User login
Jeff Evans has been editor of Rheumatology News/MDedge Rheumatology and the EULAR Congress News since 2013. He started at Frontline Medical Communications in 2001 and was a reporter for 8 years before serving as editor of Clinical Neurology News and World Neurology, and briefly as editor of GI & Hepatology News. He graduated cum laude from Cornell University (New York) with a BA in biological sciences, concentrating in neurobiology and behavior.
MS trial tracks patient-reported benefits of switching from an injectable to fingolimod
Patients with relapsing-remitting multiple sclerosis who switched from an injectable disease-modifying therapy to orally administered fingolimod reported significantly greater treatment satisfaction as well as functional and quality of life outcomes than did those who took the same or a different injectable therapy in a phase IV, open-label, randomized trial.
The results of this trial, called EPOC (Evaluate Patient Outcomes), were similar to those reported for changes in activities of daily living according to the PRIMUS (Patient Reported Indices for Multiple Sclerosis) Activities scale in the double-blind, randomized TRANSFORMS trial that compared fingolimod (Gilenya) to intramuscular interferon beta-1a. However, these measurements did not reach statistical significance in EPOC, possibly because its 6-month duration was not long enough to detect differences, Dr. Edward Fox of Central Texas Neurology Consultants, Round Rock, and his colleagues reported on behalf of the EPOC study investigators.
"Our findings are consistent with those of studies in other diseases, such as thromboembolism prophylaxis, iron chelation, and oncology, which have also found greater levels of patient satisfaction and improved QOL associated with oral versus injectable therapies," the investigators wrote.
For the EPOC trial, investigators from 158 centers in the United States and Canada randomized 1,053 patients with relapsing-remitting MS to either fingolimod 0.5 mg once daily (790 patients) or an injectable disease-modifying therapy (iDMT; 263 patients). The iDMT options included interferon beta-1b subcutaneous 0.25 mg every other day (Betaseron), interferon beta-1a intramuscular 30 mcg once per week (Avonex), interferon beta-1a subcutaneous three times per week (Rebif), or glatiramer acetate subcutaneous 20 mg once daily (Copaxone). The patients had taken an iDMT for at least 6 months and had no washout period before making a switch. They had a mean duration of MS symptoms of 12 years and a mean Expanded Disability Status Scale score of 2.4 (Mult. Scler. Relat. Disord. 2014 July 3 [doi: 10.1016/j.msard.2014.06.005]).
At the time of randomization, most patients were taking glatiramer acetate (33%-35%), followed by intramuscular interferon beta-1a (23%-26%), subcutaneous interferon beta-1a (25% in each), and interferon beta-1b (16%-18%). The patients’ most commonly cited reasons for wishing to switch to a different therapy included mode of administration in 61%-62%, tolerability issues in 13%-14%, and physician-determined lack of efficacy in 12%-16%. Of the 263 patients who stayed on an iDMT, 26 switched to a different drug.
By 6 months, fingolimod-treated patients had significantly higher treatment satisfaction than did those who remained on an iDMT, based on the primary endpoint of difference between the groups on the change in the Global Satisfaction subscale score of the Treatment Satisfaction Questionnaire for Medication. They also reported significant improvements over patients who remained on an iDMT on secondary endpoints that measured changes on the Fatigue Severity Scale, the Beck Depression Inventory II, and the 36-item Short-Form Health Survey’s domains of physical health, bodily pain, vitality, social functioning, role limitation due to emotional problems, and general mental health. The two groups reported no statistically significant differences on changes in perceived activity limitations as measured by the PRIMUS Activities scale.
The physician-rated Clinical Global Impression of Improvement scores significantly favored fingolimod over iDMT (3.2 vs. 3.9, respectively), and although these scores were "in the range of only minimal improvement to no change in both the fingolimod and the iDMT groups, it is worth noting that the effect was measured early in the course of fingolimod treatment in this cohort of patients with long-standing MS," Dr. Fox and his associates wrote.
Adverse events occurred more often among patients who took fingolimod than in those who took an iDMT (79% vs. 62%, respectively), and the most common of these for fingolimod-treated patients were headache and fatigue at 12% each. There was no difference in the incidence of serious adverse events between patients who took fingolimod or an iDMT (4% vs. 2%, respectively), including MS relapse (0.6% vs. 0.8%) and lymphopenia (0.3% vs. 0%). Overall, 41 fingolimod-treated patients discontinued treatment because of an adverse event, compared with 4 iDMT-treated patients.
"Notably, the absence of a washout period between cessation of the iDMT and initiation of fingolimod did not appear to be associated with deleterious additive immune system effects," the investigators wrote.
The study was funded by Novartis, which markets fingolimod. Many authors reported financial relationships with Novartis as well as with the marketers of Betaseron (Bayer HealthCare), Avonex (Biogen Idec), Rebif (EMD Serono and Pfizer), and Copaxone (Teva Neuroscience). Four authors are or were employees of Novartis.
Patients with relapsing-remitting multiple sclerosis who switched from an injectable disease-modifying therapy to orally administered fingolimod reported significantly greater treatment satisfaction as well as functional and quality of life outcomes than did those who took the same or a different injectable therapy in a phase IV, open-label, randomized trial.
The results of this trial, called EPOC (Evaluate Patient Outcomes), were similar to those reported for changes in activities of daily living according to the PRIMUS (Patient Reported Indices for Multiple Sclerosis) Activities scale in the double-blind, randomized TRANSFORMS trial that compared fingolimod (Gilenya) to intramuscular interferon beta-1a. However, these measurements did not reach statistical significance in EPOC, possibly because its 6-month duration was not long enough to detect differences, Dr. Edward Fox of Central Texas Neurology Consultants, Round Rock, and his colleagues reported on behalf of the EPOC study investigators.
"Our findings are consistent with those of studies in other diseases, such as thromboembolism prophylaxis, iron chelation, and oncology, which have also found greater levels of patient satisfaction and improved QOL associated with oral versus injectable therapies," the investigators wrote.
For the EPOC trial, investigators from 158 centers in the United States and Canada randomized 1,053 patients with relapsing-remitting MS to either fingolimod 0.5 mg once daily (790 patients) or an injectable disease-modifying therapy (iDMT; 263 patients). The iDMT options included interferon beta-1b subcutaneous 0.25 mg every other day (Betaseron), interferon beta-1a intramuscular 30 mcg once per week (Avonex), interferon beta-1a subcutaneous three times per week (Rebif), or glatiramer acetate subcutaneous 20 mg once daily (Copaxone). The patients had taken an iDMT for at least 6 months and had no washout period before making a switch. They had a mean duration of MS symptoms of 12 years and a mean Expanded Disability Status Scale score of 2.4 (Mult. Scler. Relat. Disord. 2014 July 3 [doi: 10.1016/j.msard.2014.06.005]).
At the time of randomization, most patients were taking glatiramer acetate (33%-35%), followed by intramuscular interferon beta-1a (23%-26%), subcutaneous interferon beta-1a (25% in each), and interferon beta-1b (16%-18%). The patients’ most commonly cited reasons for wishing to switch to a different therapy included mode of administration in 61%-62%, tolerability issues in 13%-14%, and physician-determined lack of efficacy in 12%-16%. Of the 263 patients who stayed on an iDMT, 26 switched to a different drug.
By 6 months, fingolimod-treated patients had significantly higher treatment satisfaction than did those who remained on an iDMT, based on the primary endpoint of difference between the groups on the change in the Global Satisfaction subscale score of the Treatment Satisfaction Questionnaire for Medication. They also reported significant improvements over patients who remained on an iDMT on secondary endpoints that measured changes on the Fatigue Severity Scale, the Beck Depression Inventory II, and the 36-item Short-Form Health Survey’s domains of physical health, bodily pain, vitality, social functioning, role limitation due to emotional problems, and general mental health. The two groups reported no statistically significant differences on changes in perceived activity limitations as measured by the PRIMUS Activities scale.
The physician-rated Clinical Global Impression of Improvement scores significantly favored fingolimod over iDMT (3.2 vs. 3.9, respectively), and although these scores were "in the range of only minimal improvement to no change in both the fingolimod and the iDMT groups, it is worth noting that the effect was measured early in the course of fingolimod treatment in this cohort of patients with long-standing MS," Dr. Fox and his associates wrote.
Adverse events occurred more often among patients who took fingolimod than in those who took an iDMT (79% vs. 62%, respectively), and the most common of these for fingolimod-treated patients were headache and fatigue at 12% each. There was no difference in the incidence of serious adverse events between patients who took fingolimod or an iDMT (4% vs. 2%, respectively), including MS relapse (0.6% vs. 0.8%) and lymphopenia (0.3% vs. 0%). Overall, 41 fingolimod-treated patients discontinued treatment because of an adverse event, compared with 4 iDMT-treated patients.
"Notably, the absence of a washout period between cessation of the iDMT and initiation of fingolimod did not appear to be associated with deleterious additive immune system effects," the investigators wrote.
The study was funded by Novartis, which markets fingolimod. Many authors reported financial relationships with Novartis as well as with the marketers of Betaseron (Bayer HealthCare), Avonex (Biogen Idec), Rebif (EMD Serono and Pfizer), and Copaxone (Teva Neuroscience). Four authors are or were employees of Novartis.
Patients with relapsing-remitting multiple sclerosis who switched from an injectable disease-modifying therapy to orally administered fingolimod reported significantly greater treatment satisfaction as well as functional and quality of life outcomes than did those who took the same or a different injectable therapy in a phase IV, open-label, randomized trial.
The results of this trial, called EPOC (Evaluate Patient Outcomes), were similar to those reported for changes in activities of daily living according to the PRIMUS (Patient Reported Indices for Multiple Sclerosis) Activities scale in the double-blind, randomized TRANSFORMS trial that compared fingolimod (Gilenya) to intramuscular interferon beta-1a. However, these measurements did not reach statistical significance in EPOC, possibly because its 6-month duration was not long enough to detect differences, Dr. Edward Fox of Central Texas Neurology Consultants, Round Rock, and his colleagues reported on behalf of the EPOC study investigators.
"Our findings are consistent with those of studies in other diseases, such as thromboembolism prophylaxis, iron chelation, and oncology, which have also found greater levels of patient satisfaction and improved QOL associated with oral versus injectable therapies," the investigators wrote.
For the EPOC trial, investigators from 158 centers in the United States and Canada randomized 1,053 patients with relapsing-remitting MS to either fingolimod 0.5 mg once daily (790 patients) or an injectable disease-modifying therapy (iDMT; 263 patients). The iDMT options included interferon beta-1b subcutaneous 0.25 mg every other day (Betaseron), interferon beta-1a intramuscular 30 mcg once per week (Avonex), interferon beta-1a subcutaneous three times per week (Rebif), or glatiramer acetate subcutaneous 20 mg once daily (Copaxone). The patients had taken an iDMT for at least 6 months and had no washout period before making a switch. They had a mean duration of MS symptoms of 12 years and a mean Expanded Disability Status Scale score of 2.4 (Mult. Scler. Relat. Disord. 2014 July 3 [doi: 10.1016/j.msard.2014.06.005]).
At the time of randomization, most patients were taking glatiramer acetate (33%-35%), followed by intramuscular interferon beta-1a (23%-26%), subcutaneous interferon beta-1a (25% in each), and interferon beta-1b (16%-18%). The patients’ most commonly cited reasons for wishing to switch to a different therapy included mode of administration in 61%-62%, tolerability issues in 13%-14%, and physician-determined lack of efficacy in 12%-16%. Of the 263 patients who stayed on an iDMT, 26 switched to a different drug.
By 6 months, fingolimod-treated patients had significantly higher treatment satisfaction than did those who remained on an iDMT, based on the primary endpoint of difference between the groups on the change in the Global Satisfaction subscale score of the Treatment Satisfaction Questionnaire for Medication. They also reported significant improvements over patients who remained on an iDMT on secondary endpoints that measured changes on the Fatigue Severity Scale, the Beck Depression Inventory II, and the 36-item Short-Form Health Survey’s domains of physical health, bodily pain, vitality, social functioning, role limitation due to emotional problems, and general mental health. The two groups reported no statistically significant differences on changes in perceived activity limitations as measured by the PRIMUS Activities scale.
The physician-rated Clinical Global Impression of Improvement scores significantly favored fingolimod over iDMT (3.2 vs. 3.9, respectively), and although these scores were "in the range of only minimal improvement to no change in both the fingolimod and the iDMT groups, it is worth noting that the effect was measured early in the course of fingolimod treatment in this cohort of patients with long-standing MS," Dr. Fox and his associates wrote.
Adverse events occurred more often among patients who took fingolimod than in those who took an iDMT (79% vs. 62%, respectively), and the most common of these for fingolimod-treated patients were headache and fatigue at 12% each. There was no difference in the incidence of serious adverse events between patients who took fingolimod or an iDMT (4% vs. 2%, respectively), including MS relapse (0.6% vs. 0.8%) and lymphopenia (0.3% vs. 0%). Overall, 41 fingolimod-treated patients discontinued treatment because of an adverse event, compared with 4 iDMT-treated patients.
"Notably, the absence of a washout period between cessation of the iDMT and initiation of fingolimod did not appear to be associated with deleterious additive immune system effects," the investigators wrote.
The study was funded by Novartis, which markets fingolimod. Many authors reported financial relationships with Novartis as well as with the marketers of Betaseron (Bayer HealthCare), Avonex (Biogen Idec), Rebif (EMD Serono and Pfizer), and Copaxone (Teva Neuroscience). Four authors are or were employees of Novartis.
FROM MULTIPLE SCLEROSIS AND RELATED DISORDERS
Key clinical point: Patients who switched from an injectable DMT reported greater treatment satisfaction and improvements in fatigue, depression, and quality of life.
Major finding: The physician-rated Clinical Global Impression of Improvement scores significantly favored fingolimod over injectable DMT (3.2 vs. 3.9, respectively).
Data source: A phase IV, open-label, randomized trial of 1,053 patients with relapsing-remitting MS.
Disclosures: The study was funded by Novartis, which markets fingolimod. Many authors reported financial relationships with Novartis as well as with the marketers of Betaseron (Bayer HealthCare), Avonex (Biogen Idec), Rebif (EMD Serono and Pfizer), and Copaxone (Teva Neuroscience). Four authors are or were employees of Novartis.
VIDEO: Does obesity’s effect on RA support different treatment goals?
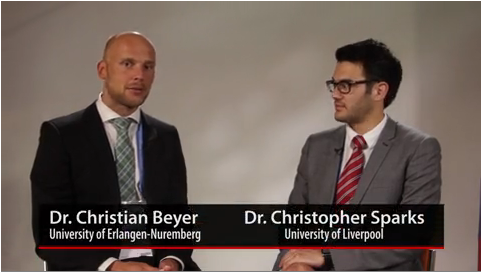
PARIS – Treat-to-target goals for obese patients with rheumatoid arthritis should take into account new research indicating that they already have a higher level of systemic inflammation and higher Disease Activity Scores than do normal-weight patients, according to Dr. Christopher Sparks of the University of Liverpool, England.
In an international sample of 3,534 patients with rheumatoid arthritis (RA), Dr. Sparks and his colleagues found that those with a body mass index of 30-34.9 kg/m2 (obese) or 35 kg/m2 or greater (obese II) had higher 28-joint Disease Activity Scores (DAS28) than did normal-weight patients, a difference that was largely driven by higher erythrocyte sedimentation rates and higher Visual Analog Scale scores, rather than higher tender and swollen joint counts. The data were reported at the annual European Congress of Rheumatology.
It cannot be known whether obese patients with higher DAS28 scores have clinically more severe disease, so it leads one to wonder, "Is it just an inflated DAS28 score that’s really driving this? And so potentially it brings up the question, Is obesity a confounding factor when looking at DAS28 scores in the RA population?" Dr. Sparks said in a video interview with Dr. Christian Beyer of the University of Erlangen-Nuremberg, Germany.
"If there is an artificial rise in the DAS28 in all of our obese patients out there, should the treatment goals in terms of remission or response be potentially slightly different for obese patients, compared to normal-weight patients, because they have a baseline higher DAS28?"
PARIS – Treat-to-target goals for obese patients with rheumatoid arthritis should take into account new research indicating that they already have a higher level of systemic inflammation and higher Disease Activity Scores than do normal-weight patients, according to Dr. Christopher Sparks of the University of Liverpool, England.
In an international sample of 3,534 patients with rheumatoid arthritis (RA), Dr. Sparks and his colleagues found that those with a body mass index of 30-34.9 kg/m2 (obese) or 35 kg/m2 or greater (obese II) had higher 28-joint Disease Activity Scores (DAS28) than did normal-weight patients, a difference that was largely driven by higher erythrocyte sedimentation rates and higher Visual Analog Scale scores, rather than higher tender and swollen joint counts. The data were reported at the annual European Congress of Rheumatology.
It cannot be known whether obese patients with higher DAS28 scores have clinically more severe disease, so it leads one to wonder, "Is it just an inflated DAS28 score that’s really driving this? And so potentially it brings up the question, Is obesity a confounding factor when looking at DAS28 scores in the RA population?" Dr. Sparks said in a video interview with Dr. Christian Beyer of the University of Erlangen-Nuremberg, Germany.
"If there is an artificial rise in the DAS28 in all of our obese patients out there, should the treatment goals in terms of remission or response be potentially slightly different for obese patients, compared to normal-weight patients, because they have a baseline higher DAS28?"
PARIS – Treat-to-target goals for obese patients with rheumatoid arthritis should take into account new research indicating that they already have a higher level of systemic inflammation and higher Disease Activity Scores than do normal-weight patients, according to Dr. Christopher Sparks of the University of Liverpool, England.
In an international sample of 3,534 patients with rheumatoid arthritis (RA), Dr. Sparks and his colleagues found that those with a body mass index of 30-34.9 kg/m2 (obese) or 35 kg/m2 or greater (obese II) had higher 28-joint Disease Activity Scores (DAS28) than did normal-weight patients, a difference that was largely driven by higher erythrocyte sedimentation rates and higher Visual Analog Scale scores, rather than higher tender and swollen joint counts. The data were reported at the annual European Congress of Rheumatology.
It cannot be known whether obese patients with higher DAS28 scores have clinically more severe disease, so it leads one to wonder, "Is it just an inflated DAS28 score that’s really driving this? And so potentially it brings up the question, Is obesity a confounding factor when looking at DAS28 scores in the RA population?" Dr. Sparks said in a video interview with Dr. Christian Beyer of the University of Erlangen-Nuremberg, Germany.
"If there is an artificial rise in the DAS28 in all of our obese patients out there, should the treatment goals in terms of remission or response be potentially slightly different for obese patients, compared to normal-weight patients, because they have a baseline higher DAS28?"
AT THE EULAR CONGRESS 2014
Increased risk of heart attack confirmed in Sjögren’s syndrome patients
PARIS – Patients with Sjögren’s syndrome appear to have a higher risk of myocardial infarction, but not stroke or transient ischemic attack, than does the general population, based on an analysis of administrative health data from British Columbia.
"Our findings support monitoring for coronary artery disease in addition to management and modification of cardiovascular disease risk factors to reduce the risk of MI in Sjögren’s syndrome patients," Dr. Marko Yurkovich said at the annual European Congress of Rheumatology.
An increased risk of MI and cerebrovascular accident (CVA) is already well known in more common rheumatic diseases such as rheumatoid arthritis and systemic lupus erythematosus, but the risk for either MI or CVA at the general population level in patients with Sjögren’s syndrome remains unknown, said Dr. Yurkovich, an internal medicine resident at the University of British Columbia, Vancouver.
The investigators used administrative health data from all people in British Columbia who had an outpatient visit or hospitalization during 1990-2010 to locate those who were older than 18 years and met the study’s criteria for a diagnosis of Sjögren’s syndrome. The criteria were two or more ICD-9-CM codes for the disease 2 or more months apart but within a 2-year period that were made by nonrheumatologists or one or more ICD-9-CM codes for the disease that were made by a rheumatologist or during a hospitalization.
The study excluded patients who had diagnoses of other inflammatory arthritides after the Sjögren’s diagnosis or cases in which an initial diagnosis of Sjögren’s by a nonrheumatologist was not confirmed by a rheumatologist at a later visit. MI and CVA diagnoses were defined from ICD-9-CM codes from hospital or death certificates.
"This diagnostic algorithm has been validated in a Canadian context and shown to have quite a high specificity and sensitivity, both over 95%," Dr. Yurkovich said.
In 1,176 patients with Sjögren’s syndrome, the investigators identified 28 MIs for an incidence rate of 7.7 per 1,000 person-years, compared with 138 MIs in 11,879 control individuals from the general population matched for birth year, sex, and calendar year of exposure. Patients with Sjögren’s had a significantly elevated risk for MI, compared with controls, with an incidence rate ratio of 2.19 (95% confidence interval, 1.40-3.31) and a multivariate relative risk of 2.36 (95% CI, 1.48-3.78), based on incidence rates of 7.7 per 1,000 person-years in patients with Sjögren’s and 3.4 per 1,000 person-years in controls. The relative risk for MI did not differ when the results were stratified by sex.
However, risk of incident CVA was not significantly higher among patients with Sjögren’s than in the controls, based on an incidence rate of 5.1 per 1,000 person-years among patients and 3.4 per 1,000 person-years in controls that yielded an incidence rate ratio of 1.49 and multivariable relative risk of 1.64.
The multivariable analysis controlled for angina, chronic obstructive pulmonary disease, obesity, Charlson Comorbidity Index, the number of hospitalizations in the year before the index date, and number of medications, including oral glucocorticoids, cardiovascular drugs, antidiabetic medication, hormone replacement therapy, contraceptives, fibrates, statins, NSAIDs, and COX-2 inhibitors.
"We found that the risk of MI was even further elevated within 1 year following initial diagnosis," with an incidence rate ratio of 3.6 before 1 year of follow-up, which decreased to 1.7 during 1-5 years of follow-up and 1.9 and after 5 years, Dr. Yurkovich said. He suggested that the increased risk of MI in the first year might occur because the acute inflammatory state in Sjögren’s is highest at the time of diagnosis or because the most susceptible patients had an MI early on and were no longer included in subsequent analyses.
The results remained statistically significant for MI and not for CVA after the investigators performed sensitivity analyses for unmeasured confounding variables using hypothetical low and high prevalences of 10% and 20% and low and high strengths of association in terms of odds ratios (1.3 and 3.0).
A member of the audience wondered whether antiphospholipid antibodies could have contributed to the increased risk for MI because patients with Sjögren’s syndrome typically don’t use medications such as glucocorticoids that increase atherosclerosis, and inflammation is not typically elevated in the condition, with normal C-reactive protein levels. Dr. Yurkovich replied that the cohort used glucocorticoids at a significantly higher rate than did the control group (20% vs. 4%), perhaps because they were put on them initially around the time of diagnosis, but nevertheless, the medication was one of the variables they adjusted for in the multivariable analysis. He noted that the investigators did not have antiphospholipid antibody data but they hope to obtain those and other autoantibody profiles for subsequent analyses.
Dr. Yurkovich had nothing to disclose.
PARIS – Patients with Sjögren’s syndrome appear to have a higher risk of myocardial infarction, but not stroke or transient ischemic attack, than does the general population, based on an analysis of administrative health data from British Columbia.
"Our findings support monitoring for coronary artery disease in addition to management and modification of cardiovascular disease risk factors to reduce the risk of MI in Sjögren’s syndrome patients," Dr. Marko Yurkovich said at the annual European Congress of Rheumatology.
An increased risk of MI and cerebrovascular accident (CVA) is already well known in more common rheumatic diseases such as rheumatoid arthritis and systemic lupus erythematosus, but the risk for either MI or CVA at the general population level in patients with Sjögren’s syndrome remains unknown, said Dr. Yurkovich, an internal medicine resident at the University of British Columbia, Vancouver.
The investigators used administrative health data from all people in British Columbia who had an outpatient visit or hospitalization during 1990-2010 to locate those who were older than 18 years and met the study’s criteria for a diagnosis of Sjögren’s syndrome. The criteria were two or more ICD-9-CM codes for the disease 2 or more months apart but within a 2-year period that were made by nonrheumatologists or one or more ICD-9-CM codes for the disease that were made by a rheumatologist or during a hospitalization.
The study excluded patients who had diagnoses of other inflammatory arthritides after the Sjögren’s diagnosis or cases in which an initial diagnosis of Sjögren’s by a nonrheumatologist was not confirmed by a rheumatologist at a later visit. MI and CVA diagnoses were defined from ICD-9-CM codes from hospital or death certificates.
"This diagnostic algorithm has been validated in a Canadian context and shown to have quite a high specificity and sensitivity, both over 95%," Dr. Yurkovich said.
In 1,176 patients with Sjögren’s syndrome, the investigators identified 28 MIs for an incidence rate of 7.7 per 1,000 person-years, compared with 138 MIs in 11,879 control individuals from the general population matched for birth year, sex, and calendar year of exposure. Patients with Sjögren’s had a significantly elevated risk for MI, compared with controls, with an incidence rate ratio of 2.19 (95% confidence interval, 1.40-3.31) and a multivariate relative risk of 2.36 (95% CI, 1.48-3.78), based on incidence rates of 7.7 per 1,000 person-years in patients with Sjögren’s and 3.4 per 1,000 person-years in controls. The relative risk for MI did not differ when the results were stratified by sex.
However, risk of incident CVA was not significantly higher among patients with Sjögren’s than in the controls, based on an incidence rate of 5.1 per 1,000 person-years among patients and 3.4 per 1,000 person-years in controls that yielded an incidence rate ratio of 1.49 and multivariable relative risk of 1.64.
The multivariable analysis controlled for angina, chronic obstructive pulmonary disease, obesity, Charlson Comorbidity Index, the number of hospitalizations in the year before the index date, and number of medications, including oral glucocorticoids, cardiovascular drugs, antidiabetic medication, hormone replacement therapy, contraceptives, fibrates, statins, NSAIDs, and COX-2 inhibitors.
"We found that the risk of MI was even further elevated within 1 year following initial diagnosis," with an incidence rate ratio of 3.6 before 1 year of follow-up, which decreased to 1.7 during 1-5 years of follow-up and 1.9 and after 5 years, Dr. Yurkovich said. He suggested that the increased risk of MI in the first year might occur because the acute inflammatory state in Sjögren’s is highest at the time of diagnosis or because the most susceptible patients had an MI early on and were no longer included in subsequent analyses.
The results remained statistically significant for MI and not for CVA after the investigators performed sensitivity analyses for unmeasured confounding variables using hypothetical low and high prevalences of 10% and 20% and low and high strengths of association in terms of odds ratios (1.3 and 3.0).
A member of the audience wondered whether antiphospholipid antibodies could have contributed to the increased risk for MI because patients with Sjögren’s syndrome typically don’t use medications such as glucocorticoids that increase atherosclerosis, and inflammation is not typically elevated in the condition, with normal C-reactive protein levels. Dr. Yurkovich replied that the cohort used glucocorticoids at a significantly higher rate than did the control group (20% vs. 4%), perhaps because they were put on them initially around the time of diagnosis, but nevertheless, the medication was one of the variables they adjusted for in the multivariable analysis. He noted that the investigators did not have antiphospholipid antibody data but they hope to obtain those and other autoantibody profiles for subsequent analyses.
Dr. Yurkovich had nothing to disclose.
PARIS – Patients with Sjögren’s syndrome appear to have a higher risk of myocardial infarction, but not stroke or transient ischemic attack, than does the general population, based on an analysis of administrative health data from British Columbia.
"Our findings support monitoring for coronary artery disease in addition to management and modification of cardiovascular disease risk factors to reduce the risk of MI in Sjögren’s syndrome patients," Dr. Marko Yurkovich said at the annual European Congress of Rheumatology.
An increased risk of MI and cerebrovascular accident (CVA) is already well known in more common rheumatic diseases such as rheumatoid arthritis and systemic lupus erythematosus, but the risk for either MI or CVA at the general population level in patients with Sjögren’s syndrome remains unknown, said Dr. Yurkovich, an internal medicine resident at the University of British Columbia, Vancouver.
The investigators used administrative health data from all people in British Columbia who had an outpatient visit or hospitalization during 1990-2010 to locate those who were older than 18 years and met the study’s criteria for a diagnosis of Sjögren’s syndrome. The criteria were two or more ICD-9-CM codes for the disease 2 or more months apart but within a 2-year period that were made by nonrheumatologists or one or more ICD-9-CM codes for the disease that were made by a rheumatologist or during a hospitalization.
The study excluded patients who had diagnoses of other inflammatory arthritides after the Sjögren’s diagnosis or cases in which an initial diagnosis of Sjögren’s by a nonrheumatologist was not confirmed by a rheumatologist at a later visit. MI and CVA diagnoses were defined from ICD-9-CM codes from hospital or death certificates.
"This diagnostic algorithm has been validated in a Canadian context and shown to have quite a high specificity and sensitivity, both over 95%," Dr. Yurkovich said.
In 1,176 patients with Sjögren’s syndrome, the investigators identified 28 MIs for an incidence rate of 7.7 per 1,000 person-years, compared with 138 MIs in 11,879 control individuals from the general population matched for birth year, sex, and calendar year of exposure. Patients with Sjögren’s had a significantly elevated risk for MI, compared with controls, with an incidence rate ratio of 2.19 (95% confidence interval, 1.40-3.31) and a multivariate relative risk of 2.36 (95% CI, 1.48-3.78), based on incidence rates of 7.7 per 1,000 person-years in patients with Sjögren’s and 3.4 per 1,000 person-years in controls. The relative risk for MI did not differ when the results were stratified by sex.
However, risk of incident CVA was not significantly higher among patients with Sjögren’s than in the controls, based on an incidence rate of 5.1 per 1,000 person-years among patients and 3.4 per 1,000 person-years in controls that yielded an incidence rate ratio of 1.49 and multivariable relative risk of 1.64.
The multivariable analysis controlled for angina, chronic obstructive pulmonary disease, obesity, Charlson Comorbidity Index, the number of hospitalizations in the year before the index date, and number of medications, including oral glucocorticoids, cardiovascular drugs, antidiabetic medication, hormone replacement therapy, contraceptives, fibrates, statins, NSAIDs, and COX-2 inhibitors.
"We found that the risk of MI was even further elevated within 1 year following initial diagnosis," with an incidence rate ratio of 3.6 before 1 year of follow-up, which decreased to 1.7 during 1-5 years of follow-up and 1.9 and after 5 years, Dr. Yurkovich said. He suggested that the increased risk of MI in the first year might occur because the acute inflammatory state in Sjögren’s is highest at the time of diagnosis or because the most susceptible patients had an MI early on and were no longer included in subsequent analyses.
The results remained statistically significant for MI and not for CVA after the investigators performed sensitivity analyses for unmeasured confounding variables using hypothetical low and high prevalences of 10% and 20% and low and high strengths of association in terms of odds ratios (1.3 and 3.0).
A member of the audience wondered whether antiphospholipid antibodies could have contributed to the increased risk for MI because patients with Sjögren’s syndrome typically don’t use medications such as glucocorticoids that increase atherosclerosis, and inflammation is not typically elevated in the condition, with normal C-reactive protein levels. Dr. Yurkovich replied that the cohort used glucocorticoids at a significantly higher rate than did the control group (20% vs. 4%), perhaps because they were put on them initially around the time of diagnosis, but nevertheless, the medication was one of the variables they adjusted for in the multivariable analysis. He noted that the investigators did not have antiphospholipid antibody data but they hope to obtain those and other autoantibody profiles for subsequent analyses.
Dr. Yurkovich had nothing to disclose.
AT THE EULAR CONGRESS 2014
Key clinical point: The findings support monitoring for coronary artery disease in patients with Sjögren’s syndrome in addition to management and modification of cardiovascular disease risk factors to reduce the risk of MI.
Major finding: Patients with Sjögren’s had a significantly elevated risk for MI, compared with controls, with an incidence rate ratio of 2.19 (95% CI, 1.40-3.31) and a multivariate relative risk of 2.36 (95% CI, 1.48-3.78).
Data source: A case-control study of 1,167 patients with Sjögren’s syndrome and 11,879 control individuals within an administrative health database.
Disclosures: Dr. Yurkovich had nothing to disclose.
VIDEO: Gene profiling could signal start of personalized medicine in RA
PARIS – A set of genetic polymorphisms is beginning to allow researchers to predict which patients with rheumatoid arthritis will have a severe disease course, as well as determine their response to treatment and risk of death.
Changes in amino acids at positions 71 and 74 of the HLA-DRB1 gene, which are a part of the "shared epitope" that is already known to increase genetic susceptibility for rheumatoid arthritis, as well as a new polymorphism at position 11 of the HLA-DRB1 gene that is outside the shared epitope, are key to this effort. These polymorphisms predicted the radiologic outcome of rheumatoid arthritis patients, response to anti-tumor necrosis factor therapy, and mortality in an analysis of blood samples from three independent multicenter, prospective cohort studies. The three polymorphisms defined 16 haplotypes whose effects on RA susceptibility range from protective to increasing risk and were perfectly correlated with the observed levels of disease susceptibility.
Further studies will be necessary to validate the associations observed with the sets of polymorphisms, said Dr. Sebastien Viatte, first author of the study and a research fellow at the Centre for Musculoskeletal Research at the University of Manchester (England). Nonetheless, the results are an important step in showing that "genetics can be used to predict disease outcomes and is ... likely to enter the clinic within 5-10 years," he said in a video interview at the annual European Congress of Rheumatology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
PARIS – A set of genetic polymorphisms is beginning to allow researchers to predict which patients with rheumatoid arthritis will have a severe disease course, as well as determine their response to treatment and risk of death.
Changes in amino acids at positions 71 and 74 of the HLA-DRB1 gene, which are a part of the "shared epitope" that is already known to increase genetic susceptibility for rheumatoid arthritis, as well as a new polymorphism at position 11 of the HLA-DRB1 gene that is outside the shared epitope, are key to this effort. These polymorphisms predicted the radiologic outcome of rheumatoid arthritis patients, response to anti-tumor necrosis factor therapy, and mortality in an analysis of blood samples from three independent multicenter, prospective cohort studies. The three polymorphisms defined 16 haplotypes whose effects on RA susceptibility range from protective to increasing risk and were perfectly correlated with the observed levels of disease susceptibility.
Further studies will be necessary to validate the associations observed with the sets of polymorphisms, said Dr. Sebastien Viatte, first author of the study and a research fellow at the Centre for Musculoskeletal Research at the University of Manchester (England). Nonetheless, the results are an important step in showing that "genetics can be used to predict disease outcomes and is ... likely to enter the clinic within 5-10 years," he said in a video interview at the annual European Congress of Rheumatology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
PARIS – A set of genetic polymorphisms is beginning to allow researchers to predict which patients with rheumatoid arthritis will have a severe disease course, as well as determine their response to treatment and risk of death.
Changes in amino acids at positions 71 and 74 of the HLA-DRB1 gene, which are a part of the "shared epitope" that is already known to increase genetic susceptibility for rheumatoid arthritis, as well as a new polymorphism at position 11 of the HLA-DRB1 gene that is outside the shared epitope, are key to this effort. These polymorphisms predicted the radiologic outcome of rheumatoid arthritis patients, response to anti-tumor necrosis factor therapy, and mortality in an analysis of blood samples from three independent multicenter, prospective cohort studies. The three polymorphisms defined 16 haplotypes whose effects on RA susceptibility range from protective to increasing risk and were perfectly correlated with the observed levels of disease susceptibility.
Further studies will be necessary to validate the associations observed with the sets of polymorphisms, said Dr. Sebastien Viatte, first author of the study and a research fellow at the Centre for Musculoskeletal Research at the University of Manchester (England). Nonetheless, the results are an important step in showing that "genetics can be used to predict disease outcomes and is ... likely to enter the clinic within 5-10 years," he said in a video interview at the annual European Congress of Rheumatology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE EULAR CONGRESS 2014
VIDEO: Micro RNA biomarkers hint at potential for predicting OA
PARIS – Newly discovered micro RNA molecules found in the blood of patients before they developed severe osteoarthritis may help predict the disease and potentially identify those who would most benefit from preventive interventions.
That finding emerged from a longitudinal study presented at the annual European Congress of Rheumatology.
By using a screening technique to search a broad array of micro RNAs (miRNAs), Dr. Christian Beyer of the University of Erlangen-Nuremberg (Germany) and his colleagues found several miRNAs that were differentially expressed in the blood of 67 people who developed severe osteoarthritis and underwent at least one total joint replacement and 749 who did not.
One miRNA molecule in particular, known as let-7e, appeared to be "very promising" in this regard, he said. Let-7e is inversely associated with the development of osteoarthritis, with levels correlating with the risk of developing OA, Dr. Beyer said in a video interview with Dr. Christopher Sparks of the University of Liverpool (England).
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
PARIS – Newly discovered micro RNA molecules found in the blood of patients before they developed severe osteoarthritis may help predict the disease and potentially identify those who would most benefit from preventive interventions.
That finding emerged from a longitudinal study presented at the annual European Congress of Rheumatology.
By using a screening technique to search a broad array of micro RNAs (miRNAs), Dr. Christian Beyer of the University of Erlangen-Nuremberg (Germany) and his colleagues found several miRNAs that were differentially expressed in the blood of 67 people who developed severe osteoarthritis and underwent at least one total joint replacement and 749 who did not.
One miRNA molecule in particular, known as let-7e, appeared to be "very promising" in this regard, he said. Let-7e is inversely associated with the development of osteoarthritis, with levels correlating with the risk of developing OA, Dr. Beyer said in a video interview with Dr. Christopher Sparks of the University of Liverpool (England).
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
PARIS – Newly discovered micro RNA molecules found in the blood of patients before they developed severe osteoarthritis may help predict the disease and potentially identify those who would most benefit from preventive interventions.
That finding emerged from a longitudinal study presented at the annual European Congress of Rheumatology.
By using a screening technique to search a broad array of micro RNAs (miRNAs), Dr. Christian Beyer of the University of Erlangen-Nuremberg (Germany) and his colleagues found several miRNAs that were differentially expressed in the blood of 67 people who developed severe osteoarthritis and underwent at least one total joint replacement and 749 who did not.
One miRNA molecule in particular, known as let-7e, appeared to be "very promising" in this regard, he said. Let-7e is inversely associated with the development of osteoarthritis, with levels correlating with the risk of developing OA, Dr. Beyer said in a video interview with Dr. Christopher Sparks of the University of Liverpool (England).
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE EULAR CONGRESS 2014
VIDEO: ACR, EULAR collaborate on first polymyalgia rheumatica treatment guidelines
PARIS – New guidelines aim to narrow the wide variation that has been observed across specialties and countries in the treatment of patients with polymyalgia rheumatica.
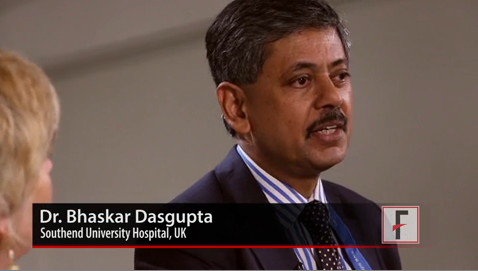
The guidelines, the first ever developed in a joint collaboration between the American College of Rheumatology and the European League Against Rheumatism, brought together "probably the biggest and most diverse guidelines panel" ever assembled for a rheumatologic condition – with 51 members, including patients, according to Dr. Bhaskar Dasgupta, primary author of the guidelines and leader of the guidelines study group.
Within the guidelines is a flowchart for the treatment of polymyalgia rheumatica that outlines overarching principles for care involving clinical, laboratory, and imaging assessments, as well as patient views, said Dr. Dasgupta, head of the department of rheumatology at Southend University Hospital, Essex, England.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
PARIS – New guidelines aim to narrow the wide variation that has been observed across specialties and countries in the treatment of patients with polymyalgia rheumatica.
The guidelines, the first ever developed in a joint collaboration between the American College of Rheumatology and the European League Against Rheumatism, brought together "probably the biggest and most diverse guidelines panel" ever assembled for a rheumatologic condition – with 51 members, including patients, according to Dr. Bhaskar Dasgupta, primary author of the guidelines and leader of the guidelines study group.
Within the guidelines is a flowchart for the treatment of polymyalgia rheumatica that outlines overarching principles for care involving clinical, laboratory, and imaging assessments, as well as patient views, said Dr. Dasgupta, head of the department of rheumatology at Southend University Hospital, Essex, England.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
PARIS – New guidelines aim to narrow the wide variation that has been observed across specialties and countries in the treatment of patients with polymyalgia rheumatica.
The guidelines, the first ever developed in a joint collaboration between the American College of Rheumatology and the European League Against Rheumatism, brought together "probably the biggest and most diverse guidelines panel" ever assembled for a rheumatologic condition – with 51 members, including patients, according to Dr. Bhaskar Dasgupta, primary author of the guidelines and leader of the guidelines study group.
Within the guidelines is a flowchart for the treatment of polymyalgia rheumatica that outlines overarching principles for care involving clinical, laboratory, and imaging assessments, as well as patient views, said Dr. Dasgupta, head of the department of rheumatology at Southend University Hospital, Essex, England.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE EULAR CONGRESS 2014
Studies hint at safety, efficacy of spinal muscular atrophy drug
PHILADELPHIA – The latest interim results from open-label studies of the investigational antisense oligonucleotide therapy ISIS-SMNRx for the treatment of patients with type 1, 2, or 3 spinal muscular atrophy support its safety and are starting to show its potential efficacy in treating the range of severity seen in the disease.
In two ongoing studies with up to 9 months of follow-up data, no safety or tolerability concerns arose with total doses of up to 18 mg in patients with type 2 or 3 spinal muscular atrophy (SMA) and in total doses of up to 48 mg in infants with type 1 SMA. Children aged 2-15 years with type 2 or 3 SMA had a dose- and time-dependent improvement in scores on the Hammersmith Functional Motor Scale-Expanded (HFMSE) that also correlated well with levels of SMN protein in cerebrospinal fluid. Infants with type 1 SMA achieved motor milestones on the Hammersmith Infant Neurological Exam that were consistent with increases in motor function test scores, according to investigators who presented the results at the annual meeting of the American Academy of Neurology.
"It’s very encouraging that we can do this safely and that the children tolerate the lumbar punctures, and there’s hope that the measures [used in the studies] are sensitive to change," said primary investigator Dr. Claudia Chiriboga, who presented the interim results of a study in patients with SMA types 2 or 3.
In that study, ISIS-SMNRx, an antisense oligonucleotide that promotes transcription of the full-length SMN protein from the SMN2 gene, was administered in an intrathecal bolus via lumbar puncture at points during a 3-month period; patients were then followed for 6 months. A total of eight patients received 3 mg at each dose (total dose, 9 mg); eight received 6 mg at each dose (total dose, 18 mg); and nine received 9 mg at each dose (18 mg total). Later, investigators added a 12-mg dose cohort that currently has eight patients enrolled, but results in that cohort are not yet available, said Dr. Chiriboga of the division of child neurology at Columbia University, New York.
The SMA type 2 and 3 patients included 10 patients with type 2 and 15 with type 3. They were medically stable and 2-15 years old, with a mean age of 7.5 years. Most (20) had three copies of the SMN2 gene; 4 had four copies and 1 had two copies. A majority of the patients (16) were nonambulatory.
None of the adverse events reported were considered related to the study drug, and most of the 143 adverse events were mild or moderate, the investigators found. Two severe adverse events were back pain and myalgia. Most of the adverse events were related to the lumbar punctures.
Scores on the HFMSE improved from baseline by a mean of 1.5 points in the 3-mg group, 2.3 points in the 6-mg group, and a statistically significant 3.7 points in the 9-mg group. SMN levels in cerebrospinal fluid at day 85 increased from baseline in all groups but were significantly increased in the 9-mg group only.
Additional secondary endpoints showed nonsignificant improvement of 22.7 m at 9 months on the 6-minute walk test in those who could walk, and an improvement of 2.3 points on an 18-point scale measuring upper limb function in weaker nonambulatory patients, but the open-label nature of the study and small numbers of patients make it difficult to interpret such findings, Dr. Chiriboga said.
"The feeling is that when there’s chronicity, like end-stage type of changes – severe scoliosis, for example – that those individuals don’t do as well. ... It’s not so much the age," Dr. Chiriboga said in an interview. Patients with type 3 disease also do better because they have more SMN2 to begin with, she said.
Similarly, in the ongoing open-label study of infants with type 1 SMA, ISIS-SMNRx was administered to 4 patients in 6-mg doses at days 1, 15, 85, and 253, and in 12-mg doses to 11 patients at the same time points. These infants were all aged 7 months or younger. Their mean age at symptom onset was 7 weeks, and they were enrolled in the study at a mean age of 18-21 weeks. All but one patient had two copies of the SMN2 gene, reported primary investigator Dr. Richard S. Finkel.
None of the adverse events in the infants were deemed to be related to ISIS-SMNRx. Of 14 severe adverse events, 11 were respiratory infections, and all were considered to be consistent with severe infant SMA, said Dr. Finkel, chief of the division of neurology at Nemours Children’s Hospital and professor of neurology at the University of Central Florida, both in Orlando.
One patient in the 6-mg group died accidentally, and another underwent permanent ventilation. Two of 11 patients in the 12-mg group died of pulmonary infection, and 1 required permanent ventilation (16 or more hours per day continuously for more than 2 weeks in the absence of an acute reversible illness), although 4 of the patients in this group have not yet received all their doses. At the last follow-up, or at the time of death or permanent ventilation, the median age was 14 months in the 6-mg group and 9.6 months in the 12-mg group (which has not been followed as long).
Scores on the Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP-INTEND) showed increases in 8 of 11 infants who had completed treatment and evaluation. The scores increased by a mean of 5.4 points overall and by 8.3 points in those in the 12-mg group. Incremental milestones on the Hammersmith Infant Neurological Exam were achieved by 9 of 11 infants, including 6 of 7 in the 12-mg group.
The median age at death or need for permanent ventilation is 10.5 months in infants with two SMN2 gene copies, and 85% reach this endpoint at 18 months. Scores on the CHOP-INTEND also declined by 1.27 points per year, according to a study of the natural history of type 1 SMA in 34 patients by Dr. Finkel and his colleagues that is under review for publication.
Compound muscle action potentials measured in the ulnar nerve–innervated abductor digiti minimi and peroneal nerve–innervated anterior tibialis were stable or increased in most infants, he said.
These encouraging results with ISIS-SMNRx have led Isis to begin plans for phase III trials in patients with SMA types 1-3, the investigators said.
The studies are funded by Isis Pharmaceuticals, the Department of Defense, and the National Institute of Neurological Disorders and Stroke. Neither Dr. Finkel nor Dr. Chiriboga had conflicts of interest. Some of the coauthors in each study were employees of Isis.
PHILADELPHIA – The latest interim results from open-label studies of the investigational antisense oligonucleotide therapy ISIS-SMNRx for the treatment of patients with type 1, 2, or 3 spinal muscular atrophy support its safety and are starting to show its potential efficacy in treating the range of severity seen in the disease.
In two ongoing studies with up to 9 months of follow-up data, no safety or tolerability concerns arose with total doses of up to 18 mg in patients with type 2 or 3 spinal muscular atrophy (SMA) and in total doses of up to 48 mg in infants with type 1 SMA. Children aged 2-15 years with type 2 or 3 SMA had a dose- and time-dependent improvement in scores on the Hammersmith Functional Motor Scale-Expanded (HFMSE) that also correlated well with levels of SMN protein in cerebrospinal fluid. Infants with type 1 SMA achieved motor milestones on the Hammersmith Infant Neurological Exam that were consistent with increases in motor function test scores, according to investigators who presented the results at the annual meeting of the American Academy of Neurology.
"It’s very encouraging that we can do this safely and that the children tolerate the lumbar punctures, and there’s hope that the measures [used in the studies] are sensitive to change," said primary investigator Dr. Claudia Chiriboga, who presented the interim results of a study in patients with SMA types 2 or 3.
In that study, ISIS-SMNRx, an antisense oligonucleotide that promotes transcription of the full-length SMN protein from the SMN2 gene, was administered in an intrathecal bolus via lumbar puncture at points during a 3-month period; patients were then followed for 6 months. A total of eight patients received 3 mg at each dose (total dose, 9 mg); eight received 6 mg at each dose (total dose, 18 mg); and nine received 9 mg at each dose (18 mg total). Later, investigators added a 12-mg dose cohort that currently has eight patients enrolled, but results in that cohort are not yet available, said Dr. Chiriboga of the division of child neurology at Columbia University, New York.
The SMA type 2 and 3 patients included 10 patients with type 2 and 15 with type 3. They were medically stable and 2-15 years old, with a mean age of 7.5 years. Most (20) had three copies of the SMN2 gene; 4 had four copies and 1 had two copies. A majority of the patients (16) were nonambulatory.
None of the adverse events reported were considered related to the study drug, and most of the 143 adverse events were mild or moderate, the investigators found. Two severe adverse events were back pain and myalgia. Most of the adverse events were related to the lumbar punctures.
Scores on the HFMSE improved from baseline by a mean of 1.5 points in the 3-mg group, 2.3 points in the 6-mg group, and a statistically significant 3.7 points in the 9-mg group. SMN levels in cerebrospinal fluid at day 85 increased from baseline in all groups but were significantly increased in the 9-mg group only.
Additional secondary endpoints showed nonsignificant improvement of 22.7 m at 9 months on the 6-minute walk test in those who could walk, and an improvement of 2.3 points on an 18-point scale measuring upper limb function in weaker nonambulatory patients, but the open-label nature of the study and small numbers of patients make it difficult to interpret such findings, Dr. Chiriboga said.
"The feeling is that when there’s chronicity, like end-stage type of changes – severe scoliosis, for example – that those individuals don’t do as well. ... It’s not so much the age," Dr. Chiriboga said in an interview. Patients with type 3 disease also do better because they have more SMN2 to begin with, she said.
Similarly, in the ongoing open-label study of infants with type 1 SMA, ISIS-SMNRx was administered to 4 patients in 6-mg doses at days 1, 15, 85, and 253, and in 12-mg doses to 11 patients at the same time points. These infants were all aged 7 months or younger. Their mean age at symptom onset was 7 weeks, and they were enrolled in the study at a mean age of 18-21 weeks. All but one patient had two copies of the SMN2 gene, reported primary investigator Dr. Richard S. Finkel.
None of the adverse events in the infants were deemed to be related to ISIS-SMNRx. Of 14 severe adverse events, 11 were respiratory infections, and all were considered to be consistent with severe infant SMA, said Dr. Finkel, chief of the division of neurology at Nemours Children’s Hospital and professor of neurology at the University of Central Florida, both in Orlando.
One patient in the 6-mg group died accidentally, and another underwent permanent ventilation. Two of 11 patients in the 12-mg group died of pulmonary infection, and 1 required permanent ventilation (16 or more hours per day continuously for more than 2 weeks in the absence of an acute reversible illness), although 4 of the patients in this group have not yet received all their doses. At the last follow-up, or at the time of death or permanent ventilation, the median age was 14 months in the 6-mg group and 9.6 months in the 12-mg group (which has not been followed as long).
Scores on the Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP-INTEND) showed increases in 8 of 11 infants who had completed treatment and evaluation. The scores increased by a mean of 5.4 points overall and by 8.3 points in those in the 12-mg group. Incremental milestones on the Hammersmith Infant Neurological Exam were achieved by 9 of 11 infants, including 6 of 7 in the 12-mg group.
The median age at death or need for permanent ventilation is 10.5 months in infants with two SMN2 gene copies, and 85% reach this endpoint at 18 months. Scores on the CHOP-INTEND also declined by 1.27 points per year, according to a study of the natural history of type 1 SMA in 34 patients by Dr. Finkel and his colleagues that is under review for publication.
Compound muscle action potentials measured in the ulnar nerve–innervated abductor digiti minimi and peroneal nerve–innervated anterior tibialis were stable or increased in most infants, he said.
These encouraging results with ISIS-SMNRx have led Isis to begin plans for phase III trials in patients with SMA types 1-3, the investigators said.
The studies are funded by Isis Pharmaceuticals, the Department of Defense, and the National Institute of Neurological Disorders and Stroke. Neither Dr. Finkel nor Dr. Chiriboga had conflicts of interest. Some of the coauthors in each study were employees of Isis.
PHILADELPHIA – The latest interim results from open-label studies of the investigational antisense oligonucleotide therapy ISIS-SMNRx for the treatment of patients with type 1, 2, or 3 spinal muscular atrophy support its safety and are starting to show its potential efficacy in treating the range of severity seen in the disease.
In two ongoing studies with up to 9 months of follow-up data, no safety or tolerability concerns arose with total doses of up to 18 mg in patients with type 2 or 3 spinal muscular atrophy (SMA) and in total doses of up to 48 mg in infants with type 1 SMA. Children aged 2-15 years with type 2 or 3 SMA had a dose- and time-dependent improvement in scores on the Hammersmith Functional Motor Scale-Expanded (HFMSE) that also correlated well with levels of SMN protein in cerebrospinal fluid. Infants with type 1 SMA achieved motor milestones on the Hammersmith Infant Neurological Exam that were consistent with increases in motor function test scores, according to investigators who presented the results at the annual meeting of the American Academy of Neurology.
"It’s very encouraging that we can do this safely and that the children tolerate the lumbar punctures, and there’s hope that the measures [used in the studies] are sensitive to change," said primary investigator Dr. Claudia Chiriboga, who presented the interim results of a study in patients with SMA types 2 or 3.
In that study, ISIS-SMNRx, an antisense oligonucleotide that promotes transcription of the full-length SMN protein from the SMN2 gene, was administered in an intrathecal bolus via lumbar puncture at points during a 3-month period; patients were then followed for 6 months. A total of eight patients received 3 mg at each dose (total dose, 9 mg); eight received 6 mg at each dose (total dose, 18 mg); and nine received 9 mg at each dose (18 mg total). Later, investigators added a 12-mg dose cohort that currently has eight patients enrolled, but results in that cohort are not yet available, said Dr. Chiriboga of the division of child neurology at Columbia University, New York.
The SMA type 2 and 3 patients included 10 patients with type 2 and 15 with type 3. They were medically stable and 2-15 years old, with a mean age of 7.5 years. Most (20) had three copies of the SMN2 gene; 4 had four copies and 1 had two copies. A majority of the patients (16) were nonambulatory.
None of the adverse events reported were considered related to the study drug, and most of the 143 adverse events were mild or moderate, the investigators found. Two severe adverse events were back pain and myalgia. Most of the adverse events were related to the lumbar punctures.
Scores on the HFMSE improved from baseline by a mean of 1.5 points in the 3-mg group, 2.3 points in the 6-mg group, and a statistically significant 3.7 points in the 9-mg group. SMN levels in cerebrospinal fluid at day 85 increased from baseline in all groups but were significantly increased in the 9-mg group only.
Additional secondary endpoints showed nonsignificant improvement of 22.7 m at 9 months on the 6-minute walk test in those who could walk, and an improvement of 2.3 points on an 18-point scale measuring upper limb function in weaker nonambulatory patients, but the open-label nature of the study and small numbers of patients make it difficult to interpret such findings, Dr. Chiriboga said.
"The feeling is that when there’s chronicity, like end-stage type of changes – severe scoliosis, for example – that those individuals don’t do as well. ... It’s not so much the age," Dr. Chiriboga said in an interview. Patients with type 3 disease also do better because they have more SMN2 to begin with, she said.
Similarly, in the ongoing open-label study of infants with type 1 SMA, ISIS-SMNRx was administered to 4 patients in 6-mg doses at days 1, 15, 85, and 253, and in 12-mg doses to 11 patients at the same time points. These infants were all aged 7 months or younger. Their mean age at symptom onset was 7 weeks, and they were enrolled in the study at a mean age of 18-21 weeks. All but one patient had two copies of the SMN2 gene, reported primary investigator Dr. Richard S. Finkel.
None of the adverse events in the infants were deemed to be related to ISIS-SMNRx. Of 14 severe adverse events, 11 were respiratory infections, and all were considered to be consistent with severe infant SMA, said Dr. Finkel, chief of the division of neurology at Nemours Children’s Hospital and professor of neurology at the University of Central Florida, both in Orlando.
One patient in the 6-mg group died accidentally, and another underwent permanent ventilation. Two of 11 patients in the 12-mg group died of pulmonary infection, and 1 required permanent ventilation (16 or more hours per day continuously for more than 2 weeks in the absence of an acute reversible illness), although 4 of the patients in this group have not yet received all their doses. At the last follow-up, or at the time of death or permanent ventilation, the median age was 14 months in the 6-mg group and 9.6 months in the 12-mg group (which has not been followed as long).
Scores on the Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP-INTEND) showed increases in 8 of 11 infants who had completed treatment and evaluation. The scores increased by a mean of 5.4 points overall and by 8.3 points in those in the 12-mg group. Incremental milestones on the Hammersmith Infant Neurological Exam were achieved by 9 of 11 infants, including 6 of 7 in the 12-mg group.
The median age at death or need for permanent ventilation is 10.5 months in infants with two SMN2 gene copies, and 85% reach this endpoint at 18 months. Scores on the CHOP-INTEND also declined by 1.27 points per year, according to a study of the natural history of type 1 SMA in 34 patients by Dr. Finkel and his colleagues that is under review for publication.
Compound muscle action potentials measured in the ulnar nerve–innervated abductor digiti minimi and peroneal nerve–innervated anterior tibialis were stable or increased in most infants, he said.
These encouraging results with ISIS-SMNRx have led Isis to begin plans for phase III trials in patients with SMA types 1-3, the investigators said.
The studies are funded by Isis Pharmaceuticals, the Department of Defense, and the National Institute of Neurological Disorders and Stroke. Neither Dr. Finkel nor Dr. Chiriboga had conflicts of interest. Some of the coauthors in each study were employees of Isis.
AT THE AAN 2014 ANNUAL MEETING
Eteplirsen showed safety, efficacy over 2 years in Duchenne muscular dystrophy
PHILADELPHIA – Eteplirsen safely maintained its beneficial effect on walking speeds for certain patients with Duchenne muscular dystrophy for more than 2 years and was the first drug to show stabilized diaphragm function over the same period.
The investigational gene therapy drug had a stabilizing effect on patients’ walking speed over the course of 120 weeks regardless of whether they started it earlier or later in the open-label extension of the initial 24-week, randomized trial, but those who started treatment earlier maintained a higher walking speed, Dr. Jerry R. Mendell said at the annual meeting of the American Academy of Neurology.
Coinvestigator Dr. Edward M. Kaye of Sarepta Therapeutics, the study sponsor, presented in-depth safety and pharmacokinetics data that showed no significant treatment-related adverse events with eteplirsen in the small study. No patients have discontinued or disrupted treatment, and no laboratory evidence of toxicity has been seen to date.
The initial 24-week, double-blind, randomized, placebo-controlled study involved four patients who were given the equivalent of 30 mg/kg eteplirsen weekly, four given 50 mg/kg eteplirsen weekly, and four given placebo. At the start of the open-label extension study, two of the placebo-treated patients were transitioned to 30 mg/kg weekly and two to 50 mg/kg weekly.
All patients underwent muscle biopsy at baseline, followed by biopsies at 12 weeks in two of the high-dose patients and in two of the placebo-treated patients, and at 24 weeks in the four patients taking 30 mg/kg weekly and in two placebo-treated patients.
"This design permitted a comparison between high dose over a shorter time interval and low dose over a longer time interval," said Dr. Mendell of the Center for Gene Therapy at Nationwide Children’s Hospital, Columbus, Ohio.
All patients underwent a biopsy at 1 year (48 weeks) in the open-label extension (Ann. Neurol. 2013;74:637-47).
By 48 weeks, the mean percentage of dystrophin-positive muscle fibers was 34% in the placebo/30 mg/kg delayed-treatment group, 43% in the placebo/50 mg/kg delayed-treatment group, 42% in the 50-mg/kg group, and 52% in the 30-mg/kg group.
Distance traveled on the 6-minute walk test began to diverge between active and placebo-treated patients at 12 weeks and stabilized at 24 weeks among those who received active treatment, but continued to decline until 36 weeks for placebo-treated patients who started treatment at 24 weeks.
At 120 weeks, the patients who had received continuous treatment with eteplirsen declined by a mean of 14 m on the 6-minute walk test since baseline, compared with a decline of 79 m in those who underwent delayed treatment with eteplirsen. In comparison, studies of the natural history of Duchenne muscular dystrophy (DMD) have shown steady declines on the 6-minute walk test, ranging from 30 to 115 m at 1 year and 97 to 125 m at 2 years.
There was "remarkable stability" in diaphragm function over the course of treatment with eteplirsen, "which is quite different from the natural history of untreated patients," Dr. Mendell said. In all 12 patients, maximal expiratory pressure remained stable, going from a mean of 90% of predicted at baseline to 95% at 120 weeks. The same was true for maximal inspiratory pressure, moving from 79% of expected at baseline to 80% at 120 weeks. In contrast, the natural history of untreated DMD shows that pulmonary function declines substantially over time: by 3.9%/year for maximal inspiratory pressure after starting at 90% of predicted at 9 years of age, and by 3.6%/year after starting at 45% of predicted at 9 years of age (Pediatr. Pulmonol. 2014;49:473-81).
Eteplirsen was created to help patients with a deletion of exon 51 in dystrophin, which causes a nonfunctional dystrophin protein. The drug is a charge-neutral phosphorodiamidate morpholino oligomer (PMO) that targets the 13% of DMD patients with this mutation by directing alternative splicing of the dystrophin gene through its ability to bind to exon 51 of dystrophin pre-mRNA. This restores transcription and translation of a truncated, yet functional, dystrophin protein, such as those found in Becker muscular dystrophy.
The neutral charge of eteplirsen helps it to avoid binding to serum proteins, which is one of the problems that have occurred with phosphorothioate antisense oligonucleotide-based drugs. Phosphorothioate antisense drugs have also been linked to immune activation, hepatotoxicity, thrombocytopenia, coagulopathy, renal toxicity, and proteinuria. But Dr. Kaye reported that eteplirsen is mainly excreted by the kidneys, has a half-life of about 3 hours, and showed no evidence of those problems. Only 13 of 453 urine protein assessments tested positive, but all were low, transient, and resolved spontaneously.
"The reason that this is important is there has actually been very little human data until recently, despite the fact that [PMOs have] been around for over 30 years. Most of the work has been done in animals and in research laboratories, and it’s only until recently that people have been exposed to the drug," Dr. Kaye said.
The study was funded by Sarepta Therapeutics. Dr. Kaye and two coauthors are employees of the company. Dr. Mendell had no relevant disclosures.
PHILADELPHIA – Eteplirsen safely maintained its beneficial effect on walking speeds for certain patients with Duchenne muscular dystrophy for more than 2 years and was the first drug to show stabilized diaphragm function over the same period.
The investigational gene therapy drug had a stabilizing effect on patients’ walking speed over the course of 120 weeks regardless of whether they started it earlier or later in the open-label extension of the initial 24-week, randomized trial, but those who started treatment earlier maintained a higher walking speed, Dr. Jerry R. Mendell said at the annual meeting of the American Academy of Neurology.
Coinvestigator Dr. Edward M. Kaye of Sarepta Therapeutics, the study sponsor, presented in-depth safety and pharmacokinetics data that showed no significant treatment-related adverse events with eteplirsen in the small study. No patients have discontinued or disrupted treatment, and no laboratory evidence of toxicity has been seen to date.
The initial 24-week, double-blind, randomized, placebo-controlled study involved four patients who were given the equivalent of 30 mg/kg eteplirsen weekly, four given 50 mg/kg eteplirsen weekly, and four given placebo. At the start of the open-label extension study, two of the placebo-treated patients were transitioned to 30 mg/kg weekly and two to 50 mg/kg weekly.
All patients underwent muscle biopsy at baseline, followed by biopsies at 12 weeks in two of the high-dose patients and in two of the placebo-treated patients, and at 24 weeks in the four patients taking 30 mg/kg weekly and in two placebo-treated patients.
"This design permitted a comparison between high dose over a shorter time interval and low dose over a longer time interval," said Dr. Mendell of the Center for Gene Therapy at Nationwide Children’s Hospital, Columbus, Ohio.
All patients underwent a biopsy at 1 year (48 weeks) in the open-label extension (Ann. Neurol. 2013;74:637-47).
By 48 weeks, the mean percentage of dystrophin-positive muscle fibers was 34% in the placebo/30 mg/kg delayed-treatment group, 43% in the placebo/50 mg/kg delayed-treatment group, 42% in the 50-mg/kg group, and 52% in the 30-mg/kg group.
Distance traveled on the 6-minute walk test began to diverge between active and placebo-treated patients at 12 weeks and stabilized at 24 weeks among those who received active treatment, but continued to decline until 36 weeks for placebo-treated patients who started treatment at 24 weeks.
At 120 weeks, the patients who had received continuous treatment with eteplirsen declined by a mean of 14 m on the 6-minute walk test since baseline, compared with a decline of 79 m in those who underwent delayed treatment with eteplirsen. In comparison, studies of the natural history of Duchenne muscular dystrophy (DMD) have shown steady declines on the 6-minute walk test, ranging from 30 to 115 m at 1 year and 97 to 125 m at 2 years.
There was "remarkable stability" in diaphragm function over the course of treatment with eteplirsen, "which is quite different from the natural history of untreated patients," Dr. Mendell said. In all 12 patients, maximal expiratory pressure remained stable, going from a mean of 90% of predicted at baseline to 95% at 120 weeks. The same was true for maximal inspiratory pressure, moving from 79% of expected at baseline to 80% at 120 weeks. In contrast, the natural history of untreated DMD shows that pulmonary function declines substantially over time: by 3.9%/year for maximal inspiratory pressure after starting at 90% of predicted at 9 years of age, and by 3.6%/year after starting at 45% of predicted at 9 years of age (Pediatr. Pulmonol. 2014;49:473-81).
Eteplirsen was created to help patients with a deletion of exon 51 in dystrophin, which causes a nonfunctional dystrophin protein. The drug is a charge-neutral phosphorodiamidate morpholino oligomer (PMO) that targets the 13% of DMD patients with this mutation by directing alternative splicing of the dystrophin gene through its ability to bind to exon 51 of dystrophin pre-mRNA. This restores transcription and translation of a truncated, yet functional, dystrophin protein, such as those found in Becker muscular dystrophy.
The neutral charge of eteplirsen helps it to avoid binding to serum proteins, which is one of the problems that have occurred with phosphorothioate antisense oligonucleotide-based drugs. Phosphorothioate antisense drugs have also been linked to immune activation, hepatotoxicity, thrombocytopenia, coagulopathy, renal toxicity, and proteinuria. But Dr. Kaye reported that eteplirsen is mainly excreted by the kidneys, has a half-life of about 3 hours, and showed no evidence of those problems. Only 13 of 453 urine protein assessments tested positive, but all were low, transient, and resolved spontaneously.
"The reason that this is important is there has actually been very little human data until recently, despite the fact that [PMOs have] been around for over 30 years. Most of the work has been done in animals and in research laboratories, and it’s only until recently that people have been exposed to the drug," Dr. Kaye said.
The study was funded by Sarepta Therapeutics. Dr. Kaye and two coauthors are employees of the company. Dr. Mendell had no relevant disclosures.
PHILADELPHIA – Eteplirsen safely maintained its beneficial effect on walking speeds for certain patients with Duchenne muscular dystrophy for more than 2 years and was the first drug to show stabilized diaphragm function over the same period.
The investigational gene therapy drug had a stabilizing effect on patients’ walking speed over the course of 120 weeks regardless of whether they started it earlier or later in the open-label extension of the initial 24-week, randomized trial, but those who started treatment earlier maintained a higher walking speed, Dr. Jerry R. Mendell said at the annual meeting of the American Academy of Neurology.
Coinvestigator Dr. Edward M. Kaye of Sarepta Therapeutics, the study sponsor, presented in-depth safety and pharmacokinetics data that showed no significant treatment-related adverse events with eteplirsen in the small study. No patients have discontinued or disrupted treatment, and no laboratory evidence of toxicity has been seen to date.
The initial 24-week, double-blind, randomized, placebo-controlled study involved four patients who were given the equivalent of 30 mg/kg eteplirsen weekly, four given 50 mg/kg eteplirsen weekly, and four given placebo. At the start of the open-label extension study, two of the placebo-treated patients were transitioned to 30 mg/kg weekly and two to 50 mg/kg weekly.
All patients underwent muscle biopsy at baseline, followed by biopsies at 12 weeks in two of the high-dose patients and in two of the placebo-treated patients, and at 24 weeks in the four patients taking 30 mg/kg weekly and in two placebo-treated patients.
"This design permitted a comparison between high dose over a shorter time interval and low dose over a longer time interval," said Dr. Mendell of the Center for Gene Therapy at Nationwide Children’s Hospital, Columbus, Ohio.
All patients underwent a biopsy at 1 year (48 weeks) in the open-label extension (Ann. Neurol. 2013;74:637-47).
By 48 weeks, the mean percentage of dystrophin-positive muscle fibers was 34% in the placebo/30 mg/kg delayed-treatment group, 43% in the placebo/50 mg/kg delayed-treatment group, 42% in the 50-mg/kg group, and 52% in the 30-mg/kg group.
Distance traveled on the 6-minute walk test began to diverge between active and placebo-treated patients at 12 weeks and stabilized at 24 weeks among those who received active treatment, but continued to decline until 36 weeks for placebo-treated patients who started treatment at 24 weeks.
At 120 weeks, the patients who had received continuous treatment with eteplirsen declined by a mean of 14 m on the 6-minute walk test since baseline, compared with a decline of 79 m in those who underwent delayed treatment with eteplirsen. In comparison, studies of the natural history of Duchenne muscular dystrophy (DMD) have shown steady declines on the 6-minute walk test, ranging from 30 to 115 m at 1 year and 97 to 125 m at 2 years.
There was "remarkable stability" in diaphragm function over the course of treatment with eteplirsen, "which is quite different from the natural history of untreated patients," Dr. Mendell said. In all 12 patients, maximal expiratory pressure remained stable, going from a mean of 90% of predicted at baseline to 95% at 120 weeks. The same was true for maximal inspiratory pressure, moving from 79% of expected at baseline to 80% at 120 weeks. In contrast, the natural history of untreated DMD shows that pulmonary function declines substantially over time: by 3.9%/year for maximal inspiratory pressure after starting at 90% of predicted at 9 years of age, and by 3.6%/year after starting at 45% of predicted at 9 years of age (Pediatr. Pulmonol. 2014;49:473-81).
Eteplirsen was created to help patients with a deletion of exon 51 in dystrophin, which causes a nonfunctional dystrophin protein. The drug is a charge-neutral phosphorodiamidate morpholino oligomer (PMO) that targets the 13% of DMD patients with this mutation by directing alternative splicing of the dystrophin gene through its ability to bind to exon 51 of dystrophin pre-mRNA. This restores transcription and translation of a truncated, yet functional, dystrophin protein, such as those found in Becker muscular dystrophy.
The neutral charge of eteplirsen helps it to avoid binding to serum proteins, which is one of the problems that have occurred with phosphorothioate antisense oligonucleotide-based drugs. Phosphorothioate antisense drugs have also been linked to immune activation, hepatotoxicity, thrombocytopenia, coagulopathy, renal toxicity, and proteinuria. But Dr. Kaye reported that eteplirsen is mainly excreted by the kidneys, has a half-life of about 3 hours, and showed no evidence of those problems. Only 13 of 453 urine protein assessments tested positive, but all were low, transient, and resolved spontaneously.
"The reason that this is important is there has actually been very little human data until recently, despite the fact that [PMOs have] been around for over 30 years. Most of the work has been done in animals and in research laboratories, and it’s only until recently that people have been exposed to the drug," Dr. Kaye said.
The study was funded by Sarepta Therapeutics. Dr. Kaye and two coauthors are employees of the company. Dr. Mendell had no relevant disclosures.
AT THE AAN 2014 ANNUAL MEETING
Key clinical point: Treatment with eteplirsen stabilized key clinical features of DMD and had no significant adverse events over 2 years.
Major finding: At 120 weeks, patients who received continuous treatment with eteplirsen had a mean decline of 14 m on the 6-minute walk test since baseline, compared with a decline of 79 m in those who underwent delayed treatment with eteplirsen.
Data source: An open-label extension of a 24-week, randomized, double-blind, placebo-controlled trial out to 120 weeks in 12 patients with DMD.
Disclosures: The study is funded by Sarepta Therapeutics. Dr. Kaye and two coauthors are employees of the company. Dr. Mendell had no relevant disclosures.
Spinal fluid may help flesh out natalizumab-associated PML diagnosis
Measuring anti–JC virus antibody levels in cerebrospinal fluid may complement testing for viral DNA in cerebrospinal fluid and provide earlier detection of progressive multifocal leukoencephalopathy cases, according to the results of a retrospective case-control study of natalizumab-treated multiple sclerosis patients.
Cerebrospinal fluid (CSF) measurements of anti–JC virus (JCV) antibodies could help to identify patients with natalizumab (Tysabri)-associated progressive multifocal leukoencephalopathy (PML) who have repetitively undetectable levels of JC virus DNA in cerebrospinal fluid and often have levels less than 100 copies/mL at the time of PML diagnosis. Such measurements could help to avoid a delayed diagnosis or the need for brain biopsy to confirm the clinical suspicion of PML, said Dr. Clemens Warnke of the department of neurology at Heinrich-Heine-University, Düsseldorf, Germany, and his colleagues (Ann. Neurol. 2014 April 11 [doi:10.1002/ana.24153]).
The investigators compared serums and CSF samples taken from 37 multiple sclerosis patients with natalizumab-associated PML and 89 multiple sclerosis patients treated with natalizumab who did not have PML and calculated their cerebrospinal fluid JCV antibody index (AIJCV) as a measure of intrathecal synthesis of anti–JC virus antibodies. An AIJCV greater than 1.5 was observed in 26 (70%) of the 37 patients with natalizumab-associated PML, but not in any of the 89 control patients. In 20 patients with samples available from the time of diagnosis of natalizumab-associated PML, 11 (55%) had an AIJCV greater than 1.5. A total of 14 of the 20 patients had JC virus DNA levels less than 100 copies/mL, including 8 with an AIJCV greater than 1.5.
The calculation of an AIJCV greater than 1.5 – which provides clear evidence of intrathecal synthesis of anti-JCV antibodies – allowed the investigators to "exclude pure diffusion and disturbance of blood-CSF barrier function as a putative explanation of increased levels of anti-JCV antibodies in CSF in cases of PML."
The necessity of a lumbar puncture to determine the AIJCV – just as with screening CSF for JC virus DNA – makes the technique "unsuitable for PML risk prediction," but because other promising methods for risk prediction have not been prospectively validated, the investigators "propose the determination of the CSF AIJCV in addition to the JCV-DNA PCR [polymerase chain reaction] in CSF in cases of possible or probable PML (based on clinical, MRI-, and/or JCV-DNA PCR findings) in patients at risk."
The investigators’ work was supported by various sources, including grants from Heinrich-Heine University, the German Ministry for Education and Research, and the Innovative Medicines Initiative Joint Undertaking within the European Union’s Seventh Framework Program, as well as a fellowship stipend from the European Committee for Treatment and Research in MS.
Measuring anti–JC virus antibody levels in cerebrospinal fluid may complement testing for viral DNA in cerebrospinal fluid and provide earlier detection of progressive multifocal leukoencephalopathy cases, according to the results of a retrospective case-control study of natalizumab-treated multiple sclerosis patients.
Cerebrospinal fluid (CSF) measurements of anti–JC virus (JCV) antibodies could help to identify patients with natalizumab (Tysabri)-associated progressive multifocal leukoencephalopathy (PML) who have repetitively undetectable levels of JC virus DNA in cerebrospinal fluid and often have levels less than 100 copies/mL at the time of PML diagnosis. Such measurements could help to avoid a delayed diagnosis or the need for brain biopsy to confirm the clinical suspicion of PML, said Dr. Clemens Warnke of the department of neurology at Heinrich-Heine-University, Düsseldorf, Germany, and his colleagues (Ann. Neurol. 2014 April 11 [doi:10.1002/ana.24153]).
The investigators compared serums and CSF samples taken from 37 multiple sclerosis patients with natalizumab-associated PML and 89 multiple sclerosis patients treated with natalizumab who did not have PML and calculated their cerebrospinal fluid JCV antibody index (AIJCV) as a measure of intrathecal synthesis of anti–JC virus antibodies. An AIJCV greater than 1.5 was observed in 26 (70%) of the 37 patients with natalizumab-associated PML, but not in any of the 89 control patients. In 20 patients with samples available from the time of diagnosis of natalizumab-associated PML, 11 (55%) had an AIJCV greater than 1.5. A total of 14 of the 20 patients had JC virus DNA levels less than 100 copies/mL, including 8 with an AIJCV greater than 1.5.
The calculation of an AIJCV greater than 1.5 – which provides clear evidence of intrathecal synthesis of anti-JCV antibodies – allowed the investigators to "exclude pure diffusion and disturbance of blood-CSF barrier function as a putative explanation of increased levels of anti-JCV antibodies in CSF in cases of PML."
The necessity of a lumbar puncture to determine the AIJCV – just as with screening CSF for JC virus DNA – makes the technique "unsuitable for PML risk prediction," but because other promising methods for risk prediction have not been prospectively validated, the investigators "propose the determination of the CSF AIJCV in addition to the JCV-DNA PCR [polymerase chain reaction] in CSF in cases of possible or probable PML (based on clinical, MRI-, and/or JCV-DNA PCR findings) in patients at risk."
The investigators’ work was supported by various sources, including grants from Heinrich-Heine University, the German Ministry for Education and Research, and the Innovative Medicines Initiative Joint Undertaking within the European Union’s Seventh Framework Program, as well as a fellowship stipend from the European Committee for Treatment and Research in MS.
Measuring anti–JC virus antibody levels in cerebrospinal fluid may complement testing for viral DNA in cerebrospinal fluid and provide earlier detection of progressive multifocal leukoencephalopathy cases, according to the results of a retrospective case-control study of natalizumab-treated multiple sclerosis patients.
Cerebrospinal fluid (CSF) measurements of anti–JC virus (JCV) antibodies could help to identify patients with natalizumab (Tysabri)-associated progressive multifocal leukoencephalopathy (PML) who have repetitively undetectable levels of JC virus DNA in cerebrospinal fluid and often have levels less than 100 copies/mL at the time of PML diagnosis. Such measurements could help to avoid a delayed diagnosis or the need for brain biopsy to confirm the clinical suspicion of PML, said Dr. Clemens Warnke of the department of neurology at Heinrich-Heine-University, Düsseldorf, Germany, and his colleagues (Ann. Neurol. 2014 April 11 [doi:10.1002/ana.24153]).
The investigators compared serums and CSF samples taken from 37 multiple sclerosis patients with natalizumab-associated PML and 89 multiple sclerosis patients treated with natalizumab who did not have PML and calculated their cerebrospinal fluid JCV antibody index (AIJCV) as a measure of intrathecal synthesis of anti–JC virus antibodies. An AIJCV greater than 1.5 was observed in 26 (70%) of the 37 patients with natalizumab-associated PML, but not in any of the 89 control patients. In 20 patients with samples available from the time of diagnosis of natalizumab-associated PML, 11 (55%) had an AIJCV greater than 1.5. A total of 14 of the 20 patients had JC virus DNA levels less than 100 copies/mL, including 8 with an AIJCV greater than 1.5.
The calculation of an AIJCV greater than 1.5 – which provides clear evidence of intrathecal synthesis of anti-JCV antibodies – allowed the investigators to "exclude pure diffusion and disturbance of blood-CSF barrier function as a putative explanation of increased levels of anti-JCV antibodies in CSF in cases of PML."
The necessity of a lumbar puncture to determine the AIJCV – just as with screening CSF for JC virus DNA – makes the technique "unsuitable for PML risk prediction," but because other promising methods for risk prediction have not been prospectively validated, the investigators "propose the determination of the CSF AIJCV in addition to the JCV-DNA PCR [polymerase chain reaction] in CSF in cases of possible or probable PML (based on clinical, MRI-, and/or JCV-DNA PCR findings) in patients at risk."
The investigators’ work was supported by various sources, including grants from Heinrich-Heine University, the German Ministry for Education and Research, and the Innovative Medicines Initiative Joint Undertaking within the European Union’s Seventh Framework Program, as well as a fellowship stipend from the European Committee for Treatment and Research in MS.
FROM ANNALS OF NEUROLOGY
Current smoking strongly predicts rheumatoid arthritis radiographic progression
Current smoking at the time of early rheumatoid arthritis diagnosis was a strong risk factor for rapid radiographic progression in early rheumatoid arthritis, according to an analysis of patients in the multicenter, randomized SWEFOT trial.
Smoking emerged as an independent risk factor despite initial treatment with methotrexate, with or without subsequent adjunctive treatment with additional synthetic disease-modifying antirheumatic drugs or a biologic in nonresponders, wrote Dr. Saedis Saevarsdottir of the Karolinska Institute, Stockholm, and her associates in the trial’s study group.
The finding may be "perhaps not so surprising, since several older studies have previously reported an association between cigarette smoking and a more severe RA. ... [But] smoking habits have not been included in any of the recent studies on risk matrix of radiographic progression," she said.
The investigators identified 79 patients with radiographic progression among 311 patients in the trial who had radiographic data available at baseline and 1 year. Those who achieved a 28-joint disease activity score of less than 3.2 by 3-4 months remained on methotrexate (147 patients), whereas others were randomized again to add either infliximab (Remicade, 128 patients) or both sulfasalazine and hydroxychloroquine (130 patients). A total of 269 patients were included in a multivariable model (72 with radiographic progression) that included 80 who remained on methotrexate monotherapy, 94 who added infliximab, and 95 on triple therapy. The researchers defined radiographic progression as an increase in Sharp-van der Heijde score of 5 or greater after 1 year.
In the multivariable model, current smoking was the strongest baseline predictor of rapid radiographic progression (odds ratio, 2.78), and other independent predictors included erosions (OR, 2.21), C-reactive protein (CRP) level (OR, 1.49), and erythrocyte sedimentation rate (OR, 1.62) – all of which have been previously reported as baseline predictors of radiographic progression (Ann. Rheum. Dis. 2014 April 4 [doi:10.1136/annrheumdis-2013-204601]).
The investigators constructed a three-dimensional risk matrix for rapid radiographic progression based on their results with current smoking status, baseline erosions, and CRP level. At 1 year, 63% of patients who had all the predictors developed radiographic progression, compared with 12% of those without these three baseline predictors. The results were similar for men and women and within the different treatment subgroups. "Thus, the matrix may help to identify at baseline those patients at risk of radiographic progression, irrespective of which treatment is chosen on clinical grounds," the researchers wrote.
Two variables that had been significant predictors of radiographic progression in other studies and used in other risk matrices – swollen joint count and autoantibody positivity (rheumatoid factor and anti-cyclic citrullinated peptide antibodies [anti-CCP]) – were not independent predictors in the current study. In an exploratory analysis, the use of a lower Sharp-van der Heijde score cutoff of 1 or greater found both rheumatoid factor and anti-CCP positivity to be significant predictors of radiographic progression in unadjusted, but not adjusted, analyses. Tests of the current study’s risk matrix in anti-CCP negative and positive patients showed that current smokers with erosive disease had high risk for radiographic progression in both subsets of patients.
Data from the current study also performed "reasonably well" in a previous clinical trial–based risk matrix that had included autoantibody status instead of smoking status (Ann. Rheum. Dis. 2010;69:1333-7).
The study’s strength was its inclusion within a clinical trial of unselected early RA patients that reflects the common standard of care: giving methotrexate first, then adding a biologic or two additional synthetic disease-modifying antirheumatic drugs if low disease activity had not been achieved after 3-4 months of methotrexate monotherapy. Although patients’ pack-years of smoking were not available, the investigators noted that a previous study did not affect outcome when the smoking intensity was evaluated based on actual smoking status of current, past, or never smoker, "indicating that the actual smoking habits have most impact."
The study received no specific external funding. There were no relevant financial disclosures.
Current smoking at the time of early rheumatoid arthritis diagnosis was a strong risk factor for rapid radiographic progression in early rheumatoid arthritis, according to an analysis of patients in the multicenter, randomized SWEFOT trial.
Smoking emerged as an independent risk factor despite initial treatment with methotrexate, with or without subsequent adjunctive treatment with additional synthetic disease-modifying antirheumatic drugs or a biologic in nonresponders, wrote Dr. Saedis Saevarsdottir of the Karolinska Institute, Stockholm, and her associates in the trial’s study group.
The finding may be "perhaps not so surprising, since several older studies have previously reported an association between cigarette smoking and a more severe RA. ... [But] smoking habits have not been included in any of the recent studies on risk matrix of radiographic progression," she said.
The investigators identified 79 patients with radiographic progression among 311 patients in the trial who had radiographic data available at baseline and 1 year. Those who achieved a 28-joint disease activity score of less than 3.2 by 3-4 months remained on methotrexate (147 patients), whereas others were randomized again to add either infliximab (Remicade, 128 patients) or both sulfasalazine and hydroxychloroquine (130 patients). A total of 269 patients were included in a multivariable model (72 with radiographic progression) that included 80 who remained on methotrexate monotherapy, 94 who added infliximab, and 95 on triple therapy. The researchers defined radiographic progression as an increase in Sharp-van der Heijde score of 5 or greater after 1 year.
In the multivariable model, current smoking was the strongest baseline predictor of rapid radiographic progression (odds ratio, 2.78), and other independent predictors included erosions (OR, 2.21), C-reactive protein (CRP) level (OR, 1.49), and erythrocyte sedimentation rate (OR, 1.62) – all of which have been previously reported as baseline predictors of radiographic progression (Ann. Rheum. Dis. 2014 April 4 [doi:10.1136/annrheumdis-2013-204601]).
The investigators constructed a three-dimensional risk matrix for rapid radiographic progression based on their results with current smoking status, baseline erosions, and CRP level. At 1 year, 63% of patients who had all the predictors developed radiographic progression, compared with 12% of those without these three baseline predictors. The results were similar for men and women and within the different treatment subgroups. "Thus, the matrix may help to identify at baseline those patients at risk of radiographic progression, irrespective of which treatment is chosen on clinical grounds," the researchers wrote.
Two variables that had been significant predictors of radiographic progression in other studies and used in other risk matrices – swollen joint count and autoantibody positivity (rheumatoid factor and anti-cyclic citrullinated peptide antibodies [anti-CCP]) – were not independent predictors in the current study. In an exploratory analysis, the use of a lower Sharp-van der Heijde score cutoff of 1 or greater found both rheumatoid factor and anti-CCP positivity to be significant predictors of radiographic progression in unadjusted, but not adjusted, analyses. Tests of the current study’s risk matrix in anti-CCP negative and positive patients showed that current smokers with erosive disease had high risk for radiographic progression in both subsets of patients.
Data from the current study also performed "reasonably well" in a previous clinical trial–based risk matrix that had included autoantibody status instead of smoking status (Ann. Rheum. Dis. 2010;69:1333-7).
The study’s strength was its inclusion within a clinical trial of unselected early RA patients that reflects the common standard of care: giving methotrexate first, then adding a biologic or two additional synthetic disease-modifying antirheumatic drugs if low disease activity had not been achieved after 3-4 months of methotrexate monotherapy. Although patients’ pack-years of smoking were not available, the investigators noted that a previous study did not affect outcome when the smoking intensity was evaluated based on actual smoking status of current, past, or never smoker, "indicating that the actual smoking habits have most impact."
The study received no specific external funding. There were no relevant financial disclosures.
Current smoking at the time of early rheumatoid arthritis diagnosis was a strong risk factor for rapid radiographic progression in early rheumatoid arthritis, according to an analysis of patients in the multicenter, randomized SWEFOT trial.
Smoking emerged as an independent risk factor despite initial treatment with methotrexate, with or without subsequent adjunctive treatment with additional synthetic disease-modifying antirheumatic drugs or a biologic in nonresponders, wrote Dr. Saedis Saevarsdottir of the Karolinska Institute, Stockholm, and her associates in the trial’s study group.
The finding may be "perhaps not so surprising, since several older studies have previously reported an association between cigarette smoking and a more severe RA. ... [But] smoking habits have not been included in any of the recent studies on risk matrix of radiographic progression," she said.
The investigators identified 79 patients with radiographic progression among 311 patients in the trial who had radiographic data available at baseline and 1 year. Those who achieved a 28-joint disease activity score of less than 3.2 by 3-4 months remained on methotrexate (147 patients), whereas others were randomized again to add either infliximab (Remicade, 128 patients) or both sulfasalazine and hydroxychloroquine (130 patients). A total of 269 patients were included in a multivariable model (72 with radiographic progression) that included 80 who remained on methotrexate monotherapy, 94 who added infliximab, and 95 on triple therapy. The researchers defined radiographic progression as an increase in Sharp-van der Heijde score of 5 or greater after 1 year.
In the multivariable model, current smoking was the strongest baseline predictor of rapid radiographic progression (odds ratio, 2.78), and other independent predictors included erosions (OR, 2.21), C-reactive protein (CRP) level (OR, 1.49), and erythrocyte sedimentation rate (OR, 1.62) – all of which have been previously reported as baseline predictors of radiographic progression (Ann. Rheum. Dis. 2014 April 4 [doi:10.1136/annrheumdis-2013-204601]).
The investigators constructed a three-dimensional risk matrix for rapid radiographic progression based on their results with current smoking status, baseline erosions, and CRP level. At 1 year, 63% of patients who had all the predictors developed radiographic progression, compared with 12% of those without these three baseline predictors. The results were similar for men and women and within the different treatment subgroups. "Thus, the matrix may help to identify at baseline those patients at risk of radiographic progression, irrespective of which treatment is chosen on clinical grounds," the researchers wrote.
Two variables that had been significant predictors of radiographic progression in other studies and used in other risk matrices – swollen joint count and autoantibody positivity (rheumatoid factor and anti-cyclic citrullinated peptide antibodies [anti-CCP]) – were not independent predictors in the current study. In an exploratory analysis, the use of a lower Sharp-van der Heijde score cutoff of 1 or greater found both rheumatoid factor and anti-CCP positivity to be significant predictors of radiographic progression in unadjusted, but not adjusted, analyses. Tests of the current study’s risk matrix in anti-CCP negative and positive patients showed that current smokers with erosive disease had high risk for radiographic progression in both subsets of patients.
Data from the current study also performed "reasonably well" in a previous clinical trial–based risk matrix that had included autoantibody status instead of smoking status (Ann. Rheum. Dis. 2010;69:1333-7).
The study’s strength was its inclusion within a clinical trial of unselected early RA patients that reflects the common standard of care: giving methotrexate first, then adding a biologic or two additional synthetic disease-modifying antirheumatic drugs if low disease activity had not been achieved after 3-4 months of methotrexate monotherapy. Although patients’ pack-years of smoking were not available, the investigators noted that a previous study did not affect outcome when the smoking intensity was evaluated based on actual smoking status of current, past, or never smoker, "indicating that the actual smoking habits have most impact."
The study received no specific external funding. There were no relevant financial disclosures.
FROM ANNALS OF THE RHEUMATIC DISEASES
Major finding: At 1 year, 63% of patients who were current smokers and had erosive disease and high CRP levels at baseline developed radiographic progression, compared with 12% of those without the three baseline predictors.
Data source: An analysis of data from the multicenter, randomized SWEFOT study of patients with early RA.
Disclosures: The study received no specific external funding. There were no relevant financial disclosures.