User login
Jeff Evans has been editor of Rheumatology News/MDedge Rheumatology and the EULAR Congress News since 2013. He started at Frontline Medical Communications in 2001 and was a reporter for 8 years before serving as editor of Clinical Neurology News and World Neurology, and briefly as editor of GI & Hepatology News. He graduated cum laude from Cornell University (New York) with a BA in biological sciences, concentrating in neurobiology and behavior.
Fatty Liver Disease Cited for Rise in Hepatocellular Carcinoma
BOSTON – Nonalcoholic fatty liver disease without cirrhosis appears to be a significant contributor to the rise in the incidence of hepatocellular carcinoma in the past two decades, according to a study linking population-based data from the National Cancer Institute with Medicare enrollment and claim files during 1993-2007.
"Our data suggest a unique underlying pathophysiology for development of HCC [hepatocellular carcinoma] in noncirrhotic NAFLD [nonalcoholic fatty liver disease]," Dr. Rubayat Rahman said at the annual meeting of the American Association for the Study of Liver Diseases.
The finding may help to explain the rise in the incidence of the malignancy, which has had no clear explanation, added Dr. Rahman of the division of gastroenterology and hepatology at the University of Missouri, Columbia.
Of 17,895 HCC cases in the linked Surveillance, Epidemiology and End Results (SEER)-Medicare database, 2,863 (16%) had only NAFLD without any other risk factors or etiologies for HCC. The linked database covers 30% of the U.S. Medicare population. SEER itself contains data from 18 cancer registries covering 28% of the U.S. population, and 93% of the individuals in the database who are at least 65 years are matched to a Medicare enrollment file.
At 16%, NAFLD was third most common risk factor for HCC after infection (44%) and alcoholic diseases (19%) – 21% were other causes – but it was the second most common risk factor after infection in Asians and Pacific islanders, said Dr. Rahman.
Cirrhosis was not present in 36% of the NAFLD-related HCC cases; of those, 18% had only steatosis and not nonalcoholic steatohepatitis (NASH) or other adverse changes in pathology. Patients without cirrhosis more often had early-stage disease (stage I or II) than did those with cirrhosis (62% vs. 44%), as well as favorable grades (I or II, 76% vs. 56%).
Although the annual percentage change of NAFLD-related HCC with cirrhosis has increased since 1993, 1999 marked a point when the annual growth of NAFLD-related cases of HCC without cirrhosis outpaced those with cirrhosis. The average number of cases per year of NAFLD-related HCC without cirrhosis grew significantly from 51 in 1993-2000 to 88 in 2001-2007, compared with no change in cases with cirrhosis.
Three components of the metabolic syndrome – body-mass index greater than 30 kg/m2, type 2 diabetes mellitus, and dyslipidemia – occurred in significantly greater proportions of patients with NAFLD-related HCC without cirrhosis than in those with cirrhosis. The odds of developing HCC for each of those three components among noncirrhotic NAFLD patients was higher than for those with cirrhotic NAFLD, although the overall odds of developing HCC were higher among patients with cirrhotic NAFLD (odds ratio, 16.5) than in those with noncirrhotic NAFLD (OR, 3.1).
One audience member expressed concern about determining the presence of cirrhosis or NASH in the data without a systematic assessment of histopathology performed in a centralized way, but Dr. Rahman noted that the Medicare-matched SEER data have CPT procedural codes and ICD-9 diagnostic codes for diagnoses made through liver biopsy. Another attendee also suggested caution in calling NAFLD a risk factor for cancer alone because the people in the database have a higher rate of cancer, and the database does not include the peak of NASH at age 55 years.
None of the study investigators had relevant financial disclosures.
BOSTON – Nonalcoholic fatty liver disease without cirrhosis appears to be a significant contributor to the rise in the incidence of hepatocellular carcinoma in the past two decades, according to a study linking population-based data from the National Cancer Institute with Medicare enrollment and claim files during 1993-2007.
"Our data suggest a unique underlying pathophysiology for development of HCC [hepatocellular carcinoma] in noncirrhotic NAFLD [nonalcoholic fatty liver disease]," Dr. Rubayat Rahman said at the annual meeting of the American Association for the Study of Liver Diseases.
The finding may help to explain the rise in the incidence of the malignancy, which has had no clear explanation, added Dr. Rahman of the division of gastroenterology and hepatology at the University of Missouri, Columbia.
Of 17,895 HCC cases in the linked Surveillance, Epidemiology and End Results (SEER)-Medicare database, 2,863 (16%) had only NAFLD without any other risk factors or etiologies for HCC. The linked database covers 30% of the U.S. Medicare population. SEER itself contains data from 18 cancer registries covering 28% of the U.S. population, and 93% of the individuals in the database who are at least 65 years are matched to a Medicare enrollment file.
At 16%, NAFLD was third most common risk factor for HCC after infection (44%) and alcoholic diseases (19%) – 21% were other causes – but it was the second most common risk factor after infection in Asians and Pacific islanders, said Dr. Rahman.
Cirrhosis was not present in 36% of the NAFLD-related HCC cases; of those, 18% had only steatosis and not nonalcoholic steatohepatitis (NASH) or other adverse changes in pathology. Patients without cirrhosis more often had early-stage disease (stage I or II) than did those with cirrhosis (62% vs. 44%), as well as favorable grades (I or II, 76% vs. 56%).
Although the annual percentage change of NAFLD-related HCC with cirrhosis has increased since 1993, 1999 marked a point when the annual growth of NAFLD-related cases of HCC without cirrhosis outpaced those with cirrhosis. The average number of cases per year of NAFLD-related HCC without cirrhosis grew significantly from 51 in 1993-2000 to 88 in 2001-2007, compared with no change in cases with cirrhosis.
Three components of the metabolic syndrome – body-mass index greater than 30 kg/m2, type 2 diabetes mellitus, and dyslipidemia – occurred in significantly greater proportions of patients with NAFLD-related HCC without cirrhosis than in those with cirrhosis. The odds of developing HCC for each of those three components among noncirrhotic NAFLD patients was higher than for those with cirrhotic NAFLD, although the overall odds of developing HCC were higher among patients with cirrhotic NAFLD (odds ratio, 16.5) than in those with noncirrhotic NAFLD (OR, 3.1).
One audience member expressed concern about determining the presence of cirrhosis or NASH in the data without a systematic assessment of histopathology performed in a centralized way, but Dr. Rahman noted that the Medicare-matched SEER data have CPT procedural codes and ICD-9 diagnostic codes for diagnoses made through liver biopsy. Another attendee also suggested caution in calling NAFLD a risk factor for cancer alone because the people in the database have a higher rate of cancer, and the database does not include the peak of NASH at age 55 years.
None of the study investigators had relevant financial disclosures.
BOSTON – Nonalcoholic fatty liver disease without cirrhosis appears to be a significant contributor to the rise in the incidence of hepatocellular carcinoma in the past two decades, according to a study linking population-based data from the National Cancer Institute with Medicare enrollment and claim files during 1993-2007.
"Our data suggest a unique underlying pathophysiology for development of HCC [hepatocellular carcinoma] in noncirrhotic NAFLD [nonalcoholic fatty liver disease]," Dr. Rubayat Rahman said at the annual meeting of the American Association for the Study of Liver Diseases.
The finding may help to explain the rise in the incidence of the malignancy, which has had no clear explanation, added Dr. Rahman of the division of gastroenterology and hepatology at the University of Missouri, Columbia.
Of 17,895 HCC cases in the linked Surveillance, Epidemiology and End Results (SEER)-Medicare database, 2,863 (16%) had only NAFLD without any other risk factors or etiologies for HCC. The linked database covers 30% of the U.S. Medicare population. SEER itself contains data from 18 cancer registries covering 28% of the U.S. population, and 93% of the individuals in the database who are at least 65 years are matched to a Medicare enrollment file.
At 16%, NAFLD was third most common risk factor for HCC after infection (44%) and alcoholic diseases (19%) – 21% were other causes – but it was the second most common risk factor after infection in Asians and Pacific islanders, said Dr. Rahman.
Cirrhosis was not present in 36% of the NAFLD-related HCC cases; of those, 18% had only steatosis and not nonalcoholic steatohepatitis (NASH) or other adverse changes in pathology. Patients without cirrhosis more often had early-stage disease (stage I or II) than did those with cirrhosis (62% vs. 44%), as well as favorable grades (I or II, 76% vs. 56%).
Although the annual percentage change of NAFLD-related HCC with cirrhosis has increased since 1993, 1999 marked a point when the annual growth of NAFLD-related cases of HCC without cirrhosis outpaced those with cirrhosis. The average number of cases per year of NAFLD-related HCC without cirrhosis grew significantly from 51 in 1993-2000 to 88 in 2001-2007, compared with no change in cases with cirrhosis.
Three components of the metabolic syndrome – body-mass index greater than 30 kg/m2, type 2 diabetes mellitus, and dyslipidemia – occurred in significantly greater proportions of patients with NAFLD-related HCC without cirrhosis than in those with cirrhosis. The odds of developing HCC for each of those three components among noncirrhotic NAFLD patients was higher than for those with cirrhotic NAFLD, although the overall odds of developing HCC were higher among patients with cirrhotic NAFLD (odds ratio, 16.5) than in those with noncirrhotic NAFLD (OR, 3.1).
One audience member expressed concern about determining the presence of cirrhosis or NASH in the data without a systematic assessment of histopathology performed in a centralized way, but Dr. Rahman noted that the Medicare-matched SEER data have CPT procedural codes and ICD-9 diagnostic codes for diagnoses made through liver biopsy. Another attendee also suggested caution in calling NAFLD a risk factor for cancer alone because the people in the database have a higher rate of cancer, and the database does not include the peak of NASH at age 55 years.
None of the study investigators had relevant financial disclosures.
AT THE ANNUAL MEETING OF THE AMERICAN ASSOCIATION FOR THE STUDY OF LIVER DISEASES
Major Finding: The average number of cases per year of NAFLD-related hepatocellular carcinoma without cirrhosis grew significantly from 51 in 1993-2000 to 88 in 2001-2007, compared with no change in cases with cirrhosis.
Data Source: This population-based study used data from the SEER-Medicare database from 1993 to 2007.
Disclosures: None of the study investigators had relevant financial disclosures.
Novel Drug for Partial Onset Seizures Approved
The Food and Drug Administration added another arrow to the quiver of epilepsy specialists on Oct. 22 by approving perampanel for the treatment of partial onset seizures in patients with epilepsy aged 12 years or older.
Perampanel, which will be marketed by Eisai Inc. under the brand name Fycompa, was the first antiepileptic drug (AED) to be submitted to the agency on the basis of an excitation mechanism rather than membrane inhibition or stabilization, according to Dr. Jacqueline French of New York University, who was an investigator in clinical studies of the drug. The AED is a highly selective, noncompetitive, alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor antagonist.
"Some people with epilepsy do not achieve satisfactory seizure control from treatments they are currently using," said Dr. Russell Katz, director of the Division of Neurology Products in the FDA’s Center for Drug Evaluation and Research. "It is important to have a variety of treatment options available for patients with epilepsy."
Three phase III trials of the drug have reported 28-day reductions in seizure frequency relative to placebo in patients who were taking one to three other AEDs.
The drug’s label will include a boxed warning about the risk of serious (and in some cases life-threatening) neuropsychiatric events that were recorded during clinical trials, including irritability, aggression, anger, anxiety, paranoia, euphoric mood, agitation, and mental status changes. The agency noted that health care professionals should "closely monitor patients during the titration period when higher doses are used."
The drug is already approved in the European Union.
The Food and Drug Administration added another arrow to the quiver of epilepsy specialists on Oct. 22 by approving perampanel for the treatment of partial onset seizures in patients with epilepsy aged 12 years or older.
Perampanel, which will be marketed by Eisai Inc. under the brand name Fycompa, was the first antiepileptic drug (AED) to be submitted to the agency on the basis of an excitation mechanism rather than membrane inhibition or stabilization, according to Dr. Jacqueline French of New York University, who was an investigator in clinical studies of the drug. The AED is a highly selective, noncompetitive, alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor antagonist.
"Some people with epilepsy do not achieve satisfactory seizure control from treatments they are currently using," said Dr. Russell Katz, director of the Division of Neurology Products in the FDA’s Center for Drug Evaluation and Research. "It is important to have a variety of treatment options available for patients with epilepsy."
Three phase III trials of the drug have reported 28-day reductions in seizure frequency relative to placebo in patients who were taking one to three other AEDs.
The drug’s label will include a boxed warning about the risk of serious (and in some cases life-threatening) neuropsychiatric events that were recorded during clinical trials, including irritability, aggression, anger, anxiety, paranoia, euphoric mood, agitation, and mental status changes. The agency noted that health care professionals should "closely monitor patients during the titration period when higher doses are used."
The drug is already approved in the European Union.
The Food and Drug Administration added another arrow to the quiver of epilepsy specialists on Oct. 22 by approving perampanel for the treatment of partial onset seizures in patients with epilepsy aged 12 years or older.
Perampanel, which will be marketed by Eisai Inc. under the brand name Fycompa, was the first antiepileptic drug (AED) to be submitted to the agency on the basis of an excitation mechanism rather than membrane inhibition or stabilization, according to Dr. Jacqueline French of New York University, who was an investigator in clinical studies of the drug. The AED is a highly selective, noncompetitive, alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor antagonist.
"Some people with epilepsy do not achieve satisfactory seizure control from treatments they are currently using," said Dr. Russell Katz, director of the Division of Neurology Products in the FDA’s Center for Drug Evaluation and Research. "It is important to have a variety of treatment options available for patients with epilepsy."
Three phase III trials of the drug have reported 28-day reductions in seizure frequency relative to placebo in patients who were taking one to three other AEDs.
The drug’s label will include a boxed warning about the risk of serious (and in some cases life-threatening) neuropsychiatric events that were recorded during clinical trials, including irritability, aggression, anger, anxiety, paranoia, euphoric mood, agitation, and mental status changes. The agency noted that health care professionals should "closely monitor patients during the titration period when higher doses are used."
The drug is already approved in the European Union.
Fungal Meningitis Outbreak Exposes Lack of Regulatory Oversight
The compounding pharmacy at the heart of the ongoing federal and Massachusetts state investigation into contaminated lots of methylprednisolone acetate has been accused of illegally selling the medication in large batches across state lines, a development that prompted officials during an Oct. 11 telebriefing on the meningitis outbreak to call for improved oversight of such pharmacies at the federal level.
The actions of the New England Compounding Center (NECC) in Framingham, Mass., the pharmacy that has been the focus of the investigation into contaminated preparations of the steroid, would constitute it to be a drug manufacturer under Massachusetts law, which allows compounding only on the receipt of a patient-specific prescription. The NECC had sold the contaminated lots to clinics in 23 states before the Food and Drug Administration worked with the pharmacy to recall the steroid on Sept. 26, and then on Oct. 6 voluntarily recalled all of the compounded products in circulation that had been made at its Framingham, Mass., facility. The company voluntarily shut down its operations Oct. 3.
Fungal meningitis cases and deaths in people who received epidural injections of the contaminated methylprednisolone acetate continue to rise, but investigators at the FDA and the Centers for Disease Control and Prevention have begun to determine which fungal species are present in patients and in contaminated vials of the steroid. Many tests are still unfinished.
Cultures of cerebrospinal fluid and histopathologic analysis of specimens have so far indicated fungal infection with Aspergillus species in 1 patient and Exserohilum species in 10 patients. The FDA has found fungal contamination by direct microscopic examination of foreign matter taken from sealed vials of the steroid collected from the NECC and from clinics that received the recalled lots.
"We know that fungus such as Exserohilum can be difficult to detect in samples from patients. Patients and their physicians should not assume fungal testing that is negative means there is no infection," Dr. J. Todd Weber, the CDC’s incident manager of the multistate meningitis outbreak, said during the telebriefing. In these cases, patients should still be treated for fungal meningitis. He noted that Exserohilum previously has not been observed to cause fungal meningitis, which historically is itself "very rare."
Dr. Weber reported that of the nearly 14,000 people who received an epidural or intra-articular injection with the steroid from recalled lots, more than 12,000 have now been contacted and those with symptoms of meningitis or possible joint infection have been referred to their physicians.
As of Oct. 11, the outbreak has caused 169 cases of meningitis and 14 deaths in 11 states: Florida (7 cases, including 2 deaths), Indiana (21 cases, including 1 death), Tennessee (49 cases, including 6 deaths), Maryland (13 cases, including 1 death), Michigan, (38 cases, including 3 deaths), North Carolina (2 cases), Virginia (30 cases, including 1 death), Ohio (3 cases), New Jersey (2 cases), Minnesota (3 cases), and Idaho (1 case). The meningitis cases have all been linked to epidural injections with methylprednisolone acetate compounded by the NECC. Production lots of the steroid at doses of 40 mg/mL and 80 mg/mL have been recalled, as well as all other products currently in circulation that were compounded at and distributed from the facility in Framingham.
The CDC is currently investigating one case of an ankle joint infection associated with an injection of the steroid in Michigan, but the laboratory results have not been completed to confirm whether or not it was caused by fungal infection, according to Dr. Weber. "We expect that through additional patient notification efforts, we may see additional patients come forward with infections of the joints. These patients may present with symptoms, including fever [and] increased pain, redness, warmth, or swelling in the joint where they received the injection or at the injection site."
In addition to identifying and assisting patients who may have been potentially exposed to the contaminated drug, the Massachusetts Department of Pharmacy and the FDA are reviewing the processes and procedures of the NECC. The FDA is also looking into the source of the ingredients used to make the compounded methylprednisolone acetate, said Deborah M. Autor, J.D., deputy commissioner for global regulatory operations and policy at the FDA.
FDA investigations during Oct. 2-10 ruled out Busse Hospital Disposables Inc. in New York as a possible source of at least one case at a Tennessee pain clinic as well as epidural trays from a New Orleans pain clinic that had been central to the outbreak, Dr. Autor said.
More than 50 sealed vials from the NECC and from samples collected from the recalled lots around the country have tested positive for fungal contaminants, but tests have not yet determined whether or not they are the same as the organisms found in patients.
On Oct. 10, the FDA and the Massachusetts Department of Pharmacy launched a joint inspection of Ameridose, a compounding pharmacy that was founded by the same people who opened the NECC, Dr. Autor said. The agency could not be sure that the same practices that were present at the NECC were also present at Ameridose, which voluntarily ceased operations until at least Oct. 22. Drugs produced by Ameridose and its partnering distributor, Alantos Pharmaceuticals, as well as other pharmacies under joint ownership, have ceased distribution of compounded drugs, but there has been no direct evidence to suggest that compounded products produced by Ameridose have been contaminated and no products have been recalled, said Dr. Madeleine Biondolillo, director of the Massachusetts Department of Public Health and Human Services’ Bureau of Health Care Safety and Quality.
The Massachusetts Department of Public Health on Oct. 10 sent a letter to compounding pharmacies in the state to reinforce the rules they must abide by, and the Board of Pharmacy issued an order for all compounding pharmacies to sign an affidavit "attesting compliance with all pertinent laws and regulations," Dr. Biondolillo said.
The recent FDA investigation is not the first for the NECC, which received a warning letter from the agency in 2006 for acting like a drug manufacturer in distributing and selling large quantities of compounded drugs.
The NECC apparently did not stop distributing its compounded drugs across state lines in large quantities despite the warning letter, previous inspections, and being under a consent agreement with Massachusetts. "The enforcement in this case and other compounding cases is complicated greatly by litigation and a lack of clarity in the law, and while we have done some with clear authority, we cannot do all," said Dr. Autor, noting that no federal or state agency is tracking the volume of medications that are prepared and distributed in compounding pharmacies.
"Once the immediate crisis is contained, we want to work with Congress, compounders, states, and all other stakeholders to try to prevent tragedies like this in the future," Dr. Autor said.
Dr. Biondolillo agreed. "We urge Congress to act quickly to address the need for new laws on the federal level to fill in the regulatory gaps so that there is clear authority over regulating these practices."
The Association of American Physicians and Surgeons, however, cautioned against taking any legislative action too quickly because the nature of the NECC’s business was different from most compounding pharmacies. "The CDC has not released any proof that the small outbreak was caused by anything improper done by a compounding pharmacy," the association said in a statement, noting that "most compounding pharmacies focus on customized preparations with tight quality controls, and they should remain under state, not federal, regulation."
Now that Exserohilum is one of the potential causes of the outbreak, the CDC has broadened the scope of treatment guidance to recommend treating patients with confirmed fungal meningitis with two antifungal drugs, voriconazole and liposomal amphotericin B, which are "very strong and can be very difficult for patients to tolerate over a long period of time," said Dr. Weber, noting that the CDC is working with clinical experts to determine the best dose and the best length of time to treat patients.
The CDC notes that infected patients have presented approximately 1-4 weeks (median of 2 weeks, with the longest being 42 days) following injection with a variety of symptoms, including fever, new or worsening headache, nausea, and new neurologic deficit (consistent with deep brain stroke). The potentially contaminated injections were given starting May 21, 2012.
"We want to emphasize that fungal infections can be slow to develop, and if there are indeed reports of longer periods of time between injection and onset to symptoms ... patients and their doctors will need to be vigilant for at least several months following the injection," said Dr. Weber, who is also chief of the prevention and response branch in the CDC’s Division of Healthcare Quality Promotion.
Nearly all patients have reported headache, whereas about half of patients have had back pain, fever, or nausea, most of which have been mild in nature. CSF obtained from these patients has typically shown elevated white cell count (with a predominance of neutrophils), low glucose, and elevated protein, according to the CDC.
The 23 states that received the implicated lots of medication are California, Connecticut, Florida, Georgia, Idaho, Illinois, Indiana, Maryland, Michigan, Minnesota, North Carolina, New Hampshire, New Jersey, Nevada, New York, Ohio, Pennsylvania, Rhode Island, South Carolina, Tennessee, Texas, Virginia, and West Virginia.
All reports of complaints or problems associated with these products should be made at the MedWatch website, the FDA’s voluntary reporting program, or by calling 800-FDA-1088.
The compounding pharmacy at the heart of the ongoing federal and Massachusetts state investigation into contaminated lots of methylprednisolone acetate has been accused of illegally selling the medication in large batches across state lines, a development that prompted officials during an Oct. 11 telebriefing on the meningitis outbreak to call for improved oversight of such pharmacies at the federal level.
The actions of the New England Compounding Center (NECC) in Framingham, Mass., the pharmacy that has been the focus of the investigation into contaminated preparations of the steroid, would constitute it to be a drug manufacturer under Massachusetts law, which allows compounding only on the receipt of a patient-specific prescription. The NECC had sold the contaminated lots to clinics in 23 states before the Food and Drug Administration worked with the pharmacy to recall the steroid on Sept. 26, and then on Oct. 6 voluntarily recalled all of the compounded products in circulation that had been made at its Framingham, Mass., facility. The company voluntarily shut down its operations Oct. 3.
Fungal meningitis cases and deaths in people who received epidural injections of the contaminated methylprednisolone acetate continue to rise, but investigators at the FDA and the Centers for Disease Control and Prevention have begun to determine which fungal species are present in patients and in contaminated vials of the steroid. Many tests are still unfinished.
Cultures of cerebrospinal fluid and histopathologic analysis of specimens have so far indicated fungal infection with Aspergillus species in 1 patient and Exserohilum species in 10 patients. The FDA has found fungal contamination by direct microscopic examination of foreign matter taken from sealed vials of the steroid collected from the NECC and from clinics that received the recalled lots.
"We know that fungus such as Exserohilum can be difficult to detect in samples from patients. Patients and their physicians should not assume fungal testing that is negative means there is no infection," Dr. J. Todd Weber, the CDC’s incident manager of the multistate meningitis outbreak, said during the telebriefing. In these cases, patients should still be treated for fungal meningitis. He noted that Exserohilum previously has not been observed to cause fungal meningitis, which historically is itself "very rare."
Dr. Weber reported that of the nearly 14,000 people who received an epidural or intra-articular injection with the steroid from recalled lots, more than 12,000 have now been contacted and those with symptoms of meningitis or possible joint infection have been referred to their physicians.
As of Oct. 11, the outbreak has caused 169 cases of meningitis and 14 deaths in 11 states: Florida (7 cases, including 2 deaths), Indiana (21 cases, including 1 death), Tennessee (49 cases, including 6 deaths), Maryland (13 cases, including 1 death), Michigan, (38 cases, including 3 deaths), North Carolina (2 cases), Virginia (30 cases, including 1 death), Ohio (3 cases), New Jersey (2 cases), Minnesota (3 cases), and Idaho (1 case). The meningitis cases have all been linked to epidural injections with methylprednisolone acetate compounded by the NECC. Production lots of the steroid at doses of 40 mg/mL and 80 mg/mL have been recalled, as well as all other products currently in circulation that were compounded at and distributed from the facility in Framingham.
The CDC is currently investigating one case of an ankle joint infection associated with an injection of the steroid in Michigan, but the laboratory results have not been completed to confirm whether or not it was caused by fungal infection, according to Dr. Weber. "We expect that through additional patient notification efforts, we may see additional patients come forward with infections of the joints. These patients may present with symptoms, including fever [and] increased pain, redness, warmth, or swelling in the joint where they received the injection or at the injection site."
In addition to identifying and assisting patients who may have been potentially exposed to the contaminated drug, the Massachusetts Department of Pharmacy and the FDA are reviewing the processes and procedures of the NECC. The FDA is also looking into the source of the ingredients used to make the compounded methylprednisolone acetate, said Deborah M. Autor, J.D., deputy commissioner for global regulatory operations and policy at the FDA.
FDA investigations during Oct. 2-10 ruled out Busse Hospital Disposables Inc. in New York as a possible source of at least one case at a Tennessee pain clinic as well as epidural trays from a New Orleans pain clinic that had been central to the outbreak, Dr. Autor said.
More than 50 sealed vials from the NECC and from samples collected from the recalled lots around the country have tested positive for fungal contaminants, but tests have not yet determined whether or not they are the same as the organisms found in patients.
On Oct. 10, the FDA and the Massachusetts Department of Pharmacy launched a joint inspection of Ameridose, a compounding pharmacy that was founded by the same people who opened the NECC, Dr. Autor said. The agency could not be sure that the same practices that were present at the NECC were also present at Ameridose, which voluntarily ceased operations until at least Oct. 22. Drugs produced by Ameridose and its partnering distributor, Alantos Pharmaceuticals, as well as other pharmacies under joint ownership, have ceased distribution of compounded drugs, but there has been no direct evidence to suggest that compounded products produced by Ameridose have been contaminated and no products have been recalled, said Dr. Madeleine Biondolillo, director of the Massachusetts Department of Public Health and Human Services’ Bureau of Health Care Safety and Quality.
The Massachusetts Department of Public Health on Oct. 10 sent a letter to compounding pharmacies in the state to reinforce the rules they must abide by, and the Board of Pharmacy issued an order for all compounding pharmacies to sign an affidavit "attesting compliance with all pertinent laws and regulations," Dr. Biondolillo said.
The recent FDA investigation is not the first for the NECC, which received a warning letter from the agency in 2006 for acting like a drug manufacturer in distributing and selling large quantities of compounded drugs.
The NECC apparently did not stop distributing its compounded drugs across state lines in large quantities despite the warning letter, previous inspections, and being under a consent agreement with Massachusetts. "The enforcement in this case and other compounding cases is complicated greatly by litigation and a lack of clarity in the law, and while we have done some with clear authority, we cannot do all," said Dr. Autor, noting that no federal or state agency is tracking the volume of medications that are prepared and distributed in compounding pharmacies.
"Once the immediate crisis is contained, we want to work with Congress, compounders, states, and all other stakeholders to try to prevent tragedies like this in the future," Dr. Autor said.
Dr. Biondolillo agreed. "We urge Congress to act quickly to address the need for new laws on the federal level to fill in the regulatory gaps so that there is clear authority over regulating these practices."
The Association of American Physicians and Surgeons, however, cautioned against taking any legislative action too quickly because the nature of the NECC’s business was different from most compounding pharmacies. "The CDC has not released any proof that the small outbreak was caused by anything improper done by a compounding pharmacy," the association said in a statement, noting that "most compounding pharmacies focus on customized preparations with tight quality controls, and they should remain under state, not federal, regulation."
Now that Exserohilum is one of the potential causes of the outbreak, the CDC has broadened the scope of treatment guidance to recommend treating patients with confirmed fungal meningitis with two antifungal drugs, voriconazole and liposomal amphotericin B, which are "very strong and can be very difficult for patients to tolerate over a long period of time," said Dr. Weber, noting that the CDC is working with clinical experts to determine the best dose and the best length of time to treat patients.
The CDC notes that infected patients have presented approximately 1-4 weeks (median of 2 weeks, with the longest being 42 days) following injection with a variety of symptoms, including fever, new or worsening headache, nausea, and new neurologic deficit (consistent with deep brain stroke). The potentially contaminated injections were given starting May 21, 2012.
"We want to emphasize that fungal infections can be slow to develop, and if there are indeed reports of longer periods of time between injection and onset to symptoms ... patients and their doctors will need to be vigilant for at least several months following the injection," said Dr. Weber, who is also chief of the prevention and response branch in the CDC’s Division of Healthcare Quality Promotion.
Nearly all patients have reported headache, whereas about half of patients have had back pain, fever, or nausea, most of which have been mild in nature. CSF obtained from these patients has typically shown elevated white cell count (with a predominance of neutrophils), low glucose, and elevated protein, according to the CDC.
The 23 states that received the implicated lots of medication are California, Connecticut, Florida, Georgia, Idaho, Illinois, Indiana, Maryland, Michigan, Minnesota, North Carolina, New Hampshire, New Jersey, Nevada, New York, Ohio, Pennsylvania, Rhode Island, South Carolina, Tennessee, Texas, Virginia, and West Virginia.
All reports of complaints or problems associated with these products should be made at the MedWatch website, the FDA’s voluntary reporting program, or by calling 800-FDA-1088.
The compounding pharmacy at the heart of the ongoing federal and Massachusetts state investigation into contaminated lots of methylprednisolone acetate has been accused of illegally selling the medication in large batches across state lines, a development that prompted officials during an Oct. 11 telebriefing on the meningitis outbreak to call for improved oversight of such pharmacies at the federal level.
The actions of the New England Compounding Center (NECC) in Framingham, Mass., the pharmacy that has been the focus of the investigation into contaminated preparations of the steroid, would constitute it to be a drug manufacturer under Massachusetts law, which allows compounding only on the receipt of a patient-specific prescription. The NECC had sold the contaminated lots to clinics in 23 states before the Food and Drug Administration worked with the pharmacy to recall the steroid on Sept. 26, and then on Oct. 6 voluntarily recalled all of the compounded products in circulation that had been made at its Framingham, Mass., facility. The company voluntarily shut down its operations Oct. 3.
Fungal meningitis cases and deaths in people who received epidural injections of the contaminated methylprednisolone acetate continue to rise, but investigators at the FDA and the Centers for Disease Control and Prevention have begun to determine which fungal species are present in patients and in contaminated vials of the steroid. Many tests are still unfinished.
Cultures of cerebrospinal fluid and histopathologic analysis of specimens have so far indicated fungal infection with Aspergillus species in 1 patient and Exserohilum species in 10 patients. The FDA has found fungal contamination by direct microscopic examination of foreign matter taken from sealed vials of the steroid collected from the NECC and from clinics that received the recalled lots.
"We know that fungus such as Exserohilum can be difficult to detect in samples from patients. Patients and their physicians should not assume fungal testing that is negative means there is no infection," Dr. J. Todd Weber, the CDC’s incident manager of the multistate meningitis outbreak, said during the telebriefing. In these cases, patients should still be treated for fungal meningitis. He noted that Exserohilum previously has not been observed to cause fungal meningitis, which historically is itself "very rare."
Dr. Weber reported that of the nearly 14,000 people who received an epidural or intra-articular injection with the steroid from recalled lots, more than 12,000 have now been contacted and those with symptoms of meningitis or possible joint infection have been referred to their physicians.
As of Oct. 11, the outbreak has caused 169 cases of meningitis and 14 deaths in 11 states: Florida (7 cases, including 2 deaths), Indiana (21 cases, including 1 death), Tennessee (49 cases, including 6 deaths), Maryland (13 cases, including 1 death), Michigan, (38 cases, including 3 deaths), North Carolina (2 cases), Virginia (30 cases, including 1 death), Ohio (3 cases), New Jersey (2 cases), Minnesota (3 cases), and Idaho (1 case). The meningitis cases have all been linked to epidural injections with methylprednisolone acetate compounded by the NECC. Production lots of the steroid at doses of 40 mg/mL and 80 mg/mL have been recalled, as well as all other products currently in circulation that were compounded at and distributed from the facility in Framingham.
The CDC is currently investigating one case of an ankle joint infection associated with an injection of the steroid in Michigan, but the laboratory results have not been completed to confirm whether or not it was caused by fungal infection, according to Dr. Weber. "We expect that through additional patient notification efforts, we may see additional patients come forward with infections of the joints. These patients may present with symptoms, including fever [and] increased pain, redness, warmth, or swelling in the joint where they received the injection or at the injection site."
In addition to identifying and assisting patients who may have been potentially exposed to the contaminated drug, the Massachusetts Department of Pharmacy and the FDA are reviewing the processes and procedures of the NECC. The FDA is also looking into the source of the ingredients used to make the compounded methylprednisolone acetate, said Deborah M. Autor, J.D., deputy commissioner for global regulatory operations and policy at the FDA.
FDA investigations during Oct. 2-10 ruled out Busse Hospital Disposables Inc. in New York as a possible source of at least one case at a Tennessee pain clinic as well as epidural trays from a New Orleans pain clinic that had been central to the outbreak, Dr. Autor said.
More than 50 sealed vials from the NECC and from samples collected from the recalled lots around the country have tested positive for fungal contaminants, but tests have not yet determined whether or not they are the same as the organisms found in patients.
On Oct. 10, the FDA and the Massachusetts Department of Pharmacy launched a joint inspection of Ameridose, a compounding pharmacy that was founded by the same people who opened the NECC, Dr. Autor said. The agency could not be sure that the same practices that were present at the NECC were also present at Ameridose, which voluntarily ceased operations until at least Oct. 22. Drugs produced by Ameridose and its partnering distributor, Alantos Pharmaceuticals, as well as other pharmacies under joint ownership, have ceased distribution of compounded drugs, but there has been no direct evidence to suggest that compounded products produced by Ameridose have been contaminated and no products have been recalled, said Dr. Madeleine Biondolillo, director of the Massachusetts Department of Public Health and Human Services’ Bureau of Health Care Safety and Quality.
The Massachusetts Department of Public Health on Oct. 10 sent a letter to compounding pharmacies in the state to reinforce the rules they must abide by, and the Board of Pharmacy issued an order for all compounding pharmacies to sign an affidavit "attesting compliance with all pertinent laws and regulations," Dr. Biondolillo said.
The recent FDA investigation is not the first for the NECC, which received a warning letter from the agency in 2006 for acting like a drug manufacturer in distributing and selling large quantities of compounded drugs.
The NECC apparently did not stop distributing its compounded drugs across state lines in large quantities despite the warning letter, previous inspections, and being under a consent agreement with Massachusetts. "The enforcement in this case and other compounding cases is complicated greatly by litigation and a lack of clarity in the law, and while we have done some with clear authority, we cannot do all," said Dr. Autor, noting that no federal or state agency is tracking the volume of medications that are prepared and distributed in compounding pharmacies.
"Once the immediate crisis is contained, we want to work with Congress, compounders, states, and all other stakeholders to try to prevent tragedies like this in the future," Dr. Autor said.
Dr. Biondolillo agreed. "We urge Congress to act quickly to address the need for new laws on the federal level to fill in the regulatory gaps so that there is clear authority over regulating these practices."
The Association of American Physicians and Surgeons, however, cautioned against taking any legislative action too quickly because the nature of the NECC’s business was different from most compounding pharmacies. "The CDC has not released any proof that the small outbreak was caused by anything improper done by a compounding pharmacy," the association said in a statement, noting that "most compounding pharmacies focus on customized preparations with tight quality controls, and they should remain under state, not federal, regulation."
Now that Exserohilum is one of the potential causes of the outbreak, the CDC has broadened the scope of treatment guidance to recommend treating patients with confirmed fungal meningitis with two antifungal drugs, voriconazole and liposomal amphotericin B, which are "very strong and can be very difficult for patients to tolerate over a long period of time," said Dr. Weber, noting that the CDC is working with clinical experts to determine the best dose and the best length of time to treat patients.
The CDC notes that infected patients have presented approximately 1-4 weeks (median of 2 weeks, with the longest being 42 days) following injection with a variety of symptoms, including fever, new or worsening headache, nausea, and new neurologic deficit (consistent with deep brain stroke). The potentially contaminated injections were given starting May 21, 2012.
"We want to emphasize that fungal infections can be slow to develop, and if there are indeed reports of longer periods of time between injection and onset to symptoms ... patients and their doctors will need to be vigilant for at least several months following the injection," said Dr. Weber, who is also chief of the prevention and response branch in the CDC’s Division of Healthcare Quality Promotion.
Nearly all patients have reported headache, whereas about half of patients have had back pain, fever, or nausea, most of which have been mild in nature. CSF obtained from these patients has typically shown elevated white cell count (with a predominance of neutrophils), low glucose, and elevated protein, according to the CDC.
The 23 states that received the implicated lots of medication are California, Connecticut, Florida, Georgia, Idaho, Illinois, Indiana, Maryland, Michigan, Minnesota, North Carolina, New Hampshire, New Jersey, Nevada, New York, Ohio, Pennsylvania, Rhode Island, South Carolina, Tennessee, Texas, Virginia, and West Virginia.
All reports of complaints or problems associated with these products should be made at the MedWatch website, the FDA’s voluntary reporting program, or by calling 800-FDA-1088.
Fungal Meningitis Cases, Deaths Continue to Rise
Health officials from 23 states in which contaminated methylprednisolone acetate products have been shipped continue to watch for additional cases of fungal meningitis tied to epidural injections given with the contaminated medication.*
As of Oct. 10, the outbreak is responsible for 137 cases and 12 deaths in 10 states, including Florida (6 cases, including one death), Indiana (15 cases), Tennessee (44 cases, including six deaths), Maryland (9 cases, including one death), Michigan, (28 cases, including three deaths), North Carolina (2 cases), Virginia (27 cases, including one death), Ohio (1 case), New Jersey (2 cases), and Minnesota (3 cases). The cases have all been linked to epidural injections with methylprednisolone acetate compounded by the New England Compounding Center (NECC) in Framingham, Mass. Production lots of the steroid at doses of 40 mg/mL and 80 mg/mL have been recalled, as well as all other products currently in circulation that were compounded at and distributed from its facility in Framingham.
The Food and Drug Administration found fungal contamination by direct microscopic examination of foreign matter taken from a sealed vial of the steroid collected from NECC, which voluntarily shut down its operations Oct. 3. The agency is in the process of conducting additional microbial testing to confirm the exact species of the fungus and is working closely with Centers of Disease Control and Prevention and state authorities to determine whether this sample taken from the product matches the organism found in patients. So far, cultures of cerebrospinal fluid (CSF) and histopathologic analysis of specimens have indicated fungal infection in nine patients, including Aspergillus and Exserohilum species.
The CDC notes that "Infected patients have presented approximately 1-4 weeks following their injection with a variety of symptoms, including fever, new or worsening headache, nausea, and new neurological deficit (consistent with deep brain stroke). Some of these patients’ symptoms were very mild in nature. CSF obtained from these patients has typically shown elevated white cell count (with a predominance of neutrophils), low glucose, and elevated protein."
"Physicians should contact patients who have had an injection (e.g., spinal, joint) using any of the three lots of methylprednisolone acetate ... to determine if they are having any symptoms. Although all cases detected to date occurred after injections with products from these three lots, out of an abundance of caution, CDC and FDA recommend that health care professionals cease use of any product produced by the New England Compounding Center until further information is available," the CDC said in a statement. The potentially contaminated injections were given starting May 21, 2012.
The CDC has provided instructions for clinicians on diagnostic testing and specimen submission.
The 23 states that received the implicated lots of medication are California, Connecticut, Florida, Georgia, Idaho, Illinois, Indiana, Maryland, Michigan, Minnesota, North Carolina, New Hampshire, New Jersey, Nevada, New York, Ohio, Pennsylvania, Rhode Island, South Carolina, Tennessee, Texas, Virginia, and West Virginia.
All reports of complaints or problems associated with these products should be made at the MedWatch website, the FDA’s voluntary reporting program, or by calling 800-FDA-1088.
* Story updated October 10, 2012.
Health officials from 23 states in which contaminated methylprednisolone acetate products have been shipped continue to watch for additional cases of fungal meningitis tied to epidural injections given with the contaminated medication.*
As of Oct. 10, the outbreak is responsible for 137 cases and 12 deaths in 10 states, including Florida (6 cases, including one death), Indiana (15 cases), Tennessee (44 cases, including six deaths), Maryland (9 cases, including one death), Michigan, (28 cases, including three deaths), North Carolina (2 cases), Virginia (27 cases, including one death), Ohio (1 case), New Jersey (2 cases), and Minnesota (3 cases). The cases have all been linked to epidural injections with methylprednisolone acetate compounded by the New England Compounding Center (NECC) in Framingham, Mass. Production lots of the steroid at doses of 40 mg/mL and 80 mg/mL have been recalled, as well as all other products currently in circulation that were compounded at and distributed from its facility in Framingham.
The Food and Drug Administration found fungal contamination by direct microscopic examination of foreign matter taken from a sealed vial of the steroid collected from NECC, which voluntarily shut down its operations Oct. 3. The agency is in the process of conducting additional microbial testing to confirm the exact species of the fungus and is working closely with Centers of Disease Control and Prevention and state authorities to determine whether this sample taken from the product matches the organism found in patients. So far, cultures of cerebrospinal fluid (CSF) and histopathologic analysis of specimens have indicated fungal infection in nine patients, including Aspergillus and Exserohilum species.
The CDC notes that "Infected patients have presented approximately 1-4 weeks following their injection with a variety of symptoms, including fever, new or worsening headache, nausea, and new neurological deficit (consistent with deep brain stroke). Some of these patients’ symptoms were very mild in nature. CSF obtained from these patients has typically shown elevated white cell count (with a predominance of neutrophils), low glucose, and elevated protein."
"Physicians should contact patients who have had an injection (e.g., spinal, joint) using any of the three lots of methylprednisolone acetate ... to determine if they are having any symptoms. Although all cases detected to date occurred after injections with products from these three lots, out of an abundance of caution, CDC and FDA recommend that health care professionals cease use of any product produced by the New England Compounding Center until further information is available," the CDC said in a statement. The potentially contaminated injections were given starting May 21, 2012.
The CDC has provided instructions for clinicians on diagnostic testing and specimen submission.
The 23 states that received the implicated lots of medication are California, Connecticut, Florida, Georgia, Idaho, Illinois, Indiana, Maryland, Michigan, Minnesota, North Carolina, New Hampshire, New Jersey, Nevada, New York, Ohio, Pennsylvania, Rhode Island, South Carolina, Tennessee, Texas, Virginia, and West Virginia.
All reports of complaints or problems associated with these products should be made at the MedWatch website, the FDA’s voluntary reporting program, or by calling 800-FDA-1088.
* Story updated October 10, 2012.
Health officials from 23 states in which contaminated methylprednisolone acetate products have been shipped continue to watch for additional cases of fungal meningitis tied to epidural injections given with the contaminated medication.*
As of Oct. 10, the outbreak is responsible for 137 cases and 12 deaths in 10 states, including Florida (6 cases, including one death), Indiana (15 cases), Tennessee (44 cases, including six deaths), Maryland (9 cases, including one death), Michigan, (28 cases, including three deaths), North Carolina (2 cases), Virginia (27 cases, including one death), Ohio (1 case), New Jersey (2 cases), and Minnesota (3 cases). The cases have all been linked to epidural injections with methylprednisolone acetate compounded by the New England Compounding Center (NECC) in Framingham, Mass. Production lots of the steroid at doses of 40 mg/mL and 80 mg/mL have been recalled, as well as all other products currently in circulation that were compounded at and distributed from its facility in Framingham.
The Food and Drug Administration found fungal contamination by direct microscopic examination of foreign matter taken from a sealed vial of the steroid collected from NECC, which voluntarily shut down its operations Oct. 3. The agency is in the process of conducting additional microbial testing to confirm the exact species of the fungus and is working closely with Centers of Disease Control and Prevention and state authorities to determine whether this sample taken from the product matches the organism found in patients. So far, cultures of cerebrospinal fluid (CSF) and histopathologic analysis of specimens have indicated fungal infection in nine patients, including Aspergillus and Exserohilum species.
The CDC notes that "Infected patients have presented approximately 1-4 weeks following their injection with a variety of symptoms, including fever, new or worsening headache, nausea, and new neurological deficit (consistent with deep brain stroke). Some of these patients’ symptoms were very mild in nature. CSF obtained from these patients has typically shown elevated white cell count (with a predominance of neutrophils), low glucose, and elevated protein."
"Physicians should contact patients who have had an injection (e.g., spinal, joint) using any of the three lots of methylprednisolone acetate ... to determine if they are having any symptoms. Although all cases detected to date occurred after injections with products from these three lots, out of an abundance of caution, CDC and FDA recommend that health care professionals cease use of any product produced by the New England Compounding Center until further information is available," the CDC said in a statement. The potentially contaminated injections were given starting May 21, 2012.
The CDC has provided instructions for clinicians on diagnostic testing and specimen submission.
The 23 states that received the implicated lots of medication are California, Connecticut, Florida, Georgia, Idaho, Illinois, Indiana, Maryland, Michigan, Minnesota, North Carolina, New Hampshire, New Jersey, Nevada, New York, Ohio, Pennsylvania, Rhode Island, South Carolina, Tennessee, Texas, Virginia, and West Virginia.
All reports of complaints or problems associated with these products should be made at the MedWatch website, the FDA’s voluntary reporting program, or by calling 800-FDA-1088.
* Story updated October 10, 2012.
Drug Dosage, Gender Affect Impulsivity Prognosis in Parkinson's
The development and resolution of impulse control disorders in patients with Parkinson’s disease appears to be largely reliant on gender, daily dose of dopamine agonists, and the presence of depressive symptoms, according to a prospective study.
Persistence of impulse control disorders (ICDs) among Parkinson’s disease patients from baseline to a mean follow-up of about 15 months were significantly associated with male gender and a higher daily dose of dopamine agonists. However, worsening depressive symptoms was the only factor associated with the development of an ICD during follow-up, reported Dr. Juho Joutsa of the University of Turku (Finland) and his colleagues (Parkinsonism Relat. Disord. 2012 [doi:10.1016/j.parkreldis.2012.06.005]). Their study is the first large-scale, prospective study to assess risk factors for the development and resolution of ICDs at two time points.
The investigators used self-reports from 290 survey participants who had had Parkinson’s disease for a median of 6 years and were a median age of 64 years at baseline. Among 270 patients who answered questions about ICDs on the Questionnaire for Impulsive-Compulsive Behaviors in Parkinson's Disease at both baseline and follow-up, 22 had a new-onset ICD at follow-up, 31 had their ICDs resolve at follow-up, 135 had no ICD at both baseline and follow-up, and 82 reported stable ICDs at both time points.
In the patients with ICDs at baseline, multivariate binary logistic regression analyses showed significantly higher odds for the presence of ICDs at follow-up if the patient was a man (odds ratio, 6.10).
"The present results demonstrate that ICDs are not only more common in men but also approximately six times more unlikely to be resolved, compared to women during a 15-month follow-up. Thus, the results also suggest that the difference in prevalence rates between the sexes found in cross-sectional studies should not be interpreted only as higher incidence of ICDs in men. However, future studies are required to clarify whether ICDs in male patients are more treatment resistant or if men are more reluctant to seek treatment for ICDs," the investigators wrote.
The multivariate analysis also found significantly higher odds for the presence of ICDs at follow-up among patients who had a higher dopamine agonist levodopa-equivalent daily dose at baseline (OR 2.25 for every 100-mg increase). However, the effect of dopamine agonist dose did not differ between genders.
"Although the baseline dopamine agonist dose was associated with ICD outcome, we did not observe significant effect of the change in the agonist dose," Dr. Joutsa and his associates wrote.
The investigators found that the optimal levodopa equivalent daily dose cut off at baseline for predicting a poor ICD outcome was 161 mg or greater, resulting in a 46% sensitivity, 90% specificity, 93% positive predictive value, and 37% negative predictive value. That level of levodopa equivalent daily dose, which corresponds to 1.6 mg pramipexole and 8 mg ropinirole, "is quite low compared to the maximal doses of the drugs, and to the doses needed for efficacious motor symptom treatment. The relatively low cut-off values suggest that ICDs are associated with dopamine agonists even with low daily doses, when the first motor benefit is reached," they wrote.
A rise in score on the Beck Depression Inventory (BDI) proved to be the only significant predictor of ICDs at follow-up among patients without ICDs at baseline, increasing the odds of developing an ICD by nearly 10% for each 1-point increase on the scale. Those who developed new-onset ICDs at follow-up had a mean BDI score of 8.4 at baseline and 12 at follow-up. Overall, patients with ICDs at either time point had a higher mean BDI score than did those without ICDs, with baseline scores of 14.2 vs. 8.9, respectively, and follow-up scores of 12.9 vs. 9.8.
However, patients who had resolved their ICDs at follow-up did not improve their depressive symptoms to the level seen in patients who never had ICDs, showing that "depressive symptoms at baseline did not predict the ICD outcome at follow-up," Dr. Joutsa and his colleagues wrote. "Therefore, these results could be interpreted to favor ICDs as a causal factor for depression rather than vice versa, although the design of the study does not allow definitive conclusions about the causality."
The research was funded by the Finnish Alcohol Research Foundation, the Finnish Medical Foundation, the Turku University Hospital, the Turku University Foundation, the Paulo Foundation, and the Finnish Parkinson Foundation. All but one of the authors declared having financial relationships with Boehringer-Ingelheim, GlaxoSmithKline, UCB Pharma, and Orion Pharma, Abbott, and Lundbeck.
The development and resolution of impulse control disorders in patients with Parkinson’s disease appears to be largely reliant on gender, daily dose of dopamine agonists, and the presence of depressive symptoms, according to a prospective study.
Persistence of impulse control disorders (ICDs) among Parkinson’s disease patients from baseline to a mean follow-up of about 15 months were significantly associated with male gender and a higher daily dose of dopamine agonists. However, worsening depressive symptoms was the only factor associated with the development of an ICD during follow-up, reported Dr. Juho Joutsa of the University of Turku (Finland) and his colleagues (Parkinsonism Relat. Disord. 2012 [doi:10.1016/j.parkreldis.2012.06.005]). Their study is the first large-scale, prospective study to assess risk factors for the development and resolution of ICDs at two time points.
The investigators used self-reports from 290 survey participants who had had Parkinson’s disease for a median of 6 years and were a median age of 64 years at baseline. Among 270 patients who answered questions about ICDs on the Questionnaire for Impulsive-Compulsive Behaviors in Parkinson's Disease at both baseline and follow-up, 22 had a new-onset ICD at follow-up, 31 had their ICDs resolve at follow-up, 135 had no ICD at both baseline and follow-up, and 82 reported stable ICDs at both time points.
In the patients with ICDs at baseline, multivariate binary logistic regression analyses showed significantly higher odds for the presence of ICDs at follow-up if the patient was a man (odds ratio, 6.10).
"The present results demonstrate that ICDs are not only more common in men but also approximately six times more unlikely to be resolved, compared to women during a 15-month follow-up. Thus, the results also suggest that the difference in prevalence rates between the sexes found in cross-sectional studies should not be interpreted only as higher incidence of ICDs in men. However, future studies are required to clarify whether ICDs in male patients are more treatment resistant or if men are more reluctant to seek treatment for ICDs," the investigators wrote.
The multivariate analysis also found significantly higher odds for the presence of ICDs at follow-up among patients who had a higher dopamine agonist levodopa-equivalent daily dose at baseline (OR 2.25 for every 100-mg increase). However, the effect of dopamine agonist dose did not differ between genders.
"Although the baseline dopamine agonist dose was associated with ICD outcome, we did not observe significant effect of the change in the agonist dose," Dr. Joutsa and his associates wrote.
The investigators found that the optimal levodopa equivalent daily dose cut off at baseline for predicting a poor ICD outcome was 161 mg or greater, resulting in a 46% sensitivity, 90% specificity, 93% positive predictive value, and 37% negative predictive value. That level of levodopa equivalent daily dose, which corresponds to 1.6 mg pramipexole and 8 mg ropinirole, "is quite low compared to the maximal doses of the drugs, and to the doses needed for efficacious motor symptom treatment. The relatively low cut-off values suggest that ICDs are associated with dopamine agonists even with low daily doses, when the first motor benefit is reached," they wrote.
A rise in score on the Beck Depression Inventory (BDI) proved to be the only significant predictor of ICDs at follow-up among patients without ICDs at baseline, increasing the odds of developing an ICD by nearly 10% for each 1-point increase on the scale. Those who developed new-onset ICDs at follow-up had a mean BDI score of 8.4 at baseline and 12 at follow-up. Overall, patients with ICDs at either time point had a higher mean BDI score than did those without ICDs, with baseline scores of 14.2 vs. 8.9, respectively, and follow-up scores of 12.9 vs. 9.8.
However, patients who had resolved their ICDs at follow-up did not improve their depressive symptoms to the level seen in patients who never had ICDs, showing that "depressive symptoms at baseline did not predict the ICD outcome at follow-up," Dr. Joutsa and his colleagues wrote. "Therefore, these results could be interpreted to favor ICDs as a causal factor for depression rather than vice versa, although the design of the study does not allow definitive conclusions about the causality."
The research was funded by the Finnish Alcohol Research Foundation, the Finnish Medical Foundation, the Turku University Hospital, the Turku University Foundation, the Paulo Foundation, and the Finnish Parkinson Foundation. All but one of the authors declared having financial relationships with Boehringer-Ingelheim, GlaxoSmithKline, UCB Pharma, and Orion Pharma, Abbott, and Lundbeck.
The development and resolution of impulse control disorders in patients with Parkinson’s disease appears to be largely reliant on gender, daily dose of dopamine agonists, and the presence of depressive symptoms, according to a prospective study.
Persistence of impulse control disorders (ICDs) among Parkinson’s disease patients from baseline to a mean follow-up of about 15 months were significantly associated with male gender and a higher daily dose of dopamine agonists. However, worsening depressive symptoms was the only factor associated with the development of an ICD during follow-up, reported Dr. Juho Joutsa of the University of Turku (Finland) and his colleagues (Parkinsonism Relat. Disord. 2012 [doi:10.1016/j.parkreldis.2012.06.005]). Their study is the first large-scale, prospective study to assess risk factors for the development and resolution of ICDs at two time points.
The investigators used self-reports from 290 survey participants who had had Parkinson’s disease for a median of 6 years and were a median age of 64 years at baseline. Among 270 patients who answered questions about ICDs on the Questionnaire for Impulsive-Compulsive Behaviors in Parkinson's Disease at both baseline and follow-up, 22 had a new-onset ICD at follow-up, 31 had their ICDs resolve at follow-up, 135 had no ICD at both baseline and follow-up, and 82 reported stable ICDs at both time points.
In the patients with ICDs at baseline, multivariate binary logistic regression analyses showed significantly higher odds for the presence of ICDs at follow-up if the patient was a man (odds ratio, 6.10).
"The present results demonstrate that ICDs are not only more common in men but also approximately six times more unlikely to be resolved, compared to women during a 15-month follow-up. Thus, the results also suggest that the difference in prevalence rates between the sexes found in cross-sectional studies should not be interpreted only as higher incidence of ICDs in men. However, future studies are required to clarify whether ICDs in male patients are more treatment resistant or if men are more reluctant to seek treatment for ICDs," the investigators wrote.
The multivariate analysis also found significantly higher odds for the presence of ICDs at follow-up among patients who had a higher dopamine agonist levodopa-equivalent daily dose at baseline (OR 2.25 for every 100-mg increase). However, the effect of dopamine agonist dose did not differ between genders.
"Although the baseline dopamine agonist dose was associated with ICD outcome, we did not observe significant effect of the change in the agonist dose," Dr. Joutsa and his associates wrote.
The investigators found that the optimal levodopa equivalent daily dose cut off at baseline for predicting a poor ICD outcome was 161 mg or greater, resulting in a 46% sensitivity, 90% specificity, 93% positive predictive value, and 37% negative predictive value. That level of levodopa equivalent daily dose, which corresponds to 1.6 mg pramipexole and 8 mg ropinirole, "is quite low compared to the maximal doses of the drugs, and to the doses needed for efficacious motor symptom treatment. The relatively low cut-off values suggest that ICDs are associated with dopamine agonists even with low daily doses, when the first motor benefit is reached," they wrote.
A rise in score on the Beck Depression Inventory (BDI) proved to be the only significant predictor of ICDs at follow-up among patients without ICDs at baseline, increasing the odds of developing an ICD by nearly 10% for each 1-point increase on the scale. Those who developed new-onset ICDs at follow-up had a mean BDI score of 8.4 at baseline and 12 at follow-up. Overall, patients with ICDs at either time point had a higher mean BDI score than did those without ICDs, with baseline scores of 14.2 vs. 8.9, respectively, and follow-up scores of 12.9 vs. 9.8.
However, patients who had resolved their ICDs at follow-up did not improve their depressive symptoms to the level seen in patients who never had ICDs, showing that "depressive symptoms at baseline did not predict the ICD outcome at follow-up," Dr. Joutsa and his colleagues wrote. "Therefore, these results could be interpreted to favor ICDs as a causal factor for depression rather than vice versa, although the design of the study does not allow definitive conclusions about the causality."
The research was funded by the Finnish Alcohol Research Foundation, the Finnish Medical Foundation, the Turku University Hospital, the Turku University Foundation, the Paulo Foundation, and the Finnish Parkinson Foundation. All but one of the authors declared having financial relationships with Boehringer-Ingelheim, GlaxoSmithKline, UCB Pharma, and Orion Pharma, Abbott, and Lundbeck.
FROM PARKINSONISM AND RELATED DISORDERS
Major Finding: The optimal levodopa equivalent daily dose cut off at baseline for predicting a poor impulse control disorder outcome was 161 mg or greater, resulting in a 46% sensitivity, 90% specificity, 93% positive predictive value, and 37% negative predictive value.
Data Source: This was a prospective, cross-sectional, cohort study of 290 patients with Parkinson’s disease, including 119 with impulse control disorders.
Disclosures: The research was funded by the Finnish Alcohol Research Foundation, the Finnish Medical Foundation, the Turku University Hospital, the Turku University Foundation, the Paulo Foundation, and the Finnish Parkinson Foundation. All but one of the authors declared having financial relationships with Boehringer-Ingelheim, GlaxoSmithKline, UCB Pharma, and Orion Pharma, Abbott, and Lundbeck.
Closed-Loop Device Controlled Seizures Transcranially
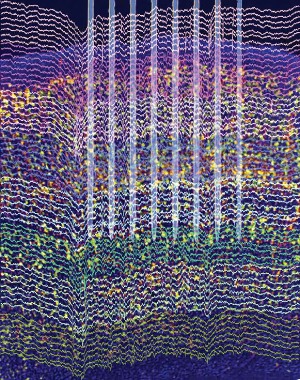
A closed-loop feedback transcranial electrical stimulation system has proved its ability to detect and suppress spike-and-wave episodes from a rat model of generalized petit mal epilepsy, providing evidence that it may be possible to avoid the adverse effects of invasive deep brain stimulation while also reducing seizures.
It is the first study to report controlling seizures through the use of transcranial electrical stimulation (TES), a development made possible by reducing TES-induced artifacts via a closed-loop feedback system with recording of spike-and-wave discharges from a chronically implanted tripolar electrode. Spike-and-wave patterns are the hallmark of generalized petit mal epilepsy.
The importance of the findings is that the researchers were "able to both record and stimulate simultaneously" and use TES as the form of electrical stimulation, Dr. Paul R. Carney said in an interview. Dr. Carney is chief of the division of pediatric neurology and director of the pediatric comprehensive epilepsy program at the University of Florida, Gainesville. He was not involved with the study.
In the study, led by György Buzsáki, Ph.D., of New York University, brain-activity timed feedback of TES at 1 Hz significantly shortened the spike-and-wave discharges of nine rats. In seven of the rats, the percentage of time spent having spike-and-wave episodes in a session also declined significantly. The device led to a greater than 60% decline in the duration and the fraction of session time spent in spike-and-wave episodes (Science 2012;337:735-7).
An investigational device currently in clinical trials, the NeuroPace Responsive Neurostimulation System, uses the same concept of closed-loop feedback stimulation but involves the placement of deep brain electrodes at the focal point of a patient’s complex partial seizures. When the NeuroPace device detects the onset of a seizure, it delivers a current through the same electrode that detected the onset, irrespective of the dynamics of the seizure.
The difference with the TES device is that it detects and responds to every single spike-and-wave episode, dampening the episode with every current discharge, not just a train of stimulation events, Dr. Buzsáki said in an interview. The high noise-to-signal ratio with devices that measure and deliver current through the same electrode, such as the NeuroPace Responsive Neurostimulation System, makes it difficult to detect ongoing seizure activity, he said.
To apply the study results of closed-loop TES successfully in a clinical situation without the use of a chronically implanted deep brain electrode, the investigators noted that they first would need to identify and record from the causal pathophysiological brain network activity. This could be accomplished in generalized spike-and-wave episodes and focal cortical epilepsies through the use of subdural or epidural electrodes, or electrodes inserted into the skull. However, for complex partial seizures, which make up the majority of drug-resistant epilepsies, the investigators wrote that "deep-electrode recordings are required for accurate detection of abnormal patterns."
Another requirement for clinical application will be the development of intra–skull plate electrodes, possibly in multiple locations, to provide closed-loop feedback TES of the target brain circuits. These implants would be much lighter and more cosmetically acceptable than what could be produced using the large and heavy coils needed for transcranial magnetic stimulation, according to the researchers.
The next step for the research group is to study patients who already have subdurally implanted titanium plates used to repair a skull defect following surgery for epilepsy. With a little modification of these plates, Dr. Buzsáki and his colleagues plan to stimulate the brain and see if they can get an adequate signal-to-noise ratio for detecting seizure activity.
The research was supported by grants from the National Institutes of Health, the Human Frontier Science Program, and the J.D. McDonnell Foundation. Neither the researchers nor Dr. Carney had relevant disclosures.
A closed-loop feedback transcranial electrical stimulation system has proved its ability to detect and suppress spike-and-wave episodes from a rat model of generalized petit mal epilepsy, providing evidence that it may be possible to avoid the adverse effects of invasive deep brain stimulation while also reducing seizures.
It is the first study to report controlling seizures through the use of transcranial electrical stimulation (TES), a development made possible by reducing TES-induced artifacts via a closed-loop feedback system with recording of spike-and-wave discharges from a chronically implanted tripolar electrode. Spike-and-wave patterns are the hallmark of generalized petit mal epilepsy.
The importance of the findings is that the researchers were "able to both record and stimulate simultaneously" and use TES as the form of electrical stimulation, Dr. Paul R. Carney said in an interview. Dr. Carney is chief of the division of pediatric neurology and director of the pediatric comprehensive epilepsy program at the University of Florida, Gainesville. He was not involved with the study.
In the study, led by György Buzsáki, Ph.D., of New York University, brain-activity timed feedback of TES at 1 Hz significantly shortened the spike-and-wave discharges of nine rats. In seven of the rats, the percentage of time spent having spike-and-wave episodes in a session also declined significantly. The device led to a greater than 60% decline in the duration and the fraction of session time spent in spike-and-wave episodes (Science 2012;337:735-7).
An investigational device currently in clinical trials, the NeuroPace Responsive Neurostimulation System, uses the same concept of closed-loop feedback stimulation but involves the placement of deep brain electrodes at the focal point of a patient’s complex partial seizures. When the NeuroPace device detects the onset of a seizure, it delivers a current through the same electrode that detected the onset, irrespective of the dynamics of the seizure.
The difference with the TES device is that it detects and responds to every single spike-and-wave episode, dampening the episode with every current discharge, not just a train of stimulation events, Dr. Buzsáki said in an interview. The high noise-to-signal ratio with devices that measure and deliver current through the same electrode, such as the NeuroPace Responsive Neurostimulation System, makes it difficult to detect ongoing seizure activity, he said.
To apply the study results of closed-loop TES successfully in a clinical situation without the use of a chronically implanted deep brain electrode, the investigators noted that they first would need to identify and record from the causal pathophysiological brain network activity. This could be accomplished in generalized spike-and-wave episodes and focal cortical epilepsies through the use of subdural or epidural electrodes, or electrodes inserted into the skull. However, for complex partial seizures, which make up the majority of drug-resistant epilepsies, the investigators wrote that "deep-electrode recordings are required for accurate detection of abnormal patterns."
Another requirement for clinical application will be the development of intra–skull plate electrodes, possibly in multiple locations, to provide closed-loop feedback TES of the target brain circuits. These implants would be much lighter and more cosmetically acceptable than what could be produced using the large and heavy coils needed for transcranial magnetic stimulation, according to the researchers.
The next step for the research group is to study patients who already have subdurally implanted titanium plates used to repair a skull defect following surgery for epilepsy. With a little modification of these plates, Dr. Buzsáki and his colleagues plan to stimulate the brain and see if they can get an adequate signal-to-noise ratio for detecting seizure activity.
The research was supported by grants from the National Institutes of Health, the Human Frontier Science Program, and the J.D. McDonnell Foundation. Neither the researchers nor Dr. Carney had relevant disclosures.
A closed-loop feedback transcranial electrical stimulation system has proved its ability to detect and suppress spike-and-wave episodes from a rat model of generalized petit mal epilepsy, providing evidence that it may be possible to avoid the adverse effects of invasive deep brain stimulation while also reducing seizures.
It is the first study to report controlling seizures through the use of transcranial electrical stimulation (TES), a development made possible by reducing TES-induced artifacts via a closed-loop feedback system with recording of spike-and-wave discharges from a chronically implanted tripolar electrode. Spike-and-wave patterns are the hallmark of generalized petit mal epilepsy.
The importance of the findings is that the researchers were "able to both record and stimulate simultaneously" and use TES as the form of electrical stimulation, Dr. Paul R. Carney said in an interview. Dr. Carney is chief of the division of pediatric neurology and director of the pediatric comprehensive epilepsy program at the University of Florida, Gainesville. He was not involved with the study.
In the study, led by György Buzsáki, Ph.D., of New York University, brain-activity timed feedback of TES at 1 Hz significantly shortened the spike-and-wave discharges of nine rats. In seven of the rats, the percentage of time spent having spike-and-wave episodes in a session also declined significantly. The device led to a greater than 60% decline in the duration and the fraction of session time spent in spike-and-wave episodes (Science 2012;337:735-7).
An investigational device currently in clinical trials, the NeuroPace Responsive Neurostimulation System, uses the same concept of closed-loop feedback stimulation but involves the placement of deep brain electrodes at the focal point of a patient’s complex partial seizures. When the NeuroPace device detects the onset of a seizure, it delivers a current through the same electrode that detected the onset, irrespective of the dynamics of the seizure.
The difference with the TES device is that it detects and responds to every single spike-and-wave episode, dampening the episode with every current discharge, not just a train of stimulation events, Dr. Buzsáki said in an interview. The high noise-to-signal ratio with devices that measure and deliver current through the same electrode, such as the NeuroPace Responsive Neurostimulation System, makes it difficult to detect ongoing seizure activity, he said.
To apply the study results of closed-loop TES successfully in a clinical situation without the use of a chronically implanted deep brain electrode, the investigators noted that they first would need to identify and record from the causal pathophysiological brain network activity. This could be accomplished in generalized spike-and-wave episodes and focal cortical epilepsies through the use of subdural or epidural electrodes, or electrodes inserted into the skull. However, for complex partial seizures, which make up the majority of drug-resistant epilepsies, the investigators wrote that "deep-electrode recordings are required for accurate detection of abnormal patterns."
Another requirement for clinical application will be the development of intra–skull plate electrodes, possibly in multiple locations, to provide closed-loop feedback TES of the target brain circuits. These implants would be much lighter and more cosmetically acceptable than what could be produced using the large and heavy coils needed for transcranial magnetic stimulation, according to the researchers.
The next step for the research group is to study patients who already have subdurally implanted titanium plates used to repair a skull defect following surgery for epilepsy. With a little modification of these plates, Dr. Buzsáki and his colleagues plan to stimulate the brain and see if they can get an adequate signal-to-noise ratio for detecting seizure activity.
The research was supported by grants from the National Institutes of Health, the Human Frontier Science Program, and the J.D. McDonnell Foundation. Neither the researchers nor Dr. Carney had relevant disclosures.
FROM SCIENCE
Studies of Neurological Disease Via Stem Cells Gather Steam
Induced pluripotent stem cell lines that are created from the somatic cells of people with neurological diseases are beginning to pave a pathway for researchers to understand pathophysiology and screen for new drug candidates.
Recent work has characterized disease phenotypes and, in some cases, tested therapies in neurons that were differentiated from induced pluripotent stem cells (iPSCs) derived from patients with sporadic and familial Alzheimer’s disease, Huntington’s disease, and familial Parkinson’s disease.
These are heady times for this field in general and for neurological disease in particular. "It is really exciting when you start to be able to see relevant pathophysiologies in the dish," said Matthew Huentelman, Ph.D., head of the neurobehavioral research unit in the neurogenomics division at the Translational Genomics Research Institute, Phoenix.
Other investigators are trying to avoid one of the problems that has been plaguing this line of research: cell teratoma formation in vivo iPSC and embryonic stem cells. Their current approach is to reprogram fibroblasts into multipotent induced neural stem cells (iNSCs) that can then be differentiated into whatever neural progenitor cells the investigators wish to culture (neurons, oligodendroglia, and astroglia). This method also may lead to more efficient differentiation into the desired cell type, according to Dr. Yadong Huang, an associate investigator at the Gladstone Institute of Neurological Disease at the University of California, San Francisco.
Dr. Huang was the senior author on a study that used a single factor, rather than three or more, to directly reprogram human fibroblasts into multipotent iNSCs that can self-renew for 40-50 generations. This self-renewal ability, which is not present in the direct reprogramming of fibroblasts into neurons, makes them better to use for pathophysiology and drug testing studies (Cell Stem Cell 2012;11:100-9).
Only in the past year or so have people begun working in this middle ground between iPSCs and directly induced neuronal cells, so "it is important for studies in all three techniques to go forward now in parallel because each has advantages and disadvantages, at least at this point," Dr. Huang said in an interview.
"These initial studies ... are really encouraging because [they set us up] to dream about how we might leverage these cellular technologies to perform high-throughput drug screening," noted Dr. Huentelman.
In one study of Alzheimer’s disease, investigators developed iPSC cell lines from one patient with sporadic disease and two patients with familial disease caused by a mutation in the gene for amyloid precursor protein (APP). In each case, the investigators observed three major biochemical markers of Alzheimer’s (Nature 2012 Jan. 25 [doi:10.1038/nature10821]).
A separate study of familial Alzheimer’s disease with patients with presenilin-1 mutations, which was recently presented at the Alzheimer’s Association International Conference 2012, found substantial phenotypic differences between control neurons derived from iPSCs from unaffected family members, such as an increased ratio of amyloid-beta-42 to amyloid-beta-40 and an enhanced cell death response to apoptotic stimuli.
Even greater success has been reported with human Huntington’s disease neurons derived from iPSCs. In one study, researchers correlated the severity of phenotype in neurons with the number of CAG expansions observed in the huntingtin gene, as is observed in humans. However, some of the phenotypes were observed only in cell lines with the longest CAG expansion (Cell Stem Cell 2012 June 28 [doi:10.1016/j.stem.2012.04.027]). A separate group of researchers reported success in replacing the CAG expansion with a normal repeat length, which corrected the Huntington’s disease phenotype of mitochondrial deficits, lower levels of brain-derived neurotrophic factor, and altered cell signaling proteins (Cell Stem Cell 2012 June 28 [doi:10.1016/j.stem.2012.04.026]).
Another group of investigators reported the pharmacologic rescue of mitochondrial deficits in iPSC-derived neural cells from patients with familial Parkinson’s disease caused by mutations in either PINK1 or LRRK2. Neural cells with PINK1 mutations that received low doses of mitochondrial stressors were rescued by both the antioxidant coenzyme Q10 and an LRRK2 inhibitor, whereas those with an LRRK2 mutation were partially rescued by rapamycin or an LRRK2 inhibitor. The researchers said that they hope to apply their observations on cellular responses to stress in mutation-carrying, iPSC-derived neural cells to sporadic forms of Parkinson’s disease (Sci. Transl. Med. 2012 July 4 [doi:10.1126/scitranslmed.3003985]).
"I think where the real excitement might come is how relevant these models systems are in helping us dissect the more sporadic forms of these diseases, because, frankly, that’s where we need some additional ability to control our investigations," Dr. Huentelman said.
However, he noted that iPSC-derived cell lines take a lot of time and money to produce. In addition, all of the studies reported so far suffer from a small sample size – mostly one or two patients – as well as the potential for diagnostic inaccuracy. "Accuracy is good for these disorders, but it is not 100%," he said.
"Most researchers are taking biopsies from living patients with a diagnosis. That’s fine, maybe, with familial cases of [Parkinson’s disease, Alzheimer’s disease], and Huntington’s because you see the family structure and you can follow the genetics," but it becomes more difficult with sporadic cases, he added.
Researchers at Dr. Huentelman’s institution have partnered with the Banner Sun Health Brain and Tissue Bank to make iPSCs from whole-body donors to that program, so that their cell studies are backed with a neuropathological diagnosis.
Neither Dr. Huang nor Dr. Huentelman had relevant disclosures.
Induced pluripotent stem cell lines that are created from the somatic cells of people with neurological diseases are beginning to pave a pathway for researchers to understand pathophysiology and screen for new drug candidates.
Recent work has characterized disease phenotypes and, in some cases, tested therapies in neurons that were differentiated from induced pluripotent stem cells (iPSCs) derived from patients with sporadic and familial Alzheimer’s disease, Huntington’s disease, and familial Parkinson’s disease.
These are heady times for this field in general and for neurological disease in particular. "It is really exciting when you start to be able to see relevant pathophysiologies in the dish," said Matthew Huentelman, Ph.D., head of the neurobehavioral research unit in the neurogenomics division at the Translational Genomics Research Institute, Phoenix.
Other investigators are trying to avoid one of the problems that has been plaguing this line of research: cell teratoma formation in vivo iPSC and embryonic stem cells. Their current approach is to reprogram fibroblasts into multipotent induced neural stem cells (iNSCs) that can then be differentiated into whatever neural progenitor cells the investigators wish to culture (neurons, oligodendroglia, and astroglia). This method also may lead to more efficient differentiation into the desired cell type, according to Dr. Yadong Huang, an associate investigator at the Gladstone Institute of Neurological Disease at the University of California, San Francisco.
Dr. Huang was the senior author on a study that used a single factor, rather than three or more, to directly reprogram human fibroblasts into multipotent iNSCs that can self-renew for 40-50 generations. This self-renewal ability, which is not present in the direct reprogramming of fibroblasts into neurons, makes them better to use for pathophysiology and drug testing studies (Cell Stem Cell 2012;11:100-9).
Only in the past year or so have people begun working in this middle ground between iPSCs and directly induced neuronal cells, so "it is important for studies in all three techniques to go forward now in parallel because each has advantages and disadvantages, at least at this point," Dr. Huang said in an interview.
"These initial studies ... are really encouraging because [they set us up] to dream about how we might leverage these cellular technologies to perform high-throughput drug screening," noted Dr. Huentelman.
In one study of Alzheimer’s disease, investigators developed iPSC cell lines from one patient with sporadic disease and two patients with familial disease caused by a mutation in the gene for amyloid precursor protein (APP). In each case, the investigators observed three major biochemical markers of Alzheimer’s (Nature 2012 Jan. 25 [doi:10.1038/nature10821]).
A separate study of familial Alzheimer’s disease with patients with presenilin-1 mutations, which was recently presented at the Alzheimer’s Association International Conference 2012, found substantial phenotypic differences between control neurons derived from iPSCs from unaffected family members, such as an increased ratio of amyloid-beta-42 to amyloid-beta-40 and an enhanced cell death response to apoptotic stimuli.
Even greater success has been reported with human Huntington’s disease neurons derived from iPSCs. In one study, researchers correlated the severity of phenotype in neurons with the number of CAG expansions observed in the huntingtin gene, as is observed in humans. However, some of the phenotypes were observed only in cell lines with the longest CAG expansion (Cell Stem Cell 2012 June 28 [doi:10.1016/j.stem.2012.04.027]). A separate group of researchers reported success in replacing the CAG expansion with a normal repeat length, which corrected the Huntington’s disease phenotype of mitochondrial deficits, lower levels of brain-derived neurotrophic factor, and altered cell signaling proteins (Cell Stem Cell 2012 June 28 [doi:10.1016/j.stem.2012.04.026]).
Another group of investigators reported the pharmacologic rescue of mitochondrial deficits in iPSC-derived neural cells from patients with familial Parkinson’s disease caused by mutations in either PINK1 or LRRK2. Neural cells with PINK1 mutations that received low doses of mitochondrial stressors were rescued by both the antioxidant coenzyme Q10 and an LRRK2 inhibitor, whereas those with an LRRK2 mutation were partially rescued by rapamycin or an LRRK2 inhibitor. The researchers said that they hope to apply their observations on cellular responses to stress in mutation-carrying, iPSC-derived neural cells to sporadic forms of Parkinson’s disease (Sci. Transl. Med. 2012 July 4 [doi:10.1126/scitranslmed.3003985]).
"I think where the real excitement might come is how relevant these models systems are in helping us dissect the more sporadic forms of these diseases, because, frankly, that’s where we need some additional ability to control our investigations," Dr. Huentelman said.
However, he noted that iPSC-derived cell lines take a lot of time and money to produce. In addition, all of the studies reported so far suffer from a small sample size – mostly one or two patients – as well as the potential for diagnostic inaccuracy. "Accuracy is good for these disorders, but it is not 100%," he said.
"Most researchers are taking biopsies from living patients with a diagnosis. That’s fine, maybe, with familial cases of [Parkinson’s disease, Alzheimer’s disease], and Huntington’s because you see the family structure and you can follow the genetics," but it becomes more difficult with sporadic cases, he added.
Researchers at Dr. Huentelman’s institution have partnered with the Banner Sun Health Brain and Tissue Bank to make iPSCs from whole-body donors to that program, so that their cell studies are backed with a neuropathological diagnosis.
Neither Dr. Huang nor Dr. Huentelman had relevant disclosures.
Induced pluripotent stem cell lines that are created from the somatic cells of people with neurological diseases are beginning to pave a pathway for researchers to understand pathophysiology and screen for new drug candidates.
Recent work has characterized disease phenotypes and, in some cases, tested therapies in neurons that were differentiated from induced pluripotent stem cells (iPSCs) derived from patients with sporadic and familial Alzheimer’s disease, Huntington’s disease, and familial Parkinson’s disease.
These are heady times for this field in general and for neurological disease in particular. "It is really exciting when you start to be able to see relevant pathophysiologies in the dish," said Matthew Huentelman, Ph.D., head of the neurobehavioral research unit in the neurogenomics division at the Translational Genomics Research Institute, Phoenix.
Other investigators are trying to avoid one of the problems that has been plaguing this line of research: cell teratoma formation in vivo iPSC and embryonic stem cells. Their current approach is to reprogram fibroblasts into multipotent induced neural stem cells (iNSCs) that can then be differentiated into whatever neural progenitor cells the investigators wish to culture (neurons, oligodendroglia, and astroglia). This method also may lead to more efficient differentiation into the desired cell type, according to Dr. Yadong Huang, an associate investigator at the Gladstone Institute of Neurological Disease at the University of California, San Francisco.
Dr. Huang was the senior author on a study that used a single factor, rather than three or more, to directly reprogram human fibroblasts into multipotent iNSCs that can self-renew for 40-50 generations. This self-renewal ability, which is not present in the direct reprogramming of fibroblasts into neurons, makes them better to use for pathophysiology and drug testing studies (Cell Stem Cell 2012;11:100-9).
Only in the past year or so have people begun working in this middle ground between iPSCs and directly induced neuronal cells, so "it is important for studies in all three techniques to go forward now in parallel because each has advantages and disadvantages, at least at this point," Dr. Huang said in an interview.
"These initial studies ... are really encouraging because [they set us up] to dream about how we might leverage these cellular technologies to perform high-throughput drug screening," noted Dr. Huentelman.
In one study of Alzheimer’s disease, investigators developed iPSC cell lines from one patient with sporadic disease and two patients with familial disease caused by a mutation in the gene for amyloid precursor protein (APP). In each case, the investigators observed three major biochemical markers of Alzheimer’s (Nature 2012 Jan. 25 [doi:10.1038/nature10821]).
A separate study of familial Alzheimer’s disease with patients with presenilin-1 mutations, which was recently presented at the Alzheimer’s Association International Conference 2012, found substantial phenotypic differences between control neurons derived from iPSCs from unaffected family members, such as an increased ratio of amyloid-beta-42 to amyloid-beta-40 and an enhanced cell death response to apoptotic stimuli.
Even greater success has been reported with human Huntington’s disease neurons derived from iPSCs. In one study, researchers correlated the severity of phenotype in neurons with the number of CAG expansions observed in the huntingtin gene, as is observed in humans. However, some of the phenotypes were observed only in cell lines with the longest CAG expansion (Cell Stem Cell 2012 June 28 [doi:10.1016/j.stem.2012.04.027]). A separate group of researchers reported success in replacing the CAG expansion with a normal repeat length, which corrected the Huntington’s disease phenotype of mitochondrial deficits, lower levels of brain-derived neurotrophic factor, and altered cell signaling proteins (Cell Stem Cell 2012 June 28 [doi:10.1016/j.stem.2012.04.026]).
Another group of investigators reported the pharmacologic rescue of mitochondrial deficits in iPSC-derived neural cells from patients with familial Parkinson’s disease caused by mutations in either PINK1 or LRRK2. Neural cells with PINK1 mutations that received low doses of mitochondrial stressors were rescued by both the antioxidant coenzyme Q10 and an LRRK2 inhibitor, whereas those with an LRRK2 mutation were partially rescued by rapamycin or an LRRK2 inhibitor. The researchers said that they hope to apply their observations on cellular responses to stress in mutation-carrying, iPSC-derived neural cells to sporadic forms of Parkinson’s disease (Sci. Transl. Med. 2012 July 4 [doi:10.1126/scitranslmed.3003985]).
"I think where the real excitement might come is how relevant these models systems are in helping us dissect the more sporadic forms of these diseases, because, frankly, that’s where we need some additional ability to control our investigations," Dr. Huentelman said.
However, he noted that iPSC-derived cell lines take a lot of time and money to produce. In addition, all of the studies reported so far suffer from a small sample size – mostly one or two patients – as well as the potential for diagnostic inaccuracy. "Accuracy is good for these disorders, but it is not 100%," he said.
"Most researchers are taking biopsies from living patients with a diagnosis. That’s fine, maybe, with familial cases of [Parkinson’s disease, Alzheimer’s disease], and Huntington’s because you see the family structure and you can follow the genetics," but it becomes more difficult with sporadic cases, he added.
Researchers at Dr. Huentelman’s institution have partnered with the Banner Sun Health Brain and Tissue Bank to make iPSCs from whole-body donors to that program, so that their cell studies are backed with a neuropathological diagnosis.
Neither Dr. Huang nor Dr. Huentelman had relevant disclosures.
PET Radiotracer Identifies Glioma Treatment Response
A PET imaging protocol using an amino acid analog radiotracer in patients with recurrent high-grade gliomas identified responses to treatment with bevacizumab as early as 2 weeks after starting therapy in a prospective study.
In the 28-patient pilot study, the metabolic tumor volume measured in follow-up PET scans with 6-18F-fluoro-L-DOPA (18F-FDOPA) at 2 and 6 weeks after the baseline scan proved to be the most significant predictor of survival with the method.
There is currently no reliable way to predict treatment response noninvasively in patients with malignant glioma, which has only 6% overall survival at 5 years. Chemotherapeutics have toxic side effects and are expensive, "so from the patient’s point of view, if a treatment doesn’t work, it’s important to get that information as early as possible," said the senior investigator of the study, Dr. Wei Chen of the division of molecular and medical pharmacology at the University of California, Los Angeles.
The ability to detect treatment response only 2 weeks after the start of treatment is the shortest interval yet reported, Dr. Chen said. In a study published last year, she and her colleagues reported that another PET radiotracer, 3´-deoxy-3´-[18F]-fluorothymidine (18F-FLT), could be used to monitor the response of recurrent high-grade gliomas to treatment (J. Nucl. Med. 2012;53:29-36). However, change in response to treatment with bevacizumab (Avastin) could not be detected with 18F-FLT until 6 weeks after starting therapy in that study, compared with 2 weeks for 18F-FDOPA in the current study.
The 18F-FDOPA technique of assessing metabolic tumor volume as early as 2 weeks after starting bevacizumab proved to be a significant predictor of overall and progression-free survival. The 17 metabolic responders survived a median of 12.1 months, compared with 3.5 months for 11 nonresponders. In comparison, when MRI was used to determine response, the survival difference shrank (12.9 months vs. 9.0 months). All patients in the study eventually died.
The investigators chose to use bevacizumab because "it is the most effective treatment," with a significant treatment response of 50% instead of 5%-10% with other drugs, Dr. Chen said in an interview. "But in terms of monitoring, it doesn’t matter which agent is used for treatment."
18F-FDOPA is normally used to assess the striatal dopaminergic system in patients with movement disorders. But it works in assessing tumor treatment response because the higher metabolic rate of cancer cells causes greater uptake of the tracer through a phenylalanine and tyrosine transporter. Conventional MRI assessments for tumor recurrence cannot distinguish tumor from scar tissue left by surgery or radiation, and cannot determine the amount of change until 1.5 to 3 months, according to Dr. Chen.
In eight of nine discrepant cases between PET and MRI, 18F-FDOPA PET demonstrated treatment response earlier than MRI.
The study was supported by grants from the National Cancer Institute and the Department of Energy. Dr. Chen had no relevant disclosures.
A PET imaging protocol using an amino acid analog radiotracer in patients with recurrent high-grade gliomas identified responses to treatment with bevacizumab as early as 2 weeks after starting therapy in a prospective study.
In the 28-patient pilot study, the metabolic tumor volume measured in follow-up PET scans with 6-18F-fluoro-L-DOPA (18F-FDOPA) at 2 and 6 weeks after the baseline scan proved to be the most significant predictor of survival with the method.
There is currently no reliable way to predict treatment response noninvasively in patients with malignant glioma, which has only 6% overall survival at 5 years. Chemotherapeutics have toxic side effects and are expensive, "so from the patient’s point of view, if a treatment doesn’t work, it’s important to get that information as early as possible," said the senior investigator of the study, Dr. Wei Chen of the division of molecular and medical pharmacology at the University of California, Los Angeles.
The ability to detect treatment response only 2 weeks after the start of treatment is the shortest interval yet reported, Dr. Chen said. In a study published last year, she and her colleagues reported that another PET radiotracer, 3´-deoxy-3´-[18F]-fluorothymidine (18F-FLT), could be used to monitor the response of recurrent high-grade gliomas to treatment (J. Nucl. Med. 2012;53:29-36). However, change in response to treatment with bevacizumab (Avastin) could not be detected with 18F-FLT until 6 weeks after starting therapy in that study, compared with 2 weeks for 18F-FDOPA in the current study.
The 18F-FDOPA technique of assessing metabolic tumor volume as early as 2 weeks after starting bevacizumab proved to be a significant predictor of overall and progression-free survival. The 17 metabolic responders survived a median of 12.1 months, compared with 3.5 months for 11 nonresponders. In comparison, when MRI was used to determine response, the survival difference shrank (12.9 months vs. 9.0 months). All patients in the study eventually died.
The investigators chose to use bevacizumab because "it is the most effective treatment," with a significant treatment response of 50% instead of 5%-10% with other drugs, Dr. Chen said in an interview. "But in terms of monitoring, it doesn’t matter which agent is used for treatment."
18F-FDOPA is normally used to assess the striatal dopaminergic system in patients with movement disorders. But it works in assessing tumor treatment response because the higher metabolic rate of cancer cells causes greater uptake of the tracer through a phenylalanine and tyrosine transporter. Conventional MRI assessments for tumor recurrence cannot distinguish tumor from scar tissue left by surgery or radiation, and cannot determine the amount of change until 1.5 to 3 months, according to Dr. Chen.
In eight of nine discrepant cases between PET and MRI, 18F-FDOPA PET demonstrated treatment response earlier than MRI.
The study was supported by grants from the National Cancer Institute and the Department of Energy. Dr. Chen had no relevant disclosures.
A PET imaging protocol using an amino acid analog radiotracer in patients with recurrent high-grade gliomas identified responses to treatment with bevacizumab as early as 2 weeks after starting therapy in a prospective study.
In the 28-patient pilot study, the metabolic tumor volume measured in follow-up PET scans with 6-18F-fluoro-L-DOPA (18F-FDOPA) at 2 and 6 weeks after the baseline scan proved to be the most significant predictor of survival with the method.
There is currently no reliable way to predict treatment response noninvasively in patients with malignant glioma, which has only 6% overall survival at 5 years. Chemotherapeutics have toxic side effects and are expensive, "so from the patient’s point of view, if a treatment doesn’t work, it’s important to get that information as early as possible," said the senior investigator of the study, Dr. Wei Chen of the division of molecular and medical pharmacology at the University of California, Los Angeles.
The ability to detect treatment response only 2 weeks after the start of treatment is the shortest interval yet reported, Dr. Chen said. In a study published last year, she and her colleagues reported that another PET radiotracer, 3´-deoxy-3´-[18F]-fluorothymidine (18F-FLT), could be used to monitor the response of recurrent high-grade gliomas to treatment (J. Nucl. Med. 2012;53:29-36). However, change in response to treatment with bevacizumab (Avastin) could not be detected with 18F-FLT until 6 weeks after starting therapy in that study, compared with 2 weeks for 18F-FDOPA in the current study.
The 18F-FDOPA technique of assessing metabolic tumor volume as early as 2 weeks after starting bevacizumab proved to be a significant predictor of overall and progression-free survival. The 17 metabolic responders survived a median of 12.1 months, compared with 3.5 months for 11 nonresponders. In comparison, when MRI was used to determine response, the survival difference shrank (12.9 months vs. 9.0 months). All patients in the study eventually died.
The investigators chose to use bevacizumab because "it is the most effective treatment," with a significant treatment response of 50% instead of 5%-10% with other drugs, Dr. Chen said in an interview. "But in terms of monitoring, it doesn’t matter which agent is used for treatment."
18F-FDOPA is normally used to assess the striatal dopaminergic system in patients with movement disorders. But it works in assessing tumor treatment response because the higher metabolic rate of cancer cells causes greater uptake of the tracer through a phenylalanine and tyrosine transporter. Conventional MRI assessments for tumor recurrence cannot distinguish tumor from scar tissue left by surgery or radiation, and cannot determine the amount of change until 1.5 to 3 months, according to Dr. Chen.
In eight of nine discrepant cases between PET and MRI, 18F-FDOPA PET demonstrated treatment response earlier than MRI.
The study was supported by grants from the National Cancer Institute and the Department of Energy. Dr. Chen had no relevant disclosures.
AT THE ANNUAL MEETING OF THE SOCIETY OF NUCLEAR MEDICINE AND MOLECULAR IMAGING
Major Finding: The 17 patients identified as responders to bevacizumab by 18F-FDOPA PET after 2 weeks of treatment survived a median of 12.1 months, compared with 3.5 months for 11 nonresponders.
Data Source: This pilot study involved 28 patients with recurrent high-grade gliomas who underwent MRI and 18F-FDOPA PET at baseline and after 2 and 6 weeks.
Disclosures: The study was supported by grants from the National Cancer Institute and the Department of Energy. Dr. Chen had no relevant disclosures.
Planning for Alzheimer's: A Special Podcast
The overarching goal of the National Plan to Address Alzheimer's Disease is to prevent and effectively treat Alzheimer's disease by 2025. As a part of the plan, the federal government is investing money for research and support for caregivers and families and has launched a website, www.alzheimers.gov, which contains information and support services for patients and their families.
One of the biggest challenges facing the government and insurance companies is figuring out which services to provide and how to pay for them. Mayo Clinic neurologist and Alzheimer’s disease specialist Dr. Richard Caselli argues that some of the available methods for helping to diagnose the disease, such as PET amyloid imaging, are most applicable to young dementia patients.
Check out this special Alzheimer’s edition of the Policy & Practice Podcast to learn more.
The overarching goal of the National Plan to Address Alzheimer's Disease is to prevent and effectively treat Alzheimer's disease by 2025. As a part of the plan, the federal government is investing money for research and support for caregivers and families and has launched a website, www.alzheimers.gov, which contains information and support services for patients and their families.
One of the biggest challenges facing the government and insurance companies is figuring out which services to provide and how to pay for them. Mayo Clinic neurologist and Alzheimer’s disease specialist Dr. Richard Caselli argues that some of the available methods for helping to diagnose the disease, such as PET amyloid imaging, are most applicable to young dementia patients.
Check out this special Alzheimer’s edition of the Policy & Practice Podcast to learn more.
The overarching goal of the National Plan to Address Alzheimer's Disease is to prevent and effectively treat Alzheimer's disease by 2025. As a part of the plan, the federal government is investing money for research and support for caregivers and families and has launched a website, www.alzheimers.gov, which contains information and support services for patients and their families.
One of the biggest challenges facing the government and insurance companies is figuring out which services to provide and how to pay for them. Mayo Clinic neurologist and Alzheimer’s disease specialist Dr. Richard Caselli argues that some of the available methods for helping to diagnose the disease, such as PET amyloid imaging, are most applicable to young dementia patients.
Check out this special Alzheimer’s edition of the Policy & Practice Podcast to learn more.
Amyloid Imaging Studies Track Dementia Development
Longitudinal tracking of the deposition of beta-amyloid over a period of 2-3 years with the use of PET imaging radiotracers in patients with mild cognitive impairment can help predict progression to Alzheimer’s disease or reliably rule it out as a diagnosis.
Those results, reported in two studies of the investigational agents 11C-Pittsburgh compound B (PiB) and 18F-florbetaben at the annual meeting of the Society of Nuclear Medicine and Molecular Imaging in Miami, showed that the tracers could be used to predict progression to Alzheimer’s in 66%-75% of those with elevated binding of the agents to beta-amyloid plaques in the brains of individuals with mild cognitive impairment (MCI). In cases where an individual tested negative for elevated beta-amyloid binding, fewer than 20% progressed to another type of dementia.
Results such as these show that detecting beta-amyloid burden in the brain "can help lead to diagnosis of Alzheimer’s disease when a patient has mild symptoms rather than wait until they have established dementia as is the current clinical practice. This may have important benefits for the patient, for their family, and for society," said Dr. Christopher Rowe, the lead investigator on the PiB study and senior investigator on the florbetaben study.
The PiB study, called the Australian Imaging, Biomarkers, and Lifestyle Flagship Study of Ageing, included 92 patients with MCI. At baseline, Dr. Rowe and his colleagues detected high PiB binding in 65% of MCI patients. After 3 years, 66% of MCI patients who had a positive scan for high beta-amyloid burden at baseline had been diagnosed with Alzheimer’s, compared with only 7% of MCI patients with a negative scan.
Other studies of PiB out to 6 years of follow-up have shown that patients accrue beta-amyloid at "an incredibly slow" rate of about 2% per year. "If you have a negative scan, you can be reassured that you’re not going to have Alzheimer’s disease for at least the next 10 years," Dr. Rowe said at a press conference at the meeting.
Because the half-life of PiB is only 20 minutes, it is not suitable for clinical use. The 2-hour half-life of florbetaben and other 18F amyloid radiotracers make them much more cost effective for clinical use, noted Dr. Rowe, director of the department of nuclear medicine and the center for PET and a consultant neurologist to the memory disorders clinic at the Austin Hospital in Melbourne, Australia.
The florbetaben study involved 45 patients with MCI who received PET imaging with the compound. At baseline, 53% had a high level of neocortical binding, and binding increased 3% after 2 years in those with already high levels. Overall, 75% of patients with elevated florbetaben binding progressed to Alzheimer’s disease, whereas 19% of those with a low level of binding progressed to other kinds of dementias.
MR imaging in the same individuals indicated that 53% of patients with hippocampal atrophy at baseline had progressed to Alzheimer’s. When the combination of high florbetaben binding and hippocampal atrophy was present, 80% had progressed to Alzheimer’s after 2 years.
"We don’t say that everybody who’s got Alzheimer’s disease should have these scans because I don’t think that would be cost effective. But in selected scenarios, when they’ve seen a memory specialist, I think these can be very useful clinically," Dr. Rowe said.
He said amyloid imaging agents such as florbetaben have two potential uses:
• In patients with established dementia when there is uncertainty about whether the patient has Alzheimer’s disease or frontotemporal dementia. He noted that this has therapeutic implications because some of the medications used to treat Alzheimer’s, such as cholinesterase inhibitors, can make behavioral symptoms worse in frontotemporal dementia.
• In patients with MCI who have seen a specialist who is not sure what the cause of the symptoms might be and wants to see if it might be Alzheimer’s instead of waiting years for dementia to develop. Because about 40% with MCI do not go on to develop dementia, amyloid imaging studies would be reassuring to those patients, Dr. Rowe said.
Guidelines from the Alzheimer’s Association and the Society of Nuclear Medicine and Molecular Imaging (which recently changed its name from the Society of Nuclear Medicine) will soon be available on the appropriate use of the imaging agents, Dr. Rowe said in an interview.
He disclosed that he has served as an investigator on studies for many of the companies developing amyloid imaging products, including Avid Radiopharmaceuticals, Bayer Schering Pharma, GE Healthcare, and AstraZeneca. He has also received payments for consulting for Bayer and GE.
Longitudinal tracking of the deposition of beta-amyloid over a period of 2-3 years with the use of PET imaging radiotracers in patients with mild cognitive impairment can help predict progression to Alzheimer’s disease or reliably rule it out as a diagnosis.
Those results, reported in two studies of the investigational agents 11C-Pittsburgh compound B (PiB) and 18F-florbetaben at the annual meeting of the Society of Nuclear Medicine and Molecular Imaging in Miami, showed that the tracers could be used to predict progression to Alzheimer’s in 66%-75% of those with elevated binding of the agents to beta-amyloid plaques in the brains of individuals with mild cognitive impairment (MCI). In cases where an individual tested negative for elevated beta-amyloid binding, fewer than 20% progressed to another type of dementia.
Results such as these show that detecting beta-amyloid burden in the brain "can help lead to diagnosis of Alzheimer’s disease when a patient has mild symptoms rather than wait until they have established dementia as is the current clinical practice. This may have important benefits for the patient, for their family, and for society," said Dr. Christopher Rowe, the lead investigator on the PiB study and senior investigator on the florbetaben study.
The PiB study, called the Australian Imaging, Biomarkers, and Lifestyle Flagship Study of Ageing, included 92 patients with MCI. At baseline, Dr. Rowe and his colleagues detected high PiB binding in 65% of MCI patients. After 3 years, 66% of MCI patients who had a positive scan for high beta-amyloid burden at baseline had been diagnosed with Alzheimer’s, compared with only 7% of MCI patients with a negative scan.
Other studies of PiB out to 6 years of follow-up have shown that patients accrue beta-amyloid at "an incredibly slow" rate of about 2% per year. "If you have a negative scan, you can be reassured that you’re not going to have Alzheimer’s disease for at least the next 10 years," Dr. Rowe said at a press conference at the meeting.
Because the half-life of PiB is only 20 minutes, it is not suitable for clinical use. The 2-hour half-life of florbetaben and other 18F amyloid radiotracers make them much more cost effective for clinical use, noted Dr. Rowe, director of the department of nuclear medicine and the center for PET and a consultant neurologist to the memory disorders clinic at the Austin Hospital in Melbourne, Australia.
The florbetaben study involved 45 patients with MCI who received PET imaging with the compound. At baseline, 53% had a high level of neocortical binding, and binding increased 3% after 2 years in those with already high levels. Overall, 75% of patients with elevated florbetaben binding progressed to Alzheimer’s disease, whereas 19% of those with a low level of binding progressed to other kinds of dementias.
MR imaging in the same individuals indicated that 53% of patients with hippocampal atrophy at baseline had progressed to Alzheimer’s. When the combination of high florbetaben binding and hippocampal atrophy was present, 80% had progressed to Alzheimer’s after 2 years.
"We don’t say that everybody who’s got Alzheimer’s disease should have these scans because I don’t think that would be cost effective. But in selected scenarios, when they’ve seen a memory specialist, I think these can be very useful clinically," Dr. Rowe said.
He said amyloid imaging agents such as florbetaben have two potential uses:
• In patients with established dementia when there is uncertainty about whether the patient has Alzheimer’s disease or frontotemporal dementia. He noted that this has therapeutic implications because some of the medications used to treat Alzheimer’s, such as cholinesterase inhibitors, can make behavioral symptoms worse in frontotemporal dementia.
• In patients with MCI who have seen a specialist who is not sure what the cause of the symptoms might be and wants to see if it might be Alzheimer’s instead of waiting years for dementia to develop. Because about 40% with MCI do not go on to develop dementia, amyloid imaging studies would be reassuring to those patients, Dr. Rowe said.
Guidelines from the Alzheimer’s Association and the Society of Nuclear Medicine and Molecular Imaging (which recently changed its name from the Society of Nuclear Medicine) will soon be available on the appropriate use of the imaging agents, Dr. Rowe said in an interview.
He disclosed that he has served as an investigator on studies for many of the companies developing amyloid imaging products, including Avid Radiopharmaceuticals, Bayer Schering Pharma, GE Healthcare, and AstraZeneca. He has also received payments for consulting for Bayer and GE.
Longitudinal tracking of the deposition of beta-amyloid over a period of 2-3 years with the use of PET imaging radiotracers in patients with mild cognitive impairment can help predict progression to Alzheimer’s disease or reliably rule it out as a diagnosis.
Those results, reported in two studies of the investigational agents 11C-Pittsburgh compound B (PiB) and 18F-florbetaben at the annual meeting of the Society of Nuclear Medicine and Molecular Imaging in Miami, showed that the tracers could be used to predict progression to Alzheimer’s in 66%-75% of those with elevated binding of the agents to beta-amyloid plaques in the brains of individuals with mild cognitive impairment (MCI). In cases where an individual tested negative for elevated beta-amyloid binding, fewer than 20% progressed to another type of dementia.
Results such as these show that detecting beta-amyloid burden in the brain "can help lead to diagnosis of Alzheimer’s disease when a patient has mild symptoms rather than wait until they have established dementia as is the current clinical practice. This may have important benefits for the patient, for their family, and for society," said Dr. Christopher Rowe, the lead investigator on the PiB study and senior investigator on the florbetaben study.
The PiB study, called the Australian Imaging, Biomarkers, and Lifestyle Flagship Study of Ageing, included 92 patients with MCI. At baseline, Dr. Rowe and his colleagues detected high PiB binding in 65% of MCI patients. After 3 years, 66% of MCI patients who had a positive scan for high beta-amyloid burden at baseline had been diagnosed with Alzheimer’s, compared with only 7% of MCI patients with a negative scan.
Other studies of PiB out to 6 years of follow-up have shown that patients accrue beta-amyloid at "an incredibly slow" rate of about 2% per year. "If you have a negative scan, you can be reassured that you’re not going to have Alzheimer’s disease for at least the next 10 years," Dr. Rowe said at a press conference at the meeting.
Because the half-life of PiB is only 20 minutes, it is not suitable for clinical use. The 2-hour half-life of florbetaben and other 18F amyloid radiotracers make them much more cost effective for clinical use, noted Dr. Rowe, director of the department of nuclear medicine and the center for PET and a consultant neurologist to the memory disorders clinic at the Austin Hospital in Melbourne, Australia.
The florbetaben study involved 45 patients with MCI who received PET imaging with the compound. At baseline, 53% had a high level of neocortical binding, and binding increased 3% after 2 years in those with already high levels. Overall, 75% of patients with elevated florbetaben binding progressed to Alzheimer’s disease, whereas 19% of those with a low level of binding progressed to other kinds of dementias.
MR imaging in the same individuals indicated that 53% of patients with hippocampal atrophy at baseline had progressed to Alzheimer’s. When the combination of high florbetaben binding and hippocampal atrophy was present, 80% had progressed to Alzheimer’s after 2 years.
"We don’t say that everybody who’s got Alzheimer’s disease should have these scans because I don’t think that would be cost effective. But in selected scenarios, when they’ve seen a memory specialist, I think these can be very useful clinically," Dr. Rowe said.
He said amyloid imaging agents such as florbetaben have two potential uses:
• In patients with established dementia when there is uncertainty about whether the patient has Alzheimer’s disease or frontotemporal dementia. He noted that this has therapeutic implications because some of the medications used to treat Alzheimer’s, such as cholinesterase inhibitors, can make behavioral symptoms worse in frontotemporal dementia.
• In patients with MCI who have seen a specialist who is not sure what the cause of the symptoms might be and wants to see if it might be Alzheimer’s instead of waiting years for dementia to develop. Because about 40% with MCI do not go on to develop dementia, amyloid imaging studies would be reassuring to those patients, Dr. Rowe said.
Guidelines from the Alzheimer’s Association and the Society of Nuclear Medicine and Molecular Imaging (which recently changed its name from the Society of Nuclear Medicine) will soon be available on the appropriate use of the imaging agents, Dr. Rowe said in an interview.
He disclosed that he has served as an investigator on studies for many of the companies developing amyloid imaging products, including Avid Radiopharmaceuticals, Bayer Schering Pharma, GE Healthcare, and AstraZeneca. He has also received payments for consulting for Bayer and GE.
FROM THE ANNUAL MEETING OF THE SOCIETY OF NUCLEAR MEDICINE AND MOLECULAR IMAGING
Major Finding: Longitudinal tracking of the deposition of beta-amyloid over a period of 2-3 years with the use of PET imaging radiotracers in patients with mild cognitive impairment can help predict progression to Alzheimer’s disease or reliably rule it out as a diagnosis.
Data Source: Data were taken from two studies of the investigational agents 11C-Pittsburgh compound B (PiB) and 18F-florbetaben.
Disclosures: Dr. Rowe disclosed that he has served as an investigator on studies for many of the companies developing amyloid imaging products, including Avid Radiopharmaceuticals, Bayer Schering Pharma, GE Healthcare, and AstraZeneca. He has also received payments for consulting for Bayer and GE.