User login
Do prophylactic antipyretics reduce vaccination-associated symptoms in children?
EVIDENCE SUMMARY
A systematic review of 13 RCTs (5077 patients) compared the effects of a prophylactic antipyretic (acetaminophen or ibuprofen, doses and schedules not described) with placebo in healthy children 6 years or younger undergoing routine childhood immunizations.1 Trials examined various schedules and combinations of vaccines. Researchers defined febrile reactions as a temperature of 38°C or higher and categorized pain as: none, mild (reaction to touch over vaccine site), moderate (protesting to limb movement), or severe (resisting limb movement).
Acetaminophen works better than ibuprofen for both fever and pain
Acetaminophen prophylaxis resulted in fewer febrile reactions in the first 24 to 48 hours after vaccine administration than placebo following both primary (odds ratio [OR] = 0.35; 95% confidence interval [CI], 0.26-0.48) and booster vaccinations (OR = 0.60; 95% CI, 0.39-0.93). Acetaminophen also reduced pain of all grades (primary vaccination: OR = 0.57; 95% CI, 0.47-0.7; booster vaccination: OR = 0.64; 95% CI, 0.48-0.84).
In contrast, ibuprofen prophylaxis had no effect on early febrile reactions for either primary or booster vaccinations. It reduced pain of all grades after primary vaccination (OR = 0.66; 95% CI, 0.49-0.88) but not after boosters (OR = 1.03; 95% CI, 0.59-1.81).
Reduced antibody response doesn’t affect seroprotective levels
Acetaminophen also generally reduced the antibody response compared with placebo (assessed using the geometric mean concentration [GMC], a statistical technique for comparing values that change logarithmically).1 GMC results are difficult to interpret clinically, however, and they differed by vaccine, antigen, and primary or booster vaccination status.
Nevertheless, patients mounted seroprotective antibody levels with or without acetaminophen prophylaxis, and the nasopharyngeal carriage rates of Streptococcus pneumoniae and Haemophilus influenzae didn’t change. Researchers didn’t publish the antibody responses to ibuprofen, nor did they track actual infection rates.
How do antipyretics work with newer combination vaccines?
A subsequent trial evaluated the immune response in 908 children receiving newer combination vaccines (DTaP/HBV/IPV/Hib and PCV13) who were randomized to 5 groups: acetaminophen 15 mg/kg at vaccination and 6 to 8 hours later; acetaminophen 15 mg/kg starting 6 to 8 hours after vaccination with a second dose 6 to 8 hours later; ibuprofen 10 mg/kg/dose at vaccination with a second dose 6 to 8 hours later; ibuprofen 10 mg/kg starting 6 to 8 hours after vaccination with a second dose 6 to 8 hours later; and placebo.2
Patients received age-appropriate vaccination and their assigned antipyretic (or placebo) at 2, 3, 4 and 12 months of age. Researchers measured the immune response at 5 and 13 months of age.
Continue to: Overall, 5% to 10% of the prophylaxis group...
Overall, 5% to 10% of the prophylaxis group had fever on Day 1 or 2 after vaccination, compared with 10% to 20% of the placebo group (no P value given). Antipyretic use produced lower antibody GMC responses for antipertussis and antitetanus vaccines at 5 months but not at 13 months. Patients achieved the prespecified effective antibody levels at both 5 and 13 months, regardless of intervention.
Antipyretics don’t affect immune response with inactivated flu vaccine
A 2017 RCT investigated the effect of either prophylactic acetaminophen (15 mg/kg every 4 to 6 hours for 24 hours) or ibuprofen (10 mg/kg every 4 to 6 hours for 24 hours) on immune response in children receiving inactivated influenza vaccine.3 Researchers randomized 142 children into 3 treatment groups (acetaminophen, 59 children; ibuprofen, 24 children; placebo, 59 children). They defined seroconversion as a hemagglutinin inhibition assay titer of 1:40 postvaccination (if baseline titer was less than 1:10) or a 4-fold rise (if the baseline titer was ≥ 1:10).
All interventions resulted in similar seroconversion rates for all A or B influenza strains investigated. Vaccine protection-level responses ranged from 9% for B/Phuket to 100% for A/Switzerland. The trial didn’t report febrile reactions or infection rates.
RECOMMENDATIONS
In 2017, the Advisory Committee on Immunization Practices (ACIP) issued guidelines generally discouraging the use of antipyretics at the time of vaccination, but allowing their use later for local discomfort or fever that might arise after vaccination. The guidelines also noted that antipyretics at the time of vaccination didn’t reduce the risk of febrile seizures.4
Editor’s takeaway
Although ACIP doesn’t encourage giving antipyretics with vaccines, moderate-quality evidence suggests that prophylactic acetaminophen reduces fever and pain after immunizations by a reasonable amount without an apparent clinical downside.
1. Das RR, Panigrahi I, Naik SS. The effect of prophylactic antipyretic administration on post-vaccination adverse reactions and antibody response in children: a systematic review. PLoS One. 2014;9:e106629.
2. Wysocki J, Center, KJ, Brzostek J, et al. A randomized study of fever prophylaxis and the immunogenicity of routine pediatric vaccinations. Vaccine. 2017;35:1926-1935.
3. Walter EB, Hornok CP, Grohskopf L, et al. The effect of antipyretics on immune response and fever following receipt of inactivated influenza vaccine in young children. Vaccine. 2017;35:6664–6671.
4. Kroger AT, Duchin J, Vázquez M. General Best Practice Guidelines for Immunization. Best Practices Guidance of the Advisory Committee on Immunization Practices (ACIP). Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2017.
EVIDENCE SUMMARY
A systematic review of 13 RCTs (5077 patients) compared the effects of a prophylactic antipyretic (acetaminophen or ibuprofen, doses and schedules not described) with placebo in healthy children 6 years or younger undergoing routine childhood immunizations.1 Trials examined various schedules and combinations of vaccines. Researchers defined febrile reactions as a temperature of 38°C or higher and categorized pain as: none, mild (reaction to touch over vaccine site), moderate (protesting to limb movement), or severe (resisting limb movement).
Acetaminophen works better than ibuprofen for both fever and pain
Acetaminophen prophylaxis resulted in fewer febrile reactions in the first 24 to 48 hours after vaccine administration than placebo following both primary (odds ratio [OR] = 0.35; 95% confidence interval [CI], 0.26-0.48) and booster vaccinations (OR = 0.60; 95% CI, 0.39-0.93). Acetaminophen also reduced pain of all grades (primary vaccination: OR = 0.57; 95% CI, 0.47-0.7; booster vaccination: OR = 0.64; 95% CI, 0.48-0.84).
In contrast, ibuprofen prophylaxis had no effect on early febrile reactions for either primary or booster vaccinations. It reduced pain of all grades after primary vaccination (OR = 0.66; 95% CI, 0.49-0.88) but not after boosters (OR = 1.03; 95% CI, 0.59-1.81).
Reduced antibody response doesn’t affect seroprotective levels
Acetaminophen also generally reduced the antibody response compared with placebo (assessed using the geometric mean concentration [GMC], a statistical technique for comparing values that change logarithmically).1 GMC results are difficult to interpret clinically, however, and they differed by vaccine, antigen, and primary or booster vaccination status.
Nevertheless, patients mounted seroprotective antibody levels with or without acetaminophen prophylaxis, and the nasopharyngeal carriage rates of Streptococcus pneumoniae and Haemophilus influenzae didn’t change. Researchers didn’t publish the antibody responses to ibuprofen, nor did they track actual infection rates.
How do antipyretics work with newer combination vaccines?
A subsequent trial evaluated the immune response in 908 children receiving newer combination vaccines (DTaP/HBV/IPV/Hib and PCV13) who were randomized to 5 groups: acetaminophen 15 mg/kg at vaccination and 6 to 8 hours later; acetaminophen 15 mg/kg starting 6 to 8 hours after vaccination with a second dose 6 to 8 hours later; ibuprofen 10 mg/kg/dose at vaccination with a second dose 6 to 8 hours later; ibuprofen 10 mg/kg starting 6 to 8 hours after vaccination with a second dose 6 to 8 hours later; and placebo.2
Patients received age-appropriate vaccination and their assigned antipyretic (or placebo) at 2, 3, 4 and 12 months of age. Researchers measured the immune response at 5 and 13 months of age.
Continue to: Overall, 5% to 10% of the prophylaxis group...
Overall, 5% to 10% of the prophylaxis group had fever on Day 1 or 2 after vaccination, compared with 10% to 20% of the placebo group (no P value given). Antipyretic use produced lower antibody GMC responses for antipertussis and antitetanus vaccines at 5 months but not at 13 months. Patients achieved the prespecified effective antibody levels at both 5 and 13 months, regardless of intervention.
Antipyretics don’t affect immune response with inactivated flu vaccine
A 2017 RCT investigated the effect of either prophylactic acetaminophen (15 mg/kg every 4 to 6 hours for 24 hours) or ibuprofen (10 mg/kg every 4 to 6 hours for 24 hours) on immune response in children receiving inactivated influenza vaccine.3 Researchers randomized 142 children into 3 treatment groups (acetaminophen, 59 children; ibuprofen, 24 children; placebo, 59 children). They defined seroconversion as a hemagglutinin inhibition assay titer of 1:40 postvaccination (if baseline titer was less than 1:10) or a 4-fold rise (if the baseline titer was ≥ 1:10).
All interventions resulted in similar seroconversion rates for all A or B influenza strains investigated. Vaccine protection-level responses ranged from 9% for B/Phuket to 100% for A/Switzerland. The trial didn’t report febrile reactions or infection rates.
RECOMMENDATIONS
In 2017, the Advisory Committee on Immunization Practices (ACIP) issued guidelines generally discouraging the use of antipyretics at the time of vaccination, but allowing their use later for local discomfort or fever that might arise after vaccination. The guidelines also noted that antipyretics at the time of vaccination didn’t reduce the risk of febrile seizures.4
Editor’s takeaway
Although ACIP doesn’t encourage giving antipyretics with vaccines, moderate-quality evidence suggests that prophylactic acetaminophen reduces fever and pain after immunizations by a reasonable amount without an apparent clinical downside.
EVIDENCE SUMMARY
A systematic review of 13 RCTs (5077 patients) compared the effects of a prophylactic antipyretic (acetaminophen or ibuprofen, doses and schedules not described) with placebo in healthy children 6 years or younger undergoing routine childhood immunizations.1 Trials examined various schedules and combinations of vaccines. Researchers defined febrile reactions as a temperature of 38°C or higher and categorized pain as: none, mild (reaction to touch over vaccine site), moderate (protesting to limb movement), or severe (resisting limb movement).
Acetaminophen works better than ibuprofen for both fever and pain
Acetaminophen prophylaxis resulted in fewer febrile reactions in the first 24 to 48 hours after vaccine administration than placebo following both primary (odds ratio [OR] = 0.35; 95% confidence interval [CI], 0.26-0.48) and booster vaccinations (OR = 0.60; 95% CI, 0.39-0.93). Acetaminophen also reduced pain of all grades (primary vaccination: OR = 0.57; 95% CI, 0.47-0.7; booster vaccination: OR = 0.64; 95% CI, 0.48-0.84).
In contrast, ibuprofen prophylaxis had no effect on early febrile reactions for either primary or booster vaccinations. It reduced pain of all grades after primary vaccination (OR = 0.66; 95% CI, 0.49-0.88) but not after boosters (OR = 1.03; 95% CI, 0.59-1.81).
Reduced antibody response doesn’t affect seroprotective levels
Acetaminophen also generally reduced the antibody response compared with placebo (assessed using the geometric mean concentration [GMC], a statistical technique for comparing values that change logarithmically).1 GMC results are difficult to interpret clinically, however, and they differed by vaccine, antigen, and primary or booster vaccination status.
Nevertheless, patients mounted seroprotective antibody levels with or without acetaminophen prophylaxis, and the nasopharyngeal carriage rates of Streptococcus pneumoniae and Haemophilus influenzae didn’t change. Researchers didn’t publish the antibody responses to ibuprofen, nor did they track actual infection rates.
How do antipyretics work with newer combination vaccines?
A subsequent trial evaluated the immune response in 908 children receiving newer combination vaccines (DTaP/HBV/IPV/Hib and PCV13) who were randomized to 5 groups: acetaminophen 15 mg/kg at vaccination and 6 to 8 hours later; acetaminophen 15 mg/kg starting 6 to 8 hours after vaccination with a second dose 6 to 8 hours later; ibuprofen 10 mg/kg/dose at vaccination with a second dose 6 to 8 hours later; ibuprofen 10 mg/kg starting 6 to 8 hours after vaccination with a second dose 6 to 8 hours later; and placebo.2
Patients received age-appropriate vaccination and their assigned antipyretic (or placebo) at 2, 3, 4 and 12 months of age. Researchers measured the immune response at 5 and 13 months of age.
Continue to: Overall, 5% to 10% of the prophylaxis group...
Overall, 5% to 10% of the prophylaxis group had fever on Day 1 or 2 after vaccination, compared with 10% to 20% of the placebo group (no P value given). Antipyretic use produced lower antibody GMC responses for antipertussis and antitetanus vaccines at 5 months but not at 13 months. Patients achieved the prespecified effective antibody levels at both 5 and 13 months, regardless of intervention.
Antipyretics don’t affect immune response with inactivated flu vaccine
A 2017 RCT investigated the effect of either prophylactic acetaminophen (15 mg/kg every 4 to 6 hours for 24 hours) or ibuprofen (10 mg/kg every 4 to 6 hours for 24 hours) on immune response in children receiving inactivated influenza vaccine.3 Researchers randomized 142 children into 3 treatment groups (acetaminophen, 59 children; ibuprofen, 24 children; placebo, 59 children). They defined seroconversion as a hemagglutinin inhibition assay titer of 1:40 postvaccination (if baseline titer was less than 1:10) or a 4-fold rise (if the baseline titer was ≥ 1:10).
All interventions resulted in similar seroconversion rates for all A or B influenza strains investigated. Vaccine protection-level responses ranged from 9% for B/Phuket to 100% for A/Switzerland. The trial didn’t report febrile reactions or infection rates.
RECOMMENDATIONS
In 2017, the Advisory Committee on Immunization Practices (ACIP) issued guidelines generally discouraging the use of antipyretics at the time of vaccination, but allowing their use later for local discomfort or fever that might arise after vaccination. The guidelines also noted that antipyretics at the time of vaccination didn’t reduce the risk of febrile seizures.4
Editor’s takeaway
Although ACIP doesn’t encourage giving antipyretics with vaccines, moderate-quality evidence suggests that prophylactic acetaminophen reduces fever and pain after immunizations by a reasonable amount without an apparent clinical downside.
1. Das RR, Panigrahi I, Naik SS. The effect of prophylactic antipyretic administration on post-vaccination adverse reactions and antibody response in children: a systematic review. PLoS One. 2014;9:e106629.
2. Wysocki J, Center, KJ, Brzostek J, et al. A randomized study of fever prophylaxis and the immunogenicity of routine pediatric vaccinations. Vaccine. 2017;35:1926-1935.
3. Walter EB, Hornok CP, Grohskopf L, et al. The effect of antipyretics on immune response and fever following receipt of inactivated influenza vaccine in young children. Vaccine. 2017;35:6664–6671.
4. Kroger AT, Duchin J, Vázquez M. General Best Practice Guidelines for Immunization. Best Practices Guidance of the Advisory Committee on Immunization Practices (ACIP). Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2017.
1. Das RR, Panigrahi I, Naik SS. The effect of prophylactic antipyretic administration on post-vaccination adverse reactions and antibody response in children: a systematic review. PLoS One. 2014;9:e106629.
2. Wysocki J, Center, KJ, Brzostek J, et al. A randomized study of fever prophylaxis and the immunogenicity of routine pediatric vaccinations. Vaccine. 2017;35:1926-1935.
3. Walter EB, Hornok CP, Grohskopf L, et al. The effect of antipyretics on immune response and fever following receipt of inactivated influenza vaccine in young children. Vaccine. 2017;35:6664–6671.
4. Kroger AT, Duchin J, Vázquez M. General Best Practice Guidelines for Immunization. Best Practices Guidance of the Advisory Committee on Immunization Practices (ACIP). Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2017.
EVIDENCE-BASED ANSWER:
Yes for acetaminophen, not so much for ibuprofen. Prophylactic acetaminophen reduces the odds of febrile reactions in the first 48 hours after vaccination by 40% to 65% and pain of all grades by 36% to 43%. In contrast, prophylactic ibuprofen reduces pain of all grades by 34% only after primary vaccination and doesn’t alter pain after boosters. Nor does it alter early febrile reactions (strength of recommendation [SOR]: B, meta-analysis of randomized clinical trials [RCTs] with moderate-to-high risk of bias).
Prophylactic administration of acetaminophen or ibuprofen is associated with a reduction in antibody response to the primary vaccine series and to influenza vaccine, but antibody responses still achieve seroprotective levels (SOR: C, bench research).
How effective and safe is fecal microbial transplant in preventing C difficile recurrence?
EVIDENCE SUMMARY
An open-label RCT enrolled 41 immunocompetent older adults who had relapsed CDI after at least one course of antibiotic therapy.1 Investigators randomized patients to 3 treatment groups:
- vancomycin therapy, bowel lavage (with 4 L nasogastric polyethylene glycol solution), and nasogastric-infused fresh donor feces;
- vancomycin with nasogastric bowel lavage without donor feces; or
- vancomycin alone.
Researchers defined cure as the absence of diarrhea or 3 negative stool samples (if patients continued to have persistent diarrhea) at 10 weeks without relapse.
Thirteen of 16 patients (81%) in the donor feces infusion group were cured with the first infusion. Two of the 3 remaining patients were cured after a second donor transplant. FMT produced higher total cure rates than those of vancomycin (94% vs 27%; P<.001; number needed to treat [NNT]=2). Bowel lavage had no effect on outcome.
FMT cures more patients than vancomycin alone
An open-label RCT of 39 patients compared healthy-donor, fresh FMT given via colonoscopy with vancomycin alone for recurrent CDIs.2 Researchers recruited immunocompetent adults who had recurrent CDIs after at least one course of vancomycin or metronidazole.
Patients in the treatment group received a short course of vancomycin, followed by bowel cleansing and fecal transplant via colonoscopy. Clinicians repeated the fecal transplant every 3 days until resolution for patients with pseudomembranous colitis. Patients in the control group were treated with vancomycin for at least 3 weeks. Researchers defined cure as the absence of diarrhea or 2 negative stool samples (if patients continued to have diarrhea) at 10 weeks without relapse.
Thirteen of 20 patients in the FMT group (65%) achieved cure after the first fecal infusion. The 7 remaining patients received multiple infusions; 5 were cured. Overall, FMT cured more patients than vancomycin alone (90% vs 26%; odds ratio=25.2; 99.9% confidence interval [CI], 1.26-502; NNT=2).
Continue to: Fresh and frozen stool are equally effective
Fresh and frozen stool are equally effective
A randomized, double-blind noninferiority trial compared the effectiveness of frozen and thawed FMT with that of fresh FMT in 219 patients ≥18 years of age with recurrent or refractory CDIs.3 Researchers prescribed suppressive antibiotics, which were discontinued within 24 to 48 hours of FMT, and then administered 50 mL of either fresh or frozen stool by enema. They repeated the FMT with the same donor stool if symptoms didn’t improve within 4 days. Any patient still unresponsive was offered repeat FMT or antibiotic therapy.
Researchers defined a 15% difference in outcome as a clinically important effect. Intention-to-treat analysis yielded no significant difference in clinical resolution between groups (75% frozen vs 70.3% fresh; P=.01 for noninferiority).
Nasogastric delivery works as well as colonoscopy
An open-label RCT (not included in the reviews described previously) evaluated the effectiveness of colonoscopically administered FMT compared with that of nasogastric administration in 20 patients with recurrent or refractory CDIs.4 Patients had experienced either a minimum of 3 episodes of mild-to-moderate CDI with a failed 6- to 8-week taper of vancomycin or 2 episodes of severe CDI resulting in hospitalization. Researchers offered patients from both groups a second FMT if symptoms didn’t improve with the initial administration.
Eight patients in the colonoscopy group and 6 in the nasogastric group were cured (all symptoms resolved) after the first FMT. One patient in the nasogastric group refused subsequent administration. All 5 remaining participants chose to have subsequent nasogastric administration (80% cure rate). Both methods of administering FMT produced comparable cure rates (80% in the initial nasogastric group vs 100% in the initial colonoscopy group; P=.53).
Continue to: A third of patients suffer adverse effects, but serious harms are rare
A third of patients suffer adverse effects, but serious harms are rare
A systematic review analyzed 50 trials (16 case series, 9 case reports, 4 RCTs, 21 unreported type; 1089 FMT-treated patients) for adverse effects of FMT.5 Most patients (831) had CDIs, 235 had inflammatory bowel disease, and 106 had both conditions. Donor screening tests for FMT included viral screenings (hepatitis A, B, and C; Epstein-Barr virus; human immunodeficiency virus; Treponema pallidum; and cytomegalovirus), stool tests for C difficile toxin, and routine bacterial culture for enteric pathogens (Escherichia coli, Salmonella, Shigella, Yersinia, Campylobacter), ova, and parasites.
Overall, 28.5% of patients receiving FMT experienced adverse events. Upper gastrointestinal (GI) administration resulted in more total adverse events than did lower GI delivery (43.6% vs 20.6%; P value not given), mostly abdominal discomfort. However, upper GI delivery was associated with fewer serious adverse events than was lower GI delivery (2% vs 6%; P value not given). FMT possibly or probably produced serious infections in 0.7% of patients, and there was one colonoscopy-associated death caused by aspiration (0.1% mortality).
RECOMMENDATIONS
Guidelines published by the American College of Gastroenterology in 2013 listed FMT as a treatment option for patients who have had 3 episodes of CDI and vancomycin therapy (based on moderate quality evidence).6
1. van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368:407-415.
2. Cammarota G, Masucci L, Ianiro G, et al. Randomised clinical trial: faecal microbiota transplantation by colonoscopy vs. vancomycin for the treatment of recurrent Clostridium difficile infection. Aliment Pharmacol Ther. 2015;41:835-843.
3. Lee CH, Steiner T, Petrof EO, et al. Frozen vs fresh fecal microbiota transplantation and clinical resolution of diarrhea in patients with recurrent Clostridium difficile infection. JAMA. 2016;315:142-149.
4. Youngster I, Sauk J, Pindar C, et al. Fecal microbiota transplant for relapsing Clostridium difficile infection using a frozen inoculum from unrelated donors: a randomized, open-label, controlled pilot study. Clin Infect Dis. 2014;58:1515-1522.
5. Wang S, Xu M, Wang W, et al. Systematic review: adverse events of fecal microbiota transplantation. PLoS One. 2016;11:e0161174.
6. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108:478-498.
EVIDENCE SUMMARY
An open-label RCT enrolled 41 immunocompetent older adults who had relapsed CDI after at least one course of antibiotic therapy.1 Investigators randomized patients to 3 treatment groups:
- vancomycin therapy, bowel lavage (with 4 L nasogastric polyethylene glycol solution), and nasogastric-infused fresh donor feces;
- vancomycin with nasogastric bowel lavage without donor feces; or
- vancomycin alone.
Researchers defined cure as the absence of diarrhea or 3 negative stool samples (if patients continued to have persistent diarrhea) at 10 weeks without relapse.
Thirteen of 16 patients (81%) in the donor feces infusion group were cured with the first infusion. Two of the 3 remaining patients were cured after a second donor transplant. FMT produced higher total cure rates than those of vancomycin (94% vs 27%; P<.001; number needed to treat [NNT]=2). Bowel lavage had no effect on outcome.
FMT cures more patients than vancomycin alone
An open-label RCT of 39 patients compared healthy-donor, fresh FMT given via colonoscopy with vancomycin alone for recurrent CDIs.2 Researchers recruited immunocompetent adults who had recurrent CDIs after at least one course of vancomycin or metronidazole.
Patients in the treatment group received a short course of vancomycin, followed by bowel cleansing and fecal transplant via colonoscopy. Clinicians repeated the fecal transplant every 3 days until resolution for patients with pseudomembranous colitis. Patients in the control group were treated with vancomycin for at least 3 weeks. Researchers defined cure as the absence of diarrhea or 2 negative stool samples (if patients continued to have diarrhea) at 10 weeks without relapse.
Thirteen of 20 patients in the FMT group (65%) achieved cure after the first fecal infusion. The 7 remaining patients received multiple infusions; 5 were cured. Overall, FMT cured more patients than vancomycin alone (90% vs 26%; odds ratio=25.2; 99.9% confidence interval [CI], 1.26-502; NNT=2).
Continue to: Fresh and frozen stool are equally effective
Fresh and frozen stool are equally effective
A randomized, double-blind noninferiority trial compared the effectiveness of frozen and thawed FMT with that of fresh FMT in 219 patients ≥18 years of age with recurrent or refractory CDIs.3 Researchers prescribed suppressive antibiotics, which were discontinued within 24 to 48 hours of FMT, and then administered 50 mL of either fresh or frozen stool by enema. They repeated the FMT with the same donor stool if symptoms didn’t improve within 4 days. Any patient still unresponsive was offered repeat FMT or antibiotic therapy.
Researchers defined a 15% difference in outcome as a clinically important effect. Intention-to-treat analysis yielded no significant difference in clinical resolution between groups (75% frozen vs 70.3% fresh; P=.01 for noninferiority).
Nasogastric delivery works as well as colonoscopy
An open-label RCT (not included in the reviews described previously) evaluated the effectiveness of colonoscopically administered FMT compared with that of nasogastric administration in 20 patients with recurrent or refractory CDIs.4 Patients had experienced either a minimum of 3 episodes of mild-to-moderate CDI with a failed 6- to 8-week taper of vancomycin or 2 episodes of severe CDI resulting in hospitalization. Researchers offered patients from both groups a second FMT if symptoms didn’t improve with the initial administration.
Eight patients in the colonoscopy group and 6 in the nasogastric group were cured (all symptoms resolved) after the first FMT. One patient in the nasogastric group refused subsequent administration. All 5 remaining participants chose to have subsequent nasogastric administration (80% cure rate). Both methods of administering FMT produced comparable cure rates (80% in the initial nasogastric group vs 100% in the initial colonoscopy group; P=.53).
Continue to: A third of patients suffer adverse effects, but serious harms are rare
A third of patients suffer adverse effects, but serious harms are rare
A systematic review analyzed 50 trials (16 case series, 9 case reports, 4 RCTs, 21 unreported type; 1089 FMT-treated patients) for adverse effects of FMT.5 Most patients (831) had CDIs, 235 had inflammatory bowel disease, and 106 had both conditions. Donor screening tests for FMT included viral screenings (hepatitis A, B, and C; Epstein-Barr virus; human immunodeficiency virus; Treponema pallidum; and cytomegalovirus), stool tests for C difficile toxin, and routine bacterial culture for enteric pathogens (Escherichia coli, Salmonella, Shigella, Yersinia, Campylobacter), ova, and parasites.
Overall, 28.5% of patients receiving FMT experienced adverse events. Upper gastrointestinal (GI) administration resulted in more total adverse events than did lower GI delivery (43.6% vs 20.6%; P value not given), mostly abdominal discomfort. However, upper GI delivery was associated with fewer serious adverse events than was lower GI delivery (2% vs 6%; P value not given). FMT possibly or probably produced serious infections in 0.7% of patients, and there was one colonoscopy-associated death caused by aspiration (0.1% mortality).
RECOMMENDATIONS
Guidelines published by the American College of Gastroenterology in 2013 listed FMT as a treatment option for patients who have had 3 episodes of CDI and vancomycin therapy (based on moderate quality evidence).6
EVIDENCE SUMMARY
An open-label RCT enrolled 41 immunocompetent older adults who had relapsed CDI after at least one course of antibiotic therapy.1 Investigators randomized patients to 3 treatment groups:
- vancomycin therapy, bowel lavage (with 4 L nasogastric polyethylene glycol solution), and nasogastric-infused fresh donor feces;
- vancomycin with nasogastric bowel lavage without donor feces; or
- vancomycin alone.
Researchers defined cure as the absence of diarrhea or 3 negative stool samples (if patients continued to have persistent diarrhea) at 10 weeks without relapse.
Thirteen of 16 patients (81%) in the donor feces infusion group were cured with the first infusion. Two of the 3 remaining patients were cured after a second donor transplant. FMT produced higher total cure rates than those of vancomycin (94% vs 27%; P<.001; number needed to treat [NNT]=2). Bowel lavage had no effect on outcome.
FMT cures more patients than vancomycin alone
An open-label RCT of 39 patients compared healthy-donor, fresh FMT given via colonoscopy with vancomycin alone for recurrent CDIs.2 Researchers recruited immunocompetent adults who had recurrent CDIs after at least one course of vancomycin or metronidazole.
Patients in the treatment group received a short course of vancomycin, followed by bowel cleansing and fecal transplant via colonoscopy. Clinicians repeated the fecal transplant every 3 days until resolution for patients with pseudomembranous colitis. Patients in the control group were treated with vancomycin for at least 3 weeks. Researchers defined cure as the absence of diarrhea or 2 negative stool samples (if patients continued to have diarrhea) at 10 weeks without relapse.
Thirteen of 20 patients in the FMT group (65%) achieved cure after the first fecal infusion. The 7 remaining patients received multiple infusions; 5 were cured. Overall, FMT cured more patients than vancomycin alone (90% vs 26%; odds ratio=25.2; 99.9% confidence interval [CI], 1.26-502; NNT=2).
Continue to: Fresh and frozen stool are equally effective
Fresh and frozen stool are equally effective
A randomized, double-blind noninferiority trial compared the effectiveness of frozen and thawed FMT with that of fresh FMT in 219 patients ≥18 years of age with recurrent or refractory CDIs.3 Researchers prescribed suppressive antibiotics, which were discontinued within 24 to 48 hours of FMT, and then administered 50 mL of either fresh or frozen stool by enema. They repeated the FMT with the same donor stool if symptoms didn’t improve within 4 days. Any patient still unresponsive was offered repeat FMT or antibiotic therapy.
Researchers defined a 15% difference in outcome as a clinically important effect. Intention-to-treat analysis yielded no significant difference in clinical resolution between groups (75% frozen vs 70.3% fresh; P=.01 for noninferiority).
Nasogastric delivery works as well as colonoscopy
An open-label RCT (not included in the reviews described previously) evaluated the effectiveness of colonoscopically administered FMT compared with that of nasogastric administration in 20 patients with recurrent or refractory CDIs.4 Patients had experienced either a minimum of 3 episodes of mild-to-moderate CDI with a failed 6- to 8-week taper of vancomycin or 2 episodes of severe CDI resulting in hospitalization. Researchers offered patients from both groups a second FMT if symptoms didn’t improve with the initial administration.
Eight patients in the colonoscopy group and 6 in the nasogastric group were cured (all symptoms resolved) after the first FMT. One patient in the nasogastric group refused subsequent administration. All 5 remaining participants chose to have subsequent nasogastric administration (80% cure rate). Both methods of administering FMT produced comparable cure rates (80% in the initial nasogastric group vs 100% in the initial colonoscopy group; P=.53).
Continue to: A third of patients suffer adverse effects, but serious harms are rare
A third of patients suffer adverse effects, but serious harms are rare
A systematic review analyzed 50 trials (16 case series, 9 case reports, 4 RCTs, 21 unreported type; 1089 FMT-treated patients) for adverse effects of FMT.5 Most patients (831) had CDIs, 235 had inflammatory bowel disease, and 106 had both conditions. Donor screening tests for FMT included viral screenings (hepatitis A, B, and C; Epstein-Barr virus; human immunodeficiency virus; Treponema pallidum; and cytomegalovirus), stool tests for C difficile toxin, and routine bacterial culture for enteric pathogens (Escherichia coli, Salmonella, Shigella, Yersinia, Campylobacter), ova, and parasites.
Overall, 28.5% of patients receiving FMT experienced adverse events. Upper gastrointestinal (GI) administration resulted in more total adverse events than did lower GI delivery (43.6% vs 20.6%; P value not given), mostly abdominal discomfort. However, upper GI delivery was associated with fewer serious adverse events than was lower GI delivery (2% vs 6%; P value not given). FMT possibly or probably produced serious infections in 0.7% of patients, and there was one colonoscopy-associated death caused by aspiration (0.1% mortality).
RECOMMENDATIONS
Guidelines published by the American College of Gastroenterology in 2013 listed FMT as a treatment option for patients who have had 3 episodes of CDI and vancomycin therapy (based on moderate quality evidence).6
1. van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368:407-415.
2. Cammarota G, Masucci L, Ianiro G, et al. Randomised clinical trial: faecal microbiota transplantation by colonoscopy vs. vancomycin for the treatment of recurrent Clostridium difficile infection. Aliment Pharmacol Ther. 2015;41:835-843.
3. Lee CH, Steiner T, Petrof EO, et al. Frozen vs fresh fecal microbiota transplantation and clinical resolution of diarrhea in patients with recurrent Clostridium difficile infection. JAMA. 2016;315:142-149.
4. Youngster I, Sauk J, Pindar C, et al. Fecal microbiota transplant for relapsing Clostridium difficile infection using a frozen inoculum from unrelated donors: a randomized, open-label, controlled pilot study. Clin Infect Dis. 2014;58:1515-1522.
5. Wang S, Xu M, Wang W, et al. Systematic review: adverse events of fecal microbiota transplantation. PLoS One. 2016;11:e0161174.
6. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108:478-498.
1. van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368:407-415.
2. Cammarota G, Masucci L, Ianiro G, et al. Randomised clinical trial: faecal microbiota transplantation by colonoscopy vs. vancomycin for the treatment of recurrent Clostridium difficile infection. Aliment Pharmacol Ther. 2015;41:835-843.
3. Lee CH, Steiner T, Petrof EO, et al. Frozen vs fresh fecal microbiota transplantation and clinical resolution of diarrhea in patients with recurrent Clostridium difficile infection. JAMA. 2016;315:142-149.
4. Youngster I, Sauk J, Pindar C, et al. Fecal microbiota transplant for relapsing Clostridium difficile infection using a frozen inoculum from unrelated donors: a randomized, open-label, controlled pilot study. Clin Infect Dis. 2014;58:1515-1522.
5. Wang S, Xu M, Wang W, et al. Systematic review: adverse events of fecal microbiota transplantation. PLoS One. 2016;11:e0161174.
6. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108:478-498.
EVIDENCE-BASED ANSWER:
Fecal microbial transplant (FMT) is reasonably safe and effective. In patients who have had multiple Clostridium difficile infections (CDIs), FMT results in a 65% to 80% cure rate with one treatment and 90% to 95% cure rate with repeated treatments compared with a 25% to 27% cure rate for antibiotics (strength of recommendation [SOR]: B, small open-label randomized controlled trials [RCTs]).
Fresh and frozen donor feces, administered by either nasogastric tube or colonoscope, produce equal results (SOR B, RCTs).
FMT has an overall adverse event rate of 30%, primarily involving abdominal discomfort, but also, rarely, severe infections (0.7%) and death (0.1%) (SOR: B, systematic review not limited to RCTs).
Which interventions are effective in managing parental vaccine refusal?
EVIDENCE SUMMARY
A systematic review analyzed 30 predominantly US studies with more than 8000 patients published between 1990 and 2012 (4 RCTs, 7 nonrandomized clinical trials, 13 before/after intervention trials, and 6 evaluation studies) to evaluate interventions that decreased parental vaccine refusal and hesitancy.1 Interventions included: change in state law, changes in state and school policies, and family-centered education initiatives.
Four studies that evaluated the impact of state laws concerning personal exemption (in addition to religious exemption) consistently found that total nonmedical exemption rates were higher in states that allowed personal exemptions. One nationwide survey found that total nonmedical exemption rates were 2.54 times higher (95% confidence interval [CI], 1.68-3.83) in states that allowed personal exemption than in states where only religious nonmedical exemption was allowed.
Fifteen studies evaluated the impact of educational initiatives on parental attitude towards vaccination; 8 of them reported statistically significant changes. None of the studies demonstrated a change in vaccination rates, however. Citing the generally low quality of the studies, the review authors concluded that they didn’t have convincing evidence that educational interventions reduced vaccine hesitancy.
Herd immunity is an iffy motivator
A systematic review analyzed 29 studies from western nations (17 qualitative and 12 quantitative, 4650 patients) regarding willingness to immunize children for the benefit of the community.2 Of the 17 qualitative studies, only 2 (164 patients) identified benefit to others as a motivating factor in parents’ decisions to immunize their children. In the 12 quantitative studies, a wide range of parents (1% to 60%) rated the concept of benefit to others as a reason for immunization. Overall, approximately one-third of parents listed herd immunity as a motivating reason. The authors concluded that the high heterogeneity of the studies made it unclear whether herd immunity was a motivating factor in childhood immunizations.
Multifaceted interventions, education, and tailored approaches may all work
A systematic review of international studies published between 2007 and 2013 investigated interventions to increase uptake of routinely recommended immunizations in groups with vaccine hesitancy and reduced use.3 Authors identified 189 articles (trial types and number of patients not given) that provided outcome measures.
Interventions that resulted in at least a 25% increase in vaccine uptake were primarily multifaceted, including elements of: targeting undervaccinated populations, improving access or convenience, educational initiatives, and mandates. Interventions that produced a greater than 20% increase in knowledge were generally educational interventions embedded in routine processes such as clinic visits.
The authors noted wide variation between studies in effect size, settings, and target populations. They concluded that interventions tailored to specific populations and concerns were likely to work best.
Corrective information doesn’t help with the most worried parents
A subsequent RCT tested whether correcting the myth that the flu vaccine can give people the flu would reduce belief in the misconception, increase perceptions that the flu vaccine is safe, and increase vaccination intent.4 Respondents to a national online poll of 1000 people received one of 3 interventions: correctional education (information debunking the myth), risk education (information about the risks of influenza infection), or no additional education.
Corrective information about the flu vaccine reduced the false belief that the vaccine can cause the flu by 15% to 20% and that the flu vaccine is unsafe by 5% to 10% (data from graphs; P<.05 for both effects). However, corrective information actually decreased parental intention to vaccinate among the group most concerned about the adverse effects of the vaccine (data from graph and text: +5% in the low-concern group vs −18% in the high-concern group; P<.05).
A presumptive approach works—but at a cost
A subsequent observational study videotaped 111 patient-provider vaccine discussions.5 Researchers categorized the initiation of the vaccine discussion as presumptive (eg, “We have to do some shots.”) or participatory (eg, “What do you want to do about shots?”). Using a presumptive style was more likely to result in acceptance of all recommended vaccines by the end of the visit (90% vs 17%; P<.05), but it decreased the chance of a highly rated visit experience (63% vs 95%; P<.05).
RECOMMENDATIONS
The 2015 Centers for Disease Control and Prevention (CDC) Pink Book recommends a combination of strategies, aimed at both providers and the public, for increasing and maintaining high immunization rates. The Pink Book advises providers to be ready to address vaccine safety concerns raised by parents.6
In a 2012 guideline, the CDC encouraged providers to listen attentively, be ready with scientific information and reliable resources, and use appropriate anecdotes in communicating with vaccine-hesitant parents.7 The guideline recommended against excluding families who refuse vaccination from the practice.
1. Sadaf A, Richards JL, Glanz J, et al. A systematic review of interventions for reducing parental vaccine refusal and vaccine hesitancy. Vaccine. 2013;31:4293-42304.
2. Quadri-Sheriff M, Hendrix K, Downs S, et al. The role of herd immunity in parents’ decision to vaccinate children: a systematic review. Pediatrics. 2012;130:522-530.
3. Jarrett C, Wilson R, O’Leary M, et al. Strategies for addressing vaccine hesitancy: a systematic review. Vaccine. 2015;33:4180-4190.
4. Nyhan B, Reifler J. Does correcting myths about the flu vaccine work? An experimental evaluation of the effects of corrective information. Vaccine. 2015;33:459-464.
5. Opel DJ, Mangione-Smith R, Robinson JD, et al. The influence of provider communication behaviors on parental vaccine acceptance and visit experience. Am J Public Health. 2015;105:1998-2004.
6. Centers for Disease Control and Prevention. Immunization Strategies for Healthcare Practices and Providers. Available at: http://www.cdc.gov/vaccines/pubs/pinkbook/strat.html. Accessed May 11, 2016.
7. Centers for Disease Control and Prevention. Provider Resources for Vaccine Conversations with Parents. Available at: http://www.cdc.gov/vaccines/hcp/conversations/about-vacc-conversations.html. Accessed May 11, 2016.
EVIDENCE SUMMARY
A systematic review analyzed 30 predominantly US studies with more than 8000 patients published between 1990 and 2012 (4 RCTs, 7 nonrandomized clinical trials, 13 before/after intervention trials, and 6 evaluation studies) to evaluate interventions that decreased parental vaccine refusal and hesitancy.1 Interventions included: change in state law, changes in state and school policies, and family-centered education initiatives.
Four studies that evaluated the impact of state laws concerning personal exemption (in addition to religious exemption) consistently found that total nonmedical exemption rates were higher in states that allowed personal exemptions. One nationwide survey found that total nonmedical exemption rates were 2.54 times higher (95% confidence interval [CI], 1.68-3.83) in states that allowed personal exemption than in states where only religious nonmedical exemption was allowed.
Fifteen studies evaluated the impact of educational initiatives on parental attitude towards vaccination; 8 of them reported statistically significant changes. None of the studies demonstrated a change in vaccination rates, however. Citing the generally low quality of the studies, the review authors concluded that they didn’t have convincing evidence that educational interventions reduced vaccine hesitancy.
Herd immunity is an iffy motivator
A systematic review analyzed 29 studies from western nations (17 qualitative and 12 quantitative, 4650 patients) regarding willingness to immunize children for the benefit of the community.2 Of the 17 qualitative studies, only 2 (164 patients) identified benefit to others as a motivating factor in parents’ decisions to immunize their children. In the 12 quantitative studies, a wide range of parents (1% to 60%) rated the concept of benefit to others as a reason for immunization. Overall, approximately one-third of parents listed herd immunity as a motivating reason. The authors concluded that the high heterogeneity of the studies made it unclear whether herd immunity was a motivating factor in childhood immunizations.
Multifaceted interventions, education, and tailored approaches may all work
A systematic review of international studies published between 2007 and 2013 investigated interventions to increase uptake of routinely recommended immunizations in groups with vaccine hesitancy and reduced use.3 Authors identified 189 articles (trial types and number of patients not given) that provided outcome measures.
Interventions that resulted in at least a 25% increase in vaccine uptake were primarily multifaceted, including elements of: targeting undervaccinated populations, improving access or convenience, educational initiatives, and mandates. Interventions that produced a greater than 20% increase in knowledge were generally educational interventions embedded in routine processes such as clinic visits.
The authors noted wide variation between studies in effect size, settings, and target populations. They concluded that interventions tailored to specific populations and concerns were likely to work best.
Corrective information doesn’t help with the most worried parents
A subsequent RCT tested whether correcting the myth that the flu vaccine can give people the flu would reduce belief in the misconception, increase perceptions that the flu vaccine is safe, and increase vaccination intent.4 Respondents to a national online poll of 1000 people received one of 3 interventions: correctional education (information debunking the myth), risk education (information about the risks of influenza infection), or no additional education.
Corrective information about the flu vaccine reduced the false belief that the vaccine can cause the flu by 15% to 20% and that the flu vaccine is unsafe by 5% to 10% (data from graphs; P<.05 for both effects). However, corrective information actually decreased parental intention to vaccinate among the group most concerned about the adverse effects of the vaccine (data from graph and text: +5% in the low-concern group vs −18% in the high-concern group; P<.05).
A presumptive approach works—but at a cost
A subsequent observational study videotaped 111 patient-provider vaccine discussions.5 Researchers categorized the initiation of the vaccine discussion as presumptive (eg, “We have to do some shots.”) or participatory (eg, “What do you want to do about shots?”). Using a presumptive style was more likely to result in acceptance of all recommended vaccines by the end of the visit (90% vs 17%; P<.05), but it decreased the chance of a highly rated visit experience (63% vs 95%; P<.05).
RECOMMENDATIONS
The 2015 Centers for Disease Control and Prevention (CDC) Pink Book recommends a combination of strategies, aimed at both providers and the public, for increasing and maintaining high immunization rates. The Pink Book advises providers to be ready to address vaccine safety concerns raised by parents.6
In a 2012 guideline, the CDC encouraged providers to listen attentively, be ready with scientific information and reliable resources, and use appropriate anecdotes in communicating with vaccine-hesitant parents.7 The guideline recommended against excluding families who refuse vaccination from the practice.
EVIDENCE SUMMARY
A systematic review analyzed 30 predominantly US studies with more than 8000 patients published between 1990 and 2012 (4 RCTs, 7 nonrandomized clinical trials, 13 before/after intervention trials, and 6 evaluation studies) to evaluate interventions that decreased parental vaccine refusal and hesitancy.1 Interventions included: change in state law, changes in state and school policies, and family-centered education initiatives.
Four studies that evaluated the impact of state laws concerning personal exemption (in addition to religious exemption) consistently found that total nonmedical exemption rates were higher in states that allowed personal exemptions. One nationwide survey found that total nonmedical exemption rates were 2.54 times higher (95% confidence interval [CI], 1.68-3.83) in states that allowed personal exemption than in states where only religious nonmedical exemption was allowed.
Fifteen studies evaluated the impact of educational initiatives on parental attitude towards vaccination; 8 of them reported statistically significant changes. None of the studies demonstrated a change in vaccination rates, however. Citing the generally low quality of the studies, the review authors concluded that they didn’t have convincing evidence that educational interventions reduced vaccine hesitancy.
Herd immunity is an iffy motivator
A systematic review analyzed 29 studies from western nations (17 qualitative and 12 quantitative, 4650 patients) regarding willingness to immunize children for the benefit of the community.2 Of the 17 qualitative studies, only 2 (164 patients) identified benefit to others as a motivating factor in parents’ decisions to immunize their children. In the 12 quantitative studies, a wide range of parents (1% to 60%) rated the concept of benefit to others as a reason for immunization. Overall, approximately one-third of parents listed herd immunity as a motivating reason. The authors concluded that the high heterogeneity of the studies made it unclear whether herd immunity was a motivating factor in childhood immunizations.
Multifaceted interventions, education, and tailored approaches may all work
A systematic review of international studies published between 2007 and 2013 investigated interventions to increase uptake of routinely recommended immunizations in groups with vaccine hesitancy and reduced use.3 Authors identified 189 articles (trial types and number of patients not given) that provided outcome measures.
Interventions that resulted in at least a 25% increase in vaccine uptake were primarily multifaceted, including elements of: targeting undervaccinated populations, improving access or convenience, educational initiatives, and mandates. Interventions that produced a greater than 20% increase in knowledge were generally educational interventions embedded in routine processes such as clinic visits.
The authors noted wide variation between studies in effect size, settings, and target populations. They concluded that interventions tailored to specific populations and concerns were likely to work best.
Corrective information doesn’t help with the most worried parents
A subsequent RCT tested whether correcting the myth that the flu vaccine can give people the flu would reduce belief in the misconception, increase perceptions that the flu vaccine is safe, and increase vaccination intent.4 Respondents to a national online poll of 1000 people received one of 3 interventions: correctional education (information debunking the myth), risk education (information about the risks of influenza infection), or no additional education.
Corrective information about the flu vaccine reduced the false belief that the vaccine can cause the flu by 15% to 20% and that the flu vaccine is unsafe by 5% to 10% (data from graphs; P<.05 for both effects). However, corrective information actually decreased parental intention to vaccinate among the group most concerned about the adverse effects of the vaccine (data from graph and text: +5% in the low-concern group vs −18% in the high-concern group; P<.05).
A presumptive approach works—but at a cost
A subsequent observational study videotaped 111 patient-provider vaccine discussions.5 Researchers categorized the initiation of the vaccine discussion as presumptive (eg, “We have to do some shots.”) or participatory (eg, “What do you want to do about shots?”). Using a presumptive style was more likely to result in acceptance of all recommended vaccines by the end of the visit (90% vs 17%; P<.05), but it decreased the chance of a highly rated visit experience (63% vs 95%; P<.05).
RECOMMENDATIONS
The 2015 Centers for Disease Control and Prevention (CDC) Pink Book recommends a combination of strategies, aimed at both providers and the public, for increasing and maintaining high immunization rates. The Pink Book advises providers to be ready to address vaccine safety concerns raised by parents.6
In a 2012 guideline, the CDC encouraged providers to listen attentively, be ready with scientific information and reliable resources, and use appropriate anecdotes in communicating with vaccine-hesitant parents.7 The guideline recommended against excluding families who refuse vaccination from the practice.
1. Sadaf A, Richards JL, Glanz J, et al. A systematic review of interventions for reducing parental vaccine refusal and vaccine hesitancy. Vaccine. 2013;31:4293-42304.
2. Quadri-Sheriff M, Hendrix K, Downs S, et al. The role of herd immunity in parents’ decision to vaccinate children: a systematic review. Pediatrics. 2012;130:522-530.
3. Jarrett C, Wilson R, O’Leary M, et al. Strategies for addressing vaccine hesitancy: a systematic review. Vaccine. 2015;33:4180-4190.
4. Nyhan B, Reifler J. Does correcting myths about the flu vaccine work? An experimental evaluation of the effects of corrective information. Vaccine. 2015;33:459-464.
5. Opel DJ, Mangione-Smith R, Robinson JD, et al. The influence of provider communication behaviors on parental vaccine acceptance and visit experience. Am J Public Health. 2015;105:1998-2004.
6. Centers for Disease Control and Prevention. Immunization Strategies for Healthcare Practices and Providers. Available at: http://www.cdc.gov/vaccines/pubs/pinkbook/strat.html. Accessed May 11, 2016.
7. Centers for Disease Control and Prevention. Provider Resources for Vaccine Conversations with Parents. Available at: http://www.cdc.gov/vaccines/hcp/conversations/about-vacc-conversations.html. Accessed May 11, 2016.
1. Sadaf A, Richards JL, Glanz J, et al. A systematic review of interventions for reducing parental vaccine refusal and vaccine hesitancy. Vaccine. 2013;31:4293-42304.
2. Quadri-Sheriff M, Hendrix K, Downs S, et al. The role of herd immunity in parents’ decision to vaccinate children: a systematic review. Pediatrics. 2012;130:522-530.
3. Jarrett C, Wilson R, O’Leary M, et al. Strategies for addressing vaccine hesitancy: a systematic review. Vaccine. 2015;33:4180-4190.
4. Nyhan B, Reifler J. Does correcting myths about the flu vaccine work? An experimental evaluation of the effects of corrective information. Vaccine. 2015;33:459-464.
5. Opel DJ, Mangione-Smith R, Robinson JD, et al. The influence of provider communication behaviors on parental vaccine acceptance and visit experience. Am J Public Health. 2015;105:1998-2004.
6. Centers for Disease Control and Prevention. Immunization Strategies for Healthcare Practices and Providers. Available at: http://www.cdc.gov/vaccines/pubs/pinkbook/strat.html. Accessed May 11, 2016.
7. Centers for Disease Control and Prevention. Provider Resources for Vaccine Conversations with Parents. Available at: http://www.cdc.gov/vaccines/hcp/conversations/about-vacc-conversations.html. Accessed May 11, 2016.
Evidence-based answers from the Family Physicians Inquiries Network
EVIDENCE-BASED ANSWER:
It’s unclear whether educational initiatives alone alter vaccine refusal. Although about a third of parents cite herd immunity as motivation for vaccination, its efficacy in addressing vaccine hesitancy isn’t clear (strength of recommendation [SOR]: B, systematic reviews not limited to randomized controlled trials [RCTs]).
Multifaceted interventions (encompassing improved access to vaccines, immunization mandates, and patient education) may produce a ≥25% increase in vaccine uptake in groups with vaccine hesitancy and low utilization (SOR: B, extrapolated from a meta-analysis across diverse cultures).
Correcting false information about influenza vaccination improves perceptions about the vaccine, but may decrease intention to vaccinate in parents who already have strong concerns about safety (SOR: C, low-quality RCT).
Discussions about vaccines that are more paternalistic (presumptive rather than participatory) are associated with higher vaccination rates, but lower visit satisfaction (SOR: C, observational study).
Providers should thoroughly address patient concerns about safety and encourage vaccine use (SOR: C, expert opinion).
Do pedometers increase activity and improve health outcomes?
EVIDENCE SUMMARY
A systematic review and meta-analysis identified 26 studies evaluating activity and health outcomes with the use of pedometers.1 The studies included 8 RCTs and 18 observational studies with 2767 patients (mean body mass index [BMI]: 30 kg/m2; mean age: 49 years; 85% women). The studies ranged from 3 to 104 weeks. From the RCT data, patients using pedometers had an increase of 2491 steps per day (about one mile) more than control group patients (8 trials, n=305; 95% confidence interval [CI], 1098-3885 steps/day; P<.001).
Across all of the observational studies, pedometer users had a 26.9% increase from their baseline physical activity (P=.001). When data from all of the studies were combined, the researchers found a decrease from baseline BMI (18 studies, n=562; mean difference [MD]=0.38 kg/m2; 95% CI, 0.05-0.72; P=.03) and a decrease in systolic BP (12 studies, n=468; MD=3.8 mm Hg; 95% CI, 1.7-5.9 mm Hg; P<.001). No statistically significant change was noted in cholesterol or fasting glucose levels. Weaknesses of this review include the heterogeneity of the interventions, relatively small study sizes, and short study durations.
A systematic review and meta-analysis of 11 RCTs (N=1258) evaluated pedometer effects in overweight patients with type 2 diabetes.2 (One RCT was included in the above meta-analysis.) Studies ran from 6 to 48 weeks, and mean enrollment BMI (where reported) was 30 kg/m2 or more in at least one treatment arm. Compared to controls, patients using pedometers had greater reductions in weight (weighted mean difference [WMD]= -0.65 kg; 95% CI, -1.12 to -0.17 kg) and BMI (WMD= -0.15 kg/m2; 95% CI, -0.29 to -0.02 kg/m2). The effect persisted in the subset of studies in which the intervention and control groups both received dietary counseling (WMD weight= -0.86 kg; 95% CI, -1.45 to -0.27 kg; WMD BMI= -0.30 kg/m2; 95% CI, -0.50 to -0.10 kg/m2). Study quality was low to moderate, and 5 studies used per-protocol analysis instead of intention-to-treat analysis.
Pedometer use benefits patients with musculoskeletal diseases, too
A systematic review and meta-analysis examined the use of pedometers in patients with musculoskeletal diseases.3 It included 7 RCTs lasting 4 weeks to one year with 484 adults, 40 to 82 years of age, with musculoskeletal disorders (eg, back pain, knee pain, hip pain). (One RCT was also included in the diabetes meta-analysis.) Pedometer use resulted in a mean increase in physical activity of 1950 steps per day above baseline (range=818-2829 steps/day; P<.05). The authors noted that 4 of the 7 studies also demonstrated significant improvement in pain scores and physical function. BMI data were not tracked in this review.
Pedometers increase walking in older patients
A RCT compared the effects of pedometer-based activity prescriptions with standard time-based activity prescriptions in 330 patients ≥65 years of age with baseline low activity levels.4 All patients received an initial physician visit followed by 3 telephone counseling sessions encouraging increased activity. The pedometer group was counseled on increasing steps (without specific targets), while the standard activity prescription group received time-related activity goals.
At one year, “leisure walking” had increased more for the pedometer group than for the standard group (mean 50 minutes/week vs 28 minutes/week; P=.03), although both groups equally increased their amount of “total activity.”
1. Bravata DM, Smith-Spangler C, Sundaram V, et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007;298:2296-2304.
2. Cai X, Qiu SH, Yin H, et al. Pedometer intervention and weight loss in overweight and obese adults with type 2 diabetes: a meta-analysis. Diabet Med. 2016;33:1035-1044.
3. Mansi S, Milosavljevic S, Baxter GD, et al. A systematic review of studies using pedometers as an intervention for musculoskeletal diseases. BMC Musculoskeletal Disorders. 2014;15:231.
4. Kolt GS, Schofield GM, Kerse N, et al. Healthy Steps trial: pedometer-based advice and physical activity for low-active older adults. Ann Fam Med. 2012;10:206-212.
EVIDENCE SUMMARY
A systematic review and meta-analysis identified 26 studies evaluating activity and health outcomes with the use of pedometers.1 The studies included 8 RCTs and 18 observational studies with 2767 patients (mean body mass index [BMI]: 30 kg/m2; mean age: 49 years; 85% women). The studies ranged from 3 to 104 weeks. From the RCT data, patients using pedometers had an increase of 2491 steps per day (about one mile) more than control group patients (8 trials, n=305; 95% confidence interval [CI], 1098-3885 steps/day; P<.001).
Across all of the observational studies, pedometer users had a 26.9% increase from their baseline physical activity (P=.001). When data from all of the studies were combined, the researchers found a decrease from baseline BMI (18 studies, n=562; mean difference [MD]=0.38 kg/m2; 95% CI, 0.05-0.72; P=.03) and a decrease in systolic BP (12 studies, n=468; MD=3.8 mm Hg; 95% CI, 1.7-5.9 mm Hg; P<.001). No statistically significant change was noted in cholesterol or fasting glucose levels. Weaknesses of this review include the heterogeneity of the interventions, relatively small study sizes, and short study durations.
A systematic review and meta-analysis of 11 RCTs (N=1258) evaluated pedometer effects in overweight patients with type 2 diabetes.2 (One RCT was included in the above meta-analysis.) Studies ran from 6 to 48 weeks, and mean enrollment BMI (where reported) was 30 kg/m2 or more in at least one treatment arm. Compared to controls, patients using pedometers had greater reductions in weight (weighted mean difference [WMD]= -0.65 kg; 95% CI, -1.12 to -0.17 kg) and BMI (WMD= -0.15 kg/m2; 95% CI, -0.29 to -0.02 kg/m2). The effect persisted in the subset of studies in which the intervention and control groups both received dietary counseling (WMD weight= -0.86 kg; 95% CI, -1.45 to -0.27 kg; WMD BMI= -0.30 kg/m2; 95% CI, -0.50 to -0.10 kg/m2). Study quality was low to moderate, and 5 studies used per-protocol analysis instead of intention-to-treat analysis.
Pedometer use benefits patients with musculoskeletal diseases, too
A systematic review and meta-analysis examined the use of pedometers in patients with musculoskeletal diseases.3 It included 7 RCTs lasting 4 weeks to one year with 484 adults, 40 to 82 years of age, with musculoskeletal disorders (eg, back pain, knee pain, hip pain). (One RCT was also included in the diabetes meta-analysis.) Pedometer use resulted in a mean increase in physical activity of 1950 steps per day above baseline (range=818-2829 steps/day; P<.05). The authors noted that 4 of the 7 studies also demonstrated significant improvement in pain scores and physical function. BMI data were not tracked in this review.
Pedometers increase walking in older patients
A RCT compared the effects of pedometer-based activity prescriptions with standard time-based activity prescriptions in 330 patients ≥65 years of age with baseline low activity levels.4 All patients received an initial physician visit followed by 3 telephone counseling sessions encouraging increased activity. The pedometer group was counseled on increasing steps (without specific targets), while the standard activity prescription group received time-related activity goals.
At one year, “leisure walking” had increased more for the pedometer group than for the standard group (mean 50 minutes/week vs 28 minutes/week; P=.03), although both groups equally increased their amount of “total activity.”
EVIDENCE SUMMARY
A systematic review and meta-analysis identified 26 studies evaluating activity and health outcomes with the use of pedometers.1 The studies included 8 RCTs and 18 observational studies with 2767 patients (mean body mass index [BMI]: 30 kg/m2; mean age: 49 years; 85% women). The studies ranged from 3 to 104 weeks. From the RCT data, patients using pedometers had an increase of 2491 steps per day (about one mile) more than control group patients (8 trials, n=305; 95% confidence interval [CI], 1098-3885 steps/day; P<.001).
Across all of the observational studies, pedometer users had a 26.9% increase from their baseline physical activity (P=.001). When data from all of the studies were combined, the researchers found a decrease from baseline BMI (18 studies, n=562; mean difference [MD]=0.38 kg/m2; 95% CI, 0.05-0.72; P=.03) and a decrease in systolic BP (12 studies, n=468; MD=3.8 mm Hg; 95% CI, 1.7-5.9 mm Hg; P<.001). No statistically significant change was noted in cholesterol or fasting glucose levels. Weaknesses of this review include the heterogeneity of the interventions, relatively small study sizes, and short study durations.
A systematic review and meta-analysis of 11 RCTs (N=1258) evaluated pedometer effects in overweight patients with type 2 diabetes.2 (One RCT was included in the above meta-analysis.) Studies ran from 6 to 48 weeks, and mean enrollment BMI (where reported) was 30 kg/m2 or more in at least one treatment arm. Compared to controls, patients using pedometers had greater reductions in weight (weighted mean difference [WMD]= -0.65 kg; 95% CI, -1.12 to -0.17 kg) and BMI (WMD= -0.15 kg/m2; 95% CI, -0.29 to -0.02 kg/m2). The effect persisted in the subset of studies in which the intervention and control groups both received dietary counseling (WMD weight= -0.86 kg; 95% CI, -1.45 to -0.27 kg; WMD BMI= -0.30 kg/m2; 95% CI, -0.50 to -0.10 kg/m2). Study quality was low to moderate, and 5 studies used per-protocol analysis instead of intention-to-treat analysis.
Pedometer use benefits patients with musculoskeletal diseases, too
A systematic review and meta-analysis examined the use of pedometers in patients with musculoskeletal diseases.3 It included 7 RCTs lasting 4 weeks to one year with 484 adults, 40 to 82 years of age, with musculoskeletal disorders (eg, back pain, knee pain, hip pain). (One RCT was also included in the diabetes meta-analysis.) Pedometer use resulted in a mean increase in physical activity of 1950 steps per day above baseline (range=818-2829 steps/day; P<.05). The authors noted that 4 of the 7 studies also demonstrated significant improvement in pain scores and physical function. BMI data were not tracked in this review.
Pedometers increase walking in older patients
A RCT compared the effects of pedometer-based activity prescriptions with standard time-based activity prescriptions in 330 patients ≥65 years of age with baseline low activity levels.4 All patients received an initial physician visit followed by 3 telephone counseling sessions encouraging increased activity. The pedometer group was counseled on increasing steps (without specific targets), while the standard activity prescription group received time-related activity goals.
At one year, “leisure walking” had increased more for the pedometer group than for the standard group (mean 50 minutes/week vs 28 minutes/week; P=.03), although both groups equally increased their amount of “total activity.”
1. Bravata DM, Smith-Spangler C, Sundaram V, et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007;298:2296-2304.
2. Cai X, Qiu SH, Yin H, et al. Pedometer intervention and weight loss in overweight and obese adults with type 2 diabetes: a meta-analysis. Diabet Med. 2016;33:1035-1044.
3. Mansi S, Milosavljevic S, Baxter GD, et al. A systematic review of studies using pedometers as an intervention for musculoskeletal diseases. BMC Musculoskeletal Disorders. 2014;15:231.
4. Kolt GS, Schofield GM, Kerse N, et al. Healthy Steps trial: pedometer-based advice and physical activity for low-active older adults. Ann Fam Med. 2012;10:206-212.
1. Bravata DM, Smith-Spangler C, Sundaram V, et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007;298:2296-2304.
2. Cai X, Qiu SH, Yin H, et al. Pedometer intervention and weight loss in overweight and obese adults with type 2 diabetes: a meta-analysis. Diabet Med. 2016;33:1035-1044.
3. Mansi S, Milosavljevic S, Baxter GD, et al. A systematic review of studies using pedometers as an intervention for musculoskeletal diseases. BMC Musculoskeletal Disorders. 2014;15:231.
4. Kolt GS, Schofield GM, Kerse N, et al. Healthy Steps trial: pedometer-based advice and physical activity for low-active older adults. Ann Fam Med. 2012;10:206-212.
Evidence-based answers from the Family Physicians Inquiries Network
EVIDENCE-BASED ANSWER:
Yes. In overweight and obese patients, exercise interventions using a pedometer increase steps by about a mile per day over the same interventions without access to pedometer information (strength of recommendation [SOR]: A, meta-analysis of randomized controlled trials [RCTs]) and are associated with a modest 4 mm Hg reduction in systolic blood pressure (BP) over baseline (SOR: B, meta-analysis of RCTs and cohort studies). In overweight patients with diabetes, pedometer use with nutritional counseling is associated with 0.86 kg greater weight loss than nutritional counseling alone (SOR: B, meta-analysis of lower quality RCTs).
Pedometers increase activity in patients with various musculoskeletal conditions and may help reduce pain (SOR: B, meta-analysis of RCTs with heterogeneous outcomes). In low-activity elderly patients, pedometers do not appear to increase total activity when added to an exercise program, but they do appear to increase walking (SOR: B, RCT).
There is no evidence concerning the impact of pedometers on cardiovascular outcomes.
Is arthroscopic subacromial decompression effective for shoulder impingement?
It’s impossible to say for certain in the absence of randomized controlled trials. However, in patients whose impingement symptoms don’t improve after 3 to 6 months, arthroscopic subacromial decompression (ASD) is associated with modest (about 10%) long-term improvement in pain and function compared with open acromioplasty or baseline (strength of recommendation [SOR]: B, cohort studies).
Patients older than 57 years may do better with surgery than physical therapy (SOR: B, single cohort study).
EVIDENCE SUMMARY
Six cohort studies found that patients who underwent ASD for subacromial impingement had improved pain and function scores at 4.5 to 12 years after surgery (TABLE1-7). Weaknesses of the overall data set include use of heterogeneous outcome measures across studies, lack of sham surgical controls, and lack of blinding.
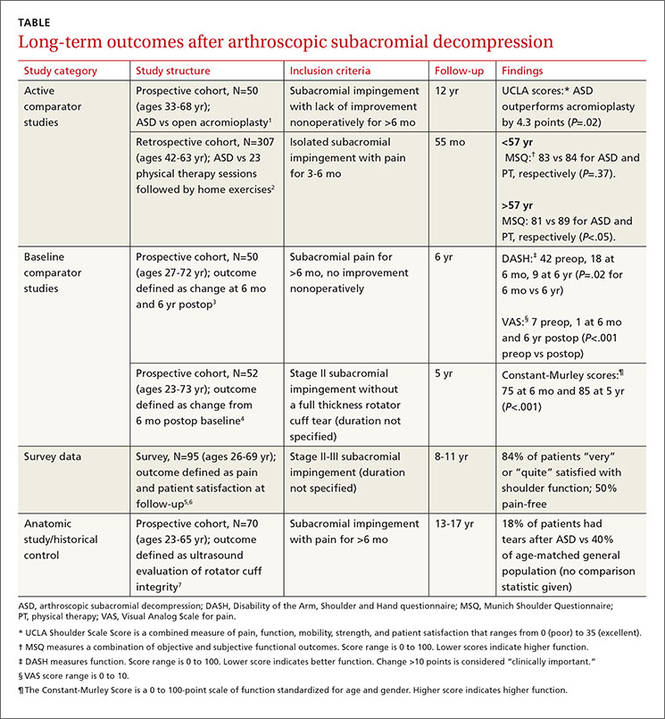
ASD improves pain and function slightly more than other treatments
One prospective and one retrospective cohort trial compared ASD with another intervention. In the prospective trial, ASD was associated with a 10% better combined pain and function score than open acromioplasty at 12 years.1 In the retrospective trial, ASD was also associated with a 10% better combined pain and function score than prolonged physical therapy in patients older than 57 years (the median age of study participants) but not patients younger than 57 years.2
Two other studies found improvements in pain and function
Two other prospective cohort studies didn’t use a comparison group but followed changes in standardized shoulder pain and function scores for 5 to 6 years after ASD. In one study, pain decreased 6 points on a 10-point visual analog scale by 6 months postop (P<.001).3 In both studies, a 9% to 10% improvement in function was seen between 6 months and 5 to 6 years after surgery.3,4
A third cohort study that asked patients about overall pain and satisfaction 8 to 11 years after ASD found that most were “very” or “quite” satisfied and half were pain-free.5,6
Rotator cuff tears found less likely with ASD
An anatomic study obtained ultrasounds of patients 13 to 17 years after ASD and compared the findings to rotator cuff ultrasounds of the general population.7 Patients who had ASD were 22% less likely to demonstrate rotator cuff tears at the end of the study (no statistics were reported to measure significance).
RECOMMENDATIONS
Guidelines from the Washington State Department of Labor and Industry state that patients who should undergo isolated subacromial decompression (with or without acromioplasty) need to have documented subacromial impingement syndrome with magnetic resonance imaging evidence of rotator cuff tendonopathy or tear, have undergone 12 weeks of conservative therapy (including at least active assisted range of motion and home-based exercises), and have had a subacromial injection with a local anesthetic that has provided documented relief of pain.8
No current guidelines are available from national or international orthopedic or sports medicine organizations.
1. Odenbring S, Wagner P, Atroshi I. Long-term outcomes of arthroscopic acromioplasty of chronic shoulder impingement syndrome: a prospective cohort study with a minimum of 12 years’ follow-up. Arthroscopy. 2008;24:1092–1098.
2. Biberthaler P, Beirer M, Kirchhoff S, et al. Significant benefit for older patients after arthroscopic subacromial decompression: a long-term follow-up study. Int Orthop. 2013;37:457–462.
3. Lunsjo K, Bengtsson M, Nordqvist A, et al. Patients with shoulder impingement remain satisfied 6 years after arthroscopic subacromial decompression. Acta Orthop. 2011;82:711–713.
4. Dom K, Van Glabbeek F, Van Riet RP, et al. Arthroscopic subacromial decompression for advanced (stage II) impingement syndrome: a study of 52 patients with 5 year follow-up. Acta Orthop Belg. 2003;69:13–17.
5. Klintberg IH, Karlsson J, Svantesson U. Health-related quality of life, patient satisfaction, and physical activity 8–11 years after arthroscopic subacromial decompression. J Shoulder Elbow Surg. 2011;20:598–608.
6. Klintberg IH, Svantesson U, Karlsson J. Long-term patient satisfaction and functional outcome 8-11 years after subacromial decompression. Knee Surg Sports Traumatol Arthrosc. 2010;18:394–403.
7. Bjornsson H, Norlin R, Knutsson A, et al. Fewer rotator cuff tears fifteen years after arthroscopic subacromial decompression. J Shoulder Elbow Surg. 2010;19:111–115.
8. Washington State Department of Labor and Industries. Shoulder Conditions Diagnosis and Treatment Guideline. Available at: http://www.lni.wa.gov/ClaimsIns/Files/OMD/MedTreat/FINALguidelineShoulderConditionsOct242013.pdf. Accessed October 20, 2015.
It’s impossible to say for certain in the absence of randomized controlled trials. However, in patients whose impingement symptoms don’t improve after 3 to 6 months, arthroscopic subacromial decompression (ASD) is associated with modest (about 10%) long-term improvement in pain and function compared with open acromioplasty or baseline (strength of recommendation [SOR]: B, cohort studies).
Patients older than 57 years may do better with surgery than physical therapy (SOR: B, single cohort study).
EVIDENCE SUMMARY
Six cohort studies found that patients who underwent ASD for subacromial impingement had improved pain and function scores at 4.5 to 12 years after surgery (TABLE1-7). Weaknesses of the overall data set include use of heterogeneous outcome measures across studies, lack of sham surgical controls, and lack of blinding.

ASD improves pain and function slightly more than other treatments
One prospective and one retrospective cohort trial compared ASD with another intervention. In the prospective trial, ASD was associated with a 10% better combined pain and function score than open acromioplasty at 12 years.1 In the retrospective trial, ASD was also associated with a 10% better combined pain and function score than prolonged physical therapy in patients older than 57 years (the median age of study participants) but not patients younger than 57 years.2
Two other studies found improvements in pain and function
Two other prospective cohort studies didn’t use a comparison group but followed changes in standardized shoulder pain and function scores for 5 to 6 years after ASD. In one study, pain decreased 6 points on a 10-point visual analog scale by 6 months postop (P<.001).3 In both studies, a 9% to 10% improvement in function was seen between 6 months and 5 to 6 years after surgery.3,4
A third cohort study that asked patients about overall pain and satisfaction 8 to 11 years after ASD found that most were “very” or “quite” satisfied and half were pain-free.5,6
Rotator cuff tears found less likely with ASD
An anatomic study obtained ultrasounds of patients 13 to 17 years after ASD and compared the findings to rotator cuff ultrasounds of the general population.7 Patients who had ASD were 22% less likely to demonstrate rotator cuff tears at the end of the study (no statistics were reported to measure significance).
RECOMMENDATIONS
Guidelines from the Washington State Department of Labor and Industry state that patients who should undergo isolated subacromial decompression (with or without acromioplasty) need to have documented subacromial impingement syndrome with magnetic resonance imaging evidence of rotator cuff tendonopathy or tear, have undergone 12 weeks of conservative therapy (including at least active assisted range of motion and home-based exercises), and have had a subacromial injection with a local anesthetic that has provided documented relief of pain.8
No current guidelines are available from national or international orthopedic or sports medicine organizations.
It’s impossible to say for certain in the absence of randomized controlled trials. However, in patients whose impingement symptoms don’t improve after 3 to 6 months, arthroscopic subacromial decompression (ASD) is associated with modest (about 10%) long-term improvement in pain and function compared with open acromioplasty or baseline (strength of recommendation [SOR]: B, cohort studies).
Patients older than 57 years may do better with surgery than physical therapy (SOR: B, single cohort study).
EVIDENCE SUMMARY
Six cohort studies found that patients who underwent ASD for subacromial impingement had improved pain and function scores at 4.5 to 12 years after surgery (TABLE1-7). Weaknesses of the overall data set include use of heterogeneous outcome measures across studies, lack of sham surgical controls, and lack of blinding.

ASD improves pain and function slightly more than other treatments
One prospective and one retrospective cohort trial compared ASD with another intervention. In the prospective trial, ASD was associated with a 10% better combined pain and function score than open acromioplasty at 12 years.1 In the retrospective trial, ASD was also associated with a 10% better combined pain and function score than prolonged physical therapy in patients older than 57 years (the median age of study participants) but not patients younger than 57 years.2
Two other studies found improvements in pain and function
Two other prospective cohort studies didn’t use a comparison group but followed changes in standardized shoulder pain and function scores for 5 to 6 years after ASD. In one study, pain decreased 6 points on a 10-point visual analog scale by 6 months postop (P<.001).3 In both studies, a 9% to 10% improvement in function was seen between 6 months and 5 to 6 years after surgery.3,4
A third cohort study that asked patients about overall pain and satisfaction 8 to 11 years after ASD found that most were “very” or “quite” satisfied and half were pain-free.5,6
Rotator cuff tears found less likely with ASD
An anatomic study obtained ultrasounds of patients 13 to 17 years after ASD and compared the findings to rotator cuff ultrasounds of the general population.7 Patients who had ASD were 22% less likely to demonstrate rotator cuff tears at the end of the study (no statistics were reported to measure significance).
RECOMMENDATIONS
Guidelines from the Washington State Department of Labor and Industry state that patients who should undergo isolated subacromial decompression (with or without acromioplasty) need to have documented subacromial impingement syndrome with magnetic resonance imaging evidence of rotator cuff tendonopathy or tear, have undergone 12 weeks of conservative therapy (including at least active assisted range of motion and home-based exercises), and have had a subacromial injection with a local anesthetic that has provided documented relief of pain.8
No current guidelines are available from national or international orthopedic or sports medicine organizations.
1. Odenbring S, Wagner P, Atroshi I. Long-term outcomes of arthroscopic acromioplasty of chronic shoulder impingement syndrome: a prospective cohort study with a minimum of 12 years’ follow-up. Arthroscopy. 2008;24:1092–1098.
2. Biberthaler P, Beirer M, Kirchhoff S, et al. Significant benefit for older patients after arthroscopic subacromial decompression: a long-term follow-up study. Int Orthop. 2013;37:457–462.
3. Lunsjo K, Bengtsson M, Nordqvist A, et al. Patients with shoulder impingement remain satisfied 6 years after arthroscopic subacromial decompression. Acta Orthop. 2011;82:711–713.
4. Dom K, Van Glabbeek F, Van Riet RP, et al. Arthroscopic subacromial decompression for advanced (stage II) impingement syndrome: a study of 52 patients with 5 year follow-up. Acta Orthop Belg. 2003;69:13–17.
5. Klintberg IH, Karlsson J, Svantesson U. Health-related quality of life, patient satisfaction, and physical activity 8–11 years after arthroscopic subacromial decompression. J Shoulder Elbow Surg. 2011;20:598–608.
6. Klintberg IH, Svantesson U, Karlsson J. Long-term patient satisfaction and functional outcome 8-11 years after subacromial decompression. Knee Surg Sports Traumatol Arthrosc. 2010;18:394–403.
7. Bjornsson H, Norlin R, Knutsson A, et al. Fewer rotator cuff tears fifteen years after arthroscopic subacromial decompression. J Shoulder Elbow Surg. 2010;19:111–115.
8. Washington State Department of Labor and Industries. Shoulder Conditions Diagnosis and Treatment Guideline. Available at: http://www.lni.wa.gov/ClaimsIns/Files/OMD/MedTreat/FINALguidelineShoulderConditionsOct242013.pdf. Accessed October 20, 2015.
1. Odenbring S, Wagner P, Atroshi I. Long-term outcomes of arthroscopic acromioplasty of chronic shoulder impingement syndrome: a prospective cohort study with a minimum of 12 years’ follow-up. Arthroscopy. 2008;24:1092–1098.
2. Biberthaler P, Beirer M, Kirchhoff S, et al. Significant benefit for older patients after arthroscopic subacromial decompression: a long-term follow-up study. Int Orthop. 2013;37:457–462.
3. Lunsjo K, Bengtsson M, Nordqvist A, et al. Patients with shoulder impingement remain satisfied 6 years after arthroscopic subacromial decompression. Acta Orthop. 2011;82:711–713.
4. Dom K, Van Glabbeek F, Van Riet RP, et al. Arthroscopic subacromial decompression for advanced (stage II) impingement syndrome: a study of 52 patients with 5 year follow-up. Acta Orthop Belg. 2003;69:13–17.
5. Klintberg IH, Karlsson J, Svantesson U. Health-related quality of life, patient satisfaction, and physical activity 8–11 years after arthroscopic subacromial decompression. J Shoulder Elbow Surg. 2011;20:598–608.
6. Klintberg IH, Svantesson U, Karlsson J. Long-term patient satisfaction and functional outcome 8-11 years after subacromial decompression. Knee Surg Sports Traumatol Arthrosc. 2010;18:394–403.
7. Bjornsson H, Norlin R, Knutsson A, et al. Fewer rotator cuff tears fifteen years after arthroscopic subacromial decompression. J Shoulder Elbow Surg. 2010;19:111–115.
8. Washington State Department of Labor and Industries. Shoulder Conditions Diagnosis and Treatment Guideline. Available at: http://www.lni.wa.gov/ClaimsIns/Files/OMD/MedTreat/FINALguidelineShoulderConditionsOct242013.pdf. Accessed October 20, 2015.
Evidence-based answers from the Family Physicians Inquiries Network
Do statins increase the risk of developing diabetes?
Yes. Statin therapy produces a small increase in the incidence of diabetes: one additional case per 255 patients taking statins over 4 years (strength of recommendation [SOR]: A, meta-analysis). Intensive statin therapy, compared with moderate therapy, produces an additional 2 cases of diabetes per 1000 patient years (SOR: B, meta-analysis with significant heterogeneity among trials).
EVIDENCE SUMMARY
A meta-analysis of 13 randomized, placebo or standard of care-controlled statin trials (113,148 patients, 81% without diabetes at enrollment, mean ages 55-76 years) found that statin therapy increased the incidence of diabetes by 9% over 4 years (odds ratio [OR]=1.09; 95% confidence interval [CI], 1.02-1.17), or one additional case per 255 patients.1 The increased risk was similar for lipophilic (pravastatin, rosuvastatin) and hydrophilic (atorvastatin, simvastatin, lovastatin) statins, although the analysis wasn’t adjusted for doses used.
In a meta-regression analysis, baseline body mass index or percentage change in low-density lipoprotein cholesterol didn’t appear to confer additional risk. The risk of diabetes with statins was generally higher in studies with older patients (data given graphically).
Higher statin doses mean higher risk
A meta-analysis of 5 placebo and standard-of-care randomized controlled trials (39,612 patients, 83% without diabetes at enrollment, mean age 58-64 years) found that the risk of diabetes was higher with higher-dose statins.2 Therapy with atorvastatin 80 mg or simvastatin 40 to 80 mg was defined as intensive. Treatment with simvastatin 20 to 40 mg, atorvastatin 10 mg, or pravastatin 40 mg was defined as moderate.
At a mean follow-up of 4.9 years, intensive statin therapy was associated with a higher risk of developing diabetes than moderate therapy (OR=1.12; 95% CI, 1.04-1.22) with 2 additional cases of diabetes per 1000 patient-years in the intensive therapy group. The authors noted significant heterogeneity between trials with regard to major cardiovascular events.
Similar results were found in a subsequent population-based cohort study of 471,250 nondiabetic patients older than 66 years who were newly prescribed a statin.3 The study authors used the incidence of new diabetes in patients taking pravastatin as the baseline, since it had been associated with reduced rates of diabetes in a large cardiovascular prevention trial.4 Without adjusting for dose, patients were at significantly higher risk of diabetes if prescribed atorvastatin (hazard ratio [HR]=1.22; 95% CI, 1.15-1.29), rosuvastatin (HR=1.18; 95% CI, 1.10-1.26), or simvastatin (HR=1.10; 95% CI, 1.04-1.17) compared with pravastatin. The risk with fluvastatin and lovastatin was similar to pravastatin.
A subanalysis that compared moderate- and high-dose statin therapy with low-dose therapy (atorvastatin <20 mg, rosuvastatin <10 mg, simvastatin <80 mg, or any dose of fluvastatin, lovastatin, or pravastatin) found a 22% increased risk of diabetes (HR=1.22; 95% CI, 1.19-1.26) for moderate-dose therapy (atorvastatin 20-79 mg, rosuvastatin 10-39 mg, or simvastatin >80 mg) and a 30% increased risk (HR=1.3; 95% CI, 1.2-1.4) for high-dose therapy (atorvastatin ≥80 mg or rosuvastatin ≥40 mg).
A cohort trial also shows increased diabetes risk
A smaller subsequent cohort trial based on data from Taiwan National Health Insurance records compared 8412 nondiabetic adult patients (mean age 63 years) taking statins with 33,648 age- and risk-matched controls not taking statins over a mean duration of 7.2 years.5 Statin use was associated with a 15% higher risk of developing diabetes (HR=1.15; 95% CI, 1.08-1.22).
RECOMMENDATIONS
The 2013 American College of Cardiology/American Heart Association guidelines for lipid-lowering therapy recommend that patients taking statins be screened for diabetes according to current screening recommendations.6 The guidelines advise encouraging patients who develop diabetes while on statin therapy to adhere to a heart-healthy dietary pattern, engage in physical activity, achieve and maintain a healthy body weight, cease tobacco use, and continue statin therapy to reduce the risk of cardiovascular events.
1. Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735-742.
2. Preiss D, Seshasai SR, Welsh P, et al. Risk of incident diabetes with intensive-dose compared with moderatedose statin therapy: a meta-analysis. JAMA. 2011;305:2556-2564.
3. Carter AA, Gomes T, Camacho X, et al. Risk of incident diabetes among patients treated with statins: population-based study. BMJ. 2013;346:f2610.
4. Freeman DJ, Morrie J, Sattar N, et al. Pravastatin and the development of diabetes mellitus: evidence for a protective treatment effect in the West of Scotland Coronary Prevention Study. Circulation. 2001;103:357-362.
5. Wang KL, Liu CJ, Chao TF, et al. Statins, risk of diabetes and implications on outcomes in the general population. J Am Coll Cardiol. 2012;60:1231-1238.
6. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1-S45.
Yes. Statin therapy produces a small increase in the incidence of diabetes: one additional case per 255 patients taking statins over 4 years (strength of recommendation [SOR]: A, meta-analysis). Intensive statin therapy, compared with moderate therapy, produces an additional 2 cases of diabetes per 1000 patient years (SOR: B, meta-analysis with significant heterogeneity among trials).
EVIDENCE SUMMARY
A meta-analysis of 13 randomized, placebo or standard of care-controlled statin trials (113,148 patients, 81% without diabetes at enrollment, mean ages 55-76 years) found that statin therapy increased the incidence of diabetes by 9% over 4 years (odds ratio [OR]=1.09; 95% confidence interval [CI], 1.02-1.17), or one additional case per 255 patients.1 The increased risk was similar for lipophilic (pravastatin, rosuvastatin) and hydrophilic (atorvastatin, simvastatin, lovastatin) statins, although the analysis wasn’t adjusted for doses used.
In a meta-regression analysis, baseline body mass index or percentage change in low-density lipoprotein cholesterol didn’t appear to confer additional risk. The risk of diabetes with statins was generally higher in studies with older patients (data given graphically).
Higher statin doses mean higher risk
A meta-analysis of 5 placebo and standard-of-care randomized controlled trials (39,612 patients, 83% without diabetes at enrollment, mean age 58-64 years) found that the risk of diabetes was higher with higher-dose statins.2 Therapy with atorvastatin 80 mg or simvastatin 40 to 80 mg was defined as intensive. Treatment with simvastatin 20 to 40 mg, atorvastatin 10 mg, or pravastatin 40 mg was defined as moderate.
At a mean follow-up of 4.9 years, intensive statin therapy was associated with a higher risk of developing diabetes than moderate therapy (OR=1.12; 95% CI, 1.04-1.22) with 2 additional cases of diabetes per 1000 patient-years in the intensive therapy group. The authors noted significant heterogeneity between trials with regard to major cardiovascular events.
Similar results were found in a subsequent population-based cohort study of 471,250 nondiabetic patients older than 66 years who were newly prescribed a statin.3 The study authors used the incidence of new diabetes in patients taking pravastatin as the baseline, since it had been associated with reduced rates of diabetes in a large cardiovascular prevention trial.4 Without adjusting for dose, patients were at significantly higher risk of diabetes if prescribed atorvastatin (hazard ratio [HR]=1.22; 95% CI, 1.15-1.29), rosuvastatin (HR=1.18; 95% CI, 1.10-1.26), or simvastatin (HR=1.10; 95% CI, 1.04-1.17) compared with pravastatin. The risk with fluvastatin and lovastatin was similar to pravastatin.
A subanalysis that compared moderate- and high-dose statin therapy with low-dose therapy (atorvastatin <20 mg, rosuvastatin <10 mg, simvastatin <80 mg, or any dose of fluvastatin, lovastatin, or pravastatin) found a 22% increased risk of diabetes (HR=1.22; 95% CI, 1.19-1.26) for moderate-dose therapy (atorvastatin 20-79 mg, rosuvastatin 10-39 mg, or simvastatin >80 mg) and a 30% increased risk (HR=1.3; 95% CI, 1.2-1.4) for high-dose therapy (atorvastatin ≥80 mg or rosuvastatin ≥40 mg).
A cohort trial also shows increased diabetes risk
A smaller subsequent cohort trial based on data from Taiwan National Health Insurance records compared 8412 nondiabetic adult patients (mean age 63 years) taking statins with 33,648 age- and risk-matched controls not taking statins over a mean duration of 7.2 years.5 Statin use was associated with a 15% higher risk of developing diabetes (HR=1.15; 95% CI, 1.08-1.22).
RECOMMENDATIONS
The 2013 American College of Cardiology/American Heart Association guidelines for lipid-lowering therapy recommend that patients taking statins be screened for diabetes according to current screening recommendations.6 The guidelines advise encouraging patients who develop diabetes while on statin therapy to adhere to a heart-healthy dietary pattern, engage in physical activity, achieve and maintain a healthy body weight, cease tobacco use, and continue statin therapy to reduce the risk of cardiovascular events.
Yes. Statin therapy produces a small increase in the incidence of diabetes: one additional case per 255 patients taking statins over 4 years (strength of recommendation [SOR]: A, meta-analysis). Intensive statin therapy, compared with moderate therapy, produces an additional 2 cases of diabetes per 1000 patient years (SOR: B, meta-analysis with significant heterogeneity among trials).
EVIDENCE SUMMARY
A meta-analysis of 13 randomized, placebo or standard of care-controlled statin trials (113,148 patients, 81% without diabetes at enrollment, mean ages 55-76 years) found that statin therapy increased the incidence of diabetes by 9% over 4 years (odds ratio [OR]=1.09; 95% confidence interval [CI], 1.02-1.17), or one additional case per 255 patients.1 The increased risk was similar for lipophilic (pravastatin, rosuvastatin) and hydrophilic (atorvastatin, simvastatin, lovastatin) statins, although the analysis wasn’t adjusted for doses used.
In a meta-regression analysis, baseline body mass index or percentage change in low-density lipoprotein cholesterol didn’t appear to confer additional risk. The risk of diabetes with statins was generally higher in studies with older patients (data given graphically).
Higher statin doses mean higher risk
A meta-analysis of 5 placebo and standard-of-care randomized controlled trials (39,612 patients, 83% without diabetes at enrollment, mean age 58-64 years) found that the risk of diabetes was higher with higher-dose statins.2 Therapy with atorvastatin 80 mg or simvastatin 40 to 80 mg was defined as intensive. Treatment with simvastatin 20 to 40 mg, atorvastatin 10 mg, or pravastatin 40 mg was defined as moderate.
At a mean follow-up of 4.9 years, intensive statin therapy was associated with a higher risk of developing diabetes than moderate therapy (OR=1.12; 95% CI, 1.04-1.22) with 2 additional cases of diabetes per 1000 patient-years in the intensive therapy group. The authors noted significant heterogeneity between trials with regard to major cardiovascular events.
Similar results were found in a subsequent population-based cohort study of 471,250 nondiabetic patients older than 66 years who were newly prescribed a statin.3 The study authors used the incidence of new diabetes in patients taking pravastatin as the baseline, since it had been associated with reduced rates of diabetes in a large cardiovascular prevention trial.4 Without adjusting for dose, patients were at significantly higher risk of diabetes if prescribed atorvastatin (hazard ratio [HR]=1.22; 95% CI, 1.15-1.29), rosuvastatin (HR=1.18; 95% CI, 1.10-1.26), or simvastatin (HR=1.10; 95% CI, 1.04-1.17) compared with pravastatin. The risk with fluvastatin and lovastatin was similar to pravastatin.
A subanalysis that compared moderate- and high-dose statin therapy with low-dose therapy (atorvastatin <20 mg, rosuvastatin <10 mg, simvastatin <80 mg, or any dose of fluvastatin, lovastatin, or pravastatin) found a 22% increased risk of diabetes (HR=1.22; 95% CI, 1.19-1.26) for moderate-dose therapy (atorvastatin 20-79 mg, rosuvastatin 10-39 mg, or simvastatin >80 mg) and a 30% increased risk (HR=1.3; 95% CI, 1.2-1.4) for high-dose therapy (atorvastatin ≥80 mg or rosuvastatin ≥40 mg).
A cohort trial also shows increased diabetes risk
A smaller subsequent cohort trial based on data from Taiwan National Health Insurance records compared 8412 nondiabetic adult patients (mean age 63 years) taking statins with 33,648 age- and risk-matched controls not taking statins over a mean duration of 7.2 years.5 Statin use was associated with a 15% higher risk of developing diabetes (HR=1.15; 95% CI, 1.08-1.22).
RECOMMENDATIONS
The 2013 American College of Cardiology/American Heart Association guidelines for lipid-lowering therapy recommend that patients taking statins be screened for diabetes according to current screening recommendations.6 The guidelines advise encouraging patients who develop diabetes while on statin therapy to adhere to a heart-healthy dietary pattern, engage in physical activity, achieve and maintain a healthy body weight, cease tobacco use, and continue statin therapy to reduce the risk of cardiovascular events.
1. Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735-742.
2. Preiss D, Seshasai SR, Welsh P, et al. Risk of incident diabetes with intensive-dose compared with moderatedose statin therapy: a meta-analysis. JAMA. 2011;305:2556-2564.
3. Carter AA, Gomes T, Camacho X, et al. Risk of incident diabetes among patients treated with statins: population-based study. BMJ. 2013;346:f2610.
4. Freeman DJ, Morrie J, Sattar N, et al. Pravastatin and the development of diabetes mellitus: evidence for a protective treatment effect in the West of Scotland Coronary Prevention Study. Circulation. 2001;103:357-362.
5. Wang KL, Liu CJ, Chao TF, et al. Statins, risk of diabetes and implications on outcomes in the general population. J Am Coll Cardiol. 2012;60:1231-1238.
6. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1-S45.
1. Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735-742.
2. Preiss D, Seshasai SR, Welsh P, et al. Risk of incident diabetes with intensive-dose compared with moderatedose statin therapy: a meta-analysis. JAMA. 2011;305:2556-2564.
3. Carter AA, Gomes T, Camacho X, et al. Risk of incident diabetes among patients treated with statins: population-based study. BMJ. 2013;346:f2610.
4. Freeman DJ, Morrie J, Sattar N, et al. Pravastatin and the development of diabetes mellitus: evidence for a protective treatment effect in the West of Scotland Coronary Prevention Study. Circulation. 2001;103:357-362.
5. Wang KL, Liu CJ, Chao TF, et al. Statins, risk of diabetes and implications on outcomes in the general population. J Am Coll Cardiol. 2012;60:1231-1238.
6. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1-S45.
Evidence-based answers from the Family Physicians Inquiries Network
What therapies alleviate symptoms of polycystic ovary syndrome?
Treatment of polycystic ovary syndrome (PCOS) in women not actively seeking to become pregnant is symptom-specific. Lifestyle modification (LSM) reduces body weight by 3.5 kg (strength of recommendation [SOR]: A, meta-analysis) and metformin reduces it by 3 kg (SOR B, cohort trial).
LSM may be better tolerated; adding metformin to LSM doesn’t lead to additional weight loss (SOR: B, randomized controlled trial [RCT]).
Spironolactone improves hirsutism scores by an absolute 8% to 22% (SOR: A, multiple RCTs); adding metformin to spironolactone improves Ferriman-Gallwey (FG) hirsutism scores an additional absolute 1.4% (SOR: B, RCT). Oral contraceptive pills (OCPs) are 12 times more likely to result in complete menstrual regularity than metformin (SOR: A, meta-analysis). Combining OCPs with metformin improves hirsutism scores by 8% over using an OCP alone (SOR: A, meta-analysis).
Statin medications don’t alter weight, hirsutism, or menstruation (SOR: B, small meta-analysis).
EVIDENCE SUMMARY
Women with PCOS who are not seeking pregnancy commonly have symptoms such as excessive weight, hirsutism, and menstrual irregularities. This review focuses on interventions to manage those symptoms. The TABLE summarizes the results of the interventions.
Lifestyle modification improves symptoms; no benefit to adding metformin
A Cochrane meta-analysis of 6 RCTs with 164 patients compared LSM (with diet and exercise) and no or minimal intervention. LSM reduced weight more than minimal intervention (mean difference [MD]=-3.5 kg; 95% confidence interval [CI], -4.5 to -2.0).1 It also improved hirsutism, assessed with the 36-point FG score, where a lower score corresponds to less hirsutism (MD=-1.2 points, 95% CI, -2.4 to -0.1). No data were available on menstrual regularity.
A double-blind RCT comparing LSM alone with LSM plus metformin in 114 patients with PCOS found no difference in mean weight reduction (-2- to -3 kg, data from graph), ovulation rate, or androgen levels at 6 months.2 Six patients dropped out of the LSM-with-metformin group, whereas no patients dropped out of the LSM-alone group.
Metformin decreases BMI more than thiazolidinediones
In a meta-analysis of 10 RCTs (459 patients) comparing the effects of metformin and thiazolidinediones (TZDs), metformin reduced body mass index (BMI) more than TZDs at 3 months (weighted mean difference [WMD]=-2.5 kg/m2; 95% CI, -3.3 to -.6) and 6 months (WMD=-0.70 kg/m2; 95% CI, -0.76 to -0.65).3
In a prospective cohort dose-comparison study, 201 women with PCOS received either metformin 1000 mg or 1500 to 1700 mg daily for 6 months. Patients were asked not to modify their diet or exercise routines. In both dosage groups, patients lost weight from baseline (-3 kg; P<.01), and the number of menstrual cycles increased (0.7 per 6 months; P<.001).4 No clear dose-response relationship was observed.
Spironolactone can significantly reduce hirsutism
A systematic review identified 4 studies (132 patients) of antiandrogen therapy for hirsutism in PCOS. The 3 studies that used the FG score as an outcome all showed significant reductions in hirsutism after 6 to 12 months of treatment with spironolactone.5
A 6-month RCT of 198 patients with PCOS compared outcomes for spironolactone (50 mg/d), metformin (1000 mg/d), or both. Combined therapy was marginally better than either agent alone for reducing the FG score (end score for combined therapy 9.1 vs 9.6 for spironolactone and 9.7 for metformin, an absolute difference for combined therapy vs spironolactone of -0.5 FG points or -1.4%; P<.05).6
OCPs normalize menstrual cycles and reduce hirsutism
A Cochrane review evaluating the effects of OCPs on patients with PCOS included 4 RCTs (104 patients) that compared OCPs with metformin (1500-2000 mg/d) and 2 RCTs (70 patients) that compared the combination of an OCP and metformin with the OCP alone. Use of an OCP was much more likely to normalize menstrual cycling than metformin alone (2 trials, N=35; odds ratio [OR]=12; 95% CI, 2.2-100). Combining an OCP with metformin resulted in slightly better FG scores than an OCP alone (1 trial, N=40; WMD=-2.8 points; 95% CI, -5.4 to -0.17).7 There was no difference in the final BMI between patients taking an OCP alone, metformin alone, or both.
An RCT of 35 patients compared the effect on insulin levels of an OCP with rosiglitazone 4 mg/d and also looked at menstrual cycling as a secondary outcome. The study found no difference in effect on insulin levels in the 2 groups. All patients taking the OCP reported regular menstrual cycles at the end of the study compared with 75% of the patients taking rosiglitazone (P=.7).8 The study was underpowered to find a difference, however.
Statins alone don’t affect hirsutism, menstruation, or BMI
A Cochrane review identified 4 RCTs (244 women, ages 18-39 years) that compared a statin alone with placebo, another agent, or another agent plus a statin.9 One RCT of 48 patients found that a statin combined with an OCP improved hirsutism compared with a statin alone. Two RCTs (85 patients) found that statins didn’t lead to resumption of regular menstrual cycles. Statins also didn’t alter BMI in 3 studies of 105 patients.
Trials report no adverse effects, but VTE may be a concern with OCPs
A meta-analysis evaluated the safety of metformin, OCPs, and antiandrogens in 22 clinical trials with 1335 patients, primarily PCOS patients. The trials reported no cases of lactic acidosis with metformin, no drug-induced liver injury with antiandrogens, and no venous thromboembolism (VTE) with OCPs. The meta-analysis authors noted, however, that in a cohort trial of 1.6 million Danish women followed for 15 years, OCPs were associated with a 2- to 3-fold increase in risk of VTE, with higher risks linked to higher ethinyl estradiol content.10
RECOMMENDATIONS
A 2009 practice bulletin from The American College of Obestetrics and Gynecology (ACOG) recommends OCPs, progestin, metformin, and TZDs for anovulation and amenorrhea in patients with PCOS. OCPs, antiandrogens, metformin, eflornithine, and mechanical hair removal are recommended for hirsutism. ACOG advocates LSM, insulin-sensitizing agents (such as metformin), and statins to prevent cardiovascular disease and diabetes.11
1. Moran LJ, Hutchison SK, Norman RJ, et al. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst Rev. 2011;(2):CD007506.
2. Ladson G, Dodson WC, Sweet SD, et al. The effects of metformin with lifestyle therapy in polycystic ovary syndrome: a randomized double-blind study. Fertil Steril. 2011;95:1059-1066.
3. Li XJ, Yu YX, Liu CQ, et al. Metformin vs thiazolidinediones for treatment of clinical, hormonal and metabolic characteristics of polycystic ovary syndrome: a meta-analysis. Clin Endocrinol (Oxf). 2011;74:332-339.
4. Fulghesu AM, Romualdi D, Di Florio C, et al. Is there a doseresponse relationship of metformin treatment in patients with polycystic ovary syndrome? Results from a multicentric study. Hum Reprod. 2012; 27:3057-3066.
5. Christy NA, Franks AS, Cross LB. Spironolactone for hirsutism in polycystic ovary syndrome. Ann Pharmacother. 2005;39:1517-1521.
6. Ganie MA, Khurana ML, Nisar S, et al. Improved efficacy of low-dose spironolactone and metformin combination than either drug alone in the management of women with polycystic ovary syndrome (PCOS): a six-month, open-label randomized study. J Clin Endocrinol Metab. 2013;98:3599-3607.
7. Costello M, Shrestha B, Eden J, et al. Insulin-sensitising drugs versus the combined oral contraceptive pill for hirsutism, acne and risk of diabetes, cardiovascular disease, and endometrial cancer in polycystic ovary syndrome. Cochrane Database Syst Rev. 2007;(1):CD005552.
8. Tfayli H, Ulnach JW, Lee S, et al. Drospirenone/ethinyl estradiol versus rosiglitazone treatment in overweight adolescents with polycystic ovary syndrome: comparison of metabolic, hormonal, and cardiovascular risk factors. J Clin Endocrinol Metab. 2011;96:1311-1319.
9. Raval AD, Hunter T, Stuckey B, et al. Statins for women with polycystic ovary syndrome not actively trying to conceive. Cochrane Database Syst Rev. 2011;(10):CD008565.
10. Domecq JP, Prutsky G, Mullan RJ, et al. Adverse effects of the common treatments for polycystic ovary syndrome: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2013;98:4646-4654.
11. ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No. 108: Polycystic ovary syndrome. Obstet Gynecol. 2009;114:936-949.
Treatment of polycystic ovary syndrome (PCOS) in women not actively seeking to become pregnant is symptom-specific. Lifestyle modification (LSM) reduces body weight by 3.5 kg (strength of recommendation [SOR]: A, meta-analysis) and metformin reduces it by 3 kg (SOR B, cohort trial).
LSM may be better tolerated; adding metformin to LSM doesn’t lead to additional weight loss (SOR: B, randomized controlled trial [RCT]).
Spironolactone improves hirsutism scores by an absolute 8% to 22% (SOR: A, multiple RCTs); adding metformin to spironolactone improves Ferriman-Gallwey (FG) hirsutism scores an additional absolute 1.4% (SOR: B, RCT). Oral contraceptive pills (OCPs) are 12 times more likely to result in complete menstrual regularity than metformin (SOR: A, meta-analysis). Combining OCPs with metformin improves hirsutism scores by 8% over using an OCP alone (SOR: A, meta-analysis).
Statin medications don’t alter weight, hirsutism, or menstruation (SOR: B, small meta-analysis).
EVIDENCE SUMMARY
Women with PCOS who are not seeking pregnancy commonly have symptoms such as excessive weight, hirsutism, and menstrual irregularities. This review focuses on interventions to manage those symptoms. The TABLE summarizes the results of the interventions.
Lifestyle modification improves symptoms; no benefit to adding metformin
A Cochrane meta-analysis of 6 RCTs with 164 patients compared LSM (with diet and exercise) and no or minimal intervention. LSM reduced weight more than minimal intervention (mean difference [MD]=-3.5 kg; 95% confidence interval [CI], -4.5 to -2.0).1 It also improved hirsutism, assessed with the 36-point FG score, where a lower score corresponds to less hirsutism (MD=-1.2 points, 95% CI, -2.4 to -0.1). No data were available on menstrual regularity.
A double-blind RCT comparing LSM alone with LSM plus metformin in 114 patients with PCOS found no difference in mean weight reduction (-2- to -3 kg, data from graph), ovulation rate, or androgen levels at 6 months.2 Six patients dropped out of the LSM-with-metformin group, whereas no patients dropped out of the LSM-alone group.

Metformin decreases BMI more than thiazolidinediones
In a meta-analysis of 10 RCTs (459 patients) comparing the effects of metformin and thiazolidinediones (TZDs), metformin reduced body mass index (BMI) more than TZDs at 3 months (weighted mean difference [WMD]=-2.5 kg/m2; 95% CI, -3.3 to -.6) and 6 months (WMD=-0.70 kg/m2; 95% CI, -0.76 to -0.65).3
In a prospective cohort dose-comparison study, 201 women with PCOS received either metformin 1000 mg or 1500 to 1700 mg daily for 6 months. Patients were asked not to modify their diet or exercise routines. In both dosage groups, patients lost weight from baseline (-3 kg; P<.01), and the number of menstrual cycles increased (0.7 per 6 months; P<.001).4 No clear dose-response relationship was observed.
Spironolactone can significantly reduce hirsutism
A systematic review identified 4 studies (132 patients) of antiandrogen therapy for hirsutism in PCOS. The 3 studies that used the FG score as an outcome all showed significant reductions in hirsutism after 6 to 12 months of treatment with spironolactone.5
A 6-month RCT of 198 patients with PCOS compared outcomes for spironolactone (50 mg/d), metformin (1000 mg/d), or both. Combined therapy was marginally better than either agent alone for reducing the FG score (end score for combined therapy 9.1 vs 9.6 for spironolactone and 9.7 for metformin, an absolute difference for combined therapy vs spironolactone of -0.5 FG points or -1.4%; P<.05).6
OCPs normalize menstrual cycles and reduce hirsutism
A Cochrane review evaluating the effects of OCPs on patients with PCOS included 4 RCTs (104 patients) that compared OCPs with metformin (1500-2000 mg/d) and 2 RCTs (70 patients) that compared the combination of an OCP and metformin with the OCP alone. Use of an OCP was much more likely to normalize menstrual cycling than metformin alone (2 trials, N=35; odds ratio [OR]=12; 95% CI, 2.2-100). Combining an OCP with metformin resulted in slightly better FG scores than an OCP alone (1 trial, N=40; WMD=-2.8 points; 95% CI, -5.4 to -0.17).7 There was no difference in the final BMI between patients taking an OCP alone, metformin alone, or both.
An RCT of 35 patients compared the effect on insulin levels of an OCP with rosiglitazone 4 mg/d and also looked at menstrual cycling as a secondary outcome. The study found no difference in effect on insulin levels in the 2 groups. All patients taking the OCP reported regular menstrual cycles at the end of the study compared with 75% of the patients taking rosiglitazone (P=.7).8 The study was underpowered to find a difference, however.
Statins alone don’t affect hirsutism, menstruation, or BMI
A Cochrane review identified 4 RCTs (244 women, ages 18-39 years) that compared a statin alone with placebo, another agent, or another agent plus a statin.9 One RCT of 48 patients found that a statin combined with an OCP improved hirsutism compared with a statin alone. Two RCTs (85 patients) found that statins didn’t lead to resumption of regular menstrual cycles. Statins also didn’t alter BMI in 3 studies of 105 patients.
Trials report no adverse effects, but VTE may be a concern with OCPs
A meta-analysis evaluated the safety of metformin, OCPs, and antiandrogens in 22 clinical trials with 1335 patients, primarily PCOS patients. The trials reported no cases of lactic acidosis with metformin, no drug-induced liver injury with antiandrogens, and no venous thromboembolism (VTE) with OCPs. The meta-analysis authors noted, however, that in a cohort trial of 1.6 million Danish women followed for 15 years, OCPs were associated with a 2- to 3-fold increase in risk of VTE, with higher risks linked to higher ethinyl estradiol content.10
RECOMMENDATIONS
A 2009 practice bulletin from The American College of Obestetrics and Gynecology (ACOG) recommends OCPs, progestin, metformin, and TZDs for anovulation and amenorrhea in patients with PCOS. OCPs, antiandrogens, metformin, eflornithine, and mechanical hair removal are recommended for hirsutism. ACOG advocates LSM, insulin-sensitizing agents (such as metformin), and statins to prevent cardiovascular disease and diabetes.11
Treatment of polycystic ovary syndrome (PCOS) in women not actively seeking to become pregnant is symptom-specific. Lifestyle modification (LSM) reduces body weight by 3.5 kg (strength of recommendation [SOR]: A, meta-analysis) and metformin reduces it by 3 kg (SOR B, cohort trial).
LSM may be better tolerated; adding metformin to LSM doesn’t lead to additional weight loss (SOR: B, randomized controlled trial [RCT]).
Spironolactone improves hirsutism scores by an absolute 8% to 22% (SOR: A, multiple RCTs); adding metformin to spironolactone improves Ferriman-Gallwey (FG) hirsutism scores an additional absolute 1.4% (SOR: B, RCT). Oral contraceptive pills (OCPs) are 12 times more likely to result in complete menstrual regularity than metformin (SOR: A, meta-analysis). Combining OCPs with metformin improves hirsutism scores by 8% over using an OCP alone (SOR: A, meta-analysis).
Statin medications don’t alter weight, hirsutism, or menstruation (SOR: B, small meta-analysis).
EVIDENCE SUMMARY
Women with PCOS who are not seeking pregnancy commonly have symptoms such as excessive weight, hirsutism, and menstrual irregularities. This review focuses on interventions to manage those symptoms. The TABLE summarizes the results of the interventions.
Lifestyle modification improves symptoms; no benefit to adding metformin
A Cochrane meta-analysis of 6 RCTs with 164 patients compared LSM (with diet and exercise) and no or minimal intervention. LSM reduced weight more than minimal intervention (mean difference [MD]=-3.5 kg; 95% confidence interval [CI], -4.5 to -2.0).1 It also improved hirsutism, assessed with the 36-point FG score, where a lower score corresponds to less hirsutism (MD=-1.2 points, 95% CI, -2.4 to -0.1). No data were available on menstrual regularity.
A double-blind RCT comparing LSM alone with LSM plus metformin in 114 patients with PCOS found no difference in mean weight reduction (-2- to -3 kg, data from graph), ovulation rate, or androgen levels at 6 months.2 Six patients dropped out of the LSM-with-metformin group, whereas no patients dropped out of the LSM-alone group.

Metformin decreases BMI more than thiazolidinediones
In a meta-analysis of 10 RCTs (459 patients) comparing the effects of metformin and thiazolidinediones (TZDs), metformin reduced body mass index (BMI) more than TZDs at 3 months (weighted mean difference [WMD]=-2.5 kg/m2; 95% CI, -3.3 to -.6) and 6 months (WMD=-0.70 kg/m2; 95% CI, -0.76 to -0.65).3
In a prospective cohort dose-comparison study, 201 women with PCOS received either metformin 1000 mg or 1500 to 1700 mg daily for 6 months. Patients were asked not to modify their diet or exercise routines. In both dosage groups, patients lost weight from baseline (-3 kg; P<.01), and the number of menstrual cycles increased (0.7 per 6 months; P<.001).4 No clear dose-response relationship was observed.
Spironolactone can significantly reduce hirsutism
A systematic review identified 4 studies (132 patients) of antiandrogen therapy for hirsutism in PCOS. The 3 studies that used the FG score as an outcome all showed significant reductions in hirsutism after 6 to 12 months of treatment with spironolactone.5
A 6-month RCT of 198 patients with PCOS compared outcomes for spironolactone (50 mg/d), metformin (1000 mg/d), or both. Combined therapy was marginally better than either agent alone for reducing the FG score (end score for combined therapy 9.1 vs 9.6 for spironolactone and 9.7 for metformin, an absolute difference for combined therapy vs spironolactone of -0.5 FG points or -1.4%; P<.05).6
OCPs normalize menstrual cycles and reduce hirsutism
A Cochrane review evaluating the effects of OCPs on patients with PCOS included 4 RCTs (104 patients) that compared OCPs with metformin (1500-2000 mg/d) and 2 RCTs (70 patients) that compared the combination of an OCP and metformin with the OCP alone. Use of an OCP was much more likely to normalize menstrual cycling than metformin alone (2 trials, N=35; odds ratio [OR]=12; 95% CI, 2.2-100). Combining an OCP with metformin resulted in slightly better FG scores than an OCP alone (1 trial, N=40; WMD=-2.8 points; 95% CI, -5.4 to -0.17).7 There was no difference in the final BMI between patients taking an OCP alone, metformin alone, or both.
An RCT of 35 patients compared the effect on insulin levels of an OCP with rosiglitazone 4 mg/d and also looked at menstrual cycling as a secondary outcome. The study found no difference in effect on insulin levels in the 2 groups. All patients taking the OCP reported regular menstrual cycles at the end of the study compared with 75% of the patients taking rosiglitazone (P=.7).8 The study was underpowered to find a difference, however.
Statins alone don’t affect hirsutism, menstruation, or BMI
A Cochrane review identified 4 RCTs (244 women, ages 18-39 years) that compared a statin alone with placebo, another agent, or another agent plus a statin.9 One RCT of 48 patients found that a statin combined with an OCP improved hirsutism compared with a statin alone. Two RCTs (85 patients) found that statins didn’t lead to resumption of regular menstrual cycles. Statins also didn’t alter BMI in 3 studies of 105 patients.
Trials report no adverse effects, but VTE may be a concern with OCPs
A meta-analysis evaluated the safety of metformin, OCPs, and antiandrogens in 22 clinical trials with 1335 patients, primarily PCOS patients. The trials reported no cases of lactic acidosis with metformin, no drug-induced liver injury with antiandrogens, and no venous thromboembolism (VTE) with OCPs. The meta-analysis authors noted, however, that in a cohort trial of 1.6 million Danish women followed for 15 years, OCPs were associated with a 2- to 3-fold increase in risk of VTE, with higher risks linked to higher ethinyl estradiol content.10
RECOMMENDATIONS
A 2009 practice bulletin from The American College of Obestetrics and Gynecology (ACOG) recommends OCPs, progestin, metformin, and TZDs for anovulation and amenorrhea in patients with PCOS. OCPs, antiandrogens, metformin, eflornithine, and mechanical hair removal are recommended for hirsutism. ACOG advocates LSM, insulin-sensitizing agents (such as metformin), and statins to prevent cardiovascular disease and diabetes.11
1. Moran LJ, Hutchison SK, Norman RJ, et al. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst Rev. 2011;(2):CD007506.
2. Ladson G, Dodson WC, Sweet SD, et al. The effects of metformin with lifestyle therapy in polycystic ovary syndrome: a randomized double-blind study. Fertil Steril. 2011;95:1059-1066.
3. Li XJ, Yu YX, Liu CQ, et al. Metformin vs thiazolidinediones for treatment of clinical, hormonal and metabolic characteristics of polycystic ovary syndrome: a meta-analysis. Clin Endocrinol (Oxf). 2011;74:332-339.
4. Fulghesu AM, Romualdi D, Di Florio C, et al. Is there a doseresponse relationship of metformin treatment in patients with polycystic ovary syndrome? Results from a multicentric study. Hum Reprod. 2012; 27:3057-3066.
5. Christy NA, Franks AS, Cross LB. Spironolactone for hirsutism in polycystic ovary syndrome. Ann Pharmacother. 2005;39:1517-1521.
6. Ganie MA, Khurana ML, Nisar S, et al. Improved efficacy of low-dose spironolactone and metformin combination than either drug alone in the management of women with polycystic ovary syndrome (PCOS): a six-month, open-label randomized study. J Clin Endocrinol Metab. 2013;98:3599-3607.
7. Costello M, Shrestha B, Eden J, et al. Insulin-sensitising drugs versus the combined oral contraceptive pill for hirsutism, acne and risk of diabetes, cardiovascular disease, and endometrial cancer in polycystic ovary syndrome. Cochrane Database Syst Rev. 2007;(1):CD005552.
8. Tfayli H, Ulnach JW, Lee S, et al. Drospirenone/ethinyl estradiol versus rosiglitazone treatment in overweight adolescents with polycystic ovary syndrome: comparison of metabolic, hormonal, and cardiovascular risk factors. J Clin Endocrinol Metab. 2011;96:1311-1319.
9. Raval AD, Hunter T, Stuckey B, et al. Statins for women with polycystic ovary syndrome not actively trying to conceive. Cochrane Database Syst Rev. 2011;(10):CD008565.
10. Domecq JP, Prutsky G, Mullan RJ, et al. Adverse effects of the common treatments for polycystic ovary syndrome: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2013;98:4646-4654.
11. ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No. 108: Polycystic ovary syndrome. Obstet Gynecol. 2009;114:936-949.
1. Moran LJ, Hutchison SK, Norman RJ, et al. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst Rev. 2011;(2):CD007506.
2. Ladson G, Dodson WC, Sweet SD, et al. The effects of metformin with lifestyle therapy in polycystic ovary syndrome: a randomized double-blind study. Fertil Steril. 2011;95:1059-1066.
3. Li XJ, Yu YX, Liu CQ, et al. Metformin vs thiazolidinediones for treatment of clinical, hormonal and metabolic characteristics of polycystic ovary syndrome: a meta-analysis. Clin Endocrinol (Oxf). 2011;74:332-339.
4. Fulghesu AM, Romualdi D, Di Florio C, et al. Is there a doseresponse relationship of metformin treatment in patients with polycystic ovary syndrome? Results from a multicentric study. Hum Reprod. 2012; 27:3057-3066.
5. Christy NA, Franks AS, Cross LB. Spironolactone for hirsutism in polycystic ovary syndrome. Ann Pharmacother. 2005;39:1517-1521.
6. Ganie MA, Khurana ML, Nisar S, et al. Improved efficacy of low-dose spironolactone and metformin combination than either drug alone in the management of women with polycystic ovary syndrome (PCOS): a six-month, open-label randomized study. J Clin Endocrinol Metab. 2013;98:3599-3607.
7. Costello M, Shrestha B, Eden J, et al. Insulin-sensitising drugs versus the combined oral contraceptive pill for hirsutism, acne and risk of diabetes, cardiovascular disease, and endometrial cancer in polycystic ovary syndrome. Cochrane Database Syst Rev. 2007;(1):CD005552.
8. Tfayli H, Ulnach JW, Lee S, et al. Drospirenone/ethinyl estradiol versus rosiglitazone treatment in overweight adolescents with polycystic ovary syndrome: comparison of metabolic, hormonal, and cardiovascular risk factors. J Clin Endocrinol Metab. 2011;96:1311-1319.
9. Raval AD, Hunter T, Stuckey B, et al. Statins for women with polycystic ovary syndrome not actively trying to conceive. Cochrane Database Syst Rev. 2011;(10):CD008565.
10. Domecq JP, Prutsky G, Mullan RJ, et al. Adverse effects of the common treatments for polycystic ovary syndrome: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2013;98:4646-4654.
11. ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No. 108: Polycystic ovary syndrome. Obstet Gynecol. 2009;114:936-949.
Evidence-based answers from the Family Physicians Inquiries Network
How do hydrochlorothiazide and chlorthalidone compare for treating hypertension?
Both medications reduce theincidence of cardiovascular events in patients with hypertension, but chlorthalidone may confer additional cardiovascular risk reduction (strength of recommendation [SOR]: B, conflicting network meta-analysis and cohort studies). (No head-to-head studies of hydrochlorothiazide [HCTZ] and chlorthalidone have been done.)
Serious hypokalemia and hyponatremia can occur with either medication; it is unclear if the rates of these adverse effects are the same at equivalent doses. Patients taking chlorthalidone are less likely to need a second antihypertensive medication but more likely to be nonadherent than patients taking HCTZ (SOR: B, cohort studies).
EVIDENCE SUMMARY
A network meta-analysis—designed to compare 2 interventions that haven’t been studied head-to-head—examined 9 trials that evaluated cardiovascular outcomes in 18,000 patients taking HCTZ and 60,000 patients taking chlorthalidone against outcomes for placebo or other antihypertensive agents.1 Daily doses ranged from 12.5 to 25 mg for HCTZ and 12.5 to 100 mg for chlorthalidone (although most patients taking chlorthalidone were on 12.5-25 mg).
In a drug-adjusted analysis using shared comparator medications, chlorthalidone proved superior to HCTZ in reducing the risk of both heart failure (relative risk [RR]=0.77; 95% confidence interval [CI], 0.61-0.98) and combined cardiovascular events—myocardial infarction (MI), stroke, a new diagnosis of coronary artery disease, and new-onset congestive heart failure (RR=0.79; 95% CI, 0.72-0.88).
After adjusting for achieved blood pressure, chlorthalidone was still associated with lower rates of cardiovascular events than HCTZ (RR=0.82; 95% CI, 0.70-0.97). Relative to HCTZ, the number needed to treat with chlorthalidone to prevent 1 additional cardiovascular event over 5 years was 27. Because network meta-analyses draw from a wider body of research than standard meta-analyses, they may be weakened by increased variability in study design and patient demographics.
But another study shows no significant difference in cardiovascular outcomes
A subsequent retrospective cohort study didn’t find a significant difference in cardiovascular outcomes between HCTZ and chlorthalidone. The study compared pooled cardiovascular outcomes (MI, heart failure, and stroke) in 10,400 patients recently started on chlorthalidone and 19,500 started on HCTZ.2 Initial doses were typically either 25 mg chlorthalidone (70% of patients on chlorthalidone) or 12.5 mg HCTZ (67% of patients on HCTZ). The median follow-up was about a year, but lasted as long as 5 years in some cases.
The 2 groups showed no significant difference in cardiovascular events (3.2 events per 100 person-years for chlorthalidone compared with 3.4 for HCTZ; adjusted hazard ratio [aHR]=0.93; 95% CI, 0.81-1.06).
Serious hypokalemia and hyponatremia are risks
Patients taking chlorthalidone were more likely to be hospitalized for hypokalemia (0.69 per 100 person-years vs 0.27 for HCTZ; aHR=3.1; 95% CI, 2.0-4.6; number needed to harm [NNH]=238 in 1 year) or hyponatremia (0.69 per 100 person-years vs 0.49 for HCTZ; aHR=1.7; 95% CI, 1.2-2.3; NNH=434 in 1 year).2 However, the all-cause hospitalization rates for the 2 drugs were the same (aHR=1.0; 95% CI, 0.93-1.07).
Lower systolic BP and serum potassium found with chlorthalidone
A smaller retrospective cohort analysis (6441 participants who received either chlorthalidone or HCTZ starting at 50 mg and stepped once to 100 mg) also assessed the difference in cardiovascular events between patients taking the 2 drugs.3 (Cardiovascular events were defined as pooled MIs, onset of angina or peripheral artery occlusive disease, or need for coronary artery bypass.) Although significant reductions in pooled events occurred in both groups over the 7-year study, these reductions were significantly lower in the chlorthalidone group than in the HCTZ group (aHR=0.79; 95% CI, 0.68-0.92).
Systolic blood pressures were statistically lower in the chlorthalidone group during Years 1 through 5 but not in Years 6 and 7 (difference 2-4 mm Hg). Serum potassium was also lower in patients taking chlorthalidone (3.8 mEq/L on chlorthalidone vs 4.0 mEq/L on HCTZ after 7 years; P<.05).
Chlorthalidone users more responsive, but less adherent than HCTZ users
A retrospective cohort study investigated medication tolerance in veterans who had recently started either HCTZ (120,000 patients) or chlorthalidone (2200 patients) and were followed for a year.4 Most received doses between 12.5 and 25 mg of active drug.
One primary outcome was “nonpersistence,” defined as failure to refill the medication after double the number of days as the initial prescription. The other was “insufficient response,” defined as the need to start another antihypertensive medication. Chlorthalidone users were less likely than HCTZ users to have an insufficient response (odds ratio [OR]=0.71; 95% CI, 0.63-0.80) but more likely to exhibit nonpersistence (OR=1.6; 95% CI, 1.5-1.8).
RECOMMENDATIONS
For primary hypertension, the United Kingdom’s National Institute for Health and Care Excellence (NICE) recommends diuretic monotherapy in patients older than 55 years who are poor candidates for calcium channel blockers.5 If a diuretic is to be initiated or changed, NICE recommends chlorthalidone (12.5-25 mg daily) or indapamide (1.5-2.5 mg daily) in preference to HCTZ. The guideline set forth in the eighth annual report of the United States Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure makes no distinction between chlorthalidone and HCTZ; it refers only to “thiazidetype diuretics.” Thiazide-type diuretics are listed as one option (along with angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and calcium channel blockers) for initial monotherapy in nonblack patients.6
1. Roush GC, Holford TR, Guddati AK. Chlorthalidone compared with hydrochlorothiazide in reducing cardiovascular events: systematic review and network meta-analyses. Hypertension. 2012;59:1110–1117.
2. Dhalla IA, Gomes T, Yao Z, et al. Chlorthalidone versus hydrochlorothiazide for the treatment of hypertension in older adults: a population-based cohort study. Ann Intern Med. 2013;158:447–455.
3. Dorsh MP, Gillespie BW, Erickson SR, et al. Chlorthalidone reduces cardiovascular events compared with hydrochlorothiazide: a retrospective cohort analysis. Hypertension. 2011;57:689–694.
4. Lund BC, Ernst ME. The comparative effectiveness of hydrochlorothiazide and chlorthalidone in an observational cohort of veterans. J Clin Hypertension. 2012;14:623–629.
5. Hypertension: clinical management of primary hypertension in adults. (NICE Clinical Guideline 127). National Institute for Health and Care Excellence Web site. London, UK: National Institute for Health and Care Excellence; 2011. Available at: www.nice.org.UK/guidance/CG127. Accessed December 16, 2013.
6. James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC8). JAMA. 2014;311:507-520.
Both medications reduce theincidence of cardiovascular events in patients with hypertension, but chlorthalidone may confer additional cardiovascular risk reduction (strength of recommendation [SOR]: B, conflicting network meta-analysis and cohort studies). (No head-to-head studies of hydrochlorothiazide [HCTZ] and chlorthalidone have been done.)
Serious hypokalemia and hyponatremia can occur with either medication; it is unclear if the rates of these adverse effects are the same at equivalent doses. Patients taking chlorthalidone are less likely to need a second antihypertensive medication but more likely to be nonadherent than patients taking HCTZ (SOR: B, cohort studies).
EVIDENCE SUMMARY
A network meta-analysis—designed to compare 2 interventions that haven’t been studied head-to-head—examined 9 trials that evaluated cardiovascular outcomes in 18,000 patients taking HCTZ and 60,000 patients taking chlorthalidone against outcomes for placebo or other antihypertensive agents.1 Daily doses ranged from 12.5 to 25 mg for HCTZ and 12.5 to 100 mg for chlorthalidone (although most patients taking chlorthalidone were on 12.5-25 mg).
In a drug-adjusted analysis using shared comparator medications, chlorthalidone proved superior to HCTZ in reducing the risk of both heart failure (relative risk [RR]=0.77; 95% confidence interval [CI], 0.61-0.98) and combined cardiovascular events—myocardial infarction (MI), stroke, a new diagnosis of coronary artery disease, and new-onset congestive heart failure (RR=0.79; 95% CI, 0.72-0.88).
After adjusting for achieved blood pressure, chlorthalidone was still associated with lower rates of cardiovascular events than HCTZ (RR=0.82; 95% CI, 0.70-0.97). Relative to HCTZ, the number needed to treat with chlorthalidone to prevent 1 additional cardiovascular event over 5 years was 27. Because network meta-analyses draw from a wider body of research than standard meta-analyses, they may be weakened by increased variability in study design and patient demographics.
But another study shows no significant difference in cardiovascular outcomes
A subsequent retrospective cohort study didn’t find a significant difference in cardiovascular outcomes between HCTZ and chlorthalidone. The study compared pooled cardiovascular outcomes (MI, heart failure, and stroke) in 10,400 patients recently started on chlorthalidone and 19,500 started on HCTZ.2 Initial doses were typically either 25 mg chlorthalidone (70% of patients on chlorthalidone) or 12.5 mg HCTZ (67% of patients on HCTZ). The median follow-up was about a year, but lasted as long as 5 years in some cases.
The 2 groups showed no significant difference in cardiovascular events (3.2 events per 100 person-years for chlorthalidone compared with 3.4 for HCTZ; adjusted hazard ratio [aHR]=0.93; 95% CI, 0.81-1.06).
Serious hypokalemia and hyponatremia are risks
Patients taking chlorthalidone were more likely to be hospitalized for hypokalemia (0.69 per 100 person-years vs 0.27 for HCTZ; aHR=3.1; 95% CI, 2.0-4.6; number needed to harm [NNH]=238 in 1 year) or hyponatremia (0.69 per 100 person-years vs 0.49 for HCTZ; aHR=1.7; 95% CI, 1.2-2.3; NNH=434 in 1 year).2 However, the all-cause hospitalization rates for the 2 drugs were the same (aHR=1.0; 95% CI, 0.93-1.07).
Lower systolic BP and serum potassium found with chlorthalidone
A smaller retrospective cohort analysis (6441 participants who received either chlorthalidone or HCTZ starting at 50 mg and stepped once to 100 mg) also assessed the difference in cardiovascular events between patients taking the 2 drugs.3 (Cardiovascular events were defined as pooled MIs, onset of angina or peripheral artery occlusive disease, or need for coronary artery bypass.) Although significant reductions in pooled events occurred in both groups over the 7-year study, these reductions were significantly lower in the chlorthalidone group than in the HCTZ group (aHR=0.79; 95% CI, 0.68-0.92).
Systolic blood pressures were statistically lower in the chlorthalidone group during Years 1 through 5 but not in Years 6 and 7 (difference 2-4 mm Hg). Serum potassium was also lower in patients taking chlorthalidone (3.8 mEq/L on chlorthalidone vs 4.0 mEq/L on HCTZ after 7 years; P<.05).
Chlorthalidone users more responsive, but less adherent than HCTZ users
A retrospective cohort study investigated medication tolerance in veterans who had recently started either HCTZ (120,000 patients) or chlorthalidone (2200 patients) and were followed for a year.4 Most received doses between 12.5 and 25 mg of active drug.
One primary outcome was “nonpersistence,” defined as failure to refill the medication after double the number of days as the initial prescription. The other was “insufficient response,” defined as the need to start another antihypertensive medication. Chlorthalidone users were less likely than HCTZ users to have an insufficient response (odds ratio [OR]=0.71; 95% CI, 0.63-0.80) but more likely to exhibit nonpersistence (OR=1.6; 95% CI, 1.5-1.8).
RECOMMENDATIONS
For primary hypertension, the United Kingdom’s National Institute for Health and Care Excellence (NICE) recommends diuretic monotherapy in patients older than 55 years who are poor candidates for calcium channel blockers.5 If a diuretic is to be initiated or changed, NICE recommends chlorthalidone (12.5-25 mg daily) or indapamide (1.5-2.5 mg daily) in preference to HCTZ. The guideline set forth in the eighth annual report of the United States Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure makes no distinction between chlorthalidone and HCTZ; it refers only to “thiazidetype diuretics.” Thiazide-type diuretics are listed as one option (along with angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and calcium channel blockers) for initial monotherapy in nonblack patients.6
Both medications reduce theincidence of cardiovascular events in patients with hypertension, but chlorthalidone may confer additional cardiovascular risk reduction (strength of recommendation [SOR]: B, conflicting network meta-analysis and cohort studies). (No head-to-head studies of hydrochlorothiazide [HCTZ] and chlorthalidone have been done.)
Serious hypokalemia and hyponatremia can occur with either medication; it is unclear if the rates of these adverse effects are the same at equivalent doses. Patients taking chlorthalidone are less likely to need a second antihypertensive medication but more likely to be nonadherent than patients taking HCTZ (SOR: B, cohort studies).
EVIDENCE SUMMARY
A network meta-analysis—designed to compare 2 interventions that haven’t been studied head-to-head—examined 9 trials that evaluated cardiovascular outcomes in 18,000 patients taking HCTZ and 60,000 patients taking chlorthalidone against outcomes for placebo or other antihypertensive agents.1 Daily doses ranged from 12.5 to 25 mg for HCTZ and 12.5 to 100 mg for chlorthalidone (although most patients taking chlorthalidone were on 12.5-25 mg).
In a drug-adjusted analysis using shared comparator medications, chlorthalidone proved superior to HCTZ in reducing the risk of both heart failure (relative risk [RR]=0.77; 95% confidence interval [CI], 0.61-0.98) and combined cardiovascular events—myocardial infarction (MI), stroke, a new diagnosis of coronary artery disease, and new-onset congestive heart failure (RR=0.79; 95% CI, 0.72-0.88).
After adjusting for achieved blood pressure, chlorthalidone was still associated with lower rates of cardiovascular events than HCTZ (RR=0.82; 95% CI, 0.70-0.97). Relative to HCTZ, the number needed to treat with chlorthalidone to prevent 1 additional cardiovascular event over 5 years was 27. Because network meta-analyses draw from a wider body of research than standard meta-analyses, they may be weakened by increased variability in study design and patient demographics.
But another study shows no significant difference in cardiovascular outcomes
A subsequent retrospective cohort study didn’t find a significant difference in cardiovascular outcomes between HCTZ and chlorthalidone. The study compared pooled cardiovascular outcomes (MI, heart failure, and stroke) in 10,400 patients recently started on chlorthalidone and 19,500 started on HCTZ.2 Initial doses were typically either 25 mg chlorthalidone (70% of patients on chlorthalidone) or 12.5 mg HCTZ (67% of patients on HCTZ). The median follow-up was about a year, but lasted as long as 5 years in some cases.
The 2 groups showed no significant difference in cardiovascular events (3.2 events per 100 person-years for chlorthalidone compared with 3.4 for HCTZ; adjusted hazard ratio [aHR]=0.93; 95% CI, 0.81-1.06).
Serious hypokalemia and hyponatremia are risks
Patients taking chlorthalidone were more likely to be hospitalized for hypokalemia (0.69 per 100 person-years vs 0.27 for HCTZ; aHR=3.1; 95% CI, 2.0-4.6; number needed to harm [NNH]=238 in 1 year) or hyponatremia (0.69 per 100 person-years vs 0.49 for HCTZ; aHR=1.7; 95% CI, 1.2-2.3; NNH=434 in 1 year).2 However, the all-cause hospitalization rates for the 2 drugs were the same (aHR=1.0; 95% CI, 0.93-1.07).
Lower systolic BP and serum potassium found with chlorthalidone
A smaller retrospective cohort analysis (6441 participants who received either chlorthalidone or HCTZ starting at 50 mg and stepped once to 100 mg) also assessed the difference in cardiovascular events between patients taking the 2 drugs.3 (Cardiovascular events were defined as pooled MIs, onset of angina or peripheral artery occlusive disease, or need for coronary artery bypass.) Although significant reductions in pooled events occurred in both groups over the 7-year study, these reductions were significantly lower in the chlorthalidone group than in the HCTZ group (aHR=0.79; 95% CI, 0.68-0.92).
Systolic blood pressures were statistically lower in the chlorthalidone group during Years 1 through 5 but not in Years 6 and 7 (difference 2-4 mm Hg). Serum potassium was also lower in patients taking chlorthalidone (3.8 mEq/L on chlorthalidone vs 4.0 mEq/L on HCTZ after 7 years; P<.05).
Chlorthalidone users more responsive, but less adherent than HCTZ users
A retrospective cohort study investigated medication tolerance in veterans who had recently started either HCTZ (120,000 patients) or chlorthalidone (2200 patients) and were followed for a year.4 Most received doses between 12.5 and 25 mg of active drug.
One primary outcome was “nonpersistence,” defined as failure to refill the medication after double the number of days as the initial prescription. The other was “insufficient response,” defined as the need to start another antihypertensive medication. Chlorthalidone users were less likely than HCTZ users to have an insufficient response (odds ratio [OR]=0.71; 95% CI, 0.63-0.80) but more likely to exhibit nonpersistence (OR=1.6; 95% CI, 1.5-1.8).
RECOMMENDATIONS
For primary hypertension, the United Kingdom’s National Institute for Health and Care Excellence (NICE) recommends diuretic monotherapy in patients older than 55 years who are poor candidates for calcium channel blockers.5 If a diuretic is to be initiated or changed, NICE recommends chlorthalidone (12.5-25 mg daily) or indapamide (1.5-2.5 mg daily) in preference to HCTZ. The guideline set forth in the eighth annual report of the United States Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure makes no distinction between chlorthalidone and HCTZ; it refers only to “thiazidetype diuretics.” Thiazide-type diuretics are listed as one option (along with angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and calcium channel blockers) for initial monotherapy in nonblack patients.6
1. Roush GC, Holford TR, Guddati AK. Chlorthalidone compared with hydrochlorothiazide in reducing cardiovascular events: systematic review and network meta-analyses. Hypertension. 2012;59:1110–1117.
2. Dhalla IA, Gomes T, Yao Z, et al. Chlorthalidone versus hydrochlorothiazide for the treatment of hypertension in older adults: a population-based cohort study. Ann Intern Med. 2013;158:447–455.
3. Dorsh MP, Gillespie BW, Erickson SR, et al. Chlorthalidone reduces cardiovascular events compared with hydrochlorothiazide: a retrospective cohort analysis. Hypertension. 2011;57:689–694.
4. Lund BC, Ernst ME. The comparative effectiveness of hydrochlorothiazide and chlorthalidone in an observational cohort of veterans. J Clin Hypertension. 2012;14:623–629.
5. Hypertension: clinical management of primary hypertension in adults. (NICE Clinical Guideline 127). National Institute for Health and Care Excellence Web site. London, UK: National Institute for Health and Care Excellence; 2011. Available at: www.nice.org.UK/guidance/CG127. Accessed December 16, 2013.
6. James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC8). JAMA. 2014;311:507-520.
1. Roush GC, Holford TR, Guddati AK. Chlorthalidone compared with hydrochlorothiazide in reducing cardiovascular events: systematic review and network meta-analyses. Hypertension. 2012;59:1110–1117.
2. Dhalla IA, Gomes T, Yao Z, et al. Chlorthalidone versus hydrochlorothiazide for the treatment of hypertension in older adults: a population-based cohort study. Ann Intern Med. 2013;158:447–455.
3. Dorsh MP, Gillespie BW, Erickson SR, et al. Chlorthalidone reduces cardiovascular events compared with hydrochlorothiazide: a retrospective cohort analysis. Hypertension. 2011;57:689–694.
4. Lund BC, Ernst ME. The comparative effectiveness of hydrochlorothiazide and chlorthalidone in an observational cohort of veterans. J Clin Hypertension. 2012;14:623–629.
5. Hypertension: clinical management of primary hypertension in adults. (NICE Clinical Guideline 127). National Institute for Health and Care Excellence Web site. London, UK: National Institute for Health and Care Excellence; 2011. Available at: www.nice.org.UK/guidance/CG127. Accessed December 16, 2013.
6. James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC8). JAMA. 2014;311:507-520.
Evidence-based answers from the Family Physicians Inquiries Network
Do venlafaxine and gabapentin control hot flashes in women with a history of breast cancer?
YES. Venlafaxine reduces hot flashes more than placebo in women with a history of breast cancer; adverse effects include dry mouth and constipation (strength of recommendation [SOR]: B, randomized clinical trials [RCTs] with heterogeneous outcomes).
Gabapentin also reduces hot flashes more than placebo (SOR: B, a single RCT); adverse effects include dizziness and somnolence (SOR: C, standard reference). After having tried both medications, women tend to prefer venlafaxine (SOR: C, open-label crossover trial).
Treating hot flashes is an off-label use for both drugs.
Evidence summary
A double-blind RCT of 191 women who had been treated for breast cancer (two-thirds were taking tamoxifen) and were having at least 14 hot flashes per week randomized the women to one of 4 groups: once-daily extended-release venlafaxine at 37.5, 75, or 150 mg, or placebo.1 Higher doses of venlafaxine produced greater reductions in hot flash scores from baseline than did the lowest dose or placebo: 150 mg lowered scores by 61% (95% confidence interval [CI], 48%-75%); 75 mg also decreased scores by 61% (95% CI, 50%-68%); 37.5 mg reduced scores by 37% (95% CI, 26%-54%); and placebo lowered scores by 27% (95% CI, 11%-34%).
At the 75-and 150-mg doses, the number needed to treat to reduce the number of hot flashes by 50% was about 3. However, these doses caused dose-dependent adverse effects, including dry mouth, constipation, nausea, and decreased appetite, whereas the lowest dose produced an adverse-effect rate equal to placebo (no statistics supplied for these comparisons).
Venlafaxine significantly decreased the frequency of hot flashes
A pair of double-blind RCTs, reported together, evaluated hot flash frequency among 77 breast cancer survivors with at least 7 hot flashes a week who took either extended- release venlafaxine (37.5 or 75 mg daily) or placebo for 6 weeks.2 Patients then crossed over to take either venlafaxine or placebo for another 6 weeks. Compared with placebo, venlafaxine 37.5 mg reduced the mean daily hot flash frequency by 22% (P<.001); venlafaxine 75 mg reduced it by 14% (P<.013).
In an open-label case series, 40 breast cancer patients with at least 20 hot flashes per week received extended-release venlafaxine 37.5 mg once daily for 8 weeks.3 Patients reported a 53% reduction in hot flash frequency compared with baseline (P<.001).
Gabapentin reduces hot flashes at 300 mg tid
A double-blind RCT of 420 women with breast cancer who had at least 14 hot flashes a week compared the effectiveness of gabapentin 100 mg tid, 300 mg tid, or placebo for 8 weeks.4 Investigators found no significant difference between the 100 mg tid dosing and placebo. However, gabapentin at 300 mg tid reduced hot flashes by 44% (an average of 2 fewer per day) compared with placebo (P<.007).
The study didn’t assess adverse effects, but noted that the withdrawal rates were similar between the groups receiving gabapentin and placebo (12%-17%). An authoritative online reference lists the 2 most common adverse effects of gabapentin as dizziness (11%-28%) and somnolence (5.5%-25%).5
Women prefer venlafaxine to gabapentin
A multicenter, open-label crossover trial involving 66 women with a history of breast cancer who had at least 14 hot flashes per week evaluated patient preference when treated with extended-release venlafaxine 75 mg daily and gabapentin 300 mg tid, each for 1 month.6 More patients preferred taking venlafaxine than gabapentin (68% vs 32%; P<.01), although both treatments reduced the numbers of hot flashes.
Recommendations
In a patient education statement, the American Cancer Society states that women with premature menopause caused by cancer treatment may do well with exercise and relaxation techniques alone. The statement mentions venlafaxine, fluoxetine, and paroxetine as adjunct therapy.7
1. Loprinzi CL, Kugler JW, Sloan JA, et al. Venlafaxine in management of hot flashes in survivors of breast cancer: a randomised controlled trial. Lancet. 2000;356:2059-2063.
2. Carpenter JS, Storniolo AM, Johns S, et al. Randomized double-blind placebo-controlled crossover trial of venlafaxine for hot flashes after breast cancer. Oncologist. 2007;12:124-135.
3. Biglia N, Torta R, Roagna R, et al. Evaluation of low-dose venlafaxine for the therapy of hot flushes in breast cancer survivors. Maturitas. 2005;52:78-85.
4. Pandya KJ, Morrow GR, Roscoe JA, et al. Gabapentin for hot flashes in 420 women with breast cancer: a randomized placebo-controlled trial. Lancet. 2005;366:818-824.
5. Data for gabapentin side effects from MicroMedX. Available at: http://www.thomsonhc.com/micromedex2/librarian. Accessed May 16, 2013.
6. Bordeleau L, Pritchard KI, Loprinzi CL, et al. Multicenter, randomized, cross-over clinical trial of venlafaxine vs. gabapentin for the management of hot flashes in breast cancer survivors. J Clin Oncol. 2010;28:5147-5152.
7. American Cancer Society. Sexuality for the woman with cancer: premature menopause. Available at: www.cancer.org/Treatment/TreatmentsandSideEffects/PhysicalSideEffects/SexualSideEffectsinWomen/SexualityfortheWoman/sexuality-for-women-with-cancer-early-menopause. Accessed April 24, 2012.
YES. Venlafaxine reduces hot flashes more than placebo in women with a history of breast cancer; adverse effects include dry mouth and constipation (strength of recommendation [SOR]: B, randomized clinical trials [RCTs] with heterogeneous outcomes).
Gabapentin also reduces hot flashes more than placebo (SOR: B, a single RCT); adverse effects include dizziness and somnolence (SOR: C, standard reference). After having tried both medications, women tend to prefer venlafaxine (SOR: C, open-label crossover trial).
Treating hot flashes is an off-label use for both drugs.
Evidence summary
A double-blind RCT of 191 women who had been treated for breast cancer (two-thirds were taking tamoxifen) and were having at least 14 hot flashes per week randomized the women to one of 4 groups: once-daily extended-release venlafaxine at 37.5, 75, or 150 mg, or placebo.1 Higher doses of venlafaxine produced greater reductions in hot flash scores from baseline than did the lowest dose or placebo: 150 mg lowered scores by 61% (95% confidence interval [CI], 48%-75%); 75 mg also decreased scores by 61% (95% CI, 50%-68%); 37.5 mg reduced scores by 37% (95% CI, 26%-54%); and placebo lowered scores by 27% (95% CI, 11%-34%).
At the 75-and 150-mg doses, the number needed to treat to reduce the number of hot flashes by 50% was about 3. However, these doses caused dose-dependent adverse effects, including dry mouth, constipation, nausea, and decreased appetite, whereas the lowest dose produced an adverse-effect rate equal to placebo (no statistics supplied for these comparisons).
Venlafaxine significantly decreased the frequency of hot flashes
A pair of double-blind RCTs, reported together, evaluated hot flash frequency among 77 breast cancer survivors with at least 7 hot flashes a week who took either extended- release venlafaxine (37.5 or 75 mg daily) or placebo for 6 weeks.2 Patients then crossed over to take either venlafaxine or placebo for another 6 weeks. Compared with placebo, venlafaxine 37.5 mg reduced the mean daily hot flash frequency by 22% (P<.001); venlafaxine 75 mg reduced it by 14% (P<.013).
In an open-label case series, 40 breast cancer patients with at least 20 hot flashes per week received extended-release venlafaxine 37.5 mg once daily for 8 weeks.3 Patients reported a 53% reduction in hot flash frequency compared with baseline (P<.001).
Gabapentin reduces hot flashes at 300 mg tid
A double-blind RCT of 420 women with breast cancer who had at least 14 hot flashes a week compared the effectiveness of gabapentin 100 mg tid, 300 mg tid, or placebo for 8 weeks.4 Investigators found no significant difference between the 100 mg tid dosing and placebo. However, gabapentin at 300 mg tid reduced hot flashes by 44% (an average of 2 fewer per day) compared with placebo (P<.007).
The study didn’t assess adverse effects, but noted that the withdrawal rates were similar between the groups receiving gabapentin and placebo (12%-17%). An authoritative online reference lists the 2 most common adverse effects of gabapentin as dizziness (11%-28%) and somnolence (5.5%-25%).5
Women prefer venlafaxine to gabapentin
A multicenter, open-label crossover trial involving 66 women with a history of breast cancer who had at least 14 hot flashes per week evaluated patient preference when treated with extended-release venlafaxine 75 mg daily and gabapentin 300 mg tid, each for 1 month.6 More patients preferred taking venlafaxine than gabapentin (68% vs 32%; P<.01), although both treatments reduced the numbers of hot flashes.
Recommendations
In a patient education statement, the American Cancer Society states that women with premature menopause caused by cancer treatment may do well with exercise and relaxation techniques alone. The statement mentions venlafaxine, fluoxetine, and paroxetine as adjunct therapy.7
YES. Venlafaxine reduces hot flashes more than placebo in women with a history of breast cancer; adverse effects include dry mouth and constipation (strength of recommendation [SOR]: B, randomized clinical trials [RCTs] with heterogeneous outcomes).
Gabapentin also reduces hot flashes more than placebo (SOR: B, a single RCT); adverse effects include dizziness and somnolence (SOR: C, standard reference). After having tried both medications, women tend to prefer venlafaxine (SOR: C, open-label crossover trial).
Treating hot flashes is an off-label use for both drugs.
Evidence summary
A double-blind RCT of 191 women who had been treated for breast cancer (two-thirds were taking tamoxifen) and were having at least 14 hot flashes per week randomized the women to one of 4 groups: once-daily extended-release venlafaxine at 37.5, 75, or 150 mg, or placebo.1 Higher doses of venlafaxine produced greater reductions in hot flash scores from baseline than did the lowest dose or placebo: 150 mg lowered scores by 61% (95% confidence interval [CI], 48%-75%); 75 mg also decreased scores by 61% (95% CI, 50%-68%); 37.5 mg reduced scores by 37% (95% CI, 26%-54%); and placebo lowered scores by 27% (95% CI, 11%-34%).
At the 75-and 150-mg doses, the number needed to treat to reduce the number of hot flashes by 50% was about 3. However, these doses caused dose-dependent adverse effects, including dry mouth, constipation, nausea, and decreased appetite, whereas the lowest dose produced an adverse-effect rate equal to placebo (no statistics supplied for these comparisons).
Venlafaxine significantly decreased the frequency of hot flashes
A pair of double-blind RCTs, reported together, evaluated hot flash frequency among 77 breast cancer survivors with at least 7 hot flashes a week who took either extended- release venlafaxine (37.5 or 75 mg daily) or placebo for 6 weeks.2 Patients then crossed over to take either venlafaxine or placebo for another 6 weeks. Compared with placebo, venlafaxine 37.5 mg reduced the mean daily hot flash frequency by 22% (P<.001); venlafaxine 75 mg reduced it by 14% (P<.013).
In an open-label case series, 40 breast cancer patients with at least 20 hot flashes per week received extended-release venlafaxine 37.5 mg once daily for 8 weeks.3 Patients reported a 53% reduction in hot flash frequency compared with baseline (P<.001).
Gabapentin reduces hot flashes at 300 mg tid
A double-blind RCT of 420 women with breast cancer who had at least 14 hot flashes a week compared the effectiveness of gabapentin 100 mg tid, 300 mg tid, or placebo for 8 weeks.4 Investigators found no significant difference between the 100 mg tid dosing and placebo. However, gabapentin at 300 mg tid reduced hot flashes by 44% (an average of 2 fewer per day) compared with placebo (P<.007).
The study didn’t assess adverse effects, but noted that the withdrawal rates were similar between the groups receiving gabapentin and placebo (12%-17%). An authoritative online reference lists the 2 most common adverse effects of gabapentin as dizziness (11%-28%) and somnolence (5.5%-25%).5
Women prefer venlafaxine to gabapentin
A multicenter, open-label crossover trial involving 66 women with a history of breast cancer who had at least 14 hot flashes per week evaluated patient preference when treated with extended-release venlafaxine 75 mg daily and gabapentin 300 mg tid, each for 1 month.6 More patients preferred taking venlafaxine than gabapentin (68% vs 32%; P<.01), although both treatments reduced the numbers of hot flashes.
Recommendations
In a patient education statement, the American Cancer Society states that women with premature menopause caused by cancer treatment may do well with exercise and relaxation techniques alone. The statement mentions venlafaxine, fluoxetine, and paroxetine as adjunct therapy.7
1. Loprinzi CL, Kugler JW, Sloan JA, et al. Venlafaxine in management of hot flashes in survivors of breast cancer: a randomised controlled trial. Lancet. 2000;356:2059-2063.
2. Carpenter JS, Storniolo AM, Johns S, et al. Randomized double-blind placebo-controlled crossover trial of venlafaxine for hot flashes after breast cancer. Oncologist. 2007;12:124-135.
3. Biglia N, Torta R, Roagna R, et al. Evaluation of low-dose venlafaxine for the therapy of hot flushes in breast cancer survivors. Maturitas. 2005;52:78-85.
4. Pandya KJ, Morrow GR, Roscoe JA, et al. Gabapentin for hot flashes in 420 women with breast cancer: a randomized placebo-controlled trial. Lancet. 2005;366:818-824.
5. Data for gabapentin side effects from MicroMedX. Available at: http://www.thomsonhc.com/micromedex2/librarian. Accessed May 16, 2013.
6. Bordeleau L, Pritchard KI, Loprinzi CL, et al. Multicenter, randomized, cross-over clinical trial of venlafaxine vs. gabapentin for the management of hot flashes in breast cancer survivors. J Clin Oncol. 2010;28:5147-5152.
7. American Cancer Society. Sexuality for the woman with cancer: premature menopause. Available at: www.cancer.org/Treatment/TreatmentsandSideEffects/PhysicalSideEffects/SexualSideEffectsinWomen/SexualityfortheWoman/sexuality-for-women-with-cancer-early-menopause. Accessed April 24, 2012.
1. Loprinzi CL, Kugler JW, Sloan JA, et al. Venlafaxine in management of hot flashes in survivors of breast cancer: a randomised controlled trial. Lancet. 2000;356:2059-2063.
2. Carpenter JS, Storniolo AM, Johns S, et al. Randomized double-blind placebo-controlled crossover trial of venlafaxine for hot flashes after breast cancer. Oncologist. 2007;12:124-135.
3. Biglia N, Torta R, Roagna R, et al. Evaluation of low-dose venlafaxine for the therapy of hot flushes in breast cancer survivors. Maturitas. 2005;52:78-85.
4. Pandya KJ, Morrow GR, Roscoe JA, et al. Gabapentin for hot flashes in 420 women with breast cancer: a randomized placebo-controlled trial. Lancet. 2005;366:818-824.
5. Data for gabapentin side effects from MicroMedX. Available at: http://www.thomsonhc.com/micromedex2/librarian. Accessed May 16, 2013.
6. Bordeleau L, Pritchard KI, Loprinzi CL, et al. Multicenter, randomized, cross-over clinical trial of venlafaxine vs. gabapentin for the management of hot flashes in breast cancer survivors. J Clin Oncol. 2010;28:5147-5152.
7. American Cancer Society. Sexuality for the woman with cancer: premature menopause. Available at: www.cancer.org/Treatment/TreatmentsandSideEffects/PhysicalSideEffects/SexualSideEffectsinWomen/SexualityfortheWoman/sexuality-for-women-with-cancer-early-menopause. Accessed April 24, 2012.
Evidence-based answers from the Family Physicians Inquiries Network
Multivitamins for healthy children: What are the true benefits?
THE BENEFITS APPEAR TO BE LIMITED. It’s doubtful that multivitamin with mineral (MVM) supplementation improves IQ in healthy, low-risk children (strength of recommendation [SOR]: B, conflicting randomized clinical trials [RCTs]).
However, MVM supplementation decreased the incidence and severity of common infectious diseases among children in peri-urban India (SOR: B, RCT).
Multivitamin (MV) use doesn’t have consistently reported harms (SOR: C, conflicting cohort studies). An association between MV use and higher rates of asthma and food allergy has been reported, but studies conflict and any such effect is small.
Evidence summary
An RCT found that MVM supplementation for one academic year didn’t improve academic achievement more than placebo in 640 children, 8 to 12 years of age, from low-income urban families.1 Scores on the Terra Nova academic achievement test of reading, math, language, science, and social sciences didn’t differ between students taking MVM supplements or placebo.
Another RCT that compared MVM supplementation with placebo among 245 children between 6 and 12 years of age found no clinically significant improvements in IQ scores overall. However, within a small subset, more children who took MVM showed a clinical increase in IQ than children who took placebo.2
Investigators randomized children to daily MVM supplementation (50% of the US recommended daily allowance) or placebo for 3 months, then measured their Wechsler IQ scores. Overall, the MVM group scored 2.5 points higher (95% confidence interval [CI], 1.85-3.15) than the placebo group (a 15-point change is clinically significant).
More children taking MVM supplements (44) than placebo (25) showed increases in nonverbal IQ scores of 15 or more points (35% compared with 21%; P<.01). The authors speculate that this result may be attributable to the fact that one in 7 schoolchildren was undernourished. A major weakness of the study was its 16% attrition rate.
Fortified milk reduced disease in young children in India
A community-based, double-blind RCT found that milk fortified with vitamins A, C, and E plus minerals reduced common illnesses over the course of a year more than unfortified milk among 633 children 1 to 3 years of age living in a peri-urban area of India.3
Children who drank fortified milk had fewer days of fever (9.1 compared with 9.7 days for placebo; P=.005), a lower incidence of diarrhea (odds ratio [OR]=0.82; 95% CI, 0.73-0.93), and a decreased rate of lower respiratory illness (OR=0.74; 95% CI, 0.57-0.97). Children 2 years and younger showed the greatest effect.
Asthma and food allergies: The data are mixed
An inception cohort study found an association between early MV use and a higher risk of asthma and food allergies.4 Investigators evaluated more than 8000 American women and their newborns over the first 3 years of life. The study population included more families with low socioeconomic status (50%), blacks (51%), and infants born before 37 weeks’ gestation (23%) than the general US population.
Exclusively formula-fed infants who took MV in the first 6 months were more likely to develop asthma (OR=1.27; 95% CI, 1.04-1.56) and food allergies (OR=1.6; 95% CI, 1.2-2.2) than formula-fed infants who didn’t take MV.
However, a birth cohort study of 2470 Swedish children that analyzed health data from parental questionnaires and compared serum immunoglobulin E (IgE) concentrations at 8 years of age found no association between MV use within the past 12 months and clinical allergic disease or specific IgE concentrations.5 Children who took MV at age 4 years or earlier had lower rates of IgE sensitization to food allergens at 8 years (OR=0.61; 95% CI, 0.39-0.97).
Recommendations
According to the American Academy of Pediatrics Committee on Nutrition,6 healthy children who are growing normally and consume a varied diet don’t need routine supplementation with vitamins and minerals. The Committee states that if parents wish to give their children supplements, a standard pediatric multivitamin generally poses no risk.
1. Perlman AI, Worobey J, O‘Sullivan Maillet J, et al. Multivitamin/mineral supplementation does not affect standardized assessment of academic performance in elementary school children. J Am Diet Assoc. 2010;110:1089-1093.
2. Schoenthaler SJ, Bier ID, Young K, et al. The effect of vitamin-mineral supplementation on the intelligence of American schoolchildren: a randomized, double-blind, placebo-controlled trial. J Altern Complement Med. 2000;6:19-29.
3. Sazawal S, Dhingra U, Dhingra P, et al. Effects of fortified milk on morbidity in young children in north India: community based, randomised, double masked, placebo controlled trial. BMJ. 2007;334:140.-
4. Milner JD, Stein DM, McCarter R, et al. Early infant multivitamin supplementation is associated with increased risk for food allergy and asthma. Pediatrics. 2004;114:27-32.
5. Marmsjö K, Rosenlund H, Kull I, et al. Use of multivitamin supplements in relation to allergic disease in 8-year-old children. Am J Clin Nutr. 2009;90:1693-1698.
6. Committee on Nutrition, American Academy of Pediatrics. Feeding the child. In: Kleinman RE, ed. Pediatric Nutrition Handbook. 6th ed. Elk Grove Village, Ill: American Academy of Pediatrics; 2009:145–174.
THE BENEFITS APPEAR TO BE LIMITED. It’s doubtful that multivitamin with mineral (MVM) supplementation improves IQ in healthy, low-risk children (strength of recommendation [SOR]: B, conflicting randomized clinical trials [RCTs]).
However, MVM supplementation decreased the incidence and severity of common infectious diseases among children in peri-urban India (SOR: B, RCT).
Multivitamin (MV) use doesn’t have consistently reported harms (SOR: C, conflicting cohort studies). An association between MV use and higher rates of asthma and food allergy has been reported, but studies conflict and any such effect is small.
Evidence summary
An RCT found that MVM supplementation for one academic year didn’t improve academic achievement more than placebo in 640 children, 8 to 12 years of age, from low-income urban families.1 Scores on the Terra Nova academic achievement test of reading, math, language, science, and social sciences didn’t differ between students taking MVM supplements or placebo.
Another RCT that compared MVM supplementation with placebo among 245 children between 6 and 12 years of age found no clinically significant improvements in IQ scores overall. However, within a small subset, more children who took MVM showed a clinical increase in IQ than children who took placebo.2
Investigators randomized children to daily MVM supplementation (50% of the US recommended daily allowance) or placebo for 3 months, then measured their Wechsler IQ scores. Overall, the MVM group scored 2.5 points higher (95% confidence interval [CI], 1.85-3.15) than the placebo group (a 15-point change is clinically significant).
More children taking MVM supplements (44) than placebo (25) showed increases in nonverbal IQ scores of 15 or more points (35% compared with 21%; P<.01). The authors speculate that this result may be attributable to the fact that one in 7 schoolchildren was undernourished. A major weakness of the study was its 16% attrition rate.
Fortified milk reduced disease in young children in India
A community-based, double-blind RCT found that milk fortified with vitamins A, C, and E plus minerals reduced common illnesses over the course of a year more than unfortified milk among 633 children 1 to 3 years of age living in a peri-urban area of India.3
Children who drank fortified milk had fewer days of fever (9.1 compared with 9.7 days for placebo; P=.005), a lower incidence of diarrhea (odds ratio [OR]=0.82; 95% CI, 0.73-0.93), and a decreased rate of lower respiratory illness (OR=0.74; 95% CI, 0.57-0.97). Children 2 years and younger showed the greatest effect.
Asthma and food allergies: The data are mixed
An inception cohort study found an association between early MV use and a higher risk of asthma and food allergies.4 Investigators evaluated more than 8000 American women and their newborns over the first 3 years of life. The study population included more families with low socioeconomic status (50%), blacks (51%), and infants born before 37 weeks’ gestation (23%) than the general US population.
Exclusively formula-fed infants who took MV in the first 6 months were more likely to develop asthma (OR=1.27; 95% CI, 1.04-1.56) and food allergies (OR=1.6; 95% CI, 1.2-2.2) than formula-fed infants who didn’t take MV.
However, a birth cohort study of 2470 Swedish children that analyzed health data from parental questionnaires and compared serum immunoglobulin E (IgE) concentrations at 8 years of age found no association between MV use within the past 12 months and clinical allergic disease or specific IgE concentrations.5 Children who took MV at age 4 years or earlier had lower rates of IgE sensitization to food allergens at 8 years (OR=0.61; 95% CI, 0.39-0.97).
Recommendations
According to the American Academy of Pediatrics Committee on Nutrition,6 healthy children who are growing normally and consume a varied diet don’t need routine supplementation with vitamins and minerals. The Committee states that if parents wish to give their children supplements, a standard pediatric multivitamin generally poses no risk.
THE BENEFITS APPEAR TO BE LIMITED. It’s doubtful that multivitamin with mineral (MVM) supplementation improves IQ in healthy, low-risk children (strength of recommendation [SOR]: B, conflicting randomized clinical trials [RCTs]).
However, MVM supplementation decreased the incidence and severity of common infectious diseases among children in peri-urban India (SOR: B, RCT).
Multivitamin (MV) use doesn’t have consistently reported harms (SOR: C, conflicting cohort studies). An association between MV use and higher rates of asthma and food allergy has been reported, but studies conflict and any such effect is small.
Evidence summary
An RCT found that MVM supplementation for one academic year didn’t improve academic achievement more than placebo in 640 children, 8 to 12 years of age, from low-income urban families.1 Scores on the Terra Nova academic achievement test of reading, math, language, science, and social sciences didn’t differ between students taking MVM supplements or placebo.
Another RCT that compared MVM supplementation with placebo among 245 children between 6 and 12 years of age found no clinically significant improvements in IQ scores overall. However, within a small subset, more children who took MVM showed a clinical increase in IQ than children who took placebo.2
Investigators randomized children to daily MVM supplementation (50% of the US recommended daily allowance) or placebo for 3 months, then measured their Wechsler IQ scores. Overall, the MVM group scored 2.5 points higher (95% confidence interval [CI], 1.85-3.15) than the placebo group (a 15-point change is clinically significant).
More children taking MVM supplements (44) than placebo (25) showed increases in nonverbal IQ scores of 15 or more points (35% compared with 21%; P<.01). The authors speculate that this result may be attributable to the fact that one in 7 schoolchildren was undernourished. A major weakness of the study was its 16% attrition rate.
Fortified milk reduced disease in young children in India
A community-based, double-blind RCT found that milk fortified with vitamins A, C, and E plus minerals reduced common illnesses over the course of a year more than unfortified milk among 633 children 1 to 3 years of age living in a peri-urban area of India.3
Children who drank fortified milk had fewer days of fever (9.1 compared with 9.7 days for placebo; P=.005), a lower incidence of diarrhea (odds ratio [OR]=0.82; 95% CI, 0.73-0.93), and a decreased rate of lower respiratory illness (OR=0.74; 95% CI, 0.57-0.97). Children 2 years and younger showed the greatest effect.
Asthma and food allergies: The data are mixed
An inception cohort study found an association between early MV use and a higher risk of asthma and food allergies.4 Investigators evaluated more than 8000 American women and their newborns over the first 3 years of life. The study population included more families with low socioeconomic status (50%), blacks (51%), and infants born before 37 weeks’ gestation (23%) than the general US population.
Exclusively formula-fed infants who took MV in the first 6 months were more likely to develop asthma (OR=1.27; 95% CI, 1.04-1.56) and food allergies (OR=1.6; 95% CI, 1.2-2.2) than formula-fed infants who didn’t take MV.
However, a birth cohort study of 2470 Swedish children that analyzed health data from parental questionnaires and compared serum immunoglobulin E (IgE) concentrations at 8 years of age found no association between MV use within the past 12 months and clinical allergic disease or specific IgE concentrations.5 Children who took MV at age 4 years or earlier had lower rates of IgE sensitization to food allergens at 8 years (OR=0.61; 95% CI, 0.39-0.97).
Recommendations
According to the American Academy of Pediatrics Committee on Nutrition,6 healthy children who are growing normally and consume a varied diet don’t need routine supplementation with vitamins and minerals. The Committee states that if parents wish to give their children supplements, a standard pediatric multivitamin generally poses no risk.
1. Perlman AI, Worobey J, O‘Sullivan Maillet J, et al. Multivitamin/mineral supplementation does not affect standardized assessment of academic performance in elementary school children. J Am Diet Assoc. 2010;110:1089-1093.
2. Schoenthaler SJ, Bier ID, Young K, et al. The effect of vitamin-mineral supplementation on the intelligence of American schoolchildren: a randomized, double-blind, placebo-controlled trial. J Altern Complement Med. 2000;6:19-29.
3. Sazawal S, Dhingra U, Dhingra P, et al. Effects of fortified milk on morbidity in young children in north India: community based, randomised, double masked, placebo controlled trial. BMJ. 2007;334:140.-
4. Milner JD, Stein DM, McCarter R, et al. Early infant multivitamin supplementation is associated with increased risk for food allergy and asthma. Pediatrics. 2004;114:27-32.
5. Marmsjö K, Rosenlund H, Kull I, et al. Use of multivitamin supplements in relation to allergic disease in 8-year-old children. Am J Clin Nutr. 2009;90:1693-1698.
6. Committee on Nutrition, American Academy of Pediatrics. Feeding the child. In: Kleinman RE, ed. Pediatric Nutrition Handbook. 6th ed. Elk Grove Village, Ill: American Academy of Pediatrics; 2009:145–174.
1. Perlman AI, Worobey J, O‘Sullivan Maillet J, et al. Multivitamin/mineral supplementation does not affect standardized assessment of academic performance in elementary school children. J Am Diet Assoc. 2010;110:1089-1093.
2. Schoenthaler SJ, Bier ID, Young K, et al. The effect of vitamin-mineral supplementation on the intelligence of American schoolchildren: a randomized, double-blind, placebo-controlled trial. J Altern Complement Med. 2000;6:19-29.
3. Sazawal S, Dhingra U, Dhingra P, et al. Effects of fortified milk on morbidity in young children in north India: community based, randomised, double masked, placebo controlled trial. BMJ. 2007;334:140.-
4. Milner JD, Stein DM, McCarter R, et al. Early infant multivitamin supplementation is associated with increased risk for food allergy and asthma. Pediatrics. 2004;114:27-32.
5. Marmsjö K, Rosenlund H, Kull I, et al. Use of multivitamin supplements in relation to allergic disease in 8-year-old children. Am J Clin Nutr. 2009;90:1693-1698.
6. Committee on Nutrition, American Academy of Pediatrics. Feeding the child. In: Kleinman RE, ed. Pediatric Nutrition Handbook. 6th ed. Elk Grove Village, Ill: American Academy of Pediatrics; 2009:145–174.
Evidence-based answers from the Family Physicians Inquiries Network