characteristics of patients with childhood primary small vessel CNS vasculitis. Another three studies of JIA patients examine the efficacy of tocilizumab in a small case series of patients with refractory uveitis, a pilot study of mesenchymal stromal cell treatment, how often patients meet the varying clinical definitions of inactive disease or minimal disease activity.
Wednesday, Nov. 11
7:30-8:30 a.m. New Adult and Juvenile Myositis Response Criteria. Learn how to apply the new response criteria in both clinical and research settings in this session. The new criteria use cut points to define minimal, moderate, and major levels of treatment response and are based on absolute changes in the measurements of improvement, rather than relative changes.
9:00-10:30 a.m. Chronic Nonbacterial Osteomyelitis (CNO): From the Bedside to the Bench. Presenters will offer a broad overview of the clinical features and management of this sterile, inflammatory bone disorder of unknown etiology, as well as growing knowledge of the genetics of the disease. Evidence will be presented that suggests the microbiome may play a role in the clinical manifestations of the disease.
FDA advisory committee recommends approval of gout drug lesinurad, with caveats
This article was updated on 10/27/15.
Members of the Food and Drug Administration’s Arthritis Advisory Committee have voted in favor of recommending approval of the investigational uricosuric drug lesinurad at 200 mg daily for the proposed indication of treatment of hyperuricemia associated with gout in combination with a xanthine oxidase inhibitor, such as allopurinol or febuxostat.
The committee’s 10-4 vote on recommending approval came with much discussion over the benefit-to-risk analysis of lesinurad. Members voted 14-0 that the data in the four pivotal phase III trials submitted to the FDA contained enough evidence of a clinically meaningful beneficial effect of lesinurad in combination with a xanthine oxidase inhibitor (XOI) in the treatment of hyperuricemia associated with gout. However, committee members were much less certain of lesinurad’s safety profile, which indicated a narrow therapeutic index, and voted 7-6 in favor of adequate safety data to support approval, with 1 abstention.
Lesinurad 200 mg daily lowered serum uric acid (sUA) levels 1.1-1.3 mg/dL on top of what is already achieved with an XOI in its pivotal phase III trials, but it did not consistently improve patient outcomes, such as reducing gout flares, resolving tophi, and improving Health Assessment Questionnaire-Disability Index scores.
The FDA and the developer of lesinurad, Ardea Biosciences, agreed before the advisory committee meeting not to consider a 400-mg dose of the drug for marketing because it was associated with an increased incidence of renal adverse events (including serious renal AEs, serum creatinine elevations, and kidney stone AEs), compared with placebo. Although, lesinurad 200 mg was not associated with an increased incidence of serious renal or kidney stone AEs, it was associated with a smaller increase in the incidence of overall renal AE and serum creatinine elevations, which suggested dose-dependent toxicity. An imbalance in major adverse cardiovascular event incidence and exposure-adjusted incidence also was observed with lesinurad 400 mg, compared with 200 mg or placebo, although there were a small number of events.
Overall, the average concentration of lesinurad in the studies was higher with 400 mg, compared with 200 mg, but the exposure for the two doses was largely overlapping, raising questions about whether the safety profile of the 200-mg dose will be consistent if used in a larger population with more variability, the FDA said.
Two of the four pivotal phase III clinical trials of lesinurad involved patients who had been taking at least 300 mg/day allopurinol (200 mg/day in patients with estimated creatinine clearance (CrCl) of less than 60 mL/min at baseline) for at least 8 weeks and still had a sUA level of 6.5 mg/dL or greater at the screening visit (and greater than 6.0 mg/dL at the day 7 visit) and also had at least two gout flares in the preceding 12 months. They were randomized to receive placebo, lesinurad 200 mg, or lesinurad 400 mg daily in addition to their background allopurinol for 12 months.
The third study involved the same doses of lesinurad and placebo over a 12-month time frame but instead had lesinurad added on to a background of febuxostat 80 mg and required that sUA had to be at least 8 mg/dL in patients not taking urate lowering therapy (ULT) and at least 6 mg/dL in patients who were on ULT previously. Patients also had to have at least one measurable tophus on the hands/wrists and/or feet/ankles at least 5 mm in width and up to 20 mm in length.
The fourth study was a 6-month study of lesinurad 400 mg monotherapy, compared with placebo, in subjects with gout who had intolerance or contraindication to treatment with an XOI.
Committee members who voted in favor of recommending approval called for postmarketing studies that showed that the drug did indeed improve clinical outcomes such as reducing gout flares and resolution of tophi; long-term safety studies of renal and cardiovascular adverse events, including studies in patients with comorbidities commonly occurring in patients with gout; and structured, specific guidance to providers on who should receive the drug and how prescribers should handle increases in serum creatinine levels during treatment.
Lesinurad inhibits the function of multiple carrier proteins that transport uric acid in the renal proximal tubule epithelium, including uric acid transporter 1 (URAT1) and organic anion transporters 1, 3 and 4.
The FDA is not obligated to follow the recommendations of its advisory committee panels.
This article was updated on 10/27/15.
Members of the Food and Drug Administration’s Arthritis Advisory Committee have voted in favor of recommending approval of the investigational uricosuric drug lesinurad at 200 mg daily for the proposed indication of treatment of hyperuricemia associated with gout in combination with a xanthine oxidase inhibitor, such as allopurinol or febuxostat.
The committee’s 10-4 vote on recommending approval came with much discussion over the benefit-to-risk analysis of lesinurad. Members voted 14-0 that the data in the four pivotal phase III trials submitted to the FDA contained enough evidence of a clinically meaningful beneficial effect of lesinurad in combination with a xanthine oxidase inhibitor (XOI) in the treatment of hyperuricemia associated with gout. However, committee members were much less certain of lesinurad’s safety profile, which indicated a narrow therapeutic index, and voted 7-6 in favor of adequate safety data to support approval, with 1 abstention.
Lesinurad 200 mg daily lowered serum uric acid (sUA) levels 1.1-1.3 mg/dL on top of what is already achieved with an XOI in its pivotal phase III trials, but it did not consistently improve patient outcomes, such as reducing gout flares, resolving tophi, and improving Health Assessment Questionnaire-Disability Index scores.
The FDA and the developer of lesinurad, Ardea Biosciences, agreed before the advisory committee meeting not to consider a 400-mg dose of the drug for marketing because it was associated with an increased incidence of renal adverse events (including serious renal AEs, serum creatinine elevations, and kidney stone AEs), compared with placebo. Although, lesinurad 200 mg was not associated with an increased incidence of serious renal or kidney stone AEs, it was associated with a smaller increase in the incidence of overall renal AE and serum creatinine elevations, which suggested dose-dependent toxicity. An imbalance in major adverse cardiovascular event incidence and exposure-adjusted incidence also was observed with lesinurad 400 mg, compared with 200 mg or placebo, although there were a small number of events.
Overall, the average concentration of lesinurad in the studies was higher with 400 mg, compared with 200 mg, but the exposure for the two doses was largely overlapping, raising questions about whether the safety profile of the 200-mg dose will be consistent if used in a larger population with more variability, the FDA said.
Two of the four pivotal phase III clinical trials of lesinurad involved patients who had been taking at least 300 mg/day allopurinol (200 mg/day in patients with estimated creatinine clearance (CrCl) of less than 60 mL/min at baseline) for at least 8 weeks and still had a sUA level of 6.5 mg/dL or greater at the screening visit (and greater than 6.0 mg/dL at the day 7 visit) and also had at least two gout flares in the preceding 12 months. They were randomized to receive placebo, lesinurad 200 mg, or lesinurad 400 mg daily in addition to their background allopurinol for 12 months.
The third study involved the same doses of lesinurad and placebo over a 12-month time frame but instead had lesinurad added on to a background of febuxostat 80 mg and required that sUA had to be at least 8 mg/dL in patients not taking urate lowering therapy (ULT) and at least 6 mg/dL in patients who were on ULT previously. Patients also had to have at least one measurable tophus on the hands/wrists and/or feet/ankles at least 5 mm in width and up to 20 mm in length.
The fourth study was a 6-month study of lesinurad 400 mg monotherapy, compared with placebo, in subjects with gout who had intolerance or contraindication to treatment with an XOI.
Committee members who voted in favor of recommending approval called for postmarketing studies that showed that the drug did indeed improve clinical outcomes such as reducing gout flares and resolution of tophi; long-term safety studies of renal and cardiovascular adverse events, including studies in patients with comorbidities commonly occurring in patients with gout; and structured, specific guidance to providers on who should receive the drug and how prescribers should handle increases in serum creatinine levels during treatment.
Lesinurad inhibits the function of multiple carrier proteins that transport uric acid in the renal proximal tubule epithelium, including uric acid transporter 1 (URAT1) and organic anion transporters 1, 3 and 4.
The FDA is not obligated to follow the recommendations of its advisory committee panels.
This article was updated on 10/27/15.
Members of the Food and Drug Administration’s Arthritis Advisory Committee have voted in favor of recommending approval of the investigational uricosuric drug lesinurad at 200 mg daily for the proposed indication of treatment of hyperuricemia associated with gout in combination with a xanthine oxidase inhibitor, such as allopurinol or febuxostat.
The committee’s 10-4 vote on recommending approval came with much discussion over the benefit-to-risk analysis of lesinurad. Members voted 14-0 that the data in the four pivotal phase III trials submitted to the FDA contained enough evidence of a clinically meaningful beneficial effect of lesinurad in combination with a xanthine oxidase inhibitor (XOI) in the treatment of hyperuricemia associated with gout. However, committee members were much less certain of lesinurad’s safety profile, which indicated a narrow therapeutic index, and voted 7-6 in favor of adequate safety data to support approval, with 1 abstention.
Lesinurad 200 mg daily lowered serum uric acid (sUA) levels 1.1-1.3 mg/dL on top of what is already achieved with an XOI in its pivotal phase III trials, but it did not consistently improve patient outcomes, such as reducing gout flares, resolving tophi, and improving Health Assessment Questionnaire-Disability Index scores.
The FDA and the developer of lesinurad, Ardea Biosciences, agreed before the advisory committee meeting not to consider a 400-mg dose of the drug for marketing because it was associated with an increased incidence of renal adverse events (including serious renal AEs, serum creatinine elevations, and kidney stone AEs), compared with placebo. Although, lesinurad 200 mg was not associated with an increased incidence of serious renal or kidney stone AEs, it was associated with a smaller increase in the incidence of overall renal AE and serum creatinine elevations, which suggested dose-dependent toxicity. An imbalance in major adverse cardiovascular event incidence and exposure-adjusted incidence also was observed with lesinurad 400 mg, compared with 200 mg or placebo, although there were a small number of events.
Overall, the average concentration of lesinurad in the studies was higher with 400 mg, compared with 200 mg, but the exposure for the two doses was largely overlapping, raising questions about whether the safety profile of the 200-mg dose will be consistent if used in a larger population with more variability, the FDA said.
Two of the four pivotal phase III clinical trials of lesinurad involved patients who had been taking at least 300 mg/day allopurinol (200 mg/day in patients with estimated creatinine clearance (CrCl) of less than 60 mL/min at baseline) for at least 8 weeks and still had a sUA level of 6.5 mg/dL or greater at the screening visit (and greater than 6.0 mg/dL at the day 7 visit) and also had at least two gout flares in the preceding 12 months. They were randomized to receive placebo, lesinurad 200 mg, or lesinurad 400 mg daily in addition to their background allopurinol for 12 months.
The third study involved the same doses of lesinurad and placebo over a 12-month time frame but instead had lesinurad added on to a background of febuxostat 80 mg and required that sUA had to be at least 8 mg/dL in patients not taking urate lowering therapy (ULT) and at least 6 mg/dL in patients who were on ULT previously. Patients also had to have at least one measurable tophus on the hands/wrists and/or feet/ankles at least 5 mm in width and up to 20 mm in length.
The fourth study was a 6-month study of lesinurad 400 mg monotherapy, compared with placebo, in subjects with gout who had intolerance or contraindication to treatment with an XOI.
Committee members who voted in favor of recommending approval called for postmarketing studies that showed that the drug did indeed improve clinical outcomes such as reducing gout flares and resolution of tophi; long-term safety studies of renal and cardiovascular adverse events, including studies in patients with comorbidities commonly occurring in patients with gout; and structured, specific guidance to providers on who should receive the drug and how prescribers should handle increases in serum creatinine levels during treatment.
Lesinurad inhibits the function of multiple carrier proteins that transport uric acid in the renal proximal tubule epithelium, including uric acid transporter 1 (URAT1) and organic anion transporters 1, 3 and 4.
The FDA is not obligated to follow the recommendations of its advisory committee panels.
Physician- and patient-based fibromyalgia criteria differ little for most patients
Physician-based and patient-based reporting of fibromyalgia symptoms yielded similar rates of fibromyalgia diagnosis in an analysis of rheumatology patient cases reported to the National Data Bank for Rheumatic Diseases.
The analysis provides evidence that the 2010 American College of Rheumatology diagnostic criteria and the 2011 modified version give consistent results, which has been a concern of researchers and clinicians because the 2010 criteria use only physician-based assessment of patient-reported symptoms, whereas the 2011 modified criteria use only questionnaire data from patient self-reports of symptoms.
The clinical importance of the study is that it reminds clinicians that “for the most part, patients and physicians agree in their assessment of symptoms,” study coauthor Dr. Brian Walitt said in an interview. It also shows, he noted, that “the scores and criteria used in physicians’ offices or in pharmacological research where patients are being evaluated and scored likely represent the same phenomenon that we would see in an epidemiological study.”
Although the findings indicate that the 2010 and 2011 criteria give acceptable agreement between fibromyalgia diagnosis and scores on the polysymptomatic distress scale (PSD) for research purposes, they don’t sufficiently agree for clinical decision making and diagnosis, so physicians and patients together must judge the best course of action, the researchers said (Arthritis Care Res. 2015 Sep 28. doi: 10.1002/acr.22742).
The researchers used a data set from the National Data Bank for Rheumatic Diseases in which they randomly selected 30 participating rheumatologists who contributed 514 rheumatology patients: 281 with a diagnosis of fibromyalgia prior to the study assessment and 233 control subjects with previously diagnosed noninflammatory painful disorders. The study incorporated all the physician-based variables involved in the 2010 criteria, including the Widespread Pain Index (WPI), widespread pain, the Symptom Severity scales (SS), the Polysymptomatic Distress scale (PSD), and fibromyalgia diagnosis, and patients separately completed the same study variables on their own.
A fibromyalgia diagnosis can be made in both the 2010 and 2011 criteria when the WPI is 7 or greater and the SS is 5 or greater, or when the WPI is 3-6 and the SS is 9 or greater. The creation of the PSD in the 2011 criteria by adding the scores on the WPI and SS (yielding a range of 0-31) defined a fibromyalgia diagnosis as a score 12 or higher. The PSD scale also allows measurement of “how close the patient is to satisfying the criteria, how much above the criteria cut point the patient’s severity is and, in this comparative study, the degree of agreement in physician and patient assessments,” the authors wrote.
In the 514 patients, patient-based criteria led to 225 diagnoses of fibromyalgia, compared with 215 diagnoses with physician-based criteria. There were 84 patients with discordant results for fibromyalgia diagnosis, including 56% who were positive by patient but not by physician measures and 44% who were positive by physician but not by patient measures. The overall diagnostic agreement in all patients was 84%, which had a kappa statistic for agreement beyond chance of 0.67, which is considered to indicate substantial agreement.
The mean PSD score was virtually the same using physician measures (12.3) or patient measures (12.8), but the level of agreement between physician and patient PSD scores dropped as the cut point for fibromyalgia diagnosis (PSD score of 12) approached.
About 85% of the physician and patient PSD scores differed by less than 5 units, but 15% were outliers where the patient and physician assessments were widely discordant. “They’re not really common, but it reminds us that it happens, too, and that’s something that’s interesting to understand on its own,” said Dr. Walitt, director of clinical pain research at the National Center for Complementary and Integrative Health and medical officer at the National Institute of Nursing Research.
“Where the discordance comes from is really right at the cut point. And so if you use the cut point religiously, you’ll have a problem because some people who are right at that margin will be classified one way or the other when the truth is that they’re all moderately symptomatic. In some ways, fibromyalgia is a measure of symptom severity. To have fibromyalgia means not just to have symptoms but those symptoms have to be severe enough to be recognized as fibromyalgia. Not everybody with aches and pains qualifies, so if you’re right around the cut point, it’s a good thing to talk to the patient to get a feel for them and understand their narrative in order to make the diagnosis regardless of which side of the cut point they’re on,” he said.
Several of the authors reported financial ties to pharmaceutical companies, including Pfizer, Abbott Germany, Grünenthal, Janssen-Cilag, and Lilly, or institutional support for fibromyalgia-related research.
Physician-based and patient-based reporting of fibromyalgia symptoms yielded similar rates of fibromyalgia diagnosis in an analysis of rheumatology patient cases reported to the National Data Bank for Rheumatic Diseases.
The analysis provides evidence that the 2010 American College of Rheumatology diagnostic criteria and the 2011 modified version give consistent results, which has been a concern of researchers and clinicians because the 2010 criteria use only physician-based assessment of patient-reported symptoms, whereas the 2011 modified criteria use only questionnaire data from patient self-reports of symptoms.
The clinical importance of the study is that it reminds clinicians that “for the most part, patients and physicians agree in their assessment of symptoms,” study coauthor Dr. Brian Walitt said in an interview. It also shows, he noted, that “the scores and criteria used in physicians’ offices or in pharmacological research where patients are being evaluated and scored likely represent the same phenomenon that we would see in an epidemiological study.”
Although the findings indicate that the 2010 and 2011 criteria give acceptable agreement between fibromyalgia diagnosis and scores on the polysymptomatic distress scale (PSD) for research purposes, they don’t sufficiently agree for clinical decision making and diagnosis, so physicians and patients together must judge the best course of action, the researchers said (Arthritis Care Res. 2015 Sep 28. doi: 10.1002/acr.22742).
The researchers used a data set from the National Data Bank for Rheumatic Diseases in which they randomly selected 30 participating rheumatologists who contributed 514 rheumatology patients: 281 with a diagnosis of fibromyalgia prior to the study assessment and 233 control subjects with previously diagnosed noninflammatory painful disorders. The study incorporated all the physician-based variables involved in the 2010 criteria, including the Widespread Pain Index (WPI), widespread pain, the Symptom Severity scales (SS), the Polysymptomatic Distress scale (PSD), and fibromyalgia diagnosis, and patients separately completed the same study variables on their own.
A fibromyalgia diagnosis can be made in both the 2010 and 2011 criteria when the WPI is 7 or greater and the SS is 5 or greater, or when the WPI is 3-6 and the SS is 9 or greater. The creation of the PSD in the 2011 criteria by adding the scores on the WPI and SS (yielding a range of 0-31) defined a fibromyalgia diagnosis as a score 12 or higher. The PSD scale also allows measurement of “how close the patient is to satisfying the criteria, how much above the criteria cut point the patient’s severity is and, in this comparative study, the degree of agreement in physician and patient assessments,” the authors wrote.
In the 514 patients, patient-based criteria led to 225 diagnoses of fibromyalgia, compared with 215 diagnoses with physician-based criteria. There were 84 patients with discordant results for fibromyalgia diagnosis, including 56% who were positive by patient but not by physician measures and 44% who were positive by physician but not by patient measures. The overall diagnostic agreement in all patients was 84%, which had a kappa statistic for agreement beyond chance of 0.67, which is considered to indicate substantial agreement.
The mean PSD score was virtually the same using physician measures (12.3) or patient measures (12.8), but the level of agreement between physician and patient PSD scores dropped as the cut point for fibromyalgia diagnosis (PSD score of 12) approached.
About 85% of the physician and patient PSD scores differed by less than 5 units, but 15% were outliers where the patient and physician assessments were widely discordant. “They’re not really common, but it reminds us that it happens, too, and that’s something that’s interesting to understand on its own,” said Dr. Walitt, director of clinical pain research at the National Center for Complementary and Integrative Health and medical officer at the National Institute of Nursing Research.
“Where the discordance comes from is really right at the cut point. And so if you use the cut point religiously, you’ll have a problem because some people who are right at that margin will be classified one way or the other when the truth is that they’re all moderately symptomatic. In some ways, fibromyalgia is a measure of symptom severity. To have fibromyalgia means not just to have symptoms but those symptoms have to be severe enough to be recognized as fibromyalgia. Not everybody with aches and pains qualifies, so if you’re right around the cut point, it’s a good thing to talk to the patient to get a feel for them and understand their narrative in order to make the diagnosis regardless of which side of the cut point they’re on,” he said.
Several of the authors reported financial ties to pharmaceutical companies, including Pfizer, Abbott Germany, Grünenthal, Janssen-Cilag, and Lilly, or institutional support for fibromyalgia-related research.
Physician-based and patient-based reporting of fibromyalgia symptoms yielded similar rates of fibromyalgia diagnosis in an analysis of rheumatology patient cases reported to the National Data Bank for Rheumatic Diseases.
The analysis provides evidence that the 2010 American College of Rheumatology diagnostic criteria and the 2011 modified version give consistent results, which has been a concern of researchers and clinicians because the 2010 criteria use only physician-based assessment of patient-reported symptoms, whereas the 2011 modified criteria use only questionnaire data from patient self-reports of symptoms.
The clinical importance of the study is that it reminds clinicians that “for the most part, patients and physicians agree in their assessment of symptoms,” study coauthor Dr. Brian Walitt said in an interview. It also shows, he noted, that “the scores and criteria used in physicians’ offices or in pharmacological research where patients are being evaluated and scored likely represent the same phenomenon that we would see in an epidemiological study.”
Although the findings indicate that the 2010 and 2011 criteria give acceptable agreement between fibromyalgia diagnosis and scores on the polysymptomatic distress scale (PSD) for research purposes, they don’t sufficiently agree for clinical decision making and diagnosis, so physicians and patients together must judge the best course of action, the researchers said (Arthritis Care Res. 2015 Sep 28. doi: 10.1002/acr.22742).
The researchers used a data set from the National Data Bank for Rheumatic Diseases in which they randomly selected 30 participating rheumatologists who contributed 514 rheumatology patients: 281 with a diagnosis of fibromyalgia prior to the study assessment and 233 control subjects with previously diagnosed noninflammatory painful disorders. The study incorporated all the physician-based variables involved in the 2010 criteria, including the Widespread Pain Index (WPI), widespread pain, the Symptom Severity scales (SS), the Polysymptomatic Distress scale (PSD), and fibromyalgia diagnosis, and patients separately completed the same study variables on their own.
A fibromyalgia diagnosis can be made in both the 2010 and 2011 criteria when the WPI is 7 or greater and the SS is 5 or greater, or when the WPI is 3-6 and the SS is 9 or greater. The creation of the PSD in the 2011 criteria by adding the scores on the WPI and SS (yielding a range of 0-31) defined a fibromyalgia diagnosis as a score 12 or higher. The PSD scale also allows measurement of “how close the patient is to satisfying the criteria, how much above the criteria cut point the patient’s severity is and, in this comparative study, the degree of agreement in physician and patient assessments,” the authors wrote.
In the 514 patients, patient-based criteria led to 225 diagnoses of fibromyalgia, compared with 215 diagnoses with physician-based criteria. There were 84 patients with discordant results for fibromyalgia diagnosis, including 56% who were positive by patient but not by physician measures and 44% who were positive by physician but not by patient measures. The overall diagnostic agreement in all patients was 84%, which had a kappa statistic for agreement beyond chance of 0.67, which is considered to indicate substantial agreement.
The mean PSD score was virtually the same using physician measures (12.3) or patient measures (12.8), but the level of agreement between physician and patient PSD scores dropped as the cut point for fibromyalgia diagnosis (PSD score of 12) approached.
About 85% of the physician and patient PSD scores differed by less than 5 units, but 15% were outliers where the patient and physician assessments were widely discordant. “They’re not really common, but it reminds us that it happens, too, and that’s something that’s interesting to understand on its own,” said Dr. Walitt, director of clinical pain research at the National Center for Complementary and Integrative Health and medical officer at the National Institute of Nursing Research.
“Where the discordance comes from is really right at the cut point. And so if you use the cut point religiously, you’ll have a problem because some people who are right at that margin will be classified one way or the other when the truth is that they’re all moderately symptomatic. In some ways, fibromyalgia is a measure of symptom severity. To have fibromyalgia means not just to have symptoms but those symptoms have to be severe enough to be recognized as fibromyalgia. Not everybody with aches and pains qualifies, so if you’re right around the cut point, it’s a good thing to talk to the patient to get a feel for them and understand their narrative in order to make the diagnosis regardless of which side of the cut point they’re on,” he said.
Several of the authors reported financial ties to pharmaceutical companies, including Pfizer, Abbott Germany, Grünenthal, Janssen-Cilag, and Lilly, or institutional support for fibromyalgia-related research.
FROM ARTHRITIS CARE & RESEARCH
Key clinical point: Physician-based and patient-based reporting of fibromyalgia symptoms yield similar rates of fibromyalgia diagnosis.
Major finding: The mean polysymptomatic distress scale score was virtually the same for physician measures (12.3) and patient measures (12.8).
Data source: A prospective cohort study of 514 patients: 281 with a previous diagnosis of fibromyalgia and 233 control subjects with previously diagnosed noninflammatory painful disorders.
Disclosures: Several of the authors reported financial ties to pharmaceutical companies, including Pfizer, Abbott Germany, Grünenthal, Janssen-Cilag, and Lilly, or institutional support for fibromyalgia-related research.
Caution advised for viewers of YouTube peripheral neuropathy videos
Less than half of YouTube videos that provide information about peripheral neuropathy discuss evidence-based treatment options, and only half are from health care professionals, according to a cross-sectional, systematic review of 200 videos on the website.
“Caution should be exercised when YouTube videos are used as a source of information for the treatment of neuropathy,” first author Dr. Harsh V. Gupta of the department of neurology at the University of Arkansas, Little Rock, said in a written statement.
He and his colleagues searched YouTube during Sept. 19-21, 2014, for the terms neuropathy, peripheral neuropathy, diabetic neuropathy, neuropathy causes, and neuropathy treatment. Of the more than 2,000 videos found in the search results, they analyzed the first 200 that were less than 10 minutes in duration, available on the first 10 pages of search results, and in the English language (Muscle Nerve. 2015 Oct 21. doi: 10.1002/mus.24916).
The videos most often featured health care professionals (51%), followed by patients (26%), and others (23%). Overall, 92 videos discussed treatment of neuropathy, which also was the only predictor of a favorable response (number of “likes”) or increased viewership. A total of 27 discussed more than one treatment modality. Health care professionals accounted for 41 of the videos discussing treatment, with most (18) from chiropractors and 7 from physicians (only 1 from a neurologist).
The treatment discussions most often cited alternative medicine (37%), devices (26%), or pharmacologic treatments (16%). “Despite having AAN [American Academy of Neurology] practice parameters for neuropathic pain management, only approximately 30% of treatment discussion in our cohort (43 of 145) mentioned the use of antidepressants, anticonvulsants, or percutaneous electrical nerve stimulation, which have level A or B evidence for efficacy,” the investigators noted.
While most videos (161) discussed symptoms of neuropathy, only 89 discussed causes, 16 discussed complications, 15 discussed types, and 9 discussed diagnostic investigations. Although more videos from health care professionals discussed diagnostic investigations or the complications of neuropathy, the differences were not statistically significant.
The study had no outside funding.
Less than half of YouTube videos that provide information about peripheral neuropathy discuss evidence-based treatment options, and only half are from health care professionals, according to a cross-sectional, systematic review of 200 videos on the website.
“Caution should be exercised when YouTube videos are used as a source of information for the treatment of neuropathy,” first author Dr. Harsh V. Gupta of the department of neurology at the University of Arkansas, Little Rock, said in a written statement.
He and his colleagues searched YouTube during Sept. 19-21, 2014, for the terms neuropathy, peripheral neuropathy, diabetic neuropathy, neuropathy causes, and neuropathy treatment. Of the more than 2,000 videos found in the search results, they analyzed the first 200 that were less than 10 minutes in duration, available on the first 10 pages of search results, and in the English language (Muscle Nerve. 2015 Oct 21. doi: 10.1002/mus.24916).
The videos most often featured health care professionals (51%), followed by patients (26%), and others (23%). Overall, 92 videos discussed treatment of neuropathy, which also was the only predictor of a favorable response (number of “likes”) or increased viewership. A total of 27 discussed more than one treatment modality. Health care professionals accounted for 41 of the videos discussing treatment, with most (18) from chiropractors and 7 from physicians (only 1 from a neurologist).
The treatment discussions most often cited alternative medicine (37%), devices (26%), or pharmacologic treatments (16%). “Despite having AAN [American Academy of Neurology] practice parameters for neuropathic pain management, only approximately 30% of treatment discussion in our cohort (43 of 145) mentioned the use of antidepressants, anticonvulsants, or percutaneous electrical nerve stimulation, which have level A or B evidence for efficacy,” the investigators noted.
While most videos (161) discussed symptoms of neuropathy, only 89 discussed causes, 16 discussed complications, 15 discussed types, and 9 discussed diagnostic investigations. Although more videos from health care professionals discussed diagnostic investigations or the complications of neuropathy, the differences were not statistically significant.
The study had no outside funding.
Less than half of YouTube videos that provide information about peripheral neuropathy discuss evidence-based treatment options, and only half are from health care professionals, according to a cross-sectional, systematic review of 200 videos on the website.
“Caution should be exercised when YouTube videos are used as a source of information for the treatment of neuropathy,” first author Dr. Harsh V. Gupta of the department of neurology at the University of Arkansas, Little Rock, said in a written statement.
He and his colleagues searched YouTube during Sept. 19-21, 2014, for the terms neuropathy, peripheral neuropathy, diabetic neuropathy, neuropathy causes, and neuropathy treatment. Of the more than 2,000 videos found in the search results, they analyzed the first 200 that were less than 10 minutes in duration, available on the first 10 pages of search results, and in the English language (Muscle Nerve. 2015 Oct 21. doi: 10.1002/mus.24916).
The videos most often featured health care professionals (51%), followed by patients (26%), and others (23%). Overall, 92 videos discussed treatment of neuropathy, which also was the only predictor of a favorable response (number of “likes”) or increased viewership. A total of 27 discussed more than one treatment modality. Health care professionals accounted for 41 of the videos discussing treatment, with most (18) from chiropractors and 7 from physicians (only 1 from a neurologist).
The treatment discussions most often cited alternative medicine (37%), devices (26%), or pharmacologic treatments (16%). “Despite having AAN [American Academy of Neurology] practice parameters for neuropathic pain management, only approximately 30% of treatment discussion in our cohort (43 of 145) mentioned the use of antidepressants, anticonvulsants, or percutaneous electrical nerve stimulation, which have level A or B evidence for efficacy,” the investigators noted.
While most videos (161) discussed symptoms of neuropathy, only 89 discussed causes, 16 discussed complications, 15 discussed types, and 9 discussed diagnostic investigations. Although more videos from health care professionals discussed diagnostic investigations or the complications of neuropathy, the differences were not statistically significant.
The study had no outside funding.
FROM MUSCLE & NERVE
New treatment recommendations issued for ankylosing spondylitis, nonradiographic axial SpA
New recommendations for the treatment of patients with ankylosing spondylitis and nonradiographic axial spondyloarthritis largely echo each other in many respective clinical circumstances that are addressed for active and stable disease, including strongly advising the use of nonsteroidal anti-inflammatory drugs and physical therapy as initial therapy.
The recommendations, issued by the American College of Rheumatology (ACR), the Spondylitis Association of America, and the Spondyloarthritis Research and Treatment Network, are the same as those outlined at the 2014 ACR annual meeting in advance of their simultaneous publication Sept. 24 in Arthritis & Rheumatology (doi: 10.1002/art.39298) and Arthritis Care & Research (doi: 10.1002/acr.22708).
The main difference in recommendations for the treatment of active ankylosing spondylitis (AS) and nonradiographic axial spondyloarthritis (SpA) is that in patients with active AS despite treatment with NSAIDs, treatment with a tumor necrosis factor inhibitor (TNFi) is strongly advised, whereas for patients with nonradiographic axial SpA, a TNFi is conditionally recommended over no treatment with a TNFi. The authors noted that this treatment question “had the highest level of evidence among those for nonradiographic axial SpA.”
The authors also said that the recommendations are limited in that they “did not examine the full range of treatment alternatives for patients with active peripheral arthritis or enthesitis, advanced options for patients who do not respond to first- and second-level systemic treatments, use of analgesics, or the use of imaging in disease monitoring.” They also did not address the cost-effectiveness of treatment options.
New recommendations for the treatment of patients with ankylosing spondylitis and nonradiographic axial spondyloarthritis largely echo each other in many respective clinical circumstances that are addressed for active and stable disease, including strongly advising the use of nonsteroidal anti-inflammatory drugs and physical therapy as initial therapy.
The recommendations, issued by the American College of Rheumatology (ACR), the Spondylitis Association of America, and the Spondyloarthritis Research and Treatment Network, are the same as those outlined at the 2014 ACR annual meeting in advance of their simultaneous publication Sept. 24 in Arthritis & Rheumatology (doi: 10.1002/art.39298) and Arthritis Care & Research (doi: 10.1002/acr.22708).
The main difference in recommendations for the treatment of active ankylosing spondylitis (AS) and nonradiographic axial spondyloarthritis (SpA) is that in patients with active AS despite treatment with NSAIDs, treatment with a tumor necrosis factor inhibitor (TNFi) is strongly advised, whereas for patients with nonradiographic axial SpA, a TNFi is conditionally recommended over no treatment with a TNFi. The authors noted that this treatment question “had the highest level of evidence among those for nonradiographic axial SpA.”
The authors also said that the recommendations are limited in that they “did not examine the full range of treatment alternatives for patients with active peripheral arthritis or enthesitis, advanced options for patients who do not respond to first- and second-level systemic treatments, use of analgesics, or the use of imaging in disease monitoring.” They also did not address the cost-effectiveness of treatment options.
New recommendations for the treatment of patients with ankylosing spondylitis and nonradiographic axial spondyloarthritis largely echo each other in many respective clinical circumstances that are addressed for active and stable disease, including strongly advising the use of nonsteroidal anti-inflammatory drugs and physical therapy as initial therapy.
The recommendations, issued by the American College of Rheumatology (ACR), the Spondylitis Association of America, and the Spondyloarthritis Research and Treatment Network, are the same as those outlined at the 2014 ACR annual meeting in advance of their simultaneous publication Sept. 24 in Arthritis & Rheumatology (doi: 10.1002/art.39298) and Arthritis Care & Research (doi: 10.1002/acr.22708).
The main difference in recommendations for the treatment of active ankylosing spondylitis (AS) and nonradiographic axial spondyloarthritis (SpA) is that in patients with active AS despite treatment with NSAIDs, treatment with a tumor necrosis factor inhibitor (TNFi) is strongly advised, whereas for patients with nonradiographic axial SpA, a TNFi is conditionally recommended over no treatment with a TNFi. The authors noted that this treatment question “had the highest level of evidence among those for nonradiographic axial SpA.”
The authors also said that the recommendations are limited in that they “did not examine the full range of treatment alternatives for patients with active peripheral arthritis or enthesitis, advanced options for patients who do not respond to first- and second-level systemic treatments, use of analgesics, or the use of imaging in disease monitoring.” They also did not address the cost-effectiveness of treatment options.