User login
Jeff Evans has been editor of Rheumatology News/MDedge Rheumatology and the EULAR Congress News since 2013. He started at Frontline Medical Communications in 2001 and was a reporter for 8 years before serving as editor of Clinical Neurology News and World Neurology, and briefly as editor of GI & Hepatology News. He graduated cum laude from Cornell University (New York) with a BA in biological sciences, concentrating in neurobiology and behavior.
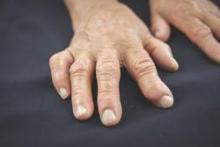
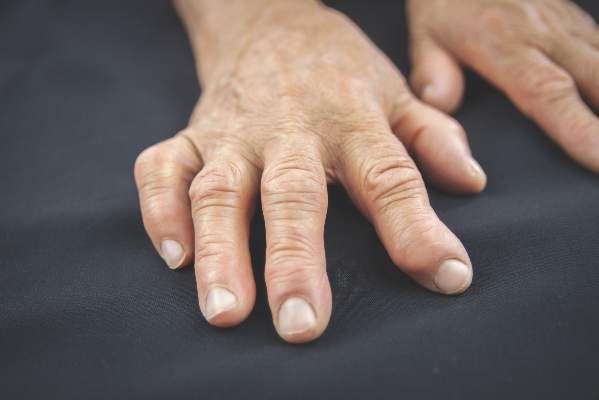
Biomarker test couldn’t predict RA transformation from undifferentiated arthritis
Baseline assessment of patients with undifferentiated arthritis at an early disease stage with the multibiomarker disease activity score did not discriminate patients who continued to have undifferentiated disease at 2 years from those who met classification criteria for rheumatoid arthritis in a prospective cohort study.
Tender joint count based on 68 joints at baseline and female sex, but not the multibiomarker disease activity (MBDA) score, were significant predictors of developing rheumatoid arthritis (RA) in the study of 45 patients who had undifferentiated arthritis (UA) and 81 who had RA based on 2010 American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) classification criteria, Dr. Karen I. Maijer of the University of Amsterdam and her colleagues reported.
At baseline, all the patients had one or more swollen joints, had clinical symptoms for less than 1 year, and had never taken disease-modifying antirheumatic drugs or corticosteroids. Following evaluation and serum collection, the patients were treated according to EULAR guidelines. Of the 45 with UA, 29 continued to have UA at 2-year follow-up and 16 developed RA.
The results of the study indicated, however, that the baseline MBDA scores were higher in patients with an initial RA diagnosis than in those with UA, which is consistent with its validation in tracking disease activity in RA patients.
Read the full study in Annals of the Rheumatic Diseases (2015 Sept. 2 doi: 10.1136/annrheumdis-2015-207911).
Baseline assessment of patients with undifferentiated arthritis at an early disease stage with the multibiomarker disease activity score did not discriminate patients who continued to have undifferentiated disease at 2 years from those who met classification criteria for rheumatoid arthritis in a prospective cohort study.
Tender joint count based on 68 joints at baseline and female sex, but not the multibiomarker disease activity (MBDA) score, were significant predictors of developing rheumatoid arthritis (RA) in the study of 45 patients who had undifferentiated arthritis (UA) and 81 who had RA based on 2010 American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) classification criteria, Dr. Karen I. Maijer of the University of Amsterdam and her colleagues reported.
At baseline, all the patients had one or more swollen joints, had clinical symptoms for less than 1 year, and had never taken disease-modifying antirheumatic drugs or corticosteroids. Following evaluation and serum collection, the patients were treated according to EULAR guidelines. Of the 45 with UA, 29 continued to have UA at 2-year follow-up and 16 developed RA.
The results of the study indicated, however, that the baseline MBDA scores were higher in patients with an initial RA diagnosis than in those with UA, which is consistent with its validation in tracking disease activity in RA patients.
Read the full study in Annals of the Rheumatic Diseases (2015 Sept. 2 doi: 10.1136/annrheumdis-2015-207911).
Baseline assessment of patients with undifferentiated arthritis at an early disease stage with the multibiomarker disease activity score did not discriminate patients who continued to have undifferentiated disease at 2 years from those who met classification criteria for rheumatoid arthritis in a prospective cohort study.
Tender joint count based on 68 joints at baseline and female sex, but not the multibiomarker disease activity (MBDA) score, were significant predictors of developing rheumatoid arthritis (RA) in the study of 45 patients who had undifferentiated arthritis (UA) and 81 who had RA based on 2010 American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) classification criteria, Dr. Karen I. Maijer of the University of Amsterdam and her colleagues reported.
At baseline, all the patients had one or more swollen joints, had clinical symptoms for less than 1 year, and had never taken disease-modifying antirheumatic drugs or corticosteroids. Following evaluation and serum collection, the patients were treated according to EULAR guidelines. Of the 45 with UA, 29 continued to have UA at 2-year follow-up and 16 developed RA.
The results of the study indicated, however, that the baseline MBDA scores were higher in patients with an initial RA diagnosis than in those with UA, which is consistent with its validation in tracking disease activity in RA patients.
Read the full study in Annals of the Rheumatic Diseases (2015 Sept. 2 doi: 10.1136/annrheumdis-2015-207911).
Infliximab biosimilar SB2 shows efficacy, safety similar to Remicade for RA
The investigational infliximab biosimilar drug SB2 provided efficacy and safety equivalent to the originator drug Remicade in patients with moderate to severe rheumatoid arthritis in a phase III study that studied the drugs in combination with methotrexate.
Treatment with SB2, which is under development by Samsung Bioepis, met the prespecified definition of equivalence to Remicade on the primary efficacy endpoint of American College of Rheumatology 20 response rate at 30 weeks by staying within a margin of −15% and +15% on the 95% confidence interval of the ACR20 rate difference (64.1% for SB2 vs. 66.0%; 95% CI, –10.26% to 6.51%). Other efficacy outcomes also showed similarity between SB2 and Remicade, including disease activity score based on 28 joints, simplified disease activity index, clinical disease activity index, and European League Against Rheumatism (EULAR) response, Dr. Jung-Yoon Choe of Daegu Catholic University Medical Center, South Korea, and colleagues reported.
Treatment-emergent adverse events occurred in 58% of patients in both groups, including 9% with at least one serious event in each group. Latent tuberculosis (TB) infections occurred in 6%-7% of patients, and none developed active TB after prophylaxis. Serious infection or active TB occurred in 3.1% of SB2 and 2.0% of Remicade patients. Only one patient in each group developed active TB, and neither had latent TB at screening.
The trial involved 584 patients who were randomized double blind to 3 mg/kg IV infusion of either drug at week 0, week 2, week 6, week 14, week 22, and week 30. The patients took 10-25 mg/week oral or parenteral methotrexate along with 5-10 mg/week folic acid. The investigators allowed nonsteroidal anti-inflammatory drugs and corticosteroids at doses equivalent to 10 mg or less prednisolone if they had been taken on a stable dose for 4 weeks before randomization. Each patient had to have had RA for at least 6 months with least six tender joints and six swollen joints, as well as an erythrocyte sedimentation rate of 28 mm/h or greater or a C-reactive protein level of 1.0 mg/dL or higher. Patients had to take methotrexate for at least 6 months with a stable dose for the 4 weeks before randomization.
Read the full study in Annals of the Rheumatic Diseases (2015 Aug. 28 doi: 10.1136/annrheumdis-2015-207764).
The investigational infliximab biosimilar drug SB2 provided efficacy and safety equivalent to the originator drug Remicade in patients with moderate to severe rheumatoid arthritis in a phase III study that studied the drugs in combination with methotrexate.
Treatment with SB2, which is under development by Samsung Bioepis, met the prespecified definition of equivalence to Remicade on the primary efficacy endpoint of American College of Rheumatology 20 response rate at 30 weeks by staying within a margin of −15% and +15% on the 95% confidence interval of the ACR20 rate difference (64.1% for SB2 vs. 66.0%; 95% CI, –10.26% to 6.51%). Other efficacy outcomes also showed similarity between SB2 and Remicade, including disease activity score based on 28 joints, simplified disease activity index, clinical disease activity index, and European League Against Rheumatism (EULAR) response, Dr. Jung-Yoon Choe of Daegu Catholic University Medical Center, South Korea, and colleagues reported.
Treatment-emergent adverse events occurred in 58% of patients in both groups, including 9% with at least one serious event in each group. Latent tuberculosis (TB) infections occurred in 6%-7% of patients, and none developed active TB after prophylaxis. Serious infection or active TB occurred in 3.1% of SB2 and 2.0% of Remicade patients. Only one patient in each group developed active TB, and neither had latent TB at screening.
The trial involved 584 patients who were randomized double blind to 3 mg/kg IV infusion of either drug at week 0, week 2, week 6, week 14, week 22, and week 30. The patients took 10-25 mg/week oral or parenteral methotrexate along with 5-10 mg/week folic acid. The investigators allowed nonsteroidal anti-inflammatory drugs and corticosteroids at doses equivalent to 10 mg or less prednisolone if they had been taken on a stable dose for 4 weeks before randomization. Each patient had to have had RA for at least 6 months with least six tender joints and six swollen joints, as well as an erythrocyte sedimentation rate of 28 mm/h or greater or a C-reactive protein level of 1.0 mg/dL or higher. Patients had to take methotrexate for at least 6 months with a stable dose for the 4 weeks before randomization.
Read the full study in Annals of the Rheumatic Diseases (2015 Aug. 28 doi: 10.1136/annrheumdis-2015-207764).
The investigational infliximab biosimilar drug SB2 provided efficacy and safety equivalent to the originator drug Remicade in patients with moderate to severe rheumatoid arthritis in a phase III study that studied the drugs in combination with methotrexate.
Treatment with SB2, which is under development by Samsung Bioepis, met the prespecified definition of equivalence to Remicade on the primary efficacy endpoint of American College of Rheumatology 20 response rate at 30 weeks by staying within a margin of −15% and +15% on the 95% confidence interval of the ACR20 rate difference (64.1% for SB2 vs. 66.0%; 95% CI, –10.26% to 6.51%). Other efficacy outcomes also showed similarity between SB2 and Remicade, including disease activity score based on 28 joints, simplified disease activity index, clinical disease activity index, and European League Against Rheumatism (EULAR) response, Dr. Jung-Yoon Choe of Daegu Catholic University Medical Center, South Korea, and colleagues reported.
Treatment-emergent adverse events occurred in 58% of patients in both groups, including 9% with at least one serious event in each group. Latent tuberculosis (TB) infections occurred in 6%-7% of patients, and none developed active TB after prophylaxis. Serious infection or active TB occurred in 3.1% of SB2 and 2.0% of Remicade patients. Only one patient in each group developed active TB, and neither had latent TB at screening.
The trial involved 584 patients who were randomized double blind to 3 mg/kg IV infusion of either drug at week 0, week 2, week 6, week 14, week 22, and week 30. The patients took 10-25 mg/week oral or parenteral methotrexate along with 5-10 mg/week folic acid. The investigators allowed nonsteroidal anti-inflammatory drugs and corticosteroids at doses equivalent to 10 mg or less prednisolone if they had been taken on a stable dose for 4 weeks before randomization. Each patient had to have had RA for at least 6 months with least six tender joints and six swollen joints, as well as an erythrocyte sedimentation rate of 28 mm/h or greater or a C-reactive protein level of 1.0 mg/dL or higher. Patients had to take methotrexate for at least 6 months with a stable dose for the 4 weeks before randomization.
Read the full study in Annals of the Rheumatic Diseases (2015 Aug. 28 doi: 10.1136/annrheumdis-2015-207764).
Evidence builds for benefits of exercise in pediatric MS
Physical activity in children with multiple sclerosis is associated with lower disease activity and fewer symptoms, both of which appear to accrue with greater levels of activity, according to findings from a cross-sectional study of 110 consecutive patients attending a specialized pediatric MS clinic,
Stephanie A. Grover and her colleagues at the Hospital for Sick Children in Toronto found that self-reported physical activity was significantly greater on average for the 79 children with monophasic acquired demyelinating syndrome (mono-ADS; age range 5-18 years) than for the 31 patients with multiple sclerosis (MS; age range 11-18 years). While greater amounts of moderate physical activity in MS patients was negatively correlated with severity of sleep/rest fatigue symptoms as well as general fatigue, strenuous levels of physical activity had an even greater negative correlation with total T2 lesion volumes and annualized relapse rates.
The differences in physical activity between mono-ADS and MS patients was not because of between-group differences in severity of clinical disability, sex, or age.
The “cross-sectional study design does not allow determination of causality” to know whether less physical activity worsens fatigue and depression or whether fatigue and depression contribute to reduced physical activity, noted Dr. Maria A. Rocca and her colleagues in an editorial accompanying the report, but it agrees with findings from adults with MS.
The generalized effect of regular physical exercise on reducing fatigue and depression – which occurred in one-fifth of MS patients in this study – might occur through an “effect on structural MRI measures of white matter architecture integrity as well as increased volume of specific gray matter structures, including the hippocampus,” Dr. Rocca and her associates wrote, as well as lessening chronic low-grade inflammation.
“A strong message from this study is that implementing physical activity may represent an easy approach that could favorably influence disease outcome in the long term,” Dr. Rocca and her coauthors concluded.
You can read the full study in Neurology (2015 Aug. 12 doi: 10.1212/WNL.0000000000001939), as well as the editorial (Neurology 2015 Aug. 12 doi: 10.1212/WNL.0000000000001941).
Physical activity in children with multiple sclerosis is associated with lower disease activity and fewer symptoms, both of which appear to accrue with greater levels of activity, according to findings from a cross-sectional study of 110 consecutive patients attending a specialized pediatric MS clinic,
Stephanie A. Grover and her colleagues at the Hospital for Sick Children in Toronto found that self-reported physical activity was significantly greater on average for the 79 children with monophasic acquired demyelinating syndrome (mono-ADS; age range 5-18 years) than for the 31 patients with multiple sclerosis (MS; age range 11-18 years). While greater amounts of moderate physical activity in MS patients was negatively correlated with severity of sleep/rest fatigue symptoms as well as general fatigue, strenuous levels of physical activity had an even greater negative correlation with total T2 lesion volumes and annualized relapse rates.
The differences in physical activity between mono-ADS and MS patients was not because of between-group differences in severity of clinical disability, sex, or age.
The “cross-sectional study design does not allow determination of causality” to know whether less physical activity worsens fatigue and depression or whether fatigue and depression contribute to reduced physical activity, noted Dr. Maria A. Rocca and her colleagues in an editorial accompanying the report, but it agrees with findings from adults with MS.
The generalized effect of regular physical exercise on reducing fatigue and depression – which occurred in one-fifth of MS patients in this study – might occur through an “effect on structural MRI measures of white matter architecture integrity as well as increased volume of specific gray matter structures, including the hippocampus,” Dr. Rocca and her associates wrote, as well as lessening chronic low-grade inflammation.
“A strong message from this study is that implementing physical activity may represent an easy approach that could favorably influence disease outcome in the long term,” Dr. Rocca and her coauthors concluded.
You can read the full study in Neurology (2015 Aug. 12 doi: 10.1212/WNL.0000000000001939), as well as the editorial (Neurology 2015 Aug. 12 doi: 10.1212/WNL.0000000000001941).
Physical activity in children with multiple sclerosis is associated with lower disease activity and fewer symptoms, both of which appear to accrue with greater levels of activity, according to findings from a cross-sectional study of 110 consecutive patients attending a specialized pediatric MS clinic,
Stephanie A. Grover and her colleagues at the Hospital for Sick Children in Toronto found that self-reported physical activity was significantly greater on average for the 79 children with monophasic acquired demyelinating syndrome (mono-ADS; age range 5-18 years) than for the 31 patients with multiple sclerosis (MS; age range 11-18 years). While greater amounts of moderate physical activity in MS patients was negatively correlated with severity of sleep/rest fatigue symptoms as well as general fatigue, strenuous levels of physical activity had an even greater negative correlation with total T2 lesion volumes and annualized relapse rates.
The differences in physical activity between mono-ADS and MS patients was not because of between-group differences in severity of clinical disability, sex, or age.
The “cross-sectional study design does not allow determination of causality” to know whether less physical activity worsens fatigue and depression or whether fatigue and depression contribute to reduced physical activity, noted Dr. Maria A. Rocca and her colleagues in an editorial accompanying the report, but it agrees with findings from adults with MS.
The generalized effect of regular physical exercise on reducing fatigue and depression – which occurred in one-fifth of MS patients in this study – might occur through an “effect on structural MRI measures of white matter architecture integrity as well as increased volume of specific gray matter structures, including the hippocampus,” Dr. Rocca and her associates wrote, as well as lessening chronic low-grade inflammation.
“A strong message from this study is that implementing physical activity may represent an easy approach that could favorably influence disease outcome in the long term,” Dr. Rocca and her coauthors concluded.
You can read the full study in Neurology (2015 Aug. 12 doi: 10.1212/WNL.0000000000001939), as well as the editorial (Neurology 2015 Aug. 12 doi: 10.1212/WNL.0000000000001941).
FROM NEUROLOGY
Secukinumab proves successful against psoriatic arthritis in FUTURE 2 trial
Treatment of psoriatic arthritis over 24 weeks with the human anti-interleukin-17A monoclonal antibody secukinumab allowed a significantly higher percentage of patients who received 150-mg and 300-mg doses to achieve at least 20% improvement in the American College of Rheumatology response criteria than did placebo.
The results of the multicenter, double-blind, randomized, placebo-controlled FUTURE 2 trial hinted at the effectiveness of the 300-mg dosing regimen in treating patients who previously had not responded to treatment with tumor necrosis factor (TNF) inhibitors and did not show differences in effectiveness when used together with methotrexate. The drug did not have any particularly worrisome treatment-related adverse events, Dr. Iain McInnes of the University of Glasgow (Scotland) and his colleagues reported (Lancet 2015 June 28 [doi:10.1016/S0140-6736(15)61134-5]).
At week 24, ACR20 responses occurred in 54 of 100 patients who received secukinumab 300 mg, 51 of 100 who took the 150-mg dosing regimen, 29 of 99 on the 75-mg dosing regimen, and 15 of 98 on placebo. The odds for achieving ACR20 at 24 weeks were nearly sevenfold higher for the 300-mg dose (odds ratio, 6.81; 95% confidence interval, 3.42-13.56; P < .0001) and 150-mg doses (OR, 6.52; 95% CI, 3.25-13.08; P < .0001) than with placebo.
In an editorial accompanying the report, Dr. Philip Helliwell and Dr. Laura Coates of the University of Leeds (England) said that it is important to note that responses at week 24 were similar to those observed at weeks 12 and 16 so that patients and physicians can “get an idea of response (or non-response) at an early timepoint” (Lancet 2015 June 28 [doi:10.1016/S0140-6736(15)61170-9]).
Although radiographic progression wasn’t assessed in the FUTURE 2 trial, Dr. Helliwell and Dr. Coates noted that secukinumab inhibited radiographic damage in the unpublished FUTURE-1 trial, so “in view of its position in the cytokine hierarchy, interleukin-17 inhibition might prevent both of these features, and future studies with this class of drug should investigate this issue in detail.”
The lower response that seemed to have occurred in the secukinumab-treated patients who had previously used TNF inhibitors vs. those who had not “is in keeping with data for other treatments such as ustekinumab,” they wrote. The fact that there appeared to be a dose-response effect between the patients who received 150 mg secukinumab vs. 300 mg and had previously used TNF inhibitors suggests that the 300-mg dose might be more appropriate for patients who did not respond to TNF inhibitors and/or have moderate to severe psoriasis, for whom the 300-mg dose is already approved and recommended.
Read our meeting coverage of the FUTURE 2 trial at ACR 2014 here.
Treatment of psoriatic arthritis over 24 weeks with the human anti-interleukin-17A monoclonal antibody secukinumab allowed a significantly higher percentage of patients who received 150-mg and 300-mg doses to achieve at least 20% improvement in the American College of Rheumatology response criteria than did placebo.
The results of the multicenter, double-blind, randomized, placebo-controlled FUTURE 2 trial hinted at the effectiveness of the 300-mg dosing regimen in treating patients who previously had not responded to treatment with tumor necrosis factor (TNF) inhibitors and did not show differences in effectiveness when used together with methotrexate. The drug did not have any particularly worrisome treatment-related adverse events, Dr. Iain McInnes of the University of Glasgow (Scotland) and his colleagues reported (Lancet 2015 June 28 [doi:10.1016/S0140-6736(15)61134-5]).
At week 24, ACR20 responses occurred in 54 of 100 patients who received secukinumab 300 mg, 51 of 100 who took the 150-mg dosing regimen, 29 of 99 on the 75-mg dosing regimen, and 15 of 98 on placebo. The odds for achieving ACR20 at 24 weeks were nearly sevenfold higher for the 300-mg dose (odds ratio, 6.81; 95% confidence interval, 3.42-13.56; P < .0001) and 150-mg doses (OR, 6.52; 95% CI, 3.25-13.08; P < .0001) than with placebo.
In an editorial accompanying the report, Dr. Philip Helliwell and Dr. Laura Coates of the University of Leeds (England) said that it is important to note that responses at week 24 were similar to those observed at weeks 12 and 16 so that patients and physicians can “get an idea of response (or non-response) at an early timepoint” (Lancet 2015 June 28 [doi:10.1016/S0140-6736(15)61170-9]).
Although radiographic progression wasn’t assessed in the FUTURE 2 trial, Dr. Helliwell and Dr. Coates noted that secukinumab inhibited radiographic damage in the unpublished FUTURE-1 trial, so “in view of its position in the cytokine hierarchy, interleukin-17 inhibition might prevent both of these features, and future studies with this class of drug should investigate this issue in detail.”
The lower response that seemed to have occurred in the secukinumab-treated patients who had previously used TNF inhibitors vs. those who had not “is in keeping with data for other treatments such as ustekinumab,” they wrote. The fact that there appeared to be a dose-response effect between the patients who received 150 mg secukinumab vs. 300 mg and had previously used TNF inhibitors suggests that the 300-mg dose might be more appropriate for patients who did not respond to TNF inhibitors and/or have moderate to severe psoriasis, for whom the 300-mg dose is already approved and recommended.
Read our meeting coverage of the FUTURE 2 trial at ACR 2014 here.
Treatment of psoriatic arthritis over 24 weeks with the human anti-interleukin-17A monoclonal antibody secukinumab allowed a significantly higher percentage of patients who received 150-mg and 300-mg doses to achieve at least 20% improvement in the American College of Rheumatology response criteria than did placebo.
The results of the multicenter, double-blind, randomized, placebo-controlled FUTURE 2 trial hinted at the effectiveness of the 300-mg dosing regimen in treating patients who previously had not responded to treatment with tumor necrosis factor (TNF) inhibitors and did not show differences in effectiveness when used together with methotrexate. The drug did not have any particularly worrisome treatment-related adverse events, Dr. Iain McInnes of the University of Glasgow (Scotland) and his colleagues reported (Lancet 2015 June 28 [doi:10.1016/S0140-6736(15)61134-5]).
At week 24, ACR20 responses occurred in 54 of 100 patients who received secukinumab 300 mg, 51 of 100 who took the 150-mg dosing regimen, 29 of 99 on the 75-mg dosing regimen, and 15 of 98 on placebo. The odds for achieving ACR20 at 24 weeks were nearly sevenfold higher for the 300-mg dose (odds ratio, 6.81; 95% confidence interval, 3.42-13.56; P < .0001) and 150-mg doses (OR, 6.52; 95% CI, 3.25-13.08; P < .0001) than with placebo.
In an editorial accompanying the report, Dr. Philip Helliwell and Dr. Laura Coates of the University of Leeds (England) said that it is important to note that responses at week 24 were similar to those observed at weeks 12 and 16 so that patients and physicians can “get an idea of response (or non-response) at an early timepoint” (Lancet 2015 June 28 [doi:10.1016/S0140-6736(15)61170-9]).
Although radiographic progression wasn’t assessed in the FUTURE 2 trial, Dr. Helliwell and Dr. Coates noted that secukinumab inhibited radiographic damage in the unpublished FUTURE-1 trial, so “in view of its position in the cytokine hierarchy, interleukin-17 inhibition might prevent both of these features, and future studies with this class of drug should investigate this issue in detail.”
The lower response that seemed to have occurred in the secukinumab-treated patients who had previously used TNF inhibitors vs. those who had not “is in keeping with data for other treatments such as ustekinumab,” they wrote. The fact that there appeared to be a dose-response effect between the patients who received 150 mg secukinumab vs. 300 mg and had previously used TNF inhibitors suggests that the 300-mg dose might be more appropriate for patients who did not respond to TNF inhibitors and/or have moderate to severe psoriasis, for whom the 300-mg dose is already approved and recommended.
Read our meeting coverage of the FUTURE 2 trial at ACR 2014 here.
FDA will strengthen heart attack, stroke risk warnings for all NSAIDs
The Food and Drug Administration has taken new action to strengthen existing warning labels about the increased risk of heart attack or stroke with the use of prescription and over-the-counter nonaspirin nonsteroidal anti-inflammatory drugs.
In a July 9 drug safety communication, the agency did not provide the exact language that will be used on NSAID labels, but said that they “will be revised to reflect” information describing that:
• The risk of heart attack or stroke can occur as early as the first weeks of using an NSAID.
• The risk may increase with longer use and at higher doses of the NSAID.
• The drugs can increase the risk of heart attack or stroke even in patients without heart disease or risk factors for heart disease, but patients with heart disease or risk factors for it have a greater likelihood of heart attack or stroke following NSAID use.
• Treatment with NSAIDs following a first heart attack increases the risk of death in the first year after the heart attack, compared with patients who were not treated with NSAIDs after their first heart attack.
• NSAID use increases the risk of heart failure.
The new wording will also note that although newer information may suggest that the risk for heart attack or stroke is not the same for all NSAIDs, it “is not sufficient for us to determine that the risk of any particular NSAID is definitely higher or lower than that of any other particular NSAID.”
*The update to NSAID labels follows the recommendations given by panel members from a joint meeting of the FDA’s Arthritis Advisory Committee and the Drug Safety and Risk Management Advisory Committee in February 2014 in which there was a split vote (14-11) that was slightly in favor of rewording the warning labeling for NSAIDs in regard to the drug class’s current labeling, which implies that the cardiovascular thrombotic risk is not substantial with short treatment courses. At that meeting, the panelists also voted 16-9 that there were not enough data to suggest that naproxen presented a substantially lower risk of CV events than did either ibuprofen or selective NSAIDs, such as cyclooxygenase-2 inhibitors.
The FDA made its decision based on a comprehensive review of the data presented during that meeting.
*Correction, 7/16/2015: An earlier version of this story misstated the FDA panels’ recommendation for labeling changes.
The Food and Drug Administration has taken new action to strengthen existing warning labels about the increased risk of heart attack or stroke with the use of prescription and over-the-counter nonaspirin nonsteroidal anti-inflammatory drugs.
In a July 9 drug safety communication, the agency did not provide the exact language that will be used on NSAID labels, but said that they “will be revised to reflect” information describing that:
• The risk of heart attack or stroke can occur as early as the first weeks of using an NSAID.
• The risk may increase with longer use and at higher doses of the NSAID.
• The drugs can increase the risk of heart attack or stroke even in patients without heart disease or risk factors for heart disease, but patients with heart disease or risk factors for it have a greater likelihood of heart attack or stroke following NSAID use.
• Treatment with NSAIDs following a first heart attack increases the risk of death in the first year after the heart attack, compared with patients who were not treated with NSAIDs after their first heart attack.
• NSAID use increases the risk of heart failure.
The new wording will also note that although newer information may suggest that the risk for heart attack or stroke is not the same for all NSAIDs, it “is not sufficient for us to determine that the risk of any particular NSAID is definitely higher or lower than that of any other particular NSAID.”
*The update to NSAID labels follows the recommendations given by panel members from a joint meeting of the FDA’s Arthritis Advisory Committee and the Drug Safety and Risk Management Advisory Committee in February 2014 in which there was a split vote (14-11) that was slightly in favor of rewording the warning labeling for NSAIDs in regard to the drug class’s current labeling, which implies that the cardiovascular thrombotic risk is not substantial with short treatment courses. At that meeting, the panelists also voted 16-9 that there were not enough data to suggest that naproxen presented a substantially lower risk of CV events than did either ibuprofen or selective NSAIDs, such as cyclooxygenase-2 inhibitors.
The FDA made its decision based on a comprehensive review of the data presented during that meeting.
*Correction, 7/16/2015: An earlier version of this story misstated the FDA panels’ recommendation for labeling changes.
The Food and Drug Administration has taken new action to strengthen existing warning labels about the increased risk of heart attack or stroke with the use of prescription and over-the-counter nonaspirin nonsteroidal anti-inflammatory drugs.
In a July 9 drug safety communication, the agency did not provide the exact language that will be used on NSAID labels, but said that they “will be revised to reflect” information describing that:
• The risk of heart attack or stroke can occur as early as the first weeks of using an NSAID.
• The risk may increase with longer use and at higher doses of the NSAID.
• The drugs can increase the risk of heart attack or stroke even in patients without heart disease or risk factors for heart disease, but patients with heart disease or risk factors for it have a greater likelihood of heart attack or stroke following NSAID use.
• Treatment with NSAIDs following a first heart attack increases the risk of death in the first year after the heart attack, compared with patients who were not treated with NSAIDs after their first heart attack.
• NSAID use increases the risk of heart failure.
The new wording will also note that although newer information may suggest that the risk for heart attack or stroke is not the same for all NSAIDs, it “is not sufficient for us to determine that the risk of any particular NSAID is definitely higher or lower than that of any other particular NSAID.”
*The update to NSAID labels follows the recommendations given by panel members from a joint meeting of the FDA’s Arthritis Advisory Committee and the Drug Safety and Risk Management Advisory Committee in February 2014 in which there was a split vote (14-11) that was slightly in favor of rewording the warning labeling for NSAIDs in regard to the drug class’s current labeling, which implies that the cardiovascular thrombotic risk is not substantial with short treatment courses. At that meeting, the panelists also voted 16-9 that there were not enough data to suggest that naproxen presented a substantially lower risk of CV events than did either ibuprofen or selective NSAIDs, such as cyclooxygenase-2 inhibitors.
The FDA made its decision based on a comprehensive review of the data presented during that meeting.
*Correction, 7/16/2015: An earlier version of this story misstated the FDA panels’ recommendation for labeling changes.
VIDEO: Could a deep Koebner phenomenon trigger psoriatic arthritis?
ROME – Psoriasis patients with a past history of bone or joint trauma had about 50% higher risk of later developing psoriatic arthritis than did those without a history of trauma in a longitudinal, population-based study of more than 70,000 psoriasis patients in the United Kingdom.
The relationship between trauma and later development of psoriatic arthritis could involve a deep Koebner phenomenon similar to what is observed with the Koebner phenomenon in the skin, suggested lead investigator Dr. Thorvardur Löve in an interview at the European Congress of Rheumatology.
Based on these findings, “one of the things that we are very excited about is the potential to think of strategies and test strategies that might be used in psoriasis patients once they are injured. So should we do anything different in an injured psoriasis patient, for instance, some sort of preventive treatment?” said Dr. Löve of Landspitali University Hospital in Reykjavik, Iceland.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ROME – Psoriasis patients with a past history of bone or joint trauma had about 50% higher risk of later developing psoriatic arthritis than did those without a history of trauma in a longitudinal, population-based study of more than 70,000 psoriasis patients in the United Kingdom.
The relationship between trauma and later development of psoriatic arthritis could involve a deep Koebner phenomenon similar to what is observed with the Koebner phenomenon in the skin, suggested lead investigator Dr. Thorvardur Löve in an interview at the European Congress of Rheumatology.
Based on these findings, “one of the things that we are very excited about is the potential to think of strategies and test strategies that might be used in psoriasis patients once they are injured. So should we do anything different in an injured psoriasis patient, for instance, some sort of preventive treatment?” said Dr. Löve of Landspitali University Hospital in Reykjavik, Iceland.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ROME – Psoriasis patients with a past history of bone or joint trauma had about 50% higher risk of later developing psoriatic arthritis than did those without a history of trauma in a longitudinal, population-based study of more than 70,000 psoriasis patients in the United Kingdom.
The relationship between trauma and later development of psoriatic arthritis could involve a deep Koebner phenomenon similar to what is observed with the Koebner phenomenon in the skin, suggested lead investigator Dr. Thorvardur Löve in an interview at the European Congress of Rheumatology.
Based on these findings, “one of the things that we are very excited about is the potential to think of strategies and test strategies that might be used in psoriasis patients once they are injured. So should we do anything different in an injured psoriasis patient, for instance, some sort of preventive treatment?” said Dr. Löve of Landspitali University Hospital in Reykjavik, Iceland.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE EULAR 2015 CONGRESS
VIDEO: JIA study details impact of biologics on adverse events
ROME – The rate of adverse events in patients with juvenile idiopathic arthritis appears to climb with the use of more than one biologic agent over time, Dr. Joost Swart reported at the European Congress of Rheumatology.
When used with methotrexate, the rate of adverse events doubled among users of one biologic agent when compared against patients who used methotrexate alone, and tripled among users of more than one biologic, according to the study of nearly 6,000 patients in the Pharmachild registry.
Dr. Swart, a pediatric rheumatologist/immunologist in the department of pediatric immunology and rheumatology in the Wilhelmina Children’s Hospital at University Medical Center Utrecht (the Netherlands), said in an interview that while patients who took a biologic had a higher rate of ever using systemic corticosteroids, it’s not clear whether that contributed to the difference in adverse events.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ROME – The rate of adverse events in patients with juvenile idiopathic arthritis appears to climb with the use of more than one biologic agent over time, Dr. Joost Swart reported at the European Congress of Rheumatology.
When used with methotrexate, the rate of adverse events doubled among users of one biologic agent when compared against patients who used methotrexate alone, and tripled among users of more than one biologic, according to the study of nearly 6,000 patients in the Pharmachild registry.
Dr. Swart, a pediatric rheumatologist/immunologist in the department of pediatric immunology and rheumatology in the Wilhelmina Children’s Hospital at University Medical Center Utrecht (the Netherlands), said in an interview that while patients who took a biologic had a higher rate of ever using systemic corticosteroids, it’s not clear whether that contributed to the difference in adverse events.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ROME – The rate of adverse events in patients with juvenile idiopathic arthritis appears to climb with the use of more than one biologic agent over time, Dr. Joost Swart reported at the European Congress of Rheumatology.
When used with methotrexate, the rate of adverse events doubled among users of one biologic agent when compared against patients who used methotrexate alone, and tripled among users of more than one biologic, according to the study of nearly 6,000 patients in the Pharmachild registry.
Dr. Swart, a pediatric rheumatologist/immunologist in the department of pediatric immunology and rheumatology in the Wilhelmina Children’s Hospital at University Medical Center Utrecht (the Netherlands), said in an interview that while patients who took a biologic had a higher rate of ever using systemic corticosteroids, it’s not clear whether that contributed to the difference in adverse events.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE EULAR 2015 CONGRESS
VIDEO: Predicting anti-TNF failure in psoriatic arthritis
ROME – Changes in the methylation status of particular genes in psoriatic arthritis patients might provide the ability to predict failure to respond to tumor necrosis factor-alpha inhibitors, according to preliminary research in 41 psoriatic arthritis patients.
Two genes stood out to the researchers from Memorial University of Newfoundland, St. John’s: TNFRSF1B and CD70. Patients who had methylation changes to those genes were more likely to have secondary failure of TNF inhibitors, said Dr. Proton Rahman, professor of internal medicine at the university and coinvestigator on the study.
It will be necessary to conduct validation studies of the results in larger numbers of patients, as well as functional studies of the effects of methylation changes on the expression of those genes and their proteins, he said in a video interview at the European Congress of Rheumatology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ROME – Changes in the methylation status of particular genes in psoriatic arthritis patients might provide the ability to predict failure to respond to tumor necrosis factor-alpha inhibitors, according to preliminary research in 41 psoriatic arthritis patients.
Two genes stood out to the researchers from Memorial University of Newfoundland, St. John’s: TNFRSF1B and CD70. Patients who had methylation changes to those genes were more likely to have secondary failure of TNF inhibitors, said Dr. Proton Rahman, professor of internal medicine at the university and coinvestigator on the study.
It will be necessary to conduct validation studies of the results in larger numbers of patients, as well as functional studies of the effects of methylation changes on the expression of those genes and their proteins, he said in a video interview at the European Congress of Rheumatology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ROME – Changes in the methylation status of particular genes in psoriatic arthritis patients might provide the ability to predict failure to respond to tumor necrosis factor-alpha inhibitors, according to preliminary research in 41 psoriatic arthritis patients.
Two genes stood out to the researchers from Memorial University of Newfoundland, St. John’s: TNFRSF1B and CD70. Patients who had methylation changes to those genes were more likely to have secondary failure of TNF inhibitors, said Dr. Proton Rahman, professor of internal medicine at the university and coinvestigator on the study.
It will be necessary to conduct validation studies of the results in larger numbers of patients, as well as functional studies of the effects of methylation changes on the expression of those genes and their proteins, he said in a video interview at the European Congress of Rheumatology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE EULAR 2015 CONGRESS
EULAR: Serious adverse events rise with number of biologics used in JIA
ROME – Biologic drug use in combination with methotrexate appears to carry the greatest risk for serious adverse events in patients with juvenile idiopathic arthritis when different biologics are tried sequentially, according to findings from a study of nearly 6,000 patients in the Pharmachild registry.
The results from the registry, the largest international pharmacovigilance juvenile idiopathic arthritis (JIA) database in the world, showed that patients who had used more than one biologic while on methotrexate had nearly twice the rate of adverse events as did those on methotrexate alone and more than three times the rate of serious adverse events, Dr. Joost F. Swart reported at the European Congress of Rheumatology.
The timing of “when to switch to a biological or even to a second one should not only be based on the activity of the joints as it is recommended nowadays, but should also take into account the different risk profiles of the drugs,” Dr. Swart, a pediatric rheumatologist/immunologist in the department of pediatric immunology and rheumatology in the Wilhelmina Children’s Hospital at University Medical Center Utrecht (the Netherlands), said in an interview. He received a clinical research abstract award for the research at the congress.
The study involved 5,862 JIA patients in the registry who continued methotrexate and either stayed on it alone (1,674) or also added one (3,025) or more biologics over time (1,163). The registry includes 93 centers of the Paediatric Rheumatology International Trials Organization (PRINTO) from more than 30 countries.
Most of the patients who had been treated with one biologic in addition to methotrexate had taken etanercept (66%), followed by adalimumab (19%), infliximab/tocilizumab (about 5% each), and other biologic agents (5%). Treatment experience with multiple biologics was most often with etanercept (30%), adalimumab (27%), infliximab (14%), tocilizumab (9%), abatacept (7%), anakinra (4%), golimumab (4%), and other biologic agents (5%). A total of 40% of children also were treated with corticosteroids (82% of those with systemic JIA); another 20% received other disease-modifying antirheumatic drugs. The patients’ JIA subtypes included systemic (10%), persistent oligoarticular (20%), polyarticular rheumatoid factor positive or negative (50%), and other JIA categories (20%).
Dr. Swart and his coauthors determined that the overall adverse event incidence rate per 100 patient-years was lowest in JIA patients who took methotrexate alone (10.7), intermediate in those who took methotrexate plus one biologic (13.9), and highest among patients who took methotrexate in combination with more than one biologic in sequence over time (19.5). Similar trends were observed for serious adverse events with methotrexate alone (3.0), with one biologic (4.7), and with more than one biologic (9.2).
Although the infection rate was higher for patients who took one biologic (4.6) in comparison with methotrexate alone (2.9), the rate did not increase significantly with more than one biologic (4.8). The same trend applied for serious infections (0.7, 1.4, and 2.0, respectively).
The median disease duration increased from methotrexate-only users (3.6 years) to those who used one biologic with methotrexate (5.4 years) and those who used more than one biologic (7.6 years). The investigators tracked more patient-years of use of one biologic (about 9,000) than two (about 2,800). The number of patient-years on methotrexate for those who took a biologic were not counted in the study.
The proportion of patients who discontinued treatment because of an adverse event or drug intolerance rose along with exposure to greater numbers of drugs, from 4% of methotrexate-only users to 16% of those exposed to a single biologic and 18% of those who took more than one biologic.
The group treated with more than one biologic had higher incidence rates for injury, poisoning and procedural complications, blood and lymphatic system disorders, and eye disorders than did the other groups, whereas methotrexate-only users had a higher incidence of hepatobiliary disorders.
“In due time, we will be able to predict which patients [age, sex, subcategory of JIA, disease activity score, which drugs/drug-combinations, etc.] have the highest risk for certain adverse events and make prediction rules for that. In that way, you might be able to prevent adverse events by specifically avoiding some drugs or drug combinations in certain patient categories or by taking precautionary measures” such as vaccination and antibiotic prophylaxis, Dr. Swart said in an interview.
Analyses are in progress for determining the relationships between individual biologics and adverse events and for differences in adverse events according to geographic area, he said.
Dr. Swart had no conflicts of interest to disclose, but many of his 28 coauthors disclosed relationships with companies that market the drugs used by patients in the registry.
ROME – Biologic drug use in combination with methotrexate appears to carry the greatest risk for serious adverse events in patients with juvenile idiopathic arthritis when different biologics are tried sequentially, according to findings from a study of nearly 6,000 patients in the Pharmachild registry.
The results from the registry, the largest international pharmacovigilance juvenile idiopathic arthritis (JIA) database in the world, showed that patients who had used more than one biologic while on methotrexate had nearly twice the rate of adverse events as did those on methotrexate alone and more than three times the rate of serious adverse events, Dr. Joost F. Swart reported at the European Congress of Rheumatology.
The timing of “when to switch to a biological or even to a second one should not only be based on the activity of the joints as it is recommended nowadays, but should also take into account the different risk profiles of the drugs,” Dr. Swart, a pediatric rheumatologist/immunologist in the department of pediatric immunology and rheumatology in the Wilhelmina Children’s Hospital at University Medical Center Utrecht (the Netherlands), said in an interview. He received a clinical research abstract award for the research at the congress.
The study involved 5,862 JIA patients in the registry who continued methotrexate and either stayed on it alone (1,674) or also added one (3,025) or more biologics over time (1,163). The registry includes 93 centers of the Paediatric Rheumatology International Trials Organization (PRINTO) from more than 30 countries.
Most of the patients who had been treated with one biologic in addition to methotrexate had taken etanercept (66%), followed by adalimumab (19%), infliximab/tocilizumab (about 5% each), and other biologic agents (5%). Treatment experience with multiple biologics was most often with etanercept (30%), adalimumab (27%), infliximab (14%), tocilizumab (9%), abatacept (7%), anakinra (4%), golimumab (4%), and other biologic agents (5%). A total of 40% of children also were treated with corticosteroids (82% of those with systemic JIA); another 20% received other disease-modifying antirheumatic drugs. The patients’ JIA subtypes included systemic (10%), persistent oligoarticular (20%), polyarticular rheumatoid factor positive or negative (50%), and other JIA categories (20%).
Dr. Swart and his coauthors determined that the overall adverse event incidence rate per 100 patient-years was lowest in JIA patients who took methotrexate alone (10.7), intermediate in those who took methotrexate plus one biologic (13.9), and highest among patients who took methotrexate in combination with more than one biologic in sequence over time (19.5). Similar trends were observed for serious adverse events with methotrexate alone (3.0), with one biologic (4.7), and with more than one biologic (9.2).
Although the infection rate was higher for patients who took one biologic (4.6) in comparison with methotrexate alone (2.9), the rate did not increase significantly with more than one biologic (4.8). The same trend applied for serious infections (0.7, 1.4, and 2.0, respectively).
The median disease duration increased from methotrexate-only users (3.6 years) to those who used one biologic with methotrexate (5.4 years) and those who used more than one biologic (7.6 years). The investigators tracked more patient-years of use of one biologic (about 9,000) than two (about 2,800). The number of patient-years on methotrexate for those who took a biologic were not counted in the study.
The proportion of patients who discontinued treatment because of an adverse event or drug intolerance rose along with exposure to greater numbers of drugs, from 4% of methotrexate-only users to 16% of those exposed to a single biologic and 18% of those who took more than one biologic.
The group treated with more than one biologic had higher incidence rates for injury, poisoning and procedural complications, blood and lymphatic system disorders, and eye disorders than did the other groups, whereas methotrexate-only users had a higher incidence of hepatobiliary disorders.
“In due time, we will be able to predict which patients [age, sex, subcategory of JIA, disease activity score, which drugs/drug-combinations, etc.] have the highest risk for certain adverse events and make prediction rules for that. In that way, you might be able to prevent adverse events by specifically avoiding some drugs or drug combinations in certain patient categories or by taking precautionary measures” such as vaccination and antibiotic prophylaxis, Dr. Swart said in an interview.
Analyses are in progress for determining the relationships between individual biologics and adverse events and for differences in adverse events according to geographic area, he said.
Dr. Swart had no conflicts of interest to disclose, but many of his 28 coauthors disclosed relationships with companies that market the drugs used by patients in the registry.
ROME – Biologic drug use in combination with methotrexate appears to carry the greatest risk for serious adverse events in patients with juvenile idiopathic arthritis when different biologics are tried sequentially, according to findings from a study of nearly 6,000 patients in the Pharmachild registry.
The results from the registry, the largest international pharmacovigilance juvenile idiopathic arthritis (JIA) database in the world, showed that patients who had used more than one biologic while on methotrexate had nearly twice the rate of adverse events as did those on methotrexate alone and more than three times the rate of serious adverse events, Dr. Joost F. Swart reported at the European Congress of Rheumatology.
The timing of “when to switch to a biological or even to a second one should not only be based on the activity of the joints as it is recommended nowadays, but should also take into account the different risk profiles of the drugs,” Dr. Swart, a pediatric rheumatologist/immunologist in the department of pediatric immunology and rheumatology in the Wilhelmina Children’s Hospital at University Medical Center Utrecht (the Netherlands), said in an interview. He received a clinical research abstract award for the research at the congress.
The study involved 5,862 JIA patients in the registry who continued methotrexate and either stayed on it alone (1,674) or also added one (3,025) or more biologics over time (1,163). The registry includes 93 centers of the Paediatric Rheumatology International Trials Organization (PRINTO) from more than 30 countries.
Most of the patients who had been treated with one biologic in addition to methotrexate had taken etanercept (66%), followed by adalimumab (19%), infliximab/tocilizumab (about 5% each), and other biologic agents (5%). Treatment experience with multiple biologics was most often with etanercept (30%), adalimumab (27%), infliximab (14%), tocilizumab (9%), abatacept (7%), anakinra (4%), golimumab (4%), and other biologic agents (5%). A total of 40% of children also were treated with corticosteroids (82% of those with systemic JIA); another 20% received other disease-modifying antirheumatic drugs. The patients’ JIA subtypes included systemic (10%), persistent oligoarticular (20%), polyarticular rheumatoid factor positive or negative (50%), and other JIA categories (20%).
Dr. Swart and his coauthors determined that the overall adverse event incidence rate per 100 patient-years was lowest in JIA patients who took methotrexate alone (10.7), intermediate in those who took methotrexate plus one biologic (13.9), and highest among patients who took methotrexate in combination with more than one biologic in sequence over time (19.5). Similar trends were observed for serious adverse events with methotrexate alone (3.0), with one biologic (4.7), and with more than one biologic (9.2).
Although the infection rate was higher for patients who took one biologic (4.6) in comparison with methotrexate alone (2.9), the rate did not increase significantly with more than one biologic (4.8). The same trend applied for serious infections (0.7, 1.4, and 2.0, respectively).
The median disease duration increased from methotrexate-only users (3.6 years) to those who used one biologic with methotrexate (5.4 years) and those who used more than one biologic (7.6 years). The investigators tracked more patient-years of use of one biologic (about 9,000) than two (about 2,800). The number of patient-years on methotrexate for those who took a biologic were not counted in the study.
The proportion of patients who discontinued treatment because of an adverse event or drug intolerance rose along with exposure to greater numbers of drugs, from 4% of methotrexate-only users to 16% of those exposed to a single biologic and 18% of those who took more than one biologic.
The group treated with more than one biologic had higher incidence rates for injury, poisoning and procedural complications, blood and lymphatic system disorders, and eye disorders than did the other groups, whereas methotrexate-only users had a higher incidence of hepatobiliary disorders.
“In due time, we will be able to predict which patients [age, sex, subcategory of JIA, disease activity score, which drugs/drug-combinations, etc.] have the highest risk for certain adverse events and make prediction rules for that. In that way, you might be able to prevent adverse events by specifically avoiding some drugs or drug combinations in certain patient categories or by taking precautionary measures” such as vaccination and antibiotic prophylaxis, Dr. Swart said in an interview.
Analyses are in progress for determining the relationships between individual biologics and adverse events and for differences in adverse events according to geographic area, he said.
Dr. Swart had no conflicts of interest to disclose, but many of his 28 coauthors disclosed relationships with companies that market the drugs used by patients in the registry.
AT THE EULAR 2015 CONGRESS
Key clinical point: JIA patients who had used more than one biologic while on methotrexate had nearly twice the rate of adverse events as did those on methotrexate alone and more than three times the rate of serious adverse events.
Major finding: The serious adverse event rate per 100 patient-years was lowest in JIA patients who took methotrexate alone (3.0), intermediate in those who took methotrexate plus one biologic (4.7), and highest among patients who took methotrexate in combination with more than one biologic in sequence over time (9.2).
Data source: A study of 5,862 JIA patients in the Pharmachild registry
Disclosures: Dr. Swart had no conflicts of interest to disclose, but many of his 28 coauthors disclosed relationships with companies that market the drugs used by patients in the registry.
VIDEO: Do pathogenic intestinal bacteria drive scleroderma GI symptoms?
ROME – Patients with systemic sclerosis who have a range of gastointestinal symptoms and severity have higher amounts of pathogenic bacteria than do normal healthy control patients, according to Dr. Elizabeth Volkmann and her colleagues.
They examined the bacterial populations found in the cecal and sigmoid portion of the colon in 17 patients with systemic sclerosis (SSc) and found a large increase in bacteria known to perpetuate inflammation in other autoimmune diseases, particularly inflammatory bowel disease, as well as a decrease in healthy commensal bacteria that are thought to decrease inflammation. The levels of both commensal and pathogenic bacteria also correlated with the severity of symptoms that patients described, said Dr. Volkmann, a rheumatologist and clinical instructor at the University of California, Los Angeles.
Bifidobacterium and Lactobacillus, two species normally found at lower levels in chronic inflammatory conditions, were increased in SSc. “This was a rather unique feature of systemic sclerosis,” she said in an interview at the European Congress of Rheumatology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ROME – Patients with systemic sclerosis who have a range of gastointestinal symptoms and severity have higher amounts of pathogenic bacteria than do normal healthy control patients, according to Dr. Elizabeth Volkmann and her colleagues.
They examined the bacterial populations found in the cecal and sigmoid portion of the colon in 17 patients with systemic sclerosis (SSc) and found a large increase in bacteria known to perpetuate inflammation in other autoimmune diseases, particularly inflammatory bowel disease, as well as a decrease in healthy commensal bacteria that are thought to decrease inflammation. The levels of both commensal and pathogenic bacteria also correlated with the severity of symptoms that patients described, said Dr. Volkmann, a rheumatologist and clinical instructor at the University of California, Los Angeles.
Bifidobacterium and Lactobacillus, two species normally found at lower levels in chronic inflammatory conditions, were increased in SSc. “This was a rather unique feature of systemic sclerosis,” she said in an interview at the European Congress of Rheumatology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ROME – Patients with systemic sclerosis who have a range of gastointestinal symptoms and severity have higher amounts of pathogenic bacteria than do normal healthy control patients, according to Dr. Elizabeth Volkmann and her colleagues.
They examined the bacterial populations found in the cecal and sigmoid portion of the colon in 17 patients with systemic sclerosis (SSc) and found a large increase in bacteria known to perpetuate inflammation in other autoimmune diseases, particularly inflammatory bowel disease, as well as a decrease in healthy commensal bacteria that are thought to decrease inflammation. The levels of both commensal and pathogenic bacteria also correlated with the severity of symptoms that patients described, said Dr. Volkmann, a rheumatologist and clinical instructor at the University of California, Los Angeles.
Bifidobacterium and Lactobacillus, two species normally found at lower levels in chronic inflammatory conditions, were increased in SSc. “This was a rather unique feature of systemic sclerosis,” she said in an interview at the European Congress of Rheumatology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE EULAR 2015 CONGRESS